User login
Patient-Reported Outcomes of Knotted and Knotless Glenohumeral Labral Repairs Are Equivalent
Take-Home Points
- There is no difference in PROMs following knotless or knotted labral repair.
- Operative time is shorter for knotless compared to knotted glenoid labral tears.
- Knotless constructs may be more predictable than knotted constructs biomechanically.
Orthopedic surgeons often encounter labral pathology, and labral tears historically have required open techniques.1-3 Arthroscopy allows for advanced visualization and treatment of shoulder lesions,4,5 including anterior, posterior, and superior labrum anterior to posterior (SLAP) lesions.6
The goal of arthroscopic labral repair is to restore joint stability while maintaining range of motion. Arthroscopically repairing the labrum with suture anchors has become the standard technique, and several studies have reported satisfactory biomechanical and clinical results.1,7-12 Surgeons traditionally have been required to tie knots for these anchors, but knot security varies significantly among experienced arthroscopic surgeons.13 In addition, knots can migrate,14 and bulky knots can cause chondral abrasion.15,16 Several manufacturers have introduced knotless anchors for soft-tissue fixation.15,17 The knotless technique provides a low-profile repair with potentially less operating time.8 These factors may warrant switching from knotted to knotless techniques if outcomes are clinically acceptable. However, few studies have compared knotted and knotless techniques for glenohumeral labral repair.8,15,18-21
We conducted a study to compare the clinical results and operative times of knotless and knotted fixation of anterior and posterior glenohumeral labral repairs and SLAP repairs. We hypothesized there would be no difference in patient-reported outcome measures (PROMs) between knotted and knotless techniques.
Methods
We retrospectively evaluated data that had been prospectively collected between 2012 and 2016 in a Surgical Outcomes System (SOS; Arthrex) database. Participation in this registry is elective, and enrollment can occur on a case-by-case basis. The database stores data on basic demographics, PROMs, and operative time. Data for our specific analysis were available for surgeries performed by 115 different surgeons. Inclusion criteria included primary isolated arthroscopic anterior, isolated posterior, and isolated SLAP repair with completely knotted or completely knotless labral repair and minimum 1-year follow-up. Exclusion criteria included hybrid knotted–knotless repair, rotator cuff repair, revision surgery, open surgery, and lack of complete follow-up data.
SOS is a proprietary registry that allows for the collection of basic patient demographics, diagnostic and operative data, and PROMs. PROMs in the SOS shoulder arthroscopy module include Veterans RAND 12-Item Health Survey (VR-12) mental health and physical health component summary scores, visual analog scale (VAS) pain scores, and American Shoulder and Elbow Surgeons (ASES) scores. For this study, PROMs were reviewed before surgery and 6 and 12 months after surgery. In addition, operative times of all procedures were collected.
For the analysis, completely knotted and completely knotless techniques were compared for anterior repair, posterior repair, and SLAP repair. A t test was used to compare the techniques on PROMs, and χ2 test was used to evaluate proportion differences. Statistical significance was set at P < .05.
Results
Anterior Labral Repairs
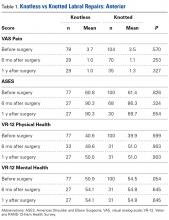
Of the 102 knotted anterior labral repairs that met the study criteria, 26 (25%) had minimum 1-year follow-up. Of the 122 knotless labral repairs, 33 (27%) had minimum 1-year follow-up. Seventy-five percent of knotted repairs and 80% of knotless repairs were performed in men. Mean (SD) age was 25.3 (11.7) years for the knotted group and 26.9 (10.6) years for the knotless group (P = .109). Anterior labral repairs did not differ in PROMs at any point (Table 1).
A mean of 2.8 anchors was used for knotted repairs, and a mean of 3.1 anchors was used for knotless repairs. Mean operative time was 75.8 minutes for knotted repairs and 67.5 minutes for knotless repairs. Mean (SD) time per anchor was 30.9 (13.9) minutes for knotted repairs and 25.6 (19.5) minutes for knotless repairs (P = .021).
Posterior Labral Repairs
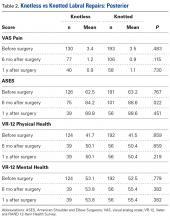
Of the 165 knotted posterior labral repairs that met the study criteria, 39 (29%) had minimum 1-year follow-up. Of the 229 knotless labral repairs, 56 (24%) had minimum 1-year follow-up. Eighty-five percent of knotted repairs and 74% of knotless repairs were performed in men. Mean (SD) age was 29.1 (12.0) years for the knotted group and 27.5 (11.9) years for the knotless group (P = .148). Posterior labral repairs did not differ in PROMs before surgery or 1 year after surgery; 6 months after surgery, these repairs differed only in ASES scores (Table 2).
A mean of 3.6 anchors was used for knotted repairs, and a mean of 3.0 anchors was used for knotless repairs. Mean operative time was 67.0 minutes for knotted repairs and 43.1 minutes for knotless repairs. Mean (SD) time per anchor was 21.1 (10.7) minutes for knotted repairs and 17.5 (14.7) minutes for knotless repairs (P = .031).
SLAP Repairs
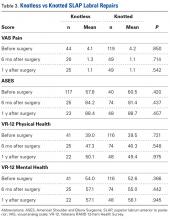
Of the 54 knotted SLAP repairs that met the study criteria, 24 (44%) had minimum 1-year follow-up. Of the 138 knotless SLAP repairs, 48 (35%) had minimum 1-year follow-up. Seventy-two percent of knotted repairs and 72% of knotless repairs were performed in men. Mean (SD) age was 32.1 (11.6) years for the knotted group and 35.0 (12.8) years for the knotless group (P = .246). SLAP repairs did not differ in PROMs at any point (Table 3).
A mean of 1.9 anchors was used for knotted repairs, and a mean of 2.1 anchors was used for knotless repairs. Mean operative time was 59.0 minutes for knotted repairs and 40.9 minutes for knotless repairs. Mean (SD) time per anchor was 36.6 (22.4) minutes for knotted repairs and 26.3 (14.0) minutes for knotless repairs (P = .080).
Discussion
Our hypothesis that there would be no difference in PROMs between knotted and knotless labral repairs was confirmed. Our findings are important because this study compared the gold standard of knotted suture anchor with the alternative knotless suture anchor in glenohumeral labral repair. These findings have several important implications for labral repair.
Knot tying traditionally has been used to achieve fixation with an anchor. Although simple in concept, knot tying can be challenging and its quality variable. Thal15 wrote that good-quality arthroscopic suture anchor repair is difficult to achieve because satisfactory knot tying requires significant practice with certain devices designed specifically for knot tying. Multiple surgeons have noted a significant learning curve associated with knot tying, and there is no agreement on which knot is superior.22-26 Leedle and Miller17 even suggested that, because knot tying is difficult, tying knots arthroscopically can lead to knot failure. In their study, they concluded that the knot is consistently the weakest link in suture repair of an anterior labrum construct. In a controlled laboratory study, Hanypsiak and colleagues13 found considerable knot-strength variability among expert arthroscopists. Only 65 (18%) of 365 knots tied fell within 20% of the mean for ultimate load failure, and only 128 (36%) of 365 fell within 20% of the mean for clinical failure (3 mm of displacement). These data suggested expert arthroscopists were unable to tie 5 consecutive knots of the same type consistently. Even among experts, it seems, knot strength varies significantly, and knot-strength issues may affect the rates of labral repair failure.
Multiple authors have also reported that bulky knots can cause chondral abrasion or that knots can migrate.25,27 Rhee and Ha27 reported that, when another knot (eg, a half-hitch knot) is tied to prevent knot failure, the resulting overall knot can be too bulky for a limited space, and chondral abrasion can result. In addition, regardless of size, a knot can migrate and, in its new position, start rubbing against the head of the humerus. Kim and colleagues14 found that, even when a knot is placed away from the humeral head, migration and repeated contact with the head are possible. Park and colleagues28 found that a significant number of knotted SLAP repairs required arthroscopic knot removal for relief of knot-induced pain and clicking.
Knotless constructs have several theoretical advantages over knotted constructs. Compared with a knotted technique, a knotless technique appears to provide more predictable strength, as variability in knot tying is eliminated (unpublished data). A knotless repair also has a lower profile,8 which should lead to less contact with the humeral head.19 Last, a knotless repair is more efficient—it takes less time to perform. In our study, operative time was reduced by a mean of 5.3 minutes per anchor for anterior labral repair. Assuming a mean of 3 anchors, this reduction equates to 16 minutes per case. Therefore, a surgeon who performs 25 labral repairs a year can save 6.7 hours a year. Reduced operative time benefits the patient (ie, lower risk of infection and other complications29), the surgeon, and the healthcare system (ie, cost savings). Macario30 found that operating room costs averaged $62 per minute (range, $22-$133 per minute). Therefore, saving 16 minutes per case could lead to saving $992 per case. In summary, a knotless technique appears to be clinically and financially advantageous as long as its results are the same as or better than those of a knotted technique.
A few other studies have compared knotted and knotless techniques. In a cadaveric study, Slabaugh and colleagues20 found no difference in labral height between traditional and knotless suture anchors. Leedle and Miller17 found that knotless constructs are biomechanically stronger than knotted constructs in anterior labral repair. In a level 3 clinical study, Yang and colleagues21 compared a conventional vertical knot with a knotless horizontal mattress suture in 41 patients who underwent SLAP repair. Functional outcome was no different between the 2 groups, but postoperative range of motion was improved in the knotless group. Ng and Kumar31 compared 45 patients who had knotted Bankart repair with 42 patients who had knotless Bankart repair and found no difference in functional outcome or rate of recurrent dislocation. Similarly, Kocaoglu and colleagues22 found no difference in recurrence rate between 18 patients who underwent a knotted technique for arthroscopic Bankart repair and 20 patients who underwent a knotless technique. Our findings corroborate the findings of these studies and further support the idea that there is no difference between knotted and knotless constructs with respect to PROMs.
Study Limitations
The major strength of this study was its large cohort and large population of surgeons. However, there were several study limitations. First, we could not detail specific repair techniques, such as simple or horizontal mattress orientation, and rehabilitation protocols and other variables are likely as well. Second, the repair technique was not randomized, and therefore there may have been a selection bias based on tissue quality. Although we cannot prove no bias, we think it was unlikely given that the groups were similar in age. Third, our data did not include information on range of motion or recurrent instability. Our goal was simply to evaluate PROMs among multiple surgeons using the 2 techniques. Fourth, there was substantial follow-up loss, which introduced potential selection bias. Last, there may have been conditions under which a hybrid technique with inferior knot tying, combined with a hybrid knotless construct, could have proved advantageous.
Conclusion
Our data showed that the advantages of knotless repair are not compromised in clinical situations. Although the data showed no significant difference in clinical outcomes, knotless repairs may provide surgeons with shorter surgeries, simpler constructs, less potential for chondral damage, and more consistent suture tensioning. Additional studies may further confirm these results.
1. Levy DM, Cole BJ, Bach BR Jr. History of surgical intervention of anterior shoulder instability. J Shoulder Elbow Surg. 2016;25(6):e139-e150.
2. Gill TJ, Zarins B. Open repairs for the treatment of anterior shoulder instability. Am J Sports Med. 2003;31(1):142-153.
3. Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419-432.
4. Stein DA, Jazrawi L, Bartolozzi AR. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18(8):912-924.
5. Kim SH, Ha KI, Kim SH. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755-763.
6. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
7. Hantes M, Raoulis V. Arthroscopic findings in anterior shoulder instability. Open Orthop J. 2017;11:119-132.
8. Sileo MJ, Lee SJ, Kremenic IJ, et al. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy. 2009;25(4):348-354.
9. Iqbal S, Jacobs U, Akhtar A, Macfarlane RJ, Waseem M. A history of shoulder surgery. Open Orthop J. 2013;7:305-309.
10. Garofalo R, Mocci A, Moretti B, et al. Arthroscopic treatment of anterior shoulder instability using knotless suture anchors. Arthroscopy. 2005;21(11):1283-1289.
11. Kersten AD, Fabing M, Ensminger S, et al. Suture capsulorrhaphy versus capsulolabral advancement for shoulder instability. Arthroscopy. 2012;28(10):1344-1351.
12. Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19-48.
13. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984.
14. Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am. 2003;85(8):1479-1487.
15. Thal R. Knotless suture anchor. Clin Orthop Relat Res. 2001;(390):42-51.
16. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
17. Leedle BP, Miller MD. Pullout strength of knotless suture anchors. Arthroscopy. 2005;21(1):81-85.
18. Caldwell PE 3rd, Pearson SE, D’Angelo MS. Arthroscopic knotless repair of the posterior labrum using LabralTape. Arthrosc Tech. 2016;5(2):e315-e320.
19. Tennent D, Concina C, Pearse E. Arthroscopic posterior stabilization of the shoulder using a percutaneous knotless mattress suture technique. Arthrosc Tech. 2014;3(1):e161-e164.
20. Slabaugh MA, Friel NA, Wang VM, Cole BJ. Restoring the labral height for treatment of Bankart lesions: a comparison of suture anchor constructs. Arthroscopy. 2010;26(5):587-591.
21. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849.
23. Aboalata M, Halawa A, Basyoni Y. The double Bankart bridge: a technique for restoration of the labral footprint in arthroscopic shoulder instability repair. Arthrosc Tech. 2017;6(1):e43-e47.
24. Rhee SM, Kang SY, Jang EC, Kim JY, Ha YC. Clinical outcomes after arthroscopic acetabular labral repair using knot-tying or knotless suture technique. Arch Orthop Trauma Surg. 2016;136(10):1411-1416.
25. Oh JH, Lee HK, Kim JY, Kim SH, Gong HS. Clinical and radiologic outcomes of arthroscopic glenoid labrum repair with the BioKnotless suture anchor. Am J Sports Med. 2009;37(12):2340-2348.
26. Yian E, Wang C, Millett PJ, Warner JJ. Arthroscopic repair of SLAP lesions with a BioKnotless suture anchor. Arthroscopy. 2004;20(5):547-551.
27. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: case report. J Shoulder Elbow Surg. 2006;15(3):391-393.
28. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic knot removal for failed superior labrum anterior-posterior repair secondary to knot-induced pain. Am J Sports Med. 2017;45(11):2563-2568.
29. Wang DS. Re: how slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Urol. 2016;195(5):1510-1511.
30. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
31. Ng DZ, Kumar VP. Arthroscopic Bankart repair using knot-tying versus knotless suture anchors: is there a difference? Arthroscopy. 2014;30(4):422-427.
Take-Home Points
- There is no difference in PROMs following knotless or knotted labral repair.
- Operative time is shorter for knotless compared to knotted glenoid labral tears.
- Knotless constructs may be more predictable than knotted constructs biomechanically.
Orthopedic surgeons often encounter labral pathology, and labral tears historically have required open techniques.1-3 Arthroscopy allows for advanced visualization and treatment of shoulder lesions,4,5 including anterior, posterior, and superior labrum anterior to posterior (SLAP) lesions.6
The goal of arthroscopic labral repair is to restore joint stability while maintaining range of motion. Arthroscopically repairing the labrum with suture anchors has become the standard technique, and several studies have reported satisfactory biomechanical and clinical results.1,7-12 Surgeons traditionally have been required to tie knots for these anchors, but knot security varies significantly among experienced arthroscopic surgeons.13 In addition, knots can migrate,14 and bulky knots can cause chondral abrasion.15,16 Several manufacturers have introduced knotless anchors for soft-tissue fixation.15,17 The knotless technique provides a low-profile repair with potentially less operating time.8 These factors may warrant switching from knotted to knotless techniques if outcomes are clinically acceptable. However, few studies have compared knotted and knotless techniques for glenohumeral labral repair.8,15,18-21
We conducted a study to compare the clinical results and operative times of knotless and knotted fixation of anterior and posterior glenohumeral labral repairs and SLAP repairs. We hypothesized there would be no difference in patient-reported outcome measures (PROMs) between knotted and knotless techniques.
Methods
We retrospectively evaluated data that had been prospectively collected between 2012 and 2016 in a Surgical Outcomes System (SOS; Arthrex) database. Participation in this registry is elective, and enrollment can occur on a case-by-case basis. The database stores data on basic demographics, PROMs, and operative time. Data for our specific analysis were available for surgeries performed by 115 different surgeons. Inclusion criteria included primary isolated arthroscopic anterior, isolated posterior, and isolated SLAP repair with completely knotted or completely knotless labral repair and minimum 1-year follow-up. Exclusion criteria included hybrid knotted–knotless repair, rotator cuff repair, revision surgery, open surgery, and lack of complete follow-up data.
SOS is a proprietary registry that allows for the collection of basic patient demographics, diagnostic and operative data, and PROMs. PROMs in the SOS shoulder arthroscopy module include Veterans RAND 12-Item Health Survey (VR-12) mental health and physical health component summary scores, visual analog scale (VAS) pain scores, and American Shoulder and Elbow Surgeons (ASES) scores. For this study, PROMs were reviewed before surgery and 6 and 12 months after surgery. In addition, operative times of all procedures were collected.
For the analysis, completely knotted and completely knotless techniques were compared for anterior repair, posterior repair, and SLAP repair. A t test was used to compare the techniques on PROMs, and χ2 test was used to evaluate proportion differences. Statistical significance was set at P < .05.
Results
Anterior Labral Repairs
Of the 102 knotted anterior labral repairs that met the study criteria, 26 (25%) had minimum 1-year follow-up. Of the 122 knotless labral repairs, 33 (27%) had minimum 1-year follow-up. Seventy-five percent of knotted repairs and 80% of knotless repairs were performed in men. Mean (SD) age was 25.3 (11.7) years for the knotted group and 26.9 (10.6) years for the knotless group (P = .109). Anterior labral repairs did not differ in PROMs at any point (Table 1).
A mean of 2.8 anchors was used for knotted repairs, and a mean of 3.1 anchors was used for knotless repairs. Mean operative time was 75.8 minutes for knotted repairs and 67.5 minutes for knotless repairs. Mean (SD) time per anchor was 30.9 (13.9) minutes for knotted repairs and 25.6 (19.5) minutes for knotless repairs (P = .021).
Posterior Labral Repairs
Of the 165 knotted posterior labral repairs that met the study criteria, 39 (29%) had minimum 1-year follow-up. Of the 229 knotless labral repairs, 56 (24%) had minimum 1-year follow-up. Eighty-five percent of knotted repairs and 74% of knotless repairs were performed in men. Mean (SD) age was 29.1 (12.0) years for the knotted group and 27.5 (11.9) years for the knotless group (P = .148). Posterior labral repairs did not differ in PROMs before surgery or 1 year after surgery; 6 months after surgery, these repairs differed only in ASES scores (Table 2).
A mean of 3.6 anchors was used for knotted repairs, and a mean of 3.0 anchors was used for knotless repairs. Mean operative time was 67.0 minutes for knotted repairs and 43.1 minutes for knotless repairs. Mean (SD) time per anchor was 21.1 (10.7) minutes for knotted repairs and 17.5 (14.7) minutes for knotless repairs (P = .031).
SLAP Repairs
Of the 54 knotted SLAP repairs that met the study criteria, 24 (44%) had minimum 1-year follow-up. Of the 138 knotless SLAP repairs, 48 (35%) had minimum 1-year follow-up. Seventy-two percent of knotted repairs and 72% of knotless repairs were performed in men. Mean (SD) age was 32.1 (11.6) years for the knotted group and 35.0 (12.8) years for the knotless group (P = .246). SLAP repairs did not differ in PROMs at any point (Table 3).
A mean of 1.9 anchors was used for knotted repairs, and a mean of 2.1 anchors was used for knotless repairs. Mean operative time was 59.0 minutes for knotted repairs and 40.9 minutes for knotless repairs. Mean (SD) time per anchor was 36.6 (22.4) minutes for knotted repairs and 26.3 (14.0) minutes for knotless repairs (P = .080).
Discussion
Our hypothesis that there would be no difference in PROMs between knotted and knotless labral repairs was confirmed. Our findings are important because this study compared the gold standard of knotted suture anchor with the alternative knotless suture anchor in glenohumeral labral repair. These findings have several important implications for labral repair.
Knot tying traditionally has been used to achieve fixation with an anchor. Although simple in concept, knot tying can be challenging and its quality variable. Thal15 wrote that good-quality arthroscopic suture anchor repair is difficult to achieve because satisfactory knot tying requires significant practice with certain devices designed specifically for knot tying. Multiple surgeons have noted a significant learning curve associated with knot tying, and there is no agreement on which knot is superior.22-26 Leedle and Miller17 even suggested that, because knot tying is difficult, tying knots arthroscopically can lead to knot failure. In their study, they concluded that the knot is consistently the weakest link in suture repair of an anterior labrum construct. In a controlled laboratory study, Hanypsiak and colleagues13 found considerable knot-strength variability among expert arthroscopists. Only 65 (18%) of 365 knots tied fell within 20% of the mean for ultimate load failure, and only 128 (36%) of 365 fell within 20% of the mean for clinical failure (3 mm of displacement). These data suggested expert arthroscopists were unable to tie 5 consecutive knots of the same type consistently. Even among experts, it seems, knot strength varies significantly, and knot-strength issues may affect the rates of labral repair failure.
Multiple authors have also reported that bulky knots can cause chondral abrasion or that knots can migrate.25,27 Rhee and Ha27 reported that, when another knot (eg, a half-hitch knot) is tied to prevent knot failure, the resulting overall knot can be too bulky for a limited space, and chondral abrasion can result. In addition, regardless of size, a knot can migrate and, in its new position, start rubbing against the head of the humerus. Kim and colleagues14 found that, even when a knot is placed away from the humeral head, migration and repeated contact with the head are possible. Park and colleagues28 found that a significant number of knotted SLAP repairs required arthroscopic knot removal for relief of knot-induced pain and clicking.
Knotless constructs have several theoretical advantages over knotted constructs. Compared with a knotted technique, a knotless technique appears to provide more predictable strength, as variability in knot tying is eliminated (unpublished data). A knotless repair also has a lower profile,8 which should lead to less contact with the humeral head.19 Last, a knotless repair is more efficient—it takes less time to perform. In our study, operative time was reduced by a mean of 5.3 minutes per anchor for anterior labral repair. Assuming a mean of 3 anchors, this reduction equates to 16 minutes per case. Therefore, a surgeon who performs 25 labral repairs a year can save 6.7 hours a year. Reduced operative time benefits the patient (ie, lower risk of infection and other complications29), the surgeon, and the healthcare system (ie, cost savings). Macario30 found that operating room costs averaged $62 per minute (range, $22-$133 per minute). Therefore, saving 16 minutes per case could lead to saving $992 per case. In summary, a knotless technique appears to be clinically and financially advantageous as long as its results are the same as or better than those of a knotted technique.
A few other studies have compared knotted and knotless techniques. In a cadaveric study, Slabaugh and colleagues20 found no difference in labral height between traditional and knotless suture anchors. Leedle and Miller17 found that knotless constructs are biomechanically stronger than knotted constructs in anterior labral repair. In a level 3 clinical study, Yang and colleagues21 compared a conventional vertical knot with a knotless horizontal mattress suture in 41 patients who underwent SLAP repair. Functional outcome was no different between the 2 groups, but postoperative range of motion was improved in the knotless group. Ng and Kumar31 compared 45 patients who had knotted Bankart repair with 42 patients who had knotless Bankart repair and found no difference in functional outcome or rate of recurrent dislocation. Similarly, Kocaoglu and colleagues22 found no difference in recurrence rate between 18 patients who underwent a knotted technique for arthroscopic Bankart repair and 20 patients who underwent a knotless technique. Our findings corroborate the findings of these studies and further support the idea that there is no difference between knotted and knotless constructs with respect to PROMs.
Study Limitations
The major strength of this study was its large cohort and large population of surgeons. However, there were several study limitations. First, we could not detail specific repair techniques, such as simple or horizontal mattress orientation, and rehabilitation protocols and other variables are likely as well. Second, the repair technique was not randomized, and therefore there may have been a selection bias based on tissue quality. Although we cannot prove no bias, we think it was unlikely given that the groups were similar in age. Third, our data did not include information on range of motion or recurrent instability. Our goal was simply to evaluate PROMs among multiple surgeons using the 2 techniques. Fourth, there was substantial follow-up loss, which introduced potential selection bias. Last, there may have been conditions under which a hybrid technique with inferior knot tying, combined with a hybrid knotless construct, could have proved advantageous.
Conclusion
Our data showed that the advantages of knotless repair are not compromised in clinical situations. Although the data showed no significant difference in clinical outcomes, knotless repairs may provide surgeons with shorter surgeries, simpler constructs, less potential for chondral damage, and more consistent suture tensioning. Additional studies may further confirm these results.
Take-Home Points
- There is no difference in PROMs following knotless or knotted labral repair.
- Operative time is shorter for knotless compared to knotted glenoid labral tears.
- Knotless constructs may be more predictable than knotted constructs biomechanically.
Orthopedic surgeons often encounter labral pathology, and labral tears historically have required open techniques.1-3 Arthroscopy allows for advanced visualization and treatment of shoulder lesions,4,5 including anterior, posterior, and superior labrum anterior to posterior (SLAP) lesions.6
The goal of arthroscopic labral repair is to restore joint stability while maintaining range of motion. Arthroscopically repairing the labrum with suture anchors has become the standard technique, and several studies have reported satisfactory biomechanical and clinical results.1,7-12 Surgeons traditionally have been required to tie knots for these anchors, but knot security varies significantly among experienced arthroscopic surgeons.13 In addition, knots can migrate,14 and bulky knots can cause chondral abrasion.15,16 Several manufacturers have introduced knotless anchors for soft-tissue fixation.15,17 The knotless technique provides a low-profile repair with potentially less operating time.8 These factors may warrant switching from knotted to knotless techniques if outcomes are clinically acceptable. However, few studies have compared knotted and knotless techniques for glenohumeral labral repair.8,15,18-21
We conducted a study to compare the clinical results and operative times of knotless and knotted fixation of anterior and posterior glenohumeral labral repairs and SLAP repairs. We hypothesized there would be no difference in patient-reported outcome measures (PROMs) between knotted and knotless techniques.
Methods
We retrospectively evaluated data that had been prospectively collected between 2012 and 2016 in a Surgical Outcomes System (SOS; Arthrex) database. Participation in this registry is elective, and enrollment can occur on a case-by-case basis. The database stores data on basic demographics, PROMs, and operative time. Data for our specific analysis were available for surgeries performed by 115 different surgeons. Inclusion criteria included primary isolated arthroscopic anterior, isolated posterior, and isolated SLAP repair with completely knotted or completely knotless labral repair and minimum 1-year follow-up. Exclusion criteria included hybrid knotted–knotless repair, rotator cuff repair, revision surgery, open surgery, and lack of complete follow-up data.
SOS is a proprietary registry that allows for the collection of basic patient demographics, diagnostic and operative data, and PROMs. PROMs in the SOS shoulder arthroscopy module include Veterans RAND 12-Item Health Survey (VR-12) mental health and physical health component summary scores, visual analog scale (VAS) pain scores, and American Shoulder and Elbow Surgeons (ASES) scores. For this study, PROMs were reviewed before surgery and 6 and 12 months after surgery. In addition, operative times of all procedures were collected.
For the analysis, completely knotted and completely knotless techniques were compared for anterior repair, posterior repair, and SLAP repair. A t test was used to compare the techniques on PROMs, and χ2 test was used to evaluate proportion differences. Statistical significance was set at P < .05.
Results
Anterior Labral Repairs
Of the 102 knotted anterior labral repairs that met the study criteria, 26 (25%) had minimum 1-year follow-up. Of the 122 knotless labral repairs, 33 (27%) had minimum 1-year follow-up. Seventy-five percent of knotted repairs and 80% of knotless repairs were performed in men. Mean (SD) age was 25.3 (11.7) years for the knotted group and 26.9 (10.6) years for the knotless group (P = .109). Anterior labral repairs did not differ in PROMs at any point (Table 1).
A mean of 2.8 anchors was used for knotted repairs, and a mean of 3.1 anchors was used for knotless repairs. Mean operative time was 75.8 minutes for knotted repairs and 67.5 minutes for knotless repairs. Mean (SD) time per anchor was 30.9 (13.9) minutes for knotted repairs and 25.6 (19.5) minutes for knotless repairs (P = .021).
Posterior Labral Repairs
Of the 165 knotted posterior labral repairs that met the study criteria, 39 (29%) had minimum 1-year follow-up. Of the 229 knotless labral repairs, 56 (24%) had minimum 1-year follow-up. Eighty-five percent of knotted repairs and 74% of knotless repairs were performed in men. Mean (SD) age was 29.1 (12.0) years for the knotted group and 27.5 (11.9) years for the knotless group (P = .148). Posterior labral repairs did not differ in PROMs before surgery or 1 year after surgery; 6 months after surgery, these repairs differed only in ASES scores (Table 2).
A mean of 3.6 anchors was used for knotted repairs, and a mean of 3.0 anchors was used for knotless repairs. Mean operative time was 67.0 minutes for knotted repairs and 43.1 minutes for knotless repairs. Mean (SD) time per anchor was 21.1 (10.7) minutes for knotted repairs and 17.5 (14.7) minutes for knotless repairs (P = .031).
SLAP Repairs
Of the 54 knotted SLAP repairs that met the study criteria, 24 (44%) had minimum 1-year follow-up. Of the 138 knotless SLAP repairs, 48 (35%) had minimum 1-year follow-up. Seventy-two percent of knotted repairs and 72% of knotless repairs were performed in men. Mean (SD) age was 32.1 (11.6) years for the knotted group and 35.0 (12.8) years for the knotless group (P = .246). SLAP repairs did not differ in PROMs at any point (Table 3).
A mean of 1.9 anchors was used for knotted repairs, and a mean of 2.1 anchors was used for knotless repairs. Mean operative time was 59.0 minutes for knotted repairs and 40.9 minutes for knotless repairs. Mean (SD) time per anchor was 36.6 (22.4) minutes for knotted repairs and 26.3 (14.0) minutes for knotless repairs (P = .080).
Discussion
Our hypothesis that there would be no difference in PROMs between knotted and knotless labral repairs was confirmed. Our findings are important because this study compared the gold standard of knotted suture anchor with the alternative knotless suture anchor in glenohumeral labral repair. These findings have several important implications for labral repair.
Knot tying traditionally has been used to achieve fixation with an anchor. Although simple in concept, knot tying can be challenging and its quality variable. Thal15 wrote that good-quality arthroscopic suture anchor repair is difficult to achieve because satisfactory knot tying requires significant practice with certain devices designed specifically for knot tying. Multiple surgeons have noted a significant learning curve associated with knot tying, and there is no agreement on which knot is superior.22-26 Leedle and Miller17 even suggested that, because knot tying is difficult, tying knots arthroscopically can lead to knot failure. In their study, they concluded that the knot is consistently the weakest link in suture repair of an anterior labrum construct. In a controlled laboratory study, Hanypsiak and colleagues13 found considerable knot-strength variability among expert arthroscopists. Only 65 (18%) of 365 knots tied fell within 20% of the mean for ultimate load failure, and only 128 (36%) of 365 fell within 20% of the mean for clinical failure (3 mm of displacement). These data suggested expert arthroscopists were unable to tie 5 consecutive knots of the same type consistently. Even among experts, it seems, knot strength varies significantly, and knot-strength issues may affect the rates of labral repair failure.
Multiple authors have also reported that bulky knots can cause chondral abrasion or that knots can migrate.25,27 Rhee and Ha27 reported that, when another knot (eg, a half-hitch knot) is tied to prevent knot failure, the resulting overall knot can be too bulky for a limited space, and chondral abrasion can result. In addition, regardless of size, a knot can migrate and, in its new position, start rubbing against the head of the humerus. Kim and colleagues14 found that, even when a knot is placed away from the humeral head, migration and repeated contact with the head are possible. Park and colleagues28 found that a significant number of knotted SLAP repairs required arthroscopic knot removal for relief of knot-induced pain and clicking.
Knotless constructs have several theoretical advantages over knotted constructs. Compared with a knotted technique, a knotless technique appears to provide more predictable strength, as variability in knot tying is eliminated (unpublished data). A knotless repair also has a lower profile,8 which should lead to less contact with the humeral head.19 Last, a knotless repair is more efficient—it takes less time to perform. In our study, operative time was reduced by a mean of 5.3 minutes per anchor for anterior labral repair. Assuming a mean of 3 anchors, this reduction equates to 16 minutes per case. Therefore, a surgeon who performs 25 labral repairs a year can save 6.7 hours a year. Reduced operative time benefits the patient (ie, lower risk of infection and other complications29), the surgeon, and the healthcare system (ie, cost savings). Macario30 found that operating room costs averaged $62 per minute (range, $22-$133 per minute). Therefore, saving 16 minutes per case could lead to saving $992 per case. In summary, a knotless technique appears to be clinically and financially advantageous as long as its results are the same as or better than those of a knotted technique.
A few other studies have compared knotted and knotless techniques. In a cadaveric study, Slabaugh and colleagues20 found no difference in labral height between traditional and knotless suture anchors. Leedle and Miller17 found that knotless constructs are biomechanically stronger than knotted constructs in anterior labral repair. In a level 3 clinical study, Yang and colleagues21 compared a conventional vertical knot with a knotless horizontal mattress suture in 41 patients who underwent SLAP repair. Functional outcome was no different between the 2 groups, but postoperative range of motion was improved in the knotless group. Ng and Kumar31 compared 45 patients who had knotted Bankart repair with 42 patients who had knotless Bankart repair and found no difference in functional outcome or rate of recurrent dislocation. Similarly, Kocaoglu and colleagues22 found no difference in recurrence rate between 18 patients who underwent a knotted technique for arthroscopic Bankart repair and 20 patients who underwent a knotless technique. Our findings corroborate the findings of these studies and further support the idea that there is no difference between knotted and knotless constructs with respect to PROMs.
Study Limitations
The major strength of this study was its large cohort and large population of surgeons. However, there were several study limitations. First, we could not detail specific repair techniques, such as simple or horizontal mattress orientation, and rehabilitation protocols and other variables are likely as well. Second, the repair technique was not randomized, and therefore there may have been a selection bias based on tissue quality. Although we cannot prove no bias, we think it was unlikely given that the groups were similar in age. Third, our data did not include information on range of motion or recurrent instability. Our goal was simply to evaluate PROMs among multiple surgeons using the 2 techniques. Fourth, there was substantial follow-up loss, which introduced potential selection bias. Last, there may have been conditions under which a hybrid technique with inferior knot tying, combined with a hybrid knotless construct, could have proved advantageous.
