User login
Bariatric surgery comes with some risk of complications
that should acknowledged by clinicians and understood by patients, a large cohort study has shown.
Gunn Signe Jakobsen, MD, of Vestfold Hospital Trust, Tønsberg, Norway, and her colleagues wrote in an article published in JAMA, “Few studies report long-term complication rates. ... No large-scale clinical practice–based study has compared the long-term association of bariatric surgery and specialized medical obesity treatment with obesity-related somatic and mental comorbidities, nor the irrespective complication rates.”
The investigators compared outcomes from 932 patients who underwent bariatric surgery and 956 who underwent specialized medical treatment that involved either individual or group lifestyle intervention programs. The study population included 1,249 women and 639 men with an average age of 44 years and an average baseline body mass index of 44 kg/m2.
The surgery patients were more likely than the medical treatment patients to have hypertension remission (absolute risk 32% vs. 12%, respectively), and less likely to develop new-onset hypertension (absolute risk 4% vs. 12%, respectively). Diabetes remission was significantly higher among surgery patients, compared with medical treatment patients (58% vs. 13%) as was the likelihood of dyslipidemia remission (43% vs. 13%). Surgery patients also were less likely to develop new-onset diabetes or dyslipidemia than the medical treatment patients.
However, more patients who underwent bariatric surgery had low ferritin levels, compared with the medical treatment patients (26% vs. 12%). The surgery patients were significantly more likely than the medical treatment patients to develop new-onset depression (adjusted relative risk, 1.5; 95% confidence interval, 1.4-1.7), anxiety and sleep disorders (aRR, 1.3; 95% CI, 1.2-1.5), and treatment with opioids (aRR, 1.3; 95% CI, 1.2-1.4). In addition, bariatric patients were more likely to have at least one additional gastrointestinal surgical procedure (aRR, 2.0; 95% CI, 1.7-2.4), an operation for intestinal obstruction (aRR, 10.5; 95% CI, 5.1-21.5), abdominal pain (aRR, 1.9; 95% CI, 1.6-2.3), and gastroduodenal ulcers (aRR, 3.4; 95% CI 2.0-5.6).
The study was limited by several factors, including selection bias of younger, heavier patients in the bariatric surgery group, the lack of data on actual weight loss, incomplete laboratory data, and a relatively homogeneous white population, the researchers noted. However, the nearly 100% follow-up over approximately 6 years adds to the strength of the findings, which suggest that “the risk for complications should be considered in the decision-making process,” for obese patients considering bariatric surgery, they said.
Dr. Jakobsen was supported by the Vestfold Hospital Trust, with no financial conflicts to disclose.
SOURCE: Jakobsen G et al. JAMA. 2018 Jan 16;319(3):291-301.
that should acknowledged by clinicians and understood by patients, a large cohort study has shown.
Gunn Signe Jakobsen, MD, of Vestfold Hospital Trust, Tønsberg, Norway, and her colleagues wrote in an article published in JAMA, “Few studies report long-term complication rates. ... No large-scale clinical practice–based study has compared the long-term association of bariatric surgery and specialized medical obesity treatment with obesity-related somatic and mental comorbidities, nor the irrespective complication rates.”
The investigators compared outcomes from 932 patients who underwent bariatric surgery and 956 who underwent specialized medical treatment that involved either individual or group lifestyle intervention programs. The study population included 1,249 women and 639 men with an average age of 44 years and an average baseline body mass index of 44 kg/m2.
The surgery patients were more likely than the medical treatment patients to have hypertension remission (absolute risk 32% vs. 12%, respectively), and less likely to develop new-onset hypertension (absolute risk 4% vs. 12%, respectively). Diabetes remission was significantly higher among surgery patients, compared with medical treatment patients (58% vs. 13%) as was the likelihood of dyslipidemia remission (43% vs. 13%). Surgery patients also were less likely to develop new-onset diabetes or dyslipidemia than the medical treatment patients.
However, more patients who underwent bariatric surgery had low ferritin levels, compared with the medical treatment patients (26% vs. 12%). The surgery patients were significantly more likely than the medical treatment patients to develop new-onset depression (adjusted relative risk, 1.5; 95% confidence interval, 1.4-1.7), anxiety and sleep disorders (aRR, 1.3; 95% CI, 1.2-1.5), and treatment with opioids (aRR, 1.3; 95% CI, 1.2-1.4). In addition, bariatric patients were more likely to have at least one additional gastrointestinal surgical procedure (aRR, 2.0; 95% CI, 1.7-2.4), an operation for intestinal obstruction (aRR, 10.5; 95% CI, 5.1-21.5), abdominal pain (aRR, 1.9; 95% CI, 1.6-2.3), and gastroduodenal ulcers (aRR, 3.4; 95% CI 2.0-5.6).
The study was limited by several factors, including selection bias of younger, heavier patients in the bariatric surgery group, the lack of data on actual weight loss, incomplete laboratory data, and a relatively homogeneous white population, the researchers noted. However, the nearly 100% follow-up over approximately 6 years adds to the strength of the findings, which suggest that “the risk for complications should be considered in the decision-making process,” for obese patients considering bariatric surgery, they said.
Dr. Jakobsen was supported by the Vestfold Hospital Trust, with no financial conflicts to disclose.
SOURCE: Jakobsen G et al. JAMA. 2018 Jan 16;319(3):291-301.
that should acknowledged by clinicians and understood by patients, a large cohort study has shown.
Gunn Signe Jakobsen, MD, of Vestfold Hospital Trust, Tønsberg, Norway, and her colleagues wrote in an article published in JAMA, “Few studies report long-term complication rates. ... No large-scale clinical practice–based study has compared the long-term association of bariatric surgery and specialized medical obesity treatment with obesity-related somatic and mental comorbidities, nor the irrespective complication rates.”
The investigators compared outcomes from 932 patients who underwent bariatric surgery and 956 who underwent specialized medical treatment that involved either individual or group lifestyle intervention programs. The study population included 1,249 women and 639 men with an average age of 44 years and an average baseline body mass index of 44 kg/m2.
The surgery patients were more likely than the medical treatment patients to have hypertension remission (absolute risk 32% vs. 12%, respectively), and less likely to develop new-onset hypertension (absolute risk 4% vs. 12%, respectively). Diabetes remission was significantly higher among surgery patients, compared with medical treatment patients (58% vs. 13%) as was the likelihood of dyslipidemia remission (43% vs. 13%). Surgery patients also were less likely to develop new-onset diabetes or dyslipidemia than the medical treatment patients.
However, more patients who underwent bariatric surgery had low ferritin levels, compared with the medical treatment patients (26% vs. 12%). The surgery patients were significantly more likely than the medical treatment patients to develop new-onset depression (adjusted relative risk, 1.5; 95% confidence interval, 1.4-1.7), anxiety and sleep disorders (aRR, 1.3; 95% CI, 1.2-1.5), and treatment with opioids (aRR, 1.3; 95% CI, 1.2-1.4). In addition, bariatric patients were more likely to have at least one additional gastrointestinal surgical procedure (aRR, 2.0; 95% CI, 1.7-2.4), an operation for intestinal obstruction (aRR, 10.5; 95% CI, 5.1-21.5), abdominal pain (aRR, 1.9; 95% CI, 1.6-2.3), and gastroduodenal ulcers (aRR, 3.4; 95% CI 2.0-5.6).
The study was limited by several factors, including selection bias of younger, heavier patients in the bariatric surgery group, the lack of data on actual weight loss, incomplete laboratory data, and a relatively homogeneous white population, the researchers noted. However, the nearly 100% follow-up over approximately 6 years adds to the strength of the findings, which suggest that “the risk for complications should be considered in the decision-making process,” for obese patients considering bariatric surgery, they said.
Dr. Jakobsen was supported by the Vestfold Hospital Trust, with no financial conflicts to disclose.
SOURCE: Jakobsen G et al. JAMA. 2018 Jan 16;319(3):291-301.
FROM JAMA
Key clinical point: Bariatric surgery was associated with reduced hypertension but more complications, including iron deficiency and ulcers.
Major finding: Obese adults who had bariatric surgery were at greater risk for new-onset depression (aRR, 1.5), anxiety and sleep disorders (aRR, 1.3), and ulcers (aRR 3.4).
Study details: A cohort study of 1,888 adults treated with bariatric surgery or medical therapy.
Disclosures: Dr. Jakobsen was supported by the Vestfold Hospital Trust, with no financial conflicts to disclose.
Source: Jakobsen G et al. JAMA. 2018 Jan 16;319(3):291-301.
You are an integral part of the epilepsy care team
CHICAGO – While neurologists treat children’s seizure conditions, you or an emergency physician usually sees the child first and must determine whether a seizure has occurred and how to proceed.
Knowing the characteristics of seizures – and their imitators – helps you appropriately evaluate and treat these children, said Sucheta Joshi, MD, of the University of Michigan, Ann Arbor, and Linda C. Laux, MD, the medical director of the Comprehensive Epilepsy Center at the Ann & Robert H. Lurie Children’s Hospital of Chicago. You also must consider the long-term management and well-being of a child with epilepsy.
A primer on seizures
Abnormal electrical discharges in the brain cause seizures, and various acute conditions can cause them, including fevers, infections, trauma, and metabolic abnormalities. But an epilepsy diagnosis requires at least two unprovoked seizures occurring more than 1 day apart. More than two dozen different epilepsy syndromes exist, determined based on age of onset, seizure type, the child’s development, and EEG patterns.
You also should be aware of what seizure imitators to rule out: movement disorders such as tics and Sandifer’s syndrome, daydreaming and inattention, fainting, migraines, panic attacks, psychogenic nonepileptic seizures (PNES), self-stimulatory behaviors, periods of the child holding her breath, and sleep parasomnias, such as night terrors, sleepwalking, and sleep myoclonus.
“It’s often difficult to tell if it’s a seizure or a nonepileptic paroxysmal event,” said Dr. Joshi. An interictal EEG can be helpful, but “there’s no reliable test to differentiate the two.”
Knowing the environment where the incident occurred, what provoking factors might have been present, what the seizure looked like, how long it lasted, and what happened afterward can help you differentiate paroxysmal spells from seizures.
Febrile seizures
About 4% of all children experience febrile seizures, particularly between 6 months and 6 years of age, Dr. Joshi said. The two types are simple and complex. A simple febrile seizure is generalized and brief, lasting less than 15 minutes, and is not followed by another within 24 hours. The child may have a family history of epilepsy but appear normal. Complex febrile seizures are focal, last more than 15 minutes, and occur more than once within 24 hours.
Although antipyretics may help the child feel better, fever control won’t always prevent seizures. Rectal or sometimes oral diazepam can prevent recurrent prolonged febrile seizures if necessary. You also may consider oral clonazepam as a rescue medication.
However, children with only simple febrile seizures are at no greater risk of developing epilepsy by age 7 years than are children in the general population, about 1%, according to the AAP’s clinical practice guideline for long-term management of children with simple febrile seizures (Pediatrics. 2008 Jun;121(6):1281-6.)
If a child has a family history of epilepsy, has their first febrile before 12 months of age, and has multiple simple febrile seizures, however, their risk of epilepsy more than doubles. An estimated 2.4% of these children will develop epilepsy by age 25 years.
“No study has demonstrated that successful treatment of simple febrile seizures can prevent this later development of epilepsy, and there currently is no evidence that simple febrile seizures cause structural damage to the brain,” the practice guideline states. “Indeed, it is most likely that the increased risk of epilepsy in this population is the result of genetic predisposition.”
Determining seizure causes
If the child’s seizure is not clearly febrile with a known cause, you should run through other possibilities. Did the child have head trauma? A central nervous system infection? Are metabolic abnormalities present, such as renal or hepatic disease or an electrolyte abnormality? Has the patient ingested something, such as a recreational drug or other toxic substance?
Lab work is unlikely to offer much information without clinical signs or symptoms present, but you may consider glucose, electrolytes, serum alcohol level, and a toxicology drug screen on a case-by-case basis: A child’s first unprovoked seizure should not lead to a lumbar puncture, Dr. Laux said. But if you suspect a CNS infection or the child is under 6 months old and does not return to baseline, you should consider a lumbar puncture. Modest increases in cerebrospinal fluid cell count (pleocytosis) occur after a seizure, but a “CSF above 20 WBC/mm3 or above 10 PMN/mm3 should not be attributed to a seizure,” she said.
An outpatient EEG, preferably performed within 24-48 hours, shows abnormalities 70% of the time after a seizure, but a normal EEG cannot rule out a seizure. EEG data also may suggest recurrence risk or a specific epilepsy syndrome and long-term prognosis.
Epilepsy management
After a second unprovoked seizure occurrs more than 24 hours after the first, you should diagnose new onset epilepsy, order an EEG and head MRI, and refer the child to a neurologist. Metabolic or genetic tests may be indicated depending on signs and symptoms.
Managing epilepsy requires much more than just treating seizures, Dr. Laux emphasized, so you play an important role in educating the family, considering safety issues, monitoring bone and reproductive health, and considering the condition’s effect on learning and mental, behavioral, and physical health.
Children with epilepsy and normal cognitive development have no greater rate of injuries than children without epilepsy, but risk increases as seizures increase, and if the child has ADHD, intellectual disability, or generalized-onset seizures, that can lead to falls.
Still, children with epilepsy can play contact sports such as soccer or volleyball without worrying it will cause a seizure. They also should always wear a helmet while bicycling, rollerblading, skating, and using scooters or anything else with wheels.
Swimming, water sports, harnessed rock climbing, horseback riding, and gymnastics also are fine with appropriate supervision. Showers are preferred to baths because of the risk of drowning should a seizure occur in the bathtub. Bathing and swimming require a specified supervisor.
Unsafe activities include free climbing, sky-diving, hang-gliding, and scuba diving. Parents should supervise their children around irons, hairdryers, curling irons, stove tops, camp fires, BBQs, and playground equipment. TV and video games are fine if children do not sit close to the screen and have ambient light in the room.
A teen with uncontrolled seizures should not drive, and pediatricians should be aware of their state’s laws related to epilepsy and driving (www.epilepsy.com/driving-laws). Pennsylvania, California, Delaware, Nevada, New Jersey, and Oregon, for example, have physician reporting laws.
