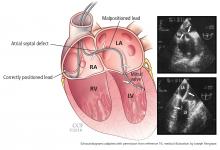
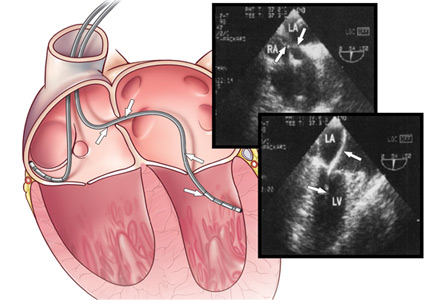
User login
Hypothermia and severe first-degree heart block
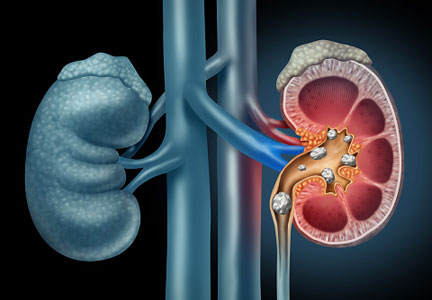
A 96-year-old woman with hypertension, diabetes, and dementia was found unresponsive in her nursing home and was transferred to the hospital.
At presentation to the hospital, her blood pressure was 76/43 mm Hg, heart rate 42 beats per minute, rectal temperature 31.6°C (88.8°F), and blood glucose 36 mg/dL.
Causes of secondary hypothermia were sought. Blood and urine cultures were negative. Computed tomography of the head showed no acute intracranial abnormalities. Tests for adrenal insufficiency and hypothyroidism were negative.
HYPOTHERMIA AND THE ECG
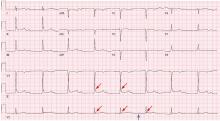
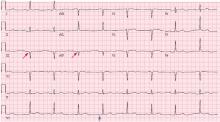
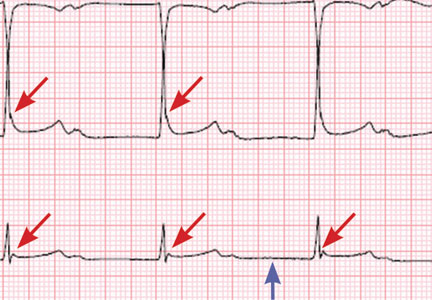
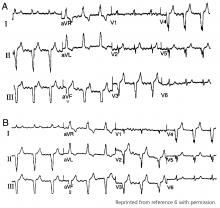
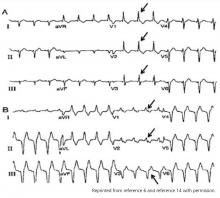
Hypothermia can produce a number of changes on the ECG. At the start of hypothermia, a stress reaction is induced, resulting in sinus tachycardia. But when the temperature goes below 32°C, sinus bradycardia ensues,1 resulting in various degrees of heart block.2 In our patient, a severely prolonged PR interval resulted in first-degree heart block.
Other findings on ECG associated with hypothermia include atrial fibrillation, widening of the P and T waves, prolonging of the QT interval, and widening of the QRS interval. Progressive widening of the QRS interval can predispose to ventricular fibrillation.1,3
An Osborn or J wave is a wave found between the end of the QRS and the beginning of the ST segment and is usually seen on the inferior and lateral precordial leads. It is found in as many as 80% of patients when the body temperature is below 30°C.1,3,4
Although Osborn waves are a common finding in hypothermia, they are also seen in electrolyte imbalances such as hypercalcemia and in central nervous system diseases.5,6 Hypothermia-associated changes on ECG are usually readily reversible with rewarming.1
TAKE-HOME MESSAGES
The ECG should always be interpreted in the proper clinical context and, whenever possible, compared with a previous ECG. It is prudent to always consider potentially reversible triggers of hypothermia other than environmental exposure such as hypothyroidism, infection, adrenal insufficiency, ketoacidosis, medication side effects, and alcohol use.
Hypothermia, especially in elderly patients with multiple comorbidities, can lead to bradycardia and varying degrees of heart block.
- Alhaddad IA, Khalil M, Brown EJ Jr. Osborn waves of hypothermia. Circulation 2000; 101:E233–E244.
- Bashour TT, Gualberto A, Ryan C. Atrioventricular block in accidental hypothermia—a case report. Angiology 1989; 40:63–66.
- Okada M, Nishimura F, Yoshino H, Kimura M, Ogino T. The J wave in accidental hypothermia. J Electrocardiol 1983; 16:23–28.
- Kukla P, Baranchuk A, Jastrzebski M, Zabojszcz M, Bryniarski L. Electrocardiographic landmarks of hypothermia. Kardiol Pol 2013; 71:1188–1189.
- Maruyama M, Kobayashi Y, Kodani E, et al. Osborn waves: history and significance. Indian Pacing Electrophysiol J 2004; 4:33–39.
- Sheikh AM, Hurst JW. Osborn waves in the electrocardiogram, hypothermia not due to exposure, and death due to diabetic ketoacidosis. Clin Cardiol 2003; 26:555–560.
A 96-year-old woman with hypertension, diabetes, and dementia was found unresponsive in her nursing home and was transferred to the hospital.
At presentation to the hospital, her blood pressure was 76/43 mm Hg, heart rate 42 beats per minute, rectal temperature 31.6°C (88.8°F), and blood glucose 36 mg/dL.
Causes of secondary hypothermia were sought. Blood and urine cultures were negative. Computed tomography of the head showed no acute intracranial abnormalities. Tests for adrenal insufficiency and hypothyroidism were negative.
HYPOTHERMIA AND THE ECG
Hypothermia can produce a number of changes on the ECG. At the start of hypothermia, a stress reaction is induced, resulting in sinus tachycardia. But when the temperature goes below 32°C, sinus bradycardia ensues,1 resulting in various degrees of heart block.2 In our patient, a severely prolonged PR interval resulted in first-degree heart block.
Other findings on ECG associated with hypothermia include atrial fibrillation, widening of the P and T waves, prolonging of the QT interval, and widening of the QRS interval. Progressive widening of the QRS interval can predispose to ventricular fibrillation.1,3
An Osborn or J wave is a wave found between the end of the QRS and the beginning of the ST segment and is usually seen on the inferior and lateral precordial leads. It is found in as many as 80% of patients when the body temperature is below 30°C.1,3,4
Although Osborn waves are a common finding in hypothermia, they are also seen in electrolyte imbalances such as hypercalcemia and in central nervous system diseases.5,6 Hypothermia-associated changes on ECG are usually readily reversible with rewarming.1
TAKE-HOME MESSAGES
The ECG should always be interpreted in the proper clinical context and, whenever possible, compared with a previous ECG. It is prudent to always consider potentially reversible triggers of hypothermia other than environmental exposure such as hypothyroidism, infection, adrenal insufficiency, ketoacidosis, medication side effects, and alcohol use.
Hypothermia, especially in elderly patients with multiple comorbidities, can lead to bradycardia and varying degrees of heart block.
A 96-year-old woman with hypertension, diabetes, and dementia was found unresponsive in her nursing home and was transferred to the hospital.
At presentation to the hospital, her blood pressure was 76/43 mm Hg, heart rate 42 beats per minute, rectal temperature 31.6°C (88.8°F), and blood glucose 36 mg/dL.
Causes of secondary hypothermia were sought. Blood and urine cultures were negative. Computed tomography of the head showed no acute intracranial abnormalities. Tests for adrenal insufficiency and hypothyroidism were negative.
HYPOTHERMIA AND THE ECG
Hypothermia can produce a number of changes on the ECG. At the start of hypothermia, a stress reaction is induced, resulting in sinus tachycardia. But when the temperature goes below 32°C, sinus bradycardia ensues,1 resulting in various degrees of heart block.2 In our patient, a severely prolonged PR interval resulted in first-degree heart block.
Other findings on ECG associated with hypothermia include atrial fibrillation, widening of the P and T waves, prolonging of the QT interval, and widening of the QRS interval. Progressive widening of the QRS interval can predispose to ventricular fibrillation.1,3
An Osborn or J wave is a wave found between the end of the QRS and the beginning of the ST segment and is usually seen on the inferior and lateral precordial leads. It is found in as many as 80% of patients when the body temperature is below 30°C.1,3,4
Although Osborn waves are a common finding in hypothermia, they are also seen in electrolyte imbalances such as hypercalcemia and in central nervous system diseases.5,6 Hypothermia-associated changes on ECG are usually readily reversible with rewarming.1
TAKE-HOME MESSAGES
The ECG should always be interpreted in the proper clinical context and, whenever possible, compared with a previous ECG. It is prudent to always consider potentially reversible triggers of hypothermia other than environmental exposure such as hypothyroidism, infection, adrenal insufficiency, ketoacidosis, medication side effects, and alcohol use.
Hypothermia, especially in elderly patients with multiple comorbidities, can lead to bradycardia and varying degrees of heart block.
- Alhaddad IA, Khalil M, Brown EJ Jr. Osborn waves of hypothermia. Circulation 2000; 101:E233–E244.
- Bashour TT, Gualberto A, Ryan C. Atrioventricular block in accidental hypothermia—a case report. Angiology 1989; 40:63–66.
- Okada M, Nishimura F, Yoshino H, Kimura M, Ogino T. The J wave in accidental hypothermia. J Electrocardiol 1983; 16:23–28.
- Kukla P, Baranchuk A, Jastrzebski M, Zabojszcz M, Bryniarski L. Electrocardiographic landmarks of hypothermia. Kardiol Pol 2013; 71:1188–1189.
- Maruyama M, Kobayashi Y, Kodani E, et al. Osborn waves: history and significance. Indian Pacing Electrophysiol J 2004; 4:33–39.
- Sheikh AM, Hurst JW. Osborn waves in the electrocardiogram, hypothermia not due to exposure, and death due to diabetic ketoacidosis. Clin Cardiol 2003; 26:555–560.
- Alhaddad IA, Khalil M, Brown EJ Jr. Osborn waves of hypothermia. Circulation 2000; 101:E233–E244.
- Bashour TT, Gualberto A, Ryan C. Atrioventricular block in accidental hypothermia—a case report. Angiology 1989; 40:63–66.
- Okada M, Nishimura F, Yoshino H, Kimura M, Ogino T. The J wave in accidental hypothermia. J Electrocardiol 1983; 16:23–28.
- Kukla P, Baranchuk A, Jastrzebski M, Zabojszcz M, Bryniarski L. Electrocardiographic landmarks of hypothermia. Kardiol Pol 2013; 71:1188–1189.
- Maruyama M, Kobayashi Y, Kodani E, et al. Osborn waves: history and significance. Indian Pacing Electrophysiol J 2004; 4:33–39.
- Sheikh AM, Hurst JW. Osborn waves in the electrocardiogram, hypothermia not due to exposure, and death due to diabetic ketoacidosis. Clin Cardiol 2003; 26:555–560.
BTK inhibitor zanubrutinib active in non-Hodgkin lymphomas
ATLANTA – , according to data presented at the annual meeting of the American Society of Hematology.
Response rates ranged from 31% to 88% depending on the lymphoma subtype. Overall, approximately 10% of patients discontinued the drug because of adverse events, reported Constantine S. Tam, MBBS, MD, of Peter MacCallum Cancer Centre & St. Vincent’s Hospital, Melbourne.
“There was encouraging activity against all the spectrum of indolent and aggressive NHL subtypes … and durable responses were observed across a variety of histologies,” Dr. Tam said.
Zanubrutinib is a second-generation BTK inhibitor that, based on biochemical assays, has higher selectivity against BTK than ibrutinib, Dr. Tam said.
He presented results of an open-label, multicenter, phase 1b study of daily or twice-daily zanubrutinib in patients with B-cell malignancies, most of them relapsed or refractory to prior therapies. The lymphoma subtypes he presented included diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL).
For 34 patients with indolent lymphomas (FL and MZL), the most frequent adverse events were petechiae/purpura/contusion and upper respiratory tract infection. Eleven grade 3-5 adverse events were reported, including neutropenia, infection, nausea, urinary tract infection, and abdominal pain.
Atrial fibrillation was observed in two patients in the aggressive NHL cohort, for an overall AF rate of approximately 2%, Dr. Tam said.
For 65 patients with aggressive lymphomas (DLBCL and MCL), the most frequent adverse events were petechiae/purpura/contusion and diarrhea; 27 grade 3-5 adverse events were reported, including neutropenia, pneumonia, and anemia.
The highest overall response rate reported was for MCL, at 88% (28 of 32 patients) followed by MZL at 78% (7 of 9 patients), FL at 41% (7 of 17 patients), and DLBCL 31% (8 of 26 patients).
The recommended phase 2 dose for zanubrutinib is either 320 mg/day once daily or a split dose of 160 mg twice daily, Dr. Tam said.
Based on this experience, investigators started a registration trial of zanubrutinib in combination with obinutuzumab for FL, and additional trials are planned, according to Dr. Tam.
There are also registration trials in Waldenstrom macroglobulinemia and chronic lymphocytic leukemia based on other data suggesting activity of zanubrutinib in those disease types, he added.
Zanubrutinib is a product of BeiGene. Dr. Tam reported disclosures related to Roche, Janssen Cilag, Abbvie, Celgene, Pharmacyclics, Onyx, and Amgen.
SOURCE: Tam C et al, ASH 2017, Abstract 152
ATLANTA – , according to data presented at the annual meeting of the American Society of Hematology.
Response rates ranged from 31% to 88% depending on the lymphoma subtype. Overall, approximately 10% of patients discontinued the drug because of adverse events, reported Constantine S. Tam, MBBS, MD, of Peter MacCallum Cancer Centre & St. Vincent’s Hospital, Melbourne.
“There was encouraging activity against all the spectrum of indolent and aggressive NHL subtypes … and durable responses were observed across a variety of histologies,” Dr. Tam said.
Zanubrutinib is a second-generation BTK inhibitor that, based on biochemical assays, has higher selectivity against BTK than ibrutinib, Dr. Tam said.
He presented results of an open-label, multicenter, phase 1b study of daily or twice-daily zanubrutinib in patients with B-cell malignancies, most of them relapsed or refractory to prior therapies. The lymphoma subtypes he presented included diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL).
For 34 patients with indolent lymphomas (FL and MZL), the most frequent adverse events were petechiae/purpura/contusion and upper respiratory tract infection. Eleven grade 3-5 adverse events were reported, including neutropenia, infection, nausea, urinary tract infection, and abdominal pain.
Atrial fibrillation was observed in two patients in the aggressive NHL cohort, for an overall AF rate of approximately 2%, Dr. Tam said.
For 65 patients with aggressive lymphomas (DLBCL and MCL), the most frequent adverse events were petechiae/purpura/contusion and diarrhea; 27 grade 3-5 adverse events were reported, including neutropenia, pneumonia, and anemia.
The highest overall response rate reported was for MCL, at 88% (28 of 32 patients) followed by MZL at 78% (7 of 9 patients), FL at 41% (7 of 17 patients), and DLBCL 31% (8 of 26 patients).
The recommended phase 2 dose for zanubrutinib is either 320 mg/day once daily or a split dose of 160 mg twice daily, Dr. Tam said.
Based on this experience, investigators started a registration trial of zanubrutinib in combination with obinutuzumab for FL, and additional trials are planned, according to Dr. Tam.
There are also registration trials in Waldenstrom macroglobulinemia and chronic lymphocytic leukemia based on other data suggesting activity of zanubrutinib in those disease types, he added.
Zanubrutinib is a product of BeiGene. Dr. Tam reported disclosures related to Roche, Janssen Cilag, Abbvie, Celgene, Pharmacyclics, Onyx, and Amgen.
SOURCE: Tam C et al, ASH 2017, Abstract 152
ATLANTA – , according to data presented at the annual meeting of the American Society of Hematology.
Response rates ranged from 31% to 88% depending on the lymphoma subtype. Overall, approximately 10% of patients discontinued the drug because of adverse events, reported Constantine S. Tam, MBBS, MD, of Peter MacCallum Cancer Centre & St. Vincent’s Hospital, Melbourne.
“There was encouraging activity against all the spectrum of indolent and aggressive NHL subtypes … and durable responses were observed across a variety of histologies,” Dr. Tam said.
Zanubrutinib is a second-generation BTK inhibitor that, based on biochemical assays, has higher selectivity against BTK than ibrutinib, Dr. Tam said.
He presented results of an open-label, multicenter, phase 1b study of daily or twice-daily zanubrutinib in patients with B-cell malignancies, most of them relapsed or refractory to prior therapies. The lymphoma subtypes he presented included diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL).
For 34 patients with indolent lymphomas (FL and MZL), the most frequent adverse events were petechiae/purpura/contusion and upper respiratory tract infection. Eleven grade 3-5 adverse events were reported, including neutropenia, infection, nausea, urinary tract infection, and abdominal pain.
Atrial fibrillation was observed in two patients in the aggressive NHL cohort, for an overall AF rate of approximately 2%, Dr. Tam said.
For 65 patients with aggressive lymphomas (DLBCL and MCL), the most frequent adverse events were petechiae/purpura/contusion and diarrhea; 27 grade 3-5 adverse events were reported, including neutropenia, pneumonia, and anemia.
The highest overall response rate reported was for MCL, at 88% (28 of 32 patients) followed by MZL at 78% (7 of 9 patients), FL at 41% (7 of 17 patients), and DLBCL 31% (8 of 26 patients).
The recommended phase 2 dose for zanubrutinib is either 320 mg/day once daily or a split dose of 160 mg twice daily, Dr. Tam said.
Based on this experience, investigators started a registration trial of zanubrutinib in combination with obinutuzumab for FL, and additional trials are planned, according to Dr. Tam.
There are also registration trials in Waldenstrom macroglobulinemia and chronic lymphocytic leukemia based on other data suggesting activity of zanubrutinib in those disease types, he added.
Zanubrutinib is a product of BeiGene. Dr. Tam reported disclosures related to Roche, Janssen Cilag, Abbvie, Celgene, Pharmacyclics, Onyx, and Amgen.
SOURCE: Tam C et al, ASH 2017, Abstract 152
REPORTING FROM ASH 2017
Key clinical point: Monotherapy with the BTK inhibitor zanubrutinib (BGB-3111) was active and well tolerated in patients with a variety of non-Hodgkin lymphoma (NHL) subtypes.
Major finding: Response rates ranged from 31% to 88% depending on the lymphoma subtype.
Data source: Preliminary results of an open-label, multicenter, phase 1b study including 99 patients with relapsed or refractory diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, or marginal zone lymphoma.
Disclosures: Zanubrutinib is a product of BeiGene. Constantine S. Tam, MBBS, MD, reported disclosures related to Roche, Janssen Cilag, Abbvie, Celgene, Pharmacyclics, Onyx, and Amgen.
Source: Tam C et al. ASH 2017, Abstract 152.
Dysmorphic red blood cell formation
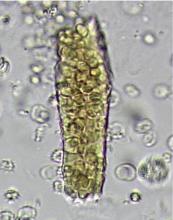
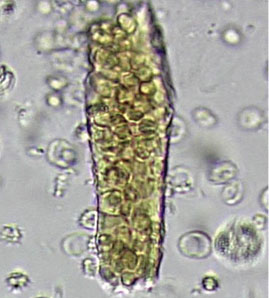
A 23-year-old woman presented with hematuria. Her blood pressure was normal, and she had no rash, joint pain, or other symptoms. Urinalysis was positive for proteinuria and hematuria, and urinary sediment analysis showed dysmorphic red blood cells (RBCs) and red cell casts, leading to a diagnosis of glomerulonephritis. She had proteinuria of 1.2 g/24 hours. Laboratory tests for systemic diseases were negative. Renal biopsy study revealed stage III immunoglobulin A (IgA) nephropathy.
GLOMERULAR HEMATURIA
Glomerular hematuria may represent an immune-mediated injury to the glomerular capillary wall, but it can also be present in noninflammatory glomerulopathies.1
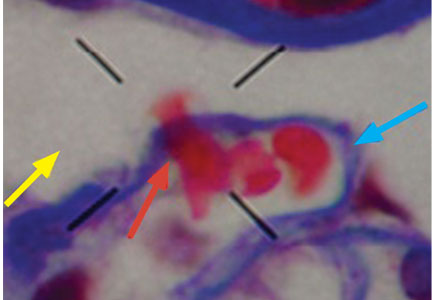
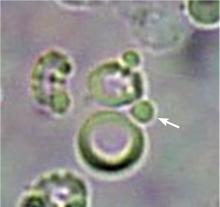
The type of dysmorphic RBCs (crenated or misshapen cells, acanthocytes) may be of diagnostic importance. In particular, dysmorphic red cells alone may be predictive of only renal bleeding, while acanthocytes (ring-shaped RBCs with vesicle-shaped protrusions best seen on phase-contrast microscopy) appear to be most predictive of glomerular disease.2 For example, in 1 study,3 the presence of acanthocytes comprising at least 5% of excreted RBCs had a sensitivity of 52% for glomerular disease and a specificity of 98%.3
- Collar JE, Ladva S, Cairns TD, Cattell V. Red cell traverse through thin glomerular basement membranes. Kidney Int 2001; 59:2069–2072.
- Fogazzi GB, Ponticelli C, Ritz E. The Urinary Sediment: An Integrated View. 2nd ed. Oxford: Oxford University Press; 1999:30.
- Köhler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int 1991; 40:115–120.
- Fogazzi GB. The Urinary Sediment: An Integrated View. 3rd ed. France: Elsevier; 2010.
- Briner VA, Reinhart WH. In vitro production of ‘glomerular red cells’: role of pH and osmolality. Nephron 1990; 56:13–18.
- Schramek P, Moritsch A, Haschkowitz H, Binder BR, Maier M. In vitro generation of dysmorphic erythrocytes. Kidney Int 1989; 36:72–77.
- Pollock C, Liu PL, Györy AZ, et al. Dysmorphism of urinary red blood cells—value in diagnosis. Kidney Int 1989; 36:1045–1049.
- Shichiri M, Hosoda K, Nishio Y, et al. Red-cell-volume distribution curves in diagnosis of glomerular and non-glomerular haematuria. Lancet 1988; 1:908–911.
A 23-year-old woman presented with hematuria. Her blood pressure was normal, and she had no rash, joint pain, or other symptoms. Urinalysis was positive for proteinuria and hematuria, and urinary sediment analysis showed dysmorphic red blood cells (RBCs) and red cell casts, leading to a diagnosis of glomerulonephritis. She had proteinuria of 1.2 g/24 hours. Laboratory tests for systemic diseases were negative. Renal biopsy study revealed stage III immunoglobulin A (IgA) nephropathy.
GLOMERULAR HEMATURIA
Glomerular hematuria may represent an immune-mediated injury to the glomerular capillary wall, but it can also be present in noninflammatory glomerulopathies.1
The type of dysmorphic RBCs (crenated or misshapen cells, acanthocytes) may be of diagnostic importance. In particular, dysmorphic red cells alone may be predictive of only renal bleeding, while acanthocytes (ring-shaped RBCs with vesicle-shaped protrusions best seen on phase-contrast microscopy) appear to be most predictive of glomerular disease.2 For example, in 1 study,3 the presence of acanthocytes comprising at least 5% of excreted RBCs had a sensitivity of 52% for glomerular disease and a specificity of 98%.3
A 23-year-old woman presented with hematuria. Her blood pressure was normal, and she had no rash, joint pain, or other symptoms. Urinalysis was positive for proteinuria and hematuria, and urinary sediment analysis showed dysmorphic red blood cells (RBCs) and red cell casts, leading to a diagnosis of glomerulonephritis. She had proteinuria of 1.2 g/24 hours. Laboratory tests for systemic diseases were negative. Renal biopsy study revealed stage III immunoglobulin A (IgA) nephropathy.
GLOMERULAR HEMATURIA
Glomerular hematuria may represent an immune-mediated injury to the glomerular capillary wall, but it can also be present in noninflammatory glomerulopathies.1
The type of dysmorphic RBCs (crenated or misshapen cells, acanthocytes) may be of diagnostic importance. In particular, dysmorphic red cells alone may be predictive of only renal bleeding, while acanthocytes (ring-shaped RBCs with vesicle-shaped protrusions best seen on phase-contrast microscopy) appear to be most predictive of glomerular disease.2 For example, in 1 study,3 the presence of acanthocytes comprising at least 5% of excreted RBCs had a sensitivity of 52% for glomerular disease and a specificity of 98%.3
- Collar JE, Ladva S, Cairns TD, Cattell V. Red cell traverse through thin glomerular basement membranes. Kidney Int 2001; 59:2069–2072.
- Fogazzi GB, Ponticelli C, Ritz E. The Urinary Sediment: An Integrated View. 2nd ed. Oxford: Oxford University Press; 1999:30.
- Köhler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int 1991; 40:115–120.
- Fogazzi GB. The Urinary Sediment: An Integrated View. 3rd ed. France: Elsevier; 2010.
- Briner VA, Reinhart WH. In vitro production of ‘glomerular red cells’: role of pH and osmolality. Nephron 1990; 56:13–18.
- Schramek P, Moritsch A, Haschkowitz H, Binder BR, Maier M. In vitro generation of dysmorphic erythrocytes. Kidney Int 1989; 36:72–77.
- Pollock C, Liu PL, Györy AZ, et al. Dysmorphism of urinary red blood cells—value in diagnosis. Kidney Int 1989; 36:1045–1049.
- Shichiri M, Hosoda K, Nishio Y, et al. Red-cell-volume distribution curves in diagnosis of glomerular and non-glomerular haematuria. Lancet 1988; 1:908–911.
- Collar JE, Ladva S, Cairns TD, Cattell V. Red cell traverse through thin glomerular basement membranes. Kidney Int 2001; 59:2069–2072.
- Fogazzi GB, Ponticelli C, Ritz E. The Urinary Sediment: An Integrated View. 2nd ed. Oxford: Oxford University Press; 1999:30.
- Köhler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int 1991; 40:115–120.
- Fogazzi GB. The Urinary Sediment: An Integrated View. 3rd ed. France: Elsevier; 2010.
- Briner VA, Reinhart WH. In vitro production of ‘glomerular red cells’: role of pH and osmolality. Nephron 1990; 56:13–18.
- Schramek P, Moritsch A, Haschkowitz H, Binder BR, Maier M. In vitro generation of dysmorphic erythrocytes. Kidney Int 1989; 36:72–77.
- Pollock C, Liu PL, Györy AZ, et al. Dysmorphism of urinary red blood cells—value in diagnosis. Kidney Int 1989; 36:1045–1049.
- Shichiri M, Hosoda K, Nishio Y, et al. Red-cell-volume distribution curves in diagnosis of glomerular and non-glomerular haematuria. Lancet 1988; 1:908–911.
Quality in urine microscopy: The eyes of the beholder
The urine is the window to the kidney.This oft-repeated adage impresses upon medical students and residents the importance of urine microscopy in the evaluation of patients with renal disorders.
While this phrase is likely an adaptation of the idea in ancient times that the urine reflected on humors or the quality of the soul, the understanding of the relevance of urine findings to the state of the kidneys likely rests with the pioneers of urine microscopy. As reviewed by Fogazzi and Cameron,1,2 the origins of direct inspection of urine under a microscope lie in the 17th century, with industrious physicians who used rudimentary microscopes to identify basic structures in the urine and correlated them to clinical presentations.1 At first, only larger structures could be seen, mostly crystals in patients with nephrolithiasis. As microscopes advanced, smaller structures such as “corpuscles” and “cylinders” could be seen that described cells and casts.1
In correlating these findings to patient presentations, a rudimentary understanding of renal pathology evolved long before the advent of the kidney biopsy. Lipid droplets were seen1 in patients swollen from dropsy, and later known to have nephrotic syndromes. In 1872, Harley first described the altered morphology of dysmorphic red blood cells in patients with Bright disease or glomerulonephritis.1,3 In 1979, Birch and Fairley recognized that the presence of acanthocytes differentiated glomerular from nonglomerular hematuria.4
DYSMORPHIC RED BLOOD CELLS: TYPES AND SIGNIFICANCE
URINE MICROSCOPY: THE NEPHROLOGIST’S ROLE
The tools used in urine microscopy have advanced significantly since its advent. But not all advances have led to improved patient care. Laboratories have trained technicians to perform urine microscopy. Analyzers can identify basic urinary structures using algorithms to compare them against stored reference images. More important, urine microscopy has been categorized by accreditation and inspection bodies as a “test” rather than a physician-performed competency. As such, definitions of quality in urine microscopy have shifted from the application of urinary findings to the care of the patient to the reproducibility of identifying individual structures in ways that can be documented with quality checks performed by nonclinicians. And since the governing bodies require laboratories to adhere to burdensome procedures to maintain accreditation (eg, the US Food and Drug Administration’s Clinical Laboratory Improvement Amendments), many hospitals have closed nephrologist-based urine laboratories.
This would be acceptable if laboratory-generated reports provided information equivalent to that obtained by the nephrologist. But such reports rarely include anything beyond the most rudimentary findings. In these reports, the red blood cell is not differentiated as dysmorphic or monomorphic. All casts are granular. Crystals are often the highlight of the report, usually an incidental finding of little relevance. Phase contrast and polarization are never performed.
Despite the poor quality of data provided in these reports, because of increasing regulations and time restrictions, a dwindling number of nephrologists perform urine microscopy even at teaching institutions. In an informal 2009 survey of nephrology fellowship program directors, 79% of responding programs relied solely on lab-generated reports for microscopic findings (verbal communication, Perazella, 2017).
There is general concern among medical educators about the surrendering of the physical examination and other techniques to technology.7,8 However, in many cases, such changes may improve the ability to make a correct diagnosis. When performed properly, urine microscopy can help determine the need for kidney biopsy, differentiate causes of acute kidney injury, and help guide decisions about therapy. Perazella showed that urine microscopy could reliably differentiate acute tubular necrosis from prerenal azotemia.9 Further, the severity of findings on urine microscopy has been associated with worse renal outcomes.10 At our institution, nephrologist-performed urine microscopy resulted in a change in cause of acute kidney injury in 25% of cases and a concrete change in management in 12% of patients (unpublished data).
With this in mind, it is concerning that the only evidence in the literature on this topic demonstrated that laboratory-based urine microscopy is actually a hindrance to its underlying purpose in acute kidney injury, which is to help identify the cause of the injury. Tsai et al11 showed that nephrologists identified the cause of acute kidney injury correctly 90% of the time when they performed their own urine microscopy, but this dropped to 23% when they relied on a laboratory-generated report. Interestingly, knowing the patient’s clinical history when performing the microscopy was important, as the accuracy was 69% when a report of another nephrologist’s microscopy findings was used.11
APPLYING RESULTS TO THE PATIENT
The purpose of urine microscopy in clinical care is to identify and understand the findings as they apply to the patient. When viewed from this perspective, the renal patient is clearly best served when the nephrologist familiar with the case performs urine microscopy, rather than a technician or analyzer in remote parts of the hospital with no connection to the patient.
Advances in technology or streamlining of hospital services do not always produce improvements in patient care, and how we define quality is integral to identifying when this is the case. Quality checklists can serve as guides to safe patient care but should not replace clinical decision-making. Direct physician involvement with our patients has concrete benefits, whether taking a history, performing a physical examination, reviewing radiologic images, or looking at specimens such as urine. It allows us to experience the amazing pathophysiology of human illness and to understand the nuances unique to each of our patients.
But most important, it reinforces the need for the direct bond, both emotional and physical, between us as healers and our patients.
- Fogazzi GB, Cameron JS. Urinary microscopy from the seventeenth century to the present day. Kidney Int 1996; 50:1058–1068.
- Cameron JS. A history of urine microscopy. Clin Chem Lab Med 2015; 53(suppl 2):s1453–s1464.
- Harley G. The Urine and Its Derangements. London: J and A Churchill, 1872:178–179.
- Birch DF, Fairley K. Hematuria: glomerular or non-glomerular? Lancet 1979; 314:845–846.
- Köhler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int 1991; 40:115–120.
- Daza JL, De Rosa M, De Rosa G. Dysmorphic red blood cells. Cleve Clin J Med 2018; 85:12–13.
- Jauhar S. The demise of the physical exam. N Engl J Med 2006; 354:548–551.
- Mangione S. When the tail wags the dog: clinical skills in the age of technology. Cleve Clin J Med 2017; 84:278–280.
- Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2008; 3:1615–1619.
- Perazella MA, Coca SG, Hall IE, Iyanam U, Koraishy M, Parikh CR. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2010; 5:402–408.
- Tsai JJ, Yeun JY, Kumar VA, Don BR. Comparison and interpretation of urinalysis performed by a nephrologist versus a hospital-based clinical laboratory. Am J Kidney Dis 2005; 46:820–829.
Additional Reading
Fogazzi GB, Garigali G, Pirovano B, Muratore MT, Raimondi S, Berti S. How to improve the teaching of urine microscopy. Clin Chem Lab Med 2007; 45:407–412.
Fogazzi GB, Secchiero S. The role of nephrologists in teaching urinary sediment examination. Am J Kidney Dis 2006; 47:713.
Fogazzi GB, Verdesca S, Garigali G. Urinalysis: core curriculum 2008. Am J Kidney Dis 2008; 51:1052–1067.
The urine is the window to the kidney.This oft-repeated adage impresses upon medical students and residents the importance of urine microscopy in the evaluation of patients with renal disorders.
While this phrase is likely an adaptation of the idea in ancient times that the urine reflected on humors or the quality of the soul, the understanding of the relevance of urine findings to the state of the kidneys likely rests with the pioneers of urine microscopy. As reviewed by Fogazzi and Cameron,1,2 the origins of direct inspection of urine under a microscope lie in the 17th century, with industrious physicians who used rudimentary microscopes to identify basic structures in the urine and correlated them to clinical presentations.1 At first, only larger structures could be seen, mostly crystals in patients with nephrolithiasis. As microscopes advanced, smaller structures such as “corpuscles” and “cylinders” could be seen that described cells and casts.1
In correlating these findings to patient presentations, a rudimentary understanding of renal pathology evolved long before the advent of the kidney biopsy. Lipid droplets were seen1 in patients swollen from dropsy, and later known to have nephrotic syndromes. In 1872, Harley first described the altered morphology of dysmorphic red blood cells in patients with Bright disease or glomerulonephritis.1,3 In 1979, Birch and Fairley recognized that the presence of acanthocytes differentiated glomerular from nonglomerular hematuria.4
DYSMORPHIC RED BLOOD CELLS: TYPES AND SIGNIFICANCE
URINE MICROSCOPY: THE NEPHROLOGIST’S ROLE
The tools used in urine microscopy have advanced significantly since its advent. But not all advances have led to improved patient care. Laboratories have trained technicians to perform urine microscopy. Analyzers can identify basic urinary structures using algorithms to compare them against stored reference images. More important, urine microscopy has been categorized by accreditation and inspection bodies as a “test” rather than a physician-performed competency. As such, definitions of quality in urine microscopy have shifted from the application of urinary findings to the care of the patient to the reproducibility of identifying individual structures in ways that can be documented with quality checks performed by nonclinicians. And since the governing bodies require laboratories to adhere to burdensome procedures to maintain accreditation (eg, the US Food and Drug Administration’s Clinical Laboratory Improvement Amendments), many hospitals have closed nephrologist-based urine laboratories.
This would be acceptable if laboratory-generated reports provided information equivalent to that obtained by the nephrologist. But such reports rarely include anything beyond the most rudimentary findings. In these reports, the red blood cell is not differentiated as dysmorphic or monomorphic. All casts are granular. Crystals are often the highlight of the report, usually an incidental finding of little relevance. Phase contrast and polarization are never performed.
Despite the poor quality of data provided in these reports, because of increasing regulations and time restrictions, a dwindling number of nephrologists perform urine microscopy even at teaching institutions. In an informal 2009 survey of nephrology fellowship program directors, 79% of responding programs relied solely on lab-generated reports for microscopic findings (verbal communication, Perazella, 2017).
There is general concern among medical educators about the surrendering of the physical examination and other techniques to technology.7,8 However, in many cases, such changes may improve the ability to make a correct diagnosis. When performed properly, urine microscopy can help determine the need for kidney biopsy, differentiate causes of acute kidney injury, and help guide decisions about therapy. Perazella showed that urine microscopy could reliably differentiate acute tubular necrosis from prerenal azotemia.9 Further, the severity of findings on urine microscopy has been associated with worse renal outcomes.10 At our institution, nephrologist-performed urine microscopy resulted in a change in cause of acute kidney injury in 25% of cases and a concrete change in management in 12% of patients (unpublished data).
With this in mind, it is concerning that the only evidence in the literature on this topic demonstrated that laboratory-based urine microscopy is actually a hindrance to its underlying purpose in acute kidney injury, which is to help identify the cause of the injury. Tsai et al11 showed that nephrologists identified the cause of acute kidney injury correctly 90% of the time when they performed their own urine microscopy, but this dropped to 23% when they relied on a laboratory-generated report. Interestingly, knowing the patient’s clinical history when performing the microscopy was important, as the accuracy was 69% when a report of another nephrologist’s microscopy findings was used.11
APPLYING RESULTS TO THE PATIENT
The purpose of urine microscopy in clinical care is to identify and understand the findings as they apply to the patient. When viewed from this perspective, the renal patient is clearly best served when the nephrologist familiar with the case performs urine microscopy, rather than a technician or analyzer in remote parts of the hospital with no connection to the patient.
Advances in technology or streamlining of hospital services do not always produce improvements in patient care, and how we define quality is integral to identifying when this is the case. Quality checklists can serve as guides to safe patient care but should not replace clinical decision-making. Direct physician involvement with our patients has concrete benefits, whether taking a history, performing a physical examination, reviewing radiologic images, or looking at specimens such as urine. It allows us to experience the amazing pathophysiology of human illness and to understand the nuances unique to each of our patients.
But most important, it reinforces the need for the direct bond, both emotional and physical, between us as healers and our patients.
The urine is the window to the kidney.This oft-repeated adage impresses upon medical students and residents the importance of urine microscopy in the evaluation of patients with renal disorders.
While this phrase is likely an adaptation of the idea in ancient times that the urine reflected on humors or the quality of the soul, the understanding of the relevance of urine findings to the state of the kidneys likely rests with the pioneers of urine microscopy. As reviewed by Fogazzi and Cameron,1,2 the origins of direct inspection of urine under a microscope lie in the 17th century, with industrious physicians who used rudimentary microscopes to identify basic structures in the urine and correlated them to clinical presentations.1 At first, only larger structures could be seen, mostly crystals in patients with nephrolithiasis. As microscopes advanced, smaller structures such as “corpuscles” and “cylinders” could be seen that described cells and casts.1
In correlating these findings to patient presentations, a rudimentary understanding of renal pathology evolved long before the advent of the kidney biopsy. Lipid droplets were seen1 in patients swollen from dropsy, and later known to have nephrotic syndromes. In 1872, Harley first described the altered morphology of dysmorphic red blood cells in patients with Bright disease or glomerulonephritis.1,3 In 1979, Birch and Fairley recognized that the presence of acanthocytes differentiated glomerular from nonglomerular hematuria.4
DYSMORPHIC RED BLOOD CELLS: TYPES AND SIGNIFICANCE
URINE MICROSCOPY: THE NEPHROLOGIST’S ROLE
The tools used in urine microscopy have advanced significantly since its advent. But not all advances have led to improved patient care. Laboratories have trained technicians to perform urine microscopy. Analyzers can identify basic urinary structures using algorithms to compare them against stored reference images. More important, urine microscopy has been categorized by accreditation and inspection bodies as a “test” rather than a physician-performed competency. As such, definitions of quality in urine microscopy have shifted from the application of urinary findings to the care of the patient to the reproducibility of identifying individual structures in ways that can be documented with quality checks performed by nonclinicians. And since the governing bodies require laboratories to adhere to burdensome procedures to maintain accreditation (eg, the US Food and Drug Administration’s Clinical Laboratory Improvement Amendments), many hospitals have closed nephrologist-based urine laboratories.
