User login
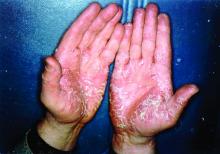
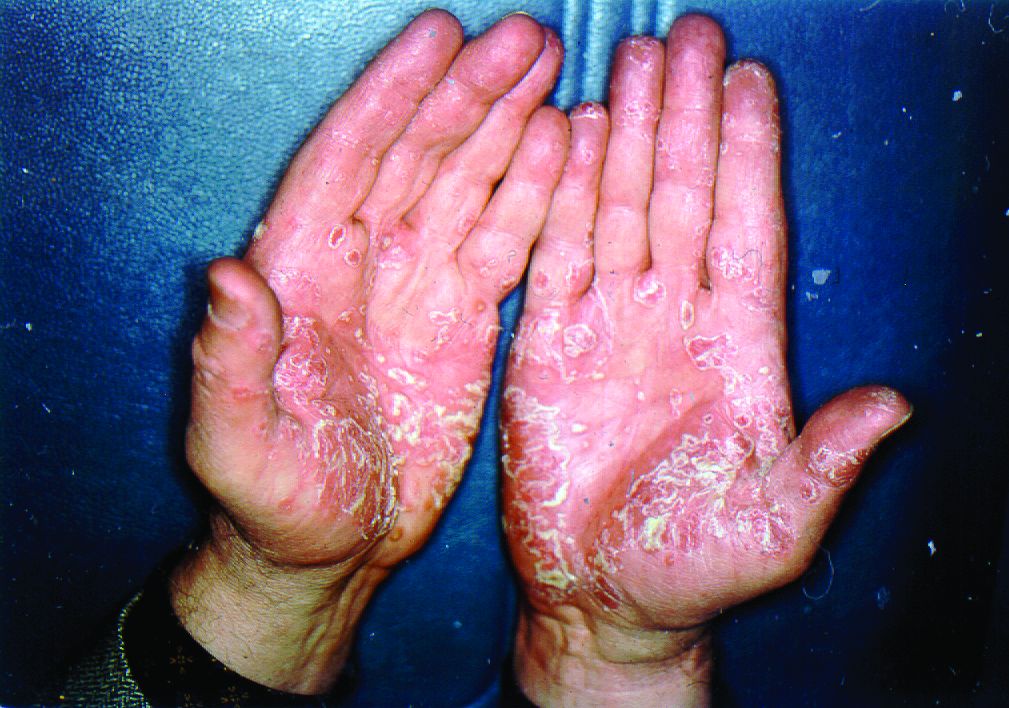
Biologics have best chance of achieving PASI 90 in psoriasis
The biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab pegol, and ustekinumab provide the best chances for achieving Psoriasis Area and Severity Index (PASI) 90 when compared with placebo in patients with moderate to severe psoriasis, according to Emilie Sbidian, MD, and her associates.
Dr. Sbidian of Henri Mondor Hospital, Créteil, France, and her colleagues conducted a network meta-analysis of 109 randomized, controlled trials that collectively had a total of 39,882 participants. The results showed that all of the interventions appeared superior to placebo in terms of reaching PASI 90.
The investigators also found that there was no significant difference between the three anti–IL-17 agents (brodalumab, ixekizumab, and secukinumab) and the two anti–IL-23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. However, all of the anti–IL-17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti–IL-23) were significantly more effective than three anti–TNF-alpha agents (infliximab, adalimumab, and etanercept).
Additionally, in the network meta-analysis, anti–IL-17 drugs were the best for achieving PASI 90, compared with placebo (RR, 30.81), followed by anti–IL-12/23 (RR, 23.16), anti–IL-23 (RR, 16.53), and anti–TNF-alpha (RR, 11.58). At the individual drug level, results showed ixekizumab was the best treatment for attaining PASI 90 when compared with placebo (RR, 32.45), followed by secukinumab (RR, 26.55), brodalumab (RR, 25.45), guselkumab (RR, 21.03), certolizumab pegol (RR, 24.58), and ustekinumab (RR, 19.91). The investigators found that “there was no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects.”
“Our main results do not reflect the way patients are managed in ‘real life,’ ” the researchers concluded. “Currently, biological treatments have been positioned as third-line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents).”
SOURCE: Sbidian E et al. Cochrane Database Syst Rev. 2017 Dec 22. doi: 10.1002/14651858.CD011535.pub2.
The biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab pegol, and ustekinumab provide the best chances for achieving Psoriasis Area and Severity Index (PASI) 90 when compared with placebo in patients with moderate to severe psoriasis, according to Emilie Sbidian, MD, and her associates.
Dr. Sbidian of Henri Mondor Hospital, Créteil, France, and her colleagues conducted a network meta-analysis of 109 randomized, controlled trials that collectively had a total of 39,882 participants. The results showed that all of the interventions appeared superior to placebo in terms of reaching PASI 90.
The investigators also found that there was no significant difference between the three anti–IL-17 agents (brodalumab, ixekizumab, and secukinumab) and the two anti–IL-23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. However, all of the anti–IL-17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti–IL-23) were significantly more effective than three anti–TNF-alpha agents (infliximab, adalimumab, and etanercept).
Additionally, in the network meta-analysis, anti–IL-17 drugs were the best for achieving PASI 90, compared with placebo (RR, 30.81), followed by anti–IL-12/23 (RR, 23.16), anti–IL-23 (RR, 16.53), and anti–TNF-alpha (RR, 11.58). At the individual drug level, results showed ixekizumab was the best treatment for attaining PASI 90 when compared with placebo (RR, 32.45), followed by secukinumab (RR, 26.55), brodalumab (RR, 25.45), guselkumab (RR, 21.03), certolizumab pegol (RR, 24.58), and ustekinumab (RR, 19.91). The investigators found that “there was no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects.”
“Our main results do not reflect the way patients are managed in ‘real life,’ ” the researchers concluded. “Currently, biological treatments have been positioned as third-line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents).”
SOURCE: Sbidian E et al. Cochrane Database Syst Rev. 2017 Dec 22. doi: 10.1002/14651858.CD011535.pub2.
The biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab pegol, and ustekinumab provide the best chances for achieving Psoriasis Area and Severity Index (PASI) 90 when compared with placebo in patients with moderate to severe psoriasis, according to Emilie Sbidian, MD, and her associates.
Dr. Sbidian of Henri Mondor Hospital, Créteil, France, and her colleagues conducted a network meta-analysis of 109 randomized, controlled trials that collectively had a total of 39,882 participants. The results showed that all of the interventions appeared superior to placebo in terms of reaching PASI 90.
The investigators also found that there was no significant difference between the three anti–IL-17 agents (brodalumab, ixekizumab, and secukinumab) and the two anti–IL-23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. However, all of the anti–IL-17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti–IL-23) were significantly more effective than three anti–TNF-alpha agents (infliximab, adalimumab, and etanercept).
Additionally, in the network meta-analysis, anti–IL-17 drugs were the best for achieving PASI 90, compared with placebo (RR, 30.81), followed by anti–IL-12/23 (RR, 23.16), anti–IL-23 (RR, 16.53), and anti–TNF-alpha (RR, 11.58). At the individual drug level, results showed ixekizumab was the best treatment for attaining PASI 90 when compared with placebo (RR, 32.45), followed by secukinumab (RR, 26.55), brodalumab (RR, 25.45), guselkumab (RR, 21.03), certolizumab pegol (RR, 24.58), and ustekinumab (RR, 19.91). The investigators found that “there was no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects.”
“Our main results do not reflect the way patients are managed in ‘real life,’ ” the researchers concluded. “Currently, biological treatments have been positioned as third-line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents).”
SOURCE: Sbidian E et al. Cochrane Database Syst Rev. 2017 Dec 22. doi: 10.1002/14651858.CD011535.pub2.
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Evaluating Dermatology Apps for Patient Education
VIDEO: SPF 100 sunscreen outperformed SPF 50 in Vail study
SAN DIEGO – a finding that might interest consumers and prompt the Food and Drug Administration to continue to allow sunscreens to have labels listing sun protection factors greater than 50.*
“Our study results show pretty definitively that SPF 100 did significantly better than SPF 50 in a real world environment,” Darrell S. Rigel, MD, said at the annual meeting of the American Academy of Dermatology.
Dr. Rigel cited data that he and his associates recently published from 199 adults skiing on a sunny March day in Colorado. Participants applied a blinded sunscreen rated at SPF 50 to one side of their face all day and an SPF 100 sunscreen to the other side all day, and the researchers then ran a blinded assessment of images taken of each side at the end of the day. The sunburn on the SPF 50 side exceeded the other side in 55% of skiers, the two sides matched in 40%, and in 5% the sunburn was worse on the SPF 100 side (J Am Acad Dermatol. 2017 Dec 29. doi: 10.1016/j.jaad.2017.12.062).
“The SPF 50 side of the face was 11 times more likely to be sunburned than the SPF 100 side,” and for all the secondary endpoints and different ways of analyzing the data, the SPF 50 was not as effective as SPF 100, Dr. Rigel said in a video interview. Erythema appeared on 41% of the SPF 50–treated sides of participants faces, compared with 14% of the sides treated with SPF 100 sunscreen.
The results followed-up on a report from Dr. Rigel and his associates from 8 years ago that ran a similar comparison of two sunscreen potencies, SPF 85 and SPF 50, in 56 skiers, with similar results showing greater sunburn protection from the higher SPF sunscreen (J Am Acad Dermatol. 2010 Feb;62[2]:348-9). In 2011, the FDA proposed a new rule for SPF labeling that would cap the maximum SPF potency possible of 50, which created a label 50+ to designate unspecified SPF above 50. According to Dr. Rigel, the FDA rejected his 2010 study as documentation of incremental benefit above SPF 50 because of several flaws the agency found with that study, including not tracking sunscreen use by weight. He specifically designed the new, 199-subject study to address that and the FDA’s other concerns.
