User login
Insulin delivery devices now covered under Part D
Older Americans will now have access to insulin delivery devices under Part D of Medicare coverage, according to guidance issued by the Centers for Medicare & Medicaid Services (CMS). The CMS guidance clarified that devices not previously covered under Medicare Part B will be covered under Part D of the prescription drug program. As a result, older Americans will now have access to a wider range of insulin delivery devices.
Insulin delivery devices help patients manage blood sugar levels effectively. The devices prevent dangerous blood sugar fluctuations that can lead to complications like hypoglycemia.
In the guidance, the CMS wrote: “With the introduction of new insulin delivery devices to the market, questions have arisen about Part D coverage for these products. Specifically, we have been asked whether newer insulin delivery devices that are not covered under Medicare Part B meet the Part D definition of ‘medical supplies associated with the injection of insulin. … The examples that were previously provided were never intended to be an exhaustive list of products that could be covered under Part D. Instead, they represented our understanding of the types of medical supplies associated with the injection of insulin that were available at the time.”
Since then, new delivery devices have been introduced to market “in the form of both mechanical and electronic insulin pumps” that are not covered under the Medicare Part B durable medical equipment (DME) benefit.
“We expect that technology will continue to advance and that ‘medical supplies associated with the injection of insulin’ will become significantly more sophisticated,” wrote the CMS. “As new products become available, Part D sponsors may evaluate these products for formulary placement and medical necessity and, subject to Part D coverage determination and appeals requirements, allow access and restrict use accordingly.”
According to the guidance, Part D will cover supplies “that are alternatives to insulin syringes,” and the CMS will not require insurers who offer Part D plan coverage to include these on their formularies. If these alternatives are included on formularies, sponsors may use utilization management criteria for the products.
In a statement praising the CMS’s decision, the Endocrine Society wrote: “This opens the door for older Americans to gain coverage for devices such as the Omnipod insulin management system, a popular insulin pump system. Until the new guidance was issued, Omnipod was the only FDA [Food and Drug Administration]–approved insulin pump system not covered by Medicare. Previously, people with diabetes who qualified for Medicare at age 65 had to pay out of pocket to continue using the Omnipod, and many lost access to the device.”
Robert Lash, MD, chief professional and clinical affairs officer for the Endocrine Society, said in an interview that the new CMS guidance "gives physicians and people with diabetes access to a wider range of technology options, since some people with type 1 diabetes prefer the Omnipod’s tubing-free design because it makes it easier to participate in sports and safer to work in certain environments.
“Before this guidance was issued, those patients had to switch pumps or revert to insulin injections when they turned 65 years old. We are pleased this guidance will open the door to greater choice” for patients, said Dr. Lash.
The CMS’s decision on insulin delivery devices follows a decision last year to cover continuous glucose monitors (CGMs) through Medicare. Last week, the CMS also announced it will provide coverage for the Abbott Freestyle Libre CGM, a welcome move for patients seeking additional choices.
This article was revised 1/18/18.
Older Americans will now have access to insulin delivery devices under Part D of Medicare coverage, according to guidance issued by the Centers for Medicare & Medicaid Services (CMS). The CMS guidance clarified that devices not previously covered under Medicare Part B will be covered under Part D of the prescription drug program. As a result, older Americans will now have access to a wider range of insulin delivery devices.
Insulin delivery devices help patients manage blood sugar levels effectively. The devices prevent dangerous blood sugar fluctuations that can lead to complications like hypoglycemia.
In the guidance, the CMS wrote: “With the introduction of new insulin delivery devices to the market, questions have arisen about Part D coverage for these products. Specifically, we have been asked whether newer insulin delivery devices that are not covered under Medicare Part B meet the Part D definition of ‘medical supplies associated with the injection of insulin. … The examples that were previously provided were never intended to be an exhaustive list of products that could be covered under Part D. Instead, they represented our understanding of the types of medical supplies associated with the injection of insulin that were available at the time.”
Since then, new delivery devices have been introduced to market “in the form of both mechanical and electronic insulin pumps” that are not covered under the Medicare Part B durable medical equipment (DME) benefit.
“We expect that technology will continue to advance and that ‘medical supplies associated with the injection of insulin’ will become significantly more sophisticated,” wrote the CMS. “As new products become available, Part D sponsors may evaluate these products for formulary placement and medical necessity and, subject to Part D coverage determination and appeals requirements, allow access and restrict use accordingly.”
According to the guidance, Part D will cover supplies “that are alternatives to insulin syringes,” and the CMS will not require insurers who offer Part D plan coverage to include these on their formularies. If these alternatives are included on formularies, sponsors may use utilization management criteria for the products.
In a statement praising the CMS’s decision, the Endocrine Society wrote: “This opens the door for older Americans to gain coverage for devices such as the Omnipod insulin management system, a popular insulin pump system. Until the new guidance was issued, Omnipod was the only FDA [Food and Drug Administration]–approved insulin pump system not covered by Medicare. Previously, people with diabetes who qualified for Medicare at age 65 had to pay out of pocket to continue using the Omnipod, and many lost access to the device.”
Robert Lash, MD, chief professional and clinical affairs officer for the Endocrine Society, said in an interview that the new CMS guidance "gives physicians and people with diabetes access to a wider range of technology options, since some people with type 1 diabetes prefer the Omnipod’s tubing-free design because it makes it easier to participate in sports and safer to work in certain environments.
“Before this guidance was issued, those patients had to switch pumps or revert to insulin injections when they turned 65 years old. We are pleased this guidance will open the door to greater choice” for patients, said Dr. Lash.
The CMS’s decision on insulin delivery devices follows a decision last year to cover continuous glucose monitors (CGMs) through Medicare. Last week, the CMS also announced it will provide coverage for the Abbott Freestyle Libre CGM, a welcome move for patients seeking additional choices.
This article was revised 1/18/18.
Older Americans will now have access to insulin delivery devices under Part D of Medicare coverage, according to guidance issued by the Centers for Medicare & Medicaid Services (CMS). The CMS guidance clarified that devices not previously covered under Medicare Part B will be covered under Part D of the prescription drug program. As a result, older Americans will now have access to a wider range of insulin delivery devices.
Insulin delivery devices help patients manage blood sugar levels effectively. The devices prevent dangerous blood sugar fluctuations that can lead to complications like hypoglycemia.
In the guidance, the CMS wrote: “With the introduction of new insulin delivery devices to the market, questions have arisen about Part D coverage for these products. Specifically, we have been asked whether newer insulin delivery devices that are not covered under Medicare Part B meet the Part D definition of ‘medical supplies associated with the injection of insulin. … The examples that were previously provided were never intended to be an exhaustive list of products that could be covered under Part D. Instead, they represented our understanding of the types of medical supplies associated with the injection of insulin that were available at the time.”
Since then, new delivery devices have been introduced to market “in the form of both mechanical and electronic insulin pumps” that are not covered under the Medicare Part B durable medical equipment (DME) benefit.
“We expect that technology will continue to advance and that ‘medical supplies associated with the injection of insulin’ will become significantly more sophisticated,” wrote the CMS. “As new products become available, Part D sponsors may evaluate these products for formulary placement and medical necessity and, subject to Part D coverage determination and appeals requirements, allow access and restrict use accordingly.”
According to the guidance, Part D will cover supplies “that are alternatives to insulin syringes,” and the CMS will not require insurers who offer Part D plan coverage to include these on their formularies. If these alternatives are included on formularies, sponsors may use utilization management criteria for the products.
In a statement praising the CMS’s decision, the Endocrine Society wrote: “This opens the door for older Americans to gain coverage for devices such as the Omnipod insulin management system, a popular insulin pump system. Until the new guidance was issued, Omnipod was the only FDA [Food and Drug Administration]–approved insulin pump system not covered by Medicare. Previously, people with diabetes who qualified for Medicare at age 65 had to pay out of pocket to continue using the Omnipod, and many lost access to the device.”
Robert Lash, MD, chief professional and clinical affairs officer for the Endocrine Society, said in an interview that the new CMS guidance "gives physicians and people with diabetes access to a wider range of technology options, since some people with type 1 diabetes prefer the Omnipod’s tubing-free design because it makes it easier to participate in sports and safer to work in certain environments.
“Before this guidance was issued, those patients had to switch pumps or revert to insulin injections when they turned 65 years old. We are pleased this guidance will open the door to greater choice” for patients, said Dr. Lash.
The CMS’s decision on insulin delivery devices follows a decision last year to cover continuous glucose monitors (CGMs) through Medicare. Last week, the CMS also announced it will provide coverage for the Abbott Freestyle Libre CGM, a welcome move for patients seeking additional choices.
This article was revised 1/18/18.
Pathological video game use can be ‘life-dominating’
SAN DIEGO – As a medical student, David L. Atkinson, MD, learned about a group of five adult men who played EverQuest, which bills itself as a 3D online world that “offers endless excitement, adventure, battle, and discovery.” They shared an apartment in Austin, Tex., and rotated which one would hold a full-time job while the other four spent their waking hours playing EverQuest.
“It was a little concerning,” recalled Dr. Atkinson, now a psychiatrist and the medical director of the teen recovery program at Children’s Health, Dallas. “EverQuest had a button in the game where you could order a pizza without interrupting your game play. Pathological video game use can be incredibly life-dominating.”
Many terms are used for pathological video game use, including problematic video game use, gaming disorder, and Internet gaming disorder, which is the term used in section III of DSM-5. Whether chronic video game use is a societal problem or an individual problem “is a very big question,” Dr. Atkinson said. “When we look at some of the prevalence data from Monitoring the Future, we have seen reductions in all kinds of substance use. We have seen reductions in teenage motor vehicle accidents and in teen pregnancy. If kids are playing video games and they’re all getting out of shape, that’s a cultural challenge. A clinician, though, may advocate against it as part of good health care.
“For instance, underage drinking in some American subcultures is normative. It doesn’t mean it’s a good idea.”