This would be acceptable if laboratory-generated reports provided information equivalent to that obtained by the nephrologist. But such reports rarely include anything beyond the most rudimentary findings. In these reports, the red blood cell is not differentiated as dysmorphic or monomorphic. All casts are granular. Crystals are often the highlight of the report, usually an incidental finding of little relevance. Phase contrast and polarization are never performed.
Despite the poor quality of data provided in these reports, because of increasing regulations and time restrictions, a dwindling number of nephrologists perform urine microscopy even at teaching institutions. In an informal 2009 survey of nephrology fellowship program directors, 79% of responding programs relied solely on lab-generated reports for microscopic findings (verbal communication, Perazella, 2017).
There is general concern among medical educators about the surrendering of the physical examination and other techniques to technology.7,8 However, in many cases, such changes may improve the ability to make a correct diagnosis. When performed properly, urine microscopy can help determine the need for kidney biopsy, differentiate causes of acute kidney injury, and help guide decisions about therapy. Perazella showed that urine microscopy could reliably differentiate acute tubular necrosis from prerenal azotemia.9 Further, the severity of findings on urine microscopy has been associated with worse renal outcomes.10 At our institution, nephrologist-performed urine microscopy resulted in a change in cause of acute kidney injury in 25% of cases and a concrete change in management in 12% of patients (unpublished data).
With this in mind, it is concerning that the only evidence in the literature on this topic demonstrated that laboratory-based urine microscopy is actually a hindrance to its underlying purpose in acute kidney injury, which is to help identify the cause of the injury. Tsai et al11 showed that nephrologists identified the cause of acute kidney injury correctly 90% of the time when they performed their own urine microscopy, but this dropped to 23% when they relied on a laboratory-generated report. Interestingly, knowing the patient’s clinical history when performing the microscopy was important, as the accuracy was 69% when a report of another nephrologist’s microscopy findings was used.11
APPLYING RESULTS TO THE PATIENT
The purpose of urine microscopy in clinical care is to identify and understand the findings as they apply to the patient. When viewed from this perspective, the renal patient is clearly best served when the nephrologist familiar with the case performs urine microscopy, rather than a technician or analyzer in remote parts of the hospital with no connection to the patient.
Advances in technology or streamlining of hospital services do not always produce improvements in patient care, and how we define quality is integral to identifying when this is the case. Quality checklists can serve as guides to safe patient care but should not replace clinical decision-making. Direct physician involvement with our patients has concrete benefits, whether taking a history, performing a physical examination, reviewing radiologic images, or looking at specimens such as urine. It allows us to experience the amazing pathophysiology of human illness and to understand the nuances unique to each of our patients.
But most important, it reinforces the need for the direct bond, both emotional and physical, between us as healers and our patients.
- Fogazzi GB, Cameron JS. Urinary microscopy from the seventeenth century to the present day. Kidney Int 1996; 50:1058–1068.
- Cameron JS. A history of urine microscopy. Clin Chem Lab Med 2015; 53(suppl 2):s1453–s1464.
- Harley G. The Urine and Its Derangements. London: J and A Churchill, 1872:178–179.
- Birch DF, Fairley K. Hematuria: glomerular or non-glomerular? Lancet 1979; 314:845–846.
- Köhler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int 1991; 40:115–120.
- Daza JL, De Rosa M, De Rosa G. Dysmorphic red blood cells. Cleve Clin J Med 2018; 85:12–13.
- Jauhar S. The demise of the physical exam. N Engl J Med 2006; 354:548–551.
- Mangione S. When the tail wags the dog: clinical skills in the age of technology. Cleve Clin J Med 2017; 84:278–280.
- Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2008; 3:1615–1619.
- Perazella MA, Coca SG, Hall IE, Iyanam U, Koraishy M, Parikh CR. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2010; 5:402–408.
- Tsai JJ, Yeun JY, Kumar VA, Don BR. Comparison and interpretation of urinalysis performed by a nephrologist versus a hospital-based clinical laboratory. Am J Kidney Dis 2005; 46:820–829.
Additional Reading
Fogazzi GB, Garigali G, Pirovano B, Muratore MT, Raimondi S, Berti S. How to improve the teaching of urine microscopy. Clin Chem Lab Med 2007; 45:407–412.
Fogazzi GB, Secchiero S. The role of nephrologists in teaching urinary sediment examination. Am J Kidney Dis 2006; 47:713.
Fogazzi GB, Verdesca S, Garigali G. Urinalysis: core curriculum 2008. Am J Kidney Dis 2008; 51:1052–1067.
- Fogazzi GB, Cameron JS. Urinary microscopy from the seventeenth century to the present day. Kidney Int 1996; 50:1058–1068.
- Cameron JS. A history of urine microscopy. Clin Chem Lab Med 2015; 53(suppl 2):s1453–s1464.
- Harley G. The Urine and Its Derangements. London: J and A Churchill, 1872:178–179.
- Birch DF, Fairley K. Hematuria: glomerular or non-glomerular? Lancet 1979; 314:845–846.
- Köhler H, Wandel E, Brunck B. Acanthocyturia—a characteristic marker for glomerular bleeding. Kidney Int 1991; 40:115–120.
- Daza JL, De Rosa M, De Rosa G. Dysmorphic red blood cells. Cleve Clin J Med 2018; 85:12–13.
- Jauhar S. The demise of the physical exam. N Engl J Med 2006; 354:548–551.
- Mangione S. When the tail wags the dog: clinical skills in the age of technology. Cleve Clin J Med 2017; 84:278–280.
- Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2008; 3:1615–1619.
- Perazella MA, Coca SG, Hall IE, Iyanam U, Koraishy M, Parikh CR. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2010; 5:402–408.
- Tsai JJ, Yeun JY, Kumar VA, Don BR. Comparison and interpretation of urinalysis performed by a nephrologist versus a hospital-based clinical laboratory. Am J Kidney Dis 2005; 46:820–829.
Additional Reading
Fogazzi GB, Garigali G, Pirovano B, Muratore MT, Raimondi S, Berti S. How to improve the teaching of urine microscopy. Clin Chem Lab Med 2007; 45:407–412.
Fogazzi GB, Secchiero S. The role of nephrologists in teaching urinary sediment examination. Am J Kidney Dis 2006; 47:713.
Fogazzi GB, Verdesca S, Garigali G. Urinalysis: core curriculum 2008. Am J Kidney Dis 2008; 51:1052–1067.
A 50-year-old woman with new-onset seizure
A 50-year-old woman presented to the emergency department after a witnessed loss of consciousness and seizurelike activity. She reported that she had been sitting outside her home, drinking coffee in the morning, but became very lightheaded when she went back into her house. At that time she felt could not focus and had a sense of impending doom. She sat down in a chair and her symptoms worsened.
According to her family, her eyes rolled back and she became rigid. The family helped her to the floor. Her body then made jerking movements that lasted for about 1 minute. She regained consciousness but was very confused for about 10 minutes until emergency medical services personnel arrived. She had no recollection of passing out. She said nothing like this had ever happened to her before.
On arrival in the emergency department, she complained of generalized headache and muscle soreness. She said the headache had been present for 1 week and was constant and dull. There were no aggravating or alleviating factors associated with the headache, and she denied fever, chills, nausea, numbness, tingling, incontinence, tongue biting, tremor, poor balance, ringing in ears, speech difficulty, or weakness.
Medical history: Multiple problems, medications
The patient’s medical history included depression, hypertension, anxiety, osteoarthritis, and asthma. She was allergic to penicillin. She had undergone carpal tunnel surgery on her right hand 5 years previously. She was perimenopausal with no children.
She denied using illicit drugs. She said she had smoked a half pack of cigarettes per day for more than 10 years and was a current smoker but was actively trying to quit. She said she occasionally used alcohol but had not consumed any alcohol in the last 2 weeks.
She had no history of central nervous system infection. She did report an episode of head trauma in grade school when a portable basketball hoop fell, striking her on the top of the head and causing her to briefly lose consciousness, but she did not seek medical attention.
She had no family history of seizure or neurologic disease.
Her current medications included atenolol, naproxen, gabapentin, venlafaxine, zolpidem, lorazepam, bupropion, and meloxicam. The bupropion and lorazepam had been prescribed recently for her anxiety. She reported that she had been given only 10 tablets of lorazepam and had taken the last tablet 48 hours previously. She had been taking the bupropion for 7 days. She reported an increase in stress lately and had been taking zolpidem due to an altered sleep pattern.
PHYSICAL EXAMINATION, INITIAL TESTS
On examination, the patient did not appear to be in acute distress. Her blood pressure was 107/22 mm Hg, pulse 100 beats per minute, respiratory rate 16 breaths per minute, temperature 37.1°C (98.8°F), and oxygen saturation 98% on room air.
Examination of her head, eyes, mouth, and neck were unremarkable. Cardiovascular, pulmonary, and abdominal examinations were normal. She had no neurologic deficits and was fully alert and oriented. She had no visible injuries.
Blood and urine samples were obtained about 15 minutes after her arrival to the emergency department. Results showed:
- Glucose 73 mg/dL (reference range 74–99)
- Sodium 142 mmol/L (136–144)
- Blood urea nitrogen 12 mg/dL (7–21)
- Creatinine 0.95 mg/dL (0.58–0.96)
- Chloride 97 mmol/L (97–105)
- Carbon dioxide (bicarbonate) 16 mmol/L (22–30)
- Prolactin 50.9 ng/mL (4.5–26.8)
- Anion gap 29 mmol/L (9–18)
- Ethanol undetectable
- White blood cell count 11.03 × 109/L (3.70–11.00)
- Creatine kinase 89 U/L (30–220)
- Urinalysis normal, specific gravity 1.010 (1.005–1.030), no detectable ketones, and no crystals seen on microscopic evaluation.
Electrocardiography showed normal sinus rhythm with no ectopy and no ST-segment changes. Chest radiography was negative for any acute process.
The patient was transferred to the 23-hour observation unit in stable condition for further evaluation, monitoring, and management.
SIGNS AND SYMPTOMS OF SEIZURE
1. What findings are consistent with seizure?
- Jerking movements
- Confusion following the event
- Tongue-biting
- Focal motor weakness
- Urinary incontinence
- Aura before the event
All of the above findings are consistent with seizure.
The first consideration in evaluating a patient who presents with a possible seizure is whether the patient’s recollections of the event—and those of the witnesses—are consistent with the symptoms of seizure.1
In generalized tonic-clonic or grand mal seizure, the patient may experience an aura or subjective sensations before the onset. These vary greatly among patients.2 There may be an initial vocalization at the onset of the seizure, such as crying out or unintelligible speech. The patient’s eyes may roll back in the head. This is followed by loss of muscle tone, and if the patient is standing, he or she may fall to the ground. The patient becomes unresponsive and may go into respiratory arrest. There is tonic stiffening of the limbs and body, followed by clonic movements typically lasting 1 to 2 minutes, or sometimes longer.1,3,4 The patient will then relax and experience a period of unconsciousness or confusion (postictal state).
Urinary incontinence and tongue-biting strongly suggest seizure activity, and turning the head to one side and posturing may also be seen.3,5 After the event, the patient may report headache, generalized muscle soreness, exhaustion, or periods of transient focal weakness, also known as Todd paralysis.2,5
Our patient had aura-like symptoms at the outset. She felt very lightheaded, had difficulty focusing, and felt a sense of impending doom. She did not make any vocalizations at the onset, but her eyes did roll backward and she became rigid (tonic). She then lost muscle tone and became unresponsive. Her family had to help her to the floor. Jerking (clonic) movements were witnessed.
She regained consciousness but was described as being confused (postictal) for 10 minutes. Additionally, she denied ever having had symptoms like this previously. On arrival in the emergency department, she reported generalized headache and muscle soreness, but no tongue-biting or urinary incontinence. Her event did not last for more than 1 to 2 minutes according to her family.
Her symptoms strongly suggest new-onset tonic-clonic or grand mal seizure, though this is not completely certain.
LABORATORY FINDINGS IN SEIZURES
2. What laboratory results are consistent with seizure?
- Prolactin elevation
- Anion gap acidosis
- Leukocytosis
As noted above, the patient had an elevated prolactin level and elevated anion gap. Both of these findings can be used, with caution, in evaluating seizure activity.
Prolactin testing is controversial
Prolactin testing in diagnosing seizure activity is controversial. The exact mechanism of prolactin release in seizures is not fully understood. Generalized tonic-clonic seizures and complex partial seizures have both been shown to elevate prolactin. Prolactin levels after these types of seizures should rise within 30 minutes of the event and normalize 1 hour later.6
However, other events and conditions that mimic seizure have been shown to cause a rise in prolactin; these include syncope, transient ischemic attack, cardiac dysrhythmia, migraine, and other epilepsy-like variants. This effect has not been adequately studied. Therefore, an elevated prolactin level alone cannot diagnose or exclude seizure.7
For the prolactin level to be helpful, the blood sample must be drawn within 10 to 20 minutes after a possible seizure. Even if the prolactin level remains normal, it does not rule out seizure. Prolactin levels should therefore be used in combination with other testing to make a definitive diagnosis or exclusion of seizure.8
Anion gap and Denver Seizure Score
The anion gap has also been shown to rise after generalized seizure due to the metabolic acidosis that occurs. With a bicarbonate level of 16 mmol/L, an elevated anion gap, and normal breathing, our patient very likely had metabolic acidosis.
It is sometimes difficult to differentiate syncope from seizure, as they share several features.
The Denver Seizure Score can help differentiate these two conditions. It is based on the patient’s anion gap and bicarbonate level and is calculated as follows:
(24 – bicarbonate) + [2 × (anion gap – 12)]
A score above 20 strongly indicates seizure activity. However, this is not a definitive tool for diagnosis. Like an elevated prolactin level, the Denver Seizure Score should be used in combination with other testing to move toward a definitive diagnosis.9
Our patient’s anion gap was 29 mmol/L and her bicarbonate level was 16 mmol/L. Her Denver Seizure Score was therefore 42, which supports this being an episode of generalized seizure activity.
Leukocytosis
The patient had a white blood cell count of 11.03 × 109/L, which was mildly elevated. She had no history of fever and no source of infection by history.
Leukocytosis is common following generalized tonic-clonic seizure. A fever may lower the seizure threshold; however, our patient was not febrile and clinically had no factors that raised concern for an underlying infection.
ANION GAP ACIDOSIS AND SEIZURE
3. Which of the following can cause both anion gap acidosis and seizure?
- Ethylene glycol
- Salicylate overdose
- Ethanol withdrawal without ketosis
- Alcoholic ketoacidosis
- Methanol
All of the above except for ethanol withdrawal without ketosis can cause both anion gap acidosis and seizure.
Ethylene glycol can cause seizure and an elevated anion gap acidosis. However, this patient had no history of ingesting antifreeze (the most common source of ethylene glycol in the home) and no evidence of calcium oxalate crystals in the urine, which would be a sign of ethylene glycol toxicity. Additional testing for ethylene glycol may include serum ethylene glycol levels and ultraviolet light testing of the urine to detect fluorescein, which is commonly added to automotive antifreeze to help mechanics find fluid leaks in engines.
Salicylate overdose can cause seizure and an elevated anion gap acidosis. However, this patient has no history of aspirin ingestion, and a serum aspirin level was later ordered and found to be negative. In addition, the acid-base disorder in salicylate overdose may be respiratory alkalosis from direct stimulation of respiratory centers in conjunction with metabolic acidosis.
Ethanol withdrawal can cause seizure and may result in ketoacidosis, which would appear as anion gap acidosis. The undetectable ethanol level in this patient would be consistent with withdrawal from ethanol, which may also lead to ketoacidosis.
Alcoholic ketoacidosis is a late finding in patients who have been drinking ethanol and is thus a possible cause of an elevated anion gap in this patient. However, the absence of ketones in her urine speaks against this diagnosis.
Methanol can cause seizure and acidosis, but laboratory testing would reveal a normal anion gap and an elevated osmolar gap. This was not likely in this patient.
The presence of anion gap acidosis is important in forming a differential diagnosis. Several causes of anion gap acidosis may also cause seizure. These include salicylates, ethanol withdrawal with ketosis, methanol, and isoniazid. None of these appears to be a factor in this patient’s case.
DIFFERENTIAL DIAGNOSIS IN OUR PATIENT
4. What is the most likely cause of this patient’s seizure?
- Bupropion side effect
- Benzodiazepine withdrawal
- Ethanol withdrawal
- Brain lesion
- Central nervous system infection
- Unprovoked seizure (new-onset epilepsy)
Bupropion, an inhibitor of neuronal reuptake of norepinephrine and dopamine, has been used in the United States since 1989 to treat major depression.10 At therapeutic doses, it lowers the seizure threshold; in cases of acute overdose, seizures typically occur within hours of the dose, or up to 24 hours in patients taking extended-release formulations.11
Bupropion should be used with caution or avoided in patients taking other medications that also lower the seizure threshold, or during withdrawal from alcohol, benzodiazepines, or barbiturates.10
Benzodiazepine withdrawal. Abrupt cessation of benzodiazepines also lowers the seizure threshold, and seizures are commonly seen in benzodiazepine withdrawal syndrome. The use of benzodiazepines is controversial in many situations, and discontinuing them may prove problematic for both the patient and physician, as the potential for abuse and addiction is significant.
Seizures have occurred during withdrawal from even short-term benzodiazepine use. Other factors, such as concomitant use of other medications that lower the seizure threshold, may play a more significant role in causing withdrawal seizures than the duration of benzodiazepine therapy.12
Medications shown to be useful in managing withdrawal from benzodiazepines include carbamazepine, imipramine, valproate, and trazodone. Paroxetine has also been shown to be helpful in patients with major depression who are being taken off a benzodiazepine.13
Ethanol withdrawal is common in patients presenting to emergency departments, and seizures are frequently seen in these patients. This patient reported social drinking but not drinking ethanol daily, although many patients are not forthcoming about alcohol or drug use when talking with a physician or other healthcare provider.
Alcohol withdrawal seizures may accompany delirium tremens or major withdrawal syndrome, but they are seen more often in the absence of major withdrawal or delirium tremens. Seizures are typically single or occur in a short grouping over a brief period of time and mostly occur in chronic alcoholism. The role of anticonvulsants in patients with alcohol withdrawal seizure has not been established.14
Brain lesion. A previously undiagnosed brain tumor is not a common cause of new-onset seizure, although it is not unusual for a brain tumor to cause new-onset seizure. In 1 study, 6% of patients with new-onset seizure had a clinically significant lesion on brain imaging.15 In addition, 15% to 30% of patients with a previously undiagnosed brain tumor present with seizure as the first symptom.16 Patients with abnormal findings on neurologic examination after the seizure activity are more likely to have a structural lesion that may be identified by computed tomography (CT) or magnetic resonance imaging. (MRI)15
Unprovoked seizure occurs without an identifiable precipitating factor, or from a central nervous system insult that occurred more than 7 days earlier. Patients who may have recurrent unprovoked seizure will likely be diagnosed with epilepsy.15 Patients with a first-time unprovoked seizure have a 30% or higher likelihood of having another unprovoked seizure within 5 years.17
It is most likely that bupropion is the key factor in lowering the seizure threshold in this patient. However, patients sometimes underreport the amount of alcohol they consume, and though less likely, our patient’s report of not drinking for 2 weeks may also be unreliable. Ethanol withdrawal, though unlikely, may also be a consideration with this case.
FURTHER TESTING FOR OUR PATIENT
5. Which tests may be helpful in this patient’s workup?
- CT of the brain
- Lumbar puncture for spinal fluid analysis
- MRI of the brain
- Electroencephalography (EEG)
This patient had had a headache for 1 week before presenting to the emergency department. Indications for neuroimaging in a patient with headache include sudden onset of severe headache, neurologic deficits, human immunodeficiency virus infection, loss of consciousness, immunosuppression, pregnancy, malignancy, and age over 50 with a new type of headache.18,19 Therefore, she should undergo some form of neuroimaging, either CT or MRI.
CT is the most readily available and fastest imaging study for the central nervous system available to emergency physicians. CT is limited, however, due to its decreased sensitivity in detecting some brain lesions. Therefore, many patients with first-time seizure may eventually require MRI.15 Furthermore, patients with focal onset of the seizure activity are more likely to have a structural lesion precipitating the seizure. MRI may have a higher yield than CT in these cases.15,20
Lumbar puncture for spinal fluid analysis is helpful in evaluating a patient with a suspected central nervous system infection such as meningitis or encephalitis, or subarachnoid hemorrhage.
This patient had a normal neurologic examination, no fever, and no meningeal signs, and central nervous system infection was very unlikely. Also, because she had had a headache for 1 week before the presentation with seizurelike activity, subarachnoid hemorrhage was very unlikely, and emergency lumbar puncture was not indicated.
MRI is less readily available than CT in a timely fashion in most emergency departments in the United States. It offers a higher yield than CT in diagnosing pathology such as acute stroke, brain tumor, and plaques seen in multiple sclerosis. CT is superior to MRI in diagnosing bony abnormalities and is very sensitive for detecting acute bleeding.
If MRI is performed, it should follow a specific protocol that includes high-resolution images for epilepsy evaluation rather than the more commonly ordered stroke protocol. The stroke protocol is more likely to be ordered in the emergency department.
EEG is well established in evaluating new-onset seizure in pediatric patients. Studies also demonstrate its utility in evaluating first-time seizure in adults, providing evidence that both epileptiform and nonepileptiform abnormalities seen on EEG are associated with a higher risk of recurrent seizure activity than in patients with normal findings on EEG.1
EEG may be difficult to interpret in adults. According to Benbadis,5 as many as one-third of adult patients diagnosed with epilepsy on EEG did not have epilepsy. This is because of normal variants, simple fluctuations of background rhythms, or fragmented alpha activity that can have a similar appearance to epileptiform patterns. EEG must always be interpreted in the context of the patient’s history and symptoms.5
Though EEG has limitations, it remains a crucial tool for identifying epilepsy. Following a single seizure, the decision to prescribe antiepileptic drugs is highly influenced by patterns on EEG associated with a risk of recurrence. In fact, a patient experiencing a single, idiopathic seizure and exhibiting an EEG pattern of spike wave discharges is likely to have recurrent seizure activity.21 Also, the appropriate use of EEG after even a single unprovoked seizure can identify patients with epilepsy and a risk of recurrent seizure greater than 60%.21,22
NO FURTHER SEIZURES
The patient was admitted to the observation unit from the emergency department after undergoing CT without intravenous contrast. While in observation, she had no additional episodes, and her vital signs remained within normal limits.
She underwent MRI and EEG as well as repeat laboratory studies and consultation by a neurologist. CT showed no structural abnormality, MRI results were read as normal, and EEG showed no epileptiform spikes or abnormal slow waves or other abnormality consistent with seizure. The repeat laboratory studies revealed normalization of the prolactin level at 11.3 ng/mL (reference range 2.0–17.4).
The final impression of the neurology consultant was that the patient had had a seizure that was most likely due to recently starting bupropion in combination with the withdrawal of the benzodiazepine, which lowered the seizure threshold. The neurologist also believed that our patient had no findings or symptoms other than the seizure that would suggest benzodiazepine withdrawal syndrome. According to the patient’s social history, it was unlikely that she had the pattern of alcohol consumption that would result in ethanol withdrawal seizure.
Seizures are common. In fact, every year, 180,000 US adults have their first seizure, and 10% of Americans will experience at least 1 seizure during their lifetime. However, only 20% to 25% of seizures are generalized tonic-clonic seizures as in our patient.23
As this patient had an identifiable cause for the seizure, there was no need to initiate anticonvulsant therapy at the time of discharge. She was discharged to home without any anticonvulsant, the bupropion was discontinued, and the lorazepam was not restarted. When contacted by telephone at 1 month and 18 months after discharge, she reported she had not experienced any additional seizures and has not needed antiepileptic medications.
- Seneviratne U. Management of the first seizure: an evidence based approach. Postgrad Med J 2009; 85:667–673.
- Krumholz A, Wiebe S, Gronseth G, et al; Quality Standards Subcommittee of the American Academy of Neurology; American Epilepsy Society. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2007; 67:1996–2007.
- Gram L. Epileptic seizures and syndromes. Lancet 1990; 336:161–163.
- Smith PE, Cossburn MD. Seizures: assessment and management in the emergency unit. Clin Med (Lond) 2004; 4:118–122.
- Benbadis S. The differential diagnosis of epilepsy: a critical review. Epilepsy Behav 2009; 15:15–21.
- Lusic I, Pintaric I, Hozo I, Boic L, Capkun V. Serum prolactin levels after seizure and syncopal attacks. Seizure 1999; 8:218–222.
- Chen DK, So YT, Fisher RS; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005; 65:668–675.
- Ben-Menachem E. Is prolactin a clinically useful measure of epilepsy? Epilepsy Curr 2006; 6:78–79.
- Bakes KM, Faragher J, Markovchick VJ, Donahoe K, Haukoos JS. The Denver Seizure Score: anion gap metabolic acidosis predicts generalized seizure. Am J Emerg Med 2011; 29:1097–1102.
- Jefferson JW, Pradok JF, Muir KT. Bupropion for major depressive disorder: pharmacokinetic and formulation considerations. Clin Ther 2005; 27:1685–1695.
- Stall N, Godwin J, Juurlink D. Bupropion abuse and overdose. CMAJ 2014; 186:1015.
- Fialip J, Aumaitre O, Eschalier A, Maradeix B, Dordain G, Lavarenne J. Benzodiazepine withdrawal seizures: analysis of 48 case reports. Clin Neuropharmacol 1987; 10:538–544.
- Lader M, Tylee A, Donoghue J. Withdrawing benzodiazepines in primary care. CNS Drugs 2009; 23:19–34.
- Chance JF. Emergency department treatment of alcohol withdrawal seizures with phenytoin. Ann Emerg Med 1991; 20:520–522.
- ACEP Clinical Policies Committee; Clinical Policies Subcommittee on Seizures. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med 2004; 43:605–625.
- Sperling MR, Ko J. Seizures and brain tumors. Semin Oncol 2006; 33:333–341.
- Musicco M, Beghi E, Solari A, Viani F. Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. First Seizure Trial Group (FIRST Group). Neurology 1997; 49:991–998.
- Edlow JA, Panagos PD, Godwin SA, Thomas TL, Decker WW; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med 2008; 52:407–436.
- Kaniecki R. Headache assessment and management. JAMA 2003; 289:1430–1433.
- Harden CL, Huff JS, Schwartz TH, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2007; 69:1772–1780.
- Bergey GK. Management of a first seizure. Continuum (Minneap Minn) 2016; 22:38–50.
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55:475–482.
- Ko DY. Generalized tonic-clonic seizures. Medscape. http://emedicine.medscape.com/article/1184608-overview. Accessed December 5, 2017.
A 50-year-old woman presented to the emergency department after a witnessed loss of consciousness and seizurelike activity. She reported that she had been sitting outside her home, drinking coffee in the morning, but became very lightheaded when she went back into her house. At that time she felt could not focus and had a sense of impending doom. She sat down in a chair and her symptoms worsened.
According to her family, her eyes rolled back and she became rigid. The family helped her to the floor. Her body then made jerking movements that lasted for about 1 minute. She regained consciousness but was very confused for about 10 minutes until emergency medical services personnel arrived. She had no recollection of passing out. She said nothing like this had ever happened to her before.
On arrival in the emergency department, she complained of generalized headache and muscle soreness. She said the headache had been present for 1 week and was constant and dull. There were no aggravating or alleviating factors associated with the headache, and she denied fever, chills, nausea, numbness, tingling, incontinence, tongue biting, tremor, poor balance, ringing in ears, speech difficulty, or weakness.
Medical history: Multiple problems, medications
The patient’s medical history included depression, hypertension, anxiety, osteoarthritis, and asthma. She was allergic to penicillin. She had undergone carpal tunnel surgery on her right hand 5 years previously. She was perimenopausal with no children.
She denied using illicit drugs. She said she had smoked a half pack of cigarettes per day for more than 10 years and was a current smoker but was actively trying to quit. She said she occasionally used alcohol but had not consumed any alcohol in the last 2 weeks.
She had no history of central nervous system infection. She did report an episode of head trauma in grade school when a portable basketball hoop fell, striking her on the top of the head and causing her to briefly lose consciousness, but she did not seek medical attention.
She had no family history of seizure or neurologic disease.
Her current medications included atenolol, naproxen, gabapentin, venlafaxine, zolpidem, lorazepam, bupropion, and meloxicam. The bupropion and lorazepam had been prescribed recently for her anxiety. She reported that she had been given only 10 tablets of lorazepam and had taken the last tablet 48 hours previously. She had been taking the bupropion for 7 days. She reported an increase in stress lately and had been taking zolpidem due to an altered sleep pattern.
PHYSICAL EXAMINATION, INITIAL TESTS
On examination, the patient did not appear to be in acute distress. Her blood pressure was 107/22 mm Hg, pulse 100 beats per minute, respiratory rate 16 breaths per minute, temperature 37.1°C (98.8°F), and oxygen saturation 98% on room air.
Examination of her head, eyes, mouth, and neck were unremarkable. Cardiovascular, pulmonary, and abdominal examinations were normal. She had no neurologic deficits and was fully alert and oriented. She had no visible injuries.
Blood and urine samples were obtained about 15 minutes after her arrival to the emergency department. Results showed:
- Glucose 73 mg/dL (reference range 74–99)
- Sodium 142 mmol/L (136–144)
- Blood urea nitrogen 12 mg/dL (7–21)
- Creatinine 0.95 mg/dL (0.58–0.96)
- Chloride 97 mmol/L (97–105)
- Carbon dioxide (bicarbonate) 16 mmol/L (22–30)
- Prolactin 50.9 ng/mL (4.5–26.8)
- Anion gap 29 mmol/L (9–18)
- Ethanol undetectable
- White blood cell count 11.03 × 109/L (3.70–11.00)
- Creatine kinase 89 U/L (30–220)
- Urinalysis normal, specific gravity 1.010 (1.005–1.030), no detectable ketones, and no crystals seen on microscopic evaluation.
Electrocardiography showed normal sinus rhythm with no ectopy and no ST-segment changes. Chest radiography was negative for any acute process.
The patient was transferred to the 23-hour observation unit in stable condition for further evaluation, monitoring, and management.
SIGNS AND SYMPTOMS OF SEIZURE
1. What findings are consistent with seizure?
- Jerking movements
- Confusion following the event
- Tongue-biting
- Focal motor weakness
- Urinary incontinence
- Aura before the event
All of the above findings are consistent with seizure.
The first consideration in evaluating a patient who presents with a possible seizure is whether the patient’s recollections of the event—and those of the witnesses—are consistent with the symptoms of seizure.1
In generalized tonic-clonic or grand mal seizure, the patient may experience an aura or subjective sensations before the onset. These vary greatly among patients.2 There may be an initial vocalization at the onset of the seizure, such as crying out or unintelligible speech. The patient’s eyes may roll back in the head. This is followed by loss of muscle tone, and if the patient is standing, he or she may fall to the ground. The patient becomes unresponsive and may go into respiratory arrest. There is tonic stiffening of the limbs and body, followed by clonic movements typically lasting 1 to 2 minutes, or sometimes longer.1,3,4 The patient will then relax and experience a period of unconsciousness or confusion (postictal state).
Urinary incontinence and tongue-biting strongly suggest seizure activity, and turning the head to one side and posturing may also be seen.3,5 After the event, the patient may report headache, generalized muscle soreness, exhaustion, or periods of transient focal weakness, also known as Todd paralysis.2,5
Our patient had aura-like symptoms at the outset. She felt very lightheaded, had difficulty focusing, and felt a sense of impending doom. She did not make any vocalizations at the onset, but her eyes did roll backward and she became rigid (tonic). She then lost muscle tone and became unresponsive. Her family had to help her to the floor. Jerking (clonic) movements were witnessed.
She regained consciousness but was described as being confused (postictal) for 10 minutes. Additionally, she denied ever having had symptoms like this previously. On arrival in the emergency department, she reported generalized headache and muscle soreness, but no tongue-biting or urinary incontinence. Her event did not last for more than 1 to 2 minutes according to her family.
Her symptoms strongly suggest new-onset tonic-clonic or grand mal seizure, though this is not completely certain.
LABORATORY FINDINGS IN SEIZURES
2. What laboratory results are consistent with seizure?
- Prolactin elevation
- Anion gap acidosis
- Leukocytosis
As noted above, the patient had an elevated prolactin level and elevated anion gap. Both of these findings can be used, with caution, in evaluating seizure activity.
Prolactin testing is controversial
Prolactin testing in diagnosing seizure activity is controversial. The exact mechanism of prolactin release in seizures is not fully understood. Generalized tonic-clonic seizures and complex partial seizures have both been shown to elevate prolactin. Prolactin levels after these types of seizures should rise within 30 minutes of the event and normalize 1 hour later.6
However, other events and conditions that mimic seizure have been shown to cause a rise in prolactin; these include syncope, transient ischemic attack, cardiac dysrhythmia, migraine, and other epilepsy-like variants. This effect has not been adequately studied. Therefore, an elevated prolactin level alone cannot diagnose or exclude seizure.7
For the prolactin level to be helpful, the blood sample must be drawn within 10 to 20 minutes after a possible seizure. Even if the prolactin level remains normal, it does not rule out seizure. Prolactin levels should therefore be used in combination with other testing to make a definitive diagnosis or exclusion of seizure.8
Anion gap and Denver Seizure Score
The anion gap has also been shown to rise after generalized seizure due to the metabolic acidosis that occurs. With a bicarbonate level of 16 mmol/L, an elevated anion gap, and normal breathing, our patient very likely had metabolic acidosis.
It is sometimes difficult to differentiate syncope from seizure, as they share several features.
The Denver Seizure Score can help differentiate these two conditions. It is based on the patient’s anion gap and bicarbonate level and is calculated as follows:
(24 – bicarbonate) + [2 × (anion gap – 12)]
A score above 20 strongly indicates seizure activity. However, this is not a definitive tool for diagnosis. Like an elevated prolactin level, the Denver Seizure Score should be used in combination with other testing to move toward a definitive diagnosis.9
Our patient’s anion gap was 29 mmol/L and her bicarbonate level was 16 mmol/L. Her Denver Seizure Score was therefore 42, which supports this being an episode of generalized seizure activity.
Leukocytosis
The patient had a white blood cell count of 11.03 × 109/L, which was mildly elevated. She had no history of fever and no source of infection by history.
Leukocytosis is common following generalized tonic-clonic seizure. A fever may lower the seizure threshold; however, our patient was not febrile and clinically had no factors that raised concern for an underlying infection.
ANION GAP ACIDOSIS AND SEIZURE
3. Which of the following can cause both anion gap acidosis and seizure?
- Ethylene glycol
- Salicylate overdose
- Ethanol withdrawal without ketosis
- Alcoholic ketoacidosis
- Methanol
All of the above except for ethanol withdrawal without ketosis can cause both anion gap acidosis and seizure.
Ethylene glycol can cause seizure and an elevated anion gap acidosis. However, this patient had no history of ingesting antifreeze (the most common source of ethylene glycol in the home) and no evidence of calcium oxalate crystals in the urine, which would be a sign of ethylene glycol toxicity. Additional testing for ethylene glycol may include serum ethylene glycol levels and ultraviolet light testing of the urine to detect fluorescein, which is commonly added to automotive antifreeze to help mechanics find fluid leaks in engines.
Salicylate overdose can cause seizure and an elevated anion gap acidosis. However, this patient has no history of aspirin ingestion, and a serum aspirin level was later ordered and found to be negative. In addition, the acid-base disorder in salicylate overdose may be respiratory alkalosis from direct stimulation of respiratory centers in conjunction with metabolic acidosis.
Ethanol withdrawal can cause seizure and may result in ketoacidosis, which would appear as anion gap acidosis. The undetectable ethanol level in this patient would be consistent with withdrawal from ethanol, which may also lead to ketoacidosis.
Alcoholic ketoacidosis is a late finding in patients who have been drinking ethanol and is thus a possible cause of an elevated anion gap in this patient. However, the absence of ketones in her urine speaks against this diagnosis.
Methanol can cause seizure and acidosis, but laboratory testing would reveal a normal anion gap and an elevated osmolar gap. This was not likely in this patient.
The presence of anion gap acidosis is important in forming a differential diagnosis. Several causes of anion gap acidosis may also cause seizure. These include salicylates, ethanol withdrawal with ketosis, methanol, and isoniazid. None of these appears to be a factor in this patient’s case.
DIFFERENTIAL DIAGNOSIS IN OUR PATIENT
4. What is the most likely cause of this patient’s seizure?
- Bupropion side effect
- Benzodiazepine withdrawal
- Ethanol withdrawal
- Brain lesion
- Central nervous system infection
- Unprovoked seizure (new-onset epilepsy)
Bupropion, an inhibitor of neuronal reuptake of norepinephrine and dopamine, has been used in the United States since 1989 to treat major depression.10 At therapeutic doses, it lowers the seizure threshold; in cases of acute overdose, seizures typically occur within hours of the dose, or up to 24 hours in patients taking extended-release formulations.11
Bupropion should be used with caution or avoided in patients taking other medications that also lower the seizure threshold, or during withdrawal from alcohol, benzodiazepines, or barbiturates.10
Benzodiazepine withdrawal. Abrupt cessation of benzodiazepines also lowers the seizure threshold, and seizures are commonly seen in benzodiazepine withdrawal syndrome. The use of benzodiazepines is controversial in many situations, and discontinuing them may prove problematic for both the patient and physician, as the potential for abuse and addiction is significant.
Seizures have occurred during withdrawal from even short-term benzodiazepine use. Other factors, such as concomitant use of other medications that lower the seizure threshold, may play a more significant role in causing withdrawal seizures than the duration of benzodiazepine therapy.12
Medications shown to be useful in managing withdrawal from benzodiazepines include carbamazepine, imipramine, valproate, and trazodone. Paroxetine has also been shown to be helpful in patients with major depression who are being taken off a benzodiazepine.13
Ethanol withdrawal is common in patients presenting to emergency departments, and seizures are frequently seen in these patients. This patient reported social drinking but not drinking ethanol daily, although many patients are not forthcoming about alcohol or drug use when talking with a physician or other healthcare provider.
Alcohol withdrawal seizures may accompany delirium tremens or major withdrawal syndrome, but they are seen more often in the absence of major withdrawal or delirium tremens. Seizures are typically single or occur in a short grouping over a brief period of time and mostly occur in chronic alcoholism. The role of anticonvulsants in patients with alcohol withdrawal seizure has not been established.14
Brain lesion. A previously undiagnosed brain tumor is not a common cause of new-onset seizure, although it is not unusual for a brain tumor to cause new-onset seizure. In 1 study, 6% of patients with new-onset seizure had a clinically significant lesion on brain imaging.15 In addition, 15% to 30% of patients with a previously undiagnosed brain tumor present with seizure as the first symptom.16 Patients with abnormal findings on neurologic examination after the seizure activity are more likely to have a structural lesion that may be identified by computed tomography (CT) or magnetic resonance imaging. (MRI)15
Unprovoked seizure occurs without an identifiable precipitating factor, or from a central nervous system insult that occurred more than 7 days earlier. Patients who may have recurrent unprovoked seizure will likely be diagnosed with epilepsy.15 Patients with a first-time unprovoked seizure have a 30% or higher likelihood of having another unprovoked seizure within 5 years.17
It is most likely that bupropion is the key factor in lowering the seizure threshold in this patient. However, patients sometimes underreport the amount of alcohol they consume, and though less likely, our patient’s report of not drinking for 2 weeks may also be unreliable. Ethanol withdrawal, though unlikely, may also be a consideration with this case.
