User login
A simplified risk prediction model for patients presenting with acute pulmonary embolism
Clinical question: Is there a simplified risk prediction model to identify those with low risk pulmonary embolism (PE) who can be treated as outpatients?
Background: Existing prognostic models for patients with acute PE are dependent on comorbidities, which can be challenging to use in a scoring system. Models that make use of acute clinical variables to predict morbidity or mortality may be of greater clinical utility.
Study design: Retrospective chart review with derivation and validation analysis.
Setting: Tertiary care hospital in Chennai, India.
Synopsis: The authors identified 400 patients with acute PE who met inclusion criteria. Using logistic regression and readily accessible clinical variables previously shown to be associated with acute PE mortality, the authors created the HOPPE prediction score: heart rate, PaO2, systolic blood pressure, diastolic blood pressure, and ECG score. Each variable was classified into three groups and assigned a point value that could be summed to a cumulative 30-day mortality risk score. In the derivation and validation cohorts, the low, intermediate, and high HOPPE scores were associated with a 30-day mortality of 0%, 7.5-8.5%, and 18.2-18.8%, respectively, with similar trends for secondary outcomes including right ventricular dysfunction, nonfatal cardiogenic shock, and cardiorespiratory arrest.
In comparison with the previously validated PESI score, the HOPPE score had significantly higher sensitivity, specificity, and discriminative power. The conclusions from this study were limited by its single institutional design.
Bottom line: The HOPPE score provides a risk assessment tool to identify those patients with acute PE who are at lowest and highest risk for morbidity and mortality.
Citation: Subramanian M et al. Derivation and validation of a novel prediction model to identify low-risk patients with acute pulmonary embolism. Am J Cardiol. 2017;120(4):676-81.
Dr. Pizza is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Is there a simplified risk prediction model to identify those with low risk pulmonary embolism (PE) who can be treated as outpatients?
Background: Existing prognostic models for patients with acute PE are dependent on comorbidities, which can be challenging to use in a scoring system. Models that make use of acute clinical variables to predict morbidity or mortality may be of greater clinical utility.
Study design: Retrospective chart review with derivation and validation analysis.
Setting: Tertiary care hospital in Chennai, India.
Synopsis: The authors identified 400 patients with acute PE who met inclusion criteria. Using logistic regression and readily accessible clinical variables previously shown to be associated with acute PE mortality, the authors created the HOPPE prediction score: heart rate, PaO2, systolic blood pressure, diastolic blood pressure, and ECG score. Each variable was classified into three groups and assigned a point value that could be summed to a cumulative 30-day mortality risk score. In the derivation and validation cohorts, the low, intermediate, and high HOPPE scores were associated with a 30-day mortality of 0%, 7.5-8.5%, and 18.2-18.8%, respectively, with similar trends for secondary outcomes including right ventricular dysfunction, nonfatal cardiogenic shock, and cardiorespiratory arrest.
In comparison with the previously validated PESI score, the HOPPE score had significantly higher sensitivity, specificity, and discriminative power. The conclusions from this study were limited by its single institutional design.
Bottom line: The HOPPE score provides a risk assessment tool to identify those patients with acute PE who are at lowest and highest risk for morbidity and mortality.
Citation: Subramanian M et al. Derivation and validation of a novel prediction model to identify low-risk patients with acute pulmonary embolism. Am J Cardiol. 2017;120(4):676-81.
Dr. Pizza is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Is there a simplified risk prediction model to identify those with low risk pulmonary embolism (PE) who can be treated as outpatients?
Background: Existing prognostic models for patients with acute PE are dependent on comorbidities, which can be challenging to use in a scoring system. Models that make use of acute clinical variables to predict morbidity or mortality may be of greater clinical utility.
Study design: Retrospective chart review with derivation and validation analysis.
Setting: Tertiary care hospital in Chennai, India.
Synopsis: The authors identified 400 patients with acute PE who met inclusion criteria. Using logistic regression and readily accessible clinical variables previously shown to be associated with acute PE mortality, the authors created the HOPPE prediction score: heart rate, PaO2, systolic blood pressure, diastolic blood pressure, and ECG score. Each variable was classified into three groups and assigned a point value that could be summed to a cumulative 30-day mortality risk score. In the derivation and validation cohorts, the low, intermediate, and high HOPPE scores were associated with a 30-day mortality of 0%, 7.5-8.5%, and 18.2-18.8%, respectively, with similar trends for secondary outcomes including right ventricular dysfunction, nonfatal cardiogenic shock, and cardiorespiratory arrest.
In comparison with the previously validated PESI score, the HOPPE score had significantly higher sensitivity, specificity, and discriminative power. The conclusions from this study were limited by its single institutional design.
Bottom line: The HOPPE score provides a risk assessment tool to identify those patients with acute PE who are at lowest and highest risk for morbidity and mortality.
Citation: Subramanian M et al. Derivation and validation of a novel prediction model to identify low-risk patients with acute pulmonary embolism. Am J Cardiol. 2017;120(4):676-81.
Dr. Pizza is a hospitalist, Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
RA associated with higher risk of psychiatric disorders
The incidence and prevalence of anxiety disorder, depression, and bipolar disorder are higher among patients with rheumatoid arthritis than individuals from the general population, according to findings from a Canadian retrospective matched cohort study.
In the study of 10,206 rheumatoid arthritis (RA) patients and 50,960 individuals matched from the general population of Manitoba between 1989 and 2012, depression incidence was higher in the RA group, compared with the matched group, when adjusted for factors including age, sex, year, region of residence, and socioeconomic status (incidence rate ratio = 1.46; 95% confidence interval, 1.35-1.58). Incidence of anxiety disorder (IRR = 1.24; 95% CI, 1.15-1.34) and bipolar disorder (IRR = 1.21; 95% CI, 1.00-1.47) were also higher in the RA group. The incidence of schizophrenia did not differ between groups (IRR = 0.96; 95% CI, 0.61-1.50), Ruth Ann Marrie, MD, PhD, of the University of Manitoba, Winnipeg, and her coauthors reported in Arthritis Care & Research.
To estimate psychiatric disorder incidence after RA diagnosis (or the index date in the matched population), the first claim had to occur after the index date, and had to be preceded by a 5-year period with no claims for that psychiatric disorder. To estimate lifetime prevalence, once a patient met the case definition for a disorder, he or she was considered affected in all subsequent years if alive and a Manitoba resident. To account for varying periods of remission, however, annual period prevalence was defined as a patient having one or more hospital claims or two or more physician claims for the disorder in that year, Dr. Marrie and her colleagues wrote.
The adjusted lifetime prevalence was also higher in the RA group for both depression (PR = 1.35; 95% CI, 1.26-1.45) and anxiety disorder (PR = 1.20; 95% CI, 1.13-1.27), as was the annual period prevalence of depression (PR = 1.36; 95% CI, 1.26-1.47) and anxiety disorder (PR = 1.30; 95% CI, 1.19-1.41). Neither lifetime prevalence of bipolar disorder (PR = 1.13; 95% CI, 0.95-1.36) and schizophrenia (PR = 1.02; 95% CI, 0.72-1.43) nor annual period prevalence of bipolar disorder (PR = 1.06; 95% CI, 0.86-1.31) and schizophrenia (PR = 0.68; 95% CI, 0.40-1.15) differed between the RA and matched cohorts, the authors reported.
Female sex was associated with risk of psychiatric disease, as was lower socioeconomic status and living in an urban area, the authors reported.
Although the study had strengths including a large study population and use of population-based data, it did not evaluate psychiatric multimorbidity, a “common and clinically relevant issue which may affect outcomes,” Dr. Marrie and her coauthors said in the report. Additionally, the use of administrative data makes it difficult to account for care provided by nonphysician providers, such as psychologists, and for conditions that cause symptoms but do not meet diagnostic criteria, the authors noted.
“Future studies should explore these issues in population-based clinical cohorts which comprehensively evaluate multiple psychiatric disorders,” they concluded.
The study was funded by the Canadian Institutes of Health Research and the Waugh Family Chair in Multiple Sclerosis. Dr. Marrie has conducted clinical trials for Sanofi Aventis. Two other authors disclosed financial ties to pharmaceutical companies.
SOURCE: Marrie R et al. Arthritis Care Res. 2018 Feb 13. doi: 10.1002/acr.23539.
The incidence and prevalence of anxiety disorder, depression, and bipolar disorder are higher among patients with rheumatoid arthritis than individuals from the general population, according to findings from a Canadian retrospective matched cohort study.
In the study of 10,206 rheumatoid arthritis (RA) patients and 50,960 individuals matched from the general population of Manitoba between 1989 and 2012, depression incidence was higher in the RA group, compared with the matched group, when adjusted for factors including age, sex, year, region of residence, and socioeconomic status (incidence rate ratio = 1.46; 95% confidence interval, 1.35-1.58). Incidence of anxiety disorder (IRR = 1.24; 95% CI, 1.15-1.34) and bipolar disorder (IRR = 1.21; 95% CI, 1.00-1.47) were also higher in the RA group. The incidence of schizophrenia did not differ between groups (IRR = 0.96; 95% CI, 0.61-1.50), Ruth Ann Marrie, MD, PhD, of the University of Manitoba, Winnipeg, and her coauthors reported in Arthritis Care & Research.
To estimate psychiatric disorder incidence after RA diagnosis (or the index date in the matched population), the first claim had to occur after the index date, and had to be preceded by a 5-year period with no claims for that psychiatric disorder. To estimate lifetime prevalence, once a patient met the case definition for a disorder, he or she was considered affected in all subsequent years if alive and a Manitoba resident. To account for varying periods of remission, however, annual period prevalence was defined as a patient having one or more hospital claims or two or more physician claims for the disorder in that year, Dr. Marrie and her colleagues wrote.
The adjusted lifetime prevalence was also higher in the RA group for both depression (PR = 1.35; 95% CI, 1.26-1.45) and anxiety disorder (PR = 1.20; 95% CI, 1.13-1.27), as was the annual period prevalence of depression (PR = 1.36; 95% CI, 1.26-1.47) and anxiety disorder (PR = 1.30; 95% CI, 1.19-1.41). Neither lifetime prevalence of bipolar disorder (PR = 1.13; 95% CI, 0.95-1.36) and schizophrenia (PR = 1.02; 95% CI, 0.72-1.43) nor annual period prevalence of bipolar disorder (PR = 1.06; 95% CI, 0.86-1.31) and schizophrenia (PR = 0.68; 95% CI, 0.40-1.15) differed between the RA and matched cohorts, the authors reported.
Female sex was associated with risk of psychiatric disease, as was lower socioeconomic status and living in an urban area, the authors reported.
Although the study had strengths including a large study population and use of population-based data, it did not evaluate psychiatric multimorbidity, a “common and clinically relevant issue which may affect outcomes,” Dr. Marrie and her coauthors said in the report. Additionally, the use of administrative data makes it difficult to account for care provided by nonphysician providers, such as psychologists, and for conditions that cause symptoms but do not meet diagnostic criteria, the authors noted.
“Future studies should explore these issues in population-based clinical cohorts which comprehensively evaluate multiple psychiatric disorders,” they concluded.
The study was funded by the Canadian Institutes of Health Research and the Waugh Family Chair in Multiple Sclerosis. Dr. Marrie has conducted clinical trials for Sanofi Aventis. Two other authors disclosed financial ties to pharmaceutical companies.
SOURCE: Marrie R et al. Arthritis Care Res. 2018 Feb 13. doi: 10.1002/acr.23539.
The incidence and prevalence of anxiety disorder, depression, and bipolar disorder are higher among patients with rheumatoid arthritis than individuals from the general population, according to findings from a Canadian retrospective matched cohort study.
