User login
DDW® 2018 general registration is now open
General registration and housing for Digestive Disease Week® (DDW) 2018 are now open. Registering during the early-bird period (until April 18) guarantees a savings of at least $80 on your registration.
Why DDW?
Just as monumental as this year’s host city, Washington, D.C., DDW is the premier meeting for GI professionals. Come to DDW 2018, taking place June 2-5, to:
- • Choose from an extensive program of high-quality education presented in a variety of learning formats.
- • Explore research unveiled in more than 4,000 poster presentations and over 1,000 abstract presentations.
- • Network and share capital ideas with more than 14,000 other attendees from around the world.
- • Browse an extensive Exhibit Hall featuring the latest products and services in gastroenterology and related fields.
Whether your area of expertise is in patient care, research, education, or administration, DDW has something for you. Register today
General registration and housing for Digestive Disease Week® (DDW) 2018 are now open. Registering during the early-bird period (until April 18) guarantees a savings of at least $80 on your registration.
Why DDW?
Just as monumental as this year’s host city, Washington, D.C., DDW is the premier meeting for GI professionals. Come to DDW 2018, taking place June 2-5, to:
- • Choose from an extensive program of high-quality education presented in a variety of learning formats.
- • Explore research unveiled in more than 4,000 poster presentations and over 1,000 abstract presentations.
- • Network and share capital ideas with more than 14,000 other attendees from around the world.
- • Browse an extensive Exhibit Hall featuring the latest products and services in gastroenterology and related fields.
Whether your area of expertise is in patient care, research, education, or administration, DDW has something for you. Register today
General registration and housing for Digestive Disease Week® (DDW) 2018 are now open. Registering during the early-bird period (until April 18) guarantees a savings of at least $80 on your registration.
Why DDW?
Just as monumental as this year’s host city, Washington, D.C., DDW is the premier meeting for GI professionals. Come to DDW 2018, taking place June 2-5, to:
- • Choose from an extensive program of high-quality education presented in a variety of learning formats.
- • Explore research unveiled in more than 4,000 poster presentations and over 1,000 abstract presentations.
- • Network and share capital ideas with more than 14,000 other attendees from around the world.
- • Browse an extensive Exhibit Hall featuring the latest products and services in gastroenterology and related fields.
Whether your area of expertise is in patient care, research, education, or administration, DDW has something for you. Register today
AGA’s Fecal Microbiota Transplantation National Registry enrolls first patient
The AGA Fecal Microbiota Transplantation (FMT) National Registry is officially underway! The first patient enrolled in the FMT National Registry received a fecal transplant through the Gastroenterology Center of Connecticut/Medical Research Center of Connecticut by Paul Feuerstadt, MD. The patient being treated had experienced multiple recurrences of C. difficile infection. As part of the registry, Dr. Feuerstadt will follow up with the patient four times over the next 2 years and report back on the patient’s health post-FMT. The patient will also provide yearly reports for up to 10 years.
The AGA FMT National Registry, a program of the AGA Center for Gut Microbiome Research and Education, was established in August 2016 after receiving funding from the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH (award number R24AI118629). The registry aims to enroll 75 sites and track 4,000 patients for 5-10 years after their FMT procedure. The data collected from this registry will guide physicians in determining when to use FMT on their patients and will provide much-needed information on the potential risks associated with stool transplants.
If you’re interested in participating in the registry, email [email protected].
New registry collaborators
AGA will collaborate with the American Gut Project – an academic effort run by the laboratory of Rob Knight, PhD, professor and director of the Center for Microbiome Innovation at the University of California, San Diego – to build a biobank of stool samples from participants in the FMT National Registry. American Gut will receive stool samples from registry participants before and after their FMT. The microbiota will be sequenced in each sample, and remaining material will be frozen to be made available for future research. Eventually, this information could help doctors screen and select the best donor samples for individual patients.
AGA will also collaborate with OpenBiome, a public stool bank and nonprofit research organization that provides clinicians with rigorously screened, ready-to-use stool preparations for fecal transplant procedures. As the only public stool bank in the country, OpenBiome serves as the source of stool preparations for nearly 1,000 clinical partners performing FMT across the U.S. For patients enrolled in the registry who receive OpenBiome FMT material, OpenBiome will provide screening information and samples to support the registry’s research analyses. Learn more at www.gastro.org/FMTRegistry.
The AGA Fecal Microbiota Transplantation (FMT) National Registry is officially underway! The first patient enrolled in the FMT National Registry received a fecal transplant through the Gastroenterology Center of Connecticut/Medical Research Center of Connecticut by Paul Feuerstadt, MD. The patient being treated had experienced multiple recurrences of C. difficile infection. As part of the registry, Dr. Feuerstadt will follow up with the patient four times over the next 2 years and report back on the patient’s health post-FMT. The patient will also provide yearly reports for up to 10 years.
The AGA FMT National Registry, a program of the AGA Center for Gut Microbiome Research and Education, was established in August 2016 after receiving funding from the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH (award number R24AI118629). The registry aims to enroll 75 sites and track 4,000 patients for 5-10 years after their FMT procedure. The data collected from this registry will guide physicians in determining when to use FMT on their patients and will provide much-needed information on the potential risks associated with stool transplants.
If you’re interested in participating in the registry, email [email protected].
New registry collaborators
AGA will collaborate with the American Gut Project – an academic effort run by the laboratory of Rob Knight, PhD, professor and director of the Center for Microbiome Innovation at the University of California, San Diego – to build a biobank of stool samples from participants in the FMT National Registry. American Gut will receive stool samples from registry participants before and after their FMT. The microbiota will be sequenced in each sample, and remaining material will be frozen to be made available for future research. Eventually, this information could help doctors screen and select the best donor samples for individual patients.
AGA will also collaborate with OpenBiome, a public stool bank and nonprofit research organization that provides clinicians with rigorously screened, ready-to-use stool preparations for fecal transplant procedures. As the only public stool bank in the country, OpenBiome serves as the source of stool preparations for nearly 1,000 clinical partners performing FMT across the U.S. For patients enrolled in the registry who receive OpenBiome FMT material, OpenBiome will provide screening information and samples to support the registry’s research analyses. Learn more at www.gastro.org/FMTRegistry.
The AGA Fecal Microbiota Transplantation (FMT) National Registry is officially underway! The first patient enrolled in the FMT National Registry received a fecal transplant through the Gastroenterology Center of Connecticut/Medical Research Center of Connecticut by Paul Feuerstadt, MD. The patient being treated had experienced multiple recurrences of C. difficile infection. As part of the registry, Dr. Feuerstadt will follow up with the patient four times over the next 2 years and report back on the patient’s health post-FMT. The patient will also provide yearly reports for up to 10 years.
The AGA FMT National Registry, a program of the AGA Center for Gut Microbiome Research and Education, was established in August 2016 after receiving funding from the National Institute of Allergy and Infectious Diseases (NIAID) of the NIH (award number R24AI118629). The registry aims to enroll 75 sites and track 4,000 patients for 5-10 years after their FMT procedure. The data collected from this registry will guide physicians in determining when to use FMT on their patients and will provide much-needed information on the potential risks associated with stool transplants.
If you’re interested in participating in the registry, email [email protected].
New registry collaborators
AGA will collaborate with the American Gut Project – an academic effort run by the laboratory of Rob Knight, PhD, professor and director of the Center for Microbiome Innovation at the University of California, San Diego – to build a biobank of stool samples from participants in the FMT National Registry. American Gut will receive stool samples from registry participants before and after their FMT. The microbiota will be sequenced in each sample, and remaining material will be frozen to be made available for future research. Eventually, this information could help doctors screen and select the best donor samples for individual patients.
AGA will also collaborate with OpenBiome, a public stool bank and nonprofit research organization that provides clinicians with rigorously screened, ready-to-use stool preparations for fecal transplant procedures. As the only public stool bank in the country, OpenBiome serves as the source of stool preparations for nearly 1,000 clinical partners performing FMT across the U.S. For patients enrolled in the registry who receive OpenBiome FMT material, OpenBiome will provide screening information and samples to support the registry’s research analyses. Learn more at www.gastro.org/FMTRegistry.
Long-term effects of ketamine uncertain
LAS VEGAS – Low doses of ketamine, an N-methyl-D-asparate glutamate receptor antagonist, can produce benefits that are unprecedented in the history of treating major depression, according to David Feifel, MD, PhD.
“When have we ever had the ability to be sitting in front of a patient who is extremely suicidal and have an intervention that will work within [an] hour that will remediate that?” he asked at an annual psychopharmacology update held by the Nevada Psychiatric Association. “Ketamine is that tool. If nothing else, that’s what ketamine already presents to us in this field.”
Then there’s the potential for abuse. he asked. “What about other long-term side effects?”
A recent consensus statement authored by Gerard Sanacora, PhD, MD, and associates acknowledged that data on the long-term effects of using ketamine in psychiatry practice are limited or nonexistent (JAMA Psychiatry. 2017 Apr 1;74[4]:399-405), even though an increasing number of clinicians are providing off-label ketamine for depression and other psychiatric disorders. First synthesized in the 1960s, ketamine’s primary site of action in the central nervous system seems to be the thalamocortical projection system, said Dr. Feifel, who has been providing the drug to patients since 2008. In a handout that accompanied his talk, he wrote that ketamine “selectively depresses neuronal function in parts of the cortex and thalamus while stimulating parts of the limbic system, including the hippocampus. This process creates what is termed a functional disorganization of nonspecific pathways in midbrain and thalamic areas.”
Ketamine creates acute subjective experiences that vary with doses, including a sense of “melting into people or things” at lower doses, and visions and hallucinations at higher doses. “Patients will tell me that they deliberately tried to move their hands and feet, to make sure they were still connected to their bodies,” Dr. Feifel said. “There’s often a sensation of moving through space. At higher doses, they often experience a profound sense of connection to all things, an ineffable universal unity that can change their perspective on themselves and their depression. It’s very profound.”
Antidepressant benefits with ketamine are usually dramatic, said Dr. Feifel, who also is founder and director of the Kadima Neuropsychiatry Institute in La Jolla, Calif. The drug is most commonly administered as an IV infusion over a 40 minute period at a dosage of 0.5 mg/kg. It also can be administered orally, intranasally, and intramuscularly. “Patients may achieve response and even remission of depression within a day, even when the depression was previously medication refractory,” he said. “The benefits are usually lost in 3-21 days. Maintenance treatment with ketamine, scheduled once every 2-4 weeks, can maintain the treatment gains. Dissociative and psychotomimetic effects are common but very seldom problematic.”
Dr. Feifel noted that ketamine’s remarkable results have piqued the interest of pharmaceutical companies. “But it is off patent – non proprietary,” he said. “Several novel agents by different pharmaceutical companies quickly have been developed and are in development. Some have modified pharmacology and are claimed to produce less acute dissociative/psychedelic effects. Janssen is developing intranasal esketamine for acutely suicidal patients that has been fast-tracked by the FDA.”
Dr. Feifel reported having no financial disclosures.
LAS VEGAS – Low doses of ketamine, an N-methyl-D-asparate glutamate receptor antagonist, can produce benefits that are unprecedented in the history of treating major depression, according to David Feifel, MD, PhD.
“When have we ever had the ability to be sitting in front of a patient who is extremely suicidal and have an intervention that will work within [an] hour that will remediate that?” he asked at an annual psychopharmacology update held by the Nevada Psychiatric Association. “Ketamine is that tool. If nothing else, that’s what ketamine already presents to us in this field.”
Then there’s the potential for abuse. he asked. “What about other long-term side effects?”
A recent consensus statement authored by Gerard Sanacora, PhD, MD, and associates acknowledged that data on the long-term effects of using ketamine in psychiatry practice are limited or nonexistent (JAMA Psychiatry. 2017 Apr 1;74[4]:399-405), even though an increasing number of clinicians are providing off-label ketamine for depression and other psychiatric disorders. First synthesized in the 1960s, ketamine’s primary site of action in the central nervous system seems to be the thalamocortical projection system, said Dr. Feifel, who has been providing the drug to patients since 2008. In a handout that accompanied his talk, he wrote that ketamine “selectively depresses neuronal function in parts of the cortex and thalamus while stimulating parts of the limbic system, including the hippocampus. This process creates what is termed a functional disorganization of nonspecific pathways in midbrain and thalamic areas.”
