User login
Once Retired, Now Just Tired
ANSWER
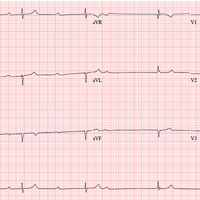
The correct interpretation includes marked sinus bradycardia with a second-degree atrioventricular (AV) block (Mobitz I) and occasional junctional escape, left-axis deviation, and evidence of an inferior MI. Poor R-wave progression is also noted in the precordial leads.
Marked sinus bradycardia is seen in the first three and the last four P waves of the rhythm strip. The P-P intervals have a rate of 50 beats/min—different than the overall rate of the QRS complex (remember, “sinus” is synonymous with “P wave”!). The rate of the QRS complex (38 beats/min) is slower than the atrial rate, signifying a block of some sort.
Second-degree AV block is evident from the lengthening PR interval in the first two P-QRS complexes, followed by a P wave with no associated QRS complex. Although the fourth QRS complex in the rhythm strip is narrow and similar in appearance to the others, it’s too far from the previous P wave to have been conducted from the atrium—indicating a junctional escape beat arising from the AV node. (The rhythm returns to second-degree AV block for the remainder of the beats seen on the ECG.)
Left-axis deviation is signified by the R axis of –78° (lower than the normal limit of –30°). The Q waves in leads II, III, and aVF indicate a prior inferior MI. Finally, poor R-wave progression is seen in the precordial leads, with no significant transition between leads V1 and V6.
Although second-degree AV block is not an indication for permanent pacing, symptomatic bradycardia that persists despite medical management is. Because this patient was symptomatic while taking an AV nodal blocking agent (metoprolol), a permanent pacemaker was recommended.
ANSWER
The correct interpretation includes marked sinus bradycardia with a second-degree atrioventricular (AV) block (Mobitz I) and occasional junctional escape, left-axis deviation, and evidence of an inferior MI. Poor R-wave progression is also noted in the precordial leads.
Marked sinus bradycardia is seen in the first three and the last four P waves of the rhythm strip. The P-P intervals have a rate of 50 beats/min—different than the overall rate of the QRS complex (remember, “sinus” is synonymous with “P wave”!). The rate of the QRS complex (38 beats/min) is slower than the atrial rate, signifying a block of some sort.
Second-degree AV block is evident from the lengthening PR interval in the first two P-QRS complexes, followed by a P wave with no associated QRS complex. Although the fourth QRS complex in the rhythm strip is narrow and similar in appearance to the others, it’s too far from the previous P wave to have been conducted from the atrium—indicating a junctional escape beat arising from the AV node. (The rhythm returns to second-degree AV block for the remainder of the beats seen on the ECG.)
Left-axis deviation is signified by the R axis of –78° (lower than the normal limit of –30°). The Q waves in leads II, III, and aVF indicate a prior inferior MI. Finally, poor R-wave progression is seen in the precordial leads, with no significant transition between leads V1 and V6.
Although second-degree AV block is not an indication for permanent pacing, symptomatic bradycardia that persists despite medical management is. Because this patient was symptomatic while taking an AV nodal blocking agent (metoprolol), a permanent pacemaker was recommended.
ANSWER
The correct interpretation includes marked sinus bradycardia with a second-degree atrioventricular (AV) block (Mobitz I) and occasional junctional escape, left-axis deviation, and evidence of an inferior MI. Poor R-wave progression is also noted in the precordial leads.
Marked sinus bradycardia is seen in the first three and the last four P waves of the rhythm strip. The P-P intervals have a rate of 50 beats/min—different than the overall rate of the QRS complex (remember, “sinus” is synonymous with “P wave”!). The rate of the QRS complex (38 beats/min) is slower than the atrial rate, signifying a block of some sort.
Second-degree AV block is evident from the lengthening PR interval in the first two P-QRS complexes, followed by a P wave with no associated QRS complex. Although the fourth QRS complex in the rhythm strip is narrow and similar in appearance to the others, it’s too far from the previous P wave to have been conducted from the atrium—indicating a junctional escape beat arising from the AV node. (The rhythm returns to second-degree AV block for the remainder of the beats seen on the ECG.)
Left-axis deviation is signified by the R axis of –78° (lower than the normal limit of –30°). The Q waves in leads II, III, and aVF indicate a prior inferior MI. Finally, poor R-wave progression is seen in the precordial leads, with no significant transition between leads V1 and V6.
Although second-degree AV block is not an indication for permanent pacing, symptomatic bradycardia that persists despite medical management is. Because this patient was symptomatic while taking an AV nodal blocking agent (metoprolol), a permanent pacemaker was recommended.
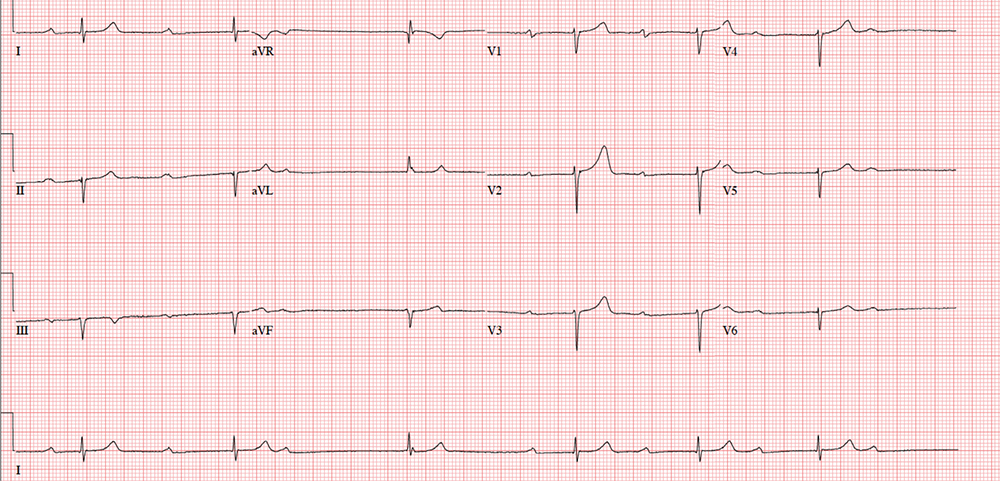
A 72-year-old
Medical history is remarkable for type 2 diabetes, hypercholesterolemia, coronary artery disease, and a remote inferior myocardial infarction (MI) 14 years ago. Surgical history includes a cholecystectomy and an open reduction and internal fixation of a left high ankle fracture.
His current medication list includes metformin, isosorbide dinitrate, metoprolol, and atorvastatin. He has an anaphylactic allergy to sulfa. He cannot give a family history, as he was adopted and does not know his biological family.
The patient, a retired welder, is a recovering alcoholic; he attends Alcoholics Anonymous meetings regularly and has been sober for more than 12 years. He smoked a half-pack of cigarettes each day as a teenager but quit when he got married 52 years ago. He is a widower. His son died in an automobile accident at age 32; his daughter lives nearby and checks on him every day.
Review of systems is remarkable for neuropathic foot pain due to diabetes, recurrent constipation, corrective lenses, and hearing aids. The remainder of the review is noncontributory.
Vital signs include a blood pressure of 112/56 mm Hg; pulse, 40 beats/min; respiratory rate, 16 breaths/min-1; and temperature, 99.2°F. His height is 76 in and his weight, 194 lb. Physical exam reveals an elderly but otherwise healthy-looking male in no distress.
The HEENT exam is remarkable for early cataract formation but is otherwise normal. His dentition is in remarkably excellent health. There is no thyromegaly or jugular venous distention, and the lungs are clear in all fields without wheezes or crackles.
Cardiac exam reveals a roughly normal rate of 40 beats/min. It is difficult to determine whether there is respiratory variation, given the slower rate. There is an early grade II/VI systolic murmur of aortic sclerosis best heard at the left upper sternal border. It does not radiate elsewhere. There are no extra heart sounds or rubs.
The abdomen has well-healed surgical scars with no palpable organomegaly. Bowel sounds are present in all quadrants. A rectal exam reveals impaction of firm stool; the prostate is not palpable given the amount of firm stool present.
The extremities have full range of motion without clubbing, cyanosis, or edema. Peripheral pulses are full bilaterally in both upper and lower extremities. A well-healed surgical scar is present on the left lateral lower extremity, and a plate is palpable beneath the skin. Skin sensitivity testing with 2-point pinprick of the soles and toes of both feet reveals extensive paresthesias. Apart from this, the neurologic exam is grossly normal. There is no evidence of diabetic foot ulcers.
An ECG shows a ventricular rate of 38 beats/min; no discernable PR interval; QRS duration, 78 ms; QT/QTc interval, 434/345 ms; P axis, 25°; R axis, –78°; and T axis, 13°. What is your interpretation?
Bedside Test Helps Protect Against Infant Deaths
According to researchers from William Paterson University, Emory University, and the CDC, screening for CCHD could save at least 120 babies a year.
Congenital heart disease accounted for 6% of U.S. infant deaths from 1999 to 2006. Almost 1 in every 4 babies born with a congenital heart defect has critical congenital heart disease (CCHD) and will need surgery or other procedures in the first year. About 7,200 babies born in the U.S .each year have 1 of 7 CCHDs. But some babies can seem healthy and be sent home before the heart defect is detected.
In 2011, CCHD was added to the U.S. Recommended Uniform Screening Panel for newborns. As of June 2013, 8 states had implemented mandatory screening policies, 5 had voluntary screening policies, and 9 had adopted but not yet implemented mandates.
The study was conducted in 2013 and involved data for nearly 27 million births. Between 2007 and 2013, 2,734 infants died due to CCHD; 3,967 died of other or unspecified causes.
The study, which is the first look at the impact of state policies to require or recommend screening for CCHD at birth, found that states with screening requirements saw the most significant drop in numbers of infant deaths. Voluntary policies or mandated policies not yet implemented were not associated with reductions. However, 47 states and DC now have mandatory screening policies in place.
According to researchers from William Paterson University, Emory University, and the CDC, screening for CCHD could save at least 120 babies a year.
Congenital heart disease accounted for 6% of U.S. infant deaths from 1999 to 2006. Almost 1 in every 4 babies born with a congenital heart defect has critical congenital heart disease (CCHD) and will need surgery or other procedures in the first year. About 7,200 babies born in the U.S .each year have 1 of 7 CCHDs. But some babies can seem healthy and be sent home before the heart defect is detected.
In 2011, CCHD was added to the U.S. Recommended Uniform Screening Panel for newborns. As of June 2013, 8 states had implemented mandatory screening policies, 5 had voluntary screening policies, and 9 had adopted but not yet implemented mandates.
The study was conducted in 2013 and involved data for nearly 27 million births. Between 2007 and 2013, 2,734 infants died due to CCHD; 3,967 died of other or unspecified causes.
The study, which is the first look at the impact of state policies to require or recommend screening for CCHD at birth, found that states with screening requirements saw the most significant drop in numbers of infant deaths. Voluntary policies or mandated policies not yet implemented were not associated with reductions. However, 47 states and DC now have mandatory screening policies in place.
According to researchers from William Paterson University, Emory University, and the CDC, screening for CCHD could save at least 120 babies a year.
Congenital heart disease accounted for 6% of U.S. infant deaths from 1999 to 2006. Almost 1 in every 4 babies born with a congenital heart defect has critical congenital heart disease (CCHD) and will need surgery or other procedures in the first year. About 7,200 babies born in the U.S .each year have 1 of 7 CCHDs. But some babies can seem healthy and be sent home before the heart defect is detected.
In 2011, CCHD was added to the U.S. Recommended Uniform Screening Panel for newborns. As of June 2013, 8 states had implemented mandatory screening policies, 5 had voluntary screening policies, and 9 had adopted but not yet implemented mandates.
