User login
Intervention helps kids stay active after cancer treatment
ORLANDO—Results of a pilot study suggest a web-based, reward-driven intervention can motivate adolescent cancer survivors to stay physically active.
Time spent performing moderate-to-vigorous physical activity (MVPA) increased by an average of 5 minutes a week for subjects who were randomized to the intervention.
For control subjects, MVPA decreased by an average of 24 minutes a week.
These findings were presented at the 2018 Cancer Survivorship Symposium (abstract 102).
“Compared to the general population, childhood cancer survivors have an increased risk for obesity and metabolic syndrome, conditions that can lead to heart disease, stroke, and diabetes, so it is really important that they are physically active,” said study investigator Carrie R. Howell, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“By intervening in this young age group, we hope to help kids develop healthy exercise habits for life.”
Dr Howell and her colleagues studied cancer survivors, ages 11 to 15, who were no longer receiving cancer treatment and were physically active less than 60 minutes a day.
The subjects were randomized to the intervention or to a control group. Controls received a wearable activity monitor and an educational handout with information about the importance of physical activity and examples of activities.
The intervention group received the handout and activity monitor but also had access to an interactive website. On at least a weekly basis, subjects would connect their monitor to a computer and log their activity through the website. Upon achieving certain thresholds of activity, they received rewards, such as T-shirts and gift cards by mail.
At the beginning and end of the study, participants visited St. Jude for an assessment of their physical fitness (strength, flexibility, and endurance) and neurocognitive measures (attention and memory), as well as health-related quality of life (assessed using the Pediatric Quality of Life Inventory questionnaire).
Results
Seventy-eight cancer survivors completed the 24-week study, 53 of them in the intervention group and 25 in the control group.
MVPA increased by an average of 4.7 minutes per week in the intervention group and decreased by an average of 24.3 minutes per week in the control group.
“In this age group, it is common to see a decrease in physical activity over time, even among healthy kids,” Dr Howell said. “Therefore, we are encouraged that our intervention was successful at maintaining physical activity levels, but a longer program may be needed to create lasting exercise habits.”
In addition to increases in MVPA, the intervention group had the following improvements in fitness:
- Increase in hand grip strength from an average of 19.9 kg to 21.0 kg
- Increase in number of push-ups from an average of 15 to 18
- Increase in number of sit-ups from an average of 11 to 14.
Furthermore, subjects in the intervention group saw their verbal fluency z-score increase by an average of 0.13 points and their general cognition z-score increase by an average of 0.23 points.
Their quality of life scores increased as well. Both overall quality of life and physical-function-related quality of life scores increased from an average of 74.2 to 78.0.
Control subjects had no significant changes in fitness, neurocognitive measures, or quality of life.
This study was supported by the National Cancer Institute, the American Lebanese Syrian Associated Charities, and HopeLab.
Based on the results of this study, the investigators have designed a larger trial (ALTE1631) to test a web-based physical activity intervention. They hope to enroll 384 survivors of childhood acute lymphoblastic leukemia at institutions across the US. The intervention will last a year, with follow-up at 18 months.
Further down the line, the investigators plan to explore the relationship between physical activity and cognition.
ORLANDO—Results of a pilot study suggest a web-based, reward-driven intervention can motivate adolescent cancer survivors to stay physically active.
Time spent performing moderate-to-vigorous physical activity (MVPA) increased by an average of 5 minutes a week for subjects who were randomized to the intervention.
For control subjects, MVPA decreased by an average of 24 minutes a week.
These findings were presented at the 2018 Cancer Survivorship Symposium (abstract 102).
“Compared to the general population, childhood cancer survivors have an increased risk for obesity and metabolic syndrome, conditions that can lead to heart disease, stroke, and diabetes, so it is really important that they are physically active,” said study investigator Carrie R. Howell, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“By intervening in this young age group, we hope to help kids develop healthy exercise habits for life.”
Dr Howell and her colleagues studied cancer survivors, ages 11 to 15, who were no longer receiving cancer treatment and were physically active less than 60 minutes a day.
The subjects were randomized to the intervention or to a control group. Controls received a wearable activity monitor and an educational handout with information about the importance of physical activity and examples of activities.
The intervention group received the handout and activity monitor but also had access to an interactive website. On at least a weekly basis, subjects would connect their monitor to a computer and log their activity through the website. Upon achieving certain thresholds of activity, they received rewards, such as T-shirts and gift cards by mail.
At the beginning and end of the study, participants visited St. Jude for an assessment of their physical fitness (strength, flexibility, and endurance) and neurocognitive measures (attention and memory), as well as health-related quality of life (assessed using the Pediatric Quality of Life Inventory questionnaire).
Results
Seventy-eight cancer survivors completed the 24-week study, 53 of them in the intervention group and 25 in the control group.
MVPA increased by an average of 4.7 minutes per week in the intervention group and decreased by an average of 24.3 minutes per week in the control group.
“In this age group, it is common to see a decrease in physical activity over time, even among healthy kids,” Dr Howell said. “Therefore, we are encouraged that our intervention was successful at maintaining physical activity levels, but a longer program may be needed to create lasting exercise habits.”
In addition to increases in MVPA, the intervention group had the following improvements in fitness:
- Increase in hand grip strength from an average of 19.9 kg to 21.0 kg
- Increase in number of push-ups from an average of 15 to 18
- Increase in number of sit-ups from an average of 11 to 14.
Furthermore, subjects in the intervention group saw their verbal fluency z-score increase by an average of 0.13 points and their general cognition z-score increase by an average of 0.23 points.
Their quality of life scores increased as well. Both overall quality of life and physical-function-related quality of life scores increased from an average of 74.2 to 78.0.
Control subjects had no significant changes in fitness, neurocognitive measures, or quality of life.
This study was supported by the National Cancer Institute, the American Lebanese Syrian Associated Charities, and HopeLab.
Based on the results of this study, the investigators have designed a larger trial (ALTE1631) to test a web-based physical activity intervention. They hope to enroll 384 survivors of childhood acute lymphoblastic leukemia at institutions across the US. The intervention will last a year, with follow-up at 18 months.
Further down the line, the investigators plan to explore the relationship between physical activity and cognition.
ORLANDO—Results of a pilot study suggest a web-based, reward-driven intervention can motivate adolescent cancer survivors to stay physically active.
Time spent performing moderate-to-vigorous physical activity (MVPA) increased by an average of 5 minutes a week for subjects who were randomized to the intervention.
For control subjects, MVPA decreased by an average of 24 minutes a week.
These findings were presented at the 2018 Cancer Survivorship Symposium (abstract 102).
“Compared to the general population, childhood cancer survivors have an increased risk for obesity and metabolic syndrome, conditions that can lead to heart disease, stroke, and diabetes, so it is really important that they are physically active,” said study investigator Carrie R. Howell, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“By intervening in this young age group, we hope to help kids develop healthy exercise habits for life.”
Dr Howell and her colleagues studied cancer survivors, ages 11 to 15, who were no longer receiving cancer treatment and were physically active less than 60 minutes a day.
The subjects were randomized to the intervention or to a control group. Controls received a wearable activity monitor and an educational handout with information about the importance of physical activity and examples of activities.
The intervention group received the handout and activity monitor but also had access to an interactive website. On at least a weekly basis, subjects would connect their monitor to a computer and log their activity through the website. Upon achieving certain thresholds of activity, they received rewards, such as T-shirts and gift cards by mail.
At the beginning and end of the study, participants visited St. Jude for an assessment of their physical fitness (strength, flexibility, and endurance) and neurocognitive measures (attention and memory), as well as health-related quality of life (assessed using the Pediatric Quality of Life Inventory questionnaire).
Results
Seventy-eight cancer survivors completed the 24-week study, 53 of them in the intervention group and 25 in the control group.
MVPA increased by an average of 4.7 minutes per week in the intervention group and decreased by an average of 24.3 minutes per week in the control group.
“In this age group, it is common to see a decrease in physical activity over time, even among healthy kids,” Dr Howell said. “Therefore, we are encouraged that our intervention was successful at maintaining physical activity levels, but a longer program may be needed to create lasting exercise habits.”
In addition to increases in MVPA, the intervention group had the following improvements in fitness:
- Increase in hand grip strength from an average of 19.9 kg to 21.0 kg
- Increase in number of push-ups from an average of 15 to 18
- Increase in number of sit-ups from an average of 11 to 14.
Furthermore, subjects in the intervention group saw their verbal fluency z-score increase by an average of 0.13 points and their general cognition z-score increase by an average of 0.23 points.
Their quality of life scores increased as well. Both overall quality of life and physical-function-related quality of life scores increased from an average of 74.2 to 78.0.
Control subjects had no significant changes in fitness, neurocognitive measures, or quality of life.
This study was supported by the National Cancer Institute, the American Lebanese Syrian Associated Charities, and HopeLab.
Based on the results of this study, the investigators have designed a larger trial (ALTE1631) to test a web-based physical activity intervention. They hope to enroll 384 survivors of childhood acute lymphoblastic leukemia at institutions across the US. The intervention will last a year, with follow-up at 18 months.
Further down the line, the investigators plan to explore the relationship between physical activity and cognition.
Two-Toned Toes
1. A 22-year-old woman dropped an iron on her toe yesterday. Today, the toe is painful at rest and worse with movement.
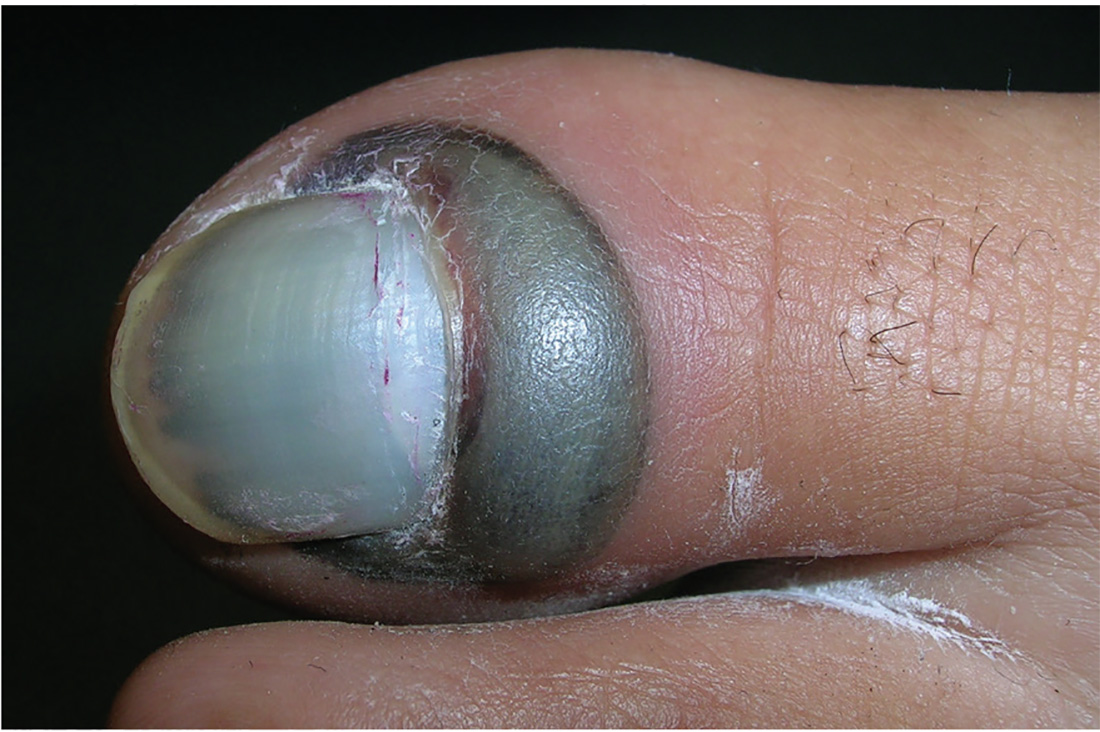
Diagnosis: The patient was diagnosed with a subungual hematoma and a possible fracture of the distal phalanx. In this case, the clinician offered to drain the hematoma but did not have access to an electrocautery unit. The patient consented to any procedure that would relieve the pain. An open paperclip, held in a hemostat and heated with a torch, was used to pierce the patient’s nail plate and drain the blood, providing immediate relief. Citing lack of insurance, the patient declined an x-ray, despite the possible fracture. The toe was bandaged, and the patient was instructed to keep it elevated and avoid weight-bearing activity. Her toe healed well, and no radiographs were taken.
For more information, see “Painful toe.” J Fam Pract. 2011;60(12).
2. For the past month, a man in his 50s has had a discolored right foot with increasing tenderness, as well as livedo reticularis on the sole and lateral aspect. He has a history of right-arm arterial thrombosis, multiple deep vein thromboses of the legs, ischemic stroke, atrial fibrillation, peripheral arterial disease, and long-term warfarin treatment. Pulses are palpable on exam.
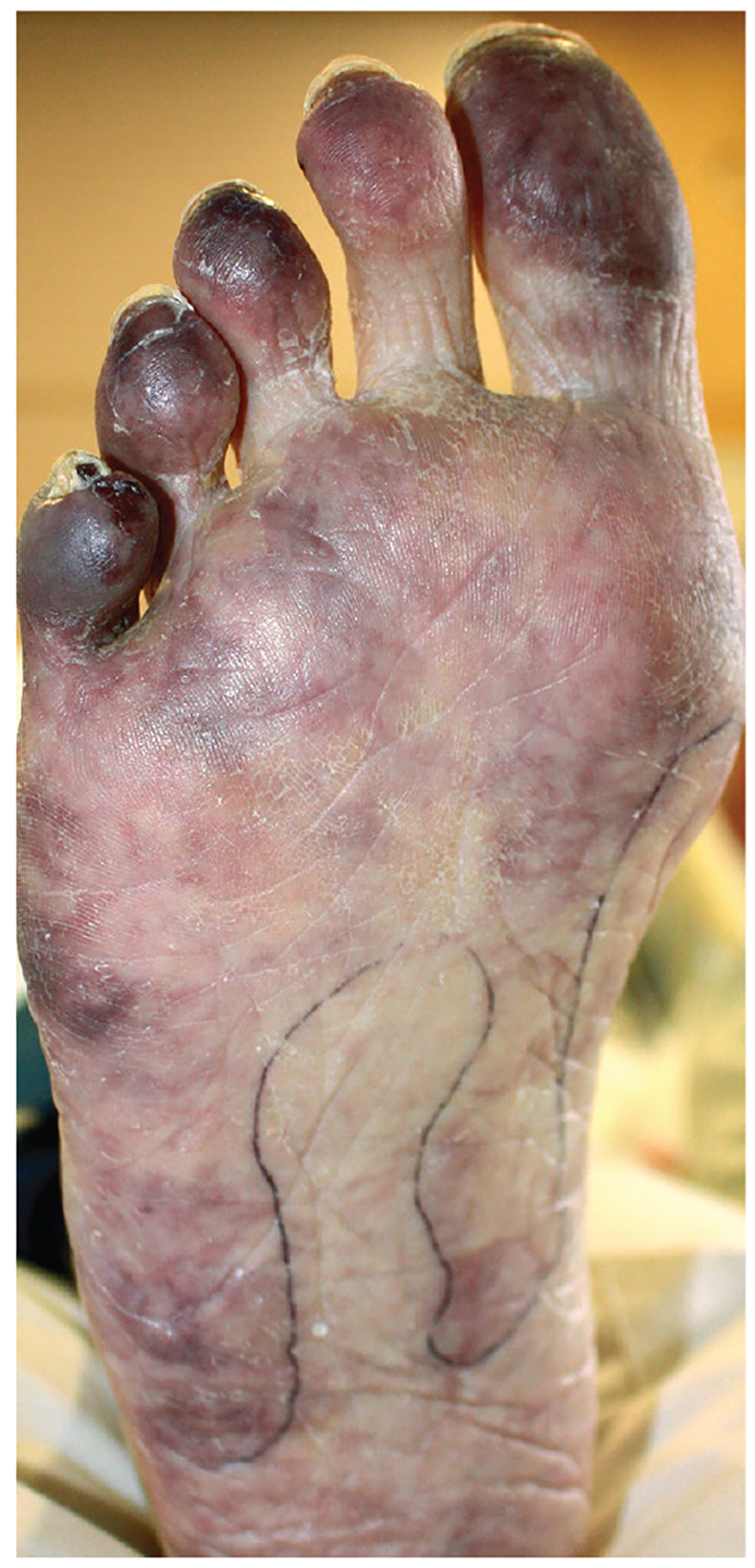
Diagnosis: The patient was diagnosed with antiphospholipid syndrome. He remained on inpatient anticoagulation therapy with fondaparinux and was treated with pulse-dose IV corticosteroids followed by a slow oral taper: daily plasmapheresis for one week, three doses of IV immunoglobulin (0.5 g/kg), and four weekly doses of rituximab (375 mg/m2). His cutaneous findings slowly improved over the next several weeks.
For more information, see “Cyanosis of the Foot.” Cutis. 2017;100(4):206, 209-210.
3. A 35-year-old woman presents in January with purplish toes that are markedly tender to pressure. Recurrent over three successive winters, the initial symptom is an itchy, burning sensation in her toes. The discoloration and other symptoms are constant, not episodic. The condition resolves each year in late spring. Her distal pulses are normal.
