User login
Register for 2018 ACS General Surgery Coding Workshops
Registration is now open to attend American College of Surgeons (ACS) 2018 General Surgery Coding Workshops. With Medicare and third-party payor policy and coding changes taking effect this year, it is imperative that surgeons have accurate and up-to-date information to protect reimbursement from Medicare and other payors and to optimize efficiency.
During the coding workshop, you will learn how to report surgical procedures and medical services and will have access to the tools necessary to succeed, including a coding workbook to keep for future reference with checklists, resource guides, templates, and examples. Physicians receive up to 6.5 AMA PRA Category 1 Credits™ for each day of participation. In addition, each day of the workshop meets AAPC guidelines for 6.5 continuing education units.
The ACS will offer the following seven coding workshops in 2018:
• Southlake, TX, January 25¬–26
• Las Vegas, NV, February 8–10
• Orlando, FL, February 22–23
• Chicago, IL, April 12–13
• New York, NY, May 17–19
• Nashville, TN, August 9–10
• Chicago, IL, November 1–3
For the first time, the ACS also will offer a three-day course, including a day devoted to trauma and critical care coding at the Las Vegas, New York City, and (November) Chicago workshops.
To register, visit www.karenzupko.com/workshops2/gensurg-workshops. For more details, visit the ACS website at www.facs.org/advocacy/practmanagement/workshops or e-mail [email protected].
Registration is now open to attend American College of Surgeons (ACS) 2018 General Surgery Coding Workshops. With Medicare and third-party payor policy and coding changes taking effect this year, it is imperative that surgeons have accurate and up-to-date information to protect reimbursement from Medicare and other payors and to optimize efficiency.
During the coding workshop, you will learn how to report surgical procedures and medical services and will have access to the tools necessary to succeed, including a coding workbook to keep for future reference with checklists, resource guides, templates, and examples. Physicians receive up to 6.5 AMA PRA Category 1 Credits™ for each day of participation. In addition, each day of the workshop meets AAPC guidelines for 6.5 continuing education units.
The ACS will offer the following seven coding workshops in 2018:
• Southlake, TX, January 25¬–26
• Las Vegas, NV, February 8–10
• Orlando, FL, February 22–23
• Chicago, IL, April 12–13
• New York, NY, May 17–19
• Nashville, TN, August 9–10
• Chicago, IL, November 1–3
For the first time, the ACS also will offer a three-day course, including a day devoted to trauma and critical care coding at the Las Vegas, New York City, and (November) Chicago workshops.
To register, visit www.karenzupko.com/workshops2/gensurg-workshops. For more details, visit the ACS website at www.facs.org/advocacy/practmanagement/workshops or e-mail [email protected].
Registration is now open to attend American College of Surgeons (ACS) 2018 General Surgery Coding Workshops. With Medicare and third-party payor policy and coding changes taking effect this year, it is imperative that surgeons have accurate and up-to-date information to protect reimbursement from Medicare and other payors and to optimize efficiency.
During the coding workshop, you will learn how to report surgical procedures and medical services and will have access to the tools necessary to succeed, including a coding workbook to keep for future reference with checklists, resource guides, templates, and examples. Physicians receive up to 6.5 AMA PRA Category 1 Credits™ for each day of participation. In addition, each day of the workshop meets AAPC guidelines for 6.5 continuing education units.
The ACS will offer the following seven coding workshops in 2018:
• Southlake, TX, January 25¬–26
• Las Vegas, NV, February 8–10
• Orlando, FL, February 22–23
• Chicago, IL, April 12–13
• New York, NY, May 17–19
• Nashville, TN, August 9–10
• Chicago, IL, November 1–3
For the first time, the ACS also will offer a three-day course, including a day devoted to trauma and critical care coding at the Las Vegas, New York City, and (November) Chicago workshops.
To register, visit www.karenzupko.com/workshops2/gensurg-workshops. For more details, visit the ACS website at www.facs.org/advocacy/practmanagement/workshops or e-mail [email protected].
Opioids a focus as HHS Secretary Azar defends White House budget proposal
The opioid abuse epidemic took center stage during a hearing of the House Ways and Means Committee, during which Alex Azar, secretary of the Department of Health & Human Services, defended the White House budget proposal for fiscal year 2019.
The president’s budget, released Feb. 13, allocates $5 billion in new resources over the next 5 years to combat the opioid abuse epidemic
During his Feb. 14 testimony, Mr. Azar said that the budget proposal “brings a new level of commitment to fighting the crisis of opioid addiction and overdose that is stealing more than 100 American lives from us every single day.”
He noted that part of the funding request includes spending $500 million to launch public-private partnership with the National Institutes of Health “to development new addiction treatments, new overdose-reversing drugs, and nonaddictive approaches to pain.”
Mr. Azar called for greater use of nonopioid pain management techniques and better communication between the HHS and the Drug Enforcement Agency to identify doctors who overprescribe opioids.
“I really want to focus on that entry point to working with DEA on ways that we can control pill mills and even just bad practice that has become part of our culture of medicine of giving people excessive numbers of pills when they do not need them,” Mr. Azar said.
The administration’s proposals for addressing the opioid crisis revealed partisan divisions among members of the Ways and Means Committee, as Republican members of the committee hailed the White House proposals while Democrats argued that the budget proposal could make it more difficult for people to fight the crisis.
Noting that many who have opioid use disorders rely on Medicaid for health insurance, Rep. Richard Neal (D-Mass.), the ranking member on the committee, noted that the proposed budget cuts $1.4 trillion from Medicaid and $500 billion from Medicare.
Physician groups were quick to point out that the budget proposal calls for addressing health care issues while simultaneously cutting the necessary funding.
The Infectious Diseases Society of America said in a statement that “while we appreciate continued funding for antimicrobial resistance research and development through the Biomedical Advanced Research and Development Authority, the current investment is insufficient, as evidenced by an antibiotic pipeline that falls far short of projected needs, while pharmaceutical company investments in antibiotic research continue to diminish. Also, the plan would limit CDC [Centers for Disease Control and Prevention] efforts to address and prevent growing resistance to existing antimicrobial drugs with a nearly $25 million cut.”
The White House budget proposal also tackles drug pricing and payment, including a new Medicaid demonstration program that would allow up to five participating states to test the use of drug formularies in the Medicaid program, speed the access to generic medications, and modify the Medicare Part D prescription drug program. Mr. Azar also reiterated his openness to government price negotiation for drugs administered in the physician office under Medicare Part B.
The proposal on drug discounts was praised by the Community Oncology Alliance (COA).
“The White House budget proposal for sharing manufacturer rebates and discounts with seniors in Medicare Part D is also a great idea,” COA said in a statement. “Pharmacy benefit managers have been enriching themselves with these rebates for too long, and their growing scale has resulted in higher drug costs for everyone. COA believes that the proposed Part D change to share rebates and discounts proposed will lower costs for patients, taxpayers, and the government.”
The budget proposal reflects President Trump’s desire to see the Affordable Care Act repealed and is built on that premise, but questions linger as to whether Congress will take up health care legislation again this year.
The opioid abuse epidemic took center stage during a hearing of the House Ways and Means Committee, during which Alex Azar, secretary of the Department of Health & Human Services, defended the White House budget proposal for fiscal year 2019.
The president’s budget, released Feb. 13, allocates $5 billion in new resources over the next 5 years to combat the opioid abuse epidemic
During his Feb. 14 testimony, Mr. Azar said that the budget proposal “brings a new level of commitment to fighting the crisis of opioid addiction and overdose that is stealing more than 100 American lives from us every single day.”
He noted that part of the funding request includes spending $500 million to launch public-private partnership with the National Institutes of Health “to development new addiction treatments, new overdose-reversing drugs, and nonaddictive approaches to pain.”
Mr. Azar called for greater use of nonopioid pain management techniques and better communication between the HHS and the Drug Enforcement Agency to identify doctors who overprescribe opioids.
“I really want to focus on that entry point to working with DEA on ways that we can control pill mills and even just bad practice that has become part of our culture of medicine of giving people excessive numbers of pills when they do not need them,” Mr. Azar said.
The administration’s proposals for addressing the opioid crisis revealed partisan divisions among members of the Ways and Means Committee, as Republican members of the committee hailed the White House proposals while Democrats argued that the budget proposal could make it more difficult for people to fight the crisis.
Noting that many who have opioid use disorders rely on Medicaid for health insurance, Rep. Richard Neal (D-Mass.), the ranking member on the committee, noted that the proposed budget cuts $1.4 trillion from Medicaid and $500 billion from Medicare.
Physician groups were quick to point out that the budget proposal calls for addressing health care issues while simultaneously cutting the necessary funding.
The Infectious Diseases Society of America said in a statement that “while we appreciate continued funding for antimicrobial resistance research and development through the Biomedical Advanced Research and Development Authority, the current investment is insufficient, as evidenced by an antibiotic pipeline that falls far short of projected needs, while pharmaceutical company investments in antibiotic research continue to diminish. Also, the plan would limit CDC [Centers for Disease Control and Prevention] efforts to address and prevent growing resistance to existing antimicrobial drugs with a nearly $25 million cut.”
