User login
Extended-interval dosing of natalizumab linked to lower risk of PML
SAN DIEGO – A large industry-funded study of the multiple sclerosis drug natalizumab suggests that physicians can dramatically lower the likelihood of progressive multifocal leukoencephalopathy in patients at higher risk of the condition by increasing the interval between doses.
Previous research has suggested that natalizumab (Tysabri) doesn’t lose efficacy when given less frequently.
Dr. Zhovtis Ryerson presented the study findings at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
As a 2014 report explains, natalizumab is a “highly effective disease-modifying therapy for the treatment of relapsing forms of multiple sclerosis,” but the risk of progressive multifocal leukoencephalopathy (PML) “has largely contributed to it being relegated to a second-line position” (Ther Adv Chronic Dis. 2014 Mar;5[2]:62-8).
Patients previously exposed to John Cunningham (JC) virus are at higher risk of PML, a rare viral brain disease that can be severely disabling or deadly. A 2017 report estimated the combined cumulative PML probability at 2.7% (with previous immunosuppressant use) and 1.7% (no previous immunosuppressant use) over 6 years in patients with signs of exposure to the JC virus (Lancet Neurol. 2017 Nov;16[11]:925-33). According to Dr. Zhovtis Ryerson, natalizumab manufacturer Biogen reported in December 2017 that it has confirmed 756 cases of PML in natalizumab-treated patients, all except for 3 in MS patients.
Doctors are wary in the at-risk patient population. “In general,” she said, “clinicians treating patients who are JC virus positive, and therefore have a risk for PML, may not utilize the drug or may take the patient off it after 2 years, which is when the risk goes up.”
Some physicians have experimented with longer intervals between treatments of 300 mg given intravenously, administering it every 5-8 weeks instead of every 4 weeks, with an eye on not extending the interval for too long “because MS disease activity returns after 12 weeks of withholding therapy,” Dr. Zhovtis Ryerson said.
In 2016, Dr. Zhovtis Ryerson and colleagues reported in a retrospective analysis that natalizumab dosing intervals of up to 8 weeks, 5 days didn’t affect the drug’s efficacy (J Neurol Neurosurg Psychiatry. 2016 Aug;87[8]:885-9).
For the primary analyses in the new study – whether dosing history in the last 18 months of natalizumab treatment affects PML – researchers tracked 1,988 patients who took the drug via an extended interval schedule and 13,132 who took it via a standard interval. The patients were tracked in the mandated TOUCH Prescribing Program; all participants showed signs of exposure to the JC virus.
The two groups were similar at about 68% female, an average age at first infusion of about 43, and a median number of about 48 natalizumab infusions.
For patients without previous immunosuppressant use who had received 49-60 doses, the researchers estimated the incidence of PML per 1,000 patients as 1.23 in the extended-interval group and 3.96 in the standard-interval group. The numbers were slightly higher for those who received 61-72 doses. At 48 or fewer doses, there were no PML cases in patients on extended-interval dosing.
“The data showed highly significant risk reductions of up to 94%,” Dr. Zhovtis Ryerson said.
Moving forward, “in collaboration with Biogen, we have more sensitivity analysis to be done to assure that our conclusions on this are correct,” she said. “Furthermore, more evidence of efficacy of extended-interval dosing natalizumab is needed, and we hope to move forward with a prospective, randomized, controlled trial to give clinicians the highest level of evidence we can.”
The study was funded by Biogen. Dr. Zhovtis Ryerson reported personal compensation from Teva, speaker/advisory board activities for Biogen, and research support from Biogen. Seven other authors reported being Biogen employees. Other authors reported no disclosures or various disclosures, including connections to Biogen.
SOURCE: Zhovtis R et al. ACTRIMS Forum 2018 Abstract LB250.
SAN DIEGO – A large industry-funded study of the multiple sclerosis drug natalizumab suggests that physicians can dramatically lower the likelihood of progressive multifocal leukoencephalopathy in patients at higher risk of the condition by increasing the interval between doses.
Previous research has suggested that natalizumab (Tysabri) doesn’t lose efficacy when given less frequently.
Dr. Zhovtis Ryerson presented the study findings at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
As a 2014 report explains, natalizumab is a “highly effective disease-modifying therapy for the treatment of relapsing forms of multiple sclerosis,” but the risk of progressive multifocal leukoencephalopathy (PML) “has largely contributed to it being relegated to a second-line position” (Ther Adv Chronic Dis. 2014 Mar;5[2]:62-8).
Patients previously exposed to John Cunningham (JC) virus are at higher risk of PML, a rare viral brain disease that can be severely disabling or deadly. A 2017 report estimated the combined cumulative PML probability at 2.7% (with previous immunosuppressant use) and 1.7% (no previous immunosuppressant use) over 6 years in patients with signs of exposure to the JC virus (Lancet Neurol. 2017 Nov;16[11]:925-33). According to Dr. Zhovtis Ryerson, natalizumab manufacturer Biogen reported in December 2017 that it has confirmed 756 cases of PML in natalizumab-treated patients, all except for 3 in MS patients.
Doctors are wary in the at-risk patient population. “In general,” she said, “clinicians treating patients who are JC virus positive, and therefore have a risk for PML, may not utilize the drug or may take the patient off it after 2 years, which is when the risk goes up.”
Some physicians have experimented with longer intervals between treatments of 300 mg given intravenously, administering it every 5-8 weeks instead of every 4 weeks, with an eye on not extending the interval for too long “because MS disease activity returns after 12 weeks of withholding therapy,” Dr. Zhovtis Ryerson said.
In 2016, Dr. Zhovtis Ryerson and colleagues reported in a retrospective analysis that natalizumab dosing intervals of up to 8 weeks, 5 days didn’t affect the drug’s efficacy (J Neurol Neurosurg Psychiatry. 2016 Aug;87[8]:885-9).
For the primary analyses in the new study – whether dosing history in the last 18 months of natalizumab treatment affects PML – researchers tracked 1,988 patients who took the drug via an extended interval schedule and 13,132 who took it via a standard interval. The patients were tracked in the mandated TOUCH Prescribing Program; all participants showed signs of exposure to the JC virus.
The two groups were similar at about 68% female, an average age at first infusion of about 43, and a median number of about 48 natalizumab infusions.
For patients without previous immunosuppressant use who had received 49-60 doses, the researchers estimated the incidence of PML per 1,000 patients as 1.23 in the extended-interval group and 3.96 in the standard-interval group. The numbers were slightly higher for those who received 61-72 doses. At 48 or fewer doses, there were no PML cases in patients on extended-interval dosing.
“The data showed highly significant risk reductions of up to 94%,” Dr. Zhovtis Ryerson said.
Moving forward, “in collaboration with Biogen, we have more sensitivity analysis to be done to assure that our conclusions on this are correct,” she said. “Furthermore, more evidence of efficacy of extended-interval dosing natalizumab is needed, and we hope to move forward with a prospective, randomized, controlled trial to give clinicians the highest level of evidence we can.”
The study was funded by Biogen. Dr. Zhovtis Ryerson reported personal compensation from Teva, speaker/advisory board activities for Biogen, and research support from Biogen. Seven other authors reported being Biogen employees. Other authors reported no disclosures or various disclosures, including connections to Biogen.
SOURCE: Zhovtis R et al. ACTRIMS Forum 2018 Abstract LB250.
SAN DIEGO – A large industry-funded study of the multiple sclerosis drug natalizumab suggests that physicians can dramatically lower the likelihood of progressive multifocal leukoencephalopathy in patients at higher risk of the condition by increasing the interval between doses.
Previous research has suggested that natalizumab (Tysabri) doesn’t lose efficacy when given less frequently.
Dr. Zhovtis Ryerson presented the study findings at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
As a 2014 report explains, natalizumab is a “highly effective disease-modifying therapy for the treatment of relapsing forms of multiple sclerosis,” but the risk of progressive multifocal leukoencephalopathy (PML) “has largely contributed to it being relegated to a second-line position” (Ther Adv Chronic Dis. 2014 Mar;5[2]:62-8).
Patients previously exposed to John Cunningham (JC) virus are at higher risk of PML, a rare viral brain disease that can be severely disabling or deadly. A 2017 report estimated the combined cumulative PML probability at 2.7% (with previous immunosuppressant use) and 1.7% (no previous immunosuppressant use) over 6 years in patients with signs of exposure to the JC virus (Lancet Neurol. 2017 Nov;16[11]:925-33). According to Dr. Zhovtis Ryerson, natalizumab manufacturer Biogen reported in December 2017 that it has confirmed 756 cases of PML in natalizumab-treated patients, all except for 3 in MS patients.
Doctors are wary in the at-risk patient population. “In general,” she said, “clinicians treating patients who are JC virus positive, and therefore have a risk for PML, may not utilize the drug or may take the patient off it after 2 years, which is when the risk goes up.”
Some physicians have experimented with longer intervals between treatments of 300 mg given intravenously, administering it every 5-8 weeks instead of every 4 weeks, with an eye on not extending the interval for too long “because MS disease activity returns after 12 weeks of withholding therapy,” Dr. Zhovtis Ryerson said.
In 2016, Dr. Zhovtis Ryerson and colleagues reported in a retrospective analysis that natalizumab dosing intervals of up to 8 weeks, 5 days didn’t affect the drug’s efficacy (J Neurol Neurosurg Psychiatry. 2016 Aug;87[8]:885-9).
For the primary analyses in the new study – whether dosing history in the last 18 months of natalizumab treatment affects PML – researchers tracked 1,988 patients who took the drug via an extended interval schedule and 13,132 who took it via a standard interval. The patients were tracked in the mandated TOUCH Prescribing Program; all participants showed signs of exposure to the JC virus.
The two groups were similar at about 68% female, an average age at first infusion of about 43, and a median number of about 48 natalizumab infusions.
For patients without previous immunosuppressant use who had received 49-60 doses, the researchers estimated the incidence of PML per 1,000 patients as 1.23 in the extended-interval group and 3.96 in the standard-interval group. The numbers were slightly higher for those who received 61-72 doses. At 48 or fewer doses, there were no PML cases in patients on extended-interval dosing.
“The data showed highly significant risk reductions of up to 94%,” Dr. Zhovtis Ryerson said.
Moving forward, “in collaboration with Biogen, we have more sensitivity analysis to be done to assure that our conclusions on this are correct,” she said. “Furthermore, more evidence of efficacy of extended-interval dosing natalizumab is needed, and we hope to move forward with a prospective, randomized, controlled trial to give clinicians the highest level of evidence we can.”
The study was funded by Biogen. Dr. Zhovtis Ryerson reported personal compensation from Teva, speaker/advisory board activities for Biogen, and research support from Biogen. Seven other authors reported being Biogen employees. Other authors reported no disclosures or various disclosures, including connections to Biogen.
SOURCE: Zhovtis R et al. ACTRIMS Forum 2018 Abstract LB250.
REPORTING FROM ACTRIMS FORUM 2018
Key clinical point: , compared with standard-interval dosing in patients who showed signs of exposure to the JC virus.
Major finding: Estimated incidence of PML per 1,000 patients was 1.23 in an extended-interval group and 3.96 in a standard-interval group among those who had received 49-60 natalizumab doses.
Study details: 18-month analysis of multiple sclerosis patients on natalizumab who showed signs of exposure to the JC virus: 1,988 on extended-interval dosing and 13,132 on standard-interval dosing.
Disclosures: The study was funded by Biogen, and multiple study authors report being employees of Biogen or having other relationships to the company.
Source: Zhovtis R et al. ACTRIMS Forum 2018, Abstract LB250.
‘Remarkable’ survival seen with pretransplant JAK inhibitor
SALT LAKE CITY – The use of Janus kinase (JAK) inhibitor therapy prior to hematopoietic stem cell transplantation is safe and may improve posttransplant survival in patients with myelofibrosis, according to findings from an ongoing prospective phase 2 study.
The 1-year overall survival rate among the 28 initial patients in the single-center study of JAK inhibitor therapy followed by myeloablative or reduced-intensity hematopoietic cell transplantation (HCT) was 93%. The 2-year survival rate was 89%, compared with 54% in a closely matched historical cohort of intermediate-2–risk patients who did not receive pre-HCT JAK inhibitor therapy, Rachel B. Salit, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“So this is pretty exciting for us,” she added, explaining that allogeneic HCT, which is currently indicated only for patients with intermediate-2– or high-risk disease on the Dynamic International Prognostic Scoring System (DIPSS), remains the only curative treatment option for patients with myelofibrosis.
“Transplant outcomes have been associated with DIPSS and DIPSS+ risk scores,” she said, adding that a study demonstrating that association showed a 54% survival rate at 3 years in patients with intermediate-2–risk disease. “Then JAK-2 inhibitors came down the pipe, and they are also indicated for patients with DIPSS intermediate-2– and high-risk disease. They’ve been shown to decrease spleen size, increase quality of life scores, decrease cytokine levels, and improve constitutional symptoms.”
Posttransplant benefits of pre-HCT JAK inhibitor therapy could include improved graft function because of decreased spleen size and cytokines, potentially decreased severe graft-versus-host disease (GVHD), and decreased nonrelapse mortality.
“Our hypothesis was that the use of JAK inhibitors before transplant in patients with myelofibrosis – by decreasing inflammation, improving constitutional symptoms, and reducing splenomegaly – would result in a decreased DIPSS score” and improved posttransplant survival, Dr. Salit said.
