User login
Questions value of ACOG/SMFM guidelines
FOR THE MANAGEMENT OF LABOR, PATIENCE IS A VIRTUE
ROBERT L. BARBIERI, MD (EDITORIAL; AUGUST 2017)
Questions value of ACOG/SMFM guidelines
The labor management guidelines recommended by the American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal-Fetal Medicine (SMFM) are terrible. Now retired, I trained in 1959–1963. In my career as an obstetrician, my primary cesarean delivery rate was 10% or less, and part of that was external pressure from people who did not know how to deliver a baby. Persistent occiput posterior position is a problem of inadequate flexion of the head, often due to ineffective contractions earlier. In such a situation, “pit” early! Rotate the head if you must, and teach residents how, please. The guidelines do not discuss the exhausted mother who goes home after a long labor or hours of pushing. I have interviewed new obstetricians in my community as early as 1980 who did not know what deep transverse arrest was. There, I am done voicing my disgust with obstetrics as it is practiced today.
James Honig, MD
Merritt Island, Florida
Managing difficult labor scenarios
I concur with Dr. Barbieri’s views on labor management that watchful waiting and giving the patient adequate time to progress naturally is the key to increase the chances of vaginal delivery. After all, labor is a physiologic process and should progress naturally. Having said that, I would like to know Dr. Barbieri’s views on handling certain circumstances in which patients these days land in the labor room, including 1) postdatedpregnancy with reduced fetal movements and not in labor; 2) full-term/postterm pregnancy with free-floating head and poor Bishop score; 3) full-term pregnancy with niggling pains for more than 1 week; and many such conditions that place you in the dilemma of whether to induce, knowing that chances of failure are high.
Manju Hotchandani, MD
New Delhi, India
Midwives always use patience to guide labor
As a Certified Nurse-Midwife since 1985 (now retired), “patience” in managing labor has always been my guide, as it has been for my midwifery colleagues. This is another example of ACOG finally acknowledging the truths we women have always known, without crediting the wisdom of midwives over the centuries. Lamaze International’s 6 Healthy Birth Practices also must have been their guide. “Evolving concepts of normal labor progress,” as though this was new information, would be humorous if it were not so frustrating!
Marsha Kelly, CNM
Charlotte, North Carolina
Dr. Barbieri responds
The readers of OBG
Every clinician involved in the birth process is deeply committed to a safe delivery for both mother and baby. Clinicians guide the birth process based on the unique characteristics and needs of each woman. Dr. Honig advocates for the active management of the labor process, while Ms. Kelly advocates for less intervention. Both approaches to labor management may be optimal depending on the unique clinical needs of each woman. Dr. Hotchandani inquires about managing common obstetric presentations. In my practice, induction is recommended for all women postterm who report consistently reduced fetal movement with the goal of reducing the risk of sudden intrauterine fetal demise. For healthy women at term with painful contractions and reassuring fetal status, but no cervical change, we support and counsel the patient and offer therapeutic rest with morphine. For women at term with a floating head and poor Bishop score, we would not intervene, until 41 weeks’ gestation when we would initiate gentle cervical ripening with mechanical or pharmacologic treatment.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
FOR THE MANAGEMENT OF LABOR, PATIENCE IS A VIRTUE
ROBERT L. BARBIERI, MD (EDITORIAL; AUGUST 2017)
Questions value of ACOG/SMFM guidelines
The labor management guidelines recommended by the American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal-Fetal Medicine (SMFM) are terrible. Now retired, I trained in 1959–1963. In my career as an obstetrician, my primary cesarean delivery rate was 10% or less, and part of that was external pressure from people who did not know how to deliver a baby. Persistent occiput posterior position is a problem of inadequate flexion of the head, often due to ineffective contractions earlier. In such a situation, “pit” early! Rotate the head if you must, and teach residents how, please. The guidelines do not discuss the exhausted mother who goes home after a long labor or hours of pushing. I have interviewed new obstetricians in my community as early as 1980 who did not know what deep transverse arrest was. There, I am done voicing my disgust with obstetrics as it is practiced today.
James Honig, MD
Merritt Island, Florida
Managing difficult labor scenarios
I concur with Dr. Barbieri’s views on labor management that watchful waiting and giving the patient adequate time to progress naturally is the key to increase the chances of vaginal delivery. After all, labor is a physiologic process and should progress naturally. Having said that, I would like to know Dr. Barbieri’s views on handling certain circumstances in which patients these days land in the labor room, including 1) postdatedpregnancy with reduced fetal movements and not in labor; 2) full-term/postterm pregnancy with free-floating head and poor Bishop score; 3) full-term pregnancy with niggling pains for more than 1 week; and many such conditions that place you in the dilemma of whether to induce, knowing that chances of failure are high.
Manju Hotchandani, MD
New Delhi, India
Midwives always use patience to guide labor
As a Certified Nurse-Midwife since 1985 (now retired), “patience” in managing labor has always been my guide, as it has been for my midwifery colleagues. This is another example of ACOG finally acknowledging the truths we women have always known, without crediting the wisdom of midwives over the centuries. Lamaze International’s 6 Healthy Birth Practices also must have been their guide. “Evolving concepts of normal labor progress,” as though this was new information, would be humorous if it were not so frustrating!
Marsha Kelly, CNM
Charlotte, North Carolina
Dr. Barbieri responds
The readers of OBG
Every clinician involved in the birth process is deeply committed to a safe delivery for both mother and baby. Clinicians guide the birth process based on the unique characteristics and needs of each woman. Dr. Honig advocates for the active management of the labor process, while Ms. Kelly advocates for less intervention. Both approaches to labor management may be optimal depending on the unique clinical needs of each woman. Dr. Hotchandani inquires about managing common obstetric presentations. In my practice, induction is recommended for all women postterm who report consistently reduced fetal movement with the goal of reducing the risk of sudden intrauterine fetal demise. For healthy women at term with painful contractions and reassuring fetal status, but no cervical change, we support and counsel the patient and offer therapeutic rest with morphine. For women at term with a floating head and poor Bishop score, we would not intervene, until 41 weeks’ gestation when we would initiate gentle cervical ripening with mechanical or pharmacologic treatment.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
FOR THE MANAGEMENT OF LABOR, PATIENCE IS A VIRTUE
ROBERT L. BARBIERI, MD (EDITORIAL; AUGUST 2017)
Questions value of ACOG/SMFM guidelines
The labor management guidelines recommended by the American College of Obstetricians and Gynecologists (ACOG) and the Society of Maternal-Fetal Medicine (SMFM) are terrible. Now retired, I trained in 1959–1963. In my career as an obstetrician, my primary cesarean delivery rate was 10% or less, and part of that was external pressure from people who did not know how to deliver a baby. Persistent occiput posterior position is a problem of inadequate flexion of the head, often due to ineffective contractions earlier. In such a situation, “pit” early! Rotate the head if you must, and teach residents how, please. The guidelines do not discuss the exhausted mother who goes home after a long labor or hours of pushing. I have interviewed new obstetricians in my community as early as 1980 who did not know what deep transverse arrest was. There, I am done voicing my disgust with obstetrics as it is practiced today.
James Honig, MD
Merritt Island, Florida
Managing difficult labor scenarios
I concur with Dr. Barbieri’s views on labor management that watchful waiting and giving the patient adequate time to progress naturally is the key to increase the chances of vaginal delivery. After all, labor is a physiologic process and should progress naturally. Having said that, I would like to know Dr. Barbieri’s views on handling certain circumstances in which patients these days land in the labor room, including 1) postdatedpregnancy with reduced fetal movements and not in labor; 2) full-term/postterm pregnancy with free-floating head and poor Bishop score; 3) full-term pregnancy with niggling pains for more than 1 week; and many such conditions that place you in the dilemma of whether to induce, knowing that chances of failure are high.
Manju Hotchandani, MD
New Delhi, India
Midwives always use patience to guide labor
As a Certified Nurse-Midwife since 1985 (now retired), “patience” in managing labor has always been my guide, as it has been for my midwifery colleagues. This is another example of ACOG finally acknowledging the truths we women have always known, without crediting the wisdom of midwives over the centuries. Lamaze International’s 6 Healthy Birth Practices also must have been their guide. “Evolving concepts of normal labor progress,” as though this was new information, would be humorous if it were not so frustrating!
Marsha Kelly, CNM
Charlotte, North Carolina
Dr. Barbieri responds
The readers of OBG
Every clinician involved in the birth process is deeply committed to a safe delivery for both mother and baby. Clinicians guide the birth process based on the unique characteristics and needs of each woman. Dr. Honig advocates for the active management of the labor process, while Ms. Kelly advocates for less intervention. Both approaches to labor management may be optimal depending on the unique clinical needs of each woman. Dr. Hotchandani inquires about managing common obstetric presentations. In my practice, induction is recommended for all women postterm who report consistently reduced fetal movement with the goal of reducing the risk of sudden intrauterine fetal demise. For healthy women at term with painful contractions and reassuring fetal status, but no cervical change, we support and counsel the patient and offer therapeutic rest with morphine. For women at term with a floating head and poor Bishop score, we would not intervene, until 41 weeks’ gestation when we would initiate gentle cervical ripening with mechanical or pharmacologic treatment.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Snake in the grass
Most accomplished public speakers will tell you that critical to their success is the ability to understand and adapt to their audiences. It turns out that even chimpanzees accept and use this cornerstone of effective communication.
In a study published in Science Advances (2017 Nov 15;3[11]:e1701742), three scientists working in Uganda reported that chimpanzees who encounter a potential threat in the form of a realistic snake model will vocalize significantly fewer alert hoots if they hear other alert calls coming from the jungle in the vicinity. In other words, the chimp is saying to himself, “Why should I bother wasting my time and lung power hooting to warn my troop mates? Those guys already know about the snake.”
To select which anticipatory guidance topics to include and still be effective communicators, we have to know the families we are trying to help. Gaining this more nuanced picture of a family takes time and is fostered by continuity. Seeing a different provider at each visit doesn’t work very well here. What are this unique family’s concerns, regardless of what some committee thinks we should be asking?
How much can we rely on the media and groups such as the American Academy of Pediatrics and the Centers for Disease Control and Prevention to get out the messages that we have decided to skip over to address this family’s special concerns? Is the message about the benefits of breastfeeding so widely known that we will be wasting our limited office time repeating it? Is the same true for gun safety and seat belts? This is where research can help us decide where to target messages on a national level. But large population studies don’t always apply to our communities and the families we serve.
Where does our role as primary care physicians fit into the bigger picture of health education? The warning messages issued on a national level may have little relevance for our individual patients’ concerns. Is it our role to echo the message, or are we the ones who must do the fine-tuning?
And then there are the recent depressing and counterintuitive findings that for hot-button topics like immunization, education has little if any value. Those families with firmly held beliefs might acknowledge the rationale of our reasoning, but then quickly slide to another argument to tighten their grips on their original position.
Finally, we must be careful to avoid being labeled as the folks whose message is all about what parents should be afraid of. There are plenty of snakes out there in the jungle, but the chimpanzees have realized that when enough of the population is aware of the threat, then it is time to adjust their message. Certainly enough health problems exist on a national level to warrant continued messaging from the large groups in organized medicine. However, it is up to us out in the jungle to learn enough about our patients to know when we should echo those alerts and when it’s time to save our breath. We can’t hoot about every snake in the grass.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Most accomplished public speakers will tell you that critical to their success is the ability to understand and adapt to their audiences. It turns out that even chimpanzees accept and use this cornerstone of effective communication.
In a study published in Science Advances (2017 Nov 15;3[11]:e1701742), three scientists working in Uganda reported that chimpanzees who encounter a potential threat in the form of a realistic snake model will vocalize significantly fewer alert hoots if they hear other alert calls coming from the jungle in the vicinity. In other words, the chimp is saying to himself, “Why should I bother wasting my time and lung power hooting to warn my troop mates? Those guys already know about the snake.”
To select which anticipatory guidance topics to include and still be effective communicators, we have to know the families we are trying to help. Gaining this more nuanced picture of a family takes time and is fostered by continuity. Seeing a different provider at each visit doesn’t work very well here. What are this unique family’s concerns, regardless of what some committee thinks we should be asking?
How much can we rely on the media and groups such as the American Academy of Pediatrics and the Centers for Disease Control and Prevention to get out the messages that we have decided to skip over to address this family’s special concerns? Is the message about the benefits of breastfeeding so widely known that we will be wasting our limited office time repeating it? Is the same true for gun safety and seat belts? This is where research can help us decide where to target messages on a national level. But large population studies don’t always apply to our communities and the families we serve.