He and his associates decided to do the study because the FDA said in the monograph that, if the concerns were met, “they would accept the study as definitive,” said Dr. Rigel, a dermatologist at New York University.
The greater protection from SPF 100 sunscreen probably occurs because it’s “more forgiving” when used with inadequate application, he suggested. Allowing labeling that specifies SPF levels greater than 50 would help consumers pick sunscreen formulations that give greater protection, and it would encourage manufacturers to market sunscreens with higher SPF levels.
Dr. Rigel has been a consultant to Castle Biosciences, DermTech, Ferndale, Myriad, Neutrogena, and Novascan and has received research support from Castle and Neutrogena.
Correction, 2/22/18: Due to an editing error, an earlier version of this article implied incorrectly that sunscreen labels listing SPFs over 50 had been banned .
SAN DIEGO – a finding that might interest consumers and prompt the Food and Drug Administration to continue to allow sunscreens to have labels listing sun protection factors greater than 50.*
“Our study results show pretty definitively that SPF 100 did significantly better than SPF 50 in a real world environment,” Darrell S. Rigel, MD, said at the annual meeting of the American Academy of Dermatology.
Dr. Rigel cited data that he and his associates recently published from 199 adults skiing on a sunny March day in Colorado. Participants applied a blinded sunscreen rated at SPF 50 to one side of their face all day and an SPF 100 sunscreen to the other side all day, and the researchers then ran a blinded assessment of images taken of each side at the end of the day. The sunburn on the SPF 50 side exceeded the other side in 55% of skiers, the two sides matched in 40%, and in 5% the sunburn was worse on the SPF 100 side (J Am Acad Dermatol. 2017 Dec 29. doi: 10.1016/j.jaad.2017.12.062).
“The SPF 50 side of the face was 11 times more likely to be sunburned than the SPF 100 side,” and for all the secondary endpoints and different ways of analyzing the data, the SPF 50 was not as effective as SPF 100, Dr. Rigel said in a video interview. Erythema appeared on 41% of the SPF 50–treated sides of participants faces, compared with 14% of the sides treated with SPF 100 sunscreen.
The results followed-up on a report from Dr. Rigel and his associates from 8 years ago that ran a similar comparison of two sunscreen potencies, SPF 85 and SPF 50, in 56 skiers, with similar results showing greater sunburn protection from the higher SPF sunscreen (J Am Acad Dermatol. 2010 Feb;62[2]:348-9). In 2011, the FDA proposed a new rule for SPF labeling that would cap the maximum SPF potency possible of 50, which created a label 50+ to designate unspecified SPF above 50. According to Dr. Rigel, the FDA rejected his 2010 study as documentation of incremental benefit above SPF 50 because of several flaws the agency found with that study, including not tracking sunscreen use by weight. He specifically designed the new, 199-subject study to address that and the FDA’s other concerns.
He and his associates decided to do the study because the FDA said in the monograph that, if the concerns were met, “they would accept the study as definitive,” said Dr. Rigel, a dermatologist at New York University.
The greater protection from SPF 100 sunscreen probably occurs because it’s “more forgiving” when used with inadequate application, he suggested. Allowing labeling that specifies SPF levels greater than 50 would help consumers pick sunscreen formulations that give greater protection, and it would encourage manufacturers to market sunscreens with higher SPF levels.
Dr. Rigel has been a consultant to Castle Biosciences, DermTech, Ferndale, Myriad, Neutrogena, and Novascan and has received research support from Castle and Neutrogena.
Correction, 2/22/18: Due to an editing error, an earlier version of this article implied incorrectly that sunscreen labels listing SPFs over 50 had been banned .
SAN DIEGO – a finding that might interest consumers and prompt the Food and Drug Administration to continue to allow sunscreens to have labels listing sun protection factors greater than 50.*
“Our study results show pretty definitively that SPF 100 did significantly better than SPF 50 in a real world environment,” Darrell S. Rigel, MD, said at the annual meeting of the American Academy of Dermatology.
Dr. Rigel cited data that he and his associates recently published from 199 adults skiing on a sunny March day in Colorado. Participants applied a blinded sunscreen rated at SPF 50 to one side of their face all day and an SPF 100 sunscreen to the other side all day, and the researchers then ran a blinded assessment of images taken of each side at the end of the day. The sunburn on the SPF 50 side exceeded the other side in 55% of skiers, the two sides matched in 40%, and in 5% the sunburn was worse on the SPF 100 side (J Am Acad Dermatol. 2017 Dec 29. doi: 10.1016/j.jaad.2017.12.062).
“The SPF 50 side of the face was 11 times more likely to be sunburned than the SPF 100 side,” and for all the secondary endpoints and different ways of analyzing the data, the SPF 50 was not as effective as SPF 100, Dr. Rigel said in a video interview. Erythema appeared on 41% of the SPF 50–treated sides of participants faces, compared with 14% of the sides treated with SPF 100 sunscreen.
The results followed-up on a report from Dr. Rigel and his associates from 8 years ago that ran a similar comparison of two sunscreen potencies, SPF 85 and SPF 50, in 56 skiers, with similar results showing greater sunburn protection from the higher SPF sunscreen (J Am Acad Dermatol. 2010 Feb;62[2]:348-9). In 2011, the FDA proposed a new rule for SPF labeling that would cap the maximum SPF potency possible of 50, which created a label 50+ to designate unspecified SPF above 50. According to Dr. Rigel, the FDA rejected his 2010 study as documentation of incremental benefit above SPF 50 because of several flaws the agency found with that study, including not tracking sunscreen use by weight. He specifically designed the new, 199-subject study to address that and the FDA’s other concerns.
He and his associates decided to do the study because the FDA said in the monograph that, if the concerns were met, “they would accept the study as definitive,” said Dr. Rigel, a dermatologist at New York University.
The greater protection from SPF 100 sunscreen probably occurs because it’s “more forgiving” when used with inadequate application, he suggested. Allowing labeling that specifies SPF levels greater than 50 would help consumers pick sunscreen formulations that give greater protection, and it would encourage manufacturers to market sunscreens with higher SPF levels.
Dr. Rigel has been a consultant to Castle Biosciences, DermTech, Ferndale, Myriad, Neutrogena, and Novascan and has received research support from Castle and Neutrogena.
Correction, 2/22/18: Due to an editing error, an earlier version of this article implied incorrectly that sunscreen labels listing SPFs over 50 had been banned .
EXPERT ANALYSIS FROM AAD 18
House cleaning linked to lung function decline
that has found accelerated decline in lung function among women regularly engaged in cleaning activities.
The longitudinal population-based cohort study, published online Feb. 16 in the American Journal of Respiratory and Critical Care Medicine, looked at the lung health of 6,230 people who were followed for more than 20 years as part of the European Community Respiratory Health Survey.
Analysis based on questionnaires about cleaning practices revealed that women who were responsible for cleaning at home or who worked as professional cleaners showed significantly greater declines in maximum forced vital capacity (FVC) and maximum forced expiratory volume in 1 second (FEV1), compared with women who said they did not regularly clean.
However, there was no association between cleaning practices in men – either professional or domestic – and accelerated lung function decline. The authors suggested that the exposures experienced by men who worked as cleaners may have been different from the exposures experienced by women. They also noted that the small numbers of male cleaners meant the study wasn’t powered to pick up greater declines in lung function.
The study also showed a significant association between use of cleaning products and decline in lung function. Women who used sprays or other cleaning agents at least once a week showed significantly greater declines in FEV1 and FVC, compared with women who didn’t use cleaning products. Again, this effect was not significant in men.
“One possible mechanism for the accelerated decline in cleaners is the repetitive exposure to low-grade irritative cleaning agents over time, thereby causing persistent changes in the airways,” the authors wrote. “Repeated exposure could lead to remodelling of the airways, thereby over time causing an accelerated decline in FVC and FEV1.”
The analysis found no significant increases in the incidence of chronic airway obstruction among regular cleaners, nor among those who used cleaning products. The authors noted that while previous studies had suggested an increase in chronic obstructive pulmonary disease among occupational cleaners, their study reported relatively few cases of COPD.
While the prevalence of asthma was slightly higher in the two groups of women exposed to regular cleaning (12.3% and 13.7%, versus 9.6%), adjustment for asthma in the analysis did not change the associations. This suggests that the declines in lung function seen in regular cleaners were not mediated by cleaning-related asthma, the researchers noted.
They also noted that the women who reported not engaging in any cleaning may represent a particular socioeconomic group, but adjustment for socioeconomic status did not alter the associations.
The European Community Respiratory Health Survey is supported by the European Union, the European Commission, and the Medical Research Council. No conflicts of interest were reported.
SOURCE: Svanes Ø et al. Am J Resp Crit Care Med. 2018 Feb 16. doi: 10.1164/rccm.201706-1311OC.
that has found accelerated decline in lung function among women regularly engaged in cleaning activities.
The longitudinal population-based cohort study, published online Feb. 16 in the American Journal of Respiratory and Critical Care Medicine, looked at the lung health of 6,230 people who were followed for more than 20 years as part of the European Community Respiratory Health Survey.
Analysis based on questionnaires about cleaning practices revealed that women who were responsible for cleaning at home or who worked as professional cleaners showed significantly greater declines in maximum forced vital capacity (FVC) and maximum forced expiratory volume in 1 second (FEV1), compared with women who said they did not regularly clean.
