User login
Social recovery therapy, early intervention ‘superior’ in first-episode psychosis
Adding social recovery therapy to early intervention services significantly improved social function, compared with early intervention alone for young first-episode psychosis patients with extreme social withdrawal, according to data from 155 patients.
“New interventions targeting functional and social recovery are needed in people with first-episode psychosis,” wrote David Fowler of the psychology department at the University of Sussex, Brighton, England, and his colleagues.
In a study known as SUPEREDEN3, published in The Lancet Psychiatry, the researchers randomized 76 patients aged 16-35 years to social recovery therapy plus early intervention and 79 to early intervention alone. The study participants were selected between Oct. 1, 2012, and June 20, 2014, and suffered from extreme social withdrawal as well as complex comorbidities, including anxiety and depression, hopelessness, and residual and treatment-resistant positive psychotic symptoms.
The social recovery therapy, delivered in three stages, included working with the patients to identify new activities and to get them engaged in those pursuits. “Therapists adopt an assertive outreach style of contact, most frequently visiting people at home or in community settings,” the researchers wrote. “Therapists are also encouraged to work systematically with family members, employers, and educational providers to discuss and overcome potential problems that could impede social recovery.”
. Structured activity was defined as time spent over the previous month on activities, including work, education, volunteering, leisure activities, sports, housework or other chores, and child care. No adverse events related to the intervention were reported.
“Our findings show that social recovery therapy plus early intervention services is superior to early intervention services alone on the primary outcome of time spent in structured activity,” Mr. Fowler and his colleagues wrote.
The findings were limited by the lack of data from secondary outcomes, in part because of the challenges of following up with a withdrawn study population, the researchers said. However, they said, the study is the first to show benefits of social recovery therapy in this challenging group.
The results offer “encouragement for practitioners in early intervention services to focus on this subgroup who are often neglected. Our results also suggest that social recovery therapy techniques could be a useful addition in this group,” the researchers said.
The National Institute for Health Research funded the study. The researchers had no financial conflicts to disclose.
SOURCE: Fowler D et al. Lancet Psychiatry. 2018 Jan;5(1):41-50.
Helping patients with first-episode psychosis improve their social function remains a challenge, Nikolai Albert, MD, and his coauthors wrote in an accompanying editorial. Social recovery therapy could help those patients but must be approached respectfully, they noted.
“The focus on everyday life in social recovery therapy has some promising elements, and seemingly can serve as a supplement to other established forms of individual support,” they wrote.
Social recovery therapy could be a tool to help guide patients with severe social withdrawal back to community living, said Dr. Albert and his coauthors. Despite the small sample size and absence of adequate 15-month follow-up data to show whether the effects of the therapy persist, the findings remain statistically significant and clinically relevant – and offer a promising option for a severely debilitated group of patients, they added (Lancet Psychiatry. 2018 Jan;5[1]:3-4).
Dr. Albert is affiliated with Mental Health Centre Copenhagen at the University of Copenhagen. The authors had no financial conflicts to disclose.
Helping patients with first-episode psychosis improve their social function remains a challenge, Nikolai Albert, MD, and his coauthors wrote in an accompanying editorial. Social recovery therapy could help those patients but must be approached respectfully, they noted.
“The focus on everyday life in social recovery therapy has some promising elements, and seemingly can serve as a supplement to other established forms of individual support,” they wrote.
Social recovery therapy could be a tool to help guide patients with severe social withdrawal back to community living, said Dr. Albert and his coauthors. Despite the small sample size and absence of adequate 15-month follow-up data to show whether the effects of the therapy persist, the findings remain statistically significant and clinically relevant – and offer a promising option for a severely debilitated group of patients, they added (Lancet Psychiatry. 2018 Jan;5[1]:3-4).
Dr. Albert is affiliated with Mental Health Centre Copenhagen at the University of Copenhagen. The authors had no financial conflicts to disclose.
Helping patients with first-episode psychosis improve their social function remains a challenge, Nikolai Albert, MD, and his coauthors wrote in an accompanying editorial. Social recovery therapy could help those patients but must be approached respectfully, they noted.
“The focus on everyday life in social recovery therapy has some promising elements, and seemingly can serve as a supplement to other established forms of individual support,” they wrote.
Social recovery therapy could be a tool to help guide patients with severe social withdrawal back to community living, said Dr. Albert and his coauthors. Despite the small sample size and absence of adequate 15-month follow-up data to show whether the effects of the therapy persist, the findings remain statistically significant and clinically relevant – and offer a promising option for a severely debilitated group of patients, they added (Lancet Psychiatry. 2018 Jan;5[1]:3-4).
Dr. Albert is affiliated with Mental Health Centre Copenhagen at the University of Copenhagen. The authors had no financial conflicts to disclose.
Adding social recovery therapy to early intervention services significantly improved social function, compared with early intervention alone for young first-episode psychosis patients with extreme social withdrawal, according to data from 155 patients.
“New interventions targeting functional and social recovery are needed in people with first-episode psychosis,” wrote David Fowler of the psychology department at the University of Sussex, Brighton, England, and his colleagues.
In a study known as SUPEREDEN3, published in The Lancet Psychiatry, the researchers randomized 76 patients aged 16-35 years to social recovery therapy plus early intervention and 79 to early intervention alone. The study participants were selected between Oct. 1, 2012, and June 20, 2014, and suffered from extreme social withdrawal as well as complex comorbidities, including anxiety and depression, hopelessness, and residual and treatment-resistant positive psychotic symptoms.
The social recovery therapy, delivered in three stages, included working with the patients to identify new activities and to get them engaged in those pursuits. “Therapists adopt an assertive outreach style of contact, most frequently visiting people at home or in community settings,” the researchers wrote. “Therapists are also encouraged to work systematically with family members, employers, and educational providers to discuss and overcome potential problems that could impede social recovery.”
. Structured activity was defined as time spent over the previous month on activities, including work, education, volunteering, leisure activities, sports, housework or other chores, and child care. No adverse events related to the intervention were reported.
“Our findings show that social recovery therapy plus early intervention services is superior to early intervention services alone on the primary outcome of time spent in structured activity,” Mr. Fowler and his colleagues wrote.
The findings were limited by the lack of data from secondary outcomes, in part because of the challenges of following up with a withdrawn study population, the researchers said. However, they said, the study is the first to show benefits of social recovery therapy in this challenging group.
The results offer “encouragement for practitioners in early intervention services to focus on this subgroup who are often neglected. Our results also suggest that social recovery therapy techniques could be a useful addition in this group,” the researchers said.
The National Institute for Health Research funded the study. The researchers had no financial conflicts to disclose.
SOURCE: Fowler D et al. Lancet Psychiatry. 2018 Jan;5(1):41-50.
Adding social recovery therapy to early intervention services significantly improved social function, compared with early intervention alone for young first-episode psychosis patients with extreme social withdrawal, according to data from 155 patients.
“New interventions targeting functional and social recovery are needed in people with first-episode psychosis,” wrote David Fowler of the psychology department at the University of Sussex, Brighton, England, and his colleagues.
In a study known as SUPEREDEN3, published in The Lancet Psychiatry, the researchers randomized 76 patients aged 16-35 years to social recovery therapy plus early intervention and 79 to early intervention alone. The study participants were selected between Oct. 1, 2012, and June 20, 2014, and suffered from extreme social withdrawal as well as complex comorbidities, including anxiety and depression, hopelessness, and residual and treatment-resistant positive psychotic symptoms.
The social recovery therapy, delivered in three stages, included working with the patients to identify new activities and to get them engaged in those pursuits. “Therapists adopt an assertive outreach style of contact, most frequently visiting people at home or in community settings,” the researchers wrote. “Therapists are also encouraged to work systematically with family members, employers, and educational providers to discuss and overcome potential problems that could impede social recovery.”
. Structured activity was defined as time spent over the previous month on activities, including work, education, volunteering, leisure activities, sports, housework or other chores, and child care. No adverse events related to the intervention were reported.
“Our findings show that social recovery therapy plus early intervention services is superior to early intervention services alone on the primary outcome of time spent in structured activity,” Mr. Fowler and his colleagues wrote.
The findings were limited by the lack of data from secondary outcomes, in part because of the challenges of following up with a withdrawn study population, the researchers said. However, they said, the study is the first to show benefits of social recovery therapy in this challenging group.
The results offer “encouragement for practitioners in early intervention services to focus on this subgroup who are often neglected. Our results also suggest that social recovery therapy techniques could be a useful addition in this group,” the researchers said.
The National Institute for Health Research funded the study. The researchers had no financial conflicts to disclose.
SOURCE: Fowler D et al. Lancet Psychiatry. 2018 Jan;5(1):41-50.
FROM THE LANCET PSYCHIATRY
Key clinical point: Adding social recovery therapy significantly improved function in first-episode psychosis patients, compared with early intervention alone.
Major finding: After 9 months, the intervention group averaged 8 more hours of structured activity compared with controls.
Study details: A randomized trial of 155 patients aged 16-35 years.
Disclosures: The National Institute for Health Research funded the study. The investigators had no financial conflicts to disclose.
Source: Fowler D et al. Lancet Psychiatry 2018 Jan;5:41-50.
Pre–bariatric surgery weight loss improves outcomes
Preoperative weight loss improves bariatric surgery outcomes, according to findings from a single-institution retrospective analysis. The weight loss came from following a 4-week low-calorie diet (LCD) and was of greatest benefit to patients who lost 8% or more of their excess weight. These patients had a greater loss of excess weight in the 12 months following surgery, as well as shorter average hospital length of stay.
The results appeared online in the Journal of the American College of Surgeons.
Preliminary studies indicated that short-term weight loss before surgery might reduce surgical complexity by reducing the size of the liver and intra-abdominal fat mass, but it remained uncertain what effect weight loss might have on long-term outcomes.
The LCD included 1,200 kcal/day (45% carbohydrates, 35% protein, 20% fat), which were consumed through five meal-replacement products and one food-based meal. Liquids included at least 80 ounces of calorie-free, caffeine-free, carbonation-free beverages per day. Patients were also instructed to conduct 30 minutes of moderate to vigorous activity per day.
Deborah A. Hutcheon, DCN, and her fellow researchers analyzed data from their own institution, where a presurgical 4-week LCD with a target loss of 8% or more of excess weight had been standard policy already. The population included 355 patients who underwent sleeve gastrectomy (n = 167) or Roux-en-Y gastric bypass (n = 188) between January 2014 and January 2016.
Almost two-thirds (63.3%) of patients achieved the target weight loss before surgery. There were some differences between the two groups. The group that achieved the target contained a greater proportion of men than did the other group (25.5% vs. 13.7%, respectively; P = .013), a higher proportion of white patients (84.8% vs. 74.1%; P = .011), and a higher proportion of patients taking antihypertensive medications (68.3% vs. 57.3%; P = .048). The two groups had similar rates of preoperative comorbidities and surgery types.
Those who achieved the target weight loss had a shorter hospital length of stay (1.8 days vs. 2.1 days; P = .006). They also had a higher percentage loss of excess weight at 3 months (42.3% vs. 36.1%; P less than .001), 6 months (56.0% vs. 47.5%; P less than .001), and at 12 months (65.1% vs. 55.7%; P = .003).
After controlling for patient characteristics, insurance status, 12-month diet compliance, and surgery type, successful presurgery weight loss was associated with greater weight loss at 12 months.
The AGA Obesity Practice Guide was created to provide a comprehensive, multidisciplinary process to personalize innovative obesity care for safe and effective weight management, including nonsurgical and endoscopic management of obesity. Learn more at www.gastro.org/obesity.
SOURCE: Hutcheon DA et al. J Am Coll Surgeons. 2018 Jan 31. doi: 10.1016/j.jamcollsurg.2017.12.032.
Preoperative weight loss improves bariatric surgery outcomes, according to findings from a single-institution retrospective analysis. The weight loss came from following a 4-week low-calorie diet (LCD) and was of greatest benefit to patients who lost 8% or more of their excess weight. These patients had a greater loss of excess weight in the 12 months following surgery, as well as shorter average hospital length of stay.
The results appeared online in the Journal of the American College of Surgeons.
Preliminary studies indicated that short-term weight loss before surgery might reduce surgical complexity by reducing the size of the liver and intra-abdominal fat mass, but it remained uncertain what effect weight loss might have on long-term outcomes.
The LCD included 1,200 kcal/day (45% carbohydrates, 35% protein, 20% fat), which were consumed through five meal-replacement products and one food-based meal. Liquids included at least 80 ounces of calorie-free, caffeine-free, carbonation-free beverages per day. Patients were also instructed to conduct 30 minutes of moderate to vigorous activity per day.
Deborah A. Hutcheon, DCN, and her fellow researchers analyzed data from their own institution, where a presurgical 4-week LCD with a target loss of 8% or more of excess weight had been standard policy already. The population included 355 patients who underwent sleeve gastrectomy (n = 167) or Roux-en-Y gastric bypass (n = 188) between January 2014 and January 2016.
Almost two-thirds (63.3%) of patients achieved the target weight loss before surgery. There were some differences between the two groups. The group that achieved the target contained a greater proportion of men than did the other group (25.5% vs. 13.7%, respectively; P = .013), a higher proportion of white patients (84.8% vs. 74.1%; P = .011), and a higher proportion of patients taking antihypertensive medications (68.3% vs. 57.3%; P = .048). The two groups had similar rates of preoperative comorbidities and surgery types.
Those who achieved the target weight loss had a shorter hospital length of stay (1.8 days vs. 2.1 days; P = .006). They also had a higher percentage loss of excess weight at 3 months (42.3% vs. 36.1%; P less than .001), 6 months (56.0% vs. 47.5%; P less than .001), and at 12 months (65.1% vs. 55.7%; P = .003).
After controlling for patient characteristics, insurance status, 12-month diet compliance, and surgery type, successful presurgery weight loss was associated with greater weight loss at 12 months.
The AGA Obesity Practice Guide was created to provide a comprehensive, multidisciplinary process to personalize innovative obesity care for safe and effective weight management, including nonsurgical and endoscopic management of obesity. Learn more at www.gastro.org/obesity.
SOURCE: Hutcheon DA et al. J Am Coll Surgeons. 2018 Jan 31. doi: 10.1016/j.jamcollsurg.2017.12.032.
Preoperative weight loss improves bariatric surgery outcomes, according to findings from a single-institution retrospective analysis. The weight loss came from following a 4-week low-calorie diet (LCD) and was of greatest benefit to patients who lost 8% or more of their excess weight. These patients had a greater loss of excess weight in the 12 months following surgery, as well as shorter average hospital length of stay.
The results appeared online in the Journal of the American College of Surgeons.