Where does our role as primary care physicians fit into the bigger picture of health education? The warning messages issued on a national level may have little relevance for our individual patients’ concerns. Is it our role to echo the message, or are we the ones who must do the fine-tuning?
And then there are the recent depressing and counterintuitive findings that for hot-button topics like immunization, education has little if any value. Those families with firmly held beliefs might acknowledge the rationale of our reasoning, but then quickly slide to another argument to tighten their grips on their original position.
Finally, we must be careful to avoid being labeled as the folks whose message is all about what parents should be afraid of. There are plenty of snakes out there in the jungle, but the chimpanzees have realized that when enough of the population is aware of the threat, then it is time to adjust their message. Certainly enough health problems exist on a national level to warrant continued messaging from the large groups in organized medicine. However, it is up to us out in the jungle to learn enough about our patients to know when we should echo those alerts and when it’s time to save our breath. We can’t hoot about every snake in the grass.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
Most accomplished public speakers will tell you that critical to their success is the ability to understand and adapt to their audiences. It turns out that even chimpanzees accept and use this cornerstone of effective communication.
In a study published in Science Advances (2017 Nov 15;3[11]:e1701742), three scientists working in Uganda reported that chimpanzees who encounter a potential threat in the form of a realistic snake model will vocalize significantly fewer alert hoots if they hear other alert calls coming from the jungle in the vicinity. In other words, the chimp is saying to himself, “Why should I bother wasting my time and lung power hooting to warn my troop mates? Those guys already know about the snake.”
To select which anticipatory guidance topics to include and still be effective communicators, we have to know the families we are trying to help. Gaining this more nuanced picture of a family takes time and is fostered by continuity. Seeing a different provider at each visit doesn’t work very well here. What are this unique family’s concerns, regardless of what some committee thinks we should be asking?
How much can we rely on the media and groups such as the American Academy of Pediatrics and the Centers for Disease Control and Prevention to get out the messages that we have decided to skip over to address this family’s special concerns? Is the message about the benefits of breastfeeding so widely known that we will be wasting our limited office time repeating it? Is the same true for gun safety and seat belts? This is where research can help us decide where to target messages on a national level. But large population studies don’t always apply to our communities and the families we serve.
Where does our role as primary care physicians fit into the bigger picture of health education? The warning messages issued on a national level may have little relevance for our individual patients’ concerns. Is it our role to echo the message, or are we the ones who must do the fine-tuning?
And then there are the recent depressing and counterintuitive findings that for hot-button topics like immunization, education has little if any value. Those families with firmly held beliefs might acknowledge the rationale of our reasoning, but then quickly slide to another argument to tighten their grips on their original position.
Finally, we must be careful to avoid being labeled as the folks whose message is all about what parents should be afraid of. There are plenty of snakes out there in the jungle, but the chimpanzees have realized that when enough of the population is aware of the threat, then it is time to adjust their message. Certainly enough health problems exist on a national level to warrant continued messaging from the large groups in organized medicine. However, it is up to us out in the jungle to learn enough about our patients to know when we should echo those alerts and when it’s time to save our breath. We can’t hoot about every snake in the grass.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.”
U.S. autism rates edge up from 2014-2016
The prevalence of autism spectrum disorder (ASD) in U.S. children and adolescents climbed to 2.41% from 2014 to 2016, Guifeng Xu, MD, and her associates, wrote in a research letter. However, the increased prevalence of ASD over the 3-year period was not statistically significant.
Males were far more likely to be diagnosed with ASD than females, with respective prevalence rates of 3.54% and 1.22%, from 2014 to 2016.
using data from the Autism and Developmental Disabilities Monitoring Network. The ADDM reported a rate of 1.46%, compared with the NHIS rate of 2.41%. Differences in study design and participant characteristics are likely the cause of this difference; the ADDM is conducted at specific sites, while the NHIS is considered to be a nationally representative sample.
“Changes in nonetiologic factors (such as diagnostic criteria, public awareness, and referral), as well as in etiologic factors (including genetic and environmental risk factors), have been postulated to account for the previously observed increase in ASD prevalence,” concluded Dr. Xu, of the department of epidemiology at the University of Iowa, Iowa City, and her associates. “Continued monitoring of the prevalence and investigation of changes in risk factors are warranted.”
Find the full research letter in JAMA (2018;319[1]:81-2. doi: 10.1001/jama.2017.17812).
The prevalence of autism spectrum disorder (ASD) in U.S. children and adolescents climbed to 2.41% from 2014 to 2016, Guifeng Xu, MD, and her associates, wrote in a research letter. However, the increased prevalence of ASD over the 3-year period was not statistically significant.
Males were far more likely to be diagnosed with ASD than females, with respective prevalence rates of 3.54% and 1.22%, from 2014 to 2016.
using data from the Autism and Developmental Disabilities Monitoring Network. The ADDM reported a rate of 1.46%, compared with the NHIS rate of 2.41%. Differences in study design and participant characteristics are likely the cause of this difference; the ADDM is conducted at specific sites, while the NHIS is considered to be a nationally representative sample.
“Changes in nonetiologic factors (such as diagnostic criteria, public awareness, and referral), as well as in etiologic factors (including genetic and environmental risk factors), have been postulated to account for the previously observed increase in ASD prevalence,” concluded Dr. Xu, of the department of epidemiology at the University of Iowa, Iowa City, and her associates. “Continued monitoring of the prevalence and investigation of changes in risk factors are warranted.”
Find the full research letter in JAMA (2018;319[1]:81-2. doi: 10.1001/jama.2017.17812).
The prevalence of autism spectrum disorder (ASD) in U.S. children and adolescents climbed to 2.41% from 2014 to 2016, Guifeng Xu, MD, and her associates, wrote in a research letter. However, the increased prevalence of ASD over the 3-year period was not statistically significant.
Males were far more likely to be diagnosed with ASD than females, with respective prevalence rates of 3.54% and 1.22%, from 2014 to 2016.
using data from the Autism and Developmental Disabilities Monitoring Network. The ADDM reported a rate of 1.46%, compared with the NHIS rate of 2.41%. Differences in study design and participant characteristics are likely the cause of this difference; the ADDM is conducted at specific sites, while the NHIS is considered to be a nationally representative sample.
“Changes in nonetiologic factors (such as diagnostic criteria, public awareness, and referral), as well as in etiologic factors (including genetic and environmental risk factors), have been postulated to account for the previously observed increase in ASD prevalence,” concluded Dr. Xu, of the department of epidemiology at the University of Iowa, Iowa City, and her associates. “Continued monitoring of the prevalence and investigation of changes in risk factors are warranted.”
Find the full research letter in JAMA (2018;319[1]:81-2. doi: 10.1001/jama.2017.17812).
FROM JAMA
Brachial plexus injury: permanent disability
Brachial plexus injury: permanent disability
After concerning test results, a woman went to the hospital for induction of labor. During vaginal delivery, a shoulder dystocia was encountered. The baby was born within 60 seconds using the McRoberts maneuver and suprapubic pressure. The ObGyn charted mild shoulder dystocia.
The child has decreased mobility of his left arm. MRI studies and surgical findings confirmed brachial plexus rupture and avulsion at C5-C7. Despite nerve grafting, the child has a significant disability to his left arm and shoulder.
PARENT'S CLAIM: The ObGyn negligently applied excessive lateral traction, improperly used lateral traction as a maneuver, and instructed the mother to continuously push.
PHYSICIAN'S DEFENSE: Shoulder dystocia was properly diagnosed and resolved using standard maneuvers. Traction and pushing are needed during shoulder dystocia management to determine whether the maneuvers are successful. Brachial plexus injuries can occur because of the normal forces of labor and delivery.
VERDICT: An Illinois defense verdict was returned.
Mother claims PTSD after twin's stillbirth
Expecting twins, a 23-year-old woman at 33.5 weeks' gestation reported pain. The ObGyn noted that her cervix was 4-cm dilated, 1 twin was in breech position, and that labor had begun. He recommended that the patient go to the hospital for cesarean delivery but told her that she could go home, shower, and gather her belongings first. When the mother arrived at the hospital 2.5 hours later, the fetal heart-rate (FHR) monitor indicated that one twin's heart was not active. An emergency cesarean delivery was performed. One twin was safely born, but the other died.
PARENT'S CLAIM: The ObGyn failed to properly address the onset of labor. The twin died because of compression of the umbilical cord. If the mother had gone directly to the hospital, FHR abnormalities would have been apparent and timely intervention could have been taken.
The stillbirth caused the onset of severe emotional distress in the mother leading to posttraumatic stress disorder (PTSD). She had extensive counseling. Her psychologist reported that the patient also suffered from complex grief disorder.
PHYSICIAN'S DEFENSE: The ObGyn's actions did not cause the injury. The twins' hearts were monitored at the last prenatal examination and were normal. It was appropriate for the ObGyn to allow the patient to return home before going to the hospital; the situation was urgent but not emergent. The stillbirth resulted from chorioamnionitis, a microscopic condition that is difficult to detect. A pathologist confirmed the diagnosis after examining the placenta.
The extent of the patient's grief was contested. An expert psychiatrist reported that complex grief disorder is not a recognized medical condition, and that, upon his examination, the patient did not exhibit PTSD symptoms.
VERDICT: A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Brachial plexus injury: permanent disability
After concerning test results, a woman went to the hospital for induction of labor. During vaginal delivery, a shoulder dystocia was encountered. The baby was born within 60 seconds using the McRoberts maneuver and suprapubic pressure. The ObGyn charted mild shoulder dystocia.
The child has decreased mobility of his left arm. MRI studies and surgical findings confirmed brachial plexus rupture and avulsion at C5-C7. Despite nerve grafting, the child has a significant disability to his left arm and shoulder.
PARENT'S CLAIM: The ObGyn negligently applied excessive lateral traction, improperly used lateral traction as a maneuver, and instructed the mother to continuously push.
PHYSICIAN'S DEFENSE: Shoulder dystocia was properly diagnosed and resolved using standard maneuvers. Traction and pushing are needed during shoulder dystocia management to determine whether the maneuvers are successful. Brachial plexus injuries can occur because of the normal forces of labor and delivery.
VERDICT: An Illinois defense verdict was returned.
Mother claims PTSD after twin's stillbirth
Expecting twins, a 23-year-old woman at 33.5 weeks' gestation reported pain. The ObGyn noted that her cervix was 4-cm dilated, 1 twin was in breech position, and that labor had begun. He recommended that the patient go to the hospital for cesarean delivery but told her that she could go home, shower, and gather her belongings first. When the mother arrived at the hospital 2.5 hours later, the fetal heart-rate (FHR) monitor indicated that one twin's heart was not active. An emergency cesarean delivery was performed. One twin was safely born, but the other died.
PARENT'S CLAIM: The ObGyn failed to properly address the onset of labor. The twin died because of compression of the umbilical cord. If the mother had gone directly to the hospital, FHR abnormalities would have been apparent and timely intervention could have been taken.
The stillbirth caused the onset of severe emotional distress in the mother leading to posttraumatic stress disorder (PTSD). She had extensive counseling. Her psychologist reported that the patient also suffered from complex grief disorder.
PHYSICIAN'S DEFENSE: The ObGyn's actions did not cause the injury. The twins' hearts were monitored at the last prenatal examination and were normal. It was appropriate for the ObGyn to allow the patient to return home before going to the hospital; the situation was urgent but not emergent. The stillbirth resulted from chorioamnionitis, a microscopic condition that is difficult to detect. A pathologist confirmed the diagnosis after examining the placenta.
The extent of the patient's grief was contested. An expert psychiatrist reported that complex grief disorder is not a recognized medical condition, and that, upon his examination, the patient did not exhibit PTSD symptoms.
VERDICT: A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Brachial plexus injury: permanent disability
After concerning test results, a woman went to the hospital for induction of labor. During vaginal delivery, a shoulder dystocia was encountered. The baby was born within 60 seconds using the McRoberts maneuver and suprapubic pressure. The ObGyn charted mild shoulder dystocia.
The child has decreased mobility of his left arm. MRI studies and surgical findings confirmed brachial plexus rupture and avulsion at C5-C7. Despite nerve grafting, the child has a significant disability to his left arm and shoulder.
PARENT'S CLAIM: The ObGyn negligently applied excessive lateral traction, improperly used lateral traction as a maneuver, and instructed the mother to continuously push.