Most youth do not develop addictive behavior from playing games like EverQuest. “Substance use and gaming are different,” Dr. Atkinson said. “The amount of time spent at the expense of other things is one of the primary harms of video gaming, but financial concerns are not irrelevant. The new Star Wars Battlefront game would cost $2,100 if someone were to buy all of the available extras for the video game. Otherwise, it would take several hundred hours of game play to achieve all of these unlocked features.” While video games do not induce supraphysiologic dopamine release in the way drugs like cocaine do, the addictive potential is measured by an equation of reward versus effort. Obtaining a video game is not dependent upon social interactions, unlike drug use in states where the drug in question is illegal. In fact, the fewer social connections, the greater the risk of developing a video game use disorder.
“The perception of harm of video game addiction is very low, and parents do not consider the potential for developing an addiction before they buy a computer, handheld device, or video game console,” he said. “It is viewed as something that has to be limited ... not as something that is impossible to limit.” However, when parents begin to detect problems, they often find themselves unable to control their children’s or teens’ use of gaming, according to Dr. Atkinson.
In the DSM-5, Internet gaming disorder is defined as being preoccupied with games and withdrawn when not playing them, including irritability, anxiety, and sadness. Tolerance manifests as needing to spend more time playing the game. Typically, gamers cannot reduce their use despite effort, and there is a loss of interest in other activities and hobbies, Dr. Atkinson said. They may continue to engage in overuse of games despite knowing it’s a problem; they may lie about usage, may use games to escape anxiety or guilt, and may have lost or risked lose or risk relationships or career opportunities because of games.
“Not all gamers will do all of these things,” he emphasized. “For example, some gamers have disordered use and lose interest in other things, but don’t lie about it.” DSM-5 criteria also note that the video gaming itself must cause clinically significant impairment and must not be a manifestation of another disorder.
Tools aimed at helping in the diagnosis include the Problem Video Game Playing Questionnaire, the Internet Gaming Disorder Scale, the Internet Gaming Disorder Scale–Short-Form, the Problematic Online Gaming Questionnaire, the Game Addiction Scale, and the Electronic Gaming Motives Questionnaire, which measures enhancement, coping, social, and self-gratification motives.
According to Dr. Atkinson, 90% of children in Japan, Korea, North America, and Europe play video games. However, the prevalence of Internet gaming disorder is estimated to be 1% in the United States, 1.14% in Germany, and 5.9% in South Korea. Males have higher rates of pathological video game use, while afflicted females tend to have more problems. Pathological gaming use is associated with high levels of previous truancy and few leisure activities. It’s also associated with depression, poor impulse control, narcissistic traits, high anxiety, poor social competence, and less religiosity.
“The overlap with depression is very interesting,” Dr. Atkinson said. “ When they get rejected in a peer group or for a job, they tend to take it harder than people who don’t game. The gaming world is a place where you can be safe from rejection. If your credit card goes through, you’re allowed in.”
Anhedonia is another factor within the clinical syndrome of depression that is associated with video game use. A nationwide community sample of individuals in Korea showed that gaming and depression have their overlap most strongly with the “escape from negative emotions” model (J Nerv Ment Dis. 2017;205[7]:568-73). Other associated problems include greater obesity; metabolic indicators, such as high triglycerides and cholesterol; and sleep deprivation. Chronic gamers also tend to have less social support, less health promotion, and heightened social phobia. “When you’re gaming all the time, you’re going to have less opportunity to engage in an exposure paradigm to help you get over your social phobia,” Dr. Atkinson said. “Problem gamers are also more likely to have pathological use of pornography, poor impulse control, and ADHD symptoms.”
Studies of biobehavioral characteristics of those with pathological video game use suggest that there is a decreased dopamine striatal response (Neurosci Biobehav Rev. 2017 Apr;75:314-30). They also suggest decreased functional connectivity across areas of the brain, including decreased resting-state functional connectivity between ventral tegmental area and the nucleus accumbens, and lower tonic dopamine firing.
Parental management training can be successful at setting gaming limits in children under 12 years of age, he said. Pathological video game use is associated with physiologic stress in the family problem-solving task. One study of a brief 3-week family therapy intervention as measured by functional MRI showed that improvement in perceived family cohesion was associated with an increase in the activity of the caudate nucleus in response to the gamer’s viewing images of family cohesion and was inversely correlated with changes in online game playing time (Psychiatry Res. 2012 May 31;202[2]:126-31). “Bringing the family together may give them something to do besides gaming,” Dr. Atkinson said. “That can help them put games in a more balanced perspective.”
The largest evidence base supports cognitive-behavioral therapy for Internet gaming disorder, but there is insufficient evidence to make a clear statement of benefit (Clin Psychol Rev. 2017 Jun;54:123-33). Gaming-related cognitions accounted for a large portion of the variance in treatment response.
“Does the gaming cause the thoughts? Or do the thoughts cause the gaming?” Dr. Atkinson asked. “The cognitive model of CBT would tell you there’s a bidirectional relationship.”
As for medications, bupropion has been shown to reduce online gaming in depressed individuals, and escitalopram also may be efficacious. One comparative analysis showed that there were greater effects from using bupropion than for using escitalopram (Clin Psychopharmacol Neurosci. 2017 Nov 30;15[4]:361-8). Methylphenidate also has been shown to reduce online gaming (Compr Psychiatry. 2009 May-Jun;50[3]:251-6).
Parents who take video games away from their children often are met with a burst of aggression. “There’s an attempt to reestablish dominance in the situation, to obtain the old reinforcer or to reestablish control,” Dr. Atkinson said. “It’s different from tapering a drug; this is something that you have to plan for. Tapering video games is difficult to do. If the kid plays longer than they’re supposed to, what do you do then? You may have a fight to discontinue the video game. That’s one of the practical problems.”
Dr. Atkinson reported having no financial disclosures.
SOURCE: Atkinson DL. AAAP 2017.
SAN DIEGO – As a medical student, David L. Atkinson, MD, learned about a group of five adult men who played EverQuest, which bills itself as a 3D online world that “offers endless excitement, adventure, battle, and discovery.” They shared an apartment in Austin, Tex., and rotated which one would hold a full-time job while the other four spent their waking hours playing EverQuest.
“It was a little concerning,” recalled Dr. Atkinson, now a psychiatrist and the medical director of the teen recovery program at Children’s Health, Dallas. “EverQuest had a button in the game where you could order a pizza without interrupting your game play. Pathological video game use can be incredibly life-dominating.”
Many terms are used for pathological video game use, including problematic video game use, gaming disorder, and Internet gaming disorder, which is the term used in section III of DSM-5. Whether chronic video game use is a societal problem or an individual problem “is a very big question,” Dr. Atkinson said. “When we look at some of the prevalence data from Monitoring the Future, we have seen reductions in all kinds of substance use. We have seen reductions in teenage motor vehicle accidents and in teen pregnancy. If kids are playing video games and they’re all getting out of shape, that’s a cultural challenge. A clinician, though, may advocate against it as part of good health care.
“For instance, underage drinking in some American subcultures is normative. It doesn’t mean it’s a good idea.”
Most youth do not develop addictive behavior from playing games like EverQuest. “Substance use and gaming are different,” Dr. Atkinson said. “The amount of time spent at the expense of other things is one of the primary harms of video gaming, but financial concerns are not irrelevant. The new Star Wars Battlefront game would cost $2,100 if someone were to buy all of the available extras for the video game. Otherwise, it would take several hundred hours of game play to achieve all of these unlocked features.” While video games do not induce supraphysiologic dopamine release in the way drugs like cocaine do, the addictive potential is measured by an equation of reward versus effort. Obtaining a video game is not dependent upon social interactions, unlike drug use in states where the drug in question is illegal. In fact, the fewer social connections, the greater the risk of developing a video game use disorder.
“The perception of harm of video game addiction is very low, and parents do not consider the potential for developing an addiction before they buy a computer, handheld device, or video game console,” he said. “It is viewed as something that has to be limited ... not as something that is impossible to limit.” However, when parents begin to detect problems, they often find themselves unable to control their children’s or teens’ use of gaming, according to Dr. Atkinson.
In the DSM-5, Internet gaming disorder is defined as being preoccupied with games and withdrawn when not playing them, including irritability, anxiety, and sadness. Tolerance manifests as needing to spend more time playing the game. Typically, gamers cannot reduce their use despite effort, and there is a loss of interest in other activities and hobbies, Dr. Atkinson said. They may continue to engage in overuse of games despite knowing it’s a problem; they may lie about usage, may use games to escape anxiety or guilt, and may have lost or risked lose or risk relationships or career opportunities because of games.
“Not all gamers will do all of these things,” he emphasized. “For example, some gamers have disordered use and lose interest in other things, but don’t lie about it.” DSM-5 criteria also note that the video gaming itself must cause clinically significant impairment and must not be a manifestation of another disorder.
Tools aimed at helping in the diagnosis include the Problem Video Game Playing Questionnaire, the Internet Gaming Disorder Scale, the Internet Gaming Disorder Scale–Short-Form, the Problematic Online Gaming Questionnaire, the Game Addiction Scale, and the Electronic Gaming Motives Questionnaire, which measures enhancement, coping, social, and self-gratification motives.
According to Dr. Atkinson, 90% of children in Japan, Korea, North America, and Europe play video games. However, the prevalence of Internet gaming disorder is estimated to be 1% in the United States, 1.14% in Germany, and 5.9% in South Korea. Males have higher rates of pathological video game use, while afflicted females tend to have more problems. Pathological gaming use is associated with high levels of previous truancy and few leisure activities. It’s also associated with depression, poor impulse control, narcissistic traits, high anxiety, poor social competence, and less religiosity.
“The overlap with depression is very interesting,” Dr. Atkinson said. “ When they get rejected in a peer group or for a job, they tend to take it harder than people who don’t game. The gaming world is a place where you can be safe from rejection. If your credit card goes through, you’re allowed in.”