FURTHER TESTING FOR OUR PATIENT
5. Which tests may be helpful in this patient’s workup?
- CT of the brain
- Lumbar puncture for spinal fluid analysis
- MRI of the brain
- Electroencephalography (EEG)
This patient had had a headache for 1 week before presenting to the emergency department. Indications for neuroimaging in a patient with headache include sudden onset of severe headache, neurologic deficits, human immunodeficiency virus infection, loss of consciousness, immunosuppression, pregnancy, malignancy, and age over 50 with a new type of headache.18,19 Therefore, she should undergo some form of neuroimaging, either CT or MRI.
CT is the most readily available and fastest imaging study for the central nervous system available to emergency physicians. CT is limited, however, due to its decreased sensitivity in detecting some brain lesions. Therefore, many patients with first-time seizure may eventually require MRI.15 Furthermore, patients with focal onset of the seizure activity are more likely to have a structural lesion precipitating the seizure. MRI may have a higher yield than CT in these cases.15,20
Lumbar puncture for spinal fluid analysis is helpful in evaluating a patient with a suspected central nervous system infection such as meningitis or encephalitis, or subarachnoid hemorrhage.
This patient had a normal neurologic examination, no fever, and no meningeal signs, and central nervous system infection was very unlikely. Also, because she had had a headache for 1 week before the presentation with seizurelike activity, subarachnoid hemorrhage was very unlikely, and emergency lumbar puncture was not indicated.
MRI is less readily available than CT in a timely fashion in most emergency departments in the United States. It offers a higher yield than CT in diagnosing pathology such as acute stroke, brain tumor, and plaques seen in multiple sclerosis. CT is superior to MRI in diagnosing bony abnormalities and is very sensitive for detecting acute bleeding.
If MRI is performed, it should follow a specific protocol that includes high-resolution images for epilepsy evaluation rather than the more commonly ordered stroke protocol. The stroke protocol is more likely to be ordered in the emergency department.
EEG is well established in evaluating new-onset seizure in pediatric patients. Studies also demonstrate its utility in evaluating first-time seizure in adults, providing evidence that both epileptiform and nonepileptiform abnormalities seen on EEG are associated with a higher risk of recurrent seizure activity than in patients with normal findings on EEG.1
EEG may be difficult to interpret in adults. According to Benbadis,5 as many as one-third of adult patients diagnosed with epilepsy on EEG did not have epilepsy. This is because of normal variants, simple fluctuations of background rhythms, or fragmented alpha activity that can have a similar appearance to epileptiform patterns. EEG must always be interpreted in the context of the patient’s history and symptoms.5
Though EEG has limitations, it remains a crucial tool for identifying epilepsy. Following a single seizure, the decision to prescribe antiepileptic drugs is highly influenced by patterns on EEG associated with a risk of recurrence. In fact, a patient experiencing a single, idiopathic seizure and exhibiting an EEG pattern of spike wave discharges is likely to have recurrent seizure activity.21 Also, the appropriate use of EEG after even a single unprovoked seizure can identify patients with epilepsy and a risk of recurrent seizure greater than 60%.21,22
NO FURTHER SEIZURES
The patient was admitted to the observation unit from the emergency department after undergoing CT without intravenous contrast. While in observation, she had no additional episodes, and her vital signs remained within normal limits.
She underwent MRI and EEG as well as repeat laboratory studies and consultation by a neurologist. CT showed no structural abnormality, MRI results were read as normal, and EEG showed no epileptiform spikes or abnormal slow waves or other abnormality consistent with seizure. The repeat laboratory studies revealed normalization of the prolactin level at 11.3 ng/mL (reference range 2.0–17.4).
The final impression of the neurology consultant was that the patient had had a seizure that was most likely due to recently starting bupropion in combination with the withdrawal of the benzodiazepine, which lowered the seizure threshold. The neurologist also believed that our patient had no findings or symptoms other than the seizure that would suggest benzodiazepine withdrawal syndrome. According to the patient’s social history, it was unlikely that she had the pattern of alcohol consumption that would result in ethanol withdrawal seizure.
Seizures are common. In fact, every year, 180,000 US adults have their first seizure, and 10% of Americans will experience at least 1 seizure during their lifetime. However, only 20% to 25% of seizures are generalized tonic-clonic seizures as in our patient.23
As this patient had an identifiable cause for the seizure, there was no need to initiate anticonvulsant therapy at the time of discharge. She was discharged to home without any anticonvulsant, the bupropion was discontinued, and the lorazepam was not restarted. When contacted by telephone at 1 month and 18 months after discharge, she reported she had not experienced any additional seizures and has not needed antiepileptic medications.
A 50-year-old woman presented to the emergency department after a witnessed loss of consciousness and seizurelike activity. She reported that she had been sitting outside her home, drinking coffee in the morning, but became very lightheaded when she went back into her house. At that time she felt could not focus and had a sense of impending doom. She sat down in a chair and her symptoms worsened.
According to her family, her eyes rolled back and she became rigid. The family helped her to the floor. Her body then made jerking movements that lasted for about 1 minute. She regained consciousness but was very confused for about 10 minutes until emergency medical services personnel arrived. She had no recollection of passing out. She said nothing like this had ever happened to her before.
On arrival in the emergency department, she complained of generalized headache and muscle soreness. She said the headache had been present for 1 week and was constant and dull. There were no aggravating or alleviating factors associated with the headache, and she denied fever, chills, nausea, numbness, tingling, incontinence, tongue biting, tremor, poor balance, ringing in ears, speech difficulty, or weakness.
Medical history: Multiple problems, medications
The patient’s medical history included depression, hypertension, anxiety, osteoarthritis, and asthma. She was allergic to penicillin. She had undergone carpal tunnel surgery on her right hand 5 years previously. She was perimenopausal with no children.
She denied using illicit drugs. She said she had smoked a half pack of cigarettes per day for more than 10 years and was a current smoker but was actively trying to quit. She said she occasionally used alcohol but had not consumed any alcohol in the last 2 weeks.
She had no history of central nervous system infection. She did report an episode of head trauma in grade school when a portable basketball hoop fell, striking her on the top of the head and causing her to briefly lose consciousness, but she did not seek medical attention.
She had no family history of seizure or neurologic disease.
Her current medications included atenolol, naproxen, gabapentin, venlafaxine, zolpidem, lorazepam, bupropion, and meloxicam. The bupropion and lorazepam had been prescribed recently for her anxiety. She reported that she had been given only 10 tablets of lorazepam and had taken the last tablet 48 hours previously. She had been taking the bupropion for 7 days. She reported an increase in stress lately and had been taking zolpidem due to an altered sleep pattern.
PHYSICAL EXAMINATION, INITIAL TESTS
On examination, the patient did not appear to be in acute distress. Her blood pressure was 107/22 mm Hg, pulse 100 beats per minute, respiratory rate 16 breaths per minute, temperature 37.1°C (98.8°F), and oxygen saturation 98% on room air.
Examination of her head, eyes, mouth, and neck were unremarkable. Cardiovascular, pulmonary, and abdominal examinations were normal. She had no neurologic deficits and was fully alert and oriented. She had no visible injuries.
Blood and urine samples were obtained about 15 minutes after her arrival to the emergency department. Results showed:
- Glucose 73 mg/dL (reference range 74–99)
- Sodium 142 mmol/L (136–144)
- Blood urea nitrogen 12 mg/dL (7–21)
- Creatinine 0.95 mg/dL (0.58–0.96)
- Chloride 97 mmol/L (97–105)
- Carbon dioxide (bicarbonate) 16 mmol/L (22–30)
- Prolactin 50.9 ng/mL (4.5–26.8)
- Anion gap 29 mmol/L (9–18)
- Ethanol undetectable
- White blood cell count 11.03 × 109/L (3.70–11.00)
- Creatine kinase 89 U/L (30–220)
- Urinalysis normal, specific gravity 1.010 (1.005–1.030), no detectable ketones, and no crystals seen on microscopic evaluation.
Electrocardiography showed normal sinus rhythm with no ectopy and no ST-segment changes. Chest radiography was negative for any acute process.
The patient was transferred to the 23-hour observation unit in stable condition for further evaluation, monitoring, and management.
SIGNS AND SYMPTOMS OF SEIZURE
1. What findings are consistent with seizure?
- Jerking movements
- Confusion following the event
- Tongue-biting
- Focal motor weakness
- Urinary incontinence
- Aura before the event
All of the above findings are consistent with seizure.
The first consideration in evaluating a patient who presents with a possible seizure is whether the patient’s recollections of the event—and those of the witnesses—are consistent with the symptoms of seizure.1
In generalized tonic-clonic or grand mal seizure, the patient may experience an aura or subjective sensations before the onset. These vary greatly among patients.2 There may be an initial vocalization at the onset of the seizure, such as crying out or unintelligible speech. The patient’s eyes may roll back in the head. This is followed by loss of muscle tone, and if the patient is standing, he or she may fall to the ground. The patient becomes unresponsive and may go into respiratory arrest. There is tonic stiffening of the limbs and body, followed by clonic movements typically lasting 1 to 2 minutes, or sometimes longer.1,3,4 The patient will then relax and experience a period of unconsciousness or confusion (postictal state).
Urinary incontinence and tongue-biting strongly suggest seizure activity, and turning the head to one side and posturing may also be seen.3,5 After the event, the patient may report headache, generalized muscle soreness, exhaustion, or periods of transient focal weakness, also known as Todd paralysis.2,5
Our patient had aura-like symptoms at the outset. She felt very lightheaded, had difficulty focusing, and felt a sense of impending doom. She did not make any vocalizations at the onset, but her eyes did roll backward and she became rigid (tonic). She then lost muscle tone and became unresponsive. Her family had to help her to the floor. Jerking (clonic) movements were witnessed.
She regained consciousness but was described as being confused (postictal) for 10 minutes. Additionally, she denied ever having had symptoms like this previously. On arrival in the emergency department, she reported generalized headache and muscle soreness, but no tongue-biting or urinary incontinence. Her event did not last for more than 1 to 2 minutes according to her family.
Her symptoms strongly suggest new-onset tonic-clonic or grand mal seizure, though this is not completely certain.
LABORATORY FINDINGS IN SEIZURES
2. What laboratory results are consistent with seizure?
- Prolactin elevation
- Anion gap acidosis
- Leukocytosis
As noted above, the patient had an elevated prolactin level and elevated anion gap. Both of these findings can be used, with caution, in evaluating seizure activity.
Prolactin testing is controversial
Prolactin testing in diagnosing seizure activity is controversial. The exact mechanism of prolactin release in seizures is not fully understood. Generalized tonic-clonic seizures and complex partial seizures have both been shown to elevate prolactin. Prolactin levels after these types of seizures should rise within 30 minutes of the event and normalize 1 hour later.6
However, other events and conditions that mimic seizure have been shown to cause a rise in prolactin; these include syncope, transient ischemic attack, cardiac dysrhythmia, migraine, and other epilepsy-like variants. This effect has not been adequately studied. Therefore, an elevated prolactin level alone cannot diagnose or exclude seizure.7
For the prolactin level to be helpful, the blood sample must be drawn within 10 to 20 minutes after a possible seizure. Even if the prolactin level remains normal, it does not rule out seizure. Prolactin levels should therefore be used in combination with other testing to make a definitive diagnosis or exclusion of seizure.8
Anion gap and Denver Seizure Score
The anion gap has also been shown to rise after generalized seizure due to the metabolic acidosis that occurs. With a bicarbonate level of 16 mmol/L, an elevated anion gap, and normal breathing, our patient very likely had metabolic acidosis.
It is sometimes difficult to differentiate syncope from seizure, as they share several features.
The Denver Seizure Score can help differentiate these two conditions. It is based on the patient’s anion gap and bicarbonate level and is calculated as follows:
(24 – bicarbonate) + [2 × (anion gap – 12)]
A score above 20 strongly indicates seizure activity. However, this is not a definitive tool for diagnosis. Like an elevated prolactin level, the Denver Seizure Score should be used in combination with other testing to move toward a definitive diagnosis.9
Our patient’s anion gap was 29 mmol/L and her bicarbonate level was 16 mmol/L. Her Denver Seizure Score was therefore 42, which supports this being an episode of generalized seizure activity.
Leukocytosis
The patient had a white blood cell count of 11.03 × 109/L, which was mildly elevated. She had no history of fever and no source of infection by history.
Leukocytosis is common following generalized tonic-clonic seizure. A fever may lower the seizure threshold; however, our patient was not febrile and clinically had no factors that raised concern for an underlying infection.
ANION GAP ACIDOSIS AND SEIZURE
3. Which of the following can cause both anion gap acidosis and seizure?
- Ethylene glycol
- Salicylate overdose
- Ethanol withdrawal without ketosis
- Alcoholic ketoacidosis
- Methanol
All of the above except for ethanol withdrawal without ketosis can cause both anion gap acidosis and seizure.
Ethylene glycol can cause seizure and an elevated anion gap acidosis. However, this patient had no history of ingesting antifreeze (the most common source of ethylene glycol in the home) and no evidence of calcium oxalate crystals in the urine, which would be a sign of ethylene glycol toxicity. Additional testing for ethylene glycol may include serum ethylene glycol levels and ultraviolet light testing of the urine to detect fluorescein, which is commonly added to automotive antifreeze to help mechanics find fluid leaks in engines.
Salicylate overdose can cause seizure and an elevated anion gap acidosis. However, this patient has no history of aspirin ingestion, and a serum aspirin level was later ordered and found to be negative. In addition, the acid-base disorder in salicylate overdose may be respiratory alkalosis from direct stimulation of respiratory centers in conjunction with metabolic acidosis.
Ethanol withdrawal can cause seizure and may result in ketoacidosis, which would appear as anion gap acidosis. The undetectable ethanol level in this patient would be consistent with withdrawal from ethanol, which may also lead to ketoacidosis.
Alcoholic ketoacidosis is a late finding in patients who have been drinking ethanol and is thus a possible cause of an elevated anion gap in this patient. However, the absence of ketones in her urine speaks against this diagnosis.
Methanol can cause seizure and acidosis, but laboratory testing would reveal a normal anion gap and an elevated osmolar gap. This was not likely in this patient.
The presence of anion gap acidosis is important in forming a differential diagnosis. Several causes of anion gap acidosis may also cause seizure. These include salicylates, ethanol withdrawal with ketosis, methanol, and isoniazid. None of these appears to be a factor in this patient’s case.
DIFFERENTIAL DIAGNOSIS IN OUR PATIENT
4. What is the most likely cause of this patient’s seizure?
- Bupropion side effect
- Benzodiazepine withdrawal
- Ethanol withdrawal
- Brain lesion
- Central nervous system infection
- Unprovoked seizure (new-onset epilepsy)
Bupropion, an inhibitor of neuronal reuptake of norepinephrine and dopamine, has been used in the United States since 1989 to treat major depression.10 At therapeutic doses, it lowers the seizure threshold; in cases of acute overdose, seizures typically occur within hours of the dose, or up to 24 hours in patients taking extended-release formulations.11
Bupropion should be used with caution or avoided in patients taking other medications that also lower the seizure threshold, or during withdrawal from alcohol, benzodiazepines, or barbiturates.10
Benzodiazepine withdrawal. Abrupt cessation of benzodiazepines also lowers the seizure threshold, and seizures are commonly seen in benzodiazepine withdrawal syndrome. The use of benzodiazepines is controversial in many situations, and discontinuing them may prove problematic for both the patient and physician, as the potential for abuse and addiction is significant.
Seizures have occurred during withdrawal from even short-term benzodiazepine use. Other factors, such as concomitant use of other medications that lower the seizure threshold, may play a more significant role in causing withdrawal seizures than the duration of benzodiazepine therapy.12
Medications shown to be useful in managing withdrawal from benzodiazepines include carbamazepine, imipramine, valproate, and trazodone. Paroxetine has also been shown to be helpful in patients with major depression who are being taken off a benzodiazepine.13
Ethanol withdrawal is common in patients presenting to emergency departments, and seizures are frequently seen in these patients. This patient reported social drinking but not drinking ethanol daily, although many patients are not forthcoming about alcohol or drug use when talking with a physician or other healthcare provider.
Alcohol withdrawal seizures may accompany delirium tremens or major withdrawal syndrome, but they are seen more often in the absence of major withdrawal or delirium tremens. Seizures are typically single or occur in a short grouping over a brief period of time and mostly occur in chronic alcoholism. The role of anticonvulsants in patients with alcohol withdrawal seizure has not been established.14
Brain lesion. A previously undiagnosed brain tumor is not a common cause of new-onset seizure, although it is not unusual for a brain tumor to cause new-onset seizure. In 1 study, 6% of patients with new-onset seizure had a clinically significant lesion on brain imaging.15 In addition, 15% to 30% of patients with a previously undiagnosed brain tumor present with seizure as the first symptom.16 Patients with abnormal findings on neurologic examination after the seizure activity are more likely to have a structural lesion that may be identified by computed tomography (CT) or magnetic resonance imaging. (MRI)15
Unprovoked seizure occurs without an identifiable precipitating factor, or from a central nervous system insult that occurred more than 7 days earlier. Patients who may have recurrent unprovoked seizure will likely be diagnosed with epilepsy.15 Patients with a first-time unprovoked seizure have a 30% or higher likelihood of having another unprovoked seizure within 5 years.17
It is most likely that bupropion is the key factor in lowering the seizure threshold in this patient. However, patients sometimes underreport the amount of alcohol they consume, and though less likely, our patient’s report of not drinking for 2 weeks may also be unreliable. Ethanol withdrawal, though unlikely, may also be a consideration with this case.
FURTHER TESTING FOR OUR PATIENT
5. Which tests may be helpful in this patient’s workup?
- CT of the brain
- Lumbar puncture for spinal fluid analysis
- MRI of the brain
- Electroencephalography (EEG)
This patient had had a headache for 1 week before presenting to the emergency department. Indications for neuroimaging in a patient with headache include sudden onset of severe headache, neurologic deficits, human immunodeficiency virus infection, loss of consciousness, immunosuppression, pregnancy, malignancy, and age over 50 with a new type of headache.18,19 Therefore, she should undergo some form of neuroimaging, either CT or MRI.
CT is the most readily available and fastest imaging study for the central nervous system available to emergency physicians. CT is limited, however, due to its decreased sensitivity in detecting some brain lesions. Therefore, many patients with first-time seizure may eventually require MRI.15 Furthermore, patients with focal onset of the seizure activity are more likely to have a structural lesion precipitating the seizure. MRI may have a higher yield than CT in these cases.15,20
Lumbar puncture for spinal fluid analysis is helpful in evaluating a patient with a suspected central nervous system infection such as meningitis or encephalitis, or subarachnoid hemorrhage.
This patient had a normal neurologic examination, no fever, and no meningeal signs, and central nervous system infection was very unlikely. Also, because she had had a headache for 1 week before the presentation with seizurelike activity, subarachnoid hemorrhage was very unlikely, and emergency lumbar puncture was not indicated.
MRI is less readily available than CT in a timely fashion in most emergency departments in the United States. It offers a higher yield than CT in diagnosing pathology such as acute stroke, brain tumor, and plaques seen in multiple sclerosis. CT is superior to MRI in diagnosing bony abnormalities and is very sensitive for detecting acute bleeding.
If MRI is performed, it should follow a specific protocol that includes high-resolution images for epilepsy evaluation rather than the more commonly ordered stroke protocol. The stroke protocol is more likely to be ordered in the emergency department.
EEG is well established in evaluating new-onset seizure in pediatric patients. Studies also demonstrate its utility in evaluating first-time seizure in adults, providing evidence that both epileptiform and nonepileptiform abnormalities seen on EEG are associated with a higher risk of recurrent seizure activity than in patients with normal findings on EEG.1
EEG may be difficult to interpret in adults. According to Benbadis,5 as many as one-third of adult patients diagnosed with epilepsy on EEG did not have epilepsy. This is because of normal variants, simple fluctuations of background rhythms, or fragmented alpha activity that can have a similar appearance to epileptiform patterns. EEG must always be interpreted in the context of the patient’s history and symptoms.5
Though EEG has limitations, it remains a crucial tool for identifying epilepsy. Following a single seizure, the decision to prescribe antiepileptic drugs is highly influenced by patterns on EEG associated with a risk of recurrence. In fact, a patient experiencing a single, idiopathic seizure and exhibiting an EEG pattern of spike wave discharges is likely to have recurrent seizure activity.21 Also, the appropriate use of EEG after even a single unprovoked seizure can identify patients with epilepsy and a risk of recurrent seizure greater than 60%.21,22
NO FURTHER SEIZURES
The patient was admitted to the observation unit from the emergency department after undergoing CT without intravenous contrast. While in observation, she had no additional episodes, and her vital signs remained within normal limits.
She underwent MRI and EEG as well as repeat laboratory studies and consultation by a neurologist. CT showed no structural abnormality, MRI results were read as normal, and EEG showed no epileptiform spikes or abnormal slow waves or other abnormality consistent with seizure. The repeat laboratory studies revealed normalization of the prolactin level at 11.3 ng/mL (reference range 2.0–17.4).
The final impression of the neurology consultant was that the patient had had a seizure that was most likely due to recently starting bupropion in combination with the withdrawal of the benzodiazepine, which lowered the seizure threshold. The neurologist also believed that our patient had no findings or symptoms other than the seizure that would suggest benzodiazepine withdrawal syndrome. According to the patient’s social history, it was unlikely that she had the pattern of alcohol consumption that would result in ethanol withdrawal seizure.
Seizures are common. In fact, every year, 180,000 US adults have their first seizure, and 10% of Americans will experience at least 1 seizure during their lifetime. However, only 20% to 25% of seizures are generalized tonic-clonic seizures as in our patient.23
As this patient had an identifiable cause for the seizure, there was no need to initiate anticonvulsant therapy at the time of discharge. She was discharged to home without any anticonvulsant, the bupropion was discontinued, and the lorazepam was not restarted. When contacted by telephone at 1 month and 18 months after discharge, she reported she had not experienced any additional seizures and has not needed antiepileptic medications.
- Seneviratne U. Management of the first seizure: an evidence based approach. Postgrad Med J 2009; 85:667–673.
- Krumholz A, Wiebe S, Gronseth G, et al; Quality Standards Subcommittee of the American Academy of Neurology; American Epilepsy Society. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2007; 67:1996–2007.
- Gram L. Epileptic seizures and syndromes. Lancet 1990; 336:161–163.
- Smith PE, Cossburn MD. Seizures: assessment and management in the emergency unit. Clin Med (Lond) 2004; 4:118–122.
- Benbadis S. The differential diagnosis of epilepsy: a critical review. Epilepsy Behav 2009; 15:15–21.
- Lusic I, Pintaric I, Hozo I, Boic L, Capkun V. Serum prolactin levels after seizure and syncopal attacks. Seizure 1999; 8:218–222.
- Chen DK, So YT, Fisher RS; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005; 65:668–675.
- Ben-Menachem E. Is prolactin a clinically useful measure of epilepsy? Epilepsy Curr 2006; 6:78–79.
- Bakes KM, Faragher J, Markovchick VJ, Donahoe K, Haukoos JS. The Denver Seizure Score: anion gap metabolic acidosis predicts generalized seizure. Am J Emerg Med 2011; 29:1097–1102.
- Jefferson JW, Pradok JF, Muir KT. Bupropion for major depressive disorder: pharmacokinetic and formulation considerations. Clin Ther 2005; 27:1685–1695.
- Stall N, Godwin J, Juurlink D. Bupropion abuse and overdose. CMAJ 2014; 186:1015.
- Fialip J, Aumaitre O, Eschalier A, Maradeix B, Dordain G, Lavarenne J. Benzodiazepine withdrawal seizures: analysis of 48 case reports. Clin Neuropharmacol 1987; 10:538–544.
- Lader M, Tylee A, Donoghue J. Withdrawing benzodiazepines in primary care. CNS Drugs 2009; 23:19–34.
- Chance JF. Emergency department treatment of alcohol withdrawal seizures with phenytoin. Ann Emerg Med 1991; 20:520–522.
- ACEP Clinical Policies Committee; Clinical Policies Subcommittee on Seizures. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med 2004; 43:605–625.
- Sperling MR, Ko J. Seizures and brain tumors. Semin Oncol 2006; 33:333–341.
- Musicco M, Beghi E, Solari A, Viani F. Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. First Seizure Trial Group (FIRST Group). Neurology 1997; 49:991–998.
- Edlow JA, Panagos PD, Godwin SA, Thomas TL, Decker WW; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med 2008; 52:407–436.
- Kaniecki R. Headache assessment and management. JAMA 2003; 289:1430–1433.
- Harden CL, Huff JS, Schwartz TH, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2007; 69:1772–1780.
- Bergey GK. Management of a first seizure. Continuum (Minneap Minn) 2016; 22:38–50.
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55:475–482.
- Ko DY. Generalized tonic-clonic seizures. Medscape. http://emedicine.medscape.com/article/1184608-overview. Accessed December 5, 2017.
- Seneviratne U. Management of the first seizure: an evidence based approach. Postgrad Med J 2009; 85:667–673.
- Krumholz A, Wiebe S, Gronseth G, et al; Quality Standards Subcommittee of the American Academy of Neurology; American Epilepsy Society. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2007; 67:1996–2007.
- Gram L. Epileptic seizures and syndromes. Lancet 1990; 336:161–163.
- Smith PE, Cossburn MD. Seizures: assessment and management in the emergency unit. Clin Med (Lond) 2004; 4:118–122.
- Benbadis S. The differential diagnosis of epilepsy: a critical review. Epilepsy Behav 2009; 15:15–21.
- Lusic I, Pintaric I, Hozo I, Boic L, Capkun V. Serum prolactin levels after seizure and syncopal attacks. Seizure 1999; 8:218–222.
- Chen DK, So YT, Fisher RS; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2005; 65:668–675.
- Ben-Menachem E. Is prolactin a clinically useful measure of epilepsy? Epilepsy Curr 2006; 6:78–79.
- Bakes KM, Faragher J, Markovchick VJ, Donahoe K, Haukoos JS. The Denver Seizure Score: anion gap metabolic acidosis predicts generalized seizure. Am J Emerg Med 2011; 29:1097–1102.
- Jefferson JW, Pradok JF, Muir KT. Bupropion for major depressive disorder: pharmacokinetic and formulation considerations. Clin Ther 2005; 27:1685–1695.
- Stall N, Godwin J, Juurlink D. Bupropion abuse and overdose. CMAJ 2014; 186:1015.
- Fialip J, Aumaitre O, Eschalier A, Maradeix B, Dordain G, Lavarenne J. Benzodiazepine withdrawal seizures: analysis of 48 case reports. Clin Neuropharmacol 1987; 10:538–544.
- Lader M, Tylee A, Donoghue J. Withdrawing benzodiazepines in primary care. CNS Drugs 2009; 23:19–34.
- Chance JF. Emergency department treatment of alcohol withdrawal seizures with phenytoin. Ann Emerg Med 1991; 20:520–522.
- ACEP Clinical Policies Committee; Clinical Policies Subcommittee on Seizures. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with seizures. Ann Emerg Med 2004; 43:605–625.
- Sperling MR, Ko J. Seizures and brain tumors. Semin Oncol 2006; 33:333–341.
- Musicco M, Beghi E, Solari A, Viani F. Treatment of first tonic-clonic seizure does not improve the prognosis of epilepsy. First Seizure Trial Group (FIRST Group). Neurology 1997; 49:991–998.
- Edlow JA, Panagos PD, Godwin SA, Thomas TL, Decker WW; American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med 2008; 52:407–436.
- Kaniecki R. Headache assessment and management. JAMA 2003; 289:1430–1433.
- Harden CL, Huff JS, Schwartz TH, et al; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Reassessment: neuroimaging in the emergency patient presenting with seizure (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2007; 69:1772–1780.
- Bergey GK. Management of a first seizure. Continuum (Minneap Minn) 2016; 22:38–50.
- Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014; 55:475–482.
- Ko DY. Generalized tonic-clonic seizures. Medscape. http://emedicine.medscape.com/article/1184608-overview. Accessed December 5, 2017.
Do cardiac risk stratification indexes accurately estimate perioperative risk in noncardiac surgery patients?
Neither of the two cardiac risk assessment indexes most commonly used (Table 1)1,2 is completely accurate, nor is one superior to the other. To provide the most accurate assessment of cardiac risk, practitioners need to select the index most applicable to the circumstances of the individual patient.
CARDIAC COMPLICATIONS ARE INCREASING
CARDIAC RISK ASSESSMENT INDEXES
The 2 risk assessment indexes most often used are:
- The Revised Cardiac Risk Index (RCRI)1
- The National Surgical Quality Improvement Program (NSQIP) risk index, also known as the Gupta index.2
Both are endorsed by the American College of Cardiology (ACC) and the American Heart Association (AHA).5 The RCRI, introduced in 1999, is more commonly used, but the NSQIP, introduced in 2011, is based on a larger sample size.
Both indexes consider various factors in estimating the risk, with some overlap. The main outcome assessed in both indexes is the risk of a major cardiac event, ie, myocardial infarction or cardiac arrest. The RCRI outcome also includes ventricular fibrillation, complete heart block, and pulmonary edema, which may be sequelae to cardiac arrest and myocardial infarction. This difference in defined outcomes between the indexes is not likely to account for a significant variation in the prediction of risk; however, this is difficult to prove.
Each index defines myocardial infarction differently. The current clinical definition6 includes detection of a rise or fall of cardiac biomarker values (preferably cardiac troponins) with at least 1 value above the 99th percentile upper reference limit and at least 1 of the following:
- Symptoms of ischemia
- New ST-T wave changes or new left bundle branch block
- New pathologic Q waves
- Imaging evidence of new loss of viable myocardium tissue or new regional wall- motion abnormality
- Finding of an intracoronary thrombus.
As seen in Table 1, the definition of myocardial infarction in NSQIP was one of the following: ST-segment elevation, new left bundle branch block, Q waves, or a troponin level greater than 3 times normal. Patients may have mild troponin leak of unknown significance without chest pain after surgery. This suggests that NSQIP may have overdiagnosed myocardial infarction.
USE IN CLINICAL PRACTICE
In clinical practice, which risk index is more accurate? Should clinicians become familiar with one index and keep using it? The 2014 ACC/AHA guidelines5 do not recommend one over the other, nor do they define the clinical situations that could lead to significant underestimation of risk.
The following are cases in which the indexes provide contradictory risk assessments.
Case 1. A 60-year-old man scheduled for surgery has diabetes mellitus, for which he takes insulin, and stable heart failure (left ventricular ejection fraction 40%). His RCRI score is 2, indicating an elevated 7% risk of cardiac complications; however, his NSQIP index is 0.31%. In this case, the NSQIP index probably underestimates the risk, as insulin-dependent diabetes and heart failure are not variables in the NSQIP index.
Case 2. A 60-year-old man who is partially functionally dependent and is on oxygen for severe chronic obstructive pulmonary disease is scheduled for craniotomy. His RCRI score is 0 (low risk), but his NSQIP index score (4.87%) indicates an elevated risk of cardiac complications based on his functional status, symptomatic chronic obstructive pulmonary disease, and high-risk surgery. In this case, the RCRI probably underestimates the risk.
These cases show that practitioners should not rely on just one index, but should rather decide which index to apply case by case. This avoids underestimating the risk. In patients with poor functional status and higher American Society of Anesthesiology class, the NSQIP index may provide a more accurate risk estimation than the RCRI. Patients with cardiomyopathy as well as those with insulin-dependent diabetes may be well assessed by the RCRI.
The following situations require additional caution when using these indexes, to avoid over- and underestimating cardiac risk.
PATIENTS WITH SEVERE AORTIC STENOSIS
Neither index lists severe aortic stenosis as a risk factor. The RCRI derivation and validation studies had only 5 patients with severe aortic stenosis, and the NSQIP validation study did not include any patients with aortic stenosis. Nevertheless, severe aortic stenosis increases the risk of cardiac complications in the perioperative period,7 making it important to consider in these patients.
Although patients with severe symptomatic aortic stenosis need valvular intervention before the surgery, patients who have asymptomatic severe aortic stenosis without associated cardiac dysfunction do not. Close hemodynamic monitoring during surgery is reasonable in the latter group.5,7
PATIENTS WITH RECENT STROKE
What would be the cardiac risk for a patient scheduled for elective hip surgery who has had a stroke within the last 3 months? If one applies both indexes, the cardiac risk comes to less than 1% (low risk) in both cases. However, this could be deceiving. A large study8 published in 2014 showed an elevated risk of cardiac complications in patients undergoing noncardiac surgery who had had an ischemic stroke within the previous 6 months; in the first 3 months, the odds ratio of developing a major adverse cardiovascular event was 14.23.This clearly overrides the traditional expert opinion-based evidence, which is that a time lapse of only 1 month after an ischemic stroke is safe for surgery.
PATIENTS WITH DIASTOLIC DYSFUNCTION
A 2016 meta-analysis and systematic review found that preoperative diastolic dysfunction was associated with higher rates of postoperative mortality and major adverse cardiac events, regardless of the left ventricular ejection fraction.9 However, the studies investigated included mostly patients undergoing cardiovascular surgeries. This raises the question of whether asymptomatic patients need echocardiography before surgery.
In a patient who has diastolic dysfunction, one should maintain adequate blood pressure control and euvolemia before the surgery and avoid hypertensive spikes in the immediate perioperative period, as hypertension is the worst enemy of those with diastolic dysfunction. Patients with atrial fibrillation may need more stringent heart rate control.
In a prospective study involving 1,005 consecutive vascular surgery patients, the 30-day cardiovascular event rate was highest in patients with symptomatic heart failure (49%), followed by those with asymptomatic systolic left ventricular dysfunction (23%), asymptomatic diastolic left ventricular dysfunction (18%), and normal left ventricular function (10%).10
Further studies are needed to determine whether the data obtained from the assessment of ventricular function in patients without signs or symptoms are significant enough to require updates to the criteria.
WHAT ABOUT THE ROLE OF BNP?
In a meta-analysis of 15 noncardiac surgery studies in 850 patients, preoperative B-type natriuretic peptide (BNP) levels independently predicted major adverse cardiac events, with levels greater than 372 pg/mL having a 36.7% incidence of major adverse cardiac events.11
A recent publication by the Canadian Cardiovascular Society12 strongly recommended measuring N-terminal-proBNP or BNP before noncardiac surgery to enhance perioperative cardiac risk estimation in patients who are age 65 or older, patients who are age 45 to 64 with significant cardiovascular disease, or patients who have an RCRI score of 1 or higher.
Further prospective randomized studies are needed to assess the utility of measuring BNP for preoperative cardiac risk evaluation.
PATIENTS WITH OBSTRUCTIVE SLEEP APNEA
Patients with obstructive sleep apnea scheduled for surgery under anesthesia have a higher risk of perioperative complications than patients without the disease, including higher rates of cardiac complications and atrial fibrillation. However, the evidence is insufficient to support canceling or delaying surgery in patients with suspected obstructive sleep apnea.
After comorbid conditions are optimally treated, patients with obstructive sleep apnea can proceed to surgery, provided strategies for mitigating complications are implemented.13
TO STRESS OR NOT TO STRESS?
A common question is whether to perform a stress test before surgery. Based on the ACC/AHA guidelines,5 preoperative stress testing is not indicated solely to assess surgical risk if there is no other indication for it.
Stress testing can be used to determine whether the patient needs coronary revascularization. However, routine coronary revascularization is not recommended before noncardiac surgery exclusively to reduce perioperative cardiac events.
This conclusion is based on a landmark trial in which revascularization had no significant effect on outcomes.14 That trial included high-risk patients undergoing major vascular surgery who had greater than 70% stenosis of 1 or more major coronary arteries on angiography, randomized to either revascularization or no revascularization. It excluded patients with severe left main artery disease, ejection fraction less than 20%, and severe aortic stenosis. Results showed no differences in the rates of postoperative death, myocardial infarction, and stroke between the 2 groups. Furthermore, there was no postoperative survival difference during 5 years of follow-up.
Stress testing may be considered for patients with elevated risk and whose functional capacity is poor (< 4 metabolic equivalents) or unknown if it will change the management strategy. Another consideration affecting whether to perform stress testing is whether the surgery can be deferred for a month if the stress test is positive and a bare-metal coronary stent is placed, to allow for completion of dual antiplatelet therapy.
SHOULD WE ROUTINELY MONITOR TROPONIN AFTER SURGERY IN ASYMPTOMATIC PATIENTS?
Currently, the role of routine monitoring of troponin postoperatively in asymptomatic patients is unclear. The Canadian Cardiovascular Society12 recommends monitoring troponin in selected group of patients, eg, those with an RCRI score of 1 or higher, age 65 or older, a significant cardiac history, or elevated BNP preoperatively. However, at this point we do not have strong evidence regarding the implications of mild asymptomatic troponin elevation postoperatively and how to manage it. Two currently ongoing randomized controlled trials will answer those questions:
- The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial, comparing the use of dabigatran and omeprazole vs placebo in myocardial injury postoperatively
- The Study of Ticagrelor Versus Aspirin Treatment in Patients With Myocardial Injury Post Major Non-cardiac Surgery (INTREPID).
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med 2015; 373:2258–2269.
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2:181–187.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:e77–e137 [Simultaneous publication: Circulation 2014; 130:2215–2245].
- Thygesen K, Alpert JS, Jaffe AS, et al, for the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation 2012; 126:2020–2035.
- Tashiro T, Pislaru SV, Blustin JM, et al. Perioperative risk of major non-cardiac surgery in patients with severe aortic stenosis: a reappraisal in contemporary practice. Eur Heart J 2014; 35:2372–2381.
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277.
- Kaw R, Hernandez AV, Pasupuleti V, et al; Cardiovascular Meta-analyses Research Group. Effect of diastolic dysfunction on postoperative outcomes after cardiovascular surgery: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2016; 152:1142–1153.
- Flu WJ, van Kuijk JP, Hoeks SE, et al. Prognostic implications of asymptomatic left ventricular dysfunction in patients undergoing vascular surgery. Anesthesiology 2010; 112:1316–1324.
- Rodseth R, Lurati Buse G, Bolliger D, et al. The predictive ability of pre-operative B-type natriuretic peptide in vascular patients for major adverse cardiac events: an individual patient data meta-analysis. J Am Coll Cardiol 2011; 58:522–529.
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society Guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33:17–32.
- Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016; 123:452–473.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351:2795–2804.
Neither of the two cardiac risk assessment indexes most commonly used (Table 1)1,2 is completely accurate, nor is one superior to the other. To provide the most accurate assessment of cardiac risk, practitioners need to select the index most applicable to the circumstances of the individual patient.
CARDIAC COMPLICATIONS ARE INCREASING
CARDIAC RISK ASSESSMENT INDEXES
The 2 risk assessment indexes most often used are:
- The Revised Cardiac Risk Index (RCRI)1
- The National Surgical Quality Improvement Program (NSQIP) risk index, also known as the Gupta index.2
Both are endorsed by the American College of Cardiology (ACC) and the American Heart Association (AHA).5 The RCRI, introduced in 1999, is more commonly used, but the NSQIP, introduced in 2011, is based on a larger sample size.
