User login
High users of healthcare: Strategies to improve care, reduce costs
Emergency departments are not primary care clinics, but some patients use them that way. This relatively small group of patients consumes a disproportionate share of healthcare at great cost, earning them the label of “high users.” Mostly poor and often burdened with mental illness and addiction, they are not necessarily sicker than other patients, and they do not enjoy better outcomes from the extra money spent on them. (Another subset of high users, those with end-stage chronic disease, is outside the scope of this review.)
Herein lies an opportunity. If—and this is a big if—we could manage their care in a systematic way instead of haphazardly, proactively instead of reactively, with continuity of care instead of episodically, and in a way that is convenient for the patient, we might be able to improve quality and save money.
A DISPROPORTIONATE SHARE OF COSTS
In the United States in 2012, the 5% of the population who were the highest users were responsible for 50% of healthcare costs.1 The mean cost per person in this group was more than $43,000 annually. The top 1% of users accounted for nearly 23% of all expenditures, averaging nearly $98,000 per patient per year—10 times more than the average yearly cost per patient.
CARE IS OFTEN INAPPROPRIATE AND UNNECESSARY
In addition to being disproportionately expensive, the care that these patients receive is often inappropriate and unnecessary for the severity of their disease.
A 2007–2009 study2 of 1,969 patients who had visited the emergency department 10 or more times in a year found they received more than twice as many computed tomography (CT) scans as a control group of infrequent users (< 3 visits/year). This occurred even though they were not as sick as infrequent users, based on significantly lower hospital admission rates (11.1% vs 17.9%; P < .001) and mortality rates (0.7% vs 1.5%; P < .002).2
This inverse relationship between emergency department use and illness severity was even more exaggerated at the upper extreme of the use curve. The highest users (> 29 visits to the emergency department in a year) had the lowest triage acuity and hospital admission rates but the highest number of CT scans. Charges per visit were lower among frequent users, but total charges rose steadily with increasing emergency department use, accounting for significantly more costs per year.2
We believe that one reason these patients receive more medical care than necessary is because their medical records are too large and complex for the average physician to distill effectively in a 20-minute physician-patient encounter. Physicians therefore simply order more tests, procedures, and admissions, which are often medically unnecessary and redundant.
WHAT DRIVES HIGH COST?
Mental illness and chemical dependence
Drug addiction, mental illness, and poverty frequently accompany (and influence) high-use behavior, particularly in patients without end-stage diseases.
Szekendi et al,3 in a study of 28,291 patients who had been admitted at least 5 times in a year in a Chicago health system, found that these high users were 2 to 3 times more likely to suffer from comorbid depression (40% vs 13%), psychosis (18% vs 5%), recreational drug dependence (20% vs 7%), and alcohol abuse (16% vs 7%) than non-high-use hospitalized patients.3
Mercer et al4 conducted a study at Duke University Medical Center, Durham, NC, aimed at reducing emergency department visits and hospital admissions among 24 of its highest users. They found that 23 (96%) were either addicted to drugs or mentally ill, and 20 (83%) suffered from chronic pain.4
Drug abuse among high users is becoming even more relevant as the opioid epidemic worsens. Given that most patients requiring high levels of care suffer from chronic pain and many of them develop an opioid addiction while treating their pain, physicians have a moral imperative to reduce the prevalence of drug abuse in this population.
Low socioeconomic status
Low socioeconomic status is an important factor among high users, as it is highly associated with greater disease severity, which usually increases cost without any guarantee of an associated increase in quality. Data suggest that patients of low socioeconomic status are twice as likely to require urgent emergency department visits, 4 times as likely to require admission to the hospital, and, importantly, about half as likely to use ambulatory care compared with patients of higher socioeconomic status.5 While this pattern of low-quality, high-cost spending in acute care settings reflects spending in the healthcare system at large, the pattern is greatly exaggerated among high users.
Lost to follow-up
Low socioeconomic status also complicates communication and follow-up. In a 2013 study, physician researchers in St. Paul, MN, documented attempts to interview 64 recently discharged high users. They could not reach 47 (73%) of them, for reasons largely attributable to low socioeconomic status, such as disconnected phone lines and changes in address.6
Clearly, the usual contact methods for follow-up care after discharge, such as phone calls and mailings, are unlikely to be effective in coordinating the outpatient care of these individuals.
Additionally, we must find ways of making primary care more convenient, gaining our patients’ trust, and finding ways to engage patients in follow-up without relying on traditional means of communication.
Do high users have medical insurance?
Surprisingly, most high users of the emergency department have health insurance. The Chicago health system study3 found that most (72.4%) of their high users had either Medicare or private health insurance, while 27.6% had either Medicaid or no insurance (compared with 21.6% in the general population). Other studies also found that most of the frequent emergency department users are insured,7 although the overall percentage who rely on publicly paid insurance is greater than in the population at large.
Many prefer acute care over primary care
Although one might think that high users go to the emergency department because they have nowhere else to go for care, a report published in 2013 by Kangovi et al5 suggests another reason—they prefer the emergency department.5 They interviewed 40 urban patients of low socioeconomic status who consistently cited the 24-hour, no-appointment-necessary structure of the emergency department as an advantage over primary care. The flexibility of emergency access to healthcare makes sense if one reflects on how difficult it is for even high-functioning individuals to schedule and keep medical appointments.
Specific reasons for preferring the emergency department included the following:
Affordability. Even if their insurance fully paid for visits to their primary care physicians, the primary care physician was likely to refer them to specialists, whose visits required a copay, and which required taking another day off of work. The emergency department is cheaper for the patient and it is a “one-stop shop.” Patients appreciated the emergency department guarantee of seeing a physician regardless of proof of insurance, a policy not guaranteed in primary care and specialist offices.
Accessibility. For those without a car, public transportation and even patient transportation services are inconvenient and unreliable, whereas emergency medical services will take you to the emergency department.
Accommodations. Although medical centers may tout their same-day appointments, often same-day appointments are all that they have—and you have no choice about the time. You have to call first thing in the morning and stay on hold for a long time, and then when you finally get through, all the same-day appointments are gone.
Availability. Patients said they often had a hard time getting timely medical advice from their primary care physicians. When they could get through to their primary care physicians on the phone, they would be told to go to the emergency department.
Acceptability. Men, especially, feel they need to be very sick indeed to seek medical care, so going to the emergency department is more acceptable.
Trust in the provider. For reasons that were not entirely clear, patients felt that acute care providers were more trustworthy, competent, and compassionate than primary care physicians.5
None of these reasons for using the emergency department has anything to do with disease severity, which supports the findings that high users of the emergency department were not as sick as their normal-use peers.2
QUALITY IMPROVEMENT AND COST-REDUCTION STRATEGIES
Efforts are being made to reduce the cost of healthcare for high users while improving the quality of their care. Promising strategies focus on coordinating care management, creating individualized patient care plans, and improving the components and instructions of discharge summaries.
Care management organizations
A care management organization (CMO) model has emerged as a strategy for quality improvement and cost reduction in the high-use population. In this model, social workers, health coaches, nurses, mid-level providers, and physicians collaborate on designing individualized care plans to meet the specific needs of patients.
Teams typically work in stepwise fashion, first identifying and engaging patients at high risk of poor outcomes and unnecessary care, often using sophisticated quantitative, risk-prediction tools. Then, they perform health assessments and identify potential interventions aimed at preventing expensive acute-care medical interventions. Third, they work with patients to rapidly identify and effectively respond to changes in their conditions and direct them to the most appropriate medical setting, typically primary or urgent care.
Effective models
In 1998, the Camden (NJ) Coalition of Healthcare Providers established a model for CMO care plans. Starting with the first 36 patients enrolled in the program, hospital admissions and emergency department visits were cut by 47% (from 62 to 37 per month), and collective hospital costs were cut by 56% (from $1.2 million to about $500,000 per month).8 It should be noted that this was a small, nonrandomized study and these preliminary numbers did not take into account the cost of outpatient physician visits or new medications. Thus, how much money this program actually saves is not clear.
Similar programs have had similar results. A nurse-led care coordination program in Doylestown, PA, showed an impressive 25% reduction in annual mortality and a 36% reduction in overall costs during a 10-year period.9
A program in Atlantic City, NJ, combined the typical CMO model with a primary care clinic to provide high users with unlimited access, while paying its providers in a capitation model (as opposed to fee for service). It achieved a 40% reduction in yearly emergency department visits and hospital admissions.8
Patient care plans
Individualized patient care plans for high users are among the most promising tools for reducing costs and improving quality in this group. They are low-cost and relatively easy to implement. The goal of these care plans is to provide practitioners with a concise care summary to help them make rational and consistent medical decisions.
Typically, a care plan is written by an interdisciplinary committee composed of physicians, nurses, and social workers. It is based on the patient’s pertinent medical and psychiatric history, which may include recent imaging results or other relevant diagnostic tests. It provides suggestions for managing complex chronic issues, such as drug abuse, that lead to high use of healthcare resources.
These care plans provide a rational and prespecified approach to workup and management, typically including a narcotic prescription protocol, regardless of the setting or the number of providers who see the patient. Practitioners guided by effective care plans are much more likely to effectively navigate a complex patient encounter as opposed to looking through extensive medical notes and hoping to find relevant information.
Effective models
Data show these plans can be effective. For example, Regions Hospital in St. Paul, MN, implemented patient care plans in 2010. During the first 4 months, hospital admissions in the first 94 patients were reduced by 67%.10
A study of high users at Duke University Medical Center reported similar results. One year after starting care plans, inpatient admissions had decreased by 50.5%, readmissions had decreased by 51.5%, and variable direct costs per admission were reduced by 35.8%. Paradoxically, emergency department visits went up, but this anomaly was driven by 134 visits incurred by a single dialysis patient. After removing this patient from the data, emergency department visits were relatively stable.4
Better discharge summaries
Although improving discharge summaries is not a novel concept, changing the summary from a historical document to a proactive discharge plan has the potential to prevent readmissions and promote a durable de-escalation in care acuity.
For example, when moving a patient to a subacute care facility, providing a concise summary of which treatments worked and which did not, a list of comorbidities, and a list of medications and strategies to consider, can help the next providers to better target their plan of care. Studies have shown that nearly half of discharge statements lack important information on treatments and tests.11
Improvement can be as simple as encouraging practitioners to construct their summaries in an “if-then” format. Instead of noting for instance that “Mr. Smith was treated for pneumonia with antibiotics and discharged to a rehab facility,” the following would be more useful: “Family would like to see if Mr. Smith can get back to his functional baseline after his acute pneumonia. If he clinically does not do well over the next 1 to 2 weeks and has a poor quality of life, then family would like to pursue hospice.”
In addition to shifting the philosophy, we believe that providing timely discharge summaries is a fundamental, high-yield aspect of ensuring their effectiveness. As an example, patients being discharged to a skilled nursing facility should have a discharge summary completed and in hand before leaving the hospital.
Evidence suggests that timely writing of discharge summaries improves their quality. In a retrospective cohort study published in 2012, discharge summaries created more than 24 hours after discharge were less likely to include important plan-of-care components.12
FUTURE NEEDS
Randomized trials
Although initial results have been promising for the strategies outlined above, much of the apparent cost reduction of these interventions may be at least partially related to the study design as opposed to the interventions themselves.
For example, Hong et al13 examined 18 of the more promising CMOs that had reported initial cost savings. Of these, only 4 had conducted randomized controlled trials. When broken down further, the initial cost reduction reported by most of these randomized controlled trials was generated primarily by small subgroups.14
These results, however, do not necessarily reflect an inherent failure in the system. We contend that they merely demonstrate that CMOs and care plan administrators need to be more selective about whom they enroll, either by targeting patients at the extremes of the usage curve or by identifying patient characteristics and usage parameters amenable to cost reduction and quality improvement strategies.
Better social infrastructure
Although patient care plans and CMOs have been effective in managing high users, we believe that the most promising quality improvement and cost-reduction strategy involves redirecting much of the expensive healthcare spending to the social determinants of health (eg, homelessness, mental illness, low socioeconomic status).
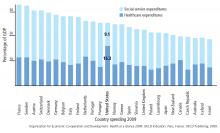
Among developed countries, the United States has the highest healthcare spending and the lowest social service spending as a percentage of its gross domestic product (Figure 1).15 Although seemingly discouraging, these data can actually be interpreted as hopeful, as they support the notion that the inefficiencies of our current system are not part of an inescapable reality, but rather reflect a system that has evolved uniquely in this country.
Using the available social programs
Exemplifying this medical and social services balance is a high user who visited her local emergency department 450 times in 1 year for reasons primarily related to homelessness.16 Each time, the medical system (as it is currently designed to do) applied a short-term medical solution to this patient’s problems and discharged her home, ie, back to the street.
But this patient’s high use was really a manifestation of a deeper social issue: homelessness. When the medical staff eventually noted how much this lack of stable shelter was contributing to her pattern of use, she was referred to appropriate social resources and provided with the housing she needed. Her hospital visits decreased from 450 to 12 in the subsequent year, amounting to a huge cost reduction and a clear improvement in her quality of life.
Similar encouraging results have resulted when available social programs are applied to the high-use population at large, which is particularly reassuring given this population’s preponderance of low socioeconomic status, mental illness, and homelessness. (The prevalence of homelessness is roughly 20%, depending on the definition of a high user).
New York Medicaid, for example, has a housing program that provides stable shelter outside of acute care medical settings for patients at a rate as low as $50 per day, compared with area hospital costs that often exceed $2,200 daily.17 A similar program in Westchester County, NY, reported a 45.9% reduction in inpatient costs and a 15.4% reduction in emergency department visits among 61 of its highest users after 2 years of enrollment.17
Need to reform privacy laws
Although legally daunting, reform of the Health Insurance Portability and Accountability Act (HIPAA) and other privacy laws in favor of a more open model of information sharing, particularly for high-risk patients, holds great opportunity for quality improvement. For patients who obtain their care from several healthcare facilities, the documentation is often inscrutable. If some of the HIPAA regulations and other patient privacy laws were exchanged for rules more akin to the current model of narcotic prescription tracking, for example, physicians would be better equipped to provide safe, organized, and efficient medical care for high-use patients.
Need to reform the system
A fundamental flaw in our healthcare system, which is largely based on a fee-for-service model, is that it was not designed for patients who use the system at the highest frequency and greatest cost. Also, it does not account for the psychosocial factors that beset many high-use patients. As such, it is imperative for the safety of our patients as well as the viability of the healthcare system that we change our historical way of thinking and reform this system that provides high users with care that is high-cost, low-quality, and not patient-centered.
IMPROVING QUALITY, REDUCING COST
High users of emergency services are a medically and socially complex group, predominantly characterized by low socioeconomic status and high rates of mental illness and drug dependency. Despite their increased healthcare use, they do not have better outcomes even though they are not sicker. Improving those outcomes requires both medical and social efforts.
Among the effective medical efforts are strategies aimed at creating individualized patient care plans, using coordinated care teams, and improving discharge summaries. Addressing patients’ social factors, such as homelessness, is more difficult, but healthcare systems can help patients navigate the available social programs. These strategies are part of a comprehensive care plan that can help reduce the cost and improve the quality of healthcare for high users.
- Cohen SB; Agency for Healthcare Research and Quality. Statistical Brief #359. The concentration of health care expenditures and related expenses for costly medical conditions, 2009. http://meps.ahrq.gov/mepsweb/data_files/publications/st359/stat359.pdf. Accessed December 18, 2017.
- Oostema J, Troost J, Schurr K, Waller R. High and low frequency emergency department users: a comparative analysis of morbidity, diagnostic testing, and health care costs. Ann Emerg Med 2011; 58:S225. Abstract 142.
- Szekendi MK, Williams MV, Carrier D, Hensley L, Thomas S, Cerese J. The characteristics of patients frequently admitted to academic medical centers in the United States. J Hosp Med 2015; 10:563–568.
- Mercer T, Bae J, Kipnes J, Velazquez M, Thomas S, Setji N. The highest utilizers of care: individualized care plans to coordinate care, improve healthcare service utilization, and reduce costs at an academic tertiary care center. J Hosp Med 2015; 10:419–424.
- Kangovi S, Barg FK, Carter T, Long JA, Shannon R, Grande D. Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood) 2013; 32:1196–1203.
- Melander I, Winkelman T, Hilger R. Analysis of high utilizers’ experience with specialized care plans. J Hosp Med 2014; 9(suppl 2):Abstract 229.
- LaCalle EJ, Rabin EJ, Genes NG. High-frequency users of emergency department care. J Emerg Med 2013; 44:1167–1173.
- Gawande A. The Hot Spotters. The New Yorker 2011. www.newyorker.com/magazine/2011/01/24/the-hot-spotters. Accessed December 18, 2017.
- Coburn KD, Marcantonio S, Lazansky R, Keller M, Davis N. Effect of a community-based nursing intervention on mortality in chronically ill older adults: a randomized controlled trial. PLoS Med 2012; 9:e1001265.
- Hilger R, Melander I, Winkelman T. Is specialized care plan work sustainable? A follow-up on healthpartners’ experience with patients who are high-utilizers. Society of Hospital Medicine Annual Meeting, Las Vegas, NV. March 24-27, 2014. www.shmabstracts.com/abstract/is-specialized-care-plan-work-sustainable-a-followup-on-healthpartners-experience-with-patients-who-are-highutilizers. Accessed December 18, 2017.
- Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA 2007; 297:831–841.
- Kind AJ, Thorpe CT, Sattin JA, Walz SE, Smith MA. Provider characteristics, clinical-work processes and their relationship to discharge summary quality for sub-acute care patients. J Gen Intern Med 2012; 27:78–84.
- Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonwealth Fund) 2014; 19:1–19.
- Williams B. Limited effects of care management for high utilizers on total healthcare costs. Am J Managed Care 2015; 21:e244–e246.
- Organization for Economic Co-operation and Development. Health at a Glance 2009: OECD Indicators. Paris, France: OECD Publishing; 2009.
- Emeche U. Is a strategy focused on super-utilizers equal to the task of health care system transformation? Yes. Ann Fam Med 2015; 13:6–7.
- Burns J. Do we overspend on healthcare, underspend on social needs? Managed Care. http://ghli.yale.edu/news/do-we-overspend-health-care-underspend-social-needs. Accessed December 18, 2017.
Emergency departments are not primary care clinics, but some patients use them that way. This relatively small group of patients consumes a disproportionate share of healthcare at great cost, earning them the label of “high users.” Mostly poor and often burdened with mental illness and addiction, they are not necessarily sicker than other patients, and they do not enjoy better outcomes from the extra money spent on them. (Another subset of high users, those with end-stage chronic disease, is outside the scope of this review.)
Herein lies an opportunity. If—and this is a big if—we could manage their care in a systematic way instead of haphazardly, proactively instead of reactively, with continuity of care instead of episodically, and in a way that is convenient for the patient, we might be able to improve quality and save money.
A DISPROPORTIONATE SHARE OF COSTS
In the United States in 2012, the 5% of the population who were the highest users were responsible for 50% of healthcare costs.1 The mean cost per person in this group was more than $43,000 annually. The top 1% of users accounted for nearly 23% of all expenditures, averaging nearly $98,000 per patient per year—10 times more than the average yearly cost per patient.
CARE IS OFTEN INAPPROPRIATE AND UNNECESSARY
In addition to being disproportionately expensive, the care that these patients receive is often inappropriate and unnecessary for the severity of their disease.
A 2007–2009 study2 of 1,969 patients who had visited the emergency department 10 or more times in a year found they received more than twice as many computed tomography (CT) scans as a control group of infrequent users (< 3 visits/year). This occurred even though they were not as sick as infrequent users, based on significantly lower hospital admission rates (11.1% vs 17.9%; P < .001) and mortality rates (0.7% vs 1.5%; P < .002).2
This inverse relationship between emergency department use and illness severity was even more exaggerated at the upper extreme of the use curve. The highest users (> 29 visits to the emergency department in a year) had the lowest triage acuity and hospital admission rates but the highest number of CT scans. Charges per visit were lower among frequent users, but total charges rose steadily with increasing emergency department use, accounting for significantly more costs per year.2
We believe that one reason these patients receive more medical care than necessary is because their medical records are too large and complex for the average physician to distill effectively in a 20-minute physician-patient encounter. Physicians therefore simply order more tests, procedures, and admissions, which are often medically unnecessary and redundant.
WHAT DRIVES HIGH COST?
Mental illness and chemical dependence
Drug addiction, mental illness, and poverty frequently accompany (and influence) high-use behavior, particularly in patients without end-stage diseases.
Szekendi et al,3 in a study of 28,291 patients who had been admitted at least 5 times in a year in a Chicago health system, found that these high users were 2 to 3 times more likely to suffer from comorbid depression (40% vs 13%), psychosis (18% vs 5%), recreational drug dependence (20% vs 7%), and alcohol abuse (16% vs 7%) than non-high-use hospitalized patients.3
Mercer et al4 conducted a study at Duke University Medical Center, Durham, NC, aimed at reducing emergency department visits and hospital admissions among 24 of its highest users. They found that 23 (96%) were either addicted to drugs or mentally ill, and 20 (83%) suffered from chronic pain.4
Drug abuse among high users is becoming even more relevant as the opioid epidemic worsens. Given that most patients requiring high levels of care suffer from chronic pain and many of them develop an opioid addiction while treating their pain, physicians have a moral imperative to reduce the prevalence of drug abuse in this population.
Low socioeconomic status
Low socioeconomic status is an important factor among high users, as it is highly associated with greater disease severity, which usually increases cost without any guarantee of an associated increase in quality. Data suggest that patients of low socioeconomic status are twice as likely to require urgent emergency department visits, 4 times as likely to require admission to the hospital, and, importantly, about half as likely to use ambulatory care compared with patients of higher socioeconomic status.5 While this pattern of low-quality, high-cost spending in acute care settings reflects spending in the healthcare system at large, the pattern is greatly exaggerated among high users.
Lost to follow-up
Low socioeconomic status also complicates communication and follow-up. In a 2013 study, physician researchers in St. Paul, MN, documented attempts to interview 64 recently discharged high users. They could not reach 47 (73%) of them, for reasons largely attributable to low socioeconomic status, such as disconnected phone lines and changes in address.6
Clearly, the usual contact methods for follow-up care after discharge, such as phone calls and mailings, are unlikely to be effective in coordinating the outpatient care of these individuals.
Additionally, we must find ways of making primary care more convenient, gaining our patients’ trust, and finding ways to engage patients in follow-up without relying on traditional means of communication.
Do high users have medical insurance?
Surprisingly, most high users of the emergency department have health insurance. The Chicago health system study3 found that most (72.4%) of their high users had either Medicare or private health insurance, while 27.6% had either Medicaid or no insurance (compared with 21.6% in the general population). Other studies also found that most of the frequent emergency department users are insured,7 although the overall percentage who rely on publicly paid insurance is greater than in the population at large.