Anhedonia is another factor within the clinical syndrome of depression that is associated with video game use. A nationwide community sample of individuals in Korea showed that gaming and depression have their overlap most strongly with the “escape from negative emotions” model (J Nerv Ment Dis. 2017;205[7]:568-73). Other associated problems include greater obesity; metabolic indicators, such as high triglycerides and cholesterol; and sleep deprivation. Chronic gamers also tend to have less social support, less health promotion, and heightened social phobia. “When you’re gaming all the time, you’re going to have less opportunity to engage in an exposure paradigm to help you get over your social phobia,” Dr. Atkinson said. “Problem gamers are also more likely to have pathological use of pornography, poor impulse control, and ADHD symptoms.”
Studies of biobehavioral characteristics of those with pathological video game use suggest that there is a decreased dopamine striatal response (Neurosci Biobehav Rev. 2017 Apr;75:314-30). They also suggest decreased functional connectivity across areas of the brain, including decreased resting-state functional connectivity between ventral tegmental area and the nucleus accumbens, and lower tonic dopamine firing.
Parental management training can be successful at setting gaming limits in children under 12 years of age, he said. Pathological video game use is associated with physiologic stress in the family problem-solving task. One study of a brief 3-week family therapy intervention as measured by functional MRI showed that improvement in perceived family cohesion was associated with an increase in the activity of the caudate nucleus in response to the gamer’s viewing images of family cohesion and was inversely correlated with changes in online game playing time (Psychiatry Res. 2012 May 31;202[2]:126-31). “Bringing the family together may give them something to do besides gaming,” Dr. Atkinson said. “That can help them put games in a more balanced perspective.”
The largest evidence base supports cognitive-behavioral therapy for Internet gaming disorder, but there is insufficient evidence to make a clear statement of benefit (Clin Psychol Rev. 2017 Jun;54:123-33). Gaming-related cognitions accounted for a large portion of the variance in treatment response.
“Does the gaming cause the thoughts? Or do the thoughts cause the gaming?” Dr. Atkinson asked. “The cognitive model of CBT would tell you there’s a bidirectional relationship.”
As for medications, bupropion has been shown to reduce online gaming in depressed individuals, and escitalopram also may be efficacious. One comparative analysis showed that there were greater effects from using bupropion than for using escitalopram (Clin Psychopharmacol Neurosci. 2017 Nov 30;15[4]:361-8). Methylphenidate also has been shown to reduce online gaming (Compr Psychiatry. 2009 May-Jun;50[3]:251-6).
Parents who take video games away from their children often are met with a burst of aggression. “There’s an attempt to reestablish dominance in the situation, to obtain the old reinforcer or to reestablish control,” Dr. Atkinson said. “It’s different from tapering a drug; this is something that you have to plan for. Tapering video games is difficult to do. If the kid plays longer than they’re supposed to, what do you do then? You may have a fight to discontinue the video game. That’s one of the practical problems.”
Dr. Atkinson reported having no financial disclosures.
SOURCE: Atkinson DL. AAAP 2017.
SAN DIEGO – As a medical student, David L. Atkinson, MD, learned about a group of five adult men who played EverQuest, which bills itself as a 3D online world that “offers endless excitement, adventure, battle, and discovery.” They shared an apartment in Austin, Tex., and rotated which one would hold a full-time job while the other four spent their waking hours playing EverQuest.
“It was a little concerning,” recalled Dr. Atkinson, now a psychiatrist and the medical director of the teen recovery program at Children’s Health, Dallas. “EverQuest had a button in the game where you could order a pizza without interrupting your game play. Pathological video game use can be incredibly life-dominating.”
Many terms are used for pathological video game use, including problematic video game use, gaming disorder, and Internet gaming disorder, which is the term used in section III of DSM-5. Whether chronic video game use is a societal problem or an individual problem “is a very big question,” Dr. Atkinson said. “When we look at some of the prevalence data from Monitoring the Future, we have seen reductions in all kinds of substance use. We have seen reductions in teenage motor vehicle accidents and in teen pregnancy. If kids are playing video games and they’re all getting out of shape, that’s a cultural challenge. A clinician, though, may advocate against it as part of good health care.
“For instance, underage drinking in some American subcultures is normative. It doesn’t mean it’s a good idea.”
Most youth do not develop addictive behavior from playing games like EverQuest. “Substance use and gaming are different,” Dr. Atkinson said. “The amount of time spent at the expense of other things is one of the primary harms of video gaming, but financial concerns are not irrelevant. The new Star Wars Battlefront game would cost $2,100 if someone were to buy all of the available extras for the video game. Otherwise, it would take several hundred hours of game play to achieve all of these unlocked features.” While video games do not induce supraphysiologic dopamine release in the way drugs like cocaine do, the addictive potential is measured by an equation of reward versus effort. Obtaining a video game is not dependent upon social interactions, unlike drug use in states where the drug in question is illegal. In fact, the fewer social connections, the greater the risk of developing a video game use disorder.
“The perception of harm of video game addiction is very low, and parents do not consider the potential for developing an addiction before they buy a computer, handheld device, or video game console,” he said. “It is viewed as something that has to be limited ... not as something that is impossible to limit.” However, when parents begin to detect problems, they often find themselves unable to control their children’s or teens’ use of gaming, according to Dr. Atkinson.
In the DSM-5, Internet gaming disorder is defined as being preoccupied with games and withdrawn when not playing them, including irritability, anxiety, and sadness. Tolerance manifests as needing to spend more time playing the game. Typically, gamers cannot reduce their use despite effort, and there is a loss of interest in other activities and hobbies, Dr. Atkinson said. They may continue to engage in overuse of games despite knowing it’s a problem; they may lie about usage, may use games to escape anxiety or guilt, and may have lost or risked lose or risk relationships or career opportunities because of games.
“Not all gamers will do all of these things,” he emphasized. “For example, some gamers have disordered use and lose interest in other things, but don’t lie about it.” DSM-5 criteria also note that the video gaming itself must cause clinically significant impairment and must not be a manifestation of another disorder.
Tools aimed at helping in the diagnosis include the Problem Video Game Playing Questionnaire, the Internet Gaming Disorder Scale, the Internet Gaming Disorder Scale–Short-Form, the Problematic Online Gaming Questionnaire, the Game Addiction Scale, and the Electronic Gaming Motives Questionnaire, which measures enhancement, coping, social, and self-gratification motives.
According to Dr. Atkinson, 90% of children in Japan, Korea, North America, and Europe play video games. However, the prevalence of Internet gaming disorder is estimated to be 1% in the United States, 1.14% in Germany, and 5.9% in South Korea. Males have higher rates of pathological video game use, while afflicted females tend to have more problems. Pathological gaming use is associated with high levels of previous truancy and few leisure activities. It’s also associated with depression, poor impulse control, narcissistic traits, high anxiety, poor social competence, and less religiosity.
“The overlap with depression is very interesting,” Dr. Atkinson said. “ When they get rejected in a peer group or for a job, they tend to take it harder than people who don’t game. The gaming world is a place where you can be safe from rejection. If your credit card goes through, you’re allowed in.”
Anhedonia is another factor within the clinical syndrome of depression that is associated with video game use. A nationwide community sample of individuals in Korea showed that gaming and depression have their overlap most strongly with the “escape from negative emotions” model (J Nerv Ment Dis. 2017;205[7]:568-73). Other associated problems include greater obesity; metabolic indicators, such as high triglycerides and cholesterol; and sleep deprivation. Chronic gamers also tend to have less social support, less health promotion, and heightened social phobia. “When you’re gaming all the time, you’re going to have less opportunity to engage in an exposure paradigm to help you get over your social phobia,” Dr. Atkinson said. “Problem gamers are also more likely to have pathological use of pornography, poor impulse control, and ADHD symptoms.”
Studies of biobehavioral characteristics of those with pathological video game use suggest that there is a decreased dopamine striatal response (Neurosci Biobehav Rev. 2017 Apr;75:314-30). They also suggest decreased functional connectivity across areas of the brain, including decreased resting-state functional connectivity between ventral tegmental area and the nucleus accumbens, and lower tonic dopamine firing.
Parental management training can be successful at setting gaming limits in children under 12 years of age, he said. Pathological video game use is associated with physiologic stress in the family problem-solving task. One study of a brief 3-week family therapy intervention as measured by functional MRI showed that improvement in perceived family cohesion was associated with an increase in the activity of the caudate nucleus in response to the gamer’s viewing images of family cohesion and was inversely correlated with changes in online game playing time (Psychiatry Res. 2012 May 31;202[2]:126-31). “Bringing the family together may give them something to do besides gaming,” Dr. Atkinson said. “That can help them put games in a more balanced perspective.”
The largest evidence base supports cognitive-behavioral therapy for Internet gaming disorder, but there is insufficient evidence to make a clear statement of benefit (Clin Psychol Rev. 2017 Jun;54:123-33). Gaming-related cognitions accounted for a large portion of the variance in treatment response.
“Does the gaming cause the thoughts? Or do the thoughts cause the gaming?” Dr. Atkinson asked. “The cognitive model of CBT would tell you there’s a bidirectional relationship.”
As for medications, bupropion has been shown to reduce online gaming in depressed individuals, and escitalopram also may be efficacious. One comparative analysis showed that there were greater effects from using bupropion than for using escitalopram (Clin Psychopharmacol Neurosci. 2017 Nov 30;15[4]:361-8). Methylphenidate also has been shown to reduce online gaming (Compr Psychiatry. 2009 May-Jun;50[3]:251-6).
Parents who take video games away from their children often are met with a burst of aggression. “There’s an attempt to reestablish dominance in the situation, to obtain the old reinforcer or to reestablish control,” Dr. Atkinson said. “It’s different from tapering a drug; this is something that you have to plan for. Tapering video games is difficult to do. If the kid plays longer than they’re supposed to, what do you do then? You may have a fight to discontinue the video game. That’s one of the practical problems.”
Dr. Atkinson reported having no financial disclosures.
SOURCE: Atkinson DL. AAAP 2017.
REPORTING FROM AAAP
Generic Glatiramer Acetate Remains Safe and Effective for Two Years
Generic glatiramer acetate remains effective and safe over two years of treatment for patients with relapsing-remitting multiple sclerosis (MS), according to data published in the December 2017 issue of Multiple Sclerosis Journal. The data also indicate that switching from branded glatiramer acetate to a generic formulation is safe and well tolerated.