However, there was no association between cleaning practices in men – either professional or domestic – and accelerated lung function decline. The authors suggested that the exposures experienced by men who worked as cleaners may have been different from the exposures experienced by women. They also noted that the small numbers of male cleaners meant the study wasn’t powered to pick up greater declines in lung function.
The study also showed a significant association between use of cleaning products and decline in lung function. Women who used sprays or other cleaning agents at least once a week showed significantly greater declines in FEV1 and FVC, compared with women who didn’t use cleaning products. Again, this effect was not significant in men.
“One possible mechanism for the accelerated decline in cleaners is the repetitive exposure to low-grade irritative cleaning agents over time, thereby causing persistent changes in the airways,” the authors wrote. “Repeated exposure could lead to remodelling of the airways, thereby over time causing an accelerated decline in FVC and FEV1.”
The analysis found no significant increases in the incidence of chronic airway obstruction among regular cleaners, nor among those who used cleaning products. The authors noted that while previous studies had suggested an increase in chronic obstructive pulmonary disease among occupational cleaners, their study reported relatively few cases of COPD.
While the prevalence of asthma was slightly higher in the two groups of women exposed to regular cleaning (12.3% and 13.7%, versus 9.6%), adjustment for asthma in the analysis did not change the associations. This suggests that the declines in lung function seen in regular cleaners were not mediated by cleaning-related asthma, the researchers noted.
They also noted that the women who reported not engaging in any cleaning may represent a particular socioeconomic group, but adjustment for socioeconomic status did not alter the associations.
The European Community Respiratory Health Survey is supported by the European Union, the European Commission, and the Medical Research Council. No conflicts of interest were reported.
SOURCE: Svanes Ø et al. Am J Resp Crit Care Med. 2018 Feb 16. doi: 10.1164/rccm.201706-1311OC.
that has found accelerated decline in lung function among women regularly engaged in cleaning activities.
The longitudinal population-based cohort study, published online Feb. 16 in the American Journal of Respiratory and Critical Care Medicine, looked at the lung health of 6,230 people who were followed for more than 20 years as part of the European Community Respiratory Health Survey.
Analysis based on questionnaires about cleaning practices revealed that women who were responsible for cleaning at home or who worked as professional cleaners showed significantly greater declines in maximum forced vital capacity (FVC) and maximum forced expiratory volume in 1 second (FEV1), compared with women who said they did not regularly clean.
However, there was no association between cleaning practices in men – either professional or domestic – and accelerated lung function decline. The authors suggested that the exposures experienced by men who worked as cleaners may have been different from the exposures experienced by women. They also noted that the small numbers of male cleaners meant the study wasn’t powered to pick up greater declines in lung function.
The study also showed a significant association between use of cleaning products and decline in lung function. Women who used sprays or other cleaning agents at least once a week showed significantly greater declines in FEV1 and FVC, compared with women who didn’t use cleaning products. Again, this effect was not significant in men.
“One possible mechanism for the accelerated decline in cleaners is the repetitive exposure to low-grade irritative cleaning agents over time, thereby causing persistent changes in the airways,” the authors wrote. “Repeated exposure could lead to remodelling of the airways, thereby over time causing an accelerated decline in FVC and FEV1.”
The analysis found no significant increases in the incidence of chronic airway obstruction among regular cleaners, nor among those who used cleaning products. The authors noted that while previous studies had suggested an increase in chronic obstructive pulmonary disease among occupational cleaners, their study reported relatively few cases of COPD.
While the prevalence of asthma was slightly higher in the two groups of women exposed to regular cleaning (12.3% and 13.7%, versus 9.6%), adjustment for asthma in the analysis did not change the associations. This suggests that the declines in lung function seen in regular cleaners were not mediated by cleaning-related asthma, the researchers noted.
They also noted that the women who reported not engaging in any cleaning may represent a particular socioeconomic group, but adjustment for socioeconomic status did not alter the associations.
The European Community Respiratory Health Survey is supported by the European Union, the European Commission, and the Medical Research Council. No conflicts of interest were reported.
SOURCE: Svanes Ø et al. Am J Resp Crit Care Med. 2018 Feb 16. doi: 10.1164/rccm.201706-1311OC.
FROM AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Key clinical point: Women – but not men – who regularly clean homes either domestically or professionally show accelerated declines in lung function.
Major finding: Women who work as cleaners or clean their own homes regularly show greater declines in FEV1 and FVC, compared with women who do not clean regularly.
Data source: Longitudinal cohort study of 6,230 individuals in the European Community Respiratory Health Survey.
Disclosures: The European Community Respiratory Health Survey is supported by the European Union, the European Commission, and the Medical Research Council. No conflicts of interest were provided.
Source: Svanes Ø et al. Am J Resp Crit Care Med. 2018 Feb. 16. doi: 10.1164/rccm.201706-1311OC.
Upadacitinib calms itch, clears skin in moderate to severe atopic dermatitis
SAN DIEGO – Upadacitinib, a selective inhibitor of the Janus kinase 1 enzyme, affected up to 90% skin clearance in a phase 2 study in patients with moderate to severe atopic dermatitis (AD).
The molecule significantly outperformed placebo with all three of the doses tested, with patients experiencing improvement in itch and skin are involved in the first week of use. During the 16-week trial, there were none of the thromboembolic events that have proven a concern with other inhibitors of the Janus kinase family of enzymes, Emma Guttman, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
The study enrolled adults (mean age, 40 years) with moderate to severe atopic dermatitis of about 30 years’ duration. Their mean Eczema Area and Severity Index (EASI) score at baseline was about 30, and mean Body Surface Area score ranged from 42-50. The mean pruritus numerical rating scale score was about 6.5.
After a month-long washout period that excluded all medications except a topical emollient, patients were randomized to placebo (40 patients) or to daily upadacitinib at 7.5-mg, 15-mg, or 30-mg doses, taken orally (42 in each group). The 16-week placebo-controlled period is being followed by a 72-week blinded extension study in which the placebo patients will be switched to upadacitinib 30 mg, and half of each upadacitinib group will be switched to placebo. Dr. Guttman reported only the 16-week results.
The study’s primary endpoint was the mean percent EASI improvement from baseline. Dr. Guttman reported several of the key secondary endpoints as well.
On the primary endpoint, all three doses of upadacitinib separated from placebo quickly and dramatically. Patients began to see some skin clearance after 1 week; by 2 weeks, the 7.5-mg group had achieved a 39% improvement and the 15- and 30-mg groups, 56% and 59% improvement, respectively.
Efficacy in the 7.5-mg group peaked at a 50% improvement by week 4, and then trailed off somewhat, with a mean 39% improvement by week 16.
Efficacy in the two higher dose groups also peaked around 4 weeks, with a 66% improvement in the 15-mg group and a 78% improvement in the 30-mg group. There was some tailing off of effect in each of these groups as well. By 16 weeks, the mean EASI improvement was 61.7% in the 15-mg group and 74.4% in the 30-mg group.
A good number of patients in each dosing group even achieved 90% skin clearance, Dr. Guttman noted.
“We generally say that we like to see an EASI 50, but now we are talking about EASI 75 and even 90. More than 60% on the highest dose achieved an EASI 75 and 60% an EASI 90. Even the 15-mg group achieved a nice EASI 90 response, at around 26%.”
Itching also responded well to upadacitinib, Dr. Guttman said. Within 1 week, all three active groups had separated significantly from the placebo group. By 2 weeks, the 7.5-mg group had a mean 29% improvement on the pruritus numerical rating scale. The improvement was 46% in the 15-mg group and 57.6% in the 30-mg group. By 16 weeks, the improvements were 39.6%, 48%, and 68.9%, respectively, compared with 9.7% among those on placebo.
The most common treatment-emergent adverse event was upper respiratory infection, which Dr. Guttman noted has been seen with JAK inhibitors. This occurred in 10% of those taking placebo and in 16.7% of the 7.5-mg group and 11.9% of both the 15-mg and 30-mg groups. A few patients experienced exacerbation of their AD on the medication (16.7%, 7% and 11.9% of the active groups, compared with 7.5% of those on placebo). New acne developed in 2.5% of placebo patients and in 9.5%, 4.8%, and 14.3% of those taking upadacitinib. The acne was on the body, considered mild, and resolved, Dr. Guttman said.
Two patients taking 7.5 mg and one taking 15 mg developed a serious infection, although she did not elaborate on the illnesses. Two taking 15 mg developed a liver disorder. There were four cases of neutropenia and one lymphopenia, all among the active groups. Creatine phosphokinase elevations occurred in one taking placebo and seven taking the study drug.
There were no pulmonary or deep vein thromboses; no opportunistic infections or malignancies; and no cases of herpes zoster, tuberculosis, or renal dysfunction. There were no deaths during the trial.
Phase 3 studies of upadacitinib in patients with rheumatoid arthritis, psoriatic arthritis, and Crohn’s disease are underway, according to AbbVie.
AbbVie sponsored the AD study. Dr. Guttman is a consultant for the company.
SOURCE: Guttman E et al. AAD late-breaking clinical trials, Abstract 6533
SAN DIEGO – Upadacitinib, a selective inhibitor of the Janus kinase 1 enzyme, affected up to 90% skin clearance in a phase 2 study in patients with moderate to severe atopic dermatitis (AD).
The molecule significantly outperformed placebo with all three of the doses tested, with patients experiencing improvement in itch and skin are involved in the first week of use. During the 16-week trial, there were none of the thromboembolic events that have proven a concern with other inhibitors of the Janus kinase family of enzymes, Emma Guttman, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
The study enrolled adults (mean age, 40 years) with moderate to severe atopic dermatitis of about 30 years’ duration. Their mean Eczema Area and Severity Index (EASI) score at baseline was about 30, and mean Body Surface Area score ranged from 42-50. The mean pruritus numerical rating scale score was about 6.5.