The White House budget proposal also tackles drug pricing and payment, including a new Medicaid demonstration program that would allow up to five participating states to test the use of drug formularies in the Medicaid program, speed the access to generic medications, and modify the Medicare Part D prescription drug program. Mr. Azar also reiterated his openness to government price negotiation for drugs administered in the physician office under Medicare Part B.
The proposal on drug discounts was praised by the Community Oncology Alliance (COA).
“The White House budget proposal for sharing manufacturer rebates and discounts with seniors in Medicare Part D is also a great idea,” COA said in a statement. “Pharmacy benefit managers have been enriching themselves with these rebates for too long, and their growing scale has resulted in higher drug costs for everyone. COA believes that the proposed Part D change to share rebates and discounts proposed will lower costs for patients, taxpayers, and the government.”
The budget proposal reflects President Trump’s desire to see the Affordable Care Act repealed and is built on that premise, but questions linger as to whether Congress will take up health care legislation again this year.
The opioid abuse epidemic took center stage during a hearing of the House Ways and Means Committee, during which Alex Azar, secretary of the Department of Health & Human Services, defended the White House budget proposal for fiscal year 2019.
The president’s budget, released Feb. 13, allocates $5 billion in new resources over the next 5 years to combat the opioid abuse epidemic
During his Feb. 14 testimony, Mr. Azar said that the budget proposal “brings a new level of commitment to fighting the crisis of opioid addiction and overdose that is stealing more than 100 American lives from us every single day.”
He noted that part of the funding request includes spending $500 million to launch public-private partnership with the National Institutes of Health “to development new addiction treatments, new overdose-reversing drugs, and nonaddictive approaches to pain.”
Mr. Azar called for greater use of nonopioid pain management techniques and better communication between the HHS and the Drug Enforcement Agency to identify doctors who overprescribe opioids.
“I really want to focus on that entry point to working with DEA on ways that we can control pill mills and even just bad practice that has become part of our culture of medicine of giving people excessive numbers of pills when they do not need them,” Mr. Azar said.
The administration’s proposals for addressing the opioid crisis revealed partisan divisions among members of the Ways and Means Committee, as Republican members of the committee hailed the White House proposals while Democrats argued that the budget proposal could make it more difficult for people to fight the crisis.
Noting that many who have opioid use disorders rely on Medicaid for health insurance, Rep. Richard Neal (D-Mass.), the ranking member on the committee, noted that the proposed budget cuts $1.4 trillion from Medicaid and $500 billion from Medicare.
Physician groups were quick to point out that the budget proposal calls for addressing health care issues while simultaneously cutting the necessary funding.
The Infectious Diseases Society of America said in a statement that “while we appreciate continued funding for antimicrobial resistance research and development through the Biomedical Advanced Research and Development Authority, the current investment is insufficient, as evidenced by an antibiotic pipeline that falls far short of projected needs, while pharmaceutical company investments in antibiotic research continue to diminish. Also, the plan would limit CDC [Centers for Disease Control and Prevention] efforts to address and prevent growing resistance to existing antimicrobial drugs with a nearly $25 million cut.”
The White House budget proposal also tackles drug pricing and payment, including a new Medicaid demonstration program that would allow up to five participating states to test the use of drug formularies in the Medicaid program, speed the access to generic medications, and modify the Medicare Part D prescription drug program. Mr. Azar also reiterated his openness to government price negotiation for drugs administered in the physician office under Medicare Part B.
The proposal on drug discounts was praised by the Community Oncology Alliance (COA).
“The White House budget proposal for sharing manufacturer rebates and discounts with seniors in Medicare Part D is also a great idea,” COA said in a statement. “Pharmacy benefit managers have been enriching themselves with these rebates for too long, and their growing scale has resulted in higher drug costs for everyone. COA believes that the proposed Part D change to share rebates and discounts proposed will lower costs for patients, taxpayers, and the government.”
The budget proposal reflects President Trump’s desire to see the Affordable Care Act repealed and is built on that premise, but questions linger as to whether Congress will take up health care legislation again this year.
REPORTING FROM a HOUSE WAYS AND MEANS HEARING
FDA approves apalutamide for castration-resistant nonmetastatic prostate cancer
The Food and Drug Administration has approved apalutamide for the treatment of patients with castration-resistant nonmetastatic prostate cancer.
Approval was based on a median metastasis-free survival for patients taking apalutamide of 40.5 months, compared with 16.2 months for patients taking a placebo in a randomized clinical trial of 1,207 patients with nonmetastatic, castration-resistant prostate cancer. All patients also received hormone therapy, either with gonadotropin-releasing hormone analogue therapy or with surgical castration.
Common side effects of apalutamide include fatigue, hypertension, rash, diarrhea, nausea, weight loss, arthralgia, falls, hot flush, decreased appetite, fractures, and peripheral edema.
Severe side effects of apalutamide include falls, fractures. and seizures, the FDA said.
Apalutamide is marketed as Erleada by Janssen Biotech.
The Food and Drug Administration has approved apalutamide for the treatment of patients with castration-resistant nonmetastatic prostate cancer.
Approval was based on a median metastasis-free survival for patients taking apalutamide of 40.5 months, compared with 16.2 months for patients taking a placebo in a randomized clinical trial of 1,207 patients with nonmetastatic, castration-resistant prostate cancer. All patients also received hormone therapy, either with gonadotropin-releasing hormone analogue therapy or with surgical castration.
Common side effects of apalutamide include fatigue, hypertension, rash, diarrhea, nausea, weight loss, arthralgia, falls, hot flush, decreased appetite, fractures, and peripheral edema.
Severe side effects of apalutamide include falls, fractures. and seizures, the FDA said.
Apalutamide is marketed as Erleada by Janssen Biotech.
The Food and Drug Administration has approved apalutamide for the treatment of patients with castration-resistant nonmetastatic prostate cancer.
Approval was based on a median metastasis-free survival for patients taking apalutamide of 40.5 months, compared with 16.2 months for patients taking a placebo in a randomized clinical trial of 1,207 patients with nonmetastatic, castration-resistant prostate cancer. All patients also received hormone therapy, either with gonadotropin-releasing hormone analogue therapy or with surgical castration.
Common side effects of apalutamide include fatigue, hypertension, rash, diarrhea, nausea, weight loss, arthralgia, falls, hot flush, decreased appetite, fractures, and peripheral edema.
Severe side effects of apalutamide include falls, fractures. and seizures, the FDA said.
Apalutamide is marketed as Erleada by Janssen Biotech.
Poor health literacy raises readmission risk
JACKSONVILLE, FLA. – Low health literacy is a common problem in Veterans Affairs health systems, and patients with low health literacy scores are 50% more likely to return to the hospital within 30 days of discharge after surgery than patients with high health literacy, investigators found in a study of surgery patients at VA medical centers.
“Low health literacy is prevalent among VA surgery patients and is associated with surgical readmissions,” said Samantha Baker, MD, of the University of Alabama at Birmingham and the VA Birmingham Healthcare System. She presented the findings at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
This study used an instrument developed by Lisa Chew, MD, at the University of Washington to determine health literacy scores (Fam Med. 2004;36:588-94). The instrument uses three questions: how often patients have someone else help them read hospital materials; whether they have problems learning about a medical condition because they have difficulty understanding written information; and how confident they are in filling out their own medical forms. Answers are given on a scale of 0-4, with 12 points being the highest score for poor health literacy. This study considered adequate health literacy to be a score of 0-3, and 4-12 as “possibly inadequate health literacy.”
“Of the 722 patients who took the survey, 39.2% had a score of 0; 33.2% had a score of 4 or more,” Dr. Baker said.
The adequate health literacy group had significantly lower rates of unplanned readmissions and a trend toward lower emergency department visits than the possibly inadequate health literacy group, 11.7% vs. 22.5% (P = .003) for the former and 18.7% vs. 24.2% (P = .08) for the latter, Dr. Baker said.
She noted that the ethnic makeup of the groups was similar and the differences in health literacy among the ethnic groups were not statistically significant.
She also mentioned that those with adequate health literacy tended to be younger – 64 vs. 66.9 years – and more likely to be women (“but our number is low for females in the VA,” Dr. Baker said). She added that married patients tended to have lower heath literacy than did single patients.”
The 30-day surgical readmission rate was 13.7% for patients with high health literacy and 22.5% for those with low health literacy. Patients with inadequate health literacy were 53% more likely to be readmitted to the hospital within 30 days of their index operation, she said. Each one-unit increase in health literacy scores – meaning an increase in inadequate health literacy – increased an individual’s risk of readmission by about 6% on an adjusted basis, Dr. Baker said.
“Future work is going to be focused on identifying these patients and developing the interventions to educate and empower this vulnerable population before they are discharged,” Dr. Baker said.
Dr. Baker and coauthors reported having no financial disclosures.
SOURCE: Baker S et al. Academic Surgical Congress.
