User login
Nivolumab linked to CNS disorder in case report
Autoimmune encephalitis may be a potentially severe complication of immune checkpoint inhibitor therapy, a case report suggests.
The recently published report describes a 53-year-old man with B-cell non-Hodgkin lymphoma who presented with double vision, ataxia, impaired speech, and mild cognitive dysfunction following treatment with the immune checkpoint inhibitor nivolumab.
Neuropathologic examination of a biopsied brain lesion found on cranial MRI showed a T cell–dominated inflammatory process thought to be autoimmune in origin, according to Herwig Strik, MD, of the department of neurology at Philipps University of Marburg (Germany), and his colleagues (Eur J Cancer. 2017 Oct 16. doi: 10.1016/j.ejca.2017.09.026).
After the patient stopped taking nivolumab and the inflammatory process was treated, his “clinical neurological and radiological status remained stable but disabling with fluctuating dysarthria and ataxia,” Dr. Strik and his colleagues wrote.
“Since these novel anticancer agents are increasingly used, this severe complication should be recognized soon and treatment should be terminated to avoid chronification,” they said in the report.
Nivolumab and other checkpoint inhibitors are known to have autoimmune side effects in some cases that can affect the pulmonary, gastrointestinal, and endocrine systems, the authors said.
Several previous case reports have detailed encephalitis occurring in cancer patients receiving nivolumab, the combination of nivolumab plus the immune checkpoint inhibitor ipilimumab, or ipilimumab alone. The authors said they believe that this case report is the first to describe multifocal CNS inflammation following nivolumab treatment for systemic lymphoma.
The patient was diagnosed with B-cell non-Hodgkin lymphoma in 2005, according to the case report. He was first treated in 2009 with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone), followed by stem cell apheresis, radioimmunotherapy, and rituximab; he then received R-DHAP (rituximab, dexamethasone, high-dose cytarabine, and cisplatin) in August 2014, followed by autologous stem cell transplantation in October of that year. The patient started nivolumab maintenance therapy in February 2015 but started experiencing neurological symptoms that eventually led to ending nivolumab treatment in September 2015.
The patient’s lymphoma relapsed in June 2016. “The disabling neurological symptoms and his personal situation, however, worsened the patient’s depressive symptoms so severely that he went abroad to commit assisted suicide,” wrote Dr. Strik and his colleagues.
The authors proposed the term “immune checkpoint inhibitor–associated CNS autoimmune disorder (ICICAD)” to describe the inflammatory condition described in the case report.
They declared no conflicts of interest related to the case report and did not receive grant support for conducting the research described in it.
Autoimmune encephalitis may be a potentially severe complication of immune checkpoint inhibitor therapy, a case report suggests.
The recently published report describes a 53-year-old man with B-cell non-Hodgkin lymphoma who presented with double vision, ataxia, impaired speech, and mild cognitive dysfunction following treatment with the immune checkpoint inhibitor nivolumab.
Neuropathologic examination of a biopsied brain lesion found on cranial MRI showed a T cell–dominated inflammatory process thought to be autoimmune in origin, according to Herwig Strik, MD, of the department of neurology at Philipps University of Marburg (Germany), and his colleagues (Eur J Cancer. 2017 Oct 16. doi: 10.1016/j.ejca.2017.09.026).
After the patient stopped taking nivolumab and the inflammatory process was treated, his “clinical neurological and radiological status remained stable but disabling with fluctuating dysarthria and ataxia,” Dr. Strik and his colleagues wrote.
“Since these novel anticancer agents are increasingly used, this severe complication should be recognized soon and treatment should be terminated to avoid chronification,” they said in the report.
Nivolumab and other checkpoint inhibitors are known to have autoimmune side effects in some cases that can affect the pulmonary, gastrointestinal, and endocrine systems, the authors said.
Several previous case reports have detailed encephalitis occurring in cancer patients receiving nivolumab, the combination of nivolumab plus the immune checkpoint inhibitor ipilimumab, or ipilimumab alone. The authors said they believe that this case report is the first to describe multifocal CNS inflammation following nivolumab treatment for systemic lymphoma.
The patient was diagnosed with B-cell non-Hodgkin lymphoma in 2005, according to the case report. He was first treated in 2009 with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone), followed by stem cell apheresis, radioimmunotherapy, and rituximab; he then received R-DHAP (rituximab, dexamethasone, high-dose cytarabine, and cisplatin) in August 2014, followed by autologous stem cell transplantation in October of that year. The patient started nivolumab maintenance therapy in February 2015 but started experiencing neurological symptoms that eventually led to ending nivolumab treatment in September 2015.
The patient’s lymphoma relapsed in June 2016. “The disabling neurological symptoms and his personal situation, however, worsened the patient’s depressive symptoms so severely that he went abroad to commit assisted suicide,” wrote Dr. Strik and his colleagues.
The authors proposed the term “immune checkpoint inhibitor–associated CNS autoimmune disorder (ICICAD)” to describe the inflammatory condition described in the case report.
They declared no conflicts of interest related to the case report and did not receive grant support for conducting the research described in it.
Autoimmune encephalitis may be a potentially severe complication of immune checkpoint inhibitor therapy, a case report suggests.
The recently published report describes a 53-year-old man with B-cell non-Hodgkin lymphoma who presented with double vision, ataxia, impaired speech, and mild cognitive dysfunction following treatment with the immune checkpoint inhibitor nivolumab.
Neuropathologic examination of a biopsied brain lesion found on cranial MRI showed a T cell–dominated inflammatory process thought to be autoimmune in origin, according to Herwig Strik, MD, of the department of neurology at Philipps University of Marburg (Germany), and his colleagues (Eur J Cancer. 2017 Oct 16. doi: 10.1016/j.ejca.2017.09.026).
After the patient stopped taking nivolumab and the inflammatory process was treated, his “clinical neurological and radiological status remained stable but disabling with fluctuating dysarthria and ataxia,” Dr. Strik and his colleagues wrote.
“Since these novel anticancer agents are increasingly used, this severe complication should be recognized soon and treatment should be terminated to avoid chronification,” they said in the report.
Nivolumab and other checkpoint inhibitors are known to have autoimmune side effects in some cases that can affect the pulmonary, gastrointestinal, and endocrine systems, the authors said.
Several previous case reports have detailed encephalitis occurring in cancer patients receiving nivolumab, the combination of nivolumab plus the immune checkpoint inhibitor ipilimumab, or ipilimumab alone. The authors said they believe that this case report is the first to describe multifocal CNS inflammation following nivolumab treatment for systemic lymphoma.
The patient was diagnosed with B-cell non-Hodgkin lymphoma in 2005, according to the case report. He was first treated in 2009 with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone), followed by stem cell apheresis, radioimmunotherapy, and rituximab; he then received R-DHAP (rituximab, dexamethasone, high-dose cytarabine, and cisplatin) in August 2014, followed by autologous stem cell transplantation in October of that year. The patient started nivolumab maintenance therapy in February 2015 but started experiencing neurological symptoms that eventually led to ending nivolumab treatment in September 2015.
The patient’s lymphoma relapsed in June 2016. “The disabling neurological symptoms and his personal situation, however, worsened the patient’s depressive symptoms so severely that he went abroad to commit assisted suicide,” wrote Dr. Strik and his colleagues.
The authors proposed the term “immune checkpoint inhibitor–associated CNS autoimmune disorder (ICICAD)” to describe the inflammatory condition described in the case report.
They declared no conflicts of interest related to the case report and did not receive grant support for conducting the research described in it.
FROM THE EUROPEAN JOURNAL OF CANCER
Key clinical point: Autoimmune encephalitis may be a potential complication of checkpoint inhibitor therapy.
Major finding: A patient with B-cell non-Hodgkin lymphoma presented with double vision, ataxia, impaired speech, and mild cognitive dysfunction following treatment with nivolumab. Examination of a brain lesion showed a T cell–dominated inflammatory process thought to be autoimmune in origin.
Data source: A case report of a 53-year-old man with B-cell non-Hodgkin lymphoma (B-NHL) who received nivolumab maintenance treatment.
Disclosures: The authors declared no conflicts of interest and did not receive grant support for the research.
Atypical Disseminated Herpes Zoster: Management Guidelines in Immunocompromised Patients
Well-known for its typical presentation, classic herpes zoster (HZ) presents as a dermatomal eruption of painful erythematous papules that evolve into grouped vesicles or bullae.1,2 Thereafter, the lesions can become pustular or hemorrhagic.1 Although the diagnosis most often is made clinically, confirmatory techniques for diagnosis include viral culture, direct fluorescent antibody testing, or polymerase chain reaction (PCR) assay.1,3
The main risk factor for HZ is advanced age, most commonly affecting elderly patients.4 It is hypothesized that a physiological decline in varicella-zoster virus (VZV)–specific cell-mediated immunity among elderly individuals helps trigger reactivation of the virus within the dorsal root ganglion.1,5 Similarly affected are immunocompromised individuals, including those with human immunodeficiency virus (HIV) infection, due to suppression of T cells immune to VZV,1,5 as well as immunosuppressed transplant recipients who have diminished VZV-specific cellular responses and VZV IgG antibody avidity.6
Secondary complications of VZV infection (eg, postherpetic neuralgia, bacterial superinfection progressing to cellulitis) lead to increased morbidity.7,8 Disseminated cutaneous HZ is another grave complication of VZV infection and almost exclusively occurs with immunosuppression.1,8 It manifests as an eruption of at least 20 widespread vesiculobullous lesions outside the primary and adjacent dermatomes.6 Immunocompromised patients also are at increased risk for visceral involvement of VZV infection, which may affect vital organs such as the brain, liver, or lungs.7,8 Given the atypical presentation of VZV infection among some immunocompromised individuals, these patients are at increased risk for diagnostic delay and morbidity in the absence of high clinical suspicion for disseminated HZ.
Case Reports
Patient 1
A 52-year-old man developed a painless nonpruritic rash on the left leg of 4 days’ duration. It initially appeared as an erythematous maculopapular rash on the medial aspect of the left knee without any prodromal symptoms. Over the next 4 days, erythematous vesicles developed that progressed to pustules, and the rash spread both proximally and distally along the left leg. Shortly following hospital admission, he developed a fever (temperature, 38.4°C). His medical history included alcoholic liver cirrhosis and AIDS, with a CD4 count of 174 cells/µL (reference range, 500–1500 cells/µL). He had been taking antiretroviral therapy (abacavir-lamivudine and dolutegravir) and prophylaxis against opportunistic infections (dapsone and itraconazole).
Physical examination was remarkable for an extensive rash consisting of multiple 1-cm clusters of approximately 40 pustules each scattered in a nondermatomal distribution along the left leg (Figure 1). Many of the vesicles were confluent with an erythematous base and were in different stages of evolution with some crusted and others emanating a thin liquid exudate. The lesions were nontender and without notable induration. The leg was warm and edematous.
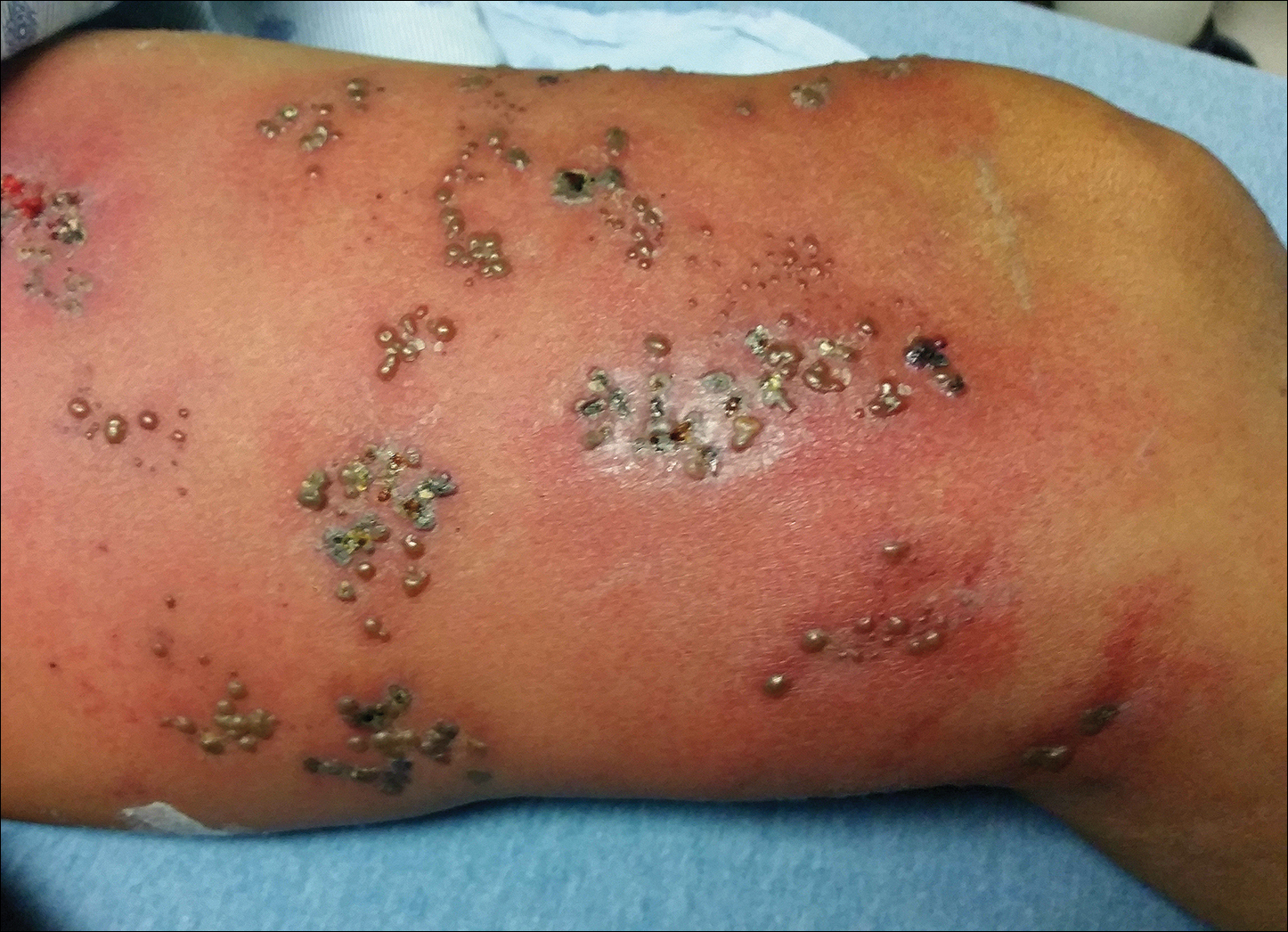
Clinically, the differential diagnosis included disseminated HZ with bacterial superinfection, Vibrio vulnificus infection, and herpes simplex virus (HSV) infection. The patient was treated with intravenous vancomycin, levofloxacin, and acyclovir, and no new lesions developed throughout the course of treatment. On this regimen, his fever resolved after 1 day, the active lesions began to crust, and the edema and erythema diminished. Results of bacterial cultures and plasma PCR and IgM for HSV types 1 and 2 were negative. Viral culture results were negative, but a PCR assay for VZV was positive, reflective of acute reactivation of VZV.
Patient 2
A 63-year-old man developed a pruritic burning rash involving the face, trunk, arms, and legs of 6 days’ duration. His medical history included a heart transplant 6 months prior to presentation, type 2 diabetes mellitus, and chronic kidney disease. He was taking antirejection therapy with mycophenolate mofetil (MMF), prednisone, and tacrolimus.
Physical examination was remarkable for an extensive rash consisting of clusters of 1- to 2-mm vesicles scattered in a nondermatomal pattern. Isolated vesicles involved the forehead, nose, and left ear, and diffuse vesicles with a relatively symmetric distribution were scattered across the back, chest, and proximal and distal arms and legs (Figure 2). Many of the vesicles had an associated overlying crust with hemorrhage. Some of the vesicles coalesced with central necrotic plaques.
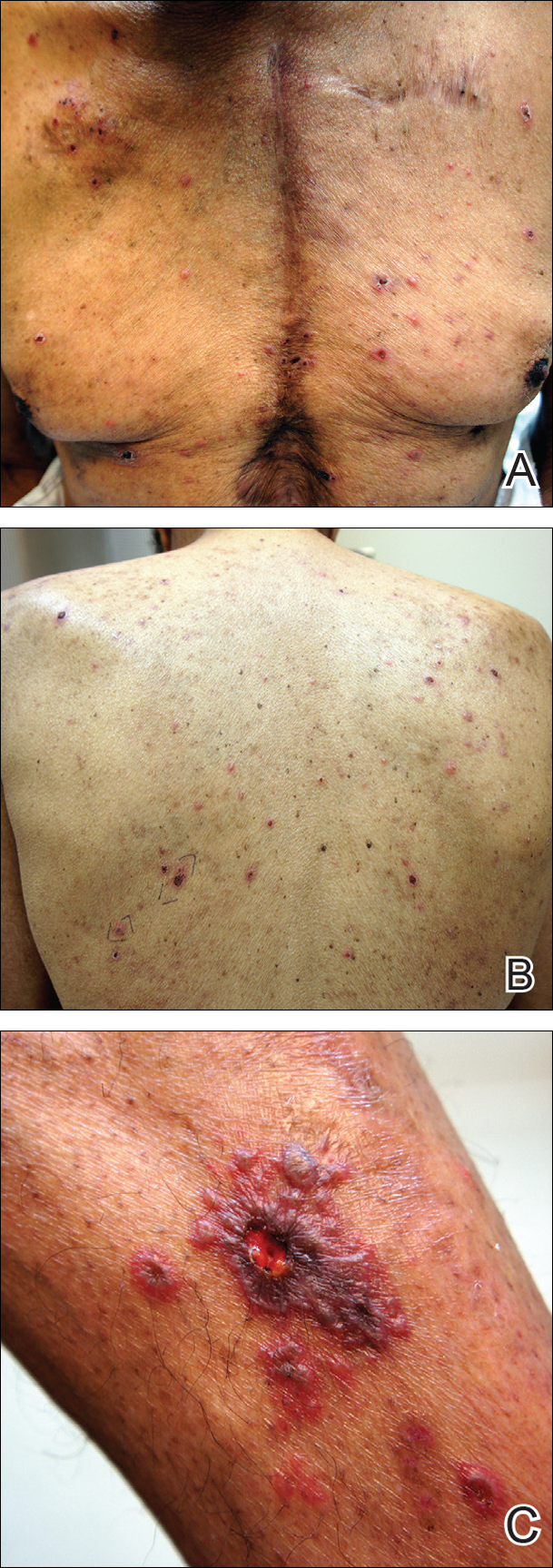
Given a clinical suspicion for disseminated HZ, therapy with oral valacyclovir was initiated. Two punch biopsies were consistent with herpesvirus cytopathic changes. Multiple sections demonstrated ulceration as well as acantholysis and necrosis of keratinocytes with multinucleation and margination of chromatin. There was an intense lichenoid and perivascular lymphocytic infiltrate in the dermis. Immunohistochemistry staining was positive for VZV and negative for HSV, indicating acute reactivation of VZV (Figure 3). Upon completion of an antiviral regimen, the patient returned to clinic with healed crusted lesions.
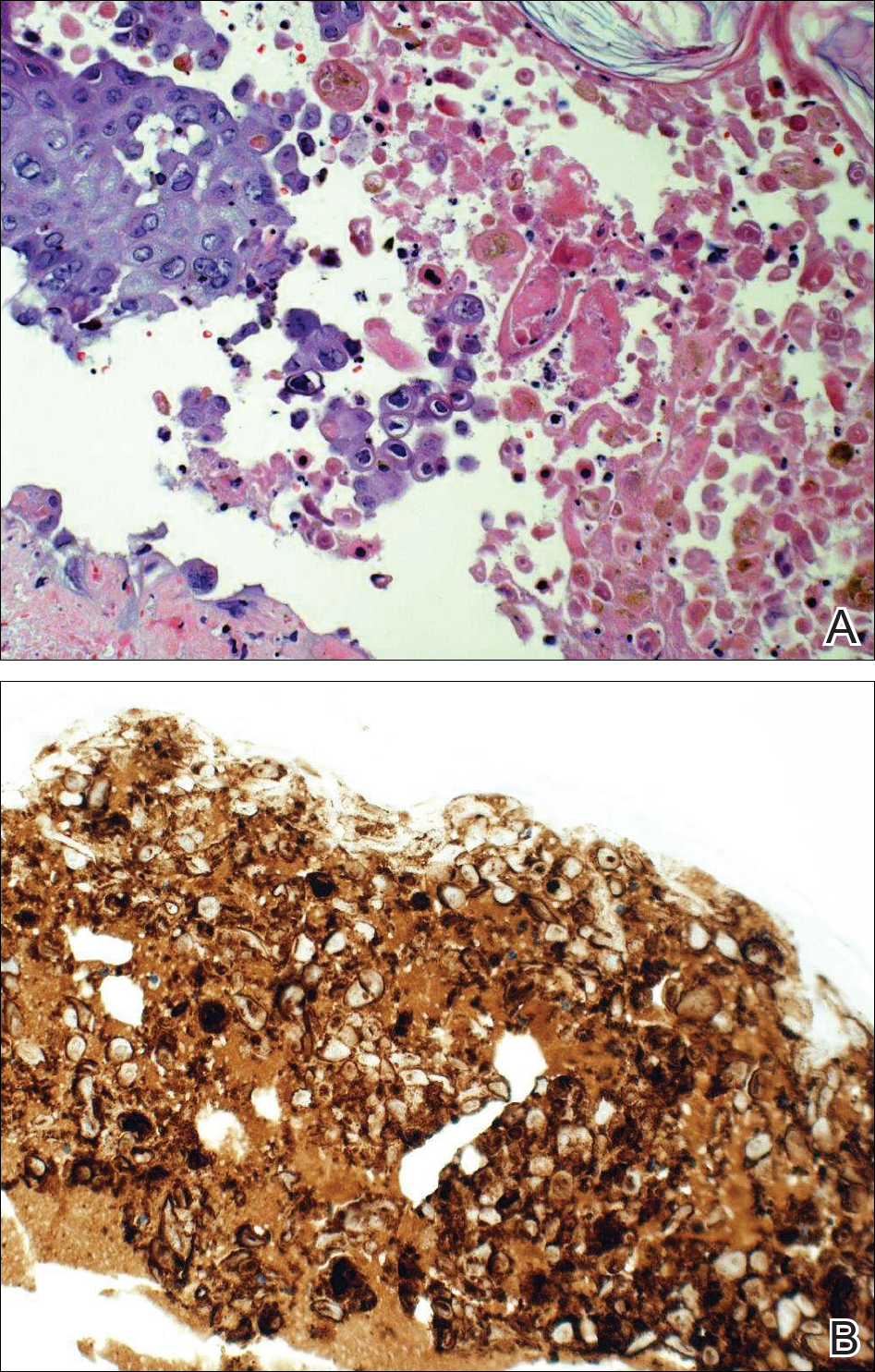
Comment
Frequently, the clinical features of HZ in immunocompromised patients mirror those in immunocompetent hosts.8 However, each of our 2 patients developed an unusual presentation of atypical generalized HZ.7 In this clinical variant, lesions develop along a single dermatome, then a diffuse vesicular eruption subsequently develops without dermatomal localization. These lesions can be chronic, persisting for months or years.7
The classic clinical presentation of HZ is distinct and often is readily diagnosed by visual inspection.7 However, atypical presentations and their associated complications can pose diagnostic and therapeutic challenges.7 Painless HZ lesions in a nondermatomal pattern were described in a patient who also had AIDS.9 Interestingly, multiple reports have found that patients with a severe but painless rash are less likely to have experienced a viral prodrome consisting of hyperesthesia, paresthesia, or pruritus.2,10 This observation suggests that lack of a prodrome, as in the case of patient 1 in our report, may aid in the recognition of painless HZ. Because of these atypical presentations, laboratory testing is even more important than in immunocompetent hosts, as diagnosis may be more difficult to establish on clinical presentation alone.
Several studies11-32 have evaluated modalities for treatment and prophylaxis for disseminated HZ in immunocompromised hosts, given its increased risk and potentially fatal complications in this population. The current guidelines in patients with HIV/AIDS, solid organ transplantation (SOT), and hematopoietic stem cell transplantation (HSCT) are summarized in the eTable.
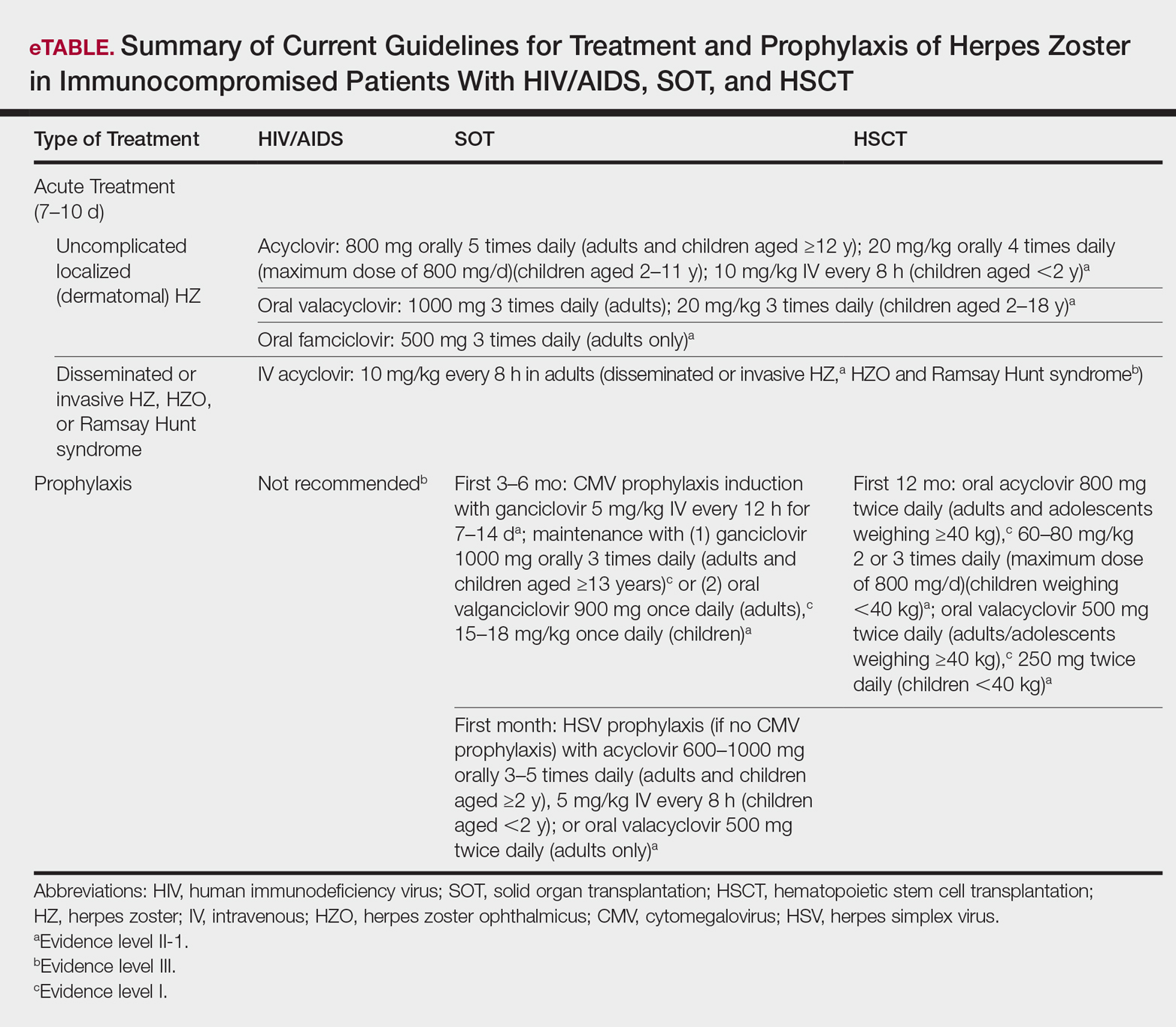
HIV/AIDS Patients
Given their efficacy and low rate of toxicity, oral acyclovir, valacyclovir, and famciclovir are recommended treatment options for HIV patients with localized, mild, dermatomal HZ.11 Two exceptions include HZ ophthalmicus and Ramsay Hunt syndrome for which some experts recommend intravenous acyclovir given the risk for vision loss and facial palsy, respectively. Intravenous acyclovir often is the drug of choice for treating complicated, disseminated, or severe HZ in HIV-infected patients, though prospective efficacy data remain limited.11
With regard to prevention of infection, a large randomized trial in 2016 found that acyclovir prophylaxis resulted in a 68% reduction in HZ over 2 years among HIV patients.12 Despite data that acyclovir may be effective for this purpose, long-term antiviral prophylaxis is not routinely recommended for HZ,11,13 as it has been linked to rare cases of acyclovir-resistant HZ in HIV patients.14,15 However, antiviral prophylaxis against HSV type 2 reactivation in HIV patients also confers protection against VZV reactivation.11,12
Solid Organ Transplantation
Localized, mild to moderately severe dermatomal HZ can be treated with oral acyclovir, valacyclovir, or famciclovir. As in HIV patients, SOT patients with severe, disseminated, or complicated HZ should receive IV acyclovir.11 In the first 3 to 6 months following the procedure, SOT patients receive cytomegalovirus prophylaxis with ganciclovir or valgan-ciclovir, which also provides protection against HZ.13-18 For patients not receiving cytomegalovirus prophylaxis, HSV prophylaxis with oral acyclovir or valacyclovir is given for at least the first month after transplantation, which also confers protection against HZ.16,19 Antiviral therapy is critical during the early posttransplantation period when patients are most severely immunosuppressed and thus have the highest risk for VZV-associated complications.20 Although immunosuppression is lifelong in most SOT recipients, there is insufficient evidence for extending prophylaxis beyond 6 months.16,21
As a possible risk factor for HZ,22 MMF use is another consideration among SOT patients, similar to patient 2 in our report. A 2003 observational study supported withdrawal of MMF therapy during active VZV infection due to clinical observation of an association with HZ.23 However, a multicenter, randomized, controlled trial reported no cases of HZ in renal transplant recipients on MMF.24 Additionally, MMF has been observed to enhance the antiviral activity of acyclovir, at least in vitro.25 Given the lack of evidence of MMF as a risk factor for HZ, there is insufficient evidence for cessation of use during VZVreactivation in SOT patients.
Hematopoietic Stem Cell Transplantation
The preferred agents for treatment of localized mild dermatomal HZ are oral acyclovir or valacyclovir, as data on the safety and efficacy of famciclovir among HSCT recipients are limited.13,26 Patients should receive antiviral prophylaxis with one of these agents during the first year following allogeneic or autologous HSCT. This 1-year course has proven highly effective in reducing HZ in the first year following transplantation when most severe cases occur,21,26-29 and it has been associated with a persistently decreased risk for HZ even after discontinuation.21 Prophylaxis may be continued beyond 1 year in allogeneic HSCT recipients experiencing graft-versus-host disease who should receive acyclovir until 6 months after the end of immunosuppressive therapy.21,26
Vaccination remains a potential strategy to reduce the incidence of HZ in this patient population. A heat-inactivated vaccine administered within the first 3 months after the procedure has been shown to be safe among autologous and allogeneic HSCT patients.30,31 The vaccine notably reduced the incidence of HZ in patients who underwent autologous HSCT,32 but no known data are available on its clinical efficacy in allogeneic HSCT patients. Accordingly, there are no known official recommendations to date regarding vaccine use in these patient populations.26
Conclusion
It is incumbent upon clinicians to recognize the spectrum of atypical presentations of HZ and maintain a low threshold for performing appropriate diagnostic or confirmatory studies among at-risk patients with impaired immune function. Disseminated HZ can have potentially life-threatening visceral complications such as encephalitis, hepatitis, or pneumonitis.7,8 As such, an understanding of prevention and treatment modalities for VZV infection among immunocompromised patients is critical. Because the morbidity associated with complications of VZV infection is substantial and the risks associated with antiviral agents are minimal, antiviral prophylaxis is recommended for 6 months following SOT or 1 year following HSCT, and prompt treatment is warranted in cases of reasonable clinical suspicion for HZ.
Acknowledgment
The authors gratefully acknowledge the generosity of our patients in permitting photography of their skin findings for the furthering of medical education.
- McCrary ML, Severson J, Tyring SK. Varicella zoster virus. J Am Acad Dermatol. 1999;41:1-16.
- Nagasako EM, Johnson RW, Griffin DR, et al. Rash severity in herpes zoster: correlates and relationship to postherpetic neuralgia. J Am Acad Dermatol. 2002;46:834-839.
- Leung J, Harpaz R, Baughman AL, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis. 2010;51:23-32.
- Herpes Zoster and Functional Decline Consortium. Functional decline and herpes zoster in older people: an interplay of multiple factors. Aging Clin Exp Res. 2015;27:757-765.
- Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341-357.
- Prelog M, Schonlaub J, Jeller V, et al. Reduced varicella-zoster-virus (VZV)-specific lymphocytes and IgG antibody avidity in solid organ transplant recipients. Vaccine. 2013;31:2420-2426.
- Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91-S98.
- Glesby MJ, Moore RD, Chaisson RE. Clinical spectrum of herpes zoster in adults infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:370-375.
- Blankenship W, Herchline T, Hockley A. Asymptomatic vesicles in a patient with the acquired immunodeficiency syndrome. disseminated varicella-zoster virus (VZV) infection. Arch Dermatol. 1994;130:1193, 1196.
- Katz J, Cooper EM, Walther RR, et al. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis. 2004;39:342-348.
- Gnann JW. Antiviral therapy of varicella-zoster virus infections. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al, eds. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge, United Kingdom: Cambridge University Press; 2007:1175-1191.
- Barnabas RV, Baeten JM, Lingappa JR, et al. Acyclovir prophylaxis reduces the incidence of herpes zoster among HIV-infected individuals: results of a randomized clinical trial. J Infect Dis. 2016;213:551-555.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
- Jacobson MA, Berger TG, Fikrig S, et al. Acyclovir-resistant varicella zoster virus infection after chronic oral acyclovir therapy in patients with the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1990;112:187-191.
- Linnemann CC Jr, Biron KK, Hoppenjans WG, et al. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS. 1990;4:577-579.
- Pergam SA, Limaye AP; AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV) in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S108-S115.
- Preiksaitis JK, Brennan DC, Fishman J, et al. Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am J Transplant. 2005;5:218-227.
- Fishman JA, Doran MT, Volpicelli SA, et al. Dosing of intravenous ganciclovir for the prophylaxis and treatment of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2000;69:389-394.
- Zuckerman R, Wald A; AST Infectious Diseases Community of Practice. Herpes simplex virus infections in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S104-S107.
- Arness T, Pedersen R, Dierkhising R, et al. Varicella zoster virus-associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis. 2008;10:260-268.
- Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007;110:3071-3077.
- Rothwell WS, Gloor JM, Morgenstern BZ, et al. Disseminated varicella infection in pediatric renal transplant recipients treated with mycophenolate mofetil. Transplantation. 1999;68:158-161.
- Lauzurica R, Bayés B, Frías C, et al. Disseminated varicella infection in adult renal allograft recipients: role of mycophenolate mofetil. Transplant Proc. 2003;35:1758-1759.
- A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. TheTricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation. 1996;61:1029-1037.
- Neyts J, De Clercq E. Mycophenolate mofetil strongly potentiates the anti-herpesvirus activity of acyclovir. Antiviral Res. 1998;40:53-56.
- Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143-1238.
- Boeckh M, Kim HW, Flowers ME, et al. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation—a randomized double-blind placebo-controlled study. Blood. 2006;107:1800-1805.
- Kawamura K, Hayakawa J, Akahoshi Y, et al. Low-dose acyclovir prophylaxis for the prevention of herpes simplex virus and varicella zoster virus diseases after autologous hematopoietic stem cell transplantation. Int J Hematol. 2015;102:230-237.
- Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance. Long-term follow-up after hematopoietic stem cell transplant general guidelines for referring physicians. Fred Hutchinson Cancer Research Center website. https://www.fredhutch.org/content/dam/public/Treatment-Suport/Long-Term-Follow-Up/physician.pdf. Published July 17, 2014. Accessed October 19, 2017.
- Kussmaul SC, Horn BN, Dvorak CC, et al. Safety of the live, attenuated varicella vaccine in pediatric recipients of hematopoietic SCTs. Bone Marrow Transplant. 2010;45:1602-1606.
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347:26-34.
- Issa NC, Marty FM, Leblebjian H, et al. Live attenuated varicella-zoster vaccine in hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2014;20:285-287.
Well-known for its typical presentation, classic herpes zoster (HZ) presents as a dermatomal eruption of painful erythematous papules that evolve into grouped vesicles or bullae.1,2 Thereafter, the lesions can become pustular or hemorrhagic.1 Although the diagnosis most often is made clinically, confirmatory techniques for diagnosis include viral culture, direct fluorescent antibody testing, or polymerase chain reaction (PCR) assay.1,3
The main risk factor for HZ is advanced age, most commonly affecting elderly patients.4 It is hypothesized that a physiological decline in varicella-zoster virus (VZV)–specific cell-mediated immunity among elderly individuals helps trigger reactivation of the virus within the dorsal root ganglion.1,5 Similarly affected are immunocompromised individuals, including those with human immunodeficiency virus (HIV) infection, due to suppression of T cells immune to VZV,1,5 as well as immunosuppressed transplant recipients who have diminished VZV-specific cellular responses and VZV IgG antibody avidity.6
Secondary complications of VZV infection (eg, postherpetic neuralgia, bacterial superinfection progressing to cellulitis) lead to increased morbidity.7,8 Disseminated cutaneous HZ is another grave complication of VZV infection and almost exclusively occurs with immunosuppression.1,8 It manifests as an eruption of at least 20 widespread vesiculobullous lesions outside the primary and adjacent dermatomes.6 Immunocompromised patients also are at increased risk for visceral involvement of VZV infection, which may affect vital organs such as the brain, liver, or lungs.7,8 Given the atypical presentation of VZV infection among some immunocompromised individuals, these patients are at increased risk for diagnostic delay and morbidity in the absence of high clinical suspicion for disseminated HZ.
Case Reports
Patient 1
A 52-year-old man developed a painless nonpruritic rash on the left leg of 4 days’ duration. It initially appeared as an erythematous maculopapular rash on the medial aspect of the left knee without any prodromal symptoms. Over the next 4 days, erythematous vesicles developed that progressed to pustules, and the rash spread both proximally and distally along the left leg. Shortly following hospital admission, he developed a fever (temperature, 38.4°C). His medical history included alcoholic liver cirrhosis and AIDS, with a CD4 count of 174 cells/µL (reference range, 500–1500 cells/µL). He had been taking antiretroviral therapy (abacavir-lamivudine and dolutegravir) and prophylaxis against opportunistic infections (dapsone and itraconazole).
Physical examination was remarkable for an extensive rash consisting of multiple 1-cm clusters of approximately 40 pustules each scattered in a nondermatomal distribution along the left leg (Figure 1). Many of the vesicles were confluent with an erythematous base and were in different stages of evolution with some crusted and others emanating a thin liquid exudate. The lesions were nontender and without notable induration. The leg was warm and edematous.