Both indexes consider various factors in estimating the risk, with some overlap. The main outcome assessed in both indexes is the risk of a major cardiac event, ie, myocardial infarction or cardiac arrest. The RCRI outcome also includes ventricular fibrillation, complete heart block, and pulmonary edema, which may be sequelae to cardiac arrest and myocardial infarction. This difference in defined outcomes between the indexes is not likely to account for a significant variation in the prediction of risk; however, this is difficult to prove.
Each index defines myocardial infarction differently. The current clinical definition6 includes detection of a rise or fall of cardiac biomarker values (preferably cardiac troponins) with at least 1 value above the 99th percentile upper reference limit and at least 1 of the following:
- Symptoms of ischemia
- New ST-T wave changes or new left bundle branch block
- New pathologic Q waves
- Imaging evidence of new loss of viable myocardium tissue or new regional wall- motion abnormality
- Finding of an intracoronary thrombus.
As seen in Table 1, the definition of myocardial infarction in NSQIP was one of the following: ST-segment elevation, new left bundle branch block, Q waves, or a troponin level greater than 3 times normal. Patients may have mild troponin leak of unknown significance without chest pain after surgery. This suggests that NSQIP may have overdiagnosed myocardial infarction.
USE IN CLINICAL PRACTICE
In clinical practice, which risk index is more accurate? Should clinicians become familiar with one index and keep using it? The 2014 ACC/AHA guidelines5 do not recommend one over the other, nor do they define the clinical situations that could lead to significant underestimation of risk.
The following are cases in which the indexes provide contradictory risk assessments.
Case 1. A 60-year-old man scheduled for surgery has diabetes mellitus, for which he takes insulin, and stable heart failure (left ventricular ejection fraction 40%). His RCRI score is 2, indicating an elevated 7% risk of cardiac complications; however, his NSQIP index is 0.31%. In this case, the NSQIP index probably underestimates the risk, as insulin-dependent diabetes and heart failure are not variables in the NSQIP index.
Case 2. A 60-year-old man who is partially functionally dependent and is on oxygen for severe chronic obstructive pulmonary disease is scheduled for craniotomy. His RCRI score is 0 (low risk), but his NSQIP index score (4.87%) indicates an elevated risk of cardiac complications based on his functional status, symptomatic chronic obstructive pulmonary disease, and high-risk surgery. In this case, the RCRI probably underestimates the risk.
These cases show that practitioners should not rely on just one index, but should rather decide which index to apply case by case. This avoids underestimating the risk. In patients with poor functional status and higher American Society of Anesthesiology class, the NSQIP index may provide a more accurate risk estimation than the RCRI. Patients with cardiomyopathy as well as those with insulin-dependent diabetes may be well assessed by the RCRI.
The following situations require additional caution when using these indexes, to avoid over- and underestimating cardiac risk.
PATIENTS WITH SEVERE AORTIC STENOSIS
Neither index lists severe aortic stenosis as a risk factor. The RCRI derivation and validation studies had only 5 patients with severe aortic stenosis, and the NSQIP validation study did not include any patients with aortic stenosis. Nevertheless, severe aortic stenosis increases the risk of cardiac complications in the perioperative period,7 making it important to consider in these patients.
Although patients with severe symptomatic aortic stenosis need valvular intervention before the surgery, patients who have asymptomatic severe aortic stenosis without associated cardiac dysfunction do not. Close hemodynamic monitoring during surgery is reasonable in the latter group.5,7
PATIENTS WITH RECENT STROKE
What would be the cardiac risk for a patient scheduled for elective hip surgery who has had a stroke within the last 3 months? If one applies both indexes, the cardiac risk comes to less than 1% (low risk) in both cases. However, this could be deceiving. A large study8 published in 2014 showed an elevated risk of cardiac complications in patients undergoing noncardiac surgery who had had an ischemic stroke within the previous 6 months; in the first 3 months, the odds ratio of developing a major adverse cardiovascular event was 14.23.This clearly overrides the traditional expert opinion-based evidence, which is that a time lapse of only 1 month after an ischemic stroke is safe for surgery.
PATIENTS WITH DIASTOLIC DYSFUNCTION
A 2016 meta-analysis and systematic review found that preoperative diastolic dysfunction was associated with higher rates of postoperative mortality and major adverse cardiac events, regardless of the left ventricular ejection fraction.9 However, the studies investigated included mostly patients undergoing cardiovascular surgeries. This raises the question of whether asymptomatic patients need echocardiography before surgery.
In a patient who has diastolic dysfunction, one should maintain adequate blood pressure control and euvolemia before the surgery and avoid hypertensive spikes in the immediate perioperative period, as hypertension is the worst enemy of those with diastolic dysfunction. Patients with atrial fibrillation may need more stringent heart rate control.
In a prospective study involving 1,005 consecutive vascular surgery patients, the 30-day cardiovascular event rate was highest in patients with symptomatic heart failure (49%), followed by those with asymptomatic systolic left ventricular dysfunction (23%), asymptomatic diastolic left ventricular dysfunction (18%), and normal left ventricular function (10%).10
Further studies are needed to determine whether the data obtained from the assessment of ventricular function in patients without signs or symptoms are significant enough to require updates to the criteria.
WHAT ABOUT THE ROLE OF BNP?
In a meta-analysis of 15 noncardiac surgery studies in 850 patients, preoperative B-type natriuretic peptide (BNP) levels independently predicted major adverse cardiac events, with levels greater than 372 pg/mL having a 36.7% incidence of major adverse cardiac events.11
A recent publication by the Canadian Cardiovascular Society12 strongly recommended measuring N-terminal-proBNP or BNP before noncardiac surgery to enhance perioperative cardiac risk estimation in patients who are age 65 or older, patients who are age 45 to 64 with significant cardiovascular disease, or patients who have an RCRI score of 1 or higher.
Further prospective randomized studies are needed to assess the utility of measuring BNP for preoperative cardiac risk evaluation.
PATIENTS WITH OBSTRUCTIVE SLEEP APNEA
Patients with obstructive sleep apnea scheduled for surgery under anesthesia have a higher risk of perioperative complications than patients without the disease, including higher rates of cardiac complications and atrial fibrillation. However, the evidence is insufficient to support canceling or delaying surgery in patients with suspected obstructive sleep apnea.
After comorbid conditions are optimally treated, patients with obstructive sleep apnea can proceed to surgery, provided strategies for mitigating complications are implemented.13
TO STRESS OR NOT TO STRESS?
A common question is whether to perform a stress test before surgery. Based on the ACC/AHA guidelines,5 preoperative stress testing is not indicated solely to assess surgical risk if there is no other indication for it.
Stress testing can be used to determine whether the patient needs coronary revascularization. However, routine coronary revascularization is not recommended before noncardiac surgery exclusively to reduce perioperative cardiac events.
This conclusion is based on a landmark trial in which revascularization had no significant effect on outcomes.14 That trial included high-risk patients undergoing major vascular surgery who had greater than 70% stenosis of 1 or more major coronary arteries on angiography, randomized to either revascularization or no revascularization. It excluded patients with severe left main artery disease, ejection fraction less than 20%, and severe aortic stenosis. Results showed no differences in the rates of postoperative death, myocardial infarction, and stroke between the 2 groups. Furthermore, there was no postoperative survival difference during 5 years of follow-up.
Stress testing may be considered for patients with elevated risk and whose functional capacity is poor (< 4 metabolic equivalents) or unknown if it will change the management strategy. Another consideration affecting whether to perform stress testing is whether the surgery can be deferred for a month if the stress test is positive and a bare-metal coronary stent is placed, to allow for completion of dual antiplatelet therapy.
SHOULD WE ROUTINELY MONITOR TROPONIN AFTER SURGERY IN ASYMPTOMATIC PATIENTS?
Currently, the role of routine monitoring of troponin postoperatively in asymptomatic patients is unclear. The Canadian Cardiovascular Society12 recommends monitoring troponin in selected group of patients, eg, those with an RCRI score of 1 or higher, age 65 or older, a significant cardiac history, or elevated BNP preoperatively. However, at this point we do not have strong evidence regarding the implications of mild asymptomatic troponin elevation postoperatively and how to manage it. Two currently ongoing randomized controlled trials will answer those questions:
- The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial, comparing the use of dabigatran and omeprazole vs placebo in myocardial injury postoperatively
- The Study of Ticagrelor Versus Aspirin Treatment in Patients With Myocardial Injury Post Major Non-cardiac Surgery (INTREPID).
Neither of the two cardiac risk assessment indexes most commonly used (Table 1)1,2 is completely accurate, nor is one superior to the other. To provide the most accurate assessment of cardiac risk, practitioners need to select the index most applicable to the circumstances of the individual patient.
CARDIAC COMPLICATIONS ARE INCREASING
CARDIAC RISK ASSESSMENT INDEXES
The 2 risk assessment indexes most often used are:
- The Revised Cardiac Risk Index (RCRI)1
- The National Surgical Quality Improvement Program (NSQIP) risk index, also known as the Gupta index.2
Both are endorsed by the American College of Cardiology (ACC) and the American Heart Association (AHA).5 The RCRI, introduced in 1999, is more commonly used, but the NSQIP, introduced in 2011, is based on a larger sample size.
Both indexes consider various factors in estimating the risk, with some overlap. The main outcome assessed in both indexes is the risk of a major cardiac event, ie, myocardial infarction or cardiac arrest. The RCRI outcome also includes ventricular fibrillation, complete heart block, and pulmonary edema, which may be sequelae to cardiac arrest and myocardial infarction. This difference in defined outcomes between the indexes is not likely to account for a significant variation in the prediction of risk; however, this is difficult to prove.
Each index defines myocardial infarction differently. The current clinical definition6 includes detection of a rise or fall of cardiac biomarker values (preferably cardiac troponins) with at least 1 value above the 99th percentile upper reference limit and at least 1 of the following:
- Symptoms of ischemia
- New ST-T wave changes or new left bundle branch block
- New pathologic Q waves
- Imaging evidence of new loss of viable myocardium tissue or new regional wall- motion abnormality
- Finding of an intracoronary thrombus.
As seen in Table 1, the definition of myocardial infarction in NSQIP was one of the following: ST-segment elevation, new left bundle branch block, Q waves, or a troponin level greater than 3 times normal. Patients may have mild troponin leak of unknown significance without chest pain after surgery. This suggests that NSQIP may have overdiagnosed myocardial infarction.
USE IN CLINICAL PRACTICE
In clinical practice, which risk index is more accurate? Should clinicians become familiar with one index and keep using it? The 2014 ACC/AHA guidelines5 do not recommend one over the other, nor do they define the clinical situations that could lead to significant underestimation of risk.
The following are cases in which the indexes provide contradictory risk assessments.
Case 1. A 60-year-old man scheduled for surgery has diabetes mellitus, for which he takes insulin, and stable heart failure (left ventricular ejection fraction 40%). His RCRI score is 2, indicating an elevated 7% risk of cardiac complications; however, his NSQIP index is 0.31%. In this case, the NSQIP index probably underestimates the risk, as insulin-dependent diabetes and heart failure are not variables in the NSQIP index.
Case 2. A 60-year-old man who is partially functionally dependent and is on oxygen for severe chronic obstructive pulmonary disease is scheduled for craniotomy. His RCRI score is 0 (low risk), but his NSQIP index score (4.87%) indicates an elevated risk of cardiac complications based on his functional status, symptomatic chronic obstructive pulmonary disease, and high-risk surgery. In this case, the RCRI probably underestimates the risk.
These cases show that practitioners should not rely on just one index, but should rather decide which index to apply case by case. This avoids underestimating the risk. In patients with poor functional status and higher American Society of Anesthesiology class, the NSQIP index may provide a more accurate risk estimation than the RCRI. Patients with cardiomyopathy as well as those with insulin-dependent diabetes may be well assessed by the RCRI.
The following situations require additional caution when using these indexes, to avoid over- and underestimating cardiac risk.
PATIENTS WITH SEVERE AORTIC STENOSIS
Neither index lists severe aortic stenosis as a risk factor. The RCRI derivation and validation studies had only 5 patients with severe aortic stenosis, and the NSQIP validation study did not include any patients with aortic stenosis. Nevertheless, severe aortic stenosis increases the risk of cardiac complications in the perioperative period,7 making it important to consider in these patients.
Although patients with severe symptomatic aortic stenosis need valvular intervention before the surgery, patients who have asymptomatic severe aortic stenosis without associated cardiac dysfunction do not. Close hemodynamic monitoring during surgery is reasonable in the latter group.5,7
PATIENTS WITH RECENT STROKE
What would be the cardiac risk for a patient scheduled for elective hip surgery who has had a stroke within the last 3 months? If one applies both indexes, the cardiac risk comes to less than 1% (low risk) in both cases. However, this could be deceiving. A large study8 published in 2014 showed an elevated risk of cardiac complications in patients undergoing noncardiac surgery who had had an ischemic stroke within the previous 6 months; in the first 3 months, the odds ratio of developing a major adverse cardiovascular event was 14.23.This clearly overrides the traditional expert opinion-based evidence, which is that a time lapse of only 1 month after an ischemic stroke is safe for surgery.
PATIENTS WITH DIASTOLIC DYSFUNCTION
A 2016 meta-analysis and systematic review found that preoperative diastolic dysfunction was associated with higher rates of postoperative mortality and major adverse cardiac events, regardless of the left ventricular ejection fraction.9 However, the studies investigated included mostly patients undergoing cardiovascular surgeries. This raises the question of whether asymptomatic patients need echocardiography before surgery.
In a patient who has diastolic dysfunction, one should maintain adequate blood pressure control and euvolemia before the surgery and avoid hypertensive spikes in the immediate perioperative period, as hypertension is the worst enemy of those with diastolic dysfunction. Patients with atrial fibrillation may need more stringent heart rate control.
In a prospective study involving 1,005 consecutive vascular surgery patients, the 30-day cardiovascular event rate was highest in patients with symptomatic heart failure (49%), followed by those with asymptomatic systolic left ventricular dysfunction (23%), asymptomatic diastolic left ventricular dysfunction (18%), and normal left ventricular function (10%).10
Further studies are needed to determine whether the data obtained from the assessment of ventricular function in patients without signs or symptoms are significant enough to require updates to the criteria.
WHAT ABOUT THE ROLE OF BNP?
In a meta-analysis of 15 noncardiac surgery studies in 850 patients, preoperative B-type natriuretic peptide (BNP) levels independently predicted major adverse cardiac events, with levels greater than 372 pg/mL having a 36.7% incidence of major adverse cardiac events.11
A recent publication by the Canadian Cardiovascular Society12 strongly recommended measuring N-terminal-proBNP or BNP before noncardiac surgery to enhance perioperative cardiac risk estimation in patients who are age 65 or older, patients who are age 45 to 64 with significant cardiovascular disease, or patients who have an RCRI score of 1 or higher.
Further prospective randomized studies are needed to assess the utility of measuring BNP for preoperative cardiac risk evaluation.
PATIENTS WITH OBSTRUCTIVE SLEEP APNEA
Patients with obstructive sleep apnea scheduled for surgery under anesthesia have a higher risk of perioperative complications than patients without the disease, including higher rates of cardiac complications and atrial fibrillation. However, the evidence is insufficient to support canceling or delaying surgery in patients with suspected obstructive sleep apnea.
After comorbid conditions are optimally treated, patients with obstructive sleep apnea can proceed to surgery, provided strategies for mitigating complications are implemented.13
TO STRESS OR NOT TO STRESS?
A common question is whether to perform a stress test before surgery. Based on the ACC/AHA guidelines,5 preoperative stress testing is not indicated solely to assess surgical risk if there is no other indication for it.
Stress testing can be used to determine whether the patient needs coronary revascularization. However, routine coronary revascularization is not recommended before noncardiac surgery exclusively to reduce perioperative cardiac events.
This conclusion is based on a landmark trial in which revascularization had no significant effect on outcomes.14 That trial included high-risk patients undergoing major vascular surgery who had greater than 70% stenosis of 1 or more major coronary arteries on angiography, randomized to either revascularization or no revascularization. It excluded patients with severe left main artery disease, ejection fraction less than 20%, and severe aortic stenosis. Results showed no differences in the rates of postoperative death, myocardial infarction, and stroke between the 2 groups. Furthermore, there was no postoperative survival difference during 5 years of follow-up.
Stress testing may be considered for patients with elevated risk and whose functional capacity is poor (< 4 metabolic equivalents) or unknown if it will change the management strategy. Another consideration affecting whether to perform stress testing is whether the surgery can be deferred for a month if the stress test is positive and a bare-metal coronary stent is placed, to allow for completion of dual antiplatelet therapy.
SHOULD WE ROUTINELY MONITOR TROPONIN AFTER SURGERY IN ASYMPTOMATIC PATIENTS?
Currently, the role of routine monitoring of troponin postoperatively in asymptomatic patients is unclear. The Canadian Cardiovascular Society12 recommends monitoring troponin in selected group of patients, eg, those with an RCRI score of 1 or higher, age 65 or older, a significant cardiac history, or elevated BNP preoperatively. However, at this point we do not have strong evidence regarding the implications of mild asymptomatic troponin elevation postoperatively and how to manage it. Two currently ongoing randomized controlled trials will answer those questions:
- The Management of Myocardial Injury After Noncardiac Surgery (MANAGE) trial, comparing the use of dabigatran and omeprazole vs placebo in myocardial injury postoperatively
- The Study of Ticagrelor Versus Aspirin Treatment in Patients With Myocardial Injury Post Major Non-cardiac Surgery (INTREPID).
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med 2015; 373:2258–2269.
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2:181–187.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:e77–e137 [Simultaneous publication: Circulation 2014; 130:2215–2245].
- Thygesen K, Alpert JS, Jaffe AS, et al, for the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation 2012; 126:2020–2035.
- Tashiro T, Pislaru SV, Blustin JM, et al. Perioperative risk of major non-cardiac surgery in patients with severe aortic stenosis: a reappraisal in contemporary practice. Eur Heart J 2014; 35:2372–2381.
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277.
- Kaw R, Hernandez AV, Pasupuleti V, et al; Cardiovascular Meta-analyses Research Group. Effect of diastolic dysfunction on postoperative outcomes after cardiovascular surgery: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2016; 152:1142–1153.
- Flu WJ, van Kuijk JP, Hoeks SE, et al. Prognostic implications of asymptomatic left ventricular dysfunction in patients undergoing vascular surgery. Anesthesiology 2010; 112:1316–1324.
- Rodseth R, Lurati Buse G, Bolliger D, et al. The predictive ability of pre-operative B-type natriuretic peptide in vascular patients for major adverse cardiac events: an individual patient data meta-analysis. J Am Coll Cardiol 2011; 58:522–529.
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society Guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33:17–32.
- Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016; 123:452–473.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351:2795–2804.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation 2011; 124:381–387.
- Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med 2015; 373:2258–2269.
- Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative major adverse cardiovascular and cerebrovascular events associated with noncardiac surgery. JAMA Cardiol 2017; 2:181–187.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al; American College of Cardiology; American Heart Association. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64:e77–e137 [Simultaneous publication: Circulation 2014; 130:2215–2245].
- Thygesen K, Alpert JS, Jaffe AS, et al, for the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation 2012; 126:2020–2035.
- Tashiro T, Pislaru SV, Blustin JM, et al. Perioperative risk of major non-cardiac surgery in patients with severe aortic stenosis: a reappraisal in contemporary practice. Eur Heart J 2014; 35:2372–2381.
- Jørgensen ME, Torp-Pedersen C, Gislason GH, et al. Time elapsed after ischemic stroke and risk of adverse cardiovascular events and mortality following elective noncardiac surgery. JAMA 2014; 312:269–277.
- Kaw R, Hernandez AV, Pasupuleti V, et al; Cardiovascular Meta-analyses Research Group. Effect of diastolic dysfunction on postoperative outcomes after cardiovascular surgery: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2016; 152:1142–1153.
- Flu WJ, van Kuijk JP, Hoeks SE, et al. Prognostic implications of asymptomatic left ventricular dysfunction in patients undergoing vascular surgery. Anesthesiology 2010; 112:1316–1324.
- Rodseth R, Lurati Buse G, Bolliger D, et al. The predictive ability of pre-operative B-type natriuretic peptide in vascular patients for major adverse cardiac events: an individual patient data meta-analysis. J Am Coll Cardiol 2011; 58:522–529.
- Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society Guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017; 33:17–32.
- Chung F, Memtsoudis SG, Ramachandran SK, et al. Society of Anesthesia and Sleep Medicine guidelines on preoperative screening and assessment of adult patients with obstructive sleep apnea. Anesth Analg 2016; 123:452–473.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351:2795–2804.
Idiopathic hypercalciuria: Can we prevent stones and protect bones?
A 65-year-old woman was recently diagnosed with osteoporosis after a screening bone mineral density test. She has hypertension (treated with lisinopril), and she had an episode of passing a kidney stone 10 years ago. A 24-hour urine study reveals an elevated urinary calcium level.
What should the physician keep in mind in managing this patient?
IDIOPATHIC HYPERCALCIURIA
Many potential causes of secondary hypercalciuria must be ruled out before deciding that a patient has idiopathic hypercalciuria, which was first noted as a distinct entity by Albright et al in 1953.1 Causes of secondary hypercalciuria include primary hyperparathyroidism, hyperthyroidism, Paget disease, myeloma, malignancy, immobility, accelerated osteoporosis, sarcoidosis, renal tubular acidosis, and drug-induced urinary calcium loss such as that seen with loop diuretics.
Idiopathic hypercalciuria is identified by the following:
- Persistent hypercalciuria despite normal or restricted calcium intake2,3
- Normal levels of parathyroid hormone (PTH), phosphorus, and 1,25-dihydroxy-vitamin D (the active form of vitamin D, also called calcitriol) in the presence of hypercalciuria; serum calcium levels are also normal.
An alias for idiopathic hypercalciuria is “fasting hypercalciuria,” as increased urinary calcium persists and sometimes worsens while fasting or on a low-calcium diet, with increased bone turnover, reduced bone density, and normal serum PTH levels.4,5
Mineral loss from bone predominates in idiopathic hypercalciuria, but there is also a minor component of intestinal hyperabsorption of calcium and reduced renal calcium reabsorption.6 Distinguishing among intestinal hyperabsorptive hypercalciuria, renal leak hypercalciuria, and idiopathic or fasting hypercalciuria can be difficult and subtle. It has been argued that differentiating among hypercalciuric subtypes (hyperabsorptive, renal leak, idiopathic) is not useful; in general clinical practice, it is impractical to collect multiple 24-hour urine samples in the setting of controlled high- vs low-calcium diets.
COMPLICATIONS OF IDIOPATHIC HYPERCALCIURIA
Calcium is an important component in many physiologic processes, including coagulation, cell membrane transfer, hormone release, neuromuscular activation, and myocardial contraction. A sophisticated system of hormonally mediated interactions normally maintains stable extracellular calcium levels. Calcium is vital for bone strength, but the bones are the body’s calcium “bank,” and withdrawals from this bank are made at the expense of bone strength and integrity.
Renal stones
Patients with idiopathic hypercalciuria have a high incidence of renal stones. Conversely, 40% to 50% of patients with recurrent kidney stones have evidence of idiopathic hypercalciuria, the most common metabolic abnormality in “stone-formers.”7,8 Further, 35% to 40% of first- and second-degree relatives of stone-formers who have idiopathic hypercalciuria also have the condition.9 In the general population without kidney stones and without first-degree relatives with stones, the prevalence is approximately 5% to 10%.10,11
Bone loss
People with idiopathic hypercalciuria have lower bone density and a higher incidence of fracture than their normocalciuric peers. This relationship has been observed in both sexes and all ages. Idiopathic hypercalciuria has been noted in 10% to 19% of otherwise healthy men with low bone mass, in postmenopausal women with osteoporosis,10–12 and in up to 40% of postmenopausal women with osteoporotic fractures and no history of kidney stones.13
LABORATORY DEFINITION
Urinary calcium excretion
Heaney et al14 measured 24-hour urinary calcium excretion in a group of early postmenopausal women, whom he divided into 3 groups by dietary calcium intake:
- Low intake (< 500 mg/day)
- Moderate intake (500–1,000 mg/day)
- High intake (> 1,000 mg/day).
In the women who were estrogen-deprived (ie, postmenopausal and not on estrogen replacement therapy), the 95% probability ranges for urinary calcium excretion were:
- 32–252 mg/day (0.51–4.06 mg/kg/day) with low calcium intake
- 36–286 mg/day (0.57–4.52 mg/kg/day) with moderate calcium intake
- 45–357 mg/day (0.69–5.47 mg/kg/day) with high calcium intake.
For estrogen-replete women (perimenopausal or postmenopausal on estrogen replacement), using the same categories of dietary calcium intake, calcium excretion was:
- 39–194 mg/day (0.65–3.23 mg/kg/day) with low calcium intake
- 54–269 mg/day (0.77–3.84 mg/kg/day) with moderate calcium intake
- 66–237 mg/day (0.98–4.89 mg/kg/day) with high calcium intake.
In the estrogen-deprived group, urinary calcium excretion increased by only 55 mg/day per 1,000-mg increase in dietary intake, though there was individual variability. These data suggest that hypercalciuria should be defined as:
- Greater than 250 mg/day (> 4.1 mg/kg/day) in estrogen-replete women
- Greater than 300 mg/day (> 5.0 mg/kg/day) in estrogen-deprived women.
Urinary calcium-to-creatinine ratio
Use of a spot urinary calcium-to-creatinine ratio has been advocated as an alternative to the more labor-intensive 24-hour urine collection.15 However, the spot urine calcium-creatinine ratio correlates poorly with 24-hour urine criteria for hypercalciuria whether by absolute, weight-based, or menopausal and calcium-adjusted definitions.
Importantly, spot urine measurements show poor sensitivity and specificity for hypercalciuria. Spot urine samples underestimate the 24-hour urinary calcium (Bland-Altman bias –71 mg/24 hours), and postprandial sampling overestimates it (Bland-Altman bias +61 mg/24 hours).15
WHAT IS THE MECHANISM OF IDIOPATHIC HYPERCALCIURIA?
The pathophysiology of idiopathic hypercalciuria has been difficult to establish.
Increased sensitivity to vitamin D? In the hyperabsorbing population, activated vitamin D levels are often robust, but a few studies of rats with hyperabsorbing, hyperexcreting physiology have shown normal calcitriol levels, suggesting an increased sensitivity to the actions of 1,25-dihydroxyvitamin D.16
Another study found that hypercalciuric stone-forming rats have more 1,25-dihydroxyvitamin D receptors than do controls.17
These changes have not been demonstrated in patients with idiopathic hypercalciuria.
High sodium intake has been proposed as the cause of idiopathic hypercalciuria. High sodium intake leads to increased urinary sodium excretion, and the increased tubular sodium load can decrease tubular calcium reabsorption, possibly favoring a reduction in bone mineral density over time.18–20
In healthy people, urine calcium excretion increases by about 0.6 mmol/day (20–40 mg/day) for each 100-mmol (2,300 mg) increment in daily sodium ingestion.21,22 But high sodium intake is seldom the principal cause of idiopathic hypercalciuria.
High protein intake, often observed in patients with nephrolithiasis, increases dietary acid load, stimulating release of calcium from bone and inhibiting renal reabsorption of calcium.23,24 Increasing dietary protein from 0.5 to 2.0 mg/kg/day can double the urinary calcium output.25
In mice, induction of metabolic acidosis, thought to mimic a high-protein diet, inhibits osteoblastic alkaline phosphatase activity while stimulating prostaglandin E2 production.26 This in turn increases osteoblastic expression of receptor activator for nuclear factor kappa b (RANK) ligand, thereby potentially contributing to osteoclastogenesis and osteoclast activity.26
Decreasing dietary protein decreases the recurrence of nephrolithiasis in established stone-formers.27 Still, urine calcium levels are higher in those with idiopathic hypercalciuria than in normal controls at comparable levels of acid excretion, so while protein ingestion could potentially exacerbate the hypercalciuria, it is unlikely to be the sole cause.
Renal calcium leak? The frequent finding of low to low-normal PTH levels in patients with idiopathic hypercalciuria contradicts the potential etiologic mechanism of renal calcium “leak.” In idiopathic hypercalciuria, the PTH response to an oral calcium load is abnormal. If given an oral calcium load, the PTH level should decline if this were due to renal leak, but in the setting of idiopathic hypercalciuria, no clinically meaningful change in PTH occurs. This lack of response of PTH to oral calcium load has been seen in both rat and human studies. Patients also excrete normal to high amounts of urine calcium after prolonged fasting or a low-calcium diet. Low-calcium diets do not induce hyperparathyroidism in these patients, and so the source of the elevated calcium in the urine must be primarily from bone. Increased levels of 1,25-dihydroxyvitamin D in patients with idiopathic hypercalciuria have been noted.28,29
Whether the cytokine milieu also contributes to the calcitriol levels is unclear, but the high or high-normal plasma level of 1,25-dihydroxyvitamin D may be the reason that the PTH is unperturbed.
IMPACT ON BONE HEALTH
Nephrolithiasis is strongly linked to fracture risk.
The bone mineral density of trabecular bone is more affected by calcium excretion than that of cortical bone.18,20,30 However, lumbar spine bone mineral density has not been consistently found to be lower in patients with hyperabsorptive hypercalciuria. Rather, bone mineral density is correlated inversely with urine calcium excretion in men and women who form stones, but not in patients without nephrolithiasis.
In children
In children, idiopathic hypercalciuria is well known to be linked to osteopenia. This is an important group to study, as adult idiopathic hypercalciuria often begins in childhood. However, the trajectory of bone loss vs gain in children is fraught with variables such as growth, puberty, and body mass index, making this a difficult group from which to extrapolate conclusions to adults.
In men
There is more information on the relationship between hypercalciuria and osteoporosis in men than in women.
In 1998, Melton et al31 published the findings of a 25-year population-based cohort study of 624 patients, 442 (71%) of whom were men, referred for new-onset urolithiasis. The incidence of vertebral fracture was 4 times higher in this group than in patients without stone disease, but there was no difference in the rate of hip, forearm, or nonvertebral fractures. This is consistent with earlier data that report a loss of predominantly cancellous bone associated with urolithiasis.
National Health and Nutrition Examination Survey III data in 2001 focused on a potential relationship between kidney stones and bone mineral density or prevalent spine or wrist fracture.32 More than 14,000 people had hip bone mineral density measurements, of whom 793 (477 men, 316 women) had kidney stones. Men with previous nephrolithiasis had lower femoral neck bone mineral density than those without. Men with kidney stones were also more likely to report prevalent wrist and spine fractures. In women, no difference was noted between those with or without stone disease with respect to femoral neck bone mineral density or fracture incidence.
Cauley et al33 also evaluated a relationship between kidney stones and bone mineral density in the Osteoporotic Fractures in Men (MrOS) study. Of approximately 6,000 men, 13.2% reported a history of kidney stones. These men had lower spine and total hip bone mineral density than controls who had not had kidney stones, and the difference persisted after adjusting for age, race, weight, and other variables. However, further data from this cohort revealed that so few men with osteoporosis had hypercalciuria that its routine measurement was not recommended.34
In women
The relationship between idiopathic hypercalciuria and fractures has been more difficult to establish in women.
Sowers et al35 performed an observational study of 1,309 women ages 20 to 92 with a history of nephrolithiasis. No association was noted between stone disease and reduced bone mineral density in the femoral neck, lumbar spine, or radius.
These epidemiologic studies did not include the cause of the kidney stones (eg, whether or not there was associated hypercalciuria or primary hyperparathyroidism), and typically a diagnosis of idiopathic hypercalciuria was not established.
The difference in association between low bone mineral density or fracture with nephrolithiasis between men and women is not well understood, but the most consistent hypothesis is that the influence of hypoestrogenemia in women is much stronger than that of the hypercalciuria.20
Does the degree of hypercalciuria influence the amount of bone loss?
A few trials have tried to determine whether the amount of calcium in the urine influences the magnitude of bone loss.
In 2003, Asplin et al36 reported that bone mineral density Z-scores differed significantly by urinary calcium excretion, but only in stone-formers. In patients without stone disease, there was no difference in Z-scores according to the absolute value of hypercalciuria. This may be due to a self-selection bias in which stone-formers avoid calcium in the diet and those without stone disease do not.
Three studies looking solely at men with idiopathic hypercalciuria also did not detect a significant difference in bone mineral loss according to degree of hypercalciuria.20,30,37
A POLYGENIC DISORDER?
The potential contribution of genetic changes to the development of idiopathic hypercalciuria has been studied. While there is an increased risk of idiopathic hypercalciuria in first-degree relatives of patients with nephrolithiasis, most experts believe that idiopathic hypercalciuria is likely a polygenic disorder.9,38
EVALUATION AND TREATMENT
The 2014 revised version of the National Osteoporosis Foundation’s “Clinician’s guide to prevention and treatment of osteoporosis”39 noted that hypercalciuria is a risk factor that contributes to the development of osteoporosis and possibly osteoporotic fractures, and that consideration should be given to evaluating for hypercalciuria, but only in selected cases. In patients with kidney stones, the link between hypercalciuria and bone loss and fracture is recognized and should be explored in both women and men at risk of osteoporosis, as 45% to 50% of patients who form calcium stones have hypercalciuria.
Patients with kidney stones who have low bone mass and idiopathic hypercalciuria should increase their daily fluid intake, follow a low-salt and low-animal-protein diet, and take thiazide diuretics to reduce the incidence of further calcium stones. Whether this approach also improves bone mass and strength and reduces the risk of fractures within this cohort requires further study.
Dietary interventions
Don’t restrict calcium intake. Despite the connection between hypercalciuria and nephrolithiasis, restriction of dietary calcium to prevent relapse of nephrolithiasis is a risk factor for negative calcium balance and bone demineralization. Observational studies and prospective clinical trials have demonstrated an increased risk of stone formation with low calcium intake.27,30 Nevertheless, this practice seems logical to many patients with kidney stones, and this process may independently contribute to lower bone mineral density.
A low-sodium, low-animal-protein diet is beneficial. Though increased intake of sodium or protein is not the main cause of idiopathic hypercalciuria, pharmacologic therapy, especially with thiazide diuretics, is more likely to be successful in the setting of a low-sodium, low-protein diet.
Borghi et al27 studied 2 diets in men with nephrolithiasis and idiopathic hypercalciuria: a low-calcium diet and a low-salt, low-animal-protein, normal-calcium diet. Men on the latter diet experienced a greater reduction in urinary calcium excretion than those on the low-calcium diet.
Breslau et al40 found that urinary calcium excretion fell by 50% in 15 people when they switched from an animal-based to a plant-based protein diet.
Thiazide diuretics
Several epidemiologic and randomized studies41–45 found that thiazide therapy decreased the likelihood of hip fracture in postmenopausal women, men, and premenopausal women. Doses ranged from 12.5 to 50 mg of hydrochlorothiazide. Bone density increased in the radius, total body, total hip, and lumbar spine. One prospective trial noted that fracture risk declined with longer duration of thiazide use, with the largest reduction in those who used thiazides for 8 or more years.46
Thiazides have anticalciuric actions.47 In addition, they have positive effects on osteoblastic cell proliferation and activity, inhibiting osteocalcin expression by osteoblasts, thereby possibly improving bone formation and mineralization.48 The effects of thiazides on bone was reviewed by Sakhaee et al.49
However, fewer studies have looked at thiazides in patients with idiopathic hypercalciuria.
García-Nieto et al50 looked retrospectively at 22 children (average age 11.7) with idiopathic hypercalciuria and osteopenia who had received thiazides (19 received chlorthalidone 25 mg daily, and 3 received hydrochlorothiazide 25 mg daily) for an average of 2.4 years, and at 32 similar patients who had not received thiazides. Twelve (55%) of the patients receiving thiazides had an improvement in bone mineral density Z-scores, compared with 23 (72%) of the controls. This finding is confounded by growth that occurred during the study, and both groups demonstrated a significantly increased body mass index and bone mineral apparent density at the end of the trial.
Bushinsky and Favus51 evaluated whether chlorthalidone improved bone quality or structure in rats that were genetically prone to hypercalciuric stones. These rats are uniformly stone-formers, and while they have components of calcium hyperabsorption, they also demonstrate renal hyperexcretion (leak) and enhanced bone mineral resorption.51 When fed a high-calcium diet, they maintain a reduction in bone mineral density and bone strength. Study rats were given chlorthalidone 4 to 5 mg/kg/day. After 18 weeks of therapy, significant improvements were observed in trabecular thickness and connectivity as well as increased vertebral compressive strength.52 No difference in cortical bone was noted.
No randomized, blinded, placebo-controlled trial has yet been done to study the impact of thiazides on bone mineral density or fracture risk in patients with idiopathic hypercalciuria.
In practice, many physicians choose chlorthalidone over hydrochlorothiazide because of chlorthalidone’s longer half-life. Combinations of a thiazide diuretic and potassium-sparing medications are also employed, such as hydrochlorothiazide plus either triamterene or spironolactone to reduce the number of pills the patient has to take.
Potassium citrate
When prescribing thiazide diuretics, one should also consider prescribing potassium citrate, as this agent not only prevents hypokalemia but also increases urinary citrate excretion, which can help to inhibit crystallization of calcium salts.6
In a longitudinal study of 28 patients with hypercalciuria,53 combined therapy with a thiazide or indapamide and potassium citrate over a mean of 7 years increased bone density of the lumbar spine by 7.1% and of the femoral neck by 4.1%, compared with treatment in age- and sex-matched normocalcemic peers. In the same study, daily urinary calcium excretion decreased and urinary pH and citrate levels increased; urinary saturation of calcium oxalate decreased by 46%, and stone formation was decreased.
Another trial evaluated 120 patients with idiopathic calcium nephrolithiasis, half of whom were given potassium citrate. Those given potassium citrate experienced an increase in distal radius bone mineral density over 2 years.54 It is theorized that alkalinization may decrease bone turnover in these patients.
Bisphosphonates
As one of the proposed main mechanisms of bone loss in idiopathic hypercalciuria is direct bone resorption, a potential target for therapy is the osteoclast, which bisphosphonates inhibit.
Ruml et al55 studied the impact of alendronate vs placebo in 16 normal men undergoing 3 weeks of strict bedrest. Compared with the placebo group, those who received alendronate had significantly lower 24-hour urine calcium excretion and higher levels of PTH and 1,25-dihydroxyvitamin D.
Weisinger et al56 evaluated the effects of alendronate 10 mg daily in 10 patients who had stone disease with documented idiopathic hypercalciuria and also in 8 normocalciuric patients without stone disease. Alendronate resulted in a sustained reduction of calcium in the urine in the patients with idiopathic hypercalciuria but not in the normocalciuric patients.
Data are somewhat scant as to the effect of bisphosphonates on bone health in the setting of idiopathic hypercalciuria,57,58 and therapy with bisphosphonates is not recommended in patients with idiopathic hypercalciuria outside the realm of postmenopausal osteoporosis or other indications for bisphosphonates approved by the US Food and Drug Administration (FDA).
Calcimimetics
Calcium-sensing receptors are found not only in parathyroid tissue but also in the intestines and kidneys. Locally, elevated plasma calcium in the kidney causes activation of the calcium-sensing receptor, diminishing further calcium reabsorption.59 Agents that increase the sensitivity of the calcium-sensing receptors are classified as calcimimetics.