The study was conducted in 2013 and involved data for nearly 27 million births. Between 2007 and 2013, 2,734 infants died due to CCHD; 3,967 died of other or unspecified causes.
The study, which is the first look at the impact of state policies to require or recommend screening for CCHD at birth, found that states with screening requirements saw the most significant drop in numbers of infant deaths. Voluntary policies or mandated policies not yet implemented were not associated with reductions. However, 47 states and DC now have mandatory screening policies in place.
First month of LABA/LAMA ups cardiovascular risk
New use of inhaled long-acting beta-2 agonists (LABAs) or long-acting antimuscarinic antagonists (LAMAs) was associated with a 1.5-fold increased cardiovascular risk within 30 days of initiation in patients with chronic obstructive pulmonary disease, irrespective of prior cardiovascular disease status and history of exacerbations, according to a review of more than 280,000 COPD patients in Taiwan.
The relationship between cardiovascular disease (CVD) and LABAs and LAMAs in chronic obstructive pulmonary disease (COPD) has long been debated. The new study addressed some limitations of previous studies, which had found conflicting results ranging from no increased risk to up to a 4.5-fold increased risk of cardiovascular events when the medications were used for COPD.
Previous randomized trials haven’t raised much concern, but they included prior users who may have developed tolerance to the heart effects and excluded patients with baseline CVD. “We caution physicians to closely monitor new users of LABAs or LAMAs for cardiovascular symptoms.” Health care professionals should be vigilant for any cardiovascular symptoms during the first 30 days of inhalation therapy, said investigators led by Meng-Ting Wang, PhD, of the National Defense Medical Center, Taipei.
“We suspect that there may exist a subgroup of patients with COPD who are particularly at risk of CVD with initial exposure to LABAs or LAMAs ... we suggest that the use of inhaled long-acting bronchodilators in COPD needs to be carefully assessed, and a thorough cardiovascular physical examination, especially heart rate measurement and electrocardiograms, needs to be performed” before prescribing LABAs and LAMAs, they wrote in an article in JAMA Internal Medicine.
The team identified 284,220 COPD patients in the Taiwan National Health Insurance Research Database during 2007-2011 who were new to the medications. During a mean follow-up of 2 years, 37,719 developed severe CVD requiring hospitalization or emergency care, including coronary artery disease, heart failure, ischemic stroke, and arrhythmia.
The team compared their CVD subjects with controls who did not have a heart event and found that new LABA and LAMA use in COPD was associated with a 1.50-fold (95% confidence interval, 1.35-1.67; P less than .001) and a 1.52-fold (95% CI, 1.28-1.80; P less than .001) increased cardiovascular risk within 30 days of initiation, respectively.
One severe CVD event requiring hospitalization or ED care occurred for every 406 (95% CI, 303-580) new LABA users and 391 (95% CI, 254-725) new LAMA users during the first 30 days of therapy.
The LABA- and LAMA-associated CVD risk remained significant, regardless of patients’ CVD history and COPD exacerbations. Analyses of individual CVD outcomes revealed increased risks of coronary artery disease and heart failure with LABA and LAMA treatment, and an increased risk for cardiac arrhythmias with LAMA therapy.
The cardiovascular risks peaked at around the 30th day of treatment, waned from 31-60 days of treatment, and reduced to a level lower than the baseline risk from 71-240 days.
“Given that CVD is highly prevalent among patients with COPD, clinicians should also pay attention to the management of CVD risk factors throughout the duration of LABA or LAMA therapy ... if needed, a preventive therapy for CVD should be considered during the initial treatment of inhaled long-acting bronchodilators,” the investigators said.
LABAs and LAMAs are believed to cause sympathetic overactivation by activating sympathetic beta-2 adrenergic receptors and suppressing parasympathetic muscarinic-3 receptors, which could contribute to the CVD risk. Also, LABA and LAMA use in COPD has been observed to increase inflammatory cytokine levels, which might also play a role.
The subjects were 40 years or older; the mean age was 71.4 years and 68.9% of the participants were men.
The work was supported by Taiwan’s Ministry of Science and Technology. The investigators had no disclosures.
Eli Zimmerman contributed to this report.
SOURCE: Wang MT et al. JAMA Intern Med. 2018 Jan 2. doi: 10.1001/jamainternmed.2017.7720.
Daniel R. Ouellette, MD, FCCP, comments: Long acting beta agonists (LABA) and long acting muscarinic antagonists (LAMA) are agents commonly used to treat patients with chronic obstructive pulmonary disease (COPD). These inhaled medications have been generally considered to be safe and have a favorable side-effect profile. Although there has been some speculative data that suggest that these agents may be associated with increased cardiovascular risk, prospective, controlled studies have generally suggested that the cardiovascular risk is not increased with the use of these medicines.
One strength of this study is the size of the database, which is robust, and the novel treatment that this study uses to address the research question. Weaknesses include the study's necessarily retrospective design, and the fact that the population is from a single geographic area. Further research will be needed to understand whether or not the initiation of LABA and LAMA medications in COPD patients is associated with increased cardiovascular risk.
Daniel R. Ouellette, MD, FCCP, comments: Long acting beta agonists (LABA) and long acting muscarinic antagonists (LAMA) are agents commonly used to treat patients with chronic obstructive pulmonary disease (COPD). These inhaled medications have been generally considered to be safe and have a favorable side-effect profile. Although there has been some speculative data that suggest that these agents may be associated with increased cardiovascular risk, prospective, controlled studies have generally suggested that the cardiovascular risk is not increased with the use of these medicines.
One strength of this study is the size of the database, which is robust, and the novel treatment that this study uses to address the research question. Weaknesses include the study's necessarily retrospective design, and the fact that the population is from a single geographic area. Further research will be needed to understand whether or not the initiation of LABA and LAMA medications in COPD patients is associated with increased cardiovascular risk.
Daniel R. Ouellette, MD, FCCP, comments: Long acting beta agonists (LABA) and long acting muscarinic antagonists (LAMA) are agents commonly used to treat patients with chronic obstructive pulmonary disease (COPD). These inhaled medications have been generally considered to be safe and have a favorable side-effect profile. Although there has been some speculative data that suggest that these agents may be associated with increased cardiovascular risk, prospective, controlled studies have generally suggested that the cardiovascular risk is not increased with the use of these medicines.
One strength of this study is the size of the database, which is robust, and the novel treatment that this study uses to address the research question. Weaknesses include the study's necessarily retrospective design, and the fact that the population is from a single geographic area. Further research will be needed to understand whether or not the initiation of LABA and LAMA medications in COPD patients is associated with increased cardiovascular risk.
New use of inhaled long-acting beta-2 agonists (LABAs) or long-acting antimuscarinic antagonists (LAMAs) was associated with a 1.5-fold increased cardiovascular risk within 30 days of initiation in patients with chronic obstructive pulmonary disease, irrespective of prior cardiovascular disease status and history of exacerbations, according to a review of more than 280,000 COPD patients in Taiwan.
The relationship between cardiovascular disease (CVD) and LABAs and LAMAs in chronic obstructive pulmonary disease (COPD) has long been debated. The new study addressed some limitations of previous studies, which had found conflicting results ranging from no increased risk to up to a 4.5-fold increased risk of cardiovascular events when the medications were used for COPD.
Previous randomized trials haven’t raised much concern, but they included prior users who may have developed tolerance to the heart effects and excluded patients with baseline CVD. “We caution physicians to closely monitor new users of LABAs or LAMAs for cardiovascular symptoms.” Health care professionals should be vigilant for any cardiovascular symptoms during the first 30 days of inhalation therapy, said investigators led by Meng-Ting Wang, PhD, of the National Defense Medical Center, Taipei.
“We suspect that there may exist a subgroup of patients with COPD who are particularly at risk of CVD with initial exposure to LABAs or LAMAs ... we suggest that the use of inhaled long-acting bronchodilators in COPD needs to be carefully assessed, and a thorough cardiovascular physical examination, especially heart rate measurement and electrocardiograms, needs to be performed” before prescribing LABAs and LAMAs, they wrote in an article in JAMA Internal Medicine.
The team identified 284,220 COPD patients in the Taiwan National Health Insurance Research Database during 2007-2011 who were new to the medications. During a mean follow-up of 2 years, 37,719 developed severe CVD requiring hospitalization or emergency care, including coronary artery disease, heart failure, ischemic stroke, and arrhythmia.
The team compared their CVD subjects with controls who did not have a heart event and found that new LABA and LAMA use in COPD was associated with a 1.50-fold (95% confidence interval, 1.35-1.67; P less than .001) and a 1.52-fold (95% CI, 1.28-1.80; P less than .001) increased cardiovascular risk within 30 days of initiation, respectively.
One severe CVD event requiring hospitalization or ED care occurred for every 406 (95% CI, 303-580) new LABA users and 391 (95% CI, 254-725) new LAMA users during the first 30 days of therapy.
The LABA- and LAMA-associated CVD risk remained significant, regardless of patients’ CVD history and COPD exacerbations. Analyses of individual CVD outcomes revealed increased risks of coronary artery disease and heart failure with LABA and LAMA treatment, and an increased risk for cardiac arrhythmias with LAMA therapy.
The cardiovascular risks peaked at around the 30th day of treatment, waned from 31-60 days of treatment, and reduced to a level lower than the baseline risk from 71-240 days.
“Given that CVD is highly prevalent among patients with COPD, clinicians should also pay attention to the management of CVD risk factors throughout the duration of LABA or LAMA therapy ... if needed, a preventive therapy for CVD should be considered during the initial treatment of inhaled long-acting bronchodilators,” the investigators said.
LABAs and LAMAs are believed to cause sympathetic overactivation by activating sympathetic beta-2 adrenergic receptors and suppressing parasympathetic muscarinic-3 receptors, which could contribute to the CVD risk. Also, LABA and LAMA use in COPD has been observed to increase inflammatory cytokine levels, which might also play a role.
The subjects were 40 years or older; the mean age was 71.4 years and 68.9% of the participants were men.
The work was supported by Taiwan’s Ministry of Science and Technology. The investigators had no disclosures.
Eli Zimmerman contributed to this report.
SOURCE: Wang MT et al. JAMA Intern Med. 2018 Jan 2. doi: 10.1001/jamainternmed.2017.7720.
New use of inhaled long-acting beta-2 agonists (LABAs) or long-acting antimuscarinic antagonists (LAMAs) was associated with a 1.5-fold increased cardiovascular risk within 30 days of initiation in patients with chronic obstructive pulmonary disease, irrespective of prior cardiovascular disease status and history of exacerbations, according to a review of more than 280,000 COPD patients in Taiwan.
The relationship between cardiovascular disease (CVD) and LABAs and LAMAs in chronic obstructive pulmonary disease (COPD) has long been debated. The new study addressed some limitations of previous studies, which had found conflicting results ranging from no increased risk to up to a 4.5-fold increased risk of cardiovascular events when the medications were used for COPD.
Previous randomized trials haven’t raised much concern, but they included prior users who may have developed tolerance to the heart effects and excluded patients with baseline CVD. “We caution physicians to closely monitor new users of LABAs or LAMAs for cardiovascular symptoms.” Health care professionals should be vigilant for any cardiovascular symptoms during the first 30 days of inhalation therapy, said investigators led by Meng-Ting Wang, PhD, of the National Defense Medical Center, Taipei.
“We suspect that there may exist a subgroup of patients with COPD who are particularly at risk of CVD with initial exposure to LABAs or LAMAs ... we suggest that the use of inhaled long-acting bronchodilators in COPD needs to be carefully assessed, and a thorough cardiovascular physical examination, especially heart rate measurement and electrocardiograms, needs to be performed” before prescribing LABAs and LAMAs, they wrote in an article in JAMA Internal Medicine.