Study participants were patients aged 18 years and older (median of 53 years) with primary or secondary myelofibrosis (14 each). About two-thirds of the study participants were men. Prior to JAK inhibitor therapy, 2 patients were low risk, 10 patients were intermediate-1 risk, and 16 patients were intermediate-2 risk according to the DIPSS.
They were given ruxolitinib (Jakafi) for at least 8 weeks prior to HCT (median of 7 months, but up to 3 years), and the treatment was tapered over 1-2 weeks through day 2 or 3 of conditioning chemotherapy, depending on the conditioning regimen. Conditioning regimens were determined by the physician based on donor type.
“After the ruxolitinib, we had 6 patients who were intermediate-1 and 21 who were intermediate-2, so in that way they failed to meet that hypothesis,” Dr. Salit said. This happened because the patients became anemic, so they gained points on their DIPSS scores for declining constitutional symptoms.
Of the 28 participants, 23 received myeloablative HCT, and 5 received reduced-intensity HCT.
“We had a mixture of related, unrelated, and cord blood recipients,” she noted.
At a median follow-up of just over 1 year, and at up to 3 years in some patients, no cases of cytokine release syndrome have occurred with the overlap of ruxolitinib and conditioning chemotherapy, and there have been no graft failures. All patients, including cord blood recipients, engrafted. The median time to engraftment was 19 days, but the range went as high as 35 days. “The 35 was one of our cord blood recipients,” she noted.
Median time to platelet engraftment was 20 days, and median CD3 and CD33 chimerism at day 80 were 88% and 100%, respectively.
Acute grades II-IV GVHD occurred in 70% of patients, but just 15% of these cases were grades III or IV, she said.
Chronic GVHD occurred in 35% of patients, including two severe cases. Two patients relapsed, including one at 6 months with marrow blast and one at 2 years with myeloid sarcoma. Two treatment-related deaths occurred, including one each at days 54 and 81.
The strategy of overlapping a JAK inhibitor with conditioning chemotherapy was safe, and “better in that we haven’t seen any cytokine release syndrome,” she concluded, adding that no graft failures occurred and grades III-IV acute and severe chronic GVHD were encouragingly infrequent.
Future studies should look at the optimal amount of time for JAK inhibitor therapy prior to transplant and whether it’s safe to continue JAK inhibitors through transplant, she said.
“Some of the work in Germany has shown that JAK inhibitors are safe to include up to day 28 through transplant, and that’s something that we’re looking to explore,” she noted.
Dr. Salit reported having no financial disclosures. This study was sponsored by the Fred Hutchinson Cancer Research Center.
SOURCE: Salit R et al. BMT Tandem Meetings, Abstract 17.
SALT LAKE CITY – The use of Janus kinase (JAK) inhibitor therapy prior to hematopoietic stem cell transplantation is safe and may improve posttransplant survival in patients with myelofibrosis, according to findings from an ongoing prospective phase 2 study.
The 1-year overall survival rate among the 28 initial patients in the single-center study of JAK inhibitor therapy followed by myeloablative or reduced-intensity hematopoietic cell transplantation (HCT) was 93%. The 2-year survival rate was 89%, compared with 54% in a closely matched historical cohort of intermediate-2–risk patients who did not receive pre-HCT JAK inhibitor therapy, Rachel B. Salit, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“So this is pretty exciting for us,” she added, explaining that allogeneic HCT, which is currently indicated only for patients with intermediate-2– or high-risk disease on the Dynamic International Prognostic Scoring System (DIPSS), remains the only curative treatment option for patients with myelofibrosis.
“Transplant outcomes have been associated with DIPSS and DIPSS+ risk scores,” she said, adding that a study demonstrating that association showed a 54% survival rate at 3 years in patients with intermediate-2–risk disease. “Then JAK-2 inhibitors came down the pipe, and they are also indicated for patients with DIPSS intermediate-2– and high-risk disease. They’ve been shown to decrease spleen size, increase quality of life scores, decrease cytokine levels, and improve constitutional symptoms.”
Posttransplant benefits of pre-HCT JAK inhibitor therapy could include improved graft function because of decreased spleen size and cytokines, potentially decreased severe graft-versus-host disease (GVHD), and decreased nonrelapse mortality.
“Our hypothesis was that the use of JAK inhibitors before transplant in patients with myelofibrosis – by decreasing inflammation, improving constitutional symptoms, and reducing splenomegaly – would result in a decreased DIPSS score” and improved posttransplant survival, Dr. Salit said.
Study participants were patients aged 18 years and older (median of 53 years) with primary or secondary myelofibrosis (14 each). About two-thirds of the study participants were men. Prior to JAK inhibitor therapy, 2 patients were low risk, 10 patients were intermediate-1 risk, and 16 patients were intermediate-2 risk according to the DIPSS.
They were given ruxolitinib (Jakafi) for at least 8 weeks prior to HCT (median of 7 months, but up to 3 years), and the treatment was tapered over 1-2 weeks through day 2 or 3 of conditioning chemotherapy, depending on the conditioning regimen. Conditioning regimens were determined by the physician based on donor type.
“After the ruxolitinib, we had 6 patients who were intermediate-1 and 21 who were intermediate-2, so in that way they failed to meet that hypothesis,” Dr. Salit said. This happened because the patients became anemic, so they gained points on their DIPSS scores for declining constitutional symptoms.
Of the 28 participants, 23 received myeloablative HCT, and 5 received reduced-intensity HCT.
“We had a mixture of related, unrelated, and cord blood recipients,” she noted.
At a median follow-up of just over 1 year, and at up to 3 years in some patients, no cases of cytokine release syndrome have occurred with the overlap of ruxolitinib and conditioning chemotherapy, and there have been no graft failures. All patients, including cord blood recipients, engrafted. The median time to engraftment was 19 days, but the range went as high as 35 days. “The 35 was one of our cord blood recipients,” she noted.
Median time to platelet engraftment was 20 days, and median CD3 and CD33 chimerism at day 80 were 88% and 100%, respectively.
Acute grades II-IV GVHD occurred in 70% of patients, but just 15% of these cases were grades III or IV, she said.
Chronic GVHD occurred in 35% of patients, including two severe cases. Two patients relapsed, including one at 6 months with marrow blast and one at 2 years with myeloid sarcoma. Two treatment-related deaths occurred, including one each at days 54 and 81.
The strategy of overlapping a JAK inhibitor with conditioning chemotherapy was safe, and “better in that we haven’t seen any cytokine release syndrome,” she concluded, adding that no graft failures occurred and grades III-IV acute and severe chronic GVHD were encouragingly infrequent.
Future studies should look at the optimal amount of time for JAK inhibitor therapy prior to transplant and whether it’s safe to continue JAK inhibitors through transplant, she said.
“Some of the work in Germany has shown that JAK inhibitors are safe to include up to day 28 through transplant, and that’s something that we’re looking to explore,” she noted.
Dr. Salit reported having no financial disclosures. This study was sponsored by the Fred Hutchinson Cancer Research Center.
SOURCE: Salit R et al. BMT Tandem Meetings, Abstract 17.
SALT LAKE CITY – The use of Janus kinase (JAK) inhibitor therapy prior to hematopoietic stem cell transplantation is safe and may improve posttransplant survival in patients with myelofibrosis, according to findings from an ongoing prospective phase 2 study.
The 1-year overall survival rate among the 28 initial patients in the single-center study of JAK inhibitor therapy followed by myeloablative or reduced-intensity hematopoietic cell transplantation (HCT) was 93%. The 2-year survival rate was 89%, compared with 54% in a closely matched historical cohort of intermediate-2–risk patients who did not receive pre-HCT JAK inhibitor therapy, Rachel B. Salit, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
“So this is pretty exciting for us,” she added, explaining that allogeneic HCT, which is currently indicated only for patients with intermediate-2– or high-risk disease on the Dynamic International Prognostic Scoring System (DIPSS), remains the only curative treatment option for patients with myelofibrosis.
“Transplant outcomes have been associated with DIPSS and DIPSS+ risk scores,” she said, adding that a study demonstrating that association showed a 54% survival rate at 3 years in patients with intermediate-2–risk disease. “Then JAK-2 inhibitors came down the pipe, and they are also indicated for patients with DIPSS intermediate-2– and high-risk disease. They’ve been shown to decrease spleen size, increase quality of life scores, decrease cytokine levels, and improve constitutional symptoms.”
Posttransplant benefits of pre-HCT JAK inhibitor therapy could include improved graft function because of decreased spleen size and cytokines, potentially decreased severe graft-versus-host disease (GVHD), and decreased nonrelapse mortality.
“Our hypothesis was that the use of JAK inhibitors before transplant in patients with myelofibrosis – by decreasing inflammation, improving constitutional symptoms, and reducing splenomegaly – would result in a decreased DIPSS score” and improved posttransplant survival, Dr. Salit said.
Study participants were patients aged 18 years and older (median of 53 years) with primary or secondary myelofibrosis (14 each). About two-thirds of the study participants were men. Prior to JAK inhibitor therapy, 2 patients were low risk, 10 patients were intermediate-1 risk, and 16 patients were intermediate-2 risk according to the DIPSS.
They were given ruxolitinib (Jakafi) for at least 8 weeks prior to HCT (median of 7 months, but up to 3 years), and the treatment was tapered over 1-2 weeks through day 2 or 3 of conditioning chemotherapy, depending on the conditioning regimen. Conditioning regimens were determined by the physician based on donor type.
“After the ruxolitinib, we had 6 patients who were intermediate-1 and 21 who were intermediate-2, so in that way they failed to meet that hypothesis,” Dr. Salit said. This happened because the patients became anemic, so they gained points on their DIPSS scores for declining constitutional symptoms.
Of the 28 participants, 23 received myeloablative HCT, and 5 received reduced-intensity HCT.
“We had a mixture of related, unrelated, and cord blood recipients,” she noted.
At a median follow-up of just over 1 year, and at up to 3 years in some patients, no cases of cytokine release syndrome have occurred with the overlap of ruxolitinib and conditioning chemotherapy, and there have been no graft failures. All patients, including cord blood recipients, engrafted. The median time to engraftment was 19 days, but the range went as high as 35 days. “The 35 was one of our cord blood recipients,” she noted.
Median time to platelet engraftment was 20 days, and median CD3 and CD33 chimerism at day 80 were 88% and 100%, respectively.
Acute grades II-IV GVHD occurred in 70% of patients, but just 15% of these cases were grades III or IV, she said.
Chronic GVHD occurred in 35% of patients, including two severe cases. Two patients relapsed, including one at 6 months with marrow blast and one at 2 years with myeloid sarcoma. Two treatment-related deaths occurred, including one each at days 54 and 81.
The strategy of overlapping a JAK inhibitor with conditioning chemotherapy was safe, and “better in that we haven’t seen any cytokine release syndrome,” she concluded, adding that no graft failures occurred and grades III-IV acute and severe chronic GVHD were encouragingly infrequent.
Future studies should look at the optimal amount of time for JAK inhibitor therapy prior to transplant and whether it’s safe to continue JAK inhibitors through transplant, she said.
“Some of the work in Germany has shown that JAK inhibitors are safe to include up to day 28 through transplant, and that’s something that we’re looking to explore,” she noted.
Dr. Salit reported having no financial disclosures. This study was sponsored by the Fred Hutchinson Cancer Research Center.
SOURCE: Salit R et al. BMT Tandem Meetings, Abstract 17.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: The 2-year overall survival rate was 89%.
Study details: Findings in 28 patients from an ongoing prospective phase 2 study.
Disclosures: Dr. Salit reported having no financial disclosures. The study was sponsored by the Fred Hutchinson Cancer Research Center in Seattle.
Source: Salit R et al. BMT Tandem Meetings, Abstract 17.
Poor sleep, suicide risk linked in college students
Suicidal behaviors are associated with poor sleep in college students, even after controlling for depression, a cross-sectional analysis shows. Specifically, students were 6.54 times as likely to be classified with suicide risk if they were depressed and 2.70 times as likely if they had poor quality of sleep.
“Our findings add to a growing body of literature pointing to sleep as an important component to include in screening and intervention efforts to prevent suicidal ideation and attempts on college campuses,” wrote Stephen P. Becker, PhD, a pediatric psychologist affiliated with Cincinnati Children’s Hospital Medical Center, and his colleagues.
Of the 1,700 college students included in this study (J Psychiatr Res. 2018 Jan 12:99:122-8), 82.7% of those classified with suicide risk had poor sleep quality, but only 31.3% of those with poor sleep quality were classified with suicide risk.
Most previous studies had looked only at insomnia or bad dreams rather than other aspects of poor sleep quality, and because this study used the Pittsburgh Sleep Quality Index, it was able to evaluate additional well-validated components of sleep. so that screening and intervention based on sleep quality can be more effective. This is important, because suicide is one of the leading causes of death among young adults.
The researchers declared no conflicts of interest.
Read more about this study in the Journal of Psychiatric Research.
Suicidal behaviors are associated with poor sleep in college students, even after controlling for depression, a cross-sectional analysis shows. Specifically, students were 6.54 times as likely to be classified with suicide risk if they were depressed and 2.70 times as likely if they had poor quality of sleep.
“Our findings add to a growing body of literature pointing to sleep as an important component to include in screening and intervention efforts to prevent suicidal ideation and attempts on college campuses,” wrote Stephen P. Becker, PhD, a pediatric psychologist affiliated with Cincinnati Children’s Hospital Medical Center, and his colleagues.
Of the 1,700 college students included in this study (J Psychiatr Res. 2018 Jan 12:99:122-8), 82.7% of those classified with suicide risk had poor sleep quality, but only 31.3% of those with poor sleep quality were classified with suicide risk.