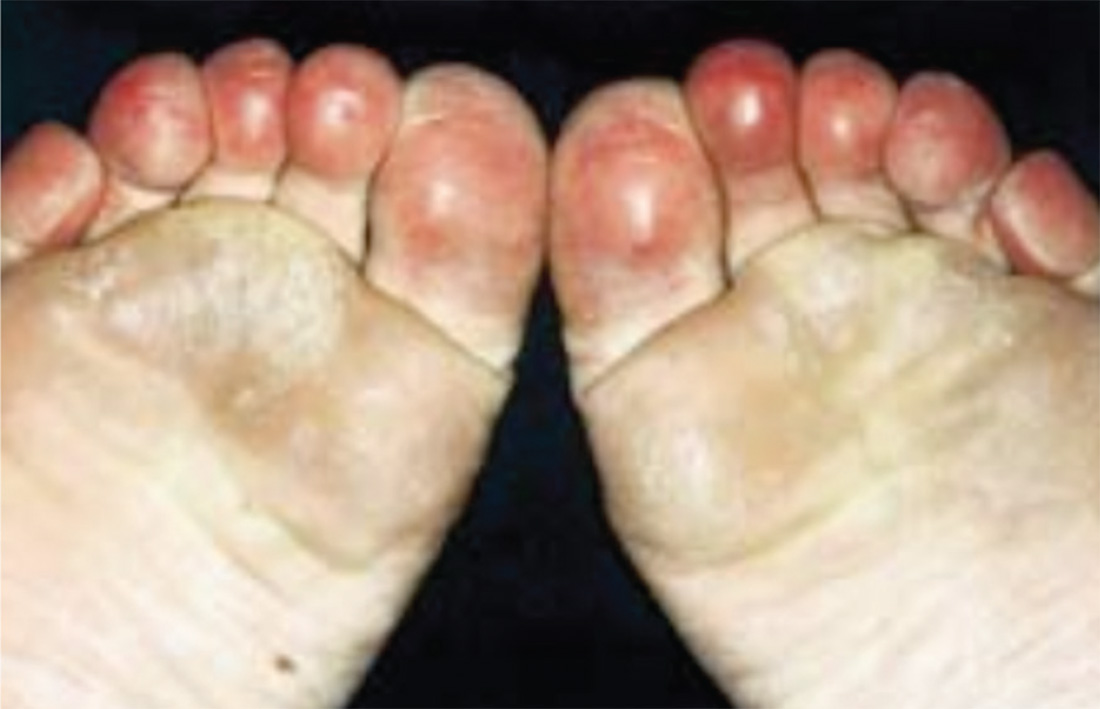
Diagnosis: Pernio—also called perniosis or chilblains—is a common dermatologic condition associated with a cold, humid climate. The inflammatory lesions of pernio may be pruritic, painful, erythematous to violaceous plaques, papules, or nodules, which may have overlying blisters or ulcerations. The condition is frequently misdiagnosed; proper diagnosis relies on patient history and clinical picture. Histologic examination is typically not needed or definitive. This patient had moderately disabling pernio, which responded promptly to therapy with a calcium channel blocker.
For more information, see “Erythrocyanotic Discoloration of the Toes.” Cutis. 2000;65(4):223-226.
4. For three days, a 63-year-old man has had severe, sudden-onset pain in the right hallux and fifth toe. The patient has hypertension and hyperlipidemia and has not undergone any vascular procedures. Physical exam reveals cyanotic change with remarkable coldness on the affected toes and livedo reticularis on the underside of the toes. Pulses are palpable. A biopsy of the fifth toe reveals thrombotic arterioles with cholesterol clefts.
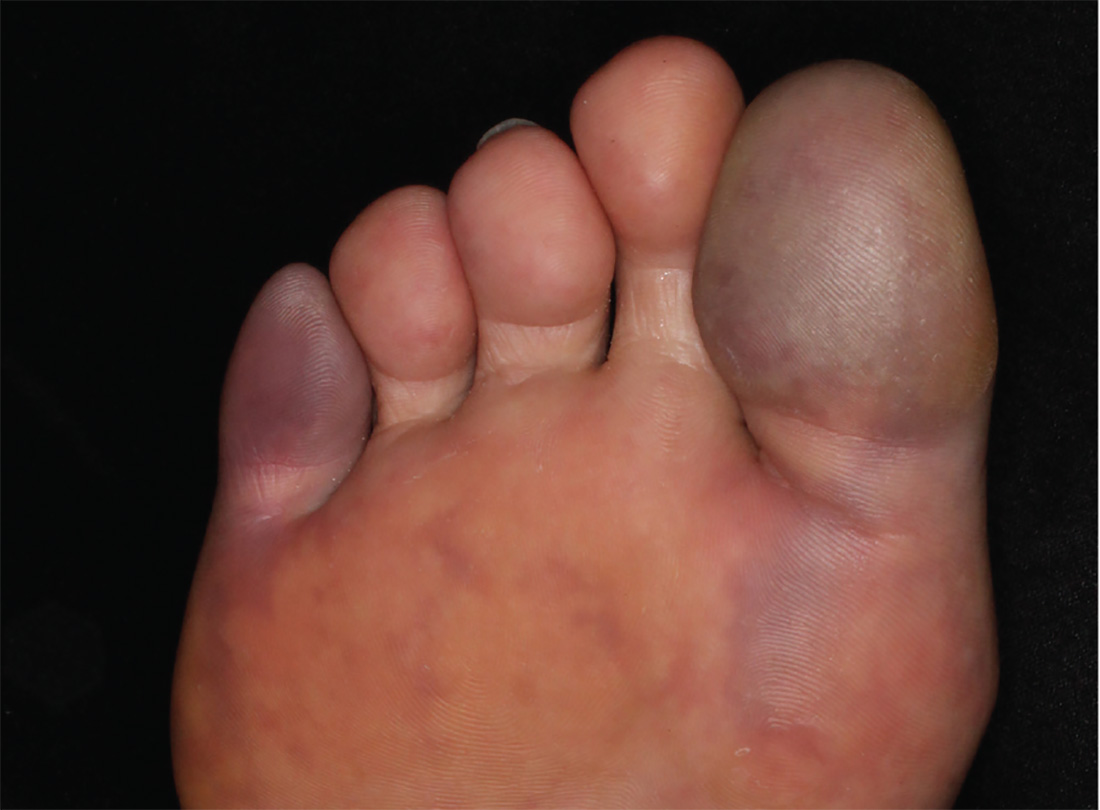
Diagnosis: There are a variety of causes for blue toe syndrome, including embolism, thrombosis, vasoconstrictive disorders, infectious and noninfectious inflammation, extensive venous thrombosis, and abnormal blood circulation. Among them, only emboli from atherosclerotic plaques give rise to cholesterol clefts on biopsy. Such atheroemboli are often an iatrogenic complication, especially those caused by invasive percutaneous procedures or damage to the arterial walls from vascular surgery. However, spontaneous plaque hemorrhage or shearing forces of the circulating blood can disrupt atheromatous plaques and cause embolization of cholesterol crystals—which was likely the case with this patient, since no preceding events were noted.
For more information, see “Painful Purple Toes.” Cutis. 2016;98(3):E8-E10.
1. A 22-year-old woman dropped an iron on her toe yesterday. Today, the toe is painful at rest and worse with movement.

Diagnosis: The patient was diagnosed with a subungual hematoma and a possible fracture of the distal phalanx. In this case, the clinician offered to drain the hematoma but did not have access to an electrocautery unit. The patient consented to any procedure that would relieve the pain. An open paperclip, held in a hemostat and heated with a torch, was used to pierce the patient’s nail plate and drain the blood, providing immediate relief. Citing lack of insurance, the patient declined an x-ray, despite the possible fracture. The toe was bandaged, and the patient was instructed to keep it elevated and avoid weight-bearing activity. Her toe healed well, and no radiographs were taken.
For more information, see “Painful toe.” J Fam Pract. 2011;60(12).
2. For the past month, a man in his 50s has had a discolored right foot with increasing tenderness, as well as livedo reticularis on the sole and lateral aspect. He has a history of right-arm arterial thrombosis, multiple deep vein thromboses of the legs, ischemic stroke, atrial fibrillation, peripheral arterial disease, and long-term warfarin treatment. Pulses are palpable on exam.

Diagnosis: The patient was diagnosed with antiphospholipid syndrome. He remained on inpatient anticoagulation therapy with fondaparinux and was treated with pulse-dose IV corticosteroids followed by a slow oral taper: daily plasmapheresis for one week, three doses of IV immunoglobulin (0.5 g/kg), and four weekly doses of rituximab (375 mg/m2). His cutaneous findings slowly improved over the next several weeks.
For more information, see “Cyanosis of the Foot.” Cutis. 2017;100(4):206, 209-210.
3. A 35-year-old woman presents in January with purplish toes that are markedly tender to pressure. Recurrent over three successive winters, the initial symptom is an itchy, burning sensation in her toes. The discoloration and other symptoms are constant, not episodic. The condition resolves each year in late spring. Her distal pulses are normal.

Diagnosis: Pernio—also called perniosis or chilblains—is a common dermatologic condition associated with a cold, humid climate. The inflammatory lesions of pernio may be pruritic, painful, erythematous to violaceous plaques, papules, or nodules, which may have overlying blisters or ulcerations. The condition is frequently misdiagnosed; proper diagnosis relies on patient history and clinical picture. Histologic examination is typically not needed or definitive. This patient had moderately disabling pernio, which responded promptly to therapy with a calcium channel blocker.
For more information, see “Erythrocyanotic Discoloration of the Toes.” Cutis. 2000;65(4):223-226.
4. For three days, a 63-year-old man has had severe, sudden-onset pain in the right hallux and fifth toe. The patient has hypertension and hyperlipidemia and has not undergone any vascular procedures. Physical exam reveals cyanotic change with remarkable coldness on the affected toes and livedo reticularis on the underside of the toes. Pulses are palpable. A biopsy of the fifth toe reveals thrombotic arterioles with cholesterol clefts.

Diagnosis: There are a variety of causes for blue toe syndrome, including embolism, thrombosis, vasoconstrictive disorders, infectious and noninfectious inflammation, extensive venous thrombosis, and abnormal blood circulation. Among them, only emboli from atherosclerotic plaques give rise to cholesterol clefts on biopsy. Such atheroemboli are often an iatrogenic complication, especially those caused by invasive percutaneous procedures or damage to the arterial walls from vascular surgery. However, spontaneous plaque hemorrhage or shearing forces of the circulating blood can disrupt atheromatous plaques and cause embolization of cholesterol crystals—which was likely the case with this patient, since no preceding events were noted.
For more information, see “Painful Purple Toes.” Cutis. 2016;98(3):E8-E10.
1. A 22-year-old woman dropped an iron on her toe yesterday. Today, the toe is painful at rest and worse with movement.

Diagnosis: The patient was diagnosed with a subungual hematoma and a possible fracture of the distal phalanx. In this case, the clinician offered to drain the hematoma but did not have access to an electrocautery unit. The patient consented to any procedure that would relieve the pain. An open paperclip, held in a hemostat and heated with a torch, was used to pierce the patient’s nail plate and drain the blood, providing immediate relief. Citing lack of insurance, the patient declined an x-ray, despite the possible fracture. The toe was bandaged, and the patient was instructed to keep it elevated and avoid weight-bearing activity. Her toe healed well, and no radiographs were taken.
For more information, see “Painful toe.” J Fam Pract. 2011;60(12).
2. For the past month, a man in his 50s has had a discolored right foot with increasing tenderness, as well as livedo reticularis on the sole and lateral aspect. He has a history of right-arm arterial thrombosis, multiple deep vein thromboses of the legs, ischemic stroke, atrial fibrillation, peripheral arterial disease, and long-term warfarin treatment. Pulses are palpable on exam.

Diagnosis: The patient was diagnosed with antiphospholipid syndrome. He remained on inpatient anticoagulation therapy with fondaparinux and was treated with pulse-dose IV corticosteroids followed by a slow oral taper: daily plasmapheresis for one week, three doses of IV immunoglobulin (0.5 g/kg), and four weekly doses of rituximab (375 mg/m2). His cutaneous findings slowly improved over the next several weeks.
For more information, see “Cyanosis of the Foot.” Cutis. 2017;100(4):206, 209-210.
3. A 35-year-old woman presents in January with purplish toes that are markedly tender to pressure. Recurrent over three successive winters, the initial symptom is an itchy, burning sensation in her toes. The discoloration and other symptoms are constant, not episodic. The condition resolves each year in late spring. Her distal pulses are normal.

Diagnosis: Pernio—also called perniosis or chilblains—is a common dermatologic condition associated with a cold, humid climate. The inflammatory lesions of pernio may be pruritic, painful, erythematous to violaceous plaques, papules, or nodules, which may have overlying blisters or ulcerations. The condition is frequently misdiagnosed; proper diagnosis relies on patient history and clinical picture. Histologic examination is typically not needed or definitive. This patient had moderately disabling pernio, which responded promptly to therapy with a calcium channel blocker.
For more information, see “Erythrocyanotic Discoloration of the Toes.” Cutis. 2000;65(4):223-226.
4. For three days, a 63-year-old man has had severe, sudden-onset pain in the right hallux and fifth toe. The patient has hypertension and hyperlipidemia and has not undergone any vascular procedures. Physical exam reveals cyanotic change with remarkable coldness on the affected toes and livedo reticularis on the underside of the toes. Pulses are palpable. A biopsy of the fifth toe reveals thrombotic arterioles with cholesterol clefts.

Diagnosis: There are a variety of causes for blue toe syndrome, including embolism, thrombosis, vasoconstrictive disorders, infectious and noninfectious inflammation, extensive venous thrombosis, and abnormal blood circulation. Among them, only emboli from atherosclerotic plaques give rise to cholesterol clefts on biopsy. Such atheroemboli are often an iatrogenic complication, especially those caused by invasive percutaneous procedures or damage to the arterial walls from vascular surgery. However, spontaneous plaque hemorrhage or shearing forces of the circulating blood can disrupt atheromatous plaques and cause embolization of cholesterol crystals—which was likely the case with this patient, since no preceding events were noted.
For more information, see “Painful Purple Toes.” Cutis. 2016;98(3):E8-E10.
You, Me, and Your A1C
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
This video was filmed at Metabolic & Endocrine Disease Summit (MEDS). Click here to learn more.
Checkpoint inhibitors forge new treatment paradigm for metastatic bladder cancer
Last spring, the US Food and Drug Administration (FDA) granted accelerated approval to 3 different immune checkpoint inhibitors for the treatment of patients with metastatic urothelial carcinoma in the second-line setting, bringing the total number of approved members of this drug class for this indication to 5.
Avelumab and durvalumab, like atezolizumab, are monoclonal antibodies that target the programmed cell death protein ligand-1 (PD-L1) and prevent it from binding to and activating the programmed cell death protein-1 (PD-1) and CD80 receptors, which transmit inhibitory signals into T cells. In this way, it is hypothesized that their use reactivates the anti-tumor immune response conducted by tumor-infiltrating T cells. Both drugs were approved for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are refractory to platinum-based chemotherapy, and the approvals provide additional treatment options for this group of patients who typically have poor prognosis.1,2
Avelumab trial findings
The approval of avelumab was based on the urothelial cancer cohorts of the JAVELIN Solid Tumor trial, a phase 1, open-label, dose-escalation study.3 Patients aged 18 years and older, with an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1 (range, 0-5; 0, fully active, and 5, dead), life expectancy of at least 3 months, and cytologically or histologically confirmed metastatic or locally advanced solid tumors were eligible.
Patients were excluded from the study if they had a history of or active central nervous system metastases, had other malignancies within the previous 5 years, had undergone organ transplant, had conditions requiring immune suppression, had active HIV or hepatitis B or C infection, or had autoimmune diseases other than type 1 diabetes, vitiligo, psoriasis, or thyroid disease that does not require immunosuppressive treatment.
Patients were also required to have adequate end organ function (white blood cell count, ≥3 x 109 cells/L; absolute neutrophil count, ≥1.5 x 109 cells/L; lymphocyte count, ≥0.5 x 109 cells/L; platelet count, ≥100 x 109 platelets/L; hemoglobin, ≥9 g/dL; total bilirubin concentration, ≤1.5 x upper limit of normal [ULN] range; aspartate- and alanine- aminotransferase (ALT/AST) concentrations, ≤2.5 x ULN); and estimated creatinine clearance, >50 mL/min.
A total of 242 patients were treated with a 10 mg/kg intravenous dose of avelumab every 2 weeks until disease progression or unacceptable toxicity. Before avelumab infusion, all patients received premedication with an antihistamine and acetaminophen.
The primary endpoint was objective response rate (ORR), which was 13.3% among 226 patients followed for at least 13 weeks, including 4% complete response (CR) rate, and 16.1% among 161 patients followed for at least 6 months, including 5.6% CR rate. The median time to response was 2 months and the median response duration had not been reached at the time of data cut-off. PD-L1 expression was evaluable in 84% of patients and there was no discernable variation in the response rates according to the levels of PD-L1 expression on the tumor.
The most common adverse events (AEs) that occurred in at least 20% of patients included fatigue, infusion-related reaction, musculoskeletal pain, nausea, decreased appetite, and urinary tract infection (UTI). Serious AEs occurred in 41% of patients and most commonly involved UTI, abdominal pain, musculoskeletal pain, creatinine increase/renal failure, dehydration, hematuria, intestinal obstruction, and pyrexia. Deaths owing to AEs occurred in 6% of patients and were related to pneumonitis, respiratory failure, sepsis/urosepsis, cerebrovascular accident, or gastrointestinal AEs.
Durvalumab approval
The agency’s approval of durvalumab rested on the results of an ongoing single-arm phase 1/2 trial (Study 1108).4 Eligibility criteria were the same as for the avelumab study. Patients were ineligible for the trial if they had received any immunotherapy within the previous 4 weeks, any monoclonal antibody within the previous 6 weeks, or had received concurrent chemotherapy, immunotherapy, biologic, or hormonal therapy.
Durvalumab was administered as an intravenous infusion at a dose of 10 mg/kg every 2 weeks, for up to 12 months or until disease progression or unacceptable toxicity. PD-L1 expression was evaluated by immunohistochemistry in tumor tissue obtained before treatment using the Ventana PD-L1 (SP263) assay (Ventana Medical Systems), which was approved by the FDA alongside durvalumab as a companion diagnostic. The first 20 patients were enrolled regardless of their PD-L1 expression, and the subsequent 43 patients were required to have PD-L1 expression of at least 5% of their tumor cells, but that requirement was removed at an interim analysis when objective responses occurred in patients with a PD-L1 expression of lessthan 5%.