In the study of 10,206 rheumatoid arthritis (RA) patients and 50,960 individuals matched from the general population of Manitoba between 1989 and 2012, depression incidence was higher in the RA group, compared with the matched group, when adjusted for factors including age, sex, year, region of residence, and socioeconomic status (incidence rate ratio = 1.46; 95% confidence interval, 1.35-1.58). Incidence of anxiety disorder (IRR = 1.24; 95% CI, 1.15-1.34) and bipolar disorder (IRR = 1.21; 95% CI, 1.00-1.47) were also higher in the RA group. The incidence of schizophrenia did not differ between groups (IRR = 0.96; 95% CI, 0.61-1.50), Ruth Ann Marrie, MD, PhD, of the University of Manitoba, Winnipeg, and her coauthors reported in Arthritis Care & Research.
To estimate psychiatric disorder incidence after RA diagnosis (or the index date in the matched population), the first claim had to occur after the index date, and had to be preceded by a 5-year period with no claims for that psychiatric disorder. To estimate lifetime prevalence, once a patient met the case definition for a disorder, he or she was considered affected in all subsequent years if alive and a Manitoba resident. To account for varying periods of remission, however, annual period prevalence was defined as a patient having one or more hospital claims or two or more physician claims for the disorder in that year, Dr. Marrie and her colleagues wrote.
The adjusted lifetime prevalence was also higher in the RA group for both depression (PR = 1.35; 95% CI, 1.26-1.45) and anxiety disorder (PR = 1.20; 95% CI, 1.13-1.27), as was the annual period prevalence of depression (PR = 1.36; 95% CI, 1.26-1.47) and anxiety disorder (PR = 1.30; 95% CI, 1.19-1.41). Neither lifetime prevalence of bipolar disorder (PR = 1.13; 95% CI, 0.95-1.36) and schizophrenia (PR = 1.02; 95% CI, 0.72-1.43) nor annual period prevalence of bipolar disorder (PR = 1.06; 95% CI, 0.86-1.31) and schizophrenia (PR = 0.68; 95% CI, 0.40-1.15) differed between the RA and matched cohorts, the authors reported.
Female sex was associated with risk of psychiatric disease, as was lower socioeconomic status and living in an urban area, the authors reported.
Although the study had strengths including a large study population and use of population-based data, it did not evaluate psychiatric multimorbidity, a “common and clinically relevant issue which may affect outcomes,” Dr. Marrie and her coauthors said in the report. Additionally, the use of administrative data makes it difficult to account for care provided by nonphysician providers, such as psychologists, and for conditions that cause symptoms but do not meet diagnostic criteria, the authors noted.
“Future studies should explore these issues in population-based clinical cohorts which comprehensively evaluate multiple psychiatric disorders,” they concluded.
The study was funded by the Canadian Institutes of Health Research and the Waugh Family Chair in Multiple Sclerosis. Dr. Marrie has conducted clinical trials for Sanofi Aventis. Two other authors disclosed financial ties to pharmaceutical companies.
SOURCE: Marrie R et al. Arthritis Care Res. 2018 Feb 13. doi: 10.1002/acr.23539.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Incidence of depression (IRR = 1.46; 95% CI, 1.35-1.58), anxiety disorder (IRR = 1.24; 95% CI, 1.15-1.34), and bipolar disorder (IRR = 1.21; 95% CI, 1.00-1.47) were higher in the RA group than in the matched group.
Data source: A retrospective matched cohort study of 10,206 RA patients and 50,960 matched individuals from the general population between 1989 and 2012.
Disclosures: The study was funded by the Canadian Institutes of Health Research and the Waugh Family Chair in Multiple Sclerosis. Dr. Marrie has conducted clinical trials for Sanofi Aventis. Two other authors disclosed financial ties to pharmaceutical companies.
Source: Marrie R et al. Arthritis Care Res. 2018 Feb 13. doi: 10.1002/acr.23539
Inflammatory markers predict vaccine response in HCV, HIV
In patients with chronic hepatitis C (HCV) and HIV infection, blood protein markers showing evidence of systemic inflammation were associated with a poor immune response to hepatitis A/hepatitis B vaccination, according to a study of blood samples obtained in two small clinical trials.
Prevaccination plasma levels of inflammatory proteins IP10, IL-6, and sCD14 were elevated in both HCV- and HIV-infected patients, while sCD163 was also elevated in HCV-infected patients, according to the report in Vaccine.
Fifteen HCV-infected, 24 HIV-infected, and 10 uninfected control patients followed an appropriate vaccination course for a combined hepatitis A–hepatitis B vaccine. Antibody levels against the challenging vaccine proteins were assessed and quantified by ELISA, according to Carey L. Shive, PhD, of Louis Stokes Cleveland VA Medical Center, and her colleagues.
After HAV/HBV vaccination, HCV- and HIV-infected patients had lower and less durable HAV and HBV antibody responses than those of uninfected control patients. This was inversely correlated with the level of the inflammatory proteins seen in HCV-infected patients. The level of the HAV/HBV antibody response was too low in the HIV-infected patients to assess correlations with the inflammatory protein levels.
The researchers speculated that the elevated blood inflammatory markers indicated similar elevation in lymph node tissues, where high levels of the proteins may effect the survival and function of T follicular helper cells that may influence the generation of B cell antibody response and B cell memory activation to vaccination.
“Understanding mechanisms underlying immune impairment during chronic viral infection is needed to guide strategies to improve immune health during these morbid infections,” the researchers concluded.
The authors reported having no conflicts. The study was funded by U.S. government grants.
Source: Shive, CL et al. Vaccine 2018;38:453-60.
In patients with chronic hepatitis C (HCV) and HIV infection, blood protein markers showing evidence of systemic inflammation were associated with a poor immune response to hepatitis A/hepatitis B vaccination, according to a study of blood samples obtained in two small clinical trials.
Prevaccination plasma levels of inflammatory proteins IP10, IL-6, and sCD14 were elevated in both HCV- and HIV-infected patients, while sCD163 was also elevated in HCV-infected patients, according to the report in Vaccine.
Fifteen HCV-infected, 24 HIV-infected, and 10 uninfected control patients followed an appropriate vaccination course for a combined hepatitis A–hepatitis B vaccine. Antibody levels against the challenging vaccine proteins were assessed and quantified by ELISA, according to Carey L. Shive, PhD, of Louis Stokes Cleveland VA Medical Center, and her colleagues.
After HAV/HBV vaccination, HCV- and HIV-infected patients had lower and less durable HAV and HBV antibody responses than those of uninfected control patients. This was inversely correlated with the level of the inflammatory proteins seen in HCV-infected patients. The level of the HAV/HBV antibody response was too low in the HIV-infected patients to assess correlations with the inflammatory protein levels.
The researchers speculated that the elevated blood inflammatory markers indicated similar elevation in lymph node tissues, where high levels of the proteins may effect the survival and function of T follicular helper cells that may influence the generation of B cell antibody response and B cell memory activation to vaccination.
“Understanding mechanisms underlying immune impairment during chronic viral infection is needed to guide strategies to improve immune health during these morbid infections,” the researchers concluded.
The authors reported having no conflicts. The study was funded by U.S. government grants.
Source: Shive, CL et al. Vaccine 2018;38:453-60.
In patients with chronic hepatitis C (HCV) and HIV infection, blood protein markers showing evidence of systemic inflammation were associated with a poor immune response to hepatitis A/hepatitis B vaccination, according to a study of blood samples obtained in two small clinical trials.
Prevaccination plasma levels of inflammatory proteins IP10, IL-6, and sCD14 were elevated in both HCV- and HIV-infected patients, while sCD163 was also elevated in HCV-infected patients, according to the report in Vaccine.
Fifteen HCV-infected, 24 HIV-infected, and 10 uninfected control patients followed an appropriate vaccination course for a combined hepatitis A–hepatitis B vaccine. Antibody levels against the challenging vaccine proteins were assessed and quantified by ELISA, according to Carey L. Shive, PhD, of Louis Stokes Cleveland VA Medical Center, and her colleagues.
After HAV/HBV vaccination, HCV- and HIV-infected patients had lower and less durable HAV and HBV antibody responses than those of uninfected control patients. This was inversely correlated with the level of the inflammatory proteins seen in HCV-infected patients. The level of the HAV/HBV antibody response was too low in the HIV-infected patients to assess correlations with the inflammatory protein levels.
The researchers speculated that the elevated blood inflammatory markers indicated similar elevation in lymph node tissues, where high levels of the proteins may effect the survival and function of T follicular helper cells that may influence the generation of B cell antibody response and B cell memory activation to vaccination.
“Understanding mechanisms underlying immune impairment during chronic viral infection is needed to guide strategies to improve immune health during these morbid infections,” the researchers concluded.
The authors reported having no conflicts. The study was funded by U.S. government grants.
Source: Shive, CL et al. Vaccine 2018;38:453-60.
FROM VACCINE
Key clinical point:
Major finding: HCV/HIV-associated inflammatory markers reflect immune dysfunction and poor performance of subsequent vaccinations.
Study details: Clinical trials comparing 15 HCV-infected, 24 HIV-infected, and 10 uninfected control patients.
Disclosures: The authors reported having no conflicts. The study was funded by U.S. government grants.
Source: Shive, CL et al. Vaccine 2018;38:453-60.
Telling her she has cancer: A patient-centered approach to breaking bad news
Hearing a diagnosis of cancer is one of the most significant moments of a patient’s life and informing a patient of her diagnosis is an emotionally and technically challenging task for an obstetrician gynecologist who is frequently on the front line of making this diagnosis. In this column, we will explore some patient-centered strategies to perform this difficult task well so that patients come away informed but with the highest chance for positive emotional adjustment.
Fewer than 10% of physicians report receiving formal training in techniques of breaking bad news. For the majority of clinicians concerns are centered on being honest and not taking away hope, and in responding to a patient’s emotions.1 The SPIKES approach was developed to arm physicians with strategies to discuss a cancer diagnosis with their patients. This approach includes six key elements to incorporate during the encounter. These strategies are not meant to be formulaic but rather consistent principles that can be adjusted for individual patient needs.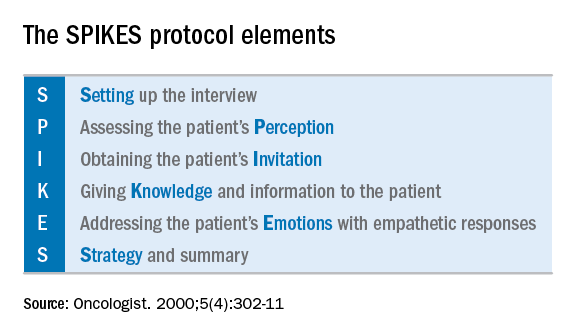
Setting up the discussion
Breaking bad news should not be a one-size-fits-all approach. Age, educational level, culture, religion, race and ethnicity, and socioeconomic opportunities each affects what and how patients may want to have this kind of information communicated to them. So how do you know how best to deliver a patient-centered approach for your patient? I recommend this simple strategy: Ask her. When ordering a test or performing a biopsy, let the patient know then why you are ordering the test and inform her of the possibility that the results may show cancer. Ask her how she would like for you to communicate that result. Would she like to be called by phone, the benefit of which is quick dissemination of information? Or would she like to receive the information face to face in the office? Research supports that most patients prefer to learn the result in the office.2 If so, I recommend scheduling a follow-up appointment in advance to prevent delays. Ask her if she would like a family member or a supportive friend to be present for the conveying of results so that she will have time to make these arrangements. Ask her if she would prefer for an alternate person to be provided with the results on her behalf.
When preparing to speak with the patient, it is valuable to mentally rehearse the words that you’ll use. Arrange for privacy and manage time constraints and interruptions (silence pagers and phones, ensure there is adequate time allocated in the schedule). Sit down to deliver the news and make a connection with eye contact and, if appropriate, touch.