Most previous studies had looked only at insomnia or bad dreams rather than other aspects of poor sleep quality, and because this study used the Pittsburgh Sleep Quality Index, it was able to evaluate additional well-validated components of sleep. so that screening and intervention based on sleep quality can be more effective. This is important, because suicide is one of the leading causes of death among young adults.
The researchers declared no conflicts of interest.
Read more about this study in the Journal of Psychiatric Research.
Suicidal behaviors are associated with poor sleep in college students, even after controlling for depression, a cross-sectional analysis shows. Specifically, students were 6.54 times as likely to be classified with suicide risk if they were depressed and 2.70 times as likely if they had poor quality of sleep.
“Our findings add to a growing body of literature pointing to sleep as an important component to include in screening and intervention efforts to prevent suicidal ideation and attempts on college campuses,” wrote Stephen P. Becker, PhD, a pediatric psychologist affiliated with Cincinnati Children’s Hospital Medical Center, and his colleagues.
Of the 1,700 college students included in this study (J Psychiatr Res. 2018 Jan 12:99:122-8), 82.7% of those classified with suicide risk had poor sleep quality, but only 31.3% of those with poor sleep quality were classified with suicide risk.
Most previous studies had looked only at insomnia or bad dreams rather than other aspects of poor sleep quality, and because this study used the Pittsburgh Sleep Quality Index, it was able to evaluate additional well-validated components of sleep. so that screening and intervention based on sleep quality can be more effective. This is important, because suicide is one of the leading causes of death among young adults.
The researchers declared no conflicts of interest.
Read more about this study in the Journal of Psychiatric Research.
FROM THE JOURNAL OF PSYCHIATRIC RESEARCH
Will Indiana Medicaid work requirements pass legal muster?
The recent federal waiver allowing work requirements for Indiana’s Medicaid program has sparked controversy from all sides and led to a debate that analysts say is likely to end in court.
Indiana’s waiver, green-lighted by the U.S. Department of Health & Human Services in February, requires certain Medicaid recipients to work an average of 20 hours a week, be enrolled in school, or participate in a job search/training program in order to qualify for assistance. Proponents say the requirements are a practical approach to improving self-sufficiency for able-bodied Medicaid patients by incentivizing work and community engagement. Critics argue the requirements are burdensome for the state’s most vulnerable populations and jeopardize medical care for some 30,000 recipients. But will the requirements pass legal muster in a court of law?
Typically, when arguments arise over state Medicaid program provisions, they center on whether the requirements help or hinder the program, Mr. Salo said in an interview.
“There isn’t really a clear-cut, black-and-white definition of the purposes of Medicaid written down in the statute,” he said. “Practically speaking, it’s a fairly subjective term. Clearly, the Obama administration said, ‘We don’t think [work requirement] policies further the objectives of the Medicaid program.’ I don’t think there’s anything that would preclude a new administration from saying, ‘We have a slightly different opinion of what the purposes of the Medicaid program are.’ ”
However, Leonardo Cuello, director of health policy for the National Health Law Program, says Indiana’s new requirements are illegal and clearly run contrary to the purpose of the Medicaid program. While the program’s work requirements are taking the spotlight, he notes that the new provisions also include lockout periods if paperwork is late and removes transportation assistance for medical care.
The National Health Law Program is a coplaintiff in a lawsuit challenging similar Medicaid requirements in Kentucky. Like Indiana’s waiver, Kentucky’s approved plan includes lockout provisions for failing to pay premiums on time and has work requirements for able-bodied adult enrollees. Mr. Cuello said his organization is looking closely at taking legal action against Indiana’s waiver as well.
In a statement, HHS Secretary Alex Azar said the approval of Indiana’s expanded Medicaid program is in line with a Trump administration policy to support state efforts to “improve Medicaid enrollee health outcomes and promote independence by incentivizing community engagement among able-bodied, working-age Medicaid beneficiaries.” The policy responds to numerous state requests to test programs through Medicaid demonstration projects under which work and other types of community engagement would be a condition of Medicaid coverage, according to the announcement.
“We look forward to collaborating with Indiana on this next evolution of [the Healthy Indiana Plan], which serves as another example of the Trump administration’s support of state-led efforts and innovative reforms to make our HHS programs really work for Americans,” Mr. Azar said in the statement.
Another legal question that may arise against Indiana’s work requirements for Medicaid is whether the provisions are part of a true “experiment.”
“These waivers are at the broad discretion of the government,” Mr. Blumstein said in an interview. “I think [Indiana’s plan] is compatible with an overall welfare strategy that’s designed to cut costs, to get folks jobs, and then ultimately, into private sector health insurance. In principal, these things are good to try.”
Mr. Blumstein said that he believes HHS has a strong legal position in defending Indiana’s waiver program, but that doesn’t mean a judge will agree, he said.
“Will a court somewhere find against the government? Against Indiana? I can’t say,” he said. “There’s a lot of hostility to change, and some judges share that hostility to change. But based on my TennCare experience, courts should bend over backward to give flexibility to the states to experiment in this way, if they’re approved by the federal government.”
Mr. Cuello of the National Health Law Program said the Indiana and Kentucky waivers both fail to demonstrate any experimental value. Studies show that when such requirements are enforced, patients drop out of Medicaid or forgo medical care.
“These waivers fail a legality test because they’re not part of any experiment,” he said. “An experiment would be: Here’s an innovative way we have for how to provide health care. These are not experiments.”
At press time, eight states had pending waiver requests at the Centers for Medicare & Medicaid Services that would require work as a condition of eligibility for expansion adults and/or traditional populations.
The recent federal waiver allowing work requirements for Indiana’s Medicaid program has sparked controversy from all sides and led to a debate that analysts say is likely to end in court.
Indiana’s waiver, green-lighted by the U.S. Department of Health & Human Services in February, requires certain Medicaid recipients to work an average of 20 hours a week, be enrolled in school, or participate in a job search/training program in order to qualify for assistance. Proponents say the requirements are a practical approach to improving self-sufficiency for able-bodied Medicaid patients by incentivizing work and community engagement. Critics argue the requirements are burdensome for the state’s most vulnerable populations and jeopardize medical care for some 30,000 recipients. But will the requirements pass legal muster in a court of law?
Typically, when arguments arise over state Medicaid program provisions, they center on whether the requirements help or hinder the program, Mr. Salo said in an interview.
“There isn’t really a clear-cut, black-and-white definition of the purposes of Medicaid written down in the statute,” he said. “Practically speaking, it’s a fairly subjective term. Clearly, the Obama administration said, ‘We don’t think [work requirement] policies further the objectives of the Medicaid program.’ I don’t think there’s anything that would preclude a new administration from saying, ‘We have a slightly different opinion of what the purposes of the Medicaid program are.’ ”
However, Leonardo Cuello, director of health policy for the National Health Law Program, says Indiana’s new requirements are illegal and clearly run contrary to the purpose of the Medicaid program. While the program’s work requirements are taking the spotlight, he notes that the new provisions also include lockout periods if paperwork is late and removes transportation assistance for medical care.
The National Health Law Program is a coplaintiff in a lawsuit challenging similar Medicaid requirements in Kentucky. Like Indiana’s waiver, Kentucky’s approved plan includes lockout provisions for failing to pay premiums on time and has work requirements for able-bodied adult enrollees. Mr. Cuello said his organization is looking closely at taking legal action against Indiana’s waiver as well.
In a statement, HHS Secretary Alex Azar said the approval of Indiana’s expanded Medicaid program is in line with a Trump administration policy to support state efforts to “improve Medicaid enrollee health outcomes and promote independence by incentivizing community engagement among able-bodied, working-age Medicaid beneficiaries.” The policy responds to numerous state requests to test programs through Medicaid demonstration projects under which work and other types of community engagement would be a condition of Medicaid coverage, according to the announcement.
“We look forward to collaborating with Indiana on this next evolution of [the Healthy Indiana Plan], which serves as another example of the Trump administration’s support of state-led efforts and innovative reforms to make our HHS programs really work for Americans,” Mr. Azar said in the statement.
Another legal question that may arise against Indiana’s work requirements for Medicaid is whether the provisions are part of a true “experiment.”
“These waivers are at the broad discretion of the government,” Mr. Blumstein said in an interview. “I think [Indiana’s plan] is compatible with an overall welfare strategy that’s designed to cut costs, to get folks jobs, and then ultimately, into private sector health insurance. In principal, these things are good to try.”
Mr. Blumstein said that he believes HHS has a strong legal position in defending Indiana’s waiver program, but that doesn’t mean a judge will agree, he said.
“Will a court somewhere find against the government? Against Indiana? I can’t say,” he said. “There’s a lot of hostility to change, and some judges share that hostility to change. But based on my TennCare experience, courts should bend over backward to give flexibility to the states to experiment in this way, if they’re approved by the federal government.”
Mr. Cuello of the National Health Law Program said the Indiana and Kentucky waivers both fail to demonstrate any experimental value. Studies show that when such requirements are enforced, patients drop out of Medicaid or forgo medical care.
“These waivers fail a legality test because they’re not part of any experiment,” he said. “An experiment would be: Here’s an innovative way we have for how to provide health care. These are not experiments.”
At press time, eight states had pending waiver requests at the Centers for Medicare & Medicaid Services that would require work as a condition of eligibility for expansion adults and/or traditional populations.
The recent federal waiver allowing work requirements for Indiana’s Medicaid program has sparked controversy from all sides and led to a debate that analysts say is likely to end in court.
Indiana’s waiver, green-lighted by the U.S. Department of Health & Human Services in February, requires certain Medicaid recipients to work an average of 20 hours a week, be enrolled in school, or participate in a job search/training program in order to qualify for assistance. Proponents say the requirements are a practical approach to improving self-sufficiency for able-bodied Medicaid patients by incentivizing work and community engagement. Critics argue the requirements are burdensome for the state’s most vulnerable populations and jeopardize medical care for some 30,000 recipients. But will the requirements pass legal muster in a court of law?
Typically, when arguments arise over state Medicaid program provisions, they center on whether the requirements help or hinder the program, Mr. Salo said in an interview.
“There isn’t really a clear-cut, black-and-white definition of the purposes of Medicaid written down in the statute,” he said. “Practically speaking, it’s a fairly subjective term. Clearly, the Obama administration said, ‘We don’t think [work requirement] policies further the objectives of the Medicaid program.’ I don’t think there’s anything that would preclude a new administration from saying, ‘We have a slightly different opinion of what the purposes of the Medicaid program are.’ ”
However, Leonardo Cuello, director of health policy for the National Health Law Program, says Indiana’s new requirements are illegal and clearly run contrary to the purpose of the Medicaid program. While the program’s work requirements are taking the spotlight, he notes that the new provisions also include lockout periods if paperwork is late and removes transportation assistance for medical care.
The National Health Law Program is a coplaintiff in a lawsuit challenging similar Medicaid requirements in Kentucky. Like Indiana’s waiver, Kentucky’s approved plan includes lockout provisions for failing to pay premiums on time and has work requirements for able-bodied adult enrollees. Mr. Cuello said his organization is looking closely at taking legal action against Indiana’s waiver as well.
In a statement, HHS Secretary Alex Azar said the approval of Indiana’s expanded Medicaid program is in line with a Trump administration policy to support state efforts to “improve Medicaid enrollee health outcomes and promote independence by incentivizing community engagement among able-bodied, working-age Medicaid beneficiaries.” The policy responds to numerous state requests to test programs through Medicaid demonstration projects under which work and other types of community engagement would be a condition of Medicaid coverage, according to the announcement.
“We look forward to collaborating with Indiana on this next evolution of [the Healthy Indiana Plan], which serves as another example of the Trump administration’s support of state-led efforts and innovative reforms to make our HHS programs really work for Americans,” Mr. Azar said in the statement.
Another legal question that may arise against Indiana’s work requirements for Medicaid is whether the provisions are part of a true “experiment.”
“These waivers are at the broad discretion of the government,” Mr. Blumstein said in an interview. “I think [Indiana’s plan] is compatible with an overall welfare strategy that’s designed to cut costs, to get folks jobs, and then ultimately, into private sector health insurance. In principal, these things are good to try.”
Mr. Blumstein said that he believes HHS has a strong legal position in defending Indiana’s waiver program, but that doesn’t mean a judge will agree, he said.
“Will a court somewhere find against the government? Against Indiana? I can’t say,” he said. “There’s a lot of hostility to change, and some judges share that hostility to change. But based on my TennCare experience, courts should bend over backward to give flexibility to the states to experiment in this way, if they’re approved by the federal government.”
Mr. Cuello of the National Health Law Program said the Indiana and Kentucky waivers both fail to demonstrate any experimental value. Studies show that when such requirements are enforced, patients drop out of Medicaid or forgo medical care.
“These waivers fail a legality test because they’re not part of any experiment,” he said. “An experiment would be: Here’s an innovative way we have for how to provide health care. These are not experiments.”
At press time, eight states had pending waiver requests at the Centers for Medicare & Medicaid Services that would require work as a condition of eligibility for expansion adults and/or traditional populations.
Is MS caused by one-two punch of pinworm and Epstein-Barr virus?
SAN DIEGO – What causes multiple sclerosis (MS)? A team of Scottish researchers offers a new theory that it’s triggered in part by a one-two punch of infection with pinworm – a common condition in the United States, especially among children – and the Epstein-Barr virus (EBV).
The theory identifies pinworm as the prime suspect to be the “missing link” that explains why EBV and MS are so tightly connected, said Patrick Kearns, MBChB, a graduate student at Harvard T.H. Chan School of Public Health in Boston.