After a month-long washout period that excluded all medications except a topical emollient, patients were randomized to placebo (40 patients) or to daily upadacitinib at 7.5-mg, 15-mg, or 30-mg doses, taken orally (42 in each group). The 16-week placebo-controlled period is being followed by a 72-week blinded extension study in which the placebo patients will be switched to upadacitinib 30 mg, and half of each upadacitinib group will be switched to placebo. Dr. Guttman reported only the 16-week results.
The study’s primary endpoint was the mean percent EASI improvement from baseline. Dr. Guttman reported several of the key secondary endpoints as well.
On the primary endpoint, all three doses of upadacitinib separated from placebo quickly and dramatically. Patients began to see some skin clearance after 1 week; by 2 weeks, the 7.5-mg group had achieved a 39% improvement and the 15- and 30-mg groups, 56% and 59% improvement, respectively.
Efficacy in the 7.5-mg group peaked at a 50% improvement by week 4, and then trailed off somewhat, with a mean 39% improvement by week 16.
Efficacy in the two higher dose groups also peaked around 4 weeks, with a 66% improvement in the 15-mg group and a 78% improvement in the 30-mg group. There was some tailing off of effect in each of these groups as well. By 16 weeks, the mean EASI improvement was 61.7% in the 15-mg group and 74.4% in the 30-mg group.
A good number of patients in each dosing group even achieved 90% skin clearance, Dr. Guttman noted.
“We generally say that we like to see an EASI 50, but now we are talking about EASI 75 and even 90. More than 60% on the highest dose achieved an EASI 75 and 60% an EASI 90. Even the 15-mg group achieved a nice EASI 90 response, at around 26%.”
Itching also responded well to upadacitinib, Dr. Guttman said. Within 1 week, all three active groups had separated significantly from the placebo group. By 2 weeks, the 7.5-mg group had a mean 29% improvement on the pruritus numerical rating scale. The improvement was 46% in the 15-mg group and 57.6% in the 30-mg group. By 16 weeks, the improvements were 39.6%, 48%, and 68.9%, respectively, compared with 9.7% among those on placebo.
The most common treatment-emergent adverse event was upper respiratory infection, which Dr. Guttman noted has been seen with JAK inhibitors. This occurred in 10% of those taking placebo and in 16.7% of the 7.5-mg group and 11.9% of both the 15-mg and 30-mg groups. A few patients experienced exacerbation of their AD on the medication (16.7%, 7% and 11.9% of the active groups, compared with 7.5% of those on placebo). New acne developed in 2.5% of placebo patients and in 9.5%, 4.8%, and 14.3% of those taking upadacitinib. The acne was on the body, considered mild, and resolved, Dr. Guttman said.
Two patients taking 7.5 mg and one taking 15 mg developed a serious infection, although she did not elaborate on the illnesses. Two taking 15 mg developed a liver disorder. There were four cases of neutropenia and one lymphopenia, all among the active groups. Creatine phosphokinase elevations occurred in one taking placebo and seven taking the study drug.
There were no pulmonary or deep vein thromboses; no opportunistic infections or malignancies; and no cases of herpes zoster, tuberculosis, or renal dysfunction. There were no deaths during the trial.
Phase 3 studies of upadacitinib in patients with rheumatoid arthritis, psoriatic arthritis, and Crohn’s disease are underway, according to AbbVie.
AbbVie sponsored the AD study. Dr. Guttman is a consultant for the company.
SOURCE: Guttman E et al. AAD late-breaking clinical trials, Abstract 6533
SAN DIEGO – Upadacitinib, a selective inhibitor of the Janus kinase 1 enzyme, affected up to 90% skin clearance in a phase 2 study in patients with moderate to severe atopic dermatitis (AD).
The molecule significantly outperformed placebo with all three of the doses tested, with patients experiencing improvement in itch and skin are involved in the first week of use. During the 16-week trial, there were none of the thromboembolic events that have proven a concern with other inhibitors of the Janus kinase family of enzymes, Emma Guttman, MD, PhD, said at the annual meeting of the American Academy of Dermatology.
The study enrolled adults (mean age, 40 years) with moderate to severe atopic dermatitis of about 30 years’ duration. Their mean Eczema Area and Severity Index (EASI) score at baseline was about 30, and mean Body Surface Area score ranged from 42-50. The mean pruritus numerical rating scale score was about 6.5.
After a month-long washout period that excluded all medications except a topical emollient, patients were randomized to placebo (40 patients) or to daily upadacitinib at 7.5-mg, 15-mg, or 30-mg doses, taken orally (42 in each group). The 16-week placebo-controlled period is being followed by a 72-week blinded extension study in which the placebo patients will be switched to upadacitinib 30 mg, and half of each upadacitinib group will be switched to placebo. Dr. Guttman reported only the 16-week results.
The study’s primary endpoint was the mean percent EASI improvement from baseline. Dr. Guttman reported several of the key secondary endpoints as well.
On the primary endpoint, all three doses of upadacitinib separated from placebo quickly and dramatically. Patients began to see some skin clearance after 1 week; by 2 weeks, the 7.5-mg group had achieved a 39% improvement and the 15- and 30-mg groups, 56% and 59% improvement, respectively.
Efficacy in the 7.5-mg group peaked at a 50% improvement by week 4, and then trailed off somewhat, with a mean 39% improvement by week 16.
Efficacy in the two higher dose groups also peaked around 4 weeks, with a 66% improvement in the 15-mg group and a 78% improvement in the 30-mg group. There was some tailing off of effect in each of these groups as well. By 16 weeks, the mean EASI improvement was 61.7% in the 15-mg group and 74.4% in the 30-mg group.
A good number of patients in each dosing group even achieved 90% skin clearance, Dr. Guttman noted.
“We generally say that we like to see an EASI 50, but now we are talking about EASI 75 and even 90. More than 60% on the highest dose achieved an EASI 75 and 60% an EASI 90. Even the 15-mg group achieved a nice EASI 90 response, at around 26%.”
Itching also responded well to upadacitinib, Dr. Guttman said. Within 1 week, all three active groups had separated significantly from the placebo group. By 2 weeks, the 7.5-mg group had a mean 29% improvement on the pruritus numerical rating scale. The improvement was 46% in the 15-mg group and 57.6% in the 30-mg group. By 16 weeks, the improvements were 39.6%, 48%, and 68.9%, respectively, compared with 9.7% among those on placebo.
The most common treatment-emergent adverse event was upper respiratory infection, which Dr. Guttman noted has been seen with JAK inhibitors. This occurred in 10% of those taking placebo and in 16.7% of the 7.5-mg group and 11.9% of both the 15-mg and 30-mg groups. A few patients experienced exacerbation of their AD on the medication (16.7%, 7% and 11.9% of the active groups, compared with 7.5% of those on placebo). New acne developed in 2.5% of placebo patients and in 9.5%, 4.8%, and 14.3% of those taking upadacitinib. The acne was on the body, considered mild, and resolved, Dr. Guttman said.
Two patients taking 7.5 mg and one taking 15 mg developed a serious infection, although she did not elaborate on the illnesses. Two taking 15 mg developed a liver disorder. There were four cases of neutropenia and one lymphopenia, all among the active groups. Creatine phosphokinase elevations occurred in one taking placebo and seven taking the study drug.
There were no pulmonary or deep vein thromboses; no opportunistic infections or malignancies; and no cases of herpes zoster, tuberculosis, or renal dysfunction. There were no deaths during the trial.
Phase 3 studies of upadacitinib in patients with rheumatoid arthritis, psoriatic arthritis, and Crohn’s disease are underway, according to AbbVie.
AbbVie sponsored the AD study. Dr. Guttman is a consultant for the company.
SOURCE: Guttman E et al. AAD late-breaking clinical trials, Abstract 6533
REPORTING FROM AAD 2018
Key clinical point: Upadacitinib had significant effects on reducing itch and clearing skin in patients with moderate to severe atopic dermatitis
Major finding: By 16 weeks, the mean EASI improvement was 61.7% in the 15-mg group and 74.4% in the 30-mg group.
Study details: In the dose-ranging, phase 2b randomized, placebo-controlled study, 126 patients with moderate to severe AD were treated with one of 3 upadacitinib doses, and 40 received placebo for 16 weeks.
Disclosures: AbbVie sponsored the study. Dr. Guttman is a consultant for the company.
Source: Guttman E et al. AAD late-breaking clinical trials, Abstract 6533
CPAP adherence linked to reduced readmissions
Hospitalized patients with obstructive sleep apnea (OSA) who were nonadherent to continuous positive airway pressure (CPAP) treatment were more than three times as likely to be readmitted for complications, according to a study.
Since preventable causes of readmission like congestive heart failure, obstructive lung disease, and diabetes are connected to OSA, boosting adherence rates to sleep apnea treatment could be an effective way to mitigate these risks.
Investigators gathered data for 345 hospitalized patients with OSA who were admitted to the VA Long Beach (Calif.) Healthcare System between January 2007 and December 2015.
Both the adherent and nonadherent groups were mostly white males. The 183 adherent patients were, on average, slightly older than the patients in the nonadherent group (66.3 vs. 62.3 years), while the nonadherent group had a larger proportion of African Americans (19.1%) than did the adherent group (10.4%).
In an analysis of both groups, 28% of nonadherent patients were readmitted within 30 days of discharge, compared with 10.2% of those in the adherent group (P less than .001). Readmission rates were significantly higher for nonadherent patients brought in for all-causes (adjusted odds ratio, 3.52; P less than .001), as were their rates of cardiovascular-related readmission (AOR, 2.31; P = .02).