The European Medicines Agency required clinical trial data to support the authorization of generic glatiramer acetate. Krzysztof Selmaj, MD, a neurologist at the Neurology Center Lodz in Poland, and colleagues conducted a nine-month study to assess the equivalence of generic glatiramer acetate with that of Copaxone, a branded formulation of the drug. The double-blind, phase III GATE trial suggested that the drugs had equivalent efficacy, safety, and tolerability.
An Open-Label Extension
Patients who completed the nine-month trial were eligible to continue into a 15-month open-label extension on generic glatiramer acetate. The goals of the extension were to evaluate the effects of long-term exposure to the drug and to assess whether switching from branded to generic glatiramer acetate influenced drug safety and efficacy.
The researchers enrolled 796 patients from 17 countries into the double-blind study. Eligible patients were between ages 18 and 55, had relapsing-remitting MS, and had an Expanded Disability Status Scale (EDSS) score of 0 to 5.5. Patients were randomized to receive 20 mg/mL/day of generic glatiramer acetate, 20 mg/mL/day of branded glatiramer acetate, or matching placebo.
The investigators performed safety evaluations at months 12, 15, 18, 21, and 24. They conducted EDSS scoring and brain MRI scans at months 12, 18, and 24. To assess glatiramer acetate antidrug antibodies, the researchers collected serum samples at baseline and months 1, 3, 6, 9, 12, 18, and 24.
Branded and Generic Formulations Produced Similar Outcomes
In all, 735 participants completed the double-blind study. In addition, 728 patients entered the open-label extension, and 670 completed it.
The proportion of patients completing the trial was 93.8% among patients who received generic treatment throughout, 92.9% among patients who switched from branded to generic, and 81.5% among patients who switched from placebo to generic glatiramer acetate.
The mean number of gadolinium-enhancing lesions was similar at months 12, 18, and 24 for patients who had started the blinded study on generic glatiramer acetate and those who had started on branded glatiramer acetate. The changes in the other MRI outcomes were similar for these two groups.
The estimated annualized relapse rates in the extension study were 0.21 for patients who took generic glatiramer acetate throughout, 0.24 for patients who switched from branded to generic glatiramer acetate, and 0.23 for patients who switched from placebo to generic glatiramer acetate.
The rate of adverse events was similar for patients who took generic glatiramer acetate throughout (33.3%) and those who switched from branded to generic treatment (36.5%). The rate of adverse events was 43.2% among patients who switched from placebo to generic glatiramer acetate. Severe and serious adverse events were uncommon and occurred at similar rates among patients who started on generic treatment and those who started on branded treatment.
During the blinded phase, antidrug antibodies formed with comparable frequency among patients who received generic and branded glatiramer treatment. During the open-label extension, the antidrug antibody titers in the group switching from branded to generic glatiramer treatment remained similar to that of the group continuing on generic treatment.
“These data should help patients and prescribers to positively consider generic glatiramer acetate as an alternative to branded glatiramer acetate,” said Dr. Selmaj.
—Erik Greb
Suggested Reading
Selmaj K, Barkhof F, Belova AN, et al. Switching from branded to generic glatiramer acetate: 15-month GATE trial extension results. Mult Scler. 2017;23(14):1909-1917.
Generic glatiramer acetate remains effective and safe over two years of treatment for patients with relapsing-remitting multiple sclerosis (MS), according to data published in the December 2017 issue of Multiple Sclerosis Journal. The data also indicate that switching from branded glatiramer acetate to a generic formulation is safe and well tolerated.
The European Medicines Agency required clinical trial data to support the authorization of generic glatiramer acetate. Krzysztof Selmaj, MD, a neurologist at the Neurology Center Lodz in Poland, and colleagues conducted a nine-month study to assess the equivalence of generic glatiramer acetate with that of Copaxone, a branded formulation of the drug. The double-blind, phase III GATE trial suggested that the drugs had equivalent efficacy, safety, and tolerability.
An Open-Label Extension
Patients who completed the nine-month trial were eligible to continue into a 15-month open-label extension on generic glatiramer acetate. The goals of the extension were to evaluate the effects of long-term exposure to the drug and to assess whether switching from branded to generic glatiramer acetate influenced drug safety and efficacy.
The researchers enrolled 796 patients from 17 countries into the double-blind study. Eligible patients were between ages 18 and 55, had relapsing-remitting MS, and had an Expanded Disability Status Scale (EDSS) score of 0 to 5.5. Patients were randomized to receive 20 mg/mL/day of generic glatiramer acetate, 20 mg/mL/day of branded glatiramer acetate, or matching placebo.
The investigators performed safety evaluations at months 12, 15, 18, 21, and 24. They conducted EDSS scoring and brain MRI scans at months 12, 18, and 24. To assess glatiramer acetate antidrug antibodies, the researchers collected serum samples at baseline and months 1, 3, 6, 9, 12, 18, and 24.
Branded and Generic Formulations Produced Similar Outcomes
In all, 735 participants completed the double-blind study. In addition, 728 patients entered the open-label extension, and 670 completed it.
The proportion of patients completing the trial was 93.8% among patients who received generic treatment throughout, 92.9% among patients who switched from branded to generic, and 81.5% among patients who switched from placebo to generic glatiramer acetate.
The mean number of gadolinium-enhancing lesions was similar at months 12, 18, and 24 for patients who had started the blinded study on generic glatiramer acetate and those who had started on branded glatiramer acetate. The changes in the other MRI outcomes were similar for these two groups.
The estimated annualized relapse rates in the extension study were 0.21 for patients who took generic glatiramer acetate throughout, 0.24 for patients who switched from branded to generic glatiramer acetate, and 0.23 for patients who switched from placebo to generic glatiramer acetate.
The rate of adverse events was similar for patients who took generic glatiramer acetate throughout (33.3%) and those who switched from branded to generic treatment (36.5%). The rate of adverse events was 43.2% among patients who switched from placebo to generic glatiramer acetate. Severe and serious adverse events were uncommon and occurred at similar rates among patients who started on generic treatment and those who started on branded treatment.
During the blinded phase, antidrug antibodies formed with comparable frequency among patients who received generic and branded glatiramer treatment. During the open-label extension, the antidrug antibody titers in the group switching from branded to generic glatiramer treatment remained similar to that of the group continuing on generic treatment.
“These data should help patients and prescribers to positively consider generic glatiramer acetate as an alternative to branded glatiramer acetate,” said Dr. Selmaj.
—Erik Greb
Suggested Reading
Selmaj K, Barkhof F, Belova AN, et al. Switching from branded to generic glatiramer acetate: 15-month GATE trial extension results. Mult Scler. 2017;23(14):1909-1917.
Generic glatiramer acetate remains effective and safe over two years of treatment for patients with relapsing-remitting multiple sclerosis (MS), according to data published in the December 2017 issue of Multiple Sclerosis Journal. The data also indicate that switching from branded glatiramer acetate to a generic formulation is safe and well tolerated.
The European Medicines Agency required clinical trial data to support the authorization of generic glatiramer acetate. Krzysztof Selmaj, MD, a neurologist at the Neurology Center Lodz in Poland, and colleagues conducted a nine-month study to assess the equivalence of generic glatiramer acetate with that of Copaxone, a branded formulation of the drug. The double-blind, phase III GATE trial suggested that the drugs had equivalent efficacy, safety, and tolerability.
An Open-Label Extension
Patients who completed the nine-month trial were eligible to continue into a 15-month open-label extension on generic glatiramer acetate. The goals of the extension were to evaluate the effects of long-term exposure to the drug and to assess whether switching from branded to generic glatiramer acetate influenced drug safety and efficacy.
The researchers enrolled 796 patients from 17 countries into the double-blind study. Eligible patients were between ages 18 and 55, had relapsing-remitting MS, and had an Expanded Disability Status Scale (EDSS) score of 0 to 5.5. Patients were randomized to receive 20 mg/mL/day of generic glatiramer acetate, 20 mg/mL/day of branded glatiramer acetate, or matching placebo.
The investigators performed safety evaluations at months 12, 15, 18, 21, and 24. They conducted EDSS scoring and brain MRI scans at months 12, 18, and 24. To assess glatiramer acetate antidrug antibodies, the researchers collected serum samples at baseline and months 1, 3, 6, 9, 12, 18, and 24.
Branded and Generic Formulations Produced Similar Outcomes
In all, 735 participants completed the double-blind study. In addition, 728 patients entered the open-label extension, and 670 completed it.
The proportion of patients completing the trial was 93.8% among patients who received generic treatment throughout, 92.9% among patients who switched from branded to generic, and 81.5% among patients who switched from placebo to generic glatiramer acetate.
The mean number of gadolinium-enhancing lesions was similar at months 12, 18, and 24 for patients who had started the blinded study on generic glatiramer acetate and those who had started on branded glatiramer acetate. The changes in the other MRI outcomes were similar for these two groups.
The estimated annualized relapse rates in the extension study were 0.21 for patients who took generic glatiramer acetate throughout, 0.24 for patients who switched from branded to generic glatiramer acetate, and 0.23 for patients who switched from placebo to generic glatiramer acetate.
The rate of adverse events was similar for patients who took generic glatiramer acetate throughout (33.3%) and those who switched from branded to generic treatment (36.5%). The rate of adverse events was 43.2% among patients who switched from placebo to generic glatiramer acetate. Severe and serious adverse events were uncommon and occurred at similar rates among patients who started on generic treatment and those who started on branded treatment.
During the blinded phase, antidrug antibodies formed with comparable frequency among patients who received generic and branded glatiramer treatment. During the open-label extension, the antidrug antibody titers in the group switching from branded to generic glatiramer treatment remained similar to that of the group continuing on generic treatment.
“These data should help patients and prescribers to positively consider generic glatiramer acetate as an alternative to branded glatiramer acetate,” said Dr. Selmaj.