Dr. Kearns is the lead author of two reports about the possible role of pinworm that were presented at ACTRIMS Forum 2018, which is held by the Americas Committee for Treatment and Research in Multiple Sclerosis. He spoke in an interview.
Dr. Kearns and his colleagues focused on a well-known cluster of MS cases that began to appear in the Faroe Islands – a Danish possession in the North Atlantic – during World War II. The cases began to appear after British troops occupied the islands.
“Many of the occupation soldiers were from the Scottish Highlands, where the MS prevalence is quite high: 90 cases per 100,000, comparable to the northern U.S.,” according to a National MS Society summary about MS clusters.
In a theory that spawned controversy, the late neurologist John Kurtzke, MD, speculated that the British soldiers brought a transmissible agent to the islands, which triggered MS cases.
Could the agent be EBV alone? The authors of the new studies don’t think so, although they note that EBV is “robustly linked” to MS. Indeed, a 2012 meta-analysis reported that the virus “appears to be present in 100% of MS patients,” based on studies considered to be the strongest (Mult Scler. 2013 Feb;19[2]:162-6).
The authors of the new reports note that, while EBV infection “appears necessary,” it is “clearly not sufficient” to cause the disease on its own.
“Certainly almost everyone gets EBV eventually,” Dr. Kearns said. “So mere presence of the virus is certainly not sufficient for causing the disease. But it seems still to be necessary, and timing of infection might be everything.”
So what’s the missing piece of the puzzle?
Dr. Kearns began to think it might be the lowly pinworm after helping a colleague by analyzing data from appendicitis samples in children. He noted that uninflamed samples often had pinworms in them, but the inflamed samples often didn’t, which suggested that “the rate of pinworms in normal appendices must be very high in the healthy pediatric population at any given time.”
More data confirmed this to be true, and medical literature told Dr. Kearns that pinworms were common in high latitudes – places where people often are especially prone to MS.
“Most remarkably, they are known to have very little migration and stay spatially stable in populations over long periods of time,” Dr. Kearns said, “and typically everyone in an affected population will encounter them because their eggs are transmitted in household dust.”
And, he said, “they are known to be common in soldiers who live in military accommodation.”
According to the Centers for Disease Control and Prevention, pinworm prevalence can be as high as 50% in at-risk groups – children, caregivers of infected children, and people who live in institutions. Pinworms, which are spread through ingestion, are often asymptomatic but may cause anal itching and trigger bacterial infections.
The researchers suggest that pinworm infection comes first, followed by EBV infection. This makes sense because “late EBV infection in the form of infectious mononucleosis is known to be a risk factor for MS,” Dr. Kearns said.
The one-two punch of pinworm and then EBV is a plausible theory “because EBV lives in memory B cells, which are known to be important in MS and could be specific for the previous exposure to pinworm,” Dr. Kearns said. “However, this is very speculative and some researchers will argue this is very unlikely to be the case. But I think there is a chance it could explain some of the epidemiology, so I’m keen to try and test the theory if I can.”
What’s next? Dr. Kearns wants to explore data from Scotland in search of areas of high and low MS incidence that could offer insight into environmental triggers.
He added that the development of a serological blood test to prove a history of pinworm infection would be “the most effective way to prove or disprove this theory.”
“I have approached an investigator who has a track record of doing this for other infections and have been encouraged that he thinks that it would be achievable,” he said. “But this will definitely take time and funding.”
No specific funding was reported. The study authors reported no relevant disclosures.
SOURCE: Kearns P et al. ACTRIMS Forum 2018, Abstracts LB257 and LB264.
SAN DIEGO – What causes multiple sclerosis (MS)? A team of Scottish researchers offers a new theory that it’s triggered in part by a one-two punch of infection with pinworm – a common condition in the United States, especially among children – and the Epstein-Barr virus (EBV).
The theory identifies pinworm as the prime suspect to be the “missing link” that explains why EBV and MS are so tightly connected, said Patrick Kearns, MBChB, a graduate student at Harvard T.H. Chan School of Public Health in Boston.
Dr. Kearns is the lead author of two reports about the possible role of pinworm that were presented at ACTRIMS Forum 2018, which is held by the Americas Committee for Treatment and Research in Multiple Sclerosis. He spoke in an interview.
Dr. Kearns and his colleagues focused on a well-known cluster of MS cases that began to appear in the Faroe Islands – a Danish possession in the North Atlantic – during World War II. The cases began to appear after British troops occupied the islands.
“Many of the occupation soldiers were from the Scottish Highlands, where the MS prevalence is quite high: 90 cases per 100,000, comparable to the northern U.S.,” according to a National MS Society summary about MS clusters.
In a theory that spawned controversy, the late neurologist John Kurtzke, MD, speculated that the British soldiers brought a transmissible agent to the islands, which triggered MS cases.
Could the agent be EBV alone? The authors of the new studies don’t think so, although they note that EBV is “robustly linked” to MS. Indeed, a 2012 meta-analysis reported that the virus “appears to be present in 100% of MS patients,” based on studies considered to be the strongest (Mult Scler. 2013 Feb;19[2]:162-6).
The authors of the new reports note that, while EBV infection “appears necessary,” it is “clearly not sufficient” to cause the disease on its own.
“Certainly almost everyone gets EBV eventually,” Dr. Kearns said. “So mere presence of the virus is certainly not sufficient for causing the disease. But it seems still to be necessary, and timing of infection might be everything.”
So what’s the missing piece of the puzzle?
Dr. Kearns began to think it might be the lowly pinworm after helping a colleague by analyzing data from appendicitis samples in children. He noted that uninflamed samples often had pinworms in them, but the inflamed samples often didn’t, which suggested that “the rate of pinworms in normal appendices must be very high in the healthy pediatric population at any given time.”
More data confirmed this to be true, and medical literature told Dr. Kearns that pinworms were common in high latitudes – places where people often are especially prone to MS.
“Most remarkably, they are known to have very little migration and stay spatially stable in populations over long periods of time,” Dr. Kearns said, “and typically everyone in an affected population will encounter them because their eggs are transmitted in household dust.”
And, he said, “they are known to be common in soldiers who live in military accommodation.”
According to the Centers for Disease Control and Prevention, pinworm prevalence can be as high as 50% in at-risk groups – children, caregivers of infected children, and people who live in institutions. Pinworms, which are spread through ingestion, are often asymptomatic but may cause anal itching and trigger bacterial infections.
The researchers suggest that pinworm infection comes first, followed by EBV infection. This makes sense because “late EBV infection in the form of infectious mononucleosis is known to be a risk factor for MS,” Dr. Kearns said.
The one-two punch of pinworm and then EBV is a plausible theory “because EBV lives in memory B cells, which are known to be important in MS and could be specific for the previous exposure to pinworm,” Dr. Kearns said. “However, this is very speculative and some researchers will argue this is very unlikely to be the case. But I think there is a chance it could explain some of the epidemiology, so I’m keen to try and test the theory if I can.”
What’s next? Dr. Kearns wants to explore data from Scotland in search of areas of high and low MS incidence that could offer insight into environmental triggers.
He added that the development of a serological blood test to prove a history of pinworm infection would be “the most effective way to prove or disprove this theory.”
“I have approached an investigator who has a track record of doing this for other infections and have been encouraged that he thinks that it would be achievable,” he said. “But this will definitely take time and funding.”
No specific funding was reported. The study authors reported no relevant disclosures.
SOURCE: Kearns P et al. ACTRIMS Forum 2018, Abstracts LB257 and LB264.
SAN DIEGO – What causes multiple sclerosis (MS)? A team of Scottish researchers offers a new theory that it’s triggered in part by a one-two punch of infection with pinworm – a common condition in the United States, especially among children – and the Epstein-Barr virus (EBV).
The theory identifies pinworm as the prime suspect to be the “missing link” that explains why EBV and MS are so tightly connected, said Patrick Kearns, MBChB, a graduate student at Harvard T.H. Chan School of Public Health in Boston.
Dr. Kearns is the lead author of two reports about the possible role of pinworm that were presented at ACTRIMS Forum 2018, which is held by the Americas Committee for Treatment and Research in Multiple Sclerosis. He spoke in an interview.
Dr. Kearns and his colleagues focused on a well-known cluster of MS cases that began to appear in the Faroe Islands – a Danish possession in the North Atlantic – during World War II. The cases began to appear after British troops occupied the islands.
“Many of the occupation soldiers were from the Scottish Highlands, where the MS prevalence is quite high: 90 cases per 100,000, comparable to the northern U.S.,” according to a National MS Society summary about MS clusters.
In a theory that spawned controversy, the late neurologist John Kurtzke, MD, speculated that the British soldiers brought a transmissible agent to the islands, which triggered MS cases.
Could the agent be EBV alone? The authors of the new studies don’t think so, although they note that EBV is “robustly linked” to MS. Indeed, a 2012 meta-analysis reported that the virus “appears to be present in 100% of MS patients,” based on studies considered to be the strongest (Mult Scler. 2013 Feb;19[2]:162-6).
The authors of the new reports note that, while EBV infection “appears necessary,” it is “clearly not sufficient” to cause the disease on its own.
“Certainly almost everyone gets EBV eventually,” Dr. Kearns said. “So mere presence of the virus is certainly not sufficient for causing the disease. But it seems still to be necessary, and timing of infection might be everything.”
So what’s the missing piece of the puzzle?
Dr. Kearns began to think it might be the lowly pinworm after helping a colleague by analyzing data from appendicitis samples in children. He noted that uninflamed samples often had pinworms in them, but the inflamed samples often didn’t, which suggested that “the rate of pinworms in normal appendices must be very high in the healthy pediatric population at any given time.”
More data confirmed this to be true, and medical literature told Dr. Kearns that pinworms were common in high latitudes – places where people often are especially prone to MS.
“Most remarkably, they are known to have very little migration and stay spatially stable in populations over long periods of time,” Dr. Kearns said, “and typically everyone in an affected population will encounter them because their eggs are transmitted in household dust.”
And, he said, “they are known to be common in soldiers who live in military accommodation.”
According to the Centers for Disease Control and Prevention, pinworm prevalence can be as high as 50% in at-risk groups – children, caregivers of infected children, and people who live in institutions. Pinworms, which are spread through ingestion, are often asymptomatic but may cause anal itching and trigger bacterial infections.
The researchers suggest that pinworm infection comes first, followed by EBV infection. This makes sense because “late EBV infection in the form of infectious mononucleosis is known to be a risk factor for MS,” Dr. Kearns said.
The one-two punch of pinworm and then EBV is a plausible theory “because EBV lives in memory B cells, which are known to be important in MS and could be specific for the previous exposure to pinworm,” Dr. Kearns said. “However, this is very speculative and some researchers will argue this is very unlikely to be the case. But I think there is a chance it could explain some of the epidemiology, so I’m keen to try and test the theory if I can.”
What’s next? Dr. Kearns wants to explore data from Scotland in search of areas of high and low MS incidence that could offer insight into environmental triggers.
He added that the development of a serological blood test to prove a history of pinworm infection would be “the most effective way to prove or disprove this theory.”
“I have approached an investigator who has a track record of doing this for other infections and have been encouraged that he thinks that it would be achievable,” he said. “But this will definitely take time and funding.”
No specific funding was reported. The study authors reported no relevant disclosures.
SOURCE: Kearns P et al. ACTRIMS Forum 2018, Abstracts LB257 and LB264.
REPORTING FROM ACTRIMS FORUM 2018
Asymptomatic Erythematous Plaques on the Scalp and Face
The Diagnosis: Granuloma Faciale
A biopsy from a scalp lesion showed an intense mixed inflammatory infiltrate mainly consisting of eosinophils, but lymphocytes, histiocytes, neutrophils, and plasma cells also were present. A grenz zone was observed between the dermal infiltrate and epidermis. Perivascular infiltrates were penetrating vessel walls, and hyalinization of the vessel walls also was seen (Figure 1). Direct immunofluorescence demonstrated IgG positivity on vessel walls (Figure 2). A diagnosis of granuloma faciale with extrafacial lesions was made. Twice daily application of tacrolimus ointment 0.1% was started, but after a 10-month course of treatment, there was no notable difference in the lesions.