PHYSICIAN'S DEFENSE: Shoulder dystocia was properly diagnosed and resolved using standard maneuvers. Traction and pushing are needed during shoulder dystocia management to determine whether the maneuvers are successful. Brachial plexus injuries can occur because of the normal forces of labor and delivery.
VERDICT: An Illinois defense verdict was returned.
Mother claims PTSD after twin's stillbirth
Expecting twins, a 23-year-old woman at 33.5 weeks' gestation reported pain. The ObGyn noted that her cervix was 4-cm dilated, 1 twin was in breech position, and that labor had begun. He recommended that the patient go to the hospital for cesarean delivery but told her that she could go home, shower, and gather her belongings first. When the mother arrived at the hospital 2.5 hours later, the fetal heart-rate (FHR) monitor indicated that one twin's heart was not active. An emergency cesarean delivery was performed. One twin was safely born, but the other died.
PARENT'S CLAIM: The ObGyn failed to properly address the onset of labor. The twin died because of compression of the umbilical cord. If the mother had gone directly to the hospital, FHR abnormalities would have been apparent and timely intervention could have been taken.
The stillbirth caused the onset of severe emotional distress in the mother leading to posttraumatic stress disorder (PTSD). She had extensive counseling. Her psychologist reported that the patient also suffered from complex grief disorder.
PHYSICIAN'S DEFENSE: The ObGyn's actions did not cause the injury. The twins' hearts were monitored at the last prenatal examination and were normal. It was appropriate for the ObGyn to allow the patient to return home before going to the hospital; the situation was urgent but not emergent. The stillbirth resulted from chorioamnionitis, a microscopic condition that is difficult to detect. A pathologist confirmed the diagnosis after examining the placenta.
The extent of the patient's grief was contested. An expert psychiatrist reported that complex grief disorder is not a recognized medical condition, and that, upon his examination, the patient did not exhibit PTSD symptoms.
VERDICT: A New York defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Unnecessary laparotomy: $625,000 award
Unnecessary laparotomy: $625,000 award
A woman in her 20s reported cramping and rectal bleeding to her ObGyn. Pelvic and rectal examinations were normal. Her family physician's exam and a gastroenterologist's rectal exam and colonoscopy were all normal. A radiologist (Dr. A) identified a 3-cm by 6-cm mass on transvaginal ultrasonography. A computed tomography (CT) scan read by another radiologist (Dr. B) confirmed the mass. After receiving the radiologists' reports, the ObGyn told the patient that she had a small tumor that needed immediate removal. No mass was found during exploratory laparotomy.
Three years postsurgery, after trying to conceive, the patient underwent exploratory laparoscopy to evaluate her fallopian tubes. A surgeon found significant pelvic adhesions occluding the left fallopian tube. He lysed the adhesions and resected the left fallopian tube.
PATIENT'S CLAIM: The patient sued the ObGyn and both radiologists, alleging that the unnecessary surgeries resulted in reduced fertility.
Postoperatively, the ObGyn told the patient that the surgery, performed for "nothing," was the radiologists' fault, and that she would have no trouble conceiving. He later blamed her fallopian tube damage on a diagnosis of chlamydia that was successfully treated years earlier with no evidence of reinfection.
The ObGyn disregarded Dr. A's recommendation for a CT scan with rectal contrast; instead he ordered oral contrast. The ObGyn also ignored Dr. B's recommendation for magnetic resonance imaging (MRI).
The mass misidentified by the radiologists was described in 2 different places on the anterior wall of the bowel, both outside the purview of a gynecologist. Given the uncertain diagnosis, referral to a general surgeon was mandated; exploratory laparotomy was not indicated. The ObGyn never referred the patient to a general surgeon for evaluation or sent records or films to the surgeon whom he claimed to have consulted before surgery. The general surgeon denied that any such discussion occurred. The surgeon's first contact with the patient occurred when he was called into the operating room because the ObGyn could not find a mass; the patient was under anesthesia and her abdomen was open.
DEFENDANTS' DEFENSE: The ObGyn claimed that he had developed a plan with the general surgeon before surgery: if the mass was a uterine fibroid, he would remove it, but if the mass was mesenteric, the surgeon would operate.
The ObGyn was justified in performing surgery based on the patient's complaints and the radiologists' findings.
The radiologists contended that, since neither of them expressed certainty, both requested further studies, and neither suggested surgery, their treatment was consistent with the standard of care.
VERDICT: A $625,000 Pennsylvania verdict was returned, finding the ObGyn 100% liable.
Both ureters injured during TAH
A 49-year-old woman underwent total abdominal hysterectomy (TAH) for removal of a uterine fibroid performed by her gynecologist and a surgical assistant. The patient had limited urine output immediately after surgery, no urinary output overnight, and abdominal pain. The gynecologist ordered a urology consultation. A CT scan showed bilateral ureteral obstruction; an interventional radiology study confirmed a blockage due to severance of both ureters. A nephrostomy was performed and, 6 weeks later, the ureters were reimplanted.
PATIENT'S CLAIM: The severing of both ureters was a negligent surgical error. While the risk of injuring a single ureter is a recognized complication of TAH, it is unacceptable that both ureters were severed.
DEFENDANTS' DEFENSE: Standard of care was met: bilateral ureteral injury is a known risk of TAH. Before surgery, the patient was fully informed of the risks and signed a consent agreement. There was no intraoperative evidence that the ureters had been damaged. The injuries were detected as soon as medically possible and timely and successfully treated.
VERDICT: An Illinois defense verdict was returned.
Failure to detect breast cancer: $21.9M verdict against radiologist
A woman went to a diagnostic imaging service for ultrasonography (US) after an earlier US was suspicious for a breast mass. She had a history of left breast pain and swelling that had been treated with antibiotics. The radiologist interpreted the second ultrasound as showing no masses; he noted skin thickening and a lymph node abnormality.
Nine months after initial US, the patient had a breast biopsy performed in another state. She was diagnosed with stage 3 breast cancer.
PATIENT'S CLAIM: The radiologist failed to properly interpret the findings of the second ultrasound.
PHYSICIAN'S DEFENSE: The radiologist contended that he was not liable because the technologist failed to place the transducer over the breast lump. The first US films were not provided for comparison.
VERDICT: A $21.9 million Florida verdict was returned.
Vesicovaginal fistula after hysterectomy
A 39-year-old woman with a history of 4 cesarean deliveries and an enlarged fibroid uterus underwent TAH. She subsequently developed urinary incontinence.
PATIENT'S CLAIM: The ObGyn used an inappropriate dissection technique to remove the uterus, causing a bladder injury. He also sutured the vaginal cuff to the bladder, causing the formation of a vesicovaginal fistula. Repair surgeries were unsuccessful and the patient now is permanently incontinent.
PHYSICIAN'S DEFENSE: The standard of care was met. The patient had a pre-existing bladder weakness due to the size of her uterus and prior surgeries. The bladder injury is a known complication of the surgery. The vaginal cuff adhered to the bladder due to postsurgical scarring or fibrosis.
VERDICT: A Michigan defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Unnecessary laparotomy: $625,000 award
A woman in her 20s reported cramping and rectal bleeding to her ObGyn. Pelvic and rectal examinations were normal. Her family physician's exam and a gastroenterologist's rectal exam and colonoscopy were all normal. A radiologist (Dr. A) identified a 3-cm by 6-cm mass on transvaginal ultrasonography. A computed tomography (CT) scan read by another radiologist (Dr. B) confirmed the mass. After receiving the radiologists' reports, the ObGyn told the patient that she had a small tumor that needed immediate removal. No mass was found during exploratory laparotomy.
Three years postsurgery, after trying to conceive, the patient underwent exploratory laparoscopy to evaluate her fallopian tubes. A surgeon found significant pelvic adhesions occluding the left fallopian tube. He lysed the adhesions and resected the left fallopian tube.
PATIENT'S CLAIM: The patient sued the ObGyn and both radiologists, alleging that the unnecessary surgeries resulted in reduced fertility.
Postoperatively, the ObGyn told the patient that the surgery, performed for "nothing," was the radiologists' fault, and that she would have no trouble conceiving. He later blamed her fallopian tube damage on a diagnosis of chlamydia that was successfully treated years earlier with no evidence of reinfection.
The ObGyn disregarded Dr. A's recommendation for a CT scan with rectal contrast; instead he ordered oral contrast. The ObGyn also ignored Dr. B's recommendation for magnetic resonance imaging (MRI).
The mass misidentified by the radiologists was described in 2 different places on the anterior wall of the bowel, both outside the purview of a gynecologist. Given the uncertain diagnosis, referral to a general surgeon was mandated; exploratory laparotomy was not indicated. The ObGyn never referred the patient to a general surgeon for evaluation or sent records or films to the surgeon whom he claimed to have consulted before surgery. The general surgeon denied that any such discussion occurred. The surgeon's first contact with the patient occurred when he was called into the operating room because the ObGyn could not find a mass; the patient was under anesthesia and her abdomen was open.
DEFENDANTS' DEFENSE: The ObGyn claimed that he had developed a plan with the general surgeon before surgery: if the mass was a uterine fibroid, he would remove it, but if the mass was mesenteric, the surgeon would operate.
The ObGyn was justified in performing surgery based on the patient's complaints and the radiologists' findings.
The radiologists contended that, since neither of them expressed certainty, both requested further studies, and neither suggested surgery, their treatment was consistent with the standard of care.
VERDICT: A $625,000 Pennsylvania verdict was returned, finding the ObGyn 100% liable.
Both ureters injured during TAH
A 49-year-old woman underwent total abdominal hysterectomy (TAH) for removal of a uterine fibroid performed by her gynecologist and a surgical assistant. The patient had limited urine output immediately after surgery, no urinary output overnight, and abdominal pain. The gynecologist ordered a urology consultation. A CT scan showed bilateral ureteral obstruction; an interventional radiology study confirmed a blockage due to severance of both ureters. A nephrostomy was performed and, 6 weeks later, the ureters were reimplanted.
PATIENT'S CLAIM: The severing of both ureters was a negligent surgical error. While the risk of injuring a single ureter is a recognized complication of TAH, it is unacceptable that both ureters were severed.
DEFENDANTS' DEFENSE: Standard of care was met: bilateral ureteral injury is a known risk of TAH. Before surgery, the patient was fully informed of the risks and signed a consent agreement. There was no intraoperative evidence that the ureters had been damaged. The injuries were detected as soon as medically possible and timely and successfully treated.
VERDICT: An Illinois defense verdict was returned.
Failure to detect breast cancer: $21.9M verdict against radiologist
A woman went to a diagnostic imaging service for ultrasonography (US) after an earlier US was suspicious for a breast mass. She had a history of left breast pain and swelling that had been treated with antibiotics. The radiologist interpreted the second ultrasound as showing no masses; he noted skin thickening and a lymph node abnormality.
Nine months after initial US, the patient had a breast biopsy performed in another state. She was diagnosed with stage 3 breast cancer.
PATIENT'S CLAIM: The radiologist failed to properly interpret the findings of the second ultrasound.
PHYSICIAN'S DEFENSE: The radiologist contended that he was not liable because the technologist failed to place the transducer over the breast lump. The first US films were not provided for comparison.
VERDICT: A $21.9 million Florida verdict was returned.
Vesicovaginal fistula after hysterectomy
A 39-year-old woman with a history of 4 cesarean deliveries and an enlarged fibroid uterus underwent TAH. She subsequently developed urinary incontinence.
PATIENT'S CLAIM: The ObGyn used an inappropriate dissection technique to remove the uterus, causing a bladder injury. He also sutured the vaginal cuff to the bladder, causing the formation of a vesicovaginal fistula. Repair surgeries were unsuccessful and the patient now is permanently incontinent.
PHYSICIAN'S DEFENSE: The standard of care was met. The patient had a pre-existing bladder weakness due to the size of her uterus and prior surgeries. The bladder injury is a known complication of the surgery. The vaginal cuff adhered to the bladder due to postsurgical scarring or fibrosis.
VERDICT: A Michigan defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Unnecessary laparotomy: $625,000 award
A woman in her 20s reported cramping and rectal bleeding to her ObGyn. Pelvic and rectal examinations were normal. Her family physician's exam and a gastroenterologist's rectal exam and colonoscopy were all normal. A radiologist (Dr. A) identified a 3-cm by 6-cm mass on transvaginal ultrasonography. A computed tomography (CT) scan read by another radiologist (Dr. B) confirmed the mass. After receiving the radiologists' reports, the ObGyn told the patient that she had a small tumor that needed immediate removal. No mass was found during exploratory laparotomy.
