User login
Pseudomyogenic Hemangioendothelioma
Pseudomyogenic hemangioendothelioma (PMHE), also referred to as epithelioid sarcoma–like hemangioendothelioma,1 is a rare soft tissue tumor that was described in 1992 by Mirra et al2 as a fibromalike variant of epithelioid sarcoma. It predominantly affects males between the second and fifth decades of life and most commonly presents as multiple nodules that may involve either the superficial or deep soft tissues of the legs and less often the arms. It also can arise on the trunk. We present a case of PMHE occurring in a young man and briefly review the literature on clinical presentation and histologic differentiation of this unique tumor, comparing these findings to its mimickers.
Case Report
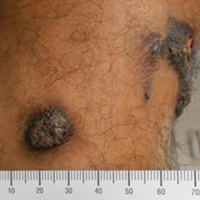
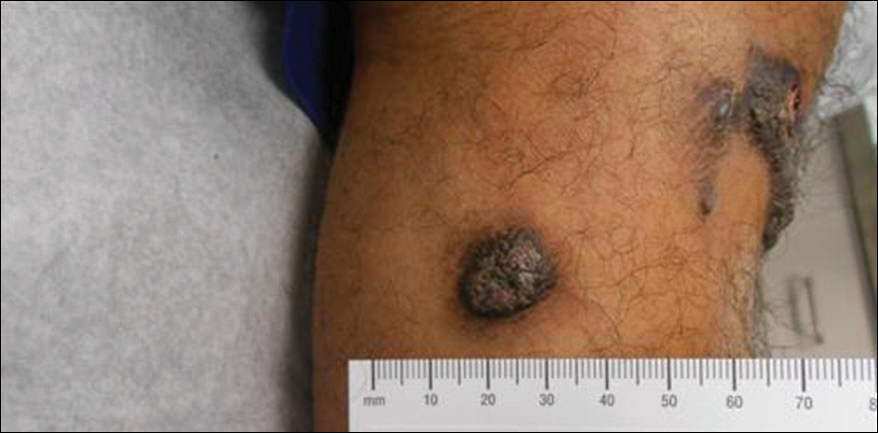
A 20-year-old man presented with skin lesions on the left leg that had been present for 1 year. The patient described the lesions as tender pimples that would drain yellow discharge on occasion but had now transformed into large brown plaques. Physical examination showed 4 verrucous plaques ranging in size from 1 to 3 cm with hyperpigmentation and a central crust (Figure 1). Initially, the patient thought the lesions appeared due to shaving his legs for sports. He presented to the emergency department multiple times over the past year; pain control was provided and local skin care was recommended. Culture of the discharge had been performed 6 months prior to biopsy with negative results. No biopsy was performed on initial presentation and the lesions were diagnosed in the emergency department clinically as boils.
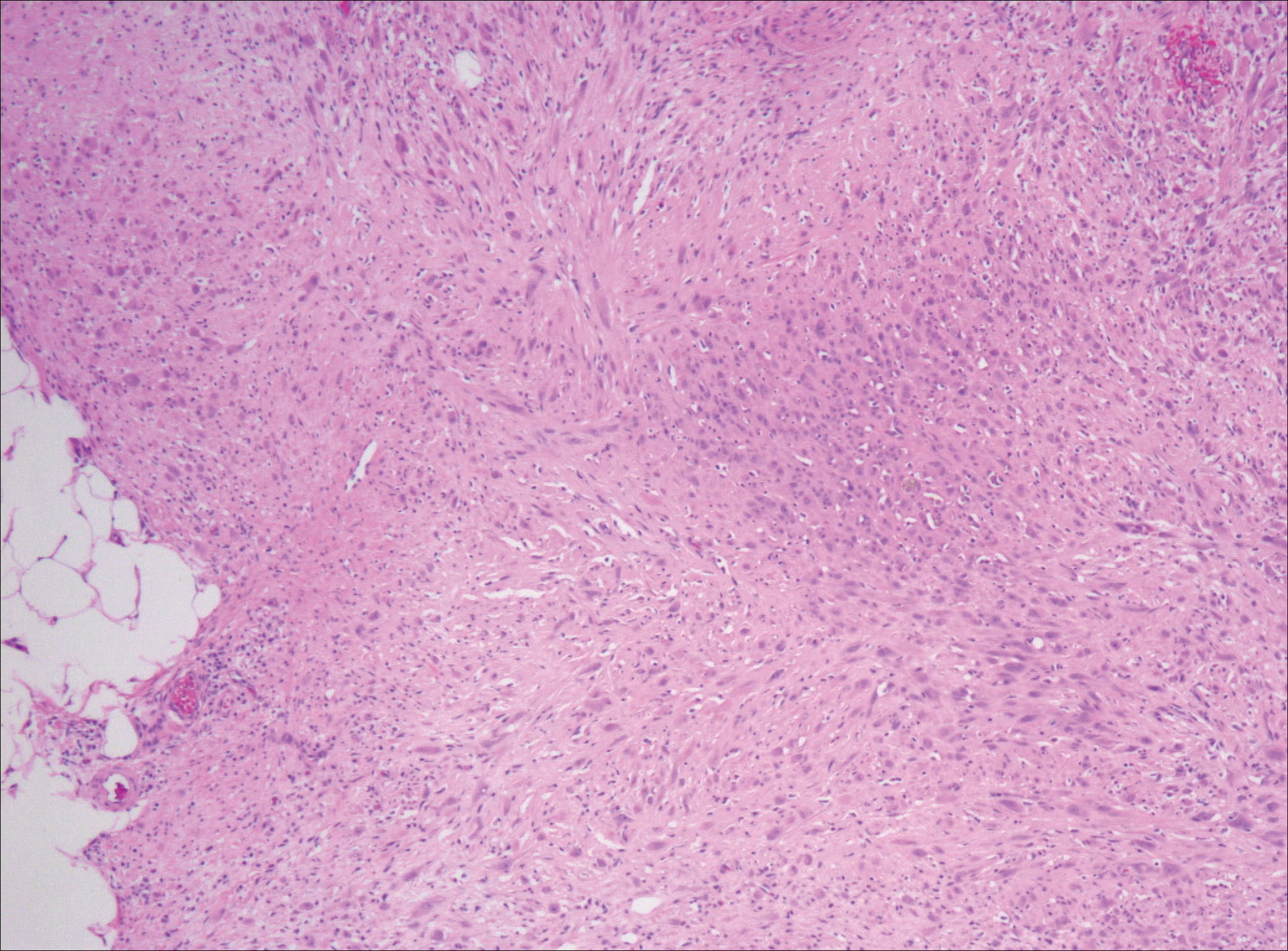
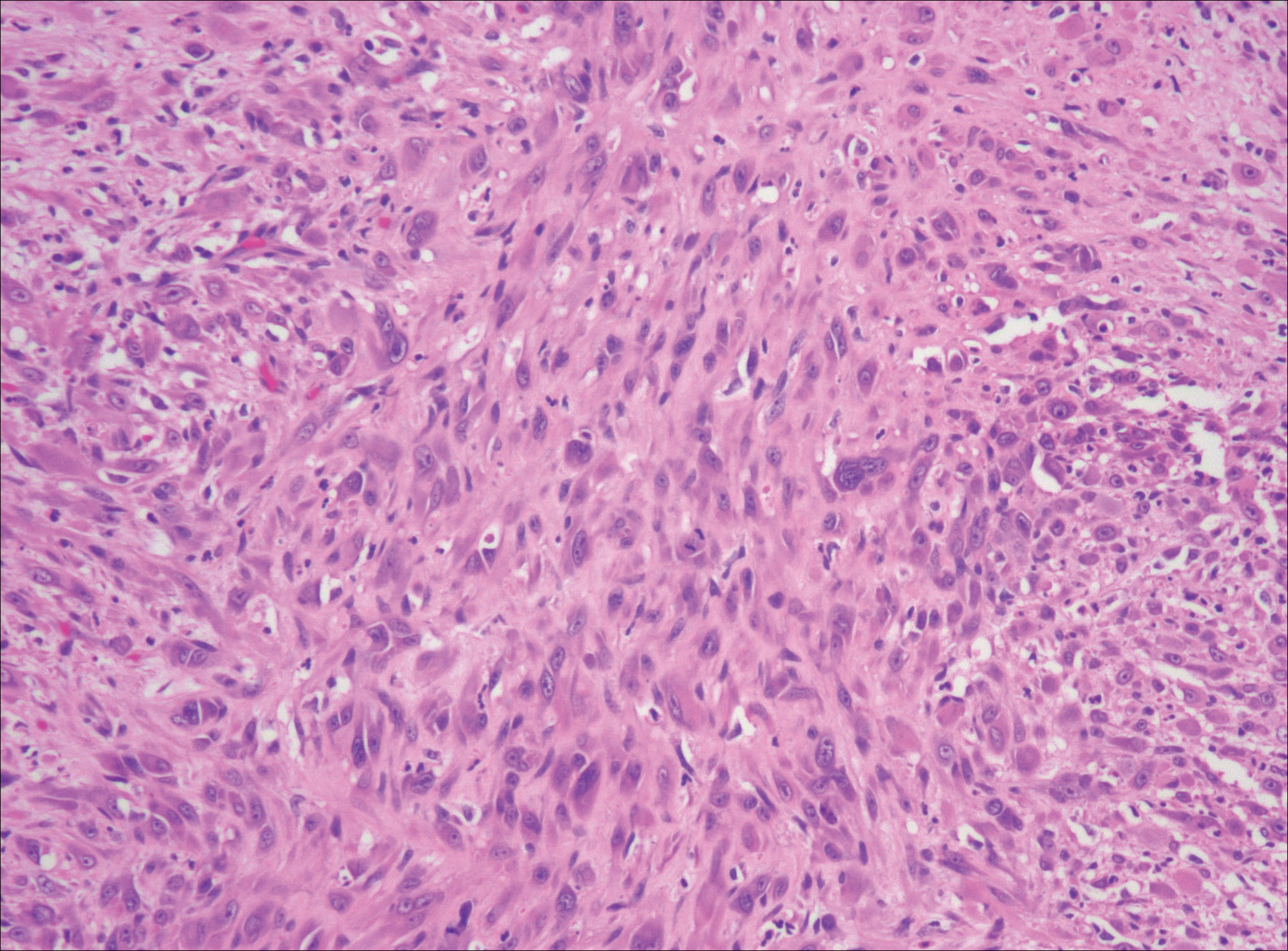
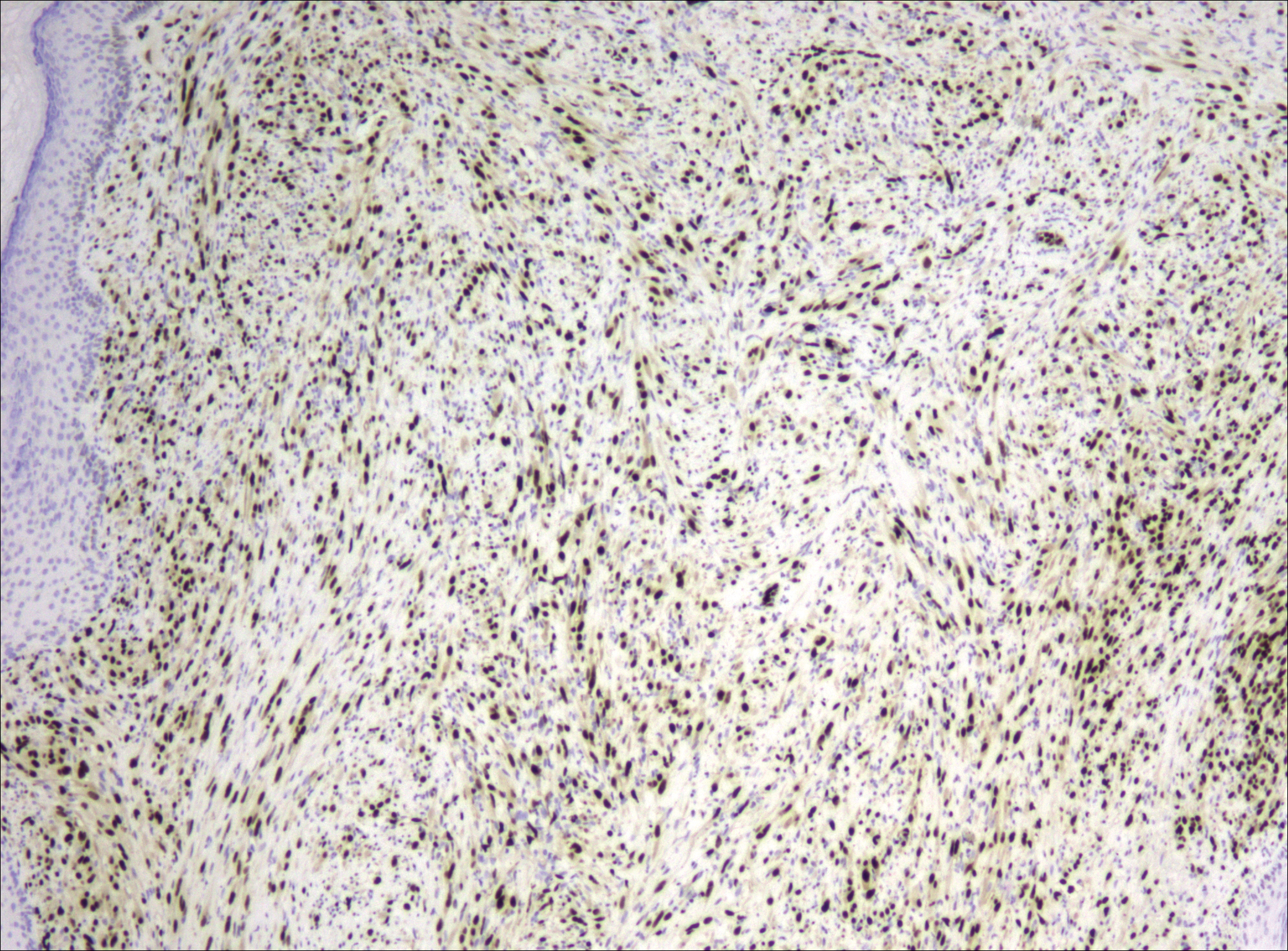
After failing to improve, the patient was seen by an outside dermatologist and the clinical differential diagnosis included deep fungal infection, atypical mycobacterial infection, and keloids. A 4-mm punch biopsy was taken from the periphery of one of the lesions and demonstrated hyperkeratosis, papillomatosis, and acanthosis (Figure 2). Within the superficial and deep dermis and focally extending into the subcutaneous tissue, there were sheets of spindled to epithelioid-appearing cells with moderate cytologic atypia (Figure 3). The tumor showed infiltrative margins. There was moderate cellularity. The individual cells had a rhabdoid appearance with large eccentric vesicular nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm (Figure 4). No definitive evidence of glandular, squamous, or vascular differentiation was present. There was an associated moderate inflammatory host response composed of neutrophils and lymphocytes. Occasional extravasated red blood cells were present. Immunohistochemistry staining was performed and the atypical cells demonstrated diffuse positive staining for friend leukemia integration 1 transcription factor (FLI-1), erythroblastosis virus E26 transforming sequence-related gene (ERG)(Figure 5), CD31, and CD68. There was patchy positive staining for cytokeratin AE1/AE3, CD10, and factor VIII. There was no remarkable staining for human herpesvirus 8, epithelial membrane antigen, S-100, CD34, cytokeratin 903, and desmin. Overall, the histologic features in conjunction with the immunohistochemistry staining were consistent with a diagnosis of PMHE.

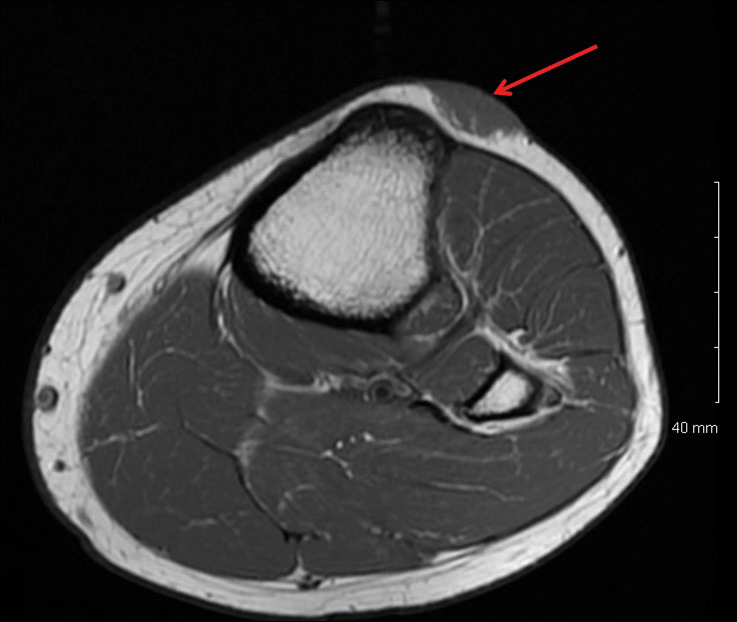
Magnetic resonance imaging was then performed to evaluate the depth and extent of the lesions for surgical excision planning (Figure 6), which showed 5 nodular lesions within the dermis and subcutis adjacent to the proximal aspect of the left tibia and medial aspect of the left knee. An additional lesion was noted between the sartorius and semimembranosus muscles, which was thought to represent either a lymph node or an additional neoplastic lesion. Chest computed tomography also displayed indeterminate lesions in the lungs.
Excision of the superficial lesions was performed. All of the lesions demonstrated similar histologic changes to the previously described biopsy specimen. The tumor was limited to the dermis and subcutaneous tissue. The patient was lost to follow-up and the etiology of the lung lesions was unknown.
Comment
Nomenclature
Pseudomyogenic hemangioendothelioma is a relatively new type of vascular tumor that has been included in the updated 2013 edition of the World Health Organization classification as an intermediate malignant tumor that rarely metastasizes.3 It typically involves multiple tissue planes, most notably the dermis and subcutaneous layers but also muscle and bone.4 The term pseudomyogenic refers to the histologic resemblance of some of the cells to rhabdomyoblasts; however, these tumors are negative for all immunohistochemical muscle markers, most notably myogenin, desmin, and α-smooth muscle actin.5
Clinical Presentation
Gross features of PMHE typically include multiple firm nodules with ill-defined margins. The tumor was initially described in 1992 by Mirra et al2 as a fibromalike variant of epithelioid sarcoma. In 2003, a series of 7 cases of PMHE was reported by Billings et al6 under the term epithelioid sarcomalike hemangioendothelioma. Other than the predominance of an epithelioid morphology, the cases reported as epithelioid sarcomalike hemangioendothelioma had similar clinical features and immunophenotype to what has been reported as PMHE.
Based on a PubMed search of articles indexed for MEDLINE using the term pseudomyogenic hemangioendothelioma, the 2 largest case series were reported by Pradhan et al7 (N=8) in 2017 and Hornick and Fletcher4 (N=50) in 2011. Hornick and Fletcher4 reported a male (41/50 [82.0%]) to female (9/50 [18.0%]) ratio of 4.6 to 1, and an average age at presentation of 31 years with 82% (41/50) of patients 40 years or younger. Pradhan et al7 also reported a male predominance (7/8 [87.5%]) with a similar average age at presentation of 29 years (age range, 9–62 years). The size of individual tumors ranged from 0.3 to 5.5 cm (mean size, 1.9 cm) in the series by Hornick and Fletcher4 and 0.3 to 6.0 com in the series by Pradhan et al.7 Hornick and Fletcher4 reported the most common site of involvement was the leg (27/50 [54.0%]), followed by the arm (12/50 [24.0%]), trunk (9/50 [18.0%]), and head and neck (2/50 [4.0%]). The leg (6/8 [75.0%]) also was the most common site of involvement in the series by Pradhan et al,7 with 2 cases occurring on the arm. In the series by Hornick and Fletcher,4 the tumors typically involved the dermis and subcutaneous tissue (26/50 [52%]) with a smaller number involving skeletal muscle (17/50 [34%]) and bone (7/50 [14%]). They reported 66% of their patients (33/50) had multifocal disease at presentation.4 Pradhan et al7 also reported 2 (25.0%) cases being limited to the superficial soft tissue, 2 (25.0%) being limited to the deep soft tissue, and 4 (50.0%) involving the bone; 5 (62.5%) patients had multifocal disease at presentation. The presentation of our patient in regards to gender, age, and tumor characteristics is consistent with other published cases.5-10
Histopathology
Microscopic features of PMHE include sheets of spindled to epithelioid-appearing cells with mild to moderate nuclear atypia and eosinophilic cytoplasm. The tumor has an infiltrative growth pattern. Some of the cells may resemble rhabdomyoblastlike cells, hence the moniker pseudomyogenic. There is no recapitulation of vascular structures or remarkable cytoplasmic vacuolization. Mitotic rate is low and there is no tumor necrosis.4 The tumor cells do not appear to arise from a vessel or display an angiocentric growth pattern. Many cases report the presence of an inflammatory infiltrate containing neutrophils interspersed within the tumor.4,5,7 The overlying epidermis will commonly show hyperkeratosis, epidermal hyperplasia, and acanthosis.4,11
Differential Diagnosis
The histopathologic differential diagnosis would include epithelioid sarcoma, epithelioid hemangioendothelioma, and to a lesser extent dermatofibrosarcoma protuberans (DFSP) and rhabdomyosarcoma. Dermatofibrosarcoma protuberans is the most commonly encountered of these tumors. Histologically, DFSP is characterized by a cellular proliferation of small spindle cells with plump nuclei arranged in a storiform or cartwheel pattern. Dermatofibrosarcoma protuberans tends to be limited to the dermis and subcutaneous tissue and only rarely involves underlying skeletal muscle. The presence of the storiform growth pattern in conjunction with the lack of rhabdoid changes would favor a diagnosis of DFSP. Another characteristic histologic finding typically only associated with DFSP is the interdigitating growth pattern of the spindle cells within the lobules of the subcutaneous tissue, creating a lacelike or honeycomb appearance.