In the most up-to-date analysis, published after FDA approval, a total of 191 patients had been treated. The ORR as assessed by blinded independent central review per RECIST-1.1, was 17.8%, including 7 CRs (3.7%). In patients with high PD-L1 expression, the ORR was 27.6%, compared with 5.1% in those with low or no PD-L1 expression. Responses were observed across all subgroups, including patients with a poor prognosis. The ORRs in patients with visceral and liver metastases were 15.3% and 7.3%, respectively. The median time to response was 1.41 months, and the median duration of response had not yet been reached.
The most common AEs experienced by patients treated with durvalumab included fatigue, musculoskeletal pain, constipation, decreased appetite, nausea, peripheral edema, and UTI. Serious treatment-related AEs occurred in 4.7% of patients, and treatment-related AEs leading to death occurred in 2 patients owing to autoimmune hepatitis and pneumonitis.
Toxicities and warnings for both therapies
Avelumab is marketed as Bavencio by EMD Serono, and durvalumab as Imfinzi by AstraZeneca. According to the prescribing information for both drugs, the recommended dose is 10 mg/kg administered as an intravenous infusion over 60 minutes every 2 weeks.5,6
Both drugs are associated with serious or potentially life-threatening toxicities for which warnings and precautions are detailed in the prescribing information, predominantly for immune-mediated toxicities such as pneumonitis, hepatitis, colitis, nephritis, and endocrinpathy. Patients should be monitored for signs and symptoms of these toxicities and managed appropriately. Avelumab and durvalumab should both be withheld for grade 2 or higher pneumonitis, hepatitis, colitis, severe or life-threatening adrenal insufficiency, thyroid disorders or hyperglycemia, and moderate or severe nephritis or renal dysfunction.
These drugs should be permanently discontinued in the event of life-threatening or recurrent AEs. Immune-mediated pneumonitis, colitis, and hepatitis and adrenal insufficiency can be managed with corticosteroids; hypothyroidism, with hormone-replacement therapy; and hyperglycemia, with hyperglycemics or insulin.
To manage infusion-related reactions, patients should be premedicated with antihistamines and acetaminophen before the first 4 infusions and closely monitored for symptoms such as pyrexia, chills, flushing, hypotension, and dyspnea. Infusion can be interrupted or slowed for mild to moderate infusion-related reactions, but should be stopped and the drug discontinued for severe or life-threatening reactions.
Durvalumab is also associated with a risk of infection and patients should be monitored for signs and symptoms of infection and treated with anti-infectives. Durvalumab should be withheld for grade 3 infections. Patients being treated with durvalumab or avelumab should also be warned of the potential for embryofetal toxicity and advised to take appropriate precautions.
1. United States Food and Drug Administration. FDA grants accelerated approval to avelumab for urothelial carcinoma. US FDA Web site. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm557162.htm. Last updated May 9, 2017. Accessed September 15, 2017.
2. United States Food and Drug Administration. Durvalumab (Imfinzi). US FDA Web site. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm555930.htm. Last updated May 1, 2017. Accessed September 15, 2017
3. Heery CR, O’Sullivan-Coyne G, Madan RA, Cordes L, et al. Avelumab for metastatic or locally advanced previously treated solid tumors (JAVELIN Solid Tumos): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18(5):587-598.
4. Powles T, O’Donnell PH, Massard C, Arkenau H-T, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(8):e172411.
5. Bavencio (avelumab) injection, for intravenous use. Prescribing information. EMD Serono Inc. https://www.bavencio.com/en_US/document/Prescribing-Information.pdf. Revised October 2017. Accessed September 18th, 2017.
6. Imfinzi (durvalumab) injection, for intravenous use. Prescribing information. AstraZeneca Pharmaceuticals. https://www.azpicentral.com/imfinzi/imfinzi.pdf#page=1. Revised May 2017. Accessed September 18, 2017.
Last spring, the US Food and Drug Administration (FDA) granted accelerated approval to 3 different immune checkpoint inhibitors for the treatment of patients with metastatic urothelial carcinoma in the second-line setting, bringing the total number of approved members of this drug class for this indication to 5.
Avelumab and durvalumab, like atezolizumab, are monoclonal antibodies that target the programmed cell death protein ligand-1 (PD-L1) and prevent it from binding to and activating the programmed cell death protein-1 (PD-1) and CD80 receptors, which transmit inhibitory signals into T cells. In this way, it is hypothesized that their use reactivates the anti-tumor immune response conducted by tumor-infiltrating T cells. Both drugs were approved for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are refractory to platinum-based chemotherapy, and the approvals provide additional treatment options for this group of patients who typically have poor prognosis.1,2
Avelumab trial findings
The approval of avelumab was based on the urothelial cancer cohorts of the JAVELIN Solid Tumor trial, a phase 1, open-label, dose-escalation study.3 Patients aged 18 years and older, with an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1 (range, 0-5; 0, fully active, and 5, dead), life expectancy of at least 3 months, and cytologically or histologically confirmed metastatic or locally advanced solid tumors were eligible.
Patients were excluded from the study if they had a history of or active central nervous system metastases, had other malignancies within the previous 5 years, had undergone organ transplant, had conditions requiring immune suppression, had active HIV or hepatitis B or C infection, or had autoimmune diseases other than type 1 diabetes, vitiligo, psoriasis, or thyroid disease that does not require immunosuppressive treatment.
Patients were also required to have adequate end organ function (white blood cell count, ≥3 x 109 cells/L; absolute neutrophil count, ≥1.5 x 109 cells/L; lymphocyte count, ≥0.5 x 109 cells/L; platelet count, ≥100 x 109 platelets/L; hemoglobin, ≥9 g/dL; total bilirubin concentration, ≤1.5 x upper limit of normal [ULN] range; aspartate- and alanine- aminotransferase (ALT/AST) concentrations, ≤2.5 x ULN); and estimated creatinine clearance, >50 mL/min.
A total of 242 patients were treated with a 10 mg/kg intravenous dose of avelumab every 2 weeks until disease progression or unacceptable toxicity. Before avelumab infusion, all patients received premedication with an antihistamine and acetaminophen.
The primary endpoint was objective response rate (ORR), which was 13.3% among 226 patients followed for at least 13 weeks, including 4% complete response (CR) rate, and 16.1% among 161 patients followed for at least 6 months, including 5.6% CR rate. The median time to response was 2 months and the median response duration had not been reached at the time of data cut-off. PD-L1 expression was evaluable in 84% of patients and there was no discernable variation in the response rates according to the levels of PD-L1 expression on the tumor.
The most common adverse events (AEs) that occurred in at least 20% of patients included fatigue, infusion-related reaction, musculoskeletal pain, nausea, decreased appetite, and urinary tract infection (UTI). Serious AEs occurred in 41% of patients and most commonly involved UTI, abdominal pain, musculoskeletal pain, creatinine increase/renal failure, dehydration, hematuria, intestinal obstruction, and pyrexia. Deaths owing to AEs occurred in 6% of patients and were related to pneumonitis, respiratory failure, sepsis/urosepsis, cerebrovascular accident, or gastrointestinal AEs.
Durvalumab approval
The agency’s approval of durvalumab rested on the results of an ongoing single-arm phase 1/2 trial (Study 1108).4 Eligibility criteria were the same as for the avelumab study. Patients were ineligible for the trial if they had received any immunotherapy within the previous 4 weeks, any monoclonal antibody within the previous 6 weeks, or had received concurrent chemotherapy, immunotherapy, biologic, or hormonal therapy.
Durvalumab was administered as an intravenous infusion at a dose of 10 mg/kg every 2 weeks, for up to 12 months or until disease progression or unacceptable toxicity. PD-L1 expression was evaluated by immunohistochemistry in tumor tissue obtained before treatment using the Ventana PD-L1 (SP263) assay (Ventana Medical Systems), which was approved by the FDA alongside durvalumab as a companion diagnostic. The first 20 patients were enrolled regardless of their PD-L1 expression, and the subsequent 43 patients were required to have PD-L1 expression of at least 5% of their tumor cells, but that requirement was removed at an interim analysis when objective responses occurred in patients with a PD-L1 expression of lessthan 5%.
In the most up-to-date analysis, published after FDA approval, a total of 191 patients had been treated. The ORR as assessed by blinded independent central review per RECIST-1.1, was 17.8%, including 7 CRs (3.7%). In patients with high PD-L1 expression, the ORR was 27.6%, compared with 5.1% in those with low or no PD-L1 expression. Responses were observed across all subgroups, including patients with a poor prognosis. The ORRs in patients with visceral and liver metastases were 15.3% and 7.3%, respectively. The median time to response was 1.41 months, and the median duration of response had not yet been reached.
The most common AEs experienced by patients treated with durvalumab included fatigue, musculoskeletal pain, constipation, decreased appetite, nausea, peripheral edema, and UTI. Serious treatment-related AEs occurred in 4.7% of patients, and treatment-related AEs leading to death occurred in 2 patients owing to autoimmune hepatitis and pneumonitis.
Toxicities and warnings for both therapies
Avelumab is marketed as Bavencio by EMD Serono, and durvalumab as Imfinzi by AstraZeneca. According to the prescribing information for both drugs, the recommended dose is 10 mg/kg administered as an intravenous infusion over 60 minutes every 2 weeks.5,6
Both drugs are associated with serious or potentially life-threatening toxicities for which warnings and precautions are detailed in the prescribing information, predominantly for immune-mediated toxicities such as pneumonitis, hepatitis, colitis, nephritis, and endocrinpathy. Patients should be monitored for signs and symptoms of these toxicities and managed appropriately. Avelumab and durvalumab should both be withheld for grade 2 or higher pneumonitis, hepatitis, colitis, severe or life-threatening adrenal insufficiency, thyroid disorders or hyperglycemia, and moderate or severe nephritis or renal dysfunction.
These drugs should be permanently discontinued in the event of life-threatening or recurrent AEs. Immune-mediated pneumonitis, colitis, and hepatitis and adrenal insufficiency can be managed with corticosteroids; hypothyroidism, with hormone-replacement therapy; and hyperglycemia, with hyperglycemics or insulin.
To manage infusion-related reactions, patients should be premedicated with antihistamines and acetaminophen before the first 4 infusions and closely monitored for symptoms such as pyrexia, chills, flushing, hypotension, and dyspnea. Infusion can be interrupted or slowed for mild to moderate infusion-related reactions, but should be stopped and the drug discontinued for severe or life-threatening reactions.
Durvalumab is also associated with a risk of infection and patients should be monitored for signs and symptoms of infection and treated with anti-infectives. Durvalumab should be withheld for grade 3 infections. Patients being treated with durvalumab or avelumab should also be warned of the potential for embryofetal toxicity and advised to take appropriate precautions.
Last spring, the US Food and Drug Administration (FDA) granted accelerated approval to 3 different immune checkpoint inhibitors for the treatment of patients with metastatic urothelial carcinoma in the second-line setting, bringing the total number of approved members of this drug class for this indication to 5.
Avelumab and durvalumab, like atezolizumab, are monoclonal antibodies that target the programmed cell death protein ligand-1 (PD-L1) and prevent it from binding to and activating the programmed cell death protein-1 (PD-1) and CD80 receptors, which transmit inhibitory signals into T cells. In this way, it is hypothesized that their use reactivates the anti-tumor immune response conducted by tumor-infiltrating T cells. Both drugs were approved for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are refractory to platinum-based chemotherapy, and the approvals provide additional treatment options for this group of patients who typically have poor prognosis.1,2
Avelumab trial findings
The approval of avelumab was based on the urothelial cancer cohorts of the JAVELIN Solid Tumor trial, a phase 1, open-label, dose-escalation study.3 Patients aged 18 years and older, with an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1 (range, 0-5; 0, fully active, and 5, dead), life expectancy of at least 3 months, and cytologically or histologically confirmed metastatic or locally advanced solid tumors were eligible.
Patients were excluded from the study if they had a history of or active central nervous system metastases, had other malignancies within the previous 5 years, had undergone organ transplant, had conditions requiring immune suppression, had active HIV or hepatitis B or C infection, or had autoimmune diseases other than type 1 diabetes, vitiligo, psoriasis, or thyroid disease that does not require immunosuppressive treatment.
Patients were also required to have adequate end organ function (white blood cell count, ≥3 x 109 cells/L; absolute neutrophil count, ≥1.5 x 109 cells/L; lymphocyte count, ≥0.5 x 109 cells/L; platelet count, ≥100 x 109 platelets/L; hemoglobin, ≥9 g/dL; total bilirubin concentration, ≤1.5 x upper limit of normal [ULN] range; aspartate- and alanine- aminotransferase (ALT/AST) concentrations, ≤2.5 x ULN); and estimated creatinine clearance, >50 mL/min.
A total of 242 patients were treated with a 10 mg/kg intravenous dose of avelumab every 2 weeks until disease progression or unacceptable toxicity. Before avelumab infusion, all patients received premedication with an antihistamine and acetaminophen.
The primary endpoint was objective response rate (ORR), which was 13.3% among 226 patients followed for at least 13 weeks, including 4% complete response (CR) rate, and 16.1% among 161 patients followed for at least 6 months, including 5.6% CR rate. The median time to response was 2 months and the median response duration had not been reached at the time of data cut-off. PD-L1 expression was evaluable in 84% of patients and there was no discernable variation in the response rates according to the levels of PD-L1 expression on the tumor.
The most common adverse events (AEs) that occurred in at least 20% of patients included fatigue, infusion-related reaction, musculoskeletal pain, nausea, decreased appetite, and urinary tract infection (UTI). Serious AEs occurred in 41% of patients and most commonly involved UTI, abdominal pain, musculoskeletal pain, creatinine increase/renal failure, dehydration, hematuria, intestinal obstruction, and pyrexia. Deaths owing to AEs occurred in 6% of patients and were related to pneumonitis, respiratory failure, sepsis/urosepsis, cerebrovascular accident, or gastrointestinal AEs.
Durvalumab approval
The agency’s approval of durvalumab rested on the results of an ongoing single-arm phase 1/2 trial (Study 1108).4 Eligibility criteria were the same as for the avelumab study. Patients were ineligible for the trial if they had received any immunotherapy within the previous 4 weeks, any monoclonal antibody within the previous 6 weeks, or had received concurrent chemotherapy, immunotherapy, biologic, or hormonal therapy.
Durvalumab was administered as an intravenous infusion at a dose of 10 mg/kg every 2 weeks, for up to 12 months or until disease progression or unacceptable toxicity. PD-L1 expression was evaluated by immunohistochemistry in tumor tissue obtained before treatment using the Ventana PD-L1 (SP263) assay (Ventana Medical Systems), which was approved by the FDA alongside durvalumab as a companion diagnostic. The first 20 patients were enrolled regardless of their PD-L1 expression, and the subsequent 43 patients were required to have PD-L1 expression of at least 5% of their tumor cells, but that requirement was removed at an interim analysis when objective responses occurred in patients with a PD-L1 expression of lessthan 5%.
In the most up-to-date analysis, published after FDA approval, a total of 191 patients had been treated. The ORR as assessed by blinded independent central review per RECIST-1.1, was 17.8%, including 7 CRs (3.7%). In patients with high PD-L1 expression, the ORR was 27.6%, compared with 5.1% in those with low or no PD-L1 expression. Responses were observed across all subgroups, including patients with a poor prognosis. The ORRs in patients with visceral and liver metastases were 15.3% and 7.3%, respectively. The median time to response was 1.41 months, and the median duration of response had not yet been reached.
The most common AEs experienced by patients treated with durvalumab included fatigue, musculoskeletal pain, constipation, decreased appetite, nausea, peripheral edema, and UTI. Serious treatment-related AEs occurred in 4.7% of patients, and treatment-related AEs leading to death occurred in 2 patients owing to autoimmune hepatitis and pneumonitis.
Toxicities and warnings for both therapies
Avelumab is marketed as Bavencio by EMD Serono, and durvalumab as Imfinzi by AstraZeneca. According to the prescribing information for both drugs, the recommended dose is 10 mg/kg administered as an intravenous infusion over 60 minutes every 2 weeks.5,6
Both drugs are associated with serious or potentially life-threatening toxicities for which warnings and precautions are detailed in the prescribing information, predominantly for immune-mediated toxicities such as pneumonitis, hepatitis, colitis, nephritis, and endocrinpathy. Patients should be monitored for signs and symptoms of these toxicities and managed appropriately. Avelumab and durvalumab should both be withheld for grade 2 or higher pneumonitis, hepatitis, colitis, severe or life-threatening adrenal insufficiency, thyroid disorders or hyperglycemia, and moderate or severe nephritis or renal dysfunction.
These drugs should be permanently discontinued in the event of life-threatening or recurrent AEs. Immune-mediated pneumonitis, colitis, and hepatitis and adrenal insufficiency can be managed with corticosteroids; hypothyroidism, with hormone-replacement therapy; and hyperglycemia, with hyperglycemics or insulin.
To manage infusion-related reactions, patients should be premedicated with antihistamines and acetaminophen before the first 4 infusions and closely monitored for symptoms such as pyrexia, chills, flushing, hypotension, and dyspnea. Infusion can be interrupted or slowed for mild to moderate infusion-related reactions, but should be stopped and the drug discontinued for severe or life-threatening reactions.
Durvalumab is also associated with a risk of infection and patients should be monitored for signs and symptoms of infection and treated with anti-infectives. Durvalumab should be withheld for grade 3 infections. Patients being treated with durvalumab or avelumab should also be warned of the potential for embryofetal toxicity and advised to take appropriate precautions.
1. United States Food and Drug Administration. FDA grants accelerated approval to avelumab for urothelial carcinoma. US FDA Web site. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm557162.htm. Last updated May 9, 2017. Accessed September 15, 2017.
