User login
Rare neurological complication linked to Waldenstrom disease
Bilateral facial nerve palsy has been associated with underlying Waldenstrom disease in only one other known published case report, which was published in 2014. In a more recent case report published in the Journal of Clinical Neuroscience, Gabriel Torrealba-Acosta, MD, and colleagues in the department of neurology at Massachusetts General Hospital, Boston, described a second case involving a 67-year-old Hispanic man with a history of Waldenstrom disease who presented with subacute onset of bilateral facial weakness.
The patient, who had longstanding painful neuropathy, had presented to urgent care with a new-onset left facial nerve palsy, was then diagnosed with left Bell’s palsy, and began treatment with valacyclovir and prednisone.
The left-sided facial weakness gradually progressed to total paralysis of the left lower face and inability to close the left eye, and 2 weeks later, he developed right facial weakness that ran a similar course. The patient had a complicated clinical course that included symptomatic acute-on-chronic subdural hematoma, among other complications; eventually the patient’s symptoms stabilized and cranial neuropathies gradually improved, according to the report.
Bilateral facial nerve palsy is an extremely rare condition, occurring in just 0.3%-2% of all facial nerve palsy cases, according to the authors. By contrast, unilateral facial nerve palsy (or Bell’s palsy) is far more common, but it still occurs in only 25 patients per 100,000 population, they said.
Most cases of bilateral facial nerve palsy are caused by underlying Guillain-Barré syndrome, though some are congenital, related to trauma, or caused by etiologies that are metabolic, immunologic, or neoplastic in nature. While various types of neurological disturbances – from ischemic and hemorrhagic events to meningoencephalitis – have been documented to occur in up to a quarter of patients with Waldenstrom disease.
“Given the large differential that comprises the assessment of a bilateral facial nerve palsy, it warrants for an extensive work-up, and Waldenstrom’s macroglobulinemia should be sought as an additional possible etiology,” the authors wrote.
Dr. Torrealba-Acosta and coauthors reported having no financial disclosures.
SOURCE: Torrealba-Acosta G et al. J Clin Neurosci. 2017. doi: 10.1016/j.jocn.2017.10.081.
Bilateral facial nerve palsy has been associated with underlying Waldenstrom disease in only one other known published case report, which was published in 2014. In a more recent case report published in the Journal of Clinical Neuroscience, Gabriel Torrealba-Acosta, MD, and colleagues in the department of neurology at Massachusetts General Hospital, Boston, described a second case involving a 67-year-old Hispanic man with a history of Waldenstrom disease who presented with subacute onset of bilateral facial weakness.
The patient, who had longstanding painful neuropathy, had presented to urgent care with a new-onset left facial nerve palsy, was then diagnosed with left Bell’s palsy, and began treatment with valacyclovir and prednisone.
The left-sided facial weakness gradually progressed to total paralysis of the left lower face and inability to close the left eye, and 2 weeks later, he developed right facial weakness that ran a similar course. The patient had a complicated clinical course that included symptomatic acute-on-chronic subdural hematoma, among other complications; eventually the patient’s symptoms stabilized and cranial neuropathies gradually improved, according to the report.
Bilateral facial nerve palsy is an extremely rare condition, occurring in just 0.3%-2% of all facial nerve palsy cases, according to the authors. By contrast, unilateral facial nerve palsy (or Bell’s palsy) is far more common, but it still occurs in only 25 patients per 100,000 population, they said.
Most cases of bilateral facial nerve palsy are caused by underlying Guillain-Barré syndrome, though some are congenital, related to trauma, or caused by etiologies that are metabolic, immunologic, or neoplastic in nature. While various types of neurological disturbances – from ischemic and hemorrhagic events to meningoencephalitis – have been documented to occur in up to a quarter of patients with Waldenstrom disease.
“Given the large differential that comprises the assessment of a bilateral facial nerve palsy, it warrants for an extensive work-up, and Waldenstrom’s macroglobulinemia should be sought as an additional possible etiology,” the authors wrote.
Dr. Torrealba-Acosta and coauthors reported having no financial disclosures.
SOURCE: Torrealba-Acosta G et al. J Clin Neurosci. 2017. doi: 10.1016/j.jocn.2017.10.081.
Bilateral facial nerve palsy has been associated with underlying Waldenstrom disease in only one other known published case report, which was published in 2014. In a more recent case report published in the Journal of Clinical Neuroscience, Gabriel Torrealba-Acosta, MD, and colleagues in the department of neurology at Massachusetts General Hospital, Boston, described a second case involving a 67-year-old Hispanic man with a history of Waldenstrom disease who presented with subacute onset of bilateral facial weakness.
The patient, who had longstanding painful neuropathy, had presented to urgent care with a new-onset left facial nerve palsy, was then diagnosed with left Bell’s palsy, and began treatment with valacyclovir and prednisone.
The left-sided facial weakness gradually progressed to total paralysis of the left lower face and inability to close the left eye, and 2 weeks later, he developed right facial weakness that ran a similar course. The patient had a complicated clinical course that included symptomatic acute-on-chronic subdural hematoma, among other complications; eventually the patient’s symptoms stabilized and cranial neuropathies gradually improved, according to the report.
Bilateral facial nerve palsy is an extremely rare condition, occurring in just 0.3%-2% of all facial nerve palsy cases, according to the authors. By contrast, unilateral facial nerve palsy (or Bell’s palsy) is far more common, but it still occurs in only 25 patients per 100,000 population, they said.
Most cases of bilateral facial nerve palsy are caused by underlying Guillain-Barré syndrome, though some are congenital, related to trauma, or caused by etiologies that are metabolic, immunologic, or neoplastic in nature. While various types of neurological disturbances – from ischemic and hemorrhagic events to meningoencephalitis – have been documented to occur in up to a quarter of patients with Waldenstrom disease.
“Given the large differential that comprises the assessment of a bilateral facial nerve palsy, it warrants for an extensive work-up, and Waldenstrom’s macroglobulinemia should be sought as an additional possible etiology,” the authors wrote.
Dr. Torrealba-Acosta and coauthors reported having no financial disclosures.
SOURCE: Torrealba-Acosta G et al. J Clin Neurosci. 2017. doi: 10.1016/j.jocn.2017.10.081.
FROM THE JOURNAL OF CLINICAL NEUROSCIENCE
Postmenopausal women who shed pounds see lower breast cancer risk
SAN ANTONIO – Postmenopausal women may be able to lower their risk of invasive breast cancer by simply losing some weight, according to an analysis of the large prospective Women’s Health Initiative Observational Study.
“While obesity is an established risk factor for postmenopausal breast cancer, studies of weight loss and breast cancer provide inconsistent results. It’s been very, very difficult to show that losing weight changes breast cancer incidence,” said lead investigator Rowan T. Chlebowski, MD, PhD, research professor in the department of medical oncology and therapeutics research at City of Hope in Duarte, Calif. “Consequently, the current public health message is limited to ‘avoid body fatness’ [N Engl J Med. 2016;375:794-8]. That’s not a very strong public health message.”
Results showed that compared with peers whose weight remained stable, women who lost at least 5% of their body weight (an average of about 17-20 pounds) had a significant 12% reduction in breast cancer risk, Dr. Chlebowski reported in a session and press briefing at the San Antonio Breast Cancer Symposium. Benefit was similar whether the weight loss was intentional or not, and whether women were normal weight, overweight, or obese at baseline.
“These findings suggest that interventions in postmenopausal women designed to generate weight loss may reduce breast cancer risk. I feel these are very optimistic findings in that they provide a lesson to postmenopausal women that even a moderate degree of weight loss may be associated with health benefits,” he said. “This is a relatively new finding, and I think it should have public health implications.”
Parsing the findings
“I hope that you can present this to general doctors rather than oncologists because those are the ones seeing healthy women, by and large,” said press briefing moderator C. Kent Osborne, MD, codirector of SABCS and director of the Dan L. Duncan Cancer Center at Baylor College of Medicine in Houston. “But in my patients, I’ve always suggested that they lose weight. Most of them with breast cancer are overweight, it seems. And I always had to do it because of diabetes and other factors. Now we can do it because we have a breast cancer endpoint that also suggests that losing weight will help with that as well.”
Additional results from the study showed that women who experienced a weight gain of at least 5% did not have a significantly elevated risk of breast cancer overall.
“Do you think that the women who gained a little bit of weight didn’t have an increase in incidence because they were already overweight?” Dr. Osborne asked.
“When we go over all these very complex mechanisms and the different drivers, there’s probably a threshold. So obesity’s association with inflammatory factors, I think that’s a driver to a certain degree. But then something else takes over, or maybe the main driver takes over,” Dr. Chlebowski replied. “We don’t have enough data to decide exactly what that threshold is, but that’s the ongoing hypothesis.”
In the session, attendee Daniel McGrail, PhD, of the MD Anderson Cancer Center, Houston, asked, “Since this [risk reduction with weight loss] occurred regardless of initial [body mass index], and you have all these covariates accounted for, does that imply that actually caloric deficits or decreasing caloric intake could be preventing breast cancer risk regardless of initial weight or any other parameters?”
“In all these western diseases, what we take for normal is probably less normal than it might have been 250 years ago or thousands of years ago when we were eating berries and being chased by animals,” Dr. Chlebowski replied. “So this raises a question as to whether the normal weight cutoff should be an ideal weight for western cancer prevention.”
Study details
The Women’s Health Initiative Observational Study recruited 93,676 postmenopausal women aged 50-79 years from 40 U.S. clinical centers during 1993-1998. The women had measurements taken of height and weight at baseline and at year 3 for calculation of BMI, and were asked about intentionality of any weight loss during that period.
Analyses were based on 61,335 women who had normal or higher body weight and were cancer free at baseline, survived at least 3 years, and had adequate data.
Overall, about 13.3% of women lost at least 5% of their body weight between baseline and year 3; 7.9% did so intentionally, losing an average of 19.6 pounds, and 5.5% did so unintentionally, losing an average of 16.9 pounds.
“We used the 5% decrease because this level has been shown to change some biochemical markers potentially associated with cancer. And it has been shown in a different study population, a randomized trial, to reduce the frequency of diabetes,” Dr. Chlebowski noted.
“There was nothing that the study did to induce the weight loss. And based on a partial look at the data, it doesn’t look like many of the women went to some kind of program to lose weight. So it was probably self-directed weight loss,” he noted. “Interestingly, the body mass index of this group was 29.9, so they were on the verge of going into obesity. We wondered in retrospect whether that was a motivating factor for them.”
In multivariate analysis, relative to peers having a stable weight over time, the women losing at least 5% of their weight had a lower risk of breast cancer (hazard ratio, 0.88; 95% confidence interval, 0.78-0.98; P = .02).
Findings were unchanged after further adjustment for mammography frequency. In addition, risk reduction was statistically indistinguishable whether the weight loss was intentional or not (P = .2 for interaction), and whether women were normal weight, overweight, or obese at baseline (P = .4 for interaction).
The 19.6% of women who had a weight gain of at least 5% did not have a significantly elevated risk of breast cancer overall, but they did have a significantly elevated risk of triple-negative breast cancer (HR, 1.54; 95% CI, 1.16-2.05). “We really don’t have a good explanation for this,” Dr. Chlebowski said.
Tackling the global obesity epidemic by conventional means to reduce cancer risk is an uphill battle, he concluded. “We are going to be looking for possible mediating factors. If you can find the mediating factors, pharmacologic intervention would have a much greater chance of success.”
Dr. Chlebowski disclosed that he had no relevant conflicts of interest. Research was supported by the National Heart, Lung and Blood Institute; National Institutes of Health; Department of Health and Human Services; and American Institute for Cancer Research.
SOURCE: Chlebowski et al., SABCS 2017 Abstract GS5-07
SAN ANTONIO – Postmenopausal women may be able to lower their risk of invasive breast cancer by simply losing some weight, according to an analysis of the large prospective Women’s Health Initiative Observational Study.
“While obesity is an established risk factor for postmenopausal breast cancer, studies of weight loss and breast cancer provide inconsistent results. It’s been very, very difficult to show that losing weight changes breast cancer incidence,” said lead investigator Rowan T. Chlebowski, MD, PhD, research professor in the department of medical oncology and therapeutics research at City of Hope in Duarte, Calif. “Consequently, the current public health message is limited to ‘avoid body fatness’ [N Engl J Med. 2016;375:794-8]. That’s not a very strong public health message.”
Results showed that compared with peers whose weight remained stable, women who lost at least 5% of their body weight (an average of about 17-20 pounds) had a significant 12% reduction in breast cancer risk, Dr. Chlebowski reported in a session and press briefing at the San Antonio Breast Cancer Symposium. Benefit was similar whether the weight loss was intentional or not, and whether women were normal weight, overweight, or obese at baseline.
“These findings suggest that interventions in postmenopausal women designed to generate weight loss may reduce breast cancer risk. I feel these are very optimistic findings in that they provide a lesson to postmenopausal women that even a moderate degree of weight loss may be associated with health benefits,” he said. “This is a relatively new finding, and I think it should have public health implications.”
Parsing the findings
“I hope that you can present this to general doctors rather than oncologists because those are the ones seeing healthy women, by and large,” said press briefing moderator C. Kent Osborne, MD, codirector of SABCS and director of the Dan L. Duncan Cancer Center at Baylor College of Medicine in Houston. “But in my patients, I’ve always suggested that they lose weight. Most of them with breast cancer are overweight, it seems. And I always had to do it because of diabetes and other factors. Now we can do it because we have a breast cancer endpoint that also suggests that losing weight will help with that as well.”
Additional results from the study showed that women who experienced a weight gain of at least 5% did not have a significantly elevated risk of breast cancer overall.
“Do you think that the women who gained a little bit of weight didn’t have an increase in incidence because they were already overweight?” Dr. Osborne asked.
“When we go over all these very complex mechanisms and the different drivers, there’s probably a threshold. So obesity’s association with inflammatory factors, I think that’s a driver to a certain degree. But then something else takes over, or maybe the main driver takes over,” Dr. Chlebowski replied. “We don’t have enough data to decide exactly what that threshold is, but that’s the ongoing hypothesis.”
In the session, attendee Daniel McGrail, PhD, of the MD Anderson Cancer Center, Houston, asked, “Since this [risk reduction with weight loss] occurred regardless of initial [body mass index], and you have all these covariates accounted for, does that imply that actually caloric deficits or decreasing caloric intake could be preventing breast cancer risk regardless of initial weight or any other parameters?”
“In all these western diseases, what we take for normal is probably less normal than it might have been 250 years ago or thousands of years ago when we were eating berries and being chased by animals,” Dr. Chlebowski replied. “So this raises a question as to whether the normal weight cutoff should be an ideal weight for western cancer prevention.”
Study details
The Women’s Health Initiative Observational Study recruited 93,676 postmenopausal women aged 50-79 years from 40 U.S. clinical centers during 1993-1998. The women had measurements taken of height and weight at baseline and at year 3 for calculation of BMI, and were asked about intentionality of any weight loss during that period.
Analyses were based on 61,335 women who had normal or higher body weight and were cancer free at baseline, survived at least 3 years, and had adequate data.
Overall, about 13.3% of women lost at least 5% of their body weight between baseline and year 3; 7.9% did so intentionally, losing an average of 19.6 pounds, and 5.5% did so unintentionally, losing an average of 16.9 pounds.
“We used the 5% decrease because this level has been shown to change some biochemical markers potentially associated with cancer. And it has been shown in a different study population, a randomized trial, to reduce the frequency of diabetes,” Dr. Chlebowski noted.