JACKSONVILLE, FLA. – Low health literacy is a common problem in Veterans Affairs health systems, and patients with low health literacy scores are 50% more likely to return to the hospital within 30 days of discharge after surgery than patients with high health literacy, investigators found in a study of surgery patients at VA medical centers.
“Low health literacy is prevalent among VA surgery patients and is associated with surgical readmissions,” said Samantha Baker, MD, of the University of Alabama at Birmingham and the VA Birmingham Healthcare System. She presented the findings at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
This study used an instrument developed by Lisa Chew, MD, at the University of Washington to determine health literacy scores (Fam Med. 2004;36:588-94). The instrument uses three questions: how often patients have someone else help them read hospital materials; whether they have problems learning about a medical condition because they have difficulty understanding written information; and how confident they are in filling out their own medical forms. Answers are given on a scale of 0-4, with 12 points being the highest score for poor health literacy. This study considered adequate health literacy to be a score of 0-3, and 4-12 as “possibly inadequate health literacy.”
“Of the 722 patients who took the survey, 39.2% had a score of 0; 33.2% had a score of 4 or more,” Dr. Baker said.
The adequate health literacy group had significantly lower rates of unplanned readmissions and a trend toward lower emergency department visits than the possibly inadequate health literacy group, 11.7% vs. 22.5% (P = .003) for the former and 18.7% vs. 24.2% (P = .08) for the latter, Dr. Baker said.
She noted that the ethnic makeup of the groups was similar and the differences in health literacy among the ethnic groups were not statistically significant.
She also mentioned that those with adequate health literacy tended to be younger – 64 vs. 66.9 years – and more likely to be women (“but our number is low for females in the VA,” Dr. Baker said). She added that married patients tended to have lower heath literacy than did single patients.”
The 30-day surgical readmission rate was 13.7% for patients with high health literacy and 22.5% for those with low health literacy. Patients with inadequate health literacy were 53% more likely to be readmitted to the hospital within 30 days of their index operation, she said. Each one-unit increase in health literacy scores – meaning an increase in inadequate health literacy – increased an individual’s risk of readmission by about 6% on an adjusted basis, Dr. Baker said.
“Future work is going to be focused on identifying these patients and developing the interventions to educate and empower this vulnerable population before they are discharged,” Dr. Baker said.
Dr. Baker and coauthors reported having no financial disclosures.
SOURCE: Baker S et al. Academic Surgical Congress.
JACKSONVILLE, FLA. – Low health literacy is a common problem in Veterans Affairs health systems, and patients with low health literacy scores are 50% more likely to return to the hospital within 30 days of discharge after surgery than patients with high health literacy, investigators found in a study of surgery patients at VA medical centers.
“Low health literacy is prevalent among VA surgery patients and is associated with surgical readmissions,” said Samantha Baker, MD, of the University of Alabama at Birmingham and the VA Birmingham Healthcare System. She presented the findings at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
This study used an instrument developed by Lisa Chew, MD, at the University of Washington to determine health literacy scores (Fam Med. 2004;36:588-94). The instrument uses three questions: how often patients have someone else help them read hospital materials; whether they have problems learning about a medical condition because they have difficulty understanding written information; and how confident they are in filling out their own medical forms. Answers are given on a scale of 0-4, with 12 points being the highest score for poor health literacy. This study considered adequate health literacy to be a score of 0-3, and 4-12 as “possibly inadequate health literacy.”
“Of the 722 patients who took the survey, 39.2% had a score of 0; 33.2% had a score of 4 or more,” Dr. Baker said.
The adequate health literacy group had significantly lower rates of unplanned readmissions and a trend toward lower emergency department visits than the possibly inadequate health literacy group, 11.7% vs. 22.5% (P = .003) for the former and 18.7% vs. 24.2% (P = .08) for the latter, Dr. Baker said.
She noted that the ethnic makeup of the groups was similar and the differences in health literacy among the ethnic groups were not statistically significant.
She also mentioned that those with adequate health literacy tended to be younger – 64 vs. 66.9 years – and more likely to be women (“but our number is low for females in the VA,” Dr. Baker said). She added that married patients tended to have lower heath literacy than did single patients.”
The 30-day surgical readmission rate was 13.7% for patients with high health literacy and 22.5% for those with low health literacy. Patients with inadequate health literacy were 53% more likely to be readmitted to the hospital within 30 days of their index operation, she said. Each one-unit increase in health literacy scores – meaning an increase in inadequate health literacy – increased an individual’s risk of readmission by about 6% on an adjusted basis, Dr. Baker said.
“Future work is going to be focused on identifying these patients and developing the interventions to educate and empower this vulnerable population before they are discharged,” Dr. Baker said.
Dr. Baker and coauthors reported having no financial disclosures.
SOURCE: Baker S et al. Academic Surgical Congress.
REPORTING FROM THE ACADEMIC SURGICAL CONGRESS
Key clinical point:
Major finding: The 30-day surgical readmission rate was 13.7% for patients with high health literacy and 22.5% for those with low health literacy.
Data source: Analysis of 722 patients who had general, vascular, or thoracic surgery at four VA Medical Centers from August 2015 to June 2017.
Disclosures: Dr. Baker and coauthors reported having no financial disclosures.
Source: Baker S et al. Academic Surgical Congress.
Laparoscopic procedure safer for SBO in elderly patients
JACKSONVILLE, FLA. – Octogenarians with small-bowel obstruction are about seven times more likely to have open than laparoscopic surgery, but the minimally invasive approach in these patients has been found to reduce their hospital stays and risk of pneumonia afterward, according to results of an observational study of data from the American College of Surgeons National Surgical Quality Improvement Program database.
Dr. Chang said, “Our study was able to show that age and the presence of preoperative sepsis are associated with mortality rather than procedure type, and that there are procedure-type risks associated with open procedures.”
The observational study analyzed 103 laparoscopic and 692 open operations for small-bowel obstruction (SBO) in patients 80 and older from 2006 to 2014. Characteristics of the open and laparoscopic group – age, gender, body mass index, and race – were similar, although the open group had higher American Society of Anesthesiologists classification and incidence of preoperative sepsis, Dr. Chang said.
“Unadjusted outcomes showed longer length of stay [and] higher postoperative mortality and rates of postoperative pneumonia in the open cases vs. laparoscopic,” she said. “But after we made adjustments for preoperative risk variables, age and the presence of preoperative sepsis were associated with mortality, not the operative approach.” Length of stay was 4 days for the laparoscopic patients vs. 8 days for open (P less than .0001).
The researchers performed logistical regression analysis and found that mortality risk rose slightly with age (odds ratio, 1.11; P = .0311) but almost quadrupled with preoperative sepsis (OR, 3.77; P = .0287) regardless of open or laparoscopic approach. For postoperative pneumonia, risk factors were male gender (OR, 2.68; P = .0003) and open procedure (OR, 5.03; P = .0282).
“Our study elucidates that the octogenarian with small-bowel obstruction due to adhesive disease may benefit from an initial laparoscopic approach,” Dr. Change said. “Further prospective studies are warranted.”
Dr. Chang and coauthors reported having no financial disclosures.
SOURCE: Chang E et al. Academic Surgical Congress.
JACKSONVILLE, FLA. – Octogenarians with small-bowel obstruction are about seven times more likely to have open than laparoscopic surgery, but the minimally invasive approach in these patients has been found to reduce their hospital stays and risk of pneumonia afterward, according to results of an observational study of data from the American College of Surgeons National Surgical Quality Improvement Program database.
Dr. Chang said, “Our study was able to show that age and the presence of preoperative sepsis are associated with mortality rather than procedure type, and that there are procedure-type risks associated with open procedures.”
The observational study analyzed 103 laparoscopic and 692 open operations for small-bowel obstruction (SBO) in patients 80 and older from 2006 to 2014. Characteristics of the open and laparoscopic group – age, gender, body mass index, and race – were similar, although the open group had higher American Society of Anesthesiologists classification and incidence of preoperative sepsis, Dr. Chang said.
“Unadjusted outcomes showed longer length of stay [and] higher postoperative mortality and rates of postoperative pneumonia in the open cases vs. laparoscopic,” she said. “But after we made adjustments for preoperative risk variables, age and the presence of preoperative sepsis were associated with mortality, not the operative approach.” Length of stay was 4 days for the laparoscopic patients vs. 8 days for open (P less than .0001).
The researchers performed logistical regression analysis and found that mortality risk rose slightly with age (odds ratio, 1.11; P = .0311) but almost quadrupled with preoperative sepsis (OR, 3.77; P = .0287) regardless of open or laparoscopic approach. For postoperative pneumonia, risk factors were male gender (OR, 2.68; P = .0003) and open procedure (OR, 5.03; P = .0282).
“Our study elucidates that the octogenarian with small-bowel obstruction due to adhesive disease may benefit from an initial laparoscopic approach,” Dr. Change said. “Further prospective studies are warranted.”
Dr. Chang and coauthors reported having no financial disclosures.