2. United States Food and Drug Administration. Durvalumab (Imfinzi). US FDA Web site. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm555930.htm. Last updated May 1, 2017. Accessed September 15, 2017
3. Heery CR, O’Sullivan-Coyne G, Madan RA, Cordes L, et al. Avelumab for metastatic or locally advanced previously treated solid tumors (JAVELIN Solid Tumos): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18(5):587-598.
4. Powles T, O’Donnell PH, Massard C, Arkenau H-T, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(8):e172411.
5. Bavencio (avelumab) injection, for intravenous use. Prescribing information. EMD Serono Inc. https://www.bavencio.com/en_US/document/Prescribing-Information.pdf. Revised October 2017. Accessed September 18th, 2017.
6. Imfinzi (durvalumab) injection, for intravenous use. Prescribing information. AstraZeneca Pharmaceuticals. https://www.azpicentral.com/imfinzi/imfinzi.pdf#page=1. Revised May 2017. Accessed September 18, 2017.
1. United States Food and Drug Administration. FDA grants accelerated approval to avelumab for urothelial carcinoma. US FDA Web site. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm557162.htm. Last updated May 9, 2017. Accessed September 15, 2017.
2. United States Food and Drug Administration. Durvalumab (Imfinzi). US FDA Web site. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm555930.htm. Last updated May 1, 2017. Accessed September 15, 2017
3. Heery CR, O’Sullivan-Coyne G, Madan RA, Cordes L, et al. Avelumab for metastatic or locally advanced previously treated solid tumors (JAVELIN Solid Tumos): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017;18(5):587-598.
4. Powles T, O’Donnell PH, Massard C, Arkenau H-T, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(8):e172411.
5. Bavencio (avelumab) injection, for intravenous use. Prescribing information. EMD Serono Inc. https://www.bavencio.com/en_US/document/Prescribing-Information.pdf. Revised October 2017. Accessed September 18th, 2017.
6. Imfinzi (durvalumab) injection, for intravenous use. Prescribing information. AstraZeneca Pharmaceuticals. https://www.azpicentral.com/imfinzi/imfinzi.pdf#page=1. Revised May 2017. Accessed September 18, 2017.
Concurrent ipilimumab and CMV colitis refractory to oral steroids
Immune checkpoint inhibitors, including anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA4) and anti-programmed cell death protein-1 (anti-PD-1) antibodies, have demonstrated clinical and survival benefits in a variety of malignancies, which has led to an expansion in their role in oncology. In melanoma, the anti-CTLA-4 antibody, ipilimumab, has demonstrated a survival benefit in patients with advanced metastatic melanoma and in patients with resectable disease with lymph node involvement.1,2
Ipilimumab exerts its effect by binding CTLA-4 on conventional and regulatory T cells, thus blocking inhibitory signals on T cells, which leads to an antitumor response.3 The increased immune response counteracts the immune-evading mechanisms of the tumor. With increased use of these agents, immune-related adverse events (irAEs) have become more prevalent. The most common irAEs secondary to ipilimumab are skin rash, colitis/diarrhea, hepatitis, pneumonitis, and various endocrinopathies.4 In a phase 3 trial of adjuvant ipilimumab in patients with resected stage III melanoma, grade 3 or 4 adverse events occurred in 54.1% of participants in the ipilimumab arm, the most common being diarrhea and colitis (9.8% and 6.8%, respectively).2Recognition and management of irAEs has led to the implementation of treatment guidelines.4,5 Management of irAEs includes checkpoint inhibitor discontinuation and reversal of the immune response by institution of immunosuppression with corticosteroids.
Case presentation and summary
A 40-year-old white woman with stage IIIB BRAF V600E-positive melanoma presented with diarrhea refractory to high-dose prednisone (1 mg/kg BID). She had recently undergone wide local excision and sentinel node biopsy and received her inaugural dose of ipilimumab (10 mg/kg).
The patient first presented with loose, watery stools that had begun 8 days after she had received her first dose of adjuvant ipilimumab. She was admitted to the hospital, and intravenous methylprednisolone was initiated along with empiric ciprofloxacin (400 mg, IVPB Q12h) and metronidazole (500 mg, IVPB Q8h) as infectious causes were concurrently ruled out. During this initial admission, the patient’s stool was negative for Clostridium difficile toxin, ova, and parasites, as well as enteric pathogens by culture. After infectious causes were excluded, she was diagnosed with ipilimumab-induced colitis. Antibiotics were discontinued, and the patient ultimately noted improvement in her symptoms. On hospital day 7, she was experiencing only 2 bowel movements a day and was discharged on 80 mg of prednisone twice daily.
After discharge the patient noted persistence of her symptoms. At her follow-up, 9 days after discharge, the patient noted continued symptoms of low-grade diarrhea. She failed a trial of steroid tapering due to exacerbation of her abdominal pain and frequency of diarrhea. Further investigation was negative for C. diff toxin and a computed-tomography scan was consistent with continuing colitis. The patient’s symptoms continued to worsen, with recurrence of grade 3 diarrhea, and she was ultimately readmitted 17 days after her earlier discharge (36 days after her first ipilimumab dosing).
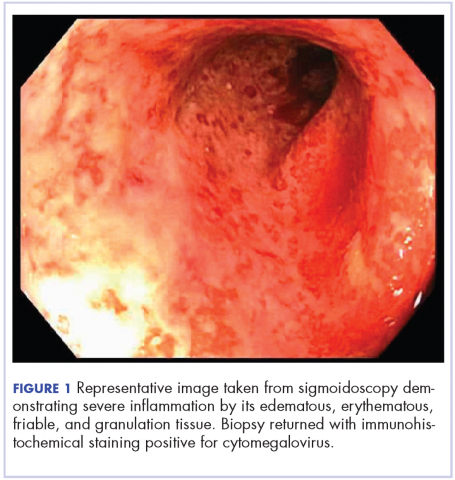
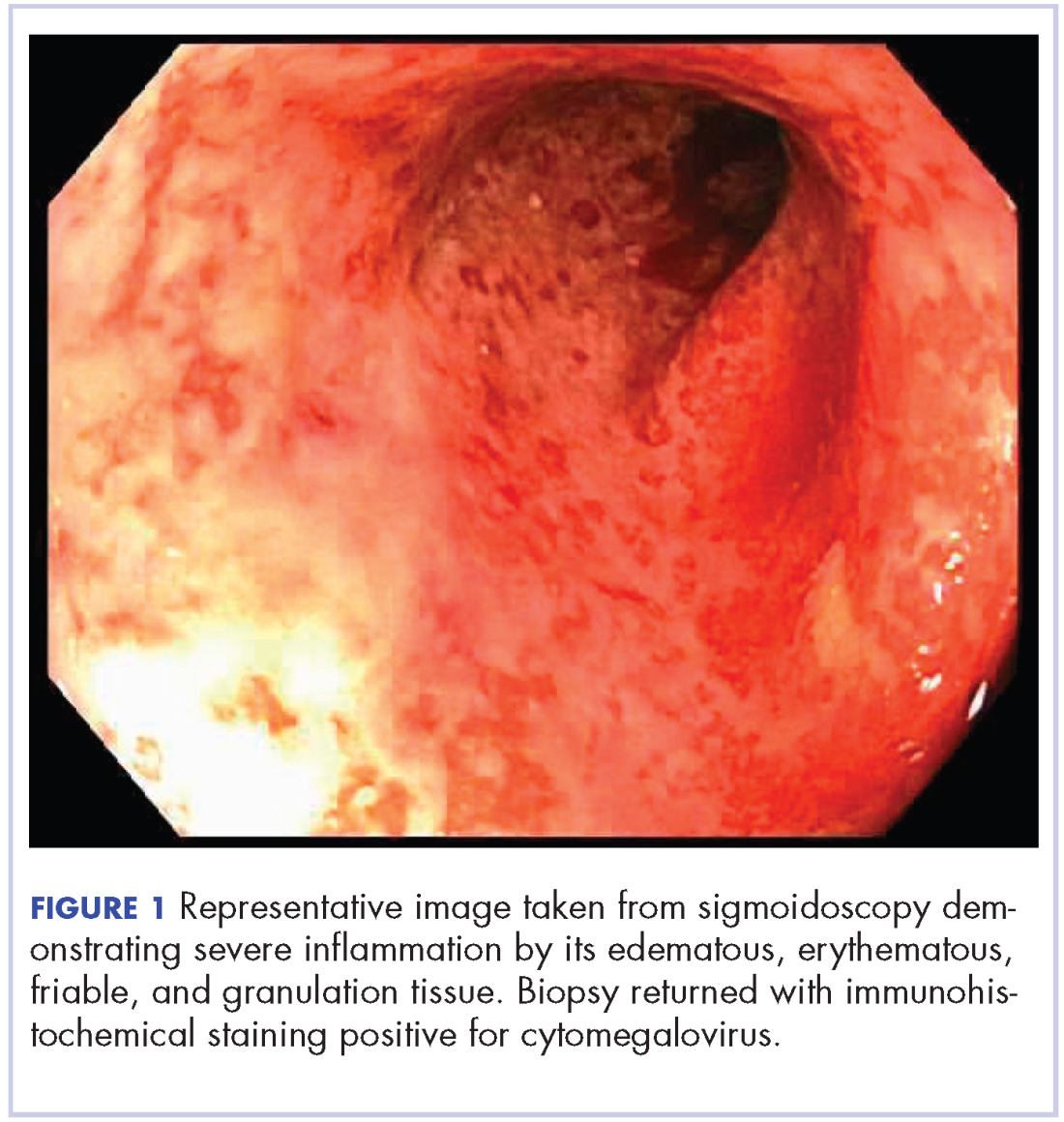
On re-admission, the patient was again given intravenous methylprednisolone and experienced interval improvement in the frequency of diarrhea. A gastroenterology expert was consulted, and the patient underwent a flexible sigmoidoscopy that demonstrated findings of diffuse and severe inflammation and biopsies were obtained (Figure 1). After several days of continued symptoms, the patient received infliximab 5 mg/kg for treatment of her adverse autoimmune reaction. After administration, the patient noted improvement in the frequency and volume of diarrhea, however, her symptoms still persisted.
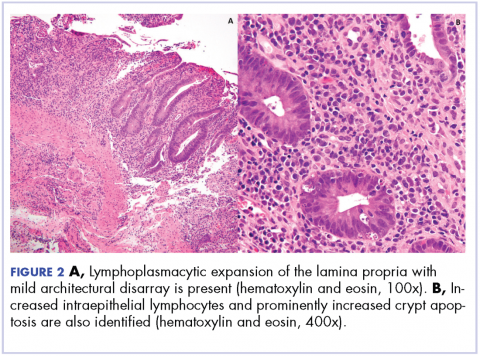
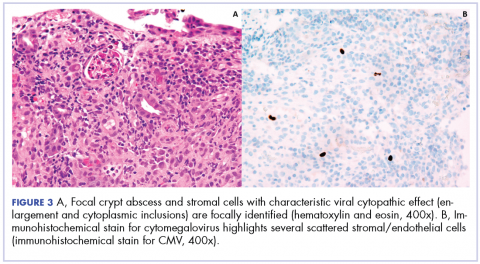
Biopsy results subsequently revealed findings compatible with ipilimumab-induced colitis, and immunohistochemical staining demonstrated positivity for cytomegalovirus (CMV). Specifically, histologic examination showed lymphoplasmacytic expansion of the lamina propria, some architectural distortion, and increased crypt apoptosis. Scattered cryptitis and crypt abscesses were also noted, as were rare stromal and endothelial cells with characteristic CMV inclusions (Figure 2 and Figure 3).
Serum CMV polymerase chain reaction (PCR) was also positive at 652,000 IU/mL (lower limit of detection 100 IU/mL). Induction dosing of ganciclovir (5 mg/kg IV Q12h) was initiated. The combined treatment with intravenous methylprednisone and ganciclovir led to an improvement in diarrhea frequency and resolution of blood in the stool. She was transitioned to oral prednisone, but it resulted in redevelopment of grade 3 diarrhea. The patient was therefore resumed on and discharged on daily intravenous methylprednisolone.
After discharge, the patient was started on budesonide 9 mg daily. Her serum CMV PCR level reduced and she was transitioned to oral valgancyclovir (900 mg daily) for maintenance. Another unsuccessful attempt was made to switch her to oral prednisone.
About 14 weeks after the initial ipilimumab dosing, the patient underwent another flexible sigmoidoscopy that again demonstrated severe colitis from the rectum to sigmoid colon. Biopsies were negative for CMV. Patient was readmitted for recurrence of diarrhea the following week. Treatment with IV methylprednisone (1mg/kg BID) and infliximab (5 mg/kg) again led to an improvement of symptoms. She was again discharged on IV methylprednisone (1 mg/kg BID) with a taper.
In the 15th week after her initial ipilimumab dose, the patient presented with a perforated bowel, requiring a subtotal colectomy and end ileostomy. She continued on a slow taper of oral prednisone (50 mg daily and decrease by 10 mg every 5 days).
At her last documented follow-up, 8 months after her first ipilimumab dose, she was having normal output from her ileostomy. She developed secondary adrenal insufficiency because of the long-term steroids and continued to take prednisone 5 mg daily.
Discussion
Diarrhea and colitis are common irAEs attributable to checkpoint-inhibitor therapy used for the treatment of melanoma. This case of ipilimumab-induced colitis refractory to high-dose oral steroids demonstrates the risks associated with management of anti-CTLA-4 induced colitis. In particular, the high-dose corticosteroids required to treat the autoimmune component of this patient’s colitis increased her susceptibility to CMV reactivation.
The diagnosis of colitis secondary to ipilimumab is made primarily in the appropriate clinical setting, and typically onsets during the induction period (within 12 weeks of initial dosing) and most resolve within 6-8 weeks.6 Histopathologically, there is lymphoplasmacytic expansion of lamina propria, increased intraepithelial lymphocytes, and increased epithelial apoptosis of crypts. One can also see acute cryptitis and crypt abscesses. Reactive epithelial changes with mucin depletion are also often seen in epithelial cells.
Findings from immunohistochemical studies have shown the increased intraepithelial lymphocytes to be predominantly CD8-positive T cells, while the lamina propria contains an increase in the mixture of CD4- and CD8-positive T cells. In addition, small intestinal samples show villous blunting. There is an absence of significant architectural distortion and well-developed basal lymphoplasmacytic infiltrates characteristic of chronic mucosal injury, such as idiopathic inflammatory bowel disease.7 Granulomas are also absent in most series, though they have been reported in some cases.8 The features are similar to those seen in autoimmune enteropathy, but goblet and endocrine cells remain preserved. Graft-versus-host disease has similar histologic features, however, the clinical setting usually makes the distinction between these obvious.
Current treatment algorithms for ipilimumab-related diarrhea, begin with immediate treatment with intravenous methylprednisolone (125 mg once). This is followed with oral prednisone at a dose of 1-2 mg/kg tapered over 4 to 8 weeks.4 In patients with persistent symptoms despite adequate doses of corticosteroids, infliximab (5 mg/kg every 2 weeks) is recommended until the resolution of symptoms, and a longer taper of prednisone is often necessary.
Institution of high-dose corticosteroids to treat grade 3 or 4 irAEs can increase the risk for infection, including opportunistic infections. One retrospective review of patients administered checkpoint inhibitors at a single institution revealed that 7.3% of 740 patients developed a severe infection that lead to hospitalization or treatment with intravenous antibiotics.9 In that patient cohort, only 0.6% had a serious infection secondary to a viral etiology, and 1 patient developed CMV enterocolitis. Most patients who developed an infection in this cohort had received corticosteroids (46/54 patients, 85%) and/or infliximab (13/54 patients, 24%).9
CMV is a member of the Herpesviridae family. After a primary infection, which can often go unrecognized in an immunocompetent host, CMV can persist in a latent state.10 In a study by Bate and colleagues, the age-adjusted seropositivity of CMV was found to be 50.4%.11 Based on those results, immunosuppression in a patient who has previously been infected with CMV can lead to a risk of reactivation or even reinfection. In the era of checkpoint-inhibitor therapy, reactivation of CMV has been described previously in a case of CMV hepatitis and a report of CMV colitis.12,13 Immunosuppression, such as that caused by corticosteroids, is a risk factor for CMV infection.14 Colitis caused by CMV usually presents with abdominal pain, diarrhea, and bloody diarrhea.15 In suspected cases of CMV colitis, endoscopy should be pursued with biopsy for tissue examination. A tissue diagnosis is required for CMV colitis because serum PCR can be negative in isolated cases of gastrointestinal CMV infection.15
Conclusion
Despite appropriate treatment with ganciclovir and the noted response in the patient’s serum CMV PCR, symptom exacerbation was observed with the transition to oral prednisone. The requirement for intravenous corticosteroids in the present case demonstrates the prolonged effects exerted by irAEs secondary to checkpoint-inhibitor therapy. Those effects are attributable to the design of the antibody – ipilimumab is a fully humanized monoclonal antibody and has a plasma half-life of about 15 days.1,4
By the identification of CMV histopathologically, this case, along with the case presented by Lankes and colleagues,13 illustrates the importance of considering CMV colitis in patients who are being treated with ipilimumab and who develop persistent or worsening diarrhea after initial treatment with high-dose steroids.
Early recognition of possible coexistent CMV colitis in patients with a history of treatment with ipilimumab can have important clinical consequences. It can lead to quicker implementation of proper antiviral therapy and minimization of immune suppression to levels required to maintain control of the patient’s symptoms.
1. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845-1855.
3. Glassman PM, Balthasar JP. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med. 2014;11(1):20-33.
4. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
5. Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18(6):733-743.
6. Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675-1682.
7. Oble DA, Mino-Kenudson M, Goldsmith J, et al. Alpha-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol. 2008;32(8):1130-1137.
8. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289.
9. Del Castillo M, Romero FA, Arguello E, Kyi C, Postow MA, Redelman-Sidi G. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis. 2016;63(11):1490-1493.
