User login
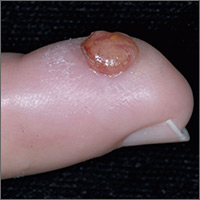
Growth on finger
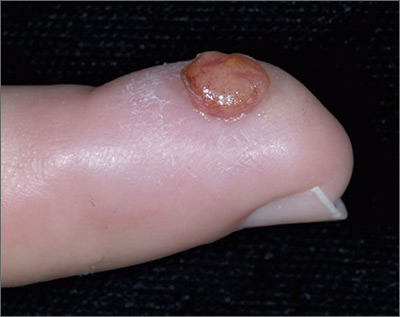
The FP diagnosed pyogenic granuloma (PG), a common, benign, acquired vascular lesion of the skin and mucous membranes. PGs are erythematous, dome-shaped papules or nodules that bleed easily. They are prone to ulceration, erosion, and crusting, and rapid growth may occur over a period of weeks. The etiology for PG is unknown, but may be the result of trauma, infection, or preceding dermatoses. A more up-to-date and appropriate term for PG is lobular capillary hemangioma, because these lesions are neither pyogenic nor granulomas.
PGs are most often found on the fingers, lips, and hands. They may resemble a number of malignancies including basal cell carcinoma, Kaposi’s sarcoma, metastatic cutaneous lesions, squamous cell carcinoma, and amelanotic melanoma. For that reason, it’s especially important to send the excised lesion for pathology to ensure that malignancy isn’t missed.
The patient was eager to have the PG removed at this visit, as a number of previous visits to urgent care centers resulted in courses of antibiotics that didn’t help. After performing a digital block, the FP removed the PG with a shave excision, followed by electrodesiccation and curettage. The electrodesiccation and curettage are important to prevent recurrence. In this case, the pathology confirmed the diagnosis, and the PG did not recur.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Usatine R. Pyogenic Granuloma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 940-944.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed pyogenic granuloma (PG), a common, benign, acquired vascular lesion of the skin and mucous membranes. PGs are erythematous, dome-shaped papules or nodules that bleed easily. They are prone to ulceration, erosion, and crusting, and rapid growth may occur over a period of weeks. The etiology for PG is unknown, but may be the result of trauma, infection, or preceding dermatoses. A more up-to-date and appropriate term for PG is lobular capillary hemangioma, because these lesions are neither pyogenic nor granulomas.
PGs are most often found on the fingers, lips, and hands. They may resemble a number of malignancies including basal cell carcinoma, Kaposi’s sarcoma, metastatic cutaneous lesions, squamous cell carcinoma, and amelanotic melanoma. For that reason, it’s especially important to send the excised lesion for pathology to ensure that malignancy isn’t missed.
The patient was eager to have the PG removed at this visit, as a number of previous visits to urgent care centers resulted in courses of antibiotics that didn’t help. After performing a digital block, the FP removed the PG with a shave excision, followed by electrodesiccation and curettage. The electrodesiccation and curettage are important to prevent recurrence. In this case, the pathology confirmed the diagnosis, and the PG did not recur.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Usatine R. Pyogenic Granuloma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 940-944.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP diagnosed pyogenic granuloma (PG), a common, benign, acquired vascular lesion of the skin and mucous membranes. PGs are erythematous, dome-shaped papules or nodules that bleed easily. They are prone to ulceration, erosion, and crusting, and rapid growth may occur over a period of weeks. The etiology for PG is unknown, but may be the result of trauma, infection, or preceding dermatoses. A more up-to-date and appropriate term for PG is lobular capillary hemangioma, because these lesions are neither pyogenic nor granulomas.
PGs are most often found on the fingers, lips, and hands. They may resemble a number of malignancies including basal cell carcinoma, Kaposi’s sarcoma, metastatic cutaneous lesions, squamous cell carcinoma, and amelanotic melanoma. For that reason, it’s especially important to send the excised lesion for pathology to ensure that malignancy isn’t missed.
The patient was eager to have the PG removed at this visit, as a number of previous visits to urgent care centers resulted in courses of antibiotics that didn’t help. After performing a digital block, the FP removed the PG with a shave excision, followed by electrodesiccation and curettage. The electrodesiccation and curettage are important to prevent recurrence. In this case, the pathology confirmed the diagnosis, and the PG did not recur.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Usatine R. Pyogenic Granuloma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 940-944.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
CPR decision support videos can serve as a supplement to CPR preference discussions for inpatients
Clinical question: Does the use of a CPR decision support video impact patients’ code status preferences?
Background: Discussions about cardiopulmonary resuscitation are an important aspect of inpatient care but may be difficult to complete for several reasons, including poor patient understanding of the CPR process and its expected outcomes. This study sought to evaluate the impact of a CPR decision support video on patient CPR preferences.
Setting: General medicine wards at the Minneapolis Veterans Affairs from Sept. 28, 2015, to Oct. 23, 2015.
Synopsis: One hundred and nineteen patients older than 65 were randomized to receive standard care plus viewing a CPR decision support video or standard care alone. The primary outcome was patient code status preference. Patients completed a survey assessing trust in their care team.
Among the patients who viewed the video, 37% chose full code, compared with 71% of patients in the usual care arm. Patients who viewed the video were more likely to choose DNR/DNI (56%, compared with 17% in the control group). There was no significant difference in patient trust of the care team.
Study conclusions are limited by a study population consisting predominantly of white males. Though the study was randomized, it was not blinded.
Bottom line: A CPR decision support video led to a decrease in full code preference and an increase in DNR/DNI preference among hospitalized patients.
Citation: Merino AM et al. A randomized controlled trial of a CPR decision support video for patients admitted to the general medicine service. J Hosp Med. 2017;12(9):700-4.
Dr. Rodriguez is a hospitalist and a clinical informatics fellow, Beth Israel Deaconess Medical Center, Boston.
Clinical question: Does the use of a CPR decision support video impact patients’ code status preferences?
Background: Discussions about cardiopulmonary resuscitation are an important aspect of inpatient care but may be difficult to complete for several reasons, including poor patient understanding of the CPR process and its expected outcomes. This study sought to evaluate the impact of a CPR decision support video on patient CPR preferences.
Setting: General medicine wards at the Minneapolis Veterans Affairs from Sept. 28, 2015, to Oct. 23, 2015.
Synopsis: One hundred and nineteen patients older than 65 were randomized to receive standard care plus viewing a CPR decision support video or standard care alone. The primary outcome was patient code status preference. Patients completed a survey assessing trust in their care team.
Among the patients who viewed the video, 37% chose full code, compared with 71% of patients in the usual care arm. Patients who viewed the video were more likely to choose DNR/DNI (56%, compared with 17% in the control group). There was no significant difference in patient trust of the care team.
Study conclusions are limited by a study population consisting predominantly of white males. Though the study was randomized, it was not blinded.
Bottom line: A CPR decision support video led to a decrease in full code preference and an increase in DNR/DNI preference among hospitalized patients.
Citation: Merino AM et al. A randomized controlled trial of a CPR decision support video for patients admitted to the general medicine service. J Hosp Med. 2017;12(9):700-4.
Dr. Rodriguez is a hospitalist and a clinical informatics fellow, Beth Israel Deaconess Medical Center, Boston.
Clinical question: Does the use of a CPR decision support video impact patients’ code status preferences?
Background: Discussions about cardiopulmonary resuscitation are an important aspect of inpatient care but may be difficult to complete for several reasons, including poor patient understanding of the CPR process and its expected outcomes. This study sought to evaluate the impact of a CPR decision support video on patient CPR preferences.
Setting: General medicine wards at the Minneapolis Veterans Affairs from Sept. 28, 2015, to Oct. 23, 2015.
Synopsis: One hundred and nineteen patients older than 65 were randomized to receive standard care plus viewing a CPR decision support video or standard care alone. The primary outcome was patient code status preference. Patients completed a survey assessing trust in their care team.
Among the patients who viewed the video, 37% chose full code, compared with 71% of patients in the usual care arm. Patients who viewed the video were more likely to choose DNR/DNI (56%, compared with 17% in the control group). There was no significant difference in patient trust of the care team.
Study conclusions are limited by a study population consisting predominantly of white males. Though the study was randomized, it was not blinded.
Bottom line: A CPR decision support video led to a decrease in full code preference and an increase in DNR/DNI preference among hospitalized patients.
Citation: Merino AM et al. A randomized controlled trial of a CPR decision support video for patients admitted to the general medicine service. J Hosp Med. 2017;12(9):700-4.
Dr. Rodriguez is a hospitalist and a clinical informatics fellow, Beth Israel Deaconess Medical Center, Boston.
From the ACS President: The joy and privilege of a surgical career
As a Fellow of the American College of Surgeons (ACS), only you can recall the personal sacrifices you have made to attain the skills and knowledge necessary to enjoy the privilege of being a surgeon – years of missed time with family and friends, sleepless nights, and endless formative hours of deep experiential learning in the hospital. Someone else could have been there instead; you could have made a different career choice. But, no – surgery chose you, and you dove in. Thank you, thank you.
I hope you never lose sight of the lives you touched during those “lost” times – injured people, previously unknown neighbors with deadly diseases, or simply patients needing a little “repair.” People with a surgical disease are experiencing a rare life event: an operation. Never forget that each of those individuals, each patient, came to you – you personally – to help them.
Challenges
Regrettably, however, at times the cherished bond between a surgeon and a patient can get lost in our busy, burdened lives. It can get lost in physical fatigue, regulatory hoops, frustrations of the electronic health record, contract negotiations, challenging reimbursement policies, and on and on.
Add to that list other challenges that will surely arise in the course of your career: You will face various forms of threatened obsolescence in knowledge, skills, and technology. You will age. You will suffer personal tragedy and loss. You will become ill. That you may stumble when facing such challenges is not a sign of weakness. It is life.
I believe there is a bit of light ahead as our health care industry begins to recognize that this thing we call burnout is not a personal failing, but rather a function of our flawed work environments – and a significant threat not only to the surgeon, but also to patient safety, quality of care, and institutional financial stability. An active voice and actions in these essential domains of our work environment are mission critical for our College, as are efforts that are pursued on many fronts by Fellows and professionals in the Division of Advocacy and Health Policy in Washington, DC, and the Divisions of Research and Optimal Patient Care, Education, and Member Services in Chicago, IL. Fostering an environment to optimally support the care of the surgical patient – and surgeons – is core business in all we do in the ACS.
Let’s tackle a few other challenges. First, consider your personal and society’s investment in surgical training. Getting you to this skilled and knowledgeable point reflects an investment of more than $1 million dollars in costs of medical school and graduate medical education, and inestimable time and effort.