Many prefer acute care over primary care
Although one might think that high users go to the emergency department because they have nowhere else to go for care, a report published in 2013 by Kangovi et al5 suggests another reason—they prefer the emergency department.5 They interviewed 40 urban patients of low socioeconomic status who consistently cited the 24-hour, no-appointment-necessary structure of the emergency department as an advantage over primary care. The flexibility of emergency access to healthcare makes sense if one reflects on how difficult it is for even high-functioning individuals to schedule and keep medical appointments.
Specific reasons for preferring the emergency department included the following:
Affordability. Even if their insurance fully paid for visits to their primary care physicians, the primary care physician was likely to refer them to specialists, whose visits required a copay, and which required taking another day off of work. The emergency department is cheaper for the patient and it is a “one-stop shop.” Patients appreciated the emergency department guarantee of seeing a physician regardless of proof of insurance, a policy not guaranteed in primary care and specialist offices.
Accessibility. For those without a car, public transportation and even patient transportation services are inconvenient and unreliable, whereas emergency medical services will take you to the emergency department.
Accommodations. Although medical centers may tout their same-day appointments, often same-day appointments are all that they have—and you have no choice about the time. You have to call first thing in the morning and stay on hold for a long time, and then when you finally get through, all the same-day appointments are gone.
Availability. Patients said they often had a hard time getting timely medical advice from their primary care physicians. When they could get through to their primary care physicians on the phone, they would be told to go to the emergency department.
Acceptability. Men, especially, feel they need to be very sick indeed to seek medical care, so going to the emergency department is more acceptable.
Trust in the provider. For reasons that were not entirely clear, patients felt that acute care providers were more trustworthy, competent, and compassionate than primary care physicians.5
None of these reasons for using the emergency department has anything to do with disease severity, which supports the findings that high users of the emergency department were not as sick as their normal-use peers.2
QUALITY IMPROVEMENT AND COST-REDUCTION STRATEGIES
Efforts are being made to reduce the cost of healthcare for high users while improving the quality of their care. Promising strategies focus on coordinating care management, creating individualized patient care plans, and improving the components and instructions of discharge summaries.
Care management organizations
A care management organization (CMO) model has emerged as a strategy for quality improvement and cost reduction in the high-use population. In this model, social workers, health coaches, nurses, mid-level providers, and physicians collaborate on designing individualized care plans to meet the specific needs of patients.
Teams typically work in stepwise fashion, first identifying and engaging patients at high risk of poor outcomes and unnecessary care, often using sophisticated quantitative, risk-prediction tools. Then, they perform health assessments and identify potential interventions aimed at preventing expensive acute-care medical interventions. Third, they work with patients to rapidly identify and effectively respond to changes in their conditions and direct them to the most appropriate medical setting, typically primary or urgent care.
Effective models
In 1998, the Camden (NJ) Coalition of Healthcare Providers established a model for CMO care plans. Starting with the first 36 patients enrolled in the program, hospital admissions and emergency department visits were cut by 47% (from 62 to 37 per month), and collective hospital costs were cut by 56% (from $1.2 million to about $500,000 per month).8 It should be noted that this was a small, nonrandomized study and these preliminary numbers did not take into account the cost of outpatient physician visits or new medications. Thus, how much money this program actually saves is not clear.
Similar programs have had similar results. A nurse-led care coordination program in Doylestown, PA, showed an impressive 25% reduction in annual mortality and a 36% reduction in overall costs during a 10-year period.9
A program in Atlantic City, NJ, combined the typical CMO model with a primary care clinic to provide high users with unlimited access, while paying its providers in a capitation model (as opposed to fee for service). It achieved a 40% reduction in yearly emergency department visits and hospital admissions.8
Patient care plans
Individualized patient care plans for high users are among the most promising tools for reducing costs and improving quality in this group. They are low-cost and relatively easy to implement. The goal of these care plans is to provide practitioners with a concise care summary to help them make rational and consistent medical decisions.
Typically, a care plan is written by an interdisciplinary committee composed of physicians, nurses, and social workers. It is based on the patient’s pertinent medical and psychiatric history, which may include recent imaging results or other relevant diagnostic tests. It provides suggestions for managing complex chronic issues, such as drug abuse, that lead to high use of healthcare resources.
These care plans provide a rational and prespecified approach to workup and management, typically including a narcotic prescription protocol, regardless of the setting or the number of providers who see the patient. Practitioners guided by effective care plans are much more likely to effectively navigate a complex patient encounter as opposed to looking through extensive medical notes and hoping to find relevant information.
Effective models
Data show these plans can be effective. For example, Regions Hospital in St. Paul, MN, implemented patient care plans in 2010. During the first 4 months, hospital admissions in the first 94 patients were reduced by 67%.10
A study of high users at Duke University Medical Center reported similar results. One year after starting care plans, inpatient admissions had decreased by 50.5%, readmissions had decreased by 51.5%, and variable direct costs per admission were reduced by 35.8%. Paradoxically, emergency department visits went up, but this anomaly was driven by 134 visits incurred by a single dialysis patient. After removing this patient from the data, emergency department visits were relatively stable.4
Better discharge summaries
Although improving discharge summaries is not a novel concept, changing the summary from a historical document to a proactive discharge plan has the potential to prevent readmissions and promote a durable de-escalation in care acuity.
For example, when moving a patient to a subacute care facility, providing a concise summary of which treatments worked and which did not, a list of comorbidities, and a list of medications and strategies to consider, can help the next providers to better target their plan of care. Studies have shown that nearly half of discharge statements lack important information on treatments and tests.11
Improvement can be as simple as encouraging practitioners to construct their summaries in an “if-then” format. Instead of noting for instance that “Mr. Smith was treated for pneumonia with antibiotics and discharged to a rehab facility,” the following would be more useful: “Family would like to see if Mr. Smith can get back to his functional baseline after his acute pneumonia. If he clinically does not do well over the next 1 to 2 weeks and has a poor quality of life, then family would like to pursue hospice.”
In addition to shifting the philosophy, we believe that providing timely discharge summaries is a fundamental, high-yield aspect of ensuring their effectiveness. As an example, patients being discharged to a skilled nursing facility should have a discharge summary completed and in hand before leaving the hospital.
Evidence suggests that timely writing of discharge summaries improves their quality. In a retrospective cohort study published in 2012, discharge summaries created more than 24 hours after discharge were less likely to include important plan-of-care components.12
FUTURE NEEDS
Randomized trials
Although initial results have been promising for the strategies outlined above, much of the apparent cost reduction of these interventions may be at least partially related to the study design as opposed to the interventions themselves.
For example, Hong et al13 examined 18 of the more promising CMOs that had reported initial cost savings. Of these, only 4 had conducted randomized controlled trials. When broken down further, the initial cost reduction reported by most of these randomized controlled trials was generated primarily by small subgroups.14
These results, however, do not necessarily reflect an inherent failure in the system. We contend that they merely demonstrate that CMOs and care plan administrators need to be more selective about whom they enroll, either by targeting patients at the extremes of the usage curve or by identifying patient characteristics and usage parameters amenable to cost reduction and quality improvement strategies.
Better social infrastructure
Although patient care plans and CMOs have been effective in managing high users, we believe that the most promising quality improvement and cost-reduction strategy involves redirecting much of the expensive healthcare spending to the social determinants of health (eg, homelessness, mental illness, low socioeconomic status).
Among developed countries, the United States has the highest healthcare spending and the lowest social service spending as a percentage of its gross domestic product (Figure 1).15 Although seemingly discouraging, these data can actually be interpreted as hopeful, as they support the notion that the inefficiencies of our current system are not part of an inescapable reality, but rather reflect a system that has evolved uniquely in this country.
Using the available social programs
Exemplifying this medical and social services balance is a high user who visited her local emergency department 450 times in 1 year for reasons primarily related to homelessness.16 Each time, the medical system (as it is currently designed to do) applied a short-term medical solution to this patient’s problems and discharged her home, ie, back to the street.
But this patient’s high use was really a manifestation of a deeper social issue: homelessness. When the medical staff eventually noted how much this lack of stable shelter was contributing to her pattern of use, she was referred to appropriate social resources and provided with the housing she needed. Her hospital visits decreased from 450 to 12 in the subsequent year, amounting to a huge cost reduction and a clear improvement in her quality of life.
Similar encouraging results have resulted when available social programs are applied to the high-use population at large, which is particularly reassuring given this population’s preponderance of low socioeconomic status, mental illness, and homelessness. (The prevalence of homelessness is roughly 20%, depending on the definition of a high user).
New York Medicaid, for example, has a housing program that provides stable shelter outside of acute care medical settings for patients at a rate as low as $50 per day, compared with area hospital costs that often exceed $2,200 daily.17 A similar program in Westchester County, NY, reported a 45.9% reduction in inpatient costs and a 15.4% reduction in emergency department visits among 61 of its highest users after 2 years of enrollment.17
Need to reform privacy laws
Although legally daunting, reform of the Health Insurance Portability and Accountability Act (HIPAA) and other privacy laws in favor of a more open model of information sharing, particularly for high-risk patients, holds great opportunity for quality improvement. For patients who obtain their care from several healthcare facilities, the documentation is often inscrutable. If some of the HIPAA regulations and other patient privacy laws were exchanged for rules more akin to the current model of narcotic prescription tracking, for example, physicians would be better equipped to provide safe, organized, and efficient medical care for high-use patients.
Need to reform the system
A fundamental flaw in our healthcare system, which is largely based on a fee-for-service model, is that it was not designed for patients who use the system at the highest frequency and greatest cost. Also, it does not account for the psychosocial factors that beset many high-use patients. As such, it is imperative for the safety of our patients as well as the viability of the healthcare system that we change our historical way of thinking and reform this system that provides high users with care that is high-cost, low-quality, and not patient-centered.
IMPROVING QUALITY, REDUCING COST
High users of emergency services are a medically and socially complex group, predominantly characterized by low socioeconomic status and high rates of mental illness and drug dependency. Despite their increased healthcare use, they do not have better outcomes even though they are not sicker. Improving those outcomes requires both medical and social efforts.
Among the effective medical efforts are strategies aimed at creating individualized patient care plans, using coordinated care teams, and improving discharge summaries. Addressing patients’ social factors, such as homelessness, is more difficult, but healthcare systems can help patients navigate the available social programs. These strategies are part of a comprehensive care plan that can help reduce the cost and improve the quality of healthcare for high users.
Emergency departments are not primary care clinics, but some patients use them that way. This relatively small group of patients consumes a disproportionate share of healthcare at great cost, earning them the label of “high users.” Mostly poor and often burdened with mental illness and addiction, they are not necessarily sicker than other patients, and they do not enjoy better outcomes from the extra money spent on them. (Another subset of high users, those with end-stage chronic disease, is outside the scope of this review.)
Herein lies an opportunity. If—and this is a big if—we could manage their care in a systematic way instead of haphazardly, proactively instead of reactively, with continuity of care instead of episodically, and in a way that is convenient for the patient, we might be able to improve quality and save money.
A DISPROPORTIONATE SHARE OF COSTS
In the United States in 2012, the 5% of the population who were the highest users were responsible for 50% of healthcare costs.1 The mean cost per person in this group was more than $43,000 annually. The top 1% of users accounted for nearly 23% of all expenditures, averaging nearly $98,000 per patient per year—10 times more than the average yearly cost per patient.
CARE IS OFTEN INAPPROPRIATE AND UNNECESSARY
In addition to being disproportionately expensive, the care that these patients receive is often inappropriate and unnecessary for the severity of their disease.
A 2007–2009 study2 of 1,969 patients who had visited the emergency department 10 or more times in a year found they received more than twice as many computed tomography (CT) scans as a control group of infrequent users (< 3 visits/year). This occurred even though they were not as sick as infrequent users, based on significantly lower hospital admission rates (11.1% vs 17.9%; P < .001) and mortality rates (0.7% vs 1.5%; P < .002).2
This inverse relationship between emergency department use and illness severity was even more exaggerated at the upper extreme of the use curve. The highest users (> 29 visits to the emergency department in a year) had the lowest triage acuity and hospital admission rates but the highest number of CT scans. Charges per visit were lower among frequent users, but total charges rose steadily with increasing emergency department use, accounting for significantly more costs per year.2
We believe that one reason these patients receive more medical care than necessary is because their medical records are too large and complex for the average physician to distill effectively in a 20-minute physician-patient encounter. Physicians therefore simply order more tests, procedures, and admissions, which are often medically unnecessary and redundant.
WHAT DRIVES HIGH COST?
Mental illness and chemical dependence
Drug addiction, mental illness, and poverty frequently accompany (and influence) high-use behavior, particularly in patients without end-stage diseases.
Szekendi et al,3 in a study of 28,291 patients who had been admitted at least 5 times in a year in a Chicago health system, found that these high users were 2 to 3 times more likely to suffer from comorbid depression (40% vs 13%), psychosis (18% vs 5%), recreational drug dependence (20% vs 7%), and alcohol abuse (16% vs 7%) than non-high-use hospitalized patients.3
Mercer et al4 conducted a study at Duke University Medical Center, Durham, NC, aimed at reducing emergency department visits and hospital admissions among 24 of its highest users. They found that 23 (96%) were either addicted to drugs or mentally ill, and 20 (83%) suffered from chronic pain.4
Drug abuse among high users is becoming even more relevant as the opioid epidemic worsens. Given that most patients requiring high levels of care suffer from chronic pain and many of them develop an opioid addiction while treating their pain, physicians have a moral imperative to reduce the prevalence of drug abuse in this population.
Low socioeconomic status
Low socioeconomic status is an important factor among high users, as it is highly associated with greater disease severity, which usually increases cost without any guarantee of an associated increase in quality. Data suggest that patients of low socioeconomic status are twice as likely to require urgent emergency department visits, 4 times as likely to require admission to the hospital, and, importantly, about half as likely to use ambulatory care compared with patients of higher socioeconomic status.5 While this pattern of low-quality, high-cost spending in acute care settings reflects spending in the healthcare system at large, the pattern is greatly exaggerated among high users.
Lost to follow-up
Low socioeconomic status also complicates communication and follow-up. In a 2013 study, physician researchers in St. Paul, MN, documented attempts to interview 64 recently discharged high users. They could not reach 47 (73%) of them, for reasons largely attributable to low socioeconomic status, such as disconnected phone lines and changes in address.6
Clearly, the usual contact methods for follow-up care after discharge, such as phone calls and mailings, are unlikely to be effective in coordinating the outpatient care of these individuals.
Additionally, we must find ways of making primary care more convenient, gaining our patients’ trust, and finding ways to engage patients in follow-up without relying on traditional means of communication.
Do high users have medical insurance?
Surprisingly, most high users of the emergency department have health insurance. The Chicago health system study3 found that most (72.4%) of their high users had either Medicare or private health insurance, while 27.6% had either Medicaid or no insurance (compared with 21.6% in the general population). Other studies also found that most of the frequent emergency department users are insured,7 although the overall percentage who rely on publicly paid insurance is greater than in the population at large.
Many prefer acute care over primary care
Although one might think that high users go to the emergency department because they have nowhere else to go for care, a report published in 2013 by Kangovi et al5 suggests another reason—they prefer the emergency department.5 They interviewed 40 urban patients of low socioeconomic status who consistently cited the 24-hour, no-appointment-necessary structure of the emergency department as an advantage over primary care. The flexibility of emergency access to healthcare makes sense if one reflects on how difficult it is for even high-functioning individuals to schedule and keep medical appointments.
Specific reasons for preferring the emergency department included the following:
Affordability. Even if their insurance fully paid for visits to their primary care physicians, the primary care physician was likely to refer them to specialists, whose visits required a copay, and which required taking another day off of work. The emergency department is cheaper for the patient and it is a “one-stop shop.” Patients appreciated the emergency department guarantee of seeing a physician regardless of proof of insurance, a policy not guaranteed in primary care and specialist offices.
Accessibility. For those without a car, public transportation and even patient transportation services are inconvenient and unreliable, whereas emergency medical services will take you to the emergency department.
Accommodations. Although medical centers may tout their same-day appointments, often same-day appointments are all that they have—and you have no choice about the time. You have to call first thing in the morning and stay on hold for a long time, and then when you finally get through, all the same-day appointments are gone.
Availability. Patients said they often had a hard time getting timely medical advice from their primary care physicians. When they could get through to their primary care physicians on the phone, they would be told to go to the emergency department.
Acceptability. Men, especially, feel they need to be very sick indeed to seek medical care, so going to the emergency department is more acceptable.
Trust in the provider. For reasons that were not entirely clear, patients felt that acute care providers were more trustworthy, competent, and compassionate than primary care physicians.5
None of these reasons for using the emergency department has anything to do with disease severity, which supports the findings that high users of the emergency department were not as sick as their normal-use peers.2
QUALITY IMPROVEMENT AND COST-REDUCTION STRATEGIES
Efforts are being made to reduce the cost of healthcare for high users while improving the quality of their care. Promising strategies focus on coordinating care management, creating individualized patient care plans, and improving the components and instructions of discharge summaries.
Care management organizations
A care management organization (CMO) model has emerged as a strategy for quality improvement and cost reduction in the high-use population. In this model, social workers, health coaches, nurses, mid-level providers, and physicians collaborate on designing individualized care plans to meet the specific needs of patients.
Teams typically work in stepwise fashion, first identifying and engaging patients at high risk of poor outcomes and unnecessary care, often using sophisticated quantitative, risk-prediction tools. Then, they perform health assessments and identify potential interventions aimed at preventing expensive acute-care medical interventions. Third, they work with patients to rapidly identify and effectively respond to changes in their conditions and direct them to the most appropriate medical setting, typically primary or urgent care.
Effective models
In 1998, the Camden (NJ) Coalition of Healthcare Providers established a model for CMO care plans. Starting with the first 36 patients enrolled in the program, hospital admissions and emergency department visits were cut by 47% (from 62 to 37 per month), and collective hospital costs were cut by 56% (from $1.2 million to about $500,000 per month).8 It should be noted that this was a small, nonrandomized study and these preliminary numbers did not take into account the cost of outpatient physician visits or new medications. Thus, how much money this program actually saves is not clear.
Similar programs have had similar results. A nurse-led care coordination program in Doylestown, PA, showed an impressive 25% reduction in annual mortality and a 36% reduction in overall costs during a 10-year period.9
A program in Atlantic City, NJ, combined the typical CMO model with a primary care clinic to provide high users with unlimited access, while paying its providers in a capitation model (as opposed to fee for service). It achieved a 40% reduction in yearly emergency department visits and hospital admissions.8
Patient care plans
Individualized patient care plans for high users are among the most promising tools for reducing costs and improving quality in this group. They are low-cost and relatively easy to implement. The goal of these care plans is to provide practitioners with a concise care summary to help them make rational and consistent medical decisions.
Typically, a care plan is written by an interdisciplinary committee composed of physicians, nurses, and social workers. It is based on the patient’s pertinent medical and psychiatric history, which may include recent imaging results or other relevant diagnostic tests. It provides suggestions for managing complex chronic issues, such as drug abuse, that lead to high use of healthcare resources.
These care plans provide a rational and prespecified approach to workup and management, typically including a narcotic prescription protocol, regardless of the setting or the number of providers who see the patient. Practitioners guided by effective care plans are much more likely to effectively navigate a complex patient encounter as opposed to looking through extensive medical notes and hoping to find relevant information.
Effective models
Data show these plans can be effective. For example, Regions Hospital in St. Paul, MN, implemented patient care plans in 2010. During the first 4 months, hospital admissions in the first 94 patients were reduced by 67%.10
A study of high users at Duke University Medical Center reported similar results. One year after starting care plans, inpatient admissions had decreased by 50.5%, readmissions had decreased by 51.5%, and variable direct costs per admission were reduced by 35.8%. Paradoxically, emergency department visits went up, but this anomaly was driven by 134 visits incurred by a single dialysis patient. After removing this patient from the data, emergency department visits were relatively stable.4
Better discharge summaries
Although improving discharge summaries is not a novel concept, changing the summary from a historical document to a proactive discharge plan has the potential to prevent readmissions and promote a durable de-escalation in care acuity.
For example, when moving a patient to a subacute care facility, providing a concise summary of which treatments worked and which did not, a list of comorbidities, and a list of medications and strategies to consider, can help the next providers to better target their plan of care. Studies have shown that nearly half of discharge statements lack important information on treatments and tests.11
Improvement can be as simple as encouraging practitioners to construct their summaries in an “if-then” format. Instead of noting for instance that “Mr. Smith was treated for pneumonia with antibiotics and discharged to a rehab facility,” the following would be more useful: “Family would like to see if Mr. Smith can get back to his functional baseline after his acute pneumonia. If he clinically does not do well over the next 1 to 2 weeks and has a poor quality of life, then family would like to pursue hospice.”
In addition to shifting the philosophy, we believe that providing timely discharge summaries is a fundamental, high-yield aspect of ensuring their effectiveness. As an example, patients being discharged to a skilled nursing facility should have a discharge summary completed and in hand before leaving the hospital.
Evidence suggests that timely writing of discharge summaries improves their quality. In a retrospective cohort study published in 2012, discharge summaries created more than 24 hours after discharge were less likely to include important plan-of-care components.12
FUTURE NEEDS
Randomized trials
Although initial results have been promising for the strategies outlined above, much of the apparent cost reduction of these interventions may be at least partially related to the study design as opposed to the interventions themselves.
For example, Hong et al13 examined 18 of the more promising CMOs that had reported initial cost savings. Of these, only 4 had conducted randomized controlled trials. When broken down further, the initial cost reduction reported by most of these randomized controlled trials was generated primarily by small subgroups.14
These results, however, do not necessarily reflect an inherent failure in the system. We contend that they merely demonstrate that CMOs and care plan administrators need to be more selective about whom they enroll, either by targeting patients at the extremes of the usage curve or by identifying patient characteristics and usage parameters amenable to cost reduction and quality improvement strategies.
Better social infrastructure
Although patient care plans and CMOs have been effective in managing high users, we believe that the most promising quality improvement and cost-reduction strategy involves redirecting much of the expensive healthcare spending to the social determinants of health (eg, homelessness, mental illness, low socioeconomic status).
Among developed countries, the United States has the highest healthcare spending and the lowest social service spending as a percentage of its gross domestic product (Figure 1).15 Although seemingly discouraging, these data can actually be interpreted as hopeful, as they support the notion that the inefficiencies of our current system are not part of an inescapable reality, but rather reflect a system that has evolved uniquely in this country.