10. Pillet S, Pozzetto B, Roblin X. Cytomegalovirus and ulcerative colitis: place of antiviral therapy. World J Gastroenterol. 2016;22(6):2030-2045.
11. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439-1447.
12. Uslu U, Agaimy A, Hundorfean G, Harrer T, Schuler G, Heinzerling L. autoimmune colitis and subsequent CMV-induced hepatitis after treatment with ipilimumab. J Immunother. 2015;38(5):212-215.
13. Lankes K, Hundorfean G, Harrer T, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016;5(6):e1128611.
14. Ko JH, Peck KR, Lee WJ, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60(6):e20-26.
15. You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14(4):334-342.
Immune checkpoint inhibitors, including anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA4) and anti-programmed cell death protein-1 (anti-PD-1) antibodies, have demonstrated clinical and survival benefits in a variety of malignancies, which has led to an expansion in their role in oncology. In melanoma, the anti-CTLA-4 antibody, ipilimumab, has demonstrated a survival benefit in patients with advanced metastatic melanoma and in patients with resectable disease with lymph node involvement.1,2
Ipilimumab exerts its effect by binding CTLA-4 on conventional and regulatory T cells, thus blocking inhibitory signals on T cells, which leads to an antitumor response.3 The increased immune response counteracts the immune-evading mechanisms of the tumor. With increased use of these agents, immune-related adverse events (irAEs) have become more prevalent. The most common irAEs secondary to ipilimumab are skin rash, colitis/diarrhea, hepatitis, pneumonitis, and various endocrinopathies.4 In a phase 3 trial of adjuvant ipilimumab in patients with resected stage III melanoma, grade 3 or 4 adverse events occurred in 54.1% of participants in the ipilimumab arm, the most common being diarrhea and colitis (9.8% and 6.8%, respectively).2Recognition and management of irAEs has led to the implementation of treatment guidelines.4,5 Management of irAEs includes checkpoint inhibitor discontinuation and reversal of the immune response by institution of immunosuppression with corticosteroids.
Case presentation and summary
A 40-year-old white woman with stage IIIB BRAF V600E-positive melanoma presented with diarrhea refractory to high-dose prednisone (1 mg/kg BID). She had recently undergone wide local excision and sentinel node biopsy and received her inaugural dose of ipilimumab (10 mg/kg).
The patient first presented with loose, watery stools that had begun 8 days after she had received her first dose of adjuvant ipilimumab. She was admitted to the hospital, and intravenous methylprednisolone was initiated along with empiric ciprofloxacin (400 mg, IVPB Q12h) and metronidazole (500 mg, IVPB Q8h) as infectious causes were concurrently ruled out. During this initial admission, the patient’s stool was negative for Clostridium difficile toxin, ova, and parasites, as well as enteric pathogens by culture. After infectious causes were excluded, she was diagnosed with ipilimumab-induced colitis. Antibiotics were discontinued, and the patient ultimately noted improvement in her symptoms. On hospital day 7, she was experiencing only 2 bowel movements a day and was discharged on 80 mg of prednisone twice daily.
After discharge the patient noted persistence of her symptoms. At her follow-up, 9 days after discharge, the patient noted continued symptoms of low-grade diarrhea. She failed a trial of steroid tapering due to exacerbation of her abdominal pain and frequency of diarrhea. Further investigation was negative for C. diff toxin and a computed-tomography scan was consistent with continuing colitis. The patient’s symptoms continued to worsen, with recurrence of grade 3 diarrhea, and she was ultimately readmitted 17 days after her earlier discharge (36 days after her first ipilimumab dosing).
On re-admission, the patient was again given intravenous methylprednisolone and experienced interval improvement in the frequency of diarrhea. A gastroenterology expert was consulted, and the patient underwent a flexible sigmoidoscopy that demonstrated findings of diffuse and severe inflammation and biopsies were obtained (Figure 1). After several days of continued symptoms, the patient received infliximab 5 mg/kg for treatment of her adverse autoimmune reaction. After administration, the patient noted improvement in the frequency and volume of diarrhea, however, her symptoms still persisted.
Biopsy results subsequently revealed findings compatible with ipilimumab-induced colitis, and immunohistochemical staining demonstrated positivity for cytomegalovirus (CMV). Specifically, histologic examination showed lymphoplasmacytic expansion of the lamina propria, some architectural distortion, and increased crypt apoptosis. Scattered cryptitis and crypt abscesses were also noted, as were rare stromal and endothelial cells with characteristic CMV inclusions (Figure 2 and Figure 3).
Serum CMV polymerase chain reaction (PCR) was also positive at 652,000 IU/mL (lower limit of detection 100 IU/mL). Induction dosing of ganciclovir (5 mg/kg IV Q12h) was initiated. The combined treatment with intravenous methylprednisone and ganciclovir led to an improvement in diarrhea frequency and resolution of blood in the stool. She was transitioned to oral prednisone, but it resulted in redevelopment of grade 3 diarrhea. The patient was therefore resumed on and discharged on daily intravenous methylprednisolone.
After discharge, the patient was started on budesonide 9 mg daily. Her serum CMV PCR level reduced and she was transitioned to oral valgancyclovir (900 mg daily) for maintenance. Another unsuccessful attempt was made to switch her to oral prednisone.
About 14 weeks after the initial ipilimumab dosing, the patient underwent another flexible sigmoidoscopy that again demonstrated severe colitis from the rectum to sigmoid colon. Biopsies were negative for CMV. Patient was readmitted for recurrence of diarrhea the following week. Treatment with IV methylprednisone (1mg/kg BID) and infliximab (5 mg/kg) again led to an improvement of symptoms. She was again discharged on IV methylprednisone (1 mg/kg BID) with a taper.
In the 15th week after her initial ipilimumab dose, the patient presented with a perforated bowel, requiring a subtotal colectomy and end ileostomy. She continued on a slow taper of oral prednisone (50 mg daily and decrease by 10 mg every 5 days).
At her last documented follow-up, 8 months after her first ipilimumab dose, she was having normal output from her ileostomy. She developed secondary adrenal insufficiency because of the long-term steroids and continued to take prednisone 5 mg daily.
Discussion
Diarrhea and colitis are common irAEs attributable to checkpoint-inhibitor therapy used for the treatment of melanoma. This case of ipilimumab-induced colitis refractory to high-dose oral steroids demonstrates the risks associated with management of anti-CTLA-4 induced colitis. In particular, the high-dose corticosteroids required to treat the autoimmune component of this patient’s colitis increased her susceptibility to CMV reactivation.
The diagnosis of colitis secondary to ipilimumab is made primarily in the appropriate clinical setting, and typically onsets during the induction period (within 12 weeks of initial dosing) and most resolve within 6-8 weeks.6 Histopathologically, there is lymphoplasmacytic expansion of lamina propria, increased intraepithelial lymphocytes, and increased epithelial apoptosis of crypts. One can also see acute cryptitis and crypt abscesses. Reactive epithelial changes with mucin depletion are also often seen in epithelial cells.
Findings from immunohistochemical studies have shown the increased intraepithelial lymphocytes to be predominantly CD8-positive T cells, while the lamina propria contains an increase in the mixture of CD4- and CD8-positive T cells. In addition, small intestinal samples show villous blunting. There is an absence of significant architectural distortion and well-developed basal lymphoplasmacytic infiltrates characteristic of chronic mucosal injury, such as idiopathic inflammatory bowel disease.7 Granulomas are also absent in most series, though they have been reported in some cases.8 The features are similar to those seen in autoimmune enteropathy, but goblet and endocrine cells remain preserved. Graft-versus-host disease has similar histologic features, however, the clinical setting usually makes the distinction between these obvious.
Current treatment algorithms for ipilimumab-related diarrhea, begin with immediate treatment with intravenous methylprednisolone (125 mg once). This is followed with oral prednisone at a dose of 1-2 mg/kg tapered over 4 to 8 weeks.4 In patients with persistent symptoms despite adequate doses of corticosteroids, infliximab (5 mg/kg every 2 weeks) is recommended until the resolution of symptoms, and a longer taper of prednisone is often necessary.
Institution of high-dose corticosteroids to treat grade 3 or 4 irAEs can increase the risk for infection, including opportunistic infections. One retrospective review of patients administered checkpoint inhibitors at a single institution revealed that 7.3% of 740 patients developed a severe infection that lead to hospitalization or treatment with intravenous antibiotics.9 In that patient cohort, only 0.6% had a serious infection secondary to a viral etiology, and 1 patient developed CMV enterocolitis. Most patients who developed an infection in this cohort had received corticosteroids (46/54 patients, 85%) and/or infliximab (13/54 patients, 24%).9
CMV is a member of the Herpesviridae family. After a primary infection, which can often go unrecognized in an immunocompetent host, CMV can persist in a latent state.10 In a study by Bate and colleagues, the age-adjusted seropositivity of CMV was found to be 50.4%.11 Based on those results, immunosuppression in a patient who has previously been infected with CMV can lead to a risk of reactivation or even reinfection. In the era of checkpoint-inhibitor therapy, reactivation of CMV has been described previously in a case of CMV hepatitis and a report of CMV colitis.12,13 Immunosuppression, such as that caused by corticosteroids, is a risk factor for CMV infection.14 Colitis caused by CMV usually presents with abdominal pain, diarrhea, and bloody diarrhea.15 In suspected cases of CMV colitis, endoscopy should be pursued with biopsy for tissue examination. A tissue diagnosis is required for CMV colitis because serum PCR can be negative in isolated cases of gastrointestinal CMV infection.15
Conclusion
Despite appropriate treatment with ganciclovir and the noted response in the patient’s serum CMV PCR, symptom exacerbation was observed with the transition to oral prednisone. The requirement for intravenous corticosteroids in the present case demonstrates the prolonged effects exerted by irAEs secondary to checkpoint-inhibitor therapy. Those effects are attributable to the design of the antibody – ipilimumab is a fully humanized monoclonal antibody and has a plasma half-life of about 15 days.1,4
By the identification of CMV histopathologically, this case, along with the case presented by Lankes and colleagues,13 illustrates the importance of considering CMV colitis in patients who are being treated with ipilimumab and who develop persistent or worsening diarrhea after initial treatment with high-dose steroids.
Early recognition of possible coexistent CMV colitis in patients with a history of treatment with ipilimumab can have important clinical consequences. It can lead to quicker implementation of proper antiviral therapy and minimization of immune suppression to levels required to maintain control of the patient’s symptoms.
Immune checkpoint inhibitors, including anti-cytotoxic T-lymphocyte antigen 4 (anti-CTLA4) and anti-programmed cell death protein-1 (anti-PD-1) antibodies, have demonstrated clinical and survival benefits in a variety of malignancies, which has led to an expansion in their role in oncology. In melanoma, the anti-CTLA-4 antibody, ipilimumab, has demonstrated a survival benefit in patients with advanced metastatic melanoma and in patients with resectable disease with lymph node involvement.1,2
Ipilimumab exerts its effect by binding CTLA-4 on conventional and regulatory T cells, thus blocking inhibitory signals on T cells, which leads to an antitumor response.3 The increased immune response counteracts the immune-evading mechanisms of the tumor. With increased use of these agents, immune-related adverse events (irAEs) have become more prevalent. The most common irAEs secondary to ipilimumab are skin rash, colitis/diarrhea, hepatitis, pneumonitis, and various endocrinopathies.4 In a phase 3 trial of adjuvant ipilimumab in patients with resected stage III melanoma, grade 3 or 4 adverse events occurred in 54.1% of participants in the ipilimumab arm, the most common being diarrhea and colitis (9.8% and 6.8%, respectively).2Recognition and management of irAEs has led to the implementation of treatment guidelines.4,5 Management of irAEs includes checkpoint inhibitor discontinuation and reversal of the immune response by institution of immunosuppression with corticosteroids.
Case presentation and summary
A 40-year-old white woman with stage IIIB BRAF V600E-positive melanoma presented with diarrhea refractory to high-dose prednisone (1 mg/kg BID). She had recently undergone wide local excision and sentinel node biopsy and received her inaugural dose of ipilimumab (10 mg/kg).
The patient first presented with loose, watery stools that had begun 8 days after she had received her first dose of adjuvant ipilimumab. She was admitted to the hospital, and intravenous methylprednisolone was initiated along with empiric ciprofloxacin (400 mg, IVPB Q12h) and metronidazole (500 mg, IVPB Q8h) as infectious causes were concurrently ruled out. During this initial admission, the patient’s stool was negative for Clostridium difficile toxin, ova, and parasites, as well as enteric pathogens by culture. After infectious causes were excluded, she was diagnosed with ipilimumab-induced colitis. Antibiotics were discontinued, and the patient ultimately noted improvement in her symptoms. On hospital day 7, she was experiencing only 2 bowel movements a day and was discharged on 80 mg of prednisone twice daily.
After discharge the patient noted persistence of her symptoms. At her follow-up, 9 days after discharge, the patient noted continued symptoms of low-grade diarrhea. She failed a trial of steroid tapering due to exacerbation of her abdominal pain and frequency of diarrhea. Further investigation was negative for C. diff toxin and a computed-tomography scan was consistent with continuing colitis. The patient’s symptoms continued to worsen, with recurrence of grade 3 diarrhea, and she was ultimately readmitted 17 days after her earlier discharge (36 days after her first ipilimumab dosing).
On re-admission, the patient was again given intravenous methylprednisolone and experienced interval improvement in the frequency of diarrhea. A gastroenterology expert was consulted, and the patient underwent a flexible sigmoidoscopy that demonstrated findings of diffuse and severe inflammation and biopsies were obtained (Figure 1). After several days of continued symptoms, the patient received infliximab 5 mg/kg for treatment of her adverse autoimmune reaction. After administration, the patient noted improvement in the frequency and volume of diarrhea, however, her symptoms still persisted.
Biopsy results subsequently revealed findings compatible with ipilimumab-induced colitis, and immunohistochemical staining demonstrated positivity for cytomegalovirus (CMV). Specifically, histologic examination showed lymphoplasmacytic expansion of the lamina propria, some architectural distortion, and increased crypt apoptosis. Scattered cryptitis and crypt abscesses were also noted, as were rare stromal and endothelial cells with characteristic CMV inclusions (Figure 2 and Figure 3).
Serum CMV polymerase chain reaction (PCR) was also positive at 652,000 IU/mL (lower limit of detection 100 IU/mL). Induction dosing of ganciclovir (5 mg/kg IV Q12h) was initiated. The combined treatment with intravenous methylprednisone and ganciclovir led to an improvement in diarrhea frequency and resolution of blood in the stool. She was transitioned to oral prednisone, but it resulted in redevelopment of grade 3 diarrhea. The patient was therefore resumed on and discharged on daily intravenous methylprednisolone.
After discharge, the patient was started on budesonide 9 mg daily. Her serum CMV PCR level reduced and she was transitioned to oral valgancyclovir (900 mg daily) for maintenance. Another unsuccessful attempt was made to switch her to oral prednisone.
About 14 weeks after the initial ipilimumab dosing, the patient underwent another flexible sigmoidoscopy that again demonstrated severe colitis from the rectum to sigmoid colon. Biopsies were negative for CMV. Patient was readmitted for recurrence of diarrhea the following week. Treatment with IV methylprednisone (1mg/kg BID) and infliximab (5 mg/kg) again led to an improvement of symptoms. She was again discharged on IV methylprednisone (1 mg/kg BID) with a taper.
In the 15th week after her initial ipilimumab dose, the patient presented with a perforated bowel, requiring a subtotal colectomy and end ileostomy. She continued on a slow taper of oral prednisone (50 mg daily and decrease by 10 mg every 5 days).
At her last documented follow-up, 8 months after her first ipilimumab dose, she was having normal output from her ileostomy. She developed secondary adrenal insufficiency because of the long-term steroids and continued to take prednisone 5 mg daily.
Discussion
Diarrhea and colitis are common irAEs attributable to checkpoint-inhibitor therapy used for the treatment of melanoma. This case of ipilimumab-induced colitis refractory to high-dose oral steroids demonstrates the risks associated with management of anti-CTLA-4 induced colitis. In particular, the high-dose corticosteroids required to treat the autoimmune component of this patient’s colitis increased her susceptibility to CMV reactivation.
The diagnosis of colitis secondary to ipilimumab is made primarily in the appropriate clinical setting, and typically onsets during the induction period (within 12 weeks of initial dosing) and most resolve within 6-8 weeks.6 Histopathologically, there is lymphoplasmacytic expansion of lamina propria, increased intraepithelial lymphocytes, and increased epithelial apoptosis of crypts. One can also see acute cryptitis and crypt abscesses. Reactive epithelial changes with mucin depletion are also often seen in epithelial cells.
Findings from immunohistochemical studies have shown the increased intraepithelial lymphocytes to be predominantly CD8-positive T cells, while the lamina propria contains an increase in the mixture of CD4- and CD8-positive T cells. In addition, small intestinal samples show villous blunting. There is an absence of significant architectural distortion and well-developed basal lymphoplasmacytic infiltrates characteristic of chronic mucosal injury, such as idiopathic inflammatory bowel disease.7 Granulomas are also absent in most series, though they have been reported in some cases.8 The features are similar to those seen in autoimmune enteropathy, but goblet and endocrine cells remain preserved. Graft-versus-host disease has similar histologic features, however, the clinical setting usually makes the distinction between these obvious.
Current treatment algorithms for ipilimumab-related diarrhea, begin with immediate treatment with intravenous methylprednisolone (125 mg once). This is followed with oral prednisone at a dose of 1-2 mg/kg tapered over 4 to 8 weeks.4 In patients with persistent symptoms despite adequate doses of corticosteroids, infliximab (5 mg/kg every 2 weeks) is recommended until the resolution of symptoms, and a longer taper of prednisone is often necessary.