Second, the dire anticipated shortage of surgeons of many disciplines – general surgeons, orthopedists, urologists – appears to be real. If we are to keep our surgical pipeline full, we need to offer careers that are attractive to men and women equally. The U.S. general surgery pathway has entering classes of 40% women; however, other high-demand disciplines, such as neurosurgery, orthopedics, and cardiothoracic surgery, have not yet attracted women to their ranks in sufficient numbers, despite the fact that 50% of our medical school graduates are women. We need to examine the pathways to those surgical disciplines to ensure that gender- and ethnicity-based barriers are receding. Efforts are underway to address these challenges by the leadership in these disciplines that our College can help with.
Although much has changed for women in surgery in recent years, there are still differences in the lives of many female surgeons compared to their male colleagues. They remain at risk for pay inequity, being in aggregate compensated 10-17% less than their male colleagues for equal work. Despite a mature gender pipeline in some surgical specialties, women are still less likely to rise to leadership roles in their group practices, hospital structures, professional organizations, and academic institutions. The ACS can serve as a professional home to develop strategies to highlight and remedy these imbalances.
Parenting, to engage as fully and successfully as one may wish, is a challenge for many who choose our careers, regardless of gender. However, for most female surgeons beginning a family, the first step often comes with pregnancy and infant care, conditions that we have yet to embrace and support as a societal good rather than an individual’s gauntlet to run. Given our long and arduous educational pathways, these women often find themselves starting a family, be it by pregnancy or adoption, at the same moment they are beginning their busy early years of practice. Policies and practices to support surgeons who choose parenthood in the workforce are sorely needed and will, in fact, benefit all in the long run.
Our College, with guidance from the Women in Surgery Committee and the Association of Women Surgeons, has advanced that goal, issuing a statement that acknowledges the need for appropriate pregnancy and parental leave and that clearly articulates that the choice to become a parent in no way diminishes a surgeon’s commitment to career. The next steps will be building the institutional, financial, and community infrastructure to foster success in both career and parenting for all.
Retooling reimagined
Let’s ponder another challenge: the need to add to your repertoire a new, potentially transformative skill. How do we safely retool?
Twenty years ago, in the flawed early adoption of laparoscopic surgery, the ACS Committee on Emerging Surgical Technology Education articulated the principles of new skill acquisition: didactic learning, coupled with simulation-based training, and then proctored early experience, leading to independent practice and assessment of outcomes.
Subsequently, the College took the visionary step of establishing the ACS Accredited Education Institutes (AEI) program to develop a network of centers that would leverage emerging simulation technologies to enhance surgical training. The 96 national and international AEIs now serve as both educational and research centers to teach technical and nontechnical skills to surgeons and other health care professionals. At Houston Methodist Hospital, for example, we have built MITIE (Methodist Institute for Technology Innovation and Education), a comprehensive center with a focus on retooling surgeons in practice. We have hosted more than 13,000 surgeons in practice for retooling hands-on courses, along with more than 30,000 other health care professionals.
To ensure our surgical workforce stays at the top of their performance over a 40-year career, our College has convened a group of stakeholders, including payers, consumers, liability carriers, surgical technology industries, hospital executives, and, of course, surgeons, to define the infrastructure – facilities, faculty, curricula, assessment tools, and finances – needed to incorporate retooling and retraining into our health care system. Work to do.
Shape your future
The retooling reimagined initiative is but one example of how we can shape our professional futures. Remember, the ACS was founded nearly 105 years ago by surgeons who sought to improve the care of the surgical patient. Since then, individual surgeons, banding together within our College, have created some of the most effective systems in the world to improve surgical care – including the formation of the Committee on Trauma and the Commission on Cancer, which have led initiatives that have vastly improved care for their respective patient populations.
The ACS National Surgical Quality Improvement Program, born of the vision of Shukri Khuri, MD, FACS, who, when tasked with resolving a perceived problem in surgical care in the Veterans Affairs Health Care System, launched a research study to measure quality. Soon thereafter, he led an army of surgeons to improve surgical care in their own hospitals, founding a nationwide movement that now flourishes in thousands of hospitals as the world’s most effective surgical quality measurement and improvement system.
We can go on and on. A surgeon identifies a gap and with a good idea, and coupled to abundant College focus and the engagement of our Fellows, a valuable new program is launched. These initiatives were not delivered from on high. They were created by regular surgeons, like you and me, who saw gaps in their professional worlds and took steps to effect meaningful change.
Caring for each other
I have one more request: I want you to be aware of your colleagues. I want you to watch them for signs of stress and disturbances in their forces. And if you see something, ask a supportive question or offer needed assistance. Be aware of help that is available in your institution; know how to move a concern up the chain with sensitivity, but also with efficacy, coupled with compassionate concern for your colleague.
These are not easy discussions and may prove fruitless, but they are worth the effort to try, for we surgeons are a high-risk group for depression, substance abuse, and suicide – and for failing to seek assistance. This situation must change, but doing so will require that we destigmatize these conditions in ourselves and our colleagues, and destigmatize seeking assistance.
But, for now, on a joyful or a challenging day in your surgical life, I hope you are proud of your Fellowship in the ACS and your FACS initials that signify your commitment to the values of our profession. I hope you will draw endless support and friendship from those around you and that you will contribute more than you receive. And I hope that you will forever treasure your opportunity to practice as a surgeon, an exceptional joy and privilege.
Dr. Bass is the John F. and Carolyn Bookout Presidential Endowed Chair, professor of surgery and chair, department of surgery, Houston Methodist Hospital, TX, and the President of the American College of Surgeons (ACS).
As a Fellow of the American College of Surgeons (ACS), only you can recall the personal sacrifices you have made to attain the skills and knowledge necessary to enjoy the privilege of being a surgeon – years of missed time with family and friends, sleepless nights, and endless formative hours of deep experiential learning in the hospital. Someone else could have been there instead; you could have made a different career choice. But, no – surgery chose you, and you dove in. Thank you, thank you.
I hope you never lose sight of the lives you touched during those “lost” times – injured people, previously unknown neighbors with deadly diseases, or simply patients needing a little “repair.” People with a surgical disease are experiencing a rare life event: an operation. Never forget that each of those individuals, each patient, came to you – you personally – to help them.
Challenges
Regrettably, however, at times the cherished bond between a surgeon and a patient can get lost in our busy, burdened lives. It can get lost in physical fatigue, regulatory hoops, frustrations of the electronic health record, contract negotiations, challenging reimbursement policies, and on and on.
Add to that list other challenges that will surely arise in the course of your career: You will face various forms of threatened obsolescence in knowledge, skills, and technology. You will age. You will suffer personal tragedy and loss. You will become ill. That you may stumble when facing such challenges is not a sign of weakness. It is life.
I believe there is a bit of light ahead as our health care industry begins to recognize that this thing we call burnout is not a personal failing, but rather a function of our flawed work environments – and a significant threat not only to the surgeon, but also to patient safety, quality of care, and institutional financial stability. An active voice and actions in these essential domains of our work environment are mission critical for our College, as are efforts that are pursued on many fronts by Fellows and professionals in the Division of Advocacy and Health Policy in Washington, DC, and the Divisions of Research and Optimal Patient Care, Education, and Member Services in Chicago, IL. Fostering an environment to optimally support the care of the surgical patient – and surgeons – is core business in all we do in the ACS.
Let’s tackle a few other challenges. First, consider your personal and society’s investment in surgical training. Getting you to this skilled and knowledgeable point reflects an investment of more than $1 million dollars in costs of medical school and graduate medical education, and inestimable time and effort.
Second, the dire anticipated shortage of surgeons of many disciplines – general surgeons, orthopedists, urologists – appears to be real. If we are to keep our surgical pipeline full, we need to offer careers that are attractive to men and women equally. The U.S. general surgery pathway has entering classes of 40% women; however, other high-demand disciplines, such as neurosurgery, orthopedics, and cardiothoracic surgery, have not yet attracted women to their ranks in sufficient numbers, despite the fact that 50% of our medical school graduates are women. We need to examine the pathways to those surgical disciplines to ensure that gender- and ethnicity-based barriers are receding. Efforts are underway to address these challenges by the leadership in these disciplines that our College can help with.
Although much has changed for women in surgery in recent years, there are still differences in the lives of many female surgeons compared to their male colleagues. They remain at risk for pay inequity, being in aggregate compensated 10-17% less than their male colleagues for equal work. Despite a mature gender pipeline in some surgical specialties, women are still less likely to rise to leadership roles in their group practices, hospital structures, professional organizations, and academic institutions. The ACS can serve as a professional home to develop strategies to highlight and remedy these imbalances.
Parenting, to engage as fully and successfully as one may wish, is a challenge for many who choose our careers, regardless of gender. However, for most female surgeons beginning a family, the first step often comes with pregnancy and infant care, conditions that we have yet to embrace and support as a societal good rather than an individual’s gauntlet to run. Given our long and arduous educational pathways, these women often find themselves starting a family, be it by pregnancy or adoption, at the same moment they are beginning their busy early years of practice. Policies and practices to support surgeons who choose parenthood in the workforce are sorely needed and will, in fact, benefit all in the long run.
Our College, with guidance from the Women in Surgery Committee and the Association of Women Surgeons, has advanced that goal, issuing a statement that acknowledges the need for appropriate pregnancy and parental leave and that clearly articulates that the choice to become a parent in no way diminishes a surgeon’s commitment to career. The next steps will be building the institutional, financial, and community infrastructure to foster success in both career and parenting for all.
Retooling reimagined
Let’s ponder another challenge: the need to add to your repertoire a new, potentially transformative skill. How do we safely retool?
Twenty years ago, in the flawed early adoption of laparoscopic surgery, the ACS Committee on Emerging Surgical Technology Education articulated the principles of new skill acquisition: didactic learning, coupled with simulation-based training, and then proctored early experience, leading to independent practice and assessment of outcomes.
Subsequently, the College took the visionary step of establishing the ACS Accredited Education Institutes (AEI) program to develop a network of centers that would leverage emerging simulation technologies to enhance surgical training. The 96 national and international AEIs now serve as both educational and research centers to teach technical and nontechnical skills to surgeons and other health care professionals. At Houston Methodist Hospital, for example, we have built MITIE (Methodist Institute for Technology Innovation and Education), a comprehensive center with a focus on retooling surgeons in practice. We have hosted more than 13,000 surgeons in practice for retooling hands-on courses, along with more than 30,000 other health care professionals.
To ensure our surgical workforce stays at the top of their performance over a 40-year career, our College has convened a group of stakeholders, including payers, consumers, liability carriers, surgical technology industries, hospital executives, and, of course, surgeons, to define the infrastructure – facilities, faculty, curricula, assessment tools, and finances – needed to incorporate retooling and retraining into our health care system. Work to do.
Shape your future
The retooling reimagined initiative is but one example of how we can shape our professional futures. Remember, the ACS was founded nearly 105 years ago by surgeons who sought to improve the care of the surgical patient. Since then, individual surgeons, banding together within our College, have created some of the most effective systems in the world to improve surgical care – including the formation of the Committee on Trauma and the Commission on Cancer, which have led initiatives that have vastly improved care for their respective patient populations.