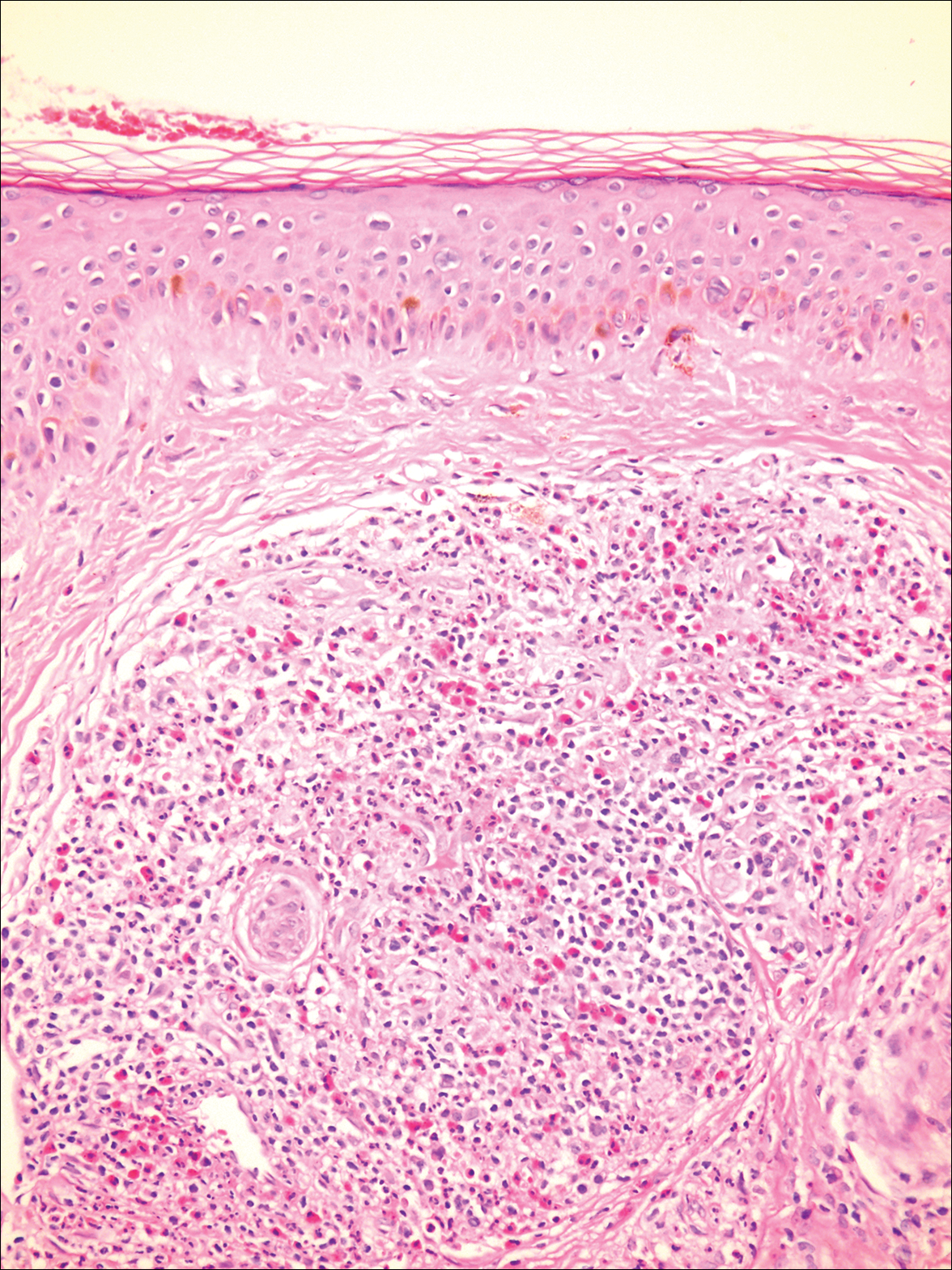
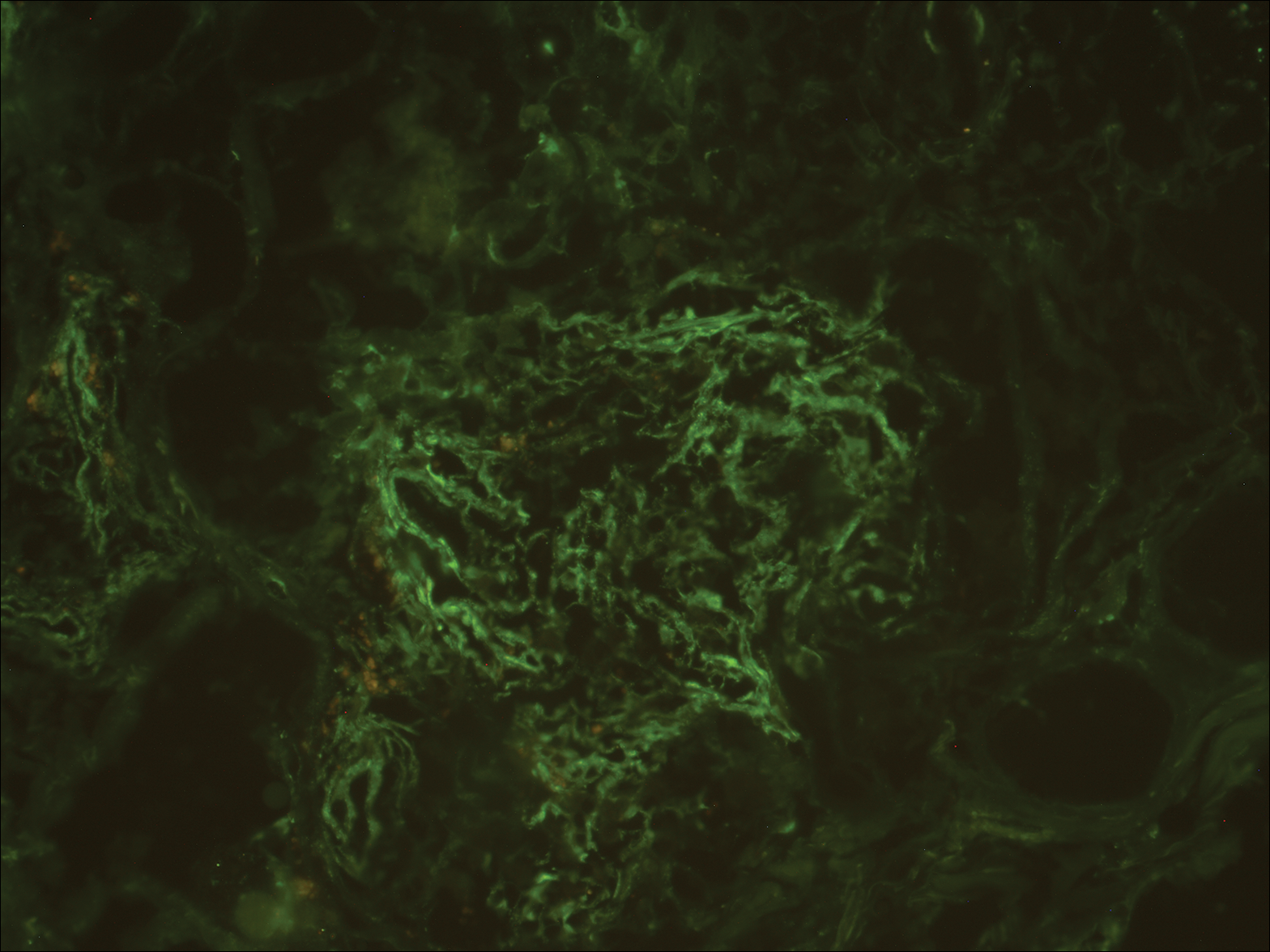
Granuloma faciale (GF) is an uncommon benign dermatosis of unknown pathogenesis characterized by erythematous, brown, or violaceous papules, plaques, or nodules. Granuloma faciale lesions can be solitary or multiple as well as disseminated and most often occur on the face. Predilection sites include the nose, periauricular area, cheeks, forehead, eyelids, and ears; however, lesions also have been reported to occur in extrafacial areas such as the trunk, arms, and legs.1-4 In our patient, multiple plaques were seen on the scalp. Facial lesions usually precede extrafacial lesions, which may present months to several years after the appearance of facial disease; however, according to our patient's history his scalp lesions appeared before the facial lesions.
The differential diagnoses for GF mainly include erythema elevatum diutinum, cutaneous sarcoidosis, cutaneous lymphoma, lupus, basal cell carcinoma, and cutaneous pseudolymphoma.5 Diagnosis may be established based on a combination of clinical features and skin biopsy results. On histopathologic examination, small-vessel vasculitis usually is present with an infiltrate predominantly consisting of neutrophils and eosinophils.6
It has been suggested that actinic damage plays a role in the etiology of GF.7 The pathogenesis is uncertain, but it is thought that immunophenotypic and molecular analysis of the dermal infiltrate in GF reveals that most lymphocytes are clonally expanded and the process is mediated by interferon gamma.7 Tacrolimus acts by binding and inactivating calcineurin and thus blocking T-cell activation and proliferation, so it is not surprising that topical tacrolimus has been shown to be useful in the management of this condition.8
- Leite I, Moreira A, Guedes R, et al. Granuloma faciale of the scalp. Dermatol Online J. 2011;17:6.
- De D, Kanwar AJ, Radotra BD, et al. Extrafacial granuloma faciale: report of a case. J Eur Acad Dermatol Venereol. 2007;21:1284-1286.
- Castellano-Howard L, Fairbee SI, Hogan DJ, et al. Extrafacial granuloma faciale: report of a case and response to treatment. Cutis. 2001;67:413-415.
- Inanir I, Alvur Y. Granuloma faciale with extrafacial lesions. Br J Dermatol. 2001;145:360-362.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Koplon BS, Wood MG. Granuloma faciale. first reported case in a Negro. Arch Dermatol. 1967;96:188-192.
- Ludwig E, Allam JP, Bieber T, et al. New treatment modalities for granuloma faciale. Br J Dermatol. 2003;149:634-637.
The Diagnosis: Granuloma Faciale
A biopsy from a scalp lesion showed an intense mixed inflammatory infiltrate mainly consisting of eosinophils, but lymphocytes, histiocytes, neutrophils, and plasma cells also were present. A grenz zone was observed between the dermal infiltrate and epidermis. Perivascular infiltrates were penetrating vessel walls, and hyalinization of the vessel walls also was seen (Figure 1). Direct immunofluorescence demonstrated IgG positivity on vessel walls (Figure 2). A diagnosis of granuloma faciale with extrafacial lesions was made. Twice daily application of tacrolimus ointment 0.1% was started, but after a 10-month course of treatment, there was no notable difference in the lesions.


Granuloma faciale (GF) is an uncommon benign dermatosis of unknown pathogenesis characterized by erythematous, brown, or violaceous papules, plaques, or nodules. Granuloma faciale lesions can be solitary or multiple as well as disseminated and most often occur on the face. Predilection sites include the nose, periauricular area, cheeks, forehead, eyelids, and ears; however, lesions also have been reported to occur in extrafacial areas such as the trunk, arms, and legs.1-4 In our patient, multiple plaques were seen on the scalp. Facial lesions usually precede extrafacial lesions, which may present months to several years after the appearance of facial disease; however, according to our patient's history his scalp lesions appeared before the facial lesions.
The differential diagnoses for GF mainly include erythema elevatum diutinum, cutaneous sarcoidosis, cutaneous lymphoma, lupus, basal cell carcinoma, and cutaneous pseudolymphoma.5 Diagnosis may be established based on a combination of clinical features and skin biopsy results. On histopathologic examination, small-vessel vasculitis usually is present with an infiltrate predominantly consisting of neutrophils and eosinophils.6
It has been suggested that actinic damage plays a role in the etiology of GF.7 The pathogenesis is uncertain, but it is thought that immunophenotypic and molecular analysis of the dermal infiltrate in GF reveals that most lymphocytes are clonally expanded and the process is mediated by interferon gamma.7 Tacrolimus acts by binding and inactivating calcineurin and thus blocking T-cell activation and proliferation, so it is not surprising that topical tacrolimus has been shown to be useful in the management of this condition.8
The Diagnosis: Granuloma Faciale
A biopsy from a scalp lesion showed an intense mixed inflammatory infiltrate mainly consisting of eosinophils, but lymphocytes, histiocytes, neutrophils, and plasma cells also were present. A grenz zone was observed between the dermal infiltrate and epidermis. Perivascular infiltrates were penetrating vessel walls, and hyalinization of the vessel walls also was seen (Figure 1). Direct immunofluorescence demonstrated IgG positivity on vessel walls (Figure 2). A diagnosis of granuloma faciale with extrafacial lesions was made. Twice daily application of tacrolimus ointment 0.1% was started, but after a 10-month course of treatment, there was no notable difference in the lesions.


Granuloma faciale (GF) is an uncommon benign dermatosis of unknown pathogenesis characterized by erythematous, brown, or violaceous papules, plaques, or nodules. Granuloma faciale lesions can be solitary or multiple as well as disseminated and most often occur on the face. Predilection sites include the nose, periauricular area, cheeks, forehead, eyelids, and ears; however, lesions also have been reported to occur in extrafacial areas such as the trunk, arms, and legs.1-4 In our patient, multiple plaques were seen on the scalp. Facial lesions usually precede extrafacial lesions, which may present months to several years after the appearance of facial disease; however, according to our patient's history his scalp lesions appeared before the facial lesions.
The differential diagnoses for GF mainly include erythema elevatum diutinum, cutaneous sarcoidosis, cutaneous lymphoma, lupus, basal cell carcinoma, and cutaneous pseudolymphoma.5 Diagnosis may be established based on a combination of clinical features and skin biopsy results. On histopathologic examination, small-vessel vasculitis usually is present with an infiltrate predominantly consisting of neutrophils and eosinophils.6
It has been suggested that actinic damage plays a role in the etiology of GF.7 The pathogenesis is uncertain, but it is thought that immunophenotypic and molecular analysis of the dermal infiltrate in GF reveals that most lymphocytes are clonally expanded and the process is mediated by interferon gamma.7 Tacrolimus acts by binding and inactivating calcineurin and thus blocking T-cell activation and proliferation, so it is not surprising that topical tacrolimus has been shown to be useful in the management of this condition.8
- Leite I, Moreira A, Guedes R, et al. Granuloma faciale of the scalp. Dermatol Online J. 2011;17:6.
- De D, Kanwar AJ, Radotra BD, et al. Extrafacial granuloma faciale: report of a case. J Eur Acad Dermatol Venereol. 2007;21:1284-1286.
- Castellano-Howard L, Fairbee SI, Hogan DJ, et al. Extrafacial granuloma faciale: report of a case and response to treatment. Cutis. 2001;67:413-415.