Conclusion
Our data showed that the advantages of knotless repair are not compromised in clinical situations. Although the data showed no significant difference in clinical outcomes, knotless repairs may provide surgeons with shorter surgeries, simpler constructs, less potential for chondral damage, and more consistent suture tensioning. Additional studies may further confirm these results.
1. Levy DM, Cole BJ, Bach BR Jr. History of surgical intervention of anterior shoulder instability. J Shoulder Elbow Surg. 2016;25(6):e139-e150.
2. Gill TJ, Zarins B. Open repairs for the treatment of anterior shoulder instability. Am J Sports Med. 2003;31(1):142-153.
3. Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419-432.
4. Stein DA, Jazrawi L, Bartolozzi AR. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18(8):912-924.
5. Kim SH, Ha KI, Kim SH. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755-763.
6. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
7. Hantes M, Raoulis V. Arthroscopic findings in anterior shoulder instability. Open Orthop J. 2017;11:119-132.
8. Sileo MJ, Lee SJ, Kremenic IJ, et al. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy. 2009;25(4):348-354.
9. Iqbal S, Jacobs U, Akhtar A, Macfarlane RJ, Waseem M. A history of shoulder surgery. Open Orthop J. 2013;7:305-309.
10. Garofalo R, Mocci A, Moretti B, et al. Arthroscopic treatment of anterior shoulder instability using knotless suture anchors. Arthroscopy. 2005;21(11):1283-1289.
11. Kersten AD, Fabing M, Ensminger S, et al. Suture capsulorrhaphy versus capsulolabral advancement for shoulder instability. Arthroscopy. 2012;28(10):1344-1351.
12. Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19-48.
13. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984.
14. Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am. 2003;85(8):1479-1487.
15. Thal R. Knotless suture anchor. Clin Orthop Relat Res. 2001;(390):42-51.
16. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
17. Leedle BP, Miller MD. Pullout strength of knotless suture anchors. Arthroscopy. 2005;21(1):81-85.
18. Caldwell PE 3rd, Pearson SE, D’Angelo MS. Arthroscopic knotless repair of the posterior labrum using LabralTape. Arthrosc Tech. 2016;5(2):e315-e320.
19. Tennent D, Concina C, Pearse E. Arthroscopic posterior stabilization of the shoulder using a percutaneous knotless mattress suture technique. Arthrosc Tech. 2014;3(1):e161-e164.
20. Slabaugh MA, Friel NA, Wang VM, Cole BJ. Restoring the labral height for treatment of Bankart lesions: a comparison of suture anchor constructs. Arthroscopy. 2010;26(5):587-591.
21. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849.
23. Aboalata M, Halawa A, Basyoni Y. The double Bankart bridge: a technique for restoration of the labral footprint in arthroscopic shoulder instability repair. Arthrosc Tech. 2017;6(1):e43-e47.
24. Rhee SM, Kang SY, Jang EC, Kim JY, Ha YC. Clinical outcomes after arthroscopic acetabular labral repair using knot-tying or knotless suture technique. Arch Orthop Trauma Surg. 2016;136(10):1411-1416.
25. Oh JH, Lee HK, Kim JY, Kim SH, Gong HS. Clinical and radiologic outcomes of arthroscopic glenoid labrum repair with the BioKnotless suture anchor. Am J Sports Med. 2009;37(12):2340-2348.
26. Yian E, Wang C, Millett PJ, Warner JJ. Arthroscopic repair of SLAP lesions with a BioKnotless suture anchor. Arthroscopy. 2004;20(5):547-551.
27. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: case report. J Shoulder Elbow Surg. 2006;15(3):391-393.
28. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic knot removal for failed superior labrum anterior-posterior repair secondary to knot-induced pain. Am J Sports Med. 2017;45(11):2563-2568.
29. Wang DS. Re: how slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Urol. 2016;195(5):1510-1511.
30. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
31. Ng DZ, Kumar VP. Arthroscopic Bankart repair using knot-tying versus knotless suture anchors: is there a difference? Arthroscopy. 2014;30(4):422-427.
1. Levy DM, Cole BJ, Bach BR Jr. History of surgical intervention of anterior shoulder instability. J Shoulder Elbow Surg. 2016;25(6):e139-e150.
2. Gill TJ, Zarins B. Open repairs for the treatment of anterior shoulder instability. Am J Sports Med. 2003;31(1):142-153.
3. Millett PJ, Clavert P, Warner JJ. Open operative treatment for anterior shoulder instability: when and why? J Bone Joint Surg Am. 2005;87(2):419-432.
4. Stein DA, Jazrawi L, Bartolozzi AR. Arthroscopic stabilization of anterior shoulder instability: a review of the literature. Arthroscopy. 2002;18(8):912-924.
5. Kim SH, Ha KI, Kim SH. Bankart repair in traumatic anterior shoulder instability: open versus arthroscopic technique. Arthroscopy. 2002;18(7):755-763.
6. Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6(4):274-279.
7. Hantes M, Raoulis V. Arthroscopic findings in anterior shoulder instability. Open Orthop J. 2017;11:119-132.
8. Sileo MJ, Lee SJ, Kremenic IJ, et al. Biomechanical comparison of a knotless suture anchor with standard suture anchor in the repair of type II SLAP tears. Arthroscopy. 2009;25(4):348-354.
9. Iqbal S, Jacobs U, Akhtar A, Macfarlane RJ, Waseem M. A history of shoulder surgery. Open Orthop J. 2013;7:305-309.
10. Garofalo R, Mocci A, Moretti B, et al. Arthroscopic treatment of anterior shoulder instability using knotless suture anchors. Arthroscopy. 2005;21(11):1283-1289.
11. Kersten AD, Fabing M, Ensminger S, et al. Suture capsulorrhaphy versus capsulolabral advancement for shoulder instability. Arthroscopy. 2012;28(10):1344-1351.
12. Cole BJ, Warner JJ. Arthroscopic versus open Bankart repair for traumatic anterior shoulder instability. Clin Sports Med. 2000;19(1):19-48.
13. Hanypsiak BT, DeLong JM, Simmons L, Lowe W, Burkhart S. Knot strength varies widely among expert arthroscopists. Am J Sports Med. 2014;42(8):1978-1984.
14. Kim SH, Ha KI, Park JH, et al. Arthroscopic posterior labral repair and capsular shift for traumatic unidirectional recurrent posterior subluxation of the shoulder. J Bone Joint Surg Am. 2003;85(8):1479-1487.
15. Thal R. Knotless suture anchor. Clin Orthop Relat Res. 2001;(390):42-51.
16. Loutzenheiser TD, Harryman DT 2nd, Yung SW, France MP, Sidles JA. Optimizing arthroscopic knots. Arthroscopy. 1995;11(2):199-206.
17. Leedle BP, Miller MD. Pullout strength of knotless suture anchors. Arthroscopy. 2005;21(1):81-85.
18. Caldwell PE 3rd, Pearson SE, D’Angelo MS. Arthroscopic knotless repair of the posterior labrum using LabralTape. Arthrosc Tech. 2016;5(2):e315-e320.
19. Tennent D, Concina C, Pearse E. Arthroscopic posterior stabilization of the shoulder using a percutaneous knotless mattress suture technique. Arthrosc Tech. 2014;3(1):e161-e164.
20. Slabaugh MA, Friel NA, Wang VM, Cole BJ. Restoring the labral height for treatment of Bankart lesions: a comparison of suture anchor constructs. Arthroscopy. 2010;26(5):587-591.
21. Yang HJ, Yoon K, Jin H, Song HS. Clinical outcome of arthroscopic SLAP repair: conventional vertical knot versus knotless horizontal mattress sutures. Knee Surg Sports Traumatol Arthrosc. 2016;24(2):464-469.
22. Kocaoglu B, Guven O, Nalbantoglu U, Aydin N, Haklar U. No difference between knotless sutures and suture anchors in arthroscopic repair of Bankart lesions in collision athletes. Knee Surg Sports Traumatol Arthrosc. 2009;17(7):844-849.
23. Aboalata M, Halawa A, Basyoni Y. The double Bankart bridge: a technique for restoration of the labral footprint in arthroscopic shoulder instability repair. Arthrosc Tech. 2017;6(1):e43-e47.
24. Rhee SM, Kang SY, Jang EC, Kim JY, Ha YC. Clinical outcomes after arthroscopic acetabular labral repair using knot-tying or knotless suture technique. Arch Orthop Trauma Surg. 2016;136(10):1411-1416.
25. Oh JH, Lee HK, Kim JY, Kim SH, Gong HS. Clinical and radiologic outcomes of arthroscopic glenoid labrum repair with the BioKnotless suture anchor. Am J Sports Med. 2009;37(12):2340-2348.
26. Yian E, Wang C, Millett PJ, Warner JJ. Arthroscopic repair of SLAP lesions with a BioKnotless suture anchor. Arthroscopy. 2004;20(5):547-551.
27. Rhee YG, Ha JH. Knot-induced glenoid erosion after arthroscopic fixation for unstable superior labrum anterior-posterior lesion: case report. J Shoulder Elbow Surg. 2006;15(3):391-393.
28. Park JG, Cho NS, Kim JY, Song JH, Hong SJ, Rhee YG. Arthroscopic knot removal for failed superior labrum anterior-posterior repair secondary to knot-induced pain. Am J Sports Med. 2017;45(11):2563-2568.
29. Wang DS. Re: how slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Urol. 2016;195(5):1510-1511.
30. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
31. Ng DZ, Kumar VP. Arthroscopic Bankart repair using knot-tying versus knotless suture anchors: is there a difference? Arthroscopy. 2014;30(4):422-427.
Update on Internet-Based Orthopedic Registries
Take-Home Points
- PRO data collection can provide feedback for improvements in patient care and physician performance.
- Many options exist for orthopedic physicians to establish clinical data registries.
- Registry systems can help improve patient follow-up with system monitoring and patient reminders.
- Clinical registries can offer many advantages to observational research.
- With registry use becoming more prevalent, work needs to be done to establish standards for validity and reliability.
In a 2012 review of database tools, Lubowitz and Smith1 examined Internet-based applications that arthroscopic surgeons could use to record and monitor patient-reported outcome (PRO) data and potential adverse effects. In this article, we update orthopedic surgeons on the registries and monitoring software mentioned in that earlier publication and in other publications that have since become available.
Most orthopedic surgery candidates are seeking pain relief and improved function. Many patients expect their pain to be completely relieved by surgical intervention and their function to return to what it was before they became stricken.2,3 Therefore, PRO measures (PROMs) are now standard in post-orthopedic surgery outcome reporting.4 PROMs, which include any measurement that assesses a patient’s health, illness, or benefits from the perspective of the patient, are often administered as a questionnaire or survey.5 The collection of PROMs continues to increase and evolve, creating a need for data storage and analysis. Registries, large collections of patient information and outcomes, allow for evaluation of patient outcomes, monitoring of adverse effects, identification of procedure incidence, understanding of predictors of prognosis, generation of feedback for quality of care, monitoring of the safety of implantable devices, and the conducting of hypothesis-driven scientific research.6-9
Orthopedic surgery has registries at regional, national, and international levels. Although the United States has fallen well behind other countries in establishing a national registry,9 it has made some recent progress. The United States now has several national registries, including the American Joint Replacement Registry (AJRR), Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement (FORCE-TJR), the Kaiser Permanente National Total Joint Replacement Registry (TJRR), the Veterans Affairs (VA) and American College of Surgeons (ACS) National Surgical Quality Improvement Programs (NSQIPs), and the National Trauma Data Bank (NTDB).9 AJRR currently has 960 hospitals participating and is tracking 1,084,664 hip and knee replacements.10
These orthopedic registries, however, are limited in 2 ways. First, the majority are joint replacement registries. Second, though registries are established to determine patterns of care and predict patient outcomes, many are not set up to report care data back to healthcare providers.7 For procedures other than joint arthroplasty and for providers interested in tracking their patients’ PROs, systems are available for establishing clinical quality registries in orthopedics.
Registry Systems
CareSense
CareSense (Medtrak) is an Internet-based care management and data collection system designed for patient engagement, which results in fewer missed appointments, increased patient adherence, enhanced patient education, and improved patient satisfaction.11 CareSense features email/text reminders for data entry, custom and standard reports, import and export of electronic medical record (EMR) information, and tools for running research studies.12 CareSense emphasizes care navigation by helping hospitals educate and guide patients through their care by sending exercise videos to patients for home rehabilitation, transferring messages from post-acute care facilities to surgeons and caregivers, and alerting the care team to any potential readmission symptoms.11,13 CareSense is also a Centers for Medicare & Medicaid Services (CMS) approved qualified clinical data registry (QCDR). QCDRs collect data for Merit-Based Incentive Payment System (MIPS) clinicians and submit the data to CMS.12
KareOutcomes
KareOutcomes, a healthcare technology and support firm founded in 2009, advocates transparency and trust among providers and patients, and aims to optimize PROs.14 The KareOutcomes team incorporates patient follow-up personnel, administrators, engineers, physicians, software developers, and technicians. The KareOutcomes software, which is backed by a 6-month guarantee, includes system design and implementation, data collection and entry, methods of submitting data to statewide or nationwide registries and sending standardized and customized surveys, and accessible and meaningful data presentation. KareOutcomes allows patient follow-up through automated reminders by telephone, SMS text message, and email. Patients can respond to surveys or questionnaires whichever way is most convenient—by telephone, Internet, SMS text message, or on paper, either in the office or by mail.
Oberd
Oberd (Universal Research Solutions) offers a comprehensive package of solutions for collecting optimal PRO data. The package has several modules: outcomes, education, registry, operative notes, data import and export, and data reporting.15 Oberd Outcomes allows convenient and engaging data collection. For example, users can send both standardized and customized forms. Oberd Education allows patients to receive information in an interactive, narrated format that is specific to their physician’s techniques and practices. Oberd Registry allows users to input multiple datasets into a registry, compare data, and generate reports with visuals. Like CareSense, Oberd is a CMS-approved QCDR. Oberd’s MIPS Dashboard helps providers collect and report patients’ reported outcomes, and use that information to modify and improve their practice.
Ortech
Ortech is a web-based data registry system that allows physicians and administrators to mine the data they own, track key metrics in their data, and improve reporting.16 Users can collect PROMs, use them to measure and analyze patient progress, and add to their collection of information that helps support their evidence-based decision making. They can capture intraoperative and implant data through barcode scanning, which then registers the data in an implant product code library that allows quick identification of patients with a specific implant in the event of a product recall. Ortech also allows automatic generation of customized operative reports on data entered from the operating room and populated into the EMR. Ortech offers 2 versions of its data collection platform, phiDB and phiDB Lite. The phiDB Lite version is for smaller practices and focuses mainly on PROMs but lacks many of the other features that phiDB offers, such as operating room modules, automated operative reports, barcode scanning, and unlimited data reporting.
Socrates
Socrates (Standardised Orthopaedic Clinical Research and Treatment Evaluation Software; Ortholink) is dedicated orthopedic software that facilitates following patient outcomes and conducting high-quality research.17 Socrates is fully customizable to fit each user’s needs. It allows for tracking of outcome scores, intraoperative details, nonoperative procedures, clinical examinations, therapies, and adverse effects. Users can also create reports from this information, which is inputted to Socrates and can be exported into EMR. Socrates data are stored on the user’s server, on site; the software generates patient summaries, collective summaries, and follow-up reports through its built-in descriptive statistics module. Raw data can be extracted for statistical analysis. Socrates can catalogue images, radiographs, documents, and videos.
Surgical Outcomes System
Surgical Outcomes System (SOS; Arthrex) is a cloud-based orthopedic and sports medicine global registry that focuses on monitoring and evaluating the outcomes of various orthopedic and sports medicine surgical procedures, as well as nonoperative interventions, to contribute to evidence-based protocols for patient treatment.18 SOS can be fully customized with desired PROMs for arthroplasty and for surgical procedures for extremity joints and even the spine. SOS includes real-time reporting on PROs for individual patients, summary PROMs for all of the physician’s patients who are receiving the same treatment, and comparisons with all registry patients (from global de-identified registry data) who had the same treatment or surgery. This real-time analysis provides immediate patient and physician feedback on treatments and products used. A patient portal for education on surgical procedures is also available. SOS is approved for use in 21 countries and is a benefit included with Arthroscopy Association of North America (AANA) membership. SOS is listed on the National Quality Registry Network (NQRN) website and, as a specialized registry as defined by CMS, can accept data generated by EMR technology.
Discussion
Delaunay19 indicated that successful registry management depends on several factors, including “use of a single identifier for each patient to ensure full traceability of all procedures related to a given implant; a long-term funding source; a contemporary, rapid, Internet-based data collection method; and the collection of exhaustive data, at least for innovative implants.” The registry systems reviewed in this article are Internet based and allow healthcare providers to monitor the clinical outcomes of their patients in the hope of improving clinical decision-making and overall patient care. From the provider perspective, many registry systems allow for integration of outcome data reporting into EMRs, including generation of operative reports. In turn, registries can improve documentation efficiency, as it was estimated that a US physician without a registry spends more than 15 hours a week reporting quality measures,20 or almost 800 hours and $15 billion each year.20,21 It remains to be seen whether registry systems will optimize the documentation process, but there is potential improvement in time and cost-efficiency with registry use.
Although the factors involved in management are important, clinical data registries must have systems in place to help ensure patient adherence and minimize selection bias, as adherence is crucial in data accuracy.3 What helps with adherence is the ability to send automated email or SMS text message reminders to patients. According to a review, email reminders increased the completion of PROM datasets by 26%.22 When the new national quality register (NQR) HAKIR (Handkirurgiskt kvalitetsregister) was established in Sweden, it was found that when only 1 type of reminder was used (SMS text message, in this case), only about 30% of participants completed their questionnaires.23 However, after the system was changed to send both SMS text message and email reminders, the response rate increased from 50% to 60%. Using 2 types of automated reminders might minimize lost data more effectively than 1 type alone.
Another benefit of outcome monitoring through a registry is potential reduction of interviewer- related errors. Interviewer bias can occur in many different ways. Interviewers might not follow the same instructions or administer questionnaires or surveys the same way for different patients,24 the interviewer’s presence might cause the patient to alter responses based on social norms,25 and the patient might report better outcomes in the presence of a physician or interviewer.26,27 Given that clinical registries allow electronic capture of self-administered surveys, interviewer bias is reduced because all patients receive a standardized set of questions and instructions. In addition, electronic questionnaires and surveys prompt users to add or fix missed or incorrectly completed items, further reducing potential data inaccuracies.
Healthcare costs continue to rise in the United States. In 2015, the total cost of healthcare expenditure in the United States was $3.2 trillion, or almost 18% of the US gross domestic product.28 In addition, in the first half of 2016, an estimated 16.2% of people under age 65 years were in families that were struggling to pay medical bills.29,30 Healthcare reform provides a financial incentive to healthcare providers to collaborate to reduce unnecessary costs and procedures and improve the quality of healthcare.31 Porter and Teisberg32 defined value as health outcomes achieved per dollar spent. Registry monitoring of PROMs, which are the numerator in this critical value formula, allows providers to track patient outcomes over time to determine which interventions produce the best outcomes.22 Therefore, clinical registries play an important role in improving health outcomes and reducing the cost of healthcare.7
Since the Swedish Knee Arthroplasty Register (SKAR) was established 40 years ago, NQRs have been commonplace in Scandinavian countries, Australia, and the United Kingdom.23 Between 2001 and 2014, the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) documented a decline in the financial burden of hip and knee arthroplasty revision in Australia—in comparison with the United States, which did not have a full national registry at the time and showed a revision rate increase.24 The economic benefit of reducing hip and knee arthroplasty revisions in Australia during that period was an estimated $65 million to $143 million.24 Besides having financial benefits, national registries allow early identification of flawed implantation products and methods, leading to a further reduction in the burden associated with recall and future use of such defective implants—including patient harm.
In addition to monitoring existing techniques and devices, registries can also follow new techniques and, compared with publication in clinical journals, more expeditiously provide clinical data for outcome expectations and treatment methods. This timeliness is particularly valuable given that publication of clinical trials with the usual mandatory 2-year follow-up can take 4 years or longer.33,34 For instance, in the expanding field of hip arthroscopy, data from registries in both Sweden and Denmark are being analyzed.35,36 These data are important in new fields such as hip arthroscopy, in which clinical indications and treatment techniques may vary considerably between locations.35 In 2012, the Danish Hip Arthroscopy Registry (DHAR) was started as a web-based prospective registry.36 Between 2012 and December 2014, DHAR added 2000 procedures, which included all hip arthroscopy procedures performed at 11 centers in Denmark.36 DHAR tracks PROM, surgical procedure, operative, and radiologic data.
Increased use of clinical registries has led to use of their data in clinical research. Registry-based randomized controlled trials (RCTs) are lower in cost than other types of research, allow for rapid enrollment of patients, offer larger population sizes and multi-institutional sampling, and can provide a more diverse patient population.19,37 Although nonregistry RCTs remain the gold standard of clinical research, registry RCTs have several advantages given the abilities and structure of registries. Because of resources and cost, nonregistry RCTs are usually limited in the number of examined exposures and typically focus on only 2.6 Registry RCTs, on the other hand, can monitor multiple exposures, typically at minimal cost difference.6 Another disadvantage of nonregistry RCTs is that they are often performed at institutions providing care that might not be indicative of the quality most patients expect, as these institutions might be selected for a specific clinician or specialty service.
Registry RCTs also have their limitations with respect to clinical research. A major one is their lack of validity standards or accepted benchmarks for accuracy, adherence rates, registry completeness, and data collection.37,38 In addition, lack of standardization across national and international registries could produce conflicting data. Another limitation is that data in most registries are not subjected to any third-party checks or independent auditing.9,39 Furthermore, evaluating the impact of registries is difficult because it is difficult to find comparable outcome data on nonregistry patients.40 A final limitation involves the ethics of including registry data in RCTs. Although data are often added to a registry without patient consent, should the same data be used for research without patient consent? Should patients be able to disallow use of their data for research, or require a notification each time their data are used? These issues must be addressed.
Review Limitations
One limitation of this review of clinical Internet-based outcome systems is that it might not have identified comparable systems. In addition, specific costs associated with each system were not addressed, as they depend on PROM licensing fees, total institutional access, other proprietary costs, and other variables. Another limitation, in terms of creating a national or international registry, continues to be Internet access. The Pew Research Center estimated that 84% of US adults used the Internet in 2015.41 Although 84% represents most of the adult population, the other 16% typically is over age 65 years, where only 58% of adults reported using the Internet, or come from lower income households, where access was <75%. For registries in European countries and North America, where Internet usage typically is >70%, this is not a significant problem. However, worldwide, only 47% of the population used the Internet in 2016.42 Internet usage by Asian and Arab states citizens was 41.6% and 41.9%, respectively, and usage by African citizens was only 25.1%. As a significant benefit of registry use is that researchers can obtain larger sample sizes, it is a problem that some populations—elderly people, people of lower socioeconomic standing, people living where the Internet is unavailable—might be underrepresented in registry data.
As mentioned, patient adherence is an ongoing issue for clinical registries. As adherence tends to decrease as more time passes after a patient’s treatment date, it is important to account for and encourage continued patient participation with outcome monitoring. Missing data lessen the validity and accuracy of a registry, increasing the likelihood that certain groups will be underrepresented. Although registry systems can reduce the cost of following PROMs, doing so requires monitoring and following up on issues of patient adherence. In other words, many clinicians will need the help of a research assistant. Makhni and colleagues21 found that adding a research assistant for this task increased survey adherence from 65% to 94% before surgery, from 65% to 72% 6 months after surgery, and from 38% to 56% 12 months after surgery.
Even though studies continue to use clinical data from registries, there is not much research on the impact of these registries on improvement in healthcare. Again, many factors are involved: lack of standardized benchmarks for accuracy and adherence, lack of an accepted method of data auditing and validation, and difficulty evaluating the impact of registries owing to the difficulty obtaining comparable data on nonregistry patients. Registries must adopt accepted forms of standardization in order to allow better comparisons of registries, because comparing data across registries can be useful in determining the strengths and weaknesses of different registries.27,43 As registries support decision making at clinical, institutional, and governmental levels, it is vital that their clinical data be accurate and reliable.38
Conclusion
Rising healthcare costs, and government and third-party pressures are making patient outcomes collection a standard of care. Going forward, orthopedic surgeons must be proactive, and Internet -based registries provide technological advances that facilitate the process.
1. Lubowitz JH, Smith PA. Current concepts in clinical research: web-based, automated, arthroscopic surgery prospective database registry. Arthroscopy. 2012;28(3):425-428.
2. Ayers DC, Bozic KJ. The importance of outcome measurement in orthopaedics. Clin Orthop Relat Res. 2013;471(11):3409-3411.
3. Nwachukwu BU, Fields K, Chang B, Nawabi DH, Kelly BT, Ranawat AS. Preoperative outcome scores are predictive of achieving the minimal clinically important difference after arthroscopic treatment of femoroacetabular impingement. Am J Sports Med. 2017;45(3):612-619.
4. Breckenridge K, Bekker HL, Gibbons E, et al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant. 2015;30(10):1605-1614.
5. Inacio MC, Paxton EW, Dillon MT. Understanding orthopaedic registry studies: a comparison with clinical studies. J Bone Joint Surg Am. 2016;98(1):e3.
6. Hoque DME, Kumari V, Hoque M, Ruseckaite R, Romero L, Evans SM. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS One. 2017;12(9):e0183667.
7. Physician Consortium for Performance Improvement. National Quality Registry Network. http://www.thepcpi.org/programs-initiatives/national-quality-registry-network/. Accessed October 5, 2017.
8. Hickey GL, Grant SW, Cosgriff R, et al. Clinical registries: governance, management, analysis and applications. Eur J Cardiothorac Surg. 2013;44(4):605-614.
9. Pugely AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopaedic surgery: part 2: clinical registry data. J Bone Joint Surg Am. 2015;97(21):1799-1808.
10. American Joint Replacement Registry. http://www.ajrr.net/. Accessed October 5, 2017.
11. CareSense. https://www.caresense.com/. Accessed October 4, 2017.
12. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, Quality Payment Program. Merit-Based Incentive Payment System (MIPS): 2017 CMS-Approved Qualified Clinical Data Registries (QCDRs). https://qpp.cms.gov/docs/QPP_2017_CMS_Approved_QCDRs.pdf. Accessed October 9, 2017.
13. Johnson & Johnson. Johnson & Johnson Medical Devices Companies introduce Orthopaedic Episode of Care Approach, leveraging CareAdvantage capabilities to support better clinical outcomes and reduce the cost of care. https://www.jnj.com/media-center/press-releases/johnson-johnson-medical-devices-companies-introduce-orthopaedic-episode-of-care-approach-leveraging-careadvantage-capabilities-to-support-better-clinical-outcomes-and-reduce-the-cost-of-care. Published January 9, 2017. Accessed October 4, 2017.
14. KareOutcomes. http://www.kareoutcomes.com/. Accessed October 4, 2017.
15. Oberd. http://www.oberd.com/. Accessed October 4, 2017.
16. Ortech Systems. http://www.ortechsystems.com/. Accessed October 4, 2017.
17. Socrates. http://www.socratesortho.com/. Accessed October 4, 2017.
18. Surgical Outcomes System. https://www.surgicaloutcomesystem.com/. Accessed October 4, 2017.
19. Delaunay C. Registries in orthopaedics. Orthop Traumatol Surg Res. 2015;101(1 suppl):S69-S75.
20. Bryan S, Davis J, Broesch J, Doyle-Waters MM, Lewis S, McGrail K. Choosing your partner for the PROM: a review of evidence on patient-reported outcome measures for use in primary and community care. Healthc Policy. 2014;10(2):38-51.
21. Makhni EC, Higgins JD, Hamamoto JT, Cole BJ, Romeo AA, Verma NN. Patient compliance with electronic patient reported outcomes following shoulder arthroscopy [published online ahead of print September 25, 2017]. Arthroscopy. doi:10.1016/j.arthro.2017.06.016.
22. Triplet JJ, Momoh E, Kurowicki J, Villarroel LD, Law T, Levy JC. E-mail reminders improve completion rates of patient-reported outcome measures. JSES Open Access. 2017;1:25-28.
23. Arner M. Developing a national quality registry for hand surgery: challenges and opportunities. EFORT Open Rev. 2016;1(4):100-106.
24. Ngongo CJ, Frick KD, Hightower AW, Mathingau FA, Burke H, Breiman RF. The perils of straying from protocol: sampling bias and interviewer effects. PLoS One. 2015;10(2):e0118025.
25. Hammarstedt JE, Redmond JM, Gupta A, Dunne KF, Vemula SP, Domb BG. Survey mode influence on patient-reported outcome scores in orthopaedic surgery: telephone results may be positively biased. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):50-54.
26. Hoher J, Bach T, Munster A, et al. Does the mode of data collection change results in a subjective knee score? Self-administration versus interview. Am J Sports Med. 1997;25(5):642-647.
27. Lacny S, Bohm E, Hawker G, Powell J, Marshall DA. Assessing the comparability of hip arthroplasty registries in order to improve the recording and monitoring of outcome. Bone Joint J. 2016;98-B(4):442-451.
28. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-Term Trends in Health. Hyattsville, MD: National Center for Health Statistics, Centers for Medicare & Medicaid Services, US Dept of Health and Human Services; 2017. DHHS Publication 2017-1232. https://www.cdc.gov/nchs/data/hus/hus16.pdf. Published May 2017. Accessed October 9, 2017.
29. Cohen RA, Zammitti EP. Problems paying medical bills among persons under age 65: early release of estimates from the National Health Interview Survey, 2011-June 2016. National Health Interview Survey Early Release Program, Division of Health Interview Statistics, National Center for Health Statistics, Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. https://www.cdc.gov/nchs/data/nhis/earlyrelease/probs_paying_medical_bills_jan_2011_jun_2016.pdf. Published November 2016. Accessed October 9, 2017.
30. National Center for Health Statistics. National Health Interview Survey. http://www.cdc.gov/nchs/nhis/releases.htm. Accessed October 5, 2017.
31. Karhade AV, Larsen AMG, Cote DJ, Dubois HM, Smith TR. National databases for neurosurgical outcomes research: options, strengths, and limitations [published online ahead of print August 5, 2017]. Neurosurgery. https://doi.org/10.1093/neuros/nyx408.
32. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
33. Chen R, Desai NR, Ross JS, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ. 2016;352:i637.
34. Counsell N, Biri D, Fraczek J, Hackshaw A. Publishing interim results of randomised clinical trials in peer-reviewed journals. Clin Trials. 2017;14(1):67-77.
35. Sansone M, Ahldén M, Jonasson P, et al. A Swedish hip arthroscopy registry: demographics and development. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):774-780.
36. Mygind-Klavsen B, Grønbech Nielsen T, Maagaard N, et al. Danish Hip Arthroscopy Registry: an epidemiologic and perioperative description of the first 2000 procedures. J Hip Preserv Surg. 2016;3(2):138-145.
37. Li G, Sajobi TT, Menon BK, et al; 2016 Symposium on Registry-Based Randomized Controlled Trials in Calgary. Registry-based randomized controlled trials—what are the advantages, challenges, and areas for future research? J Clin Epidemiol. 2016;80:16-24.
38. Bautista MP, Bonilla GA, Mieth KW. Data quality in institutional arthroplasty registries: description of model of validation and report of preliminary results. J Arthroplasty. 2017;32(7):2065-2069.
39. Tevaearai H, Carrel T. Clinical registries: yes, but then appropriately! Eur J Cardiothorac Surg. 2013;44(4):614-615.
40. Australian Commission on Safety and Quality in Health Care. Economic evaluation of clinical quality registries: final report. Sydney, Australia: ACSQHC; 2016.
41. Perrin A, Duggan M. Americans’ internet access: 2000-2015. Pew Research Center website. http://www.pewinternet.org/2015/06/26/americans-internet-access-2000-2015/. Published June 26, 2015. Accessed October 5, 2017.
42. Taylor A. 47 percent of the world’s population now use the internet, study says. https://www.washingtonpost.com/news/worldviews/wp/2016/11/22/47-percent-of-the-worlds-population-now-use-the-internet-users-study-says/. Published November 22, 2016. Accessed October 5, 2017.
43. Romero L, Nieuwenhuijse M, Carr A, Sedrakyan A. Review of clinical outcomes-based anchors of minimum clinically important differences in hip and knee registry–based reports and publications. J Bone Joint Surg Am. 2014;96(suppl 1):98-103.
Take-Home Points
- PRO data collection can provide feedback for improvements in patient care and physician performance.
- Many options exist for orthopedic physicians to establish clinical data registries.
- Registry systems can help improve patient follow-up with system monitoring and patient reminders.
- Clinical registries can offer many advantages to observational research.
- With registry use becoming more prevalent, work needs to be done to establish standards for validity and reliability.
In a 2012 review of database tools, Lubowitz and Smith1 examined Internet-based applications that arthroscopic surgeons could use to record and monitor patient-reported outcome (PRO) data and potential adverse effects. In this article, we update orthopedic surgeons on the registries and monitoring software mentioned in that earlier publication and in other publications that have since become available.
Most orthopedic surgery candidates are seeking pain relief and improved function. Many patients expect their pain to be completely relieved by surgical intervention and their function to return to what it was before they became stricken.2,3 Therefore, PRO measures (PROMs) are now standard in post-orthopedic surgery outcome reporting.4 PROMs, which include any measurement that assesses a patient’s health, illness, or benefits from the perspective of the patient, are often administered as a questionnaire or survey.5 The collection of PROMs continues to increase and evolve, creating a need for data storage and analysis. Registries, large collections of patient information and outcomes, allow for evaluation of patient outcomes, monitoring of adverse effects, identification of procedure incidence, understanding of predictors of prognosis, generation of feedback for quality of care, monitoring of the safety of implantable devices, and the conducting of hypothesis-driven scientific research.6-9
Orthopedic surgery has registries at regional, national, and international levels. Although the United States has fallen well behind other countries in establishing a national registry,9 it has made some recent progress. The United States now has several national registries, including the American Joint Replacement Registry (AJRR), Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement (FORCE-TJR), the Kaiser Permanente National Total Joint Replacement Registry (TJRR), the Veterans Affairs (VA) and American College of Surgeons (ACS) National Surgical Quality Improvement Programs (NSQIPs), and the National Trauma Data Bank (NTDB).9 AJRR currently has 960 hospitals participating and is tracking 1,084,664 hip and knee replacements.10
These orthopedic registries, however, are limited in 2 ways. First, the majority are joint replacement registries. Second, though registries are established to determine patterns of care and predict patient outcomes, many are not set up to report care data back to healthcare providers.7 For procedures other than joint arthroplasty and for providers interested in tracking their patients’ PROs, systems are available for establishing clinical quality registries in orthopedics.