Assessing the patient’s perception. Before you tell, ask. For example, “what is your understanding about why we did the biopsy?” This will guide you in where her head and heart are and can ensure you meet her wherever she is.
Obtaining the patient’s invitation. Ask the patient what she would like to be told and how much information. What would she like you to focus on? What does she not want to hear?
Giving knowledge and information to the patient. Especially now, it is important to avoid jargon and use nontechnical terms. However, do not shy away from using specific words like “cancer” by substituting them for more vague and confusing terms such as “malignancy” or “tumor.” It is important to find the balance between expressing information without being overly emotive, while avoiding excessive bluntness. Word choice is critical. Communication styles in the breaking of bad news can be separated broadly into three styles: disease centered, emotion centered, and patient-centered.3 The patient-centered approach is achieved by balancing emotional connection, information sharing, nondominance, and conveying hope. (For example, “I have some disappointing news to share. Shall we talk about the next steps in treatment? I understand this is that this is difficult for you.”) In general, this approach is most valued by patients and is associated with better information recall.
Addressing the patient’s emotions with empathetic responses. It is important that physicians take a moment to pause after communicating the test result. Even if prepared, most patients will still have a moment of shock, and their minds will likely spin through a multitude of thoughts preventing them from being able to “hear” and focus on the subsequent information. This is a moment to reflect on her reactions, her body language, and nonverbal communications to guide you on how to approach the rest of the encounter. Offer her your comfort and condolence in whichever way feels appropriate for you and her.
Beware of your own inclinations to “soften the blow.” It is a natural, compassionate instinct to follow-up giving a bad piece of information by balancing a good piece of information. For example, after just telling a woman that she has endometrial cancer, following with a statement such as “but it’s just stage 1 and is curable with surgery.” While this certainly may have immediate comforting effects, it has a couple of unintended consequences. First, it can result in difficulties later adjusting to a change in diagnosis when more information comes in (for example, upstaging after surgery or imaging). It is better to be honest and tell patients only what you know for sure in these immediate first moments of diagnosis when complete information is lacking. A more general statement such as “for most women, this is found at an early stage and is highly treatable” may be more appropriate and still provide some comfort. Second, attempts to soften the blow with a qualifying statement of positivity, such as “this is a good kind of cancer to have” might be interpreted by some patients as failing to acknowledge their devastation. She may feel that you are minimizing her condition and not allowing her to grieve or be distressed.
Strategy and summary. Patients who leave the encounter with some kind of plan for the future feel less distressed and anxious. The direction at this point of the encounter should be led by the patient. What are her greatest concerns (such as mortality, loss of fertility, time off work for treatment), and what does she want to know right now? Most patients express a desire to know more about treatment or prognosis.2,4 Unfortunately, it often is not possible to furnish this yet, particularly if this falls into the realm of a subspecialist, and prognostication typically requires more information than a provider has at initial diagnosis. However, leaving these questions unanswered is likely to result in a patient feeling helpless. For example, if an ob.gyn. discovers an apparent advanced ovarian cancer on a CT scan, tell her that, despite its apparent advanced case, it is usually treatable and that a gynecologic oncologist will discuss those best treatment options with her. Assure her that you will expeditiously refer her to a specialist who will provide her with those specifics.
The aftermath
That interval between initial diagnosis and specialist consultation is extraordinarily difficult and a high anxiety time. It is not unreasonable, in such cases, to recommend the patient to reputable online information sources, such as the Society of Gynecologic Oncology or American Cancer Society websites so that she and her family can do some research prior to that visit in order to prepare them better and give them a sense of understanding in their disease.
It is a particularly compassionate touch to reach out to the patient in the days following her cancer diagnosis, even if she has moved on to a specialist. Patients often tell me that they felt enormous reassurance and appreciation when their ob.gyn. reached out to them to “check on how they are doing.” This can usually reasonably be done by phone. This second contact serves another critical purpose: it allows for repetition of the diagnosis and initial plan, and the ability to fill in the blanks of what the patient may have missed during the prior visit, if her mind was, naturally, elsewhere. It also, quite simply, shows that you care.
Ultimately, none of us can break bad news perfectly every time. We all need to be insightful with each of these encounters as to what we did well, what we did not, and how we can adjust in the future. With respect to the SPIKES approach, patients report that physicians struggle most with the “perception,” “invitation,” and “strategy and summary” components.5 Our objective should be keeping the patient’s needs in mind, rather than our own, to maximize the chance of doing a good job. If this task is done well, not only are patients more likely to have positive emotional adjustments to their diagnosis but also more adherence with future therapies.4 In the end, it is the patient who has the final say on whether it was done well or not.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reports no relevant financial disclosures.
References
1. Baile WF et al. Oncologist. 2000;5(4):302-11.
2. Girgis A et al. Behav Med. 1999 Summer;25(2):69-77.
3. Schmid MM et al. Patient Educ Couns. 2005 Sep;58(3):244-51.
4. Girgis A et al. J Clin Oncol. 1995 Sep;13(9):2449-56.
5. Marscholiek P et al. J Cancer Educ. 2018 Feb 5. doi: 10.1007/s13187-017-1315-3.
Hearing a diagnosis of cancer is one of the most significant moments of a patient’s life and informing a patient of her diagnosis is an emotionally and technically challenging task for an obstetrician gynecologist who is frequently on the front line of making this diagnosis. In this column, we will explore some patient-centered strategies to perform this difficult task well so that patients come away informed but with the highest chance for positive emotional adjustment.
Fewer than 10% of physicians report receiving formal training in techniques of breaking bad news. For the majority of clinicians concerns are centered on being honest and not taking away hope, and in responding to a patient’s emotions.1 The SPIKES approach was developed to arm physicians with strategies to discuss a cancer diagnosis with their patients. This approach includes six key elements to incorporate during the encounter. These strategies are not meant to be formulaic but rather consistent principles that can be adjusted for individual patient needs.
Setting up the discussion
Breaking bad news should not be a one-size-fits-all approach. Age, educational level, culture, religion, race and ethnicity, and socioeconomic opportunities each affects what and how patients may want to have this kind of information communicated to them. So how do you know how best to deliver a patient-centered approach for your patient? I recommend this simple strategy: Ask her. When ordering a test or performing a biopsy, let the patient know then why you are ordering the test and inform her of the possibility that the results may show cancer. Ask her how she would like for you to communicate that result. Would she like to be called by phone, the benefit of which is quick dissemination of information? Or would she like to receive the information face to face in the office? Research supports that most patients prefer to learn the result in the office.2 If so, I recommend scheduling a follow-up appointment in advance to prevent delays. Ask her if she would like a family member or a supportive friend to be present for the conveying of results so that she will have time to make these arrangements. Ask her if she would prefer for an alternate person to be provided with the results on her behalf.
When preparing to speak with the patient, it is valuable to mentally rehearse the words that you’ll use. Arrange for privacy and manage time constraints and interruptions (silence pagers and phones, ensure there is adequate time allocated in the schedule). Sit down to deliver the news and make a connection with eye contact and, if appropriate, touch.
Assessing the patient’s perception. Before you tell, ask. For example, “what is your understanding about why we did the biopsy?” This will guide you in where her head and heart are and can ensure you meet her wherever she is.
Obtaining the patient’s invitation. Ask the patient what she would like to be told and how much information. What would she like you to focus on? What does she not want to hear?
Giving knowledge and information to the patient. Especially now, it is important to avoid jargon and use nontechnical terms. However, do not shy away from using specific words like “cancer” by substituting them for more vague and confusing terms such as “malignancy” or “tumor.” It is important to find the balance between expressing information without being overly emotive, while avoiding excessive bluntness. Word choice is critical. Communication styles in the breaking of bad news can be separated broadly into three styles: disease centered, emotion centered, and patient-centered.3 The patient-centered approach is achieved by balancing emotional connection, information sharing, nondominance, and conveying hope. (For example, “I have some disappointing news to share. Shall we talk about the next steps in treatment? I understand this is that this is difficult for you.”) In general, this approach is most valued by patients and is associated with better information recall.
Addressing the patient’s emotions with empathetic responses. It is important that physicians take a moment to pause after communicating the test result. Even if prepared, most patients will still have a moment of shock, and their minds will likely spin through a multitude of thoughts preventing them from being able to “hear” and focus on the subsequent information. This is a moment to reflect on her reactions, her body language, and nonverbal communications to guide you on how to approach the rest of the encounter. Offer her your comfort and condolence in whichever way feels appropriate for you and her.
Beware of your own inclinations to “soften the blow.” It is a natural, compassionate instinct to follow-up giving a bad piece of information by balancing a good piece of information. For example, after just telling a woman that she has endometrial cancer, following with a statement such as “but it’s just stage 1 and is curable with surgery.” While this certainly may have immediate comforting effects, it has a couple of unintended consequences. First, it can result in difficulties later adjusting to a change in diagnosis when more information comes in (for example, upstaging after surgery or imaging). It is better to be honest and tell patients only what you know for sure in these immediate first moments of diagnosis when complete information is lacking. A more general statement such as “for most women, this is found at an early stage and is highly treatable” may be more appropriate and still provide some comfort. Second, attempts to soften the blow with a qualifying statement of positivity, such as “this is a good kind of cancer to have” might be interpreted by some patients as failing to acknowledge their devastation. She may feel that you are minimizing her condition and not allowing her to grieve or be distressed.
Strategy and summary. Patients who leave the encounter with some kind of plan for the future feel less distressed and anxious. The direction at this point of the encounter should be led by the patient. What are her greatest concerns (such as mortality, loss of fertility, time off work for treatment), and what does she want to know right now? Most patients express a desire to know more about treatment or prognosis.2,4 Unfortunately, it often is not possible to furnish this yet, particularly if this falls into the realm of a subspecialist, and prognostication typically requires more information than a provider has at initial diagnosis. However, leaving these questions unanswered is likely to result in a patient feeling helpless. For example, if an ob.gyn. discovers an apparent advanced ovarian cancer on a CT scan, tell her that, despite its apparent advanced case, it is usually treatable and that a gynecologic oncologist will discuss those best treatment options with her. Assure her that you will expeditiously refer her to a specialist who will provide her with those specifics.
The aftermath
That interval between initial diagnosis and specialist consultation is extraordinarily difficult and a high anxiety time. It is not unreasonable, in such cases, to recommend the patient to reputable online information sources, such as the Society of Gynecologic Oncology or American Cancer Society websites so that she and her family can do some research prior to that visit in order to prepare them better and give them a sense of understanding in their disease.
It is a particularly compassionate touch to reach out to the patient in the days following her cancer diagnosis, even if she has moved on to a specialist. Patients often tell me that they felt enormous reassurance and appreciation when their ob.gyn. reached out to them to “check on how they are doing.” This can usually reasonably be done by phone. This second contact serves another critical purpose: it allows for repetition of the diagnosis and initial plan, and the ability to fill in the blanks of what the patient may have missed during the prior visit, if her mind was, naturally, elsewhere. It also, quite simply, shows that you care.
Ultimately, none of us can break bad news perfectly every time. We all need to be insightful with each of these encounters as to what we did well, what we did not, and how we can adjust in the future. With respect to the SPIKES approach, patients report that physicians struggle most with the “perception,” “invitation,” and “strategy and summary” components.5 Our objective should be keeping the patient’s needs in mind, rather than our own, to maximize the chance of doing a good job. If this task is done well, not only are patients more likely to have positive emotional adjustments to their diagnosis but also more adherence with future therapies.4 In the end, it is the patient who has the final say on whether it was done well or not.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reports no relevant financial disclosures.