The ACS National Surgical Quality Improvement Program, born of the vision of Shukri Khuri, MD, FACS, who, when tasked with resolving a perceived problem in surgical care in the Veterans Affairs Health Care System, launched a research study to measure quality. Soon thereafter, he led an army of surgeons to improve surgical care in their own hospitals, founding a nationwide movement that now flourishes in thousands of hospitals as the world’s most effective surgical quality measurement and improvement system.
We can go on and on. A surgeon identifies a gap and with a good idea, and coupled to abundant College focus and the engagement of our Fellows, a valuable new program is launched. These initiatives were not delivered from on high. They were created by regular surgeons, like you and me, who saw gaps in their professional worlds and took steps to effect meaningful change.
Caring for each other
I have one more request: I want you to be aware of your colleagues. I want you to watch them for signs of stress and disturbances in their forces. And if you see something, ask a supportive question or offer needed assistance. Be aware of help that is available in your institution; know how to move a concern up the chain with sensitivity, but also with efficacy, coupled with compassionate concern for your colleague.
These are not easy discussions and may prove fruitless, but they are worth the effort to try, for we surgeons are a high-risk group for depression, substance abuse, and suicide – and for failing to seek assistance. This situation must change, but doing so will require that we destigmatize these conditions in ourselves and our colleagues, and destigmatize seeking assistance.
But, for now, on a joyful or a challenging day in your surgical life, I hope you are proud of your Fellowship in the ACS and your FACS initials that signify your commitment to the values of our profession. I hope you will draw endless support and friendship from those around you and that you will contribute more than you receive. And I hope that you will forever treasure your opportunity to practice as a surgeon, an exceptional joy and privilege.
Dr. Bass is the John F. and Carolyn Bookout Presidential Endowed Chair, professor of surgery and chair, department of surgery, Houston Methodist Hospital, TX, and the President of the American College of Surgeons (ACS).
As a Fellow of the American College of Surgeons (ACS), only you can recall the personal sacrifices you have made to attain the skills and knowledge necessary to enjoy the privilege of being a surgeon – years of missed time with family and friends, sleepless nights, and endless formative hours of deep experiential learning in the hospital. Someone else could have been there instead; you could have made a different career choice. But, no – surgery chose you, and you dove in. Thank you, thank you.
I hope you never lose sight of the lives you touched during those “lost” times – injured people, previously unknown neighbors with deadly diseases, or simply patients needing a little “repair.” People with a surgical disease are experiencing a rare life event: an operation. Never forget that each of those individuals, each patient, came to you – you personally – to help them.
Challenges
Regrettably, however, at times the cherished bond between a surgeon and a patient can get lost in our busy, burdened lives. It can get lost in physical fatigue, regulatory hoops, frustrations of the electronic health record, contract negotiations, challenging reimbursement policies, and on and on.
Add to that list other challenges that will surely arise in the course of your career: You will face various forms of threatened obsolescence in knowledge, skills, and technology. You will age. You will suffer personal tragedy and loss. You will become ill. That you may stumble when facing such challenges is not a sign of weakness. It is life.
I believe there is a bit of light ahead as our health care industry begins to recognize that this thing we call burnout is not a personal failing, but rather a function of our flawed work environments – and a significant threat not only to the surgeon, but also to patient safety, quality of care, and institutional financial stability. An active voice and actions in these essential domains of our work environment are mission critical for our College, as are efforts that are pursued on many fronts by Fellows and professionals in the Division of Advocacy and Health Policy in Washington, DC, and the Divisions of Research and Optimal Patient Care, Education, and Member Services in Chicago, IL. Fostering an environment to optimally support the care of the surgical patient – and surgeons – is core business in all we do in the ACS.
Let’s tackle a few other challenges. First, consider your personal and society’s investment in surgical training. Getting you to this skilled and knowledgeable point reflects an investment of more than $1 million dollars in costs of medical school and graduate medical education, and inestimable time and effort.
Second, the dire anticipated shortage of surgeons of many disciplines – general surgeons, orthopedists, urologists – appears to be real. If we are to keep our surgical pipeline full, we need to offer careers that are attractive to men and women equally. The U.S. general surgery pathway has entering classes of 40% women; however, other high-demand disciplines, such as neurosurgery, orthopedics, and cardiothoracic surgery, have not yet attracted women to their ranks in sufficient numbers, despite the fact that 50% of our medical school graduates are women. We need to examine the pathways to those surgical disciplines to ensure that gender- and ethnicity-based barriers are receding. Efforts are underway to address these challenges by the leadership in these disciplines that our College can help with.
Although much has changed for women in surgery in recent years, there are still differences in the lives of many female surgeons compared to their male colleagues. They remain at risk for pay inequity, being in aggregate compensated 10-17% less than their male colleagues for equal work. Despite a mature gender pipeline in some surgical specialties, women are still less likely to rise to leadership roles in their group practices, hospital structures, professional organizations, and academic institutions. The ACS can serve as a professional home to develop strategies to highlight and remedy these imbalances.
Parenting, to engage as fully and successfully as one may wish, is a challenge for many who choose our careers, regardless of gender. However, for most female surgeons beginning a family, the first step often comes with pregnancy and infant care, conditions that we have yet to embrace and support as a societal good rather than an individual’s gauntlet to run. Given our long and arduous educational pathways, these women often find themselves starting a family, be it by pregnancy or adoption, at the same moment they are beginning their busy early years of practice. Policies and practices to support surgeons who choose parenthood in the workforce are sorely needed and will, in fact, benefit all in the long run.
Our College, with guidance from the Women in Surgery Committee and the Association of Women Surgeons, has advanced that goal, issuing a statement that acknowledges the need for appropriate pregnancy and parental leave and that clearly articulates that the choice to become a parent in no way diminishes a surgeon’s commitment to career. The next steps will be building the institutional, financial, and community infrastructure to foster success in both career and parenting for all.
Retooling reimagined
Let’s ponder another challenge: the need to add to your repertoire a new, potentially transformative skill. How do we safely retool?
Twenty years ago, in the flawed early adoption of laparoscopic surgery, the ACS Committee on Emerging Surgical Technology Education articulated the principles of new skill acquisition: didactic learning, coupled with simulation-based training, and then proctored early experience, leading to independent practice and assessment of outcomes.
Subsequently, the College took the visionary step of establishing the ACS Accredited Education Institutes (AEI) program to develop a network of centers that would leverage emerging simulation technologies to enhance surgical training. The 96 national and international AEIs now serve as both educational and research centers to teach technical and nontechnical skills to surgeons and other health care professionals. At Houston Methodist Hospital, for example, we have built MITIE (Methodist Institute for Technology Innovation and Education), a comprehensive center with a focus on retooling surgeons in practice. We have hosted more than 13,000 surgeons in practice for retooling hands-on courses, along with more than 30,000 other health care professionals.
To ensure our surgical workforce stays at the top of their performance over a 40-year career, our College has convened a group of stakeholders, including payers, consumers, liability carriers, surgical technology industries, hospital executives, and, of course, surgeons, to define the infrastructure – facilities, faculty, curricula, assessment tools, and finances – needed to incorporate retooling and retraining into our health care system. Work to do.
Shape your future
The retooling reimagined initiative is but one example of how we can shape our professional futures. Remember, the ACS was founded nearly 105 years ago by surgeons who sought to improve the care of the surgical patient. Since then, individual surgeons, banding together within our College, have created some of the most effective systems in the world to improve surgical care – including the formation of the Committee on Trauma and the Commission on Cancer, which have led initiatives that have vastly improved care for their respective patient populations.
The ACS National Surgical Quality Improvement Program, born of the vision of Shukri Khuri, MD, FACS, who, when tasked with resolving a perceived problem in surgical care in the Veterans Affairs Health Care System, launched a research study to measure quality. Soon thereafter, he led an army of surgeons to improve surgical care in their own hospitals, founding a nationwide movement that now flourishes in thousands of hospitals as the world’s most effective surgical quality measurement and improvement system.
We can go on and on. A surgeon identifies a gap and with a good idea, and coupled to abundant College focus and the engagement of our Fellows, a valuable new program is launched. These initiatives were not delivered from on high. They were created by regular surgeons, like you and me, who saw gaps in their professional worlds and took steps to effect meaningful change.
Caring for each other
I have one more request: I want you to be aware of your colleagues. I want you to watch them for signs of stress and disturbances in their forces. And if you see something, ask a supportive question or offer needed assistance. Be aware of help that is available in your institution; know how to move a concern up the chain with sensitivity, but also with efficacy, coupled with compassionate concern for your colleague.
These are not easy discussions and may prove fruitless, but they are worth the effort to try, for we surgeons are a high-risk group for depression, substance abuse, and suicide – and for failing to seek assistance. This situation must change, but doing so will require that we destigmatize these conditions in ourselves and our colleagues, and destigmatize seeking assistance.
But, for now, on a joyful or a challenging day in your surgical life, I hope you are proud of your Fellowship in the ACS and your FACS initials that signify your commitment to the values of our profession. I hope you will draw endless support and friendship from those around you and that you will contribute more than you receive. And I hope that you will forever treasure your opportunity to practice as a surgeon, an exceptional joy and privilege.
Dr. Bass is the John F. and Carolyn Bookout Presidential Endowed Chair, professor of surgery and chair, department of surgery, Houston Methodist Hospital, TX, and the President of the American College of Surgeons (ACS).
From the Editors: An unexpected call to action
In the waning weeks of 2017, still another disaster was added to the long list of natural and man-made tragedies of the year: the derailment of an Amtrak train near Tacoma, Washington. Although the cause of this event has not yet been determined and will not be for at least several months, we do know that safety equipment that has been recommended for years had not been installed in the train. I can only hope that this accident reminds our governmental leaders and institutional officials of the costs in lives and injury of ignoring deteriorating infrastructure and neglecting known safety measures.
An eyewitness and participant in the response to the accident was the Oregon Health & Science University Chair of Neurological Surgery, Nathan Selden, MD, PhD, FACS, who was driving north on Interstate 5 that morning with his 18-year-old son. I spoke recently with Dr. Selden to obtain his first-hand impressions of the experience.
They came upon the scene of the derailment shortly after it had occurred. He recognized immediately the horrifying potential for serious injuries and fatalities. First responders were already arriving on the scene and Dr. Selden offered his services to assist the injured. The first responders eagerly accepted his offer, and he spent the next two hours working with another MD and one RN from nearby Joint Base Lewis-McChord and a large number of EMTs and firefighters mobilized from nearby communities. The team of emergency workers removed almost 80 victims from precariously dangling train cars, provided first aid and basic trauma care, and triaged the victims to the most appropriate next site for treatment. Dr. Selden was most impressed by the courage of the firefighters who climbed into two train cars hanging off the highway overpass. He commented, “They were awesome, working in incredibly risky conditions.”