Registry Systems
CareSense
CareSense (Medtrak) is an Internet-based care management and data collection system designed for patient engagement, which results in fewer missed appointments, increased patient adherence, enhanced patient education, and improved patient satisfaction.11 CareSense features email/text reminders for data entry, custom and standard reports, import and export of electronic medical record (EMR) information, and tools for running research studies.12 CareSense emphasizes care navigation by helping hospitals educate and guide patients through their care by sending exercise videos to patients for home rehabilitation, transferring messages from post-acute care facilities to surgeons and caregivers, and alerting the care team to any potential readmission symptoms.11,13 CareSense is also a Centers for Medicare & Medicaid Services (CMS) approved qualified clinical data registry (QCDR). QCDRs collect data for Merit-Based Incentive Payment System (MIPS) clinicians and submit the data to CMS.12
KareOutcomes
KareOutcomes, a healthcare technology and support firm founded in 2009, advocates transparency and trust among providers and patients, and aims to optimize PROs.14 The KareOutcomes team incorporates patient follow-up personnel, administrators, engineers, physicians, software developers, and technicians. The KareOutcomes software, which is backed by a 6-month guarantee, includes system design and implementation, data collection and entry, methods of submitting data to statewide or nationwide registries and sending standardized and customized surveys, and accessible and meaningful data presentation. KareOutcomes allows patient follow-up through automated reminders by telephone, SMS text message, and email. Patients can respond to surveys or questionnaires whichever way is most convenient—by telephone, Internet, SMS text message, or on paper, either in the office or by mail.
Oberd
Oberd (Universal Research Solutions) offers a comprehensive package of solutions for collecting optimal PRO data. The package has several modules: outcomes, education, registry, operative notes, data import and export, and data reporting.15 Oberd Outcomes allows convenient and engaging data collection. For example, users can send both standardized and customized forms. Oberd Education allows patients to receive information in an interactive, narrated format that is specific to their physician’s techniques and practices. Oberd Registry allows users to input multiple datasets into a registry, compare data, and generate reports with visuals. Like CareSense, Oberd is a CMS-approved QCDR. Oberd’s MIPS Dashboard helps providers collect and report patients’ reported outcomes, and use that information to modify and improve their practice.
Ortech
Ortech is a web-based data registry system that allows physicians and administrators to mine the data they own, track key metrics in their data, and improve reporting.16 Users can collect PROMs, use them to measure and analyze patient progress, and add to their collection of information that helps support their evidence-based decision making. They can capture intraoperative and implant data through barcode scanning, which then registers the data in an implant product code library that allows quick identification of patients with a specific implant in the event of a product recall. Ortech also allows automatic generation of customized operative reports on data entered from the operating room and populated into the EMR. Ortech offers 2 versions of its data collection platform, phiDB and phiDB Lite. The phiDB Lite version is for smaller practices and focuses mainly on PROMs but lacks many of the other features that phiDB offers, such as operating room modules, automated operative reports, barcode scanning, and unlimited data reporting.
Socrates
Socrates (Standardised Orthopaedic Clinical Research and Treatment Evaluation Software; Ortholink) is dedicated orthopedic software that facilitates following patient outcomes and conducting high-quality research.17 Socrates is fully customizable to fit each user’s needs. It allows for tracking of outcome scores, intraoperative details, nonoperative procedures, clinical examinations, therapies, and adverse effects. Users can also create reports from this information, which is inputted to Socrates and can be exported into EMR. Socrates data are stored on the user’s server, on site; the software generates patient summaries, collective summaries, and follow-up reports through its built-in descriptive statistics module. Raw data can be extracted for statistical analysis. Socrates can catalogue images, radiographs, documents, and videos.
Surgical Outcomes System
Surgical Outcomes System (SOS; Arthrex) is a cloud-based orthopedic and sports medicine global registry that focuses on monitoring and evaluating the outcomes of various orthopedic and sports medicine surgical procedures, as well as nonoperative interventions, to contribute to evidence-based protocols for patient treatment.18 SOS can be fully customized with desired PROMs for arthroplasty and for surgical procedures for extremity joints and even the spine. SOS includes real-time reporting on PROs for individual patients, summary PROMs for all of the physician’s patients who are receiving the same treatment, and comparisons with all registry patients (from global de-identified registry data) who had the same treatment or surgery. This real-time analysis provides immediate patient and physician feedback on treatments and products used. A patient portal for education on surgical procedures is also available. SOS is approved for use in 21 countries and is a benefit included with Arthroscopy Association of North America (AANA) membership. SOS is listed on the National Quality Registry Network (NQRN) website and, as a specialized registry as defined by CMS, can accept data generated by EMR technology.
Discussion
Delaunay19 indicated that successful registry management depends on several factors, including “use of a single identifier for each patient to ensure full traceability of all procedures related to a given implant; a long-term funding source; a contemporary, rapid, Internet-based data collection method; and the collection of exhaustive data, at least for innovative implants.” The registry systems reviewed in this article are Internet based and allow healthcare providers to monitor the clinical outcomes of their patients in the hope of improving clinical decision-making and overall patient care. From the provider perspective, many registry systems allow for integration of outcome data reporting into EMRs, including generation of operative reports. In turn, registries can improve documentation efficiency, as it was estimated that a US physician without a registry spends more than 15 hours a week reporting quality measures,20 or almost 800 hours and $15 billion each year.20,21 It remains to be seen whether registry systems will optimize the documentation process, but there is potential improvement in time and cost-efficiency with registry use.
Although the factors involved in management are important, clinical data registries must have systems in place to help ensure patient adherence and minimize selection bias, as adherence is crucial in data accuracy.3 What helps with adherence is the ability to send automated email or SMS text message reminders to patients. According to a review, email reminders increased the completion of PROM datasets by 26%.22 When the new national quality register (NQR) HAKIR (Handkirurgiskt kvalitetsregister) was established in Sweden, it was found that when only 1 type of reminder was used (SMS text message, in this case), only about 30% of participants completed their questionnaires.23 However, after the system was changed to send both SMS text message and email reminders, the response rate increased from 50% to 60%. Using 2 types of automated reminders might minimize lost data more effectively than 1 type alone.
Another benefit of outcome monitoring through a registry is potential reduction of interviewer- related errors. Interviewer bias can occur in many different ways. Interviewers might not follow the same instructions or administer questionnaires or surveys the same way for different patients,24 the interviewer’s presence might cause the patient to alter responses based on social norms,25 and the patient might report better outcomes in the presence of a physician or interviewer.26,27 Given that clinical registries allow electronic capture of self-administered surveys, interviewer bias is reduced because all patients receive a standardized set of questions and instructions. In addition, electronic questionnaires and surveys prompt users to add or fix missed or incorrectly completed items, further reducing potential data inaccuracies.
Healthcare costs continue to rise in the United States. In 2015, the total cost of healthcare expenditure in the United States was $3.2 trillion, or almost 18% of the US gross domestic product.28 In addition, in the first half of 2016, an estimated 16.2% of people under age 65 years were in families that were struggling to pay medical bills.29,30 Healthcare reform provides a financial incentive to healthcare providers to collaborate to reduce unnecessary costs and procedures and improve the quality of healthcare.31 Porter and Teisberg32 defined value as health outcomes achieved per dollar spent. Registry monitoring of PROMs, which are the numerator in this critical value formula, allows providers to track patient outcomes over time to determine which interventions produce the best outcomes.22 Therefore, clinical registries play an important role in improving health outcomes and reducing the cost of healthcare.7
Since the Swedish Knee Arthroplasty Register (SKAR) was established 40 years ago, NQRs have been commonplace in Scandinavian countries, Australia, and the United Kingdom.23 Between 2001 and 2014, the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) documented a decline in the financial burden of hip and knee arthroplasty revision in Australia—in comparison with the United States, which did not have a full national registry at the time and showed a revision rate increase.24 The economic benefit of reducing hip and knee arthroplasty revisions in Australia during that period was an estimated $65 million to $143 million.24 Besides having financial benefits, national registries allow early identification of flawed implantation products and methods, leading to a further reduction in the burden associated with recall and future use of such defective implants—including patient harm.
In addition to monitoring existing techniques and devices, registries can also follow new techniques and, compared with publication in clinical journals, more expeditiously provide clinical data for outcome expectations and treatment methods. This timeliness is particularly valuable given that publication of clinical trials with the usual mandatory 2-year follow-up can take 4 years or longer.33,34 For instance, in the expanding field of hip arthroscopy, data from registries in both Sweden and Denmark are being analyzed.35,36 These data are important in new fields such as hip arthroscopy, in which clinical indications and treatment techniques may vary considerably between locations.35 In 2012, the Danish Hip Arthroscopy Registry (DHAR) was started as a web-based prospective registry.36 Between 2012 and December 2014, DHAR added 2000 procedures, which included all hip arthroscopy procedures performed at 11 centers in Denmark.36 DHAR tracks PROM, surgical procedure, operative, and radiologic data.
Increased use of clinical registries has led to use of their data in clinical research. Registry-based randomized controlled trials (RCTs) are lower in cost than other types of research, allow for rapid enrollment of patients, offer larger population sizes and multi-institutional sampling, and can provide a more diverse patient population.19,37 Although nonregistry RCTs remain the gold standard of clinical research, registry RCTs have several advantages given the abilities and structure of registries. Because of resources and cost, nonregistry RCTs are usually limited in the number of examined exposures and typically focus on only 2.6 Registry RCTs, on the other hand, can monitor multiple exposures, typically at minimal cost difference.6 Another disadvantage of nonregistry RCTs is that they are often performed at institutions providing care that might not be indicative of the quality most patients expect, as these institutions might be selected for a specific clinician or specialty service.
Registry RCTs also have their limitations with respect to clinical research. A major one is their lack of validity standards or accepted benchmarks for accuracy, adherence rates, registry completeness, and data collection.37,38 In addition, lack of standardization across national and international registries could produce conflicting data. Another limitation is that data in most registries are not subjected to any third-party checks or independent auditing.9,39 Furthermore, evaluating the impact of registries is difficult because it is difficult to find comparable outcome data on nonregistry patients.40 A final limitation involves the ethics of including registry data in RCTs. Although data are often added to a registry without patient consent, should the same data be used for research without patient consent? Should patients be able to disallow use of their data for research, or require a notification each time their data are used? These issues must be addressed.
Review Limitations
One limitation of this review of clinical Internet-based outcome systems is that it might not have identified comparable systems. In addition, specific costs associated with each system were not addressed, as they depend on PROM licensing fees, total institutional access, other proprietary costs, and other variables. Another limitation, in terms of creating a national or international registry, continues to be Internet access. The Pew Research Center estimated that 84% of US adults used the Internet in 2015.41 Although 84% represents most of the adult population, the other 16% typically is over age 65 years, where only 58% of adults reported using the Internet, or come from lower income households, where access was <75%. For registries in European countries and North America, where Internet usage typically is >70%, this is not a significant problem. However, worldwide, only 47% of the population used the Internet in 2016.42 Internet usage by Asian and Arab states citizens was 41.6% and 41.9%, respectively, and usage by African citizens was only 25.1%. As a significant benefit of registry use is that researchers can obtain larger sample sizes, it is a problem that some populations—elderly people, people of lower socioeconomic standing, people living where the Internet is unavailable—might be underrepresented in registry data.
As mentioned, patient adherence is an ongoing issue for clinical registries. As adherence tends to decrease as more time passes after a patient’s treatment date, it is important to account for and encourage continued patient participation with outcome monitoring. Missing data lessen the validity and accuracy of a registry, increasing the likelihood that certain groups will be underrepresented. Although registry systems can reduce the cost of following PROMs, doing so requires monitoring and following up on issues of patient adherence. In other words, many clinicians will need the help of a research assistant. Makhni and colleagues21 found that adding a research assistant for this task increased survey adherence from 65% to 94% before surgery, from 65% to 72% 6 months after surgery, and from 38% to 56% 12 months after surgery.
Even though studies continue to use clinical data from registries, there is not much research on the impact of these registries on improvement in healthcare. Again, many factors are involved: lack of standardized benchmarks for accuracy and adherence, lack of an accepted method of data auditing and validation, and difficulty evaluating the impact of registries owing to the difficulty obtaining comparable data on nonregistry patients. Registries must adopt accepted forms of standardization in order to allow better comparisons of registries, because comparing data across registries can be useful in determining the strengths and weaknesses of different registries.27,43 As registries support decision making at clinical, institutional, and governmental levels, it is vital that their clinical data be accurate and reliable.38
Conclusion
Rising healthcare costs, and government and third-party pressures are making patient outcomes collection a standard of care. Going forward, orthopedic surgeons must be proactive, and Internet -based registries provide technological advances that facilitate the process.
Take-Home Points
- PRO data collection can provide feedback for improvements in patient care and physician performance.
- Many options exist for orthopedic physicians to establish clinical data registries.
- Registry systems can help improve patient follow-up with system monitoring and patient reminders.
- Clinical registries can offer many advantages to observational research.
- With registry use becoming more prevalent, work needs to be done to establish standards for validity and reliability.
In a 2012 review of database tools, Lubowitz and Smith1 examined Internet-based applications that arthroscopic surgeons could use to record and monitor patient-reported outcome (PRO) data and potential adverse effects. In this article, we update orthopedic surgeons on the registries and monitoring software mentioned in that earlier publication and in other publications that have since become available.
Most orthopedic surgery candidates are seeking pain relief and improved function. Many patients expect their pain to be completely relieved by surgical intervention and their function to return to what it was before they became stricken.2,3 Therefore, PRO measures (PROMs) are now standard in post-orthopedic surgery outcome reporting.4 PROMs, which include any measurement that assesses a patient’s health, illness, or benefits from the perspective of the patient, are often administered as a questionnaire or survey.5 The collection of PROMs continues to increase and evolve, creating a need for data storage and analysis. Registries, large collections of patient information and outcomes, allow for evaluation of patient outcomes, monitoring of adverse effects, identification of procedure incidence, understanding of predictors of prognosis, generation of feedback for quality of care, monitoring of the safety of implantable devices, and the conducting of hypothesis-driven scientific research.6-9
Orthopedic surgery has registries at regional, national, and international levels. Although the United States has fallen well behind other countries in establishing a national registry,9 it has made some recent progress. The United States now has several national registries, including the American Joint Replacement Registry (AJRR), Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement (FORCE-TJR), the Kaiser Permanente National Total Joint Replacement Registry (TJRR), the Veterans Affairs (VA) and American College of Surgeons (ACS) National Surgical Quality Improvement Programs (NSQIPs), and the National Trauma Data Bank (NTDB).9 AJRR currently has 960 hospitals participating and is tracking 1,084,664 hip and knee replacements.10
These orthopedic registries, however, are limited in 2 ways. First, the majority are joint replacement registries. Second, though registries are established to determine patterns of care and predict patient outcomes, many are not set up to report care data back to healthcare providers.7 For procedures other than joint arthroplasty and for providers interested in tracking their patients’ PROs, systems are available for establishing clinical quality registries in orthopedics.
Registry Systems
CareSense
CareSense (Medtrak) is an Internet-based care management and data collection system designed for patient engagement, which results in fewer missed appointments, increased patient adherence, enhanced patient education, and improved patient satisfaction.11 CareSense features email/text reminders for data entry, custom and standard reports, import and export of electronic medical record (EMR) information, and tools for running research studies.12 CareSense emphasizes care navigation by helping hospitals educate and guide patients through their care by sending exercise videos to patients for home rehabilitation, transferring messages from post-acute care facilities to surgeons and caregivers, and alerting the care team to any potential readmission symptoms.11,13 CareSense is also a Centers for Medicare & Medicaid Services (CMS) approved qualified clinical data registry (QCDR). QCDRs collect data for Merit-Based Incentive Payment System (MIPS) clinicians and submit the data to CMS.12
KareOutcomes
KareOutcomes, a healthcare technology and support firm founded in 2009, advocates transparency and trust among providers and patients, and aims to optimize PROs.14 The KareOutcomes team incorporates patient follow-up personnel, administrators, engineers, physicians, software developers, and technicians. The KareOutcomes software, which is backed by a 6-month guarantee, includes system design and implementation, data collection and entry, methods of submitting data to statewide or nationwide registries and sending standardized and customized surveys, and accessible and meaningful data presentation. KareOutcomes allows patient follow-up through automated reminders by telephone, SMS text message, and email. Patients can respond to surveys or questionnaires whichever way is most convenient—by telephone, Internet, SMS text message, or on paper, either in the office or by mail.
Oberd
Oberd (Universal Research Solutions) offers a comprehensive package of solutions for collecting optimal PRO data. The package has several modules: outcomes, education, registry, operative notes, data import and export, and data reporting.15 Oberd Outcomes allows convenient and engaging data collection. For example, users can send both standardized and customized forms. Oberd Education allows patients to receive information in an interactive, narrated format that is specific to their physician’s techniques and practices. Oberd Registry allows users to input multiple datasets into a registry, compare data, and generate reports with visuals. Like CareSense, Oberd is a CMS-approved QCDR. Oberd’s MIPS Dashboard helps providers collect and report patients’ reported outcomes, and use that information to modify and improve their practice.
Ortech
Ortech is a web-based data registry system that allows physicians and administrators to mine the data they own, track key metrics in their data, and improve reporting.16 Users can collect PROMs, use them to measure and analyze patient progress, and add to their collection of information that helps support their evidence-based decision making. They can capture intraoperative and implant data through barcode scanning, which then registers the data in an implant product code library that allows quick identification of patients with a specific implant in the event of a product recall. Ortech also allows automatic generation of customized operative reports on data entered from the operating room and populated into the EMR. Ortech offers 2 versions of its data collection platform, phiDB and phiDB Lite. The phiDB Lite version is for smaller practices and focuses mainly on PROMs but lacks many of the other features that phiDB offers, such as operating room modules, automated operative reports, barcode scanning, and unlimited data reporting.
Socrates
Socrates (Standardised Orthopaedic Clinical Research and Treatment Evaluation Software; Ortholink) is dedicated orthopedic software that facilitates following patient outcomes and conducting high-quality research.17 Socrates is fully customizable to fit each user’s needs. It allows for tracking of outcome scores, intraoperative details, nonoperative procedures, clinical examinations, therapies, and adverse effects. Users can also create reports from this information, which is inputted to Socrates and can be exported into EMR. Socrates data are stored on the user’s server, on site; the software generates patient summaries, collective summaries, and follow-up reports through its built-in descriptive statistics module. Raw data can be extracted for statistical analysis. Socrates can catalogue images, radiographs, documents, and videos.
Surgical Outcomes System
Surgical Outcomes System (SOS; Arthrex) is a cloud-based orthopedic and sports medicine global registry that focuses on monitoring and evaluating the outcomes of various orthopedic and sports medicine surgical procedures, as well as nonoperative interventions, to contribute to evidence-based protocols for patient treatment.18 SOS can be fully customized with desired PROMs for arthroplasty and for surgical procedures for extremity joints and even the spine. SOS includes real-time reporting on PROs for individual patients, summary PROMs for all of the physician’s patients who are receiving the same treatment, and comparisons with all registry patients (from global de-identified registry data) who had the same treatment or surgery. This real-time analysis provides immediate patient and physician feedback on treatments and products used. A patient portal for education on surgical procedures is also available. SOS is approved for use in 21 countries and is a benefit included with Arthroscopy Association of North America (AANA) membership. SOS is listed on the National Quality Registry Network (NQRN) website and, as a specialized registry as defined by CMS, can accept data generated by EMR technology.
Discussion
Delaunay19 indicated that successful registry management depends on several factors, including “use of a single identifier for each patient to ensure full traceability of all procedures related to a given implant; a long-term funding source; a contemporary, rapid, Internet-based data collection method; and the collection of exhaustive data, at least for innovative implants.” The registry systems reviewed in this article are Internet based and allow healthcare providers to monitor the clinical outcomes of their patients in the hope of improving clinical decision-making and overall patient care. From the provider perspective, many registry systems allow for integration of outcome data reporting into EMRs, including generation of operative reports. In turn, registries can improve documentation efficiency, as it was estimated that a US physician without a registry spends more than 15 hours a week reporting quality measures,20 or almost 800 hours and $15 billion each year.20,21 It remains to be seen whether registry systems will optimize the documentation process, but there is potential improvement in time and cost-efficiency with registry use.
Although the factors involved in management are important, clinical data registries must have systems in place to help ensure patient adherence and minimize selection bias, as adherence is crucial in data accuracy.3 What helps with adherence is the ability to send automated email or SMS text message reminders to patients. According to a review, email reminders increased the completion of PROM datasets by 26%.22 When the new national quality register (NQR) HAKIR (Handkirurgiskt kvalitetsregister) was established in Sweden, it was found that when only 1 type of reminder was used (SMS text message, in this case), only about 30% of participants completed their questionnaires.23 However, after the system was changed to send both SMS text message and email reminders, the response rate increased from 50% to 60%. Using 2 types of automated reminders might minimize lost data more effectively than 1 type alone.
Another benefit of outcome monitoring through a registry is potential reduction of interviewer- related errors. Interviewer bias can occur in many different ways. Interviewers might not follow the same instructions or administer questionnaires or surveys the same way for different patients,24 the interviewer’s presence might cause the patient to alter responses based on social norms,25 and the patient might report better outcomes in the presence of a physician or interviewer.26,27 Given that clinical registries allow electronic capture of self-administered surveys, interviewer bias is reduced because all patients receive a standardized set of questions and instructions. In addition, electronic questionnaires and surveys prompt users to add or fix missed or incorrectly completed items, further reducing potential data inaccuracies.
Healthcare costs continue to rise in the United States. In 2015, the total cost of healthcare expenditure in the United States was $3.2 trillion, or almost 18% of the US gross domestic product.28 In addition, in the first half of 2016, an estimated 16.2% of people under age 65 years were in families that were struggling to pay medical bills.29,30 Healthcare reform provides a financial incentive to healthcare providers to collaborate to reduce unnecessary costs and procedures and improve the quality of healthcare.31 Porter and Teisberg32 defined value as health outcomes achieved per dollar spent. Registry monitoring of PROMs, which are the numerator in this critical value formula, allows providers to track patient outcomes over time to determine which interventions produce the best outcomes.22 Therefore, clinical registries play an important role in improving health outcomes and reducing the cost of healthcare.7
Since the Swedish Knee Arthroplasty Register (SKAR) was established 40 years ago, NQRs have been commonplace in Scandinavian countries, Australia, and the United Kingdom.23 Between 2001 and 2014, the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) documented a decline in the financial burden of hip and knee arthroplasty revision in Australia—in comparison with the United States, which did not have a full national registry at the time and showed a revision rate increase.24 The economic benefit of reducing hip and knee arthroplasty revisions in Australia during that period was an estimated $65 million to $143 million.24 Besides having financial benefits, national registries allow early identification of flawed implantation products and methods, leading to a further reduction in the burden associated with recall and future use of such defective implants—including patient harm.
In addition to monitoring existing techniques and devices, registries can also follow new techniques and, compared with publication in clinical journals, more expeditiously provide clinical data for outcome expectations and treatment methods. This timeliness is particularly valuable given that publication of clinical trials with the usual mandatory 2-year follow-up can take 4 years or longer.33,34 For instance, in the expanding field of hip arthroscopy, data from registries in both Sweden and Denmark are being analyzed.35,36 These data are important in new fields such as hip arthroscopy, in which clinical indications and treatment techniques may vary considerably between locations.35 In 2012, the Danish Hip Arthroscopy Registry (DHAR) was started as a web-based prospective registry.36 Between 2012 and December 2014, DHAR added 2000 procedures, which included all hip arthroscopy procedures performed at 11 centers in Denmark.36 DHAR tracks PROM, surgical procedure, operative, and radiologic data.
Increased use of clinical registries has led to use of their data in clinical research. Registry-based randomized controlled trials (RCTs) are lower in cost than other types of research, allow for rapid enrollment of patients, offer larger population sizes and multi-institutional sampling, and can provide a more diverse patient population.19,37 Although nonregistry RCTs remain the gold standard of clinical research, registry RCTs have several advantages given the abilities and structure of registries. Because of resources and cost, nonregistry RCTs are usually limited in the number of examined exposures and typically focus on only 2.6 Registry RCTs, on the other hand, can monitor multiple exposures, typically at minimal cost difference.6 Another disadvantage of nonregistry RCTs is that they are often performed at institutions providing care that might not be indicative of the quality most patients expect, as these institutions might be selected for a specific clinician or specialty service.
Registry RCTs also have their limitations with respect to clinical research. A major one is their lack of validity standards or accepted benchmarks for accuracy, adherence rates, registry completeness, and data collection.37,38 In addition, lack of standardization across national and international registries could produce conflicting data. Another limitation is that data in most registries are not subjected to any third-party checks or independent auditing.9,39 Furthermore, evaluating the impact of registries is difficult because it is difficult to find comparable outcome data on nonregistry patients.40 A final limitation involves the ethics of including registry data in RCTs. Although data are often added to a registry without patient consent, should the same data be used for research without patient consent? Should patients be able to disallow use of their data for research, or require a notification each time their data are used? These issues must be addressed.
Review Limitations
One limitation of this review of clinical Internet-based outcome systems is that it might not have identified comparable systems. In addition, specific costs associated with each system were not addressed, as they depend on PROM licensing fees, total institutional access, other proprietary costs, and other variables. Another limitation, in terms of creating a national or international registry, continues to be Internet access. The Pew Research Center estimated that 84% of US adults used the Internet in 2015.41 Although 84% represents most of the adult population, the other 16% typically is over age 65 years, where only 58% of adults reported using the Internet, or come from lower income households, where access was <75%. For registries in European countries and North America, where Internet usage typically is >70%, this is not a significant problem. However, worldwide, only 47% of the population used the Internet in 2016.42 Internet usage by Asian and Arab states citizens was 41.6% and 41.9%, respectively, and usage by African citizens was only 25.1%. As a significant benefit of registry use is that researchers can obtain larger sample sizes, it is a problem that some populations—elderly people, people of lower socioeconomic standing, people living where the Internet is unavailable—might be underrepresented in registry data.
As mentioned, patient adherence is an ongoing issue for clinical registries. As adherence tends to decrease as more time passes after a patient’s treatment date, it is important to account for and encourage continued patient participation with outcome monitoring. Missing data lessen the validity and accuracy of a registry, increasing the likelihood that certain groups will be underrepresented. Although registry systems can reduce the cost of following PROMs, doing so requires monitoring and following up on issues of patient adherence. In other words, many clinicians will need the help of a research assistant. Makhni and colleagues21 found that adding a research assistant for this task increased survey adherence from 65% to 94% before surgery, from 65% to 72% 6 months after surgery, and from 38% to 56% 12 months after surgery.
Even though studies continue to use clinical data from registries, there is not much research on the impact of these registries on improvement in healthcare. Again, many factors are involved: lack of standardized benchmarks for accuracy and adherence, lack of an accepted method of data auditing and validation, and difficulty evaluating the impact of registries owing to the difficulty obtaining comparable data on nonregistry patients. Registries must adopt accepted forms of standardization in order to allow better comparisons of registries, because comparing data across registries can be useful in determining the strengths and weaknesses of different registries.27,43 As registries support decision making at clinical, institutional, and governmental levels, it is vital that their clinical data be accurate and reliable.38
Conclusion
Rising healthcare costs, and government and third-party pressures are making patient outcomes collection a standard of care. Going forward, orthopedic surgeons must be proactive, and Internet -based registries provide technological advances that facilitate the process.
1. Lubowitz JH, Smith PA. Current concepts in clinical research: web-based, automated, arthroscopic surgery prospective database registry. Arthroscopy. 2012;28(3):425-428.
2. Ayers DC, Bozic KJ. The importance of outcome measurement in orthopaedics. Clin Orthop Relat Res. 2013;471(11):3409-3411.
3. Nwachukwu BU, Fields K, Chang B, Nawabi DH, Kelly BT, Ranawat AS. Preoperative outcome scores are predictive of achieving the minimal clinically important difference after arthroscopic treatment of femoroacetabular impingement. Am J Sports Med. 2017;45(3):612-619.
4. Breckenridge K, Bekker HL, Gibbons E, et al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant. 2015;30(10):1605-1614.
5. Inacio MC, Paxton EW, Dillon MT. Understanding orthopaedic registry studies: a comparison with clinical studies. J Bone Joint Surg Am. 2016;98(1):e3.
6. Hoque DME, Kumari V, Hoque M, Ruseckaite R, Romero L, Evans SM. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS One. 2017;12(9):e0183667.
7. Physician Consortium for Performance Improvement. National Quality Registry Network. http://www.thepcpi.org/programs-initiatives/national-quality-registry-network/. Accessed October 5, 2017.
8. Hickey GL, Grant SW, Cosgriff R, et al. Clinical registries: governance, management, analysis and applications. Eur J Cardiothorac Surg. 2013;44(4):605-614.
9. Pugely AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopaedic surgery: part 2: clinical registry data. J Bone Joint Surg Am. 2015;97(21):1799-1808.
10. American Joint Replacement Registry. http://www.ajrr.net/. Accessed October 5, 2017.
11. CareSense. https://www.caresense.com/. Accessed October 4, 2017.
12. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, Quality Payment Program. Merit-Based Incentive Payment System (MIPS): 2017 CMS-Approved Qualified Clinical Data Registries (QCDRs). https://qpp.cms.gov/docs/QPP_2017_CMS_Approved_QCDRs.pdf. Accessed October 9, 2017.
13. Johnson & Johnson. Johnson & Johnson Medical Devices Companies introduce Orthopaedic Episode of Care Approach, leveraging CareAdvantage capabilities to support better clinical outcomes and reduce the cost of care. https://www.jnj.com/media-center/press-releases/johnson-johnson-medical-devices-companies-introduce-orthopaedic-episode-of-care-approach-leveraging-careadvantage-capabilities-to-support-better-clinical-outcomes-and-reduce-the-cost-of-care. Published January 9, 2017. Accessed October 4, 2017.
14. KareOutcomes. http://www.kareoutcomes.com/. Accessed October 4, 2017.
15. Oberd. http://www.oberd.com/. Accessed October 4, 2017.
16. Ortech Systems. http://www.ortechsystems.com/. Accessed October 4, 2017.
17. Socrates. http://www.socratesortho.com/. Accessed October 4, 2017.
18. Surgical Outcomes System. https://www.surgicaloutcomesystem.com/. Accessed October 4, 2017.
19. Delaunay C. Registries in orthopaedics. Orthop Traumatol Surg Res. 2015;101(1 suppl):S69-S75.
20. Bryan S, Davis J, Broesch J, Doyle-Waters MM, Lewis S, McGrail K. Choosing your partner for the PROM: a review of evidence on patient-reported outcome measures for use in primary and community care. Healthc Policy. 2014;10(2):38-51.
21. Makhni EC, Higgins JD, Hamamoto JT, Cole BJ, Romeo AA, Verma NN. Patient compliance with electronic patient reported outcomes following shoulder arthroscopy [published online ahead of print September 25, 2017]. Arthroscopy. doi:10.1016/j.arthro.2017.06.016.
22. Triplet JJ, Momoh E, Kurowicki J, Villarroel LD, Law T, Levy JC. E-mail reminders improve completion rates of patient-reported outcome measures. JSES Open Access. 2017;1:25-28.
23. Arner M. Developing a national quality registry for hand surgery: challenges and opportunities. EFORT Open Rev. 2016;1(4):100-106.
24. Ngongo CJ, Frick KD, Hightower AW, Mathingau FA, Burke H, Breiman RF. The perils of straying from protocol: sampling bias and interviewer effects. PLoS One. 2015;10(2):e0118025.
25. Hammarstedt JE, Redmond JM, Gupta A, Dunne KF, Vemula SP, Domb BG. Survey mode influence on patient-reported outcome scores in orthopaedic surgery: telephone results may be positively biased. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):50-54.
26. Hoher J, Bach T, Munster A, et al. Does the mode of data collection change results in a subjective knee score? Self-administration versus interview. Am J Sports Med. 1997;25(5):642-647.
27. Lacny S, Bohm E, Hawker G, Powell J, Marshall DA. Assessing the comparability of hip arthroplasty registries in order to improve the recording and monitoring of outcome. Bone Joint J. 2016;98-B(4):442-451.
28. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-Term Trends in Health. Hyattsville, MD: National Center for Health Statistics, Centers for Medicare & Medicaid Services, US Dept of Health and Human Services; 2017. DHHS Publication 2017-1232. https://www.cdc.gov/nchs/data/hus/hus16.pdf. Published May 2017. Accessed October 9, 2017.
29. Cohen RA, Zammitti EP. Problems paying medical bills among persons under age 65: early release of estimates from the National Health Interview Survey, 2011-June 2016. National Health Interview Survey Early Release Program, Division of Health Interview Statistics, National Center for Health Statistics, Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. https://www.cdc.gov/nchs/data/nhis/earlyrelease/probs_paying_medical_bills_jan_2011_jun_2016.pdf. Published November 2016. Accessed October 9, 2017.
30. National Center for Health Statistics. National Health Interview Survey. http://www.cdc.gov/nchs/nhis/releases.htm. Accessed October 5, 2017.
31. Karhade AV, Larsen AMG, Cote DJ, Dubois HM, Smith TR. National databases for neurosurgical outcomes research: options, strengths, and limitations [published online ahead of print August 5, 2017]. Neurosurgery. https://doi.org/10.1093/neuros/nyx408.
32. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
33. Chen R, Desai NR, Ross JS, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ. 2016;352:i637.
34. Counsell N, Biri D, Fraczek J, Hackshaw A. Publishing interim results of randomised clinical trials in peer-reviewed journals. Clin Trials. 2017;14(1):67-77.
35. Sansone M, Ahldén M, Jonasson P, et al. A Swedish hip arthroscopy registry: demographics and development. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):774-780.
36. Mygind-Klavsen B, Grønbech Nielsen T, Maagaard N, et al. Danish Hip Arthroscopy Registry: an epidemiologic and perioperative description of the first 2000 procedures. J Hip Preserv Surg. 2016;3(2):138-145.
37. Li G, Sajobi TT, Menon BK, et al; 2016 Symposium on Registry-Based Randomized Controlled Trials in Calgary. Registry-based randomized controlled trials—what are the advantages, challenges, and areas for future research? J Clin Epidemiol. 2016;80:16-24.
38. Bautista MP, Bonilla GA, Mieth KW. Data quality in institutional arthroplasty registries: description of model of validation and report of preliminary results. J Arthroplasty. 2017;32(7):2065-2069.
39. Tevaearai H, Carrel T. Clinical registries: yes, but then appropriately! Eur J Cardiothorac Surg. 2013;44(4):614-615.