—Erik Greb
Suggested Reading
Selmaj K, Barkhof F, Belova AN, et al. Switching from branded to generic glatiramer acetate: 15-month GATE trial extension results. Mult Scler. 2017;23(14):1909-1917.
Hydroxychloroquine use rising in SLE pregnancies
but its use “remains low, and that is concerning for maternal and fetal well-being,” said investigators who analyzed one public and one private database.
The two databases showed increases of somewhat different scale. According to Medicaid data on 3,045 pregnancies among SLE women, initiation of hydroxychloroquine rose from 2.7% in 2001 to 7.5% (P = .0002) in 2010. The analysis of data for 2,255 SLE pregnancies from a large commercial insurance database (Optum Clinformatics) showed an increase from 4.9% in 2003 to 13.6% (P = .0001) in 2015, wrote Bonnie L. Bermas, MD, of Brigham and Women’s Hospital, Boston, and her associates. The report was published in Lupus.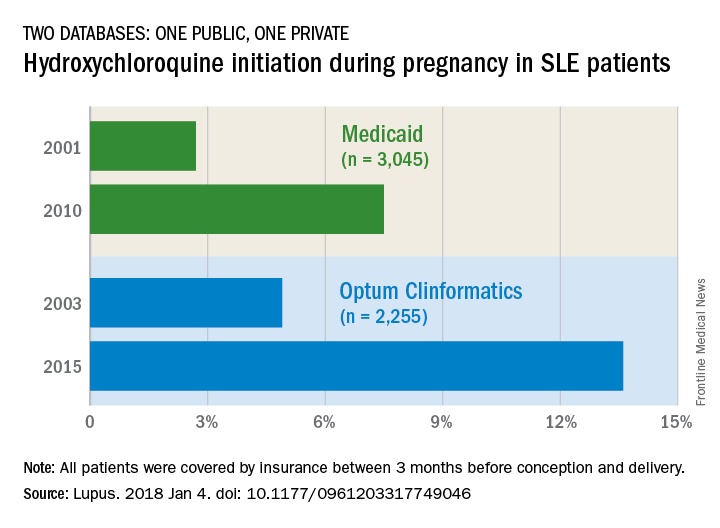
The study was funded by Brigham and Women’s Hospital and Harvard Medical School. Dr. Bermas did not report any conflicts. Her associates reported unrelated projects with a number of pharmaceutical companies.
SOURCE: Bermas BL et al. Lupus. 2018 Jan 4. doi: 10.1177/0961203317749046.
but its use “remains low, and that is concerning for maternal and fetal well-being,” said investigators who analyzed one public and one private database.
The two databases showed increases of somewhat different scale. According to Medicaid data on 3,045 pregnancies among SLE women, initiation of hydroxychloroquine rose from 2.7% in 2001 to 7.5% (P = .0002) in 2010. The analysis of data for 2,255 SLE pregnancies from a large commercial insurance database (Optum Clinformatics) showed an increase from 4.9% in 2003 to 13.6% (P = .0001) in 2015, wrote Bonnie L. Bermas, MD, of Brigham and Women’s Hospital, Boston, and her associates. The report was published in Lupus.
The study was funded by Brigham and Women’s Hospital and Harvard Medical School. Dr. Bermas did not report any conflicts. Her associates reported unrelated projects with a number of pharmaceutical companies.
SOURCE: Bermas BL et al. Lupus. 2018 Jan 4. doi: 10.1177/0961203317749046.
but its use “remains low, and that is concerning for maternal and fetal well-being,” said investigators who analyzed one public and one private database.
The two databases showed increases of somewhat different scale. According to Medicaid data on 3,045 pregnancies among SLE women, initiation of hydroxychloroquine rose from 2.7% in 2001 to 7.5% (P = .0002) in 2010. The analysis of data for 2,255 SLE pregnancies from a large commercial insurance database (Optum Clinformatics) showed an increase from 4.9% in 2003 to 13.6% (P = .0001) in 2015, wrote Bonnie L. Bermas, MD, of Brigham and Women’s Hospital, Boston, and her associates. The report was published in Lupus.
The study was funded by Brigham and Women’s Hospital and Harvard Medical School. Dr. Bermas did not report any conflicts. Her associates reported unrelated projects with a number of pharmaceutical companies.
SOURCE: Bermas BL et al. Lupus. 2018 Jan 4. doi: 10.1177/0961203317749046.
FROM LUPUS
FDA: LifeVest wearable defibrillator has safety issue
The Zoll LifeVest 4000, a wearable defibrillator, could fail to deliver a treatment shock after displaying the message “Call for service: Device has a problem that may require service. Call ZOLL for service, Message Code 102,” according to the FDA.
“Failure to contact Zoll and immediately replace the device after Message Code 102 appears on the device screen may result in serious patient harm or death of the patient because the device may fail to deliver therapy appropriately when needed” according to an FDA press release.
Only one death associated with “Message Code 102” malfunction of LifeVest has been reported, but about 0.1% of devices have displayed the “Message Code 102” error. According to Zoll, roughly 33,670 devices have been distributed as of Nov. 14, 2017, with nearly 75% of them distributed in the United States.
The FDA has indicated that it will continue to work with Zoll to monitor adverse events associated with the “Message Code 102” error and work on finding a permanent solution to this problem. Recommendations for physicians, caregivers, and patients regarding how to respond to error messages can be found here.
The Zoll LifeVest 4000, a wearable defibrillator, could fail to deliver a treatment shock after displaying the message “Call for service: Device has a problem that may require service. Call ZOLL for service, Message Code 102,” according to the FDA.
“Failure to contact Zoll and immediately replace the device after Message Code 102 appears on the device screen may result in serious patient harm or death of the patient because the device may fail to deliver therapy appropriately when needed” according to an FDA press release.
Only one death associated with “Message Code 102” malfunction of LifeVest has been reported, but about 0.1% of devices have displayed the “Message Code 102” error. According to Zoll, roughly 33,670 devices have been distributed as of Nov. 14, 2017, with nearly 75% of them distributed in the United States.
The FDA has indicated that it will continue to work with Zoll to monitor adverse events associated with the “Message Code 102” error and work on finding a permanent solution to this problem. Recommendations for physicians, caregivers, and patients regarding how to respond to error messages can be found here.
The Zoll LifeVest 4000, a wearable defibrillator, could fail to deliver a treatment shock after displaying the message “Call for service: Device has a problem that may require service. Call ZOLL for service, Message Code 102,” according to the FDA.
“Failure to contact Zoll and immediately replace the device after Message Code 102 appears on the device screen may result in serious patient harm or death of the patient because the device may fail to deliver therapy appropriately when needed” according to an FDA press release.
Only one death associated with “Message Code 102” malfunction of LifeVest has been reported, but about 0.1% of devices have displayed the “Message Code 102” error. According to Zoll, roughly 33,670 devices have been distributed as of Nov. 14, 2017, with nearly 75% of them distributed in the United States.
The FDA has indicated that it will continue to work with Zoll to monitor adverse events associated with the “Message Code 102” error and work on finding a permanent solution to this problem. Recommendations for physicians, caregivers, and patients regarding how to respond to error messages can be found here.
When cloth diapers cause diaper dermatitis, think calcineurin inhibitors
, a reemerging complication of diaper dermatitis associated with use of cloth reusable diapers, wrote Rita Ramos Pinheiro, MD, of Hospital Santo António dos Capuchos, Lisbon, and her associates.
An otherwise healthy 18-month-old girl was referred to the dermatology clinic with a 9-month history of severe relapsing diaper dermatitis, which responded to topical clotrimazole and zinc oxide cream. Nondisposable cloth diapers were used. She returned with a rash unresponsive to barrier creams, topical antibiotics and antifungals, and a variety of topical corticosteroids.
Clinically, a diagnosis of granuloma gluteale infantum was suspected; this was confirmed by a skin biopsy of one of the nodules.
The parents declined to stop using cloth diapers, so general measures to alleviate diaper dermatitis were tried, including frequent diaper-free periods. All previous topical treatments were stopped. A 0.1% pimecrolimus cream applied daily for 1 month was well tolerated, and a more potent 0.03% tacrolimus cream then was applied; both medications are topical calcineurin inhibitors.
At 4 weeks, there was complete regression of the ulcerated lesions, with later thinning of the lesions. At the last examination, there remained only slightly hypopigmented residual patches.
The same general measures were continued, including use of barrier creams. The patient had a relapse at her 9-month follow-up, with three nodules occurring on the gluteal region; these resolved with application of topical tacrolimus cream for 1 week.
Although reusable cloth diapers are considered better for the environment and cheaper than disposable diapers, “purportedly” they are less absorbent than disposable diapers, the investigators said.
Characteristics of the clinical diagnosis of granuloma gluteale infantum are “red-purple to red-brown, round to oval, deep, firm nodules with central ulceration,” with a “classic distribution over the convexities of the gluteal region, sparing the inguinal folds,” Dr. Pinheiro and her associates observed. The case study is the first reporting the use of topical calcineurin inhibitors for treating granuloma gluteale infantum, the researchers asserted.
Read more at Pediatrics (2017 Jan 3. doi: 10.1542/peds.2016-2064).
, a reemerging complication of diaper dermatitis associated with use of cloth reusable diapers, wrote Rita Ramos Pinheiro, MD, of Hospital Santo António dos Capuchos, Lisbon, and her associates.
An otherwise healthy 18-month-old girl was referred to the dermatology clinic with a 9-month history of severe relapsing diaper dermatitis, which responded to topical clotrimazole and zinc oxide cream. Nondisposable cloth diapers were used. She returned with a rash unresponsive to barrier creams, topical antibiotics and antifungals, and a variety of topical corticosteroids.
Clinically, a diagnosis of granuloma gluteale infantum was suspected; this was confirmed by a skin biopsy of one of the nodules.
The parents declined to stop using cloth diapers, so general measures to alleviate diaper dermatitis were tried, including frequent diaper-free periods. All previous topical treatments were stopped. A 0.1% pimecrolimus cream applied daily for 1 month was well tolerated, and a more potent 0.03% tacrolimus cream then was applied; both medications are topical calcineurin inhibitors.