SOURCE: Chang E et al. Academic Surgical Congress.
JACKSONVILLE, FLA. – Octogenarians with small-bowel obstruction are about seven times more likely to have open than laparoscopic surgery, but the minimally invasive approach in these patients has been found to reduce their hospital stays and risk of pneumonia afterward, according to results of an observational study of data from the American College of Surgeons National Surgical Quality Improvement Program database.
Dr. Chang said, “Our study was able to show that age and the presence of preoperative sepsis are associated with mortality rather than procedure type, and that there are procedure-type risks associated with open procedures.”
The observational study analyzed 103 laparoscopic and 692 open operations for small-bowel obstruction (SBO) in patients 80 and older from 2006 to 2014. Characteristics of the open and laparoscopic group – age, gender, body mass index, and race – were similar, although the open group had higher American Society of Anesthesiologists classification and incidence of preoperative sepsis, Dr. Chang said.
“Unadjusted outcomes showed longer length of stay [and] higher postoperative mortality and rates of postoperative pneumonia in the open cases vs. laparoscopic,” she said. “But after we made adjustments for preoperative risk variables, age and the presence of preoperative sepsis were associated with mortality, not the operative approach.” Length of stay was 4 days for the laparoscopic patients vs. 8 days for open (P less than .0001).
The researchers performed logistical regression analysis and found that mortality risk rose slightly with age (odds ratio, 1.11; P = .0311) but almost quadrupled with preoperative sepsis (OR, 3.77; P = .0287) regardless of open or laparoscopic approach. For postoperative pneumonia, risk factors were male gender (OR, 2.68; P = .0003) and open procedure (OR, 5.03; P = .0282).
“Our study elucidates that the octogenarian with small-bowel obstruction due to adhesive disease may benefit from an initial laparoscopic approach,” Dr. Change said. “Further prospective studies are warranted.”
Dr. Chang and coauthors reported having no financial disclosures.
SOURCE: Chang E et al. Academic Surgical Congress.
REPORTING FROM THE ACADEMIC SURGICAL CONGRESS
Key clinical point: and older.
Major finding: The open procedure had an odds ratio five times greater than laparoscopic surgery for risk of pneumonia after the operation in this age group (OR, 5.03; P =.0282).
Data source: Observational study of 103 laparoscopic and 692 open cases of surgery for SBO in the ACS NSQIP database from 2006 to 2014.
Disclosures: Dr. Chang and coauthors reported having no financial disclosures.
Source: Chang E et al. Academic Surgical Congress.
Major depression identified in almost 21% of U.S. adults
Major depressive disorder (MDD) was identified in 21% of adults in the United States during their lifetimes and 10% over 12 months, according to data published Feb. 14 from the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions III (NESARC-III).
Research shows that the prevalence of depression in adolescents and adults in the United States has increased over the last 25 years. However, epidemiologic data on MDD prevalence since the 2013 publication of the DSM-5 have been limited, wrote Deborah S. Hasin, PhD, of Columbia University, New York, and her colleagues.
In a study published in JAMA Psychiatry, Dr. Hasin and her colleagues reviewed data from 36,309 adult participants in the NESARC-III who reflected DSM-5 criteria. Major depressive disorder was defined as at least 2 weeks of persistent depressed mood, anhedonia, or hopelessness reported by the individual or others observing the individual.
Overall, the 12-month and lifetime prevalences of MDD were 10.4% and 20.6%, respectively. Factors associated with a more likely 12-month diagnosis of MDD included younger age (18-29 years) and lower income (less than $19,999 per year). In addition, MDD was significantly less likely in men than it was in women (odds ratio, 0.5). Compared with the likelihood among white adults, MDD was less likely among adults who were African American (OR, 0.6), Asian/Pacific Islander (OR, 0.6), and Hispanic (OR, 0.7).
Generalized anxiety disorder was the most common comorbidity associated with MDD (adjusted OR, 5.7). Any drug disorder was three times more likely in MDD patients (aOR, 3.0), and alcohol use disorder was nearly twice as likely (aOR, 1.8). Approximately 70% of patients with lifetime MDD received some treatment. But patients with substance use disorders and depression are less likely to receive treatment for major depression disorder. “Therefore, clinician education and training in dual-disorder screening and treatment should be prioritized,” Dr. Hasin and her colleagues wrote.
The study is the first to include data on two new major depression specifiers from the DSM-5, the researchers noted. “That almost three-quarters of those with MDD had the anxious/distressed specifier confirms clinical observation and research,” they said. “In patient samples, the anxious/distressed specifier predicts a poor course of MDD.”
The study was limited by several factors, including its cross-sectional design and the potentially inconsistent differentiation of MDD from normal bereavement in patients who had been diagnosed with MDD shortly after the death of a loved one, the researchers said. However, the findings provide the first nationally representative information on MDD since the advent of the DSM-5 and highlight the high prevalence of MDD in the U.S. population and the need for further intervention, they said.
The researchers had no financial conflicts to disclose. The NESARC-III was supported by several entities, including the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the New York State Psychiatric Institute.
SOURCE: Hasin D et al. JAMA Psychiatry. 2018 Feb 14. doi: 10.1001/jamapsychiatry.2017.4602.
Major depressive disorder (MDD) was identified in 21% of adults in the United States during their lifetimes and 10% over 12 months, according to data published Feb. 14 from the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions III (NESARC-III).
Research shows that the prevalence of depression in adolescents and adults in the United States has increased over the last 25 years. However, epidemiologic data on MDD prevalence since the 2013 publication of the DSM-5 have been limited, wrote Deborah S. Hasin, PhD, of Columbia University, New York, and her colleagues.
In a study published in JAMA Psychiatry, Dr. Hasin and her colleagues reviewed data from 36,309 adult participants in the NESARC-III who reflected DSM-5 criteria. Major depressive disorder was defined as at least 2 weeks of persistent depressed mood, anhedonia, or hopelessness reported by the individual or others observing the individual.
Overall, the 12-month and lifetime prevalences of MDD were 10.4% and 20.6%, respectively. Factors associated with a more likely 12-month diagnosis of MDD included younger age (18-29 years) and lower income (less than $19,999 per year). In addition, MDD was significantly less likely in men than it was in women (odds ratio, 0.5). Compared with the likelihood among white adults, MDD was less likely among adults who were African American (OR, 0.6), Asian/Pacific Islander (OR, 0.6), and Hispanic (OR, 0.7).
Generalized anxiety disorder was the most common comorbidity associated with MDD (adjusted OR, 5.7). Any drug disorder was three times more likely in MDD patients (aOR, 3.0), and alcohol use disorder was nearly twice as likely (aOR, 1.8). Approximately 70% of patients with lifetime MDD received some treatment. But patients with substance use disorders and depression are less likely to receive treatment for major depression disorder. “Therefore, clinician education and training in dual-disorder screening and treatment should be prioritized,” Dr. Hasin and her colleagues wrote.
The study is the first to include data on two new major depression specifiers from the DSM-5, the researchers noted. “That almost three-quarters of those with MDD had the anxious/distressed specifier confirms clinical observation and research,” they said. “In patient samples, the anxious/distressed specifier predicts a poor course of MDD.”
The study was limited by several factors, including its cross-sectional design and the potentially inconsistent differentiation of MDD from normal bereavement in patients who had been diagnosed with MDD shortly after the death of a loved one, the researchers said. However, the findings provide the first nationally representative information on MDD since the advent of the DSM-5 and highlight the high prevalence of MDD in the U.S. population and the need for further intervention, they said.
The researchers had no financial conflicts to disclose. The NESARC-III was supported by several entities, including the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the New York State Psychiatric Institute.
SOURCE: Hasin D et al. JAMA Psychiatry. 2018 Feb 14. doi: 10.1001/jamapsychiatry.2017.4602.
Major depressive disorder (MDD) was identified in 21% of adults in the United States during their lifetimes and 10% over 12 months, according to data published Feb. 14 from the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions III (NESARC-III).
Research shows that the prevalence of depression in adolescents and adults in the United States has increased over the last 25 years. However, epidemiologic data on MDD prevalence since the 2013 publication of the DSM-5 have been limited, wrote Deborah S. Hasin, PhD, of Columbia University, New York, and her colleagues.
In a study published in JAMA Psychiatry, Dr. Hasin and her colleagues reviewed data from 36,309 adult participants in the NESARC-III who reflected DSM-5 criteria. Major depressive disorder was defined as at least 2 weeks of persistent depressed mood, anhedonia, or hopelessness reported by the individual or others observing the individual.
Overall, the 12-month and lifetime prevalences of MDD were 10.4% and 20.6%, respectively. Factors associated with a more likely 12-month diagnosis of MDD included younger age (18-29 years) and lower income (less than $19,999 per year). In addition, MDD was significantly less likely in men than it was in women (odds ratio, 0.5). Compared with the likelihood among white adults, MDD was less likely among adults who were African American (OR, 0.6), Asian/Pacific Islander (OR, 0.6), and Hispanic (OR, 0.7).