Three years postsurgery, after trying to conceive, the patient underwent exploratory laparoscopy to evaluate her fallopian tubes. A surgeon found significant pelvic adhesions occluding the left fallopian tube. He lysed the adhesions and resected the left fallopian tube.
PATIENT'S CLAIM: The patient sued the ObGyn and both radiologists, alleging that the unnecessary surgeries resulted in reduced fertility.
Postoperatively, the ObGyn told the patient that the surgery, performed for "nothing," was the radiologists' fault, and that she would have no trouble conceiving. He later blamed her fallopian tube damage on a diagnosis of chlamydia that was successfully treated years earlier with no evidence of reinfection.
The ObGyn disregarded Dr. A's recommendation for a CT scan with rectal contrast; instead he ordered oral contrast. The ObGyn also ignored Dr. B's recommendation for magnetic resonance imaging (MRI).
The mass misidentified by the radiologists was described in 2 different places on the anterior wall of the bowel, both outside the purview of a gynecologist. Given the uncertain diagnosis, referral to a general surgeon was mandated; exploratory laparotomy was not indicated. The ObGyn never referred the patient to a general surgeon for evaluation or sent records or films to the surgeon whom he claimed to have consulted before surgery. The general surgeon denied that any such discussion occurred. The surgeon's first contact with the patient occurred when he was called into the operating room because the ObGyn could not find a mass; the patient was under anesthesia and her abdomen was open.
DEFENDANTS' DEFENSE: The ObGyn claimed that he had developed a plan with the general surgeon before surgery: if the mass was a uterine fibroid, he would remove it, but if the mass was mesenteric, the surgeon would operate.
The ObGyn was justified in performing surgery based on the patient's complaints and the radiologists' findings.
The radiologists contended that, since neither of them expressed certainty, both requested further studies, and neither suggested surgery, their treatment was consistent with the standard of care.
VERDICT: A $625,000 Pennsylvania verdict was returned, finding the ObGyn 100% liable.
Both ureters injured during TAH
A 49-year-old woman underwent total abdominal hysterectomy (TAH) for removal of a uterine fibroid performed by her gynecologist and a surgical assistant. The patient had limited urine output immediately after surgery, no urinary output overnight, and abdominal pain. The gynecologist ordered a urology consultation. A CT scan showed bilateral ureteral obstruction; an interventional radiology study confirmed a blockage due to severance of both ureters. A nephrostomy was performed and, 6 weeks later, the ureters were reimplanted.
PATIENT'S CLAIM: The severing of both ureters was a negligent surgical error. While the risk of injuring a single ureter is a recognized complication of TAH, it is unacceptable that both ureters were severed.
DEFENDANTS' DEFENSE: Standard of care was met: bilateral ureteral injury is a known risk of TAH. Before surgery, the patient was fully informed of the risks and signed a consent agreement. There was no intraoperative evidence that the ureters had been damaged. The injuries were detected as soon as medically possible and timely and successfully treated.
VERDICT: An Illinois defense verdict was returned.
Failure to detect breast cancer: $21.9M verdict against radiologist
A woman went to a diagnostic imaging service for ultrasonography (US) after an earlier US was suspicious for a breast mass. She had a history of left breast pain and swelling that had been treated with antibiotics. The radiologist interpreted the second ultrasound as showing no masses; he noted skin thickening and a lymph node abnormality.
Nine months after initial US, the patient had a breast biopsy performed in another state. She was diagnosed with stage 3 breast cancer.
PATIENT'S CLAIM: The radiologist failed to properly interpret the findings of the second ultrasound.
PHYSICIAN'S DEFENSE: The radiologist contended that he was not liable because the technologist failed to place the transducer over the breast lump. The first US films were not provided for comparison.
VERDICT: A $21.9 million Florida verdict was returned.
Vesicovaginal fistula after hysterectomy
A 39-year-old woman with a history of 4 cesarean deliveries and an enlarged fibroid uterus underwent TAH. She subsequently developed urinary incontinence.
PATIENT'S CLAIM: The ObGyn used an inappropriate dissection technique to remove the uterus, causing a bladder injury. He also sutured the vaginal cuff to the bladder, causing the formation of a vesicovaginal fistula. Repair surgeries were unsuccessful and the patient now is permanently incontinent.
PHYSICIAN'S DEFENSE: The standard of care was met. The patient had a pre-existing bladder weakness due to the size of her uterus and prior surgeries. The bladder injury is a known complication of the surgery. The vaginal cuff adhered to the bladder due to postsurgical scarring or fibrosis.
VERDICT: A Michigan defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Thrombectomy at Six to 24 Hours After Stroke May Improve Outcomes
Compared with standard care alone, standard care plus endovascular thrombectomy at six to 24 hours after stroke onset appears to reduce disability and increase functional independence among patients with a mismatch between clinical deficit and infarct. These findings, which were published online ahead of print November 11 in the New England Journal of Medicine, could benefit patients with stroke who arrive at the hospital after the current six-hour treatment window has closed, said the authors.
“When the irreversibly damaged brain area affected by the stroke is small, we see that clot removal can make a significant positive difference, even if performed outside the six-hour window,” said Tudor Jovin, MD, Director of the University of Pittsburgh Medical Center Stroke Institute. “However, this does not diminish the urgency with which patients must be rushed to the emergency room in the event of a stroke. The mantra ‘time is brain’ still holds true.”
The DAWN Trial Examined Late Thrombectomy
Previous research has indicated that thrombectomy provides clinical benefits for patients with acute ischemic stroke when it is performed within six hours of symptom onset. The benefit of treatment appeared to decrease as the time to treatment increased. Nonrandomized studies, however, have shown that reperfusion of occluded proximal anterior cerebral vessels improves outcomes in patients with a mismatch between the volume of brain tissue that may be salvaged and the volume of infarcted tissue, even if performed more than six hours after the patient was last known to be well.
Dr. Jovin and colleagues conducted the DAWN trial, a multicenter, prospective, randomized, open-label study, to evaluate the effects of late thrombectomy. Eligible patients had an occlusion of the intracranial internal carotid artery or proximal middle cerebral artery and had last been known to be well six to 24 hours earlier. Patients either did not meet criteria for treatment with IV alteplase because of late presentation, or had persistent vessel occlusion despite treatment with IV alteplase.
Participants also had a mismatch between the severity of the clinical deficit and the infarct volume, which was assessed using diffusion-weighted MRI or perfusion CT. The investigators sorted mismatches into three groups. Group A included patients age 80 or older with an NIH Stroke Scale (NIHSS) score of 10 or higher and an infarct volume of less than 21 mL. Group B included patients younger than 80 with an NIHSS score of 10 or higher and an infarct volume of less than 31 mL. Group C included patients younger than 80 with an NIHSS score of 20 or higher and had an infarct volume between 31 mL and 51 mL.
The investigators randomized participants to thrombectomy plus standard care or standard care alone. The first primary end point was the mean score for disability on the utility-weighted modified Rankin scale, which ranges from 0 (ie, death) to 10 (ie, no symptoms or disability). The second was the rate of functional independence (ie, a score of 0–2 on the modified Rankin scale) at 90 days. The main safety end point was stroke-related death at 90 days.
Enrollment Was Halted Early
Dr. Jovin and colleagues enrolled 206 patients in the trial. Enrollment was stopped at 31 months, because an interim analysis found that thrombectomy plus standard care was 95% likely to be superior to standard care alone for the first primary end point. In all, 107 patients received thrombectomy plus standard care, and 99 received standard care alone.
Participants’ mean age was 70, and approximately 46% of participants were men. Median NIHSS score was 17, and median infarct volume was 8.25 mL. The treatment arms were generally balanced, except for three factors. History of atrial fibrillation and wake-up stroke were more common in the thrombectomy group, and treatment with IV alteplase was more common in the control group.
The mean utility-weighted modified Rankin scale score at 90 days was 5.5 in the thrombectomy group and 3.4 in the control group. The rate of functional independence at 90 days was 49% in the thrombectomy group and 13% in the control group. The superiority of thrombectomy plus standard treatment to standard treatment alone for both end points remained significant in post hoc sensitivity analyses that adjusted for between-group differences in baseline characteristics.
The rate of stroke-related death at 90 days did not differ significantly between the treatment groups. Nor did the rate of death from any cause at 90 days or the rate of symptomatic intracerebral hemorrhage differ significantly between groups. The rate of neurologic deterioration was lower in the thrombectomy group than in the control group.
Should the Time Window Be Expanded?
By selecting patients who had a region of brain that was poorly perfused, but not yet infarcted, Dr. Jovin and colleagues replaced the conventional six-hour time window for stroke treatment with a “tissue window,” said Werner Hacke, MD, PhD, Chair of the Department of Neurology at the University of Heidelberg in Germany, in an accompanying editorial. The rate of functional independence at 90 days in the DAWN trial (49%) was similar to that in a previous meta-analysis of several trials of mechanical thrombectomy (46%). “These similar findings suggest that the use of a ‘tissue window’ in choosing patients for thrombectomy is as good as the use of a time window,” said Dr. Hacke. “However, it is also worth emphasizing that the 13% rate of functional independence in the control group in the DAWN trial was lower than the 26% rate in the control group in the pooled analysis.” This low rate of functional independence “is probably the best we can expect” for patients who have not had recanalization by 24 hours after stroke onset.
The DAWN trial provides reason to expect that trials investigating late IV thrombolysis that require the presence of ischemic tissue might have positive outcomes. But “the results of the DAWN trial do not support a general liberalization of the time window for thrombectomy or thrombolysis,” Dr. Hacke continued. “Reducing the time from the onset of stroke to treatment remains essential and results in the best outcomes.”
—Erik Greb
Suggested Reading
Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2017 Nov 11 [Epub ahead of print].
Hacke W. A new DAWN for imaging-based selection in the treatment of acute stroke. N Engl J Med. 2017 Nov 11 [Epub ahead of print].
Compared with standard care alone, standard care plus endovascular thrombectomy at six to 24 hours after stroke onset appears to reduce disability and increase functional independence among patients with a mismatch between clinical deficit and infarct. These findings, which were published online ahead of print November 11 in the New England Journal of Medicine, could benefit patients with stroke who arrive at the hospital after the current six-hour treatment window has closed, said the authors.
“When the irreversibly damaged brain area affected by the stroke is small, we see that clot removal can make a significant positive difference, even if performed outside the six-hour window,” said Tudor Jovin, MD, Director of the University of Pittsburgh Medical Center Stroke Institute. “However, this does not diminish the urgency with which patients must be rushed to the emergency room in the event of a stroke. The mantra ‘time is brain’ still holds true.”
The DAWN Trial Examined Late Thrombectomy
Previous research has indicated that thrombectomy provides clinical benefits for patients with acute ischemic stroke when it is performed within six hours of symptom onset. The benefit of treatment appeared to decrease as the time to treatment increased. Nonrandomized studies, however, have shown that reperfusion of occluded proximal anterior cerebral vessels improves outcomes in patients with a mismatch between the volume of brain tissue that may be salvaged and the volume of infarcted tissue, even if performed more than six hours after the patient was last known to be well.
Dr. Jovin and colleagues conducted the DAWN trial, a multicenter, prospective, randomized, open-label study, to evaluate the effects of late thrombectomy. Eligible patients had an occlusion of the intracranial internal carotid artery or proximal middle cerebral artery and had last been known to be well six to 24 hours earlier. Patients either did not meet criteria for treatment with IV alteplase because of late presentation, or had persistent vessel occlusion despite treatment with IV alteplase.
Participants also had a mismatch between the severity of the clinical deficit and the infarct volume, which was assessed using diffusion-weighted MRI or perfusion CT. The investigators sorted mismatches into three groups. Group A included patients age 80 or older with an NIH Stroke Scale (NIHSS) score of 10 or higher and an infarct volume of less than 21 mL. Group B included patients younger than 80 with an NIHSS score of 10 or higher and an infarct volume of less than 31 mL. Group C included patients younger than 80 with an NIHSS score of 20 or higher and had an infarct volume between 31 mL and 51 mL.
The investigators randomized participants to thrombectomy plus standard care or standard care alone. The first primary end point was the mean score for disability on the utility-weighted modified Rankin scale, which ranges from 0 (ie, death) to 10 (ie, no symptoms or disability). The second was the rate of functional independence (ie, a score of 0–2 on the modified Rankin scale) at 90 days. The main safety end point was stroke-related death at 90 days.
Enrollment Was Halted Early
Dr. Jovin and colleagues enrolled 206 patients in the trial. Enrollment was stopped at 31 months, because an interim analysis found that thrombectomy plus standard care was 95% likely to be superior to standard care alone for the first primary end point. In all, 107 patients received thrombectomy plus standard care, and 99 received standard care alone.