Immunohistochemistry staining is necessary to help differentiate PMHE from other tumors in the differential diagnosis. Pseudomyogenic hemangioendothelioma stains positive for cytokeratin AE1/AE3; integrase interactor 1; and vascular markers FLI-1, CD31, and ERG, and negative for CD34.4,6,12-15 In contrast to epithelioid hemangioendothelioma, DFSP, and to a lesser extent epithelioid sarcoma, all of which are positive for CD34, epithelioid sarcoma is negative for both CD31 and integrase interactor 1. Dermatofibrosarcoma protuberans is negative for cytokeratin AE1/AE3. Rhabdomyosarcomas are positive for myogenic markers such as MyoD1 and myogenin, unlike any of the other tumors mentioned. Histologically, epithelioid sarcomas will tend to have a granulomalike growth pattern with central necrosis, unlike PMHE.12 Epithelioid hemangioendothelioma often will have a cordlike growth pattern in a myxochondroid background. Unlike PMHE, these tumors often will appear to be arising from vessels, and intracytoplasmic vacuoles are common. Three cases of PMHE have been reported to have a t(7;19)(q22;q13) chromosomal anomaly, which is not consistent with every case.16
Treatment Options
Standard treatment typically includes wide excision of the lesions, as was done in our case. Because of the substantial risk of local recurrence, which was up to 58% in the series by Hornick and Fletcher,4 adjuvant therapy may be considered if positive margins are found on excision. Metastasis to lymph nodes and the lungs has been reported but is rare.2,4 Most cases have been shown to have a favorable prognosis; however, local recurrence seems to be common. Rarely, amputation of the limb may be required.5 In contrast, epithelioid sarcomas have been found to spread to lymph nodes and the lungs in up to 50% of cases with a 5-year survival rate of 10% to 30%.13
Conclusion
In summary, we describe a case of PMHE involving the lower leg in a 20-year-old man. These tumors often are multinodular and multiplanar, with the dermis and subcutaneous tissues being the most common areas affected. It has a high rate of local recurrence but rarely has distant metastasis. Pseudomyogenic hemangioendothelioma, similar to other soft tissue tumors, can be difficult to diagnose on shave biopsy or superficial punch biopsy not extending into subcutaneous tissue. Deep incisional or punch biopsies are required to more definitively diagnose these types of tumors. The diagnosis of PMHE versus other soft tissue tumors requires correlation of histology and immunohistochemistry staining with clinical information and radiographic findings.
- Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma (pseudomyogenic hemangioendothelioma). Am J Surg Pathol. 2011;35:1088; author reply 1088-1089.
- Mirra JM, Kessler S, Bhuta S, et al. The fibroma-like variant of epithelioid sarcoma. a fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69:1382-1395.
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95-104.
- Hornick JL, Fletcher CD. Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol. 2011;35:190-201.
- Sheng W, Pan Y, Wang J. Pseudomyogenic hemangioendothelioma: report of an additional case with aggressive clinical course. Am J Dermatopathol. 2013;35:597-600.
- Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma. Am J Surg Pathol. 2003;27:48-57.
- Pradhan D, Schoedel K, McGough RL, et al. Pseudomyogenic hemangioendothelioma of skin, bone and soft tissue—a clinicopathological, immunohistochemical and fluorescence in situ hybridization study [published online November 2, 2017]. Hum Pathol. 2017. doi:0.1016/j.humpath.2017.10.023.
- Requena L, Santonja C, Martinez-Amo JL, et al. Cutaneous epithelioid sarcoma like (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149:459-465.
- McGinity M, Bartanusz V, Dengler B, et al. Pseudomyogenic hemangioendothelioma (epithelioid sarcoma-like hemangioendothelioma, fibroma-like variant of epithelioid sarcoma) of the thoracic spine. Eur Spine J. 2013;22(suppl 3):S506-S511.
- Stuart LN, Gardner JM, Lauer SR, et al. Epithelioid sarcoma-like (pseudomyogenic) hemangioendothelioma, clinically mimicking dermatofibroma, diagnosed by skin biopsy in a 30-year-old man. J Cutan Pathol. 2013;40:909-913.
- Amary MF, O’Donnell P, Berisha F, et al. Pseudomyogenic (epithelioid sarcoma-like) hemangioendothelioma: characterization of five cases. Skeletal Radiol. 2013;42:947-957.
- Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542-550.
- Chbani L, Guillou L, Terrier P, et al. Epithelioid sarcoma: a clinicopathologic and immunohistochemical analysis of 106 cases from the French Sarcoma Group. Am J Clin Pathol. 2009;131:222-227.
- Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
- Requena L, Santonja C, Martinez-Amo JL, et al. Cutaneous epithelioid sarcoma like (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149:459-465.
- Trombetta D, Magnusson L, von Steyern FV, et al. Translocation t(7;19)(q22;q13)—a recurrent chromosome aberration in pseudomyogenic hemangioendothelioma? Cancer Genet. 2011;204:211-215.
Pseudomyogenic hemangioendothelioma (PMHE), also referred to as epithelioid sarcoma–like hemangioendothelioma,1 is a rare soft tissue tumor that was described in 1992 by Mirra et al2 as a fibromalike variant of epithelioid sarcoma. It predominantly affects males between the second and fifth decades of life and most commonly presents as multiple nodules that may involve either the superficial or deep soft tissues of the legs and less often the arms. It also can arise on the trunk. We present a case of PMHE occurring in a young man and briefly review the literature on clinical presentation and histologic differentiation of this unique tumor, comparing these findings to its mimickers.
Case Report
A 20-year-old man presented with skin lesions on the left leg that had been present for 1 year. The patient described the lesions as tender pimples that would drain yellow discharge on occasion but had now transformed into large brown plaques. Physical examination showed 4 verrucous plaques ranging in size from 1 to 3 cm with hyperpigmentation and a central crust (Figure 1). Initially, the patient thought the lesions appeared due to shaving his legs for sports. He presented to the emergency department multiple times over the past year; pain control was provided and local skin care was recommended. Culture of the discharge had been performed 6 months prior to biopsy with negative results. No biopsy was performed on initial presentation and the lesions were diagnosed in the emergency department clinically as boils.

After failing to improve, the patient was seen by an outside dermatologist and the clinical differential diagnosis included deep fungal infection, atypical mycobacterial infection, and keloids. A 4-mm punch biopsy was taken from the periphery of one of the lesions and demonstrated hyperkeratosis, papillomatosis, and acanthosis (Figure 2). Within the superficial and deep dermis and focally extending into the subcutaneous tissue, there were sheets of spindled to epithelioid-appearing cells with moderate cytologic atypia (Figure 3). The tumor showed infiltrative margins. There was moderate cellularity. The individual cells had a rhabdoid appearance with large eccentric vesicular nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm (Figure 4). No definitive evidence of glandular, squamous, or vascular differentiation was present. There was an associated moderate inflammatory host response composed of neutrophils and lymphocytes. Occasional extravasated red blood cells were present. Immunohistochemistry staining was performed and the atypical cells demonstrated diffuse positive staining for friend leukemia integration 1 transcription factor (FLI-1), erythroblastosis virus E26 transforming sequence-related gene (ERG)(Figure 5), CD31, and CD68. There was patchy positive staining for cytokeratin AE1/AE3, CD10, and factor VIII. There was no remarkable staining for human herpesvirus 8, epithelial membrane antigen, S-100, CD34, cytokeratin 903, and desmin. Overall, the histologic features in conjunction with the immunohistochemistry staining were consistent with a diagnosis of PMHE.




Magnetic resonance imaging was then performed to evaluate the depth and extent of the lesions for surgical excision planning (Figure 6), which showed 5 nodular lesions within the dermis and subcutis adjacent to the proximal aspect of the left tibia and medial aspect of the left knee. An additional lesion was noted between the sartorius and semimembranosus muscles, which was thought to represent either a lymph node or an additional neoplastic lesion. Chest computed tomography also displayed indeterminate lesions in the lungs.

Excision of the superficial lesions was performed. All of the lesions demonstrated similar histologic changes to the previously described biopsy specimen. The tumor was limited to the dermis and subcutaneous tissue. The patient was lost to follow-up and the etiology of the lung lesions was unknown.
Comment
Nomenclature
Pseudomyogenic hemangioendothelioma is a relatively new type of vascular tumor that has been included in the updated 2013 edition of the World Health Organization classification as an intermediate malignant tumor that rarely metastasizes.3 It typically involves multiple tissue planes, most notably the dermis and subcutaneous layers but also muscle and bone.4 The term pseudomyogenic refers to the histologic resemblance of some of the cells to rhabdomyoblasts; however, these tumors are negative for all immunohistochemical muscle markers, most notably myogenin, desmin, and α-smooth muscle actin.5
Clinical Presentation
Gross features of PMHE typically include multiple firm nodules with ill-defined margins. The tumor was initially described in 1992 by Mirra et al2 as a fibromalike variant of epithelioid sarcoma. In 2003, a series of 7 cases of PMHE was reported by Billings et al6 under the term epithelioid sarcomalike hemangioendothelioma. Other than the predominance of an epithelioid morphology, the cases reported as epithelioid sarcomalike hemangioendothelioma had similar clinical features and immunophenotype to what has been reported as PMHE.
Based on a PubMed search of articles indexed for MEDLINE using the term pseudomyogenic hemangioendothelioma, the 2 largest case series were reported by Pradhan et al7 (N=8) in 2017 and Hornick and Fletcher4 (N=50) in 2011. Hornick and Fletcher4 reported a male (41/50 [82.0%]) to female (9/50 [18.0%]) ratio of 4.6 to 1, and an average age at presentation of 31 years with 82% (41/50) of patients 40 years or younger. Pradhan et al7 also reported a male predominance (7/8 [87.5%]) with a similar average age at presentation of 29 years (age range, 9–62 years). The size of individual tumors ranged from 0.3 to 5.5 cm (mean size, 1.9 cm) in the series by Hornick and Fletcher4 and 0.3 to 6.0 com in the series by Pradhan et al.7 Hornick and Fletcher4 reported the most common site of involvement was the leg (27/50 [54.0%]), followed by the arm (12/50 [24.0%]), trunk (9/50 [18.0%]), and head and neck (2/50 [4.0%]). The leg (6/8 [75.0%]) also was the most common site of involvement in the series by Pradhan et al,7 with 2 cases occurring on the arm. In the series by Hornick and Fletcher,4 the tumors typically involved the dermis and subcutaneous tissue (26/50 [52%]) with a smaller number involving skeletal muscle (17/50 [34%]) and bone (7/50 [14%]). They reported 66% of their patients (33/50) had multifocal disease at presentation.4 Pradhan et al7 also reported 2 (25.0%) cases being limited to the superficial soft tissue, 2 (25.0%) being limited to the deep soft tissue, and 4 (50.0%) involving the bone; 5 (62.5%) patients had multifocal disease at presentation. The presentation of our patient in regards to gender, age, and tumor characteristics is consistent with other published cases.5-10
Histopathology
Microscopic features of PMHE include sheets of spindled to epithelioid-appearing cells with mild to moderate nuclear atypia and eosinophilic cytoplasm. The tumor has an infiltrative growth pattern. Some of the cells may resemble rhabdomyoblastlike cells, hence the moniker pseudomyogenic. There is no recapitulation of vascular structures or remarkable cytoplasmic vacuolization. Mitotic rate is low and there is no tumor necrosis.4 The tumor cells do not appear to arise from a vessel or display an angiocentric growth pattern. Many cases report the presence of an inflammatory infiltrate containing neutrophils interspersed within the tumor.4,5,7 The overlying epidermis will commonly show hyperkeratosis, epidermal hyperplasia, and acanthosis.4,11
Differential Diagnosis
The histopathologic differential diagnosis would include epithelioid sarcoma, epithelioid hemangioendothelioma, and to a lesser extent dermatofibrosarcoma protuberans (DFSP) and rhabdomyosarcoma. Dermatofibrosarcoma protuberans is the most commonly encountered of these tumors. Histologically, DFSP is characterized by a cellular proliferation of small spindle cells with plump nuclei arranged in a storiform or cartwheel pattern. Dermatofibrosarcoma protuberans tends to be limited to the dermis and subcutaneous tissue and only rarely involves underlying skeletal muscle. The presence of the storiform growth pattern in conjunction with the lack of rhabdoid changes would favor a diagnosis of DFSP. Another characteristic histologic finding typically only associated with DFSP is the interdigitating growth pattern of the spindle cells within the lobules of the subcutaneous tissue, creating a lacelike or honeycomb appearance.
Immunohistochemistry staining is necessary to help differentiate PMHE from other tumors in the differential diagnosis. Pseudomyogenic hemangioendothelioma stains positive for cytokeratin AE1/AE3; integrase interactor 1; and vascular markers FLI-1, CD31, and ERG, and negative for CD34.4,6,12-15 In contrast to epithelioid hemangioendothelioma, DFSP, and to a lesser extent epithelioid sarcoma, all of which are positive for CD34, epithelioid sarcoma is negative for both CD31 and integrase interactor 1. Dermatofibrosarcoma protuberans is negative for cytokeratin AE1/AE3. Rhabdomyosarcomas are positive for myogenic markers such as MyoD1 and myogenin, unlike any of the other tumors mentioned. Histologically, epithelioid sarcomas will tend to have a granulomalike growth pattern with central necrosis, unlike PMHE.12 Epithelioid hemangioendothelioma often will have a cordlike growth pattern in a myxochondroid background. Unlike PMHE, these tumors often will appear to be arising from vessels, and intracytoplasmic vacuoles are common. Three cases of PMHE have been reported to have a t(7;19)(q22;q13) chromosomal anomaly, which is not consistent with every case.16
Treatment Options
Standard treatment typically includes wide excision of the lesions, as was done in our case. Because of the substantial risk of local recurrence, which was up to 58% in the series by Hornick and Fletcher,4 adjuvant therapy may be considered if positive margins are found on excision. Metastasis to lymph nodes and the lungs has been reported but is rare.2,4 Most cases have been shown to have a favorable prognosis; however, local recurrence seems to be common. Rarely, amputation of the limb may be required.5 In contrast, epithelioid sarcomas have been found to spread to lymph nodes and the lungs in up to 50% of cases with a 5-year survival rate of 10% to 30%.13
Conclusion
In summary, we describe a case of PMHE involving the lower leg in a 20-year-old man. These tumors often are multinodular and multiplanar, with the dermis and subcutaneous tissues being the most common areas affected. It has a high rate of local recurrence but rarely has distant metastasis. Pseudomyogenic hemangioendothelioma, similar to other soft tissue tumors, can be difficult to diagnose on shave biopsy or superficial punch biopsy not extending into subcutaneous tissue. Deep incisional or punch biopsies are required to more definitively diagnose these types of tumors. The diagnosis of PMHE versus other soft tissue tumors requires correlation of histology and immunohistochemistry staining with clinical information and radiographic findings.
Pseudomyogenic hemangioendothelioma (PMHE), also referred to as epithelioid sarcoma–like hemangioendothelioma,1 is a rare soft tissue tumor that was described in 1992 by Mirra et al2 as a fibromalike variant of epithelioid sarcoma. It predominantly affects males between the second and fifth decades of life and most commonly presents as multiple nodules that may involve either the superficial or deep soft tissues of the legs and less often the arms. It also can arise on the trunk. We present a case of PMHE occurring in a young man and briefly review the literature on clinical presentation and histologic differentiation of this unique tumor, comparing these findings to its mimickers.
Case Report
A 20-year-old man presented with skin lesions on the left leg that had been present for 1 year. The patient described the lesions as tender pimples that would drain yellow discharge on occasion but had now transformed into large brown plaques. Physical examination showed 4 verrucous plaques ranging in size from 1 to 3 cm with hyperpigmentation and a central crust (Figure 1). Initially, the patient thought the lesions appeared due to shaving his legs for sports. He presented to the emergency department multiple times over the past year; pain control was provided and local skin care was recommended. Culture of the discharge had been performed 6 months prior to biopsy with negative results. No biopsy was performed on initial presentation and the lesions were diagnosed in the emergency department clinically as boils.

After failing to improve, the patient was seen by an outside dermatologist and the clinical differential diagnosis included deep fungal infection, atypical mycobacterial infection, and keloids. A 4-mm punch biopsy was taken from the periphery of one of the lesions and demonstrated hyperkeratosis, papillomatosis, and acanthosis (Figure 2). Within the superficial and deep dermis and focally extending into the subcutaneous tissue, there were sheets of spindled to epithelioid-appearing cells with moderate cytologic atypia (Figure 3). The tumor showed infiltrative margins. There was moderate cellularity. The individual cells had a rhabdoid appearance with large eccentric vesicular nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm (Figure 4). No definitive evidence of glandular, squamous, or vascular differentiation was present. There was an associated moderate inflammatory host response composed of neutrophils and lymphocytes. Occasional extravasated red blood cells were present. Immunohistochemistry staining was performed and the atypical cells demonstrated diffuse positive staining for friend leukemia integration 1 transcription factor (FLI-1), erythroblastosis virus E26 transforming sequence-related gene (ERG)(Figure 5), CD31, and CD68. There was patchy positive staining for cytokeratin AE1/AE3, CD10, and factor VIII. There was no remarkable staining for human herpesvirus 8, epithelial membrane antigen, S-100, CD34, cytokeratin 903, and desmin. Overall, the histologic features in conjunction with the immunohistochemistry staining were consistent with a diagnosis of PMHE.




Magnetic resonance imaging was then performed to evaluate the depth and extent of the lesions for surgical excision planning (Figure 6), which showed 5 nodular lesions within the dermis and subcutis adjacent to the proximal aspect of the left tibia and medial aspect of the left knee. An additional lesion was noted between the sartorius and semimembranosus muscles, which was thought to represent either a lymph node or an additional neoplastic lesion. Chest computed tomography also displayed indeterminate lesions in the lungs.

Excision of the superficial lesions was performed. All of the lesions demonstrated similar histologic changes to the previously described biopsy specimen. The tumor was limited to the dermis and subcutaneous tissue. The patient was lost to follow-up and the etiology of the lung lesions was unknown.
Comment
Nomenclature
Pseudomyogenic hemangioendothelioma is a relatively new type of vascular tumor that has been included in the updated 2013 edition of the World Health Organization classification as an intermediate malignant tumor that rarely metastasizes.3 It typically involves multiple tissue planes, most notably the dermis and subcutaneous layers but also muscle and bone.4 The term pseudomyogenic refers to the histologic resemblance of some of the cells to rhabdomyoblasts; however, these tumors are negative for all immunohistochemical muscle markers, most notably myogenin, desmin, and α-smooth muscle actin.5
Clinical Presentation
Gross features of PMHE typically include multiple firm nodules with ill-defined margins. The tumor was initially described in 1992 by Mirra et al2 as a fibromalike variant of epithelioid sarcoma. In 2003, a series of 7 cases of PMHE was reported by Billings et al6 under the term epithelioid sarcomalike hemangioendothelioma. Other than the predominance of an epithelioid morphology, the cases reported as epithelioid sarcomalike hemangioendothelioma had similar clinical features and immunophenotype to what has been reported as PMHE.
