User login
Cyclophosphamide extends PFS in elderly HER2+ breast cancer patients
by 7 months – with acceptable toxicity – vs. dual HER2 blockade alone in a randomized, open-label, phase 2 trial.
After a median follow-up of 20.7 months, the median progression-free survival (PFS) among 41 women who received trastuzumab and pertuzumab plus metronomic oral cyclophosphamide was 12.7 months, compared with 5.6 months among 39 women who received only trastuzumab and pertuzumab. Estimated PFS at 6 months was 73.4% and 46.2% in the groups, respectively (hazard ratio, 0.65), Hans Wildiers, MD, PhD, of University Hospitals Leuven, Belgium, and his colleagues reported in Lancet Oncology.
The most frequent grade 3-4 adverse events occurring in each group, respectively, were hypertension (12% and 15%), diarrhea (12% and 10%), dyspnea (10% and 5%), fatigue (5% and 8%), and pain (5% in each group). Thromboembolic events occurred in four patients (10%) receiving cyclophosphamide, while none occurred in the dual HER2 blockade-only group. Severe cardiac toxicities were occasionally observed in both groups.
Study subjects were women aged at least 70 years, or at least 60 years with confirmed functional restrictions. All had confirmed HER2-positive metastatic breast cancer and no prior chemotherapy for metastatic disease. They received either intravenous trastuzumab at a loading dose of 8 mg/kg, followed by 6 mg/kg every 3 weeks, and intravenous pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks (median of 6 cycles), or those same doses of trastuzumab and pertuzumab plus metronomic oral cyclophosphamide at a dose of 50 mg daily (median of 13 cycles). Subsequent treatment with trastuzumab emtansine in 29 patients who progressed during the study was active and well tolerated; the overall PFS at 6 months in this group of patients was 49.5% and median PFS was 5 months after starting trastuzumab emtansine.
“The results of this study indicate that the benefit of avoiding the side effects of chemotherapy with the use of dual anti–HER2 blockade only does not compensate for an important loss of activity in the metastatic setting,” the investigators wrote, concluding that “trastuzumab and pertuzumab plus metronomic oral cyclophosphamide, potentially followed by trastuzumab emtansine after progression, might delay the need for, or supersede, the use of taxane-based chemotherapy in this population.”
Further evaluation of this combination in a randomized phase 3 study is warranted, as the phase 2 findings do not provide “robust justification for a change in practice.” They do, however, provide a scientific framework for supporting more specific trials in older patients, who compromise nearly a third of breast cancer patients worldwide, and up to 50% in high-income countries, they said.
This study was funded by F Hoffmann-La Roche. Dr. Wildiers has received research grants from Roche, and personal fees to his institute from Roche, Amgen, Novartis, Pfizer, Puma, and Celldex. Other authors also reported receiving research or other support from Roche Products, Eisai, Novartis Pharmaceuticals UK, Astellas/Medivation, Astra Zeneca, Celgene, Daiichi-Sankyo, GE Oncology, Genentech, GlaxoSmithKline, Macrogenics, Merck Sharp & Dohme, Merus BV, Mylan, Novartis, Pfizer, Pierre Fabre, Sanofi, and Teva.
SOURCE: Wildiers H et al. Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30083-4.
The findings by Wildiers et al. regarding the benefits of adding oral cyclophosphamide to dual HER2 blockade should be evaluated further, according to Charles E. Geyer Jr., MD, who applauded the investigators’ demonstration of a framework for much-needed clinical trials in frail elderly patients with breast cancer.
In an editorial, Dr. Geyer wrote that the trial results provide sufficient evidence for consideration of trastuzumab and pertuzumab plus oral cyclophosphamide in frail elderly patients at increased risk of adverse events from taxane-based therapies, but he also encouraged additional study (Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30084-6).
An important consideration in future studies will be the choice of a comparator group; he suggested a version of the CLEOPATRA study to look at trastuzumab plus metronomic oral cyclophosphamide with and without pertuzumab. A prior small study suggested that the problematic risk of diarrhea seen in patients in the current study did not occur in patients pretreated with trastuzumab who showed activity with trastuzumab and metronomic oral cyclophosphamide chemotherapy, he noted.
Dr. Geyer is with the Massey Cancer Center at Virginia Commonwealth University, Richmond. He reported receiving personal fees from Myriad and Heron Therapeutics for advisory board participation, and travel support from AstraZeneca, Genentech, and Macrogenics, outside the submitted work.
The findings by Wildiers et al. regarding the benefits of adding oral cyclophosphamide to dual HER2 blockade should be evaluated further, according to Charles E. Geyer Jr., MD, who applauded the investigators’ demonstration of a framework for much-needed clinical trials in frail elderly patients with breast cancer.
In an editorial, Dr. Geyer wrote that the trial results provide sufficient evidence for consideration of trastuzumab and pertuzumab plus oral cyclophosphamide in frail elderly patients at increased risk of adverse events from taxane-based therapies, but he also encouraged additional study (Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30084-6).
An important consideration in future studies will be the choice of a comparator group; he suggested a version of the CLEOPATRA study to look at trastuzumab plus metronomic oral cyclophosphamide with and without pertuzumab. A prior small study suggested that the problematic risk of diarrhea seen in patients in the current study did not occur in patients pretreated with trastuzumab who showed activity with trastuzumab and metronomic oral cyclophosphamide chemotherapy, he noted.
Dr. Geyer is with the Massey Cancer Center at Virginia Commonwealth University, Richmond. He reported receiving personal fees from Myriad and Heron Therapeutics for advisory board participation, and travel support from AstraZeneca, Genentech, and Macrogenics, outside the submitted work.
The findings by Wildiers et al. regarding the benefits of adding oral cyclophosphamide to dual HER2 blockade should be evaluated further, according to Charles E. Geyer Jr., MD, who applauded the investigators’ demonstration of a framework for much-needed clinical trials in frail elderly patients with breast cancer.
In an editorial, Dr. Geyer wrote that the trial results provide sufficient evidence for consideration of trastuzumab and pertuzumab plus oral cyclophosphamide in frail elderly patients at increased risk of adverse events from taxane-based therapies, but he also encouraged additional study (Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30084-6).
An important consideration in future studies will be the choice of a comparator group; he suggested a version of the CLEOPATRA study to look at trastuzumab plus metronomic oral cyclophosphamide with and without pertuzumab. A prior small study suggested that the problematic risk of diarrhea seen in patients in the current study did not occur in patients pretreated with trastuzumab who showed activity with trastuzumab and metronomic oral cyclophosphamide chemotherapy, he noted.
Dr. Geyer is with the Massey Cancer Center at Virginia Commonwealth University, Richmond. He reported receiving personal fees from Myriad and Heron Therapeutics for advisory board participation, and travel support from AstraZeneca, Genentech, and Macrogenics, outside the submitted work.
by 7 months – with acceptable toxicity – vs. dual HER2 blockade alone in a randomized, open-label, phase 2 trial.
After a median follow-up of 20.7 months, the median progression-free survival (PFS) among 41 women who received trastuzumab and pertuzumab plus metronomic oral cyclophosphamide was 12.7 months, compared with 5.6 months among 39 women who received only trastuzumab and pertuzumab. Estimated PFS at 6 months was 73.4% and 46.2% in the groups, respectively (hazard ratio, 0.65), Hans Wildiers, MD, PhD, of University Hospitals Leuven, Belgium, and his colleagues reported in Lancet Oncology.
The most frequent grade 3-4 adverse events occurring in each group, respectively, were hypertension (12% and 15%), diarrhea (12% and 10%), dyspnea (10% and 5%), fatigue (5% and 8%), and pain (5% in each group). Thromboembolic events occurred in four patients (10%) receiving cyclophosphamide, while none occurred in the dual HER2 blockade-only group. Severe cardiac toxicities were occasionally observed in both groups.
Study subjects were women aged at least 70 years, or at least 60 years with confirmed functional restrictions. All had confirmed HER2-positive metastatic breast cancer and no prior chemotherapy for metastatic disease. They received either intravenous trastuzumab at a loading dose of 8 mg/kg, followed by 6 mg/kg every 3 weeks, and intravenous pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks (median of 6 cycles), or those same doses of trastuzumab and pertuzumab plus metronomic oral cyclophosphamide at a dose of 50 mg daily (median of 13 cycles). Subsequent treatment with trastuzumab emtansine in 29 patients who progressed during the study was active and well tolerated; the overall PFS at 6 months in this group of patients was 49.5% and median PFS was 5 months after starting trastuzumab emtansine.
“The results of this study indicate that the benefit of avoiding the side effects of chemotherapy with the use of dual anti–HER2 blockade only does not compensate for an important loss of activity in the metastatic setting,” the investigators wrote, concluding that “trastuzumab and pertuzumab plus metronomic oral cyclophosphamide, potentially followed by trastuzumab emtansine after progression, might delay the need for, or supersede, the use of taxane-based chemotherapy in this population.”
Further evaluation of this combination in a randomized phase 3 study is warranted, as the phase 2 findings do not provide “robust justification for a change in practice.” They do, however, provide a scientific framework for supporting more specific trials in older patients, who compromise nearly a third of breast cancer patients worldwide, and up to 50% in high-income countries, they said.
This study was funded by F Hoffmann-La Roche. Dr. Wildiers has received research grants from Roche, and personal fees to his institute from Roche, Amgen, Novartis, Pfizer, Puma, and Celldex. Other authors also reported receiving research or other support from Roche Products, Eisai, Novartis Pharmaceuticals UK, Astellas/Medivation, Astra Zeneca, Celgene, Daiichi-Sankyo, GE Oncology, Genentech, GlaxoSmithKline, Macrogenics, Merck Sharp & Dohme, Merus BV, Mylan, Novartis, Pfizer, Pierre Fabre, Sanofi, and Teva.
SOURCE: Wildiers H et al. Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30083-4.
by 7 months – with acceptable toxicity – vs. dual HER2 blockade alone in a randomized, open-label, phase 2 trial.
After a median follow-up of 20.7 months, the median progression-free survival (PFS) among 41 women who received trastuzumab and pertuzumab plus metronomic oral cyclophosphamide was 12.7 months, compared with 5.6 months among 39 women who received only trastuzumab and pertuzumab. Estimated PFS at 6 months was 73.4% and 46.2% in the groups, respectively (hazard ratio, 0.65), Hans Wildiers, MD, PhD, of University Hospitals Leuven, Belgium, and his colleagues reported in Lancet Oncology.
The most frequent grade 3-4 adverse events occurring in each group, respectively, were hypertension (12% and 15%), diarrhea (12% and 10%), dyspnea (10% and 5%), fatigue (5% and 8%), and pain (5% in each group). Thromboembolic events occurred in four patients (10%) receiving cyclophosphamide, while none occurred in the dual HER2 blockade-only group. Severe cardiac toxicities were occasionally observed in both groups.
Study subjects were women aged at least 70 years, or at least 60 years with confirmed functional restrictions. All had confirmed HER2-positive metastatic breast cancer and no prior chemotherapy for metastatic disease. They received either intravenous trastuzumab at a loading dose of 8 mg/kg, followed by 6 mg/kg every 3 weeks, and intravenous pertuzumab at a loading dose of 840 mg followed by 420 mg every 3 weeks (median of 6 cycles), or those same doses of trastuzumab and pertuzumab plus metronomic oral cyclophosphamide at a dose of 50 mg daily (median of 13 cycles). Subsequent treatment with trastuzumab emtansine in 29 patients who progressed during the study was active and well tolerated; the overall PFS at 6 months in this group of patients was 49.5% and median PFS was 5 months after starting trastuzumab emtansine.
“The results of this study indicate that the benefit of avoiding the side effects of chemotherapy with the use of dual anti–HER2 blockade only does not compensate for an important loss of activity in the metastatic setting,” the investigators wrote, concluding that “trastuzumab and pertuzumab plus metronomic oral cyclophosphamide, potentially followed by trastuzumab emtansine after progression, might delay the need for, or supersede, the use of taxane-based chemotherapy in this population.”
Further evaluation of this combination in a randomized phase 3 study is warranted, as the phase 2 findings do not provide “robust justification for a change in practice.” They do, however, provide a scientific framework for supporting more specific trials in older patients, who compromise nearly a third of breast cancer patients worldwide, and up to 50% in high-income countries, they said.
This study was funded by F Hoffmann-La Roche. Dr. Wildiers has received research grants from Roche, and personal fees to his institute from Roche, Amgen, Novartis, Pfizer, Puma, and Celldex. Other authors also reported receiving research or other support from Roche Products, Eisai, Novartis Pharmaceuticals UK, Astellas/Medivation, Astra Zeneca, Celgene, Daiichi-Sankyo, GE Oncology, Genentech, GlaxoSmithKline, Macrogenics, Merck Sharp & Dohme, Merus BV, Mylan, Novartis, Pfizer, Pierre Fabre, Sanofi, and Teva.
SOURCE: Wildiers H et al. Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30083-4.
FROM THE LANCET ONCOLOGY
Key clinical point: Adding oral cyclophosphamide to trastuzumab and pertuzumab benefits frail elderly breast cancer patients.
Major finding: PFS was 12.7 months vs. 5.6 months with trastuzumab and pertuzumab plus metronomic oral cyclophosphamide vs. trastuzumab and pertuzumab alone.
Study details: A randomized phase 2 trial in 80 patients.
Disclosures: This study was funded by F Hoffmann-La Roche. Dr. Wildiers has received research grants from Roche, and personal fees to his institute from Roche, Amgen, Novartis, Pfizer, Puma, and Celldex. Other authors also reported receiving research or other support from Roche Products, Eisai, Novartis Pharmaceuticals UK, Astellas/Medivation, AstraZeneca, Celgene, Daiichi-Sankyo, GE Oncology, Genentech, GlaxoSmithKline, MacroGenics, Merck Sharp & Dohme, Merus BV, Mylan, Novartis, Pfizer, Pierre Fabre, Sanofi, and Teva.
Source: Wildiers Hans et al. Lancet Oncol. 2018 Feb 9. doi: 10.1016/S1470-2045(18)30083-4.
Allscripts’ charges for sending, refilling prescriptions
How much is $9 worth? Not much. Probably less than most people spend on coffee in a given week.
And yet, that $9 is really irritating to me.
For the last few weeks, when signing into Allscripts to send and refill prescriptions, I’ve encountered this:
I know that $9 a month doesn’t seem like much: It’s $108 a year. But still, it’s irritating.
I understand Allscripts, and every other health care company, is here to make a living. Heck, so am I. Software development isn’t cheap. Neither are the servers hosting it or the security software needed, or the buildings to house them, and a million other things. I get that. None of these things are free.
But, at the same time, it’s part of a general trend of modern health care. Our landlords and vendors can arbitrarily raise prices to keep up with their costs, but we can’t do the same to keep up with ours.
The majority of doctors aren’t in a position to raise our prices to account for these things. We’re stuck with insurance companies and government agencies that tell us to accept a given amount or eat rocks.
There are, of course, concierge practices that can raise their prices, but they’re mostly boutique-level general care with wealthy patients who can afford them. Most small specialists aren’t in that position. We can’t afford to put Keurigs in the lobby.
The few revenue streams most of us have for which we can increase prices, such as legal work and cash patients, are typically not enough of the practice where it would make a difference to overcome it. In fact, the lien company I see patients for recently told me they were lowering their reimbursements to me to compensate for their own higher expenses.
Some people may see the $9 a month as a minor issue and move on. But to a small practice, it’s now another $108 in revenue that I have to bring in each year to cover. And, in a field in which, unlike every other product or service people pay for, I’m not allowed to control my own prices to make up for it.
That doesn’t seem fair, does it?
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
How much is $9 worth? Not much. Probably less than most people spend on coffee in a given week.
And yet, that $9 is really irritating to me.
For the last few weeks, when signing into Allscripts to send and refill prescriptions, I’ve encountered this:
I know that $9 a month doesn’t seem like much: It’s $108 a year. But still, it’s irritating.
I understand Allscripts, and every other health care company, is here to make a living. Heck, so am I. Software development isn’t cheap. Neither are the servers hosting it or the security software needed, or the buildings to house them, and a million other things. I get that. None of these things are free.
But, at the same time, it’s part of a general trend of modern health care. Our landlords and vendors can arbitrarily raise prices to keep up with their costs, but we can’t do the same to keep up with ours.
The majority of doctors aren’t in a position to raise our prices to account for these things. We’re stuck with insurance companies and government agencies that tell us to accept a given amount or eat rocks.
There are, of course, concierge practices that can raise their prices, but they’re mostly boutique-level general care with wealthy patients who can afford them. Most small specialists aren’t in that position. We can’t afford to put Keurigs in the lobby.
The few revenue streams most of us have for which we can increase prices, such as legal work and cash patients, are typically not enough of the practice where it would make a difference to overcome it. In fact, the lien company I see patients for recently told me they were lowering their reimbursements to me to compensate for their own higher expenses.
Some people may see the $9 a month as a minor issue and move on. But to a small practice, it’s now another $108 in revenue that I have to bring in each year to cover. And, in a field in which, unlike every other product or service people pay for, I’m not allowed to control my own prices to make up for it.
That doesn’t seem fair, does it?
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
How much is $9 worth? Not much. Probably less than most people spend on coffee in a given week.
And yet, that $9 is really irritating to me.
For the last few weeks, when signing into Allscripts to send and refill prescriptions, I’ve encountered this:
I know that $9 a month doesn’t seem like much: It’s $108 a year. But still, it’s irritating.
I understand Allscripts, and every other health care company, is here to make a living. Heck, so am I. Software development isn’t cheap. Neither are the servers hosting it or the security software needed, or the buildings to house them, and a million other things. I get that. None of these things are free.
But, at the same time, it’s part of a general trend of modern health care. Our landlords and vendors can arbitrarily raise prices to keep up with their costs, but we can’t do the same to keep up with ours.
The majority of doctors aren’t in a position to raise our prices to account for these things. We’re stuck with insurance companies and government agencies that tell us to accept a given amount or eat rocks.
There are, of course, concierge practices that can raise their prices, but they’re mostly boutique-level general care with wealthy patients who can afford them. Most small specialists aren’t in that position. We can’t afford to put Keurigs in the lobby.
The few revenue streams most of us have for which we can increase prices, such as legal work and cash patients, are typically not enough of the practice where it would make a difference to overcome it. In fact, the lien company I see patients for recently told me they were lowering their reimbursements to me to compensate for their own higher expenses.
Some people may see the $9 a month as a minor issue and move on. But to a small practice, it’s now another $108 in revenue that I have to bring in each year to cover. And, in a field in which, unlike every other product or service people pay for, I’m not allowed to control my own prices to make up for it.
That doesn’t seem fair, does it?
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Blood test approved for patients with concussions
The Food and Drug Administration approved a new blood test on Feb. 14 – the Banyan Brain Trauma Indicator – for assessing patients with mild traumatic brain injuries, also known as concussions.
Most traumatic brain injuries are classified as “mild,” and the majority of patients have negative CT scans, according to an FDA announcement. Within a matter of hours, this test can help predict which patients will have negative scans by measuring certain proteins released by brain tissue, thereby potentially eliminating unnecessary imaging – and the costs and radiation exposure that would go along with it.
Read more in the FDA’s press release.
The Food and Drug Administration approved a new blood test on Feb. 14 – the Banyan Brain Trauma Indicator – for assessing patients with mild traumatic brain injuries, also known as concussions.
Most traumatic brain injuries are classified as “mild,” and the majority of patients have negative CT scans, according to an FDA announcement. Within a matter of hours, this test can help predict which patients will have negative scans by measuring certain proteins released by brain tissue, thereby potentially eliminating unnecessary imaging – and the costs and radiation exposure that would go along with it.
Read more in the FDA’s press release.
The Food and Drug Administration approved a new blood test on Feb. 14 – the Banyan Brain Trauma Indicator – for assessing patients with mild traumatic brain injuries, also known as concussions.
Most traumatic brain injuries are classified as “mild,” and the majority of patients have negative CT scans, according to an FDA announcement. Within a matter of hours, this test can help predict which patients will have negative scans by measuring certain proteins released by brain tissue, thereby potentially eliminating unnecessary imaging – and the costs and radiation exposure that would go along with it.
Read more in the FDA’s press release.
MMWR: Current flu vaccine does not protect elderly
, according to the Feb. 16 issue of Morbidity and Mortality Weekly Report.
The elderly are not among them. Although the vaccine was somewhat protective in children and adults up to 49 years old, “no statistically significant protection was observed in other age groups,” including people 65 years and older, reported investigators led by Brendan Flannery, PhD, of the Centers for Disease Control and Prevention influenza division.
They also reported that the cumulative hospitalization rate attributed to laboratory-confirmed influenza for the week ending Feb. 3, 2018 (59.9/100,000), exceeded the rate for the same week in 2014-2015 (50.9/100,000), an A(H3N2) virus–predominant season, and is the highest rate observed for this week since the system expanded to include adults during the 2005-2006 season.
This year’s overall effectiveness rating was in contrast to the 2016-2017 seasonal effectiveness of 48% (MMWR. 2017 Feb 17;66[6];167-71).
The CDC noted that influenza is going to be active for several more weeks, so “vaccination is still recommended,” but “treatment with influenza antiviral medications, where appropriate, is especially important this season.” Meanwhile, “influenza vaccines with improved effectiveness are needed,” the CDC said.
The estimates are based on 4,562 patients 6 months to over 65 years old presenting with acute respiratory illness in 2018 from Nov. 2 to Feb. 3 at five outpatient medical clinics scattered across the United States. Nasal and oropharyngeal swabs were tested with reverse transcription polymerase chain reaction for the presence of influenza viruses; 413 subjects were 65 years or older.
Vaccine effectiveness against the less common virus A(H1N1)pdm09 was 67%, and 42% against the even rarer influenza B viruses. Estimates were adjusted for a range of confounders, including study site, age, general health, and week of illness. Vaccination rates ranged from 45% to 59% across the study sites; 38% of the subjects tested positive for influenza, most for type A viruses. The shot didn’t work too well: 43% of the influenza cases had gotten it.
The 25% effectiveness against A(H3N2) is a bit higher than recent reports of 17% from Canada and 10% from Australia, but similar to the 32% efficacy reported in the United States for the 2016-2017 season.
“These interim estimates reflect ongoing challenges with the A(H3N2) vaccine component since the 2011-12 season,” the investigators wrote. “Multiple factors might be contributing to the reported [vaccine effectiveness] against A(H3N2) viruses this season. … Genetic changes in the vaccine virus hemagglutinin protein that arise during passage in eggs might result in a vaccine immune response that is less effective against circulating viruses.”
On a related note, on Feb. 18, Senators Edward J. Markey (D-Mass.), Richard Blumenthal (D-Conn.), and Amy Klobuchar (D-Minn.) held a press conference to announce they were introducing the Flu Vaccine Bill to dedicate $1 billion over a 5-year period in order to develop a flu vaccine that could provide lifetime protection.
The investigators had no conflicts of interest.
SOURCE: Flannery B. et al. MMWR. 2018 Feb 16;67(6):180-5; Budd A. et al. MMWR. 2018 Feb 16;67(6):169-79.
, according to the Feb. 16 issue of Morbidity and Mortality Weekly Report.
The elderly are not among them. Although the vaccine was somewhat protective in children and adults up to 49 years old, “no statistically significant protection was observed in other age groups,” including people 65 years and older, reported investigators led by Brendan Flannery, PhD, of the Centers for Disease Control and Prevention influenza division.
They also reported that the cumulative hospitalization rate attributed to laboratory-confirmed influenza for the week ending Feb. 3, 2018 (59.9/100,000), exceeded the rate for the same week in 2014-2015 (50.9/100,000), an A(H3N2) virus–predominant season, and is the highest rate observed for this week since the system expanded to include adults during the 2005-2006 season.