40. Australian Commission on Safety and Quality in Health Care. Economic evaluation of clinical quality registries: final report. Sydney, Australia: ACSQHC; 2016.
41. Perrin A, Duggan M. Americans’ internet access: 2000-2015. Pew Research Center website. http://www.pewinternet.org/2015/06/26/americans-internet-access-2000-2015/. Published June 26, 2015. Accessed October 5, 2017.
42. Taylor A. 47 percent of the world’s population now use the internet, study says. https://www.washingtonpost.com/news/worldviews/wp/2016/11/22/47-percent-of-the-worlds-population-now-use-the-internet-users-study-says/. Published November 22, 2016. Accessed October 5, 2017.
43. Romero L, Nieuwenhuijse M, Carr A, Sedrakyan A. Review of clinical outcomes-based anchors of minimum clinically important differences in hip and knee registry–based reports and publications. J Bone Joint Surg Am. 2014;96(suppl 1):98-103.
1. Lubowitz JH, Smith PA. Current concepts in clinical research: web-based, automated, arthroscopic surgery prospective database registry. Arthroscopy. 2012;28(3):425-428.
2. Ayers DC, Bozic KJ. The importance of outcome measurement in orthopaedics. Clin Orthop Relat Res. 2013;471(11):3409-3411.
3. Nwachukwu BU, Fields K, Chang B, Nawabi DH, Kelly BT, Ranawat AS. Preoperative outcome scores are predictive of achieving the minimal clinically important difference after arthroscopic treatment of femoroacetabular impingement. Am J Sports Med. 2017;45(3):612-619.
4. Breckenridge K, Bekker HL, Gibbons E, et al. How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting. Nephrol Dial Transplant. 2015;30(10):1605-1614.
5. Inacio MC, Paxton EW, Dillon MT. Understanding orthopaedic registry studies: a comparison with clinical studies. J Bone Joint Surg Am. 2016;98(1):e3.
6. Hoque DME, Kumari V, Hoque M, Ruseckaite R, Romero L, Evans SM. Impact of clinical registries on quality of patient care and clinical outcomes: a systematic review. PLoS One. 2017;12(9):e0183667.
7. Physician Consortium for Performance Improvement. National Quality Registry Network. http://www.thepcpi.org/programs-initiatives/national-quality-registry-network/. Accessed October 5, 2017.
8. Hickey GL, Grant SW, Cosgriff R, et al. Clinical registries: governance, management, analysis and applications. Eur J Cardiothorac Surg. 2013;44(4):605-614.
9. Pugely AJ, Martin CT, Harwood J, Ong KL, Bozic KJ, Callaghan JJ. Database and registry research in orthopaedic surgery: part 2: clinical registry data. J Bone Joint Surg Am. 2015;97(21):1799-1808.
10. American Joint Replacement Registry. http://www.ajrr.net/. Accessed October 5, 2017.
11. CareSense. https://www.caresense.com/. Accessed October 4, 2017.
12. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, Quality Payment Program. Merit-Based Incentive Payment System (MIPS): 2017 CMS-Approved Qualified Clinical Data Registries (QCDRs). https://qpp.cms.gov/docs/QPP_2017_CMS_Approved_QCDRs.pdf. Accessed October 9, 2017.
13. Johnson & Johnson. Johnson & Johnson Medical Devices Companies introduce Orthopaedic Episode of Care Approach, leveraging CareAdvantage capabilities to support better clinical outcomes and reduce the cost of care. https://www.jnj.com/media-center/press-releases/johnson-johnson-medical-devices-companies-introduce-orthopaedic-episode-of-care-approach-leveraging-careadvantage-capabilities-to-support-better-clinical-outcomes-and-reduce-the-cost-of-care. Published January 9, 2017. Accessed October 4, 2017.
14. KareOutcomes. http://www.kareoutcomes.com/. Accessed October 4, 2017.
15. Oberd. http://www.oberd.com/. Accessed October 4, 2017.
16. Ortech Systems. http://www.ortechsystems.com/. Accessed October 4, 2017.
17. Socrates. http://www.socratesortho.com/. Accessed October 4, 2017.
18. Surgical Outcomes System. https://www.surgicaloutcomesystem.com/. Accessed October 4, 2017.
19. Delaunay C. Registries in orthopaedics. Orthop Traumatol Surg Res. 2015;101(1 suppl):S69-S75.
20. Bryan S, Davis J, Broesch J, Doyle-Waters MM, Lewis S, McGrail K. Choosing your partner for the PROM: a review of evidence on patient-reported outcome measures for use in primary and community care. Healthc Policy. 2014;10(2):38-51.
21. Makhni EC, Higgins JD, Hamamoto JT, Cole BJ, Romeo AA, Verma NN. Patient compliance with electronic patient reported outcomes following shoulder arthroscopy [published online ahead of print September 25, 2017]. Arthroscopy. doi:10.1016/j.arthro.2017.06.016.
22. Triplet JJ, Momoh E, Kurowicki J, Villarroel LD, Law T, Levy JC. E-mail reminders improve completion rates of patient-reported outcome measures. JSES Open Access. 2017;1:25-28.
23. Arner M. Developing a national quality registry for hand surgery: challenges and opportunities. EFORT Open Rev. 2016;1(4):100-106.
24. Ngongo CJ, Frick KD, Hightower AW, Mathingau FA, Burke H, Breiman RF. The perils of straying from protocol: sampling bias and interviewer effects. PLoS One. 2015;10(2):e0118025.
25. Hammarstedt JE, Redmond JM, Gupta A, Dunne KF, Vemula SP, Domb BG. Survey mode influence on patient-reported outcome scores in orthopaedic surgery: telephone results may be positively biased. Knee Surg Sports Traumatol Arthrosc. 2017;25(1):50-54.
26. Hoher J, Bach T, Munster A, et al. Does the mode of data collection change results in a subjective knee score? Self-administration versus interview. Am J Sports Med. 1997;25(5):642-647.
27. Lacny S, Bohm E, Hawker G, Powell J, Marshall DA. Assessing the comparability of hip arthroplasty registries in order to improve the recording and monitoring of outcome. Bone Joint J. 2016;98-B(4):442-451.
28. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-Term Trends in Health. Hyattsville, MD: National Center for Health Statistics, Centers for Medicare & Medicaid Services, US Dept of Health and Human Services; 2017. DHHS Publication 2017-1232. https://www.cdc.gov/nchs/data/hus/hus16.pdf. Published May 2017. Accessed October 9, 2017.
29. Cohen RA, Zammitti EP. Problems paying medical bills among persons under age 65: early release of estimates from the National Health Interview Survey, 2011-June 2016. National Health Interview Survey Early Release Program, Division of Health Interview Statistics, National Center for Health Statistics, Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. https://www.cdc.gov/nchs/data/nhis/earlyrelease/probs_paying_medical_bills_jan_2011_jun_2016.pdf. Published November 2016. Accessed October 9, 2017.
30. National Center for Health Statistics. National Health Interview Survey. http://www.cdc.gov/nchs/nhis/releases.htm. Accessed October 5, 2017.
31. Karhade AV, Larsen AMG, Cote DJ, Dubois HM, Smith TR. National databases for neurosurgical outcomes research: options, strengths, and limitations [published online ahead of print August 5, 2017]. Neurosurgery. https://doi.org/10.1093/neuros/nyx408.
32. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
33. Chen R, Desai NR, Ross JS, et al. Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers. BMJ. 2016;352:i637.
34. Counsell N, Biri D, Fraczek J, Hackshaw A. Publishing interim results of randomised clinical trials in peer-reviewed journals. Clin Trials. 2017;14(1):67-77.
35. Sansone M, Ahldén M, Jonasson P, et al. A Swedish hip arthroscopy registry: demographics and development. Knee Surg Sports Traumatol Arthrosc. 2014;22(4):774-780.
36. Mygind-Klavsen B, Grønbech Nielsen T, Maagaard N, et al. Danish Hip Arthroscopy Registry: an epidemiologic and perioperative description of the first 2000 procedures. J Hip Preserv Surg. 2016;3(2):138-145.
37. Li G, Sajobi TT, Menon BK, et al; 2016 Symposium on Registry-Based Randomized Controlled Trials in Calgary. Registry-based randomized controlled trials—what are the advantages, challenges, and areas for future research? J Clin Epidemiol. 2016;80:16-24.
38. Bautista MP, Bonilla GA, Mieth KW. Data quality in institutional arthroplasty registries: description of model of validation and report of preliminary results. J Arthroplasty. 2017;32(7):2065-2069.
39. Tevaearai H, Carrel T. Clinical registries: yes, but then appropriately! Eur J Cardiothorac Surg. 2013;44(4):614-615.
40. Australian Commission on Safety and Quality in Health Care. Economic evaluation of clinical quality registries: final report. Sydney, Australia: ACSQHC; 2016.
41. Perrin A, Duggan M. Americans’ internet access: 2000-2015. Pew Research Center website. http://www.pewinternet.org/2015/06/26/americans-internet-access-2000-2015/. Published June 26, 2015. Accessed October 5, 2017.
42. Taylor A. 47 percent of the world’s population now use the internet, study says. https://www.washingtonpost.com/news/worldviews/wp/2016/11/22/47-percent-of-the-worlds-population-now-use-the-internet-users-study-says/. Published November 22, 2016. Accessed October 5, 2017.
43. Romero L, Nieuwenhuijse M, Carr A, Sedrakyan A. Review of clinical outcomes-based anchors of minimum clinically important differences in hip and knee registry–based reports and publications. J Bone Joint Surg Am. 2014;96(suppl 1):98-103.
Implementing Patient-Reported Outcome Measures in Your Practice: Pearls and Pitfalls
Take-Home Points
- Systematic use of PROMs allows physicians to review data on pain, physical function, and psychological status to aid in clinical decision-making and best practices.
- PROMs should include both general outcome measures (VAS, SF-36, or EQ-5D) and reliable, valid, and responsive disease specific measures.
- PROM questionnaires should collect pertinent information while limiting the length to maximize patient compliance and reliability.
- PROMIS has been developed to standardize questionnaires, but generality for specific orthopedic procedures may result in less effective measures.
- PROMs can also be used for predictive modeling, which has the potential to help develop more cost-effective care and predict expected outcomes and recovery trajectories for individual patients.
Owing to their unique ability to recognize patients as stakeholders in their own healthcare, patient-reported outcome measures (PROMs) are becoming increasingly popular in the assessment of medical and surgical outcomes.1 PROMs are an outcome measures subset in which patients complete questionnaires about their perceptions of their overall health status and specific health limitations. By systematically using PROMs before and after a clearly defined episode of care, clinicians can collect data on perceived pain level, physical function, and psychological status and use the data to validate use of surgical procedures and shape clinical decisions about best practices.2-4 Although mortality and morbidity rates and other traditional measures are valuable in assessing outcomes, they do not represent or communicate the larger impact of an episode of care. As many orthopedic procedures are elective, and some are low-risk, the evaluation of changes in quality of life and self-reported functional improvement is an important addition to morbidity and mortality rates in capturing the true impact of a surgical procedure and recovery. The patient’s preoperative and postoperative perspectives on his or her health status have become important as well; our healthcare system has been placing more emphasis on patient-centered quality care.2,5
Although PROMs have many benefits, implementation in an orthopedic surgery practice has its challenges. With so many PROMs available, selecting those that fit the patient population for a specialized orthopedic surgery practice can be difficult. In addition, although PROM data are essential for research and for measuring individual or institutional recovery trajectories for surgical procedures, in a busy practice getting patients to provide these data can be difficult.
PROMs are heavily used for outcomes assessment in the orthopedics literature, but there are few resources for orthopedic surgeons who want to implement PROMs in their practices. In this article, we review the literature on the challenges of effectively implementing PROMs in an orthopedic surgery practice.
PROM Selection Considerations
PROMs can be categorized as either generic or disease-specific,4 but together they are used to adequately capture the impact, both broad and local, of an orthopedic condition.
Generic Outcome Measures
Generic outcome measures apply to a range of subspecialties or anatomical regions, allowing for evaluation of a patient’s overall health or quality of life. The most widely accepted measure of pain is the visual analog scale (VAS). The VAS for pain quantifies the level of pain a patient experiences at a given time on a graphic sliding scale from 0 (no pain) to 10 (worst possible pain). The VAS is used in clinical evaluation of pain and in reported outcomes literature.6,7
Many generic PROMs assess mental health status in addition to physical limitations. Poor preoperative mental health status has been recognized as a predictor of worse outcomes across a variety of orthopedic procedures.8,9 Therefore, to assess the overall influence of an orthopedic condition, it is important to include at least 1 generic PROM that assesses mental health status before and after an episode of care. Generic PROMs commonly used in orthopedic surgery include the 36-Item Short Form Health Survey (SF-36), the shorter SF-12, the Veterans RAND 12-Item Health Survey (VR-12), the World Health Organization Disability Assessment Schedule (WHODAS), the European Quality of Life-5 Dimensions (EQ-5D) index, and the 10-item Patient-Reported Outcomes Measurement Information System Global Health (PROMIS-10) scale.10-14
Some generic outcome measures (eg, the EQ-5D index) offer the “utility” calculation, which represents a preference for a patient’s desired health status. Such utilities allow for a measurement of quality of life, represented by quality-adjusted life years (QALY), which is a standardized measure of disease burden. Calculated QALY from measures such as the EQ-5D can be used in cost-effectiveness analyses of surgical interventions and have been used to validate use of procedures, particularly in arthroplasty.15-17
Disease-Specific Outcome Measures
Likewise, there is a range of disease-specific PROMs validated for use in orthopedic surgery, and providers select PROMs that fit their scope of practice. In anatomical regions such as the knee, hip, and shoulder, disease-specific outcome measures vary significantly by subspecialty and patient population. When selecting disease-specific PROMs, providers must consider tools such as reliability, validity, responsiveness, and available population norms. One study used Evaluating Measures of Patient-Reported Outcomes (EMPRO) to assess the quality of a PROM in shoulders and concluded that the American Shoulder and Elbow Surgeons (ASES) index, the Simple Shoulder Test (SST), and the Oxford Shoulder Score (OSS) were all supported for use in practice.18 It is important to note that reliability, validity, and responsiveness of a PROM may vary with the diagnosis or the patient population studied. For example, the SST was found to be responsive in assessing rotator cuff injury but not as useful in assessing shoulder instability or arthritis.19 Variable responsiveness highlights the need for a diagnosis-based level of PROM customization. For example, patients who undergo a surgical intervention for shoulder instability are given a customized survey, which includes PROMs specific to their condition, such as the Western Ontario Shoulder Instability (WOSI) index.20 For patients with knee instability, similar considerations apply; specific measures such as the Lysholm score and the Tenger Activity Scale capture the impact of injury in physically demanding activities.21 When selecting disease-specific PROMs, providers should consult articles like those by Davidson and Keating22 and Bent and colleagues,23 who present provider-friendly tools that can be used to examine the effectiveness of a PROM, and provide additional background information on selecting disease-specific PROMs. For hip and knee arthroplasty subspecialties, the International Society of Arthroplasty Registries (ISAR) created a working group that determines best practices for PROM collection and identifies PROMs most commonly reported in arthroplasty.24
Questionnaire Length Considerations
When PROMs are used in a practice, a balance must be struck between gathering enough information to determine functionality and limiting the patient burden of questionnaire length. A decision to use several PROMs all at once, at a single data collection point, can lengthen the questionnaire significantly. One study found that, with use of longer questionnaires, patients may lose interest, resulting in decreased reliability and compliance.25 For example, providers who use the long (42-item) Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire to assess knee function are often limited in what other PROMs they may administer at the same time. Efforts to shorten this questionnaire while still capturing necessary information led to the development of the 7-item KOOS Jr, which was validated for use in knee arthroplasty and had its 7 items drawn from the original 42.26 Similarly, the 40-item Hip Disability and Osteoarthritis Outcome Score (HOOS) questionnaire was shortened to the 6-item HOOS Jr, which was validated for use in hip arthroplasty,27 and the generic SF-36 was shortened to the SF-12.11 Providers trying to build an outcomes database while minimizing patient burden should consider using the shorter versions of these questionnaires but should also consider their validity, as KOOS Jr and HOOS Jr have been validated for use only in knee and hip arthroplasty and not in other knee and hip conditions.
PROM Data Collection Considerations
Comprehensive collection of longitudinal PROM data poses many challenges for providers and patients. For providers, the greatest challenges are infrastructure, technology, and the personnel needed to administer and store paper or electronic surveys. For patients, the most common survey completion barriers are questionnaire length, confusing or irrelevant content, and, in the case of some older adults, inability to complete surveys electronically.25
Identifying a nonresponsive or noncompliant patient population is an important issue in collecting PROM data for research or other purposes. A study of factors associated with higher nonresponse rates in elective surgery patients (N = 135,474) found that noncompliance was higher for males, patients under age 55 years, nonwhites, patients in the lowest socioeconomic quintile, patients living alone, patients needing assistance in completing questionnaires, and patients who previously underwent surgery for their condition.28 In a systematic review of methods that increased the response rates of postal and electronic surveys, Edwards and colleagues29 found significantly higher odds of response for patients who were prenotified of the survey, given shorter questionnaires, or given a deadline for survey completion. Of note, response rates were lower when the word survey was used in the subject line of an email.
PROM distribution has evolved with the rise of technological advances that allow for electronic survey distribution and data capture. Several studies have found that electronically administered PROMs have high response rates.3,30,31 In a study of patients who underwent total hip arthroplasty, Rolfson and colleagues32 found that response rates were significantly higher for those who were surveyed on paper than for those surveyed over the internet. A randomized controlled study found that, compared with paper surveys, digital tablet surveys effectively and reliably collected PROM data; in addition, digital tablets provided instant data storage, and improved survey completion by requiring that all questions be answered before the survey could be submitted.33 However, age, race/ethnicity, and income disparities in technology use must be considered when administering internet-based follow-up surveys and analyzing data collected with web-based methods.34 A study of total joint arthroplasty candidates found that several groups were less likely to complete electronic PROM questionnaires: patients over age 75 years, Hispanic or black patients, patients with Medicare or Medicaid, patients who previously underwent orthopedic surgery, patients undergoing revision total joint arthroplasty, patients with other comorbidities, and patients whose primary language was not English.35 Providers interested in implementing PROMs must consider their patient population when selecting a method for survey distribution and follow-up. A study found that a majority of PROMs were written at a level many patients may not have understood, because of their literacy level or age; this lack of understanding created a barrier to compliance in many patient populations.36
PROM Limitations and PROMIS Use
Use of PROMs has its limitations. The large variety of PROMs available for use in orthopedic surgery has led to several standardization initiatives. The National Institutes of Health funded the development of PROMIS, a person-centered measures database that evaluates and monitors the physical, social, and emotional health of adults and children.37 The goal of PROMIS is to develop a standardized method of selecting PROMs, so that all medical disciplines and subspecialties can choose an applicable set of questions from the PROMIS question bank and use it in practice. Orthopedic surgery can use questions pertaining to physical functioning of the lower and upper extremities as well as quality of life and mental health. PROMIS physical function questions have been validated for use in several areas of orthopedic surgery.38-40 A disadvantage of PROMIS is the overgenerality of its questions, which may not be as effective in capturing the implications of specific diagnoses. For example, it is difficult to use generalized questions to determine the implications of a diagnosis such as shoulder instability, which may affect only higher functioning activities or sports. More research on best PROM selection practices is needed in order to either standardize PROMs or move toward use of a single database such as PROMIS.
Future Directions in PROM Applications
PROMs are being used for research and patient engagement, but there are many other applications on the horizon. As already mentioned, predictive modeling is of particular interest. The existence of vast collaborative PROM databases that capture a diverse patient population introduces the possibility of creating models capable of predicting a patient outcome and enhancing shared decision-making.3 Predicting good or excellent patient outcomes for specific patient populations may allow elimination of certain postoperative visits, thereby creating more cost-effective care and reducing the burden of unnecessary clinic visits for both patients and physicians.
As with other healthcare areas, PROM data collection technology is rapidly advancing. Not only has electronic technology almost entirely replaced paper-and-pencil collection methods, but a new method of outcome data collection has been developed: computerized adaptive testing (CAT). CAT uses item-response theory to minimize the number of questions patients must answer in order for validated and reliable outcome scores to be calculated. According to multiple studies, CAT used across several questionnaires has reliably assessed PROMs while minimizing floor and ceiling effects, eliminating irrelevant questions, and shortening survey completion time.41-43
Besides becoming more patient-friendly and accessible across multiple interfaces (mobile devices and computers), PROMs are also beginning to be integrated into the electronic medical record, allowing easier access to information during chart reviews. Use of statistical and predictive modeling, as described by Chang,3 could give PROMs a role in clinical decision-making. Informing patients of their expected outcome and recovery trajectory—based on demographics, comorbidities, preoperative functional status, and other factors—could influence their decision to undergo surgical intervention. As Halawi and colleagues44 pointed out, it is important to discuss patient expectations before surgery, as unrealistic ones can negatively affect outcomes and lead to dissatisfaction. With clinicians having ready access to statistics and models in patient charts, we may see a transformation in clinical practices and surgical decision-making.
Conclusion
PROMs offer many ways to improve research and clinical care in orthopedic surgery. However, implementing PROMs in practice is not without challenges. Interested orthopedic surgeons should select the PROMs that are most appropriate—reliable, validated, and responsive to their patient population. Electronic distribution of PROM questionnaires is effective and allows data to be stored on entry, but orthopedic surgeons must consider their patient population to ensure accurate data capture and compliance in longitudinal surveys. Proper implementation of PROMs in a practice can allow clinicians to formulate expectations for postoperative recovery and set reasonable postoperative goals while engaging patients in improving quality of care.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Haywood KL. Patient-reported outcome I: measuring what matters in musculoskeletal care. Musculoskeletal Care. 2006;4(4):187-203.
3. Chang CH. Patient-reported outcomes measurement and management with innovative methodologies and technologies. Qual Life Res. 2007;16(suppl 1):157-166.
4. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
5. Porter ME. A strategy for health care reform—toward a value-based system. N Engl J Med. 2009;361(2):109-112.
6. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175-184.
7. de Nies F, Fidler MW. Visual analog scale for the assessment of total hip arthroplasty. J Arthroplasty. 1997;12(4):416-419.
8. Ayers DC, Franklin PD, Ring DC. The role of emotional health in functional outcomes after orthopaedic surgery: extending the biopsychosocial model to orthopaedics: AOA critical issues. J Bone Joint Surg Am. 2013;95(21):e165.
9. Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14(4):307-311.
10. Patel AA, Donegan D, Albert T. The 36-Item Short Form. J Am Acad Orthop Surg. 2007;15(2):126-134.
11. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
12. About the VR-36, VR-12 and VR-6D. Boston University School of Public Health website. http://www.bu.edu/sph/research/research-landing-page/vr-36-vr-12-and-vr-6d/. Accessed October 4, 2017.
13. Jansson KA, Granath F. Health-related quality of life (EQ-5D) before and after orthopedic surgery. Acta Orthop. 2011;82(1):82-89.
14. Oak SR, Strnad GJ, Bena J, et al. Responsiveness comparison of the EQ-5D, PROMIS Global Health, and VR-12 questionnaires in knee arthroscopy. Orthop J Sports Med. 2016;4(12):2325967116674714.
15. Lavernia CJ, Iacobelli DA, Brooks L, Villa JM. The cost-utility of total hip arthroplasty: earlier intervention, improved economics. J Arthroplasty. 2015;30(6):945-949.
16. Mather RC 3rd, Watters TS, Orlando LA, Bolognesi MP, Moorman CT 3rd. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(3):325-334.
17. Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87(6):1253-1259.
18. Schmidt S, Ferrer M, González M, et al; EMPRO Group. Evaluation of shoulder-specific patient-reported outcome measures: a systematic and standardized comparison of available evidence. J Shoulder Elbow Surg. 2014;23(3):434-444.
19. Godfrey J, Hamman R, Lowenstein S, Briggs K, Kocher M. Reliability, validity, and responsiveness of the Simple Shoulder Test: psychometric properties by age and injury type. J Shoulder Elbow Surg. 2007;16(3):260-267.
20. Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med. 1998;26(6):764-772.
21. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner Activity Scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897.
22. Davidson M, Keating J. Patient-reported outcome measures (PROMs): how should I interpret reports of measurement properties? A practical guide for clinicians and researchers who are not biostatisticians. Br J Sports Med. 2014;48(9):792-796.
23. Bent NP, Wright CC, Rushton AB, Batt ME. Selecting outcome measures in sports medicine: a guide for practitioners using the example of anterior cruciate ligament rehabilitation. Br J Sports Med. 2009;43(13):1006-1012.
24. Rolfson O, Eresian Chenok K, Bohm E, et al; Patient-Reported Outcome Measures Working Group of the International Society of Arthroplasty Registries. Patient-reported outcome measures in arthroplasty registries. Acta Orthop. 2016;87(suppl 1):3-8.
25. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(suppl 1):104-109.
26. Lyman S, Lee YY, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474(6):1461-1471.
27. Lyman S, Lee YY, Franklin PD, Li W, Mayman DJ, Padgett DE. Validation of the HOOS, JR: a short-form hip replacement survey. Clin Orthop Relat Res. 2016;474(6):1472-1482.
28. Hutchings A, Neuburger J, Grosse Frie K, Black N, van der Meulen J. Factors associated with non-response in routine use of patient reported outcome measures after elective surgery in England. Health Qual Life Outcomes. 2012;10:34.
29. Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009;(3):MR000008.
30. Gakhar H, McConnell B, Apostolopoulos AP, Lewis P. A pilot study investigating the use of at-home, web-based questionnaires compiling patient-reported outcome measures following total hip and knee replacement surgeries. J Long Term Eff Med Implants. 2013;23(1):39-43.
31. Bojcic JL, Sue VM, Huon TS, Maletis GB, Inacio MC. Comparison of paper and electronic surveys for measuring patient-reported outcomes after anterior cruciate ligament reconstruction. Perm J. 2014;18(3):22-26.
32. Rolfson O, Salomonsson R, Dahlberg LE, Garellick G. Internet-based follow-up questionnaire for measuring patient-reported outcome after total hip replacement surgery—reliability and response rate. Value Health. 2011;14(2):316-321.
33. Shah KN, Hofmann MR, Schwarzkopf R, et al. Patient-reported outcome measures: how do digital tablets stack up to paper forms? A randomized, controlled study. Am J Orthop. 2016;45(7):E451-E457.
34. Kaiser Family Foundation. The Digital Divide and Access to Health Information Online. http://kff.org/disparities-policy/poll-finding/the-digital-divide-and-access-to-health/. Published April 1, 2011. Accessed October 4, 2017.
35. Schamber EM, Takemoto SK, Chenok KE, Bozic KJ. Barriers to completion of patient reported outcome measures. J Arthroplasty. 2013;28(9):1449-1453.
36. El-Daly I, Ibraheim H, Rajakulendran K, Culpan P, Bates P. Are patient-reported outcome measures in orthopaedics easily read by patients? Clin Orthop Relat Res. 2016;474(1):246-255.
37. Intro to PROMIS. 2016. Health Measures website. http://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis. Accessed October 4, 2017.
38. Hung M, Baumhauer JF, Latt LD, Saltzman CL, SooHoo NF, Hunt KJ; National Orthopaedic Foot & Ankle Outcomes Research Network. Validation of PROMIS ® Physical Function computerized adaptive tests for orthopaedic foot and ankle outcome research. Clin Orthop Relat Res. 2013;471(11):3466-3474.
39. Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS Physical Function item bank in orthopaedic patients. J Orthop Res. 2011;29(6):947-953.
40. Tyser AR, Beckmann J, Franklin JD, et al. Evaluation of the PROMIS Physical Function computer adaptive test in the upper extremity. J Hand Surg Am. 2014;39(10):2047-2051.e4.
41. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS Physical Function item bank reduces test burden with less ceiling effects compared with the Short Musculoskeletal Function Assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443.
42. Hung M, Clegg DO, Greene T, Weir C, Saltzman CL. A lower extremity physical function computerized adaptive testing instrument for orthopaedic patients. Foot Ankle Int. 2012;33(4):326-335.
43. Döring AC, Nota SP, Hageman MG, Ring DC. Measurement of upper extremity disability using the Patient-Reported Outcomes Measurement Information System. J Hand Surg Am. 2014;39(6):1160-1165.
44. Halawi MJ, Greene K, Barsoum WK. Optimizing outcomes of total joint arthroplasty under the comprehensive care for joint replacement model. Am J Orthop. 2016;45(3):E112-E113.
Take-Home Points
- Systematic use of PROMs allows physicians to review data on pain, physical function, and psychological status to aid in clinical decision-making and best practices.
- PROMs should include both general outcome measures (VAS, SF-36, or EQ-5D) and reliable, valid, and responsive disease specific measures.
- PROM questionnaires should collect pertinent information while limiting the length to maximize patient compliance and reliability.
- PROMIS has been developed to standardize questionnaires, but generality for specific orthopedic procedures may result in less effective measures.
- PROMs can also be used for predictive modeling, which has the potential to help develop more cost-effective care and predict expected outcomes and recovery trajectories for individual patients.
Owing to their unique ability to recognize patients as stakeholders in their own healthcare, patient-reported outcome measures (PROMs) are becoming increasingly popular in the assessment of medical and surgical outcomes.1 PROMs are an outcome measures subset in which patients complete questionnaires about their perceptions of their overall health status and specific health limitations. By systematically using PROMs before and after a clearly defined episode of care, clinicians can collect data on perceived pain level, physical function, and psychological status and use the data to validate use of surgical procedures and shape clinical decisions about best practices.2-4 Although mortality and morbidity rates and other traditional measures are valuable in assessing outcomes, they do not represent or communicate the larger impact of an episode of care. As many orthopedic procedures are elective, and some are low-risk, the evaluation of changes in quality of life and self-reported functional improvement is an important addition to morbidity and mortality rates in capturing the true impact of a surgical procedure and recovery. The patient’s preoperative and postoperative perspectives on his or her health status have become important as well; our healthcare system has been placing more emphasis on patient-centered quality care.2,5
Although PROMs have many benefits, implementation in an orthopedic surgery practice has its challenges. With so many PROMs available, selecting those that fit the patient population for a specialized orthopedic surgery practice can be difficult. In addition, although PROM data are essential for research and for measuring individual or institutional recovery trajectories for surgical procedures, in a busy practice getting patients to provide these data can be difficult.
PROMs are heavily used for outcomes assessment in the orthopedics literature, but there are few resources for orthopedic surgeons who want to implement PROMs in their practices. In this article, we review the literature on the challenges of effectively implementing PROMs in an orthopedic surgery practice.
PROM Selection Considerations
PROMs can be categorized as either generic or disease-specific,4 but together they are used to adequately capture the impact, both broad and local, of an orthopedic condition.
Generic Outcome Measures
Generic outcome measures apply to a range of subspecialties or anatomical regions, allowing for evaluation of a patient’s overall health or quality of life. The most widely accepted measure of pain is the visual analog scale (VAS). The VAS for pain quantifies the level of pain a patient experiences at a given time on a graphic sliding scale from 0 (no pain) to 10 (worst possible pain). The VAS is used in clinical evaluation of pain and in reported outcomes literature.6,7
Many generic PROMs assess mental health status in addition to physical limitations. Poor preoperative mental health status has been recognized as a predictor of worse outcomes across a variety of orthopedic procedures.8,9 Therefore, to assess the overall influence of an orthopedic condition, it is important to include at least 1 generic PROM that assesses mental health status before and after an episode of care. Generic PROMs commonly used in orthopedic surgery include the 36-Item Short Form Health Survey (SF-36), the shorter SF-12, the Veterans RAND 12-Item Health Survey (VR-12), the World Health Organization Disability Assessment Schedule (WHODAS), the European Quality of Life-5 Dimensions (EQ-5D) index, and the 10-item Patient-Reported Outcomes Measurement Information System Global Health (PROMIS-10) scale.10-14
Some generic outcome measures (eg, the EQ-5D index) offer the “utility” calculation, which represents a preference for a patient’s desired health status. Such utilities allow for a measurement of quality of life, represented by quality-adjusted life years (QALY), which is a standardized measure of disease burden. Calculated QALY from measures such as the EQ-5D can be used in cost-effectiveness analyses of surgical interventions and have been used to validate use of procedures, particularly in arthroplasty.15-17
Disease-Specific Outcome Measures
Likewise, there is a range of disease-specific PROMs validated for use in orthopedic surgery, and providers select PROMs that fit their scope of practice. In anatomical regions such as the knee, hip, and shoulder, disease-specific outcome measures vary significantly by subspecialty and patient population. When selecting disease-specific PROMs, providers must consider tools such as reliability, validity, responsiveness, and available population norms. One study used Evaluating Measures of Patient-Reported Outcomes (EMPRO) to assess the quality of a PROM in shoulders and concluded that the American Shoulder and Elbow Surgeons (ASES) index, the Simple Shoulder Test (SST), and the Oxford Shoulder Score (OSS) were all supported for use in practice.18 It is important to note that reliability, validity, and responsiveness of a PROM may vary with the diagnosis or the patient population studied. For example, the SST was found to be responsive in assessing rotator cuff injury but not as useful in assessing shoulder instability or arthritis.19 Variable responsiveness highlights the need for a diagnosis-based level of PROM customization. For example, patients who undergo a surgical intervention for shoulder instability are given a customized survey, which includes PROMs specific to their condition, such as the Western Ontario Shoulder Instability (WOSI) index.20 For patients with knee instability, similar considerations apply; specific measures such as the Lysholm score and the Tenger Activity Scale capture the impact of injury in physically demanding activities.21 When selecting disease-specific PROMs, providers should consult articles like those by Davidson and Keating22 and Bent and colleagues,23 who present provider-friendly tools that can be used to examine the effectiveness of a PROM, and provide additional background information on selecting disease-specific PROMs. For hip and knee arthroplasty subspecialties, the International Society of Arthroplasty Registries (ISAR) created a working group that determines best practices for PROM collection and identifies PROMs most commonly reported in arthroplasty.24
Questionnaire Length Considerations
When PROMs are used in a practice, a balance must be struck between gathering enough information to determine functionality and limiting the patient burden of questionnaire length. A decision to use several PROMs all at once, at a single data collection point, can lengthen the questionnaire significantly. One study found that, with use of longer questionnaires, patients may lose interest, resulting in decreased reliability and compliance.25 For example, providers who use the long (42-item) Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire to assess knee function are often limited in what other PROMs they may administer at the same time. Efforts to shorten this questionnaire while still capturing necessary information led to the development of the 7-item KOOS Jr, which was validated for use in knee arthroplasty and had its 7 items drawn from the original 42.26 Similarly, the 40-item Hip Disability and Osteoarthritis Outcome Score (HOOS) questionnaire was shortened to the 6-item HOOS Jr, which was validated for use in hip arthroplasty,27 and the generic SF-36 was shortened to the SF-12.11 Providers trying to build an outcomes database while minimizing patient burden should consider using the shorter versions of these questionnaires but should also consider their validity, as KOOS Jr and HOOS Jr have been validated for use only in knee and hip arthroplasty and not in other knee and hip conditions.