Based on a PubMed search of articles indexed for MEDLINE using the term pseudomyogenic hemangioendothelioma, the 2 largest case series were reported by Pradhan et al7 (N=8) in 2017 and Hornick and Fletcher4 (N=50) in 2011. Hornick and Fletcher4 reported a male (41/50 [82.0%]) to female (9/50 [18.0%]) ratio of 4.6 to 1, and an average age at presentation of 31 years with 82% (41/50) of patients 40 years or younger. Pradhan et al7 also reported a male predominance (7/8 [87.5%]) with a similar average age at presentation of 29 years (age range, 9–62 years). The size of individual tumors ranged from 0.3 to 5.5 cm (mean size, 1.9 cm) in the series by Hornick and Fletcher4 and 0.3 to 6.0 com in the series by Pradhan et al.7 Hornick and Fletcher4 reported the most common site of involvement was the leg (27/50 [54.0%]), followed by the arm (12/50 [24.0%]), trunk (9/50 [18.0%]), and head and neck (2/50 [4.0%]). The leg (6/8 [75.0%]) also was the most common site of involvement in the series by Pradhan et al,7 with 2 cases occurring on the arm. In the series by Hornick and Fletcher,4 the tumors typically involved the dermis and subcutaneous tissue (26/50 [52%]) with a smaller number involving skeletal muscle (17/50 [34%]) and bone (7/50 [14%]). They reported 66% of their patients (33/50) had multifocal disease at presentation.4 Pradhan et al7 also reported 2 (25.0%) cases being limited to the superficial soft tissue, 2 (25.0%) being limited to the deep soft tissue, and 4 (50.0%) involving the bone; 5 (62.5%) patients had multifocal disease at presentation. The presentation of our patient in regards to gender, age, and tumor characteristics is consistent with other published cases.5-10
Histopathology
Microscopic features of PMHE include sheets of spindled to epithelioid-appearing cells with mild to moderate nuclear atypia and eosinophilic cytoplasm. The tumor has an infiltrative growth pattern. Some of the cells may resemble rhabdomyoblastlike cells, hence the moniker pseudomyogenic. There is no recapitulation of vascular structures or remarkable cytoplasmic vacuolization. Mitotic rate is low and there is no tumor necrosis.4 The tumor cells do not appear to arise from a vessel or display an angiocentric growth pattern. Many cases report the presence of an inflammatory infiltrate containing neutrophils interspersed within the tumor.4,5,7 The overlying epidermis will commonly show hyperkeratosis, epidermal hyperplasia, and acanthosis.4,11
Differential Diagnosis
The histopathologic differential diagnosis would include epithelioid sarcoma, epithelioid hemangioendothelioma, and to a lesser extent dermatofibrosarcoma protuberans (DFSP) and rhabdomyosarcoma. Dermatofibrosarcoma protuberans is the most commonly encountered of these tumors. Histologically, DFSP is characterized by a cellular proliferation of small spindle cells with plump nuclei arranged in a storiform or cartwheel pattern. Dermatofibrosarcoma protuberans tends to be limited to the dermis and subcutaneous tissue and only rarely involves underlying skeletal muscle. The presence of the storiform growth pattern in conjunction with the lack of rhabdoid changes would favor a diagnosis of DFSP. Another characteristic histologic finding typically only associated with DFSP is the interdigitating growth pattern of the spindle cells within the lobules of the subcutaneous tissue, creating a lacelike or honeycomb appearance.
Immunohistochemistry staining is necessary to help differentiate PMHE from other tumors in the differential diagnosis. Pseudomyogenic hemangioendothelioma stains positive for cytokeratin AE1/AE3; integrase interactor 1; and vascular markers FLI-1, CD31, and ERG, and negative for CD34.4,6,12-15 In contrast to epithelioid hemangioendothelioma, DFSP, and to a lesser extent epithelioid sarcoma, all of which are positive for CD34, epithelioid sarcoma is negative for both CD31 and integrase interactor 1. Dermatofibrosarcoma protuberans is negative for cytokeratin AE1/AE3. Rhabdomyosarcomas are positive for myogenic markers such as MyoD1 and myogenin, unlike any of the other tumors mentioned. Histologically, epithelioid sarcomas will tend to have a granulomalike growth pattern with central necrosis, unlike PMHE.12 Epithelioid hemangioendothelioma often will have a cordlike growth pattern in a myxochondroid background. Unlike PMHE, these tumors often will appear to be arising from vessels, and intracytoplasmic vacuoles are common. Three cases of PMHE have been reported to have a t(7;19)(q22;q13) chromosomal anomaly, which is not consistent with every case.16
Treatment Options
Standard treatment typically includes wide excision of the lesions, as was done in our case. Because of the substantial risk of local recurrence, which was up to 58% in the series by Hornick and Fletcher,4 adjuvant therapy may be considered if positive margins are found on excision. Metastasis to lymph nodes and the lungs has been reported but is rare.2,4 Most cases have been shown to have a favorable prognosis; however, local recurrence seems to be common. Rarely, amputation of the limb may be required.5 In contrast, epithelioid sarcomas have been found to spread to lymph nodes and the lungs in up to 50% of cases with a 5-year survival rate of 10% to 30%.13
Conclusion
In summary, we describe a case of PMHE involving the lower leg in a 20-year-old man. These tumors often are multinodular and multiplanar, with the dermis and subcutaneous tissues being the most common areas affected. It has a high rate of local recurrence but rarely has distant metastasis. Pseudomyogenic hemangioendothelioma, similar to other soft tissue tumors, can be difficult to diagnose on shave biopsy or superficial punch biopsy not extending into subcutaneous tissue. Deep incisional or punch biopsies are required to more definitively diagnose these types of tumors. The diagnosis of PMHE versus other soft tissue tumors requires correlation of histology and immunohistochemistry staining with clinical information and radiographic findings.
- Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma (pseudomyogenic hemangioendothelioma). Am J Surg Pathol. 2011;35:1088; author reply 1088-1089.
- Mirra JM, Kessler S, Bhuta S, et al. The fibroma-like variant of epithelioid sarcoma. a fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69:1382-1395.
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95-104.
- Hornick JL, Fletcher CD. Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol. 2011;35:190-201.
- Sheng W, Pan Y, Wang J. Pseudomyogenic hemangioendothelioma: report of an additional case with aggressive clinical course. Am J Dermatopathol. 2013;35:597-600.
- Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma. Am J Surg Pathol. 2003;27:48-57.
- Pradhan D, Schoedel K, McGough RL, et al. Pseudomyogenic hemangioendothelioma of skin, bone and soft tissue—a clinicopathological, immunohistochemical and fluorescence in situ hybridization study [published online November 2, 2017]. Hum Pathol. 2017. doi:0.1016/j.humpath.2017.10.023.
- Requena L, Santonja C, Martinez-Amo JL, et al. Cutaneous epithelioid sarcoma like (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149:459-465.
- McGinity M, Bartanusz V, Dengler B, et al. Pseudomyogenic hemangioendothelioma (epithelioid sarcoma-like hemangioendothelioma, fibroma-like variant of epithelioid sarcoma) of the thoracic spine. Eur Spine J. 2013;22(suppl 3):S506-S511.
- Stuart LN, Gardner JM, Lauer SR, et al. Epithelioid sarcoma-like (pseudomyogenic) hemangioendothelioma, clinically mimicking dermatofibroma, diagnosed by skin biopsy in a 30-year-old man. J Cutan Pathol. 2013;40:909-913.
- Amary MF, O’Donnell P, Berisha F, et al. Pseudomyogenic (epithelioid sarcoma-like) hemangioendothelioma: characterization of five cases. Skeletal Radiol. 2013;42:947-957.
- Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542-550.
- Chbani L, Guillou L, Terrier P, et al. Epithelioid sarcoma: a clinicopathologic and immunohistochemical analysis of 106 cases from the French Sarcoma Group. Am J Clin Pathol. 2009;131:222-227.
- Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
- Requena L, Santonja C, Martinez-Amo JL, et al. Cutaneous epithelioid sarcoma like (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149:459-465.
- Trombetta D, Magnusson L, von Steyern FV, et al. Translocation t(7;19)(q22;q13)—a recurrent chromosome aberration in pseudomyogenic hemangioendothelioma? Cancer Genet. 2011;204:211-215.
- Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma (pseudomyogenic hemangioendothelioma). Am J Surg Pathol. 2011;35:1088; author reply 1088-1089.
- Mirra JM, Kessler S, Bhuta S, et al. The fibroma-like variant of epithelioid sarcoma. a fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69:1382-1395.
- Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46:95-104.
- Hornick JL, Fletcher CD. Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol. 2011;35:190-201.
- Sheng W, Pan Y, Wang J. Pseudomyogenic hemangioendothelioma: report of an additional case with aggressive clinical course. Am J Dermatopathol. 2013;35:597-600.
- Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma. Am J Surg Pathol. 2003;27:48-57.
- Pradhan D, Schoedel K, McGough RL, et al. Pseudomyogenic hemangioendothelioma of skin, bone and soft tissue—a clinicopathological, immunohistochemical and fluorescence in situ hybridization study [published online November 2, 2017]. Hum Pathol. 2017. doi:0.1016/j.humpath.2017.10.023.
- Requena L, Santonja C, Martinez-Amo JL, et al. Cutaneous epithelioid sarcoma like (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149:459-465.
- McGinity M, Bartanusz V, Dengler B, et al. Pseudomyogenic hemangioendothelioma (epithelioid sarcoma-like hemangioendothelioma, fibroma-like variant of epithelioid sarcoma) of the thoracic spine. Eur Spine J. 2013;22(suppl 3):S506-S511.
- Stuart LN, Gardner JM, Lauer SR, et al. Epithelioid sarcoma-like (pseudomyogenic) hemangioendothelioma, clinically mimicking dermatofibroma, diagnosed by skin biopsy in a 30-year-old man. J Cutan Pathol. 2013;40:909-913.
- Amary MF, O’Donnell P, Berisha F, et al. Pseudomyogenic (epithelioid sarcoma-like) hemangioendothelioma: characterization of five cases. Skeletal Radiol. 2013;42:947-957.
- Hornick JL, Dal Cin P, Fletcher CD. Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol. 2009;33:542-550.
- Chbani L, Guillou L, Terrier P, et al. Epithelioid sarcoma: a clinicopathologic and immunohistochemical analysis of 106 cases from the French Sarcoma Group. Am J Clin Pathol. 2009;131:222-227.
- Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
- Requena L, Santonja C, Martinez-Amo JL, et al. Cutaneous epithelioid sarcoma like (pseudomyogenic) hemangioendothelioma: a little-known low-grade cutaneous vascular neoplasm. JAMA Dermatol. 2013;149:459-465.
- Trombetta D, Magnusson L, von Steyern FV, et al. Translocation t(7;19)(q22;q13)—a recurrent chromosome aberration in pseudomyogenic hemangioendothelioma? Cancer Genet. 2011;204:211-215.
Practice Points
- Pseudomyogenic hemangioendothelioma (PMHE) is an uncommon vascular tumor that most often presents as multiple distinct nodules on the legs in young men.
- Pseudomyogenic hemangioendothelioma has an unusual immunohistochemistry staining pattern, with positive staining for cytokeratin AE1/AE3, CD31, and ERG but negative for CD34.
- Although local reoccurrence is common, PMHE metastasis is very uncommon.
Sequential chemotherapy and radiotherapy may be best in locally advanced NSCLC with negative margins
Chemotherapy followed by radiation resulted in superior outcomes compared with concurrent chemoradiotherapy in patients with non–small-cell lung cancer (NSCLC) who had negative margins after surgery and pN2 disease, according to results of a retrospective analysis.
“We also found that sequential therapy is becoming the more frequent practice pattern over time for patients with R0 pN2 disease, and our results support this practice,” wrote Samual Francis, MD, of the University of Utah Huntsman Cancer Institute, Salt Lake City, and his coauthors.
By contrast, there was no clear association between treatment sequence and survival among patients who had positive margins after surgery in this analysis, authors of the study reported (J Clin Oncol. 2017 Dec 13. doi: 10.1200/JCO.2017.74.4771).
The study included 1,024 patients in National Cancer Database who received a diagnosis of nonmetastatic NSCLC and received chemotherapy and radiation concurrently or sequentially after surgery. Of those patients, 747 had R0 resections and pN2 nodal status, and the remainder had R1 or R2 resections and pN0-N2 disease.
For patients with R0 pN2 NSCLC, median overall survival was 58.8 months for sequential chemotherapy followed by radiation, versus 40.4 months for concurrent chemoradiotherapy (log-rank P less than .001), data show.
That association between sequential treatment and improved overall survival remained significant even after propensity score matching (hazard ratio [HR], 1.35; P = .019), investigators reported.
However, for the remaining cohort of patients with positive margins regardless of nodal status, there was no significant difference in overall survival favoring either approach (42.6 months for sequential versus 38.5 months for concurrent; log-rank P = .42), they added, and no clear association between treatment approaches (HR 1.35; P = .19).
The analysis covered patients diagnosed between 2006 and 2012, the most recent data period available.
Investigators were also able to identify changes in practice patterns over time using this data set. Between 2006 and 2012, there was an increase in the use of sequential chemotherapy and radiotherapy for patients with R0 resection and pN2 disease, and a decrease in the use of concurrent chemoradiotherapy.
Conversely, they found that for patients with positive margins, there was an increase over time in use of the concurrent approach and decrease in the sequential approach.
“These findings suggest practice patterns have shifted toward concordance with consensus guideline recommendations over time,” Dr. Francis and his colleagues said in their report.
Current guidelines advocate for chemotherapy followed by postoperative radiotherapy for patients with negative margins and pN2 disease, but suggest concurrent chemoradiotherapy for patients with positive margins, they added.
Dr. Francis had no relationships to disclose. Study coauthors reported disclosures related to Elekta, ProLung, Merit Medical Systems, and Bard Medical.
Chemotherapy followed by radiation resulted in superior outcomes compared with concurrent chemoradiotherapy in patients with non–small-cell lung cancer (NSCLC) who had negative margins after surgery and pN2 disease, according to results of a retrospective analysis.
“We also found that sequential therapy is becoming the more frequent practice pattern over time for patients with R0 pN2 disease, and our results support this practice,” wrote Samual Francis, MD, of the University of Utah Huntsman Cancer Institute, Salt Lake City, and his coauthors.
By contrast, there was no clear association between treatment sequence and survival among patients who had positive margins after surgery in this analysis, authors of the study reported (J Clin Oncol. 2017 Dec 13. doi: 10.1200/JCO.2017.74.4771).
The study included 1,024 patients in National Cancer Database who received a diagnosis of nonmetastatic NSCLC and received chemotherapy and radiation concurrently or sequentially after surgery. Of those patients, 747 had R0 resections and pN2 nodal status, and the remainder had R1 or R2 resections and pN0-N2 disease.
For patients with R0 pN2 NSCLC, median overall survival was 58.8 months for sequential chemotherapy followed by radiation, versus 40.4 months for concurrent chemoradiotherapy (log-rank P less than .001), data show.
That association between sequential treatment and improved overall survival remained significant even after propensity score matching (hazard ratio [HR], 1.35; P = .019), investigators reported.
However, for the remaining cohort of patients with positive margins regardless of nodal status, there was no significant difference in overall survival favoring either approach (42.6 months for sequential versus 38.5 months for concurrent; log-rank P = .42), they added, and no clear association between treatment approaches (HR 1.35; P = .19).
The analysis covered patients diagnosed between 2006 and 2012, the most recent data period available.
Investigators were also able to identify changes in practice patterns over time using this data set. Between 2006 and 2012, there was an increase in the use of sequential chemotherapy and radiotherapy for patients with R0 resection and pN2 disease, and a decrease in the use of concurrent chemoradiotherapy.
Conversely, they found that for patients with positive margins, there was an increase over time in use of the concurrent approach and decrease in the sequential approach.
“These findings suggest practice patterns have shifted toward concordance with consensus guideline recommendations over time,” Dr. Francis and his colleagues said in their report.
Current guidelines advocate for chemotherapy followed by postoperative radiotherapy for patients with negative margins and pN2 disease, but suggest concurrent chemoradiotherapy for patients with positive margins, they added.
Dr. Francis had no relationships to disclose. Study coauthors reported disclosures related to Elekta, ProLung, Merit Medical Systems, and Bard Medical.
Chemotherapy followed by radiation resulted in superior outcomes compared with concurrent chemoradiotherapy in patients with non–small-cell lung cancer (NSCLC) who had negative margins after surgery and pN2 disease, according to results of a retrospective analysis.
“We also found that sequential therapy is becoming the more frequent practice pattern over time for patients with R0 pN2 disease, and our results support this practice,” wrote Samual Francis, MD, of the University of Utah Huntsman Cancer Institute, Salt Lake City, and his coauthors.
By contrast, there was no clear association between treatment sequence and survival among patients who had positive margins after surgery in this analysis, authors of the study reported (J Clin Oncol. 2017 Dec 13. doi: 10.1200/JCO.2017.74.4771).
The study included 1,024 patients in National Cancer Database who received a diagnosis of nonmetastatic NSCLC and received chemotherapy and radiation concurrently or sequentially after surgery. Of those patients, 747 had R0 resections and pN2 nodal status, and the remainder had R1 or R2 resections and pN0-N2 disease.
For patients with R0 pN2 NSCLC, median overall survival was 58.8 months for sequential chemotherapy followed by radiation, versus 40.4 months for concurrent chemoradiotherapy (log-rank P less than .001), data show.
That association between sequential treatment and improved overall survival remained significant even after propensity score matching (hazard ratio [HR], 1.35; P = .019), investigators reported.
However, for the remaining cohort of patients with positive margins regardless of nodal status, there was no significant difference in overall survival favoring either approach (42.6 months for sequential versus 38.5 months for concurrent; log-rank P = .42), they added, and no clear association between treatment approaches (HR 1.35; P = .19).
The analysis covered patients diagnosed between 2006 and 2012, the most recent data period available.
Investigators were also able to identify changes in practice patterns over time using this data set. Between 2006 and 2012, there was an increase in the use of sequential chemotherapy and radiotherapy for patients with R0 resection and pN2 disease, and a decrease in the use of concurrent chemoradiotherapy.
Conversely, they found that for patients with positive margins, there was an increase over time in use of the concurrent approach and decrease in the sequential approach.
“These findings suggest practice patterns have shifted toward concordance with consensus guideline recommendations over time,” Dr. Francis and his colleagues said in their report.
Current guidelines advocate for chemotherapy followed by postoperative radiotherapy for patients with negative margins and pN2 disease, but suggest concurrent chemoradiotherapy for patients with positive margins, they added.
Dr. Francis had no relationships to disclose. Study coauthors reported disclosures related to Elekta, ProLung, Merit Medical Systems, and Bard Medical.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: In patients with pN2 non–small-cell lung cancer who have negative margins after surgery, sequential chemotherapy and radiotherapy provided superior outcomes compared with concurrent chemoradiotherapy.
Major finding: Median overall survival was 58.8 months for the sequential approach and 40.4 months for concurrent (log-rank P less than .001).
Data source: A retrospective analysis including 1,024 patients with nonmetastatic NSCLC who received chemotherapy and radiation concurrently or sequentially after surgery.
Disclosures: Study authors reported disclosures related to Elekta, ProLung, Merit Medical Systems, and Bard Medical.
Sleepless in adolescence
One thing that constantly surprises me about adolescent sleep is that neither the teen nor the parent is as concerned about it as I am. Instead, they complain about irritability, dropping grades, anxiety, depression, obesity, oppositionality, fatigue, and even substance use – all documented effects of sleep debt.
Inadequate sleep changes the brain, resulting in thinner gray matter, less neuroplasticity, poorer higher-level cognitive abilities (attention, working memory, inhibition, judgment, decision-making), lower motivation, and poorer academic functioning. None of these are losses teens can afford!
While sleep problems are more common in those with mental health disorders, poor sleep precedes anxiety and depression more than the reverse. Sleep problems increase the risk of depression, and depression relapses. Insomnia predicts risk behaviors – drinking and driving, smoking, delinquency. Getting less than 8 hours of sleep is associated with a threefold higher risk of suicide attempts.
Despite these pervasive threats to health and development, instead of concern, I find a lot of resistance in families and teens to taking action to improve sleep.