The cardiovascular-related readmissions were most often caused by atrial fibrillation (29%), myocardial ischemia (22.5%), and congestive heart failure (19.3%) in the group who were not using CPAP. In this same group, urologic problems (10.7%), infections (8.0%), and psychiatric issues (5.3%) were the most common causes for hospital readmissions.
Investigators were surprised to find that the rate of pulmonary-related readmissions was not higher among nonadherent patients, considering the shared characteristics of OSA and COPD.
While nonadherent patients had an adjusted rate of pulmonary-related readmissions of 3.66, the difference between nonadherent and adherent patients was not significant.
“Those with OSA and COPD are considered to have overlap syndrome and, without CPAP therapy, are at higher risk for COPD exacerbation requiring hospitalization, pulmonary hypertension, and mortality,” according to Dr. Truong and her colleagues. “However, the number of patients with pulmonary readmissions was very small, and analysis did not reach statistical or clinical significance.”
Given the single-center nature of the study, these findings have limited generalizability. The study may also have been underpowered to uncover certain differences between the two groups because of the small population size.
Investigators reported no relevant financial disclosures.
SOURCE: K. Truong et al. J Clin Sleep Med. 2018;14(2):183–9.
The comorbidities associated with obstructive sleep apnea (OSA), such as heart failure, coronary artery disease, diabetes, and stroke, can be detrimental to patients’ care and commonly lead to hospitalization. Not only are these diseases interfering with successful treatment, but financial penalties linked to 30-day readmissions have economic implications for hospitals as well. Increasing CPAP adherence, therefore, may be a low-cost tool to improve hospital outcomes. Dr. Truong and her colleagues find compelling data showing the association of CPAP adherence and reduced 30-day readmissions. However, more work is needed before we can fully back the idea that CPAP adherence will prevent readmissions. While many studies have shown associations between OSA and cardiovascular events, there are no large, randomized trials that show the cardiovascular benefit of CPAP. The current theory is that patients who are adherent to CPAP are more likely to be healthier individuals, which makes them less likely to exhibit the comorbidities that would cause readmissions. A large randomized trial is the next logical step, and with OSA costs estimated at $2,000 annually per patient, it is a step worth pursuing.
Lucas M. Donovan, MD, is a pulmonologist at the University of Washington, Seattle. Martha E. Billings, MD, is an assistant professor in the division of pulmonary and critical care medicine at the University of Washington, Seattle. They reported no conflicts of interest.
The comorbidities associated with obstructive sleep apnea (OSA), such as heart failure, coronary artery disease, diabetes, and stroke, can be detrimental to patients’ care and commonly lead to hospitalization. Not only are these diseases interfering with successful treatment, but financial penalties linked to 30-day readmissions have economic implications for hospitals as well. Increasing CPAP adherence, therefore, may be a low-cost tool to improve hospital outcomes. Dr. Truong and her colleagues find compelling data showing the association of CPAP adherence and reduced 30-day readmissions. However, more work is needed before we can fully back the idea that CPAP adherence will prevent readmissions. While many studies have shown associations between OSA and cardiovascular events, there are no large, randomized trials that show the cardiovascular benefit of CPAP. The current theory is that patients who are adherent to CPAP are more likely to be healthier individuals, which makes them less likely to exhibit the comorbidities that would cause readmissions. A large randomized trial is the next logical step, and with OSA costs estimated at $2,000 annually per patient, it is a step worth pursuing.
Lucas M. Donovan, MD, is a pulmonologist at the University of Washington, Seattle. Martha E. Billings, MD, is an assistant professor in the division of pulmonary and critical care medicine at the University of Washington, Seattle. They reported no conflicts of interest.
The comorbidities associated with obstructive sleep apnea (OSA), such as heart failure, coronary artery disease, diabetes, and stroke, can be detrimental to patients’ care and commonly lead to hospitalization. Not only are these diseases interfering with successful treatment, but financial penalties linked to 30-day readmissions have economic implications for hospitals as well. Increasing CPAP adherence, therefore, may be a low-cost tool to improve hospital outcomes. Dr. Truong and her colleagues find compelling data showing the association of CPAP adherence and reduced 30-day readmissions. However, more work is needed before we can fully back the idea that CPAP adherence will prevent readmissions. While many studies have shown associations between OSA and cardiovascular events, there are no large, randomized trials that show the cardiovascular benefit of CPAP. The current theory is that patients who are adherent to CPAP are more likely to be healthier individuals, which makes them less likely to exhibit the comorbidities that would cause readmissions. A large randomized trial is the next logical step, and with OSA costs estimated at $2,000 annually per patient, it is a step worth pursuing.
Lucas M. Donovan, MD, is a pulmonologist at the University of Washington, Seattle. Martha E. Billings, MD, is an assistant professor in the division of pulmonary and critical care medicine at the University of Washington, Seattle. They reported no conflicts of interest.
Hospitalized patients with obstructive sleep apnea (OSA) who were nonadherent to continuous positive airway pressure (CPAP) treatment were more than three times as likely to be readmitted for complications, according to a study.
Since preventable causes of readmission like congestive heart failure, obstructive lung disease, and diabetes are connected to OSA, boosting adherence rates to sleep apnea treatment could be an effective way to mitigate these risks.
Investigators gathered data for 345 hospitalized patients with OSA who were admitted to the VA Long Beach (Calif.) Healthcare System between January 2007 and December 2015.
Both the adherent and nonadherent groups were mostly white males. The 183 adherent patients were, on average, slightly older than the patients in the nonadherent group (66.3 vs. 62.3 years), while the nonadherent group had a larger proportion of African Americans (19.1%) than did the adherent group (10.4%).
In an analysis of both groups, 28% of nonadherent patients were readmitted within 30 days of discharge, compared with 10.2% of those in the adherent group (P less than .001). Readmission rates were significantly higher for nonadherent patients brought in for all-causes (adjusted odds ratio, 3.52; P less than .001), as were their rates of cardiovascular-related readmission (AOR, 2.31; P = .02).
The cardiovascular-related readmissions were most often caused by atrial fibrillation (29%), myocardial ischemia (22.5%), and congestive heart failure (19.3%) in the group who were not using CPAP. In this same group, urologic problems (10.7%), infections (8.0%), and psychiatric issues (5.3%) were the most common causes for hospital readmissions.
Investigators were surprised to find that the rate of pulmonary-related readmissions was not higher among nonadherent patients, considering the shared characteristics of OSA and COPD.
While nonadherent patients had an adjusted rate of pulmonary-related readmissions of 3.66, the difference between nonadherent and adherent patients was not significant.
“Those with OSA and COPD are considered to have overlap syndrome and, without CPAP therapy, are at higher risk for COPD exacerbation requiring hospitalization, pulmonary hypertension, and mortality,” according to Dr. Truong and her colleagues. “However, the number of patients with pulmonary readmissions was very small, and analysis did not reach statistical or clinical significance.”
Given the single-center nature of the study, these findings have limited generalizability. The study may also have been underpowered to uncover certain differences between the two groups because of the small population size.
Investigators reported no relevant financial disclosures.
SOURCE: K. Truong et al. J Clin Sleep Med. 2018;14(2):183–9.
Hospitalized patients with obstructive sleep apnea (OSA) who were nonadherent to continuous positive airway pressure (CPAP) treatment were more than three times as likely to be readmitted for complications, according to a study.
Since preventable causes of readmission like congestive heart failure, obstructive lung disease, and diabetes are connected to OSA, boosting adherence rates to sleep apnea treatment could be an effective way to mitigate these risks.
Investigators gathered data for 345 hospitalized patients with OSA who were admitted to the VA Long Beach (Calif.) Healthcare System between January 2007 and December 2015.
Both the adherent and nonadherent groups were mostly white males. The 183 adherent patients were, on average, slightly older than the patients in the nonadherent group (66.3 vs. 62.3 years), while the nonadherent group had a larger proportion of African Americans (19.1%) than did the adherent group (10.4%).
In an analysis of both groups, 28% of nonadherent patients were readmitted within 30 days of discharge, compared with 10.2% of those in the adherent group (P less than .001). Readmission rates were significantly higher for nonadherent patients brought in for all-causes (adjusted odds ratio, 3.52; P less than .001), as were their rates of cardiovascular-related readmission (AOR, 2.31; P = .02).
The cardiovascular-related readmissions were most often caused by atrial fibrillation (29%), myocardial ischemia (22.5%), and congestive heart failure (19.3%) in the group who were not using CPAP. In this same group, urologic problems (10.7%), infections (8.0%), and psychiatric issues (5.3%) were the most common causes for hospital readmissions.
Investigators were surprised to find that the rate of pulmonary-related readmissions was not higher among nonadherent patients, considering the shared characteristics of OSA and COPD.
While nonadherent patients had an adjusted rate of pulmonary-related readmissions of 3.66, the difference between nonadherent and adherent patients was not significant.
“Those with OSA and COPD are considered to have overlap syndrome and, without CPAP therapy, are at higher risk for COPD exacerbation requiring hospitalization, pulmonary hypertension, and mortality,” according to Dr. Truong and her colleagues. “However, the number of patients with pulmonary readmissions was very small, and analysis did not reach statistical or clinical significance.”
Given the single-center nature of the study, these findings have limited generalizability. The study may also have been underpowered to uncover certain differences between the two groups because of the small population size.
Investigators reported no relevant financial disclosures.
SOURCE: K. Truong et al. J Clin Sleep Med. 2018;14(2):183–9.
FROM THE JOURNAL OF CLINICAL SLEEP MEDICINE
Key clinical point:
Major finding: CPAP-nonadherent patients were 3.5 times more likely to be readmitted within 30 days.
Study details: A retrospective study of 345 patients with obstructive sleep apnea who were hospitalized at a Veterans Affairs hospital between Jan. 1, 2007, and Dec. 31, 2015.