A pediatric neurosurgeon in his daily work, Dr. Selden is not in the habit of performing the duties that he did that day, but he used his expertise in trauma to assess the victims’ injuries, listing their problems on tags hung around their necks and advising the scene commander about what kind of specialist each patient would likely need. The commander could then direct ambulances to the most appropriate nearby facility for definitive care.
Although most of the hastily assembled emergency response team were strangers to one another, Dr. Selden remarked that “they all worked together efficiently” at a scene that he described as “orderly, purposeful chaos” to stop bleeding, bandage cuts, splint fractures, apply cervical collars, place the injured on backboards, and reassure and calm the victims, who were understandably scared and in shock. Dr. Selden modestly downplayed his role at the scene, and praised the EMTs, firefighters, and police for their leadership and professionalism in organizing and coordinating everyone efficiently and expertly. His comments about the experience focused on the effective way that the caregivers at the scene did their jobs and emphasized how long the road to healing will be for many of the injured. These victims will be in need of support and healing long after the public’s attention has moved on from the drama of that remarkably devastating event. For many of them and their families, he soberly noted, “their lives will be changed forever.” His comments reflect his deep understanding of the implications of the victims’ injuries, many of which involved his area of expertise, neurosurgical trauma.
Few of us will be called on during our careers to step out of our comfort zone to provide emergency care in a situation as far from our normal daily environment as this one was. But if we are, we would do well to follow the lead exemplified by Dr. Selden: call on the basic skills that we as surgeons all possess, work collaboratively with those trained to be first responders and rescuers, and acknowledge the profound and long-lasting effect such a calamity has on all of those who experience it.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
In the waning weeks of 2017, still another disaster was added to the long list of natural and man-made tragedies of the year: the derailment of an Amtrak train near Tacoma, Washington. Although the cause of this event has not yet been determined and will not be for at least several months, we do know that safety equipment that has been recommended for years had not been installed in the train. I can only hope that this accident reminds our governmental leaders and institutional officials of the costs in lives and injury of ignoring deteriorating infrastructure and neglecting known safety measures.
An eyewitness and participant in the response to the accident was the Oregon Health & Science University Chair of Neurological Surgery, Nathan Selden, MD, PhD, FACS, who was driving north on Interstate 5 that morning with his 18-year-old son. I spoke recently with Dr. Selden to obtain his first-hand impressions of the experience.
They came upon the scene of the derailment shortly after it had occurred. He recognized immediately the horrifying potential for serious injuries and fatalities. First responders were already arriving on the scene and Dr. Selden offered his services to assist the injured. The first responders eagerly accepted his offer, and he spent the next two hours working with another MD and one RN from nearby Joint Base Lewis-McChord and a large number of EMTs and firefighters mobilized from nearby communities. The team of emergency workers removed almost 80 victims from precariously dangling train cars, provided first aid and basic trauma care, and triaged the victims to the most appropriate next site for treatment. Dr. Selden was most impressed by the courage of the firefighters who climbed into two train cars hanging off the highway overpass. He commented, “They were awesome, working in incredibly risky conditions.”
A pediatric neurosurgeon in his daily work, Dr. Selden is not in the habit of performing the duties that he did that day, but he used his expertise in trauma to assess the victims’ injuries, listing their problems on tags hung around their necks and advising the scene commander about what kind of specialist each patient would likely need. The commander could then direct ambulances to the most appropriate nearby facility for definitive care.
Although most of the hastily assembled emergency response team were strangers to one another, Dr. Selden remarked that “they all worked together efficiently” at a scene that he described as “orderly, purposeful chaos” to stop bleeding, bandage cuts, splint fractures, apply cervical collars, place the injured on backboards, and reassure and calm the victims, who were understandably scared and in shock. Dr. Selden modestly downplayed his role at the scene, and praised the EMTs, firefighters, and police for their leadership and professionalism in organizing and coordinating everyone efficiently and expertly. His comments about the experience focused on the effective way that the caregivers at the scene did their jobs and emphasized how long the road to healing will be for many of the injured. These victims will be in need of support and healing long after the public’s attention has moved on from the drama of that remarkably devastating event. For many of them and their families, he soberly noted, “their lives will be changed forever.” His comments reflect his deep understanding of the implications of the victims’ injuries, many of which involved his area of expertise, neurosurgical trauma.
Few of us will be called on during our careers to step out of our comfort zone to provide emergency care in a situation as far from our normal daily environment as this one was. But if we are, we would do well to follow the lead exemplified by Dr. Selden: call on the basic skills that we as surgeons all possess, work collaboratively with those trained to be first responders and rescuers, and acknowledge the profound and long-lasting effect such a calamity has on all of those who experience it.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
In the waning weeks of 2017, still another disaster was added to the long list of natural and man-made tragedies of the year: the derailment of an Amtrak train near Tacoma, Washington. Although the cause of this event has not yet been determined and will not be for at least several months, we do know that safety equipment that has been recommended for years had not been installed in the train. I can only hope that this accident reminds our governmental leaders and institutional officials of the costs in lives and injury of ignoring deteriorating infrastructure and neglecting known safety measures.
An eyewitness and participant in the response to the accident was the Oregon Health & Science University Chair of Neurological Surgery, Nathan Selden, MD, PhD, FACS, who was driving north on Interstate 5 that morning with his 18-year-old son. I spoke recently with Dr. Selden to obtain his first-hand impressions of the experience.
They came upon the scene of the derailment shortly after it had occurred. He recognized immediately the horrifying potential for serious injuries and fatalities. First responders were already arriving on the scene and Dr. Selden offered his services to assist the injured. The first responders eagerly accepted his offer, and he spent the next two hours working with another MD and one RN from nearby Joint Base Lewis-McChord and a large number of EMTs and firefighters mobilized from nearby communities. The team of emergency workers removed almost 80 victims from precariously dangling train cars, provided first aid and basic trauma care, and triaged the victims to the most appropriate next site for treatment. Dr. Selden was most impressed by the courage of the firefighters who climbed into two train cars hanging off the highway overpass. He commented, “They were awesome, working in incredibly risky conditions.”
A pediatric neurosurgeon in his daily work, Dr. Selden is not in the habit of performing the duties that he did that day, but he used his expertise in trauma to assess the victims’ injuries, listing their problems on tags hung around their necks and advising the scene commander about what kind of specialist each patient would likely need. The commander could then direct ambulances to the most appropriate nearby facility for definitive care.
Although most of the hastily assembled emergency response team were strangers to one another, Dr. Selden remarked that “they all worked together efficiently” at a scene that he described as “orderly, purposeful chaos” to stop bleeding, bandage cuts, splint fractures, apply cervical collars, place the injured on backboards, and reassure and calm the victims, who were understandably scared and in shock. Dr. Selden modestly downplayed his role at the scene, and praised the EMTs, firefighters, and police for their leadership and professionalism in organizing and coordinating everyone efficiently and expertly. His comments about the experience focused on the effective way that the caregivers at the scene did their jobs and emphasized how long the road to healing will be for many of the injured. These victims will be in need of support and healing long after the public’s attention has moved on from the drama of that remarkably devastating event. For many of them and their families, he soberly noted, “their lives will be changed forever.” His comments reflect his deep understanding of the implications of the victims’ injuries, many of which involved his area of expertise, neurosurgical trauma.
Few of us will be called on during our careers to step out of our comfort zone to provide emergency care in a situation as far from our normal daily environment as this one was. But if we are, we would do well to follow the lead exemplified by Dr. Selden: call on the basic skills that we as surgeons all possess, work collaboratively with those trained to be first responders and rescuers, and acknowledge the profound and long-lasting effect such a calamity has on all of those who experience it.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
From the Washington Office: MIPS 2018 … Determining your status, making your plan
Surgeons’ practice situations vary. Therefore, for various reasons, surgeons may not be required to participate in MIPS, or they may not be eligible to do so. For 2018, the Centers for Medicare & Medicaid Services (CMS) estimates that approximately 40 percent of eligible clinicians will be required to submit data under MIPS. Furthermore, many providers (particularly those who are employed or are in large group practices) will have data submitted on their behalf by their groups, institutions, or employers. It is thus imperative that surgeons determine whether they are exempt from participating in the MIPS program. If not exempt, they should then determine if their practice situation necessitates that they report their own individual MIPS data for 2018 or, alternatively, if data will be reported for their groups, institutions, or employers.
If you are a Qualifying Participant (QP) in an Advanced Alternative Payment Model (APM), you are exempt from reporting MIPS data. If you are unsure of your status as such a participant, you can use you NPI number to determine your status at data.cms.gov/qplookup. QPs are not only exempt from reporting MIPS data, but could receive a 5 percent bonus in 2020 for participation in 2018.
For 2018, CMS increased the low-volume threshold. The low-volume threshold is now set at less than or equal to $90,000 in Medicare Part B allowable charges or 200 or fewer Medicare Part B patients seen during the period selected by CMS. Because this threshold represents an increase compared to 2017, it will result in even more providers being exempted from participating in MIPS. It should be noted that failure to meet either of these thresholds, $90,000 in allowable charges or 200 Medicare Part B patients, is sufficient to exclude one from reporting MIPS data in 2018. As was the case in 2017, we anticipate that CMS will notify those providers who are exempt based on the low-volume threshold within the first or second quarter of 2018. Alternatively, surgeons may use their NPI to check their status relative to MIPS reporting at qpp.cms.gov/participation-lookup.
To determine whether MIPS applies to your practice circumstances, you can use the following simple steps:
1) Are you a participant in a qualified advanced alternative payment model (A-APM)? If you are unsure, you can use your National Provider Identifier (NPI) number to look up your status at data.cms.gov/qplookup. If you are a qualified participant in an A-APM, you are not only exempt from reporting MIPS data, but you could also receive a 5 percent bonus in 2020 for your participation in 2018.
2) Are you exempt from participating in MIPS based on the low-volume threshold? As mentioned above, for 2018, the increase in the low-volume threshold is expected to exclude a significant number of providers. To determine if you are exempt based on the low-volume threshold, use your NPI number at qpp.cms.gov/participation-lookup.
3) Are your MIPS data reported for you by your institution, your employer, or your group? If your data are being reported for you then you need take no further action. You should contact your institution, employer, or group to confirm that data are being reported for you.
If MIPS applies to your practice, you need to make a choice between:
1) Submitting the minimum data necessary to avoid a penalty, and accept a freeze in your payments in 2020 for the 2018 performance period.
or
2) Submitting data in an effort to compete for a positive update.
If you prefer to submit the minimum amount of data necessary to avoid a penalty, your best option is to complete the requirements for the Improvement Activities (IA) component of MIPS. By achieving full credit in this category of MIPS, you will acquire enough points (15) to reach the performance threshold in your MIPS final score to avoid a 5 percent penalty in your Medicare payments in 2020 based on your performance and participation in 2018.