“There was nothing that the study did to induce the weight loss. And based on a partial look at the data, it doesn’t look like many of the women went to some kind of program to lose weight. So it was probably self-directed weight loss,” he noted. “Interestingly, the body mass index of this group was 29.9, so they were on the verge of going into obesity. We wondered in retrospect whether that was a motivating factor for them.”
In multivariate analysis, relative to peers having a stable weight over time, the women losing at least 5% of their weight had a lower risk of breast cancer (hazard ratio, 0.88; 95% confidence interval, 0.78-0.98; P = .02).
Findings were unchanged after further adjustment for mammography frequency. In addition, risk reduction was statistically indistinguishable whether the weight loss was intentional or not (P = .2 for interaction), and whether women were normal weight, overweight, or obese at baseline (P = .4 for interaction).
The 19.6% of women who had a weight gain of at least 5% did not have a significantly elevated risk of breast cancer overall, but they did have a significantly elevated risk of triple-negative breast cancer (HR, 1.54; 95% CI, 1.16-2.05). “We really don’t have a good explanation for this,” Dr. Chlebowski said.
Tackling the global obesity epidemic by conventional means to reduce cancer risk is an uphill battle, he concluded. “We are going to be looking for possible mediating factors. If you can find the mediating factors, pharmacologic intervention would have a much greater chance of success.”
Dr. Chlebowski disclosed that he had no relevant conflicts of interest. Research was supported by the National Heart, Lung and Blood Institute; National Institutes of Health; Department of Health and Human Services; and American Institute for Cancer Research.
SOURCE: Chlebowski et al., SABCS 2017 Abstract GS5-07
SAN ANTONIO – Postmenopausal women may be able to lower their risk of invasive breast cancer by simply losing some weight, according to an analysis of the large prospective Women’s Health Initiative Observational Study.
“While obesity is an established risk factor for postmenopausal breast cancer, studies of weight loss and breast cancer provide inconsistent results. It’s been very, very difficult to show that losing weight changes breast cancer incidence,” said lead investigator Rowan T. Chlebowski, MD, PhD, research professor in the department of medical oncology and therapeutics research at City of Hope in Duarte, Calif. “Consequently, the current public health message is limited to ‘avoid body fatness’ [N Engl J Med. 2016;375:794-8]. That’s not a very strong public health message.”
Results showed that compared with peers whose weight remained stable, women who lost at least 5% of their body weight (an average of about 17-20 pounds) had a significant 12% reduction in breast cancer risk, Dr. Chlebowski reported in a session and press briefing at the San Antonio Breast Cancer Symposium. Benefit was similar whether the weight loss was intentional or not, and whether women were normal weight, overweight, or obese at baseline.
“These findings suggest that interventions in postmenopausal women designed to generate weight loss may reduce breast cancer risk. I feel these are very optimistic findings in that they provide a lesson to postmenopausal women that even a moderate degree of weight loss may be associated with health benefits,” he said. “This is a relatively new finding, and I think it should have public health implications.”
Parsing the findings
“I hope that you can present this to general doctors rather than oncologists because those are the ones seeing healthy women, by and large,” said press briefing moderator C. Kent Osborne, MD, codirector of SABCS and director of the Dan L. Duncan Cancer Center at Baylor College of Medicine in Houston. “But in my patients, I’ve always suggested that they lose weight. Most of them with breast cancer are overweight, it seems. And I always had to do it because of diabetes and other factors. Now we can do it because we have a breast cancer endpoint that also suggests that losing weight will help with that as well.”
Additional results from the study showed that women who experienced a weight gain of at least 5% did not have a significantly elevated risk of breast cancer overall.
“Do you think that the women who gained a little bit of weight didn’t have an increase in incidence because they were already overweight?” Dr. Osborne asked.
“When we go over all these very complex mechanisms and the different drivers, there’s probably a threshold. So obesity’s association with inflammatory factors, I think that’s a driver to a certain degree. But then something else takes over, or maybe the main driver takes over,” Dr. Chlebowski replied. “We don’t have enough data to decide exactly what that threshold is, but that’s the ongoing hypothesis.”
In the session, attendee Daniel McGrail, PhD, of the MD Anderson Cancer Center, Houston, asked, “Since this [risk reduction with weight loss] occurred regardless of initial [body mass index], and you have all these covariates accounted for, does that imply that actually caloric deficits or decreasing caloric intake could be preventing breast cancer risk regardless of initial weight or any other parameters?”
“In all these western diseases, what we take for normal is probably less normal than it might have been 250 years ago or thousands of years ago when we were eating berries and being chased by animals,” Dr. Chlebowski replied. “So this raises a question as to whether the normal weight cutoff should be an ideal weight for western cancer prevention.”
Study details
The Women’s Health Initiative Observational Study recruited 93,676 postmenopausal women aged 50-79 years from 40 U.S. clinical centers during 1993-1998. The women had measurements taken of height and weight at baseline and at year 3 for calculation of BMI, and were asked about intentionality of any weight loss during that period.
Analyses were based on 61,335 women who had normal or higher body weight and were cancer free at baseline, survived at least 3 years, and had adequate data.
Overall, about 13.3% of women lost at least 5% of their body weight between baseline and year 3; 7.9% did so intentionally, losing an average of 19.6 pounds, and 5.5% did so unintentionally, losing an average of 16.9 pounds.
“We used the 5% decrease because this level has been shown to change some biochemical markers potentially associated with cancer. And it has been shown in a different study population, a randomized trial, to reduce the frequency of diabetes,” Dr. Chlebowski noted.
“There was nothing that the study did to induce the weight loss. And based on a partial look at the data, it doesn’t look like many of the women went to some kind of program to lose weight. So it was probably self-directed weight loss,” he noted. “Interestingly, the body mass index of this group was 29.9, so they were on the verge of going into obesity. We wondered in retrospect whether that was a motivating factor for them.”
In multivariate analysis, relative to peers having a stable weight over time, the women losing at least 5% of their weight had a lower risk of breast cancer (hazard ratio, 0.88; 95% confidence interval, 0.78-0.98; P = .02).
Findings were unchanged after further adjustment for mammography frequency. In addition, risk reduction was statistically indistinguishable whether the weight loss was intentional or not (P = .2 for interaction), and whether women were normal weight, overweight, or obese at baseline (P = .4 for interaction).
The 19.6% of women who had a weight gain of at least 5% did not have a significantly elevated risk of breast cancer overall, but they did have a significantly elevated risk of triple-negative breast cancer (HR, 1.54; 95% CI, 1.16-2.05). “We really don’t have a good explanation for this,” Dr. Chlebowski said.
Tackling the global obesity epidemic by conventional means to reduce cancer risk is an uphill battle, he concluded. “We are going to be looking for possible mediating factors. If you can find the mediating factors, pharmacologic intervention would have a much greater chance of success.”
Dr. Chlebowski disclosed that he had no relevant conflicts of interest. Research was supported by the National Heart, Lung and Blood Institute; National Institutes of Health; Department of Health and Human Services; and American Institute for Cancer Research.
SOURCE: Chlebowski et al., SABCS 2017 Abstract GS5-07
REPORTING FROM SABCS 2017
Key clinical point:
Major finding: Compared with peers who had stable weight, women who lost at least 5% of their body weight had a lower risk of invasive breast cancer (hazard ratio, 0.88; P = .02).
Data source: A prospective cohort study of 93,676 postmenopausal women from the Women’s Health Initiative Observational Study.
Disclosures: Dr. Chlebowski disclosed that he had no relevant conflicts of interest. Research was supported by the National Heart, Lung, and Blood Institute; National Institutes of Health; Department of Health and Human Services; and American Institute for Cancer Research.
Source: Chlebowski et al. SABCS 2017 Abstract GS5-07.
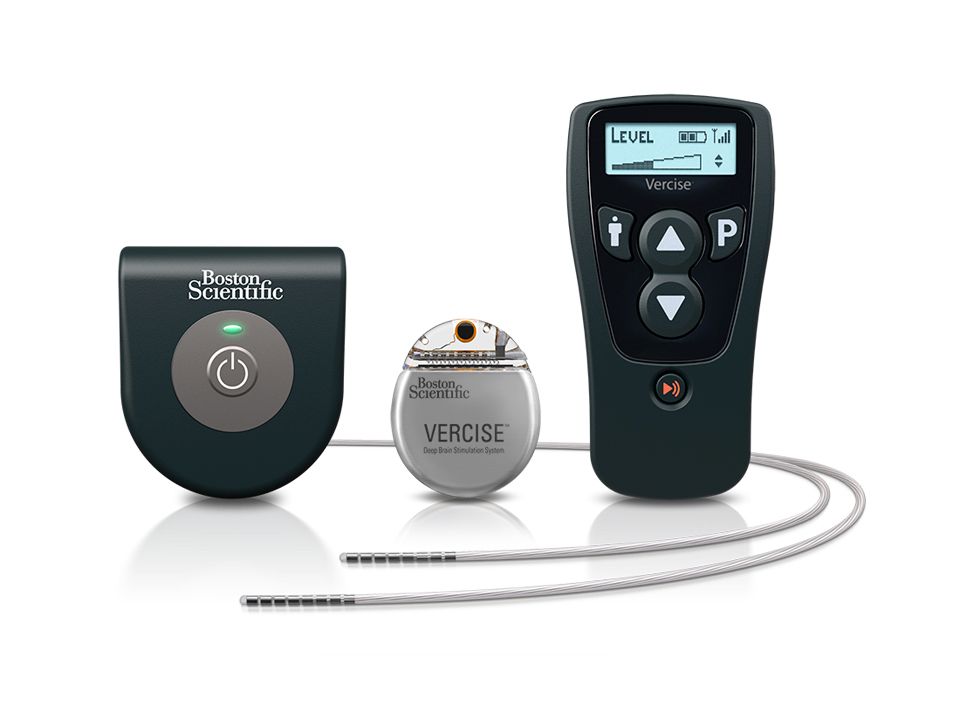
New DBS device gains approval for Parkinson’s disease
The approval is based on as-yet unpublished results of the INTREPID trial, which successfully met its primary endpoint of mean change in waking hours with good symptom control, according to an announcement from the device’s manufacturer, Boston Scientific. The INTREPID study is the first multicenter, prospective, double-blind, randomized, sham-controlled study of DBS for Parkinson’s disease in the United States and enrolled in 292 patients at 23 sites.
The implantable pulse generator of the device is “the smallest, rechargeable DBS device available in the U.S.,” according to Boston Scientific, and can independently control the amount of current delivered by each of the electrodes on the implanted leads, allowing them “to work together to address common challenges in DBS therapy such as fluctuations in symptoms and the progressive nature of the condition by offering more adaptable delivery of stimulation.”
“The Vercise DBS System changes the landscape of what physicians can do to help improve the quality of life for people living with Parkinson’s disease,” Jerry Vitek, MD, PhD, professor and chair of the department of neurology at the University of Minnesota, Minneapolis, and coordinating principal investigator for the INTREPID study said in the Boston Scientific announcement. “This system provides an ability to sculpt the current field in the DBS target using novel technology that offers flexibility in programming. This flexibility allows us to target different regions of the subthalamic nucleus, which we believe will improve outcomes while reducing side effects.”
The approval is based on as-yet unpublished results of the INTREPID trial, which successfully met its primary endpoint of mean change in waking hours with good symptom control, according to an announcement from the device’s manufacturer, Boston Scientific. The INTREPID study is the first multicenter, prospective, double-blind, randomized, sham-controlled study of DBS for Parkinson’s disease in the United States and enrolled in 292 patients at 23 sites.
The implantable pulse generator of the device is “the smallest, rechargeable DBS device available in the U.S.,” according to Boston Scientific, and can independently control the amount of current delivered by each of the electrodes on the implanted leads, allowing them “to work together to address common challenges in DBS therapy such as fluctuations in symptoms and the progressive nature of the condition by offering more adaptable delivery of stimulation.”
“The Vercise DBS System changes the landscape of what physicians can do to help improve the quality of life for people living with Parkinson’s disease,” Jerry Vitek, MD, PhD, professor and chair of the department of neurology at the University of Minnesota, Minneapolis, and coordinating principal investigator for the INTREPID study said in the Boston Scientific announcement. “This system provides an ability to sculpt the current field in the DBS target using novel technology that offers flexibility in programming. This flexibility allows us to target different regions of the subthalamic nucleus, which we believe will improve outcomes while reducing side effects.”
The approval is based on as-yet unpublished results of the INTREPID trial, which successfully met its primary endpoint of mean change in waking hours with good symptom control, according to an announcement from the device’s manufacturer, Boston Scientific. The INTREPID study is the first multicenter, prospective, double-blind, randomized, sham-controlled study of DBS for Parkinson’s disease in the United States and enrolled in 292 patients at 23 sites.
The implantable pulse generator of the device is “the smallest, rechargeable DBS device available in the U.S.,” according to Boston Scientific, and can independently control the amount of current delivered by each of the electrodes on the implanted leads, allowing them “to work together to address common challenges in DBS therapy such as fluctuations in symptoms and the progressive nature of the condition by offering more adaptable delivery of stimulation.”
“The Vercise DBS System changes the landscape of what physicians can do to help improve the quality of life for people living with Parkinson’s disease,” Jerry Vitek, MD, PhD, professor and chair of the department of neurology at the University of Minnesota, Minneapolis, and coordinating principal investigator for the INTREPID study said in the Boston Scientific announcement. “This system provides an ability to sculpt the current field in the DBS target using novel technology that offers flexibility in programming. This flexibility allows us to target different regions of the subthalamic nucleus, which we believe will improve outcomes while reducing side effects.”
Hospitals will feel the squeeze of DSH payment changes
Earlier this year, the Centers for Medicare and Medicaid Services finalized fundamental changes to how it reimburses hospitals for uncompensated care costs. When first proposed, the move raised alarm among physicians, hospitals, health systems, state health departments, and others around the country, and even prompted a lawsuit in New Hampshire.
In the months since the official adoption by the CMS, it remains unclear how the change will affect hospitals around the country, particularly the safety net hospitals that rely on these payments most.
In its final rule issued in April 2017 and finalized on August 2, 2017, the federal agency said the intent of the change is to more fairly distribute a fixed amount of DSH funds to the hospitals most in need. It also argued the change is a more consistent interpretation of the existing statute [Section 1923(g)], provides clarification around language that has been the subject of inquiry over the last decade, and promotes what it calls “fiscal integrity.”
“These allotments essentially establish a finite pool of available federal DSH funds that states use to pay the federal portion of payments to all qualifying hospitals in each state,” the final rule reads. “As states often use most or all of their federal DSH allotment, in practice, if one hospital gets more DSH funding, other DSH-eligible hospitals in the state may get less.”
This is not, however, the way all parties see it. For instance, in a comment submitted to the CMS in September 2016, the National Association of Urban Hospitals expressed its concern that DSH payments already are inadequate to cover the financial burden associated with providing care in low-income communities, such as translation services and the costs of employing physicians to practice in more challenged settings.2
In a letter to the CMS, the Minnesota Department of Human Services said it agrees with the agency that DSH payments should not be used to “subsidize costs that have been paid by Medicare and other insurers” but disagrees with the agency’s approach. Its argument includes a challenge to the CMS’ statutory authority to change the formula based on existing language.3
“I think the reason it’s contentious is because when you’re dealing with a fixed dollar amount and you’re talking about redistributing dollars, someone is going to lose,” said John McHugh, PhD, professor of health management at the Mailman School of Public Health at Columbia University. “A facility receiving DSH payments is already dealing with high levels of uncompensated care; the hospitals are operating on very thin margins. They are very often getting by because of these payments.”