Clinically, the differential diagnosis included disseminated HZ with bacterial superinfection, Vibrio vulnificus infection, and herpes simplex virus (HSV) infection. The patient was treated with intravenous vancomycin, levofloxacin, and acyclovir, and no new lesions developed throughout the course of treatment. On this regimen, his fever resolved after 1 day, the active lesions began to crust, and the edema and erythema diminished. Results of bacterial cultures and plasma PCR and IgM for HSV types 1 and 2 were negative. Viral culture results were negative, but a PCR assay for VZV was positive, reflective of acute reactivation of VZV.
Patient 2
A 63-year-old man developed a pruritic burning rash involving the face, trunk, arms, and legs of 6 days’ duration. His medical history included a heart transplant 6 months prior to presentation, type 2 diabetes mellitus, and chronic kidney disease. He was taking antirejection therapy with mycophenolate mofetil (MMF), prednisone, and tacrolimus.
Physical examination was remarkable for an extensive rash consisting of clusters of 1- to 2-mm vesicles scattered in a nondermatomal pattern. Isolated vesicles involved the forehead, nose, and left ear, and diffuse vesicles with a relatively symmetric distribution were scattered across the back, chest, and proximal and distal arms and legs (Figure 2). Many of the vesicles had an associated overlying crust with hemorrhage. Some of the vesicles coalesced with central necrotic plaques.

Given a clinical suspicion for disseminated HZ, therapy with oral valacyclovir was initiated. Two punch biopsies were consistent with herpesvirus cytopathic changes. Multiple sections demonstrated ulceration as well as acantholysis and necrosis of keratinocytes with multinucleation and margination of chromatin. There was an intense lichenoid and perivascular lymphocytic infiltrate in the dermis. Immunohistochemistry staining was positive for VZV and negative for HSV, indicating acute reactivation of VZV (Figure 3). Upon completion of an antiviral regimen, the patient returned to clinic with healed crusted lesions.

Comment
Frequently, the clinical features of HZ in immunocompromised patients mirror those in immunocompetent hosts.8 However, each of our 2 patients developed an unusual presentation of atypical generalized HZ.7 In this clinical variant, lesions develop along a single dermatome, then a diffuse vesicular eruption subsequently develops without dermatomal localization. These lesions can be chronic, persisting for months or years.7
The classic clinical presentation of HZ is distinct and often is readily diagnosed by visual inspection.7 However, atypical presentations and their associated complications can pose diagnostic and therapeutic challenges.7 Painless HZ lesions in a nondermatomal pattern were described in a patient who also had AIDS.9 Interestingly, multiple reports have found that patients with a severe but painless rash are less likely to have experienced a viral prodrome consisting of hyperesthesia, paresthesia, or pruritus.2,10 This observation suggests that lack of a prodrome, as in the case of patient 1 in our report, may aid in the recognition of painless HZ. Because of these atypical presentations, laboratory testing is even more important than in immunocompetent hosts, as diagnosis may be more difficult to establish on clinical presentation alone.
Several studies11-32 have evaluated modalities for treatment and prophylaxis for disseminated HZ in immunocompromised hosts, given its increased risk and potentially fatal complications in this population. The current guidelines in patients with HIV/AIDS, solid organ transplantation (SOT), and hematopoietic stem cell transplantation (HSCT) are summarized in the eTable.

HIV/AIDS Patients
Given their efficacy and low rate of toxicity, oral acyclovir, valacyclovir, and famciclovir are recommended treatment options for HIV patients with localized, mild, dermatomal HZ.11 Two exceptions include HZ ophthalmicus and Ramsay Hunt syndrome for which some experts recommend intravenous acyclovir given the risk for vision loss and facial palsy, respectively. Intravenous acyclovir often is the drug of choice for treating complicated, disseminated, or severe HZ in HIV-infected patients, though prospective efficacy data remain limited.11
With regard to prevention of infection, a large randomized trial in 2016 found that acyclovir prophylaxis resulted in a 68% reduction in HZ over 2 years among HIV patients.12 Despite data that acyclovir may be effective for this purpose, long-term antiviral prophylaxis is not routinely recommended for HZ,11,13 as it has been linked to rare cases of acyclovir-resistant HZ in HIV patients.14,15 However, antiviral prophylaxis against HSV type 2 reactivation in HIV patients also confers protection against VZV reactivation.11,12
Solid Organ Transplantation
Localized, mild to moderately severe dermatomal HZ can be treated with oral acyclovir, valacyclovir, or famciclovir. As in HIV patients, SOT patients with severe, disseminated, or complicated HZ should receive IV acyclovir.11 In the first 3 to 6 months following the procedure, SOT patients receive cytomegalovirus prophylaxis with ganciclovir or valgan-ciclovir, which also provides protection against HZ.13-18 For patients not receiving cytomegalovirus prophylaxis, HSV prophylaxis with oral acyclovir or valacyclovir is given for at least the first month after transplantation, which also confers protection against HZ.16,19 Antiviral therapy is critical during the early posttransplantation period when patients are most severely immunosuppressed and thus have the highest risk for VZV-associated complications.20 Although immunosuppression is lifelong in most SOT recipients, there is insufficient evidence for extending prophylaxis beyond 6 months.16,21
As a possible risk factor for HZ,22 MMF use is another consideration among SOT patients, similar to patient 2 in our report. A 2003 observational study supported withdrawal of MMF therapy during active VZV infection due to clinical observation of an association with HZ.23 However, a multicenter, randomized, controlled trial reported no cases of HZ in renal transplant recipients on MMF.24 Additionally, MMF has been observed to enhance the antiviral activity of acyclovir, at least in vitro.25 Given the lack of evidence of MMF as a risk factor for HZ, there is insufficient evidence for cessation of use during VZVreactivation in SOT patients.
Hematopoietic Stem Cell Transplantation
The preferred agents for treatment of localized mild dermatomal HZ are oral acyclovir or valacyclovir, as data on the safety and efficacy of famciclovir among HSCT recipients are limited.13,26 Patients should receive antiviral prophylaxis with one of these agents during the first year following allogeneic or autologous HSCT. This 1-year course has proven highly effective in reducing HZ in the first year following transplantation when most severe cases occur,21,26-29 and it has been associated with a persistently decreased risk for HZ even after discontinuation.21 Prophylaxis may be continued beyond 1 year in allogeneic HSCT recipients experiencing graft-versus-host disease who should receive acyclovir until 6 months after the end of immunosuppressive therapy.21,26
Vaccination remains a potential strategy to reduce the incidence of HZ in this patient population. A heat-inactivated vaccine administered within the first 3 months after the procedure has been shown to be safe among autologous and allogeneic HSCT patients.30,31 The vaccine notably reduced the incidence of HZ in patients who underwent autologous HSCT,32 but no known data are available on its clinical efficacy in allogeneic HSCT patients. Accordingly, there are no known official recommendations to date regarding vaccine use in these patient populations.26
Conclusion
It is incumbent upon clinicians to recognize the spectrum of atypical presentations of HZ and maintain a low threshold for performing appropriate diagnostic or confirmatory studies among at-risk patients with impaired immune function. Disseminated HZ can have potentially life-threatening visceral complications such as encephalitis, hepatitis, or pneumonitis.7,8 As such, an understanding of prevention and treatment modalities for VZV infection among immunocompromised patients is critical. Because the morbidity associated with complications of VZV infection is substantial and the risks associated with antiviral agents are minimal, antiviral prophylaxis is recommended for 6 months following SOT or 1 year following HSCT, and prompt treatment is warranted in cases of reasonable clinical suspicion for HZ.
Acknowledgment
The authors gratefully acknowledge the generosity of our patients in permitting photography of their skin findings for the furthering of medical education.
Well-known for its typical presentation, classic herpes zoster (HZ) presents as a dermatomal eruption of painful erythematous papules that evolve into grouped vesicles or bullae.1,2 Thereafter, the lesions can become pustular or hemorrhagic.1 Although the diagnosis most often is made clinically, confirmatory techniques for diagnosis include viral culture, direct fluorescent antibody testing, or polymerase chain reaction (PCR) assay.1,3
The main risk factor for HZ is advanced age, most commonly affecting elderly patients.4 It is hypothesized that a physiological decline in varicella-zoster virus (VZV)–specific cell-mediated immunity among elderly individuals helps trigger reactivation of the virus within the dorsal root ganglion.1,5 Similarly affected are immunocompromised individuals, including those with human immunodeficiency virus (HIV) infection, due to suppression of T cells immune to VZV,1,5 as well as immunosuppressed transplant recipients who have diminished VZV-specific cellular responses and VZV IgG antibody avidity.6
Secondary complications of VZV infection (eg, postherpetic neuralgia, bacterial superinfection progressing to cellulitis) lead to increased morbidity.7,8 Disseminated cutaneous HZ is another grave complication of VZV infection and almost exclusively occurs with immunosuppression.1,8 It manifests as an eruption of at least 20 widespread vesiculobullous lesions outside the primary and adjacent dermatomes.6 Immunocompromised patients also are at increased risk for visceral involvement of VZV infection, which may affect vital organs such as the brain, liver, or lungs.7,8 Given the atypical presentation of VZV infection among some immunocompromised individuals, these patients are at increased risk for diagnostic delay and morbidity in the absence of high clinical suspicion for disseminated HZ.
Case Reports
Patient 1
A 52-year-old man developed a painless nonpruritic rash on the left leg of 4 days’ duration. It initially appeared as an erythematous maculopapular rash on the medial aspect of the left knee without any prodromal symptoms. Over the next 4 days, erythematous vesicles developed that progressed to pustules, and the rash spread both proximally and distally along the left leg. Shortly following hospital admission, he developed a fever (temperature, 38.4°C). His medical history included alcoholic liver cirrhosis and AIDS, with a CD4 count of 174 cells/µL (reference range, 500–1500 cells/µL). He had been taking antiretroviral therapy (abacavir-lamivudine and dolutegravir) and prophylaxis against opportunistic infections (dapsone and itraconazole).
Physical examination was remarkable for an extensive rash consisting of multiple 1-cm clusters of approximately 40 pustules each scattered in a nondermatomal distribution along the left leg (Figure 1). Many of the vesicles were confluent with an erythematous base and were in different stages of evolution with some crusted and others emanating a thin liquid exudate. The lesions were nontender and without notable induration. The leg was warm and edematous.

Clinically, the differential diagnosis included disseminated HZ with bacterial superinfection, Vibrio vulnificus infection, and herpes simplex virus (HSV) infection. The patient was treated with intravenous vancomycin, levofloxacin, and acyclovir, and no new lesions developed throughout the course of treatment. On this regimen, his fever resolved after 1 day, the active lesions began to crust, and the edema and erythema diminished. Results of bacterial cultures and plasma PCR and IgM for HSV types 1 and 2 were negative. Viral culture results were negative, but a PCR assay for VZV was positive, reflective of acute reactivation of VZV.
Patient 2
A 63-year-old man developed a pruritic burning rash involving the face, trunk, arms, and legs of 6 days’ duration. His medical history included a heart transplant 6 months prior to presentation, type 2 diabetes mellitus, and chronic kidney disease. He was taking antirejection therapy with mycophenolate mofetil (MMF), prednisone, and tacrolimus.
Physical examination was remarkable for an extensive rash consisting of clusters of 1- to 2-mm vesicles scattered in a nondermatomal pattern. Isolated vesicles involved the forehead, nose, and left ear, and diffuse vesicles with a relatively symmetric distribution were scattered across the back, chest, and proximal and distal arms and legs (Figure 2). Many of the vesicles had an associated overlying crust with hemorrhage. Some of the vesicles coalesced with central necrotic plaques.

Given a clinical suspicion for disseminated HZ, therapy with oral valacyclovir was initiated. Two punch biopsies were consistent with herpesvirus cytopathic changes. Multiple sections demonstrated ulceration as well as acantholysis and necrosis of keratinocytes with multinucleation and margination of chromatin. There was an intense lichenoid and perivascular lymphocytic infiltrate in the dermis. Immunohistochemistry staining was positive for VZV and negative for HSV, indicating acute reactivation of VZV (Figure 3). Upon completion of an antiviral regimen, the patient returned to clinic with healed crusted lesions.

Comment
Frequently, the clinical features of HZ in immunocompromised patients mirror those in immunocompetent hosts.8 However, each of our 2 patients developed an unusual presentation of atypical generalized HZ.7 In this clinical variant, lesions develop along a single dermatome, then a diffuse vesicular eruption subsequently develops without dermatomal localization. These lesions can be chronic, persisting for months or years.7
The classic clinical presentation of HZ is distinct and often is readily diagnosed by visual inspection.7 However, atypical presentations and their associated complications can pose diagnostic and therapeutic challenges.7 Painless HZ lesions in a nondermatomal pattern were described in a patient who also had AIDS.9 Interestingly, multiple reports have found that patients with a severe but painless rash are less likely to have experienced a viral prodrome consisting of hyperesthesia, paresthesia, or pruritus.2,10 This observation suggests that lack of a prodrome, as in the case of patient 1 in our report, may aid in the recognition of painless HZ. Because of these atypical presentations, laboratory testing is even more important than in immunocompetent hosts, as diagnosis may be more difficult to establish on clinical presentation alone.
Several studies11-32 have evaluated modalities for treatment and prophylaxis for disseminated HZ in immunocompromised hosts, given its increased risk and potentially fatal complications in this population. The current guidelines in patients with HIV/AIDS, solid organ transplantation (SOT), and hematopoietic stem cell transplantation (HSCT) are summarized in the eTable.