Within the IA category, each activity is assigned either a high (20 points) or medium (10 points) weight. To receive full credit in the IA component, most surgeons must select and attest to having completed between two and four activities for a total of 40 points in the IA category. For small practices or rural practices to achieve full credit, only one high-value or two medium-value activities are required. CMS defines small practices as those consisting of 15 or fewer eligible clinicians. The Centers for Medicare and Medicaid Services defines rural practices as those where more than 75 percent of the NPIs billing under the individual MIPS eligible clinician or group’s Tax Identification Number (TIN) are designated in a ZIP code as a rural area or Health Professional Shortage Area (HPSA), based on the most recent Health Resources and Services Administration Area Health Resource File data set.
Those who fulfill these requirements will receive the maximum score and full credit in the IA category toward their MIPS Final Score (15 points). Because the performance threshold for 2018 is set at 15 points, those who wish to avoid a payment penalty can acquire the required number of points to avoid a penalty for their performance in 2018 by simply fulfilling the IA requirements.
Attestation to having completed the Improvement Activities can be accomplished via a qualified registry, qualified clinical data registry (QCDR), an electronic health record (EHR), or the QPP Data Submission System (accessible at qpp.cms.gov/login). The American College of Surgeons (ACS) has two QCDRs, the Surgeon Specific Registry (SSR) and the Metabolic and Bariatric Surgery Quality Improvement Program (MBSAQIP) Data Registry. Both can be used to attest to completing the IAs.
If you plan to compete for a positive update in 2020 based on your performance in 2018, you should ideally report on the Quality, Advancing Care Information (ACI), and Improvement Activities categories of MIPS. If this is your intent, we recommend you obtain a copy of our new 2018 MACRA manual, visit facs.org/qpp, and make your plan for 2018.
Regardless of your choice, ACS staff in the DC office and the SSR are here to help and can be reached at 202-337-2701 (DC) or 312-202-5408 (SSR).
Until next month …
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
Surgeons’ practice situations vary. Therefore, for various reasons, surgeons may not be required to participate in MIPS, or they may not be eligible to do so. For 2018, the Centers for Medicare & Medicaid Services (CMS) estimates that approximately 40 percent of eligible clinicians will be required to submit data under MIPS. Furthermore, many providers (particularly those who are employed or are in large group practices) will have data submitted on their behalf by their groups, institutions, or employers. It is thus imperative that surgeons determine whether they are exempt from participating in the MIPS program. If not exempt, they should then determine if their practice situation necessitates that they report their own individual MIPS data for 2018 or, alternatively, if data will be reported for their groups, institutions, or employers.
If you are a Qualifying Participant (QP) in an Advanced Alternative Payment Model (APM), you are exempt from reporting MIPS data. If you are unsure of your status as such a participant, you can use you NPI number to determine your status at data.cms.gov/qplookup. QPs are not only exempt from reporting MIPS data, but could receive a 5 percent bonus in 2020 for participation in 2018.
For 2018, CMS increased the low-volume threshold. The low-volume threshold is now set at less than or equal to $90,000 in Medicare Part B allowable charges or 200 or fewer Medicare Part B patients seen during the period selected by CMS. Because this threshold represents an increase compared to 2017, it will result in even more providers being exempted from participating in MIPS. It should be noted that failure to meet either of these thresholds, $90,000 in allowable charges or 200 Medicare Part B patients, is sufficient to exclude one from reporting MIPS data in 2018. As was the case in 2017, we anticipate that CMS will notify those providers who are exempt based on the low-volume threshold within the first or second quarter of 2018. Alternatively, surgeons may use their NPI to check their status relative to MIPS reporting at qpp.cms.gov/participation-lookup.
To determine whether MIPS applies to your practice circumstances, you can use the following simple steps:
1) Are you a participant in a qualified advanced alternative payment model (A-APM)? If you are unsure, you can use your National Provider Identifier (NPI) number to look up your status at data.cms.gov/qplookup. If you are a qualified participant in an A-APM, you are not only exempt from reporting MIPS data, but you could also receive a 5 percent bonus in 2020 for your participation in 2018.
2) Are you exempt from participating in MIPS based on the low-volume threshold? As mentioned above, for 2018, the increase in the low-volume threshold is expected to exclude a significant number of providers. To determine if you are exempt based on the low-volume threshold, use your NPI number at qpp.cms.gov/participation-lookup.
3) Are your MIPS data reported for you by your institution, your employer, or your group? If your data are being reported for you then you need take no further action. You should contact your institution, employer, or group to confirm that data are being reported for you.
If MIPS applies to your practice, you need to make a choice between:
1) Submitting the minimum data necessary to avoid a penalty, and accept a freeze in your payments in 2020 for the 2018 performance period.
or
2) Submitting data in an effort to compete for a positive update.
If you prefer to submit the minimum amount of data necessary to avoid a penalty, your best option is to complete the requirements for the Improvement Activities (IA) component of MIPS. By achieving full credit in this category of MIPS, you will acquire enough points (15) to reach the performance threshold in your MIPS final score to avoid a 5 percent penalty in your Medicare payments in 2020 based on your performance and participation in 2018.
Within the IA category, each activity is assigned either a high (20 points) or medium (10 points) weight. To receive full credit in the IA component, most surgeons must select and attest to having completed between two and four activities for a total of 40 points in the IA category. For small practices or rural practices to achieve full credit, only one high-value or two medium-value activities are required. CMS defines small practices as those consisting of 15 or fewer eligible clinicians. The Centers for Medicare and Medicaid Services defines rural practices as those where more than 75 percent of the NPIs billing under the individual MIPS eligible clinician or group’s Tax Identification Number (TIN) are designated in a ZIP code as a rural area or Health Professional Shortage Area (HPSA), based on the most recent Health Resources and Services Administration Area Health Resource File data set.
Those who fulfill these requirements will receive the maximum score and full credit in the IA category toward their MIPS Final Score (15 points). Because the performance threshold for 2018 is set at 15 points, those who wish to avoid a payment penalty can acquire the required number of points to avoid a penalty for their performance in 2018 by simply fulfilling the IA requirements.
Attestation to having completed the Improvement Activities can be accomplished via a qualified registry, qualified clinical data registry (QCDR), an electronic health record (EHR), or the QPP Data Submission System (accessible at qpp.cms.gov/login). The American College of Surgeons (ACS) has two QCDRs, the Surgeon Specific Registry (SSR) and the Metabolic and Bariatric Surgery Quality Improvement Program (MBSAQIP) Data Registry. Both can be used to attest to completing the IAs.
If you plan to compete for a positive update in 2020 based on your performance in 2018, you should ideally report on the Quality, Advancing Care Information (ACI), and Improvement Activities categories of MIPS. If this is your intent, we recommend you obtain a copy of our new 2018 MACRA manual, visit facs.org/qpp, and make your plan for 2018.
Regardless of your choice, ACS staff in the DC office and the SSR are here to help and can be reached at 202-337-2701 (DC) or 312-202-5408 (SSR).
Until next month …
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
Surgeons’ practice situations vary. Therefore, for various reasons, surgeons may not be required to participate in MIPS, or they may not be eligible to do so. For 2018, the Centers for Medicare & Medicaid Services (CMS) estimates that approximately 40 percent of eligible clinicians will be required to submit data under MIPS. Furthermore, many providers (particularly those who are employed or are in large group practices) will have data submitted on their behalf by their groups, institutions, or employers. It is thus imperative that surgeons determine whether they are exempt from participating in the MIPS program. If not exempt, they should then determine if their practice situation necessitates that they report their own individual MIPS data for 2018 or, alternatively, if data will be reported for their groups, institutions, or employers.
If you are a Qualifying Participant (QP) in an Advanced Alternative Payment Model (APM), you are exempt from reporting MIPS data. If you are unsure of your status as such a participant, you can use you NPI number to determine your status at data.cms.gov/qplookup. QPs are not only exempt from reporting MIPS data, but could receive a 5 percent bonus in 2020 for participation in 2018.
For 2018, CMS increased the low-volume threshold. The low-volume threshold is now set at less than or equal to $90,000 in Medicare Part B allowable charges or 200 or fewer Medicare Part B patients seen during the period selected by CMS. Because this threshold represents an increase compared to 2017, it will result in even more providers being exempted from participating in MIPS. It should be noted that failure to meet either of these thresholds, $90,000 in allowable charges or 200 Medicare Part B patients, is sufficient to exclude one from reporting MIPS data in 2018. As was the case in 2017, we anticipate that CMS will notify those providers who are exempt based on the low-volume threshold within the first or second quarter of 2018. Alternatively, surgeons may use their NPI to check their status relative to MIPS reporting at qpp.cms.gov/participation-lookup.
To determine whether MIPS applies to your practice circumstances, you can use the following simple steps:
1) Are you a participant in a qualified advanced alternative payment model (A-APM)? If you are unsure, you can use your National Provider Identifier (NPI) number to look up your status at data.cms.gov/qplookup. If you are a qualified participant in an A-APM, you are not only exempt from reporting MIPS data, but you could also receive a 5 percent bonus in 2020 for your participation in 2018.
2) Are you exempt from participating in MIPS based on the low-volume threshold? As mentioned above, for 2018, the increase in the low-volume threshold is expected to exclude a significant number of providers. To determine if you are exempt based on the low-volume threshold, use your NPI number at qpp.cms.gov/participation-lookup.
3) Are your MIPS data reported for you by your institution, your employer, or your group? If your data are being reported for you then you need take no further action. You should contact your institution, employer, or group to confirm that data are being reported for you.
If MIPS applies to your practice, you need to make a choice between:
1) Submitting the minimum data necessary to avoid a penalty, and accept a freeze in your payments in 2020 for the 2018 performance period.
or
2) Submitting data in an effort to compete for a positive update.
If you prefer to submit the minimum amount of data necessary to avoid a penalty, your best option is to complete the requirements for the Improvement Activities (IA) component of MIPS. By achieving full credit in this category of MIPS, you will acquire enough points (15) to reach the performance threshold in your MIPS final score to avoid a 5 percent penalty in your Medicare payments in 2020 based on your performance and participation in 2018.
Within the IA category, each activity is assigned either a high (20 points) or medium (10 points) weight. To receive full credit in the IA component, most surgeons must select and attest to having completed between two and four activities for a total of 40 points in the IA category. For small practices or rural practices to achieve full credit, only one high-value or two medium-value activities are required. CMS defines small practices as those consisting of 15 or fewer eligible clinicians. The Centers for Medicare and Medicaid Services defines rural practices as those where more than 75 percent of the NPIs billing under the individual MIPS eligible clinician or group’s Tax Identification Number (TIN) are designated in a ZIP code as a rural area or Health Professional Shortage Area (HPSA), based on the most recent Health Resources and Services Administration Area Health Resource File data set.