Institution of high-dose corticosteroids to treat grade 3 or 4 irAEs can increase the risk for infection, including opportunistic infections. One retrospective review of patients administered checkpoint inhibitors at a single institution revealed that 7.3% of 740 patients developed a severe infection that lead to hospitalization or treatment with intravenous antibiotics.9 In that patient cohort, only 0.6% had a serious infection secondary to a viral etiology, and 1 patient developed CMV enterocolitis. Most patients who developed an infection in this cohort had received corticosteroids (46/54 patients, 85%) and/or infliximab (13/54 patients, 24%).9
CMV is a member of the Herpesviridae family. After a primary infection, which can often go unrecognized in an immunocompetent host, CMV can persist in a latent state.10 In a study by Bate and colleagues, the age-adjusted seropositivity of CMV was found to be 50.4%.11 Based on those results, immunosuppression in a patient who has previously been infected with CMV can lead to a risk of reactivation or even reinfection. In the era of checkpoint-inhibitor therapy, reactivation of CMV has been described previously in a case of CMV hepatitis and a report of CMV colitis.12,13 Immunosuppression, such as that caused by corticosteroids, is a risk factor for CMV infection.14 Colitis caused by CMV usually presents with abdominal pain, diarrhea, and bloody diarrhea.15 In suspected cases of CMV colitis, endoscopy should be pursued with biopsy for tissue examination. A tissue diagnosis is required for CMV colitis because serum PCR can be negative in isolated cases of gastrointestinal CMV infection.15
Conclusion
Despite appropriate treatment with ganciclovir and the noted response in the patient’s serum CMV PCR, symptom exacerbation was observed with the transition to oral prednisone. The requirement for intravenous corticosteroids in the present case demonstrates the prolonged effects exerted by irAEs secondary to checkpoint-inhibitor therapy. Those effects are attributable to the design of the antibody – ipilimumab is a fully humanized monoclonal antibody and has a plasma half-life of about 15 days.1,4
By the identification of CMV histopathologically, this case, along with the case presented by Lankes and colleagues,13 illustrates the importance of considering CMV colitis in patients who are being treated with ipilimumab and who develop persistent or worsening diarrhea after initial treatment with high-dose steroids.
Early recognition of possible coexistent CMV colitis in patients with a history of treatment with ipilimumab can have important clinical consequences. It can lead to quicker implementation of proper antiviral therapy and minimization of immune suppression to levels required to maintain control of the patient’s symptoms.
1. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845-1855.
3. Glassman PM, Balthasar JP. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med. 2014;11(1):20-33.
4. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
5. Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18(6):733-743.
6. Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675-1682.
7. Oble DA, Mino-Kenudson M, Goldsmith J, et al. Alpha-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol. 2008;32(8):1130-1137.
8. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289.
9. Del Castillo M, Romero FA, Arguello E, Kyi C, Postow MA, Redelman-Sidi G. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis. 2016;63(11):1490-1493.
10. Pillet S, Pozzetto B, Roblin X. Cytomegalovirus and ulcerative colitis: place of antiviral therapy. World J Gastroenterol. 2016;22(6):2030-2045.
11. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439-1447.
12. Uslu U, Agaimy A, Hundorfean G, Harrer T, Schuler G, Heinzerling L. autoimmune colitis and subsequent CMV-induced hepatitis after treatment with ipilimumab. J Immunother. 2015;38(5):212-215.
13. Lankes K, Hundorfean G, Harrer T, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016;5(6):e1128611.
14. Ko JH, Peck KR, Lee WJ, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60(6):e20-26.
15. You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14(4):334-342.
1. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711-723.
2. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375(19):1845-1855.
3. Glassman PM, Balthasar JP. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol Med. 2014;11(1):20-33.
4. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-2697.
5. Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18(6):733-743.
6. Weber JS, Dummer R, de Pril V, Lebbe C, Hodi FS, Investigators MDX. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675-1682.
7. Oble DA, Mino-Kenudson M, Goldsmith J, et al. Alpha-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol. 2008;32(8):1130-1137.
8. Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283-2289.
9. Del Castillo M, Romero FA, Arguello E, Kyi C, Postow MA, Redelman-Sidi G. The spectrum of serious infections among patients receiving immune checkpoint blockade for the treatment of melanoma. Clin Infect Dis. 2016;63(11):1490-1493.
10. Pillet S, Pozzetto B, Roblin X. Cytomegalovirus and ulcerative colitis: place of antiviral therapy. World J Gastroenterol. 2016;22(6):2030-2045.
11. Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439-1447.
12. Uslu U, Agaimy A, Hundorfean G, Harrer T, Schuler G, Heinzerling L. autoimmune colitis and subsequent CMV-induced hepatitis after treatment with ipilimumab. J Immunother. 2015;38(5):212-215.
13. Lankes K, Hundorfean G, Harrer T, et al. Anti-TNF-refractory colitis after checkpoint inhibitor therapy: possible role of CMV-mediated immunopathogenesis. Oncoimmunology. 2016;5(6):e1128611.
14. Ko JH, Peck KR, Lee WJ, et al. Clinical presentation and risk factors for cytomegalovirus colitis in immunocompetent adult patients. Clin Infect Dis. 2015;60(6):e20-26.
15. You DM, Johnson MD. Cytomegalovirus infection and the gastrointestinal tract. Curr Gastroenterol Rep. 2012;14(4):334-342.
Recurrent head and neck cancer presenting as a large retroperitoneal mass
Worldwide, head and neck cancers account for more than half a million cases annually and nearly 400,000 deaths.1 Although the exact incidence of metastatic disease of these primarily squamous cell tumors is difficult to determine, the incidence is thought to be much lower than that of other solid tumors.2 When the different sites of metastatic disease of these tumors have been studied previously, the most common have been (in descending order of frequency) the lungs, bones, liver, skin, mediastinum, and bone marrow.2,3 It is extremely rare area for head and neck squamous cell cancers to metastasize to the retroperitoneum. To our knowledge, only 2 other such cases have been reported in the literature.4,5 In those two cases, the metastatic recurrence occurred at 6 and 13 months after definitive treatment of the primary cancer.
Case presentation and summary
The patient in this case is a 60-year-old man with a history of stage IV moderately differentiated invasive squamous cell carcinoma (p16 negative, Bcl-2 negative, EGFR positive) of the hypopharynx that had been initially diagnosed in 2012. At that time, he underwent a total laryngectomy, partial pharyngectomy, and total thyroidectomy. A 2-centimeter mediastinal mass was also identified on a computed-tomography scan of the thorax and resected during the initial curative surgery. Final surgical pathology on the primary hypopharygeal tumor revealed a 4.1-cm moderately differentiated squamous cell carcinoma with negative margins, but positive lymphovascular invasion (Figure 1). The 2-cm mediastinal mass also revealed the same squamous cell carcinoma as the hypopharyngeal primary. Final surgical margins were negative.
The patient went on to receive adjuvant treatment in the form of concurrent chemoradiation with cisplatin (100 mg/m2 every 21 days for 3 doses, with 70 Gy of radiation]. After his initial treatment, he was followed closely by a multidisciplinary team, including otolaryngology, radiation oncology, and medical oncology specialists. He underwent a positron-emission tomography–CT scan 1 year after the conclusion of adjuvant therapy that showed no evidence of local or distant disease. The patient underwent 12 fiberoptic pharyngoscopy procedures over the course of 4 years without any evidence of local disease recurrence. He underwent a CT scan of the neck in October of 2016 without any evidence of local disease recurrence.
In early 2017, the patient presented with fatigue, abdominal pain, and back pain during the previous month. CT imaging revealed a left retroperitoneal mass of 8.8 x 4.0 x 6.6 cm, with bony destruction of L3-L4 causing left hydronephrosis (Figure 2 and Figure 3). Other staging work-up and imaging did not reveal any other distant disease or locoregional disease recurrence in the head and neck. Lab work was significant for an acute kidney injury that was likely secondary to mass effect from the tumor.
The mass was biopsied, with pathology revealing squamous cell carcinoma consistent with metastatic, recurrent disease from the previously known head and neck primary, and it was also p16 negative, Bcl-2 negative, and EGFR positive (Figure 4).
After a multidisciplinary discussion it was determined that the best front-line treatment option would be to treat with definitive concurrent chemoradiation. However, due to the size and location of the mass, it was not possible to deliver an effective therapeutic dose of radiation without unacceptable toxicity to the adjacent structures. Therefore, palliative systemic therapy was the only option. These treatment options, including systemic chemotherapy and immunotherapy, were discussed with the patient. However, he did not want to pursue any further cancer treatment and wanted instead to focus on palliation (pain control, antiemetics and nephrostomy to relieve obstruction) and hospice. He passed away 3 months later.
Discussion
Masses of the retroperitoneum have a wide differential diagnosis.6 Primary malignancies including lymphomas, sarcomas, neurogenic tumors, and germ cell tumors may all present primarily as retroperitoneal masses.6,7 Nonmalignant processes such as retroperitoneal fibrosis may also present in this manner.7 Certain tumors are known to metastasize to the retroperitoneum, namely carcinomas of the gastrointestinal tract and ovary as well as lung cancer or melanoma.5,8 Some primary retroperitoneal masses in women have been described in the literature as being HPV-associated squamous cell cancers of unknown primaries.9
When head and neck cancers metastasize they typically metastasize to the lungs, bone, liver, mediastinum, skin, and bone marrow. Most metastasis is pulmonary in origin, with the literature indicating it accounts for 52%-66% of head and neck cancer metastases, with bone metastases next in frequency at 12%-22%.2,3,10 In general, the incidence of distant metastatic disease in head and neck cancers is not as common as its other solid tumor counterparts, and even metastasis to other lymph node groups other than locoregional cervical nodes is rare.11 Furthermore, late metastasis occurring more than 2 years after definitive treatment is also an infrequent occurrence.12
When discussing distant metastatic disease in head and neck cancer, previous literature has described an increasing likelihood of distant metastases when there is locoregional disease recurrence.13 Moreover, the retroperitoneum is an exceedingly rare site of distant metastatic disease for head and neck cancer. There have been only 2 previous cases that have described this phenomenon, and in both cases the metastases occurred within or close to 1 year of definitive locoregional treatment.4,5
Conclusion
We present our case to present an exceedingly rare case of distant metastatic, recurrent disease from head and neck cancer to the retroperitoneum (without locoregional recurrence) that occurred 4 years after definitive treatment. We believe this to be the first case of its kind to be described when taking into consideration the site of metastases, when the metastatic recurrence occurred and that it happened without loco-regional disease recurrence. This case highlights the importance of keeping a wide differential diagnosis when encountering a retroperitoneal mass in a patient with even a remote history of head and neck cancer.
Acknowledgments
The authors thank the following members of the Department of Pathology at the University of Texas Medical Branch: Asad Ahmad, MD; Eduardo Eyzaguirre, MD; Timothy C Allen, MD, JD, FACP; and Suimmin Qiu, MD, PHD.
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
2. Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202-207.
3. Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499-5502.
4. Hofmann U, O’Connor JP, Biyani CS, Harnden P, Selby P, Weston PM. Retroperitoneal metastatic squamous cell carcinoma of the tonsil (with elevated beta human chorionic gonadotrophin): a misdiagnosis as extra-gonadal germ cell tumour. J Laryngol Otol. 2006;120:885-887.
5. Purkayastha A, Sharma N, Suhag V. Extremely rare and unusual case of retroperitoneal and pelvic metastasis from squamous cell carcinoma of vallecula. Int J Cancer Ther Oncol. 2016;4(2):1-4.
6. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-976.
7. Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40:1887-1903.
8. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
9. Isbell A, Fields EC. Three cases of women with HPV-related squamous cell carcinoma of unknown primary in the pelvis and retroperitoneum: a case series. Gynecol Oncol Rep. 2016;16:5-8.
10. León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680-686.
11. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE. Distant lymphatic metastasis from head and neck cancer. Ann Otol Rhinol Laryngol. 1999;108:860-863.
12. Krishnatry R, Gupta T, Murthy V, et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J Cancer. 2014;51:231-235.
13. Goodwin WJ. Distant metastases from oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:222-223.
Worldwide, head and neck cancers account for more than half a million cases annually and nearly 400,000 deaths.1 Although the exact incidence of metastatic disease of these primarily squamous cell tumors is difficult to determine, the incidence is thought to be much lower than that of other solid tumors.2 When the different sites of metastatic disease of these tumors have been studied previously, the most common have been (in descending order of frequency) the lungs, bones, liver, skin, mediastinum, and bone marrow.2,3 It is extremely rare area for head and neck squamous cell cancers to metastasize to the retroperitoneum. To our knowledge, only 2 other such cases have been reported in the literature.4,5 In those two cases, the metastatic recurrence occurred at 6 and 13 months after definitive treatment of the primary cancer.
Case presentation and summary
The patient in this case is a 60-year-old man with a history of stage IV moderately differentiated invasive squamous cell carcinoma (p16 negative, Bcl-2 negative, EGFR positive) of the hypopharynx that had been initially diagnosed in 2012. At that time, he underwent a total laryngectomy, partial pharyngectomy, and total thyroidectomy. A 2-centimeter mediastinal mass was also identified on a computed-tomography scan of the thorax and resected during the initial curative surgery. Final surgical pathology on the primary hypopharygeal tumor revealed a 4.1-cm moderately differentiated squamous cell carcinoma with negative margins, but positive lymphovascular invasion (Figure 1). The 2-cm mediastinal mass also revealed the same squamous cell carcinoma as the hypopharyngeal primary. Final surgical margins were negative.
The patient went on to receive adjuvant treatment in the form of concurrent chemoradiation with cisplatin (100 mg/m2 every 21 days for 3 doses, with 70 Gy of radiation]. After his initial treatment, he was followed closely by a multidisciplinary team, including otolaryngology, radiation oncology, and medical oncology specialists. He underwent a positron-emission tomography–CT scan 1 year after the conclusion of adjuvant therapy that showed no evidence of local or distant disease. The patient underwent 12 fiberoptic pharyngoscopy procedures over the course of 4 years without any evidence of local disease recurrence. He underwent a CT scan of the neck in October of 2016 without any evidence of local disease recurrence.
In early 2017, the patient presented with fatigue, abdominal pain, and back pain during the previous month. CT imaging revealed a left retroperitoneal mass of 8.8 x 4.0 x 6.6 cm, with bony destruction of L3-L4 causing left hydronephrosis (Figure 2 and Figure 3). Other staging work-up and imaging did not reveal any other distant disease or locoregional disease recurrence in the head and neck. Lab work was significant for an acute kidney injury that was likely secondary to mass effect from the tumor.
The mass was biopsied, with pathology revealing squamous cell carcinoma consistent with metastatic, recurrent disease from the previously known head and neck primary, and it was also p16 negative, Bcl-2 negative, and EGFR positive (Figure 4).
After a multidisciplinary discussion it was determined that the best front-line treatment option would be to treat with definitive concurrent chemoradiation. However, due to the size and location of the mass, it was not possible to deliver an effective therapeutic dose of radiation without unacceptable toxicity to the adjacent structures. Therefore, palliative systemic therapy was the only option. These treatment options, including systemic chemotherapy and immunotherapy, were discussed with the patient. However, he did not want to pursue any further cancer treatment and wanted instead to focus on palliation (pain control, antiemetics and nephrostomy to relieve obstruction) and hospice. He passed away 3 months later.
Discussion
Masses of the retroperitoneum have a wide differential diagnosis.6 Primary malignancies including lymphomas, sarcomas, neurogenic tumors, and germ cell tumors may all present primarily as retroperitoneal masses.6,7 Nonmalignant processes such as retroperitoneal fibrosis may also present in this manner.7 Certain tumors are known to metastasize to the retroperitoneum, namely carcinomas of the gastrointestinal tract and ovary as well as lung cancer or melanoma.5,8 Some primary retroperitoneal masses in women have been described in the literature as being HPV-associated squamous cell cancers of unknown primaries.9
When head and neck cancers metastasize they typically metastasize to the lungs, bone, liver, mediastinum, skin, and bone marrow. Most metastasis is pulmonary in origin, with the literature indicating it accounts for 52%-66% of head and neck cancer metastases, with bone metastases next in frequency at 12%-22%.2,3,10 In general, the incidence of distant metastatic disease in head and neck cancers is not as common as its other solid tumor counterparts, and even metastasis to other lymph node groups other than locoregional cervical nodes is rare.11 Furthermore, late metastasis occurring more than 2 years after definitive treatment is also an infrequent occurrence.12
When discussing distant metastatic disease in head and neck cancer, previous literature has described an increasing likelihood of distant metastases when there is locoregional disease recurrence.13 Moreover, the retroperitoneum is an exceedingly rare site of distant metastatic disease for head and neck cancer. There have been only 2 previous cases that have described this phenomenon, and in both cases the metastases occurred within or close to 1 year of definitive locoregional treatment.4,5
Conclusion
We present our case to present an exceedingly rare case of distant metastatic, recurrent disease from head and neck cancer to the retroperitoneum (without locoregional recurrence) that occurred 4 years after definitive treatment. We believe this to be the first case of its kind to be described when taking into consideration the site of metastases, when the metastatic recurrence occurred and that it happened without loco-regional disease recurrence. This case highlights the importance of keeping a wide differential diagnosis when encountering a retroperitoneal mass in a patient with even a remote history of head and neck cancer.
Acknowledgments
The authors thank the following members of the Department of Pathology at the University of Texas Medical Branch: Asad Ahmad, MD; Eduardo Eyzaguirre, MD; Timothy C Allen, MD, JD, FACP; and Suimmin Qiu, MD, PHD.
Worldwide, head and neck cancers account for more than half a million cases annually and nearly 400,000 deaths.1 Although the exact incidence of metastatic disease of these primarily squamous cell tumors is difficult to determine, the incidence is thought to be much lower than that of other solid tumors.2 When the different sites of metastatic disease of these tumors have been studied previously, the most common have been (in descending order of frequency) the lungs, bones, liver, skin, mediastinum, and bone marrow.2,3 It is extremely rare area for head and neck squamous cell cancers to metastasize to the retroperitoneum. To our knowledge, only 2 other such cases have been reported in the literature.4,5 In those two cases, the metastatic recurrence occurred at 6 and 13 months after definitive treatment of the primary cancer.
