User login
FDA grants ‘Breakthrough Therapy Designation’ for upadacitinib for atopic dermatitis
The Food and Drug Administration has granted “Breakthrough Therapy Designation” for the investigational, once-daily oral Janus kinase 1 (JAK1)-selective inhibitor upadacitinib (ABT-494) in adult patients with moderate to severe atopic dermatitis who are candidates for systemic therapy.
The Breakthrough Therapy Designation is based on positive phase 2b results announced in Sept. 2017. The study found that patients treated with upadacitinib achieved statistically significant improvements in the primary endpoint (greater mean percentage change from baseline in Eczema Area and Severity Index score) and in all skin- and itch-specific secondary endpoints across all doses (30 mg, 15mg, or 7.5 mg once-daily) at week 16, compared with placebo (P less than .05). Reduction in itch was observed within the first week and improvement in skin within the first 2 weeks (P less than .001 across all doses). Of patients receiving the 30 mg once-daily dose of upadacitinib, 50% had clear or almost clear skin, according to a press release. There were 42 patients in each of the three treatment groups and 41 patients in the placebo group in this randomized, double-blind, parallel-group study sponsored by AbbVie, which discovered and developed upadacitinib.
The phase 3 clinical program is expected to begin in the first half of 2018, according to AbbVie. Any additional information on the clinical trials for upadacitinib is available at clinicaltrials.gov.
SOURCE: Prnewswire.com.
The Food and Drug Administration has granted “Breakthrough Therapy Designation” for the investigational, once-daily oral Janus kinase 1 (JAK1)-selective inhibitor upadacitinib (ABT-494) in adult patients with moderate to severe atopic dermatitis who are candidates for systemic therapy.
The Breakthrough Therapy Designation is based on positive phase 2b results announced in Sept. 2017. The study found that patients treated with upadacitinib achieved statistically significant improvements in the primary endpoint (greater mean percentage change from baseline in Eczema Area and Severity Index score) and in all skin- and itch-specific secondary endpoints across all doses (30 mg, 15mg, or 7.5 mg once-daily) at week 16, compared with placebo (P less than .05). Reduction in itch was observed within the first week and improvement in skin within the first 2 weeks (P less than .001 across all doses). Of patients receiving the 30 mg once-daily dose of upadacitinib, 50% had clear or almost clear skin, according to a press release. There were 42 patients in each of the three treatment groups and 41 patients in the placebo group in this randomized, double-blind, parallel-group study sponsored by AbbVie, which discovered and developed upadacitinib.
The phase 3 clinical program is expected to begin in the first half of 2018, according to AbbVie. Any additional information on the clinical trials for upadacitinib is available at clinicaltrials.gov.
SOURCE: Prnewswire.com.
The Food and Drug Administration has granted “Breakthrough Therapy Designation” for the investigational, once-daily oral Janus kinase 1 (JAK1)-selective inhibitor upadacitinib (ABT-494) in adult patients with moderate to severe atopic dermatitis who are candidates for systemic therapy.
The Breakthrough Therapy Designation is based on positive phase 2b results announced in Sept. 2017. The study found that patients treated with upadacitinib achieved statistically significant improvements in the primary endpoint (greater mean percentage change from baseline in Eczema Area and Severity Index score) and in all skin- and itch-specific secondary endpoints across all doses (30 mg, 15mg, or 7.5 mg once-daily) at week 16, compared with placebo (P less than .05). Reduction in itch was observed within the first week and improvement in skin within the first 2 weeks (P less than .001 across all doses). Of patients receiving the 30 mg once-daily dose of upadacitinib, 50% had clear or almost clear skin, according to a press release. There were 42 patients in each of the three treatment groups and 41 patients in the placebo group in this randomized, double-blind, parallel-group study sponsored by AbbVie, which discovered and developed upadacitinib.
The phase 3 clinical program is expected to begin in the first half of 2018, according to AbbVie. Any additional information on the clinical trials for upadacitinib is available at clinicaltrials.gov.
SOURCE: Prnewswire.com.
Our fascination with medication compliance
As a forensic psychiatrist, I follow news relating to mental illness and crime. Like many of the judges and lawyers with whom I work, the media appear to have an obsession with medication compliance in those with mental illness. Being “off medications” has become a threatening term suggestive of unbridled impulsivity and violence – a term that can explain any behavior, implying that without medication, humans are routinely capable of all things without warning.
The year 2018 already has provided two stark examples of this phenomenon. During the first week of the year, two articles with the following headlines were published: “ ‘He was off his meds’: Son charged in mother’s murder”1 and “A man lost his life in the subway after telling a teen off of his medication to ‘get away.’ ”2 Those two articles describe awful events that, as the headlines suggest, are best explained by a lack of compliance with a psychotropic medication regimen.
“A man lost his life in the subway after telling a teen off of his medication to ‘get away’ ” reports on a 65-year-old man who was pushed onto New York’s subway tracks after interacting with an 18-year-old who “did not take medication that day for his mental illness.” This article provides some limited details on the state of mind of the defendant, indicating that he had been “talking to himself.” However, to reinforce the message, the article informs the reader that he had been prescribed three psychotropics – insinuating a multiplier effect for the role of noncompliance. Furthermore, the article implies that missing a single day of psychotropics is an explanation for the incident.
The media routinely use this bias in favor of the medication explanation in its analysis – or lack thereof – of violent behavior in people with mental illness. Recent stories include a man killing his nephew3 and a man killing his girlfriend4, and both were incidents apparently best explained by medication noncompliance. Another story reports of police officers who were charged with assault after an altercation with a patient with mental illness led to the patient’s death. In the latter case, the article suggests that the simple fact that the victim had been acting erratically warrants the comment that he was “likely was off his medications.”
My work in the jail system also has been tainted by this overreliance on the unquestioned dogma of medication compliance. Discussions pertaining to punishment and privileges of inmates with mental illness often would lead to the question: “Is he taking his meds?” I have witnessed countless times when crucial decisions about placement in solitary confinement were predicated on questions of medication compliance. My answer was always the same: “Why does it matter? If the inmate is following the rules and behaving respectfully, how does taking a pill provide more important information?”
What was once thought to be a predictor of relapse risk, despite limited evidence, has become the outcome itself. As a society, we have falsely equated mental illness with violence; we have furthermore falsely equated remission and safety with medication compliance. The consequences of those beliefs are severe as we have limited attention, and our focus on medications blinds us to much clearer risk factors. 5
The court system is equally riveted with this question. Judges and lawyers associate medication compliance with legal competency, safety in probation or parole, and general well-being. I have witnessed agitated patients being reprimanded for their lack of medication adherence, leaving me to remind lawyers that the patient has been compliant. Conversely, patients are congratulated for their medication adherence when appearing well, until I remind the lawyers that the patient missed his last two visits for long-acting injectables.
As our field is reconciling new evidence questioning the long-term role of antipsychotics in schizophrenia, I am questioning whether society has accepted their value as a foregone conclusion. Lex Wunderink, MD, PhD, and his associates challenged accepted dogma when conducting a long-term, randomized trial of antipsychotics, in which patients on a dose reduction and discontinuation arm did better at 7 years than the patients on the continuation arm.6 The then National Institute of Mental Health director, Thomas Insel, MD, wrote in his blog that for some schizophrenia patients, “remaining on medication long term might impede a full return to wellness.”7
A Cochrane review of the literature found that, over time, antipsychotics had a diminishing effect on relapse prevention. After 2 years, the effect approached zero.8 In 2016, Nancy L. Sohler, PhD, and her colleagues looked at the literature on antipsychotic use in longer trials. The data were of poor quality and inconclusive.9 However, stories in the popular press suggest that the best explanation for violent behavior in the mentally ill population is medication noncompliance.
Inextricably bound up with this dogma regarding noncompliance is the incorporation of the pharmaceutical industry into mainstream psychiatry. The promise of psychopharmacology to treat mental illness was adopted in a wholesale manner for financial and practical reasons as well as a desperate optimism to relieve seemingly intractable problems. Sadly, the failure of psychopharmacology to produce on said promises has not produced a backlash. Instead, there is a doubling down on this belief, which can be seen, for example, in the creation of Abilify MyCite – with its promise to keep clinicians informed about their patients’ medication compliance.10 The alternative to this prescribing culture would be an attentive reckoning of the ongoing limitations inherent in the treatment of those with mental illness. If noncompliance cannot explain violence in people with mental illness, we are left with the same complex and subtle issues surrounding violence that frustrate easy journalistic explanations, and relatively cheap and easy interventions for the care of this population. It feels better and is more cost effective to blame the patients for not fitting our biological models by being drug nonresponders or noncompliant.