Despite the CMS’ seemingly good intentions, Bradley Flansbaum, DO, MPH, MHM, a hospitalist at Geisinger Health System and member of the SHM Public Policy Committee, remains skeptical that the hospitals that need and deserve DSH payments will actually see more redistributed in their favor.
If hospitals in need see fewer DSH dollars, Dr. McHugh noted, they will feel the squeeze.
“It’s not easy to operate safety net hospitals,” he said. “And on top of that, hospitals have been operating under a certain assumption and it’s changing, and it takes time to incorporate those changes. There will probably be some fallout for the first couple of years as hospitals are adapting their practices. It could mean loss of services. It could mean the loss of quality physicians and quality staffing, and that can impact patient care.”
How will hospitals adapt?
The CMS did not give hospitals transition time. The reinterpretation became effective in June 2017, just 60 days after the agency issued the final rule. Dr. McHugh said he is not sure why the agency did not build in time for hospitals to adapt, particularly given the uncertainty around the national uninsured rate going forward, with so many potential changes to the American health care system under a new administration.
How any of these changes trickle down to hospitalists remains to be seen, said Dr. Flansbaum. Dr. McHugh believes it could lead to increased patient loads, higher turnover and churn, and fewer experienced physicians in safety net hospitals as younger doctors are hired and burn out. “At the end of the day, that feeds into patient care and patient satisfaction and quality,” he said.
However, hospitals across the country have been living with this “slow burn” for a long time, said Dr. Flansbaum, though not necessarily due to inadequate DSH payments. At least in some areas, reimbursements have gone down, hospital occupancy rates have declined, rural hospitals have closed, hospitals have consolidated, and people have been laid off.
It’s important to ensure the hospitals providing care for high levels of uninsured or underinsured patients receive the help they need, he said, and it’s also important to examine the role hospitals play as a whole in the American health care system.
“It’s an expensive system,” he said. “We have we created a system where, unlike other countries that have developed more vigorous primary or outpatient care, we have created an inpatient health system.”
With the CMS’ change, the government is the only entity that seems to win across the board, Dr. McHugh said. He said he would not be surprised if analysts looked to see how hospitals were affected by it in coming months.
But, he remains optimistic. In fact, the final rule also came with an $800 million increase in the amount of uncompensated care payments for acute care hospitals in fiscal year 2018, the CMS says.4
“Hospitals are adaptable,” Dr. McHugh said. “I think what you’ll see is this will spur some innovation in terms of patient care maybe a few years down the road. It may hit some stumbling blocks in the early going but there may be some positive changes in the future.”
References
1. Medicaid Program; Disproportionate Share Hospital Payments –Treatment of Third Party Payers in Calculating Uncompensated Care Costs. Centers for Medicare and Medicaid Services final rule. Citation 82 FR 16114. Published April 3, 2017. Last accessed August 14, 2017. https://www.federalregister.gov/documents/2017/04/03/2017-06538/medicaid-program-disproportionate-share-hospital-payments-treatment-of-third-party-payers-in
2. Kugler E. 2016-09-14 NAUH Medicaid Program DSH Payments – Treatment of Third Party Payer in Calculating Uncompensated Care Costs. September 14, 2016. Last accessed August 14, 2017. https://www.regulations.gov/document?D=CMS-2016-0144-0020
3. Berg A. Proposed Rule on Disproportionate Share Hospital Payments – Treatment of Third Party Payers in Calculating Uncompensated Care Costs, CMS-2399-P. September 14, 2016. Last accessed August 14, 2017. https://www.regulations.gov/document?D=CMS-2016-0144-0046
4. CMS finalizes 2018 payment and policy updates for Medicare hospital admissions. Published August 2, 2017. Last accessed August 14, 2017. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2017-Press-releases-items/2017-08-02.html
Earlier this year, the Centers for Medicare and Medicaid Services finalized fundamental changes to how it reimburses hospitals for uncompensated care costs. When first proposed, the move raised alarm among physicians, hospitals, health systems, state health departments, and others around the country, and even prompted a lawsuit in New Hampshire.
In the months since the official adoption by the CMS, it remains unclear how the change will affect hospitals around the country, particularly the safety net hospitals that rely on these payments most.
In its final rule issued in April 2017 and finalized on August 2, 2017, the federal agency said the intent of the change is to more fairly distribute a fixed amount of DSH funds to the hospitals most in need. It also argued the change is a more consistent interpretation of the existing statute [Section 1923(g)], provides clarification around language that has been the subject of inquiry over the last decade, and promotes what it calls “fiscal integrity.”
“These allotments essentially establish a finite pool of available federal DSH funds that states use to pay the federal portion of payments to all qualifying hospitals in each state,” the final rule reads. “As states often use most or all of their federal DSH allotment, in practice, if one hospital gets more DSH funding, other DSH-eligible hospitals in the state may get less.”
This is not, however, the way all parties see it. For instance, in a comment submitted to the CMS in September 2016, the National Association of Urban Hospitals expressed its concern that DSH payments already are inadequate to cover the financial burden associated with providing care in low-income communities, such as translation services and the costs of employing physicians to practice in more challenged settings.2
In a letter to the CMS, the Minnesota Department of Human Services said it agrees with the agency that DSH payments should not be used to “subsidize costs that have been paid by Medicare and other insurers” but disagrees with the agency’s approach. Its argument includes a challenge to the CMS’ statutory authority to change the formula based on existing language.3
“I think the reason it’s contentious is because when you’re dealing with a fixed dollar amount and you’re talking about redistributing dollars, someone is going to lose,” said John McHugh, PhD, professor of health management at the Mailman School of Public Health at Columbia University. “A facility receiving DSH payments is already dealing with high levels of uncompensated care; the hospitals are operating on very thin margins. They are very often getting by because of these payments.”
Despite the CMS’ seemingly good intentions, Bradley Flansbaum, DO, MPH, MHM, a hospitalist at Geisinger Health System and member of the SHM Public Policy Committee, remains skeptical that the hospitals that need and deserve DSH payments will actually see more redistributed in their favor.
If hospitals in need see fewer DSH dollars, Dr. McHugh noted, they will feel the squeeze.
“It’s not easy to operate safety net hospitals,” he said. “And on top of that, hospitals have been operating under a certain assumption and it’s changing, and it takes time to incorporate those changes. There will probably be some fallout for the first couple of years as hospitals are adapting their practices. It could mean loss of services. It could mean the loss of quality physicians and quality staffing, and that can impact patient care.”
How will hospitals adapt?
The CMS did not give hospitals transition time. The reinterpretation became effective in June 2017, just 60 days after the agency issued the final rule. Dr. McHugh said he is not sure why the agency did not build in time for hospitals to adapt, particularly given the uncertainty around the national uninsured rate going forward, with so many potential changes to the American health care system under a new administration.
How any of these changes trickle down to hospitalists remains to be seen, said Dr. Flansbaum. Dr. McHugh believes it could lead to increased patient loads, higher turnover and churn, and fewer experienced physicians in safety net hospitals as younger doctors are hired and burn out. “At the end of the day, that feeds into patient care and patient satisfaction and quality,” he said.
However, hospitals across the country have been living with this “slow burn” for a long time, said Dr. Flansbaum, though not necessarily due to inadequate DSH payments. At least in some areas, reimbursements have gone down, hospital occupancy rates have declined, rural hospitals have closed, hospitals have consolidated, and people have been laid off.
It’s important to ensure the hospitals providing care for high levels of uninsured or underinsured patients receive the help they need, he said, and it’s also important to examine the role hospitals play as a whole in the American health care system.
“It’s an expensive system,” he said. “We have we created a system where, unlike other countries that have developed more vigorous primary or outpatient care, we have created an inpatient health system.”
With the CMS’ change, the government is the only entity that seems to win across the board, Dr. McHugh said. He said he would not be surprised if analysts looked to see how hospitals were affected by it in coming months.
But, he remains optimistic. In fact, the final rule also came with an $800 million increase in the amount of uncompensated care payments for acute care hospitals in fiscal year 2018, the CMS says.4
“Hospitals are adaptable,” Dr. McHugh said. “I think what you’ll see is this will spur some innovation in terms of patient care maybe a few years down the road. It may hit some stumbling blocks in the early going but there may be some positive changes in the future.”
References
1. Medicaid Program; Disproportionate Share Hospital Payments –Treatment of Third Party Payers in Calculating Uncompensated Care Costs. Centers for Medicare and Medicaid Services final rule. Citation 82 FR 16114. Published April 3, 2017. Last accessed August 14, 2017. https://www.federalregister.gov/documents/2017/04/03/2017-06538/medicaid-program-disproportionate-share-hospital-payments-treatment-of-third-party-payers-in
2. Kugler E. 2016-09-14 NAUH Medicaid Program DSH Payments – Treatment of Third Party Payer in Calculating Uncompensated Care Costs. September 14, 2016. Last accessed August 14, 2017. https://www.regulations.gov/document?D=CMS-2016-0144-0020
3. Berg A. Proposed Rule on Disproportionate Share Hospital Payments – Treatment of Third Party Payers in Calculating Uncompensated Care Costs, CMS-2399-P. September 14, 2016. Last accessed August 14, 2017. https://www.regulations.gov/document?D=CMS-2016-0144-0046
4. CMS finalizes 2018 payment and policy updates for Medicare hospital admissions. Published August 2, 2017. Last accessed August 14, 2017. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2017-Press-releases-items/2017-08-02.html
Earlier this year, the Centers for Medicare and Medicaid Services finalized fundamental changes to how it reimburses hospitals for uncompensated care costs. When first proposed, the move raised alarm among physicians, hospitals, health systems, state health departments, and others around the country, and even prompted a lawsuit in New Hampshire.
In the months since the official adoption by the CMS, it remains unclear how the change will affect hospitals around the country, particularly the safety net hospitals that rely on these payments most.
In its final rule issued in April 2017 and finalized on August 2, 2017, the federal agency said the intent of the change is to more fairly distribute a fixed amount of DSH funds to the hospitals most in need. It also argued the change is a more consistent interpretation of the existing statute [Section 1923(g)], provides clarification around language that has been the subject of inquiry over the last decade, and promotes what it calls “fiscal integrity.”
“These allotments essentially establish a finite pool of available federal DSH funds that states use to pay the federal portion of payments to all qualifying hospitals in each state,” the final rule reads. “As states often use most or all of their federal DSH allotment, in practice, if one hospital gets more DSH funding, other DSH-eligible hospitals in the state may get less.”
This is not, however, the way all parties see it. For instance, in a comment submitted to the CMS in September 2016, the National Association of Urban Hospitals expressed its concern that DSH payments already are inadequate to cover the financial burden associated with providing care in low-income communities, such as translation services and the costs of employing physicians to practice in more challenged settings.2
In a letter to the CMS, the Minnesota Department of Human Services said it agrees with the agency that DSH payments should not be used to “subsidize costs that have been paid by Medicare and other insurers” but disagrees with the agency’s approach. Its argument includes a challenge to the CMS’ statutory authority to change the formula based on existing language.3
“I think the reason it’s contentious is because when you’re dealing with a fixed dollar amount and you’re talking about redistributing dollars, someone is going to lose,” said John McHugh, PhD, professor of health management at the Mailman School of Public Health at Columbia University. “A facility receiving DSH payments is already dealing with high levels of uncompensated care; the hospitals are operating on very thin margins. They are very often getting by because of these payments.”
Despite the CMS’ seemingly good intentions, Bradley Flansbaum, DO, MPH, MHM, a hospitalist at Geisinger Health System and member of the SHM Public Policy Committee, remains skeptical that the hospitals that need and deserve DSH payments will actually see more redistributed in their favor.
If hospitals in need see fewer DSH dollars, Dr. McHugh noted, they will feel the squeeze.
“It’s not easy to operate safety net hospitals,” he said. “And on top of that, hospitals have been operating under a certain assumption and it’s changing, and it takes time to incorporate those changes. There will probably be some fallout for the first couple of years as hospitals are adapting their practices. It could mean loss of services. It could mean the loss of quality physicians and quality staffing, and that can impact patient care.”
How will hospitals adapt?
The CMS did not give hospitals transition time. The reinterpretation became effective in June 2017, just 60 days after the agency issued the final rule. Dr. McHugh said he is not sure why the agency did not build in time for hospitals to adapt, particularly given the uncertainty around the national uninsured rate going forward, with so many potential changes to the American health care system under a new administration.
How any of these changes trickle down to hospitalists remains to be seen, said Dr. Flansbaum. Dr. McHugh believes it could lead to increased patient loads, higher turnover and churn, and fewer experienced physicians in safety net hospitals as younger doctors are hired and burn out. “At the end of the day, that feeds into patient care and patient satisfaction and quality,” he said.
However, hospitals across the country have been living with this “slow burn” for a long time, said Dr. Flansbaum, though not necessarily due to inadequate DSH payments. At least in some areas, reimbursements have gone down, hospital occupancy rates have declined, rural hospitals have closed, hospitals have consolidated, and people have been laid off.
It’s important to ensure the hospitals providing care for high levels of uninsured or underinsured patients receive the help they need, he said, and it’s also important to examine the role hospitals play as a whole in the American health care system.
“It’s an expensive system,” he said. “We have we created a system where, unlike other countries that have developed more vigorous primary or outpatient care, we have created an inpatient health system.”
With the CMS’ change, the government is the only entity that seems to win across the board, Dr. McHugh said. He said he would not be surprised if analysts looked to see how hospitals were affected by it in coming months.
But, he remains optimistic. In fact, the final rule also came with an $800 million increase in the amount of uncompensated care payments for acute care hospitals in fiscal year 2018, the CMS says.4
“Hospitals are adaptable,” Dr. McHugh said. “I think what you’ll see is this will spur some innovation in terms of patient care maybe a few years down the road. It may hit some stumbling blocks in the early going but there may be some positive changes in the future.”
References
1. Medicaid Program; Disproportionate Share Hospital Payments –Treatment of Third Party Payers in Calculating Uncompensated Care Costs. Centers for Medicare and Medicaid Services final rule. Citation 82 FR 16114. Published April 3, 2017. Last accessed August 14, 2017. https://www.federalregister.gov/documents/2017/04/03/2017-06538/medicaid-program-disproportionate-share-hospital-payments-treatment-of-third-party-payers-in
2. Kugler E. 2016-09-14 NAUH Medicaid Program DSH Payments – Treatment of Third Party Payer in Calculating Uncompensated Care Costs. September 14, 2016. Last accessed August 14, 2017. https://www.regulations.gov/document?D=CMS-2016-0144-0020
3. Berg A. Proposed Rule on Disproportionate Share Hospital Payments – Treatment of Third Party Payers in Calculating Uncompensated Care Costs, CMS-2399-P. September 14, 2016. Last accessed August 14, 2017. https://www.regulations.gov/document?D=CMS-2016-0144-0046
4. CMS finalizes 2018 payment and policy updates for Medicare hospital admissions. Published August 2, 2017. Last accessed August 14, 2017. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2017-Press-releases-items/2017-08-02.html
Leflunomide use in pregnancy shows little impact on newborns
Leflunomide, prescribed in Canada to treat active rheumatoid arthritis, was previously classified as a category X pregnancy medication because it is embryotoxic and teratogenic in rats and rabbits in doses similar to those used in humans, wrote Anick Bérard, MD, of CHU Sainte Justine, Montreal, and her colleagues, in a study published in Annals of the Rheumatic Diseases.
However, data on the impact of leflunomide on a developing human embryo are limited, so the researchers analyzed the Quebec Pregnancy Cohort, an ongoing study of all pregnancies in Quebec, Canada, between Jan. 1, 1998, and Dec. 31, 2015.
The findings are consistent with those from previous studies and suggest that continued caution is warranted for women of childbearing age who are taking or considering leflunomide, the researchers concluded.
They also examined the potential impact of several categories of other antirheumatic drugs to account for indication bias: other conventional disease-modifying antirheumatic drugs, biologic agents, nonsteroidal anti-inflammatory drugs, oral corticosteroids, and gold salts. Oral corticosteroid use in the first trimester was associated with an increased risk of major congenital malformations (aOR 1.31; 95% CI, 1.06-1.61), and the risk of prematurity also was significant with their use in the second or third trimester (aOR 1.32; 95% CI, 1.09 to 1.60). The risk of major congenital malformations was significantly higher with the use of NSAIDs in the first trimester (aOR 1.15; 95% CI, 1.03-1.29). Any use of disease-modifying antirheumatic drugs overall between the first day of gestation and the index date increased the odds for spontaneous abortion (aOR, 1.54; 95% CI, 1.06-2.22).
Cholestyramine may lower the blood level of the active metabolite of leflunomide to a safe level, the researchers noted, but the study population showed no evidence of cholestyramine or charcoal use for leflunomide washout, and any cholestyramine exposures during pregnancy were not concurrent with leflunomide exposure. “In three first-trimester leflunomide-exposed pregnancies, cholestyramine was introduced in monotherapy in the third trimester,” they wrote.
The results were limited by the small number of women exposed to leflunomide, despite the population-based study being the largest of its kind published to date, the researchers said.
The study was supported in part by the Fonds de la Recherche du Québec-Santé and by Sanofi. Two authors are employees of Sanofi.
SOURCE: Bérard A et al., Ann Rheum Dis. 2017 Dec 8. doi: 10.1136/annrheumdis-2017-212078
Leflunomide, prescribed in Canada to treat active rheumatoid arthritis, was previously classified as a category X pregnancy medication because it is embryotoxic and teratogenic in rats and rabbits in doses similar to those used in humans, wrote Anick Bérard, MD, of CHU Sainte Justine, Montreal, and her colleagues, in a study published in Annals of the Rheumatic Diseases.
However, data on the impact of leflunomide on a developing human embryo are limited, so the researchers analyzed the Quebec Pregnancy Cohort, an ongoing study of all pregnancies in Quebec, Canada, between Jan. 1, 1998, and Dec. 31, 2015.
The findings are consistent with those from previous studies and suggest that continued caution is warranted for women of childbearing age who are taking or considering leflunomide, the researchers concluded.
They also examined the potential impact of several categories of other antirheumatic drugs to account for indication bias: other conventional disease-modifying antirheumatic drugs, biologic agents, nonsteroidal anti-inflammatory drugs, oral corticosteroids, and gold salts. Oral corticosteroid use in the first trimester was associated with an increased risk of major congenital malformations (aOR 1.31; 95% CI, 1.06-1.61), and the risk of prematurity also was significant with their use in the second or third trimester (aOR 1.32; 95% CI, 1.09 to 1.60). The risk of major congenital malformations was significantly higher with the use of NSAIDs in the first trimester (aOR 1.15; 95% CI, 1.03-1.29). Any use of disease-modifying antirheumatic drugs overall between the first day of gestation and the index date increased the odds for spontaneous abortion (aOR, 1.54; 95% CI, 1.06-2.22).
Cholestyramine may lower the blood level of the active metabolite of leflunomide to a safe level, the researchers noted, but the study population showed no evidence of cholestyramine or charcoal use for leflunomide washout, and any cholestyramine exposures during pregnancy were not concurrent with leflunomide exposure. “In three first-trimester leflunomide-exposed pregnancies, cholestyramine was introduced in monotherapy in the third trimester,” they wrote.
The results were limited by the small number of women exposed to leflunomide, despite the population-based study being the largest of its kind published to date, the researchers said.
The study was supported in part by the Fonds de la Recherche du Québec-Santé and by Sanofi. Two authors are employees of Sanofi.
SOURCE: Bérard A et al., Ann Rheum Dis. 2017 Dec 8. doi: 10.1136/annrheumdis-2017-212078
Leflunomide, prescribed in Canada to treat active rheumatoid arthritis, was previously classified as a category X pregnancy medication because it is embryotoxic and teratogenic in rats and rabbits in doses similar to those used in humans, wrote Anick Bérard, MD, of CHU Sainte Justine, Montreal, and her colleagues, in a study published in Annals of the Rheumatic Diseases.
However, data on the impact of leflunomide on a developing human embryo are limited, so the researchers analyzed the Quebec Pregnancy Cohort, an ongoing study of all pregnancies in Quebec, Canada, between Jan. 1, 1998, and Dec. 31, 2015.
The findings are consistent with those from previous studies and suggest that continued caution is warranted for women of childbearing age who are taking or considering leflunomide, the researchers concluded.
They also examined the potential impact of several categories of other antirheumatic drugs to account for indication bias: other conventional disease-modifying antirheumatic drugs, biologic agents, nonsteroidal anti-inflammatory drugs, oral corticosteroids, and gold salts. Oral corticosteroid use in the first trimester was associated with an increased risk of major congenital malformations (aOR 1.31; 95% CI, 1.06-1.61), and the risk of prematurity also was significant with their use in the second or third trimester (aOR 1.32; 95% CI, 1.09 to 1.60). The risk of major congenital malformations was significantly higher with the use of NSAIDs in the first trimester (aOR 1.15; 95% CI, 1.03-1.29). Any use of disease-modifying antirheumatic drugs overall between the first day of gestation and the index date increased the odds for spontaneous abortion (aOR, 1.54; 95% CI, 1.06-2.22).
Cholestyramine may lower the blood level of the active metabolite of leflunomide to a safe level, the researchers noted, but the study population showed no evidence of cholestyramine or charcoal use for leflunomide washout, and any cholestyramine exposures during pregnancy were not concurrent with leflunomide exposure. “In three first-trimester leflunomide-exposed pregnancies, cholestyramine was introduced in monotherapy in the third trimester,” they wrote.
The results were limited by the small number of women exposed to leflunomide, despite the population-based study being the largest of its kind published to date, the researchers said.
The study was supported in part by the Fonds de la Recherche du Québec-Santé and by Sanofi. Two authors are employees of Sanofi.
SOURCE: Bérard A et al., Ann Rheum Dis. 2017 Dec 8. doi: 10.1136/annrheumdis-2017-212078
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Exposure to leflunomide during pregnancy was not associated with significantly increased risk of major congenital malformations, low birth weight, premature birth, or spontaneous abortions.
Major finding: No significant association was seen between leflunomide use in the first trimester and an increased risk of major congenital malformations based on five cases (adjusted odds ratio, 0.97).
Data source: A population-based cohort study of 289,688 pregnancies in Canada between 1998 and 2015.
Disclosures: The study was supported in part by the Fonds de la Recherche du Québec-Santé and by Sanofi. Two authors are employees of Sanofi.
Source: Bérard A et al., Ann Rheum Dis. 2017 Dec 8. doi: 10.1136/annrheumdis-2017-212078
MRI-guided focused ultrasound shows promise for subcortical epilepsy
WASHINGTON – MRI-guided focused ultrasound (FUS) is now being employed on an experimental basis to treat deep subcortical lesions, such as hypothalamic hamartoma, to control intractable epilepsy, according to an expert summary of a “hot topic” presented at the American Epilepsy Society annual meeting.
“If the risk of FUS is as low as we expect, it could change our paradigm,” reported Nathan B. Fountain, MD, director of the F.E. Dreifuss Comprehensive Epilepsy Program at the University of Virginia, Charlottesville.
FUS has been used clinically for the treatment of uterine fibroids since 2004, according to an overview provided by Dr. Fountain. Clinical studies of MRI-guided FUS for lesions in the brain began in 2009. The approval of MRI-guided FUS thalamotomy for essential tumor in 2016 was based on a pivotal trial led by Jeffrey Elias, MD, a colleague of Dr. Fountain’s at the University of Virginia (N Engl J Med. 2016;375:730-9). Many of the principles for treating subcortical lesions causing epilepsy are the same as those for treating essential tremor.
Under MRI guidance, FUS is delivered via a helmet with 1,024 transducers. These focus sound waves to a highly targeted area of the brain, resulting in thermal ablation. The treatment is noninvasive in the sense that no craniotomy is involved. It can be delivered without anesthesia. When used to treat essential tremor in awake patients, MRI-guided FUS confirms the target when the tremor resolves.
“There is no injury to the brain as far as we can tell,” reported Dr. Fountain, referring to the tremor studies.
Because the thermal ablation is delivered by sound waves, this approach appears to be safer to structures surrounding the lesion than would be anticipated with energy delivered by radiation. For treatment of lesions in the hypothalamus, where surrounding tissue is responsible for important brain functions, the apparent low risk of collateral damage is a major potential advantage, according to Dr. Fountain.
Although Dr. Fountain conceded that the term “subcortical” is not commonly used to describe epilepsy lesions, he considers it appropriate to explain the role of MRI-guided FUS. Without technical advancements, this tool is not appropriate for the cortical lesions that are responsible for the majority of epileptic seizures. Rather, lesions must be positioned deep in the skull to be in the “envelope” where energy can be concentrated. Lesions in the temporal or hippocampal areas of the brain, for example, will not be suitable without technical advances.
Due to its position in the brain, “hypothalamic hamartoma is the prototype lesion,” Dr. Fountain reported. Importantly, these and other lesions within the envelope where energy can be targeted are the most difficult to treat with other options. Due to the need to transverse much of the brain to reach these areas, open surgery is often not practical. Even though Dr. Fountain acknowledged that MRI-guided stereotactic laser has been proposed for these types of lesions, the laser must also transverse vulnerable structures of the brain that can be avoided with MR-guided FUS.
Results on the first patient in a planned pediatric treatment series with MRI-guided FUS were presented at the AES annual meeting by Travis Tierney, MD, PhD, a neurosurgeon associated with Nicklaus Children’s Hospital in Miami. According to the data presented by Dr. Tierney, the 21-year-old patient was treated for a hypothalamic hamartoma. She was rendered seizure free and had no complications.
An adult series is now recruiting candidates, according to Dr. Fountain. He reported that adults of at least 18 years of age with intractable epilepsy due to subcortical lesions in the central envelope suitable for MRI-guided FUS are eligible if they have at least three seizures per month while taking at least two antiepileptic drugs. He encouraged referrals.
“The primary outcome will be just to demonstrate that a lesion can be created,” Dr. Fountain said. He reported that the planned enrollment of 15 subjects would not be sufficient to draw conclusions about efficacy “unless, of course, we eliminate everyone’s seizures – and that would be useful – but that is still a secondary outcome,”
There are a number of applications in neurology beyond treatment of tremors and epilepsy that are also being considered for MRI-guided FUS, Dr. Fountain reported. This could include, for example, clot lysis in stroke, but he indicated that there are a number of reasons to be particularly optimistic about its potential role in the treatment intractable epilepsy due to subcortical lesions. This strategy seems feasible in a condition with limited treatment options.
WASHINGTON – MRI-guided focused ultrasound (FUS) is now being employed on an experimental basis to treat deep subcortical lesions, such as hypothalamic hamartoma, to control intractable epilepsy, according to an expert summary of a “hot topic” presented at the American Epilepsy Society annual meeting.
“If the risk of FUS is as low as we expect, it could change our paradigm,” reported Nathan B. Fountain, MD, director of the F.E. Dreifuss Comprehensive Epilepsy Program at the University of Virginia, Charlottesville.
FUS has been used clinically for the treatment of uterine fibroids since 2004, according to an overview provided by Dr. Fountain. Clinical studies of MRI-guided FUS for lesions in the brain began in 2009. The approval of MRI-guided FUS thalamotomy for essential tumor in 2016 was based on a pivotal trial led by Jeffrey Elias, MD, a colleague of Dr. Fountain’s at the University of Virginia (N Engl J Med. 2016;375:730-9). Many of the principles for treating subcortical lesions causing epilepsy are the same as those for treating essential tremor.
Under MRI guidance, FUS is delivered via a helmet with 1,024 transducers. These focus sound waves to a highly targeted area of the brain, resulting in thermal ablation. The treatment is noninvasive in the sense that no craniotomy is involved. It can be delivered without anesthesia. When used to treat essential tremor in awake patients, MRI-guided FUS confirms the target when the tremor resolves.
“There is no injury to the brain as far as we can tell,” reported Dr. Fountain, referring to the tremor studies.
Because the thermal ablation is delivered by sound waves, this approach appears to be safer to structures surrounding the lesion than would be anticipated with energy delivered by radiation. For treatment of lesions in the hypothalamus, where surrounding tissue is responsible for important brain functions, the apparent low risk of collateral damage is a major potential advantage, according to Dr. Fountain.
Although Dr. Fountain conceded that the term “subcortical” is not commonly used to describe epilepsy lesions, he considers it appropriate to explain the role of MRI-guided FUS. Without technical advancements, this tool is not appropriate for the cortical lesions that are responsible for the majority of epileptic seizures. Rather, lesions must be positioned deep in the skull to be in the “envelope” where energy can be concentrated. Lesions in the temporal or hippocampal areas of the brain, for example, will not be suitable without technical advances.
Due to its position in the brain, “hypothalamic hamartoma is the prototype lesion,” Dr. Fountain reported. Importantly, these and other lesions within the envelope where energy can be targeted are the most difficult to treat with other options. Due to the need to transverse much of the brain to reach these areas, open surgery is often not practical. Even though Dr. Fountain acknowledged that MRI-guided stereotactic laser has been proposed for these types of lesions, the laser must also transverse vulnerable structures of the brain that can be avoided with MR-guided FUS.
Results on the first patient in a planned pediatric treatment series with MRI-guided FUS were presented at the AES annual meeting by Travis Tierney, MD, PhD, a neurosurgeon associated with Nicklaus Children’s Hospital in Miami. According to the data presented by Dr. Tierney, the 21-year-old patient was treated for a hypothalamic hamartoma. She was rendered seizure free and had no complications.
An adult series is now recruiting candidates, according to Dr. Fountain. He reported that adults of at least 18 years of age with intractable epilepsy due to subcortical lesions in the central envelope suitable for MRI-guided FUS are eligible if they have at least three seizures per month while taking at least two antiepileptic drugs. He encouraged referrals.
“The primary outcome will be just to demonstrate that a lesion can be created,” Dr. Fountain said. He reported that the planned enrollment of 15 subjects would not be sufficient to draw conclusions about efficacy “unless, of course, we eliminate everyone’s seizures – and that would be useful – but that is still a secondary outcome,”
There are a number of applications in neurology beyond treatment of tremors and epilepsy that are also being considered for MRI-guided FUS, Dr. Fountain reported. This could include, for example, clot lysis in stroke, but he indicated that there are a number of reasons to be particularly optimistic about its potential role in the treatment intractable epilepsy due to subcortical lesions. This strategy seems feasible in a condition with limited treatment options.
WASHINGTON – MRI-guided focused ultrasound (FUS) is now being employed on an experimental basis to treat deep subcortical lesions, such as hypothalamic hamartoma, to control intractable epilepsy, according to an expert summary of a “hot topic” presented at the American Epilepsy Society annual meeting.
“If the risk of FUS is as low as we expect, it could change our paradigm,” reported Nathan B. Fountain, MD, director of the F.E. Dreifuss Comprehensive Epilepsy Program at the University of Virginia, Charlottesville.