Ketamine creates acute subjective experiences that vary with doses, including a sense of “melting into people or things” at lower doses, and visions and hallucinations at higher doses. “Patients will tell me that they deliberately tried to move their hands and feet, to make sure they were still connected to their bodies,” Dr. Feifel said. “There’s often a sensation of moving through space. At higher doses, they often experience a profound sense of connection to all things, an ineffable universal unity that can change their perspective on themselves and their depression. It’s very profound.”
Antidepressant benefits with ketamine are usually dramatic, said Dr. Feifel, who also is founder and director of the Kadima Neuropsychiatry Institute in La Jolla, Calif. The drug is most commonly administered as an IV infusion over a 40 minute period at a dosage of 0.5 mg/kg. It also can be administered orally, intranasally, and intramuscularly. “Patients may achieve response and even remission of depression within a day, even when the depression was previously medication refractory,” he said. “The benefits are usually lost in 3-21 days. Maintenance treatment with ketamine, scheduled once every 2-4 weeks, can maintain the treatment gains. Dissociative and psychotomimetic effects are common but very seldom problematic.”
Dr. Feifel noted that ketamine’s remarkable results have piqued the interest of pharmaceutical companies. “But it is off patent – non proprietary,” he said. “Several novel agents by different pharmaceutical companies quickly have been developed and are in development. Some have modified pharmacology and are claimed to produce less acute dissociative/psychedelic effects. Janssen is developing intranasal esketamine for acutely suicidal patients that has been fast-tracked by the FDA.”
Dr. Feifel reported having no financial disclosures.
LAS VEGAS – Low doses of ketamine, an N-methyl-D-asparate glutamate receptor antagonist, can produce benefits that are unprecedented in the history of treating major depression, according to David Feifel, MD, PhD.
“When have we ever had the ability to be sitting in front of a patient who is extremely suicidal and have an intervention that will work within [an] hour that will remediate that?” he asked at an annual psychopharmacology update held by the Nevada Psychiatric Association. “Ketamine is that tool. If nothing else, that’s what ketamine already presents to us in this field.”
Then there’s the potential for abuse. he asked. “What about other long-term side effects?”
A recent consensus statement authored by Gerard Sanacora, PhD, MD, and associates acknowledged that data on the long-term effects of using ketamine in psychiatry practice are limited or nonexistent (JAMA Psychiatry. 2017 Apr 1;74[4]:399-405), even though an increasing number of clinicians are providing off-label ketamine for depression and other psychiatric disorders. First synthesized in the 1960s, ketamine’s primary site of action in the central nervous system seems to be the thalamocortical projection system, said Dr. Feifel, who has been providing the drug to patients since 2008. In a handout that accompanied his talk, he wrote that ketamine “selectively depresses neuronal function in parts of the cortex and thalamus while stimulating parts of the limbic system, including the hippocampus. This process creates what is termed a functional disorganization of nonspecific pathways in midbrain and thalamic areas.”
Ketamine creates acute subjective experiences that vary with doses, including a sense of “melting into people or things” at lower doses, and visions and hallucinations at higher doses. “Patients will tell me that they deliberately tried to move their hands and feet, to make sure they were still connected to their bodies,” Dr. Feifel said. “There’s often a sensation of moving through space. At higher doses, they often experience a profound sense of connection to all things, an ineffable universal unity that can change their perspective on themselves and their depression. It’s very profound.”
Antidepressant benefits with ketamine are usually dramatic, said Dr. Feifel, who also is founder and director of the Kadima Neuropsychiatry Institute in La Jolla, Calif. The drug is most commonly administered as an IV infusion over a 40 minute period at a dosage of 0.5 mg/kg. It also can be administered orally, intranasally, and intramuscularly. “Patients may achieve response and even remission of depression within a day, even when the depression was previously medication refractory,” he said. “The benefits are usually lost in 3-21 days. Maintenance treatment with ketamine, scheduled once every 2-4 weeks, can maintain the treatment gains. Dissociative and psychotomimetic effects are common but very seldom problematic.”
Dr. Feifel noted that ketamine’s remarkable results have piqued the interest of pharmaceutical companies. “But it is off patent – non proprietary,” he said. “Several novel agents by different pharmaceutical companies quickly have been developed and are in development. Some have modified pharmacology and are claimed to produce less acute dissociative/psychedelic effects. Janssen is developing intranasal esketamine for acutely suicidal patients that has been fast-tracked by the FDA.”
Dr. Feifel reported having no financial disclosures.
REPORTING FROM NPA 2018
Embrace the complexity of marijuana use in adolescents
Las Vegas – When talking with adolescents and their families about marijuana use, Kevin M. Gray, MD, recommends embracing the complexity of the issue.
“Avoid polarizing this topic and avoid vilifying cannabis,” he advised at an annual psychopharmacology update held by the Nevada Psychiatric Association. “Take an interest in what they have to say about cannabis. Work in a gentle, nonconfrontational way where you avoid polarization and find some common ground where you can agree that maybe there’s some good and some bad [about cannabis], but the overwhelming evidence in adolescents is that there’s more harm than good with cannabis use, particularly recreationally.”
Dr. Gray, professor and director of child and adolescent psychiatry at the Medical University of South Carolina, Charleston, acknowledged that clinicians face a delicate balance between risk and benefit, even among Food and Drug Administration–approved medications. However, teens and families may struggle with these nuances, especially in light of the term “medical marijuana.” Some assume that “medical” implies “beneficial.” Others may equate “marijuana” with “natural,” which they may, in turn, equate with being “harmless.”
“Perception is critically important,” Dr. Gray said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
Cannabis initiation typically occurs during adolescence, and rates of initiation and use are increasing. According to Dr. Gray, 55% of U.S. high school seniors have used marijuana, 23% use currently, and 6% use daily. “Those are the ones who have adverse outcomes,” he said. Young users are particularly prone to dependence symptoms and an inability to cut back their use. The odds of meeting criteria for cannabis use disorder are substantially greater in adolescent users than they are in adults regardless of time frame or intensity of use.
“In a dose-dependent manner, adolescent cannabis use is associated with adverse academic, occupational, cognitive, psychiatric, and substance use outcomes,” Dr. Gray said, adding that the average potency of delta-9-tetrahydrocannabinol (THC) in seized cannabis increased from 3% in 1992 to 11% in 2010. “Cannabis use in adolescence is associated with increased incidence and worsened course of psychotic, mood, and anxiety disorders. Serious cannabis-associated risks are well recognized and are particularly striking in adolescents. Adult-onset cannabis users may experience fewer adverse effects.”
Evidence-based psychosocial approaches for adolescents with cannabis use disorder include motivational enhancement therapy, which involves building rapport in a gentle way, with phrasing such as “Tell me what you like about marijuana use” and “What don’t you like about it?” Dr. Gray described motivational enhancement therapy as “a gentle nudge for behavior change” that serves as a bridge to cognitive-behavioral therapy, family therapy, and contingency management. “That said, long-term abstinence outcomes are generally poor,” he said. “People tend to go back to use.”
N-acetylcysteine (NAC) shows promise as a medication for adolescents with cannabis use disorder. NAC activates the cystine/glutamate exchanger and upregulates the GLT-1 receptor, which leads to reduction in reinstatement of drug seeking in animal models. One trial of NAC supported efficacy in 116 cannabis-dependent adolescents (Am J Psychiatry. 2012 Aug;169[8]:805-12). Led by Dr. Gray, the trial consisted of 8 weeks of active treatment on placebo or NAC 1,200 mg BID. All participants received weekly brief cessation counseling and twice-weekly contingency management. The researchers found that adolescents in the NAC group were more than twice as likely to submit a negative urine specimen during treatment than were their counterparts in the placebo group (odds ratio, 2.4; P = .029). In addition, those in the NAC group also were significantly more likely than were those in the placebo group to achieve end-of-treatment abstinence, which was defined as self-reported abstinence confirmed by negative urine testing throughout the last 2 weeks of treatment (OR, 2.3; P = .054).
A similarly designed adult trial indicated that adolescent findings did not translate to adults (Drug Alcohol Depend. 2017 Aug 1;177:249-57). “Whether this may be due to developmental differences in the course and phenomenology of cannabis use disorder, we don’t know,” Dr. Gray said. “For now, NAC remains the only pharmacotherapy with positive published intent-to-treat clinical trial abstinence findings for cannabis use disorder in adolescents. Positive adolescent findings must be replicated, but the necessary behavioral treatment platform must be clarified to translate successfully to real-world practice.”
Dr. Gray disclosed that he receives research funding from the National Institutes of Health.
[email protected]
Las Vegas – When talking with adolescents and their families about marijuana use, Kevin M. Gray, MD, recommends embracing the complexity of the issue.
“Avoid polarizing this topic and avoid vilifying cannabis,” he advised at an annual psychopharmacology update held by the Nevada Psychiatric Association. “Take an interest in what they have to say about cannabis. Work in a gentle, nonconfrontational way where you avoid polarization and find some common ground where you can agree that maybe there’s some good and some bad [about cannabis], but the overwhelming evidence in adolescents is that there’s more harm than good with cannabis use, particularly recreationally.”
Dr. Gray, professor and director of child and adolescent psychiatry at the Medical University of South Carolina, Charleston, acknowledged that clinicians face a delicate balance between risk and benefit, even among Food and Drug Administration–approved medications. However, teens and families may struggle with these nuances, especially in light of the term “medical marijuana.” Some assume that “medical” implies “beneficial.” Others may equate “marijuana” with “natural,” which they may, in turn, equate with being “harmless.”
“Perception is critically important,” Dr. Gray said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
Cannabis initiation typically occurs during adolescence, and rates of initiation and use are increasing. According to Dr. Gray, 55% of U.S. high school seniors have used marijuana, 23% use currently, and 6% use daily. “Those are the ones who have adverse outcomes,” he said. Young users are particularly prone to dependence symptoms and an inability to cut back their use. The odds of meeting criteria for cannabis use disorder are substantially greater in adolescent users than they are in adults regardless of time frame or intensity of use.
“In a dose-dependent manner, adolescent cannabis use is associated with adverse academic, occupational, cognitive, psychiatric, and substance use outcomes,” Dr. Gray said, adding that the average potency of delta-9-tetrahydrocannabinol (THC) in seized cannabis increased from 3% in 1992 to 11% in 2010. “Cannabis use in adolescence is associated with increased incidence and worsened course of psychotic, mood, and anxiety disorders. Serious cannabis-associated risks are well recognized and are particularly striking in adolescents. Adult-onset cannabis users may experience fewer adverse effects.”
Evidence-based psychosocial approaches for adolescents with cannabis use disorder include motivational enhancement therapy, which involves building rapport in a gentle way, with phrasing such as “Tell me what you like about marijuana use” and “What don’t you like about it?” Dr. Gray described motivational enhancement therapy as “a gentle nudge for behavior change” that serves as a bridge to cognitive-behavioral therapy, family therapy, and contingency management. “That said, long-term abstinence outcomes are generally poor,” he said. “People tend to go back to use.”
N-acetylcysteine (NAC) shows promise as a medication for adolescents with cannabis use disorder. NAC activates the cystine/glutamate exchanger and upregulates the GLT-1 receptor, which leads to reduction in reinstatement of drug seeking in animal models. One trial of NAC supported efficacy in 116 cannabis-dependent adolescents (Am J Psychiatry. 2012 Aug;169[8]:805-12). Led by Dr. Gray, the trial consisted of 8 weeks of active treatment on placebo or NAC 1,200 mg BID. All participants received weekly brief cessation counseling and twice-weekly contingency management. The researchers found that adolescents in the NAC group were more than twice as likely to submit a negative urine specimen during treatment than were their counterparts in the placebo group (odds ratio, 2.4; P = .029). In addition, those in the NAC group also were significantly more likely than were those in the placebo group to achieve end-of-treatment abstinence, which was defined as self-reported abstinence confirmed by negative urine testing throughout the last 2 weeks of treatment (OR, 2.3; P = .054).