The team identified 284,220 COPD patients in the Taiwan National Health Insurance Research Database during 2007-2011 who were new to the medications. During a mean follow-up of 2 years, 37,719 developed severe CVD requiring hospitalization or emergency care, including coronary artery disease, heart failure, ischemic stroke, and arrhythmia.
The team compared their CVD subjects with controls who did not have a heart event and found that new LABA and LAMA use in COPD was associated with a 1.50-fold (95% confidence interval, 1.35-1.67; P less than .001) and a 1.52-fold (95% CI, 1.28-1.80; P less than .001) increased cardiovascular risk within 30 days of initiation, respectively.
One severe CVD event requiring hospitalization or ED care occurred for every 406 (95% CI, 303-580) new LABA users and 391 (95% CI, 254-725) new LAMA users during the first 30 days of therapy.
The LABA- and LAMA-associated CVD risk remained significant, regardless of patients’ CVD history and COPD exacerbations. Analyses of individual CVD outcomes revealed increased risks of coronary artery disease and heart failure with LABA and LAMA treatment, and an increased risk for cardiac arrhythmias with LAMA therapy.
The cardiovascular risks peaked at around the 30th day of treatment, waned from 31-60 days of treatment, and reduced to a level lower than the baseline risk from 71-240 days.
“Given that CVD is highly prevalent among patients with COPD, clinicians should also pay attention to the management of CVD risk factors throughout the duration of LABA or LAMA therapy ... if needed, a preventive therapy for CVD should be considered during the initial treatment of inhaled long-acting bronchodilators,” the investigators said.
LABAs and LAMAs are believed to cause sympathetic overactivation by activating sympathetic beta-2 adrenergic receptors and suppressing parasympathetic muscarinic-3 receptors, which could contribute to the CVD risk. Also, LABA and LAMA use in COPD has been observed to increase inflammatory cytokine levels, which might also play a role.
The subjects were 40 years or older; the mean age was 71.4 years and 68.9% of the participants were men.
The work was supported by Taiwan’s Ministry of Science and Technology. The investigators had no disclosures.
Eli Zimmerman contributed to this report.
SOURCE: Wang MT et al. JAMA Intern Med. 2018 Jan 2. doi: 10.1001/jamainternmed.2017.7720.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Researchers recommend patients receive a thorough cardiovascular physical examination before they are prescribed LABAs and LAMAs.
Major finding: New irrespective of prior cardiovascular disease status and history of exacerbations.
Study details: The findings are from a review of 284,220 COPD patients in the Taiwan National Health Insurance Research Database.
Disclosures: The work was supported by Taiwan’s Ministry of Science and Technology. The investigators had no disclosures.
Source: Wang MT et al. JAMA Intern Med. 2018 Jan 2. doi: 10.1001/jamainternmed.2017.7720.
Age of Seizure Onset Is Related to Menarche in Women With Epilepsy
WASHINGTON, DC—Age of seizure onset is significantly associated with age of menarche, according to research presented at the 71st Annual Meeting of the American Epilepsy Society. Seizure onset, however, is also common in the years before and after menarche, suggesting an impact of increased production of neuroactive steroids.
“The levels of some hormones in the blood increase tenfold during this time of life, including neuroactive steroids that affect the brain and make a seizure more likely,” said Andrew G. Herzog, MD, Professor of Neurology at Harvard Medical School and Director of the Harvard Neuroendocrine Unit of Beth Israel Deaconess Medical Center in Boston. “Clearly more seizures develop in girls during that period of time, so we need to begin looking at risk factors as well as potential treatments.”
Previous research has suggested that seizure onset during late childhood may be related to menarche, but study results regarding this relationship have been inconsistent, said Dr. Herzog. To further examine the relationship between seizure onset and age of menarche, Dr. Herzog and colleagues analyzed data from an Epilepsy Birth Control Registry web-based survey of 1,144 women with epilepsy. The women were ages 18 to 47 and provided demographic, epilepsy, antiepileptic drug, reproductive, and contraceptive data.
The average age of menarche was 12.55, which was similar to that for women in the general population. Mean age of menarche was significantly greater in women with epilepsy who had seizure onset before menarche versus after menarche (12.70 vs 12.42). The average age of seizure onset was 14.13.
Overall, more women with epilepsy had seizure onset during the year of menarche than during any other year, significantly more than would have been expected by chance. Approximately 49% of seizure onset occurred over the span of two years before menarche and six years after menarche.
Girls who may be at risk of developing seizures (eg, those who have had febrile seizures or a head injury) may warrant closer monitoring and potential treatment with steroids or other medications to inhibit neuroactivity that may cause seizures, Dr. Herzog said.
“We need to look at the whole puberty process and this massive increase in hormone production,” said Dr. Herzog. “If we have a better idea of how hormones are acting on the brain, we can develop appropriate treatment.”
—Erica Tricarico
WASHINGTON, DC—Age of seizure onset is significantly associated with age of menarche, according to research presented at the 71st Annual Meeting of the American Epilepsy Society. Seizure onset, however, is also common in the years before and after menarche, suggesting an impact of increased production of neuroactive steroids.
“The levels of some hormones in the blood increase tenfold during this time of life, including neuroactive steroids that affect the brain and make a seizure more likely,” said Andrew G. Herzog, MD, Professor of Neurology at Harvard Medical School and Director of the Harvard Neuroendocrine Unit of Beth Israel Deaconess Medical Center in Boston. “Clearly more seizures develop in girls during that period of time, so we need to begin looking at risk factors as well as potential treatments.”
Previous research has suggested that seizure onset during late childhood may be related to menarche, but study results regarding this relationship have been inconsistent, said Dr. Herzog. To further examine the relationship between seizure onset and age of menarche, Dr. Herzog and colleagues analyzed data from an Epilepsy Birth Control Registry web-based survey of 1,144 women with epilepsy. The women were ages 18 to 47 and provided demographic, epilepsy, antiepileptic drug, reproductive, and contraceptive data.
The average age of menarche was 12.55, which was similar to that for women in the general population. Mean age of menarche was significantly greater in women with epilepsy who had seizure onset before menarche versus after menarche (12.70 vs 12.42). The average age of seizure onset was 14.13.
Overall, more women with epilepsy had seizure onset during the year of menarche than during any other year, significantly more than would have been expected by chance. Approximately 49% of seizure onset occurred over the span of two years before menarche and six years after menarche.
Girls who may be at risk of developing seizures (eg, those who have had febrile seizures or a head injury) may warrant closer monitoring and potential treatment with steroids or other medications to inhibit neuroactivity that may cause seizures, Dr. Herzog said.
“We need to look at the whole puberty process and this massive increase in hormone production,” said Dr. Herzog. “If we have a better idea of how hormones are acting on the brain, we can develop appropriate treatment.”
—Erica Tricarico
WASHINGTON, DC—Age of seizure onset is significantly associated with age of menarche, according to research presented at the 71st Annual Meeting of the American Epilepsy Society. Seizure onset, however, is also common in the years before and after menarche, suggesting an impact of increased production of neuroactive steroids.
“The levels of some hormones in the blood increase tenfold during this time of life, including neuroactive steroids that affect the brain and make a seizure more likely,” said Andrew G. Herzog, MD, Professor of Neurology at Harvard Medical School and Director of the Harvard Neuroendocrine Unit of Beth Israel Deaconess Medical Center in Boston. “Clearly more seizures develop in girls during that period of time, so we need to begin looking at risk factors as well as potential treatments.”
Previous research has suggested that seizure onset during late childhood may be related to menarche, but study results regarding this relationship have been inconsistent, said Dr. Herzog. To further examine the relationship between seizure onset and age of menarche, Dr. Herzog and colleagues analyzed data from an Epilepsy Birth Control Registry web-based survey of 1,144 women with epilepsy. The women were ages 18 to 47 and provided demographic, epilepsy, antiepileptic drug, reproductive, and contraceptive data.
The average age of menarche was 12.55, which was similar to that for women in the general population. Mean age of menarche was significantly greater in women with epilepsy who had seizure onset before menarche versus after menarche (12.70 vs 12.42). The average age of seizure onset was 14.13.
Overall, more women with epilepsy had seizure onset during the year of menarche than during any other year, significantly more than would have been expected by chance. Approximately 49% of seizure onset occurred over the span of two years before menarche and six years after menarche.
Girls who may be at risk of developing seizures (eg, those who have had febrile seizures or a head injury) may warrant closer monitoring and potential treatment with steroids or other medications to inhibit neuroactivity that may cause seizures, Dr. Herzog said.
“We need to look at the whole puberty process and this massive increase in hormone production,” said Dr. Herzog. “If we have a better idea of how hormones are acting on the brain, we can develop appropriate treatment.”
—Erica Tricarico
Fenfluramine Reduces Convulsive Seizure Frequency in Dravet Syndrome
WASHINGTON, DC—Among patients with Dravet syndrome, adjunctive treatment with fenfluramine hydrochloride oral solution significantly reduces convulsive seizure frequency, compared with placebo, according to data presented at the 71st Annual Meeting of the American Epilepsy Society. The treatment is generally well tolerated at doses of 30 mg/day or less, and no clinical or echocardiographic signs of cardiac valvulopathy or pulmonary hypertension were observed in a phase III trial, investigators said.
Fenfluramine has been used at higher doses for weight loss, but the drug was withdrawn from US and European markets because it was associated with cardiac valvulopathy and pulmonary hypertension. Investigators in Belgium, however, continued exploratory studies of low-dose fenfluramine for refractory pediatric epilepsy. The studies suggested that fenfluramine reduced seizure frequency. Zogenix, based in Emeryville, California, is developing the drug, known as ZX008.
No FDA-approved treatments for seizures associated with Dravet syndrome exist, and 45% of patients with Dravet syndrome have four or more tonic-clonic seizures per month despite polytherapy with antiepileptic drugs (AEDs), said the investigators.
A Placebo-Controlled Trial
Lieven Lagae, MD, PhD, Professor at the University of Leuven in Belgium and Head of the Pediatric Neurology Department and Director of the Childhood Epilepsy Program at the University of Leuven Hospitals, and colleagues conducted a phase III, randomized, placebo-controlled, double-blind, 14-week, fixed-dose clinical trial at sites in the United States, Canada, Europe, and Australia.
The researchers included patients with Dravet syndrome ages 2 to 18 whose seizures were not completely controlled by their current AED regimen and whose medications and interventions (eg, ketogenic diet and vagal nerve stimulation) had been stable for at least four weeks before screening. The researchers excluded patients with a history of cardiovascular or cerebrovascular disease, concomitant serotonergic or cannabinoid treatment, treatment with stiripentol in the 21 days before screening, or any diagnosis that might alter the risk–benefit ratio or impede participation in the trial.
Patients underwent six weeks of baseline observation before they were randomized 1:1:1 to receive ZX008 (0.8 mg/kg/day), ZX008 (0.2 mg/kg/day), or placebo. The treatment period included a two-week titration period followed by a 12-week maintenance period. The maximum daily dose was 30 mg. The primary efficacy end point was change in mean convulsive seizure frequency from the baseline period to the 14-week treatment period between ZX008 (0.8 mg/kg/day) and placebo.
Frequency of Seizures
The study included 119 patients. Patients had a mean age of 9, and about 54% were male. Average baseline convulsive seizure frequency was about 42 seizures per 28 days.
Patients who received ZX008 (0.8 mg/kg/day) had a 63.9% reduction in mean monthly convulsive seizures, compared with placebo. The median percent reduction in monthly convulsive seizure frequency was 72.4% among patients who received ZX008 (0.8 mg/kg/day), compared with 17.4% among patients who received placebo.
Significantly more patients who received either dose of ZX008 had at least a 25%, 50%, or 75% reduction in convulsive seizures during treatment, compared with patients who received placebo. The median longest seizure-free interval was significantly longer in patients treated with ZX008 (0.8 mg/kg/day) or ZX008 (0.2 mg/kg/day), compared with patients who received placebo (20.5 days, 14 days, and nine days, respectively).