References
1. Baile WF et al. Oncologist. 2000;5(4):302-11.
2. Girgis A et al. Behav Med. 1999 Summer;25(2):69-77.
3. Schmid MM et al. Patient Educ Couns. 2005 Sep;58(3):244-51.
4. Girgis A et al. J Clin Oncol. 1995 Sep;13(9):2449-56.
5. Marscholiek P et al. J Cancer Educ. 2018 Feb 5. doi: 10.1007/s13187-017-1315-3.
Hearing a diagnosis of cancer is one of the most significant moments of a patient’s life and informing a patient of her diagnosis is an emotionally and technically challenging task for an obstetrician gynecologist who is frequently on the front line of making this diagnosis. In this column, we will explore some patient-centered strategies to perform this difficult task well so that patients come away informed but with the highest chance for positive emotional adjustment.
Fewer than 10% of physicians report receiving formal training in techniques of breaking bad news. For the majority of clinicians concerns are centered on being honest and not taking away hope, and in responding to a patient’s emotions.1 The SPIKES approach was developed to arm physicians with strategies to discuss a cancer diagnosis with their patients. This approach includes six key elements to incorporate during the encounter. These strategies are not meant to be formulaic but rather consistent principles that can be adjusted for individual patient needs.
Setting up the discussion
Breaking bad news should not be a one-size-fits-all approach. Age, educational level, culture, religion, race and ethnicity, and socioeconomic opportunities each affects what and how patients may want to have this kind of information communicated to them. So how do you know how best to deliver a patient-centered approach for your patient? I recommend this simple strategy: Ask her. When ordering a test or performing a biopsy, let the patient know then why you are ordering the test and inform her of the possibility that the results may show cancer. Ask her how she would like for you to communicate that result. Would she like to be called by phone, the benefit of which is quick dissemination of information? Or would she like to receive the information face to face in the office? Research supports that most patients prefer to learn the result in the office.2 If so, I recommend scheduling a follow-up appointment in advance to prevent delays. Ask her if she would like a family member or a supportive friend to be present for the conveying of results so that she will have time to make these arrangements. Ask her if she would prefer for an alternate person to be provided with the results on her behalf.
When preparing to speak with the patient, it is valuable to mentally rehearse the words that you’ll use. Arrange for privacy and manage time constraints and interruptions (silence pagers and phones, ensure there is adequate time allocated in the schedule). Sit down to deliver the news and make a connection with eye contact and, if appropriate, touch.
Assessing the patient’s perception. Before you tell, ask. For example, “what is your understanding about why we did the biopsy?” This will guide you in where her head and heart are and can ensure you meet her wherever she is.
Obtaining the patient’s invitation. Ask the patient what she would like to be told and how much information. What would she like you to focus on? What does she not want to hear?
Giving knowledge and information to the patient. Especially now, it is important to avoid jargon and use nontechnical terms. However, do not shy away from using specific words like “cancer” by substituting them for more vague and confusing terms such as “malignancy” or “tumor.” It is important to find the balance between expressing information without being overly emotive, while avoiding excessive bluntness. Word choice is critical. Communication styles in the breaking of bad news can be separated broadly into three styles: disease centered, emotion centered, and patient-centered.3 The patient-centered approach is achieved by balancing emotional connection, information sharing, nondominance, and conveying hope. (For example, “I have some disappointing news to share. Shall we talk about the next steps in treatment? I understand this is that this is difficult for you.”) In general, this approach is most valued by patients and is associated with better information recall.
Addressing the patient’s emotions with empathetic responses. It is important that physicians take a moment to pause after communicating the test result. Even if prepared, most patients will still have a moment of shock, and their minds will likely spin through a multitude of thoughts preventing them from being able to “hear” and focus on the subsequent information. This is a moment to reflect on her reactions, her body language, and nonverbal communications to guide you on how to approach the rest of the encounter. Offer her your comfort and condolence in whichever way feels appropriate for you and her.
Beware of your own inclinations to “soften the blow.” It is a natural, compassionate instinct to follow-up giving a bad piece of information by balancing a good piece of information. For example, after just telling a woman that she has endometrial cancer, following with a statement such as “but it’s just stage 1 and is curable with surgery.” While this certainly may have immediate comforting effects, it has a couple of unintended consequences. First, it can result in difficulties later adjusting to a change in diagnosis when more information comes in (for example, upstaging after surgery or imaging). It is better to be honest and tell patients only what you know for sure in these immediate first moments of diagnosis when complete information is lacking. A more general statement such as “for most women, this is found at an early stage and is highly treatable” may be more appropriate and still provide some comfort. Second, attempts to soften the blow with a qualifying statement of positivity, such as “this is a good kind of cancer to have” might be interpreted by some patients as failing to acknowledge their devastation. She may feel that you are minimizing her condition and not allowing her to grieve or be distressed.
Strategy and summary. Patients who leave the encounter with some kind of plan for the future feel less distressed and anxious. The direction at this point of the encounter should be led by the patient. What are her greatest concerns (such as mortality, loss of fertility, time off work for treatment), and what does she want to know right now? Most patients express a desire to know more about treatment or prognosis.2,4 Unfortunately, it often is not possible to furnish this yet, particularly if this falls into the realm of a subspecialist, and prognostication typically requires more information than a provider has at initial diagnosis. However, leaving these questions unanswered is likely to result in a patient feeling helpless. For example, if an ob.gyn. discovers an apparent advanced ovarian cancer on a CT scan, tell her that, despite its apparent advanced case, it is usually treatable and that a gynecologic oncologist will discuss those best treatment options with her. Assure her that you will expeditiously refer her to a specialist who will provide her with those specifics.
The aftermath
That interval between initial diagnosis and specialist consultation is extraordinarily difficult and a high anxiety time. It is not unreasonable, in such cases, to recommend the patient to reputable online information sources, such as the Society of Gynecologic Oncology or American Cancer Society websites so that she and her family can do some research prior to that visit in order to prepare them better and give them a sense of understanding in their disease.
It is a particularly compassionate touch to reach out to the patient in the days following her cancer diagnosis, even if she has moved on to a specialist. Patients often tell me that they felt enormous reassurance and appreciation when their ob.gyn. reached out to them to “check on how they are doing.” This can usually reasonably be done by phone. This second contact serves another critical purpose: it allows for repetition of the diagnosis and initial plan, and the ability to fill in the blanks of what the patient may have missed during the prior visit, if her mind was, naturally, elsewhere. It also, quite simply, shows that you care.
Ultimately, none of us can break bad news perfectly every time. We all need to be insightful with each of these encounters as to what we did well, what we did not, and how we can adjust in the future. With respect to the SPIKES approach, patients report that physicians struggle most with the “perception,” “invitation,” and “strategy and summary” components.5 Our objective should be keeping the patient’s needs in mind, rather than our own, to maximize the chance of doing a good job. If this task is done well, not only are patients more likely to have positive emotional adjustments to their diagnosis but also more adherence with future therapies.4 In the end, it is the patient who has the final say on whether it was done well or not.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She reports no relevant financial disclosures.
References
1. Baile WF et al. Oncologist. 2000;5(4):302-11.
2. Girgis A et al. Behav Med. 1999 Summer;25(2):69-77.
3. Schmid MM et al. Patient Educ Couns. 2005 Sep;58(3):244-51.
4. Girgis A et al. J Clin Oncol. 1995 Sep;13(9):2449-56.
5. Marscholiek P et al. J Cancer Educ. 2018 Feb 5. doi: 10.1007/s13187-017-1315-3.
MDedge Daily News: The flu may be slowing down
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The flu may be slowing down, there’s a potential breakthrough in the treatment of melasma, what happened in rheumatology last year, and the AMA takes a stand on gun violence.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The flu may be slowing down, there’s a potential breakthrough in the treatment of melasma, what happened in rheumatology last year, and the AMA takes a stand on gun violence.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The flu may be slowing down, there’s a potential breakthrough in the treatment of melasma, what happened in rheumatology last year, and the AMA takes a stand on gun violence.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Call for Articles on Hematology/Oncology
Federal Practitioner articles are now available in PubMed Central, which provides full text access to any reader and is included in all PubMed online searches.
Federal Practitioner is inviting VA, DoD, and PHS health care providers and researchers to contribute to the May 2018 and August 2018 Advances in Hematology and Oncology special issues. The special issues are produced in cooperation with the Association of VA Hematology/Oncology (AVAHO). The journal is especially interested in articles on lung, prostate, and head and neck cancers; melanoma and skin cancers; survivorship; patient navigation; lymphomas; leukemias; multiple myeloma; neuroendocrine tumors; and immunotherapies.
Interested authors can send a brief 2 to 3 sentence abstract to [email protected] by March 15, 2018, or submit a completed article directly into Editorial Manager, a web-based manuscript submission and review system. Federal Practitioner welcomes case studies, literature reviews, original research, program profiles, guest editorials, and other evidence-based articles. The updated and complete submission guidelines, including details about the style and format, can be found here:
http://www.mdedge.com/fedprac/page/submission-guidelines
All manuscripts submitted to Federal Practitioner for both special and regular issues will be subject to peer review. Peer reviews are conducted in a double-blind fashion, and the reviewers are asked to comment on the manuscript’s importance, accuracy, relevance, clarity, timeliness, balance, and reference citation. Final decisions on all submitted manuscripts are made by the Editorial Advisory Association Hematology/Oncology special issue advisory board.
Federal Practitioner articles are now available in PubMed Central, which provides full text access to any reader and is included in all PubMed online searches.
Federal Practitioner is inviting VA, DoD, and PHS health care providers and researchers to contribute to the May 2018 and August 2018 Advances in Hematology and Oncology special issues. The special issues are produced in cooperation with the Association of VA Hematology/Oncology (AVAHO). The journal is especially interested in articles on lung, prostate, and head and neck cancers; melanoma and skin cancers; survivorship; patient navigation; lymphomas; leukemias; multiple myeloma; neuroendocrine tumors; and immunotherapies.
Interested authors can send a brief 2 to 3 sentence abstract to [email protected] by March 15, 2018, or submit a completed article directly into Editorial Manager, a web-based manuscript submission and review system. Federal Practitioner welcomes case studies, literature reviews, original research, program profiles, guest editorials, and other evidence-based articles. The updated and complete submission guidelines, including details about the style and format, can be found here:
http://www.mdedge.com/fedprac/page/submission-guidelines
All manuscripts submitted to Federal Practitioner for both special and regular issues will be subject to peer review. Peer reviews are conducted in a double-blind fashion, and the reviewers are asked to comment on the manuscript’s importance, accuracy, relevance, clarity, timeliness, balance, and reference citation. Final decisions on all submitted manuscripts are made by the Editorial Advisory Association Hematology/Oncology special issue advisory board.
Federal Practitioner articles are now available in PubMed Central, which provides full text access to any reader and is included in all PubMed online searches.
Federal Practitioner is inviting VA, DoD, and PHS health care providers and researchers to contribute to the May 2018 and August 2018 Advances in Hematology and Oncology special issues. The special issues are produced in cooperation with the Association of VA Hematology/Oncology (AVAHO). The journal is especially interested in articles on lung, prostate, and head and neck cancers; melanoma and skin cancers; survivorship; patient navigation; lymphomas; leukemias; multiple myeloma; neuroendocrine tumors; and immunotherapies.
Interested authors can send a brief 2 to 3 sentence abstract to [email protected] by March 15, 2018, or submit a completed article directly into Editorial Manager, a web-based manuscript submission and review system. Federal Practitioner welcomes case studies, literature reviews, original research, program profiles, guest editorials, and other evidence-based articles. The updated and complete submission guidelines, including details about the style and format, can be found here:
http://www.mdedge.com/fedprac/page/submission-guidelines
All manuscripts submitted to Federal Practitioner for both special and regular issues will be subject to peer review. Peer reviews are conducted in a double-blind fashion, and the reviewers are asked to comment on the manuscript’s importance, accuracy, relevance, clarity, timeliness, balance, and reference citation. Final decisions on all submitted manuscripts are made by the Editorial Advisory Association Hematology/Oncology special issue advisory board.