A similarly designed adult trial indicated that adolescent findings did not translate to adults (Drug Alcohol Depend. 2017 Aug 1;177:249-57). “Whether this may be due to developmental differences in the course and phenomenology of cannabis use disorder, we don’t know,” Dr. Gray said. “For now, NAC remains the only pharmacotherapy with positive published intent-to-treat clinical trial abstinence findings for cannabis use disorder in adolescents. Positive adolescent findings must be replicated, but the necessary behavioral treatment platform must be clarified to translate successfully to real-world practice.”
Dr. Gray disclosed that he receives research funding from the National Institutes of Health.
[email protected]
Las Vegas – When talking with adolescents and their families about marijuana use, Kevin M. Gray, MD, recommends embracing the complexity of the issue.
“Avoid polarizing this topic and avoid vilifying cannabis,” he advised at an annual psychopharmacology update held by the Nevada Psychiatric Association. “Take an interest in what they have to say about cannabis. Work in a gentle, nonconfrontational way where you avoid polarization and find some common ground where you can agree that maybe there’s some good and some bad [about cannabis], but the overwhelming evidence in adolescents is that there’s more harm than good with cannabis use, particularly recreationally.”
Dr. Gray, professor and director of child and adolescent psychiatry at the Medical University of South Carolina, Charleston, acknowledged that clinicians face a delicate balance between risk and benefit, even among Food and Drug Administration–approved medications. However, teens and families may struggle with these nuances, especially in light of the term “medical marijuana.” Some assume that “medical” implies “beneficial.” Others may equate “marijuana” with “natural,” which they may, in turn, equate with being “harmless.”
“Perception is critically important,” Dr. Gray said at an annual psychopharmacology update held by the Nevada Psychiatric Association.
Cannabis initiation typically occurs during adolescence, and rates of initiation and use are increasing. According to Dr. Gray, 55% of U.S. high school seniors have used marijuana, 23% use currently, and 6% use daily. “Those are the ones who have adverse outcomes,” he said. Young users are particularly prone to dependence symptoms and an inability to cut back their use. The odds of meeting criteria for cannabis use disorder are substantially greater in adolescent users than they are in adults regardless of time frame or intensity of use.
“In a dose-dependent manner, adolescent cannabis use is associated with adverse academic, occupational, cognitive, psychiatric, and substance use outcomes,” Dr. Gray said, adding that the average potency of delta-9-tetrahydrocannabinol (THC) in seized cannabis increased from 3% in 1992 to 11% in 2010. “Cannabis use in adolescence is associated with increased incidence and worsened course of psychotic, mood, and anxiety disorders. Serious cannabis-associated risks are well recognized and are particularly striking in adolescents. Adult-onset cannabis users may experience fewer adverse effects.”
Evidence-based psychosocial approaches for adolescents with cannabis use disorder include motivational enhancement therapy, which involves building rapport in a gentle way, with phrasing such as “Tell me what you like about marijuana use” and “What don’t you like about it?” Dr. Gray described motivational enhancement therapy as “a gentle nudge for behavior change” that serves as a bridge to cognitive-behavioral therapy, family therapy, and contingency management. “That said, long-term abstinence outcomes are generally poor,” he said. “People tend to go back to use.”
N-acetylcysteine (NAC) shows promise as a medication for adolescents with cannabis use disorder. NAC activates the cystine/glutamate exchanger and upregulates the GLT-1 receptor, which leads to reduction in reinstatement of drug seeking in animal models. One trial of NAC supported efficacy in 116 cannabis-dependent adolescents (Am J Psychiatry. 2012 Aug;169[8]:805-12). Led by Dr. Gray, the trial consisted of 8 weeks of active treatment on placebo or NAC 1,200 mg BID. All participants received weekly brief cessation counseling and twice-weekly contingency management. The researchers found that adolescents in the NAC group were more than twice as likely to submit a negative urine specimen during treatment than were their counterparts in the placebo group (odds ratio, 2.4; P = .029). In addition, those in the NAC group also were significantly more likely than were those in the placebo group to achieve end-of-treatment abstinence, which was defined as self-reported abstinence confirmed by negative urine testing throughout the last 2 weeks of treatment (OR, 2.3; P = .054).
A similarly designed adult trial indicated that adolescent findings did not translate to adults (Drug Alcohol Depend. 2017 Aug 1;177:249-57). “Whether this may be due to developmental differences in the course and phenomenology of cannabis use disorder, we don’t know,” Dr. Gray said. “For now, NAC remains the only pharmacotherapy with positive published intent-to-treat clinical trial abstinence findings for cannabis use disorder in adolescents. Positive adolescent findings must be replicated, but the necessary behavioral treatment platform must be clarified to translate successfully to real-world practice.”
Dr. Gray disclosed that he receives research funding from the National Institutes of Health.
[email protected]
REPORTING FROM NPA 2018
NF-kappaB pathway could help solve resistance problem in mantle cell lymphoma
B-cell receptor (BCR) resistance is a significant treatment obstacle in mantle cell lymphoma (MCL), but a new study highlights the potential protective role for cells expressing specific ligands.
Hilka Rauert-Wunderlich, of the University of Würzburg and Comprehensive Cancer Center, Germany, and her colleagues stimulated the REC-1, MAVER-1, and L-929 cell lines to show the role of the alternative nuclear factor-kappa B (NF-kappaB) pathway and the tumor necrosis factor ligand CD40L.
Viability assays showed a protective effect of CD40L prestimulation on BCR inhibitor treatment. The effect was detectable and significant in the REC-1 cell line for both ibrutinib and sotrastaurin at “clinically relevant concentrations” and in the resistant MAVER-1 cell line at “nonphysiologically high” sotrastaurin concentrations. CD40L stimulation also induced alternative NF-kappaB pathway signaling in both REC-1 and MAVER-1 cell lines.
“The data presented in this study argue for the protective potential of microenvironmentally mediated activation of the alternative [NF-kappaB] pathway in MCL cell against BCR signaling-associated drugs, which might represent a physiologic niche for MCL relapse. Additionally, these data provide evidence for the potential of the alternative [NF-kappaB] pathway as a possible therapeutic target in MCL,” the researchers wrote in Cell Death & Disease.
The researchers reported having no conflicts of interest.
SOURCE: Rauert-Wunderlich H et al. Cell Death Dis. 2018 Jan 24. doi: 10.1038/s41419-017-0157-6.
B-cell receptor (BCR) resistance is a significant treatment obstacle in mantle cell lymphoma (MCL), but a new study highlights the potential protective role for cells expressing specific ligands.
Hilka Rauert-Wunderlich, of the University of Würzburg and Comprehensive Cancer Center, Germany, and her colleagues stimulated the REC-1, MAVER-1, and L-929 cell lines to show the role of the alternative nuclear factor-kappa B (NF-kappaB) pathway and the tumor necrosis factor ligand CD40L.
Viability assays showed a protective effect of CD40L prestimulation on BCR inhibitor treatment. The effect was detectable and significant in the REC-1 cell line for both ibrutinib and sotrastaurin at “clinically relevant concentrations” and in the resistant MAVER-1 cell line at “nonphysiologically high” sotrastaurin concentrations. CD40L stimulation also induced alternative NF-kappaB pathway signaling in both REC-1 and MAVER-1 cell lines.
“The data presented in this study argue for the protective potential of microenvironmentally mediated activation of the alternative [NF-kappaB] pathway in MCL cell against BCR signaling-associated drugs, which might represent a physiologic niche for MCL relapse. Additionally, these data provide evidence for the potential of the alternative [NF-kappaB] pathway as a possible therapeutic target in MCL,” the researchers wrote in Cell Death & Disease.
The researchers reported having no conflicts of interest.
SOURCE: Rauert-Wunderlich H et al. Cell Death Dis. 2018 Jan 24. doi: 10.1038/s41419-017-0157-6.
B-cell receptor (BCR) resistance is a significant treatment obstacle in mantle cell lymphoma (MCL), but a new study highlights the potential protective role for cells expressing specific ligands.
Hilka Rauert-Wunderlich, of the University of Würzburg and Comprehensive Cancer Center, Germany, and her colleagues stimulated the REC-1, MAVER-1, and L-929 cell lines to show the role of the alternative nuclear factor-kappa B (NF-kappaB) pathway and the tumor necrosis factor ligand CD40L.
Viability assays showed a protective effect of CD40L prestimulation on BCR inhibitor treatment. The effect was detectable and significant in the REC-1 cell line for both ibrutinib and sotrastaurin at “clinically relevant concentrations” and in the resistant MAVER-1 cell line at “nonphysiologically high” sotrastaurin concentrations. CD40L stimulation also induced alternative NF-kappaB pathway signaling in both REC-1 and MAVER-1 cell lines.
“The data presented in this study argue for the protective potential of microenvironmentally mediated activation of the alternative [NF-kappaB] pathway in MCL cell against BCR signaling-associated drugs, which might represent a physiologic niche for MCL relapse. Additionally, these data provide evidence for the potential of the alternative [NF-kappaB] pathway as a possible therapeutic target in MCL,” the researchers wrote in Cell Death & Disease.
The researchers reported having no conflicts of interest.
SOURCE: Rauert-Wunderlich H et al. Cell Death Dis. 2018 Jan 24. doi: 10.1038/s41419-017-0157-6.
FROM CELL DEATH & DISEASE
Axitinib/pembrolizumab combo safe, effective against mRCC
A combination of the tyrosine kinase inhibitor axitinib (Inlyta) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was associated with acceptable toxicities and showed promising activity against advanced renal cell carcinoma (RCC) in the first-line setting, results of a phase 1b trial indicate.
Of 11 patients enrolled in a dose-finding study and 41 enrolled in the expansion phase of that study, 38 had an objective response (complete or partial response), for an overall response rate of 73%, reported Michael B. Atkins, MD, of Georgetown-Lombardi Comprehensive Cancer Center, Washington, and his colleagues.
“This phase 1b study showed that the combination of axitinib and pembrolizumab at nearly the full planned doses of each drug is tolerable in patients with treatment-naive advanced renal cell carcinoma,” they wrote. The report was published in The Lancet Oncology.
Previous studies of programmed death-1 (PD-1) checkpoint inhibitors such as pembrolizumab or nivolumab (Opdivo) combined with inhibitors of vascular endothelial growth factor (VEGF) have resulted in excessive toxicities attributed to off-target effects of the VEGF inhibitors used.
The investigators reasoned that because axitinib is more selective and specific for targets in the VEGF pathway, it might make a safer and more effective partner to a PD-1 inhibitor than the multikinase inhibitors sunitinib (Sutent) or pazopanib (Votrient).
“A formal systematic review was not done before doing this trial because most of the work combining VEGF pathway inhibitors with checkpoint inhibitors is new and not yet published,” Dr. Atkins and his associates explained.
As of the March 31, 2017, data cutoff, 52 patients from 10 U.S. centers had been treated with the same dose and schedule and were included in the analysis. All patients had tumors with clear cell renal carcinoma histologies; one also had sarcomatoid features.
There were three investigator-assessed dose-limiting toxicities (DLT, the primary endpoint) in the 11 patients treated in the dose-finding phase. One of the patients had a transient ischemic attack and two completed less than 75% of the planned axitinib dose because of treatment-related toxicities.
Of the 52 patients, 25 were still on treatment at the time of data cutoff: 22 who were still receiving both axitinib and pembrolizumab and 3 who were receiving only the PD-1 inhibitor. Eight of the patients continued on therapy despite disease progression.
Of the 27 patients who discontinued both drugs, 10 did so because of adverse events, 9 for disease progression, and others for various reasons such as mixed adverse events and disease progression, investigator discretion, global deterioration, or protocol violation.
Grade 3 or greater adverse events occurred in 34 patients (65%), and included hypertension, diarrhea, fatigue, and elevated alanine aminostransferase (ALT) levels.
The most common potentially immune-related adverse events were diarrhea, ALT elevations, hypothyroidism, and fatigue.
At a median follow-up of 20.4 months, 4 patients had a complete response, and 34 had a partial response. An additional eight patients had stable disease. Responses were seen in 18 of 24 patients with favorable-risk disease and in 18 of 26 patients with intermediate- or poor-risk disease. The median time to response was 2.8 months, and the median duration of response was 18.6 months.