Using the available social programs
Exemplifying this medical and social services balance is a high user who visited her local emergency department 450 times in 1 year for reasons primarily related to homelessness.16 Each time, the medical system (as it is currently designed to do) applied a short-term medical solution to this patient’s problems and discharged her home, ie, back to the street.
But this patient’s high use was really a manifestation of a deeper social issue: homelessness. When the medical staff eventually noted how much this lack of stable shelter was contributing to her pattern of use, she was referred to appropriate social resources and provided with the housing she needed. Her hospital visits decreased from 450 to 12 in the subsequent year, amounting to a huge cost reduction and a clear improvement in her quality of life.
Similar encouraging results have resulted when available social programs are applied to the high-use population at large, which is particularly reassuring given this population’s preponderance of low socioeconomic status, mental illness, and homelessness. (The prevalence of homelessness is roughly 20%, depending on the definition of a high user).
New York Medicaid, for example, has a housing program that provides stable shelter outside of acute care medical settings for patients at a rate as low as $50 per day, compared with area hospital costs that often exceed $2,200 daily.17 A similar program in Westchester County, NY, reported a 45.9% reduction in inpatient costs and a 15.4% reduction in emergency department visits among 61 of its highest users after 2 years of enrollment.17
Need to reform privacy laws
Although legally daunting, reform of the Health Insurance Portability and Accountability Act (HIPAA) and other privacy laws in favor of a more open model of information sharing, particularly for high-risk patients, holds great opportunity for quality improvement. For patients who obtain their care from several healthcare facilities, the documentation is often inscrutable. If some of the HIPAA regulations and other patient privacy laws were exchanged for rules more akin to the current model of narcotic prescription tracking, for example, physicians would be better equipped to provide safe, organized, and efficient medical care for high-use patients.
Need to reform the system
A fundamental flaw in our healthcare system, which is largely based on a fee-for-service model, is that it was not designed for patients who use the system at the highest frequency and greatest cost. Also, it does not account for the psychosocial factors that beset many high-use patients. As such, it is imperative for the safety of our patients as well as the viability of the healthcare system that we change our historical way of thinking and reform this system that provides high users with care that is high-cost, low-quality, and not patient-centered.
IMPROVING QUALITY, REDUCING COST
High users of emergency services are a medically and socially complex group, predominantly characterized by low socioeconomic status and high rates of mental illness and drug dependency. Despite their increased healthcare use, they do not have better outcomes even though they are not sicker. Improving those outcomes requires both medical and social efforts.
Among the effective medical efforts are strategies aimed at creating individualized patient care plans, using coordinated care teams, and improving discharge summaries. Addressing patients’ social factors, such as homelessness, is more difficult, but healthcare systems can help patients navigate the available social programs. These strategies are part of a comprehensive care plan that can help reduce the cost and improve the quality of healthcare for high users.
- Cohen SB; Agency for Healthcare Research and Quality. Statistical Brief #359. The concentration of health care expenditures and related expenses for costly medical conditions, 2009. http://meps.ahrq.gov/mepsweb/data_files/publications/st359/stat359.pdf. Accessed December 18, 2017.
- Oostema J, Troost J, Schurr K, Waller R. High and low frequency emergency department users: a comparative analysis of morbidity, diagnostic testing, and health care costs. Ann Emerg Med 2011; 58:S225. Abstract 142.
- Szekendi MK, Williams MV, Carrier D, Hensley L, Thomas S, Cerese J. The characteristics of patients frequently admitted to academic medical centers in the United States. J Hosp Med 2015; 10:563–568.
- Mercer T, Bae J, Kipnes J, Velazquez M, Thomas S, Setji N. The highest utilizers of care: individualized care plans to coordinate care, improve healthcare service utilization, and reduce costs at an academic tertiary care center. J Hosp Med 2015; 10:419–424.
- Kangovi S, Barg FK, Carter T, Long JA, Shannon R, Grande D. Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood) 2013; 32:1196–1203.
- Melander I, Winkelman T, Hilger R. Analysis of high utilizers’ experience with specialized care plans. J Hosp Med 2014; 9(suppl 2):Abstract 229.
- LaCalle EJ, Rabin EJ, Genes NG. High-frequency users of emergency department care. J Emerg Med 2013; 44:1167–1173.
- Gawande A. The Hot Spotters. The New Yorker 2011. www.newyorker.com/magazine/2011/01/24/the-hot-spotters. Accessed December 18, 2017.
- Coburn KD, Marcantonio S, Lazansky R, Keller M, Davis N. Effect of a community-based nursing intervention on mortality in chronically ill older adults: a randomized controlled trial. PLoS Med 2012; 9:e1001265.
- Hilger R, Melander I, Winkelman T. Is specialized care plan work sustainable? A follow-up on healthpartners’ experience with patients who are high-utilizers. Society of Hospital Medicine Annual Meeting, Las Vegas, NV. March 24-27, 2014. www.shmabstracts.com/abstract/is-specialized-care-plan-work-sustainable-a-followup-on-healthpartners-experience-with-patients-who-are-highutilizers. Accessed December 18, 2017.
- Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA 2007; 297:831–841.
- Kind AJ, Thorpe CT, Sattin JA, Walz SE, Smith MA. Provider characteristics, clinical-work processes and their relationship to discharge summary quality for sub-acute care patients. J Gen Intern Med 2012; 27:78–84.
- Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonwealth Fund) 2014; 19:1–19.
- Williams B. Limited effects of care management for high utilizers on total healthcare costs. Am J Managed Care 2015; 21:e244–e246.
- Organization for Economic Co-operation and Development. Health at a Glance 2009: OECD Indicators. Paris, France: OECD Publishing; 2009.
- Emeche U. Is a strategy focused on super-utilizers equal to the task of health care system transformation? Yes. Ann Fam Med 2015; 13:6–7.
- Burns J. Do we overspend on healthcare, underspend on social needs? Managed Care. http://ghli.yale.edu/news/do-we-overspend-health-care-underspend-social-needs. Accessed December 18, 2017.
- Cohen SB; Agency for Healthcare Research and Quality. Statistical Brief #359. The concentration of health care expenditures and related expenses for costly medical conditions, 2009. http://meps.ahrq.gov/mepsweb/data_files/publications/st359/stat359.pdf. Accessed December 18, 2017.
- Oostema J, Troost J, Schurr K, Waller R. High and low frequency emergency department users: a comparative analysis of morbidity, diagnostic testing, and health care costs. Ann Emerg Med 2011; 58:S225. Abstract 142.
- Szekendi MK, Williams MV, Carrier D, Hensley L, Thomas S, Cerese J. The characteristics of patients frequently admitted to academic medical centers in the United States. J Hosp Med 2015; 10:563–568.
- Mercer T, Bae J, Kipnes J, Velazquez M, Thomas S, Setji N. The highest utilizers of care: individualized care plans to coordinate care, improve healthcare service utilization, and reduce costs at an academic tertiary care center. J Hosp Med 2015; 10:419–424.
- Kangovi S, Barg FK, Carter T, Long JA, Shannon R, Grande D. Understanding why patients of low socioeconomic status prefer hospitals over ambulatory care. Health Aff (Millwood) 2013; 32:1196–1203.
- Melander I, Winkelman T, Hilger R. Analysis of high utilizers’ experience with specialized care plans. J Hosp Med 2014; 9(suppl 2):Abstract 229.
- LaCalle EJ, Rabin EJ, Genes NG. High-frequency users of emergency department care. J Emerg Med 2013; 44:1167–1173.
- Gawande A. The Hot Spotters. The New Yorker 2011. www.newyorker.com/magazine/2011/01/24/the-hot-spotters. Accessed December 18, 2017.
- Coburn KD, Marcantonio S, Lazansky R, Keller M, Davis N. Effect of a community-based nursing intervention on mortality in chronically ill older adults: a randomized controlled trial. PLoS Med 2012; 9:e1001265.
- Hilger R, Melander I, Winkelman T. Is specialized care plan work sustainable? A follow-up on healthpartners’ experience with patients who are high-utilizers. Society of Hospital Medicine Annual Meeting, Las Vegas, NV. March 24-27, 2014. www.shmabstracts.com/abstract/is-specialized-care-plan-work-sustainable-a-followup-on-healthpartners-experience-with-patients-who-are-highutilizers. Accessed December 18, 2017.
- Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA 2007; 297:831–841.
- Kind AJ, Thorpe CT, Sattin JA, Walz SE, Smith MA. Provider characteristics, clinical-work processes and their relationship to discharge summary quality for sub-acute care patients. J Gen Intern Med 2012; 27:78–84.
- Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonwealth Fund) 2014; 19:1–19.
- Williams B. Limited effects of care management for high utilizers on total healthcare costs. Am J Managed Care 2015; 21:e244–e246.
- Organization for Economic Co-operation and Development. Health at a Glance 2009: OECD Indicators. Paris, France: OECD Publishing; 2009.
- Emeche U. Is a strategy focused on super-utilizers equal to the task of health care system transformation? Yes. Ann Fam Med 2015; 13:6–7.
- Burns J. Do we overspend on healthcare, underspend on social needs? Managed Care. http://ghli.yale.edu/news/do-we-overspend-health-care-underspend-social-needs. Accessed December 18, 2017.
KEY POINTS
- The top 5% of the population in terms of healthcare use account for 50% of costs. The top 1% account for 23% of all expenditures and cost 10 times more per year than the average patient.
- Drug addiction, mental illness, and poverty often accompany and underlie high-use behavior, particularly in patients without end-stage medical conditions.
- Comprehensive patient care plans and care management organizations are among the most effective strategies for cost reduction and quality improvement.
Drug price increases far outpaced inflation in 2015
The retail price for a set of 768 prescription drugs rose by 6.4% in 2015, while the general inflation rate increased by just 0.1%, according to the AARP Public Policy Institute and the PRIME Institute at the University of Minnesota in Minneapolis.
One year, of course, does not make a trend, but how about 10 years? The average increase in the price of the “market basket” of 768 drugs widely used by older Americans has exceeded the rate of inflation every year since the AARP started tracking costs in 2004. This is “attributable entirely to drug price growth among brand name and specialty drugs, which more than offset often substantial price decreases among generic drugs,” Leigh Purvis of AARP and Stephen Schondelmeyer, PharmD, PhD, of the Prime Institute, said in an Rx Price Watch report.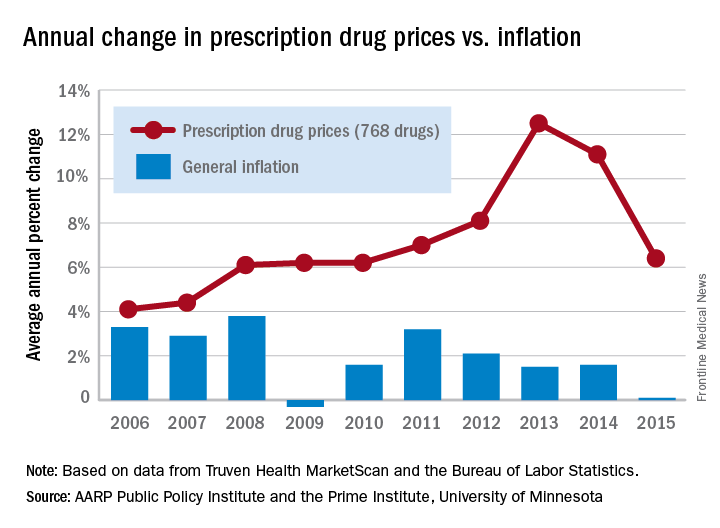
In terms of actual cost, however, the specialty drugs were far ahead of the other two segments. The average cost of a year of treatment with a specialty drug was more than $52,000 in 2015, which was nine times higher than the brand-name drugs ($5,800) and 100 times higher than the generics ($523), they said.
The Rx Price Watch reports are based on retail-level prescription prices from the Truven Health MarketScan Research Databases. The general inflation rate is based on the Consumer Price Index–All Urban Consumers for All Items, which is measured by the Bureau of Labor Statistics.
The retail price for a set of 768 prescription drugs rose by 6.4% in 2015, while the general inflation rate increased by just 0.1%, according to the AARP Public Policy Institute and the PRIME Institute at the University of Minnesota in Minneapolis.
One year, of course, does not make a trend, but how about 10 years? The average increase in the price of the “market basket” of 768 drugs widely used by older Americans has exceeded the rate of inflation every year since the AARP started tracking costs in 2004. This is “attributable entirely to drug price growth among brand name and specialty drugs, which more than offset often substantial price decreases among generic drugs,” Leigh Purvis of AARP and Stephen Schondelmeyer, PharmD, PhD, of the Prime Institute, said in an Rx Price Watch report.
In terms of actual cost, however, the specialty drugs were far ahead of the other two segments. The average cost of a year of treatment with a specialty drug was more than $52,000 in 2015, which was nine times higher than the brand-name drugs ($5,800) and 100 times higher than the generics ($523), they said.
The Rx Price Watch reports are based on retail-level prescription prices from the Truven Health MarketScan Research Databases. The general inflation rate is based on the Consumer Price Index–All Urban Consumers for All Items, which is measured by the Bureau of Labor Statistics.
The retail price for a set of 768 prescription drugs rose by 6.4% in 2015, while the general inflation rate increased by just 0.1%, according to the AARP Public Policy Institute and the PRIME Institute at the University of Minnesota in Minneapolis.
One year, of course, does not make a trend, but how about 10 years? The average increase in the price of the “market basket” of 768 drugs widely used by older Americans has exceeded the rate of inflation every year since the AARP started tracking costs in 2004. This is “attributable entirely to drug price growth among brand name and specialty drugs, which more than offset often substantial price decreases among generic drugs,” Leigh Purvis of AARP and Stephen Schondelmeyer, PharmD, PhD, of the Prime Institute, said in an Rx Price Watch report.
In terms of actual cost, however, the specialty drugs were far ahead of the other two segments. The average cost of a year of treatment with a specialty drug was more than $52,000 in 2015, which was nine times higher than the brand-name drugs ($5,800) and 100 times higher than the generics ($523), they said.
The Rx Price Watch reports are based on retail-level prescription prices from the Truven Health MarketScan Research Databases. The general inflation rate is based on the Consumer Price Index–All Urban Consumers for All Items, which is measured by the Bureau of Labor Statistics.
Majority of influenza-related deaths among hospitalized patients occur after discharge
SAN DIEGO – Over half of hospitalized, influenza-related deaths occurred within 30 days of discharge, according to a study presented at an annual scientific meeting on infectious diseases.
As physicians and pharmaceutical companies attempt to measure the burden of seasonal influenza, discharged patients are currently not considered as much as they should be, according to investigators.
Among 968 deceased patients studied, 444 (46%) died in hospital, while 524 (54%) died within 30 days of discharge.
Investigators conducted a retrospective study of 15,562 patients hospitalized for influenza-related cases between 2014 and 2015, as recorded in Influenza-Associated Hospitalizations Surveillance (FluSurv-NET), a database of the Centers for Disease Control and Prevention.
The majority of the studied patients were women (55%) and the majority were white.
Those who died were more likely to have been admitted to the hospital immediately after influenza onset, with 26% of those who died after discharge and 22% of those who died in hospital having been admitted the same day. In contrast, 13% of those who lived past 30 days were admitted immediately after onset.
A total of 46% of those who died after hospitalization had a length of stay longer than 1 week, compared to 15% of those who lived.
Among patients who died after discharge, 356 (68%) died within 2 weeks of discharge, with the highest number of deaths occurring within the first few days, according to presenter Craig McGowan of the Influenza Division of the CDC in Atlanta.
Age also seemed to be a possible mortality predictor, according to Mr. McGowan and his fellow investigators. “Those who died were more likely to be elderly, and those who died after discharge were even more likely to be 85 [years or older] than those who died during their influenza-related hospitalizations,” said Mr. McGowan, who added that patients aged 85 years and older made up more than half of those who died after discharge.
Patients who died in hospital were significantly more likely to have influenza listed as a cause of death. Overall, influenza-related and non–influenza-related respiratory issues were the two most common causes of death listed on death certificates of patients who died during hospitalization or within 14 days of discharge, while cardiovascular or other symptoms were listed for those who died between 15 and 30 days after discharge.
Admission and discharge locations among patients who did not die were almost 80% from a private residence to a private residence, while observations of those who died revealed a different pattern. “Those individuals who died after discharge were almost evenly split between admission from a nursing home or a private residence,” Mr. McGowan said. “Those who were admitted from the nursing home were almost exclusively discharged to either hospice care or back to a nursing home.”
Mr. McGowan noted rehospitalization to be a significant factor among those who died, with 34% of deaths occurring back in the hospital after initial discharge.
Influenza testing of studied patients was given at clinicians’ discretion, which may make the sample not generalizable to the overall influenza population, and the investigators included only bivariate associations, which means there were likely confounding effects that could not be accounted for.
Mr. McGowan and his fellow investigators plan to expand their research by determining underlying causes of death in these patients, to create more accurate estimates of influenza-associated mortality.
Mr. McGowan reported no relevant financial disclosures.
SOURCE: McGowan, C., et al., ID Week 2017, Abstract 951.
SAN DIEGO – Over half of hospitalized, influenza-related deaths occurred within 30 days of discharge, according to a study presented at an annual scientific meeting on infectious diseases.
As physicians and pharmaceutical companies attempt to measure the burden of seasonal influenza, discharged patients are currently not considered as much as they should be, according to investigators.
Among 968 deceased patients studied, 444 (46%) died in hospital, while 524 (54%) died within 30 days of discharge.
Investigators conducted a retrospective study of 15,562 patients hospitalized for influenza-related cases between 2014 and 2015, as recorded in Influenza-Associated Hospitalizations Surveillance (FluSurv-NET), a database of the Centers for Disease Control and Prevention.
The majority of the studied patients were women (55%) and the majority were white.
Those who died were more likely to have been admitted to the hospital immediately after influenza onset, with 26% of those who died after discharge and 22% of those who died in hospital having been admitted the same day. In contrast, 13% of those who lived past 30 days were admitted immediately after onset.
A total of 46% of those who died after hospitalization had a length of stay longer than 1 week, compared to 15% of those who lived.
Among patients who died after discharge, 356 (68%) died within 2 weeks of discharge, with the highest number of deaths occurring within the first few days, according to presenter Craig McGowan of the Influenza Division of the CDC in Atlanta.
Age also seemed to be a possible mortality predictor, according to Mr. McGowan and his fellow investigators. “Those who died were more likely to be elderly, and those who died after discharge were even more likely to be 85 [years or older] than those who died during their influenza-related hospitalizations,” said Mr. McGowan, who added that patients aged 85 years and older made up more than half of those who died after discharge.
Patients who died in hospital were significantly more likely to have influenza listed as a cause of death. Overall, influenza-related and non–influenza-related respiratory issues were the two most common causes of death listed on death certificates of patients who died during hospitalization or within 14 days of discharge, while cardiovascular or other symptoms were listed for those who died between 15 and 30 days after discharge.
Admission and discharge locations among patients who did not die were almost 80% from a private residence to a private residence, while observations of those who died revealed a different pattern. “Those individuals who died after discharge were almost evenly split between admission from a nursing home or a private residence,” Mr. McGowan said. “Those who were admitted from the nursing home were almost exclusively discharged to either hospice care or back to a nursing home.”
Mr. McGowan noted rehospitalization to be a significant factor among those who died, with 34% of deaths occurring back in the hospital after initial discharge.
Influenza testing of studied patients was given at clinicians’ discretion, which may make the sample not generalizable to the overall influenza population, and the investigators included only bivariate associations, which means there were likely confounding effects that could not be accounted for.
Mr. McGowan and his fellow investigators plan to expand their research by determining underlying causes of death in these patients, to create more accurate estimates of influenza-associated mortality.
Mr. McGowan reported no relevant financial disclosures.
SOURCE: McGowan, C., et al., ID Week 2017, Abstract 951.
SAN DIEGO – Over half of hospitalized, influenza-related deaths occurred within 30 days of discharge, according to a study presented at an annual scientific meeting on infectious diseases.
As physicians and pharmaceutical companies attempt to measure the burden of seasonal influenza, discharged patients are currently not considered as much as they should be, according to investigators.
Among 968 deceased patients studied, 444 (46%) died in hospital, while 524 (54%) died within 30 days of discharge.
Investigators conducted a retrospective study of 15,562 patients hospitalized for influenza-related cases between 2014 and 2015, as recorded in Influenza-Associated Hospitalizations Surveillance (FluSurv-NET), a database of the Centers for Disease Control and Prevention.
The majority of the studied patients were women (55%) and the majority were white.
Those who died were more likely to have been admitted to the hospital immediately after influenza onset, with 26% of those who died after discharge and 22% of those who died in hospital having been admitted the same day. In contrast, 13% of those who lived past 30 days were admitted immediately after onset.
A total of 46% of those who died after hospitalization had a length of stay longer than 1 week, compared to 15% of those who lived.
Among patients who died after discharge, 356 (68%) died within 2 weeks of discharge, with the highest number of deaths occurring within the first few days, according to presenter Craig McGowan of the Influenza Division of the CDC in Atlanta.
Age also seemed to be a possible mortality predictor, according to Mr. McGowan and his fellow investigators. “Those who died were more likely to be elderly, and those who died after discharge were even more likely to be 85 [years or older] than those who died during their influenza-related hospitalizations,” said Mr. McGowan, who added that patients aged 85 years and older made up more than half of those who died after discharge.
Patients who died in hospital were significantly more likely to have influenza listed as a cause of death. Overall, influenza-related and non–influenza-related respiratory issues were the two most common causes of death listed on death certificates of patients who died during hospitalization or within 14 days of discharge, while cardiovascular or other symptoms were listed for those who died between 15 and 30 days after discharge.
Admission and discharge locations among patients who did not die were almost 80% from a private residence to a private residence, while observations of those who died revealed a different pattern. “Those individuals who died after discharge were almost evenly split between admission from a nursing home or a private residence,” Mr. McGowan said. “Those who were admitted from the nursing home were almost exclusively discharged to either hospice care or back to a nursing home.”
Mr. McGowan noted rehospitalization to be a significant factor among those who died, with 34% of deaths occurring back in the hospital after initial discharge.
Influenza testing of studied patients was given at clinicians’ discretion, which may make the sample not generalizable to the overall influenza population, and the investigators included only bivariate associations, which means there were likely confounding effects that could not be accounted for.
Mr. McGowan and his fellow investigators plan to expand their research by determining underlying causes of death in these patients, to create more accurate estimates of influenza-associated mortality.
Mr. McGowan reported no relevant financial disclosures.
SOURCE: McGowan, C., et al., ID Week 2017, Abstract 951.
AT IDWEEK 2017
Key clinical point:
Major finding: Among patients who died with confirmed influenza, 46% died in hospital, while 54% died within 30 days of discharge.