HIV/AIDS Patients
Given their efficacy and low rate of toxicity, oral acyclovir, valacyclovir, and famciclovir are recommended treatment options for HIV patients with localized, mild, dermatomal HZ.11 Two exceptions include HZ ophthalmicus and Ramsay Hunt syndrome for which some experts recommend intravenous acyclovir given the risk for vision loss and facial palsy, respectively. Intravenous acyclovir often is the drug of choice for treating complicated, disseminated, or severe HZ in HIV-infected patients, though prospective efficacy data remain limited.11
With regard to prevention of infection, a large randomized trial in 2016 found that acyclovir prophylaxis resulted in a 68% reduction in HZ over 2 years among HIV patients.12 Despite data that acyclovir may be effective for this purpose, long-term antiviral prophylaxis is not routinely recommended for HZ,11,13 as it has been linked to rare cases of acyclovir-resistant HZ in HIV patients.14,15 However, antiviral prophylaxis against HSV type 2 reactivation in HIV patients also confers protection against VZV reactivation.11,12
Solid Organ Transplantation
Localized, mild to moderately severe dermatomal HZ can be treated with oral acyclovir, valacyclovir, or famciclovir. As in HIV patients, SOT patients with severe, disseminated, or complicated HZ should receive IV acyclovir.11 In the first 3 to 6 months following the procedure, SOT patients receive cytomegalovirus prophylaxis with ganciclovir or valgan-ciclovir, which also provides protection against HZ.13-18 For patients not receiving cytomegalovirus prophylaxis, HSV prophylaxis with oral acyclovir or valacyclovir is given for at least the first month after transplantation, which also confers protection against HZ.16,19 Antiviral therapy is critical during the early posttransplantation period when patients are most severely immunosuppressed and thus have the highest risk for VZV-associated complications.20 Although immunosuppression is lifelong in most SOT recipients, there is insufficient evidence for extending prophylaxis beyond 6 months.16,21
As a possible risk factor for HZ,22 MMF use is another consideration among SOT patients, similar to patient 2 in our report. A 2003 observational study supported withdrawal of MMF therapy during active VZV infection due to clinical observation of an association with HZ.23 However, a multicenter, randomized, controlled trial reported no cases of HZ in renal transplant recipients on MMF.24 Additionally, MMF has been observed to enhance the antiviral activity of acyclovir, at least in vitro.25 Given the lack of evidence of MMF as a risk factor for HZ, there is insufficient evidence for cessation of use during VZVreactivation in SOT patients.
Hematopoietic Stem Cell Transplantation
The preferred agents for treatment of localized mild dermatomal HZ are oral acyclovir or valacyclovir, as data on the safety and efficacy of famciclovir among HSCT recipients are limited.13,26 Patients should receive antiviral prophylaxis with one of these agents during the first year following allogeneic or autologous HSCT. This 1-year course has proven highly effective in reducing HZ in the first year following transplantation when most severe cases occur,21,26-29 and it has been associated with a persistently decreased risk for HZ even after discontinuation.21 Prophylaxis may be continued beyond 1 year in allogeneic HSCT recipients experiencing graft-versus-host disease who should receive acyclovir until 6 months after the end of immunosuppressive therapy.21,26
Vaccination remains a potential strategy to reduce the incidence of HZ in this patient population. A heat-inactivated vaccine administered within the first 3 months after the procedure has been shown to be safe among autologous and allogeneic HSCT patients.30,31 The vaccine notably reduced the incidence of HZ in patients who underwent autologous HSCT,32 but no known data are available on its clinical efficacy in allogeneic HSCT patients. Accordingly, there are no known official recommendations to date regarding vaccine use in these patient populations.26
Conclusion
It is incumbent upon clinicians to recognize the spectrum of atypical presentations of HZ and maintain a low threshold for performing appropriate diagnostic or confirmatory studies among at-risk patients with impaired immune function. Disseminated HZ can have potentially life-threatening visceral complications such as encephalitis, hepatitis, or pneumonitis.7,8 As such, an understanding of prevention and treatment modalities for VZV infection among immunocompromised patients is critical. Because the morbidity associated with complications of VZV infection is substantial and the risks associated with antiviral agents are minimal, antiviral prophylaxis is recommended for 6 months following SOT or 1 year following HSCT, and prompt treatment is warranted in cases of reasonable clinical suspicion for HZ.
Acknowledgment
The authors gratefully acknowledge the generosity of our patients in permitting photography of their skin findings for the furthering of medical education.
- McCrary ML, Severson J, Tyring SK. Varicella zoster virus. J Am Acad Dermatol. 1999;41:1-16.
- Nagasako EM, Johnson RW, Griffin DR, et al. Rash severity in herpes zoster: correlates and relationship to postherpetic neuralgia. J Am Acad Dermatol. 2002;46:834-839.
- Leung J, Harpaz R, Baughman AL, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis. 2010;51:23-32.
- Herpes Zoster and Functional Decline Consortium. Functional decline and herpes zoster in older people: an interplay of multiple factors. Aging Clin Exp Res. 2015;27:757-765.
- Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341-357.
- Prelog M, Schonlaub J, Jeller V, et al. Reduced varicella-zoster-virus (VZV)-specific lymphocytes and IgG antibody avidity in solid organ transplant recipients. Vaccine. 2013;31:2420-2426.
- Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91-S98.
- Glesby MJ, Moore RD, Chaisson RE. Clinical spectrum of herpes zoster in adults infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:370-375.
- Blankenship W, Herchline T, Hockley A. Asymptomatic vesicles in a patient with the acquired immunodeficiency syndrome. disseminated varicella-zoster virus (VZV) infection. Arch Dermatol. 1994;130:1193, 1196.
- Katz J, Cooper EM, Walther RR, et al. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis. 2004;39:342-348.
- Gnann JW. Antiviral therapy of varicella-zoster virus infections. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al, eds. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge, United Kingdom: Cambridge University Press; 2007:1175-1191.
- Barnabas RV, Baeten JM, Lingappa JR, et al. Acyclovir prophylaxis reduces the incidence of herpes zoster among HIV-infected individuals: results of a randomized clinical trial. J Infect Dis. 2016;213:551-555.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
- Jacobson MA, Berger TG, Fikrig S, et al. Acyclovir-resistant varicella zoster virus infection after chronic oral acyclovir therapy in patients with the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1990;112:187-191.
- Linnemann CC Jr, Biron KK, Hoppenjans WG, et al. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS. 1990;4:577-579.
- Pergam SA, Limaye AP; AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV) in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S108-S115.
- Preiksaitis JK, Brennan DC, Fishman J, et al. Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am J Transplant. 2005;5:218-227.
- Fishman JA, Doran MT, Volpicelli SA, et al. Dosing of intravenous ganciclovir for the prophylaxis and treatment of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2000;69:389-394.
- Zuckerman R, Wald A; AST Infectious Diseases Community of Practice. Herpes simplex virus infections in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S104-S107.
- Arness T, Pedersen R, Dierkhising R, et al. Varicella zoster virus-associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis. 2008;10:260-268.
- Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007;110:3071-3077.
- Rothwell WS, Gloor JM, Morgenstern BZ, et al. Disseminated varicella infection in pediatric renal transplant recipients treated with mycophenolate mofetil. Transplantation. 1999;68:158-161.
- Lauzurica R, Bayés B, Frías C, et al. Disseminated varicella infection in adult renal allograft recipients: role of mycophenolate mofetil. Transplant Proc. 2003;35:1758-1759.
- A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. TheTricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation. 1996;61:1029-1037.
- Neyts J, De Clercq E. Mycophenolate mofetil strongly potentiates the anti-herpesvirus activity of acyclovir. Antiviral Res. 1998;40:53-56.
- Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143-1238.
- Boeckh M, Kim HW, Flowers ME, et al. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation—a randomized double-blind placebo-controlled study. Blood. 2006;107:1800-1805.
- Kawamura K, Hayakawa J, Akahoshi Y, et al. Low-dose acyclovir prophylaxis for the prevention of herpes simplex virus and varicella zoster virus diseases after autologous hematopoietic stem cell transplantation. Int J Hematol. 2015;102:230-237.
- Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance. Long-term follow-up after hematopoietic stem cell transplant general guidelines for referring physicians. Fred Hutchinson Cancer Research Center website. https://www.fredhutch.org/content/dam/public/Treatment-Suport/Long-Term-Follow-Up/physician.pdf. Published July 17, 2014. Accessed October 19, 2017.
- Kussmaul SC, Horn BN, Dvorak CC, et al. Safety of the live, attenuated varicella vaccine in pediatric recipients of hematopoietic SCTs. Bone Marrow Transplant. 2010;45:1602-1606.
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347:26-34.
- Issa NC, Marty FM, Leblebjian H, et al. Live attenuated varicella-zoster vaccine in hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2014;20:285-287.
- McCrary ML, Severson J, Tyring SK. Varicella zoster virus. J Am Acad Dermatol. 1999;41:1-16.
- Nagasako EM, Johnson RW, Griffin DR, et al. Rash severity in herpes zoster: correlates and relationship to postherpetic neuralgia. J Am Acad Dermatol. 2002;46:834-839.
- Leung J, Harpaz R, Baughman AL, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis. 2010;51:23-32.
- Herpes Zoster and Functional Decline Consortium. Functional decline and herpes zoster in older people: an interplay of multiple factors. Aging Clin Exp Res. 2015;27:757-765.
- Weinberg A, Levin MJ. VZV T cell-mediated immunity. Curr Top Microbiol Immunol. 2010;342:341-357.
- Prelog M, Schonlaub J, Jeller V, et al. Reduced varicella-zoster-virus (VZV)-specific lymphocytes and IgG antibody avidity in solid organ transplant recipients. Vaccine. 2013;31:2420-2426.
- Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002;186(suppl 1):S91-S98.
- Glesby MJ, Moore RD, Chaisson RE. Clinical spectrum of herpes zoster in adults infected with human immunodeficiency virus. Clin Infect Dis. 1995;21:370-375.
- Blankenship W, Herchline T, Hockley A. Asymptomatic vesicles in a patient with the acquired immunodeficiency syndrome. disseminated varicella-zoster virus (VZV) infection. Arch Dermatol. 1994;130:1193, 1196.
- Katz J, Cooper EM, Walther RR, et al. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis. 2004;39:342-348.
- Gnann JW. Antiviral therapy of varicella-zoster virus infections. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al, eds. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge, United Kingdom: Cambridge University Press; 2007:1175-1191.
- Barnabas RV, Baeten JM, Lingappa JR, et al. Acyclovir prophylaxis reduces the incidence of herpes zoster among HIV-infected individuals: results of a randomized clinical trial. J Infect Dis. 2016;213:551-555.
- Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
- Jacobson MA, Berger TG, Fikrig S, et al. Acyclovir-resistant varicella zoster virus infection after chronic oral acyclovir therapy in patients with the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1990;112:187-191.
- Linnemann CC Jr, Biron KK, Hoppenjans WG, et al. Emergence of acyclovir-resistant varicella zoster virus in an AIDS patient on prolonged acyclovir therapy. AIDS. 1990;4:577-579.
- Pergam SA, Limaye AP; AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV) in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S108-S115.
- Preiksaitis JK, Brennan DC, Fishman J, et al. Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am J Transplant. 2005;5:218-227.
- Fishman JA, Doran MT, Volpicelli SA, et al. Dosing of intravenous ganciclovir for the prophylaxis and treatment of cytomegalovirus infection in solid organ transplant recipients. Transplantation. 2000;69:389-394.
- Zuckerman R, Wald A; AST Infectious Diseases Community of Practice. Herpes simplex virus infections in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S104-S107.
- Arness T, Pedersen R, Dierkhising R, et al. Varicella zoster virus-associated disease in adult kidney transplant recipients: incidence and risk-factor analysis. Transpl Infect Dis. 2008;10:260-268.
- Erard V, Guthrie KA, Varley C, et al. One-year acyclovir prophylaxis for preventing varicella-zoster virus disease after hematopoietic cell transplantation: no evidence of rebound varicella-zoster virus disease after drug discontinuation. Blood. 2007;110:3071-3077.
- Rothwell WS, Gloor JM, Morgenstern BZ, et al. Disseminated varicella infection in pediatric renal transplant recipients treated with mycophenolate mofetil. Transplantation. 1999;68:158-161.
- Lauzurica R, Bayés B, Frías C, et al. Disseminated varicella infection in adult renal allograft recipients: role of mycophenolate mofetil. Transplant Proc. 2003;35:1758-1759.
- A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. TheTricontinental Mycophenolate Mofetil Renal Transplantation Study Group. Transplantation. 1996;61:1029-1037.
- Neyts J, De Clercq E. Mycophenolate mofetil strongly potentiates the anti-herpesvirus activity of acyclovir. Antiviral Res. 1998;40:53-56.
- Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143-1238.
- Boeckh M, Kim HW, Flowers ME, et al. Long-term acyclovir for prevention of varicella zoster virus disease after allogeneic hematopoietic cell transplantation—a randomized double-blind placebo-controlled study. Blood. 2006;107:1800-1805.
- Kawamura K, Hayakawa J, Akahoshi Y, et al. Low-dose acyclovir prophylaxis for the prevention of herpes simplex virus and varicella zoster virus diseases after autologous hematopoietic stem cell transplantation. Int J Hematol. 2015;102:230-237.
- Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance. Long-term follow-up after hematopoietic stem cell transplant general guidelines for referring physicians. Fred Hutchinson Cancer Research Center website. https://www.fredhutch.org/content/dam/public/Treatment-Suport/Long-Term-Follow-Up/physician.pdf. Published July 17, 2014. Accessed October 19, 2017.
- Kussmaul SC, Horn BN, Dvorak CC, et al. Safety of the live, attenuated varicella vaccine in pediatric recipients of hematopoietic SCTs. Bone Marrow Transplant. 2010;45:1602-1606.
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347:26-34.
- Issa NC, Marty FM, Leblebjian H, et al. Live attenuated varicella-zoster vaccine in hematopoietic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2014;20:285-287.
Practice Points
- Clinician awareness of management guidelines for the prevention and treatment of varicella-zoster virus infection in immunocompromised individuals is critical to minimize the risk for disease and associated morbidity.
- Antiviral prophylaxis is recommended for 6 months following solid organ transplantation or 1 year following hematopoietic stem cell transplantation, and prompt treatment is warranted in cases of reasonable clinical suspicion for herpes zoster.
Mycobacterium marinum Remains an Unrecognized Cause of Indolent Skin Infections
An environmental pathogen, Mycobacterium marinum can cause cutaneous infection when traumatized skin is exposed to fresh, brackish, or salt water. Fishing, aquarium cleaning, and aquatic recreational activities are risk factors for infection.1,2 Diagnosis often is delayed and is made several weeks or even months after initial symptoms appear.3 Due to the protracted clinical course, patients may not recall the initial exposure, contributing to the delay in diagnosis and initiation of appropriate treatment. It is not uncommon for patients with M marinum infection to be initially treated with antibiotics or antifungal drugs.
We present a review of 5 patients who were diagnosed with M marinum infection at our institution between January 2003 and March 2013.
Methods
This study was conducted at Henry Ford Hospital, a 900-bed tertiary care center in Detroit, Michigan. Patients who had cultures positive for M marinum between January 2003 and March 2013 were identified using the institution’s laboratory database. Medical records were reviewed, and relevant demographic, epidemiologic, and clinical data, including initial clinical presentation, alternative diagnoses, time between initial presentation and definitive diagnosis, and specific treatment, were recorded.
Results
We identified 5 patients who were diagnosed with culture-confirmed M marinum skin infections during the study period: 3 men and 2 women aged 43 to 72 years (Table 1). Two patients had diabetes mellitus and 1 had hepatitis C virus. None had classic immunosuppression. On repeated questioning after the diagnosis was established, all 5 patients reported that they kept a home aquarium, and all recalled mild trauma to the hand prior to the onset of symptoms; however, none of the patients initially linked the minor skin injury to the subsequent infection.
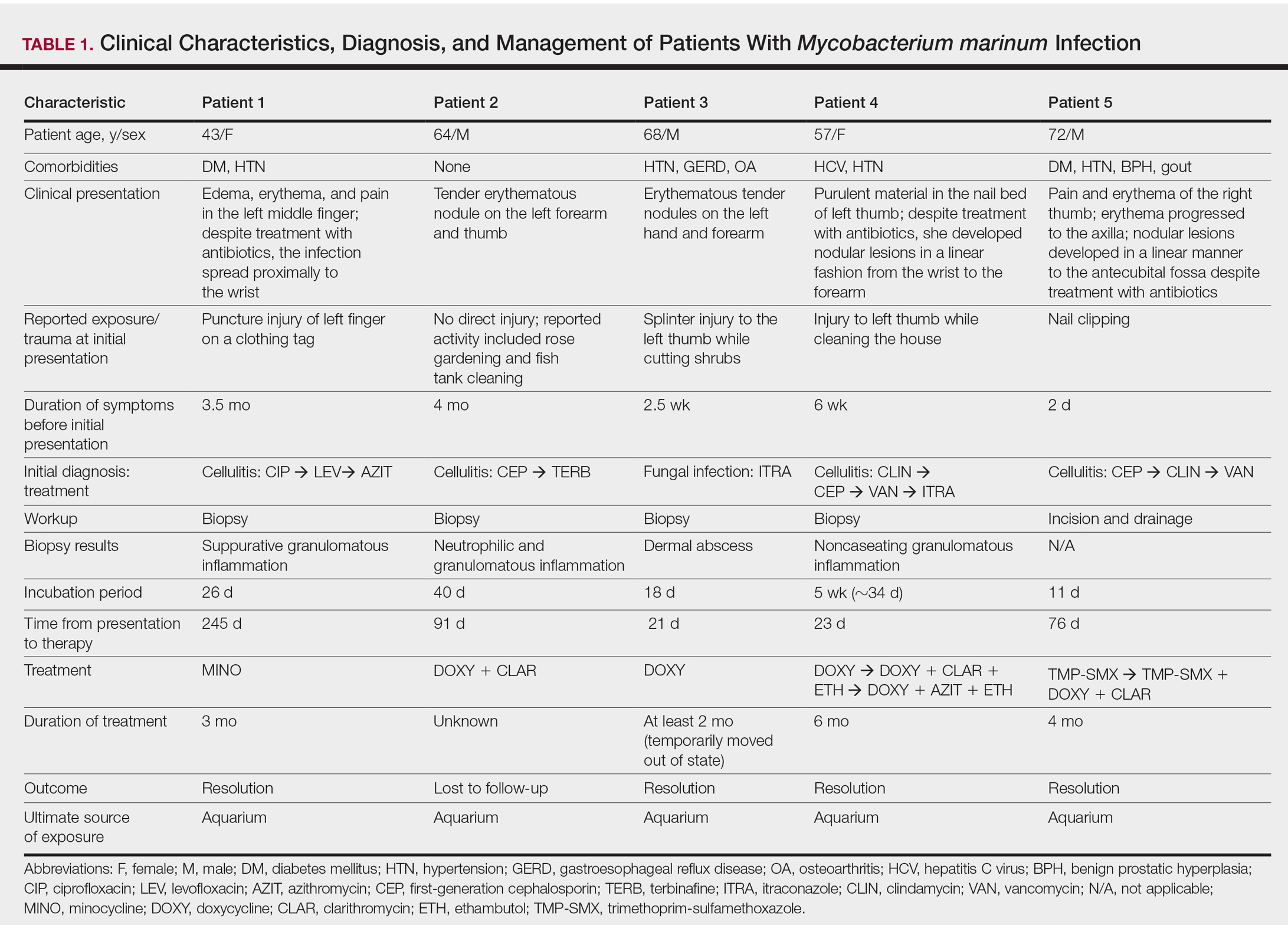
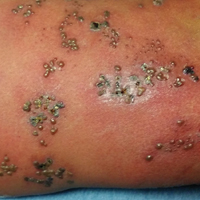
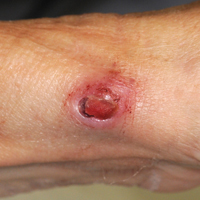
All 5 patients initially presented with erythema and swelling at the site of the injury, which evolved into inflammatory nodules that progressed proximally up to the arm despite empiric treatment with antibiotics active against streptococci and staphylococci (Figures 1 and 2). Three patients also received empiric antifungal therapy due to suspicion of sporotrichosis.
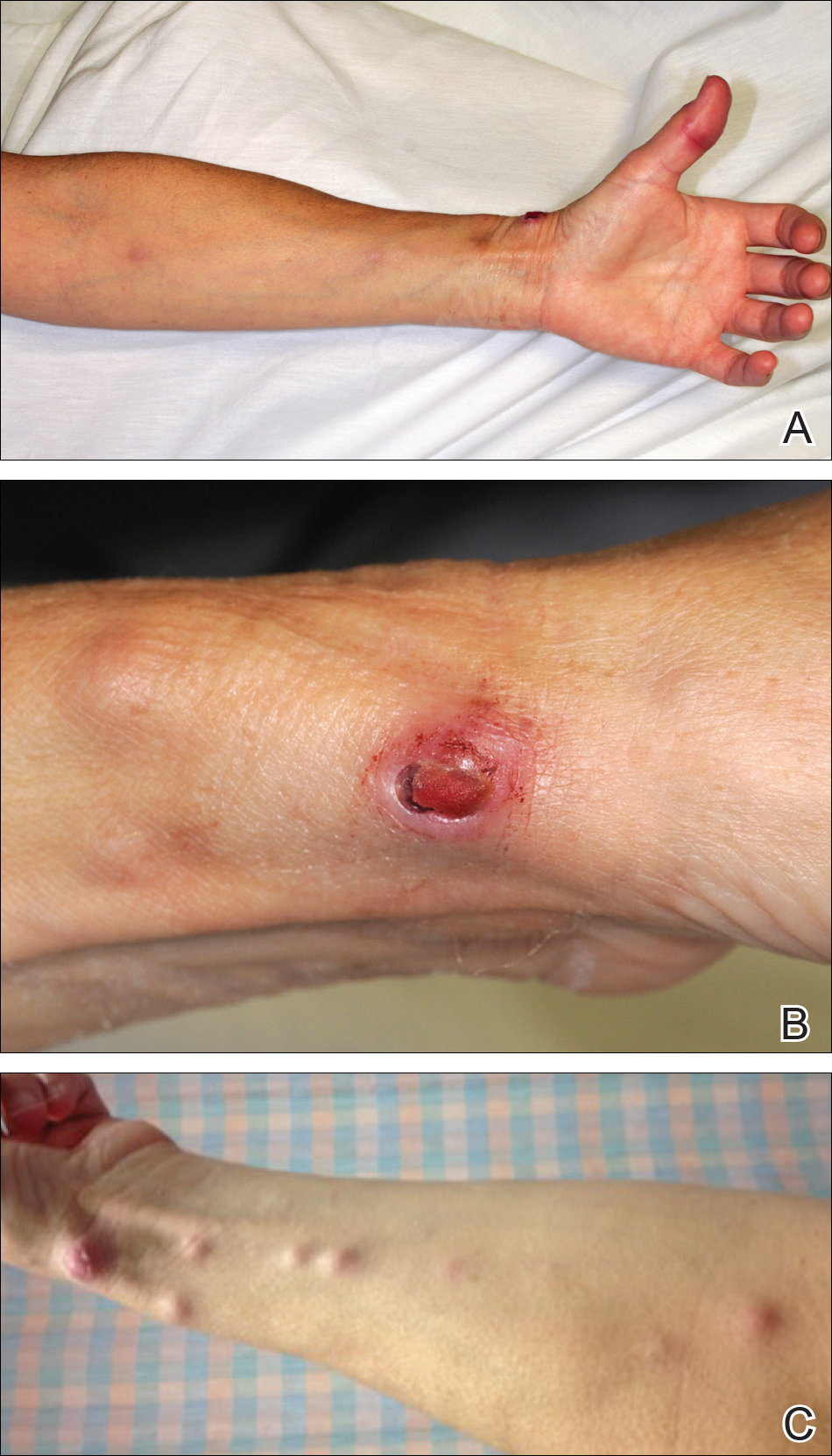
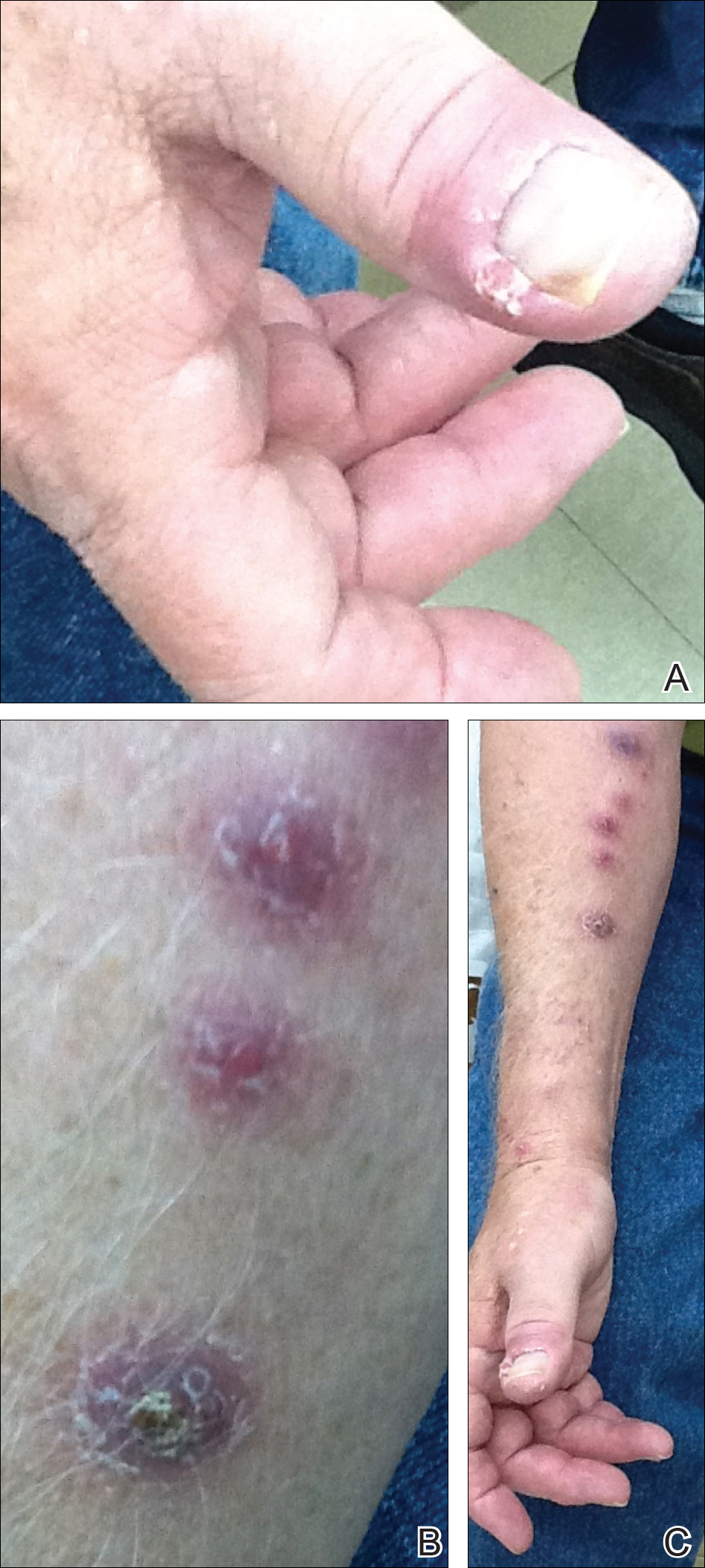
Skin biopsies were performed on 4 patients, and incision and drainage of purulent material was performed on the fifth patient. Histopathologic examination revealed granulomatous inflammation in 3 patients. Stains for acid-fast bacilli were positive in all 5 patients. Definitive diagnosis of the organism was confirmed by growth of M marinum within 11 to 40 days from the tissue in 4 patients and purulent material in the fifth patient. Susceptibility testing was performed on only 1 of the 5 isolates and showed that the organism was susceptible to amikacin, clarithromycin, doxycycline, ethambutol, rifampin, and trimethoprim-sulfamethoxazole (TMP-SMX).
The mean time from initial presentation to initiation of appropriate therapy for M marinum infection was 91 days (range, 21–245 days). Several different treatment regimens were used. All patients received either doxycycline or minocycline with or without a macrolide. Two also received other agents (TMP-SMX or ethambutol). Treatment duration varied from 2 to 6 months in 4 patients, and all 4 had complete resolution of the lesions; 1 patient was lost to follow-up.
Comment
Diagnosing the Infection
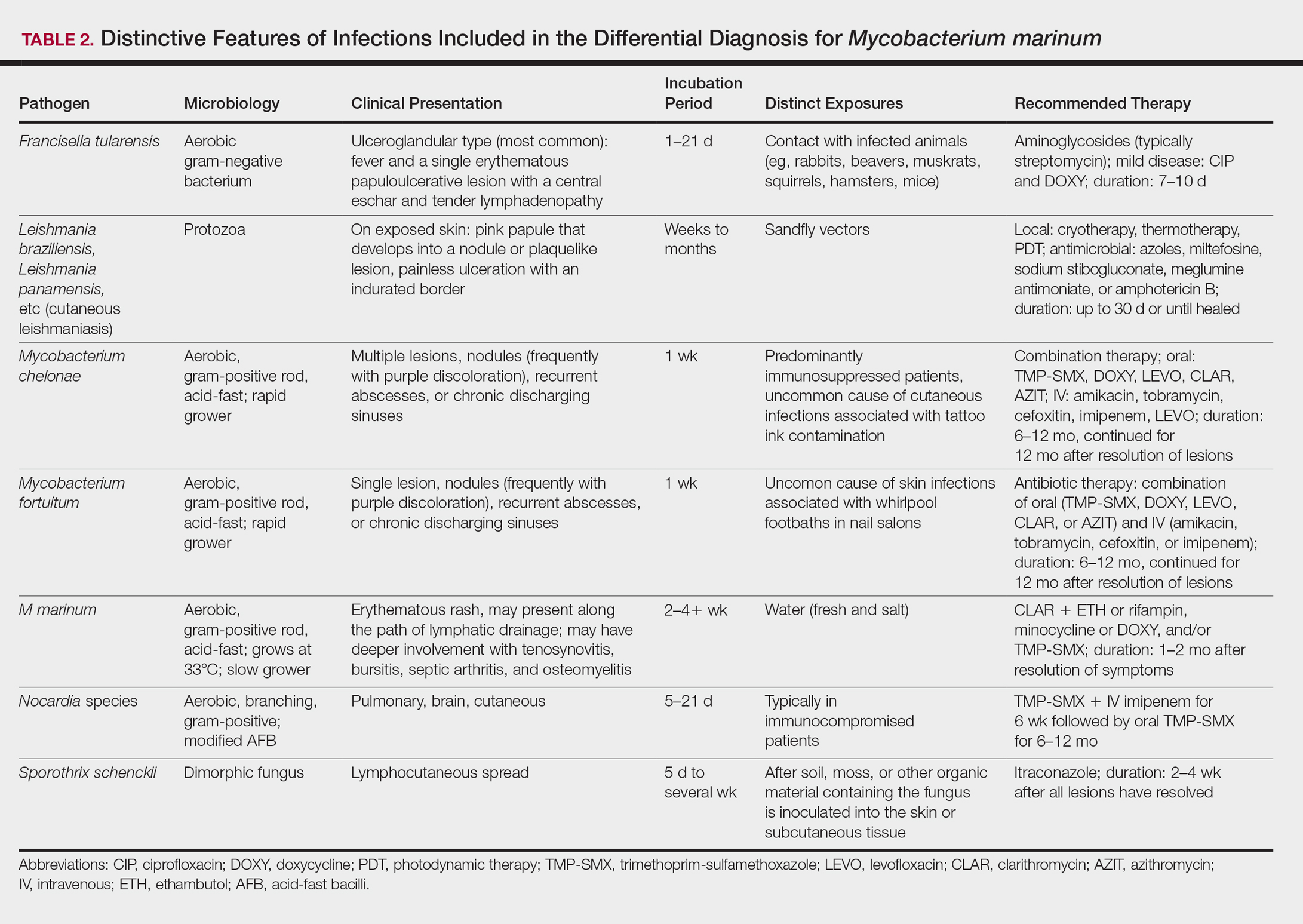
Diagnosis of M marinum infection remains problematic. In the 5 patients included in this study, the time between initial onset of symptoms and diagnosis of M marinum infection was delayed, as has been noted in other reports.4-7 Delays as long as 2 years before the diagnosis is made have been described.7 The clinical presentation of cutaneous infection with M marinum varies, which may delay diagnosis. Nodular lymphangitis is classic, but papules, pustules, ulcers, inflammatory plaques, and single nodules also can occur.1,2 Lymphadenopathy may or may not be present.4,8,9 The differential diagnosis is broad and includes infection by other nontuberculous mycobacteria such as Mycobacterium chelonae; Mycobacterium fortuitum; Nocardia species, especially Nocardia brasiliensis; Francisella tularensis; Sporothrix schenckii; and Leishmania species. It is not surprising that 4 patients in our study were initially treated for a gram-positive bacterial infection and 3 were treated for a fungal infection before the diagnosis of M marinum was made. Distinctive features that may help to differentiate these infections are summarized in Table 2.
We found that the main cause of delayed diagnosis was the failure of physicians to obtain a thorough history regarding patients’ recreational activities and animal exposure. Patients often do not associate a remote aquatic exposure with their symptoms and will not volunteer this information unless directly asked.2,10 It was only after repeated questioning in all of these patients that they recounted prior trauma to the involved hand related to the aquarium.
Biopsy and Culture
Histopathologic examination of material from a biopsied lesion can give an early clue that a mycobacterial infection might be involved. Biopsy can reveal either noncaseating or necrotizing granulomas that have larger numbers of neutrophils in addition to lymphocytes and macrophages. Giant cells often are noted.5,9,11 Organisms can be seen with the use of a tissue acid-fast stain, but species cannot be differentiated by acid-fast staining.12 However, the sensitivity of acid-fast stains on biopsy material is low.3,13,14
Culture of the involved tissue is crucial for establishing the diagnosis of this infection. However, the rate of growth of M marinum is slow. Temperature requirements for incubation and delay in transporting specimens to the laboratory can lead to bacterial overgrowth, resulting in the inability to recover M marinum from the culture.13Mycobacterium marinum grows preferentially between 28°C and 32°C, and growth is limited at temperatures above 33°C.13,15,16 As illustrated in the cases presented, recovery of the organism may not be accomplished from the first culture performed, and additional biopsy material for culture may be needed. Liquid media generally is more sensitive and produces more rapid results than solid media (eg, Löwenstein-Jensen, Middlebrook 7H10/7H11 agar). However, solid media carry the advantage of allowing observation of morphology and estimation of the number of organisms.12,17
Rapid Detection
Advancements in molecular methods have allowed for more definitive and rapid identification of M marinum, substantially reducing the delay in diagnosis. Commercial molecular assays utilize in-solution hybridization or solid-format reverse-hybridization assays to allow mycobacterial detection as soon as growth appears.18 Use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry can substantially shorten the time to species identification.19,20 Nonculture-based tests that have been developed for the rapid detection of M marinum infection include polymerase chain reaction-restriction fragment length polymorphism and polymerase chain reaction amplification of the 16S RNA gene.21 It should be noted, however, that M marinum and Mycobacterium ulcerans have a very homologous 16S ribosomal RNA gene sequence, differing by only 1 nucleotide; thus, distinguishing between M marinum and M ulcerans using this method may be challenging.22,23
Management
Treatment depends on the extent of the disease. Generally, localized cutaneous disease can be treated with monotherapy with agents such as doxycycline, clarithromycin, or TMP-SMX. Extensive disease typically requires a combination of 2 antimycobacterial agents, typically clarithromycin-rifampin, clarithromycin-ethambutol, or rifampin-ethambutol.12 Amikacin has been used in combination with other agents such as rifampin and clarithromycin in refractory cases.22,24 The use of ciprofloxacin is not encouraged because some isolates are resistant; however, other fluoroquinolones, such as moxifloxacin, may be options for combination therapy. Isoniazid, pyrazinamide, and streptomycin are not effective to treat M marinum.
Susceptibility testing of M marinum usually is performed to guide antimicrobial therapy in cases of poor clinical response or intolerance to first-line antimicrobials such as macrolides.25 The likelihood of M marinum developing resistance to the agents used for treatment appears to be low. Unfortunately, in vitro antimicrobial susceptibility tests do not correlate well with treatment efficiency.10
The duration of therapy is not standardized but usually is 5 to 6 months,7,10,26 with therapy often continuing 1 to 2 months after lesions appear to have resolved.12 However, in some cases (usually those who have more extensive disease), therapy has been extended to as long as 1 to 2 years.10 The ideal length of therapy in immunocompromised individuals has not been established27; however, a treatment duration of 6 to 9 months was reported in one study.28 Surgical debridement may be necessary in some patients who have involvement of deep structures of the hand or knee, those with persistent pain, or those who fail to respond to a prolonged period of medical therapy.29 Successful use of less conventional therapeutic approaches, including cryotherapy, radiation therapy, electrodesiccation, photodynamic therapy, curettage, and local hyperthermic therapy has been reported.30-32
Conclusion
Diagnosis and management of M marinum infection is difficult. Patients presenting with indolent nodular skin infections affecting the upper extremities should be asked about aquatic exposure. Tissue biopsy for histopathologic examination and culture is essential to establish an early diagnosis and promptly initiate appropriate therapy.
Acknowledgment
We would like to thank Carol A. Kauffman, MD (Ann Arbor, Michigan), for her thoughtful comments that greatly improved this manuscript.
- Lewis FM, Marsh BJ, von Reyn CF. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis. 2003;37:390-397.
- Jernigan JA, Farr BM. Incubation period and sources of exposure for cutaneous Mycobacterium marinum infection: case report and review of the literature. Clin Infect Dis. 2000;31:439-443.
- Edelstein H. Mycobacterium marinum skin infections. report of 31 cases and review of the literature. Arch Intern Med. 1994;154:1359-1364.
- Janik JP, Bang RH, Palmer CH. Case reports: successful treatment of Mycobacterium marinum infection with minocycline after complication of disease by delayed diagnosis and systemic steroids. J Drugs Dermatol. 2005;4:621-624.
- Jolly HW Jr, Seabury JH. Infections with Myocbacterium marinum. Arch Dermatol. 1972;106:32-36.
- Sette CS, Wachholz PA, Masuda PY, et al. Mycobacterium marinum infection: a case report. J Venom Anim Toxins Incl Trop Dis. 2015;21:7.
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662.
- Eberst E, Dereure O, Guillot B, et al. Epidemiological, clinical, and therapeutic pattern of Mycobacterium marinum infection: a retrospective series of 35 cases from southern France. J Am Acad Dermatol. 2012;66:E15-E16.
- Philpott JA Jr, Woodburne AR, Philpott OS, et al. Swimming pool granuloma. a study of 290 cases. Arch Dermatol. 1963;88:158-162.
- Aubry A, Chosidow O, Caumes E, et al. Sixty-three cases of Mycobacterium marinum infection: clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med. 2002;162:1746-1752.
- Feng Y, Xu H, Wang H, et al. Outbreak of a cutaneous Mycobacterium marinum infection in Jiangsu Haian, China. Diagn Microbiol Infect Dis. 2011;71:267-272.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of non-tuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Ang P, Rattana-Apiromyakij N, Goh CL. Retrospective study of Mycobacterium marinum skin infections. Int J Dermatol. 2000;39:343-347.
- Wu TS, Chiu CH, Yang CH, et al. Fish tank granuloma caused by Mycobacterium marinum. PLoS One. 2012;7:e41296.
- Ho WL, Chuang WY, Kuo AJ, et al. Nasal fish tank granuloma: an uncommon cause for epistaxis. Am J Trop Med Hyg. 2011;85:195-196.
- Dobos KM, Quinn FD, Ashford DA, et al. Emergence of a unique group of necrotizing mycobacterial diseases. Emerg Infect Dis. 1999;5:367-378.
- van Ingen J. Diagnosis of non-tuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109.
- Piersimoni C, Scarparo C. Extrapulmonary infections associated with non-tuberculous mycobacteria in immunocompetent persons. Emerg Infect Dis. 2009;15:1351-1358; quiz 1544.
- Saleeb PG, Drake SK, Murray PR, et al. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49:1790-1794.
- Adams LL, Salee P, Dionne K, et al. A novel protein extraction method for identification of mycobacteria using MALDI-ToF MS. J Microbiol Methods. 2015;119:1-3.
- Posteraro B, Sanguinetti M, Garcovich A, et al. Polymerase chain reaction-reverse cross-blot hybridization assay in the diagnosis of sporotrichoid Mycobacterium marinum infection. Br J Dermatol. 1998;139:872-876.
- Lau SK, Curreem SO, Ngan AH, et al. First report of disseminated Mycobacterium skin infections in two liver transplant recipients and rapid diagnosis by hsp65 gene sequencing. J Clin Microbiol. 2011;49:3733-3738.
- Hofer M, Hirschel B, Kirschner P, et al. Brief report: disseminated osteomyelitis from Mycobacterium ulcerans after a snakebite. N Engl J Med. 1993;328:1007-1009.
- Huang Y, Xu X, Liu Y, et al. Successful treatment of refractory cutaneous infection caused by Mycobacterium marinum with a combined regimen containing amikacin. Clin Interv Aging. 2012;7:533-538.
- Woods GL. Susceptibility testing for mycobacteria. Clin Infect Dis. 2000;31:1209-1215.
- Balaqué N, Uçkay I, Vostrel P, et al. Non-tuberculous mycobacterial infections of the hand. Chir Main. 2015;34:18-23.
- Pandian TK, Deziel PJ, Otley CC, et al. Mycobacterium marinum infections in transplant recipients: case report and review of the literature. Transpl Infect Dis. 2008;10:358-363.
- Jacobs S, George A, Papanicolaou GA, et al. Disseminated Mycobacterium marinum infection in a hematopoietic stem cell transplant recipient. Transpl Infect Dis. 2012;14:410-414.
- Chow SP, Ip FK, Lau JH, et al. Mycobacterium marinum infection of the hand and wrist. results of conservative treatment in twenty-four cases. J Bone Joint Surg Am. 1987;69:1161-1168.
- Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965-2978.
- Nenoff P, Klapper BM, Mayser P, et al. Infections due to Mycobacterium marinum: a review. Hautarzt. 2011;62:266-271.
- Prevost E, Walker EM Jr, Kreutner A Jr, et al. Mycobacterium marinum infections: diagnosis and treatment. South Med J. 1982;75:1349-1352.
An environmental pathogen, Mycobacterium marinum can cause cutaneous infection when traumatized skin is exposed to fresh, brackish, or salt water. Fishing, aquarium cleaning, and aquatic recreational activities are risk factors for infection.1,2 Diagnosis often is delayed and is made several weeks or even months after initial symptoms appear.3 Due to the protracted clinical course, patients may not recall the initial exposure, contributing to the delay in diagnosis and initiation of appropriate treatment. It is not uncommon for patients with M marinum infection to be initially treated with antibiotics or antifungal drugs.
We present a review of 5 patients who were diagnosed with M marinum infection at our institution between January 2003 and March 2013.
Methods
This study was conducted at Henry Ford Hospital, a 900-bed tertiary care center in Detroit, Michigan. Patients who had cultures positive for M marinum between January 2003 and March 2013 were identified using the institution’s laboratory database. Medical records were reviewed, and relevant demographic, epidemiologic, and clinical data, including initial clinical presentation, alternative diagnoses, time between initial presentation and definitive diagnosis, and specific treatment, were recorded.
Results
We identified 5 patients who were diagnosed with culture-confirmed M marinum skin infections during the study period: 3 men and 2 women aged 43 to 72 years (Table 1). Two patients had diabetes mellitus and 1 had hepatitis C virus. None had classic immunosuppression. On repeated questioning after the diagnosis was established, all 5 patients reported that they kept a home aquarium, and all recalled mild trauma to the hand prior to the onset of symptoms; however, none of the patients initially linked the minor skin injury to the subsequent infection.

All 5 patients initially presented with erythema and swelling at the site of the injury, which evolved into inflammatory nodules that progressed proximally up to the arm despite empiric treatment with antibiotics active against streptococci and staphylococci (Figures 1 and 2). Three patients also received empiric antifungal therapy due to suspicion of sporotrichosis.