“Future research should focus on investigating the mechanism of the potential synergistic effects of axitinib and pembrolizumab, and whether an immunotherapy-only approach (including combinations) enriched by the appropriate biomarkers, followed by VEGFR TKI salvage, might produce more durable off-treatment responses or whether administering VEGFR TKI monotherapy followed by PD-1 and PD-L1 pathway blockade might produce superior or equivalent results,” the investigators concluded.
Pfizer, in collaboration with Merck, sponsored the study. Dr. Atkins and several coauthors disclosed consulting fees from Pfizer, Merck, and other companies. Four of the coauthors are Pfizer employees and stockholders.
SOURCE: Atkins MB et al. Lancet Oncol 2018 Feb. 10. doi: 10.1016/S1470-2045(18)30081-0.
This phase 1 trial highlights some issues that merit discussion. First, the eligibility criteria of the study population should be considered. Renal cell carcinoma is a very heterogeneous disease with a natural history that could range from an indolent clinical course to a slow progressive or an aggressive behavior. The prognostic models proposed by Memorial Sloan-Kettering Cancer Center and the International Metastatic Renal Cell Carcinoma Database Consortium are now validated for stratification of patients in clinical trials. By contrast, patients treated in clinical practice are often excluded from clinical trials and have a poor prognosis and derive less benefit from standard treatments. Atkins and colleagues treated a small number of patients from a highly selected study population, with no patients with poor clinical conditions or who had not undergone nephrectomy. Considering these aspects, how have the favorable prognostic features of the population affected the feasibility and efficacy of the treatment proposed? In this regard, only assessment of a larger number of patients in a less selected population can confirm these activity results.
Second, the absence of central radiological review in the trial could represent a major limitation in the interpretation of treatment response and the evaluation of progression-free survival. The Response Evaluation Criteria in Solid Tumors version 1.1 criteria used to assess response are often unable to distinguish between pseudoprogression, hyperprogression, and late response to immunotherapies. Therefore, the assessment of response during treatment with immune checkpoint inhibitors remains debated, and physicians often carefully consider the opportunity to continue treatment in cases with doubtful or mixed response and must decide whether to change the therapy on the basis of the clinical benefit being received by patients.
Third, another issue concerns the potential benefit of the combination therapy proposed. Axitinib is a multikinase inhibitor able to act on a broad spectrum of kinases related to angiogenesis. Axitinib monotherapy is considered a therapeutic option after an angiogenesis inhibitor for patients with metastatic renal cell carcinoma. Pembrolizumab is a PD-1 inhibitor under evaluation in combination strategies for the treatment of metastatic renal cell carcinoma. The combination of pembrolizumab and axitinib was safe and feasible, which is in contrast to results previously reported for other combinations, such as nivolumab plus pazopanib or sunitinib and pembrolizumab plus pazopanib. This evidence suggests that similar drugs might have different toxicity profiles when used in combination, and axitinib remains one the most tolerable tyrosine kinase inhibitors.
In summary, the combination of pembrolizumab and axitinib is very promising and the outcomes of Atkins and colleagues’ study could become the first evidence in favor of a combination of two drugs with different mechanisms of action for the treatment of metastatic renal cell carcinoma. Future research should attempt to select more patients who will respond to treatment on the basis of their clinical and molecular features.
Giuseppe Procopio, MD, Raffaele Ratta, MD, Filippo de Braud, MD, and Elena Verzoni, MD, are with the medical oncology department of Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, and the medical oncology department of the University of Milan. The commentary was adapted and condensed from an editorial (Lancet Oncol. 2018 Feb 10. doi: 10.1016/S1470-2045[18]30092-5).
This phase 1 trial highlights some issues that merit discussion. First, the eligibility criteria of the study population should be considered. Renal cell carcinoma is a very heterogeneous disease with a natural history that could range from an indolent clinical course to a slow progressive or an aggressive behavior. The prognostic models proposed by Memorial Sloan-Kettering Cancer Center and the International Metastatic Renal Cell Carcinoma Database Consortium are now validated for stratification of patients in clinical trials. By contrast, patients treated in clinical practice are often excluded from clinical trials and have a poor prognosis and derive less benefit from standard treatments. Atkins and colleagues treated a small number of patients from a highly selected study population, with no patients with poor clinical conditions or who had not undergone nephrectomy. Considering these aspects, how have the favorable prognostic features of the population affected the feasibility and efficacy of the treatment proposed? In this regard, only assessment of a larger number of patients in a less selected population can confirm these activity results.
Second, the absence of central radiological review in the trial could represent a major limitation in the interpretation of treatment response and the evaluation of progression-free survival. The Response Evaluation Criteria in Solid Tumors version 1.1 criteria used to assess response are often unable to distinguish between pseudoprogression, hyperprogression, and late response to immunotherapies. Therefore, the assessment of response during treatment with immune checkpoint inhibitors remains debated, and physicians often carefully consider the opportunity to continue treatment in cases with doubtful or mixed response and must decide whether to change the therapy on the basis of the clinical benefit being received by patients.
Third, another issue concerns the potential benefit of the combination therapy proposed. Axitinib is a multikinase inhibitor able to act on a broad spectrum of kinases related to angiogenesis. Axitinib monotherapy is considered a therapeutic option after an angiogenesis inhibitor for patients with metastatic renal cell carcinoma. Pembrolizumab is a PD-1 inhibitor under evaluation in combination strategies for the treatment of metastatic renal cell carcinoma. The combination of pembrolizumab and axitinib was safe and feasible, which is in contrast to results previously reported for other combinations, such as nivolumab plus pazopanib or sunitinib and pembrolizumab plus pazopanib. This evidence suggests that similar drugs might have different toxicity profiles when used in combination, and axitinib remains one the most tolerable tyrosine kinase inhibitors.
In summary, the combination of pembrolizumab and axitinib is very promising and the outcomes of Atkins and colleagues’ study could become the first evidence in favor of a combination of two drugs with different mechanisms of action for the treatment of metastatic renal cell carcinoma. Future research should attempt to select more patients who will respond to treatment on the basis of their clinical and molecular features.
Giuseppe Procopio, MD, Raffaele Ratta, MD, Filippo de Braud, MD, and Elena Verzoni, MD, are with the medical oncology department of Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, and the medical oncology department of the University of Milan. The commentary was adapted and condensed from an editorial (Lancet Oncol. 2018 Feb 10. doi: 10.1016/S1470-2045[18]30092-5).
This phase 1 trial highlights some issues that merit discussion. First, the eligibility criteria of the study population should be considered. Renal cell carcinoma is a very heterogeneous disease with a natural history that could range from an indolent clinical course to a slow progressive or an aggressive behavior. The prognostic models proposed by Memorial Sloan-Kettering Cancer Center and the International Metastatic Renal Cell Carcinoma Database Consortium are now validated for stratification of patients in clinical trials. By contrast, patients treated in clinical practice are often excluded from clinical trials and have a poor prognosis and derive less benefit from standard treatments. Atkins and colleagues treated a small number of patients from a highly selected study population, with no patients with poor clinical conditions or who had not undergone nephrectomy. Considering these aspects, how have the favorable prognostic features of the population affected the feasibility and efficacy of the treatment proposed? In this regard, only assessment of a larger number of patients in a less selected population can confirm these activity results.
Second, the absence of central radiological review in the trial could represent a major limitation in the interpretation of treatment response and the evaluation of progression-free survival. The Response Evaluation Criteria in Solid Tumors version 1.1 criteria used to assess response are often unable to distinguish between pseudoprogression, hyperprogression, and late response to immunotherapies. Therefore, the assessment of response during treatment with immune checkpoint inhibitors remains debated, and physicians often carefully consider the opportunity to continue treatment in cases with doubtful or mixed response and must decide whether to change the therapy on the basis of the clinical benefit being received by patients.
Third, another issue concerns the potential benefit of the combination therapy proposed. Axitinib is a multikinase inhibitor able to act on a broad spectrum of kinases related to angiogenesis. Axitinib monotherapy is considered a therapeutic option after an angiogenesis inhibitor for patients with metastatic renal cell carcinoma. Pembrolizumab is a PD-1 inhibitor under evaluation in combination strategies for the treatment of metastatic renal cell carcinoma. The combination of pembrolizumab and axitinib was safe and feasible, which is in contrast to results previously reported for other combinations, such as nivolumab plus pazopanib or sunitinib and pembrolizumab plus pazopanib. This evidence suggests that similar drugs might have different toxicity profiles when used in combination, and axitinib remains one the most tolerable tyrosine kinase inhibitors.
In summary, the combination of pembrolizumab and axitinib is very promising and the outcomes of Atkins and colleagues’ study could become the first evidence in favor of a combination of two drugs with different mechanisms of action for the treatment of metastatic renal cell carcinoma. Future research should attempt to select more patients who will respond to treatment on the basis of their clinical and molecular features.
Giuseppe Procopio, MD, Raffaele Ratta, MD, Filippo de Braud, MD, and Elena Verzoni, MD, are with the medical oncology department of Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, and the medical oncology department of the University of Milan. The commentary was adapted and condensed from an editorial (Lancet Oncol. 2018 Feb 10. doi: 10.1016/S1470-2045[18]30092-5).
A combination of the tyrosine kinase inhibitor axitinib (Inlyta) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was associated with acceptable toxicities and showed promising activity against advanced renal cell carcinoma (RCC) in the first-line setting, results of a phase 1b trial indicate.
Of 11 patients enrolled in a dose-finding study and 41 enrolled in the expansion phase of that study, 38 had an objective response (complete or partial response), for an overall response rate of 73%, reported Michael B. Atkins, MD, of Georgetown-Lombardi Comprehensive Cancer Center, Washington, and his colleagues.
“This phase 1b study showed that the combination of axitinib and pembrolizumab at nearly the full planned doses of each drug is tolerable in patients with treatment-naive advanced renal cell carcinoma,” they wrote. The report was published in The Lancet Oncology.
Previous studies of programmed death-1 (PD-1) checkpoint inhibitors such as pembrolizumab or nivolumab (Opdivo) combined with inhibitors of vascular endothelial growth factor (VEGF) have resulted in excessive toxicities attributed to off-target effects of the VEGF inhibitors used.
The investigators reasoned that because axitinib is more selective and specific for targets in the VEGF pathway, it might make a safer and more effective partner to a PD-1 inhibitor than the multikinase inhibitors sunitinib (Sutent) or pazopanib (Votrient).
“A formal systematic review was not done before doing this trial because most of the work combining VEGF pathway inhibitors with checkpoint inhibitors is new and not yet published,” Dr. Atkins and his associates explained.
As of the March 31, 2017, data cutoff, 52 patients from 10 U.S. centers had been treated with the same dose and schedule and were included in the analysis. All patients had tumors with clear cell renal carcinoma histologies; one also had sarcomatoid features.
There were three investigator-assessed dose-limiting toxicities (DLT, the primary endpoint) in the 11 patients treated in the dose-finding phase. One of the patients had a transient ischemic attack and two completed less than 75% of the planned axitinib dose because of treatment-related toxicities.
Of the 52 patients, 25 were still on treatment at the time of data cutoff: 22 who were still receiving both axitinib and pembrolizumab and 3 who were receiving only the PD-1 inhibitor. Eight of the patients continued on therapy despite disease progression.
Of the 27 patients who discontinued both drugs, 10 did so because of adverse events, 9 for disease progression, and others for various reasons such as mixed adverse events and disease progression, investigator discretion, global deterioration, or protocol violation.
Grade 3 or greater adverse events occurred in 34 patients (65%), and included hypertension, diarrhea, fatigue, and elevated alanine aminostransferase (ALT) levels.
The most common potentially immune-related adverse events were diarrhea, ALT elevations, hypothyroidism, and fatigue.
At a median follow-up of 20.4 months, 4 patients had a complete response, and 34 had a partial response. An additional eight patients had stable disease. Responses were seen in 18 of 24 patients with favorable-risk disease and in 18 of 26 patients with intermediate- or poor-risk disease. The median time to response was 2.8 months, and the median duration of response was 18.6 months.