Physical health and learning differences
Epilepsy increases risk of poor bone mineralization, and seizures can lead to falls and fractures. You therefore should keep tabs on the child’s vitamin D intake, physical activity levels, neuromotor dysfunction, and overall nutrition. Vitamin D insufficiency is more common in those with epilepsy than in the general population, particularly females and those with obesity. Evidence suggests both anticonvulsants and epilepsy syndromes contribute to low vitamin D levels, so daily supplements may be wise.
Antiepileptic drugs also reduce the effectiveness of hormonal contraception, and teens with epilepsy already have a higher risk for unplanned pregnancy. You should ensure that sexually active female teens get folic acid daily and educate them on valproate’s increased risk of causing birth defects. For pregnant teens, levetiracetam and lamotrigine present less risk to a fetus.
You should ask about the patient’s school performance and consider requesting an Individualized Education Plan or a 504 plan at school if it seems needed. Even in youths with a normal IQ and well-managed seizures, lower academic achievement and difficulties with memory and behavior are more likely. Risk increases in disorganized or unsupportive homes and with comorbidities. ADHD occurs in 38% of children with epilepsy, but stimulants such as methylphenidate are not contraindicated with epilepsy medications.
Epilepsy affects the whole family: About half of all mothers of children with epilepsy have depression – which can adversely affect her children – and risk increases for younger moms with lower education and income. Siblings have added burdens, too: In one study, 95% of siblings had witnessed a seizure, and 79% believed their siblings suffered during seizures. More than two-thirds (68%) say their sibling with epilepsy gets more attention, and 42% feel responsible for their sibling, often restricting their own activities. You should be considering all these factors in managing the well-being of a child with epilepsy.
Dr. Joshi summed up the complex management of epilepsy with an acrostic that may be helpful to share with parents:
• Education
• Parenting
• Independence (including driving)
• Learning
• Eating (nutrition and bone health)
• Pharmacotherapy (anticonvulsants)
• School
• You (the child’s caretakers).
Dr. Joshi and Dr. Laux reported having no relevant financial disclosures and no external funding.
CHICAGO – While neurologists treat children’s seizure conditions, you or an emergency physician usually sees the child first and must determine whether a seizure has occurred and how to proceed.
Knowing the characteristics of seizures – and their imitators – helps you appropriately evaluate and treat these children, said Sucheta Joshi, MD, of the University of Michigan, Ann Arbor, and Linda C. Laux, MD, the medical director of the Comprehensive Epilepsy Center at the Ann & Robert H. Lurie Children’s Hospital of Chicago. You also must consider the long-term management and well-being of a child with epilepsy.
A primer on seizures
Abnormal electrical discharges in the brain cause seizures, and various acute conditions can cause them, including fevers, infections, trauma, and metabolic abnormalities. But an epilepsy diagnosis requires at least two unprovoked seizures occurring more than 1 day apart. More than two dozen different epilepsy syndromes exist, determined based on age of onset, seizure type, the child’s development, and EEG patterns.
You also should be aware of what seizure imitators to rule out: movement disorders such as tics and Sandifer’s syndrome, daydreaming and inattention, fainting, migraines, panic attacks, psychogenic nonepileptic seizures (PNES), self-stimulatory behaviors, periods of the child holding her breath, and sleep parasomnias, such as night terrors, sleepwalking, and sleep myoclonus.
“It’s often difficult to tell if it’s a seizure or a nonepileptic paroxysmal event,” said Dr. Joshi. An interictal EEG can be helpful, but “there’s no reliable test to differentiate the two.”
Knowing the environment where the incident occurred, what provoking factors might have been present, what the seizure looked like, how long it lasted, and what happened afterward can help you differentiate paroxysmal spells from seizures.
Febrile seizures
About 4% of all children experience febrile seizures, particularly between 6 months and 6 years of age, Dr. Joshi said. The two types are simple and complex. A simple febrile seizure is generalized and brief, lasting less than 15 minutes, and is not followed by another within 24 hours. The child may have a family history of epilepsy but appear normal. Complex febrile seizures are focal, last more than 15 minutes, and occur more than once within 24 hours.
Although antipyretics may help the child feel better, fever control won’t always prevent seizures. Rectal or sometimes oral diazepam can prevent recurrent prolonged febrile seizures if necessary. You also may consider oral clonazepam as a rescue medication.
However, children with only simple febrile seizures are at no greater risk of developing epilepsy by age 7 years than are children in the general population, about 1%, according to the AAP’s clinical practice guideline for long-term management of children with simple febrile seizures (Pediatrics. 2008 Jun;121(6):1281-6.)
If a child has a family history of epilepsy, has their first febrile before 12 months of age, and has multiple simple febrile seizures, however, their risk of epilepsy more than doubles. An estimated 2.4% of these children will develop epilepsy by age 25 years.
“No study has demonstrated that successful treatment of simple febrile seizures can prevent this later development of epilepsy, and there currently is no evidence that simple febrile seizures cause structural damage to the brain,” the practice guideline states. “Indeed, it is most likely that the increased risk of epilepsy in this population is the result of genetic predisposition.”
Determining seizure causes
If the child’s seizure is not clearly febrile with a known cause, you should run through other possibilities. Did the child have head trauma? A central nervous system infection? Are metabolic abnormalities present, such as renal or hepatic disease or an electrolyte abnormality? Has the patient ingested something, such as a recreational drug or other toxic substance?
Lab work is unlikely to offer much information without clinical signs or symptoms present, but you may consider glucose, electrolytes, serum alcohol level, and a toxicology drug screen on a case-by-case basis: A child’s first unprovoked seizure should not lead to a lumbar puncture, Dr. Laux said. But if you suspect a CNS infection or the child is under 6 months old and does not return to baseline, you should consider a lumbar puncture. Modest increases in cerebrospinal fluid cell count (pleocytosis) occur after a seizure, but a “CSF above 20 WBC/mm3 or above 10 PMN/mm3 should not be attributed to a seizure,” she said.
An outpatient EEG, preferably performed within 24-48 hours, shows abnormalities 70% of the time after a seizure, but a normal EEG cannot rule out a seizure. EEG data also may suggest recurrence risk or a specific epilepsy syndrome and long-term prognosis.
Epilepsy management
After a second unprovoked seizure occurrs more than 24 hours after the first, you should diagnose new onset epilepsy, order an EEG and head MRI, and refer the child to a neurologist. Metabolic or genetic tests may be indicated depending on signs and symptoms.
Managing epilepsy requires much more than just treating seizures, Dr. Laux emphasized, so you play an important role in educating the family, considering safety issues, monitoring bone and reproductive health, and considering the condition’s effect on learning and mental, behavioral, and physical health.
Children with epilepsy and normal cognitive development have no greater rate of injuries than children without epilepsy, but risk increases as seizures increase, and if the child has ADHD, intellectual disability, or generalized-onset seizures, that can lead to falls.
Still, children with epilepsy can play contact sports such as soccer or volleyball without worrying it will cause a seizure. They also should always wear a helmet while bicycling, rollerblading, skating, and using scooters or anything else with wheels.
Swimming, water sports, harnessed rock climbing, horseback riding, and gymnastics also are fine with appropriate supervision. Showers are preferred to baths because of the risk of drowning should a seizure occur in the bathtub. Bathing and swimming require a specified supervisor.
Unsafe activities include free climbing, sky-diving, hang-gliding, and scuba diving. Parents should supervise their children around irons, hairdryers, curling irons, stove tops, camp fires, BBQs, and playground equipment. TV and video games are fine if children do not sit close to the screen and have ambient light in the room.
A teen with uncontrolled seizures should not drive, and pediatricians should be aware of their state’s laws related to epilepsy and driving (www.epilepsy.com/driving-laws). Pennsylvania, California, Delaware, Nevada, New Jersey, and Oregon, for example, have physician reporting laws.
Physical health and learning differences
Epilepsy increases risk of poor bone mineralization, and seizures can lead to falls and fractures. You therefore should keep tabs on the child’s vitamin D intake, physical activity levels, neuromotor dysfunction, and overall nutrition. Vitamin D insufficiency is more common in those with epilepsy than in the general population, particularly females and those with obesity. Evidence suggests both anticonvulsants and epilepsy syndromes contribute to low vitamin D levels, so daily supplements may be wise.
Antiepileptic drugs also reduce the effectiveness of hormonal contraception, and teens with epilepsy already have a higher risk for unplanned pregnancy. You should ensure that sexually active female teens get folic acid daily and educate them on valproate’s increased risk of causing birth defects. For pregnant teens, levetiracetam and lamotrigine present less risk to a fetus.
You should ask about the patient’s school performance and consider requesting an Individualized Education Plan or a 504 plan at school if it seems needed. Even in youths with a normal IQ and well-managed seizures, lower academic achievement and difficulties with memory and behavior are more likely. Risk increases in disorganized or unsupportive homes and with comorbidities. ADHD occurs in 38% of children with epilepsy, but stimulants such as methylphenidate are not contraindicated with epilepsy medications.
Epilepsy affects the whole family: About half of all mothers of children with epilepsy have depression – which can adversely affect her children – and risk increases for younger moms with lower education and income. Siblings have added burdens, too: In one study, 95% of siblings had witnessed a seizure, and 79% believed their siblings suffered during seizures. More than two-thirds (68%) say their sibling with epilepsy gets more attention, and 42% feel responsible for their sibling, often restricting their own activities. You should be considering all these factors in managing the well-being of a child with epilepsy.
Dr. Joshi summed up the complex management of epilepsy with an acrostic that may be helpful to share with parents:
• Education
• Parenting
• Independence (including driving)
• Learning
• Eating (nutrition and bone health)
• Pharmacotherapy (anticonvulsants)
• School
• You (the child’s caretakers).
Dr. Joshi and Dr. Laux reported having no relevant financial disclosures and no external funding.
CHICAGO – While neurologists treat children’s seizure conditions, you or an emergency physician usually sees the child first and must determine whether a seizure has occurred and how to proceed.
Knowing the characteristics of seizures – and their imitators – helps you appropriately evaluate and treat these children, said Sucheta Joshi, MD, of the University of Michigan, Ann Arbor, and Linda C. Laux, MD, the medical director of the Comprehensive Epilepsy Center at the Ann & Robert H. Lurie Children’s Hospital of Chicago. You also must consider the long-term management and well-being of a child with epilepsy.
A primer on seizures
Abnormal electrical discharges in the brain cause seizures, and various acute conditions can cause them, including fevers, infections, trauma, and metabolic abnormalities. But an epilepsy diagnosis requires at least two unprovoked seizures occurring more than 1 day apart. More than two dozen different epilepsy syndromes exist, determined based on age of onset, seizure type, the child’s development, and EEG patterns.
You also should be aware of what seizure imitators to rule out: movement disorders such as tics and Sandifer’s syndrome, daydreaming and inattention, fainting, migraines, panic attacks, psychogenic nonepileptic seizures (PNES), self-stimulatory behaviors, periods of the child holding her breath, and sleep parasomnias, such as night terrors, sleepwalking, and sleep myoclonus.
“It’s often difficult to tell if it’s a seizure or a nonepileptic paroxysmal event,” said Dr. Joshi. An interictal EEG can be helpful, but “there’s no reliable test to differentiate the two.”
Knowing the environment where the incident occurred, what provoking factors might have been present, what the seizure looked like, how long it lasted, and what happened afterward can help you differentiate paroxysmal spells from seizures.
Febrile seizures
About 4% of all children experience febrile seizures, particularly between 6 months and 6 years of age, Dr. Joshi said. The two types are simple and complex. A simple febrile seizure is generalized and brief, lasting less than 15 minutes, and is not followed by another within 24 hours. The child may have a family history of epilepsy but appear normal. Complex febrile seizures are focal, last more than 15 minutes, and occur more than once within 24 hours.
Although antipyretics may help the child feel better, fever control won’t always prevent seizures. Rectal or sometimes oral diazepam can prevent recurrent prolonged febrile seizures if necessary. You also may consider oral clonazepam as a rescue medication.
However, children with only simple febrile seizures are at no greater risk of developing epilepsy by age 7 years than are children in the general population, about 1%, according to the AAP’s clinical practice guideline for long-term management of children with simple febrile seizures (Pediatrics. 2008 Jun;121(6):1281-6.)
If a child has a family history of epilepsy, has their first febrile before 12 months of age, and has multiple simple febrile seizures, however, their risk of epilepsy more than doubles. An estimated 2.4% of these children will develop epilepsy by age 25 years.
“No study has demonstrated that successful treatment of simple febrile seizures can prevent this later development of epilepsy, and there currently is no evidence that simple febrile seizures cause structural damage to the brain,” the practice guideline states. “Indeed, it is most likely that the increased risk of epilepsy in this population is the result of genetic predisposition.”
Determining seizure causes
If the child’s seizure is not clearly febrile with a known cause, you should run through other possibilities. Did the child have head trauma? A central nervous system infection? Are metabolic abnormalities present, such as renal or hepatic disease or an electrolyte abnormality? Has the patient ingested something, such as a recreational drug or other toxic substance?
Lab work is unlikely to offer much information without clinical signs or symptoms present, but you may consider glucose, electrolytes, serum alcohol level, and a toxicology drug screen on a case-by-case basis: A child’s first unprovoked seizure should not lead to a lumbar puncture, Dr. Laux said. But if you suspect a CNS infection or the child is under 6 months old and does not return to baseline, you should consider a lumbar puncture. Modest increases in cerebrospinal fluid cell count (pleocytosis) occur after a seizure, but a “CSF above 20 WBC/mm3 or above 10 PMN/mm3 should not be attributed to a seizure,” she said.
An outpatient EEG, preferably performed within 24-48 hours, shows abnormalities 70% of the time after a seizure, but a normal EEG cannot rule out a seizure. EEG data also may suggest recurrence risk or a specific epilepsy syndrome and long-term prognosis.
Epilepsy management
After a second unprovoked seizure occurrs more than 24 hours after the first, you should diagnose new onset epilepsy, order an EEG and head MRI, and refer the child to a neurologist. Metabolic or genetic tests may be indicated depending on signs and symptoms.
Managing epilepsy requires much more than just treating seizures, Dr. Laux emphasized, so you play an important role in educating the family, considering safety issues, monitoring bone and reproductive health, and considering the condition’s effect on learning and mental, behavioral, and physical health.