Blaming pills is facile. It is tangible and easier to measure than looking into someone’s mind. Psychiatrists have promoted this idea by teaching the public about chemical imbalances and by focusing on medication management. However, when the consequences are as severe as placing people in solitary confinement or explaining murder, the evidence needs to be equally solid as the severity of the punishment. This must start with psychiatrists reeducating the public on the role, the power, and the limitations of psychotropics.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre mentors residents on projects, including reduction in the use of solitary confinement of patients with mental illness and examination of the mentally ill offender. Dr. Badre can be reached at Badremd.com.
References
1. Worcester Patch. Jan. 2, 2018.
2. Rare News. Jan. 4, 2018.
3. WTSP.com. Dec. 31, 2017.
4. Wavy.com, Dec. 20, 2017.
5. U.S. Attorney General. 2016. U.S. Department of Justice Report and Recommendations Concerning the Use of Restrictive Housing.
6. JAMA Psychiatry. 2013 Sep;70(9):913-20.
7. Blogpost, by Thomas Insel, MD. Aug. 28, 2013.
8. Cochrane Database Syst Rev. 2012 May 16. doi: 10.1002/14651858.CD008016.pub2.
9. Am J Orthopsychiatry. 2016;86(5):477-85.
10. New York Times. Nov. 13, 2017.
As a forensic psychiatrist, I follow news relating to mental illness and crime. Like many of the judges and lawyers with whom I work, the media appear to have an obsession with medication compliance in those with mental illness. Being “off medications” has become a threatening term suggestive of unbridled impulsivity and violence – a term that can explain any behavior, implying that without medication, humans are routinely capable of all things without warning.
The year 2018 already has provided two stark examples of this phenomenon. During the first week of the year, two articles with the following headlines were published: “ ‘He was off his meds’: Son charged in mother’s murder”1 and “A man lost his life in the subway after telling a teen off of his medication to ‘get away.’ ”2 Those two articles describe awful events that, as the headlines suggest, are best explained by a lack of compliance with a psychotropic medication regimen.
“A man lost his life in the subway after telling a teen off of his medication to ‘get away’ ” reports on a 65-year-old man who was pushed onto New York’s subway tracks after interacting with an 18-year-old who “did not take medication that day for his mental illness.” This article provides some limited details on the state of mind of the defendant, indicating that he had been “talking to himself.” However, to reinforce the message, the article informs the reader that he had been prescribed three psychotropics – insinuating a multiplier effect for the role of noncompliance. Furthermore, the article implies that missing a single day of psychotropics is an explanation for the incident.
The media routinely use this bias in favor of the medication explanation in its analysis – or lack thereof – of violent behavior in people with mental illness. Recent stories include a man killing his nephew3 and a man killing his girlfriend4, and both were incidents apparently best explained by medication noncompliance. Another story reports of police officers who were charged with assault after an altercation with a patient with mental illness led to the patient’s death. In the latter case, the article suggests that the simple fact that the victim had been acting erratically warrants the comment that he was “likely was off his medications.”
My work in the jail system also has been tainted by this overreliance on the unquestioned dogma of medication compliance. Discussions pertaining to punishment and privileges of inmates with mental illness often would lead to the question: “Is he taking his meds?” I have witnessed countless times when crucial decisions about placement in solitary confinement were predicated on questions of medication compliance. My answer was always the same: “Why does it matter? If the inmate is following the rules and behaving respectfully, how does taking a pill provide more important information?”
What was once thought to be a predictor of relapse risk, despite limited evidence, has become the outcome itself. As a society, we have falsely equated mental illness with violence; we have furthermore falsely equated remission and safety with medication compliance. The consequences of those beliefs are severe as we have limited attention, and our focus on medications blinds us to much clearer risk factors. 5
The court system is equally riveted with this question. Judges and lawyers associate medication compliance with legal competency, safety in probation or parole, and general well-being. I have witnessed agitated patients being reprimanded for their lack of medication adherence, leaving me to remind lawyers that the patient has been compliant. Conversely, patients are congratulated for their medication adherence when appearing well, until I remind the lawyers that the patient missed his last two visits for long-acting injectables.
As our field is reconciling new evidence questioning the long-term role of antipsychotics in schizophrenia, I am questioning whether society has accepted their value as a foregone conclusion. Lex Wunderink, MD, PhD, and his associates challenged accepted dogma when conducting a long-term, randomized trial of antipsychotics, in which patients on a dose reduction and discontinuation arm did better at 7 years than the patients on the continuation arm.6 The then National Institute of Mental Health director, Thomas Insel, MD, wrote in his blog that for some schizophrenia patients, “remaining on medication long term might impede a full return to wellness.”7
A Cochrane review of the literature found that, over time, antipsychotics had a diminishing effect on relapse prevention. After 2 years, the effect approached zero.8 In 2016, Nancy L. Sohler, PhD, and her colleagues looked at the literature on antipsychotic use in longer trials. The data were of poor quality and inconclusive.9 However, stories in the popular press suggest that the best explanation for violent behavior in the mentally ill population is medication noncompliance.
Inextricably bound up with this dogma regarding noncompliance is the incorporation of the pharmaceutical industry into mainstream psychiatry. The promise of psychopharmacology to treat mental illness was adopted in a wholesale manner for financial and practical reasons as well as a desperate optimism to relieve seemingly intractable problems. Sadly, the failure of psychopharmacology to produce on said promises has not produced a backlash. Instead, there is a doubling down on this belief, which can be seen, for example, in the creation of Abilify MyCite – with its promise to keep clinicians informed about their patients’ medication compliance.10 The alternative to this prescribing culture would be an attentive reckoning of the ongoing limitations inherent in the treatment of those with mental illness. If noncompliance cannot explain violence in people with mental illness, we are left with the same complex and subtle issues surrounding violence that frustrate easy journalistic explanations, and relatively cheap and easy interventions for the care of this population. It feels better and is more cost effective to blame the patients for not fitting our biological models by being drug nonresponders or noncompliant.
Blaming pills is facile. It is tangible and easier to measure than looking into someone’s mind. Psychiatrists have promoted this idea by teaching the public about chemical imbalances and by focusing on medication management. However, when the consequences are as severe as placing people in solitary confinement or explaining murder, the evidence needs to be equally solid as the severity of the punishment. This must start with psychiatrists reeducating the public on the role, the power, and the limitations of psychotropics.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre mentors residents on projects, including reduction in the use of solitary confinement of patients with mental illness and examination of the mentally ill offender. Dr. Badre can be reached at Badremd.com.
References
1. Worcester Patch. Jan. 2, 2018.
2. Rare News. Jan. 4, 2018.
3. WTSP.com. Dec. 31, 2017.
4. Wavy.com, Dec. 20, 2017.
5. U.S. Attorney General. 2016. U.S. Department of Justice Report and Recommendations Concerning the Use of Restrictive Housing.
6. JAMA Psychiatry. 2013 Sep;70(9):913-20.
7. Blogpost, by Thomas Insel, MD. Aug. 28, 2013.
8. Cochrane Database Syst Rev. 2012 May 16. doi: 10.1002/14651858.CD008016.pub2.
9. Am J Orthopsychiatry. 2016;86(5):477-85.
10. New York Times. Nov. 13, 2017.
As a forensic psychiatrist, I follow news relating to mental illness and crime. Like many of the judges and lawyers with whom I work, the media appear to have an obsession with medication compliance in those with mental illness. Being “off medications” has become a threatening term suggestive of unbridled impulsivity and violence – a term that can explain any behavior, implying that without medication, humans are routinely capable of all things without warning.
The year 2018 already has provided two stark examples of this phenomenon. During the first week of the year, two articles with the following headlines were published: “ ‘He was off his meds’: Son charged in mother’s murder”1 and “A man lost his life in the subway after telling a teen off of his medication to ‘get away.’ ”2 Those two articles describe awful events that, as the headlines suggest, are best explained by a lack of compliance with a psychotropic medication regimen.
“A man lost his life in the subway after telling a teen off of his medication to ‘get away’ ” reports on a 65-year-old man who was pushed onto New York’s subway tracks after interacting with an 18-year-old who “did not take medication that day for his mental illness.” This article provides some limited details on the state of mind of the defendant, indicating that he had been “talking to himself.” However, to reinforce the message, the article informs the reader that he had been prescribed three psychotropics – insinuating a multiplier effect for the role of noncompliance. Furthermore, the article implies that missing a single day of psychotropics is an explanation for the incident.
The media routinely use this bias in favor of the medication explanation in its analysis – or lack thereof – of violent behavior in people with mental illness. Recent stories include a man killing his nephew3 and a man killing his girlfriend4, and both were incidents apparently best explained by medication noncompliance. Another story reports of police officers who were charged with assault after an altercation with a patient with mental illness led to the patient’s death. In the latter case, the article suggests that the simple fact that the victim had been acting erratically warrants the comment that he was “likely was off his medications.”