Skin biopsies were performed on 4 patients, and incision and drainage of purulent material was performed on the fifth patient. Histopathologic examination revealed granulomatous inflammation in 3 patients. Stains for acid-fast bacilli were positive in all 5 patients. Definitive diagnosis of the organism was confirmed by growth of M marinum within 11 to 40 days from the tissue in 4 patients and purulent material in the fifth patient. Susceptibility testing was performed on only 1 of the 5 isolates and showed that the organism was susceptible to amikacin, clarithromycin, doxycycline, ethambutol, rifampin, and trimethoprim-sulfamethoxazole (TMP-SMX).
The mean time from initial presentation to initiation of appropriate therapy for M marinum infection was 91 days (range, 21–245 days). Several different treatment regimens were used. All patients received either doxycycline or minocycline with or without a macrolide. Two also received other agents (TMP-SMX or ethambutol). Treatment duration varied from 2 to 6 months in 4 patients, and all 4 had complete resolution of the lesions; 1 patient was lost to follow-up.
Comment
Diagnosing the Infection
Diagnosis of M marinum infection remains problematic. In the 5 patients included in this study, the time between initial onset of symptoms and diagnosis of M marinum infection was delayed, as has been noted in other reports.4-7 Delays as long as 2 years before the diagnosis is made have been described.7 The clinical presentation of cutaneous infection with M marinum varies, which may delay diagnosis. Nodular lymphangitis is classic, but papules, pustules, ulcers, inflammatory plaques, and single nodules also can occur.1,2 Lymphadenopathy may or may not be present.4,8,9 The differential diagnosis is broad and includes infection by other nontuberculous mycobacteria such as Mycobacterium chelonae; Mycobacterium fortuitum; Nocardia species, especially Nocardia brasiliensis; Francisella tularensis; Sporothrix schenckii; and Leishmania species. It is not surprising that 4 patients in our study were initially treated for a gram-positive bacterial infection and 3 were treated for a fungal infection before the diagnosis of M marinum was made. Distinctive features that may help to differentiate these infections are summarized in Table 2.

We found that the main cause of delayed diagnosis was the failure of physicians to obtain a thorough history regarding patients’ recreational activities and animal exposure. Patients often do not associate a remote aquatic exposure with their symptoms and will not volunteer this information unless directly asked.2,10 It was only after repeated questioning in all of these patients that they recounted prior trauma to the involved hand related to the aquarium.
Biopsy and Culture
Histopathologic examination of material from a biopsied lesion can give an early clue that a mycobacterial infection might be involved. Biopsy can reveal either noncaseating or necrotizing granulomas that have larger numbers of neutrophils in addition to lymphocytes and macrophages. Giant cells often are noted.5,9,11 Organisms can be seen with the use of a tissue acid-fast stain, but species cannot be differentiated by acid-fast staining.12 However, the sensitivity of acid-fast stains on biopsy material is low.3,13,14
Culture of the involved tissue is crucial for establishing the diagnosis of this infection. However, the rate of growth of M marinum is slow. Temperature requirements for incubation and delay in transporting specimens to the laboratory can lead to bacterial overgrowth, resulting in the inability to recover M marinum from the culture.13Mycobacterium marinum grows preferentially between 28°C and 32°C, and growth is limited at temperatures above 33°C.13,15,16 As illustrated in the cases presented, recovery of the organism may not be accomplished from the first culture performed, and additional biopsy material for culture may be needed. Liquid media generally is more sensitive and produces more rapid results than solid media (eg, Löwenstein-Jensen, Middlebrook 7H10/7H11 agar). However, solid media carry the advantage of allowing observation of morphology and estimation of the number of organisms.12,17
Rapid Detection
Advancements in molecular methods have allowed for more definitive and rapid identification of M marinum, substantially reducing the delay in diagnosis. Commercial molecular assays utilize in-solution hybridization or solid-format reverse-hybridization assays to allow mycobacterial detection as soon as growth appears.18 Use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry can substantially shorten the time to species identification.19,20 Nonculture-based tests that have been developed for the rapid detection of M marinum infection include polymerase chain reaction-restriction fragment length polymorphism and polymerase chain reaction amplification of the 16S RNA gene.21 It should be noted, however, that M marinum and Mycobacterium ulcerans have a very homologous 16S ribosomal RNA gene sequence, differing by only 1 nucleotide; thus, distinguishing between M marinum and M ulcerans using this method may be challenging.22,23
Management
Treatment depends on the extent of the disease. Generally, localized cutaneous disease can be treated with monotherapy with agents such as doxycycline, clarithromycin, or TMP-SMX. Extensive disease typically requires a combination of 2 antimycobacterial agents, typically clarithromycin-rifampin, clarithromycin-ethambutol, or rifampin-ethambutol.12 Amikacin has been used in combination with other agents such as rifampin and clarithromycin in refractory cases.22,24 The use of ciprofloxacin is not encouraged because some isolates are resistant; however, other fluoroquinolones, such as moxifloxacin, may be options for combination therapy. Isoniazid, pyrazinamide, and streptomycin are not effective to treat M marinum.
Susceptibility testing of M marinum usually is performed to guide antimicrobial therapy in cases of poor clinical response or intolerance to first-line antimicrobials such as macrolides.25 The likelihood of M marinum developing resistance to the agents used for treatment appears to be low. Unfortunately, in vitro antimicrobial susceptibility tests do not correlate well with treatment efficiency.10
The duration of therapy is not standardized but usually is 5 to 6 months,7,10,26 with therapy often continuing 1 to 2 months after lesions appear to have resolved.12 However, in some cases (usually those who have more extensive disease), therapy has been extended to as long as 1 to 2 years.10 The ideal length of therapy in immunocompromised individuals has not been established27; however, a treatment duration of 6 to 9 months was reported in one study.28 Surgical debridement may be necessary in some patients who have involvement of deep structures of the hand or knee, those with persistent pain, or those who fail to respond to a prolonged period of medical therapy.29 Successful use of less conventional therapeutic approaches, including cryotherapy, radiation therapy, electrodesiccation, photodynamic therapy, curettage, and local hyperthermic therapy has been reported.30-32
Conclusion
Diagnosis and management of M marinum infection is difficult. Patients presenting with indolent nodular skin infections affecting the upper extremities should be asked about aquatic exposure. Tissue biopsy for histopathologic examination and culture is essential to establish an early diagnosis and promptly initiate appropriate therapy.
Acknowledgment
We would like to thank Carol A. Kauffman, MD (Ann Arbor, Michigan), for her thoughtful comments that greatly improved this manuscript.
An environmental pathogen, Mycobacterium marinum can cause cutaneous infection when traumatized skin is exposed to fresh, brackish, or salt water. Fishing, aquarium cleaning, and aquatic recreational activities are risk factors for infection.1,2 Diagnosis often is delayed and is made several weeks or even months after initial symptoms appear.3 Due to the protracted clinical course, patients may not recall the initial exposure, contributing to the delay in diagnosis and initiation of appropriate treatment. It is not uncommon for patients with M marinum infection to be initially treated with antibiotics or antifungal drugs.
We present a review of 5 patients who were diagnosed with M marinum infection at our institution between January 2003 and March 2013.
Methods
This study was conducted at Henry Ford Hospital, a 900-bed tertiary care center in Detroit, Michigan. Patients who had cultures positive for M marinum between January 2003 and March 2013 were identified using the institution’s laboratory database. Medical records were reviewed, and relevant demographic, epidemiologic, and clinical data, including initial clinical presentation, alternative diagnoses, time between initial presentation and definitive diagnosis, and specific treatment, were recorded.
Results
We identified 5 patients who were diagnosed with culture-confirmed M marinum skin infections during the study period: 3 men and 2 women aged 43 to 72 years (Table 1). Two patients had diabetes mellitus and 1 had hepatitis C virus. None had classic immunosuppression. On repeated questioning after the diagnosis was established, all 5 patients reported that they kept a home aquarium, and all recalled mild trauma to the hand prior to the onset of symptoms; however, none of the patients initially linked the minor skin injury to the subsequent infection.

All 5 patients initially presented with erythema and swelling at the site of the injury, which evolved into inflammatory nodules that progressed proximally up to the arm despite empiric treatment with antibiotics active against streptococci and staphylococci (Figures 1 and 2). Three patients also received empiric antifungal therapy due to suspicion of sporotrichosis.


Skin biopsies were performed on 4 patients, and incision and drainage of purulent material was performed on the fifth patient. Histopathologic examination revealed granulomatous inflammation in 3 patients. Stains for acid-fast bacilli were positive in all 5 patients. Definitive diagnosis of the organism was confirmed by growth of M marinum within 11 to 40 days from the tissue in 4 patients and purulent material in the fifth patient. Susceptibility testing was performed on only 1 of the 5 isolates and showed that the organism was susceptible to amikacin, clarithromycin, doxycycline, ethambutol, rifampin, and trimethoprim-sulfamethoxazole (TMP-SMX).
The mean time from initial presentation to initiation of appropriate therapy for M marinum infection was 91 days (range, 21–245 days). Several different treatment regimens were used. All patients received either doxycycline or minocycline with or without a macrolide. Two also received other agents (TMP-SMX or ethambutol). Treatment duration varied from 2 to 6 months in 4 patients, and all 4 had complete resolution of the lesions; 1 patient was lost to follow-up.
Comment
Diagnosing the Infection
Diagnosis of M marinum infection remains problematic. In the 5 patients included in this study, the time between initial onset of symptoms and diagnosis of M marinum infection was delayed, as has been noted in other reports.4-7 Delays as long as 2 years before the diagnosis is made have been described.7 The clinical presentation of cutaneous infection with M marinum varies, which may delay diagnosis. Nodular lymphangitis is classic, but papules, pustules, ulcers, inflammatory plaques, and single nodules also can occur.1,2 Lymphadenopathy may or may not be present.4,8,9 The differential diagnosis is broad and includes infection by other nontuberculous mycobacteria such as Mycobacterium chelonae; Mycobacterium fortuitum; Nocardia species, especially Nocardia brasiliensis; Francisella tularensis; Sporothrix schenckii; and Leishmania species. It is not surprising that 4 patients in our study were initially treated for a gram-positive bacterial infection and 3 were treated for a fungal infection before the diagnosis of M marinum was made. Distinctive features that may help to differentiate these infections are summarized in Table 2.