Generalized anxiety disorder was the most common comorbidity associated with MDD (adjusted OR, 5.7). Any drug disorder was three times more likely in MDD patients (aOR, 3.0), and alcohol use disorder was nearly twice as likely (aOR, 1.8). Approximately 70% of patients with lifetime MDD received some treatment. But patients with substance use disorders and depression are less likely to receive treatment for major depression disorder. “Therefore, clinician education and training in dual-disorder screening and treatment should be prioritized,” Dr. Hasin and her colleagues wrote.
The study is the first to include data on two new major depression specifiers from the DSM-5, the researchers noted. “That almost three-quarters of those with MDD had the anxious/distressed specifier confirms clinical observation and research,” they said. “In patient samples, the anxious/distressed specifier predicts a poor course of MDD.”
The study was limited by several factors, including its cross-sectional design and the potentially inconsistent differentiation of MDD from normal bereavement in patients who had been diagnosed with MDD shortly after the death of a loved one, the researchers said. However, the findings provide the first nationally representative information on MDD since the advent of the DSM-5 and highlight the high prevalence of MDD in the U.S. population and the need for further intervention, they said.
The researchers had no financial conflicts to disclose. The NESARC-III was supported by several entities, including the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the New York State Psychiatric Institute.
SOURCE: Hasin D et al. JAMA Psychiatry. 2018 Feb 14. doi: 10.1001/jamapsychiatry.2017.4602.
FROM JAMA PSYCHIATRY
Key clinical point: Clinicians should prioritize education and training in treating patients with comorbid MDD and substance use disorders.
Major finding: Among adults in the United States, the 12-month and lifetime prevalences of MDD were 10.4% and 20.6%, respectively.
Data source: The data come from the National Epidemiologic Survey on Alcohol and Related Conditions III (NESARC-III) for 2012-2013 and includes 36,309 adults.
Disclosures: The researchers had no financial conflicts to disclose. The National Epidemiologic Survey on Alcohol and Related Conditions III (NESARC-III) was supported by several entities, including the National Institute on Alcohol Abuse and Alcoholism, the National Institute on Drug Abuse, and the New York State Psychiatric Institute.
Source: Hasin D et al. JAMA Psychiatry. doi: 10.1001/jamapsychiatry.2017.4602.
‘Real-world’ study finds treat-to-target benefits out to 5 years
A treat-to-target (T2T) strategy in daily clinical practice for patients with early rheumatoid arthritis proved successful in maintaining good disease- and patient-related outcomes over a 5-year period at two rheumatology clinics in the Netherlands.
The observational study builds on previous research on the long-term results of continuous application of T2T strategies in rheumatoid arthritis, for which there have been few published studies. “Long-term data from more recent randomized controlled clinical trials, using a T2T approach and biologicals, have shown good clinical outcomes. However, the generalizability of these results is hampered by the selection of specific patient groups in clinical trials and strict exclusion criteria. Patients seen in real-life practice may differ substantially from those in randomized clinical trials,” first author Letty G.A. Versteeg of Medisch Spectrum Twente, Enschede, the Netherlands, and her colleagues wrote in Clinical Rheumatology.
The investigators examined outcomes for 229 patients with very early RA who enrolled in the Dutch Rheumatoid Arthritis Monitoring (DREAM) remission induction cohort during 2006-2009, which included 5 years of follow-up for 171 of the patients. These patients underwent a protocoled T2T strategy aimed at remission, defined as a 28-joint Disease Activity Score (DAS28) of less than 2.6.
“In previous publications on the [DREAM] remission induction cohort, successful implementation of T2T in daily clinical practice was demonstrated. Achieving remission within the first year of treatment was shown to be a realistic goal for an important proportion of patients,” the authors wrote.
All patients started methotrexate monotherapy at an initial dosage of 15 mg/week that could be increased to a maximum dosage of 25 mg/week in week 8. Patients took folic acid on the second day after methotrexate. By week 12, those with persistent disease activity added sulfasalazine, starting at 2,000 mg/day and increasing if necessary to a maximum of 3,000 mg/day at week 20. Patients whose DAS28 remained at 3.2 or greater at week 24 received a tumor necrosis factor inhibitor. Those who reached remission had no change in medication, and when remission lasted for at least 6 months, medication was gradually tapered and eventually discontinued. Patients who had flares in which disease activity increased to a DAS28 of 2.6 and higher restarted their last effective medication or dosage, which could subsequently be intensified if necessary. Patients with comorbidities and contraindications for medication were not excluded because deviations from the protocol were allowed. The protocol also allowed concomitant treatment with NSAIDs, prednisolone at a dosage of less than 10 mg/day, and intra-articular corticosteroid injections.
The rate of DAS28-defined remission rose to 63% (126 of 199 patients) by the end of the first year, and only 5% had high disease activity at 24 weeks. The rate of remission remained stable over the next 4 years. This rate of remission was reflected as a drop from an overall mean DAS28 of 4.93 at baseline to 2.49 at 5 years. The majority of the drop in DAS28 occurred during the first 3 months (–1.63 points), and by the end of the first year of treatment, mean disease activity stayed below 2.6 on the DAS28.
The investigators saw a sustained remission at least once in 144 of the 171 patients with 5-year outcome data available, including sustained remission for 1 year or longer in 115. Median time to the first sustained remission proved to be 50 weeks, and half had this last less than 97 weeks and half more than 97 weeks.
During the 5-year follow-up, 17% of patients received treatment with biologics, with a median start of their first biologic at about 54 weeks after baseline. This first biologic was used continuously for a median of 29 weeks, and close to one-third of patients who started a biologic switched to a second biologic after a median duration of 41 weeks on the first. About two-thirds did not need a second biologic. A total of 66% of patients who took a biologic had at least one period of sustained remission.
Functional disability improved overall at 5 years as determined by Health Assessment Questionnaire (HAQ) scores that were available for 107 patients. HAQ scores decreased from a median of 1.125 at baseline to 0.375 after 24 weeks (P less than .001), where they remained stable throughout the rest of follow-up. Overall, nearly 70% of the patients with available 5-year data had a change in their individual HAQ score that was clinically meaningful from baseline to 24 weeks.
“Our study describes long-term outcome of implementation and continuous application of T2T to RA patients in daily clinical practice. The outcomes are similar to or even better than the results of T2T randomized clinical trials, in which strict selection of patients and controlled conditions were followed. These ‘real-life data’ are of important additional value in the evidence for the effectiveness of a T2T approach in RA patients,” the investigators concluded.
They had no disclosures to report.
SOURCE: Versteeg G et al. Clin Rheumatol. 2018 Feb 1. doi: 10.1007/s10067-017-3962-5.
A treat-to-target (T2T) strategy in daily clinical practice for patients with early rheumatoid arthritis proved successful in maintaining good disease- and patient-related outcomes over a 5-year period at two rheumatology clinics in the Netherlands.
The observational study builds on previous research on the long-term results of continuous application of T2T strategies in rheumatoid arthritis, for which there have been few published studies. “Long-term data from more recent randomized controlled clinical trials, using a T2T approach and biologicals, have shown good clinical outcomes. However, the generalizability of these results is hampered by the selection of specific patient groups in clinical trials and strict exclusion criteria. Patients seen in real-life practice may differ substantially from those in randomized clinical trials,” first author Letty G.A. Versteeg of Medisch Spectrum Twente, Enschede, the Netherlands, and her colleagues wrote in Clinical Rheumatology.
The investigators examined outcomes for 229 patients with very early RA who enrolled in the Dutch Rheumatoid Arthritis Monitoring (DREAM) remission induction cohort during 2006-2009, which included 5 years of follow-up for 171 of the patients. These patients underwent a protocoled T2T strategy aimed at remission, defined as a 28-joint Disease Activity Score (DAS28) of less than 2.6.
“In previous publications on the [DREAM] remission induction cohort, successful implementation of T2T in daily clinical practice was demonstrated. Achieving remission within the first year of treatment was shown to be a realistic goal for an important proportion of patients,” the authors wrote.
All patients started methotrexate monotherapy at an initial dosage of 15 mg/week that could be increased to a maximum dosage of 25 mg/week in week 8. Patients took folic acid on the second day after methotrexate. By week 12, those with persistent disease activity added sulfasalazine, starting at 2,000 mg/day and increasing if necessary to a maximum of 3,000 mg/day at week 20. Patients whose DAS28 remained at 3.2 or greater at week 24 received a tumor necrosis factor inhibitor. Those who reached remission had no change in medication, and when remission lasted for at least 6 months, medication was gradually tapered and eventually discontinued. Patients who had flares in which disease activity increased to a DAS28 of 2.6 and higher restarted their last effective medication or dosage, which could subsequently be intensified if necessary. Patients with comorbidities and contraindications for medication were not excluded because deviations from the protocol were allowed. The protocol also allowed concomitant treatment with NSAIDs, prednisolone at a dosage of less than 10 mg/day, and intra-articular corticosteroid injections.