My work in the jail system also has been tainted by this overreliance on the unquestioned dogma of medication compliance. Discussions pertaining to punishment and privileges of inmates with mental illness often would lead to the question: “Is he taking his meds?” I have witnessed countless times when crucial decisions about placement in solitary confinement were predicated on questions of medication compliance. My answer was always the same: “Why does it matter? If the inmate is following the rules and behaving respectfully, how does taking a pill provide more important information?”
What was once thought to be a predictor of relapse risk, despite limited evidence, has become the outcome itself. As a society, we have falsely equated mental illness with violence; we have furthermore falsely equated remission and safety with medication compliance. The consequences of those beliefs are severe as we have limited attention, and our focus on medications blinds us to much clearer risk factors. 5
The court system is equally riveted with this question. Judges and lawyers associate medication compliance with legal competency, safety in probation or parole, and general well-being. I have witnessed agitated patients being reprimanded for their lack of medication adherence, leaving me to remind lawyers that the patient has been compliant. Conversely, patients are congratulated for their medication adherence when appearing well, until I remind the lawyers that the patient missed his last two visits for long-acting injectables.
As our field is reconciling new evidence questioning the long-term role of antipsychotics in schizophrenia, I am questioning whether society has accepted their value as a foregone conclusion. Lex Wunderink, MD, PhD, and his associates challenged accepted dogma when conducting a long-term, randomized trial of antipsychotics, in which patients on a dose reduction and discontinuation arm did better at 7 years than the patients on the continuation arm.6 The then National Institute of Mental Health director, Thomas Insel, MD, wrote in his blog that for some schizophrenia patients, “remaining on medication long term might impede a full return to wellness.”7
A Cochrane review of the literature found that, over time, antipsychotics had a diminishing effect on relapse prevention. After 2 years, the effect approached zero.8 In 2016, Nancy L. Sohler, PhD, and her colleagues looked at the literature on antipsychotic use in longer trials. The data were of poor quality and inconclusive.9 However, stories in the popular press suggest that the best explanation for violent behavior in the mentally ill population is medication noncompliance.
Inextricably bound up with this dogma regarding noncompliance is the incorporation of the pharmaceutical industry into mainstream psychiatry. The promise of psychopharmacology to treat mental illness was adopted in a wholesale manner for financial and practical reasons as well as a desperate optimism to relieve seemingly intractable problems. Sadly, the failure of psychopharmacology to produce on said promises has not produced a backlash. Instead, there is a doubling down on this belief, which can be seen, for example, in the creation of Abilify MyCite – with its promise to keep clinicians informed about their patients’ medication compliance.10 The alternative to this prescribing culture would be an attentive reckoning of the ongoing limitations inherent in the treatment of those with mental illness. If noncompliance cannot explain violence in people with mental illness, we are left with the same complex and subtle issues surrounding violence that frustrate easy journalistic explanations, and relatively cheap and easy interventions for the care of this population. It feels better and is more cost effective to blame the patients for not fitting our biological models by being drug nonresponders or noncompliant.
Blaming pills is facile. It is tangible and easier to measure than looking into someone’s mind. Psychiatrists have promoted this idea by teaching the public about chemical imbalances and by focusing on medication management. However, when the consequences are as severe as placing people in solitary confinement or explaining murder, the evidence needs to be equally solid as the severity of the punishment. This must start with psychiatrists reeducating the public on the role, the power, and the limitations of psychotropics.
Dr. Badre is a forensic psychiatrist in San Diego and an expert in correctional mental health. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre mentors residents on projects, including reduction in the use of solitary confinement of patients with mental illness and examination of the mentally ill offender. Dr. Badre can be reached at Badremd.com.
References
1. Worcester Patch. Jan. 2, 2018.
2. Rare News. Jan. 4, 2018.
3. WTSP.com. Dec. 31, 2017.
4. Wavy.com, Dec. 20, 2017.
5. U.S. Attorney General. 2016. U.S. Department of Justice Report and Recommendations Concerning the Use of Restrictive Housing.
6. JAMA Psychiatry. 2013 Sep;70(9):913-20.
7. Blogpost, by Thomas Insel, MD. Aug. 28, 2013.
8. Cochrane Database Syst Rev. 2012 May 16. doi: 10.1002/14651858.CD008016.pub2.
9. Am J Orthopsychiatry. 2016;86(5):477-85.
10. New York Times. Nov. 13, 2017.
Putting a number on biologic DMARD costs
Annual medical costs for patients with rheumatoid arthritis were almost three times higher for those who used biologic disease-modifying antirheumatic drugs (bDMARDs), compared with those who used any treatment regimen, according to meta-analysis of 12 studies conducted since bDMARDs were introduced in 1999.
RA patients who used bDMARDs had an average direct cost of $36,053 per year, which was 2.9 times higher than the $12,509 in annual direct medical costs for all RA patients on any treatment regimen. Proportionately, the difference was even greater for RA-specific care, with the annual cost of bDMARD care ($20,262) 5.4 times higher than that of all treatment regimens ($3,723), reported Andrew Hresko and his associates at Brigham and Women’s Hospital, Boston.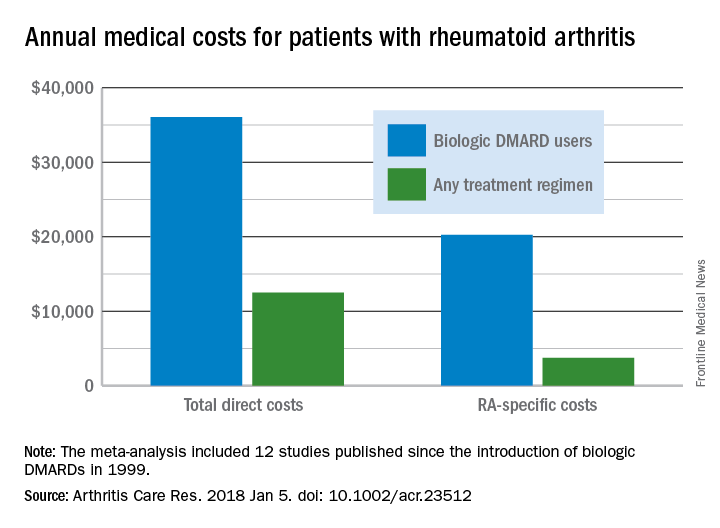
Funding for the study was supported through a grant from the National Institutes of Health. Mr. Hresko was supported by a fellowship from Tufts University. One of his associates receives research support from grants to his hospital from Amgen, Pfizer, Eli Lilly, AstraZeneca, Genentech, Bristol Myers Squibb, and Corrona. The third investigator is now an employee of Amgen but was not during the study.
SOURCE: Hresko A et al. Arthritis Care Res. 2018 Jan 5. doi: 10.1002/acr.23512.
Annual medical costs for patients with rheumatoid arthritis were almost three times higher for those who used biologic disease-modifying antirheumatic drugs (bDMARDs), compared with those who used any treatment regimen, according to meta-analysis of 12 studies conducted since bDMARDs were introduced in 1999.
RA patients who used bDMARDs had an average direct cost of $36,053 per year, which was 2.9 times higher than the $12,509 in annual direct medical costs for all RA patients on any treatment regimen. Proportionately, the difference was even greater for RA-specific care, with the annual cost of bDMARD care ($20,262) 5.4 times higher than that of all treatment regimens ($3,723), reported Andrew Hresko and his associates at Brigham and Women’s Hospital, Boston.
Funding for the study was supported through a grant from the National Institutes of Health. Mr. Hresko was supported by a fellowship from Tufts University. One of his associates receives research support from grants to his hospital from Amgen, Pfizer, Eli Lilly, AstraZeneca, Genentech, Bristol Myers Squibb, and Corrona. The third investigator is now an employee of Amgen but was not during the study.
SOURCE: Hresko A et al. Arthritis Care Res. 2018 Jan 5. doi: 10.1002/acr.23512.
Annual medical costs for patients with rheumatoid arthritis were almost three times higher for those who used biologic disease-modifying antirheumatic drugs (bDMARDs), compared with those who used any treatment regimen, according to meta-analysis of 12 studies conducted since bDMARDs were introduced in 1999.
RA patients who used bDMARDs had an average direct cost of $36,053 per year, which was 2.9 times higher than the $12,509 in annual direct medical costs for all RA patients on any treatment regimen. Proportionately, the difference was even greater for RA-specific care, with the annual cost of bDMARD care ($20,262) 5.4 times higher than that of all treatment regimens ($3,723), reported Andrew Hresko and his associates at Brigham and Women’s Hospital, Boston.