Teens don’t believe in problems from inadequate sleep. After all, they say, their peers are “all” getting the same amount of sleep. And they are largely correct – 75% of U.S. 12th graders get less than 8 hours of sleep. But the data are clear that children aged 12-18 years need 8.25-9.25 hours of sleep.
Parents generally are not aware of how little sleep their teens are getting because they go to bed on their own. If parents do check, any teenagers worth the label can growl their way out of supervision, “promise” to shut off the lights, or feign sleep. Having the house, pantry, and electronics to themselves at night is worth the risk of a consequence, especially for those who would rather avoid interacting.
The social forces keeping teens up at night are their “life”: the hours required for homework can be the reason for inadequate sleep. In subgroups of teens, sports practices, employment, or family responsibilities may extend the day past a bedtime needed for optimal sleep.
But use of electronics – the lifeline of adolescents – is responsible for much of their sleep debt. Electronic devices both delay sleep onset and reduce sleep duration. After 9:00 p.m., 34% of children aged older than 12 years are text messaging, 44% are talking, 55% are online, and 24% are playing computer games. Use of a TV or tablet at bedtime results in reduced sleep, and increased poor quality of sleep. Three or more hours of TV result not only in difficulty falling asleep and frequent awakenings, but also sleep issues later as adults. Shooter video games result in lower sleepiness, longer sleep latency, and shorter REM sleep. Even the low level light from electronic devices alters circadian rhythm and suppresses nocturnal melatonin secretion.
Keep in mind the biological reasons teens go to bed later. One is the typical emotional hyperarousal of being a teen. But other biological forces are at work in adolescence, such as reduction in the accumulation of sleep pressure during wakefulness and delaying the melatonin release that produces sleepiness. Teens (and parents) think sleeping in on weekends takes care of inadequate weekday sleep, but this so-called “recovery sleep” tends to occur at an inappropriate time in the circadian phase and further delays melatonin production, as well as reducing sleep pressure, making it even harder to fall asleep.
In some cases, medications we prescribe – such as stimulants, theophylline, antihistamines, or anticonvulsants – are at fault for delaying or disturbing sleep. But more often it is self-administered substances that are part of the teen’s attempt to stay awake – including nicotine, alcohol, and caffeine – that produce shorter sleep duration, increased latency to sleep, more wake time during sleep, and increased daytime sleepiness; it results in a vicious cycle. Sleep disruption may explain the association of these substances with less memory consolidation, poorer academic performance, and higher rates of risk behaviors.
We adults also are a cause of teen sleep debt. We are the ones allowing the early school start times for teens, primarily to allow for after school sports programs that glorify the school and bring kudos to some at the expense of all the students. A 65-minute earlier start in 10th grade resulted in less than half of students getting 7 hours of sleep or more. The level of resulting sleepiness is equal to that of narcolepsy.
As primary care clinicians, we can and need to detect, educate about, and treat sleep debt and sleep disorders. Sleep questionnaires can help. Treatment of sleep includes coaching for: having a cool, dark room used mainly for sleep; a regular schedule 7 days per week; avoiding exercise within 2 hours of bedtime; avoiding stimulants such as caffeine, tea, nicotine, and medications at least 3 hours before bedtime; keeping to a routine with no daytime naps; and especially no media in the bedroom! For teens already not able to sleep until early morning, you can recommend that they work bedtime back or forward by 1 hour per day until hitting a time that will allow 9 hours of sleep. Alternatively, have them stay up all night to reset their biological clock. Subsequently, the sleep schedule has to stay within 1 hour for sleep and waking 7 days per week. Anxious teens, besides needing therapy, may need a soothing routine, no visible clock, and a plan to get back up for 1 hour every time it takes longer than 10 minutes to fall asleep.
If sleepy teens report adequate time in bed, then we need to understand pathologies such as obstructive sleep apnea, restless legs syndrome, menstruation-related or primary hypersomnias, and narcolepsy to diagnose and resolve the problem.
Parents may have given up protecting their teens from inadequate sleep so we as health providers need to do so.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at [email protected].
One thing that constantly surprises me about adolescent sleep is that neither the teen nor the parent is as concerned about it as I am. Instead, they complain about irritability, dropping grades, anxiety, depression, obesity, oppositionality, fatigue, and even substance use – all documented effects of sleep debt.
Inadequate sleep changes the brain, resulting in thinner gray matter, less neuroplasticity, poorer higher-level cognitive abilities (attention, working memory, inhibition, judgment, decision-making), lower motivation, and poorer academic functioning. None of these are losses teens can afford!
While sleep problems are more common in those with mental health disorders, poor sleep precedes anxiety and depression more than the reverse. Sleep problems increase the risk of depression, and depression relapses. Insomnia predicts risk behaviors – drinking and driving, smoking, delinquency. Getting less than 8 hours of sleep is associated with a threefold higher risk of suicide attempts.
Despite these pervasive threats to health and development, instead of concern, I find a lot of resistance in families and teens to taking action to improve sleep.
Teens don’t believe in problems from inadequate sleep. After all, they say, their peers are “all” getting the same amount of sleep. And they are largely correct – 75% of U.S. 12th graders get less than 8 hours of sleep. But the data are clear that children aged 12-18 years need 8.25-9.25 hours of sleep.
Parents generally are not aware of how little sleep their teens are getting because they go to bed on their own. If parents do check, any teenagers worth the label can growl their way out of supervision, “promise” to shut off the lights, or feign sleep. Having the house, pantry, and electronics to themselves at night is worth the risk of a consequence, especially for those who would rather avoid interacting.
The social forces keeping teens up at night are their “life”: the hours required for homework can be the reason for inadequate sleep. In subgroups of teens, sports practices, employment, or family responsibilities may extend the day past a bedtime needed for optimal sleep.
But use of electronics – the lifeline of adolescents – is responsible for much of their sleep debt. Electronic devices both delay sleep onset and reduce sleep duration. After 9:00 p.m., 34% of children aged older than 12 years are text messaging, 44% are talking, 55% are online, and 24% are playing computer games. Use of a TV or tablet at bedtime results in reduced sleep, and increased poor quality of sleep. Three or more hours of TV result not only in difficulty falling asleep and frequent awakenings, but also sleep issues later as adults. Shooter video games result in lower sleepiness, longer sleep latency, and shorter REM sleep. Even the low level light from electronic devices alters circadian rhythm and suppresses nocturnal melatonin secretion.
Keep in mind the biological reasons teens go to bed later. One is the typical emotional hyperarousal of being a teen. But other biological forces are at work in adolescence, such as reduction in the accumulation of sleep pressure during wakefulness and delaying the melatonin release that produces sleepiness. Teens (and parents) think sleeping in on weekends takes care of inadequate weekday sleep, but this so-called “recovery sleep” tends to occur at an inappropriate time in the circadian phase and further delays melatonin production, as well as reducing sleep pressure, making it even harder to fall asleep.
In some cases, medications we prescribe – such as stimulants, theophylline, antihistamines, or anticonvulsants – are at fault for delaying or disturbing sleep. But more often it is self-administered substances that are part of the teen’s attempt to stay awake – including nicotine, alcohol, and caffeine – that produce shorter sleep duration, increased latency to sleep, more wake time during sleep, and increased daytime sleepiness; it results in a vicious cycle. Sleep disruption may explain the association of these substances with less memory consolidation, poorer academic performance, and higher rates of risk behaviors.
We adults also are a cause of teen sleep debt. We are the ones allowing the early school start times for teens, primarily to allow for after school sports programs that glorify the school and bring kudos to some at the expense of all the students. A 65-minute earlier start in 10th grade resulted in less than half of students getting 7 hours of sleep or more. The level of resulting sleepiness is equal to that of narcolepsy.
As primary care clinicians, we can and need to detect, educate about, and treat sleep debt and sleep disorders. Sleep questionnaires can help. Treatment of sleep includes coaching for: having a cool, dark room used mainly for sleep; a regular schedule 7 days per week; avoiding exercise within 2 hours of bedtime; avoiding stimulants such as caffeine, tea, nicotine, and medications at least 3 hours before bedtime; keeping to a routine with no daytime naps; and especially no media in the bedroom! For teens already not able to sleep until early morning, you can recommend that they work bedtime back or forward by 1 hour per day until hitting a time that will allow 9 hours of sleep. Alternatively, have them stay up all night to reset their biological clock. Subsequently, the sleep schedule has to stay within 1 hour for sleep and waking 7 days per week. Anxious teens, besides needing therapy, may need a soothing routine, no visible clock, and a plan to get back up for 1 hour every time it takes longer than 10 minutes to fall asleep.
If sleepy teens report adequate time in bed, then we need to understand pathologies such as obstructive sleep apnea, restless legs syndrome, menstruation-related or primary hypersomnias, and narcolepsy to diagnose and resolve the problem.
Parents may have given up protecting their teens from inadequate sleep so we as health providers need to do so.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at [email protected].
One thing that constantly surprises me about adolescent sleep is that neither the teen nor the parent is as concerned about it as I am. Instead, they complain about irritability, dropping grades, anxiety, depression, obesity, oppositionality, fatigue, and even substance use – all documented effects of sleep debt.
Inadequate sleep changes the brain, resulting in thinner gray matter, less neuroplasticity, poorer higher-level cognitive abilities (attention, working memory, inhibition, judgment, decision-making), lower motivation, and poorer academic functioning. None of these are losses teens can afford!
While sleep problems are more common in those with mental health disorders, poor sleep precedes anxiety and depression more than the reverse. Sleep problems increase the risk of depression, and depression relapses. Insomnia predicts risk behaviors – drinking and driving, smoking, delinquency. Getting less than 8 hours of sleep is associated with a threefold higher risk of suicide attempts.
Despite these pervasive threats to health and development, instead of concern, I find a lot of resistance in families and teens to taking action to improve sleep.
Teens don’t believe in problems from inadequate sleep. After all, they say, their peers are “all” getting the same amount of sleep. And they are largely correct – 75% of U.S. 12th graders get less than 8 hours of sleep. But the data are clear that children aged 12-18 years need 8.25-9.25 hours of sleep.
Parents generally are not aware of how little sleep their teens are getting because they go to bed on their own. If parents do check, any teenagers worth the label can growl their way out of supervision, “promise” to shut off the lights, or feign sleep. Having the house, pantry, and electronics to themselves at night is worth the risk of a consequence, especially for those who would rather avoid interacting.
The social forces keeping teens up at night are their “life”: the hours required for homework can be the reason for inadequate sleep. In subgroups of teens, sports practices, employment, or family responsibilities may extend the day past a bedtime needed for optimal sleep.
But use of electronics – the lifeline of adolescents – is responsible for much of their sleep debt. Electronic devices both delay sleep onset and reduce sleep duration. After 9:00 p.m., 34% of children aged older than 12 years are text messaging, 44% are talking, 55% are online, and 24% are playing computer games. Use of a TV or tablet at bedtime results in reduced sleep, and increased poor quality of sleep. Three or more hours of TV result not only in difficulty falling asleep and frequent awakenings, but also sleep issues later as adults. Shooter video games result in lower sleepiness, longer sleep latency, and shorter REM sleep. Even the low level light from electronic devices alters circadian rhythm and suppresses nocturnal melatonin secretion.
Keep in mind the biological reasons teens go to bed later. One is the typical emotional hyperarousal of being a teen. But other biological forces are at work in adolescence, such as reduction in the accumulation of sleep pressure during wakefulness and delaying the melatonin release that produces sleepiness. Teens (and parents) think sleeping in on weekends takes care of inadequate weekday sleep, but this so-called “recovery sleep” tends to occur at an inappropriate time in the circadian phase and further delays melatonin production, as well as reducing sleep pressure, making it even harder to fall asleep.
In some cases, medications we prescribe – such as stimulants, theophylline, antihistamines, or anticonvulsants – are at fault for delaying or disturbing sleep. But more often it is self-administered substances that are part of the teen’s attempt to stay awake – including nicotine, alcohol, and caffeine – that produce shorter sleep duration, increased latency to sleep, more wake time during sleep, and increased daytime sleepiness; it results in a vicious cycle. Sleep disruption may explain the association of these substances with less memory consolidation, poorer academic performance, and higher rates of risk behaviors.
We adults also are a cause of teen sleep debt. We are the ones allowing the early school start times for teens, primarily to allow for after school sports programs that glorify the school and bring kudos to some at the expense of all the students. A 65-minute earlier start in 10th grade resulted in less than half of students getting 7 hours of sleep or more. The level of resulting sleepiness is equal to that of narcolepsy.
As primary care clinicians, we can and need to detect, educate about, and treat sleep debt and sleep disorders. Sleep questionnaires can help. Treatment of sleep includes coaching for: having a cool, dark room used mainly for sleep; a regular schedule 7 days per week; avoiding exercise within 2 hours of bedtime; avoiding stimulants such as caffeine, tea, nicotine, and medications at least 3 hours before bedtime; keeping to a routine with no daytime naps; and especially no media in the bedroom! For teens already not able to sleep until early morning, you can recommend that they work bedtime back or forward by 1 hour per day until hitting a time that will allow 9 hours of sleep. Alternatively, have them stay up all night to reset their biological clock. Subsequently, the sleep schedule has to stay within 1 hour for sleep and waking 7 days per week. Anxious teens, besides needing therapy, may need a soothing routine, no visible clock, and a plan to get back up for 1 hour every time it takes longer than 10 minutes to fall asleep.
If sleepy teens report adequate time in bed, then we need to understand pathologies such as obstructive sleep apnea, restless legs syndrome, menstruation-related or primary hypersomnias, and narcolepsy to diagnose and resolve the problem.
Parents may have given up protecting their teens from inadequate sleep so we as health providers need to do so.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to Frontline Medical News. E-mail her at [email protected].
Management of Acute Coronary Syndromes in Patients with Diabetes
HERCULES: Caplacizumab improved platelet response in aTTP
ATLANTA – Adding caplacizumab to standard therapy for acquired thrombotic thrombocytopenic purpura (aTTP) significantly improved platelet response and prevented recurrent episodes in an international, randomized, double-blind, phase 3 placebo-controlled trial.
“Patients were 55% more likely to achieve normalization of platelet count in the caplacizumab group, and this was highly significant,” Marie Scully, MD, said during a late-breaking presentation at the annual meeting of the American Society of Hematology.
“So far, phase 2 and phase 3 studies confirm no deaths in patients treated with caplacizumab,” said Dr. Scully of University College London Hospitals NHS Trust.
Acquired TTP is an acute, life-threatening thrombotic microangiopathy that occurs when inhibitory autoantibodies cause a deficiency of ADAMTS13, an enzyme that cleaves von Willebrand factor (vWF). Inadequate levels of ADAMTS13 activity lead to the formation of ultra-large vWF multimers, platelet strings, and microthrombi. Caplacizumab is a humanized immunoglobulin that helps stop this process by binding the A1 domain of vWF.
“Caplacizumab addresses the pathophysiological platelet aggregation that leads to the formation of microthrombi,” Dr. Scully said.
In this phase 3 trial (HERCULES), 145 patients who had received plasma exchange (PE) at least once for an acute episode of aTTP were randomly assigned to receive caplacizumab or placebo injections plus daily PE and corticosteroids. Caplacizumab (10-mg) therapy consisted of one IV bolus followed by subcutaneous treatment for 30 days. Patients whose ADAMTS13 activity remained below 10% were able to continue treatment for another 28 days, after which all patients were followed for another 28 days without treatment.
At any given time, platelet normalization was 55% more likely with caplacizumab (10 mg) than placebo (platelet normalization rate ratio, 1.55; 95% confidence interval, 1.10-2.20; P less than .01).
Rates of a key combined secondary endpoint – aTTP-related death/recurrence/major thrombotic events – were 12.7% with caplacizumab and 49.3% with placebo (P less than .0001).
Rates of major thrombotic events were 8% in each arm, and deaths were rare overall, meaning that the difference in rates of recurrence drove most of the effect on the combined secondary endpoint. However, patients who received caplacizumab also received 41% less plasma and had 38% fewer days of PE, 65% fewer days in the intensive care unit, and 31% fewer days in the hospital than patients in the placebo arm.
Refractory aTTP affected 4.2% of placebo patients and no caplacizumab patients, which trended toward statistical significance (P = .057). Caplacizumab therapy also led to a faster normalization of several markers of organ damage, including lactate dehydrogenase, cardiac troponin 1, and serum creatinine.
Safety findings reflected earlier-phase studies and caplacizumab’s mechanism of action, Dr. Scully said. Nearly all trial participants experienced at least one treatment-emergent adverse event. Such events led 7% of patients to stop caplacizumab and 12% to stop placebo. Caplacizumab was associated with a range of bleeding-related events, most commonly epistaxis (23.9 vs. 1.4% with placebo), gingival bleeding (11.3% vs. 0%), bruising (7.0% vs. 4.1%), and hematuria (5.6% vs. 1.4%).
Patients in both arms tended to be in their 40s and two-thirds were female. At baseline, about 85% had less than 10% ADAMTS13 activity, and about 40% had severe aTTP (French severity scores of at least 3 or severe neurologic or cardiac involvement).
“Most [66%] patients in the caplacizumab group presented with de novo aTTP, and this is relevant because patients who are in their first episode are much harder to treat,” Dr. Scully said. About half of placebo patients had de novo disease.
In July 2017, caplacizumab received a fast-track designation from the Food and Drug Administration for aTTP after a phase 2 trial showed that platelet counts normalized 39% faster with caplacizumab versus placebo plus standard of care (P = .005). Of eight patients in that study who relapsed within a month of stopping caplacizumab, seven still had less than 10% ADAMTS13 activity, “suggesting unresolved autoimmune activity,” the investigators wrote at the time in the New England Journal of Medicine (2016;374:511-22).
Dr. Scully reported similar findings in the phase 3 HERCULES trial, noting that all patients had ADAMTS13 levels less than 5% on stopping caplacizumab.
Ablynx provided funding for the study. Dr. Scully reported financial relationships with Ablynx, Shire, Novartis, and Alexion.
SOURCE: Scully M et al., ASH 2017 Abstract LBA-1.
ATLANTA – Adding caplacizumab to standard therapy for acquired thrombotic thrombocytopenic purpura (aTTP) significantly improved platelet response and prevented recurrent episodes in an international, randomized, double-blind, phase 3 placebo-controlled trial.
“Patients were 55% more likely to achieve normalization of platelet count in the caplacizumab group, and this was highly significant,” Marie Scully, MD, said during a late-breaking presentation at the annual meeting of the American Society of Hematology.
“So far, phase 2 and phase 3 studies confirm no deaths in patients treated with caplacizumab,” said Dr. Scully of University College London Hospitals NHS Trust.
Acquired TTP is an acute, life-threatening thrombotic microangiopathy that occurs when inhibitory autoantibodies cause a deficiency of ADAMTS13, an enzyme that cleaves von Willebrand factor (vWF). Inadequate levels of ADAMTS13 activity lead to the formation of ultra-large vWF multimers, platelet strings, and microthrombi. Caplacizumab is a humanized immunoglobulin that helps stop this process by binding the A1 domain of vWF.
“Caplacizumab addresses the pathophysiological platelet aggregation that leads to the formation of microthrombi,” Dr. Scully said.
In this phase 3 trial (HERCULES), 145 patients who had received plasma exchange (PE) at least once for an acute episode of aTTP were randomly assigned to receive caplacizumab or placebo injections plus daily PE and corticosteroids. Caplacizumab (10-mg) therapy consisted of one IV bolus followed by subcutaneous treatment for 30 days. Patients whose ADAMTS13 activity remained below 10% were able to continue treatment for another 28 days, after which all patients were followed for another 28 days without treatment.
At any given time, platelet normalization was 55% more likely with caplacizumab (10 mg) than placebo (platelet normalization rate ratio, 1.55; 95% confidence interval, 1.10-2.20; P less than .01).
Rates of a key combined secondary endpoint – aTTP-related death/recurrence/major thrombotic events – were 12.7% with caplacizumab and 49.3% with placebo (P less than .0001).
Rates of major thrombotic events were 8% in each arm, and deaths were rare overall, meaning that the difference in rates of recurrence drove most of the effect on the combined secondary endpoint. However, patients who received caplacizumab also received 41% less plasma and had 38% fewer days of PE, 65% fewer days in the intensive care unit, and 31% fewer days in the hospital than patients in the placebo arm.
Refractory aTTP affected 4.2% of placebo patients and no caplacizumab patients, which trended toward statistical significance (P = .057). Caplacizumab therapy also led to a faster normalization of several markers of organ damage, including lactate dehydrogenase, cardiac troponin 1, and serum creatinine.