This year’s overall effectiveness rating was in contrast to the 2016-2017 seasonal effectiveness of 48% (MMWR. 2017 Feb 17;66[6];167-71).
The CDC noted that influenza is going to be active for several more weeks, so “vaccination is still recommended,” but “treatment with influenza antiviral medications, where appropriate, is especially important this season.” Meanwhile, “influenza vaccines with improved effectiveness are needed,” the CDC said.
The estimates are based on 4,562 patients 6 months to over 65 years old presenting with acute respiratory illness in 2018 from Nov. 2 to Feb. 3 at five outpatient medical clinics scattered across the United States. Nasal and oropharyngeal swabs were tested with reverse transcription polymerase chain reaction for the presence of influenza viruses; 413 subjects were 65 years or older.
Vaccine effectiveness against the less common virus A(H1N1)pdm09 was 67%, and 42% against the even rarer influenza B viruses. Estimates were adjusted for a range of confounders, including study site, age, general health, and week of illness. Vaccination rates ranged from 45% to 59% across the study sites; 38% of the subjects tested positive for influenza, most for type A viruses. The shot didn’t work too well: 43% of the influenza cases had gotten it.
The 25% effectiveness against A(H3N2) is a bit higher than recent reports of 17% from Canada and 10% from Australia, but similar to the 32% efficacy reported in the United States for the 2016-2017 season.
“These interim estimates reflect ongoing challenges with the A(H3N2) vaccine component since the 2011-12 season,” the investigators wrote. “Multiple factors might be contributing to the reported [vaccine effectiveness] against A(H3N2) viruses this season. … Genetic changes in the vaccine virus hemagglutinin protein that arise during passage in eggs might result in a vaccine immune response that is less effective against circulating viruses.”
On a related note, on Feb. 18, Senators Edward J. Markey (D-Mass.), Richard Blumenthal (D-Conn.), and Amy Klobuchar (D-Minn.) held a press conference to announce they were introducing the Flu Vaccine Bill to dedicate $1 billion over a 5-year period in order to develop a flu vaccine that could provide lifetime protection.
The investigators had no conflicts of interest.
SOURCE: Flannery B. et al. MMWR. 2018 Feb 16;67(6):180-5; Budd A. et al. MMWR. 2018 Feb 16;67(6):169-79.
, according to the Feb. 16 issue of Morbidity and Mortality Weekly Report.
The elderly are not among them. Although the vaccine was somewhat protective in children and adults up to 49 years old, “no statistically significant protection was observed in other age groups,” including people 65 years and older, reported investigators led by Brendan Flannery, PhD, of the Centers for Disease Control and Prevention influenza division.
They also reported that the cumulative hospitalization rate attributed to laboratory-confirmed influenza for the week ending Feb. 3, 2018 (59.9/100,000), exceeded the rate for the same week in 2014-2015 (50.9/100,000), an A(H3N2) virus–predominant season, and is the highest rate observed for this week since the system expanded to include adults during the 2005-2006 season.
This year’s overall effectiveness rating was in contrast to the 2016-2017 seasonal effectiveness of 48% (MMWR. 2017 Feb 17;66[6];167-71).
The CDC noted that influenza is going to be active for several more weeks, so “vaccination is still recommended,” but “treatment with influenza antiviral medications, where appropriate, is especially important this season.” Meanwhile, “influenza vaccines with improved effectiveness are needed,” the CDC said.
The estimates are based on 4,562 patients 6 months to over 65 years old presenting with acute respiratory illness in 2018 from Nov. 2 to Feb. 3 at five outpatient medical clinics scattered across the United States. Nasal and oropharyngeal swabs were tested with reverse transcription polymerase chain reaction for the presence of influenza viruses; 413 subjects were 65 years or older.
Vaccine effectiveness against the less common virus A(H1N1)pdm09 was 67%, and 42% against the even rarer influenza B viruses. Estimates were adjusted for a range of confounders, including study site, age, general health, and week of illness. Vaccination rates ranged from 45% to 59% across the study sites; 38% of the subjects tested positive for influenza, most for type A viruses. The shot didn’t work too well: 43% of the influenza cases had gotten it.
The 25% effectiveness against A(H3N2) is a bit higher than recent reports of 17% from Canada and 10% from Australia, but similar to the 32% efficacy reported in the United States for the 2016-2017 season.
“These interim estimates reflect ongoing challenges with the A(H3N2) vaccine component since the 2011-12 season,” the investigators wrote. “Multiple factors might be contributing to the reported [vaccine effectiveness] against A(H3N2) viruses this season. … Genetic changes in the vaccine virus hemagglutinin protein that arise during passage in eggs might result in a vaccine immune response that is less effective against circulating viruses.”
On a related note, on Feb. 18, Senators Edward J. Markey (D-Mass.), Richard Blumenthal (D-Conn.), and Amy Klobuchar (D-Minn.) held a press conference to announce they were introducing the Flu Vaccine Bill to dedicate $1 billion over a 5-year period in order to develop a flu vaccine that could provide lifetime protection.
The investigators had no conflicts of interest.
SOURCE: Flannery B. et al. MMWR. 2018 Feb 16;67(6):180-5; Budd A. et al. MMWR. 2018 Feb 16;67(6):169-79.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Opioids and hospital medicine: What can we do?
I recently attended a local Charleston (S.C.) Medical Society meeting, the theme of which was the opioid crisis. Although at the time I did not see a perfect relevance of this crisis to hospital medicine, I attended anyway, hoping to gain some pearls of wisdom regarding what my role in this epidemic could be.
I was already certainly aware of the extent of the opioid epidemic, including some startling statistics. For example, the burden of the crisis totaled $95 billion in the United States in 2016 from lost productivity and health care and criminal justice system expenses.1 But I was still not certain what my specific role could be in doing something about it.
The main speaker at the meeting was Nanci Steadman Shipman, the mother of a 19-year-old college student who had accidentally overdosed on heroin the year prior. She told the story of his upbringing, which was in an upper-middle-class suburban neighborhood, full of family, friends, and loving support. When her son was 15 years old, he suffered a leg injury during his lacrosse season, which led to a hospital stay, a surgery, and a prolonged recovery. It was during this period of time that, unbeknownst to his family, he became addicted to opioids.
Over the years, Nanci’s son found ever more creative mechanisms to procure various opioids, eventually resorting to heroin, which was remarkably cheap and easy to find. All the while in high school, he maintained good grades, remained active in sports, and had a normal social circle of friends. It was not until his first year of college that his mother started to worry that something might be wrong. In less than a year, her son quit sports, and his grades spiraled. Despite ongoing family support and extensive rehab, he suffered more than one setback and accidentally overdosed.
After her son’s death, Nanci started a nonprofit organization, Wake Up Carolina.2 Its mission is to fight drug abuse among adolescents and young adults. They use a combination of education, awareness, prevention, and recovery tactics to achieve their task. In the meantime, they try to diminish the shame and secrecy among families suffering from opioid addiction. During Nanci’s presentation at the medical society meeting, the message she conveyed to us – an audience full of physicians – was simple: We can either be part of the problem or part of the solution; we all have a duty to help and a role to play in this crisis. Whether a patient is exposed first inside the hospital or outside of it, for a short period of time or for a long one, every opioid exposure comes with a risk. Nanci’s story was incredibly affecting and made me rethink my personal role in this epidemic; how might I have contributed to this, and what could I do differently?
Shortly after her son died, her younger son suffered a femur fracture during a football game. You can imagine the horror her family felt knowing that he would need some pain medication for his acute injury. Nanci and her family were able to work with the medical and surgical teams, and through multimodal pain regimens, her son received little to no opioids during the hospital stay and was able to recover from the fracture with reasonable pain levels. She expressed gratitude that the hospital teams were willing to listen to her and her family’s concerns and offer both pharmacological and nonpharmacological therapies for her son’s recovery, which allayed their fears about opioids.
From this incredibly powerful and moving story of one family’s experience, I was able to gather some very meaningful, evidence-based, and tangible practices that I could implement in my own organization. These might even help other hospitalists take an active role in stemming this sadly growing epidemic.
As hospitalists, we should work on the following:
- Improve our personal knowledge of and skills in utilizing multimodal pain therapies regardless of the etiology of the pain. These should include both pharmacologic and nonpharmacologic interventions.
- Use consultants to assist us with difficult cases. Depending on our practice setting, we should utilize consultants who can give us good advice on nonopioid pain management regimens, such as palliative care.
- Try to influence the practice of surgeons and other specialties that consult us, to help shape prescribing patterns that include nonopioid medical regimens, and to get doctors used to entertaining nonpharmacologic pain-reducing interventions.
- Limit the volume of prescription opioids written to our patients at the time of hospital discharge. There is mounting literature that suggests leftover prescriptions can be the start of an opioid addiction for a family member.
- Educate ourselves and our patients about any local “take back” programs that allow for safe, secure, and anonymous drops of prescription medications. This may reduce opioids getting into the hands of someone who might later become addicted.
- Find out whether our hospitals or health systems have a pain or opioid oversight group or team, and if not, see whether there is interest in starting one.
- Look into local community activist programs to partner with for education, awareness, prevention, or treatment options (such as Wake Up Carolina).
- Work with local resources (for example, case management, social workers, psychiatrists) to learn about and utilize local options for rehabilitation. We should actively and openly discuss these options with any patients known or suspected to be addicted.
- Make a valiant attempt to remove any unconscious bias against people who have become addicted to opioids. Continuing the social stigma of addiction only spurs the shame and secrecy.
Please share other ideas or suggestions you may have regarding the role hospitalists can have in curbing this growing epidemic.
References
1. “Burden of opioid crisis reached $95 billion in 2016; private sector hit hardest.” Altarum press release. Nov 16, 2017.
2. Wake Up Carolina website.
I recently attended a local Charleston (S.C.) Medical Society meeting, the theme of which was the opioid crisis. Although at the time I did not see a perfect relevance of this crisis to hospital medicine, I attended anyway, hoping to gain some pearls of wisdom regarding what my role in this epidemic could be.
I was already certainly aware of the extent of the opioid epidemic, including some startling statistics. For example, the burden of the crisis totaled $95 billion in the United States in 2016 from lost productivity and health care and criminal justice system expenses.1 But I was still not certain what my specific role could be in doing something about it.
The main speaker at the meeting was Nanci Steadman Shipman, the mother of a 19-year-old college student who had accidentally overdosed on heroin the year prior. She told the story of his upbringing, which was in an upper-middle-class suburban neighborhood, full of family, friends, and loving support. When her son was 15 years old, he suffered a leg injury during his lacrosse season, which led to a hospital stay, a surgery, and a prolonged recovery. It was during this period of time that, unbeknownst to his family, he became addicted to opioids.
Over the years, Nanci’s son found ever more creative mechanisms to procure various opioids, eventually resorting to heroin, which was remarkably cheap and easy to find. All the while in high school, he maintained good grades, remained active in sports, and had a normal social circle of friends. It was not until his first year of college that his mother started to worry that something might be wrong. In less than a year, her son quit sports, and his grades spiraled. Despite ongoing family support and extensive rehab, he suffered more than one setback and accidentally overdosed.
After her son’s death, Nanci started a nonprofit organization, Wake Up Carolina.2 Its mission is to fight drug abuse among adolescents and young adults. They use a combination of education, awareness, prevention, and recovery tactics to achieve their task. In the meantime, they try to diminish the shame and secrecy among families suffering from opioid addiction. During Nanci’s presentation at the medical society meeting, the message she conveyed to us – an audience full of physicians – was simple: We can either be part of the problem or part of the solution; we all have a duty to help and a role to play in this crisis. Whether a patient is exposed first inside the hospital or outside of it, for a short period of time or for a long one, every opioid exposure comes with a risk. Nanci’s story was incredibly affecting and made me rethink my personal role in this epidemic; how might I have contributed to this, and what could I do differently?
Shortly after her son died, her younger son suffered a femur fracture during a football game. You can imagine the horror her family felt knowing that he would need some pain medication for his acute injury. Nanci and her family were able to work with the medical and surgical teams, and through multimodal pain regimens, her son received little to no opioids during the hospital stay and was able to recover from the fracture with reasonable pain levels. She expressed gratitude that the hospital teams were willing to listen to her and her family’s concerns and offer both pharmacological and nonpharmacological therapies for her son’s recovery, which allayed their fears about opioids.
From this incredibly powerful and moving story of one family’s experience, I was able to gather some very meaningful, evidence-based, and tangible practices that I could implement in my own organization. These might even help other hospitalists take an active role in stemming this sadly growing epidemic.
As hospitalists, we should work on the following:
- Improve our personal knowledge of and skills in utilizing multimodal pain therapies regardless of the etiology of the pain. These should include both pharmacologic and nonpharmacologic interventions.
- Use consultants to assist us with difficult cases. Depending on our practice setting, we should utilize consultants who can give us good advice on nonopioid pain management regimens, such as palliative care.
- Try to influence the practice of surgeons and other specialties that consult us, to help shape prescribing patterns that include nonopioid medical regimens, and to get doctors used to entertaining nonpharmacologic pain-reducing interventions.
- Limit the volume of prescription opioids written to our patients at the time of hospital discharge. There is mounting literature that suggests leftover prescriptions can be the start of an opioid addiction for a family member.
- Educate ourselves and our patients about any local “take back” programs that allow for safe, secure, and anonymous drops of prescription medications. This may reduce opioids getting into the hands of someone who might later become addicted.
- Find out whether our hospitals or health systems have a pain or opioid oversight group or team, and if not, see whether there is interest in starting one.
- Look into local community activist programs to partner with for education, awareness, prevention, or treatment options (such as Wake Up Carolina).
- Work with local resources (for example, case management, social workers, psychiatrists) to learn about and utilize local options for rehabilitation. We should actively and openly discuss these options with any patients known or suspected to be addicted.
- Make a valiant attempt to remove any unconscious bias against people who have become addicted to opioids. Continuing the social stigma of addiction only spurs the shame and secrecy.
Please share other ideas or suggestions you may have regarding the role hospitalists can have in curbing this growing epidemic.
References
1. “Burden of opioid crisis reached $95 billion in 2016; private sector hit hardest.” Altarum press release. Nov 16, 2017.
2. Wake Up Carolina website.
I recently attended a local Charleston (S.C.) Medical Society meeting, the theme of which was the opioid crisis. Although at the time I did not see a perfect relevance of this crisis to hospital medicine, I attended anyway, hoping to gain some pearls of wisdom regarding what my role in this epidemic could be.
I was already certainly aware of the extent of the opioid epidemic, including some startling statistics. For example, the burden of the crisis totaled $95 billion in the United States in 2016 from lost productivity and health care and criminal justice system expenses.1 But I was still not certain what my specific role could be in doing something about it.
The main speaker at the meeting was Nanci Steadman Shipman, the mother of a 19-year-old college student who had accidentally overdosed on heroin the year prior. She told the story of his upbringing, which was in an upper-middle-class suburban neighborhood, full of family, friends, and loving support. When her son was 15 years old, he suffered a leg injury during his lacrosse season, which led to a hospital stay, a surgery, and a prolonged recovery. It was during this period of time that, unbeknownst to his family, he became addicted to opioids.
Over the years, Nanci’s son found ever more creative mechanisms to procure various opioids, eventually resorting to heroin, which was remarkably cheap and easy to find. All the while in high school, he maintained good grades, remained active in sports, and had a normal social circle of friends. It was not until his first year of college that his mother started to worry that something might be wrong. In less than a year, her son quit sports, and his grades spiraled. Despite ongoing family support and extensive rehab, he suffered more than one setback and accidentally overdosed.
After her son’s death, Nanci started a nonprofit organization, Wake Up Carolina.2 Its mission is to fight drug abuse among adolescents and young adults. They use a combination of education, awareness, prevention, and recovery tactics to achieve their task. In the meantime, they try to diminish the shame and secrecy among families suffering from opioid addiction. During Nanci’s presentation at the medical society meeting, the message she conveyed to us – an audience full of physicians – was simple: We can either be part of the problem or part of the solution; we all have a duty to help and a role to play in this crisis. Whether a patient is exposed first inside the hospital or outside of it, for a short period of time or for a long one, every opioid exposure comes with a risk. Nanci’s story was incredibly affecting and made me rethink my personal role in this epidemic; how might I have contributed to this, and what could I do differently?
Shortly after her son died, her younger son suffered a femur fracture during a football game. You can imagine the horror her family felt knowing that he would need some pain medication for his acute injury. Nanci and her family were able to work with the medical and surgical teams, and through multimodal pain regimens, her son received little to no opioids during the hospital stay and was able to recover from the fracture with reasonable pain levels. She expressed gratitude that the hospital teams were willing to listen to her and her family’s concerns and offer both pharmacological and nonpharmacological therapies for her son’s recovery, which allayed their fears about opioids.
From this incredibly powerful and moving story of one family’s experience, I was able to gather some very meaningful, evidence-based, and tangible practices that I could implement in my own organization. These might even help other hospitalists take an active role in stemming this sadly growing epidemic.
As hospitalists, we should work on the following:
- Improve our personal knowledge of and skills in utilizing multimodal pain therapies regardless of the etiology of the pain. These should include both pharmacologic and nonpharmacologic interventions.
- Use consultants to assist us with difficult cases. Depending on our practice setting, we should utilize consultants who can give us good advice on nonopioid pain management regimens, such as palliative care.
- Try to influence the practice of surgeons and other specialties that consult us, to help shape prescribing patterns that include nonopioid medical regimens, and to get doctors used to entertaining nonpharmacologic pain-reducing interventions.
- Limit the volume of prescription opioids written to our patients at the time of hospital discharge. There is mounting literature that suggests leftover prescriptions can be the start of an opioid addiction for a family member.
- Educate ourselves and our patients about any local “take back” programs that allow for safe, secure, and anonymous drops of prescription medications. This may reduce opioids getting into the hands of someone who might later become addicted.
- Find out whether our hospitals or health systems have a pain or opioid oversight group or team, and if not, see whether there is interest in starting one.
- Look into local community activist programs to partner with for education, awareness, prevention, or treatment options (such as Wake Up Carolina).
- Work with local resources (for example, case management, social workers, psychiatrists) to learn about and utilize local options for rehabilitation. We should actively and openly discuss these options with any patients known or suspected to be addicted.
- Make a valiant attempt to remove any unconscious bias against people who have become addicted to opioids. Continuing the social stigma of addiction only spurs the shame and secrecy.
Please share other ideas or suggestions you may have regarding the role hospitalists can have in curbing this growing epidemic.
References
1. “Burden of opioid crisis reached $95 billion in 2016; private sector hit hardest.” Altarum press release. Nov 16, 2017.
2. Wake Up Carolina website.
Application Deadline Extended for ACS and MacLean Center Fellowships in Surgical Ethics
The American College of Surgeons (ACS) Division of Education is offering fellowships in surgical ethics with the MacLean Center for Clinical Medical Ethics, University of Chicago, IL. The MacLean Center will prepare two surgeons for careers that combine clinical surgery with scholarly studies in surgical ethics, beginning with a five-week, full-time course in Chicago in July and August 2018. From September 2018 to June 2019, fellowship recipients will meet weekly for a structured ethics curriculum. In addition, fellows will participate in an ethics consultation service and complete a research project.
For additional information about this fellowship, contact Patrice Gabler Blair, MPH, Associate Director, ACS Division of Education, at [email protected]. Application materials are now due March 15.
The American College of Surgeons (ACS) Division of Education is offering fellowships in surgical ethics with the MacLean Center for Clinical Medical Ethics, University of Chicago, IL. The MacLean Center will prepare two surgeons for careers that combine clinical surgery with scholarly studies in surgical ethics, beginning with a five-week, full-time course in Chicago in July and August 2018. From September 2018 to June 2019, fellowship recipients will meet weekly for a structured ethics curriculum. In addition, fellows will participate in an ethics consultation service and complete a research project.
For additional information about this fellowship, contact Patrice Gabler Blair, MPH, Associate Director, ACS Division of Education, at [email protected]. Application materials are now due March 15.
The American College of Surgeons (ACS) Division of Education is offering fellowships in surgical ethics with the MacLean Center for Clinical Medical Ethics, University of Chicago, IL. The MacLean Center will prepare two surgeons for careers that combine clinical surgery with scholarly studies in surgical ethics, beginning with a five-week, full-time course in Chicago in July and August 2018. From September 2018 to June 2019, fellowship recipients will meet weekly for a structured ethics curriculum. In addition, fellows will participate in an ethics consultation service and complete a research project.
For additional information about this fellowship, contact Patrice Gabler Blair, MPH, Associate Director, ACS Division of Education, at [email protected]. Application materials are now due March 15.
Register for 2018 ACS Residents as Teachers and Leaders Course
Registration is open for the 12th annual Residents as Teachers and Leaders Course hosted by the American College of Surgeons (ACS) Division of Education. The 2018 program, April 13−15 at the ACS headquarters in Chicago, IL, is designed specifically for surgery residents and will address the essential nonclinical skills—teaching and leading—that are required for success as surgeons and members of the health care team.
The course faculty, all experts in resident education, will provide an interactive learning environment. Residents will learn to lead a team more effectively, resolve conflict, be better teachers, give constructive feedback, and apply these skills during and after residency. The number of participants is limited to allow ample interaction with faculty and to facilitate networking. This course is targeted at mid- to senior-level residents, but all are welcome to attend.
Registration information and a brochure are available on the course web page at www.facs.org/education/division-of-education/courses/residents-as-teachers; the advance registration discount ends March 16. Note that last year’s course was oversubscribed, so register soon if you are interested in attending. Contact Kim Echert at [email protected] or at 312-202-5488 with any questions.
Registration is open for the 12th annual Residents as Teachers and Leaders Course hosted by the American College of Surgeons (ACS) Division of Education. The 2018 program, April 13−15 at the ACS headquarters in Chicago, IL, is designed specifically for surgery residents and will address the essential nonclinical skills—teaching and leading—that are required for success as surgeons and members of the health care team.
The course faculty, all experts in resident education, will provide an interactive learning environment. Residents will learn to lead a team more effectively, resolve conflict, be better teachers, give constructive feedback, and apply these skills during and after residency. The number of participants is limited to allow ample interaction with faculty and to facilitate networking. This course is targeted at mid- to senior-level residents, but all are welcome to attend.
Registration information and a brochure are available on the course web page at www.facs.org/education/division-of-education/courses/residents-as-teachers; the advance registration discount ends March 16. Note that last year’s course was oversubscribed, so register soon if you are interested in attending. Contact Kim Echert at [email protected] or at 312-202-5488 with any questions.
Registration is open for the 12th annual Residents as Teachers and Leaders Course hosted by the American College of Surgeons (ACS) Division of Education. The 2018 program, April 13−15 at the ACS headquarters in Chicago, IL, is designed specifically for surgery residents and will address the essential nonclinical skills—teaching and leading—that are required for success as surgeons and members of the health care team.
The course faculty, all experts in resident education, will provide an interactive learning environment. Residents will learn to lead a team more effectively, resolve conflict, be better teachers, give constructive feedback, and apply these skills during and after residency. The number of participants is limited to allow ample interaction with faculty and to facilitate networking. This course is targeted at mid- to senior-level residents, but all are welcome to attend.
Registration information and a brochure are available on the course web page at www.facs.org/education/division-of-education/courses/residents-as-teachers; the advance registration discount ends March 16. Note that last year’s course was oversubscribed, so register soon if you are interested in attending. Contact Kim Echert at [email protected] or at 312-202-5488 with any questions.