Case presentation and summary
The patient in this case is a 60-year-old man with a history of stage IV moderately differentiated invasive squamous cell carcinoma (p16 negative, Bcl-2 negative, EGFR positive) of the hypopharynx that had been initially diagnosed in 2012. At that time, he underwent a total laryngectomy, partial pharyngectomy, and total thyroidectomy. A 2-centimeter mediastinal mass was also identified on a computed-tomography scan of the thorax and resected during the initial curative surgery. Final surgical pathology on the primary hypopharygeal tumor revealed a 4.1-cm moderately differentiated squamous cell carcinoma with negative margins, but positive lymphovascular invasion (Figure 1). The 2-cm mediastinal mass also revealed the same squamous cell carcinoma as the hypopharyngeal primary. Final surgical margins were negative.
The patient went on to receive adjuvant treatment in the form of concurrent chemoradiation with cisplatin (100 mg/m2 every 21 days for 3 doses, with 70 Gy of radiation]. After his initial treatment, he was followed closely by a multidisciplinary team, including otolaryngology, radiation oncology, and medical oncology specialists. He underwent a positron-emission tomography–CT scan 1 year after the conclusion of adjuvant therapy that showed no evidence of local or distant disease. The patient underwent 12 fiberoptic pharyngoscopy procedures over the course of 4 years without any evidence of local disease recurrence. He underwent a CT scan of the neck in October of 2016 without any evidence of local disease recurrence.
In early 2017, the patient presented with fatigue, abdominal pain, and back pain during the previous month. CT imaging revealed a left retroperitoneal mass of 8.8 x 4.0 x 6.6 cm, with bony destruction of L3-L4 causing left hydronephrosis (Figure 2 and Figure 3). Other staging work-up and imaging did not reveal any other distant disease or locoregional disease recurrence in the head and neck. Lab work was significant for an acute kidney injury that was likely secondary to mass effect from the tumor.
The mass was biopsied, with pathology revealing squamous cell carcinoma consistent with metastatic, recurrent disease from the previously known head and neck primary, and it was also p16 negative, Bcl-2 negative, and EGFR positive (Figure 4).
After a multidisciplinary discussion it was determined that the best front-line treatment option would be to treat with definitive concurrent chemoradiation. However, due to the size and location of the mass, it was not possible to deliver an effective therapeutic dose of radiation without unacceptable toxicity to the adjacent structures. Therefore, palliative systemic therapy was the only option. These treatment options, including systemic chemotherapy and immunotherapy, were discussed with the patient. However, he did not want to pursue any further cancer treatment and wanted instead to focus on palliation (pain control, antiemetics and nephrostomy to relieve obstruction) and hospice. He passed away 3 months later.
Discussion
Masses of the retroperitoneum have a wide differential diagnosis.6 Primary malignancies including lymphomas, sarcomas, neurogenic tumors, and germ cell tumors may all present primarily as retroperitoneal masses.6,7 Nonmalignant processes such as retroperitoneal fibrosis may also present in this manner.7 Certain tumors are known to metastasize to the retroperitoneum, namely carcinomas of the gastrointestinal tract and ovary as well as lung cancer or melanoma.5,8 Some primary retroperitoneal masses in women have been described in the literature as being HPV-associated squamous cell cancers of unknown primaries.9
When head and neck cancers metastasize they typically metastasize to the lungs, bone, liver, mediastinum, skin, and bone marrow. Most metastasis is pulmonary in origin, with the literature indicating it accounts for 52%-66% of head and neck cancer metastases, with bone metastases next in frequency at 12%-22%.2,3,10 In general, the incidence of distant metastatic disease in head and neck cancers is not as common as its other solid tumor counterparts, and even metastasis to other lymph node groups other than locoregional cervical nodes is rare.11 Furthermore, late metastasis occurring more than 2 years after definitive treatment is also an infrequent occurrence.12
When discussing distant metastatic disease in head and neck cancer, previous literature has described an increasing likelihood of distant metastases when there is locoregional disease recurrence.13 Moreover, the retroperitoneum is an exceedingly rare site of distant metastatic disease for head and neck cancer. There have been only 2 previous cases that have described this phenomenon, and in both cases the metastases occurred within or close to 1 year of definitive locoregional treatment.4,5
Conclusion
We present our case to present an exceedingly rare case of distant metastatic, recurrent disease from head and neck cancer to the retroperitoneum (without locoregional recurrence) that occurred 4 years after definitive treatment. We believe this to be the first case of its kind to be described when taking into consideration the site of metastases, when the metastatic recurrence occurred and that it happened without loco-regional disease recurrence. This case highlights the importance of keeping a wide differential diagnosis when encountering a retroperitoneal mass in a patient with even a remote history of head and neck cancer.
Acknowledgments
The authors thank the following members of the Department of Pathology at the University of Texas Medical Branch: Asad Ahmad, MD; Eduardo Eyzaguirre, MD; Timothy C Allen, MD, JD, FACP; and Suimmin Qiu, MD, PHD.
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
2. Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202-207.
3. Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499-5502.
4. Hofmann U, O’Connor JP, Biyani CS, Harnden P, Selby P, Weston PM. Retroperitoneal metastatic squamous cell carcinoma of the tonsil (with elevated beta human chorionic gonadotrophin): a misdiagnosis as extra-gonadal germ cell tumour. J Laryngol Otol. 2006;120:885-887.
5. Purkayastha A, Sharma N, Suhag V. Extremely rare and unusual case of retroperitoneal and pelvic metastasis from squamous cell carcinoma of vallecula. Int J Cancer Ther Oncol. 2016;4(2):1-4.
6. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-976.
7. Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40:1887-1903.
8. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
9. Isbell A, Fields EC. Three cases of women with HPV-related squamous cell carcinoma of unknown primary in the pelvis and retroperitoneum: a case series. Gynecol Oncol Rep. 2016;16:5-8.
10. León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680-686.
11. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE. Distant lymphatic metastasis from head and neck cancer. Ann Otol Rhinol Laryngol. 1999;108:860-863.
12. Krishnatry R, Gupta T, Murthy V, et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J Cancer. 2014;51:231-235.
13. Goodwin WJ. Distant metastases from oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:222-223.
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548.
2. Ferlito A, Shaha AR, Silver CE, Rinaldo A, Mondin V. Incidence and sites of distant metastases from head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:202-207.
3. Wiegand S, Zimmermann A, Wilhelm T, Werner JA. Survival after distant metastasis in head and neck cancer. Anticancer Res. 2015;35:5499-5502.
4. Hofmann U, O’Connor JP, Biyani CS, Harnden P, Selby P, Weston PM. Retroperitoneal metastatic squamous cell carcinoma of the tonsil (with elevated beta human chorionic gonadotrophin): a misdiagnosis as extra-gonadal germ cell tumour. J Laryngol Otol. 2006;120:885-887.
5. Purkayastha A, Sharma N, Suhag V. Extremely rare and unusual case of retroperitoneal and pelvic metastasis from squamous cell carcinoma of vallecula. Int J Cancer Ther Oncol. 2016;4(2):1-4.
6. Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-976.
7. Scali EP, Chandler TM, Heffernan EJ, Coyle J, Harris AC, Chang SD. Primary retroperitoneal masses: what is the differential diagnosis? Abdom Imaging. 2015;40:1887-1903.
8. Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009;29:347-373.
9. Isbell A, Fields EC. Three cases of women with HPV-related squamous cell carcinoma of unknown primary in the pelvis and retroperitoneum: a case series. Gynecol Oncol Rep. 2016;16:5-8.
10. León X, Quer M, Orús C, del Prado Venegas M, López M. Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck. 2000;22:680-686.
11. Alavi S, Namazie A, Sercarz JA, Wang MB, Blackwell KE. Distant lymphatic metastasis from head and neck cancer. Ann Otol Rhinol Laryngol. 1999;108:860-863.
12. Krishnatry R, Gupta T, Murthy V, et al. Factors predicting ‘time to distant metastasis’ in radically treated head and neck cancer. Indian J Cancer. 2014;51:231-235.
13. Goodwin WJ. Distant metastases from oropharyngeal cancer. ORL J Otorhinolaryngol Relat Spec. 2001;63:222-223.
VIDEO: Painful skin conditions need pain management by dermatologists
Patients with painful skin conditions need pain management that is provided by their dermatologists, Robert G. Micheletti, MD, contended in a presentation at the annual meeting of the American Academy of Dermatology.
Dermatologists are the experts when it comes to treating painful skin conditions like pyoderma gangrenosum, hidradenitis suppurativa, calciphylaxis, and vasculopathies. “We should be willing to treat the pain that goes with (these conditions), at least within our scope of practice,” said Dr. Micheletti, co-director of the Inpatient Dermatology Consult Service at the University of Pennsylvania, Philadelphia. “At the same time, we know opioids should be prescribed only when necessary, at the lowest effective dose, and for the shortest possible duration.”
In our exclusive video interview, Dr. Micheletti outlined the keys to successful care of patients with painful skin disease. He described patient characteristics that influence prescribing choices and tips for accurately assessing pain needs with a preference for a conservative regimen that utilizes non-opioids and avoids over-reliance on narcotics.
Source: Micheletti, R., Session F013
Patients with painful skin conditions need pain management that is provided by their dermatologists, Robert G. Micheletti, MD, contended in a presentation at the annual meeting of the American Academy of Dermatology.
Dermatologists are the experts when it comes to treating painful skin conditions like pyoderma gangrenosum, hidradenitis suppurativa, calciphylaxis, and vasculopathies. “We should be willing to treat the pain that goes with (these conditions), at least within our scope of practice,” said Dr. Micheletti, co-director of the Inpatient Dermatology Consult Service at the University of Pennsylvania, Philadelphia. “At the same time, we know opioids should be prescribed only when necessary, at the lowest effective dose, and for the shortest possible duration.”
In our exclusive video interview, Dr. Micheletti outlined the keys to successful care of patients with painful skin disease. He described patient characteristics that influence prescribing choices and tips for accurately assessing pain needs with a preference for a conservative regimen that utilizes non-opioids and avoids over-reliance on narcotics.
Source: Micheletti, R., Session F013
Patients with painful skin conditions need pain management that is provided by their dermatologists, Robert G. Micheletti, MD, contended in a presentation at the annual meeting of the American Academy of Dermatology.
Dermatologists are the experts when it comes to treating painful skin conditions like pyoderma gangrenosum, hidradenitis suppurativa, calciphylaxis, and vasculopathies. “We should be willing to treat the pain that goes with (these conditions), at least within our scope of practice,” said Dr. Micheletti, co-director of the Inpatient Dermatology Consult Service at the University of Pennsylvania, Philadelphia. “At the same time, we know opioids should be prescribed only when necessary, at the lowest effective dose, and for the shortest possible duration.”
In our exclusive video interview, Dr. Micheletti outlined the keys to successful care of patients with painful skin disease. He described patient characteristics that influence prescribing choices and tips for accurately assessing pain needs with a preference for a conservative regimen that utilizes non-opioids and avoids over-reliance on narcotics.
Source: Micheletti, R., Session F013
VIDEO: Delusional parasitosis? Try these real solutions
SAN DIEGO – The path to successful treatment of patients with imagined skin disorders is paved with compassion, according to John Koo, MD, a dermatologist and psychiatrist with the University of California at San Francisco.
When a patient presents with delusional parasitosis -- horror stories about imagined infestations of parasites or bugs – the key to successful treatment is a positive attitude and validation, not denial, Dr. Koo said in a presentation at the annual meeting of the American Academy of Dermatology.
"I cannot afford to go in (the exam room) with a long face," he said. "If I go in and I’m not looking happy, things can deteriorate quickly. So I make sure I go in with the biggest smile on my face like I'm meeting my favorite Hollywood star."
"When I say something like 'It's like a living hell, isn't it,' patients are really touched, he said. The patient’s response is typically 'You're the first dermatologist to understand what I'm going through.' You cannot endorse their delusion, but you can endorse their suffering."
In our video interview, Dr. Koo delved into techniques for the successful work-up and evaluation of patients with delusional parasitosis, the varying degrees of the condition, medications used for treatment, and the prospects for eventual drug-free relief.
Dr. Koo reports no relevant financial disclosures.
SAN DIEGO – The path to successful treatment of patients with imagined skin disorders is paved with compassion, according to John Koo, MD, a dermatologist and psychiatrist with the University of California at San Francisco.
When a patient presents with delusional parasitosis -- horror stories about imagined infestations of parasites or bugs – the key to successful treatment is a positive attitude and validation, not denial, Dr. Koo said in a presentation at the annual meeting of the American Academy of Dermatology.
"I cannot afford to go in (the exam room) with a long face," he said. "If I go in and I’m not looking happy, things can deteriorate quickly. So I make sure I go in with the biggest smile on my face like I'm meeting my favorite Hollywood star."
"When I say something like 'It's like a living hell, isn't it,' patients are really touched, he said. The patient’s response is typically 'You're the first dermatologist to understand what I'm going through.' You cannot endorse their delusion, but you can endorse their suffering."
In our video interview, Dr. Koo delved into techniques for the successful work-up and evaluation of patients with delusional parasitosis, the varying degrees of the condition, medications used for treatment, and the prospects for eventual drug-free relief.
Dr. Koo reports no relevant financial disclosures.
SAN DIEGO – The path to successful treatment of patients with imagined skin disorders is paved with compassion, according to John Koo, MD, a dermatologist and psychiatrist with the University of California at San Francisco.
When a patient presents with delusional parasitosis -- horror stories about imagined infestations of parasites or bugs – the key to successful treatment is a positive attitude and validation, not denial, Dr. Koo said in a presentation at the annual meeting of the American Academy of Dermatology.
"I cannot afford to go in (the exam room) with a long face," he said. "If I go in and I’m not looking happy, things can deteriorate quickly. So I make sure I go in with the biggest smile on my face like I'm meeting my favorite Hollywood star."
"When I say something like 'It's like a living hell, isn't it,' patients are really touched, he said. The patient’s response is typically 'You're the first dermatologist to understand what I'm going through.' You cannot endorse their delusion, but you can endorse their suffering."
In our video interview, Dr. Koo delved into techniques for the successful work-up and evaluation of patients with delusional parasitosis, the varying degrees of the condition, medications used for treatment, and the prospects for eventual drug-free relief.
Dr. Koo reports no relevant financial disclosures.
REPORTING FROM AAD 18
Balancing quality and cost of care with patient well-being
Welcome to the first issue of The Journal of Community and Supportive Oncology for this year. 2017 was a rollercoaster year for the oncology community, literally from day 1. January 1 saw the kick-off for participation in the MACRA [Medicare Access and CHIP Reauthorization Act] Quality Payment Program, and soon after came the growing concern and uncertainty around the future of President Barack Obama’s Affordable Health Care Act. Attempts during the year to repeal the ACA failed, but with the December passage of the tax bill came Medicare cuts and the repeal of the individual mandate, which will effectively sever crucial revenue sources for the ACA. Nevertheless, against that backdrop, there was a slew of exciting therapeutic approvals – some of them landmark, as my fellow Editor, Linda Bosserman, noted in her year-end editorial (JCSO 2017;15[6]:e283-e290). As often happens, and as noted in the editorial, such advances come with concerns about the high cost of the therapies and their related toxicities, and the combined negative impact of those on quality and cost of care and patient quality of life. (QoL).
In this issue, 2 research articles examine bone metastasis in late-stage disease and their findings underscore the aforementioned importance of care cost and quality and patient QoL. Bone metastases are a common cause of pain in patients with advanced cancer. That pain is often associated with higher rates of depression, anxiety, and fatigue, and patient QoL will diminish if the pain is not adequately treated. Although radiotherapy is effective in palliating painful bone metastases, relief may be delayed and interim analgesic management needed. Garcia and colleagues (p. e8) examined the frequency of analgesic regimen assessment and intervention during radiation oncology consultations for bone metastases and evaluated the impact on analgesic management before and after implementation of a dedicated palliative radiation oncology service. They found that pain assessment and intervention were not common in the radiation oncology setting before establishment of the service and suggest that integrating palliative care within radiation oncology could improve the quality of pain management and by extension, patient well-being.
Patients with bone metastases are also at greater risk of bone fracture, for which they often are hospitalized at great cost. Nikkel and colleagues sought to determine the primary tumors in patients hospitalized with metastatic disease and who sustained pathologic and nonpathologic fractures, and to estimate the costs and lengths of stay for those hospitalizations (p. e14). The most common primary cancers in these patients were lung, breast, prostate, kidney, and colorectal – a novel finding in this study was that there were almost 4 times as many pathologic fractures from colorectal than from thyroid carcinoma. Patients hospitalized for pathologic fracture had higher billed costs and longer length of stay. The authors emphasize the importance of identifying patients at risk for pathological fracture based on primary tumor type, age, and socio-economic group; improving surveillance; and doing timely osteoporosis screening.
Therapeutic advances and the ensuing new options and combination possibilities are the substrate for our daily engagement with our patients. On page e53, Dr David Henry, the JCSO Editor-in-Chief, talks with Dr Kenneth Anderson of Harvard Medical School about advances in multiple myeloma therapies and how numerous therapy approvals have pushed the disease closer to becoming a manageable, chronic disease. On page e47, Jane de Lartigue describes the latest developments in the therapeutic targeting of altered metabolic pathways in cancer cells.
Also in this issue are new approval updates for abemaciclib as the first CDK inhibitor for breast cancer (p. e2) and the checkpoint inhibitors avelumab and durvalumab for metastatic bladder cancer (p. e5), a brief report on whether patient navigators’ personal experience with cancer has any effect on patient experience (p. e43), a research article on physical activity and sedentary behavior in survivors of breast cancer, and Case Reports (pp. e30-e42).