At 4 weeks, there was complete regression of the ulcerated lesions, with later thinning of the lesions. At the last examination, there remained only slightly hypopigmented residual patches.
The same general measures were continued, including use of barrier creams. The patient had a relapse at her 9-month follow-up, with three nodules occurring on the gluteal region; these resolved with application of topical tacrolimus cream for 1 week.
Although reusable cloth diapers are considered better for the environment and cheaper than disposable diapers, “purportedly” they are less absorbent than disposable diapers, the investigators said.
Characteristics of the clinical diagnosis of granuloma gluteale infantum are “red-purple to red-brown, round to oval, deep, firm nodules with central ulceration,” with a “classic distribution over the convexities of the gluteal region, sparing the inguinal folds,” Dr. Pinheiro and her associates observed. The case study is the first reporting the use of topical calcineurin inhibitors for treating granuloma gluteale infantum, the researchers asserted.
Read more at Pediatrics (2017 Jan 3. doi: 10.1542/peds.2016-2064).
, a reemerging complication of diaper dermatitis associated with use of cloth reusable diapers, wrote Rita Ramos Pinheiro, MD, of Hospital Santo António dos Capuchos, Lisbon, and her associates.
An otherwise healthy 18-month-old girl was referred to the dermatology clinic with a 9-month history of severe relapsing diaper dermatitis, which responded to topical clotrimazole and zinc oxide cream. Nondisposable cloth diapers were used. She returned with a rash unresponsive to barrier creams, topical antibiotics and antifungals, and a variety of topical corticosteroids.
Clinically, a diagnosis of granuloma gluteale infantum was suspected; this was confirmed by a skin biopsy of one of the nodules.
The parents declined to stop using cloth diapers, so general measures to alleviate diaper dermatitis were tried, including frequent diaper-free periods. All previous topical treatments were stopped. A 0.1% pimecrolimus cream applied daily for 1 month was well tolerated, and a more potent 0.03% tacrolimus cream then was applied; both medications are topical calcineurin inhibitors.
At 4 weeks, there was complete regression of the ulcerated lesions, with later thinning of the lesions. At the last examination, there remained only slightly hypopigmented residual patches.
The same general measures were continued, including use of barrier creams. The patient had a relapse at her 9-month follow-up, with three nodules occurring on the gluteal region; these resolved with application of topical tacrolimus cream for 1 week.
Although reusable cloth diapers are considered better for the environment and cheaper than disposable diapers, “purportedly” they are less absorbent than disposable diapers, the investigators said.
Characteristics of the clinical diagnosis of granuloma gluteale infantum are “red-purple to red-brown, round to oval, deep, firm nodules with central ulceration,” with a “classic distribution over the convexities of the gluteal region, sparing the inguinal folds,” Dr. Pinheiro and her associates observed. The case study is the first reporting the use of topical calcineurin inhibitors for treating granuloma gluteale infantum, the researchers asserted.
Read more at Pediatrics (2017 Jan 3. doi: 10.1542/peds.2016-2064).
FROM PEDIATRICS
Tracheobronchial tree size changes may predict IPF outcomes
Changes in tracheobronchial tree size may serve as a practical and noninvasive method for predicting disease severity in patients diagnosed with idiopathic pulmonary fibrosis, according to data from 150 adults.
To determine the potential predictive value of tracheobronchial tree changes on mortality, Ankush Ratwani, MD, of Georgetown University, Washington, and colleagues reviewed data from adults with IPF seen at a single center between March 2012 and December 2016. The findings were presented at the CHEST annual meeting.
The researchers measured the tracheal diameters of the patients and used the GAP index, an established system for predicting mortality in IPF patients, to determine a relationship. Overall, they found a significant correlation between GAP index scores and increasing tracheobronchial tree size across eight measurements of different levels along the tracheobronchial tree “with an increase in GAP index stage for every level of increase in tracheal measurements (P less than .005),” they noted.
Measurements included the anterior-posterior diameter at the subglottic level, aortic arch, carina, right main stem bronchus, and left main stem bronchus, as well as transverse diameter assessment at the subglottis, aortic arch, and carina. The average anterior-posterior tracheal diameters were 21.77 mm for the subglottis, 21.84 mm for the aortic arch, 20.47 mm for the carina, 15.19 for the right main stem bronchus, and 14.21 mm for the left main stem bronchus.
No correlation appeared between tracheal size and lung volume, which suggests that enlargement of the trachea is likely caused by other factors beyond fibrosis, and next steps for research should determine whether tracheal size is an independent predictor of mortality in IPF patients, the investigators noted.
“With the field of treatment and management changing for IPF over the last few years, it has becoming increasingly important to prognose these patients in order to find where they fit in the spectrum for treatment or lung transplant,” Dr. Ratwani said in an interview. “Additionally, there needs to be a noninvasive measure to show disease progression, such as with using CT scans, and correlate with other prognostic indicators to hopefully create a regression formula that encompasses multiple parameters,” he explained.
“The results were surprising in that there was a correlation of a radiographic measure that has not been looked at previously with a validated measure of prognostication in IPF (GAP Index),” Dr. Ratwani said.
Although the findings do not imply more than a correlation, the results serve as “a good start to validate the theory that as the distal airways enlarge (traction bronchiectasis) in later stages of IPF, so may the proximal airways, which may be used to easily measure disease progression and guide the conversation for transplant or treatment,” Dr. Ratwani noted. His next steps for research include studying transplant-free survival in correlation with tracheal size, as well as serial changes between CT scans with correlations of lung volumes and survival.
Changes in tracheobronchial tree size may serve as a practical and noninvasive method for predicting disease severity in patients diagnosed with idiopathic pulmonary fibrosis, according to data from 150 adults.
To determine the potential predictive value of tracheobronchial tree changes on mortality, Ankush Ratwani, MD, of Georgetown University, Washington, and colleagues reviewed data from adults with IPF seen at a single center between March 2012 and December 2016. The findings were presented at the CHEST annual meeting.
The researchers measured the tracheal diameters of the patients and used the GAP index, an established system for predicting mortality in IPF patients, to determine a relationship. Overall, they found a significant correlation between GAP index scores and increasing tracheobronchial tree size across eight measurements of different levels along the tracheobronchial tree “with an increase in GAP index stage for every level of increase in tracheal measurements (P less than .005),” they noted.
Measurements included the anterior-posterior diameter at the subglottic level, aortic arch, carina, right main stem bronchus, and left main stem bronchus, as well as transverse diameter assessment at the subglottis, aortic arch, and carina. The average anterior-posterior tracheal diameters were 21.77 mm for the subglottis, 21.84 mm for the aortic arch, 20.47 mm for the carina, 15.19 for the right main stem bronchus, and 14.21 mm for the left main stem bronchus.
No correlation appeared between tracheal size and lung volume, which suggests that enlargement of the trachea is likely caused by other factors beyond fibrosis, and next steps for research should determine whether tracheal size is an independent predictor of mortality in IPF patients, the investigators noted.
“With the field of treatment and management changing for IPF over the last few years, it has becoming increasingly important to prognose these patients in order to find where they fit in the spectrum for treatment or lung transplant,” Dr. Ratwani said in an interview. “Additionally, there needs to be a noninvasive measure to show disease progression, such as with using CT scans, and correlate with other prognostic indicators to hopefully create a regression formula that encompasses multiple parameters,” he explained.
“The results were surprising in that there was a correlation of a radiographic measure that has not been looked at previously with a validated measure of prognostication in IPF (GAP Index),” Dr. Ratwani said.
Although the findings do not imply more than a correlation, the results serve as “a good start to validate the theory that as the distal airways enlarge (traction bronchiectasis) in later stages of IPF, so may the proximal airways, which may be used to easily measure disease progression and guide the conversation for transplant or treatment,” Dr. Ratwani noted. His next steps for research include studying transplant-free survival in correlation with tracheal size, as well as serial changes between CT scans with correlations of lung volumes and survival.
Changes in tracheobronchial tree size may serve as a practical and noninvasive method for predicting disease severity in patients diagnosed with idiopathic pulmonary fibrosis, according to data from 150 adults.
To determine the potential predictive value of tracheobronchial tree changes on mortality, Ankush Ratwani, MD, of Georgetown University, Washington, and colleagues reviewed data from adults with IPF seen at a single center between March 2012 and December 2016. The findings were presented at the CHEST annual meeting.
The researchers measured the tracheal diameters of the patients and used the GAP index, an established system for predicting mortality in IPF patients, to determine a relationship. Overall, they found a significant correlation between GAP index scores and increasing tracheobronchial tree size across eight measurements of different levels along the tracheobronchial tree “with an increase in GAP index stage for every level of increase in tracheal measurements (P less than .005),” they noted.
Measurements included the anterior-posterior diameter at the subglottic level, aortic arch, carina, right main stem bronchus, and left main stem bronchus, as well as transverse diameter assessment at the subglottis, aortic arch, and carina. The average anterior-posterior tracheal diameters were 21.77 mm for the subglottis, 21.84 mm for the aortic arch, 20.47 mm for the carina, 15.19 for the right main stem bronchus, and 14.21 mm for the left main stem bronchus.
No correlation appeared between tracheal size and lung volume, which suggests that enlargement of the trachea is likely caused by other factors beyond fibrosis, and next steps for research should determine whether tracheal size is an independent predictor of mortality in IPF patients, the investigators noted.
“With the field of treatment and management changing for IPF over the last few years, it has becoming increasingly important to prognose these patients in order to find where they fit in the spectrum for treatment or lung transplant,” Dr. Ratwani said in an interview. “Additionally, there needs to be a noninvasive measure to show disease progression, such as with using CT scans, and correlate with other prognostic indicators to hopefully create a regression formula that encompasses multiple parameters,” he explained.
“The results were surprising in that there was a correlation of a radiographic measure that has not been looked at previously with a validated measure of prognostication in IPF (GAP Index),” Dr. Ratwani said.