The rate of DAS28-defined remission rose to 63% (126 of 199 patients) by the end of the first year, and only 5% had high disease activity at 24 weeks. The rate of remission remained stable over the next 4 years. This rate of remission was reflected as a drop from an overall mean DAS28 of 4.93 at baseline to 2.49 at 5 years. The majority of the drop in DAS28 occurred during the first 3 months (–1.63 points), and by the end of the first year of treatment, mean disease activity stayed below 2.6 on the DAS28.
The investigators saw a sustained remission at least once in 144 of the 171 patients with 5-year outcome data available, including sustained remission for 1 year or longer in 115. Median time to the first sustained remission proved to be 50 weeks, and half had this last less than 97 weeks and half more than 97 weeks.
During the 5-year follow-up, 17% of patients received treatment with biologics, with a median start of their first biologic at about 54 weeks after baseline. This first biologic was used continuously for a median of 29 weeks, and close to one-third of patients who started a biologic switched to a second biologic after a median duration of 41 weeks on the first. About two-thirds did not need a second biologic. A total of 66% of patients who took a biologic had at least one period of sustained remission.
Functional disability improved overall at 5 years as determined by Health Assessment Questionnaire (HAQ) scores that were available for 107 patients. HAQ scores decreased from a median of 1.125 at baseline to 0.375 after 24 weeks (P less than .001), where they remained stable throughout the rest of follow-up. Overall, nearly 70% of the patients with available 5-year data had a change in their individual HAQ score that was clinically meaningful from baseline to 24 weeks.
“Our study describes long-term outcome of implementation and continuous application of T2T to RA patients in daily clinical practice. The outcomes are similar to or even better than the results of T2T randomized clinical trials, in which strict selection of patients and controlled conditions were followed. These ‘real-life data’ are of important additional value in the evidence for the effectiveness of a T2T approach in RA patients,” the investigators concluded.
They had no disclosures to report.
SOURCE: Versteeg G et al. Clin Rheumatol. 2018 Feb 1. doi: 10.1007/s10067-017-3962-5.
A treat-to-target (T2T) strategy in daily clinical practice for patients with early rheumatoid arthritis proved successful in maintaining good disease- and patient-related outcomes over a 5-year period at two rheumatology clinics in the Netherlands.
The observational study builds on previous research on the long-term results of continuous application of T2T strategies in rheumatoid arthritis, for which there have been few published studies. “Long-term data from more recent randomized controlled clinical trials, using a T2T approach and biologicals, have shown good clinical outcomes. However, the generalizability of these results is hampered by the selection of specific patient groups in clinical trials and strict exclusion criteria. Patients seen in real-life practice may differ substantially from those in randomized clinical trials,” first author Letty G.A. Versteeg of Medisch Spectrum Twente, Enschede, the Netherlands, and her colleagues wrote in Clinical Rheumatology.
The investigators examined outcomes for 229 patients with very early RA who enrolled in the Dutch Rheumatoid Arthritis Monitoring (DREAM) remission induction cohort during 2006-2009, which included 5 years of follow-up for 171 of the patients. These patients underwent a protocoled T2T strategy aimed at remission, defined as a 28-joint Disease Activity Score (DAS28) of less than 2.6.
“In previous publications on the [DREAM] remission induction cohort, successful implementation of T2T in daily clinical practice was demonstrated. Achieving remission within the first year of treatment was shown to be a realistic goal for an important proportion of patients,” the authors wrote.
All patients started methotrexate monotherapy at an initial dosage of 15 mg/week that could be increased to a maximum dosage of 25 mg/week in week 8. Patients took folic acid on the second day after methotrexate. By week 12, those with persistent disease activity added sulfasalazine, starting at 2,000 mg/day and increasing if necessary to a maximum of 3,000 mg/day at week 20. Patients whose DAS28 remained at 3.2 or greater at week 24 received a tumor necrosis factor inhibitor. Those who reached remission had no change in medication, and when remission lasted for at least 6 months, medication was gradually tapered and eventually discontinued. Patients who had flares in which disease activity increased to a DAS28 of 2.6 and higher restarted their last effective medication or dosage, which could subsequently be intensified if necessary. Patients with comorbidities and contraindications for medication were not excluded because deviations from the protocol were allowed. The protocol also allowed concomitant treatment with NSAIDs, prednisolone at a dosage of less than 10 mg/day, and intra-articular corticosteroid injections.
The rate of DAS28-defined remission rose to 63% (126 of 199 patients) by the end of the first year, and only 5% had high disease activity at 24 weeks. The rate of remission remained stable over the next 4 years. This rate of remission was reflected as a drop from an overall mean DAS28 of 4.93 at baseline to 2.49 at 5 years. The majority of the drop in DAS28 occurred during the first 3 months (–1.63 points), and by the end of the first year of treatment, mean disease activity stayed below 2.6 on the DAS28.
The investigators saw a sustained remission at least once in 144 of the 171 patients with 5-year outcome data available, including sustained remission for 1 year or longer in 115. Median time to the first sustained remission proved to be 50 weeks, and half had this last less than 97 weeks and half more than 97 weeks.
During the 5-year follow-up, 17% of patients received treatment with biologics, with a median start of their first biologic at about 54 weeks after baseline. This first biologic was used continuously for a median of 29 weeks, and close to one-third of patients who started a biologic switched to a second biologic after a median duration of 41 weeks on the first. About two-thirds did not need a second biologic. A total of 66% of patients who took a biologic had at least one period of sustained remission.
Functional disability improved overall at 5 years as determined by Health Assessment Questionnaire (HAQ) scores that were available for 107 patients. HAQ scores decreased from a median of 1.125 at baseline to 0.375 after 24 weeks (P less than .001), where they remained stable throughout the rest of follow-up. Overall, nearly 70% of the patients with available 5-year data had a change in their individual HAQ score that was clinically meaningful from baseline to 24 weeks.
“Our study describes long-term outcome of implementation and continuous application of T2T to RA patients in daily clinical practice. The outcomes are similar to or even better than the results of T2T randomized clinical trials, in which strict selection of patients and controlled conditions were followed. These ‘real-life data’ are of important additional value in the evidence for the effectiveness of a T2T approach in RA patients,” the investigators concluded.
They had no disclosures to report.
SOURCE: Versteeg G et al. Clin Rheumatol. 2018 Feb 1. doi: 10.1007/s10067-017-3962-5.
FROM CLINICAL RHEUMATOLOGY
Key clinical point:
Major finding: The rate of DAS28 remission rose to 63% by the end of the first year and remained stable over the next 4 years.
Study details: An observational cohort study of 171 patients with 5 years of follow-up data.
Disclosures: The investigators had no disclosures to report.
Source: Versteeg G et al. Clin Rheumatol. 2018 Feb 1. doi: 10.1007/s10067-017-3962-5.
Colorectal cancer deaths projected for 2018
Approximately 50,630 deaths from colorectal cancer are predicted for the year in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics.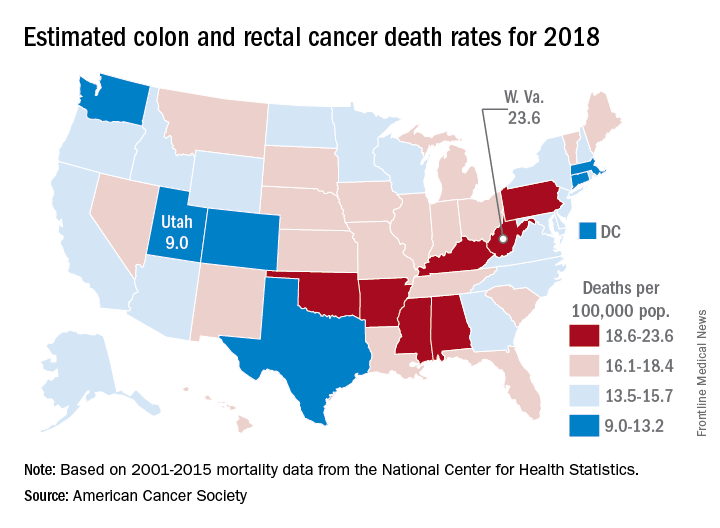
March is Colorectal Cancer Awareness Month. Visit www.gastro.org/CRC for tools you can use to help educate your patients about the importance of colorectal cancer screening.
Approximately 50,630 deaths from colorectal cancer are predicted for the year in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics.
March is Colorectal Cancer Awareness Month. Visit www.gastro.org/CRC for tools you can use to help educate your patients about the importance of colorectal cancer screening.
Approximately 50,630 deaths from colorectal cancer are predicted for the year in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics.
March is Colorectal Cancer Awareness Month. Visit www.gastro.org/CRC for tools you can use to help educate your patients about the importance of colorectal cancer screening.
Man's Condition Gets Out of Hand
This 46-year-old man’s skin disease has gotten so serious that he is essentially disabled. The problem started about six months ago, with joint pain that particularly affected his left ankle. Now, his hands are fissured and swollen to the point that he is unable to button his shirt or hold a fork. He is referred to dermatology by his attorney, who is helping him pursue possible disability benefits, for evaluation and treatment.