Data source: Retrospective study of 15,562 influenza patients hospitalized or within 30 days of discharge between 2014 and 2015, recorded in Influenza-Associated Hospitalizations Surveillance (FluSurv-NET).
Disclosures: Mr. McGowen reported no relevant financial disclosures.
GERD linked to upper aerodigestive tract cancers in elderly
The risk for gastroesophageal reflux disease and cancer of the larynx, tonsils, and other areas of the upper aerodigestive tract was strongly associated in a longitudinal-based population study of the U.S. elderly population.
A total of 13,805 cases involving gastroesophageal reflux disease (GERD) and malignancies of the upper aerodigestive tract (UADT) and 13,805 GERD cases with no UADT from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER)-Medicare linked database in patients aged 66 years and older from 2003 through 2011 were examined. Only those who had no malignancy before they were diagnosed with GERD were included in the study, which was published in JAMA Otolaryngology–Head & Neck Surgery (doi: 10.1001/jamaoto.2017.2561.
Lead author Charles A. Riley, MD, of Tulane University in New Orleans, and his coauthors noted that previous studies had drawn conflicting conclusions about the link between GERD and UADT malignancies. To their knowledge, this is the first study to investigate UADT malignancies specifically in the elderly in the United States.
“The increased relative risk for laryngeal and pharyngeal cancers in this population suggests an opportunity for earlier detection and intervention,” Dr. Riley and his colleagues said.
For the study, they calculated the adjusted odds ratios (aOR) of cancer in six areas of the UADT in patients with GERD vs. patients who never had GERD: larynx (2.86), hypopharynx (2.54), oropharynx (2.47), tonsil (2.14), nasopharynx (2.04), and paranasal sinuses (1.40).
The study also evaluated the relative risk of malignancy with GERD and without GERD. “These data suggest that elderly patients with GERD in the United States are 3.47, 3.23, 2.88, and 2.37 times as likely as those without GERD to be diagnosed with laryngeal, hypopharyngeal, oropharyngeal and tonsillar cancers, respectively,” Dr. Riley and his associates wrote.
These findings may point to a need for a paradigm shift like that which led to the use of screening esophagogastroduodenoscopy for patients at risk of Barrett esophagus and esophageal cancer. “A similar screening platform may benefit those patients at higher risk for the development of malignancy of the UADT, though further research is necessary,” they said.
Dr. Riley and his coauthors reported having no financial disclosures.
Source: Riley C et al. JAMA Otolaryngol Head Neck Surg. 2017 Dec 21. doi: 10.1001/jamaoto.2017.2561.
The risk for gastroesophageal reflux disease and cancer of the larynx, tonsils, and other areas of the upper aerodigestive tract was strongly associated in a longitudinal-based population study of the U.S. elderly population.
A total of 13,805 cases involving gastroesophageal reflux disease (GERD) and malignancies of the upper aerodigestive tract (UADT) and 13,805 GERD cases with no UADT from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER)-Medicare linked database in patients aged 66 years and older from 2003 through 2011 were examined. Only those who had no malignancy before they were diagnosed with GERD were included in the study, which was published in JAMA Otolaryngology–Head & Neck Surgery (doi: 10.1001/jamaoto.2017.2561.
Lead author Charles A. Riley, MD, of Tulane University in New Orleans, and his coauthors noted that previous studies had drawn conflicting conclusions about the link between GERD and UADT malignancies. To their knowledge, this is the first study to investigate UADT malignancies specifically in the elderly in the United States.
“The increased relative risk for laryngeal and pharyngeal cancers in this population suggests an opportunity for earlier detection and intervention,” Dr. Riley and his colleagues said.
For the study, they calculated the adjusted odds ratios (aOR) of cancer in six areas of the UADT in patients with GERD vs. patients who never had GERD: larynx (2.86), hypopharynx (2.54), oropharynx (2.47), tonsil (2.14), nasopharynx (2.04), and paranasal sinuses (1.40).
The study also evaluated the relative risk of malignancy with GERD and without GERD. “These data suggest that elderly patients with GERD in the United States are 3.47, 3.23, 2.88, and 2.37 times as likely as those without GERD to be diagnosed with laryngeal, hypopharyngeal, oropharyngeal and tonsillar cancers, respectively,” Dr. Riley and his associates wrote.
These findings may point to a need for a paradigm shift like that which led to the use of screening esophagogastroduodenoscopy for patients at risk of Barrett esophagus and esophageal cancer. “A similar screening platform may benefit those patients at higher risk for the development of malignancy of the UADT, though further research is necessary,” they said.
Dr. Riley and his coauthors reported having no financial disclosures.
Source: Riley C et al. JAMA Otolaryngol Head Neck Surg. 2017 Dec 21. doi: 10.1001/jamaoto.2017.2561.
The risk for gastroesophageal reflux disease and cancer of the larynx, tonsils, and other areas of the upper aerodigestive tract was strongly associated in a longitudinal-based population study of the U.S. elderly population.
A total of 13,805 cases involving gastroesophageal reflux disease (GERD) and malignancies of the upper aerodigestive tract (UADT) and 13,805 GERD cases with no UADT from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER)-Medicare linked database in patients aged 66 years and older from 2003 through 2011 were examined. Only those who had no malignancy before they were diagnosed with GERD were included in the study, which was published in JAMA Otolaryngology–Head & Neck Surgery (doi: 10.1001/jamaoto.2017.2561.
Lead author Charles A. Riley, MD, of Tulane University in New Orleans, and his coauthors noted that previous studies had drawn conflicting conclusions about the link between GERD and UADT malignancies. To their knowledge, this is the first study to investigate UADT malignancies specifically in the elderly in the United States.
“The increased relative risk for laryngeal and pharyngeal cancers in this population suggests an opportunity for earlier detection and intervention,” Dr. Riley and his colleagues said.
For the study, they calculated the adjusted odds ratios (aOR) of cancer in six areas of the UADT in patients with GERD vs. patients who never had GERD: larynx (2.86), hypopharynx (2.54), oropharynx (2.47), tonsil (2.14), nasopharynx (2.04), and paranasal sinuses (1.40).
The study also evaluated the relative risk of malignancy with GERD and without GERD. “These data suggest that elderly patients with GERD in the United States are 3.47, 3.23, 2.88, and 2.37 times as likely as those without GERD to be diagnosed with laryngeal, hypopharyngeal, oropharyngeal and tonsillar cancers, respectively,” Dr. Riley and his associates wrote.
These findings may point to a need for a paradigm shift like that which led to the use of screening esophagogastroduodenoscopy for patients at risk of Barrett esophagus and esophageal cancer. “A similar screening platform may benefit those patients at higher risk for the development of malignancy of the UADT, though further research is necessary,” they said.
Dr. Riley and his coauthors reported having no financial disclosures.
Source: Riley C et al. JAMA Otolaryngol Head Neck Surg. 2017 Dec 21. doi: 10.1001/jamaoto.2017.2561.
FROM JAMA OTOLARYNGOLOGY
Key clinical point: Gastroesophageal reflux disease (GERD) is associated with malignancies of the upper aerodigestive tract (UADT) in U.S. patients aged 66 years and older.
Major finding: GERD was associated with a 2.86 adjusted odds ratio for developing malignancy of the larynx.
Data source: 13,805 cases with UADT malignancies and 13.805 cases without disease from the National Cancer Institute’s Surveillance, Epidemiology and End Results-Medicare linked database queried from January 2003 to December 2011.
Disclosures: Dr. Riley and his coauthors reported having no financial disclosures.
Source: Riley C et al. JAMA Otolaryngol Head Neck Surg. 2017 Dec 21. doi: 10.1001/jamaoto.2017.2561.
Project improves noninvasive IUC alternatives
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-18 year, offering two options for students to receive funding and engage in scholarly work during their first, second and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
It truly has been a rewarding experience participating in a quality improvement project and I am excited to see what the future holds. Our project, “Reducing CAUTI with Noninvasive UC Alternatives and Measure-vention,” aimed to combat catheter associated urinary tract infections, with a three-pronged approach: by reducing UC placement, performing proper maintenance of IUC, and ensuring prompt removal of unnecessary UC.
To date, our project has demonstrated qualitative success. Specifically, we have implemented a pipeline to perform “measure-vention,” or real-time monitoring and correction of defects. The surgical care intensive unit (SICU) was identified as an appropriate candidate for a pilot partnership due to its high utilization of UC. A daily report of patients with UC is generated and then checked against the EMR for UC necessity. Subsequently, we contact the unit RN for details and physicians for removal orders, when possible. Simultaneously, this enables us to reinforce our management bundle in real time. This protocol is being effectively implemented in the SICU and we are hoping to expand to other units as well. Quantitative data collection is still ongoing and hopefully forthcoming.
Previous CAUTI reduction efforts have had variable and partial success. We are very excited to have improved noninvasive IUC alternatives that address staff concerns about incontinence workload, urine output monitoring, and patient comfort. We hope to protect our patients from harm and eventually publicize our experience to help other health care facilities reduce IUC use and CAUTI.
It has been a rewarding experience to participate in a quality improvement project and I am enjoying the challenges of collaborating with a diverse team of medical professionals to improve the patient experience.
Victor Ekuta is a third-year medical student at UC San Diego.
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-18 year, offering two options for students to receive funding and engage in scholarly work during their first, second and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
It truly has been a rewarding experience participating in a quality improvement project and I am excited to see what the future holds. Our project, “Reducing CAUTI with Noninvasive UC Alternatives and Measure-vention,” aimed to combat catheter associated urinary tract infections, with a three-pronged approach: by reducing UC placement, performing proper maintenance of IUC, and ensuring prompt removal of unnecessary UC.
To date, our project has demonstrated qualitative success. Specifically, we have implemented a pipeline to perform “measure-vention,” or real-time monitoring and correction of defects. The surgical care intensive unit (SICU) was identified as an appropriate candidate for a pilot partnership due to its high utilization of UC. A daily report of patients with UC is generated and then checked against the EMR for UC necessity. Subsequently, we contact the unit RN for details and physicians for removal orders, when possible. Simultaneously, this enables us to reinforce our management bundle in real time. This protocol is being effectively implemented in the SICU and we are hoping to expand to other units as well. Quantitative data collection is still ongoing and hopefully forthcoming.
Previous CAUTI reduction efforts have had variable and partial success. We are very excited to have improved noninvasive IUC alternatives that address staff concerns about incontinence workload, urine output monitoring, and patient comfort. We hope to protect our patients from harm and eventually publicize our experience to help other health care facilities reduce IUC use and CAUTI.
It has been a rewarding experience to participate in a quality improvement project and I am enjoying the challenges of collaborating with a diverse team of medical professionals to improve the patient experience.
Victor Ekuta is a third-year medical student at UC San Diego.
Editor’s note: The Society of Hospital Medicine’s (SHM’s) Physician in Training Committee launched a scholarship program in 2015 for medical students to help transform health care and revolutionize patient care. The program has been expanded for the 2017-18 year, offering two options for students to receive funding and engage in scholarly work during their first, second and third years of medical school. As a part of the longitudinal (18-month) program, recipients are required to write about their experience on a monthly basis.
It truly has been a rewarding experience participating in a quality improvement project and I am excited to see what the future holds. Our project, “Reducing CAUTI with Noninvasive UC Alternatives and Measure-vention,” aimed to combat catheter associated urinary tract infections, with a three-pronged approach: by reducing UC placement, performing proper maintenance of IUC, and ensuring prompt removal of unnecessary UC.
To date, our project has demonstrated qualitative success. Specifically, we have implemented a pipeline to perform “measure-vention,” or real-time monitoring and correction of defects. The surgical care intensive unit (SICU) was identified as an appropriate candidate for a pilot partnership due to its high utilization of UC. A daily report of patients with UC is generated and then checked against the EMR for UC necessity. Subsequently, we contact the unit RN for details and physicians for removal orders, when possible. Simultaneously, this enables us to reinforce our management bundle in real time. This protocol is being effectively implemented in the SICU and we are hoping to expand to other units as well. Quantitative data collection is still ongoing and hopefully forthcoming.
Previous CAUTI reduction efforts have had variable and partial success. We are very excited to have improved noninvasive IUC alternatives that address staff concerns about incontinence workload, urine output monitoring, and patient comfort. We hope to protect our patients from harm and eventually publicize our experience to help other health care facilities reduce IUC use and CAUTI.
It has been a rewarding experience to participate in a quality improvement project and I am enjoying the challenges of collaborating with a diverse team of medical professionals to improve the patient experience.
Victor Ekuta is a third-year medical student at UC San Diego.
Daratumumab looks good in light chain amyloidosis
ATLANTA – In patients with previously treated immunoglobulin light chain (AL) amyloidosis, daratumumab monotherapy produced deep, rapid hematologic responses, based on initial results from a phase 2 trial.
So far, the response rate is about twice the rate seen with daratumumab in relapsed/refractory multiple myeloma, Murielle Roussel, MD, of IUCT-Oncopole, Toulouse, France, said at the annual meeting of the American Society of Hematology. “We observed deep and rapid clonal responses, even after the first infusion.”
“Daratumumab also had a good safety profile characterized by nonsevere adverse events after initial infusion. There was only one drug-related serious adverse event, grade 3 lymphopenia,” she said.
In a second study, the risk for daratumumab infusion reactions was low when patients received a prophylactic regimen initiated about an hour before daratumumab infusion.
Daratumumab, a novel, fully humanized IgG1-kappa monoclonal antibody with high affinity for CD38, is approved for treating relapsed/refractory multiple myeloma. In AL amyloidosis, as in myeloma, monoclonal light chains nearly always originate from plasma cells that consistently express CD38.
Data from small studies indicate that daratumumab effectively treats AL amyloidosis. To further evaluate safety and efficacy, 36 adults with previously treated disease received 28-day cycles of daratumumab (16 mg/kg IV) weekly for two cycles and then every other week for four cycles. Most patients had received three prior lines of therapy, about two-thirds had cardiac involvement (median baseline NT-proBNP 1,118 ng/L; range, 60-6,825), and about 60% had renal involvement.
At data cutoff in mid-November 2017, fifteen patients had completed all six treatment cycles. Three stopped treatment because of progression. Two died, one of progressive cardiac amyloidosis and one of unrelated lung cancer.
Eleven patients had grade 1-2 infusion reactions at first injection. Among 17 grade 3 or higher adverse events, only lymphopenia was deemed treatment related.
At 6 months, 15 of 32 evaluable patients (44%) had a very good partial response (VGPR; at least a 40% drop in baseline difference in involved and uninvolved free light chains (dFLC). Another 16% had a partial response, and 41% did not respond.
Patients with durable responses tended to have about a 70% drop in dFLC after the first daratumumab dose. Baseline variables did not seem to predict durability of response, Dr. Roussel said. “Further studies in amyloidosis are warranted in relapsed or refractory patients and also in the frontline setting.”
The second trial focused on preventing infusion reactions to daratumumab. In early trials of daratumumab for relapsed/recalcitrant multiple myeloma, patients developed moderate to severe bronchospasm, laryngeal or pulmonary edema, hypoxia, and hypertension, noted Vaishali Sanchorawala, MD, of Boston Medical Center. Since those trials, prophylactic therapies have been used to reduce the risk of infusion reactions.
Dr. Sanchorawala’s study enrolled 12 patients with previously treated AL amyloidosis and cardiac biomarker stage II or stage III disease. About 60% of patients were refractory to their last treatment. Median NT-proBNP level was 1,357 pg/mL (range, 469-3,962), median urine protein excretion was 0.44 g (0-10.1), and median dFLC was 105 mg/dL (3.8-854).
Patients received 16 mg/kg daratumumab IV weekly for 8 weeks, then every 2 weeks for 16 weeks, and then every 4 weeks for up to 24 months. About an hour before infusion, they received acetaminophen, diphenhydramine, loratadine famotidine, montelukast, and methylprednisolone (100 mg for two infusions; 60 mg thereafter). Ondansetron also was added to control mild nausea and vomiting. Two hours into the infusion, patients received diphenhydramine, famotidine, and methylprednisolone (40 mg). They received methylprednisolone (20 mg) and montelukast 1-2 days after the first two infusions, after which montelukast was optional. All received prophylactic acyclovir.
At the Nov. 15, 2017 data cutoff, 11 patients remained on study and one left after disease progressed. This patient’s disease was refractory to many prior therapies and had a complete response to autologous stem cell transplant, said Dr. Sanchorawala.
There were no grade 3-4 infusion reactions. Nine evaluable patients at 3 months had two complete hematologic responses, six VGPRs (at least a 65% drop in dFLC), and one partial response. One-third had at least a 30% improvement in NT-proBNP at 3 months, as did three of four evaluable patients at 6 months. About half had least a 30% drop in urine protein excretion at 6 months.
First infusions lasted a median of 7 hours, making them doable during a clinic day if bloods are drawn beforehand, Dr. Sanchorawala said. Second and subsequent infusions took about 4 hours.
“Preliminary data suggest a rapid hematologic response after one dose of daratumumab and high rates of response at 3 and 6 months, ” she concluded. “Since the plasma cell clone is so low in amyloidosis, single-agent daratumumab has a very positive, strong effect. We may not need to combine other agents with this therapy.”
Both presentations sparked substantial interest during the discussion period after the presentations, especially because daratumumab was given as monotherapy. “This would be a new indication for daratumumab,” said session moderator Dan Vogl, MD, director of the Abramson Cancer Center Clinical Research Unit, University of Pennsylvania, Philadelphia.
Janssen makes daratumumab and provided partial funding for both studies. Dr. Sanchorawala had no conflicts of interest. Dr. Roussel disclosed honoraria and research funding from Janssen.
SOURCES: Sanchorawala V et al. ASH 2017 Abstract 507; Roussel M et al. ASH 2017 Abstract 508.
ATLANTA – In patients with previously treated immunoglobulin light chain (AL) amyloidosis, daratumumab monotherapy produced deep, rapid hematologic responses, based on initial results from a phase 2 trial.
So far, the response rate is about twice the rate seen with daratumumab in relapsed/refractory multiple myeloma, Murielle Roussel, MD, of IUCT-Oncopole, Toulouse, France, said at the annual meeting of the American Society of Hematology. “We observed deep and rapid clonal responses, even after the first infusion.”
“Daratumumab also had a good safety profile characterized by nonsevere adverse events after initial infusion. There was only one drug-related serious adverse event, grade 3 lymphopenia,” she said.
In a second study, the risk for daratumumab infusion reactions was low when patients received a prophylactic regimen initiated about an hour before daratumumab infusion.
Daratumumab, a novel, fully humanized IgG1-kappa monoclonal antibody with high affinity for CD38, is approved for treating relapsed/refractory multiple myeloma. In AL amyloidosis, as in myeloma, monoclonal light chains nearly always originate from plasma cells that consistently express CD38.
Data from small studies indicate that daratumumab effectively treats AL amyloidosis. To further evaluate safety and efficacy, 36 adults with previously treated disease received 28-day cycles of daratumumab (16 mg/kg IV) weekly for two cycles and then every other week for four cycles. Most patients had received three prior lines of therapy, about two-thirds had cardiac involvement (median baseline NT-proBNP 1,118 ng/L; range, 60-6,825), and about 60% had renal involvement.
At data cutoff in mid-November 2017, fifteen patients had completed all six treatment cycles. Three stopped treatment because of progression. Two died, one of progressive cardiac amyloidosis and one of unrelated lung cancer.
Eleven patients had grade 1-2 infusion reactions at first injection. Among 17 grade 3 or higher adverse events, only lymphopenia was deemed treatment related.
At 6 months, 15 of 32 evaluable patients (44%) had a very good partial response (VGPR; at least a 40% drop in baseline difference in involved and uninvolved free light chains (dFLC). Another 16% had a partial response, and 41% did not respond.
Patients with durable responses tended to have about a 70% drop in dFLC after the first daratumumab dose. Baseline variables did not seem to predict durability of response, Dr. Roussel said. “Further studies in amyloidosis are warranted in relapsed or refractory patients and also in the frontline setting.”
The second trial focused on preventing infusion reactions to daratumumab. In early trials of daratumumab for relapsed/recalcitrant multiple myeloma, patients developed moderate to severe bronchospasm, laryngeal or pulmonary edema, hypoxia, and hypertension, noted Vaishali Sanchorawala, MD, of Boston Medical Center. Since those trials, prophylactic therapies have been used to reduce the risk of infusion reactions.
Dr. Sanchorawala’s study enrolled 12 patients with previously treated AL amyloidosis and cardiac biomarker stage II or stage III disease. About 60% of patients were refractory to their last treatment. Median NT-proBNP level was 1,357 pg/mL (range, 469-3,962), median urine protein excretion was 0.44 g (0-10.1), and median dFLC was 105 mg/dL (3.8-854).
Patients received 16 mg/kg daratumumab IV weekly for 8 weeks, then every 2 weeks for 16 weeks, and then every 4 weeks for up to 24 months. About an hour before infusion, they received acetaminophen, diphenhydramine, loratadine famotidine, montelukast, and methylprednisolone (100 mg for two infusions; 60 mg thereafter). Ondansetron also was added to control mild nausea and vomiting. Two hours into the infusion, patients received diphenhydramine, famotidine, and methylprednisolone (40 mg). They received methylprednisolone (20 mg) and montelukast 1-2 days after the first two infusions, after which montelukast was optional. All received prophylactic acyclovir.
At the Nov. 15, 2017 data cutoff, 11 patients remained on study and one left after disease progressed. This patient’s disease was refractory to many prior therapies and had a complete response to autologous stem cell transplant, said Dr. Sanchorawala.
There were no grade 3-4 infusion reactions. Nine evaluable patients at 3 months had two complete hematologic responses, six VGPRs (at least a 65% drop in dFLC), and one partial response. One-third had at least a 30% improvement in NT-proBNP at 3 months, as did three of four evaluable patients at 6 months. About half had least a 30% drop in urine protein excretion at 6 months.
First infusions lasted a median of 7 hours, making them doable during a clinic day if bloods are drawn beforehand, Dr. Sanchorawala said. Second and subsequent infusions took about 4 hours.
“Preliminary data suggest a rapid hematologic response after one dose of daratumumab and high rates of response at 3 and 6 months, ” she concluded. “Since the plasma cell clone is so low in amyloidosis, single-agent daratumumab has a very positive, strong effect. We may not need to combine other agents with this therapy.”
Both presentations sparked substantial interest during the discussion period after the presentations, especially because daratumumab was given as monotherapy. “This would be a new indication for daratumumab,” said session moderator Dan Vogl, MD, director of the Abramson Cancer Center Clinical Research Unit, University of Pennsylvania, Philadelphia.
Janssen makes daratumumab and provided partial funding for both studies. Dr. Sanchorawala had no conflicts of interest. Dr. Roussel disclosed honoraria and research funding from Janssen.
SOURCES: Sanchorawala V et al. ASH 2017 Abstract 507; Roussel M et al. ASH 2017 Abstract 508.
ATLANTA – In patients with previously treated immunoglobulin light chain (AL) amyloidosis, daratumumab monotherapy produced deep, rapid hematologic responses, based on initial results from a phase 2 trial.
So far, the response rate is about twice the rate seen with daratumumab in relapsed/refractory multiple myeloma, Murielle Roussel, MD, of IUCT-Oncopole, Toulouse, France, said at the annual meeting of the American Society of Hematology. “We observed deep and rapid clonal responses, even after the first infusion.”
“Daratumumab also had a good safety profile characterized by nonsevere adverse events after initial infusion. There was only one drug-related serious adverse event, grade 3 lymphopenia,” she said.
In a second study, the risk for daratumumab infusion reactions was low when patients received a prophylactic regimen initiated about an hour before daratumumab infusion.
Daratumumab, a novel, fully humanized IgG1-kappa monoclonal antibody with high affinity for CD38, is approved for treating relapsed/refractory multiple myeloma. In AL amyloidosis, as in myeloma, monoclonal light chains nearly always originate from plasma cells that consistently express CD38.
Data from small studies indicate that daratumumab effectively treats AL amyloidosis. To further evaluate safety and efficacy, 36 adults with previously treated disease received 28-day cycles of daratumumab (16 mg/kg IV) weekly for two cycles and then every other week for four cycles. Most patients had received three prior lines of therapy, about two-thirds had cardiac involvement (median baseline NT-proBNP 1,118 ng/L; range, 60-6,825), and about 60% had renal involvement.
At data cutoff in mid-November 2017, fifteen patients had completed all six treatment cycles. Three stopped treatment because of progression. Two died, one of progressive cardiac amyloidosis and one of unrelated lung cancer.
Eleven patients had grade 1-2 infusion reactions at first injection. Among 17 grade 3 or higher adverse events, only lymphopenia was deemed treatment related.
At 6 months, 15 of 32 evaluable patients (44%) had a very good partial response (VGPR; at least a 40% drop in baseline difference in involved and uninvolved free light chains (dFLC). Another 16% had a partial response, and 41% did not respond.
Patients with durable responses tended to have about a 70% drop in dFLC after the first daratumumab dose. Baseline variables did not seem to predict durability of response, Dr. Roussel said. “Further studies in amyloidosis are warranted in relapsed or refractory patients and also in the frontline setting.”
The second trial focused on preventing infusion reactions to daratumumab. In early trials of daratumumab for relapsed/recalcitrant multiple myeloma, patients developed moderate to severe bronchospasm, laryngeal or pulmonary edema, hypoxia, and hypertension, noted Vaishali Sanchorawala, MD, of Boston Medical Center. Since those trials, prophylactic therapies have been used to reduce the risk of infusion reactions.
Dr. Sanchorawala’s study enrolled 12 patients with previously treated AL amyloidosis and cardiac biomarker stage II or stage III disease. About 60% of patients were refractory to their last treatment. Median NT-proBNP level was 1,357 pg/mL (range, 469-3,962), median urine protein excretion was 0.44 g (0-10.1), and median dFLC was 105 mg/dL (3.8-854).
Patients received 16 mg/kg daratumumab IV weekly for 8 weeks, then every 2 weeks for 16 weeks, and then every 4 weeks for up to 24 months. About an hour before infusion, they received acetaminophen, diphenhydramine, loratadine famotidine, montelukast, and methylprednisolone (100 mg for two infusions; 60 mg thereafter). Ondansetron also was added to control mild nausea and vomiting. Two hours into the infusion, patients received diphenhydramine, famotidine, and methylprednisolone (40 mg). They received methylprednisolone (20 mg) and montelukast 1-2 days after the first two infusions, after which montelukast was optional. All received prophylactic acyclovir.
At the Nov. 15, 2017 data cutoff, 11 patients remained on study and one left after disease progressed. This patient’s disease was refractory to many prior therapies and had a complete response to autologous stem cell transplant, said Dr. Sanchorawala.
There were no grade 3-4 infusion reactions. Nine evaluable patients at 3 months had two complete hematologic responses, six VGPRs (at least a 65% drop in dFLC), and one partial response. One-third had at least a 30% improvement in NT-proBNP at 3 months, as did three of four evaluable patients at 6 months. About half had least a 30% drop in urine protein excretion at 6 months.
First infusions lasted a median of 7 hours, making them doable during a clinic day if bloods are drawn beforehand, Dr. Sanchorawala said. Second and subsequent infusions took about 4 hours.
“Preliminary data suggest a rapid hematologic response after one dose of daratumumab and high rates of response at 3 and 6 months, ” she concluded. “Since the plasma cell clone is so low in amyloidosis, single-agent daratumumab has a very positive, strong effect. We may not need to combine other agents with this therapy.”
Both presentations sparked substantial interest during the discussion period after the presentations, especially because daratumumab was given as monotherapy. “This would be a new indication for daratumumab,” said session moderator Dan Vogl, MD, director of the Abramson Cancer Center Clinical Research Unit, University of Pennsylvania, Philadelphia.
Janssen makes daratumumab and provided partial funding for both studies. Dr. Sanchorawala had no conflicts of interest. Dr. Roussel disclosed honoraria and research funding from Janssen.
SOURCES: Sanchorawala V et al. ASH 2017 Abstract 507; Roussel M et al. ASH 2017 Abstract 508.
REPORTING FROM ASH 2017
Key clinical point: Major finding: Rates of very good partial response or complete response were 44% and 33%, respectively, at 6 months.
Data source: Two phase 2 trials of daratumumab monotherapy in patients with previously treated light chain amyloidosis (NCT02816476 [36 patients] and NCT02841033 [12 patients]).
Disclosures: Janssen makes daratumumab and provided partial funding for both studies. Dr. Roussel disclosed honoraria and research funding from Janssen. Dr. Sanchorawala had no conflicts of interest.
Sources: Sanchorawala V et al. ASH 2017 Abstract 507; Roussel M et al. ASH 2017 Abstract 508.
2018 Directory of VA and DoD Facilities
January 2018 Digital Edition
Click here to access the January 2018 Digital Edition.
Table of Contents
- Impact of Drug Shortages on Patient Safety and Pharmacy Operation Costs
- Pulmonary Mucormycosis in a Patient With Uncontrolled Diabetes
- Pilot Inpatient Pain Pharmacist Consult Service at the West Palm Beach VAMC
- The Right, and Now the Wrong of 2017
- Risk Factors Associated With Multidrug-Resistant Pneumonia in Nonhospitalized Patients
- Major General David N.W. Grant: An Early Leader in Air Force Medicine

Click here to access the January 2018 Digital Edition.
Table of Contents
- Impact of Drug Shortages on Patient Safety and Pharmacy Operation Costs
- Pulmonary Mucormycosis in a Patient With Uncontrolled Diabetes
- Pilot Inpatient Pain Pharmacist Consult Service at the West Palm Beach VAMC
- The Right, and Now the Wrong of 2017
- Risk Factors Associated With Multidrug-Resistant Pneumonia in Nonhospitalized Patients
- Major General David N.W. Grant: An Early Leader in Air Force Medicine

Click here to access the January 2018 Digital Edition.
Table of Contents
- Impact of Drug Shortages on Patient Safety and Pharmacy Operation Costs
- Pulmonary Mucormycosis in a Patient With Uncontrolled Diabetes
- Pilot Inpatient Pain Pharmacist Consult Service at the West Palm Beach VAMC
- The Right, and Now the Wrong of 2017
- Risk Factors Associated With Multidrug-Resistant Pneumonia in Nonhospitalized Patients
- Major General David N.W. Grant: An Early Leader in Air Force Medicine

Disorders of diminished motivation: What they are, and how to treat them
Disorders of diminished motivation (DDM)—including apathy, abulia, and akinetic mutism—are characterized by impairment in goal-directed behavior, thought, and emotion.1 These disorders can be observed clinically as a gross underproduction of speech, movement, and emotional response.
DDM are not classified as disorders within DSM-5, and it remains unclear if they are distinct disorders or symptoms that overlap in other conditions. Some sources support distinct diagnoses, while the traditional position is that DDM are variations along a spectrum, with apathy as the mildest form and akinetic mutism as the most severe form (Figure).1-3 DDM can result from various neurologic, medical, psychiatric, socioeconomic, and drug-induced pathologies, and may represent differing severity of the same underlying pathology.1,4 It is postulated that DDM arise from disruptions in the dopaminergic frontal-subcortical-mesolimbic networks.1,4
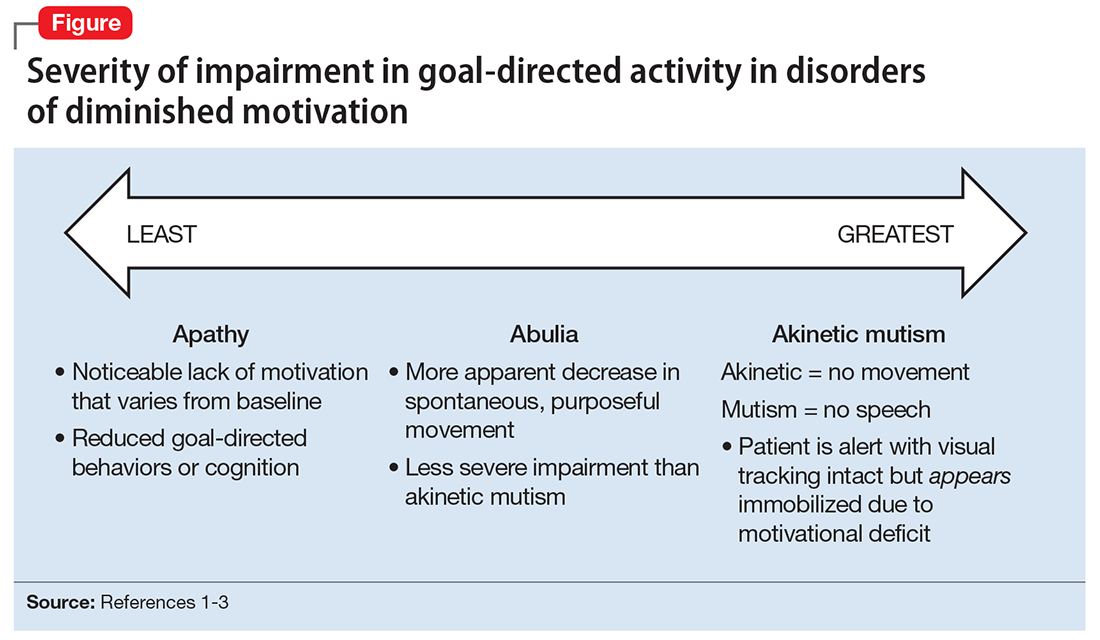
We present 2 cases of patients who developed distinct phenotypes within DDM. Despite differences in presentation and symptom severity, both patients showed clinical improvement on methylphenidate (not the only treatment option) as assessed by the Neuropsychiatric Inventory (NPI),5 a scale used to measure dementia-related behavioral symptoms that includes an Apathy/Indifference (A/I) subscale.
CASE 1
Apathy secondary to glioblastoma multiforme
Ms. E, age 59, presents with wound drainage 3 weeks after a repeat right craniotomy for recurrent glioblastoma multiforme (GBM) of the temporal lobe. Her medical history is not believed to have contributed to her current presentation.
On hospital day 2, Ms. E undergoes debridement and reclosure at the craniotomy site. Prior to the procedure, the patient was noted to have anhedonia and flat affect. Her family reports that she seems to get little enjoyment from life and “only slept and ate.” Psychiatry is consulted on hospital day 3 for evaluation and management of a perceived depressed mood.
On initial psychiatric evaluation, Ms. E continues to have a constricted affect with delayed psychomotor processing speed. However, she denies dysphoria or anhedonia. Richmond Agitation-Sedation Scale6 score is 0 (alert and calm) and test of sustained attention (‘Vigilant A’) is intact (ie, based on the Confusion Assessment Method for the Intensive Care Unit [CAM-ICU],7 Ms. E does not have delirium). The NPI A/I frequency score is 15, with a severity score of 3, for a total score of 45, indicating moderate behavioral disturbance on the NPI A/I subsection. A diagnosis of neuropsychiatric apathy due to recurrent GBM or craniotomy is made, although substance-induced mood disorder due to concurrent dexamethasone and opiate use is considered. Methylphenidate, 2.5 mg/d, is started, and Ms. E’s blood pressure remains stable with the initial dose.
Methylphenidate is titrated to 5 mg, twice daily, over a 1-week period. Ms. E’s NPI A/I subscale score improves to 3 (mild behavioral problem), with 3 points for frequency and a multiplier of 1 for mild severity, reflecting an improvement in neuropsychiatric apathy, and she is transferred to a long-term care rehabilitation center.
CASE 2
Akinetic mutism secondary to subarachnoid hemorrhage
Ms. G, age 47, is brought to an outside hospital with syncope and a severe headache radiating to her neck. Upon arrival, she is unconscious and requires intubation. A non-contrast head CT scan shows diffuse subarachnoid hemorrhage, 6 mm right midline shift, and a small left frontal subdural hematoma. A CT angiography of her head and neck reveals a 0.7 cm anterior paraclinoid left internal carotid artery aneurysm with ophthalmic involvement. Evidence of underlying left and right carotid fibromuscular dysplasia is also seen. Ms. G is transferred to our facility for neurosurgical intervention.
Neurosurgery proceeds with aneurysm coiling, followed by left craniotomy with subdural evacuation and ventriculostomy placement. Her postoperative course is complicated by prolonged nasogastric hyperalimentation, mild hypernatremia and hyperglycemia, tracheostomy, and recurrent central fever. She also develops persistent vasospasm, which requires balloon angioplasty of the left middle cerebral artery.
The psychiatry team is consulted on postoperative day 29 to assess for delirium. The CAM-ICU is positive for delirium, with nocturnal accentuation of agitation. Ms. G demonstrates paucity of speech and minimal verbal comprehension. She starts oral ziprasidone, 5 mg/d at bedtime. In addition to her original CNS insult, scopolamine patch, 1.5 mg, to decrease respiratory secretions, and IV metronidazole, 500 mg every 8 hours, for skin-site infection, may have been contributing to her delirium.
Ms. G’s delirium quickly resolves; however, on day 32 she continues to demonstrate behavioral and cognitive slowing; The NPI A/I frequency score is 28, with a severity score of 3, for a total score of 84, indicating severe behavioral disturbance on the NPI A/I subsection. Methylphenidate, 2.5 mg/d, is started and the next day is increased to 5 mg twice a day to treat severe akinetic mutism. Ms. G also is switched from ziprasidone to olanzapine, 2.5 mg/d at night.
By day 37, the tracheostomy is decannulated, and Ms. G demonstrates a full level of alertness, awareness, and attention. Her affect is full range and appropriate; however, she demonstrates residual language deficits, including dysnomia. On day 38, Ms. G is discharged with an NPI A/I subscale score of 5, indicating a mild behavioral problem.
What these cases demonstrate about DDM
These 2 cases are part of a larger, emerging conversation about the role of dopamine in DDM. Although not fully elucidated, the pathophysiology of abulia, apathy, and akinetic mutism is thought to be related to multiple neurotransmitters—especially dopamine—involved in the cortico-striatal-pallidal-thalamic network.1,8 This position has been supported by reports of clinical improvement in patients with DDM who are given dopaminergic agonists (Table 1).3,9-32
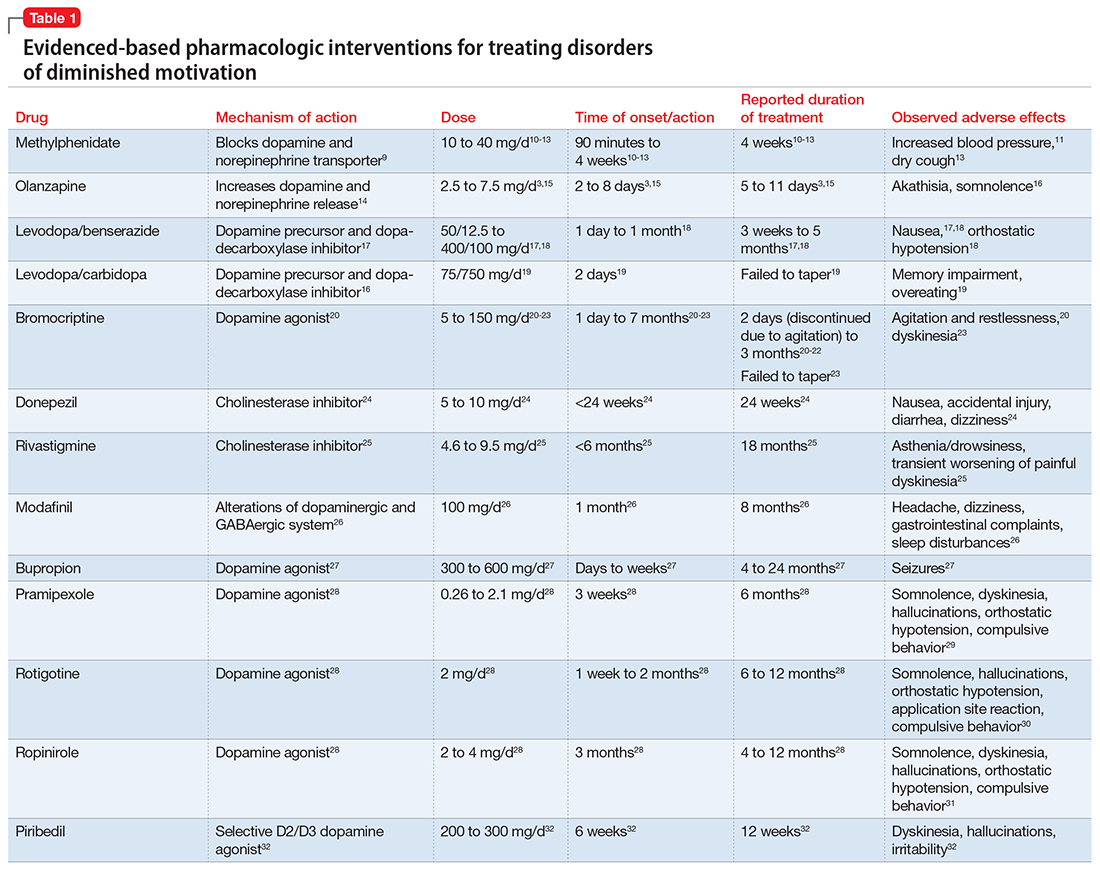
The clinical improvement seen in both of our patients after initiating methylphenidate is consistent with previous reports.10-13 Methylphenidate was selected because of its favorable adverse effect profile and potentially rapid onset of action in DDM.10-13 In cases where oral medication cannot be administered, such as in patients with akinetic mutism, short-term adjunctive IM olanzapine may be helpful, although it is not a first-line treatment.3,15
Interestingly, both of our patients showed improvement with low doses of methylphenidate. Ms. E showed rapid improvement at 2.5 mg/d, but eventually was increased to 10 mg/d. For Ms. G, who demonstrated severe akinetic mutism, rapid improvement was noted after the initial 2.5 mg/d dose; however, because of reports of efficacy of olanzapine in treating akinetic mutism, it is possible that these medications worked synergistically. The proposed mechanism of action of olanzapine in akinetic mutism is through increased dopamine transmission in the medial prefrontal cortex.3,15 Ms. G’s methylphenidate dose was increased to 5 mg/d, which was still “subtherapeutic,” because most reports have used dosages ranging from 10 to 40 mg/d.10-13 Although there were favorable acute results in both patients, their long-term requirements are unknown because of a lack of follow-up. Our findings are also limited by the fact that both patients were recovering from neurosurgical procedures, which could lead to natural improvement in symptoms over time.
Prevalence of DDM in psychiatric disorders
The successful treatment of DDM with dopaminergic drugs is meaningful because of the coexistence of DDM in various neuropsychiatric conditions. In Alzheimer’s disease (AD), disturbances in the dopaminergic system may explain the high comorbidity of apathy, which ranges from 47% in mild AD to 80% in moderate AD.33 In the dopamine-reduced states of cocaine and amphetamine withdrawal, 67% of patients report apathy and lack of motivation.8,34 Additionally, the prevalence of apathy is reported at 27% in Parkinson’s disease, 43% in mild cognitive impairment, 70% in mixed dementia, 94% in a major depressive episode, and 53% in schizophrenia.35 In schizophrenia with predominately negative symptoms, in vivo and postmortem studies have found reduced dopamine receptors.8 Meanwhile, the high rate of akinetic mutism in Creutzfeldt-Jakob disease allows for its use as a reliable diagnostic criteria in this disorder.36
However, the prevalence of DDM is best documented as it relates to stroke and traumatic brain injury (TBI). For instance, after experiencing a stroke, 20% to 25% of patients suffer from apathy.37 Many case reports describe abulia and akinetic mutism after cerebral infarction or hemorrhage, although the incidence of these disorders is unknown.2,38-40 Apathy following TBI is common, especially in younger patients who have sustained a severe injury.41 One study evaluated the prevalence of apathy after TBI among 83 consecutive patients in a neuropsychiatric clinic. Of the 83 patients, 10.84% had apathy without depression, and an equal number were depressed without apathy; another 60% of patients exhibited both apathy and depression. Younger patients (mean age, 29 years) were more likely to be apathetic than older patients, who were more likely to be depressed or depressed and apathetic (mean age, 42 and 38 years, respectively).41 Interestingly, DDM often are associated with cerebral lesions in distinct and distant anatomical locations that are not clearly connected to the neural circuits of motivational pathways. This phenomenon may be explained by the concept of diaschisis, which states that injury to one part of an interconnected neural network can affect other, separate parts of that network.2 If this concept is accurate, it may broaden the impact of DDM, especially as it relates to stroke and TBI.
The differential diagnosis of DDM includes depression and hypokinetic delirium (Table 21,3,42-50). A potential overlapping but confounding condition is stuporous catatonia, with symptoms that include psychomotor slowing such as immobility, staring, and stupor.47 It is important to differentiate these disorders because the treatment for each differs. As previously discussed, there is a clear role for dopamine receptor agonists in the treatment of DDM.