Safety findings reflected earlier-phase studies and caplacizumab’s mechanism of action, Dr. Scully said. Nearly all trial participants experienced at least one treatment-emergent adverse event. Such events led 7% of patients to stop caplacizumab and 12% to stop placebo. Caplacizumab was associated with a range of bleeding-related events, most commonly epistaxis (23.9 vs. 1.4% with placebo), gingival bleeding (11.3% vs. 0%), bruising (7.0% vs. 4.1%), and hematuria (5.6% vs. 1.4%).
Patients in both arms tended to be in their 40s and two-thirds were female. At baseline, about 85% had less than 10% ADAMTS13 activity, and about 40% had severe aTTP (French severity scores of at least 3 or severe neurologic or cardiac involvement).
“Most [66%] patients in the caplacizumab group presented with de novo aTTP, and this is relevant because patients who are in their first episode are much harder to treat,” Dr. Scully said. About half of placebo patients had de novo disease.
In July 2017, caplacizumab received a fast-track designation from the Food and Drug Administration for aTTP after a phase 2 trial showed that platelet counts normalized 39% faster with caplacizumab versus placebo plus standard of care (P = .005). Of eight patients in that study who relapsed within a month of stopping caplacizumab, seven still had less than 10% ADAMTS13 activity, “suggesting unresolved autoimmune activity,” the investigators wrote at the time in the New England Journal of Medicine (2016;374:511-22).
Dr. Scully reported similar findings in the phase 3 HERCULES trial, noting that all patients had ADAMTS13 levels less than 5% on stopping caplacizumab.
Ablynx provided funding for the study. Dr. Scully reported financial relationships with Ablynx, Shire, Novartis, and Alexion.
SOURCE: Scully M et al., ASH 2017 Abstract LBA-1.
ATLANTA – Adding caplacizumab to standard therapy for acquired thrombotic thrombocytopenic purpura (aTTP) significantly improved platelet response and prevented recurrent episodes in an international, randomized, double-blind, phase 3 placebo-controlled trial.
“Patients were 55% more likely to achieve normalization of platelet count in the caplacizumab group, and this was highly significant,” Marie Scully, MD, said during a late-breaking presentation at the annual meeting of the American Society of Hematology.
“So far, phase 2 and phase 3 studies confirm no deaths in patients treated with caplacizumab,” said Dr. Scully of University College London Hospitals NHS Trust.
Acquired TTP is an acute, life-threatening thrombotic microangiopathy that occurs when inhibitory autoantibodies cause a deficiency of ADAMTS13, an enzyme that cleaves von Willebrand factor (vWF). Inadequate levels of ADAMTS13 activity lead to the formation of ultra-large vWF multimers, platelet strings, and microthrombi. Caplacizumab is a humanized immunoglobulin that helps stop this process by binding the A1 domain of vWF.
“Caplacizumab addresses the pathophysiological platelet aggregation that leads to the formation of microthrombi,” Dr. Scully said.
In this phase 3 trial (HERCULES), 145 patients who had received plasma exchange (PE) at least once for an acute episode of aTTP were randomly assigned to receive caplacizumab or placebo injections plus daily PE and corticosteroids. Caplacizumab (10-mg) therapy consisted of one IV bolus followed by subcutaneous treatment for 30 days. Patients whose ADAMTS13 activity remained below 10% were able to continue treatment for another 28 days, after which all patients were followed for another 28 days without treatment.
At any given time, platelet normalization was 55% more likely with caplacizumab (10 mg) than placebo (platelet normalization rate ratio, 1.55; 95% confidence interval, 1.10-2.20; P less than .01).
Rates of a key combined secondary endpoint – aTTP-related death/recurrence/major thrombotic events – were 12.7% with caplacizumab and 49.3% with placebo (P less than .0001).
Rates of major thrombotic events were 8% in each arm, and deaths were rare overall, meaning that the difference in rates of recurrence drove most of the effect on the combined secondary endpoint. However, patients who received caplacizumab also received 41% less plasma and had 38% fewer days of PE, 65% fewer days in the intensive care unit, and 31% fewer days in the hospital than patients in the placebo arm.
Refractory aTTP affected 4.2% of placebo patients and no caplacizumab patients, which trended toward statistical significance (P = .057). Caplacizumab therapy also led to a faster normalization of several markers of organ damage, including lactate dehydrogenase, cardiac troponin 1, and serum creatinine.
Safety findings reflected earlier-phase studies and caplacizumab’s mechanism of action, Dr. Scully said. Nearly all trial participants experienced at least one treatment-emergent adverse event. Such events led 7% of patients to stop caplacizumab and 12% to stop placebo. Caplacizumab was associated with a range of bleeding-related events, most commonly epistaxis (23.9 vs. 1.4% with placebo), gingival bleeding (11.3% vs. 0%), bruising (7.0% vs. 4.1%), and hematuria (5.6% vs. 1.4%).
Patients in both arms tended to be in their 40s and two-thirds were female. At baseline, about 85% had less than 10% ADAMTS13 activity, and about 40% had severe aTTP (French severity scores of at least 3 or severe neurologic or cardiac involvement).
“Most [66%] patients in the caplacizumab group presented with de novo aTTP, and this is relevant because patients who are in their first episode are much harder to treat,” Dr. Scully said. About half of placebo patients had de novo disease.
In July 2017, caplacizumab received a fast-track designation from the Food and Drug Administration for aTTP after a phase 2 trial showed that platelet counts normalized 39% faster with caplacizumab versus placebo plus standard of care (P = .005). Of eight patients in that study who relapsed within a month of stopping caplacizumab, seven still had less than 10% ADAMTS13 activity, “suggesting unresolved autoimmune activity,” the investigators wrote at the time in the New England Journal of Medicine (2016;374:511-22).
Dr. Scully reported similar findings in the phase 3 HERCULES trial, noting that all patients had ADAMTS13 levels less than 5% on stopping caplacizumab.
Ablynx provided funding for the study. Dr. Scully reported financial relationships with Ablynx, Shire, Novartis, and Alexion.
SOURCE: Scully M et al., ASH 2017 Abstract LBA-1.
REPORTING FROM ASH 2017
Key clinical point:
Major finding: The rate of platelet normalization was 55% faster for caplacizumab vs. placebo plus standard of care (platelet normalization rate ratio, 1.55; P less than .01).
Study details: A randomized double-blind phase 3 trial of 145 patients.
Disclosures: Ablynx provided funding for the study. Dr. Scully reported financial relationships with Ablynx, Shire, Novartis, and Alexion.
Source: Scully M et al. ASH 2017 Abstract LBA-1.
Head injury linked to amyloid deposition decades later
SAN DIEGO – , according to PET imaging in 329 older adults without dementia.
Previous studies have found an association between head injury and later dementia, but the mechanism isn’t understood. The importance of the new investigation is that it “hints at pathophysiology. We were very excited” to find amyloid in the frontal cortex, “which is also associated with Alzheimer’s disease,” said lead investigator Andrea Schneider, MD, PhD, a neurology resident at Johns Hopkins University, Baltimore.
Increased amyloid deposition was not found in other areas associated with the disease, but frontal cortex involvement “does suggest perhaps a common” denominator, she noted.
The older adults in the study were all participants in the Atherosclerosis Risk in Communities (ARIC) cohort, a group of over 15,000 community-dwelling adults who have been followed since the late 1980s. All 329 had brain amyloid deposition assessed by florbetapir (Amyvid) PET scans in 2011-2013; mean age was 76 years.
Sixty-six (20%) reported a history of head trauma at a median of about 25 years before the scan, with self-reports correlating well with hospitalization billing codes collected as part of ARIC. The cause of the head trauma was not known.
Head injury was associated with elevated standardized uptake value ratios (SUVRs, greater than 1.2). Head injury patients had a 1.31-fold increased prevalence of elevated global amyloid deposition (95% confidence interval, 1.07-1.60) as well as a 1.24-fold increased prevalence of elevated deposition in the orbitofrontal cortex (95% CI, 1.02-1.50) and prefrontal cortex (95% CI, 1.03-1.49), and 1.29-fold increased prevalence in the superior frontal cortex (95% CI, 1.06-1.56).
There were no differences in the prevalence of elevated SUVRs in the anterior cingulate, posterior cingulate, precuneus, or the lateral temporal, parietal, or occipital lobes (all P greater than .05) Dr. Schneider reported.
The model was adjusted for age, sex, race, total intracranial volume, and cardiovascular disease, among other things.
Thirty percent of the participants had either one or two APOE4 alleles, and 27% had mild cognitive impairment; neither correlated with increased amyloid deposition.
Head injuries were more common among participants who were men (43%) or white (57%). There was a trend for a slightly stronger link between head injury and increased amyloid deposition among black individuals (P for interaction 0.169).
Dr. Schneider is continuing her work to illuminate the connection between head trauma and dementia. She plans to integrate more detailed cognitive data from ARIC into the analysis, and perhaps emergency department and outpatient data. She also wants to look at more acute imaging after head trauma.
The work was funded by the National Institutes of Health. Avid Radiopharmaceuticals provided the florbetapir. Dr. Schneider did not have any relevant disclosures.
SOURCE: Schneider A et al. ANA 2017 abstract M336WIP
SAN DIEGO – , according to PET imaging in 329 older adults without dementia.
Previous studies have found an association between head injury and later dementia, but the mechanism isn’t understood. The importance of the new investigation is that it “hints at pathophysiology. We were very excited” to find amyloid in the frontal cortex, “which is also associated with Alzheimer’s disease,” said lead investigator Andrea Schneider, MD, PhD, a neurology resident at Johns Hopkins University, Baltimore.
Increased amyloid deposition was not found in other areas associated with the disease, but frontal cortex involvement “does suggest perhaps a common” denominator, she noted.
The older adults in the study were all participants in the Atherosclerosis Risk in Communities (ARIC) cohort, a group of over 15,000 community-dwelling adults who have been followed since the late 1980s. All 329 had brain amyloid deposition assessed by florbetapir (Amyvid) PET scans in 2011-2013; mean age was 76 years.
Sixty-six (20%) reported a history of head trauma at a median of about 25 years before the scan, with self-reports correlating well with hospitalization billing codes collected as part of ARIC. The cause of the head trauma was not known.
Head injury was associated with elevated standardized uptake value ratios (SUVRs, greater than 1.2). Head injury patients had a 1.31-fold increased prevalence of elevated global amyloid deposition (95% confidence interval, 1.07-1.60) as well as a 1.24-fold increased prevalence of elevated deposition in the orbitofrontal cortex (95% CI, 1.02-1.50) and prefrontal cortex (95% CI, 1.03-1.49), and 1.29-fold increased prevalence in the superior frontal cortex (95% CI, 1.06-1.56).
There were no differences in the prevalence of elevated SUVRs in the anterior cingulate, posterior cingulate, precuneus, or the lateral temporal, parietal, or occipital lobes (all P greater than .05) Dr. Schneider reported.
The model was adjusted for age, sex, race, total intracranial volume, and cardiovascular disease, among other things.
Thirty percent of the participants had either one or two APOE4 alleles, and 27% had mild cognitive impairment; neither correlated with increased amyloid deposition.
Head injuries were more common among participants who were men (43%) or white (57%). There was a trend for a slightly stronger link between head injury and increased amyloid deposition among black individuals (P for interaction 0.169).
Dr. Schneider is continuing her work to illuminate the connection between head trauma and dementia. She plans to integrate more detailed cognitive data from ARIC into the analysis, and perhaps emergency department and outpatient data. She also wants to look at more acute imaging after head trauma.
The work was funded by the National Institutes of Health. Avid Radiopharmaceuticals provided the florbetapir. Dr. Schneider did not have any relevant disclosures.
SOURCE: Schneider A et al. ANA 2017 abstract M336WIP
SAN DIEGO – , according to PET imaging in 329 older adults without dementia.
Previous studies have found an association between head injury and later dementia, but the mechanism isn’t understood. The importance of the new investigation is that it “hints at pathophysiology. We were very excited” to find amyloid in the frontal cortex, “which is also associated with Alzheimer’s disease,” said lead investigator Andrea Schneider, MD, PhD, a neurology resident at Johns Hopkins University, Baltimore.
Increased amyloid deposition was not found in other areas associated with the disease, but frontal cortex involvement “does suggest perhaps a common” denominator, she noted.
The older adults in the study were all participants in the Atherosclerosis Risk in Communities (ARIC) cohort, a group of over 15,000 community-dwelling adults who have been followed since the late 1980s. All 329 had brain amyloid deposition assessed by florbetapir (Amyvid) PET scans in 2011-2013; mean age was 76 years.
Sixty-six (20%) reported a history of head trauma at a median of about 25 years before the scan, with self-reports correlating well with hospitalization billing codes collected as part of ARIC. The cause of the head trauma was not known.
Head injury was associated with elevated standardized uptake value ratios (SUVRs, greater than 1.2). Head injury patients had a 1.31-fold increased prevalence of elevated global amyloid deposition (95% confidence interval, 1.07-1.60) as well as a 1.24-fold increased prevalence of elevated deposition in the orbitofrontal cortex (95% CI, 1.02-1.50) and prefrontal cortex (95% CI, 1.03-1.49), and 1.29-fold increased prevalence in the superior frontal cortex (95% CI, 1.06-1.56).
There were no differences in the prevalence of elevated SUVRs in the anterior cingulate, posterior cingulate, precuneus, or the lateral temporal, parietal, or occipital lobes (all P greater than .05) Dr. Schneider reported.
The model was adjusted for age, sex, race, total intracranial volume, and cardiovascular disease, among other things.
Thirty percent of the participants had either one or two APOE4 alleles, and 27% had mild cognitive impairment; neither correlated with increased amyloid deposition.
Head injuries were more common among participants who were men (43%) or white (57%). There was a trend for a slightly stronger link between head injury and increased amyloid deposition among black individuals (P for interaction 0.169).
Dr. Schneider is continuing her work to illuminate the connection between head trauma and dementia. She plans to integrate more detailed cognitive data from ARIC into the analysis, and perhaps emergency department and outpatient data. She also wants to look at more acute imaging after head trauma.
The work was funded by the National Institutes of Health. Avid Radiopharmaceuticals provided the florbetapir. Dr. Schneider did not have any relevant disclosures.
SOURCE: Schneider A et al. ANA 2017 abstract M336WIP
REPORTING FROM ANA 2017
Key clinical point: Head injury is associated with increased brain amyloid deposition globally and in the frontal cortex, which may help explain the association between head trauma and dementia.
Major finding: Head injury patients had a 1.31-fold increased prevalence of elevated global amyloid deposition (95% CI, 1.07-1.60) as well as a 1.24-fold increased prevalence of elevated deposition in the orbitofrontal cortex (95% CI, 1.02-1.50) and prefrontal cortex (95% CI, 1.03-1.49) and 1.29-fold increased prevalence in the superior frontal cortex (95% CI, 1.06-1.56).
Data source: PET imaging in 329 older adults without dementia.
Disclosures: The work was funded by the National Institutes of Health. Avid Radiopharmaceuticals provided the florbetapir. The lead author did not have any relevant disclosures.
Source: Schneider A et al. ANA 2017 abstract M336WIP.
Practice changing events of 2017
Members of the Pediatric News Editorial Advisory Board share some of the events and findings of 2017 that they believe have or will have the most impact on pediatric practice.
Francis Rushton Jr., MD, practiced pediatrics in Beaufort, S.C. for 32 years, and currently is the medical director of S.C. Quality through Technology and Innovation in Pediatrics (QTIP), funded by the South Carolina Department of Health and Human Services.
Dropping the human papillomavirus (HPV) regimen to two shots from three shots, as recommended by the Centers for Disease Control and Prevention, appears to have really improved uptake of HPV immunization.
Preventive oral health in the pediatrician’s office is not really a new recommendation from 2017; we have been talking about fluoride varnish for over a decade. What is new is that we gradually are seeing fluoride varnish move into practice, up from 1,000 applications in pediatric offices in 2011 to close to 20,000 applications in South Carolina alone.
Pediatricians are being asked to screen more and more. We’re asked to do developmental screening, postpartum depression screening, autism screening, behavioral health screening, social determinants of health screening, parental concerns screening, etc. As a result, we now have multiple different screens with different schedules. The Survey of Well-Being of Young Children screening tool does it all – one screen at each preschool well visit from birth to age 5 years.
A different approach is to use CHADIS (Child Health and Development Interactive System), a for-profit venture where all the screens are loaded electronically.
Howard Smart, MD, is chairman of pediatrics at Sharp Rees-Stealy Medical Group, San Diego.
The switch to a two-dose schedule for HPV vaccination has improved both acceptance of the vaccine and the likelihood of timely completion of the HPV series.
Kelly Curran, MD, MA, is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, practicing adolescent medicine.
The news from Australia is that the older meningitis B vaccine (MeNZB) provides some protection against Neisseria gonorrhoeae, as reported in the Lancet (2017 July 10. doi: 10.1016/S0140-6736[17]31449-6)! The newer version of the meningitis B vaccine Bexsero also contains the same outer membrane vesicle antigen. Given increasing bacterial resistance – and pan-resistant gonorrhea organisms already in some parts of the world – this is exciting news for the future!
The increased use of the reverse screening algorithm for syphilis is exciting. Although this has been “available” for several years, increasingly more physicians/laboratories are using this in practice. Our academic center – in a relatively high prevalence area for syphilis – recently switched to this screening method.
M. Susan Jay, MD, is a professor of pediatrics and section chief of adolescent medicine at the Medical College of Wisconsin and program director of adolescent health and medicine at the Children’s Hospital of Wisconsin, both in Milwaukee.
In adolescent medicine, the addition of long-acting reversible contraceptives has been wonderful as an aid to both menstrual management and contraception. Specifically, Liletta, a new IUD that is smaller in size and remains in place for 5 years as well as being considerably more cost effective, has changed care for adolescent females.
Suzanne C. Boulter, MD, is adjunct professor of pediatrics and community and family medicine at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
I was very impressed with the recent American Academy of Pediatrics policy on human trafficking published in Pediatrics (2017 November. doi: 10.1542/peds.2017-3138), and think this is a new area of knowledge of which pediatricians need to be aware.
With all the news and social media about sexual misconduct by persons in power, I’m a bit concerned that there could be a fallout on pediatricians performing appropriate examinations on their patients that could be interpreted as something else.
Timothy J. Joos, MD, MPH, is a practicing clinician in combined internal medicine/pediatrics in Seattle. For the last decade, he has worked at a federally qualified community health center in Seattle serving a largely low-income and immigrant population.
With regard to practice-changing events for 2017, I don’t wish to downplay the numerous research advances over the year, but the advances cannot be made without funding, and they are not going to be practice-changing if they can’t reach the patients. We can’t ignore the uncertainty that the current political situation in 2017 has caused for our patients and their families, as well as for research and for the health care industry in general.
It is impossible to deny the important role government health care programs play in the health of our own patients and the health of the whole country. According to numbers from the Kaiser Family Foundation website, currently 38% of the estimated 74 million kids in this country are covered by Medicaid and CHIP programs. The numbers of uninsured children are at all-time lows at 5% (adults 10%). The current uncertainty of government funding is felt strongly by safety net providers such as community health centers that have traditionally seen the uninsured patients. The community health center where I work went from 35% of its patients being uninsured before the Affordable Care Act to about 15% now.
Efforts to dismantle the Affordable Care Act and reverse Medicaid expansions, as well as delays on funding to the CHIP program, have created uncertainty and anxiety across health care from the administrators and insurance companies to us – the providers – and the families we take care of. In addition, National Institutes of Health funding is threatened to be cut by 20%. 2017 will go down in history as the year of health care toxic stress (that is, unless 2018 is worse). As we celebrate the end of the year, we all deserve a Xanax and a Zantac.
Members of the Pediatric News Editorial Advisory Board share some of the events and findings of 2017 that they believe have or will have the most impact on pediatric practice.
Francis Rushton Jr., MD, practiced pediatrics in Beaufort, S.C. for 32 years, and currently is the medical director of S.C. Quality through Technology and Innovation in Pediatrics (QTIP), funded by the South Carolina Department of Health and Human Services.
Dropping the human papillomavirus (HPV) regimen to two shots from three shots, as recommended by the Centers for Disease Control and Prevention, appears to have really improved uptake of HPV immunization.