Funding for the study was supported through a grant from the National Institutes of Health. Mr. Hresko was supported by a fellowship from Tufts University. One of his associates receives research support from grants to his hospital from Amgen, Pfizer, Eli Lilly, AstraZeneca, Genentech, Bristol Myers Squibb, and Corrona. The third investigator is now an employee of Amgen but was not during the study.
SOURCE: Hresko A et al. Arthritis Care Res. 2018 Jan 5. doi: 10.1002/acr.23512.
FROM ARTHRITIS CARE & RESEARCH
Finance Committee votes on Azar HHS nomination
With a Senate Finance Committee vote of 15 to 12, Alex Azar’s nomination for secretary of Health & Human Services has been sent to the full Senate for consideration.
Finance Committee Chairman Orrin Hatch (R-Utah) said at a Jan. 17 hearing that “by any objective account, Mr. Azar is very well qualified for this important position. He has close to two decades of experience, the right expertise, and sound judgment.”
Most recently, Mr. Azar served as president of Eli Lilly’s U.S. operations from 2012 to 2017 after joining the company in 2007. His drug industry ties have raised concerns that the agency’s regulatory actions would be favorable to pharmaceutical manufacturers at the expense of patients. However, at his confirmation hearing, Mr. Azar noted that he would be willing to investigate government drug price negotiations for Medicare Part B drugs.
The top Democrat on the Finance Committee, Sen. Ron Wyden (D-Ore.) voted against Mr. Azar’s nomination, noting that President Trump “famously said, his words, in the 2016 campaign, ‘price-hiking drug companies were getting away with murder.’ The President has now nominated a drug company executive with a documented history of raising drug prices.”
Sen. Wyden noted that prices of many commonly prescribed drugs “more than doubled under [Mr. Azar’s] watch” while no drugs saw a decline in pricing.
With a Senate Finance Committee vote of 15 to 12, Alex Azar’s nomination for secretary of Health & Human Services has been sent to the full Senate for consideration.
Finance Committee Chairman Orrin Hatch (R-Utah) said at a Jan. 17 hearing that “by any objective account, Mr. Azar is very well qualified for this important position. He has close to two decades of experience, the right expertise, and sound judgment.”
Most recently, Mr. Azar served as president of Eli Lilly’s U.S. operations from 2012 to 2017 after joining the company in 2007. His drug industry ties have raised concerns that the agency’s regulatory actions would be favorable to pharmaceutical manufacturers at the expense of patients. However, at his confirmation hearing, Mr. Azar noted that he would be willing to investigate government drug price negotiations for Medicare Part B drugs.
The top Democrat on the Finance Committee, Sen. Ron Wyden (D-Ore.) voted against Mr. Azar’s nomination, noting that President Trump “famously said, his words, in the 2016 campaign, ‘price-hiking drug companies were getting away with murder.’ The President has now nominated a drug company executive with a documented history of raising drug prices.”
Sen. Wyden noted that prices of many commonly prescribed drugs “more than doubled under [Mr. Azar’s] watch” while no drugs saw a decline in pricing.
With a Senate Finance Committee vote of 15 to 12, Alex Azar’s nomination for secretary of Health & Human Services has been sent to the full Senate for consideration.
Finance Committee Chairman Orrin Hatch (R-Utah) said at a Jan. 17 hearing that “by any objective account, Mr. Azar is very well qualified for this important position. He has close to two decades of experience, the right expertise, and sound judgment.”
Most recently, Mr. Azar served as president of Eli Lilly’s U.S. operations from 2012 to 2017 after joining the company in 2007. His drug industry ties have raised concerns that the agency’s regulatory actions would be favorable to pharmaceutical manufacturers at the expense of patients. However, at his confirmation hearing, Mr. Azar noted that he would be willing to investigate government drug price negotiations for Medicare Part B drugs.
The top Democrat on the Finance Committee, Sen. Ron Wyden (D-Ore.) voted against Mr. Azar’s nomination, noting that President Trump “famously said, his words, in the 2016 campaign, ‘price-hiking drug companies were getting away with murder.’ The President has now nominated a drug company executive with a documented history of raising drug prices.”
Sen. Wyden noted that prices of many commonly prescribed drugs “more than doubled under [Mr. Azar’s] watch” while no drugs saw a decline in pricing.
REPORTING FROM A SENATE FINANCE COMMITTEE HEARING
Mood changes reported in cases of methotrexate use for dermatologic disease
, said Trisha Bhat and Carrie C. Coughlin, MD, both of Washington University, St. Louis.
Neurotoxicity with low-dose methotrexate often has been described when used to treat rheumatologic disease, the investigators said, but not in the dermatologic literature – although CNS symptoms such as dizziness and headache have been described in both.
An 8-year-old girl with psoriasis vulgaris and no prior psychiatric history began weekly subcutaneous methotrexate 12.5 mg (0.23 mg/kg) with folic acid supplementation 6 days per week after failing topical therapy. Her parents noticed severe irritability right away and fluctuating mood changes over the next 2 months. At first, she was angry and unsettled, with depressed mood; her irritability became less frequent later, but she said she wanted to hurt someone else. After stopping methotrexate, her mood returned to normal within 2 weeks.
It is unclear how methotrexate affects mood, but recent evidence suggests that abnormalities in synaptic plasticity are involved in mood changes, and there is evidence that mice treated with methotrexate show changes in synaptic plasticity. Methotrexate also increases extracellular adenosine, which affects neuronal excitability and synaptic plasticity, Ms. Bhat and Dr. Coughlin observed. Methotrexate also affects glucose metabolism in rats, and regional metabolic disturbances occur in a number of psychiatric disorders.
Read more at Pediatric Dermatology (2018 Jan 9. doi: 10.1111/pde.13406).
, said Trisha Bhat and Carrie C. Coughlin, MD, both of Washington University, St. Louis.
Neurotoxicity with low-dose methotrexate often has been described when used to treat rheumatologic disease, the investigators said, but not in the dermatologic literature – although CNS symptoms such as dizziness and headache have been described in both.
An 8-year-old girl with psoriasis vulgaris and no prior psychiatric history began weekly subcutaneous methotrexate 12.5 mg (0.23 mg/kg) with folic acid supplementation 6 days per week after failing topical therapy. Her parents noticed severe irritability right away and fluctuating mood changes over the next 2 months. At first, she was angry and unsettled, with depressed mood; her irritability became less frequent later, but she said she wanted to hurt someone else. After stopping methotrexate, her mood returned to normal within 2 weeks.
It is unclear how methotrexate affects mood, but recent evidence suggests that abnormalities in synaptic plasticity are involved in mood changes, and there is evidence that mice treated with methotrexate show changes in synaptic plasticity. Methotrexate also increases extracellular adenosine, which affects neuronal excitability and synaptic plasticity, Ms. Bhat and Dr. Coughlin observed. Methotrexate also affects glucose metabolism in rats, and regional metabolic disturbances occur in a number of psychiatric disorders.
Read more at Pediatric Dermatology (2018 Jan 9. doi: 10.1111/pde.13406).
, said Trisha Bhat and Carrie C. Coughlin, MD, both of Washington University, St. Louis.
Neurotoxicity with low-dose methotrexate often has been described when used to treat rheumatologic disease, the investigators said, but not in the dermatologic literature – although CNS symptoms such as dizziness and headache have been described in both.
An 8-year-old girl with psoriasis vulgaris and no prior psychiatric history began weekly subcutaneous methotrexate 12.5 mg (0.23 mg/kg) with folic acid supplementation 6 days per week after failing topical therapy. Her parents noticed severe irritability right away and fluctuating mood changes over the next 2 months. At first, she was angry and unsettled, with depressed mood; her irritability became less frequent later, but she said she wanted to hurt someone else. After stopping methotrexate, her mood returned to normal within 2 weeks.
It is unclear how methotrexate affects mood, but recent evidence suggests that abnormalities in synaptic plasticity are involved in mood changes, and there is evidence that mice treated with methotrexate show changes in synaptic plasticity. Methotrexate also increases extracellular adenosine, which affects neuronal excitability and synaptic plasticity, Ms. Bhat and Dr. Coughlin observed. Methotrexate also affects glucose metabolism in rats, and regional metabolic disturbances occur in a number of psychiatric disorders.
Read more at Pediatric Dermatology (2018 Jan 9. doi: 10.1111/pde.13406).
FROM PEDIATRIC DERMATOLOGY
FDA grants priority review to CAR T-cell therapy for DLBCL
The Food and Drug Administration has granted a priority review for the CAR T-cell therapy tisagenlecleucel suspension, formerly CTL019, for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma who are ineligible for or relapsed after autologous stem cell transplant.