- Inanir I, Alvur Y. Granuloma faciale with extrafacial lesions. Br J Dermatol. 2001;145:360-362.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Koplon BS, Wood MG. Granuloma faciale. first reported case in a Negro. Arch Dermatol. 1967;96:188-192.
- Ludwig E, Allam JP, Bieber T, et al. New treatment modalities for granuloma faciale. Br J Dermatol. 2003;149:634-637.
- Leite I, Moreira A, Guedes R, et al. Granuloma faciale of the scalp. Dermatol Online J. 2011;17:6.
- De D, Kanwar AJ, Radotra BD, et al. Extrafacial granuloma faciale: report of a case. J Eur Acad Dermatol Venereol. 2007;21:1284-1286.
- Castellano-Howard L, Fairbee SI, Hogan DJ, et al. Extrafacial granuloma faciale: report of a case and response to treatment. Cutis. 2001;67:413-415.
- Inanir I, Alvur Y. Granuloma faciale with extrafacial lesions. Br J Dermatol. 2001;145:360-362.
- Ortonne N, Wechsler J, Bagot M, et al. Granuloma faciale: a clinicopathologic study of 66 patients. J Am Acad Dermatol. 2005;53:1002-1009.
- LeBoit PE. Granuloma faciale: a diagnosis deserving of dignity. Am J Dermatopathol. 2002;24:440-443.
- Koplon BS, Wood MG. Granuloma faciale. first reported case in a Negro. Arch Dermatol. 1967;96:188-192.
- Ludwig E, Allam JP, Bieber T, et al. New treatment modalities for granuloma faciale. Br J Dermatol. 2003;149:634-637.
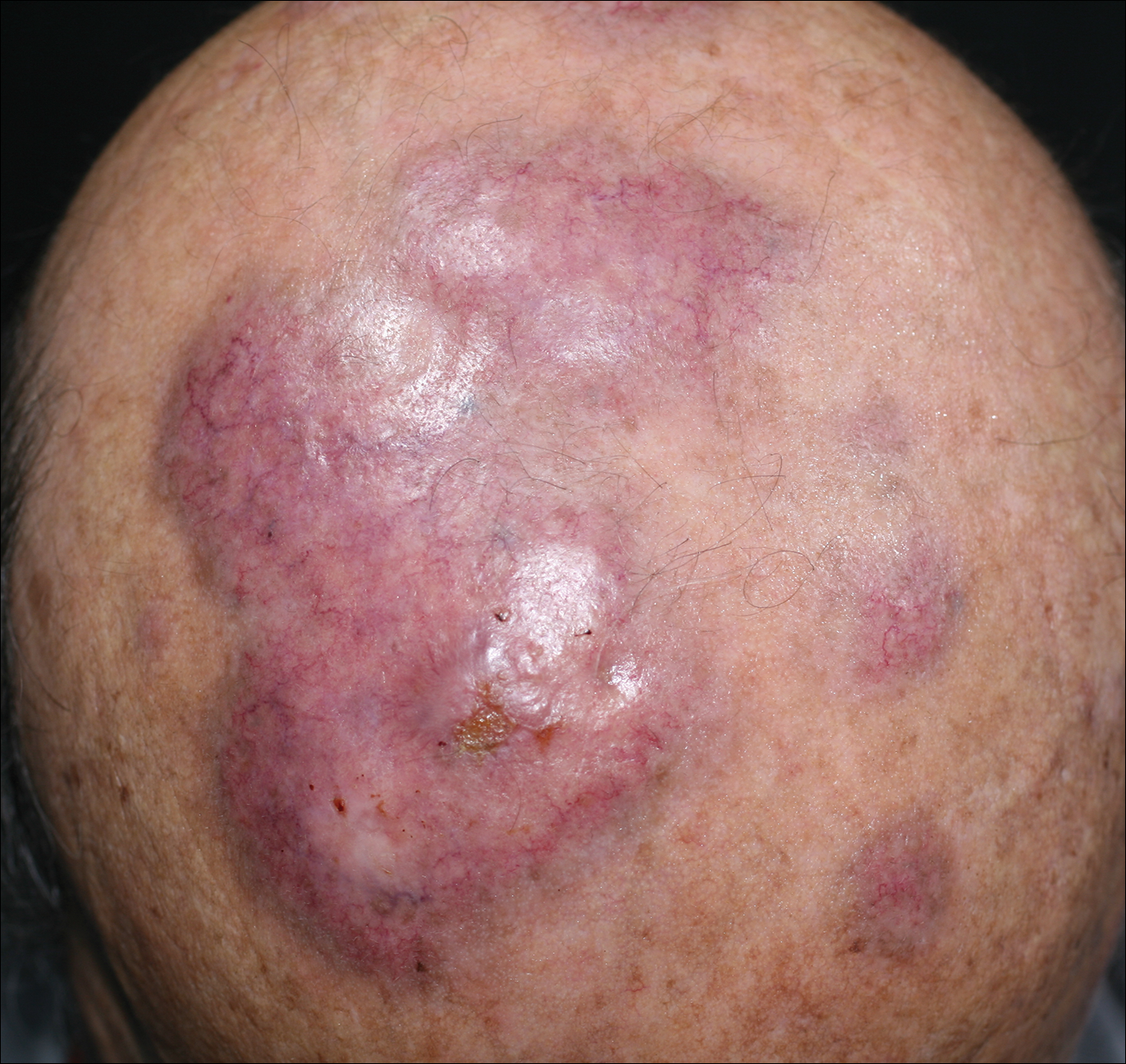
An 84-year-old man presented with gradually enlarging, asymptomatic, erythematous to violaceous plaques on the face and scalp of 11 years' duration ranging in size from 0.5×0.5 cm to 10×8 cm. The plaques were unresponsive to treatment with topical steroids. The lesions were nontender with no associated bleeding, burning, or pruritus. The patient denied any trauma to the sites or systemic symptoms. He had a history of essential hypertension and benign prostatic hyperplasia and had been taking ramipril, tamsulosin, and dutasteride for 5 years. His medical history was otherwise unremarkable, and routine laboratory findings were within normal range.
Guidelines update best practices for hemorrhoid treatment
Each year, more than 2.2 million patients in the United States undergo evaluations for symptoms of hemorrhoids, according to updated guidelines on the management of hemorrhoids issued by the American Society of Colon and Rectal Surgeons.
“As a result, it is important to identify symptomatic hemorrhoids as the underlying source of the anorectal symptom and to have a clear understanding of the evaluation and management of this disease process,” wrote Bradley R. Davis, MD, FACS, chief of colon and rectal surgery at the Carolinas Medical Center, Charlotte, N.C., and the fellow members of the Clinical Practice Guidelines Committee of the ASCRS.
The guidelines recommend evaluation of hemorrhoids based on a disease-specific history, and a physical that emphasizes the degree and duration of symptoms and identifies risk factors. But the guideline writers note that the recommendation is a grade 1C because the supporting data mainly come from observational or case studies.
“The cardinal signs of internal hemorrhoids are painless bleeding with bowel movements with intermittent protrusion,” the committee said, also emphasizing that patients should be evaluated for fecal incontinence, which could inform surgical decision making.
In addition, the guidelines call for a complete endoscopic evaluation of the colon for patients who present with symptomatic hemorrhoids and rectal bleeding; this recommendation is based on moderately strong evidence, and presented with a grade of 1B.
Medical management of hemorrhoids may include office-based procedures or surgery, according to the guidelines.
“Most patients with grade I and II and select patients with grade III internal hemorrhoidal disease who fail medical treatment can be effectively treated with office-based procedures, such as banding, sclerotherapy, and infrared coagulation,” the committee wrote, and medical office treatment received a strong grade 1A recommendation based on high-quality evidence. Although office procedures are generally well tolerated, the condition can recur. Bleeding is the most common complication, and it is more likely after rubber-band ligation than other office-based options, the guidelines state.
The guidelines offer a weak recommendation of 2C, based on the lack of quality evidence, for the use of early surgical excision to treat patients with thrombosed external hemorrhoids. “Although most patients treated nonoperatively will experience eventual resolution of their symptoms, excision of thrombosed external hemorrhoids may result in more rapid symptom resolution, lower incidence of recurrence, and longer remission intervals,” the committee noted.
Surgical hemorrhoidectomy received the strongest possible recommendation (1A, based on high-quality evidence) for the treatment of patients with external hemorrhoids or a combination of internal and external hemorrhoids with prolapse.
Surgical options described in the recommendations include surgical excision (hemorrhoidectomy), hemorrhoidopexy, and Doppler-guided hemorrhoidectomy, with citations of studies on each procedure. Data from a meta-analysis of 18 randomized prospective studies comparing hemorrhoidectomy with office-based procedures showed that hemorrhoidectomy was “the most effective treatment for patients with grade III hemorrhoids,” but it was associated with greater pain and complication rates, according to the guidelines.
However, complications in general are low after surgical hemorrhoidectomy, with reported complication rates of 1%-2% for the most common complication of postprocedure hemorrhage, the guidelines state. After surgery, the guidelines recommend with a 1B grade (moderate quality evidence) that patients use “a multimodality pain regimen to reduce narcotic usage and promote a faster recovery.”
The committee members had no financial conflicts to disclose.
SOURCE: Davis BR et al. Dis Colon Rectum. 2018; 61:284-92.
Each year, more than 2.2 million patients in the United States undergo evaluations for symptoms of hemorrhoids, according to updated guidelines on the management of hemorrhoids issued by the American Society of Colon and Rectal Surgeons.
“As a result, it is important to identify symptomatic hemorrhoids as the underlying source of the anorectal symptom and to have a clear understanding of the evaluation and management of this disease process,” wrote Bradley R. Davis, MD, FACS, chief of colon and rectal surgery at the Carolinas Medical Center, Charlotte, N.C., and the fellow members of the Clinical Practice Guidelines Committee of the ASCRS.
The guidelines recommend evaluation of hemorrhoids based on a disease-specific history, and a physical that emphasizes the degree and duration of symptoms and identifies risk factors. But the guideline writers note that the recommendation is a grade 1C because the supporting data mainly come from observational or case studies.
“The cardinal signs of internal hemorrhoids are painless bleeding with bowel movements with intermittent protrusion,” the committee said, also emphasizing that patients should be evaluated for fecal incontinence, which could inform surgical decision making.
In addition, the guidelines call for a complete endoscopic evaluation of the colon for patients who present with symptomatic hemorrhoids and rectal bleeding; this recommendation is based on moderately strong evidence, and presented with a grade of 1B.
Medical management of hemorrhoids may include office-based procedures or surgery, according to the guidelines.
“Most patients with grade I and II and select patients with grade III internal hemorrhoidal disease who fail medical treatment can be effectively treated with office-based procedures, such as banding, sclerotherapy, and infrared coagulation,” the committee wrote, and medical office treatment received a strong grade 1A recommendation based on high-quality evidence. Although office procedures are generally well tolerated, the condition can recur. Bleeding is the most common complication, and it is more likely after rubber-band ligation than other office-based options, the guidelines state.
The guidelines offer a weak recommendation of 2C, based on the lack of quality evidence, for the use of early surgical excision to treat patients with thrombosed external hemorrhoids. “Although most patients treated nonoperatively will experience eventual resolution of their symptoms, excision of thrombosed external hemorrhoids may result in more rapid symptom resolution, lower incidence of recurrence, and longer remission intervals,” the committee noted.
Surgical hemorrhoidectomy received the strongest possible recommendation (1A, based on high-quality evidence) for the treatment of patients with external hemorrhoids or a combination of internal and external hemorrhoids with prolapse.
Surgical options described in the recommendations include surgical excision (hemorrhoidectomy), hemorrhoidopexy, and Doppler-guided hemorrhoidectomy, with citations of studies on each procedure. Data from a meta-analysis of 18 randomized prospective studies comparing hemorrhoidectomy with office-based procedures showed that hemorrhoidectomy was “the most effective treatment for patients with grade III hemorrhoids,” but it was associated with greater pain and complication rates, according to the guidelines.
However, complications in general are low after surgical hemorrhoidectomy, with reported complication rates of 1%-2% for the most common complication of postprocedure hemorrhage, the guidelines state. After surgery, the guidelines recommend with a 1B grade (moderate quality evidence) that patients use “a multimodality pain regimen to reduce narcotic usage and promote a faster recovery.”
The committee members had no financial conflicts to disclose.
SOURCE: Davis BR et al. Dis Colon Rectum. 2018; 61:284-92.
Each year, more than 2.2 million patients in the United States undergo evaluations for symptoms of hemorrhoids, according to updated guidelines on the management of hemorrhoids issued by the American Society of Colon and Rectal Surgeons.
“As a result, it is important to identify symptomatic hemorrhoids as the underlying source of the anorectal symptom and to have a clear understanding of the evaluation and management of this disease process,” wrote Bradley R. Davis, MD, FACS, chief of colon and rectal surgery at the Carolinas Medical Center, Charlotte, N.C., and the fellow members of the Clinical Practice Guidelines Committee of the ASCRS.
The guidelines recommend evaluation of hemorrhoids based on a disease-specific history, and a physical that emphasizes the degree and duration of symptoms and identifies risk factors. But the guideline writers note that the recommendation is a grade 1C because the supporting data mainly come from observational or case studies.
“The cardinal signs of internal hemorrhoids are painless bleeding with bowel movements with intermittent protrusion,” the committee said, also emphasizing that patients should be evaluated for fecal incontinence, which could inform surgical decision making.
In addition, the guidelines call for a complete endoscopic evaluation of the colon for patients who present with symptomatic hemorrhoids and rectal bleeding; this recommendation is based on moderately strong evidence, and presented with a grade of 1B.
