User login
Fentanyl: A Major Culprit in Opioid Overdoses
Researchers examined 5,152 people who died due to opioid overdose in Maine, Massachusetts, Missouri, New Hampshire, New Mexico, Ohio, Oklahoma, Rhode Island, West Virginia, and Wisconsin and found that nearly 3,000 were fentanyl positive. In addition, > 700 tested positive for drugs with similar chemical structures to fentanyl, including an extremely potent analog, carfentanil, which is used to sedate large animals.
The findings are from the first report on data from the State Unintentional Drug Overdose Reporting System (SUDORS). According to the CDC, SUDORS makes it possible to use toxicology and death scene investigation data previously unavailable across states to provide insights into specific substances and circumstances driving overdoses. That information can help pinpoint changes in the opioid epidemic and inform interventions.
Starting in late 2017, the CDC’s Enhanced State Opioid Overdose Surveillance program is funding expanded forensic toxicology testing of opioid overdose deaths to detect fentanyl analogs and other illicitly manufactured synthetic opioid drugs.
Researchers examined 5,152 people who died due to opioid overdose in Maine, Massachusetts, Missouri, New Hampshire, New Mexico, Ohio, Oklahoma, Rhode Island, West Virginia, and Wisconsin and found that nearly 3,000 were fentanyl positive. In addition, > 700 tested positive for drugs with similar chemical structures to fentanyl, including an extremely potent analog, carfentanil, which is used to sedate large animals.
The findings are from the first report on data from the State Unintentional Drug Overdose Reporting System (SUDORS). According to the CDC, SUDORS makes it possible to use toxicology and death scene investigation data previously unavailable across states to provide insights into specific substances and circumstances driving overdoses. That information can help pinpoint changes in the opioid epidemic and inform interventions.
Starting in late 2017, the CDC’s Enhanced State Opioid Overdose Surveillance program is funding expanded forensic toxicology testing of opioid overdose deaths to detect fentanyl analogs and other illicitly manufactured synthetic opioid drugs.
Researchers examined 5,152 people who died due to opioid overdose in Maine, Massachusetts, Missouri, New Hampshire, New Mexico, Ohio, Oklahoma, Rhode Island, West Virginia, and Wisconsin and found that nearly 3,000 were fentanyl positive. In addition, > 700 tested positive for drugs with similar chemical structures to fentanyl, including an extremely potent analog, carfentanil, which is used to sedate large animals.
The findings are from the first report on data from the State Unintentional Drug Overdose Reporting System (SUDORS). According to the CDC, SUDORS makes it possible to use toxicology and death scene investigation data previously unavailable across states to provide insights into specific substances and circumstances driving overdoses. That information can help pinpoint changes in the opioid epidemic and inform interventions.
Starting in late 2017, the CDC’s Enhanced State Opioid Overdose Surveillance program is funding expanded forensic toxicology testing of opioid overdose deaths to detect fentanyl analogs and other illicitly manufactured synthetic opioid drugs.
Maternal Weight Impacts Childhood Asthma
Extreme weight gain during pregnancy may contribute to risk factors for early childhood asthma, say researchers from University of South Carolina. They found obesity nearly doubled a woman’s chances of having a child who developed asthma by age 4. Extreme-low weight gain (<5 kg) and extreme-high weight gain (≥25 kg) were both associated with increased risk of asthma.
At 9 months, 2 years, and 4 years, 5%, 8%, and 12% of children, respectively, were diagnosed with asthma. Overall, 15% of children were diagnosed with asthma by age 4. Every 1.0 unit increase in maternal body mass index was associated with increased odds of asthma in children.
Childhood asthma already is believed to have inutero origins, the researchers say. They cite a study that found 40% of children with a diagnosis of asthma by age 7 had reduced airflow and bronchial responsiveness as neonates. Research suggests that maternal weight and gestational weight gain may change the intrauterine environment and affect the development of asthma.
The researchers say, to their knowledge, no studies have examined the association between gestational weight gain and asthma in offspring in a nationally representative sample of children in the U.S. Moreover, previous studies did not account for gestational age, which can affect the amount of weight gained.
Although the researchers note that no single risk factor can entirely account for childhood asthma, maternal obesity is one that is modifiable.
Extreme weight gain during pregnancy may contribute to risk factors for early childhood asthma, say researchers from University of South Carolina. They found obesity nearly doubled a woman’s chances of having a child who developed asthma by age 4. Extreme-low weight gain (<5 kg) and extreme-high weight gain (≥25 kg) were both associated with increased risk of asthma.
At 9 months, 2 years, and 4 years, 5%, 8%, and 12% of children, respectively, were diagnosed with asthma. Overall, 15% of children were diagnosed with asthma by age 4. Every 1.0 unit increase in maternal body mass index was associated with increased odds of asthma in children.
Childhood asthma already is believed to have inutero origins, the researchers say. They cite a study that found 40% of children with a diagnosis of asthma by age 7 had reduced airflow and bronchial responsiveness as neonates. Research suggests that maternal weight and gestational weight gain may change the intrauterine environment and affect the development of asthma.
The researchers say, to their knowledge, no studies have examined the association between gestational weight gain and asthma in offspring in a nationally representative sample of children in the U.S. Moreover, previous studies did not account for gestational age, which can affect the amount of weight gained.
Although the researchers note that no single risk factor can entirely account for childhood asthma, maternal obesity is one that is modifiable.
Extreme weight gain during pregnancy may contribute to risk factors for early childhood asthma, say researchers from University of South Carolina. They found obesity nearly doubled a woman’s chances of having a child who developed asthma by age 4. Extreme-low weight gain (<5 kg) and extreme-high weight gain (≥25 kg) were both associated with increased risk of asthma.
At 9 months, 2 years, and 4 years, 5%, 8%, and 12% of children, respectively, were diagnosed with asthma. Overall, 15% of children were diagnosed with asthma by age 4. Every 1.0 unit increase in maternal body mass index was associated with increased odds of asthma in children.
Childhood asthma already is believed to have inutero origins, the researchers say. They cite a study that found 40% of children with a diagnosis of asthma by age 7 had reduced airflow and bronchial responsiveness as neonates. Research suggests that maternal weight and gestational weight gain may change the intrauterine environment and affect the development of asthma.
The researchers say, to their knowledge, no studies have examined the association between gestational weight gain and asthma in offspring in a nationally representative sample of children in the U.S. Moreover, previous studies did not account for gestational age, which can affect the amount of weight gained.
Although the researchers note that no single risk factor can entirely account for childhood asthma, maternal obesity is one that is modifiable.
Woman Gets Heated ... Literally
Recently, a 57-year-old woman was informed by her husband that she has a discolored area of skin on her back. This discovery followed a prolonged period of back pain, for which she was prescribed an NSAID. When this yielded no relief, she started sleeping with an electric heating pad, held in place by an elastic bandage, to ease the pain.
She reports mild itching in the affected area. She claims to be in good health otherwise and is not taking any medication.
EXAMINATION
The patient is in no acute distress but has obvious difficulty walking without pain. Covering her back, from the bra strap down, is modest erythema, a reticular pattern of modest hyperpigmentation, and focal areas of mild scaling.
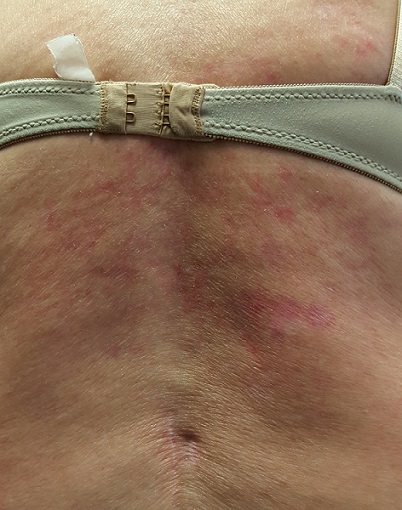
No tenderness or increased warmth is detected on palpation. No notable changes are seen elsewhere on her skin.
What is the diagnosis?
DISCUSSION
This condition, termed erythema ab igne (EAI), was first described in 18th century women who worked while seated in front of a fire all day—hence the name, which translates to “redness from fire.” Over time, this exposure produced permanent changes on the anterior portions of their legs—similar to those seen in this patient.
Today, the condition can develop in a variety of contexts, all of which involve prolonged, repeated exposure to infrared radiation (eg, electric blankets, hot water bottles, electric space heaters, laptop computers). EAI can also affect the faces and arms of bakers, chefs, welders, and silversmiths working in close proximity to radiation.
This type of heat increases vasodilation in the superficial venous plexus (located in the papillary dermis), which causes hemosiderin to leak into the superficial dermis over time. This permanently stains the skin with a characteristic reticular pattern that mimics the affected vascular pattern. Generally, the darker the patient’s skin, the darker the pattern appears.
EAI more commonly affects women than men, and its diagnosis should prompt further investigation for underlying conditions. Musculoskeletal problems, chronic infection, anemia, or hypothyroidism could be involved.
Treatment, besides avoidance of radiation, includes topical application of retinoids or use of lasers.
In this case, the changes were discovered early enough to be at least partially reversible. In more advanced cases, focal areas of scaling can coalesce into papules or nodules that can undergo malignant transformation to squamous cell carcinoma.
TAKE-HOME LEARNING POINTS
- Erythema ab igne (EAI) is a fairly common condition caused by close proximity to infrared radiation (eg, heating pads, laptop computers, electric space heaters).
- This radiation causes chronic vasodilatation of the superficial venous plexus, which leads to leakage of hemosiderin pigment, permanently staining the skin in a reticular pattern.
- EAI can affect the faces and arms of bakers, welders, chefs, and silversmiths.
- Treatment can be attempted with topical application of retinoids or with use of lasers.
Recently, a 57-year-old woman was informed by her husband that she has a discolored area of skin on her back. This discovery followed a prolonged period of back pain, for which she was prescribed an NSAID. When this yielded no relief, she started sleeping with an electric heating pad, held in place by an elastic bandage, to ease the pain.
She reports mild itching in the affected area. She claims to be in good health otherwise and is not taking any medication.
EXAMINATION
The patient is in no acute distress but has obvious difficulty walking without pain. Covering her back, from the bra strap down, is modest erythema, a reticular pattern of modest hyperpigmentation, and focal areas of mild scaling.

No tenderness or increased warmth is detected on palpation. No notable changes are seen elsewhere on her skin.
What is the diagnosis?
DISCUSSION
This condition, termed erythema ab igne (EAI), was first described in 18th century women who worked while seated in front of a fire all day—hence the name, which translates to “redness from fire.” Over time, this exposure produced permanent changes on the anterior portions of their legs—similar to those seen in this patient.
Today, the condition can develop in a variety of contexts, all of which involve prolonged, repeated exposure to infrared radiation (eg, electric blankets, hot water bottles, electric space heaters, laptop computers). EAI can also affect the faces and arms of bakers, chefs, welders, and silversmiths working in close proximity to radiation.
This type of heat increases vasodilation in the superficial venous plexus (located in the papillary dermis), which causes hemosiderin to leak into the superficial dermis over time. This permanently stains the skin with a characteristic reticular pattern that mimics the affected vascular pattern. Generally, the darker the patient’s skin, the darker the pattern appears.
EAI more commonly affects women than men, and its diagnosis should prompt further investigation for underlying conditions. Musculoskeletal problems, chronic infection, anemia, or hypothyroidism could be involved.
Treatment, besides avoidance of radiation, includes topical application of retinoids or use of lasers.
In this case, the changes were discovered early enough to be at least partially reversible. In more advanced cases, focal areas of scaling can coalesce into papules or nodules that can undergo malignant transformation to squamous cell carcinoma.
TAKE-HOME LEARNING POINTS
- Erythema ab igne (EAI) is a fairly common condition caused by close proximity to infrared radiation (eg, heating pads, laptop computers, electric space heaters).
- This radiation causes chronic vasodilatation of the superficial venous plexus, which leads to leakage of hemosiderin pigment, permanently staining the skin in a reticular pattern.
- EAI can affect the faces and arms of bakers, welders, chefs, and silversmiths.
- Treatment can be attempted with topical application of retinoids or with use of lasers.
Recently, a 57-year-old woman was informed by her husband that she has a discolored area of skin on her back. This discovery followed a prolonged period of back pain, for which she was prescribed an NSAID. When this yielded no relief, she started sleeping with an electric heating pad, held in place by an elastic bandage, to ease the pain.
She reports mild itching in the affected area. She claims to be in good health otherwise and is not taking any medication.
EXAMINATION
The patient is in no acute distress but has obvious difficulty walking without pain. Covering her back, from the bra strap down, is modest erythema, a reticular pattern of modest hyperpigmentation, and focal areas of mild scaling.

No tenderness or increased warmth is detected on palpation. No notable changes are seen elsewhere on her skin.
What is the diagnosis?
DISCUSSION
This condition, termed erythema ab igne (EAI), was first described in 18th century women who worked while seated in front of a fire all day—hence the name, which translates to “redness from fire.” Over time, this exposure produced permanent changes on the anterior portions of their legs—similar to those seen in this patient.
Today, the condition can develop in a variety of contexts, all of which involve prolonged, repeated exposure to infrared radiation (eg, electric blankets, hot water bottles, electric space heaters, laptop computers). EAI can also affect the faces and arms of bakers, chefs, welders, and silversmiths working in close proximity to radiation.
This type of heat increases vasodilation in the superficial venous plexus (located in the papillary dermis), which causes hemosiderin to leak into the superficial dermis over time. This permanently stains the skin with a characteristic reticular pattern that mimics the affected vascular pattern. Generally, the darker the patient’s skin, the darker the pattern appears.
EAI more commonly affects women than men, and its diagnosis should prompt further investigation for underlying conditions. Musculoskeletal problems, chronic infection, anemia, or hypothyroidism could be involved.
Treatment, besides avoidance of radiation, includes topical application of retinoids or use of lasers.
In this case, the changes were discovered early enough to be at least partially reversible. In more advanced cases, focal areas of scaling can coalesce into papules or nodules that can undergo malignant transformation to squamous cell carcinoma.
TAKE-HOME LEARNING POINTS
- Erythema ab igne (EAI) is a fairly common condition caused by close proximity to infrared radiation (eg, heating pads, laptop computers, electric space heaters).
- This radiation causes chronic vasodilatation of the superficial venous plexus, which leads to leakage of hemosiderin pigment, permanently staining the skin in a reticular pattern.
- EAI can affect the faces and arms of bakers, welders, chefs, and silversmiths.
- Treatment can be attempted with topical application of retinoids or with use of lasers.
Drug allows for treatment-free periods in PV
ATLANTA—Results of a phase 1 study suggest patients with polycythemia vera (PV) can achieve extended treatment-free periods after receiving idasanutlin.
Six of 12 patients who received the drug were able to have a treatment holiday—4 patients for 1 month, 1 for 2 to 3 consecutive months, and 1 for more than 3 consecutive months.
Four patients achieved a complete response (CR) and 3 a partial response (PR), for an overall response rate (ORR) of 58%.
There were no dose-limiting toxicities (DLTs) in the trial.
John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York, reported these results at the 2017 ASH Annual Meeting as abstract 254.*
The study was supported by Roche and funded by the National Cancer Institute, PDRC, and a grant from the Leukemia and Lymphoma Society.
Study rationale
Patients with PV have higher levels of MDM2 in their CD34+ cells compared to normal CD34+ cells. Nutlins block the interaction between p53 and MDM2, thus activating the p53 pathway.
Investigators previously found that low doses of nutlin and pegylated IFNα 2a promoted apoptosis in PV CD34+ cells.
And treatment with the combination reduced the numbers of JAK2V617-positive cells transplanted into immune-deficient NOD/SCID mice.
So Dr Mascarenhas and his colleagues undertook a study (NCT02407080) to evaluate the toxicity, safety, and tolerability of the MDM2 antagonist idasanutlin in patients with PV and essential thrombocythemia (ET).
The investigators hypothesized that since overexpression of MDM2 negatively regulates wild-type p53 function in primary PV cells, idasanutlin therapy, either alone or in combination with low-dose peg-IFN, could result in selective reduction or elimination of the myeloproliferative neoplasm cells in PV patients.
Study design
The investigators evaluated 2 dose levels of idasanutlin—100 mg daily and 150 mg daily on days 1 to 5, repeated every 28 days. The first cycle was 56 days to allow investigators to evaluate any DLTs.
Dr Mascarenhas pointed out that the dose is 1/6 of that being evaluated in acute myeloid leukemia.
Investigators defined a DLT as a non-hematologic adverse event (AE) of grade 3 or higher or a hematologic AE of grade 2 or higher thrombocytopenia or grade 3 or higher neutropenia or anemia.
If patients did not achieve at least a PR by the end of cycle 3 of single-agent therapy, they could proceed to Part B of the study and receive idasanutlin with pegylated IFN.
After cycle 3, dosing was dependent upon patients hitting a hematocrit greater than 42% and/or platelet counts greater than 400,000.
“So you had to meet parameters, which means that if you did meet parameters, you could get a treatment holiday,” Dr Mascarenhas explained.
Patients were eligible if they had JAK2V617F-positive PV or ET confirmed by WHO diagnostic criteria.
They had to have high-risk disease, be older than 60, and have a history of thrombosis. They also had to be either intolerant or resistant to at least one prior treatment, including hydroxyurea, interferon, or anagrelide.
Patients were excluded if they had post-ET/PV myelofibrosis, blast phase disease, acute thrombosis within 3 months of screening, or uncontrolled inter-current illness.
Baseline patient characteristics
Eleven of the 12 patients enrolled had PV, and 1 had ET. Their median age was 63.5, and 7 were female. Their median duration of disease was 43.9 months (range, 14.9–154.3), 3 had previous thrombosis, and 10 had prior hydroxyurea therapy.
They had a median leukocyte count of 11.3 x 109/L, median hemoglobin levels of 13.6 g/dL, median hematocrit of 42.3%, and median platelet levels of 443.5 x 109/L.
They all had the JAK2V617F mutation. Some patients had additional mutations, including TET2, DNMT3A, ASXL1, CBL, and EZH2, among others.