Those who fulfill these requirements will receive the maximum score and full credit in the IA category toward their MIPS Final Score (15 points). Because the performance threshold for 2018 is set at 15 points, those who wish to avoid a payment penalty can acquire the required number of points to avoid a penalty for their performance in 2018 by simply fulfilling the IA requirements.
Attestation to having completed the Improvement Activities can be accomplished via a qualified registry, qualified clinical data registry (QCDR), an electronic health record (EHR), or the QPP Data Submission System (accessible at qpp.cms.gov/login). The American College of Surgeons (ACS) has two QCDRs, the Surgeon Specific Registry (SSR) and the Metabolic and Bariatric Surgery Quality Improvement Program (MBSAQIP) Data Registry. Both can be used to attest to completing the IAs.
If you plan to compete for a positive update in 2020 based on your performance in 2018, you should ideally report on the Quality, Advancing Care Information (ACI), and Improvement Activities categories of MIPS. If this is your intent, we recommend you obtain a copy of our new 2018 MACRA manual, visit facs.org/qpp, and make your plan for 2018.
Regardless of your choice, ACS staff in the DC office and the SSR are here to help and can be reached at 202-337-2701 (DC) or 312-202-5408 (SSR).
Until next month …
Dr. Bailey is a pediatric surgeon and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
Leigh A. Neumayer, MD, MS, FACS, elected Chair of ACS Board of Regents
Leigh A. Neumayer, MD, MS, FACS, Tucson, AZ, is the 2017–2018 Chair of the Board of Regents (B/R) of the American College of Surgeons (ACS). She was elected at the Annual Business Meeting of Members, October 25, 2017, in San Diego, CA.
A general surgeon, Dr. Neumayer is professor and chair, department of surgery, and the Margaret and Fenton Maynard Endowed Chair in Breast Cancer Research, University of Arizona (UA) College of Medicine, Tucson. She also is the interim senior vice-president for UA Health Sciences. Before accepting these positions in Arizona, Dr. Neumayer was professor, department of surgery, University of Utah Health Sciences Center, and the Jon and Karen Huntsman Presidential Professor, University of Utah/Huntsman Cancer Institute, Salt Lake City.
Previous leadership roles
A Fellow of the ACS since 1994 and a member of the B/R since 2009, Dr. Neumayer has served in many leadership roles within the organization. She was Chair of the Committee on Medical Student Education (2001–2003), Vice-Chair of the Surgical Research Committee (2015–2016), a Governor for the ACS Utah Chapter (2002–2008), and Vice-Chair of the Board of Regents (2016–2017). Dr. Neumayer also was Vice-Chair of the Nominating Committee of the Board of Governors (2004–2006) and a Board of Governors Executive Committee Member (2008–2011).
Nationally, Dr. Neumayer has served on the board of directors of the American Board of Surgery (2005–2011) and as the President of the Association of Women Surgeons (1997–1998), the Association for Surgical Education (2001–2002), the Association of Veterans Administration Surgeons (2002–2003), and the Society of Clinical Surgery (2012–2014).
At present, she serves on the editorial boards for the Journal of the American College of Surgeons and Annals of Surgery.
Dr. Neumayer’s most recent work is focused on the diagnosis and treatment of breast cancer via innovative technology and clinical trials. She has led investigations in hernia repair techniques, breast cancer treatment, surgical quality and outcomes, and surgical education techniques. Dr. Neumayer has mentored students, residents, and colleagues in these and other pursuits.
Dr. Neumayer studied biomedical engineering at Colorado State University, Fort Collins, before getting her medical degree from Baylor College of Medicine, Houston, TX. She trained in general surgery at the University of California, San Francisco, and at the University of Arizona, Tuscon. Dr. Neumayer then studied clinical research design and statistical analysis at the University of Michigan, Ann Arbor.
Dr. Schwartz elected Vice-Chair
A Fellow of the College since 1982, Dr. Schwartz has been a Regent since 2009 and has served on many ACS Committees. He was Chair of the Advisory Council Chairs (2004–2008), the Advisory Council for Pediatric Surgery (2004–2008), and the Health Policy and Advocacy Group (2014–2017). At present, he is Chair of the Comprehensive Communications Committee and a Member of the Surgical History Group Executive Committee.
Leigh A. Neumayer, MD, MS, FACS, Tucson, AZ, is the 2017–2018 Chair of the Board of Regents (B/R) of the American College of Surgeons (ACS). She was elected at the Annual Business Meeting of Members, October 25, 2017, in San Diego, CA.
A general surgeon, Dr. Neumayer is professor and chair, department of surgery, and the Margaret and Fenton Maynard Endowed Chair in Breast Cancer Research, University of Arizona (UA) College of Medicine, Tucson. She also is the interim senior vice-president for UA Health Sciences. Before accepting these positions in Arizona, Dr. Neumayer was professor, department of surgery, University of Utah Health Sciences Center, and the Jon and Karen Huntsman Presidential Professor, University of Utah/Huntsman Cancer Institute, Salt Lake City.
Previous leadership roles
A Fellow of the ACS since 1994 and a member of the B/R since 2009, Dr. Neumayer has served in many leadership roles within the organization. She was Chair of the Committee on Medical Student Education (2001–2003), Vice-Chair of the Surgical Research Committee (2015–2016), a Governor for the ACS Utah Chapter (2002–2008), and Vice-Chair of the Board of Regents (2016–2017). Dr. Neumayer also was Vice-Chair of the Nominating Committee of the Board of Governors (2004–2006) and a Board of Governors Executive Committee Member (2008–2011).
Nationally, Dr. Neumayer has served on the board of directors of the American Board of Surgery (2005–2011) and as the President of the Association of Women Surgeons (1997–1998), the Association for Surgical Education (2001–2002), the Association of Veterans Administration Surgeons (2002–2003), and the Society of Clinical Surgery (2012–2014).
At present, she serves on the editorial boards for the Journal of the American College of Surgeons and Annals of Surgery.
Dr. Neumayer’s most recent work is focused on the diagnosis and treatment of breast cancer via innovative technology and clinical trials. She has led investigations in hernia repair techniques, breast cancer treatment, surgical quality and outcomes, and surgical education techniques. Dr. Neumayer has mentored students, residents, and colleagues in these and other pursuits.
Dr. Neumayer studied biomedical engineering at Colorado State University, Fort Collins, before getting her medical degree from Baylor College of Medicine, Houston, TX. She trained in general surgery at the University of California, San Francisco, and at the University of Arizona, Tuscon. Dr. Neumayer then studied clinical research design and statistical analysis at the University of Michigan, Ann Arbor.
Dr. Schwartz elected Vice-Chair
A Fellow of the College since 1982, Dr. Schwartz has been a Regent since 2009 and has served on many ACS Committees. He was Chair of the Advisory Council Chairs (2004–2008), the Advisory Council for Pediatric Surgery (2004–2008), and the Health Policy and Advocacy Group (2014–2017). At present, he is Chair of the Comprehensive Communications Committee and a Member of the Surgical History Group Executive Committee.
Leigh A. Neumayer, MD, MS, FACS, Tucson, AZ, is the 2017–2018 Chair of the Board of Regents (B/R) of the American College of Surgeons (ACS). She was elected at the Annual Business Meeting of Members, October 25, 2017, in San Diego, CA.
A general surgeon, Dr. Neumayer is professor and chair, department of surgery, and the Margaret and Fenton Maynard Endowed Chair in Breast Cancer Research, University of Arizona (UA) College of Medicine, Tucson. She also is the interim senior vice-president for UA Health Sciences. Before accepting these positions in Arizona, Dr. Neumayer was professor, department of surgery, University of Utah Health Sciences Center, and the Jon and Karen Huntsman Presidential Professor, University of Utah/Huntsman Cancer Institute, Salt Lake City.
Previous leadership roles
A Fellow of the ACS since 1994 and a member of the B/R since 2009, Dr. Neumayer has served in many leadership roles within the organization. She was Chair of the Committee on Medical Student Education (2001–2003), Vice-Chair of the Surgical Research Committee (2015–2016), a Governor for the ACS Utah Chapter (2002–2008), and Vice-Chair of the Board of Regents (2016–2017). Dr. Neumayer also was Vice-Chair of the Nominating Committee of the Board of Governors (2004–2006) and a Board of Governors Executive Committee Member (2008–2011).
Nationally, Dr. Neumayer has served on the board of directors of the American Board of Surgery (2005–2011) and as the President of the Association of Women Surgeons (1997–1998), the Association for Surgical Education (2001–2002), the Association of Veterans Administration Surgeons (2002–2003), and the Society of Clinical Surgery (2012–2014).
At present, she serves on the editorial boards for the Journal of the American College of Surgeons and Annals of Surgery.
Dr. Neumayer’s most recent work is focused on the diagnosis and treatment of breast cancer via innovative technology and clinical trials. She has led investigations in hernia repair techniques, breast cancer treatment, surgical quality and outcomes, and surgical education techniques. Dr. Neumayer has mentored students, residents, and colleagues in these and other pursuits.
Dr. Neumayer studied biomedical engineering at Colorado State University, Fort Collins, before getting her medical degree from Baylor College of Medicine, Houston, TX. She trained in general surgery at the University of California, San Francisco, and at the University of Arizona, Tuscon. Dr. Neumayer then studied clinical research design and statistical analysis at the University of Michigan, Ann Arbor.
Dr. Schwartz elected Vice-Chair
A Fellow of the College since 1982, Dr. Schwartz has been a Regent since 2009 and has served on many ACS Committees. He was Chair of the Advisory Council Chairs (2004–2008), the Advisory Council for Pediatric Surgery (2004–2008), and the Health Policy and Advocacy Group (2014–2017). At present, he is Chair of the Comprehensive Communications Committee and a Member of the Surgical History Group Executive Committee.
Nominations for the 2018 Surgical Volunteerism and Humanitarian Awards due February 28
The American College of Surgeons (ACS), in association with Pfizer, Inc., is accepting nominations for the 2018 Surgical Volunteerism and Humanitarian Awards. These annual awards recognize surgeons who have made significant contributions to communities in need of surgical aid, be that through organized volunteer activities or through the dedication of a significant portion of their surgical career to the underserved or a retirement characterized by surgical outreach.
Submit nominations today at www.facs.org/ogb/award-winners/nominations. All nominations must be received by February 28, 2018. For more information, contact [email protected] or visit the awards web page.
The American College of Surgeons (ACS), in association with Pfizer, Inc., is accepting nominations for the 2018 Surgical Volunteerism and Humanitarian Awards. These annual awards recognize surgeons who have made significant contributions to communities in need of surgical aid, be that through organized volunteer activities or through the dedication of a significant portion of their surgical career to the underserved or a retirement characterized by surgical outreach.
Submit nominations today at www.facs.org/ogb/award-winners/nominations. All nominations must be received by February 28, 2018. For more information, contact [email protected] or visit the awards web page.