The current application is based on a 6-month primary analysis from the single-arm, phase 2 JULIET clinical trial in adult patients with relapsed or refractory diffuse large B-cell lymphoma. According to results presented at ASH 2017, among 81 patients followed for at least 3 months before data cutoff, best overall response rate was 53%, and 40% had a complete response. Cytokine release syndrome (all grades) occurred in 58% of infused patients. Other grade 3 or 4 adverse events included neurologic toxicities, cytopenias lasting more than 28 days, infections, and febrile neutropenia.
Tisagenlecleucel suspension is marketed as Kymriah by Novartis.
The Food and Drug Administration has granted a priority review for the CAR T-cell therapy tisagenlecleucel suspension, formerly CTL019, for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma who are ineligible for or relapsed after autologous stem cell transplant.
The current application is based on a 6-month primary analysis from the single-arm, phase 2 JULIET clinical trial in adult patients with relapsed or refractory diffuse large B-cell lymphoma. According to results presented at ASH 2017, among 81 patients followed for at least 3 months before data cutoff, best overall response rate was 53%, and 40% had a complete response. Cytokine release syndrome (all grades) occurred in 58% of infused patients. Other grade 3 or 4 adverse events included neurologic toxicities, cytopenias lasting more than 28 days, infections, and febrile neutropenia.
Tisagenlecleucel suspension is marketed as Kymriah by Novartis.
The Food and Drug Administration has granted a priority review for the CAR T-cell therapy tisagenlecleucel suspension, formerly CTL019, for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma who are ineligible for or relapsed after autologous stem cell transplant.
The current application is based on a 6-month primary analysis from the single-arm, phase 2 JULIET clinical trial in adult patients with relapsed or refractory diffuse large B-cell lymphoma. According to results presented at ASH 2017, among 81 patients followed for at least 3 months before data cutoff, best overall response rate was 53%, and 40% had a complete response. Cytokine release syndrome (all grades) occurred in 58% of infused patients. Other grade 3 or 4 adverse events included neurologic toxicities, cytopenias lasting more than 28 days, infections, and febrile neutropenia.
Tisagenlecleucel suspension is marketed as Kymriah by Novartis.
FDA approves injection treatment for low-risk APL
The Food and Drug Administration announced the approval of arsenic trioxide injection (Trisenox) in combination with tretinoin for the treatment of adults with newly diagnosed, low-risk acute promyelocytic leukemia (APL) characterized by t(15;17) translocation or PML/RAR-alpha gene expression.
The expanded indication was granted by the FDA on Jan. 12 after priority review. It is based on published studies and a review of Teva’s global safety database for arsenic trioxide.
A recent randomized, phase 3 trial compared tretinoin plus arsenic trioxide with tretinoin plus chemotherapy as first-line treatment for APL (J Clin Oncol. 2017 Feb 20;35[6]:605-12). It found that 100% of 127 patients in the tretinoin plus arsenic trioxide arm achieved complete remission, compared with 97% of 136 patients in the tretinoin plus chemotherapy arm. After a median follow-up of 40.6 months, the event-free survival at 50 months for patients in the tretinoin/arsenic trioxide arm was 97.3% vs. 80% for tretinoin/chemotherapy (P = .001).
The arsenic trioxide injection carries a boxed warning for differentiation syndrome and cardiac conduction abnormalities.
The Food and Drug Administration announced the approval of arsenic trioxide injection (Trisenox) in combination with tretinoin for the treatment of adults with newly diagnosed, low-risk acute promyelocytic leukemia (APL) characterized by t(15;17) translocation or PML/RAR-alpha gene expression.
The expanded indication was granted by the FDA on Jan. 12 after priority review. It is based on published studies and a review of Teva’s global safety database for arsenic trioxide.
A recent randomized, phase 3 trial compared tretinoin plus arsenic trioxide with tretinoin plus chemotherapy as first-line treatment for APL (J Clin Oncol. 2017 Feb 20;35[6]:605-12). It found that 100% of 127 patients in the tretinoin plus arsenic trioxide arm achieved complete remission, compared with 97% of 136 patients in the tretinoin plus chemotherapy arm. After a median follow-up of 40.6 months, the event-free survival at 50 months for patients in the tretinoin/arsenic trioxide arm was 97.3% vs. 80% for tretinoin/chemotherapy (P = .001).
The arsenic trioxide injection carries a boxed warning for differentiation syndrome and cardiac conduction abnormalities.
The Food and Drug Administration announced the approval of arsenic trioxide injection (Trisenox) in combination with tretinoin for the treatment of adults with newly diagnosed, low-risk acute promyelocytic leukemia (APL) characterized by t(15;17) translocation or PML/RAR-alpha gene expression.
The expanded indication was granted by the FDA on Jan. 12 after priority review. It is based on published studies and a review of Teva’s global safety database for arsenic trioxide.
A recent randomized, phase 3 trial compared tretinoin plus arsenic trioxide with tretinoin plus chemotherapy as first-line treatment for APL (J Clin Oncol. 2017 Feb 20;35[6]:605-12). It found that 100% of 127 patients in the tretinoin plus arsenic trioxide arm achieved complete remission, compared with 97% of 136 patients in the tretinoin plus chemotherapy arm. After a median follow-up of 40.6 months, the event-free survival at 50 months for patients in the tretinoin/arsenic trioxide arm was 97.3% vs. 80% for tretinoin/chemotherapy (P = .001).
The arsenic trioxide injection carries a boxed warning for differentiation syndrome and cardiac conduction abnormalities.
Clinical rule decreased pediatric trauma CT scans
ORLANDO – A according to a study presented at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
With values for five clinical variables, the prediction rule would eliminate the need to subject some patients to unwarranted radiation exposure, which has become a growing health and financial concern for medical institutions.
“CT utilization rates in pediatric blunt trauma are very high, at a rate of 40%-60%, despite a relatively low incidence of intra-abdominal injury after abdominal trauma,” according to presenter Chase A. Arbra, MD, of the department of surgery at the Medical University of South Carolina, Charleston. “With increasing concerns regarding the cost and radiation exposure in children, our group is focusing on research to safely avoid these unnecessary scans.”
The rule, developed by the Pediatric Surgery Research Collaborative (PedSRC), evaluates abdominal wall trauma and tenderness, complaint of abdominal pain, aspartate aminotransferase level greater than 200 U/L, abnormal pancreatic enzymes, and abnormal chest x-rays to determine a patient’s risk of having an intra-abdominal injury (IAI). If none of the five variables in a patient is abnormal, the finding is considered negative and the patient is considered to be at very low risk for having an IAI or an IAI requiring acute intervention (IAI-I).
Investigators studied 2,435 pediatric blunt trauma patients with all five clinical variables documented within 6 hours of arrival, using data gathered from the Pediatric Emergency Care Applied Research Network.
Patients were an average of 9.4 years old, with an IAI rate of 9.7% (n = 235) and an IAI-I rate of 2.5% (n = 60); 61.1% of the patients had a CT scan.
Prediction sensitivity of the method was 97.5% for IAI and 100% for IAI-I, said Dr. Arbra. Negative predictive value for the model was 99.3% for IAI and 100% for IAI-I.
Patients who were found to have aspartate aminotransferase level greater than 200 U/L were at the highest risk of IAI (52.6%) and IAI-I (11.9%), according to investigators. One-third of the test population was found to be at very low risk after using the prediction model, according to Dr. Arbra, with 46.8% of them still undergoing a CT scan. Of those tested, six patients had IAI that was not predicted by the model, three of whom were intubated. Because CT scans were not required and there was no follow-up after discharge, investigators are not able to determine if any minor IAI was missed.
Despite these limitations, the highly sensitive rule shows great promise, according to Dr. Arbra.
“Patients with 0-5 variables, even patients who were involved in a high impact mechanism, could potentially forgo CT scans safely.”
A closer look at the 26 patients who only had abdominal pain showed that only 1 had IAI, suggesting that patients with only abdominal pain could be safely observed with only serial exams, according to Dr. Arbra.
Investigators plan to conduct a prospective study that will include older patients.
Dr. Arbra concluded, “The rule could potentially help centers to determine who could avoid imaging prior to transfer and potentially could one day be used to see who could be discharged.”
Dr. Arbra reported no relevant financial disclosures.
SOURCE: Arbra CA. EAST Scientific Assembly 2018, paper #7.
ORLANDO – A according to a study presented at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
With values for five clinical variables, the prediction rule would eliminate the need to subject some patients to unwarranted radiation exposure, which has become a growing health and financial concern for medical institutions.