Although the findings do not imply more than a correlation, the results serve as “a good start to validate the theory that as the distal airways enlarge (traction bronchiectasis) in later stages of IPF, so may the proximal airways, which may be used to easily measure disease progression and guide the conversation for transplant or treatment,” Dr. Ratwani noted. His next steps for research include studying transplant-free survival in correlation with tracheal size, as well as serial changes between CT scans with correlations of lung volumes and survival.
FROM CHEST 2017
More savings available from generic oral contraceptives
, according to an analysis of almost 20,000 OC-prescribing events.
Brand OCs with available generics represented 44% of expenditures for all OCs in both 2010 and 2014, while generics had 56% and 55% of spending in 2010 and 2014 and brand names without generics took 0% and 1%, respectively, Mark Chee and his associates wrote in a report published in JAMA Internal Medicine.
For the whole 4-year period, brand OCs accounted for 24% of all prescriptions and 42% of all expenditures for the 19,944 OC prescribing events included in the analysis of data from the Medical Expenditure Panel Survey.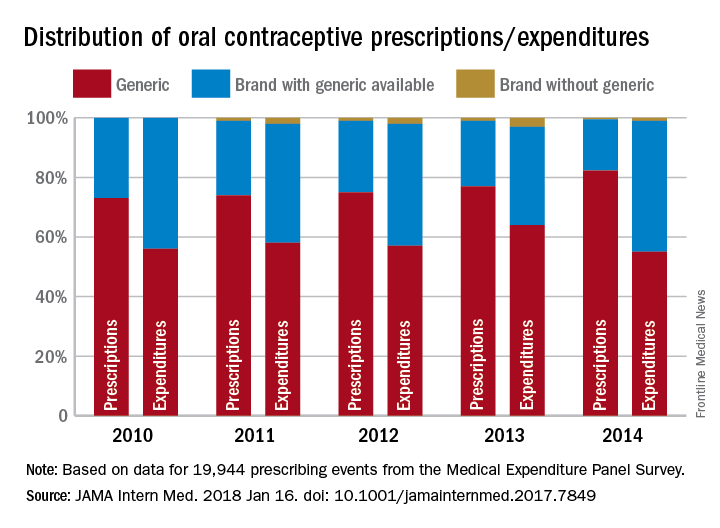
The study was funded by a National Institutes of Health grant to Mr. Chee. All of his five associates have received a grant from the Food and Drug Administration to improve prescription of generic drugs.
SOURCE: Chee M. et al. JAMA Intern Med. 2018 Jan 16. doi: 10.1001/jamainternmed.2017.7849.
, according to an analysis of almost 20,000 OC-prescribing events.
Brand OCs with available generics represented 44% of expenditures for all OCs in both 2010 and 2014, while generics had 56% and 55% of spending in 2010 and 2014 and brand names without generics took 0% and 1%, respectively, Mark Chee and his associates wrote in a report published in JAMA Internal Medicine.
For the whole 4-year period, brand OCs accounted for 24% of all prescriptions and 42% of all expenditures for the 19,944 OC prescribing events included in the analysis of data from the Medical Expenditure Panel Survey.
The study was funded by a National Institutes of Health grant to Mr. Chee. All of his five associates have received a grant from the Food and Drug Administration to improve prescription of generic drugs.
SOURCE: Chee M. et al. JAMA Intern Med. 2018 Jan 16. doi: 10.1001/jamainternmed.2017.7849.
, according to an analysis of almost 20,000 OC-prescribing events.
Brand OCs with available generics represented 44% of expenditures for all OCs in both 2010 and 2014, while generics had 56% and 55% of spending in 2010 and 2014 and brand names without generics took 0% and 1%, respectively, Mark Chee and his associates wrote in a report published in JAMA Internal Medicine.
For the whole 4-year period, brand OCs accounted for 24% of all prescriptions and 42% of all expenditures for the 19,944 OC prescribing events included in the analysis of data from the Medical Expenditure Panel Survey.
The study was funded by a National Institutes of Health grant to Mr. Chee. All of his five associates have received a grant from the Food and Drug Administration to improve prescription of generic drugs.
SOURCE: Chee M. et al. JAMA Intern Med. 2018 Jan 16. doi: 10.1001/jamainternmed.2017.7849.
FROM JAMA INTERNAL MEDICINE
Breastfeeding lowers later diabetes risk in women
Breastfeeding may reduce a woman’s risk of developing diabetes, with a prospective cohort study showing a strong, inverse association between lactation duration and risk of diabetes.
In a report published in the Jan. 16 online edition of JAMA Internal Medicine, researchers analyzed data from the Coronary Artery Risk Development Study in Young Adults (CARDIA), which followed 1,238 women aged 18-30 for 30 years, with multiple assessments of glucose tolerance over the course of the study.
Women who breastfed for 6-12 months had a 48% reduction in the risk of diabetes (95% CI, 0.31-0.87), and those who breastfed for up to 6 months had a 25% lower risk (95% CI, 0.51-1.09), with the trend being significant.
In women with a history of gestational diabetes, those who did not breastfeed at all had a 2.08% higher excess risk of incident diabetes per year, compared with women who breastfed for at least 12 months. The increase in excess risk for the same comparison in women without a history of gestational diabetes was 0.48% per year.
Erica P. Gunderson, PhD, of Kaiser Permanente Northern California, Oakland, and her coauthors noted that previous meta-analyses of the effect of lactation on diabetes incidence or prevalence pointed to protective summary estimates of 9%-11% per year of lactation.
“Lactating women have lower circulating glucose in both fasting and post absorptive states, as well as lower insulin secretion, despite increased glucose production rates,” the authors wrote. “About 50 g of glucose per 24 hours is diverted into the mammary gland for milk synthesis via non–insulin mediated pathways.”
Studies in mice have also suggested that lactating animals have greater pancreatic beta-cell proliferation.
While the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists recommend breastfeeding for 1 year, only 55% of women in the United States are still breastfeeding at 6 months and 33% are still breastfeeding at 1 year after birth. Black women are also less likely to breastfeed, regardless of socioeconomic status or body size.
“Lactation is a natural biological process with the enormous potential to provide long-term benefits to maternal health, but has been underappreciated as a potential key strategy for early primary prevention of metabolic diseases in women across the childbearing years and beyond.”
The study and analyses were supported by the National Institute of Diabetes and Digestive and Kidney Diseases, and two authors declared funding from pharmaceutical companies.
SOURCE: JAMA Intern Med. 2016 Jan 16. doi: 10.1001/jamainternmed.2017.7978.
Breastfeeding may reduce a woman’s risk of developing diabetes, with a prospective cohort study showing a strong, inverse association between lactation duration and risk of diabetes.
In a report published in the Jan. 16 online edition of JAMA Internal Medicine, researchers analyzed data from the Coronary Artery Risk Development Study in Young Adults (CARDIA), which followed 1,238 women aged 18-30 for 30 years, with multiple assessments of glucose tolerance over the course of the study.
Women who breastfed for 6-12 months had a 48% reduction in the risk of diabetes (95% CI, 0.31-0.87), and those who breastfed for up to 6 months had a 25% lower risk (95% CI, 0.51-1.09), with the trend being significant.
In women with a history of gestational diabetes, those who did not breastfeed at all had a 2.08% higher excess risk of incident diabetes per year, compared with women who breastfed for at least 12 months. The increase in excess risk for the same comparison in women without a history of gestational diabetes was 0.48% per year.
Erica P. Gunderson, PhD, of Kaiser Permanente Northern California, Oakland, and her coauthors noted that previous meta-analyses of the effect of lactation on diabetes incidence or prevalence pointed to protective summary estimates of 9%-11% per year of lactation.
“Lactating women have lower circulating glucose in both fasting and post absorptive states, as well as lower insulin secretion, despite increased glucose production rates,” the authors wrote. “About 50 g of glucose per 24 hours is diverted into the mammary gland for milk synthesis via non–insulin mediated pathways.”
Studies in mice have also suggested that lactating animals have greater pancreatic beta-cell proliferation.
While the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists recommend breastfeeding for 1 year, only 55% of women in the United States are still breastfeeding at 6 months and 33% are still breastfeeding at 1 year after birth. Black women are also less likely to breastfeed, regardless of socioeconomic status or body size.
“Lactation is a natural biological process with the enormous potential to provide long-term benefits to maternal health, but has been underappreciated as a potential key strategy for early primary prevention of metabolic diseases in women across the childbearing years and beyond.”
The study and analyses were supported by the National Institute of Diabetes and Digestive and Kidney Diseases, and two authors declared funding from pharmaceutical companies.
SOURCE: JAMA Intern Med. 2016 Jan 16. doi: 10.1001/jamainternmed.2017.7978.
Breastfeeding may reduce a woman’s risk of developing diabetes, with a prospective cohort study showing a strong, inverse association between lactation duration and risk of diabetes.
In a report published in the Jan. 16 online edition of JAMA Internal Medicine, researchers analyzed data from the Coronary Artery Risk Development Study in Young Adults (CARDIA), which followed 1,238 women aged 18-30 for 30 years, with multiple assessments of glucose tolerance over the course of the study.
Women who breastfed for 6-12 months had a 48% reduction in the risk of diabetes (95% CI, 0.31-0.87), and those who breastfed for up to 6 months had a 25% lower risk (95% CI, 0.51-1.09), with the trend being significant.
In women with a history of gestational diabetes, those who did not breastfeed at all had a 2.08% higher excess risk of incident diabetes per year, compared with women who breastfed for at least 12 months. The increase in excess risk for the same comparison in women without a history of gestational diabetes was 0.48% per year.
Erica P. Gunderson, PhD, of Kaiser Permanente Northern California, Oakland, and her coauthors noted that previous meta-analyses of the effect of lactation on diabetes incidence or prevalence pointed to protective summary estimates of 9%-11% per year of lactation.
“Lactating women have lower circulating glucose in both fasting and post absorptive states, as well as lower insulin secretion, despite increased glucose production rates,” the authors wrote. “About 50 g of glucose per 24 hours is diverted into the mammary gland for milk synthesis via non–insulin mediated pathways.”
Studies in mice have also suggested that lactating animals have greater pancreatic beta-cell proliferation.