Cognitive impairment in HSCT recipients
New research suggests the risk of cognitive impairment after hematopoietic stem cell transplant (HSCT) is greatest among recipients of myeloablative allogeneic (allo) HSCTs.
Compared to healthy controls, patients who received myeloablative allo-HSCT had a significantly higher risk of global cognitive deficit at a few time points after transplant.
There was a trend toward increased global cognitive deficit in recipients of allo-HSCT who had reduced-intensity conditioning (RIC), but there was no increased risk of global cognitive deficit in recipients of autologous (auto) HSCT.
Researchers reported these findings in the Journal of Clinical Oncology.
“With this research from our longitudinal prospective assessment, we are able to deduce that a significant population of allogeneic [HSCT] survivors will experience cognitive impairment that can and will impact different aspects of their lives moving forward,” said study author Noha Sharafeldin, MD, PhD, of the University of Alabama at Birmingham.
“And it’s critical that we as clinicians develop interventions for these patients. This research is just the beginning of our figuring out how we can best care for [HSCT] survivors and enable them to live healthy lives.”
This study included 477 patients who underwent HSCT between 2004 and 2014. There were 236 auto-HSCTs, 128 RIC allo-HSCTs, and 113 myeloablative allo-HSCTs.
Patients underwent standardized neuropsychological testing before HSCT as well as at 6 months, 1 year, 2 years, and 3 years after transplant.
There were 429 patients who completed pre-HSCT testing (89.9%), 341 (81.6%) who underwent testing at 6 months after HSCT, 308 (81.5%) at 1 year, 247 (80.7%) at 2 years, and 227 (81.4%) at 3 years.
Testing was conducted on 8 cognitive domains—executive function, verbal fluency and speed, processing speed, working memory, visual and auditory memory, and fine motor dexterity.
The researchers conducted this testing in 99 healthy control subjects as well.
Before and after HSCT
Prior to HSCT, there were no significant differences in the cognitive domains tested between auto-HSCT recipients and controls or between RIC allo-HSCT recipients and controls.
Recipients of myeloablative allo-HSCT had significantly lower pre-HSCT scores for processing speed (P=0.001), as compared to controls.
After HSCT, there were no significant differences in overall scores between auto-HSCT recipients and controls or between RIC allo-HSCT recipients and controls.
However, recipients of myeloablative allo-HSCT had significantly lower scores than controls for executive function, verbal speed, processing speed, auditory memory, and fine motor dexterity (P≤0.003 for all).
Outcomes over time
For auto-HSCT recipients, there was a significant improvement from pre-HSCT to 6 months post-HSCT in verbal fluency (P<0.001). Meanwhile, there was a significant decrease in verbal processing and fine motor dexterity (P<0.001 for both).
At 3 years, auto-HSCT recipients had a significant increase in verbal fluency (P<0.001) but a significant decrease in visual memory (P=0.001) and fine motor dexterity (P<0.001).
For RIC allo-HSCT recipients, there was a significant decrease from pre-HSCT to 3 years post-HSCT in executive functioning (P=0.003), verbal fluency (P<0.001), and working memory (P<0.001). There were no significant differences between pre-HSCT and 6-month scores.
For patients who received myeloablative allo-HSCT, the only significant difference from pre-HSCT to 6 months or 3 years was a decrease in fine motor dexterity (P<0.001 for both time points).
Global cognitive deficit
There was no significant difference in the prevalence of global cognitive deficit between auto-HSCT recipients and controls before HSCT (22.5% vs 17.2%; P=0.28) or at any time point after—6 months (26.1% vs 16.5%; P=0.07), 1 year (21.4% vs 19.5%; P=0.73), 2 years (21.1% vs 16.4%; P=0.43), and 3 years (18.7% vs 8.7%, P=0.11).
There was no significant difference in the prevalence of global cognitive deficit between RIC allo-HSCT recipients and controls before HSCT (17.2% for both; P=1.0), at 6 months (22.0% vs 16.5%; P=0.35), 1 year (24.1% vs 19.5%; P=0.46), or 2 years (30.6% vs 16.4%; P=0.05) after HSCT.
However, the prevalence was significantly higher for RIC allo-HSCT recipients 3 years after HSCT (35.4% vs 8.7%; P=0.0012).
There was no significant difference in the prevalence of global cognitive deficit between myeloablative allo-HSCT recipients and controls before HSCT (22.3% vs 17.2%; P=0.37) or at 1 year after (28.4% vs 19.5%; P=0.20).
However, the prevalence was significantly higher for myeloablative allo-HSCT recipients at 6 months (31.1% vs 16.5%; P=0.03), 2 years (34.6% vs 16.4%; P=0.02), and 3 years after HSCT (36.0% vs 8.7%; P=0.0015).
“From this data, it’s clear that we have to make strides in supporting allogeneic [HSCT] recipients in their recovery to ensure that we are educating patients and their families on signs of cognitive impairment,” Dr Sharafeldin said. “This data will help us identify patients at highest risk of cognitive impairment and inform the development of interventions that facilitate a patient’s recovery and return to normal life.”
New research suggests the risk of cognitive impairment after hematopoietic stem cell transplant (HSCT) is greatest among recipients of myeloablative allogeneic (allo) HSCTs.
Compared to healthy controls, patients who received myeloablative allo-HSCT had a significantly higher risk of global cognitive deficit at a few time points after transplant.
There was a trend toward increased global cognitive deficit in recipients of allo-HSCT who had reduced-intensity conditioning (RIC), but there was no increased risk of global cognitive deficit in recipients of autologous (auto) HSCT.
Researchers reported these findings in the Journal of Clinical Oncology.
“With this research from our longitudinal prospective assessment, we are able to deduce that a significant population of allogeneic [HSCT] survivors will experience cognitive impairment that can and will impact different aspects of their lives moving forward,” said study author Noha Sharafeldin, MD, PhD, of the University of Alabama at Birmingham.
“And it’s critical that we as clinicians develop interventions for these patients. This research is just the beginning of our figuring out how we can best care for [HSCT] survivors and enable them to live healthy lives.”
This study included 477 patients who underwent HSCT between 2004 and 2014. There were 236 auto-HSCTs, 128 RIC allo-HSCTs, and 113 myeloablative allo-HSCTs.
Patients underwent standardized neuropsychological testing before HSCT as well as at 6 months, 1 year, 2 years, and 3 years after transplant.
There were 429 patients who completed pre-HSCT testing (89.9%), 341 (81.6%) who underwent testing at 6 months after HSCT, 308 (81.5%) at 1 year, 247 (80.7%) at 2 years, and 227 (81.4%) at 3 years.
Testing was conducted on 8 cognitive domains—executive function, verbal fluency and speed, processing speed, working memory, visual and auditory memory, and fine motor dexterity.
The researchers conducted this testing in 99 healthy control subjects as well.
Before and after HSCT
Prior to HSCT, there were no significant differences in the cognitive domains tested between auto-HSCT recipients and controls or between RIC allo-HSCT recipients and controls.
Recipients of myeloablative allo-HSCT had significantly lower pre-HSCT scores for processing speed (P=0.001), as compared to controls.
After HSCT, there were no significant differences in overall scores between auto-HSCT recipients and controls or between RIC allo-HSCT recipients and controls.
However, recipients of myeloablative allo-HSCT had significantly lower scores than controls for executive function, verbal speed, processing speed, auditory memory, and fine motor dexterity (P≤0.003 for all).
Outcomes over time
For auto-HSCT recipients, there was a significant improvement from pre-HSCT to 6 months post-HSCT in verbal fluency (P<0.001). Meanwhile, there was a significant decrease in verbal processing and fine motor dexterity (P<0.001 for both).
At 3 years, auto-HSCT recipients had a significant increase in verbal fluency (P<0.001) but a significant decrease in visual memory (P=0.001) and fine motor dexterity (P<0.001).
For RIC allo-HSCT recipients, there was a significant decrease from pre-HSCT to 3 years post-HSCT in executive functioning (P=0.003), verbal fluency (P<0.001), and working memory (P<0.001). There were no significant differences between pre-HSCT and 6-month scores.
For patients who received myeloablative allo-HSCT, the only significant difference from pre-HSCT to 6 months or 3 years was a decrease in fine motor dexterity (P<0.001 for both time points).
Global cognitive deficit
There was no significant difference in the prevalence of global cognitive deficit between auto-HSCT recipients and controls before HSCT (22.5% vs 17.2%; P=0.28) or at any time point after—6 months (26.1% vs 16.5%; P=0.07), 1 year (21.4% vs 19.5%; P=0.73), 2 years (21.1% vs 16.4%; P=0.43), and 3 years (18.7% vs 8.7%, P=0.11).
There was no significant difference in the prevalence of global cognitive deficit between RIC allo-HSCT recipients and controls before HSCT (17.2% for both; P=1.0), at 6 months (22.0% vs 16.5%; P=0.35), 1 year (24.1% vs 19.5%; P=0.46), or 2 years (30.6% vs 16.4%; P=0.05) after HSCT.
However, the prevalence was significantly higher for RIC allo-HSCT recipients 3 years after HSCT (35.4% vs 8.7%; P=0.0012).
There was no significant difference in the prevalence of global cognitive deficit between myeloablative allo-HSCT recipients and controls before HSCT (22.3% vs 17.2%; P=0.37) or at 1 year after (28.4% vs 19.5%; P=0.20).
However, the prevalence was significantly higher for myeloablative allo-HSCT recipients at 6 months (31.1% vs 16.5%; P=0.03), 2 years (34.6% vs 16.4%; P=0.02), and 3 years after HSCT (36.0% vs 8.7%; P=0.0015).
“From this data, it’s clear that we have to make strides in supporting allogeneic [HSCT] recipients in their recovery to ensure that we are educating patients and their families on signs of cognitive impairment,” Dr Sharafeldin said. “This data will help us identify patients at highest risk of cognitive impairment and inform the development of interventions that facilitate a patient’s recovery and return to normal life.”
New research suggests the risk of cognitive impairment after hematopoietic stem cell transplant (HSCT) is greatest among recipients of myeloablative allogeneic (allo) HSCTs.
Compared to healthy controls, patients who received myeloablative allo-HSCT had a significantly higher risk of global cognitive deficit at a few time points after transplant.
There was a trend toward increased global cognitive deficit in recipients of allo-HSCT who had reduced-intensity conditioning (RIC), but there was no increased risk of global cognitive deficit in recipients of autologous (auto) HSCT.
Researchers reported these findings in the Journal of Clinical Oncology.
“With this research from our longitudinal prospective assessment, we are able to deduce that a significant population of allogeneic [HSCT] survivors will experience cognitive impairment that can and will impact different aspects of their lives moving forward,” said study author Noha Sharafeldin, MD, PhD, of the University of Alabama at Birmingham.
“And it’s critical that we as clinicians develop interventions for these patients. This research is just the beginning of our figuring out how we can best care for [HSCT] survivors and enable them to live healthy lives.”
This study included 477 patients who underwent HSCT between 2004 and 2014. There were 236 auto-HSCTs, 128 RIC allo-HSCTs, and 113 myeloablative allo-HSCTs.
Patients underwent standardized neuropsychological testing before HSCT as well as at 6 months, 1 year, 2 years, and 3 years after transplant.
There were 429 patients who completed pre-HSCT testing (89.9%), 341 (81.6%) who underwent testing at 6 months after HSCT, 308 (81.5%) at 1 year, 247 (80.7%) at 2 years, and 227 (81.4%) at 3 years.