Preliminary studies indicated that short-term weight loss before surgery might reduce surgical complexity by reducing the size of the liver and intra-abdominal fat mass, but it remained uncertain what effect weight loss might have on long-term outcomes.
The LCD included 1,200 kcal/day (45% carbohydrates, 35% protein, 20% fat), which were consumed through five meal-replacement products and one food-based meal. Liquids included at least 80 ounces of calorie-free, caffeine-free, carbonation-free beverages per day. Patients were also instructed to conduct 30 minutes of moderate to vigorous activity per day.
Deborah A. Hutcheon, DCN, and her fellow researchers analyzed data from their own institution, where a presurgical 4-week LCD with a target loss of 8% or more of excess weight had been standard policy already. The population included 355 patients who underwent sleeve gastrectomy (n = 167) or Roux-en-Y gastric bypass (n = 188) between January 2014 and January 2016.
Almost two-thirds (63.3%) of patients achieved the target weight loss before surgery. There were some differences between the two groups. The group that achieved the target contained a greater proportion of men than did the other group (25.5% vs. 13.7%, respectively; P = .013), a higher proportion of white patients (84.8% vs. 74.1%; P = .011), and a higher proportion of patients taking antihypertensive medications (68.3% vs. 57.3%; P = .048). The two groups had similar rates of preoperative comorbidities and surgery types.
Those who achieved the target weight loss had a shorter hospital length of stay (1.8 days vs. 2.1 days; P = .006). They also had a higher percentage loss of excess weight at 3 months (42.3% vs. 36.1%; P less than .001), 6 months (56.0% vs. 47.5%; P less than .001), and at 12 months (65.1% vs. 55.7%; P = .003).
After controlling for patient characteristics, insurance status, 12-month diet compliance, and surgery type, successful presurgery weight loss was associated with greater weight loss at 12 months.
The AGA Obesity Practice Guide was created to provide a comprehensive, multidisciplinary process to personalize innovative obesity care for safe and effective weight management, including nonsurgical and endoscopic management of obesity. Learn more at www.gastro.org/obesity.
SOURCE: Hutcheon DA et al. J Am Coll Surgeons. 2018 Jan 31. doi: 10.1016/j.jamcollsurg.2017.12.032.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Key clinical point: Weight loss before bariatric surgery boosts results.
Major finding: Patients who lost at least 8% of excess body weight had an average of 65.1% loss of excess weight at 12 months, compared with the 55.7% seen in those who did not.
Data source: Retrospective, single-center analysis (n = 355).
Disclosures: No source of funding was disclosed.
Source: Hutcheon DA et al. J Am Coll Surgeons. 2018 Jan 31. doi: 10.1016/j.jamcollsurg.2017.12.032.
Transfusion threshold and bleeding risk in malignancy-related thrombocytopenia
Background: The association between platelet counts, risk of bleeding, and transfusions in patients with thrombocytopenia related to stem cell transplant (SCT) or chemotherapy is not clear, except at very low platelet counts.
Study design: Secondary analysis of a multicenter, randomized controlled trial, stratified by cause of thrombocytopenia: autologous or syngeneic SCT (AUTO), allogeneic SCT (ALLO), or chemotherapy for hematologic malignancy without SCT (CHEMO).
Setting: Twenty-six hospitals from 2004 to 2007.
Synopsis: The PLADO trial enrolled more than 1,200 patients aged 18 years and older expected to experience a period of hypoproliferative thrombocytopenia as a result of chemotherapy or SCT, and randomized them to low, medium, or high doses of prophylactic platelets. This secondary analysis assessed laboratory predictors of bleeding, and the effect of transfusion.
Of 1,077 patients who received platelet transfusions, there were no differences between dose groups for any bleeding outcomes. Over a wide range of platelet counts, the ALLO stratum had a higher risk of bleeding than other strata, with clinically significant bleeding on 21% of patient-days in the ALLO stratum, compared with 19% in the AUTO stratum and 11% in the CHEMO stratum (P less than .001). Risk for bleeding was significantly higher at platelet counts of equal to or less than 5x109/L compared with platelet counts greater than or equal to 81x109/L. Higher aPTT and INR were also associated with higher risk of clinically significant bleeding. In a multipredictor model, only hematocrit was significantly associated with more severe bleeding. Neither platelet transfusion nor RBC transfusion reduced the risk of bleeding on the following day, although the authors note some possibility of confounding by indication.
Bottom line: Predictors of overall increased risk for bleeding in patients with secondary hypoproliferative thrombocytopenia were treatment stratum, platelet counts less than or equal to 5x109/L, hematocrit less than 25%, INR greater than 1.2, and aPTT greater than 30 seconds. This study challenges the conventional wisdom that transfusions reduce bleeding risk in patients with secondary hypoproliferative thrombocytopenia.
Citation: Uhl L, Assmann SF, Hamza TH, et al. Laboratory predictors of bleeding and the effect of platelet and RBC transfusions on bleeding outcomes in the PLADO trial. Blood. 2017;130(10):1247-58.
Background: The association between platelet counts, risk of bleeding, and transfusions in patients with thrombocytopenia related to stem cell transplant (SCT) or chemotherapy is not clear, except at very low platelet counts.
Study design: Secondary analysis of a multicenter, randomized controlled trial, stratified by cause of thrombocytopenia: autologous or syngeneic SCT (AUTO), allogeneic SCT (ALLO), or chemotherapy for hematologic malignancy without SCT (CHEMO).
Setting: Twenty-six hospitals from 2004 to 2007.
Synopsis: The PLADO trial enrolled more than 1,200 patients aged 18 years and older expected to experience a period of hypoproliferative thrombocytopenia as a result of chemotherapy or SCT, and randomized them to low, medium, or high doses of prophylactic platelets. This secondary analysis assessed laboratory predictors of bleeding, and the effect of transfusion.
Of 1,077 patients who received platelet transfusions, there were no differences between dose groups for any bleeding outcomes. Over a wide range of platelet counts, the ALLO stratum had a higher risk of bleeding than other strata, with clinically significant bleeding on 21% of patient-days in the ALLO stratum, compared with 19% in the AUTO stratum and 11% in the CHEMO stratum (P less than .001). Risk for bleeding was significantly higher at platelet counts of equal to or less than 5x109/L compared with platelet counts greater than or equal to 81x109/L. Higher aPTT and INR were also associated with higher risk of clinically significant bleeding. In a multipredictor model, only hematocrit was significantly associated with more severe bleeding. Neither platelet transfusion nor RBC transfusion reduced the risk of bleeding on the following day, although the authors note some possibility of confounding by indication.
Bottom line: Predictors of overall increased risk for bleeding in patients with secondary hypoproliferative thrombocytopenia were treatment stratum, platelet counts less than or equal to 5x109/L, hematocrit less than 25%, INR greater than 1.2, and aPTT greater than 30 seconds. This study challenges the conventional wisdom that transfusions reduce bleeding risk in patients with secondary hypoproliferative thrombocytopenia.
Citation: Uhl L, Assmann SF, Hamza TH, et al. Laboratory predictors of bleeding and the effect of platelet and RBC transfusions on bleeding outcomes in the PLADO trial. Blood. 2017;130(10):1247-58.
Background: The association between platelet counts, risk of bleeding, and transfusions in patients with thrombocytopenia related to stem cell transplant (SCT) or chemotherapy is not clear, except at very low platelet counts.
Study design: Secondary analysis of a multicenter, randomized controlled trial, stratified by cause of thrombocytopenia: autologous or syngeneic SCT (AUTO), allogeneic SCT (ALLO), or chemotherapy for hematologic malignancy without SCT (CHEMO).
Setting: Twenty-six hospitals from 2004 to 2007.
Synopsis: The PLADO trial enrolled more than 1,200 patients aged 18 years and older expected to experience a period of hypoproliferative thrombocytopenia as a result of chemotherapy or SCT, and randomized them to low, medium, or high doses of prophylactic platelets. This secondary analysis assessed laboratory predictors of bleeding, and the effect of transfusion.
Of 1,077 patients who received platelet transfusions, there were no differences between dose groups for any bleeding outcomes. Over a wide range of platelet counts, the ALLO stratum had a higher risk of bleeding than other strata, with clinically significant bleeding on 21% of patient-days in the ALLO stratum, compared with 19% in the AUTO stratum and 11% in the CHEMO stratum (P less than .001). Risk for bleeding was significantly higher at platelet counts of equal to or less than 5x109/L compared with platelet counts greater than or equal to 81x109/L. Higher aPTT and INR were also associated with higher risk of clinically significant bleeding. In a multipredictor model, only hematocrit was significantly associated with more severe bleeding. Neither platelet transfusion nor RBC transfusion reduced the risk of bleeding on the following day, although the authors note some possibility of confounding by indication.
Bottom line: Predictors of overall increased risk for bleeding in patients with secondary hypoproliferative thrombocytopenia were treatment stratum, platelet counts less than or equal to 5x109/L, hematocrit less than 25%, INR greater than 1.2, and aPTT greater than 30 seconds. This study challenges the conventional wisdom that transfusions reduce bleeding risk in patients with secondary hypoproliferative thrombocytopenia.
Citation: Uhl L, Assmann SF, Hamza TH, et al. Laboratory predictors of bleeding and the effect of platelet and RBC transfusions on bleeding outcomes in the PLADO trial. Blood. 2017;130(10):1247-58.
Is 17-OHPC effective for reducing risk of preterm birth?
In 2003, the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medicine Units (MFMU) Network reported on a placebo-controlled randomized study of 17–alpha hydroxyprogesterone caproate (17-OHPC) in women with a history of spontaneous preterm delivery. The study demonstrated a 33% reduction in recurrent preterm birth after weekly treatment with 17-OHPC, which was initiated at 16-20 weeks of gestation.
This landmark study, led by Paul Meis, MD, validated what had been suggested in an earlier meta-analysis (1990) by Mark Keirse, MD – and it quickly altered clinical practice. It set into motion a string of studies on the use of 17-OHPC and other progestational compounds in women with a variety of conditions associated with an increased risk for preterm birth.
It is not surprising, then, that the literature has become muddied and full of contradictory findings since publication of the Meis study and the initial studies on vaginal progesterone in women with a midtrimester short cervix. Further confounding our ability to judge a treatment’s effectiveness is the fact that spontaneous preterm birth is increasingly understood to be a multifactorial, highly heterogeneous condition. We cannot, with a broad stroke, say that all women with a prior preterm birth, for instance, will respond to progestogens in a similar manner or are at the same level of risk of recurrent spontaneous preterm birth (sPTB).
The number of large, randomized clinical trials evaluating progestins is actually quite small but opinions abound about the data from these studies. Below, I have categorized these treatments according to my view at this time of the currently available data.
Consensus
One area in which there is agreement concerns the use of 17-OHPC intramuscular injections in multifetal gestations. Two randomized clinical trials undertaken by the MFMU Network – one in twins and one in triplets – concluded that 17-OHPC is ineffective in reducing the rate of preterm birth. Moreover, in another, more recent MFMU Network study, there was a negative linear relationship between concentrations of 17-OHPC and gestational age at delivery. Women with twin gestations who had higher concentrations of 17-OHPC delivered at earlier gestational ages than women with lower concentrations (Am J Obstet Gynecol. 2012;207[5]:396.e1-8).
Other investigators have similarly shown in clinical trials that the preterm birth rate actually seems to be worsened in multifetal gestations when 17-OHPC is used. There is now widespread agreement that the compound should not be used in these patients.
In addition, an MFMU Network study led by William A. Grobman, MD, demonstrated that 17-OHPC (250-mg injections) does not provide any benefit to nulliparous women with a sonographic cervical length less than 30 mm (Am J Obstet Gynecol. 2012;207[5]:390.e1-8). Other studies utilizing higher doses of 17-OHPC similarly found no benefit. There is also agreement that 17-OHPC has no benefit in treating women with preterm premature rupture of the membranes, preterm labor, or as a maintenance treatment after an episode of preterm labor.
General agreement without consensus
There is general agreement that women with a singleton gestation and a prior spontaneous preterm birth should be offered 17-OHPC, and that women with a singleton gestation and a midtrimester shortened cervical length should be offered vaginal progesterone and not 17-OHPC. However, even in these populations, there are questions about efficacy, dosing, and other issues.
In the Meis study (N Engl J Med. 2003;348:2379-85), treatment with 17-OHPC in women with a singleton gestation and a prior preterm delivery significantly reduced the risk of another preterm birth at less than 37 weeks’ gestation (36.3% in the progesterone group vs. 54.9% in the placebo group; relative risk, 0.66), at less than 35 weeks’ gestation (RR, 0.67), and at less than 32 weeks’ gestation (RR, 0.58). The exceptionally high rate of preterm delivery in the placebo group, however, prompted other investigators to express concern in published correspondence that the study was potentially flawed.
We reported an inverse relationship between 17-OHPC concentration and spontaneous preterm birth as part of a study conducted with the MFMU Network and the Obstetrical-Fetal Pharmacology Research Units Network. All women in the study had singleton gestations and received 250 mg weekly 17-OHPC (the broader study was designed to evaluate the benefit of omega-3 supplementation). We measured plasma concentrations of 17-OHPC and found that women with concentrations in the lowest quartile had a significantly higher risk of preterm birth and delivered at significantly earlier gestational ages than did women in the second through fourth quartiles (Am J Obstet Gynecol. 2014;210[2]:128.e1-6).
Other studies/abstracts similarly evaluating the relationship between 17-OHPC concentrations and preterm birth have reported mixed results, with both validation and refutation of our findings.
Research underway may help settle the controversy. In an ongoing, open-label pharmacology study being conducted by the Obstetrical-Fetal Pharmacology Research Units Network, women with singleton pregnancies and a history of prior preterm birth are being randomly assigned to receive either 250 mg (the empirically chosen, currently recommended dose) or 500 mg 17-OHPC. A relationship between the plasma concentration of 17-OHPC at 26-30 weeks’ gestation and the incidence of preterm birth would offer proof of efficacy and could help elucidate the therapeutic dosing; if there is no relationship, we revert to the question of whether the agent really works. Based on current evidence, both the Society for Maternal-Fetal Medicine (SMFM) and the American College of Obstetricians and Gynecologists (ACOG) support the use of 17-OHPC for prevention of sPTB in women with a prior sPTB.