While the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists recommend breastfeeding for 1 year, only 55% of women in the United States are still breastfeeding at 6 months and 33% are still breastfeeding at 1 year after birth. Black women are also less likely to breastfeed, regardless of socioeconomic status or body size.
“Lactation is a natural biological process with the enormous potential to provide long-term benefits to maternal health, but has been underappreciated as a potential key strategy for early primary prevention of metabolic diseases in women across the childbearing years and beyond.”
The study and analyses were supported by the National Institute of Diabetes and Digestive and Kidney Diseases, and two authors declared funding from pharmaceutical companies.
SOURCE: JAMA Intern Med. 2016 Jan 16. doi: 10.1001/jamainternmed.2017.7978.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Duration of breastfeeding is inversely associated with risk of developing diabetes.
Major finding: Women who breastfeed for 12 months or more have a 47% lower risk of developing diabetes compared with women who do not breastfeed at all.
Data source: Analysis of data from the CARDIA population-based prospective cohort study in 1,238 women.
Disclosures: The study and analyses were supported by the National Institute of Diabetes and Digestive and Kidney Diseases, and two authors declared funding from pharmaceutical companies.
Source: JAMA Intern Med. 2016 Jan 16. doi: 10.1001/jamainternmed.2017.7978.
EC approves product to treat hemophilia A
The European Commission (EC) has granted marketing authorization for rurioctocog alfa pegol (Adynovi).
Rurioctocog alfa pegol (formerly BAX 855) is a pegylated, full-length, recombinant factor VIII product built on a previously licensed recombinant factor VIII product (Advate).
Rurioctocog alfa pegol is now EC-approved for on-demand and prophylactic use in patients age 12 and older with hemophilia A.
With the EC’s approval, Shire is authorized to market rurioctocog alfa pegol in all member states of the European Union as well as in Iceland, Liechtenstein, and Norway.
The marketing authorization for rurioctocog alfa pegol is based on outcomes from three phase 3 trials—a study of adolescents and adults, a trial of patients undergoing surgical procedures, and a study of pediatric patients.
Trial of adolescents/adults
This study included 137 patients, ages 12 to 58, with previously treated hemophilia A. They were assigned to either twice-weekly prophylaxis (40-50 IU/kg, n=120) or on-demand treatment (10-60 IU/kg, n=17) with rurioctocog alfa pegol. One hundred and twenty-six patients completed the study.
Patients who received prophylaxis had a 90% reduction in annualized bleeding rate (ABR) compared to those who received on-demand treatment. The median ABR was 1.9 in the prophylactic arm and 41.5 in the on-demand arm.
There were 7 adverse events (occurring in 6 patients) that were considered possibly related to rurioctocog alfa pegol. These included diarrhea, nausea, headache, and flushing.
These results were published in Blood in July 2015.
Perioperative study
This study included 15 patients with severe hemophilia A who were undergoing surgical procedures (11 of them major and 4 minor).
The patients received rurioctocog alfa pegol at varying doses and schedules, depending on each patient’s pharmacokinetic profile for major procedures or rurioctocog alfa pegol incremental recovery for minor procedures.
Intra-operative and peri-operative hemostatic efficacy of rurioctocog alfa pegol was deemed “excellent” for all 15 patients. The “excellent” rating meant that blood loss was less than or equal to that expected for the type of procedure performed in a non-hemophilic population.
Post-operatively (day 1 after the procedure), hemostatic efficacy was rated “good” for 1 procedure and “excellent” for the rest. The “good” rating meant that post-operative blood loss was up to 50% more than expected for the type of procedure performed in a non-hemophilic population.
There were no treatment-related adverse events or signs of immunogenicity in this trial.
Results were published in Haemophilia in June 2016.
Pediatric trial
This study included 66 patients, ages 1 to 11, who had previously treated hemophilia A. They received twice-weekly prophylaxis with rurioctocog alfa pegol (50 ± 10 IU/kg) for at least 6 months or 50 exposure days, whichever occurred last.
The median ABR was 2.0, and 38% of patients did not have any bleeding episodes.
None of the patients developed inhibitory antibodies, and there were no treatment-related adverse events.
Results from this trial were presented at the World Federation of Hemophilia 2016 World Congress in July 2016 and published in Haemophilia in November 2016. ![]()
The European Commission (EC) has granted marketing authorization for rurioctocog alfa pegol (Adynovi).
Rurioctocog alfa pegol (formerly BAX 855) is a pegylated, full-length, recombinant factor VIII product built on a previously licensed recombinant factor VIII product (Advate).
Rurioctocog alfa pegol is now EC-approved for on-demand and prophylactic use in patients age 12 and older with hemophilia A.
With the EC’s approval, Shire is authorized to market rurioctocog alfa pegol in all member states of the European Union as well as in Iceland, Liechtenstein, and Norway.
The marketing authorization for rurioctocog alfa pegol is based on outcomes from three phase 3 trials—a study of adolescents and adults, a trial of patients undergoing surgical procedures, and a study of pediatric patients.
Trial of adolescents/adults
This study included 137 patients, ages 12 to 58, with previously treated hemophilia A. They were assigned to either twice-weekly prophylaxis (40-50 IU/kg, n=120) or on-demand treatment (10-60 IU/kg, n=17) with rurioctocog alfa pegol. One hundred and twenty-six patients completed the study.
Patients who received prophylaxis had a 90% reduction in annualized bleeding rate (ABR) compared to those who received on-demand treatment. The median ABR was 1.9 in the prophylactic arm and 41.5 in the on-demand arm.
There were 7 adverse events (occurring in 6 patients) that were considered possibly related to rurioctocog alfa pegol. These included diarrhea, nausea, headache, and flushing.
These results were published in Blood in July 2015.
Perioperative study
This study included 15 patients with severe hemophilia A who were undergoing surgical procedures (11 of them major and 4 minor).
The patients received rurioctocog alfa pegol at varying doses and schedules, depending on each patient’s pharmacokinetic profile for major procedures or rurioctocog alfa pegol incremental recovery for minor procedures.
Intra-operative and peri-operative hemostatic efficacy of rurioctocog alfa pegol was deemed “excellent” for all 15 patients. The “excellent” rating meant that blood loss was less than or equal to that expected for the type of procedure performed in a non-hemophilic population.
Post-operatively (day 1 after the procedure), hemostatic efficacy was rated “good” for 1 procedure and “excellent” for the rest. The “good” rating meant that post-operative blood loss was up to 50% more than expected for the type of procedure performed in a non-hemophilic population.
There were no treatment-related adverse events or signs of immunogenicity in this trial.
Results were published in Haemophilia in June 2016.
Pediatric trial
This study included 66 patients, ages 1 to 11, who had previously treated hemophilia A. They received twice-weekly prophylaxis with rurioctocog alfa pegol (50 ± 10 IU/kg) for at least 6 months or 50 exposure days, whichever occurred last.
The median ABR was 2.0, and 38% of patients did not have any bleeding episodes.
None of the patients developed inhibitory antibodies, and there were no treatment-related adverse events.
Results from this trial were presented at the World Federation of Hemophilia 2016 World Congress in July 2016 and published in Haemophilia in November 2016. ![]()
The European Commission (EC) has granted marketing authorization for rurioctocog alfa pegol (Adynovi).
Rurioctocog alfa pegol (formerly BAX 855) is a pegylated, full-length, recombinant factor VIII product built on a previously licensed recombinant factor VIII product (Advate).
Rurioctocog alfa pegol is now EC-approved for on-demand and prophylactic use in patients age 12 and older with hemophilia A.
With the EC’s approval, Shire is authorized to market rurioctocog alfa pegol in all member states of the European Union as well as in Iceland, Liechtenstein, and Norway.
The marketing authorization for rurioctocog alfa pegol is based on outcomes from three phase 3 trials—a study of adolescents and adults, a trial of patients undergoing surgical procedures, and a study of pediatric patients.
Trial of adolescents/adults
This study included 137 patients, ages 12 to 58, with previously treated hemophilia A. They were assigned to either twice-weekly prophylaxis (40-50 IU/kg, n=120) or on-demand treatment (10-60 IU/kg, n=17) with rurioctocog alfa pegol. One hundred and twenty-six patients completed the study.
Patients who received prophylaxis had a 90% reduction in annualized bleeding rate (ABR) compared to those who received on-demand treatment. The median ABR was 1.9 in the prophylactic arm and 41.5 in the on-demand arm.
There were 7 adverse events (occurring in 6 patients) that were considered possibly related to rurioctocog alfa pegol. These included diarrhea, nausea, headache, and flushing.
These results were published in Blood in July 2015.
Perioperative study
This study included 15 patients with severe hemophilia A who were undergoing surgical procedures (11 of them major and 4 minor).
The patients received rurioctocog alfa pegol at varying doses and schedules, depending on each patient’s pharmacokinetic profile for major procedures or rurioctocog alfa pegol incremental recovery for minor procedures.
Intra-operative and peri-operative hemostatic efficacy of rurioctocog alfa pegol was deemed “excellent” for all 15 patients. The “excellent” rating meant that blood loss was less than or equal to that expected for the type of procedure performed in a non-hemophilic population.
Post-operatively (day 1 after the procedure), hemostatic efficacy was rated “good” for 1 procedure and “excellent” for the rest. The “good” rating meant that post-operative blood loss was up to 50% more than expected for the type of procedure performed in a non-hemophilic population.
There were no treatment-related adverse events or signs of immunogenicity in this trial.
Results were published in Haemophilia in June 2016.
Pediatric trial
This study included 66 patients, ages 1 to 11, who had previously treated hemophilia A. They received twice-weekly prophylaxis with rurioctocog alfa pegol (50 ± 10 IU/kg) for at least 6 months or 50 exposure days, whichever occurred last.
The median ABR was 2.0, and 38% of patients did not have any bleeding episodes.
None of the patients developed inhibitory antibodies, and there were no treatment-related adverse events.
Results from this trial were presented at the World Federation of Hemophilia 2016 World Congress in July 2016 and published in Haemophilia in November 2016. ![]()