Preventive oral health in the pediatrician’s office is not really a new recommendation from 2017; we have been talking about fluoride varnish for over a decade. What is new is that we gradually are seeing fluoride varnish move into practice, up from 1,000 applications in pediatric offices in 2011 to close to 20,000 applications in South Carolina alone.
Pediatricians are being asked to screen more and more. We’re asked to do developmental screening, postpartum depression screening, autism screening, behavioral health screening, social determinants of health screening, parental concerns screening, etc. As a result, we now have multiple different screens with different schedules. The Survey of Well-Being of Young Children screening tool does it all – one screen at each preschool well visit from birth to age 5 years.
A different approach is to use CHADIS (Child Health and Development Interactive System), a for-profit venture where all the screens are loaded electronically.
Howard Smart, MD, is chairman of pediatrics at Sharp Rees-Stealy Medical Group, San Diego.
The switch to a two-dose schedule for HPV vaccination has improved both acceptance of the vaccine and the likelihood of timely completion of the HPV series.
Kelly Curran, MD, MA, is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, practicing adolescent medicine.
The news from Australia is that the older meningitis B vaccine (MeNZB) provides some protection against Neisseria gonorrhoeae, as reported in the Lancet (2017 July 10. doi: 10.1016/S0140-6736[17]31449-6)! The newer version of the meningitis B vaccine Bexsero also contains the same outer membrane vesicle antigen. Given increasing bacterial resistance – and pan-resistant gonorrhea organisms already in some parts of the world – this is exciting news for the future!
The increased use of the reverse screening algorithm for syphilis is exciting. Although this has been “available” for several years, increasingly more physicians/laboratories are using this in practice. Our academic center – in a relatively high prevalence area for syphilis – recently switched to this screening method.
M. Susan Jay, MD, is a professor of pediatrics and section chief of adolescent medicine at the Medical College of Wisconsin and program director of adolescent health and medicine at the Children’s Hospital of Wisconsin, both in Milwaukee.
In adolescent medicine, the addition of long-acting reversible contraceptives has been wonderful as an aid to both menstrual management and contraception. Specifically, Liletta, a new IUD that is smaller in size and remains in place for 5 years as well as being considerably more cost effective, has changed care for adolescent females.
Suzanne C. Boulter, MD, is adjunct professor of pediatrics and community and family medicine at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
I was very impressed with the recent American Academy of Pediatrics policy on human trafficking published in Pediatrics (2017 November. doi: 10.1542/peds.2017-3138), and think this is a new area of knowledge of which pediatricians need to be aware.
With all the news and social media about sexual misconduct by persons in power, I’m a bit concerned that there could be a fallout on pediatricians performing appropriate examinations on their patients that could be interpreted as something else.
Timothy J. Joos, MD, MPH, is a practicing clinician in combined internal medicine/pediatrics in Seattle. For the last decade, he has worked at a federally qualified community health center in Seattle serving a largely low-income and immigrant population.
With regard to practice-changing events for 2017, I don’t wish to downplay the numerous research advances over the year, but the advances cannot be made without funding, and they are not going to be practice-changing if they can’t reach the patients. We can’t ignore the uncertainty that the current political situation in 2017 has caused for our patients and their families, as well as for research and for the health care industry in general.
It is impossible to deny the important role government health care programs play in the health of our own patients and the health of the whole country. According to numbers from the Kaiser Family Foundation website, currently 38% of the estimated 74 million kids in this country are covered by Medicaid and CHIP programs. The numbers of uninsured children are at all-time lows at 5% (adults 10%). The current uncertainty of government funding is felt strongly by safety net providers such as community health centers that have traditionally seen the uninsured patients. The community health center where I work went from 35% of its patients being uninsured before the Affordable Care Act to about 15% now.
Efforts to dismantle the Affordable Care Act and reverse Medicaid expansions, as well as delays on funding to the CHIP program, have created uncertainty and anxiety across health care from the administrators and insurance companies to us – the providers – and the families we take care of. In addition, National Institutes of Health funding is threatened to be cut by 20%. 2017 will go down in history as the year of health care toxic stress (that is, unless 2018 is worse). As we celebrate the end of the year, we all deserve a Xanax and a Zantac.
Members of the Pediatric News Editorial Advisory Board share some of the events and findings of 2017 that they believe have or will have the most impact on pediatric practice.
Francis Rushton Jr., MD, practiced pediatrics in Beaufort, S.C. for 32 years, and currently is the medical director of S.C. Quality through Technology and Innovation in Pediatrics (QTIP), funded by the South Carolina Department of Health and Human Services.
Dropping the human papillomavirus (HPV) regimen to two shots from three shots, as recommended by the Centers for Disease Control and Prevention, appears to have really improved uptake of HPV immunization.
Preventive oral health in the pediatrician’s office is not really a new recommendation from 2017; we have been talking about fluoride varnish for over a decade. What is new is that we gradually are seeing fluoride varnish move into practice, up from 1,000 applications in pediatric offices in 2011 to close to 20,000 applications in South Carolina alone.
Pediatricians are being asked to screen more and more. We’re asked to do developmental screening, postpartum depression screening, autism screening, behavioral health screening, social determinants of health screening, parental concerns screening, etc. As a result, we now have multiple different screens with different schedules. The Survey of Well-Being of Young Children screening tool does it all – one screen at each preschool well visit from birth to age 5 years.
A different approach is to use CHADIS (Child Health and Development Interactive System), a for-profit venture where all the screens are loaded electronically.
Howard Smart, MD, is chairman of pediatrics at Sharp Rees-Stealy Medical Group, San Diego.
The switch to a two-dose schedule for HPV vaccination has improved both acceptance of the vaccine and the likelihood of timely completion of the HPV series.
Kelly Curran, MD, MA, is an assistant professor of pediatrics at the University of Oklahoma Health Sciences Center, Oklahoma City, practicing adolescent medicine.
The news from Australia is that the older meningitis B vaccine (MeNZB) provides some protection against Neisseria gonorrhoeae, as reported in the Lancet (2017 July 10. doi: 10.1016/S0140-6736[17]31449-6)! The newer version of the meningitis B vaccine Bexsero also contains the same outer membrane vesicle antigen. Given increasing bacterial resistance – and pan-resistant gonorrhea organisms already in some parts of the world – this is exciting news for the future!
The increased use of the reverse screening algorithm for syphilis is exciting. Although this has been “available” for several years, increasingly more physicians/laboratories are using this in practice. Our academic center – in a relatively high prevalence area for syphilis – recently switched to this screening method.
M. Susan Jay, MD, is a professor of pediatrics and section chief of adolescent medicine at the Medical College of Wisconsin and program director of adolescent health and medicine at the Children’s Hospital of Wisconsin, both in Milwaukee.
In adolescent medicine, the addition of long-acting reversible contraceptives has been wonderful as an aid to both menstrual management and contraception. Specifically, Liletta, a new IUD that is smaller in size and remains in place for 5 years as well as being considerably more cost effective, has changed care for adolescent females.
Suzanne C. Boulter, MD, is adjunct professor of pediatrics and community and family medicine at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
I was very impressed with the recent American Academy of Pediatrics policy on human trafficking published in Pediatrics (2017 November. doi: 10.1542/peds.2017-3138), and think this is a new area of knowledge of which pediatricians need to be aware.
With all the news and social media about sexual misconduct by persons in power, I’m a bit concerned that there could be a fallout on pediatricians performing appropriate examinations on their patients that could be interpreted as something else.
Timothy J. Joos, MD, MPH, is a practicing clinician in combined internal medicine/pediatrics in Seattle. For the last decade, he has worked at a federally qualified community health center in Seattle serving a largely low-income and immigrant population.
With regard to practice-changing events for 2017, I don’t wish to downplay the numerous research advances over the year, but the advances cannot be made without funding, and they are not going to be practice-changing if they can’t reach the patients. We can’t ignore the uncertainty that the current political situation in 2017 has caused for our patients and their families, as well as for research and for the health care industry in general.
It is impossible to deny the important role government health care programs play in the health of our own patients and the health of the whole country. According to numbers from the Kaiser Family Foundation website, currently 38% of the estimated 74 million kids in this country are covered by Medicaid and CHIP programs. The numbers of uninsured children are at all-time lows at 5% (adults 10%). The current uncertainty of government funding is felt strongly by safety net providers such as community health centers that have traditionally seen the uninsured patients. The community health center where I work went from 35% of its patients being uninsured before the Affordable Care Act to about 15% now.
Efforts to dismantle the Affordable Care Act and reverse Medicaid expansions, as well as delays on funding to the CHIP program, have created uncertainty and anxiety across health care from the administrators and insurance companies to us – the providers – and the families we take care of. In addition, National Institutes of Health funding is threatened to be cut by 20%. 2017 will go down in history as the year of health care toxic stress (that is, unless 2018 is worse). As we celebrate the end of the year, we all deserve a Xanax and a Zantac.
When should I transfuse a patient who has anemia?
Clinical case
A 48-year-old man with cirrhosis and esophageal varices presents to the emergency department with hematochezia and hematemesis. Upon arrival to the floor, he has another bout of hematochezia and hematemesis. He appears pale. His pulse is 90 beats per minute, his blood pressure is 100/60 mmHg, and his respiratory rate is 14 breaths per minute. A complete blood count reveals a hemoglobin level of 7.8 g/dL and hematocrit of 23.5%. Should he receive a blood transfusion?
Introduction
Anemia is one of the most frequent conditions in hospitalized patients. Anemia is variably associated with morbidity and mortality depending on chronicity, etiology, and associated comorbidities. Before the 1980s, standard practice was to transfuse all patients to a hemoglobin level greater than 10 g/dL and/or a hematocrit greater than 30%. However, with concerns about the potential adverse effects and cost of transfusions, the safety and effectiveness of liberal versus restrictive transfusion thresholds became the subject of many studies.
The 2016 the AABB (formerly American Association of Blood Banks) guidelines focused on the evidence for hemodynamically stable and asymptomatic hospitalized patients. The guidelines are based on randomized controlled trials that measured mortality as the primary endpoint. Most trials and guidelines reinforce that if a patient is symptomatic or hemodynamically unstable from anemia or hemorrhage, RBC transfusion is appropriate irrespective of hemoglobin level.
Overview of the data
Critically ill patients
The Transfusion Requirements in Critical Care (TRICC) trial, published in 1999, was the first large clinical trial examining the safety of restrictive transfusion thresholds in critically ill patients.3
The subsequent Transfusion Requirements in Septic Shock (TRISS) study involved patients with septic shock and similarly found that patients assigned to a restrictive strategy (transfusion for hemoglobin less than 7 g/dL) had similar outcomes to patients assigned to a liberal strategy (transfusion for hemoglobin less than 9 g/dL). The patients in the restrictive group received fewer transfusions, but had similar rates of 90-day mortality, use of life support, and number of days alive and out of the hospital.4
These large randomized controlled trials in critically ill patients served as the basis for subsequent studies in patient populations outside of the ICU.
Acute upper GI bleed
Acute upper gastrointestinal bleeding (UGIB) is one of the most common indications for RBC transfusion.
The TRIGGER trial, a cluster randomized multicenter study published in 2015, also found no difference in clinical outcomes, including mortality, between a restrictive strategy and liberal strategy for transfusion of patients with UGIB.6
Perioperative patients
Transfusion thresholds have been studied in large randomized trials for perioperative patients undergoing cardiac and orthopedic surgery.
The Transfusion Requirements after Cardiac Surgery (TRACS) trial, published in 2010, randomized patients undergoing cardiac surgery at a single center to a liberal strategy of blood transfusion (to maintain a hematocrit 30% or greater) or a restrictive strategy (hematocrit 24% or greater).7 Mortality and severe morbidity rates were noninferior in the restrictive strategy group. Mean hemoglobin concentrations were 10.5 g/dL in the liberal-strategy group and 9.1 g/dL in the restrictive-strategy group. Independent of transfusion strategy, the number of transfused red blood cell units was an independent risk factor for clinical complications and death at 30 days.
Based on these two trials, as well as other smaller randomized controlled trials and observational studies, the AABB guidelines recommend a restrictive RBC transfusion threshold of 8 g/dL for patients undergoing cardiac or orthopedic surgery.1
Acute coronary syndrome, stable coronary artery disease, and congestive heart failure
Anemia is an independent predictor of major adverse cardiovascular events in patients with acute coronary syndrome.9 However, it remains controversial if transfusion has benefit or causes harm in patients with acute coronary syndrome. No randomized controlled trials have yet been published on this topic, and observational studies and subgroups from randomized controlled trials have yielded mixed results.
Similarly, there are no randomized controlled trials examining liberal versus restrictive transfusion goals for asymptomatic hospitalized patients with stable coronary artery disease (CAD). However, patients with CAD were included in the TRICC and FOCUS trials.3,8 Of patients enrolled in the TRICC trial, 26% had a primary or secondary diagnosis of cardiac disease; subgroup analysis found no significant differences in 30-day mortality between treatment groups, similar to that of the entire study population.3 In a subgroup analysis of patients with “cardiovascular disease” the FOCUS trial found no difference in outcomes between a restrictive and liberal transfusion strategy, although there was a marginally higher incidence of myocardial infarction in the restrictive arm in the entire study population.8
Although some studies have included patients with congestive heart failure (CHF) as a subgroup, these subgroups are often not powered to show clinically meaningful differences. The risk of volume overload is one explanation for why transfusions may be harmful for patients with CHF.
Based on these limited available data, the AABB guidelines recommend a “restrictive RBC transfusion threshold (hemoglobin level of 8 g/dL)” for patients “with preexisting cardiovascular disease,” without distinguishing among patients with acute coronary syndrome, stable CAD, and CHF, but adds that the “threshold of 7 g/dL is likely comparable with 8 g/dL, but randomized controlled trial evidence is not available for all patient categories.”1
Of note, certain patient populations, such as those with end-stage renal disease, oncology patients (especially those undergoing active chemotherapy), and those with comorbid conditions such as thrombocytopenia and coagulopathy are specifically excluded from the AABB guidelines. The reader is referred to guidelines from respective specialty societies for further guidance.
Back to our case
This 48-year-old man with cirrhosis and esophageal varices presenting with ongoing blood loss due to hematochezia and hematemesis with a hemoglobin of 7.8 g/dL should be transfused due to his active bleeding; his hemoglobin level should not be a determining factor under such circumstances. This distinction is one of the most fundamental in interpreting the guidelines, that is, patients with hemodynamic insult, symptoms, and/or ongoing bleeding should be evaluated clinically, independent of their hemoglobin level.
Bottom line
Although recent guidelines generally favor a more restrictive hemoglobin goal threshold, in the presence of active blood loss or hemodynamic instability, blood transfusion should be considered independent of the initial hemoglobin level.
Key Points
- The AABB guidelines recommend transfusing stable general medical inpatients to a hemoglobin level of 7 g/dL.
- For patients undergoing orthopedic surgery, cardiac surgery, and those with preexisting cardiovascular disease, a transfusion threshold of 8 g/dL is recommended.
- Patients with hemodynamic instability or active blood loss should be transfused based on clinical criteria, and not absolute hemoglobin levels.
- Patients with acute coronary syndrome, severe thrombocytopenia, and chronic transfusion–dependent anemia are excluded from these recommendations.
- Further studies are needed to further refine these recommendations to specific patient populations in maximizing the benefits and minimizing the risks of blood transfusions.
Quiz
In which of the following scenarios is RBC transfusion LEAST indicated?
- A. Active diverticular bleed, heart rate 125 beats/minute, blood pressure 85/65 mm HG, hemoglobin 10.5 g/dL
- B. Septic shock in the ICU, hemoglobin 6.7 g/dL
- C. Pulmonary embolism in the setting of stable coronary artery disease and hemoglobin 8.7 g/dL
- D. Lymphoma on chemotherapy, hemoglobin 7.5 g/d, patient becomes dizzy when standing
Answer: C – A hemoglobin transfusion goal of 8.0 g/dL is recommended for patients with cardiovascular disease. The patient in answer choice A has active blood loss, tachycardia, and hypotension, all pointing to the need for blood transfusion irrespective of an initial hemoglobin level greater than 8.0 g/dL. The patient in answer choice B has a hemoglobin level less than 7.0 g/dL, and the patient in choice D is symptomatic, possibly from anemia, as well as on chemotherapy, therefore making transfusion a reasonable option.
Dr. Sampat, Dr. Berger, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. Carson JL et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-35.
2. Dwyre DM et al. Hepatitis B, hepatitis C and HIV transfusion-transmitted infections in the 21st century. Vox Sang. 2011;100:92-8.
3. Hébert PC et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-17.
4. Holst LB et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-91.
5. Villanueva C et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.
6. Jairath V et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015;386:137-44.
7. Hajjar LA et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559-67.
8. Carson JL et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-62.
9. Sabatine MS et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042-9.
Clinical case
A 48-year-old man with cirrhosis and esophageal varices presents to the emergency department with hematochezia and hematemesis. Upon arrival to the floor, he has another bout of hematochezia and hematemesis. He appears pale. His pulse is 90 beats per minute, his blood pressure is 100/60 mmHg, and his respiratory rate is 14 breaths per minute. A complete blood count reveals a hemoglobin level of 7.8 g/dL and hematocrit of 23.5%. Should he receive a blood transfusion?
Introduction
Anemia is one of the most frequent conditions in hospitalized patients. Anemia is variably associated with morbidity and mortality depending on chronicity, etiology, and associated comorbidities. Before the 1980s, standard practice was to transfuse all patients to a hemoglobin level greater than 10 g/dL and/or a hematocrit greater than 30%. However, with concerns about the potential adverse effects and cost of transfusions, the safety and effectiveness of liberal versus restrictive transfusion thresholds became the subject of many studies.
The 2016 the AABB (formerly American Association of Blood Banks) guidelines focused on the evidence for hemodynamically stable and asymptomatic hospitalized patients. The guidelines are based on randomized controlled trials that measured mortality as the primary endpoint. Most trials and guidelines reinforce that if a patient is symptomatic or hemodynamically unstable from anemia or hemorrhage, RBC transfusion is appropriate irrespective of hemoglobin level.
Overview of the data
Critically ill patients
The Transfusion Requirements in Critical Care (TRICC) trial, published in 1999, was the first large clinical trial examining the safety of restrictive transfusion thresholds in critically ill patients.3
The subsequent Transfusion Requirements in Septic Shock (TRISS) study involved patients with septic shock and similarly found that patients assigned to a restrictive strategy (transfusion for hemoglobin less than 7 g/dL) had similar outcomes to patients assigned to a liberal strategy (transfusion for hemoglobin less than 9 g/dL). The patients in the restrictive group received fewer transfusions, but had similar rates of 90-day mortality, use of life support, and number of days alive and out of the hospital.4
These large randomized controlled trials in critically ill patients served as the basis for subsequent studies in patient populations outside of the ICU.
Acute upper GI bleed
Acute upper gastrointestinal bleeding (UGIB) is one of the most common indications for RBC transfusion.
The TRIGGER trial, a cluster randomized multicenter study published in 2015, also found no difference in clinical outcomes, including mortality, between a restrictive strategy and liberal strategy for transfusion of patients with UGIB.6
Perioperative patients
Transfusion thresholds have been studied in large randomized trials for perioperative patients undergoing cardiac and orthopedic surgery.
The Transfusion Requirements after Cardiac Surgery (TRACS) trial, published in 2010, randomized patients undergoing cardiac surgery at a single center to a liberal strategy of blood transfusion (to maintain a hematocrit 30% or greater) or a restrictive strategy (hematocrit 24% or greater).7 Mortality and severe morbidity rates were noninferior in the restrictive strategy group. Mean hemoglobin concentrations were 10.5 g/dL in the liberal-strategy group and 9.1 g/dL in the restrictive-strategy group. Independent of transfusion strategy, the number of transfused red blood cell units was an independent risk factor for clinical complications and death at 30 days.
Based on these two trials, as well as other smaller randomized controlled trials and observational studies, the AABB guidelines recommend a restrictive RBC transfusion threshold of 8 g/dL for patients undergoing cardiac or orthopedic surgery.1
Acute coronary syndrome, stable coronary artery disease, and congestive heart failure
Anemia is an independent predictor of major adverse cardiovascular events in patients with acute coronary syndrome.9 However, it remains controversial if transfusion has benefit or causes harm in patients with acute coronary syndrome. No randomized controlled trials have yet been published on this topic, and observational studies and subgroups from randomized controlled trials have yielded mixed results.