Noncardiovascular treatment-emergent adverse events included diarrhea, fatigue, lethargy, and decreased weight and appetite. “No cases of FDA-defined cardiac valvulopathy were observed during the trial,” the researchers said. “No echocardiographic findings or clinical symptoms suggesting pulmonary hypertension were noted.”
Caregivers and clinicians rated a greater proportion of patients who received ZX008 to be very much or much improved following treatment, compared with patients who received placebo. Results from another phase III trial are expected in 2018.
“Patients treated with ZX008 achieved a clinically meaningful reduction in seizure frequency,” said Dr. Lagae. “If approved, ZX008 could play an important role in changing the treatment paradigm for patients and their families whose lives have been greatly impacted by the lack of effective seizure control provided by current treatment options.”
WASHINGTON, DC—Among patients with Dravet syndrome, adjunctive treatment with fenfluramine hydrochloride oral solution significantly reduces convulsive seizure frequency, compared with placebo, according to data presented at the 71st Annual Meeting of the American Epilepsy Society. The treatment is generally well tolerated at doses of 30 mg/day or less, and no clinical or echocardiographic signs of cardiac valvulopathy or pulmonary hypertension were observed in a phase III trial, investigators said.
Fenfluramine has been used at higher doses for weight loss, but the drug was withdrawn from US and European markets because it was associated with cardiac valvulopathy and pulmonary hypertension. Investigators in Belgium, however, continued exploratory studies of low-dose fenfluramine for refractory pediatric epilepsy. The studies suggested that fenfluramine reduced seizure frequency. Zogenix, based in Emeryville, California, is developing the drug, known as ZX008.
No FDA-approved treatments for seizures associated with Dravet syndrome exist, and 45% of patients with Dravet syndrome have four or more tonic-clonic seizures per month despite polytherapy with antiepileptic drugs (AEDs), said the investigators.
A Placebo-Controlled Trial
Lieven Lagae, MD, PhD, Professor at the University of Leuven in Belgium and Head of the Pediatric Neurology Department and Director of the Childhood Epilepsy Program at the University of Leuven Hospitals, and colleagues conducted a phase III, randomized, placebo-controlled, double-blind, 14-week, fixed-dose clinical trial at sites in the United States, Canada, Europe, and Australia.
The researchers included patients with Dravet syndrome ages 2 to 18 whose seizures were not completely controlled by their current AED regimen and whose medications and interventions (eg, ketogenic diet and vagal nerve stimulation) had been stable for at least four weeks before screening. The researchers excluded patients with a history of cardiovascular or cerebrovascular disease, concomitant serotonergic or cannabinoid treatment, treatment with stiripentol in the 21 days before screening, or any diagnosis that might alter the risk–benefit ratio or impede participation in the trial.
Patients underwent six weeks of baseline observation before they were randomized 1:1:1 to receive ZX008 (0.8 mg/kg/day), ZX008 (0.2 mg/kg/day), or placebo. The treatment period included a two-week titration period followed by a 12-week maintenance period. The maximum daily dose was 30 mg. The primary efficacy end point was change in mean convulsive seizure frequency from the baseline period to the 14-week treatment period between ZX008 (0.8 mg/kg/day) and placebo.
Frequency of Seizures
The study included 119 patients. Patients had a mean age of 9, and about 54% were male. Average baseline convulsive seizure frequency was about 42 seizures per 28 days.
Patients who received ZX008 (0.8 mg/kg/day) had a 63.9% reduction in mean monthly convulsive seizures, compared with placebo. The median percent reduction in monthly convulsive seizure frequency was 72.4% among patients who received ZX008 (0.8 mg/kg/day), compared with 17.4% among patients who received placebo.
Significantly more patients who received either dose of ZX008 had at least a 25%, 50%, or 75% reduction in convulsive seizures during treatment, compared with patients who received placebo. The median longest seizure-free interval was significantly longer in patients treated with ZX008 (0.8 mg/kg/day) or ZX008 (0.2 mg/kg/day), compared with patients who received placebo (20.5 days, 14 days, and nine days, respectively).
Noncardiovascular treatment-emergent adverse events included diarrhea, fatigue, lethargy, and decreased weight and appetite. “No cases of FDA-defined cardiac valvulopathy were observed during the trial,” the researchers said. “No echocardiographic findings or clinical symptoms suggesting pulmonary hypertension were noted.”
Caregivers and clinicians rated a greater proportion of patients who received ZX008 to be very much or much improved following treatment, compared with patients who received placebo. Results from another phase III trial are expected in 2018.
“Patients treated with ZX008 achieved a clinically meaningful reduction in seizure frequency,” said Dr. Lagae. “If approved, ZX008 could play an important role in changing the treatment paradigm for patients and their families whose lives have been greatly impacted by the lack of effective seizure control provided by current treatment options.”
WASHINGTON, DC—Among patients with Dravet syndrome, adjunctive treatment with fenfluramine hydrochloride oral solution significantly reduces convulsive seizure frequency, compared with placebo, according to data presented at the 71st Annual Meeting of the American Epilepsy Society. The treatment is generally well tolerated at doses of 30 mg/day or less, and no clinical or echocardiographic signs of cardiac valvulopathy or pulmonary hypertension were observed in a phase III trial, investigators said.
Fenfluramine has been used at higher doses for weight loss, but the drug was withdrawn from US and European markets because it was associated with cardiac valvulopathy and pulmonary hypertension. Investigators in Belgium, however, continued exploratory studies of low-dose fenfluramine for refractory pediatric epilepsy. The studies suggested that fenfluramine reduced seizure frequency. Zogenix, based in Emeryville, California, is developing the drug, known as ZX008.
No FDA-approved treatments for seizures associated with Dravet syndrome exist, and 45% of patients with Dravet syndrome have four or more tonic-clonic seizures per month despite polytherapy with antiepileptic drugs (AEDs), said the investigators.
A Placebo-Controlled Trial
Lieven Lagae, MD, PhD, Professor at the University of Leuven in Belgium and Head of the Pediatric Neurology Department and Director of the Childhood Epilepsy Program at the University of Leuven Hospitals, and colleagues conducted a phase III, randomized, placebo-controlled, double-blind, 14-week, fixed-dose clinical trial at sites in the United States, Canada, Europe, and Australia.
The researchers included patients with Dravet syndrome ages 2 to 18 whose seizures were not completely controlled by their current AED regimen and whose medications and interventions (eg, ketogenic diet and vagal nerve stimulation) had been stable for at least four weeks before screening. The researchers excluded patients with a history of cardiovascular or cerebrovascular disease, concomitant serotonergic or cannabinoid treatment, treatment with stiripentol in the 21 days before screening, or any diagnosis that might alter the risk–benefit ratio or impede participation in the trial.
Patients underwent six weeks of baseline observation before they were randomized 1:1:1 to receive ZX008 (0.8 mg/kg/day), ZX008 (0.2 mg/kg/day), or placebo. The treatment period included a two-week titration period followed by a 12-week maintenance period. The maximum daily dose was 30 mg. The primary efficacy end point was change in mean convulsive seizure frequency from the baseline period to the 14-week treatment period between ZX008 (0.8 mg/kg/day) and placebo.
Frequency of Seizures
The study included 119 patients. Patients had a mean age of 9, and about 54% were male. Average baseline convulsive seizure frequency was about 42 seizures per 28 days.
Patients who received ZX008 (0.8 mg/kg/day) had a 63.9% reduction in mean monthly convulsive seizures, compared with placebo. The median percent reduction in monthly convulsive seizure frequency was 72.4% among patients who received ZX008 (0.8 mg/kg/day), compared with 17.4% among patients who received placebo.
Significantly more patients who received either dose of ZX008 had at least a 25%, 50%, or 75% reduction in convulsive seizures during treatment, compared with patients who received placebo. The median longest seizure-free interval was significantly longer in patients treated with ZX008 (0.8 mg/kg/day) or ZX008 (0.2 mg/kg/day), compared with patients who received placebo (20.5 days, 14 days, and nine days, respectively).
Noncardiovascular treatment-emergent adverse events included diarrhea, fatigue, lethargy, and decreased weight and appetite. “No cases of FDA-defined cardiac valvulopathy were observed during the trial,” the researchers said. “No echocardiographic findings or clinical symptoms suggesting pulmonary hypertension were noted.”
Caregivers and clinicians rated a greater proportion of patients who received ZX008 to be very much or much improved following treatment, compared with patients who received placebo. Results from another phase III trial are expected in 2018.
“Patients treated with ZX008 achieved a clinically meaningful reduction in seizure frequency,” said Dr. Lagae. “If approved, ZX008 could play an important role in changing the treatment paradigm for patients and their families whose lives have been greatly impacted by the lack of effective seizure control provided by current treatment options.”
Ibudilast May Slow Brain Atrophy in Progressive MS
PARIS—Ibudilast decreases the rate of brain atrophy, compared with placebo, in patients with progressive multiple sclerosis (MS), according to top-line results presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. The drug appears to be safe and tolerable.
Ibudilast is an orally bioavailable small-molecule drug that is available in Japan for treating asthma and post stroke dizziness. In a phase II trial that included patients with relapsing-remitting MS, ibudilast slowed brain atrophy progression in a dose-dependent manner. In addition, animal models suggest that the drug may promote neuroprotection.
Examining Ibudilast in a Phase II Trial
Robert Fox, MD, a neurologist at the Cleveland Clinic, and colleagues conducted a phase II clinical trial at 28 sites to analyze ibudilast’s effects in patients with primary or secondary progressive MS. Eligible patients were between ages 18 and 65, had a maximum Expanded Disability Status Scale (EDSS) score of 6.5, had disability progression in the previous two years, and were allowed to receive concurrent treatment with interferon beta or glatiramer acetate.
Patients were randomized in equal groups to placebo or ibudilast. The intervention group received 60 mg/day, 80 mg/day, or 100 mg/day of ibudilast as tolerated. Randomization was stratified by primary or secondary progressive MS and by whether the subjects received injectable disease-modifying therapies. For the first three months of treatment, patients presented for monthly safety evaluations. From the third month onward, patients returned quarterly for safety assessments and every six months for efficacy assessments, which included clinical disability and 3-T MRI measures. The treatment period was two years.
The trial’s primary end points were brain atrophy, as measured by brain parenchymal fraction; safety; and tolerability. Secondary outcomes included magnetization transfer ratio (MTR), diffusion tensor imaging (DTI), optical coherence tomography of the retinal nerve fiber layer, and cortical atrophy.
Researchers Observed Gastrointestinal Side Effects
The investigators enrolled 255 patients into the study. The population’s mean age was approximately 56, and mean EDSS was about 5. The study sample had approximately equal numbers of patients with primary progressive MS and secondary progressive MS. Eleven participants withdrew from the study before the first efficacy assessment, and another 24 participants discontinued the trial before week 96. The trial’s retention rate was 86%.
In the primary analysis, ibudilast was associated with a 48% reduction in the progression of brain atrophy over two years, compared with placebo. A per-protocol sensitivity analysis confirmed that ibudilast reduced the progression of brain atrophy by half, compared with placebo.
Treatment-related adverse events included gastrointestinal side effects such as nausea, vomiting, diarrhea, and abdominal pain. Patients treated with ibudilast also had higher rates of rash, depression, and fatigue than did controls. The rate of serious adverse events was similar in both groups. There were no related and unanticipated serious adverse events.
There was no significant difference in the rates of treatment discontinuation, early study termination, and early drug withdrawal between the two study arms. Approximately 25% of controls discontinued treatment during the study, compared with 30% of the ibudilast group.
Compared with placebo, ibudilast was associated with a significant slowing of the progression of MTR in normal-appearing brain tissue and normal-appearing gray matter. The researchers did not find a significant difference between treatment groups on DTI. Additional analyses of the secondary and tertiary outcomes are forthcoming, said Dr. Fox.