“Future research should focus on investigating the mechanism of the potential synergistic effects of axitinib and pembrolizumab, and whether an immunotherapy-only approach (including combinations) enriched by the appropriate biomarkers, followed by VEGFR TKI salvage, might produce more durable off-treatment responses or whether administering VEGFR TKI monotherapy followed by PD-1 and PD-L1 pathway blockade might produce superior or equivalent results,” the investigators concluded.
Pfizer, in collaboration with Merck, sponsored the study. Dr. Atkins and several coauthors disclosed consulting fees from Pfizer, Merck, and other companies. Four of the coauthors are Pfizer employees and stockholders.
SOURCE: Atkins MB et al. Lancet Oncol 2018 Feb. 10. doi: 10.1016/S1470-2045(18)30081-0.
A combination of the tyrosine kinase inhibitor axitinib (Inlyta) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was associated with acceptable toxicities and showed promising activity against advanced renal cell carcinoma (RCC) in the first-line setting, results of a phase 1b trial indicate.
Of 11 patients enrolled in a dose-finding study and 41 enrolled in the expansion phase of that study, 38 had an objective response (complete or partial response), for an overall response rate of 73%, reported Michael B. Atkins, MD, of Georgetown-Lombardi Comprehensive Cancer Center, Washington, and his colleagues.
“This phase 1b study showed that the combination of axitinib and pembrolizumab at nearly the full planned doses of each drug is tolerable in patients with treatment-naive advanced renal cell carcinoma,” they wrote. The report was published in The Lancet Oncology.
Previous studies of programmed death-1 (PD-1) checkpoint inhibitors such as pembrolizumab or nivolumab (Opdivo) combined with inhibitors of vascular endothelial growth factor (VEGF) have resulted in excessive toxicities attributed to off-target effects of the VEGF inhibitors used.
The investigators reasoned that because axitinib is more selective and specific for targets in the VEGF pathway, it might make a safer and more effective partner to a PD-1 inhibitor than the multikinase inhibitors sunitinib (Sutent) or pazopanib (Votrient).
“A formal systematic review was not done before doing this trial because most of the work combining VEGF pathway inhibitors with checkpoint inhibitors is new and not yet published,” Dr. Atkins and his associates explained.
As of the March 31, 2017, data cutoff, 52 patients from 10 U.S. centers had been treated with the same dose and schedule and were included in the analysis. All patients had tumors with clear cell renal carcinoma histologies; one also had sarcomatoid features.
There were three investigator-assessed dose-limiting toxicities (DLT, the primary endpoint) in the 11 patients treated in the dose-finding phase. One of the patients had a transient ischemic attack and two completed less than 75% of the planned axitinib dose because of treatment-related toxicities.
Of the 52 patients, 25 were still on treatment at the time of data cutoff: 22 who were still receiving both axitinib and pembrolizumab and 3 who were receiving only the PD-1 inhibitor. Eight of the patients continued on therapy despite disease progression.
Of the 27 patients who discontinued both drugs, 10 did so because of adverse events, 9 for disease progression, and others for various reasons such as mixed adverse events and disease progression, investigator discretion, global deterioration, or protocol violation.
Grade 3 or greater adverse events occurred in 34 patients (65%), and included hypertension, diarrhea, fatigue, and elevated alanine aminostransferase (ALT) levels.
The most common potentially immune-related adverse events were diarrhea, ALT elevations, hypothyroidism, and fatigue.
At a median follow-up of 20.4 months, 4 patients had a complete response, and 34 had a partial response. An additional eight patients had stable disease. Responses were seen in 18 of 24 patients with favorable-risk disease and in 18 of 26 patients with intermediate- or poor-risk disease. The median time to response was 2.8 months, and the median duration of response was 18.6 months.
“Future research should focus on investigating the mechanism of the potential synergistic effects of axitinib and pembrolizumab, and whether an immunotherapy-only approach (including combinations) enriched by the appropriate biomarkers, followed by VEGFR TKI salvage, might produce more durable off-treatment responses or whether administering VEGFR TKI monotherapy followed by PD-1 and PD-L1 pathway blockade might produce superior or equivalent results,” the investigators concluded.
Pfizer, in collaboration with Merck, sponsored the study. Dr. Atkins and several coauthors disclosed consulting fees from Pfizer, Merck, and other companies. Four of the coauthors are Pfizer employees and stockholders.
SOURCE: Atkins MB et al. Lancet Oncol 2018 Feb. 10. doi: 10.1016/S1470-2045(18)30081-0.
FROM THE LANCET ONCOLOGY
Key clinical point: A combination of axitinib and pembrolizumab was tolerable and showed activity against advanced/metastatic renal cell carcinoma.
Major finding: At a median of 20.4 months of follow-up, the overall response rate was 73%.
Study details: Open-label phase 1b dose-finding trial and expansion in 52 patients with advanced RCC.
Disclosures: Pfizer, in collaboration with Merck, sponsored the study. Dr. Atkins and several coauthors disclosed consulting fees from Pfizer, Merck, and other companies. Four of the coauthors are Pfizer employees and stockholders.
Source: Atkins MB et al. Lancet Oncol 2018 Feb 10. doi: 10.1016/S1470-2045(18)30081-0.
Sexual aids not available to cancer survivors despite recommendations
ORLANDO – Therapeutic aids for sexual rehabilitation were not available at most major cancer centers, according to results of a structured telephone survey presented at the Cancer Survivorship Symposium.
Of the centers reached, 87% said they had no sexual aids available for men, and 72% said they had no such aids for women, said lead study author Sharon Bober, PhD, a psychologist at the Dana-Farber Cancer Institute in Boston, Massachusetts.
“I think the scarcity of all of these products really underscores the cultural taboos around sexual dysfunction, as did some of the discomfort of the staff responding to our calls,” Dr. Bober said in a press conference at the symposium, which was sponsored by the American Academy of Family Physicians, the American College of Physicians, and the American Society of Clinical Oncology.
Cancer treatment guidelines from the National Comprehensive Cancer Network (NCCN) recommend therapeutic aids for sexual health rehabilitation including vaginal dilators, moisturizers, and vacuum erection devices, Dr. Bober said.
Dr. Bober and her colleagues surveyed 25 NCI-designated Cancer Centers/National Comprehensive Cancer Network–member institutions about on-site availability of sexual aids and resources for cancer survivors.
After conducting internet searches and phone calls designed to identify potential sources of sexual aids at each center, study staff posed as relatives of patients and used a structured script to query cancer center staff about on-site availability of sexual aids.
Separate calls were conducted to query on availability of men and women’s sexual aids.
Of 23 centers that responded about men, 87% reported having no sexual aids, and of 22 centers that responded about women, 72% reported having no sexual aids, Dr. Bober reported at the symposium.
The lack of sexual aids was particularly notable given the wide availability of wigs, prosthetics, sunscreen, and other cancer care products at leading cancer centers, she added.
“Only one center of the 25 had an extensive list of products and resources for both men and women, which may well serve as a model when we think about the needs for cancer survivors in general,” said Dr. Bober.
These results suggest that leading cancer centers are not meeting the needs of cancer survivors in terms of recommended sexual therapeutic aids and informational resources, according to Timothy Gilligan, MD, an American Society of Clinical Oncology expert and member of the Cancer Survivorship news planning team.
“You sort of wonder where a cancer patient’s supposed to go to get this information if not at the Cancer Center,” said Dr. Gilligan, who moderated the press conference. “We’re really kind of leaving them shortchanged here, and the good news is I think we could easily do better if we just decide that we want to.”
The study was funded by Dana-Farber Cancer Institute. Dr. Bober reported research funding from Apex Neuro.
SOURCE: Bober S. et al. Cancer Survivorship Symposium Abstract #134
ORLANDO – Therapeutic aids for sexual rehabilitation were not available at most major cancer centers, according to results of a structured telephone survey presented at the Cancer Survivorship Symposium.
Of the centers reached, 87% said they had no sexual aids available for men, and 72% said they had no such aids for women, said lead study author Sharon Bober, PhD, a psychologist at the Dana-Farber Cancer Institute in Boston, Massachusetts.
“I think the scarcity of all of these products really underscores the cultural taboos around sexual dysfunction, as did some of the discomfort of the staff responding to our calls,” Dr. Bober said in a press conference at the symposium, which was sponsored by the American Academy of Family Physicians, the American College of Physicians, and the American Society of Clinical Oncology.
Cancer treatment guidelines from the National Comprehensive Cancer Network (NCCN) recommend therapeutic aids for sexual health rehabilitation including vaginal dilators, moisturizers, and vacuum erection devices, Dr. Bober said.
Dr. Bober and her colleagues surveyed 25 NCI-designated Cancer Centers/National Comprehensive Cancer Network–member institutions about on-site availability of sexual aids and resources for cancer survivors.
After conducting internet searches and phone calls designed to identify potential sources of sexual aids at each center, study staff posed as relatives of patients and used a structured script to query cancer center staff about on-site availability of sexual aids.
Separate calls were conducted to query on availability of men and women’s sexual aids.
Of 23 centers that responded about men, 87% reported having no sexual aids, and of 22 centers that responded about women, 72% reported having no sexual aids, Dr. Bober reported at the symposium.
The lack of sexual aids was particularly notable given the wide availability of wigs, prosthetics, sunscreen, and other cancer care products at leading cancer centers, she added.
“Only one center of the 25 had an extensive list of products and resources for both men and women, which may well serve as a model when we think about the needs for cancer survivors in general,” said Dr. Bober.
These results suggest that leading cancer centers are not meeting the needs of cancer survivors in terms of recommended sexual therapeutic aids and informational resources, according to Timothy Gilligan, MD, an American Society of Clinical Oncology expert and member of the Cancer Survivorship news planning team.
“You sort of wonder where a cancer patient’s supposed to go to get this information if not at the Cancer Center,” said Dr. Gilligan, who moderated the press conference. “We’re really kind of leaving them shortchanged here, and the good news is I think we could easily do better if we just decide that we want to.”
The study was funded by Dana-Farber Cancer Institute. Dr. Bober reported research funding from Apex Neuro.
SOURCE: Bober S. et al. Cancer Survivorship Symposium Abstract #134
ORLANDO – Therapeutic aids for sexual rehabilitation were not available at most major cancer centers, according to results of a structured telephone survey presented at the Cancer Survivorship Symposium.
Of the centers reached, 87% said they had no sexual aids available for men, and 72% said they had no such aids for women, said lead study author Sharon Bober, PhD, a psychologist at the Dana-Farber Cancer Institute in Boston, Massachusetts.
“I think the scarcity of all of these products really underscores the cultural taboos around sexual dysfunction, as did some of the discomfort of the staff responding to our calls,” Dr. Bober said in a press conference at the symposium, which was sponsored by the American Academy of Family Physicians, the American College of Physicians, and the American Society of Clinical Oncology.
Cancer treatment guidelines from the National Comprehensive Cancer Network (NCCN) recommend therapeutic aids for sexual health rehabilitation including vaginal dilators, moisturizers, and vacuum erection devices, Dr. Bober said.
Dr. Bober and her colleagues surveyed 25 NCI-designated Cancer Centers/National Comprehensive Cancer Network–member institutions about on-site availability of sexual aids and resources for cancer survivors.
After conducting internet searches and phone calls designed to identify potential sources of sexual aids at each center, study staff posed as relatives of patients and used a structured script to query cancer center staff about on-site availability of sexual aids.
Separate calls were conducted to query on availability of men and women’s sexual aids.
Of 23 centers that responded about men, 87% reported having no sexual aids, and of 22 centers that responded about women, 72% reported having no sexual aids, Dr. Bober reported at the symposium.
The lack of sexual aids was particularly notable given the wide availability of wigs, prosthetics, sunscreen, and other cancer care products at leading cancer centers, she added.
“Only one center of the 25 had an extensive list of products and resources for both men and women, which may well serve as a model when we think about the needs for cancer survivors in general,” said Dr. Bober.
These results suggest that leading cancer centers are not meeting the needs of cancer survivors in terms of recommended sexual therapeutic aids and informational resources, according to Timothy Gilligan, MD, an American Society of Clinical Oncology expert and member of the Cancer Survivorship news planning team.