Children with epilepsy and normal cognitive development have no greater rate of injuries than children without epilepsy, but risk increases as seizures increase, and if the child has ADHD, intellectual disability, or generalized-onset seizures, that can lead to falls.
Still, children with epilepsy can play contact sports such as soccer or volleyball without worrying it will cause a seizure. They also should always wear a helmet while bicycling, rollerblading, skating, and using scooters or anything else with wheels.
Swimming, water sports, harnessed rock climbing, horseback riding, and gymnastics also are fine with appropriate supervision. Showers are preferred to baths because of the risk of drowning should a seizure occur in the bathtub. Bathing and swimming require a specified supervisor.
Unsafe activities include free climbing, sky-diving, hang-gliding, and scuba diving. Parents should supervise their children around irons, hairdryers, curling irons, stove tops, camp fires, BBQs, and playground equipment. TV and video games are fine if children do not sit close to the screen and have ambient light in the room.
A teen with uncontrolled seizures should not drive, and pediatricians should be aware of their state’s laws related to epilepsy and driving (www.epilepsy.com/driving-laws). Pennsylvania, California, Delaware, Nevada, New Jersey, and Oregon, for example, have physician reporting laws.
Physical health and learning differences
Epilepsy increases risk of poor bone mineralization, and seizures can lead to falls and fractures. You therefore should keep tabs on the child’s vitamin D intake, physical activity levels, neuromotor dysfunction, and overall nutrition. Vitamin D insufficiency is more common in those with epilepsy than in the general population, particularly females and those with obesity. Evidence suggests both anticonvulsants and epilepsy syndromes contribute to low vitamin D levels, so daily supplements may be wise.
Antiepileptic drugs also reduce the effectiveness of hormonal contraception, and teens with epilepsy already have a higher risk for unplanned pregnancy. You should ensure that sexually active female teens get folic acid daily and educate them on valproate’s increased risk of causing birth defects. For pregnant teens, levetiracetam and lamotrigine present less risk to a fetus.
You should ask about the patient’s school performance and consider requesting an Individualized Education Plan or a 504 plan at school if it seems needed. Even in youths with a normal IQ and well-managed seizures, lower academic achievement and difficulties with memory and behavior are more likely. Risk increases in disorganized or unsupportive homes and with comorbidities. ADHD occurs in 38% of children with epilepsy, but stimulants such as methylphenidate are not contraindicated with epilepsy medications.
Epilepsy affects the whole family: About half of all mothers of children with epilepsy have depression – which can adversely affect her children – and risk increases for younger moms with lower education and income. Siblings have added burdens, too: In one study, 95% of siblings had witnessed a seizure, and 79% believed their siblings suffered during seizures. More than two-thirds (68%) say their sibling with epilepsy gets more attention, and 42% feel responsible for their sibling, often restricting their own activities. You should be considering all these factors in managing the well-being of a child with epilepsy.
Dr. Joshi summed up the complex management of epilepsy with an acrostic that may be helpful to share with parents:
• Education
• Parenting
• Independence (including driving)
• Learning
• Eating (nutrition and bone health)
• Pharmacotherapy (anticonvulsants)
• School
• You (the child’s caretakers).
Dr. Joshi and Dr. Laux reported having no relevant financial disclosures and no external funding.
EXPERT ANALYSIS FROM AAP 2017
DAPT duration: How low can you go?
DENVER – Six months of dual-antiplatelet therapy proved equivalent in terms of safety, efficacy, and bleeding risk to the guideline-recommended standard 12 months in ST-elevation MI patients after primary PCI with a second-generation drug-eluting stent in the randomized DAPT-STEMI trial.
“This trial, for the first time, showed that in the modern DES [drug-eluting stent] era, event-free STEMI patients do not benefit from a prolonged DAPT beyond 6 months, as currently recommended, and sets the stage for further dedicated research in this important topic,” Elvin Kedhi, MD, PhD, declared in presenting the DAPT-STEMI results at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The final analysis took place at 24 months post STEMI; that is, 18 months post randomization. Among the 861 completers, the composite primary outcome of death, MI, revascularization, stroke, and major bleeding during months 6-24 occurred in 4.8% of the SAPT group, a 27% relative risk reduction compared with the 6.6% rate in the DAPT group. Thus, 6 months of DAPT met the prespecified endpoint of noninferiority compared to the standard 12 months of DAPT, reported Dr. Kedhi, head of interventional cardiology and clinical research and innovation at the Isala Heart Center in Zwolle, The Netherlands.
The secondary composite endpoint of death, MI, stroke, stent thrombosis, or TIMI major bleeding occurred in 3.2% of the SAPT group and 4.3% of the DAPT group, for a 25% relative risk reduction.
All individual components of the composite endpoints occurred at the same or lower rate in the SAPT group compared with the DAPT arm, he noted at the meeting, which was sponsored by the Cardiovascular Research Foundation.
At a press conference where Dr. Kedhi presented the DAPT-STEMI results, discussant Dean J. Kereiakes, MD, explained why he didn’t find the study results surprising.
Press conference moderator Gary S. Mintz, MD, put the DAPT-STEMI findings in perspective: “The need for DAPT has decreased along with all the stent-related complications. There’s always been a greater focus on DAPT for preventing events and a relatively lesser focus on the adverse consequences of DAPT. And anybody who’s a clinician who takes care of patients knows that drug-related bleeding after stent implantation is not a trivial occurrence,” observed Dr. Mintz, chief medical officer at the Cardiovascular Research Foundation in Washington.
DAPT-STEMI isn’t the final word on DAPT duration
At a late-breaking clinical trials session, comoderator Eric D. Peterson, MD, noted that in earlier megatrials such as PEGASUS, DAPT, and PLATO, there were signals that extending DAPT beyond 12 months might be even more beneficial than the guideline-recommended 12 months.
“It seems somewhat counterintuitive that now you have better results with less. Any speculation as to why?” asked Dr. Peterson, executive director of the Duke Clinical Research Institute and professor of medicine at Duke University in Durham, N.C.
“It’s true that DAPT reduces the general risk of thromboembolic events, but it does so at a relative risk reduction rate of about 20%, while it augments the bleeding risk by over 200%. And ask yourself, what is the benefit of this 6 months of extra DAPT on the lifelong process of atherosclerosis? It’s almost invisible,” Dr. Kedhi explained.
Dmitriy N. Feldman, MD, of Cornell University in New York, was one of several discussants to note that DAPT-STEMI was statistically underpowered to reach definitive conclusions. But he nonetheless found the results encouraging.
“It’s very reassuring that the stent thrombosis rates are quite low: 0.7% and 0.9%. And with this DES system and 42% of patients receiving clopidogrel rather than ticagrelor or prasugrel we still see low event rates. This is a very select group – patients had to tolerate the first 6 months of DAPT without MACE events or bleeding. But it is reassuring that in patients who are able to do well at 6 months, this is an option,” the interventional cardiologist said.
Session moderator Gregg W. Stone, MD, called DAPT-STEMI “hypothesis-generating” in light of its limited size and statistical power.
“At least it raises the concept of shorter-duration DAPT, whereas I’d say before today it was not a concept. We were always talking about prolonging DAPT in the highest-thrombotic risk STEMI patients, and now we can at least think about shortening it, whether for all patients or for higher-bleeding-risk patients,” observed Dr. Stone, professor of medicine at Columbia University in New York.
As a matter of fact, DAPT durations even briefer than 6 months are under active investigation. Dr. Kedhi is co-principal investigator in the Onyx ONE clinical trial, a new prospective, 85-center, randomized, single-blind trial of a mere 1 month of DAPT in 2,000 high-bleeding-risk CAD patients undergoing PCI with the Resolute Onyx DES or the BioFreedom drug-coated stent.
The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. Dr. Kedhi reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
SOURCE: Kedhi, E. no abst.
DENVER – Six months of dual-antiplatelet therapy proved equivalent in terms of safety, efficacy, and bleeding risk to the guideline-recommended standard 12 months in ST-elevation MI patients after primary PCI with a second-generation drug-eluting stent in the randomized DAPT-STEMI trial.
“This trial, for the first time, showed that in the modern DES [drug-eluting stent] era, event-free STEMI patients do not benefit from a prolonged DAPT beyond 6 months, as currently recommended, and sets the stage for further dedicated research in this important topic,” Elvin Kedhi, MD, PhD, declared in presenting the DAPT-STEMI results at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The final analysis took place at 24 months post STEMI; that is, 18 months post randomization. Among the 861 completers, the composite primary outcome of death, MI, revascularization, stroke, and major bleeding during months 6-24 occurred in 4.8% of the SAPT group, a 27% relative risk reduction compared with the 6.6% rate in the DAPT group. Thus, 6 months of DAPT met the prespecified endpoint of noninferiority compared to the standard 12 months of DAPT, reported Dr. Kedhi, head of interventional cardiology and clinical research and innovation at the Isala Heart Center in Zwolle, The Netherlands.
The secondary composite endpoint of death, MI, stroke, stent thrombosis, or TIMI major bleeding occurred in 3.2% of the SAPT group and 4.3% of the DAPT group, for a 25% relative risk reduction.
All individual components of the composite endpoints occurred at the same or lower rate in the SAPT group compared with the DAPT arm, he noted at the meeting, which was sponsored by the Cardiovascular Research Foundation.
At a press conference where Dr. Kedhi presented the DAPT-STEMI results, discussant Dean J. Kereiakes, MD, explained why he didn’t find the study results surprising.
Press conference moderator Gary S. Mintz, MD, put the DAPT-STEMI findings in perspective: “The need for DAPT has decreased along with all the stent-related complications. There’s always been a greater focus on DAPT for preventing events and a relatively lesser focus on the adverse consequences of DAPT. And anybody who’s a clinician who takes care of patients knows that drug-related bleeding after stent implantation is not a trivial occurrence,” observed Dr. Mintz, chief medical officer at the Cardiovascular Research Foundation in Washington.
DAPT-STEMI isn’t the final word on DAPT duration
At a late-breaking clinical trials session, comoderator Eric D. Peterson, MD, noted that in earlier megatrials such as PEGASUS, DAPT, and PLATO, there were signals that extending DAPT beyond 12 months might be even more beneficial than the guideline-recommended 12 months.
“It seems somewhat counterintuitive that now you have better results with less. Any speculation as to why?” asked Dr. Peterson, executive director of the Duke Clinical Research Institute and professor of medicine at Duke University in Durham, N.C.
“It’s true that DAPT reduces the general risk of thromboembolic events, but it does so at a relative risk reduction rate of about 20%, while it augments the bleeding risk by over 200%. And ask yourself, what is the benefit of this 6 months of extra DAPT on the lifelong process of atherosclerosis? It’s almost invisible,” Dr. Kedhi explained.
Dmitriy N. Feldman, MD, of Cornell University in New York, was one of several discussants to note that DAPT-STEMI was statistically underpowered to reach definitive conclusions. But he nonetheless found the results encouraging.
“It’s very reassuring that the stent thrombosis rates are quite low: 0.7% and 0.9%. And with this DES system and 42% of patients receiving clopidogrel rather than ticagrelor or prasugrel we still see low event rates. This is a very select group – patients had to tolerate the first 6 months of DAPT without MACE events or bleeding. But it is reassuring that in patients who are able to do well at 6 months, this is an option,” the interventional cardiologist said.
Session moderator Gregg W. Stone, MD, called DAPT-STEMI “hypothesis-generating” in light of its limited size and statistical power.
“At least it raises the concept of shorter-duration DAPT, whereas I’d say before today it was not a concept. We were always talking about prolonging DAPT in the highest-thrombotic risk STEMI patients, and now we can at least think about shortening it, whether for all patients or for higher-bleeding-risk patients,” observed Dr. Stone, professor of medicine at Columbia University in New York.
As a matter of fact, DAPT durations even briefer than 6 months are under active investigation. Dr. Kedhi is co-principal investigator in the Onyx ONE clinical trial, a new prospective, 85-center, randomized, single-blind trial of a mere 1 month of DAPT in 2,000 high-bleeding-risk CAD patients undergoing PCI with the Resolute Onyx DES or the BioFreedom drug-coated stent.
The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. Dr. Kedhi reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
SOURCE: Kedhi, E. no abst.
DENVER – Six months of dual-antiplatelet therapy proved equivalent in terms of safety, efficacy, and bleeding risk to the guideline-recommended standard 12 months in ST-elevation MI patients after primary PCI with a second-generation drug-eluting stent in the randomized DAPT-STEMI trial.
“This trial, for the first time, showed that in the modern DES [drug-eluting stent] era, event-free STEMI patients do not benefit from a prolonged DAPT beyond 6 months, as currently recommended, and sets the stage for further dedicated research in this important topic,” Elvin Kedhi, MD, PhD, declared in presenting the DAPT-STEMI results at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The final analysis took place at 24 months post STEMI; that is, 18 months post randomization. Among the 861 completers, the composite primary outcome of death, MI, revascularization, stroke, and major bleeding during months 6-24 occurred in 4.8% of the SAPT group, a 27% relative risk reduction compared with the 6.6% rate in the DAPT group. Thus, 6 months of DAPT met the prespecified endpoint of noninferiority compared to the standard 12 months of DAPT, reported Dr. Kedhi, head of interventional cardiology and clinical research and innovation at the Isala Heart Center in Zwolle, The Netherlands.
The secondary composite endpoint of death, MI, stroke, stent thrombosis, or TIMI major bleeding occurred in 3.2% of the SAPT group and 4.3% of the DAPT group, for a 25% relative risk reduction.
All individual components of the composite endpoints occurred at the same or lower rate in the SAPT group compared with the DAPT arm, he noted at the meeting, which was sponsored by the Cardiovascular Research Foundation.
At a press conference where Dr. Kedhi presented the DAPT-STEMI results, discussant Dean J. Kereiakes, MD, explained why he didn’t find the study results surprising.
Press conference moderator Gary S. Mintz, MD, put the DAPT-STEMI findings in perspective: “The need for DAPT has decreased along with all the stent-related complications. There’s always been a greater focus on DAPT for preventing events and a relatively lesser focus on the adverse consequences of DAPT. And anybody who’s a clinician who takes care of patients knows that drug-related bleeding after stent implantation is not a trivial occurrence,” observed Dr. Mintz, chief medical officer at the Cardiovascular Research Foundation in Washington.