—Erik Greb
Suggested Reading
Barkhof F, Hulst HE, Drulovic J, et al. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology. 2010;74(13):1033-1040.
Fox RJ, Coffey CS, Cudkowicz ME, et al. Design, rationale, and baseline characteristics of the randomized double-blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemp Clin Trials. 2016;50:166-177.
PARIS—Ibudilast decreases the rate of brain atrophy, compared with placebo, in patients with progressive multiple sclerosis (MS), according to top-line results presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. The drug appears to be safe and tolerable.
Ibudilast is an orally bioavailable small-molecule drug that is available in Japan for treating asthma and post stroke dizziness. In a phase II trial that included patients with relapsing-remitting MS, ibudilast slowed brain atrophy progression in a dose-dependent manner. In addition, animal models suggest that the drug may promote neuroprotection.
Examining Ibudilast in a Phase II Trial
Robert Fox, MD, a neurologist at the Cleveland Clinic, and colleagues conducted a phase II clinical trial at 28 sites to analyze ibudilast’s effects in patients with primary or secondary progressive MS. Eligible patients were between ages 18 and 65, had a maximum Expanded Disability Status Scale (EDSS) score of 6.5, had disability progression in the previous two years, and were allowed to receive concurrent treatment with interferon beta or glatiramer acetate.
Patients were randomized in equal groups to placebo or ibudilast. The intervention group received 60 mg/day, 80 mg/day, or 100 mg/day of ibudilast as tolerated. Randomization was stratified by primary or secondary progressive MS and by whether the subjects received injectable disease-modifying therapies. For the first three months of treatment, patients presented for monthly safety evaluations. From the third month onward, patients returned quarterly for safety assessments and every six months for efficacy assessments, which included clinical disability and 3-T MRI measures. The treatment period was two years.
The trial’s primary end points were brain atrophy, as measured by brain parenchymal fraction; safety; and tolerability. Secondary outcomes included magnetization transfer ratio (MTR), diffusion tensor imaging (DTI), optical coherence tomography of the retinal nerve fiber layer, and cortical atrophy.
Researchers Observed Gastrointestinal Side Effects
The investigators enrolled 255 patients into the study. The population’s mean age was approximately 56, and mean EDSS was about 5. The study sample had approximately equal numbers of patients with primary progressive MS and secondary progressive MS. Eleven participants withdrew from the study before the first efficacy assessment, and another 24 participants discontinued the trial before week 96. The trial’s retention rate was 86%.
In the primary analysis, ibudilast was associated with a 48% reduction in the progression of brain atrophy over two years, compared with placebo. A per-protocol sensitivity analysis confirmed that ibudilast reduced the progression of brain atrophy by half, compared with placebo.
Treatment-related adverse events included gastrointestinal side effects such as nausea, vomiting, diarrhea, and abdominal pain. Patients treated with ibudilast also had higher rates of rash, depression, and fatigue than did controls. The rate of serious adverse events was similar in both groups. There were no related and unanticipated serious adverse events.
There was no significant difference in the rates of treatment discontinuation, early study termination, and early drug withdrawal between the two study arms. Approximately 25% of controls discontinued treatment during the study, compared with 30% of the ibudilast group.
Compared with placebo, ibudilast was associated with a significant slowing of the progression of MTR in normal-appearing brain tissue and normal-appearing gray matter. The researchers did not find a significant difference between treatment groups on DTI. Additional analyses of the secondary and tertiary outcomes are forthcoming, said Dr. Fox.
—Erik Greb
Suggested Reading
Barkhof F, Hulst HE, Drulovic J, et al. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology. 2010;74(13):1033-1040.
Fox RJ, Coffey CS, Cudkowicz ME, et al. Design, rationale, and baseline characteristics of the randomized double-blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemp Clin Trials. 2016;50:166-177.
PARIS—Ibudilast decreases the rate of brain atrophy, compared with placebo, in patients with progressive multiple sclerosis (MS), according to top-line results presented at the Seventh Joint ECTRIMS–ACTRIMS Meeting. The drug appears to be safe and tolerable.
Ibudilast is an orally bioavailable small-molecule drug that is available in Japan for treating asthma and post stroke dizziness. In a phase II trial that included patients with relapsing-remitting MS, ibudilast slowed brain atrophy progression in a dose-dependent manner. In addition, animal models suggest that the drug may promote neuroprotection.
Examining Ibudilast in a Phase II Trial
Robert Fox, MD, a neurologist at the Cleveland Clinic, and colleagues conducted a phase II clinical trial at 28 sites to analyze ibudilast’s effects in patients with primary or secondary progressive MS. Eligible patients were between ages 18 and 65, had a maximum Expanded Disability Status Scale (EDSS) score of 6.5, had disability progression in the previous two years, and were allowed to receive concurrent treatment with interferon beta or glatiramer acetate.
Patients were randomized in equal groups to placebo or ibudilast. The intervention group received 60 mg/day, 80 mg/day, or 100 mg/day of ibudilast as tolerated. Randomization was stratified by primary or secondary progressive MS and by whether the subjects received injectable disease-modifying therapies. For the first three months of treatment, patients presented for monthly safety evaluations. From the third month onward, patients returned quarterly for safety assessments and every six months for efficacy assessments, which included clinical disability and 3-T MRI measures. The treatment period was two years.
The trial’s primary end points were brain atrophy, as measured by brain parenchymal fraction; safety; and tolerability. Secondary outcomes included magnetization transfer ratio (MTR), diffusion tensor imaging (DTI), optical coherence tomography of the retinal nerve fiber layer, and cortical atrophy.
Researchers Observed Gastrointestinal Side Effects
The investigators enrolled 255 patients into the study. The population’s mean age was approximately 56, and mean EDSS was about 5. The study sample had approximately equal numbers of patients with primary progressive MS and secondary progressive MS. Eleven participants withdrew from the study before the first efficacy assessment, and another 24 participants discontinued the trial before week 96. The trial’s retention rate was 86%.
In the primary analysis, ibudilast was associated with a 48% reduction in the progression of brain atrophy over two years, compared with placebo. A per-protocol sensitivity analysis confirmed that ibudilast reduced the progression of brain atrophy by half, compared with placebo.
Treatment-related adverse events included gastrointestinal side effects such as nausea, vomiting, diarrhea, and abdominal pain. Patients treated with ibudilast also had higher rates of rash, depression, and fatigue than did controls. The rate of serious adverse events was similar in both groups. There were no related and unanticipated serious adverse events.
There was no significant difference in the rates of treatment discontinuation, early study termination, and early drug withdrawal between the two study arms. Approximately 25% of controls discontinued treatment during the study, compared with 30% of the ibudilast group.
Compared with placebo, ibudilast was associated with a significant slowing of the progression of MTR in normal-appearing brain tissue and normal-appearing gray matter. The researchers did not find a significant difference between treatment groups on DTI. Additional analyses of the secondary and tertiary outcomes are forthcoming, said Dr. Fox.
—Erik Greb
Suggested Reading
Barkhof F, Hulst HE, Drulovic J, et al. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology. 2010;74(13):1033-1040.
Fox RJ, Coffey CS, Cudkowicz ME, et al. Design, rationale, and baseline characteristics of the randomized double-blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemp Clin Trials. 2016;50:166-177.
Conference News Roundup—American Heart Association
Does Cholesterol Testing Reduce Risk of Recurrent Stroke?
When a patient has a heart attack or stroke, it is critical for his or her clinician to perform a follow-up cholesterol test, according to a study conducted at the Intermountain Medical Center Heart Institute in Salt Lake City. This additional measure is significantly associated with reduced risk of having another serious cardiovascular episode.
Investigators found that patients who do not follow up with their doctor by getting a low-density lipoprotein (LDL) cholesterol test after a heart attack or stroke are significantly more likely to have a recurrence. They also found a significant and clinically meaningful difference in major adverse outcomes—including death, heart attack, stroke, and a vascular bypass or an angioplasty—based on whether or not a patient has a follow-up measurement of his or her LDL cholesterol.
“It is clear that anyone with a previous heart problem caused by clogged arteries should be taking a cholesterol-lowering medication,” said Kirk U. Knowlton, MD, Director of Cardiovascular Research at the Intermountain Medical Center Heart Institute.
The study of more than 60,000 patients with known heart disease, cerebrovascular disease, or peripheral artery disease, including patients with stroke and heart attack, showed that the major adverse clinical event rate was lower in patients who took cholesterol-lowering statins and in those who did not take them if their LDL was measured.
“The large difference is surprising. The risk of dying after three years with no LDL follow-up is 21% versus 5.9% for patients who have an LDL follow-up,” said Dr. Knowlton.
Researchers reviewed Intermountain Healthcare’s enterprise data warehouse to identify all adults who came to one of Intermountain’s 22 hospitals for the first time with a heart attack or stroke. These data included patients with coronary artery disease, cerebrovascular disease, and peripheral arterial disease admitted between January 1, 1999, and December 31, 2013.
Investigators observed patients who survived and were followed for three years or more or until their death. Patient demographics, history, prescribed medications, and whether LDL was measured was analyzed.
The study compared 62,070 patients in the database who met the study criteria. The mean age was 66, and 65% of patients were male. Of those who met the criteria, 69.3% had coronary artery disease, 18.6% had cerebrovascular disease, and 12.1% had peripheral arterial disease when they came to the hospital with their first heart attack or stroke.
Researchers found that the risk of a patient having a secondary event or dying decreased in patients who had a follow-up LDL test before a subsequent adverse outcome or before the end of their follow-up.
Coffee Is Associated With Lower Risk of Heart Failure and Stroke
Drinking coffee may be associated with a decreased risk of heart failure or stroke, according to researchers.
Investigators used machine learning to analyze data from the long-running Framingham Heart Study, which includes information about what people eat and their cardiovascular health. They found that every additional cup of coffee consumed per week was associated with a 7% decreased risk of heart failure and an 8% reduced risk of stroke, compared with non-coffee drinkers.
The researchers further investigated the machine learning results using traditional analysis in two studies with similar sets of data: the Cardiovascular Heart Study and the Atherosclerosis Risk In Communities Study. The association between drinking coffee and a decreased risk of heart failure and stroke was observed consistently in all three studies.
Another potential risk factor identified by machine-learning analysis was red meat consumption. The association between red meat consumption and heart failure or stroke was less clear, however. Eating red meat was associated with decreased risk of heart failure and stroke in the Framingham Heart Study, but validating the finding in comparable studies is more challenging due to differences in the definitions of red meat between studies, said the researchers. Further investigation to better determine how red meat consumption affects risk for heart failure and stroke is ongoing.
The researchers also built a predictive model using known risk factors from the Framingham Risk Score such as blood pressure, age, and other patient characteristics associated with cardiovascular disease. “By including coffee in the model, the prediction accuracy increased by 4%. Machine learning may be a useful addition to the way we look at data and may help us to find new ways to lower the risk of heart failure and strokes,” said David Kao, MD, Assistant Professor of Medicine in the Division of Cardiology at the University of Colorado School of Medicine in Aurora.
Statins May Improve Stroke Outcome
Patients with a prior history of heart attack or stroke have better outcomes when cholesterol-lowering medications are used after they are discharged from the hospital, according to researchers.
Prior surveys in hospitals found that statins are not being used consistently in patients who have been admitted to the hospital following a heart attack or stroke. Researchers also found that when the medication is prescribed, dosing is likely not as high as it should be to provide optimal benefits.
Researchers examined more than 62,000 records of patients from the Intermountain Healthcare system between 1999 and 2013 who survived an initial atherosclerotic cardiovascular disease event, such as a heart attack or stroke. They were then followed for three years or until death to identify the effectiveness of statin use prescribed at the time of their discharge.
“Patients who were prescribed a statin medication following an initial heart attack or stroke reduced their risk of a future adverse event such as a future heart attack, stroke, revascularization, or death by almost 25%—the rate dropped from 34% to 26%,” said Jeffrey L. Anderson, MD, a cardiovascular researcher at the Intermountain Medical Center Heart Institute. “The patients who were discharged on what is considered a high-intensity dose of a statin saw a 21% reduction in their risk,” compared with those discharged on a low-intensity statin dose.”
Investigators found that 30% of patients in the study who were discharged from the hospital following a heart attack or stroke were not prescribed a statin. This factor led to worse outcomes for those patients.
Researchers also found that only 13% of patients were given a high-intensity dose of statins, but noted that patients on those higher doses experienced fewer heart attacks or strokes. For patients younger than age 76, a high-intensity statin is indicated, according to American Heart Association guidelines. Only 17.7% of these patients were discharged on a high-intensity dose, however.
Does Cholesterol Testing Reduce Risk of Recurrent Stroke?
When a patient has a heart attack or stroke, it is critical for his or her clinician to perform a follow-up cholesterol test, according to a study conducted at the Intermountain Medical Center Heart Institute in Salt Lake City. This additional measure is significantly associated with reduced risk of having another serious cardiovascular episode.
Investigators found that patients who do not follow up with their doctor by getting a low-density lipoprotein (LDL) cholesterol test after a heart attack or stroke are significantly more likely to have a recurrence. They also found a significant and clinically meaningful difference in major adverse outcomes—including death, heart attack, stroke, and a vascular bypass or an angioplasty—based on whether or not a patient has a follow-up measurement of his or her LDL cholesterol.
“It is clear that anyone with a previous heart problem caused by clogged arteries should be taking a cholesterol-lowering medication,” said Kirk U. Knowlton, MD, Director of Cardiovascular Research at the Intermountain Medical Center Heart Institute.
The study of more than 60,000 patients with known heart disease, cerebrovascular disease, or peripheral artery disease, including patients with stroke and heart attack, showed that the major adverse clinical event rate was lower in patients who took cholesterol-lowering statins and in those who did not take them if their LDL was measured.
“The large difference is surprising. The risk of dying after three years with no LDL follow-up is 21% versus 5.9% for patients who have an LDL follow-up,” said Dr. Knowlton.
Researchers reviewed Intermountain Healthcare’s enterprise data warehouse to identify all adults who came to one of Intermountain’s 22 hospitals for the first time with a heart attack or stroke. These data included patients with coronary artery disease, cerebrovascular disease, and peripheral arterial disease admitted between January 1, 1999, and December 31, 2013.
Investigators observed patients who survived and were followed for three years or more or until their death. Patient demographics, history, prescribed medications, and whether LDL was measured was analyzed.
The study compared 62,070 patients in the database who met the study criteria. The mean age was 66, and 65% of patients were male. Of those who met the criteria, 69.3% had coronary artery disease, 18.6% had cerebrovascular disease, and 12.1% had peripheral arterial disease when they came to the hospital with their first heart attack or stroke.
Researchers found that the risk of a patient having a secondary event or dying decreased in patients who had a follow-up LDL test before a subsequent adverse outcome or before the end of their follow-up.
Coffee Is Associated With Lower Risk of Heart Failure and Stroke
Drinking coffee may be associated with a decreased risk of heart failure or stroke, according to researchers.
Investigators used machine learning to analyze data from the long-running Framingham Heart Study, which includes information about what people eat and their cardiovascular health. They found that every additional cup of coffee consumed per week was associated with a 7% decreased risk of heart failure and an 8% reduced risk of stroke, compared with non-coffee drinkers.
The researchers further investigated the machine learning results using traditional analysis in two studies with similar sets of data: the Cardiovascular Heart Study and the Atherosclerosis Risk In Communities Study. The association between drinking coffee and a decreased risk of heart failure and stroke was observed consistently in all three studies.
Another potential risk factor identified by machine-learning analysis was red meat consumption. The association between red meat consumption and heart failure or stroke was less clear, however. Eating red meat was associated with decreased risk of heart failure and stroke in the Framingham Heart Study, but validating the finding in comparable studies is more challenging due to differences in the definitions of red meat between studies, said the researchers. Further investigation to better determine how red meat consumption affects risk for heart failure and stroke is ongoing.
The researchers also built a predictive model using known risk factors from the Framingham Risk Score such as blood pressure, age, and other patient characteristics associated with cardiovascular disease. “By including coffee in the model, the prediction accuracy increased by 4%. Machine learning may be a useful addition to the way we look at data and may help us to find new ways to lower the risk of heart failure and strokes,” said David Kao, MD, Assistant Professor of Medicine in the Division of Cardiology at the University of Colorado School of Medicine in Aurora.
Statins May Improve Stroke Outcome
Patients with a prior history of heart attack or stroke have better outcomes when cholesterol-lowering medications are used after they are discharged from the hospital, according to researchers.
Prior surveys in hospitals found that statins are not being used consistently in patients who have been admitted to the hospital following a heart attack or stroke. Researchers also found that when the medication is prescribed, dosing is likely not as high as it should be to provide optimal benefits.
Researchers examined more than 62,000 records of patients from the Intermountain Healthcare system between 1999 and 2013 who survived an initial atherosclerotic cardiovascular disease event, such as a heart attack or stroke. They were then followed for three years or until death to identify the effectiveness of statin use prescribed at the time of their discharge.
“Patients who were prescribed a statin medication following an initial heart attack or stroke reduced their risk of a future adverse event such as a future heart attack, stroke, revascularization, or death by almost 25%—the rate dropped from 34% to 26%,” said Jeffrey L. Anderson, MD, a cardiovascular researcher at the Intermountain Medical Center Heart Institute. “The patients who were discharged on what is considered a high-intensity dose of a statin saw a 21% reduction in their risk,” compared with those discharged on a low-intensity statin dose.”
Investigators found that 30% of patients in the study who were discharged from the hospital following a heart attack or stroke were not prescribed a statin. This factor led to worse outcomes for those patients.
Researchers also found that only 13% of patients were given a high-intensity dose of statins, but noted that patients on those higher doses experienced fewer heart attacks or strokes. For patients younger than age 76, a high-intensity statin is indicated, according to American Heart Association guidelines. Only 17.7% of these patients were discharged on a high-intensity dose, however.
Does Cholesterol Testing Reduce Risk of Recurrent Stroke?
When a patient has a heart attack or stroke, it is critical for his or her clinician to perform a follow-up cholesterol test, according to a study conducted at the Intermountain Medical Center Heart Institute in Salt Lake City. This additional measure is significantly associated with reduced risk of having another serious cardiovascular episode.
Investigators found that patients who do not follow up with their doctor by getting a low-density lipoprotein (LDL) cholesterol test after a heart attack or stroke are significantly more likely to have a recurrence. They also found a significant and clinically meaningful difference in major adverse outcomes—including death, heart attack, stroke, and a vascular bypass or an angioplasty—based on whether or not a patient has a follow-up measurement of his or her LDL cholesterol.
“It is clear that anyone with a previous heart problem caused by clogged arteries should be taking a cholesterol-lowering medication,” said Kirk U. Knowlton, MD, Director of Cardiovascular Research at the Intermountain Medical Center Heart Institute.
The study of more than 60,000 patients with known heart disease, cerebrovascular disease, or peripheral artery disease, including patients with stroke and heart attack, showed that the major adverse clinical event rate was lower in patients who took cholesterol-lowering statins and in those who did not take them if their LDL was measured.
“The large difference is surprising. The risk of dying after three years with no LDL follow-up is 21% versus 5.9% for patients who have an LDL follow-up,” said Dr. Knowlton.
Researchers reviewed Intermountain Healthcare’s enterprise data warehouse to identify all adults who came to one of Intermountain’s 22 hospitals for the first time with a heart attack or stroke. These data included patients with coronary artery disease, cerebrovascular disease, and peripheral arterial disease admitted between January 1, 1999, and December 31, 2013.
Investigators observed patients who survived and were followed for three years or more or until their death. Patient demographics, history, prescribed medications, and whether LDL was measured was analyzed.
The study compared 62,070 patients in the database who met the study criteria. The mean age was 66, and 65% of patients were male. Of those who met the criteria, 69.3% had coronary artery disease, 18.6% had cerebrovascular disease, and 12.1% had peripheral arterial disease when they came to the hospital with their first heart attack or stroke.
Researchers found that the risk of a patient having a secondary event or dying decreased in patients who had a follow-up LDL test before a subsequent adverse outcome or before the end of their follow-up.
Coffee Is Associated With Lower Risk of Heart Failure and Stroke
Drinking coffee may be associated with a decreased risk of heart failure or stroke, according to researchers.
Investigators used machine learning to analyze data from the long-running Framingham Heart Study, which includes information about what people eat and their cardiovascular health. They found that every additional cup of coffee consumed per week was associated with a 7% decreased risk of heart failure and an 8% reduced risk of stroke, compared with non-coffee drinkers.
The researchers further investigated the machine learning results using traditional analysis in two studies with similar sets of data: the Cardiovascular Heart Study and the Atherosclerosis Risk In Communities Study. The association between drinking coffee and a decreased risk of heart failure and stroke was observed consistently in all three studies.
Another potential risk factor identified by machine-learning analysis was red meat consumption. The association between red meat consumption and heart failure or stroke was less clear, however. Eating red meat was associated with decreased risk of heart failure and stroke in the Framingham Heart Study, but validating the finding in comparable studies is more challenging due to differences in the definitions of red meat between studies, said the researchers. Further investigation to better determine how red meat consumption affects risk for heart failure and stroke is ongoing.
The researchers also built a predictive model using known risk factors from the Framingham Risk Score such as blood pressure, age, and other patient characteristics associated with cardiovascular disease. “By including coffee in the model, the prediction accuracy increased by 4%. Machine learning may be a useful addition to the way we look at data and may help us to find new ways to lower the risk of heart failure and strokes,” said David Kao, MD, Assistant Professor of Medicine in the Division of Cardiology at the University of Colorado School of Medicine in Aurora.
Statins May Improve Stroke Outcome
Patients with a prior history of heart attack or stroke have better outcomes when cholesterol-lowering medications are used after they are discharged from the hospital, according to researchers.
Prior surveys in hospitals found that statins are not being used consistently in patients who have been admitted to the hospital following a heart attack or stroke. Researchers also found that when the medication is prescribed, dosing is likely not as high as it should be to provide optimal benefits.
Researchers examined more than 62,000 records of patients from the Intermountain Healthcare system between 1999 and 2013 who survived an initial atherosclerotic cardiovascular disease event, such as a heart attack or stroke. They were then followed for three years or until death to identify the effectiveness of statin use prescribed at the time of their discharge.
“Patients who were prescribed a statin medication following an initial heart attack or stroke reduced their risk of a future adverse event such as a future heart attack, stroke, revascularization, or death by almost 25%—the rate dropped from 34% to 26%,” said Jeffrey L. Anderson, MD, a cardiovascular researcher at the Intermountain Medical Center Heart Institute. “The patients who were discharged on what is considered a high-intensity dose of a statin saw a 21% reduction in their risk,” compared with those discharged on a low-intensity statin dose.”
Investigators found that 30% of patients in the study who were discharged from the hospital following a heart attack or stroke were not prescribed a statin. This factor led to worse outcomes for those patients.
Researchers also found that only 13% of patients were given a high-intensity dose of statins, but noted that patients on those higher doses experienced fewer heart attacks or strokes. For patients younger than age 76, a high-intensity statin is indicated, according to American Heart Association guidelines. Only 17.7% of these patients were discharged on a high-intensity dose, however.
Withholding elective surgery in smokers, obese patients
No one will argue that obesity and tobacco aren’t serious public health issues, underlying many causes of morbidity and mortality. As a result, they’re driving factors behind a fair amount of health care spending.