FUS has been used clinically for the treatment of uterine fibroids since 2004, according to an overview provided by Dr. Fountain. Clinical studies of MRI-guided FUS for lesions in the brain began in 2009. The approval of MRI-guided FUS thalamotomy for essential tumor in 2016 was based on a pivotal trial led by Jeffrey Elias, MD, a colleague of Dr. Fountain’s at the University of Virginia (N Engl J Med. 2016;375:730-9). Many of the principles for treating subcortical lesions causing epilepsy are the same as those for treating essential tremor.
Under MRI guidance, FUS is delivered via a helmet with 1,024 transducers. These focus sound waves to a highly targeted area of the brain, resulting in thermal ablation. The treatment is noninvasive in the sense that no craniotomy is involved. It can be delivered without anesthesia. When used to treat essential tremor in awake patients, MRI-guided FUS confirms the target when the tremor resolves.
“There is no injury to the brain as far as we can tell,” reported Dr. Fountain, referring to the tremor studies.
Because the thermal ablation is delivered by sound waves, this approach appears to be safer to structures surrounding the lesion than would be anticipated with energy delivered by radiation. For treatment of lesions in the hypothalamus, where surrounding tissue is responsible for important brain functions, the apparent low risk of collateral damage is a major potential advantage, according to Dr. Fountain.
Although Dr. Fountain conceded that the term “subcortical” is not commonly used to describe epilepsy lesions, he considers it appropriate to explain the role of MRI-guided FUS. Without technical advancements, this tool is not appropriate for the cortical lesions that are responsible for the majority of epileptic seizures. Rather, lesions must be positioned deep in the skull to be in the “envelope” where energy can be concentrated. Lesions in the temporal or hippocampal areas of the brain, for example, will not be suitable without technical advances.
Due to its position in the brain, “hypothalamic hamartoma is the prototype lesion,” Dr. Fountain reported. Importantly, these and other lesions within the envelope where energy can be targeted are the most difficult to treat with other options. Due to the need to transverse much of the brain to reach these areas, open surgery is often not practical. Even though Dr. Fountain acknowledged that MRI-guided stereotactic laser has been proposed for these types of lesions, the laser must also transverse vulnerable structures of the brain that can be avoided with MR-guided FUS.
Results on the first patient in a planned pediatric treatment series with MRI-guided FUS were presented at the AES annual meeting by Travis Tierney, MD, PhD, a neurosurgeon associated with Nicklaus Children’s Hospital in Miami. According to the data presented by Dr. Tierney, the 21-year-old patient was treated for a hypothalamic hamartoma. She was rendered seizure free and had no complications.
An adult series is now recruiting candidates, according to Dr. Fountain. He reported that adults of at least 18 years of age with intractable epilepsy due to subcortical lesions in the central envelope suitable for MRI-guided FUS are eligible if they have at least three seizures per month while taking at least two antiepileptic drugs. He encouraged referrals.
“The primary outcome will be just to demonstrate that a lesion can be created,” Dr. Fountain said. He reported that the planned enrollment of 15 subjects would not be sufficient to draw conclusions about efficacy “unless, of course, we eliminate everyone’s seizures – and that would be useful – but that is still a secondary outcome,”
There are a number of applications in neurology beyond treatment of tremors and epilepsy that are also being considered for MRI-guided FUS, Dr. Fountain reported. This could include, for example, clot lysis in stroke, but he indicated that there are a number of reasons to be particularly optimistic about its potential role in the treatment intractable epilepsy due to subcortical lesions. This strategy seems feasible in a condition with limited treatment options.
EXPERT ANALYSIS FROM AES 2017
Early intervention key to treating substance use disorders
SAN DIEGO – Intervening early can positively affect outcomes for patients struggling with substance use disorders, regardless of their current stage of development and place in the life cycle, speaker after speaker said at the annual meeting and scientific symposium of the American Academy of Addiction Psychiatry.
The prenatal/perinatal period represents a high-risk window for the kindling and precipitation of substance use disorders (SUDs), because of the stress of pregnancy, delivery, recovery, and the newly assumed parenting role. “Offspring who are exposed in utero have an earlier age of initiation of substance use, as well as a greater risk of substance use disorders later on in life,” said Justine Wittenaur Welsh, MD, director of the Emory Adolescent Substance Use Treatment Services, Atlanta. “We as treatment providers need to start to think about how to screen for these individuals. What types of early intervention can we provide, and how do we integrate this into our treatment planning?”
According to data on current illicit drug use among pregnant women from the Substance Abuse and Mental Health Services Administration, the risk is highest among those aged 15-17 years (14.6%), followed by those aged 18-25 years (8.6%), and 26-44 years (3.2%). “We also know that rates of substance use are higher in the first trimester, compared with the second and the third,” said Dr. Welsh, who holds a faculty position in the department of psychiatry and behavioral sciences at Emory University. “But unfortunately, a significant number of pregnant women return to substance use after they deliver. This is really important for treatment providers, to intervene early before a return to substance use. We know that improving the postnatal environment does reduce the risk of substance use, even in individuals who have in utero exposures.”
SUDs confer a host of adverse effects to both the baby and the mother. “With tobacco, although there are a significant number of poisonous chemicals in cigarettes that the baby would be exposed to, we believe it’s the carbon monoxide and the nicotine exposure that confers the most known damage,” Dr. Welsh said. In utero exposure to cigarettes increases the likelihood to initiate smoking during adolescence by twofold (Neurotoxicol Teratol. 2005;27[2]:267-77), and a dose-response relationship has been observed between prenatal smoking and psychiatric hospitalization for substance abuse in offspring (Am J Psychiatry. 2002;159[1]:48-54). In utero exposure to cigarettes also increases the risk for offspring cigarette use. “The exposure to greater than a half-pack of cigarettes per day has a 5.5-fold increased risk for early cigarette experimentation, while exposure to one pack per day or more is associated with twice the risk of developing nicotine dependence,” she said.
As for in utero alcohol exposure, consuming greater than or equal to three drinks is associated with an alcohol disorder at age 21 (odds ratio, 2.95) if the exposure occurred in early pregnancy; Arch Gen Psychiatry. 2006;63[9]:1009-16). In utero alcohol exposure also has been more predictive of adolescent alcohol use than family history. “It’s also been associated with an increased number of abuse/dependency symptoms in offspring, for nicotine, alcohol, and illicit drugs,” Dr. Welsh said. “It has also been associated with an increased risk of cigarette use and substance use disorders in offspring.”
In utero cannabis exposure has been associated with a twofold increased risk of tobacco and marijuana use later in life (Addiction 2006;101[9]:1313-22), as well as with a twofold increased risk of smoking cigarettes daily and of using marijuana during adolescence. In utero cocaine exposure has been associated with a twofold increased risk of using tobacco, a 2.2-fold increased risk of using alcohol, and a 1.8-fold risk of using marijuana, as well as a 2.1-fold increased risk of having an SUD by age 17. “Prenatal exposure and postnatal parent/caregiver cocaine use is uniquely related to teen use of cocaine at age 14,” Dr. Welsh said. “In addition, exposure in the first trimester is associated with earlier marijuana and alcohol initiation.”
Real-world barriers to SUD treatment in adolescents include a lack of coordination between treatment resources, fear of losing custody of children, shame and stigma, concerns about criminal prosecution, domestic violence, and other obstacles such as transportation, finances, and child care. Dr. Welsh pointed out that pregnant women with SUDs usually receive prenatal care and addiction treatment from different providers. “We should be thinking about an integrated treatment model to improve maternal and prenatal care for patients using substances prior to or during pregnancy,” she said. “Some sites have developed combined maternity care units as a way to reduce some of these barriers to treatment. We also need to be thinking about the infants themselves. In order to counter the drug-exposed child’s early disadvantages, service providers must be prepared to intervene early, focusing on things like nutrition, psychomotor assessments, early educational needs assessments, as well as modification of existing treatment services for people with in utero exposures. This can be a difficult population to treat.”
One resource for addressing fetal alcohol spectrum disorders that she recommended is Treatment Improvement Protocol 58, published by Substance Abuse and Mental Health Services Administration. She described one young adult with significant substance use issues who was diagnosed with fetal alcohol syndrome as a child. “This young adult didn’t know about the FAS diagnosis,” Dr. Welsh said. “I expressed to the mother that, ‘this is an ethical dilemma. I’m treating someone and I know this directly impacts my treatment planning. They have a real-world diagnosis that I feel they have a right to know about.’ It made me think: If I think it’s so important that this young adult knows the diagnosis, why are we not screening for it in our general patient populations more often?
“As child psychiatrists we often ask, ‘Were there any in utero exposures?’ When treating adults, I’m asking about family history, but I’m not asking about in utero exposures. This may not be information that they’re privy to, but it’s worth starting a conversation about.”
, a 14-session, evidence-based curriculum that addresses parenting skills, children’s social skills, and family life skills training, are among the treatment options aimed at decreasing the risk of substance use progression in offspring.
‘Adolescents indeed care about their health’
During a separate presentation, Peter Jackson, MD, a psychiatrist at the University of Vermont Medical Center, Burlington, noted that an estimated 3.4 million adolescents in the United States meet criteria for an SUD, but fewer than 10% enter treatment each year. Of those who do enter treatment, 50% relapse within 6 months.
“It’s not the same story for illicit drugs, unfortunately, and that’s largely due to marijuana,” Dr. Jackson said. He noted that adolescent cannabis use is inversely proportional to how dangerous they perceive it to be. “This is evidence that adolescents indeed care about their health,” he said. “They use when they think it’s safe, and they use less when they think it’s unsafe.” Recent Monitoring the Future data also demonstrate that e-cigarette use is outpacing cigarette use among 8th, 10th, and 12th graders, while the past-year misuse of acetaminophen/hydrocodone (Vicodin) among 12th graders has dropped dramatically in the past 5 years. So has misuse of all prescription opioids among 12th graders despite high opioid overdose rates among adults.
Adolescents are more likely to be secretive about their substance use, compared with older adults. “Also, with many substances, especially alcohol, they are more likely to use in a binge pattern,” Dr. Jackson said. “They haven’t accumulated as many negative consequences from their use, so voluntarily seeking treatment is less common than in adults. They’re most often referred through the justice department or legal channels.”
Screening instruments to consider using include the CRAFFT (Car, Relax, Alone, Forget, Friends, Trouble), which has been validated as an adolescent-specific screening instrument. A score of two or more has a strong correlation with meeting criteria for an SUD. The American Academy of Pediatrics recommends the Screening, Brief Intervention, and Referral to Treatment (SBIRT), though this intervention has not been as rigorously evaluated in adolescents, compared with adults.
Dr. Jackson frowned on the notion perpetuated by some parents and caregivers that novelty seeking behavior involving alcohol and other substances is okay if it’s controlled somehow, with statements such as, “I just make sure they’ve given up their car keys and make sure it stays in my own basement. That way nobody leaves here drunk.”
“I think we can do better than that,” Dr. Jackson said.
Well-validated behavioral treatment approaches for adolescent patients include the adolescent community reinforcement approach (A-CRA); cognitive-behavioral therapy (particularly in groups); motivational enhancement therapy (MET); contingency management, and 12-step facilitation.
“Colloquially, in pop culture, we’ve been pressed on this message at times that adolescents don’t care what their parents think; they only care what their peers think,” Dr. Jackson said. “That’s not true from literature we’ve seen, so we need to put the family back in its important place.”
In a meta-analysis of promising behavioral approaches for adolescent SUD, five out of six found to have promising to excellent empirical support were family-based therapies: multidimensional family therapy, functional family therapy, multisystemic family therapy, behavioral strategic family therapy, and family behavior therapy (J Child and Adolesc Psychology. 2008;37[1]:236-59). “That’s a huge take-home point when working with adolescents: Involve the family,” Dr. Jackson said. “As providers, do we have a tendency to side only with the parent or side only with the adolescent? If we do, we should be cautious and thoughtful, because we can very effectively work with both parties. If you have an adolescent who shows up [for counseling] but then walks right out the door, you still have a parent, maybe two parents or other concerned loved ones sitting there. You still have a target for intervention.”
Even if the adolescent never returns to your office, he continued, clinicians can do an intervention with the family to statistically decrease the SUD for the adolescent. “That’s really promising,” he said. In adults, the best evidence for treatment engagement of a loved one following these family-specific interventions is for the Community Reinforcement and Family Training (CRAFT) method (64%-74%), followed by the Johnson Intervention (23%-30%) and Al-Anon/Nar-Anon facilitation (13%-29%). These interventions are being studied more specifically in adolescents and young adults.
A key tip for parents of teens coping with substance abuse is the old adage actions speak louder than words. “You can tell your child not to smoke marijuana until you’re blue in the face, but then if you go out on the back porch and smoke marijuana, that’s a really bad message for behavior change,” Dr. Jackson said. “Words also speak louder than no words. Some parents have never told their adolescent children what their opinion is, and that they’re concerned. Maybe those parents have been convinced that adolescents don’t care what they think. In fact, they do care what they think.”
Once engaged, older adults do well
Among older adults, triggers for SUDs vary considerably from that of their younger counterparts. “We tend to think of this as a population that carries a lot of wisdom and has the coping skills to deal with life,” Olivera J. Bogunovic, MD, a psychiatrist who is medical director of ambulatory services in the division of alcohol and drug abuse at McClean Hospital, Belmont, Mass., said during a separate presentation. “In fact, this is a very vulnerable population at increased risk of mood disorders. For example, many men spend their careers working 60 or 80 hours a week, then they stop working. What is there for them? They sometimes turn to substance abuse. Similarly, older women who lose a loved one may turn to drinking. There’s a lot of room for prevention. The good news is that this group of patients is very responsive to treatment. Once engaged, they do very well.”
Nicotine is the chief addictive substance affecting older adults (17%), followed by alcohol (12% in a binge capacity, dependence 1%), illicit drugs (1.8%), and medications (1.6% for pain medications and 12% on benzodiazepines). Alcohol use disorders encompass a spectrum of problems for this patient population, including at-risk drinking, problem drinking, and dependence. Presentations, complications, and consequences are wide-ranging. “We sometimes miss the symptoms of alcohol use disorders when patients present in emergency rooms,” Dr. Bogunovic said. “Unfortunately, if it’s not treated initially it results in serious medical comorbidities and complications.”
The National Institute on Drug Abuse recommends that adults consume no more than two drinks per day, but the quantity is even lower for elderly. “For men it is no more than one drink per day, while for women it’s questionable if that one drink per day is even recommended,” Dr. Bogunovic said. The prevalence of at-risk drinking among older adults ranges from 3% to 25% and the rates for problem drinking range from 2.2% to 9.6%. Alcohol dependence rates, meanwhile, range from 2% to 3% among men and less than 1% among women.
Cross-sectional data indicate a low prevalence of illicit drug use in older adults, but longitudinal data from the National Survey on Drug Abuse and others suggest an increased use of cannabis and prescription opioids. “A lot of people do not think about opioid use disorders in the elderly,” she said. “The prevalence rate in methadone clinics is less than 2%. However, with the epidemic crisis we’re seeing opioid use disorders in the elderly. What’s important to know is that they also respond to medications for treatment, more so with suboxone than with naltrexone, as we know those patients sometimes have complications that need to be treated with pain medication.”
Elderly patients constitute 13% of the population yet they account for 30% of prescriptions, mainly benzodiazepines and prescription opioids. “Two-thirds of patients who are struggling with SUDs struggled with an SUD at some point in their youth,” said Dr. Bogunovic, who holds a faculty position in the department of psychiatry at Harvard Medical School, Boston. “They continue to use for longer periods of time, with some intermittent periods of sobriety. Of those 15% who do have an alcohol use problem, they are prescribed benzodiazepines. That can be a lethal combination, because there is a combined effect of both alcohol and benzodiazepines that can result in hip fractures, subdural hematomas, and other medical comorbidity.”
Risk factors for development of alcohol use disorder in elderly include physiologic changes in the way alcohol is metabolized; gender, family history, or prior personal history of alcohol use disorder; having a concomitant psychiatric disorder; and having a chronic medical illness, such as severe arthritis. Compared with their male peers, older women generally drink less often and less heavily, but they are more likely to start drinking heavily later in life. Older men face an increased risk of alcohol-related problems tied to cumulative use over the years.
“Social factors also play a role, so it’s important to perform a skilled diagnostic interview and a psychiatric evaluation but also to evaluate the motivational stage of change, because that’s the right moment to do the intervention, knowing what the patient’s values are,” she said. Recommended screenings include the Short Michigan Alcoholism Screening Instrument–Geriatric Version (SMAST-G), the SBIRT, the CAGE-AID, and the Opioid Risk Tool. Compliance with treatment tends to be greater in older adults, compared with their younger counterparts. “They don’t like a confrontational approach, so it’s important to communicate with empathy in a straightforward manner and pay attention to what is important to patients and motivate them,” she noted. “Involve family members or other social support whenever possible.”
Relapses are common, but clinicians should encourage patients to continue treatment, “because they can do very well,” Dr. Bogunovic emphasized. “And we can offer them a good life after that.”
Dr. Welsh disclosed that she has consulted for GW Pharmaceuticals and that she has received training fees from Chestnut Health Systems. Dr. Jackson and Dr. Bogunovic reported having no financial disclosures.
SAN DIEGO – Intervening early can positively affect outcomes for patients struggling with substance use disorders, regardless of their current stage of development and place in the life cycle, speaker after speaker said at the annual meeting and scientific symposium of the American Academy of Addiction Psychiatry.
The prenatal/perinatal period represents a high-risk window for the kindling and precipitation of substance use disorders (SUDs), because of the stress of pregnancy, delivery, recovery, and the newly assumed parenting role. “Offspring who are exposed in utero have an earlier age of initiation of substance use, as well as a greater risk of substance use disorders later on in life,” said Justine Wittenaur Welsh, MD, director of the Emory Adolescent Substance Use Treatment Services, Atlanta. “We as treatment providers need to start to think about how to screen for these individuals. What types of early intervention can we provide, and how do we integrate this into our treatment planning?”
According to data on current illicit drug use among pregnant women from the Substance Abuse and Mental Health Services Administration, the risk is highest among those aged 15-17 years (14.6%), followed by those aged 18-25 years (8.6%), and 26-44 years (3.2%). “We also know that rates of substance use are higher in the first trimester, compared with the second and the third,” said Dr. Welsh, who holds a faculty position in the department of psychiatry and behavioral sciences at Emory University. “But unfortunately, a significant number of pregnant women return to substance use after they deliver. This is really important for treatment providers, to intervene early before a return to substance use. We know that improving the postnatal environment does reduce the risk of substance use, even in individuals who have in utero exposures.”
SUDs confer a host of adverse effects to both the baby and the mother. “With tobacco, although there are a significant number of poisonous chemicals in cigarettes that the baby would be exposed to, we believe it’s the carbon monoxide and the nicotine exposure that confers the most known damage,” Dr. Welsh said. In utero exposure to cigarettes increases the likelihood to initiate smoking during adolescence by twofold (Neurotoxicol Teratol. 2005;27[2]:267-77), and a dose-response relationship has been observed between prenatal smoking and psychiatric hospitalization for substance abuse in offspring (Am J Psychiatry. 2002;159[1]:48-54). In utero exposure to cigarettes also increases the risk for offspring cigarette use. “The exposure to greater than a half-pack of cigarettes per day has a 5.5-fold increased risk for early cigarette experimentation, while exposure to one pack per day or more is associated with twice the risk of developing nicotine dependence,” she said.
As for in utero alcohol exposure, consuming greater than or equal to three drinks is associated with an alcohol disorder at age 21 (odds ratio, 2.95) if the exposure occurred in early pregnancy; Arch Gen Psychiatry. 2006;63[9]:1009-16). In utero alcohol exposure also has been more predictive of adolescent alcohol use than family history. “It’s also been associated with an increased number of abuse/dependency symptoms in offspring, for nicotine, alcohol, and illicit drugs,” Dr. Welsh said. “It has also been associated with an increased risk of cigarette use and substance use disorders in offspring.”
In utero cannabis exposure has been associated with a twofold increased risk of tobacco and marijuana use later in life (Addiction 2006;101[9]:1313-22), as well as with a twofold increased risk of smoking cigarettes daily and of using marijuana during adolescence. In utero cocaine exposure has been associated with a twofold increased risk of using tobacco, a 2.2-fold increased risk of using alcohol, and a 1.8-fold risk of using marijuana, as well as a 2.1-fold increased risk of having an SUD by age 17. “Prenatal exposure and postnatal parent/caregiver cocaine use is uniquely related to teen use of cocaine at age 14,” Dr. Welsh said. “In addition, exposure in the first trimester is associated with earlier marijuana and alcohol initiation.”
Real-world barriers to SUD treatment in adolescents include a lack of coordination between treatment resources, fear of losing custody of children, shame and stigma, concerns about criminal prosecution, domestic violence, and other obstacles such as transportation, finances, and child care. Dr. Welsh pointed out that pregnant women with SUDs usually receive prenatal care and addiction treatment from different providers. “We should be thinking about an integrated treatment model to improve maternal and prenatal care for patients using substances prior to or during pregnancy,” she said. “Some sites have developed combined maternity care units as a way to reduce some of these barriers to treatment. We also need to be thinking about the infants themselves. In order to counter the drug-exposed child’s early disadvantages, service providers must be prepared to intervene early, focusing on things like nutrition, psychomotor assessments, early educational needs assessments, as well as modification of existing treatment services for people with in utero exposures. This can be a difficult population to treat.”
One resource for addressing fetal alcohol spectrum disorders that she recommended is Treatment Improvement Protocol 58, published by Substance Abuse and Mental Health Services Administration. She described one young adult with significant substance use issues who was diagnosed with fetal alcohol syndrome as a child. “This young adult didn’t know about the FAS diagnosis,” Dr. Welsh said. “I expressed to the mother that, ‘this is an ethical dilemma. I’m treating someone and I know this directly impacts my treatment planning. They have a real-world diagnosis that I feel they have a right to know about.’ It made me think: If I think it’s so important that this young adult knows the diagnosis, why are we not screening for it in our general patient populations more often?
“As child psychiatrists we often ask, ‘Were there any in utero exposures?’ When treating adults, I’m asking about family history, but I’m not asking about in utero exposures. This may not be information that they’re privy to, but it’s worth starting a conversation about.”
, a 14-session, evidence-based curriculum that addresses parenting skills, children’s social skills, and family life skills training, are among the treatment options aimed at decreasing the risk of substance use progression in offspring.
‘Adolescents indeed care about their health’
During a separate presentation, Peter Jackson, MD, a psychiatrist at the University of Vermont Medical Center, Burlington, noted that an estimated 3.4 million adolescents in the United States meet criteria for an SUD, but fewer than 10% enter treatment each year. Of those who do enter treatment, 50% relapse within 6 months.
“It’s not the same story for illicit drugs, unfortunately, and that’s largely due to marijuana,” Dr. Jackson said. He noted that adolescent cannabis use is inversely proportional to how dangerous they perceive it to be. “This is evidence that adolescents indeed care about their health,” he said. “They use when they think it’s safe, and they use less when they think it’s unsafe.” Recent Monitoring the Future data also demonstrate that e-cigarette use is outpacing cigarette use among 8th, 10th, and 12th graders, while the past-year misuse of acetaminophen/hydrocodone (Vicodin) among 12th graders has dropped dramatically in the past 5 years. So has misuse of all prescription opioids among 12th graders despite high opioid overdose rates among adults.
Adolescents are more likely to be secretive about their substance use, compared with older adults. “Also, with many substances, especially alcohol, they are more likely to use in a binge pattern,” Dr. Jackson said. “They haven’t accumulated as many negative consequences from their use, so voluntarily seeking treatment is less common than in adults. They’re most often referred through the justice department or legal channels.”
Screening instruments to consider using include the CRAFFT (Car, Relax, Alone, Forget, Friends, Trouble), which has been validated as an adolescent-specific screening instrument. A score of two or more has a strong correlation with meeting criteria for an SUD. The American Academy of Pediatrics recommends the Screening, Brief Intervention, and Referral to Treatment (SBIRT), though this intervention has not been as rigorously evaluated in adolescents, compared with adults.
Dr. Jackson frowned on the notion perpetuated by some parents and caregivers that novelty seeking behavior involving alcohol and other substances is okay if it’s controlled somehow, with statements such as, “I just make sure they’ve given up their car keys and make sure it stays in my own basement. That way nobody leaves here drunk.”
“I think we can do better than that,” Dr. Jackson said.
Well-validated behavioral treatment approaches for adolescent patients include the adolescent community reinforcement approach (A-CRA); cognitive-behavioral therapy (particularly in groups); motivational enhancement therapy (MET); contingency management, and 12-step facilitation.
“Colloquially, in pop culture, we’ve been pressed on this message at times that adolescents don’t care what their parents think; they only care what their peers think,” Dr. Jackson said. “That’s not true from literature we’ve seen, so we need to put the family back in its important place.”
In a meta-analysis of promising behavioral approaches for adolescent SUD, five out of six found to have promising to excellent empirical support were family-based therapies: multidimensional family therapy, functional family therapy, multisystemic family therapy, behavioral strategic family therapy, and family behavior therapy (J Child and Adolesc Psychology. 2008;37[1]:236-59). “That’s a huge take-home point when working with adolescents: Involve the family,” Dr. Jackson said. “As providers, do we have a tendency to side only with the parent or side only with the adolescent? If we do, we should be cautious and thoughtful, because we can very effectively work with both parties. If you have an adolescent who shows up [for counseling] but then walks right out the door, you still have a parent, maybe two parents or other concerned loved ones sitting there. You still have a target for intervention.”
Even if the adolescent never returns to your office, he continued, clinicians can do an intervention with the family to statistically decrease the SUD for the adolescent. “That’s really promising,” he said. In adults, the best evidence for treatment engagement of a loved one following these family-specific interventions is for the Community Reinforcement and Family Training (CRAFT) method (64%-74%), followed by the Johnson Intervention (23%-30%) and Al-Anon/Nar-Anon facilitation (13%-29%). These interventions are being studied more specifically in adolescents and young adults.
A key tip for parents of teens coping with substance abuse is the old adage actions speak louder than words. “You can tell your child not to smoke marijuana until you’re blue in the face, but then if you go out on the back porch and smoke marijuana, that’s a really bad message for behavior change,” Dr. Jackson said. “Words also speak louder than no words. Some parents have never told their adolescent children what their opinion is, and that they’re concerned. Maybe those parents have been convinced that adolescents don’t care what they think. In fact, they do care what they think.”
Once engaged, older adults do well
Among older adults, triggers for SUDs vary considerably from that of their younger counterparts. “We tend to think of this as a population that carries a lot of wisdom and has the coping skills to deal with life,” Olivera J. Bogunovic, MD, a psychiatrist who is medical director of ambulatory services in the division of alcohol and drug abuse at McClean Hospital, Belmont, Mass., said during a separate presentation. “In fact, this is a very vulnerable population at increased risk of mood disorders. For example, many men spend their careers working 60 or 80 hours a week, then they stop working. What is there for them? They sometimes turn to substance abuse. Similarly, older women who lose a loved one may turn to drinking. There’s a lot of room for prevention. The good news is that this group of patients is very responsive to treatment. Once engaged, they do very well.”
Nicotine is the chief addictive substance affecting older adults (17%), followed by alcohol (12% in a binge capacity, dependence 1%), illicit drugs (1.8%), and medications (1.6% for pain medications and 12% on benzodiazepines). Alcohol use disorders encompass a spectrum of problems for this patient population, including at-risk drinking, problem drinking, and dependence. Presentations, complications, and consequences are wide-ranging. “We sometimes miss the symptoms of alcohol use disorders when patients present in emergency rooms,” Dr. Bogunovic said. “Unfortunately, if it’s not treated initially it results in serious medical comorbidities and complications.”
The National Institute on Drug Abuse recommends that adults consume no more than two drinks per day, but the quantity is even lower for elderly. “For men it is no more than one drink per day, while for women it’s questionable if that one drink per day is even recommended,” Dr. Bogunovic said. The prevalence of at-risk drinking among older adults ranges from 3% to 25% and the rates for problem drinking range from 2.2% to 9.6%. Alcohol dependence rates, meanwhile, range from 2% to 3% among men and less than 1% among women.
Cross-sectional data indicate a low prevalence of illicit drug use in older adults, but longitudinal data from the National Survey on Drug Abuse and others suggest an increased use of cannabis and prescription opioids. “A lot of people do not think about opioid use disorders in the elderly,” she said. “The prevalence rate in methadone clinics is less than 2%. However, with the epidemic crisis we’re seeing opioid use disorders in the elderly. What’s important to know is that they also respond to medications for treatment, more so with suboxone than with naltrexone, as we know those patients sometimes have complications that need to be treated with pain medication.”
Elderly patients constitute 13% of the population yet they account for 30% of prescriptions, mainly benzodiazepines and prescription opioids. “Two-thirds of patients who are struggling with SUDs struggled with an SUD at some point in their youth,” said Dr. Bogunovic, who holds a faculty position in the department of psychiatry at Harvard Medical School, Boston. “They continue to use for longer periods of time, with some intermittent periods of sobriety. Of those 15% who do have an alcohol use problem, they are prescribed benzodiazepines. That can be a lethal combination, because there is a combined effect of both alcohol and benzodiazepines that can result in hip fractures, subdural hematomas, and other medical comorbidity.”
Risk factors for development of alcohol use disorder in elderly include physiologic changes in the way alcohol is metabolized; gender, family history, or prior personal history of alcohol use disorder; having a concomitant psychiatric disorder; and having a chronic medical illness, such as severe arthritis. Compared with their male peers, older women generally drink less often and less heavily, but they are more likely to start drinking heavily later in life. Older men face an increased risk of alcohol-related problems tied to cumulative use over the years.
“Social factors also play a role, so it’s important to perform a skilled diagnostic interview and a psychiatric evaluation but also to evaluate the motivational stage of change, because that’s the right moment to do the intervention, knowing what the patient’s values are,” she said. Recommended screenings include the Short Michigan Alcoholism Screening Instrument–Geriatric Version (SMAST-G), the SBIRT, the CAGE-AID, and the Opioid Risk Tool. Compliance with treatment tends to be greater in older adults, compared with their younger counterparts. “They don’t like a confrontational approach, so it’s important to communicate with empathy in a straightforward manner and pay attention to what is important to patients and motivate them,” she noted. “Involve family members or other social support whenever possible.”
Relapses are common, but clinicians should encourage patients to continue treatment, “because they can do very well,” Dr. Bogunovic emphasized. “And we can offer them a good life after that.”
Dr. Welsh disclosed that she has consulted for GW Pharmaceuticals and that she has received training fees from Chestnut Health Systems. Dr. Jackson and Dr. Bogunovic reported having no financial disclosures.
SAN DIEGO – Intervening early can positively affect outcomes for patients struggling with substance use disorders, regardless of their current stage of development and place in the life cycle, speaker after speaker said at the annual meeting and scientific symposium of the American Academy of Addiction Psychiatry.
The prenatal/perinatal period represents a high-risk window for the kindling and precipitation of substance use disorders (SUDs), because of the stress of pregnancy, delivery, recovery, and the newly assumed parenting role. “Offspring who are exposed in utero have an earlier age of initiation of substance use, as well as a greater risk of substance use disorders later on in life,” said Justine Wittenaur Welsh, MD, director of the Emory Adolescent Substance Use Treatment Services, Atlanta. “We as treatment providers need to start to think about how to screen for these individuals. What types of early intervention can we provide, and how do we integrate this into our treatment planning?”
According to data on current illicit drug use among pregnant women from the Substance Abuse and Mental Health Services Administration, the risk is highest among those aged 15-17 years (14.6%), followed by those aged 18-25 years (8.6%), and 26-44 years (3.2%). “We also know that rates of substance use are higher in the first trimester, compared with the second and the third,” said Dr. Welsh, who holds a faculty position in the department of psychiatry and behavioral sciences at Emory University. “But unfortunately, a significant number of pregnant women return to substance use after they deliver. This is really important for treatment providers, to intervene early before a return to substance use. We know that improving the postnatal environment does reduce the risk of substance use, even in individuals who have in utero exposures.”
SUDs confer a host of adverse effects to both the baby and the mother. “With tobacco, although there are a significant number of poisonous chemicals in cigarettes that the baby would be exposed to, we believe it’s the carbon monoxide and the nicotine exposure that confers the most known damage,” Dr. Welsh said. In utero exposure to cigarettes increases the likelihood to initiate smoking during adolescence by twofold (Neurotoxicol Teratol. 2005;27[2]:267-77), and a dose-response relationship has been observed between prenatal smoking and psychiatric hospitalization for substance abuse in offspring (Am J Psychiatry. 2002;159[1]:48-54). In utero exposure to cigarettes also increases the risk for offspring cigarette use. “The exposure to greater than a half-pack of cigarettes per day has a 5.5-fold increased risk for early cigarette experimentation, while exposure to one pack per day or more is associated with twice the risk of developing nicotine dependence,” she said.
As for in utero alcohol exposure, consuming greater than or equal to three drinks is associated with an alcohol disorder at age 21 (odds ratio, 2.95) if the exposure occurred in early pregnancy; Arch Gen Psychiatry. 2006;63[9]:1009-16). In utero alcohol exposure also has been more predictive of adolescent alcohol use than family history. “It’s also been associated with an increased number of abuse/dependency symptoms in offspring, for nicotine, alcohol, and illicit drugs,” Dr. Welsh said. “It has also been associated with an increased risk of cigarette use and substance use disorders in offspring.”
In utero cannabis exposure has been associated with a twofold increased risk of tobacco and marijuana use later in life (Addiction 2006;101[9]:1313-22), as well as with a twofold increased risk of smoking cigarettes daily and of using marijuana during adolescence. In utero cocaine exposure has been associated with a twofold increased risk of using tobacco, a 2.2-fold increased risk of using alcohol, and a 1.8-fold risk of using marijuana, as well as a 2.1-fold increased risk of having an SUD by age 17. “Prenatal exposure and postnatal parent/caregiver cocaine use is uniquely related to teen use of cocaine at age 14,” Dr. Welsh said. “In addition, exposure in the first trimester is associated with earlier marijuana and alcohol initiation.”
Real-world barriers to SUD treatment in adolescents include a lack of coordination between treatment resources, fear of losing custody of children, shame and stigma, concerns about criminal prosecution, domestic violence, and other obstacles such as transportation, finances, and child care. Dr. Welsh pointed out that pregnant women with SUDs usually receive prenatal care and addiction treatment from different providers. “We should be thinking about an integrated treatment model to improve maternal and prenatal care for patients using substances prior to or during pregnancy,” she said. “Some sites have developed combined maternity care units as a way to reduce some of these barriers to treatment. We also need to be thinking about the infants themselves. In order to counter the drug-exposed child’s early disadvantages, service providers must be prepared to intervene early, focusing on things like nutrition, psychomotor assessments, early educational needs assessments, as well as modification of existing treatment services for people with in utero exposures. This can be a difficult population to treat.”
One resource for addressing fetal alcohol spectrum disorders that she recommended is Treatment Improvement Protocol 58, published by Substance Abuse and Mental Health Services Administration. She described one young adult with significant substance use issues who was diagnosed with fetal alcohol syndrome as a child. “This young adult didn’t know about the FAS diagnosis,” Dr. Welsh said. “I expressed to the mother that, ‘this is an ethical dilemma. I’m treating someone and I know this directly impacts my treatment planning. They have a real-world diagnosis that I feel they have a right to know about.’ It made me think: If I think it’s so important that this young adult knows the diagnosis, why are we not screening for it in our general patient populations more often?
“As child psychiatrists we often ask, ‘Were there any in utero exposures?’ When treating adults, I’m asking about family history, but I’m not asking about in utero exposures. This may not be information that they’re privy to, but it’s worth starting a conversation about.”
, a 14-session, evidence-based curriculum that addresses parenting skills, children’s social skills, and family life skills training, are among the treatment options aimed at decreasing the risk of substance use progression in offspring.
‘Adolescents indeed care about their health’
During a separate presentation, Peter Jackson, MD, a psychiatrist at the University of Vermont Medical Center, Burlington, noted that an estimated 3.4 million adolescents in the United States meet criteria for an SUD, but fewer than 10% enter treatment each year. Of those who do enter treatment, 50% relapse within 6 months.
“It’s not the same story for illicit drugs, unfortunately, and that’s largely due to marijuana,” Dr. Jackson said. He noted that adolescent cannabis use is inversely proportional to how dangerous they perceive it to be. “This is evidence that adolescents indeed care about their health,” he said. “They use when they think it’s safe, and they use less when they think it’s unsafe.” Recent Monitoring the Future data also demonstrate that e-cigarette use is outpacing cigarette use among 8th, 10th, and 12th graders, while the past-year misuse of acetaminophen/hydrocodone (Vicodin) among 12th graders has dropped dramatically in the past 5 years. So has misuse of all prescription opioids among 12th graders despite high opioid overdose rates among adults.
Adolescents are more likely to be secretive about their substance use, compared with older adults. “Also, with many substances, especially alcohol, they are more likely to use in a binge pattern,” Dr. Jackson said. “They haven’t accumulated as many negative consequences from their use, so voluntarily seeking treatment is less common than in adults. They’re most often referred through the justice department or legal channels.”
Screening instruments to consider using include the CRAFFT (Car, Relax, Alone, Forget, Friends, Trouble), which has been validated as an adolescent-specific screening instrument. A score of two or more has a strong correlation with meeting criteria for an SUD. The American Academy of Pediatrics recommends the Screening, Brief Intervention, and Referral to Treatment (SBIRT), though this intervention has not been as rigorously evaluated in adolescents, compared with adults.
Dr. Jackson frowned on the notion perpetuated by some parents and caregivers that novelty seeking behavior involving alcohol and other substances is okay if it’s controlled somehow, with statements such as, “I just make sure they’ve given up their car keys and make sure it stays in my own basement. That way nobody leaves here drunk.”
“I think we can do better than that,” Dr. Jackson said.
Well-validated behavioral treatment approaches for adolescent patients include the adolescent community reinforcement approach (A-CRA); cognitive-behavioral therapy (particularly in groups); motivational enhancement therapy (MET); contingency management, and 12-step facilitation.
“Colloquially, in pop culture, we’ve been pressed on this message at times that adolescents don’t care what their parents think; they only care what their peers think,” Dr. Jackson said. “That’s not true from literature we’ve seen, so we need to put the family back in its important place.”
In a meta-analysis of promising behavioral approaches for adolescent SUD, five out of six found to have promising to excellent empirical support were family-based therapies: multidimensional family therapy, functional family therapy, multisystemic family therapy, behavioral strategic family therapy, and family behavior therapy (J Child and Adolesc Psychology. 2008;37[1]:236-59). “That’s a huge take-home point when working with adolescents: Involve the family,” Dr. Jackson said. “As providers, do we have a tendency to side only with the parent or side only with the adolescent? If we do, we should be cautious and thoughtful, because we can very effectively work with both parties. If you have an adolescent who shows up [for counseling] but then walks right out the door, you still have a parent, maybe two parents or other concerned loved ones sitting there. You still have a target for intervention.”
Even if the adolescent never returns to your office, he continued, clinicians can do an intervention with the family to statistically decrease the SUD for the adolescent. “That’s really promising,” he said. In adults, the best evidence for treatment engagement of a loved one following these family-specific interventions is for the Community Reinforcement and Family Training (CRAFT) method (64%-74%), followed by the Johnson Intervention (23%-30%) and Al-Anon/Nar-Anon facilitation (13%-29%). These interventions are being studied more specifically in adolescents and young adults.
A key tip for parents of teens coping with substance abuse is the old adage actions speak louder than words. “You can tell your child not to smoke marijuana until you’re blue in the face, but then if you go out on the back porch and smoke marijuana, that’s a really bad message for behavior change,” Dr. Jackson said. “Words also speak louder than no words. Some parents have never told their adolescent children what their opinion is, and that they’re concerned. Maybe those parents have been convinced that adolescents don’t care what they think. In fact, they do care what they think.”
Once engaged, older adults do well
Among older adults, triggers for SUDs vary considerably from that of their younger counterparts. “We tend to think of this as a population that carries a lot of wisdom and has the coping skills to deal with life,” Olivera J. Bogunovic, MD, a psychiatrist who is medical director of ambulatory services in the division of alcohol and drug abuse at McClean Hospital, Belmont, Mass., said during a separate presentation. “In fact, this is a very vulnerable population at increased risk of mood disorders. For example, many men spend their careers working 60 or 80 hours a week, then they stop working. What is there for them? They sometimes turn to substance abuse. Similarly, older women who lose a loved one may turn to drinking. There’s a lot of room for prevention. The good news is that this group of patients is very responsive to treatment. Once engaged, they do very well.”
Nicotine is the chief addictive substance affecting older adults (17%), followed by alcohol (12% in a binge capacity, dependence 1%), illicit drugs (1.8%), and medications (1.6% for pain medications and 12% on benzodiazepines). Alcohol use disorders encompass a spectrum of problems for this patient population, including at-risk drinking, problem drinking, and dependence. Presentations, complications, and consequences are wide-ranging. “We sometimes miss the symptoms of alcohol use disorders when patients present in emergency rooms,” Dr. Bogunovic said. “Unfortunately, if it’s not treated initially it results in serious medical comorbidities and complications.”
The National Institute on Drug Abuse recommends that adults consume no more than two drinks per day, but the quantity is even lower for elderly. “For men it is no more than one drink per day, while for women it’s questionable if that one drink per day is even recommended,” Dr. Bogunovic said. The prevalence of at-risk drinking among older adults ranges from 3% to 25% and the rates for problem drinking range from 2.2% to 9.6%. Alcohol dependence rates, meanwhile, range from 2% to 3% among men and less than 1% among women.
Cross-sectional data indicate a low prevalence of illicit drug use in older adults, but longitudinal data from the National Survey on Drug Abuse and others suggest an increased use of cannabis and prescription opioids. “A lot of people do not think about opioid use disorders in the elderly,” she said. “The prevalence rate in methadone clinics is less than 2%. However, with the epidemic crisis we’re seeing opioid use disorders in the elderly. What’s important to know is that they also respond to medications for treatment, more so with suboxone than with naltrexone, as we know those patients sometimes have complications that need to be treated with pain medication.”
Elderly patients constitute 13% of the population yet they account for 30% of prescriptions, mainly benzodiazepines and prescription opioids. “Two-thirds of patients who are struggling with SUDs struggled with an SUD at some point in their youth,” said Dr. Bogunovic, who holds a faculty position in the department of psychiatry at Harvard Medical School, Boston. “They continue to use for longer periods of time, with some intermittent periods of sobriety. Of those 15% who do have an alcohol use problem, they are prescribed benzodiazepines. That can be a lethal combination, because there is a combined effect of both alcohol and benzodiazepines that can result in hip fractures, subdural hematomas, and other medical comorbidity.”
Risk factors for development of alcohol use disorder in elderly include physiologic changes in the way alcohol is metabolized; gender, family history, or prior personal history of alcohol use disorder; having a concomitant psychiatric disorder; and having a chronic medical illness, such as severe arthritis. Compared with their male peers, older women generally drink less often and less heavily, but they are more likely to start drinking heavily later in life. Older men face an increased risk of alcohol-related problems tied to cumulative use over the years.
“Social factors also play a role, so it’s important to perform a skilled diagnostic interview and a psychiatric evaluation but also to evaluate the motivational stage of change, because that’s the right moment to do the intervention, knowing what the patient’s values are,” she said. Recommended screenings include the Short Michigan Alcoholism Screening Instrument–Geriatric Version (SMAST-G), the SBIRT, the CAGE-AID, and the Opioid Risk Tool. Compliance with treatment tends to be greater in older adults, compared with their younger counterparts. “They don’t like a confrontational approach, so it’s important to communicate with empathy in a straightforward manner and pay attention to what is important to patients and motivate them,” she noted. “Involve family members or other social support whenever possible.”
Relapses are common, but clinicians should encourage patients to continue treatment, “because they can do very well,” Dr. Bogunovic emphasized. “And we can offer them a good life after that.”
Dr. Welsh disclosed that she has consulted for GW Pharmaceuticals and that she has received training fees from Chestnut Health Systems. Dr. Jackson and Dr. Bogunovic reported having no financial disclosures.
EXPERT ANALYSIS FROM AAAP
High rate of arm morbidity in young breast cancer survivors
SAN ANTONIO – as compared with having a sentinel lymph node biopsy (SLNB), according to new findings.
In a large prospective cohort study that included 1,302 breast cancer patients aged 40 or younger, the incidence of arm swelling 1 year after diagnosis among women who underwent breast-conserving surgery was 6% for the SLNB group versus 24% for those who had ALND. Among patients who had a mastectomy, the rates were similar; 6% versus 23% for SLNB or ALND, respectively.
“Young breast cancer survivors report high rates of arm morbidity in the first year of follow-up,” said lead author Anne Kuijer, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston. “Axillary node dissection, increased BMI [body mass index] and socioeconomic status were all independently associated with an increased risk of arm swelling,” she said at the San Antonio Breast Cancer Symposium.
She noted that patients who received mastectomy with radiation therapy were twice as likely to have decreased range of motion at 1 year, compared with patients treated with breast-conserving treatment.
In this study, the authors evaluated the incidence of arm morbidity associated with both ALND and SLNB in patients who were enrolled in the Young Women’s Breast Cancer Study. This multicenter prospective cohort study was designed to explore biological, medical, and psychosocial issues experienced by young breast cancer patients.
Within this large cohort, 55% had undergone an SLNB only, and 41% an ALND. The remaining patients did not undergo either procedure.
The primary endpoint of this study was to examine the incidence of patient-reported arm swelling or decreased range of motion at 1 year after their breast cancer diagnosis. Patients used the Cancer Rehabilitation Evaluation System (CARES-SF) to measure their symptoms.
Overall, at 1 year, 13% of the cohort reported arm swelling, and 40% reported decreased range of motion in the ipsilateral arm.
Several factors were associated with a higher risk of arm morbidity. Patients with a BMI of greater than 25 were more likely to report arm swelling vs. those with lower BMI (odds ratio, 1.7; P = .03) as well as have less range of motion (OR, 1.5; P = .05). Women who reported feeling financially comfortable were 40% less likely to report swelling (P = .02) and 90% less likely to report decreased range of motion (P = .67).