DAPT-STEMI isn’t the final word on DAPT duration
At a late-breaking clinical trials session, comoderator Eric D. Peterson, MD, noted that in earlier megatrials such as PEGASUS, DAPT, and PLATO, there were signals that extending DAPT beyond 12 months might be even more beneficial than the guideline-recommended 12 months.
“It seems somewhat counterintuitive that now you have better results with less. Any speculation as to why?” asked Dr. Peterson, executive director of the Duke Clinical Research Institute and professor of medicine at Duke University in Durham, N.C.
“It’s true that DAPT reduces the general risk of thromboembolic events, but it does so at a relative risk reduction rate of about 20%, while it augments the bleeding risk by over 200%. And ask yourself, what is the benefit of this 6 months of extra DAPT on the lifelong process of atherosclerosis? It’s almost invisible,” Dr. Kedhi explained.
Dmitriy N. Feldman, MD, of Cornell University in New York, was one of several discussants to note that DAPT-STEMI was statistically underpowered to reach definitive conclusions. But he nonetheless found the results encouraging.
“It’s very reassuring that the stent thrombosis rates are quite low: 0.7% and 0.9%. And with this DES system and 42% of patients receiving clopidogrel rather than ticagrelor or prasugrel we still see low event rates. This is a very select group – patients had to tolerate the first 6 months of DAPT without MACE events or bleeding. But it is reassuring that in patients who are able to do well at 6 months, this is an option,” the interventional cardiologist said.
Session moderator Gregg W. Stone, MD, called DAPT-STEMI “hypothesis-generating” in light of its limited size and statistical power.
“At least it raises the concept of shorter-duration DAPT, whereas I’d say before today it was not a concept. We were always talking about prolonging DAPT in the highest-thrombotic risk STEMI patients, and now we can at least think about shortening it, whether for all patients or for higher-bleeding-risk patients,” observed Dr. Stone, professor of medicine at Columbia University in New York.
As a matter of fact, DAPT durations even briefer than 6 months are under active investigation. Dr. Kedhi is co-principal investigator in the Onyx ONE clinical trial, a new prospective, 85-center, randomized, single-blind trial of a mere 1 month of DAPT in 2,000 high-bleeding-risk CAD patients undergoing PCI with the Resolute Onyx DES or the BioFreedom drug-coated stent.
The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. Dr. Kedhi reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
SOURCE: Kedhi, E. no abst.
REPORTING FROM TCT 2017
Key clinical point:
Major finding: The composite endpoint of death, MI, stroke, revascularization, and major bleeding 24 months after primary PCI with a second-generation DES was 6.6% in patients who got the standard 12 months of DAPT and 4.8% in those who got 6.
Study details: A prospective randomized international study that enrolled 1,100 STEMI patients who underwent primary PCI.
Disclosures: The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. The presenter reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
Source: Kedhi, E. No abstract.
How to set up your own RSS feed
In my last column, I reviewed the reasons why RSS news feeds can be a useful tool for keeping abreast on frequently updated information, including blog entries, news headlines, audio, and video, without having to check multiple Web pages every day.
This can help increase readership on your website, publicize a podcast, or keep your patients up to date on the latest treatments and procedures in your practice. And if your name appears in news or gossip sites, you will be alerted immediately.
Alternatively, many organizations that publish their own articles and news stories use a content management system (CMS) to organize, store, and publish their material, including RSS feeds. Examples include Drupal and Plone, which are both free, open source programs. Stand-alone RSS creation programs also exist; one popular example is RSS Builder, also a free and open source.
Disadvantages of free systems include advertisements (which can sometimes be removed for a monthly fee) and little or no technical support – and you will probably be limited to a single feed. You’ll also have to add and update headlines, links, and descriptive text manually. Your free feed can become quite expensive if you or staffers are forced to spend an inordinate amount of time maintaining it. Paid RSS editors like FeedForAll and NewzAlert Composer allow easier and less time-consuming content creation and maintenance.
Once you have picked a service or application, you can create your first feed, a process that will be different from program to program. But all feeds will need some basic data: a name (which should be the same as your practice or website); the URL for your website, to help viewers link back to your home page; and a description – a sentence or two describing the general content on the feed.
The next step is to populate the feed with content. Enter the title of each article, blog post, podcast episode, etc.; the URL that links directly to that content; and the publishing date. Each entry should have its own short, interesting description, which is what potential readers will see before they choose to click your entry in their RSS readers, and a global unique identifier (GUID), which the RSS readers use to detect changes or updates.
When all of your content is entered, all that remains is to export your feed to an extensible markup language (XML) file, which will allow visitors to subscribe to it. Upload the XML file to your website, place it on your home page, and click the “publish feed” button.
Once your feed is live, you’ll want to list it on some of the many RSS feed directories to maximize its visibility on search engines. There are literally hundreds of such directories; look for medically oriented ones that do not charge fees, and do not require a reciprocal link back to their website. Add each directory’s URL to your XML file.
Addendum: In my December 2017 column (“Your Online Reputation”), I suggested encouraging your most devoted patients to post favorable reviews about you on the “rating” websites. Several readers (including a practice consultant) have suggested making a laptop or tablet available in your office for that purpose. While that sounds like a great idea, most rating portals track incoming IP addresses, and automatically reject multiple reviews originating from the same computer.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
In my last column, I reviewed the reasons why RSS news feeds can be a useful tool for keeping abreast on frequently updated information, including blog entries, news headlines, audio, and video, without having to check multiple Web pages every day.
This can help increase readership on your website, publicize a podcast, or keep your patients up to date on the latest treatments and procedures in your practice. And if your name appears in news or gossip sites, you will be alerted immediately.
Alternatively, many organizations that publish their own articles and news stories use a content management system (CMS) to organize, store, and publish their material, including RSS feeds. Examples include Drupal and Plone, which are both free, open source programs. Stand-alone RSS creation programs also exist; one popular example is RSS Builder, also a free and open source.
Disadvantages of free systems include advertisements (which can sometimes be removed for a monthly fee) and little or no technical support – and you will probably be limited to a single feed. You’ll also have to add and update headlines, links, and descriptive text manually. Your free feed can become quite expensive if you or staffers are forced to spend an inordinate amount of time maintaining it. Paid RSS editors like FeedForAll and NewzAlert Composer allow easier and less time-consuming content creation and maintenance.
Once you have picked a service or application, you can create your first feed, a process that will be different from program to program. But all feeds will need some basic data: a name (which should be the same as your practice or website); the URL for your website, to help viewers link back to your home page; and a description – a sentence or two describing the general content on the feed.
The next step is to populate the feed with content. Enter the title of each article, blog post, podcast episode, etc.; the URL that links directly to that content; and the publishing date. Each entry should have its own short, interesting description, which is what potential readers will see before they choose to click your entry in their RSS readers, and a global unique identifier (GUID), which the RSS readers use to detect changes or updates.
When all of your content is entered, all that remains is to export your feed to an extensible markup language (XML) file, which will allow visitors to subscribe to it. Upload the XML file to your website, place it on your home page, and click the “publish feed” button.
Once your feed is live, you’ll want to list it on some of the many RSS feed directories to maximize its visibility on search engines. There are literally hundreds of such directories; look for medically oriented ones that do not charge fees, and do not require a reciprocal link back to their website. Add each directory’s URL to your XML file.
Addendum: In my December 2017 column (“Your Online Reputation”), I suggested encouraging your most devoted patients to post favorable reviews about you on the “rating” websites. Several readers (including a practice consultant) have suggested making a laptop or tablet available in your office for that purpose. While that sounds like a great idea, most rating portals track incoming IP addresses, and automatically reject multiple reviews originating from the same computer.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
In my last column, I reviewed the reasons why RSS news feeds can be a useful tool for keeping abreast on frequently updated information, including blog entries, news headlines, audio, and video, without having to check multiple Web pages every day.
This can help increase readership on your website, publicize a podcast, or keep your patients up to date on the latest treatments and procedures in your practice. And if your name appears in news or gossip sites, you will be alerted immediately.
Alternatively, many organizations that publish their own articles and news stories use a content management system (CMS) to organize, store, and publish their material, including RSS feeds. Examples include Drupal and Plone, which are both free, open source programs. Stand-alone RSS creation programs also exist; one popular example is RSS Builder, also a free and open source.
Disadvantages of free systems include advertisements (which can sometimes be removed for a monthly fee) and little or no technical support – and you will probably be limited to a single feed. You’ll also have to add and update headlines, links, and descriptive text manually. Your free feed can become quite expensive if you or staffers are forced to spend an inordinate amount of time maintaining it. Paid RSS editors like FeedForAll and NewzAlert Composer allow easier and less time-consuming content creation and maintenance.
Once you have picked a service or application, you can create your first feed, a process that will be different from program to program. But all feeds will need some basic data: a name (which should be the same as your practice or website); the URL for your website, to help viewers link back to your home page; and a description – a sentence or two describing the general content on the feed.
The next step is to populate the feed with content. Enter the title of each article, blog post, podcast episode, etc.; the URL that links directly to that content; and the publishing date. Each entry should have its own short, interesting description, which is what potential readers will see before they choose to click your entry in their RSS readers, and a global unique identifier (GUID), which the RSS readers use to detect changes or updates.
When all of your content is entered, all that remains is to export your feed to an extensible markup language (XML) file, which will allow visitors to subscribe to it. Upload the XML file to your website, place it on your home page, and click the “publish feed” button.
Once your feed is live, you’ll want to list it on some of the many RSS feed directories to maximize its visibility on search engines. There are literally hundreds of such directories; look for medically oriented ones that do not charge fees, and do not require a reciprocal link back to their website. Add each directory’s URL to your XML file.
Addendum: In my December 2017 column (“Your Online Reputation”), I suggested encouraging your most devoted patients to post favorable reviews about you on the “rating” websites. Several readers (including a practice consultant) have suggested making a laptop or tablet available in your office for that purpose. While that sounds like a great idea, most rating portals track incoming IP addresses, and automatically reject multiple reviews originating from the same computer.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News.
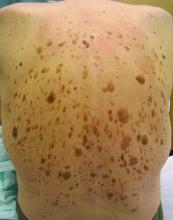
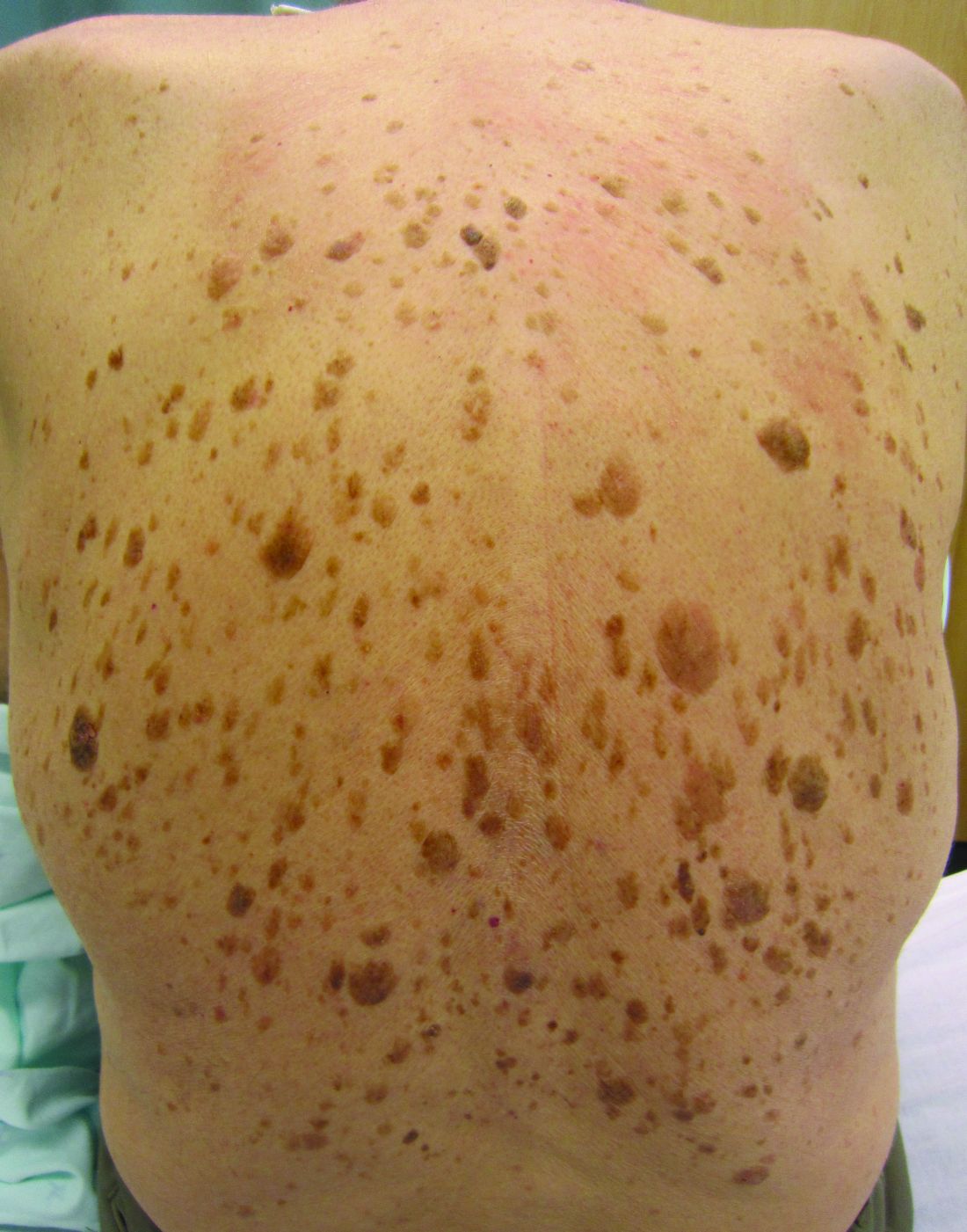
High-dose hydrogen peroxide for SKs
Formerly known as A-101, . It is a 40% hydrogen peroxide topical solution that is applied to raised SKs as an in-office procedure. As previously reported, SKs are composed of hyperadherent senescent cells that are arrested in the G1 phase of the cell cycle. They exhibit decreased apoptotic cell death, compared with normal skin.