Questions about vaginal progesterone have also been somewhat unsettled. Eduardo B. Fonseca, MD, reported in 2007 that asymptomatic women with a short cervix (defined as 15 mm or less) who were randomized to receive vaginal progesterone at a median of 22 weeks’ gestation had a significantly lower rate of preterm birth before 34 weeks’ gestation than those who received placebo (RR, 0.56; N Engl J Med. 2007;357[5]:462-9). Research that followed offered mixed conclusions, with a study by Sonia S. Hassan, MD, showing benefit and a study by Jane E. Norman, MD, showing no benefit. Notably, in 2012, the Food and Drug Administration voted against approval of a sustained-release progesterone vaginal gel, citing research results that were not sufficiently compelling.
Still, vaginal progesterone has been endorsed by both ACOG and by the SMFM for women with a short cervical length in the midtrimester. This is supported by a new review and meta-analysis of individual patient data by Roberto Romero, MD, in which vaginal progesterone was found to significantly decrease the risk of preterm birth in singleton gestations with a midtrimester cervical length of 25 mm or less. The reduction occurred over a wide range of gestational ages, including at less than 33 weeks of gestation (RR, 0.62; Am J Obstet Gynecol. 2018 Feb;218[2]:161-80).
Disagreement
Some have argued that vaginal progesterone should be offered to women with a history of prior spontaneous preterm birth, but the largest study to look at this application – a randomized multinational trial reported by John M. O’Brien, MD, and his colleagues in 2007 – found that use of the compound did not reduce the frequency of recurrent preterm birth at or before 32 weeks. Others have argued that vaginal progesterone is of benefit in this group of women based on a combination of multiple subgroup analyses. There is disagreement between ACOG and SMFM on this issue. ACOG supports the use of vaginal progesterone for women with a prior preterm birth but the SMFM strongly rejects this treatment and only endorses 17-OHPC for this indication.
Unresolved
The value of vaginal progesterone supplementation in reducing preterm births in women with twin gestations is under continuing investigation, including a study of women with twin gestation and a short cervix. This MFMU Network randomized trial, now underway, is evaluating the effectiveness of vaginal progesterone or pessary, compared with placebo, in preventing early preterm birth in women carrying twins who have a cervical length less than 30 mm.
Another question about the use of progesterone concerns the woman who delivered preterm during a twin gestation and is now pregnant with a singleton gestation. Should anything be offered to her? This is a question that has not yet been addressed in the literature.
What does seem clear is that spontaneous preterm birth is a multifactorial condition with numerous causes, and quite possibly an interaction between genetics, maternal characteristics, and the environment surrounding each pregnancy (Semin Perinatol. 2016;40[5]:273-80). Certainly, there are different pathways and mechanisms at play in patients who deliver at 35-36 weeks, for instance, compared with those who deliver at 25-26 weeks.
We recently obtained cervical fluid from pregnant women with prior preterm births and analyzed the samples for concentrations of cytokines and matrix metalloproteinases. Women with a prior early preterm delivery at less than 26 weeks had elevations in five cervical cytokines – an inflammatory signature, in essence – while those whose prior preterm birth occurred at a later gestational age had no elevations of these cytokines (Am J Perinatol. 2017 Nov 15. doi: 10.1055/s-0037-1608631).
Hopefully, we soon will be able to identify subpopulations of pregnant women who will benefit more from progesterone supplementation. More research needs to be done at a granular level, with more narrowly defined populations – and with consideration of various pharmacologic, genetic and environmental factors – in order to develop a more specific treatment approach. In the meantime, it is important to appreciate the unknowns that underlie the highly variable clinical responses and outcomes seen in our clinical trials.
Dr. Caritis is professor of obstetrics, gynecology, and reproductive sciences at Magee-Womens Hospital, University of Pittsburgh. He has no disclosures relevant to this Master Class.
In 2003, the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medicine Units (MFMU) Network reported on a placebo-controlled randomized study of 17–alpha hydroxyprogesterone caproate (17-OHPC) in women with a history of spontaneous preterm delivery. The study demonstrated a 33% reduction in recurrent preterm birth after weekly treatment with 17-OHPC, which was initiated at 16-20 weeks of gestation.
This landmark study, led by Paul Meis, MD, validated what had been suggested in an earlier meta-analysis (1990) by Mark Keirse, MD – and it quickly altered clinical practice. It set into motion a string of studies on the use of 17-OHPC and other progestational compounds in women with a variety of conditions associated with an increased risk for preterm birth.
It is not surprising, then, that the literature has become muddied and full of contradictory findings since publication of the Meis study and the initial studies on vaginal progesterone in women with a midtrimester short cervix. Further confounding our ability to judge a treatment’s effectiveness is the fact that spontaneous preterm birth is increasingly understood to be a multifactorial, highly heterogeneous condition. We cannot, with a broad stroke, say that all women with a prior preterm birth, for instance, will respond to progestogens in a similar manner or are at the same level of risk of recurrent spontaneous preterm birth (sPTB).
The number of large, randomized clinical trials evaluating progestins is actually quite small but opinions abound about the data from these studies. Below, I have categorized these treatments according to my view at this time of the currently available data.
Consensus
One area in which there is agreement concerns the use of 17-OHPC intramuscular injections in multifetal gestations. Two randomized clinical trials undertaken by the MFMU Network – one in twins and one in triplets – concluded that 17-OHPC is ineffective in reducing the rate of preterm birth. Moreover, in another, more recent MFMU Network study, there was a negative linear relationship between concentrations of 17-OHPC and gestational age at delivery. Women with twin gestations who had higher concentrations of 17-OHPC delivered at earlier gestational ages than women with lower concentrations (Am J Obstet Gynecol. 2012;207[5]:396.e1-8).
Other investigators have similarly shown in clinical trials that the preterm birth rate actually seems to be worsened in multifetal gestations when 17-OHPC is used. There is now widespread agreement that the compound should not be used in these patients.
In addition, an MFMU Network study led by William A. Grobman, MD, demonstrated that 17-OHPC (250-mg injections) does not provide any benefit to nulliparous women with a sonographic cervical length less than 30 mm (Am J Obstet Gynecol. 2012;207[5]:390.e1-8). Other studies utilizing higher doses of 17-OHPC similarly found no benefit. There is also agreement that 17-OHPC has no benefit in treating women with preterm premature rupture of the membranes, preterm labor, or as a maintenance treatment after an episode of preterm labor.
General agreement without consensus
There is general agreement that women with a singleton gestation and a prior spontaneous preterm birth should be offered 17-OHPC, and that women with a singleton gestation and a midtrimester shortened cervical length should be offered vaginal progesterone and not 17-OHPC. However, even in these populations, there are questions about efficacy, dosing, and other issues.
In the Meis study (N Engl J Med. 2003;348:2379-85), treatment with 17-OHPC in women with a singleton gestation and a prior preterm delivery significantly reduced the risk of another preterm birth at less than 37 weeks’ gestation (36.3% in the progesterone group vs. 54.9% in the placebo group; relative risk, 0.66), at less than 35 weeks’ gestation (RR, 0.67), and at less than 32 weeks’ gestation (RR, 0.58). The exceptionally high rate of preterm delivery in the placebo group, however, prompted other investigators to express concern in published correspondence that the study was potentially flawed.
We reported an inverse relationship between 17-OHPC concentration and spontaneous preterm birth as part of a study conducted with the MFMU Network and the Obstetrical-Fetal Pharmacology Research Units Network. All women in the study had singleton gestations and received 250 mg weekly 17-OHPC (the broader study was designed to evaluate the benefit of omega-3 supplementation). We measured plasma concentrations of 17-OHPC and found that women with concentrations in the lowest quartile had a significantly higher risk of preterm birth and delivered at significantly earlier gestational ages than did women in the second through fourth quartiles (Am J Obstet Gynecol. 2014;210[2]:128.e1-6).
Other studies/abstracts similarly evaluating the relationship between 17-OHPC concentrations and preterm birth have reported mixed results, with both validation and refutation of our findings.
Research underway may help settle the controversy. In an ongoing, open-label pharmacology study being conducted by the Obstetrical-Fetal Pharmacology Research Units Network, women with singleton pregnancies and a history of prior preterm birth are being randomly assigned to receive either 250 mg (the empirically chosen, currently recommended dose) or 500 mg 17-OHPC. A relationship between the plasma concentration of 17-OHPC at 26-30 weeks’ gestation and the incidence of preterm birth would offer proof of efficacy and could help elucidate the therapeutic dosing; if there is no relationship, we revert to the question of whether the agent really works. Based on current evidence, both the Society for Maternal-Fetal Medicine (SMFM) and the American College of Obstetricians and Gynecologists (ACOG) support the use of 17-OHPC for prevention of sPTB in women with a prior sPTB.
Questions about vaginal progesterone have also been somewhat unsettled. Eduardo B. Fonseca, MD, reported in 2007 that asymptomatic women with a short cervix (defined as 15 mm or less) who were randomized to receive vaginal progesterone at a median of 22 weeks’ gestation had a significantly lower rate of preterm birth before 34 weeks’ gestation than those who received placebo (RR, 0.56; N Engl J Med. 2007;357[5]:462-9). Research that followed offered mixed conclusions, with a study by Sonia S. Hassan, MD, showing benefit and a study by Jane E. Norman, MD, showing no benefit. Notably, in 2012, the Food and Drug Administration voted against approval of a sustained-release progesterone vaginal gel, citing research results that were not sufficiently compelling.
Still, vaginal progesterone has been endorsed by both ACOG and by the SMFM for women with a short cervical length in the midtrimester. This is supported by a new review and meta-analysis of individual patient data by Roberto Romero, MD, in which vaginal progesterone was found to significantly decrease the risk of preterm birth in singleton gestations with a midtrimester cervical length of 25 mm or less. The reduction occurred over a wide range of gestational ages, including at less than 33 weeks of gestation (RR, 0.62; Am J Obstet Gynecol. 2018 Feb;218[2]:161-80).
Disagreement
Some have argued that vaginal progesterone should be offered to women with a history of prior spontaneous preterm birth, but the largest study to look at this application – a randomized multinational trial reported by John M. O’Brien, MD, and his colleagues in 2007 – found that use of the compound did not reduce the frequency of recurrent preterm birth at or before 32 weeks. Others have argued that vaginal progesterone is of benefit in this group of women based on a combination of multiple subgroup analyses. There is disagreement between ACOG and SMFM on this issue. ACOG supports the use of vaginal progesterone for women with a prior preterm birth but the SMFM strongly rejects this treatment and only endorses 17-OHPC for this indication.
Unresolved
The value of vaginal progesterone supplementation in reducing preterm births in women with twin gestations is under continuing investigation, including a study of women with twin gestation and a short cervix. This MFMU Network randomized trial, now underway, is evaluating the effectiveness of vaginal progesterone or pessary, compared with placebo, in preventing early preterm birth in women carrying twins who have a cervical length less than 30 mm.
Another question about the use of progesterone concerns the woman who delivered preterm during a twin gestation and is now pregnant with a singleton gestation. Should anything be offered to her? This is a question that has not yet been addressed in the literature.
What does seem clear is that spontaneous preterm birth is a multifactorial condition with numerous causes, and quite possibly an interaction between genetics, maternal characteristics, and the environment surrounding each pregnancy (Semin Perinatol. 2016;40[5]:273-80). Certainly, there are different pathways and mechanisms at play in patients who deliver at 35-36 weeks, for instance, compared with those who deliver at 25-26 weeks.
We recently obtained cervical fluid from pregnant women with prior preterm births and analyzed the samples for concentrations of cytokines and matrix metalloproteinases. Women with a prior early preterm delivery at less than 26 weeks had elevations in five cervical cytokines – an inflammatory signature, in essence – while those whose prior preterm birth occurred at a later gestational age had no elevations of these cytokines (Am J Perinatol. 2017 Nov 15. doi: 10.1055/s-0037-1608631).
Hopefully, we soon will be able to identify subpopulations of pregnant women who will benefit more from progesterone supplementation. More research needs to be done at a granular level, with more narrowly defined populations – and with consideration of various pharmacologic, genetic and environmental factors – in order to develop a more specific treatment approach. In the meantime, it is important to appreciate the unknowns that underlie the highly variable clinical responses and outcomes seen in our clinical trials.
Dr. Caritis is professor of obstetrics, gynecology, and reproductive sciences at Magee-Womens Hospital, University of Pittsburgh. He has no disclosures relevant to this Master Class.
In 2003, the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medicine Units (MFMU) Network reported on a placebo-controlled randomized study of 17–alpha hydroxyprogesterone caproate (17-OHPC) in women with a history of spontaneous preterm delivery. The study demonstrated a 33% reduction in recurrent preterm birth after weekly treatment with 17-OHPC, which was initiated at 16-20 weeks of gestation.
This landmark study, led by Paul Meis, MD, validated what had been suggested in an earlier meta-analysis (1990) by Mark Keirse, MD – and it quickly altered clinical practice. It set into motion a string of studies on the use of 17-OHPC and other progestational compounds in women with a variety of conditions associated with an increased risk for preterm birth.
It is not surprising, then, that the literature has become muddied and full of contradictory findings since publication of the Meis study and the initial studies on vaginal progesterone in women with a midtrimester short cervix. Further confounding our ability to judge a treatment’s effectiveness is the fact that spontaneous preterm birth is increasingly understood to be a multifactorial, highly heterogeneous condition. We cannot, with a broad stroke, say that all women with a prior preterm birth, for instance, will respond to progestogens in a similar manner or are at the same level of risk of recurrent spontaneous preterm birth (sPTB).
The number of large, randomized clinical trials evaluating progestins is actually quite small but opinions abound about the data from these studies. Below, I have categorized these treatments according to my view at this time of the currently available data.
Consensus
One area in which there is agreement concerns the use of 17-OHPC intramuscular injections in multifetal gestations. Two randomized clinical trials undertaken by the MFMU Network – one in twins and one in triplets – concluded that 17-OHPC is ineffective in reducing the rate of preterm birth. Moreover, in another, more recent MFMU Network study, there was a negative linear relationship between concentrations of 17-OHPC and gestational age at delivery. Women with twin gestations who had higher concentrations of 17-OHPC delivered at earlier gestational ages than women with lower concentrations (Am J Obstet Gynecol. 2012;207[5]:396.e1-8).
Other investigators have similarly shown in clinical trials that the preterm birth rate actually seems to be worsened in multifetal gestations when 17-OHPC is used. There is now widespread agreement that the compound should not be used in these patients.
In addition, an MFMU Network study led by William A. Grobman, MD, demonstrated that 17-OHPC (250-mg injections) does not provide any benefit to nulliparous women with a sonographic cervical length less than 30 mm (Am J Obstet Gynecol. 2012;207[5]:390.e1-8). Other studies utilizing higher doses of 17-OHPC similarly found no benefit. There is also agreement that 17-OHPC has no benefit in treating women with preterm premature rupture of the membranes, preterm labor, or as a maintenance treatment after an episode of preterm labor.