Cinacalcet is a calcimimetic approved by the FDA for treatment of secondary hyperparathyroidism in patients with chronic kidney disease on dialysis, for the treatment of hypercalcemia in patients with parathyroid carcinoma, and for patients with primary hyperparathyroidism who are unable to undergo parathyroidectomy. In an uncontrolled 5-year study of cinacalcet in patients with primary hyperparathyroidism, there was no significant change in bone density.60
Anti-inflammatory drugs
The role of cytokines in stimulating bone resorption in idiopathic hypercalciuria has led to the investigation of several anti-inflammatory drugs (eg, diclofenac, indomethacin) as potential treatments, but studies have been limited in number and scope.61,62
Omega-3 fatty acids
Omega-3 fatty acids are thought to alter prostaglandin metabolism and to potentially reduce stone formation.63
A retrospective study of 29 patients with stone disease found that, combined with dietary counseling, omega-3 fatty acids could potentially reduce urinary calcium and oxalate excretion and increase urinary citrate in hypercalciuric stone-formers.64
A review of published randomized controlled trials of omega-3 fatty acids in skeletal health discovered that 4 studies found positive effects on bone mineral density or bone turnover markers, whereas 5 studies reported no differences. All trials were small, and none evaluated fracture outcome.65
- Albright F, Henneman P, Benedict PH, Forbes AP. Idiopathic hypercalciuria: a preliminary report. Proc R Soc Med 1953; 46:1077–1081.
- Pak CY. Pathophysiology of calcium nephrolithiasis. In: Seldin DW, Giebiscg G, eds. The Kidney: Physiology and Pathophysiology. New York, NY: Raven Press; 1992:2461–2480.
- Frick KK, Bushinsky DA. Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol 2003; 14:1082–1095.
- Pacifici R, Rothstein M, Rifas L, et al. Increased monocyte interleukin-1 activity and decreased vertebral bone density in patients with fasting idiopathic hypercalciuria. J Clin Endocrinol Metab 1990; 71:138–145.
- Messa P, Mioni G, Montanaro D, et al. About a primitive osseous origin of the so-called ‘renal hypercalciuria.’ Contrib Nephrol 1987; 58:106–110.
- Zerwekh JE. Bone disease and idiopathic hypercalciuria. Semin Nephrol 2008; 28:133–142.
- Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med 1977; 87:404–410.
- Lemann J Jr. Pathogenesis of idiopathic hypercalciuria and nephrolithiasis. In: Coe FL, Favus MJ, eds. Disorders of Bone and Mineral Metabolism. New York, NY: Raven Press; 1992:685-706.
- Coe FL, Parks JH, Moore ES. Familial idiopathic hypercalciuria. N Engl J Med 1979; 300:337–340.
- Giannini S, Nobile M, Dalle Carbonare L, et al. Hypercalciuria is a common and important finding in postmenopausal women with osteoporosis. Eur J Endocrinol 2003; 149:209–213.
- Tannenbaum C, Clark J, Schwartzman K, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 2002; 87:4431–4437.
- Cerda Gabaroi D, Peris P, Monegal A, et al. Search for hidden secondary causes in postmenopausal women with osteoporosis. Menopause 2010; 17:135–139.
- Rull MA, Cano-García Mdel C, Arrabal-Martín M, Arrabal-Polo MA. The importance of urinary calcium in postmenopausal women with osteoporotic fracture. Can Urol Assoc J 2015; 9:E183–E186.
- Heaney RP, Recker RR, Ryan RA. Urinary calcium in perimenopausal women: normative values. Osteoporos Int 1999; 9:13–18.
- Bleich HL, Moore MJ, Lemann J Jr, Adams ND, Gray RW. Urinary calcium excretion in human beings. N Engl J Med 1979; 301:535–541.
- Li XQ, Tembe V, Horwitz GM, Bushinsky DA, Favus MJ. Increased intestinal vitamin D receptor in genetic hypercalciuric rats. A cause of intestinal calcium hyperabsorption. J Clin Invest 1993; 91:661–667.
- Yao J, Kathpalia P, Bushinsky DA, Favus MJ. Hyperresponsiveness of vitamin D receptor gene expression to 1,25-dihydroxyvitamin D3. A new characteristic of genetic hypercalciuric stone-forming rats. J Clin Invest 1998; 101:2223–2232.
- Pietschmann F, Breslau NA, Pak CY. Reduced vertebral bone density in hypercalciuric nephrolithiasis. J Bone Miner Res 1992; 7:1383–1388.
- Jaeger P, Lippuner K, Casez JP, Hess B, Ackermann D, Hug C. Low bone mass in idiopathic renal stone formers: magnitude and significance. J Bone Miner Res 1994; 9:1525–1532.
- Vezzoli G, Soldati L, Arcidiacono T, et al. Urinary calcium is a determinant of bone mineral density in elderly men participating in the InCHIANTI study. Kidney Int 2005; 67:2006–2014.
- Lemann J Jr, Worcester EM, Gray RW. Hypercalciuria and stones. Am J Kidney Dis 1991; 17:386–391.
- Gokce C, Gokce O, Baydinc C, et al. Use of random urine samples to estimate total urinary calcium and phosphate excretion. Arch Intern Med 1991; 151:1587–1588.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993; 328:833–838.
- Siener R, Schade N, Nicolay C, von Unruh GE, Hesse A. The efficacy of dietary intervention on urinary risk factors for stone formation in recurrent calcium oxalate stone patients. J Urol 2005; 173:1601–1605.
- Jones AN, Shafer MM, Keuler NS, Crone EM, Hansen KE. Fasting and postprandial spot urine calcium-to-creatinine ratios do not detect hypercalciuria. Osteoporos Int 2012; 23:553–562.
- Frick KK, Bushinsky DA. Metabolic acidosis stimulates RANKL RNA expression in bone through a cyclo-oxygenase-dependent mechanism. J Bone Miner Res 2003; 18:1317–1325.
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346:77–84.
- Ghazali A, Fuentes V, Desaint C, et al. Low bone mineral density and peripheral blood monocyte activation profile in calcium stone formers with idiopathic hypercalciuria. J Clin Endocrinol Metab 1997; 82:32–38.
- Broadus AE, Insogna KL, Lang R, Ellison AF, Dreyer BE. Evidence for disordered control of 1,25-dihydroxyvitamin D production in absorptive hypercalciuria. N Engl J Med 1984; 311:73–80.
- Tasca A, Cacciola A, Ferrarese P, et al. Bone alterations in patients with idiopathic hypercalciuria and calcium nephrolithiasis. Urology 2002; 59:865–869.
- Melton LJ 3rd, Crowson CS, Khosla S, Wilson DM, O’Fallon WM. Fracture risk among patients with urolithiasis: a population-based cohort study. Kidney Int 1998; 53:459–464.
- Lauderdale DS, Thisted RA, Wen M, Favus MJ. Bone mineral density and fracture among prevalent kidney stone cases in the Third National Health and Nutrition Examination Survey. J Bone Miner Res 2001; 16:1893–1898.
- Cauley JA, Fullman RL, Stone KL, et al; MrOS Research Group. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int 2005; 16:1525–1537.
- Fink HA, Litwack-Harrison S, Taylor BC, et al; Osteoporotic Fractures in Men (MrOS) Study Group. Clinical utility of routine laboratory testing to identify possible secondary causes in older men with osteoporosis: the Osteoporotic Fractures in Men (MrOS) Study. Osteoporos Int 2016: 27:331–338.
- Sowers MR, Jannausch M, Wood C, Pope SK, Lachance LL, Peterson B. Prevalence of renal stones in a population-based study with dietary calcium, oxalate and medication exposures. Am J Epidemiol 1998; 147:914–920.
- Asplin JR, Bauer KA, Kinder J, et al. Bone mineral density and urine calcium excretion among subjects with and without nephrolithiasis. Kidney Int 2003; 63:662–669.
- Letavernier E, Traxer O, Daudon M, et al. Determinants of osteopenia in male renal-stone-disease patients with idiopathic hypercalciuria. Clin J Am Soc Nephrol 2011; 6:1149–1154.
- Vezzoli G, Soldati L, Gambaro G. Update on primary hypercalciuria from a genetic perspective. J Urol 2008; 179:1676–1682.
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014: 25:2359–2381.
- Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab 1988; 66:140–146.
- Reid IR, Ames RW, Orr-Walker BJ, et al. Hydrochlorothiazide reduces loss of cortical bone in normal postmenopausal women: a randomized controlled trial. Am J Med 2000; 109:362–370.
- Bolland MJ, Ames RW, Horne AM, Orr-Walker BJ, Gamble GD, Reid IR. The effect of treatment with a thiazide diuretic for 4 years on bone density in normal postmenopausal women. Osteoporos Int 2007; 18:479–486.
- LaCroix AZ, Ott SM, Ichikawa L, Scholes D, Barlow WE. Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. Ann Intern Med 2000; 133:516–526.
- Wasnich RD, Davis JW, He YF, Petrovich H, Ross PD. A randomized, double-masked, placebo-controlled trial of chlorthalidone and bone loss in elderly women. Osteoporos Int 1995; 5:247–251.
- Adams JS, Song CF, Kantorovich V. Rapid recovery of bone mass in hypercalciuric, osteoporotic men treated with hydrochlorothiazide. Ann Intern Med 1999; 130:658–660.
- Feskanich D, Willett WC, Stampfer MJ, Colditz GA. A prospective study of thiazide use and fractures in women. Osteoporos Int 1997; 7:79–84.
- Lamberg BA, Kuhlback B. Effect of chlorothiazide and hydrochlorothiazide on the excretion of calcium in the urine. Scand J Clin Lab Invest 1959; 11:351–357.
- Lajeunesse D, Delalandre A, Guggino SE. Thiazide diuretics affect osteocalcin production in human osteoblasts at the transcription level without affecting vitamin D3 receptors. J Bone Miner Res 2000; 15:894–901.
- Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int 2001; 79:393–403.
- García-Nieto V, Monge-Zamorano M, González-García M, Luis-Yanes MI. Effect of thiazides on bone mineral density in children with idiopathic hypercalciuria. Pediatr Nephrol 2012; 27:261–268.
- Bushinsky DA, Favus MJ. Mechanism of hypercalciuria in genetic hypercalciuric rats. Inherited defect in intestinal calcium transport. J Clin Invest 1988; 82:1585–1591.
- Bushinsky DA, Willett T, Asplin JR, Culbertson C, Che SP, Grynpas M. Chlorthalidone improves vertebral bone quality in genetic hypercalciuric stone-forming rats. J Bone Miner Res 2011; 26:1904–1912.
- Pak CY, Heller HJ, Pearle MS, Odvina CV, Poindexter JR, Peterson RD. Prevention of stone formation and bone loss in absorptive hypercalciuria by combined dietary and pharmacological interventions. J Urol 2003; 169:465–469.
- Vescini F, Buffa A, LaManna G, et al. Long-term potassium citrate therapy and bone mineral density in idiopathic calcium stone formers. J Endocrinol Invest 2005; 28:218–222.
- Ruml LA, Dubois SK, Roberts ML, Pak CY. Prevention of hypercalciuria and stone-forming propensity during prolonged bedrest by alendronate. J Bone Miner Res 1995; 10:655–662.
- Weisinger JR, Alonzo E, Machado C, et al. Role of bones in the physiopathology of idiopathic hypercalciuria: effect of amino-bisphosphonate alendronate. Medicina (B Aires) 1997; 57(suppl 1):45–48. Spanish.
- Heilberg IP, Martini LA, Teixeira SH, et al. Effect of etidronate treatment on bone mass of male nephrolithiasis patients with idiopathic hypercalciuria and osteopenia. Nephron 1998; 79:430–437.
- Bushinsky DA, Neumann KJ, Asplin J, Krieger NS. Alendronate decreases urine calcium and supersaturation in genetic hypercalciuric rats. Kidney Int 1999; 55:234–243.
- Riccardi D, Park J, Lee WS, Gamba G, Brown EM, Hebert SC. Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc Natl Acad Sci USA 1995; 92:131–145.
- Peacock M, Bolognese MA, Borofsky M, et al. Cinacalcet treatment of primary hyperparathyroidism: biochemical and bone densitometric outcomes in a five-year study. J Clin Endocrinol Metab 2009; 94:4860–4867.
- Filipponi P, Mannarelli C, Pacifici R, et al. Evidence for a prostaglandin-mediated bone resorptive mechanism in subjects with fasting hypercalciuria. Calcif Tissue Int 1988; 43:61–66.
- Gomaa AA, Hassan HA, Ghaneimah SA. Effect of aspirin and indomethacin on the serum and urinary calcium, magnesium and phosphate. Pharmacol Res 1990; 22:59–70.
- Buck AC, Davies RL, Harrison T. The protective role of eicosapentaenoic acid (EPA) in the pathogenesis of nephrolithiasis. J Urol 1991; 146:188–194.
- Ortiz-Alvarado O, Miyaoka R, Kriedberg C, et al. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid in the management of hypercalciuric stone formers. Urology 2012; 79:282–286.
- Orchard TS, Pan X, Cheek F, Ing SW, Jackson RD. A systematic review of omega-3 fatty acids and osteoporosis. Br J Nutr 2012; 107(suppl 2):S253–S260.
A 65-year-old woman was recently diagnosed with osteoporosis after a screening bone mineral density test. She has hypertension (treated with lisinopril), and she had an episode of passing a kidney stone 10 years ago. A 24-hour urine study reveals an elevated urinary calcium level.
What should the physician keep in mind in managing this patient?
IDIOPATHIC HYPERCALCIURIA
Many potential causes of secondary hypercalciuria must be ruled out before deciding that a patient has idiopathic hypercalciuria, which was first noted as a distinct entity by Albright et al in 1953.1 Causes of secondary hypercalciuria include primary hyperparathyroidism, hyperthyroidism, Paget disease, myeloma, malignancy, immobility, accelerated osteoporosis, sarcoidosis, renal tubular acidosis, and drug-induced urinary calcium loss such as that seen with loop diuretics.
Idiopathic hypercalciuria is identified by the following:
- Persistent hypercalciuria despite normal or restricted calcium intake2,3
- Normal levels of parathyroid hormone (PTH), phosphorus, and 1,25-dihydroxy-vitamin D (the active form of vitamin D, also called calcitriol) in the presence of hypercalciuria; serum calcium levels are also normal.
An alias for idiopathic hypercalciuria is “fasting hypercalciuria,” as increased urinary calcium persists and sometimes worsens while fasting or on a low-calcium diet, with increased bone turnover, reduced bone density, and normal serum PTH levels.4,5
Mineral loss from bone predominates in idiopathic hypercalciuria, but there is also a minor component of intestinal hyperabsorption of calcium and reduced renal calcium reabsorption.6 Distinguishing among intestinal hyperabsorptive hypercalciuria, renal leak hypercalciuria, and idiopathic or fasting hypercalciuria can be difficult and subtle. It has been argued that differentiating among hypercalciuric subtypes (hyperabsorptive, renal leak, idiopathic) is not useful; in general clinical practice, it is impractical to collect multiple 24-hour urine samples in the setting of controlled high- vs low-calcium diets.
COMPLICATIONS OF IDIOPATHIC HYPERCALCIURIA
Calcium is an important component in many physiologic processes, including coagulation, cell membrane transfer, hormone release, neuromuscular activation, and myocardial contraction. A sophisticated system of hormonally mediated interactions normally maintains stable extracellular calcium levels. Calcium is vital for bone strength, but the bones are the body’s calcium “bank,” and withdrawals from this bank are made at the expense of bone strength and integrity.
Renal stones
Patients with idiopathic hypercalciuria have a high incidence of renal stones. Conversely, 40% to 50% of patients with recurrent kidney stones have evidence of idiopathic hypercalciuria, the most common metabolic abnormality in “stone-formers.”7,8 Further, 35% to 40% of first- and second-degree relatives of stone-formers who have idiopathic hypercalciuria also have the condition.9 In the general population without kidney stones and without first-degree relatives with stones, the prevalence is approximately 5% to 10%.10,11
Bone loss
People with idiopathic hypercalciuria have lower bone density and a higher incidence of fracture than their normocalciuric peers. This relationship has been observed in both sexes and all ages. Idiopathic hypercalciuria has been noted in 10% to 19% of otherwise healthy men with low bone mass, in postmenopausal women with osteoporosis,10–12 and in up to 40% of postmenopausal women with osteoporotic fractures and no history of kidney stones.13
LABORATORY DEFINITION
Urinary calcium excretion
Heaney et al14 measured 24-hour urinary calcium excretion in a group of early postmenopausal women, whom he divided into 3 groups by dietary calcium intake:
- Low intake (< 500 mg/day)
- Moderate intake (500–1,000 mg/day)
- High intake (> 1,000 mg/day).
In the women who were estrogen-deprived (ie, postmenopausal and not on estrogen replacement therapy), the 95% probability ranges for urinary calcium excretion were:
- 32–252 mg/day (0.51–4.06 mg/kg/day) with low calcium intake
- 36–286 mg/day (0.57–4.52 mg/kg/day) with moderate calcium intake
- 45–357 mg/day (0.69–5.47 mg/kg/day) with high calcium intake.
For estrogen-replete women (perimenopausal or postmenopausal on estrogen replacement), using the same categories of dietary calcium intake, calcium excretion was:
- 39–194 mg/day (0.65–3.23 mg/kg/day) with low calcium intake
- 54–269 mg/day (0.77–3.84 mg/kg/day) with moderate calcium intake
- 66–237 mg/day (0.98–4.89 mg/kg/day) with high calcium intake.
In the estrogen-deprived group, urinary calcium excretion increased by only 55 mg/day per 1,000-mg increase in dietary intake, though there was individual variability. These data suggest that hypercalciuria should be defined as:
- Greater than 250 mg/day (> 4.1 mg/kg/day) in estrogen-replete women
- Greater than 300 mg/day (> 5.0 mg/kg/day) in estrogen-deprived women.
Urinary calcium-to-creatinine ratio
Use of a spot urinary calcium-to-creatinine ratio has been advocated as an alternative to the more labor-intensive 24-hour urine collection.15 However, the spot urine calcium-creatinine ratio correlates poorly with 24-hour urine criteria for hypercalciuria whether by absolute, weight-based, or menopausal and calcium-adjusted definitions.
Importantly, spot urine measurements show poor sensitivity and specificity for hypercalciuria. Spot urine samples underestimate the 24-hour urinary calcium (Bland-Altman bias –71 mg/24 hours), and postprandial sampling overestimates it (Bland-Altman bias +61 mg/24 hours).15
WHAT IS THE MECHANISM OF IDIOPATHIC HYPERCALCIURIA?
The pathophysiology of idiopathic hypercalciuria has been difficult to establish.
Increased sensitivity to vitamin D? In the hyperabsorbing population, activated vitamin D levels are often robust, but a few studies of rats with hyperabsorbing, hyperexcreting physiology have shown normal calcitriol levels, suggesting an increased sensitivity to the actions of 1,25-dihydroxyvitamin D.16
Another study found that hypercalciuric stone-forming rats have more 1,25-dihydroxyvitamin D receptors than do controls.17
These changes have not been demonstrated in patients with idiopathic hypercalciuria.
High sodium intake has been proposed as the cause of idiopathic hypercalciuria. High sodium intake leads to increased urinary sodium excretion, and the increased tubular sodium load can decrease tubular calcium reabsorption, possibly favoring a reduction in bone mineral density over time.18–20
In healthy people, urine calcium excretion increases by about 0.6 mmol/day (20–40 mg/day) for each 100-mmol (2,300 mg) increment in daily sodium ingestion.21,22 But high sodium intake is seldom the principal cause of idiopathic hypercalciuria.
High protein intake, often observed in patients with nephrolithiasis, increases dietary acid load, stimulating release of calcium from bone and inhibiting renal reabsorption of calcium.23,24 Increasing dietary protein from 0.5 to 2.0 mg/kg/day can double the urinary calcium output.25
In mice, induction of metabolic acidosis, thought to mimic a high-protein diet, inhibits osteoblastic alkaline phosphatase activity while stimulating prostaglandin E2 production.26 This in turn increases osteoblastic expression of receptor activator for nuclear factor kappa b (RANK) ligand, thereby potentially contributing to osteoclastogenesis and osteoclast activity.26
Decreasing dietary protein decreases the recurrence of nephrolithiasis in established stone-formers.27 Still, urine calcium levels are higher in those with idiopathic hypercalciuria than in normal controls at comparable levels of acid excretion, so while protein ingestion could potentially exacerbate the hypercalciuria, it is unlikely to be the sole cause.
Renal calcium leak? The frequent finding of low to low-normal PTH levels in patients with idiopathic hypercalciuria contradicts the potential etiologic mechanism of renal calcium “leak.” In idiopathic hypercalciuria, the PTH response to an oral calcium load is abnormal. If given an oral calcium load, the PTH level should decline if this were due to renal leak, but in the setting of idiopathic hypercalciuria, no clinically meaningful change in PTH occurs. This lack of response of PTH to oral calcium load has been seen in both rat and human studies. Patients also excrete normal to high amounts of urine calcium after prolonged fasting or a low-calcium diet. Low-calcium diets do not induce hyperparathyroidism in these patients, and so the source of the elevated calcium in the urine must be primarily from bone. Increased levels of 1,25-dihydroxyvitamin D in patients with idiopathic hypercalciuria have been noted.28,29
Whether the cytokine milieu also contributes to the calcitriol levels is unclear, but the high or high-normal plasma level of 1,25-dihydroxyvitamin D may be the reason that the PTH is unperturbed.
IMPACT ON BONE HEALTH
Nephrolithiasis is strongly linked to fracture risk.
The bone mineral density of trabecular bone is more affected by calcium excretion than that of cortical bone.18,20,30 However, lumbar spine bone mineral density has not been consistently found to be lower in patients with hyperabsorptive hypercalciuria. Rather, bone mineral density is correlated inversely with urine calcium excretion in men and women who form stones, but not in patients without nephrolithiasis.
In children
In children, idiopathic hypercalciuria is well known to be linked to osteopenia. This is an important group to study, as adult idiopathic hypercalciuria often begins in childhood. However, the trajectory of bone loss vs gain in children is fraught with variables such as growth, puberty, and body mass index, making this a difficult group from which to extrapolate conclusions to adults.
In men
There is more information on the relationship between hypercalciuria and osteoporosis in men than in women.
In 1998, Melton et al31 published the findings of a 25-year population-based cohort study of 624 patients, 442 (71%) of whom were men, referred for new-onset urolithiasis. The incidence of vertebral fracture was 4 times higher in this group than in patients without stone disease, but there was no difference in the rate of hip, forearm, or nonvertebral fractures. This is consistent with earlier data that report a loss of predominantly cancellous bone associated with urolithiasis.
National Health and Nutrition Examination Survey III data in 2001 focused on a potential relationship between kidney stones and bone mineral density or prevalent spine or wrist fracture.32 More than 14,000 people had hip bone mineral density measurements, of whom 793 (477 men, 316 women) had kidney stones. Men with previous nephrolithiasis had lower femoral neck bone mineral density than those without. Men with kidney stones were also more likely to report prevalent wrist and spine fractures. In women, no difference was noted between those with or without stone disease with respect to femoral neck bone mineral density or fracture incidence.
Cauley et al33 also evaluated a relationship between kidney stones and bone mineral density in the Osteoporotic Fractures in Men (MrOS) study. Of approximately 6,000 men, 13.2% reported a history of kidney stones. These men had lower spine and total hip bone mineral density than controls who had not had kidney stones, and the difference persisted after adjusting for age, race, weight, and other variables. However, further data from this cohort revealed that so few men with osteoporosis had hypercalciuria that its routine measurement was not recommended.34
In women
The relationship between idiopathic hypercalciuria and fractures has been more difficult to establish in women.
Sowers et al35 performed an observational study of 1,309 women ages 20 to 92 with a history of nephrolithiasis. No association was noted between stone disease and reduced bone mineral density in the femoral neck, lumbar spine, or radius.
These epidemiologic studies did not include the cause of the kidney stones (eg, whether or not there was associated hypercalciuria or primary hyperparathyroidism), and typically a diagnosis of idiopathic hypercalciuria was not established.
The difference in association between low bone mineral density or fracture with nephrolithiasis between men and women is not well understood, but the most consistent hypothesis is that the influence of hypoestrogenemia in women is much stronger than that of the hypercalciuria.20
Does the degree of hypercalciuria influence the amount of bone loss?
A few trials have tried to determine whether the amount of calcium in the urine influences the magnitude of bone loss.
In 2003, Asplin et al36 reported that bone mineral density Z-scores differed significantly by urinary calcium excretion, but only in stone-formers. In patients without stone disease, there was no difference in Z-scores according to the absolute value of hypercalciuria. This may be due to a self-selection bias in which stone-formers avoid calcium in the diet and those without stone disease do not.
Three studies looking solely at men with idiopathic hypercalciuria also did not detect a significant difference in bone mineral loss according to degree of hypercalciuria.20,30,37
A POLYGENIC DISORDER?
The potential contribution of genetic changes to the development of idiopathic hypercalciuria has been studied. While there is an increased risk of idiopathic hypercalciuria in first-degree relatives of patients with nephrolithiasis, most experts believe that idiopathic hypercalciuria is likely a polygenic disorder.9,38
EVALUATION AND TREATMENT
The 2014 revised version of the National Osteoporosis Foundation’s “Clinician’s guide to prevention and treatment of osteoporosis”39 noted that hypercalciuria is a risk factor that contributes to the development of osteoporosis and possibly osteoporotic fractures, and that consideration should be given to evaluating for hypercalciuria, but only in selected cases. In patients with kidney stones, the link between hypercalciuria and bone loss and fracture is recognized and should be explored in both women and men at risk of osteoporosis, as 45% to 50% of patients who form calcium stones have hypercalciuria.
Patients with kidney stones who have low bone mass and idiopathic hypercalciuria should increase their daily fluid intake, follow a low-salt and low-animal-protein diet, and take thiazide diuretics to reduce the incidence of further calcium stones. Whether this approach also improves bone mass and strength and reduces the risk of fractures within this cohort requires further study.
Dietary interventions
Don’t restrict calcium intake. Despite the connection between hypercalciuria and nephrolithiasis, restriction of dietary calcium to prevent relapse of nephrolithiasis is a risk factor for negative calcium balance and bone demineralization. Observational studies and prospective clinical trials have demonstrated an increased risk of stone formation with low calcium intake.27,30 Nevertheless, this practice seems logical to many patients with kidney stones, and this process may independently contribute to lower bone mineral density.
A low-sodium, low-animal-protein diet is beneficial. Though increased intake of sodium or protein is not the main cause of idiopathic hypercalciuria, pharmacologic therapy, especially with thiazide diuretics, is more likely to be successful in the setting of a low-sodium, low-protein diet.
Borghi et al27 studied 2 diets in men with nephrolithiasis and idiopathic hypercalciuria: a low-calcium diet and a low-salt, low-animal-protein, normal-calcium diet. Men on the latter diet experienced a greater reduction in urinary calcium excretion than those on the low-calcium diet.
Breslau et al40 found that urinary calcium excretion fell by 50% in 15 people when they switched from an animal-based to a plant-based protein diet.
Thiazide diuretics
Several epidemiologic and randomized studies41–45 found that thiazide therapy decreased the likelihood of hip fracture in postmenopausal women, men, and premenopausal women. Doses ranged from 12.5 to 50 mg of hydrochlorothiazide. Bone density increased in the radius, total body, total hip, and lumbar spine. One prospective trial noted that fracture risk declined with longer duration of thiazide use, with the largest reduction in those who used thiazides for 8 or more years.46
Thiazides have anticalciuric actions.47 In addition, they have positive effects on osteoblastic cell proliferation and activity, inhibiting osteocalcin expression by osteoblasts, thereby possibly improving bone formation and mineralization.48 The effects of thiazides on bone was reviewed by Sakhaee et al.49
However, fewer studies have looked at thiazides in patients with idiopathic hypercalciuria.
García-Nieto et al50 looked retrospectively at 22 children (average age 11.7) with idiopathic hypercalciuria and osteopenia who had received thiazides (19 received chlorthalidone 25 mg daily, and 3 received hydrochlorothiazide 25 mg daily) for an average of 2.4 years, and at 32 similar patients who had not received thiazides. Twelve (55%) of the patients receiving thiazides had an improvement in bone mineral density Z-scores, compared with 23 (72%) of the controls. This finding is confounded by growth that occurred during the study, and both groups demonstrated a significantly increased body mass index and bone mineral apparent density at the end of the trial.
Bushinsky and Favus51 evaluated whether chlorthalidone improved bone quality or structure in rats that were genetically prone to hypercalciuric stones. These rats are uniformly stone-formers, and while they have components of calcium hyperabsorption, they also demonstrate renal hyperexcretion (leak) and enhanced bone mineral resorption.51 When fed a high-calcium diet, they maintain a reduction in bone mineral density and bone strength. Study rats were given chlorthalidone 4 to 5 mg/kg/day. After 18 weeks of therapy, significant improvements were observed in trabecular thickness and connectivity as well as increased vertebral compressive strength.52 No difference in cortical bone was noted.
No randomized, blinded, placebo-controlled trial has yet been done to study the impact of thiazides on bone mineral density or fracture risk in patients with idiopathic hypercalciuria.
In practice, many physicians choose chlorthalidone over hydrochlorothiazide because of chlorthalidone’s longer half-life. Combinations of a thiazide diuretic and potassium-sparing medications are also employed, such as hydrochlorothiazide plus either triamterene or spironolactone to reduce the number of pills the patient has to take.
Potassium citrate
When prescribing thiazide diuretics, one should also consider prescribing potassium citrate, as this agent not only prevents hypokalemia but also increases urinary citrate excretion, which can help to inhibit crystallization of calcium salts.6
In a longitudinal study of 28 patients with hypercalciuria,53 combined therapy with a thiazide or indapamide and potassium citrate over a mean of 7 years increased bone density of the lumbar spine by 7.1% and of the femoral neck by 4.1%, compared with treatment in age- and sex-matched normocalcemic peers. In the same study, daily urinary calcium excretion decreased and urinary pH and citrate levels increased; urinary saturation of calcium oxalate decreased by 46%, and stone formation was decreased.
Another trial evaluated 120 patients with idiopathic calcium nephrolithiasis, half of whom were given potassium citrate. Those given potassium citrate experienced an increase in distal radius bone mineral density over 2 years.54 It is theorized that alkalinization may decrease bone turnover in these patients.
Bisphosphonates
As one of the proposed main mechanisms of bone loss in idiopathic hypercalciuria is direct bone resorption, a potential target for therapy is the osteoclast, which bisphosphonates inhibit.
Ruml et al55 studied the impact of alendronate vs placebo in 16 normal men undergoing 3 weeks of strict bedrest. Compared with the placebo group, those who received alendronate had significantly lower 24-hour urine calcium excretion and higher levels of PTH and 1,25-dihydroxyvitamin D.
Weisinger et al56 evaluated the effects of alendronate 10 mg daily in 10 patients who had stone disease with documented idiopathic hypercalciuria and also in 8 normocalciuric patients without stone disease. Alendronate resulted in a sustained reduction of calcium in the urine in the patients with idiopathic hypercalciuria but not in the normocalciuric patients.
Data are somewhat scant as to the effect of bisphosphonates on bone health in the setting of idiopathic hypercalciuria,57,58 and therapy with bisphosphonates is not recommended in patients with idiopathic hypercalciuria outside the realm of postmenopausal osteoporosis or other indications for bisphosphonates approved by the US Food and Drug Administration (FDA).
Calcimimetics
Calcium-sensing receptors are found not only in parathyroid tissue but also in the intestines and kidneys. Locally, elevated plasma calcium in the kidney causes activation of the calcium-sensing receptor, diminishing further calcium reabsorption.59 Agents that increase the sensitivity of the calcium-sensing receptors are classified as calcimimetics.
Cinacalcet is a calcimimetic approved by the FDA for treatment of secondary hyperparathyroidism in patients with chronic kidney disease on dialysis, for the treatment of hypercalcemia in patients with parathyroid carcinoma, and for patients with primary hyperparathyroidism who are unable to undergo parathyroidectomy. In an uncontrolled 5-year study of cinacalcet in patients with primary hyperparathyroidism, there was no significant change in bone density.60
Anti-inflammatory drugs
The role of cytokines in stimulating bone resorption in idiopathic hypercalciuria has led to the investigation of several anti-inflammatory drugs (eg, diclofenac, indomethacin) as potential treatments, but studies have been limited in number and scope.61,62
Omega-3 fatty acids
Omega-3 fatty acids are thought to alter prostaglandin metabolism and to potentially reduce stone formation.63
A retrospective study of 29 patients with stone disease found that, combined with dietary counseling, omega-3 fatty acids could potentially reduce urinary calcium and oxalate excretion and increase urinary citrate in hypercalciuric stone-formers.64
A review of published randomized controlled trials of omega-3 fatty acids in skeletal health discovered that 4 studies found positive effects on bone mineral density or bone turnover markers, whereas 5 studies reported no differences. All trials were small, and none evaluated fracture outcome.65
A 65-year-old woman was recently diagnosed with osteoporosis after a screening bone mineral density test. She has hypertension (treated with lisinopril), and she had an episode of passing a kidney stone 10 years ago. A 24-hour urine study reveals an elevated urinary calcium level.
What should the physician keep in mind in managing this patient?
IDIOPATHIC HYPERCALCIURIA
Many potential causes of secondary hypercalciuria must be ruled out before deciding that a patient has idiopathic hypercalciuria, which was first noted as a distinct entity by Albright et al in 1953.1 Causes of secondary hypercalciuria include primary hyperparathyroidism, hyperthyroidism, Paget disease, myeloma, malignancy, immobility, accelerated osteoporosis, sarcoidosis, renal tubular acidosis, and drug-induced urinary calcium loss such as that seen with loop diuretics.
Idiopathic hypercalciuria is identified by the following:
- Persistent hypercalciuria despite normal or restricted calcium intake2,3
- Normal levels of parathyroid hormone (PTH), phosphorus, and 1,25-dihydroxy-vitamin D (the active form of vitamin D, also called calcitriol) in the presence of hypercalciuria; serum calcium levels are also normal.
An alias for idiopathic hypercalciuria is “fasting hypercalciuria,” as increased urinary calcium persists and sometimes worsens while fasting or on a low-calcium diet, with increased bone turnover, reduced bone density, and normal serum PTH levels.4,5
Mineral loss from bone predominates in idiopathic hypercalciuria, but there is also a minor component of intestinal hyperabsorption of calcium and reduced renal calcium reabsorption.6 Distinguishing among intestinal hyperabsorptive hypercalciuria, renal leak hypercalciuria, and idiopathic or fasting hypercalciuria can be difficult and subtle. It has been argued that differentiating among hypercalciuric subtypes (hyperabsorptive, renal leak, idiopathic) is not useful; in general clinical practice, it is impractical to collect multiple 24-hour urine samples in the setting of controlled high- vs low-calcium diets.
COMPLICATIONS OF IDIOPATHIC HYPERCALCIURIA
Calcium is an important component in many physiologic processes, including coagulation, cell membrane transfer, hormone release, neuromuscular activation, and myocardial contraction. A sophisticated system of hormonally mediated interactions normally maintains stable extracellular calcium levels. Calcium is vital for bone strength, but the bones are the body’s calcium “bank,” and withdrawals from this bank are made at the expense of bone strength and integrity.
Renal stones
Patients with idiopathic hypercalciuria have a high incidence of renal stones. Conversely, 40% to 50% of patients with recurrent kidney stones have evidence of idiopathic hypercalciuria, the most common metabolic abnormality in “stone-formers.”7,8 Further, 35% to 40% of first- and second-degree relatives of stone-formers who have idiopathic hypercalciuria also have the condition.9 In the general population without kidney stones and without first-degree relatives with stones, the prevalence is approximately 5% to 10%.10,11
Bone loss
People with idiopathic hypercalciuria have lower bone density and a higher incidence of fracture than their normocalciuric peers. This relationship has been observed in both sexes and all ages. Idiopathic hypercalciuria has been noted in 10% to 19% of otherwise healthy men with low bone mass, in postmenopausal women with osteoporosis,10–12 and in up to 40% of postmenopausal women with osteoporotic fractures and no history of kidney stones.13
LABORATORY DEFINITION
Urinary calcium excretion
Heaney et al14 measured 24-hour urinary calcium excretion in a group of early postmenopausal women, whom he divided into 3 groups by dietary calcium intake:
- Low intake (< 500 mg/day)
- Moderate intake (500–1,000 mg/day)
- High intake (> 1,000 mg/day).
In the women who were estrogen-deprived (ie, postmenopausal and not on estrogen replacement therapy), the 95% probability ranges for urinary calcium excretion were:
- 32–252 mg/day (0.51–4.06 mg/kg/day) with low calcium intake
- 36–286 mg/day (0.57–4.52 mg/kg/day) with moderate calcium intake
- 45–357 mg/day (0.69–5.47 mg/kg/day) with high calcium intake.
For estrogen-replete women (perimenopausal or postmenopausal on estrogen replacement), using the same categories of dietary calcium intake, calcium excretion was:
- 39–194 mg/day (0.65–3.23 mg/kg/day) with low calcium intake
- 54–269 mg/day (0.77–3.84 mg/kg/day) with moderate calcium intake
- 66–237 mg/day (0.98–4.89 mg/kg/day) with high calcium intake.
In the estrogen-deprived group, urinary calcium excretion increased by only 55 mg/day per 1,000-mg increase in dietary intake, though there was individual variability. These data suggest that hypercalciuria should be defined as:
- Greater than 250 mg/day (> 4.1 mg/kg/day) in estrogen-replete women
- Greater than 300 mg/day (> 5.0 mg/kg/day) in estrogen-deprived women.
Urinary calcium-to-creatinine ratio
Use of a spot urinary calcium-to-creatinine ratio has been advocated as an alternative to the more labor-intensive 24-hour urine collection.15 However, the spot urine calcium-creatinine ratio correlates poorly with 24-hour urine criteria for hypercalciuria whether by absolute, weight-based, or menopausal and calcium-adjusted definitions.
Importantly, spot urine measurements show poor sensitivity and specificity for hypercalciuria. Spot urine samples underestimate the 24-hour urinary calcium (Bland-Altman bias –71 mg/24 hours), and postprandial sampling overestimates it (Bland-Altman bias +61 mg/24 hours).15
WHAT IS THE MECHANISM OF IDIOPATHIC HYPERCALCIURIA?
The pathophysiology of idiopathic hypercalciuria has been difficult to establish.
Increased sensitivity to vitamin D? In the hyperabsorbing population, activated vitamin D levels are often robust, but a few studies of rats with hyperabsorbing, hyperexcreting physiology have shown normal calcitriol levels, suggesting an increased sensitivity to the actions of 1,25-dihydroxyvitamin D.16
Another study found that hypercalciuric stone-forming rats have more 1,25-dihydroxyvitamin D receptors than do controls.17
These changes have not been demonstrated in patients with idiopathic hypercalciuria.
High sodium intake has been proposed as the cause of idiopathic hypercalciuria. High sodium intake leads to increased urinary sodium excretion, and the increased tubular sodium load can decrease tubular calcium reabsorption, possibly favoring a reduction in bone mineral density over time.18–20
In healthy people, urine calcium excretion increases by about 0.6 mmol/day (20–40 mg/day) for each 100-mmol (2,300 mg) increment in daily sodium ingestion.21,22 But high sodium intake is seldom the principal cause of idiopathic hypercalciuria.