Testing was conducted on 8 cognitive domains—executive function, verbal fluency and speed, processing speed, working memory, visual and auditory memory, and fine motor dexterity.
The researchers conducted this testing in 99 healthy control subjects as well.
Before and after HSCT
Prior to HSCT, there were no significant differences in the cognitive domains tested between auto-HSCT recipients and controls or between RIC allo-HSCT recipients and controls.
Recipients of myeloablative allo-HSCT had significantly lower pre-HSCT scores for processing speed (P=0.001), as compared to controls.
After HSCT, there were no significant differences in overall scores between auto-HSCT recipients and controls or between RIC allo-HSCT recipients and controls.
However, recipients of myeloablative allo-HSCT had significantly lower scores than controls for executive function, verbal speed, processing speed, auditory memory, and fine motor dexterity (P≤0.003 for all).
Outcomes over time
For auto-HSCT recipients, there was a significant improvement from pre-HSCT to 6 months post-HSCT in verbal fluency (P<0.001). Meanwhile, there was a significant decrease in verbal processing and fine motor dexterity (P<0.001 for both).
At 3 years, auto-HSCT recipients had a significant increase in verbal fluency (P<0.001) but a significant decrease in visual memory (P=0.001) and fine motor dexterity (P<0.001).
For RIC allo-HSCT recipients, there was a significant decrease from pre-HSCT to 3 years post-HSCT in executive functioning (P=0.003), verbal fluency (P<0.001), and working memory (P<0.001). There were no significant differences between pre-HSCT and 6-month scores.
For patients who received myeloablative allo-HSCT, the only significant difference from pre-HSCT to 6 months or 3 years was a decrease in fine motor dexterity (P<0.001 for both time points).
Global cognitive deficit
There was no significant difference in the prevalence of global cognitive deficit between auto-HSCT recipients and controls before HSCT (22.5% vs 17.2%; P=0.28) or at any time point after—6 months (26.1% vs 16.5%; P=0.07), 1 year (21.4% vs 19.5%; P=0.73), 2 years (21.1% vs 16.4%; P=0.43), and 3 years (18.7% vs 8.7%, P=0.11).
There was no significant difference in the prevalence of global cognitive deficit between RIC allo-HSCT recipients and controls before HSCT (17.2% for both; P=1.0), at 6 months (22.0% vs 16.5%; P=0.35), 1 year (24.1% vs 19.5%; P=0.46), or 2 years (30.6% vs 16.4%; P=0.05) after HSCT.
However, the prevalence was significantly higher for RIC allo-HSCT recipients 3 years after HSCT (35.4% vs 8.7%; P=0.0012).
There was no significant difference in the prevalence of global cognitive deficit between myeloablative allo-HSCT recipients and controls before HSCT (22.3% vs 17.2%; P=0.37) or at 1 year after (28.4% vs 19.5%; P=0.20).
However, the prevalence was significantly higher for myeloablative allo-HSCT recipients at 6 months (31.1% vs 16.5%; P=0.03), 2 years (34.6% vs 16.4%; P=0.02), and 3 years after HSCT (36.0% vs 8.7%; P=0.0015).
“From this data, it’s clear that we have to make strides in supporting allogeneic [HSCT] recipients in their recovery to ensure that we are educating patients and their families on signs of cognitive impairment,” Dr Sharafeldin said. “This data will help us identify patients at highest risk of cognitive impairment and inform the development of interventions that facilitate a patient’s recovery and return to normal life.”
Gait freezing relieved by spinal cord, transcranial direct-current stimulation
The application of transcranial direct-current stimulation and spinal cord stimulation alleviated freezing of gait in two separate studies of patients with idiopathic Parkinson’s disease published online in Movement Disorders.
In the first study, Moria Dagan of Tel Aviv University and her colleagues reported that transcranial direct-current stimulation (tDCS) applied simultaneously to the primary motor cortex (M1) and the left dorsolateral prefrontal cortex (DLPFC) improved freezing of gait (FOG) more than M1 stimulation alone or sham stimulation in a double-blind, randomized trial of 20 patients. The second study, reported by Olivia Samotus and her associates at the London (Ont.) Health Sciences Centre, found that open-label, midthoracic epidural spinal cord stimulation (SCS) in five patients produced significantly reduced FOG episodes and also provided sustained improvement in gait measurements.
The tDCS study used a crossover design to show that, over the course of four separate, 20-minute tDCS sessions, FOG-provoking test scores improved in 15 of 17 patients who received multitarget stimulation and were significantly improved on average, whereas the scores of patients who had M1 stimulation alone or sham stimulation did not improve. Improvement in FOG severity nearly reached statistical significance when compared with M1 only and sham stimulation (P = .06). Three patients were unable to complete the FOG-provoking test and were excluded from the analysis.
The investigators tested simultaneous tDCS of the M1 and left DLPFC because a previous study showed improvement in FOG after M1 stimulation, and other evidence suggests that FOG might also arise through deficits in executive function mediated by the DLPFC. Previous research in other patient populations has also shown that tDCS improves cognition, gait, and postural control, and a prior study using transcranial magnetic stimulation of the M1 and left DLPFC improved gait and cognition in patients with FOG.
“We suggest that the results move this emerging approach a key step forward, as they support the idea that the cognitive executive circuit plays a role in FOG and the possibility that multitarget stimulation may have value as an intervention for ameliorating FOG,” Ms. Dagan and her colleagues wrote.
In a separate, nonrandomized pilot trial, SCS produced improvements in stride velocity, step length, and single- and double-support phases in follow-up visits out to 6 months post surgery, in addition to significantly decreasing FOG episodes. The study is the first to use objective gait technology to assess the efficacy of SCS for gait in advanced Parkinson’s disease patients. Ms. Samotus and her associates observed these changes after testing a range of pulse width and frequency combinations in each of the five participants to determine which settings best improved gait for each participant. Overall, the results suggest that pulse widths of “300-400 microseconds and frequencies of 30-130 Hz may be safe and possibly effective for reducing FOG frequency and improving gait dysfunction in advanced Parkinson’s disease patients.”
The tDCS trial was supported by the Michael J. Fox Foundation for Parkinson’s Research. One investigator disclosed that he is cofounder and shareholder of Neuroelectrics, which makes brain stimulation technologies such as the ones used in the study. No outside funding was reported for the SCS study. One investigator in the SCS study reported ties to pharmaceutical companies and device manufacturers.
SOURCES: Dagan M et al. Mov Disord. 2018 Feb 13. doi: 10.1002/mds.27300; Samotus O et al. Mov Disord. 2018 Feb 14. doi: 10.1002/mds.27299.
The application of transcranial direct-current stimulation and spinal cord stimulation alleviated freezing of gait in two separate studies of patients with idiopathic Parkinson’s disease published online in Movement Disorders.
In the first study, Moria Dagan of Tel Aviv University and her colleagues reported that transcranial direct-current stimulation (tDCS) applied simultaneously to the primary motor cortex (M1) and the left dorsolateral prefrontal cortex (DLPFC) improved freezing of gait (FOG) more than M1 stimulation alone or sham stimulation in a double-blind, randomized trial of 20 patients. The second study, reported by Olivia Samotus and her associates at the London (Ont.) Health Sciences Centre, found that open-label, midthoracic epidural spinal cord stimulation (SCS) in five patients produced significantly reduced FOG episodes and also provided sustained improvement in gait measurements.
The tDCS study used a crossover design to show that, over the course of four separate, 20-minute tDCS sessions, FOG-provoking test scores improved in 15 of 17 patients who received multitarget stimulation and were significantly improved on average, whereas the scores of patients who had M1 stimulation alone or sham stimulation did not improve. Improvement in FOG severity nearly reached statistical significance when compared with M1 only and sham stimulation (P = .06). Three patients were unable to complete the FOG-provoking test and were excluded from the analysis.
The investigators tested simultaneous tDCS of the M1 and left DLPFC because a previous study showed improvement in FOG after M1 stimulation, and other evidence suggests that FOG might also arise through deficits in executive function mediated by the DLPFC. Previous research in other patient populations has also shown that tDCS improves cognition, gait, and postural control, and a prior study using transcranial magnetic stimulation of the M1 and left DLPFC improved gait and cognition in patients with FOG.
“We suggest that the results move this emerging approach a key step forward, as they support the idea that the cognitive executive circuit plays a role in FOG and the possibility that multitarget stimulation may have value as an intervention for ameliorating FOG,” Ms. Dagan and her colleagues wrote.
In a separate, nonrandomized pilot trial, SCS produced improvements in stride velocity, step length, and single- and double-support phases in follow-up visits out to 6 months post surgery, in addition to significantly decreasing FOG episodes. The study is the first to use objective gait technology to assess the efficacy of SCS for gait in advanced Parkinson’s disease patients. Ms. Samotus and her associates observed these changes after testing a range of pulse width and frequency combinations in each of the five participants to determine which settings best improved gait for each participant. Overall, the results suggest that pulse widths of “300-400 microseconds and frequencies of 30-130 Hz may be safe and possibly effective for reducing FOG frequency and improving gait dysfunction in advanced Parkinson’s disease patients.”
The tDCS trial was supported by the Michael J. Fox Foundation for Parkinson’s Research. One investigator disclosed that he is cofounder and shareholder of Neuroelectrics, which makes brain stimulation technologies such as the ones used in the study. No outside funding was reported for the SCS study. One investigator in the SCS study reported ties to pharmaceutical companies and device manufacturers.
SOURCES: Dagan M et al. Mov Disord. 2018 Feb 13. doi: 10.1002/mds.27300; Samotus O et al. Mov Disord. 2018 Feb 14. doi: 10.1002/mds.27299.
The application of transcranial direct-current stimulation and spinal cord stimulation alleviated freezing of gait in two separate studies of patients with idiopathic Parkinson’s disease published online in Movement Disorders.
In the first study, Moria Dagan of Tel Aviv University and her colleagues reported that transcranial direct-current stimulation (tDCS) applied simultaneously to the primary motor cortex (M1) and the left dorsolateral prefrontal cortex (DLPFC) improved freezing of gait (FOG) more than M1 stimulation alone or sham stimulation in a double-blind, randomized trial of 20 patients. The second study, reported by Olivia Samotus and her associates at the London (Ont.) Health Sciences Centre, found that open-label, midthoracic epidural spinal cord stimulation (SCS) in five patients produced significantly reduced FOG episodes and also provided sustained improvement in gait measurements.
The tDCS study used a crossover design to show that, over the course of four separate, 20-minute tDCS sessions, FOG-provoking test scores improved in 15 of 17 patients who received multitarget stimulation and were significantly improved on average, whereas the scores of patients who had M1 stimulation alone or sham stimulation did not improve. Improvement in FOG severity nearly reached statistical significance when compared with M1 only and sham stimulation (P = .06). Three patients were unable to complete the FOG-provoking test and were excluded from the analysis.
The investigators tested simultaneous tDCS of the M1 and left DLPFC because a previous study showed improvement in FOG after M1 stimulation, and other evidence suggests that FOG might also arise through deficits in executive function mediated by the DLPFC. Previous research in other patient populations has also shown that tDCS improves cognition, gait, and postural control, and a prior study using transcranial magnetic stimulation of the M1 and left DLPFC improved gait and cognition in patients with FOG.
“We suggest that the results move this emerging approach a key step forward, as they support the idea that the cognitive executive circuit plays a role in FOG and the possibility that multitarget stimulation may have value as an intervention for ameliorating FOG,” Ms. Dagan and her colleagues wrote.