We found that the main cause of delayed diagnosis was the failure of physicians to obtain a thorough history regarding patients’ recreational activities and animal exposure. Patients often do not associate a remote aquatic exposure with their symptoms and will not volunteer this information unless directly asked.2,10 It was only after repeated questioning in all of these patients that they recounted prior trauma to the involved hand related to the aquarium.
Biopsy and Culture
Histopathologic examination of material from a biopsied lesion can give an early clue that a mycobacterial infection might be involved. Biopsy can reveal either noncaseating or necrotizing granulomas that have larger numbers of neutrophils in addition to lymphocytes and macrophages. Giant cells often are noted.5,9,11 Organisms can be seen with the use of a tissue acid-fast stain, but species cannot be differentiated by acid-fast staining.12 However, the sensitivity of acid-fast stains on biopsy material is low.3,13,14
Culture of the involved tissue is crucial for establishing the diagnosis of this infection. However, the rate of growth of M marinum is slow. Temperature requirements for incubation and delay in transporting specimens to the laboratory can lead to bacterial overgrowth, resulting in the inability to recover M marinum from the culture.13Mycobacterium marinum grows preferentially between 28°C and 32°C, and growth is limited at temperatures above 33°C.13,15,16 As illustrated in the cases presented, recovery of the organism may not be accomplished from the first culture performed, and additional biopsy material for culture may be needed. Liquid media generally is more sensitive and produces more rapid results than solid media (eg, Löwenstein-Jensen, Middlebrook 7H10/7H11 agar). However, solid media carry the advantage of allowing observation of morphology and estimation of the number of organisms.12,17
Rapid Detection
Advancements in molecular methods have allowed for more definitive and rapid identification of M marinum, substantially reducing the delay in diagnosis. Commercial molecular assays utilize in-solution hybridization or solid-format reverse-hybridization assays to allow mycobacterial detection as soon as growth appears.18 Use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry can substantially shorten the time to species identification.19,20 Nonculture-based tests that have been developed for the rapid detection of M marinum infection include polymerase chain reaction-restriction fragment length polymorphism and polymerase chain reaction amplification of the 16S RNA gene.21 It should be noted, however, that M marinum and Mycobacterium ulcerans have a very homologous 16S ribosomal RNA gene sequence, differing by only 1 nucleotide; thus, distinguishing between M marinum and M ulcerans using this method may be challenging.22,23
Management
Treatment depends on the extent of the disease. Generally, localized cutaneous disease can be treated with monotherapy with agents such as doxycycline, clarithromycin, or TMP-SMX. Extensive disease typically requires a combination of 2 antimycobacterial agents, typically clarithromycin-rifampin, clarithromycin-ethambutol, or rifampin-ethambutol.12 Amikacin has been used in combination with other agents such as rifampin and clarithromycin in refractory cases.22,24 The use of ciprofloxacin is not encouraged because some isolates are resistant; however, other fluoroquinolones, such as moxifloxacin, may be options for combination therapy. Isoniazid, pyrazinamide, and streptomycin are not effective to treat M marinum.
Susceptibility testing of M marinum usually is performed to guide antimicrobial therapy in cases of poor clinical response or intolerance to first-line antimicrobials such as macrolides.25 The likelihood of M marinum developing resistance to the agents used for treatment appears to be low. Unfortunately, in vitro antimicrobial susceptibility tests do not correlate well with treatment efficiency.10
The duration of therapy is not standardized but usually is 5 to 6 months,7,10,26 with therapy often continuing 1 to 2 months after lesions appear to have resolved.12 However, in some cases (usually those who have more extensive disease), therapy has been extended to as long as 1 to 2 years.10 The ideal length of therapy in immunocompromised individuals has not been established27; however, a treatment duration of 6 to 9 months was reported in one study.28 Surgical debridement may be necessary in some patients who have involvement of deep structures of the hand or knee, those with persistent pain, or those who fail to respond to a prolonged period of medical therapy.29 Successful use of less conventional therapeutic approaches, including cryotherapy, radiation therapy, electrodesiccation, photodynamic therapy, curettage, and local hyperthermic therapy has been reported.30-32
Conclusion
Diagnosis and management of M marinum infection is difficult. Patients presenting with indolent nodular skin infections affecting the upper extremities should be asked about aquatic exposure. Tissue biopsy for histopathologic examination and culture is essential to establish an early diagnosis and promptly initiate appropriate therapy.
Acknowledgment
We would like to thank Carol A. Kauffman, MD (Ann Arbor, Michigan), for her thoughtful comments that greatly improved this manuscript.
- Lewis FM, Marsh BJ, von Reyn CF. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis. 2003;37:390-397.
- Jernigan JA, Farr BM. Incubation period and sources of exposure for cutaneous Mycobacterium marinum infection: case report and review of the literature. Clin Infect Dis. 2000;31:439-443.
- Edelstein H. Mycobacterium marinum skin infections. report of 31 cases and review of the literature. Arch Intern Med. 1994;154:1359-1364.
- Janik JP, Bang RH, Palmer CH. Case reports: successful treatment of Mycobacterium marinum infection with minocycline after complication of disease by delayed diagnosis and systemic steroids. J Drugs Dermatol. 2005;4:621-624.
- Jolly HW Jr, Seabury JH. Infections with Myocbacterium marinum. Arch Dermatol. 1972;106:32-36.
- Sette CS, Wachholz PA, Masuda PY, et al. Mycobacterium marinum infection: a case report. J Venom Anim Toxins Incl Trop Dis. 2015;21:7.
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662.
- Eberst E, Dereure O, Guillot B, et al. Epidemiological, clinical, and therapeutic pattern of Mycobacterium marinum infection: a retrospective series of 35 cases from southern France. J Am Acad Dermatol. 2012;66:E15-E16.
- Philpott JA Jr, Woodburne AR, Philpott OS, et al. Swimming pool granuloma. a study of 290 cases. Arch Dermatol. 1963;88:158-162.
- Aubry A, Chosidow O, Caumes E, et al. Sixty-three cases of Mycobacterium marinum infection: clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med. 2002;162:1746-1752.
- Feng Y, Xu H, Wang H, et al. Outbreak of a cutaneous Mycobacterium marinum infection in Jiangsu Haian, China. Diagn Microbiol Infect Dis. 2011;71:267-272.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of non-tuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Ang P, Rattana-Apiromyakij N, Goh CL. Retrospective study of Mycobacterium marinum skin infections. Int J Dermatol. 2000;39:343-347.
- Wu TS, Chiu CH, Yang CH, et al. Fish tank granuloma caused by Mycobacterium marinum. PLoS One. 2012;7:e41296.
- Ho WL, Chuang WY, Kuo AJ, et al. Nasal fish tank granuloma: an uncommon cause for epistaxis. Am J Trop Med Hyg. 2011;85:195-196.
- Dobos KM, Quinn FD, Ashford DA, et al. Emergence of a unique group of necrotizing mycobacterial diseases. Emerg Infect Dis. 1999;5:367-378.
- van Ingen J. Diagnosis of non-tuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109.
- Piersimoni C, Scarparo C. Extrapulmonary infections associated with non-tuberculous mycobacteria in immunocompetent persons. Emerg Infect Dis. 2009;15:1351-1358; quiz 1544.
- Saleeb PG, Drake SK, Murray PR, et al. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49:1790-1794.
- Adams LL, Salee P, Dionne K, et al. A novel protein extraction method for identification of mycobacteria using MALDI-ToF MS. J Microbiol Methods. 2015;119:1-3.
- Posteraro B, Sanguinetti M, Garcovich A, et al. Polymerase chain reaction-reverse cross-blot hybridization assay in the diagnosis of sporotrichoid Mycobacterium marinum infection. Br J Dermatol. 1998;139:872-876.
- Lau SK, Curreem SO, Ngan AH, et al. First report of disseminated Mycobacterium skin infections in two liver transplant recipients and rapid diagnosis by hsp65 gene sequencing. J Clin Microbiol. 2011;49:3733-3738.
- Hofer M, Hirschel B, Kirschner P, et al. Brief report: disseminated osteomyelitis from Mycobacterium ulcerans after a snakebite. N Engl J Med. 1993;328:1007-1009.
- Huang Y, Xu X, Liu Y, et al. Successful treatment of refractory cutaneous infection caused by Mycobacterium marinum with a combined regimen containing amikacin. Clin Interv Aging. 2012;7:533-538.
- Woods GL. Susceptibility testing for mycobacteria. Clin Infect Dis. 2000;31:1209-1215.
- Balaqué N, Uçkay I, Vostrel P, et al. Non-tuberculous mycobacterial infections of the hand. Chir Main. 2015;34:18-23.
- Pandian TK, Deziel PJ, Otley CC, et al. Mycobacterium marinum infections in transplant recipients: case report and review of the literature. Transpl Infect Dis. 2008;10:358-363.
- Jacobs S, George A, Papanicolaou GA, et al. Disseminated Mycobacterium marinum infection in a hematopoietic stem cell transplant recipient. Transpl Infect Dis. 2012;14:410-414.
- Chow SP, Ip FK, Lau JH, et al. Mycobacterium marinum infection of the hand and wrist. results of conservative treatment in twenty-four cases. J Bone Joint Surg Am. 1987;69:1161-1168.
- Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965-2978.
- Nenoff P, Klapper BM, Mayser P, et al. Infections due to Mycobacterium marinum: a review. Hautarzt. 2011;62:266-271.
- Prevost E, Walker EM Jr, Kreutner A Jr, et al. Mycobacterium marinum infections: diagnosis and treatment. South Med J. 1982;75:1349-1352.
- Lewis FM, Marsh BJ, von Reyn CF. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin Infect Dis. 2003;37:390-397.
- Jernigan JA, Farr BM. Incubation period and sources of exposure for cutaneous Mycobacterium marinum infection: case report and review of the literature. Clin Infect Dis. 2000;31:439-443.
- Edelstein H. Mycobacterium marinum skin infections. report of 31 cases and review of the literature. Arch Intern Med. 1994;154:1359-1364.
- Janik JP, Bang RH, Palmer CH. Case reports: successful treatment of Mycobacterium marinum infection with minocycline after complication of disease by delayed diagnosis and systemic steroids. J Drugs Dermatol. 2005;4:621-624.
- Jolly HW Jr, Seabury JH. Infections with Myocbacterium marinum. Arch Dermatol. 1972;106:32-36.
- Sette CS, Wachholz PA, Masuda PY, et al. Mycobacterium marinum infection: a case report. J Venom Anim Toxins Incl Trop Dis. 2015;21:7.
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662.
- Eberst E, Dereure O, Guillot B, et al. Epidemiological, clinical, and therapeutic pattern of Mycobacterium marinum infection: a retrospective series of 35 cases from southern France. J Am Acad Dermatol. 2012;66:E15-E16.
- Philpott JA Jr, Woodburne AR, Philpott OS, et al. Swimming pool granuloma. a study of 290 cases. Arch Dermatol. 1963;88:158-162.
- Aubry A, Chosidow O, Caumes E, et al. Sixty-three cases of Mycobacterium marinum infection: clinical features, treatment, and antibiotic susceptibility of causative isolates. Arch Intern Med. 2002;162:1746-1752.
- Feng Y, Xu H, Wang H, et al. Outbreak of a cutaneous Mycobacterium marinum infection in Jiangsu Haian, China. Diagn Microbiol Infect Dis. 2011;71:267-272.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of non-tuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- Ang P, Rattana-Apiromyakij N, Goh CL. Retrospective study of Mycobacterium marinum skin infections. Int J Dermatol. 2000;39:343-347.
- Wu TS, Chiu CH, Yang CH, et al. Fish tank granuloma caused by Mycobacterium marinum. PLoS One. 2012;7:e41296.
- Ho WL, Chuang WY, Kuo AJ, et al. Nasal fish tank granuloma: an uncommon cause for epistaxis. Am J Trop Med Hyg. 2011;85:195-196.
- Dobos KM, Quinn FD, Ashford DA, et al. Emergence of a unique group of necrotizing mycobacterial diseases. Emerg Infect Dis. 1999;5:367-378.
- van Ingen J. Diagnosis of non-tuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103-109.
- Piersimoni C, Scarparo C. Extrapulmonary infections associated with non-tuberculous mycobacteria in immunocompetent persons. Emerg Infect Dis. 2009;15:1351-1358; quiz 1544.
- Saleeb PG, Drake SK, Murray PR, et al. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49:1790-1794.
- Adams LL, Salee P, Dionne K, et al. A novel protein extraction method for identification of mycobacteria using MALDI-ToF MS. J Microbiol Methods. 2015;119:1-3.
- Posteraro B, Sanguinetti M, Garcovich A, et al. Polymerase chain reaction-reverse cross-blot hybridization assay in the diagnosis of sporotrichoid Mycobacterium marinum infection. Br J Dermatol. 1998;139:872-876.
- Lau SK, Curreem SO, Ngan AH, et al. First report of disseminated Mycobacterium skin infections in two liver transplant recipients and rapid diagnosis by hsp65 gene sequencing. J Clin Microbiol. 2011;49:3733-3738.
- Hofer M, Hirschel B, Kirschner P, et al. Brief report: disseminated osteomyelitis from Mycobacterium ulcerans after a snakebite. N Engl J Med. 1993;328:1007-1009.
- Huang Y, Xu X, Liu Y, et al. Successful treatment of refractory cutaneous infection caused by Mycobacterium marinum with a combined regimen containing amikacin. Clin Interv Aging. 2012;7:533-538.
- Woods GL. Susceptibility testing for mycobacteria. Clin Infect Dis. 2000;31:1209-1215.
- Balaqué N, Uçkay I, Vostrel P, et al. Non-tuberculous mycobacterial infections of the hand. Chir Main. 2015;34:18-23.
- Pandian TK, Deziel PJ, Otley CC, et al. Mycobacterium marinum infections in transplant recipients: case report and review of the literature. Transpl Infect Dis. 2008;10:358-363.
- Jacobs S, George A, Papanicolaou GA, et al. Disseminated Mycobacterium marinum infection in a hematopoietic stem cell transplant recipient. Transpl Infect Dis. 2012;14:410-414.
- Chow SP, Ip FK, Lau JH, et al. Mycobacterium marinum infection of the hand and wrist. results of conservative treatment in twenty-four cases. J Bone Joint Surg Am. 1987;69:1161-1168.
- Rallis E, Koumantaki-Mathioudaki E. Treatment of Mycobacterium marinum cutaneous infections. Expert Opin Pharmacother. 2007;8:2965-2978.
- Nenoff P, Klapper BM, Mayser P, et al. Infections due to Mycobacterium marinum: a review. Hautarzt. 2011;62:266-271.
- Prevost E, Walker EM Jr, Kreutner A Jr, et al. Mycobacterium marinum infections: diagnosis and treatment. South Med J. 1982;75:1349-1352.
Practice Points
- Mycobacterium marinum infection should be suspected in patients with skin/soft tissue infections that fail to respond or progress despite treatment with antibiotics active against streptococci and staphylococci.
- Inquiring about environmental exposure prior to the onset of the symptoms is key to elaborate a differential diagnosis list.
- Biopsy for pathology evaluation and acid-fast bacilli smear and culture are key to establish the diagnosis of M marinum infection.
Coordinating data collection in a QI project
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-18 year, offering two options for students to receive funding and engage in scholarly work during their first, second and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
I am currently working with my mentor, Dr. Ian Jenkins, an attending in the Division of Hospital Medicine at the University of California, San Diego, to start piloting data collection on our project to cut catheter-associated urinary tract infections (CAUTI). We have contacted a number of potential units to recruit for CAUTI prevention efforts, but we are hoping to do a preliminary trial of data collection to better estimate the amount of time it takes to gather data for an individual unit.
I am quickly learning that conducting a successful quality improvement project requires one to be forward-looking in an attempt to identify challenges before they arise. With respect to coordinating data collection, it may have been helpful for us to initially meet with hospital staff to identify the best staff for coordinating data collection efforts (i.e. physician, nurse, trainee) within each individual unit. This could potentially have helped us to also better communicate and recruit individuals to partner with us for our project.
I am continuing to enjoy the challenges of performing a quality improvement project. One skill that I have developed is learning how to be forward-thinking in my approach to research in an attempt to handle challenges prospectively, as opposed to retrospectively. This has helped me improve everything from how I think about data collection to how I think about displaying results. I am truly grateful to my mentor Dr. Jenkins for his help in this regard.
Victor Ekuta is a third-year medical student at UC San Diego.
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-18 year, offering two options for students to receive funding and engage in scholarly work during their first, second and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
I am currently working with my mentor, Dr. Ian Jenkins, an attending in the Division of Hospital Medicine at the University of California, San Diego, to start piloting data collection on our project to cut catheter-associated urinary tract infections (CAUTI). We have contacted a number of potential units to recruit for CAUTI prevention efforts, but we are hoping to do a preliminary trial of data collection to better estimate the amount of time it takes to gather data for an individual unit.
I am quickly learning that conducting a successful quality improvement project requires one to be forward-looking in an attempt to identify challenges before they arise. With respect to coordinating data collection, it may have been helpful for us to initially meet with hospital staff to identify the best staff for coordinating data collection efforts (i.e. physician, nurse, trainee) within each individual unit. This could potentially have helped us to also better communicate and recruit individuals to partner with us for our project.
I am continuing to enjoy the challenges of performing a quality improvement project. One skill that I have developed is learning how to be forward-thinking in my approach to research in an attempt to handle challenges prospectively, as opposed to retrospectively. This has helped me improve everything from how I think about data collection to how I think about displaying results. I am truly grateful to my mentor Dr. Jenkins for his help in this regard.
Victor Ekuta is a third-year medical student at UC San Diego.
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-18 year, offering two options for students to receive funding and engage in scholarly work during their first, second and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
I am currently working with my mentor, Dr. Ian Jenkins, an attending in the Division of Hospital Medicine at the University of California, San Diego, to start piloting data collection on our project to cut catheter-associated urinary tract infections (CAUTI). We have contacted a number of potential units to recruit for CAUTI prevention efforts, but we are hoping to do a preliminary trial of data collection to better estimate the amount of time it takes to gather data for an individual unit.
I am quickly learning that conducting a successful quality improvement project requires one to be forward-looking in an attempt to identify challenges before they arise. With respect to coordinating data collection, it may have been helpful for us to initially meet with hospital staff to identify the best staff for coordinating data collection efforts (i.e. physician, nurse, trainee) within each individual unit. This could potentially have helped us to also better communicate and recruit individuals to partner with us for our project.
I am continuing to enjoy the challenges of performing a quality improvement project. One skill that I have developed is learning how to be forward-thinking in my approach to research in an attempt to handle challenges prospectively, as opposed to retrospectively. This has helped me improve everything from how I think about data collection to how I think about displaying results. I am truly grateful to my mentor Dr. Jenkins for his help in this regard.
Victor Ekuta is a third-year medical student at UC San Diego.
From the Washington Office: Taking action
As we head into the last few weeks of the first session of the 115th Congress, it is likely that several pieces of “must pass” legislation will move through the process of becoming law. This “must pass” legislation can serve as a vehicle onto which other bills are attached and thus, also move successfully through the process for passage. I have highlighted below three such bills from the Action Alert section of the SurgeonsVoice website (www.surgeonsvoice.com) which could, with less than 5 minutes of your time, develop enough forward momentum to so move.
Ensuring Access to General Surgery Act
Increasing evidence indicates a current and growing shortage of surgeons available to serve our nation’s population. A shortage of general surgeons is a critical component of the crisis in health care workforce because only surgeons are uniquely trained and qualified to provide certain necessary, lifesaving procedures. Accordingly, the American College of Surgeons (ACS) is urging policy makers to recognize, through the designation of a formal surgical shortage area, that surgeons are an essential component of a community based health care system.
Unlike other key providers of the community-based health care system, general surgeons do not currently have a formal workforce shortage area designation. In light of growing evidence demonstrating a shortage of general surgeons, ACS believes that more accurate and actionable workforce data are necessary to determine exactly what constitutes a surgical shortage area for general surgery, and where these areas exist. Identifying where patients lack access to surgical services will provide HRSA with a valuable new tool for increasing access to the full spectrum of high-quality health care services. Determining what constitutes and defines a surgical shortage area is an important first step in guaranteeing all Medicare beneficiaries, regardless of geographic location, have access to quality surgical care.
Mission Zero Act
It has long been a priority of the ACS to establish and maintain high-quality and adequately-funded trauma systems throughout the U.S., including within the Armed Forces. The Mission Zero Act, introduced by Chairman of the House Energy and Commerce Health Subcommittee, Michael Burgess, MD (R-TX), Representatives Gene Green (D-TX), Richard Hudson (R-NC), and Kathy Castor (D-FL) in the House of Representatives and Senators Johnny Isakson (R-GA), John Cornyn (R-TX), and Tammy Duckworth (D-IL) in the Senate, would provide HHS grant funding to assist civilian trauma centers in partnering with military trauma professionals to establish a pathway to provide patients with the highest quality trauma care. As a result of these partnerships, military trauma care teams and providers will gain exposure treating critically injured patients and increase readiness for future deployments. Not only will this serve to maintain readiness among military providers, but it will facilitate the promulgation of the trauma lessons learned from the military theatres of conflict to the civilian world and potentially alleviate staffing shortages in civilian centers.
CHIP Funding
The Children’s Health Insurance Program (CHIP) is a joint federal and state program that provides health coverage to uninsured children from low-income families. In 2015, the CHIP program provided coverage to over 8 million children in the United States. In sum, CHIP ensures that these children have access to care. The ACS is very supportive of the CHIP program. The CHIP program ensures that a child’s health care concerns are addressed in a timely manner. Contrary to popular belief, many children currently covered by CHIP are not eligible to be covered under Medicaid and would therefore, be left uninsured if CHIP funding is not continued. The most recent reauthorization of this program extended funding for the CHIP program through Sept. 30, 2017, and funding for the program expired on that date. Urgent Congressional action is needed to reauthorize funding and thus, ensure that the children covered by CHIP continue to have access to the health care services they need.
The ACS strongly urges Congress to continue to make children’s health care a priority issue and accordingly, implores Congress to take action to reauthorize CHIP funding prior to concluding the business of the current session.
The SurgeonsVoice website provides an easy and efficient platform for surgeons to use to contact their senators and their representative to let them know of their support of these issues. Taking action on all three of these items would require the investment of less than 5 minutes of one’s valuable time. Our ability “to petition the government for a redress of grievances” is guaranteed by the First Amendment. I urge all Fellows to visit the SurgeonsVoice website and use it as a tool to exercise that right.
Until next month ….
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
As we head into the last few weeks of the first session of the 115th Congress, it is likely that several pieces of “must pass” legislation will move through the process of becoming law. This “must pass” legislation can serve as a vehicle onto which other bills are attached and thus, also move successfully through the process for passage. I have highlighted below three such bills from the Action Alert section of the SurgeonsVoice website (www.surgeonsvoice.com) which could, with less than 5 minutes of your time, develop enough forward momentum to so move.
Ensuring Access to General Surgery Act
Increasing evidence indicates a current and growing shortage of surgeons available to serve our nation’s population. A shortage of general surgeons is a critical component of the crisis in health care workforce because only surgeons are uniquely trained and qualified to provide certain necessary, lifesaving procedures. Accordingly, the American College of Surgeons (ACS) is urging policy makers to recognize, through the designation of a formal surgical shortage area, that surgeons are an essential component of a community based health care system.
Unlike other key providers of the community-based health care system, general surgeons do not currently have a formal workforce shortage area designation. In light of growing evidence demonstrating a shortage of general surgeons, ACS believes that more accurate and actionable workforce data are necessary to determine exactly what constitutes a surgical shortage area for general surgery, and where these areas exist. Identifying where patients lack access to surgical services will provide HRSA with a valuable new tool for increasing access to the full spectrum of high-quality health care services. Determining what constitutes and defines a surgical shortage area is an important first step in guaranteeing all Medicare beneficiaries, regardless of geographic location, have access to quality surgical care.
Mission Zero Act
It has long been a priority of the ACS to establish and maintain high-quality and adequately-funded trauma systems throughout the U.S., including within the Armed Forces. The Mission Zero Act, introduced by Chairman of the House Energy and Commerce Health Subcommittee, Michael Burgess, MD (R-TX), Representatives Gene Green (D-TX), Richard Hudson (R-NC), and Kathy Castor (D-FL) in the House of Representatives and Senators Johnny Isakson (R-GA), John Cornyn (R-TX), and Tammy Duckworth (D-IL) in the Senate, would provide HHS grant funding to assist civilian trauma centers in partnering with military trauma professionals to establish a pathway to provide patients with the highest quality trauma care. As a result of these partnerships, military trauma care teams and providers will gain exposure treating critically injured patients and increase readiness for future deployments. Not only will this serve to maintain readiness among military providers, but it will facilitate the promulgation of the trauma lessons learned from the military theatres of conflict to the civilian world and potentially alleviate staffing shortages in civilian centers.
CHIP Funding
The Children’s Health Insurance Program (CHIP) is a joint federal and state program that provides health coverage to uninsured children from low-income families. In 2015, the CHIP program provided coverage to over 8 million children in the United States. In sum, CHIP ensures that these children have access to care. The ACS is very supportive of the CHIP program. The CHIP program ensures that a child’s health care concerns are addressed in a timely manner. Contrary to popular belief, many children currently covered by CHIP are not eligible to be covered under Medicaid and would therefore, be left uninsured if CHIP funding is not continued. The most recent reauthorization of this program extended funding for the CHIP program through Sept. 30, 2017, and funding for the program expired on that date. Urgent Congressional action is needed to reauthorize funding and thus, ensure that the children covered by CHIP continue to have access to the health care services they need.
The ACS strongly urges Congress to continue to make children’s health care a priority issue and accordingly, implores Congress to take action to reauthorize CHIP funding prior to concluding the business of the current session.
The SurgeonsVoice website provides an easy and efficient platform for surgeons to use to contact their senators and their representative to let them know of their support of these issues. Taking action on all three of these items would require the investment of less than 5 minutes of one’s valuable time. Our ability “to petition the government for a redress of grievances” is guaranteed by the First Amendment. I urge all Fellows to visit the SurgeonsVoice website and use it as a tool to exercise that right.
Until next month ….
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
As we head into the last few weeks of the first session of the 115th Congress, it is likely that several pieces of “must pass” legislation will move through the process of becoming law. This “must pass” legislation can serve as a vehicle onto which other bills are attached and thus, also move successfully through the process for passage. I have highlighted below three such bills from the Action Alert section of the SurgeonsVoice website (www.surgeonsvoice.com) which could, with less than 5 minutes of your time, develop enough forward momentum to so move.
Ensuring Access to General Surgery Act
Increasing evidence indicates a current and growing shortage of surgeons available to serve our nation’s population. A shortage of general surgeons is a critical component of the crisis in health care workforce because only surgeons are uniquely trained and qualified to provide certain necessary, lifesaving procedures. Accordingly, the American College of Surgeons (ACS) is urging policy makers to recognize, through the designation of a formal surgical shortage area, that surgeons are an essential component of a community based health care system.
Unlike other key providers of the community-based health care system, general surgeons do not currently have a formal workforce shortage area designation. In light of growing evidence demonstrating a shortage of general surgeons, ACS believes that more accurate and actionable workforce data are necessary to determine exactly what constitutes a surgical shortage area for general surgery, and where these areas exist. Identifying where patients lack access to surgical services will provide HRSA with a valuable new tool for increasing access to the full spectrum of high-quality health care services. Determining what constitutes and defines a surgical shortage area is an important first step in guaranteeing all Medicare beneficiaries, regardless of geographic location, have access to quality surgical care.
Mission Zero Act
It has long been a priority of the ACS to establish and maintain high-quality and adequately-funded trauma systems throughout the U.S., including within the Armed Forces. The Mission Zero Act, introduced by Chairman of the House Energy and Commerce Health Subcommittee, Michael Burgess, MD (R-TX), Representatives Gene Green (D-TX), Richard Hudson (R-NC), and Kathy Castor (D-FL) in the House of Representatives and Senators Johnny Isakson (R-GA), John Cornyn (R-TX), and Tammy Duckworth (D-IL) in the Senate, would provide HHS grant funding to assist civilian trauma centers in partnering with military trauma professionals to establish a pathway to provide patients with the highest quality trauma care. As a result of these partnerships, military trauma care teams and providers will gain exposure treating critically injured patients and increase readiness for future deployments. Not only will this serve to maintain readiness among military providers, but it will facilitate the promulgation of the trauma lessons learned from the military theatres of conflict to the civilian world and potentially alleviate staffing shortages in civilian centers.
CHIP Funding
The Children’s Health Insurance Program (CHIP) is a joint federal and state program that provides health coverage to uninsured children from low-income families. In 2015, the CHIP program provided coverage to over 8 million children in the United States. In sum, CHIP ensures that these children have access to care. The ACS is very supportive of the CHIP program. The CHIP program ensures that a child’s health care concerns are addressed in a timely manner. Contrary to popular belief, many children currently covered by CHIP are not eligible to be covered under Medicaid and would therefore, be left uninsured if CHIP funding is not continued. The most recent reauthorization of this program extended funding for the CHIP program through Sept. 30, 2017, and funding for the program expired on that date. Urgent Congressional action is needed to reauthorize funding and thus, ensure that the children covered by CHIP continue to have access to the health care services they need.
The ACS strongly urges Congress to continue to make children’s health care a priority issue and accordingly, implores Congress to take action to reauthorize CHIP funding prior to concluding the business of the current session.
The SurgeonsVoice website provides an easy and efficient platform for surgeons to use to contact their senators and their representative to let them know of their support of these issues. Taking action on all three of these items would require the investment of less than 5 minutes of one’s valuable time. Our ability “to petition the government for a redress of grievances” is guaranteed by the First Amendment. I urge all Fellows to visit the SurgeonsVoice website and use it as a tool to exercise that right.
Until next month ….
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
One-step GDM diagnosis: Research moves closer
WASHINGTON – in the United States.
The American College of Obstetricians and Gynecologists now acknowledges this approach as an option, yet “tremendous controversy persists,” according to Mark Landon, MD.
“In the U.S., we continue to be the principal purveyors of a two-step method with a 100-g [oral glucose tolerance test] diagnostic approach, which is in contrast to much of the rest of the world,” he said the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“At this time, if we’re going to [turn nationally] to the one-step approach, we have to lower the cost of diagnosis and treatment, and we may need some upwards adjustments in the [International Association of Diabetes and Pregnancy Study Groups] criteria in order to achieve consensus,” said Dr. Landon, professor and chair of the department of obstetrics and gynecology at Ohio State University, Columbus.
The International Association of Diabetes in Pregnancy Study Groups (IADPSG) created a stir in the American obstetrics community when it recommended in 2010 that a universal 75-g, 2-hour oral glucose tolerance test (OGTT) be performed during pregnancy and that gestational diabetes mellitus (GDM) be diagnosed when any single measurement threshold – a fasting value of 92 mg/dL, a 1-hour value of 180 mg/dL, or a 2-hour value of 153 mg/dL – is met or exceeded (Diabetes Care 2010 Mar; 33[3]:676-82).
The consensus group made its recommendation based largely on published associations of maternal glycemia with perinatal and long-term outcomes in offspring. Chief among the studies was the landmark Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, which found continuous linear relationships between maternal glucose levels – including levels that had been viewed as normal – and adverse fetal outcomes such as high fetal birth weight, cord-blood serum C-peptide level (an index of fetal beta-cell function and fetal hyperinsulinemia), and clinical neonatal hypoglycemia. Maternal glucose tolerance was measured in the study with the 75-g 2-hour OGTT.
The IADPSG chose its cut-off points to convey an odds ratio for adverse outcomes of 1.75. But use of the criteria meant that 16%-18% of pregnant women in the United States would be identified as having GDM – a doubling, at least.
In 2013, a National Institute of Child Health and Human Development Consensus Development Conference recommended against adoption of the new criteria, citing uncertainties regarding the benefits of treating so many additional cases of GDM, as well as the costs and additional burden on patients, providers, and the health care system.
In an updated Practice Bulletin on GDM, ACOG recommends that the suggested changes be studied “before they are proposed at a national level.” But ACOG noted that “individual practices and institutions may choose to use the IADPSG’s recommendation, if appropriate, for the population they serve” (Obstet Gynecol. 2017;130[1]:e17-37).
Since the IADPSG proposal came out, Dr. Landon said, at least a half-dozen published studies have attempted to clarify the additional benefit of their proposed criteria, analyzing the risk of adverse maternal and fetal outcomes in women who are diagnosed using IADPSG criteria and not treated, versus those with a normal glucose tolerance test. In these analyses, researchers have excluded women who would also meet usual diagnostic criteria, such as the Carpenter-Coustan criteria, in order to hone in on those with the mildest levels of GDM – the new diagnoses.
Research published “in the last 5-6 years has almost exclusively shown that, in using the IADPSG criteria, and excluding other usual criteria, you see graded, increased frequencies in large babies, preeclampsia, [neonatal] hypoglycemia” and other adverse outcomes, Dr. Landon said. “I know of only one study that refutes these associations.”
A secondary analysis of HAPO study data, for instance, grouped women into three categories: those with no GDM, GDM based on traditional Carpenter-Coustan criteria, and GDM based on IADPSG criteria but not the Carpenter-Coustan thresholds. A 3-hour OGTT result was not used in this analysis since the HAPO study did not collect this.
Compared with cases with no GDM, those with GDM based on IADPSG criteria (but not the Carpenter-Coustan criteria) were nearly twice as likely to have birth weights above the 90th percentile, newborn percentage fat over the 90th percentile, and preeclampsia, for instance (Diabetes Care 2016;39[12];2204-10).
Other researchers are trying to tease apart risk levels according to thresholds that differ slightly from traditional criteria. A retrospective cohort study from Kaiser Permanente Southern California, for instance, chose two strata of women whose GDM was in the lower levels of the IADPSG-defined spectrum for glucose intolerance and found that, in those with the lesser degree of hyperglycemia, only birth weight and large-for-gestational-age was significantly greater than in women with no GDM (Obstet Gynecol. 2015;126[1]:67-73).
“This study is interesting because it raises the question of whether there might be differential treatment effects based on the level of hyperglycemia within the IADPSG category,” Dr. Landon said.
Dr. Landon served as the principal investigator of a large national, randomized controlled trial that showed a reduction in the risk of fetal overgrowth, shoulder dystocia, cesarean delivery, and hypertensive disorders in women who were treated for mild gestational diabetes (N Engl J Med. 2009;361:1339-48). But this study defined mild gestational diabetes according to the Carpenter-Coustan criteria.
“What about the women who meet the [even lower thresholds] of the IADPSG criteria? One would expect that the treatment benefit would not be as great, but will they still benefit from treatment? To date, this is simply unknown,” he said in an interview.
Research in the last 5 years has also begun to look at the financial implications of the IADPSG criteria and strategies for reducing the cost of implementation. Dr. Landon noted that investigators in Brazil, for instance, have determined that an alternative strategy of using a fasting plasma glucose value of 92 mg/gL or greater to rule in GDM, and a fasting value of 80 mg/dL or less to rule out GDM, eliminates the need for 61% of oral glucose challenges and has 96.9% sensitivity for diagnosing GDM (Diabetes Res Clin Pract. 2015 May;108[2]:288-95).
Dr. Landon reported having no relevant financial disclosures.
WASHINGTON – in the United States.
The American College of Obstetricians and Gynecologists now acknowledges this approach as an option, yet “tremendous controversy persists,” according to Mark Landon, MD.
“In the U.S., we continue to be the principal purveyors of a two-step method with a 100-g [oral glucose tolerance test] diagnostic approach, which is in contrast to much of the rest of the world,” he said the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“At this time, if we’re going to [turn nationally] to the one-step approach, we have to lower the cost of diagnosis and treatment, and we may need some upwards adjustments in the [International Association of Diabetes and Pregnancy Study Groups] criteria in order to achieve consensus,” said Dr. Landon, professor and chair of the department of obstetrics and gynecology at Ohio State University, Columbus.
The International Association of Diabetes in Pregnancy Study Groups (IADPSG) created a stir in the American obstetrics community when it recommended in 2010 that a universal 75-g, 2-hour oral glucose tolerance test (OGTT) be performed during pregnancy and that gestational diabetes mellitus (GDM) be diagnosed when any single measurement threshold – a fasting value of 92 mg/dL, a 1-hour value of 180 mg/dL, or a 2-hour value of 153 mg/dL – is met or exceeded (Diabetes Care 2010 Mar; 33[3]:676-82).
The consensus group made its recommendation based largely on published associations of maternal glycemia with perinatal and long-term outcomes in offspring. Chief among the studies was the landmark Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, which found continuous linear relationships between maternal glucose levels – including levels that had been viewed as normal – and adverse fetal outcomes such as high fetal birth weight, cord-blood serum C-peptide level (an index of fetal beta-cell function and fetal hyperinsulinemia), and clinical neonatal hypoglycemia. Maternal glucose tolerance was measured in the study with the 75-g 2-hour OGTT.
The IADPSG chose its cut-off points to convey an odds ratio for adverse outcomes of 1.75. But use of the criteria meant that 16%-18% of pregnant women in the United States would be identified as having GDM – a doubling, at least.
In 2013, a National Institute of Child Health and Human Development Consensus Development Conference recommended against adoption of the new criteria, citing uncertainties regarding the benefits of treating so many additional cases of GDM, as well as the costs and additional burden on patients, providers, and the health care system.
In an updated Practice Bulletin on GDM, ACOG recommends that the suggested changes be studied “before they are proposed at a national level.” But ACOG noted that “individual practices and institutions may choose to use the IADPSG’s recommendation, if appropriate, for the population they serve” (Obstet Gynecol. 2017;130[1]:e17-37).
Since the IADPSG proposal came out, Dr. Landon said, at least a half-dozen published studies have attempted to clarify the additional benefit of their proposed criteria, analyzing the risk of adverse maternal and fetal outcomes in women who are diagnosed using IADPSG criteria and not treated, versus those with a normal glucose tolerance test. In these analyses, researchers have excluded women who would also meet usual diagnostic criteria, such as the Carpenter-Coustan criteria, in order to hone in on those with the mildest levels of GDM – the new diagnoses.
Research published “in the last 5-6 years has almost exclusively shown that, in using the IADPSG criteria, and excluding other usual criteria, you see graded, increased frequencies in large babies, preeclampsia, [neonatal] hypoglycemia” and other adverse outcomes, Dr. Landon said. “I know of only one study that refutes these associations.”
A secondary analysis of HAPO study data, for instance, grouped women into three categories: those with no GDM, GDM based on traditional Carpenter-Coustan criteria, and GDM based on IADPSG criteria but not the Carpenter-Coustan thresholds. A 3-hour OGTT result was not used in this analysis since the HAPO study did not collect this.
Compared with cases with no GDM, those with GDM based on IADPSG criteria (but not the Carpenter-Coustan criteria) were nearly twice as likely to have birth weights above the 90th percentile, newborn percentage fat over the 90th percentile, and preeclampsia, for instance (Diabetes Care 2016;39[12];2204-10).
Other researchers are trying to tease apart risk levels according to thresholds that differ slightly from traditional criteria. A retrospective cohort study from Kaiser Permanente Southern California, for instance, chose two strata of women whose GDM was in the lower levels of the IADPSG-defined spectrum for glucose intolerance and found that, in those with the lesser degree of hyperglycemia, only birth weight and large-for-gestational-age was significantly greater than in women with no GDM (Obstet Gynecol. 2015;126[1]:67-73).
“This study is interesting because it raises the question of whether there might be differential treatment effects based on the level of hyperglycemia within the IADPSG category,” Dr. Landon said.
Dr. Landon served as the principal investigator of a large national, randomized controlled trial that showed a reduction in the risk of fetal overgrowth, shoulder dystocia, cesarean delivery, and hypertensive disorders in women who were treated for mild gestational diabetes (N Engl J Med. 2009;361:1339-48). But this study defined mild gestational diabetes according to the Carpenter-Coustan criteria.
“What about the women who meet the [even lower thresholds] of the IADPSG criteria? One would expect that the treatment benefit would not be as great, but will they still benefit from treatment? To date, this is simply unknown,” he said in an interview.
Research in the last 5 years has also begun to look at the financial implications of the IADPSG criteria and strategies for reducing the cost of implementation. Dr. Landon noted that investigators in Brazil, for instance, have determined that an alternative strategy of using a fasting plasma glucose value of 92 mg/gL or greater to rule in GDM, and a fasting value of 80 mg/dL or less to rule out GDM, eliminates the need for 61% of oral glucose challenges and has 96.9% sensitivity for diagnosing GDM (Diabetes Res Clin Pract. 2015 May;108[2]:288-95).
Dr. Landon reported having no relevant financial disclosures.
WASHINGTON – in the United States.
The American College of Obstetricians and Gynecologists now acknowledges this approach as an option, yet “tremendous controversy persists,” according to Mark Landon, MD.
“In the U.S., we continue to be the principal purveyors of a two-step method with a 100-g [oral glucose tolerance test] diagnostic approach, which is in contrast to much of the rest of the world,” he said the biennial meeting of the Diabetes in Pregnancy Study Group of North America.
“At this time, if we’re going to [turn nationally] to the one-step approach, we have to lower the cost of diagnosis and treatment, and we may need some upwards adjustments in the [International Association of Diabetes and Pregnancy Study Groups] criteria in order to achieve consensus,” said Dr. Landon, professor and chair of the department of obstetrics and gynecology at Ohio State University, Columbus.
The International Association of Diabetes in Pregnancy Study Groups (IADPSG) created a stir in the American obstetrics community when it recommended in 2010 that a universal 75-g, 2-hour oral glucose tolerance test (OGTT) be performed during pregnancy and that gestational diabetes mellitus (GDM) be diagnosed when any single measurement threshold – a fasting value of 92 mg/dL, a 1-hour value of 180 mg/dL, or a 2-hour value of 153 mg/dL – is met or exceeded (Diabetes Care 2010 Mar; 33[3]:676-82).
The consensus group made its recommendation based largely on published associations of maternal glycemia with perinatal and long-term outcomes in offspring. Chief among the studies was the landmark Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, which found continuous linear relationships between maternal glucose levels – including levels that had been viewed as normal – and adverse fetal outcomes such as high fetal birth weight, cord-blood serum C-peptide level (an index of fetal beta-cell function and fetal hyperinsulinemia), and clinical neonatal hypoglycemia. Maternal glucose tolerance was measured in the study with the 75-g 2-hour OGTT.
The IADPSG chose its cut-off points to convey an odds ratio for adverse outcomes of 1.75. But use of the criteria meant that 16%-18% of pregnant women in the United States would be identified as having GDM – a doubling, at least.
In 2013, a National Institute of Child Health and Human Development Consensus Development Conference recommended against adoption of the new criteria, citing uncertainties regarding the benefits of treating so many additional cases of GDM, as well as the costs and additional burden on patients, providers, and the health care system.
In an updated Practice Bulletin on GDM, ACOG recommends that the suggested changes be studied “before they are proposed at a national level.” But ACOG noted that “individual practices and institutions may choose to use the IADPSG’s recommendation, if appropriate, for the population they serve” (Obstet Gynecol. 2017;130[1]:e17-37).
Since the IADPSG proposal came out, Dr. Landon said, at least a half-dozen published studies have attempted to clarify the additional benefit of their proposed criteria, analyzing the risk of adverse maternal and fetal outcomes in women who are diagnosed using IADPSG criteria and not treated, versus those with a normal glucose tolerance test. In these analyses, researchers have excluded women who would also meet usual diagnostic criteria, such as the Carpenter-Coustan criteria, in order to hone in on those with the mildest levels of GDM – the new diagnoses.
Research published “in the last 5-6 years has almost exclusively shown that, in using the IADPSG criteria, and excluding other usual criteria, you see graded, increased frequencies in large babies, preeclampsia, [neonatal] hypoglycemia” and other adverse outcomes, Dr. Landon said. “I know of only one study that refutes these associations.”
A secondary analysis of HAPO study data, for instance, grouped women into three categories: those with no GDM, GDM based on traditional Carpenter-Coustan criteria, and GDM based on IADPSG criteria but not the Carpenter-Coustan thresholds. A 3-hour OGTT result was not used in this analysis since the HAPO study did not collect this.
Compared with cases with no GDM, those with GDM based on IADPSG criteria (but not the Carpenter-Coustan criteria) were nearly twice as likely to have birth weights above the 90th percentile, newborn percentage fat over the 90th percentile, and preeclampsia, for instance (Diabetes Care 2016;39[12];2204-10).
Other researchers are trying to tease apart risk levels according to thresholds that differ slightly from traditional criteria. A retrospective cohort study from Kaiser Permanente Southern California, for instance, chose two strata of women whose GDM was in the lower levels of the IADPSG-defined spectrum for glucose intolerance and found that, in those with the lesser degree of hyperglycemia, only birth weight and large-for-gestational-age was significantly greater than in women with no GDM (Obstet Gynecol. 2015;126[1]:67-73).
“This study is interesting because it raises the question of whether there might be differential treatment effects based on the level of hyperglycemia within the IADPSG category,” Dr. Landon said.
Dr. Landon served as the principal investigator of a large national, randomized controlled trial that showed a reduction in the risk of fetal overgrowth, shoulder dystocia, cesarean delivery, and hypertensive disorders in women who were treated for mild gestational diabetes (N Engl J Med. 2009;361:1339-48). But this study defined mild gestational diabetes according to the Carpenter-Coustan criteria.
“What about the women who meet the [even lower thresholds] of the IADPSG criteria? One would expect that the treatment benefit would not be as great, but will they still benefit from treatment? To date, this is simply unknown,” he said in an interview.
Research in the last 5 years has also begun to look at the financial implications of the IADPSG criteria and strategies for reducing the cost of implementation. Dr. Landon noted that investigators in Brazil, for instance, have determined that an alternative strategy of using a fasting plasma glucose value of 92 mg/gL or greater to rule in GDM, and a fasting value of 80 mg/dL or less to rule out GDM, eliminates the need for 61% of oral glucose challenges and has 96.9% sensitivity for diagnosing GDM (Diabetes Res Clin Pract. 2015 May;108[2]:288-95).
Dr. Landon reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM DPSG-NA 2017
Optimal Treatments for Cardiovascular Diseases
Click here to access Optimal Treatments for Cardiovascular Diseases
Table of Contents
- Roundtable Discussion : Anticoagulation Management
- The Pharmacist’s Role in Medication Optimization for Patients With Chronic Heart Failure
- Pharmacist Interventions to Reduce Modifiable Bleeding Risk Factors Using HAS-BLED in Patients Taking Warfarin

Click here to access Optimal Treatments for Cardiovascular Diseases
Table of Contents
- Roundtable Discussion : Anticoagulation Management
- The Pharmacist’s Role in Medication Optimization for Patients With Chronic Heart Failure
- Pharmacist Interventions to Reduce Modifiable Bleeding Risk Factors Using HAS-BLED in Patients Taking Warfarin

Click here to access Optimal Treatments for Cardiovascular Diseases
Table of Contents
- Roundtable Discussion : Anticoagulation Management
- The Pharmacist’s Role in Medication Optimization for Patients With Chronic Heart Failure
- Pharmacist Interventions to Reduce Modifiable Bleeding Risk Factors Using HAS-BLED in Patients Taking Warfarin