“CT utilization rates in pediatric blunt trauma are very high, at a rate of 40%-60%, despite a relatively low incidence of intra-abdominal injury after abdominal trauma,” according to presenter Chase A. Arbra, MD, of the department of surgery at the Medical University of South Carolina, Charleston. “With increasing concerns regarding the cost and radiation exposure in children, our group is focusing on research to safely avoid these unnecessary scans.”
The rule, developed by the Pediatric Surgery Research Collaborative (PedSRC), evaluates abdominal wall trauma and tenderness, complaint of abdominal pain, aspartate aminotransferase level greater than 200 U/L, abnormal pancreatic enzymes, and abnormal chest x-rays to determine a patient’s risk of having an intra-abdominal injury (IAI). If none of the five variables in a patient is abnormal, the finding is considered negative and the patient is considered to be at very low risk for having an IAI or an IAI requiring acute intervention (IAI-I).
Investigators studied 2,435 pediatric blunt trauma patients with all five clinical variables documented within 6 hours of arrival, using data gathered from the Pediatric Emergency Care Applied Research Network.
Patients were an average of 9.4 years old, with an IAI rate of 9.7% (n = 235) and an IAI-I rate of 2.5% (n = 60); 61.1% of the patients had a CT scan.
Prediction sensitivity of the method was 97.5% for IAI and 100% for IAI-I, said Dr. Arbra. Negative predictive value for the model was 99.3% for IAI and 100% for IAI-I.
Patients who were found to have aspartate aminotransferase level greater than 200 U/L were at the highest risk of IAI (52.6%) and IAI-I (11.9%), according to investigators. One-third of the test population was found to be at very low risk after using the prediction model, according to Dr. Arbra, with 46.8% of them still undergoing a CT scan. Of those tested, six patients had IAI that was not predicted by the model, three of whom were intubated. Because CT scans were not required and there was no follow-up after discharge, investigators are not able to determine if any minor IAI was missed.
Despite these limitations, the highly sensitive rule shows great promise, according to Dr. Arbra.
“Patients with 0-5 variables, even patients who were involved in a high impact mechanism, could potentially forgo CT scans safely.”
A closer look at the 26 patients who only had abdominal pain showed that only 1 had IAI, suggesting that patients with only abdominal pain could be safely observed with only serial exams, according to Dr. Arbra.
Investigators plan to conduct a prospective study that will include older patients.
Dr. Arbra concluded, “The rule could potentially help centers to determine who could avoid imaging prior to transfer and potentially could one day be used to see who could be discharged.”
Dr. Arbra reported no relevant financial disclosures.
SOURCE: Arbra CA. EAST Scientific Assembly 2018, paper #7.
ORLANDO – A according to a study presented at the annual scientific assembly of the Eastern Association for the Surgery of Trauma.
With values for five clinical variables, the prediction rule would eliminate the need to subject some patients to unwarranted radiation exposure, which has become a growing health and financial concern for medical institutions.
“CT utilization rates in pediatric blunt trauma are very high, at a rate of 40%-60%, despite a relatively low incidence of intra-abdominal injury after abdominal trauma,” according to presenter Chase A. Arbra, MD, of the department of surgery at the Medical University of South Carolina, Charleston. “With increasing concerns regarding the cost and radiation exposure in children, our group is focusing on research to safely avoid these unnecessary scans.”
The rule, developed by the Pediatric Surgery Research Collaborative (PedSRC), evaluates abdominal wall trauma and tenderness, complaint of abdominal pain, aspartate aminotransferase level greater than 200 U/L, abnormal pancreatic enzymes, and abnormal chest x-rays to determine a patient’s risk of having an intra-abdominal injury (IAI). If none of the five variables in a patient is abnormal, the finding is considered negative and the patient is considered to be at very low risk for having an IAI or an IAI requiring acute intervention (IAI-I).
Investigators studied 2,435 pediatric blunt trauma patients with all five clinical variables documented within 6 hours of arrival, using data gathered from the Pediatric Emergency Care Applied Research Network.
Patients were an average of 9.4 years old, with an IAI rate of 9.7% (n = 235) and an IAI-I rate of 2.5% (n = 60); 61.1% of the patients had a CT scan.
Prediction sensitivity of the method was 97.5% for IAI and 100% for IAI-I, said Dr. Arbra. Negative predictive value for the model was 99.3% for IAI and 100% for IAI-I.
Patients who were found to have aspartate aminotransferase level greater than 200 U/L were at the highest risk of IAI (52.6%) and IAI-I (11.9%), according to investigators. One-third of the test population was found to be at very low risk after using the prediction model, according to Dr. Arbra, with 46.8% of them still undergoing a CT scan. Of those tested, six patients had IAI that was not predicted by the model, three of whom were intubated. Because CT scans were not required and there was no follow-up after discharge, investigators are not able to determine if any minor IAI was missed.
Despite these limitations, the highly sensitive rule shows great promise, according to Dr. Arbra.
“Patients with 0-5 variables, even patients who were involved in a high impact mechanism, could potentially forgo CT scans safely.”
A closer look at the 26 patients who only had abdominal pain showed that only 1 had IAI, suggesting that patients with only abdominal pain could be safely observed with only serial exams, according to Dr. Arbra.
Investigators plan to conduct a prospective study that will include older patients.
Dr. Arbra concluded, “The rule could potentially help centers to determine who could avoid imaging prior to transfer and potentially could one day be used to see who could be discharged.”
Dr. Arbra reported no relevant financial disclosures.
SOURCE: Arbra CA. EAST Scientific Assembly 2018, paper #7.
REPORTING FROM EAST 2018
Key clinical point: New prediction model successfully identified patients with intra-abdominal injury (IAI) and IAI patients who require acute intervention (IAI-I).
Major finding: The test had a negative predictive value of 99.3% in IAI patients and 100% in IAI-I patients when either had no abnormalities.
Study details: Prospective study of 2,345 pediatric patients with IAI or IAI-I, the data for which was collected from the Pediatric Emergency Care Applied Research Network.
Disclosures: Dr. Arbra reported no relevant financial disclosures.
Source: Arbra CA. EAST Scientific Assembly 2018, paper #7.
Influenza vaccination of pregnant women needs surveillance
Seasonal influenza vaccine is specifically recommended for women who are or who might become pregnant in the flu season. This special population is targeted for vaccination because pregnant women are at increased risks of serious complications if infected with influenza virus. Despite this recommendation, recent evidence indicates that still fewer than 50% of women in the United States are vaccinated during pregnancy (MMWR Morb Mortal Wkly Rep. 2016 Dec 9;65[48]:1370-3).
Potential reasons for this lack of uptake are concerns about safety of the vaccine for mothers and fetuses (Vaccine. 2012 Dec 17;31[1]:213-8). This has highlighted the need for systematic safety surveillance for influenza vaccination with each subsequent seasonal formulation. To that end, season-specific studies of birth and infant outcomes since the 2009 season have been conducted; findings have been generally reassuring (Vaccine. 2016 Aug 17;34[37]:4443-9; Vaccine. 2016 Aug 17;34[37]:4450-9).
The authors found a doubling of risk for spontaneous abortion within that 28-day exposure window, but no association if the vaccination took place outside that period. This was in contrast to null findings for a similar analysis that the same group had conducted for vaccination in the 2005-2006 and 2006-2007 seasons. Of further interest, the authors noted even higher risks among women who had also been vaccinated for influenza in the previous season (adjusted odds ratio, 7.7; 95% confidence interval, 2.2-27.3). The highest odds ratios were among women who had been vaccinated in the 2010-2011 season and had also been vaccinated with monovalent pandemic H1N1 vaccine in the 2009-2010 season (aOR, 32.5; 95% CI, 2.9-359.0).
The VSD findings raise interesting questions about the biologic plausibility of strain-specific risks for spontaneous abortion, and risks of receiving a second vaccine containing the same strain in a subsequent season. However, this study should be interpreted with caution. With respect to the overall finding of a doubling of risk for spontaneous abortion, this is inconsistent with previous studies. A systematic review of 19 observational studies, 14 of which included exposure to the 2009 monovalent pandemic H1N1 strain, noted hazard ratios or odds ratios for spontaneous abortion ranging from 0.45 to 1.23 and 95% confidence intervals that crossed or were below the null (Vaccine. 2015 Apr 27;33[18]:2108-17). More recently, the Vaccines and Medications in Pregnancy Surveillance System investigators evaluated spontaneous abortion in pregnancies exposed to influenza vaccine over four seasons from 2010 to 2014 and found an overall hazard ratio of 1.09 (95% CI, 0.49-2.40).