In a separate, nonrandomized pilot trial, SCS produced improvements in stride velocity, step length, and single- and double-support phases in follow-up visits out to 6 months post surgery, in addition to significantly decreasing FOG episodes. The study is the first to use objective gait technology to assess the efficacy of SCS for gait in advanced Parkinson’s disease patients. Ms. Samotus and her associates observed these changes after testing a range of pulse width and frequency combinations in each of the five participants to determine which settings best improved gait for each participant. Overall, the results suggest that pulse widths of “300-400 microseconds and frequencies of 30-130 Hz may be safe and possibly effective for reducing FOG frequency and improving gait dysfunction in advanced Parkinson’s disease patients.”
The tDCS trial was supported by the Michael J. Fox Foundation for Parkinson’s Research. One investigator disclosed that he is cofounder and shareholder of Neuroelectrics, which makes brain stimulation technologies such as the ones used in the study. No outside funding was reported for the SCS study. One investigator in the SCS study reported ties to pharmaceutical companies and device manufacturers.
SOURCES: Dagan M et al. Mov Disord. 2018 Feb 13. doi: 10.1002/mds.27300; Samotus O et al. Mov Disord. 2018 Feb 14. doi: 10.1002/mds.27299.
FROM MOVEMENT DISORDERS
Key clinical point:
Major finding: Freezing of gait–provoking test scores improved in 15 of 17 patients who received simultaneous tDCS to the primary motor cortex and the left dorsolateral prefrontal cortex.
Study details: A double-blind, randomized trial of tDCS in 20 Parkinson’s disease patients and a nonrandomized, open-label study of spinal cord stimulation in 5 Parkinson’s patients.
Disclosures: The tDCS trial was supported by the Michael J. Fox Foundation for Parkinson’s Research. One investigator disclosed that he is cofounder and shareholder of Neuroelectrics, which makes brain stimulation technologies such as the ones used in the study. No outside funding was reported for the SCS study. One investigator in the SCS study reported ties to pharmaceutical companies and device manufacturers.
Sources: Dagan M et al. Mov Disord. 2018 Feb 13. doi: 10.1002/mds.27300; Samotus O et al. Mov Disord. 2018 Feb 14. doi: 10.1002/mds.27299.
Trump administration proposes rule to loosen curbs on short-term health plans
Insurers will again be able to sell short-term health insurance good for up to 12 months under a proposed rule released Feb. 20 by the Trump administration that could further roil the marketplace.
“We want to open up affordable alternatives to unaffordable Affordable Care Act policies,” said Health and Human Services Secretary Alex Azar. “This is one step in the direction of providing Americans health insurance options that are more affordable and more suitable to individual and family circumstances.”
The proposed rule said short-term plans could add more choices to the market at lower cost and may offer broader provider networks than Affordable Care Act plans in rural areas.
But most short-term coverage requires answering a string of medical questions, and insurers can reject applicants with preexisting medical problems, which ACA plans cannot do. As a result, the proposed rule also noted that some people who switch to them from ACA coverage may see “reduced access to some services,” and “increased out of pocket costs, possibly leading to financial hardship.”
The directive follows an executive order issued in October to roll back restrictions put in place during the Obama administration that limited these plans to 3 months. The rule comes on the heels of Congress’ approval of tax legislation that in 2019 will end the penalty for people who opt not to carry insurance coverage.
The administration also issued separate regulations Jan. 4 that would make it easier to form “association health plans,” which are offered to small businesses through membership organizations.
Together, the proposed regulations and the elimination of the so-called individual mandate by Congress could further undermine the Affordable Care Act marketplace, critics say.
Seema Verma, who now heads the Centers for Medicare & Medicaid Services, which oversees the marketplaces, told reporters Feb. 20 that federal officials believe that between 100,000 and 200,000 “healthy people” now buying insurance through those federal exchanges would switch to the short-term plans, as well as others who are now uninsured.
The new rule is expected to entice younger and healthier people from the general insurance pool by allowing a range of lower-cost options that don’t include all the benefits required by the federal law – including plans that can reject people with preexisting medical conditions. Most short-term coverage excludes benefits for maternity care, preventive care, mental health services, or substance abuse treatment.
“It’s deeply concerning to me, considering the tragedy in Florida and national opioid crisis, that the administration would be encouraging the sale of policies that don’t have to cover mental health and substance abuse,” said Kevin Lucia, a research professor and project director at Georgetown University’s Health Policy Institute.
Over time, those remaining in ACA plans will increasingly be those who qualify for premium tax credit subsidies and the sick, who can’t get an alternative like a short-term plan, predict Mr. Lucia and other experts. That, in turn, would drive up ACA premiums further.
“If consumers think Obamacare premiums are high today, wait until people flood into these short-term and association health plans,” said industry consultant Robert Laszewski. “The Trump administration will bring rates down substantially for healthy people, but woe unto those who get a condition and have to go back into Obamacare.”
If 100,000-200,000 people shift from ACA-compliant plans in 2019, this would cause “average monthly individual market premiums … to increase,” the proposed rule states. That, in turn, would cause subsidies for eligible policyholders in the ACA market to rise, costing the government $96 million–$168 million.
Supporters said the rules are needed because the ACA plans have already become too costly for people who don’t receive a government subsidy to help them purchase the coverage. “The current system is failing too many,” said Ms. Verma.
And, many supporters don’t think the change is as significant as skeptics fear.
“It simply reverts back to where the short-term plan rules were prior to Obama limiting those plans,” said Christopher Condeluci, a benefits attorney who also served as tax counsel to the U.S. Senate Finance Committee. “While these plans might not be the best answer, people do need a choice, and this new proposal provides needed choice to a certain subsection of the population.”
But, in their call with reporters, CMS officials said the proposed rule seeks comment on whether there are ways to guarantee renewability of the plans, which currently cannot be renewed. Instead, policyholders must reapply and answer medical questions again. The proposal also seeks comments on whether the plans should be allowed for longer than 12-month periods.
The comment period for the proposed rule runs for 60 days. Ms. Verma said CMS hopes to get final rules out “as quickly as possible,” so insurers could start offering the longer duration plans.
Short-term plans had been designed as temporary coverage, lasting for a few months while, for instance, a worker is between jobs and employer-sponsored insurances. They provide some protection to those who enroll, generally paying a percentage of hospital and doctor bills after the policyholder meets a deductible.
They are generally less expensive than ACA plans, because they cover less. For example, they set annual and lifetime caps on benefits, and few cover prescription drugs.
Most require applicants to pass a medical questionnaire – and they can also exclude coverage for preexisting medical conditions.
The plans are appealing to consumers because they are cheaper than Obamacare plans. They are also attractive to brokers, because they often pay higher commissions than ACA plans. Insurers like them because their profit margins are relatively high – and are not held to the ACA requirement that they spend at least 80 percent of premium revenue on plan members’ medical care.
Extending short-term plans to a full year could be a benefit to consumers because they must pass the health questionnaire only once. Still, if a consumer develops a health condition during the contract’s term, that person would likely be rejected if he or she tried to renew.
Both supporters and critics of short-term plans say consumers who do develop health problems could then sign up for an ACA plan during the next open enrollment because the ACA bars insurers from rejecting people with preexisting conditions.
“We’re going to have two different markets, a Wild West frontier called short-term medical … and a high-risk pool called Obamacare,” said Laszewski.
KHN senior correspondent Phil Galewitz contributed to this article. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Insurers will again be able to sell short-term health insurance good for up to 12 months under a proposed rule released Feb. 20 by the Trump administration that could further roil the marketplace.
“We want to open up affordable alternatives to unaffordable Affordable Care Act policies,” said Health and Human Services Secretary Alex Azar. “This is one step in the direction of providing Americans health insurance options that are more affordable and more suitable to individual and family circumstances.”
The proposed rule said short-term plans could add more choices to the market at lower cost and may offer broader provider networks than Affordable Care Act plans in rural areas.
But most short-term coverage requires answering a string of medical questions, and insurers can reject applicants with preexisting medical problems, which ACA plans cannot do. As a result, the proposed rule also noted that some people who switch to them from ACA coverage may see “reduced access to some services,” and “increased out of pocket costs, possibly leading to financial hardship.”
The directive follows an executive order issued in October to roll back restrictions put in place during the Obama administration that limited these plans to 3 months. The rule comes on the heels of Congress’ approval of tax legislation that in 2019 will end the penalty for people who opt not to carry insurance coverage.
The administration also issued separate regulations Jan. 4 that would make it easier to form “association health plans,” which are offered to small businesses through membership organizations.
Together, the proposed regulations and the elimination of the so-called individual mandate by Congress could further undermine the Affordable Care Act marketplace, critics say.
Seema Verma, who now heads the Centers for Medicare & Medicaid Services, which oversees the marketplaces, told reporters Feb. 20 that federal officials believe that between 100,000 and 200,000 “healthy people” now buying insurance through those federal exchanges would switch to the short-term plans, as well as others who are now uninsured.
The new rule is expected to entice younger and healthier people from the general insurance pool by allowing a range of lower-cost options that don’t include all the benefits required by the federal law – including plans that can reject people with preexisting medical conditions. Most short-term coverage excludes benefits for maternity care, preventive care, mental health services, or substance abuse treatment.
“It’s deeply concerning to me, considering the tragedy in Florida and national opioid crisis, that the administration would be encouraging the sale of policies that don’t have to cover mental health and substance abuse,” said Kevin Lucia, a research professor and project director at Georgetown University’s Health Policy Institute.
Over time, those remaining in ACA plans will increasingly be those who qualify for premium tax credit subsidies and the sick, who can’t get an alternative like a short-term plan, predict Mr. Lucia and other experts. That, in turn, would drive up ACA premiums further.
“If consumers think Obamacare premiums are high today, wait until people flood into these short-term and association health plans,” said industry consultant Robert Laszewski. “The Trump administration will bring rates down substantially for healthy people, but woe unto those who get a condition and have to go back into Obamacare.”
If 100,000-200,000 people shift from ACA-compliant plans in 2019, this would cause “average monthly individual market premiums … to increase,” the proposed rule states. That, in turn, would cause subsidies for eligible policyholders in the ACA market to rise, costing the government $96 million–$168 million.
Supporters said the rules are needed because the ACA plans have already become too costly for people who don’t receive a government subsidy to help them purchase the coverage. “The current system is failing too many,” said Ms. Verma.
And, many supporters don’t think the change is as significant as skeptics fear.
“It simply reverts back to where the short-term plan rules were prior to Obama limiting those plans,” said Christopher Condeluci, a benefits attorney who also served as tax counsel to the U.S. Senate Finance Committee. “While these plans might not be the best answer, people do need a choice, and this new proposal provides needed choice to a certain subsection of the population.”
But, in their call with reporters, CMS officials said the proposed rule seeks comment on whether there are ways to guarantee renewability of the plans, which currently cannot be renewed. Instead, policyholders must reapply and answer medical questions again. The proposal also seeks comments on whether the plans should be allowed for longer than 12-month periods.
The comment period for the proposed rule runs for 60 days. Ms. Verma said CMS hopes to get final rules out “as quickly as possible,” so insurers could start offering the longer duration plans.
Short-term plans had been designed as temporary coverage, lasting for a few months while, for instance, a worker is between jobs and employer-sponsored insurances. They provide some protection to those who enroll, generally paying a percentage of hospital and doctor bills after the policyholder meets a deductible.
They are generally less expensive than ACA plans, because they cover less. For example, they set annual and lifetime caps on benefits, and few cover prescription drugs.
Most require applicants to pass a medical questionnaire – and they can also exclude coverage for preexisting medical conditions.
The plans are appealing to consumers because they are cheaper than Obamacare plans. They are also attractive to brokers, because they often pay higher commissions than ACA plans. Insurers like them because their profit margins are relatively high – and are not held to the ACA requirement that they spend at least 80 percent of premium revenue on plan members’ medical care.
Extending short-term plans to a full year could be a benefit to consumers because they must pass the health questionnaire only once. Still, if a consumer develops a health condition during the contract’s term, that person would likely be rejected if he or she tried to renew.