Participants’ mean age was 70, and approximately 46% of participants were men. Median NIHSS score was 17, and median infarct volume was 8.25 mL. The treatment arms were generally balanced, except for three factors. History of atrial fibrillation and wake-up stroke were more common in the thrombectomy group, and treatment with IV alteplase was more common in the control group.
The mean utility-weighted modified Rankin scale score at 90 days was 5.5 in the thrombectomy group and 3.4 in the control group. The rate of functional independence at 90 days was 49% in the thrombectomy group and 13% in the control group. The superiority of thrombectomy plus standard treatment to standard treatment alone for both end points remained significant in post hoc sensitivity analyses that adjusted for between-group differences in baseline characteristics.
The rate of stroke-related death at 90 days did not differ significantly between the treatment groups. Nor did the rate of death from any cause at 90 days or the rate of symptomatic intracerebral hemorrhage differ significantly between groups. The rate of neurologic deterioration was lower in the thrombectomy group than in the control group.
Should the Time Window Be Expanded?
By selecting patients who had a region of brain that was poorly perfused, but not yet infarcted, Dr. Jovin and colleagues replaced the conventional six-hour time window for stroke treatment with a “tissue window,” said Werner Hacke, MD, PhD, Chair of the Department of Neurology at the University of Heidelberg in Germany, in an accompanying editorial. The rate of functional independence at 90 days in the DAWN trial (49%) was similar to that in a previous meta-analysis of several trials of mechanical thrombectomy (46%). “These similar findings suggest that the use of a ‘tissue window’ in choosing patients for thrombectomy is as good as the use of a time window,” said Dr. Hacke. “However, it is also worth emphasizing that the 13% rate of functional independence in the control group in the DAWN trial was lower than the 26% rate in the control group in the pooled analysis.” This low rate of functional independence “is probably the best we can expect” for patients who have not had recanalization by 24 hours after stroke onset.
The DAWN trial provides reason to expect that trials investigating late IV thrombolysis that require the presence of ischemic tissue might have positive outcomes. But “the results of the DAWN trial do not support a general liberalization of the time window for thrombectomy or thrombolysis,” Dr. Hacke continued. “Reducing the time from the onset of stroke to treatment remains essential and results in the best outcomes.”
—Erik Greb
Suggested Reading
Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2017 Nov 11 [Epub ahead of print].
Hacke W. A new DAWN for imaging-based selection in the treatment of acute stroke. N Engl J Med. 2017 Nov 11 [Epub ahead of print].
Compared with standard care alone, standard care plus endovascular thrombectomy at six to 24 hours after stroke onset appears to reduce disability and increase functional independence among patients with a mismatch between clinical deficit and infarct. These findings, which were published online ahead of print November 11 in the New England Journal of Medicine, could benefit patients with stroke who arrive at the hospital after the current six-hour treatment window has closed, said the authors.
“When the irreversibly damaged brain area affected by the stroke is small, we see that clot removal can make a significant positive difference, even if performed outside the six-hour window,” said Tudor Jovin, MD, Director of the University of Pittsburgh Medical Center Stroke Institute. “However, this does not diminish the urgency with which patients must be rushed to the emergency room in the event of a stroke. The mantra ‘time is brain’ still holds true.”
The DAWN Trial Examined Late Thrombectomy
Previous research has indicated that thrombectomy provides clinical benefits for patients with acute ischemic stroke when it is performed within six hours of symptom onset. The benefit of treatment appeared to decrease as the time to treatment increased. Nonrandomized studies, however, have shown that reperfusion of occluded proximal anterior cerebral vessels improves outcomes in patients with a mismatch between the volume of brain tissue that may be salvaged and the volume of infarcted tissue, even if performed more than six hours after the patient was last known to be well.
Dr. Jovin and colleagues conducted the DAWN trial, a multicenter, prospective, randomized, open-label study, to evaluate the effects of late thrombectomy. Eligible patients had an occlusion of the intracranial internal carotid artery or proximal middle cerebral artery and had last been known to be well six to 24 hours earlier. Patients either did not meet criteria for treatment with IV alteplase because of late presentation, or had persistent vessel occlusion despite treatment with IV alteplase.
Participants also had a mismatch between the severity of the clinical deficit and the infarct volume, which was assessed using diffusion-weighted MRI or perfusion CT. The investigators sorted mismatches into three groups. Group A included patients age 80 or older with an NIH Stroke Scale (NIHSS) score of 10 or higher and an infarct volume of less than 21 mL. Group B included patients younger than 80 with an NIHSS score of 10 or higher and an infarct volume of less than 31 mL. Group C included patients younger than 80 with an NIHSS score of 20 or higher and had an infarct volume between 31 mL and 51 mL.
The investigators randomized participants to thrombectomy plus standard care or standard care alone. The first primary end point was the mean score for disability on the utility-weighted modified Rankin scale, which ranges from 0 (ie, death) to 10 (ie, no symptoms or disability). The second was the rate of functional independence (ie, a score of 0–2 on the modified Rankin scale) at 90 days. The main safety end point was stroke-related death at 90 days.
Enrollment Was Halted Early
Dr. Jovin and colleagues enrolled 206 patients in the trial. Enrollment was stopped at 31 months, because an interim analysis found that thrombectomy plus standard care was 95% likely to be superior to standard care alone for the first primary end point. In all, 107 patients received thrombectomy plus standard care, and 99 received standard care alone.
Participants’ mean age was 70, and approximately 46% of participants were men. Median NIHSS score was 17, and median infarct volume was 8.25 mL. The treatment arms were generally balanced, except for three factors. History of atrial fibrillation and wake-up stroke were more common in the thrombectomy group, and treatment with IV alteplase was more common in the control group.
The mean utility-weighted modified Rankin scale score at 90 days was 5.5 in the thrombectomy group and 3.4 in the control group. The rate of functional independence at 90 days was 49% in the thrombectomy group and 13% in the control group. The superiority of thrombectomy plus standard treatment to standard treatment alone for both end points remained significant in post hoc sensitivity analyses that adjusted for between-group differences in baseline characteristics.
The rate of stroke-related death at 90 days did not differ significantly between the treatment groups. Nor did the rate of death from any cause at 90 days or the rate of symptomatic intracerebral hemorrhage differ significantly between groups. The rate of neurologic deterioration was lower in the thrombectomy group than in the control group.
Should the Time Window Be Expanded?
By selecting patients who had a region of brain that was poorly perfused, but not yet infarcted, Dr. Jovin and colleagues replaced the conventional six-hour time window for stroke treatment with a “tissue window,” said Werner Hacke, MD, PhD, Chair of the Department of Neurology at the University of Heidelberg in Germany, in an accompanying editorial. The rate of functional independence at 90 days in the DAWN trial (49%) was similar to that in a previous meta-analysis of several trials of mechanical thrombectomy (46%). “These similar findings suggest that the use of a ‘tissue window’ in choosing patients for thrombectomy is as good as the use of a time window,” said Dr. Hacke. “However, it is also worth emphasizing that the 13% rate of functional independence in the control group in the DAWN trial was lower than the 26% rate in the control group in the pooled analysis.” This low rate of functional independence “is probably the best we can expect” for patients who have not had recanalization by 24 hours after stroke onset.
The DAWN trial provides reason to expect that trials investigating late IV thrombolysis that require the presence of ischemic tissue might have positive outcomes. But “the results of the DAWN trial do not support a general liberalization of the time window for thrombectomy or thrombolysis,” Dr. Hacke continued. “Reducing the time from the onset of stroke to treatment remains essential and results in the best outcomes.”
—Erik Greb
Suggested Reading
Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2017 Nov 11 [Epub ahead of print].
Hacke W. A new DAWN for imaging-based selection in the treatment of acute stroke. N Engl J Med. 2017 Nov 11 [Epub ahead of print].
Product Update: MyoSure MANUAL; Rivanna's Accuro 3D; PeriGen; Instavit
HYSTEROSCOPY TISSUE REMOVAL DEVICE

Hologic, Inc, has introduced the MyoSure® MANUAL Tissue Removal Device for resecting and removing tissue during in-office hysteroscopic intrauterine procedures. When used with the MyoSure hysteroscope, the MyoSure MANUAL device has a fully integrated vacuum that does not require external suction and can be operated using a 1-L saline bag. The clear tissue trap allows for visual co
This new Hologic product joins the MyoSure suite of gynecologic surgical products that includes the MyoSure, MyoSure REACH, MyoSure XL, and MyoSure LITE devices.
FOR MORE INFORMATION, VISIT: http://myosure.com/
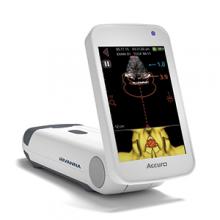
SPINAL NAVIGATION TECHNOLOGY FOR EPIDURAL ANESTHESIA
The Accuro® 3D image-guided spinal navigation technology by Rivanna Medical® is a handheld, lightweight, untethered ultrasound-based system designed to help apply spinal and epidural anesthesia. Ultrasonography is the imaging modality of choice for epidurals in expectant mothers who must avoid the radiation involved in other imaging procedures, according to Rivanna Medical.
In a recent trial, Accuro identified the appropriate epidural injection sites along the lower spine and calculated the depth to the epidural space. Actual epidural depth was confirmed by measuring needle penetration during successful epidural delivery by anesthesia providers. Accuro predicted this depth within an average of 0.61 cm, reports Rivanna Medical. In addition, Accuro identified the appropriate spinal interspace for needle insertion in 94% of patients and enabled 87% success in first-attempt epidural administration.
FOR MORE INFORMATION, VISIT: https://rivannamedical.com/
SOFTWARE AND HUB HELP IDENTIFY CRITICALLY ILL L&D PATIENTS
PeriGen, Inc, a software-solutions company, has launched PeriWatch™ HUB™, new perinatal software and a dashboard for labor and delivery (L&D) units.
PeriGen says that its PeriWatch modules provide state-of-the-art L&D documentation and fetal surveillance coupled with analytics and an electronic critical-condition dashboard for hospital maternity units.
HUB is an intelligent perinatal dashboard designed to facilitate the timely recognition of maternity patients who develop critical illness. Using PeriGen’s proprietary algorithms, it prioritizes patients based on physician-chosen threshold settings for vital signs, labor progress, and fetal heart rate patterns, and consolidates that data into an easy-to-read interactive dashboard.
FOR MORE INFORMATION, VISIT: http://perigen.com/
ORAL SPRAY VITAMINS: ALTERNATIVE TO PILLS

Instavit® Spray Vitamins offer an alternative to patients who have difficulty swallowing pills. Instavit says that its oral vitamins, sprayed directly into the mouth, are sugar-free, tasty, gluten-free, and contain zero calories. The sprays are manufactured to the highest standard in cGMP, FDA approved facilities in the United States. Each Instavit spray provides an exact and measured amount of liquid, allowing for correct dosing and also permitting individualization of intake. A 14-oz spray bottle contains about 28 doses.
The Instavit line includes: “Prenatal Care,” “Vitamin B12,” “Instant Energy,” “Vitamin D,” “Daily Health,” “Sweet Dreams,” “Immune Strength,” “Clearer Thinking,” and “Instavit for Kids.” Instavit products are available in retail stores in North America.
FOR MORE INFORMATION, VISIT: http://www.instavit.com/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
HYSTEROSCOPY TISSUE REMOVAL DEVICE

Hologic, Inc, has introduced the MyoSure® MANUAL Tissue Removal Device for resecting and removing tissue during in-office hysteroscopic intrauterine procedures. When used with the MyoSure hysteroscope, the MyoSure MANUAL device has a fully integrated vacuum that does not require external suction and can be operated using a 1-L saline bag. The clear tissue trap allows for visual co
This new Hologic product joins the MyoSure suite of gynecologic surgical products that includes the MyoSure, MyoSure REACH, MyoSure XL, and MyoSure LITE devices.
FOR MORE INFORMATION, VISIT: http://myosure.com/
SPINAL NAVIGATION TECHNOLOGY FOR EPIDURAL ANESTHESIA
The Accuro® 3D image-guided spinal navigation technology by Rivanna Medical® is a handheld, lightweight, untethered ultrasound-based system designed to help apply spinal and epidural anesthesia. Ultrasonography is the imaging modality of choice for epidurals in expectant mothers who must avoid the radiation involved in other imaging procedures, according to Rivanna Medical.