Humeral Bone Loss in Revision Shoulder Arthroplasty
ABSTRACT
Revision shoulder arthroplasty is becoming more prevalent as the rate of primary shoulder arthroplasty in the US continues to increase. The management of proximal humeral bone loss in the revision setting presents a difficult problem without a clear solution. Different preoperative diagnoses often lead to distinctly different patterns of bone loss. Successful management of these cases requires a clear understanding of the normal anatomy of the proximal humerus, as well as structural limitations imposed by significant bone loss and the effect this loss has on component fixation. Our preferred technique differs depending on the pattern of bone loss encountered. The use of allograft-prosthetic composites, the cement-within-cement technique, and combinations of these strategies comprise the mainstay of our treatment algorithm. This article focuses on indications, surgical techniques, and some of the published outcomes using these strategies in the management of proximal humeral bone loss.
Continue to: The demand for shoulder arthroplasty...
The demand for shoulder arthroplasty (SA) has increased significantly over the past decade, with a 200% increase witnessed from 2011 to 2015.1 SA performed in patients younger than 55 years is expected to increase 333% between 2011 to 2030.2 With increasing rates of SA being performed in younger patient populations, rates of revision SA also can be expected to climb. Revision to reverse shoulder arthroplasty (RSA) has arisen as a viable option in these patients, and multiple studies demonstrate excellent outcomes that can be obtained with RSA.3-11
Despite significant improvements obtained in revision SA since the mainstream acceptance of RSA, bone loss remains a problematic issue. Loss of humeral bone stock, in particular, can be a challenging problem to solve with multiple clinical implications. Biomechanical studies have demonstrated that if bone loss is left unaddressed, increased bending and torsional forces on the prosthesis result, which ultimately contribute to increased micromotion and eventual component failure.12 In addition, existing challenges are associated with the lack of attachment sites for both multiple muscles and tendons. Also, there is a loss of the normal lateralized pull of the deltoid, which results in a decreased amount of force generated by this muscle.13,14 Ultimately, the increased loss of bone can lead to a devastating situation where there is not enough bone to provide adequate fixation while maintaining the appropriate humeral length necessary to achieve stability of the articulation, which will inevitably lead to instability.4,15 Therefore, techniques are needed to address proximal humeral bone loss while maintaining as much native humeral bone as possible.
PROXIMAL HUMERUS: ANATOMICAL CONSIDERATIONS
The anatomy of the proximal humerus has been studied in great detail and reported in a number of different studies.16-23 The average humeral head thickness (24 mm in men and 19 mm in women) and offset relative to the humeral shaft (2.1 mm posterior and 6.6 mm medial) act to tension the rotator cuff musculature appropriately and contribute to a wrapping effect that allows the deltoid to function more effectively.13,14 Knowledge regarding the rotator cuff footprint has advanced over the past 10 years, specifically with regard to the supraspinatus and infraspinatus.24 The current belief is that the supraspinatus has a triangular insertion onto the most anterior aspect of the greater tuberosity, with a maximum medial-to-lateral length of 6.9 mm and a maximum anterior-to-posterior width of 12.6 mm. The infraspinatus insertion has a trapezoidal insertion, with a maximum medial-to-lateral length of 10.2 mm and anterior-to-posterior width of 32.7 mm. The subscapularis, by far the largest of all the rotator cuff muscles, has a complex geometry with regard to its insertion on the lesser tuberosity, with 4 different insertion points and an overall lateral footprint measuring 37.6 mm and a medial footprint measuring 40.7 mm.25 Finally, the teres minor, with the smallest volume of all the rotator cuff muscles, inserts immediately inferior to the infraspinatus along the inferior facet of the greater tuberosity.26
Aside from the rotator cuff, there are various other muscles and tendons that insert about the proximal humerus and are essential for normal function. The deltoid, which inserts at a point approximately 6 cm from the greater tuberosity along the length of the humerus, with an insertion length between 5 cm to 7 cm,13,27 is the primary mover of the shoulder and essential for proper function after RSA.28,29 The pectoralis major tendon, which begins inserting at a point approximately 5.6 cm from the humeral head and spans a distance of 7.7 cm along the length of the humerus,30-32 is important not only for function but as an anatomical landmark in reconstruction. Lastly, the latissimus dorsi and teres major, which share a role in extension, adduction, and internal rotation of the glenohumeral joint, insert along the floor and medial lip of the intertubercular groove of the humerus, respectively.33,34 In addition to their role in tendon transfer procedures because of treating irreparable posterosuperior cuff and subscapularis tears,35,36 it has been suggested that these tendons may play some role in glenohumeral joint stability.37
In addition to the loss of muscular attachments, the absence of proximal humeral bone stock, in and of itself, can have deleterious effects on fixation of the humeral component. RSA is a semiconstrained device, which results in increased transmission of forces to the interface between the humeral implant and its surrounding structures, including cement (when present) and the bone itself. When there is the absence of significant amounts of bone, the remaining bone must now account for an even higher proportion of these forces. A previous biomechanical study showed that cemented humeral stems demonstrated significantly increased micromotion in the presence of proximal humeral bone loss, particularly when a modular humeral component was used.12
Continue to: TYPES OF BONE LOSS
TYPES OF BONE LOSS
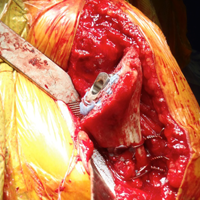
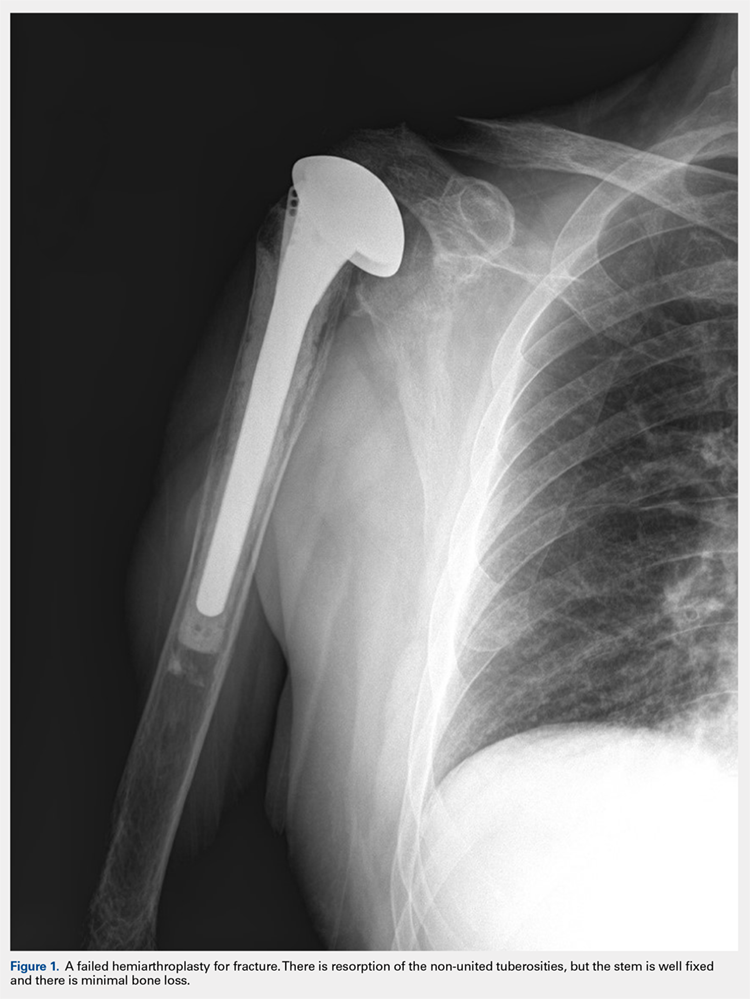
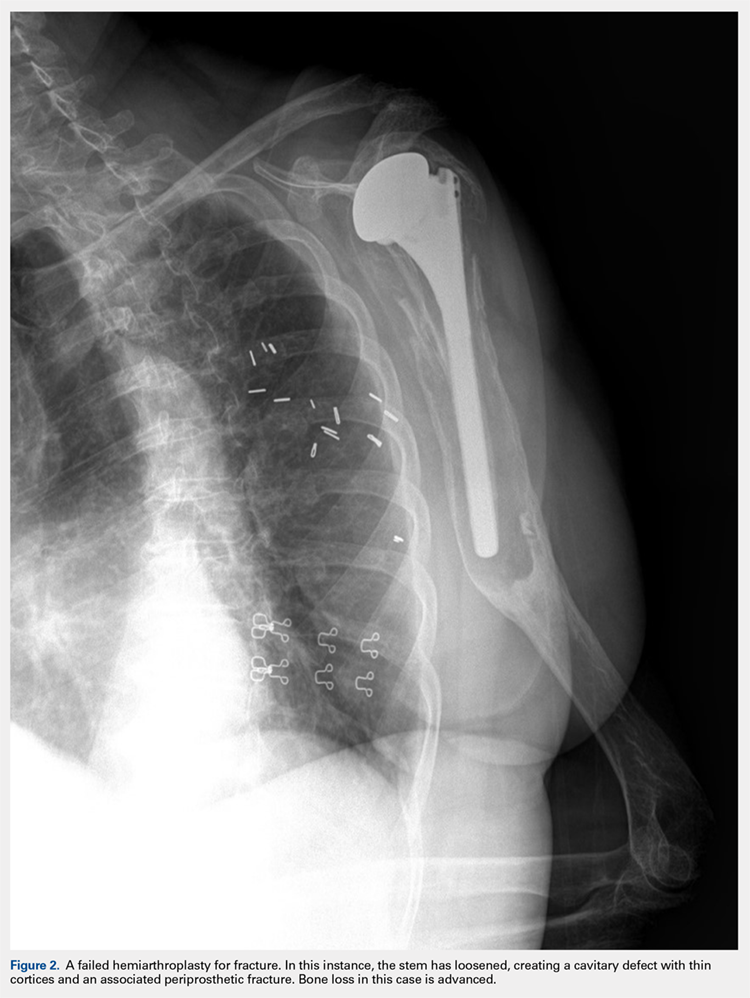
There are a variety of different etiologies of proximal humeral bone loss that result in distinctly different clinical presentations. These can be fairly mild, as is the case of isolated resorption of the greater tuberosity in a non-united proximal humerus fracture (Figure 1). Alternatively, they can be severe, as seen in a grossly loose cemented long-stemmed component that is freely mobile, creating a windshield-wiper effect throughout the length of the humerus (Figure 2). This can be somewhat deceiving, however, as the amount of bone loss, as well as the pathophysiologic process that led to the bone loss, are important factors to determine ideal reconstructive methods. In the case of a failed open reduction internal fixation, where the tuberosity has failed to unite or has been resected, there is much less of a biologic response in comparison with implant loosening associated with osteolysis. This latter condition will be associated with a much more destructive inflammatory response resulting in poor tissue quality and often dramatic thinning of the cortex. If one simply measured the distance from the most proximal remaining bone stock to the area where the greater tuberosity should be, a loose stem with subsidence and ballooning of the cortices may appear to have a similar amount of bone loss as a failed hemiarthroplasty for fracture with a well-fixed stem. However, intraoperatively, one will find that the bone that appeared to be present radiographically in the case of the loose stem is of such poor quality that it cannot reasonably provide any beneficial fixation. In light of this, different treatment modalities are recommended for different types of bone loss, and the revision surgeon must be able to anticipate this and possess a full armamentarium of options to treat these challenging cases successfully.
INDICATIONS
Our technique to manage proximal humeral bone loss is dependent on both the quantity of bone loss, which can be measured radiographically, as well as the anticipated inflammatory response described above. As both the destructive process and the amount of bone loss increase, the importance of more advanced reconstructive procedures that will sustain implant security and soft-tissue management becomes apparent. In the least destructive cases with <5 cm of bone loss, successful revision can typically be accomplished with stem removal and placement of a new monoblock humeral stem. In cases where more advanced destructive pathology is present, and bone loss is >5 cm, an allograft-prosthetic composite (APC) is typically used. In both scenarios, if the stem being revised is cemented and the cement mantle remains intact, and of reasonable length, consideration is given to the cement-within-cement technique. Finally, in the most destructive cases where bone loss exceeds 10 cm and a large biological response is anticipated (eg, periprosthetic fractures with humeral loosening), the use of a longer diaphyseal-incorporating APC is often necessary. This prosthetic composite can be combined with a cement-within-cement technique as well.
It is important to comment on the implications of using modular stems in this setting. With advanced bone loss, a situation is often encountered where the newly implanted stem geometry and working length may be insufficient to acquire adequate rotational stability. In this setting, if a modular junction is positioned close to the stem and cement/bone interface, it will be exposed to very high stress concentrations which can lead to component fracture38 as well as taper corrosion, also referred to as trunnionosis. This latter phenomenon, which has been well studied in the total hip arthroplasty literature with the use of modular components,39 is especially relevant given the high torsional loads imparted at the modular junction. Ultimately, high torsional loads lead to micromotion and electrochemical ion release via degradation of the passivation layer, initiating the process of mechanically assisted crevice corrosion.40 For these reasons, when a modular stem must be used in the presence of mild to moderate bone loss, using a proximal humeral allograft to protect the junction or to provide additional fixation may be implemented with a lower threshold than when using a monoblock stem.
SURGICAL TECHNIQUE: ALLOGRAFT-PROSTHETIC COMPOSITES
A standard deltopectoral approach is used, taking care to preserve all viable muscular attachments to the proximal humerus. After removal of the prosthetic humeral head, the decision to proceed with removal of the stem at this juncture is based on several factors. If the remaining proximal humeral bone is so compromised that it might not be able to withstand the forces exerted upon it during retraction for glenoid exposure, the component is left in place. Additionally, if there is consideration that the glenoid-sided bone loss may be so severe that a glenoid baseplate cannot be implanted, and the stem remains well fixed, it is retained so that it can be converted to a hemiarthroplasty.
If neither of the above issues is present, the humeral stem is removed. If a well-fixed press-fit stem is in place, it is typically removed using a combination of burrs and osteotomes to disrupt the bone-implant interface, and the stem is then carefully removed using an impactor and mallet. If a cemented stem is present, the stem is removed in a similar manner, and the cement mantle is left in place if stable, in anticipation of a cement-within-cement technique. If the mantle is disrupted, standard cement removal instruments are used to remove all cement from the canal meticulously.
Continue to: Management of the glenoid...
Management of the glenoid can have significant implications with regard to the humerus. Most notably, the size of the glenosphere has direct implications on the fixation of the humeral component. Use of larger diameter glenospheres result in increased contact area between the glenosphere and humerosocket, adding constraint to the articulation and further increasing the stresses at the implant-bone interface. As such, the use of larger glenospheres to prevent instability must be balanced with the resulting implications on humeral component fixation, especially in cases of severe bone loss.
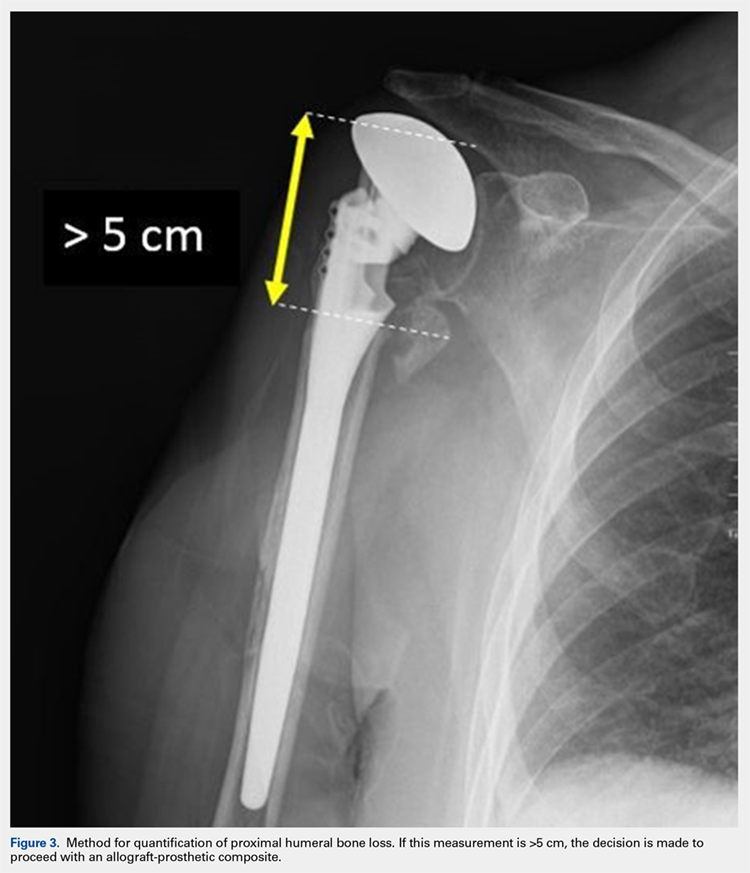
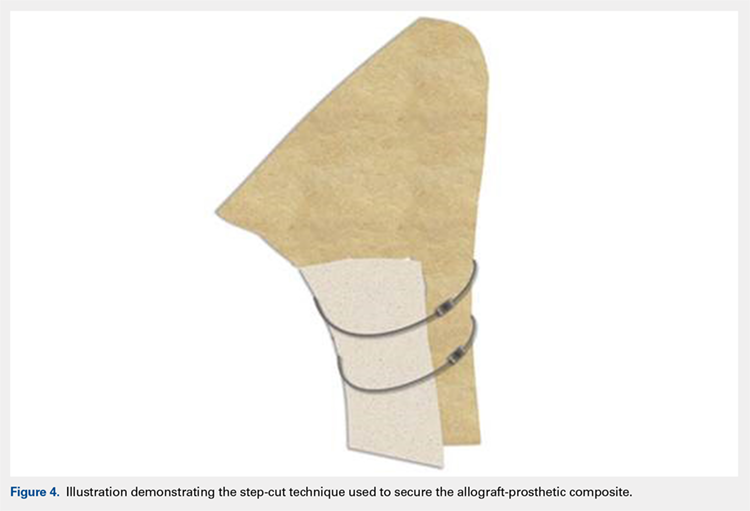
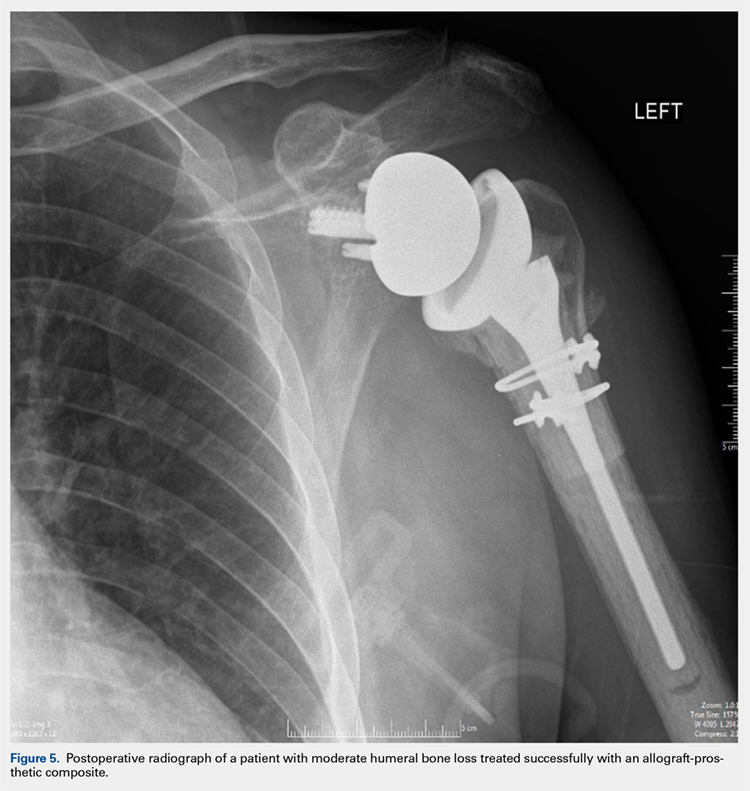
After implanting the appropriate glenosphere, attention is then turned back to the humerus. Trial implants are sequentially used to obtain adequate humeral length and stability. Once this is accomplished, the amount of humeral bone loss is quantified by measuring the distance from the superior aspect of the medial humeral tray to the medial humeral shaft. If this number is >5 cm (Figure 3), the decision is made to proceed with an APC. The allograft humeral head is cut, cancellous bone is removed, and a step-cut is performed, with the medial portion of the allograft measuring the same length as that of bone loss and the lateral plate extending an additional several centimeters distally (Figure 4). Additional soft tissue is removed from the allograft, leaving the subscapularis stump intact for later repair with the patient’s native tissue. The allograft is secured to the patient’s proximal humerus using multiple cerclage wires, and the humeral stem is cemented into place. The final construct is shown in Figure 5.
ADDITIONAL CONSIDERATIONS: CASES OF ADVANCED BONE LOSS
In cases of advanced humeral bone loss, as is often seen when revising loose humeral stems, larger allografts that span a significant length of the diaphysis are often required. This type of bone loss has implications with regard to how the deltoid insertion is managed. Interestingly, even in situations when the vast majority of the remaining diaphysis consists of ectatic egg-shell bone, the deltoid tuberosity remains of fairly substantial quality due to the continued pull of the muscular insertion on this area. This fragment is isolated, carefully mobilized, and subsequently repaired back on top of the allograft using cables.
POSTOPERATIVE CARE
Patients are kept in a shoulder immobilizer for 6 weeks after surgery to facilitate allograft incorporation and subscapularis tendon healing. During this time, pendulum exercises are initiated. Active assisted range of motion (ROM) exercises begin after 6 weeks, consisting of supine forward elevation. A sling is given to be used in public. Light strengthening exercises begin at 3 months postoperatively.
DISCUSSION
In cases of mild to moderate proximal humeral bone loss, RSA using a long-stem humeral component without allograft augmentation is a viable option. Budge and colleagues38 demonstrated excellent results in a population of 15 patients with an average of 38 mm of proximal humeral bone loss without use of allografts. Interestingly, they noted 1 case of component fracture in a modular prosthesis and therefore concluded that monoblock humeral stems should be used in the absence of allograft augmentation.
Continue to: In more advanced cases of bone loss...
In more advanced cases of bone loss, our data shows that use of APCs can result in equally satisfactory results. In a series of 25 patients with an average bone loss of 54 mm, patients were able to achieve statistically significant improvements in pain, ROM, and function with high rates of allograft incorporation.9 Overall, a low rate of complications was noted, including 1 infection. This finding is consistent with an additional study looking specifically at factors associated with infection in revision SA, which found that the use of allografts was not associated with increased risk of infection.41
As stated previously, the size of allograft needed for the APC construct is related to the distinct pathology encountered. In our experience, we have noted that well-fixed stems can be treated with short metaphyseal APCs in 85% of cases. On the other hand, loose stems require long allografts measuring >10 cm in 90% of cases. As such, these cases typically require mobilization of the deltoid insertion as described above, and therefore it is important that the surgeon is prepared for this aspect of the procedure preoperatively.
Finally, the cement-within-cement technique, originally popularized for use in revision total hip arthroplasty, has demonstrated reliable results when utilized in revision SA.42 To date, there are no recommendations regarding the minimal length of existing cement mantle that is needed to perform this technique. In situations in which the length of the cement mantle is questionable, our preference is to combine the cement-within-cement technique with an APC when possible.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi:10.1016/j.jse.2010.02.009.
2. Padegimas EM, Maltenfort M, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res. 2015;473(6):1860-1867. doi:10.1007/s11999-015-4231-z.
3. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522. doi:10.1016/j.jse.2011.03.006.
4. Levy JC, Virani N, Pupello D, et al. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195. doi:10.1302/0301-620X.89B2.
5. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
6. Deutsch A, Abboud JA, Kelly J, et al. Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg. 2007;16(6):706-716. doi:10.1016/j.jse.2007.01.007.
7. Kelly JD, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
8. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJP. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042. doi:10.1016/j.jse.2014.02.019.
9. Composite P, Chacon BA, Virani N, et al. Revision arthroplasty with use of a reverse shoulder. J Bone Joint Surg. 2009;1:119-127. doi:10.2106/JBJS.H.00094.
10. Klein SM, Dunning P, Mulieri P, Pupello D, Downes K, Frankle MA. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1144-1154. doi:10.2106/JBJS.I.00778.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
12. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
13. Morgan SJ, Furry K, Parekh A, Agudelo JF, Smith WR. The deltoid muscle: an anatomic description of the deltoid insertion to the proximal humerus. J Orthop Trauma. 2006;20(1):19-21. doi:10.1097/01.bot.0000187063.43267.18.
14. Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop Relat Res. 2000;375:250-257. doi:10.1097/00003086-200006000-00030.
15. De Wilde L, Plasschaert F. Prosthetic treatment and functional recovery of the shoulder after tumor resection 10 years ago: a case report. J Shoulder Elbow Surg. 2005;14(6):645-649. doi:10.1016/j.jse.2004.11.001.
16. Wataru S, Kazuomi S, Yoshikazu N, Hiroaki I, Takaharu Y, Hideki Y. Three-dimensional morphological analysis of humeral heads: a study in cadavers. Acta Orthop. 2005;76(3):392-396. doi:10.1080/00016470510030878.
17. Tillett E, Smith M, Fulcher M, Shanklin J. Anatomic determination of humeral head retroversion: the relationship of the central axis of the humeral head to the bicipital groove. J Shoulder Elbow Surg. 1993;2(5):255-256. doi:10.1016/S1058-2746(09)80085-2.
18. Doyle AJ, Burks RT. Comparison of humeral head retroversion with the humeral axis/biceps groove relationship: a study in live subjects and cadavers. J Shoulder Elbow Surg. 1998;7(5):453-457. doi:10.1016/S1058-2746(98)90193-8.
19. Johnson JW, Thostenson JD, Suva LJ, Hasan SA. Relationship of bicipital groove rotation with humeral head retroversion: a three-dimensional computed tomographic analysis. J Bone Joint Surg Am. 2013;95(8):719-724. doi:10.2106/JBJS.J.00085.
20. Hromádka R, Kuběna AA, Pokorný D, Popelka S, Jahoda D, Sosna A. Lesser tuberosity is more reliable than bicipital groove when determining orientation of humeral head in primary shoulder arthroplasty. Surg Radiol Anat. 2010;32(1):31-37. doi:10.1007/s00276-009-0543-6.
21. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338. doi:10.1067/mse.2002.124429.
22. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(suppl 1):99-104. doi:10.1016/j.jse.2004.09.025.
23. Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg Am. 2000;82-A(11):1594-1602.
24. Mochizuki T, Sugaya H, Uomizu M, et al. Humeral insertion of the supraspinatus and infraspinatus. J Bone Joint Surg Am. 2008;90(5):962-969. doi:10.2106/JBJS.G.00427.
25. Arai R, Sugaya H, Mochizuki T, Nimura A, Moriishi J, Akita K. Subscapularis tendon tear: an anatomic and clinical investigation. Arthroscopy. 2008;24(9):997-1004. doi:10.1016/j.arthro.2008.04.076.
26. Nimura A, Kato A, Yamaguchi K, et al. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg. 2012;21(7):867-872. doi:10.1016/j.jse.2011.04.034.
27. Rispoli DM, Athwal GS, Sperling JW, Cofield RH. The anatomy of the deltoid insertion. J Shoulder Elbow Surg. 2009;18(3):386-390. doi:10.1016/j.jse.2008.10.012.
28. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364. doi:10.1016/j.jse.2012.02.002.
29. Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clinical Orthop Relat Res. 2011;469(9):2440-2451. doi:10.1007/s11999-011-1892-0.
30. Torrens C, Corrales M, Melendo E, Solano A, Rodríguez-Baeza A, Cáceres E. The pectoralis major tendon as a reference for restoring humeral length and retroversion with hemiarthroplasty for fracture. J Shoulder Elbow Surg. 2008;17(6):947-950. doi:10.1016/j.jse.2008.05.041.
31. Ponce BA, Thompson KJ, Rosenzweig SD, et al. Re-evaluation of pectoralis major height as an anatomic reference for humeral height in fracture hemiarthroplasty. J Shoulder Elbow Surg. 2013;22(11):1567-1572. doi:10.1016/j.jse.2013.01.039.
32. LaFrance R, Madsen W, Yaseen Z, Giordano B, Maloney M, Voloshin I. Relevant anatomic landmarks and measurements for biceps tenodesis. Am J Sports Med. 2013;41(6):1395-1399. doi:10.1177/0363546513482297.
33. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
34. Bhatt CR, Prajapati B, Patil DS, Patel VD, Singh BGP, Mehta CD. Variation in the insertion of the latissimus dorsi & its clinical importance. J Orthop. 2013;10(1):25-28. doi:10.1016/j.jor.2013.01.002.
35. Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg. 2006;88(1):113-120. doi:10.2106/JBJS.E.00282.
36. Elhassan B, Christensen TJ, Wagner ER. Feasibility of latissimus and teres major transfer to reconstruct irreparable subscapularis tendon tear: an anatomic study. J Shoulder Elbow Surg. 2014;23(4):492-499. doi:10.1016/j.jse.2013.07.046.
37. Pouliart N, Gagey O. Significance of the latissimus dorsi for shoulder instability. II. Its influence on dislocation behavior in a sequential cutting protocol of the glenohumeral capsule. Clin Anat. 2005;18(7):500-509. doi:10.1002/ca.20181.
38. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
39. Weiser MC, Lavernia CJ. Trunnionosis in total hip arthroplasty. J Bone Joint Surg Am. 2017;99(17):27-29. doi:10.2106/JBJS.17.00345.
40. Cohen J. Current concepts review. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(10):1554.
41. Meijer ST, Paulino Pereira NR, Nota SPFT, Ferrone ML, Schwab JH, Lozano Calderón SA. Factors associated with infection after reconstructive shoulder surgery for proximal humerus tumors. J Shoulder Elbow Surg. 2017;26(6):931-938. doi:10.1016/j.jse.2016.10.014.
42. Wagner ER, Houdek MT, Hernandez NM, Cofield RH, Sánchez-Sotelo J, Sperling JW. Cement-within-cement technique in revision reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(8):1448-1453. doi:10.1016/j.jse.2017.01.013.
ABSTRACT
Revision shoulder arthroplasty is becoming more prevalent as the rate of primary shoulder arthroplasty in the US continues to increase. The management of proximal humeral bone loss in the revision setting presents a difficult problem without a clear solution. Different preoperative diagnoses often lead to distinctly different patterns of bone loss. Successful management of these cases requires a clear understanding of the normal anatomy of the proximal humerus, as well as structural limitations imposed by significant bone loss and the effect this loss has on component fixation. Our preferred technique differs depending on the pattern of bone loss encountered. The use of allograft-prosthetic composites, the cement-within-cement technique, and combinations of these strategies comprise the mainstay of our treatment algorithm. This article focuses on indications, surgical techniques, and some of the published outcomes using these strategies in the management of proximal humeral bone loss.
Continue to: The demand for shoulder arthroplasty...
The demand for shoulder arthroplasty (SA) has increased significantly over the past decade, with a 200% increase witnessed from 2011 to 2015.1 SA performed in patients younger than 55 years is expected to increase 333% between 2011 to 2030.2 With increasing rates of SA being performed in younger patient populations, rates of revision SA also can be expected to climb. Revision to reverse shoulder arthroplasty (RSA) has arisen as a viable option in these patients, and multiple studies demonstrate excellent outcomes that can be obtained with RSA.3-11
Despite significant improvements obtained in revision SA since the mainstream acceptance of RSA, bone loss remains a problematic issue. Loss of humeral bone stock, in particular, can be a challenging problem to solve with multiple clinical implications. Biomechanical studies have demonstrated that if bone loss is left unaddressed, increased bending and torsional forces on the prosthesis result, which ultimately contribute to increased micromotion and eventual component failure.12 In addition, existing challenges are associated with the lack of attachment sites for both multiple muscles and tendons. Also, there is a loss of the normal lateralized pull of the deltoid, which results in a decreased amount of force generated by this muscle.13,14 Ultimately, the increased loss of bone can lead to a devastating situation where there is not enough bone to provide adequate fixation while maintaining the appropriate humeral length necessary to achieve stability of the articulation, which will inevitably lead to instability.4,15 Therefore, techniques are needed to address proximal humeral bone loss while maintaining as much native humeral bone as possible.
PROXIMAL HUMERUS: ANATOMICAL CONSIDERATIONS
The anatomy of the proximal humerus has been studied in great detail and reported in a number of different studies.16-23 The average humeral head thickness (24 mm in men and 19 mm in women) and offset relative to the humeral shaft (2.1 mm posterior and 6.6 mm medial) act to tension the rotator cuff musculature appropriately and contribute to a wrapping effect that allows the deltoid to function more effectively.13,14 Knowledge regarding the rotator cuff footprint has advanced over the past 10 years, specifically with regard to the supraspinatus and infraspinatus.24 The current belief is that the supraspinatus has a triangular insertion onto the most anterior aspect of the greater tuberosity, with a maximum medial-to-lateral length of 6.9 mm and a maximum anterior-to-posterior width of 12.6 mm. The infraspinatus insertion has a trapezoidal insertion, with a maximum medial-to-lateral length of 10.2 mm and anterior-to-posterior width of 32.7 mm. The subscapularis, by far the largest of all the rotator cuff muscles, has a complex geometry with regard to its insertion on the lesser tuberosity, with 4 different insertion points and an overall lateral footprint measuring 37.6 mm and a medial footprint measuring 40.7 mm.25 Finally, the teres minor, with the smallest volume of all the rotator cuff muscles, inserts immediately inferior to the infraspinatus along the inferior facet of the greater tuberosity.26
Aside from the rotator cuff, there are various other muscles and tendons that insert about the proximal humerus and are essential for normal function. The deltoid, which inserts at a point approximately 6 cm from the greater tuberosity along the length of the humerus, with an insertion length between 5 cm to 7 cm,13,27 is the primary mover of the shoulder and essential for proper function after RSA.28,29 The pectoralis major tendon, which begins inserting at a point approximately 5.6 cm from the humeral head and spans a distance of 7.7 cm along the length of the humerus,30-32 is important not only for function but as an anatomical landmark in reconstruction. Lastly, the latissimus dorsi and teres major, which share a role in extension, adduction, and internal rotation of the glenohumeral joint, insert along the floor and medial lip of the intertubercular groove of the humerus, respectively.33,34 In addition to their role in tendon transfer procedures because of treating irreparable posterosuperior cuff and subscapularis tears,35,36 it has been suggested that these tendons may play some role in glenohumeral joint stability.37
In addition to the loss of muscular attachments, the absence of proximal humeral bone stock, in and of itself, can have deleterious effects on fixation of the humeral component. RSA is a semiconstrained device, which results in increased transmission of forces to the interface between the humeral implant and its surrounding structures, including cement (when present) and the bone itself. When there is the absence of significant amounts of bone, the remaining bone must now account for an even higher proportion of these forces. A previous biomechanical study showed that cemented humeral stems demonstrated significantly increased micromotion in the presence of proximal humeral bone loss, particularly when a modular humeral component was used.12
Continue to: TYPES OF BONE LOSS
TYPES OF BONE LOSS
There are a variety of different etiologies of proximal humeral bone loss that result in distinctly different clinical presentations. These can be fairly mild, as is the case of isolated resorption of the greater tuberosity in a non-united proximal humerus fracture (Figure 1). Alternatively, they can be severe, as seen in a grossly loose cemented long-stemmed component that is freely mobile, creating a windshield-wiper effect throughout the length of the humerus (Figure 2). This can be somewhat deceiving, however, as the amount of bone loss, as well as the pathophysiologic process that led to the bone loss, are important factors to determine ideal reconstructive methods. In the case of a failed open reduction internal fixation, where the tuberosity has failed to unite or has been resected, there is much less of a biologic response in comparison with implant loosening associated with osteolysis. This latter condition will be associated with a much more destructive inflammatory response resulting in poor tissue quality and often dramatic thinning of the cortex. If one simply measured the distance from the most proximal remaining bone stock to the area where the greater tuberosity should be, a loose stem with subsidence and ballooning of the cortices may appear to have a similar amount of bone loss as a failed hemiarthroplasty for fracture with a well-fixed stem. However, intraoperatively, one will find that the bone that appeared to be present radiographically in the case of the loose stem is of such poor quality that it cannot reasonably provide any beneficial fixation. In light of this, different treatment modalities are recommended for different types of bone loss, and the revision surgeon must be able to anticipate this and possess a full armamentarium of options to treat these challenging cases successfully.

INDICATIONS
Our technique to manage proximal humeral bone loss is dependent on both the quantity of bone loss, which can be measured radiographically, as well as the anticipated inflammatory response described above. As both the destructive process and the amount of bone loss increase, the importance of more advanced reconstructive procedures that will sustain implant security and soft-tissue management becomes apparent. In the least destructive cases with <5 cm of bone loss, successful revision can typically be accomplished with stem removal and placement of a new monoblock humeral stem. In cases where more advanced destructive pathology is present, and bone loss is >5 cm, an allograft-prosthetic composite (APC) is typically used. In both scenarios, if the stem being revised is cemented and the cement mantle remains intact, and of reasonable length, consideration is given to the cement-within-cement technique. Finally, in the most destructive cases where bone loss exceeds 10 cm and a large biological response is anticipated (eg, periprosthetic fractures with humeral loosening), the use of a longer diaphyseal-incorporating APC is often necessary. This prosthetic composite can be combined with a cement-within-cement technique as well.

It is important to comment on the implications of using modular stems in this setting. With advanced bone loss, a situation is often encountered where the newly implanted stem geometry and working length may be insufficient to acquire adequate rotational stability. In this setting, if a modular junction is positioned close to the stem and cement/bone interface, it will be exposed to very high stress concentrations which can lead to component fracture38 as well as taper corrosion, also referred to as trunnionosis. This latter phenomenon, which has been well studied in the total hip arthroplasty literature with the use of modular components,39 is especially relevant given the high torsional loads imparted at the modular junction. Ultimately, high torsional loads lead to micromotion and electrochemical ion release via degradation of the passivation layer, initiating the process of mechanically assisted crevice corrosion.40 For these reasons, when a modular stem must be used in the presence of mild to moderate bone loss, using a proximal humeral allograft to protect the junction or to provide additional fixation may be implemented with a lower threshold than when using a monoblock stem.
SURGICAL TECHNIQUE: ALLOGRAFT-PROSTHETIC COMPOSITES
A standard deltopectoral approach is used, taking care to preserve all viable muscular attachments to the proximal humerus. After removal of the prosthetic humeral head, the decision to proceed with removal of the stem at this juncture is based on several factors. If the remaining proximal humeral bone is so compromised that it might not be able to withstand the forces exerted upon it during retraction for glenoid exposure, the component is left in place. Additionally, if there is consideration that the glenoid-sided bone loss may be so severe that a glenoid baseplate cannot be implanted, and the stem remains well fixed, it is retained so that it can be converted to a hemiarthroplasty.
If neither of the above issues is present, the humeral stem is removed. If a well-fixed press-fit stem is in place, it is typically removed using a combination of burrs and osteotomes to disrupt the bone-implant interface, and the stem is then carefully removed using an impactor and mallet. If a cemented stem is present, the stem is removed in a similar manner, and the cement mantle is left in place if stable, in anticipation of a cement-within-cement technique. If the mantle is disrupted, standard cement removal instruments are used to remove all cement from the canal meticulously.
Continue to: Management of the glenoid...
Management of the glenoid can have significant implications with regard to the humerus. Most notably, the size of the glenosphere has direct implications on the fixation of the humeral component. Use of larger diameter glenospheres result in increased contact area between the glenosphere and humerosocket, adding constraint to the articulation and further increasing the stresses at the implant-bone interface. As such, the use of larger glenospheres to prevent instability must be balanced with the resulting implications on humeral component fixation, especially in cases of severe bone loss.

After implanting the appropriate glenosphere, attention is then turned back to the humerus. Trial implants are sequentially used to obtain adequate humeral length and stability. Once this is accomplished, the amount of humeral bone loss is quantified by measuring the distance from the superior aspect of the medial humeral tray to the medial humeral shaft. If this number is >5 cm (Figure 3), the decision is made to proceed with an APC. The allograft humeral head is cut, cancellous bone is removed, and a step-cut is performed, with the medial portion of the allograft measuring the same length as that of bone loss and the lateral plate extending an additional several centimeters distally (Figure 4). Additional soft tissue is removed from the allograft, leaving the subscapularis stump intact for later repair with the patient’s native tissue. The allograft is secured to the patient’s proximal humerus using multiple cerclage wires, and the humeral stem is cemented into place. The final construct is shown in Figure 5.

ADDITIONAL CONSIDERATIONS: CASES OF ADVANCED BONE LOSS
In cases of advanced humeral bone loss, as is often seen when revising loose humeral stems, larger allografts that span a significant length of the diaphysis are often required. This type of bone loss has implications with regard to how the deltoid insertion is managed. Interestingly, even in situations when the vast majority of the remaining diaphysis consists of ectatic egg-shell bone, the deltoid tuberosity remains of fairly substantial quality due to the continued pull of the muscular insertion on this area. This fragment is isolated, carefully mobilized, and subsequently repaired back on top of the allograft using cables.

POSTOPERATIVE CARE
Patients are kept in a shoulder immobilizer for 6 weeks after surgery to facilitate allograft incorporation and subscapularis tendon healing. During this time, pendulum exercises are initiated. Active assisted range of motion (ROM) exercises begin after 6 weeks, consisting of supine forward elevation. A sling is given to be used in public. Light strengthening exercises begin at 3 months postoperatively.
DISCUSSION
In cases of mild to moderate proximal humeral bone loss, RSA using a long-stem humeral component without allograft augmentation is a viable option. Budge and colleagues38 demonstrated excellent results in a population of 15 patients with an average of 38 mm of proximal humeral bone loss without use of allografts. Interestingly, they noted 1 case of component fracture in a modular prosthesis and therefore concluded that monoblock humeral stems should be used in the absence of allograft augmentation.
Continue to: In more advanced cases of bone loss...
In more advanced cases of bone loss, our data shows that use of APCs can result in equally satisfactory results. In a series of 25 patients with an average bone loss of 54 mm, patients were able to achieve statistically significant improvements in pain, ROM, and function with high rates of allograft incorporation.9 Overall, a low rate of complications was noted, including 1 infection. This finding is consistent with an additional study looking specifically at factors associated with infection in revision SA, which found that the use of allografts was not associated with increased risk of infection.41
As stated previously, the size of allograft needed for the APC construct is related to the distinct pathology encountered. In our experience, we have noted that well-fixed stems can be treated with short metaphyseal APCs in 85% of cases. On the other hand, loose stems require long allografts measuring >10 cm in 90% of cases. As such, these cases typically require mobilization of the deltoid insertion as described above, and therefore it is important that the surgeon is prepared for this aspect of the procedure preoperatively.
Finally, the cement-within-cement technique, originally popularized for use in revision total hip arthroplasty, has demonstrated reliable results when utilized in revision SA.42 To date, there are no recommendations regarding the minimal length of existing cement mantle that is needed to perform this technique. In situations in which the length of the cement mantle is questionable, our preference is to combine the cement-within-cement technique with an APC when possible.
ABSTRACT
Revision shoulder arthroplasty is becoming more prevalent as the rate of primary shoulder arthroplasty in the US continues to increase. The management of proximal humeral bone loss in the revision setting presents a difficult problem without a clear solution. Different preoperative diagnoses often lead to distinctly different patterns of bone loss. Successful management of these cases requires a clear understanding of the normal anatomy of the proximal humerus, as well as structural limitations imposed by significant bone loss and the effect this loss has on component fixation. Our preferred technique differs depending on the pattern of bone loss encountered. The use of allograft-prosthetic composites, the cement-within-cement technique, and combinations of these strategies comprise the mainstay of our treatment algorithm. This article focuses on indications, surgical techniques, and some of the published outcomes using these strategies in the management of proximal humeral bone loss.
Continue to: The demand for shoulder arthroplasty...
The demand for shoulder arthroplasty (SA) has increased significantly over the past decade, with a 200% increase witnessed from 2011 to 2015.1 SA performed in patients younger than 55 years is expected to increase 333% between 2011 to 2030.2 With increasing rates of SA being performed in younger patient populations, rates of revision SA also can be expected to climb. Revision to reverse shoulder arthroplasty (RSA) has arisen as a viable option in these patients, and multiple studies demonstrate excellent outcomes that can be obtained with RSA.3-11
Despite significant improvements obtained in revision SA since the mainstream acceptance of RSA, bone loss remains a problematic issue. Loss of humeral bone stock, in particular, can be a challenging problem to solve with multiple clinical implications. Biomechanical studies have demonstrated that if bone loss is left unaddressed, increased bending and torsional forces on the prosthesis result, which ultimately contribute to increased micromotion and eventual component failure.12 In addition, existing challenges are associated with the lack of attachment sites for both multiple muscles and tendons. Also, there is a loss of the normal lateralized pull of the deltoid, which results in a decreased amount of force generated by this muscle.13,14 Ultimately, the increased loss of bone can lead to a devastating situation where there is not enough bone to provide adequate fixation while maintaining the appropriate humeral length necessary to achieve stability of the articulation, which will inevitably lead to instability.4,15 Therefore, techniques are needed to address proximal humeral bone loss while maintaining as much native humeral bone as possible.