Two double-blind vehicle-controlled studies demonstrated that more patients were clear or near clear of SKs after use of the 40% concentration solution than those in the vehicle group, according to the prescribing information. In the studies, patients with four raised SKs of the face, trunk or extremities were treated with Eskata at baseline and 3 weeks later, if necessary, or a vehicle. None of those in the vehicle group were cleared at follow-up (day 106), compared with 4% and 8% of those treated with Eskata, and at least three of the four lesions treated with Eskata had cleared in 13% and 23% at follow-up. Local skin reactions were mostly mild and transient, the most common being itching, stinging, crusting, swelling, redness, and scaling at the application site.
In the clinical studies, the solution was applied up to two times, on day 0 and again 3 weeks later on day 22. The lesion should first be cleansed with alcohol, and appropriate measures should be taken to ensure Eskata does not come in contact with the eyes. Nitrile or vinyl examination gloves should be used for application. Eskata is applied to the SKs with a pen-like applicator. The solution is applied uniformly in a circular motion with excess and the surrounding area patted dry with an absorbent wipe. Cotton gauze, tips, paper towels, or tissue should not be used as organic compounds can react with high concentrations of hydrogen peroxide. In a treatment session, one lesion may be treated up to four times, 1 minute apart. The applicator is used only once and may be discarded after lesions are treated. The packages may be stored at controlled room temperature (68° F to 77° F).
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Dr. Wesley has served on an advisory board panel for Aclaris, the manufacturer of Eskata. Dr. Talakoub had no related disclosures. Write to them at [email protected].
Formerly known as A-101, . It is a 40% hydrogen peroxide topical solution that is applied to raised SKs as an in-office procedure. As previously reported, SKs are composed of hyperadherent senescent cells that are arrested in the G1 phase of the cell cycle. They exhibit decreased apoptotic cell death, compared with normal skin.
Two double-blind vehicle-controlled studies demonstrated that more patients were clear or near clear of SKs after use of the 40% concentration solution than those in the vehicle group, according to the prescribing information. In the studies, patients with four raised SKs of the face, trunk or extremities were treated with Eskata at baseline and 3 weeks later, if necessary, or a vehicle. None of those in the vehicle group were cleared at follow-up (day 106), compared with 4% and 8% of those treated with Eskata, and at least three of the four lesions treated with Eskata had cleared in 13% and 23% at follow-up. Local skin reactions were mostly mild and transient, the most common being itching, stinging, crusting, swelling, redness, and scaling at the application site.
In the clinical studies, the solution was applied up to two times, on day 0 and again 3 weeks later on day 22. The lesion should first be cleansed with alcohol, and appropriate measures should be taken to ensure Eskata does not come in contact with the eyes. Nitrile or vinyl examination gloves should be used for application. Eskata is applied to the SKs with a pen-like applicator. The solution is applied uniformly in a circular motion with excess and the surrounding area patted dry with an absorbent wipe. Cotton gauze, tips, paper towels, or tissue should not be used as organic compounds can react with high concentrations of hydrogen peroxide. In a treatment session, one lesion may be treated up to four times, 1 minute apart. The applicator is used only once and may be discarded after lesions are treated. The packages may be stored at controlled room temperature (68° F to 77° F).
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Dr. Wesley has served on an advisory board panel for Aclaris, the manufacturer of Eskata. Dr. Talakoub had no related disclosures. Write to them at [email protected].
Formerly known as A-101, . It is a 40% hydrogen peroxide topical solution that is applied to raised SKs as an in-office procedure. As previously reported, SKs are composed of hyperadherent senescent cells that are arrested in the G1 phase of the cell cycle. They exhibit decreased apoptotic cell death, compared with normal skin.
Two double-blind vehicle-controlled studies demonstrated that more patients were clear or near clear of SKs after use of the 40% concentration solution than those in the vehicle group, according to the prescribing information. In the studies, patients with four raised SKs of the face, trunk or extremities were treated with Eskata at baseline and 3 weeks later, if necessary, or a vehicle. None of those in the vehicle group were cleared at follow-up (day 106), compared with 4% and 8% of those treated with Eskata, and at least three of the four lesions treated with Eskata had cleared in 13% and 23% at follow-up. Local skin reactions were mostly mild and transient, the most common being itching, stinging, crusting, swelling, redness, and scaling at the application site.
In the clinical studies, the solution was applied up to two times, on day 0 and again 3 weeks later on day 22. The lesion should first be cleansed with alcohol, and appropriate measures should be taken to ensure Eskata does not come in contact with the eyes. Nitrile or vinyl examination gloves should be used for application. Eskata is applied to the SKs with a pen-like applicator. The solution is applied uniformly in a circular motion with excess and the surrounding area patted dry with an absorbent wipe. Cotton gauze, tips, paper towels, or tissue should not be used as organic compounds can react with high concentrations of hydrogen peroxide. In a treatment session, one lesion may be treated up to four times, 1 minute apart. The applicator is used only once and may be discarded after lesions are treated. The packages may be stored at controlled room temperature (68° F to 77° F).
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Dr. Wesley has served on an advisory board panel for Aclaris, the manufacturer of Eskata. Dr. Talakoub had no related disclosures. Write to them at [email protected].
MedPAC recommends scrapping MIPS, gets pushback from doctors
WASHINGTON – As unpopular as the new Quality Payment Program may be, repealing all or part of it at this stage will be a tough sell.
That was the message heard by members of the Medicare Payment Advisory Commission (MedPAC) during public comments at their Jan. 11 meeting. Comments followed a 14-2 vote by commissioners in favor of recommending that Congress scrap the Merit-Based Incentive Payment System (MIPS) track of the QPP.
“We do agree that there are problems with MIPS,” said Sharon McIlrath, assistant director of federal affairs and coalitions at the American Medical Association. “We would like to fix it rather than kill it, and partly that’s because we don’t want to send shifting messages to physicians. Are they going to invest in building an infrastructure on shifting ground?”
She also questioned whether this proposal could gain any traction at all in Congress.
“We don’t think that it is politically viable to think that you are going to go up there and get the Hill to kill MIPS,” she said.
The Alliance of Specialty Medicine in a Jan. 9 letter to MedPAC also voiced its objections to the commission’s plan to recommend the end of MIPS.
“Our efforts to work with CMS and congressional leaders to improve MIPS and allow for more meaningful and robust engagement are ongoing. We urge you to withdraw your forthcoming recommendation, which diminishes the important role of specialty medicine in Medicare,” alliance members wrote to MedPAC Chairman Francis J. Crosson, MD. “Instead, the commission and staff, under your leadership, should work toward a new recommendation that would improve aspects of the MIPS program that remain a challenge for all clinicians.”
MedPAC had been working on its recommendations regarding MIPS since the program was launched, but ultimately came to the conclusion that it was not fixable. During a presentation on the draft recommendation, MedPAC staff listed a variety of reasons why MIPS “cannot succeed,” including how it replicates flaws of previous value-based purchasing plans and is burdensome and complex, the information reported is not meaningful, scores are not comparable across clinicians, and the payment adjustments, while minimal early on, could vary widely from year to year with even the smallest of MIPS score changes.
Instead, current draft MedPAC recommendations put forward a voluntary value program (VVP) to replace MIPS. VVP would withhold a specific percentage of Medicare pay for physicians who are not involved in a QPP advanced Alternative Payment Model (APM).
Physicians would be able to earn back the withheld pay, plus be eligible for potential bonuses by voluntarily participating in virtual groups. Those groups would be scored on population-based measures.
As was the case across previous meetings where this was discussed, commissioners David Nerenz, PhD, of the Henry Ford Health System of Detroit, and Alice Coombs, MD, of South Shore Hospital, Weymouth, Mass., continued to voice their objections about repealing MIPS and ultimately voted against the recommendation.
The MIPS recommendation will be included in MedPAC’s June report to Congress; it then will be up to Congress to decide whether to act on it.
WASHINGTON – As unpopular as the new Quality Payment Program may be, repealing all or part of it at this stage will be a tough sell.
That was the message heard by members of the Medicare Payment Advisory Commission (MedPAC) during public comments at their Jan. 11 meeting. Comments followed a 14-2 vote by commissioners in favor of recommending that Congress scrap the Merit-Based Incentive Payment System (MIPS) track of the QPP.
“We do agree that there are problems with MIPS,” said Sharon McIlrath, assistant director of federal affairs and coalitions at the American Medical Association. “We would like to fix it rather than kill it, and partly that’s because we don’t want to send shifting messages to physicians. Are they going to invest in building an infrastructure on shifting ground?”
She also questioned whether this proposal could gain any traction at all in Congress.
“We don’t think that it is politically viable to think that you are going to go up there and get the Hill to kill MIPS,” she said.
The Alliance of Specialty Medicine in a Jan. 9 letter to MedPAC also voiced its objections to the commission’s plan to recommend the end of MIPS.
“Our efforts to work with CMS and congressional leaders to improve MIPS and allow for more meaningful and robust engagement are ongoing. We urge you to withdraw your forthcoming recommendation, which diminishes the important role of specialty medicine in Medicare,” alliance members wrote to MedPAC Chairman Francis J. Crosson, MD. “Instead, the commission and staff, under your leadership, should work toward a new recommendation that would improve aspects of the MIPS program that remain a challenge for all clinicians.”
MedPAC had been working on its recommendations regarding MIPS since the program was launched, but ultimately came to the conclusion that it was not fixable. During a presentation on the draft recommendation, MedPAC staff listed a variety of reasons why MIPS “cannot succeed,” including how it replicates flaws of previous value-based purchasing plans and is burdensome and complex, the information reported is not meaningful, scores are not comparable across clinicians, and the payment adjustments, while minimal early on, could vary widely from year to year with even the smallest of MIPS score changes.
Instead, current draft MedPAC recommendations put forward a voluntary value program (VVP) to replace MIPS. VVP would withhold a specific percentage of Medicare pay for physicians who are not involved in a QPP advanced Alternative Payment Model (APM).
Physicians would be able to earn back the withheld pay, plus be eligible for potential bonuses by voluntarily participating in virtual groups. Those groups would be scored on population-based measures.
As was the case across previous meetings where this was discussed, commissioners David Nerenz, PhD, of the Henry Ford Health System of Detroit, and Alice Coombs, MD, of South Shore Hospital, Weymouth, Mass., continued to voice their objections about repealing MIPS and ultimately voted against the recommendation.
The MIPS recommendation will be included in MedPAC’s June report to Congress; it then will be up to Congress to decide whether to act on it.
WASHINGTON – As unpopular as the new Quality Payment Program may be, repealing all or part of it at this stage will be a tough sell.
That was the message heard by members of the Medicare Payment Advisory Commission (MedPAC) during public comments at their Jan. 11 meeting. Comments followed a 14-2 vote by commissioners in favor of recommending that Congress scrap the Merit-Based Incentive Payment System (MIPS) track of the QPP.
“We do agree that there are problems with MIPS,” said Sharon McIlrath, assistant director of federal affairs and coalitions at the American Medical Association. “We would like to fix it rather than kill it, and partly that’s because we don’t want to send shifting messages to physicians. Are they going to invest in building an infrastructure on shifting ground?”
She also questioned whether this proposal could gain any traction at all in Congress.
“We don’t think that it is politically viable to think that you are going to go up there and get the Hill to kill MIPS,” she said.
The Alliance of Specialty Medicine in a Jan. 9 letter to MedPAC also voiced its objections to the commission’s plan to recommend the end of MIPS.
“Our efforts to work with CMS and congressional leaders to improve MIPS and allow for more meaningful and robust engagement are ongoing. We urge you to withdraw your forthcoming recommendation, which diminishes the important role of specialty medicine in Medicare,” alliance members wrote to MedPAC Chairman Francis J. Crosson, MD. “Instead, the commission and staff, under your leadership, should work toward a new recommendation that would improve aspects of the MIPS program that remain a challenge for all clinicians.”
MedPAC had been working on its recommendations regarding MIPS since the program was launched, but ultimately came to the conclusion that it was not fixable. During a presentation on the draft recommendation, MedPAC staff listed a variety of reasons why MIPS “cannot succeed,” including how it replicates flaws of previous value-based purchasing plans and is burdensome and complex, the information reported is not meaningful, scores are not comparable across clinicians, and the payment adjustments, while minimal early on, could vary widely from year to year with even the smallest of MIPS score changes.
Instead, current draft MedPAC recommendations put forward a voluntary value program (VVP) to replace MIPS. VVP would withhold a specific percentage of Medicare pay for physicians who are not involved in a QPP advanced Alternative Payment Model (APM).
Physicians would be able to earn back the withheld pay, plus be eligible for potential bonuses by voluntarily participating in virtual groups. Those groups would be scored on population-based measures.
As was the case across previous meetings where this was discussed, commissioners David Nerenz, PhD, of the Henry Ford Health System of Detroit, and Alice Coombs, MD, of South Shore Hospital, Weymouth, Mass., continued to voice their objections about repealing MIPS and ultimately voted against the recommendation.
The MIPS recommendation will be included in MedPAC’s June report to Congress; it then will be up to Congress to decide whether to act on it.
REPORTING FROM A MEDPAC MEETING
Debunking Atopic Dermatitis Myths: Should Patients Avoid Products With Parabens?
Myth: Parabens are dangerous
Some atopic dermatitis (AD) patients may be misinformed by reports that parabens have estrogenic and antiandrogenic effects and may be involved in carcinogenesis via endocrine modulation. Although in Europe some parabens have been banned or restricted, in the United States there are no regulations against the use of parabens in cosmetics. Dermatologists must acknowledge that their AD patients may have concerns about cosmetic products and they must be prepared to dispel any myths.
Parabens such as methylparaben, propylparaben, butylparaben, and ethylparaben are common in cosmetics such as moisturizers. Parabens have protective properties to prevent the growth of harmful bacteria and mold. According to the US Food and Drug Administration, “scientists continue to review published studies on the safety of parabens. At this time, we do not have information showing that parabens as they are used in cosmetics have an effect on human health. . . . If we determine that a health hazard exists, we will advise the industry and the public.”
Here are some important facts to note for patients, based on a research article published in Cosmetics & Toiletries in June 2017:
- Parabens are not toxic at the concentrations used in personal care products
- Parabens are not genotoxic or carcinogenic
- Parabens are readily excreted in urine and do not accumulate in tissues
In patients with chronic dermatitis, the Cosmetic Ingredient Review Expert Panel reported that parabens generally induce sensitization in less than 4% of patients. The panel concluded that they can support the safety of cosmetic products in which parabens are used as preservatives.