In England, the county of Hertfordshire recently adopted a new approach to this: a ban on routine, nonurgent surgeries for both. Those with a body mass index of 30-40 kg/m2 must lose 10% of their weight over 9 months to qualify for a procedure, while those with a BMI above 40 must lose 15%. Smokers have to go 8 weeks without a cigarette and take breath tests to prove it.
The group that formulated the plan noted that resources to help these groups achieve such goals are (and will continue to be) freely available.
Not unexpectedly, the plan is controversial. Robert West, MD, a professor of health psychology and director of tobacco studies at the University College London, told CNN that “rationing treatment on the basis of unhealthy behaviors betrays an extraordinary naivety about what drives those behaviors.”
Of course, this debate is nothing new. In December 2014, I wrote about surgeons in the United States who were refusing to do elective hernia repairs on smokers because of their higher complication rates.
A lot of this is framed in terms of money, since that’s the driving factor. Obese patients and smokers do have higher rates of surgical complications in general, with longer recoveries and, hence, higher costs. This policy tries to put greater responsibility on patients to maintain their own health and well-being. After all, financial resources are a finite, shared commodity.
Like everything in modern health care, there’s no easy answer. Insurers and doctors try to balance better outcomes vs. greater good and cost savings.
Medicine is, and always will be, an ongoing experiment, where some things work, some don’t, and we learn from time and experience.
Unfortunately, patients and their doctors are often caught in the middle, trapped between market and political forces on one side and caring for those who need us on the other. That’s never good, or easy, for those directly involved with individual patients on the front lines of medical care.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
No one will argue that obesity and tobacco aren’t serious public health issues, underlying many causes of morbidity and mortality. As a result, they’re driving factors behind a fair amount of health care spending.
In England, the county of Hertfordshire recently adopted a new approach to this: a ban on routine, nonurgent surgeries for both. Those with a body mass index of 30-40 kg/m2 must lose 10% of their weight over 9 months to qualify for a procedure, while those with a BMI above 40 must lose 15%. Smokers have to go 8 weeks without a cigarette and take breath tests to prove it.
The group that formulated the plan noted that resources to help these groups achieve such goals are (and will continue to be) freely available.
Not unexpectedly, the plan is controversial. Robert West, MD, a professor of health psychology and director of tobacco studies at the University College London, told CNN that “rationing treatment on the basis of unhealthy behaviors betrays an extraordinary naivety about what drives those behaviors.”
Of course, this debate is nothing new. In December 2014, I wrote about surgeons in the United States who were refusing to do elective hernia repairs on smokers because of their higher complication rates.
A lot of this is framed in terms of money, since that’s the driving factor. Obese patients and smokers do have higher rates of surgical complications in general, with longer recoveries and, hence, higher costs. This policy tries to put greater responsibility on patients to maintain their own health and well-being. After all, financial resources are a finite, shared commodity.
Like everything in modern health care, there’s no easy answer. Insurers and doctors try to balance better outcomes vs. greater good and cost savings.
Medicine is, and always will be, an ongoing experiment, where some things work, some don’t, and we learn from time and experience.
Unfortunately, patients and their doctors are often caught in the middle, trapped between market and political forces on one side and caring for those who need us on the other. That’s never good, or easy, for those directly involved with individual patients on the front lines of medical care.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
No one will argue that obesity and tobacco aren’t serious public health issues, underlying many causes of morbidity and mortality. As a result, they’re driving factors behind a fair amount of health care spending.
In England, the county of Hertfordshire recently adopted a new approach to this: a ban on routine, nonurgent surgeries for both. Those with a body mass index of 30-40 kg/m2 must lose 10% of their weight over 9 months to qualify for a procedure, while those with a BMI above 40 must lose 15%. Smokers have to go 8 weeks without a cigarette and take breath tests to prove it.
The group that formulated the plan noted that resources to help these groups achieve such goals are (and will continue to be) freely available.
Not unexpectedly, the plan is controversial. Robert West, MD, a professor of health psychology and director of tobacco studies at the University College London, told CNN that “rationing treatment on the basis of unhealthy behaviors betrays an extraordinary naivety about what drives those behaviors.”
Of course, this debate is nothing new. In December 2014, I wrote about surgeons in the United States who were refusing to do elective hernia repairs on smokers because of their higher complication rates.
A lot of this is framed in terms of money, since that’s the driving factor. Obese patients and smokers do have higher rates of surgical complications in general, with longer recoveries and, hence, higher costs. This policy tries to put greater responsibility on patients to maintain their own health and well-being. After all, financial resources are a finite, shared commodity.
Like everything in modern health care, there’s no easy answer. Insurers and doctors try to balance better outcomes vs. greater good and cost savings.
Medicine is, and always will be, an ongoing experiment, where some things work, some don’t, and we learn from time and experience.
Unfortunately, patients and their doctors are often caught in the middle, trapped between market and political forces on one side and caring for those who need us on the other. That’s never good, or easy, for those directly involved with individual patients on the front lines of medical care.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Is the Estrogen–CGRP Relationship Relevant to Migraine?
Calcitonin gene-related peptide (CGRP) plays a key role in migraine pathophysiology, and recent studies have identified interactions between ovarian steroid hormones, CGRP, and the trigeminovascular system, according to a review published online ahead of print October 30, 2017, in Cephalalgia.
“Numerous animal and human studies have shown that cyclic fluctuations of ovarian hormones (mainly estrogen) modulate CGRP in the peripheral and central trigeminovascular system; this [effect] is especially relevant now that novel antibodies directed against CGRP or its receptor are currently in clinical trials,” said Alejandro Labastida-Ramírez of the Division of Vascular Medicine and Pharmacology at Erasmus University Medical Center in Rotterdam, the Netherlands, and colleagues.
The relationship between estrogen and CGRP seems to be “a key factor involved in the higher prevalence of migraine in women,” the authors said. “Future studies should focus on how fluctuations of gonadal hormones influence migraine pathophysiology in both genders…. Hopefully, these sex-related differences may contribute to the development of gender-specific therapies.”
Interplay of Hormones and CGRP
A clinical study by Stevenson et al in 1986 was one of the first to discover a relationship between female sex hormones and CGRP. In this study, concentrations of immunoreactive plasma CGRP in healthy women were significantly increased throughout pregnancy and decreased after delivery. A 1990 study by Valdemarsson et al found that in healthy subjects, immunoreactive plasma CGRP levels were significantly higher in females than in males. “The use of combined contraceptive pills was associated with even higher levels of immunoreactive CGRP in plasma,” said the review authors. “Accordingly, in postmenopausal women, decreased estradiol serum levels were positively correlated with decreased plasma immunoreactive CGRP concentrations, [suggesting] that the CGRP system could be influenced directly by endogenous or exogenous ovarian steroid hormones.”
Ibrahimi et al in 2017 used an experimental model to explore gender differences in CGRP-dependent dermal blood flow in healthy subjects and migraineurs. Dermal blood flow in males did not vary over time and was comparable between healthy subjects and migraineurs. In healthy women, fluctuations of ovarian steroid hormones influenced CGRP-dependent dermal blood flow. “Interestingly, in female migraine patients, dermal blood flow responses were elevated, compared to healthy subjects, but these responses were independent of the menstrual cycle,” the review authors noted.
Therapeutic Trials
Three humanized monoclonal antibodies targeting CGRP and one fully human monoclonal antibody targeting the CGRP receptor are in development.
While trials indicate that CGRP blockade is effective for treating migraine, further studies are needed to “elucidate whether these novel drugs are safe in individuals with cardiovascular risk factors, if there are any consequences of chronic CGRP inhibition in young reproductive women with a normal menstrual cycle, and whether efficacy depends on the phase of the menstrual cycle,” the authors said.
—Jake Remaly
Suggested Reading
Ibrahimi K, Vermeersch S, Frederiks P, et al. The influence of migraine and female hormones on capsaicin-induced dermal blood flow. Cephalalgia. 2017;37(12):1164-1172.
Labastida-Ramírez A, Rubio-Beltrán E, Villalón CM, MaassenVanDenBrink A. Gender aspects of CGRP in migraine. Cephalalgia. 2017 Oct 30 [Epub ahead of print].
Stevenson JC, Macdonald DW, Warren RC, et al. Increased concentration of circulating calcitonin gene related peptide during normal human pregnancy. Br Med J (Clin Res Ed). 1986;293(6558):1329-1330.
Valdemarsson S, Edvinsson L, Hedner P, Ekman R. Hormonal influence on calcitonin gene-related peptide in man: effects of sex difference and contraceptive pills. Scand J Clin Lab Invest. 1990;50(4):385-388.
Calcitonin gene-related peptide (CGRP) plays a key role in migraine pathophysiology, and recent studies have identified interactions between ovarian steroid hormones, CGRP, and the trigeminovascular system, according to a review published online ahead of print October 30, 2017, in Cephalalgia.
“Numerous animal and human studies have shown that cyclic fluctuations of ovarian hormones (mainly estrogen) modulate CGRP in the peripheral and central trigeminovascular system; this [effect] is especially relevant now that novel antibodies directed against CGRP or its receptor are currently in clinical trials,” said Alejandro Labastida-Ramírez of the Division of Vascular Medicine and Pharmacology at Erasmus University Medical Center in Rotterdam, the Netherlands, and colleagues.
The relationship between estrogen and CGRP seems to be “a key factor involved in the higher prevalence of migraine in women,” the authors said. “Future studies should focus on how fluctuations of gonadal hormones influence migraine pathophysiology in both genders…. Hopefully, these sex-related differences may contribute to the development of gender-specific therapies.”
Interplay of Hormones and CGRP
A clinical study by Stevenson et al in 1986 was one of the first to discover a relationship between female sex hormones and CGRP. In this study, concentrations of immunoreactive plasma CGRP in healthy women were significantly increased throughout pregnancy and decreased after delivery. A 1990 study by Valdemarsson et al found that in healthy subjects, immunoreactive plasma CGRP levels were significantly higher in females than in males. “The use of combined contraceptive pills was associated with even higher levels of immunoreactive CGRP in plasma,” said the review authors. “Accordingly, in postmenopausal women, decreased estradiol serum levels were positively correlated with decreased plasma immunoreactive CGRP concentrations, [suggesting] that the CGRP system could be influenced directly by endogenous or exogenous ovarian steroid hormones.”
Ibrahimi et al in 2017 used an experimental model to explore gender differences in CGRP-dependent dermal blood flow in healthy subjects and migraineurs. Dermal blood flow in males did not vary over time and was comparable between healthy subjects and migraineurs. In healthy women, fluctuations of ovarian steroid hormones influenced CGRP-dependent dermal blood flow. “Interestingly, in female migraine patients, dermal blood flow responses were elevated, compared to healthy subjects, but these responses were independent of the menstrual cycle,” the review authors noted.
Therapeutic Trials
Three humanized monoclonal antibodies targeting CGRP and one fully human monoclonal antibody targeting the CGRP receptor are in development.
While trials indicate that CGRP blockade is effective for treating migraine, further studies are needed to “elucidate whether these novel drugs are safe in individuals with cardiovascular risk factors, if there are any consequences of chronic CGRP inhibition in young reproductive women with a normal menstrual cycle, and whether efficacy depends on the phase of the menstrual cycle,” the authors said.
—Jake Remaly
Suggested Reading
Ibrahimi K, Vermeersch S, Frederiks P, et al. The influence of migraine and female hormones on capsaicin-induced dermal blood flow. Cephalalgia. 2017;37(12):1164-1172.
Labastida-Ramírez A, Rubio-Beltrán E, Villalón CM, MaassenVanDenBrink A. Gender aspects of CGRP in migraine. Cephalalgia. 2017 Oct 30 [Epub ahead of print].