The American College of Surgeons (ACS), in association with Pfizer, Inc., is accepting nominations for the 2018 Surgical Volunteerism and Humanitarian Awards. These annual awards recognize surgeons who have made significant contributions to communities in need of surgical aid, be that through organized volunteer activities or through the dedication of a significant portion of their surgical career to the underserved or a retirement characterized by surgical outreach.
Submit nominations today at www.facs.org/ogb/award-winners/nominations. All nominations must be received by February 28, 2018. For more information, contact [email protected] or visit the awards web page.
The American College of Surgeons (ACS), in association with Pfizer, Inc., is accepting nominations for the 2018 Surgical Volunteerism and Humanitarian Awards. These annual awards recognize surgeons who have made significant contributions to communities in need of surgical aid, be that through organized volunteer activities or through the dedication of a significant portion of their surgical career to the underserved or a retirement characterized by surgical outreach.
Submit nominations today at www.facs.org/ogb/award-winners/nominations. All nominations must be received by February 28, 2018. For more information, contact [email protected] or visit the awards web page.
The American College of Surgeons (ACS), in association with Pfizer, Inc., is accepting nominations for the 2018 Surgical Volunteerism and Humanitarian Awards. These annual awards recognize surgeons who have made significant contributions to communities in need of surgical aid, be that through organized volunteer activities or through the dedication of a significant portion of their surgical career to the underserved or a retirement characterized by surgical outreach.
Submit nominations today at www.facs.org/ogb/award-winners/nominations. All nominations must be received by February 28, 2018. For more information, contact [email protected] or visit the awards web page.
The American College of Surgeons (ACS), in association with Pfizer, Inc., is accepting nominations for the 2018 Surgical Volunteerism and Humanitarian Awards. These annual awards recognize surgeons who have made significant contributions to communities in need of surgical aid, be that through organized volunteer activities or through the dedication of a significant portion of their surgical career to the underserved or a retirement characterized by surgical outreach.
Submit nominations today at www.facs.org/ogb/award-winners/nominations. All nominations must be received by February 28, 2018. For more information, contact [email protected] or visit the awards web page.
Nominations for Board of Regents, Officers-Elect due February 23
The American College of Surgeons (ACS) 2018 Nominating Committee of the Fellows (NCF) and the Nominating Committee of the Board of Governors (NCBG) will be selecting nominees for leadership positions in the College.
The 2018 NCF will select nominees for the three Officer-Elect positions of the ACS:
• President-Elect
• First Vice-President-Elect
• Second Vice-President-Elect
The 2018 NCBG will select nominees for pending vacancies on the Board of Regents to be filled at Clinical Congress 2018. Nominations are open to surgeons of all specialties, but particular consideration will be given this nomination cycle to those in the following specialties:
• Burn and critical care surgery
• Gastrointestinal surgery
• General surgery
• Surgical oncology
• Transplantation
• Trauma
• Vascular surgery
For information only, the members of the Board of Regents who will be considered for reelection in 2018 are (all MD, FACS) John L. D. Atkinson, James C. Denneny III, Timothy J. Eberlein, Henri R. Ford, Enrique Hernandez, L. Scott Levin, Linda Phillips, Anton A. Sidawy, Beth H. Sutton, and Steven D. Wexner.
Visit the Bulletin website at http://bit.ly/2l69j2Y for a list of criteria for each nominating committee, as well as further details on how to submit a nomination and the nomination process. The deadline for submitting nominations is February 23, 2018.
Nominations may be submitted to [email protected]. If you have any questions, contact Emily Kalata, Staff Liaison for the NCF and NCBG, at 312-202-5360 or [email protected].
The American College of Surgeons (ACS) 2018 Nominating Committee of the Fellows (NCF) and the Nominating Committee of the Board of Governors (NCBG) will be selecting nominees for leadership positions in the College.
The 2018 NCF will select nominees for the three Officer-Elect positions of the ACS:
• President-Elect
• First Vice-President-Elect
• Second Vice-President-Elect
The 2018 NCBG will select nominees for pending vacancies on the Board of Regents to be filled at Clinical Congress 2018. Nominations are open to surgeons of all specialties, but particular consideration will be given this nomination cycle to those in the following specialties:
• Burn and critical care surgery
• Gastrointestinal surgery
• General surgery
• Surgical oncology
• Transplantation
• Trauma
• Vascular surgery
For information only, the members of the Board of Regents who will be considered for reelection in 2018 are (all MD, FACS) John L. D. Atkinson, James C. Denneny III, Timothy J. Eberlein, Henri R. Ford, Enrique Hernandez, L. Scott Levin, Linda Phillips, Anton A. Sidawy, Beth H. Sutton, and Steven D. Wexner.
Visit the Bulletin website at http://bit.ly/2l69j2Y for a list of criteria for each nominating committee, as well as further details on how to submit a nomination and the nomination process. The deadline for submitting nominations is February 23, 2018.
Nominations may be submitted to [email protected]. If you have any questions, contact Emily Kalata, Staff Liaison for the NCF and NCBG, at 312-202-5360 or [email protected].
The American College of Surgeons (ACS) 2018 Nominating Committee of the Fellows (NCF) and the Nominating Committee of the Board of Governors (NCBG) will be selecting nominees for leadership positions in the College.
The 2018 NCF will select nominees for the three Officer-Elect positions of the ACS:
• President-Elect
• First Vice-President-Elect
• Second Vice-President-Elect
The 2018 NCBG will select nominees for pending vacancies on the Board of Regents to be filled at Clinical Congress 2018. Nominations are open to surgeons of all specialties, but particular consideration will be given this nomination cycle to those in the following specialties:
• Burn and critical care surgery
• Gastrointestinal surgery
• General surgery
• Surgical oncology
• Transplantation
• Trauma
• Vascular surgery
For information only, the members of the Board of Regents who will be considered for reelection in 2018 are (all MD, FACS) John L. D. Atkinson, James C. Denneny III, Timothy J. Eberlein, Henri R. Ford, Enrique Hernandez, L. Scott Levin, Linda Phillips, Anton A. Sidawy, Beth H. Sutton, and Steven D. Wexner.
Visit the Bulletin website at http://bit.ly/2l69j2Y for a list of criteria for each nominating committee, as well as further details on how to submit a nomination and the nomination process. The deadline for submitting nominations is February 23, 2018.
Nominations may be submitted to [email protected]. If you have any questions, contact Emily Kalata, Staff Liaison for the NCF and NCBG, at 312-202-5360 or [email protected].
Register now to participate in 2018 Leadership & Advocacy Summit
The American College of Surgeons (ACS) will host the seventh annual Leadership & Advocacy Summit May 19–22 at the Renaissance Washington, DC, Downtown Hotel. The summit is a dual meeting offering comprehensive and specialized sessions that provide volunteer leaders and advocates with the skills and tools necessary to be effective in those roles. Registration for the event is now open at facs.org/summit.
Leadership Summit
The Leadership Summit provides a venue for members to network with ACS leaders, attend professional development sessions, and engage with colleagues to determine new and innovative ways to face challenges and enhance their leadership skills. It begins Saturday evening, May 19, with a Welcome Reception open to all registrants, followed by a full day of programming on Sunday, May 20.
For more information about the Leadership Summit, contact Brian Frankel, ACS Manager, International Chapter Services and Special Initiatives, at [email protected] or 312-202-5361.
Advocacy Summit
The Advocacy Summit provides a unique opportunity to obtain the knowledge and skills necessary to become a surgeon advocate. With several legislative priorities for Congress to consider before the 2018 midterm elections, surgeons are encouraged to travel to Washington to learn about and participate in this unique political climate.
Since last year’s summit, the Division of Advocacy and Health Policy (DAHP) has been focused on an extensive list of federal legislative priorities, including reducing administrative practice burdens; modifying and implementing new physician payment reforms; improving electronic health record and health information technology interoperability; increasing funding for trauma systems; enhancing cancer care and accreditation; and addressing surgical workforce and graduate medical education issues. ACS staff also will help members and attendees navigate the many additional legislative changes that lie ahead.
The Advocacy Summit will begin after the Leadership Summit on Sunday, May 20, with a dinner and keynote address. Past speakers have included television journalist Chuck Todd, political commentator Chris Matthews, U.S. Army Gen. (Retired) Stanley A. McChrystal, author Thomas Goetz, and journalists Bob Woodward and George Will. Sessions planned for the following day will focus on the political environment in Washington, and speakers will provide updates on important health care policies and issues that detract from surgeons’ ability to provide quality patient care. Attendees will then apply this knowledge in face-to-face meetings with their senators and representatives and congressional staff. This portion of the program provides an opportunity to demonstrate surgery’s strength on Capitol Hill regarding issues of importance to surgeons and the surgical patient.
During this three-day conference, participants can expect to receive comprehensive advocacy training and learn how to use these skills throughout the year, not just in Washington. The Advocacy Summit is a great place to interact and share ideas with other surgeon advocates; meet face-to-face with key health care policymakers and legislators; and, perhaps most importantly, become the constituents their legislators know and trust to offer advice on surgical issues.
The ACS Professional Association political action committee (ACSPA-SurgeonsPAC) sponsors various events for members and SurgeonsPAC contributors. These events provide contributors with unique networking opportunities and advanced educational sessions aimed at providing an insider’s perspective on how College members can remain active participants in the political process.
In addition to raising funds to elect or re-elect congressional candidates who support a pro-surgeon, pro-patient agenda, SurgeonsPAC will host a reception at which PAC contributors will be recognized for their commitment to the surgical profession. Other SurgeonsPAC-sponsored events include an annual drawing with a grand prize valued at $3,000, a political luncheon featuring a renowned guest speaker, and presentation of the 2017 PAC awards. Resident engagement opportunities will be provided as well. In addition, the SurgeonsPAC information booth will provide attendees with a venue to meet DAHP staff to learn more about the College’s advocacy and political efforts.
For more information about the Advocacy Summit, contact Michael Carmody, ACS Congressional Affairs Coordinator, at [email protected] or 202-672-1511. For more information about SurgeonsPAC activities, e-mail [email protected] or call 202-672-1520.
The American College of Surgeons (ACS) will host the seventh annual Leadership & Advocacy Summit May 19–22 at the Renaissance Washington, DC, Downtown Hotel. The summit is a dual meeting offering comprehensive and specialized sessions that provide volunteer leaders and advocates with the skills and tools necessary to be effective in those roles. Registration for the event is now open at facs.org/summit.
Leadership Summit
The Leadership Summit provides a venue for members to network with ACS leaders, attend professional development sessions, and engage with colleagues to determine new and innovative ways to face challenges and enhance their leadership skills. It begins Saturday evening, May 19, with a Welcome Reception open to all registrants, followed by a full day of programming on Sunday, May 20.
For more information about the Leadership Summit, contact Brian Frankel, ACS Manager, International Chapter Services and Special Initiatives, at [email protected] or 312-202-5361.