In a recent trial, Accuro identified the appropriate epidural injection sites along the lower spine and calculated the depth to the epidural space. Actual epidural depth was confirmed by measuring needle penetration during successful epidural delivery by anesthesia providers. Accuro predicted this depth within an average of 0.61 cm, reports Rivanna Medical. In addition, Accuro identified the appropriate spinal interspace for needle insertion in 94% of patients and enabled 87% success in first-attempt epidural administration.
FOR MORE INFORMATION, VISIT: https://rivannamedical.com/
SOFTWARE AND HUB HELP IDENTIFY CRITICALLY ILL L&D PATIENTS
PeriGen, Inc, a software-solutions company, has launched PeriWatch™ HUB™, new perinatal software and a dashboard for labor and delivery (L&D) units.
PeriGen says that its PeriWatch modules provide state-of-the-art L&D documentation and fetal surveillance coupled with analytics and an electronic critical-condition dashboard for hospital maternity units.
HUB is an intelligent perinatal dashboard designed to facilitate the timely recognition of maternity patients who develop critical illness. Using PeriGen’s proprietary algorithms, it prioritizes patients based on physician-chosen threshold settings for vital signs, labor progress, and fetal heart rate patterns, and consolidates that data into an easy-to-read interactive dashboard.
FOR MORE INFORMATION, VISIT: http://perigen.com/
ORAL SPRAY VITAMINS: ALTERNATIVE TO PILLS

Instavit® Spray Vitamins offer an alternative to patients who have difficulty swallowing pills. Instavit says that its oral vitamins, sprayed directly into the mouth, are sugar-free, tasty, gluten-free, and contain zero calories. The sprays are manufactured to the highest standard in cGMP, FDA approved facilities in the United States. Each Instavit spray provides an exact and measured amount of liquid, allowing for correct dosing and also permitting individualization of intake. A 14-oz spray bottle contains about 28 doses.
The Instavit line includes: “Prenatal Care,” “Vitamin B12,” “Instant Energy,” “Vitamin D,” “Daily Health,” “Sweet Dreams,” “Immune Strength,” “Clearer Thinking,” and “Instavit for Kids.” Instavit products are available in retail stores in North America.
FOR MORE INFORMATION, VISIT: http://www.instavit.com/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
HYSTEROSCOPY TISSUE REMOVAL DEVICE

Hologic, Inc, has introduced the MyoSure® MANUAL Tissue Removal Device for resecting and removing tissue during in-office hysteroscopic intrauterine procedures. When used with the MyoSure hysteroscope, the MyoSure MANUAL device has a fully integrated vacuum that does not require external suction and can be operated using a 1-L saline bag. The clear tissue trap allows for visual co
This new Hologic product joins the MyoSure suite of gynecologic surgical products that includes the MyoSure, MyoSure REACH, MyoSure XL, and MyoSure LITE devices.
FOR MORE INFORMATION, VISIT: http://myosure.com/
SPINAL NAVIGATION TECHNOLOGY FOR EPIDURAL ANESTHESIA
The Accuro® 3D image-guided spinal navigation technology by Rivanna Medical® is a handheld, lightweight, untethered ultrasound-based system designed to help apply spinal and epidural anesthesia. Ultrasonography is the imaging modality of choice for epidurals in expectant mothers who must avoid the radiation involved in other imaging procedures, according to Rivanna Medical.
In a recent trial, Accuro identified the appropriate epidural injection sites along the lower spine and calculated the depth to the epidural space. Actual epidural depth was confirmed by measuring needle penetration during successful epidural delivery by anesthesia providers. Accuro predicted this depth within an average of 0.61 cm, reports Rivanna Medical. In addition, Accuro identified the appropriate spinal interspace for needle insertion in 94% of patients and enabled 87% success in first-attempt epidural administration.
FOR MORE INFORMATION, VISIT: https://rivannamedical.com/
SOFTWARE AND HUB HELP IDENTIFY CRITICALLY ILL L&D PATIENTS
PeriGen, Inc, a software-solutions company, has launched PeriWatch™ HUB™, new perinatal software and a dashboard for labor and delivery (L&D) units.
PeriGen says that its PeriWatch modules provide state-of-the-art L&D documentation and fetal surveillance coupled with analytics and an electronic critical-condition dashboard for hospital maternity units.
HUB is an intelligent perinatal dashboard designed to facilitate the timely recognition of maternity patients who develop critical illness. Using PeriGen’s proprietary algorithms, it prioritizes patients based on physician-chosen threshold settings for vital signs, labor progress, and fetal heart rate patterns, and consolidates that data into an easy-to-read interactive dashboard.
FOR MORE INFORMATION, VISIT: http://perigen.com/
ORAL SPRAY VITAMINS: ALTERNATIVE TO PILLS

Instavit® Spray Vitamins offer an alternative to patients who have difficulty swallowing pills. Instavit says that its oral vitamins, sprayed directly into the mouth, are sugar-free, tasty, gluten-free, and contain zero calories. The sprays are manufactured to the highest standard in cGMP, FDA approved facilities in the United States. Each Instavit spray provides an exact and measured amount of liquid, allowing for correct dosing and also permitting individualization of intake. A 14-oz spray bottle contains about 28 doses.
The Instavit line includes: “Prenatal Care,” “Vitamin B12,” “Instant Energy,” “Vitamin D,” “Daily Health,” “Sweet Dreams,” “Immune Strength,” “Clearer Thinking,” and “Instavit for Kids.” Instavit products are available in retail stores in North America.
FOR MORE INFORMATION, VISIT: http://www.instavit.com/
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
DLBCL survivors at greater risk of autoimmune, infectious diseases
ATLANTA—A population-based study indicates that, compared to other cancer survivors, patients who survive diffuse large B-cell lymphoma (DLBCL) have an increased risk of autoimmune and infectious diseases.
For example, investigators found the risk of being diagnosed with impaired humoral immunity was 16.2 times higher in female DLBCL survivors than in breast cancer survivors, 14.8 times higher in male DLBCL survivors than in prostate cancer survivors, and 12.5 times higher in all DLBCL survivors than in survivors of head and neck cancer.
“Most of the treatments that we give for lymphoma have profound effects on the immune system, either directly or indirectly, including many of the T-cell-directed therapies,” said Tanaya Shree, MD, PhD, of Stanford University Medical Center in California.
“There have been studies on many of the effects suffered by lymphoma survivors, but very little is known about their immune health.”
Dr Shree and her colleagues undertook this study to determine how the immune system fares in lymphoma survivors. The investigators limited their analysis to survivors of DLBCL.
Dr Shree presented the findings at the 2017 ASH Annual Meeting (abstract 198*).
Study design
Investigators pulled data from the California Cancer Registry for patients with DLBCL as their first primary cancer diagnosed between 1991 and 2012. Patients had to be 18 or older at diagnosis and have survived more than a year after diagnosis.
“Importantly, we counted only diagnoses [of autoimmune and infectious diseases] that first appeared between 1 and 10 years after cancer diagnosis,” Dr Shree explained. “So any diagnosis we saw that had also been seen prior to cancer diagnosis or even up to 1 year post-cancer diagnosis, we considered to be pre-existing and were excluded from the analysis in order to really focus on new incident cases during survivorship.”
Investigators used the same criteria for the comparator cohorts.
The survivor data was linked to statewide discharge databases, and investigators performed the incidence analysis based on ICD-9 codes.
Investigators used Poisson regression analysis to obtain incident ratios and adjusted the models for age, race, and year of diagnosis.
They graphed the incident rate ratios for all the diagnoses that were significantly different between the DLBCL cohort and the comparator cohorts.
“[W]e considered a P value of less than 0.0005 to be significant,” Dr Shree clarified.
Survivor characteristics
The cohorts comprised 802,255 survivors of DLBCL (n=21,690), breast cancer (n=337,591), prostate cancer (n=325,533), melanoma (n=73,196), and head and neck cancer (n=44,245).
“At least 75% of patients in each cohort were aged 40 to 79,” Dr Shree noted, “with a good representation of elderly patients.”
The median follow-up time was 6.1 years for DLBCL patients and ranged from 5.7 years for head and neck cancer survivors to 8.3 years for prostate cancer survivors.
About three-quarters of patients in each cohort had hospitalization data within 1 to 10 years from cancer diagnosis.
DLBCL vs breast cancer
“Interestingly, we found some familiar names amongst the top-scoring diagnoses,” Dr Shree said.
Deficiency of humoral immunity (16.2-fold), autoimmune hemolytic anemia (9.9-fold), Sicca syndrome (6.9-fold), and immune thrombocytopenia (3.1-fold) were higher in female DLBCL survivors than breast cancer survivors.
“All of these have known associations with lymphoma,” Dr Shree said. “But we also found, surprisingly, increased rates of fungal [6.0-fold] and viral pneumonia [3.3-fold], and many other codes associated with respiratory infections. We also found a 3-fold increased rate of meningitis.”
“The only diagnosis statistically more common amongst breast cancer patients was cervicitis and endocervicitis, and this likely relates to the fact that many of these patients are undergoing hormone therapy.”
DLBCL vs prostate cancer
“We saw some of the same diagnoses come up as top-scoring hits, including viral [4.5-fold] and fungal pneumonia [8.2-fold], and meningitis [3.9-fold], and, in this case, Staphylococcal meningitis [8.6-fold],” Dr Shree said.
Deficiency of humoral immunity (14.8-fold), autoimmune hemolytic anemia (8.9-fold), Sicca syndrome (8.6-fold), and immune thrombocytopenia (4.8-fold) were also higher in the male DLBCL survivors than in prostate cancer survivors.
“No diagnoses were statistically more common in the prostate cancer survivors [than in male DLBCL survivors],” Dr Shree noted.
DLBCL vs head and neck cancer
“Again, the top 4 hits were the same 4 diagnoses we have been seeing repeatedly,” Dr Shree said.
Deficiency of humoral immunity (12.5-fold), autoimmune hemolytic anemia (9.3-fold), Sicca syndrome (5.5-fold), and immune thrombocytopenia (4.5-fold) were increased for DLBCL survivors compared to survivors of head and neck cancer.
DLBCL survivors also had an increased risk of respiratory infections, especially viral (4.4-fold) and fungal pneumonias (4.0-fold), meningitis (3.0-fold), and chronic lymphocytic thyroiditis (2.8-fold), also known as Hashimoto’s thyroiditis.
On the other hand, bacterial pneumonias and skin infections were more common in the head and neck cancer survivors than in DLBCL survivors.
DLBCL vs melanoma
“Interestingly, we did not see an increased risk for immune thrombocytopenias [in DLBCL survivors] compared to melanoma survivors in this comparison, which we had in all the other comparisons,” Dr Shree noted.
“But we did see [an increased risk for] the other diagnoses that we had been tracking, including, again, fungal pneumonia [6.9-fold], viral pneumonia [4.7-fold], and miscellaneous viral infections [2.6-fold].”
The only diagnosis that was statistically more common among melanoma survivors than DLBCL survivors was vitiligo.
Risks persist over time
The investigators assessed whether the elevated risks were static over the 1- to 10-year analysis period.
They took the top diagnoses—humoral deficiency, autoimmune hemolytic anemia, Sicca syndrome, and immune thrombocytopenia—and reviewed them for all cohorts to determine the rate of new cases.
“[F]or 3 out of these 4 diagnoses [humoral deficiency, autoimmune hemolytic anemia, and Sicca syndrome], increased incident rates are highest in the first 1 to 3 years after diagnosis in the lymphoma patients,” Dr Shree said.
“But even at 5 to 10 years out, these patients continue to have increased incidence of these diagnoses compared to the other cohorts, suggesting that these risks really do remain elevated over some time.”
The investigators repeated the analysis using broader categories of diagnoses with each category encompassing many ICD-9 codes.
“[I]n 12 out of 18 broad categories that we looked at, we can still find statistically significant differences in the incident rates for these diagnoses, and they were all increased in the lymphoma patients compared to the other cohorts,” Dr Shree explained.
“[T]hese increases were seen across multiple comparisons, suggesting that this phenomenon seems to be really lymphoma-specific and not specific to any of the individual comparisons we had chosen to perform.”
The findings, she said, have a lot of implications.
“We are particularly interested in which features of patients’ treatment contribute most to these elevated risks,” Dr Shree said. “And, of course, we want to know what to be able to tell our patients and how to follow them during survivorship.”
The investigators are currently validating their findings with further analysis of the Stanford lymphoma survivors cohort of approximately 3500 patients. ![]()
*Data in the abstract differ from the presentation.