Rural Communities Have High Rates of Suicide
More than half a million people committed suicide between 2001 and 2015, according to the CDC. Rural counties consistently had higher rates than those of metropolitan areas. “While we’ve seen many causes of death come down in recent years, suicide rates have increased more than 20% from 2001 to 2015,” said Brenda Fitzgerald, MD, CDC director. “And this is especially concerning in rural areas.”
Related: Suicide Federal Health Data Trends
Suicide rates in rural counties were 17.32 per 100,000 people compared with 14.86 in small-to-medium metropolitan counties and 11.92 in large metropolitan counties. Rates for American Indian/Alaska Native non-Hispanics were the highest.
The researchers note that, at some points, different negative factors had more impact. For instance, rural communities were harder hit by housing foreclosures, poverty, and unemployment due to the recession. However, the researchers also point out that suicide rates were on the rise before the recession began.
“The trends in suicide rates…are magnified in rural areas,” said James Mercy, PhD, director of CDC’s Division of Violence Prevention. “This report underscores the need for suicide prevention strategies that are specifically tailored for these communities.” To that end, the CDC recently released a compilation of evidence-based strategies that have the greatest prevention potential. The set includes examples of programs that can be customized to fit the cultural needs of different groups. In North Dakota, for instance, the program Sources of Strength was developed for tribal communities to promote connectedness between youth and adults.
Related: Improving Veteran Engagement With Mental Health Care
The Health Resource and Service Administration (HRSA) also has developed activities to address suicide in rural areas, including epidemiologic studies, research, telemedicine, and programs addressing primary health care providers.
https://www.cdc.gov/violenceprevention/pub/technical-packages.html
More than half a million people committed suicide between 2001 and 2015, according to the CDC. Rural counties consistently had higher rates than those of metropolitan areas. “While we’ve seen many causes of death come down in recent years, suicide rates have increased more than 20% from 2001 to 2015,” said Brenda Fitzgerald, MD, CDC director. “And this is especially concerning in rural areas.”
Related: Suicide Federal Health Data Trends
Suicide rates in rural counties were 17.32 per 100,000 people compared with 14.86 in small-to-medium metropolitan counties and 11.92 in large metropolitan counties. Rates for American Indian/Alaska Native non-Hispanics were the highest.
The researchers note that, at some points, different negative factors had more impact. For instance, rural communities were harder hit by housing foreclosures, poverty, and unemployment due to the recession. However, the researchers also point out that suicide rates were on the rise before the recession began.
“The trends in suicide rates…are magnified in rural areas,” said James Mercy, PhD, director of CDC’s Division of Violence Prevention. “This report underscores the need for suicide prevention strategies that are specifically tailored for these communities.” To that end, the CDC recently released a compilation of evidence-based strategies that have the greatest prevention potential. The set includes examples of programs that can be customized to fit the cultural needs of different groups. In North Dakota, for instance, the program Sources of Strength was developed for tribal communities to promote connectedness between youth and adults.
Related: Improving Veteran Engagement With Mental Health Care
The Health Resource and Service Administration (HRSA) also has developed activities to address suicide in rural areas, including epidemiologic studies, research, telemedicine, and programs addressing primary health care providers.
https://www.cdc.gov/violenceprevention/pub/technical-packages.html
More than half a million people committed suicide between 2001 and 2015, according to the CDC. Rural counties consistently had higher rates than those of metropolitan areas. “While we’ve seen many causes of death come down in recent years, suicide rates have increased more than 20% from 2001 to 2015,” said Brenda Fitzgerald, MD, CDC director. “And this is especially concerning in rural areas.”
Related: Suicide Federal Health Data Trends
Suicide rates in rural counties were 17.32 per 100,000 people compared with 14.86 in small-to-medium metropolitan counties and 11.92 in large metropolitan counties. Rates for American Indian/Alaska Native non-Hispanics were the highest.
The researchers note that, at some points, different negative factors had more impact. For instance, rural communities were harder hit by housing foreclosures, poverty, and unemployment due to the recession. However, the researchers also point out that suicide rates were on the rise before the recession began.
“The trends in suicide rates…are magnified in rural areas,” said James Mercy, PhD, director of CDC’s Division of Violence Prevention. “This report underscores the need for suicide prevention strategies that are specifically tailored for these communities.” To that end, the CDC recently released a compilation of evidence-based strategies that have the greatest prevention potential. The set includes examples of programs that can be customized to fit the cultural needs of different groups. In North Dakota, for instance, the program Sources of Strength was developed for tribal communities to promote connectedness between youth and adults.
Related: Improving Veteran Engagement With Mental Health Care
The Health Resource and Service Administration (HRSA) also has developed activities to address suicide in rural areas, including epidemiologic studies, research, telemedicine, and programs addressing primary health care providers.
https://www.cdc.gov/violenceprevention/pub/technical-packages.html
High Deductible Health Plans: Take Accounts Receivable Action Now
If you missed the recent headlines, Why Patients Delay Medical Payments: 12 findings 1 and You think your health insurance costs too much. Try being a farmer.2, you may not be too worried about your ever-rising accounts receivables. But you should be.
The facts in these stories and the 2017 Employer Health Benefits Survey,3 released on September 19 by the non-partisan Kaiser Family Foundation and Health Research & Educational Trust (HRET), are alarming. Let’s look at some of the survey results.
Since 2007, the average family premium has increased 55% and the average worker contribution toward the premium has increased 74%.3 How does that translate into dollars and cents? Well, the average annual premiums this year are $6690 for single coverage and $18,764 for family coverage.
What does that mean exactly to the farm couple in the Crain’s story? The farmer who “will be lucky to net $75,000” on his hay crop this year has a policy premium with Blue Cross Blue Shield of Illinois that was $22,000 last year. And then there is a $5000 deductible for each him and his wife. Do the math: it means they’d spend 43% of their income before health insurance covers anything.
Do premiums vary significantly by firm size or region? Should surgeons in certain areas of the country be less concerned about these trends? No, the premiums don’t significantly vary by size or region.
The point here is not to write an essay about health insurance premiums, but rather to discuss what this economic reality means to patients who are seeing you tomorrow, next week, and next month. Given these economic realities, what is their attitude about your bill?
For insight, let’s look at an Advisory Board Company brief, “Minimizing Bad Debt: Point-of-Service Collections,”4 which states: “Patient propensity to pay decreases as patient obligation increases.” According to the brief, “Our analysis indicates that as the dollar value of a patient’s obligation increases, their propensity to pay any portion of the obligation decreases—for all patients, at all income levels.” See Figure 1.
Given that market statistics show that more than a quarter of the commercially insured patients are covered by high deductible health plans (HDHPs), your practice must adapt to these changing times.
Review Your Pay or Mix
Smart practice administrators will keep their finger on the pulse of the insurance local market as more employers move toward offering HDHPs or health savings accounts. Knowing what the largest manufacturers are offering, along with local hospitals that are typically sizable employers in most communities, is critical. The coverage for school systems, police departments, fire departments, and governments should also be the practice radar.
A recent West Corporation survey5 reveals more about the demographic profile of patients who are less likely to pay or delay payments. Their study shows that 79% of patients cite affordability as the largest healthcare problem with 93% of patients saying it costs too much. So it should be no surprise that 67% say their financial situation makes it challenging to submit timely payments. If you are not familiar with the company name, you’ll be familiar with West Corporation’s products, like Televox, which are automated tools used by practices to remind patients about appointments and copays.
Here are other relevant findings from the West Corporation survey:
- 56% delay payments of medical bills at least some of the time.
- 70% of millennials have missed medical payment deadlines.
- 42% of patients cite their HDHP as the reason for delaying their payments.
- 36% of patients said they have difficulty remembering to make timely medical payments.
It’s no surprise that orthopedic surgeons are not discussing fees with patients. Although only 9% admitted that they don’t discuss costs with patients, it is safe to estimate that few surgeons have their fee schedules memorized.
In a 2013 article, Ubel and colleagues7 said, “Because treatments can be ‘financially toxic,’ physicians need to disclose the financial consequences of treatment alternatives just as they inform patients about treatments’ side effects.”
While uniquely qualified to discuss treatment options, few orthopedic surgeons have the time or the facts to personally discuss fees, out-of-pocket expenses, uncovered services and payment plans. Detailed discussions about patients’ financial liabilities are better done by qualified staff, who verify benefits and use modern technology tools to generate an electronic “estimate of costs and benefits.”
Target Your Efforts
What steps can your practice take to help patients pay their portion, even when it’s large, as well as help your practice reduce receivables and avoid collection problems and bad debt write-offs?
Start by analyzing the top Current Procedural Terminology (CPT) codes with patient responsibility, so you can focus your efforts. One such analysis, conducted by our firm, is shown in Figure 3. Although you might think the highest percent of patient financial responsibilities are for surgical procedures, notice that 4 of the top 5 services this practice identified as having the highest amount of patient collectible dollars are rendered in the office-carpal tunnel surgery being the only exception.
Take Action
After conducting a thorough analysis and reviewing the results, here are 5 actionable steps your practice could take:
1. Make sure your patient portal has the capacity to take patient payments. Offering online payment options increases the opportunity for patients to pay. Promote this option on the patient statement.
2. Implement a system of collecting from patients before they leave the office. After a new visit, which involves a more expensive evaluation and management code, and possibly imaging and durable medical device, counsel patients to leave a credit card on file, so the minute insurance pays, their credit card can be charged.
The 2017 Navicure Patient Payment Check-Up survey8 conducted by Healthcare Information and Management Systems Society (HIMSS) Analytics shows that 78% of patients would provide a card to be charged for one time up to $200. Think about the previously illustrated collection amounts this would alleviate.
3. Provide all surgery patients with a cost estimate. Generating cost estimates has been possible for close to 10 years. It’s done through your clearinghouse and practice management software by entering the CPT codes and diagnosis codes, along with the patient’s information. Save time and avoid tying staff up on hold.
According to the Navicure Patient Payment Check-Up survey,8 75% of provider organizations are able to provide a cost estimate upon request. It makes good business sense.
4. Collect a pre-treatment or pre-surgery scheduling deposit. In the KarenZupko & Associates/American Academy of Orthopaedic Surgeons (AAOS) pre-course survey of those attending the 2017 coding and reimbursement workshops, 55% of orthopedic practices reported that they have instituted such a practice. With the proliferation of HDHPs, asking for a scheduling deposit is fast becoming a must for all surgeons.
5. Offer patients a healthcare financing option through a third party. In response to another pre-course survey question, about offering CareCredit or another healthcare credit card, 28% of orthopedic practices say they do. Still, that leaves >70% of the orthopedic patients without a financing option. Given the reality of high deductible HDHPs and the patient responsibilities going uncollected, it’s time surgeons take a look at financing. It’s a fool’s wish to believe the practice is “saving” the service fee by sending dozens of statements, having staff make calls, and ultimately writing off unpaid balances as uncollectible.
Practices that fail to change, will fail to prosper. Those who have technology-phobic staff will suffer as healthcare continues to automate. Practices led by surgeons like one recently interviewed who said, “If patients knew how much it cost, they’d never schedule” will see patient accounts receivable soar and patient online ratings sink. The first quarter of 2018 means the number of patients with HDHPs will increase and that deductibles will have to be met. It’s wise to have a full staff meeting, share the facts, and put an action plan in place.
1. Gooch K. Why patients delay medical payments: 12 findings. Becker’s ASC. https://www.beckershospitalreview.com/finance/why-patients-delay-medical-payments-12-findings.html. Published August 28, 2017.
2. Murphy HL. You think your health insurance costs too much. Try being a farmer. Crain’s Chicago Business. http://www.chicagobusiness.com/article/20170929/ISSUE01/170929835. Published September 29, 2017. Accessed October 2, 2017.
3. 2017 Employer Health Benefits Survey. The Henry J. Kaiser Family Foundation and the Health Research & Educational Trust (HRET). https://www.kff.org/health-costs/report/2017-employer-health-benefits-survey/. Published September 19, 2017.
4. Minimizing Bad Debt: Point-of-Service Collections. The Advisory Board Company. https://www.advisory.com/-/media/Advisory-com/Research/FLC/Resources/2015/CFO-Brief-POS.pdf. Published August 21, 2015.
5. Optimizing Revenue: Solving Healthcare’s Revenue Cycle Challenges Using Technology-Enabled Communications. West Corporation. https://cdn2.hubspot.net/hubfs/402746/Assets/West%20Assets/Optimizing%20Revenue%20Report/Reports%20and%20Handouts/WEST-Optimizing%20Revenue%20Report%20final.pdf?t=1508789915319. Accessed October 26, 2017.
6. Peckham C. Medscape Orthopedist Compensation Report 2016. Medscape. https://www.medscape.com/features/slideshow/compensation/2016/orthopedics. Published April 1, 2016.
7. Ubel PA, Abernathy AP, Zafar SY. Full disclosure - out-of-pocket costs as side effects. N Engl J. Med. 2013;369:1484-1486. doi:10.1056/NEJMp1306826.
8. Patient Payment Check-Up 2017. Navicure. http://info.navicure.com/rs/669-OIJ-380/images/Navicure-Survey-Report-2017-Patient-Payment-Check-Up.pdf?mkt_tok=eyJpIjoiTVdKak1HUmhObVV6WkRVeSIsInQiOiJRcFNyRGVrOXlTS0pjalwvWEw3c2s1UmRMRHJVXC9EQzRkSnBkWCs0S2FEbUt3Z1I1a2Y3d1BBY3FKY0I1QWpEdkJRWU9ibmFlUlpnYVRIbVJMcStTVmdkRVwvSTJzcHE1cDVTajBRM3B1Q25lbDQwamViWnMwWGd1c1QzVk1cL2hYdkYifQ%3D%3D. Accessed October 26, 2017.
If you missed the recent headlines, Why Patients Delay Medical Payments: 12 findings 1 and You think your health insurance costs too much. Try being a farmer.2, you may not be too worried about your ever-rising accounts receivables. But you should be.
The facts in these stories and the 2017 Employer Health Benefits Survey,3 released on September 19 by the non-partisan Kaiser Family Foundation and Health Research & Educational Trust (HRET), are alarming. Let’s look at some of the survey results.
Since 2007, the average family premium has increased 55% and the average worker contribution toward the premium has increased 74%.3 How does that translate into dollars and cents? Well, the average annual premiums this year are $6690 for single coverage and $18,764 for family coverage.
What does that mean exactly to the farm couple in the Crain’s story? The farmer who “will be lucky to net $75,000” on his hay crop this year has a policy premium with Blue Cross Blue Shield of Illinois that was $22,000 last year. And then there is a $5000 deductible for each him and his wife. Do the math: it means they’d spend 43% of their income before health insurance covers anything.
Do premiums vary significantly by firm size or region? Should surgeons in certain areas of the country be less concerned about these trends? No, the premiums don’t significantly vary by size or region.
The point here is not to write an essay about health insurance premiums, but rather to discuss what this economic reality means to patients who are seeing you tomorrow, next week, and next month. Given these economic realities, what is their attitude about your bill?
For insight, let’s look at an Advisory Board Company brief, “Minimizing Bad Debt: Point-of-Service Collections,”4 which states: “Patient propensity to pay decreases as patient obligation increases.” According to the brief, “Our analysis indicates that as the dollar value of a patient’s obligation increases, their propensity to pay any portion of the obligation decreases—for all patients, at all income levels.” See Figure 1.
Given that market statistics show that more than a quarter of the commercially insured patients are covered by high deductible health plans (HDHPs), your practice must adapt to these changing times.
Review Your Pay or Mix
Smart practice administrators will keep their finger on the pulse of the insurance local market as more employers move toward offering HDHPs or health savings accounts. Knowing what the largest manufacturers are offering, along with local hospitals that are typically sizable employers in most communities, is critical. The coverage for school systems, police departments, fire departments, and governments should also be the practice radar.
A recent West Corporation survey5 reveals more about the demographic profile of patients who are less likely to pay or delay payments. Their study shows that 79% of patients cite affordability as the largest healthcare problem with 93% of patients saying it costs too much. So it should be no surprise that 67% say their financial situation makes it challenging to submit timely payments. If you are not familiar with the company name, you’ll be familiar with West Corporation’s products, like Televox, which are automated tools used by practices to remind patients about appointments and copays.
Here are other relevant findings from the West Corporation survey:
- 56% delay payments of medical bills at least some of the time.
- 70% of millennials have missed medical payment deadlines.
- 42% of patients cite their HDHP as the reason for delaying their payments.
- 36% of patients said they have difficulty remembering to make timely medical payments.
It’s no surprise that orthopedic surgeons are not discussing fees with patients. Although only 9% admitted that they don’t discuss costs with patients, it is safe to estimate that few surgeons have their fee schedules memorized.
In a 2013 article, Ubel and colleagues7 said, “Because treatments can be ‘financially toxic,’ physicians need to disclose the financial consequences of treatment alternatives just as they inform patients about treatments’ side effects.”
While uniquely qualified to discuss treatment options, few orthopedic surgeons have the time or the facts to personally discuss fees, out-of-pocket expenses, uncovered services and payment plans. Detailed discussions about patients’ financial liabilities are better done by qualified staff, who verify benefits and use modern technology tools to generate an electronic “estimate of costs and benefits.”
Target Your Efforts
What steps can your practice take to help patients pay their portion, even when it’s large, as well as help your practice reduce receivables and avoid collection problems and bad debt write-offs?
Start by analyzing the top Current Procedural Terminology (CPT) codes with patient responsibility, so you can focus your efforts. One such analysis, conducted by our firm, is shown in Figure 3. Although you might think the highest percent of patient financial responsibilities are for surgical procedures, notice that 4 of the top 5 services this practice identified as having the highest amount of patient collectible dollars are rendered in the office-carpal tunnel surgery being the only exception.
Take Action
After conducting a thorough analysis and reviewing the results, here are 5 actionable steps your practice could take:
1. Make sure your patient portal has the capacity to take patient payments. Offering online payment options increases the opportunity for patients to pay. Promote this option on the patient statement.
2. Implement a system of collecting from patients before they leave the office. After a new visit, which involves a more expensive evaluation and management code, and possibly imaging and durable medical device, counsel patients to leave a credit card on file, so the minute insurance pays, their credit card can be charged.
The 2017 Navicure Patient Payment Check-Up survey8 conducted by Healthcare Information and Management Systems Society (HIMSS) Analytics shows that 78% of patients would provide a card to be charged for one time up to $200. Think about the previously illustrated collection amounts this would alleviate.
3. Provide all surgery patients with a cost estimate. Generating cost estimates has been possible for close to 10 years. It’s done through your clearinghouse and practice management software by entering the CPT codes and diagnosis codes, along with the patient’s information. Save time and avoid tying staff up on hold.
According to the Navicure Patient Payment Check-Up survey,8 75% of provider organizations are able to provide a cost estimate upon request. It makes good business sense.
4. Collect a pre-treatment or pre-surgery scheduling deposit. In the KarenZupko & Associates/American Academy of Orthopaedic Surgeons (AAOS) pre-course survey of those attending the 2017 coding and reimbursement workshops, 55% of orthopedic practices reported that they have instituted such a practice. With the proliferation of HDHPs, asking for a scheduling deposit is fast becoming a must for all surgeons.
5. Offer patients a healthcare financing option through a third party. In response to another pre-course survey question, about offering CareCredit or another healthcare credit card, 28% of orthopedic practices say they do. Still, that leaves >70% of the orthopedic patients without a financing option. Given the reality of high deductible HDHPs and the patient responsibilities going uncollected, it’s time surgeons take a look at financing. It’s a fool’s wish to believe the practice is “saving” the service fee by sending dozens of statements, having staff make calls, and ultimately writing off unpaid balances as uncollectible.
Practices that fail to change, will fail to prosper. Those who have technology-phobic staff will suffer as healthcare continues to automate. Practices led by surgeons like one recently interviewed who said, “If patients knew how much it cost, they’d never schedule” will see patient accounts receivable soar and patient online ratings sink. The first quarter of 2018 means the number of patients with HDHPs will increase and that deductibles will have to be met. It’s wise to have a full staff meeting, share the facts, and put an action plan in place.
If you missed the recent headlines, Why Patients Delay Medical Payments: 12 findings 1 and You think your health insurance costs too much. Try being a farmer.2, you may not be too worried about your ever-rising accounts receivables. But you should be.
The facts in these stories and the 2017 Employer Health Benefits Survey,3 released on September 19 by the non-partisan Kaiser Family Foundation and Health Research & Educational Trust (HRET), are alarming. Let’s look at some of the survey results.
Since 2007, the average family premium has increased 55% and the average worker contribution toward the premium has increased 74%.3 How does that translate into dollars and cents? Well, the average annual premiums this year are $6690 for single coverage and $18,764 for family coverage.
What does that mean exactly to the farm couple in the Crain’s story? The farmer who “will be lucky to net $75,000” on his hay crop this year has a policy premium with Blue Cross Blue Shield of Illinois that was $22,000 last year. And then there is a $5000 deductible for each him and his wife. Do the math: it means they’d spend 43% of their income before health insurance covers anything.
Do premiums vary significantly by firm size or region? Should surgeons in certain areas of the country be less concerned about these trends? No, the premiums don’t significantly vary by size or region.
The point here is not to write an essay about health insurance premiums, but rather to discuss what this economic reality means to patients who are seeing you tomorrow, next week, and next month. Given these economic realities, what is their attitude about your bill?
For insight, let’s look at an Advisory Board Company brief, “Minimizing Bad Debt: Point-of-Service Collections,”4 which states: “Patient propensity to pay decreases as patient obligation increases.” According to the brief, “Our analysis indicates that as the dollar value of a patient’s obligation increases, their propensity to pay any portion of the obligation decreases—for all patients, at all income levels.” See Figure 1.
Given that market statistics show that more than a quarter of the commercially insured patients are covered by high deductible health plans (HDHPs), your practice must adapt to these changing times.
Review Your Pay or Mix
Smart practice administrators will keep their finger on the pulse of the insurance local market as more employers move toward offering HDHPs or health savings accounts. Knowing what the largest manufacturers are offering, along with local hospitals that are typically sizable employers in most communities, is critical. The coverage for school systems, police departments, fire departments, and governments should also be the practice radar.
A recent West Corporation survey5 reveals more about the demographic profile of patients who are less likely to pay or delay payments. Their study shows that 79% of patients cite affordability as the largest healthcare problem with 93% of patients saying it costs too much. So it should be no surprise that 67% say their financial situation makes it challenging to submit timely payments. If you are not familiar with the company name, you’ll be familiar with West Corporation’s products, like Televox, which are automated tools used by practices to remind patients about appointments and copays.
Here are other relevant findings from the West Corporation survey:
- 56% delay payments of medical bills at least some of the time.
- 70% of millennials have missed medical payment deadlines.
- 42% of patients cite their HDHP as the reason for delaying their payments.
- 36% of patients said they have difficulty remembering to make timely medical payments.
It’s no surprise that orthopedic surgeons are not discussing fees with patients. Although only 9% admitted that they don’t discuss costs with patients, it is safe to estimate that few surgeons have their fee schedules memorized.
In a 2013 article, Ubel and colleagues7 said, “Because treatments can be ‘financially toxic,’ physicians need to disclose the financial consequences of treatment alternatives just as they inform patients about treatments’ side effects.”
While uniquely qualified to discuss treatment options, few orthopedic surgeons have the time or the facts to personally discuss fees, out-of-pocket expenses, uncovered services and payment plans. Detailed discussions about patients’ financial liabilities are better done by qualified staff, who verify benefits and use modern technology tools to generate an electronic “estimate of costs and benefits.”
Target Your Efforts
What steps can your practice take to help patients pay their portion, even when it’s large, as well as help your practice reduce receivables and avoid collection problems and bad debt write-offs?
Start by analyzing the top Current Procedural Terminology (CPT) codes with patient responsibility, so you can focus your efforts. One such analysis, conducted by our firm, is shown in Figure 3. Although you might think the highest percent of patient financial responsibilities are for surgical procedures, notice that 4 of the top 5 services this practice identified as having the highest amount of patient collectible dollars are rendered in the office-carpal tunnel surgery being the only exception.
Take Action
After conducting a thorough analysis and reviewing the results, here are 5 actionable steps your practice could take:
1. Make sure your patient portal has the capacity to take patient payments. Offering online payment options increases the opportunity for patients to pay. Promote this option on the patient statement.
2. Implement a system of collecting from patients before they leave the office. After a new visit, which involves a more expensive evaluation and management code, and possibly imaging and durable medical device, counsel patients to leave a credit card on file, so the minute insurance pays, their credit card can be charged.
The 2017 Navicure Patient Payment Check-Up survey8 conducted by Healthcare Information and Management Systems Society (HIMSS) Analytics shows that 78% of patients would provide a card to be charged for one time up to $200. Think about the previously illustrated collection amounts this would alleviate.
3. Provide all surgery patients with a cost estimate. Generating cost estimates has been possible for close to 10 years. It’s done through your clearinghouse and practice management software by entering the CPT codes and diagnosis codes, along with the patient’s information. Save time and avoid tying staff up on hold.
According to the Navicure Patient Payment Check-Up survey,8 75% of provider organizations are able to provide a cost estimate upon request. It makes good business sense.
4. Collect a pre-treatment or pre-surgery scheduling deposit. In the KarenZupko & Associates/American Academy of Orthopaedic Surgeons (AAOS) pre-course survey of those attending the 2017 coding and reimbursement workshops, 55% of orthopedic practices reported that they have instituted such a practice. With the proliferation of HDHPs, asking for a scheduling deposit is fast becoming a must for all surgeons.
5. Offer patients a healthcare financing option through a third party. In response to another pre-course survey question, about offering CareCredit or another healthcare credit card, 28% of orthopedic practices say they do. Still, that leaves >70% of the orthopedic patients without a financing option. Given the reality of high deductible HDHPs and the patient responsibilities going uncollected, it’s time surgeons take a look at financing. It’s a fool’s wish to believe the practice is “saving” the service fee by sending dozens of statements, having staff make calls, and ultimately writing off unpaid balances as uncollectible.
Practices that fail to change, will fail to prosper. Those who have technology-phobic staff will suffer as healthcare continues to automate. Practices led by surgeons like one recently interviewed who said, “If patients knew how much it cost, they’d never schedule” will see patient accounts receivable soar and patient online ratings sink. The first quarter of 2018 means the number of patients with HDHPs will increase and that deductibles will have to be met. It’s wise to have a full staff meeting, share the facts, and put an action plan in place.
1. Gooch K. Why patients delay medical payments: 12 findings. Becker’s ASC. https://www.beckershospitalreview.com/finance/why-patients-delay-medical-payments-12-findings.html. Published August 28, 2017.
2. Murphy HL. You think your health insurance costs too much. Try being a farmer. Crain’s Chicago Business. http://www.chicagobusiness.com/article/20170929/ISSUE01/170929835. Published September 29, 2017. Accessed October 2, 2017.
3. 2017 Employer Health Benefits Survey. The Henry J. Kaiser Family Foundation and the Health Research & Educational Trust (HRET). https://www.kff.org/health-costs/report/2017-employer-health-benefits-survey/. Published September 19, 2017.
4. Minimizing Bad Debt: Point-of-Service Collections. The Advisory Board Company. https://www.advisory.com/-/media/Advisory-com/Research/FLC/Resources/2015/CFO-Brief-POS.pdf. Published August 21, 2015.
5. Optimizing Revenue: Solving Healthcare’s Revenue Cycle Challenges Using Technology-Enabled Communications. West Corporation. https://cdn2.hubspot.net/hubfs/402746/Assets/West%20Assets/Optimizing%20Revenue%20Report/Reports%20and%20Handouts/WEST-Optimizing%20Revenue%20Report%20final.pdf?t=1508789915319. Accessed October 26, 2017.
6. Peckham C. Medscape Orthopedist Compensation Report 2016. Medscape. https://www.medscape.com/features/slideshow/compensation/2016/orthopedics. Published April 1, 2016.
7. Ubel PA, Abernathy AP, Zafar SY. Full disclosure - out-of-pocket costs as side effects. N Engl J. Med. 2013;369:1484-1486. doi:10.1056/NEJMp1306826.
8. Patient Payment Check-Up 2017. Navicure. http://info.navicure.com/rs/669-OIJ-380/images/Navicure-Survey-Report-2017-Patient-Payment-Check-Up.pdf?mkt_tok=eyJpIjoiTVdKak1HUmhObVV6WkRVeSIsInQiOiJRcFNyRGVrOXlTS0pjalwvWEw3c2s1UmRMRHJVXC9EQzRkSnBkWCs0S2FEbUt3Z1I1a2Y3d1BBY3FKY0I1QWpEdkJRWU9ibmFlUlpnYVRIbVJMcStTVmdkRVwvSTJzcHE1cDVTajBRM3B1Q25lbDQwamViWnMwWGd1c1QzVk1cL2hYdkYifQ%3D%3D. Accessed October 26, 2017.
1. Gooch K. Why patients delay medical payments: 12 findings. Becker’s ASC. https://www.beckershospitalreview.com/finance/why-patients-delay-medical-payments-12-findings.html. Published August 28, 2017.
2. Murphy HL. You think your health insurance costs too much. Try being a farmer. Crain’s Chicago Business. http://www.chicagobusiness.com/article/20170929/ISSUE01/170929835. Published September 29, 2017. Accessed October 2, 2017.
3. 2017 Employer Health Benefits Survey. The Henry J. Kaiser Family Foundation and the Health Research & Educational Trust (HRET). https://www.kff.org/health-costs/report/2017-employer-health-benefits-survey/. Published September 19, 2017.
4. Minimizing Bad Debt: Point-of-Service Collections. The Advisory Board Company. https://www.advisory.com/-/media/Advisory-com/Research/FLC/Resources/2015/CFO-Brief-POS.pdf. Published August 21, 2015.
5. Optimizing Revenue: Solving Healthcare’s Revenue Cycle Challenges Using Technology-Enabled Communications. West Corporation. https://cdn2.hubspot.net/hubfs/402746/Assets/West%20Assets/Optimizing%20Revenue%20Report/Reports%20and%20Handouts/WEST-Optimizing%20Revenue%20Report%20final.pdf?t=1508789915319. Accessed October 26, 2017.
6. Peckham C. Medscape Orthopedist Compensation Report 2016. Medscape. https://www.medscape.com/features/slideshow/compensation/2016/orthopedics. Published April 1, 2016.
7. Ubel PA, Abernathy AP, Zafar SY. Full disclosure - out-of-pocket costs as side effects. N Engl J. Med. 2013;369:1484-1486. doi:10.1056/NEJMp1306826.
8. Patient Payment Check-Up 2017. Navicure. http://info.navicure.com/rs/669-OIJ-380/images/Navicure-Survey-Report-2017-Patient-Payment-Check-Up.pdf?mkt_tok=eyJpIjoiTVdKak1HUmhObVV6WkRVeSIsInQiOiJRcFNyRGVrOXlTS0pjalwvWEw3c2s1UmRMRHJVXC9EQzRkSnBkWCs0S2FEbUt3Z1I1a2Y3d1BBY3FKY0I1QWpEdkJRWU9ibmFlUlpnYVRIbVJMcStTVmdkRVwvSTJzcHE1cDVTajBRM3B1Q25lbDQwamViWnMwWGd1c1QzVk1cL2hYdkYifQ%3D%3D. Accessed October 26, 2017.
Patella Alta: A Comprehensive Review of Current Knowledge
Take-Home Points
- Patella alta has a reduced articular area of PF contact.
- Presence of patella alta depends on the measurement method.
- Patella alta is defined as ISI >1.2 and CDI >1.2 to >1.3.
- On sagittal MRI, PTI is used most often with cutoff values of <0.125 to 0.28.
- Tibial tubercle distalization is most often used to treat patella alta. The desired postoperative patellar height is a CDI of 1.0.
Patella alta is a patella that rides abnormally high in relation to the femur, the femoral trochlea, or the tibia,1 with decreased bony stability requiring increased knee flexion angles to engage the trochlea.2,3 An abnormally high patella may therefore insufficiently engage the proximal trochlea groove both in extension and in the early phase of knee flexion—making it one of the potential risk factors for patellar instability.4-10 Accordingly, patella alta is present in 30% of patients with recurrent patellar dislocation.11 It also occurs in other disorders, such as knee extensor apparatus disorders, in patients with patellofemoral (PF) pain, chondromalacia, Sinding-Larsen-Johansson disease, Osgood-Schlatter disease, patellar tendinopathy, and osteoarthritis.1,7,8,12-18 As such, patella alta represents an important predisposing factor for patellar malalignment and PF-related complaints. On the other hand, patella alta may also be a normal variant of a person’s knee anatomy and may be well tolerated when not combined with other instability factors.4
Despite the importance of patella alta, there is no consensus on a precise definition, the most reliable measurement method, or the factors thought to be important in clinical decisions regarding treatment. To address this issue, we systematically reviewed the patella alta literature for definitions, the most common measurement methods and their patella alta cutoff values, and cutoff values for surgical correction and proposed surgical techniques.
Methods
In February 2017, using the term patella alta, we performed a systematic literature search on PubMed. Inclusion criteria were original study or review articles, publication in peer-reviewed English-language journals between 2000 and 2017, and narrative description or measurement of human patellar height on plain radiographs or magnetic resonance imaging (MRI). Excluded were abstracts and articles in languages other than English; animal and computational/biomechanical studies; case reports; and knee arthroplasties, knee extensor ruptures, and hereditary and congenital diseases. All evidence levels were included.