Similarly, there are no randomized controlled trials examining liberal versus restrictive transfusion goals for asymptomatic hospitalized patients with stable coronary artery disease (CAD). However, patients with CAD were included in the TRICC and FOCUS trials.3,8 Of patients enrolled in the TRICC trial, 26% had a primary or secondary diagnosis of cardiac disease; subgroup analysis found no significant differences in 30-day mortality between treatment groups, similar to that of the entire study population.3 In a subgroup analysis of patients with “cardiovascular disease” the FOCUS trial found no difference in outcomes between a restrictive and liberal transfusion strategy, although there was a marginally higher incidence of myocardial infarction in the restrictive arm in the entire study population.8
Although some studies have included patients with congestive heart failure (CHF) as a subgroup, these subgroups are often not powered to show clinically meaningful differences. The risk of volume overload is one explanation for why transfusions may be harmful for patients with CHF.
Based on these limited available data, the AABB guidelines recommend a “restrictive RBC transfusion threshold (hemoglobin level of 8 g/dL)” for patients “with preexisting cardiovascular disease,” without distinguishing among patients with acute coronary syndrome, stable CAD, and CHF, but adds that the “threshold of 7 g/dL is likely comparable with 8 g/dL, but randomized controlled trial evidence is not available for all patient categories.”1
Of note, certain patient populations, such as those with end-stage renal disease, oncology patients (especially those undergoing active chemotherapy), and those with comorbid conditions such as thrombocytopenia and coagulopathy are specifically excluded from the AABB guidelines. The reader is referred to guidelines from respective specialty societies for further guidance.
Back to our case
This 48-year-old man with cirrhosis and esophageal varices presenting with ongoing blood loss due to hematochezia and hematemesis with a hemoglobin of 7.8 g/dL should be transfused due to his active bleeding; his hemoglobin level should not be a determining factor under such circumstances. This distinction is one of the most fundamental in interpreting the guidelines, that is, patients with hemodynamic insult, symptoms, and/or ongoing bleeding should be evaluated clinically, independent of their hemoglobin level.
Bottom line
Although recent guidelines generally favor a more restrictive hemoglobin goal threshold, in the presence of active blood loss or hemodynamic instability, blood transfusion should be considered independent of the initial hemoglobin level.
Key Points
- The AABB guidelines recommend transfusing stable general medical inpatients to a hemoglobin level of 7 g/dL.
- For patients undergoing orthopedic surgery, cardiac surgery, and those with preexisting cardiovascular disease, a transfusion threshold of 8 g/dL is recommended.
- Patients with hemodynamic instability or active blood loss should be transfused based on clinical criteria, and not absolute hemoglobin levels.
- Patients with acute coronary syndrome, severe thrombocytopenia, and chronic transfusion–dependent anemia are excluded from these recommendations.
- Further studies are needed to further refine these recommendations to specific patient populations in maximizing the benefits and minimizing the risks of blood transfusions.
Quiz
In which of the following scenarios is RBC transfusion LEAST indicated?
- A. Active diverticular bleed, heart rate 125 beats/minute, blood pressure 85/65 mm HG, hemoglobin 10.5 g/dL
- B. Septic shock in the ICU, hemoglobin 6.7 g/dL
- C. Pulmonary embolism in the setting of stable coronary artery disease and hemoglobin 8.7 g/dL
- D. Lymphoma on chemotherapy, hemoglobin 7.5 g/d, patient becomes dizzy when standing
Answer: C – A hemoglobin transfusion goal of 8.0 g/dL is recommended for patients with cardiovascular disease. The patient in answer choice A has active blood loss, tachycardia, and hypotension, all pointing to the need for blood transfusion irrespective of an initial hemoglobin level greater than 8.0 g/dL. The patient in answer choice B has a hemoglobin level less than 7.0 g/dL, and the patient in choice D is symptomatic, possibly from anemia, as well as on chemotherapy, therefore making transfusion a reasonable option.
Dr. Sampat, Dr. Berger, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. Carson JL et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-35.
2. Dwyre DM et al. Hepatitis B, hepatitis C and HIV transfusion-transmitted infections in the 21st century. Vox Sang. 2011;100:92-8.
3. Hébert PC et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-17.
4. Holst LB et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-91.
5. Villanueva C et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.
6. Jairath V et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015;386:137-44.
7. Hajjar LA et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559-67.
8. Carson JL et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-62.
9. Sabatine MS et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042-9.
Clinical case
A 48-year-old man with cirrhosis and esophageal varices presents to the emergency department with hematochezia and hematemesis. Upon arrival to the floor, he has another bout of hematochezia and hematemesis. He appears pale. His pulse is 90 beats per minute, his blood pressure is 100/60 mmHg, and his respiratory rate is 14 breaths per minute. A complete blood count reveals a hemoglobin level of 7.8 g/dL and hematocrit of 23.5%. Should he receive a blood transfusion?
Introduction
Anemia is one of the most frequent conditions in hospitalized patients. Anemia is variably associated with morbidity and mortality depending on chronicity, etiology, and associated comorbidities. Before the 1980s, standard practice was to transfuse all patients to a hemoglobin level greater than 10 g/dL and/or a hematocrit greater than 30%. However, with concerns about the potential adverse effects and cost of transfusions, the safety and effectiveness of liberal versus restrictive transfusion thresholds became the subject of many studies.
The 2016 the AABB (formerly American Association of Blood Banks) guidelines focused on the evidence for hemodynamically stable and asymptomatic hospitalized patients. The guidelines are based on randomized controlled trials that measured mortality as the primary endpoint. Most trials and guidelines reinforce that if a patient is symptomatic or hemodynamically unstable from anemia or hemorrhage, RBC transfusion is appropriate irrespective of hemoglobin level.
Overview of the data
Critically ill patients
The Transfusion Requirements in Critical Care (TRICC) trial, published in 1999, was the first large clinical trial examining the safety of restrictive transfusion thresholds in critically ill patients.3
The subsequent Transfusion Requirements in Septic Shock (TRISS) study involved patients with septic shock and similarly found that patients assigned to a restrictive strategy (transfusion for hemoglobin less than 7 g/dL) had similar outcomes to patients assigned to a liberal strategy (transfusion for hemoglobin less than 9 g/dL). The patients in the restrictive group received fewer transfusions, but had similar rates of 90-day mortality, use of life support, and number of days alive and out of the hospital.4
These large randomized controlled trials in critically ill patients served as the basis for subsequent studies in patient populations outside of the ICU.
Acute upper GI bleed
Acute upper gastrointestinal bleeding (UGIB) is one of the most common indications for RBC transfusion.
The TRIGGER trial, a cluster randomized multicenter study published in 2015, also found no difference in clinical outcomes, including mortality, between a restrictive strategy and liberal strategy for transfusion of patients with UGIB.6
Perioperative patients
Transfusion thresholds have been studied in large randomized trials for perioperative patients undergoing cardiac and orthopedic surgery.
The Transfusion Requirements after Cardiac Surgery (TRACS) trial, published in 2010, randomized patients undergoing cardiac surgery at a single center to a liberal strategy of blood transfusion (to maintain a hematocrit 30% or greater) or a restrictive strategy (hematocrit 24% or greater).7 Mortality and severe morbidity rates were noninferior in the restrictive strategy group. Mean hemoglobin concentrations were 10.5 g/dL in the liberal-strategy group and 9.1 g/dL in the restrictive-strategy group. Independent of transfusion strategy, the number of transfused red blood cell units was an independent risk factor for clinical complications and death at 30 days.
Based on these two trials, as well as other smaller randomized controlled trials and observational studies, the AABB guidelines recommend a restrictive RBC transfusion threshold of 8 g/dL for patients undergoing cardiac or orthopedic surgery.1
Acute coronary syndrome, stable coronary artery disease, and congestive heart failure
Anemia is an independent predictor of major adverse cardiovascular events in patients with acute coronary syndrome.9 However, it remains controversial if transfusion has benefit or causes harm in patients with acute coronary syndrome. No randomized controlled trials have yet been published on this topic, and observational studies and subgroups from randomized controlled trials have yielded mixed results.
Similarly, there are no randomized controlled trials examining liberal versus restrictive transfusion goals for asymptomatic hospitalized patients with stable coronary artery disease (CAD). However, patients with CAD were included in the TRICC and FOCUS trials.3,8 Of patients enrolled in the TRICC trial, 26% had a primary or secondary diagnosis of cardiac disease; subgroup analysis found no significant differences in 30-day mortality between treatment groups, similar to that of the entire study population.3 In a subgroup analysis of patients with “cardiovascular disease” the FOCUS trial found no difference in outcomes between a restrictive and liberal transfusion strategy, although there was a marginally higher incidence of myocardial infarction in the restrictive arm in the entire study population.8
Although some studies have included patients with congestive heart failure (CHF) as a subgroup, these subgroups are often not powered to show clinically meaningful differences. The risk of volume overload is one explanation for why transfusions may be harmful for patients with CHF.
Based on these limited available data, the AABB guidelines recommend a “restrictive RBC transfusion threshold (hemoglobin level of 8 g/dL)” for patients “with preexisting cardiovascular disease,” without distinguishing among patients with acute coronary syndrome, stable CAD, and CHF, but adds that the “threshold of 7 g/dL is likely comparable with 8 g/dL, but randomized controlled trial evidence is not available for all patient categories.”1
Of note, certain patient populations, such as those with end-stage renal disease, oncology patients (especially those undergoing active chemotherapy), and those with comorbid conditions such as thrombocytopenia and coagulopathy are specifically excluded from the AABB guidelines. The reader is referred to guidelines from respective specialty societies for further guidance.
Back to our case
This 48-year-old man with cirrhosis and esophageal varices presenting with ongoing blood loss due to hematochezia and hematemesis with a hemoglobin of 7.8 g/dL should be transfused due to his active bleeding; his hemoglobin level should not be a determining factor under such circumstances. This distinction is one of the most fundamental in interpreting the guidelines, that is, patients with hemodynamic insult, symptoms, and/or ongoing bleeding should be evaluated clinically, independent of their hemoglobin level.
Bottom line
Although recent guidelines generally favor a more restrictive hemoglobin goal threshold, in the presence of active blood loss or hemodynamic instability, blood transfusion should be considered independent of the initial hemoglobin level.
Key Points
- The AABB guidelines recommend transfusing stable general medical inpatients to a hemoglobin level of 7 g/dL.
- For patients undergoing orthopedic surgery, cardiac surgery, and those with preexisting cardiovascular disease, a transfusion threshold of 8 g/dL is recommended.
- Patients with hemodynamic instability or active blood loss should be transfused based on clinical criteria, and not absolute hemoglobin levels.
- Patients with acute coronary syndrome, severe thrombocytopenia, and chronic transfusion–dependent anemia are excluded from these recommendations.
- Further studies are needed to further refine these recommendations to specific patient populations in maximizing the benefits and minimizing the risks of blood transfusions.
Quiz
In which of the following scenarios is RBC transfusion LEAST indicated?
- A. Active diverticular bleed, heart rate 125 beats/minute, blood pressure 85/65 mm HG, hemoglobin 10.5 g/dL
- B. Septic shock in the ICU, hemoglobin 6.7 g/dL
- C. Pulmonary embolism in the setting of stable coronary artery disease and hemoglobin 8.7 g/dL
- D. Lymphoma on chemotherapy, hemoglobin 7.5 g/d, patient becomes dizzy when standing
Answer: C – A hemoglobin transfusion goal of 8.0 g/dL is recommended for patients with cardiovascular disease. The patient in answer choice A has active blood loss, tachycardia, and hypotension, all pointing to the need for blood transfusion irrespective of an initial hemoglobin level greater than 8.0 g/dL. The patient in answer choice B has a hemoglobin level less than 7.0 g/dL, and the patient in choice D is symptomatic, possibly from anemia, as well as on chemotherapy, therefore making transfusion a reasonable option.
Dr. Sampat, Dr. Berger, and Dr. Manian are hospitalists at Massachusetts General Hospital, Boston.
References
1. Carson JL et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316:2025-35.
2. Dwyre DM et al. Hepatitis B, hepatitis C and HIV transfusion-transmitted infections in the 21st century. Vox Sang. 2011;100:92-8.
3. Hébert PC et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-17.
4. Holst LB et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-91.
5. Villanueva C et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11-21.
6. Jairath V et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open-label, cluster randomised feasibility trial. Lancet. 2015;386:137-44.
7. Hajjar LA et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559-67.
8. Carson JL et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-62.
9. Sabatine MS et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042-9.
Preexposure prophylaxis among LGBT youth
Every prevention effort or treatment has its own risks. Gynecologists must consider the risk for blood clots from using estrogen-containing oral contraceptives versus the risk of blood clots from pregnancy. Endocrinologists must weigh the risk of decreased bone mineral density versus premature closure of growth plates when starting pubertal blockers for children suffering from precocious puberty. Psychologists and primary care providers must consider the risk for increased suicidal thoughts while on selective serotonin reuptake inhibitors versus the risk of completed suicide if the depression remains untreated.
In the United States alone, 22% of HIV infections occur in people aged 13-24 years. Among those with HIV infection, 81% are young men who have sex with men (MSM).1 Among those new infections, young MSM of color are nearly four times as likely to have HIV, compared with white young MSM.2 Moreover, the incidence of HIV infection among transgender individuals is three times higher than the national average.3
What further hampers public health prevention efforts is the stigma and discrimination LGBT youth face in trying to prevent HIV infections: 84% of those aged 15-24 years report recognizing stigma around HIV in the United States.4 In addition, black MSM were more likely than other MSMs to report this kind of stigma.5 And it isn’t enough that LGBT youth have to face stigma and discrimination. In fact, because of it, they often face serious financial challenges. It is estimated that 50% of homeless youth identify as LGBT, and 40% of them were forced out of their homes because of their sexual orientation or gender identity.6 Also, transgender youth have difficulty finding employment because of their gender identity.7 A combination of homelessness or chronic unemployment has driven many LGBT youth to survival sex or sex for money, which puts them at higher risk for HIV infection.7,8 The risk for HIV infection is so high that we should be using all available resources, including PrEP, to address these profound health disparities.
Studies, however, are forthcoming. One study by Hosek et al. that was published in September suggested that PrEP among adolescents can be safe and well tolerated, may not increase the rate of high-risk sexual behaviors, and may not increase the risk of other STDs such as gonorrhea and chlamydia. It must be noted, however, that incidence of HIV was fairly high – the HIV seroconversion rate was 6.4 per 100 person-years. Nevertheless, researchers found the rate of HIV seroconversion was higher among those with lower levels of Truvada in their bodies, compared with the seroconversion rate in those with higher levels of the medication. This suggests that adherence is key in using PrEP to prevent HIV infection.10 Although far from definitive, this small study provides some solid evidence that PrEP is safe and effective in preventing HIV among LGBT youth. More studies that will eventually support its effectiveness and safety are on the way.11
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
Resource
CDC website on PrEP: https://www.cdc.gov/hiv/risk/prep/index.html, with provider guidelines.
References
1. Centers for Disease Control and Prevention. HIV Among Youth fact sheet, April 2017.
2. Centers for Disease Control and Prevention. HIV Surveillance Report, 2015; vol. 27.
3. Centers for Disease Control and Prevention. HIV Among Transgender People.
4. Kaiser Family Foundation. National survey of teens and young adults on HIV/AIDS, Nov. 1, 2012. .
5. J Acquir Immune Defic Syndr. 2016;73(5):547-55.
6. Serving our youth: Findings from a national survey of services providers working with lesbian, gay, bisexual and transgender youth who are homeless or at risk of becoming homeless (The Williams Institute with True Colors and The Palette Fund, 2012).
7. Injustice at every turn: A report of the national transgender discrimination survey (National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011).
8. J Acquir Immune Defic Syndr. 2010 Apr;53(5):661-4.
9. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States: A clinical practice guideline, 2014.
10. JAMA Pediatr. 2017;171(11):1063-71.
11. J Int AIDS Soc. 2016;19. doi: 10.7448/IAS.19.7.21107.
Every prevention effort or treatment has its own risks. Gynecologists must consider the risk for blood clots from using estrogen-containing oral contraceptives versus the risk of blood clots from pregnancy. Endocrinologists must weigh the risk of decreased bone mineral density versus premature closure of growth plates when starting pubertal blockers for children suffering from precocious puberty. Psychologists and primary care providers must consider the risk for increased suicidal thoughts while on selective serotonin reuptake inhibitors versus the risk of completed suicide if the depression remains untreated.
In the United States alone, 22% of HIV infections occur in people aged 13-24 years. Among those with HIV infection, 81% are young men who have sex with men (MSM).1 Among those new infections, young MSM of color are nearly four times as likely to have HIV, compared with white young MSM.2 Moreover, the incidence of HIV infection among transgender individuals is three times higher than the national average.3
What further hampers public health prevention efforts is the stigma and discrimination LGBT youth face in trying to prevent HIV infections: 84% of those aged 15-24 years report recognizing stigma around HIV in the United States.4 In addition, black MSM were more likely than other MSMs to report this kind of stigma.5 And it isn’t enough that LGBT youth have to face stigma and discrimination. In fact, because of it, they often face serious financial challenges. It is estimated that 50% of homeless youth identify as LGBT, and 40% of them were forced out of their homes because of their sexual orientation or gender identity.6 Also, transgender youth have difficulty finding employment because of their gender identity.7 A combination of homelessness or chronic unemployment has driven many LGBT youth to survival sex or sex for money, which puts them at higher risk for HIV infection.7,8 The risk for HIV infection is so high that we should be using all available resources, including PrEP, to address these profound health disparities.
Studies, however, are forthcoming. One study by Hosek et al. that was published in September suggested that PrEP among adolescents can be safe and well tolerated, may not increase the rate of high-risk sexual behaviors, and may not increase the risk of other STDs such as gonorrhea and chlamydia. It must be noted, however, that incidence of HIV was fairly high – the HIV seroconversion rate was 6.4 per 100 person-years. Nevertheless, researchers found the rate of HIV seroconversion was higher among those with lower levels of Truvada in their bodies, compared with the seroconversion rate in those with higher levels of the medication. This suggests that adherence is key in using PrEP to prevent HIV infection.10 Although far from definitive, this small study provides some solid evidence that PrEP is safe and effective in preventing HIV among LGBT youth. More studies that will eventually support its effectiveness and safety are on the way.11
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
Resource
CDC website on PrEP: https://www.cdc.gov/hiv/risk/prep/index.html, with provider guidelines.
References
1. Centers for Disease Control and Prevention. HIV Among Youth fact sheet, April 2017.
2. Centers for Disease Control and Prevention. HIV Surveillance Report, 2015; vol. 27.
3. Centers for Disease Control and Prevention. HIV Among Transgender People.
4. Kaiser Family Foundation. National survey of teens and young adults on HIV/AIDS, Nov. 1, 2012. .
5. J Acquir Immune Defic Syndr. 2016;73(5):547-55.
6. Serving our youth: Findings from a national survey of services providers working with lesbian, gay, bisexual and transgender youth who are homeless or at risk of becoming homeless (The Williams Institute with True Colors and The Palette Fund, 2012).
7. Injustice at every turn: A report of the national transgender discrimination survey (National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011).
8. J Acquir Immune Defic Syndr. 2010 Apr;53(5):661-4.
9. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States: A clinical practice guideline, 2014.
10. JAMA Pediatr. 2017;171(11):1063-71.
11. J Int AIDS Soc. 2016;19. doi: 10.7448/IAS.19.7.21107.
Every prevention effort or treatment has its own risks. Gynecologists must consider the risk for blood clots from using estrogen-containing oral contraceptives versus the risk of blood clots from pregnancy. Endocrinologists must weigh the risk of decreased bone mineral density versus premature closure of growth plates when starting pubertal blockers for children suffering from precocious puberty. Psychologists and primary care providers must consider the risk for increased suicidal thoughts while on selective serotonin reuptake inhibitors versus the risk of completed suicide if the depression remains untreated.
In the United States alone, 22% of HIV infections occur in people aged 13-24 years. Among those with HIV infection, 81% are young men who have sex with men (MSM).1 Among those new infections, young MSM of color are nearly four times as likely to have HIV, compared with white young MSM.2 Moreover, the incidence of HIV infection among transgender individuals is three times higher than the national average.3
What further hampers public health prevention efforts is the stigma and discrimination LGBT youth face in trying to prevent HIV infections: 84% of those aged 15-24 years report recognizing stigma around HIV in the United States.4 In addition, black MSM were more likely than other MSMs to report this kind of stigma.5 And it isn’t enough that LGBT youth have to face stigma and discrimination. In fact, because of it, they often face serious financial challenges. It is estimated that 50% of homeless youth identify as LGBT, and 40% of them were forced out of their homes because of their sexual orientation or gender identity.6 Also, transgender youth have difficulty finding employment because of their gender identity.7 A combination of homelessness or chronic unemployment has driven many LGBT youth to survival sex or sex for money, which puts them at higher risk for HIV infection.7,8 The risk for HIV infection is so high that we should be using all available resources, including PrEP, to address these profound health disparities.