ATLANTA—A population-based study indicates that, compared to other cancer survivors, patients who survive diffuse large B-cell lymphoma (DLBCL) have an increased risk of autoimmune and infectious diseases.
For example, investigators found the risk of being diagnosed with impaired humoral immunity was 16.2 times higher in female DLBCL survivors than in breast cancer survivors, 14.8 times higher in male DLBCL survivors than in prostate cancer survivors, and 12.5 times higher in all DLBCL survivors than in survivors of head and neck cancer.
“Most of the treatments that we give for lymphoma have profound effects on the immune system, either directly or indirectly, including many of the T-cell-directed therapies,” said Tanaya Shree, MD, PhD, of Stanford University Medical Center in California.
“There have been studies on many of the effects suffered by lymphoma survivors, but very little is known about their immune health.”
Dr Shree and her colleagues undertook this study to determine how the immune system fares in lymphoma survivors. The investigators limited their analysis to survivors of DLBCL.
Dr Shree presented the findings at the 2017 ASH Annual Meeting (abstract 198*).
Study design
Investigators pulled data from the California Cancer Registry for patients with DLBCL as their first primary cancer diagnosed between 1991 and 2012. Patients had to be 18 or older at diagnosis and have survived more than a year after diagnosis.
“Importantly, we counted only diagnoses [of autoimmune and infectious diseases] that first appeared between 1 and 10 years after cancer diagnosis,” Dr Shree explained. “So any diagnosis we saw that had also been seen prior to cancer diagnosis or even up to 1 year post-cancer diagnosis, we considered to be pre-existing and were excluded from the analysis in order to really focus on new incident cases during survivorship.”
Investigators used the same criteria for the comparator cohorts.
The survivor data was linked to statewide discharge databases, and investigators performed the incidence analysis based on ICD-9 codes.
Investigators used Poisson regression analysis to obtain incident ratios and adjusted the models for age, race, and year of diagnosis.
They graphed the incident rate ratios for all the diagnoses that were significantly different between the DLBCL cohort and the comparator cohorts.
“[W]e considered a P value of less than 0.0005 to be significant,” Dr Shree clarified.
Survivor characteristics
The cohorts comprised 802,255 survivors of DLBCL (n=21,690), breast cancer (n=337,591), prostate cancer (n=325,533), melanoma (n=73,196), and head and neck cancer (n=44,245).
“At least 75% of patients in each cohort were aged 40 to 79,” Dr Shree noted, “with a good representation of elderly patients.”
The median follow-up time was 6.1 years for DLBCL patients and ranged from 5.7 years for head and neck cancer survivors to 8.3 years for prostate cancer survivors.
About three-quarters of patients in each cohort had hospitalization data within 1 to 10 years from cancer diagnosis.
DLBCL vs breast cancer
“Interestingly, we found some familiar names amongst the top-scoring diagnoses,” Dr Shree said.
Deficiency of humoral immunity (16.2-fold), autoimmune hemolytic anemia (9.9-fold), Sicca syndrome (6.9-fold), and immune thrombocytopenia (3.1-fold) were higher in female DLBCL survivors than breast cancer survivors.
“All of these have known associations with lymphoma,” Dr Shree said. “But we also found, surprisingly, increased rates of fungal [6.0-fold] and viral pneumonia [3.3-fold], and many other codes associated with respiratory infections. We also found a 3-fold increased rate of meningitis.”
“The only diagnosis statistically more common amongst breast cancer patients was cervicitis and endocervicitis, and this likely relates to the fact that many of these patients are undergoing hormone therapy.”
DLBCL vs prostate cancer
“We saw some of the same diagnoses come up as top-scoring hits, including viral [4.5-fold] and fungal pneumonia [8.2-fold], and meningitis [3.9-fold], and, in this case, Staphylococcal meningitis [8.6-fold],” Dr Shree said.
Deficiency of humoral immunity (14.8-fold), autoimmune hemolytic anemia (8.9-fold), Sicca syndrome (8.6-fold), and immune thrombocytopenia (4.8-fold) were also higher in the male DLBCL survivors than in prostate cancer survivors.
“No diagnoses were statistically more common in the prostate cancer survivors [than in male DLBCL survivors],” Dr Shree noted.
DLBCL vs head and neck cancer
“Again, the top 4 hits were the same 4 diagnoses we have been seeing repeatedly,” Dr Shree said.
Deficiency of humoral immunity (12.5-fold), autoimmune hemolytic anemia (9.3-fold), Sicca syndrome (5.5-fold), and immune thrombocytopenia (4.5-fold) were increased for DLBCL survivors compared to survivors of head and neck cancer.
DLBCL survivors also had an increased risk of respiratory infections, especially viral (4.4-fold) and fungal pneumonias (4.0-fold), meningitis (3.0-fold), and chronic lymphocytic thyroiditis (2.8-fold), also known as Hashimoto’s thyroiditis.
On the other hand, bacterial pneumonias and skin infections were more common in the head and neck cancer survivors than in DLBCL survivors.
DLBCL vs melanoma
“Interestingly, we did not see an increased risk for immune thrombocytopenias [in DLBCL survivors] compared to melanoma survivors in this comparison, which we had in all the other comparisons,” Dr Shree noted.
“But we did see [an increased risk for] the other diagnoses that we had been tracking, including, again, fungal pneumonia [6.9-fold], viral pneumonia [4.7-fold], and miscellaneous viral infections [2.6-fold].”
The only diagnosis that was statistically more common among melanoma survivors than DLBCL survivors was vitiligo.
Risks persist over time
The investigators assessed whether the elevated risks were static over the 1- to 10-year analysis period.
They took the top diagnoses—humoral deficiency, autoimmune hemolytic anemia, Sicca syndrome, and immune thrombocytopenia—and reviewed them for all cohorts to determine the rate of new cases.
“[F]or 3 out of these 4 diagnoses [humoral deficiency, autoimmune hemolytic anemia, and Sicca syndrome], increased incident rates are highest in the first 1 to 3 years after diagnosis in the lymphoma patients,” Dr Shree said.
“But even at 5 to 10 years out, these patients continue to have increased incidence of these diagnoses compared to the other cohorts, suggesting that these risks really do remain elevated over some time.”
The investigators repeated the analysis using broader categories of diagnoses with each category encompassing many ICD-9 codes.
“[I]n 12 out of 18 broad categories that we looked at, we can still find statistically significant differences in the incident rates for these diagnoses, and they were all increased in the lymphoma patients compared to the other cohorts,” Dr Shree explained.
“[T]hese increases were seen across multiple comparisons, suggesting that this phenomenon seems to be really lymphoma-specific and not specific to any of the individual comparisons we had chosen to perform.”
The findings, she said, have a lot of implications.
“We are particularly interested in which features of patients’ treatment contribute most to these elevated risks,” Dr Shree said. “And, of course, we want to know what to be able to tell our patients and how to follow them during survivorship.”
The investigators are currently validating their findings with further analysis of the Stanford lymphoma survivors cohort of approximately 3500 patients. ![]()
*Data in the abstract differ from the presentation.
ATLANTA—A population-based study indicates that, compared to other cancer survivors, patients who survive diffuse large B-cell lymphoma (DLBCL) have an increased risk of autoimmune and infectious diseases.
For example, investigators found the risk of being diagnosed with impaired humoral immunity was 16.2 times higher in female DLBCL survivors than in breast cancer survivors, 14.8 times higher in male DLBCL survivors than in prostate cancer survivors, and 12.5 times higher in all DLBCL survivors than in survivors of head and neck cancer.
“Most of the treatments that we give for lymphoma have profound effects on the immune system, either directly or indirectly, including many of the T-cell-directed therapies,” said Tanaya Shree, MD, PhD, of Stanford University Medical Center in California.
“There have been studies on many of the effects suffered by lymphoma survivors, but very little is known about their immune health.”
Dr Shree and her colleagues undertook this study to determine how the immune system fares in lymphoma survivors. The investigators limited their analysis to survivors of DLBCL.
Dr Shree presented the findings at the 2017 ASH Annual Meeting (abstract 198*).
Study design
Investigators pulled data from the California Cancer Registry for patients with DLBCL as their first primary cancer diagnosed between 1991 and 2012. Patients had to be 18 or older at diagnosis and have survived more than a year after diagnosis.
“Importantly, we counted only diagnoses [of autoimmune and infectious diseases] that first appeared between 1 and 10 years after cancer diagnosis,” Dr Shree explained. “So any diagnosis we saw that had also been seen prior to cancer diagnosis or even up to 1 year post-cancer diagnosis, we considered to be pre-existing and were excluded from the analysis in order to really focus on new incident cases during survivorship.”
Investigators used the same criteria for the comparator cohorts.
The survivor data was linked to statewide discharge databases, and investigators performed the incidence analysis based on ICD-9 codes.
Investigators used Poisson regression analysis to obtain incident ratios and adjusted the models for age, race, and year of diagnosis.
They graphed the incident rate ratios for all the diagnoses that were significantly different between the DLBCL cohort and the comparator cohorts.
“[W]e considered a P value of less than 0.0005 to be significant,” Dr Shree clarified.
Survivor characteristics
The cohorts comprised 802,255 survivors of DLBCL (n=21,690), breast cancer (n=337,591), prostate cancer (n=325,533), melanoma (n=73,196), and head and neck cancer (n=44,245).
“At least 75% of patients in each cohort were aged 40 to 79,” Dr Shree noted, “with a good representation of elderly patients.”
The median follow-up time was 6.1 years for DLBCL patients and ranged from 5.7 years for head and neck cancer survivors to 8.3 years for prostate cancer survivors.
About three-quarters of patients in each cohort had hospitalization data within 1 to 10 years from cancer diagnosis.
DLBCL vs breast cancer
“Interestingly, we found some familiar names amongst the top-scoring diagnoses,” Dr Shree said.
Deficiency of humoral immunity (16.2-fold), autoimmune hemolytic anemia (9.9-fold), Sicca syndrome (6.9-fold), and immune thrombocytopenia (3.1-fold) were higher in female DLBCL survivors than breast cancer survivors.
“All of these have known associations with lymphoma,” Dr Shree said. “But we also found, surprisingly, increased rates of fungal [6.0-fold] and viral pneumonia [3.3-fold], and many other codes associated with respiratory infections. We also found a 3-fold increased rate of meningitis.”
“The only diagnosis statistically more common amongst breast cancer patients was cervicitis and endocervicitis, and this likely relates to the fact that many of these patients are undergoing hormone therapy.”
DLBCL vs prostate cancer
“We saw some of the same diagnoses come up as top-scoring hits, including viral [4.5-fold] and fungal pneumonia [8.2-fold], and meningitis [3.9-fold], and, in this case, Staphylococcal meningitis [8.6-fold],” Dr Shree said.
Deficiency of humoral immunity (14.8-fold), autoimmune hemolytic anemia (8.9-fold), Sicca syndrome (8.6-fold), and immune thrombocytopenia (4.8-fold) were also higher in the male DLBCL survivors than in prostate cancer survivors.
“No diagnoses were statistically more common in the prostate cancer survivors [than in male DLBCL survivors],” Dr Shree noted.
DLBCL vs head and neck cancer
“Again, the top 4 hits were the same 4 diagnoses we have been seeing repeatedly,” Dr Shree said.
Deficiency of humoral immunity (12.5-fold), autoimmune hemolytic anemia (9.3-fold), Sicca syndrome (5.5-fold), and immune thrombocytopenia (4.5-fold) were increased for DLBCL survivors compared to survivors of head and neck cancer.
DLBCL survivors also had an increased risk of respiratory infections, especially viral (4.4-fold) and fungal pneumonias (4.0-fold), meningitis (3.0-fold), and chronic lymphocytic thyroiditis (2.8-fold), also known as Hashimoto’s thyroiditis.
On the other hand, bacterial pneumonias and skin infections were more common in the head and neck cancer survivors than in DLBCL survivors.
DLBCL vs melanoma
“Interestingly, we did not see an increased risk for immune thrombocytopenias [in DLBCL survivors] compared to melanoma survivors in this comparison, which we had in all the other comparisons,” Dr Shree noted.
“But we did see [an increased risk for] the other diagnoses that we had been tracking, including, again, fungal pneumonia [6.9-fold], viral pneumonia [4.7-fold], and miscellaneous viral infections [2.6-fold].”
The only diagnosis that was statistically more common among melanoma survivors than DLBCL survivors was vitiligo.
Risks persist over time
The investigators assessed whether the elevated risks were static over the 1- to 10-year analysis period.