Both supporters and critics of short-term plans say consumers who do develop health problems could then sign up for an ACA plan during the next open enrollment because the ACA bars insurers from rejecting people with preexisting conditions.
“We’re going to have two different markets, a Wild West frontier called short-term medical … and a high-risk pool called Obamacare,” said Laszewski.
KHN senior correspondent Phil Galewitz contributed to this article. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Insurers will again be able to sell short-term health insurance good for up to 12 months under a proposed rule released Feb. 20 by the Trump administration that could further roil the marketplace.
“We want to open up affordable alternatives to unaffordable Affordable Care Act policies,” said Health and Human Services Secretary Alex Azar. “This is one step in the direction of providing Americans health insurance options that are more affordable and more suitable to individual and family circumstances.”
The proposed rule said short-term plans could add more choices to the market at lower cost and may offer broader provider networks than Affordable Care Act plans in rural areas.
But most short-term coverage requires answering a string of medical questions, and insurers can reject applicants with preexisting medical problems, which ACA plans cannot do. As a result, the proposed rule also noted that some people who switch to them from ACA coverage may see “reduced access to some services,” and “increased out of pocket costs, possibly leading to financial hardship.”
The directive follows an executive order issued in October to roll back restrictions put in place during the Obama administration that limited these plans to 3 months. The rule comes on the heels of Congress’ approval of tax legislation that in 2019 will end the penalty for people who opt not to carry insurance coverage.
The administration also issued separate regulations Jan. 4 that would make it easier to form “association health plans,” which are offered to small businesses through membership organizations.
Together, the proposed regulations and the elimination of the so-called individual mandate by Congress could further undermine the Affordable Care Act marketplace, critics say.
Seema Verma, who now heads the Centers for Medicare & Medicaid Services, which oversees the marketplaces, told reporters Feb. 20 that federal officials believe that between 100,000 and 200,000 “healthy people” now buying insurance through those federal exchanges would switch to the short-term plans, as well as others who are now uninsured.
The new rule is expected to entice younger and healthier people from the general insurance pool by allowing a range of lower-cost options that don’t include all the benefits required by the federal law – including plans that can reject people with preexisting medical conditions. Most short-term coverage excludes benefits for maternity care, preventive care, mental health services, or substance abuse treatment.
“It’s deeply concerning to me, considering the tragedy in Florida and national opioid crisis, that the administration would be encouraging the sale of policies that don’t have to cover mental health and substance abuse,” said Kevin Lucia, a research professor and project director at Georgetown University’s Health Policy Institute.
Over time, those remaining in ACA plans will increasingly be those who qualify for premium tax credit subsidies and the sick, who can’t get an alternative like a short-term plan, predict Mr. Lucia and other experts. That, in turn, would drive up ACA premiums further.
“If consumers think Obamacare premiums are high today, wait until people flood into these short-term and association health plans,” said industry consultant Robert Laszewski. “The Trump administration will bring rates down substantially for healthy people, but woe unto those who get a condition and have to go back into Obamacare.”
If 100,000-200,000 people shift from ACA-compliant plans in 2019, this would cause “average monthly individual market premiums … to increase,” the proposed rule states. That, in turn, would cause subsidies for eligible policyholders in the ACA market to rise, costing the government $96 million–$168 million.
Supporters said the rules are needed because the ACA plans have already become too costly for people who don’t receive a government subsidy to help them purchase the coverage. “The current system is failing too many,” said Ms. Verma.
And, many supporters don’t think the change is as significant as skeptics fear.
“It simply reverts back to where the short-term plan rules were prior to Obama limiting those plans,” said Christopher Condeluci, a benefits attorney who also served as tax counsel to the U.S. Senate Finance Committee. “While these plans might not be the best answer, people do need a choice, and this new proposal provides needed choice to a certain subsection of the population.”
But, in their call with reporters, CMS officials said the proposed rule seeks comment on whether there are ways to guarantee renewability of the plans, which currently cannot be renewed. Instead, policyholders must reapply and answer medical questions again. The proposal also seeks comments on whether the plans should be allowed for longer than 12-month periods.
The comment period for the proposed rule runs for 60 days. Ms. Verma said CMS hopes to get final rules out “as quickly as possible,” so insurers could start offering the longer duration plans.
Short-term plans had been designed as temporary coverage, lasting for a few months while, for instance, a worker is between jobs and employer-sponsored insurances. They provide some protection to those who enroll, generally paying a percentage of hospital and doctor bills after the policyholder meets a deductible.
They are generally less expensive than ACA plans, because they cover less. For example, they set annual and lifetime caps on benefits, and few cover prescription drugs.
Most require applicants to pass a medical questionnaire – and they can also exclude coverage for preexisting medical conditions.
The plans are appealing to consumers because they are cheaper than Obamacare plans. They are also attractive to brokers, because they often pay higher commissions than ACA plans. Insurers like them because their profit margins are relatively high – and are not held to the ACA requirement that they spend at least 80 percent of premium revenue on plan members’ medical care.
Extending short-term plans to a full year could be a benefit to consumers because they must pass the health questionnaire only once. Still, if a consumer develops a health condition during the contract’s term, that person would likely be rejected if he or she tried to renew.
Both supporters and critics of short-term plans say consumers who do develop health problems could then sign up for an ACA plan during the next open enrollment because the ACA bars insurers from rejecting people with preexisting conditions.
“We’re going to have two different markets, a Wild West frontier called short-term medical … and a high-risk pool called Obamacare,” said Laszewski.
KHN senior correspondent Phil Galewitz contributed to this article. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of the Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Gut-homing protein predicts HIV-acquisition, disease progression in women
Higher frequency of alpha4beta7 expression in the CD4 T cells in the gut was associated with increased HIV acquisition and severity, according to the results of a retrospective comparative analysis of blood samples from patients in the CAPRISA 004 study.
Researchers compared samples from patients who eventually developed HIV with samples from those who did not; they also assessed human study cohorts from Kenya and the RV254/Search 010 cohort in Thailand, according to an online report in Science Translational Medicine. In addition, they obtained data from nonhuman primates (NHPs) challenged with simian immunodeficiency virus (SIV) to compare results between primate species.
They found that alpha4beta7+ CD4+ T cells were depleted very early in HIV infection, particularly in the gut, and the initiation of antiretroviral therapy (ART) was unable to restore the normal levels of those cells even when provided at the earliest time point. Citing the literature, the researchers speculated that interactions between alpha4beta7 and the HIV env protein may assist the virus in locating its ideal target cells, and that high levels of alpha4beta7+ CD4+ T cells were associated with preferential infection by HIV-1 types containing motifs associated with higher alpha4beta7 binding, which are overrepresented in the region where the CAPRISA004 study was conducted.
“Although the association of alpha4beta7 and HIV expression was relatively modest, results were consistent in independent cohorts in two different countries and in NHPs,” according to Aida Sivro, PhD, of the Centre for the AIDS Programme of Research and her colleagues on behalf of the CAPRISA004 and RS254 study groups.
NHP studies showed some promise in this regard because, while ART alone did not lead to immune restoration of these T cells, “ART in combination with anti-alpha4beta7 did so in NHPs,” the researchers concluded, suggesting the possibility of additional therapeutic interventions in humans.
The authors reported having no disclosures. The CAPRISA 004 study was funded by the U.S. National Institutes of Health, U.S. Agency for International Development, and the South African Department of Science and Technology.
SOURCE: Sivro A et al. Sci Transl Med. 2018 Jan 24;10(425):eaam6354.
Higher frequency of alpha4beta7 expression in the CD4 T cells in the gut was associated with increased HIV acquisition and severity, according to the results of a retrospective comparative analysis of blood samples from patients in the CAPRISA 004 study.
Researchers compared samples from patients who eventually developed HIV with samples from those who did not; they also assessed human study cohorts from Kenya and the RV254/Search 010 cohort in Thailand, according to an online report in Science Translational Medicine. In addition, they obtained data from nonhuman primates (NHPs) challenged with simian immunodeficiency virus (SIV) to compare results between primate species.
They found that alpha4beta7+ CD4+ T cells were depleted very early in HIV infection, particularly in the gut, and the initiation of antiretroviral therapy (ART) was unable to restore the normal levels of those cells even when provided at the earliest time point. Citing the literature, the researchers speculated that interactions between alpha4beta7 and the HIV env protein may assist the virus in locating its ideal target cells, and that high levels of alpha4beta7+ CD4+ T cells were associated with preferential infection by HIV-1 types containing motifs associated with higher alpha4beta7 binding, which are overrepresented in the region where the CAPRISA004 study was conducted.
“Although the association of alpha4beta7 and HIV expression was relatively modest, results were consistent in independent cohorts in two different countries and in NHPs,” according to Aida Sivro, PhD, of the Centre for the AIDS Programme of Research and her colleagues on behalf of the CAPRISA004 and RS254 study groups.
NHP studies showed some promise in this regard because, while ART alone did not lead to immune restoration of these T cells, “ART in combination with anti-alpha4beta7 did so in NHPs,” the researchers concluded, suggesting the possibility of additional therapeutic interventions in humans.
The authors reported having no disclosures. The CAPRISA 004 study was funded by the U.S. National Institutes of Health, U.S. Agency for International Development, and the South African Department of Science and Technology.
SOURCE: Sivro A et al. Sci Transl Med. 2018 Jan 24;10(425):eaam6354.
Higher frequency of alpha4beta7 expression in the CD4 T cells in the gut was associated with increased HIV acquisition and severity, according to the results of a retrospective comparative analysis of blood samples from patients in the CAPRISA 004 study.
Researchers compared samples from patients who eventually developed HIV with samples from those who did not; they also assessed human study cohorts from Kenya and the RV254/Search 010 cohort in Thailand, according to an online report in Science Translational Medicine. In addition, they obtained data from nonhuman primates (NHPs) challenged with simian immunodeficiency virus (SIV) to compare results between primate species.
They found that alpha4beta7+ CD4+ T cells were depleted very early in HIV infection, particularly in the gut, and the initiation of antiretroviral therapy (ART) was unable to restore the normal levels of those cells even when provided at the earliest time point. Citing the literature, the researchers speculated that interactions between alpha4beta7 and the HIV env protein may assist the virus in locating its ideal target cells, and that high levels of alpha4beta7+ CD4+ T cells were associated with preferential infection by HIV-1 types containing motifs associated with higher alpha4beta7 binding, which are overrepresented in the region where the CAPRISA004 study was conducted.
“Although the association of alpha4beta7 and HIV expression was relatively modest, results were consistent in independent cohorts in two different countries and in NHPs,” according to Aida Sivro, PhD, of the Centre for the AIDS Programme of Research and her colleagues on behalf of the CAPRISA004 and RS254 study groups.
NHP studies showed some promise in this regard because, while ART alone did not lead to immune restoration of these T cells, “ART in combination with anti-alpha4beta7 did so in NHPs,” the researchers concluded, suggesting the possibility of additional therapeutic interventions in humans.
The authors reported having no disclosures. The CAPRISA 004 study was funded by the U.S. National Institutes of Health, U.S. Agency for International Development, and the South African Department of Science and Technology.
SOURCE: Sivro A et al. Sci Transl Med. 2018 Jan 24;10(425):eaam6354.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: CD4+ T cells expressing the protein were rapidly depleted very early in HIV infection.
Major finding: HIV outcomes and alpha4beta7 expression appear linked.
Study details: Blood samples analyzed from the CAPRISA 004 study comparing HIV-infected patients and controls.
Disclosures: The CAPRISA 004 study was funded by the U.S. National Institutes of Health, U.S. Agency for International Development, and the South African Department of Science and Technology.
Source: Sivro A et al. Sci Transl Med. 2018 Jan 24;10(425):eaam6354.