Studies, however, are forthcoming. One study by Hosek et al. that was published in September suggested that PrEP among adolescents can be safe and well tolerated, may not increase the rate of high-risk sexual behaviors, and may not increase the risk of other STDs such as gonorrhea and chlamydia. It must be noted, however, that incidence of HIV was fairly high – the HIV seroconversion rate was 6.4 per 100 person-years. Nevertheless, researchers found the rate of HIV seroconversion was higher among those with lower levels of Truvada in their bodies, compared with the seroconversion rate in those with higher levels of the medication. This suggests that adherence is key in using PrEP to prevent HIV infection.10 Although far from definitive, this small study provides some solid evidence that PrEP is safe and effective in preventing HIV among LGBT youth. More studies that will eventually support its effectiveness and safety are on the way.11
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
Resource
CDC website on PrEP: https://www.cdc.gov/hiv/risk/prep/index.html, with provider guidelines.
References
1. Centers for Disease Control and Prevention. HIV Among Youth fact sheet, April 2017.
2. Centers for Disease Control and Prevention. HIV Surveillance Report, 2015; vol. 27.
3. Centers for Disease Control and Prevention. HIV Among Transgender People.
4. Kaiser Family Foundation. National survey of teens and young adults on HIV/AIDS, Nov. 1, 2012. .
5. J Acquir Immune Defic Syndr. 2016;73(5):547-55.
6. Serving our youth: Findings from a national survey of services providers working with lesbian, gay, bisexual and transgender youth who are homeless or at risk of becoming homeless (The Williams Institute with True Colors and The Palette Fund, 2012).
7. Injustice at every turn: A report of the national transgender discrimination survey (National Center for Transgender Equality and National Gay and Lesbian Task Force, 2011).
8. J Acquir Immune Defic Syndr. 2010 Apr;53(5):661-4.
9. Centers for Disease Control and Prevention. Preexposure prophylaxis for the prevention of HIV infection in the United States: A clinical practice guideline, 2014.
10. JAMA Pediatr. 2017;171(11):1063-71.
11. J Int AIDS Soc. 2016;19. doi: 10.7448/IAS.19.7.21107.
Red Patches on a Newborn
The Diagnosis: Congenital Unilateral Nevoid Telangiectasia
Two weeks later the patches were noticeably lighter (Figures 1A and 1B). She continued to be in good health, but gynecomastia was notably present on examination (Figure 1C). At 3 months of age, all patches on the right arm, superior aspect of the chest, and superior aspect of the back had resolved, along with the gynecomastia (Figure 2).
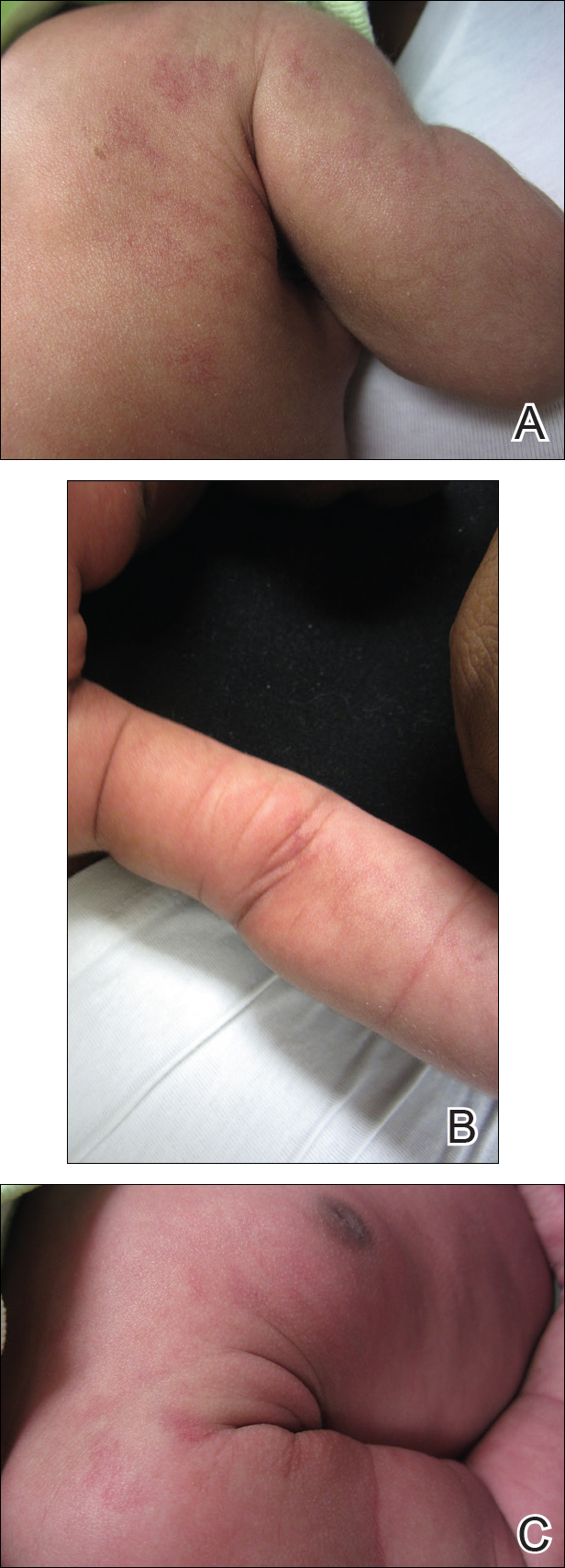
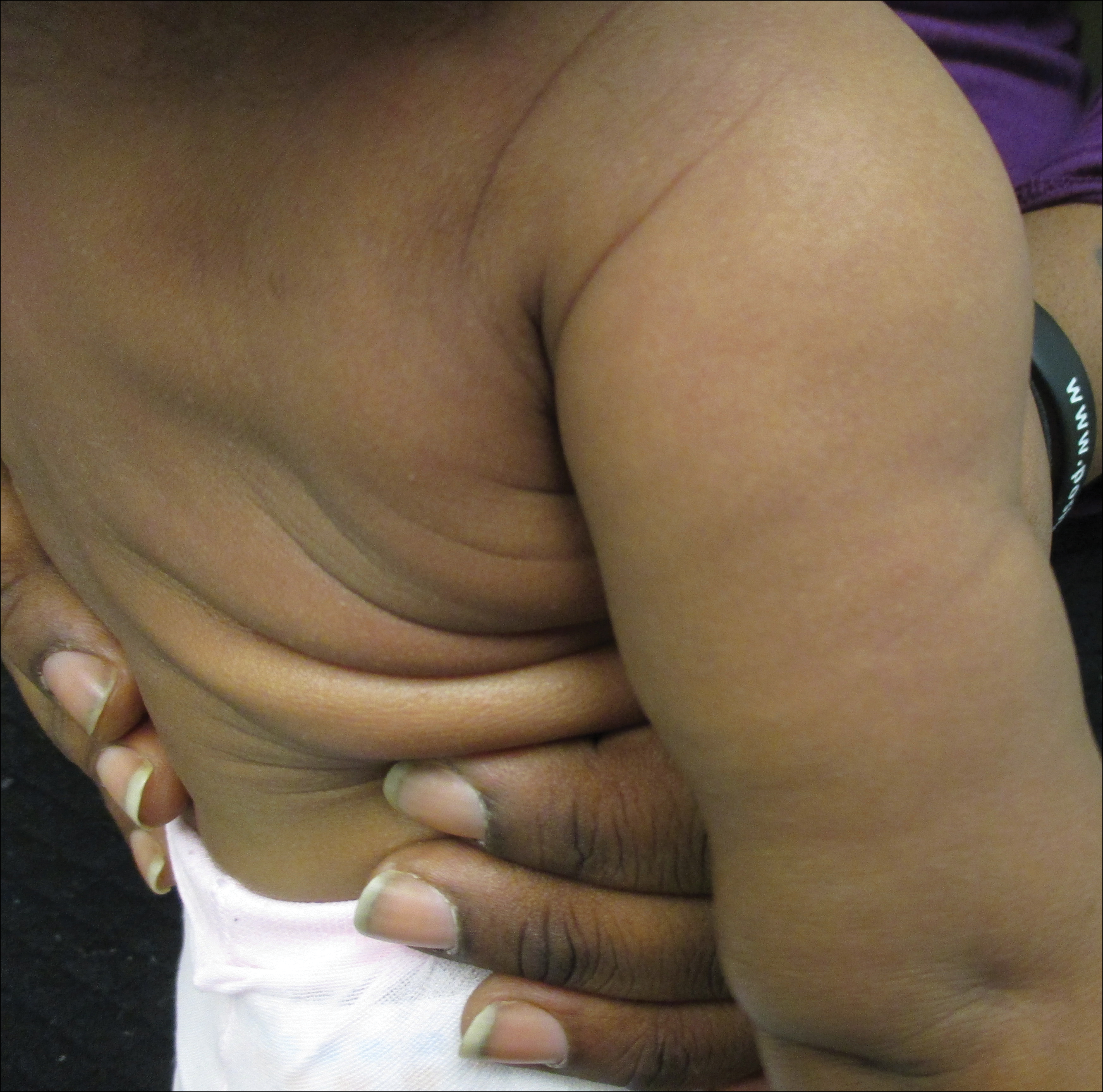
This case describes the rare condition of congenital unilateral nevoid telangiectasia (UNT). Unilateral nevoid telangiectasia is a rare cutaneous vascular condition first described by Blaschko1 in 1899. It is characterized by the presence of unilateral superficial telangiectases occurring most often in the cervical and upper thoracic dermatomes in a linear pattern.2 Females are more often affected than males (2:1 ratio), and cases of UNT are either congenital or acquired.3 Although most UNT cases are acquired and often found in females, approximately 15% of cases are congenital and are comprised largely by males. Acquired cases have been hypothesized to occur in association with hyperestrogenemic states such as pregnancy, puberty, oral contraceptive use and hormonal therapy, alcoholism, and liver disease including hepatitis B and C infections.4,5 There is conflicting evidence as to whether there is an absolute increase in the presence of estrogen and progesterone receptors in the skin, as many case reports show no increase. Instead, others hypothesize that the condition is actually a result of somatic mosaicism and that the cutaneous lesions are genetically predisposed to becoming visibly evident under conditions of elevated estrogen.2
In our case, we hypothesize that the cause was elevated maternal estrogen levels present at higher than normal levels in the fetal circulation. The presence of gynecomastia seen in our patient supports the hypothesis that increased circulating estrogen may be present in infants with UNT.
- Blaschko A. Teleangiektasien. versammlungen. Berliner Dermatologische Gesellschaft. Monatschr prakt Dermat. 1899;28:451.
- Karakas¸ M, Durdu M, Sönmezoğlu S, et al. Unilateral nevoidtelangiectasia. J Dermatol. 2004;31:109-112.
- Wenson SF, Farhana J, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Hynes LR, Shenefelt PD. Unilateral nevoid telangiectasia: occurrence in two patients with hepatitis C. J Am Acad Dermatol. 1997;36(5 pt 2):819-822.
- Guedes R, Leite L. Unilateral nevoid telangiectasia: a rare disease? Indian J Dermatol. 2012;57:138-140.
The Diagnosis: Congenital Unilateral Nevoid Telangiectasia
Two weeks later the patches were noticeably lighter (Figures 1A and 1B). She continued to be in good health, but gynecomastia was notably present on examination (Figure 1C). At 3 months of age, all patches on the right arm, superior aspect of the chest, and superior aspect of the back had resolved, along with the gynecomastia (Figure 2).


This case describes the rare condition of congenital unilateral nevoid telangiectasia (UNT). Unilateral nevoid telangiectasia is a rare cutaneous vascular condition first described by Blaschko1 in 1899. It is characterized by the presence of unilateral superficial telangiectases occurring most often in the cervical and upper thoracic dermatomes in a linear pattern.2 Females are more often affected than males (2:1 ratio), and cases of UNT are either congenital or acquired.3 Although most UNT cases are acquired and often found in females, approximately 15% of cases are congenital and are comprised largely by males. Acquired cases have been hypothesized to occur in association with hyperestrogenemic states such as pregnancy, puberty, oral contraceptive use and hormonal therapy, alcoholism, and liver disease including hepatitis B and C infections.4,5 There is conflicting evidence as to whether there is an absolute increase in the presence of estrogen and progesterone receptors in the skin, as many case reports show no increase. Instead, others hypothesize that the condition is actually a result of somatic mosaicism and that the cutaneous lesions are genetically predisposed to becoming visibly evident under conditions of elevated estrogen.2
In our case, we hypothesize that the cause was elevated maternal estrogen levels present at higher than normal levels in the fetal circulation. The presence of gynecomastia seen in our patient supports the hypothesis that increased circulating estrogen may be present in infants with UNT.
The Diagnosis: Congenital Unilateral Nevoid Telangiectasia
Two weeks later the patches were noticeably lighter (Figures 1A and 1B). She continued to be in good health, but gynecomastia was notably present on examination (Figure 1C). At 3 months of age, all patches on the right arm, superior aspect of the chest, and superior aspect of the back had resolved, along with the gynecomastia (Figure 2).


This case describes the rare condition of congenital unilateral nevoid telangiectasia (UNT). Unilateral nevoid telangiectasia is a rare cutaneous vascular condition first described by Blaschko1 in 1899. It is characterized by the presence of unilateral superficial telangiectases occurring most often in the cervical and upper thoracic dermatomes in a linear pattern.2 Females are more often affected than males (2:1 ratio), and cases of UNT are either congenital or acquired.3 Although most UNT cases are acquired and often found in females, approximately 15% of cases are congenital and are comprised largely by males. Acquired cases have been hypothesized to occur in association with hyperestrogenemic states such as pregnancy, puberty, oral contraceptive use and hormonal therapy, alcoholism, and liver disease including hepatitis B and C infections.4,5 There is conflicting evidence as to whether there is an absolute increase in the presence of estrogen and progesterone receptors in the skin, as many case reports show no increase. Instead, others hypothesize that the condition is actually a result of somatic mosaicism and that the cutaneous lesions are genetically predisposed to becoming visibly evident under conditions of elevated estrogen.2
In our case, we hypothesize that the cause was elevated maternal estrogen levels present at higher than normal levels in the fetal circulation. The presence of gynecomastia seen in our patient supports the hypothesis that increased circulating estrogen may be present in infants with UNT.
- Blaschko A. Teleangiektasien. versammlungen. Berliner Dermatologische Gesellschaft. Monatschr prakt Dermat. 1899;28:451.
- Karakas¸ M, Durdu M, Sönmezoğlu S, et al. Unilateral nevoidtelangiectasia. J Dermatol. 2004;31:109-112.
- Wenson SF, Farhana J, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Hynes LR, Shenefelt PD. Unilateral nevoid telangiectasia: occurrence in two patients with hepatitis C. J Am Acad Dermatol. 1997;36(5 pt 2):819-822.
- Guedes R, Leite L. Unilateral nevoid telangiectasia: a rare disease? Indian J Dermatol. 2012;57:138-140.
- Blaschko A. Teleangiektasien. versammlungen. Berliner Dermatologische Gesellschaft. Monatschr prakt Dermat. 1899;28:451.
- Karakas¸ M, Durdu M, Sönmezoğlu S, et al. Unilateral nevoidtelangiectasia. J Dermatol. 2004;31:109-112.
- Wenson SF, Farhana J, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Hynes LR, Shenefelt PD. Unilateral nevoid telangiectasia: occurrence in two patients with hepatitis C. J Am Acad Dermatol. 1997;36(5 pt 2):819-822.
- Guedes R, Leite L. Unilateral nevoid telangiectasia: a rare disease? Indian J Dermatol. 2012;57:138-140.
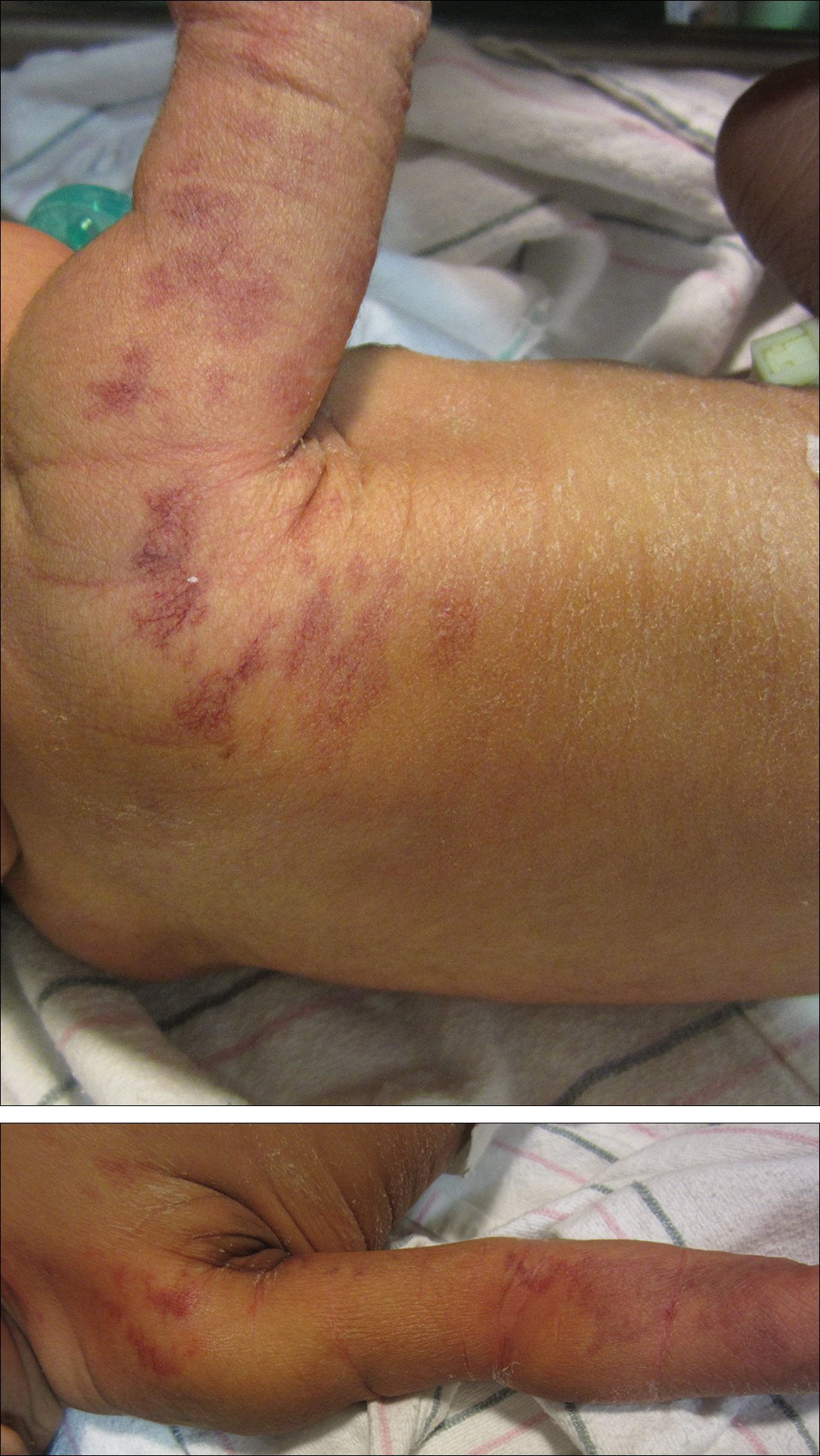
A 1-day-old female infant presented with red patches on the right arm that had been present since delivery. The patient was born to a healthy mother by spontaneous vaginal delivery without complications and with a good Apgar score. The newborn moved both arms and legs well and blood work was unremarkable. Her mother noted being healthy during pregnancy, and she had not taken any additional medications aside from prenatal vitamins. Examination of the infant revealed red blanchable reticulate patches in a dermatomal distribution extending from the posterior aspect of the right shoulder (top) down to the flexural aspect of the arm (bottom). There also were a few coalescing reticulate patches on the superior aspect of the right side of the chest and superior aspect of the right side of the back that resolved by 3 months of age.