PROXIMAL HUMERUS: ANATOMICAL CONSIDERATIONS
The anatomy of the proximal humerus has been studied in great detail and reported in a number of different studies.16-23 The average humeral head thickness (24 mm in men and 19 mm in women) and offset relative to the humeral shaft (2.1 mm posterior and 6.6 mm medial) act to tension the rotator cuff musculature appropriately and contribute to a wrapping effect that allows the deltoid to function more effectively.13,14 Knowledge regarding the rotator cuff footprint has advanced over the past 10 years, specifically with regard to the supraspinatus and infraspinatus.24 The current belief is that the supraspinatus has a triangular insertion onto the most anterior aspect of the greater tuberosity, with a maximum medial-to-lateral length of 6.9 mm and a maximum anterior-to-posterior width of 12.6 mm. The infraspinatus insertion has a trapezoidal insertion, with a maximum medial-to-lateral length of 10.2 mm and anterior-to-posterior width of 32.7 mm. The subscapularis, by far the largest of all the rotator cuff muscles, has a complex geometry with regard to its insertion on the lesser tuberosity, with 4 different insertion points and an overall lateral footprint measuring 37.6 mm and a medial footprint measuring 40.7 mm.25 Finally, the teres minor, with the smallest volume of all the rotator cuff muscles, inserts immediately inferior to the infraspinatus along the inferior facet of the greater tuberosity.26
Aside from the rotator cuff, there are various other muscles and tendons that insert about the proximal humerus and are essential for normal function. The deltoid, which inserts at a point approximately 6 cm from the greater tuberosity along the length of the humerus, with an insertion length between 5 cm to 7 cm,13,27 is the primary mover of the shoulder and essential for proper function after RSA.28,29 The pectoralis major tendon, which begins inserting at a point approximately 5.6 cm from the humeral head and spans a distance of 7.7 cm along the length of the humerus,30-32 is important not only for function but as an anatomical landmark in reconstruction. Lastly, the latissimus dorsi and teres major, which share a role in extension, adduction, and internal rotation of the glenohumeral joint, insert along the floor and medial lip of the intertubercular groove of the humerus, respectively.33,34 In addition to their role in tendon transfer procedures because of treating irreparable posterosuperior cuff and subscapularis tears,35,36 it has been suggested that these tendons may play some role in glenohumeral joint stability.37
In addition to the loss of muscular attachments, the absence of proximal humeral bone stock, in and of itself, can have deleterious effects on fixation of the humeral component. RSA is a semiconstrained device, which results in increased transmission of forces to the interface between the humeral implant and its surrounding structures, including cement (when present) and the bone itself. When there is the absence of significant amounts of bone, the remaining bone must now account for an even higher proportion of these forces. A previous biomechanical study showed that cemented humeral stems demonstrated significantly increased micromotion in the presence of proximal humeral bone loss, particularly when a modular humeral component was used.12
Continue to: TYPES OF BONE LOSS
TYPES OF BONE LOSS
There are a variety of different etiologies of proximal humeral bone loss that result in distinctly different clinical presentations. These can be fairly mild, as is the case of isolated resorption of the greater tuberosity in a non-united proximal humerus fracture (Figure 1). Alternatively, they can be severe, as seen in a grossly loose cemented long-stemmed component that is freely mobile, creating a windshield-wiper effect throughout the length of the humerus (Figure 2). This can be somewhat deceiving, however, as the amount of bone loss, as well as the pathophysiologic process that led to the bone loss, are important factors to determine ideal reconstructive methods. In the case of a failed open reduction internal fixation, where the tuberosity has failed to unite or has been resected, there is much less of a biologic response in comparison with implant loosening associated with osteolysis. This latter condition will be associated with a much more destructive inflammatory response resulting in poor tissue quality and often dramatic thinning of the cortex. If one simply measured the distance from the most proximal remaining bone stock to the area where the greater tuberosity should be, a loose stem with subsidence and ballooning of the cortices may appear to have a similar amount of bone loss as a failed hemiarthroplasty for fracture with a well-fixed stem. However, intraoperatively, one will find that the bone that appeared to be present radiographically in the case of the loose stem is of such poor quality that it cannot reasonably provide any beneficial fixation. In light of this, different treatment modalities are recommended for different types of bone loss, and the revision surgeon must be able to anticipate this and possess a full armamentarium of options to treat these challenging cases successfully.

INDICATIONS
Our technique to manage proximal humeral bone loss is dependent on both the quantity of bone loss, which can be measured radiographically, as well as the anticipated inflammatory response described above. As both the destructive process and the amount of bone loss increase, the importance of more advanced reconstructive procedures that will sustain implant security and soft-tissue management becomes apparent. In the least destructive cases with <5 cm of bone loss, successful revision can typically be accomplished with stem removal and placement of a new monoblock humeral stem. In cases where more advanced destructive pathology is present, and bone loss is >5 cm, an allograft-prosthetic composite (APC) is typically used. In both scenarios, if the stem being revised is cemented and the cement mantle remains intact, and of reasonable length, consideration is given to the cement-within-cement technique. Finally, in the most destructive cases where bone loss exceeds 10 cm and a large biological response is anticipated (eg, periprosthetic fractures with humeral loosening), the use of a longer diaphyseal-incorporating APC is often necessary. This prosthetic composite can be combined with a cement-within-cement technique as well.

It is important to comment on the implications of using modular stems in this setting. With advanced bone loss, a situation is often encountered where the newly implanted stem geometry and working length may be insufficient to acquire adequate rotational stability. In this setting, if a modular junction is positioned close to the stem and cement/bone interface, it will be exposed to very high stress concentrations which can lead to component fracture38 as well as taper corrosion, also referred to as trunnionosis. This latter phenomenon, which has been well studied in the total hip arthroplasty literature with the use of modular components,39 is especially relevant given the high torsional loads imparted at the modular junction. Ultimately, high torsional loads lead to micromotion and electrochemical ion release via degradation of the passivation layer, initiating the process of mechanically assisted crevice corrosion.40 For these reasons, when a modular stem must be used in the presence of mild to moderate bone loss, using a proximal humeral allograft to protect the junction or to provide additional fixation may be implemented with a lower threshold than when using a monoblock stem.
SURGICAL TECHNIQUE: ALLOGRAFT-PROSTHETIC COMPOSITES
A standard deltopectoral approach is used, taking care to preserve all viable muscular attachments to the proximal humerus. After removal of the prosthetic humeral head, the decision to proceed with removal of the stem at this juncture is based on several factors. If the remaining proximal humeral bone is so compromised that it might not be able to withstand the forces exerted upon it during retraction for glenoid exposure, the component is left in place. Additionally, if there is consideration that the glenoid-sided bone loss may be so severe that a glenoid baseplate cannot be implanted, and the stem remains well fixed, it is retained so that it can be converted to a hemiarthroplasty.
If neither of the above issues is present, the humeral stem is removed. If a well-fixed press-fit stem is in place, it is typically removed using a combination of burrs and osteotomes to disrupt the bone-implant interface, and the stem is then carefully removed using an impactor and mallet. If a cemented stem is present, the stem is removed in a similar manner, and the cement mantle is left in place if stable, in anticipation of a cement-within-cement technique. If the mantle is disrupted, standard cement removal instruments are used to remove all cement from the canal meticulously.
Continue to: Management of the glenoid...
Management of the glenoid can have significant implications with regard to the humerus. Most notably, the size of the glenosphere has direct implications on the fixation of the humeral component. Use of larger diameter glenospheres result in increased contact area between the glenosphere and humerosocket, adding constraint to the articulation and further increasing the stresses at the implant-bone interface. As such, the use of larger glenospheres to prevent instability must be balanced with the resulting implications on humeral component fixation, especially in cases of severe bone loss.

After implanting the appropriate glenosphere, attention is then turned back to the humerus. Trial implants are sequentially used to obtain adequate humeral length and stability. Once this is accomplished, the amount of humeral bone loss is quantified by measuring the distance from the superior aspect of the medial humeral tray to the medial humeral shaft. If this number is >5 cm (Figure 3), the decision is made to proceed with an APC. The allograft humeral head is cut, cancellous bone is removed, and a step-cut is performed, with the medial portion of the allograft measuring the same length as that of bone loss and the lateral plate extending an additional several centimeters distally (Figure 4). Additional soft tissue is removed from the allograft, leaving the subscapularis stump intact for later repair with the patient’s native tissue. The allograft is secured to the patient’s proximal humerus using multiple cerclage wires, and the humeral stem is cemented into place. The final construct is shown in Figure 5.

ADDITIONAL CONSIDERATIONS: CASES OF ADVANCED BONE LOSS
In cases of advanced humeral bone loss, as is often seen when revising loose humeral stems, larger allografts that span a significant length of the diaphysis are often required. This type of bone loss has implications with regard to how the deltoid insertion is managed. Interestingly, even in situations when the vast majority of the remaining diaphysis consists of ectatic egg-shell bone, the deltoid tuberosity remains of fairly substantial quality due to the continued pull of the muscular insertion on this area. This fragment is isolated, carefully mobilized, and subsequently repaired back on top of the allograft using cables.

POSTOPERATIVE CARE
Patients are kept in a shoulder immobilizer for 6 weeks after surgery to facilitate allograft incorporation and subscapularis tendon healing. During this time, pendulum exercises are initiated. Active assisted range of motion (ROM) exercises begin after 6 weeks, consisting of supine forward elevation. A sling is given to be used in public. Light strengthening exercises begin at 3 months postoperatively.
DISCUSSION
In cases of mild to moderate proximal humeral bone loss, RSA using a long-stem humeral component without allograft augmentation is a viable option. Budge and colleagues38 demonstrated excellent results in a population of 15 patients with an average of 38 mm of proximal humeral bone loss without use of allografts. Interestingly, they noted 1 case of component fracture in a modular prosthesis and therefore concluded that monoblock humeral stems should be used in the absence of allograft augmentation.
Continue to: In more advanced cases of bone loss...
In more advanced cases of bone loss, our data shows that use of APCs can result in equally satisfactory results. In a series of 25 patients with an average bone loss of 54 mm, patients were able to achieve statistically significant improvements in pain, ROM, and function with high rates of allograft incorporation.9 Overall, a low rate of complications was noted, including 1 infection. This finding is consistent with an additional study looking specifically at factors associated with infection in revision SA, which found that the use of allografts was not associated with increased risk of infection.41
As stated previously, the size of allograft needed for the APC construct is related to the distinct pathology encountered. In our experience, we have noted that well-fixed stems can be treated with short metaphyseal APCs in 85% of cases. On the other hand, loose stems require long allografts measuring >10 cm in 90% of cases. As such, these cases typically require mobilization of the deltoid insertion as described above, and therefore it is important that the surgeon is prepared for this aspect of the procedure preoperatively.
Finally, the cement-within-cement technique, originally popularized for use in revision total hip arthroplasty, has demonstrated reliable results when utilized in revision SA.42 To date, there are no recommendations regarding the minimal length of existing cement mantle that is needed to perform this technique. In situations in which the length of the cement mantle is questionable, our preference is to combine the cement-within-cement technique with an APC when possible.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi:10.1016/j.jse.2010.02.009.
2. Padegimas EM, Maltenfort M, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res. 2015;473(6):1860-1867. doi:10.1007/s11999-015-4231-z.
3. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522. doi:10.1016/j.jse.2011.03.006.
4. Levy JC, Virani N, Pupello D, et al. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195. doi:10.1302/0301-620X.89B2.
5. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
6. Deutsch A, Abboud JA, Kelly J, et al. Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg. 2007;16(6):706-716. doi:10.1016/j.jse.2007.01.007.
7. Kelly JD, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
8. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJP. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042. doi:10.1016/j.jse.2014.02.019.
9. Composite P, Chacon BA, Virani N, et al. Revision arthroplasty with use of a reverse shoulder. J Bone Joint Surg. 2009;1:119-127. doi:10.2106/JBJS.H.00094.
10. Klein SM, Dunning P, Mulieri P, Pupello D, Downes K, Frankle MA. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1144-1154. doi:10.2106/JBJS.I.00778.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
12. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
13. Morgan SJ, Furry K, Parekh A, Agudelo JF, Smith WR. The deltoid muscle: an anatomic description of the deltoid insertion to the proximal humerus. J Orthop Trauma. 2006;20(1):19-21. doi:10.1097/01.bot.0000187063.43267.18.
14. Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop Relat Res. 2000;375:250-257. doi:10.1097/00003086-200006000-00030.
15. De Wilde L, Plasschaert F. Prosthetic treatment and functional recovery of the shoulder after tumor resection 10 years ago: a case report. J Shoulder Elbow Surg. 2005;14(6):645-649. doi:10.1016/j.jse.2004.11.001.
16. Wataru S, Kazuomi S, Yoshikazu N, Hiroaki I, Takaharu Y, Hideki Y. Three-dimensional morphological analysis of humeral heads: a study in cadavers. Acta Orthop. 2005;76(3):392-396. doi:10.1080/00016470510030878.
17. Tillett E, Smith M, Fulcher M, Shanklin J. Anatomic determination of humeral head retroversion: the relationship of the central axis of the humeral head to the bicipital groove. J Shoulder Elbow Surg. 1993;2(5):255-256. doi:10.1016/S1058-2746(09)80085-2.
18. Doyle AJ, Burks RT. Comparison of humeral head retroversion with the humeral axis/biceps groove relationship: a study in live subjects and cadavers. J Shoulder Elbow Surg. 1998;7(5):453-457. doi:10.1016/S1058-2746(98)90193-8.
19. Johnson JW, Thostenson JD, Suva LJ, Hasan SA. Relationship of bicipital groove rotation with humeral head retroversion: a three-dimensional computed tomographic analysis. J Bone Joint Surg Am. 2013;95(8):719-724. doi:10.2106/JBJS.J.00085.
20. Hromádka R, Kuběna AA, Pokorný D, Popelka S, Jahoda D, Sosna A. Lesser tuberosity is more reliable than bicipital groove when determining orientation of humeral head in primary shoulder arthroplasty. Surg Radiol Anat. 2010;32(1):31-37. doi:10.1007/s00276-009-0543-6.
21. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338. doi:10.1067/mse.2002.124429.
22. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(suppl 1):99-104. doi:10.1016/j.jse.2004.09.025.
23. Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg Am. 2000;82-A(11):1594-1602.
24. Mochizuki T, Sugaya H, Uomizu M, et al. Humeral insertion of the supraspinatus and infraspinatus. J Bone Joint Surg Am. 2008;90(5):962-969. doi:10.2106/JBJS.G.00427.
25. Arai R, Sugaya H, Mochizuki T, Nimura A, Moriishi J, Akita K. Subscapularis tendon tear: an anatomic and clinical investigation. Arthroscopy. 2008;24(9):997-1004. doi:10.1016/j.arthro.2008.04.076.
26. Nimura A, Kato A, Yamaguchi K, et al. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg. 2012;21(7):867-872. doi:10.1016/j.jse.2011.04.034.
27. Rispoli DM, Athwal GS, Sperling JW, Cofield RH. The anatomy of the deltoid insertion. J Shoulder Elbow Surg. 2009;18(3):386-390. doi:10.1016/j.jse.2008.10.012.
28. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364. doi:10.1016/j.jse.2012.02.002.
29. Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clinical Orthop Relat Res. 2011;469(9):2440-2451. doi:10.1007/s11999-011-1892-0.
30. Torrens C, Corrales M, Melendo E, Solano A, Rodríguez-Baeza A, Cáceres E. The pectoralis major tendon as a reference for restoring humeral length and retroversion with hemiarthroplasty for fracture. J Shoulder Elbow Surg. 2008;17(6):947-950. doi:10.1016/j.jse.2008.05.041.
31. Ponce BA, Thompson KJ, Rosenzweig SD, et al. Re-evaluation of pectoralis major height as an anatomic reference for humeral height in fracture hemiarthroplasty. J Shoulder Elbow Surg. 2013;22(11):1567-1572. doi:10.1016/j.jse.2013.01.039.
32. LaFrance R, Madsen W, Yaseen Z, Giordano B, Maloney M, Voloshin I. Relevant anatomic landmarks and measurements for biceps tenodesis. Am J Sports Med. 2013;41(6):1395-1399. doi:10.1177/0363546513482297.
33. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
34. Bhatt CR, Prajapati B, Patil DS, Patel VD, Singh BGP, Mehta CD. Variation in the insertion of the latissimus dorsi & its clinical importance. J Orthop. 2013;10(1):25-28. doi:10.1016/j.jor.2013.01.002.
35. Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg. 2006;88(1):113-120. doi:10.2106/JBJS.E.00282.
36. Elhassan B, Christensen TJ, Wagner ER. Feasibility of latissimus and teres major transfer to reconstruct irreparable subscapularis tendon tear: an anatomic study. J Shoulder Elbow Surg. 2014;23(4):492-499. doi:10.1016/j.jse.2013.07.046.
37. Pouliart N, Gagey O. Significance of the latissimus dorsi for shoulder instability. II. Its influence on dislocation behavior in a sequential cutting protocol of the glenohumeral capsule. Clin Anat. 2005;18(7):500-509. doi:10.1002/ca.20181.
38. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
39. Weiser MC, Lavernia CJ. Trunnionosis in total hip arthroplasty. J Bone Joint Surg Am. 2017;99(17):27-29. doi:10.2106/JBJS.17.00345.
40. Cohen J. Current concepts review. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(10):1554.
41. Meijer ST, Paulino Pereira NR, Nota SPFT, Ferrone ML, Schwab JH, Lozano Calderón SA. Factors associated with infection after reconstructive shoulder surgery for proximal humerus tumors. J Shoulder Elbow Surg. 2017;26(6):931-938. doi:10.1016/j.jse.2016.10.014.
42. Wagner ER, Houdek MT, Hernandez NM, Cofield RH, Sánchez-Sotelo J, Sperling JW. Cement-within-cement technique in revision reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(8):1448-1453. doi:10.1016/j.jse.2017.01.013.
1. Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010;19(8):1115-1120. doi:10.1016/j.jse.2010.02.009.
2. Padegimas EM, Maltenfort M, Lazarus MD, Ramsey ML, Williams GR, Namdari S. Future patient demand for shoulder arthroplasty by younger patients: national projections. Clin Orthop Relat Res. 2015;473(6):1860-1867. doi:10.1007/s11999-015-4231-z.
3. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522. doi:10.1016/j.jse.2011.03.006.
4. Levy JC, Virani N, Pupello D, et al. Use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty in patients with glenohumeral arthritis and rotator cuff deficiency. J Bone Joint Surg Br. 2007;89(2):189-195. doi:10.1302/0301-620X.89B2.
5. Melis B, Bonnevialle N, Neyton L, et al. Glenoid loosening and failure in anatomical total shoulder arthroplasty: is revision with a reverse shoulder arthroplasty a reliable option? J Shoulder Elbow Surg. 2012;21(3):342-349. doi:10.1016/j.jse.2011.05.021.
6. Deutsch A, Abboud JA, Kelly J, et al. Clinical results of revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg. 2007;16(6):706-716. doi:10.1016/j.jse.2007.01.007.
7. Kelly JD, Zhao JX, Hobgood ER, Norris TR. Clinical results of revision shoulder arthroplasty using the reverse prosthesis. J Shoulder Elbow Surg. 2012;21(11):1516-1525. doi:10.1016/j.jse.2011.11.021.
8. Black EM, Roberts SM, Siegel E, Yannopoulos P, Higgins LD, Warner JJP. Reverse shoulder arthroplasty as salvage for failed prior arthroplasty in patients 65 years of age or younger. J Shoulder Elbow Surg. 2014;23(7):1036-1042. doi:10.1016/j.jse.2014.02.019.
9. Composite P, Chacon BA, Virani N, et al. Revision arthroplasty with use of a reverse shoulder. J Bone Joint Surg. 2009;1:119-127. doi:10.2106/JBJS.H.00094.
10. Klein SM, Dunning P, Mulieri P, Pupello D, Downes K, Frankle MA. Effects of acquired glenoid bone defects on surgical technique and clinical outcomes in reverse shoulder arthroplasty. J Bone Joint Surg Am. 2010;92(5):1144-1154. doi:10.2106/JBJS.I.00778.
11. Patel DN, Young B, Onyekwelu I, Zuckerman JD, Kwon YW. Reverse total shoulder arthroplasty for failed shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(11):1478-1483. doi:10.1016/j.jse.2011.11.004.
12. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
13. Morgan SJ, Furry K, Parekh A, Agudelo JF, Smith WR. The deltoid muscle: an anatomic description of the deltoid insertion to the proximal humerus. J Orthop Trauma. 2006;20(1):19-21. doi:10.1097/01.bot.0000187063.43267.18.
14. Gagey O, Hue E. Mechanics of the deltoid muscle. A new approach. Clin Orthop Relat Res. 2000;375:250-257. doi:10.1097/00003086-200006000-00030.
15. De Wilde L, Plasschaert F. Prosthetic treatment and functional recovery of the shoulder after tumor resection 10 years ago: a case report. J Shoulder Elbow Surg. 2005;14(6):645-649. doi:10.1016/j.jse.2004.11.001.
16. Wataru S, Kazuomi S, Yoshikazu N, Hiroaki I, Takaharu Y, Hideki Y. Three-dimensional morphological analysis of humeral heads: a study in cadavers. Acta Orthop. 2005;76(3):392-396. doi:10.1080/00016470510030878.
17. Tillett E, Smith M, Fulcher M, Shanklin J. Anatomic determination of humeral head retroversion: the relationship of the central axis of the humeral head to the bicipital groove. J Shoulder Elbow Surg. 1993;2(5):255-256. doi:10.1016/S1058-2746(09)80085-2.
18. Doyle AJ, Burks RT. Comparison of humeral head retroversion with the humeral axis/biceps groove relationship: a study in live subjects and cadavers. J Shoulder Elbow Surg. 1998;7(5):453-457. doi:10.1016/S1058-2746(98)90193-8.
19. Johnson JW, Thostenson JD, Suva LJ, Hasan SA. Relationship of bicipital groove rotation with humeral head retroversion: a three-dimensional computed tomographic analysis. J Bone Joint Surg Am. 2013;95(8):719-724. doi:10.2106/JBJS.J.00085.
20. Hromádka R, Kuběna AA, Pokorný D, Popelka S, Jahoda D, Sosna A. Lesser tuberosity is more reliable than bicipital groove when determining orientation of humeral head in primary shoulder arthroplasty. Surg Radiol Anat. 2010;32(1):31-37. doi:10.1007/s00276-009-0543-6.
21. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg. 2002;11(4):331-338. doi:10.1067/mse.2002.124429.
22. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(suppl 1):99-104. doi:10.1016/j.jse.2004.09.025.
23. Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg Am. 2000;82-A(11):1594-1602.
24. Mochizuki T, Sugaya H, Uomizu M, et al. Humeral insertion of the supraspinatus and infraspinatus. J Bone Joint Surg Am. 2008;90(5):962-969. doi:10.2106/JBJS.G.00427.
25. Arai R, Sugaya H, Mochizuki T, Nimura A, Moriishi J, Akita K. Subscapularis tendon tear: an anatomic and clinical investigation. Arthroscopy. 2008;24(9):997-1004. doi:10.1016/j.arthro.2008.04.076.
26. Nimura A, Kato A, Yamaguchi K, et al. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg. 2012;21(7):867-872. doi:10.1016/j.jse.2011.04.034.
27. Rispoli DM, Athwal GS, Sperling JW, Cofield RH. The anatomy of the deltoid insertion. J Shoulder Elbow Surg. 2009;18(3):386-390. doi:10.1016/j.jse.2008.10.012.
28. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg. 2013;22(3):357-364. doi:10.1016/j.jse.2012.02.002.
29. Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clinical Orthop Relat Res. 2011;469(9):2440-2451. doi:10.1007/s11999-011-1892-0.
30. Torrens C, Corrales M, Melendo E, Solano A, Rodríguez-Baeza A, Cáceres E. The pectoralis major tendon as a reference for restoring humeral length and retroversion with hemiarthroplasty for fracture. J Shoulder Elbow Surg. 2008;17(6):947-950. doi:10.1016/j.jse.2008.05.041.
31. Ponce BA, Thompson KJ, Rosenzweig SD, et al. Re-evaluation of pectoralis major height as an anatomic reference for humeral height in fracture hemiarthroplasty. J Shoulder Elbow Surg. 2013;22(11):1567-1572. doi:10.1016/j.jse.2013.01.039.
32. LaFrance R, Madsen W, Yaseen Z, Giordano B, Maloney M, Voloshin I. Relevant anatomic landmarks and measurements for biceps tenodesis. Am J Sports Med. 2013;41(6):1395-1399. doi:10.1177/0363546513482297.
33. Beck PA, Hoffer MM. Latissimus dorsi and teres major tendons: separate or conjoint tendons? J Pediatr Orthop. 1989;9(3):308-309.
34. Bhatt CR, Prajapati B, Patil DS, Patel VD, Singh BGP, Mehta CD. Variation in the insertion of the latissimus dorsi & its clinical importance. J Orthop. 2013;10(1):25-28. doi:10.1016/j.jor.2013.01.002.
35. Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg. 2006;88(1):113-120. doi:10.2106/JBJS.E.00282.