He has been seen by a variety of primary care providers, who have collectively prescribed topical triamcinolone 0.1% and several antifungal medications, including a two-month course of oral terbinafine. When those failed, he was treated with prednisone; at the start of the three-week course, there were signs of improvement but by the end, his hands were worse than ever.
EXAMINATION
The dorsal and palmar surfaces of the patient’s hands are covered with thick, white scales atop salmon-colored erythematous bases. Multiple fissures and marked edema can be seen. Seven of 10 fingernails are dystrophic, yellowed, and thickened.
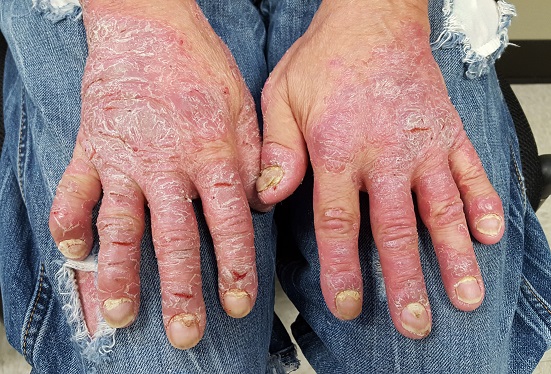
The patient’s elbows, knees, and upper intergluteal area show less impressive involvement.
There is marked tenderness on palpation of the left Achilles insertion, made worse by dorsiflexion.
What is the diagnosis?
DISCUSSION
Psoriasis can affect one or more areas, typically the hands, scalp, genitals, or feet. When it’s focused in one area, as in this case, it can be baffling to diagnose; sometimes it’s hard to see the forest for the trees. But because the condition affects almost 3% of white Americans, you’ll see it regularly—if you’re looking for it.
Nearly 25% of patients with psoriasis eventually develop psoriatic arthritis (PsA), which not only affects the joints but also can cause complications such as enthesitis, or inflammation of the entheses (the sites of insertion of the tendon into bone; eg, the Achilles). This can be confused with plantar fasciitis, which this patient had been previously diagnosed with.
This diagnosis could have been proved or disproved by a KOH prep (which would have shown evidence of fungal disease) or a biopsy (which would have shown fused rete ridges, microabscesses at the papillary tips, hyperkeratosis, and parakeratosis). Providers should first establish a firm diagnosis to dictate effective treatment. In this case, a visual diagnosis was possible.
Given the severity of the problem, the patient was started on a biologic; he showed vast improvement within a week. He was referred to rheumatology for evaluation and management of PsA, and the severity of his disease was communicated to his attorney.
TAKE-HOME LEARNING POINTS
- In some cases, psoriasis can be isolated to the hands, feet, genitals, or scalp, complicating detection of the condition.
- Almost 25% of patients with psoriasis develop psoriatic arthritis (PsA), which can manifest with dactylitis, arthritis, or enthesitis.
- Left untreated, PsA is potentially debilitating.
- Establishing a firm diagnosis with KOH prep or biopsy will dictate effective treatment.
This 46-year-old man’s skin disease has gotten so serious that he is essentially disabled. The problem started about six months ago, with joint pain that particularly affected his left ankle. Now, his hands are fissured and swollen to the point that he is unable to button his shirt or hold a fork. He is referred to dermatology by his attorney, who is helping him pursue possible disability benefits, for evaluation and treatment.
He has been seen by a variety of primary care providers, who have collectively prescribed topical triamcinolone 0.1% and several antifungal medications, including a two-month course of oral terbinafine. When those failed, he was treated with prednisone; at the start of the three-week course, there were signs of improvement but by the end, his hands were worse than ever.
EXAMINATION
The dorsal and palmar surfaces of the patient’s hands are covered with thick, white scales atop salmon-colored erythematous bases. Multiple fissures and marked edema can be seen. Seven of 10 fingernails are dystrophic, yellowed, and thickened.

The patient’s elbows, knees, and upper intergluteal area show less impressive involvement.
There is marked tenderness on palpation of the left Achilles insertion, made worse by dorsiflexion.
What is the diagnosis?
DISCUSSION
Psoriasis can affect one or more areas, typically the hands, scalp, genitals, or feet. When it’s focused in one area, as in this case, it can be baffling to diagnose; sometimes it’s hard to see the forest for the trees. But because the condition affects almost 3% of white Americans, you’ll see it regularly—if you’re looking for it.
Nearly 25% of patients with psoriasis eventually develop psoriatic arthritis (PsA), which not only affects the joints but also can cause complications such as enthesitis, or inflammation of the entheses (the sites of insertion of the tendon into bone; eg, the Achilles). This can be confused with plantar fasciitis, which this patient had been previously diagnosed with.
This diagnosis could have been proved or disproved by a KOH prep (which would have shown evidence of fungal disease) or a biopsy (which would have shown fused rete ridges, microabscesses at the papillary tips, hyperkeratosis, and parakeratosis). Providers should first establish a firm diagnosis to dictate effective treatment. In this case, a visual diagnosis was possible.
Given the severity of the problem, the patient was started on a biologic; he showed vast improvement within a week. He was referred to rheumatology for evaluation and management of PsA, and the severity of his disease was communicated to his attorney.
TAKE-HOME LEARNING POINTS
- In some cases, psoriasis can be isolated to the hands, feet, genitals, or scalp, complicating detection of the condition.
- Almost 25% of patients with psoriasis develop psoriatic arthritis (PsA), which can manifest with dactylitis, arthritis, or enthesitis.
- Left untreated, PsA is potentially debilitating.
- Establishing a firm diagnosis with KOH prep or biopsy will dictate effective treatment.
This 46-year-old man’s skin disease has gotten so serious that he is essentially disabled. The problem started about six months ago, with joint pain that particularly affected his left ankle. Now, his hands are fissured and swollen to the point that he is unable to button his shirt or hold a fork. He is referred to dermatology by his attorney, who is helping him pursue possible disability benefits, for evaluation and treatment.
He has been seen by a variety of primary care providers, who have collectively prescribed topical triamcinolone 0.1% and several antifungal medications, including a two-month course of oral terbinafine. When those failed, he was treated with prednisone; at the start of the three-week course, there were signs of improvement but by the end, his hands were worse than ever.
EXAMINATION
The dorsal and palmar surfaces of the patient’s hands are covered with thick, white scales atop salmon-colored erythematous bases. Multiple fissures and marked edema can be seen. Seven of 10 fingernails are dystrophic, yellowed, and thickened.

The patient’s elbows, knees, and upper intergluteal area show less impressive involvement.
There is marked tenderness on palpation of the left Achilles insertion, made worse by dorsiflexion.
What is the diagnosis?
DISCUSSION
Psoriasis can affect one or more areas, typically the hands, scalp, genitals, or feet. When it’s focused in one area, as in this case, it can be baffling to diagnose; sometimes it’s hard to see the forest for the trees. But because the condition affects almost 3% of white Americans, you’ll see it regularly—if you’re looking for it.
Nearly 25% of patients with psoriasis eventually develop psoriatic arthritis (PsA), which not only affects the joints but also can cause complications such as enthesitis, or inflammation of the entheses (the sites of insertion of the tendon into bone; eg, the Achilles). This can be confused with plantar fasciitis, which this patient had been previously diagnosed with.
This diagnosis could have been proved or disproved by a KOH prep (which would have shown evidence of fungal disease) or a biopsy (which would have shown fused rete ridges, microabscesses at the papillary tips, hyperkeratosis, and parakeratosis). Providers should first establish a firm diagnosis to dictate effective treatment. In this case, a visual diagnosis was possible.
Given the severity of the problem, the patient was started on a biologic; he showed vast improvement within a week. He was referred to rheumatology for evaluation and management of PsA, and the severity of his disease was communicated to his attorney.
TAKE-HOME LEARNING POINTS
- In some cases, psoriasis can be isolated to the hands, feet, genitals, or scalp, complicating detection of the condition.
- Almost 25% of patients with psoriasis develop psoriatic arthritis (PsA), which can manifest with dactylitis, arthritis, or enthesitis.
- Left untreated, PsA is potentially debilitating.
- Establishing a firm diagnosis with KOH prep or biopsy will dictate effective treatment.