Disclosures: Investigators reported no relevant financial disclosures.
Source: K. Truong et al. J Clin Sleep Med. 2018;14(2):183-9.
Learning from the 2017 Oscar fiasco
It was a “never event.” At the very end of the 2017 Academy Awards presentation, the winner for Best Picture was announced. It was wrong. Two and a half minutes later it was corrected. The true winner was “Moonlight,” not “La La Land.” But by then much damage had been done.
I watched it happen live on TV and reviewed it again on YouTube. Several news agencies investigated and reported on what happened. I don’t have any inside information beyond that, but my engineering perspective can illuminate how to reduce mistakes.
The first lesson is how quickly people seek to assign blame after something goes wrong. I saw various online news agencies say Warren Beatty had announced the wrong winner. While he opened the envelope, it was Faye Dunaway who actually made the announcement of “La La Land.” Furthermore, Warren and Faye were merely reading the card. Warren had been given the wrong envelope, as high resolution photographs prove. The envelope was a duplicate for the prize announced just before them for the Best Actress award. The card said Emma Stone and in a smaller font “La La Land,” the film in which she starred. Warren hesitated because of how this was written on the card. Faye thought he was trying to pause as a shtick to increase suspense so she glanced at the card and blurted out “La La Land.”
Experts in quality improvement have learned that the best way to reduce errors is to resist this tendency to assign blame. A better approach is to assume, absent evidence to the contrary, that everyone is acting responsibly and sincerely to help the patient. Hear both sides of the story before jumping to any conclusions. Find systemic factors that contributed to a human error. Then focus on ameliorating systemic weaknesses.
One contributing factor for the error at the Oscars was that there were two copies of the set of award envelopes, with one set available on each side of the stage. This way the presenters can enter from either side of the stage. They are handed an envelope by one of the two auditors from PricewaterhouseCoopers, who are the only ones who know the contents.
A key component of safety is having check backs. The envelopes have the name of the award on the outside. One might hope the presenter would double check that they are being given the correct envelope by the auditor. But backstage is a very nervous and hectic place for the presenters. Actors are not professionals dedicated to safety.
Medical care is different. Before giving a transfusion, one nurse reads the number on the bag of blood to another nurse, who confirms that it matches a paper form. That simple act can prevent mistakes. Perhaps the auditor handing the envelope to the Oscar presenter should ask the presenter, who knows which award s/he is scheduled to announce, to read out loud the award title on the front of the envelope.
Clearly, Warren Beatty was confused by the contents of the envelope. He was expecting a card to have the name of a film, not the name of an actress with the film’s name in small print below it. He didn’t know what action to take and hesitated. Faye Dunaway plunged forward and misinterpreted the card. A key component of quality is making it safe for anyone, if they are not confident in what is happening, to stop the proceeding, ask questions, and challenge plans. For example, there are time-outs prior to surgery. A second component is presenting information in a form less likely to be misinterpreted. Medicine has a problem with many sound-alike and look-alike drug names, so sometimes these words are spelled with particular letters capitalized, to distinguish them. I wish EHRs would present lab results in large, bold font.
Another contributing factor here was that Faye misinterpreted Warren’s behaviors as a joke. Major airlines utilize the “sterile cockpit.” During the few minutes that they are running through the preflight checklist, the pilot and copilot do not discuss last night’s football game, crack jokes, or engage in any other extraneous conversations. They avoid interruptions and distractions, focusing solely on the task. Sign outs in medicine need to adopt this habit.
There is a concern that one of the auditors tweeted a picture of Emma Stone backstage holding her Oscar at the same time the fiasco was happening on stage. In the modern world, cell phones and selfies are a key source of distraction, errors, and car accidents.
Per the Army, “Prior planning prevents poor performance.” A couple days before the Oscar fiasco, the auditors were interviewed and they revealed that they didn’t have an action plan to deal with the situation of a mistaken announcement. They figured it was extremely unlikely and that the circumstances would determine the best response.
Experience has shown that in the hours leading up to a pediatric code, there may be several opportunities to recognize the risk and intervene so that blame cannot be assigned to a single person or action. Mock codes prepare people to think on their feet. And it is important to have a clearly designated person in charge of a code. Leadership matters.
In the Oscar fiasco, the damage was quickly limited by the gracious words of a “La La Land” producer He assessed the situation, announced the mistake, beckoned the “Moonlight” cast and crew to the stage, graciously complimented them, showed the correct award envelope and card to the camera, and offered the statue to the correct producer. Then he hastened his team off the stage. These actions of responsibility, truthfulness, transparency, and grace staunched the bleeding, minimized the damage, and as best as possible, remediated the error. Movie producers are experts at dealing with crises and catastrophes. Medical staff, when revealing errors to patients, can learn from this role model.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
It was a “never event.” At the very end of the 2017 Academy Awards presentation, the winner for Best Picture was announced. It was wrong. Two and a half minutes later it was corrected. The true winner was “Moonlight,” not “La La Land.” But by then much damage had been done.
I watched it happen live on TV and reviewed it again on YouTube. Several news agencies investigated and reported on what happened. I don’t have any inside information beyond that, but my engineering perspective can illuminate how to reduce mistakes.
The first lesson is how quickly people seek to assign blame after something goes wrong. I saw various online news agencies say Warren Beatty had announced the wrong winner. While he opened the envelope, it was Faye Dunaway who actually made the announcement of “La La Land.” Furthermore, Warren and Faye were merely reading the card. Warren had been given the wrong envelope, as high resolution photographs prove. The envelope was a duplicate for the prize announced just before them for the Best Actress award. The card said Emma Stone and in a smaller font “La La Land,” the film in which she starred. Warren hesitated because of how this was written on the card. Faye thought he was trying to pause as a shtick to increase suspense so she glanced at the card and blurted out “La La Land.”
Experts in quality improvement have learned that the best way to reduce errors is to resist this tendency to assign blame. A better approach is to assume, absent evidence to the contrary, that everyone is acting responsibly and sincerely to help the patient. Hear both sides of the story before jumping to any conclusions. Find systemic factors that contributed to a human error. Then focus on ameliorating systemic weaknesses.
One contributing factor for the error at the Oscars was that there were two copies of the set of award envelopes, with one set available on each side of the stage. This way the presenters can enter from either side of the stage. They are handed an envelope by one of the two auditors from PricewaterhouseCoopers, who are the only ones who know the contents.
A key component of safety is having check backs. The envelopes have the name of the award on the outside. One might hope the presenter would double check that they are being given the correct envelope by the auditor. But backstage is a very nervous and hectic place for the presenters. Actors are not professionals dedicated to safety.
Medical care is different. Before giving a transfusion, one nurse reads the number on the bag of blood to another nurse, who confirms that it matches a paper form. That simple act can prevent mistakes. Perhaps the auditor handing the envelope to the Oscar presenter should ask the presenter, who knows which award s/he is scheduled to announce, to read out loud the award title on the front of the envelope.
Clearly, Warren Beatty was confused by the contents of the envelope. He was expecting a card to have the name of a film, not the name of an actress with the film’s name in small print below it. He didn’t know what action to take and hesitated. Faye Dunaway plunged forward and misinterpreted the card. A key component of quality is making it safe for anyone, if they are not confident in what is happening, to stop the proceeding, ask questions, and challenge plans. For example, there are time-outs prior to surgery. A second component is presenting information in a form less likely to be misinterpreted. Medicine has a problem with many sound-alike and look-alike drug names, so sometimes these words are spelled with particular letters capitalized, to distinguish them. I wish EHRs would present lab results in large, bold font.
Another contributing factor here was that Faye misinterpreted Warren’s behaviors as a joke. Major airlines utilize the “sterile cockpit.” During the few minutes that they are running through the preflight checklist, the pilot and copilot do not discuss last night’s football game, crack jokes, or engage in any other extraneous conversations. They avoid interruptions and distractions, focusing solely on the task. Sign outs in medicine need to adopt this habit.
There is a concern that one of the auditors tweeted a picture of Emma Stone backstage holding her Oscar at the same time the fiasco was happening on stage. In the modern world, cell phones and selfies are a key source of distraction, errors, and car accidents.
Per the Army, “Prior planning prevents poor performance.” A couple days before the Oscar fiasco, the auditors were interviewed and they revealed that they didn’t have an action plan to deal with the situation of a mistaken announcement. They figured it was extremely unlikely and that the circumstances would determine the best response.
Experience has shown that in the hours leading up to a pediatric code, there may be several opportunities to recognize the risk and intervene so that blame cannot be assigned to a single person or action. Mock codes prepare people to think on their feet. And it is important to have a clearly designated person in charge of a code. Leadership matters.
In the Oscar fiasco, the damage was quickly limited by the gracious words of a “La La Land” producer He assessed the situation, announced the mistake, beckoned the “Moonlight” cast and crew to the stage, graciously complimented them, showed the correct award envelope and card to the camera, and offered the statue to the correct producer. Then he hastened his team off the stage. These actions of responsibility, truthfulness, transparency, and grace staunched the bleeding, minimized the damage, and as best as possible, remediated the error. Movie producers are experts at dealing with crises and catastrophes. Medical staff, when revealing errors to patients, can learn from this role model.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
It was a “never event.” At the very end of the 2017 Academy Awards presentation, the winner for Best Picture was announced. It was wrong. Two and a half minutes later it was corrected. The true winner was “Moonlight,” not “La La Land.” But by then much damage had been done.
I watched it happen live on TV and reviewed it again on YouTube. Several news agencies investigated and reported on what happened. I don’t have any inside information beyond that, but my engineering perspective can illuminate how to reduce mistakes.