“One patient had an inactivating p53 mutation,” Dr Mascarenhas said. “I didn’t know this when we put her on study. . . , but this is an interesting part of the study. So remember, one patient had an inactivating p53 mutation in a trial where I’m using a drug that interrupts wild-type p53-MDM2 interaction.”
Efficacy
Plasma MIC-1 levels were significantly increased (P=0.004) in PV patients following treatment with idasanutlin at day 5 compared to day 1. MIC-1 is a secreted protein strongly induced by activated p53.
“Some patients didn’t need to be treated every month,” Dr Mascarenhas said. “They got treatment holidays. That’s unique. I don’t usually see that in the treatments that we give. In fact, this one person here, after 3 cycles, didn’t need to be re-treated for 9 months.”
In the 100 mg cohort (n=6), patients received a median of 8 cycles of idasanutlin (range, 7-13) and were on study for a median of 34.1 weeks (range, 29.0–127.3). One patient experienced a treatment holiday of 1 month, and another patient had a treatment holiday of more than 3 consecutive months.
In the 150 mg cohort (n=6), patients received a median of 9.5 cycles of therapy (range, 5–17) and were on study for a median of 52.1 weeks (range, 23.1–72.9). Three patients experienced a 1-month treatment holiday, and 1 patient had a 2- to 3-month treatment holiday.
In total, 6 patients continued the single-agent regimen, and 4 proceeded to the combination treatment with pegylated IFN.
Reasons for discontinuation were patient refusal and investigator decision.
The ORR (CR + PR) for both dose cohorts with single-agent idasanutlin was 58%. One patient was not evaluable, 4 had no response, 3 had a PR, and 4 had a CR.
In the combination portion of the study, the ORR was 50%. One patient was not evaluable, 1 had no response, 1 had a PR, and 1 had a CR.
“The 1 non-responder in Part B,” Dr Mascarenhas noted, “was the p53-mutated patient. Makes sense.”
The ORR for both the single-agent and combination parts of the study was 75%.
Eight of 12 patients had a 50% reduction in total symptom score from baseline, which is considered clinically meaningful, according to Dr Mascarenhas.
“What’s also interesting,” he pointed out, “[ is that] patients who didn’t obtain a response also enjoyed symptom benefit.”
Patients had a median 43% reduction in JAK2 mutation from baseline.
“One patient had nearly 92% reduction in JAK2V617F,” Dr Mascarenhas said. “One patient had a 60% increase. But guess what? That was the p53-mutated patient. Makes sense.”
Bone morphology showed reduction in marrow hypercellularity and normalization of megakaryocyte atypia and clustering.
Safety
There were no DLTs with either dose of idasanutlin.
“This was a well-tolerated drug,” Dr Mascarenhas said.
Three patients experienced grade 3 non-hematologic treatment-emergent AEs, all at 100 mg, of fatigue (1 patient), headache (1 patient), and pain (1 patient).
No grade 4 non-hematologic treatment-emergent AEs occurred at either dose, and investigators observed no hematologic AE of any grade.
Investigators also observed no grade 3–4 gastrointestinal (GI) treatment-emergent AEs. Constipation (91.7%), nausea (75%), and diarrhea (66.7%) were the most frequent grade 1 or 2 events. Patients received GI prophylaxis upfront with ondansetron, lorazepam, or dexamethasone.
Because of the safety profile and manageable GI toxicity, the higher dose of idasanutlin was chosen as the recommended phase 2 dose.
A global, multicenter, single-arm, phase 2 trial with idasanutlin in patients with hydroxyurea-resistant or -intolerant PV is underway. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Results of a phase 1 study suggest patients with polycythemia vera (PV) can achieve extended treatment-free periods after receiving idasanutlin.
Six of 12 patients who received the drug were able to have a treatment holiday—4 patients for 1 month, 1 for 2 to 3 consecutive months, and 1 for more than 3 consecutive months.
Four patients achieved a complete response (CR) and 3 a partial response (PR), for an overall response rate (ORR) of 58%.
There were no dose-limiting toxicities (DLTs) in the trial.
John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York, reported these results at the 2017 ASH Annual Meeting as abstract 254.*
The study was supported by Roche and funded by the National Cancer Institute, PDRC, and a grant from the Leukemia and Lymphoma Society.
Study rationale
Patients with PV have higher levels of MDM2 in their CD34+ cells compared to normal CD34+ cells. Nutlins block the interaction between p53 and MDM2, thus activating the p53 pathway.
Investigators previously found that low doses of nutlin and pegylated IFNα 2a promoted apoptosis in PV CD34+ cells.
And treatment with the combination reduced the numbers of JAK2V617-positive cells transplanted into immune-deficient NOD/SCID mice.
So Dr Mascarenhas and his colleagues undertook a study (NCT02407080) to evaluate the toxicity, safety, and tolerability of the MDM2 antagonist idasanutlin in patients with PV and essential thrombocythemia (ET).
The investigators hypothesized that since overexpression of MDM2 negatively regulates wild-type p53 function in primary PV cells, idasanutlin therapy, either alone or in combination with low-dose peg-IFN, could result in selective reduction or elimination of the myeloproliferative neoplasm cells in PV patients.
Study design
The investigators evaluated 2 dose levels of idasanutlin—100 mg daily and 150 mg daily on days 1 to 5, repeated every 28 days. The first cycle was 56 days to allow investigators to evaluate any DLTs.
Dr Mascarenhas pointed out that the dose is 1/6 of that being evaluated in acute myeloid leukemia.
Investigators defined a DLT as a non-hematologic adverse event (AE) of grade 3 or higher or a hematologic AE of grade 2 or higher thrombocytopenia or grade 3 or higher neutropenia or anemia.
If patients did not achieve at least a PR by the end of cycle 3 of single-agent therapy, they could proceed to Part B of the study and receive idasanutlin with pegylated IFN.
After cycle 3, dosing was dependent upon patients hitting a hematocrit greater than 42% and/or platelet counts greater than 400,000.
“So you had to meet parameters, which means that if you did meet parameters, you could get a treatment holiday,” Dr Mascarenhas explained.
Patients were eligible if they had JAK2V617F-positive PV or ET confirmed by WHO diagnostic criteria.
They had to have high-risk disease, be older than 60, and have a history of thrombosis. They also had to be either intolerant or resistant to at least one prior treatment, including hydroxyurea, interferon, or anagrelide.
Patients were excluded if they had post-ET/PV myelofibrosis, blast phase disease, acute thrombosis within 3 months of screening, or uncontrolled inter-current illness.
Baseline patient characteristics
Eleven of the 12 patients enrolled had PV, and 1 had ET. Their median age was 63.5, and 7 were female. Their median duration of disease was 43.9 months (range, 14.9–154.3), 3 had previous thrombosis, and 10 had prior hydroxyurea therapy.
They had a median leukocyte count of 11.3 x 109/L, median hemoglobin levels of 13.6 g/dL, median hematocrit of 42.3%, and median platelet levels of 443.5 x 109/L.
They all had the JAK2V617F mutation. Some patients had additional mutations, including TET2, DNMT3A, ASXL1, CBL, and EZH2, among others.
“One patient had an inactivating p53 mutation,” Dr Mascarenhas said. “I didn’t know this when we put her on study. . . , but this is an interesting part of the study. So remember, one patient had an inactivating p53 mutation in a trial where I’m using a drug that interrupts wild-type p53-MDM2 interaction.”
Efficacy
Plasma MIC-1 levels were significantly increased (P=0.004) in PV patients following treatment with idasanutlin at day 5 compared to day 1. MIC-1 is a secreted protein strongly induced by activated p53.
“Some patients didn’t need to be treated every month,” Dr Mascarenhas said. “They got treatment holidays. That’s unique. I don’t usually see that in the treatments that we give. In fact, this one person here, after 3 cycles, didn’t need to be re-treated for 9 months.”
In the 100 mg cohort (n=6), patients received a median of 8 cycles of idasanutlin (range, 7-13) and were on study for a median of 34.1 weeks (range, 29.0–127.3). One patient experienced a treatment holiday of 1 month, and another patient had a treatment holiday of more than 3 consecutive months.
In the 150 mg cohort (n=6), patients received a median of 9.5 cycles of therapy (range, 5–17) and were on study for a median of 52.1 weeks (range, 23.1–72.9). Three patients experienced a 1-month treatment holiday, and 1 patient had a 2- to 3-month treatment holiday.
In total, 6 patients continued the single-agent regimen, and 4 proceeded to the combination treatment with pegylated IFN.
Reasons for discontinuation were patient refusal and investigator decision.
The ORR (CR + PR) for both dose cohorts with single-agent idasanutlin was 58%. One patient was not evaluable, 4 had no response, 3 had a PR, and 4 had a CR.
In the combination portion of the study, the ORR was 50%. One patient was not evaluable, 1 had no response, 1 had a PR, and 1 had a CR.
“The 1 non-responder in Part B,” Dr Mascarenhas noted, “was the p53-mutated patient. Makes sense.”
The ORR for both the single-agent and combination parts of the study was 75%.
Eight of 12 patients had a 50% reduction in total symptom score from baseline, which is considered clinically meaningful, according to Dr Mascarenhas.
“What’s also interesting,” he pointed out, “[ is that] patients who didn’t obtain a response also enjoyed symptom benefit.”
Patients had a median 43% reduction in JAK2 mutation from baseline.
“One patient had nearly 92% reduction in JAK2V617F,” Dr Mascarenhas said. “One patient had a 60% increase. But guess what? That was the p53-mutated patient. Makes sense.”
Bone morphology showed reduction in marrow hypercellularity and normalization of megakaryocyte atypia and clustering.
Safety
There were no DLTs with either dose of idasanutlin.
“This was a well-tolerated drug,” Dr Mascarenhas said.
Three patients experienced grade 3 non-hematologic treatment-emergent AEs, all at 100 mg, of fatigue (1 patient), headache (1 patient), and pain (1 patient).
No grade 4 non-hematologic treatment-emergent AEs occurred at either dose, and investigators observed no hematologic AE of any grade.
Investigators also observed no grade 3–4 gastrointestinal (GI) treatment-emergent AEs. Constipation (91.7%), nausea (75%), and diarrhea (66.7%) were the most frequent grade 1 or 2 events. Patients received GI prophylaxis upfront with ondansetron, lorazepam, or dexamethasone.
Because of the safety profile and manageable GI toxicity, the higher dose of idasanutlin was chosen as the recommended phase 2 dose.
A global, multicenter, single-arm, phase 2 trial with idasanutlin in patients with hydroxyurea-resistant or -intolerant PV is underway. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Results of a phase 1 study suggest patients with polycythemia vera (PV) can achieve extended treatment-free periods after receiving idasanutlin.
Six of 12 patients who received the drug were able to have a treatment holiday—4 patients for 1 month, 1 for 2 to 3 consecutive months, and 1 for more than 3 consecutive months.
Four patients achieved a complete response (CR) and 3 a partial response (PR), for an overall response rate (ORR) of 58%.
There were no dose-limiting toxicities (DLTs) in the trial.
John Mascarenhas, MD, of Icahn School of Medicine at Mount Sinai in New York, New York, reported these results at the 2017 ASH Annual Meeting as abstract 254.*
The study was supported by Roche and funded by the National Cancer Institute, PDRC, and a grant from the Leukemia and Lymphoma Society.
Study rationale
Patients with PV have higher levels of MDM2 in their CD34+ cells compared to normal CD34+ cells. Nutlins block the interaction between p53 and MDM2, thus activating the p53 pathway.
Investigators previously found that low doses of nutlin and pegylated IFNα 2a promoted apoptosis in PV CD34+ cells.
And treatment with the combination reduced the numbers of JAK2V617-positive cells transplanted into immune-deficient NOD/SCID mice.
So Dr Mascarenhas and his colleagues undertook a study (NCT02407080) to evaluate the toxicity, safety, and tolerability of the MDM2 antagonist idasanutlin in patients with PV and essential thrombocythemia (ET).
The investigators hypothesized that since overexpression of MDM2 negatively regulates wild-type p53 function in primary PV cells, idasanutlin therapy, either alone or in combination with low-dose peg-IFN, could result in selective reduction or elimination of the myeloproliferative neoplasm cells in PV patients.
Study design
The investigators evaluated 2 dose levels of idasanutlin—100 mg daily and 150 mg daily on days 1 to 5, repeated every 28 days. The first cycle was 56 days to allow investigators to evaluate any DLTs.
Dr Mascarenhas pointed out that the dose is 1/6 of that being evaluated in acute myeloid leukemia.
Investigators defined a DLT as a non-hematologic adverse event (AE) of grade 3 or higher or a hematologic AE of grade 2 or higher thrombocytopenia or grade 3 or higher neutropenia or anemia.
If patients did not achieve at least a PR by the end of cycle 3 of single-agent therapy, they could proceed to Part B of the study and receive idasanutlin with pegylated IFN.
After cycle 3, dosing was dependent upon patients hitting a hematocrit greater than 42% and/or platelet counts greater than 400,000.
“So you had to meet parameters, which means that if you did meet parameters, you could get a treatment holiday,” Dr Mascarenhas explained.
Patients were eligible if they had JAK2V617F-positive PV or ET confirmed by WHO diagnostic criteria.
They had to have high-risk disease, be older than 60, and have a history of thrombosis. They also had to be either intolerant or resistant to at least one prior treatment, including hydroxyurea, interferon, or anagrelide.
Patients were excluded if they had post-ET/PV myelofibrosis, blast phase disease, acute thrombosis within 3 months of screening, or uncontrolled inter-current illness.
Baseline patient characteristics
Eleven of the 12 patients enrolled had PV, and 1 had ET. Their median age was 63.5, and 7 were female. Their median duration of disease was 43.9 months (range, 14.9–154.3), 3 had previous thrombosis, and 10 had prior hydroxyurea therapy.
They had a median leukocyte count of 11.3 x 109/L, median hemoglobin levels of 13.6 g/dL, median hematocrit of 42.3%, and median platelet levels of 443.5 x 109/L.
They all had the JAK2V617F mutation. Some patients had additional mutations, including TET2, DNMT3A, ASXL1, CBL, and EZH2, among others.
“One patient had an inactivating p53 mutation,” Dr Mascarenhas said. “I didn’t know this when we put her on study. . . , but this is an interesting part of the study. So remember, one patient had an inactivating p53 mutation in a trial where I’m using a drug that interrupts wild-type p53-MDM2 interaction.”
Efficacy
Plasma MIC-1 levels were significantly increased (P=0.004) in PV patients following treatment with idasanutlin at day 5 compared to day 1. MIC-1 is a secreted protein strongly induced by activated p53.
“Some patients didn’t need to be treated every month,” Dr Mascarenhas said. “They got treatment holidays. That’s unique. I don’t usually see that in the treatments that we give. In fact, this one person here, after 3 cycles, didn’t need to be re-treated for 9 months.”
In the 100 mg cohort (n=6), patients received a median of 8 cycles of idasanutlin (range, 7-13) and were on study for a median of 34.1 weeks (range, 29.0–127.3). One patient experienced a treatment holiday of 1 month, and another patient had a treatment holiday of more than 3 consecutive months.
In the 150 mg cohort (n=6), patients received a median of 9.5 cycles of therapy (range, 5–17) and were on study for a median of 52.1 weeks (range, 23.1–72.9). Three patients experienced a 1-month treatment holiday, and 1 patient had a 2- to 3-month treatment holiday.
In total, 6 patients continued the single-agent regimen, and 4 proceeded to the combination treatment with pegylated IFN.
Reasons for discontinuation were patient refusal and investigator decision.
The ORR (CR + PR) for both dose cohorts with single-agent idasanutlin was 58%. One patient was not evaluable, 4 had no response, 3 had a PR, and 4 had a CR.
In the combination portion of the study, the ORR was 50%. One patient was not evaluable, 1 had no response, 1 had a PR, and 1 had a CR.
“The 1 non-responder in Part B,” Dr Mascarenhas noted, “was the p53-mutated patient. Makes sense.”
The ORR for both the single-agent and combination parts of the study was 75%.
Eight of 12 patients had a 50% reduction in total symptom score from baseline, which is considered clinically meaningful, according to Dr Mascarenhas.
“What’s also interesting,” he pointed out, “[ is that] patients who didn’t obtain a response also enjoyed symptom benefit.”
Patients had a median 43% reduction in JAK2 mutation from baseline.
“One patient had nearly 92% reduction in JAK2V617F,” Dr Mascarenhas said. “One patient had a 60% increase. But guess what? That was the p53-mutated patient. Makes sense.”
Bone morphology showed reduction in marrow hypercellularity and normalization of megakaryocyte atypia and clustering.
Safety
There were no DLTs with either dose of idasanutlin.
“This was a well-tolerated drug,” Dr Mascarenhas said.
Three patients experienced grade 3 non-hematologic treatment-emergent AEs, all at 100 mg, of fatigue (1 patient), headache (1 patient), and pain (1 patient).
No grade 4 non-hematologic treatment-emergent AEs occurred at either dose, and investigators observed no hematologic AE of any grade.
Investigators also observed no grade 3–4 gastrointestinal (GI) treatment-emergent AEs. Constipation (91.7%), nausea (75%), and diarrhea (66.7%) were the most frequent grade 1 or 2 events. Patients received GI prophylaxis upfront with ondansetron, lorazepam, or dexamethasone.
Because of the safety profile and manageable GI toxicity, the higher dose of idasanutlin was chosen as the recommended phase 2 dose.
A global, multicenter, single-arm, phase 2 trial with idasanutlin in patients with hydroxyurea-resistant or -intolerant PV is underway. ![]()
*Data in the presentation differ from the abstract.
Risk stratification may be possible with JCAR017
ATLANTA—Data suggest a therapeutic window may exist for chimeric antigen receptor (CAR) T-cell expansion with JCAR017, according to a preliminary model.
In a core set of 67 patients with diffuse large B-cell lymphoma (DLBCL) who had received JCAR017 in the TRANSCEND NHL 001 trial, investigators observed that baseline high tumor burden and inflammatory biomarkers were associated with high CAR T-cell expansion and increased rates of cytokine release syndrome (CRS) and neurotoxicity.
If the model holds up, researchers say they could potentially identify patients at risk for low or high T-cell expansion levels and develop a strategy to enhance or limit the expansion.