High protein intake, often observed in patients with nephrolithiasis, increases dietary acid load, stimulating release of calcium from bone and inhibiting renal reabsorption of calcium.23,24 Increasing dietary protein from 0.5 to 2.0 mg/kg/day can double the urinary calcium output.25
In mice, induction of metabolic acidosis, thought to mimic a high-protein diet, inhibits osteoblastic alkaline phosphatase activity while stimulating prostaglandin E2 production.26 This in turn increases osteoblastic expression of receptor activator for nuclear factor kappa b (RANK) ligand, thereby potentially contributing to osteoclastogenesis and osteoclast activity.26
Decreasing dietary protein decreases the recurrence of nephrolithiasis in established stone-formers.27 Still, urine calcium levels are higher in those with idiopathic hypercalciuria than in normal controls at comparable levels of acid excretion, so while protein ingestion could potentially exacerbate the hypercalciuria, it is unlikely to be the sole cause.
Renal calcium leak? The frequent finding of low to low-normal PTH levels in patients with idiopathic hypercalciuria contradicts the potential etiologic mechanism of renal calcium “leak.” In idiopathic hypercalciuria, the PTH response to an oral calcium load is abnormal. If given an oral calcium load, the PTH level should decline if this were due to renal leak, but in the setting of idiopathic hypercalciuria, no clinically meaningful change in PTH occurs. This lack of response of PTH to oral calcium load has been seen in both rat and human studies. Patients also excrete normal to high amounts of urine calcium after prolonged fasting or a low-calcium diet. Low-calcium diets do not induce hyperparathyroidism in these patients, and so the source of the elevated calcium in the urine must be primarily from bone. Increased levels of 1,25-dihydroxyvitamin D in patients with idiopathic hypercalciuria have been noted.28,29
Whether the cytokine milieu also contributes to the calcitriol levels is unclear, but the high or high-normal plasma level of 1,25-dihydroxyvitamin D may be the reason that the PTH is unperturbed.
IMPACT ON BONE HEALTH
Nephrolithiasis is strongly linked to fracture risk.
The bone mineral density of trabecular bone is more affected by calcium excretion than that of cortical bone.18,20,30 However, lumbar spine bone mineral density has not been consistently found to be lower in patients with hyperabsorptive hypercalciuria. Rather, bone mineral density is correlated inversely with urine calcium excretion in men and women who form stones, but not in patients without nephrolithiasis.
In children
In children, idiopathic hypercalciuria is well known to be linked to osteopenia. This is an important group to study, as adult idiopathic hypercalciuria often begins in childhood. However, the trajectory of bone loss vs gain in children is fraught with variables such as growth, puberty, and body mass index, making this a difficult group from which to extrapolate conclusions to adults.
In men
There is more information on the relationship between hypercalciuria and osteoporosis in men than in women.
In 1998, Melton et al31 published the findings of a 25-year population-based cohort study of 624 patients, 442 (71%) of whom were men, referred for new-onset urolithiasis. The incidence of vertebral fracture was 4 times higher in this group than in patients without stone disease, but there was no difference in the rate of hip, forearm, or nonvertebral fractures. This is consistent with earlier data that report a loss of predominantly cancellous bone associated with urolithiasis.
National Health and Nutrition Examination Survey III data in 2001 focused on a potential relationship between kidney stones and bone mineral density or prevalent spine or wrist fracture.32 More than 14,000 people had hip bone mineral density measurements, of whom 793 (477 men, 316 women) had kidney stones. Men with previous nephrolithiasis had lower femoral neck bone mineral density than those without. Men with kidney stones were also more likely to report prevalent wrist and spine fractures. In women, no difference was noted between those with or without stone disease with respect to femoral neck bone mineral density or fracture incidence.
Cauley et al33 also evaluated a relationship between kidney stones and bone mineral density in the Osteoporotic Fractures in Men (MrOS) study. Of approximately 6,000 men, 13.2% reported a history of kidney stones. These men had lower spine and total hip bone mineral density than controls who had not had kidney stones, and the difference persisted after adjusting for age, race, weight, and other variables. However, further data from this cohort revealed that so few men with osteoporosis had hypercalciuria that its routine measurement was not recommended.34
In women
The relationship between idiopathic hypercalciuria and fractures has been more difficult to establish in women.
Sowers et al35 performed an observational study of 1,309 women ages 20 to 92 with a history of nephrolithiasis. No association was noted between stone disease and reduced bone mineral density in the femoral neck, lumbar spine, or radius.
These epidemiologic studies did not include the cause of the kidney stones (eg, whether or not there was associated hypercalciuria or primary hyperparathyroidism), and typically a diagnosis of idiopathic hypercalciuria was not established.
The difference in association between low bone mineral density or fracture with nephrolithiasis between men and women is not well understood, but the most consistent hypothesis is that the influence of hypoestrogenemia in women is much stronger than that of the hypercalciuria.20
Does the degree of hypercalciuria influence the amount of bone loss?
A few trials have tried to determine whether the amount of calcium in the urine influences the magnitude of bone loss.
In 2003, Asplin et al36 reported that bone mineral density Z-scores differed significantly by urinary calcium excretion, but only in stone-formers. In patients without stone disease, there was no difference in Z-scores according to the absolute value of hypercalciuria. This may be due to a self-selection bias in which stone-formers avoid calcium in the diet and those without stone disease do not.
Three studies looking solely at men with idiopathic hypercalciuria also did not detect a significant difference in bone mineral loss according to degree of hypercalciuria.20,30,37
A POLYGENIC DISORDER?
The potential contribution of genetic changes to the development of idiopathic hypercalciuria has been studied. While there is an increased risk of idiopathic hypercalciuria in first-degree relatives of patients with nephrolithiasis, most experts believe that idiopathic hypercalciuria is likely a polygenic disorder.9,38
EVALUATION AND TREATMENT
The 2014 revised version of the National Osteoporosis Foundation’s “Clinician’s guide to prevention and treatment of osteoporosis”39 noted that hypercalciuria is a risk factor that contributes to the development of osteoporosis and possibly osteoporotic fractures, and that consideration should be given to evaluating for hypercalciuria, but only in selected cases. In patients with kidney stones, the link between hypercalciuria and bone loss and fracture is recognized and should be explored in both women and men at risk of osteoporosis, as 45% to 50% of patients who form calcium stones have hypercalciuria.
Patients with kidney stones who have low bone mass and idiopathic hypercalciuria should increase their daily fluid intake, follow a low-salt and low-animal-protein diet, and take thiazide diuretics to reduce the incidence of further calcium stones. Whether this approach also improves bone mass and strength and reduces the risk of fractures within this cohort requires further study.
Dietary interventions
Don’t restrict calcium intake. Despite the connection between hypercalciuria and nephrolithiasis, restriction of dietary calcium to prevent relapse of nephrolithiasis is a risk factor for negative calcium balance and bone demineralization. Observational studies and prospective clinical trials have demonstrated an increased risk of stone formation with low calcium intake.27,30 Nevertheless, this practice seems logical to many patients with kidney stones, and this process may independently contribute to lower bone mineral density.
A low-sodium, low-animal-protein diet is beneficial. Though increased intake of sodium or protein is not the main cause of idiopathic hypercalciuria, pharmacologic therapy, especially with thiazide diuretics, is more likely to be successful in the setting of a low-sodium, low-protein diet.
Borghi et al27 studied 2 diets in men with nephrolithiasis and idiopathic hypercalciuria: a low-calcium diet and a low-salt, low-animal-protein, normal-calcium diet. Men on the latter diet experienced a greater reduction in urinary calcium excretion than those on the low-calcium diet.
Breslau et al40 found that urinary calcium excretion fell by 50% in 15 people when they switched from an animal-based to a plant-based protein diet.
Thiazide diuretics
Several epidemiologic and randomized studies41–45 found that thiazide therapy decreased the likelihood of hip fracture in postmenopausal women, men, and premenopausal women. Doses ranged from 12.5 to 50 mg of hydrochlorothiazide. Bone density increased in the radius, total body, total hip, and lumbar spine. One prospective trial noted that fracture risk declined with longer duration of thiazide use, with the largest reduction in those who used thiazides for 8 or more years.46
Thiazides have anticalciuric actions.47 In addition, they have positive effects on osteoblastic cell proliferation and activity, inhibiting osteocalcin expression by osteoblasts, thereby possibly improving bone formation and mineralization.48 The effects of thiazides on bone was reviewed by Sakhaee et al.49
However, fewer studies have looked at thiazides in patients with idiopathic hypercalciuria.
García-Nieto et al50 looked retrospectively at 22 children (average age 11.7) with idiopathic hypercalciuria and osteopenia who had received thiazides (19 received chlorthalidone 25 mg daily, and 3 received hydrochlorothiazide 25 mg daily) for an average of 2.4 years, and at 32 similar patients who had not received thiazides. Twelve (55%) of the patients receiving thiazides had an improvement in bone mineral density Z-scores, compared with 23 (72%) of the controls. This finding is confounded by growth that occurred during the study, and both groups demonstrated a significantly increased body mass index and bone mineral apparent density at the end of the trial.
Bushinsky and Favus51 evaluated whether chlorthalidone improved bone quality or structure in rats that were genetically prone to hypercalciuric stones. These rats are uniformly stone-formers, and while they have components of calcium hyperabsorption, they also demonstrate renal hyperexcretion (leak) and enhanced bone mineral resorption.51 When fed a high-calcium diet, they maintain a reduction in bone mineral density and bone strength. Study rats were given chlorthalidone 4 to 5 mg/kg/day. After 18 weeks of therapy, significant improvements were observed in trabecular thickness and connectivity as well as increased vertebral compressive strength.52 No difference in cortical bone was noted.
No randomized, blinded, placebo-controlled trial has yet been done to study the impact of thiazides on bone mineral density or fracture risk in patients with idiopathic hypercalciuria.
In practice, many physicians choose chlorthalidone over hydrochlorothiazide because of chlorthalidone’s longer half-life. Combinations of a thiazide diuretic and potassium-sparing medications are also employed, such as hydrochlorothiazide plus either triamterene or spironolactone to reduce the number of pills the patient has to take.
Potassium citrate
When prescribing thiazide diuretics, one should also consider prescribing potassium citrate, as this agent not only prevents hypokalemia but also increases urinary citrate excretion, which can help to inhibit crystallization of calcium salts.6
In a longitudinal study of 28 patients with hypercalciuria,53 combined therapy with a thiazide or indapamide and potassium citrate over a mean of 7 years increased bone density of the lumbar spine by 7.1% and of the femoral neck by 4.1%, compared with treatment in age- and sex-matched normocalcemic peers. In the same study, daily urinary calcium excretion decreased and urinary pH and citrate levels increased; urinary saturation of calcium oxalate decreased by 46%, and stone formation was decreased.
Another trial evaluated 120 patients with idiopathic calcium nephrolithiasis, half of whom were given potassium citrate. Those given potassium citrate experienced an increase in distal radius bone mineral density over 2 years.54 It is theorized that alkalinization may decrease bone turnover in these patients.
Bisphosphonates
As one of the proposed main mechanisms of bone loss in idiopathic hypercalciuria is direct bone resorption, a potential target for therapy is the osteoclast, which bisphosphonates inhibit.
Ruml et al55 studied the impact of alendronate vs placebo in 16 normal men undergoing 3 weeks of strict bedrest. Compared with the placebo group, those who received alendronate had significantly lower 24-hour urine calcium excretion and higher levels of PTH and 1,25-dihydroxyvitamin D.
Weisinger et al56 evaluated the effects of alendronate 10 mg daily in 10 patients who had stone disease with documented idiopathic hypercalciuria and also in 8 normocalciuric patients without stone disease. Alendronate resulted in a sustained reduction of calcium in the urine in the patients with idiopathic hypercalciuria but not in the normocalciuric patients.
Data are somewhat scant as to the effect of bisphosphonates on bone health in the setting of idiopathic hypercalciuria,57,58 and therapy with bisphosphonates is not recommended in patients with idiopathic hypercalciuria outside the realm of postmenopausal osteoporosis or other indications for bisphosphonates approved by the US Food and Drug Administration (FDA).
Calcimimetics
Calcium-sensing receptors are found not only in parathyroid tissue but also in the intestines and kidneys. Locally, elevated plasma calcium in the kidney causes activation of the calcium-sensing receptor, diminishing further calcium reabsorption.59 Agents that increase the sensitivity of the calcium-sensing receptors are classified as calcimimetics.
Cinacalcet is a calcimimetic approved by the FDA for treatment of secondary hyperparathyroidism in patients with chronic kidney disease on dialysis, for the treatment of hypercalcemia in patients with parathyroid carcinoma, and for patients with primary hyperparathyroidism who are unable to undergo parathyroidectomy. In an uncontrolled 5-year study of cinacalcet in patients with primary hyperparathyroidism, there was no significant change in bone density.60
Anti-inflammatory drugs
The role of cytokines in stimulating bone resorption in idiopathic hypercalciuria has led to the investigation of several anti-inflammatory drugs (eg, diclofenac, indomethacin) as potential treatments, but studies have been limited in number and scope.61,62
Omega-3 fatty acids
Omega-3 fatty acids are thought to alter prostaglandin metabolism and to potentially reduce stone formation.63
A retrospective study of 29 patients with stone disease found that, combined with dietary counseling, omega-3 fatty acids could potentially reduce urinary calcium and oxalate excretion and increase urinary citrate in hypercalciuric stone-formers.64
A review of published randomized controlled trials of omega-3 fatty acids in skeletal health discovered that 4 studies found positive effects on bone mineral density or bone turnover markers, whereas 5 studies reported no differences. All trials were small, and none evaluated fracture outcome.65
- Albright F, Henneman P, Benedict PH, Forbes AP. Idiopathic hypercalciuria: a preliminary report. Proc R Soc Med 1953; 46:1077–1081.
- Pak CY. Pathophysiology of calcium nephrolithiasis. In: Seldin DW, Giebiscg G, eds. The Kidney: Physiology and Pathophysiology. New York, NY: Raven Press; 1992:2461–2480.
- Frick KK, Bushinsky DA. Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol 2003; 14:1082–1095.
- Pacifici R, Rothstein M, Rifas L, et al. Increased monocyte interleukin-1 activity and decreased vertebral bone density in patients with fasting idiopathic hypercalciuria. J Clin Endocrinol Metab 1990; 71:138–145.
- Messa P, Mioni G, Montanaro D, et al. About a primitive osseous origin of the so-called ‘renal hypercalciuria.’ Contrib Nephrol 1987; 58:106–110.
- Zerwekh JE. Bone disease and idiopathic hypercalciuria. Semin Nephrol 2008; 28:133–142.
- Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med 1977; 87:404–410.
- Lemann J Jr. Pathogenesis of idiopathic hypercalciuria and nephrolithiasis. In: Coe FL, Favus MJ, eds. Disorders of Bone and Mineral Metabolism. New York, NY: Raven Press; 1992:685-706.
- Coe FL, Parks JH, Moore ES. Familial idiopathic hypercalciuria. N Engl J Med 1979; 300:337–340.
- Giannini S, Nobile M, Dalle Carbonare L, et al. Hypercalciuria is a common and important finding in postmenopausal women with osteoporosis. Eur J Endocrinol 2003; 149:209–213.
- Tannenbaum C, Clark J, Schwartzman K, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 2002; 87:4431–4437.
- Cerda Gabaroi D, Peris P, Monegal A, et al. Search for hidden secondary causes in postmenopausal women with osteoporosis. Menopause 2010; 17:135–139.
- Rull MA, Cano-García Mdel C, Arrabal-Martín M, Arrabal-Polo MA. The importance of urinary calcium in postmenopausal women with osteoporotic fracture. Can Urol Assoc J 2015; 9:E183–E186.
- Heaney RP, Recker RR, Ryan RA. Urinary calcium in perimenopausal women: normative values. Osteoporos Int 1999; 9:13–18.
- Bleich HL, Moore MJ, Lemann J Jr, Adams ND, Gray RW. Urinary calcium excretion in human beings. N Engl J Med 1979; 301:535–541.
- Li XQ, Tembe V, Horwitz GM, Bushinsky DA, Favus MJ. Increased intestinal vitamin D receptor in genetic hypercalciuric rats. A cause of intestinal calcium hyperabsorption. J Clin Invest 1993; 91:661–667.
- Yao J, Kathpalia P, Bushinsky DA, Favus MJ. Hyperresponsiveness of vitamin D receptor gene expression to 1,25-dihydroxyvitamin D3. A new characteristic of genetic hypercalciuric stone-forming rats. J Clin Invest 1998; 101:2223–2232.
- Pietschmann F, Breslau NA, Pak CY. Reduced vertebral bone density in hypercalciuric nephrolithiasis. J Bone Miner Res 1992; 7:1383–1388.
- Jaeger P, Lippuner K, Casez JP, Hess B, Ackermann D, Hug C. Low bone mass in idiopathic renal stone formers: magnitude and significance. J Bone Miner Res 1994; 9:1525–1532.
- Vezzoli G, Soldati L, Arcidiacono T, et al. Urinary calcium is a determinant of bone mineral density in elderly men participating in the InCHIANTI study. Kidney Int 2005; 67:2006–2014.
- Lemann J Jr, Worcester EM, Gray RW. Hypercalciuria and stones. Am J Kidney Dis 1991; 17:386–391.
- Gokce C, Gokce O, Baydinc C, et al. Use of random urine samples to estimate total urinary calcium and phosphate excretion. Arch Intern Med 1991; 151:1587–1588.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993; 328:833–838.
- Siener R, Schade N, Nicolay C, von Unruh GE, Hesse A. The efficacy of dietary intervention on urinary risk factors for stone formation in recurrent calcium oxalate stone patients. J Urol 2005; 173:1601–1605.
- Jones AN, Shafer MM, Keuler NS, Crone EM, Hansen KE. Fasting and postprandial spot urine calcium-to-creatinine ratios do not detect hypercalciuria. Osteoporos Int 2012; 23:553–562.
- Frick KK, Bushinsky DA. Metabolic acidosis stimulates RANKL RNA expression in bone through a cyclo-oxygenase-dependent mechanism. J Bone Miner Res 2003; 18:1317–1325.
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346:77–84.
- Ghazali A, Fuentes V, Desaint C, et al. Low bone mineral density and peripheral blood monocyte activation profile in calcium stone formers with idiopathic hypercalciuria. J Clin Endocrinol Metab 1997; 82:32–38.
- Broadus AE, Insogna KL, Lang R, Ellison AF, Dreyer BE. Evidence for disordered control of 1,25-dihydroxyvitamin D production in absorptive hypercalciuria. N Engl J Med 1984; 311:73–80.
- Tasca A, Cacciola A, Ferrarese P, et al. Bone alterations in patients with idiopathic hypercalciuria and calcium nephrolithiasis. Urology 2002; 59:865–869.
- Melton LJ 3rd, Crowson CS, Khosla S, Wilson DM, O’Fallon WM. Fracture risk among patients with urolithiasis: a population-based cohort study. Kidney Int 1998; 53:459–464.
- Lauderdale DS, Thisted RA, Wen M, Favus MJ. Bone mineral density and fracture among prevalent kidney stone cases in the Third National Health and Nutrition Examination Survey. J Bone Miner Res 2001; 16:1893–1898.
- Cauley JA, Fullman RL, Stone KL, et al; MrOS Research Group. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int 2005; 16:1525–1537.
- Fink HA, Litwack-Harrison S, Taylor BC, et al; Osteoporotic Fractures in Men (MrOS) Study Group. Clinical utility of routine laboratory testing to identify possible secondary causes in older men with osteoporosis: the Osteoporotic Fractures in Men (MrOS) Study. Osteoporos Int 2016: 27:331–338.
- Sowers MR, Jannausch M, Wood C, Pope SK, Lachance LL, Peterson B. Prevalence of renal stones in a population-based study with dietary calcium, oxalate and medication exposures. Am J Epidemiol 1998; 147:914–920.
- Asplin JR, Bauer KA, Kinder J, et al. Bone mineral density and urine calcium excretion among subjects with and without nephrolithiasis. Kidney Int 2003; 63:662–669.
- Letavernier E, Traxer O, Daudon M, et al. Determinants of osteopenia in male renal-stone-disease patients with idiopathic hypercalciuria. Clin J Am Soc Nephrol 2011; 6:1149–1154.
- Vezzoli G, Soldati L, Gambaro G. Update on primary hypercalciuria from a genetic perspective. J Urol 2008; 179:1676–1682.
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014: 25:2359–2381.
- Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab 1988; 66:140–146.
- Reid IR, Ames RW, Orr-Walker BJ, et al. Hydrochlorothiazide reduces loss of cortical bone in normal postmenopausal women: a randomized controlled trial. Am J Med 2000; 109:362–370.
- Bolland MJ, Ames RW, Horne AM, Orr-Walker BJ, Gamble GD, Reid IR. The effect of treatment with a thiazide diuretic for 4 years on bone density in normal postmenopausal women. Osteoporos Int 2007; 18:479–486.
- LaCroix AZ, Ott SM, Ichikawa L, Scholes D, Barlow WE. Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. Ann Intern Med 2000; 133:516–526.
- Wasnich RD, Davis JW, He YF, Petrovich H, Ross PD. A randomized, double-masked, placebo-controlled trial of chlorthalidone and bone loss in elderly women. Osteoporos Int 1995; 5:247–251.
- Adams JS, Song CF, Kantorovich V. Rapid recovery of bone mass in hypercalciuric, osteoporotic men treated with hydrochlorothiazide. Ann Intern Med 1999; 130:658–660.
- Feskanich D, Willett WC, Stampfer MJ, Colditz GA. A prospective study of thiazide use and fractures in women. Osteoporos Int 1997; 7:79–84.
- Lamberg BA, Kuhlback B. Effect of chlorothiazide and hydrochlorothiazide on the excretion of calcium in the urine. Scand J Clin Lab Invest 1959; 11:351–357.
- Lajeunesse D, Delalandre A, Guggino SE. Thiazide diuretics affect osteocalcin production in human osteoblasts at the transcription level without affecting vitamin D3 receptors. J Bone Miner Res 2000; 15:894–901.
- Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int 2001; 79:393–403.
- García-Nieto V, Monge-Zamorano M, González-García M, Luis-Yanes MI. Effect of thiazides on bone mineral density in children with idiopathic hypercalciuria. Pediatr Nephrol 2012; 27:261–268.
- Bushinsky DA, Favus MJ. Mechanism of hypercalciuria in genetic hypercalciuric rats. Inherited defect in intestinal calcium transport. J Clin Invest 1988; 82:1585–1591.
- Bushinsky DA, Willett T, Asplin JR, Culbertson C, Che SP, Grynpas M. Chlorthalidone improves vertebral bone quality in genetic hypercalciuric stone-forming rats. J Bone Miner Res 2011; 26:1904–1912.
- Pak CY, Heller HJ, Pearle MS, Odvina CV, Poindexter JR, Peterson RD. Prevention of stone formation and bone loss in absorptive hypercalciuria by combined dietary and pharmacological interventions. J Urol 2003; 169:465–469.
- Vescini F, Buffa A, LaManna G, et al. Long-term potassium citrate therapy and bone mineral density in idiopathic calcium stone formers. J Endocrinol Invest 2005; 28:218–222.
- Ruml LA, Dubois SK, Roberts ML, Pak CY. Prevention of hypercalciuria and stone-forming propensity during prolonged bedrest by alendronate. J Bone Miner Res 1995; 10:655–662.
- Weisinger JR, Alonzo E, Machado C, et al. Role of bones in the physiopathology of idiopathic hypercalciuria: effect of amino-bisphosphonate alendronate. Medicina (B Aires) 1997; 57(suppl 1):45–48. Spanish.
- Heilberg IP, Martini LA, Teixeira SH, et al. Effect of etidronate treatment on bone mass of male nephrolithiasis patients with idiopathic hypercalciuria and osteopenia. Nephron 1998; 79:430–437.
- Bushinsky DA, Neumann KJ, Asplin J, Krieger NS. Alendronate decreases urine calcium and supersaturation in genetic hypercalciuric rats. Kidney Int 1999; 55:234–243.
- Riccardi D, Park J, Lee WS, Gamba G, Brown EM, Hebert SC. Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc Natl Acad Sci USA 1995; 92:131–145.
- Peacock M, Bolognese MA, Borofsky M, et al. Cinacalcet treatment of primary hyperparathyroidism: biochemical and bone densitometric outcomes in a five-year study. J Clin Endocrinol Metab 2009; 94:4860–4867.
- Filipponi P, Mannarelli C, Pacifici R, et al. Evidence for a prostaglandin-mediated bone resorptive mechanism in subjects with fasting hypercalciuria. Calcif Tissue Int 1988; 43:61–66.
- Gomaa AA, Hassan HA, Ghaneimah SA. Effect of aspirin and indomethacin on the serum and urinary calcium, magnesium and phosphate. Pharmacol Res 1990; 22:59–70.
- Buck AC, Davies RL, Harrison T. The protective role of eicosapentaenoic acid (EPA) in the pathogenesis of nephrolithiasis. J Urol 1991; 146:188–194.
- Ortiz-Alvarado O, Miyaoka R, Kriedberg C, et al. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid in the management of hypercalciuric stone formers. Urology 2012; 79:282–286.
- Orchard TS, Pan X, Cheek F, Ing SW, Jackson RD. A systematic review of omega-3 fatty acids and osteoporosis. Br J Nutr 2012; 107(suppl 2):S253–S260.
- Albright F, Henneman P, Benedict PH, Forbes AP. Idiopathic hypercalciuria: a preliminary report. Proc R Soc Med 1953; 46:1077–1081.
- Pak CY. Pathophysiology of calcium nephrolithiasis. In: Seldin DW, Giebiscg G, eds. The Kidney: Physiology and Pathophysiology. New York, NY: Raven Press; 1992:2461–2480.
- Frick KK, Bushinsky DA. Molecular mechanisms of primary hypercalciuria. J Am Soc Nephrol 2003; 14:1082–1095.
- Pacifici R, Rothstein M, Rifas L, et al. Increased monocyte interleukin-1 activity and decreased vertebral bone density in patients with fasting idiopathic hypercalciuria. J Clin Endocrinol Metab 1990; 71:138–145.
- Messa P, Mioni G, Montanaro D, et al. About a primitive osseous origin of the so-called ‘renal hypercalciuria.’ Contrib Nephrol 1987; 58:106–110.
- Zerwekh JE. Bone disease and idiopathic hypercalciuria. Semin Nephrol 2008; 28:133–142.
- Coe FL. Treated and untreated recurrent calcium nephrolithiasis in patients with idiopathic hypercalciuria, hyperuricosuria, or no metabolic disorder. Ann Intern Med 1977; 87:404–410.
- Lemann J Jr. Pathogenesis of idiopathic hypercalciuria and nephrolithiasis. In: Coe FL, Favus MJ, eds. Disorders of Bone and Mineral Metabolism. New York, NY: Raven Press; 1992:685-706.
- Coe FL, Parks JH, Moore ES. Familial idiopathic hypercalciuria. N Engl J Med 1979; 300:337–340.
- Giannini S, Nobile M, Dalle Carbonare L, et al. Hypercalciuria is a common and important finding in postmenopausal women with osteoporosis. Eur J Endocrinol 2003; 149:209–213.
- Tannenbaum C, Clark J, Schwartzman K, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 2002; 87:4431–4437.
- Cerda Gabaroi D, Peris P, Monegal A, et al. Search for hidden secondary causes in postmenopausal women with osteoporosis. Menopause 2010; 17:135–139.
- Rull MA, Cano-García Mdel C, Arrabal-Martín M, Arrabal-Polo MA. The importance of urinary calcium in postmenopausal women with osteoporotic fracture. Can Urol Assoc J 2015; 9:E183–E186.
- Heaney RP, Recker RR, Ryan RA. Urinary calcium in perimenopausal women: normative values. Osteoporos Int 1999; 9:13–18.
- Bleich HL, Moore MJ, Lemann J Jr, Adams ND, Gray RW. Urinary calcium excretion in human beings. N Engl J Med 1979; 301:535–541.
- Li XQ, Tembe V, Horwitz GM, Bushinsky DA, Favus MJ. Increased intestinal vitamin D receptor in genetic hypercalciuric rats. A cause of intestinal calcium hyperabsorption. J Clin Invest 1993; 91:661–667.
- Yao J, Kathpalia P, Bushinsky DA, Favus MJ. Hyperresponsiveness of vitamin D receptor gene expression to 1,25-dihydroxyvitamin D3. A new characteristic of genetic hypercalciuric stone-forming rats. J Clin Invest 1998; 101:2223–2232.
- Pietschmann F, Breslau NA, Pak CY. Reduced vertebral bone density in hypercalciuric nephrolithiasis. J Bone Miner Res 1992; 7:1383–1388.
- Jaeger P, Lippuner K, Casez JP, Hess B, Ackermann D, Hug C. Low bone mass in idiopathic renal stone formers: magnitude and significance. J Bone Miner Res 1994; 9:1525–1532.
- Vezzoli G, Soldati L, Arcidiacono T, et al. Urinary calcium is a determinant of bone mineral density in elderly men participating in the InCHIANTI study. Kidney Int 2005; 67:2006–2014.
- Lemann J Jr, Worcester EM, Gray RW. Hypercalciuria and stones. Am J Kidney Dis 1991; 17:386–391.
- Gokce C, Gokce O, Baydinc C, et al. Use of random urine samples to estimate total urinary calcium and phosphate excretion. Arch Intern Med 1991; 151:1587–1588.
- Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med 1993; 328:833–838.
- Siener R, Schade N, Nicolay C, von Unruh GE, Hesse A. The efficacy of dietary intervention on urinary risk factors for stone formation in recurrent calcium oxalate stone patients. J Urol 2005; 173:1601–1605.
- Jones AN, Shafer MM, Keuler NS, Crone EM, Hansen KE. Fasting and postprandial spot urine calcium-to-creatinine ratios do not detect hypercalciuria. Osteoporos Int 2012; 23:553–562.
- Frick KK, Bushinsky DA. Metabolic acidosis stimulates RANKL RNA expression in bone through a cyclo-oxygenase-dependent mechanism. J Bone Miner Res 2003; 18:1317–1325.
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002; 346:77–84.
- Ghazali A, Fuentes V, Desaint C, et al. Low bone mineral density and peripheral blood monocyte activation profile in calcium stone formers with idiopathic hypercalciuria. J Clin Endocrinol Metab 1997; 82:32–38.
- Broadus AE, Insogna KL, Lang R, Ellison AF, Dreyer BE. Evidence for disordered control of 1,25-dihydroxyvitamin D production in absorptive hypercalciuria. N Engl J Med 1984; 311:73–80.
- Tasca A, Cacciola A, Ferrarese P, et al. Bone alterations in patients with idiopathic hypercalciuria and calcium nephrolithiasis. Urology 2002; 59:865–869.
- Melton LJ 3rd, Crowson CS, Khosla S, Wilson DM, O’Fallon WM. Fracture risk among patients with urolithiasis: a population-based cohort study. Kidney Int 1998; 53:459–464.
- Lauderdale DS, Thisted RA, Wen M, Favus MJ. Bone mineral density and fracture among prevalent kidney stone cases in the Third National Health and Nutrition Examination Survey. J Bone Miner Res 2001; 16:1893–1898.
- Cauley JA, Fullman RL, Stone KL, et al; MrOS Research Group. Factors associated with the lumbar spine and proximal femur bone mineral density in older men. Osteoporos Int 2005; 16:1525–1537.
- Fink HA, Litwack-Harrison S, Taylor BC, et al; Osteoporotic Fractures in Men (MrOS) Study Group. Clinical utility of routine laboratory testing to identify possible secondary causes in older men with osteoporosis: the Osteoporotic Fractures in Men (MrOS) Study. Osteoporos Int 2016: 27:331–338.
- Sowers MR, Jannausch M, Wood C, Pope SK, Lachance LL, Peterson B. Prevalence of renal stones in a population-based study with dietary calcium, oxalate and medication exposures. Am J Epidemiol 1998; 147:914–920.
- Asplin JR, Bauer KA, Kinder J, et al. Bone mineral density and urine calcium excretion among subjects with and without nephrolithiasis. Kidney Int 2003; 63:662–669.
- Letavernier E, Traxer O, Daudon M, et al. Determinants of osteopenia in male renal-stone-disease patients with idiopathic hypercalciuria. Clin J Am Soc Nephrol 2011; 6:1149–1154.
- Vezzoli G, Soldati L, Gambaro G. Update on primary hypercalciuria from a genetic perspective. J Urol 2008; 179:1676–1682.
- Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 2014: 25:2359–2381.
- Breslau NA, Brinkley L, Hill KD, Pak CY. Relationship of animal protein-rich diet to kidney stone formation and calcium metabolism. J Clin Endocrinol Metab 1988; 66:140–146.
- Reid IR, Ames RW, Orr-Walker BJ, et al. Hydrochlorothiazide reduces loss of cortical bone in normal postmenopausal women: a randomized controlled trial. Am J Med 2000; 109:362–370.
- Bolland MJ, Ames RW, Horne AM, Orr-Walker BJ, Gamble GD, Reid IR. The effect of treatment with a thiazide diuretic for 4 years on bone density in normal postmenopausal women. Osteoporos Int 2007; 18:479–486.
- LaCroix AZ, Ott SM, Ichikawa L, Scholes D, Barlow WE. Low-dose hydrochlorothiazide and preservation of bone mineral density in older adults. Ann Intern Med 2000; 133:516–526.
- Wasnich RD, Davis JW, He YF, Petrovich H, Ross PD. A randomized, double-masked, placebo-controlled trial of chlorthalidone and bone loss in elderly women. Osteoporos Int 1995; 5:247–251.
- Adams JS, Song CF, Kantorovich V. Rapid recovery of bone mass in hypercalciuric, osteoporotic men treated with hydrochlorothiazide. Ann Intern Med 1999; 130:658–660.
- Feskanich D, Willett WC, Stampfer MJ, Colditz GA. A prospective study of thiazide use and fractures in women. Osteoporos Int 1997; 7:79–84.
- Lamberg BA, Kuhlback B. Effect of chlorothiazide and hydrochlorothiazide on the excretion of calcium in the urine. Scand J Clin Lab Invest 1959; 11:351–357.
- Lajeunesse D, Delalandre A, Guggino SE. Thiazide diuretics affect osteocalcin production in human osteoblasts at the transcription level without affecting vitamin D3 receptors. J Bone Miner Res 2000; 15:894–901.
- Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW. Nephrolithiasis-associated bone disease: pathogenesis and treatment options. Kidney Int 2001; 79:393–403.
- García-Nieto V, Monge-Zamorano M, González-García M, Luis-Yanes MI. Effect of thiazides on bone mineral density in children with idiopathic hypercalciuria. Pediatr Nephrol 2012; 27:261–268.
- Bushinsky DA, Favus MJ. Mechanism of hypercalciuria in genetic hypercalciuric rats. Inherited defect in intestinal calcium transport. J Clin Invest 1988; 82:1585–1591.
- Bushinsky DA, Willett T, Asplin JR, Culbertson C, Che SP, Grynpas M. Chlorthalidone improves vertebral bone quality in genetic hypercalciuric stone-forming rats. J Bone Miner Res 2011; 26:1904–1912.
- Pak CY, Heller HJ, Pearle MS, Odvina CV, Poindexter JR, Peterson RD. Prevention of stone formation and bone loss in absorptive hypercalciuria by combined dietary and pharmacological interventions. J Urol 2003; 169:465–469.
- Vescini F, Buffa A, LaManna G, et al. Long-term potassium citrate therapy and bone mineral density in idiopathic calcium stone formers. J Endocrinol Invest 2005; 28:218–222.
- Ruml LA, Dubois SK, Roberts ML, Pak CY. Prevention of hypercalciuria and stone-forming propensity during prolonged bedrest by alendronate. J Bone Miner Res 1995; 10:655–662.
- Weisinger JR, Alonzo E, Machado C, et al. Role of bones in the physiopathology of idiopathic hypercalciuria: effect of amino-bisphosphonate alendronate. Medicina (B Aires) 1997; 57(suppl 1):45–48. Spanish.
- Heilberg IP, Martini LA, Teixeira SH, et al. Effect of etidronate treatment on bone mass of male nephrolithiasis patients with idiopathic hypercalciuria and osteopenia. Nephron 1998; 79:430–437.
- Bushinsky DA, Neumann KJ, Asplin J, Krieger NS. Alendronate decreases urine calcium and supersaturation in genetic hypercalciuric rats. Kidney Int 1999; 55:234–243.
- Riccardi D, Park J, Lee WS, Gamba G, Brown EM, Hebert SC. Cloning and functional expression of a rat kidney extracellular calcium/polyvalent cation-sensing receptor. Proc Natl Acad Sci USA 1995; 92:131–145.
- Peacock M, Bolognese MA, Borofsky M, et al. Cinacalcet treatment of primary hyperparathyroidism: biochemical and bone densitometric outcomes in a five-year study. J Clin Endocrinol Metab 2009; 94:4860–4867.
- Filipponi P, Mannarelli C, Pacifici R, et al. Evidence for a prostaglandin-mediated bone resorptive mechanism in subjects with fasting hypercalciuria. Calcif Tissue Int 1988; 43:61–66.
- Gomaa AA, Hassan HA, Ghaneimah SA. Effect of aspirin and indomethacin on the serum and urinary calcium, magnesium and phosphate. Pharmacol Res 1990; 22:59–70.
- Buck AC, Davies RL, Harrison T. The protective role of eicosapentaenoic acid (EPA) in the pathogenesis of nephrolithiasis. J Urol 1991; 146:188–194.
- Ortiz-Alvarado O, Miyaoka R, Kriedberg C, et al. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid in the management of hypercalciuric stone formers. Urology 2012; 79:282–286.
- Orchard TS, Pan X, Cheek F, Ing SW, Jackson RD. A systematic review of omega-3 fatty acids and osteoporosis. Br J Nutr 2012; 107(suppl 2):S253–S260.
KEY POINTS
- Idiopathic hypercalciuria is common in patients with kidney stones and is also present in up to 20% of postmenopausal women with osteoporosis but no history of kidney stones.
- Idiopathic hypercalciuria has been directly implicated as a cause of loss of trabecular bone, especially in men. But reversing the hypercalciuria in this condition has not been definitively shown to diminish fracture incidence.
- Patients with kidney stones who have low bone mass and idiopathic hypercalciuria should increase their daily fluid intake, follow a diet low in salt and animal protein, and take thiazide diuretics to reduce the risk of further calcium stone formation. Whether this approach also improves bone mass and strength and reduces fracture risk in this patient group requires further study.
What is the hepatitis B vaccination regimen in chronic kidney disease?
For patients age 16 and older with advanced chronic kidney disease (CKD), including those undergoing hemodialysis, we recommend a higher dose of hepatitis B virus (HBV) vaccine, more doses, or both. Vaccination with a higher dose may improve the immune response. The hepatitis B surface antibody (anti-HBs) titer should be monitored 1 to 2 months after completion of the vaccination schedule and annually thereafter, with a target titer of 10 IU/mL or greater. For patients who do not develop a protective antibody titer after completing the initial vaccination schedule, the vaccination schedule should be repeated.
RECOMMENDED DOSES AND SCHEDULES
Recommendation 1
Give higher vaccine doses, increase the number of doses, or both.
Recommendation 2
A 4-dose regimen may provide a better antibody response than a 3-dose regimen. (Note: This recommendation applies only to Engerix-B; 4 doses of Recombivax-HB would be an off-label use.)
Rationale. The US Centers for Disease Control and Prevention reported that after completion of a 3-dose vaccination schedule, the median proportion of patients developing a protective antibody response was 64% (range 34%–88%) vs a median of 86% (range 40%–98%) after a 4-dose schedule.3
Lacson et al5 compared antibody response rates after 3 doses of Recombivax-HB and after 4 doses of Engerix-B and found a better response rate with the 4-dose schedule. The rate of persistent protective anti-HBs titer after 1 year was 77% for Engerix-B vs 53% for Recombivax-HB.