We assessed measurement methods, reported cutoff values for patella alta, cutoff values for performing surgical correction, proposed surgical techniques, and postoperative target values. Original study articles and review articles were analyzed separately.
Results
Of 211 articles identified, 92 met the inclusion criteria for original study, and 28 for review. All their abstracts were reviewed, and 91 were excluded: 17 for language other than English, 11 for animal study, 12 for biomechanical/computational study, 20 for case report, 8 for arthroplasty, 13 for hereditary or congenital disease, 1 for extensor apparatus rupture, 3 for editorial letter, and 6 for other reasons. Full text copies of all included articles were obtained and reviewed.
Original Study Articles
Definition. Of the 92 original study articles, 17 (18.5%) defined patella alta by description alone, and 75 (81.5%) used imaging-based measurements. Patella alta was described as a patella that rides abnormally high in relation to the trochlear groove, with a reduced articular area of PF contact1,15,19-21 or decreased patella–trochlea cartilage overlap.22 With this reduced contact area, there is increased PF stress.21
With radiographic measurements, patella alta is defined as a Caton-Deschamps index (CDI) of >1.2 to >1.3, an Insall-Salvati index (ISI) of >1.2, a Blackburne-Peel index (PBI) of >1.0,4,15,18,23-25 and a patellotrochlear index (PTI) of <0.125 to 0.28.6,26
On lateral radiographs, ISI was the most common measurement (33 studies), with patella alta cutoff values ranging from >1.2 to >1.5. The second most common measurement was CDI (24 studies), with cutoff values of >1.2 to >1.3. Other indices, such as the modified ISI (6 studies), the BPI (1 study), and the Koshino index (2 studies), had their cutoff values used more consistently (>1.6 to >2.4, >1.0, and >1.2, respectively) but these indices were rarely reported in the literature (Table 1).27,28,29
Thirty-six studies defined patella alta with MRI using either the aforementioned radiographic ratios (23 studies, 3 indices, Table 2) or PF indices (13 studies, 4 methods, Table 3).30
On sagittal MRI, PTI was used most often; cutoff values were <0.125 to 0.28.
Thirteen studies defined patella alta with PTL: 5 using lateral conventional radiographs and 8 using sagittal MRI. For each type of imaging, the cutoff value was >52 mm to >56 mm. PTL was measured either independently or together with ISI on sagittal MRI (Table 4).
Review Articles
Twenty-eight review articles met the inclusion criteria.35-40 Patella alta was described mostly with ISI (75%) or CDI (64%). In up to 57% of these articles, a patella alta reference value was missing. Only 1 article mentioned different cutoff values for conventional radiographs and MRI. BPI was mentioned in 50% of studies, but only 2 indicated a cutoff value for patella alta. Eleven percent used PTI on sagittal MRI and took patella–trochlea cartilage overlap into account.
Eight review articles mentioned cutoff values for surgical correction. Only 1 of 4 articles mentioned a cutoff value for correcting patella alta in PF instability, and only 2 articles suggested an ideal postoperative patellar height (Table 6).
No review article relied on PTL to describe patella alta or to advise surgical treatment.
Discussion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Interpretation of patellar height and, particularly, patella alta is very heterogeneous. Accordingly, comparing surgical indications, surgical treatment options, and outcomes across studies can be difficult.
Measurement Methods
Radiographs. Conventional radiographs were used to describe patella alta in two-thirds of the original study articles. The most common measurement methods marked the position of the patella relative to either the femur (direct assessment) or the tibia (indirect assessment).14,25 All established measurement methods use lateral radiographs, which do not show articular cartilage.11,14 Therefore, lateral radiograph ratios of different and variable bony landmarks do not measure actual PF articular congruence. The widely variable morphologies of patella (distal patellar nose, different articulating surface), femoral trochlea (facet in height and length), proximal tibia (inherited or acquired deformities on tibial tubercle), and slope are all confounding factors that may affect measurement (Figure 1). In addition, specific variations in PF joint morphology (eg, small patellar articular surface, short trochlea) are not well represented by these ratios.11
All these methods have advantages, limitations, and different values of interobserver and intraobserver variability, reliability, and reproducibility (Table 7).29,41
Determination of both patellar height and patella alta depends on the measurement method used and is significantly affected by anatomical variants, such as extra-articular patella and femorotibial landmarks.
The ISI method is most commonly used because it does not depend on degree of knee flexion, is thought to measure the relative length of the patellar tendon the best,5 and has established MRI criteria.6 However, ISI has limitations: Its distal bony landmark is the tibial tuberosity, which can be difficult to assess14; the shape of the patella (mainly its inferior point) varies42; and the measurement does not change after distalization of the tibial tubercle.5
CDI is the most accurate diagnostic method because it relies on readily identifiable and reproducible anatomical landmarks; does not depend on radiograph quality, knee size, radiologic enlargement, or position of the tibial tubercle or patellar modification; and is unaffected by degree of knee flexion between 10° and 80°.38,43 CDI is the method that is most useful in describing patellar height after distalization of the tibial tubercle because it assesses the height of the patella relative to the tibial plateau.5 CDI is limited with respect to clear identification of the patellar and tibial articular margin.37
BPI has the lowest interobserver variability and discriminates best among patella alta, norma, and infera.41 Like CDI, BPI assesses patellar height relative to the tibial plateau, and thus is useful in describing patellar height after distalization of the tibial tubercle.5 BPI is limited in that it uses a line drawn along the tibial plateau, where variations in inclination and tibial slope produce inaccuracies,14 and depends highly on projection angles. Clinically, BPI is rarely used.
As the literature shows, the radiologic methods of determining patellar height are variable and unreliable and depend on the ratio used.26,41 These methods do not reveal precise information about the PF articular congruence, the key finding for necessary treatment.
Radiographic Indices on MRI. Some studies measured radiographic indices on sagittal MRI. Radiologic and MRI measurements are described as not significantly different or slightly different, and 0.09 to 0.13 needs to be added with ISI and BP ratios on radiographs, MRI, and computed tomography (CT).17,44-47 ISI is commonly used, and its clinical application is easy. Although classically measured on plain radiographs, ISI is reliably measured on MRI as well.17,30 The traditional 1.2 threshold for defining patella alta was used by Charles and colleagues.44 CDI is equally well measured on radiographs and MRI.47
According to the literature, 3 different measurement methods have been used to describe degree of patella–trochlea cartilage overlap: PTI, sagittal patellofemoral engagement (SPE), and patellar articular overlap (PAO).1,11,26
PTI uses the true articular cartilage patella–trochlea relationship for measurement of patellar height on sagittal MRI in extension (Figure 3). If the patellar tendon is fully out to length (no laxity), PTI reliably and precisely determines the exact articular correlation of PF joint and patellar height.6
SPE is similar to percentage of articular coverage.1,11 Patellar overlap of the trochlea is measured on 2 different sagittal MRI images: 1 with the greatest length of patellar cartilage and 1 with the greatest length of femoral trochlear cartilage. This measurement did not correlate with CDI.1 SPE was not evaluated in control studies, and normal and pathologic values are unavailable.
PAO is measured with patients positioned at rest and in standard knee coils.1 MRI was not obtained in full extension or with quadriceps activation, and knee flexion angle values are unavailable. Total patellar articular length was measured on the sagittal MRI that showed the greatest patellar length and articular cartilage thickness. The same image was used to measure articular overlap, or the length of patellar cartilage overlying the trochlear cartilage, as measured parallel to the subchondral surface of the patella. PAO correlates well with conventional patellar height measurements in the sagittal plane and shows promise as a simpler alternative to the conventional indices.1 Normal and pathologic values are unavailable.
So far, PTI is the only method that was controlled in several studies, assessed for its reliability, and compared with other patellar height measurements.6,14
Patellar Tendon Length. PTL can be measured on lateral radiographs or sagittal MRI. Radiologic and MRI measurements are described as not significantly different47 or slightly different, and 0.09 to 0.13 needs to be added with the ISI ratio on radiographs, MRI, and CT.46 PTL is reliably evaluated with ISI.41,50 There is a weak correlation of patient height and PTL: Taller people have normally longer tendons.51-53
PTL measurements revealed that the posterior surface of the patellar tendon was significantly shorter than the anterior surface. Compared with their corresponding posterior fascicles, anterior fascicles are longer; their attachment is more proximal to the patella and more distal to the tibia. In addition, posterior PTL is significantly shorter with the posterior patellar attachment (adheres to posterior aspect of the inferior patellar pole) than with the anterior attachment (adheres to anterior aspect of the inferior patellar pole).56 Moreover, the lateral and medial fascicles are longer than the central fascicles attaching at the most inferior patellar pole. This issue must be considered in image cut selection (Figures 6A, 6B). Furthermore, type of inferior pole of patella (pointed, intermediate, blunt) is important in measurement.56 Overall, mean (SD) PTL was 54.9 (1.2) mm on the anterior surface and 35.0 (0.6) mm on the posterior surface.
It is important to precisely describe the measurement method and location (sagittal cut) and to consider the shape of the inferior patellar pole and the site of the patellar tendon attachment.56
Treatments. For patella alta, the surgical treatment goals are to increase the PF contact area and improve PF articular congruence, and thereby increase PF stability.31-33 Four different procedures were used to treat patella alta (Table 5), but only 11 of the 92 original study articles and 8 of the 28 review articles included in our review mentioned a specific patellar height that required surgical correction (Tables 5, 6). Recommendations were based on CDI (>1.2 to >1.4) and less often on ISI (>1.2 to >1.4) or PTL (>56 mm).
Tibial tubercle distalization is effective in normalizing patellar height to correct the patellar index in patella alta (Figures 7A, 7B).13,18,38,55,57 Tibial tubercle distalization and patellar tendon tenodesis attached to the original insertion normalize PTL and stabilize the PF joint in patients with patella alta.5,47 In a comparison of these surgical procedures, cartilage stress was lower with distalization than with distalization and tenodesis.13
Soft-tissue methods are recommended in children, as bone procedures injure the proximal tibial physis and may cause it to close prematurely.58 The patella can be distally advanced by completely mobilizing the patellar tendon and fixating with sutures through the cartilaginous tibial tubercle. This technique is a satisfactory treatment for skeletally immature patients who present with habitual patellar dislocation associated with patella alta.58,59
CDI and BPI assess patellar height relative to the tibial plateau, and therefore are the most useful measurement methods for patellar height after distalization of the tibial tubercle.5 ISI does not change after distalization of the tibial tubercle and cannot be recommended.5,18 As PF indices (eg, PTI) can trace preoperative and postoperative values, these measurements are valuable.
Controversies
Our review found no consensus on measurement method or cutoff value. No measurement method showed clear clinical or methodologic superiority. Most published patellar height and patella alta data are based on conventional radiographs using a tibial reference point, even though a femoral or even trochlear reference point seems more reproducible, particularly in PF pathologies. Several cutoff values have been reported for each patella alta measurement method. Regarding pathologic thresholds, which might require surgical correction, very little information has been published, and scientific evidence is lacking. Numerous important aspects remain unanswered after this review, and clarification is mandatory.
Five Key Facts
1. In patella alta assessment, different morphologic, biomechanical, and functional aspects must be considered. The most relevant aspect is decreased engagement of the patella and trochlea,4-9,11,26 which results in decreased bony stability in knee extension.1-3 Therefore, patella alta is one of the potential risk factors for patellar instability with a high percentage of recurrent patellar dislocation.4-9 In early knee flexion, the patella translates more distally with better engagement of the patella in the trochlea and better stability. Therefore, measurement in extension, with the patellar tendon out to length, seems to offer a more reliable assessment of the patella–femur relationship.
2. Insufficient engagement of the patella in the trochlea is the most important aspect of patella alta.11 Therefore, direct measurement of this engagement seems logical.
3. As radiographs do not show articular cartilage, they should not be used to assess the articular patella–femur relationship.11,14,26 Patella–trochlea cartilage overlap is the most relevant factor for patella alta and should be measured on MRI.6,14,26,32
4. PTL is an important factor for patellar height and particularly patella alta. The range of normal PTL values is wide: 35 mm to 61 mm. The described cutoff values for patella alta (>52 mm to >56 mm) fall within this normal range. The cause may be the different measurement methods used.33,47,59-61 Therefore, for precise diagnostics, a standardized measurement method that includes the selected cut imaging is mandatory.
Many important aspects remain unanswered, and clarification is mandatory (Table 9).
Conclusion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Presence of patella alta depends on measurement method used. Methods cannot be used interchangeably, and they all have their advantages and limitations. Unfortunately, there is no generally accepted consensus on measurement method, patella alta cutoff value, or treatment with ideal correction. Treatment planning and outcomes assessment require clarification of these many issues.
1 Munch JL, Sullivan JP, Nguyen JT, et al. Patellar articular overlap on MRI is a simple alternative to conventional measurements of patellar height. Orthop J Sports Med. 2016;4(7):2325967116656328.
2. Elias JJ, Soehnlen NT, Guseila LM, Cosgarea AJ. Dynamic tracking influenced by anatomy in patellar instability. Knee. 2016;23(3):450-455.
3. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184.
4. Askenberger M, Janarv PM, Finnbogason T, Arendt EA. Morphology and anatomic patellar instability risk factors in first-time traumatic lateral patellar dislocations. Am J Sports Med. 2017;45(1):50-58.
5. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
6. Ali SA, Helmer R, Terk MR. Patella alta: lack of correlation between patellotrochlear cartilage congruence and commonly used patellar height ratios. AJR Am J Roentgenol. 2009;193(5):1361-1366.
7. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581.
8. Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):1749-1755.
9. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
10. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
11. Dejour D, Ferrua P, Ntagiopoulos PG, et al. The introduction of a new MRI index to evaluate sagittal patellofemoral engagement. Orthop Traumatol Surg Res. 2013;99(8 suppl):S391-S398.
12. Althani S, Shahi A, Tan TL, Al-Belooshi A. Position of the patella among Emirati adult knees. Is Insall-Salvati ratio applicable to Middle-Easterners? Arch Bone Joint Surg. 2016;4(2):137-140.
13. Yin L, Liao TC, Yang L, Powers CM. Does patella tendon tenodesis improve tibial tubercle distalization in treating patella alta? A computational study. Clin Orthop Relat Res. 2016;474(11):2451-2461.
14. Barnett AJ, Prentice M, Mandalia V, Wakeley CJ, Eldridge JD. Patellar height measurement in trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1412-1415.
15. Otsuki S, Nakajima M, Fujiwara K, et al. Influence of age on clinical outcomes of three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2392-2396.
16. Otsuki S, Nakajima M, Oda S, et al. Three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. J Orthop Sci. 2013;18(3):437-442.
17. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
18. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2545-2550.
19. Bertollo N, Pelletier MH, Walsh WR. Simulation of patella alta and the implications for in vitro patellar tracking in the ovine stifle joint. J Orthop Res. 2012;30(11):1789-1797.
20. Narkbunnam R, Chareancholvanich K. Effect of patient position on measurement of patellar height ratio. Arch Orthop Trauma Surg. 2015;135(8):1151-1156.
21. Stefanik JJ, Zhu Y, Zumwalt AC, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Care Res. 2010;62(9):1258-1265.
22. Monk AP, Doll HA, Gibbons CL, et al. The patho-anatomy of patellofemoral subluxation. J Bone Joint Surg Br. 2011;93(10):1341-1347.
23. Hirano A, Fukubayashi T, Ishii T, Ochiai N. Relationship between the patellar height and the disorder of the knee extensor mechanism in immature athletes. J Pediatr Orthop. 2001;21(4):541-544.
24. Ng JP, Cawley DT, Beecher SM, Lee MJ, Bergin D, Shannon FJ. Focal intratendinous radiolucency: a new radiographic method for diagnosing patellar tendon ruptures. Knee. 2016;23(3):482-486.
25. van Duijvenbode D, Stavenuiter M, Burger B, van Dijke C, Spermon J, Hoozemans M. The reliability of four widely used patellar height ratios. Int Orthop. 2016;40(3):493-497.
26. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
27. Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;(282):170-176.
28. Thaunat M, Erasmus PJ. The favourable anisometry: an original concept for medial patellofemoral ligament reconstruction. Knee. 2007;14(6):424-428.
29. Anagnostakos K, Lorbach O, Reiter S, Kohn D. Comparison of five patellar height measurement methods in 90 degrees knee flexion. Int Orthop. 2011;35(12):1791-1797.
30. Miller TT, Staron RB, Feldman F. Patellar height on sagittal MR imaging of the knee. AJR Am J Roentgenol. 1996;167(2):339-341.
31. Dejour D, Le Coultre B. Osteotomies in patello-femoral instabilities. Sports Med Arthrosc Rev. 2007;15(1):39-46.
32. Rhee SJ, Pavlou G, Oakley J, Barlow D, Haddad F. Modern management of patellar instability. Int Orthop. 2012;36(12):2447-2456.
33. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc Rev. 2007;15(2):61-67.
34. Caton J, Deschamps G, Chambat P, Lerat JL, Dejour H. [Patella infera. Apropos of 128 cases]. Rev Chir Orthop Reparatrice Appar Mot. 1982;68(5):317-325.
35. Meyers AB, Laor T, Sharafinski M, Zbojniewicz AM. Imaging assessment of patellar instability and its treatment in children and adolescents. Pediatr Radiol. 2016;46(5):618-636.
36. Feller JA. Distal realignment (tibial tuberosity transfer). Sports Med Arthrosc Rev. 2012;20(3):152-161.
37. Dietrich TJ, Fucentese SF, Pfirrmann CW. Imaging of individual anatomical risk factors for patellar instability. Semin Musculoskelet Radiol. 2016;20(1):65-73.
38. Dean CS, Chahla J, Serra Cruz R, Cram TR, LaPrade RF. Patellofemoral joint reconstruction for patellar instability: medial patellofemoral ligament reconstruction, trochleoplasty, and tibial tubercle osteotomy. Arthrosc Tech. 2016;5(1):e169-e175.
39. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497.
40. Weber AE, Nathani A, Dines JS, et al. An algorithmic approach to the management of recurrent lateral patellar dislocation. J Bone Joint Surg Am. 2016;98(5):417-427.
41. Seil R, Muller B, Georg T, Kohn D, Rupp S. Reliability and interobserver variability in radiological patellar height ratios. Knee Surg Sports Traumatol Arthrosc. 2000;8(4):231-236.
42. Laprade J, Culham E. Radiographic measures in subjects who are asymptomatic and subjects with patellofemoral pain syndrome. Clin Orthop Relat Res. 2003;(414):172-182.
43. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
44. Charles MD, Haloman S, Chen L, Ward SR, Fithian D, Afra R. Magnetic resonance imaging–based topographical differences between control and recurrent patellofemoral instability patients. Am J Sports Med. 2013;41(2):374-384.
45. Kurtul Yildiz H, Ekin EE. Patellar malalignment: a new method on knee MRI. Springerplus. 2016;5(1):1500.
46. Lee PP, Chalian M, Carrino JA, Eng J, Chhabra A. Multimodality correlations of patellar height measurement on x-ray, CT, and MRI. Skeletal Radiol. 2012;41(10):1309-1314.
47. Neyret P, Robinson AH, Le Coultre B, Lapra C, Chambat P. Patellar tendon length—the factor in patellar instability? Knee. 2002;9(1):3-6.
48. Diederichs G, Issever AS, Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics. 2010;30(4):961-981.
49. Earhart C, Patel DB, White EA, Gottsegen CJ, Forrester DM, Matcuk GR Jr. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20(1):11-23.
50. Aarimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446.
51. Brown DE, Alexander AH, Lichtman DM. The Elmslie-Trillat procedure: evaluation in patellar dislocation and subluxation. Am J Sports Med. 1984;12(2):104-109.
52. Goldstein JL, Verma N, McNickle AG, Zelazny A, Ghodadra N, Bach BR Jr. Avoiding mismatch in allograft anterior cruciate ligament reconstruction: correlation between patient height and patellar tendon length. Arthroscopy. 2010;26(5):643-650.
53. Navali AM, Jafarabadi MA. Is there any correlation between patient height and patellar tendon length? Arch Bone Joint Surg. 2015;3(2):99-103.
54. Park MS, Chung CY, Lee KM, Lee SH, Choi IH. Which is the best method to determine the patellar height in children and adolescents? Clin Orthop Relat Res. 2010;468(5):1344-1351.
55. Berard JB, Magnussen RA, Bonjean G, et al. Femoral tunnel enlargement after medial patellofemoral ligament reconstruction: prevalence, risk factors, and clinical effect. Am J Sports Med. 2014;42(2):297-301.
56. Edama M, Kageyama I, Nakamura M, et al. Anatomical study of the inferior patellar pole and patellar tendon [published online ahead of print February 16, 2017]. Scand J Med Sci Sports. doi:10.1111/sms.12858.
57. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Relat Res. 2002;(396):152-162.
58. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177.
59. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
60. Degnan AJ, Maldjian C, Adam RJ, Fu FH, Di Domenica M. Comparison of Insall-Salvati ratios in children with an acute anterior cruciate ligament tear and a matched control population. AJR Am J Roentgenol. 2015;204(1):161-166.
61. Wittstein JR, Bartlett EC, Easterbrook J, Byrd JC. Magnetic resonance imaging evaluation of patellofemoral malalignment. Arthroscopy. 2006;22(6):643-649.
Take-Home Points
- Patella alta has a reduced articular area of PF contact.
- Presence of patella alta depends on the measurement method.
- Patella alta is defined as ISI >1.2 and CDI >1.2 to >1.3.
- On sagittal MRI, PTI is used most often with cutoff values of <0.125 to 0.28.
- Tibial tubercle distalization is most often used to treat patella alta. The desired postoperative patellar height is a CDI of 1.0.
Patella alta is a patella that rides abnormally high in relation to the femur, the femoral trochlea, or the tibia,1 with decreased bony stability requiring increased knee flexion angles to engage the trochlea.2,3 An abnormally high patella may therefore insufficiently engage the proximal trochlea groove both in extension and in the early phase of knee flexion—making it one of the potential risk factors for patellar instability.4-10 Accordingly, patella alta is present in 30% of patients with recurrent patellar dislocation.11 It also occurs in other disorders, such as knee extensor apparatus disorders, in patients with patellofemoral (PF) pain, chondromalacia, Sinding-Larsen-Johansson disease, Osgood-Schlatter disease, patellar tendinopathy, and osteoarthritis.1,7,8,12-18 As such, patella alta represents an important predisposing factor for patellar malalignment and PF-related complaints. On the other hand, patella alta may also be a normal variant of a person’s knee anatomy and may be well tolerated when not combined with other instability factors.4
Despite the importance of patella alta, there is no consensus on a precise definition, the most reliable measurement method, or the factors thought to be important in clinical decisions regarding treatment. To address this issue, we systematically reviewed the patella alta literature for definitions, the most common measurement methods and their patella alta cutoff values, and cutoff values for surgical correction and proposed surgical techniques.
Methods
In February 2017, using the term patella alta, we performed a systematic literature search on PubMed. Inclusion criteria were original study or review articles, publication in peer-reviewed English-language journals between 2000 and 2017, and narrative description or measurement of human patellar height on plain radiographs or magnetic resonance imaging (MRI). Excluded were abstracts and articles in languages other than English; animal and computational/biomechanical studies; case reports; and knee arthroplasties, knee extensor ruptures, and hereditary and congenital diseases. All evidence levels were included.
We assessed measurement methods, reported cutoff values for patella alta, cutoff values for performing surgical correction, proposed surgical techniques, and postoperative target values. Original study articles and review articles were analyzed separately.
Results
Of 211 articles identified, 92 met the inclusion criteria for original study, and 28 for review. All their abstracts were reviewed, and 91 were excluded: 17 for language other than English, 11 for animal study, 12 for biomechanical/computational study, 20 for case report, 8 for arthroplasty, 13 for hereditary or congenital disease, 1 for extensor apparatus rupture, 3 for editorial letter, and 6 for other reasons. Full text copies of all included articles were obtained and reviewed.
Original Study Articles
Definition. Of the 92 original study articles, 17 (18.5%) defined patella alta by description alone, and 75 (81.5%) used imaging-based measurements. Patella alta was described as a patella that rides abnormally high in relation to the trochlear groove, with a reduced articular area of PF contact1,15,19-21 or decreased patella–trochlea cartilage overlap.22 With this reduced contact area, there is increased PF stress.21
With radiographic measurements, patella alta is defined as a Caton-Deschamps index (CDI) of >1.2 to >1.3, an Insall-Salvati index (ISI) of >1.2, a Blackburne-Peel index (PBI) of >1.0,4,15,18,23-25 and a patellotrochlear index (PTI) of <0.125 to 0.28.6,26
On lateral radiographs, ISI was the most common measurement (33 studies), with patella alta cutoff values ranging from >1.2 to >1.5. The second most common measurement was CDI (24 studies), with cutoff values of >1.2 to >1.3. Other indices, such as the modified ISI (6 studies), the BPI (1 study), and the Koshino index (2 studies), had their cutoff values used more consistently (>1.6 to >2.4, >1.0, and >1.2, respectively) but these indices were rarely reported in the literature (Table 1).27,28,29
Thirty-six studies defined patella alta with MRI using either the aforementioned radiographic ratios (23 studies, 3 indices, Table 2) or PF indices (13 studies, 4 methods, Table 3).30
On sagittal MRI, PTI was used most often; cutoff values were <0.125 to 0.28.
Thirteen studies defined patella alta with PTL: 5 using lateral conventional radiographs and 8 using sagittal MRI. For each type of imaging, the cutoff value was >52 mm to >56 mm. PTL was measured either independently or together with ISI on sagittal MRI (Table 4).
Review Articles
Twenty-eight review articles met the inclusion criteria.35-40 Patella alta was described mostly with ISI (75%) or CDI (64%). In up to 57% of these articles, a patella alta reference value was missing. Only 1 article mentioned different cutoff values for conventional radiographs and MRI. BPI was mentioned in 50% of studies, but only 2 indicated a cutoff value for patella alta. Eleven percent used PTI on sagittal MRI and took patella–trochlea cartilage overlap into account.
Eight review articles mentioned cutoff values for surgical correction. Only 1 of 4 articles mentioned a cutoff value for correcting patella alta in PF instability, and only 2 articles suggested an ideal postoperative patellar height (Table 6).
No review article relied on PTL to describe patella alta or to advise surgical treatment.
Discussion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Interpretation of patellar height and, particularly, patella alta is very heterogeneous. Accordingly, comparing surgical indications, surgical treatment options, and outcomes across studies can be difficult.
Measurement Methods
Radiographs. Conventional radiographs were used to describe patella alta in two-thirds of the original study articles. The most common measurement methods marked the position of the patella relative to either the femur (direct assessment) or the tibia (indirect assessment).14,25 All established measurement methods use lateral radiographs, which do not show articular cartilage.11,14 Therefore, lateral radiograph ratios of different and variable bony landmarks do not measure actual PF articular congruence. The widely variable morphologies of patella (distal patellar nose, different articulating surface), femoral trochlea (facet in height and length), proximal tibia (inherited or acquired deformities on tibial tubercle), and slope are all confounding factors that may affect measurement (Figure 1). In addition, specific variations in PF joint morphology (eg, small patellar articular surface, short trochlea) are not well represented by these ratios.11
All these methods have advantages, limitations, and different values of interobserver and intraobserver variability, reliability, and reproducibility (Table 7).29,41
Determination of both patellar height and patella alta depends on the measurement method used and is significantly affected by anatomical variants, such as extra-articular patella and femorotibial landmarks.
The ISI method is most commonly used because it does not depend on degree of knee flexion, is thought to measure the relative length of the patellar tendon the best,5 and has established MRI criteria.6 However, ISI has limitations: Its distal bony landmark is the tibial tuberosity, which can be difficult to assess14; the shape of the patella (mainly its inferior point) varies42; and the measurement does not change after distalization of the tibial tubercle.5
CDI is the most accurate diagnostic method because it relies on readily identifiable and reproducible anatomical landmarks; does not depend on radiograph quality, knee size, radiologic enlargement, or position of the tibial tubercle or patellar modification; and is unaffected by degree of knee flexion between 10° and 80°.38,43 CDI is the method that is most useful in describing patellar height after distalization of the tibial tubercle because it assesses the height of the patella relative to the tibial plateau.5 CDI is limited with respect to clear identification of the patellar and tibial articular margin.37
BPI has the lowest interobserver variability and discriminates best among patella alta, norma, and infera.41 Like CDI, BPI assesses patellar height relative to the tibial plateau, and thus is useful in describing patellar height after distalization of the tibial tubercle.5 BPI is limited in that it uses a line drawn along the tibial plateau, where variations in inclination and tibial slope produce inaccuracies,14 and depends highly on projection angles. Clinically, BPI is rarely used.
As the literature shows, the radiologic methods of determining patellar height are variable and unreliable and depend on the ratio used.26,41 These methods do not reveal precise information about the PF articular congruence, the key finding for necessary treatment.
Radiographic Indices on MRI. Some studies measured radiographic indices on sagittal MRI. Radiologic and MRI measurements are described as not significantly different or slightly different, and 0.09 to 0.13 needs to be added with ISI and BP ratios on radiographs, MRI, and computed tomography (CT).17,44-47 ISI is commonly used, and its clinical application is easy. Although classically measured on plain radiographs, ISI is reliably measured on MRI as well.17,30 The traditional 1.2 threshold for defining patella alta was used by Charles and colleagues.44 CDI is equally well measured on radiographs and MRI.47
According to the literature, 3 different measurement methods have been used to describe degree of patella–trochlea cartilage overlap: PTI, sagittal patellofemoral engagement (SPE), and patellar articular overlap (PAO).1,11,26
PTI uses the true articular cartilage patella–trochlea relationship for measurement of patellar height on sagittal MRI in extension (Figure 3). If the patellar tendon is fully out to length (no laxity), PTI reliably and precisely determines the exact articular correlation of PF joint and patellar height.6
SPE is similar to percentage of articular coverage.1,11 Patellar overlap of the trochlea is measured on 2 different sagittal MRI images: 1 with the greatest length of patellar cartilage and 1 with the greatest length of femoral trochlear cartilage. This measurement did not correlate with CDI.1 SPE was not evaluated in control studies, and normal and pathologic values are unavailable.
PAO is measured with patients positioned at rest and in standard knee coils.1 MRI was not obtained in full extension or with quadriceps activation, and knee flexion angle values are unavailable. Total patellar articular length was measured on the sagittal MRI that showed the greatest patellar length and articular cartilage thickness. The same image was used to measure articular overlap, or the length of patellar cartilage overlying the trochlear cartilage, as measured parallel to the subchondral surface of the patella. PAO correlates well with conventional patellar height measurements in the sagittal plane and shows promise as a simpler alternative to the conventional indices.1 Normal and pathologic values are unavailable.
So far, PTI is the only method that was controlled in several studies, assessed for its reliability, and compared with other patellar height measurements.6,14
Patellar Tendon Length. PTL can be measured on lateral radiographs or sagittal MRI. Radiologic and MRI measurements are described as not significantly different47 or slightly different, and 0.09 to 0.13 needs to be added with the ISI ratio on radiographs, MRI, and CT.46 PTL is reliably evaluated with ISI.41,50 There is a weak correlation of patient height and PTL: Taller people have normally longer tendons.51-53
PTL measurements revealed that the posterior surface of the patellar tendon was significantly shorter than the anterior surface. Compared with their corresponding posterior fascicles, anterior fascicles are longer; their attachment is more proximal to the patella and more distal to the tibia. In addition, posterior PTL is significantly shorter with the posterior patellar attachment (adheres to posterior aspect of the inferior patellar pole) than with the anterior attachment (adheres to anterior aspect of the inferior patellar pole).56 Moreover, the lateral and medial fascicles are longer than the central fascicles attaching at the most inferior patellar pole. This issue must be considered in image cut selection (Figures 6A, 6B). Furthermore, type of inferior pole of patella (pointed, intermediate, blunt) is important in measurement.56 Overall, mean (SD) PTL was 54.9 (1.2) mm on the anterior surface and 35.0 (0.6) mm on the posterior surface.
It is important to precisely describe the measurement method and location (sagittal cut) and to consider the shape of the inferior patellar pole and the site of the patellar tendon attachment.56
Treatments. For patella alta, the surgical treatment goals are to increase the PF contact area and improve PF articular congruence, and thereby increase PF stability.31-33 Four different procedures were used to treat patella alta (Table 5), but only 11 of the 92 original study articles and 8 of the 28 review articles included in our review mentioned a specific patellar height that required surgical correction (Tables 5, 6). Recommendations were based on CDI (>1.2 to >1.4) and less often on ISI (>1.2 to >1.4) or PTL (>56 mm).
Tibial tubercle distalization is effective in normalizing patellar height to correct the patellar index in patella alta (Figures 7A, 7B).13,18,38,55,57 Tibial tubercle distalization and patellar tendon tenodesis attached to the original insertion normalize PTL and stabilize the PF joint in patients with patella alta.5,47 In a comparison of these surgical procedures, cartilage stress was lower with distalization than with distalization and tenodesis.13
Soft-tissue methods are recommended in children, as bone procedures injure the proximal tibial physis and may cause it to close prematurely.58 The patella can be distally advanced by completely mobilizing the patellar tendon and fixating with sutures through the cartilaginous tibial tubercle. This technique is a satisfactory treatment for skeletally immature patients who present with habitual patellar dislocation associated with patella alta.58,59
CDI and BPI assess patellar height relative to the tibial plateau, and therefore are the most useful measurement methods for patellar height after distalization of the tibial tubercle.5 ISI does not change after distalization of the tibial tubercle and cannot be recommended.5,18 As PF indices (eg, PTI) can trace preoperative and postoperative values, these measurements are valuable.
Controversies
Our review found no consensus on measurement method or cutoff value. No measurement method showed clear clinical or methodologic superiority. Most published patellar height and patella alta data are based on conventional radiographs using a tibial reference point, even though a femoral or even trochlear reference point seems more reproducible, particularly in PF pathologies. Several cutoff values have been reported for each patella alta measurement method. Regarding pathologic thresholds, which might require surgical correction, very little information has been published, and scientific evidence is lacking. Numerous important aspects remain unanswered after this review, and clarification is mandatory.