General agreement without consensus
There is general agreement that women with a singleton gestation and a prior spontaneous preterm birth should be offered 17-OHPC, and that women with a singleton gestation and a midtrimester shortened cervical length should be offered vaginal progesterone and not 17-OHPC. However, even in these populations, there are questions about efficacy, dosing, and other issues.
In the Meis study (N Engl J Med. 2003;348:2379-85), treatment with 17-OHPC in women with a singleton gestation and a prior preterm delivery significantly reduced the risk of another preterm birth at less than 37 weeks’ gestation (36.3% in the progesterone group vs. 54.9% in the placebo group; relative risk, 0.66), at less than 35 weeks’ gestation (RR, 0.67), and at less than 32 weeks’ gestation (RR, 0.58). The exceptionally high rate of preterm delivery in the placebo group, however, prompted other investigators to express concern in published correspondence that the study was potentially flawed.
We reported an inverse relationship between 17-OHPC concentration and spontaneous preterm birth as part of a study conducted with the MFMU Network and the Obstetrical-Fetal Pharmacology Research Units Network. All women in the study had singleton gestations and received 250 mg weekly 17-OHPC (the broader study was designed to evaluate the benefit of omega-3 supplementation). We measured plasma concentrations of 17-OHPC and found that women with concentrations in the lowest quartile had a significantly higher risk of preterm birth and delivered at significantly earlier gestational ages than did women in the second through fourth quartiles (Am J Obstet Gynecol. 2014;210[2]:128.e1-6).
Other studies/abstracts similarly evaluating the relationship between 17-OHPC concentrations and preterm birth have reported mixed results, with both validation and refutation of our findings.
Research underway may help settle the controversy. In an ongoing, open-label pharmacology study being conducted by the Obstetrical-Fetal Pharmacology Research Units Network, women with singleton pregnancies and a history of prior preterm birth are being randomly assigned to receive either 250 mg (the empirically chosen, currently recommended dose) or 500 mg 17-OHPC. A relationship between the plasma concentration of 17-OHPC at 26-30 weeks’ gestation and the incidence of preterm birth would offer proof of efficacy and could help elucidate the therapeutic dosing; if there is no relationship, we revert to the question of whether the agent really works. Based on current evidence, both the Society for Maternal-Fetal Medicine (SMFM) and the American College of Obstetricians and Gynecologists (ACOG) support the use of 17-OHPC for prevention of sPTB in women with a prior sPTB.
Questions about vaginal progesterone have also been somewhat unsettled. Eduardo B. Fonseca, MD, reported in 2007 that asymptomatic women with a short cervix (defined as 15 mm or less) who were randomized to receive vaginal progesterone at a median of 22 weeks’ gestation had a significantly lower rate of preterm birth before 34 weeks’ gestation than those who received placebo (RR, 0.56; N Engl J Med. 2007;357[5]:462-9). Research that followed offered mixed conclusions, with a study by Sonia S. Hassan, MD, showing benefit and a study by Jane E. Norman, MD, showing no benefit. Notably, in 2012, the Food and Drug Administration voted against approval of a sustained-release progesterone vaginal gel, citing research results that were not sufficiently compelling.
Still, vaginal progesterone has been endorsed by both ACOG and by the SMFM for women with a short cervical length in the midtrimester. This is supported by a new review and meta-analysis of individual patient data by Roberto Romero, MD, in which vaginal progesterone was found to significantly decrease the risk of preterm birth in singleton gestations with a midtrimester cervical length of 25 mm or less. The reduction occurred over a wide range of gestational ages, including at less than 33 weeks of gestation (RR, 0.62; Am J Obstet Gynecol. 2018 Feb;218[2]:161-80).
Disagreement
Some have argued that vaginal progesterone should be offered to women with a history of prior spontaneous preterm birth, but the largest study to look at this application – a randomized multinational trial reported by John M. O’Brien, MD, and his colleagues in 2007 – found that use of the compound did not reduce the frequency of recurrent preterm birth at or before 32 weeks. Others have argued that vaginal progesterone is of benefit in this group of women based on a combination of multiple subgroup analyses. There is disagreement between ACOG and SMFM on this issue. ACOG supports the use of vaginal progesterone for women with a prior preterm birth but the SMFM strongly rejects this treatment and only endorses 17-OHPC for this indication.
Unresolved
The value of vaginal progesterone supplementation in reducing preterm births in women with twin gestations is under continuing investigation, including a study of women with twin gestation and a short cervix. This MFMU Network randomized trial, now underway, is evaluating the effectiveness of vaginal progesterone or pessary, compared with placebo, in preventing early preterm birth in women carrying twins who have a cervical length less than 30 mm.
Another question about the use of progesterone concerns the woman who delivered preterm during a twin gestation and is now pregnant with a singleton gestation. Should anything be offered to her? This is a question that has not yet been addressed in the literature.
What does seem clear is that spontaneous preterm birth is a multifactorial condition with numerous causes, and quite possibly an interaction between genetics, maternal characteristics, and the environment surrounding each pregnancy (Semin Perinatol. 2016;40[5]:273-80). Certainly, there are different pathways and mechanisms at play in patients who deliver at 35-36 weeks, for instance, compared with those who deliver at 25-26 weeks.
We recently obtained cervical fluid from pregnant women with prior preterm births and analyzed the samples for concentrations of cytokines and matrix metalloproteinases. Women with a prior early preterm delivery at less than 26 weeks had elevations in five cervical cytokines – an inflammatory signature, in essence – while those whose prior preterm birth occurred at a later gestational age had no elevations of these cytokines (Am J Perinatol. 2017 Nov 15. doi: 10.1055/s-0037-1608631).
Hopefully, we soon will be able to identify subpopulations of pregnant women who will benefit more from progesterone supplementation. More research needs to be done at a granular level, with more narrowly defined populations – and with consideration of various pharmacologic, genetic and environmental factors – in order to develop a more specific treatment approach. In the meantime, it is important to appreciate the unknowns that underlie the highly variable clinical responses and outcomes seen in our clinical trials.
Dr. Caritis is professor of obstetrics, gynecology, and reproductive sciences at Magee-Womens Hospital, University of Pittsburgh. He has no disclosures relevant to this Master Class.
For preterm birth, we must avoid being too quick to prescribe therapeutic measures
As ob.gyns., our decisions not only deeply affect the health and well-being of our patients, but can also dramatically impact their children and families. Perhaps nowhere else is the gravity of our medical choices more felt than in the management of premature labor. Premature birth is one of the major drivers of infant mortality, which remains a significant public health problem in the United States where the rate of infant mortality is nearly 6 of every 1,000 live births.
Therefore, when the two seminal studies were published that showed using injectable or vaginal progesterone successfully delayed labor with fewer neonatal complications, the findings were quickly embraced and applied clinically. However, subsequent studies indicated that progesterone is only beneficial to a certain subset of patients – those with singleton pregnancies and a short cervix. The variance in the results of this research highlights an important point: We must treat each patient as an individual, based on her unique medical history, circumstances, and, yes, symptoms. One size does not fit all.
Equally important is a greater need across our practice to avoid being too quick to prescribe therapeutic measures that do not treat the root of the problem. We must instead provide guidance based on rigorously conducted research and analysis. However, even very promising results should not necessarily be used to guide all of clinical practice, and certainly not without scrutiny and considerable analysis.
To dissect the available data and present the most current findings regarding progesterone use to prevent preterm labor, we have invited Steve Caritis, MD, professor of obstetrics, gynecology, and reproductive sciences at Magee-Womens Hospital, University of Pittsburgh, to be the guest author for this month’s Master Class.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
As ob.gyns., our decisions not only deeply affect the health and well-being of our patients, but can also dramatically impact their children and families. Perhaps nowhere else is the gravity of our medical choices more felt than in the management of premature labor. Premature birth is one of the major drivers of infant mortality, which remains a significant public health problem in the United States where the rate of infant mortality is nearly 6 of every 1,000 live births.
Therefore, when the two seminal studies were published that showed using injectable or vaginal progesterone successfully delayed labor with fewer neonatal complications, the findings were quickly embraced and applied clinically. However, subsequent studies indicated that progesterone is only beneficial to a certain subset of patients – those with singleton pregnancies and a short cervix. The variance in the results of this research highlights an important point: We must treat each patient as an individual, based on her unique medical history, circumstances, and, yes, symptoms. One size does not fit all.
Equally important is a greater need across our practice to avoid being too quick to prescribe therapeutic measures that do not treat the root of the problem. We must instead provide guidance based on rigorously conducted research and analysis. However, even very promising results should not necessarily be used to guide all of clinical practice, and certainly not without scrutiny and considerable analysis.
To dissect the available data and present the most current findings regarding progesterone use to prevent preterm labor, we have invited Steve Caritis, MD, professor of obstetrics, gynecology, and reproductive sciences at Magee-Womens Hospital, University of Pittsburgh, to be the guest author for this month’s Master Class.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
As ob.gyns., our decisions not only deeply affect the health and well-being of our patients, but can also dramatically impact their children and families. Perhaps nowhere else is the gravity of our medical choices more felt than in the management of premature labor. Premature birth is one of the major drivers of infant mortality, which remains a significant public health problem in the United States where the rate of infant mortality is nearly 6 of every 1,000 live births.
Therefore, when the two seminal studies were published that showed using injectable or vaginal progesterone successfully delayed labor with fewer neonatal complications, the findings were quickly embraced and applied clinically. However, subsequent studies indicated that progesterone is only beneficial to a certain subset of patients – those with singleton pregnancies and a short cervix. The variance in the results of this research highlights an important point: We must treat each patient as an individual, based on her unique medical history, circumstances, and, yes, symptoms. One size does not fit all.
Equally important is a greater need across our practice to avoid being too quick to prescribe therapeutic measures that do not treat the root of the problem. We must instead provide guidance based on rigorously conducted research and analysis. However, even very promising results should not necessarily be used to guide all of clinical practice, and certainly not without scrutiny and considerable analysis.
To dissect the available data and present the most current findings regarding progesterone use to prevent preterm labor, we have invited Steve Caritis, MD, professor of obstetrics, gynecology, and reproductive sciences at Magee-Womens Hospital, University of Pittsburgh, to be the guest author for this month’s Master Class.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Objective response rate correlates poorly with overall survival in checkpoint-inhibitor trials
Objective response rate (ORR) correlated poorly with overall survival (OS), but 6-month progression-free survival was a better predictor of 12-month OS, according to a systematic review and meta-analysis of phase 2 and phase 3 trials of checkpoint inhibitors in advanced solid cancers.
Six-month progression-free survival is recommended in place of objective response rate as an endpoint in future phase 2 checkpoint-inhibitor trials, investigators wrote. The report was published in JAMA Oncology.
Appropriate selection of a primary endpoint in phase 2 checkpoint-inhibitor trials is critical to proceed to phase 3 testing. In checkpoint inhibitor trials, the validity of ORR, as determined by RECIST, and PFS as surrogates for OS remains unclear.
The investigators conducted a systematic search of electronic databases for trial results from January 2000 to January 2017, identified through PREMEDLINE, MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials. In addition, abstracts and conference presentations on the European Society for Medical Oncology and American Society of Clinical Oncology websites were hand-searched, wrote Georgia Ritchie, MBBS, of the Cancer Care Centre, St. George Hospital, Sydney, and associates.
Inclusion criteria comprised trials that used checkpoint inhibitors in advanced solid cancers in single-arm or randomized controlled trials of phase 2 and phase 3 designs.
Within the checkpoint inhibitor arms of the trials, r correlation coefficients between ORR with 6-month PFS, ORR with 12-month OS, and 6-month PFS with 12-month OS were 0.37 (95% confidence interval, −0.06 to 0.95), 0.08 (95% confidence interval, −0.17 to 0.70), and 0.74 (95% confidence interval, 0.57-0.92), respectively, Dr. Ritchie and associates reported. To validate an OS prediction model, the investigators found a good calibration between 6-month PFS and actual and predicted 12-month OS. However, when ORR was used to predict 6-month PFS and 12-month OS rates, the actual vs. predicted rates calibrated poorly, they said.
A strength of the study is its generalizability, because of a heterogeneous population of patients with advanced cancer. “Future phase 2 trials might require a larger sample size, and more resources to report on this result than RECIST ORR,” reported the authors. Further research is required to assess the validity of milestone analysis with 6-month PFS as a potential surrogate for OS in treatment comparisons between checkpoint inhibitors and standard of care therapy, they added.
The authors reported no conflicts of interest.
SOURCE: Ritchie G et al., JAMA Oncol. 2018 Feb 22 doi: 10.1001/jamaoncol.2017.5236.
Objective response rate (ORR) correlated poorly with overall survival (OS), but 6-month progression-free survival was a better predictor of 12-month OS, according to a systematic review and meta-analysis of phase 2 and phase 3 trials of checkpoint inhibitors in advanced solid cancers.
Six-month progression-free survival is recommended in place of objective response rate as an endpoint in future phase 2 checkpoint-inhibitor trials, investigators wrote. The report was published in JAMA Oncology.
Appropriate selection of a primary endpoint in phase 2 checkpoint-inhibitor trials is critical to proceed to phase 3 testing. In checkpoint inhibitor trials, the validity of ORR, as determined by RECIST, and PFS as surrogates for OS remains unclear.
The investigators conducted a systematic search of electronic databases for trial results from January 2000 to January 2017, identified through PREMEDLINE, MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials. In addition, abstracts and conference presentations on the European Society for Medical Oncology and American Society of Clinical Oncology websites were hand-searched, wrote Georgia Ritchie, MBBS, of the Cancer Care Centre, St. George Hospital, Sydney, and associates.
Inclusion criteria comprised trials that used checkpoint inhibitors in advanced solid cancers in single-arm or randomized controlled trials of phase 2 and phase 3 designs.
Within the checkpoint inhibitor arms of the trials, r correlation coefficients between ORR with 6-month PFS, ORR with 12-month OS, and 6-month PFS with 12-month OS were 0.37 (95% confidence interval, −0.06 to 0.95), 0.08 (95% confidence interval, −0.17 to 0.70), and 0.74 (95% confidence interval, 0.57-0.92), respectively, Dr. Ritchie and associates reported. To validate an OS prediction model, the investigators found a good calibration between 6-month PFS and actual and predicted 12-month OS. However, when ORR was used to predict 6-month PFS and 12-month OS rates, the actual vs. predicted rates calibrated poorly, they said.
A strength of the study is its generalizability, because of a heterogeneous population of patients with advanced cancer. “Future phase 2 trials might require a larger sample size, and more resources to report on this result than RECIST ORR,” reported the authors. Further research is required to assess the validity of milestone analysis with 6-month PFS as a potential surrogate for OS in treatment comparisons between checkpoint inhibitors and standard of care therapy, they added.