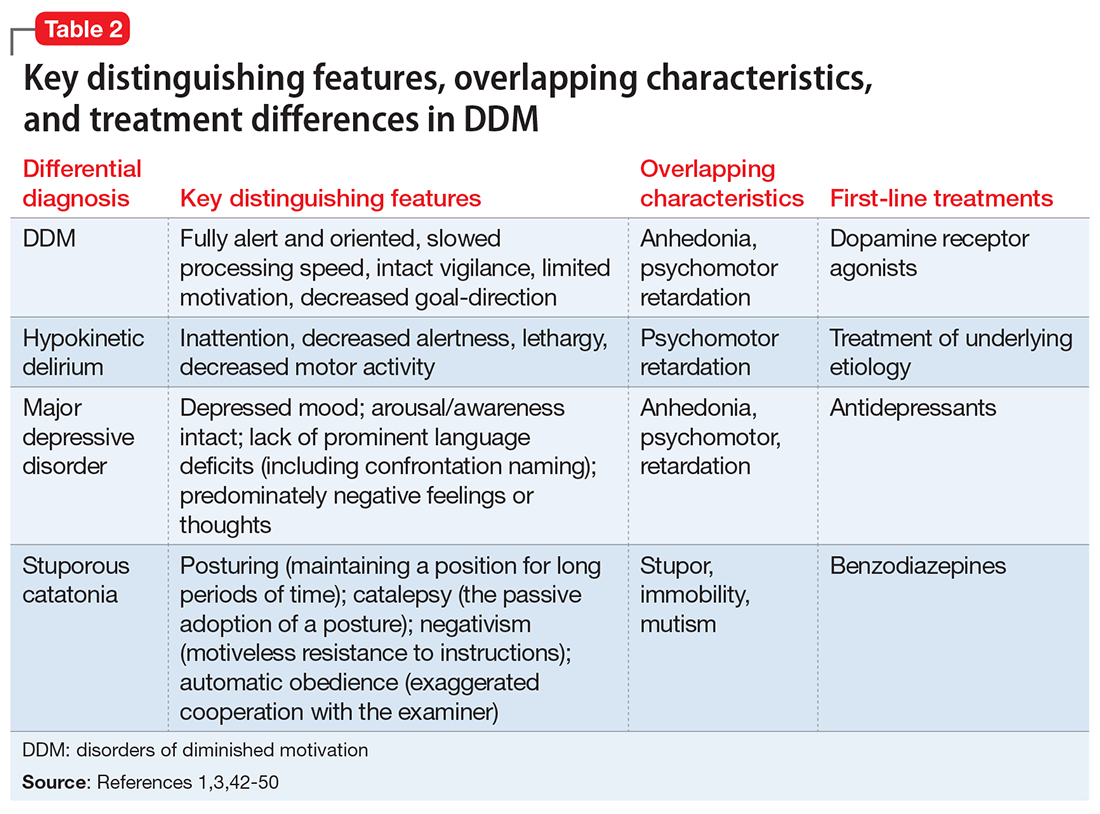
Although major depressive disorder can be treated with medications that increase dopaminergic transmission, selective serotonin reuptake inhibitors (SSRIs) are more commonly used as first-line agents.44 However, an SSRI would theoretically be contraindicated in DDM, because increased serotonin transmission decreases dopamine release from the midbrain, and therefore an SSRI may not only result in a lack of improvement but may worsen DDM.48 Finally, although delirium is treated with atypical or conventional antipsychotics vis-a-vis dopamine type 2 receptor antagonism,45 stuporous catatonia is preferentially treated with gamma-aminobutyric acid-A receptor agonists such as lorazepam.50
What to do when your patient’s presentation suggests DDM
Assessment of DDM should be structured, with input from the patient and the caregiver, and should incorporate the physician’s perspective. A history should be obtained applying recent criteria of apathy. The 3 core domains of apathy—behavior, cognition, and emotion—need to be evaluated. The revised criteria are based on the premise that change in motivation can be measured by examining a patient’s responsiveness to internal or external stimuli. Therefore, each of the 3 domains includes 2 symptoms: (1) self-initiated or “internal” behaviors, cognitions, and emotions (initiation symptom), and (2) the patient’s responsiveness to “external” stimuli (responsiveness symptom).51
One of the main diagnostic dilemmas is how to separate DDM from depression. The differentiation is difficult because of substantial overlap in the manifestation of key symptoms, such as a lack of interest, anergia, psychomotor slowing, and fatigue. Caregivers often mistakenly describe DDM as a depressive state, even though a lack of sadness, desperation, crying, and a depressive mood distinguish DDM from depression. Usually, DDM patients lack negative thoughts, emotional distress, sadness, vegetative symptoms, and somatic concerns, which are frequently observed in mood disorders.51
Several instruments have been developed for assessing neuropsychiatric symptoms. Some were specifically designed to measure apathy, whereas others were designed to provide a broader neuropsychiatric assessment. The NPI is the most widely used multidimensional instrument for assessing neuropsychiatric functioning in patients with neurocognitive disorders (NCDs). It is a valid, reliable instrument that consists of an interview of the patient’s caregiver. It is designed to assess the presence and severity of 10 symptoms, including apathy. The NPI includes both apathy and depression items, which can help clinicians distinguish the 2 conditions. Although beyond the scope of this article, more recent standardized instruments that can assess DDM include the Apathy Inventory, the Dementia Apathy Interview and Rating, and the Structured Clinical Interview for Apathy.52
As previously mentioned, researchers have proposed that DDM are simply a continuum of severity of reduced behavior, and akinetic mutism may be the extreme form. The dilemma is how to formally diagnose states of abulia and akinetic mutism, given the lack of diagnostic criteria and paucity of standardized instruments. Thus, distinguishing between abulia and akinetic mutism (and apathy) is more of a quantitative than qualitative exercise. One could hypothesize that higher scores on a standardized scale to measure apathy (ie, NPI) could imply abulia or akinetic mutism, although to the best of our knowledge, no formal “cut-off scores” exist.53
Treatment of apathy. The duration of pharmacotherapy to treat apathy is unknown and their usage is off-label. Further studies, including double-blind, randomized controlled trials (RCTs), are needed. Nonetheless, the 2 classes of medications that have the most evidence for treating apathy/DDM are psychostimulants and acetylcholinesterase inhibitors (AChEIs).
AChEIs are primarily used for treating cognitive symptoms in NCDs, but recent findings indicate that they have beneficial effects on noncognitive symptoms such as apathy. Of all medications used to treat apathy in NCDs, AChEIs have been used to treat the largest number of patients. Of 26 studies, 24 demonstrated improvement in apathy, with 21 demonstrating statistical significance. These studies ranged in duration from 8 weeks to 1 year, and most were open-label.54
Five studies (3 RCTs and 2 open-label studies) assessed the efficacy of methylphenidate for treating apathy due to AD. All the studies demonstrated at least some benefit in apathy scores after treatment with methylphenidate. These studies ranged from 5 to 12 weeks in duration. Notably, some patients reported adverse effects, including delusions and irritability.54
Although available evidence suggests AChEIs may be the most effective medications for treating apathy in NCDs, methylphenidate has been demonstrated to work faster.55 Thus, in cases where apathy can significantly affect activities of daily living or instrumental activities of daily living, a quicker response may dictate treatment with methylphenidate. It is imperative to note that safety studies and more large-scale double-blind RCTs are needed to further demonstrate the effectiveness and safety of methylphenidate.
Published in 2007, the American Psychiatric Association (APA) guidelines56 state that psychostimulants are a possible treatment option for patients with severe apathy. At the same time, clinicians are reminded that these agents—especially at higher doses—can produce various problematic adverse effects, including tachycardia, hypertension, restlessness, dyskinesia, agitation, sleep disturbances, psychosis, confusion, and decreased appetite. The APA guidelines recommend using low initial doses, with slow and careful titration. For example, methylphenidate should be started at 2.5 to 5 mg once in the morning, with daily doses not to exceed 30 to 40 mg. In our clinical experience, doses >20 mg/d have not been necessary.57
Treatment of akinetic mutism and abulia. In patients with akinetic mutism and possible abulia, for whom oral medication administration is either impossible or contraindicated (ie, due to the potential risk of aspiration pneumonia), atypical antipsychotics, such as IM olanazapine, have produced a therapeutic response in apathetic patients with NCD. However, extensive use of antipsychotics in NCD is not recommended because this class of medications has been associated with serious adverse effects, including an increased risk of death.55
Bottom Line
Apathy, abulia, and akinetic mutism have been categorized as disorders of diminished motivation (DDM). They commonly present after a stroke or traumatic brain injury, and should be differentiated from depression, hypokinetic delirium, and stuporous catatonia. DDM can be successfully treated with dopamine agonists.
Related Resources
- Barnhart WJ, Makela EH, Latocha MJ. SSRI-induced apathy syndrome: a clinical review. J Psychiatr Pract. 2004;10(3):196-199.
- Dell’Osso B, Benatti B, Altamura AC, et al. Prevalence of selective serotonin reuptake inhibitor-related apathy in patients with obsessive compulsive disorder. J Clin Psychopharmacol. 2016;36(6):725-726.
- D’Souza G, Kakoullis A, Hegde N, et al. Recognition and management of abulia in the elderly. Prog Neurol Psychiatry. 2010;14(6):24-28.
Drug Brand Names
Bromocriptine • Parlodel
Bupropion • Wellbutrin XL, Zyban
Carbidopa • Lodosyn
Dexamethasone • DexPak, Ozurde
Donepezil • Aricept
Levodopa/benserazide • Prolopa
Levodopa/carbidopa • Pacopa Rytary Sinemet
Lorazepam • Ativan
Methylphenidate • Concerta, Methylin
Metronidazole • Flagyl, Metrogel
Modafinil • Provigil
Olanzapine • Zyprexa
Pramipexole • Mirapex
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neurpro
Scopolamine • Transderm Scop
Ziprasidone • Geodon
1. Marin RS, Wilkosz PA. Disorders of diminished motivation. J Head Trauma Rehabil. 2005;20(4):377-388.
2. Ghoshal S, Gokhale S, Rebovich G, et al. The neurology of decreased activity: abulia. Rev Neurol Dis. 2011;8(3-4):e55-e67.
3. Spiegel DR, Chatterjee A. A case of abulia, status/post right middle cerebral artery territory infarct, treated successfully with olanzapine. Clin Neuropharmacol. 2014;37(6):186-189.
4. Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147(1):22-30.
5. Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314.
6. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344.
7. Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the intensive care unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379.
8. Al-Adawi S, Dawe GS, Al-Hussaini AA. Aboulia: neurobehavioural dysfunction of dopaminergic system? Med Hypotheses. 2000;54(4):523-530.
9. Volkow ND, Fowler JS, Wang G, et al. Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord. 2002;6(suppl 1):S31-S43.
10. Chatterjee A, Fahn S. Methylphenidate treats apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2002;14(4):461-462.
11. Keenan S, Mavaddat N, Iddon J, et al. Effects of methylphenidate on cognition and apathy in normal pressure hydrocephalus: a case study and review. Br J Neurosurg. 2005;19(1):46-50.
12. Padala PR, Petty F, Bhatia SC. Methylphenidate may treat apathy independent of depression. Ann Pharmacother. 2005;39(11):1947-1949.
13. Padala PR, Burke WJ, Bhatia SC, et al. Treatment of apathy with methylphenidate. J Neuropsychiatry Clin Neurosci. 2007;19(1):81-83.
14. Li XM, Perry KW, Wong DT, et al. Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacology (Berl). 1998;136(2):153-161.
15. Spiegel DR, Casella DP, Callender DM, et al. Treatment of akinetic mutism with intramuscular olanzapine: a case series. J Neuropsychiatry Clin Neurosci. 2008;20(1):93-95.
16. Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37(2):138-147.
17. Bakheit AM, Fletcher K, Brennan A. Successful treatment of severe abulia with co-beneldopa. NeuroRehabilitation. 2011;29(4):347-351.
18. Debette S, Kozlowski O, Steinling M, et al. Levodopa and bromocriptine in hypoxic brain injury. J Neurol. 2002;249(12):1678-1682.
19. Combarros O, Infante J, Berciano J. Akinetic mutism from frontal lobe damage responding to levodopa. J Neurol. 2000;247(7):568-569.
20. Echiverri HC, Tatum WO, Merens TA, et al. Akinetic mutism: pharmacologic probe of the dopaminergic mesencephalofrontal activating system. Pediatr Neurol. 1988;4(4):228-230.
21. Psarros T, Zouros A, Coimbra C. Bromocriptine-responsive akinetic mutism following endoscopy for ventricular neurocysticercosis. Case report and review of the literature. J Neurosurg. 2003;99(2):397-401.
22. Naik VD. Abulia following an episode of cardiac arrest [published online July 1, 2015]. BMJ Case Rep. doi: 10.1136/bcr-2015-209357.
23. Kim MS, Rhee JJ, Lee SJ, et al. Akinetic mutism responsive to bromocriptine following subdural hematoma evacuation in a patient with hydrocephalus. Neurol Med Chir (Tokyo). 2007;47(9):419-423.
24. Rockwood K, Black S, Bedard MA; TOPS Study Investigators. Specific symptomatic changes following donepezil treatment of Alzheimer’s disease: a multi-centre, primary care, open-label study. Int J Geriatr Psychiatry. 2007;22(4):312-319.
25. Devos D, Moreau C, Maltête D, et al. Rivastigmine in apathetic but dementia and depression-free patients with Parkinson’s disease: a double-blind, placebo-controlled, randomised clinical trial. J Neurol Neurosurg Psychiatry. 2014;85(6):668-674.
26. Camargos EF, Quintas JL. Apathy syndrome treated successfully with modafinil [published online November 15, 2011]. BMJ Case Rep. doi: 10.1136/bcr.08.2011.4652.
27. Corcoran C, Wong ML, O’Keane V. Bupropion in the management of apathy. J Psychopharmacol. 2004;18(1):133-135.
28. Blundo C, Gerace C. Dopamine agonists can improve pure apathy associated with lesions of the prefrontal-basal ganglia functional system. Neurol Sci. 2015;36(7):1197-1201.
29. Mirapex [package insert]. Ridgefield, CT: Boehringer Ingelheim International GmbH; 2016.
30. Neupro [package insert]. Smyrna, GA: UBC, Inc.; 2012.
31. Requip [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
32. Thobois S, Lhommée E, Klinger H, et al. Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with piribedil. Brain. 2013;136(pt 5):1568-1577.
33. Mitchell RA, Herrmann N, Lanctôt KL. The role of dopamine in symptoms and treatment of apathy in Alzheimer’s disease. CNS Neurosci Ther. 2011;17(5):411-427.
34. Brower KJ, Maddahian E, Blow FC, et al. A comparison of self-reported symptoms and DSM-III-R criteria for cocaine withdrawal. Am J Drug Alcohol Abuse. 1988;14(3):347-356.
35. Mulin E, Leone E, Dujardin K, et al. Diagnostic criteria for apathy in clinical practice. Int J Geriatr Psychiatry. 2011;26(2):158-165.
36. Otto A, Zerr I, Lantsch M, et al. Akinetic mutism as a classification criterion for the diagnosis of Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 1998;64(4):524-528.
37. Jorge RE, Starkstein SE, Robinson RG. Apathy following stroke. Can J Psychiatry. 2010;55(6):350-354.
38. Hastak SM, Gorawara PS, Mishra NK. Abulia: no will, no way. J Assoc Physicians India. 2005;53:814-818.
39. Nagaratnam N, Nagaratnam K, Ng K, et al. Akinetic mutism following stroke. J Clin Neurosci. 2004;11(1):25-30.
40. Freemon FR. Akinetic mutism and bilateral anterior cerebral artery occlusion. J Neurol Neurosurg Psychiatry. 1971;34(6):693-698.
41. Schwarzbold M, Diaz A, Martins ET, et al. Psychiatric disorders and traumatic brain injury. Neuropsychiatr Dis Treat. 2008;4(4):797-816.
42. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
43. Levy ML, Cummings JL, Fairbanks LA, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10(3):314-319.
44. Snow V, Lascher S, Mottur-Pilson C. Pharmacologic treatment of acute major depression and dysthymia. American College of Physicians-American Society of Internal Medicine. Ann Intern Med. 2000;132(9):738-742.
45. Schwartz AC, Fisher TJ, Greenspan HN, et al. Pharmacologic and nonpharmacologic approaches to the prevention and management of delirium. Int J Psychiatry Med. 2016;51(2):160-170.
46. Kang H, Zhao F, You L, et al. Pseudo-dementia: a neuropsychological review. Ann Indian Acad Neurol. 2014;17(2):147-154.
47. Fricchione GL, Beach SR, Huffman J, et al. Life-threatening conditions in psychiatry: catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fava M, Wilens TE, eds. Massachusetts General Hospital comprehensive clinical psychiatry. London, United Kingdom: Elsevier; 2016:608-617.
48. Rogers RD. The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacology. 2011;36(1):114-132.
49. Stransky M, Schmidt C, Ganslmeier P, et al. Hypoactive delirium after cardiac surgery as an independent risk factor for prolonged mechanical ventilation. J Cardiothorac Vasc Anesth. 2011;25(6):968-974.
50. Wilcox JA, Reid Duffy P. The syndrome of catatonia. Behav Sci (Basel). 2015;5(4):576-588.
51. Robert PH, Mulin E, Malléa P, et al. REVIEW: apathy diagnosis, assessment, and treatment in Alzheimer’s disease. CNS Neurosci Ther. 2010;16(5):263-271.
52. Cipriani G, Lucetti C, Danti S, et al. Apathy and dementia. Nosology, assessment and management. J Nerv Ment Dis. 2014;202(10):718-724.
53. Starkstein SE, Leentjens AF. The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry. 2008;79(10):1088-1092.54. Berman K, Brodaty H, Withall A, et al. Pharmacologic treatment of apathy in dementia. Am J Geriatr Psychiatry. 2012;20(2):104-122.
55. Theleritis C, Siarkos K, Katirtzoglou E, et al. Pharmacological and nonpharmacological treatment for apathy in Alzheimer disease: a systematic review across modalities. J Geriatr Psychiatry Neurol. 2017;30(1):26-49.
56. APA Work Group on Alzheimer’s Disease and other Dementias; Rabins PV, Blacker D, Rovner BW, et al. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Second edition. Am J Psychiatry. 2007;164(suppl 12):5-56.
57. Dolder CR, Davis LN, McKinsey J. Use of psychostimulants in patients with dementia. Ann Pharmacother. 2010;44(10):1624-1632.
Disorders of diminished motivation (DDM)—including apathy, abulia, and akinetic mutism—are characterized by impairment in goal-directed behavior, thought, and emotion.1 These disorders can be observed clinically as a gross underproduction of speech, movement, and emotional response.
DDM are not classified as disorders within DSM-5, and it remains unclear if they are distinct disorders or symptoms that overlap in other conditions. Some sources support distinct diagnoses, while the traditional position is that DDM are variations along a spectrum, with apathy as the mildest form and akinetic mutism as the most severe form (Figure).1-3 DDM can result from various neurologic, medical, psychiatric, socioeconomic, and drug-induced pathologies, and may represent differing severity of the same underlying pathology.1,4 It is postulated that DDM arise from disruptions in the dopaminergic frontal-subcortical-mesolimbic networks.1,4

We present 2 cases of patients who developed distinct phenotypes within DDM. Despite differences in presentation and symptom severity, both patients showed clinical improvement on methylphenidate (not the only treatment option) as assessed by the Neuropsychiatric Inventory (NPI),5 a scale used to measure dementia-related behavioral symptoms that includes an Apathy/Indifference (A/I) subscale.
CASE 1
Apathy secondary to glioblastoma multiforme
Ms. E, age 59, presents with wound drainage 3 weeks after a repeat right craniotomy for recurrent glioblastoma multiforme (GBM) of the temporal lobe. Her medical history is not believed to have contributed to her current presentation.
On hospital day 2, Ms. E undergoes debridement and reclosure at the craniotomy site. Prior to the procedure, the patient was noted to have anhedonia and flat affect. Her family reports that she seems to get little enjoyment from life and “only slept and ate.” Psychiatry is consulted on hospital day 3 for evaluation and management of a perceived depressed mood.
On initial psychiatric evaluation, Ms. E continues to have a constricted affect with delayed psychomotor processing speed. However, she denies dysphoria or anhedonia. Richmond Agitation-Sedation Scale6 score is 0 (alert and calm) and test of sustained attention (‘Vigilant A’) is intact (ie, based on the Confusion Assessment Method for the Intensive Care Unit [CAM-ICU],7 Ms. E does not have delirium). The NPI A/I frequency score is 15, with a severity score of 3, for a total score of 45, indicating moderate behavioral disturbance on the NPI A/I subsection. A diagnosis of neuropsychiatric apathy due to recurrent GBM or craniotomy is made, although substance-induced mood disorder due to concurrent dexamethasone and opiate use is considered. Methylphenidate, 2.5 mg/d, is started, and Ms. E’s blood pressure remains stable with the initial dose.
Methylphenidate is titrated to 5 mg, twice daily, over a 1-week period. Ms. E’s NPI A/I subscale score improves to 3 (mild behavioral problem), with 3 points for frequency and a multiplier of 1 for mild severity, reflecting an improvement in neuropsychiatric apathy, and she is transferred to a long-term care rehabilitation center.
CASE 2
Akinetic mutism secondary to subarachnoid hemorrhage
Ms. G, age 47, is brought to an outside hospital with syncope and a severe headache radiating to her neck. Upon arrival, she is unconscious and requires intubation. A non-contrast head CT scan shows diffuse subarachnoid hemorrhage, 6 mm right midline shift, and a small left frontal subdural hematoma. A CT angiography of her head and neck reveals a 0.7 cm anterior paraclinoid left internal carotid artery aneurysm with ophthalmic involvement. Evidence of underlying left and right carotid fibromuscular dysplasia is also seen. Ms. G is transferred to our facility for neurosurgical intervention.
Neurosurgery proceeds with aneurysm coiling, followed by left craniotomy with subdural evacuation and ventriculostomy placement. Her postoperative course is complicated by prolonged nasogastric hyperalimentation, mild hypernatremia and hyperglycemia, tracheostomy, and recurrent central fever. She also develops persistent vasospasm, which requires balloon angioplasty of the left middle cerebral artery.
The psychiatry team is consulted on postoperative day 29 to assess for delirium. The CAM-ICU is positive for delirium, with nocturnal accentuation of agitation. Ms. G demonstrates paucity of speech and minimal verbal comprehension. She starts oral ziprasidone, 5 mg/d at bedtime. In addition to her original CNS insult, scopolamine patch, 1.5 mg, to decrease respiratory secretions, and IV metronidazole, 500 mg every 8 hours, for skin-site infection, may have been contributing to her delirium.
Ms. G’s delirium quickly resolves; however, on day 32 she continues to demonstrate behavioral and cognitive slowing; The NPI A/I frequency score is 28, with a severity score of 3, for a total score of 84, indicating severe behavioral disturbance on the NPI A/I subsection. Methylphenidate, 2.5 mg/d, is started and the next day is increased to 5 mg twice a day to treat severe akinetic mutism. Ms. G also is switched from ziprasidone to olanzapine, 2.5 mg/d at night.
By day 37, the tracheostomy is decannulated, and Ms. G demonstrates a full level of alertness, awareness, and attention. Her affect is full range and appropriate; however, she demonstrates residual language deficits, including dysnomia. On day 38, Ms. G is discharged with an NPI A/I subscale score of 5, indicating a mild behavioral problem.
What these cases demonstrate about DDM
These 2 cases are part of a larger, emerging conversation about the role of dopamine in DDM. Although not fully elucidated, the pathophysiology of abulia, apathy, and akinetic mutism is thought to be related to multiple neurotransmitters—especially dopamine—involved in the cortico-striatal-pallidal-thalamic network.1,8 This position has been supported by reports of clinical improvement in patients with DDM who are given dopaminergic agonists (Table 1).3,9-32

The clinical improvement seen in both of our patients after initiating methylphenidate is consistent with previous reports.10-13 Methylphenidate was selected because of its favorable adverse effect profile and potentially rapid onset of action in DDM.10-13 In cases where oral medication cannot be administered, such as in patients with akinetic mutism, short-term adjunctive IM olanzapine may be helpful, although it is not a first-line treatment.3,15
Interestingly, both of our patients showed improvement with low doses of methylphenidate. Ms. E showed rapid improvement at 2.5 mg/d, but eventually was increased to 10 mg/d. For Ms. G, who demonstrated severe akinetic mutism, rapid improvement was noted after the initial 2.5 mg/d dose; however, because of reports of efficacy of olanzapine in treating akinetic mutism, it is possible that these medications worked synergistically. The proposed mechanism of action of olanzapine in akinetic mutism is through increased dopamine transmission in the medial prefrontal cortex.3,15 Ms. G’s methylphenidate dose was increased to 5 mg/d, which was still “subtherapeutic,” because most reports have used dosages ranging from 10 to 40 mg/d.10-13 Although there were favorable acute results in both patients, their long-term requirements are unknown because of a lack of follow-up. Our findings are also limited by the fact that both patients were recovering from neurosurgical procedures, which could lead to natural improvement in symptoms over time.
Prevalence of DDM in psychiatric disorders
The successful treatment of DDM with dopaminergic drugs is meaningful because of the coexistence of DDM in various neuropsychiatric conditions. In Alzheimer’s disease (AD), disturbances in the dopaminergic system may explain the high comorbidity of apathy, which ranges from 47% in mild AD to 80% in moderate AD.33 In the dopamine-reduced states of cocaine and amphetamine withdrawal, 67% of patients report apathy and lack of motivation.8,34 Additionally, the prevalence of apathy is reported at 27% in Parkinson’s disease, 43% in mild cognitive impairment, 70% in mixed dementia, 94% in a major depressive episode, and 53% in schizophrenia.35 In schizophrenia with predominately negative symptoms, in vivo and postmortem studies have found reduced dopamine receptors.8 Meanwhile, the high rate of akinetic mutism in Creutzfeldt-Jakob disease allows for its use as a reliable diagnostic criteria in this disorder.36
However, the prevalence of DDM is best documented as it relates to stroke and traumatic brain injury (TBI). For instance, after experiencing a stroke, 20% to 25% of patients suffer from apathy.37 Many case reports describe abulia and akinetic mutism after cerebral infarction or hemorrhage, although the incidence of these disorders is unknown.2,38-40 Apathy following TBI is common, especially in younger patients who have sustained a severe injury.41 One study evaluated the prevalence of apathy after TBI among 83 consecutive patients in a neuropsychiatric clinic. Of the 83 patients, 10.84% had apathy without depression, and an equal number were depressed without apathy; another 60% of patients exhibited both apathy and depression. Younger patients (mean age, 29 years) were more likely to be apathetic than older patients, who were more likely to be depressed or depressed and apathetic (mean age, 42 and 38 years, respectively).41 Interestingly, DDM often are associated with cerebral lesions in distinct and distant anatomical locations that are not clearly connected to the neural circuits of motivational pathways. This phenomenon may be explained by the concept of diaschisis, which states that injury to one part of an interconnected neural network can affect other, separate parts of that network.2 If this concept is accurate, it may broaden the impact of DDM, especially as it relates to stroke and TBI.
The differential diagnosis of DDM includes depression and hypokinetic delirium (Table 21,3,42-50). A potential overlapping but confounding condition is stuporous catatonia, with symptoms that include psychomotor slowing such as immobility, staring, and stupor.47 It is important to differentiate these disorders because the treatment for each differs. As previously discussed, there is a clear role for dopamine receptor agonists in the treatment of DDM.

Although major depressive disorder can be treated with medications that increase dopaminergic transmission, selective serotonin reuptake inhibitors (SSRIs) are more commonly used as first-line agents.44 However, an SSRI would theoretically be contraindicated in DDM, because increased serotonin transmission decreases dopamine release from the midbrain, and therefore an SSRI may not only result in a lack of improvement but may worsen DDM.48 Finally, although delirium is treated with atypical or conventional antipsychotics vis-a-vis dopamine type 2 receptor antagonism,45 stuporous catatonia is preferentially treated with gamma-aminobutyric acid-A receptor agonists such as lorazepam.50
What to do when your patient’s presentation suggests DDM
Assessment of DDM should be structured, with input from the patient and the caregiver, and should incorporate the physician’s perspective. A history should be obtained applying recent criteria of apathy. The 3 core domains of apathy—behavior, cognition, and emotion—need to be evaluated. The revised criteria are based on the premise that change in motivation can be measured by examining a patient’s responsiveness to internal or external stimuli. Therefore, each of the 3 domains includes 2 symptoms: (1) self-initiated or “internal” behaviors, cognitions, and emotions (initiation symptom), and (2) the patient’s responsiveness to “external” stimuli (responsiveness symptom).51
One of the main diagnostic dilemmas is how to separate DDM from depression. The differentiation is difficult because of substantial overlap in the manifestation of key symptoms, such as a lack of interest, anergia, psychomotor slowing, and fatigue. Caregivers often mistakenly describe DDM as a depressive state, even though a lack of sadness, desperation, crying, and a depressive mood distinguish DDM from depression. Usually, DDM patients lack negative thoughts, emotional distress, sadness, vegetative symptoms, and somatic concerns, which are frequently observed in mood disorders.51
Several instruments have been developed for assessing neuropsychiatric symptoms. Some were specifically designed to measure apathy, whereas others were designed to provide a broader neuropsychiatric assessment. The NPI is the most widely used multidimensional instrument for assessing neuropsychiatric functioning in patients with neurocognitive disorders (NCDs). It is a valid, reliable instrument that consists of an interview of the patient’s caregiver. It is designed to assess the presence and severity of 10 symptoms, including apathy. The NPI includes both apathy and depression items, which can help clinicians distinguish the 2 conditions. Although beyond the scope of this article, more recent standardized instruments that can assess DDM include the Apathy Inventory, the Dementia Apathy Interview and Rating, and the Structured Clinical Interview for Apathy.52
As previously mentioned, researchers have proposed that DDM are simply a continuum of severity of reduced behavior, and akinetic mutism may be the extreme form. The dilemma is how to formally diagnose states of abulia and akinetic mutism, given the lack of diagnostic criteria and paucity of standardized instruments. Thus, distinguishing between abulia and akinetic mutism (and apathy) is more of a quantitative than qualitative exercise. One could hypothesize that higher scores on a standardized scale to measure apathy (ie, NPI) could imply abulia or akinetic mutism, although to the best of our knowledge, no formal “cut-off scores” exist.53
Treatment of apathy. The duration of pharmacotherapy to treat apathy is unknown and their usage is off-label. Further studies, including double-blind, randomized controlled trials (RCTs), are needed. Nonetheless, the 2 classes of medications that have the most evidence for treating apathy/DDM are psychostimulants and acetylcholinesterase inhibitors (AChEIs).
AChEIs are primarily used for treating cognitive symptoms in NCDs, but recent findings indicate that they have beneficial effects on noncognitive symptoms such as apathy. Of all medications used to treat apathy in NCDs, AChEIs have been used to treat the largest number of patients. Of 26 studies, 24 demonstrated improvement in apathy, with 21 demonstrating statistical significance. These studies ranged in duration from 8 weeks to 1 year, and most were open-label.54
Five studies (3 RCTs and 2 open-label studies) assessed the efficacy of methylphenidate for treating apathy due to AD. All the studies demonstrated at least some benefit in apathy scores after treatment with methylphenidate. These studies ranged from 5 to 12 weeks in duration. Notably, some patients reported adverse effects, including delusions and irritability.54
Although available evidence suggests AChEIs may be the most effective medications for treating apathy in NCDs, methylphenidate has been demonstrated to work faster.55 Thus, in cases where apathy can significantly affect activities of daily living or instrumental activities of daily living, a quicker response may dictate treatment with methylphenidate. It is imperative to note that safety studies and more large-scale double-blind RCTs are needed to further demonstrate the effectiveness and safety of methylphenidate.
Published in 2007, the American Psychiatric Association (APA) guidelines56 state that psychostimulants are a possible treatment option for patients with severe apathy. At the same time, clinicians are reminded that these agents—especially at higher doses—can produce various problematic adverse effects, including tachycardia, hypertension, restlessness, dyskinesia, agitation, sleep disturbances, psychosis, confusion, and decreased appetite. The APA guidelines recommend using low initial doses, with slow and careful titration. For example, methylphenidate should be started at 2.5 to 5 mg once in the morning, with daily doses not to exceed 30 to 40 mg. In our clinical experience, doses >20 mg/d have not been necessary.57
Treatment of akinetic mutism and abulia. In patients with akinetic mutism and possible abulia, for whom oral medication administration is either impossible or contraindicated (ie, due to the potential risk of aspiration pneumonia), atypical antipsychotics, such as IM olanazapine, have produced a therapeutic response in apathetic patients with NCD. However, extensive use of antipsychotics in NCD is not recommended because this class of medications has been associated with serious adverse effects, including an increased risk of death.55
Bottom Line
Apathy, abulia, and akinetic mutism have been categorized as disorders of diminished motivation (DDM). They commonly present after a stroke or traumatic brain injury, and should be differentiated from depression, hypokinetic delirium, and stuporous catatonia. DDM can be successfully treated with dopamine agonists.
Related Resources
- Barnhart WJ, Makela EH, Latocha MJ. SSRI-induced apathy syndrome: a clinical review. J Psychiatr Pract. 2004;10(3):196-199.
- Dell’Osso B, Benatti B, Altamura AC, et al. Prevalence of selective serotonin reuptake inhibitor-related apathy in patients with obsessive compulsive disorder. J Clin Psychopharmacol. 2016;36(6):725-726.
- D’Souza G, Kakoullis A, Hegde N, et al. Recognition and management of abulia in the elderly. Prog Neurol Psychiatry. 2010;14(6):24-28.
Drug Brand Names
Bromocriptine • Parlodel
Bupropion • Wellbutrin XL, Zyban
Carbidopa • Lodosyn
Dexamethasone • DexPak, Ozurde
Donepezil • Aricept
Levodopa/benserazide • Prolopa
Levodopa/carbidopa • Pacopa Rytary Sinemet
Lorazepam • Ativan
Methylphenidate • Concerta, Methylin
Metronidazole • Flagyl, Metrogel
Modafinil • Provigil
Olanzapine • Zyprexa
Pramipexole • Mirapex
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neurpro
Scopolamine • Transderm Scop
Ziprasidone • Geodon
Disorders of diminished motivation (DDM)—including apathy, abulia, and akinetic mutism—are characterized by impairment in goal-directed behavior, thought, and emotion.1 These disorders can be observed clinically as a gross underproduction of speech, movement, and emotional response.
DDM are not classified as disorders within DSM-5, and it remains unclear if they are distinct disorders or symptoms that overlap in other conditions. Some sources support distinct diagnoses, while the traditional position is that DDM are variations along a spectrum, with apathy as the mildest form and akinetic mutism as the most severe form (Figure).1-3 DDM can result from various neurologic, medical, psychiatric, socioeconomic, and drug-induced pathologies, and may represent differing severity of the same underlying pathology.1,4 It is postulated that DDM arise from disruptions in the dopaminergic frontal-subcortical-mesolimbic networks.1,4

We present 2 cases of patients who developed distinct phenotypes within DDM. Despite differences in presentation and symptom severity, both patients showed clinical improvement on methylphenidate (not the only treatment option) as assessed by the Neuropsychiatric Inventory (NPI),5 a scale used to measure dementia-related behavioral symptoms that includes an Apathy/Indifference (A/I) subscale.
CASE 1
Apathy secondary to glioblastoma multiforme
Ms. E, age 59, presents with wound drainage 3 weeks after a repeat right craniotomy for recurrent glioblastoma multiforme (GBM) of the temporal lobe. Her medical history is not believed to have contributed to her current presentation.
On hospital day 2, Ms. E undergoes debridement and reclosure at the craniotomy site. Prior to the procedure, the patient was noted to have anhedonia and flat affect. Her family reports that she seems to get little enjoyment from life and “only slept and ate.” Psychiatry is consulted on hospital day 3 for evaluation and management of a perceived depressed mood.
On initial psychiatric evaluation, Ms. E continues to have a constricted affect with delayed psychomotor processing speed. However, she denies dysphoria or anhedonia. Richmond Agitation-Sedation Scale6 score is 0 (alert and calm) and test of sustained attention (‘Vigilant A’) is intact (ie, based on the Confusion Assessment Method for the Intensive Care Unit [CAM-ICU],7 Ms. E does not have delirium). The NPI A/I frequency score is 15, with a severity score of 3, for a total score of 45, indicating moderate behavioral disturbance on the NPI A/I subsection. A diagnosis of neuropsychiatric apathy due to recurrent GBM or craniotomy is made, although substance-induced mood disorder due to concurrent dexamethasone and opiate use is considered. Methylphenidate, 2.5 mg/d, is started, and Ms. E’s blood pressure remains stable with the initial dose.
Methylphenidate is titrated to 5 mg, twice daily, over a 1-week period. Ms. E’s NPI A/I subscale score improves to 3 (mild behavioral problem), with 3 points for frequency and a multiplier of 1 for mild severity, reflecting an improvement in neuropsychiatric apathy, and she is transferred to a long-term care rehabilitation center.
CASE 2
Akinetic mutism secondary to subarachnoid hemorrhage
Ms. G, age 47, is brought to an outside hospital with syncope and a severe headache radiating to her neck. Upon arrival, she is unconscious and requires intubation. A non-contrast head CT scan shows diffuse subarachnoid hemorrhage, 6 mm right midline shift, and a small left frontal subdural hematoma. A CT angiography of her head and neck reveals a 0.7 cm anterior paraclinoid left internal carotid artery aneurysm with ophthalmic involvement. Evidence of underlying left and right carotid fibromuscular dysplasia is also seen. Ms. G is transferred to our facility for neurosurgical intervention.
Neurosurgery proceeds with aneurysm coiling, followed by left craniotomy with subdural evacuation and ventriculostomy placement. Her postoperative course is complicated by prolonged nasogastric hyperalimentation, mild hypernatremia and hyperglycemia, tracheostomy, and recurrent central fever. She also develops persistent vasospasm, which requires balloon angioplasty of the left middle cerebral artery.
The psychiatry team is consulted on postoperative day 29 to assess for delirium. The CAM-ICU is positive for delirium, with nocturnal accentuation of agitation. Ms. G demonstrates paucity of speech and minimal verbal comprehension. She starts oral ziprasidone, 5 mg/d at bedtime. In addition to her original CNS insult, scopolamine patch, 1.5 mg, to decrease respiratory secretions, and IV metronidazole, 500 mg every 8 hours, for skin-site infection, may have been contributing to her delirium.
Ms. G’s delirium quickly resolves; however, on day 32 she continues to demonstrate behavioral and cognitive slowing; The NPI A/I frequency score is 28, with a severity score of 3, for a total score of 84, indicating severe behavioral disturbance on the NPI A/I subsection. Methylphenidate, 2.5 mg/d, is started and the next day is increased to 5 mg twice a day to treat severe akinetic mutism. Ms. G also is switched from ziprasidone to olanzapine, 2.5 mg/d at night.
By day 37, the tracheostomy is decannulated, and Ms. G demonstrates a full level of alertness, awareness, and attention. Her affect is full range and appropriate; however, she demonstrates residual language deficits, including dysnomia. On day 38, Ms. G is discharged with an NPI A/I subscale score of 5, indicating a mild behavioral problem.
What these cases demonstrate about DDM
These 2 cases are part of a larger, emerging conversation about the role of dopamine in DDM. Although not fully elucidated, the pathophysiology of abulia, apathy, and akinetic mutism is thought to be related to multiple neurotransmitters—especially dopamine—involved in the cortico-striatal-pallidal-thalamic network.1,8 This position has been supported by reports of clinical improvement in patients with DDM who are given dopaminergic agonists (Table 1).3,9-32

The clinical improvement seen in both of our patients after initiating methylphenidate is consistent with previous reports.10-13 Methylphenidate was selected because of its favorable adverse effect profile and potentially rapid onset of action in DDM.10-13 In cases where oral medication cannot be administered, such as in patients with akinetic mutism, short-term adjunctive IM olanzapine may be helpful, although it is not a first-line treatment.3,15
Interestingly, both of our patients showed improvement with low doses of methylphenidate. Ms. E showed rapid improvement at 2.5 mg/d, but eventually was increased to 10 mg/d. For Ms. G, who demonstrated severe akinetic mutism, rapid improvement was noted after the initial 2.5 mg/d dose; however, because of reports of efficacy of olanzapine in treating akinetic mutism, it is possible that these medications worked synergistically. The proposed mechanism of action of olanzapine in akinetic mutism is through increased dopamine transmission in the medial prefrontal cortex.3,15 Ms. G’s methylphenidate dose was increased to 5 mg/d, which was still “subtherapeutic,” because most reports have used dosages ranging from 10 to 40 mg/d.10-13 Although there were favorable acute results in both patients, their long-term requirements are unknown because of a lack of follow-up. Our findings are also limited by the fact that both patients were recovering from neurosurgical procedures, which could lead to natural improvement in symptoms over time.
Prevalence of DDM in psychiatric disorders
The successful treatment of DDM with dopaminergic drugs is meaningful because of the coexistence of DDM in various neuropsychiatric conditions. In Alzheimer’s disease (AD), disturbances in the dopaminergic system may explain the high comorbidity of apathy, which ranges from 47% in mild AD to 80% in moderate AD.33 In the dopamine-reduced states of cocaine and amphetamine withdrawal, 67% of patients report apathy and lack of motivation.8,34 Additionally, the prevalence of apathy is reported at 27% in Parkinson’s disease, 43% in mild cognitive impairment, 70% in mixed dementia, 94% in a major depressive episode, and 53% in schizophrenia.35 In schizophrenia with predominately negative symptoms, in vivo and postmortem studies have found reduced dopamine receptors.8 Meanwhile, the high rate of akinetic mutism in Creutzfeldt-Jakob disease allows for its use as a reliable diagnostic criteria in this disorder.36
However, the prevalence of DDM is best documented as it relates to stroke and traumatic brain injury (TBI). For instance, after experiencing a stroke, 20% to 25% of patients suffer from apathy.37 Many case reports describe abulia and akinetic mutism after cerebral infarction or hemorrhage, although the incidence of these disorders is unknown.2,38-40 Apathy following TBI is common, especially in younger patients who have sustained a severe injury.41 One study evaluated the prevalence of apathy after TBI among 83 consecutive patients in a neuropsychiatric clinic. Of the 83 patients, 10.84% had apathy without depression, and an equal number were depressed without apathy; another 60% of patients exhibited both apathy and depression. Younger patients (mean age, 29 years) were more likely to be apathetic than older patients, who were more likely to be depressed or depressed and apathetic (mean age, 42 and 38 years, respectively).41 Interestingly, DDM often are associated with cerebral lesions in distinct and distant anatomical locations that are not clearly connected to the neural circuits of motivational pathways. This phenomenon may be explained by the concept of diaschisis, which states that injury to one part of an interconnected neural network can affect other, separate parts of that network.2 If this concept is accurate, it may broaden the impact of DDM, especially as it relates to stroke and TBI.
The differential diagnosis of DDM includes depression and hypokinetic delirium (Table 21,3,42-50). A potential overlapping but confounding condition is stuporous catatonia, with symptoms that include psychomotor slowing such as immobility, staring, and stupor.47 It is important to differentiate these disorders because the treatment for each differs. As previously discussed, there is a clear role for dopamine receptor agonists in the treatment of DDM.

Although major depressive disorder can be treated with medications that increase dopaminergic transmission, selective serotonin reuptake inhibitors (SSRIs) are more commonly used as first-line agents.44 However, an SSRI would theoretically be contraindicated in DDM, because increased serotonin transmission decreases dopamine release from the midbrain, and therefore an SSRI may not only result in a lack of improvement but may worsen DDM.48 Finally, although delirium is treated with atypical or conventional antipsychotics vis-a-vis dopamine type 2 receptor antagonism,45 stuporous catatonia is preferentially treated with gamma-aminobutyric acid-A receptor agonists such as lorazepam.50
What to do when your patient’s presentation suggests DDM
Assessment of DDM should be structured, with input from the patient and the caregiver, and should incorporate the physician’s perspective. A history should be obtained applying recent criteria of apathy. The 3 core domains of apathy—behavior, cognition, and emotion—need to be evaluated. The revised criteria are based on the premise that change in motivation can be measured by examining a patient’s responsiveness to internal or external stimuli. Therefore, each of the 3 domains includes 2 symptoms: (1) self-initiated or “internal” behaviors, cognitions, and emotions (initiation symptom), and (2) the patient’s responsiveness to “external” stimuli (responsiveness symptom).51
One of the main diagnostic dilemmas is how to separate DDM from depression. The differentiation is difficult because of substantial overlap in the manifestation of key symptoms, such as a lack of interest, anergia, psychomotor slowing, and fatigue. Caregivers often mistakenly describe DDM as a depressive state, even though a lack of sadness, desperation, crying, and a depressive mood distinguish DDM from depression. Usually, DDM patients lack negative thoughts, emotional distress, sadness, vegetative symptoms, and somatic concerns, which are frequently observed in mood disorders.51
Several instruments have been developed for assessing neuropsychiatric symptoms. Some were specifically designed to measure apathy, whereas others were designed to provide a broader neuropsychiatric assessment. The NPI is the most widely used multidimensional instrument for assessing neuropsychiatric functioning in patients with neurocognitive disorders (NCDs). It is a valid, reliable instrument that consists of an interview of the patient’s caregiver. It is designed to assess the presence and severity of 10 symptoms, including apathy. The NPI includes both apathy and depression items, which can help clinicians distinguish the 2 conditions. Although beyond the scope of this article, more recent standardized instruments that can assess DDM include the Apathy Inventory, the Dementia Apathy Interview and Rating, and the Structured Clinical Interview for Apathy.52
As previously mentioned, researchers have proposed that DDM are simply a continuum of severity of reduced behavior, and akinetic mutism may be the extreme form. The dilemma is how to formally diagnose states of abulia and akinetic mutism, given the lack of diagnostic criteria and paucity of standardized instruments. Thus, distinguishing between abulia and akinetic mutism (and apathy) is more of a quantitative than qualitative exercise. One could hypothesize that higher scores on a standardized scale to measure apathy (ie, NPI) could imply abulia or akinetic mutism, although to the best of our knowledge, no formal “cut-off scores” exist.53
Treatment of apathy. The duration of pharmacotherapy to treat apathy is unknown and their usage is off-label. Further studies, including double-blind, randomized controlled trials (RCTs), are needed. Nonetheless, the 2 classes of medications that have the most evidence for treating apathy/DDM are psychostimulants and acetylcholinesterase inhibitors (AChEIs).
AChEIs are primarily used for treating cognitive symptoms in NCDs, but recent findings indicate that they have beneficial effects on noncognitive symptoms such as apathy. Of all medications used to treat apathy in NCDs, AChEIs have been used to treat the largest number of patients. Of 26 studies, 24 demonstrated improvement in apathy, with 21 demonstrating statistical significance. These studies ranged in duration from 8 weeks to 1 year, and most were open-label.54
Five studies (3 RCTs and 2 open-label studies) assessed the efficacy of methylphenidate for treating apathy due to AD. All the studies demonstrated at least some benefit in apathy scores after treatment with methylphenidate. These studies ranged from 5 to 12 weeks in duration. Notably, some patients reported adverse effects, including delusions and irritability.54
Although available evidence suggests AChEIs may be the most effective medications for treating apathy in NCDs, methylphenidate has been demonstrated to work faster.55 Thus, in cases where apathy can significantly affect activities of daily living or instrumental activities of daily living, a quicker response may dictate treatment with methylphenidate. It is imperative to note that safety studies and more large-scale double-blind RCTs are needed to further demonstrate the effectiveness and safety of methylphenidate.
Published in 2007, the American Psychiatric Association (APA) guidelines56 state that psychostimulants are a possible treatment option for patients with severe apathy. At the same time, clinicians are reminded that these agents—especially at higher doses—can produce various problematic adverse effects, including tachycardia, hypertension, restlessness, dyskinesia, agitation, sleep disturbances, psychosis, confusion, and decreased appetite. The APA guidelines recommend using low initial doses, with slow and careful titration. For example, methylphenidate should be started at 2.5 to 5 mg once in the morning, with daily doses not to exceed 30 to 40 mg. In our clinical experience, doses >20 mg/d have not been necessary.57
Treatment of akinetic mutism and abulia. In patients with akinetic mutism and possible abulia, for whom oral medication administration is either impossible or contraindicated (ie, due to the potential risk of aspiration pneumonia), atypical antipsychotics, such as IM olanazapine, have produced a therapeutic response in apathetic patients with NCD. However, extensive use of antipsychotics in NCD is not recommended because this class of medications has been associated with serious adverse effects, including an increased risk of death.55
Bottom Line
Apathy, abulia, and akinetic mutism have been categorized as disorders of diminished motivation (DDM). They commonly present after a stroke or traumatic brain injury, and should be differentiated from depression, hypokinetic delirium, and stuporous catatonia. DDM can be successfully treated with dopamine agonists.
Related Resources
- Barnhart WJ, Makela EH, Latocha MJ. SSRI-induced apathy syndrome: a clinical review. J Psychiatr Pract. 2004;10(3):196-199.
- Dell’Osso B, Benatti B, Altamura AC, et al. Prevalence of selective serotonin reuptake inhibitor-related apathy in patients with obsessive compulsive disorder. J Clin Psychopharmacol. 2016;36(6):725-726.
- D’Souza G, Kakoullis A, Hegde N, et al. Recognition and management of abulia in the elderly. Prog Neurol Psychiatry. 2010;14(6):24-28.
Drug Brand Names
Bromocriptine • Parlodel
Bupropion • Wellbutrin XL, Zyban
Carbidopa • Lodosyn
Dexamethasone • DexPak, Ozurde
Donepezil • Aricept
Levodopa/benserazide • Prolopa
Levodopa/carbidopa • Pacopa Rytary Sinemet
Lorazepam • Ativan
Methylphenidate • Concerta, Methylin
Metronidazole • Flagyl, Metrogel
Modafinil • Provigil
Olanzapine • Zyprexa
Pramipexole • Mirapex
Rivastigmine • Exelon
Ropinirole • Requip
Rotigotine • Neurpro
Scopolamine • Transderm Scop
Ziprasidone • Geodon
1. Marin RS, Wilkosz PA. Disorders of diminished motivation. J Head Trauma Rehabil. 2005;20(4):377-388.
2. Ghoshal S, Gokhale S, Rebovich G, et al. The neurology of decreased activity: abulia. Rev Neurol Dis. 2011;8(3-4):e55-e67.
3. Spiegel DR, Chatterjee A. A case of abulia, status/post right middle cerebral artery territory infarct, treated successfully with olanzapine. Clin Neuropharmacol. 2014;37(6):186-189.
4. Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147(1):22-30.
5. Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314.
6. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344.
7. Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the intensive care unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379.
8. Al-Adawi S, Dawe GS, Al-Hussaini AA. Aboulia: neurobehavioural dysfunction of dopaminergic system? Med Hypotheses. 2000;54(4):523-530.
9. Volkow ND, Fowler JS, Wang G, et al. Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord. 2002;6(suppl 1):S31-S43.
10. Chatterjee A, Fahn S. Methylphenidate treats apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2002;14(4):461-462.
11. Keenan S, Mavaddat N, Iddon J, et al. Effects of methylphenidate on cognition and apathy in normal pressure hydrocephalus: a case study and review. Br J Neurosurg. 2005;19(1):46-50.
12. Padala PR, Petty F, Bhatia SC. Methylphenidate may treat apathy independent of depression. Ann Pharmacother. 2005;39(11):1947-1949.
13. Padala PR, Burke WJ, Bhatia SC, et al. Treatment of apathy with methylphenidate. J Neuropsychiatry Clin Neurosci. 2007;19(1):81-83.
14. Li XM, Perry KW, Wong DT, et al. Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacology (Berl). 1998;136(2):153-161.
15. Spiegel DR, Casella DP, Callender DM, et al. Treatment of akinetic mutism with intramuscular olanzapine: a case series. J Neuropsychiatry Clin Neurosci. 2008;20(1):93-95.
16. Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37(2):138-147.
17. Bakheit AM, Fletcher K, Brennan A. Successful treatment of severe abulia with co-beneldopa. NeuroRehabilitation. 2011;29(4):347-351.
18. Debette S, Kozlowski O, Steinling M, et al. Levodopa and bromocriptine in hypoxic brain injury. J Neurol. 2002;249(12):1678-1682.
19. Combarros O, Infante J, Berciano J. Akinetic mutism from frontal lobe damage responding to levodopa. J Neurol. 2000;247(7):568-569.
20. Echiverri HC, Tatum WO, Merens TA, et al. Akinetic mutism: pharmacologic probe of the dopaminergic mesencephalofrontal activating system. Pediatr Neurol. 1988;4(4):228-230.
21. Psarros T, Zouros A, Coimbra C. Bromocriptine-responsive akinetic mutism following endoscopy for ventricular neurocysticercosis. Case report and review of the literature. J Neurosurg. 2003;99(2):397-401.
22. Naik VD. Abulia following an episode of cardiac arrest [published online July 1, 2015]. BMJ Case Rep. doi: 10.1136/bcr-2015-209357.
23. Kim MS, Rhee JJ, Lee SJ, et al. Akinetic mutism responsive to bromocriptine following subdural hematoma evacuation in a patient with hydrocephalus. Neurol Med Chir (Tokyo). 2007;47(9):419-423.
24. Rockwood K, Black S, Bedard MA; TOPS Study Investigators. Specific symptomatic changes following donepezil treatment of Alzheimer’s disease: a multi-centre, primary care, open-label study. Int J Geriatr Psychiatry. 2007;22(4):312-319.
25. Devos D, Moreau C, Maltête D, et al. Rivastigmine in apathetic but dementia and depression-free patients with Parkinson’s disease: a double-blind, placebo-controlled, randomised clinical trial. J Neurol Neurosurg Psychiatry. 2014;85(6):668-674.
26. Camargos EF, Quintas JL. Apathy syndrome treated successfully with modafinil [published online November 15, 2011]. BMJ Case Rep. doi: 10.1136/bcr.08.2011.4652.
27. Corcoran C, Wong ML, O’Keane V. Bupropion in the management of apathy. J Psychopharmacol. 2004;18(1):133-135.
28. Blundo C, Gerace C. Dopamine agonists can improve pure apathy associated with lesions of the prefrontal-basal ganglia functional system. Neurol Sci. 2015;36(7):1197-1201.
29. Mirapex [package insert]. Ridgefield, CT: Boehringer Ingelheim International GmbH; 2016.
30. Neupro [package insert]. Smyrna, GA: UBC, Inc.; 2012.
31. Requip [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
32. Thobois S, Lhommée E, Klinger H, et al. Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with piribedil. Brain. 2013;136(pt 5):1568-1577.
33. Mitchell RA, Herrmann N, Lanctôt KL. The role of dopamine in symptoms and treatment of apathy in Alzheimer’s disease. CNS Neurosci Ther. 2011;17(5):411-427.
34. Brower KJ, Maddahian E, Blow FC, et al. A comparison of self-reported symptoms and DSM-III-R criteria for cocaine withdrawal. Am J Drug Alcohol Abuse. 1988;14(3):347-356.
35. Mulin E, Leone E, Dujardin K, et al. Diagnostic criteria for apathy in clinical practice. Int J Geriatr Psychiatry. 2011;26(2):158-165.
36. Otto A, Zerr I, Lantsch M, et al. Akinetic mutism as a classification criterion for the diagnosis of Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 1998;64(4):524-528.
37. Jorge RE, Starkstein SE, Robinson RG. Apathy following stroke. Can J Psychiatry. 2010;55(6):350-354.
38. Hastak SM, Gorawara PS, Mishra NK. Abulia: no will, no way. J Assoc Physicians India. 2005;53:814-818.
39. Nagaratnam N, Nagaratnam K, Ng K, et al. Akinetic mutism following stroke. J Clin Neurosci. 2004;11(1):25-30.
40. Freemon FR. Akinetic mutism and bilateral anterior cerebral artery occlusion. J Neurol Neurosurg Psychiatry. 1971;34(6):693-698.
41. Schwarzbold M, Diaz A, Martins ET, et al. Psychiatric disorders and traumatic brain injury. Neuropsychiatr Dis Treat. 2008;4(4):797-816.
42. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
43. Levy ML, Cummings JL, Fairbanks LA, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10(3):314-319.
44. Snow V, Lascher S, Mottur-Pilson C. Pharmacologic treatment of acute major depression and dysthymia. American College of Physicians-American Society of Internal Medicine. Ann Intern Med. 2000;132(9):738-742.
45. Schwartz AC, Fisher TJ, Greenspan HN, et al. Pharmacologic and nonpharmacologic approaches to the prevention and management of delirium. Int J Psychiatry Med. 2016;51(2):160-170.
46. Kang H, Zhao F, You L, et al. Pseudo-dementia: a neuropsychological review. Ann Indian Acad Neurol. 2014;17(2):147-154.
47. Fricchione GL, Beach SR, Huffman J, et al. Life-threatening conditions in psychiatry: catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fava M, Wilens TE, eds. Massachusetts General Hospital comprehensive clinical psychiatry. London, United Kingdom: Elsevier; 2016:608-617.
48. Rogers RD. The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacology. 2011;36(1):114-132.
49. Stransky M, Schmidt C, Ganslmeier P, et al. Hypoactive delirium after cardiac surgery as an independent risk factor for prolonged mechanical ventilation. J Cardiothorac Vasc Anesth. 2011;25(6):968-974.
50. Wilcox JA, Reid Duffy P. The syndrome of catatonia. Behav Sci (Basel). 2015;5(4):576-588.
51. Robert PH, Mulin E, Malléa P, et al. REVIEW: apathy diagnosis, assessment, and treatment in Alzheimer’s disease. CNS Neurosci Ther. 2010;16(5):263-271.
52. Cipriani G, Lucetti C, Danti S, et al. Apathy and dementia. Nosology, assessment and management. J Nerv Ment Dis. 2014;202(10):718-724.
53. Starkstein SE, Leentjens AF. The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry. 2008;79(10):1088-1092.54. Berman K, Brodaty H, Withall A, et al. Pharmacologic treatment of apathy in dementia. Am J Geriatr Psychiatry. 2012;20(2):104-122.
55. Theleritis C, Siarkos K, Katirtzoglou E, et al. Pharmacological and nonpharmacological treatment for apathy in Alzheimer disease: a systematic review across modalities. J Geriatr Psychiatry Neurol. 2017;30(1):26-49.
56. APA Work Group on Alzheimer’s Disease and other Dementias; Rabins PV, Blacker D, Rovner BW, et al. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Second edition. Am J Psychiatry. 2007;164(suppl 12):5-56.
57. Dolder CR, Davis LN, McKinsey J. Use of psychostimulants in patients with dementia. Ann Pharmacother. 2010;44(10):1624-1632.
1. Marin RS, Wilkosz PA. Disorders of diminished motivation. J Head Trauma Rehabil. 2005;20(4):377-388.
2. Ghoshal S, Gokhale S, Rebovich G, et al. The neurology of decreased activity: abulia. Rev Neurol Dis. 2011;8(3-4):e55-e67.
3. Spiegel DR, Chatterjee A. A case of abulia, status/post right middle cerebral artery territory infarct, treated successfully with olanzapine. Clin Neuropharmacol. 2014;37(6):186-189.
4. Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147(1):22-30.
5. Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314.
6. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344.
7. Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the intensive care unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379.
8. Al-Adawi S, Dawe GS, Al-Hussaini AA. Aboulia: neurobehavioural dysfunction of dopaminergic system? Med Hypotheses. 2000;54(4):523-530.
9. Volkow ND, Fowler JS, Wang G, et al. Mechanism of action of methylphenidate: insights from PET imaging studies. J Atten Disord. 2002;6(suppl 1):S31-S43.
10. Chatterjee A, Fahn S. Methylphenidate treats apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2002;14(4):461-462.
11. Keenan S, Mavaddat N, Iddon J, et al. Effects of methylphenidate on cognition and apathy in normal pressure hydrocephalus: a case study and review. Br J Neurosurg. 2005;19(1):46-50.
12. Padala PR, Petty F, Bhatia SC. Methylphenidate may treat apathy independent of depression. Ann Pharmacother. 2005;39(11):1947-1949.
13. Padala PR, Burke WJ, Bhatia SC, et al. Treatment of apathy with methylphenidate. J Neuropsychiatry Clin Neurosci. 2007;19(1):81-83.
14. Li XM, Perry KW, Wong DT, et al. Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacology (Berl). 1998;136(2):153-161.
15. Spiegel DR, Casella DP, Callender DM, et al. Treatment of akinetic mutism with intramuscular olanzapine: a case series. J Neuropsychiatry Clin Neurosci. 2008;20(1):93-95.
16. Citrome L. Activating and sedating adverse effects of second-generation antipsychotics in the treatment of schizophrenia and major depressive disorder: absolute risk increase and number needed to harm. J Clin Psychopharmacol. 2017;37(2):138-147.
17. Bakheit AM, Fletcher K, Brennan A. Successful treatment of severe abulia with co-beneldopa. NeuroRehabilitation. 2011;29(4):347-351.
18. Debette S, Kozlowski O, Steinling M, et al. Levodopa and bromocriptine in hypoxic brain injury. J Neurol. 2002;249(12):1678-1682.
19. Combarros O, Infante J, Berciano J. Akinetic mutism from frontal lobe damage responding to levodopa. J Neurol. 2000;247(7):568-569.
20. Echiverri HC, Tatum WO, Merens TA, et al. Akinetic mutism: pharmacologic probe of the dopaminergic mesencephalofrontal activating system. Pediatr Neurol. 1988;4(4):228-230.
21. Psarros T, Zouros A, Coimbra C. Bromocriptine-responsive akinetic mutism following endoscopy for ventricular neurocysticercosis. Case report and review of the literature. J Neurosurg. 2003;99(2):397-401.
22. Naik VD. Abulia following an episode of cardiac arrest [published online July 1, 2015]. BMJ Case Rep. doi: 10.1136/bcr-2015-209357.
23. Kim MS, Rhee JJ, Lee SJ, et al. Akinetic mutism responsive to bromocriptine following subdural hematoma evacuation in a patient with hydrocephalus. Neurol Med Chir (Tokyo). 2007;47(9):419-423.
24. Rockwood K, Black S, Bedard MA; TOPS Study Investigators. Specific symptomatic changes following donepezil treatment of Alzheimer’s disease: a multi-centre, primary care, open-label study. Int J Geriatr Psychiatry. 2007;22(4):312-319.
25. Devos D, Moreau C, Maltête D, et al. Rivastigmine in apathetic but dementia and depression-free patients with Parkinson’s disease: a double-blind, placebo-controlled, randomised clinical trial. J Neurol Neurosurg Psychiatry. 2014;85(6):668-674.
26. Camargos EF, Quintas JL. Apathy syndrome treated successfully with modafinil [published online November 15, 2011]. BMJ Case Rep. doi: 10.1136/bcr.08.2011.4652.
27. Corcoran C, Wong ML, O’Keane V. Bupropion in the management of apathy. J Psychopharmacol. 2004;18(1):133-135.
28. Blundo C, Gerace C. Dopamine agonists can improve pure apathy associated with lesions of the prefrontal-basal ganglia functional system. Neurol Sci. 2015;36(7):1197-1201.
29. Mirapex [package insert]. Ridgefield, CT: Boehringer Ingelheim International GmbH; 2016.
30. Neupro [package insert]. Smyrna, GA: UBC, Inc.; 2012.
31. Requip [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
32. Thobois S, Lhommée E, Klinger H, et al. Parkinsonian apathy responds to dopaminergic stimulation of D2/D3 receptors with piribedil. Brain. 2013;136(pt 5):1568-1577.
33. Mitchell RA, Herrmann N, Lanctôt KL. The role of dopamine in symptoms and treatment of apathy in Alzheimer’s disease. CNS Neurosci Ther. 2011;17(5):411-427.
34. Brower KJ, Maddahian E, Blow FC, et al. A comparison of self-reported symptoms and DSM-III-R criteria for cocaine withdrawal. Am J Drug Alcohol Abuse. 1988;14(3):347-356.
35. Mulin E, Leone E, Dujardin K, et al. Diagnostic criteria for apathy in clinical practice. Int J Geriatr Psychiatry. 2011;26(2):158-165.
36. Otto A, Zerr I, Lantsch M, et al. Akinetic mutism as a classification criterion for the diagnosis of Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry. 1998;64(4):524-528.
37. Jorge RE, Starkstein SE, Robinson RG. Apathy following stroke. Can J Psychiatry. 2010;55(6):350-354.
38. Hastak SM, Gorawara PS, Mishra NK. Abulia: no will, no way. J Assoc Physicians India. 2005;53:814-818.
39. Nagaratnam N, Nagaratnam K, Ng K, et al. Akinetic mutism following stroke. J Clin Neurosci. 2004;11(1):25-30.
40. Freemon FR. Akinetic mutism and bilateral anterior cerebral artery occlusion. J Neurol Neurosurg Psychiatry. 1971;34(6):693-698.
41. Schwarzbold M, Diaz A, Martins ET, et al. Psychiatric disorders and traumatic brain injury. Neuropsychiatr Dis Treat. 2008;4(4):797-816.
42. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
43. Levy ML, Cummings JL, Fairbanks LA, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10(3):314-319.
44. Snow V, Lascher S, Mottur-Pilson C. Pharmacologic treatment of acute major depression and dysthymia. American College of Physicians-American Society of Internal Medicine. Ann Intern Med. 2000;132(9):738-742.
45. Schwartz AC, Fisher TJ, Greenspan HN, et al. Pharmacologic and nonpharmacologic approaches to the prevention and management of delirium. Int J Psychiatry Med. 2016;51(2):160-170.
46. Kang H, Zhao F, You L, et al. Pseudo-dementia: a neuropsychological review. Ann Indian Acad Neurol. 2014;17(2):147-154.
47. Fricchione GL, Beach SR, Huffman J, et al. Life-threatening conditions in psychiatry: catatonia, neuroleptic malignant syndrome, and serotonin syndrome. In: Stern TA, Fava M, Wilens TE, eds. Massachusetts General Hospital comprehensive clinical psychiatry. London, United Kingdom: Elsevier; 2016:608-617.
48. Rogers RD. The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacology. 2011;36(1):114-132.
49. Stransky M, Schmidt C, Ganslmeier P, et al. Hypoactive delirium after cardiac surgery as an independent risk factor for prolonged mechanical ventilation. J Cardiothorac Vasc Anesth. 2011;25(6):968-974.
50. Wilcox JA, Reid Duffy P. The syndrome of catatonia. Behav Sci (Basel). 2015;5(4):576-588.
51. Robert PH, Mulin E, Malléa P, et al. REVIEW: apathy diagnosis, assessment, and treatment in Alzheimer’s disease. CNS Neurosci Ther. 2010;16(5):263-271.
52. Cipriani G, Lucetti C, Danti S, et al. Apathy and dementia. Nosology, assessment and management. J Nerv Ment Dis. 2014;202(10):718-724.
53. Starkstein SE, Leentjens AF. The nosological position of apathy in clinical practice. J Neurol Neurosurg Psychiatry. 2008;79(10):1088-1092.54. Berman K, Brodaty H, Withall A, et al. Pharmacologic treatment of apathy in dementia. Am J Geriatr Psychiatry. 2012;20(2):104-122.
55. Theleritis C, Siarkos K, Katirtzoglou E, et al. Pharmacological and nonpharmacological treatment for apathy in Alzheimer disease: a systematic review across modalities. J Geriatr Psychiatry Neurol. 2017;30(1):26-49.
56. APA Work Group on Alzheimer’s Disease and other Dementias; Rabins PV, Blacker D, Rovner BW, et al. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Second edition. Am J Psychiatry. 2007;164(suppl 12):5-56.
57. Dolder CR, Davis LN, McKinsey J. Use of psychostimulants in patients with dementia. Ann Pharmacother. 2010;44(10):1624-1632.