“You sort of wonder where a cancer patient’s supposed to go to get this information if not at the Cancer Center,” said Dr. Gilligan, who moderated the press conference. “We’re really kind of leaving them shortchanged here, and the good news is I think we could easily do better if we just decide that we want to.”
The study was funded by Dana-Farber Cancer Institute. Dr. Bober reported research funding from Apex Neuro.
SOURCE: Bober S. et al. Cancer Survivorship Symposium Abstract #134
FROM THE CSC 2018
Key clinical point: Therapeutic aids for sexual health rehabilitation were not available at most leading cancer centers, despite clinical practice guidelines recommending their use.
Major finding: Of the centers reached, 87% said they had no sexual aids available for men, and 72% said they had no aids for women.
Data source: Analysis of responses from cancer center staff at 25 NCI-designated cancer centers to telephone queries that used a structured script.
Disclosures: Study funding came from Dana-Farber Cancer Institute. Dr. Bober reported research funding from Apex Neuro.
Source: Bober S. et al. Cancer Survivorship Symposium, Abstract #134.
Salpingectomy at cesarean feasible, but adds to operative time
DALLAS – Salpingectomy – which can reduce the risk for later ovarian cancer – was completed successfully in about two-thirds of women who desired permanent contraception at cesarean delivery and were randomized to receive this procedure rather than simple tubal ligation.
In a study of 80 patients, operative times were longer by 15 minutes for those who received salpingectomy, and neither group had adverse outcomes or serious complications, according to Akila Subramaniam, MD, who presented the findings of the single-site Salpingectomy at Cesarean Delivery for Ovarian Cancer Reduction (SCORE) trial at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The strategy to perform salpingectomy during cesarean delivery may be one way to reduce the incidence of ovarian cancer, “the most lethal gynecologic malignancy in the United States,” said Dr. Subramaniam, a maternal-fetal medicine specialist at the University of Alabama at Birmingham. “Primary prevention is the focus to reduce the ovarian cancer burden.”
However, Dr. Subramaniam said, there had been limited prospective data on salpingectomy performed at the time of cesarean delivery. One theoretical concern is an increased risk of bleeding; another is the additional operative time required for a potentially difficult dissection of the entire fallopian tube.
Dr. Subramaniam and her colleagues constructed a clinical trial that asked patients who were receiving cesarean delivery and who desired surgical sterilization to agree to randomization to complete salpingectomy or standard tubal ligation. Patient allocation was determined by computer-generated numbers, placed in a sealed envelope, and revealed to the surgeon only at the time of the opening incision for the cesarean procedure. Patients were unaware of the allocation until hospital discharge.
The single-center trial enrolled women undergoing a planned cesarean delivery at 35 or more weeks’ gestation, including those who had previous cesareans and women with multiple gestations or fetal malpresentations. Women who went on to cesarean after a trial of labor after prior cesarean were also eligible.
Patients younger than 25 years and patients with known fetal anomalies or fetal demise were excluded, as were women with previous tubal surgery and those who were anticoagulated or had immunodeficiency. The study did not enroll women who were known to carry the BRCA mutation.
Patients who were randomized to the intervention arm received a complete salpingectomy involving excision from the fimbriae to within 1 cm of the cornua, when technically feasible. The control arm participants received a standard bilateral tubal ligation using either the modified Pomeroy technique or the Parkland technique.
All study participants received routine pre- and postoperative care and instructions, and had study follow-up visits at 1 and 6 weeks post partum.
The study had two primary endpoints: rate of bilateral completion of the randomized procedure, and mean total operative time measured from skin incision to closure. Secondary outcomes included assessments of blood loss and surgical complications, followed through the 6-week postpartum visit.
The study just met the predetermined statistical power needed to detect a 10-minute difference in operative time, enrolling 80 patients and randomizing 40 to each arm.
Of the 40 patients randomized to salpingectomy, 27 (67.5%) received the intended complete salpingectomy. Three had a unilateral salpingectomy, with a tubal ligation contralaterally. Eight patients received bilateral tubal ligations, and in two patients, the surgeon was unable to perform any sterilization procedure at all.
In the tubal ligation arm, 38 patients (95%) received bilateral tubal ligation, 1 patient received a unilateral tubal ligation and a unilateral salpingectomy, and 1 patient received no procedure. The difference in success of completing the intended procedures between the two study arms was statistically significant (P = .002); in both groups, adhesions and scarring were the primary impediments to successful completion of the intended procedure, Dr. Subramaniam said.
Operative times were longer by about 15 minutes in the salpingectomy arm, with the difference accounted for by the longer duration of the sterilization procedure. No significant differences were seen in estimated blood loss or decrease in hematocrit between the two groups, and pain scores were similar.
The study, which successfully recruited 80 women from 221 approached, “may be underpowered for safety outcomes,” said Dr. Subramaniam. She also pointed out that there was no assessment of ovarian reserve, and that it’s not possible to assess the true risk reduction of salpingectomy in this study design.
Still, “It’s reasonable to consider this surgical sterilization method during cesarean as an ovarian cancer risk-reducing strategy.” One impediment to study recruitment, she said, was that after receiving education about salpingectomy, many patients desired salpingectomy and were not willing to risk randomization to simple tubal ligation.
Dr. Subramaniam reported receiving research funding for the study from the Debra Kogan Lyda Memorial Ovarian Cancer Fund.
SOURCE: Subramaniam A et al. Am J Obstet Gynecol. 2018 Jan;218:S27-8.
DALLAS – Salpingectomy – which can reduce the risk for later ovarian cancer – was completed successfully in about two-thirds of women who desired permanent contraception at cesarean delivery and were randomized to receive this procedure rather than simple tubal ligation.
In a study of 80 patients, operative times were longer by 15 minutes for those who received salpingectomy, and neither group had adverse outcomes or serious complications, according to Akila Subramaniam, MD, who presented the findings of the single-site Salpingectomy at Cesarean Delivery for Ovarian Cancer Reduction (SCORE) trial at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The strategy to perform salpingectomy during cesarean delivery may be one way to reduce the incidence of ovarian cancer, “the most lethal gynecologic malignancy in the United States,” said Dr. Subramaniam, a maternal-fetal medicine specialist at the University of Alabama at Birmingham. “Primary prevention is the focus to reduce the ovarian cancer burden.”
However, Dr. Subramaniam said, there had been limited prospective data on salpingectomy performed at the time of cesarean delivery. One theoretical concern is an increased risk of bleeding; another is the additional operative time required for a potentially difficult dissection of the entire fallopian tube.
Dr. Subramaniam and her colleagues constructed a clinical trial that asked patients who were receiving cesarean delivery and who desired surgical sterilization to agree to randomization to complete salpingectomy or standard tubal ligation. Patient allocation was determined by computer-generated numbers, placed in a sealed envelope, and revealed to the surgeon only at the time of the opening incision for the cesarean procedure. Patients were unaware of the allocation until hospital discharge.
The single-center trial enrolled women undergoing a planned cesarean delivery at 35 or more weeks’ gestation, including those who had previous cesareans and women with multiple gestations or fetal malpresentations. Women who went on to cesarean after a trial of labor after prior cesarean were also eligible.
Patients younger than 25 years and patients with known fetal anomalies or fetal demise were excluded, as were women with previous tubal surgery and those who were anticoagulated or had immunodeficiency. The study did not enroll women who were known to carry the BRCA mutation.
Patients who were randomized to the intervention arm received a complete salpingectomy involving excision from the fimbriae to within 1 cm of the cornua, when technically feasible. The control arm participants received a standard bilateral tubal ligation using either the modified Pomeroy technique or the Parkland technique.
All study participants received routine pre- and postoperative care and instructions, and had study follow-up visits at 1 and 6 weeks post partum.
The study had two primary endpoints: rate of bilateral completion of the randomized procedure, and mean total operative time measured from skin incision to closure. Secondary outcomes included assessments of blood loss and surgical complications, followed through the 6-week postpartum visit.
The study just met the predetermined statistical power needed to detect a 10-minute difference in operative time, enrolling 80 patients and randomizing 40 to each arm.
Of the 40 patients randomized to salpingectomy, 27 (67.5%) received the intended complete salpingectomy. Three had a unilateral salpingectomy, with a tubal ligation contralaterally. Eight patients received bilateral tubal ligations, and in two patients, the surgeon was unable to perform any sterilization procedure at all.
In the tubal ligation arm, 38 patients (95%) received bilateral tubal ligation, 1 patient received a unilateral tubal ligation and a unilateral salpingectomy, and 1 patient received no procedure. The difference in success of completing the intended procedures between the two study arms was statistically significant (P = .002); in both groups, adhesions and scarring were the primary impediments to successful completion of the intended procedure, Dr. Subramaniam said.
Operative times were longer by about 15 minutes in the salpingectomy arm, with the difference accounted for by the longer duration of the sterilization procedure. No significant differences were seen in estimated blood loss or decrease in hematocrit between the two groups, and pain scores were similar.
The study, which successfully recruited 80 women from 221 approached, “may be underpowered for safety outcomes,” said Dr. Subramaniam. She also pointed out that there was no assessment of ovarian reserve, and that it’s not possible to assess the true risk reduction of salpingectomy in this study design.
Still, “It’s reasonable to consider this surgical sterilization method during cesarean as an ovarian cancer risk-reducing strategy.” One impediment to study recruitment, she said, was that after receiving education about salpingectomy, many patients desired salpingectomy and were not willing to risk randomization to simple tubal ligation.
Dr. Subramaniam reported receiving research funding for the study from the Debra Kogan Lyda Memorial Ovarian Cancer Fund.
SOURCE: Subramaniam A et al. Am J Obstet Gynecol. 2018 Jan;218:S27-8.
DALLAS – Salpingectomy – which can reduce the risk for later ovarian cancer – was completed successfully in about two-thirds of women who desired permanent contraception at cesarean delivery and were randomized to receive this procedure rather than simple tubal ligation.
In a study of 80 patients, operative times were longer by 15 minutes for those who received salpingectomy, and neither group had adverse outcomes or serious complications, according to Akila Subramaniam, MD, who presented the findings of the single-site Salpingectomy at Cesarean Delivery for Ovarian Cancer Reduction (SCORE) trial at the meeting sponsored by the Society for Maternal-Fetal Medicine.
The strategy to perform salpingectomy during cesarean delivery may be one way to reduce the incidence of ovarian cancer, “the most lethal gynecologic malignancy in the United States,” said Dr. Subramaniam, a maternal-fetal medicine specialist at the University of Alabama at Birmingham. “Primary prevention is the focus to reduce the ovarian cancer burden.”
However, Dr. Subramaniam said, there had been limited prospective data on salpingectomy performed at the time of cesarean delivery. One theoretical concern is an increased risk of bleeding; another is the additional operative time required for a potentially difficult dissection of the entire fallopian tube.
Dr. Subramaniam and her colleagues constructed a clinical trial that asked patients who were receiving cesarean delivery and who desired surgical sterilization to agree to randomization to complete salpingectomy or standard tubal ligation. Patient allocation was determined by computer-generated numbers, placed in a sealed envelope, and revealed to the surgeon only at the time of the opening incision for the cesarean procedure. Patients were unaware of the allocation until hospital discharge.
The single-center trial enrolled women undergoing a planned cesarean delivery at 35 or more weeks’ gestation, including those who had previous cesareans and women with multiple gestations or fetal malpresentations. Women who went on to cesarean after a trial of labor after prior cesarean were also eligible.
Patients younger than 25 years and patients with known fetal anomalies or fetal demise were excluded, as were women with previous tubal surgery and those who were anticoagulated or had immunodeficiency. The study did not enroll women who were known to carry the BRCA mutation.
Patients who were randomized to the intervention arm received a complete salpingectomy involving excision from the fimbriae to within 1 cm of the cornua, when technically feasible. The control arm participants received a standard bilateral tubal ligation using either the modified Pomeroy technique or the Parkland technique.
All study participants received routine pre- and postoperative care and instructions, and had study follow-up visits at 1 and 6 weeks post partum.