The authors reported no conflicts of interest.
SOURCE: Ritchie G et al., JAMA Oncol. 2018 Feb 22 doi: 10.1001/jamaoncol.2017.5236.
Objective response rate (ORR) correlated poorly with overall survival (OS), but 6-month progression-free survival was a better predictor of 12-month OS, according to a systematic review and meta-analysis of phase 2 and phase 3 trials of checkpoint inhibitors in advanced solid cancers.
Six-month progression-free survival is recommended in place of objective response rate as an endpoint in future phase 2 checkpoint-inhibitor trials, investigators wrote. The report was published in JAMA Oncology.
Appropriate selection of a primary endpoint in phase 2 checkpoint-inhibitor trials is critical to proceed to phase 3 testing. In checkpoint inhibitor trials, the validity of ORR, as determined by RECIST, and PFS as surrogates for OS remains unclear.
The investigators conducted a systematic search of electronic databases for trial results from January 2000 to January 2017, identified through PREMEDLINE, MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials. In addition, abstracts and conference presentations on the European Society for Medical Oncology and American Society of Clinical Oncology websites were hand-searched, wrote Georgia Ritchie, MBBS, of the Cancer Care Centre, St. George Hospital, Sydney, and associates.
Inclusion criteria comprised trials that used checkpoint inhibitors in advanced solid cancers in single-arm or randomized controlled trials of phase 2 and phase 3 designs.
Within the checkpoint inhibitor arms of the trials, r correlation coefficients between ORR with 6-month PFS, ORR with 12-month OS, and 6-month PFS with 12-month OS were 0.37 (95% confidence interval, −0.06 to 0.95), 0.08 (95% confidence interval, −0.17 to 0.70), and 0.74 (95% confidence interval, 0.57-0.92), respectively, Dr. Ritchie and associates reported. To validate an OS prediction model, the investigators found a good calibration between 6-month PFS and actual and predicted 12-month OS. However, when ORR was used to predict 6-month PFS and 12-month OS rates, the actual vs. predicted rates calibrated poorly, they said.
A strength of the study is its generalizability, because of a heterogeneous population of patients with advanced cancer. “Future phase 2 trials might require a larger sample size, and more resources to report on this result than RECIST ORR,” reported the authors. Further research is required to assess the validity of milestone analysis with 6-month PFS as a potential surrogate for OS in treatment comparisons between checkpoint inhibitors and standard of care therapy, they added.
The authors reported no conflicts of interest.
SOURCE: Ritchie G et al., JAMA Oncol. 2018 Feb 22 doi: 10.1001/jamaoncol.2017.5236.
FROM JAMA ONCOLOGY
Key clinical point: Immune checkpoint inhibitors activate anti-tumor T-cells to detect and destroy tumor cells and have become the standard of care for many patients with advanced solid cancers. The most appropriate primary endpoint in phase 2 trials of checkpoint inhibitors remains uncertain.
Major finding: In this systematic review and meta-analysis of phase 2 and phase 3 trials of checkpoint inhibitors in advanced solid cancers, objective response rate correlated poorly with overall survival, but 6-month progression-free survival was a better predictor of 12-month overall survival.
Study details: Trials listed in electronic databases from 2000 to 2017 (PREMEDLINE, MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials).
Disclosures: None reported.
Source: Ritchie G et al. JAMA Oncol. 2018 Feb 22. doi: 10.1001/jamaoncol.2017.5236.
Hospital chemo carries higher price tag than the office
Commercial insurers are spending nearly twice as much on chemotherapy administered in hospital outpatient departments as they are for therapy administered in a physician’s office, according to an analysis of a decade of claims data.
Commercial insurance data from 283,502 patients who initiated treatment with infused chemotherapy and remained enrolled continuously for 6 months, without receiving infused chemotherapy in the preceding 6 months, revealed that spending at the drug level was “significantly lower in offices vs. in HOPDs [hospital outpatient departments],” Aaron Winn, PhD, of the Medical College of Wisconsin, Milwaukee, and his colleagues wrote in a research letter published Feb. 22 in JAMA Oncology.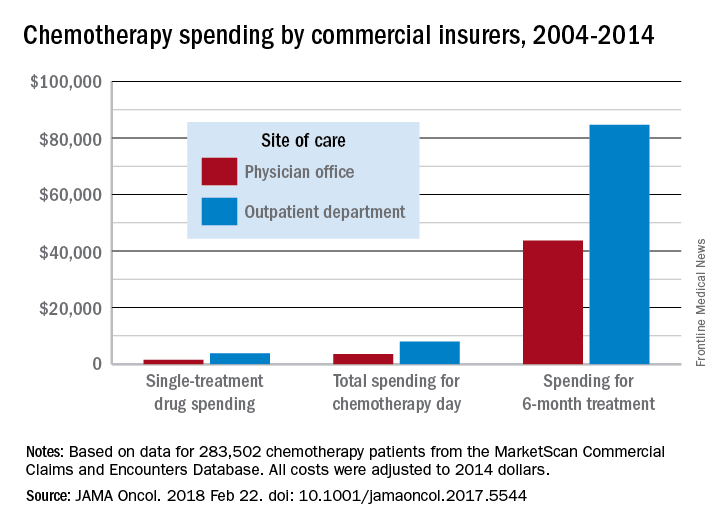
During the review period from Jan. 1, 2004 through Dec. 31, 2014, the rate of commercially-insured patients receiving chemotherapy in HOPDs grew from 6% in 2004 to 43% in 2014. The spending data was adjusted for various factors, including, sex, comorbidity, year of diagnosis, drug administered, and location.
“Shifting the provision of infused chemotherapy from physician offices to HOPDs is increasing and is associated with increased spending for chemotherapy services,” the researchers wrote. “Potential targets for reduction of excess spending can come from private insurers following Medicaid’s lead, which has started to equalize payments across sites of care.”
“I was a little surprised that the site location was converging on 50-50 across the country,” Dr. Carole Miller, director of the Cancer Institute at St. Agnes Hospital, Baltimore, said in an interview, adding that the spending figures were not a surprise.
Dr. Miller noted that another thing the claims data does not capture are the kinds of additional services that patients are receiving in their respective sites of care, which could also account for the difference in total reimbursement. For example, hospital-based cancer centers may offer more social support service given that the patients tend to be older and may have more social service needs as well as more uncompensated care.
David Henry, MD, an oncologist who practices in a community setting that is part of the University of Pennsylvania hospital system, said the data fits with his experience. “Is the care better? I don’t think so. Is the overhead bigger? Sure.”
Part of what makes the care better in the community setting is the patient experience, said Dr. Henry, who serves as editor-in-chief of the Journal of Community and Supportive Oncology, which is published by this news organization.
The office setting can often boast a streamlined experience, he added. In the hospital, the administrative elements and travel across the hospital campus can make an infusion a day-long task, versus going to a community office setting where the total infusion process, including the administrative aspects, can be handled in a few hours.
Dr. Henry acknowledged that the hospital setting does have an advantage in terms of depth of services, including specialists to deal with a variety of tumors.
Dr. Henry noted that the current analysis does not address the shift to paying for value and the move away from fee-for-service payment. Speaking about whether Medicare’s Quality Payment Program can level the playing field in some ways between the two settings of care, he said “the idea has potential” but the success will be determined by how it is implemented.
This article was updated on 2/22/18.
SOURCE: Winn A et al., JAMA Oncol. 2018 Feb 22. doi: 10.1001/jamaoncol.2017.5544.
Commercial insurers are spending nearly twice as much on chemotherapy administered in hospital outpatient departments as they are for therapy administered in a physician’s office, according to an analysis of a decade of claims data.
Commercial insurance data from 283,502 patients who initiated treatment with infused chemotherapy and remained enrolled continuously for 6 months, without receiving infused chemotherapy in the preceding 6 months, revealed that spending at the drug level was “significantly lower in offices vs. in HOPDs [hospital outpatient departments],” Aaron Winn, PhD, of the Medical College of Wisconsin, Milwaukee, and his colleagues wrote in a research letter published Feb. 22 in JAMA Oncology.
During the review period from Jan. 1, 2004 through Dec. 31, 2014, the rate of commercially-insured patients receiving chemotherapy in HOPDs grew from 6% in 2004 to 43% in 2014. The spending data was adjusted for various factors, including, sex, comorbidity, year of diagnosis, drug administered, and location.
“Shifting the provision of infused chemotherapy from physician offices to HOPDs is increasing and is associated with increased spending for chemotherapy services,” the researchers wrote. “Potential targets for reduction of excess spending can come from private insurers following Medicaid’s lead, which has started to equalize payments across sites of care.”
“I was a little surprised that the site location was converging on 50-50 across the country,” Dr. Carole Miller, director of the Cancer Institute at St. Agnes Hospital, Baltimore, said in an interview, adding that the spending figures were not a surprise.
Dr. Miller noted that another thing the claims data does not capture are the kinds of additional services that patients are receiving in their respective sites of care, which could also account for the difference in total reimbursement. For example, hospital-based cancer centers may offer more social support service given that the patients tend to be older and may have more social service needs as well as more uncompensated care.
David Henry, MD, an oncologist who practices in a community setting that is part of the University of Pennsylvania hospital system, said the data fits with his experience. “Is the care better? I don’t think so. Is the overhead bigger? Sure.”
Part of what makes the care better in the community setting is the patient experience, said Dr. Henry, who serves as editor-in-chief of the Journal of Community and Supportive Oncology, which is published by this news organization.
The office setting can often boast a streamlined experience, he added. In the hospital, the administrative elements and travel across the hospital campus can make an infusion a day-long task, versus going to a community office setting where the total infusion process, including the administrative aspects, can be handled in a few hours.
Dr. Henry acknowledged that the hospital setting does have an advantage in terms of depth of services, including specialists to deal with a variety of tumors.
Dr. Henry noted that the current analysis does not address the shift to paying for value and the move away from fee-for-service payment. Speaking about whether Medicare’s Quality Payment Program can level the playing field in some ways between the two settings of care, he said “the idea has potential” but the success will be determined by how it is implemented.
This article was updated on 2/22/18.
SOURCE: Winn A et al., JAMA Oncol. 2018 Feb 22. doi: 10.1001/jamaoncol.2017.5544.
Commercial insurers are spending nearly twice as much on chemotherapy administered in hospital outpatient departments as they are for therapy administered in a physician’s office, according to an analysis of a decade of claims data.
Commercial insurance data from 283,502 patients who initiated treatment with infused chemotherapy and remained enrolled continuously for 6 months, without receiving infused chemotherapy in the preceding 6 months, revealed that spending at the drug level was “significantly lower in offices vs. in HOPDs [hospital outpatient departments],” Aaron Winn, PhD, of the Medical College of Wisconsin, Milwaukee, and his colleagues wrote in a research letter published Feb. 22 in JAMA Oncology.
During the review period from Jan. 1, 2004 through Dec. 31, 2014, the rate of commercially-insured patients receiving chemotherapy in HOPDs grew from 6% in 2004 to 43% in 2014. The spending data was adjusted for various factors, including, sex, comorbidity, year of diagnosis, drug administered, and location.
“Shifting the provision of infused chemotherapy from physician offices to HOPDs is increasing and is associated with increased spending for chemotherapy services,” the researchers wrote. “Potential targets for reduction of excess spending can come from private insurers following Medicaid’s lead, which has started to equalize payments across sites of care.”
“I was a little surprised that the site location was converging on 50-50 across the country,” Dr. Carole Miller, director of the Cancer Institute at St. Agnes Hospital, Baltimore, said in an interview, adding that the spending figures were not a surprise.
Dr. Miller noted that another thing the claims data does not capture are the kinds of additional services that patients are receiving in their respective sites of care, which could also account for the difference in total reimbursement. For example, hospital-based cancer centers may offer more social support service given that the patients tend to be older and may have more social service needs as well as more uncompensated care.
David Henry, MD, an oncologist who practices in a community setting that is part of the University of Pennsylvania hospital system, said the data fits with his experience. “Is the care better? I don’t think so. Is the overhead bigger? Sure.”
Part of what makes the care better in the community setting is the patient experience, said Dr. Henry, who serves as editor-in-chief of the Journal of Community and Supportive Oncology, which is published by this news organization.
The office setting can often boast a streamlined experience, he added. In the hospital, the administrative elements and travel across the hospital campus can make an infusion a day-long task, versus going to a community office setting where the total infusion process, including the administrative aspects, can be handled in a few hours.
Dr. Henry acknowledged that the hospital setting does have an advantage in terms of depth of services, including specialists to deal with a variety of tumors.
Dr. Henry noted that the current analysis does not address the shift to paying for value and the move away from fee-for-service payment. Speaking about whether Medicare’s Quality Payment Program can level the playing field in some ways between the two settings of care, he said “the idea has potential” but the success will be determined by how it is implemented.
This article was updated on 2/22/18.
SOURCE: Winn A et al., JAMA Oncol. 2018 Feb 22. doi: 10.1001/jamaoncol.2017.5544.
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: Total reimbursement during the 6-month treatment episode was lower in offices ($43,700) than in hospital outpatient departments ($84,660).
Study details: An examination of claims data from 283,502 patients who initiated treatment with infused chemotherapy and remained enrolled continuously for 6 months between Jan. 1, 2004, and Dec. 31, 2014.
Disclosures: The researchers reported having no financial conflicts of interest.
Source: Winn A et al., JAMA Oncol. 2018 Feb 22. doi: 10.1001/jamaoncol.2017.5544.
Cyberliability insurance: Should you purchase a policy?
As hackers become more sophisticated, these .
In 2017, there were at least 477 publicly reported health data breaches in the United States, affecting some 5.6 million patients, up from 450 health care breaches in 2016, according to Protenus, a health care cybersecurity vendor that tracks data breaches reported to the U.S. Department of Health & Human Services.
“A breach is very expensive,” said Mr. Cohen, chair for the New York City Bar Association Committee on Medical Malpractice. “You have the fine to the Office for Civil Rights, which can be in the millions of dollars, and you’re going to have to ameliorate the breach, which can be hundreds of dollars per person, let alone deal with lawsuits from the patients.”
Cyberliability: What’s the risk?
Cyberliability refers to legal dangers arising from data breaches, privacy law violations, and ransomware/cyberextortion threats, as well as data loss and business interruption from computer system failures.
Of the 477 breaches in 2017 analyzed by Protenus, 37% were from hacking, 37% resulted from insider incidents, and 16% stemmed from data loss or theft. About 10% of cases resulted from unknown reasons, according to the report.