PROM Data Collection Considerations
Comprehensive collection of longitudinal PROM data poses many challenges for providers and patients. For providers, the greatest challenges are infrastructure, technology, and the personnel needed to administer and store paper or electronic surveys. For patients, the most common survey completion barriers are questionnaire length, confusing or irrelevant content, and, in the case of some older adults, inability to complete surveys electronically.25
Identifying a nonresponsive or noncompliant patient population is an important issue in collecting PROM data for research or other purposes. A study of factors associated with higher nonresponse rates in elective surgery patients (N = 135,474) found that noncompliance was higher for males, patients under age 55 years, nonwhites, patients in the lowest socioeconomic quintile, patients living alone, patients needing assistance in completing questionnaires, and patients who previously underwent surgery for their condition.28 In a systematic review of methods that increased the response rates of postal and electronic surveys, Edwards and colleagues29 found significantly higher odds of response for patients who were prenotified of the survey, given shorter questionnaires, or given a deadline for survey completion. Of note, response rates were lower when the word survey was used in the subject line of an email.
PROM distribution has evolved with the rise of technological advances that allow for electronic survey distribution and data capture. Several studies have found that electronically administered PROMs have high response rates.3,30,31 In a study of patients who underwent total hip arthroplasty, Rolfson and colleagues32 found that response rates were significantly higher for those who were surveyed on paper than for those surveyed over the internet. A randomized controlled study found that, compared with paper surveys, digital tablet surveys effectively and reliably collected PROM data; in addition, digital tablets provided instant data storage, and improved survey completion by requiring that all questions be answered before the survey could be submitted.33 However, age, race/ethnicity, and income disparities in technology use must be considered when administering internet-based follow-up surveys and analyzing data collected with web-based methods.34 A study of total joint arthroplasty candidates found that several groups were less likely to complete electronic PROM questionnaires: patients over age 75 years, Hispanic or black patients, patients with Medicare or Medicaid, patients who previously underwent orthopedic surgery, patients undergoing revision total joint arthroplasty, patients with other comorbidities, and patients whose primary language was not English.35 Providers interested in implementing PROMs must consider their patient population when selecting a method for survey distribution and follow-up. A study found that a majority of PROMs were written at a level many patients may not have understood, because of their literacy level or age; this lack of understanding created a barrier to compliance in many patient populations.36
PROM Limitations and PROMIS Use
Use of PROMs has its limitations. The large variety of PROMs available for use in orthopedic surgery has led to several standardization initiatives. The National Institutes of Health funded the development of PROMIS, a person-centered measures database that evaluates and monitors the physical, social, and emotional health of adults and children.37 The goal of PROMIS is to develop a standardized method of selecting PROMs, so that all medical disciplines and subspecialties can choose an applicable set of questions from the PROMIS question bank and use it in practice. Orthopedic surgery can use questions pertaining to physical functioning of the lower and upper extremities as well as quality of life and mental health. PROMIS physical function questions have been validated for use in several areas of orthopedic surgery.38-40 A disadvantage of PROMIS is the overgenerality of its questions, which may not be as effective in capturing the implications of specific diagnoses. For example, it is difficult to use generalized questions to determine the implications of a diagnosis such as shoulder instability, which may affect only higher functioning activities or sports. More research on best PROM selection practices is needed in order to either standardize PROMs or move toward use of a single database such as PROMIS.
Future Directions in PROM Applications
PROMs are being used for research and patient engagement, but there are many other applications on the horizon. As already mentioned, predictive modeling is of particular interest. The existence of vast collaborative PROM databases that capture a diverse patient population introduces the possibility of creating models capable of predicting a patient outcome and enhancing shared decision-making.3 Predicting good or excellent patient outcomes for specific patient populations may allow elimination of certain postoperative visits, thereby creating more cost-effective care and reducing the burden of unnecessary clinic visits for both patients and physicians.
As with other healthcare areas, PROM data collection technology is rapidly advancing. Not only has electronic technology almost entirely replaced paper-and-pencil collection methods, but a new method of outcome data collection has been developed: computerized adaptive testing (CAT). CAT uses item-response theory to minimize the number of questions patients must answer in order for validated and reliable outcome scores to be calculated. According to multiple studies, CAT used across several questionnaires has reliably assessed PROMs while minimizing floor and ceiling effects, eliminating irrelevant questions, and shortening survey completion time.41-43
Besides becoming more patient-friendly and accessible across multiple interfaces (mobile devices and computers), PROMs are also beginning to be integrated into the electronic medical record, allowing easier access to information during chart reviews. Use of statistical and predictive modeling, as described by Chang,3 could give PROMs a role in clinical decision-making. Informing patients of their expected outcome and recovery trajectory—based on demographics, comorbidities, preoperative functional status, and other factors—could influence their decision to undergo surgical intervention. As Halawi and colleagues44 pointed out, it is important to discuss patient expectations before surgery, as unrealistic ones can negatively affect outcomes and lead to dissatisfaction. With clinicians having ready access to statistics and models in patient charts, we may see a transformation in clinical practices and surgical decision-making.
Conclusion
PROMs offer many ways to improve research and clinical care in orthopedic surgery. However, implementing PROMs in practice is not without challenges. Interested orthopedic surgeons should select the PROMs that are most appropriate—reliable, validated, and responsive to their patient population. Electronic distribution of PROM questionnaires is effective and allows data to be stored on entry, but orthopedic surgeons must consider their patient population to ensure accurate data capture and compliance in longitudinal surveys. Proper implementation of PROMs in a practice can allow clinicians to formulate expectations for postoperative recovery and set reasonable postoperative goals while engaging patients in improving quality of care.
Take-Home Points
- Systematic use of PROMs allows physicians to review data on pain, physical function, and psychological status to aid in clinical decision-making and best practices.
- PROMs should include both general outcome measures (VAS, SF-36, or EQ-5D) and reliable, valid, and responsive disease specific measures.
- PROM questionnaires should collect pertinent information while limiting the length to maximize patient compliance and reliability.
- PROMIS has been developed to standardize questionnaires, but generality for specific orthopedic procedures may result in less effective measures.
- PROMs can also be used for predictive modeling, which has the potential to help develop more cost-effective care and predict expected outcomes and recovery trajectories for individual patients.
Owing to their unique ability to recognize patients as stakeholders in their own healthcare, patient-reported outcome measures (PROMs) are becoming increasingly popular in the assessment of medical and surgical outcomes.1 PROMs are an outcome measures subset in which patients complete questionnaires about their perceptions of their overall health status and specific health limitations. By systematically using PROMs before and after a clearly defined episode of care, clinicians can collect data on perceived pain level, physical function, and psychological status and use the data to validate use of surgical procedures and shape clinical decisions about best practices.2-4 Although mortality and morbidity rates and other traditional measures are valuable in assessing outcomes, they do not represent or communicate the larger impact of an episode of care. As many orthopedic procedures are elective, and some are low-risk, the evaluation of changes in quality of life and self-reported functional improvement is an important addition to morbidity and mortality rates in capturing the true impact of a surgical procedure and recovery. The patient’s preoperative and postoperative perspectives on his or her health status have become important as well; our healthcare system has been placing more emphasis on patient-centered quality care.2,5
Although PROMs have many benefits, implementation in an orthopedic surgery practice has its challenges. With so many PROMs available, selecting those that fit the patient population for a specialized orthopedic surgery practice can be difficult. In addition, although PROM data are essential for research and for measuring individual or institutional recovery trajectories for surgical procedures, in a busy practice getting patients to provide these data can be difficult.
PROMs are heavily used for outcomes assessment in the orthopedics literature, but there are few resources for orthopedic surgeons who want to implement PROMs in their practices. In this article, we review the literature on the challenges of effectively implementing PROMs in an orthopedic surgery practice.
PROM Selection Considerations
PROMs can be categorized as either generic or disease-specific,4 but together they are used to adequately capture the impact, both broad and local, of an orthopedic condition.
Generic Outcome Measures
Generic outcome measures apply to a range of subspecialties or anatomical regions, allowing for evaluation of a patient’s overall health or quality of life. The most widely accepted measure of pain is the visual analog scale (VAS). The VAS for pain quantifies the level of pain a patient experiences at a given time on a graphic sliding scale from 0 (no pain) to 10 (worst possible pain). The VAS is used in clinical evaluation of pain and in reported outcomes literature.6,7
Many generic PROMs assess mental health status in addition to physical limitations. Poor preoperative mental health status has been recognized as a predictor of worse outcomes across a variety of orthopedic procedures.8,9 Therefore, to assess the overall influence of an orthopedic condition, it is important to include at least 1 generic PROM that assesses mental health status before and after an episode of care. Generic PROMs commonly used in orthopedic surgery include the 36-Item Short Form Health Survey (SF-36), the shorter SF-12, the Veterans RAND 12-Item Health Survey (VR-12), the World Health Organization Disability Assessment Schedule (WHODAS), the European Quality of Life-5 Dimensions (EQ-5D) index, and the 10-item Patient-Reported Outcomes Measurement Information System Global Health (PROMIS-10) scale.10-14
Some generic outcome measures (eg, the EQ-5D index) offer the “utility” calculation, which represents a preference for a patient’s desired health status. Such utilities allow for a measurement of quality of life, represented by quality-adjusted life years (QALY), which is a standardized measure of disease burden. Calculated QALY from measures such as the EQ-5D can be used in cost-effectiveness analyses of surgical interventions and have been used to validate use of procedures, particularly in arthroplasty.15-17
Disease-Specific Outcome Measures
Likewise, there is a range of disease-specific PROMs validated for use in orthopedic surgery, and providers select PROMs that fit their scope of practice. In anatomical regions such as the knee, hip, and shoulder, disease-specific outcome measures vary significantly by subspecialty and patient population. When selecting disease-specific PROMs, providers must consider tools such as reliability, validity, responsiveness, and available population norms. One study used Evaluating Measures of Patient-Reported Outcomes (EMPRO) to assess the quality of a PROM in shoulders and concluded that the American Shoulder and Elbow Surgeons (ASES) index, the Simple Shoulder Test (SST), and the Oxford Shoulder Score (OSS) were all supported for use in practice.18 It is important to note that reliability, validity, and responsiveness of a PROM may vary with the diagnosis or the patient population studied. For example, the SST was found to be responsive in assessing rotator cuff injury but not as useful in assessing shoulder instability or arthritis.19 Variable responsiveness highlights the need for a diagnosis-based level of PROM customization. For example, patients who undergo a surgical intervention for shoulder instability are given a customized survey, which includes PROMs specific to their condition, such as the Western Ontario Shoulder Instability (WOSI) index.20 For patients with knee instability, similar considerations apply; specific measures such as the Lysholm score and the Tenger Activity Scale capture the impact of injury in physically demanding activities.21 When selecting disease-specific PROMs, providers should consult articles like those by Davidson and Keating22 and Bent and colleagues,23 who present provider-friendly tools that can be used to examine the effectiveness of a PROM, and provide additional background information on selecting disease-specific PROMs. For hip and knee arthroplasty subspecialties, the International Society of Arthroplasty Registries (ISAR) created a working group that determines best practices for PROM collection and identifies PROMs most commonly reported in arthroplasty.24
Questionnaire Length Considerations
When PROMs are used in a practice, a balance must be struck between gathering enough information to determine functionality and limiting the patient burden of questionnaire length. A decision to use several PROMs all at once, at a single data collection point, can lengthen the questionnaire significantly. One study found that, with use of longer questionnaires, patients may lose interest, resulting in decreased reliability and compliance.25 For example, providers who use the long (42-item) Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire to assess knee function are often limited in what other PROMs they may administer at the same time. Efforts to shorten this questionnaire while still capturing necessary information led to the development of the 7-item KOOS Jr, which was validated for use in knee arthroplasty and had its 7 items drawn from the original 42.26 Similarly, the 40-item Hip Disability and Osteoarthritis Outcome Score (HOOS) questionnaire was shortened to the 6-item HOOS Jr, which was validated for use in hip arthroplasty,27 and the generic SF-36 was shortened to the SF-12.11 Providers trying to build an outcomes database while minimizing patient burden should consider using the shorter versions of these questionnaires but should also consider their validity, as KOOS Jr and HOOS Jr have been validated for use only in knee and hip arthroplasty and not in other knee and hip conditions.
PROM Data Collection Considerations
Comprehensive collection of longitudinal PROM data poses many challenges for providers and patients. For providers, the greatest challenges are infrastructure, technology, and the personnel needed to administer and store paper or electronic surveys. For patients, the most common survey completion barriers are questionnaire length, confusing or irrelevant content, and, in the case of some older adults, inability to complete surveys electronically.25
Identifying a nonresponsive or noncompliant patient population is an important issue in collecting PROM data for research or other purposes. A study of factors associated with higher nonresponse rates in elective surgery patients (N = 135,474) found that noncompliance was higher for males, patients under age 55 years, nonwhites, patients in the lowest socioeconomic quintile, patients living alone, patients needing assistance in completing questionnaires, and patients who previously underwent surgery for their condition.28 In a systematic review of methods that increased the response rates of postal and electronic surveys, Edwards and colleagues29 found significantly higher odds of response for patients who were prenotified of the survey, given shorter questionnaires, or given a deadline for survey completion. Of note, response rates were lower when the word survey was used in the subject line of an email.
PROM distribution has evolved with the rise of technological advances that allow for electronic survey distribution and data capture. Several studies have found that electronically administered PROMs have high response rates.3,30,31 In a study of patients who underwent total hip arthroplasty, Rolfson and colleagues32 found that response rates were significantly higher for those who were surveyed on paper than for those surveyed over the internet. A randomized controlled study found that, compared with paper surveys, digital tablet surveys effectively and reliably collected PROM data; in addition, digital tablets provided instant data storage, and improved survey completion by requiring that all questions be answered before the survey could be submitted.33 However, age, race/ethnicity, and income disparities in technology use must be considered when administering internet-based follow-up surveys and analyzing data collected with web-based methods.34 A study of total joint arthroplasty candidates found that several groups were less likely to complete electronic PROM questionnaires: patients over age 75 years, Hispanic or black patients, patients with Medicare or Medicaid, patients who previously underwent orthopedic surgery, patients undergoing revision total joint arthroplasty, patients with other comorbidities, and patients whose primary language was not English.35 Providers interested in implementing PROMs must consider their patient population when selecting a method for survey distribution and follow-up. A study found that a majority of PROMs were written at a level many patients may not have understood, because of their literacy level or age; this lack of understanding created a barrier to compliance in many patient populations.36
PROM Limitations and PROMIS Use
Use of PROMs has its limitations. The large variety of PROMs available for use in orthopedic surgery has led to several standardization initiatives. The National Institutes of Health funded the development of PROMIS, a person-centered measures database that evaluates and monitors the physical, social, and emotional health of adults and children.37 The goal of PROMIS is to develop a standardized method of selecting PROMs, so that all medical disciplines and subspecialties can choose an applicable set of questions from the PROMIS question bank and use it in practice. Orthopedic surgery can use questions pertaining to physical functioning of the lower and upper extremities as well as quality of life and mental health. PROMIS physical function questions have been validated for use in several areas of orthopedic surgery.38-40 A disadvantage of PROMIS is the overgenerality of its questions, which may not be as effective in capturing the implications of specific diagnoses. For example, it is difficult to use generalized questions to determine the implications of a diagnosis such as shoulder instability, which may affect only higher functioning activities or sports. More research on best PROM selection practices is needed in order to either standardize PROMs or move toward use of a single database such as PROMIS.
Future Directions in PROM Applications
PROMs are being used for research and patient engagement, but there are many other applications on the horizon. As already mentioned, predictive modeling is of particular interest. The existence of vast collaborative PROM databases that capture a diverse patient population introduces the possibility of creating models capable of predicting a patient outcome and enhancing shared decision-making.3 Predicting good or excellent patient outcomes for specific patient populations may allow elimination of certain postoperative visits, thereby creating more cost-effective care and reducing the burden of unnecessary clinic visits for both patients and physicians.
As with other healthcare areas, PROM data collection technology is rapidly advancing. Not only has electronic technology almost entirely replaced paper-and-pencil collection methods, but a new method of outcome data collection has been developed: computerized adaptive testing (CAT). CAT uses item-response theory to minimize the number of questions patients must answer in order for validated and reliable outcome scores to be calculated. According to multiple studies, CAT used across several questionnaires has reliably assessed PROMs while minimizing floor and ceiling effects, eliminating irrelevant questions, and shortening survey completion time.41-43
Besides becoming more patient-friendly and accessible across multiple interfaces (mobile devices and computers), PROMs are also beginning to be integrated into the electronic medical record, allowing easier access to information during chart reviews. Use of statistical and predictive modeling, as described by Chang,3 could give PROMs a role in clinical decision-making. Informing patients of their expected outcome and recovery trajectory—based on demographics, comorbidities, preoperative functional status, and other factors—could influence their decision to undergo surgical intervention. As Halawi and colleagues44 pointed out, it is important to discuss patient expectations before surgery, as unrealistic ones can negatively affect outcomes and lead to dissatisfaction. With clinicians having ready access to statistics and models in patient charts, we may see a transformation in clinical practices and surgical decision-making.
Conclusion
PROMs offer many ways to improve research and clinical care in orthopedic surgery. However, implementing PROMs in practice is not without challenges. Interested orthopedic surgeons should select the PROMs that are most appropriate—reliable, validated, and responsive to their patient population. Electronic distribution of PROM questionnaires is effective and allows data to be stored on entry, but orthopedic surgeons must consider their patient population to ensure accurate data capture and compliance in longitudinal surveys. Proper implementation of PROMs in a practice can allow clinicians to formulate expectations for postoperative recovery and set reasonable postoperative goals while engaging patients in improving quality of care.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Haywood KL. Patient-reported outcome I: measuring what matters in musculoskeletal care. Musculoskeletal Care. 2006;4(4):187-203.
3. Chang CH. Patient-reported outcomes measurement and management with innovative methodologies and technologies. Qual Life Res. 2007;16(suppl 1):157-166.
4. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
5. Porter ME. A strategy for health care reform—toward a value-based system. N Engl J Med. 2009;361(2):109-112.
6. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175-184.
7. de Nies F, Fidler MW. Visual analog scale for the assessment of total hip arthroplasty. J Arthroplasty. 1997;12(4):416-419.
8. Ayers DC, Franklin PD, Ring DC. The role of emotional health in functional outcomes after orthopaedic surgery: extending the biopsychosocial model to orthopaedics: AOA critical issues. J Bone Joint Surg Am. 2013;95(21):e165.
9. Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14(4):307-311.
10. Patel AA, Donegan D, Albert T. The 36-Item Short Form. J Am Acad Orthop Surg. 2007;15(2):126-134.
11. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
12. About the VR-36, VR-12 and VR-6D. Boston University School of Public Health website. http://www.bu.edu/sph/research/research-landing-page/vr-36-vr-12-and-vr-6d/. Accessed October 4, 2017.
13. Jansson KA, Granath F. Health-related quality of life (EQ-5D) before and after orthopedic surgery. Acta Orthop. 2011;82(1):82-89.
14. Oak SR, Strnad GJ, Bena J, et al. Responsiveness comparison of the EQ-5D, PROMIS Global Health, and VR-12 questionnaires in knee arthroscopy. Orthop J Sports Med. 2016;4(12):2325967116674714.
15. Lavernia CJ, Iacobelli DA, Brooks L, Villa JM. The cost-utility of total hip arthroplasty: earlier intervention, improved economics. J Arthroplasty. 2015;30(6):945-949.
16. Mather RC 3rd, Watters TS, Orlando LA, Bolognesi MP, Moorman CT 3rd. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(3):325-334.
17. Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87(6):1253-1259.
18. Schmidt S, Ferrer M, González M, et al; EMPRO Group. Evaluation of shoulder-specific patient-reported outcome measures: a systematic and standardized comparison of available evidence. J Shoulder Elbow Surg. 2014;23(3):434-444.
19. Godfrey J, Hamman R, Lowenstein S, Briggs K, Kocher M. Reliability, validity, and responsiveness of the Simple Shoulder Test: psychometric properties by age and injury type. J Shoulder Elbow Surg. 2007;16(3):260-267.
20. Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med. 1998;26(6):764-772.
21. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner Activity Scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897.
22. Davidson M, Keating J. Patient-reported outcome measures (PROMs): how should I interpret reports of measurement properties? A practical guide for clinicians and researchers who are not biostatisticians. Br J Sports Med. 2014;48(9):792-796.
23. Bent NP, Wright CC, Rushton AB, Batt ME. Selecting outcome measures in sports medicine: a guide for practitioners using the example of anterior cruciate ligament rehabilitation. Br J Sports Med. 2009;43(13):1006-1012.
24. Rolfson O, Eresian Chenok K, Bohm E, et al; Patient-Reported Outcome Measures Working Group of the International Society of Arthroplasty Registries. Patient-reported outcome measures in arthroplasty registries. Acta Orthop. 2016;87(suppl 1):3-8.
25. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(suppl 1):104-109.
26. Lyman S, Lee YY, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474(6):1461-1471.
27. Lyman S, Lee YY, Franklin PD, Li W, Mayman DJ, Padgett DE. Validation of the HOOS, JR: a short-form hip replacement survey. Clin Orthop Relat Res. 2016;474(6):1472-1482.
28. Hutchings A, Neuburger J, Grosse Frie K, Black N, van der Meulen J. Factors associated with non-response in routine use of patient reported outcome measures after elective surgery in England. Health Qual Life Outcomes. 2012;10:34.
29. Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009;(3):MR000008.
30. Gakhar H, McConnell B, Apostolopoulos AP, Lewis P. A pilot study investigating the use of at-home, web-based questionnaires compiling patient-reported outcome measures following total hip and knee replacement surgeries. J Long Term Eff Med Implants. 2013;23(1):39-43.
31. Bojcic JL, Sue VM, Huon TS, Maletis GB, Inacio MC. Comparison of paper and electronic surveys for measuring patient-reported outcomes after anterior cruciate ligament reconstruction. Perm J. 2014;18(3):22-26.
32. Rolfson O, Salomonsson R, Dahlberg LE, Garellick G. Internet-based follow-up questionnaire for measuring patient-reported outcome after total hip replacement surgery—reliability and response rate. Value Health. 2011;14(2):316-321.
33. Shah KN, Hofmann MR, Schwarzkopf R, et al. Patient-reported outcome measures: how do digital tablets stack up to paper forms? A randomized, controlled study. Am J Orthop. 2016;45(7):E451-E457.
34. Kaiser Family Foundation. The Digital Divide and Access to Health Information Online. http://kff.org/disparities-policy/poll-finding/the-digital-divide-and-access-to-health/. Published April 1, 2011. Accessed October 4, 2017.
35. Schamber EM, Takemoto SK, Chenok KE, Bozic KJ. Barriers to completion of patient reported outcome measures. J Arthroplasty. 2013;28(9):1449-1453.
36. El-Daly I, Ibraheim H, Rajakulendran K, Culpan P, Bates P. Are patient-reported outcome measures in orthopaedics easily read by patients? Clin Orthop Relat Res. 2016;474(1):246-255.
37. Intro to PROMIS. 2016. Health Measures website. http://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis. Accessed October 4, 2017.
38. Hung M, Baumhauer JF, Latt LD, Saltzman CL, SooHoo NF, Hunt KJ; National Orthopaedic Foot & Ankle Outcomes Research Network. Validation of PROMIS ® Physical Function computerized adaptive tests for orthopaedic foot and ankle outcome research. Clin Orthop Relat Res. 2013;471(11):3466-3474.
39. Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS Physical Function item bank in orthopaedic patients. J Orthop Res. 2011;29(6):947-953.
40. Tyser AR, Beckmann J, Franklin JD, et al. Evaluation of the PROMIS Physical Function computer adaptive test in the upper extremity. J Hand Surg Am. 2014;39(10):2047-2051.e4.
41. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS Physical Function item bank reduces test burden with less ceiling effects compared with the Short Musculoskeletal Function Assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443.
42. Hung M, Clegg DO, Greene T, Weir C, Saltzman CL. A lower extremity physical function computerized adaptive testing instrument for orthopaedic patients. Foot Ankle Int. 2012;33(4):326-335.
43. Döring AC, Nota SP, Hageman MG, Ring DC. Measurement of upper extremity disability using the Patient-Reported Outcomes Measurement Information System. J Hand Surg Am. 2014;39(6):1160-1165.
44. Halawi MJ, Greene K, Barsoum WK. Optimizing outcomes of total joint arthroplasty under the comprehensive care for joint replacement model. Am J Orthop. 2016;45(3):E112-E113.
1. Howie L, Hirsch B, Locklear T, Abernethy AP. Assessing the value of patient-generated data to comparative effectiveness research. Health Aff (Millwood). 2014;33(7):1220-1228.
2. Haywood KL. Patient-reported outcome I: measuring what matters in musculoskeletal care. Musculoskeletal Care. 2006;4(4):187-203.
3. Chang CH. Patient-reported outcomes measurement and management with innovative methodologies and technologies. Qual Life Res. 2007;16(suppl 1):157-166.
4. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167.
5. Porter ME. A strategy for health care reform—toward a value-based system. N Engl J Med. 2009;361(2):109-112.
6. Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2(2):175-184.
7. de Nies F, Fidler MW. Visual analog scale for the assessment of total hip arthroplasty. J Arthroplasty. 1997;12(4):416-419.
8. Ayers DC, Franklin PD, Ring DC. The role of emotional health in functional outcomes after orthopaedic surgery: extending the biopsychosocial model to orthopaedics: AOA critical issues. J Bone Joint Surg Am. 2013;95(21):e165.
9. Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14(4):307-311.
10. Patel AA, Donegan D, Albert T. The 36-Item Short Form. J Am Acad Orthop Surg. 2007;15(2):126-134.
11. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
12. About the VR-36, VR-12 and VR-6D. Boston University School of Public Health website. http://www.bu.edu/sph/research/research-landing-page/vr-36-vr-12-and-vr-6d/. Accessed October 4, 2017.
13. Jansson KA, Granath F. Health-related quality of life (EQ-5D) before and after orthopedic surgery. Acta Orthop. 2011;82(1):82-89.
14. Oak SR, Strnad GJ, Bena J, et al. Responsiveness comparison of the EQ-5D, PROMIS Global Health, and VR-12 questionnaires in knee arthroscopy. Orthop J Sports Med. 2016;4(12):2325967116674714.
15. Lavernia CJ, Iacobelli DA, Brooks L, Villa JM. The cost-utility of total hip arthroplasty: earlier intervention, improved economics. J Arthroplasty. 2015;30(6):945-949.
16. Mather RC 3rd, Watters TS, Orlando LA, Bolognesi MP, Moorman CT 3rd. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(3):325-334.
17. Brauer CA, Rosen AB, Olchanski NV, Neumann PJ. Cost-utility analyses in orthopaedic surgery. J Bone Joint Surg Am. 2005;87(6):1253-1259.
18. Schmidt S, Ferrer M, González M, et al; EMPRO Group. Evaluation of shoulder-specific patient-reported outcome measures: a systematic and standardized comparison of available evidence. J Shoulder Elbow Surg. 2014;23(3):434-444.
19. Godfrey J, Hamman R, Lowenstein S, Briggs K, Kocher M. Reliability, validity, and responsiveness of the Simple Shoulder Test: psychometric properties by age and injury type. J Shoulder Elbow Surg. 2007;16(3):260-267.
20. Kirkley A, Griffin S, McLintock H, Ng L. The development and evaluation of a disease-specific quality of life measurement tool for shoulder instability. The Western Ontario Shoulder Instability Index (WOSI). Am J Sports Med. 1998;26(6):764-772.
21. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm score and Tegner Activity Scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897.
22. Davidson M, Keating J. Patient-reported outcome measures (PROMs): how should I interpret reports of measurement properties? A practical guide for clinicians and researchers who are not biostatisticians. Br J Sports Med. 2014;48(9):792-796.
23. Bent NP, Wright CC, Rushton AB, Batt ME. Selecting outcome measures in sports medicine: a guide for practitioners using the example of anterior cruciate ligament rehabilitation. Br J Sports Med. 2009;43(13):1006-1012.
24. Rolfson O, Eresian Chenok K, Bohm E, et al; Patient-Reported Outcome Measures Working Group of the International Society of Arthroplasty Registries. Patient-reported outcome measures in arthroplasty registries. Acta Orthop. 2016;87(suppl 1):3-8.
25. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(suppl 1):104-109.
26. Lyman S, Lee YY, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474(6):1461-1471.
27. Lyman S, Lee YY, Franklin PD, Li W, Mayman DJ, Padgett DE. Validation of the HOOS, JR: a short-form hip replacement survey. Clin Orthop Relat Res. 2016;474(6):1472-1482.
28. Hutchings A, Neuburger J, Grosse Frie K, Black N, van der Meulen J. Factors associated with non-response in routine use of patient reported outcome measures after elective surgery in England. Health Qual Life Outcomes. 2012;10:34.
29. Edwards PJ, Roberts I, Clarke MJ, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev. 2009;(3):MR000008.
30. Gakhar H, McConnell B, Apostolopoulos AP, Lewis P. A pilot study investigating the use of at-home, web-based questionnaires compiling patient-reported outcome measures following total hip and knee replacement surgeries. J Long Term Eff Med Implants. 2013;23(1):39-43.
31. Bojcic JL, Sue VM, Huon TS, Maletis GB, Inacio MC. Comparison of paper and electronic surveys for measuring patient-reported outcomes after anterior cruciate ligament reconstruction. Perm J. 2014;18(3):22-26.
32. Rolfson O, Salomonsson R, Dahlberg LE, Garellick G. Internet-based follow-up questionnaire for measuring patient-reported outcome after total hip replacement surgery—reliability and response rate. Value Health. 2011;14(2):316-321.
33. Shah KN, Hofmann MR, Schwarzkopf R, et al. Patient-reported outcome measures: how do digital tablets stack up to paper forms? A randomized, controlled study. Am J Orthop. 2016;45(7):E451-E457.
34. Kaiser Family Foundation. The Digital Divide and Access to Health Information Online. http://kff.org/disparities-policy/poll-finding/the-digital-divide-and-access-to-health/. Published April 1, 2011. Accessed October 4, 2017.
35. Schamber EM, Takemoto SK, Chenok KE, Bozic KJ. Barriers to completion of patient reported outcome measures. J Arthroplasty. 2013;28(9):1449-1453.
36. El-Daly I, Ibraheim H, Rajakulendran K, Culpan P, Bates P. Are patient-reported outcome measures in orthopaedics easily read by patients? Clin Orthop Relat Res. 2016;474(1):246-255.
37. Intro to PROMIS. 2016. Health Measures website. http://www.healthmeasures.net/explore-measurement-systems/promis/intro-to-promis. Accessed October 4, 2017.
38. Hung M, Baumhauer JF, Latt LD, Saltzman CL, SooHoo NF, Hunt KJ; National Orthopaedic Foot & Ankle Outcomes Research Network. Validation of PROMIS ® Physical Function computerized adaptive tests for orthopaedic foot and ankle outcome research. Clin Orthop Relat Res. 2013;471(11):3466-3474.
39. Hung M, Clegg DO, Greene T, Saltzman CL. Evaluation of the PROMIS Physical Function item bank in orthopaedic patients. J Orthop Res. 2011;29(6):947-953.
40. Tyser AR, Beckmann J, Franklin JD, et al. Evaluation of the PROMIS Physical Function computer adaptive test in the upper extremity. J Hand Surg Am. 2014;39(10):2047-2051.e4.
41. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS Physical Function item bank reduces test burden with less ceiling effects compared with the Short Musculoskeletal Function Assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443.
42. Hung M, Clegg DO, Greene T, Weir C, Saltzman CL. A lower extremity physical function computerized adaptive testing instrument for orthopaedic patients. Foot Ankle Int. 2012;33(4):326-335.
43. Döring AC, Nota SP, Hageman MG, Ring DC. Measurement of upper extremity disability using the Patient-Reported Outcomes Measurement Information System. J Hand Surg Am. 2014;39(6):1160-1165.
44. Halawi MJ, Greene K, Barsoum WK. Optimizing outcomes of total joint arthroplasty under the comprehensive care for joint replacement model. Am J Orthop. 2016;45(3):E112-E113.
Superior Capsular Reconstruction: Clinical Outcomes After Minimum 2-Year Follow-Up
Take-Home Points
- The SCR is a viable treatment option for massive, irreparable RCTs.
- Arm position and exact measurement between anchors will help ensure proper graft tensioning.
- Anterior and posterior tension and margin convergence are critical to stabilizing the graft.
- Acromial-humeral distance, ASES, and VAS scores are improved and maintained over long-term follow-up.
- The dermal allograft should be 3.0 mm or thicker.
Conventional treatments for irreparable massive rotator cuff tears (RCTs) have ranged from nonoperative care to débridement and biceps tenotomy,1,2 partial cuff repair,3,4 bridging patch grafts,5 tendon transfers,6,7 and reverse total shoulder arthroplasty (RTSA).8,9 Superior capsular reconstruction (SCR), originally described by Mihata and colleagues,10 has been developed as an alternative to these interventions. Dr. Hirahara modified the technique to use dermal allograft instead of fascia lata autograft.10,11
Biomechanical analysis has confirmed the integral role of the superior capsule in shoulder function.10,12-14 In the presence of a massive RCT, the humeral head migrates superiorly, causing significant pain and functional deficits, such as pseudoparalysis. It is theorized that reestablishing this important stabilizer—centering the humeral head in the glenoid and allowing the larger muscles to move the arm about a proper fulcrum—improves function and decreases pain.
Using ultrasonography (US), radiography, magnetic resonance imaging (MRI), clinical outcome scores, and a visual analog scale (VAS) for pain, we prospectively evaluated minimum 2-year clinical outcomes of performing SCR with dermal allograft for irreparable RCTs.
Methods
Except where noted otherwise, all products mentioned in this section were made by Arthrex.
Surgical Technique
The surgical technique used here was described by Hirahara and Adams.11 ArthroFlex dermal allograft was attached to the greater tuberosity and the glenoid, creating a superior restraint that replaced the anatomical superior capsule (Figures 1A, 1B). Some cases included biceps tenotomy, subscapularis repair, or infraspinatus repair.