The case for closing robotic surgery port sites
JACKSONVILLE, FLA. – Findings from a retrospective chart review of robotic operations performed over 6 years has identified situations in which surgeons may consider closing 8-mm port sites after robotic surgery, according to a presentation at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
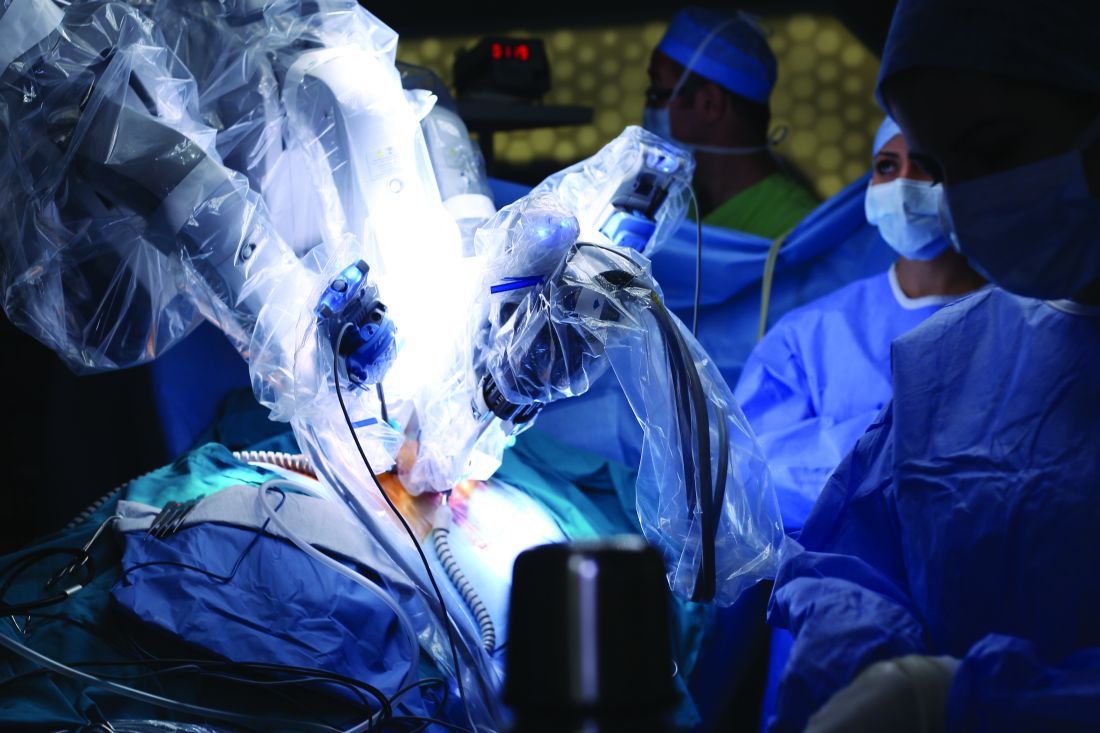
“Although the incidence of hernia through the 8-mm port sites was low, it’s still important because it’s a significant cause of morbidity in these patients,” Dr. Diez-Barroso said. Two of the three 8-mm port-site hernias required emergency surgery for small bowel incarceration.
“Both of the hernias occurred in the left lower quadrant in the lateral most port, near the anterior superior iliac spine,” he said. “The nearest site of muscle insertions was where the abdominal wall muscle layers have a limited ability to slide over one another during insufflation and desufflation and therefore have a lack of ability to seal off the port site correctly.”
These results have caused surgeons in his group to take a closer look at their own practices, Dr. Diez-Barroso said. “In our practice, now we’re considering closure of the ports in that location in the presence of known risk factors for hernia formation,” he said.
Dr. Diez-Barroso noted other scenarios when surgeons might consider closing 8-mm port sites, for example, after a prolonged operation, when significant torque has been placed on the port site, and in obese patients. The two cases of emergency surgery for port-site hernias involved obese patients: a female with a body mass index of 33 kg/m2 who had an abdominoperineal resection and a male with a BMI of 34 kg/m2 who had a right-sided ventral hernia repair.
The study had a number of limitations, Dr. Diez-Barroso said: its small sample size, retrospective nature, and short follow-up. “Moving forward, to understand better the true incidence of port-site hernias, we want further investigation with longer follow-up times and a larger sample size,” he said.
During questions, moderator Lesly Ann Dossett, MD, FACS, of the University of Michigan, Ann Arbor, asked whether there were other steps surgeons could take, such as where to place the ports or how much torque they apply, besides closing the ports.
“We’ve always placed ports with the standard approach: inserting them perpendicular to the abdominal wall,” Dr. Diez-Barroso said. “Others have theorized that the lateral sites undergo more torque, but I think that also needs further investigation.”
Dr. Diez-Barroso and coauthors reported having no financial disclosures.
Source: Diez-Barroso R. Academic Surgical Congress 2018.
JACKSONVILLE, FLA. – Findings from a retrospective chart review of robotic operations performed over 6 years has identified situations in which surgeons may consider closing 8-mm port sites after robotic surgery, according to a presentation at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.

“Although the incidence of hernia through the 8-mm port sites was low, it’s still important because it’s a significant cause of morbidity in these patients,” Dr. Diez-Barroso said. Two of the three 8-mm port-site hernias required emergency surgery for small bowel incarceration.
“Both of the hernias occurred in the left lower quadrant in the lateral most port, near the anterior superior iliac spine,” he said. “The nearest site of muscle insertions was where the abdominal wall muscle layers have a limited ability to slide over one another during insufflation and desufflation and therefore have a lack of ability to seal off the port site correctly.”
These results have caused surgeons in his group to take a closer look at their own practices, Dr. Diez-Barroso said. “In our practice, now we’re considering closure of the ports in that location in the presence of known risk factors for hernia formation,” he said.
Dr. Diez-Barroso noted other scenarios when surgeons might consider closing 8-mm port sites, for example, after a prolonged operation, when significant torque has been placed on the port site, and in obese patients. The two cases of emergency surgery for port-site hernias involved obese patients: a female with a body mass index of 33 kg/m2 who had an abdominoperineal resection and a male with a BMI of 34 kg/m2 who had a right-sided ventral hernia repair.
The study had a number of limitations, Dr. Diez-Barroso said: its small sample size, retrospective nature, and short follow-up. “Moving forward, to understand better the true incidence of port-site hernias, we want further investigation with longer follow-up times and a larger sample size,” he said.
During questions, moderator Lesly Ann Dossett, MD, FACS, of the University of Michigan, Ann Arbor, asked whether there were other steps surgeons could take, such as where to place the ports or how much torque they apply, besides closing the ports.
“We’ve always placed ports with the standard approach: inserting them perpendicular to the abdominal wall,” Dr. Diez-Barroso said. “Others have theorized that the lateral sites undergo more torque, but I think that also needs further investigation.”
Dr. Diez-Barroso and coauthors reported having no financial disclosures.
Source: Diez-Barroso R. Academic Surgical Congress 2018.
JACKSONVILLE, FLA. – Findings from a retrospective chart review of robotic operations performed over 6 years has identified situations in which surgeons may consider closing 8-mm port sites after robotic surgery, according to a presentation at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.

“Although the incidence of hernia through the 8-mm port sites was low, it’s still important because it’s a significant cause of morbidity in these patients,” Dr. Diez-Barroso said. Two of the three 8-mm port-site hernias required emergency surgery for small bowel incarceration.
“Both of the hernias occurred in the left lower quadrant in the lateral most port, near the anterior superior iliac spine,” he said. “The nearest site of muscle insertions was where the abdominal wall muscle layers have a limited ability to slide over one another during insufflation and desufflation and therefore have a lack of ability to seal off the port site correctly.”
These results have caused surgeons in his group to take a closer look at their own practices, Dr. Diez-Barroso said. “In our practice, now we’re considering closure of the ports in that location in the presence of known risk factors for hernia formation,” he said.
Dr. Diez-Barroso noted other scenarios when surgeons might consider closing 8-mm port sites, for example, after a prolonged operation, when significant torque has been placed on the port site, and in obese patients. The two cases of emergency surgery for port-site hernias involved obese patients: a female with a body mass index of 33 kg/m2 who had an abdominoperineal resection and a male with a BMI of 34 kg/m2 who had a right-sided ventral hernia repair.
The study had a number of limitations, Dr. Diez-Barroso said: its small sample size, retrospective nature, and short follow-up. “Moving forward, to understand better the true incidence of port-site hernias, we want further investigation with longer follow-up times and a larger sample size,” he said.
During questions, moderator Lesly Ann Dossett, MD, FACS, of the University of Michigan, Ann Arbor, asked whether there were other steps surgeons could take, such as where to place the ports or how much torque they apply, besides closing the ports.
“We’ve always placed ports with the standard approach: inserting them perpendicular to the abdominal wall,” Dr. Diez-Barroso said. “Others have theorized that the lateral sites undergo more torque, but I think that also needs further investigation.”
Dr. Diez-Barroso and coauthors reported having no financial disclosures.
Source: Diez-Barroso R. Academic Surgical Congress 2018.
AT THE ACADEMIC SURGICAL CONGRESS
Key clinical point: Some 8-mm robotic port sites may warrant closure under certain circumstances.
Major finding: Of 178 patients, 3 had complications caused by 8-mm robotic port sites that were not closed, 2 of which required emergency reoperation for small bowel incarceration.
Data source: Retrospective chart review of 178 patients who had robotic general and oncologic surgical procedures between July 2010 and December 2016.
Disclosures: Dr. Diez-Barroso and coauthors reported having no financial disclosures.
Source: Diez-Barroso R. Academic Surgical Congress 2018.