Data breaches caused by hackers and malware attacks are rising in the health care sector, said Katherine Keefe, global head of breach response services for Beazley, a national cyberliability insurer and risk management company. Beazley handled 2,615 data breaches in 2017, more than half of which were health care–related, Ms. Keefe said in an interview. The top three causes of health care breaches reported to Beazley in 2017 were accidental disclosure, hack or malware, and insider incidents, according to a recent report from that company
“We see an awful lot of that,” Ms. Keefe said. “There’s been a real surge in successful phishing emails and social engineering that enables criminals to identify medical practice leaders. It’s not hard to dress up an email to look like it’s coming from a specific individual. There are all kinds of increasingly sophisticated tactics to trick people into letting criminals into their systems or tricking people into forwarding money or valuable information.”
Hackers frequently use phishing emails to get employees to download a payload, the portion of malware that performs malicious actions, Mr. Cohen added. Once downloaded, payloads can do significant damage to a medical practice.
“Once you get hit with these payloads, not only can they start pulling information out of the computer system, they can also start doing things, such as turning on laptop cameras, reading emails, listening in on computer microphones,” he said. “All they need is one employee to click.”
Considering cybercoverage
To protect themselves from potential breach expenses, more medical practices are purchasing cyberliability insurance policies. A 2017 survey of 270 insurance brokers and 125 underwriters found that health care has more first-time buyers of stand-alone cyberliability insurance than does any other industry.
However, Mr. Cohen advises that practices should do their research before buying and be aware of the different types of policies, coverage limits, and insurance options.
“Be careful about what it covers,” he said. “Are they going to pay for all the amelioration for all the patients affected? Some policies will cover ‘repairing and disinfecting the system,’ but they will not likely cover all the [Office for Civil Rights] fines.”
The Doctors Company, a national medical liability insurer, provides $50,000 in cybersecurity coverage to all its insured physician members and the option to increase coverage by $1 million in additional protection, according to Crystal Brown, senior vice president of underwriting for the Doctors Company. The coverage protects against regulatory and liability claims arising from theft, loss, or accidental transmission of patient or financial information as well as the cost of data recovery. Another policy offered protects against claims arising from administrative actions pertaining to utilization, licensing, credentialing, and misconduct.
Meanwhile, national medical liability insurer ProAssurance offers health providers a basic cyberliability coverage endorsement in most states on its medical professional liability policy. The insurer also has a branded cyberprogram that allows clients to buy additional and broader coverage at a discounted premium.
“In today’s electronic environment, we are hearing about breaches occurring at both small and large health care practices,” said Melanie Tullos, vice president for ProAssurance. “Small physician practices are just as vulnerable, if not more so, to a cyberbreach and should take the necessary steps to protect patient data against an attack at all measures, including, but not limited to, purchasing cyberliability coverage.
The price of cyberliability insurance varies by risk and other factors, Ms. Tullos said. Generally, the cost of a $1 million cyberliability policy for a single physician practice is less than $1,000, whereas a group of 10 physicians can pay up to $8,000-$9,000, she said in an interview.
Beazley offers policies that cover the expenses and services associated with investigating whether a data breach has occurred, responding to breaches, and liability that may arise from the breach, said Ms. Keefe, of Beazley, which works with companies such as the Doctors Company to provide coverage and also works with state-run malpractice programs to offer a cyberliability component for a small, additional premium, she said.
Ms. Keefe stressed that cyberliability coverage can ensure that physician practices don’t run up a hefty bill in the event of a data breach by paying for separate specialists and damage control.
“One of the reasons doctors should have cyberliability coverage are the costs associated with figuring out what to do if patient records are lost or stolen,” she said. “The cost of hiring a lawyer, hiring a forensics investigator to assess the situation, the cost of notifying the patients, and taking all the steps required by HIPAA can really add up. Most practices don’t have those costs built into their annual budgets. A cyberpolicy acts as a buffer against those expenses.”
Manage risk before a breach
Of course, there is plenty that practices can do to prevent – and protect themselves from – a health data breach before it happens. Providing employee awareness training is an important step, said Craig Musgrave, chief information officer of the Doctors Company. Institute a training program for staff at all levels and go over the basics, such as refraining from opening emails from senders they don’t know, Mr. Musgrave wrote in a recent column. Updating all software regularly and backing up data is also essential. And Mr. Musgrave emphasizes the importance of “whitelisting.”
“Health care systems are fragmented in their management of systems and data,” Mr. Musgrave wrote in his column. “Their ability to patch legacy systems and employ cybersecurity staff varies enormously. Therefore, application whitelisting is essential. Rather than blacklisting known malicious software, an application whitelist prevents the launching of any executable program (known or unknown) that does not have explicit authorization. This, in combination with strong firewalls and network segmentation tools like micro-segmentation, provides stronger security.”
In addition, consider implementing data security policies and incident response protocols as well as employee training on securing patient data, ProAssurance’s Ms. Tullos said.
“A breach can also occur within a third-party vendors system and infiltrate the physician’s records, so it is important to discuss cybersecurity with those vendors and all parties should purchase cyberliability insurance,” she said.
Michael E. Nelson, MD, FCCP, comments: Being old enough to remember a paper chart and scheduling book, I can't help but marvel at the how the electronic health record (EHR) has fallen short of its expectations and added to the cost of medical care. Well, let's add cybersecurity insurance to the cost of doing business. While I love the ability to look at a chest x-ray or CT without a viewbox, I can't think of many other things that the EHR has done to make me a more efficient physician. It has, however, spawned many cottage industries that provide "must have" services with their attendant fees. The ever-increasing regulatory and administrative burdens and costs placed on physicians' practices is making it impossible for smaller practices to remain financially viable, leaving smaller communities without medical services. I don't think this was the intent when we decided to "modernize" medicine. It makes me want to go back to those Halcyon days of the paper chart - try phishing one of those, you hackers.
Michael E. Nelson, MD, FCCP, comments: Being old enough to remember a paper chart and scheduling book, I can't help but marvel at the how the electronic health record (EHR) has fallen short of its expectations and added to the cost of medical care. Well, let's add cybersecurity insurance to the cost of doing business. While I love the ability to look at a chest x-ray or CT without a viewbox, I can't think of many other things that the EHR has done to make me a more efficient physician. It has, however, spawned many cottage industries that provide "must have" services with their attendant fees. The ever-increasing regulatory and administrative burdens and costs placed on physicians' practices is making it impossible for smaller practices to remain financially viable, leaving smaller communities without medical services. I don't think this was the intent when we decided to "modernize" medicine. It makes me want to go back to those Halcyon days of the paper chart - try phishing one of those, you hackers.
Michael E. Nelson, MD, FCCP, comments: Being old enough to remember a paper chart and scheduling book, I can't help but marvel at the how the electronic health record (EHR) has fallen short of its expectations and added to the cost of medical care. Well, let's add cybersecurity insurance to the cost of doing business. While I love the ability to look at a chest x-ray or CT without a viewbox, I can't think of many other things that the EHR has done to make me a more efficient physician. It has, however, spawned many cottage industries that provide "must have" services with their attendant fees. The ever-increasing regulatory and administrative burdens and costs placed on physicians' practices is making it impossible for smaller practices to remain financially viable, leaving smaller communities without medical services. I don't think this was the intent when we decided to "modernize" medicine. It makes me want to go back to those Halcyon days of the paper chart - try phishing one of those, you hackers.
As hackers become more sophisticated, these .
In 2017, there were at least 477 publicly reported health data breaches in the United States, affecting some 5.6 million patients, up from 450 health care breaches in 2016, according to Protenus, a health care cybersecurity vendor that tracks data breaches reported to the U.S. Department of Health & Human Services.
“A breach is very expensive,” said Mr. Cohen, chair for the New York City Bar Association Committee on Medical Malpractice. “You have the fine to the Office for Civil Rights, which can be in the millions of dollars, and you’re going to have to ameliorate the breach, which can be hundreds of dollars per person, let alone deal with lawsuits from the patients.”
Cyberliability: What’s the risk?
Cyberliability refers to legal dangers arising from data breaches, privacy law violations, and ransomware/cyberextortion threats, as well as data loss and business interruption from computer system failures.
Of the 477 breaches in 2017 analyzed by Protenus, 37% were from hacking, 37% resulted from insider incidents, and 16% stemmed from data loss or theft. About 10% of cases resulted from unknown reasons, according to the report.
Data breaches caused by hackers and malware attacks are rising in the health care sector, said Katherine Keefe, global head of breach response services for Beazley, a national cyberliability insurer and risk management company. Beazley handled 2,615 data breaches in 2017, more than half of which were health care–related, Ms. Keefe said in an interview. The top three causes of health care breaches reported to Beazley in 2017 were accidental disclosure, hack or malware, and insider incidents, according to a recent report from that company
“We see an awful lot of that,” Ms. Keefe said. “There’s been a real surge in successful phishing emails and social engineering that enables criminals to identify medical practice leaders. It’s not hard to dress up an email to look like it’s coming from a specific individual. There are all kinds of increasingly sophisticated tactics to trick people into letting criminals into their systems or tricking people into forwarding money or valuable information.”
Hackers frequently use phishing emails to get employees to download a payload, the portion of malware that performs malicious actions, Mr. Cohen added. Once downloaded, payloads can do significant damage to a medical practice.
“Once you get hit with these payloads, not only can they start pulling information out of the computer system, they can also start doing things, such as turning on laptop cameras, reading emails, listening in on computer microphones,” he said. “All they need is one employee to click.”
Considering cybercoverage
To protect themselves from potential breach expenses, more medical practices are purchasing cyberliability insurance policies. A 2017 survey of 270 insurance brokers and 125 underwriters found that health care has more first-time buyers of stand-alone cyberliability insurance than does any other industry.
However, Mr. Cohen advises that practices should do their research before buying and be aware of the different types of policies, coverage limits, and insurance options.
“Be careful about what it covers,” he said. “Are they going to pay for all the amelioration for all the patients affected? Some policies will cover ‘repairing and disinfecting the system,’ but they will not likely cover all the [Office for Civil Rights] fines.”
The Doctors Company, a national medical liability insurer, provides $50,000 in cybersecurity coverage to all its insured physician members and the option to increase coverage by $1 million in additional protection, according to Crystal Brown, senior vice president of underwriting for the Doctors Company. The coverage protects against regulatory and liability claims arising from theft, loss, or accidental transmission of patient or financial information as well as the cost of data recovery. Another policy offered protects against claims arising from administrative actions pertaining to utilization, licensing, credentialing, and misconduct.
Meanwhile, national medical liability insurer ProAssurance offers health providers a basic cyberliability coverage endorsement in most states on its medical professional liability policy. The insurer also has a branded cyberprogram that allows clients to buy additional and broader coverage at a discounted premium.
“In today’s electronic environment, we are hearing about breaches occurring at both small and large health care practices,” said Melanie Tullos, vice president for ProAssurance. “Small physician practices are just as vulnerable, if not more so, to a cyberbreach and should take the necessary steps to protect patient data against an attack at all measures, including, but not limited to, purchasing cyberliability coverage.
The price of cyberliability insurance varies by risk and other factors, Ms. Tullos said. Generally, the cost of a $1 million cyberliability policy for a single physician practice is less than $1,000, whereas a group of 10 physicians can pay up to $8,000-$9,000, she said in an interview.
Beazley offers policies that cover the expenses and services associated with investigating whether a data breach has occurred, responding to breaches, and liability that may arise from the breach, said Ms. Keefe, of Beazley, which works with companies such as the Doctors Company to provide coverage and also works with state-run malpractice programs to offer a cyberliability component for a small, additional premium, she said.
Ms. Keefe stressed that cyberliability coverage can ensure that physician practices don’t run up a hefty bill in the event of a data breach by paying for separate specialists and damage control.
“One of the reasons doctors should have cyberliability coverage are the costs associated with figuring out what to do if patient records are lost or stolen,” she said. “The cost of hiring a lawyer, hiring a forensics investigator to assess the situation, the cost of notifying the patients, and taking all the steps required by HIPAA can really add up. Most practices don’t have those costs built into their annual budgets. A cyberpolicy acts as a buffer against those expenses.”
Manage risk before a breach
Of course, there is plenty that practices can do to prevent – and protect themselves from – a health data breach before it happens. Providing employee awareness training is an important step, said Craig Musgrave, chief information officer of the Doctors Company. Institute a training program for staff at all levels and go over the basics, such as refraining from opening emails from senders they don’t know, Mr. Musgrave wrote in a recent column. Updating all software regularly and backing up data is also essential. And Mr. Musgrave emphasizes the importance of “whitelisting.”
“Health care systems are fragmented in their management of systems and data,” Mr. Musgrave wrote in his column. “Their ability to patch legacy systems and employ cybersecurity staff varies enormously. Therefore, application whitelisting is essential. Rather than blacklisting known malicious software, an application whitelist prevents the launching of any executable program (known or unknown) that does not have explicit authorization. This, in combination with strong firewalls and network segmentation tools like micro-segmentation, provides stronger security.”
In addition, consider implementing data security policies and incident response protocols as well as employee training on securing patient data, ProAssurance’s Ms. Tullos said.
“A breach can also occur within a third-party vendors system and infiltrate the physician’s records, so it is important to discuss cybersecurity with those vendors and all parties should purchase cyberliability insurance,” she said.
As hackers become more sophisticated, these .
In 2017, there were at least 477 publicly reported health data breaches in the United States, affecting some 5.6 million patients, up from 450 health care breaches in 2016, according to Protenus, a health care cybersecurity vendor that tracks data breaches reported to the U.S. Department of Health & Human Services.
“A breach is very expensive,” said Mr. Cohen, chair for the New York City Bar Association Committee on Medical Malpractice. “You have the fine to the Office for Civil Rights, which can be in the millions of dollars, and you’re going to have to ameliorate the breach, which can be hundreds of dollars per person, let alone deal with lawsuits from the patients.”
Cyberliability: What’s the risk?
Cyberliability refers to legal dangers arising from data breaches, privacy law violations, and ransomware/cyberextortion threats, as well as data loss and business interruption from computer system failures.
Of the 477 breaches in 2017 analyzed by Protenus, 37% were from hacking, 37% resulted from insider incidents, and 16% stemmed from data loss or theft. About 10% of cases resulted from unknown reasons, according to the report.
Data breaches caused by hackers and malware attacks are rising in the health care sector, said Katherine Keefe, global head of breach response services for Beazley, a national cyberliability insurer and risk management company. Beazley handled 2,615 data breaches in 2017, more than half of which were health care–related, Ms. Keefe said in an interview. The top three causes of health care breaches reported to Beazley in 2017 were accidental disclosure, hack or malware, and insider incidents, according to a recent report from that company
“We see an awful lot of that,” Ms. Keefe said. “There’s been a real surge in successful phishing emails and social engineering that enables criminals to identify medical practice leaders. It’s not hard to dress up an email to look like it’s coming from a specific individual. There are all kinds of increasingly sophisticated tactics to trick people into letting criminals into their systems or tricking people into forwarding money or valuable information.”