Stevenson JC, Macdonald DW, Warren RC, et al. Increased concentration of circulating calcitonin gene related peptide during normal human pregnancy. Br Med J (Clin Res Ed). 1986;293(6558):1329-1330.
Valdemarsson S, Edvinsson L, Hedner P, Ekman R. Hormonal influence on calcitonin gene-related peptide in man: effects of sex difference and contraceptive pills. Scand J Clin Lab Invest. 1990;50(4):385-388.
Calcitonin gene-related peptide (CGRP) plays a key role in migraine pathophysiology, and recent studies have identified interactions between ovarian steroid hormones, CGRP, and the trigeminovascular system, according to a review published online ahead of print October 30, 2017, in Cephalalgia.
“Numerous animal and human studies have shown that cyclic fluctuations of ovarian hormones (mainly estrogen) modulate CGRP in the peripheral and central trigeminovascular system; this [effect] is especially relevant now that novel antibodies directed against CGRP or its receptor are currently in clinical trials,” said Alejandro Labastida-Ramírez of the Division of Vascular Medicine and Pharmacology at Erasmus University Medical Center in Rotterdam, the Netherlands, and colleagues.
The relationship between estrogen and CGRP seems to be “a key factor involved in the higher prevalence of migraine in women,” the authors said. “Future studies should focus on how fluctuations of gonadal hormones influence migraine pathophysiology in both genders…. Hopefully, these sex-related differences may contribute to the development of gender-specific therapies.”
Interplay of Hormones and CGRP
A clinical study by Stevenson et al in 1986 was one of the first to discover a relationship between female sex hormones and CGRP. In this study, concentrations of immunoreactive plasma CGRP in healthy women were significantly increased throughout pregnancy and decreased after delivery. A 1990 study by Valdemarsson et al found that in healthy subjects, immunoreactive plasma CGRP levels were significantly higher in females than in males. “The use of combined contraceptive pills was associated with even higher levels of immunoreactive CGRP in plasma,” said the review authors. “Accordingly, in postmenopausal women, decreased estradiol serum levels were positively correlated with decreased plasma immunoreactive CGRP concentrations, [suggesting] that the CGRP system could be influenced directly by endogenous or exogenous ovarian steroid hormones.”
Ibrahimi et al in 2017 used an experimental model to explore gender differences in CGRP-dependent dermal blood flow in healthy subjects and migraineurs. Dermal blood flow in males did not vary over time and was comparable between healthy subjects and migraineurs. In healthy women, fluctuations of ovarian steroid hormones influenced CGRP-dependent dermal blood flow. “Interestingly, in female migraine patients, dermal blood flow responses were elevated, compared to healthy subjects, but these responses were independent of the menstrual cycle,” the review authors noted.
Therapeutic Trials
Three humanized monoclonal antibodies targeting CGRP and one fully human monoclonal antibody targeting the CGRP receptor are in development.
While trials indicate that CGRP blockade is effective for treating migraine, further studies are needed to “elucidate whether these novel drugs are safe in individuals with cardiovascular risk factors, if there are any consequences of chronic CGRP inhibition in young reproductive women with a normal menstrual cycle, and whether efficacy depends on the phase of the menstrual cycle,” the authors said.
—Jake Remaly
Suggested Reading
Ibrahimi K, Vermeersch S, Frederiks P, et al. The influence of migraine and female hormones on capsaicin-induced dermal blood flow. Cephalalgia. 2017;37(12):1164-1172.
Labastida-Ramírez A, Rubio-Beltrán E, Villalón CM, MaassenVanDenBrink A. Gender aspects of CGRP in migraine. Cephalalgia. 2017 Oct 30 [Epub ahead of print].
Stevenson JC, Macdonald DW, Warren RC, et al. Increased concentration of circulating calcitonin gene related peptide during normal human pregnancy. Br Med J (Clin Res Ed). 1986;293(6558):1329-1330.
Valdemarsson S, Edvinsson L, Hedner P, Ekman R. Hormonal influence on calcitonin gene-related peptide in man: effects of sex difference and contraceptive pills. Scand J Clin Lab Invest. 1990;50(4):385-388.
Debunking Psoriasis Myths: How to Help Patients Who Are Afraid of Injections
Myth: Patients Are Not Willing to Give Themselves Injections
Injectable biologics target specific parts of the immune system, making them popular treatment options for psoriasis patients, with ample research on their efficacy. Performing a self-injection can be daunting for patients trying a biologic for the first time, and clinicians should be aware of the dearth of patient education material. Although patients may be fearful of self-injections, especially the first few treatments, their worries can be assuaged with proper instruction and appropriate delivery method.
Abrouk et al sought to provide an online guide and video on biologic injections to increase the success of the therapy and compliance among patients. They created a printable guide that covers the supplies needed, procedure techniques, and plans for traveling with medications. Because pain is a common concern for patients, they suggest numbing the injection area with an ice pack first. They also offer tips on dealing with injection-site reactions such as redness or bruising.
Nurse practitioners and physician assistants can be used to give psoriasis patients more personalized attention regarding the fear of injections. They can explain the injection procedures and describe differences between administration techniques. Some patients may prefer using an autoinjector versus a prefilled syringe, which may impact the treatment administered. Taking photographs to show progress with therapy also may motivate patients to tolerate therapy.
The National Psoriasis Foundation provides the following tips to make it easier for patients to self-inject and reduce the chance of an injection-site reaction:
- Pick an easy injection site, such as the top of the rights, abdomen, or back of the arms.
- Rotate injection sites from right to left.
- Numb the area.
- Warm the pen up by taking it out of the refrigerator 1.5 hours before it is used.
- Be patient and avoid moving the injection pen before the needle is finished administering the drug.
By giving psoriasis patients educational materials, you can empower them to control their disease with injectable biologics.
Expert Commentary
Most of my patients who use a biologic for the first time are undaunted by learning to inject themselves. I can think of just 1 of my ~300 biologic patients who has to come in every few weeks for their medicine to be injected by one of our nurses. Surprisingly, some patients (I'd estimate 5% of my biologic patients) actually prefer the syringe compared to the autoinjector, with some comments saying that the syringe is less painful and less abrupt. Needle phobia should not be a reason to not prescribe a biologic for a patient with severe psoriasis who needs it.
Abrouk M, Nakamura M, Zhu TH, et al. The patient’s guide to psoriasis treatment. part 3: biologic injectables. Dermatol Ther (Heidelb). 2016;6:325-331.
Aldredge LM, Young MS. Providing guidance for patients with moderate-to-severe psoriasis who are candidates for biologic therapy. J Dermatol Nurses Assoc. 2016;8:14-26.
National Psoriasis Foundation. Self-injections 101. https://www.psoriasis.org/about-psoriasis/treatments/biologics/self-injections-101. Accessed January 2, 2018.
Myth: Patients Are Not Willing to Give Themselves Injections
Injectable biologics target specific parts of the immune system, making them popular treatment options for psoriasis patients, with ample research on their efficacy. Performing a self-injection can be daunting for patients trying a biologic for the first time, and clinicians should be aware of the dearth of patient education material. Although patients may be fearful of self-injections, especially the first few treatments, their worries can be assuaged with proper instruction and appropriate delivery method.
Abrouk et al sought to provide an online guide and video on biologic injections to increase the success of the therapy and compliance among patients. They created a printable guide that covers the supplies needed, procedure techniques, and plans for traveling with medications. Because pain is a common concern for patients, they suggest numbing the injection area with an ice pack first. They also offer tips on dealing with injection-site reactions such as redness or bruising.
Nurse practitioners and physician assistants can be used to give psoriasis patients more personalized attention regarding the fear of injections. They can explain the injection procedures and describe differences between administration techniques. Some patients may prefer using an autoinjector versus a prefilled syringe, which may impact the treatment administered. Taking photographs to show progress with therapy also may motivate patients to tolerate therapy.
The National Psoriasis Foundation provides the following tips to make it easier for patients to self-inject and reduce the chance of an injection-site reaction:
- Pick an easy injection site, such as the top of the rights, abdomen, or back of the arms.
- Rotate injection sites from right to left.
- Numb the area.
- Warm the pen up by taking it out of the refrigerator 1.5 hours before it is used.
- Be patient and avoid moving the injection pen before the needle is finished administering the drug.
By giving psoriasis patients educational materials, you can empower them to control their disease with injectable biologics.
Expert Commentary
Most of my patients who use a biologic for the first time are undaunted by learning to inject themselves. I can think of just 1 of my ~300 biologic patients who has to come in every few weeks for their medicine to be injected by one of our nurses. Surprisingly, some patients (I'd estimate 5% of my biologic patients) actually prefer the syringe compared to the autoinjector, with some comments saying that the syringe is less painful and less abrupt. Needle phobia should not be a reason to not prescribe a biologic for a patient with severe psoriasis who needs it.
Myth: Patients Are Not Willing to Give Themselves Injections
Injectable biologics target specific parts of the immune system, making them popular treatment options for psoriasis patients, with ample research on their efficacy. Performing a self-injection can be daunting for patients trying a biologic for the first time, and clinicians should be aware of the dearth of patient education material. Although patients may be fearful of self-injections, especially the first few treatments, their worries can be assuaged with proper instruction and appropriate delivery method.
Abrouk et al sought to provide an online guide and video on biologic injections to increase the success of the therapy and compliance among patients. They created a printable guide that covers the supplies needed, procedure techniques, and plans for traveling with medications. Because pain is a common concern for patients, they suggest numbing the injection area with an ice pack first. They also offer tips on dealing with injection-site reactions such as redness or bruising.
Nurse practitioners and physician assistants can be used to give psoriasis patients more personalized attention regarding the fear of injections. They can explain the injection procedures and describe differences between administration techniques. Some patients may prefer using an autoinjector versus a prefilled syringe, which may impact the treatment administered. Taking photographs to show progress with therapy also may motivate patients to tolerate therapy.
The National Psoriasis Foundation provides the following tips to make it easier for patients to self-inject and reduce the chance of an injection-site reaction:
- Pick an easy injection site, such as the top of the rights, abdomen, or back of the arms.
- Rotate injection sites from right to left.
- Numb the area.
- Warm the pen up by taking it out of the refrigerator 1.5 hours before it is used.
- Be patient and avoid moving the injection pen before the needle is finished administering the drug.
By giving psoriasis patients educational materials, you can empower them to control their disease with injectable biologics.
Expert Commentary
Most of my patients who use a biologic for the first time are undaunted by learning to inject themselves. I can think of just 1 of my ~300 biologic patients who has to come in every few weeks for their medicine to be injected by one of our nurses. Surprisingly, some patients (I'd estimate 5% of my biologic patients) actually prefer the syringe compared to the autoinjector, with some comments saying that the syringe is less painful and less abrupt. Needle phobia should not be a reason to not prescribe a biologic for a patient with severe psoriasis who needs it.
Abrouk M, Nakamura M, Zhu TH, et al. The patient’s guide to psoriasis treatment. part 3: biologic injectables. Dermatol Ther (Heidelb). 2016;6:325-331.
Aldredge LM, Young MS. Providing guidance for patients with moderate-to-severe psoriasis who are candidates for biologic therapy. J Dermatol Nurses Assoc. 2016;8:14-26.
National Psoriasis Foundation. Self-injections 101. https://www.psoriasis.org/about-psoriasis/treatments/biologics/self-injections-101. Accessed January 2, 2018.
Abrouk M, Nakamura M, Zhu TH, et al. The patient’s guide to psoriasis treatment. part 3: biologic injectables. Dermatol Ther (Heidelb). 2016;6:325-331.
Aldredge LM, Young MS. Providing guidance for patients with moderate-to-severe psoriasis who are candidates for biologic therapy. J Dermatol Nurses Assoc. 2016;8:14-26.
National Psoriasis Foundation. Self-injections 101. https://www.psoriasis.org/about-psoriasis/treatments/biologics/self-injections-101. Accessed January 2, 2018.