TRANSCEND NHL 001 (NCT02631044) is a multicenter, phase 1 trial in relapsed or refractory non-Hodgkin lymphoma evaluating 2 dose levels of JCAR017, also known as lisocabtagene maraleucel, or liso-cel for short.
Liso-cel is a CD19-directed 4-1BB CAR T cell administered at precise doses of CD4+ and CD8+ CAR T cells. It had previously demonstrated high complete remission (CR) rates and low incidences of CRS and neurotoxicity.
Tanya Saddiqi, MD, of City of Hope National Medical Center in Duarte, California, presented data from the dose-finding and expansion cohorts at the 2017 ASH Annual Meeting (abstract 193*).
Study design
Patients with DLBCL after 2 lines of prior therapy or mantle cell lymphoma after 1 prior line of therapy were eligible to enroll in TRANSCEND NHL 001.
Patients with de novo DLBCL, those who transformed from follicular lymphoma, or those with high-grade B-cell lymphoma made up the pivotal or core population. All DLBCL patients enrolled on the trial comprised the full population.
Patients were screened, enrolled, and underwent apheresis. Bridging therapy was permitted while their CAR T cells were being manufactured.
Patients then had a PET scan and lab tests prior to lymphodepletion.
“This is the time point of our interest,” Dr Saddiqi said, “to see if there are any patient characteristics or biomarkers that we can identify . . . that could help us figure out which patients are at higher risk of toxicity, potentially.”
Lymphodepletion consisted of fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2 for 3 days).
Patients received the JCAR017 infusion, and, at specific time points thereafter, cytokine, pharmacokinetic (PK), and clinical lab evaluations were conducted. PK evaluation and scans were performed every 3 months for the first year after JCAR017 infusion, and safety and viral vector follow-up for 15 years.
Dose levels were 5 x 107 cells as a single or double dose (DL1S) and 1 x 108 cells as a single dose (DL2S). Dose level 2 was chosen for further study, and double dosing was discontinued.
“Double dosing was actually not pursued further,” Dr Saddiqi explained, “because it did not seem to add any benefit over single dosing.”
At the time of the presentation, 91 total patients were treated, 67 of whom were the core population.
Results
Dr Saddiqi reported that patients treated with JCAR017 achieved a relatively high best overall response rate (ORR) and high durable CR rates.
“And this seems to be especially true for the core set of patients and particularly for patients at dose level 2,” she added.
At all dose levels, the core patients had a best ORR of 84% (41/49) and a CR rate of 61% (30/49).
At follow-up of 3 months or longer, the core group had an ORR of 65% (26/40) for all dose levels, 52% (11/21) for dose level 1, and 80% (12/15) for dose level 2.
The 3-month CR rate was 53% (21/40) for all dose levels in the core group, 33% (7/21) in dose level 1, and 73% (11/15) in dose level 2.
Dr Saddiqi noted that CRS and neurotoxicity did not differ by dose level or schedule, and there were no grade 5 events of CRS or neurotoxicity.
“Among the core group, dose level change did not add to their toxicity,” she said. “And so the question is: Is it patient factors, is it tumor factors? What is it that is actually causing the toxicities in these patients?”
Dr Saddiqi focused the presentation on patient factors.
Patient factors
The data showed that tumor burden and lactose dehydrogenase (LDH) levels were higher in patients with CRS and neurotoxicity.
Univariate analysis revealed that CRS and neurotoxicity were associated with a shorter time since diagnosis.
However, prior number of therapies, patient weight, and disease stage were not associated with CRS or neurotoxicity.
Investigators were able to identify preliminary risk boundaries. Core patients with high LDH levels (≥ 500 U/L) and sum of the products of diameters (SPD) ≥ 50 cm2 at baseline had an 8-fold increase in risk of CRS and neurotoxicity.
“Inversely, if these patients did not meet the cutoff for LDH or SPD,” Dr Saddiqi pointed out, “if they were lower than that, they have significantly lower CRS and neurotoxicity events.”
Investigators also observed that baseline markers of inflammation and inflammatory cytokines trended higher in patients with CRS and neurotoxicity. For CRS, this includes ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNFα, and MIP-1β. For neurotoxicity, this includes ferritin, CRP, d-Dimer, IL-6, IL-15, TNFα, and MIP-1α.
The team also observed that tumor burden, baseline markers of inflammation, and inflammatory cytokines trended lower in core patients with durable responses.
“Interestingly, it’s inversely true that patients who did have these higher levels [of inflammation markers], and higher tumor burden, and higher LDH, actually were the ones that were either showing no response at 3 months or had lost their response by the 3-month assessment point,” Dr Saddiqi explained.
And in patients with higher baseline tumor burden and inflammatory cytokine levels, JCAR017 T-cell expansion trended higher.
“Some were deemed to be super expanders because their CAR T-cell levels were very high in their blood,” she added.
The investigators created a preliminary logistic model based on the data that suggests a therapeutic window might be able to limit toxicity and optimize efficacy.
The model indicates that patients with higher tumor burden, higher LDH, and higher inflammatory state at baseline seem to be the ones who are having more CRS and more neurotoxicity after CAR T-cell infusion.
“They are expanding their cells much more, yet their responses at 3 months seem to be affected adversely by this entire situation,” Dr Saddiqi said.
"One explanation, potentially, could be that these CAR T cells are seeing a lot of antigen when they go into the body. They have the perfect cytokine milieu to grow, expand, and go crazy in the body, if you will, and very quickly peter out as well because there’s T-cell exhaustion that happens rather rapidly and clinical responses are then then lost.”
The investigators believe that if they can identify those patients ahead of time who may be at risk of too high expansion or too low expansion of their CAR T cells, they may be able to find strategies to push expansion into the “sweet spot of CAR T-cell expansion and ultimately get the holy grail of having durable responses for all with minimal toxicity,” Dr Saddiqi concluded.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics, Inc. Dr Saddiqi has served on a steering committee for JCAR017. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Data suggest a therapeutic window may exist for chimeric antigen receptor (CAR) T-cell expansion with JCAR017, according to a preliminary model.
In a core set of 67 patients with diffuse large B-cell lymphoma (DLBCL) who had received JCAR017 in the TRANSCEND NHL 001 trial, investigators observed that baseline high tumor burden and inflammatory biomarkers were associated with high CAR T-cell expansion and increased rates of cytokine release syndrome (CRS) and neurotoxicity.
If the model holds up, researchers say they could potentially identify patients at risk for low or high T-cell expansion levels and develop a strategy to enhance or limit the expansion.
TRANSCEND NHL 001 (NCT02631044) is a multicenter, phase 1 trial in relapsed or refractory non-Hodgkin lymphoma evaluating 2 dose levels of JCAR017, also known as lisocabtagene maraleucel, or liso-cel for short.
Liso-cel is a CD19-directed 4-1BB CAR T cell administered at precise doses of CD4+ and CD8+ CAR T cells. It had previously demonstrated high complete remission (CR) rates and low incidences of CRS and neurotoxicity.
Tanya Saddiqi, MD, of City of Hope National Medical Center in Duarte, California, presented data from the dose-finding and expansion cohorts at the 2017 ASH Annual Meeting (abstract 193*).
Study design
Patients with DLBCL after 2 lines of prior therapy or mantle cell lymphoma after 1 prior line of therapy were eligible to enroll in TRANSCEND NHL 001.
Patients with de novo DLBCL, those who transformed from follicular lymphoma, or those with high-grade B-cell lymphoma made up the pivotal or core population. All DLBCL patients enrolled on the trial comprised the full population.
Patients were screened, enrolled, and underwent apheresis. Bridging therapy was permitted while their CAR T cells were being manufactured.
Patients then had a PET scan and lab tests prior to lymphodepletion.
“This is the time point of our interest,” Dr Saddiqi said, “to see if there are any patient characteristics or biomarkers that we can identify . . . that could help us figure out which patients are at higher risk of toxicity, potentially.”
Lymphodepletion consisted of fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2 for 3 days).
Patients received the JCAR017 infusion, and, at specific time points thereafter, cytokine, pharmacokinetic (PK), and clinical lab evaluations were conducted. PK evaluation and scans were performed every 3 months for the first year after JCAR017 infusion, and safety and viral vector follow-up for 15 years.
Dose levels were 5 x 107 cells as a single or double dose (DL1S) and 1 x 108 cells as a single dose (DL2S). Dose level 2 was chosen for further study, and double dosing was discontinued.
“Double dosing was actually not pursued further,” Dr Saddiqi explained, “because it did not seem to add any benefit over single dosing.”
At the time of the presentation, 91 total patients were treated, 67 of whom were the core population.
Results
Dr Saddiqi reported that patients treated with JCAR017 achieved a relatively high best overall response rate (ORR) and high durable CR rates.
“And this seems to be especially true for the core set of patients and particularly for patients at dose level 2,” she added.
At all dose levels, the core patients had a best ORR of 84% (41/49) and a CR rate of 61% (30/49).
At follow-up of 3 months or longer, the core group had an ORR of 65% (26/40) for all dose levels, 52% (11/21) for dose level 1, and 80% (12/15) for dose level 2.
The 3-month CR rate was 53% (21/40) for all dose levels in the core group, 33% (7/21) in dose level 1, and 73% (11/15) in dose level 2.
Dr Saddiqi noted that CRS and neurotoxicity did not differ by dose level or schedule, and there were no grade 5 events of CRS or neurotoxicity.
“Among the core group, dose level change did not add to their toxicity,” she said. “And so the question is: Is it patient factors, is it tumor factors? What is it that is actually causing the toxicities in these patients?”
Dr Saddiqi focused the presentation on patient factors.
Patient factors
The data showed that tumor burden and lactose dehydrogenase (LDH) levels were higher in patients with CRS and neurotoxicity.
Univariate analysis revealed that CRS and neurotoxicity were associated with a shorter time since diagnosis.
However, prior number of therapies, patient weight, and disease stage were not associated with CRS or neurotoxicity.
Investigators were able to identify preliminary risk boundaries. Core patients with high LDH levels (≥ 500 U/L) and sum of the products of diameters (SPD) ≥ 50 cm2 at baseline had an 8-fold increase in risk of CRS and neurotoxicity.
“Inversely, if these patients did not meet the cutoff for LDH or SPD,” Dr Saddiqi pointed out, “if they were lower than that, they have significantly lower CRS and neurotoxicity events.”
Investigators also observed that baseline markers of inflammation and inflammatory cytokines trended higher in patients with CRS and neurotoxicity. For CRS, this includes ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNFα, and MIP-1β. For neurotoxicity, this includes ferritin, CRP, d-Dimer, IL-6, IL-15, TNFα, and MIP-1α.
The team also observed that tumor burden, baseline markers of inflammation, and inflammatory cytokines trended lower in core patients with durable responses.
“Interestingly, it’s inversely true that patients who did have these higher levels [of inflammation markers], and higher tumor burden, and higher LDH, actually were the ones that were either showing no response at 3 months or had lost their response by the 3-month assessment point,” Dr Saddiqi explained.
And in patients with higher baseline tumor burden and inflammatory cytokine levels, JCAR017 T-cell expansion trended higher.
“Some were deemed to be super expanders because their CAR T-cell levels were very high in their blood,” she added.
The investigators created a preliminary logistic model based on the data that suggests a therapeutic window might be able to limit toxicity and optimize efficacy.
The model indicates that patients with higher tumor burden, higher LDH, and higher inflammatory state at baseline seem to be the ones who are having more CRS and more neurotoxicity after CAR T-cell infusion.
“They are expanding their cells much more, yet their responses at 3 months seem to be affected adversely by this entire situation,” Dr Saddiqi said.
"One explanation, potentially, could be that these CAR T cells are seeing a lot of antigen when they go into the body. They have the perfect cytokine milieu to grow, expand, and go crazy in the body, if you will, and very quickly peter out as well because there’s T-cell exhaustion that happens rather rapidly and clinical responses are then then lost.”
The investigators believe that if they can identify those patients ahead of time who may be at risk of too high expansion or too low expansion of their CAR T cells, they may be able to find strategies to push expansion into the “sweet spot of CAR T-cell expansion and ultimately get the holy grail of having durable responses for all with minimal toxicity,” Dr Saddiqi concluded.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics, Inc. Dr Saddiqi has served on a steering committee for JCAR017. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Data suggest a therapeutic window may exist for chimeric antigen receptor (CAR) T-cell expansion with JCAR017, according to a preliminary model.
In a core set of 67 patients with diffuse large B-cell lymphoma (DLBCL) who had received JCAR017 in the TRANSCEND NHL 001 trial, investigators observed that baseline high tumor burden and inflammatory biomarkers were associated with high CAR T-cell expansion and increased rates of cytokine release syndrome (CRS) and neurotoxicity.
If the model holds up, researchers say they could potentially identify patients at risk for low or high T-cell expansion levels and develop a strategy to enhance or limit the expansion.
TRANSCEND NHL 001 (NCT02631044) is a multicenter, phase 1 trial in relapsed or refractory non-Hodgkin lymphoma evaluating 2 dose levels of JCAR017, also known as lisocabtagene maraleucel, or liso-cel for short.
Liso-cel is a CD19-directed 4-1BB CAR T cell administered at precise doses of CD4+ and CD8+ CAR T cells. It had previously demonstrated high complete remission (CR) rates and low incidences of CRS and neurotoxicity.
Tanya Saddiqi, MD, of City of Hope National Medical Center in Duarte, California, presented data from the dose-finding and expansion cohorts at the 2017 ASH Annual Meeting (abstract 193*).
Study design
Patients with DLBCL after 2 lines of prior therapy or mantle cell lymphoma after 1 prior line of therapy were eligible to enroll in TRANSCEND NHL 001.
Patients with de novo DLBCL, those who transformed from follicular lymphoma, or those with high-grade B-cell lymphoma made up the pivotal or core population. All DLBCL patients enrolled on the trial comprised the full population.
Patients were screened, enrolled, and underwent apheresis. Bridging therapy was permitted while their CAR T cells were being manufactured.
Patients then had a PET scan and lab tests prior to lymphodepletion.
“This is the time point of our interest,” Dr Saddiqi said, “to see if there are any patient characteristics or biomarkers that we can identify . . . that could help us figure out which patients are at higher risk of toxicity, potentially.”
Lymphodepletion consisted of fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2 for 3 days).
Patients received the JCAR017 infusion, and, at specific time points thereafter, cytokine, pharmacokinetic (PK), and clinical lab evaluations were conducted. PK evaluation and scans were performed every 3 months for the first year after JCAR017 infusion, and safety and viral vector follow-up for 15 years.
Dose levels were 5 x 107 cells as a single or double dose (DL1S) and 1 x 108 cells as a single dose (DL2S). Dose level 2 was chosen for further study, and double dosing was discontinued.
“Double dosing was actually not pursued further,” Dr Saddiqi explained, “because it did not seem to add any benefit over single dosing.”
At the time of the presentation, 91 total patients were treated, 67 of whom were the core population.
Results
Dr Saddiqi reported that patients treated with JCAR017 achieved a relatively high best overall response rate (ORR) and high durable CR rates.
“And this seems to be especially true for the core set of patients and particularly for patients at dose level 2,” she added.
At all dose levels, the core patients had a best ORR of 84% (41/49) and a CR rate of 61% (30/49).
At follow-up of 3 months or longer, the core group had an ORR of 65% (26/40) for all dose levels, 52% (11/21) for dose level 1, and 80% (12/15) for dose level 2.
The 3-month CR rate was 53% (21/40) for all dose levels in the core group, 33% (7/21) in dose level 1, and 73% (11/15) in dose level 2.
Dr Saddiqi noted that CRS and neurotoxicity did not differ by dose level or schedule, and there were no grade 5 events of CRS or neurotoxicity.
“Among the core group, dose level change did not add to their toxicity,” she said. “And so the question is: Is it patient factors, is it tumor factors? What is it that is actually causing the toxicities in these patients?”
Dr Saddiqi focused the presentation on patient factors.
Patient factors
The data showed that tumor burden and lactose dehydrogenase (LDH) levels were higher in patients with CRS and neurotoxicity.
Univariate analysis revealed that CRS and neurotoxicity were associated with a shorter time since diagnosis.
However, prior number of therapies, patient weight, and disease stage were not associated with CRS or neurotoxicity.
Investigators were able to identify preliminary risk boundaries. Core patients with high LDH levels (≥ 500 U/L) and sum of the products of diameters (SPD) ≥ 50 cm2 at baseline had an 8-fold increase in risk of CRS and neurotoxicity.
“Inversely, if these patients did not meet the cutoff for LDH or SPD,” Dr Saddiqi pointed out, “if they were lower than that, they have significantly lower CRS and neurotoxicity events.”
Investigators also observed that baseline markers of inflammation and inflammatory cytokines trended higher in patients with CRS and neurotoxicity. For CRS, this includes ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNFα, and MIP-1β. For neurotoxicity, this includes ferritin, CRP, d-Dimer, IL-6, IL-15, TNFα, and MIP-1α.
The team also observed that tumor burden, baseline markers of inflammation, and inflammatory cytokines trended lower in core patients with durable responses.
“Interestingly, it’s inversely true that patients who did have these higher levels [of inflammation markers], and higher tumor burden, and higher LDH, actually were the ones that were either showing no response at 3 months or had lost their response by the 3-month assessment point,” Dr Saddiqi explained.
And in patients with higher baseline tumor burden and inflammatory cytokine levels, JCAR017 T-cell expansion trended higher.
“Some were deemed to be super expanders because their CAR T-cell levels were very high in their blood,” she added.
The investigators created a preliminary logistic model based on the data that suggests a therapeutic window might be able to limit toxicity and optimize efficacy.
The model indicates that patients with higher tumor burden, higher LDH, and higher inflammatory state at baseline seem to be the ones who are having more CRS and more neurotoxicity after CAR T-cell infusion.
“They are expanding their cells much more, yet their responses at 3 months seem to be affected adversely by this entire situation,” Dr Saddiqi said.
"One explanation, potentially, could be that these CAR T cells are seeing a lot of antigen when they go into the body. They have the perfect cytokine milieu to grow, expand, and go crazy in the body, if you will, and very quickly peter out as well because there’s T-cell exhaustion that happens rather rapidly and clinical responses are then then lost.”
The investigators believe that if they can identify those patients ahead of time who may be at risk of too high expansion or too low expansion of their CAR T cells, they may be able to find strategies to push expansion into the “sweet spot of CAR T-cell expansion and ultimately get the holy grail of having durable responses for all with minimal toxicity,” Dr Saddiqi concluded.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics, Inc. Dr Saddiqi has served on a steering committee for JCAR017. ![]()
*Data in the presentation differ from the abstract.
Transformation of Benign Giant Cell Tumor of Bone Into Epithelioid Angiosarcoma
Take-Home Points
- Malignant transformation of a benign GCT is extremely rare.
- It is difficult to distinguish between an early malignant transformation and an overlooked malignancy.
- The most common clinical presentation of transformation of GCT into malignancy is pain, often with swelling.
- Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation.
- Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the wound site.
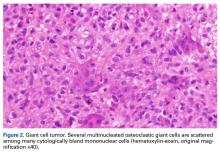
Giant cell tumors (GCTs) of bone account for about 5% of all primary bone tumors in adults, with a predominance in the third decade in life.1 Clinically, GCT of bone often presents with pain, pathologic fracture, and/or soft- tissue expansion in the epiphysis of long bones. However, GCT of bone also has been reported in non-long bones, such as the talus and the calcaneus.2,3 Histologically, GCT of bone consists of neoplastic stromal cells, mononuclear histiocytic cells, and multinucleated giant cells that resemble osteoclasts.4 The radiologic appearance of GCT is often described as a lytic, eccentrically located bony lesion that extends near the articular surface in patients with closed physes. Many GCTs have aggressive radiologic features with possible extensive bony destruction and soft-tissue extension.
Although categorized as a benign lesion, GCT can be locally aggressive, with a variable local recurrence rate of 0% to 65%, depending on treatment modality and skeletal location. Given the aggressiveness of GCT of bone, recommendations for operative intervention include intralesional curettage with adjuvant therapy (eg, cryotherapy, phenol, argon beam, electrocautery) and placement of bone void fillers (eg, bone graft polymethylmethacrylate). Wide resection is recommended when the articular surface is no longer viable for reconstruction secondary to extensive destruction. Some authors have reported that surgical margin is the only risk factor in local recurrence,5,6 and thus complete resection may be needed for tumor eradication. In addition, about 3% of GCTs demonstrate benign pulmonary implants, which have been cited as cause of death in 16% to 25% of reported cases of pulmonary spread.7,8
The literature includes few reports of primary or secondary malignant transformation of GCT. Hutter and colleagues9 defined primary malignant GCT as GCT with sarcomatous tissue juxtaposed with zones of typical benign GCT cells. Secondary malignant GCT is a sarcomatous lesion at the site of a previously documented benign GCT. Secondary malignant GCT of bone histologically has been classified as a fibrosarcoma, malignant fibrous histiocytoma, or osteosarcoma transformation.10
Most malignant transformations of GCT of bone have been attributed to previous irradiation of the lesion.11,12 However, there are some case reports of benign bone GCT malignant transformation in situ without any other medical intervention. It was reported that non-radiation-induced secondary transformations occur relatively early after GCT treatment.13 During the early stages of tumor recurrence, however, it is difficult to distinguish between malignant transformation and primary disease overlooked as a result of sampling error.
We report a case of secondary malignant transformation of GCT of bone 11 years after surgical curettage, cryotherapy, and cementation without adjuvant radiation therapy. To our knowledge, this case report is the first to describe transformation of a nonirradiated benign GCT into an aggressive, high-grade epithelioid angiosarcoma, a very rare vascular bone tumor. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
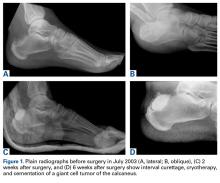
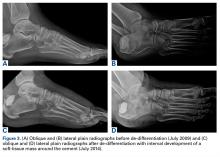
In July 2003, a 46-year-old woman presented with left heel pain of several months’ duration. Plain radiographs showed a nonaggressive-appearing lytic lesion of the superior aspect of the posterior calcaneal tuberosity with a small cortical incongruity along the superior margin of the lesion (Figures 1A-1D).
A postoperative splint was placed, and weight-bearing progressed over 6 weeks. The patient was followed at 2- to 3-month intervals over the first 5 postoperative years. She was able to work and perform activities of daily living, but her postoperative course was complicated by significant chronic pain in multiple extremities and long-term treatment by the chronic pain service. At no time did postoperative imaging—magnetic resonance imaging (MRI) at 6 years, whole-body bone scan at 7 years, plain radiographs at 10 years—show evidence of recurrence.
Radiographs showed stable postoperative changes with a small radiolucent area (with sclerotic rim) surrounding the cement-bone interface. Given its proximity to the Achilles tendon and more motion than usual at the wound site, the radiolucency likely was caused by small movements of the interface. The radiolucent area remained stable over a 15-month period.
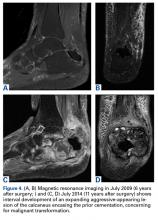
Whole-body bone scan showed a small area of osteoblastic activity in the left calcaneus, consistent with inflammation surrounding the bone- cement interface, but the uptake was minor relative to other areas of signal, and there were no significant inflammatory reactive changes on MRI (Figures 3A, 3B).
Over 11 years, regular 6- to 12-month follow-up examinations revealed no significant changes in the left foot or in plain radiographs of the chest. In addition, physical examinations revealed no evidence of a palpable mass of the left foot.
In July 2014 (11 years after curettage and cementation), the patient presented to her pain clinic appointment with severe left foot pain. She said that, over a few weeks, she experienced a significant increase in pain and developed posterolateral foot swelling, which limited her ability to ambulate. Plain radiographs showed a significant soft-tissue prominence around the posterior calcaneus, increased lucency around the bone-cement interface in the calcaneus with elevation, and a cortical break of the superior margin of the posterior calcaneus (Figures 3C, 3D). MRI showed a large lobular mass in the calcaneus and surrounding soft tissue with T1 and T2 signal heterogeneity and enhancement after administration of gadolinium (Figures 4A-4D). There was a large extraosseous extension of the calcaneus-based mass laterally and superiorly with edema in the surrounding hindfoot region (Figure 4).
Physical examination revealed exquisite tenderness along the lateral and posterior aspects of the left hindfoot. The patient was unable to bear weight and had soft-tissue swelling throughout the foot and mid calf as well as a palpable mass in the posterior heel. She was otherwise neurovascularly intact through all distributions of the left lower extremity. It was unclear if the GCT of the calcaneus had recurred or if there was a new, secondary tumor. Given her severe pain and morbidity, the patient decided to proceed with open biopsy and a pathology-pending plan for possible amputation in the near future.
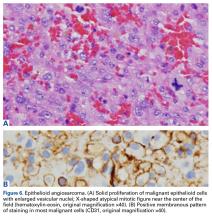
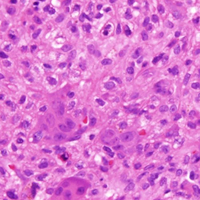
In August 2014, an open biopsy with intraoperative frozen evaluation yielded a diagnosis of malignant neoplasm not otherwise specified. Permanent sections showed a proliferation of malignant epithelioid cells with extensive necrosis, hemorrhage, and hemosiderin deposition but no multinucleated giant cells.
Transformation of the GCT into a high-grade epithelioid angiosarcoma prompted presentation of the patient’s case to a multidisciplinary board of physicians with a focused clinical practice in sarcoma management. The board included board-certified specialists in orthopedic oncology, pathology, musculoskeletal radiology, medical oncology, and radiation oncology. Although discussion included pre-resection use of neoadjuvant chemotherapy to evaluate for disease response, the patient’s severe pain led her to forgo this treatment and proceed directly to below-knee amputation.
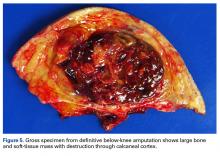
Amputation revealed a 7.7-cm hemorrhagic necrotic mass composed of a highly cellular spindle and epithelioid malignancy with abundant hemosiderin deposition (Figure 5). In addition, several atypical mitotic figures and malignant multinucleated tumor giant cells were randomly scattered throughout the neoplasm.
At first follow-up, the patient reported significant pain relief and asked to begin titrating off her chronic pain medicine. Clinical staging, which involved performing whole-body positron emission tomography/computed tomography, revealed nothing concerning for metastases. When this report was being written, the patient was being monitored for recurrent disease in accordance with National Comprehensive Cancer Network guidelines. In the absence of residual sarcoma, our medical oncology team discussed adjuvant chemotherapy options with her. Subsequently, however, she proceeded only with observation and periodic imaging.
Discussion
Malignant transformation of a benign GCT is extremely rare, especially in cases in which the tumor bed has not previously undergone radiation therapy. Although the literature includes historical case reports, primary and secondary malignant GCTs comprise <9% of all GCTs.11,13,14 Primary bone epithelioid angiosarcoma is also extremely rare, especially in the calcaneus; only 1 case is described in the literature.15 In this article, we report on a benign GCT of bone that transformed into an epithelioid angiosarcoma more than a decade after the GCT was treated with curettage and cementation.
The fact that the malignant areas of a previous tumor may have been missed because of sampling error is important for benign GCT of bone in the early postoperative period, as distinguishing between early malignant transformation and an overlooked malignancy may not be possible. However, transformation is more likely the case when a benign GCT becomes a high-grade malignancy after a long disease-free interval. Several authors have indicated that a benign GCT tumor recurring with a secondary malignancy 2 to 5 years after initial GCT treatment suggests malignant transformation.16 Grote and colleagues10 compiled reports of malignant transformation of GCT of bone and described the clinicopathologic features of secondary malignant transformation of GCTs. The data they compiled and data from several other studies indicate a poor prognosis after malignant transformation of GCT; 4 years after diagnosis, mean survival is 40% to 50%.10,16 The most common clinical presentation of transformation of GCT into malignancy is pain, often with coincident swelling of the native wound bed. However, a few cases have been identified with radiologic imaging alone and without a period of clinical symptoms.16
To our knowledge, this case report is the first to describe a longitudinal assessment of the transformation of a benign GCT of bone into an epithelioid angiosarcoma. Whereas an earlier reported GCT of bone transformed into epithelioid angiosarcoma after irradiation,12 our patient’s GCT of bone transformed without irradiation. GCTs of bone are locally aggressive benign tumors and are relatively rare. Malignant transformation of a benign bone tumor a decade after initial, definitive treatment is concerning, especially given the poor prognosis after malignant transformation in this clinical scenario. Current adjuvant treatments have not changed the prognosis. The literature includes a wide variety of histologic transformations, including high-grade sarcomas, after a long disease-free interval. Although malignant transformation of benign GCTs is rare, clinicians should be aware of the potential. Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation—pain or swelling in the wound bed—and patients should know to immediately inform their physician of any changes in pain level or local wound bed. Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the site of a previously treated GCT of bone with a disease-free interval of several years.
1. Unni KK. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
2. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1-7.
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106-114.
4. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30(6):484-489.
5 Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
6. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242.
7. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;(302):219-230.
8. Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989;(243):208-215.
9. Hutter RV, Worcester JN Jr, Francis KC, Foote FW Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653-690.
10. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169-175.
11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073-1079.
12. Mittal S, Goswami C, Kanoria N, Bhattacharya A. Post-irradiation angiosarcoma of bone. J Cancer Res Ther. 2007;3(2):96-99.
13. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520-2529.
14. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061-1070.
15. Balaji GG, Arockiaraj JS, Roy AC, Deepak B. Primary epithelioid angiosarcoma of the calcaneum: a diagnostic dilemma. J Foot Ankle Surg. 2014;53(2):239-242.
16. Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22(1):19-26.
Take-Home Points
- Malignant transformation of a benign GCT is extremely rare.
- It is difficult to distinguish between an early malignant transformation and an overlooked malignancy.
- The most common clinical presentation of transformation of GCT into malignancy is pain, often with swelling.
- Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation.
- Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the wound site.
Giant cell tumors (GCTs) of bone account for about 5% of all primary bone tumors in adults, with a predominance in the third decade in life.1 Clinically, GCT of bone often presents with pain, pathologic fracture, and/or soft- tissue expansion in the epiphysis of long bones. However, GCT of bone also has been reported in non-long bones, such as the talus and the calcaneus.2,3 Histologically, GCT of bone consists of neoplastic stromal cells, mononuclear histiocytic cells, and multinucleated giant cells that resemble osteoclasts.4 The radiologic appearance of GCT is often described as a lytic, eccentrically located bony lesion that extends near the articular surface in patients with closed physes. Many GCTs have aggressive radiologic features with possible extensive bony destruction and soft-tissue extension.
Although categorized as a benign lesion, GCT can be locally aggressive, with a variable local recurrence rate of 0% to 65%, depending on treatment modality and skeletal location. Given the aggressiveness of GCT of bone, recommendations for operative intervention include intralesional curettage with adjuvant therapy (eg, cryotherapy, phenol, argon beam, electrocautery) and placement of bone void fillers (eg, bone graft polymethylmethacrylate). Wide resection is recommended when the articular surface is no longer viable for reconstruction secondary to extensive destruction. Some authors have reported that surgical margin is the only risk factor in local recurrence,5,6 and thus complete resection may be needed for tumor eradication. In addition, about 3% of GCTs demonstrate benign pulmonary implants, which have been cited as cause of death in 16% to 25% of reported cases of pulmonary spread.7,8
The literature includes few reports of primary or secondary malignant transformation of GCT. Hutter and colleagues9 defined primary malignant GCT as GCT with sarcomatous tissue juxtaposed with zones of typical benign GCT cells. Secondary malignant GCT is a sarcomatous lesion at the site of a previously documented benign GCT. Secondary malignant GCT of bone histologically has been classified as a fibrosarcoma, malignant fibrous histiocytoma, or osteosarcoma transformation.10
Most malignant transformations of GCT of bone have been attributed to previous irradiation of the lesion.11,12 However, there are some case reports of benign bone GCT malignant transformation in situ without any other medical intervention. It was reported that non-radiation-induced secondary transformations occur relatively early after GCT treatment.13 During the early stages of tumor recurrence, however, it is difficult to distinguish between malignant transformation and primary disease overlooked as a result of sampling error.
We report a case of secondary malignant transformation of GCT of bone 11 years after surgical curettage, cryotherapy, and cementation without adjuvant radiation therapy. To our knowledge, this case report is the first to describe transformation of a nonirradiated benign GCT into an aggressive, high-grade epithelioid angiosarcoma, a very rare vascular bone tumor. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In July 2003, a 46-year-old woman presented with left heel pain of several months’ duration. Plain radiographs showed a nonaggressive-appearing lytic lesion of the superior aspect of the posterior calcaneal tuberosity with a small cortical incongruity along the superior margin of the lesion (Figures 1A-1D).
A postoperative splint was placed, and weight-bearing progressed over 6 weeks. The patient was followed at 2- to 3-month intervals over the first 5 postoperative years. She was able to work and perform activities of daily living, but her postoperative course was complicated by significant chronic pain in multiple extremities and long-term treatment by the chronic pain service. At no time did postoperative imaging—magnetic resonance imaging (MRI) at 6 years, whole-body bone scan at 7 years, plain radiographs at 10 years—show evidence of recurrence.
Radiographs showed stable postoperative changes with a small radiolucent area (with sclerotic rim) surrounding the cement-bone interface. Given its proximity to the Achilles tendon and more motion than usual at the wound site, the radiolucency likely was caused by small movements of the interface. The radiolucent area remained stable over a 15-month period.
Whole-body bone scan showed a small area of osteoblastic activity in the left calcaneus, consistent with inflammation surrounding the bone- cement interface, but the uptake was minor relative to other areas of signal, and there were no significant inflammatory reactive changes on MRI (Figures 3A, 3B).
Over 11 years, regular 6- to 12-month follow-up examinations revealed no significant changes in the left foot or in plain radiographs of the chest. In addition, physical examinations revealed no evidence of a palpable mass of the left foot.
In July 2014 (11 years after curettage and cementation), the patient presented to her pain clinic appointment with severe left foot pain. She said that, over a few weeks, she experienced a significant increase in pain and developed posterolateral foot swelling, which limited her ability to ambulate. Plain radiographs showed a significant soft-tissue prominence around the posterior calcaneus, increased lucency around the bone-cement interface in the calcaneus with elevation, and a cortical break of the superior margin of the posterior calcaneus (Figures 3C, 3D). MRI showed a large lobular mass in the calcaneus and surrounding soft tissue with T1 and T2 signal heterogeneity and enhancement after administration of gadolinium (Figures 4A-4D). There was a large extraosseous extension of the calcaneus-based mass laterally and superiorly with edema in the surrounding hindfoot region (Figure 4).
Physical examination revealed exquisite tenderness along the lateral and posterior aspects of the left hindfoot. The patient was unable to bear weight and had soft-tissue swelling throughout the foot and mid calf as well as a palpable mass in the posterior heel. She was otherwise neurovascularly intact through all distributions of the left lower extremity. It was unclear if the GCT of the calcaneus had recurred or if there was a new, secondary tumor. Given her severe pain and morbidity, the patient decided to proceed with open biopsy and a pathology-pending plan for possible amputation in the near future.
In August 2014, an open biopsy with intraoperative frozen evaluation yielded a diagnosis of malignant neoplasm not otherwise specified. Permanent sections showed a proliferation of malignant epithelioid cells with extensive necrosis, hemorrhage, and hemosiderin deposition but no multinucleated giant cells.
Transformation of the GCT into a high-grade epithelioid angiosarcoma prompted presentation of the patient’s case to a multidisciplinary board of physicians with a focused clinical practice in sarcoma management. The board included board-certified specialists in orthopedic oncology, pathology, musculoskeletal radiology, medical oncology, and radiation oncology. Although discussion included pre-resection use of neoadjuvant chemotherapy to evaluate for disease response, the patient’s severe pain led her to forgo this treatment and proceed directly to below-knee amputation.
Amputation revealed a 7.7-cm hemorrhagic necrotic mass composed of a highly cellular spindle and epithelioid malignancy with abundant hemosiderin deposition (Figure 5). In addition, several atypical mitotic figures and malignant multinucleated tumor giant cells were randomly scattered throughout the neoplasm.
At first follow-up, the patient reported significant pain relief and asked to begin titrating off her chronic pain medicine. Clinical staging, which involved performing whole-body positron emission tomography/computed tomography, revealed nothing concerning for metastases. When this report was being written, the patient was being monitored for recurrent disease in accordance with National Comprehensive Cancer Network guidelines. In the absence of residual sarcoma, our medical oncology team discussed adjuvant chemotherapy options with her. Subsequently, however, she proceeded only with observation and periodic imaging.
Discussion
Malignant transformation of a benign GCT is extremely rare, especially in cases in which the tumor bed has not previously undergone radiation therapy. Although the literature includes historical case reports, primary and secondary malignant GCTs comprise <9% of all GCTs.11,13,14 Primary bone epithelioid angiosarcoma is also extremely rare, especially in the calcaneus; only 1 case is described in the literature.15 In this article, we report on a benign GCT of bone that transformed into an epithelioid angiosarcoma more than a decade after the GCT was treated with curettage and cementation.
The fact that the malignant areas of a previous tumor may have been missed because of sampling error is important for benign GCT of bone in the early postoperative period, as distinguishing between early malignant transformation and an overlooked malignancy may not be possible. However, transformation is more likely the case when a benign GCT becomes a high-grade malignancy after a long disease-free interval. Several authors have indicated that a benign GCT tumor recurring with a secondary malignancy 2 to 5 years after initial GCT treatment suggests malignant transformation.16 Grote and colleagues10 compiled reports of malignant transformation of GCT of bone and described the clinicopathologic features of secondary malignant transformation of GCTs. The data they compiled and data from several other studies indicate a poor prognosis after malignant transformation of GCT; 4 years after diagnosis, mean survival is 40% to 50%.10,16 The most common clinical presentation of transformation of GCT into malignancy is pain, often with coincident swelling of the native wound bed. However, a few cases have been identified with radiologic imaging alone and without a period of clinical symptoms.16
To our knowledge, this case report is the first to describe a longitudinal assessment of the transformation of a benign GCT of bone into an epithelioid angiosarcoma. Whereas an earlier reported GCT of bone transformed into epithelioid angiosarcoma after irradiation,12 our patient’s GCT of bone transformed without irradiation. GCTs of bone are locally aggressive benign tumors and are relatively rare. Malignant transformation of a benign bone tumor a decade after initial, definitive treatment is concerning, especially given the poor prognosis after malignant transformation in this clinical scenario. Current adjuvant treatments have not changed the prognosis. The literature includes a wide variety of histologic transformations, including high-grade sarcomas, after a long disease-free interval. Although malignant transformation of benign GCTs is rare, clinicians should be aware of the potential. Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation—pain or swelling in the wound bed—and patients should know to immediately inform their physician of any changes in pain level or local wound bed. Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the site of a previously treated GCT of bone with a disease-free interval of several years.
Take-Home Points
- Malignant transformation of a benign GCT is extremely rare.
- It is difficult to distinguish between an early malignant transformation and an overlooked malignancy.
- The most common clinical presentation of transformation of GCT into malignancy is pain, often with swelling.
- Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation.
- Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the wound site.
Giant cell tumors (GCTs) of bone account for about 5% of all primary bone tumors in adults, with a predominance in the third decade in life.1 Clinically, GCT of bone often presents with pain, pathologic fracture, and/or soft- tissue expansion in the epiphysis of long bones. However, GCT of bone also has been reported in non-long bones, such as the talus and the calcaneus.2,3 Histologically, GCT of bone consists of neoplastic stromal cells, mononuclear histiocytic cells, and multinucleated giant cells that resemble osteoclasts.4 The radiologic appearance of GCT is often described as a lytic, eccentrically located bony lesion that extends near the articular surface in patients with closed physes. Many GCTs have aggressive radiologic features with possible extensive bony destruction and soft-tissue extension.
Although categorized as a benign lesion, GCT can be locally aggressive, with a variable local recurrence rate of 0% to 65%, depending on treatment modality and skeletal location. Given the aggressiveness of GCT of bone, recommendations for operative intervention include intralesional curettage with adjuvant therapy (eg, cryotherapy, phenol, argon beam, electrocautery) and placement of bone void fillers (eg, bone graft polymethylmethacrylate). Wide resection is recommended when the articular surface is no longer viable for reconstruction secondary to extensive destruction. Some authors have reported that surgical margin is the only risk factor in local recurrence,5,6 and thus complete resection may be needed for tumor eradication. In addition, about 3% of GCTs demonstrate benign pulmonary implants, which have been cited as cause of death in 16% to 25% of reported cases of pulmonary spread.7,8
The literature includes few reports of primary or secondary malignant transformation of GCT. Hutter and colleagues9 defined primary malignant GCT as GCT with sarcomatous tissue juxtaposed with zones of typical benign GCT cells. Secondary malignant GCT is a sarcomatous lesion at the site of a previously documented benign GCT. Secondary malignant GCT of bone histologically has been classified as a fibrosarcoma, malignant fibrous histiocytoma, or osteosarcoma transformation.10
Most malignant transformations of GCT of bone have been attributed to previous irradiation of the lesion.11,12 However, there are some case reports of benign bone GCT malignant transformation in situ without any other medical intervention. It was reported that non-radiation-induced secondary transformations occur relatively early after GCT treatment.13 During the early stages of tumor recurrence, however, it is difficult to distinguish between malignant transformation and primary disease overlooked as a result of sampling error.
We report a case of secondary malignant transformation of GCT of bone 11 years after surgical curettage, cryotherapy, and cementation without adjuvant radiation therapy. To our knowledge, this case report is the first to describe transformation of a nonirradiated benign GCT into an aggressive, high-grade epithelioid angiosarcoma, a very rare vascular bone tumor. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In July 2003, a 46-year-old woman presented with left heel pain of several months’ duration. Plain radiographs showed a nonaggressive-appearing lytic lesion of the superior aspect of the posterior calcaneal tuberosity with a small cortical incongruity along the superior margin of the lesion (Figures 1A-1D).
A postoperative splint was placed, and weight-bearing progressed over 6 weeks. The patient was followed at 2- to 3-month intervals over the first 5 postoperative years. She was able to work and perform activities of daily living, but her postoperative course was complicated by significant chronic pain in multiple extremities and long-term treatment by the chronic pain service. At no time did postoperative imaging—magnetic resonance imaging (MRI) at 6 years, whole-body bone scan at 7 years, plain radiographs at 10 years—show evidence of recurrence.
Radiographs showed stable postoperative changes with a small radiolucent area (with sclerotic rim) surrounding the cement-bone interface. Given its proximity to the Achilles tendon and more motion than usual at the wound site, the radiolucency likely was caused by small movements of the interface. The radiolucent area remained stable over a 15-month period.
Whole-body bone scan showed a small area of osteoblastic activity in the left calcaneus, consistent with inflammation surrounding the bone- cement interface, but the uptake was minor relative to other areas of signal, and there were no significant inflammatory reactive changes on MRI (Figures 3A, 3B).
Over 11 years, regular 6- to 12-month follow-up examinations revealed no significant changes in the left foot or in plain radiographs of the chest. In addition, physical examinations revealed no evidence of a palpable mass of the left foot.
In July 2014 (11 years after curettage and cementation), the patient presented to her pain clinic appointment with severe left foot pain. She said that, over a few weeks, she experienced a significant increase in pain and developed posterolateral foot swelling, which limited her ability to ambulate. Plain radiographs showed a significant soft-tissue prominence around the posterior calcaneus, increased lucency around the bone-cement interface in the calcaneus with elevation, and a cortical break of the superior margin of the posterior calcaneus (Figures 3C, 3D). MRI showed a large lobular mass in the calcaneus and surrounding soft tissue with T1 and T2 signal heterogeneity and enhancement after administration of gadolinium (Figures 4A-4D). There was a large extraosseous extension of the calcaneus-based mass laterally and superiorly with edema in the surrounding hindfoot region (Figure 4).
Physical examination revealed exquisite tenderness along the lateral and posterior aspects of the left hindfoot. The patient was unable to bear weight and had soft-tissue swelling throughout the foot and mid calf as well as a palpable mass in the posterior heel. She was otherwise neurovascularly intact through all distributions of the left lower extremity. It was unclear if the GCT of the calcaneus had recurred or if there was a new, secondary tumor. Given her severe pain and morbidity, the patient decided to proceed with open biopsy and a pathology-pending plan for possible amputation in the near future.
In August 2014, an open biopsy with intraoperative frozen evaluation yielded a diagnosis of malignant neoplasm not otherwise specified. Permanent sections showed a proliferation of malignant epithelioid cells with extensive necrosis, hemorrhage, and hemosiderin deposition but no multinucleated giant cells.
Transformation of the GCT into a high-grade epithelioid angiosarcoma prompted presentation of the patient’s case to a multidisciplinary board of physicians with a focused clinical practice in sarcoma management. The board included board-certified specialists in orthopedic oncology, pathology, musculoskeletal radiology, medical oncology, and radiation oncology. Although discussion included pre-resection use of neoadjuvant chemotherapy to evaluate for disease response, the patient’s severe pain led her to forgo this treatment and proceed directly to below-knee amputation.
Amputation revealed a 7.7-cm hemorrhagic necrotic mass composed of a highly cellular spindle and epithelioid malignancy with abundant hemosiderin deposition (Figure 5). In addition, several atypical mitotic figures and malignant multinucleated tumor giant cells were randomly scattered throughout the neoplasm.
At first follow-up, the patient reported significant pain relief and asked to begin titrating off her chronic pain medicine. Clinical staging, which involved performing whole-body positron emission tomography/computed tomography, revealed nothing concerning for metastases. When this report was being written, the patient was being monitored for recurrent disease in accordance with National Comprehensive Cancer Network guidelines. In the absence of residual sarcoma, our medical oncology team discussed adjuvant chemotherapy options with her. Subsequently, however, she proceeded only with observation and periodic imaging.
Discussion
Malignant transformation of a benign GCT is extremely rare, especially in cases in which the tumor bed has not previously undergone radiation therapy. Although the literature includes historical case reports, primary and secondary malignant GCTs comprise <9% of all GCTs.11,13,14 Primary bone epithelioid angiosarcoma is also extremely rare, especially in the calcaneus; only 1 case is described in the literature.15 In this article, we report on a benign GCT of bone that transformed into an epithelioid angiosarcoma more than a decade after the GCT was treated with curettage and cementation.
The fact that the malignant areas of a previous tumor may have been missed because of sampling error is important for benign GCT of bone in the early postoperative period, as distinguishing between early malignant transformation and an overlooked malignancy may not be possible. However, transformation is more likely the case when a benign GCT becomes a high-grade malignancy after a long disease-free interval. Several authors have indicated that a benign GCT tumor recurring with a secondary malignancy 2 to 5 years after initial GCT treatment suggests malignant transformation.16 Grote and colleagues10 compiled reports of malignant transformation of GCT of bone and described the clinicopathologic features of secondary malignant transformation of GCTs. The data they compiled and data from several other studies indicate a poor prognosis after malignant transformation of GCT; 4 years after diagnosis, mean survival is 40% to 50%.10,16 The most common clinical presentation of transformation of GCT into malignancy is pain, often with coincident swelling of the native wound bed. However, a few cases have been identified with radiologic imaging alone and without a period of clinical symptoms.16
To our knowledge, this case report is the first to describe a longitudinal assessment of the transformation of a benign GCT of bone into an epithelioid angiosarcoma. Whereas an earlier reported GCT of bone transformed into epithelioid angiosarcoma after irradiation,12 our patient’s GCT of bone transformed without irradiation. GCTs of bone are locally aggressive benign tumors and are relatively rare. Malignant transformation of a benign bone tumor a decade after initial, definitive treatment is concerning, especially given the poor prognosis after malignant transformation in this clinical scenario. Current adjuvant treatments have not changed the prognosis. The literature includes a wide variety of histologic transformations, including high-grade sarcomas, after a long disease-free interval. Although malignant transformation of benign GCTs is rare, clinicians should be aware of the potential. Interval monitoring of GCTs may be necessary in patients with symptoms concerning for malignant transformation—pain or swelling in the wound bed—and patients should know to immediately inform their physician of any changes in pain level or local wound bed. Clinicians should maintain a high clinical suspicion for malignant transformation or late recurrence of GCT in a patient with new pain at the site of a previously treated GCT of bone with a disease-free interval of several years.
1. Unni KK. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
2. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1-7.
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106-114.
4. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30(6):484-489.
5 Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
6. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242.
7. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;(302):219-230.
8. Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989;(243):208-215.
9. Hutter RV, Worcester JN Jr, Francis KC, Foote FW Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653-690.
10. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169-175.
11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073-1079.
12. Mittal S, Goswami C, Kanoria N, Bhattacharya A. Post-irradiation angiosarcoma of bone. J Cancer Res Ther. 2007;3(2):96-99.
13. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520-2529.
14. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061-1070.
15. Balaji GG, Arockiaraj JS, Roy AC, Deepak B. Primary epithelioid angiosarcoma of the calcaneum: a diagnostic dilemma. J Foot Ankle Surg. 2014;53(2):239-242.
16. Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22(1):19-26.
1. Unni KK. Dahlin’s Bone Tumors: General Aspects and Data on 11,087 Cases. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
2. Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010;36(1):1-7.
3. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106-114.
4. Werner M. Giant cell tumour of bone: morphological, biological and histogenetical aspects. Int Orthop. 2006;30(6):484-489.
5 Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187.
6. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242.
7. Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone. Six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;(302):219-230.
8. Maloney WJ, Vaughan LM, Jones HH, Ross J, Nagel DA. Benign metastasizing giant-cell tumor of bone. Report of three cases and review of the literature. Clin Orthop Relat Res. 1989;(243):208-215.
9. Hutter RV, Worcester JN Jr, Francis KC, Foote FW Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653-690.
10. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169-175.
11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073-1079.
12. Mittal S, Goswami C, Kanoria N, Bhattacharya A. Post-irradiation angiosarcoma of bone. J Cancer Res Ther. 2007;3(2):96-99.
13. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520-2529.
14. Dahlin DC, Cupps RE, Johnson EW Jr. Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061-1070.
15. Balaji GG, Arockiaraj JS, Roy AC, Deepak B. Primary epithelioid angiosarcoma of the calcaneum: a diagnostic dilemma. J Foot Ankle Surg. 2014;53(2):239-242.
16. Anract P, De Pinieux G, Cottias P, Pouillart P, Forest M, Tomeno B. Malignant giant-cell tumours of bone. Clinico-pathological types and prognosis: a review of 29 cases. Int Orthop. 1998;22(1):19-26.
Adopting the patient’s perspective
Editor’s note: “Everything We Say and Do” provides readers with thoughtful and actionable communication tactics that can positively impact patients’ experience of care. In the current series of columns, physicians share how their experiences as patients have shaped their professional approach.
I have been fortunate to have had very few major health issues throughout my life. I have, however, had three major surgical procedures in the last 10 years – two total hip arthroplasties and a cataract removal with lens implant in between. The most recent THA was October 2017. Going through each procedure helped me see things from a patient’s perspective, and that showed me how important little things are to a patient, things which we may not think are all that big a deal as a provider.
Almost all of the medical personnel who came to care for me during my stays identified themselves and why they were there, and that made me feel comfortable, knowing who they were and their role. However, there were a few who did not do this, and that made me uncomfortable, not knowing who they were and why they were in my room. Not knowing is an uncomfortable feeling for a patient.
Almost every registered nurse who came to me with medication explained what the medicine was and why they were administering it, with the exception of one preop RN I met before to my cataract procedure. She walked up to me, told me to open my eye wide, held the affected eye open, and started dripping cold drops into my eye without explanation. She then said she would be back every 10 minutes to repeat the process. I had to inquire as to what the medication was and why there was a need for this process. It was a jolting experience, and she showed no compassion toward me as a patient or a person, even after I inquired.
This was not a good experience. Although cataract surgery was a totally new experience for me, she had obviously done this many times before and had to do it many times that day. However, she acted as if I should have known what she was going to do and as if she need not explain herself to anyone – which she did not, even after being queried.
Everyone during the admission process for all three procedures was solicitous and warm except for one person. Unfortunately, this individual was the first person to greet my wife and me when we arrived for my last total hip arthroplasty. She was seated at the welcome desk with her head down. After we arrived, she kept her head down and asked “How can I help you?” without ever looking up. I did not realize how unwelcome I would feel when the first person I encountered in the surgical preop admissions area failed to make eye contact with me. Her demeanor was nice enough, but she did not even attempt to make a personal connection with me – and she was at the welcome desk!
Overall, I had tremendously good experiences at three facilities in three different parts of the United States, but as we all know, it is the things that do not go well that stand out. I choose to use those things, along with some of the good things, as “reinforcers” for many of the patient-experience behaviors we identify as best practices.
What I say and do
During each patient encounter, I make eye contact with the patient and each person in the room and identify who I am and why I am there. I sit down during each visit unless there is simply no place for me to do so. I explain the procedures that are to take place, set expectations for those procedures, and then use “teachback” to ensure that my discussion with the patient has been effective. Setting expectations is very important to me: If you do not ensure that patients have appropriate expectations, their expectations will never be met and they will never have a good experience. I explain any new medication I am ordering, what it is for, and any possible significant side effects and again use teachback. The last thing I do is ask “What questions do you have for me today?” giving the patient permission to have questions, and then I respond to those questions with plain talk and teachback.
Why I do it
Not knowing what was going on and feeling marginalized were the most uncomfortable things I experienced as a patient. Using best practices for patient experience shows courtesy and respect. These practices show a willingness to take time with the patient and demonstrate my concern that I am effectively communicating my message for that visit. All of these behaviors decrease uncertainty and/or raise the patient’s feelings of importance, thereby decreasing marginalization.
How I do it
I remind myself each day I am on a clinical shift that my goal is to treat each patient like I would want my family (or myself) to be treated, and then I go out and do it. After “forcing” myself to put these behaviors into my rounding routine, they have become second nature, and I feel better for providing this level of care because it made me feel so good when I was cared for in this manner.
Dr. Sharp is chief hospitalist with Sound Physicians at University of Florida Health in Jacksonville, Fla.
Editor’s note: “Everything We Say and Do” provides readers with thoughtful and actionable communication tactics that can positively impact patients’ experience of care. In the current series of columns, physicians share how their experiences as patients have shaped their professional approach.
I have been fortunate to have had very few major health issues throughout my life. I have, however, had three major surgical procedures in the last 10 years – two total hip arthroplasties and a cataract removal with lens implant in between. The most recent THA was October 2017. Going through each procedure helped me see things from a patient’s perspective, and that showed me how important little things are to a patient, things which we may not think are all that big a deal as a provider.
Almost all of the medical personnel who came to care for me during my stays identified themselves and why they were there, and that made me feel comfortable, knowing who they were and their role. However, there were a few who did not do this, and that made me uncomfortable, not knowing who they were and why they were in my room. Not knowing is an uncomfortable feeling for a patient.
Almost every registered nurse who came to me with medication explained what the medicine was and why they were administering it, with the exception of one preop RN I met before to my cataract procedure. She walked up to me, told me to open my eye wide, held the affected eye open, and started dripping cold drops into my eye without explanation. She then said she would be back every 10 minutes to repeat the process. I had to inquire as to what the medication was and why there was a need for this process. It was a jolting experience, and she showed no compassion toward me as a patient or a person, even after I inquired.
This was not a good experience. Although cataract surgery was a totally new experience for me, she had obviously done this many times before and had to do it many times that day. However, she acted as if I should have known what she was going to do and as if she need not explain herself to anyone – which she did not, even after being queried.
Everyone during the admission process for all three procedures was solicitous and warm except for one person. Unfortunately, this individual was the first person to greet my wife and me when we arrived for my last total hip arthroplasty. She was seated at the welcome desk with her head down. After we arrived, she kept her head down and asked “How can I help you?” without ever looking up. I did not realize how unwelcome I would feel when the first person I encountered in the surgical preop admissions area failed to make eye contact with me. Her demeanor was nice enough, but she did not even attempt to make a personal connection with me – and she was at the welcome desk!
Overall, I had tremendously good experiences at three facilities in three different parts of the United States, but as we all know, it is the things that do not go well that stand out. I choose to use those things, along with some of the good things, as “reinforcers” for many of the patient-experience behaviors we identify as best practices.
What I say and do
During each patient encounter, I make eye contact with the patient and each person in the room and identify who I am and why I am there. I sit down during each visit unless there is simply no place for me to do so. I explain the procedures that are to take place, set expectations for those procedures, and then use “teachback” to ensure that my discussion with the patient has been effective. Setting expectations is very important to me: If you do not ensure that patients have appropriate expectations, their expectations will never be met and they will never have a good experience. I explain any new medication I am ordering, what it is for, and any possible significant side effects and again use teachback. The last thing I do is ask “What questions do you have for me today?” giving the patient permission to have questions, and then I respond to those questions with plain talk and teachback.
Why I do it
Not knowing what was going on and feeling marginalized were the most uncomfortable things I experienced as a patient. Using best practices for patient experience shows courtesy and respect. These practices show a willingness to take time with the patient and demonstrate my concern that I am effectively communicating my message for that visit. All of these behaviors decrease uncertainty and/or raise the patient’s feelings of importance, thereby decreasing marginalization.
How I do it
I remind myself each day I am on a clinical shift that my goal is to treat each patient like I would want my family (or myself) to be treated, and then I go out and do it. After “forcing” myself to put these behaviors into my rounding routine, they have become second nature, and I feel better for providing this level of care because it made me feel so good when I was cared for in this manner.
Dr. Sharp is chief hospitalist with Sound Physicians at University of Florida Health in Jacksonville, Fla.
Editor’s note: “Everything We Say and Do” provides readers with thoughtful and actionable communication tactics that can positively impact patients’ experience of care. In the current series of columns, physicians share how their experiences as patients have shaped their professional approach.
I have been fortunate to have had very few major health issues throughout my life. I have, however, had three major surgical procedures in the last 10 years – two total hip arthroplasties and a cataract removal with lens implant in between. The most recent THA was October 2017. Going through each procedure helped me see things from a patient’s perspective, and that showed me how important little things are to a patient, things which we may not think are all that big a deal as a provider.
Almost all of the medical personnel who came to care for me during my stays identified themselves and why they were there, and that made me feel comfortable, knowing who they were and their role. However, there were a few who did not do this, and that made me uncomfortable, not knowing who they were and why they were in my room. Not knowing is an uncomfortable feeling for a patient.
Almost every registered nurse who came to me with medication explained what the medicine was and why they were administering it, with the exception of one preop RN I met before to my cataract procedure. She walked up to me, told me to open my eye wide, held the affected eye open, and started dripping cold drops into my eye without explanation. She then said she would be back every 10 minutes to repeat the process. I had to inquire as to what the medication was and why there was a need for this process. It was a jolting experience, and she showed no compassion toward me as a patient or a person, even after I inquired.
This was not a good experience. Although cataract surgery was a totally new experience for me, she had obviously done this many times before and had to do it many times that day. However, she acted as if I should have known what she was going to do and as if she need not explain herself to anyone – which she did not, even after being queried.
Everyone during the admission process for all three procedures was solicitous and warm except for one person. Unfortunately, this individual was the first person to greet my wife and me when we arrived for my last total hip arthroplasty. She was seated at the welcome desk with her head down. After we arrived, she kept her head down and asked “How can I help you?” without ever looking up. I did not realize how unwelcome I would feel when the first person I encountered in the surgical preop admissions area failed to make eye contact with me. Her demeanor was nice enough, but she did not even attempt to make a personal connection with me – and she was at the welcome desk!
Overall, I had tremendously good experiences at three facilities in three different parts of the United States, but as we all know, it is the things that do not go well that stand out. I choose to use those things, along with some of the good things, as “reinforcers” for many of the patient-experience behaviors we identify as best practices.
What I say and do
During each patient encounter, I make eye contact with the patient and each person in the room and identify who I am and why I am there. I sit down during each visit unless there is simply no place for me to do so. I explain the procedures that are to take place, set expectations for those procedures, and then use “teachback” to ensure that my discussion with the patient has been effective. Setting expectations is very important to me: If you do not ensure that patients have appropriate expectations, their expectations will never be met and they will never have a good experience. I explain any new medication I am ordering, what it is for, and any possible significant side effects and again use teachback. The last thing I do is ask “What questions do you have for me today?” giving the patient permission to have questions, and then I respond to those questions with plain talk and teachback.
Why I do it
Not knowing what was going on and feeling marginalized were the most uncomfortable things I experienced as a patient. Using best practices for patient experience shows courtesy and respect. These practices show a willingness to take time with the patient and demonstrate my concern that I am effectively communicating my message for that visit. All of these behaviors decrease uncertainty and/or raise the patient’s feelings of importance, thereby decreasing marginalization.
How I do it
I remind myself each day I am on a clinical shift that my goal is to treat each patient like I would want my family (or myself) to be treated, and then I go out and do it. After “forcing” myself to put these behaviors into my rounding routine, they have become second nature, and I feel better for providing this level of care because it made me feel so good when I was cared for in this manner.
Dr. Sharp is chief hospitalist with Sound Physicians at University of Florida Health in Jacksonville, Fla.
Total Knee Arthroplasty Performed With Long-Acting Liposomal Bupivacaine Versus Femoral Nerve Catheter
Take-Home Points
- At our institution, LALB has shortened our hospital stay.
- There is a trend towards decreased opioid consumption with LALB.
- With the opioid epidemic we face today, LALB can be one of many options in our toolbox towards a solution.
- As stated in prior publications, the effectiveness of LALB is definitely technique dependent.
- Additional clinical studies are warranted to better determine the efficacy and cost-effectiveness of LALB.
Almost 1 million total knee arthroplasties (TKAs) are performed in the United States each year, and the number continues to grow.1.2 For patients about to undergo TKA, a significant concern is postoperative pain.3 Fear of postoperative pain is often cited as a reason for delaying surgery.3 Recent literature suggests that patients with poor pain management during the first 48 hours after surgery have a 50% chance of gaining satisfactory long-term pain relief.4 In addition, inadequate postoperative pain management can interfere with participation in and progression of physical rehabilitation, prolong hospital stay, and increase patient dissatisfaction.5 Poorly controlled pain results in decreased range of motion (ROM), strength, stability, and ambulation thereby prolongs hospital stays, and increases costs and overall dissatisfaction with the procedure.
Post-TKA pain management has received much attention in recent years. A multimodal pain management protocol is now a key component of clinical pathways in TKA. Appropriate postoperative pain control lowers postoperative complications and accelerates recovery.6 Pain-caused loss of function makes surgical patients more susceptible to edema, deep vein thrombosis, and pulmonary embolism.4 Various oral and intravenous medications are used to lessen the pain response during the perioperative period. In addition, regional or neuraxial anesthesia is often added to blunt the immediate surgical pain response.7,8 At our institution, TKA traditionally has been performed with femoral nerve catheters (FNCs) for postoperative pain control. Although effective, this method often results in decreased quadriceps musculature function, which delays rehabilitation and increases the fall risk. Recently, there has been a shift toward using local anesthetic infusions about the knee to provide adequate pain relief and restore motor function, which is often sacrificed with use of regional nerve blocks and continuous catheter infusions.9
Many institutions have started using a new long-acting local anesthetic in their multimodal pain management pathways: Exparel (Pacira Pharmaceuticals), a liposomal membrane-bound bupivacaine with sustained release of approximately 72 hours. Several studies have verified the safety of this medication.10 A systemic review of prospective studies revealed that, compared with bupivacaine, long-acting liposomal bupivacaine (LALB) in therapeutic doses had a higher safety margin and a favorable safety profile.10 However, no study has compared the effectiveness of LALB and FNC in a matched TKA cohort with each patient serving as his or her own control.
We recently reviewed our multimodal pain management protocol for any areas in need of improvement and decided to compare the effects of the indwelling FNC protocol that was in use with the effects of injecting the local anesthetic LALB. We conducted a study to compare the 2 methods with respect to pain control, ROM, ability to ambulate, and hospital length of stay (LOS). We hypothesized that the longer acting local anesthetic would provide comparable post-TKA pain control and post-TKA opioid use but would accelerate post-TKA rehabilitation.
Materials and Methods
This retrospective, longitudinal, repeated- measures study was approved by the Greenville Hospital System Institutional Review Board and conducted at the Steadman Hawkins Clinic of the Carolinas, Greenville Health System.
Interventions
Twenty-three patients underwent separately staged bilateral TKAs between 2010 and 2013. For each TKA, a Genesis II implant (Smith & Nephew) was used, and the surgery was performed with the patient under spinal anesthesia. In each case, FNC was used for pain control after the first TKA, and periarticular injection (PAI) of LALB for pain control after the second TKA.
In the first TKAs, FNC-administered ropivacaine 0.2% (2 mg/mL) was maintained at a standard basal rate of 8 mL/h for 48 hours. In the second TKAs, LALB was administered along with bupivacaine/epinephrine. Twenty milliliters of LALB from a single-use vial was diluted in 40 mL of normal (0.9%) saline to obtain a 60-mL solution, and a 25-gauge needle was used to inject this solution into the periarticular soft tissues; another needle was used for PAI of 30 mL of bupivacaine 0.25% with epinephrine.
Continuous passive motion devices were not used. Most patients began therapy on day of surgery. Knee immobilizers were not used in the FNC group.
The same standardized multimodal pain management protocol was used for all TKAs. Non- narcotic medications, including acetaminophen, ketorolac, and celecoxib, were given on a scheduled basis. Tramadol and opioid medications were administered as needed for pain. The attending physician based patient discharge timing on pain control, ability to safely ambulate, and absence of complications.
Outcome Measures
Outcome measures were LOS; extension and flexion at discharge and 3-week follow-up; total ROM (extension plus flexion) at discharge and 3-week follow-up; per-day and total hospital stay morphine -equivalent doses (MEDs); and per-attempt walking distance during gait training.
ROM was measured with a standard goniometer. Flexion was tested with the patient supine and the hip and knee in neutral rotation. The goniometer axis was along the lateral epicondyle of the femur with the proximal arm of the goniometer parallel to the long axis of the femur and pointing at the greater trochanter and with the distal arm parallel to the long axis of the fibula and pointing at the lateral malleolus. The patient was instructed to flex the hip and knee by moving the heel toward the buttock. Expected normal ROM is 135°. The same landmarks were used for extension. The patient was instructed to push the back of the knee toward the plinth/bed, for maximal active extension. The same ROM assessment strategy was used during the hospitalization and at the 3-week follow-up.
Several opioid medications (eg, hydrocodone, oxycodone, tramadol, hydromorphone, morphine) with different dosages were used during hospitalization. Opioid doses were converted to MEDs to permit FNC–LALB comparisons. For each patient, total MEDs were divided by LOS to determine MEDs per day.
Mean per-attempt walking distance was calculated by dividing the total distance walked during hospitalization—the sum of the number of feet walked during each and every attempt, as measured by the treating physical therapist—by the total number of walking attempts.
Data Analysis
A paired-samples t test was used to calculate differences between all outcome measures: LOS; extension and flexion at discharge and 3-month follow-up; per-day and total MEDs; and mean per-attempt walking distance. P < .05 was considered significant. We elected not to adjust our α for a potential familywise error.
Results
Of the 23 patients, 14 were female and 9 were male, and 19 were white and 4 were black. Mean (SD) age was 64.4 (6.4) years for the FNC group and 66.0 (6.0) years for the LALB group. The age difference was not statistically significant.
Discussion
Poor pain control during the post-TKA period may have a significant impact on recovery rate, standard of living, psychological health, and postoperative complications.10 Inadequate postoperative pain control increases postoperative morbidity, hinders physiotherapy, increases anxiety, disrupts sleep patterns, and decreases patient satisfaction.9 There has been increased interest in PAIs. Local anesthetics are additional sources of pain control at surgical sites. However, the half-life of most local anesthetics is short. Soft-tissue infiltration of LALB into a surgical site extends the duration of active analgesia. Our study found that, compared with patients who received FNC, patients who received LALB had comparable pain control, improved knee ROM, and shorter hospital stays. In addition, the LALB group had no reports of quadriceps weakness or falls, both of which are associated with femoral nerve blocks. The FNC group had no reported falls, either. PAIs have the benefit of avoiding the invasiveness of femoral nerve blocks and possible neuritis.
Many complications are associated with or indirectly related to delayed rehabilitation and immobility during the acute post-TKA period. From prolonged hospitalization to need for manipulation, the consequences of inadequate pain control and decreased function can be numerous and costly for patients and the healthcare system. In the present study, LALB use led to a statistically significant overall decrease in mean LOS (LALB group, 2.3 days; FNC, 2.8 days). With LALB, there was a higher likelihood of discharge the day after surgery; 20% of patients in the LALB group and no patients in the FNC group went home that day.
The implication is that inadequate pain control led to decreased motion and decreased progression during postoperative rehabilitation. Local infiltration resulted in increased total ROM (extension plus flexion) at 3-week follow-up (LALB, 116.3°; FNC, 107.2°). In addition, there was an increase in walking distance per day of hospital stay (LALB, 135.9 feet; FNC, 84.2 feet). Furthermore, patients indicated LALB when asked which anesthetic they preferred. To our knowledge, this is the first study to compare LALB and FNC data in a matched TKA cohort with each patient serving as his or her own control.
Our study had several limitations. First was the retrospective design. Second was the small sample size, which made definitive conclusions difficult. However, the statistically significant differences we noted validated our conclusions. A statistically significant difference favoring LALB over FNC was found for total MEDs during hospitalization, but there was no significant difference in per-day MEDs. A possible reason for this difference is that LALB patients had shorter hospital stays, and therefore received fewer doses overall. Another possible reason is the small sample size; whereas a larger study using our protocol may find a statistically significant difference between LALB and FNC, we found only a trend. In the FNC group, anesthetic infiltration occurred with use of a computerized pump, which was removed on postoperative day 2; most of these patients were discharged home that day or the morning of postoperative day 3. As it is possible that some of these patients could have gone home sooner, our LOS data may have been affected. We do not consider this limitation significant, as one of our discharge criteria was 150 feet of ambulation, and most patients who received FNCs could not ambulate that far until after FNC removal. Furthermore, this study compared LALB only with FNC. It is possible that our improved outcomes could have resulted from the PAIs themselves, irrespective of LALB. In a recent TKA study by Bagsby and colleagues,11 pain was controlled better with the less expensive traditional PAI of ropivacaine, epinephrine, and morphine than with the PAI of liposomal bupivacaine. Last, in our study, the experience of undergoing the first TKA may have increased patients’ confidence going into the second TKA and then helped them make faster progress in rehabilitation. Regardless, the promising results of our study and the firsthand use of LALB at our institution led us to modify our intraoperative pain management protocol for surgeons who perform TKA.
As we continue to use LALB, our study numbers will increase, and we may discover other factors that, though now underpowered, will prove to be statistically significant. Additional clinical studies are needed to better determine the efficacy and cost-effectiveness of LALB and other long-acting local anesthetic formulations.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Ruiz D Jr, Koenig L, Dall TM, et al. The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J Bone Joint Surg Am. 2013;95(16):1473-1480.
3. Trousdale RT, McGrory BJ, Berry DJ, Becker MW, Harmsen WS. Patients’ concerns prior to undergoing total hip and total knee arthroplasty. Mayo Clin Proc. 1999;74(10):978-982.
4. Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses, Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2008:469-497.
5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287-333.
6. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084.
7. Stein BE, Srikumaran U, Tan EW, Freehill MT, Wilckens JH. Lower-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(22):e167.
8. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
9. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
10. Portillo J, Kamar N, Melibary S, Quevedo E, Bergese S. Safety of liposome extended-release bupivacaine for postoperative pain control. Front Pharmacol. 2014;5:90.
11. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
Take-Home Points
- At our institution, LALB has shortened our hospital stay.
- There is a trend towards decreased opioid consumption with LALB.
- With the opioid epidemic we face today, LALB can be one of many options in our toolbox towards a solution.
- As stated in prior publications, the effectiveness of LALB is definitely technique dependent.
- Additional clinical studies are warranted to better determine the efficacy and cost-effectiveness of LALB.
Almost 1 million total knee arthroplasties (TKAs) are performed in the United States each year, and the number continues to grow.1.2 For patients about to undergo TKA, a significant concern is postoperative pain.3 Fear of postoperative pain is often cited as a reason for delaying surgery.3 Recent literature suggests that patients with poor pain management during the first 48 hours after surgery have a 50% chance of gaining satisfactory long-term pain relief.4 In addition, inadequate postoperative pain management can interfere with participation in and progression of physical rehabilitation, prolong hospital stay, and increase patient dissatisfaction.5 Poorly controlled pain results in decreased range of motion (ROM), strength, stability, and ambulation thereby prolongs hospital stays, and increases costs and overall dissatisfaction with the procedure.
Post-TKA pain management has received much attention in recent years. A multimodal pain management protocol is now a key component of clinical pathways in TKA. Appropriate postoperative pain control lowers postoperative complications and accelerates recovery.6 Pain-caused loss of function makes surgical patients more susceptible to edema, deep vein thrombosis, and pulmonary embolism.4 Various oral and intravenous medications are used to lessen the pain response during the perioperative period. In addition, regional or neuraxial anesthesia is often added to blunt the immediate surgical pain response.7,8 At our institution, TKA traditionally has been performed with femoral nerve catheters (FNCs) for postoperative pain control. Although effective, this method often results in decreased quadriceps musculature function, which delays rehabilitation and increases the fall risk. Recently, there has been a shift toward using local anesthetic infusions about the knee to provide adequate pain relief and restore motor function, which is often sacrificed with use of regional nerve blocks and continuous catheter infusions.9
Many institutions have started using a new long-acting local anesthetic in their multimodal pain management pathways: Exparel (Pacira Pharmaceuticals), a liposomal membrane-bound bupivacaine with sustained release of approximately 72 hours. Several studies have verified the safety of this medication.10 A systemic review of prospective studies revealed that, compared with bupivacaine, long-acting liposomal bupivacaine (LALB) in therapeutic doses had a higher safety margin and a favorable safety profile.10 However, no study has compared the effectiveness of LALB and FNC in a matched TKA cohort with each patient serving as his or her own control.
We recently reviewed our multimodal pain management protocol for any areas in need of improvement and decided to compare the effects of the indwelling FNC protocol that was in use with the effects of injecting the local anesthetic LALB. We conducted a study to compare the 2 methods with respect to pain control, ROM, ability to ambulate, and hospital length of stay (LOS). We hypothesized that the longer acting local anesthetic would provide comparable post-TKA pain control and post-TKA opioid use but would accelerate post-TKA rehabilitation.
Materials and Methods
This retrospective, longitudinal, repeated- measures study was approved by the Greenville Hospital System Institutional Review Board and conducted at the Steadman Hawkins Clinic of the Carolinas, Greenville Health System.
Interventions
Twenty-three patients underwent separately staged bilateral TKAs between 2010 and 2013. For each TKA, a Genesis II implant (Smith & Nephew) was used, and the surgery was performed with the patient under spinal anesthesia. In each case, FNC was used for pain control after the first TKA, and periarticular injection (PAI) of LALB for pain control after the second TKA.
In the first TKAs, FNC-administered ropivacaine 0.2% (2 mg/mL) was maintained at a standard basal rate of 8 mL/h for 48 hours. In the second TKAs, LALB was administered along with bupivacaine/epinephrine. Twenty milliliters of LALB from a single-use vial was diluted in 40 mL of normal (0.9%) saline to obtain a 60-mL solution, and a 25-gauge needle was used to inject this solution into the periarticular soft tissues; another needle was used for PAI of 30 mL of bupivacaine 0.25% with epinephrine.
Continuous passive motion devices were not used. Most patients began therapy on day of surgery. Knee immobilizers were not used in the FNC group.
The same standardized multimodal pain management protocol was used for all TKAs. Non- narcotic medications, including acetaminophen, ketorolac, and celecoxib, were given on a scheduled basis. Tramadol and opioid medications were administered as needed for pain. The attending physician based patient discharge timing on pain control, ability to safely ambulate, and absence of complications.
Outcome Measures
Outcome measures were LOS; extension and flexion at discharge and 3-week follow-up; total ROM (extension plus flexion) at discharge and 3-week follow-up; per-day and total hospital stay morphine -equivalent doses (MEDs); and per-attempt walking distance during gait training.
ROM was measured with a standard goniometer. Flexion was tested with the patient supine and the hip and knee in neutral rotation. The goniometer axis was along the lateral epicondyle of the femur with the proximal arm of the goniometer parallel to the long axis of the femur and pointing at the greater trochanter and with the distal arm parallel to the long axis of the fibula and pointing at the lateral malleolus. The patient was instructed to flex the hip and knee by moving the heel toward the buttock. Expected normal ROM is 135°. The same landmarks were used for extension. The patient was instructed to push the back of the knee toward the plinth/bed, for maximal active extension. The same ROM assessment strategy was used during the hospitalization and at the 3-week follow-up.
Several opioid medications (eg, hydrocodone, oxycodone, tramadol, hydromorphone, morphine) with different dosages were used during hospitalization. Opioid doses were converted to MEDs to permit FNC–LALB comparisons. For each patient, total MEDs were divided by LOS to determine MEDs per day.
Mean per-attempt walking distance was calculated by dividing the total distance walked during hospitalization—the sum of the number of feet walked during each and every attempt, as measured by the treating physical therapist—by the total number of walking attempts.
Data Analysis
A paired-samples t test was used to calculate differences between all outcome measures: LOS; extension and flexion at discharge and 3-month follow-up; per-day and total MEDs; and mean per-attempt walking distance. P < .05 was considered significant. We elected not to adjust our α for a potential familywise error.
Results
Of the 23 patients, 14 were female and 9 were male, and 19 were white and 4 were black. Mean (SD) age was 64.4 (6.4) years for the FNC group and 66.0 (6.0) years for the LALB group. The age difference was not statistically significant.
Discussion
Poor pain control during the post-TKA period may have a significant impact on recovery rate, standard of living, psychological health, and postoperative complications.10 Inadequate postoperative pain control increases postoperative morbidity, hinders physiotherapy, increases anxiety, disrupts sleep patterns, and decreases patient satisfaction.9 There has been increased interest in PAIs. Local anesthetics are additional sources of pain control at surgical sites. However, the half-life of most local anesthetics is short. Soft-tissue infiltration of LALB into a surgical site extends the duration of active analgesia. Our study found that, compared with patients who received FNC, patients who received LALB had comparable pain control, improved knee ROM, and shorter hospital stays. In addition, the LALB group had no reports of quadriceps weakness or falls, both of which are associated with femoral nerve blocks. The FNC group had no reported falls, either. PAIs have the benefit of avoiding the invasiveness of femoral nerve blocks and possible neuritis.
Many complications are associated with or indirectly related to delayed rehabilitation and immobility during the acute post-TKA period. From prolonged hospitalization to need for manipulation, the consequences of inadequate pain control and decreased function can be numerous and costly for patients and the healthcare system. In the present study, LALB use led to a statistically significant overall decrease in mean LOS (LALB group, 2.3 days; FNC, 2.8 days). With LALB, there was a higher likelihood of discharge the day after surgery; 20% of patients in the LALB group and no patients in the FNC group went home that day.
The implication is that inadequate pain control led to decreased motion and decreased progression during postoperative rehabilitation. Local infiltration resulted in increased total ROM (extension plus flexion) at 3-week follow-up (LALB, 116.3°; FNC, 107.2°). In addition, there was an increase in walking distance per day of hospital stay (LALB, 135.9 feet; FNC, 84.2 feet). Furthermore, patients indicated LALB when asked which anesthetic they preferred. To our knowledge, this is the first study to compare LALB and FNC data in a matched TKA cohort with each patient serving as his or her own control.
Our study had several limitations. First was the retrospective design. Second was the small sample size, which made definitive conclusions difficult. However, the statistically significant differences we noted validated our conclusions. A statistically significant difference favoring LALB over FNC was found for total MEDs during hospitalization, but there was no significant difference in per-day MEDs. A possible reason for this difference is that LALB patients had shorter hospital stays, and therefore received fewer doses overall. Another possible reason is the small sample size; whereas a larger study using our protocol may find a statistically significant difference between LALB and FNC, we found only a trend. In the FNC group, anesthetic infiltration occurred with use of a computerized pump, which was removed on postoperative day 2; most of these patients were discharged home that day or the morning of postoperative day 3. As it is possible that some of these patients could have gone home sooner, our LOS data may have been affected. We do not consider this limitation significant, as one of our discharge criteria was 150 feet of ambulation, and most patients who received FNCs could not ambulate that far until after FNC removal. Furthermore, this study compared LALB only with FNC. It is possible that our improved outcomes could have resulted from the PAIs themselves, irrespective of LALB. In a recent TKA study by Bagsby and colleagues,11 pain was controlled better with the less expensive traditional PAI of ropivacaine, epinephrine, and morphine than with the PAI of liposomal bupivacaine. Last, in our study, the experience of undergoing the first TKA may have increased patients’ confidence going into the second TKA and then helped them make faster progress in rehabilitation. Regardless, the promising results of our study and the firsthand use of LALB at our institution led us to modify our intraoperative pain management protocol for surgeons who perform TKA.
As we continue to use LALB, our study numbers will increase, and we may discover other factors that, though now underpowered, will prove to be statistically significant. Additional clinical studies are needed to better determine the efficacy and cost-effectiveness of LALB and other long-acting local anesthetic formulations.
Take-Home Points
- At our institution, LALB has shortened our hospital stay.
- There is a trend towards decreased opioid consumption with LALB.
- With the opioid epidemic we face today, LALB can be one of many options in our toolbox towards a solution.
- As stated in prior publications, the effectiveness of LALB is definitely technique dependent.
- Additional clinical studies are warranted to better determine the efficacy and cost-effectiveness of LALB.
Almost 1 million total knee arthroplasties (TKAs) are performed in the United States each year, and the number continues to grow.1.2 For patients about to undergo TKA, a significant concern is postoperative pain.3 Fear of postoperative pain is often cited as a reason for delaying surgery.3 Recent literature suggests that patients with poor pain management during the first 48 hours after surgery have a 50% chance of gaining satisfactory long-term pain relief.4 In addition, inadequate postoperative pain management can interfere with participation in and progression of physical rehabilitation, prolong hospital stay, and increase patient dissatisfaction.5 Poorly controlled pain results in decreased range of motion (ROM), strength, stability, and ambulation thereby prolongs hospital stays, and increases costs and overall dissatisfaction with the procedure.
Post-TKA pain management has received much attention in recent years. A multimodal pain management protocol is now a key component of clinical pathways in TKA. Appropriate postoperative pain control lowers postoperative complications and accelerates recovery.6 Pain-caused loss of function makes surgical patients more susceptible to edema, deep vein thrombosis, and pulmonary embolism.4 Various oral and intravenous medications are used to lessen the pain response during the perioperative period. In addition, regional or neuraxial anesthesia is often added to blunt the immediate surgical pain response.7,8 At our institution, TKA traditionally has been performed with femoral nerve catheters (FNCs) for postoperative pain control. Although effective, this method often results in decreased quadriceps musculature function, which delays rehabilitation and increases the fall risk. Recently, there has been a shift toward using local anesthetic infusions about the knee to provide adequate pain relief and restore motor function, which is often sacrificed with use of regional nerve blocks and continuous catheter infusions.9
Many institutions have started using a new long-acting local anesthetic in their multimodal pain management pathways: Exparel (Pacira Pharmaceuticals), a liposomal membrane-bound bupivacaine with sustained release of approximately 72 hours. Several studies have verified the safety of this medication.10 A systemic review of prospective studies revealed that, compared with bupivacaine, long-acting liposomal bupivacaine (LALB) in therapeutic doses had a higher safety margin and a favorable safety profile.10 However, no study has compared the effectiveness of LALB and FNC in a matched TKA cohort with each patient serving as his or her own control.
We recently reviewed our multimodal pain management protocol for any areas in need of improvement and decided to compare the effects of the indwelling FNC protocol that was in use with the effects of injecting the local anesthetic LALB. We conducted a study to compare the 2 methods with respect to pain control, ROM, ability to ambulate, and hospital length of stay (LOS). We hypothesized that the longer acting local anesthetic would provide comparable post-TKA pain control and post-TKA opioid use but would accelerate post-TKA rehabilitation.
Materials and Methods
This retrospective, longitudinal, repeated- measures study was approved by the Greenville Hospital System Institutional Review Board and conducted at the Steadman Hawkins Clinic of the Carolinas, Greenville Health System.
Interventions
Twenty-three patients underwent separately staged bilateral TKAs between 2010 and 2013. For each TKA, a Genesis II implant (Smith & Nephew) was used, and the surgery was performed with the patient under spinal anesthesia. In each case, FNC was used for pain control after the first TKA, and periarticular injection (PAI) of LALB for pain control after the second TKA.
In the first TKAs, FNC-administered ropivacaine 0.2% (2 mg/mL) was maintained at a standard basal rate of 8 mL/h for 48 hours. In the second TKAs, LALB was administered along with bupivacaine/epinephrine. Twenty milliliters of LALB from a single-use vial was diluted in 40 mL of normal (0.9%) saline to obtain a 60-mL solution, and a 25-gauge needle was used to inject this solution into the periarticular soft tissues; another needle was used for PAI of 30 mL of bupivacaine 0.25% with epinephrine.
Continuous passive motion devices were not used. Most patients began therapy on day of surgery. Knee immobilizers were not used in the FNC group.
The same standardized multimodal pain management protocol was used for all TKAs. Non- narcotic medications, including acetaminophen, ketorolac, and celecoxib, were given on a scheduled basis. Tramadol and opioid medications were administered as needed for pain. The attending physician based patient discharge timing on pain control, ability to safely ambulate, and absence of complications.
Outcome Measures
Outcome measures were LOS; extension and flexion at discharge and 3-week follow-up; total ROM (extension plus flexion) at discharge and 3-week follow-up; per-day and total hospital stay morphine -equivalent doses (MEDs); and per-attempt walking distance during gait training.
ROM was measured with a standard goniometer. Flexion was tested with the patient supine and the hip and knee in neutral rotation. The goniometer axis was along the lateral epicondyle of the femur with the proximal arm of the goniometer parallel to the long axis of the femur and pointing at the greater trochanter and with the distal arm parallel to the long axis of the fibula and pointing at the lateral malleolus. The patient was instructed to flex the hip and knee by moving the heel toward the buttock. Expected normal ROM is 135°. The same landmarks were used for extension. The patient was instructed to push the back of the knee toward the plinth/bed, for maximal active extension. The same ROM assessment strategy was used during the hospitalization and at the 3-week follow-up.
Several opioid medications (eg, hydrocodone, oxycodone, tramadol, hydromorphone, morphine) with different dosages were used during hospitalization. Opioid doses were converted to MEDs to permit FNC–LALB comparisons. For each patient, total MEDs were divided by LOS to determine MEDs per day.
Mean per-attempt walking distance was calculated by dividing the total distance walked during hospitalization—the sum of the number of feet walked during each and every attempt, as measured by the treating physical therapist—by the total number of walking attempts.
Data Analysis
A paired-samples t test was used to calculate differences between all outcome measures: LOS; extension and flexion at discharge and 3-month follow-up; per-day and total MEDs; and mean per-attempt walking distance. P < .05 was considered significant. We elected not to adjust our α for a potential familywise error.
Results
Of the 23 patients, 14 were female and 9 were male, and 19 were white and 4 were black. Mean (SD) age was 64.4 (6.4) years for the FNC group and 66.0 (6.0) years for the LALB group. The age difference was not statistically significant.
Discussion
Poor pain control during the post-TKA period may have a significant impact on recovery rate, standard of living, psychological health, and postoperative complications.10 Inadequate postoperative pain control increases postoperative morbidity, hinders physiotherapy, increases anxiety, disrupts sleep patterns, and decreases patient satisfaction.9 There has been increased interest in PAIs. Local anesthetics are additional sources of pain control at surgical sites. However, the half-life of most local anesthetics is short. Soft-tissue infiltration of LALB into a surgical site extends the duration of active analgesia. Our study found that, compared with patients who received FNC, patients who received LALB had comparable pain control, improved knee ROM, and shorter hospital stays. In addition, the LALB group had no reports of quadriceps weakness or falls, both of which are associated with femoral nerve blocks. The FNC group had no reported falls, either. PAIs have the benefit of avoiding the invasiveness of femoral nerve blocks and possible neuritis.
Many complications are associated with or indirectly related to delayed rehabilitation and immobility during the acute post-TKA period. From prolonged hospitalization to need for manipulation, the consequences of inadequate pain control and decreased function can be numerous and costly for patients and the healthcare system. In the present study, LALB use led to a statistically significant overall decrease in mean LOS (LALB group, 2.3 days; FNC, 2.8 days). With LALB, there was a higher likelihood of discharge the day after surgery; 20% of patients in the LALB group and no patients in the FNC group went home that day.
The implication is that inadequate pain control led to decreased motion and decreased progression during postoperative rehabilitation. Local infiltration resulted in increased total ROM (extension plus flexion) at 3-week follow-up (LALB, 116.3°; FNC, 107.2°). In addition, there was an increase in walking distance per day of hospital stay (LALB, 135.9 feet; FNC, 84.2 feet). Furthermore, patients indicated LALB when asked which anesthetic they preferred. To our knowledge, this is the first study to compare LALB and FNC data in a matched TKA cohort with each patient serving as his or her own control.
Our study had several limitations. First was the retrospective design. Second was the small sample size, which made definitive conclusions difficult. However, the statistically significant differences we noted validated our conclusions. A statistically significant difference favoring LALB over FNC was found for total MEDs during hospitalization, but there was no significant difference in per-day MEDs. A possible reason for this difference is that LALB patients had shorter hospital stays, and therefore received fewer doses overall. Another possible reason is the small sample size; whereas a larger study using our protocol may find a statistically significant difference between LALB and FNC, we found only a trend. In the FNC group, anesthetic infiltration occurred with use of a computerized pump, which was removed on postoperative day 2; most of these patients were discharged home that day or the morning of postoperative day 3. As it is possible that some of these patients could have gone home sooner, our LOS data may have been affected. We do not consider this limitation significant, as one of our discharge criteria was 150 feet of ambulation, and most patients who received FNCs could not ambulate that far until after FNC removal. Furthermore, this study compared LALB only with FNC. It is possible that our improved outcomes could have resulted from the PAIs themselves, irrespective of LALB. In a recent TKA study by Bagsby and colleagues,11 pain was controlled better with the less expensive traditional PAI of ropivacaine, epinephrine, and morphine than with the PAI of liposomal bupivacaine. Last, in our study, the experience of undergoing the first TKA may have increased patients’ confidence going into the second TKA and then helped them make faster progress in rehabilitation. Regardless, the promising results of our study and the firsthand use of LALB at our institution led us to modify our intraoperative pain management protocol for surgeons who perform TKA.
As we continue to use LALB, our study numbers will increase, and we may discover other factors that, though now underpowered, will prove to be statistically significant. Additional clinical studies are needed to better determine the efficacy and cost-effectiveness of LALB and other long-acting local anesthetic formulations.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Ruiz D Jr, Koenig L, Dall TM, et al. The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J Bone Joint Surg Am. 2013;95(16):1473-1480.
3. Trousdale RT, McGrory BJ, Berry DJ, Becker MW, Harmsen WS. Patients’ concerns prior to undergoing total hip and total knee arthroplasty. Mayo Clin Proc. 1999;74(10):978-982.
4. Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses, Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2008:469-497.
5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287-333.
6. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084.
7. Stein BE, Srikumaran U, Tan EW, Freehill MT, Wilckens JH. Lower-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(22):e167.
8. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
9. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
10. Portillo J, Kamar N, Melibary S, Quevedo E, Bergese S. Safety of liposome extended-release bupivacaine for postoperative pain control. Front Pharmacol. 2014;5:90.
11. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Ruiz D Jr, Koenig L, Dall TM, et al. The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J Bone Joint Surg Am. 2013;95(16):1473-1480.
3. Trousdale RT, McGrory BJ, Berry DJ, Becker MW, Harmsen WS. Patients’ concerns prior to undergoing total hip and total knee arthroplasty. Mayo Clin Proc. 1999;74(10):978-982.
4. Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses, Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2008:469-497.
5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287-333.
6. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084.
7. Stein BE, Srikumaran U, Tan EW, Freehill MT, Wilckens JH. Lower-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(22):e167.
8. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
9. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
10. Portillo J, Kamar N, Melibary S, Quevedo E, Bergese S. Safety of liposome extended-release bupivacaine for postoperative pain control. Front Pharmacol. 2014;5:90.
11. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
Varenicline may reduce heavy drinking in male smokers
The smoking cessation aid varenicline tartrate is effective for reducing heavy drinking in men with alcohol use disorder and comorbid cigarette smoking, according to findings published Dec. 20, 2017. The drug also increased smoking abstinence in participants overall, reported Stephanie S. O’Malley, PhD, of the department of psychiatry at Yale University, New Haven, Conn., and her coauthors.
Women had a smaller decrease in PHDD (P = .15), and 5% had NHDD, compared with 25% of the women on placebo.
The trial was conducted between September 2012 and August 2015 at research facilities affiliated with Columbia University in New York and with Yale. The study group was made up of 92 men and 39 women aged 18-70 years who met DSM-IV-TR criteria for alcohol dependence. Most of the respondents (52.7%) identified themselves as black. They reported heavy drinking at least twice per week for the preceding 90 days, having seven or fewer consecutive days of alcohol abstinence, and smoking at least twice per week, the investigators reported.
the authors reported, whereas none of the participants on placebo quit smoking (P = .003).
The sex differences in the trial may be attributed to differences in baseline characteristics, such as greater alcohol dependence and lower nicotine dependence, Dr. O’Malley and her colleagues said.
Additionally, women were more likely to reduce or discontinue varenicline dose. “From a methodological perspective, we permitted dose reductions to minimize adherence problems because lower varenicline doses are effective for smoking cessation,” they said.
The clinical implications of the findings are important, Dr. O’Malley and her colleagues said. “Individuals treated for alcoholism are more likely to die of smoking than from alcohol-related causes,” they wrote, and “most smokers do not receive smoking-cessation assistance, yet heavy-drinking smokers see these behaviors as highly associated.”
Lastly, the trial was limited by the small sample size of women.
“Future studies should evaluate the effectiveness and safety of varenicline in women and men separately in larger samples to establish whether the observed effects are of clinical significance,” the authors concluded.
The study was funded by grants from the National Institutes of Health and from Connecticut’s Department of Mental Health and Addiction Services. Pfizer, the manufacturer of varenicline under the name Chantix, provided varenicline and placebo pills.
The authors disclosed relationships with several companies, including Pfizer.
SOURCE: O’Malley SS et al. JAMA Psychiatry. 2017 Dec 20. doi: 10.1001/jamapsychiatry.2017.3544.
Despite its limitations, this study provides an important contribution to the body of addiction research, wrote A. Eden Evins, MD, MPH, in an accompanying editorial.
“Outcome measures that take into account multiple addictions are largely unexplored,” Dr. Evins said.
In addition to demonstrating its tolerability in patients with substance use disorders, the study makes “a creative contribution toward improved treatment trials for those who use multiple addictive substances by including an exploratory mixed outcome of no heavy drinking days and no tobacco use,” she wrote.
Future research should further explore sex differences, as well as proactive treatment strategies for smokers who are not ready to quit but are willing to try medications to improve their chances of quitting, Dr. Evins concluded.
Dr. A. Eden Evins is affiliated with the Center for Addiction Medicine at the department of psychiatry at Massachusetts General Hospital and with Harvard Medical School, both in Boston. She disclosed financial relationships with Forum Pharmaceuticals, Pfizer, and Brain Solutions.
Despite its limitations, this study provides an important contribution to the body of addiction research, wrote A. Eden Evins, MD, MPH, in an accompanying editorial.
“Outcome measures that take into account multiple addictions are largely unexplored,” Dr. Evins said.
In addition to demonstrating its tolerability in patients with substance use disorders, the study makes “a creative contribution toward improved treatment trials for those who use multiple addictive substances by including an exploratory mixed outcome of no heavy drinking days and no tobacco use,” she wrote.
Future research should further explore sex differences, as well as proactive treatment strategies for smokers who are not ready to quit but are willing to try medications to improve their chances of quitting, Dr. Evins concluded.
Dr. A. Eden Evins is affiliated with the Center for Addiction Medicine at the department of psychiatry at Massachusetts General Hospital and with Harvard Medical School, both in Boston. She disclosed financial relationships with Forum Pharmaceuticals, Pfizer, and Brain Solutions.
Despite its limitations, this study provides an important contribution to the body of addiction research, wrote A. Eden Evins, MD, MPH, in an accompanying editorial.
“Outcome measures that take into account multiple addictions are largely unexplored,” Dr. Evins said.
In addition to demonstrating its tolerability in patients with substance use disorders, the study makes “a creative contribution toward improved treatment trials for those who use multiple addictive substances by including an exploratory mixed outcome of no heavy drinking days and no tobacco use,” she wrote.
Future research should further explore sex differences, as well as proactive treatment strategies for smokers who are not ready to quit but are willing to try medications to improve their chances of quitting, Dr. Evins concluded.
Dr. A. Eden Evins is affiliated with the Center for Addiction Medicine at the department of psychiatry at Massachusetts General Hospital and with Harvard Medical School, both in Boston. She disclosed financial relationships with Forum Pharmaceuticals, Pfizer, and Brain Solutions.
The smoking cessation aid varenicline tartrate is effective for reducing heavy drinking in men with alcohol use disorder and comorbid cigarette smoking, according to findings published Dec. 20, 2017. The drug also increased smoking abstinence in participants overall, reported Stephanie S. O’Malley, PhD, of the department of psychiatry at Yale University, New Haven, Conn., and her coauthors.
Women had a smaller decrease in PHDD (P = .15), and 5% had NHDD, compared with 25% of the women on placebo.
The trial was conducted between September 2012 and August 2015 at research facilities affiliated with Columbia University in New York and with Yale. The study group was made up of 92 men and 39 women aged 18-70 years who met DSM-IV-TR criteria for alcohol dependence. Most of the respondents (52.7%) identified themselves as black. They reported heavy drinking at least twice per week for the preceding 90 days, having seven or fewer consecutive days of alcohol abstinence, and smoking at least twice per week, the investigators reported.
the authors reported, whereas none of the participants on placebo quit smoking (P = .003).
The sex differences in the trial may be attributed to differences in baseline characteristics, such as greater alcohol dependence and lower nicotine dependence, Dr. O’Malley and her colleagues said.
Additionally, women were more likely to reduce or discontinue varenicline dose. “From a methodological perspective, we permitted dose reductions to minimize adherence problems because lower varenicline doses are effective for smoking cessation,” they said.
The clinical implications of the findings are important, Dr. O’Malley and her colleagues said. “Individuals treated for alcoholism are more likely to die of smoking than from alcohol-related causes,” they wrote, and “most smokers do not receive smoking-cessation assistance, yet heavy-drinking smokers see these behaviors as highly associated.”
Lastly, the trial was limited by the small sample size of women.
“Future studies should evaluate the effectiveness and safety of varenicline in women and men separately in larger samples to establish whether the observed effects are of clinical significance,” the authors concluded.
The study was funded by grants from the National Institutes of Health and from Connecticut’s Department of Mental Health and Addiction Services. Pfizer, the manufacturer of varenicline under the name Chantix, provided varenicline and placebo pills.
The authors disclosed relationships with several companies, including Pfizer.
SOURCE: O’Malley SS et al. JAMA Psychiatry. 2017 Dec 20. doi: 10.1001/jamapsychiatry.2017.3544.
The smoking cessation aid varenicline tartrate is effective for reducing heavy drinking in men with alcohol use disorder and comorbid cigarette smoking, according to findings published Dec. 20, 2017. The drug also increased smoking abstinence in participants overall, reported Stephanie S. O’Malley, PhD, of the department of psychiatry at Yale University, New Haven, Conn., and her coauthors.
Women had a smaller decrease in PHDD (P = .15), and 5% had NHDD, compared with 25% of the women on placebo.
The trial was conducted between September 2012 and August 2015 at research facilities affiliated with Columbia University in New York and with Yale. The study group was made up of 92 men and 39 women aged 18-70 years who met DSM-IV-TR criteria for alcohol dependence. Most of the respondents (52.7%) identified themselves as black. They reported heavy drinking at least twice per week for the preceding 90 days, having seven or fewer consecutive days of alcohol abstinence, and smoking at least twice per week, the investigators reported.
the authors reported, whereas none of the participants on placebo quit smoking (P = .003).
The sex differences in the trial may be attributed to differences in baseline characteristics, such as greater alcohol dependence and lower nicotine dependence, Dr. O’Malley and her colleagues said.
Additionally, women were more likely to reduce or discontinue varenicline dose. “From a methodological perspective, we permitted dose reductions to minimize adherence problems because lower varenicline doses are effective for smoking cessation,” they said.
The clinical implications of the findings are important, Dr. O’Malley and her colleagues said. “Individuals treated for alcoholism are more likely to die of smoking than from alcohol-related causes,” they wrote, and “most smokers do not receive smoking-cessation assistance, yet heavy-drinking smokers see these behaviors as highly associated.”
Lastly, the trial was limited by the small sample size of women.
“Future studies should evaluate the effectiveness and safety of varenicline in women and men separately in larger samples to establish whether the observed effects are of clinical significance,” the authors concluded.
The study was funded by grants from the National Institutes of Health and from Connecticut’s Department of Mental Health and Addiction Services. Pfizer, the manufacturer of varenicline under the name Chantix, provided varenicline and placebo pills.
The authors disclosed relationships with several companies, including Pfizer.
SOURCE: O’Malley SS et al. JAMA Psychiatry. 2017 Dec 20. doi: 10.1001/jamapsychiatry.2017.3544.
FROM JAMA PSYCHIATRY
Key clinical point: The smoking cessation aid varenicline tartrate reduced heavy drinking in men and increased smoking abstinence in men and women.
Major finding: Varenicline resulted in a decrease in heavy drinking in men (95% confidence interval, –0.09 to 1.18; P = .09) and prolonged smoking abstinence in 13% of participants overall.
Study details: A phase 2, randomized, double-blind study of 131 participants with alcohol use disorder and comorbid smoking.
Disclosures: The study was funded by grants from the National Institutes of Health and from Connecticut’s Department of Mental Health and Addiction Services. Varenicline and placebo pills were provided by Pfizer, manufacturer of varenicline under the name Chantix. The authors disclosed relationships with several companies and organizations, including Pfizer.
Source: O’Malley SS et al. JAMA Psychiatry. 2017 Dec 20. doi: 10.1001/jamapsychiatry.2017.3544.
Recurrent rash
Based on the appearance and location of the lesions, a diagnosis of recurrent herpes simplex virus-2 (HSV-2) was made. HSV-2 is generally a genital eruption but can also occur on the buttocks, especially in women. (Our patient was not aware that she’d had a genital primary HSV-2 infection.) HSV-2 infects an estimated 5% to 25% of adults in western nations. In 2012, approximately 417 million people ages 15 to 49 were living with HSV-2 worldwide, including 19 million who were newly infected.
Following a genital primary infection, HSV-2 lies dormant in the sacral nerve root ganglia, which innervate both the genitals and sacrum. Reactivation can thus result in recurrences anywhere over the sacral dermatome. The sacral area is the most common nongenital site for recurrent HSV-2. Reactivation of HSV-2 is more common and more severe in patients with human immunodeficiency virus infection.
The mainstay of treatment for HSV is antiviral therapy with acyclovir. Famciclovir and valacyclovir can be used, as well. These antivirals inhibit viral DNA replication, shorten duration of symptoms, increase lesion healing, and decrease viral shedding time. They are generally safe; the main adverse effects of oral therapy are nausea, vomiting, and diarrhea.
In general, nongenital recurrences of HSV are treated the same as genital recurrences. Dosing during prodromal symptoms or at the first sign of a recurrence is recommended for maximum efficacy. Suppressive therapy can be effective in patients who experience frequent recurrences and is generally recommended for 6 months to a year (or longer). Patients should also be warned that because of increased genital viral shedding during sacral recurrences, they should avoid sexual contact during outbreaks.
After a discussion about the benefits and risks of antiviral suppression, the patient decided she was ready to proceed with treatment. The physician prescribed oral valacyclovir 1 g daily for treatment and suppression. She had no recurrences over the next year.
Adapted from: Flowers H, Brodell RT. Recurrent vesicular rash over the sacrum. J Fam Pract. 2015;64:577-579.

Based on the appearance and location of the lesions, a diagnosis of recurrent herpes simplex virus-2 (HSV-2) was made. HSV-2 is generally a genital eruption but can also occur on the buttocks, especially in women. (Our patient was not aware that she’d had a genital primary HSV-2 infection.) HSV-2 infects an estimated 5% to 25% of adults in western nations. In 2012, approximately 417 million people ages 15 to 49 were living with HSV-2 worldwide, including 19 million who were newly infected.
Following a genital primary infection, HSV-2 lies dormant in the sacral nerve root ganglia, which innervate both the genitals and sacrum. Reactivation can thus result in recurrences anywhere over the sacral dermatome. The sacral area is the most common nongenital site for recurrent HSV-2. Reactivation of HSV-2 is more common and more severe in patients with human immunodeficiency virus infection.
The mainstay of treatment for HSV is antiviral therapy with acyclovir. Famciclovir and valacyclovir can be used, as well. These antivirals inhibit viral DNA replication, shorten duration of symptoms, increase lesion healing, and decrease viral shedding time. They are generally safe; the main adverse effects of oral therapy are nausea, vomiting, and diarrhea.
In general, nongenital recurrences of HSV are treated the same as genital recurrences. Dosing during prodromal symptoms or at the first sign of a recurrence is recommended for maximum efficacy. Suppressive therapy can be effective in patients who experience frequent recurrences and is generally recommended for 6 months to a year (or longer). Patients should also be warned that because of increased genital viral shedding during sacral recurrences, they should avoid sexual contact during outbreaks.
After a discussion about the benefits and risks of antiviral suppression, the patient decided she was ready to proceed with treatment. The physician prescribed oral valacyclovir 1 g daily for treatment and suppression. She had no recurrences over the next year.
Adapted from: Flowers H, Brodell RT. Recurrent vesicular rash over the sacrum. J Fam Pract. 2015;64:577-579.

Based on the appearance and location of the lesions, a diagnosis of recurrent herpes simplex virus-2 (HSV-2) was made. HSV-2 is generally a genital eruption but can also occur on the buttocks, especially in women. (Our patient was not aware that she’d had a genital primary HSV-2 infection.) HSV-2 infects an estimated 5% to 25% of adults in western nations. In 2012, approximately 417 million people ages 15 to 49 were living with HSV-2 worldwide, including 19 million who were newly infected.
Following a genital primary infection, HSV-2 lies dormant in the sacral nerve root ganglia, which innervate both the genitals and sacrum. Reactivation can thus result in recurrences anywhere over the sacral dermatome. The sacral area is the most common nongenital site for recurrent HSV-2. Reactivation of HSV-2 is more common and more severe in patients with human immunodeficiency virus infection.
The mainstay of treatment for HSV is antiviral therapy with acyclovir. Famciclovir and valacyclovir can be used, as well. These antivirals inhibit viral DNA replication, shorten duration of symptoms, increase lesion healing, and decrease viral shedding time. They are generally safe; the main adverse effects of oral therapy are nausea, vomiting, and diarrhea.
In general, nongenital recurrences of HSV are treated the same as genital recurrences. Dosing during prodromal symptoms or at the first sign of a recurrence is recommended for maximum efficacy. Suppressive therapy can be effective in patients who experience frequent recurrences and is generally recommended for 6 months to a year (or longer). Patients should also be warned that because of increased genital viral shedding during sacral recurrences, they should avoid sexual contact during outbreaks.
After a discussion about the benefits and risks of antiviral suppression, the patient decided she was ready to proceed with treatment. The physician prescribed oral valacyclovir 1 g daily for treatment and suppression. She had no recurrences over the next year.
Adapted from: Flowers H, Brodell RT. Recurrent vesicular rash over the sacrum. J Fam Pract. 2015;64:577-579.