In addition, those who underwent ALND were 3.4 times more likely to report swelling, compared with women who had SLNB, but it was not associated with a reduction in range of motion.
One limitation of the study is that the cohort included patients who had received treatment at large cancer centers in the Northeast, suggesting that they may have been of higher socioeconomic status and may have led more active lifestyles, compared with the general population. Another limitation is that arm morbidity was self-reported and not objectively measured.
“I think our findings highlight opportunities for preoperative counseling, early referral of patients to physical therapy, and identification of resources for support of those at increased risk,” said Dr. Kuijer.
SOURCE: Kuijer et al. SABCS Abstract GS5-03
SAN ANTONIO – as compared with having a sentinel lymph node biopsy (SLNB), according to new findings.
In a large prospective cohort study that included 1,302 breast cancer patients aged 40 or younger, the incidence of arm swelling 1 year after diagnosis among women who underwent breast-conserving surgery was 6% for the SLNB group versus 24% for those who had ALND. Among patients who had a mastectomy, the rates were similar; 6% versus 23% for SLNB or ALND, respectively.
“Young breast cancer survivors report high rates of arm morbidity in the first year of follow-up,” said lead author Anne Kuijer, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston. “Axillary node dissection, increased BMI [body mass index] and socioeconomic status were all independently associated with an increased risk of arm swelling,” she said at the San Antonio Breast Cancer Symposium.
She noted that patients who received mastectomy with radiation therapy were twice as likely to have decreased range of motion at 1 year, compared with patients treated with breast-conserving treatment.
In this study, the authors evaluated the incidence of arm morbidity associated with both ALND and SLNB in patients who were enrolled in the Young Women’s Breast Cancer Study. This multicenter prospective cohort study was designed to explore biological, medical, and psychosocial issues experienced by young breast cancer patients.
Within this large cohort, 55% had undergone an SLNB only, and 41% an ALND. The remaining patients did not undergo either procedure.
The primary endpoint of this study was to examine the incidence of patient-reported arm swelling or decreased range of motion at 1 year after their breast cancer diagnosis. Patients used the Cancer Rehabilitation Evaluation System (CARES-SF) to measure their symptoms.
Overall, at 1 year, 13% of the cohort reported arm swelling, and 40% reported decreased range of motion in the ipsilateral arm.
Several factors were associated with a higher risk of arm morbidity. Patients with a BMI of greater than 25 were more likely to report arm swelling vs. those with lower BMI (odds ratio, 1.7; P = .03) as well as have less range of motion (OR, 1.5; P = .05). Women who reported feeling financially comfortable were 40% less likely to report swelling (P = .02) and 90% less likely to report decreased range of motion (P = .67).
In addition, those who underwent ALND were 3.4 times more likely to report swelling, compared with women who had SLNB, but it was not associated with a reduction in range of motion.
One limitation of the study is that the cohort included patients who had received treatment at large cancer centers in the Northeast, suggesting that they may have been of higher socioeconomic status and may have led more active lifestyles, compared with the general population. Another limitation is that arm morbidity was self-reported and not objectively measured.
“I think our findings highlight opportunities for preoperative counseling, early referral of patients to physical therapy, and identification of resources for support of those at increased risk,” said Dr. Kuijer.
SOURCE: Kuijer et al. SABCS Abstract GS5-03
SAN ANTONIO – as compared with having a sentinel lymph node biopsy (SLNB), according to new findings.
In a large prospective cohort study that included 1,302 breast cancer patients aged 40 or younger, the incidence of arm swelling 1 year after diagnosis among women who underwent breast-conserving surgery was 6% for the SLNB group versus 24% for those who had ALND. Among patients who had a mastectomy, the rates were similar; 6% versus 23% for SLNB or ALND, respectively.
“Young breast cancer survivors report high rates of arm morbidity in the first year of follow-up,” said lead author Anne Kuijer, MD, of Dana-Farber Cancer Institute/Brigham and Women’s Hospital in Boston. “Axillary node dissection, increased BMI [body mass index] and socioeconomic status were all independently associated with an increased risk of arm swelling,” she said at the San Antonio Breast Cancer Symposium.
She noted that patients who received mastectomy with radiation therapy were twice as likely to have decreased range of motion at 1 year, compared with patients treated with breast-conserving treatment.
In this study, the authors evaluated the incidence of arm morbidity associated with both ALND and SLNB in patients who were enrolled in the Young Women’s Breast Cancer Study. This multicenter prospective cohort study was designed to explore biological, medical, and psychosocial issues experienced by young breast cancer patients.
Within this large cohort, 55% had undergone an SLNB only, and 41% an ALND. The remaining patients did not undergo either procedure.
The primary endpoint of this study was to examine the incidence of patient-reported arm swelling or decreased range of motion at 1 year after their breast cancer diagnosis. Patients used the Cancer Rehabilitation Evaluation System (CARES-SF) to measure their symptoms.
Overall, at 1 year, 13% of the cohort reported arm swelling, and 40% reported decreased range of motion in the ipsilateral arm.
Several factors were associated with a higher risk of arm morbidity. Patients with a BMI of greater than 25 were more likely to report arm swelling vs. those with lower BMI (odds ratio, 1.7; P = .03) as well as have less range of motion (OR, 1.5; P = .05). Women who reported feeling financially comfortable were 40% less likely to report swelling (P = .02) and 90% less likely to report decreased range of motion (P = .67).
In addition, those who underwent ALND were 3.4 times more likely to report swelling, compared with women who had SLNB, but it was not associated with a reduction in range of motion.
One limitation of the study is that the cohort included patients who had received treatment at large cancer centers in the Northeast, suggesting that they may have been of higher socioeconomic status and may have led more active lifestyles, compared with the general population. Another limitation is that arm morbidity was self-reported and not objectively measured.
“I think our findings highlight opportunities for preoperative counseling, early referral of patients to physical therapy, and identification of resources for support of those at increased risk,” said Dr. Kuijer.
SOURCE: Kuijer et al. SABCS Abstract GS5-03
REPORTING FROM SABCS 2017
Key clinical point: A significant rate of arm swelling and decreased range of motion was seen in young breast cancer patients 1 year after undergoing surgery.
Major finding: At 1 year, 13% of a large cohort of breast cancer patients aged 40 years or younger reported arm swelling, and 40% reported decreased range of motion in the ipsilateral arm.
Data source: Large prospective cohort study that included 1,302 breast cancer patients aged 40 or younger.
Disclosures:. This study was funded by the National Institutes of Health, the Susan G. Komen Foundation, The Pink Agenda, and the Breast Cancer Research Foundation. Dr. Kuijer and her colleagues declare no conflicts of interest.
Source: Kuijer et al. SABCS 2017 Abstract GS5-03.
Rituximab may be best choice for splenic MZL
For patients with splenic marginal zone lymphoma (MZL) requiring treatment, rituximab may be a superior choice, compared with splenectomy, according to authors of a recent review article.
Although treatment with splenectomy and rituximab both are associated with high rates of 10-year survival, splenectomy also is associated with acute surgical complications and late toxicities, mainly from infections, Christina Kalpadakis, MD, of the department of hematology at Heraklion University Hospital, University of Crete, Greece, reported in Best Practice & Research Clinical Haematology.
The review article aims to answer the question of whether rituximab monotherapy should replace splenectomy as the treatment of choice, according to the authors, who said treatment for this rare, low-grade B-cell lymphoma has not been standardized due to a lack of large, randomized trials.
Asymptomatic splenic MZL without significant cytopenias can be managed with a watch-and-wait approach, but when treatment is warranted, the choice is often between splenectomy, rituximab monotherapy, or chemoimmunotherapy.
“Based on the existing retrospective series of patients, rituximab monotherapy appears to be the best choice since it combines high efficacy with the lowest toxicity,” Dr. Kalpadakis and her colleagues wrote.
Until the early 2000s, splenectomy was the standard treatment modality for splenic MZL, offering quick amelioration of symptoms related to splenomegaly in more than 90% of patients, as well as improvements in hypersplenism-related cytopenias, the researchers reported. Among 100 patients with splenic MZL, the median progression-free survival with splenectomy was 8.25 years, and median 10-year overall survival was 67%.
However, responses to splenectomy are not complete because some patients are not good candidates for splenectomy, including those with lymphadenopathy or heavy bone marrow infiltration, according to the authors. Moreover, since it is a major surgical procedure that can be associated with complications and infections, splenectomy is not appropriate for elderly patients or patients with comorbidities with a high surgical risk, they wrote.
“Furthermore, splenectomy cannot eradicate bone marrow disease and has no impact on other extrasplenic disease localization such as lymphadenopathy,” they added.
Rituximab monotherapy, by contrast, has “minimal toxicity” with high efficacy, including complete response rates of around 50% and a 10-year overall survival of 85% reported in a series of 104 patients.
Maintenance with rituximab may further improve the quality of responses, although data are limited. One study, conducted by the review article authors, showed that rituximab maintenance increased the complete response rate from 42% after induction treatment to 71% after maintenance, along with a significant improvement in response duration, though no differences in overall survival have been observed thus far.
Rituximab plus chemotherapy has been evaluated, but with “significant toxicity without improvement in the outcome” versus rituximab monotherapy, the researchers wrote. Splenectomy is “effective” but should be reserved for patients refractory to rituximab, Dr. Kalpadakis and her colleagues wrote.
The researchers reported having no relevant financial disclosures.
SOURCE: Kalpadakis C et al. Best Pract Res Clin Haematol. Mar-Jun 2017. doi:10.1016/j.beha.2017.10.011.
For patients with splenic marginal zone lymphoma (MZL) requiring treatment, rituximab may be a superior choice, compared with splenectomy, according to authors of a recent review article.
Although treatment with splenectomy and rituximab both are associated with high rates of 10-year survival, splenectomy also is associated with acute surgical complications and late toxicities, mainly from infections, Christina Kalpadakis, MD, of the department of hematology at Heraklion University Hospital, University of Crete, Greece, reported in Best Practice & Research Clinical Haematology.
The review article aims to answer the question of whether rituximab monotherapy should replace splenectomy as the treatment of choice, according to the authors, who said treatment for this rare, low-grade B-cell lymphoma has not been standardized due to a lack of large, randomized trials.
Asymptomatic splenic MZL without significant cytopenias can be managed with a watch-and-wait approach, but when treatment is warranted, the choice is often between splenectomy, rituximab monotherapy, or chemoimmunotherapy.
“Based on the existing retrospective series of patients, rituximab monotherapy appears to be the best choice since it combines high efficacy with the lowest toxicity,” Dr. Kalpadakis and her colleagues wrote.
Until the early 2000s, splenectomy was the standard treatment modality for splenic MZL, offering quick amelioration of symptoms related to splenomegaly in more than 90% of patients, as well as improvements in hypersplenism-related cytopenias, the researchers reported. Among 100 patients with splenic MZL, the median progression-free survival with splenectomy was 8.25 years, and median 10-year overall survival was 67%.
However, responses to splenectomy are not complete because some patients are not good candidates for splenectomy, including those with lymphadenopathy or heavy bone marrow infiltration, according to the authors. Moreover, since it is a major surgical procedure that can be associated with complications and infections, splenectomy is not appropriate for elderly patients or patients with comorbidities with a high surgical risk, they wrote.
“Furthermore, splenectomy cannot eradicate bone marrow disease and has no impact on other extrasplenic disease localization such as lymphadenopathy,” they added.
Rituximab monotherapy, by contrast, has “minimal toxicity” with high efficacy, including complete response rates of around 50% and a 10-year overall survival of 85% reported in a series of 104 patients.
Maintenance with rituximab may further improve the quality of responses, although data are limited. One study, conducted by the review article authors, showed that rituximab maintenance increased the complete response rate from 42% after induction treatment to 71% after maintenance, along with a significant improvement in response duration, though no differences in overall survival have been observed thus far.
Rituximab plus chemotherapy has been evaluated, but with “significant toxicity without improvement in the outcome” versus rituximab monotherapy, the researchers wrote. Splenectomy is “effective” but should be reserved for patients refractory to rituximab, Dr. Kalpadakis and her colleagues wrote.
The researchers reported having no relevant financial disclosures.
SOURCE: Kalpadakis C et al. Best Pract Res Clin Haematol. Mar-Jun 2017. doi:10.1016/j.beha.2017.10.011.
For patients with splenic marginal zone lymphoma (MZL) requiring treatment, rituximab may be a superior choice, compared with splenectomy, according to authors of a recent review article.
Although treatment with splenectomy and rituximab both are associated with high rates of 10-year survival, splenectomy also is associated with acute surgical complications and late toxicities, mainly from infections, Christina Kalpadakis, MD, of the department of hematology at Heraklion University Hospital, University of Crete, Greece, reported in Best Practice & Research Clinical Haematology.
The review article aims to answer the question of whether rituximab monotherapy should replace splenectomy as the treatment of choice, according to the authors, who said treatment for this rare, low-grade B-cell lymphoma has not been standardized due to a lack of large, randomized trials.
Asymptomatic splenic MZL without significant cytopenias can be managed with a watch-and-wait approach, but when treatment is warranted, the choice is often between splenectomy, rituximab monotherapy, or chemoimmunotherapy.
“Based on the existing retrospective series of patients, rituximab monotherapy appears to be the best choice since it combines high efficacy with the lowest toxicity,” Dr. Kalpadakis and her colleagues wrote.
Until the early 2000s, splenectomy was the standard treatment modality for splenic MZL, offering quick amelioration of symptoms related to splenomegaly in more than 90% of patients, as well as improvements in hypersplenism-related cytopenias, the researchers reported. Among 100 patients with splenic MZL, the median progression-free survival with splenectomy was 8.25 years, and median 10-year overall survival was 67%.
However, responses to splenectomy are not complete because some patients are not good candidates for splenectomy, including those with lymphadenopathy or heavy bone marrow infiltration, according to the authors. Moreover, since it is a major surgical procedure that can be associated with complications and infections, splenectomy is not appropriate for elderly patients or patients with comorbidities with a high surgical risk, they wrote.
“Furthermore, splenectomy cannot eradicate bone marrow disease and has no impact on other extrasplenic disease localization such as lymphadenopathy,” they added.
Rituximab monotherapy, by contrast, has “minimal toxicity” with high efficacy, including complete response rates of around 50% and a 10-year overall survival of 85% reported in a series of 104 patients.
Maintenance with rituximab may further improve the quality of responses, although data are limited. One study, conducted by the review article authors, showed that rituximab maintenance increased the complete response rate from 42% after induction treatment to 71% after maintenance, along with a significant improvement in response duration, though no differences in overall survival have been observed thus far.
Rituximab plus chemotherapy has been evaluated, but with “significant toxicity without improvement in the outcome” versus rituximab monotherapy, the researchers wrote. Splenectomy is “effective” but should be reserved for patients refractory to rituximab, Dr. Kalpadakis and her colleagues wrote.
The researchers reported having no relevant financial disclosures.
SOURCE: Kalpadakis C et al. Best Pract Res Clin Haematol. Mar-Jun 2017. doi:10.1016/j.beha.2017.10.011.
FROM BEST PRACTICE & RESEARCH CLINICAL HAEMATOLOGY
Key clinical point:
Major finding: Both splenectomy and rituximab are associated with high rates of 10-year overalls survival, but splenectomy has higher rates of surgical complications and infection.
Study details: Review article of 63 publications, mostly retrospective studies of marginal zone lymphoma.
Disclosures: The researchers reported having no relevant financial disclosures.
Source: Kalpadakis C et al. Best Pract Res Clin Haematol. 2017 Mar-Jun. doi:10.1016/j.beha.2017.10.011.
Tatiana Falcone, MD
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel