User login
Impact of Hypsarrhythmia on Infantile Spasm Therapy
Infants experiencing spasms will likely respond to standard first-line treatment, whether or not they also have hypsarrhythmia, according to a multicenter study of the National Infantile Spasms Consortium.
- The investigation included infants between 2 months and 2 years who had been enrolled in the Consortium cohort with new onset spasms.
- 82% of the infants had hypsarrthythmia but the condition was not linked to the child’s average age, gender, any preexisting developmental delay or epilepsy, or their response to first line treatment.
First-line treatment, which consisted of vigabatrin, prednisolone, and adrenocorticotropic hormone (ACTH), was by far the most important variable that predicted the infants’ response to therapy, whether or not they had hypsarrhythmia.
The impact of hypsarrhythmia on infantile spasms treatment response: Observational cohort study from the National Infantile Spasms Consortium. Epilepsia. 2017;58:2098-2103.
Infants experiencing spasms will likely respond to standard first-line treatment, whether or not they also have hypsarrhythmia, according to a multicenter study of the National Infantile Spasms Consortium.
- The investigation included infants between 2 months and 2 years who had been enrolled in the Consortium cohort with new onset spasms.
- 82% of the infants had hypsarrthythmia but the condition was not linked to the child’s average age, gender, any preexisting developmental delay or epilepsy, or their response to first line treatment.
First-line treatment, which consisted of vigabatrin, prednisolone, and adrenocorticotropic hormone (ACTH), was by far the most important variable that predicted the infants’ response to therapy, whether or not they had hypsarrhythmia.
The impact of hypsarrhythmia on infantile spasms treatment response: Observational cohort study from the National Infantile Spasms Consortium. Epilepsia. 2017;58:2098-2103.
Infants experiencing spasms will likely respond to standard first-line treatment, whether or not they also have hypsarrhythmia, according to a multicenter study of the National Infantile Spasms Consortium.
- The investigation included infants between 2 months and 2 years who had been enrolled in the Consortium cohort with new onset spasms.
- 82% of the infants had hypsarrthythmia but the condition was not linked to the child’s average age, gender, any preexisting developmental delay or epilepsy, or their response to first line treatment.
First-line treatment, which consisted of vigabatrin, prednisolone, and adrenocorticotropic hormone (ACTH), was by far the most important variable that predicted the infants’ response to therapy, whether or not they had hypsarrhythmia.
The impact of hypsarrhythmia on infantile spasms treatment response: Observational cohort study from the National Infantile Spasms Consortium. Epilepsia. 2017;58:2098-2103.
Black Patients More Likely to Receive TLE Diagnosis
Black patients are almost twice as likely to be diagnosed with temporal lobe epilepsy than white patients, despite the fact that black patients make up only 20% of the patient population in the Southeastern United States, according to a recent study.
- The University of Alabama at Birmingham reviewed all the video EEG diagnoses in its seizure monitoring unit from 2000 to 2011.
- Researchers performed a statistical analysis that included multivariate logistic regression to detect factors associated with the temporal lobe epilepsy (TLE) diagnosis.
- The diagnoses in the entire patient population included 630 cases of TLE, 1150 cases of psychogenic nonepileptic seizures, 424 additional focal epilepsies, and 224 generalized epilepsies.
- The population included 77.3% white patients, 20% black patients, and 2.3% patients of other races.
- TLE was diagnosed far more often in black patients than white patients (odds ratio: 1.87).
There were more women in the study population, and black women were responsible for most of the statistical difference in TLE diagnoses.
Racial disparities in temporal lobe epilepsy. Epilepsy Res. 2017;16;140:56-60.
Black patients are almost twice as likely to be diagnosed with temporal lobe epilepsy than white patients, despite the fact that black patients make up only 20% of the patient population in the Southeastern United States, according to a recent study.
- The University of Alabama at Birmingham reviewed all the video EEG diagnoses in its seizure monitoring unit from 2000 to 2011.
- Researchers performed a statistical analysis that included multivariate logistic regression to detect factors associated with the temporal lobe epilepsy (TLE) diagnosis.
- The diagnoses in the entire patient population included 630 cases of TLE, 1150 cases of psychogenic nonepileptic seizures, 424 additional focal epilepsies, and 224 generalized epilepsies.
- The population included 77.3% white patients, 20% black patients, and 2.3% patients of other races.
- TLE was diagnosed far more often in black patients than white patients (odds ratio: 1.87).
There were more women in the study population, and black women were responsible for most of the statistical difference in TLE diagnoses.
Racial disparities in temporal lobe epilepsy. Epilepsy Res. 2017;16;140:56-60.
Black patients are almost twice as likely to be diagnosed with temporal lobe epilepsy than white patients, despite the fact that black patients make up only 20% of the patient population in the Southeastern United States, according to a recent study.
- The University of Alabama at Birmingham reviewed all the video EEG diagnoses in its seizure monitoring unit from 2000 to 2011.
- Researchers performed a statistical analysis that included multivariate logistic regression to detect factors associated with the temporal lobe epilepsy (TLE) diagnosis.
- The diagnoses in the entire patient population included 630 cases of TLE, 1150 cases of psychogenic nonepileptic seizures, 424 additional focal epilepsies, and 224 generalized epilepsies.
- The population included 77.3% white patients, 20% black patients, and 2.3% patients of other races.
- TLE was diagnosed far more often in black patients than white patients (odds ratio: 1.87).
There were more women in the study population, and black women were responsible for most of the statistical difference in TLE diagnoses.
Racial disparities in temporal lobe epilepsy. Epilepsy Res. 2017;16;140:56-60.
As Many as 47 Million Americans May Have Preclinical Alzheimer’s Disease
A treatment that would reduce the risk of developing amyloid plaques in the brain by 50% could save more than four million Americans from mild cognitive impairment (MCI) and 2.5 million Americans from Alzheimer’s disease by 2060, according to a study published online ahead of print November 29 in Alzheimer’s & Dementia.
The conclusion that modestly effective preventive therapy could greatly improve the Alzheimer’s disease outlook is especially important, given another finding in a new mathematical modeling study by Ron Brookmeyer, PhD, a biostatistician at the University of California, Los Angeles, and colleagues. They assert that 47 million cognitively normal people in the United States may have brain amyloidosis, the physical finding used to define preclinical Alzheimer’s disease.
This study is the first to quantify the number of cognitively healthy US residents who could eventually experience cognitive changes that put them at risk of developing Alzheimer’s dementia, according to a statement from the Alzheimer’s Association.
Model Predicted Increase in Preclinical Alzheimer’s Disease
“This is the first major attempt to forecast these proposed preclinical Alzheimer’s disease and [MCI] due to Alzheimer’s disease numbers. If confirmed, these data [will] provide essential information for public health planning and for informing and guiding the public and private investment in Alzheimer’s and dementia research,” said Dr. Brookmeyer. “We need more research to confirm the findings from this model, and more Alzheimer’s disease and dementia research that includes diverse populations.”
“I want to emphasize that of the 47 million [people] with these Alzheimer’s brain changes, but without clinical symptoms, most will not progress to clinical disease during their lifetimes. In fact, perhaps only one in seven will progress to full-blown dementia.” Nevertheless, the numbers represent a reality that must be confronted and managed proactively, said Dr. Brookmeyer.
The results may sound alarming, he said, “but I have every confidence in them. And they are important because they allow us to understand how many people could potentially benefit from treatment, at what point on the disease continuum it would be useful to implement treatment, and how those treatments could impact public health.”
Studies Provided Rates of Transition Between Disease States
To create predictive models, Dr. Brookmeyer used data from two prospective longitudinal cohort studies: the Mayo Clinic Study of Aging and a study conducted by Stephanie J. Vos, PhD, a postdoctoral researcher at Maastricht University in the Netherlands.
The Mayo Clinic study followed 1,541 cognitively normal older adults and provided data on the rate of transition from normal cognition to MCI. The study by Dr. Vos and her associates followed 353 patients with MCI and brain amyloid and 222 patients with late MCI as they progressed. It is the largest prospective study of progression from MCI to Alzheimer’s disease that also contains data on baseline neurodegeneration and amyloid burden.
“These studies gave us the rates of transition from one state to another,” said Dr. Brookmeyer. “For example, the Mayo Clinic study gave us rates of transition from normal [health] to amyloidosis: 3% of normal 60-year-olds will convert to this state every year.”
Dr. Vos’s study determined rates of progression from MCI to Alzheimer’s dementia, given two preclinical states: asymptomatic amyloid brain plaques alone, or plaques with evidence of neurodegeneration and cognitive signs, said Dr. Brookmeyer. Both of these transitional states were first defined in 2011 in a joint paper by the Alzheimer’s Association and the National Institute on Aging. While acknowledging that the root causes of Alzheimer’s disease are unknown, the paper hypothesized a pathophysiologic time line beginning with a three-stage preclinical phase.
Asymptomatic cerebral amyloidosis is the first stage. It entails amyloid-positive PET brain imaging with an amyloid-binding ligand, a CSF assay with a low level of amyloid-beta 42 in the presence of normal cognition, or both. Stage 2 is one of amyloid positivity and evidence of synaptic dysfunction or early neurodegeneration in the presence of normal cognition. Finally, stage 3 entails amyloid positivity with evidence of neurodegeneration in the presence of subtle cognitive decline.
“Using those definitions, and piecing together the numbers from these studies, we constructed a computer model based on US census population projections to [estimate] how many people might be in these different states of disease,” said Dr. Brookmeyer.
In 2017, six million Americans were in one of the clinical disease states (ie, MCI due to Alzheimer’s disease, early clinical Alzheimer’s disease, or late clinical Alzheimer’s disease). Dr. Brookmeyer and his colleagues predicted that that number would grow to 15 million by 2060. Similarly, in 2017, about 47 million Americans were in one of the preclinical Alzheimer’s disease states, including 22 million with amyloidosis, 8.3 million with neurodegeneration alone, and 16.2 million with both. He projects that this number will increase to 75.7 million by 2060.
Prevention Strategies
The team remodeled those numbers in three hypothetical intervention scenarios. Researchers say that a treatment that slows decline by at least 30% would have a meaningful clinical, financial, and societal impact. However, Dr. Brookmeyer modeled treatment scenarios with a greater effect.
A primary prevention method that reduced the annual risk of new amyloidosis by 50% could decrease the prevalence of MCI by about 700,000 in 2060. A secondary prevention strategy that reduced the annual risk progression to MCI by 50% would decrease the prevalence of MCI by more than two million and the prevalence of Alzheimer’s disease by about 3.8 million.
The results were more complicated with a secondary prevention strategy that would reduce annual risk of conversion from MCI to Alzheimer’s disease by 50%. In this scenario, the prevalence of MCI in 2060 would increase by 2.8 million, but the prevalence of Alzheimer’s disease would decrease by 2.5 million. These scenarios developed over different time courses, said the researchers.
“We find that the highly effective primary prevention strategy resulted in the lowest Alzheimer’s disease prevalence by the year 2060. However, [it] was associated with the largest Alzheimer’s disease prevalence in the 15 years immediately after its introduction ... The explanation for this finding is that the full benefits of delaying amyloidosis, in terms of reduced Alzheimer’s disease prevalence, are not realized for many years because of the long lag time between amyloidosis and clinical Alzheimer’s disease. A take-home message is that the full impact on disease burden of primary prevention that targets the early stages of the pathogenesis of Alzheimer’s disease may not be realized for decades.”
Decreasing preclinical conversion to MCI with a secondary prevention strategy would result in the highest Alzheimer’s disease prevalence reduction for most of the period. But the reduction resulting from the primary prevention strategy would surpass it by 2054.
The intervention targeting conversion from MCI to Alzheimer’s disease would reduce Alzheimer’s disease prevalence the quickest, with a slight decrease in the first three years after introduction. “The explanation for this finding is that MCI is proximate to clinical Alzheimer’s disease diagnosis, and thus the impact of delaying progression of MCI will be seen relatively quickly on Alzheimer’s disease prevalence, compared to interventions that delay onset of amyloidosis or MCI.
“By focusing attention on a concerning reality—that tens of millions of American adults may face the possibility of dementia due to Alzheimer’s disease—the results reported in this new article, if confirmed, illustrate and greatly amplify the need for more research to develop effective treatments and proven prevention strategies for Alzheimer’s disease,” said Dr. Brookmeyer. “This is especially true as we get better at early detection and are able to more accurately identify people who have the early brain changes associated with Alzheimer’s disease and other dementias.”
Dr. Brookmeyer reported receiving fees from Takeda for serving as a member of a data safety monitoring board.
—Michele G. Sullivan
Suggested Reading
Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 2017 Nov 29 [Epub ahead of print].
Derby CA, Katz MJ, Lipton RB, Hall CB. Trends in dementia incidence in a birth cohort analysis of the Einstein Aging Study. JAMA Neurol. 2017;74(11):1345-1351.
Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
Vos SJ, Verhey F, Frölich L, et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain. 2015;138(Pt 5):1327-1338.
A treatment that would reduce the risk of developing amyloid plaques in the brain by 50% could save more than four million Americans from mild cognitive impairment (MCI) and 2.5 million Americans from Alzheimer’s disease by 2060, according to a study published online ahead of print November 29 in Alzheimer’s & Dementia.
The conclusion that modestly effective preventive therapy could greatly improve the Alzheimer’s disease outlook is especially important, given another finding in a new mathematical modeling study by Ron Brookmeyer, PhD, a biostatistician at the University of California, Los Angeles, and colleagues. They assert that 47 million cognitively normal people in the United States may have brain amyloidosis, the physical finding used to define preclinical Alzheimer’s disease.
This study is the first to quantify the number of cognitively healthy US residents who could eventually experience cognitive changes that put them at risk of developing Alzheimer’s dementia, according to a statement from the Alzheimer’s Association.
Model Predicted Increase in Preclinical Alzheimer’s Disease
“This is the first major attempt to forecast these proposed preclinical Alzheimer’s disease and [MCI] due to Alzheimer’s disease numbers. If confirmed, these data [will] provide essential information for public health planning and for informing and guiding the public and private investment in Alzheimer’s and dementia research,” said Dr. Brookmeyer. “We need more research to confirm the findings from this model, and more Alzheimer’s disease and dementia research that includes diverse populations.”
“I want to emphasize that of the 47 million [people] with these Alzheimer’s brain changes, but without clinical symptoms, most will not progress to clinical disease during their lifetimes. In fact, perhaps only one in seven will progress to full-blown dementia.” Nevertheless, the numbers represent a reality that must be confronted and managed proactively, said Dr. Brookmeyer.
The results may sound alarming, he said, “but I have every confidence in them. And they are important because they allow us to understand how many people could potentially benefit from treatment, at what point on the disease continuum it would be useful to implement treatment, and how those treatments could impact public health.”
Studies Provided Rates of Transition Between Disease States
To create predictive models, Dr. Brookmeyer used data from two prospective longitudinal cohort studies: the Mayo Clinic Study of Aging and a study conducted by Stephanie J. Vos, PhD, a postdoctoral researcher at Maastricht University in the Netherlands.
The Mayo Clinic study followed 1,541 cognitively normal older adults and provided data on the rate of transition from normal cognition to MCI. The study by Dr. Vos and her associates followed 353 patients with MCI and brain amyloid and 222 patients with late MCI as they progressed. It is the largest prospective study of progression from MCI to Alzheimer’s disease that also contains data on baseline neurodegeneration and amyloid burden.
“These studies gave us the rates of transition from one state to another,” said Dr. Brookmeyer. “For example, the Mayo Clinic study gave us rates of transition from normal [health] to amyloidosis: 3% of normal 60-year-olds will convert to this state every year.”
Dr. Vos’s study determined rates of progression from MCI to Alzheimer’s dementia, given two preclinical states: asymptomatic amyloid brain plaques alone, or plaques with evidence of neurodegeneration and cognitive signs, said Dr. Brookmeyer. Both of these transitional states were first defined in 2011 in a joint paper by the Alzheimer’s Association and the National Institute on Aging. While acknowledging that the root causes of Alzheimer’s disease are unknown, the paper hypothesized a pathophysiologic time line beginning with a three-stage preclinical phase.
Asymptomatic cerebral amyloidosis is the first stage. It entails amyloid-positive PET brain imaging with an amyloid-binding ligand, a CSF assay with a low level of amyloid-beta 42 in the presence of normal cognition, or both. Stage 2 is one of amyloid positivity and evidence of synaptic dysfunction or early neurodegeneration in the presence of normal cognition. Finally, stage 3 entails amyloid positivity with evidence of neurodegeneration in the presence of subtle cognitive decline.
“Using those definitions, and piecing together the numbers from these studies, we constructed a computer model based on US census population projections to [estimate] how many people might be in these different states of disease,” said Dr. Brookmeyer.
In 2017, six million Americans were in one of the clinical disease states (ie, MCI due to Alzheimer’s disease, early clinical Alzheimer’s disease, or late clinical Alzheimer’s disease). Dr. Brookmeyer and his colleagues predicted that that number would grow to 15 million by 2060. Similarly, in 2017, about 47 million Americans were in one of the preclinical Alzheimer’s disease states, including 22 million with amyloidosis, 8.3 million with neurodegeneration alone, and 16.2 million with both. He projects that this number will increase to 75.7 million by 2060.
Prevention Strategies
The team remodeled those numbers in three hypothetical intervention scenarios. Researchers say that a treatment that slows decline by at least 30% would have a meaningful clinical, financial, and societal impact. However, Dr. Brookmeyer modeled treatment scenarios with a greater effect.
A primary prevention method that reduced the annual risk of new amyloidosis by 50% could decrease the prevalence of MCI by about 700,000 in 2060. A secondary prevention strategy that reduced the annual risk progression to MCI by 50% would decrease the prevalence of MCI by more than two million and the prevalence of Alzheimer’s disease by about 3.8 million.
The results were more complicated with a secondary prevention strategy that would reduce annual risk of conversion from MCI to Alzheimer’s disease by 50%. In this scenario, the prevalence of MCI in 2060 would increase by 2.8 million, but the prevalence of Alzheimer’s disease would decrease by 2.5 million. These scenarios developed over different time courses, said the researchers.
“We find that the highly effective primary prevention strategy resulted in the lowest Alzheimer’s disease prevalence by the year 2060. However, [it] was associated with the largest Alzheimer’s disease prevalence in the 15 years immediately after its introduction ... The explanation for this finding is that the full benefits of delaying amyloidosis, in terms of reduced Alzheimer’s disease prevalence, are not realized for many years because of the long lag time between amyloidosis and clinical Alzheimer’s disease. A take-home message is that the full impact on disease burden of primary prevention that targets the early stages of the pathogenesis of Alzheimer’s disease may not be realized for decades.”
Decreasing preclinical conversion to MCI with a secondary prevention strategy would result in the highest Alzheimer’s disease prevalence reduction for most of the period. But the reduction resulting from the primary prevention strategy would surpass it by 2054.
The intervention targeting conversion from MCI to Alzheimer’s disease would reduce Alzheimer’s disease prevalence the quickest, with a slight decrease in the first three years after introduction. “The explanation for this finding is that MCI is proximate to clinical Alzheimer’s disease diagnosis, and thus the impact of delaying progression of MCI will be seen relatively quickly on Alzheimer’s disease prevalence, compared to interventions that delay onset of amyloidosis or MCI.
“By focusing attention on a concerning reality—that tens of millions of American adults may face the possibility of dementia due to Alzheimer’s disease—the results reported in this new article, if confirmed, illustrate and greatly amplify the need for more research to develop effective treatments and proven prevention strategies for Alzheimer’s disease,” said Dr. Brookmeyer. “This is especially true as we get better at early detection and are able to more accurately identify people who have the early brain changes associated with Alzheimer’s disease and other dementias.”
Dr. Brookmeyer reported receiving fees from Takeda for serving as a member of a data safety monitoring board.
—Michele G. Sullivan
Suggested Reading
Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 2017 Nov 29 [Epub ahead of print].
Derby CA, Katz MJ, Lipton RB, Hall CB. Trends in dementia incidence in a birth cohort analysis of the Einstein Aging Study. JAMA Neurol. 2017;74(11):1345-1351.
Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
Vos SJ, Verhey F, Frölich L, et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain. 2015;138(Pt 5):1327-1338.
A treatment that would reduce the risk of developing amyloid plaques in the brain by 50% could save more than four million Americans from mild cognitive impairment (MCI) and 2.5 million Americans from Alzheimer’s disease by 2060, according to a study published online ahead of print November 29 in Alzheimer’s & Dementia.
The conclusion that modestly effective preventive therapy could greatly improve the Alzheimer’s disease outlook is especially important, given another finding in a new mathematical modeling study by Ron Brookmeyer, PhD, a biostatistician at the University of California, Los Angeles, and colleagues. They assert that 47 million cognitively normal people in the United States may have brain amyloidosis, the physical finding used to define preclinical Alzheimer’s disease.
This study is the first to quantify the number of cognitively healthy US residents who could eventually experience cognitive changes that put them at risk of developing Alzheimer’s dementia, according to a statement from the Alzheimer’s Association.
Model Predicted Increase in Preclinical Alzheimer’s Disease
“This is the first major attempt to forecast these proposed preclinical Alzheimer’s disease and [MCI] due to Alzheimer’s disease numbers. If confirmed, these data [will] provide essential information for public health planning and for informing and guiding the public and private investment in Alzheimer’s and dementia research,” said Dr. Brookmeyer. “We need more research to confirm the findings from this model, and more Alzheimer’s disease and dementia research that includes diverse populations.”
“I want to emphasize that of the 47 million [people] with these Alzheimer’s brain changes, but without clinical symptoms, most will not progress to clinical disease during their lifetimes. In fact, perhaps only one in seven will progress to full-blown dementia.” Nevertheless, the numbers represent a reality that must be confronted and managed proactively, said Dr. Brookmeyer.
The results may sound alarming, he said, “but I have every confidence in them. And they are important because they allow us to understand how many people could potentially benefit from treatment, at what point on the disease continuum it would be useful to implement treatment, and how those treatments could impact public health.”
Studies Provided Rates of Transition Between Disease States
To create predictive models, Dr. Brookmeyer used data from two prospective longitudinal cohort studies: the Mayo Clinic Study of Aging and a study conducted by Stephanie J. Vos, PhD, a postdoctoral researcher at Maastricht University in the Netherlands.
The Mayo Clinic study followed 1,541 cognitively normal older adults and provided data on the rate of transition from normal cognition to MCI. The study by Dr. Vos and her associates followed 353 patients with MCI and brain amyloid and 222 patients with late MCI as they progressed. It is the largest prospective study of progression from MCI to Alzheimer’s disease that also contains data on baseline neurodegeneration and amyloid burden.
“These studies gave us the rates of transition from one state to another,” said Dr. Brookmeyer. “For example, the Mayo Clinic study gave us rates of transition from normal [health] to amyloidosis: 3% of normal 60-year-olds will convert to this state every year.”
Dr. Vos’s study determined rates of progression from MCI to Alzheimer’s dementia, given two preclinical states: asymptomatic amyloid brain plaques alone, or plaques with evidence of neurodegeneration and cognitive signs, said Dr. Brookmeyer. Both of these transitional states were first defined in 2011 in a joint paper by the Alzheimer’s Association and the National Institute on Aging. While acknowledging that the root causes of Alzheimer’s disease are unknown, the paper hypothesized a pathophysiologic time line beginning with a three-stage preclinical phase.
Asymptomatic cerebral amyloidosis is the first stage. It entails amyloid-positive PET brain imaging with an amyloid-binding ligand, a CSF assay with a low level of amyloid-beta 42 in the presence of normal cognition, or both. Stage 2 is one of amyloid positivity and evidence of synaptic dysfunction or early neurodegeneration in the presence of normal cognition. Finally, stage 3 entails amyloid positivity with evidence of neurodegeneration in the presence of subtle cognitive decline.
“Using those definitions, and piecing together the numbers from these studies, we constructed a computer model based on US census population projections to [estimate] how many people might be in these different states of disease,” said Dr. Brookmeyer.
In 2017, six million Americans were in one of the clinical disease states (ie, MCI due to Alzheimer’s disease, early clinical Alzheimer’s disease, or late clinical Alzheimer’s disease). Dr. Brookmeyer and his colleagues predicted that that number would grow to 15 million by 2060. Similarly, in 2017, about 47 million Americans were in one of the preclinical Alzheimer’s disease states, including 22 million with amyloidosis, 8.3 million with neurodegeneration alone, and 16.2 million with both. He projects that this number will increase to 75.7 million by 2060.
Prevention Strategies
The team remodeled those numbers in three hypothetical intervention scenarios. Researchers say that a treatment that slows decline by at least 30% would have a meaningful clinical, financial, and societal impact. However, Dr. Brookmeyer modeled treatment scenarios with a greater effect.
A primary prevention method that reduced the annual risk of new amyloidosis by 50% could decrease the prevalence of MCI by about 700,000 in 2060. A secondary prevention strategy that reduced the annual risk progression to MCI by 50% would decrease the prevalence of MCI by more than two million and the prevalence of Alzheimer’s disease by about 3.8 million.
The results were more complicated with a secondary prevention strategy that would reduce annual risk of conversion from MCI to Alzheimer’s disease by 50%. In this scenario, the prevalence of MCI in 2060 would increase by 2.8 million, but the prevalence of Alzheimer’s disease would decrease by 2.5 million. These scenarios developed over different time courses, said the researchers.
“We find that the highly effective primary prevention strategy resulted in the lowest Alzheimer’s disease prevalence by the year 2060. However, [it] was associated with the largest Alzheimer’s disease prevalence in the 15 years immediately after its introduction ... The explanation for this finding is that the full benefits of delaying amyloidosis, in terms of reduced Alzheimer’s disease prevalence, are not realized for many years because of the long lag time between amyloidosis and clinical Alzheimer’s disease. A take-home message is that the full impact on disease burden of primary prevention that targets the early stages of the pathogenesis of Alzheimer’s disease may not be realized for decades.”
Decreasing preclinical conversion to MCI with a secondary prevention strategy would result in the highest Alzheimer’s disease prevalence reduction for most of the period. But the reduction resulting from the primary prevention strategy would surpass it by 2054.
The intervention targeting conversion from MCI to Alzheimer’s disease would reduce Alzheimer’s disease prevalence the quickest, with a slight decrease in the first three years after introduction. “The explanation for this finding is that MCI is proximate to clinical Alzheimer’s disease diagnosis, and thus the impact of delaying progression of MCI will be seen relatively quickly on Alzheimer’s disease prevalence, compared to interventions that delay onset of amyloidosis or MCI.
“By focusing attention on a concerning reality—that tens of millions of American adults may face the possibility of dementia due to Alzheimer’s disease—the results reported in this new article, if confirmed, illustrate and greatly amplify the need for more research to develop effective treatments and proven prevention strategies for Alzheimer’s disease,” said Dr. Brookmeyer. “This is especially true as we get better at early detection and are able to more accurately identify people who have the early brain changes associated with Alzheimer’s disease and other dementias.”
Dr. Brookmeyer reported receiving fees from Takeda for serving as a member of a data safety monitoring board.
—Michele G. Sullivan
Suggested Reading
Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 2017 Nov 29 [Epub ahead of print].
Derby CA, Katz MJ, Lipton RB, Hall CB. Trends in dementia incidence in a birth cohort analysis of the Einstein Aging Study. JAMA Neurol. 2017;74(11):1345-1351.
Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
Vos SJ, Verhey F, Frölich L, et al. Prevalence and prognosis of Alzheimer’s disease at the mild cognitive impairment stage. Brain. 2015;138(Pt 5):1327-1338.
Gastrointestinal complications are a key risk factor for death after AEF repair
Aortoenteric fistulas (AEFs) are an uncommon but lethal form of aortic graft infection with morbidity and mortality rates reported in the literature to range from 14% to 75%. Over a 20-year period, researchers found that nearly half of their patients undergoing repair of their aortoenteric fistulas died within 60 days. The presence of gastrointestinal complications increased the risk of mortality more than threefold, according to the results of a single-center retrospective review of consecutive AEF repairs.
The researchers assessed 50 patients who presented with AEF and had repair during 1995-2014. Sixty percent of the patients were men, and the overall median age was 70 years. The median follow-up for the entire cohort was 14 months. The duodenum was the most common location of the enteric defect, found in 80% of the infections. Overall, 23 patients (46%) died by day 60, according to the report published in the July Journal of the American College of Surgeons.
Univariate analysis showed that advanced age, chronic renal insufficiency, any complications, and GI complications in particular (occurring in 26% of patients) were all associated with an increase in overall mortality (P less than .05). But upon multivariate analysis, gastrointestinal complications (hazard ratio, 3.23; P = .015) and advanced age (HR, 1.07; P = .01) were the only independent predictors of mortality, Atish Chopra, MD, of the division of vascular surgery, Oregon Health & Science University, Portland, and his colleagues wrote.
The institution changed operative procedures in 2007, based upon an earlier assessment of the importance of GI complications performed by the researchers, with greater emphasis placed on ensuring a viable GI reconstruction, and early intervention for mesenteric ischemia. In addition, they surmised that, after 2007, there was improved adherence to achieving wide debridement of nonviable and infected tissue, and to creating a tension-free anastomosis to healthy tissue edges while optimizing nutritional, medical, and antibiotic therapy, according to the researchers.
“When comparing the patients undergoing repair before 2007 with those compared after 2007 [38 and 12 AEF patients, respectively], we found that in-hospitality mortality decreased from 37% to 8% (P = .08), 60-day mortality decreased from 53% to 8% (P less than .01), and mortality at last follow-up decreased from 55% to 17% (P = .02). Dr. Chopra and his colleagues also found that mortality after GI complications decreased from 90% for those operated on before 2007 to 33% in those operated on after 2007 (P = .01).
“Methods to decrease and improvement management of GI complications may prove most effective at improving mortality rates for this lethal pathology,” the researchers concluded.
The authors reported that they had nothing to disclose.
SOURCE: Chopra A et al. J Am Coll Surg 2017 Jul;225(1):9-18.
While secondary aortoenteric fistulas fortunately are a rare occurrence after open aortic repair (less than 1%), the reported results of treatment are disappointing (up to 75% mortality). The authors have demonstrated significant improvement in overall mortality in their series by paying more attention to the GI component of the procedure and postoperative management of the patient.
When we published our institutions experience with AEFs from 1991 to 2004, the study included 29 patients with one-third of patients presenting in shock. Extra-anatomic repair was performed in 86% (68% single stage). Mortality was 24%, with shock, blood transfusions, and suprarenal clamping associated with a worse outcome. Our 5-year survival was 61% and freedom from recurrent infection or amputation was 86% and 88% respectively. While our experience favors an extra-anatomic reconstruction, there has been increased utilization of in-situ repair in recent years. The current authors have extensive experience with the Neoaortoiliac System (NAIS) procedure and have had minimal complications associated with the extensive vein harvest necessary for NAIS; however, this is not universal. Wound complications, compartment syndrome, and chronic venous insufficiency are not uncommon complications. Improved results using cryopreserved aortoiliac allografts (CAA) were reported by The Vascular Low-Frequency Disease Consortium. In a 2014 publication on 220 patients receiving CAA for aortic reconstruction for aortic graft infection, freedom from graft-related complications, graft explant, and limb loss was 80%, 88%, and 97%, respectively, at 5 years. This compares favorably with extra-anatomic reconstruction and other techniques. In suprarenal reconstructions, allografts have been favored since the natural branches can be used to simplify multiple vessel reconstruction. In cases of low-grade infection (Staphylococcus epidermidis), excellent results have been demonstrated with wide debridement, in-situ replacement with rifampin-bonded grafts and omental coverage.
AEF can also occur after endovascular abdominal aortic aneurysm (AAA) repair and a management strategy for removing the endograft should be considered in the planning of these cases. The authors describe their technique, which involves constraining the endograft using an umbilical tape. Another technique that I prefer uses a 20-cc syringe with the tip cut off. The endograft is constrained by advancing the syringe proximally over the endograft. The syringe collapses the device and can be used to constrain the proximal fixation stents of a suprarenal graft, simplifying removal of the stent graft. Infected EVAR will likely become more common as an etiology as we continue to expand EVAR utilization for AAA repair. Another Low-Frequency Consortium paper reviewed the treatment and outcome of EVAR infection. In that report of 206 infected EVAR (endovascular aneurysm repair) and TEVAR (thoracic endovascular aortic repair) patients, 90% had in situ replacement with a 30-day mortality of 11% and morbidity of 35%.
As in previous reports on AEF, the authors highlight the importance of high clinical suspicion in making an expeditious diagnosis. Many imaging modalities can be used, but often operative exploration is required for a definitive diagnosis. Complete graft excision and wide debridement are critical to minimize the risk of recurrent infection. Optimal revascularization techniques should be determined by the experience of the operator, current experience demonstrating nearly equivalent outcomes with extra-anatomic and in situ replacement. Careful GI reconstruction and post-operative nutrition, culture-specific antibiotics, and ICU care are likely more important than the mode of reconstruction. Lifelong surveillance to detect recurrent infections also is recommended.
Murray L. Shames, MD , is professor of surgery and radiology and chief of the division of vascular surgery at the University of South Florida, Tampa, and director of the Tampa General Hospital aortic program.
While secondary aortoenteric fistulas fortunately are a rare occurrence after open aortic repair (less than 1%), the reported results of treatment are disappointing (up to 75% mortality). The authors have demonstrated significant improvement in overall mortality in their series by paying more attention to the GI component of the procedure and postoperative management of the patient.
When we published our institutions experience with AEFs from 1991 to 2004, the study included 29 patients with one-third of patients presenting in shock. Extra-anatomic repair was performed in 86% (68% single stage). Mortality was 24%, with shock, blood transfusions, and suprarenal clamping associated with a worse outcome. Our 5-year survival was 61% and freedom from recurrent infection or amputation was 86% and 88% respectively. While our experience favors an extra-anatomic reconstruction, there has been increased utilization of in-situ repair in recent years. The current authors have extensive experience with the Neoaortoiliac System (NAIS) procedure and have had minimal complications associated with the extensive vein harvest necessary for NAIS; however, this is not universal. Wound complications, compartment syndrome, and chronic venous insufficiency are not uncommon complications. Improved results using cryopreserved aortoiliac allografts (CAA) were reported by The Vascular Low-Frequency Disease Consortium. In a 2014 publication on 220 patients receiving CAA for aortic reconstruction for aortic graft infection, freedom from graft-related complications, graft explant, and limb loss was 80%, 88%, and 97%, respectively, at 5 years. This compares favorably with extra-anatomic reconstruction and other techniques. In suprarenal reconstructions, allografts have been favored since the natural branches can be used to simplify multiple vessel reconstruction. In cases of low-grade infection (Staphylococcus epidermidis), excellent results have been demonstrated with wide debridement, in-situ replacement with rifampin-bonded grafts and omental coverage.
AEF can also occur after endovascular abdominal aortic aneurysm (AAA) repair and a management strategy for removing the endograft should be considered in the planning of these cases. The authors describe their technique, which involves constraining the endograft using an umbilical tape. Another technique that I prefer uses a 20-cc syringe with the tip cut off. The endograft is constrained by advancing the syringe proximally over the endograft. The syringe collapses the device and can be used to constrain the proximal fixation stents of a suprarenal graft, simplifying removal of the stent graft. Infected EVAR will likely become more common as an etiology as we continue to expand EVAR utilization for AAA repair. Another Low-Frequency Consortium paper reviewed the treatment and outcome of EVAR infection. In that report of 206 infected EVAR (endovascular aneurysm repair) and TEVAR (thoracic endovascular aortic repair) patients, 90% had in situ replacement with a 30-day mortality of 11% and morbidity of 35%.
As in previous reports on AEF, the authors highlight the importance of high clinical suspicion in making an expeditious diagnosis. Many imaging modalities can be used, but often operative exploration is required for a definitive diagnosis. Complete graft excision and wide debridement are critical to minimize the risk of recurrent infection. Optimal revascularization techniques should be determined by the experience of the operator, current experience demonstrating nearly equivalent outcomes with extra-anatomic and in situ replacement. Careful GI reconstruction and post-operative nutrition, culture-specific antibiotics, and ICU care are likely more important than the mode of reconstruction. Lifelong surveillance to detect recurrent infections also is recommended.
Murray L. Shames, MD , is professor of surgery and radiology and chief of the division of vascular surgery at the University of South Florida, Tampa, and director of the Tampa General Hospital aortic program.
While secondary aortoenteric fistulas fortunately are a rare occurrence after open aortic repair (less than 1%), the reported results of treatment are disappointing (up to 75% mortality). The authors have demonstrated significant improvement in overall mortality in their series by paying more attention to the GI component of the procedure and postoperative management of the patient.
When we published our institutions experience with AEFs from 1991 to 2004, the study included 29 patients with one-third of patients presenting in shock. Extra-anatomic repair was performed in 86% (68% single stage). Mortality was 24%, with shock, blood transfusions, and suprarenal clamping associated with a worse outcome. Our 5-year survival was 61% and freedom from recurrent infection or amputation was 86% and 88% respectively. While our experience favors an extra-anatomic reconstruction, there has been increased utilization of in-situ repair in recent years. The current authors have extensive experience with the Neoaortoiliac System (NAIS) procedure and have had minimal complications associated with the extensive vein harvest necessary for NAIS; however, this is not universal. Wound complications, compartment syndrome, and chronic venous insufficiency are not uncommon complications. Improved results using cryopreserved aortoiliac allografts (CAA) were reported by The Vascular Low-Frequency Disease Consortium. In a 2014 publication on 220 patients receiving CAA for aortic reconstruction for aortic graft infection, freedom from graft-related complications, graft explant, and limb loss was 80%, 88%, and 97%, respectively, at 5 years. This compares favorably with extra-anatomic reconstruction and other techniques. In suprarenal reconstructions, allografts have been favored since the natural branches can be used to simplify multiple vessel reconstruction. In cases of low-grade infection (Staphylococcus epidermidis), excellent results have been demonstrated with wide debridement, in-situ replacement with rifampin-bonded grafts and omental coverage.
AEF can also occur after endovascular abdominal aortic aneurysm (AAA) repair and a management strategy for removing the endograft should be considered in the planning of these cases. The authors describe their technique, which involves constraining the endograft using an umbilical tape. Another technique that I prefer uses a 20-cc syringe with the tip cut off. The endograft is constrained by advancing the syringe proximally over the endograft. The syringe collapses the device and can be used to constrain the proximal fixation stents of a suprarenal graft, simplifying removal of the stent graft. Infected EVAR will likely become more common as an etiology as we continue to expand EVAR utilization for AAA repair. Another Low-Frequency Consortium paper reviewed the treatment and outcome of EVAR infection. In that report of 206 infected EVAR (endovascular aneurysm repair) and TEVAR (thoracic endovascular aortic repair) patients, 90% had in situ replacement with a 30-day mortality of 11% and morbidity of 35%.
As in previous reports on AEF, the authors highlight the importance of high clinical suspicion in making an expeditious diagnosis. Many imaging modalities can be used, but often operative exploration is required for a definitive diagnosis. Complete graft excision and wide debridement are critical to minimize the risk of recurrent infection. Optimal revascularization techniques should be determined by the experience of the operator, current experience demonstrating nearly equivalent outcomes with extra-anatomic and in situ replacement. Careful GI reconstruction and post-operative nutrition, culture-specific antibiotics, and ICU care are likely more important than the mode of reconstruction. Lifelong surveillance to detect recurrent infections also is recommended.
Murray L. Shames, MD , is professor of surgery and radiology and chief of the division of vascular surgery at the University of South Florida, Tampa, and director of the Tampa General Hospital aortic program.
Aortoenteric fistulas (AEFs) are an uncommon but lethal form of aortic graft infection with morbidity and mortality rates reported in the literature to range from 14% to 75%. Over a 20-year period, researchers found that nearly half of their patients undergoing repair of their aortoenteric fistulas died within 60 days. The presence of gastrointestinal complications increased the risk of mortality more than threefold, according to the results of a single-center retrospective review of consecutive AEF repairs.
The researchers assessed 50 patients who presented with AEF and had repair during 1995-2014. Sixty percent of the patients were men, and the overall median age was 70 years. The median follow-up for the entire cohort was 14 months. The duodenum was the most common location of the enteric defect, found in 80% of the infections. Overall, 23 patients (46%) died by day 60, according to the report published in the July Journal of the American College of Surgeons.
Univariate analysis showed that advanced age, chronic renal insufficiency, any complications, and GI complications in particular (occurring in 26% of patients) were all associated with an increase in overall mortality (P less than .05). But upon multivariate analysis, gastrointestinal complications (hazard ratio, 3.23; P = .015) and advanced age (HR, 1.07; P = .01) were the only independent predictors of mortality, Atish Chopra, MD, of the division of vascular surgery, Oregon Health & Science University, Portland, and his colleagues wrote.
The institution changed operative procedures in 2007, based upon an earlier assessment of the importance of GI complications performed by the researchers, with greater emphasis placed on ensuring a viable GI reconstruction, and early intervention for mesenteric ischemia. In addition, they surmised that, after 2007, there was improved adherence to achieving wide debridement of nonviable and infected tissue, and to creating a tension-free anastomosis to healthy tissue edges while optimizing nutritional, medical, and antibiotic therapy, according to the researchers.
“When comparing the patients undergoing repair before 2007 with those compared after 2007 [38 and 12 AEF patients, respectively], we found that in-hospitality mortality decreased from 37% to 8% (P = .08), 60-day mortality decreased from 53% to 8% (P less than .01), and mortality at last follow-up decreased from 55% to 17% (P = .02). Dr. Chopra and his colleagues also found that mortality after GI complications decreased from 90% for those operated on before 2007 to 33% in those operated on after 2007 (P = .01).
“Methods to decrease and improvement management of GI complications may prove most effective at improving mortality rates for this lethal pathology,” the researchers concluded.
The authors reported that they had nothing to disclose.
SOURCE: Chopra A et al. J Am Coll Surg 2017 Jul;225(1):9-18.
Aortoenteric fistulas (AEFs) are an uncommon but lethal form of aortic graft infection with morbidity and mortality rates reported in the literature to range from 14% to 75%. Over a 20-year period, researchers found that nearly half of their patients undergoing repair of their aortoenteric fistulas died within 60 days. The presence of gastrointestinal complications increased the risk of mortality more than threefold, according to the results of a single-center retrospective review of consecutive AEF repairs.
The researchers assessed 50 patients who presented with AEF and had repair during 1995-2014. Sixty percent of the patients were men, and the overall median age was 70 years. The median follow-up for the entire cohort was 14 months. The duodenum was the most common location of the enteric defect, found in 80% of the infections. Overall, 23 patients (46%) died by day 60, according to the report published in the July Journal of the American College of Surgeons.
Univariate analysis showed that advanced age, chronic renal insufficiency, any complications, and GI complications in particular (occurring in 26% of patients) were all associated with an increase in overall mortality (P less than .05). But upon multivariate analysis, gastrointestinal complications (hazard ratio, 3.23; P = .015) and advanced age (HR, 1.07; P = .01) were the only independent predictors of mortality, Atish Chopra, MD, of the division of vascular surgery, Oregon Health & Science University, Portland, and his colleagues wrote.
The institution changed operative procedures in 2007, based upon an earlier assessment of the importance of GI complications performed by the researchers, with greater emphasis placed on ensuring a viable GI reconstruction, and early intervention for mesenteric ischemia. In addition, they surmised that, after 2007, there was improved adherence to achieving wide debridement of nonviable and infected tissue, and to creating a tension-free anastomosis to healthy tissue edges while optimizing nutritional, medical, and antibiotic therapy, according to the researchers.
“When comparing the patients undergoing repair before 2007 with those compared after 2007 [38 and 12 AEF patients, respectively], we found that in-hospitality mortality decreased from 37% to 8% (P = .08), 60-day mortality decreased from 53% to 8% (P less than .01), and mortality at last follow-up decreased from 55% to 17% (P = .02). Dr. Chopra and his colleagues also found that mortality after GI complications decreased from 90% for those operated on before 2007 to 33% in those operated on after 2007 (P = .01).
“Methods to decrease and improvement management of GI complications may prove most effective at improving mortality rates for this lethal pathology,” the researchers concluded.
The authors reported that they had nothing to disclose.
SOURCE: Chopra A et al. J Am Coll Surg 2017 Jul;225(1):9-18.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: Nearly half of patients undergoing aortoenteric fistula repair died within 60 days.
Major finding: The presence of gastrointestinal complications increased the risk of mortality for AEF repair more than threefold.
Study details: A single center, retrospective review of 50 consecutive patients with AEF repairs during 1995-2014.
Disclosures: The authors reported that they had nothing to disclose.
Source: Chopra A et al. J Am Coll Surg. 2017 Jul;225:9-18.
Trying to Keep Pace With 3-D Technology
Three-dimensional printing has revolutionized the drug and device market and already has changed the lives of millions of patients. The FDA has reviewed more than 100 devices now on the market that were manufactured on 3-D printers, including knee replacements and implants “designed to fit like a missing puzzle piece into a patient’s skull for facial reconstruction,” says FDA Commissioner Scott Gottlieb, MD. The FDA also has approved the first drug produced on a 3-D printer. It has a more porous matrix than that of the drug manufactured in the traditional way, which allows it to dissolve more rapidly. But the technology advances have been moving so fast that they have threatened to outpace safeguards.
Now the FDA is preparing for a “significant wave” of new technologies, Gottlieb says, such as 3-D-printer skin cells for burn victims and is working to provide a regulatory pathway that keeps pace with those advances, helping to keep them safe and effective. To that end, the FDA has issued new guidance to help advise manufacturers on technical aspects of 3-D printing. And as more hospitals and academic centers use their 3-D printers for innovations to use in clinical studies, the FDA also is establishing a regulatory framework for applying existing laws to nontraditional manufacturers.
The Center for Drug Evaluation and Research state-of-the-art 3-D printing facility allows FDA scientists to conduct research to determine how 3-D printing of drugs, for instance, affects drug components. The Center for Devices and Radiological Health also has a 3-D printing facility to investigate the effect of design changes on safety and performance.
Gottlieb calls the technical guidance leapfrog guidance because it helps bridge current policy with innovation. It is only intended, he says, to provide “initial thoughts on an emerging technology with the understanding that our recommendations are likely to evolve as the technology develops in unexpected ways.”
Three-dimensional printing has revolutionized the drug and device market and already has changed the lives of millions of patients. The FDA has reviewed more than 100 devices now on the market that were manufactured on 3-D printers, including knee replacements and implants “designed to fit like a missing puzzle piece into a patient’s skull for facial reconstruction,” says FDA Commissioner Scott Gottlieb, MD. The FDA also has approved the first drug produced on a 3-D printer. It has a more porous matrix than that of the drug manufactured in the traditional way, which allows it to dissolve more rapidly. But the technology advances have been moving so fast that they have threatened to outpace safeguards.
Now the FDA is preparing for a “significant wave” of new technologies, Gottlieb says, such as 3-D-printer skin cells for burn victims and is working to provide a regulatory pathway that keeps pace with those advances, helping to keep them safe and effective. To that end, the FDA has issued new guidance to help advise manufacturers on technical aspects of 3-D printing. And as more hospitals and academic centers use their 3-D printers for innovations to use in clinical studies, the FDA also is establishing a regulatory framework for applying existing laws to nontraditional manufacturers.
The Center for Drug Evaluation and Research state-of-the-art 3-D printing facility allows FDA scientists to conduct research to determine how 3-D printing of drugs, for instance, affects drug components. The Center for Devices and Radiological Health also has a 3-D printing facility to investigate the effect of design changes on safety and performance.
Gottlieb calls the technical guidance leapfrog guidance because it helps bridge current policy with innovation. It is only intended, he says, to provide “initial thoughts on an emerging technology with the understanding that our recommendations are likely to evolve as the technology develops in unexpected ways.”
Three-dimensional printing has revolutionized the drug and device market and already has changed the lives of millions of patients. The FDA has reviewed more than 100 devices now on the market that were manufactured on 3-D printers, including knee replacements and implants “designed to fit like a missing puzzle piece into a patient’s skull for facial reconstruction,” says FDA Commissioner Scott Gottlieb, MD. The FDA also has approved the first drug produced on a 3-D printer. It has a more porous matrix than that of the drug manufactured in the traditional way, which allows it to dissolve more rapidly. But the technology advances have been moving so fast that they have threatened to outpace safeguards.
Now the FDA is preparing for a “significant wave” of new technologies, Gottlieb says, such as 3-D-printer skin cells for burn victims and is working to provide a regulatory pathway that keeps pace with those advances, helping to keep them safe and effective. To that end, the FDA has issued new guidance to help advise manufacturers on technical aspects of 3-D printing. And as more hospitals and academic centers use their 3-D printers for innovations to use in clinical studies, the FDA also is establishing a regulatory framework for applying existing laws to nontraditional manufacturers.
The Center for Drug Evaluation and Research state-of-the-art 3-D printing facility allows FDA scientists to conduct research to determine how 3-D printing of drugs, for instance, affects drug components. The Center for Devices and Radiological Health also has a 3-D printing facility to investigate the effect of design changes on safety and performance.
Gottlieb calls the technical guidance leapfrog guidance because it helps bridge current policy with innovation. It is only intended, he says, to provide “initial thoughts on an emerging technology with the understanding that our recommendations are likely to evolve as the technology develops in unexpected ways.”
It's A Slow Grow
For more than two years, this 40-year-old African-American woman has had a lesion on her left maxilla. While it does not cause pain or discomfort, its presence is alarming to the patient. The lesion has grown steadily without responding to various topical OTC medications (tolnaftate, clotrimazole, miconazole, 1% hydrocortisone cream), oral anti-yeast medication, and antibiotics (fluconazole, erythromycin).
The patient claims to be in excellent health otherwise. She reports a strong family history of autoimmune disease, including rheumatoid arthritis and lupus.
EXAMINATION
There is an oval, scaly, atrophic patch on the upper left maxilla, just below the left nostril. The lesion measures 1.2 cm, has well-defined margins, and appears darker than the patient’s type V skin.
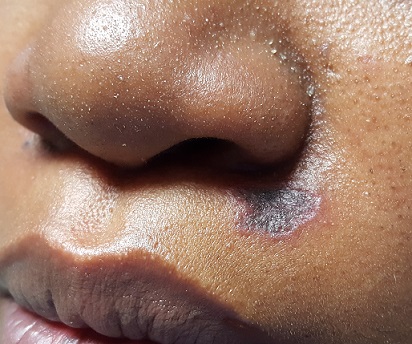
No redness or edema are seen, and no nodes are palpable in the area. Examination of the adjacent oral mucosal surface shows nothing amiss.
Under sterile conditions, and after local anesthesia (1% lidocaine with epinephrine) is administered, a 3-mm punch biopsy is obtained from the center of the lesion. The defect is closed with two interrupted nylon sutures.
What is the diagnosis?
DISCUSSION
The results showed follicular hyperkeratosis, epidermal atrophy, acanthosis with basal-layer degeneration, periadnexal lymphocytic infiltrate, and increased mucin in the dermis. These findings—along with the clinical picture and family history—are consistent with discoid lupus erythematosus (DLE).
This manifestation of an autoimmune process is especially common in younger women of color. DLE primarily affects sun-exposed areas (eg, face, ears, neck) and involves scaly, round-to-oval patches and plaques with atrophic centers and follicular accentuation.
These lesions are frequently misdiagnosed as “fungal.” The differential also includes lichen planus and Jessner lymphocytic infiltrate.
Of the three types of cutaneous lupus erythematosus (discoid, acute, subacute), DLE is the most common. The acute form is defined by a “butterfly rash” across the face, while the subacute form involves multiple round-to-oval scaly lesions in wide photodistribution.
The chronic cutaneous lupus category includes DLE, tumid (characterized by deep, painful, indurated nodules), and panniculitis, which affects large areas of adipose tissue. DLE is, once again, the most common.
DLE can be an entity unto itself or part of a larger diagnosis of systemic lupus erythematosus (SLE). The good news: Only about 15% of patients with DLE progress to SLE. It is debatable whether patients with DLE need a full workup for SLE, since corroborative findings are rarely found.
Emphasis is placed on effective treatment of DLE, which includes the use of class 3 or 4 topical steroids and oral antimalarials (eg, hydroxychloroquine). Even with treatment, DLE can take weeks or months to resolve and often leaves permanent scarring. Ongoing sun protection is necessary to prevent recurrence.
TAKE-HOME LEARNING POINTS
- Discoid lupus erythematosus (DLE) is a common form of chronic cutaneous lupus, which has an autoimmune origin but is triggered by sun exposure.
- DLE typically presents with scaly round-to-oval patches and plaques with atrophic centers and follicular accentuation, often on sun-exposed areas.
- Women of color are at increased risk for lupus, and biopsy is often needed to confirm the diagnosis.
- Only about 15% of DLE patients ever progress to systemic lupus erythematosus (SLE), but DLE can be part of a larger SLE diagnosis.
- Treatment includes topical steroids and oral antimalarials (eg, hydroxychloroquine)—and sun protection is crucial.
For more than two years, this 40-year-old African-American woman has had a lesion on her left maxilla. While it does not cause pain or discomfort, its presence is alarming to the patient. The lesion has grown steadily without responding to various topical OTC medications (tolnaftate, clotrimazole, miconazole, 1% hydrocortisone cream), oral anti-yeast medication, and antibiotics (fluconazole, erythromycin).
The patient claims to be in excellent health otherwise. She reports a strong family history of autoimmune disease, including rheumatoid arthritis and lupus.
EXAMINATION
There is an oval, scaly, atrophic patch on the upper left maxilla, just below the left nostril. The lesion measures 1.2 cm, has well-defined margins, and appears darker than the patient’s type V skin.

No redness or edema are seen, and no nodes are palpable in the area. Examination of the adjacent oral mucosal surface shows nothing amiss.
Under sterile conditions, and after local anesthesia (1% lidocaine with epinephrine) is administered, a 3-mm punch biopsy is obtained from the center of the lesion. The defect is closed with two interrupted nylon sutures.
What is the diagnosis?
DISCUSSION
The results showed follicular hyperkeratosis, epidermal atrophy, acanthosis with basal-layer degeneration, periadnexal lymphocytic infiltrate, and increased mucin in the dermis. These findings—along with the clinical picture and family history—are consistent with discoid lupus erythematosus (DLE).
This manifestation of an autoimmune process is especially common in younger women of color. DLE primarily affects sun-exposed areas (eg, face, ears, neck) and involves scaly, round-to-oval patches and plaques with atrophic centers and follicular accentuation.
These lesions are frequently misdiagnosed as “fungal.” The differential also includes lichen planus and Jessner lymphocytic infiltrate.
Of the three types of cutaneous lupus erythematosus (discoid, acute, subacute), DLE is the most common. The acute form is defined by a “butterfly rash” across the face, while the subacute form involves multiple round-to-oval scaly lesions in wide photodistribution.
The chronic cutaneous lupus category includes DLE, tumid (characterized by deep, painful, indurated nodules), and panniculitis, which affects large areas of adipose tissue. DLE is, once again, the most common.
DLE can be an entity unto itself or part of a larger diagnosis of systemic lupus erythematosus (SLE). The good news: Only about 15% of patients with DLE progress to SLE. It is debatable whether patients with DLE need a full workup for SLE, since corroborative findings are rarely found.
Emphasis is placed on effective treatment of DLE, which includes the use of class 3 or 4 topical steroids and oral antimalarials (eg, hydroxychloroquine). Even with treatment, DLE can take weeks or months to resolve and often leaves permanent scarring. Ongoing sun protection is necessary to prevent recurrence.
TAKE-HOME LEARNING POINTS
- Discoid lupus erythematosus (DLE) is a common form of chronic cutaneous lupus, which has an autoimmune origin but is triggered by sun exposure.
- DLE typically presents with scaly round-to-oval patches and plaques with atrophic centers and follicular accentuation, often on sun-exposed areas.
- Women of color are at increased risk for lupus, and biopsy is often needed to confirm the diagnosis.
- Only about 15% of DLE patients ever progress to systemic lupus erythematosus (SLE), but DLE can be part of a larger SLE diagnosis.
- Treatment includes topical steroids and oral antimalarials (eg, hydroxychloroquine)—and sun protection is crucial.
For more than two years, this 40-year-old African-American woman has had a lesion on her left maxilla. While it does not cause pain or discomfort, its presence is alarming to the patient. The lesion has grown steadily without responding to various topical OTC medications (tolnaftate, clotrimazole, miconazole, 1% hydrocortisone cream), oral anti-yeast medication, and antibiotics (fluconazole, erythromycin).
The patient claims to be in excellent health otherwise. She reports a strong family history of autoimmune disease, including rheumatoid arthritis and lupus.
EXAMINATION
There is an oval, scaly, atrophic patch on the upper left maxilla, just below the left nostril. The lesion measures 1.2 cm, has well-defined margins, and appears darker than the patient’s type V skin.

No redness or edema are seen, and no nodes are palpable in the area. Examination of the adjacent oral mucosal surface shows nothing amiss.
Under sterile conditions, and after local anesthesia (1% lidocaine with epinephrine) is administered, a 3-mm punch biopsy is obtained from the center of the lesion. The defect is closed with two interrupted nylon sutures.
What is the diagnosis?
DISCUSSION
The results showed follicular hyperkeratosis, epidermal atrophy, acanthosis with basal-layer degeneration, periadnexal lymphocytic infiltrate, and increased mucin in the dermis. These findings—along with the clinical picture and family history—are consistent with discoid lupus erythematosus (DLE).
This manifestation of an autoimmune process is especially common in younger women of color. DLE primarily affects sun-exposed areas (eg, face, ears, neck) and involves scaly, round-to-oval patches and plaques with atrophic centers and follicular accentuation.
These lesions are frequently misdiagnosed as “fungal.” The differential also includes lichen planus and Jessner lymphocytic infiltrate.
Of the three types of cutaneous lupus erythematosus (discoid, acute, subacute), DLE is the most common. The acute form is defined by a “butterfly rash” across the face, while the subacute form involves multiple round-to-oval scaly lesions in wide photodistribution.
The chronic cutaneous lupus category includes DLE, tumid (characterized by deep, painful, indurated nodules), and panniculitis, which affects large areas of adipose tissue. DLE is, once again, the most common.
DLE can be an entity unto itself or part of a larger diagnosis of systemic lupus erythematosus (SLE). The good news: Only about 15% of patients with DLE progress to SLE. It is debatable whether patients with DLE need a full workup for SLE, since corroborative findings are rarely found.
Emphasis is placed on effective treatment of DLE, which includes the use of class 3 or 4 topical steroids and oral antimalarials (eg, hydroxychloroquine). Even with treatment, DLE can take weeks or months to resolve and often leaves permanent scarring. Ongoing sun protection is necessary to prevent recurrence.
TAKE-HOME LEARNING POINTS
- Discoid lupus erythematosus (DLE) is a common form of chronic cutaneous lupus, which has an autoimmune origin but is triggered by sun exposure.
- DLE typically presents with scaly round-to-oval patches and plaques with atrophic centers and follicular accentuation, often on sun-exposed areas.
- Women of color are at increased risk for lupus, and biopsy is often needed to confirm the diagnosis.
- Only about 15% of DLE patients ever progress to systemic lupus erythematosus (SLE), but DLE can be part of a larger SLE diagnosis.
- Treatment includes topical steroids and oral antimalarials (eg, hydroxychloroquine)—and sun protection is crucial.
Combo shows promise for elderly/unfit ALL patients
ATLANTA—Results of a phase 2 trial suggest treatment with ponatinib and steroids is feasible for patients with acute lymphoblastic leukemia (ALL) who are elderly and/or unfit for intensive chemotherapy and stem cell transplant.
More than 90% of patients who received this combination had a complete hematologic response at 24 weeks, and the treatment was considered well-tolerated.
“Ponatinib plus steroids is safe and effective in this fragile patient population, which is in urgent need of better therapeutic regimens,” said Giovanni Martinelli, MD, of the University of Bologna in Italy.
Dr Martinelli presented these findings at the 2017 ASH Annual Meeting (abstract 99*).
The trial (GIMEMA LAL 1811) was designed to evaluate whether steroids and ponatinib would be safe and effective in patients with newly diagnosed, Philadelphia chromosome-positive ALL who were older than 60 or ineligible for intensive chemotherapy and stem cell transplant.
The study included 42 such patients. Their median age was 68 (range, 27-85). Nine patients were younger than 60 and unfit.
Sixty-two percent of patients had the p190 fusion transcript, 10% had p210, and 29% had p190/210 transcripts.
Patients received oral ponatinib (45 mg/day) for 8 consecutive 6-week courses. They also received steroids from day -14 to day 29 of course 1.
Patients received intrathecal methotrexate, cytarabine, and dexamethasone every 28 days for central nervous system (CNS) disease prophylaxis. If they were positive for CNS disease at diagnosis, patients received intrathecal therapy twice a week until complete remission.
The median follow-up was 17.2 months.
Results
Thirty-nine patients received steroid pretreatment, and 14 of them had a reduction in circulating blasts of 75% or more before starting ponatinib.
The study’s primary endpoint was complete hematologic response, which occurred in 95.2% of patients at 6 weeks and 90.5% at 24 weeks.
Based on test sensitivity of at least 10,000 ABL molecules, 60.6% of evaluable patients (20/33) had a complete molecular response at 24 weeks.
One patient with relapse had evidence of T315L mutations, which correlates with ponatinib resistance. No other mutations were detected in patients with relapse.
The overall survival rate was 97.6% at 6 months and 87.5% at 1 year.
“The fast and deep reduction of the disease burden in the majority of patients, the ability of ponatinib to prevent the emergence of clones harboring BCR-ABL mutations, and the synthetic lethality with steroids on the BCR-ABL, FLT3, HCK, CDK6, MCL1 pathway most likely explain the therapeutic effectiveness of this regimen,” Dr Martinelli said.
He also said steroids and ponatinib were well-tolerated, with 15 patients continuing treatment at 24 weeks.
Of 75 adverse events (AEs), 36 were related to ponatinib. There were 26 serious AEs, and 13 of them were related to ponatinib.
Serious cardiovascular AEs included arterial disorders (n=3), embolism (n=2), acute coronary syndrome (n=2), acute myocardial infarction (n=1), cardiac failure (n=1), pericardial effusion (n=1), and ischemia (n=1).
Ten deaths were reported. The causes were identified as progression (n=4), cardiovascular disease (n=3), toxicity (n=1), toxicity and progression (n=1), and unknown cause (n=1).
Dr Martinelli reported no disclosures. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Results of a phase 2 trial suggest treatment with ponatinib and steroids is feasible for patients with acute lymphoblastic leukemia (ALL) who are elderly and/or unfit for intensive chemotherapy and stem cell transplant.
More than 90% of patients who received this combination had a complete hematologic response at 24 weeks, and the treatment was considered well-tolerated.
“Ponatinib plus steroids is safe and effective in this fragile patient population, which is in urgent need of better therapeutic regimens,” said Giovanni Martinelli, MD, of the University of Bologna in Italy.
Dr Martinelli presented these findings at the 2017 ASH Annual Meeting (abstract 99*).
The trial (GIMEMA LAL 1811) was designed to evaluate whether steroids and ponatinib would be safe and effective in patients with newly diagnosed, Philadelphia chromosome-positive ALL who were older than 60 or ineligible for intensive chemotherapy and stem cell transplant.
The study included 42 such patients. Their median age was 68 (range, 27-85). Nine patients were younger than 60 and unfit.
Sixty-two percent of patients had the p190 fusion transcript, 10% had p210, and 29% had p190/210 transcripts.
Patients received oral ponatinib (45 mg/day) for 8 consecutive 6-week courses. They also received steroids from day -14 to day 29 of course 1.
Patients received intrathecal methotrexate, cytarabine, and dexamethasone every 28 days for central nervous system (CNS) disease prophylaxis. If they were positive for CNS disease at diagnosis, patients received intrathecal therapy twice a week until complete remission.
The median follow-up was 17.2 months.
Results
Thirty-nine patients received steroid pretreatment, and 14 of them had a reduction in circulating blasts of 75% or more before starting ponatinib.
The study’s primary endpoint was complete hematologic response, which occurred in 95.2% of patients at 6 weeks and 90.5% at 24 weeks.
Based on test sensitivity of at least 10,000 ABL molecules, 60.6% of evaluable patients (20/33) had a complete molecular response at 24 weeks.
One patient with relapse had evidence of T315L mutations, which correlates with ponatinib resistance. No other mutations were detected in patients with relapse.
The overall survival rate was 97.6% at 6 months and 87.5% at 1 year.
“The fast and deep reduction of the disease burden in the majority of patients, the ability of ponatinib to prevent the emergence of clones harboring BCR-ABL mutations, and the synthetic lethality with steroids on the BCR-ABL, FLT3, HCK, CDK6, MCL1 pathway most likely explain the therapeutic effectiveness of this regimen,” Dr Martinelli said.
He also said steroids and ponatinib were well-tolerated, with 15 patients continuing treatment at 24 weeks.
Of 75 adverse events (AEs), 36 were related to ponatinib. There were 26 serious AEs, and 13 of them were related to ponatinib.
Serious cardiovascular AEs included arterial disorders (n=3), embolism (n=2), acute coronary syndrome (n=2), acute myocardial infarction (n=1), cardiac failure (n=1), pericardial effusion (n=1), and ischemia (n=1).
Ten deaths were reported. The causes were identified as progression (n=4), cardiovascular disease (n=3), toxicity (n=1), toxicity and progression (n=1), and unknown cause (n=1).
Dr Martinelli reported no disclosures. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Results of a phase 2 trial suggest treatment with ponatinib and steroids is feasible for patients with acute lymphoblastic leukemia (ALL) who are elderly and/or unfit for intensive chemotherapy and stem cell transplant.
More than 90% of patients who received this combination had a complete hematologic response at 24 weeks, and the treatment was considered well-tolerated.
“Ponatinib plus steroids is safe and effective in this fragile patient population, which is in urgent need of better therapeutic regimens,” said Giovanni Martinelli, MD, of the University of Bologna in Italy.
Dr Martinelli presented these findings at the 2017 ASH Annual Meeting (abstract 99*).
The trial (GIMEMA LAL 1811) was designed to evaluate whether steroids and ponatinib would be safe and effective in patients with newly diagnosed, Philadelphia chromosome-positive ALL who were older than 60 or ineligible for intensive chemotherapy and stem cell transplant.
The study included 42 such patients. Their median age was 68 (range, 27-85). Nine patients were younger than 60 and unfit.
Sixty-two percent of patients had the p190 fusion transcript, 10% had p210, and 29% had p190/210 transcripts.
Patients received oral ponatinib (45 mg/day) for 8 consecutive 6-week courses. They also received steroids from day -14 to day 29 of course 1.
Patients received intrathecal methotrexate, cytarabine, and dexamethasone every 28 days for central nervous system (CNS) disease prophylaxis. If they were positive for CNS disease at diagnosis, patients received intrathecal therapy twice a week until complete remission.
The median follow-up was 17.2 months.
Results
Thirty-nine patients received steroid pretreatment, and 14 of them had a reduction in circulating blasts of 75% or more before starting ponatinib.
The study’s primary endpoint was complete hematologic response, which occurred in 95.2% of patients at 6 weeks and 90.5% at 24 weeks.
Based on test sensitivity of at least 10,000 ABL molecules, 60.6% of evaluable patients (20/33) had a complete molecular response at 24 weeks.
One patient with relapse had evidence of T315L mutations, which correlates with ponatinib resistance. No other mutations were detected in patients with relapse.
The overall survival rate was 97.6% at 6 months and 87.5% at 1 year.
“The fast and deep reduction of the disease burden in the majority of patients, the ability of ponatinib to prevent the emergence of clones harboring BCR-ABL mutations, and the synthetic lethality with steroids on the BCR-ABL, FLT3, HCK, CDK6, MCL1 pathway most likely explain the therapeutic effectiveness of this regimen,” Dr Martinelli said.
He also said steroids and ponatinib were well-tolerated, with 15 patients continuing treatment at 24 weeks.
Of 75 adverse events (AEs), 36 were related to ponatinib. There were 26 serious AEs, and 13 of them were related to ponatinib.
Serious cardiovascular AEs included arterial disorders (n=3), embolism (n=2), acute coronary syndrome (n=2), acute myocardial infarction (n=1), cardiac failure (n=1), pericardial effusion (n=1), and ischemia (n=1).
Ten deaths were reported. The causes were identified as progression (n=4), cardiovascular disease (n=3), toxicity (n=1), toxicity and progression (n=1), and unknown cause (n=1).
Dr Martinelli reported no disclosures. ![]()
*Data in the presentation differ from the abstract.
Obesity has negative impact on HSC compartment
Obesity can affect the long-term health of hematopoietic stem cells (HSCs), according to research published in the Journal of Experimental Medicine.
Conducted largely in models of obese mice, the research showed that obesity causes durable and harmful changes to the HSC compartment.
“Keeping this compartment healthy is essential to human health,” said study author Damien Reynaud, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
“This includes maintaining the diverse pool of [HSCs] needed to produce blood cells the body needs to function properly.”
Although still poorly understood, research is showing that age and environmental stresses can lessen the healthy diversity of cells in the hematopoietic system.
This can include skewing blood cell formation toward myeloid cells and possibly promoting pre-leukemic fates, according to Dr Reynaud and his colleagues.
With the current study, the team found that obesity-related stresses alter the cellular architecture of the HSC compartment and reduce its long-term functional fitness.
Tests in obese mice showed these effects were progressive. And some of the harmful manifestations persisted even after the researchers normalized the animals’ weight through dietary controls.
These alterations of the hematopoietic system appear to be linked to overexpression of the transcription factor Gfi1.
The researchers found that oxidative stresses in the body caused by obesity drive overexpression of Gfi1. When this happens, it produces a lasting alteration of the HSC compartment.
The researchers said their study provides groundwork to investigate how lifestyle choices, such as diet, can durably impact blood formation and may contribute to the development of blood cancers.
The study also raises questions about the use of HSCs isolated from obese transplant donors.
“Little is known about how obesity in marrow donors could affect the quality of the hematopoietic stem cell compartment,” Dr Reynaud explained.
“We want to better understand the molecular alterations in obesity to predict potential risks associated with the therapeutic use of stem cells isolated from obese donors.” ![]()
Obesity can affect the long-term health of hematopoietic stem cells (HSCs), according to research published in the Journal of Experimental Medicine.
Conducted largely in models of obese mice, the research showed that obesity causes durable and harmful changes to the HSC compartment.
“Keeping this compartment healthy is essential to human health,” said study author Damien Reynaud, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
“This includes maintaining the diverse pool of [HSCs] needed to produce blood cells the body needs to function properly.”
Although still poorly understood, research is showing that age and environmental stresses can lessen the healthy diversity of cells in the hematopoietic system.
This can include skewing blood cell formation toward myeloid cells and possibly promoting pre-leukemic fates, according to Dr Reynaud and his colleagues.
With the current study, the team found that obesity-related stresses alter the cellular architecture of the HSC compartment and reduce its long-term functional fitness.
Tests in obese mice showed these effects were progressive. And some of the harmful manifestations persisted even after the researchers normalized the animals’ weight through dietary controls.
These alterations of the hematopoietic system appear to be linked to overexpression of the transcription factor Gfi1.
The researchers found that oxidative stresses in the body caused by obesity drive overexpression of Gfi1. When this happens, it produces a lasting alteration of the HSC compartment.
The researchers said their study provides groundwork to investigate how lifestyle choices, such as diet, can durably impact blood formation and may contribute to the development of blood cancers.
The study also raises questions about the use of HSCs isolated from obese transplant donors.
“Little is known about how obesity in marrow donors could affect the quality of the hematopoietic stem cell compartment,” Dr Reynaud explained.
“We want to better understand the molecular alterations in obesity to predict potential risks associated with the therapeutic use of stem cells isolated from obese donors.” ![]()
Obesity can affect the long-term health of hematopoietic stem cells (HSCs), according to research published in the Journal of Experimental Medicine.
Conducted largely in models of obese mice, the research showed that obesity causes durable and harmful changes to the HSC compartment.
“Keeping this compartment healthy is essential to human health,” said study author Damien Reynaud, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
“This includes maintaining the diverse pool of [HSCs] needed to produce blood cells the body needs to function properly.”
Although still poorly understood, research is showing that age and environmental stresses can lessen the healthy diversity of cells in the hematopoietic system.
This can include skewing blood cell formation toward myeloid cells and possibly promoting pre-leukemic fates, according to Dr Reynaud and his colleagues.
With the current study, the team found that obesity-related stresses alter the cellular architecture of the HSC compartment and reduce its long-term functional fitness.
Tests in obese mice showed these effects were progressive. And some of the harmful manifestations persisted even after the researchers normalized the animals’ weight through dietary controls.
These alterations of the hematopoietic system appear to be linked to overexpression of the transcription factor Gfi1.
The researchers found that oxidative stresses in the body caused by obesity drive overexpression of Gfi1. When this happens, it produces a lasting alteration of the HSC compartment.
The researchers said their study provides groundwork to investigate how lifestyle choices, such as diet, can durably impact blood formation and may contribute to the development of blood cancers.
The study also raises questions about the use of HSCs isolated from obese transplant donors.
“Little is known about how obesity in marrow donors could affect the quality of the hematopoietic stem cell compartment,” Dr Reynaud explained.
“We want to better understand the molecular alterations in obesity to predict potential risks associated with the therapeutic use of stem cells isolated from obese donors.” ![]()
CX-01 receives orphan designation for AML
The US Food and Drug Administration (FDA) has granted orphan drug designation to CX-01 for the treatment of acute myeloid leukemia (AML).
CX-01 is a polysaccharide derived from heparin that is thought to enhance chemotherapy by disrupting the adhesion of leukemia cells in the bone marrow.
CX-01 inhibits the activity of HMGB1, disrupts the CXCL12/CXCR4 axis, and neutralizes the activity of platelet factor 4.
HMGB1 has been implicated in autophagy, a mechanism by which cells withstand the effects of chemotherapy. The CXCL12/CXCR4 axis is thought to be involved in protecting leukemia cells from chemotherapy. And platelet factor 4 inhibits bone marrow recovery after chemotherapy.