Agarwal et al6 evaluated response rates in patients who had mild CKD (serum creatinine levels 1.5–3.0 mg/dL), moderate CKD (creatinine 3.0–6.0 mg/dL), and severe CKD (creatinine > 6.0 mg/dL). The seroconversion rates after 3 doses of 40-μg HBV vaccine were 87.5% in those with mild CKD, 66.6% in those with moderate CKD, and 35.7% in those with severe disease. After a fourth dose, rates improved significantly to 100%, 77%, and 36.4%, respectively.
Recommendation 3
In patients with CKD, vaccination should be done early, before they become dependent on hemodialysis.
Rationale. Patients with advanced CKD may have a lower seroconversion rate. Fraser et al7 found that after a 4-dose series, the seroprotection rate in adult prehemodialysis patients with serum creatinine levels of 4 mg/dL or less was 86%, compared with 37% in patients with serum creatinine levels above 4 mg/dL, of whom 88% were on hemodialysis.7
In a 2003 prospective cohort study by DaRoza et al,8 patients with higher levels of kidney function were more likely to respond to HBV vaccination, and the level of kidney function was found to be an independent predictor of seroconversion.8
A 2012 prospective study by Ghadiani et al9 compared seroconversion rates in patients with stage 3 or 4 CKD vs patients on hemodialysis, with medical staff as controls. The authors reported seroprotection rates of 26.1% in patients on hemodialysis, 55.2% in patients with stage 3 or 4 CKD, and 96.2% in controls. They concluded that vaccination is more likely to induce seroconversion in earlier stages of kidney disease.9
MONITORING THE RESPONSE TO VACCINATION AND REVACCINATION
Testing after vaccination is recommended to determine response. Testing should be done 1 to 2 months after the last dose of the vaccination schedule.1–3 Anti-HBs levels 10 IU/mL and greater are considered protective.10
Revaccination with a full vaccination series is recommended for patients who do not develop adequate levels of protective antibodies after completion of the vaccination schedule.2 Reported response rates to revaccination have varied from 40% to 50% after 2 or 3 additional intramuscular doses of 40 µg, to 64% after 4 additional intramuscular doses of 10 µg.3 Serologic testing should be repeated after the last dose of the vaccination series, as serologic testing after only 1 or 2 additional doses appears to be no more cost-effective.2,3
To the best of our knowledge, no data exist to indicate that in nonresponders, further doses given after completion of 2 full vaccination schedules would induce an antibody response.
ANTIBODY PERSISTENCE AND BOOSTER DOSES
Antibody levels fall with time in patients on hemodialysis. Limited data suggest that in patients who respond to the primary vaccination series, antibodies remain detectable for 6 months in 80% to 100% (median 100%) of patients and for 12 months in 58% to 100% (median 70%) of patients.3 The need for booster doses should be assessed by annual monitoring.2,11 Booster doses should be given when the anti-HBs titer declines to below 10 IU/mL. Limited data indicate that nearly all such patients would respond to a booster dose.3
OTHER WAYS TO IMPROVE VACCINE RESPONSE
Other strategies to improve vaccine response, such as the addition of adjuvants or immunostimulants, have shown variable success.12 Intradermal HBV vaccination in patients on chronic hemodialysis has also been proposed. The efficacy of intradermal vaccination may be related to the dense network of immunologic dendritic cells within the dermis. After intradermal administration, the antigen is taken up by dendritic cells residing in the dermis, which mature and travel to the regional lymph node where further immunostimulation takes place.13
In a systematic review of four prospective trials with a total of 204 hemodialysis patients,13 a significantly higher proportion of patients achieved seroconversion with intradermal HBV vaccine administration than with intramuscular administration. The authors concluded that the intradermal route in primary nonresponders undergoing hemodialysis provides an effective alternative to the intramuscular route to protect against HBV infection in this highly susceptible population.
Additional well-designed, double-blinded, randomized trials are needed to establish clear guidelines on intradermal HBV vaccine dosing and vaccination schedules.
- Grzegorzewska AE. Hepatitis B vaccination in chronic kidney disease: review of evidence in non-dialyzed patients. Hepat Mon 2012; 12:e7359.
- Chi C, Patel P, Pilishvili T, Moore M, Murphy T, Strikas R. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. www.cdc.gov/dialysis/PDFs/Vaccinating_Dialysis_Patients_and_Patients_dec2012.pdf. Accessed September 6, 2017.
- Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep 2001; 50:1–43.
- Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB; Advisory Committee on Immunization Practices. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. Ann Intern Med 2017; 166:209–219.
- Lacson E, Teng M, Ong J, Vienneau L, Ofsthun N, Lazarus JM. Antibody response to Engerix-B and Recombivax-HB hepatitis B vaccination in end-stage renal disease. Hemodialysis international. Hemodial Int 2005; 9:367–375.
- Agarwal SK, Irshad M, Dash SC. Comparison of two schedules of hepatitis B vaccination in patients with mild, moderate and severe renal failure. J Assoc Physicians India 1999; 47:183–185.
- Fraser GM, Ochana N, Fenyves D, et al. Increasing serum creatinine and age reduce the response to hepatitis B vaccine in renal failure patients. J Hepatol 1994; 21:450–454.
- DaRoza G, Loewen A, Djurdjev O, et al. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis 2003; 42:1184–1192.
- Ghadiani MH, Besharati S, Mousavinasab N, Jalalzadeh M. Response rates to HB vaccine in CKD stages 3-4 and hemodialysis patients. J Res Med Sci 2012; 17:527–533.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489–492.
- Guidelines for vaccination in patients with chronic kidney disease. Indian J Nephrol 2016; 26(suppl 1):S15–S18.
- Somi MH, Hajipour B. Improving hepatitis B vaccine efficacy in end-stage renal diseases patients and role of adjuvants. ISRN Gastroenterol 2012; 2012:960413.
- Yousaf F, Gandham S, Galler M, Spinowitz B, Charytan C. Systematic review of the efficacy and safety of intradermal versus intramuscular hepatitis B vaccination in end-stage renal disease population unresponsive to primary vaccination series. Ren Fail 2015; 37:1080–1088.
For patients age 16 and older with advanced chronic kidney disease (CKD), including those undergoing hemodialysis, we recommend a higher dose of hepatitis B virus (HBV) vaccine, more doses, or both. Vaccination with a higher dose may improve the immune response. The hepatitis B surface antibody (anti-HBs) titer should be monitored 1 to 2 months after completion of the vaccination schedule and annually thereafter, with a target titer of 10 IU/mL or greater. For patients who do not develop a protective antibody titer after completing the initial vaccination schedule, the vaccination schedule should be repeated.
RECOMMENDED DOSES AND SCHEDULES
Recommendation 1
Give higher vaccine doses, increase the number of doses, or both.
Recommendation 2
A 4-dose regimen may provide a better antibody response than a 3-dose regimen. (Note: This recommendation applies only to Engerix-B; 4 doses of Recombivax-HB would be an off-label use.)
Rationale. The US Centers for Disease Control and Prevention reported that after completion of a 3-dose vaccination schedule, the median proportion of patients developing a protective antibody response was 64% (range 34%–88%) vs a median of 86% (range 40%–98%) after a 4-dose schedule.3
Lacson et al5 compared antibody response rates after 3 doses of Recombivax-HB and after 4 doses of Engerix-B and found a better response rate with the 4-dose schedule. The rate of persistent protective anti-HBs titer after 1 year was 77% for Engerix-B vs 53% for Recombivax-HB.
Agarwal et al6 evaluated response rates in patients who had mild CKD (serum creatinine levels 1.5–3.0 mg/dL), moderate CKD (creatinine 3.0–6.0 mg/dL), and severe CKD (creatinine > 6.0 mg/dL). The seroconversion rates after 3 doses of 40-μg HBV vaccine were 87.5% in those with mild CKD, 66.6% in those with moderate CKD, and 35.7% in those with severe disease. After a fourth dose, rates improved significantly to 100%, 77%, and 36.4%, respectively.
Recommendation 3
In patients with CKD, vaccination should be done early, before they become dependent on hemodialysis.
Rationale. Patients with advanced CKD may have a lower seroconversion rate. Fraser et al7 found that after a 4-dose series, the seroprotection rate in adult prehemodialysis patients with serum creatinine levels of 4 mg/dL or less was 86%, compared with 37% in patients with serum creatinine levels above 4 mg/dL, of whom 88% were on hemodialysis.7
In a 2003 prospective cohort study by DaRoza et al,8 patients with higher levels of kidney function were more likely to respond to HBV vaccination, and the level of kidney function was found to be an independent predictor of seroconversion.8
A 2012 prospective study by Ghadiani et al9 compared seroconversion rates in patients with stage 3 or 4 CKD vs patients on hemodialysis, with medical staff as controls. The authors reported seroprotection rates of 26.1% in patients on hemodialysis, 55.2% in patients with stage 3 or 4 CKD, and 96.2% in controls. They concluded that vaccination is more likely to induce seroconversion in earlier stages of kidney disease.9
MONITORING THE RESPONSE TO VACCINATION AND REVACCINATION
Testing after vaccination is recommended to determine response. Testing should be done 1 to 2 months after the last dose of the vaccination schedule.1–3 Anti-HBs levels 10 IU/mL and greater are considered protective.10
Revaccination with a full vaccination series is recommended for patients who do not develop adequate levels of protective antibodies after completion of the vaccination schedule.2 Reported response rates to revaccination have varied from 40% to 50% after 2 or 3 additional intramuscular doses of 40 µg, to 64% after 4 additional intramuscular doses of 10 µg.3 Serologic testing should be repeated after the last dose of the vaccination series, as serologic testing after only 1 or 2 additional doses appears to be no more cost-effective.2,3
To the best of our knowledge, no data exist to indicate that in nonresponders, further doses given after completion of 2 full vaccination schedules would induce an antibody response.
ANTIBODY PERSISTENCE AND BOOSTER DOSES
Antibody levels fall with time in patients on hemodialysis. Limited data suggest that in patients who respond to the primary vaccination series, antibodies remain detectable for 6 months in 80% to 100% (median 100%) of patients and for 12 months in 58% to 100% (median 70%) of patients.3 The need for booster doses should be assessed by annual monitoring.2,11 Booster doses should be given when the anti-HBs titer declines to below 10 IU/mL. Limited data indicate that nearly all such patients would respond to a booster dose.3
OTHER WAYS TO IMPROVE VACCINE RESPONSE
Other strategies to improve vaccine response, such as the addition of adjuvants or immunostimulants, have shown variable success.12 Intradermal HBV vaccination in patients on chronic hemodialysis has also been proposed. The efficacy of intradermal vaccination may be related to the dense network of immunologic dendritic cells within the dermis. After intradermal administration, the antigen is taken up by dendritic cells residing in the dermis, which mature and travel to the regional lymph node where further immunostimulation takes place.13
In a systematic review of four prospective trials with a total of 204 hemodialysis patients,13 a significantly higher proportion of patients achieved seroconversion with intradermal HBV vaccine administration than with intramuscular administration. The authors concluded that the intradermal route in primary nonresponders undergoing hemodialysis provides an effective alternative to the intramuscular route to protect against HBV infection in this highly susceptible population.
Additional well-designed, double-blinded, randomized trials are needed to establish clear guidelines on intradermal HBV vaccine dosing and vaccination schedules.
For patients age 16 and older with advanced chronic kidney disease (CKD), including those undergoing hemodialysis, we recommend a higher dose of hepatitis B virus (HBV) vaccine, more doses, or both. Vaccination with a higher dose may improve the immune response. The hepatitis B surface antibody (anti-HBs) titer should be monitored 1 to 2 months after completion of the vaccination schedule and annually thereafter, with a target titer of 10 IU/mL or greater. For patients who do not develop a protective antibody titer after completing the initial vaccination schedule, the vaccination schedule should be repeated.
RECOMMENDED DOSES AND SCHEDULES
Recommendation 1
Give higher vaccine doses, increase the number of doses, or both.
Recommendation 2
A 4-dose regimen may provide a better antibody response than a 3-dose regimen. (Note: This recommendation applies only to Engerix-B; 4 doses of Recombivax-HB would be an off-label use.)
Rationale. The US Centers for Disease Control and Prevention reported that after completion of a 3-dose vaccination schedule, the median proportion of patients developing a protective antibody response was 64% (range 34%–88%) vs a median of 86% (range 40%–98%) after a 4-dose schedule.3
Lacson et al5 compared antibody response rates after 3 doses of Recombivax-HB and after 4 doses of Engerix-B and found a better response rate with the 4-dose schedule. The rate of persistent protective anti-HBs titer after 1 year was 77% for Engerix-B vs 53% for Recombivax-HB.
Agarwal et al6 evaluated response rates in patients who had mild CKD (serum creatinine levels 1.5–3.0 mg/dL), moderate CKD (creatinine 3.0–6.0 mg/dL), and severe CKD (creatinine > 6.0 mg/dL). The seroconversion rates after 3 doses of 40-μg HBV vaccine were 87.5% in those with mild CKD, 66.6% in those with moderate CKD, and 35.7% in those with severe disease. After a fourth dose, rates improved significantly to 100%, 77%, and 36.4%, respectively.
Recommendation 3
In patients with CKD, vaccination should be done early, before they become dependent on hemodialysis.
Rationale. Patients with advanced CKD may have a lower seroconversion rate. Fraser et al7 found that after a 4-dose series, the seroprotection rate in adult prehemodialysis patients with serum creatinine levels of 4 mg/dL or less was 86%, compared with 37% in patients with serum creatinine levels above 4 mg/dL, of whom 88% were on hemodialysis.7
In a 2003 prospective cohort study by DaRoza et al,8 patients with higher levels of kidney function were more likely to respond to HBV vaccination, and the level of kidney function was found to be an independent predictor of seroconversion.8
A 2012 prospective study by Ghadiani et al9 compared seroconversion rates in patients with stage 3 or 4 CKD vs patients on hemodialysis, with medical staff as controls. The authors reported seroprotection rates of 26.1% in patients on hemodialysis, 55.2% in patients with stage 3 or 4 CKD, and 96.2% in controls. They concluded that vaccination is more likely to induce seroconversion in earlier stages of kidney disease.9
MONITORING THE RESPONSE TO VACCINATION AND REVACCINATION
Testing after vaccination is recommended to determine response. Testing should be done 1 to 2 months after the last dose of the vaccination schedule.1–3 Anti-HBs levels 10 IU/mL and greater are considered protective.10
Revaccination with a full vaccination series is recommended for patients who do not develop adequate levels of protective antibodies after completion of the vaccination schedule.2 Reported response rates to revaccination have varied from 40% to 50% after 2 or 3 additional intramuscular doses of 40 µg, to 64% after 4 additional intramuscular doses of 10 µg.3 Serologic testing should be repeated after the last dose of the vaccination series, as serologic testing after only 1 or 2 additional doses appears to be no more cost-effective.2,3
To the best of our knowledge, no data exist to indicate that in nonresponders, further doses given after completion of 2 full vaccination schedules would induce an antibody response.
ANTIBODY PERSISTENCE AND BOOSTER DOSES
Antibody levels fall with time in patients on hemodialysis. Limited data suggest that in patients who respond to the primary vaccination series, antibodies remain detectable for 6 months in 80% to 100% (median 100%) of patients and for 12 months in 58% to 100% (median 70%) of patients.3 The need for booster doses should be assessed by annual monitoring.2,11 Booster doses should be given when the anti-HBs titer declines to below 10 IU/mL. Limited data indicate that nearly all such patients would respond to a booster dose.3
OTHER WAYS TO IMPROVE VACCINE RESPONSE
Other strategies to improve vaccine response, such as the addition of adjuvants or immunostimulants, have shown variable success.12 Intradermal HBV vaccination in patients on chronic hemodialysis has also been proposed. The efficacy of intradermal vaccination may be related to the dense network of immunologic dendritic cells within the dermis. After intradermal administration, the antigen is taken up by dendritic cells residing in the dermis, which mature and travel to the regional lymph node where further immunostimulation takes place.13
In a systematic review of four prospective trials with a total of 204 hemodialysis patients,13 a significantly higher proportion of patients achieved seroconversion with intradermal HBV vaccine administration than with intramuscular administration. The authors concluded that the intradermal route in primary nonresponders undergoing hemodialysis provides an effective alternative to the intramuscular route to protect against HBV infection in this highly susceptible population.
Additional well-designed, double-blinded, randomized trials are needed to establish clear guidelines on intradermal HBV vaccine dosing and vaccination schedules.
- Grzegorzewska AE. Hepatitis B vaccination in chronic kidney disease: review of evidence in non-dialyzed patients. Hepat Mon 2012; 12:e7359.
- Chi C, Patel P, Pilishvili T, Moore M, Murphy T, Strikas R. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. www.cdc.gov/dialysis/PDFs/Vaccinating_Dialysis_Patients_and_Patients_dec2012.pdf. Accessed September 6, 2017.
- Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep 2001; 50:1–43.
- Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB; Advisory Committee on Immunization Practices. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. Ann Intern Med 2017; 166:209–219.
- Lacson E, Teng M, Ong J, Vienneau L, Ofsthun N, Lazarus JM. Antibody response to Engerix-B and Recombivax-HB hepatitis B vaccination in end-stage renal disease. Hemodialysis international. Hemodial Int 2005; 9:367–375.
- Agarwal SK, Irshad M, Dash SC. Comparison of two schedules of hepatitis B vaccination in patients with mild, moderate and severe renal failure. J Assoc Physicians India 1999; 47:183–185.
- Fraser GM, Ochana N, Fenyves D, et al. Increasing serum creatinine and age reduce the response to hepatitis B vaccine in renal failure patients. J Hepatol 1994; 21:450–454.
- DaRoza G, Loewen A, Djurdjev O, et al. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis 2003; 42:1184–1192.
- Ghadiani MH, Besharati S, Mousavinasab N, Jalalzadeh M. Response rates to HB vaccine in CKD stages 3-4 and hemodialysis patients. J Res Med Sci 2012; 17:527–533.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489–492.
- Guidelines for vaccination in patients with chronic kidney disease. Indian J Nephrol 2016; 26(suppl 1):S15–S18.
- Somi MH, Hajipour B. Improving hepatitis B vaccine efficacy in end-stage renal diseases patients and role of adjuvants. ISRN Gastroenterol 2012; 2012:960413.
- Yousaf F, Gandham S, Galler M, Spinowitz B, Charytan C. Systematic review of the efficacy and safety of intradermal versus intramuscular hepatitis B vaccination in end-stage renal disease population unresponsive to primary vaccination series. Ren Fail 2015; 37:1080–1088.
- Grzegorzewska AE. Hepatitis B vaccination in chronic kidney disease: review of evidence in non-dialyzed patients. Hepat Mon 2012; 12:e7359.
- Chi C, Patel P, Pilishvili T, Moore M, Murphy T, Strikas R. Guidelines for vaccinating kidney dialysis patients and patients with chronic kidney disease. www.cdc.gov/dialysis/PDFs/Vaccinating_Dialysis_Patients_and_Patients_dec2012.pdf. Accessed September 6, 2017.
- Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep 2001; 50:1–43.
- Kim DK, Riley LE, Harriman KH, Hunter P, Bridges CB; Advisory Committee on Immunization Practices. Recommended immunization schedule for adults aged 19 years or older, United States, 2017. Ann Intern Med 2017; 166:209–219.
- Lacson E, Teng M, Ong J, Vienneau L, Ofsthun N, Lazarus JM. Antibody response to Engerix-B and Recombivax-HB hepatitis B vaccination in end-stage renal disease. Hemodialysis international. Hemodial Int 2005; 9:367–375.
- Agarwal SK, Irshad M, Dash SC. Comparison of two schedules of hepatitis B vaccination in patients with mild, moderate and severe renal failure. J Assoc Physicians India 1999; 47:183–185.
- Fraser GM, Ochana N, Fenyves D, et al. Increasing serum creatinine and age reduce the response to hepatitis B vaccine in renal failure patients. J Hepatol 1994; 21:450–454.
- DaRoza G, Loewen A, Djurdjev O, et al. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kidney Dis 2003; 42:1184–1192.
- Ghadiani MH, Besharati S, Mousavinasab N, Jalalzadeh M. Response rates to HB vaccine in CKD stages 3-4 and hemodialysis patients. J Res Med Sci 2012; 17:527–533.
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis 1999; 179:489–492.
- Guidelines for vaccination in patients with chronic kidney disease. Indian J Nephrol 2016; 26(suppl 1):S15–S18.
- Somi MH, Hajipour B. Improving hepatitis B vaccine efficacy in end-stage renal diseases patients and role of adjuvants. ISRN Gastroenterol 2012; 2012:960413.
- Yousaf F, Gandham S, Galler M, Spinowitz B, Charytan C. Systematic review of the efficacy and safety of intradermal versus intramuscular hepatitis B vaccination in end-stage renal disease population unresponsive to primary vaccination series. Ren Fail 2015; 37:1080–1088.
Detecting and managing device leads inadvertently placed in the left ventricle
Although rare, inadvertent placement of a pacemaker or defibrillator lead in the left ventricle can have serious consequences, including arterial thromboembolism and aortic or mitral valve damage or infection.1–4
This article discusses situations in which lead malpositioning is likely to occur, how to prevent it, how to detect and correct it immediately, and how to manage cases discovered long after implantation.
RARE, BUT LIKELY UNDERREPORTED
In 2011, Rodriguez et al1 reviewed 56 reported cases in which an endocardial lead had been mistakenly placed in the left ventricle. A few more cases have been reported since then, but some cases are not reported, so how often this occurs is unknown.
A large single-center retrospective study2 reported a 3.4% incidence of inadvertent lead placement in the left side of the heart, including the cardiac veins.
HOW LEADS CAN END UP IN THE WRONG PLACE
Risk factors for lead malpositioning include abnormal thoracic anatomy, underlying congenital heart disease, and operator inexperience.2
Normally, in single- and double-lead systems, leads are inserted into a cephalic, subclavian, or axillary vein and advanced into the right atrium, right ventricle, or both. However, pacing, sensing, and defibrillation leads have inadvertently been placed in the left ventricular endocardium and even on the epicardial surface.
Leads can end up inside the left ventricle by passing through an unrecognized atrial septal defect, patent foramen ovale, or ventricular septal defect, or by perforating the interventricular septum. Another route into the left ventricle is by gaining vascular access through the axillary or subclavian artery and advancing the lead retrograde across the aortic valve.
Epicardial lead placement may result from perforating the right ventricle5 or inadvertent positioning within the main coronary sinus or in a cardiac vein.
PREVENTION IS THE BEST MANAGEMENT
The best way to manage lead malpositioning is to prevent it in the first place.
Make sure you are in a vein, not an artery! If you are working from the patient’s left side, you should see the guidewire cross the midline on fluoroscopy. Working from either the left or the right side, you can ensure that the guidewire is in the venous system by advancing it into the inferior vena cava and then all the way below the diaphragm (best seen on anteroposterior views). These observations help avoid lead placement in the left ventricle by an inadvertent retrograde aortic approach.
Suspect that you are taking the wrong route to the heart (ie, through the arterial system) if, in the anteroposterior view, the guidewire bends as it approaches the left spinal border. This sign suggests that you are going backwards through the ascending aorta and bumping up against the aortic cusps. Occasionally, the wire may pass through the aortic valve without resistance and bending. Additional advancement toward the left chest wall will make contact with the left ventricular endocardium and may result in ventricular ectopy. Placement in the left ventricle is best seen in the left anterior oblique projection; the lead will cross the spine or its distal end will point toward the spine in progressive projections from farther to the left.
Make sure you are in the right ventricle. Even if you have gone through the venous system, you are not home free. Advancing the lead into the right ventricular outflow tract (best seen in the right anterior oblique projection) is a key step in avoiding lead misplacement. In the right ventricular outflow tract, the lead tip should move freely; if it does not, it may be in the coronary sinus or middle cardiac vein.
If a lead passes through a patent foramen ovale or septal defect to the left atrium, a left anterior oblique view should also demonstrate movement toward or beyond the spine. If the lead passes beyond the left heart border, a position in a pulmonary vein is possible. This is often associated with loss of a recordable intracardiac electrogram. A position in a right pulmonary vein is possible but very, very unlikely. If a lead passes through a patent foramen ovale or septal defect to the left ventricle, it will point toward the spine in left anterior oblique projections. (See “Postoperative detection by chest radiography.”)
Ventricular paced QRS complexes should show a left bundle branch pattern on electrocardiography (ECG), not a right bundle branch pattern (more about this below). However, when inserting a pacemaker, the sterile field includes the front of the chest and therefore lead V1 is usually omitted, depriving the operator of valuable information.
Fortunately, operators may fluoroscopically view leads intended for the right ventricle in left anterior oblique projections. We recommend beginning at 40° left anterior oblique. In this view, septally positioned right ventricular leads may appear to abut the spine. A right ventricular position is confirmed in a steeper left anterior oblique projection, where the lead should be seen to be away from the spine.4
POSTOPERATIVE DETECTION BY ECG
Careful evaluation of the 12-lead electrocardiogram during ventricular pacing is important for confirming correct lead placement. If ventricular pacing is absent, eg, if the device fires only if the natural heart rate drops below a set number and the heart happens to be firing on its own when you happen to be looking at it, programming the device to pace the right ventricle 10 beats per minute faster than the intrinsic heart rate usually suffices. Temporarily disabling atrial pacing and cardiac venous pacing in biventricular devices facilitates interpretation of the paced QRS complex.
Bundle branch block patterns
The typical morphology for paced events originating from the right ventricle has a left bundle branch block pattern, ie, a dominant S wave in leads V1 and V2. Nevertheless, many patients with a safely placed lead in the right ventricle can also demonstrate right bundle branch morphology during pacing,6 ie, a dominant R wave in leads V1 and V2.
Klein et al7 reported on 8 patients who had features of right bundle branch block in leads V1 and V2 and noted that placing these leads 1 interspace lower eliminated the right bundle branch block appearance. The utility of this maneuver is demonstrated in Figure 1.
Almehairi et al8 demonstrated transition to a left bundle branch block-like pattern in V1 in 14 of 26 patients after leads V1 and V2 were moved to the fifth intercostal space. Moving these leads to the sixth intercostal space produced a left bundle branch block-like pattern in all the remaining patients. Additional study is needed to validate this precordial mapping technique.9
Although the Coman and Trohman algorithm suggests that a frontal plane axis of −90° to –180° is specific for left ventricular pacing,6 other reports have identified this axis in the presence of true right ventricular pacing.6,9–12 Therefore, Barold and Giudici9 argue that a frontal plane axis in the right superior quadrant has limited diagnostic value.
POSTOPERATIVE DETECTION BY CHEST RADIOGRAPHY
A lead in the left ventricle may be a subtle finding on an anteroposterior or posteroanterior chest radiograph. The definitive view is the lateral projection, which is also true during intraoperative fluoroscopy.13–15 The tip of a malpositioned left-ventricular lead is characteristically seen farther posterior (toward the spine) in the cardiac silhouette on the lateral view (Figure 3).2 If the lead is properly positioned, the general direction of the middle to distal portion should be away from the spine.
ECHOCARDIOGRAPHY TO CONFIRM
Two-dimensional echocardiography can help to confirm left ventricular placement via an atrial septal defect, patent foramen ovale, or perforation of the interventricular septum.16,17
Three-dimensional echocardiography can facilitate cardiac venous lead placement and assess the impact of right ventricular lead placement on tricuspid valve function.18,19 In one case report, 3-dimensional echocardiography provided a definitive diagnosis of interventricular septal perforation when findings on computed tomography (CT) were indeterminate.20
CT AND MRI: LIMITED ROLES
When echocardiographic findings are equivocal, CT can help diagnose lead perforation. Electrocardiogram-triggered cardiac CT can help visualize lead positions and potential lead perforation. Unfortunately, the precise location of the lead tip (and the diagnosis) can be missed due to streaking (“star”) artifacts and acoustic shadowing from the metallic lead.21–26 Because of these limitations, as well as radiation exposure and high costs, CT should be used sparingly, if at all, for diagnosing lead malposition.
Technological advances and the increasing use of magnetic resonance imaging (MRI) in clinical practice have led to the development of “MRI-conditional” cardiac implantable electronic devices (ie, safe for undergoing MRI), as well as more lenient regulation of MRI in patients with nonconditional devices.27,28 Although the widely held opinion that patients with a pacemaker or implantable cardioverter defibrillator are not eligible to undergo MRI has largely been abandoned, it seems unlikely that cardiac MRI will become a pivotal tool in assessing lead malposition.
MANAGING MALPOSITIONED LEADS
Inadvertent left ventricular lead placement provides a nidus for thrombus formation. When inadvertent left ventricular lead malposition is identified acutely, correction of the lead position should be performed immediately by an experienced electrophysiologist.
Treatment of left ventricular lead misplacement discovered late after implantation includes lead removal or chronic anticoagulation with warfarin to prevent thromboemboli.
Long-term anticoagulation
No thromboembolic events have been reported2 in patients with lead malposition who take warfarin and maintain an international normalized ratio of 2.5 to 3.5.
Antiplatelet agents are not enough by themselves.16
The use of direct oral anticoagulants has not been explored in this setting. Use of dabigatran in patients with mechanical heart valves was associated with increased rates of thromboembolic and bleeding complications compared with warfarin.29 Based on these results and an overall lack of evidence, we do not recommend substituting a direct oral anticoagulant for warfarin in the setting of malpositioned left ventricular leads.
Late percutaneous removal
Late lead removal is most appropriate if cardiac surgery is planned for other reasons. Although percutaneous extraction of a malpositioned left ventricular lead was first described over 25 years ago,13 the safety of this procedure remains uncertain.
Kosmidou et al17 reported two cases of percutaneous removal of inadvertent transarterial leads employing standard interventional cardiology methods for cerebral embolic protection. Distal embolic filter wires were deployed in the left and right internal carotid arteries. A covered stent was deployed at the arterial entry site simultaneously with lead removal, providing immediate and effective hemostasis. Similar protection should be considered during transvenous access and extraction via an atrial septal or patent foramen ovale.
Nevertheless, not even transesophageal echocardiography can reliably exclude adhered thrombi, and the risk of embolization of fibrous adhesions or thrombi has been cited as a pivotal contraindication to percutaneous lead extraction regardless of modality.16
- Rodriguez Y, Baltodano P, Tower A, Martinez C, Carrillo R. Management of symptomatic inadvertently placed endocardial leads in the left ventricle. Pacing Clin Electrophysiol 2011; 34:1192–1200.
- Ohlow MA, Roos M, Lauer B, Von Korn H, Geller JC. Incidence, predictors, and outcome of inadvertent malposition of transvenous pacing or defibrillation lead in the left heart. Europace 2016; 18:1049–1054.
- Madias C, Trohman RG. Cardiac resynchronization therapy: the state of the art. Expert Rev Cardiovasc Ther 2014; 12:573–587.
- Trohman RG. To the editor—comment on six uneventful years with a pacing lead in the left ventricle. Heart Rhythm 2013; 10:e81.
- Cossú SF. Unusual placement of a coronary sinus lead for resynchronization therapy resulting in late lead fracture. J Innovations Cardiac Rhythm Manage 2013; 4:1148–1153.
- Coman JA, Trohman RG. Incidence and electrocardiographic localization of safe right bundle branch block configurations during permanent ventricular pacing. Am J Cardiol 1995; 76:781–784.
- Klein HO, Beker B, Sareli P, DiSegni E, Dean H, Kaplinsky E. Unusual QRS morphology associated with transvenous pacemakers. The pseudo RBBB pattern. Chest 1985; 87:517–521.
- Almehairi M, Enriquez A, Redfearn D, et al. Right bundle branch block-like pattern during ventricular pacing: a surface electrocardiographic mapping technique to locate the ventricular lead. Can J Cardiol 2015; 31:1019–1024.
- Barold SS, Giudici MC. Renewed interest in the significance of the tall R wave in ECG lead V1 during right ventricular pacing. Expert Rev Med Devices 2016; 13:611–613.
- Almehairi M, Ali FS, Enriquez A, et al. Electrocardiographic algorithms to predict true right ventricular pacing in the presence of right bundle branch block-like pattern. Int J Cardiol 2014; 172:e403–e405.
- Tzeis S, Andrikopoulos G, Weigand S, et al. Right bundle branch block-like pattern during uncomplicated right ventricular pacing and the effect of pacing site. Am J Cardiol 2016; 117:935–939.
- Hemminger EJ, Criley JM. Right ventricular enlargement mimicking electrocardiographic left ventricular pacing. J Electrocardiol 2006; 39:180–182.
- Furman S. Chest PA and lateral. Pacing Clin Electrophysiol 1993; 16:953.
- Trohman RG, Wilkoff BL, Byrne T, Cook S. Successful percutaneous extraction of a chronic left ventricular pacing lead. Pacing Clin Electrophysiol 1991; 14:1448–1451.
- Trohman RG, Kim MH, Pinski SL. Cardiac pacing: the state of the art. Lancet 2004; 364:1701–1719.
- Van Gelder BM, Bracke FA, Oto A, et al. Diagnosis and management of inadvertently placed pacing and ICD leads in the left ventricle: a multicenter experience and review of the literature. Pacing Clin Electrophysiol 2000; 23:877–883.
- Kosmidou I, Karmpaliotis D, Kandzari DE, Dan D. Inadvertent transarterial lead placement in the left ventricle and aortic cusp: percutaneous lead removal with carotid embolic protection and stent graft placement. Indian Pacing Electrophysiol J 2012; 12:269–273.
- Villanueva FS, Heinsimer JA, Burkman MH, Fananapazir L,
- Halvorsen RA Jr, Chen JT. Echocardiographic detection of perforation of the cardiac ventricular septum by a permanent pacemaker lead. Am J Cardiol 1987; 59:370–371.
- Döring M, Braunschweig F, Eitel C, et al. Individually tailored left ventricular lead placement: lessons from multimodality integration between three-dimensional echocardiography and coronary sinus angiogram. Europace 2013; 15:718–727.
- Mediratta A, Addetia K, Yamat M, et al. 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc Imaging 2014; 7:337–347.
- Daher IN, Saeed M, Schwarz ER, Agoston I, Rahman MA, Ahmad M. Live three-dimensional echocardiography in diagnosis of interventricular septal perforation by pacemaker lead. Echocardiography 2006; 23:428–429.
- Mak GS, Truong QA. Cardiac CT: imaging of and through cardiac devices. Curr Cardiovasc Imaging Rep 2012; 5:328–336.
- Henrikson CA, Leng CT, Yuh DD, Brinker JA. Computed tomography to assess possible cardiac lead perforation. Pacing Clin Electrophysiol 2006; 29:509–511.
- Hirschl DA, Jain VR, Spindola-Franco H, Gross JN, Haramati LB. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing Clin Electrophysiol 2007; 30:28–32.
- Pang BJ, Lui EH, Joshi SB, et al. Pacing and implantable cardioverter defibrillator lead perforation as assessed by multiplanar reformatted ECG-gated cardiac computed tomography and clinical correlates. Pacing Clin Electrophysiol 2014; 37:537–545.
- Lanzman RS, Winter J, Blondin D, et al. Where does it lead? Imaging features of cardiovascular implantable electronic devices on chest radiograph and CT. Korean J Radiol 2011; 12:611–619.
- van der Graaf AW, Bhagirath P, Götte MJ. MRI and cardiac implantable electronic devices; current status and required safety conditions. Neth Heart J 2014; 22:269–276.
- European Society of Cardiology (ESC), European Heart Rhythm Association (EHRA); Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013; 15:1070–1118.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
Although rare, inadvertent placement of a pacemaker or defibrillator lead in the left ventricle can have serious consequences, including arterial thromboembolism and aortic or mitral valve damage or infection.1–4
This article discusses situations in which lead malpositioning is likely to occur, how to prevent it, how to detect and correct it immediately, and how to manage cases discovered long after implantation.
RARE, BUT LIKELY UNDERREPORTED
In 2011, Rodriguez et al1 reviewed 56 reported cases in which an endocardial lead had been mistakenly placed in the left ventricle. A few more cases have been reported since then, but some cases are not reported, so how often this occurs is unknown.
A large single-center retrospective study2 reported a 3.4% incidence of inadvertent lead placement in the left side of the heart, including the cardiac veins.
HOW LEADS CAN END UP IN THE WRONG PLACE
Risk factors for lead malpositioning include abnormal thoracic anatomy, underlying congenital heart disease, and operator inexperience.2
Normally, in single- and double-lead systems, leads are inserted into a cephalic, subclavian, or axillary vein and advanced into the right atrium, right ventricle, or both. However, pacing, sensing, and defibrillation leads have inadvertently been placed in the left ventricular endocardium and even on the epicardial surface.
Leads can end up inside the left ventricle by passing through an unrecognized atrial septal defect, patent foramen ovale, or ventricular septal defect, or by perforating the interventricular septum. Another route into the left ventricle is by gaining vascular access through the axillary or subclavian artery and advancing the lead retrograde across the aortic valve.
Epicardial lead placement may result from perforating the right ventricle5 or inadvertent positioning within the main coronary sinus or in a cardiac vein.
PREVENTION IS THE BEST MANAGEMENT
The best way to manage lead malpositioning is to prevent it in the first place.
Make sure you are in a vein, not an artery! If you are working from the patient’s left side, you should see the guidewire cross the midline on fluoroscopy. Working from either the left or the right side, you can ensure that the guidewire is in the venous system by advancing it into the inferior vena cava and then all the way below the diaphragm (best seen on anteroposterior views). These observations help avoid lead placement in the left ventricle by an inadvertent retrograde aortic approach.
Suspect that you are taking the wrong route to the heart (ie, through the arterial system) if, in the anteroposterior view, the guidewire bends as it approaches the left spinal border. This sign suggests that you are going backwards through the ascending aorta and bumping up against the aortic cusps. Occasionally, the wire may pass through the aortic valve without resistance and bending. Additional advancement toward the left chest wall will make contact with the left ventricular endocardium and may result in ventricular ectopy. Placement in the left ventricle is best seen in the left anterior oblique projection; the lead will cross the spine or its distal end will point toward the spine in progressive projections from farther to the left.
Make sure you are in the right ventricle. Even if you have gone through the venous system, you are not home free. Advancing the lead into the right ventricular outflow tract (best seen in the right anterior oblique projection) is a key step in avoiding lead misplacement. In the right ventricular outflow tract, the lead tip should move freely; if it does not, it may be in the coronary sinus or middle cardiac vein.
If a lead passes through a patent foramen ovale or septal defect to the left atrium, a left anterior oblique view should also demonstrate movement toward or beyond the spine. If the lead passes beyond the left heart border, a position in a pulmonary vein is possible. This is often associated with loss of a recordable intracardiac electrogram. A position in a right pulmonary vein is possible but very, very unlikely. If a lead passes through a patent foramen ovale or septal defect to the left ventricle, it will point toward the spine in left anterior oblique projections. (See “Postoperative detection by chest radiography.”)
Ventricular paced QRS complexes should show a left bundle branch pattern on electrocardiography (ECG), not a right bundle branch pattern (more about this below). However, when inserting a pacemaker, the sterile field includes the front of the chest and therefore lead V1 is usually omitted, depriving the operator of valuable information.