In fact, one study published in the Journal of the American Academy of Dermatology found that AD patients were not predisposed to allergies to parabens, formaldehyde, or diazolidinyl urea, but they were more likely to have allergic reactions to formaldehyde releasers. As a result, AD patients should choose moisturizers containing parabens and should have no fears about using them.
Expert Commentary
In general I recommend paraben-containing cleansers and emollients on a daily basis in practice. However, patient concerns exist due to negative online content easily accessed and fear can prevent usage of agents. Therefore, I am also open to offering options lacking in parabens, such as coconut oil.
—Nanette B. Silverberg, MD (New York, New York)
Doyle K. Some skin creams bad news for eczema. Reuters. December 12, 2013. https://www.reuters.com/article/us-skin-creams-eczema/some-skin-creams-bad-news-for-eczema-idUSBRE9BB14720131212. Accessed January 12, 2018.
Final amended report on the safety assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
Krowka JF, Loretz L, Geis PA, et al. Preserving the facts on parabens: an overview of these important tools of the trade. Cosmetics & Toiletries. June 1, 2017. http://www.cosmeticsandtoiletries.com/research/chemistry/Preserving-the-Facts-on-Parabens-An-Overview-of-These-Important-Tools-of-the-Trade-425784294.html. Accessed January 12, 2018.
Parabens in cosmetics. US Food and Drug Administration website. https://www.fda.gov/Cosmetics/ProductsIngredients/Ingredients/ucm128042.html. Accessed January 12, 2018.
Sasseville D, Alfalah M, Lacroix JP. “Parabenoia” debunked, or “who’s afraid of parabens?” Dermatitis. 2015;26:254-259.
Shaughnessy CN, Malajian D, Belsito DV. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis: reactivity to topical preservatives. J Am Acad Dermatol. 2014;70:102-107.
Myth: Parabens are dangerous
Some atopic dermatitis (AD) patients may be misinformed by reports that parabens have estrogenic and antiandrogenic effects and may be involved in carcinogenesis via endocrine modulation. Although in Europe some parabens have been banned or restricted, in the United States there are no regulations against the use of parabens in cosmetics. Dermatologists must acknowledge that their AD patients may have concerns about cosmetic products and they must be prepared to dispel any myths.
Parabens such as methylparaben, propylparaben, butylparaben, and ethylparaben are common in cosmetics such as moisturizers. Parabens have protective properties to prevent the growth of harmful bacteria and mold. According to the US Food and Drug Administration, “scientists continue to review published studies on the safety of parabens. At this time, we do not have information showing that parabens as they are used in cosmetics have an effect on human health. . . . If we determine that a health hazard exists, we will advise the industry and the public.”
Here are some important facts to note for patients, based on a research article published in Cosmetics & Toiletries in June 2017:
- Parabens are not toxic at the concentrations used in personal care products
- Parabens are not genotoxic or carcinogenic
- Parabens are readily excreted in urine and do not accumulate in tissues
In patients with chronic dermatitis, the Cosmetic Ingredient Review Expert Panel reported that parabens generally induce sensitization in less than 4% of patients. The panel concluded that they can support the safety of cosmetic products in which parabens are used as preservatives.
In fact, one study published in the Journal of the American Academy of Dermatology found that AD patients were not predisposed to allergies to parabens, formaldehyde, or diazolidinyl urea, but they were more likely to have allergic reactions to formaldehyde releasers. As a result, AD patients should choose moisturizers containing parabens and should have no fears about using them.
Expert Commentary
In general I recommend paraben-containing cleansers and emollients on a daily basis in practice. However, patient concerns exist due to negative online content easily accessed and fear can prevent usage of agents. Therefore, I am also open to offering options lacking in parabens, such as coconut oil.
—Nanette B. Silverberg, MD (New York, New York)
Myth: Parabens are dangerous
Some atopic dermatitis (AD) patients may be misinformed by reports that parabens have estrogenic and antiandrogenic effects and may be involved in carcinogenesis via endocrine modulation. Although in Europe some parabens have been banned or restricted, in the United States there are no regulations against the use of parabens in cosmetics. Dermatologists must acknowledge that their AD patients may have concerns about cosmetic products and they must be prepared to dispel any myths.
Parabens such as methylparaben, propylparaben, butylparaben, and ethylparaben are common in cosmetics such as moisturizers. Parabens have protective properties to prevent the growth of harmful bacteria and mold. According to the US Food and Drug Administration, “scientists continue to review published studies on the safety of parabens. At this time, we do not have information showing that parabens as they are used in cosmetics have an effect on human health. . . . If we determine that a health hazard exists, we will advise the industry and the public.”
Here are some important facts to note for patients, based on a research article published in Cosmetics & Toiletries in June 2017:
- Parabens are not toxic at the concentrations used in personal care products
- Parabens are not genotoxic or carcinogenic
- Parabens are readily excreted in urine and do not accumulate in tissues
In patients with chronic dermatitis, the Cosmetic Ingredient Review Expert Panel reported that parabens generally induce sensitization in less than 4% of patients. The panel concluded that they can support the safety of cosmetic products in which parabens are used as preservatives.
In fact, one study published in the Journal of the American Academy of Dermatology found that AD patients were not predisposed to allergies to parabens, formaldehyde, or diazolidinyl urea, but they were more likely to have allergic reactions to formaldehyde releasers. As a result, AD patients should choose moisturizers containing parabens and should have no fears about using them.
Expert Commentary
In general I recommend paraben-containing cleansers and emollients on a daily basis in practice. However, patient concerns exist due to negative online content easily accessed and fear can prevent usage of agents. Therefore, I am also open to offering options lacking in parabens, such as coconut oil.
—Nanette B. Silverberg, MD (New York, New York)
Doyle K. Some skin creams bad news for eczema. Reuters. December 12, 2013. https://www.reuters.com/article/us-skin-creams-eczema/some-skin-creams-bad-news-for-eczema-idUSBRE9BB14720131212. Accessed January 12, 2018.
Final amended report on the safety assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
Krowka JF, Loretz L, Geis PA, et al. Preserving the facts on parabens: an overview of these important tools of the trade. Cosmetics & Toiletries. June 1, 2017. http://www.cosmeticsandtoiletries.com/research/chemistry/Preserving-the-Facts-on-Parabens-An-Overview-of-These-Important-Tools-of-the-Trade-425784294.html. Accessed January 12, 2018.
Parabens in cosmetics. US Food and Drug Administration website. https://www.fda.gov/Cosmetics/ProductsIngredients/Ingredients/ucm128042.html. Accessed January 12, 2018.
Sasseville D, Alfalah M, Lacroix JP. “Parabenoia” debunked, or “who’s afraid of parabens?” Dermatitis. 2015;26:254-259.
Shaughnessy CN, Malajian D, Belsito DV. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis: reactivity to topical preservatives. J Am Acad Dermatol. 2014;70:102-107.
Doyle K. Some skin creams bad news for eczema. Reuters. December 12, 2013. https://www.reuters.com/article/us-skin-creams-eczema/some-skin-creams-bad-news-for-eczema-idUSBRE9BB14720131212. Accessed January 12, 2018.
Final amended report on the safety assessment of Methylparaben, Ethylparaben, Propylparaben, Isopropylparaben, Butylparaben, Isobutylparaben, and Benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27(suppl 4):1-82.
Krowka JF, Loretz L, Geis PA, et al. Preserving the facts on parabens: an overview of these important tools of the trade. Cosmetics & Toiletries. June 1, 2017. http://www.cosmeticsandtoiletries.com/research/chemistry/Preserving-the-Facts-on-Parabens-An-Overview-of-These-Important-Tools-of-the-Trade-425784294.html. Accessed January 12, 2018.
Parabens in cosmetics. US Food and Drug Administration website. https://www.fda.gov/Cosmetics/ProductsIngredients/Ingredients/ucm128042.html. Accessed January 12, 2018.
Sasseville D, Alfalah M, Lacroix JP. “Parabenoia” debunked, or “who’s afraid of parabens?” Dermatitis. 2015;26:254-259.
Shaughnessy CN, Malajian D, Belsito DV. Cutaneous delayed-type hypersensitivity in patients with atopic dermatitis: reactivity to topical preservatives. J Am Acad Dermatol. 2014;70:102-107.
POSH study: BRCA mutations did not influence survival in young onset breast cancer
For women with young-onset breast cancer, presence of a BRCA mutation did not significantly impact survival, according to results of the Prospective Outcomes in Sporadic versus Hereditary breast cancer (POSH) study.
BRCA-positive and BRCA-negative women had similar overall survival at 2 years, 5 years, and 10 years after diagnosis, according to lead author Ellen R. Copson, MD, a senior lecturer in medical oncology in the cancer sciences division, University of Southampton (England) and her study coauthors.
Currently, young women with breast cancer and a BRCA mutation frequently are offered bilateral mastectomy, the authors noted.
The prospective cohort study by Dr. Copson and her colleagues included 2,733 women with breast cancer who were aged 40 years or younger at first diagnosis. Of those patients, 338 (12%) had either a BRCA1 or BRCA2 mutation, according to investigators.
At 2 years after diagnosis, overall survival was 97.0% and 96.6% for BRCA-positive and BRCA-negative patients, respectively, the report said. Similarly, overall survival was 83.8% and 85.0% for the two groups at 5 years after diagnosis, and 73.4% vs. 70.1% at 10 years.
Multivariable analysis accounting for known prognostic factors including ethnicity and body mass index showed there was no significant difference between groups (hazard ratio, 0.96; 95% confidence interval, 0.76-1.22; P = .76), the authors wrote.
Triple-negative breast cancer patients with a BRCA mutation might have a survival advantage in the first few years following diagnosis,compared with non-BRCA carriers, the POSH study also found. Researchers reported a significant difference at 2 years (95% for BRCA-positive vs. 91% for BRCA-negative patients; P = .047), but there was no significant difference between arms at 5 or 10 years.
POSH is believed to be the largest prospective cohort study to compare breast cancer outcomes for patients with BRCA mutations to those with sporadic breast cancer. Previous studies, primarily retrospective, have suggested “better, worse, or similar outcomes” for BRCA-positive versus BRCA-negative patients, the authors wrote. Dr. Copson reported receiving honoraria from Roche, while her coauthors reported honoraria from GSK, Pfizer, AstraZeneca, and Pierre Fabre. Funding for the study was provided by the Wessex Cancer Trust, Cancer Research UK, and Breast Cancer Now.
SOURCE: Copson et al. Lancet Oncol. 2018 Jan 11 doi: 10.1016/S1470-2045(17)30891-4.
The POSH prospective cohort study, which showed no significant difference in survival for BRCA-positive versus BRCA-negative young onset breast cancer patients, has contributed to the understanding of this patient population, providing “comprehensive data about patient, tumor, and treatment characteristics, along with extensive follow-up data,” wrote Peter A. Fasching, MD, in accompanying editorial.
“Understanding prognosis in young patients is important because patients with BRCA mutations are at increased risk of developing specific conditions, such as secondary cancers,” Dr. Fasching said. “These risks determine treatment, and knowing that BRCA1 or BRCA2 mutations do not result in a different prognosis might change the therapeutic approach for these risks.”
Moreover, in retrospective analyses, bilateral mastectomy conferred an overall survival benefit for BRCA mutation carriers: “This important topic needs more prospective research, as preventive surgical measures might have an effect on what might be a very long life after a diagnosis of breast cancer at a young age,” said Dr. Fasching. “The data from POSH deliver a rationale for prospective studies to address these questions.”
Dr. Peter A. Fasching is with Friedrich-Alexander University Erlangen-Nuremberg, Germany. These comments are based on his editorial appearing in Lancet Oncology (2018 Jan 11. doi: 10.1016/S1470-2045(18)30008-1). Dr. Fasching declared grants from Novartis, along with personal fees from Novartis, Pfizer, Roche, Teva, and Amgen.
The POSH prospective cohort study, which showed no significant difference in survival for BRCA-positive versus BRCA-negative young onset breast cancer patients, has contributed to the understanding of this patient population, providing “comprehensive data about patient, tumor, and treatment characteristics, along with extensive follow-up data,” wrote Peter A. Fasching, MD, in accompanying editorial.
“Understanding prognosis in young patients is important because patients with BRCA mutations are at increased risk of developing specific conditions, such as secondary cancers,” Dr. Fasching said. “These risks determine treatment, and knowing that BRCA1 or BRCA2 mutations do not result in a different prognosis might change the therapeutic approach for these risks.”
Moreover, in retrospective analyses, bilateral mastectomy conferred an overall survival benefit for BRCA mutation carriers: “This important topic needs more prospective research, as preventive surgical measures might have an effect on what might be a very long life after a diagnosis of breast cancer at a young age,” said Dr. Fasching. “The data from POSH deliver a rationale for prospective studies to address these questions.”
Dr. Peter A. Fasching is with Friedrich-Alexander University Erlangen-Nuremberg, Germany. These comments are based on his editorial appearing in Lancet Oncology (2018 Jan 11. doi: 10.1016/S1470-2045(18)30008-1). Dr. Fasching declared grants from Novartis, along with personal fees from Novartis, Pfizer, Roche, Teva, and Amgen.
The POSH prospective cohort study, which showed no significant difference in survival for BRCA-positive versus BRCA-negative young onset breast cancer patients, has contributed to the understanding of this patient population, providing “comprehensive data about patient, tumor, and treatment characteristics, along with extensive follow-up data,” wrote Peter A. Fasching, MD, in accompanying editorial.
“Understanding prognosis in young patients is important because patients with BRCA mutations are at increased risk of developing specific conditions, such as secondary cancers,” Dr. Fasching said. “These risks determine treatment, and knowing that BRCA1 or BRCA2 mutations do not result in a different prognosis might change the therapeutic approach for these risks.”
Moreover, in retrospective analyses, bilateral mastectomy conferred an overall survival benefit for BRCA mutation carriers: “This important topic needs more prospective research, as preventive surgical measures might have an effect on what might be a very long life after a diagnosis of breast cancer at a young age,” said Dr. Fasching. “The data from POSH deliver a rationale for prospective studies to address these questions.”