36. Elhassan B, Christensen TJ, Wagner ER. Feasibility of latissimus and teres major transfer to reconstruct irreparable subscapularis tendon tear: an anatomic study. J Shoulder Elbow Surg. 2014;23(4):492-499. doi:10.1016/j.jse.2013.07.046.
37. Pouliart N, Gagey O. Significance of the latissimus dorsi for shoulder instability. II. Its influence on dislocation behavior in a sequential cutting protocol of the glenohumeral capsule. Clin Anat. 2005;18(7):500-509. doi:10.1002/ca.20181.
38. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
39. Weiser MC, Lavernia CJ. Trunnionosis in total hip arthroplasty. J Bone Joint Surg Am. 2017;99(17):27-29. doi:10.2106/JBJS.17.00345.
40. Cohen J. Current concepts review. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am. 1998;80(10):1554.
41. Meijer ST, Paulino Pereira NR, Nota SPFT, Ferrone ML, Schwab JH, Lozano Calderón SA. Factors associated with infection after reconstructive shoulder surgery for proximal humerus tumors. J Shoulder Elbow Surg. 2017;26(6):931-938. doi:10.1016/j.jse.2016.10.014.
42. Wagner ER, Houdek MT, Hernandez NM, Cofield RH, Sánchez-Sotelo J, Sperling JW. Cement-within-cement technique in revision reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(8):1448-1453. doi:10.1016/j.jse.2017.01.013.
TAKE-HOME POINTS
- Different preoperative diagnoses lead to distinct patterns of bone loss in revision shoulder arthroplasty.
- A variety of techniques should be utilized to address the specific pathologies encountered.
- Advanced proximal humeral bone loss results in limited substrate available for humeral component fixation.
- Monoblock humeral stems can be used without allografts in cases with mild humeral bone loss.
- The revision of loose humeral stems dictates the use of large diaphyseal allografts in the majority of cases.
Treating Humeral Bone Loss in Shoulder Arthroplasty: Modular Humeral Components or Allografts
ABSTRACT
Reconstructing proximal humeral bone loss in the setting of shoulder arthroplasty can be a daunting task. Proposed techniques include long-stemmed humeral components, allograft-prosthesis composites (APCs), and modular endoprosthetic reconstruction. While unsupported long-stemmed components are at high risk for component loosening, APC reconstruction techniques have been reported with success. However, graft resorption and eventual failure are significant concerns. Modular endoprosthetic systems allow bone deficiencies to be reconstructed with metal, which may allow for a more durable reconstruction.
Continue to: Shoulder arthroplasty is an established procedure...
Shoulder arthroplasty is an established procedure with good results for restoring motion and decreasing pain for a variety of indications, including arthritis, fracture, posttraumatic sequelae, and tumor resection.1-4 As the population ages, the incidence of these shoulder disorders increases, with the incidence of total shoulder arthroplasty (TSA) and reverse total shoulder arthroplasty (RTSA) increasing at faster rates than that of hemiarthroplasty.5,6 These expanding indications will, in turn, result in more revisions that would present challenges for surgeons.1,7,8
The glenoid component is much more commonly revised than the humeral component; however, the humeral component may also require revision or removal to allow exposure of the glenoid component.9 Revision of the humeral stem might be required in cases of infection, periprosthetic fracture, dislocation, or aseptic loosening.10 The survival rate of humeral stems is generally >90% at 10 years and >80% at 20-year follow-up.7 Despite these good survival rates, a revision setting the humeral component requires exchange in about half of all cases.11
Humeral bone loss or deficiency is one of the challenges encountered in both primary and revision TSA. The amount of proximal bone loss can be determined by measuring the distance from the top of the prosthesis laterally to the intact lateral cortex.12 Methods for treating bone loss may involve monoblock revision stems to bypass the deficiency, allografts to rebuild the bone stock, or modular components or endoprostheses to restore the length and stability of the extremity.
Proximal humeral bone loss may make component positioning difficult and may create problems with fixation of the humeral stem. Proper sizing and placement of components are important for improving postoperative function, decreasing component wear and instability, and restoring humeral height and offset. Determining the appropriate center of rotation is important for the function and avoidance of impingement on the acromion, as well as for the restoration of the lever arm of the deltoid without overtensioning. The selection of components with the correct size and the accurate intraoperative placement are important to restore humeral height and offset.13,14 Components must be positioned <4 mm from the height of the greater tuberosity and <8 mm of offset to avoid compromising motion.15 De Wilde and Walch16 described about 3 patients who underwent revision reverse shoulder arthroplasty after failure of the humeral implant because of inadequate proximal humeral bone stock. They concluded that treatment of the bone loss was critical to achieve a successful outcome.
LONG-STEMMED HUMERAL COMPONENTS WITHOUT GRAFTING
There is some evidence indicating that humeral bone loss can be managed without allograft or augmentation. Owens and colleagues17 evaluated the use of intermediate- or long-stemmed humeral components for primary shoulder arthroplasty in 17 patients with severe proximal humeral bone loss and in 18 patients with large humeral canals. The stems were fully cemented, cemented distally only with proximal allografting, and uncemented. Indications for fully cemented stems were loss of proximal bone that could be filled with a proximal cement mantle to ensure a secure fit. Distal cement fixation was applied when there was significant proximal bone loss and was often supplemented with cancellous or structural allograft and/or cancellous autograft. Intraoperative complications included cortical perforation or cement extrusion in 16% of patients. Excellent or satisfactory results were obtained in 21 (60%) of the 35 shoulders, 14 (78%) of the 18 shoulders with large humeral canals, and 7 (41%) of the 17 shoulders with bone loss. All the 17 components implanted in patients with proximal humeral bone loss were stable with no gross loosening at an average 6-year follow-up.
Continue to: Budge and colleagues...
Budge and colleagues12 prospectively enrolled 15 patients with substantial proximal humeral bone loss (38.4 mm) who had conversion to RTSA without allografting after a failed TSA. All patients showed improvements in terms of the American Shoulder and Elbow Surgeons (ASES) score, subjective shoulder value, Constant score, and Visual Analog Scale (VAS) pain score, as well as an improved active range of motion (ROM) and good radiographic outcomes at 2-year follow-up. Although the complication rate was high (7 of 15), most of the complications were minor, with only 2 requiring operative intervention. The only component fracture occurred in a patient with a modular prosthesis that was unsupported by bone proximally. Budge and colleagues12 suggested that concerns about prosthetic fracture can be alleviated using a nonmodular monoblock design. No prosthesis-related complications occurred in their series, leading them to recommend monoblock humeral stems in patients with severe proximal humeral bone loss.
Stephens and colleagues18 reported revision to RTSA in 32 patients with hemiarthroplasties, half of whom had proximal humeral bone loss (average 36.3 mm). Of these 16 patients, 10 were treated with monoblock stems and 6 with modular components, with cement fixation of all implants. At an average 4-year follow-up, patients with proximal bone loss had improved motion and outcomes, and decreased pain compared to their preoperative condition; however, they had lower functional and pain ratings, as well as less ROM compared to those of patients with intact proximal bone stock. Complications occurred in 5 (31%) of those with bone loss and in 1 (0.6%) of those without bone loss. Three of the 16 patients with bone loss had humeral stem loosening, with 2 of the 3 having subsidence. Only 1 patient required return to the operating room for the treatment of a periprosthetic fracture sustained in a fall. Of the 16 patients with bone loss, 14 patients demonstrated scapular notching, which was severe in 5 of them. Because patients with altered humeral length and/or standard length stems had worse outcomes, the authors recommended longer stems to improve fixation and advocated the use of a long-stemmed monoblock prosthesis over an allograft-prosthesis composite (APC).18
However, Werner and colleagues19 reported high rates of loosening and distal migration with the use of long-stemmed humeral implants in 50 patients with revision RTSA. They noted periprosthetic lucency on radiographs in 48% of patients, with more than half of them requiring revision. In 6 patients with subsidence of the humeral shaft, revision was done using custom, modular implants, with the anatomic curve being further stabilized using distal screw and cable fixation to provide rotational stability.
Using a biomechanical model, Cuff and colleagues20 compared 3 RTSA humeral designs, 2 modular designs, and 1 monoblock design in 12 intact models and in 12 models simulating 5 cm of proximal humeral bone loss. They observed that proximal humeral bone loss led to increased humeral component micromotion and rotational instability. The bone loss group had 5 failures compared to 2 in the control group. All failures occurred in those with modular components, whereas those with monoblock implants had no failures.
ALLOGRAFT-PROSTHESIS COMPOSITE
Composite treatment with a humeral stem and a metaphyseal allograft was described by Kassab and colleagues21 in 2005 and Levy and colleagues22 in 2007 (Figures 1A-1C) in patients with tumor resections21 or failed hemiarthroplasties.22 Allograft was used when there was insufficient metaphyseal bone to support the implant, and a graft was fashioned and fixed with cerclage wire before the component was cemented in place. In the 29 patients reported by Levy and colleagues,22 subjective and objective measurements trended toward better results in those with an APC than in those with RTSA alone, but this difference did not reach statistical significance. Several authors have identified a lack of proximal humeral bone support as 1 of the 4 possible causes of failure, and suggested that the allograft provides structural support, serves as an attachment for subscapularis repair, and maximizes deltoid function by increasing lateral offset and setting the moment arm of the deltoid.21-23
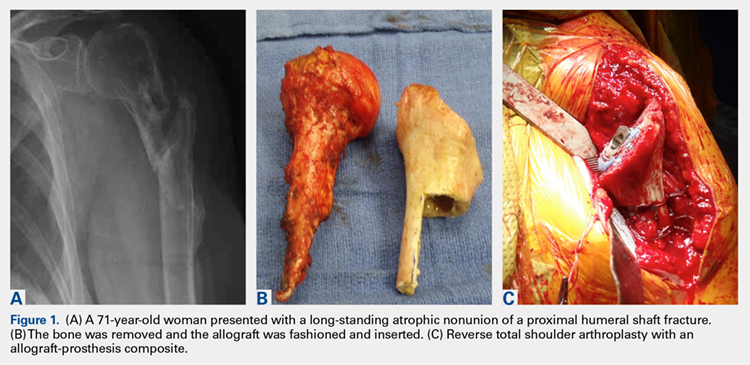
Continue to: In a prospective study of RTSA...
In a prospective study of RTSA using structural allografts for failed hemiarthroplasty in 25 patients with an average bone loss of 5 cm, 19 patients (76%) reported good or excellent results, 5 reported satisfactory results, and 1 patient reported an unsatisfactory result.1 Patients had significantly improved forward flexion, abduction, and external rotation and improved outcome scores (ASES and SST). Graft incorporation was good, with 88% and 79% incorporation in the metaphysis and diaphysis, respectively. This technique used a fresh-frozen proximal humeral allograft to fashion a custom proximal block with a lateral step-cut, which was fixed around the stem with cables. A long stem and cement were used. If there was no cement mantle remaining or if the medial portion of the graft was longer than 120 mm, the cement mantle was completely excised. The allograft stump of the subscapularis was used to repair the subscapularis tendon either end-to-end or pants-over-vest. The authors noted that the subscapularis repair provided increased stability; the only dislocation not caused by trauma did not have an identifiable tendon to repair. In this manner, APC reconstruction provided structural and rotational support to the humeral stem as well as bone stock for future revision.1,20
One significant concern with APC reconstruction is the potential for graft resorption, which can lead to humeral stem loosening, loss of contour of the tuberosity, or weakening to the point of fracture.24,25 This may be worsened by stress shielding of the allograft by distal stem cement fixation.26 Other concerns include the cost of the allograft, increased risk of de novo infection, donor-to-host infection, increased operative time and complexity, and failure of allograft incorporation.
The use of a proximal femoral allograft has been described when there is a lack of a proximal humeral allograft.1,27 Kelly and colleagues27 described good results in 2 patients in whom proximal femoral allograft was used along with bone morphogenetic protein, cemented long-stemmed revision implants, and locking plate augmentation.
ENDOPROSTHETIC RECONSTRUCTION
Various forms of prosthetic augmentation have been described to compensate for proximal humeral bone loss, with the majority of reports involving the use of endoprosthetic replacement for tumor procedures.28-31 Use of endoprostheses has also been described for revision procedures in patients with rheumatoid arthritis with massive bone loss, demonstrating modest improvements compared to severe preoperative functional limitations.32
Tumor patients, as well as revision arthroplasty patients, may present difficulties with prosthetic fixation due to massive bone loss. Chao and colleagues29 reported about the long-term outcomes after the use of implants with a porous ongrowth surface and extracortical bridging bone graft in multiple anatomic locations, including the proximal humerus, the proximal and distal femur, and the femoral diaphysis. In 3 patients with proximal humeral reconstruction, the measured ongrowth was only 30%. Given the small number of patients with a proximal humerus, no statistical significance was observed in the prosthesis location and the amount of bony ongrowth, but it was far less than that in the lower extremity.
Continue to: Endoprosthetic reconstruction...
Endoprosthetic reconstruction of the proximal humerus is commonly used for tumor resection that resulted in bone loss. Cannon and colleagues28 reported a 97.6% survival rate at a mean follow-up of 30 months in 83 patients with modular and custom reconstruction with a unipolar head. The ROM was limited, but the prosthesis provided adequate stability to allow elbow and hand function. Proximal migration of the prosthetic head was noticed with increasing frequency as the length of follow-up increased.
Use of an endoprosthesis with compressive osteointegration (Zimmer Biomet) has been described in lower extremities and more recently with follow-up on several cases, including 2 proximal humeral replacements for oncology patients to treat severe bone loss. One case was for a primary sarcoma resection, and the other was for the revision of aseptic loosening of a previous endoprosthesis. Follow-up periods for these 2 patients were 54 and 141 months, respectively. Both these patients had complications, but both retained the endoprosthesis. The authors concluded that this is a salvage operation with high risk.30 In another study, Guven and colleagues31 reported about reverse endoprosthetic reconstruction for tumor resection with bone loss. The ROM was improved, with a mean active forward elevation of 96° (range, 30°-160°), an abduction of 88° (range, 30-160°), and an external rotation of 13° (range, 0°-20°).
Modular endoprostheses have been evaluated as a method for improving bone fixation and restoring soft-tissue tension, while avoiding the complications associated with traditional endoprostheses or allografts (Figures 2A-2D). These systems allow precise adjustments of length using different trial lengths intraoperatively to obtain proper stability and deltoid tension. Of the 12 patients in a 2 center study, 11 had cementless components inserted using a press-fit technique (unpublished data, J. Feldman). At a minimum 2-year follow-up, the patients had an average improvement in forward elevation from 78° to 97°. Excluding 2 patients with loss of the deltoid tuberosity, the forward elevation averaged 109°. There were significant improvements in internal rotation (from 18° to 38°), as well as in the scores of Quick Disabilities of the Arm, Shoulder and Hand (DASH), forward elevation strength, ASES, and VAS pain. However, the overall complication rate was 41%. Therefore, despite these promising early results, longer-term studies are needed.
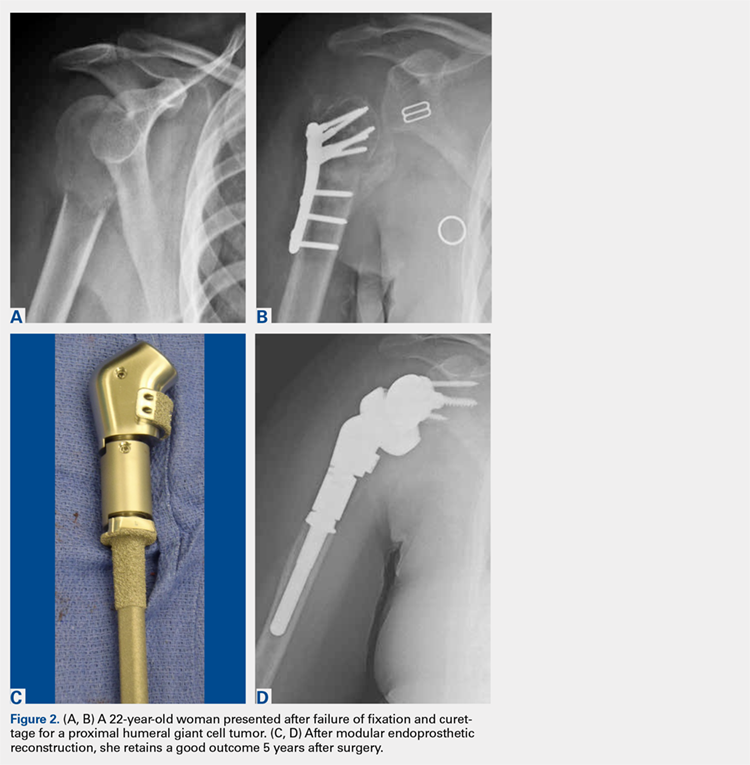
CONCLUSION
Proximal humeral bone loss remains a significant challenge for the shoulder arthroplasty surgeon. In the setting of a primary or a revision arthroplasty, the bone stock must be thoroughly evaluated during preoperative planning, and a surgical plan for addressing the deficits should be developed. Because proximal humeral bone loss may contribute to prosthetic failure, every effort should be made to reconstitute the bone stock.16 If the bone loss is less extensive, impaction grafting may be considered. Options to address massive proximal humeral bone loss include APCs and endoprosthetic reconstruction. The use of an allograft allows subscapularis repair, which may help stabilize the shoulder and restore the natural lever arm, as well as the tension of the deltoid.1,21-23 In addition, it helps avoid rotational instability and micromotion and provides bone stock for future revisions. However, concern persists regarding allograft resorption over time. More recently, modular endoprosthetic reconstruction systems have been developed to address bone deficiency with metal augmentation. Early clinical results demonstrate a high complication rate in this complex cohort of patients, not unlike those in the series of APCs, but clinical outcomes were improved compared to those in historical series. Nevertheless, longer-term clinical studies are necessary to determine the role of these modular endoprosthetic implant systems.
1. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. doi:10.2106/JBJS.H.00094.
2. Hattrup SJ, Waldrop R, Sanchez-Sotelo J. Reverse total shoulder arthroplasty for posttraumatic sequelae. J Orthop Trauma. 2016;30(2):e41-e47. doi:10.1097/BOT.0000000000000416.
3. Sewell MD, Kang SN, Al-Hadithy N, et al. Management of peri-prosthetic fracture of the humerus with severe bone loss and loosening of the humeral component after total shoulder replacement. J Bone Joint Surg Br. 2012;94(10):1382-1389. doi:10.1302/0301-620X.94B10.29248.
4. Trompeter AJ, Gupta RR. The management of complex periprosthetic humeral fractures: a case series of strut allograft augmentation, and a review of the literature. Strategies Trauma Limb Reconstr. 2013;8(1):43-51. doi:10.1007/s11751-013-0155-x.
5. Khatib O, Onyekwelu I, Yu S, Zuckerman JD. Shoulder arthroplasty in New York State, 1991 to 2010: changing patterns of utilization. J Shoulder Elbow Surg. 2015;24(10):e286-e291. doi:10.1016/j.jse.2015.05.038.
6. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
7. Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck CD, Cofield RH. Survivorship of the humeral component in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):143-150. doi:10.1016/j.jse.2009.04.011.
8. Wright TW. Revision of humeral components in shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(2 suppl):S77-S81.
9. Duquin TR, Sperling JW. Revision shoulder arthroplasty—how to manage the humerus. Oper Tech Orthop. 2011;21(1):44-51. doi:10.1053/j.oto.2010.09.008.
10. Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck C, Cofield RH. Revision of the humeral component for aseptic loosening in arthroplasty of the shoulder. J Bone Joint Surg Br. 2009;91(1):75-81. doi:10.1302/0301-620X.91B1.21094.
11. Cofield RH. Revision of hemiarthroplasty to total shoulder arthroplasty. In: Zuckerman JD, ed. Advanced Reconstruction: Shoulder. 1st edition. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2007;613-622.
12. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
13. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-865.
14. Throckmorton TW. Reconstructive procedures of the shoulder and elbow. In: Azar FM, Beaty JH, Canale ST, eds. Campbell’s Operative Orthopaedics. 13th edition. Philadelphia, PA: Elsevier; 2017;570-622.
15. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409. doi:10.1067/mse.2001.116871.
16. De Wilde L, Walch G. Humeral prosthetic failure of reversed total shoulder arthroplasty: a report of three cases. J Shoulder Elbow Surg. 2006;15(2):260-264. doi:10.1016/j.jse.2005.07.014.
17. Owens CJ, Sperling JW, Cofield RH. Utility and complications of long-stem humeral components in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):e7-e12. doi:10.1016/j.jse.2012.10.034.
18. Stephens SP, Paisley KC, Giveans MR, Wirth MA. The effect of proximal humeral bone loss on revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1519-1526. doi:10.1016/j.jse.2015.02.020.
19. Werner BS, Abdelkawi AF, Boehm D, et al. Long-term analysis of revision reverse shoulder arthroplasty using cemented long stems. J Shoulder Elbow Surg. 2017;26(2):273-278. doi:10.1016/j.jse.2016.05.015.
20. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
21. Kassab M, Dumaine V, Babinet A, Ouaknine M, Tomeno B, Anract P. Twenty nine shoulder reconstructions after resection of the proximal humerus for neoplasm with mean 7-year follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2005;91(1):15-23.
22. Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg. 2007;98(2):292-300. doi:10.2106/JBJS.E.01310.
23. Gagey O, Pourjamasb B, Court C. Revision arthroplasty of the shoulder for painful glenoid loosening: a series of 14 cases with acromial prostheses reviewed at four year follow up. Rev Chir Reparatrice Appar Mot. 2001;87(3):221-228.
24. Abdeen A, Hoang BH, Althanasina EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91(10):2406-2415. doi:10.2106/JBJS.H.00815.
25. Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am. 1999;81(8):1138-1146.
26. Chen CF, Chen WM, Cheng YC, Chiang CC, Huang CK, Chen TH. Extracorporeally irradiated autograft-prosthetic composite arthroplasty using AML® extensively porous-coated stem for proximal femur reconstruction: a clinical analysis of 14 patients. J Surg Oncol. 2009;100(5):418-422. doi:10.1002/jso.21351.
27. Kelly JD 2nd, Purchase RJ, Kam G, Norris TR. Alloprosthetic composite reconstruction using the reverse shoulder arthroplasty. Tech Shoulder Elbow Surg. 2009;10(1):5-10.
28. Cannon CP, Paraliticci GU, Lin PP, Lewis VO, Yasko AW. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J Shoulder Elbow Surg. 2009;18(5):705-710. doi:10.1016/j.jse.2008.10.011.
29. Chao EY, Fuchs B, Rowland CM, Ilstrup DM, Pritchard DJ, Sim FH. Long-term results of segmental prosthesis fixation by extracortical bone-bridging and ingrowth. J Bone Joint Surg Am. 2004;86-A(5):948-955.
30. Goulding KA, Schwartz A, Hattrup SJ, et al. Use of compressive osseointegration endoprostheses for massive bone loss from tumor and failed arthroplasty: a viable option in the upper extremity. Clin Orthop Relat Res. 2017;475(6):1702-1711. doi:10.1007/s11999-017-5258-0.
31. Guven MF, Aslan L, Botanlioglu H, Kaynak G, Kesmezacar H, Babacan M. Functional outcome of reverse shoulder tumor prosthesis in the treatment of proximal humeral tumors. J Shoulder Elbow Surg. 2016;25(1):e1-e6. doi:10.1016/j.jse.2015.06.012.