The study had two primary endpoints: rate of bilateral completion of the randomized procedure, and mean total operative time measured from skin incision to closure. Secondary outcomes included assessments of blood loss and surgical complications, followed through the 6-week postpartum visit.
The study just met the predetermined statistical power needed to detect a 10-minute difference in operative time, enrolling 80 patients and randomizing 40 to each arm.
Of the 40 patients randomized to salpingectomy, 27 (67.5%) received the intended complete salpingectomy. Three had a unilateral salpingectomy, with a tubal ligation contralaterally. Eight patients received bilateral tubal ligations, and in two patients, the surgeon was unable to perform any sterilization procedure at all.
In the tubal ligation arm, 38 patients (95%) received bilateral tubal ligation, 1 patient received a unilateral tubal ligation and a unilateral salpingectomy, and 1 patient received no procedure. The difference in success of completing the intended procedures between the two study arms was statistically significant (P = .002); in both groups, adhesions and scarring were the primary impediments to successful completion of the intended procedure, Dr. Subramaniam said.
Operative times were longer by about 15 minutes in the salpingectomy arm, with the difference accounted for by the longer duration of the sterilization procedure. No significant differences were seen in estimated blood loss or decrease in hematocrit between the two groups, and pain scores were similar.
The study, which successfully recruited 80 women from 221 approached, “may be underpowered for safety outcomes,” said Dr. Subramaniam. She also pointed out that there was no assessment of ovarian reserve, and that it’s not possible to assess the true risk reduction of salpingectomy in this study design.
Still, “It’s reasonable to consider this surgical sterilization method during cesarean as an ovarian cancer risk-reducing strategy.” One impediment to study recruitment, she said, was that after receiving education about salpingectomy, many patients desired salpingectomy and were not willing to risk randomization to simple tubal ligation.
Dr. Subramaniam reported receiving research funding for the study from the Debra Kogan Lyda Memorial Ovarian Cancer Fund.
SOURCE: Subramaniam A et al. Am J Obstet Gynecol. 2018 Jan;218:S27-8.
REPORTING FROM THE PREGNANCY MEETING
Key clinical point: Salpingectomy was successful in two-thirds of patients but added 15 minutes to operative time.
Major finding: The intended procedure was successful in 27 of 40 salpingectomy patients (67.5%) and 38 of 40 tubal ligation patients (95%; P = .02).
Study details: Randomized controlled trial of 80 women receiving bilateral salpingectomy or tubal ligation at cesarean delivery.
Disclosures: Study funding was received from the Debra Kogan Lyda Memorial Ovarian Cancer Fund.
Source: Subramaniam A et al. Am J Obstet Gynecol. 2018 Jan;218:S27-8.
Flu increase may be slowing
A bit of revisionist history has outpatient influenza activity at a lower level than was reported last week, even though it hasn’t dropped.
The proportion of outpatient visits for influenza-like illness (ILI) for the week ending Feb. 10 was 7.5%, according to the Centers for Disease Control. That is lower than the 7.7% previously reported for the week ending Feb. 3, which would seem to be a drop, but the CDC also has revised that earlier number to 7.5%, so there is no change. (This is not the first time an earlier ILI level has been retroactively lowered: The figure reported for the week ending Jan. 13 was revised in the following report from 6.3% down to 6.0%.)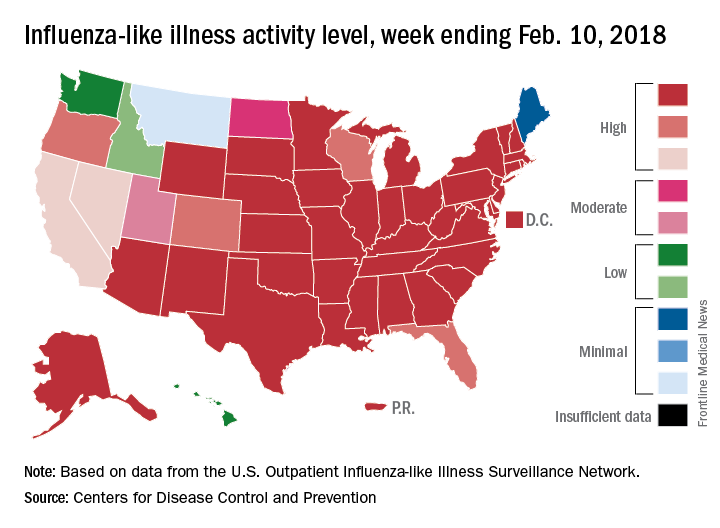
Hospital visits, however, continue to rise at record levels. The cumulative rate for the week ending Feb. 10 was 67.9 visits per 100,000 population, which is higher than the same week for the 2014-2015 (52.9 per 100,000) when flu hospitalizations for the season hit a high of 710,000. Flu-related pediatric deaths also went up, with 22 new reports; this brings the total to 84 for the 2017-2018 season.
A bit of revisionist history has outpatient influenza activity at a lower level than was reported last week, even though it hasn’t dropped.
The proportion of outpatient visits for influenza-like illness (ILI) for the week ending Feb. 10 was 7.5%, according to the Centers for Disease Control. That is lower than the 7.7% previously reported for the week ending Feb. 3, which would seem to be a drop, but the CDC also has revised that earlier number to 7.5%, so there is no change. (This is not the first time an earlier ILI level has been retroactively lowered: The figure reported for the week ending Jan. 13 was revised in the following report from 6.3% down to 6.0%.)
Hospital visits, however, continue to rise at record levels. The cumulative rate for the week ending Feb. 10 was 67.9 visits per 100,000 population, which is higher than the same week for the 2014-2015 (52.9 per 100,000) when flu hospitalizations for the season hit a high of 710,000. Flu-related pediatric deaths also went up, with 22 new reports; this brings the total to 84 for the 2017-2018 season.
A bit of revisionist history has outpatient influenza activity at a lower level than was reported last week, even though it hasn’t dropped.
The proportion of outpatient visits for influenza-like illness (ILI) for the week ending Feb. 10 was 7.5%, according to the Centers for Disease Control. That is lower than the 7.7% previously reported for the week ending Feb. 3, which would seem to be a drop, but the CDC also has revised that earlier number to 7.5%, so there is no change. (This is not the first time an earlier ILI level has been retroactively lowered: The figure reported for the week ending Jan. 13 was revised in the following report from 6.3% down to 6.0%.)
Hospital visits, however, continue to rise at record levels. The cumulative rate for the week ending Feb. 10 was 67.9 visits per 100,000 population, which is higher than the same week for the 2014-2015 (52.9 per 100,000) when flu hospitalizations for the season hit a high of 710,000. Flu-related pediatric deaths also went up, with 22 new reports; this brings the total to 84 for the 2017-2018 season.
FROM THE CDC WEEKLY U.S. INFLUENZA SURVEILLANCE REPORT
Inaccurate depictions of inpatient psychiatry foster stigma
When it comes to the portrayal of physicians in popular culture, psychiatrists are second only to surgeons.1 Far too often, these portrayals of psychiatry have misrepresented our specialty – and stigmatized our patients. Some of the content produced by entertainment giants Netflix and Marvel is a case in point.
That Netflix-Marvel collaboration has proven fruitful, resulting in six television series spanning seven seasons of entertainment to date. Last year alone saw the release of three Netflix-Marvel productions, including Marvel’s “Iron Fist” in March, “The Defenders” in August, and “The Punisher” in November. Given the popularity and ease of streaming Marvel’s Netflix productions, these series have the potential to entertain and inform a wide audience. Unfortunately, however, part of their influence might be to perpetuate stigma and fear surrounding inpatient psychiatric care. Take the inaccurate portrayal of psychiatry in “Iron Fist” compared with the reality of modern psychiatric care, for example.
While hospitalized, Danny is repeatedly shown in restraints, including four-point restraints, a belt, and a straitjacket. The use of restraints is sometimes portrayed as unprovoked, without evidence of aggression on the part of Danny. In addition, it appears that he is left in restraints for extended periods of time, as he is shown, for example, waking up in the night still restrained. While the use of restraints may be warranted in instances of extreme aggression or violence, the current culture of inpatient psychiatric care has shifted toward safely minimizing the use of restraints and seclusion.2 Straitjackets might be an icon of a bygone era of psychiatric care, but they no longer are a mainstream form of restraint used in the United States. Modern best practices would not result in a nonthreatening patient being placed in restraints and left in them for an extended period of time as shown in “Iron Fist.”
Danny is shown being forcibly administered medications, even in nonthreatening situations. These medications are given via parenteral injection as well as orally, with a psychiatric technician shown roughly inserting a tongue depressor deep in Danny’s mouth, pouring pills into his oral cavity, and manually holding his mouth shut to ensure ingestion. In truth, psychiatric patients are sometimes given intramuscular injections of calming agents when their level of agitation threatens to harm others or themselves. For patients to be given an involuntary injection when not acutely threatening, typically states require some form of legal application for forced medication (a process that does not appear to have been observed in “Iron Fist”). As we know, forced oral medications never should be undertaken given the significant risk to patient (possible choking) as well as staff (bitten fingers).
Staff supervision of patients at the fictional Birch Psychiatric Hospital is extremely poor. At one point, a fellow patient enters Danny’s room dressed in a white coat, pretending to be his doctor. As the conversation progresses, the patient grabs a fork from Danny’s food tray and holds it to his throat, threatening to kill him before being gruffly dragged off by hospital staff. At another point, Danny is shown in four-point restraints, and a patient simply walks into his room and removes the restraints for him. By contrast, in a real, modern inpatient psychiatric facility unit, staff would be routinely providing safety checks on patients throughout the day. If a patient is at risk for violence, sharp metal cutlery would not be included on accessible food trays. Patient attire would be subject to hospital inspection and approval, so it is unclear how or where a patient would have access to a physician’s coat to pull off such an impersonation. And finally, if a patient were sufficiently agitated to require the use of four-point restraints, he or she would be closely supervised and not left alone in an open area where other patients could remove the restraints.
The hospital stays described in “Iron Fist” are very long, and it is strongly implied that psychiatric diagnoses are invented to prolong inpatient care indefinitely. Referring to the duration of his initial involuntary hold, one patient tells Danny: “That what they tell you? Seventy-two hours? (laughs) Me, I had a little incident inside a pharmacy. Seventy-two hours later, I’m a bipolar with mixed affective episodes layered atop a substance abuse disorder. That was 2 years ago. Billy was living under a bridge. Seventy-two hours later, he’s a paranoid personality disorder. That was just over a year ago. And Jimmy was screaming at people in Times Square. Seventy-two hours later, he’s a schizoaffective disorder. He’s been here almost 15 years. Don’t think you’ll be any different.”
Most modern inpatient psychiatric care is designed around short-term hospitalization (days to weeks rather than months to years) with a goal of reintegrating patients back into the community with ongoing outpatient care as soon as they can safely make that transition. In addition, to insinuate that psychiatrists invent diagnoses to keep patients “locked up” insults the integrity of the many dedicated mental health workers who provide care for an ill and often overlooked population.
The most egregious examples of poor psychiatric care portrayed in “Iron Fist” involve illegal or criminal activities. Video cameras placed throughout the hospital transmit a live feed to a shadowy figure who has no role in patient care. A psychiatric technician escorts Danny to a room full of patients hired to kill him. These particular concerns are outlandish enough that perhaps they don’t even need to be directly addressed, but for the sake of completeness it is worth noting that psychiatric hospitals are subject to rigorous oversight from numerous regulatory bodies to ensure that patient care is delivered in a safe and respectable manner and that all protected health information is accessible only by those whose treatment role necessitates such access.
Marvel’s “Iron Fist” seeks to entertain its audience, but it does a poor job of showing viewers a realistic portrayal of inpatient psychiatric care. The show presents a psychiatric hospital as the setting for inappropriate use of restraints, unwarranted use of forced oral and injectable medications, a lack of supervision leading to violence between patients, and even attempted murder accommodated by a hospital employee. Also, the show strongly implies that psychiatric diagnoses are invented for the purpose of continuing inpatient care indefinitely.