Advocacy Summit
The Advocacy Summit provides a unique opportunity to obtain the knowledge and skills necessary to become a surgeon advocate. With several legislative priorities for Congress to consider before the 2018 midterm elections, surgeons are encouraged to travel to Washington to learn about and participate in this unique political climate.
Since last year’s summit, the Division of Advocacy and Health Policy (DAHP) has been focused on an extensive list of federal legislative priorities, including reducing administrative practice burdens; modifying and implementing new physician payment reforms; improving electronic health record and health information technology interoperability; increasing funding for trauma systems; enhancing cancer care and accreditation; and addressing surgical workforce and graduate medical education issues. ACS staff also will help members and attendees navigate the many additional legislative changes that lie ahead.
The Advocacy Summit will begin after the Leadership Summit on Sunday, May 20, with a dinner and keynote address. Past speakers have included television journalist Chuck Todd, political commentator Chris Matthews, U.S. Army Gen. (Retired) Stanley A. McChrystal, author Thomas Goetz, and journalists Bob Woodward and George Will. Sessions planned for the following day will focus on the political environment in Washington, and speakers will provide updates on important health care policies and issues that detract from surgeons’ ability to provide quality patient care. Attendees will then apply this knowledge in face-to-face meetings with their senators and representatives and congressional staff. This portion of the program provides an opportunity to demonstrate surgery’s strength on Capitol Hill regarding issues of importance to surgeons and the surgical patient.
During this three-day conference, participants can expect to receive comprehensive advocacy training and learn how to use these skills throughout the year, not just in Washington. The Advocacy Summit is a great place to interact and share ideas with other surgeon advocates; meet face-to-face with key health care policymakers and legislators; and, perhaps most importantly, become the constituents their legislators know and trust to offer advice on surgical issues.
The ACS Professional Association political action committee (ACSPA-SurgeonsPAC) sponsors various events for members and SurgeonsPAC contributors. These events provide contributors with unique networking opportunities and advanced educational sessions aimed at providing an insider’s perspective on how College members can remain active participants in the political process.
In addition to raising funds to elect or re-elect congressional candidates who support a pro-surgeon, pro-patient agenda, SurgeonsPAC will host a reception at which PAC contributors will be recognized for their commitment to the surgical profession. Other SurgeonsPAC-sponsored events include an annual drawing with a grand prize valued at $3,000, a political luncheon featuring a renowned guest speaker, and presentation of the 2017 PAC awards. Resident engagement opportunities will be provided as well. In addition, the SurgeonsPAC information booth will provide attendees with a venue to meet DAHP staff to learn more about the College’s advocacy and political efforts.
For more information about the Advocacy Summit, contact Michael Carmody, ACS Congressional Affairs Coordinator, at [email protected] or 202-672-1511. For more information about SurgeonsPAC activities, e-mail [email protected] or call 202-672-1520.
The American College of Surgeons (ACS) will host the seventh annual Leadership & Advocacy Summit May 19–22 at the Renaissance Washington, DC, Downtown Hotel. The summit is a dual meeting offering comprehensive and specialized sessions that provide volunteer leaders and advocates with the skills and tools necessary to be effective in those roles. Registration for the event is now open at facs.org/summit.
Leadership Summit
The Leadership Summit provides a venue for members to network with ACS leaders, attend professional development sessions, and engage with colleagues to determine new and innovative ways to face challenges and enhance their leadership skills. It begins Saturday evening, May 19, with a Welcome Reception open to all registrants, followed by a full day of programming on Sunday, May 20.
For more information about the Leadership Summit, contact Brian Frankel, ACS Manager, International Chapter Services and Special Initiatives, at [email protected] or 312-202-5361.
Advocacy Summit
The Advocacy Summit provides a unique opportunity to obtain the knowledge and skills necessary to become a surgeon advocate. With several legislative priorities for Congress to consider before the 2018 midterm elections, surgeons are encouraged to travel to Washington to learn about and participate in this unique political climate.
Since last year’s summit, the Division of Advocacy and Health Policy (DAHP) has been focused on an extensive list of federal legislative priorities, including reducing administrative practice burdens; modifying and implementing new physician payment reforms; improving electronic health record and health information technology interoperability; increasing funding for trauma systems; enhancing cancer care and accreditation; and addressing surgical workforce and graduate medical education issues. ACS staff also will help members and attendees navigate the many additional legislative changes that lie ahead.
The Advocacy Summit will begin after the Leadership Summit on Sunday, May 20, with a dinner and keynote address. Past speakers have included television journalist Chuck Todd, political commentator Chris Matthews, U.S. Army Gen. (Retired) Stanley A. McChrystal, author Thomas Goetz, and journalists Bob Woodward and George Will. Sessions planned for the following day will focus on the political environment in Washington, and speakers will provide updates on important health care policies and issues that detract from surgeons’ ability to provide quality patient care. Attendees will then apply this knowledge in face-to-face meetings with their senators and representatives and congressional staff. This portion of the program provides an opportunity to demonstrate surgery’s strength on Capitol Hill regarding issues of importance to surgeons and the surgical patient.
During this three-day conference, participants can expect to receive comprehensive advocacy training and learn how to use these skills throughout the year, not just in Washington. The Advocacy Summit is a great place to interact and share ideas with other surgeon advocates; meet face-to-face with key health care policymakers and legislators; and, perhaps most importantly, become the constituents their legislators know and trust to offer advice on surgical issues.
The ACS Professional Association political action committee (ACSPA-SurgeonsPAC) sponsors various events for members and SurgeonsPAC contributors. These events provide contributors with unique networking opportunities and advanced educational sessions aimed at providing an insider’s perspective on how College members can remain active participants in the political process.
In addition to raising funds to elect or re-elect congressional candidates who support a pro-surgeon, pro-patient agenda, SurgeonsPAC will host a reception at which PAC contributors will be recognized for their commitment to the surgical profession. Other SurgeonsPAC-sponsored events include an annual drawing with a grand prize valued at $3,000, a political luncheon featuring a renowned guest speaker, and presentation of the 2017 PAC awards. Resident engagement opportunities will be provided as well. In addition, the SurgeonsPAC information booth will provide attendees with a venue to meet DAHP staff to learn more about the College’s advocacy and political efforts.
For more information about the Advocacy Summit, contact Michael Carmody, ACS Congressional Affairs Coordinator, at [email protected] or 202-672-1511. For more information about SurgeonsPAC activities, e-mail [email protected] or call 202-672-1520.
Register for the Annual ACS Surgical Simulation Summit by March 2
The annual American College of Surgeons (ACS) Accredited Education Institutes (AEI) Surgical Simulation Summit will take place March 16−17 at the Swissôtel Chicago, IL.
Retired Army Lieutenant General James B. Peake, MD, FACS, senior vice-president, CGI Federal, and immediate past-president, American Telemedicine Association, will deliver the keynote address, which will focus on advances in technology that would affect simulation-based surgical education and training and areas for research and development to advance the field. A special panel on advances in technology will follow. Meeting sessions will include an interactive debate about industry’s role in training and credentialing of new technology, and participants will have access to interactive workshops, scientific paper presentations, posters, and networking opportunities.
New this year is a pre-meeting Simulation Research Summit on March 15, the goal of which is development of a research agenda for the future that focuses on three core areas: impact of simulation training on patient safety and outcomes, the value proposition of simulation, and simulation use for physician credentialing. David M. Gaba, MD, associate dean for immersive and simulation-based learning and professor of anesthesiology, perioperative, and pain medicine at Stanford School of Medicine, CA, will kick off the inaugural Simulation Research Summit with a keynote address. A separate registration fee applies for the pre-meeting.
Visit the ACS website at www.facs.org/education/accreditation/aei/surgical-simulation-summit to view the Surgical Simulation Summit program, register for the meeting, and reserve a hotel room. The deadline to register for the summit is March 2.
For more information about the meeting or the AEI program, contact Cathy Wojcik, Manager, Program for Accreditation of Education Institutes, at [email protected].
The annual American College of Surgeons (ACS) Accredited Education Institutes (AEI) Surgical Simulation Summit will take place March 16−17 at the Swissôtel Chicago, IL.
Retired Army Lieutenant General James B. Peake, MD, FACS, senior vice-president, CGI Federal, and immediate past-president, American Telemedicine Association, will deliver the keynote address, which will focus on advances in technology that would affect simulation-based surgical education and training and areas for research and development to advance the field. A special panel on advances in technology will follow. Meeting sessions will include an interactive debate about industry’s role in training and credentialing of new technology, and participants will have access to interactive workshops, scientific paper presentations, posters, and networking opportunities.
New this year is a pre-meeting Simulation Research Summit on March 15, the goal of which is development of a research agenda for the future that focuses on three core areas: impact of simulation training on patient safety and outcomes, the value proposition of simulation, and simulation use for physician credentialing. David M. Gaba, MD, associate dean for immersive and simulation-based learning and professor of anesthesiology, perioperative, and pain medicine at Stanford School of Medicine, CA, will kick off the inaugural Simulation Research Summit with a keynote address. A separate registration fee applies for the pre-meeting.
Visit the ACS website at www.facs.org/education/accreditation/aei/surgical-simulation-summit to view the Surgical Simulation Summit program, register for the meeting, and reserve a hotel room. The deadline to register for the summit is March 2.
For more information about the meeting or the AEI program, contact Cathy Wojcik, Manager, Program for Accreditation of Education Institutes, at [email protected].
The annual American College of Surgeons (ACS) Accredited Education Institutes (AEI) Surgical Simulation Summit will take place March 16−17 at the Swissôtel Chicago, IL.
Retired Army Lieutenant General James B. Peake, MD, FACS, senior vice-president, CGI Federal, and immediate past-president, American Telemedicine Association, will deliver the keynote address, which will focus on advances in technology that would affect simulation-based surgical education and training and areas for research and development to advance the field. A special panel on advances in technology will follow. Meeting sessions will include an interactive debate about industry’s role in training and credentialing of new technology, and participants will have access to interactive workshops, scientific paper presentations, posters, and networking opportunities.
New this year is a pre-meeting Simulation Research Summit on March 15, the goal of which is development of a research agenda for the future that focuses on three core areas: impact of simulation training on patient safety and outcomes, the value proposition of simulation, and simulation use for physician credentialing. David M. Gaba, MD, associate dean for immersive and simulation-based learning and professor of anesthesiology, perioperative, and pain medicine at Stanford School of Medicine, CA, will kick off the inaugural Simulation Research Summit with a keynote address. A separate registration fee applies for the pre-meeting.
Visit the ACS website at www.facs.org/education/accreditation/aei/surgical-simulation-summit to view the Surgical Simulation Summit program, register for the meeting, and reserve a hotel room. The deadline to register for the summit is March 2.
For more information about the meeting or the AEI program, contact Cathy Wojcik, Manager, Program for Accreditation of Education Institutes, at [email protected].