Five Key Facts
1. In patella alta assessment, different morphologic, biomechanical, and functional aspects must be considered. The most relevant aspect is decreased engagement of the patella and trochlea,4-9,11,26 which results in decreased bony stability in knee extension.1-3 Therefore, patella alta is one of the potential risk factors for patellar instability with a high percentage of recurrent patellar dislocation.4-9 In early knee flexion, the patella translates more distally with better engagement of the patella in the trochlea and better stability. Therefore, measurement in extension, with the patellar tendon out to length, seems to offer a more reliable assessment of the patella–femur relationship.
2. Insufficient engagement of the patella in the trochlea is the most important aspect of patella alta.11 Therefore, direct measurement of this engagement seems logical.
3. As radiographs do not show articular cartilage, they should not be used to assess the articular patella–femur relationship.11,14,26 Patella–trochlea cartilage overlap is the most relevant factor for patella alta and should be measured on MRI.6,14,26,32
4. PTL is an important factor for patellar height and particularly patella alta. The range of normal PTL values is wide: 35 mm to 61 mm. The described cutoff values for patella alta (>52 mm to >56 mm) fall within this normal range. The cause may be the different measurement methods used.33,47,59-61 Therefore, for precise diagnostics, a standardized measurement method that includes the selected cut imaging is mandatory.
Many important aspects remain unanswered, and clarification is mandatory (Table 9).
Conclusion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Presence of patella alta depends on measurement method used. Methods cannot be used interchangeably, and they all have their advantages and limitations. Unfortunately, there is no generally accepted consensus on measurement method, patella alta cutoff value, or treatment with ideal correction. Treatment planning and outcomes assessment require clarification of these many issues.
Take-Home Points
- Patella alta has a reduced articular area of PF contact.
- Presence of patella alta depends on the measurement method.
- Patella alta is defined as ISI >1.2 and CDI >1.2 to >1.3.
- On sagittal MRI, PTI is used most often with cutoff values of <0.125 to 0.28.
- Tibial tubercle distalization is most often used to treat patella alta. The desired postoperative patellar height is a CDI of 1.0.
Patella alta is a patella that rides abnormally high in relation to the femur, the femoral trochlea, or the tibia,1 with decreased bony stability requiring increased knee flexion angles to engage the trochlea.2,3 An abnormally high patella may therefore insufficiently engage the proximal trochlea groove both in extension and in the early phase of knee flexion—making it one of the potential risk factors for patellar instability.4-10 Accordingly, patella alta is present in 30% of patients with recurrent patellar dislocation.11 It also occurs in other disorders, such as knee extensor apparatus disorders, in patients with patellofemoral (PF) pain, chondromalacia, Sinding-Larsen-Johansson disease, Osgood-Schlatter disease, patellar tendinopathy, and osteoarthritis.1,7,8,12-18 As such, patella alta represents an important predisposing factor for patellar malalignment and PF-related complaints. On the other hand, patella alta may also be a normal variant of a person’s knee anatomy and may be well tolerated when not combined with other instability factors.4
Despite the importance of patella alta, there is no consensus on a precise definition, the most reliable measurement method, or the factors thought to be important in clinical decisions regarding treatment. To address this issue, we systematically reviewed the patella alta literature for definitions, the most common measurement methods and their patella alta cutoff values, and cutoff values for surgical correction and proposed surgical techniques.
Methods
In February 2017, using the term patella alta, we performed a systematic literature search on PubMed. Inclusion criteria were original study or review articles, publication in peer-reviewed English-language journals between 2000 and 2017, and narrative description or measurement of human patellar height on plain radiographs or magnetic resonance imaging (MRI). Excluded were abstracts and articles in languages other than English; animal and computational/biomechanical studies; case reports; and knee arthroplasties, knee extensor ruptures, and hereditary and congenital diseases. All evidence levels were included.
We assessed measurement methods, reported cutoff values for patella alta, cutoff values for performing surgical correction, proposed surgical techniques, and postoperative target values. Original study articles and review articles were analyzed separately.
Results
Of 211 articles identified, 92 met the inclusion criteria for original study, and 28 for review. All their abstracts were reviewed, and 91 were excluded: 17 for language other than English, 11 for animal study, 12 for biomechanical/computational study, 20 for case report, 8 for arthroplasty, 13 for hereditary or congenital disease, 1 for extensor apparatus rupture, 3 for editorial letter, and 6 for other reasons. Full text copies of all included articles were obtained and reviewed.
Original Study Articles
Definition. Of the 92 original study articles, 17 (18.5%) defined patella alta by description alone, and 75 (81.5%) used imaging-based measurements. Patella alta was described as a patella that rides abnormally high in relation to the trochlear groove, with a reduced articular area of PF contact1,15,19-21 or decreased patella–trochlea cartilage overlap.22 With this reduced contact area, there is increased PF stress.21
With radiographic measurements, patella alta is defined as a Caton-Deschamps index (CDI) of >1.2 to >1.3, an Insall-Salvati index (ISI) of >1.2, a Blackburne-Peel index (PBI) of >1.0,4,15,18,23-25 and a patellotrochlear index (PTI) of <0.125 to 0.28.6,26
On lateral radiographs, ISI was the most common measurement (33 studies), with patella alta cutoff values ranging from >1.2 to >1.5. The second most common measurement was CDI (24 studies), with cutoff values of >1.2 to >1.3. Other indices, such as the modified ISI (6 studies), the BPI (1 study), and the Koshino index (2 studies), had their cutoff values used more consistently (>1.6 to >2.4, >1.0, and >1.2, respectively) but these indices were rarely reported in the literature (Table 1).27,28,29
Thirty-six studies defined patella alta with MRI using either the aforementioned radiographic ratios (23 studies, 3 indices, Table 2) or PF indices (13 studies, 4 methods, Table 3).30
On sagittal MRI, PTI was used most often; cutoff values were <0.125 to 0.28.
Thirteen studies defined patella alta with PTL: 5 using lateral conventional radiographs and 8 using sagittal MRI. For each type of imaging, the cutoff value was >52 mm to >56 mm. PTL was measured either independently or together with ISI on sagittal MRI (Table 4).
Review Articles
Twenty-eight review articles met the inclusion criteria.35-40 Patella alta was described mostly with ISI (75%) or CDI (64%). In up to 57% of these articles, a patella alta reference value was missing. Only 1 article mentioned different cutoff values for conventional radiographs and MRI. BPI was mentioned in 50% of studies, but only 2 indicated a cutoff value for patella alta. Eleven percent used PTI on sagittal MRI and took patella–trochlea cartilage overlap into account.
Eight review articles mentioned cutoff values for surgical correction. Only 1 of 4 articles mentioned a cutoff value for correcting patella alta in PF instability, and only 2 articles suggested an ideal postoperative patellar height (Table 6).
No review article relied on PTL to describe patella alta or to advise surgical treatment.
Discussion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Interpretation of patellar height and, particularly, patella alta is very heterogeneous. Accordingly, comparing surgical indications, surgical treatment options, and outcomes across studies can be difficult.
Measurement Methods
Radiographs. Conventional radiographs were used to describe patella alta in two-thirds of the original study articles. The most common measurement methods marked the position of the patella relative to either the femur (direct assessment) or the tibia (indirect assessment).14,25 All established measurement methods use lateral radiographs, which do not show articular cartilage.11,14 Therefore, lateral radiograph ratios of different and variable bony landmarks do not measure actual PF articular congruence. The widely variable morphologies of patella (distal patellar nose, different articulating surface), femoral trochlea (facet in height and length), proximal tibia (inherited or acquired deformities on tibial tubercle), and slope are all confounding factors that may affect measurement (Figure 1). In addition, specific variations in PF joint morphology (eg, small patellar articular surface, short trochlea) are not well represented by these ratios.11
All these methods have advantages, limitations, and different values of interobserver and intraobserver variability, reliability, and reproducibility (Table 7).29,41
Determination of both patellar height and patella alta depends on the measurement method used and is significantly affected by anatomical variants, such as extra-articular patella and femorotibial landmarks.
The ISI method is most commonly used because it does not depend on degree of knee flexion, is thought to measure the relative length of the patellar tendon the best,5 and has established MRI criteria.6 However, ISI has limitations: Its distal bony landmark is the tibial tuberosity, which can be difficult to assess14; the shape of the patella (mainly its inferior point) varies42; and the measurement does not change after distalization of the tibial tubercle.5
CDI is the most accurate diagnostic method because it relies on readily identifiable and reproducible anatomical landmarks; does not depend on radiograph quality, knee size, radiologic enlargement, or position of the tibial tubercle or patellar modification; and is unaffected by degree of knee flexion between 10° and 80°.38,43 CDI is the method that is most useful in describing patellar height after distalization of the tibial tubercle because it assesses the height of the patella relative to the tibial plateau.5 CDI is limited with respect to clear identification of the patellar and tibial articular margin.37
BPI has the lowest interobserver variability and discriminates best among patella alta, norma, and infera.41 Like CDI, BPI assesses patellar height relative to the tibial plateau, and thus is useful in describing patellar height after distalization of the tibial tubercle.5 BPI is limited in that it uses a line drawn along the tibial plateau, where variations in inclination and tibial slope produce inaccuracies,14 and depends highly on projection angles. Clinically, BPI is rarely used.
As the literature shows, the radiologic methods of determining patellar height are variable and unreliable and depend on the ratio used.26,41 These methods do not reveal precise information about the PF articular congruence, the key finding for necessary treatment.
Radiographic Indices on MRI. Some studies measured radiographic indices on sagittal MRI. Radiologic and MRI measurements are described as not significantly different or slightly different, and 0.09 to 0.13 needs to be added with ISI and BP ratios on radiographs, MRI, and computed tomography (CT).17,44-47 ISI is commonly used, and its clinical application is easy. Although classically measured on plain radiographs, ISI is reliably measured on MRI as well.17,30 The traditional 1.2 threshold for defining patella alta was used by Charles and colleagues.44 CDI is equally well measured on radiographs and MRI.47
According to the literature, 3 different measurement methods have been used to describe degree of patella–trochlea cartilage overlap: PTI, sagittal patellofemoral engagement (SPE), and patellar articular overlap (PAO).1,11,26
PTI uses the true articular cartilage patella–trochlea relationship for measurement of patellar height on sagittal MRI in extension (Figure 3). If the patellar tendon is fully out to length (no laxity), PTI reliably and precisely determines the exact articular correlation of PF joint and patellar height.6
SPE is similar to percentage of articular coverage.1,11 Patellar overlap of the trochlea is measured on 2 different sagittal MRI images: 1 with the greatest length of patellar cartilage and 1 with the greatest length of femoral trochlear cartilage. This measurement did not correlate with CDI.1 SPE was not evaluated in control studies, and normal and pathologic values are unavailable.
PAO is measured with patients positioned at rest and in standard knee coils.1 MRI was not obtained in full extension or with quadriceps activation, and knee flexion angle values are unavailable. Total patellar articular length was measured on the sagittal MRI that showed the greatest patellar length and articular cartilage thickness. The same image was used to measure articular overlap, or the length of patellar cartilage overlying the trochlear cartilage, as measured parallel to the subchondral surface of the patella. PAO correlates well with conventional patellar height measurements in the sagittal plane and shows promise as a simpler alternative to the conventional indices.1 Normal and pathologic values are unavailable.
So far, PTI is the only method that was controlled in several studies, assessed for its reliability, and compared with other patellar height measurements.6,14
Patellar Tendon Length. PTL can be measured on lateral radiographs or sagittal MRI. Radiologic and MRI measurements are described as not significantly different47 or slightly different, and 0.09 to 0.13 needs to be added with the ISI ratio on radiographs, MRI, and CT.46 PTL is reliably evaluated with ISI.41,50 There is a weak correlation of patient height and PTL: Taller people have normally longer tendons.51-53
PTL measurements revealed that the posterior surface of the patellar tendon was significantly shorter than the anterior surface. Compared with their corresponding posterior fascicles, anterior fascicles are longer; their attachment is more proximal to the patella and more distal to the tibia. In addition, posterior PTL is significantly shorter with the posterior patellar attachment (adheres to posterior aspect of the inferior patellar pole) than with the anterior attachment (adheres to anterior aspect of the inferior patellar pole).56 Moreover, the lateral and medial fascicles are longer than the central fascicles attaching at the most inferior patellar pole. This issue must be considered in image cut selection (Figures 6A, 6B). Furthermore, type of inferior pole of patella (pointed, intermediate, blunt) is important in measurement.56 Overall, mean (SD) PTL was 54.9 (1.2) mm on the anterior surface and 35.0 (0.6) mm on the posterior surface.
It is important to precisely describe the measurement method and location (sagittal cut) and to consider the shape of the inferior patellar pole and the site of the patellar tendon attachment.56
Treatments. For patella alta, the surgical treatment goals are to increase the PF contact area and improve PF articular congruence, and thereby increase PF stability.31-33 Four different procedures were used to treat patella alta (Table 5), but only 11 of the 92 original study articles and 8 of the 28 review articles included in our review mentioned a specific patellar height that required surgical correction (Tables 5, 6). Recommendations were based on CDI (>1.2 to >1.4) and less often on ISI (>1.2 to >1.4) or PTL (>56 mm).
Tibial tubercle distalization is effective in normalizing patellar height to correct the patellar index in patella alta (Figures 7A, 7B).13,18,38,55,57 Tibial tubercle distalization and patellar tendon tenodesis attached to the original insertion normalize PTL and stabilize the PF joint in patients with patella alta.5,47 In a comparison of these surgical procedures, cartilage stress was lower with distalization than with distalization and tenodesis.13
Soft-tissue methods are recommended in children, as bone procedures injure the proximal tibial physis and may cause it to close prematurely.58 The patella can be distally advanced by completely mobilizing the patellar tendon and fixating with sutures through the cartilaginous tibial tubercle. This technique is a satisfactory treatment for skeletally immature patients who present with habitual patellar dislocation associated with patella alta.58,59
CDI and BPI assess patellar height relative to the tibial plateau, and therefore are the most useful measurement methods for patellar height after distalization of the tibial tubercle.5 ISI does not change after distalization of the tibial tubercle and cannot be recommended.5,18 As PF indices (eg, PTI) can trace preoperative and postoperative values, these measurements are valuable.
Controversies
Our review found no consensus on measurement method or cutoff value. No measurement method showed clear clinical or methodologic superiority. Most published patellar height and patella alta data are based on conventional radiographs using a tibial reference point, even though a femoral or even trochlear reference point seems more reproducible, particularly in PF pathologies. Several cutoff values have been reported for each patella alta measurement method. Regarding pathologic thresholds, which might require surgical correction, very little information has been published, and scientific evidence is lacking. Numerous important aspects remain unanswered after this review, and clarification is mandatory.
Five Key Facts
1. In patella alta assessment, different morphologic, biomechanical, and functional aspects must be considered. The most relevant aspect is decreased engagement of the patella and trochlea,4-9,11,26 which results in decreased bony stability in knee extension.1-3 Therefore, patella alta is one of the potential risk factors for patellar instability with a high percentage of recurrent patellar dislocation.4-9 In early knee flexion, the patella translates more distally with better engagement of the patella in the trochlea and better stability. Therefore, measurement in extension, with the patellar tendon out to length, seems to offer a more reliable assessment of the patella–femur relationship.
2. Insufficient engagement of the patella in the trochlea is the most important aspect of patella alta.11 Therefore, direct measurement of this engagement seems logical.
3. As radiographs do not show articular cartilage, they should not be used to assess the articular patella–femur relationship.11,14,26 Patella–trochlea cartilage overlap is the most relevant factor for patella alta and should be measured on MRI.6,14,26,32
4. PTL is an important factor for patellar height and particularly patella alta. The range of normal PTL values is wide: 35 mm to 61 mm. The described cutoff values for patella alta (>52 mm to >56 mm) fall within this normal range. The cause may be the different measurement methods used.33,47,59-61 Therefore, for precise diagnostics, a standardized measurement method that includes the selected cut imaging is mandatory.
Many important aspects remain unanswered, and clarification is mandatory (Table 9).
Conclusion
Our review revealed many variations in patella alta definitions and descriptions, measurement methods, cutoff values, and treatment options. Presence of patella alta depends on measurement method used. Methods cannot be used interchangeably, and they all have their advantages and limitations. Unfortunately, there is no generally accepted consensus on measurement method, patella alta cutoff value, or treatment with ideal correction. Treatment planning and outcomes assessment require clarification of these many issues.
1 Munch JL, Sullivan JP, Nguyen JT, et al. Patellar articular overlap on MRI is a simple alternative to conventional measurements of patellar height. Orthop J Sports Med. 2016;4(7):2325967116656328.
2. Elias JJ, Soehnlen NT, Guseila LM, Cosgarea AJ. Dynamic tracking influenced by anatomy in patellar instability. Knee. 2016;23(3):450-455.
3. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184.
4. Askenberger M, Janarv PM, Finnbogason T, Arendt EA. Morphology and anatomic patellar instability risk factors in first-time traumatic lateral patellar dislocations. Am J Sports Med. 2017;45(1):50-58.
5. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
6. Ali SA, Helmer R, Terk MR. Patella alta: lack of correlation between patellotrochlear cartilage congruence and commonly used patellar height ratios. AJR Am J Roentgenol. 2009;193(5):1361-1366.
7. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581.
8. Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):1749-1755.
9. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
10. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
11. Dejour D, Ferrua P, Ntagiopoulos PG, et al. The introduction of a new MRI index to evaluate sagittal patellofemoral engagement. Orthop Traumatol Surg Res. 2013;99(8 suppl):S391-S398.
12. Althani S, Shahi A, Tan TL, Al-Belooshi A. Position of the patella among Emirati adult knees. Is Insall-Salvati ratio applicable to Middle-Easterners? Arch Bone Joint Surg. 2016;4(2):137-140.
13. Yin L, Liao TC, Yang L, Powers CM. Does patella tendon tenodesis improve tibial tubercle distalization in treating patella alta? A computational study. Clin Orthop Relat Res. 2016;474(11):2451-2461.
14. Barnett AJ, Prentice M, Mandalia V, Wakeley CJ, Eldridge JD. Patellar height measurement in trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1412-1415.
15. Otsuki S, Nakajima M, Fujiwara K, et al. Influence of age on clinical outcomes of three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2392-2396.
16. Otsuki S, Nakajima M, Oda S, et al. Three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. J Orthop Sci. 2013;18(3):437-442.
17. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
18. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2545-2550.
19. Bertollo N, Pelletier MH, Walsh WR. Simulation of patella alta and the implications for in vitro patellar tracking in the ovine stifle joint. J Orthop Res. 2012;30(11):1789-1797.
20. Narkbunnam R, Chareancholvanich K. Effect of patient position on measurement of patellar height ratio. Arch Orthop Trauma Surg. 2015;135(8):1151-1156.
21. Stefanik JJ, Zhu Y, Zumwalt AC, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Care Res. 2010;62(9):1258-1265.
22. Monk AP, Doll HA, Gibbons CL, et al. The patho-anatomy of patellofemoral subluxation. J Bone Joint Surg Br. 2011;93(10):1341-1347.
23. Hirano A, Fukubayashi T, Ishii T, Ochiai N. Relationship between the patellar height and the disorder of the knee extensor mechanism in immature athletes. J Pediatr Orthop. 2001;21(4):541-544.
24. Ng JP, Cawley DT, Beecher SM, Lee MJ, Bergin D, Shannon FJ. Focal intratendinous radiolucency: a new radiographic method for diagnosing patellar tendon ruptures. Knee. 2016;23(3):482-486.
25. van Duijvenbode D, Stavenuiter M, Burger B, van Dijke C, Spermon J, Hoozemans M. The reliability of four widely used patellar height ratios. Int Orthop. 2016;40(3):493-497.
26. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
27. Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;(282):170-176.
28. Thaunat M, Erasmus PJ. The favourable anisometry: an original concept for medial patellofemoral ligament reconstruction. Knee. 2007;14(6):424-428.
29. Anagnostakos K, Lorbach O, Reiter S, Kohn D. Comparison of five patellar height measurement methods in 90 degrees knee flexion. Int Orthop. 2011;35(12):1791-1797.
30. Miller TT, Staron RB, Feldman F. Patellar height on sagittal MR imaging of the knee. AJR Am J Roentgenol. 1996;167(2):339-341.
31. Dejour D, Le Coultre B. Osteotomies in patello-femoral instabilities. Sports Med Arthrosc Rev. 2007;15(1):39-46.
32. Rhee SJ, Pavlou G, Oakley J, Barlow D, Haddad F. Modern management of patellar instability. Int Orthop. 2012;36(12):2447-2456.
33. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc Rev. 2007;15(2):61-67.
34. Caton J, Deschamps G, Chambat P, Lerat JL, Dejour H. [Patella infera. Apropos of 128 cases]. Rev Chir Orthop Reparatrice Appar Mot. 1982;68(5):317-325.
35. Meyers AB, Laor T, Sharafinski M, Zbojniewicz AM. Imaging assessment of patellar instability and its treatment in children and adolescents. Pediatr Radiol. 2016;46(5):618-636.
36. Feller JA. Distal realignment (tibial tuberosity transfer). Sports Med Arthrosc Rev. 2012;20(3):152-161.
37. Dietrich TJ, Fucentese SF, Pfirrmann CW. Imaging of individual anatomical risk factors for patellar instability. Semin Musculoskelet Radiol. 2016;20(1):65-73.
38. Dean CS, Chahla J, Serra Cruz R, Cram TR, LaPrade RF. Patellofemoral joint reconstruction for patellar instability: medial patellofemoral ligament reconstruction, trochleoplasty, and tibial tubercle osteotomy. Arthrosc Tech. 2016;5(1):e169-e175.
39. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497.
40. Weber AE, Nathani A, Dines JS, et al. An algorithmic approach to the management of recurrent lateral patellar dislocation. J Bone Joint Surg Am. 2016;98(5):417-427.
41. Seil R, Muller B, Georg T, Kohn D, Rupp S. Reliability and interobserver variability in radiological patellar height ratios. Knee Surg Sports Traumatol Arthrosc. 2000;8(4):231-236.
42. Laprade J, Culham E. Radiographic measures in subjects who are asymptomatic and subjects with patellofemoral pain syndrome. Clin Orthop Relat Res. 2003;(414):172-182.
43. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
44. Charles MD, Haloman S, Chen L, Ward SR, Fithian D, Afra R. Magnetic resonance imaging–based topographical differences between control and recurrent patellofemoral instability patients. Am J Sports Med. 2013;41(2):374-384.
45. Kurtul Yildiz H, Ekin EE. Patellar malalignment: a new method on knee MRI. Springerplus. 2016;5(1):1500.
46. Lee PP, Chalian M, Carrino JA, Eng J, Chhabra A. Multimodality correlations of patellar height measurement on x-ray, CT, and MRI. Skeletal Radiol. 2012;41(10):1309-1314.
47. Neyret P, Robinson AH, Le Coultre B, Lapra C, Chambat P. Patellar tendon length—the factor in patellar instability? Knee. 2002;9(1):3-6.
48. Diederichs G, Issever AS, Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics. 2010;30(4):961-981.
49. Earhart C, Patel DB, White EA, Gottsegen CJ, Forrester DM, Matcuk GR Jr. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20(1):11-23.
50. Aarimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446.
51. Brown DE, Alexander AH, Lichtman DM. The Elmslie-Trillat procedure: evaluation in patellar dislocation and subluxation. Am J Sports Med. 1984;12(2):104-109.
52. Goldstein JL, Verma N, McNickle AG, Zelazny A, Ghodadra N, Bach BR Jr. Avoiding mismatch in allograft anterior cruciate ligament reconstruction: correlation between patient height and patellar tendon length. Arthroscopy. 2010;26(5):643-650.
53. Navali AM, Jafarabadi MA. Is there any correlation between patient height and patellar tendon length? Arch Bone Joint Surg. 2015;3(2):99-103.
54. Park MS, Chung CY, Lee KM, Lee SH, Choi IH. Which is the best method to determine the patellar height in children and adolescents? Clin Orthop Relat Res. 2010;468(5):1344-1351.
55. Berard JB, Magnussen RA, Bonjean G, et al. Femoral tunnel enlargement after medial patellofemoral ligament reconstruction: prevalence, risk factors, and clinical effect. Am J Sports Med. 2014;42(2):297-301.
56. Edama M, Kageyama I, Nakamura M, et al. Anatomical study of the inferior patellar pole and patellar tendon [published online ahead of print February 16, 2017]. Scand J Med Sci Sports. doi:10.1111/sms.12858.
57. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Relat Res. 2002;(396):152-162.
58. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177.
59. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
60. Degnan AJ, Maldjian C, Adam RJ, Fu FH, Di Domenica M. Comparison of Insall-Salvati ratios in children with an acute anterior cruciate ligament tear and a matched control population. AJR Am J Roentgenol. 2015;204(1):161-166.
61. Wittstein JR, Bartlett EC, Easterbrook J, Byrd JC. Magnetic resonance imaging evaluation of patellofemoral malalignment. Arthroscopy. 2006;22(6):643-649.
1 Munch JL, Sullivan JP, Nguyen JT, et al. Patellar articular overlap on MRI is a simple alternative to conventional measurements of patellar height. Orthop J Sports Med. 2016;4(7):2325967116656328.
2. Elias JJ, Soehnlen NT, Guseila LM, Cosgarea AJ. Dynamic tracking influenced by anatomy in patellar instability. Knee. 2016;23(3):450-455.
3. Fabricant PD, Ladenhauf HN, Salvati EA, Green DW. Medial patellofemoral ligament (MPFL) reconstruction improves radiographic measures of patella alta in children. Knee. 2014;21(6):1180-1184.
4. Askenberger M, Janarv PM, Finnbogason T, Arendt EA. Morphology and anatomic patellar instability risk factors in first-time traumatic lateral patellar dislocations. Am J Sports Med. 2017;45(1):50-58.
5. Mayer C, Magnussen RA, Servien E, et al. Patellar tendon tenodesis in association with tibial tubercle distalization for the treatment of episodic patellar dislocation with patella alta. Am J Sports Med. 2012;40(2):346-351.
6. Ali SA, Helmer R, Terk MR. Patella alta: lack of correlation between patellotrochlear cartilage congruence and commonly used patellar height ratios. AJR Am J Roentgenol. 2009;193(5):1361-1366.
7. Lewallen LW, McIntosh AL, Dahm DL. Predictors of recurrent instability after acute patellofemoral dislocation in pediatric and adolescent patients. Am J Sports Med. 2013;41(3):575-581.
8. Ward SR, Terk MR, Powers CM. Patella alta: association with patellofemoral alignment and changes in contact area during weight-bearing. J Bone Joint Surg Am. 2007;89(8):1749-1755.
9. Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19-26.
10. Arendt EA, Fithian DC, Cohen E. Current concepts of lateral patella dislocation. Clin Sports Med. 2002;21(3):499-519.
11. Dejour D, Ferrua P, Ntagiopoulos PG, et al. The introduction of a new MRI index to evaluate sagittal patellofemoral engagement. Orthop Traumatol Surg Res. 2013;99(8 suppl):S391-S398.
12. Althani S, Shahi A, Tan TL, Al-Belooshi A. Position of the patella among Emirati adult knees. Is Insall-Salvati ratio applicable to Middle-Easterners? Arch Bone Joint Surg. 2016;4(2):137-140.
13. Yin L, Liao TC, Yang L, Powers CM. Does patella tendon tenodesis improve tibial tubercle distalization in treating patella alta? A computational study. Clin Orthop Relat Res. 2016;474(11):2451-2461.
14. Barnett AJ, Prentice M, Mandalia V, Wakeley CJ, Eldridge JD. Patellar height measurement in trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1412-1415.
15. Otsuki S, Nakajima M, Fujiwara K, et al. Influence of age on clinical outcomes of three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2392-2396.
16. Otsuki S, Nakajima M, Oda S, et al. Three-dimensional transfer of the tibial tuberosity for patellar instability with patella alta. J Orthop Sci. 2013;18(3):437-442.
17. Steensen RN, Bentley JC, Trinh TQ, Backes JR, Wiltfong RE. The prevalence and combined prevalences of anatomic factors associated with recurrent patellar dislocation: a magnetic resonance imaging study. Am J Sports Med. 2015;43(4):921-927.
18. Magnussen RA, De Simone V, Lustig S, Neyret P, Flanigan DC. Treatment of patella alta in patients with episodic patellar dislocation: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014;22(10):2545-2550.
19. Bertollo N, Pelletier MH, Walsh WR. Simulation of patella alta and the implications for in vitro patellar tracking in the ovine stifle joint. J Orthop Res. 2012;30(11):1789-1797.
20. Narkbunnam R, Chareancholvanich K. Effect of patient position on measurement of patellar height ratio. Arch Orthop Trauma Surg. 2015;135(8):1151-1156.
21. Stefanik JJ, Zhu Y, Zumwalt AC, et al. Association between patella alta and the prevalence and worsening of structural features of patellofemoral joint osteoarthritis: the Multicenter Osteoarthritis Study. Arthritis Care Res. 2010;62(9):1258-1265.
22. Monk AP, Doll HA, Gibbons CL, et al. The patho-anatomy of patellofemoral subluxation. J Bone Joint Surg Br. 2011;93(10):1341-1347.
23. Hirano A, Fukubayashi T, Ishii T, Ochiai N. Relationship between the patellar height and the disorder of the knee extensor mechanism in immature athletes. J Pediatr Orthop. 2001;21(4):541-544.
24. Ng JP, Cawley DT, Beecher SM, Lee MJ, Bergin D, Shannon FJ. Focal intratendinous radiolucency: a new radiographic method for diagnosing patellar tendon ruptures. Knee. 2016;23(3):482-486.
25. van Duijvenbode D, Stavenuiter M, Burger B, van Dijke C, Spermon J, Hoozemans M. The reliability of four widely used patellar height ratios. Int Orthop. 2016;40(3):493-497.
26. Biedert RM, Albrecht S. The patellotrochlear index: a new index for assessing patellar height. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):707-712.
27. Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;(282):170-176.
28. Thaunat M, Erasmus PJ. The favourable anisometry: an original concept for medial patellofemoral ligament reconstruction. Knee. 2007;14(6):424-428.
29. Anagnostakos K, Lorbach O, Reiter S, Kohn D. Comparison of five patellar height measurement methods in 90 degrees knee flexion. Int Orthop. 2011;35(12):1791-1797.
30. Miller TT, Staron RB, Feldman F. Patellar height on sagittal MR imaging of the knee. AJR Am J Roentgenol. 1996;167(2):339-341.
31. Dejour D, Le Coultre B. Osteotomies in patello-femoral instabilities. Sports Med Arthrosc Rev. 2007;15(1):39-46.
32. Rhee SJ, Pavlou G, Oakley J, Barlow D, Haddad F. Modern management of patellar instability. Int Orthop. 2012;36(12):2447-2456.
33. Servien E, Verdonk PC, Neyret P. Tibial tuberosity transfer for episodic patellar dislocation. Sports Med Arthrosc Rev. 2007;15(2):61-67.
34. Caton J, Deschamps G, Chambat P, Lerat JL, Dejour H. [Patella infera. Apropos of 128 cases]. Rev Chir Orthop Reparatrice Appar Mot. 1982;68(5):317-325.
35. Meyers AB, Laor T, Sharafinski M, Zbojniewicz AM. Imaging assessment of patellar instability and its treatment in children and adolescents. Pediatr Radiol. 2016;46(5):618-636.
36. Feller JA. Distal realignment (tibial tuberosity transfer). Sports Med Arthrosc Rev. 2012;20(3):152-161.
37. Dietrich TJ, Fucentese SF, Pfirrmann CW. Imaging of individual anatomical risk factors for patellar instability. Semin Musculoskelet Radiol. 2016;20(1):65-73.
38. Dean CS, Chahla J, Serra Cruz R, Cram TR, LaPrade RF. Patellofemoral joint reconstruction for patellar instability: medial patellofemoral ligament reconstruction, trochleoplasty, and tibial tubercle osteotomy. Arthrosc Tech. 2016;5(1):e169-e175.
39. Frosch KH, Schmeling A. A new classification system of patellar instability and patellar maltracking. Arch Orthop Trauma Surg. 2016;136(4):485-497.
40. Weber AE, Nathani A, Dines JS, et al. An algorithmic approach to the management of recurrent lateral patellar dislocation. J Bone Joint Surg Am. 2016;98(5):417-427.
41. Seil R, Muller B, Georg T, Kohn D, Rupp S. Reliability and interobserver variability in radiological patellar height ratios. Knee Surg Sports Traumatol Arthrosc. 2000;8(4):231-236.
42. Laprade J, Culham E. Radiographic measures in subjects who are asymptomatic and subjects with patellofemoral pain syndrome. Clin Orthop Relat Res. 2003;(414):172-182.
43. Caton JH, Dejour D. Tibial tubercle osteotomy in patello-femoral instability and in patellar height abnormality. Int Orthop. 2010;34(2):305-309.
44. Charles MD, Haloman S, Chen L, Ward SR, Fithian D, Afra R. Magnetic resonance imaging–based topographical differences between control and recurrent patellofemoral instability patients. Am J Sports Med. 2013;41(2):374-384.
45. Kurtul Yildiz H, Ekin EE. Patellar malalignment: a new method on knee MRI. Springerplus. 2016;5(1):1500.
46. Lee PP, Chalian M, Carrino JA, Eng J, Chhabra A. Multimodality correlations of patellar height measurement on x-ray, CT, and MRI. Skeletal Radiol. 2012;41(10):1309-1314.
47. Neyret P, Robinson AH, Le Coultre B, Lapra C, Chambat P. Patellar tendon length—the factor in patellar instability? Knee. 2002;9(1):3-6.
48. Diederichs G, Issever AS, Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics. 2010;30(4):961-981.
49. Earhart C, Patel DB, White EA, Gottsegen CJ, Forrester DM, Matcuk GR Jr. Transient lateral patellar dislocation: review of imaging findings, patellofemoral anatomy, and treatment options. Emerg Radiol. 2013;20(1):11-23.
50. Aarimaa V, Ranne J, Mattila K, Rahi K, Virolainen P, Hiltunen A. Patellar tendon shortening after treatment of patellar instability with a patellar tendon medialization procedure. Scand J Med Sci Sports. 2008;18(4):442-446.
51. Brown DE, Alexander AH, Lichtman DM. The Elmslie-Trillat procedure: evaluation in patellar dislocation and subluxation. Am J Sports Med. 1984;12(2):104-109.
52. Goldstein JL, Verma N, McNickle AG, Zelazny A, Ghodadra N, Bach BR Jr. Avoiding mismatch in allograft anterior cruciate ligament reconstruction: correlation between patient height and patellar tendon length. Arthroscopy. 2010;26(5):643-650.
53. Navali AM, Jafarabadi MA. Is there any correlation between patient height and patellar tendon length? Arch Bone Joint Surg. 2015;3(2):99-103.
54. Park MS, Chung CY, Lee KM, Lee SH, Choi IH. Which is the best method to determine the patellar height in children and adolescents? Clin Orthop Relat Res. 2010;468(5):1344-1351.
55. Berard JB, Magnussen RA, Bonjean G, et al. Femoral tunnel enlargement after medial patellofemoral ligament reconstruction: prevalence, risk factors, and clinical effect. Am J Sports Med. 2014;42(2):297-301.
56. Edama M, Kageyama I, Nakamura M, et al. Anatomical study of the inferior patellar pole and patellar tendon [published online ahead of print February 16, 2017]. Scand J Med Sci Sports. doi:10.1111/sms.12858.
57. Al-Sayyad MJ, Cameron JC. Functional outcome after tibial tubercle transfer for the painful patella alta. Clin Orthop Relat Res. 2002;(396):152-162.
58. Benoit B, Laflamme GY, Laflamme GH, Rouleau D, Delisle J, Morin B. Long-term outcome of surgically-treated habitual patellar dislocation in children with coexistent patella alta. Minimum follow-up of 11 years. J Bone Joint Surg Br. 2007;89(9):1172-1177.
59. Simmons E Jr, Cameron JC. Patella alta and recurrent dislocation of the patella. Clin Orthop Relat Res. 1992;(274):265-269.
60. Degnan AJ, Maldjian C, Adam RJ, Fu FH, Di Domenica M. Comparison of Insall-Salvati ratios in children with an acute anterior cruciate ligament tear and a matched control population. AJR Am J Roentgenol. 2015;204(1):161-166.
61. Wittstein JR, Bartlett EC, Easterbrook J, Byrd JC. Magnetic resonance imaging evaluation of patellofemoral malalignment. Arthroscopy. 2006;22(6):643-649.