Medial fixation was obtained with a PASTA (partial articular supraspinatus tendon avulsion) bridge-type construct15 that consisted of two 3.0-mm BioComposite SutureTak anchors (placed medially on the glenoid rim, medial to the labrum) and a 3.5-mm BioComposite Vented SwiveLock. In some cases, a significant amount of tissue was present medially, and the third anchor was not used; instead, a double surgeon knot was used to fixate the double pulley medially.
Posterior margin convergence (PMC) was performed in all cases. Anterior margin convergence (AMC) was performed in only 3 cases.
Clinical Evaluation
All patients who underwent SCR were followed prospectively, and all signed an informed consent form. Between 2014 and the time of this study, 9 patients had surgery with a minimum 2-year follow-up. Before surgery, all patients received a diagnosis of full-thickness RCT with decreased acromial-humeral distance (AHD). One patient had RTSA 18 months after surgery, did not reach the 2-year follow-up, and was excluded from the data analysis. Patients were clinically evaluated on the 100-point American Shoulder and Elbow Surgeons (ASES) shoulder index and on a 10-point VAS for pain—before surgery, monthly for the first 6 months after surgery, then every 6 months until 2 years after surgery, and yearly thereafter. These patients were compared with Dr. Hirahara’s historical control patients, who had undergone repair of massive RCTs. Mean graft size was calculated and reported. Cases were separated and analyzed on the basis of whether AMC was performed. Student t tests were used to determine statistical differences between study patients’ preoperative and postoperative scores, between study and historical control patients, and between patients who had AMC performed and those who did not (P < .05).
Imaging
For all SCR patients, preoperative and postoperative radiographs were obtained in 2 planes: anterior-posterior with arm in neutral rotation, and scapular Y. On anteroposterior radiographs, AHD was measured from the most proximal aspect of the humeral head in a vertical line to the most inferior portion of the acromion (Figures 2A, 2B).
Results
The Table provides an overview of the study results. Eight patients (6 men, 2 women) met the final inclusion criteria for postoperative ASES and VAS data analysis.
AHD was measured on a standard anteroposterior radiograph in neutral rotation. The Hamada grading scale16 was used to classify the massive RCTs before and after surgery. Before surgery, 4 were grade 4A, 1 grade 3, 2 grade 2, and 1 grade 1; immediately after surgery, all were grade 1 (AHD, ≥6 mm). Two years after surgery, 1 patient had an AHD of 4.6 mm after a failure caused by a fall. Mean (SD) preoperative AHD was 4.50 (2.25) mm (range, 1.7-7.9 mm). Radiographs obtained immediately (mean, 1.22 months; range, 1 day-2.73 months) after surgery showed AHD was significantly (P < .0008) increased (mean, 8.48 mm; SD, 1.25 mm; range, 6.0-10.0 mm) (Figure 5).
Mean graft size was 2.9 mm medial × 3.6 mm lateral × 5.4 mm anterior × 5.4 mm posterior. Three patients had AMC performed. There was a significant (P < .05) difference in ASES scores between patients who had AMC performed (93) and those who did not (77).
Ultrasonography
Two weeks to 2 months after surgery, all patients had an intact capsular graft and no pulsatile vessels on US. Between 4 months and 10 months, US showed the construct intact laterally in all cases, a pulsatile vessel in the graft at the tuberosity (evidence of blood flow) in 4 of 5 cases, and a pulsatile vessel hypertrophied in 2 cases (Figures 6A, 6B).
Magnetic Resonance Imaging
Before surgery, 4 patients had Goutallier17 stage 4 rotator cuff muscle degeneration, 2 had stage 3 degeneration, and 2 had stage 2 degeneration. Throughout the follow-up period, US was as effective as MRI in determining graft integrity, graft thickness, and greater tuberosity fixation. Therefore, the SCRs were assessed primarily with US. MRI was ordered only if a failure was suspected or if the patient had some form of trauma. A total of 7 MRIs were ordered for 5 of the 8 patients in the study. The graft was intact in 4 of the 5 (Figures 7A-7C) and ruptured in the fifth.
Discussion
Mihata and colleagues10 published 2-year data for their reconstructive procedure with fascia lata autograft. In a modification of their procedure, Dr. Hirahara used dermal allograft to recreate the superior capsule.11 The results of the present 2-year study mirror the clinical outcomes reported by Mihata and colleagues10 and confirm that SCR improves functional outcomes and increases AHD regardless of graft type used.
The outcomes of the SCR patients in our study were significantly better than the outcomes of the historical control patients, who underwent repair of massive RCTs. Although there was no significant difference in the 2 groups’ ASES scores, the control patients had significantly higher postoperative VAS pain scores. We think that, as more patients undergo SCR and the population sample increases, we will see a significant difference in ASES scores as well (our SCR patients already showed a trend toward improved ASES scores).
Compared with RTSA, SCR has fewer risks and fewer complications and does not limit further surgical options.8,9,18 The 9 patients who had surgery with a minimum 2-year follow-up in our study had 4 complications. Six months after surgery, 1 patient fell and tore the infraspinatus and subscapularis muscles. Outcomes continued to improve, and no issues were reported, despite a decrease in AHD, from 8 mm immediately after surgery to 4.6 mm 2 years after surgery.
Two patients were in motor vehicle accidents. In 1 case, the accident occurred about 2 months after surgery. This patient also sustained a possible injury in a fall after receiving general anesthesia for a dental procedure. After having done very well the preceding months, the patient now reported increasing pain and dysfunction. MRI showed loss of glenoid fixation. Improved ASES and VAS pain scores were maintained throughout the follow-up period. AHD was increased at 13 months and mildly decreased at 2 years. Glenoid fixation was obtained with 2 anchors and a double surgeon knot. When possible, however, it is best to add an anchor and double-row fixation, as 3 anchors and a double-row construct are biomechanically stronger.19-24
The other motor vehicle accident occurred about 23 months after surgery. Two months later, a graft rupture was found on US and MRI, but the patient was maintaining full range of motion, AHD, and improved strength. The 1.5-mm graft in this patient was thinner than the 3.5-mm grafts in the rest of the study group. This was the only patient who developed a graft rupture rather than loss of fixation.
If only patients with graft thickness >3.0 mm are included in the data analysis, mean ASES score rises to 89.76, and mean VAS pain score drops to 0. Therefore, we argue against using a graft thinner than 3.5 mm. Our excellent study results indicate that larger grafts are unnecessary. Mihata and colleagues10 used fascia lata grafts of 6 mm to 8 mm. Ultimate load to failure is significantly higher for dermal allograft than for fascia lata graft.25 In SCR, the stronger dermal allograft withstands applied forces and repeated deformations and has excellent clinical outcomes.
Only 1 patient had a failure that required RTSA. VAS pain scores were lower and ASES scores were improved the first year after surgery, but then function deteriorated. The patient said there was no specific precipitating incident. Computed tomography arthrogram, ordered to assess the construct, showed anterior and superior subluxation of the humeral head, even with an intact subscapularis tendon—an indication of underlying instability, which most likely caused the failure. Eighteen months after surgery, the patient was able to undergo RTSA. On further evaluation of this patient’s procedure, it was determined that the graft needed better fixation anteriorly.
Mihata and colleagues10,12,14 indicated that AMC was unnecessary, and our procedure did not require it. However, data in our prospective evaluation began showing improved outcomes with AMC. As dermal allograft is more elastic than fascia lata autograft,25 we concluded that graft tensioning is key to the success of this procedure. Graft tension depends on many factors, including exact measurement of the distances between the anchors to punch holes in the graft, arm position to set the relationship between the anchor distances, and AMC and PMC. We recommend placing the arm in neutral rotation, neutral flexion, and abduction with the patient at rest, based on the size of the patient’s latissimus dorsi. Too much abduction causes overtensioning, and excess rotation or flexion-extension changes the distance between the glenoid and the greater tuberosity asymmetrically, from anterior to posterior. With the arm in neutral position, distances between anchors are accurately measured, and these measurements are used to determine graft size.
Graft tension is also needed to control the amount of elasticity allowed by the graft and thereby maintain stability, as shown by the Poisson ratio, the ratio of transverse contraction to longitudinal extension on a material in the presence of a stretching force. As applied to SCR, it is the ratio of mediolateral elasticity to anteroposterior deformation or constraint. If the graft is appropriately secured in the anteroposterior direction by way of ACM and PMC, elongation in the medial-lateral direction will be limited—reducing the elasticity of the graft, improving overall stability, and ultimately producing better clinical outcomes. This issue was discussed by Burkhart and colleagues26 with respect to the “rotator cable complex,” which now might be best described as the “rotator-capsule cable complex.” In our study, this phenomenon was evident in the finding that patients who had AMC performed did significantly better than patients who did not have AMC performed. The ability of dermal allograft to deform in these dimensions without failure while allowing excellent range of motion makes dermal allograft an exceptional choice for grafting during SCR. Mihata25 also found dermal allograft had a clear advantage in providing better range of motion, whereas fascia lata autograft resulted in a stiffer construct.
Dermal allograft can also incorporate into the body and transform into host tissue. The literature has described musculoskeletal US as an effective diagnostic and interventional tool.27-31 We used it to evaluate graft size, patency, and viability. As can be seen on US, the native rotator cuff does not have any pulsatile vessels and is fed by capillary flow. Dermal allograft has native vasculature built into the tissue. After 4 months to 8 months, presence of pulsatile vessels within the graft at the greater tuberosity indicates clear revascularization and incorporation of the tissue (Figure 6B). Disappearance of pulsatile vessels on US after 1 year indicates transformation to a stabilizing structure analogous to capsule or ligament with capillary flow. US also showed graft hypertrophy after 2 years, supporting a finding of integration and growth.
Conclusion
In the past, patients with irreparable massive RCTs had few good surgical management options, RTSA being the most definitive. SCR is technically challenging and requires use of specific implantation methods but can provide patients with outstanding relief. Our clinical data showed that technically well executed SCR effectively restores the superior restraints in the glenohumeral joint and thereby increases function and decreases pain in patients with irreparable massive RCTs, even after 2 years.
1 Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27(10):1341-1350.
2. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743-748.
3. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761-768.
4. Wellmann M, Lichtenberg S, da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthroscopy. 2013;29(8):1275-1282.
5. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911-1921.
6. Gavriilidis I, Kircher J, Mogasch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010;34(5):689-694.
7. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599-607.
8. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894-1908.
9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199-1208.
10. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
11. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637-e641.
12. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
13. Mihata T, McGarry MH, Ishihara Y, et al. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43(2):439-446.
14. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
15. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions [published online ahead of print September 18, 2017]. Arthrosc Tech. http://dx.doi.org/10.1016/j.eats.2017.06.022.
16. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460.
17. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468(6):1558-1564.
18. Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562-575.
19. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519-526.
20. Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon–bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1466-1472.
21. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
22. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
23. Pauly S, Fiebig D, Kieser B, Albrecht B, Schill A, Scheibel M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2090-2097.
24. Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26(10):1281-1288.
25. Mihata T. Superior capsule reconstruction using human dermal allograft: a biomechanical cadaveric study. Presentation at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 1-5, 2016; Orlando, FL.
26. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
27. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
28. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
29. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Accepted for publication.
30. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
31. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
Take-Home Points
- The SCR is a viable treatment option for massive, irreparable RCTs.
- Arm position and exact measurement between anchors will help ensure proper graft tensioning.
- Anterior and posterior tension and margin convergence are critical to stabilizing the graft.
- Acromial-humeral distance, ASES, and VAS scores are improved and maintained over long-term follow-up.
- The dermal allograft should be 3.0 mm or thicker.
Conventional treatments for irreparable massive rotator cuff tears (RCTs) have ranged from nonoperative care to débridement and biceps tenotomy,1,2 partial cuff repair,3,4 bridging patch grafts,5 tendon transfers,6,7 and reverse total shoulder arthroplasty (RTSA).8,9 Superior capsular reconstruction (SCR), originally described by Mihata and colleagues,10 has been developed as an alternative to these interventions. Dr. Hirahara modified the technique to use dermal allograft instead of fascia lata autograft.10,11
Biomechanical analysis has confirmed the integral role of the superior capsule in shoulder function.10,12-14 In the presence of a massive RCT, the humeral head migrates superiorly, causing significant pain and functional deficits, such as pseudoparalysis. It is theorized that reestablishing this important stabilizer—centering the humeral head in the glenoid and allowing the larger muscles to move the arm about a proper fulcrum—improves function and decreases pain.
Using ultrasonography (US), radiography, magnetic resonance imaging (MRI), clinical outcome scores, and a visual analog scale (VAS) for pain, we prospectively evaluated minimum 2-year clinical outcomes of performing SCR with dermal allograft for irreparable RCTs.
Methods
Except where noted otherwise, all products mentioned in this section were made by Arthrex.
Surgical Technique
The surgical technique used here was described by Hirahara and Adams.11 ArthroFlex dermal allograft was attached to the greater tuberosity and the glenoid, creating a superior restraint that replaced the anatomical superior capsule (Figures 1A, 1B). Some cases included biceps tenotomy, subscapularis repair, or infraspinatus repair.
Medial fixation was obtained with a PASTA (partial articular supraspinatus tendon avulsion) bridge-type construct15 that consisted of two 3.0-mm BioComposite SutureTak anchors (placed medially on the glenoid rim, medial to the labrum) and a 3.5-mm BioComposite Vented SwiveLock. In some cases, a significant amount of tissue was present medially, and the third anchor was not used; instead, a double surgeon knot was used to fixate the double pulley medially.
Posterior margin convergence (PMC) was performed in all cases. Anterior margin convergence (AMC) was performed in only 3 cases.
Clinical Evaluation
All patients who underwent SCR were followed prospectively, and all signed an informed consent form. Between 2014 and the time of this study, 9 patients had surgery with a minimum 2-year follow-up. Before surgery, all patients received a diagnosis of full-thickness RCT with decreased acromial-humeral distance (AHD). One patient had RTSA 18 months after surgery, did not reach the 2-year follow-up, and was excluded from the data analysis. Patients were clinically evaluated on the 100-point American Shoulder and Elbow Surgeons (ASES) shoulder index and on a 10-point VAS for pain—before surgery, monthly for the first 6 months after surgery, then every 6 months until 2 years after surgery, and yearly thereafter. These patients were compared with Dr. Hirahara’s historical control patients, who had undergone repair of massive RCTs. Mean graft size was calculated and reported. Cases were separated and analyzed on the basis of whether AMC was performed. Student t tests were used to determine statistical differences between study patients’ preoperative and postoperative scores, between study and historical control patients, and between patients who had AMC performed and those who did not (P < .05).
Imaging
For all SCR patients, preoperative and postoperative radiographs were obtained in 2 planes: anterior-posterior with arm in neutral rotation, and scapular Y. On anteroposterior radiographs, AHD was measured from the most proximal aspect of the humeral head in a vertical line to the most inferior portion of the acromion (Figures 2A, 2B).
Results
The Table provides an overview of the study results. Eight patients (6 men, 2 women) met the final inclusion criteria for postoperative ASES and VAS data analysis.
AHD was measured on a standard anteroposterior radiograph in neutral rotation. The Hamada grading scale16 was used to classify the massive RCTs before and after surgery. Before surgery, 4 were grade 4A, 1 grade 3, 2 grade 2, and 1 grade 1; immediately after surgery, all were grade 1 (AHD, ≥6 mm). Two years after surgery, 1 patient had an AHD of 4.6 mm after a failure caused by a fall. Mean (SD) preoperative AHD was 4.50 (2.25) mm (range, 1.7-7.9 mm). Radiographs obtained immediately (mean, 1.22 months; range, 1 day-2.73 months) after surgery showed AHD was significantly (P < .0008) increased (mean, 8.48 mm; SD, 1.25 mm; range, 6.0-10.0 mm) (Figure 5).
Mean graft size was 2.9 mm medial × 3.6 mm lateral × 5.4 mm anterior × 5.4 mm posterior. Three patients had AMC performed. There was a significant (P < .05) difference in ASES scores between patients who had AMC performed (93) and those who did not (77).
Ultrasonography
Two weeks to 2 months after surgery, all patients had an intact capsular graft and no pulsatile vessels on US. Between 4 months and 10 months, US showed the construct intact laterally in all cases, a pulsatile vessel in the graft at the tuberosity (evidence of blood flow) in 4 of 5 cases, and a pulsatile vessel hypertrophied in 2 cases (Figures 6A, 6B).
Magnetic Resonance Imaging
Before surgery, 4 patients had Goutallier17 stage 4 rotator cuff muscle degeneration, 2 had stage 3 degeneration, and 2 had stage 2 degeneration. Throughout the follow-up period, US was as effective as MRI in determining graft integrity, graft thickness, and greater tuberosity fixation. Therefore, the SCRs were assessed primarily with US. MRI was ordered only if a failure was suspected or if the patient had some form of trauma. A total of 7 MRIs were ordered for 5 of the 8 patients in the study. The graft was intact in 4 of the 5 (Figures 7A-7C) and ruptured in the fifth.
Discussion
Mihata and colleagues10 published 2-year data for their reconstructive procedure with fascia lata autograft. In a modification of their procedure, Dr. Hirahara used dermal allograft to recreate the superior capsule.11 The results of the present 2-year study mirror the clinical outcomes reported by Mihata and colleagues10 and confirm that SCR improves functional outcomes and increases AHD regardless of graft type used.
The outcomes of the SCR patients in our study were significantly better than the outcomes of the historical control patients, who underwent repair of massive RCTs. Although there was no significant difference in the 2 groups’ ASES scores, the control patients had significantly higher postoperative VAS pain scores. We think that, as more patients undergo SCR and the population sample increases, we will see a significant difference in ASES scores as well (our SCR patients already showed a trend toward improved ASES scores).
Compared with RTSA, SCR has fewer risks and fewer complications and does not limit further surgical options.8,9,18 The 9 patients who had surgery with a minimum 2-year follow-up in our study had 4 complications. Six months after surgery, 1 patient fell and tore the infraspinatus and subscapularis muscles. Outcomes continued to improve, and no issues were reported, despite a decrease in AHD, from 8 mm immediately after surgery to 4.6 mm 2 years after surgery.
Two patients were in motor vehicle accidents. In 1 case, the accident occurred about 2 months after surgery. This patient also sustained a possible injury in a fall after receiving general anesthesia for a dental procedure. After having done very well the preceding months, the patient now reported increasing pain and dysfunction. MRI showed loss of glenoid fixation. Improved ASES and VAS pain scores were maintained throughout the follow-up period. AHD was increased at 13 months and mildly decreased at 2 years. Glenoid fixation was obtained with 2 anchors and a double surgeon knot. When possible, however, it is best to add an anchor and double-row fixation, as 3 anchors and a double-row construct are biomechanically stronger.19-24
The other motor vehicle accident occurred about 23 months after surgery. Two months later, a graft rupture was found on US and MRI, but the patient was maintaining full range of motion, AHD, and improved strength. The 1.5-mm graft in this patient was thinner than the 3.5-mm grafts in the rest of the study group. This was the only patient who developed a graft rupture rather than loss of fixation.
If only patients with graft thickness >3.0 mm are included in the data analysis, mean ASES score rises to 89.76, and mean VAS pain score drops to 0. Therefore, we argue against using a graft thinner than 3.5 mm. Our excellent study results indicate that larger grafts are unnecessary. Mihata and colleagues10 used fascia lata grafts of 6 mm to 8 mm. Ultimate load to failure is significantly higher for dermal allograft than for fascia lata graft.25 In SCR, the stronger dermal allograft withstands applied forces and repeated deformations and has excellent clinical outcomes.
Only 1 patient had a failure that required RTSA. VAS pain scores were lower and ASES scores were improved the first year after surgery, but then function deteriorated. The patient said there was no specific precipitating incident. Computed tomography arthrogram, ordered to assess the construct, showed anterior and superior subluxation of the humeral head, even with an intact subscapularis tendon—an indication of underlying instability, which most likely caused the failure. Eighteen months after surgery, the patient was able to undergo RTSA. On further evaluation of this patient’s procedure, it was determined that the graft needed better fixation anteriorly.
Mihata and colleagues10,12,14 indicated that AMC was unnecessary, and our procedure did not require it. However, data in our prospective evaluation began showing improved outcomes with AMC. As dermal allograft is more elastic than fascia lata autograft,25 we concluded that graft tensioning is key to the success of this procedure. Graft tension depends on many factors, including exact measurement of the distances between the anchors to punch holes in the graft, arm position to set the relationship between the anchor distances, and AMC and PMC. We recommend placing the arm in neutral rotation, neutral flexion, and abduction with the patient at rest, based on the size of the patient’s latissimus dorsi. Too much abduction causes overtensioning, and excess rotation or flexion-extension changes the distance between the glenoid and the greater tuberosity asymmetrically, from anterior to posterior. With the arm in neutral position, distances between anchors are accurately measured, and these measurements are used to determine graft size.
Graft tension is also needed to control the amount of elasticity allowed by the graft and thereby maintain stability, as shown by the Poisson ratio, the ratio of transverse contraction to longitudinal extension on a material in the presence of a stretching force. As applied to SCR, it is the ratio of mediolateral elasticity to anteroposterior deformation or constraint. If the graft is appropriately secured in the anteroposterior direction by way of ACM and PMC, elongation in the medial-lateral direction will be limited—reducing the elasticity of the graft, improving overall stability, and ultimately producing better clinical outcomes. This issue was discussed by Burkhart and colleagues26 with respect to the “rotator cable complex,” which now might be best described as the “rotator-capsule cable complex.” In our study, this phenomenon was evident in the finding that patients who had AMC performed did significantly better than patients who did not have AMC performed. The ability of dermal allograft to deform in these dimensions without failure while allowing excellent range of motion makes dermal allograft an exceptional choice for grafting during SCR. Mihata25 also found dermal allograft had a clear advantage in providing better range of motion, whereas fascia lata autograft resulted in a stiffer construct.
Dermal allograft can also incorporate into the body and transform into host tissue. The literature has described musculoskeletal US as an effective diagnostic and interventional tool.27-31 We used it to evaluate graft size, patency, and viability. As can be seen on US, the native rotator cuff does not have any pulsatile vessels and is fed by capillary flow. Dermal allograft has native vasculature built into the tissue. After 4 months to 8 months, presence of pulsatile vessels within the graft at the greater tuberosity indicates clear revascularization and incorporation of the tissue (Figure 6B). Disappearance of pulsatile vessels on US after 1 year indicates transformation to a stabilizing structure analogous to capsule or ligament with capillary flow. US also showed graft hypertrophy after 2 years, supporting a finding of integration and growth.
Conclusion
In the past, patients with irreparable massive RCTs had few good surgical management options, RTSA being the most definitive. SCR is technically challenging and requires use of specific implantation methods but can provide patients with outstanding relief. Our clinical data showed that technically well executed SCR effectively restores the superior restraints in the glenohumeral joint and thereby increases function and decreases pain in patients with irreparable massive RCTs, even after 2 years.
Take-Home Points
- The SCR is a viable treatment option for massive, irreparable RCTs.
- Arm position and exact measurement between anchors will help ensure proper graft tensioning.
- Anterior and posterior tension and margin convergence are critical to stabilizing the graft.
- Acromial-humeral distance, ASES, and VAS scores are improved and maintained over long-term follow-up.
- The dermal allograft should be 3.0 mm or thicker.
Conventional treatments for irreparable massive rotator cuff tears (RCTs) have ranged from nonoperative care to débridement and biceps tenotomy,1,2 partial cuff repair,3,4 bridging patch grafts,5 tendon transfers,6,7 and reverse total shoulder arthroplasty (RTSA).8,9 Superior capsular reconstruction (SCR), originally described by Mihata and colleagues,10 has been developed as an alternative to these interventions. Dr. Hirahara modified the technique to use dermal allograft instead of fascia lata autograft.10,11
Biomechanical analysis has confirmed the integral role of the superior capsule in shoulder function.10,12-14 In the presence of a massive RCT, the humeral head migrates superiorly, causing significant pain and functional deficits, such as pseudoparalysis. It is theorized that reestablishing this important stabilizer—centering the humeral head in the glenoid and allowing the larger muscles to move the arm about a proper fulcrum—improves function and decreases pain.
Using ultrasonography (US), radiography, magnetic resonance imaging (MRI), clinical outcome scores, and a visual analog scale (VAS) for pain, we prospectively evaluated minimum 2-year clinical outcomes of performing SCR with dermal allograft for irreparable RCTs.
Methods
Except where noted otherwise, all products mentioned in this section were made by Arthrex.
Surgical Technique
The surgical technique used here was described by Hirahara and Adams.11 ArthroFlex dermal allograft was attached to the greater tuberosity and the glenoid, creating a superior restraint that replaced the anatomical superior capsule (Figures 1A, 1B). Some cases included biceps tenotomy, subscapularis repair, or infraspinatus repair.
Medial fixation was obtained with a PASTA (partial articular supraspinatus tendon avulsion) bridge-type construct15 that consisted of two 3.0-mm BioComposite SutureTak anchors (placed medially on the glenoid rim, medial to the labrum) and a 3.5-mm BioComposite Vented SwiveLock. In some cases, a significant amount of tissue was present medially, and the third anchor was not used; instead, a double surgeon knot was used to fixate the double pulley medially.
Posterior margin convergence (PMC) was performed in all cases. Anterior margin convergence (AMC) was performed in only 3 cases.
Clinical Evaluation
All patients who underwent SCR were followed prospectively, and all signed an informed consent form. Between 2014 and the time of this study, 9 patients had surgery with a minimum 2-year follow-up. Before surgery, all patients received a diagnosis of full-thickness RCT with decreased acromial-humeral distance (AHD). One patient had RTSA 18 months after surgery, did not reach the 2-year follow-up, and was excluded from the data analysis. Patients were clinically evaluated on the 100-point American Shoulder and Elbow Surgeons (ASES) shoulder index and on a 10-point VAS for pain—before surgery, monthly for the first 6 months after surgery, then every 6 months until 2 years after surgery, and yearly thereafter. These patients were compared with Dr. Hirahara’s historical control patients, who had undergone repair of massive RCTs. Mean graft size was calculated and reported. Cases were separated and analyzed on the basis of whether AMC was performed. Student t tests were used to determine statistical differences between study patients’ preoperative and postoperative scores, between study and historical control patients, and between patients who had AMC performed and those who did not (P < .05).
Imaging
For all SCR patients, preoperative and postoperative radiographs were obtained in 2 planes: anterior-posterior with arm in neutral rotation, and scapular Y. On anteroposterior radiographs, AHD was measured from the most proximal aspect of the humeral head in a vertical line to the most inferior portion of the acromion (Figures 2A, 2B).
Results
The Table provides an overview of the study results. Eight patients (6 men, 2 women) met the final inclusion criteria for postoperative ASES and VAS data analysis.
AHD was measured on a standard anteroposterior radiograph in neutral rotation. The Hamada grading scale16 was used to classify the massive RCTs before and after surgery. Before surgery, 4 were grade 4A, 1 grade 3, 2 grade 2, and 1 grade 1; immediately after surgery, all were grade 1 (AHD, ≥6 mm). Two years after surgery, 1 patient had an AHD of 4.6 mm after a failure caused by a fall. Mean (SD) preoperative AHD was 4.50 (2.25) mm (range, 1.7-7.9 mm). Radiographs obtained immediately (mean, 1.22 months; range, 1 day-2.73 months) after surgery showed AHD was significantly (P < .0008) increased (mean, 8.48 mm; SD, 1.25 mm; range, 6.0-10.0 mm) (Figure 5).
Mean graft size was 2.9 mm medial × 3.6 mm lateral × 5.4 mm anterior × 5.4 mm posterior. Three patients had AMC performed. There was a significant (P < .05) difference in ASES scores between patients who had AMC performed (93) and those who did not (77).
Ultrasonography
Two weeks to 2 months after surgery, all patients had an intact capsular graft and no pulsatile vessels on US. Between 4 months and 10 months, US showed the construct intact laterally in all cases, a pulsatile vessel in the graft at the tuberosity (evidence of blood flow) in 4 of 5 cases, and a pulsatile vessel hypertrophied in 2 cases (Figures 6A, 6B).
Magnetic Resonance Imaging
Before surgery, 4 patients had Goutallier17 stage 4 rotator cuff muscle degeneration, 2 had stage 3 degeneration, and 2 had stage 2 degeneration. Throughout the follow-up period, US was as effective as MRI in determining graft integrity, graft thickness, and greater tuberosity fixation. Therefore, the SCRs were assessed primarily with US. MRI was ordered only if a failure was suspected or if the patient had some form of trauma. A total of 7 MRIs were ordered for 5 of the 8 patients in the study. The graft was intact in 4 of the 5 (Figures 7A-7C) and ruptured in the fifth.
Discussion
Mihata and colleagues10 published 2-year data for their reconstructive procedure with fascia lata autograft. In a modification of their procedure, Dr. Hirahara used dermal allograft to recreate the superior capsule.11 The results of the present 2-year study mirror the clinical outcomes reported by Mihata and colleagues10 and confirm that SCR improves functional outcomes and increases AHD regardless of graft type used.
The outcomes of the SCR patients in our study were significantly better than the outcomes of the historical control patients, who underwent repair of massive RCTs. Although there was no significant difference in the 2 groups’ ASES scores, the control patients had significantly higher postoperative VAS pain scores. We think that, as more patients undergo SCR and the population sample increases, we will see a significant difference in ASES scores as well (our SCR patients already showed a trend toward improved ASES scores).
Compared with RTSA, SCR has fewer risks and fewer complications and does not limit further surgical options.8,9,18 The 9 patients who had surgery with a minimum 2-year follow-up in our study had 4 complications. Six months after surgery, 1 patient fell and tore the infraspinatus and subscapularis muscles. Outcomes continued to improve, and no issues were reported, despite a decrease in AHD, from 8 mm immediately after surgery to 4.6 mm 2 years after surgery.
Two patients were in motor vehicle accidents. In 1 case, the accident occurred about 2 months after surgery. This patient also sustained a possible injury in a fall after receiving general anesthesia for a dental procedure. After having done very well the preceding months, the patient now reported increasing pain and dysfunction. MRI showed loss of glenoid fixation. Improved ASES and VAS pain scores were maintained throughout the follow-up period. AHD was increased at 13 months and mildly decreased at 2 years. Glenoid fixation was obtained with 2 anchors and a double surgeon knot. When possible, however, it is best to add an anchor and double-row fixation, as 3 anchors and a double-row construct are biomechanically stronger.19-24
The other motor vehicle accident occurred about 23 months after surgery. Two months later, a graft rupture was found on US and MRI, but the patient was maintaining full range of motion, AHD, and improved strength. The 1.5-mm graft in this patient was thinner than the 3.5-mm grafts in the rest of the study group. This was the only patient who developed a graft rupture rather than loss of fixation.
If only patients with graft thickness >3.0 mm are included in the data analysis, mean ASES score rises to 89.76, and mean VAS pain score drops to 0. Therefore, we argue against using a graft thinner than 3.5 mm. Our excellent study results indicate that larger grafts are unnecessary. Mihata and colleagues10 used fascia lata grafts of 6 mm to 8 mm. Ultimate load to failure is significantly higher for dermal allograft than for fascia lata graft.25 In SCR, the stronger dermal allograft withstands applied forces and repeated deformations and has excellent clinical outcomes.
Only 1 patient had a failure that required RTSA. VAS pain scores were lower and ASES scores were improved the first year after surgery, but then function deteriorated. The patient said there was no specific precipitating incident. Computed tomography arthrogram, ordered to assess the construct, showed anterior and superior subluxation of the humeral head, even with an intact subscapularis tendon—an indication of underlying instability, which most likely caused the failure. Eighteen months after surgery, the patient was able to undergo RTSA. On further evaluation of this patient’s procedure, it was determined that the graft needed better fixation anteriorly.
Mihata and colleagues10,12,14 indicated that AMC was unnecessary, and our procedure did not require it. However, data in our prospective evaluation began showing improved outcomes with AMC. As dermal allograft is more elastic than fascia lata autograft,25 we concluded that graft tensioning is key to the success of this procedure. Graft tension depends on many factors, including exact measurement of the distances between the anchors to punch holes in the graft, arm position to set the relationship between the anchor distances, and AMC and PMC. We recommend placing the arm in neutral rotation, neutral flexion, and abduction with the patient at rest, based on the size of the patient’s latissimus dorsi. Too much abduction causes overtensioning, and excess rotation or flexion-extension changes the distance between the glenoid and the greater tuberosity asymmetrically, from anterior to posterior. With the arm in neutral position, distances between anchors are accurately measured, and these measurements are used to determine graft size.
Graft tension is also needed to control the amount of elasticity allowed by the graft and thereby maintain stability, as shown by the Poisson ratio, the ratio of transverse contraction to longitudinal extension on a material in the presence of a stretching force. As applied to SCR, it is the ratio of mediolateral elasticity to anteroposterior deformation or constraint. If the graft is appropriately secured in the anteroposterior direction by way of ACM and PMC, elongation in the medial-lateral direction will be limited—reducing the elasticity of the graft, improving overall stability, and ultimately producing better clinical outcomes. This issue was discussed by Burkhart and colleagues26 with respect to the “rotator cable complex,” which now might be best described as the “rotator-capsule cable complex.” In our study, this phenomenon was evident in the finding that patients who had AMC performed did significantly better than patients who did not have AMC performed. The ability of dermal allograft to deform in these dimensions without failure while allowing excellent range of motion makes dermal allograft an exceptional choice for grafting during SCR. Mihata25 also found dermal allograft had a clear advantage in providing better range of motion, whereas fascia lata autograft resulted in a stiffer construct.
Dermal allograft can also incorporate into the body and transform into host tissue. The literature has described musculoskeletal US as an effective diagnostic and interventional tool.27-31 We used it to evaluate graft size, patency, and viability. As can be seen on US, the native rotator cuff does not have any pulsatile vessels and is fed by capillary flow. Dermal allograft has native vasculature built into the tissue. After 4 months to 8 months, presence of pulsatile vessels within the graft at the greater tuberosity indicates clear revascularization and incorporation of the tissue (Figure 6B). Disappearance of pulsatile vessels on US after 1 year indicates transformation to a stabilizing structure analogous to capsule or ligament with capillary flow. US also showed graft hypertrophy after 2 years, supporting a finding of integration and growth.
Conclusion
In the past, patients with irreparable massive RCTs had few good surgical management options, RTSA being the most definitive. SCR is technically challenging and requires use of specific implantation methods but can provide patients with outstanding relief. Our clinical data showed that technically well executed SCR effectively restores the superior restraints in the glenohumeral joint and thereby increases function and decreases pain in patients with irreparable massive RCTs, even after 2 years.
1 Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27(10):1341-1350.
2. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743-748.
3. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761-768.
4. Wellmann M, Lichtenberg S, da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthroscopy. 2013;29(8):1275-1282.
5. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911-1921.
6. Gavriilidis I, Kircher J, Mogasch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010;34(5):689-694.
7. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599-607.
8. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894-1908.
9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199-1208.
10. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
11. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637-e641.
12. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
13. Mihata T, McGarry MH, Ishihara Y, et al. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43(2):439-446.
14. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
15. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions [published online ahead of print September 18, 2017]. Arthrosc Tech. http://dx.doi.org/10.1016/j.eats.2017.06.022.
16. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460.
17. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468(6):1558-1564.
18. Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562-575.
19. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519-526.
20. Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon–bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1466-1472.
21. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
22. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
23. Pauly S, Fiebig D, Kieser B, Albrecht B, Schill A, Scheibel M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2090-2097.
24. Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26(10):1281-1288.
25. Mihata T. Superior capsule reconstruction using human dermal allograft: a biomechanical cadaveric study. Presentation at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 1-5, 2016; Orlando, FL.
26. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
27. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
28. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
29. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Accepted for publication.
30. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
31. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
1 Lee BG, Cho NS, Rhee YG. Results of arthroscopic decompression and tuberoplasty for irreparable massive rotator cuff tears. Arthroscopy. 2011;27(10):1341-1350.
2. Liem D, Lengers N, Dedy N, Poetzl W, Steinbeck J, Marquardt B. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24(7):743-748.
3. Kim SJ, Lee IS, Kim SH, Lee WY, Chun YM. Arthroscopic partial repair of irreparable large to massive rotator cuff tears. Arthroscopy. 2012;28(6):761-768.
4. Wellmann M, Lichtenberg S, da Silva G, Magosch P, Habermeyer P. Results of arthroscopic partial repair of large retracted rotator cuff tears. Arthroscopy. 2013;29(8):1275-1282.
5. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy. 2013;29(12):1911-1921.
6. Gavriilidis I, Kircher J, Mogasch P, Lichtenberg S, Habermeyer P. Pectoralis major transfer for the treatment of irreparable anterosuperior rotator cuff tears. Int Orthop. 2010;34(5):689-694.
7. Grimberg J, Kany J, Valenti P, Amaravathi R, Ramalingam AT. Arthroscopic-assisted latissimus dorsi tendon transfer for irreparable posterosuperior cuff tears. Arthroscopy. 2015;31(4):599-607.
8. Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92(9):1894-1908.
9. Ek ET, Neukom L, Catanzaro S, Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22(9):1199-1208.
10. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29(3):459-470.
11. Hirahara AM, Adams CR. Arthroscopic superior capsular reconstruction for treatment of massive irreparable rotator cuff tears. Arthrosc Tech. 2015;4(6):e637-e641.
12. Mihata T, McGarry MH, Kahn T, Goldberg I, Neo M, Lee TQ. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med. 2016;44(6):1423-1430.
13. Mihata T, McGarry MH, Ishihara Y, et al. Biomechanical analysis of articular-sided partial-thickness rotator cuff tear and repair. Am J Sports Med. 2015;43(2):439-446.
14. Mihata T, McGarry MH, Pirolo JM, Kinoshita M, Lee TQ. Superior capsule reconstruction to restore superior stability in irreparable rotator cuff tears: a biomechanical cadaveric study. Am J Sports Med. 2012;40(10):2248-2255.
15. Hirahara AM, Andersen WJ. The PASTA bridge: a technique for the arthroscopic repair of PASTA lesions [published online ahead of print September 18, 2017]. Arthrosc Tech. http://dx.doi.org/10.1016/j.eats.2017.06.022.
16. Hamada K, Yamanaka K, Uchiyama Y, Mikasa T, Mikasa M. A radiographic classification of massive rotator cuff tear arthritis. Clin Orthop Relat Res. 2011;469(9):2452-2460.
17. Oh JH, Kim SH, Choi JA, Kim Y, Oh CH. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2010;468(6):1558-1564.
18. Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. J Bone Joint Surg Br. 2006;88(5):562-575.
19. Apreleva M, Özbaydar M, Fitzgibbons PG, Warner JJ. Rotator cuff tears: the effect of the reconstruction method on three-dimensional repair site area. Arthroscopy. 2002;18(5):519-526.
20. Baums MH, Spahn G, Steckel H, Fischer A, Schultz W, Klinger HM. Comparative evaluation of the tendon–bone interface contact pressure in different single- versus double-row suture anchor repair techniques. Knee Surg Sports Traumatol Arthrosc. 2009;17(12):1466-1472.
21. Lo IK, Burkhart SS. Double-row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
22. Mazzocca AD, Millett PJ, Guanche CA, Santangelo SA, Arciero RA. Arthroscopic single-row versus double-row suture anchor rotator cuff repair. Am J Sports Med. 2005;33(12):1861-1868.
23. Pauly S, Fiebig D, Kieser B, Albrecht B, Schill A, Scheibel M. Biomechanical comparison of four double-row speed-bridging rotator cuff repair techniques with or without medial or lateral row enhancement. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2090-2097.
24. Pauly S, Kieser B, Schill A, Gerhardt C, Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26(10):1281-1288.
25. Mihata T. Superior capsule reconstruction using human dermal allograft: a biomechanical cadaveric study. Presentation at: Annual Meeting of the American Academy of Orthopaedic Surgeons; March 1-5, 2016; Orlando, FL.
26. Burkhart SS, Esch JC, Jolson RS. The rotator crescent and rotator cable: an anatomic description of the shoulder’s “suspension bridge.” Arthroscopy. 1993;9(6):611-616.
27. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous reconstruction of the anterolateral ligament: surgical technique and case report. Am J Orthop. 2016;45(7):418-422, 460.
28. Hirahara AM, Andersen WJ. Ultrasound-guided percutaneous repair of medial patellofemoral ligament: surgical technique and outcomes. Am J Orthop. 2017;46(3):152-157.
29. Hirahara AM, Mackay G, Andersen WJ. Ultrasound-guided InternalBrace of the medial collateral ligament. Arthrosc Tech. Accepted for publication.
30. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 3: interventional and procedural uses. Am J Orthop. 2016;45(7):440-445.
31. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016;45(4):233-238.
Boldly Going (Where No Journal Has Gone Before)
On a recent visit to my daughter’s school, I caught sight of a set of encyclopedias on the shelf. It brought me back to the days where I would open my own set to find out the information I needed to write reports for school. But my sense of nostalgia was short lived as I thought about all of the limitations of the format. If it wasn’t in the encyclopedias, I couldn’t write the report and would need to head to the library. The Internet changed all of that. Now, when I want to know something I don’t look it up in a book anymore. I ask Siri or Alexa or head to the Google home page. When one of my kids asks me a question I can’t answer, like how a tornado forms, I take out my phone and search for the answer on the Internet.
When it comes to medical information, I can’t remember the last time I opened up a journal sitting on my shelf and leafed through the contents to identify the article I needed. I simply go online and search PubMed or download the article from the AJO website. My office is no longer filled with volumes of journals, and I need only my phone to research whatever topic I’m interested in.
The way I prefer to prepare for cases has changed as well. In the past I would simply open a book or technique article and read about the best way to perform the case. Now, I prefer to watch a video or download the technique guide. I find it easier and faster than reading a book chapter or article.
When we began to change the format of the journal, we stated that AJO would be filled with practical information that would be directly impactful to your practice. That’s the number one criteria we utilize when evaluating content. We wanted to make AJO the journal you wanted to read, because it would improve your knowledge, your outcomes, and your bottom line. We have made many changes to AJO in the last 2 years of print issues. But to truly provide the experience our readers demand and deserve, we have to take a huge next step. Right now we are limited by page and word counts, printed media, and advertising pages. We receive hundreds of submissions a month, yet can only print a fraction of the great material we receive.
If you’ve been following the journal for the last 24 months, you’ve noticed that we have been testing the limits of printed media. We’ve included QR codes for videos, companion PDFs, patient information sheets, and downloadable reports to incorporate into your practice.
The way we access the journal is also changing. We’ve looked closely at our web statistics since the redesign. Our website visits have gone up by a factor of 6 with nearly half of our website traffic coming from mobile usage. It became clear that the days of the printed journal are slowly coming to an end. Surgeons don’t have time to read the journal cover to cover, and now most of our traffic comes from our eBlasts. Surgeons find an article that catches their eye and click a link to find out more. We’ve dramatically increased our eBlasts, and our website volume has been increasing exponentially.
While these small steps have been met with great success, it’s now time to make a giant leap. But unlike most journals, where the online version is just an electronic copy of the printed book, we wanted to make the new AJO something vastly different. We wanted to change the way surgeons utilized a journal and interacted with it on a daily basis. We wanted to be the electronic companion to your practice; a trusted, media rich, peer-reviewed source where you and your patients can turn to for the practical day-to-day information you can use to improve your practice.
We’ve built it, and now I’m proud to unveil it. Beginning January 1, AJO will be published exclusively online. All articles will still be PubMed cited, but will contain more photos, videos, handouts and all the information you need to replicate the findings or procedures in your practice. For example, new surgical techniques will be published with the presenting surgeon’s preference cards, rehab protocols, surgical video, and a PowerPoint presentation that can be presented to referral sources or prospective patients.
New features on our web portal will include:
An orthopedic product guide: A database organized by pathology which contains all of the relevant orthopedic products that could be used for treatment. Relevant products will be cross-referenced to articles so you can quickly identify and order equipment for new cases.
Smart article selection: You can filter the articles that match your interests and have them delivered directly to your inbox. For example, foot and ankle surgeons will no longer need to sift through hundreds of pages to find articles relevant to their practice.
A coding and billing section: Discuss and share tips and tricks with your peers and ask questions of the experts. Regular articles will present relevant codes and how to use them appropriately to get the reimbursement you deserve for your services.
Practice management and business strategies: Get advice from, and interact with, the experts in all areas of your practice.
Ask the experts: Present your cases to our editorial board and enjoy a written, peer-reviewed response. Discuss cases and mutual challenges in communities organized by subspecialty and sport. Cover a high school football team? Imagine a place where you can present your football-related injury to the world’s best football doctors and have them review and comment on the case.
These are just some of the changes you will see in the coming months. We will continuously work to improve and welcome your future suggestions as to how we can provide a truly valuable, customized journal.
Looking to the future, it is my opinion that patient-reported outcome scores will be a large part of what we do. By presenting our successful outcomes, we will ultimately justify the procedures which we perform and justify the reimbursement to third party payers. In this issue, we examine the concept of patient-reported outcome measures (PROMs), and how and why to apply them to your practice.
In our lead article, Elizabeth Matzkin and colleagues present a guideline for implementing PROMs in your practice. Patrick Smith and Corey Cook provide a review of available electronic databases, and Patrick Denard and colleagues present data obtained through an electronic PROM database to settle the question “Is knotless labral repair better than conventional anchors in the shoulder?” Alan Hirahara and colleagues present their 2-year data on superior capsular Reconstruction, and Roland Biedert and Philippe Tscholl discuss the management of patella alta.
By now you’ve realized you’re holding the last printed issue of AJO. Enjoy a moment of nostalgia for the old days, and then buckle your seatbelt. We’re taking AJO where no other journal has gone before and it’s going to be one heck of a ride.
On a recent visit to my daughter’s school, I caught sight of a set of encyclopedias on the shelf. It brought me back to the days where I would open my own set to find out the information I needed to write reports for school. But my sense of nostalgia was short lived as I thought about all of the limitations of the format. If it wasn’t in the encyclopedias, I couldn’t write the report and would need to head to the library. The Internet changed all of that. Now, when I want to know something I don’t look it up in a book anymore. I ask Siri or Alexa or head to the Google home page. When one of my kids asks me a question I can’t answer, like how a tornado forms, I take out my phone and search for the answer on the Internet.
When it comes to medical information, I can’t remember the last time I opened up a journal sitting on my shelf and leafed through the contents to identify the article I needed. I simply go online and search PubMed or download the article from the AJO website. My office is no longer filled with volumes of journals, and I need only my phone to research whatever topic I’m interested in.
The way I prefer to prepare for cases has changed as well. In the past I would simply open a book or technique article and read about the best way to perform the case. Now, I prefer to watch a video or download the technique guide. I find it easier and faster than reading a book chapter or article.
When we began to change the format of the journal, we stated that AJO would be filled with practical information that would be directly impactful to your practice. That’s the number one criteria we utilize when evaluating content. We wanted to make AJO the journal you wanted to read, because it would improve your knowledge, your outcomes, and your bottom line. We have made many changes to AJO in the last 2 years of print issues. But to truly provide the experience our readers demand and deserve, we have to take a huge next step. Right now we are limited by page and word counts, printed media, and advertising pages. We receive hundreds of submissions a month, yet can only print a fraction of the great material we receive.
If you’ve been following the journal for the last 24 months, you’ve noticed that we have been testing the limits of printed media. We’ve included QR codes for videos, companion PDFs, patient information sheets, and downloadable reports to incorporate into your practice.
The way we access the journal is also changing. We’ve looked closely at our web statistics since the redesign. Our website visits have gone up by a factor of 6 with nearly half of our website traffic coming from mobile usage. It became clear that the days of the printed journal are slowly coming to an end. Surgeons don’t have time to read the journal cover to cover, and now most of our traffic comes from our eBlasts. Surgeons find an article that catches their eye and click a link to find out more. We’ve dramatically increased our eBlasts, and our website volume has been increasing exponentially.
While these small steps have been met with great success, it’s now time to make a giant leap. But unlike most journals, where the online version is just an electronic copy of the printed book, we wanted to make the new AJO something vastly different. We wanted to change the way surgeons utilized a journal and interacted with it on a daily basis. We wanted to be the electronic companion to your practice; a trusted, media rich, peer-reviewed source where you and your patients can turn to for the practical day-to-day information you can use to improve your practice.
We’ve built it, and now I’m proud to unveil it. Beginning January 1, AJO will be published exclusively online. All articles will still be PubMed cited, but will contain more photos, videos, handouts and all the information you need to replicate the findings or procedures in your practice. For example, new surgical techniques will be published with the presenting surgeon’s preference cards, rehab protocols, surgical video, and a PowerPoint presentation that can be presented to referral sources or prospective patients.
New features on our web portal will include:
An orthopedic product guide: A database organized by pathology which contains all of the relevant orthopedic products that could be used for treatment. Relevant products will be cross-referenced to articles so you can quickly identify and order equipment for new cases.
Smart article selection: You can filter the articles that match your interests and have them delivered directly to your inbox. For example, foot and ankle surgeons will no longer need to sift through hundreds of pages to find articles relevant to their practice.
A coding and billing section: Discuss and share tips and tricks with your peers and ask questions of the experts. Regular articles will present relevant codes and how to use them appropriately to get the reimbursement you deserve for your services.
Practice management and business strategies: Get advice from, and interact with, the experts in all areas of your practice.
Ask the experts: Present your cases to our editorial board and enjoy a written, peer-reviewed response. Discuss cases and mutual challenges in communities organized by subspecialty and sport. Cover a high school football team? Imagine a place where you can present your football-related injury to the world’s best football doctors and have them review and comment on the case.
These are just some of the changes you will see in the coming months. We will continuously work to improve and welcome your future suggestions as to how we can provide a truly valuable, customized journal.
Looking to the future, it is my opinion that patient-reported outcome scores will be a large part of what we do. By presenting our successful outcomes, we will ultimately justify the procedures which we perform and justify the reimbursement to third party payers. In this issue, we examine the concept of patient-reported outcome measures (PROMs), and how and why to apply them to your practice.
In our lead article, Elizabeth Matzkin and colleagues present a guideline for implementing PROMs in your practice. Patrick Smith and Corey Cook provide a review of available electronic databases, and Patrick Denard and colleagues present data obtained through an electronic PROM database to settle the question “Is knotless labral repair better than conventional anchors in the shoulder?” Alan Hirahara and colleagues present their 2-year data on superior capsular Reconstruction, and Roland Biedert and Philippe Tscholl discuss the management of patella alta.
By now you’ve realized you’re holding the last printed issue of AJO. Enjoy a moment of nostalgia for the old days, and then buckle your seatbelt. We’re taking AJO where no other journal has gone before and it’s going to be one heck of a ride.
On a recent visit to my daughter’s school, I caught sight of a set of encyclopedias on the shelf. It brought me back to the days where I would open my own set to find out the information I needed to write reports for school. But my sense of nostalgia was short lived as I thought about all of the limitations of the format. If it wasn’t in the encyclopedias, I couldn’t write the report and would need to head to the library. The Internet changed all of that. Now, when I want to know something I don’t look it up in a book anymore. I ask Siri or Alexa or head to the Google home page. When one of my kids asks me a question I can’t answer, like how a tornado forms, I take out my phone and search for the answer on the Internet.
When it comes to medical information, I can’t remember the last time I opened up a journal sitting on my shelf and leafed through the contents to identify the article I needed. I simply go online and search PubMed or download the article from the AJO website. My office is no longer filled with volumes of journals, and I need only my phone to research whatever topic I’m interested in.
The way I prefer to prepare for cases has changed as well. In the past I would simply open a book or technique article and read about the best way to perform the case. Now, I prefer to watch a video or download the technique guide. I find it easier and faster than reading a book chapter or article.
When we began to change the format of the journal, we stated that AJO would be filled with practical information that would be directly impactful to your practice. That’s the number one criteria we utilize when evaluating content. We wanted to make AJO the journal you wanted to read, because it would improve your knowledge, your outcomes, and your bottom line. We have made many changes to AJO in the last 2 years of print issues. But to truly provide the experience our readers demand and deserve, we have to take a huge next step. Right now we are limited by page and word counts, printed media, and advertising pages. We receive hundreds of submissions a month, yet can only print a fraction of the great material we receive.
If you’ve been following the journal for the last 24 months, you’ve noticed that we have been testing the limits of printed media. We’ve included QR codes for videos, companion PDFs, patient information sheets, and downloadable reports to incorporate into your practice.
The way we access the journal is also changing. We’ve looked closely at our web statistics since the redesign. Our website visits have gone up by a factor of 6 with nearly half of our website traffic coming from mobile usage. It became clear that the days of the printed journal are slowly coming to an end. Surgeons don’t have time to read the journal cover to cover, and now most of our traffic comes from our eBlasts. Surgeons find an article that catches their eye and click a link to find out more. We’ve dramatically increased our eBlasts, and our website volume has been increasing exponentially.
While these small steps have been met with great success, it’s now time to make a giant leap. But unlike most journals, where the online version is just an electronic copy of the printed book, we wanted to make the new AJO something vastly different. We wanted to change the way surgeons utilized a journal and interacted with it on a daily basis. We wanted to be the electronic companion to your practice; a trusted, media rich, peer-reviewed source where you and your patients can turn to for the practical day-to-day information you can use to improve your practice.
We’ve built it, and now I’m proud to unveil it. Beginning January 1, AJO will be published exclusively online. All articles will still be PubMed cited, but will contain more photos, videos, handouts and all the information you need to replicate the findings or procedures in your practice. For example, new surgical techniques will be published with the presenting surgeon’s preference cards, rehab protocols, surgical video, and a PowerPoint presentation that can be presented to referral sources or prospective patients.
New features on our web portal will include:
An orthopedic product guide: A database organized by pathology which contains all of the relevant orthopedic products that could be used for treatment. Relevant products will be cross-referenced to articles so you can quickly identify and order equipment for new cases.
Smart article selection: You can filter the articles that match your interests and have them delivered directly to your inbox. For example, foot and ankle surgeons will no longer need to sift through hundreds of pages to find articles relevant to their practice.
A coding and billing section: Discuss and share tips and tricks with your peers and ask questions of the experts. Regular articles will present relevant codes and how to use them appropriately to get the reimbursement you deserve for your services.
Practice management and business strategies: Get advice from, and interact with, the experts in all areas of your practice.
Ask the experts: Present your cases to our editorial board and enjoy a written, peer-reviewed response. Discuss cases and mutual challenges in communities organized by subspecialty and sport. Cover a high school football team? Imagine a place where you can present your football-related injury to the world’s best football doctors and have them review and comment on the case.
These are just some of the changes you will see in the coming months. We will continuously work to improve and welcome your future suggestions as to how we can provide a truly valuable, customized journal.
Looking to the future, it is my opinion that patient-reported outcome scores will be a large part of what we do. By presenting our successful outcomes, we will ultimately justify the procedures which we perform and justify the reimbursement to third party payers. In this issue, we examine the concept of patient-reported outcome measures (PROMs), and how and why to apply them to your practice.
In our lead article, Elizabeth Matzkin and colleagues present a guideline for implementing PROMs in your practice. Patrick Smith and Corey Cook provide a review of available electronic databases, and Patrick Denard and colleagues present data obtained through an electronic PROM database to settle the question “Is knotless labral repair better than conventional anchors in the shoulder?” Alan Hirahara and colleagues present their 2-year data on superior capsular Reconstruction, and Roland Biedert and Philippe Tscholl discuss the management of patella alta.
By now you’ve realized you’re holding the last printed issue of AJO. Enjoy a moment of nostalgia for the old days, and then buckle your seatbelt. We’re taking AJO where no other journal has gone before and it’s going to be one heck of a ride.
The Lesion with Legs
No one in the family is certain when this 6-year-old boy first developed the red spot on his nose, but it is increasingly noticeable. And with school picture day approaching, they would like the redness to resolve.
Their primary care provider reassured them, at length, that it was benign and would eventually resolve without treatment. The lesion causes no symptoms and is purely a cosmetic concern.
The child is otherwise healthy and has been since birth.
EXAMINATION
A pinpoint red dot can be seen on the upper nasal bridge, just to the right of the midline. Tiny linear red “legs” extend from the central dot, like spokes on a wheel. In aggregate, the lesion measures about 3 mm in diameter. There is no palpable component.
However, the entire lesion is blanchable: Pinpoint pressure on the central dot causes it to blanch, and as the pressure is released, the legs of the lesion refill immediately from the center outward. When a glass slide is pressed against the lesion (a process called diascopy) and then released, the same process occurs.

What is the diagnosis?
DISCUSSION
Spider angiomas (SAs), originally called nevus araneus, are actually neither angiomas nor nevi. Instead, they are telangiectasias formed from a superficial arteriole whose surrounding sphincter muscle has failed. The “legs” are tiny veins that carry blood away from the central lesion; this is why they blanch so readily with central pressure and refill from the center outward.
SAs affect 10% to 15% of children and occur only in areas along the path of the superior vena cava. Besides the face, they can be found on the trunk, arms, and dorsal hands.
When solitary, these lesions are benign and can be either left to clear on their own or removed by laser or electrodessication. The presence of three or more lesions warrants further investigation, since multiple SAs can signify underlying disease (eg, cirrhosis of the liver, thyrotoxicosis, or systemic sclerosis).
This patient was not inclined to let us treat the lesion; within a few years, a teasing comment from a classmate or two may change his mind! But with a little luck, it will disappear on its own eventually.
TAKE-HOME LEARNING POINTS
- Spider angiomas (SAs) are actually dilated telangiectatic arterioles manifesting as blanchable, bright red, pinpoint papules with venous “legs” that extend from the center.
- SAs affect 10% to 15% of all children and are confined to areas drained by the superior vena cava.
- Pressure on the lesion with a glass slide (a process called diascopy) causes total blanching; this, along with the clinical findings, confirms the diagnosis.
- Most SAs resolve on their own eventually, but they can be destroyed by laser or electrodessication.
- The presence of more than three SAs should prompt a search for potential causes, such as liver disease, thyrotoxicosis, or systemic sclerosis.
No one in the family is certain when this 6-year-old boy first developed the red spot on his nose, but it is increasingly noticeable. And with school picture day approaching, they would like the redness to resolve.
Their primary care provider reassured them, at length, that it was benign and would eventually resolve without treatment. The lesion causes no symptoms and is purely a cosmetic concern.
The child is otherwise healthy and has been since birth.

EXAMINATION
A pinpoint red dot can be seen on the upper nasal bridge, just to the right of the midline. Tiny linear red “legs” extend from the central dot, like spokes on a wheel. In aggregate, the lesion measures about 3 mm in diameter. There is no palpable component.
However, the entire lesion is blanchable: Pinpoint pressure on the central dot causes it to blanch, and as the pressure is released, the legs of the lesion refill immediately from the center outward. When a glass slide is pressed against the lesion (a process called diascopy) and then released, the same process occurs.

What is the diagnosis?
DISCUSSION
Spider angiomas (SAs), originally called nevus araneus, are actually neither angiomas nor nevi. Instead, they are telangiectasias formed from a superficial arteriole whose surrounding sphincter muscle has failed. The “legs” are tiny veins that carry blood away from the central lesion; this is why they blanch so readily with central pressure and refill from the center outward.
SAs affect 10% to 15% of children and occur only in areas along the path of the superior vena cava. Besides the face, they can be found on the trunk, arms, and dorsal hands.
When solitary, these lesions are benign and can be either left to clear on their own or removed by laser or electrodessication. The presence of three or more lesions warrants further investigation, since multiple SAs can signify underlying disease (eg, cirrhosis of the liver, thyrotoxicosis, or systemic sclerosis).
This patient was not inclined to let us treat the lesion; within a few years, a teasing comment from a classmate or two may change his mind! But with a little luck, it will disappear on its own eventually.
TAKE-HOME LEARNING POINTS
- Spider angiomas (SAs) are actually dilated telangiectatic arterioles manifesting as blanchable, bright red, pinpoint papules with venous “legs” that extend from the center.
- SAs affect 10% to 15% of all children and are confined to areas drained by the superior vena cava.
- Pressure on the lesion with a glass slide (a process called diascopy) causes total blanching; this, along with the clinical findings, confirms the diagnosis.
- Most SAs resolve on their own eventually, but they can be destroyed by laser or electrodessication.
- The presence of more than three SAs should prompt a search for potential causes, such as liver disease, thyrotoxicosis, or systemic sclerosis.
No one in the family is certain when this 6-year-old boy first developed the red spot on his nose, but it is increasingly noticeable. And with school picture day approaching, they would like the redness to resolve.
Their primary care provider reassured them, at length, that it was benign and would eventually resolve without treatment. The lesion causes no symptoms and is purely a cosmetic concern.
The child is otherwise healthy and has been since birth.

EXAMINATION
A pinpoint red dot can be seen on the upper nasal bridge, just to the right of the midline. Tiny linear red “legs” extend from the central dot, like spokes on a wheel. In aggregate, the lesion measures about 3 mm in diameter. There is no palpable component.
However, the entire lesion is blanchable: Pinpoint pressure on the central dot causes it to blanch, and as the pressure is released, the legs of the lesion refill immediately from the center outward. When a glass slide is pressed against the lesion (a process called diascopy) and then released, the same process occurs.

What is the diagnosis?
DISCUSSION
Spider angiomas (SAs), originally called nevus araneus, are actually neither angiomas nor nevi. Instead, they are telangiectasias formed from a superficial arteriole whose surrounding sphincter muscle has failed. The “legs” are tiny veins that carry blood away from the central lesion; this is why they blanch so readily with central pressure and refill from the center outward.
SAs affect 10% to 15% of children and occur only in areas along the path of the superior vena cava. Besides the face, they can be found on the trunk, arms, and dorsal hands.
When solitary, these lesions are benign and can be either left to clear on their own or removed by laser or electrodessication. The presence of three or more lesions warrants further investigation, since multiple SAs can signify underlying disease (eg, cirrhosis of the liver, thyrotoxicosis, or systemic sclerosis).
This patient was not inclined to let us treat the lesion; within a few years, a teasing comment from a classmate or two may change his mind! But with a little luck, it will disappear on its own eventually.
TAKE-HOME LEARNING POINTS
- Spider angiomas (SAs) are actually dilated telangiectatic arterioles manifesting as blanchable, bright red, pinpoint papules with venous “legs” that extend from the center.
- SAs affect 10% to 15% of all children and are confined to areas drained by the superior vena cava.
- Pressure on the lesion with a glass slide (a process called diascopy) causes total blanching; this, along with the clinical findings, confirms the diagnosis.
- Most SAs resolve on their own eventually, but they can be destroyed by laser or electrodessication.
- The presence of more than three SAs should prompt a search for potential causes, such as liver disease, thyrotoxicosis, or systemic sclerosis.
BCMA emerging as a promising target in MM
NEW YORK, NY—The B-cell maturation antigen (BCMA) is emerging as a promising target in multiple myeloma (MM), according to Adam D. Cohen, MD, of the University of Pennsylvania in Philadelphia.
BCMA is highly expressed on MM cells and, with its 2 ligands, is responsible for maintaining normal plasma cell homeostasis.
“And it’s really not expressed on any other normal tissues of the body,” Dr Cohen said.
“Importantly, BCMA is not just sitting on the cell surface as a target but actually promotes myeloma pathogenesis.”
Dr Cohen reviewed the progress being made using BCMA as a target in MM, paying particular attention to chimeric antigen receptor (CAR) T cells. He presented the update at Lymphoma & Myeloma 2017.
NCI BCMA-specific CARs
The first CAR to specifically target BCMA in MM was developed at the National Cancer Institute (NCI). It consisted of a murine single-chain variable fragment (scFv), CD3/CD28 signaling domains, and a gamma-retroviral vector.
Investigators conducted the first-in-human trial of this CAR T-cell therapy in 12 relapsed/refractory MM patients.
All patients received a lymphodepleting conditioning regimen of cyclophosphamide and fludarabine and a single infusion of 1 of 4 doses of the CAR T-cell therapy.
At higher dose levels, the BCMA-CAR produced objective responses “even in these highly refractory patients,” Dr Cohen said. “Some responses lasted 4 to 6 months.”
Patients who had the greatest degree of expansion of CAR T cells were the ones who had the best responses.
The BCMA-CAR is associated with the same toxicities as the CD19-directed CAR T-cell therapies now approved in acute lymphoblastic leukemia and non-Hodgkin lymphoma—cytokine release syndrome (CRS) and neurotoxicity.
The NCI study (NCT02215967) is ongoing.
Penn BCMA-specific CAR
A different BCMA CAR is being investigated at the University of Pennsylvania. It is a fully human CAR that consists of a human scFv, CD3/4-1BB costimulatory domains, and a lentiviral vector.
Investigators designed the first-in-human trial* (NCT02546167) with 3 different cohorts.
Patients in cohort 1 received 5 x 108 CAR T cells without any lymphodepleting chemotherapy.
The remaining patients received cyclophosphamide, followed by 5 x 107 CAR T cells in cohort 2 and 5 x 108 CAR T cells in cohort 3.
Dr Cohen reviewed current data from cohort 1, which included 9 patients. They were a median age of 57 (range, 44-70), and 67% were male. They were heavily pretreated with a median of 9 prior lines of therapy (range, 4-11).
All had high-risk cytogenetics, 67% had deletion 17p or TP53 mutation, and they had a median of 80% bone marrow plasma cells (range, 15%-95%).
“Despite this,” Dr Cohen said, “we were able to generate, successfully, CAR T cells from all patients, although 1 patient did require a second apheresis and manufacturing attempt.”
Four of the 9 patients achieved very good partial responses, and an additional 2 patients had minimal responses.
One patient had a stringent CR (sCR) for close to 2 years without having any intervening therapy.
“[The sCR] shows the potential for this [therapy] to create a durable remission in a patient without any other therapy,” Dr Cohen said. “And this patient still has circulating CAR cells detectable.”
Most of the other patients did not have as durable a response. Responses lasted a median of 3 to 5 months before the patients relapsed.
Dr Cohen noted that the Penn data confirm the NCI experience showing proof of principle.
“You can target BCMA with these cells and get objective responses that can lead to a durable one in a subset of patients,” he added.
Dr Cohen is scheduled to report the preliminary data on the cyclophosphamide cohorts (cohorts 2 and 3) at ASH 2017. Those cohorts, he said, are showing a bit more persistence and increased frequency of responses.
Other BCMA-specific CAR trials
Another BCMA CAR, bb2121, consists of a human anti-BCMA scFv, a lentiviral vector, and a CD3/4-1BB costimulatory domain.
In the first-in-human trial of bb2121* (NCT02658929), all 21 patients received cyclophosphamide and fludarabine conditioning first.
There were “very impressive response rates in this study,” Dr Cohen said.
Once patients were dosed at the higher levels of 150 million cells or greater, every patient responded.
“Many responses are still durable,” he pointed out, “some approaching a year.”
LCAR-B38M is structurally different from the other BCMA-specific CARs. It has 2 binding sites for BCMA.
Thirty-five patients enrolled on the trial of LCAR-B38M (NCT03090659), and 19 were evaluable for response.
Patients had a median of 3 or 4 lines of prior therapy. All received cyclophosphamide alone as conditioning.
All 19 evaluable patients responded, and 14 (74%) achieved an sCR.
Dr Cohen noted the differences among the 4 trials: costimulatory domains varied, the Penn study did not require a pre-existing level of BCMA on the MM cells, other studies excluded patients who didn’t meet a certain threshold level of BCMA expression, median lines of prior therapy differed, and conditioning regimens differed as well.
All trials, however, presented data on fewer than 20 patients.
“And so I think it’s a little too early to compare head-to-head between all of these, but they all are really demonstrating promising results so far,” he observed.
Toxicities
“Unfortunately, there’s no free lunch with CAR T cells,” Dr Cohen stated, “and these extraordinary responses do come at the cost of several somewhat unique toxicities that can be serious.”
Toxicities have included:
- Tumor lysis syndrome, which is expected and manageable
- B-cell aplasia, which is not as much of an issue with BCMA as with CD19-directed CAR Ts, since most B cells don’t express BCMA
- Hypogammaglobulinemia, which can be mitigated with IVIG
- CRS, which can be alleviated with tocilizumab
- Neurotoxicity and encephalopathy, which do not occur as frequently as CRS.
“These, I think, are things we still obviously need to learn more about and try to mitigate before this [BCMA-directed CAR therapy] is expanded and brought earlier to patients,” Dr Cohen said.
“The other issues with CAR T cells are logistical. Limited access (the procedure is available at only a few centers); manufacturing can take 2 to 4 weeks, during which time it is difficult to maintain disease control; and the cost is significant.”
“We are really in the early days of CAR cells for myeloma, but, certainly, this appears very bright in terms of its future.” ![]()
* Data from the presentation differ from the abstract.
NEW YORK, NY—The B-cell maturation antigen (BCMA) is emerging as a promising target in multiple myeloma (MM), according to Adam D. Cohen, MD, of the University of Pennsylvania in Philadelphia.
BCMA is highly expressed on MM cells and, with its 2 ligands, is responsible for maintaining normal plasma cell homeostasis.