In sum, the psychiatric hospital is seen as an inhumane form of imprisonment from which one can only hope to escape with the benefit of a glowing, magical fist. Although this is fiction, these kinds of narratives can have very real consequences in perpetuating stigma against psychiatric care. Ultimately, such storylines undermine the public’s confidence in clinicians seeking to provide caring and compassionate care.
Dr. Weber is psychiatry department chair at Logan (Utah) Regional Hospital with Intermountain Healthcare.
References
1 J Nat Med Assoc. 2002 Jul. 94[7]:635-58.
2 Aggress Violent Behav. 2017;34:139-46.
When it comes to the portrayal of physicians in popular culture, psychiatrists are second only to surgeons.1 Far too often, these portrayals of psychiatry have misrepresented our specialty – and stigmatized our patients. Some of the content produced by entertainment giants Netflix and Marvel is a case in point.
That Netflix-Marvel collaboration has proven fruitful, resulting in six television series spanning seven seasons of entertainment to date. Last year alone saw the release of three Netflix-Marvel productions, including Marvel’s “Iron Fist” in March, “The Defenders” in August, and “The Punisher” in November. Given the popularity and ease of streaming Marvel’s Netflix productions, these series have the potential to entertain and inform a wide audience. Unfortunately, however, part of their influence might be to perpetuate stigma and fear surrounding inpatient psychiatric care. Take the inaccurate portrayal of psychiatry in “Iron Fist” compared with the reality of modern psychiatric care, for example.
While hospitalized, Danny is repeatedly shown in restraints, including four-point restraints, a belt, and a straitjacket. The use of restraints is sometimes portrayed as unprovoked, without evidence of aggression on the part of Danny. In addition, it appears that he is left in restraints for extended periods of time, as he is shown, for example, waking up in the night still restrained. While the use of restraints may be warranted in instances of extreme aggression or violence, the current culture of inpatient psychiatric care has shifted toward safely minimizing the use of restraints and seclusion.2 Straitjackets might be an icon of a bygone era of psychiatric care, but they no longer are a mainstream form of restraint used in the United States. Modern best practices would not result in a nonthreatening patient being placed in restraints and left in them for an extended period of time as shown in “Iron Fist.”
Danny is shown being forcibly administered medications, even in nonthreatening situations. These medications are given via parenteral injection as well as orally, with a psychiatric technician shown roughly inserting a tongue depressor deep in Danny’s mouth, pouring pills into his oral cavity, and manually holding his mouth shut to ensure ingestion. In truth, psychiatric patients are sometimes given intramuscular injections of calming agents when their level of agitation threatens to harm others or themselves. For patients to be given an involuntary injection when not acutely threatening, typically states require some form of legal application for forced medication (a process that does not appear to have been observed in “Iron Fist”). As we know, forced oral medications never should be undertaken given the significant risk to patient (possible choking) as well as staff (bitten fingers).
Staff supervision of patients at the fictional Birch Psychiatric Hospital is extremely poor. At one point, a fellow patient enters Danny’s room dressed in a white coat, pretending to be his doctor. As the conversation progresses, the patient grabs a fork from Danny’s food tray and holds it to his throat, threatening to kill him before being gruffly dragged off by hospital staff. At another point, Danny is shown in four-point restraints, and a patient simply walks into his room and removes the restraints for him. By contrast, in a real, modern inpatient psychiatric facility unit, staff would be routinely providing safety checks on patients throughout the day. If a patient is at risk for violence, sharp metal cutlery would not be included on accessible food trays. Patient attire would be subject to hospital inspection and approval, so it is unclear how or where a patient would have access to a physician’s coat to pull off such an impersonation. And finally, if a patient were sufficiently agitated to require the use of four-point restraints, he or she would be closely supervised and not left alone in an open area where other patients could remove the restraints.
The hospital stays described in “Iron Fist” are very long, and it is strongly implied that psychiatric diagnoses are invented to prolong inpatient care indefinitely. Referring to the duration of his initial involuntary hold, one patient tells Danny: “That what they tell you? Seventy-two hours? (laughs) Me, I had a little incident inside a pharmacy. Seventy-two hours later, I’m a bipolar with mixed affective episodes layered atop a substance abuse disorder. That was 2 years ago. Billy was living under a bridge. Seventy-two hours later, he’s a paranoid personality disorder. That was just over a year ago. And Jimmy was screaming at people in Times Square. Seventy-two hours later, he’s a schizoaffective disorder. He’s been here almost 15 years. Don’t think you’ll be any different.”
Most modern inpatient psychiatric care is designed around short-term hospitalization (days to weeks rather than months to years) with a goal of reintegrating patients back into the community with ongoing outpatient care as soon as they can safely make that transition. In addition, to insinuate that psychiatrists invent diagnoses to keep patients “locked up” insults the integrity of the many dedicated mental health workers who provide care for an ill and often overlooked population.
The most egregious examples of poor psychiatric care portrayed in “Iron Fist” involve illegal or criminal activities. Video cameras placed throughout the hospital transmit a live feed to a shadowy figure who has no role in patient care. A psychiatric technician escorts Danny to a room full of patients hired to kill him. These particular concerns are outlandish enough that perhaps they don’t even need to be directly addressed, but for the sake of completeness it is worth noting that psychiatric hospitals are subject to rigorous oversight from numerous regulatory bodies to ensure that patient care is delivered in a safe and respectable manner and that all protected health information is accessible only by those whose treatment role necessitates such access.
Marvel’s “Iron Fist” seeks to entertain its audience, but it does a poor job of showing viewers a realistic portrayal of inpatient psychiatric care. The show presents a psychiatric hospital as the setting for inappropriate use of restraints, unwarranted use of forced oral and injectable medications, a lack of supervision leading to violence between patients, and even attempted murder accommodated by a hospital employee. Also, the show strongly implies that psychiatric diagnoses are invented for the purpose of continuing inpatient care indefinitely.
In sum, the psychiatric hospital is seen as an inhumane form of imprisonment from which one can only hope to escape with the benefit of a glowing, magical fist. Although this is fiction, these kinds of narratives can have very real consequences in perpetuating stigma against psychiatric care. Ultimately, such storylines undermine the public’s confidence in clinicians seeking to provide caring and compassionate care.
Dr. Weber is psychiatry department chair at Logan (Utah) Regional Hospital with Intermountain Healthcare.
References
1 J Nat Med Assoc. 2002 Jul. 94[7]:635-58.
2 Aggress Violent Behav. 2017;34:139-46.
When it comes to the portrayal of physicians in popular culture, psychiatrists are second only to surgeons.1 Far too often, these portrayals of psychiatry have misrepresented our specialty – and stigmatized our patients. Some of the content produced by entertainment giants Netflix and Marvel is a case in point.
That Netflix-Marvel collaboration has proven fruitful, resulting in six television series spanning seven seasons of entertainment to date. Last year alone saw the release of three Netflix-Marvel productions, including Marvel’s “Iron Fist” in March, “The Defenders” in August, and “The Punisher” in November. Given the popularity and ease of streaming Marvel’s Netflix productions, these series have the potential to entertain and inform a wide audience. Unfortunately, however, part of their influence might be to perpetuate stigma and fear surrounding inpatient psychiatric care. Take the inaccurate portrayal of psychiatry in “Iron Fist” compared with the reality of modern psychiatric care, for example.
While hospitalized, Danny is repeatedly shown in restraints, including four-point restraints, a belt, and a straitjacket. The use of restraints is sometimes portrayed as unprovoked, without evidence of aggression on the part of Danny. In addition, it appears that he is left in restraints for extended periods of time, as he is shown, for example, waking up in the night still restrained. While the use of restraints may be warranted in instances of extreme aggression or violence, the current culture of inpatient psychiatric care has shifted toward safely minimizing the use of restraints and seclusion.2 Straitjackets might be an icon of a bygone era of psychiatric care, but they no longer are a mainstream form of restraint used in the United States. Modern best practices would not result in a nonthreatening patient being placed in restraints and left in them for an extended period of time as shown in “Iron Fist.”
Danny is shown being forcibly administered medications, even in nonthreatening situations. These medications are given via parenteral injection as well as orally, with a psychiatric technician shown roughly inserting a tongue depressor deep in Danny’s mouth, pouring pills into his oral cavity, and manually holding his mouth shut to ensure ingestion. In truth, psychiatric patients are sometimes given intramuscular injections of calming agents when their level of agitation threatens to harm others or themselves. For patients to be given an involuntary injection when not acutely threatening, typically states require some form of legal application for forced medication (a process that does not appear to have been observed in “Iron Fist”). As we know, forced oral medications never should be undertaken given the significant risk to patient (possible choking) as well as staff (bitten fingers).
Staff supervision of patients at the fictional Birch Psychiatric Hospital is extremely poor. At one point, a fellow patient enters Danny’s room dressed in a white coat, pretending to be his doctor. As the conversation progresses, the patient grabs a fork from Danny’s food tray and holds it to his throat, threatening to kill him before being gruffly dragged off by hospital staff. At another point, Danny is shown in four-point restraints, and a patient simply walks into his room and removes the restraints for him. By contrast, in a real, modern inpatient psychiatric facility unit, staff would be routinely providing safety checks on patients throughout the day. If a patient is at risk for violence, sharp metal cutlery would not be included on accessible food trays. Patient attire would be subject to hospital inspection and approval, so it is unclear how or where a patient would have access to a physician’s coat to pull off such an impersonation. And finally, if a patient were sufficiently agitated to require the use of four-point restraints, he or she would be closely supervised and not left alone in an open area where other patients could remove the restraints.
The hospital stays described in “Iron Fist” are very long, and it is strongly implied that psychiatric diagnoses are invented to prolong inpatient care indefinitely. Referring to the duration of his initial involuntary hold, one patient tells Danny: “That what they tell you? Seventy-two hours? (laughs) Me, I had a little incident inside a pharmacy. Seventy-two hours later, I’m a bipolar with mixed affective episodes layered atop a substance abuse disorder. That was 2 years ago. Billy was living under a bridge. Seventy-two hours later, he’s a paranoid personality disorder. That was just over a year ago. And Jimmy was screaming at people in Times Square. Seventy-two hours later, he’s a schizoaffective disorder. He’s been here almost 15 years. Don’t think you’ll be any different.”
Most modern inpatient psychiatric care is designed around short-term hospitalization (days to weeks rather than months to years) with a goal of reintegrating patients back into the community with ongoing outpatient care as soon as they can safely make that transition. In addition, to insinuate that psychiatrists invent diagnoses to keep patients “locked up” insults the integrity of the many dedicated mental health workers who provide care for an ill and often overlooked population.
The most egregious examples of poor psychiatric care portrayed in “Iron Fist” involve illegal or criminal activities. Video cameras placed throughout the hospital transmit a live feed to a shadowy figure who has no role in patient care. A psychiatric technician escorts Danny to a room full of patients hired to kill him. These particular concerns are outlandish enough that perhaps they don’t even need to be directly addressed, but for the sake of completeness it is worth noting that psychiatric hospitals are subject to rigorous oversight from numerous regulatory bodies to ensure that patient care is delivered in a safe and respectable manner and that all protected health information is accessible only by those whose treatment role necessitates such access.
Marvel’s “Iron Fist” seeks to entertain its audience, but it does a poor job of showing viewers a realistic portrayal of inpatient psychiatric care. The show presents a psychiatric hospital as the setting for inappropriate use of restraints, unwarranted use of forced oral and injectable medications, a lack of supervision leading to violence between patients, and even attempted murder accommodated by a hospital employee. Also, the show strongly implies that psychiatric diagnoses are invented for the purpose of continuing inpatient care indefinitely.
In sum, the psychiatric hospital is seen as an inhumane form of imprisonment from which one can only hope to escape with the benefit of a glowing, magical fist. Although this is fiction, these kinds of narratives can have very real consequences in perpetuating stigma against psychiatric care. Ultimately, such storylines undermine the public’s confidence in clinicians seeking to provide caring and compassionate care.
Dr. Weber is psychiatry department chair at Logan (Utah) Regional Hospital with Intermountain Healthcare.
References
1 J Nat Med Assoc. 2002 Jul. 94[7]:635-58.
2 Aggress Violent Behav. 2017;34:139-46.