32. Wang ML, Ballard BL, Kulidjian AA, Abrams RA. Upper extremity reconstruction with a humeral tumor endoprosthesis: a novel salvage procedure after multiple revisions of total shoulder and elbow replacement. J Shoulder Elbow Surg. 2011;20(1):e1-e8. doi:10.1016/j.jse.2010.07.018.
ABSTRACT
Reconstructing proximal humeral bone loss in the setting of shoulder arthroplasty can be a daunting task. Proposed techniques include long-stemmed humeral components, allograft-prosthesis composites (APCs), and modular endoprosthetic reconstruction. While unsupported long-stemmed components are at high risk for component loosening, APC reconstruction techniques have been reported with success. However, graft resorption and eventual failure are significant concerns. Modular endoprosthetic systems allow bone deficiencies to be reconstructed with metal, which may allow for a more durable reconstruction.
Continue to: Shoulder arthroplasty is an established procedure...
Shoulder arthroplasty is an established procedure with good results for restoring motion and decreasing pain for a variety of indications, including arthritis, fracture, posttraumatic sequelae, and tumor resection.1-4 As the population ages, the incidence of these shoulder disorders increases, with the incidence of total shoulder arthroplasty (TSA) and reverse total shoulder arthroplasty (RTSA) increasing at faster rates than that of hemiarthroplasty.5,6 These expanding indications will, in turn, result in more revisions that would present challenges for surgeons.1,7,8
The glenoid component is much more commonly revised than the humeral component; however, the humeral component may also require revision or removal to allow exposure of the glenoid component.9 Revision of the humeral stem might be required in cases of infection, periprosthetic fracture, dislocation, or aseptic loosening.10 The survival rate of humeral stems is generally >90% at 10 years and >80% at 20-year follow-up.7 Despite these good survival rates, a revision setting the humeral component requires exchange in about half of all cases.11
Humeral bone loss or deficiency is one of the challenges encountered in both primary and revision TSA. The amount of proximal bone loss can be determined by measuring the distance from the top of the prosthesis laterally to the intact lateral cortex.12 Methods for treating bone loss may involve monoblock revision stems to bypass the deficiency, allografts to rebuild the bone stock, or modular components or endoprostheses to restore the length and stability of the extremity.
Proximal humeral bone loss may make component positioning difficult and may create problems with fixation of the humeral stem. Proper sizing and placement of components are important for improving postoperative function, decreasing component wear and instability, and restoring humeral height and offset. Determining the appropriate center of rotation is important for the function and avoidance of impingement on the acromion, as well as for the restoration of the lever arm of the deltoid without overtensioning. The selection of components with the correct size and the accurate intraoperative placement are important to restore humeral height and offset.13,14 Components must be positioned <4 mm from the height of the greater tuberosity and <8 mm of offset to avoid compromising motion.15 De Wilde and Walch16 described about 3 patients who underwent revision reverse shoulder arthroplasty after failure of the humeral implant because of inadequate proximal humeral bone stock. They concluded that treatment of the bone loss was critical to achieve a successful outcome.
LONG-STEMMED HUMERAL COMPONENTS WITHOUT GRAFTING
There is some evidence indicating that humeral bone loss can be managed without allograft or augmentation. Owens and colleagues17 evaluated the use of intermediate- or long-stemmed humeral components for primary shoulder arthroplasty in 17 patients with severe proximal humeral bone loss and in 18 patients with large humeral canals. The stems were fully cemented, cemented distally only with proximal allografting, and uncemented. Indications for fully cemented stems were loss of proximal bone that could be filled with a proximal cement mantle to ensure a secure fit. Distal cement fixation was applied when there was significant proximal bone loss and was often supplemented with cancellous or structural allograft and/or cancellous autograft. Intraoperative complications included cortical perforation or cement extrusion in 16% of patients. Excellent or satisfactory results were obtained in 21 (60%) of the 35 shoulders, 14 (78%) of the 18 shoulders with large humeral canals, and 7 (41%) of the 17 shoulders with bone loss. All the 17 components implanted in patients with proximal humeral bone loss were stable with no gross loosening at an average 6-year follow-up.
Continue to: Budge and colleagues...
Budge and colleagues12 prospectively enrolled 15 patients with substantial proximal humeral bone loss (38.4 mm) who had conversion to RTSA without allografting after a failed TSA. All patients showed improvements in terms of the American Shoulder and Elbow Surgeons (ASES) score, subjective shoulder value, Constant score, and Visual Analog Scale (VAS) pain score, as well as an improved active range of motion (ROM) and good radiographic outcomes at 2-year follow-up. Although the complication rate was high (7 of 15), most of the complications were minor, with only 2 requiring operative intervention. The only component fracture occurred in a patient with a modular prosthesis that was unsupported by bone proximally. Budge and colleagues12 suggested that concerns about prosthetic fracture can be alleviated using a nonmodular monoblock design. No prosthesis-related complications occurred in their series, leading them to recommend monoblock humeral stems in patients with severe proximal humeral bone loss.
Stephens and colleagues18 reported revision to RTSA in 32 patients with hemiarthroplasties, half of whom had proximal humeral bone loss (average 36.3 mm). Of these 16 patients, 10 were treated with monoblock stems and 6 with modular components, with cement fixation of all implants. At an average 4-year follow-up, patients with proximal bone loss had improved motion and outcomes, and decreased pain compared to their preoperative condition; however, they had lower functional and pain ratings, as well as less ROM compared to those of patients with intact proximal bone stock. Complications occurred in 5 (31%) of those with bone loss and in 1 (0.6%) of those without bone loss. Three of the 16 patients with bone loss had humeral stem loosening, with 2 of the 3 having subsidence. Only 1 patient required return to the operating room for the treatment of a periprosthetic fracture sustained in a fall. Of the 16 patients with bone loss, 14 patients demonstrated scapular notching, which was severe in 5 of them. Because patients with altered humeral length and/or standard length stems had worse outcomes, the authors recommended longer stems to improve fixation and advocated the use of a long-stemmed monoblock prosthesis over an allograft-prosthesis composite (APC).18
However, Werner and colleagues19 reported high rates of loosening and distal migration with the use of long-stemmed humeral implants in 50 patients with revision RTSA. They noted periprosthetic lucency on radiographs in 48% of patients, with more than half of them requiring revision. In 6 patients with subsidence of the humeral shaft, revision was done using custom, modular implants, with the anatomic curve being further stabilized using distal screw and cable fixation to provide rotational stability.
Using a biomechanical model, Cuff and colleagues20 compared 3 RTSA humeral designs, 2 modular designs, and 1 monoblock design in 12 intact models and in 12 models simulating 5 cm of proximal humeral bone loss. They observed that proximal humeral bone loss led to increased humeral component micromotion and rotational instability. The bone loss group had 5 failures compared to 2 in the control group. All failures occurred in those with modular components, whereas those with monoblock implants had no failures.
ALLOGRAFT-PROSTHESIS COMPOSITE
Composite treatment with a humeral stem and a metaphyseal allograft was described by Kassab and colleagues21 in 2005 and Levy and colleagues22 in 2007 (Figures 1A-1C) in patients with tumor resections21 or failed hemiarthroplasties.22 Allograft was used when there was insufficient metaphyseal bone to support the implant, and a graft was fashioned and fixed with cerclage wire before the component was cemented in place. In the 29 patients reported by Levy and colleagues,22 subjective and objective measurements trended toward better results in those with an APC than in those with RTSA alone, but this difference did not reach statistical significance. Several authors have identified a lack of proximal humeral bone support as 1 of the 4 possible causes of failure, and suggested that the allograft provides structural support, serves as an attachment for subscapularis repair, and maximizes deltoid function by increasing lateral offset and setting the moment arm of the deltoid.21-23

Continue to: In a prospective study of RTSA...
In a prospective study of RTSA using structural allografts for failed hemiarthroplasty in 25 patients with an average bone loss of 5 cm, 19 patients (76%) reported good or excellent results, 5 reported satisfactory results, and 1 patient reported an unsatisfactory result.1 Patients had significantly improved forward flexion, abduction, and external rotation and improved outcome scores (ASES and SST). Graft incorporation was good, with 88% and 79% incorporation in the metaphysis and diaphysis, respectively. This technique used a fresh-frozen proximal humeral allograft to fashion a custom proximal block with a lateral step-cut, which was fixed around the stem with cables. A long stem and cement were used. If there was no cement mantle remaining or if the medial portion of the graft was longer than 120 mm, the cement mantle was completely excised. The allograft stump of the subscapularis was used to repair the subscapularis tendon either end-to-end or pants-over-vest. The authors noted that the subscapularis repair provided increased stability; the only dislocation not caused by trauma did not have an identifiable tendon to repair. In this manner, APC reconstruction provided structural and rotational support to the humeral stem as well as bone stock for future revision.1,20
One significant concern with APC reconstruction is the potential for graft resorption, which can lead to humeral stem loosening, loss of contour of the tuberosity, or weakening to the point of fracture.24,25 This may be worsened by stress shielding of the allograft by distal stem cement fixation.26 Other concerns include the cost of the allograft, increased risk of de novo infection, donor-to-host infection, increased operative time and complexity, and failure of allograft incorporation.
The use of a proximal femoral allograft has been described when there is a lack of a proximal humeral allograft.1,27 Kelly and colleagues27 described good results in 2 patients in whom proximal femoral allograft was used along with bone morphogenetic protein, cemented long-stemmed revision implants, and locking plate augmentation.
ENDOPROSTHETIC RECONSTRUCTION
Various forms of prosthetic augmentation have been described to compensate for proximal humeral bone loss, with the majority of reports involving the use of endoprosthetic replacement for tumor procedures.28-31 Use of endoprostheses has also been described for revision procedures in patients with rheumatoid arthritis with massive bone loss, demonstrating modest improvements compared to severe preoperative functional limitations.32
Tumor patients, as well as revision arthroplasty patients, may present difficulties with prosthetic fixation due to massive bone loss. Chao and colleagues29 reported about the long-term outcomes after the use of implants with a porous ongrowth surface and extracortical bridging bone graft in multiple anatomic locations, including the proximal humerus, the proximal and distal femur, and the femoral diaphysis. In 3 patients with proximal humeral reconstruction, the measured ongrowth was only 30%. Given the small number of patients with a proximal humerus, no statistical significance was observed in the prosthesis location and the amount of bony ongrowth, but it was far less than that in the lower extremity.
Continue to: Endoprosthetic reconstruction...
Endoprosthetic reconstruction of the proximal humerus is commonly used for tumor resection that resulted in bone loss. Cannon and colleagues28 reported a 97.6% survival rate at a mean follow-up of 30 months in 83 patients with modular and custom reconstruction with a unipolar head. The ROM was limited, but the prosthesis provided adequate stability to allow elbow and hand function. Proximal migration of the prosthetic head was noticed with increasing frequency as the length of follow-up increased.
Use of an endoprosthesis with compressive osteointegration (Zimmer Biomet) has been described in lower extremities and more recently with follow-up on several cases, including 2 proximal humeral replacements for oncology patients to treat severe bone loss. One case was for a primary sarcoma resection, and the other was for the revision of aseptic loosening of a previous endoprosthesis. Follow-up periods for these 2 patients were 54 and 141 months, respectively. Both these patients had complications, but both retained the endoprosthesis. The authors concluded that this is a salvage operation with high risk.30 In another study, Guven and colleagues31 reported about reverse endoprosthetic reconstruction for tumor resection with bone loss. The ROM was improved, with a mean active forward elevation of 96° (range, 30°-160°), an abduction of 88° (range, 30-160°), and an external rotation of 13° (range, 0°-20°).
Modular endoprostheses have been evaluated as a method for improving bone fixation and restoring soft-tissue tension, while avoiding the complications associated with traditional endoprostheses or allografts (Figures 2A-2D). These systems allow precise adjustments of length using different trial lengths intraoperatively to obtain proper stability and deltoid tension. Of the 12 patients in a 2 center study, 11 had cementless components inserted using a press-fit technique (unpublished data, J. Feldman). At a minimum 2-year follow-up, the patients had an average improvement in forward elevation from 78° to 97°. Excluding 2 patients with loss of the deltoid tuberosity, the forward elevation averaged 109°. There were significant improvements in internal rotation (from 18° to 38°), as well as in the scores of Quick Disabilities of the Arm, Shoulder and Hand (DASH), forward elevation strength, ASES, and VAS pain. However, the overall complication rate was 41%. Therefore, despite these promising early results, longer-term studies are needed.

CONCLUSION
Proximal humeral bone loss remains a significant challenge for the shoulder arthroplasty surgeon. In the setting of a primary or a revision arthroplasty, the bone stock must be thoroughly evaluated during preoperative planning, and a surgical plan for addressing the deficits should be developed. Because proximal humeral bone loss may contribute to prosthetic failure, every effort should be made to reconstitute the bone stock.16 If the bone loss is less extensive, impaction grafting may be considered. Options to address massive proximal humeral bone loss include APCs and endoprosthetic reconstruction. The use of an allograft allows subscapularis repair, which may help stabilize the shoulder and restore the natural lever arm, as well as the tension of the deltoid.1,21-23 In addition, it helps avoid rotational instability and micromotion and provides bone stock for future revisions. However, concern persists regarding allograft resorption over time. More recently, modular endoprosthetic reconstruction systems have been developed to address bone deficiency with metal augmentation. Early clinical results demonstrate a high complication rate in this complex cohort of patients, not unlike those in the series of APCs, but clinical outcomes were improved compared to those in historical series. Nevertheless, longer-term clinical studies are necessary to determine the role of these modular endoprosthetic implant systems.
ABSTRACT
Reconstructing proximal humeral bone loss in the setting of shoulder arthroplasty can be a daunting task. Proposed techniques include long-stemmed humeral components, allograft-prosthesis composites (APCs), and modular endoprosthetic reconstruction. While unsupported long-stemmed components are at high risk for component loosening, APC reconstruction techniques have been reported with success. However, graft resorption and eventual failure are significant concerns. Modular endoprosthetic systems allow bone deficiencies to be reconstructed with metal, which may allow for a more durable reconstruction.
Continue to: Shoulder arthroplasty is an established procedure...
Shoulder arthroplasty is an established procedure with good results for restoring motion and decreasing pain for a variety of indications, including arthritis, fracture, posttraumatic sequelae, and tumor resection.1-4 As the population ages, the incidence of these shoulder disorders increases, with the incidence of total shoulder arthroplasty (TSA) and reverse total shoulder arthroplasty (RTSA) increasing at faster rates than that of hemiarthroplasty.5,6 These expanding indications will, in turn, result in more revisions that would present challenges for surgeons.1,7,8
The glenoid component is much more commonly revised than the humeral component; however, the humeral component may also require revision or removal to allow exposure of the glenoid component.9 Revision of the humeral stem might be required in cases of infection, periprosthetic fracture, dislocation, or aseptic loosening.10 The survival rate of humeral stems is generally >90% at 10 years and >80% at 20-year follow-up.7 Despite these good survival rates, a revision setting the humeral component requires exchange in about half of all cases.11
Humeral bone loss or deficiency is one of the challenges encountered in both primary and revision TSA. The amount of proximal bone loss can be determined by measuring the distance from the top of the prosthesis laterally to the intact lateral cortex.12 Methods for treating bone loss may involve monoblock revision stems to bypass the deficiency, allografts to rebuild the bone stock, or modular components or endoprostheses to restore the length and stability of the extremity.
Proximal humeral bone loss may make component positioning difficult and may create problems with fixation of the humeral stem. Proper sizing and placement of components are important for improving postoperative function, decreasing component wear and instability, and restoring humeral height and offset. Determining the appropriate center of rotation is important for the function and avoidance of impingement on the acromion, as well as for the restoration of the lever arm of the deltoid without overtensioning. The selection of components with the correct size and the accurate intraoperative placement are important to restore humeral height and offset.13,14 Components must be positioned <4 mm from the height of the greater tuberosity and <8 mm of offset to avoid compromising motion.15 De Wilde and Walch16 described about 3 patients who underwent revision reverse shoulder arthroplasty after failure of the humeral implant because of inadequate proximal humeral bone stock. They concluded that treatment of the bone loss was critical to achieve a successful outcome.
LONG-STEMMED HUMERAL COMPONENTS WITHOUT GRAFTING
There is some evidence indicating that humeral bone loss can be managed without allograft or augmentation. Owens and colleagues17 evaluated the use of intermediate- or long-stemmed humeral components for primary shoulder arthroplasty in 17 patients with severe proximal humeral bone loss and in 18 patients with large humeral canals. The stems were fully cemented, cemented distally only with proximal allografting, and uncemented. Indications for fully cemented stems were loss of proximal bone that could be filled with a proximal cement mantle to ensure a secure fit. Distal cement fixation was applied when there was significant proximal bone loss and was often supplemented with cancellous or structural allograft and/or cancellous autograft. Intraoperative complications included cortical perforation or cement extrusion in 16% of patients. Excellent or satisfactory results were obtained in 21 (60%) of the 35 shoulders, 14 (78%) of the 18 shoulders with large humeral canals, and 7 (41%) of the 17 shoulders with bone loss. All the 17 components implanted in patients with proximal humeral bone loss were stable with no gross loosening at an average 6-year follow-up.
Continue to: Budge and colleagues...
Budge and colleagues12 prospectively enrolled 15 patients with substantial proximal humeral bone loss (38.4 mm) who had conversion to RTSA without allografting after a failed TSA. All patients showed improvements in terms of the American Shoulder and Elbow Surgeons (ASES) score, subjective shoulder value, Constant score, and Visual Analog Scale (VAS) pain score, as well as an improved active range of motion (ROM) and good radiographic outcomes at 2-year follow-up. Although the complication rate was high (7 of 15), most of the complications were minor, with only 2 requiring operative intervention. The only component fracture occurred in a patient with a modular prosthesis that was unsupported by bone proximally. Budge and colleagues12 suggested that concerns about prosthetic fracture can be alleviated using a nonmodular monoblock design. No prosthesis-related complications occurred in their series, leading them to recommend monoblock humeral stems in patients with severe proximal humeral bone loss.
Stephens and colleagues18 reported revision to RTSA in 32 patients with hemiarthroplasties, half of whom had proximal humeral bone loss (average 36.3 mm). Of these 16 patients, 10 were treated with monoblock stems and 6 with modular components, with cement fixation of all implants. At an average 4-year follow-up, patients with proximal bone loss had improved motion and outcomes, and decreased pain compared to their preoperative condition; however, they had lower functional and pain ratings, as well as less ROM compared to those of patients with intact proximal bone stock. Complications occurred in 5 (31%) of those with bone loss and in 1 (0.6%) of those without bone loss. Three of the 16 patients with bone loss had humeral stem loosening, with 2 of the 3 having subsidence. Only 1 patient required return to the operating room for the treatment of a periprosthetic fracture sustained in a fall. Of the 16 patients with bone loss, 14 patients demonstrated scapular notching, which was severe in 5 of them. Because patients with altered humeral length and/or standard length stems had worse outcomes, the authors recommended longer stems to improve fixation and advocated the use of a long-stemmed monoblock prosthesis over an allograft-prosthesis composite (APC).18
However, Werner and colleagues19 reported high rates of loosening and distal migration with the use of long-stemmed humeral implants in 50 patients with revision RTSA. They noted periprosthetic lucency on radiographs in 48% of patients, with more than half of them requiring revision. In 6 patients with subsidence of the humeral shaft, revision was done using custom, modular implants, with the anatomic curve being further stabilized using distal screw and cable fixation to provide rotational stability.
Using a biomechanical model, Cuff and colleagues20 compared 3 RTSA humeral designs, 2 modular designs, and 1 monoblock design in 12 intact models and in 12 models simulating 5 cm of proximal humeral bone loss. They observed that proximal humeral bone loss led to increased humeral component micromotion and rotational instability. The bone loss group had 5 failures compared to 2 in the control group. All failures occurred in those with modular components, whereas those with monoblock implants had no failures.
ALLOGRAFT-PROSTHESIS COMPOSITE
Composite treatment with a humeral stem and a metaphyseal allograft was described by Kassab and colleagues21 in 2005 and Levy and colleagues22 in 2007 (Figures 1A-1C) in patients with tumor resections21 or failed hemiarthroplasties.22 Allograft was used when there was insufficient metaphyseal bone to support the implant, and a graft was fashioned and fixed with cerclage wire before the component was cemented in place. In the 29 patients reported by Levy and colleagues,22 subjective and objective measurements trended toward better results in those with an APC than in those with RTSA alone, but this difference did not reach statistical significance. Several authors have identified a lack of proximal humeral bone support as 1 of the 4 possible causes of failure, and suggested that the allograft provides structural support, serves as an attachment for subscapularis repair, and maximizes deltoid function by increasing lateral offset and setting the moment arm of the deltoid.21-23

Continue to: In a prospective study of RTSA...
In a prospective study of RTSA using structural allografts for failed hemiarthroplasty in 25 patients with an average bone loss of 5 cm, 19 patients (76%) reported good or excellent results, 5 reported satisfactory results, and 1 patient reported an unsatisfactory result.1 Patients had significantly improved forward flexion, abduction, and external rotation and improved outcome scores (ASES and SST). Graft incorporation was good, with 88% and 79% incorporation in the metaphysis and diaphysis, respectively. This technique used a fresh-frozen proximal humeral allograft to fashion a custom proximal block with a lateral step-cut, which was fixed around the stem with cables. A long stem and cement were used. If there was no cement mantle remaining or if the medial portion of the graft was longer than 120 mm, the cement mantle was completely excised. The allograft stump of the subscapularis was used to repair the subscapularis tendon either end-to-end or pants-over-vest. The authors noted that the subscapularis repair provided increased stability; the only dislocation not caused by trauma did not have an identifiable tendon to repair. In this manner, APC reconstruction provided structural and rotational support to the humeral stem as well as bone stock for future revision.1,20
One significant concern with APC reconstruction is the potential for graft resorption, which can lead to humeral stem loosening, loss of contour of the tuberosity, or weakening to the point of fracture.24,25 This may be worsened by stress shielding of the allograft by distal stem cement fixation.26 Other concerns include the cost of the allograft, increased risk of de novo infection, donor-to-host infection, increased operative time and complexity, and failure of allograft incorporation.
The use of a proximal femoral allograft has been described when there is a lack of a proximal humeral allograft.1,27 Kelly and colleagues27 described good results in 2 patients in whom proximal femoral allograft was used along with bone morphogenetic protein, cemented long-stemmed revision implants, and locking plate augmentation.
ENDOPROSTHETIC RECONSTRUCTION
Various forms of prosthetic augmentation have been described to compensate for proximal humeral bone loss, with the majority of reports involving the use of endoprosthetic replacement for tumor procedures.28-31 Use of endoprostheses has also been described for revision procedures in patients with rheumatoid arthritis with massive bone loss, demonstrating modest improvements compared to severe preoperative functional limitations.32
Tumor patients, as well as revision arthroplasty patients, may present difficulties with prosthetic fixation due to massive bone loss. Chao and colleagues29 reported about the long-term outcomes after the use of implants with a porous ongrowth surface and extracortical bridging bone graft in multiple anatomic locations, including the proximal humerus, the proximal and distal femur, and the femoral diaphysis. In 3 patients with proximal humeral reconstruction, the measured ongrowth was only 30%. Given the small number of patients with a proximal humerus, no statistical significance was observed in the prosthesis location and the amount of bony ongrowth, but it was far less than that in the lower extremity.
Continue to: Endoprosthetic reconstruction...
Endoprosthetic reconstruction of the proximal humerus is commonly used for tumor resection that resulted in bone loss. Cannon and colleagues28 reported a 97.6% survival rate at a mean follow-up of 30 months in 83 patients with modular and custom reconstruction with a unipolar head. The ROM was limited, but the prosthesis provided adequate stability to allow elbow and hand function. Proximal migration of the prosthetic head was noticed with increasing frequency as the length of follow-up increased.