Medical management of hemorrhoids may include office-based procedures or surgery, according to the guidelines.
“Most patients with grade I and II and select patients with grade III internal hemorrhoidal disease who fail medical treatment can be effectively treated with office-based procedures, such as banding, sclerotherapy, and infrared coagulation,” the committee wrote, and medical office treatment received a strong grade 1A recommendation based on high-quality evidence. Although office procedures are generally well tolerated, the condition can recur. Bleeding is the most common complication, and it is more likely after rubber-band ligation than other office-based options, the guidelines state.
The guidelines offer a weak recommendation of 2C, based on the lack of quality evidence, for the use of early surgical excision to treat patients with thrombosed external hemorrhoids. “Although most patients treated nonoperatively will experience eventual resolution of their symptoms, excision of thrombosed external hemorrhoids may result in more rapid symptom resolution, lower incidence of recurrence, and longer remission intervals,” the committee noted.
Surgical hemorrhoidectomy received the strongest possible recommendation (1A, based on high-quality evidence) for the treatment of patients with external hemorrhoids or a combination of internal and external hemorrhoids with prolapse.
Surgical options described in the recommendations include surgical excision (hemorrhoidectomy), hemorrhoidopexy, and Doppler-guided hemorrhoidectomy, with citations of studies on each procedure. Data from a meta-analysis of 18 randomized prospective studies comparing hemorrhoidectomy with office-based procedures showed that hemorrhoidectomy was “the most effective treatment for patients with grade III hemorrhoids,” but it was associated with greater pain and complication rates, according to the guidelines.
However, complications in general are low after surgical hemorrhoidectomy, with reported complication rates of 1%-2% for the most common complication of postprocedure hemorrhage, the guidelines state. After surgery, the guidelines recommend with a 1B grade (moderate quality evidence) that patients use “a multimodality pain regimen to reduce narcotic usage and promote a faster recovery.”
The committee members had no financial conflicts to disclose.
SOURCE: Davis BR et al. Dis Colon Rectum. 2018; 61:284-92.
FROM DISEASES OF THE COLON & RECTUM
VIDEO: Bioimpedance provides accurate assessment of Mohs surgical margins
SAN DIEGO – In assessing tumor-free margins during Mohs micrographic surgery for skin cancer, of histologic sections, in a single-center, pilot study of bioimpedance in 151 specimens from 50 consecutive patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If the finding of high diagnostic accuracy using bioimpedance spectroscopy is confirmed in larger numbers of patients and specimens run at multiple sites, this approach could “potentially revolutionize what happens with the way Mohs sections are processed in the future” by potentially shaving many minutes off the duration of a standard procedure, Darrell S. Rigel, MD, said in a video interview during the annual meeting of the American Academy of Dermatology.
Usually, it takes 10-20 minutes to process and examine Mohs specimens at each stage of the surgical procedure to determine whether additional excision must remove residual cancer cells, said Dr. Rigel, a dermatologist at New York University. In contrast, assessment for residual cancer cells in the surgical field takes less than a minute using bioimpedance spectroscopy, which relies on differences in electrical conductivity between benign and cancerous cells to identify cancer cells remaining at the surgical margins.
The results of the study were presented in a poster at the meeting, by a research associate of Dr. Rigel’s, Ryan Svoboda, MD, of the National Society for Cutaneous Medicine, New York.
The researchers used a bioimpedance spectroscopy device made by NovaScan to assess 151 histology slides prepared during Mohs micrographic surgery on patients with nonmelanoma skin cancer, and compared the findings against the gold standard of histological slide examination. By this criterion, bioimpedance spectroscopy identified 105 true negatives and 2 false negatives, and 43 true positives and 1 false positive. Calculations showed that this equated to 95.6% sensitivity, 99.1% specificity, a 97.7% positive predictive value, and a 98.1% negative predictive value.
These may be underestimates of the accuracy of bioimpedance spectroscopy because the calculations presume that conventional histology is always correct, but Dr. Rigel noted that sometimes the histological diagnosis is wrong.
SOURCE: Svoboda R et al. Poster 7304.
SAN DIEGO – In assessing tumor-free margins during Mohs micrographic surgery for skin cancer, of histologic sections, in a single-center, pilot study of bioimpedance in 151 specimens from 50 consecutive patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If the finding of high diagnostic accuracy using bioimpedance spectroscopy is confirmed in larger numbers of patients and specimens run at multiple sites, this approach could “potentially revolutionize what happens with the way Mohs sections are processed in the future” by potentially shaving many minutes off the duration of a standard procedure, Darrell S. Rigel, MD, said in a video interview during the annual meeting of the American Academy of Dermatology.
Usually, it takes 10-20 minutes to process and examine Mohs specimens at each stage of the surgical procedure to determine whether additional excision must remove residual cancer cells, said Dr. Rigel, a dermatologist at New York University. In contrast, assessment for residual cancer cells in the surgical field takes less than a minute using bioimpedance spectroscopy, which relies on differences in electrical conductivity between benign and cancerous cells to identify cancer cells remaining at the surgical margins.
The results of the study were presented in a poster at the meeting, by a research associate of Dr. Rigel’s, Ryan Svoboda, MD, of the National Society for Cutaneous Medicine, New York.
The researchers used a bioimpedance spectroscopy device made by NovaScan to assess 151 histology slides prepared during Mohs micrographic surgery on patients with nonmelanoma skin cancer, and compared the findings against the gold standard of histological slide examination. By this criterion, bioimpedance spectroscopy identified 105 true negatives and 2 false negatives, and 43 true positives and 1 false positive. Calculations showed that this equated to 95.6% sensitivity, 99.1% specificity, a 97.7% positive predictive value, and a 98.1% negative predictive value.
These may be underestimates of the accuracy of bioimpedance spectroscopy because the calculations presume that conventional histology is always correct, but Dr. Rigel noted that sometimes the histological diagnosis is wrong.
SOURCE: Svoboda R et al. Poster 7304.
SAN DIEGO – In assessing tumor-free margins during Mohs micrographic surgery for skin cancer, of histologic sections, in a single-center, pilot study of bioimpedance in 151 specimens from 50 consecutive patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
If the finding of high diagnostic accuracy using bioimpedance spectroscopy is confirmed in larger numbers of patients and specimens run at multiple sites, this approach could “potentially revolutionize what happens with the way Mohs sections are processed in the future” by potentially shaving many minutes off the duration of a standard procedure, Darrell S. Rigel, MD, said in a video interview during the annual meeting of the American Academy of Dermatology.
Usually, it takes 10-20 minutes to process and examine Mohs specimens at each stage of the surgical procedure to determine whether additional excision must remove residual cancer cells, said Dr. Rigel, a dermatologist at New York University. In contrast, assessment for residual cancer cells in the surgical field takes less than a minute using bioimpedance spectroscopy, which relies on differences in electrical conductivity between benign and cancerous cells to identify cancer cells remaining at the surgical margins.
The results of the study were presented in a poster at the meeting, by a research associate of Dr. Rigel’s, Ryan Svoboda, MD, of the National Society for Cutaneous Medicine, New York.
The researchers used a bioimpedance spectroscopy device made by NovaScan to assess 151 histology slides prepared during Mohs micrographic surgery on patients with nonmelanoma skin cancer, and compared the findings against the gold standard of histological slide examination. By this criterion, bioimpedance spectroscopy identified 105 true negatives and 2 false negatives, and 43 true positives and 1 false positive. Calculations showed that this equated to 95.6% sensitivity, 99.1% specificity, a 97.7% positive predictive value, and a 98.1% negative predictive value.
These may be underestimates of the accuracy of bioimpedance spectroscopy because the calculations presume that conventional histology is always correct, but Dr. Rigel noted that sometimes the histological diagnosis is wrong.
SOURCE: Svoboda R et al. Poster 7304.
REPORTING FROM AAD 18
Key clinical point: Bioimpedance spectroscopy showed excellent diagnostic accuracy for cancer cells on Mohs surgical margins.
Major finding: Bioimpedance spectroscopy had a sensitivity of 95.6% and specificity of 99.1% compared with Mohs histology.
Study details: A single-center pilot study with 151 Mohs surgical specimens taken from 50 patients.
Disclosures: The study was funded by NovaScan, the company developing the device tested in the study. Dr. Rigel has been a consultant to NovaScan and to Castle Biosciences, DermTech, Ferndale, Myriad, and Neutrogena, and has received research support from Castle and Neutrogena.
Source: Svoboda R et al. Poster 7304.
Anti–IL-33 antibody stakes a first-in-class claim on moderate to severe atopic dermatitis
SAN DIEGO – and will proceed along its developmental pathway.
All 12 patients who received one intravenous infusion of ANB020 achieved at least a 50% reduction in their Eczema Area and Severity Index (EASI) by day 29, Graham Ogg, MD, said at the annual meeting of the American Academy of Dermatology. Most of the improvement occurred in the first 2 weeks, was largely sustained for 2 months, then gradually began to fade, said Dr. Ogg, professor of dermatology at Oxford University, England.
The EASI improvement, along with improvements in itch and clinical assessment, are enough to propel ANB020 into a phase 2b, placebo-controlled, randomized trial, which Dr. Ogg said would recruit 200 to 300 adults with moderate to severe atomic dermatitis (AD).
ANB020 is a selective inhibitor of interleukin-33 (IL-33), which is highly expressed in the lesions of AD, Dr. Ogg said. “IL-33 is produced by a number of cells, including keratinocytes and epithelial cells. It’s an alarm molecule predominately produced after damage to keratinocytes; for example, after an allergenic challenge to the skin.”
The antibody has already passed its phase 1 trials, which included both a fixed and ascending dose study of 10 mg to 750 mg in healthy adults. ANB020 has a long half-life of 16 days after IV infusion or subcutaneous injection, inhibits IL-33 for up to 85 days, and has no apparent dose-limiting toxicities.
The phase 2a study comprised 12 adults (mean age 40 years) with a mean EASI score of 32, Dr. Ogg reported. The mean Investigator’s Global Assessment score was 4, and the mean Severity Scoring of Atopic Dermatitis almost 65. Patients had high itch scores and scored a mean of 13 on the Dermatology Life Quality Index. All were inadequately controlled on topical corticosteroids, and about half had failed at least one systemic immunomodulator.
Following a washout period, all subjects received an initial placebo infusion, and 1 week later received a single 300-mg infusion of ANB020. The primary endpoint – at least a 50% reduction in EASI score (EASI-50) – was assessed at day 29, with close follow-up until day 140.
By day 15, nine patients (75%) had achieved EASI-50, and three (25%) had achieved a 75% reduction in EASI score (EASI-75). The average EASI score reduction was 58% at that point. By day 29, 10 (83%) had achieved EASI-50, and 4 (33%), EASI-75. This benefit was largely maintained until day 78, when it began to tail off somewhat. By day 140, five patients (42%) still had an EASI-50 and three (25%), an EASI-75.
Three patients (25%) achieved an Investigator’s Global Assessment score of 0 or 1, indicating clear or almost clear skin. On day 29, the average pruritus decrease was 32%, which dropped to 21% by day 140. The average Severity Scoring of Atopic Dermatitis reduction was 40% on day 29 and 32% on day 140. The average Dermatology Life Quality Index reduction was 45% on day 29 and 43% on day 140.
ANB020 was well tolerated with no drug-related safety signals. Dizziness occurred in 17% of subjects, and mild headache in 25%. There was one serious adverse event, a case of major depression that occurred on day 140. This was consistent with the patient’s baseline health history and deemed unrelated to the study drug.
According to an AnaptysBio press release, ANB020 is also being investigated in a double-blind, placebo-controlled study of 20 adults with severe peanut allergy. The company also is enrolling a 24-patient randomized, double-blind, placebo-controlled, phase 2a trial in adults with severe eosinophilic asthma.
Topline data from both of these studies are expected soon, the website noted.
AnaptysBio funded Dr. Ogg’s travel and registration fees for the meeting. He said he had no other financial disclosures relevant to ANB020.
SOURCE: Ogg G et al. AAD 2018. Abstract 6658.
SAN DIEGO – and will proceed along its developmental pathway.
All 12 patients who received one intravenous infusion of ANB020 achieved at least a 50% reduction in their Eczema Area and Severity Index (EASI) by day 29, Graham Ogg, MD, said at the annual meeting of the American Academy of Dermatology. Most of the improvement occurred in the first 2 weeks, was largely sustained for 2 months, then gradually began to fade, said Dr. Ogg, professor of dermatology at Oxford University, England.
The EASI improvement, along with improvements in itch and clinical assessment, are enough to propel ANB020 into a phase 2b, placebo-controlled, randomized trial, which Dr. Ogg said would recruit 200 to 300 adults with moderate to severe atomic dermatitis (AD).
ANB020 is a selective inhibitor of interleukin-33 (IL-33), which is highly expressed in the lesions of AD, Dr. Ogg said. “IL-33 is produced by a number of cells, including keratinocytes and epithelial cells. It’s an alarm molecule predominately produced after damage to keratinocytes; for example, after an allergenic challenge to the skin.”
The antibody has already passed its phase 1 trials, which included both a fixed and ascending dose study of 10 mg to 750 mg in healthy adults. ANB020 has a long half-life of 16 days after IV infusion or subcutaneous injection, inhibits IL-33 for up to 85 days, and has no apparent dose-limiting toxicities.