Welcome to the first issue of The Journal of Community and Supportive Oncology for this year. 2017 was a rollercoaster year for the oncology community, literally from day 1. January 1 saw the kick-off for participation in the MACRA [Medicare Access and CHIP Reauthorization Act] Quality Payment Program, and soon after came the growing concern and uncertainty around the future of President Barack Obama’s Affordable Health Care Act. Attempts during the year to repeal the ACA failed, but with the December passage of the tax bill came Medicare cuts and the repeal of the individual mandate, which will effectively sever crucial revenue sources for the ACA. Nevertheless, against that backdrop, there was a slew of exciting therapeutic approvals – some of them landmark, as my fellow Editor, Linda Bosserman, noted in her year-end editorial (JCSO 2017;15[6]:e283-e290). As often happens, and as noted in the editorial, such advances come with concerns about the high cost of the therapies and their related toxicities, and the combined negative impact of those on quality and cost of care and patient quality of life. (QoL).
In this issue, 2 research articles examine bone metastasis in late-stage disease and their findings underscore the aforementioned importance of care cost and quality and patient QoL. Bone metastases are a common cause of pain in patients with advanced cancer. That pain is often associated with higher rates of depression, anxiety, and fatigue, and patient QoL will diminish if the pain is not adequately treated. Although radiotherapy is effective in palliating painful bone metastases, relief may be delayed and interim analgesic management needed. Garcia and colleagues (p. e8) examined the frequency of analgesic regimen assessment and intervention during radiation oncology consultations for bone metastases and evaluated the impact on analgesic management before and after implementation of a dedicated palliative radiation oncology service. They found that pain assessment and intervention were not common in the radiation oncology setting before establishment of the service and suggest that integrating palliative care within radiation oncology could improve the quality of pain management and by extension, patient well-being.
Patients with bone metastases are also at greater risk of bone fracture, for which they often are hospitalized at great cost. Nikkel and colleagues sought to determine the primary tumors in patients hospitalized with metastatic disease and who sustained pathologic and nonpathologic fractures, and to estimate the costs and lengths of stay for those hospitalizations (p. e14). The most common primary cancers in these patients were lung, breast, prostate, kidney, and colorectal – a novel finding in this study was that there were almost 4 times as many pathologic fractures from colorectal than from thyroid carcinoma. Patients hospitalized for pathologic fracture had higher billed costs and longer length of stay. The authors emphasize the importance of identifying patients at risk for pathological fracture based on primary tumor type, age, and socio-economic group; improving surveillance; and doing timely osteoporosis screening.
Therapeutic advances and the ensuing new options and combination possibilities are the substrate for our daily engagement with our patients. On page e53, Dr David Henry, the JCSO Editor-in-Chief, talks with Dr Kenneth Anderson of Harvard Medical School about advances in multiple myeloma therapies and how numerous therapy approvals have pushed the disease closer to becoming a manageable, chronic disease. On page e47, Jane de Lartigue describes the latest developments in the therapeutic targeting of altered metabolic pathways in cancer cells.
Also in this issue are new approval updates for abemaciclib as the first CDK inhibitor for breast cancer (p. e2) and the checkpoint inhibitors avelumab and durvalumab for metastatic bladder cancer (p. e5), a brief report on whether patient navigators’ personal experience with cancer has any effect on patient experience (p. e43), a research article on physical activity and sedentary behavior in survivors of breast cancer, and Case Reports (pp. e30-e42).
Welcome to the first issue of The Journal of Community and Supportive Oncology for this year. 2017 was a rollercoaster year for the oncology community, literally from day 1. January 1 saw the kick-off for participation in the MACRA [Medicare Access and CHIP Reauthorization Act] Quality Payment Program, and soon after came the growing concern and uncertainty around the future of President Barack Obama’s Affordable Health Care Act. Attempts during the year to repeal the ACA failed, but with the December passage of the tax bill came Medicare cuts and the repeal of the individual mandate, which will effectively sever crucial revenue sources for the ACA. Nevertheless, against that backdrop, there was a slew of exciting therapeutic approvals – some of them landmark, as my fellow Editor, Linda Bosserman, noted in her year-end editorial (JCSO 2017;15[6]:e283-e290). As often happens, and as noted in the editorial, such advances come with concerns about the high cost of the therapies and their related toxicities, and the combined negative impact of those on quality and cost of care and patient quality of life. (QoL).
In this issue, 2 research articles examine bone metastasis in late-stage disease and their findings underscore the aforementioned importance of care cost and quality and patient QoL. Bone metastases are a common cause of pain in patients with advanced cancer. That pain is often associated with higher rates of depression, anxiety, and fatigue, and patient QoL will diminish if the pain is not adequately treated. Although radiotherapy is effective in palliating painful bone metastases, relief may be delayed and interim analgesic management needed. Garcia and colleagues (p. e8) examined the frequency of analgesic regimen assessment and intervention during radiation oncology consultations for bone metastases and evaluated the impact on analgesic management before and after implementation of a dedicated palliative radiation oncology service. They found that pain assessment and intervention were not common in the radiation oncology setting before establishment of the service and suggest that integrating palliative care within radiation oncology could improve the quality of pain management and by extension, patient well-being.
Patients with bone metastases are also at greater risk of bone fracture, for which they often are hospitalized at great cost. Nikkel and colleagues sought to determine the primary tumors in patients hospitalized with metastatic disease and who sustained pathologic and nonpathologic fractures, and to estimate the costs and lengths of stay for those hospitalizations (p. e14). The most common primary cancers in these patients were lung, breast, prostate, kidney, and colorectal – a novel finding in this study was that there were almost 4 times as many pathologic fractures from colorectal than from thyroid carcinoma. Patients hospitalized for pathologic fracture had higher billed costs and longer length of stay. The authors emphasize the importance of identifying patients at risk for pathological fracture based on primary tumor type, age, and socio-economic group; improving surveillance; and doing timely osteoporosis screening.
Therapeutic advances and the ensuing new options and combination possibilities are the substrate for our daily engagement with our patients. On page e53, Dr David Henry, the JCSO Editor-in-Chief, talks with Dr Kenneth Anderson of Harvard Medical School about advances in multiple myeloma therapies and how numerous therapy approvals have pushed the disease closer to becoming a manageable, chronic disease. On page e47, Jane de Lartigue describes the latest developments in the therapeutic targeting of altered metabolic pathways in cancer cells.
Also in this issue are new approval updates for abemaciclib as the first CDK inhibitor for breast cancer (p. e2) and the checkpoint inhibitors avelumab and durvalumab for metastatic bladder cancer (p. e5), a brief report on whether patient navigators’ personal experience with cancer has any effect on patient experience (p. e43), a research article on physical activity and sedentary behavior in survivors of breast cancer, and Case Reports (pp. e30-e42).
Topical anticholinergic improved hyperhidrosis in children
SAN DIEGO – A topical anticholinergic drug, glycopyrronium tosylate, was as safe and effective for treating hyperhidrosis in children 9-16 years old as it was in adults in two phase 3 trials that included 25 treated children, raising the prospect it could become the first drug to gain Food and Drug Administration approval for treating pediatric hyperhidrosis.
“Topical glycopyrronium tosylate treatment may provide a much needed treatment option for those with primary axillary hyperhidrosis, including pediatric patients,” Adelaide A. Hebert, MD, said at the annual meeting of the American Academy of Dermatology.
The data she reported from a post hoc analysis included 25 children. 9-16 years old, who received a daily, topical application of glycopyrronium tosylate to their underarms for 4 weeks and 19 children treated with vehicle only. The children were enrolled in either of a pair of phase 3 pivotal trials that together randomized 697 patients. In November 2017, Dermira, the company developing this drug, submitted an application to the FDA for marketing approval of the agent for adults and children at least 9 years old. A statement from the company said an FDA decision is expected by mid-2018.
Getting approval from the FDA for an effective pediatric hyperhidrosis treatment would be an important advance because nothing now exists in that space, said Dr. Hebert, professor of dermatology and pediatrics and director of pediatric dermatology at the University of Texas Health Sciences Center at Houston.
Based on past FDA actions, safety data from 25 children should be adequate to support pediatric labeling, she said in an interview, though she added that confirmatory safety data from a phase 4 study in children would be a welcome future addition. Hyperhydrosis in adolescents is “underappreciated, underdiagnosed, and is very impactful,” and currently has limited treatment options that are readily available for children, especially effective options for more severe hyperhidrosis.
The pediatric data came from the phase 3, randomized, double-blind, vehicle-controlled ATMOS-1 (DRM04 in Subjects With Axillary Hyperhidrosis) trial. The trial ran at several U.S. and German centers, although only the U.S. centers enrolled pediatric patients.
The two trials enrolled patients with “intolerable or barely tolerable” primary, axillary hyperhidrosis of at least 6 months' duration. After 4 weeks, patients treated with glycopyrronium tosylate had improvements in their daily diary account of axillary sweating and in sweat production. Dr. Hebert and her associates reported overall results from the two trials at various prior dermatology meetings, and the company reported some of the results in a press release, but the results have not yet been published in a journal.
The new, pediatric analysis that Dr. Hebert reported showed that the responder rate based on a 4 point or greater improvement in daily sweat diary assessments occurred in 60% of the actively treated children and in 13% of the controls. A 50% or greater reduction in sweat production occurred in 80% of the treated children and in 55% of controls. Quality of life, measured using the Children’s Dermatology Life Quality Index improved by an average of 8 points among the treated children, compared with an average 2-point improvement among the controls. This level of improvement among the glycopyrronium-treated patients would have been enough to move patients from the moderate-effect category at baseline to a no- or small-effect category.
The treatment was generally well tolerated, with no serious adverse effects reported and with treatment effects that were primarily as expected from an anticholinergic agent, including dry mouth, pupil dilation, and blurred vision. One of the 25 treated children withdrew because of these effects, which then resolved. Blood testing showed no systemic absorption of the drug, Dr. Hebert said.
The ATMOS-1 and ATMOS-2 trials were sponsored by Dermira, the company developing glycopyrronium tosylate. Dr. Hebert has been a consultant to and has received research funding from Dermira, and some of the coauthors of the study are Dermira employees. Dr. Hebert is an advisor to the editorial board of Dermatology News.
SOURCE: Hebert A et al. Annual meeting of the American Academy of Dermatology Abstract 6659.
SAN DIEGO – A topical anticholinergic drug, glycopyrronium tosylate, was as safe and effective for treating hyperhidrosis in children 9-16 years old as it was in adults in two phase 3 trials that included 25 treated children, raising the prospect it could become the first drug to gain Food and Drug Administration approval for treating pediatric hyperhidrosis.
“Topical glycopyrronium tosylate treatment may provide a much needed treatment option for those with primary axillary hyperhidrosis, including pediatric patients,” Adelaide A. Hebert, MD, said at the annual meeting of the American Academy of Dermatology.
The data she reported from a post hoc analysis included 25 children. 9-16 years old, who received a daily, topical application of glycopyrronium tosylate to their underarms for 4 weeks and 19 children treated with vehicle only. The children were enrolled in either of a pair of phase 3 pivotal trials that together randomized 697 patients. In November 2017, Dermira, the company developing this drug, submitted an application to the FDA for marketing approval of the agent for adults and children at least 9 years old. A statement from the company said an FDA decision is expected by mid-2018.
Getting approval from the FDA for an effective pediatric hyperhidrosis treatment would be an important advance because nothing now exists in that space, said Dr. Hebert, professor of dermatology and pediatrics and director of pediatric dermatology at the University of Texas Health Sciences Center at Houston.
Based on past FDA actions, safety data from 25 children should be adequate to support pediatric labeling, she said in an interview, though she added that confirmatory safety data from a phase 4 study in children would be a welcome future addition. Hyperhydrosis in adolescents is “underappreciated, underdiagnosed, and is very impactful,” and currently has limited treatment options that are readily available for children, especially effective options for more severe hyperhidrosis.
The pediatric data came from the phase 3, randomized, double-blind, vehicle-controlled ATMOS-1 (DRM04 in Subjects With Axillary Hyperhidrosis) trial. The trial ran at several U.S. and German centers, although only the U.S. centers enrolled pediatric patients.
The two trials enrolled patients with “intolerable or barely tolerable” primary, axillary hyperhidrosis of at least 6 months' duration. After 4 weeks, patients treated with glycopyrronium tosylate had improvements in their daily diary account of axillary sweating and in sweat production. Dr. Hebert and her associates reported overall results from the two trials at various prior dermatology meetings, and the company reported some of the results in a press release, but the results have not yet been published in a journal.
The new, pediatric analysis that Dr. Hebert reported showed that the responder rate based on a 4 point or greater improvement in daily sweat diary assessments occurred in 60% of the actively treated children and in 13% of the controls. A 50% or greater reduction in sweat production occurred in 80% of the treated children and in 55% of controls. Quality of life, measured using the Children’s Dermatology Life Quality Index improved by an average of 8 points among the treated children, compared with an average 2-point improvement among the controls. This level of improvement among the glycopyrronium-treated patients would have been enough to move patients from the moderate-effect category at baseline to a no- or small-effect category.
The treatment was generally well tolerated, with no serious adverse effects reported and with treatment effects that were primarily as expected from an anticholinergic agent, including dry mouth, pupil dilation, and blurred vision. One of the 25 treated children withdrew because of these effects, which then resolved. Blood testing showed no systemic absorption of the drug, Dr. Hebert said.
The ATMOS-1 and ATMOS-2 trials were sponsored by Dermira, the company developing glycopyrronium tosylate. Dr. Hebert has been a consultant to and has received research funding from Dermira, and some of the coauthors of the study are Dermira employees. Dr. Hebert is an advisor to the editorial board of Dermatology News.
SOURCE: Hebert A et al. Annual meeting of the American Academy of Dermatology Abstract 6659.
SAN DIEGO – A topical anticholinergic drug, glycopyrronium tosylate, was as safe and effective for treating hyperhidrosis in children 9-16 years old as it was in adults in two phase 3 trials that included 25 treated children, raising the prospect it could become the first drug to gain Food and Drug Administration approval for treating pediatric hyperhidrosis.
“Topical glycopyrronium tosylate treatment may provide a much needed treatment option for those with primary axillary hyperhidrosis, including pediatric patients,” Adelaide A. Hebert, MD, said at the annual meeting of the American Academy of Dermatology.
The data she reported from a post hoc analysis included 25 children. 9-16 years old, who received a daily, topical application of glycopyrronium tosylate to their underarms for 4 weeks and 19 children treated with vehicle only. The children were enrolled in either of a pair of phase 3 pivotal trials that together randomized 697 patients. In November 2017, Dermira, the company developing this drug, submitted an application to the FDA for marketing approval of the agent for adults and children at least 9 years old. A statement from the company said an FDA decision is expected by mid-2018.
Getting approval from the FDA for an effective pediatric hyperhidrosis treatment would be an important advance because nothing now exists in that space, said Dr. Hebert, professor of dermatology and pediatrics and director of pediatric dermatology at the University of Texas Health Sciences Center at Houston.
Based on past FDA actions, safety data from 25 children should be adequate to support pediatric labeling, she said in an interview, though she added that confirmatory safety data from a phase 4 study in children would be a welcome future addition. Hyperhydrosis in adolescents is “underappreciated, underdiagnosed, and is very impactful,” and currently has limited treatment options that are readily available for children, especially effective options for more severe hyperhidrosis.
The pediatric data came from the phase 3, randomized, double-blind, vehicle-controlled ATMOS-1 (DRM04 in Subjects With Axillary Hyperhidrosis) trial. The trial ran at several U.S. and German centers, although only the U.S. centers enrolled pediatric patients.
The two trials enrolled patients with “intolerable or barely tolerable” primary, axillary hyperhidrosis of at least 6 months' duration. After 4 weeks, patients treated with glycopyrronium tosylate had improvements in their daily diary account of axillary sweating and in sweat production. Dr. Hebert and her associates reported overall results from the two trials at various prior dermatology meetings, and the company reported some of the results in a press release, but the results have not yet been published in a journal.
The new, pediatric analysis that Dr. Hebert reported showed that the responder rate based on a 4 point or greater improvement in daily sweat diary assessments occurred in 60% of the actively treated children and in 13% of the controls. A 50% or greater reduction in sweat production occurred in 80% of the treated children and in 55% of controls. Quality of life, measured using the Children’s Dermatology Life Quality Index improved by an average of 8 points among the treated children, compared with an average 2-point improvement among the controls. This level of improvement among the glycopyrronium-treated patients would have been enough to move patients from the moderate-effect category at baseline to a no- or small-effect category.
The treatment was generally well tolerated, with no serious adverse effects reported and with treatment effects that were primarily as expected from an anticholinergic agent, including dry mouth, pupil dilation, and blurred vision. One of the 25 treated children withdrew because of these effects, which then resolved. Blood testing showed no systemic absorption of the drug, Dr. Hebert said.
The ATMOS-1 and ATMOS-2 trials were sponsored by Dermira, the company developing glycopyrronium tosylate. Dr. Hebert has been a consultant to and has received research funding from Dermira, and some of the coauthors of the study are Dermira employees. Dr. Hebert is an advisor to the editorial board of Dermatology News.
SOURCE: Hebert A et al. Annual meeting of the American Academy of Dermatology Abstract 6659.
REPORTING FROM AAD 18
Key clinical point: Max. Daily, topical glycopyrronium tosylate safely controlled pediatric hyperhidrosis.
Major finding: At least a 4-point improvement in the axillary sweating daily diary score occurred in 60% of treated patients and in 13% of controls.
Study details: Post hoc analysis of data from 44 children enrolled in either of two pivotal trials, ATMOS-1 and ATMOS-2.
Disclosures: The ATMOS-1 and ATMOS-2 trials were sponsored by Dermira, the company developing glycopyrronium tosylate. Dr. Hebert has been a consultant to and has received research funding from Dermira, and some of the coauthors of the study are Dermira employees. Dr. Hebert is an adviser to the editorial board of Dermatology News.
Source: Hebert A et al. AAD 2018, Abstract 6659.