Hackers frequently use phishing emails to get employees to download a payload, the portion of malware that performs malicious actions, Mr. Cohen added. Once downloaded, payloads can do significant damage to a medical practice.
“Once you get hit with these payloads, not only can they start pulling information out of the computer system, they can also start doing things, such as turning on laptop cameras, reading emails, listening in on computer microphones,” he said. “All they need is one employee to click.”
Considering cybercoverage
To protect themselves from potential breach expenses, more medical practices are purchasing cyberliability insurance policies. A 2017 survey of 270 insurance brokers and 125 underwriters found that health care has more first-time buyers of stand-alone cyberliability insurance than does any other industry.
However, Mr. Cohen advises that practices should do their research before buying and be aware of the different types of policies, coverage limits, and insurance options.
“Be careful about what it covers,” he said. “Are they going to pay for all the amelioration for all the patients affected? Some policies will cover ‘repairing and disinfecting the system,’ but they will not likely cover all the [Office for Civil Rights] fines.”
The Doctors Company, a national medical liability insurer, provides $50,000 in cybersecurity coverage to all its insured physician members and the option to increase coverage by $1 million in additional protection, according to Crystal Brown, senior vice president of underwriting for the Doctors Company. The coverage protects against regulatory and liability claims arising from theft, loss, or accidental transmission of patient or financial information as well as the cost of data recovery. Another policy offered protects against claims arising from administrative actions pertaining to utilization, licensing, credentialing, and misconduct.
Meanwhile, national medical liability insurer ProAssurance offers health providers a basic cyberliability coverage endorsement in most states on its medical professional liability policy. The insurer also has a branded cyberprogram that allows clients to buy additional and broader coverage at a discounted premium.
“In today’s electronic environment, we are hearing about breaches occurring at both small and large health care practices,” said Melanie Tullos, vice president for ProAssurance. “Small physician practices are just as vulnerable, if not more so, to a cyberbreach and should take the necessary steps to protect patient data against an attack at all measures, including, but not limited to, purchasing cyberliability coverage.
The price of cyberliability insurance varies by risk and other factors, Ms. Tullos said. Generally, the cost of a $1 million cyberliability policy for a single physician practice is less than $1,000, whereas a group of 10 physicians can pay up to $8,000-$9,000, she said in an interview.
Beazley offers policies that cover the expenses and services associated with investigating whether a data breach has occurred, responding to breaches, and liability that may arise from the breach, said Ms. Keefe, of Beazley, which works with companies such as the Doctors Company to provide coverage and also works with state-run malpractice programs to offer a cyberliability component for a small, additional premium, she said.
Ms. Keefe stressed that cyberliability coverage can ensure that physician practices don’t run up a hefty bill in the event of a data breach by paying for separate specialists and damage control.
“One of the reasons doctors should have cyberliability coverage are the costs associated with figuring out what to do if patient records are lost or stolen,” she said. “The cost of hiring a lawyer, hiring a forensics investigator to assess the situation, the cost of notifying the patients, and taking all the steps required by HIPAA can really add up. Most practices don’t have those costs built into their annual budgets. A cyberpolicy acts as a buffer against those expenses.”
Manage risk before a breach
Of course, there is plenty that practices can do to prevent – and protect themselves from – a health data breach before it happens. Providing employee awareness training is an important step, said Craig Musgrave, chief information officer of the Doctors Company. Institute a training program for staff at all levels and go over the basics, such as refraining from opening emails from senders they don’t know, Mr. Musgrave wrote in a recent column. Updating all software regularly and backing up data is also essential. And Mr. Musgrave emphasizes the importance of “whitelisting.”
“Health care systems are fragmented in their management of systems and data,” Mr. Musgrave wrote in his column. “Their ability to patch legacy systems and employ cybersecurity staff varies enormously. Therefore, application whitelisting is essential. Rather than blacklisting known malicious software, an application whitelist prevents the launching of any executable program (known or unknown) that does not have explicit authorization. This, in combination with strong firewalls and network segmentation tools like micro-segmentation, provides stronger security.”
In addition, consider implementing data security policies and incident response protocols as well as employee training on securing patient data, ProAssurance’s Ms. Tullos said.
“A breach can also occur within a third-party vendors system and infiltrate the physician’s records, so it is important to discuss cybersecurity with those vendors and all parties should purchase cyberliability insurance,” she said.
Treatment of Melasma Using Tranexamic Acid: What’s Known and What’s Next
Tranexamic acid is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule. Although the US Food and Drug Administration–approved indications for tranexamic acid include treatment of patients with menorrhagia and reduction or prevention of hemorrhage in patients with hemophilia undergoing tooth extraction, the potential efficacy of tranexamic acid in the treatment of melasma has been consistently reported since the 1980s.1
Tranexamic acid exerts effects on pigmentation via its inhibitory effects on UV light–induced plasminogen activator and plasmin activity.2 UV radiation induces the synthesis of plasminogen activator by keratinocytes, which results in increased conversion of plasminogen to plasmin. Plasminogen activator induces tyrosinase activity, resulting in increased melanin synthesis. The presence of plasmin results in increased production of both arachidonic acid and fibroblast growth factor, which stimulate melanogenesis and neovascularization, respectively.3 By inhibiting plasminogen activation, tranexamic acid mitigates UV radiation–induced melanogenesis and neovascularization. In treated guinea pig skin, application of topical tranexamic acid following UV radiation exposure inhibited the development of expected skin hyperpigmentation and also reduced tyrosinase activity.4,5
The largest study on the use of oral tranexamic acid for treatment of melasma was a retrospective chart review of 561 melasma patients treated with tranexamic acid at a single center in Singapore.6 More than 90% of patients received prior treatment of their melasma, including bleaching creams and energy-based treatment. Among patients who received oral tranexamic acid over a 4-month period, 90% of patients demonstrated improvement in their melasma severity. Side effects were experienced by 7% of patients; the most common side effects were abdominal bloating and pain (experienced by 2% of patients). Notably, 1 patient developed deep vein thrombosis during treatment and subsequently was found to have protein S deficiency.6
Although the daily doses of tranexamic acid for the treatment of menorrhagia and perioperative hemophilia patients are 3900 mg and 30 to 40 mg/kg, respectively, effective daily doses reported for the treatment of melasma have ranged from the initial report of efficacy at 750 to 1500 mg to subsequent reports of improvement at daily doses of 500 mg.1,2,6-8
Challenges to the use of tranexamic acid for melasma treatment in the United States include the medicolegal environment, specifically the risks associated with using a systemic procoagulant medication for a cosmetic indication. Patients should be screened and counseled on the risks of developing deep vein thrombosis and pulmonary embolism prior to initiating treatment. Cost and accessibility also may limit the use of tranexamic acid in the United States. Tranexamic acid is available for off-label use in the United States with a prescription in the form of 650-mg tablets that can be split by patients to approximate twice-daily 325 mg dosing. This cosmetic indication poses an out-of-pocket cost to patients of over $110 per month or as low as $48 per month with a coupon at the time of publication.9
Given the potential for serious adverse effects with the use of systemic tranexamic acid, there has been interest in formulating and evaluating topical tranexamic acid for cosmetic indications.10-13 Topical tranexamic acid has been used alone and in conjunction with modalities to increase uptake, including intradermal injection, microneedling, and fractionated CO2 laser.12-14 Although these reports show initial promise, the currently available data are limited by small sample sizes, short treatment durations, lack of dose comparisons, and lack of short-term or long-term follow-up data. In addition to addressing these knowledge gaps in our understanding of topical tranexamic acid as a treatment option for melasma, further studies on the minimum systemic dose may address the downside of cost and potential for complications that may limit use of this medication in the United States.
The potential uses for tranexamic acid extend to the treatment of postinflammatory hyperpigmentation and rosacea. Melanocytes cultured in media conditioned by fractionated CO2 laser–treated keratinocytes were found to have decreased tyrosinase activity and reduced melanin content when treated with tranexamic acid, suggesting the potential role for tranexamic acid to be used postprocedurally to reduce the risk for postinflammatory hyperpigmentation in prone skin types.15 Oral and topical tranexamic acid also have been reported to improve the appearance of erythematotelangiectatic rosacea, potentially relating to the inhibitory effects of tranexamic acid on neovascularization.3,16,17 Although larger-scale controlled studies are required for further investigation of tranexamic acid for these indications, it has shown early promise as an adjunctive treatment for several dermatologic disorders, including melasma, and warrants further characterization as a potential therapeutic option.
- Higashi N. Treatment of melasma with oral tranexamic acid. Skin Res. 1988;30:676-680.
- Tse TW, Hui E. Tranexamic acid: an important adjuvant in the treatment of melasma. J Cosmet Dermatol. 2013;12:57-66.
- Sundbeck A, Karlsson L, Lilja J, et al. Inhibition of tumour vascularization by tranexamic acid. experimental studies on possible mechanisms. Anticancer Res. 1981;1:299-304.
- Maeda K, Naganuma M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J Photochem Photobiol B. 1998;47:136-141.
- Li D, Shi Y, Li M, et al. Tranexamic acid can treat ultraviolet radiation-induced pigmentation in guinea pigs. Eur J Dermatol. 2010;20:289-292.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective analysis. J Am Acad Dermatol. 2016;75:385-392.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Perper M, Eber AE, Fayne R, et al. Tranexamic acid in the treatment of melasma: a review of the literature. Am J Clin Dermatol. 2017;18:373-381.
- Tranexamic acid. GoodRx website. https://www.goodrx.com/tranexamic-acid. Accessed February 2, 2018.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Xu Y, Ma R, Juliandri J, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore). 2017;96(19):e6897.
- Hsiao CY, Sung HC, Hu S, et al. Fractional CO2 laser treatment to enhance skin permeation of tranexamic acid with minimal skin disruption. Dermatology (Basel). 2015;230:269-275.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial [published online November 9, 2017]. J Dermatolog Treat. doi:10.1080/09546634.2017.1392476.
- Kim MS, Bang SH, Kim JH, et al. Tranexamic acid diminishes laser-induced melanogenesis. Ann Dermatol. 2015;27:250-256.
- Kim MS, Chang SE, Haw S, et al. Tranexamic acid solution soaking is an excellent approach for rosacea patients: a preliminary observation in six patients. J Dermatol. 2013;40:70-71.
- Kwon HJ, Suh JH, Ko EJ, et al. Combination treatment of propranolol, minocycline, and tranexamic acid for effective control of rosacea [published online November 26, 2017]. Dermatol Ther. doi:10.1111/dth.12439.
Tranexamic acid is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule. Although the US Food and Drug Administration–approved indications for tranexamic acid include treatment of patients with menorrhagia and reduction or prevention of hemorrhage in patients with hemophilia undergoing tooth extraction, the potential efficacy of tranexamic acid in the treatment of melasma has been consistently reported since the 1980s.1
Tranexamic acid exerts effects on pigmentation via its inhibitory effects on UV light–induced plasminogen activator and plasmin activity.2 UV radiation induces the synthesis of plasminogen activator by keratinocytes, which results in increased conversion of plasminogen to plasmin. Plasminogen activator induces tyrosinase activity, resulting in increased melanin synthesis. The presence of plasmin results in increased production of both arachidonic acid and fibroblast growth factor, which stimulate melanogenesis and neovascularization, respectively.3 By inhibiting plasminogen activation, tranexamic acid mitigates UV radiation–induced melanogenesis and neovascularization. In treated guinea pig skin, application of topical tranexamic acid following UV radiation exposure inhibited the development of expected skin hyperpigmentation and also reduced tyrosinase activity.4,5
The largest study on the use of oral tranexamic acid for treatment of melasma was a retrospective chart review of 561 melasma patients treated with tranexamic acid at a single center in Singapore.6 More than 90% of patients received prior treatment of their melasma, including bleaching creams and energy-based treatment. Among patients who received oral tranexamic acid over a 4-month period, 90% of patients demonstrated improvement in their melasma severity. Side effects were experienced by 7% of patients; the most common side effects were abdominal bloating and pain (experienced by 2% of patients). Notably, 1 patient developed deep vein thrombosis during treatment and subsequently was found to have protein S deficiency.6
Although the daily doses of tranexamic acid for the treatment of menorrhagia and perioperative hemophilia patients are 3900 mg and 30 to 40 mg/kg, respectively, effective daily doses reported for the treatment of melasma have ranged from the initial report of efficacy at 750 to 1500 mg to subsequent reports of improvement at daily doses of 500 mg.1,2,6-8
Challenges to the use of tranexamic acid for melasma treatment in the United States include the medicolegal environment, specifically the risks associated with using a systemic procoagulant medication for a cosmetic indication. Patients should be screened and counseled on the risks of developing deep vein thrombosis and pulmonary embolism prior to initiating treatment. Cost and accessibility also may limit the use of tranexamic acid in the United States. Tranexamic acid is available for off-label use in the United States with a prescription in the form of 650-mg tablets that can be split by patients to approximate twice-daily 325 mg dosing. This cosmetic indication poses an out-of-pocket cost to patients of over $110 per month or as low as $48 per month with a coupon at the time of publication.9
Given the potential for serious adverse effects with the use of systemic tranexamic acid, there has been interest in formulating and evaluating topical tranexamic acid for cosmetic indications.10-13 Topical tranexamic acid has been used alone and in conjunction with modalities to increase uptake, including intradermal injection, microneedling, and fractionated CO2 laser.12-14 Although these reports show initial promise, the currently available data are limited by small sample sizes, short treatment durations, lack of dose comparisons, and lack of short-term or long-term follow-up data. In addition to addressing these knowledge gaps in our understanding of topical tranexamic acid as a treatment option for melasma, further studies on the minimum systemic dose may address the downside of cost and potential for complications that may limit use of this medication in the United States.
The potential uses for tranexamic acid extend to the treatment of postinflammatory hyperpigmentation and rosacea. Melanocytes cultured in media conditioned by fractionated CO2 laser–treated keratinocytes were found to have decreased tyrosinase activity and reduced melanin content when treated with tranexamic acid, suggesting the potential role for tranexamic acid to be used postprocedurally to reduce the risk for postinflammatory hyperpigmentation in prone skin types.15 Oral and topical tranexamic acid also have been reported to improve the appearance of erythematotelangiectatic rosacea, potentially relating to the inhibitory effects of tranexamic acid on neovascularization.3,16,17 Although larger-scale controlled studies are required for further investigation of tranexamic acid for these indications, it has shown early promise as an adjunctive treatment for several dermatologic disorders, including melasma, and warrants further characterization as a potential therapeutic option.