The phase 2a study comprised 12 adults (mean age 40 years) with a mean EASI score of 32, Dr. Ogg reported. The mean Investigator’s Global Assessment score was 4, and the mean Severity Scoring of Atopic Dermatitis almost 65. Patients had high itch scores and scored a mean of 13 on the Dermatology Life Quality Index. All were inadequately controlled on topical corticosteroids, and about half had failed at least one systemic immunomodulator.
Following a washout period, all subjects received an initial placebo infusion, and 1 week later received a single 300-mg infusion of ANB020. The primary endpoint – at least a 50% reduction in EASI score (EASI-50) – was assessed at day 29, with close follow-up until day 140.
By day 15, nine patients (75%) had achieved EASI-50, and three (25%) had achieved a 75% reduction in EASI score (EASI-75). The average EASI score reduction was 58% at that point. By day 29, 10 (83%) had achieved EASI-50, and 4 (33%), EASI-75. This benefit was largely maintained until day 78, when it began to tail off somewhat. By day 140, five patients (42%) still had an EASI-50 and three (25%), an EASI-75.
Three patients (25%) achieved an Investigator’s Global Assessment score of 0 or 1, indicating clear or almost clear skin. On day 29, the average pruritus decrease was 32%, which dropped to 21% by day 140. The average Severity Scoring of Atopic Dermatitis reduction was 40% on day 29 and 32% on day 140. The average Dermatology Life Quality Index reduction was 45% on day 29 and 43% on day 140.
ANB020 was well tolerated with no drug-related safety signals. Dizziness occurred in 17% of subjects, and mild headache in 25%. There was one serious adverse event, a case of major depression that occurred on day 140. This was consistent with the patient’s baseline health history and deemed unrelated to the study drug.
According to an AnaptysBio press release, ANB020 is also being investigated in a double-blind, placebo-controlled study of 20 adults with severe peanut allergy. The company also is enrolling a 24-patient randomized, double-blind, placebo-controlled, phase 2a trial in adults with severe eosinophilic asthma.
Topline data from both of these studies are expected soon, the website noted.
AnaptysBio funded Dr. Ogg’s travel and registration fees for the meeting. He said he had no other financial disclosures relevant to ANB020.
SOURCE: Ogg G et al. AAD 2018. Abstract 6658.
SAN DIEGO – and will proceed along its developmental pathway.
All 12 patients who received one intravenous infusion of ANB020 achieved at least a 50% reduction in their Eczema Area and Severity Index (EASI) by day 29, Graham Ogg, MD, said at the annual meeting of the American Academy of Dermatology. Most of the improvement occurred in the first 2 weeks, was largely sustained for 2 months, then gradually began to fade, said Dr. Ogg, professor of dermatology at Oxford University, England.
The EASI improvement, along with improvements in itch and clinical assessment, are enough to propel ANB020 into a phase 2b, placebo-controlled, randomized trial, which Dr. Ogg said would recruit 200 to 300 adults with moderate to severe atomic dermatitis (AD).
ANB020 is a selective inhibitor of interleukin-33 (IL-33), which is highly expressed in the lesions of AD, Dr. Ogg said. “IL-33 is produced by a number of cells, including keratinocytes and epithelial cells. It’s an alarm molecule predominately produced after damage to keratinocytes; for example, after an allergenic challenge to the skin.”
The antibody has already passed its phase 1 trials, which included both a fixed and ascending dose study of 10 mg to 750 mg in healthy adults. ANB020 has a long half-life of 16 days after IV infusion or subcutaneous injection, inhibits IL-33 for up to 85 days, and has no apparent dose-limiting toxicities.
The phase 2a study comprised 12 adults (mean age 40 years) with a mean EASI score of 32, Dr. Ogg reported. The mean Investigator’s Global Assessment score was 4, and the mean Severity Scoring of Atopic Dermatitis almost 65. Patients had high itch scores and scored a mean of 13 on the Dermatology Life Quality Index. All were inadequately controlled on topical corticosteroids, and about half had failed at least one systemic immunomodulator.
Following a washout period, all subjects received an initial placebo infusion, and 1 week later received a single 300-mg infusion of ANB020. The primary endpoint – at least a 50% reduction in EASI score (EASI-50) – was assessed at day 29, with close follow-up until day 140.
By day 15, nine patients (75%) had achieved EASI-50, and three (25%) had achieved a 75% reduction in EASI score (EASI-75). The average EASI score reduction was 58% at that point. By day 29, 10 (83%) had achieved EASI-50, and 4 (33%), EASI-75. This benefit was largely maintained until day 78, when it began to tail off somewhat. By day 140, five patients (42%) still had an EASI-50 and three (25%), an EASI-75.
Three patients (25%) achieved an Investigator’s Global Assessment score of 0 or 1, indicating clear or almost clear skin. On day 29, the average pruritus decrease was 32%, which dropped to 21% by day 140. The average Severity Scoring of Atopic Dermatitis reduction was 40% on day 29 and 32% on day 140. The average Dermatology Life Quality Index reduction was 45% on day 29 and 43% on day 140.
ANB020 was well tolerated with no drug-related safety signals. Dizziness occurred in 17% of subjects, and mild headache in 25%. There was one serious adverse event, a case of major depression that occurred on day 140. This was consistent with the patient’s baseline health history and deemed unrelated to the study drug.
According to an AnaptysBio press release, ANB020 is also being investigated in a double-blind, placebo-controlled study of 20 adults with severe peanut allergy. The company also is enrolling a 24-patient randomized, double-blind, placebo-controlled, phase 2a trial in adults with severe eosinophilic asthma.
Topline data from both of these studies are expected soon, the website noted.
AnaptysBio funded Dr. Ogg’s travel and registration fees for the meeting. He said he had no other financial disclosures relevant to ANB020.
SOURCE: Ogg G et al. AAD 2018. Abstract 6658.
REPORTING FROM AAD 2018
Key clinical point: ANB020 improved skin involvement and itch in a small phase 2 study.
Major finding: All patients who received one infusion achieved at least a 50% reduction in their EASI scores by day 29.
Study details: The phase 2a study comprised 12 patients with moderate to severe AD.
Disclosures: AnaptysBio is developing the molecule. The company paid Dr. Ogg’s travel and meeting registration fees.
Source: Ogg G et al. AAD 2018. Abstract 6658.
Defining incisional hernia risk in IBD surgery
Open surgery for inflammatory bowel disease (IBD) has been known to carry a high risk of incisional hernia, but the risk factors have not been well understood.
A review of 1,000 operations performed over nearly 40 years at a high-volume, nationally recognized center has identified five patient factors that can raise the risk of incisional hernia in these operations by 50% or more, according to a study published in the Annals of Surgery.
The study followed the patients for an average of 8 years after their operations, which were performed between January 1976 and December 2014 at Mount Sinai Medical Center in New York. The overall incidence of incisional hernia was 20%-21% for patients with ulcerative colitis and 20% for those with Crohn’s disease.
Half of these patients developed an incisional hernia less than 2 years after the index surgery and 75% in less than 4 years.
The researchers identified the following statistically significant risk factors for incisional hernia: wound infection (hazard ratio, 3.66; P less than .001); hypoalbuminemia (HR, 2.02; P = .002); previous bowel resection (HR, 1.6; P = .003); ileostomy created at time of procedure (HR, 1.53; P = .01); and a history of smoking (HR, 1.52; P less than .013). Other risk factors to lesser degrees are body mass index at time of surgery (HR, 1.036; P = .009); age at time of surgery (HR, 1.021; P less than .001), and age at disease onset (HR, 1.018; P less than .001).
Lead author Tomas Heimann, MD, and his coauthors pointed out that this study population had severe levels of disease. Almost half of the patients had severe intractable disease that had resisted medical treatment. More than a quarter of these patients had received preoperative steroid therapy within 6 weeks, and 15% had received recent immunosuppressive therapy. Almost 80% were either anemic or had hypoalbuminemia or both. The average duration of disease was 12 years. More than half had undergone previous bowel surgery – “often lengthy and difficult” – with many patients suffering from fistulae, abscesses, and dense adhesions. “These factors were more likely to predispose patients to develop wound infections and delayed healing resulting in incisional hernia in one-fifth of our patients,” Dr. Heimann and his coauthors noted.
A somewhat unexpected finding was that immunosuppressive therapy and steroids were not linked to incisional hernia in these patients.
Prophylactic mesh placement in patients with IBD is impractical because of the risk of infection it carries, Dr. Heimann and his coauthors said.
Dr. Heimann and his coauthors reported having no financial disclosures.
SOURCE: Heimann T et al. Ann Surg. 2018 Mar;267(3):532-6.
Open surgery for inflammatory bowel disease (IBD) has been known to carry a high risk of incisional hernia, but the risk factors have not been well understood.
A review of 1,000 operations performed over nearly 40 years at a high-volume, nationally recognized center has identified five patient factors that can raise the risk of incisional hernia in these operations by 50% or more, according to a study published in the Annals of Surgery.
The study followed the patients for an average of 8 years after their operations, which were performed between January 1976 and December 2014 at Mount Sinai Medical Center in New York. The overall incidence of incisional hernia was 20%-21% for patients with ulcerative colitis and 20% for those with Crohn’s disease.
Half of these patients developed an incisional hernia less than 2 years after the index surgery and 75% in less than 4 years.
The researchers identified the following statistically significant risk factors for incisional hernia: wound infection (hazard ratio, 3.66; P less than .001); hypoalbuminemia (HR, 2.02; P = .002); previous bowel resection (HR, 1.6; P = .003); ileostomy created at time of procedure (HR, 1.53; P = .01); and a history of smoking (HR, 1.52; P less than .013). Other risk factors to lesser degrees are body mass index at time of surgery (HR, 1.036; P = .009); age at time of surgery (HR, 1.021; P less than .001), and age at disease onset (HR, 1.018; P less than .001).
Lead author Tomas Heimann, MD, and his coauthors pointed out that this study population had severe levels of disease. Almost half of the patients had severe intractable disease that had resisted medical treatment. More than a quarter of these patients had received preoperative steroid therapy within 6 weeks, and 15% had received recent immunosuppressive therapy. Almost 80% were either anemic or had hypoalbuminemia or both. The average duration of disease was 12 years. More than half had undergone previous bowel surgery – “often lengthy and difficult” – with many patients suffering from fistulae, abscesses, and dense adhesions. “These factors were more likely to predispose patients to develop wound infections and delayed healing resulting in incisional hernia in one-fifth of our patients,” Dr. Heimann and his coauthors noted.
A somewhat unexpected finding was that immunosuppressive therapy and steroids were not linked to incisional hernia in these patients.
Prophylactic mesh placement in patients with IBD is impractical because of the risk of infection it carries, Dr. Heimann and his coauthors said.
Dr. Heimann and his coauthors reported having no financial disclosures.
SOURCE: Heimann T et al. Ann Surg. 2018 Mar;267(3):532-6.
Open surgery for inflammatory bowel disease (IBD) has been known to carry a high risk of incisional hernia, but the risk factors have not been well understood.
A review of 1,000 operations performed over nearly 40 years at a high-volume, nationally recognized center has identified five patient factors that can raise the risk of incisional hernia in these operations by 50% or more, according to a study published in the Annals of Surgery.
The study followed the patients for an average of 8 years after their operations, which were performed between January 1976 and December 2014 at Mount Sinai Medical Center in New York. The overall incidence of incisional hernia was 20%-21% for patients with ulcerative colitis and 20% for those with Crohn’s disease.
Half of these patients developed an incisional hernia less than 2 years after the index surgery and 75% in less than 4 years.
The researchers identified the following statistically significant risk factors for incisional hernia: wound infection (hazard ratio, 3.66; P less than .001); hypoalbuminemia (HR, 2.02; P = .002); previous bowel resection (HR, 1.6; P = .003); ileostomy created at time of procedure (HR, 1.53; P = .01); and a history of smoking (HR, 1.52; P less than .013). Other risk factors to lesser degrees are body mass index at time of surgery (HR, 1.036; P = .009); age at time of surgery (HR, 1.021; P less than .001), and age at disease onset (HR, 1.018; P less than .001).
Lead author Tomas Heimann, MD, and his coauthors pointed out that this study population had severe levels of disease. Almost half of the patients had severe intractable disease that had resisted medical treatment. More than a quarter of these patients had received preoperative steroid therapy within 6 weeks, and 15% had received recent immunosuppressive therapy. Almost 80% were either anemic or had hypoalbuminemia or both. The average duration of disease was 12 years. More than half had undergone previous bowel surgery – “often lengthy and difficult” – with many patients suffering from fistulae, abscesses, and dense adhesions. “These factors were more likely to predispose patients to develop wound infections and delayed healing resulting in incisional hernia in one-fifth of our patients,” Dr. Heimann and his coauthors noted.
A somewhat unexpected finding was that immunosuppressive therapy and steroids were not linked to incisional hernia in these patients.
Prophylactic mesh placement in patients with IBD is impractical because of the risk of infection it carries, Dr. Heimann and his coauthors said.
Dr. Heimann and his coauthors reported having no financial disclosures.
SOURCE: Heimann T et al. Ann Surg. 2018 Mar;267(3):532-6.
FROM ANNALS OF SURGERY
Key clinical point: Patients with IBD have a high incidence of incisional hernia after open bowel resection.
Major finding: The overall incidence of incisional hernia after open bowel resection was 20%.
Data source: One thousand patients who had undergone open bowel surgery for IBD at Mount Sinai Medical Center, New York, between January 1976 and December 2014.
Disclosures: Dr. Heimann and his coauthors reported having no financial disclosures.
Source: Heimann T et al. Ann Surg. 2018 Mar;267(3):532-6.