The first lesson is how quickly people seek to assign blame after something goes wrong. I saw various online news agencies say Warren Beatty had announced the wrong winner. While he opened the envelope, it was Faye Dunaway who actually made the announcement of “La La Land.” Furthermore, Warren and Faye were merely reading the card. Warren had been given the wrong envelope, as high resolution photographs prove. The envelope was a duplicate for the prize announced just before them for the Best Actress award. The card said Emma Stone and in a smaller font “La La Land,” the film in which she starred. Warren hesitated because of how this was written on the card. Faye thought he was trying to pause as a shtick to increase suspense so she glanced at the card and blurted out “La La Land.”
Experts in quality improvement have learned that the best way to reduce errors is to resist this tendency to assign blame. A better approach is to assume, absent evidence to the contrary, that everyone is acting responsibly and sincerely to help the patient. Hear both sides of the story before jumping to any conclusions. Find systemic factors that contributed to a human error. Then focus on ameliorating systemic weaknesses.
One contributing factor for the error at the Oscars was that there were two copies of the set of award envelopes, with one set available on each side of the stage. This way the presenters can enter from either side of the stage. They are handed an envelope by one of the two auditors from PricewaterhouseCoopers, who are the only ones who know the contents.
A key component of safety is having check backs. The envelopes have the name of the award on the outside. One might hope the presenter would double check that they are being given the correct envelope by the auditor. But backstage is a very nervous and hectic place for the presenters. Actors are not professionals dedicated to safety.
Medical care is different. Before giving a transfusion, one nurse reads the number on the bag of blood to another nurse, who confirms that it matches a paper form. That simple act can prevent mistakes. Perhaps the auditor handing the envelope to the Oscar presenter should ask the presenter, who knows which award s/he is scheduled to announce, to read out loud the award title on the front of the envelope.
Clearly, Warren Beatty was confused by the contents of the envelope. He was expecting a card to have the name of a film, not the name of an actress with the film’s name in small print below it. He didn’t know what action to take and hesitated. Faye Dunaway plunged forward and misinterpreted the card. A key component of quality is making it safe for anyone, if they are not confident in what is happening, to stop the proceeding, ask questions, and challenge plans. For example, there are time-outs prior to surgery. A second component is presenting information in a form less likely to be misinterpreted. Medicine has a problem with many sound-alike and look-alike drug names, so sometimes these words are spelled with particular letters capitalized, to distinguish them. I wish EHRs would present lab results in large, bold font.
Another contributing factor here was that Faye misinterpreted Warren’s behaviors as a joke. Major airlines utilize the “sterile cockpit.” During the few minutes that they are running through the preflight checklist, the pilot and copilot do not discuss last night’s football game, crack jokes, or engage in any other extraneous conversations. They avoid interruptions and distractions, focusing solely on the task. Sign outs in medicine need to adopt this habit.
There is a concern that one of the auditors tweeted a picture of Emma Stone backstage holding her Oscar at the same time the fiasco was happening on stage. In the modern world, cell phones and selfies are a key source of distraction, errors, and car accidents.
Per the Army, “Prior planning prevents poor performance.” A couple days before the Oscar fiasco, the auditors were interviewed and they revealed that they didn’t have an action plan to deal with the situation of a mistaken announcement. They figured it was extremely unlikely and that the circumstances would determine the best response.
Experience has shown that in the hours leading up to a pediatric code, there may be several opportunities to recognize the risk and intervene so that blame cannot be assigned to a single person or action. Mock codes prepare people to think on their feet. And it is important to have a clearly designated person in charge of a code. Leadership matters.
In the Oscar fiasco, the damage was quickly limited by the gracious words of a “La La Land” producer He assessed the situation, announced the mistake, beckoned the “Moonlight” cast and crew to the stage, graciously complimented them, showed the correct award envelope and card to the camera, and offered the statue to the correct producer. Then he hastened his team off the stage. These actions of responsibility, truthfulness, transparency, and grace staunched the bleeding, minimized the damage, and as best as possible, remediated the error. Movie producers are experts at dealing with crises and catastrophes. Medical staff, when revealing errors to patients, can learn from this role model.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
Delayed treatment for psychosis can have ‘deleterious’ effects
The longer that patients with schizophrenia go without treatment for a psychotic episode, the more their hippocampus atrophies, suggests a study published Feb. 21 in JAMA Psychiatry.
To conduct the study, researchers studied 71 patients between March 5, 2013, and Oct. 8, 2014, who were experiencing their first psychotic episode and were antipsychotic naive. The research team matched those patients with 73 healthy controls, treated the patients with antipsychotics, and performed MRIs on members of both groups at baseline and 8 weeks later. The primary outcome measure was change in left and right hippocampal volume integrity, which is inversely associated with hippocampal atrophy.
Results showed that many patients with psychosis had lower median hippocampal volume integrity. Furthermore, those . The duration of untreated psychosis was correlated with both decreases, but this association was only significant with left hippocampal volume integrity.
This relationship is “consistent with a persistent, possibly deleterious, effect of untreated psychosis on brain structure,” wrote Donald C. Goff, MD, of the psychiatry department at New York University, and his associates. “Larger longitudinal studies of longer duration are needed to examine the association between [duration of untreated psychosis], hippocampal volume, and clinical outcomes.”
Dr. Goff reported receiving research support from the National Institute of Mental Health, the Stanley Medical Research Institute, and Avanir Pharmaceuticals. Another author reported receiving support from numerous entities and honoraria for serving on an advisory board for Allergan. No other disclosures were reported.
Read more at JAMA Psychiatry.
The longer that patients with schizophrenia go without treatment for a psychotic episode, the more their hippocampus atrophies, suggests a study published Feb. 21 in JAMA Psychiatry.
To conduct the study, researchers studied 71 patients between March 5, 2013, and Oct. 8, 2014, who were experiencing their first psychotic episode and were antipsychotic naive. The research team matched those patients with 73 healthy controls, treated the patients with antipsychotics, and performed MRIs on members of both groups at baseline and 8 weeks later. The primary outcome measure was change in left and right hippocampal volume integrity, which is inversely associated with hippocampal atrophy.
Results showed that many patients with psychosis had lower median hippocampal volume integrity. Furthermore, those . The duration of untreated psychosis was correlated with both decreases, but this association was only significant with left hippocampal volume integrity.
This relationship is “consistent with a persistent, possibly deleterious, effect of untreated psychosis on brain structure,” wrote Donald C. Goff, MD, of the psychiatry department at New York University, and his associates. “Larger longitudinal studies of longer duration are needed to examine the association between [duration of untreated psychosis], hippocampal volume, and clinical outcomes.”
Dr. Goff reported receiving research support from the National Institute of Mental Health, the Stanley Medical Research Institute, and Avanir Pharmaceuticals. Another author reported receiving support from numerous entities and honoraria for serving on an advisory board for Allergan. No other disclosures were reported.
Read more at JAMA Psychiatry.
The longer that patients with schizophrenia go without treatment for a psychotic episode, the more their hippocampus atrophies, suggests a study published Feb. 21 in JAMA Psychiatry.
To conduct the study, researchers studied 71 patients between March 5, 2013, and Oct. 8, 2014, who were experiencing their first psychotic episode and were antipsychotic naive. The research team matched those patients with 73 healthy controls, treated the patients with antipsychotics, and performed MRIs on members of both groups at baseline and 8 weeks later. The primary outcome measure was change in left and right hippocampal volume integrity, which is inversely associated with hippocampal atrophy.
Results showed that many patients with psychosis had lower median hippocampal volume integrity. Furthermore, those . The duration of untreated psychosis was correlated with both decreases, but this association was only significant with left hippocampal volume integrity.
This relationship is “consistent with a persistent, possibly deleterious, effect of untreated psychosis on brain structure,” wrote Donald C. Goff, MD, of the psychiatry department at New York University, and his associates. “Larger longitudinal studies of longer duration are needed to examine the association between [duration of untreated psychosis], hippocampal volume, and clinical outcomes.”
Dr. Goff reported receiving research support from the National Institute of Mental Health, the Stanley Medical Research Institute, and Avanir Pharmaceuticals. Another author reported receiving support from numerous entities and honoraria for serving on an advisory board for Allergan. No other disclosures were reported.
Read more at JAMA Psychiatry.
FROM JAMA PSYCHIATRY
Lung scan often not requested for new SSc patients
Only half of American general rheumatologists and two-thirds of global systemic sclerosis experts routinely request high-resolution CT chest scans for all their newly diagnosed systemic sclerosis patients despite their increased risk of interstitial lung disease, according to survey data from approximately 200 clinicians.
The researchers, led by Elana J. Bernstein, MD, of Columbia University, New York, conducted the survey because of a lack of data on how often rheumatologists order high-resolution CT for their newly diagnosed patients and the absence of clinical practice guidelines that recommend screening for interstitial lung disease (ILD) in systemic sclerosis (SSc).
In a study published in Arthritis & Rheumatology, the researchers surveyed 676 American College of Rheumatology members and 356 global experts on systemic sclerosis; of these, 76 ACR general rheumatologists and 135 SSc experts responded. The use of high-resolution CT varied widely by country or region: 0 of 5 respondents from Australia, 2 of 6 from Canada, 28 of 47 from the United States, 45 of 57 from Europe, 4 of 5 from Asia, and 7 of 7 from Latin America.
The researchers also found little consensus on indications for high-resolution CT in SSc patients. Among the SSc experts who do not routinely obtain screening high-resolution CTs in their SSc patients, 81% said they would request one for dyspnea on exertion, 74% would request one for an abnormal forced vital capacity less than 80% of predicted, and 52% would request one for an abnormal diffusion capacity for carbon monoxide less than 80% predicted.