Fortunately, operators may fluoroscopically view leads intended for the right ventricle in left anterior oblique projections. We recommend beginning at 40° left anterior oblique. In this view, septally positioned right ventricular leads may appear to abut the spine. A right ventricular position is confirmed in a steeper left anterior oblique projection, where the lead should be seen to be away from the spine.4
POSTOPERATIVE DETECTION BY ECG
Careful evaluation of the 12-lead electrocardiogram during ventricular pacing is important for confirming correct lead placement. If ventricular pacing is absent, eg, if the device fires only if the natural heart rate drops below a set number and the heart happens to be firing on its own when you happen to be looking at it, programming the device to pace the right ventricle 10 beats per minute faster than the intrinsic heart rate usually suffices. Temporarily disabling atrial pacing and cardiac venous pacing in biventricular devices facilitates interpretation of the paced QRS complex.
Bundle branch block patterns
The typical morphology for paced events originating from the right ventricle has a left bundle branch block pattern, ie, a dominant S wave in leads V1 and V2. Nevertheless, many patients with a safely placed lead in the right ventricle can also demonstrate right bundle branch morphology during pacing,6 ie, a dominant R wave in leads V1 and V2.
Klein et al7 reported on 8 patients who had features of right bundle branch block in leads V1 and V2 and noted that placing these leads 1 interspace lower eliminated the right bundle branch block appearance. The utility of this maneuver is demonstrated in Figure 1.
Almehairi et al8 demonstrated transition to a left bundle branch block-like pattern in V1 in 14 of 26 patients after leads V1 and V2 were moved to the fifth intercostal space. Moving these leads to the sixth intercostal space produced a left bundle branch block-like pattern in all the remaining patients. Additional study is needed to validate this precordial mapping technique.9
Although the Coman and Trohman algorithm suggests that a frontal plane axis of −90° to –180° is specific for left ventricular pacing,6 other reports have identified this axis in the presence of true right ventricular pacing.6,9–12 Therefore, Barold and Giudici9 argue that a frontal plane axis in the right superior quadrant has limited diagnostic value.
POSTOPERATIVE DETECTION BY CHEST RADIOGRAPHY
A lead in the left ventricle may be a subtle finding on an anteroposterior or posteroanterior chest radiograph. The definitive view is the lateral projection, which is also true during intraoperative fluoroscopy.13–15 The tip of a malpositioned left-ventricular lead is characteristically seen farther posterior (toward the spine) in the cardiac silhouette on the lateral view (Figure 3).2 If the lead is properly positioned, the general direction of the middle to distal portion should be away from the spine.
ECHOCARDIOGRAPHY TO CONFIRM
Two-dimensional echocardiography can help to confirm left ventricular placement via an atrial septal defect, patent foramen ovale, or perforation of the interventricular septum.16,17
Three-dimensional echocardiography can facilitate cardiac venous lead placement and assess the impact of right ventricular lead placement on tricuspid valve function.18,19 In one case report, 3-dimensional echocardiography provided a definitive diagnosis of interventricular septal perforation when findings on computed tomography (CT) were indeterminate.20
CT AND MRI: LIMITED ROLES
When echocardiographic findings are equivocal, CT can help diagnose lead perforation. Electrocardiogram-triggered cardiac CT can help visualize lead positions and potential lead perforation. Unfortunately, the precise location of the lead tip (and the diagnosis) can be missed due to streaking (“star”) artifacts and acoustic shadowing from the metallic lead.21–26 Because of these limitations, as well as radiation exposure and high costs, CT should be used sparingly, if at all, for diagnosing lead malposition.
Technological advances and the increasing use of magnetic resonance imaging (MRI) in clinical practice have led to the development of “MRI-conditional” cardiac implantable electronic devices (ie, safe for undergoing MRI), as well as more lenient regulation of MRI in patients with nonconditional devices.27,28 Although the widely held opinion that patients with a pacemaker or implantable cardioverter defibrillator are not eligible to undergo MRI has largely been abandoned, it seems unlikely that cardiac MRI will become a pivotal tool in assessing lead malposition.
MANAGING MALPOSITIONED LEADS
Inadvertent left ventricular lead placement provides a nidus for thrombus formation. When inadvertent left ventricular lead malposition is identified acutely, correction of the lead position should be performed immediately by an experienced electrophysiologist.
Treatment of left ventricular lead misplacement discovered late after implantation includes lead removal or chronic anticoagulation with warfarin to prevent thromboemboli.
Long-term anticoagulation
No thromboembolic events have been reported2 in patients with lead malposition who take warfarin and maintain an international normalized ratio of 2.5 to 3.5.
Antiplatelet agents are not enough by themselves.16
The use of direct oral anticoagulants has not been explored in this setting. Use of dabigatran in patients with mechanical heart valves was associated with increased rates of thromboembolic and bleeding complications compared with warfarin.29 Based on these results and an overall lack of evidence, we do not recommend substituting a direct oral anticoagulant for warfarin in the setting of malpositioned left ventricular leads.
Late percutaneous removal
Late lead removal is most appropriate if cardiac surgery is planned for other reasons. Although percutaneous extraction of a malpositioned left ventricular lead was first described over 25 years ago,13 the safety of this procedure remains uncertain.
Kosmidou et al17 reported two cases of percutaneous removal of inadvertent transarterial leads employing standard interventional cardiology methods for cerebral embolic protection. Distal embolic filter wires were deployed in the left and right internal carotid arteries. A covered stent was deployed at the arterial entry site simultaneously with lead removal, providing immediate and effective hemostasis. Similar protection should be considered during transvenous access and extraction via an atrial septal or patent foramen ovale.
Nevertheless, not even transesophageal echocardiography can reliably exclude adhered thrombi, and the risk of embolization of fibrous adhesions or thrombi has been cited as a pivotal contraindication to percutaneous lead extraction regardless of modality.16
Although rare, inadvertent placement of a pacemaker or defibrillator lead in the left ventricle can have serious consequences, including arterial thromboembolism and aortic or mitral valve damage or infection.1–4
This article discusses situations in which lead malpositioning is likely to occur, how to prevent it, how to detect and correct it immediately, and how to manage cases discovered long after implantation.
RARE, BUT LIKELY UNDERREPORTED
In 2011, Rodriguez et al1 reviewed 56 reported cases in which an endocardial lead had been mistakenly placed in the left ventricle. A few more cases have been reported since then, but some cases are not reported, so how often this occurs is unknown.
A large single-center retrospective study2 reported a 3.4% incidence of inadvertent lead placement in the left side of the heart, including the cardiac veins.
HOW LEADS CAN END UP IN THE WRONG PLACE
Risk factors for lead malpositioning include abnormal thoracic anatomy, underlying congenital heart disease, and operator inexperience.2
Normally, in single- and double-lead systems, leads are inserted into a cephalic, subclavian, or axillary vein and advanced into the right atrium, right ventricle, or both. However, pacing, sensing, and defibrillation leads have inadvertently been placed in the left ventricular endocardium and even on the epicardial surface.
Leads can end up inside the left ventricle by passing through an unrecognized atrial septal defect, patent foramen ovale, or ventricular septal defect, or by perforating the interventricular septum. Another route into the left ventricle is by gaining vascular access through the axillary or subclavian artery and advancing the lead retrograde across the aortic valve.
Epicardial lead placement may result from perforating the right ventricle5 or inadvertent positioning within the main coronary sinus or in a cardiac vein.
PREVENTION IS THE BEST MANAGEMENT
The best way to manage lead malpositioning is to prevent it in the first place.
Make sure you are in a vein, not an artery! If you are working from the patient’s left side, you should see the guidewire cross the midline on fluoroscopy. Working from either the left or the right side, you can ensure that the guidewire is in the venous system by advancing it into the inferior vena cava and then all the way below the diaphragm (best seen on anteroposterior views). These observations help avoid lead placement in the left ventricle by an inadvertent retrograde aortic approach.
Suspect that you are taking the wrong route to the heart (ie, through the arterial system) if, in the anteroposterior view, the guidewire bends as it approaches the left spinal border. This sign suggests that you are going backwards through the ascending aorta and bumping up against the aortic cusps. Occasionally, the wire may pass through the aortic valve without resistance and bending. Additional advancement toward the left chest wall will make contact with the left ventricular endocardium and may result in ventricular ectopy. Placement in the left ventricle is best seen in the left anterior oblique projection; the lead will cross the spine or its distal end will point toward the spine in progressive projections from farther to the left.
Make sure you are in the right ventricle. Even if you have gone through the venous system, you are not home free. Advancing the lead into the right ventricular outflow tract (best seen in the right anterior oblique projection) is a key step in avoiding lead misplacement. In the right ventricular outflow tract, the lead tip should move freely; if it does not, it may be in the coronary sinus or middle cardiac vein.
If a lead passes through a patent foramen ovale or septal defect to the left atrium, a left anterior oblique view should also demonstrate movement toward or beyond the spine. If the lead passes beyond the left heart border, a position in a pulmonary vein is possible. This is often associated with loss of a recordable intracardiac electrogram. A position in a right pulmonary vein is possible but very, very unlikely. If a lead passes through a patent foramen ovale or septal defect to the left ventricle, it will point toward the spine in left anterior oblique projections. (See “Postoperative detection by chest radiography.”)
Ventricular paced QRS complexes should show a left bundle branch pattern on electrocardiography (ECG), not a right bundle branch pattern (more about this below). However, when inserting a pacemaker, the sterile field includes the front of the chest and therefore lead V1 is usually omitted, depriving the operator of valuable information.
Fortunately, operators may fluoroscopically view leads intended for the right ventricle in left anterior oblique projections. We recommend beginning at 40° left anterior oblique. In this view, septally positioned right ventricular leads may appear to abut the spine. A right ventricular position is confirmed in a steeper left anterior oblique projection, where the lead should be seen to be away from the spine.4
POSTOPERATIVE DETECTION BY ECG
Careful evaluation of the 12-lead electrocardiogram during ventricular pacing is important for confirming correct lead placement. If ventricular pacing is absent, eg, if the device fires only if the natural heart rate drops below a set number and the heart happens to be firing on its own when you happen to be looking at it, programming the device to pace the right ventricle 10 beats per minute faster than the intrinsic heart rate usually suffices. Temporarily disabling atrial pacing and cardiac venous pacing in biventricular devices facilitates interpretation of the paced QRS complex.
Bundle branch block patterns
The typical morphology for paced events originating from the right ventricle has a left bundle branch block pattern, ie, a dominant S wave in leads V1 and V2. Nevertheless, many patients with a safely placed lead in the right ventricle can also demonstrate right bundle branch morphology during pacing,6 ie, a dominant R wave in leads V1 and V2.
Klein et al7 reported on 8 patients who had features of right bundle branch block in leads V1 and V2 and noted that placing these leads 1 interspace lower eliminated the right bundle branch block appearance. The utility of this maneuver is demonstrated in Figure 1.
Almehairi et al8 demonstrated transition to a left bundle branch block-like pattern in V1 in 14 of 26 patients after leads V1 and V2 were moved to the fifth intercostal space. Moving these leads to the sixth intercostal space produced a left bundle branch block-like pattern in all the remaining patients. Additional study is needed to validate this precordial mapping technique.9
Although the Coman and Trohman algorithm suggests that a frontal plane axis of −90° to –180° is specific for left ventricular pacing,6 other reports have identified this axis in the presence of true right ventricular pacing.6,9–12 Therefore, Barold and Giudici9 argue that a frontal plane axis in the right superior quadrant has limited diagnostic value.
POSTOPERATIVE DETECTION BY CHEST RADIOGRAPHY
A lead in the left ventricle may be a subtle finding on an anteroposterior or posteroanterior chest radiograph. The definitive view is the lateral projection, which is also true during intraoperative fluoroscopy.13–15 The tip of a malpositioned left-ventricular lead is characteristically seen farther posterior (toward the spine) in the cardiac silhouette on the lateral view (Figure 3).2 If the lead is properly positioned, the general direction of the middle to distal portion should be away from the spine.
ECHOCARDIOGRAPHY TO CONFIRM
Two-dimensional echocardiography can help to confirm left ventricular placement via an atrial septal defect, patent foramen ovale, or perforation of the interventricular septum.16,17
Three-dimensional echocardiography can facilitate cardiac venous lead placement and assess the impact of right ventricular lead placement on tricuspid valve function.18,19 In one case report, 3-dimensional echocardiography provided a definitive diagnosis of interventricular septal perforation when findings on computed tomography (CT) were indeterminate.20
CT AND MRI: LIMITED ROLES
When echocardiographic findings are equivocal, CT can help diagnose lead perforation. Electrocardiogram-triggered cardiac CT can help visualize lead positions and potential lead perforation. Unfortunately, the precise location of the lead tip (and the diagnosis) can be missed due to streaking (“star”) artifacts and acoustic shadowing from the metallic lead.21–26 Because of these limitations, as well as radiation exposure and high costs, CT should be used sparingly, if at all, for diagnosing lead malposition.
Technological advances and the increasing use of magnetic resonance imaging (MRI) in clinical practice have led to the development of “MRI-conditional” cardiac implantable electronic devices (ie, safe for undergoing MRI), as well as more lenient regulation of MRI in patients with nonconditional devices.27,28 Although the widely held opinion that patients with a pacemaker or implantable cardioverter defibrillator are not eligible to undergo MRI has largely been abandoned, it seems unlikely that cardiac MRI will become a pivotal tool in assessing lead malposition.
MANAGING MALPOSITIONED LEADS
Inadvertent left ventricular lead placement provides a nidus for thrombus formation. When inadvertent left ventricular lead malposition is identified acutely, correction of the lead position should be performed immediately by an experienced electrophysiologist.
Treatment of left ventricular lead misplacement discovered late after implantation includes lead removal or chronic anticoagulation with warfarin to prevent thromboemboli.
Long-term anticoagulation
No thromboembolic events have been reported2 in patients with lead malposition who take warfarin and maintain an international normalized ratio of 2.5 to 3.5.
Antiplatelet agents are not enough by themselves.16
The use of direct oral anticoagulants has not been explored in this setting. Use of dabigatran in patients with mechanical heart valves was associated with increased rates of thromboembolic and bleeding complications compared with warfarin.29 Based on these results and an overall lack of evidence, we do not recommend substituting a direct oral anticoagulant for warfarin in the setting of malpositioned left ventricular leads.
Late percutaneous removal
Late lead removal is most appropriate if cardiac surgery is planned for other reasons. Although percutaneous extraction of a malpositioned left ventricular lead was first described over 25 years ago,13 the safety of this procedure remains uncertain.
Kosmidou et al17 reported two cases of percutaneous removal of inadvertent transarterial leads employing standard interventional cardiology methods for cerebral embolic protection. Distal embolic filter wires were deployed in the left and right internal carotid arteries. A covered stent was deployed at the arterial entry site simultaneously with lead removal, providing immediate and effective hemostasis. Similar protection should be considered during transvenous access and extraction via an atrial septal or patent foramen ovale.
Nevertheless, not even transesophageal echocardiography can reliably exclude adhered thrombi, and the risk of embolization of fibrous adhesions or thrombi has been cited as a pivotal contraindication to percutaneous lead extraction regardless of modality.16
- Rodriguez Y, Baltodano P, Tower A, Martinez C, Carrillo R. Management of symptomatic inadvertently placed endocardial leads in the left ventricle. Pacing Clin Electrophysiol 2011; 34:1192–1200.
- Ohlow MA, Roos M, Lauer B, Von Korn H, Geller JC. Incidence, predictors, and outcome of inadvertent malposition of transvenous pacing or defibrillation lead in the left heart. Europace 2016; 18:1049–1054.
- Madias C, Trohman RG. Cardiac resynchronization therapy: the state of the art. Expert Rev Cardiovasc Ther 2014; 12:573–587.
- Trohman RG. To the editor—comment on six uneventful years with a pacing lead in the left ventricle. Heart Rhythm 2013; 10:e81.
- Cossú SF. Unusual placement of a coronary sinus lead for resynchronization therapy resulting in late lead fracture. J Innovations Cardiac Rhythm Manage 2013; 4:1148–1153.
- Coman JA, Trohman RG. Incidence and electrocardiographic localization of safe right bundle branch block configurations during permanent ventricular pacing. Am J Cardiol 1995; 76:781–784.
- Klein HO, Beker B, Sareli P, DiSegni E, Dean H, Kaplinsky E. Unusual QRS morphology associated with transvenous pacemakers. The pseudo RBBB pattern. Chest 1985; 87:517–521.
- Almehairi M, Enriquez A, Redfearn D, et al. Right bundle branch block-like pattern during ventricular pacing: a surface electrocardiographic mapping technique to locate the ventricular lead. Can J Cardiol 2015; 31:1019–1024.
- Barold SS, Giudici MC. Renewed interest in the significance of the tall R wave in ECG lead V1 during right ventricular pacing. Expert Rev Med Devices 2016; 13:611–613.
- Almehairi M, Ali FS, Enriquez A, et al. Electrocardiographic algorithms to predict true right ventricular pacing in the presence of right bundle branch block-like pattern. Int J Cardiol 2014; 172:e403–e405.
- Tzeis S, Andrikopoulos G, Weigand S, et al. Right bundle branch block-like pattern during uncomplicated right ventricular pacing and the effect of pacing site. Am J Cardiol 2016; 117:935–939.
- Hemminger EJ, Criley JM. Right ventricular enlargement mimicking electrocardiographic left ventricular pacing. J Electrocardiol 2006; 39:180–182.
- Furman S. Chest PA and lateral. Pacing Clin Electrophysiol 1993; 16:953.
- Trohman RG, Wilkoff BL, Byrne T, Cook S. Successful percutaneous extraction of a chronic left ventricular pacing lead. Pacing Clin Electrophysiol 1991; 14:1448–1451.
- Trohman RG, Kim MH, Pinski SL. Cardiac pacing: the state of the art. Lancet 2004; 364:1701–1719.
- Van Gelder BM, Bracke FA, Oto A, et al. Diagnosis and management of inadvertently placed pacing and ICD leads in the left ventricle: a multicenter experience and review of the literature. Pacing Clin Electrophysiol 2000; 23:877–883.
- Kosmidou I, Karmpaliotis D, Kandzari DE, Dan D. Inadvertent transarterial lead placement in the left ventricle and aortic cusp: percutaneous lead removal with carotid embolic protection and stent graft placement. Indian Pacing Electrophysiol J 2012; 12:269–273.
- Villanueva FS, Heinsimer JA, Burkman MH, Fananapazir L,
- Halvorsen RA Jr, Chen JT. Echocardiographic detection of perforation of the cardiac ventricular septum by a permanent pacemaker lead. Am J Cardiol 1987; 59:370–371.
- Döring M, Braunschweig F, Eitel C, et al. Individually tailored left ventricular lead placement: lessons from multimodality integration between three-dimensional echocardiography and coronary sinus angiogram. Europace 2013; 15:718–727.
- Mediratta A, Addetia K, Yamat M, et al. 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc Imaging 2014; 7:337–347.
- Daher IN, Saeed M, Schwarz ER, Agoston I, Rahman MA, Ahmad M. Live three-dimensional echocardiography in diagnosis of interventricular septal perforation by pacemaker lead. Echocardiography 2006; 23:428–429.
- Mak GS, Truong QA. Cardiac CT: imaging of and through cardiac devices. Curr Cardiovasc Imaging Rep 2012; 5:328–336.
- Henrikson CA, Leng CT, Yuh DD, Brinker JA. Computed tomography to assess possible cardiac lead perforation. Pacing Clin Electrophysiol 2006; 29:509–511.
- Hirschl DA, Jain VR, Spindola-Franco H, Gross JN, Haramati LB. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing Clin Electrophysiol 2007; 30:28–32.
- Pang BJ, Lui EH, Joshi SB, et al. Pacing and implantable cardioverter defibrillator lead perforation as assessed by multiplanar reformatted ECG-gated cardiac computed tomography and clinical correlates. Pacing Clin Electrophysiol 2014; 37:537–545.
- Lanzman RS, Winter J, Blondin D, et al. Where does it lead? Imaging features of cardiovascular implantable electronic devices on chest radiograph and CT. Korean J Radiol 2011; 12:611–619.
- van der Graaf AW, Bhagirath P, Götte MJ. MRI and cardiac implantable electronic devices; current status and required safety conditions. Neth Heart J 2014; 22:269–276.
- European Society of Cardiology (ESC), European Heart Rhythm Association (EHRA); Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013; 15:1070–1118.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Rodriguez Y, Baltodano P, Tower A, Martinez C, Carrillo R. Management of symptomatic inadvertently placed endocardial leads in the left ventricle. Pacing Clin Electrophysiol 2011; 34:1192–1200.
- Ohlow MA, Roos M, Lauer B, Von Korn H, Geller JC. Incidence, predictors, and outcome of inadvertent malposition of transvenous pacing or defibrillation lead in the left heart. Europace 2016; 18:1049–1054.
- Madias C, Trohman RG. Cardiac resynchronization therapy: the state of the art. Expert Rev Cardiovasc Ther 2014; 12:573–587.
- Trohman RG. To the editor—comment on six uneventful years with a pacing lead in the left ventricle. Heart Rhythm 2013; 10:e81.
- Cossú SF. Unusual placement of a coronary sinus lead for resynchronization therapy resulting in late lead fracture. J Innovations Cardiac Rhythm Manage 2013; 4:1148–1153.
- Coman JA, Trohman RG. Incidence and electrocardiographic localization of safe right bundle branch block configurations during permanent ventricular pacing. Am J Cardiol 1995; 76:781–784.
- Klein HO, Beker B, Sareli P, DiSegni E, Dean H, Kaplinsky E. Unusual QRS morphology associated with transvenous pacemakers. The pseudo RBBB pattern. Chest 1985; 87:517–521.
- Almehairi M, Enriquez A, Redfearn D, et al. Right bundle branch block-like pattern during ventricular pacing: a surface electrocardiographic mapping technique to locate the ventricular lead. Can J Cardiol 2015; 31:1019–1024.
- Barold SS, Giudici MC. Renewed interest in the significance of the tall R wave in ECG lead V1 during right ventricular pacing. Expert Rev Med Devices 2016; 13:611–613.
- Almehairi M, Ali FS, Enriquez A, et al. Electrocardiographic algorithms to predict true right ventricular pacing in the presence of right bundle branch block-like pattern. Int J Cardiol 2014; 172:e403–e405.
- Tzeis S, Andrikopoulos G, Weigand S, et al. Right bundle branch block-like pattern during uncomplicated right ventricular pacing and the effect of pacing site. Am J Cardiol 2016; 117:935–939.
- Hemminger EJ, Criley JM. Right ventricular enlargement mimicking electrocardiographic left ventricular pacing. J Electrocardiol 2006; 39:180–182.
- Furman S. Chest PA and lateral. Pacing Clin Electrophysiol 1993; 16:953.
- Trohman RG, Wilkoff BL, Byrne T, Cook S. Successful percutaneous extraction of a chronic left ventricular pacing lead. Pacing Clin Electrophysiol 1991; 14:1448–1451.
- Trohman RG, Kim MH, Pinski SL. Cardiac pacing: the state of the art. Lancet 2004; 364:1701–1719.
- Van Gelder BM, Bracke FA, Oto A, et al. Diagnosis and management of inadvertently placed pacing and ICD leads in the left ventricle: a multicenter experience and review of the literature. Pacing Clin Electrophysiol 2000; 23:877–883.
- Kosmidou I, Karmpaliotis D, Kandzari DE, Dan D. Inadvertent transarterial lead placement in the left ventricle and aortic cusp: percutaneous lead removal with carotid embolic protection and stent graft placement. Indian Pacing Electrophysiol J 2012; 12:269–273.
- Villanueva FS, Heinsimer JA, Burkman MH, Fananapazir L,
- Halvorsen RA Jr, Chen JT. Echocardiographic detection of perforation of the cardiac ventricular septum by a permanent pacemaker lead. Am J Cardiol 1987; 59:370–371.
- Döring M, Braunschweig F, Eitel C, et al. Individually tailored left ventricular lead placement: lessons from multimodality integration between three-dimensional echocardiography and coronary sinus angiogram. Europace 2013; 15:718–727.
- Mediratta A, Addetia K, Yamat M, et al. 3D echocardiographic location of implantable device leads and mechanism of associated tricuspid regurgitation. JACC Cardiovasc Imaging 2014; 7:337–347.
- Daher IN, Saeed M, Schwarz ER, Agoston I, Rahman MA, Ahmad M. Live three-dimensional echocardiography in diagnosis of interventricular septal perforation by pacemaker lead. Echocardiography 2006; 23:428–429.
- Mak GS, Truong QA. Cardiac CT: imaging of and through cardiac devices. Curr Cardiovasc Imaging Rep 2012; 5:328–336.
- Henrikson CA, Leng CT, Yuh DD, Brinker JA. Computed tomography to assess possible cardiac lead perforation. Pacing Clin Electrophysiol 2006; 29:509–511.
- Hirschl DA, Jain VR, Spindola-Franco H, Gross JN, Haramati LB. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. Pacing Clin Electrophysiol 2007; 30:28–32.
- Pang BJ, Lui EH, Joshi SB, et al. Pacing and implantable cardioverter defibrillator lead perforation as assessed by multiplanar reformatted ECG-gated cardiac computed tomography and clinical correlates. Pacing Clin Electrophysiol 2014; 37:537–545.
- Lanzman RS, Winter J, Blondin D, et al. Where does it lead? Imaging features of cardiovascular implantable electronic devices on chest radiograph and CT. Korean J Radiol 2011; 12:611–619.
- van der Graaf AW, Bhagirath P, Götte MJ. MRI and cardiac implantable electronic devices; current status and required safety conditions. Neth Heart J 2014; 22:269–276.
- European Society of Cardiology (ESC), European Heart Rhythm Association (EHRA); Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013; 15:1070–1118.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
KEY POINTS
- During device implantation, fluoroscopy in progressively lateral left anterior oblique views should be used to ensure correct lead position.
- After implantation, malposition can almost always be detected promptly by examining a 12-lead electrocardiogram for the paced QRS morphology and by lateral chest radiography.
- Echocardiography and computed tomography may enhance diagnostic accuracy and clarify equivocal findings.
- Late surgical correction of a malpositioned lead is best done when a patient is undergoing cardiac surgery for other reasons.
- Long-term warfarin therapy is recommended to prevent thromboembolism if malpositioning cannot be corrected.
A New Year’s transition and looking forward
Dr. Cosgrove took the leadership reins of the Clinic in 2004, the same year Dr. Mihaljevic joined the Department of Cardiothoracic Surgery. Under Dr. Cosgrove’s leadership the Clinic has grown in size, scope of practice, and international impact. His support of education has contributed enormously to the maturation of the Cleveland Clinic Lerner College of Medicine, the continued successes of our sizeable postgraduate education training program, and many other activities including our CME Center and the Cleveland Clinic Journal of Medicine. His willingness to recognize and continue to subsidize the Journal as an educational vehicle, with no direct marketing intent, has permitted the Journal to thrive in the international medical education space as a leading purveyor of sound, practical, evidence-based medical information. I speak for our editorial staff, authors, and readers when I say, “Thank you, Toby, for your support, trust, and belief in our educational mission.”
Dr. Mihaljevic is also a notable cardiothoracic surgeon, widely recognized for his skills and expertise in innovative minimally invasive and robotic-assisted cardiac valve surgery. He has returned to our Cleveland campus after several years as CEO of Cleveland Clinic Abu Dhabi. We welcome him back in his new role.
As Cleveland Clinic leadership undergoes an expected smooth transition, healthcare in the United States seems perpetually stuck trying to balance the response to a plethora of scientific and clinical advances, the rapid technologic changes in healthcare delivery systems, the cost-profit distribution within and external to expanding healthcare systems, and divergent social and political pressures. Advances in molecular medicine are changing the diagnosis and therapy of cancers and inflammatory diseases. Personalized precision medicine is evolving from the abstract to the tangible. Surgical advances on a true macro scale are leading to deliverable, effective treatments of the metabolic manifestations of diabetes, while microscopic, intravascular, and minimally invasive approaches are transforming the management of patients with structural and infiltrative disease. Understanding of the microbiome may well lead to better management of cardiovascular and inflammatory diseases. There have been advances in tissue scaffolding as well as gene and cell replacement techniques that may soon transform the therapy of several diseases. These advances provide cause for intellectual and clinical enthusiasm.
And yet, the environment in which we live and practice is increasingly divided and divisive socially and politically. Medicine has lost much of its luster. Burnout and early retirement are adversely affecting the physician workforce. The current model of financial support for medical education in the United States is being reevaluated, without a clear effective alternative. Costs of healthcare are rising at unsustainable rates, and swathes of our vulnerable, elderly, and young middle-class population are faced with serious challenges in getting and maintaining medical care because it is inaccessible and unaffordable. Even for patients of comfortable financial means, acquiring health insurance is not an activity for the weak of heart (and that weakness might be interpreted in the future as a pre-existing condition).
Who will pay for the exciting innovations I noted above, and who will deliver them? As reimbursement is shrinking, the time demands for physician electronic charting and communications with insurance companies are increasing. More physicians are employed and controlled by healthcare systems. How many will have the time and updated knowledge to discuss the appropriateness and clinical implications of these therapies between the phone calls begging for insurance company approval of coverage and payment?
As corporate taxes appear on the brink of being reduced, we can hope that this corporate financial benefit will translate to reduced drug and device costs and more affordable insurance for our more vulnerable populations. But this is not certain.
I have concerns as to how clinical science and healthcare delivery can move forward in an environment in which federal directives now prohibit our most respected federal research agencies from using such terms as “vulnerable” (populations) and “evidence-based” to justify their proposals for budgetary support for their ongoing work in population disease health and disease management.1 Even a short time spent in the hallways or emergency rooms of any of our safety-net hospitals reveals the strain that acute and chronic illness is imposing on the social fabric of families, society, and the often underfunded infrastructure of this aspect of our healthcare system. Who will be in the position to empathetically and objectively assess the value of translating these ongoing efforts in discovery to implementation?
Basic stem cell and genetic research is also under ongoing scrutiny. There remains legitimate fear that ultimate policy decisions will not be made by fully informed scientists and ethicists. The ongoing “dialogue” in the United States around climate change and global warming does not give me confidence that our current government policy-makers are up to the task of objectively dealing with these more nuanced and emotionally charged issues, particularly while avoiding the expression of any evidence-based rationales.
In 2016, the world lost the iconic musical poet Leonard Cohen. Hopefully, he got it right when he wrote:
Ring the bells that still can ring
Forget your perfect offering
There is a crack in everything
That’s how the light gets in
—“Anthem”; 1992
I and the rest of our editorial team wish you, our readers, a healthy and peaceful 2018. I am optimistic that we can all find or create at least some light.
- Sun LH, Eilperin J. CDC gets list of forbidden words: fetus, transgender, diversity. The Washington Post December 15, 2017.
Dr. Cosgrove took the leadership reins of the Clinic in 2004, the same year Dr. Mihaljevic joined the Department of Cardiothoracic Surgery. Under Dr. Cosgrove’s leadership the Clinic has grown in size, scope of practice, and international impact. His support of education has contributed enormously to the maturation of the Cleveland Clinic Lerner College of Medicine, the continued successes of our sizeable postgraduate education training program, and many other activities including our CME Center and the Cleveland Clinic Journal of Medicine. His willingness to recognize and continue to subsidize the Journal as an educational vehicle, with no direct marketing intent, has permitted the Journal to thrive in the international medical education space as a leading purveyor of sound, practical, evidence-based medical information. I speak for our editorial staff, authors, and readers when I say, “Thank you, Toby, for your support, trust, and belief in our educational mission.”
Dr. Mihaljevic is also a notable cardiothoracic surgeon, widely recognized for his skills and expertise in innovative minimally invasive and robotic-assisted cardiac valve surgery. He has returned to our Cleveland campus after several years as CEO of Cleveland Clinic Abu Dhabi. We welcome him back in his new role.
As Cleveland Clinic leadership undergoes an expected smooth transition, healthcare in the United States seems perpetually stuck trying to balance the response to a plethora of scientific and clinical advances, the rapid technologic changes in healthcare delivery systems, the cost-profit distribution within and external to expanding healthcare systems, and divergent social and political pressures. Advances in molecular medicine are changing the diagnosis and therapy of cancers and inflammatory diseases. Personalized precision medicine is evolving from the abstract to the tangible. Surgical advances on a true macro scale are leading to deliverable, effective treatments of the metabolic manifestations of diabetes, while microscopic, intravascular, and minimally invasive approaches are transforming the management of patients with structural and infiltrative disease. Understanding of the microbiome may well lead to better management of cardiovascular and inflammatory diseases. There have been advances in tissue scaffolding as well as gene and cell replacement techniques that may soon transform the therapy of several diseases. These advances provide cause for intellectual and clinical enthusiasm.
And yet, the environment in which we live and practice is increasingly divided and divisive socially and politically. Medicine has lost much of its luster. Burnout and early retirement are adversely affecting the physician workforce. The current model of financial support for medical education in the United States is being reevaluated, without a clear effective alternative. Costs of healthcare are rising at unsustainable rates, and swathes of our vulnerable, elderly, and young middle-class population are faced with serious challenges in getting and maintaining medical care because it is inaccessible and unaffordable. Even for patients of comfortable financial means, acquiring health insurance is not an activity for the weak of heart (and that weakness might be interpreted in the future as a pre-existing condition).
Who will pay for the exciting innovations I noted above, and who will deliver them? As reimbursement is shrinking, the time demands for physician electronic charting and communications with insurance companies are increasing. More physicians are employed and controlled by healthcare systems. How many will have the time and updated knowledge to discuss the appropriateness and clinical implications of these therapies between the phone calls begging for insurance company approval of coverage and payment?
As corporate taxes appear on the brink of being reduced, we can hope that this corporate financial benefit will translate to reduced drug and device costs and more affordable insurance for our more vulnerable populations. But this is not certain.
I have concerns as to how clinical science and healthcare delivery can move forward in an environment in which federal directives now prohibit our most respected federal research agencies from using such terms as “vulnerable” (populations) and “evidence-based” to justify their proposals for budgetary support for their ongoing work in population disease health and disease management.1 Even a short time spent in the hallways or emergency rooms of any of our safety-net hospitals reveals the strain that acute and chronic illness is imposing on the social fabric of families, society, and the often underfunded infrastructure of this aspect of our healthcare system. Who will be in the position to empathetically and objectively assess the value of translating these ongoing efforts in discovery to implementation?
Basic stem cell and genetic research is also under ongoing scrutiny. There remains legitimate fear that ultimate policy decisions will not be made by fully informed scientists and ethicists. The ongoing “dialogue” in the United States around climate change and global warming does not give me confidence that our current government policy-makers are up to the task of objectively dealing with these more nuanced and emotionally charged issues, particularly while avoiding the expression of any evidence-based rationales.
In 2016, the world lost the iconic musical poet Leonard Cohen. Hopefully, he got it right when he wrote:
Ring the bells that still can ring
Forget your perfect offering
There is a crack in everything
That’s how the light gets in
—“Anthem”; 1992
I and the rest of our editorial team wish you, our readers, a healthy and peaceful 2018. I am optimistic that we can all find or create at least some light.
Dr. Cosgrove took the leadership reins of the Clinic in 2004, the same year Dr. Mihaljevic joined the Department of Cardiothoracic Surgery. Under Dr. Cosgrove’s leadership the Clinic has grown in size, scope of practice, and international impact. His support of education has contributed enormously to the maturation of the Cleveland Clinic Lerner College of Medicine, the continued successes of our sizeable postgraduate education training program, and many other activities including our CME Center and the Cleveland Clinic Journal of Medicine. His willingness to recognize and continue to subsidize the Journal as an educational vehicle, with no direct marketing intent, has permitted the Journal to thrive in the international medical education space as a leading purveyor of sound, practical, evidence-based medical information. I speak for our editorial staff, authors, and readers when I say, “Thank you, Toby, for your support, trust, and belief in our educational mission.”
Dr. Mihaljevic is also a notable cardiothoracic surgeon, widely recognized for his skills and expertise in innovative minimally invasive and robotic-assisted cardiac valve surgery. He has returned to our Cleveland campus after several years as CEO of Cleveland Clinic Abu Dhabi. We welcome him back in his new role.
As Cleveland Clinic leadership undergoes an expected smooth transition, healthcare in the United States seems perpetually stuck trying to balance the response to a plethora of scientific and clinical advances, the rapid technologic changes in healthcare delivery systems, the cost-profit distribution within and external to expanding healthcare systems, and divergent social and political pressures. Advances in molecular medicine are changing the diagnosis and therapy of cancers and inflammatory diseases. Personalized precision medicine is evolving from the abstract to the tangible. Surgical advances on a true macro scale are leading to deliverable, effective treatments of the metabolic manifestations of diabetes, while microscopic, intravascular, and minimally invasive approaches are transforming the management of patients with structural and infiltrative disease. Understanding of the microbiome may well lead to better management of cardiovascular and inflammatory diseases. There have been advances in tissue scaffolding as well as gene and cell replacement techniques that may soon transform the therapy of several diseases. These advances provide cause for intellectual and clinical enthusiasm.
And yet, the environment in which we live and practice is increasingly divided and divisive socially and politically. Medicine has lost much of its luster. Burnout and early retirement are adversely affecting the physician workforce. The current model of financial support for medical education in the United States is being reevaluated, without a clear effective alternative. Costs of healthcare are rising at unsustainable rates, and swathes of our vulnerable, elderly, and young middle-class population are faced with serious challenges in getting and maintaining medical care because it is inaccessible and unaffordable. Even for patients of comfortable financial means, acquiring health insurance is not an activity for the weak of heart (and that weakness might be interpreted in the future as a pre-existing condition).
Who will pay for the exciting innovations I noted above, and who will deliver them? As reimbursement is shrinking, the time demands for physician electronic charting and communications with insurance companies are increasing. More physicians are employed and controlled by healthcare systems. How many will have the time and updated knowledge to discuss the appropriateness and clinical implications of these therapies between the phone calls begging for insurance company approval of coverage and payment?
As corporate taxes appear on the brink of being reduced, we can hope that this corporate financial benefit will translate to reduced drug and device costs and more affordable insurance for our more vulnerable populations. But this is not certain.
I have concerns as to how clinical science and healthcare delivery can move forward in an environment in which federal directives now prohibit our most respected federal research agencies from using such terms as “vulnerable” (populations) and “evidence-based” to justify their proposals for budgetary support for their ongoing work in population disease health and disease management.1 Even a short time spent in the hallways or emergency rooms of any of our safety-net hospitals reveals the strain that acute and chronic illness is imposing on the social fabric of families, society, and the often underfunded infrastructure of this aspect of our healthcare system. Who will be in the position to empathetically and objectively assess the value of translating these ongoing efforts in discovery to implementation?
Basic stem cell and genetic research is also under ongoing scrutiny. There remains legitimate fear that ultimate policy decisions will not be made by fully informed scientists and ethicists. The ongoing “dialogue” in the United States around climate change and global warming does not give me confidence that our current government policy-makers are up to the task of objectively dealing with these more nuanced and emotionally charged issues, particularly while avoiding the expression of any evidence-based rationales.
In 2016, the world lost the iconic musical poet Leonard Cohen. Hopefully, he got it right when he wrote:
Ring the bells that still can ring
Forget your perfect offering
There is a crack in everything
That’s how the light gets in
—“Anthem”; 1992
I and the rest of our editorial team wish you, our readers, a healthy and peaceful 2018. I am optimistic that we can all find or create at least some light.
- Sun LH, Eilperin J. CDC gets list of forbidden words: fetus, transgender, diversity. The Washington Post December 15, 2017.
- Sun LH, Eilperin J. CDC gets list of forbidden words: fetus, transgender, diversity. The Washington Post December 15, 2017.