Tranexamic acid is a synthetic lysine derivative that inhibits plasminogen activation by blocking lysine-binding sites on the plasminogen molecule. Although the US Food and Drug Administration–approved indications for tranexamic acid include treatment of patients with menorrhagia and reduction or prevention of hemorrhage in patients with hemophilia undergoing tooth extraction, the potential efficacy of tranexamic acid in the treatment of melasma has been consistently reported since the 1980s.1
Tranexamic acid exerts effects on pigmentation via its inhibitory effects on UV light–induced plasminogen activator and plasmin activity.2 UV radiation induces the synthesis of plasminogen activator by keratinocytes, which results in increased conversion of plasminogen to plasmin. Plasminogen activator induces tyrosinase activity, resulting in increased melanin synthesis. The presence of plasmin results in increased production of both arachidonic acid and fibroblast growth factor, which stimulate melanogenesis and neovascularization, respectively.3 By inhibiting plasminogen activation, tranexamic acid mitigates UV radiation–induced melanogenesis and neovascularization. In treated guinea pig skin, application of topical tranexamic acid following UV radiation exposure inhibited the development of expected skin hyperpigmentation and also reduced tyrosinase activity.4,5
The largest study on the use of oral tranexamic acid for treatment of melasma was a retrospective chart review of 561 melasma patients treated with tranexamic acid at a single center in Singapore.6 More than 90% of patients received prior treatment of their melasma, including bleaching creams and energy-based treatment. Among patients who received oral tranexamic acid over a 4-month period, 90% of patients demonstrated improvement in their melasma severity. Side effects were experienced by 7% of patients; the most common side effects were abdominal bloating and pain (experienced by 2% of patients). Notably, 1 patient developed deep vein thrombosis during treatment and subsequently was found to have protein S deficiency.6
Although the daily doses of tranexamic acid for the treatment of menorrhagia and perioperative hemophilia patients are 3900 mg and 30 to 40 mg/kg, respectively, effective daily doses reported for the treatment of melasma have ranged from the initial report of efficacy at 750 to 1500 mg to subsequent reports of improvement at daily doses of 500 mg.1,2,6-8
Challenges to the use of tranexamic acid for melasma treatment in the United States include the medicolegal environment, specifically the risks associated with using a systemic procoagulant medication for a cosmetic indication. Patients should be screened and counseled on the risks of developing deep vein thrombosis and pulmonary embolism prior to initiating treatment. Cost and accessibility also may limit the use of tranexamic acid in the United States. Tranexamic acid is available for off-label use in the United States with a prescription in the form of 650-mg tablets that can be split by patients to approximate twice-daily 325 mg dosing. This cosmetic indication poses an out-of-pocket cost to patients of over $110 per month or as low as $48 per month with a coupon at the time of publication.9
Given the potential for serious adverse effects with the use of systemic tranexamic acid, there has been interest in formulating and evaluating topical tranexamic acid for cosmetic indications.10-13 Topical tranexamic acid has been used alone and in conjunction with modalities to increase uptake, including intradermal injection, microneedling, and fractionated CO2 laser.12-14 Although these reports show initial promise, the currently available data are limited by small sample sizes, short treatment durations, lack of dose comparisons, and lack of short-term or long-term follow-up data. In addition to addressing these knowledge gaps in our understanding of topical tranexamic acid as a treatment option for melasma, further studies on the minimum systemic dose may address the downside of cost and potential for complications that may limit use of this medication in the United States.
The potential uses for tranexamic acid extend to the treatment of postinflammatory hyperpigmentation and rosacea. Melanocytes cultured in media conditioned by fractionated CO2 laser–treated keratinocytes were found to have decreased tyrosinase activity and reduced melanin content when treated with tranexamic acid, suggesting the potential role for tranexamic acid to be used postprocedurally to reduce the risk for postinflammatory hyperpigmentation in prone skin types.15 Oral and topical tranexamic acid also have been reported to improve the appearance of erythematotelangiectatic rosacea, potentially relating to the inhibitory effects of tranexamic acid on neovascularization.3,16,17 Although larger-scale controlled studies are required for further investigation of tranexamic acid for these indications, it has shown early promise as an adjunctive treatment for several dermatologic disorders, including melasma, and warrants further characterization as a potential therapeutic option.
- Higashi N. Treatment of melasma with oral tranexamic acid. Skin Res. 1988;30:676-680.
- Tse TW, Hui E. Tranexamic acid: an important adjuvant in the treatment of melasma. J Cosmet Dermatol. 2013;12:57-66.
- Sundbeck A, Karlsson L, Lilja J, et al. Inhibition of tumour vascularization by tranexamic acid. experimental studies on possible mechanisms. Anticancer Res. 1981;1:299-304.
- Maeda K, Naganuma M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J Photochem Photobiol B. 1998;47:136-141.
- Li D, Shi Y, Li M, et al. Tranexamic acid can treat ultraviolet radiation-induced pigmentation in guinea pigs. Eur J Dermatol. 2010;20:289-292.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective analysis. J Am Acad Dermatol. 2016;75:385-392.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Perper M, Eber AE, Fayne R, et al. Tranexamic acid in the treatment of melasma: a review of the literature. Am J Clin Dermatol. 2017;18:373-381.
- Tranexamic acid. GoodRx website. https://www.goodrx.com/tranexamic-acid. Accessed February 2, 2018.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Xu Y, Ma R, Juliandri J, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore). 2017;96(19):e6897.
- Hsiao CY, Sung HC, Hu S, et al. Fractional CO2 laser treatment to enhance skin permeation of tranexamic acid with minimal skin disruption. Dermatology (Basel). 2015;230:269-275.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial [published online November 9, 2017]. J Dermatolog Treat. doi:10.1080/09546634.2017.1392476.
- Kim MS, Bang SH, Kim JH, et al. Tranexamic acid diminishes laser-induced melanogenesis. Ann Dermatol. 2015;27:250-256.
- Kim MS, Chang SE, Haw S, et al. Tranexamic acid solution soaking is an excellent approach for rosacea patients: a preliminary observation in six patients. J Dermatol. 2013;40:70-71.
- Kwon HJ, Suh JH, Ko EJ, et al. Combination treatment of propranolol, minocycline, and tranexamic acid for effective control of rosacea [published online November 26, 2017]. Dermatol Ther. doi:10.1111/dth.12439.
- Higashi N. Treatment of melasma with oral tranexamic acid. Skin Res. 1988;30:676-680.
- Tse TW, Hui E. Tranexamic acid: an important adjuvant in the treatment of melasma. J Cosmet Dermatol. 2013;12:57-66.
- Sundbeck A, Karlsson L, Lilja J, et al. Inhibition of tumour vascularization by tranexamic acid. experimental studies on possible mechanisms. Anticancer Res. 1981;1:299-304.
- Maeda K, Naganuma M. Topical trans-4-aminomethylcyclohexanecarboxylic acid prevents ultraviolet radiation-induced pigmentation. J Photochem Photobiol B. 1998;47:136-141.
- Li D, Shi Y, Li M, et al. Tranexamic acid can treat ultraviolet radiation-induced pigmentation in guinea pigs. Eur J Dermatol. 2010;20:289-292.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective analysis. J Am Acad Dermatol. 2016;75:385-392.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Perper M, Eber AE, Fayne R, et al. Tranexamic acid in the treatment of melasma: a review of the literature. Am J Clin Dermatol. 2017;18:373-381.
- Tranexamic acid. GoodRx website. https://www.goodrx.com/tranexamic-acid. Accessed February 2, 2018.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Xu Y, Ma R, Juliandri J, et al. Efficacy of functional microarray of microneedles combined with topical tranexamic acid for melasma: a randomized, self-controlled, split-face study. Medicine (Baltimore). 2017;96(19):e6897.
- Hsiao CY, Sung HC, Hu S, et al. Fractional CO2 laser treatment to enhance skin permeation of tranexamic acid with minimal skin disruption. Dermatology (Basel). 2015;230:269-275.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial [published online November 9, 2017]. J Dermatolog Treat. doi:10.1080/09546634.2017.1392476.
- Kim MS, Bang SH, Kim JH, et al. Tranexamic acid diminishes laser-induced melanogenesis. Ann Dermatol. 2015;27:250-256.
- Kim MS, Chang SE, Haw S, et al. Tranexamic acid solution soaking is an excellent approach for rosacea patients: a preliminary observation in six patients. J Dermatol. 2013;40:70-71.
- Kwon HJ, Suh JH, Ko EJ, et al. Combination treatment of propranolol, minocycline, and tranexamic acid for effective control of rosacea [published online November 26, 2017]. Dermatol Ther. doi:10.1111/dth.12439.
Resident Pearl
- Oral tranexamic acid is an antifibrinolytic agent that can be used off-label for the treatment of melasma.
ACIP unanimously recommends HEPLISAV-B
At a meeting of the Center for Disease Control and Prevention’s Advisory Committee on Immunization Practices, members unanimously voted to include HEPLISAV-B on the ACIP list of recommended products to vaccinate adults against hepatitis B.
“I think this is a huge advance, and a step forward “ said David S. Stephens, MD, of Emory University, Atlanta, who is a voting member of ACIP.
According to Sarah Schillie, MD, of ACIP’s Hepatitis Work Group, the reduction from three doses to two also will improve vaccine series completion rates, providing more effective protection. This could be very important for health care professionals, with only about 60% of treated individuals fulfilling the three doses necessary for complete HBV protection.
Although fewer doses are needed with HEPLISAV-B, it displays similar immunogenicity to similar vaccines (90.0%-100% vs. 70.5%-90.2%). It is also more effective, compared with similar vaccines in those with type II diabetes (90.0% vs. 65.1%) and chronic kidney disease (89.9% vs. 81.1%).
HEPLISAV-B uses an adjuvant, but it appears to be safe and well tolerated. The rate of mild (45.6% vs. 45.7%) and serious (5.4% vs. 6.3%) reactions were similar among HEPLISAV-B and comparable vaccines, although cardiovascular events were more common with this vaccine than with others (0.27% vs. 0.14%).
Dr. Stephens, who supported the recommendation of HEPLISAV-B, voiced reservations about these findings. “I am concerned about the myocardial infarction signal and the use of this new adjuvant and certainly urge us to look at the postmarketing data carefully.”
While the reported incidence rate of acute HBV cases consistently fell during 2000-2015, it began to rise again at the end of 2015. This is linked with concomitant rise in injection drug use, which also is a risk factor in transmitting HBV. Over the same period, the incidence of HBV was highest in people aged 30-49 years. With these factors to consider, the vote by ACIP for HEPLISAV-B as a recommended vaccine is timely and may help reduce HBV infections in adults.
At a meeting of the Center for Disease Control and Prevention’s Advisory Committee on Immunization Practices, members unanimously voted to include HEPLISAV-B on the ACIP list of recommended products to vaccinate adults against hepatitis B.
“I think this is a huge advance, and a step forward “ said David S. Stephens, MD, of Emory University, Atlanta, who is a voting member of ACIP.
According to Sarah Schillie, MD, of ACIP’s Hepatitis Work Group, the reduction from three doses to two also will improve vaccine series completion rates, providing more effective protection. This could be very important for health care professionals, with only about 60% of treated individuals fulfilling the three doses necessary for complete HBV protection.
Although fewer doses are needed with HEPLISAV-B, it displays similar immunogenicity to similar vaccines (90.0%-100% vs. 70.5%-90.2%). It is also more effective, compared with similar vaccines in those with type II diabetes (90.0% vs. 65.1%) and chronic kidney disease (89.9% vs. 81.1%).
HEPLISAV-B uses an adjuvant, but it appears to be safe and well tolerated. The rate of mild (45.6% vs. 45.7%) and serious (5.4% vs. 6.3%) reactions were similar among HEPLISAV-B and comparable vaccines, although cardiovascular events were more common with this vaccine than with others (0.27% vs. 0.14%).
Dr. Stephens, who supported the recommendation of HEPLISAV-B, voiced reservations about these findings. “I am concerned about the myocardial infarction signal and the use of this new adjuvant and certainly urge us to look at the postmarketing data carefully.”
While the reported incidence rate of acute HBV cases consistently fell during 2000-2015, it began to rise again at the end of 2015. This is linked with concomitant rise in injection drug use, which also is a risk factor in transmitting HBV. Over the same period, the incidence of HBV was highest in people aged 30-49 years. With these factors to consider, the vote by ACIP for HEPLISAV-B as a recommended vaccine is timely and may help reduce HBV infections in adults.
At a meeting of the Center for Disease Control and Prevention’s Advisory Committee on Immunization Practices, members unanimously voted to include HEPLISAV-B on the ACIP list of recommended products to vaccinate adults against hepatitis B.
“I think this is a huge advance, and a step forward “ said David S. Stephens, MD, of Emory University, Atlanta, who is a voting member of ACIP.
According to Sarah Schillie, MD, of ACIP’s Hepatitis Work Group, the reduction from three doses to two also will improve vaccine series completion rates, providing more effective protection. This could be very important for health care professionals, with only about 60% of treated individuals fulfilling the three doses necessary for complete HBV protection.
Although fewer doses are needed with HEPLISAV-B, it displays similar immunogenicity to similar vaccines (90.0%-100% vs. 70.5%-90.2%). It is also more effective, compared with similar vaccines in those with type II diabetes (90.0% vs. 65.1%) and chronic kidney disease (89.9% vs. 81.1%).
HEPLISAV-B uses an adjuvant, but it appears to be safe and well tolerated. The rate of mild (45.6% vs. 45.7%) and serious (5.4% vs. 6.3%) reactions were similar among HEPLISAV-B and comparable vaccines, although cardiovascular events were more common with this vaccine than with others (0.27% vs. 0.14%).
Dr. Stephens, who supported the recommendation of HEPLISAV-B, voiced reservations about these findings. “I am concerned about the myocardial infarction signal and the use of this new adjuvant and certainly urge us to look at the postmarketing data carefully.”
While the reported incidence rate of acute HBV cases consistently fell during 2000-2015, it began to rise again at the end of 2015. This is linked with concomitant rise in injection drug use, which also is a risk factor in transmitting HBV. Over the same period, the incidence of HBV was highest in people aged 30-49 years. With these factors to consider, the vote by ACIP for HEPLISAV-B as a recommended vaccine is timely and may help reduce HBV infections in adults.
REPORTING FROM AN ACIP MEETING