Use of an endoprosthesis with compressive osteointegration (Zimmer Biomet) has been described in lower extremities and more recently with follow-up on several cases, including 2 proximal humeral replacements for oncology patients to treat severe bone loss. One case was for a primary sarcoma resection, and the other was for the revision of aseptic loosening of a previous endoprosthesis. Follow-up periods for these 2 patients were 54 and 141 months, respectively. Both these patients had complications, but both retained the endoprosthesis. The authors concluded that this is a salvage operation with high risk.30 In another study, Guven and colleagues31 reported about reverse endoprosthetic reconstruction for tumor resection with bone loss. The ROM was improved, with a mean active forward elevation of 96° (range, 30°-160°), an abduction of 88° (range, 30-160°), and an external rotation of 13° (range, 0°-20°).
Modular endoprostheses have been evaluated as a method for improving bone fixation and restoring soft-tissue tension, while avoiding the complications associated with traditional endoprostheses or allografts (Figures 2A-2D). These systems allow precise adjustments of length using different trial lengths intraoperatively to obtain proper stability and deltoid tension. Of the 12 patients in a 2 center study, 11 had cementless components inserted using a press-fit technique (unpublished data, J. Feldman). At a minimum 2-year follow-up, the patients had an average improvement in forward elevation from 78° to 97°. Excluding 2 patients with loss of the deltoid tuberosity, the forward elevation averaged 109°. There were significant improvements in internal rotation (from 18° to 38°), as well as in the scores of Quick Disabilities of the Arm, Shoulder and Hand (DASH), forward elevation strength, ASES, and VAS pain. However, the overall complication rate was 41%. Therefore, despite these promising early results, longer-term studies are needed.

CONCLUSION
Proximal humeral bone loss remains a significant challenge for the shoulder arthroplasty surgeon. In the setting of a primary or a revision arthroplasty, the bone stock must be thoroughly evaluated during preoperative planning, and a surgical plan for addressing the deficits should be developed. Because proximal humeral bone loss may contribute to prosthetic failure, every effort should be made to reconstitute the bone stock.16 If the bone loss is less extensive, impaction grafting may be considered. Options to address massive proximal humeral bone loss include APCs and endoprosthetic reconstruction. The use of an allograft allows subscapularis repair, which may help stabilize the shoulder and restore the natural lever arm, as well as the tension of the deltoid.1,21-23 In addition, it helps avoid rotational instability and micromotion and provides bone stock for future revisions. However, concern persists regarding allograft resorption over time. More recently, modular endoprosthetic reconstruction systems have been developed to address bone deficiency with metal augmentation. Early clinical results demonstrate a high complication rate in this complex cohort of patients, not unlike those in the series of APCs, but clinical outcomes were improved compared to those in historical series. Nevertheless, longer-term clinical studies are necessary to determine the role of these modular endoprosthetic implant systems.
1. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. doi:10.2106/JBJS.H.00094.
2. Hattrup SJ, Waldrop R, Sanchez-Sotelo J. Reverse total shoulder arthroplasty for posttraumatic sequelae. J Orthop Trauma. 2016;30(2):e41-e47. doi:10.1097/BOT.0000000000000416.
3. Sewell MD, Kang SN, Al-Hadithy N, et al. Management of peri-prosthetic fracture of the humerus with severe bone loss and loosening of the humeral component after total shoulder replacement. J Bone Joint Surg Br. 2012;94(10):1382-1389. doi:10.1302/0301-620X.94B10.29248.
4. Trompeter AJ, Gupta RR. The management of complex periprosthetic humeral fractures: a case series of strut allograft augmentation, and a review of the literature. Strategies Trauma Limb Reconstr. 2013;8(1):43-51. doi:10.1007/s11751-013-0155-x.
5. Khatib O, Onyekwelu I, Yu S, Zuckerman JD. Shoulder arthroplasty in New York State, 1991 to 2010: changing patterns of utilization. J Shoulder Elbow Surg. 2015;24(10):e286-e291. doi:10.1016/j.jse.2015.05.038.
6. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
7. Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck CD, Cofield RH. Survivorship of the humeral component in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):143-150. doi:10.1016/j.jse.2009.04.011.
8. Wright TW. Revision of humeral components in shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(2 suppl):S77-S81.
9. Duquin TR, Sperling JW. Revision shoulder arthroplasty—how to manage the humerus. Oper Tech Orthop. 2011;21(1):44-51. doi:10.1053/j.oto.2010.09.008.
10. Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck C, Cofield RH. Revision of the humeral component for aseptic loosening in arthroplasty of the shoulder. J Bone Joint Surg Br. 2009;91(1):75-81. doi:10.1302/0301-620X.91B1.21094.
11. Cofield RH. Revision of hemiarthroplasty to total shoulder arthroplasty. In: Zuckerman JD, ed. Advanced Reconstruction: Shoulder. 1st edition. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2007;613-622.
12. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
13. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-865.
14. Throckmorton TW. Reconstructive procedures of the shoulder and elbow. In: Azar FM, Beaty JH, Canale ST, eds. Campbell’s Operative Orthopaedics. 13th edition. Philadelphia, PA: Elsevier; 2017;570-622.
15. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409. doi:10.1067/mse.2001.116871.
16. De Wilde L, Walch G. Humeral prosthetic failure of reversed total shoulder arthroplasty: a report of three cases. J Shoulder Elbow Surg. 2006;15(2):260-264. doi:10.1016/j.jse.2005.07.014.
17. Owens CJ, Sperling JW, Cofield RH. Utility and complications of long-stem humeral components in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):e7-e12. doi:10.1016/j.jse.2012.10.034.
18. Stephens SP, Paisley KC, Giveans MR, Wirth MA. The effect of proximal humeral bone loss on revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1519-1526. doi:10.1016/j.jse.2015.02.020.
19. Werner BS, Abdelkawi AF, Boehm D, et al. Long-term analysis of revision reverse shoulder arthroplasty using cemented long stems. J Shoulder Elbow Surg. 2017;26(2):273-278. doi:10.1016/j.jse.2016.05.015.
20. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
21. Kassab M, Dumaine V, Babinet A, Ouaknine M, Tomeno B, Anract P. Twenty nine shoulder reconstructions after resection of the proximal humerus for neoplasm with mean 7-year follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2005;91(1):15-23.
22. Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg. 2007;98(2):292-300. doi:10.2106/JBJS.E.01310.
23. Gagey O, Pourjamasb B, Court C. Revision arthroplasty of the shoulder for painful glenoid loosening: a series of 14 cases with acromial prostheses reviewed at four year follow up. Rev Chir Reparatrice Appar Mot. 2001;87(3):221-228.
24. Abdeen A, Hoang BH, Althanasina EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91(10):2406-2415. doi:10.2106/JBJS.H.00815.
25. Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am. 1999;81(8):1138-1146.
26. Chen CF, Chen WM, Cheng YC, Chiang CC, Huang CK, Chen TH. Extracorporeally irradiated autograft-prosthetic composite arthroplasty using AML® extensively porous-coated stem for proximal femur reconstruction: a clinical analysis of 14 patients. J Surg Oncol. 2009;100(5):418-422. doi:10.1002/jso.21351.
27. Kelly JD 2nd, Purchase RJ, Kam G, Norris TR. Alloprosthetic composite reconstruction using the reverse shoulder arthroplasty. Tech Shoulder Elbow Surg. 2009;10(1):5-10.
28. Cannon CP, Paraliticci GU, Lin PP, Lewis VO, Yasko AW. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J Shoulder Elbow Surg. 2009;18(5):705-710. doi:10.1016/j.jse.2008.10.011.
29. Chao EY, Fuchs B, Rowland CM, Ilstrup DM, Pritchard DJ, Sim FH. Long-term results of segmental prosthesis fixation by extracortical bone-bridging and ingrowth. J Bone Joint Surg Am. 2004;86-A(5):948-955.
30. Goulding KA, Schwartz A, Hattrup SJ, et al. Use of compressive osseointegration endoprostheses for massive bone loss from tumor and failed arthroplasty: a viable option in the upper extremity. Clin Orthop Relat Res. 2017;475(6):1702-1711. doi:10.1007/s11999-017-5258-0.
31. Guven MF, Aslan L, Botanlioglu H, Kaynak G, Kesmezacar H, Babacan M. Functional outcome of reverse shoulder tumor prosthesis in the treatment of proximal humeral tumors. J Shoulder Elbow Surg. 2016;25(1):e1-e6. doi:10.1016/j.jse.2015.06.012.
32. Wang ML, Ballard BL, Kulidjian AA, Abrams RA. Upper extremity reconstruction with a humeral tumor endoprosthesis: a novel salvage procedure after multiple revisions of total shoulder and elbow replacement. J Shoulder Elbow Surg. 2011;20(1):e1-e8. doi:10.1016/j.jse.2010.07.018.
1. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. doi:10.2106/JBJS.H.00094.
2. Hattrup SJ, Waldrop R, Sanchez-Sotelo J. Reverse total shoulder arthroplasty for posttraumatic sequelae. J Orthop Trauma. 2016;30(2):e41-e47. doi:10.1097/BOT.0000000000000416.
3. Sewell MD, Kang SN, Al-Hadithy N, et al. Management of peri-prosthetic fracture of the humerus with severe bone loss and loosening of the humeral component after total shoulder replacement. J Bone Joint Surg Br. 2012;94(10):1382-1389. doi:10.1302/0301-620X.94B10.29248.
4. Trompeter AJ, Gupta RR. The management of complex periprosthetic humeral fractures: a case series of strut allograft augmentation, and a review of the literature. Strategies Trauma Limb Reconstr. 2013;8(1):43-51. doi:10.1007/s11751-013-0155-x.
5. Khatib O, Onyekwelu I, Yu S, Zuckerman JD. Shoulder arthroplasty in New York State, 1991 to 2010: changing patterns of utilization. J Shoulder Elbow Surg. 2015;24(10):e286-e291. doi:10.1016/j.jse.2015.05.038.
6. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
7. Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck CD, Cofield RH. Survivorship of the humeral component in shoulder arthroplasty. J Shoulder Elbow Surg. 2010;19(1):143-150. doi:10.1016/j.jse.2009.04.011.
8. Wright TW. Revision of humeral components in shoulder arthroplasty. Bull Hosp Jt Dis. 2013;71(2 suppl):S77-S81.
9. Duquin TR, Sperling JW. Revision shoulder arthroplasty—how to manage the humerus. Oper Tech Orthop. 2011;21(1):44-51. doi:10.1053/j.oto.2010.09.008.
10. Cil A, Veillette CJ, Sanchez-Sotelo J, Sperling JW, Schleck C, Cofield RH. Revision of the humeral component for aseptic loosening in arthroplasty of the shoulder. J Bone Joint Surg Br. 2009;91(1):75-81. doi:10.1302/0301-620X.91B1.21094.
11. Cofield RH. Revision of hemiarthroplasty to total shoulder arthroplasty. In: Zuckerman JD, ed. Advanced Reconstruction: Shoulder. 1st edition. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2007;613-622.
12. Budge MD, Moravek JE, Zimel MN, Nolan EM, Wiater JM. Reverse total shoulder arthroplasty for the management of failed shoulder arthroplasty with proximal humeral bone loss: is allograft augmentation necessary? J Shoulder Elbow Surg. 2013;22(6):739-744. doi:10.1016/j.jse.2012.08.008.
13. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-865.
14. Throckmorton TW. Reconstructive procedures of the shoulder and elbow. In: Azar FM, Beaty JH, Canale ST, eds. Campbell’s Operative Orthopaedics. 13th edition. Philadelphia, PA: Elsevier; 2017;570-622.
15. Williams GR Jr, Wong KL, Pepe MD, et al. The effect of articular malposition after total shoulder arthroplasty on glenohumeral translations, range of motion, and subacromial impingement. J Shoulder Elbow Surg. 2001;10(5):399-409. doi:10.1067/mse.2001.116871.
16. De Wilde L, Walch G. Humeral prosthetic failure of reversed total shoulder arthroplasty: a report of three cases. J Shoulder Elbow Surg. 2006;15(2):260-264. doi:10.1016/j.jse.2005.07.014.
17. Owens CJ, Sperling JW, Cofield RH. Utility and complications of long-stem humeral components in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(7):e7-e12. doi:10.1016/j.jse.2012.10.034.
18. Stephens SP, Paisley KC, Giveans MR, Wirth MA. The effect of proximal humeral bone loss on revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(10):1519-1526. doi:10.1016/j.jse.2015.02.020.
19. Werner BS, Abdelkawi AF, Boehm D, et al. Long-term analysis of revision reverse shoulder arthroplasty using cemented long stems. J Shoulder Elbow Surg. 2017;26(2):273-278. doi:10.1016/j.jse.2016.05.015.
20. Cuff D, Levy JC, Gutiérrez S, Frankle MA. Torsional stability of modular and non-modular reverse shoulder humeral components in a proximal humeral bone loss model. J Shoulder Elbow Surg. 2011;20(4):646-651. doi:10.1016/j.jse.2010.10.026.
21. Kassab M, Dumaine V, Babinet A, Ouaknine M, Tomeno B, Anract P. Twenty nine shoulder reconstructions after resection of the proximal humerus for neoplasm with mean 7-year follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2005;91(1):15-23.
22. Levy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. J Bone Joint Surg. 2007;98(2):292-300. doi:10.2106/JBJS.E.01310.
23. Gagey O, Pourjamasb B, Court C. Revision arthroplasty of the shoulder for painful glenoid loosening: a series of 14 cases with acromial prostheses reviewed at four year follow up. Rev Chir Reparatrice Appar Mot. 2001;87(3):221-228.
24. Abdeen A, Hoang BH, Althanasina EA, Morris CD, Boland PJ, Healey JH. Allograft-prosthesis composite reconstruction of the proximal part of the humerus: functional outcome and survivorship. J Bone Joint Surg Am. 2009;91(10):2406-2415. doi:10.2106/JBJS.H.00815.
25. Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am. 1999;81(8):1138-1146.
26. Chen CF, Chen WM, Cheng YC, Chiang CC, Huang CK, Chen TH. Extracorporeally irradiated autograft-prosthetic composite arthroplasty using AML® extensively porous-coated stem for proximal femur reconstruction: a clinical analysis of 14 patients. J Surg Oncol. 2009;100(5):418-422. doi:10.1002/jso.21351.
27. Kelly JD 2nd, Purchase RJ, Kam G, Norris TR. Alloprosthetic composite reconstruction using the reverse shoulder arthroplasty. Tech Shoulder Elbow Surg. 2009;10(1):5-10.
28. Cannon CP, Paraliticci GU, Lin PP, Lewis VO, Yasko AW. Functional outcome following endoprosthetic reconstruction of the proximal humerus. J Shoulder Elbow Surg. 2009;18(5):705-710. doi:10.1016/j.jse.2008.10.011.
29. Chao EY, Fuchs B, Rowland CM, Ilstrup DM, Pritchard DJ, Sim FH. Long-term results of segmental prosthesis fixation by extracortical bone-bridging and ingrowth. J Bone Joint Surg Am. 2004;86-A(5):948-955.
30. Goulding KA, Schwartz A, Hattrup SJ, et al. Use of compressive osseointegration endoprostheses for massive bone loss from tumor and failed arthroplasty: a viable option in the upper extremity. Clin Orthop Relat Res. 2017;475(6):1702-1711. doi:10.1007/s11999-017-5258-0.
31. Guven MF, Aslan L, Botanlioglu H, Kaynak G, Kesmezacar H, Babacan M. Functional outcome of reverse shoulder tumor prosthesis in the treatment of proximal humeral tumors. J Shoulder Elbow Surg. 2016;25(1):e1-e6. doi:10.1016/j.jse.2015.06.012.
32. Wang ML, Ballard BL, Kulidjian AA, Abrams RA. Upper extremity reconstruction with a humeral tumor endoprosthesis: a novel salvage procedure after multiple revisions of total shoulder and elbow replacement. J Shoulder Elbow Surg. 2011;20(1):e1-e8. doi:10.1016/j.jse.2010.07.018.
TAKE-HOME POINTS
- Proximal humeral bone loss presents a significant challenge for the shoulder arthroplasty surgeon.
- Unsupported long-stemmed humeral components in this setting are prone to early loosening.
- APCs can rebuild proximal humeral bone stock, but have concerns with graft resorption and long-term failure.
- Modular endoprosthetic reconstruction of proximal humeral bone loss potentially allows those deficiencies to be addressed in a more durable fashion.
- Longer-term and larger studies are needed to determine the optimal reconstruction technique for proximal humeral bone loss.
Morbid, super obesity raises laparoscopic VHR risk
JACKSONVILLE, FLA. – Super-obese patients who have laparoscopic repair for ventral hernias have complications at a rate more than twice that for overweight individuals undergoing the same operation, according to an analysis of 10-year data presented at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
“Patients with a body mass index of 40 kg/m2 or greater were found to be significantly more likely to have a complication following laparoscopic ventral hernia repair,” said Robert A. Swendiman, MD, of the University of Pennsylvania, Philadelphia.
Dr. Swendiman and his colleagues analyzed outcomes of 57,957 patients in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database who had laparoscopic ventral hernia repair (VHR) from 2005 to 2015. The dataset was stratified into seven different BMI classes, and by hernia type (reducible or strangulated) and time of repair (initial or recurrent).
The overall complication rate for the study population was 4%, ranging from 3% in overweight patients (BMI of 25-29.99 kg/m2) to 6.9% for the super obese (BMI of 50 kg/m2 or greater); 61.4% of the study population was obese. “Initial repair and reducible hernias had lower complication rates than recurrent and incarcerated/strangulated hernias,” Dr. Swendiman said. The study considered 1 of 19 different complications within 30 days of the operation.
Three weight groups had the highest odds ratios (OR) for complications: underweight patients (less than 18.5 kg/m2, OR 1.46, P = .283); morbidly obese (40-50 kg/m2, OR 1.28, P = .014); and super obese (greater than or equal to 50 kg/m2, OR 1.76, P = less than .0001). However, Dr. Swendiman noted, “Overweight patients had a lower rate of overall complications compared to normal-weight individuals.”
These findings were consistent with a prior analysis the group did that found patients with BMI greater than 30 kg/m2 was associated with increased risk of complications after open VHR, Dr. Swendiman noted (Surgery. 2017;162[6]:1320-9).
“Future studies should be considered to evaluate the role of weight reduction prior to hernia repair as a method to reduce patient risk,” Dr. Swendiman said. Laparoscopic repair may be preferable to open VHR in obese patients, depending on the clinical context, he said.
Dr. Swendiman and coauthors reported having no financial disclosures.
SOURCE: Academic Surgical Congress. Abstract 50.02.
JACKSONVILLE, FLA. – Super-obese patients who have laparoscopic repair for ventral hernias have complications at a rate more than twice that for overweight individuals undergoing the same operation, according to an analysis of 10-year data presented at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
“Patients with a body mass index of 40 kg/m2 or greater were found to be significantly more likely to have a complication following laparoscopic ventral hernia repair,” said Robert A. Swendiman, MD, of the University of Pennsylvania, Philadelphia.
Dr. Swendiman and his colleagues analyzed outcomes of 57,957 patients in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database who had laparoscopic ventral hernia repair (VHR) from 2005 to 2015. The dataset was stratified into seven different BMI classes, and by hernia type (reducible or strangulated) and time of repair (initial or recurrent).
The overall complication rate for the study population was 4%, ranging from 3% in overweight patients (BMI of 25-29.99 kg/m2) to 6.9% for the super obese (BMI of 50 kg/m2 or greater); 61.4% of the study population was obese. “Initial repair and reducible hernias had lower complication rates than recurrent and incarcerated/strangulated hernias,” Dr. Swendiman said. The study considered 1 of 19 different complications within 30 days of the operation.
Three weight groups had the highest odds ratios (OR) for complications: underweight patients (less than 18.5 kg/m2, OR 1.46, P = .283); morbidly obese (40-50 kg/m2, OR 1.28, P = .014); and super obese (greater than or equal to 50 kg/m2, OR 1.76, P = less than .0001). However, Dr. Swendiman noted, “Overweight patients had a lower rate of overall complications compared to normal-weight individuals.”
These findings were consistent with a prior analysis the group did that found patients with BMI greater than 30 kg/m2 was associated with increased risk of complications after open VHR, Dr. Swendiman noted (Surgery. 2017;162[6]:1320-9).
“Future studies should be considered to evaluate the role of weight reduction prior to hernia repair as a method to reduce patient risk,” Dr. Swendiman said. Laparoscopic repair may be preferable to open VHR in obese patients, depending on the clinical context, he said.
Dr. Swendiman and coauthors reported having no financial disclosures.
SOURCE: Academic Surgical Congress. Abstract 50.02.
JACKSONVILLE, FLA. – Super-obese patients who have laparoscopic repair for ventral hernias have complications at a rate more than twice that for overweight individuals undergoing the same operation, according to an analysis of 10-year data presented at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.
“Patients with a body mass index of 40 kg/m2 or greater were found to be significantly more likely to have a complication following laparoscopic ventral hernia repair,” said Robert A. Swendiman, MD, of the University of Pennsylvania, Philadelphia.
Dr. Swendiman and his colleagues analyzed outcomes of 57,957 patients in the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database who had laparoscopic ventral hernia repair (VHR) from 2005 to 2015. The dataset was stratified into seven different BMI classes, and by hernia type (reducible or strangulated) and time of repair (initial or recurrent).
The overall complication rate for the study population was 4%, ranging from 3% in overweight patients (BMI of 25-29.99 kg/m2) to 6.9% for the super obese (BMI of 50 kg/m2 or greater); 61.4% of the study population was obese. “Initial repair and reducible hernias had lower complication rates than recurrent and incarcerated/strangulated hernias,” Dr. Swendiman said. The study considered 1 of 19 different complications within 30 days of the operation.
Three weight groups had the highest odds ratios (OR) for complications: underweight patients (less than 18.5 kg/m2, OR 1.46, P = .283); morbidly obese (40-50 kg/m2, OR 1.28, P = .014); and super obese (greater than or equal to 50 kg/m2, OR 1.76, P = less than .0001). However, Dr. Swendiman noted, “Overweight patients had a lower rate of overall complications compared to normal-weight individuals.”
These findings were consistent with a prior analysis the group did that found patients with BMI greater than 30 kg/m2 was associated with increased risk of complications after open VHR, Dr. Swendiman noted (Surgery. 2017;162[6]:1320-9).
“Future studies should be considered to evaluate the role of weight reduction prior to hernia repair as a method to reduce patient risk,” Dr. Swendiman said. Laparoscopic repair may be preferable to open VHR in obese patients, depending on the clinical context, he said.
Dr. Swendiman and coauthors reported having no financial disclosures.
SOURCE: Academic Surgical Congress. Abstract 50.02.
REPORTING FROM THE ANNUAL ACADEMIC SURGICAL CONGRESS
Key clinical point: Laparoscopic ventral hernia repair is associated with a significantly increased risk of complications in the morbidly and super obese.
Major finding: Individuals with a body mass index in the overweight range (BMI 25 to 29.99 kg/m2) had a complication rate of 3% vs. 6.9% for those with BMI greater than or equal to 50 kg/m2.
Story details: A retrospective analysis of 57,957 patients in the NSQIP database who had laparoscopic ventral hernia repair between 2005 and 2015.
Disclosures: Dr. Swendiman and coauthors reported having no financial disclosures.
Source: Academic Surgical Congress. Abstract 50.02.