Cantex Pharmaceuticals, Inc., is conducting a randomized, phase 2b study to determine whether CX-01 can improve the efficacy of frontline chemotherapy in patients with AML.
This study builds upon results of a pilot study, which were presented at the 2015 ASCO Annual Meeting (abstract 7053).
The study enrolled 12 adults with newly diagnosed AML. They received CX-01 as a 7-day continuous infusion, along with standard induction chemotherapy (cytarabine and idarubicin, 7+3).
Eleven patients (92%), all of whom had de novo AML, had a complete response (CR) with a single induction cycle.
The median time to neutrophil recovery was 23 days, and the median time to platelet recovery was 22 days.
With a median follow-up of 14.2 months, the median event-free survival exceeded 11.6 months, and the median overall survival exceeded 13.6 months.
No adverse events related to CX-01 were reported.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to CX-01 for the treatment of acute myeloid leukemia (AML).
CX-01 is a polysaccharide derived from heparin that is thought to enhance chemotherapy by disrupting the adhesion of leukemia cells in the bone marrow.
CX-01 inhibits the activity of HMGB1, disrupts the CXCL12/CXCR4 axis, and neutralizes the activity of platelet factor 4.
HMGB1 has been implicated in autophagy, a mechanism by which cells withstand the effects of chemotherapy. The CXCL12/CXCR4 axis is thought to be involved in protecting leukemia cells from chemotherapy. And platelet factor 4 inhibits bone marrow recovery after chemotherapy.
Cantex Pharmaceuticals, Inc., is conducting a randomized, phase 2b study to determine whether CX-01 can improve the efficacy of frontline chemotherapy in patients with AML.
This study builds upon results of a pilot study, which were presented at the 2015 ASCO Annual Meeting (abstract 7053).
The study enrolled 12 adults with newly diagnosed AML. They received CX-01 as a 7-day continuous infusion, along with standard induction chemotherapy (cytarabine and idarubicin, 7+3).
Eleven patients (92%), all of whom had de novo AML, had a complete response (CR) with a single induction cycle.
The median time to neutrophil recovery was 23 days, and the median time to platelet recovery was 22 days.
With a median follow-up of 14.2 months, the median event-free survival exceeded 11.6 months, and the median overall survival exceeded 13.6 months.
No adverse events related to CX-01 were reported.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to CX-01 for the treatment of acute myeloid leukemia (AML).
CX-01 is a polysaccharide derived from heparin that is thought to enhance chemotherapy by disrupting the adhesion of leukemia cells in the bone marrow.
CX-01 inhibits the activity of HMGB1, disrupts the CXCL12/CXCR4 axis, and neutralizes the activity of platelet factor 4.
HMGB1 has been implicated in autophagy, a mechanism by which cells withstand the effects of chemotherapy. The CXCL12/CXCR4 axis is thought to be involved in protecting leukemia cells from chemotherapy. And platelet factor 4 inhibits bone marrow recovery after chemotherapy.
Cantex Pharmaceuticals, Inc., is conducting a randomized, phase 2b study to determine whether CX-01 can improve the efficacy of frontline chemotherapy in patients with AML.
This study builds upon results of a pilot study, which were presented at the 2015 ASCO Annual Meeting (abstract 7053).
The study enrolled 12 adults with newly diagnosed AML. They received CX-01 as a 7-day continuous infusion, along with standard induction chemotherapy (cytarabine and idarubicin, 7+3).
Eleven patients (92%), all of whom had de novo AML, had a complete response (CR) with a single induction cycle.
The median time to neutrophil recovery was 23 days, and the median time to platelet recovery was 22 days.
With a median follow-up of 14.2 months, the median event-free survival exceeded 11.6 months, and the median overall survival exceeded 13.6 months.
No adverse events related to CX-01 were reported.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
Analysis: Hospital pay for performance not significantly improving Medicare outcomes
for Medicare beneficiaries, according to an analysis of Medicare claims data.
“No evidence that hospitals [that were] operating under pay for performance programs for more than a decade had better process scores or lower mortality than other hospitals was found,” Igna Bonfrer, PhD, of Erasmus University, Rotterdam, the Netherlands, and colleagues wrote in a study published Jan. 4, 2018, in BMJ.
Researchers looked at Medicare claims data from nearly 1.4 million patients aged 65 years and older across 1,189 hospitals. That total included 214 hospitals that were early adopters of pay for performance (PFP) programs, including the Hospital Quality Incentive Demonstration (HQID) and the current Hospital Value-Based Purchasing (HVBP) program, and 975 hospitals that adopted the programs at a later date. The study authors examined clinical process scores and 30-day mortality rates from 2003 to 2013.
Hospitals that were early adopters of a PFP program typically started from a higher baseline process measure score (91.5), compared with late adopters (89.9).
However, improvements among the early adopters “were smaller during the HQID period, although early adopters continued to perform at a slightly higher level than the late adopters during the pre-HVBP period,” the researchers explained. “Over the HVBP period, early and late adopters no longer differed in their clinical process scores.”
Indeed, a ceiling was ultimately reached, with early and late adopters approaching the same level (98.5 vs. 98.2).
For the 30-day mortality rates, both groups “started from a similar baseline (14.9% and 14.8% for the early and late adopters in the fourth quarter of 2003) and ended at the same rate of 9.9% for both groups in the fourth quarter of 2013,” Dr. Bonfrer and colleagues wrote.
The researchers suggested that the programs did not yield better results because of small financial incentives, coupled with program complexities that made it “difficult for hospitals to meaningfully engage in the program.” They also suggested that having to wait until year end to receive any financial incentives could have limited the impact.
“We found that hospitals that have been under financial incentives for more than a decade have not been able to reduce patient mortality more than late adopters, which had only been under financial incentives for less than 3 years,” the researchers concluded. “Given its cost, policymakers in the [United States] should consider one of two things: revise the current program or potentially end it.”
The changes suggested include increasing financial incentives and focusing on process measures that matter most to patients (mortality, patient experience, and functional status), rather than the current measure set that is larger and more difficult to track.
The researchers did not report any financial conflicts of interest.
SOURCE: Dr. Igna Bonfrer et al. BMJ 2018;360:j5622.
for Medicare beneficiaries, according to an analysis of Medicare claims data.
“No evidence that hospitals [that were] operating under pay for performance programs for more than a decade had better process scores or lower mortality than other hospitals was found,” Igna Bonfrer, PhD, of Erasmus University, Rotterdam, the Netherlands, and colleagues wrote in a study published Jan. 4, 2018, in BMJ.
Researchers looked at Medicare claims data from nearly 1.4 million patients aged 65 years and older across 1,189 hospitals. That total included 214 hospitals that were early adopters of pay for performance (PFP) programs, including the Hospital Quality Incentive Demonstration (HQID) and the current Hospital Value-Based Purchasing (HVBP) program, and 975 hospitals that adopted the programs at a later date. The study authors examined clinical process scores and 30-day mortality rates from 2003 to 2013.
Hospitals that were early adopters of a PFP program typically started from a higher baseline process measure score (91.5), compared with late adopters (89.9).
However, improvements among the early adopters “were smaller during the HQID period, although early adopters continued to perform at a slightly higher level than the late adopters during the pre-HVBP period,” the researchers explained. “Over the HVBP period, early and late adopters no longer differed in their clinical process scores.”
Indeed, a ceiling was ultimately reached, with early and late adopters approaching the same level (98.5 vs. 98.2).
For the 30-day mortality rates, both groups “started from a similar baseline (14.9% and 14.8% for the early and late adopters in the fourth quarter of 2003) and ended at the same rate of 9.9% for both groups in the fourth quarter of 2013,” Dr. Bonfrer and colleagues wrote.
The researchers suggested that the programs did not yield better results because of small financial incentives, coupled with program complexities that made it “difficult for hospitals to meaningfully engage in the program.” They also suggested that having to wait until year end to receive any financial incentives could have limited the impact.
“We found that hospitals that have been under financial incentives for more than a decade have not been able to reduce patient mortality more than late adopters, which had only been under financial incentives for less than 3 years,” the researchers concluded. “Given its cost, policymakers in the [United States] should consider one of two things: revise the current program or potentially end it.”
The changes suggested include increasing financial incentives and focusing on process measures that matter most to patients (mortality, patient experience, and functional status), rather than the current measure set that is larger and more difficult to track.
The researchers did not report any financial conflicts of interest.
SOURCE: Dr. Igna Bonfrer et al. BMJ 2018;360:j5622.
for Medicare beneficiaries, according to an analysis of Medicare claims data.
“No evidence that hospitals [that were] operating under pay for performance programs for more than a decade had better process scores or lower mortality than other hospitals was found,” Igna Bonfrer, PhD, of Erasmus University, Rotterdam, the Netherlands, and colleagues wrote in a study published Jan. 4, 2018, in BMJ.
Researchers looked at Medicare claims data from nearly 1.4 million patients aged 65 years and older across 1,189 hospitals. That total included 214 hospitals that were early adopters of pay for performance (PFP) programs, including the Hospital Quality Incentive Demonstration (HQID) and the current Hospital Value-Based Purchasing (HVBP) program, and 975 hospitals that adopted the programs at a later date. The study authors examined clinical process scores and 30-day mortality rates from 2003 to 2013.
Hospitals that were early adopters of a PFP program typically started from a higher baseline process measure score (91.5), compared with late adopters (89.9).
However, improvements among the early adopters “were smaller during the HQID period, although early adopters continued to perform at a slightly higher level than the late adopters during the pre-HVBP period,” the researchers explained. “Over the HVBP period, early and late adopters no longer differed in their clinical process scores.”
Indeed, a ceiling was ultimately reached, with early and late adopters approaching the same level (98.5 vs. 98.2).
For the 30-day mortality rates, both groups “started from a similar baseline (14.9% and 14.8% for the early and late adopters in the fourth quarter of 2003) and ended at the same rate of 9.9% for both groups in the fourth quarter of 2013,” Dr. Bonfrer and colleagues wrote.
The researchers suggested that the programs did not yield better results because of small financial incentives, coupled with program complexities that made it “difficult for hospitals to meaningfully engage in the program.” They also suggested that having to wait until year end to receive any financial incentives could have limited the impact.
“We found that hospitals that have been under financial incentives for more than a decade have not been able to reduce patient mortality more than late adopters, which had only been under financial incentives for less than 3 years,” the researchers concluded. “Given its cost, policymakers in the [United States] should consider one of two things: revise the current program or potentially end it.”
The changes suggested include increasing financial incentives and focusing on process measures that matter most to patients (mortality, patient experience, and functional status), rather than the current measure set that is larger and more difficult to track.
The researchers did not report any financial conflicts of interest.
SOURCE: Dr. Igna Bonfrer et al. BMJ 2018;360:j5622.
FROM BMJ