A significant limitation of the study was the low response rate, and more research is needed on the clinical impact of high-resolution CT screening for ILD in SSc patients, the researchers noted. However, the results highlight the need for a clinical practice guideline to create a more consistent approach to identifying ILD in these patients, they said.
The researchers had no financial conflicts to disclose. Dr. Bernstein was supported by a Rheumatology Research Foundation Scientist Development Award, and two of her colleagues were funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute.
SOURCE: Bernstein E et al. Arthritis Rheumatol. 2018 Feb 9. doi: 10.1002/art.40441.
Only half of American general rheumatologists and two-thirds of global systemic sclerosis experts routinely request high-resolution CT chest scans for all their newly diagnosed systemic sclerosis patients despite their increased risk of interstitial lung disease, according to survey data from approximately 200 clinicians.
The researchers, led by Elana J. Bernstein, MD, of Columbia University, New York, conducted the survey because of a lack of data on how often rheumatologists order high-resolution CT for their newly diagnosed patients and the absence of clinical practice guidelines that recommend screening for interstitial lung disease (ILD) in systemic sclerosis (SSc).
In a study published in Arthritis & Rheumatology, the researchers surveyed 676 American College of Rheumatology members and 356 global experts on systemic sclerosis; of these, 76 ACR general rheumatologists and 135 SSc experts responded. The use of high-resolution CT varied widely by country or region: 0 of 5 respondents from Australia, 2 of 6 from Canada, 28 of 47 from the United States, 45 of 57 from Europe, 4 of 5 from Asia, and 7 of 7 from Latin America.
The researchers also found little consensus on indications for high-resolution CT in SSc patients. Among the SSc experts who do not routinely obtain screening high-resolution CTs in their SSc patients, 81% said they would request one for dyspnea on exertion, 74% would request one for an abnormal forced vital capacity less than 80% of predicted, and 52% would request one for an abnormal diffusion capacity for carbon monoxide less than 80% predicted.
A significant limitation of the study was the low response rate, and more research is needed on the clinical impact of high-resolution CT screening for ILD in SSc patients, the researchers noted. However, the results highlight the need for a clinical practice guideline to create a more consistent approach to identifying ILD in these patients, they said.
The researchers had no financial conflicts to disclose. Dr. Bernstein was supported by a Rheumatology Research Foundation Scientist Development Award, and two of her colleagues were funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute.
SOURCE: Bernstein E et al. Arthritis Rheumatol. 2018 Feb 9. doi: 10.1002/art.40441.
Only half of American general rheumatologists and two-thirds of global systemic sclerosis experts routinely request high-resolution CT chest scans for all their newly diagnosed systemic sclerosis patients despite their increased risk of interstitial lung disease, according to survey data from approximately 200 clinicians.
The researchers, led by Elana J. Bernstein, MD, of Columbia University, New York, conducted the survey because of a lack of data on how often rheumatologists order high-resolution CT for their newly diagnosed patients and the absence of clinical practice guidelines that recommend screening for interstitial lung disease (ILD) in systemic sclerosis (SSc).
In a study published in Arthritis & Rheumatology, the researchers surveyed 676 American College of Rheumatology members and 356 global experts on systemic sclerosis; of these, 76 ACR general rheumatologists and 135 SSc experts responded. The use of high-resolution CT varied widely by country or region: 0 of 5 respondents from Australia, 2 of 6 from Canada, 28 of 47 from the United States, 45 of 57 from Europe, 4 of 5 from Asia, and 7 of 7 from Latin America.
The researchers also found little consensus on indications for high-resolution CT in SSc patients. Among the SSc experts who do not routinely obtain screening high-resolution CTs in their SSc patients, 81% said they would request one for dyspnea on exertion, 74% would request one for an abnormal forced vital capacity less than 80% of predicted, and 52% would request one for an abnormal diffusion capacity for carbon monoxide less than 80% predicted.
A significant limitation of the study was the low response rate, and more research is needed on the clinical impact of high-resolution CT screening for ILD in SSc patients, the researchers noted. However, the results highlight the need for a clinical practice guideline to create a more consistent approach to identifying ILD in these patients, they said.
The researchers had no financial conflicts to disclose. Dr. Bernstein was supported by a Rheumatology Research Foundation Scientist Development Award, and two of her colleagues were funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute.
SOURCE: Bernstein E et al. Arthritis Rheumatol. 2018 Feb 9. doi: 10.1002/art.40441.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Despite the risk of interstitial lung disease in systemic sclerosis patients, the use of high-resolution CT scans of the chest is inconsistent.
Major finding: Overall, 51% of ACR general rheumatologists and 66% of global systemic sclerosis experts ordered high-resolution CTs for new SSc patients.
Study details: The data come from surveys completed by 76 ACR general rheumatologists and 135 SSc experts worldwide.
Disclosures: The researchers had no financial conflicts to disclose. Dr. Bernstein was supported by a Rheumatology Research Foundation Scientist Development Award, and two of her colleagues were funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute.
Source: Bernstein E et al. Arthritis Rheumatol. 2018 Feb 9. doi: 10.1002/art.40441.
Viremic suppression linked to decreased MACE rate in patients with HCV-cirrhosis
Hepatitis C viremic suppression was associated with a lower rate of cardiovascular events in patients with compensated HCV-related cirrhosis, compared with control patients who did not achieve a sustained virological response. In addition, predictive factors for major adverse cardiovascular events (MACEs) in compensated HCV-related cirrhosis were Asian ethnic origin, hypertension, smoking, and low serum albumin, according to a report in American Heart Journal.
A total of 878 patients with HCV-related cirrhosis were enrolled at 35 French centers. Upon enrollment, all patients received HCV treatment and were followed for MACEs, including stroke, myocardial infarction, ischemic heart disease, heart failure, peripheral arterial disease, cardiac arrest, and cardiovascular-related death, according to Patrice Cacoub, MD, of Sorbonne Universités, Paris, and his colleagues.
Five-year survival for patients presenting with a MACE was 60% vs. 88% in patients who did not have an event.
“[Our] results strengthen the systemic nature of HCV infection, a chronic disease in which cardiovascular risk must be carefully assessed. The decreased rate of MACEs after [sustained virological response] in this population should be taken into account to enable wider access to new [direct-acting antivirals]. Further studies are warranted to evaluate whether a similar benefit can be obtained in less severe patients, such as noncirrhotic HCV-infected patients,” the researchers concluded.
The authors reported having no conflicts of interest.
SOURCE: Cacoub, P et al. Am Heart J. 2018;198:4-17.
Hepatitis C viremic suppression was associated with a lower rate of cardiovascular events in patients with compensated HCV-related cirrhosis, compared with control patients who did not achieve a sustained virological response. In addition, predictive factors for major adverse cardiovascular events (MACEs) in compensated HCV-related cirrhosis were Asian ethnic origin, hypertension, smoking, and low serum albumin, according to a report in American Heart Journal.
A total of 878 patients with HCV-related cirrhosis were enrolled at 35 French centers. Upon enrollment, all patients received HCV treatment and were followed for MACEs, including stroke, myocardial infarction, ischemic heart disease, heart failure, peripheral arterial disease, cardiac arrest, and cardiovascular-related death, according to Patrice Cacoub, MD, of Sorbonne Universités, Paris, and his colleagues.
Five-year survival for patients presenting with a MACE was 60% vs. 88% in patients who did not have an event.
“[Our] results strengthen the systemic nature of HCV infection, a chronic disease in which cardiovascular risk must be carefully assessed. The decreased rate of MACEs after [sustained virological response] in this population should be taken into account to enable wider access to new [direct-acting antivirals]. Further studies are warranted to evaluate whether a similar benefit can be obtained in less severe patients, such as noncirrhotic HCV-infected patients,” the researchers concluded.
The authors reported having no conflicts of interest.
SOURCE: Cacoub, P et al. Am Heart J. 2018;198:4-17.
Hepatitis C viremic suppression was associated with a lower rate of cardiovascular events in patients with compensated HCV-related cirrhosis, compared with control patients who did not achieve a sustained virological response. In addition, predictive factors for major adverse cardiovascular events (MACEs) in compensated HCV-related cirrhosis were Asian ethnic origin, hypertension, smoking, and low serum albumin, according to a report in American Heart Journal.
A total of 878 patients with HCV-related cirrhosis were enrolled at 35 French centers. Upon enrollment, all patients received HCV treatment and were followed for MACEs, including stroke, myocardial infarction, ischemic heart disease, heart failure, peripheral arterial disease, cardiac arrest, and cardiovascular-related death, according to Patrice Cacoub, MD, of Sorbonne Universités, Paris, and his colleagues.
Five-year survival for patients presenting with a MACE was 60% vs. 88% in patients who did not have an event.
“[Our] results strengthen the systemic nature of HCV infection, a chronic disease in which cardiovascular risk must be carefully assessed. The decreased rate of MACEs after [sustained virological response] in this population should be taken into account to enable wider access to new [direct-acting antivirals]. Further studies are warranted to evaluate whether a similar benefit can be obtained in less severe patients, such as noncirrhotic HCV-infected patients,” the researchers concluded.
The authors reported having no conflicts of interest.
SOURCE: Cacoub, P et al. Am Heart J. 2018;198:4-17.
FROM AMERICAN HEART JOURNAL
Key clinical point: Predictive factors for MACE in compensated HCV-related cirrhosis were Asian ethnic origin, hypertension, smoking, and low serum albumin.
Major finding: Achieving viremic suppression was associated with a lower rate of cardiovascular events in patients with compensated HCV-related cirrhosis.
Study details: A study at 35 French centers of 878 patients with HCV-related cirrhosis.
Disclosures: The authors reported having no conflicts of interest.
Source: Cacoub, P et al. Am Heart J. 2018;198:4-17.