They took the top diagnoses—humoral deficiency, autoimmune hemolytic anemia, Sicca syndrome, and immune thrombocytopenia—and reviewed them for all cohorts to determine the rate of new cases.
“[F]or 3 out of these 4 diagnoses [humoral deficiency, autoimmune hemolytic anemia, and Sicca syndrome], increased incident rates are highest in the first 1 to 3 years after diagnosis in the lymphoma patients,” Dr Shree said.
“But even at 5 to 10 years out, these patients continue to have increased incidence of these diagnoses compared to the other cohorts, suggesting that these risks really do remain elevated over some time.”
The investigators repeated the analysis using broader categories of diagnoses with each category encompassing many ICD-9 codes.
“[I]n 12 out of 18 broad categories that we looked at, we can still find statistically significant differences in the incident rates for these diagnoses, and they were all increased in the lymphoma patients compared to the other cohorts,” Dr Shree explained.
“[T]hese increases were seen across multiple comparisons, suggesting that this phenomenon seems to be really lymphoma-specific and not specific to any of the individual comparisons we had chosen to perform.”
The findings, she said, have a lot of implications.
“We are particularly interested in which features of patients’ treatment contribute most to these elevated risks,” Dr Shree said. “And, of course, we want to know what to be able to tell our patients and how to follow them during survivorship.”
The investigators are currently validating their findings with further analysis of the Stanford lymphoma survivors cohort of approximately 3500 patients. ![]()
*Data in the abstract differ from the presentation.
Brentuximab vedotin sBLA receives priority review
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application (sBLA) for brentuximab vedotin (ADCETRIS).
With this sBLA, Seattle Genetics, Inc., is seeking approval for brentuximab vedotin in combination with chemotherapy for frontline treatment of patients with advanced classical Hodgkin lymphoma (HL).
The FDA expects to make a decision on the sBLA by May 1, 2018.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The priority review for this sBLA is based on positive results from the phase 3 ECHELON-1 trial.
The FDA previously granted brentuximab vedotin breakthrough therapy designation based on ECHELON-1 results.
Breakthrough therapy designation is intended to expedite the development and review of promising drug candidates for serious or life-threatening conditions. It is based upon clinical evidence of substantial improvement over existing therapies in one or more clinically significant endpoints.
ECHELON-1
Result from ECHELON-1 were presented at the 2017 ASH Annual Meeting and simultaneously published in NEJM.
In this trial, researchers compared brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for 1334 patients with advanced HL.
The study’s primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review facility, A+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm.
There was no significant difference between the treatment arms when it came to response rates or overall survival.
The objective response rate was 86% in the A+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The interim 2-year overall survival rate was 97% in the A+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
The overall incidence of adverse events (AEs) was 99% in the A+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with A+AVD, while pulmonary toxicity was more common with ABVD.
About brentuximab vedotin
Brentuximab vedotin is already FDA-approved to treat adults with:
- Classical HL who have failed autologous hematopoietic stem cell transplant (auto-HSCT) or, in those who are not auto-HSCT candidates, have failed at least 2 prior multi-agent chemotherapy regimens.
- Classical HL at high risk of relapse or progression as post-auto-HSCT consolidation.
- Primary cutaneous anaplastic large-cell lymphoma (ALCL) or CD30-expressing mycosis fungoides who have received prior systemic therapy.
- Systemic ALCL who have failed at least 1 prior multi-agent chemotherapy regimen. (The drug has accelerated approval for this indication, based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.)

The US Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application (sBLA) for brentuximab vedotin (ADCETRIS).
With this sBLA, Seattle Genetics, Inc., is seeking approval for brentuximab vedotin in combination with chemotherapy for frontline treatment of patients with advanced classical Hodgkin lymphoma (HL).
The FDA expects to make a decision on the sBLA by May 1, 2018.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The priority review for this sBLA is based on positive results from the phase 3 ECHELON-1 trial.
The FDA previously granted brentuximab vedotin breakthrough therapy designation based on ECHELON-1 results.
Breakthrough therapy designation is intended to expedite the development and review of promising drug candidates for serious or life-threatening conditions. It is based upon clinical evidence of substantial improvement over existing therapies in one or more clinically significant endpoints.
ECHELON-1
Result from ECHELON-1 were presented at the 2017 ASH Annual Meeting and simultaneously published in NEJM.
In this trial, researchers compared brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for 1334 patients with advanced HL.
The study’s primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review facility, A+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm.
There was no significant difference between the treatment arms when it came to response rates or overall survival.
The objective response rate was 86% in the A+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The interim 2-year overall survival rate was 97% in the A+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
The overall incidence of adverse events (AEs) was 99% in the A+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with A+AVD, while pulmonary toxicity was more common with ABVD.
About brentuximab vedotin
Brentuximab vedotin is already FDA-approved to treat adults with:
- Classical HL who have failed autologous hematopoietic stem cell transplant (auto-HSCT) or, in those who are not auto-HSCT candidates, have failed at least 2 prior multi-agent chemotherapy regimens.
- Classical HL at high risk of relapse or progression as post-auto-HSCT consolidation.
- Primary cutaneous anaplastic large-cell lymphoma (ALCL) or CD30-expressing mycosis fungoides who have received prior systemic therapy.
- Systemic ALCL who have failed at least 1 prior multi-agent chemotherapy regimen. (The drug has accelerated approval for this indication, based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.)

The US Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application (sBLA) for brentuximab vedotin (ADCETRIS).
With this sBLA, Seattle Genetics, Inc., is seeking approval for brentuximab vedotin in combination with chemotherapy for frontline treatment of patients with advanced classical Hodgkin lymphoma (HL).
The FDA expects to make a decision on the sBLA by May 1, 2018.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The priority review for this sBLA is based on positive results from the phase 3 ECHELON-1 trial.
The FDA previously granted brentuximab vedotin breakthrough therapy designation based on ECHELON-1 results.
Breakthrough therapy designation is intended to expedite the development and review of promising drug candidates for serious or life-threatening conditions. It is based upon clinical evidence of substantial improvement over existing therapies in one or more clinically significant endpoints.
ECHELON-1
Result from ECHELON-1 were presented at the 2017 ASH Annual Meeting and simultaneously published in NEJM.
In this trial, researchers compared brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for 1334 patients with advanced HL.
The study’s primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review facility, A+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm.
There was no significant difference between the treatment arms when it came to response rates or overall survival.
The objective response rate was 86% in the A+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The interim 2-year overall survival rate was 97% in the A+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
The overall incidence of adverse events (AEs) was 99% in the A+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with A+AVD, while pulmonary toxicity was more common with ABVD.
About brentuximab vedotin
Brentuximab vedotin is already FDA-approved to treat adults with:
- Classical HL who have failed autologous hematopoietic stem cell transplant (auto-HSCT) or, in those who are not auto-HSCT candidates, have failed at least 2 prior multi-agent chemotherapy regimens.
- Classical HL at high risk of relapse or progression as post-auto-HSCT consolidation.
- Primary cutaneous anaplastic large-cell lymphoma (ALCL) or CD30-expressing mycosis fungoides who have received prior systemic therapy.
- Systemic ALCL who have failed at least 1 prior multi-agent chemotherapy regimen. (The drug has accelerated approval for this indication, based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.)

FDA lifts hold on trial of SEL24 in AML
The US Food and Drug Administration (FDA) has lifted the clinical hold placed on a phase 1/2 trial of SEL24, a dual PIM/FLT3 kinase inhibitor, in patients with relapsed/refractory acute myeloid leukemia (AML).
Selvita and Menarini Group, the companies developing SEL24, agreed to continue the trial with additional provisions to its protocol.
The companies will be working with trial investigators and clinical sites to obtain institutional review board approval on the revised protocol and resume enrollment in the trial, according to Krzysztof Brzozka, PhD, chief scientific officer of Selvita.
The trial is a dose-escalation study of SEL24 in patients with relapsed and refractory AML. The study was designed to determine the maximum tolerated dose and recommended dose of SEL24.
The first patient was dosed in March 2017. The study began with a 25 mg daily dose, which was then escalated following cohort reviews.
In October, the trial was placed on full clinical hold, which meant no new patients could be enrolled on the trial, and enrolled patients could not receive SEL24 until the hold was lifted.
The hold was the result of a fatal cerebral adverse event that was considered possibly related to SEL24.
The event occurred in a patient who started treatment with a 150 mg dose of SEL24 as the third patient in this dose cohort.
The patient received 4 doses of the drug and developed a life-threatening, grade 4 venous thrombus in the brain with subsequent intracerebral hemorrhage, which required hospitalization.
The patient died in hospice 4 days later. The patient’s death was deemed possibly related to SEL24.
In response to the death, a safety report was submitted to the FDA, along with a review by the trial’s data monitoring committee.
The FDA then placed a hold on the trial and requested more safety data on patients who have received SEL24, as well as specific protocol changes and additional guidance to the study staff.
Selvita and Menarini Group complied with the FDA’s requests and agreed to revise the dose-finding scheme to a standard 3+3 design under an amended protocol. ![]()
The US Food and Drug Administration (FDA) has lifted the clinical hold placed on a phase 1/2 trial of SEL24, a dual PIM/FLT3 kinase inhibitor, in patients with relapsed/refractory acute myeloid leukemia (AML).
Selvita and Menarini Group, the companies developing SEL24, agreed to continue the trial with additional provisions to its protocol.
The companies will be working with trial investigators and clinical sites to obtain institutional review board approval on the revised protocol and resume enrollment in the trial, according to Krzysztof Brzozka, PhD, chief scientific officer of Selvita.
The trial is a dose-escalation study of SEL24 in patients with relapsed and refractory AML. The study was designed to determine the maximum tolerated dose and recommended dose of SEL24.
The first patient was dosed in March 2017. The study began with a 25 mg daily dose, which was then escalated following cohort reviews.
In October, the trial was placed on full clinical hold, which meant no new patients could be enrolled on the trial, and enrolled patients could not receive SEL24 until the hold was lifted.
The hold was the result of a fatal cerebral adverse event that was considered possibly related to SEL24.
The event occurred in a patient who started treatment with a 150 mg dose of SEL24 as the third patient in this dose cohort.
The patient received 4 doses of the drug and developed a life-threatening, grade 4 venous thrombus in the brain with subsequent intracerebral hemorrhage, which required hospitalization.
The patient died in hospice 4 days later. The patient’s death was deemed possibly related to SEL24.
In response to the death, a safety report was submitted to the FDA, along with a review by the trial’s data monitoring committee.
The FDA then placed a hold on the trial and requested more safety data on patients who have received SEL24, as well as specific protocol changes and additional guidance to the study staff.
Selvita and Menarini Group complied with the FDA’s requests and agreed to revise the dose-finding scheme to a standard 3+3 design under an amended protocol. ![]()
The US Food and Drug Administration (FDA) has lifted the clinical hold placed on a phase 1/2 trial of SEL24, a dual PIM/FLT3 kinase inhibitor, in patients with relapsed/refractory acute myeloid leukemia (AML).
Selvita and Menarini Group, the companies developing SEL24, agreed to continue the trial with additional provisions to its protocol.
The companies will be working with trial investigators and clinical sites to obtain institutional review board approval on the revised protocol and resume enrollment in the trial, according to Krzysztof Brzozka, PhD, chief scientific officer of Selvita.
The trial is a dose-escalation study of SEL24 in patients with relapsed and refractory AML. The study was designed to determine the maximum tolerated dose and recommended dose of SEL24.
The first patient was dosed in March 2017. The study began with a 25 mg daily dose, which was then escalated following cohort reviews.
In October, the trial was placed on full clinical hold, which meant no new patients could be enrolled on the trial, and enrolled patients could not receive SEL24 until the hold was lifted.
The hold was the result of a fatal cerebral adverse event that was considered possibly related to SEL24.
The event occurred in a patient who started treatment with a 150 mg dose of SEL24 as the third patient in this dose cohort.
The patient received 4 doses of the drug and developed a life-threatening, grade 4 venous thrombus in the brain with subsequent intracerebral hemorrhage, which required hospitalization.
The patient died in hospice 4 days later. The patient’s death was deemed possibly related to SEL24.
In response to the death, a safety report was submitted to the FDA, along with a review by the trial’s data monitoring committee.
The FDA then placed a hold on the trial and requested more safety data on patients who have received SEL24, as well as specific protocol changes and additional guidance to the study staff.
Selvita and Menarini Group complied with the FDA’s requests and agreed to revise the dose-finding scheme to a standard 3+3 design under an amended protocol. ![]()