Dr. Peter A. Fasching is with Friedrich-Alexander University Erlangen-Nuremberg, Germany. These comments are based on his editorial appearing in Lancet Oncology (2018 Jan 11. doi: 10.1016/S1470-2045(18)30008-1). Dr. Fasching declared grants from Novartis, along with personal fees from Novartis, Pfizer, Roche, Teva, and Amgen.
For women with young-onset breast cancer, presence of a BRCA mutation did not significantly impact survival, according to results of the Prospective Outcomes in Sporadic versus Hereditary breast cancer (POSH) study.
BRCA-positive and BRCA-negative women had similar overall survival at 2 years, 5 years, and 10 years after diagnosis, according to lead author Ellen R. Copson, MD, a senior lecturer in medical oncology in the cancer sciences division, University of Southampton (England) and her study coauthors.
Currently, young women with breast cancer and a BRCA mutation frequently are offered bilateral mastectomy, the authors noted.
The prospective cohort study by Dr. Copson and her colleagues included 2,733 women with breast cancer who were aged 40 years or younger at first diagnosis. Of those patients, 338 (12%) had either a BRCA1 or BRCA2 mutation, according to investigators.
At 2 years after diagnosis, overall survival was 97.0% and 96.6% for BRCA-positive and BRCA-negative patients, respectively, the report said. Similarly, overall survival was 83.8% and 85.0% for the two groups at 5 years after diagnosis, and 73.4% vs. 70.1% at 10 years.
Multivariable analysis accounting for known prognostic factors including ethnicity and body mass index showed there was no significant difference between groups (hazard ratio, 0.96; 95% confidence interval, 0.76-1.22; P = .76), the authors wrote.
Triple-negative breast cancer patients with a BRCA mutation might have a survival advantage in the first few years following diagnosis,compared with non-BRCA carriers, the POSH study also found. Researchers reported a significant difference at 2 years (95% for BRCA-positive vs. 91% for BRCA-negative patients; P = .047), but there was no significant difference between arms at 5 or 10 years.
POSH is believed to be the largest prospective cohort study to compare breast cancer outcomes for patients with BRCA mutations to those with sporadic breast cancer. Previous studies, primarily retrospective, have suggested “better, worse, or similar outcomes” for BRCA-positive versus BRCA-negative patients, the authors wrote. Dr. Copson reported receiving honoraria from Roche, while her coauthors reported honoraria from GSK, Pfizer, AstraZeneca, and Pierre Fabre. Funding for the study was provided by the Wessex Cancer Trust, Cancer Research UK, and Breast Cancer Now.
SOURCE: Copson et al. Lancet Oncol. 2018 Jan 11 doi: 10.1016/S1470-2045(17)30891-4.
For women with young-onset breast cancer, presence of a BRCA mutation did not significantly impact survival, according to results of the Prospective Outcomes in Sporadic versus Hereditary breast cancer (POSH) study.
BRCA-positive and BRCA-negative women had similar overall survival at 2 years, 5 years, and 10 years after diagnosis, according to lead author Ellen R. Copson, MD, a senior lecturer in medical oncology in the cancer sciences division, University of Southampton (England) and her study coauthors.
Currently, young women with breast cancer and a BRCA mutation frequently are offered bilateral mastectomy, the authors noted.
The prospective cohort study by Dr. Copson and her colleagues included 2,733 women with breast cancer who were aged 40 years or younger at first diagnosis. Of those patients, 338 (12%) had either a BRCA1 or BRCA2 mutation, according to investigators.
At 2 years after diagnosis, overall survival was 97.0% and 96.6% for BRCA-positive and BRCA-negative patients, respectively, the report said. Similarly, overall survival was 83.8% and 85.0% for the two groups at 5 years after diagnosis, and 73.4% vs. 70.1% at 10 years.
Multivariable analysis accounting for known prognostic factors including ethnicity and body mass index showed there was no significant difference between groups (hazard ratio, 0.96; 95% confidence interval, 0.76-1.22; P = .76), the authors wrote.
Triple-negative breast cancer patients with a BRCA mutation might have a survival advantage in the first few years following diagnosis,compared with non-BRCA carriers, the POSH study also found. Researchers reported a significant difference at 2 years (95% for BRCA-positive vs. 91% for BRCA-negative patients; P = .047), but there was no significant difference between arms at 5 or 10 years.
POSH is believed to be the largest prospective cohort study to compare breast cancer outcomes for patients with BRCA mutations to those with sporadic breast cancer. Previous studies, primarily retrospective, have suggested “better, worse, or similar outcomes” for BRCA-positive versus BRCA-negative patients, the authors wrote. Dr. Copson reported receiving honoraria from Roche, while her coauthors reported honoraria from GSK, Pfizer, AstraZeneca, and Pierre Fabre. Funding for the study was provided by the Wessex Cancer Trust, Cancer Research UK, and Breast Cancer Now.
SOURCE: Copson et al. Lancet Oncol. 2018 Jan 11 doi: 10.1016/S1470-2045(17)30891-4.
FROM LANCET ONCOLOGY
Key clinical point: Presence of BRCA1 or BRCA2 germline mutations did not significantly affect overall survival in women with young onset breast cancer.
Major finding: At 2 years, overall survival was 97.0% for BRCA mutation carriers and 96.6% for non-carriers, with similar results reported at 5 and 10 years.
Data source: A prospective cohort study including 2,733 women with breast cancer who were aged 40 years or younger at first diagnosis.
Disclosures: Funding for the study was provided by the Wessex Cancer Trust, Cancer Research UK, and Breast Cancer Now. Study authors declared honoraria from Roche, GSK, Pfizer, AstraZeneca, and Pierre Fabre.
Source: Copson ER et al. Lancet Oncol. 2018 Jan 11. doi: 10.1016/S1470-2045(17)30891-4.
VIDEO: Anticoagulant underprescribing common, jeopardizing atrial fib patients
ORLANDO – A high fraction of U.S. patients with atrial fibrillation receive an inappropriately low dosage of an anticoagulant for stroke prevention, often in a misguided attempt to avoid potential bleeding complications.
When physicians “reduce the dose to prevent a bleed they increase the risk for an ischemic stroke,” Elaine M. Hylek, MD, said in a video interview during the annual International AF Symposium.
Recent data on actual anticoagulant dosages prescribed to U.S. patients with atrial fibrillation show that “an unexpectedly high proportion of prescriptions for apixaban (Eliquis), dabigatran (Pradaxa), and rivaroxaban (Xarelto) are given at lower doses,” Dr. Hylek noted at the meeting. The lower-dose formulations with U.S. marketing are only appropriate for patients on apixaban with at least two of the following: serum creatinine 1.5 mg/dL or higher, age 80 years or older, and weight 60 kg or less; patients on dabigatran with moderate renal impairment or treated with dronedarone or systemic ketoconazole; or patients on rivaroxaban with a creatinine clearance of 15-50 mL/min.
For example, in the pivotal trial for apixaban only 5% of atrial fibrillation patients qualified for the lower dosage, yet recent data have shown that, in actual U.S. practice roughly a quarter of patients were on this lower dosage, said Dr. Hylek, professor of medicine at Boston University and director of the thrombosis and anticoagulation service at Boston Medical Center (Curr Med Res Opin. 2016 July;32[7]:1277-79). A second recent report showed that among U.S. patients with atrial fibrillation hospitalized for an ischemic stroke 84% had received inadequate anticoagulation with either subtherapeutic dosages of anticoagulant or no anticoagulant at all (JAMA. 2017 Mar 14;317[10]:1057-67).
Another manifestation of the underprescribing problem are patients with atrial fibrillation treated with aspirin only, an approach proven ineffective for preventing ischemic strokes in these patients, Dr. Hylek said.
Dr. Hylek has been an advisor to or has received honoraria from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Doasense, Janssen, Medtronic, Pfizer, and Portola, and she has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, and Janssen.
[email protected]
On Twitter @mitchelzoler
ORLANDO – A high fraction of U.S. patients with atrial fibrillation receive an inappropriately low dosage of an anticoagulant for stroke prevention, often in a misguided attempt to avoid potential bleeding complications.
When physicians “reduce the dose to prevent a bleed they increase the risk for an ischemic stroke,” Elaine M. Hylek, MD, said in a video interview during the annual International AF Symposium.
Recent data on actual anticoagulant dosages prescribed to U.S. patients with atrial fibrillation show that “an unexpectedly high proportion of prescriptions for apixaban (Eliquis), dabigatran (Pradaxa), and rivaroxaban (Xarelto) are given at lower doses,” Dr. Hylek noted at the meeting. The lower-dose formulations with U.S. marketing are only appropriate for patients on apixaban with at least two of the following: serum creatinine 1.5 mg/dL or higher, age 80 years or older, and weight 60 kg or less; patients on dabigatran with moderate renal impairment or treated with dronedarone or systemic ketoconazole; or patients on rivaroxaban with a creatinine clearance of 15-50 mL/min.
For example, in the pivotal trial for apixaban only 5% of atrial fibrillation patients qualified for the lower dosage, yet recent data have shown that, in actual U.S. practice roughly a quarter of patients were on this lower dosage, said Dr. Hylek, professor of medicine at Boston University and director of the thrombosis and anticoagulation service at Boston Medical Center (Curr Med Res Opin. 2016 July;32[7]:1277-79). A second recent report showed that among U.S. patients with atrial fibrillation hospitalized for an ischemic stroke 84% had received inadequate anticoagulation with either subtherapeutic dosages of anticoagulant or no anticoagulant at all (JAMA. 2017 Mar 14;317[10]:1057-67).
Another manifestation of the underprescribing problem are patients with atrial fibrillation treated with aspirin only, an approach proven ineffective for preventing ischemic strokes in these patients, Dr. Hylek said.
Dr. Hylek has been an advisor to or has received honoraria from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Doasense, Janssen, Medtronic, Pfizer, and Portola, and she has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, and Janssen.
[email protected]
On Twitter @mitchelzoler
ORLANDO – A high fraction of U.S. patients with atrial fibrillation receive an inappropriately low dosage of an anticoagulant for stroke prevention, often in a misguided attempt to avoid potential bleeding complications.
When physicians “reduce the dose to prevent a bleed they increase the risk for an ischemic stroke,” Elaine M. Hylek, MD, said in a video interview during the annual International AF Symposium.
Recent data on actual anticoagulant dosages prescribed to U.S. patients with atrial fibrillation show that “an unexpectedly high proportion of prescriptions for apixaban (Eliquis), dabigatran (Pradaxa), and rivaroxaban (Xarelto) are given at lower doses,” Dr. Hylek noted at the meeting. The lower-dose formulations with U.S. marketing are only appropriate for patients on apixaban with at least two of the following: serum creatinine 1.5 mg/dL or higher, age 80 years or older, and weight 60 kg or less; patients on dabigatran with moderate renal impairment or treated with dronedarone or systemic ketoconazole; or patients on rivaroxaban with a creatinine clearance of 15-50 mL/min.
For example, in the pivotal trial for apixaban only 5% of atrial fibrillation patients qualified for the lower dosage, yet recent data have shown that, in actual U.S. practice roughly a quarter of patients were on this lower dosage, said Dr. Hylek, professor of medicine at Boston University and director of the thrombosis and anticoagulation service at Boston Medical Center (Curr Med Res Opin. 2016 July;32[7]:1277-79). A second recent report showed that among U.S. patients with atrial fibrillation hospitalized for an ischemic stroke 84% had received inadequate anticoagulation with either subtherapeutic dosages of anticoagulant or no anticoagulant at all (JAMA. 2017 Mar 14;317[10]:1057-67).
Another manifestation of the underprescribing problem are patients with atrial fibrillation treated with aspirin only, an approach proven ineffective for preventing ischemic strokes in these patients, Dr. Hylek said.
Dr. Hylek has been an advisor to or has received honoraria from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Doasense, Janssen, Medtronic, Pfizer, and Portola, and she has received research funding from Boehringer Ingelheim, Bristol-Myers Squibb, and Janssen.
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE AF SYMPOSIUM 2018
Who fares best after successful ECT?
PARIS – , Pascal Sienaert, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
This conclusion is based on the results of two prospective studies by ResPECT – the Research in Psychiatry and ECT by the Flemish-Dutch Research Consortium – which, in turn, confirm the findings of an earlier metaanalysis of 32 studies including 702 patients conducted by investigators at Trinity College Dublin, noted Dr. Sienaert, a psychiatrist at the Catholic University of Leuven (Belgium) Academic Center for ECT and Neuromodulation.
Dr. Sienaert noted that the relapse rate in the ECT metaanalysis is nearly identical to that reported in the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial in real-world patients with major depression who achieved remission in response to second-step or later antidepressant medication.
“It’s a common misconception that relapse is higher after ECT than medication,” the psychiatrist said.
In the ECT metaanalysis, continuation ECT after induction of remission did not substantially affect the relapse risk. But that’s because the prevailing maintenance ECT strategy in the studies included in the 2013 metaanalysis relied upon a fixed-dose treatment schedule, according to Dr. Sienaert.
“In most studies, fixed-schedule maintenance ECT is used and with rather high relapse rates. Most clinicians have the experience that flexible, clinically driven on an as-needed-basis maintenance ECT has lower relapse rates,” he said. “Still, relapse remains the most pressing issue in the field, and it is very difficult for us as clinicians to predict which patients will relapse and which will not.”
That’s where the two ResPECT studies come into play.
In one of the studies, 116 patients with major depression at three tertiary psychiatric hospitals were randomized double blind to twice-weekly high-dose ultrabrief pulse (0.3-0.4 milliseconds) right unilateral or high-dose brief pulse (1.0 millisecond) right unilateral ECT. The dosing was at eight times the seizure threshold until remission as defined by a Montgomery-Åsberg Depression Rating Scale (MADRS) score below 10 or for a maximum of 6 weeks. Among the 87 completers, the remission rate was 68% in the brief pulse group, significantly higher than the 49% rate with ultrabrief ECT. Cognitive effects on semantic and lexical memory, and retrograde amnesia were the same in the two groups (J Clin Psychiatry. 2013 Nov;74[11]:e1029-36).
Dr. Sienaert and his coinvestigators then prospectively followed the 50 remitters for 6 months, during which all but one patient remained on antidepressant medication. The relapse rate, defined as rehospitalization for depression, restart of ECT, suicide, or a MADRS score above 15, was 25% at 3 months and about 40% at 6 months. The investigators found several predictors of a lower relapse rate. The strongest was early complete remission as defined by a Clinical Global Impressions Scale score of 1 out of a maximum of a possible 7 points within the first four ECT sessions: The 6-month relapse rate was 10% among those early complete remitters versus 63% in the other remitters (J Affect Disord. 2015 Sep 15;184:137-44).
“These are very small numbers in these groups, but the signal that emerges is the same as we have seen in the Irish metaanalysis: Early complete remitters were older, had shorter current episodes of depression, and showed more baseline psychotic features,” Dr. Sienaert said.
In a more recent ResPECT consortium study, the Mood Disorders in Elderly Treated With ECT (MODECT) study, 110 patients aged 55 and older with unipolar depression treated by ECT were followed with serial brain imaging studies prior to and for 6 months post treatment in an effort to gain insight into the mechanism of the particularly strong benefit of ECT in late-life depression. The response rate to ECT was significantly higher in those with onset of depression at age 55 or older than in those with disease onset before age 55, by a margin of 87% vs. 67%. The presence of baseline psychotic symptoms also was associated with a higher response rate.
In contrast, treatment response proved unrelated to changes in hippocampal volume, white matter hypersensitivities, amyloid load, or serum brain-derived neurotrophic factor, which is believed to be an important mediator of neuroplasticity. Thus, ECT’s mechanism of action in late-life depression remains elusive, the authors reported (Am J Geriatr Psychiatry. 2017 Feb;25[2]:178-89).
In a separate study, Dr. Sienaert and his colleagues found that ECT’s superior efficacy, compared with antidepressant medication in patients with late-life depression, was independent of their vascular disease burden. The study population was comprised of 81 patients in an antidepressant drug trial and 43 in an ECT trial, all of whom were inpatients with unipolar major depression. Their mean age was in the mid-70s.
The investigators gauged vascular burden by adding up each patient’s number of vascular risk factors, namely, diabetes, hypertension, smoking, hypercholesterolemia, known cardiovascular disease, and cerebrovascular disease. The depression remission rate was 80% in the ECT patients with no vascular risk factors, dropping to 58% in those with one or more. In the antidepressant drug trial participants, the remission rate was 38% in those with no vascular risk factors, compared with 32% in patients with one or more. Using different cutoffs for the number of vascular risk factors did not significantly alter the results (Int J Geriatr Psychiatry. 2017 Jun 28. doi: 10.1002/gps.4754).
At present, once a patient has achieved remission in response to ECT, most psychiatrists stop the therapy altogether. That’s often a mistake, according to session cochair Eduard Vieta, MD, PhD.
“. In many cases you need to continue ECT. Especially in patients who are refractory or treatment resistant, I don’t see a reason why maintenance ECT shouldn’t be the first choice. Yet in the guidelines, ECT is always the third- or fourth-line therapy,” said Dr. Vieta, professor of psychiatry at the University of Barcelona and scientific director of the Spanish Research Network on Mental Diseases.
Dr. Sienaert concurred, adding that he has patients who are on weekly maintenance ECT for as long as 16 years, with continued good results.
He reported having received honoraria from Mecta, a manufacturer of ECT equipment.
[email protected]
PARIS – , Pascal Sienaert, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
This conclusion is based on the results of two prospective studies by ResPECT – the Research in Psychiatry and ECT by the Flemish-Dutch Research Consortium – which, in turn, confirm the findings of an earlier metaanalysis of 32 studies including 702 patients conducted by investigators at Trinity College Dublin, noted Dr. Sienaert, a psychiatrist at the Catholic University of Leuven (Belgium) Academic Center for ECT and Neuromodulation.
Dr. Sienaert noted that the relapse rate in the ECT metaanalysis is nearly identical to that reported in the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial in real-world patients with major depression who achieved remission in response to second-step or later antidepressant medication.
“It’s a common misconception that relapse is higher after ECT than medication,” the psychiatrist said.
In the ECT metaanalysis, continuation ECT after induction of remission did not substantially affect the relapse risk. But that’s because the prevailing maintenance ECT strategy in the studies included in the 2013 metaanalysis relied upon a fixed-dose treatment schedule, according to Dr. Sienaert.
“In most studies, fixed-schedule maintenance ECT is used and with rather high relapse rates. Most clinicians have the experience that flexible, clinically driven on an as-needed-basis maintenance ECT has lower relapse rates,” he said. “Still, relapse remains the most pressing issue in the field, and it is very difficult for us as clinicians to predict which patients will relapse and which will not.”
That’s where the two ResPECT studies come into play.
In one of the studies, 116 patients with major depression at three tertiary psychiatric hospitals were randomized double blind to twice-weekly high-dose ultrabrief pulse (0.3-0.4 milliseconds) right unilateral or high-dose brief pulse (1.0 millisecond) right unilateral ECT. The dosing was at eight times the seizure threshold until remission as defined by a Montgomery-Åsberg Depression Rating Scale (MADRS) score below 10 or for a maximum of 6 weeks. Among the 87 completers, the remission rate was 68% in the brief pulse group, significantly higher than the 49% rate with ultrabrief ECT. Cognitive effects on semantic and lexical memory, and retrograde amnesia were the same in the two groups (J Clin Psychiatry. 2013 Nov;74[11]:e1029-36).
Dr. Sienaert and his coinvestigators then prospectively followed the 50 remitters for 6 months, during which all but one patient remained on antidepressant medication. The relapse rate, defined as rehospitalization for depression, restart of ECT, suicide, or a MADRS score above 15, was 25% at 3 months and about 40% at 6 months. The investigators found several predictors of a lower relapse rate. The strongest was early complete remission as defined by a Clinical Global Impressions Scale score of 1 out of a maximum of a possible 7 points within the first four ECT sessions: The 6-month relapse rate was 10% among those early complete remitters versus 63% in the other remitters (J Affect Disord. 2015 Sep 15;184:137-44).
“These are very small numbers in these groups, but the signal that emerges is the same as we have seen in the Irish metaanalysis: Early complete remitters were older, had shorter current episodes of depression, and showed more baseline psychotic features,” Dr. Sienaert said.
In a more recent ResPECT consortium study, the Mood Disorders in Elderly Treated With ECT (MODECT) study, 110 patients aged 55 and older with unipolar depression treated by ECT were followed with serial brain imaging studies prior to and for 6 months post treatment in an effort to gain insight into the mechanism of the particularly strong benefit of ECT in late-life depression. The response rate to ECT was significantly higher in those with onset of depression at age 55 or older than in those with disease onset before age 55, by a margin of 87% vs. 67%. The presence of baseline psychotic symptoms also was associated with a higher response rate.
In contrast, treatment response proved unrelated to changes in hippocampal volume, white matter hypersensitivities, amyloid load, or serum brain-derived neurotrophic factor, which is believed to be an important mediator of neuroplasticity. Thus, ECT’s mechanism of action in late-life depression remains elusive, the authors reported (Am J Geriatr Psychiatry. 2017 Feb;25[2]:178-89).
In a separate study, Dr. Sienaert and his colleagues found that ECT’s superior efficacy, compared with antidepressant medication in patients with late-life depression, was independent of their vascular disease burden. The study population was comprised of 81 patients in an antidepressant drug trial and 43 in an ECT trial, all of whom were inpatients with unipolar major depression. Their mean age was in the mid-70s.
The investigators gauged vascular burden by adding up each patient’s number of vascular risk factors, namely, diabetes, hypertension, smoking, hypercholesterolemia, known cardiovascular disease, and cerebrovascular disease. The depression remission rate was 80% in the ECT patients with no vascular risk factors, dropping to 58% in those with one or more. In the antidepressant drug trial participants, the remission rate was 38% in those with no vascular risk factors, compared with 32% in patients with one or more. Using different cutoffs for the number of vascular risk factors did not significantly alter the results (Int J Geriatr Psychiatry. 2017 Jun 28. doi: 10.1002/gps.4754).
At present, once a patient has achieved remission in response to ECT, most psychiatrists stop the therapy altogether. That’s often a mistake, according to session cochair Eduard Vieta, MD, PhD.
“. In many cases you need to continue ECT. Especially in patients who are refractory or treatment resistant, I don’t see a reason why maintenance ECT shouldn’t be the first choice. Yet in the guidelines, ECT is always the third- or fourth-line therapy,” said Dr. Vieta, professor of psychiatry at the University of Barcelona and scientific director of the Spanish Research Network on Mental Diseases.
Dr. Sienaert concurred, adding that he has patients who are on weekly maintenance ECT for as long as 16 years, with continued good results.
He reported having received honoraria from Mecta, a manufacturer of ECT equipment.
[email protected]
PARIS – , Pascal Sienaert, MD, PhD, reported at the annual congress of the European College of Neuropsychopharmacology.
This conclusion is based on the results of two prospective studies by ResPECT – the Research in Psychiatry and ECT by the Flemish-Dutch Research Consortium – which, in turn, confirm the findings of an earlier metaanalysis of 32 studies including 702 patients conducted by investigators at Trinity College Dublin, noted Dr. Sienaert, a psychiatrist at the Catholic University of Leuven (Belgium) Academic Center for ECT and Neuromodulation.
Dr. Sienaert noted that the relapse rate in the ECT metaanalysis is nearly identical to that reported in the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial in real-world patients with major depression who achieved remission in response to second-step or later antidepressant medication.
“It’s a common misconception that relapse is higher after ECT than medication,” the psychiatrist said.
In the ECT metaanalysis, continuation ECT after induction of remission did not substantially affect the relapse risk. But that’s because the prevailing maintenance ECT strategy in the studies included in the 2013 metaanalysis relied upon a fixed-dose treatment schedule, according to Dr. Sienaert.
“In most studies, fixed-schedule maintenance ECT is used and with rather high relapse rates. Most clinicians have the experience that flexible, clinically driven on an as-needed-basis maintenance ECT has lower relapse rates,” he said. “Still, relapse remains the most pressing issue in the field, and it is very difficult for us as clinicians to predict which patients will relapse and which will not.”
That’s where the two ResPECT studies come into play.
In one of the studies, 116 patients with major depression at three tertiary psychiatric hospitals were randomized double blind to twice-weekly high-dose ultrabrief pulse (0.3-0.4 milliseconds) right unilateral or high-dose brief pulse (1.0 millisecond) right unilateral ECT. The dosing was at eight times the seizure threshold until remission as defined by a Montgomery-Åsberg Depression Rating Scale (MADRS) score below 10 or for a maximum of 6 weeks. Among the 87 completers, the remission rate was 68% in the brief pulse group, significantly higher than the 49% rate with ultrabrief ECT. Cognitive effects on semantic and lexical memory, and retrograde amnesia were the same in the two groups (J Clin Psychiatry. 2013 Nov;74[11]:e1029-36).
Dr. Sienaert and his coinvestigators then prospectively followed the 50 remitters for 6 months, during which all but one patient remained on antidepressant medication. The relapse rate, defined as rehospitalization for depression, restart of ECT, suicide, or a MADRS score above 15, was 25% at 3 months and about 40% at 6 months. The investigators found several predictors of a lower relapse rate. The strongest was early complete remission as defined by a Clinical Global Impressions Scale score of 1 out of a maximum of a possible 7 points within the first four ECT sessions: The 6-month relapse rate was 10% among those early complete remitters versus 63% in the other remitters (J Affect Disord. 2015 Sep 15;184:137-44).
“These are very small numbers in these groups, but the signal that emerges is the same as we have seen in the Irish metaanalysis: Early complete remitters were older, had shorter current episodes of depression, and showed more baseline psychotic features,” Dr. Sienaert said.
In a more recent ResPECT consortium study, the Mood Disorders in Elderly Treated With ECT (MODECT) study, 110 patients aged 55 and older with unipolar depression treated by ECT were followed with serial brain imaging studies prior to and for 6 months post treatment in an effort to gain insight into the mechanism of the particularly strong benefit of ECT in late-life depression. The response rate to ECT was significantly higher in those with onset of depression at age 55 or older than in those with disease onset before age 55, by a margin of 87% vs. 67%. The presence of baseline psychotic symptoms also was associated with a higher response rate.
In contrast, treatment response proved unrelated to changes in hippocampal volume, white matter hypersensitivities, amyloid load, or serum brain-derived neurotrophic factor, which is believed to be an important mediator of neuroplasticity. Thus, ECT’s mechanism of action in late-life depression remains elusive, the authors reported (Am J Geriatr Psychiatry. 2017 Feb;25[2]:178-89).
In a separate study, Dr. Sienaert and his colleagues found that ECT’s superior efficacy, compared with antidepressant medication in patients with late-life depression, was independent of their vascular disease burden. The study population was comprised of 81 patients in an antidepressant drug trial and 43 in an ECT trial, all of whom were inpatients with unipolar major depression. Their mean age was in the mid-70s.
The investigators gauged vascular burden by adding up each patient’s number of vascular risk factors, namely, diabetes, hypertension, smoking, hypercholesterolemia, known cardiovascular disease, and cerebrovascular disease. The depression remission rate was 80% in the ECT patients with no vascular risk factors, dropping to 58% in those with one or more. In the antidepressant drug trial participants, the remission rate was 38% in those with no vascular risk factors, compared with 32% in patients with one or more. Using different cutoffs for the number of vascular risk factors did not significantly alter the results (Int J Geriatr Psychiatry. 2017 Jun 28. doi: 10.1002/gps.4754).
At present, once a patient has achieved remission in response to ECT, most psychiatrists stop the therapy altogether. That’s often a mistake, according to session cochair Eduard Vieta, MD, PhD.
“. In many cases you need to continue ECT. Especially in patients who are refractory or treatment resistant, I don’t see a reason why maintenance ECT shouldn’t be the first choice. Yet in the guidelines, ECT is always the third- or fourth-line therapy,” said Dr. Vieta, professor of psychiatry at the University of Barcelona and scientific director of the Spanish Research Network on Mental Diseases.
Dr. Sienaert concurred, adding that he has patients who are on weekly maintenance ECT for as long as 16 years, with continued good results.
He reported having received honoraria from Mecta, a manufacturer of ECT equipment.
[email protected]
REPORTING FROM THE ECNP CONGRESS