However, there are a number of limitations that must be considered. Many previous studies, including the VSD analysis, could have had misclassification of exposure, especially in recent years when vaccines are often received in nontraditional settings. The VSD study findings could have been influenced by unmeasured confounding. For example, there could be differential vaccine uptake in women with comorbidities that are also associated with spontaneous abortion, such as subfertility and psychiatric disorders.
In summary, at present the data viewed as a whole do not support a change to the current recommendation that pregnant women be vaccinated for influenza regardless of trimester. However, these data do call for continued surveillance for the safety of each seasonal formulation of influenza vaccine, and for further exploration of the association between repeat vaccination and spontaneous abortion in other datasets.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no direct conflicts of interest to disclose, but has received grant funding to study influenza vaccine from the Biomedical Advanced Research and Development Authority (BARDA) in the Department of Health and Human Services, and from Seqirus Corporation.
Seasonal influenza vaccine is specifically recommended for women who are or who might become pregnant in the flu season. This special population is targeted for vaccination because pregnant women are at increased risks of serious complications if infected with influenza virus. Despite this recommendation, recent evidence indicates that still fewer than 50% of women in the United States are vaccinated during pregnancy (MMWR Morb Mortal Wkly Rep. 2016 Dec 9;65[48]:1370-3).
Potential reasons for this lack of uptake are concerns about safety of the vaccine for mothers and fetuses (Vaccine. 2012 Dec 17;31[1]:213-8). This has highlighted the need for systematic safety surveillance for influenza vaccination with each subsequent seasonal formulation. To that end, season-specific studies of birth and infant outcomes since the 2009 season have been conducted; findings have been generally reassuring (Vaccine. 2016 Aug 17;34[37]:4443-9; Vaccine. 2016 Aug 17;34[37]:4450-9).
The authors found a doubling of risk for spontaneous abortion within that 28-day exposure window, but no association if the vaccination took place outside that period. This was in contrast to null findings for a similar analysis that the same group had conducted for vaccination in the 2005-2006 and 2006-2007 seasons. Of further interest, the authors noted even higher risks among women who had also been vaccinated for influenza in the previous season (adjusted odds ratio, 7.7; 95% confidence interval, 2.2-27.3). The highest odds ratios were among women who had been vaccinated in the 2010-2011 season and had also been vaccinated with monovalent pandemic H1N1 vaccine in the 2009-2010 season (aOR, 32.5; 95% CI, 2.9-359.0).
The VSD findings raise interesting questions about the biologic plausibility of strain-specific risks for spontaneous abortion, and risks of receiving a second vaccine containing the same strain in a subsequent season. However, this study should be interpreted with caution. With respect to the overall finding of a doubling of risk for spontaneous abortion, this is inconsistent with previous studies. A systematic review of 19 observational studies, 14 of which included exposure to the 2009 monovalent pandemic H1N1 strain, noted hazard ratios or odds ratios for spontaneous abortion ranging from 0.45 to 1.23 and 95% confidence intervals that crossed or were below the null (Vaccine. 2015 Apr 27;33[18]:2108-17). More recently, the Vaccines and Medications in Pregnancy Surveillance System investigators evaluated spontaneous abortion in pregnancies exposed to influenza vaccine over four seasons from 2010 to 2014 and found an overall hazard ratio of 1.09 (95% CI, 0.49-2.40).
However, there are a number of limitations that must be considered. Many previous studies, including the VSD analysis, could have had misclassification of exposure, especially in recent years when vaccines are often received in nontraditional settings. The VSD study findings could have been influenced by unmeasured confounding. For example, there could be differential vaccine uptake in women with comorbidities that are also associated with spontaneous abortion, such as subfertility and psychiatric disorders.
In summary, at present the data viewed as a whole do not support a change to the current recommendation that pregnant women be vaccinated for influenza regardless of trimester. However, these data do call for continued surveillance for the safety of each seasonal formulation of influenza vaccine, and for further exploration of the association between repeat vaccination and spontaneous abortion in other datasets.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no direct conflicts of interest to disclose, but has received grant funding to study influenza vaccine from the Biomedical Advanced Research and Development Authority (BARDA) in the Department of Health and Human Services, and from Seqirus Corporation.
Seasonal influenza vaccine is specifically recommended for women who are or who might become pregnant in the flu season. This special population is targeted for vaccination because pregnant women are at increased risks of serious complications if infected with influenza virus. Despite this recommendation, recent evidence indicates that still fewer than 50% of women in the United States are vaccinated during pregnancy (MMWR Morb Mortal Wkly Rep. 2016 Dec 9;65[48]:1370-3).
Potential reasons for this lack of uptake are concerns about safety of the vaccine for mothers and fetuses (Vaccine. 2012 Dec 17;31[1]:213-8). This has highlighted the need for systematic safety surveillance for influenza vaccination with each subsequent seasonal formulation. To that end, season-specific studies of birth and infant outcomes since the 2009 season have been conducted; findings have been generally reassuring (Vaccine. 2016 Aug 17;34[37]:4443-9; Vaccine. 2016 Aug 17;34[37]:4450-9).
The authors found a doubling of risk for spontaneous abortion within that 28-day exposure window, but no association if the vaccination took place outside that period. This was in contrast to null findings for a similar analysis that the same group had conducted for vaccination in the 2005-2006 and 2006-2007 seasons. Of further interest, the authors noted even higher risks among women who had also been vaccinated for influenza in the previous season (adjusted odds ratio, 7.7; 95% confidence interval, 2.2-27.3). The highest odds ratios were among women who had been vaccinated in the 2010-2011 season and had also been vaccinated with monovalent pandemic H1N1 vaccine in the 2009-2010 season (aOR, 32.5; 95% CI, 2.9-359.0).
The VSD findings raise interesting questions about the biologic plausibility of strain-specific risks for spontaneous abortion, and risks of receiving a second vaccine containing the same strain in a subsequent season. However, this study should be interpreted with caution. With respect to the overall finding of a doubling of risk for spontaneous abortion, this is inconsistent with previous studies. A systematic review of 19 observational studies, 14 of which included exposure to the 2009 monovalent pandemic H1N1 strain, noted hazard ratios or odds ratios for spontaneous abortion ranging from 0.45 to 1.23 and 95% confidence intervals that crossed or were below the null (Vaccine. 2015 Apr 27;33[18]:2108-17). More recently, the Vaccines and Medications in Pregnancy Surveillance System investigators evaluated spontaneous abortion in pregnancies exposed to influenza vaccine over four seasons from 2010 to 2014 and found an overall hazard ratio of 1.09 (95% CI, 0.49-2.40).
However, there are a number of limitations that must be considered. Many previous studies, including the VSD analysis, could have had misclassification of exposure, especially in recent years when vaccines are often received in nontraditional settings. The VSD study findings could have been influenced by unmeasured confounding. For example, there could be differential vaccine uptake in women with comorbidities that are also associated with spontaneous abortion, such as subfertility and psychiatric disorders.
In summary, at present the data viewed as a whole do not support a change to the current recommendation that pregnant women be vaccinated for influenza regardless of trimester. However, these data do call for continued surveillance for the safety of each seasonal formulation of influenza vaccine, and for further exploration of the association between repeat vaccination and spontaneous abortion in other datasets.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is also director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no direct conflicts of interest to disclose, but has received grant funding to study influenza vaccine from the Biomedical Advanced Research and Development Authority (BARDA) in the Department of Health and Human Services, and from Seqirus Corporation.
Carotid stenting isn’t safer than endarterectomy with contralateral carotid occlusion
CHICAGO – Carotid angioplasty and stenting (CAS) isn’t associated with a lower 30-day stroke risk than carotid endarterectomy (CEA) for revascularization of the internal carotid artery in patients with contralateral carotid occlusion, Leila Mureebe, MD, said at a symposium on vascular surgery sponsored by Northwestern University, Chicago.
The reported prevalence of contralateral carotid occlusion (CCO) in patients undergoing revascularization for carotid artery disease is 3%-15%. Of late Dr. Mureebe has been particularly interested in two questions regarding CCO in patients undergoing revascularization of their other carotid artery: Is CCO truly a risk factor for perioperative stroke? And if so, can this risk be mitigated by the choice of procedure?
To answer the first question, Dr. Mureebe and her coinvestigators performed a meta-analysis of eight representative studies published between 1994 and 2012; they determined that CCO in patients undergoing CEA was indeed associated with a near doubling of perioperative stroke risk, compared with that of patients without CCO.
In order to learn whether CAS mitigates this risk, she and her coworkers analyzed the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for the period between 2011 and 2015, in which they identified 15,619 fully documented CEA and 496 CAS.
“This NSQIP data is not just academic medical centers or big centers. I think it’s a pretty good look at what’s actually being done in the real world today,” according to Dr. Mureebe.