“And it’s really not expressed on any other normal tissues of the body,” Dr Cohen said.
“Importantly, BCMA is not just sitting on the cell surface as a target but actually promotes myeloma pathogenesis.”
Dr Cohen reviewed the progress being made using BCMA as a target in MM, paying particular attention to chimeric antigen receptor (CAR) T cells. He presented the update at Lymphoma & Myeloma 2017.
NCI BCMA-specific CARs
The first CAR to specifically target BCMA in MM was developed at the National Cancer Institute (NCI). It consisted of a murine single-chain variable fragment (scFv), CD3/CD28 signaling domains, and a gamma-retroviral vector.
Investigators conducted the first-in-human trial of this CAR T-cell therapy in 12 relapsed/refractory MM patients.
All patients received a lymphodepleting conditioning regimen of cyclophosphamide and fludarabine and a single infusion of 1 of 4 doses of the CAR T-cell therapy.
At higher dose levels, the BCMA-CAR produced objective responses “even in these highly refractory patients,” Dr Cohen said. “Some responses lasted 4 to 6 months.”
Patients who had the greatest degree of expansion of CAR T cells were the ones who had the best responses.
The BCMA-CAR is associated with the same toxicities as the CD19-directed CAR T-cell therapies now approved in acute lymphoblastic leukemia and non-Hodgkin lymphoma—cytokine release syndrome (CRS) and neurotoxicity.
The NCI study (NCT02215967) is ongoing.
Penn BCMA-specific CAR
A different BCMA CAR is being investigated at the University of Pennsylvania. It is a fully human CAR that consists of a human scFv, CD3/4-1BB costimulatory domains, and a lentiviral vector.
Investigators designed the first-in-human trial* (NCT02546167) with 3 different cohorts.
Patients in cohort 1 received 5 x 108 CAR T cells without any lymphodepleting chemotherapy.
The remaining patients received cyclophosphamide, followed by 5 x 107 CAR T cells in cohort 2 and 5 x 108 CAR T cells in cohort 3.
Dr Cohen reviewed current data from cohort 1, which included 9 patients. They were a median age of 57 (range, 44-70), and 67% were male. They were heavily pretreated with a median of 9 prior lines of therapy (range, 4-11).
All had high-risk cytogenetics, 67% had deletion 17p or TP53 mutation, and they had a median of 80% bone marrow plasma cells (range, 15%-95%).
“Despite this,” Dr Cohen said, “we were able to generate, successfully, CAR T cells from all patients, although 1 patient did require a second apheresis and manufacturing attempt.”
Four of the 9 patients achieved very good partial responses, and an additional 2 patients had minimal responses.
One patient had a stringent CR (sCR) for close to 2 years without having any intervening therapy.
“[The sCR] shows the potential for this [therapy] to create a durable remission in a patient without any other therapy,” Dr Cohen said. “And this patient still has circulating CAR cells detectable.”
Most of the other patients did not have as durable a response. Responses lasted a median of 3 to 5 months before the patients relapsed.
Dr Cohen noted that the Penn data confirm the NCI experience showing proof of principle.
“You can target BCMA with these cells and get objective responses that can lead to a durable one in a subset of patients,” he added.
Dr Cohen is scheduled to report the preliminary data on the cyclophosphamide cohorts (cohorts 2 and 3) at ASH 2017. Those cohorts, he said, are showing a bit more persistence and increased frequency of responses.
Other BCMA-specific CAR trials
Another BCMA CAR, bb2121, consists of a human anti-BCMA scFv, a lentiviral vector, and a CD3/4-1BB costimulatory domain.
In the first-in-human trial of bb2121* (NCT02658929), all 21 patients received cyclophosphamide and fludarabine conditioning first.
There were “very impressive response rates in this study,” Dr Cohen said.
Once patients were dosed at the higher levels of 150 million cells or greater, every patient responded.
“Many responses are still durable,” he pointed out, “some approaching a year.”
LCAR-B38M is structurally different from the other BCMA-specific CARs. It has 2 binding sites for BCMA.
Thirty-five patients enrolled on the trial of LCAR-B38M (NCT03090659), and 19 were evaluable for response.
Patients had a median of 3 or 4 lines of prior therapy. All received cyclophosphamide alone as conditioning.
All 19 evaluable patients responded, and 14 (74%) achieved an sCR.
Dr Cohen noted the differences among the 4 trials: costimulatory domains varied, the Penn study did not require a pre-existing level of BCMA on the MM cells, other studies excluded patients who didn’t meet a certain threshold level of BCMA expression, median lines of prior therapy differed, and conditioning regimens differed as well.
All trials, however, presented data on fewer than 20 patients.
“And so I think it’s a little too early to compare head-to-head between all of these, but they all are really demonstrating promising results so far,” he observed.
Toxicities
“Unfortunately, there’s no free lunch with CAR T cells,” Dr Cohen stated, “and these extraordinary responses do come at the cost of several somewhat unique toxicities that can be serious.”
Toxicities have included:
- Tumor lysis syndrome, which is expected and manageable
- B-cell aplasia, which is not as much of an issue with BCMA as with CD19-directed CAR Ts, since most B cells don’t express BCMA
- Hypogammaglobulinemia, which can be mitigated with IVIG
- CRS, which can be alleviated with tocilizumab
- Neurotoxicity and encephalopathy, which do not occur as frequently as CRS.
“These, I think, are things we still obviously need to learn more about and try to mitigate before this [BCMA-directed CAR therapy] is expanded and brought earlier to patients,” Dr Cohen said.
“The other issues with CAR T cells are logistical. Limited access (the procedure is available at only a few centers); manufacturing can take 2 to 4 weeks, during which time it is difficult to maintain disease control; and the cost is significant.”
“We are really in the early days of CAR cells for myeloma, but, certainly, this appears very bright in terms of its future.” ![]()
* Data from the presentation differ from the abstract.
NEW YORK, NY—The B-cell maturation antigen (BCMA) is emerging as a promising target in multiple myeloma (MM), according to Adam D. Cohen, MD, of the University of Pennsylvania in Philadelphia.
BCMA is highly expressed on MM cells and, with its 2 ligands, is responsible for maintaining normal plasma cell homeostasis.
“And it’s really not expressed on any other normal tissues of the body,” Dr Cohen said.
“Importantly, BCMA is not just sitting on the cell surface as a target but actually promotes myeloma pathogenesis.”
Dr Cohen reviewed the progress being made using BCMA as a target in MM, paying particular attention to chimeric antigen receptor (CAR) T cells. He presented the update at Lymphoma & Myeloma 2017.
NCI BCMA-specific CARs
The first CAR to specifically target BCMA in MM was developed at the National Cancer Institute (NCI). It consisted of a murine single-chain variable fragment (scFv), CD3/CD28 signaling domains, and a gamma-retroviral vector.
Investigators conducted the first-in-human trial of this CAR T-cell therapy in 12 relapsed/refractory MM patients.
All patients received a lymphodepleting conditioning regimen of cyclophosphamide and fludarabine and a single infusion of 1 of 4 doses of the CAR T-cell therapy.
At higher dose levels, the BCMA-CAR produced objective responses “even in these highly refractory patients,” Dr Cohen said. “Some responses lasted 4 to 6 months.”
Patients who had the greatest degree of expansion of CAR T cells were the ones who had the best responses.
The BCMA-CAR is associated with the same toxicities as the CD19-directed CAR T-cell therapies now approved in acute lymphoblastic leukemia and non-Hodgkin lymphoma—cytokine release syndrome (CRS) and neurotoxicity.
The NCI study (NCT02215967) is ongoing.
Penn BCMA-specific CAR
A different BCMA CAR is being investigated at the University of Pennsylvania. It is a fully human CAR that consists of a human scFv, CD3/4-1BB costimulatory domains, and a lentiviral vector.
Investigators designed the first-in-human trial* (NCT02546167) with 3 different cohorts.
Patients in cohort 1 received 5 x 108 CAR T cells without any lymphodepleting chemotherapy.
The remaining patients received cyclophosphamide, followed by 5 x 107 CAR T cells in cohort 2 and 5 x 108 CAR T cells in cohort 3.
Dr Cohen reviewed current data from cohort 1, which included 9 patients. They were a median age of 57 (range, 44-70), and 67% were male. They were heavily pretreated with a median of 9 prior lines of therapy (range, 4-11).
All had high-risk cytogenetics, 67% had deletion 17p or TP53 mutation, and they had a median of 80% bone marrow plasma cells (range, 15%-95%).
“Despite this,” Dr Cohen said, “we were able to generate, successfully, CAR T cells from all patients, although 1 patient did require a second apheresis and manufacturing attempt.”
Four of the 9 patients achieved very good partial responses, and an additional 2 patients had minimal responses.
One patient had a stringent CR (sCR) for close to 2 years without having any intervening therapy.
“[The sCR] shows the potential for this [therapy] to create a durable remission in a patient without any other therapy,” Dr Cohen said. “And this patient still has circulating CAR cells detectable.”
Most of the other patients did not have as durable a response. Responses lasted a median of 3 to 5 months before the patients relapsed.
Dr Cohen noted that the Penn data confirm the NCI experience showing proof of principle.
“You can target BCMA with these cells and get objective responses that can lead to a durable one in a subset of patients,” he added.
Dr Cohen is scheduled to report the preliminary data on the cyclophosphamide cohorts (cohorts 2 and 3) at ASH 2017. Those cohorts, he said, are showing a bit more persistence and increased frequency of responses.
Other BCMA-specific CAR trials
Another BCMA CAR, bb2121, consists of a human anti-BCMA scFv, a lentiviral vector, and a CD3/4-1BB costimulatory domain.
In the first-in-human trial of bb2121* (NCT02658929), all 21 patients received cyclophosphamide and fludarabine conditioning first.
There were “very impressive response rates in this study,” Dr Cohen said.
Once patients were dosed at the higher levels of 150 million cells or greater, every patient responded.
“Many responses are still durable,” he pointed out, “some approaching a year.”
LCAR-B38M is structurally different from the other BCMA-specific CARs. It has 2 binding sites for BCMA.
Thirty-five patients enrolled on the trial of LCAR-B38M (NCT03090659), and 19 were evaluable for response.
Patients had a median of 3 or 4 lines of prior therapy. All received cyclophosphamide alone as conditioning.
All 19 evaluable patients responded, and 14 (74%) achieved an sCR.
Dr Cohen noted the differences among the 4 trials: costimulatory domains varied, the Penn study did not require a pre-existing level of BCMA on the MM cells, other studies excluded patients who didn’t meet a certain threshold level of BCMA expression, median lines of prior therapy differed, and conditioning regimens differed as well.
All trials, however, presented data on fewer than 20 patients.
“And so I think it’s a little too early to compare head-to-head between all of these, but they all are really demonstrating promising results so far,” he observed.
Toxicities
“Unfortunately, there’s no free lunch with CAR T cells,” Dr Cohen stated, “and these extraordinary responses do come at the cost of several somewhat unique toxicities that can be serious.”
Toxicities have included:
- Tumor lysis syndrome, which is expected and manageable
- B-cell aplasia, which is not as much of an issue with BCMA as with CD19-directed CAR Ts, since most B cells don’t express BCMA
- Hypogammaglobulinemia, which can be mitigated with IVIG
- CRS, which can be alleviated with tocilizumab
- Neurotoxicity and encephalopathy, which do not occur as frequently as CRS.
“These, I think, are things we still obviously need to learn more about and try to mitigate before this [BCMA-directed CAR therapy] is expanded and brought earlier to patients,” Dr Cohen said.
“The other issues with CAR T cells are logistical. Limited access (the procedure is available at only a few centers); manufacturing can take 2 to 4 weeks, during which time it is difficult to maintain disease control; and the cost is significant.”
“We are really in the early days of CAR cells for myeloma, but, certainly, this appears very bright in terms of its future.” ![]()
* Data from the presentation differ from the abstract.
Study reveals misperceptions among AML patients
SAN DIEGO—A study of acute myeloid leukemia (AML) patients has revealed misperceptions about treatment risks and the likelihood of cure.
Investigators surveyed 100 AML patients receiving intensive and non-intensive chemotherapy, as well as the patients’ oncologists.
The results showed that patients tended to overestimate both the risk of dying due to treatment and the likelihood of cure.
These findings were presented at the 2017 Palliative and Supportive Care in Oncology Symposium (abstract 43).
“Patients with AML face very challenging treatment decisions that are often placed upon them within days after being diagnosed,” said study investigator Areej El-Jawahri, MD, of Massachusetts General Hospital in Boston.
“Because they face a grave decision, they need to understand what the risks of treatment are versus the possibility of a cure.”
For this study, Dr El-Jawahri and her colleagues enrolled 50 patients who were receiving intensive care for AML (which usually meant hospitalization for 4 to 6 weeks) and 50 patients who were receiving non-intensive care (often given as outpatient treatment).
The patients’ median age was 71 (range, 60-100), and 92% were white. Six percent of patients had low-risk disease, 48% had intermediate-risk, and 46% had high-risk disease.
Within 3 days of starting treatment, both the patients and their physicians were given a questionnaire to assess how they perceived the likelihood of the patient dying from treatment.
One month later, patients and physicians completed a follow-up questionnaire to assess perceptions of patient prognosis. Within that time frame, most patients received laboratory results that more definitively established the type and stage of cancer.
At 24 weeks, the investigators asked patients if they had discussed their end-of-life wishes with their oncologists.
Results
Initially, most of the patient population (91.3%) thought it was “somewhat” or “extremely” likely they would die from their treatment. However, only 22% of treating oncologists said the same.
One month later, a majority of patients in both treatment groups thought it was “somewhat” or “extremely” likely they would be cured of their AML.
Specifically, 82.1% of patients receiving non-intensive chemotherapy said it was “somewhat” or “extremely” likely they would be cured, while 10% of their oncologists said the same.
Meanwhile, 97.6% of patients receiving intensive chemotherapy said it was “somewhat” or “extremely” likely they would be cured, and 42% of their oncologists said the same.
Overall, 77.8% of patients said they had not discussed their end-of-life wishes with their oncologists at 24 weeks.
“There were several very important factors we were not able to capture in our study, including what was actually discussed between patients and their oncologists and whether patients simply misunderstood or misheard the information conveyed to them,” Dr El-Jawahri said.
“Perhaps most importantly, we did not audio-record the discussions between the patients and their physicians, which could provide additional details regarding barriers to accurate prognostic understanding in these conversations.”
Related research and next steps
Prior to this study, Dr El-Jawahri and her colleagues had looked at similar perceptions in patients with solid tumor malignancies as well as in patients with hematologic malignancies who were receiving hematopoietic stem cell transplants.
The gaps in perception of treatment risk and cure for patients compared to their physicians were not as large in those cases as in the AML patients in this study. The investigators attribute this to higher levels of distress seen in AML patients due to the urgency of their treatment decisions.
Dr El-Jawahri and her colleagues have found that early consideration of palliative care in a treatment plan for patients with solid tumors improves patients’ understanding of the prognosis. The team hopes to implement a similar study in patients with AML.
“Clearly, there are important communication gaps between oncologists and their patients,” Dr El-Jawahri said. “We need to find ways to help physicians do a better job of communicating with their patients, especially in diseases like AML where stress levels are remarkably high.” ![]()
SAN DIEGO—A study of acute myeloid leukemia (AML) patients has revealed misperceptions about treatment risks and the likelihood of cure.
Investigators surveyed 100 AML patients receiving intensive and non-intensive chemotherapy, as well as the patients’ oncologists.
The results showed that patients tended to overestimate both the risk of dying due to treatment and the likelihood of cure.
These findings were presented at the 2017 Palliative and Supportive Care in Oncology Symposium (abstract 43).
“Patients with AML face very challenging treatment decisions that are often placed upon them within days after being diagnosed,” said study investigator Areej El-Jawahri, MD, of Massachusetts General Hospital in Boston.
“Because they face a grave decision, they need to understand what the risks of treatment are versus the possibility of a cure.”
For this study, Dr El-Jawahri and her colleagues enrolled 50 patients who were receiving intensive care for AML (which usually meant hospitalization for 4 to 6 weeks) and 50 patients who were receiving non-intensive care (often given as outpatient treatment).
The patients’ median age was 71 (range, 60-100), and 92% were white. Six percent of patients had low-risk disease, 48% had intermediate-risk, and 46% had high-risk disease.
Within 3 days of starting treatment, both the patients and their physicians were given a questionnaire to assess how they perceived the likelihood of the patient dying from treatment.
One month later, patients and physicians completed a follow-up questionnaire to assess perceptions of patient prognosis. Within that time frame, most patients received laboratory results that more definitively established the type and stage of cancer.
At 24 weeks, the investigators asked patients if they had discussed their end-of-life wishes with their oncologists.
Results
Initially, most of the patient population (91.3%) thought it was “somewhat” or “extremely” likely they would die from their treatment. However, only 22% of treating oncologists said the same.
One month later, a majority of patients in both treatment groups thought it was “somewhat” or “extremely” likely they would be cured of their AML.
Specifically, 82.1% of patients receiving non-intensive chemotherapy said it was “somewhat” or “extremely” likely they would be cured, while 10% of their oncologists said the same.
Meanwhile, 97.6% of patients receiving intensive chemotherapy said it was “somewhat” or “extremely” likely they would be cured, and 42% of their oncologists said the same.
Overall, 77.8% of patients said they had not discussed their end-of-life wishes with their oncologists at 24 weeks.
“There were several very important factors we were not able to capture in our study, including what was actually discussed between patients and their oncologists and whether patients simply misunderstood or misheard the information conveyed to them,” Dr El-Jawahri said.
“Perhaps most importantly, we did not audio-record the discussions between the patients and their physicians, which could provide additional details regarding barriers to accurate prognostic understanding in these conversations.”
Related research and next steps
Prior to this study, Dr El-Jawahri and her colleagues had looked at similar perceptions in patients with solid tumor malignancies as well as in patients with hematologic malignancies who were receiving hematopoietic stem cell transplants.
The gaps in perception of treatment risk and cure for patients compared to their physicians were not as large in those cases as in the AML patients in this study. The investigators attribute this to higher levels of distress seen in AML patients due to the urgency of their treatment decisions.
Dr El-Jawahri and her colleagues have found that early consideration of palliative care in a treatment plan for patients with solid tumors improves patients’ understanding of the prognosis. The team hopes to implement a similar study in patients with AML.
“Clearly, there are important communication gaps between oncologists and their patients,” Dr El-Jawahri said. “We need to find ways to help physicians do a better job of communicating with their patients, especially in diseases like AML where stress levels are remarkably high.” ![]()
SAN DIEGO—A study of acute myeloid leukemia (AML) patients has revealed misperceptions about treatment risks and the likelihood of cure.
Investigators surveyed 100 AML patients receiving intensive and non-intensive chemotherapy, as well as the patients’ oncologists.
The results showed that patients tended to overestimate both the risk of dying due to treatment and the likelihood of cure.
These findings were presented at the 2017 Palliative and Supportive Care in Oncology Symposium (abstract 43).
“Patients with AML face very challenging treatment decisions that are often placed upon them within days after being diagnosed,” said study investigator Areej El-Jawahri, MD, of Massachusetts General Hospital in Boston.
“Because they face a grave decision, they need to understand what the risks of treatment are versus the possibility of a cure.”
For this study, Dr El-Jawahri and her colleagues enrolled 50 patients who were receiving intensive care for AML (which usually meant hospitalization for 4 to 6 weeks) and 50 patients who were receiving non-intensive care (often given as outpatient treatment).
The patients’ median age was 71 (range, 60-100), and 92% were white. Six percent of patients had low-risk disease, 48% had intermediate-risk, and 46% had high-risk disease.
Within 3 days of starting treatment, both the patients and their physicians were given a questionnaire to assess how they perceived the likelihood of the patient dying from treatment.
One month later, patients and physicians completed a follow-up questionnaire to assess perceptions of patient prognosis. Within that time frame, most patients received laboratory results that more definitively established the type and stage of cancer.
At 24 weeks, the investigators asked patients if they had discussed their end-of-life wishes with their oncologists.
Results
Initially, most of the patient population (91.3%) thought it was “somewhat” or “extremely” likely they would die from their treatment. However, only 22% of treating oncologists said the same.
One month later, a majority of patients in both treatment groups thought it was “somewhat” or “extremely” likely they would be cured of their AML.
Specifically, 82.1% of patients receiving non-intensive chemotherapy said it was “somewhat” or “extremely” likely they would be cured, while 10% of their oncologists said the same.
Meanwhile, 97.6% of patients receiving intensive chemotherapy said it was “somewhat” or “extremely” likely they would be cured, and 42% of their oncologists said the same.
Overall, 77.8% of patients said they had not discussed their end-of-life wishes with their oncologists at 24 weeks.
“There were several very important factors we were not able to capture in our study, including what was actually discussed between patients and their oncologists and whether patients simply misunderstood or misheard the information conveyed to them,” Dr El-Jawahri said.
“Perhaps most importantly, we did not audio-record the discussions between the patients and their physicians, which could provide additional details regarding barriers to accurate prognostic understanding in these conversations.”
Related research and next steps
Prior to this study, Dr El-Jawahri and her colleagues had looked at similar perceptions in patients with solid tumor malignancies as well as in patients with hematologic malignancies who were receiving hematopoietic stem cell transplants.
The gaps in perception of treatment risk and cure for patients compared to their physicians were not as large in those cases as in the AML patients in this study. The investigators attribute this to higher levels of distress seen in AML patients due to the urgency of their treatment decisions.
Dr El-Jawahri and her colleagues have found that early consideration of palliative care in a treatment plan for patients with solid tumors improves patients’ understanding of the prognosis. The team hopes to implement a similar study in patients with AML.
“Clearly, there are important communication gaps between oncologists and their patients,” Dr El-Jawahri said. “We need to find ways to help physicians do a better job of communicating with their patients, especially in diseases like AML where stress levels are remarkably high.” ![]()
Drug receives fast track designation for lower-risk MDS
The US Food and Drug Administration (FDA) has granted fast track designation to the telomerase inhibitor imetelstat.
The designation is for imetelstat as a potential treatment for adults who have transfusion-dependent anemia due to low or intermediate-1 risk myelodysplastic syndromes (MDS), do not have 5q deletion, and are refractory or resistant to treatment with an erythropoiesis-stimulating agent (ESA).
Imetelstat was initially developed by Geron Corporation and exclusively licensed to Janssen Biotech, Inc.
Janssen sponsored the application for fast track designation using preliminary data from IMerge, a trial in which researchers are studying transfusion-dependent patients with low- or intermediate-1 risk MDS who have relapsed after or are refractory to treatment with an ESA.
Part 1 of IMerge is a phase 2, single-arm trial. Part 2 is a phase 3, randomized, placebo-controlled trial.
Thirty-two patients have been enrolled in part 1 of IMerge. However, this part of the trial is expanding to enroll approximately 20 additional patients who do not have 5q deletion and are naïve to treatment with a hypomethylating agent and lenalidomide.
The expansion is based on results observed in a subset of the original 32 patients who had not received prior treatment with a hypomethylating agent or lenalidomide and did not have 5q deletion.
As of May 2017, this 13-patient subset showed an increased durability and rate of red blood cell transfusion-independence compared to the overall trial population.
Results in these patients and the rest of the original 32 patients are expected to be presented at an upcoming medical conference.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to the telomerase inhibitor imetelstat.
The designation is for imetelstat as a potential treatment for adults who have transfusion-dependent anemia due to low or intermediate-1 risk myelodysplastic syndromes (MDS), do not have 5q deletion, and are refractory or resistant to treatment with an erythropoiesis-stimulating agent (ESA).
Imetelstat was initially developed by Geron Corporation and exclusively licensed to Janssen Biotech, Inc.
Janssen sponsored the application for fast track designation using preliminary data from IMerge, a trial in which researchers are studying transfusion-dependent patients with low- or intermediate-1 risk MDS who have relapsed after or are refractory to treatment with an ESA.
Part 1 of IMerge is a phase 2, single-arm trial. Part 2 is a phase 3, randomized, placebo-controlled trial.
Thirty-two patients have been enrolled in part 1 of IMerge. However, this part of the trial is expanding to enroll approximately 20 additional patients who do not have 5q deletion and are naïve to treatment with a hypomethylating agent and lenalidomide.
The expansion is based on results observed in a subset of the original 32 patients who had not received prior treatment with a hypomethylating agent or lenalidomide and did not have 5q deletion.
As of May 2017, this 13-patient subset showed an increased durability and rate of red blood cell transfusion-independence compared to the overall trial population.
Results in these patients and the rest of the original 32 patients are expected to be presented at an upcoming medical conference.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to the telomerase inhibitor imetelstat.
The designation is for imetelstat as a potential treatment for adults who have transfusion-dependent anemia due to low or intermediate-1 risk myelodysplastic syndromes (MDS), do not have 5q deletion, and are refractory or resistant to treatment with an erythropoiesis-stimulating agent (ESA).
Imetelstat was initially developed by Geron Corporation and exclusively licensed to Janssen Biotech, Inc.
Janssen sponsored the application for fast track designation using preliminary data from IMerge, a trial in which researchers are studying transfusion-dependent patients with low- or intermediate-1 risk MDS who have relapsed after or are refractory to treatment with an ESA.
Part 1 of IMerge is a phase 2, single-arm trial. Part 2 is a phase 3, randomized, placebo-controlled trial.
Thirty-two patients have been enrolled in part 1 of IMerge. However, this part of the trial is expanding to enroll approximately 20 additional patients who do not have 5q deletion and are naïve to treatment with a hypomethylating agent and lenalidomide.
The expansion is based on results observed in a subset of the original 32 patients who had not received prior treatment with a hypomethylating agent or lenalidomide and did not have 5q deletion.
As of May 2017, this 13-patient subset showed an increased durability and rate of red blood cell transfusion-independence compared to the overall trial population.
Results in these patients and the rest of the original 32 patients are expected to be presented at an upcoming medical conference.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA. ![]()
FDA panels support two NDAs for buprenorphine subcutaneous injections
SILVER SPRING, MD. – Two Food and Drug Administration advisory panels have recommended approval of two new drug applications (NDA) for buprenorphine subcutaneous injections for the treatment of opioid dependence.
On Nov. 1, panelists recommended approval of some of the doses proposed in the NDA submitted by Braeburn Pharmaceuticals at the joint meeting of the Psychopharmacologic Drugs Advisory and the Drug Safety and Risk Management committees. The formulation, currently known as CAM2038, is intended to be used as part of a treatment plan that can include counseling and psychosocial support. The subcutaneous depot is available weekly, in 8-, 16-, 24-, and 32-mg injections, and monthly, in 64-, 96-, 128-, and 160-mg injections.
On the previous day, Oct. 31, the panelists voted 18-1 with no abstentions to recommend an NDA submitted by Indivior. This formulation is known as RBP-6000.
Both formulations must be administered by a health care provider using a prefilled syringe with a predetermined dosage. The injection forms a biodegradable subcutaneous depot that, as it degrades, releases buprenorphine at a steady and controlled pace over the course of treatment – increasing the success of treatment for opioid use disorder.
Braeburn’s NDA was based on results of a double-blind, randomized, within-subject, inpatient laboratory study of 47 patients over 14 days. Patients were randomized into two groups: 22 patients in the 24-mg group and 25 patients in the 32-mg group. Patients were administered an initial dose on day 0 and a follow-up dose on day 7. The results of the study found a complete blockade of opioids after the first injection that was sustained over the 1-week interdosing interval.
The committees said that of most of the doses should be approved, but a majority of committee members were uncomfortable with the higher doses.
Voting on Indivior’s NDA was based, in part, on the results of a randomized, double-blind, placebo-controlled, multicenter phase 3 study. The study lasted 24 weeks and randomly assigned 504 patients into one of three groups based on monthly dosing regimen of buprenorphine: 300 mg/300 mg, 300 mg/100 mg, and placebo. After randomization, the 300 mg/300 mg group had 201 patients, the 300 mg/100 mg group had 203 patients, and the placebo group had 100 patients. The study found that the primary and secondary endpoints were met, and significantly higher percentage of abstinence with subcutaneous buprenorphine were observed. Patients in both the 300 mg/300 mg and 300 mg/100 mg groups had very similar distributions of percentage of weeks patients abstained from opioid use with more than 20% of patients achieving 80%-100% abstinence from opioids during the course of the study, a significant improvement over the placebo group.
The panels’ recommendations come against the backdrop of the opioid epidemic in the United States, which President Trump has deemed a public health emergency. Many of the panel members and speakers at both meetings expressed support for the NDAs in that context and emphasized that, unlike sublingual administration of buprenorphine, these treatments do not require daily intervention. In addition, sublingual tablets are easier to abuse or more likely to lead to overdose because the patient must self-administer the medication. Expanding the toolkit of physicians who treat opioid use disorder might help stem the tide of the epidemic, some speakers said.
Usually, the FDA follows its advisory panels’ recommendations, which are not binding.
SILVER SPRING, MD. – Two Food and Drug Administration advisory panels have recommended approval of two new drug applications (NDA) for buprenorphine subcutaneous injections for the treatment of opioid dependence.
On Nov. 1, panelists recommended approval of some of the doses proposed in the NDA submitted by Braeburn Pharmaceuticals at the joint meeting of the Psychopharmacologic Drugs Advisory and the Drug Safety and Risk Management committees. The formulation, currently known as CAM2038, is intended to be used as part of a treatment plan that can include counseling and psychosocial support. The subcutaneous depot is available weekly, in 8-, 16-, 24-, and 32-mg injections, and monthly, in 64-, 96-, 128-, and 160-mg injections.
On the previous day, Oct. 31, the panelists voted 18-1 with no abstentions to recommend an NDA submitted by Indivior. This formulation is known as RBP-6000.
Both formulations must be administered by a health care provider using a prefilled syringe with a predetermined dosage. The injection forms a biodegradable subcutaneous depot that, as it degrades, releases buprenorphine at a steady and controlled pace over the course of treatment – increasing the success of treatment for opioid use disorder.
Braeburn’s NDA was based on results of a double-blind, randomized, within-subject, inpatient laboratory study of 47 patients over 14 days. Patients were randomized into two groups: 22 patients in the 24-mg group and 25 patients in the 32-mg group. Patients were administered an initial dose on day 0 and a follow-up dose on day 7. The results of the study found a complete blockade of opioids after the first injection that was sustained over the 1-week interdosing interval.
The committees said that of most of the doses should be approved, but a majority of committee members were uncomfortable with the higher doses.
Voting on Indivior’s NDA was based, in part, on the results of a randomized, double-blind, placebo-controlled, multicenter phase 3 study. The study lasted 24 weeks and randomly assigned 504 patients into one of three groups based on monthly dosing regimen of buprenorphine: 300 mg/300 mg, 300 mg/100 mg, and placebo. After randomization, the 300 mg/300 mg group had 201 patients, the 300 mg/100 mg group had 203 patients, and the placebo group had 100 patients. The study found that the primary and secondary endpoints were met, and significantly higher percentage of abstinence with subcutaneous buprenorphine were observed. Patients in both the 300 mg/300 mg and 300 mg/100 mg groups had very similar distributions of percentage of weeks patients abstained from opioid use with more than 20% of patients achieving 80%-100% abstinence from opioids during the course of the study, a significant improvement over the placebo group.
The panels’ recommendations come against the backdrop of the opioid epidemic in the United States, which President Trump has deemed a public health emergency. Many of the panel members and speakers at both meetings expressed support for the NDAs in that context and emphasized that, unlike sublingual administration of buprenorphine, these treatments do not require daily intervention. In addition, sublingual tablets are easier to abuse or more likely to lead to overdose because the patient must self-administer the medication. Expanding the toolkit of physicians who treat opioid use disorder might help stem the tide of the epidemic, some speakers said.
Usually, the FDA follows its advisory panels’ recommendations, which are not binding.
SILVER SPRING, MD. – Two Food and Drug Administration advisory panels have recommended approval of two new drug applications (NDA) for buprenorphine subcutaneous injections for the treatment of opioid dependence.
On Nov. 1, panelists recommended approval of some of the doses proposed in the NDA submitted by Braeburn Pharmaceuticals at the joint meeting of the Psychopharmacologic Drugs Advisory and the Drug Safety and Risk Management committees. The formulation, currently known as CAM2038, is intended to be used as part of a treatment plan that can include counseling and psychosocial support. The subcutaneous depot is available weekly, in 8-, 16-, 24-, and 32-mg injections, and monthly, in 64-, 96-, 128-, and 160-mg injections.
On the previous day, Oct. 31, the panelists voted 18-1 with no abstentions to recommend an NDA submitted by Indivior. This formulation is known as RBP-6000.
Both formulations must be administered by a health care provider using a prefilled syringe with a predetermined dosage. The injection forms a biodegradable subcutaneous depot that, as it degrades, releases buprenorphine at a steady and controlled pace over the course of treatment – increasing the success of treatment for opioid use disorder.
Braeburn’s NDA was based on results of a double-blind, randomized, within-subject, inpatient laboratory study of 47 patients over 14 days. Patients were randomized into two groups: 22 patients in the 24-mg group and 25 patients in the 32-mg group. Patients were administered an initial dose on day 0 and a follow-up dose on day 7. The results of the study found a complete blockade of opioids after the first injection that was sustained over the 1-week interdosing interval.
The committees said that of most of the doses should be approved, but a majority of committee members were uncomfortable with the higher doses.
Voting on Indivior’s NDA was based, in part, on the results of a randomized, double-blind, placebo-controlled, multicenter phase 3 study. The study lasted 24 weeks and randomly assigned 504 patients into one of three groups based on monthly dosing regimen of buprenorphine: 300 mg/300 mg, 300 mg/100 mg, and placebo. After randomization, the 300 mg/300 mg group had 201 patients, the 300 mg/100 mg group had 203 patients, and the placebo group had 100 patients. The study found that the primary and secondary endpoints were met, and significantly higher percentage of abstinence with subcutaneous buprenorphine were observed. Patients in both the 300 mg/300 mg and 300 mg/100 mg groups had very similar distributions of percentage of weeks patients abstained from opioid use with more than 20% of patients achieving 80%-100% abstinence from opioids during the course of the study, a significant improvement over the placebo group.
The panels’ recommendations come against the backdrop of the opioid epidemic in the United States, which President Trump has deemed a public health emergency. Many of the panel members and speakers at both meetings expressed support for the NDAs in that context and emphasized that, unlike sublingual administration of buprenorphine, these treatments do not require daily intervention. In addition, sublingual tablets are easier to abuse or more likely to lead to overdose because the patient must self-administer the medication. Expanding the toolkit of physicians who treat opioid use disorder might help stem the tide of the epidemic, some speakers said.
Usually, the FDA follows its advisory panels’ recommendations, which are not binding.