The analysis showed that CCO has already had an effect on practice. A higher proportion of patients with CCO now undergo stenting as opposed to endarterectomy. Only 4.6% of all CEAs were done in patients with CCO, compared with 11.5% of CAS procedures. Moreover, the majority of revascularizations in the setting of CCO were performed in patients with asymptomatic disease: 57% of all CEA and 53% of the CAS. The CAS finding was surprising given that reimbursement for CAS is at present limited to symptomatic patients at high surgical risk who have a significant internal carotid artery stenosis, Dr. Mureebe observed.
The 30-day stroke rate in patients with CCO was 3.22% after CEA and 1.75% after CAS, a difference that wasn’t statistically significant. In patients without CCO, the stroke rate was 2.03% after CEA and 2.96% after CAS.
Next, the investigators analyzed differences in stroke rates according to symptom status. Among patients with CCO and preprocedural transient ischemic attack, stroke, or transient monocular blindness who underwent CEA, the 30-day stroke risk associated with CEA was 5.2%, a significantly higher rate than the 2.1% rate seen in patients without symptoms. The number of patients with CCO undergoing CAS was too small to draw conclusions regarding possible differences in stroke risk based upon symptom status.
In the NSQIP database, patients with CCO had higher prevalences of heart failure, hypertension, and smoking. For this reason, Dr. Mureebe said she suspects CCO is a surrogate for greater atherosclerotic disease burden and not an independent risk factor for periprocedural stroke. If future studies of the minimally invasive transcarotid artery revascularization procedure also show a higher rate of bad outcomes in patients with CCO, that would further support the hypothesis that CCO is a marker of higher atherosclerotic disease burden, Dr. Mureebe said.
A limitation of the NSQIP database is that it captures only those CAS cases done in operating rooms. “Maybe patients undergoing CAS in the OR are different from those undergoing CAS in a radiologic suite or cath lab,” she noted.
Dr. Mureebe reported having no financial conflicts of interest regarding her presentation.
SOURCE: Mureebe L. 42nd Annual Northwestern Vascular Symposium.
CHICAGO – Carotid angioplasty and stenting (CAS) isn’t associated with a lower 30-day stroke risk than carotid endarterectomy (CEA) for revascularization of the internal carotid artery in patients with contralateral carotid occlusion, Leila Mureebe, MD, said at a symposium on vascular surgery sponsored by Northwestern University, Chicago.
The reported prevalence of contralateral carotid occlusion (CCO) in patients undergoing revascularization for carotid artery disease is 3%-15%. Of late Dr. Mureebe has been particularly interested in two questions regarding CCO in patients undergoing revascularization of their other carotid artery: Is CCO truly a risk factor for perioperative stroke? And if so, can this risk be mitigated by the choice of procedure?
To answer the first question, Dr. Mureebe and her coinvestigators performed a meta-analysis of eight representative studies published between 1994 and 2012; they determined that CCO in patients undergoing CEA was indeed associated with a near doubling of perioperative stroke risk, compared with that of patients without CCO.
In order to learn whether CAS mitigates this risk, she and her coworkers analyzed the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for the period between 2011 and 2015, in which they identified 15,619 fully documented CEA and 496 CAS.
“This NSQIP data is not just academic medical centers or big centers. I think it’s a pretty good look at what’s actually being done in the real world today,” according to Dr. Mureebe.
The analysis showed that CCO has already had an effect on practice. A higher proportion of patients with CCO now undergo stenting as opposed to endarterectomy. Only 4.6% of all CEAs were done in patients with CCO, compared with 11.5% of CAS procedures. Moreover, the majority of revascularizations in the setting of CCO were performed in patients with asymptomatic disease: 57% of all CEA and 53% of the CAS. The CAS finding was surprising given that reimbursement for CAS is at present limited to symptomatic patients at high surgical risk who have a significant internal carotid artery stenosis, Dr. Mureebe observed.
The 30-day stroke rate in patients with CCO was 3.22% after CEA and 1.75% after CAS, a difference that wasn’t statistically significant. In patients without CCO, the stroke rate was 2.03% after CEA and 2.96% after CAS.
Next, the investigators analyzed differences in stroke rates according to symptom status. Among patients with CCO and preprocedural transient ischemic attack, stroke, or transient monocular blindness who underwent CEA, the 30-day stroke risk associated with CEA was 5.2%, a significantly higher rate than the 2.1% rate seen in patients without symptoms. The number of patients with CCO undergoing CAS was too small to draw conclusions regarding possible differences in stroke risk based upon symptom status.
In the NSQIP database, patients with CCO had higher prevalences of heart failure, hypertension, and smoking. For this reason, Dr. Mureebe said she suspects CCO is a surrogate for greater atherosclerotic disease burden and not an independent risk factor for periprocedural stroke. If future studies of the minimally invasive transcarotid artery revascularization procedure also show a higher rate of bad outcomes in patients with CCO, that would further support the hypothesis that CCO is a marker of higher atherosclerotic disease burden, Dr. Mureebe said.
A limitation of the NSQIP database is that it captures only those CAS cases done in operating rooms. “Maybe patients undergoing CAS in the OR are different from those undergoing CAS in a radiologic suite or cath lab,” she noted.
Dr. Mureebe reported having no financial conflicts of interest regarding her presentation.
SOURCE: Mureebe L. 42nd Annual Northwestern Vascular Symposium.
CHICAGO – Carotid angioplasty and stenting (CAS) isn’t associated with a lower 30-day stroke risk than carotid endarterectomy (CEA) for revascularization of the internal carotid artery in patients with contralateral carotid occlusion, Leila Mureebe, MD, said at a symposium on vascular surgery sponsored by Northwestern University, Chicago.
The reported prevalence of contralateral carotid occlusion (CCO) in patients undergoing revascularization for carotid artery disease is 3%-15%. Of late Dr. Mureebe has been particularly interested in two questions regarding CCO in patients undergoing revascularization of their other carotid artery: Is CCO truly a risk factor for perioperative stroke? And if so, can this risk be mitigated by the choice of procedure?
To answer the first question, Dr. Mureebe and her coinvestigators performed a meta-analysis of eight representative studies published between 1994 and 2012; they determined that CCO in patients undergoing CEA was indeed associated with a near doubling of perioperative stroke risk, compared with that of patients without CCO.
In order to learn whether CAS mitigates this risk, she and her coworkers analyzed the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database for the period between 2011 and 2015, in which they identified 15,619 fully documented CEA and 496 CAS.
“This NSQIP data is not just academic medical centers or big centers. I think it’s a pretty good look at what’s actually being done in the real world today,” according to Dr. Mureebe.
The analysis showed that CCO has already had an effect on practice. A higher proportion of patients with CCO now undergo stenting as opposed to endarterectomy. Only 4.6% of all CEAs were done in patients with CCO, compared with 11.5% of CAS procedures. Moreover, the majority of revascularizations in the setting of CCO were performed in patients with asymptomatic disease: 57% of all CEA and 53% of the CAS. The CAS finding was surprising given that reimbursement for CAS is at present limited to symptomatic patients at high surgical risk who have a significant internal carotid artery stenosis, Dr. Mureebe observed.
The 30-day stroke rate in patients with CCO was 3.22% after CEA and 1.75% after CAS, a difference that wasn’t statistically significant. In patients without CCO, the stroke rate was 2.03% after CEA and 2.96% after CAS.
Next, the investigators analyzed differences in stroke rates according to symptom status. Among patients with CCO and preprocedural transient ischemic attack, stroke, or transient monocular blindness who underwent CEA, the 30-day stroke risk associated with CEA was 5.2%, a significantly higher rate than the 2.1% rate seen in patients without symptoms. The number of patients with CCO undergoing CAS was too small to draw conclusions regarding possible differences in stroke risk based upon symptom status.
In the NSQIP database, patients with CCO had higher prevalences of heart failure, hypertension, and smoking. For this reason, Dr. Mureebe said she suspects CCO is a surrogate for greater atherosclerotic disease burden and not an independent risk factor for periprocedural stroke. If future studies of the minimally invasive transcarotid artery revascularization procedure also show a higher rate of bad outcomes in patients with CCO, that would further support the hypothesis that CCO is a marker of higher atherosclerotic disease burden, Dr. Mureebe said.
A limitation of the NSQIP database is that it captures only those CAS cases done in operating rooms. “Maybe patients undergoing CAS in the OR are different from those undergoing CAS in a radiologic suite or cath lab,” she noted.
Dr. Mureebe reported having no financial conflicts of interest regarding her presentation.
SOURCE: Mureebe L. 42nd Annual Northwestern Vascular Symposium.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM









