User login
Failure to thrive in a 6-day-old neonate • intermittent retractions with inspiratory stridor • Dx?
THE CASE
A primiparous mother gave birth to a girl at 38 and 4/7 weeks via uncomplicated vaginal delivery. Prenatal labs were normal. Neonatal physical examination was normal and the child’s birth weight was in the 33rd percentile. APGAR scores were 8 and 9. The neonate was afebrile during hospitalization, with a heart rate of 120 to 150 beats/min and a respiratory rate of 30 to 48 breaths/min. Her preductal and postductal oxygen saturations were 100% and 98%, respectively. She was discharged on Day 2 of life, having lost only 3% of her birth weight.
The patient was seen in clinic on Day 6 of life for a well-child exam and was in the 17th percentile for weight. At another visit for a well-child exam on Day 14 of life, she had not fully regained her birth weight. At both visits, the mother reported no issues with breastfeeding and said she was supplementing with formula. The patient was seen again for follow-up on Days 16 and 21 of life and demonstrated no weight gain despite close follow-up with the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), which determined the newborn had some breastfeeding issues but seemed to be consuming adequate calories. However, WIC assessments revealed that during feeding, the child was expending too many calories and had nasal congestion. The patient was admitted to the hospital on Day 21 of life with a diagnosis of failure to thrive (FTT), at which point she was in the 12th percentile for weight.
THE DIAGNOSIS
Shortly after the infant was admitted, she showed signs of respiratory distress. On physical examination, the on-call resident noted intermittent retractions with inspiratory stridor, and the patient demonstrated intermittent severe oxygen desaturations into the 70s. She also was sucking her pacifier furiously, which appeared to provide some relief from the respiratory distress. The child’s parents noted that she had demonstrated intermittent periods of respiratory distress since shortly after birth that seemed to be increasing in frequency.
Upon careful examination, the on-call resident identified a cystic lesion at the base of the child’s tongue. The otolaryngologist on call was brought in for an urgent consultation but was unable to visualize the lesion on physical examination and did not recommend further intervention at that time. The patient continued to demonstrate respiratory distress with hypoxia and was transferred to the pediatric intensive care unit for close monitoring.
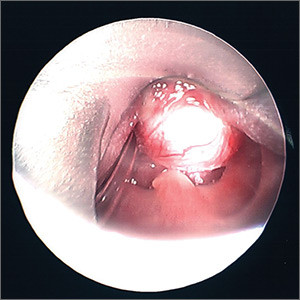
The next morning a second otolaryngology consultation was requested. A computed tomography scan of the neck demonstrated a 1.5-cm cystic-appearing mass at the base of the tongue that was obstructing the patient’s airway. Direct flexible bronchoscopy confirmed the radiographic findings. The patient underwent immediate surgical resection of the lesion using a laser. A clear and milky gray cystic fluid exuded from the cyst when the lesion was pierced. The otolaryngologist visualized a widely patent airway following excision of the lesion (FIGURE).
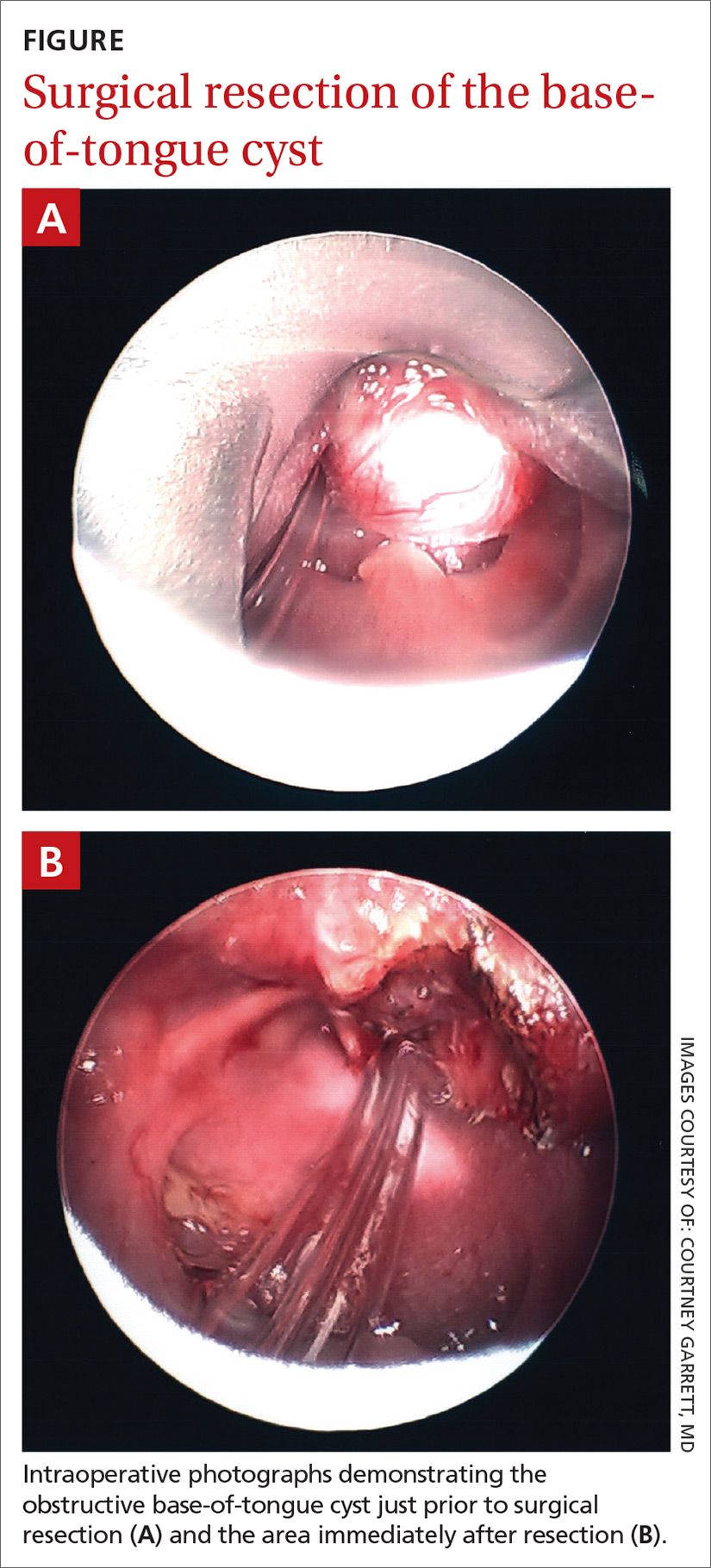
Pathology results revealed no evidence of malignancy. The final diagnosis was a simple base-of-tongue cyst.
DISCUSSION
Failure to thrive is common in neonates and occurs most often due to inadequate caloric intake; however, it also can be caused by systemic disease associated with inadequate gastrointestinal absorption or increased caloric expenditure, such as congenital heart disease, renal disease (eg, renal tubular acidosis), chronic pulmonary disease (eg, cystic fibrosis), laryngomalacia, malignancy, immunodeficiency, or thyroid disease.1
Continue to: Respiratory distress
Respiratory distress in neonates also is common but tends to occur shortly after birth.2 Conditions associated with respiratory distress in neonates include transient tachypnea of the newborn, respiratory distress syndrome, pneumothorax, persistent pulmonary hypertension of the newborn, pneumonia, and meconium aspiration syndrome.2 Interestingly, there are additional reports in the literature of FTT and respiratory distress in neonat
Base-of-tongue cysts are rare in infants. Fewer than 50 cases were reported prior to 2011, with many being described as asymptomatic nonpainful lesions.6 Given the anatomic location of base-of-tongue cysts, the differential diagnosis should also include mucoceles, thyroglossal duct cysts, dermoid cysts, epidermoid cysts, vallecular cysts, hemangiomas, cystic hygromas, lymphangiomas, thyroid remnant cysts, teratomas, and hamartomas.4,7,8 When tongue cysts are initially discovered, inspiratory stridor, FTT, swallowing deficits, oxygen desaturation, respiratory failure, and/or acute life-threatening events have been reported.6,9,10
One important clinical observation made in our case was the use of an external apparatus to relieve the neonate’s respiratory distress. During physical examination, the on-call resident noted the patient was furiously sucking her pacifier, which seemed to reduce the respiratory difficulty and desaturations. It is known that non-nutritive sucking (NNS) can provide provisions for stress relief, improve oxygenation, and provide proprioceptive positioning of key anatomical structures within the oral cavity.11 Without the use of an external apparatus like a pacifier during restful states, neonates may develop vacuum-glossoptosis syndrome, in which the dorsum of the tongue and the soft palate adhere to the posterior pharyngeal wall and obstruct the airway.12 Our patient may have used the pacifier as an NNS task to move the tongue forward and break the glossoptosis-pharyngeal seal by sucking hard and fast during periods of respiratory distress, which reduced the potential for a vacuum-glossoptosis phenomenon that was likely created by the cyst during restful states.
Our patient was seen in clinic for follow-up after surgery on Day 35 of life. She was thriving and her weight was in the 24th percentile. She was seen again on Day 67 of life for a well-child exam and was in the 43rd percentile for weight.
THE TAKEAWAY
There is a sizeable list of possible diagnoses to consider when a neonate presents with FTT and respiratory distress. It is important to consider mechanical obstruction as a possible diagnosis and one which, if identified early, may be lifesaving. Our case demonstrates a proposed mechanism by which a mechanical obstruction such as a base-of-tongue cyst can cause the vacuum-glossoptosis syndrome; it also highlights NNS as a potential means of overcoming this phenomenon.
CORRESPONDENCE
Benjamin P. Hansen, MD, Renown Medical Group, 4796 Caughlin Pkwy, Ste 108, Reno, NV 89519; [email protected]
1. Larson-Nath C, Biank VF. Clinical review of failure to thrive in pediatric patients. Pediatr Ann. 2016;45:e46-e49.
2. Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14:29-37.
3. Brennan T, Rastatter JC. Multilevel airway obstruction including rare tongue base mass presenting as severe croup in an infant. Int J Pediatr Otorhinolaryngol. 2013;77:128-129.
4. Gutiérrez JP, Berkowitz RG, Robertson CF. Vallecular cysts in newborns and young infants. Pediatr Pulmonol. 1999;27:282-285.
5. Wong KS, Huang YH, Wu CT. A vanishing tongue-base cyst. Turk J Pediatr. 2007;49:451-452.
6. Aubin A, Lescanne E, Pondaven S, et al. Stridor and lingual thyroglossal duct cyst in a newborn. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:321-323.
7. Hur JH, Byun JS, Kim JK, et al. Mucocele in the base of the tongue mimicking a thyroglossal duct cyst: a very rare location. Iran J Radiol. 2016;13:4-7.
8. Tárrega ER, Rojas SF, Portero RG, et al. Prenatal ultrasound diagnosis of a cyst of the oral cavity: an unusual case of thyroglossal duct cyst located on the tongue base [published online January 21, 2016]. 2016;2016:7816306.
9. Parelkar SV, Patel JL, Sanghvi BV, et al. An unusual presentation of vallecular cyst with near fatal respiratory distress and management using conventional laparoscopic instruments. J Surg Tech Case Rep. 2012;4:118-120.
10. Sands NB, Anand SM, Manoukian JJ. Series of congenital vallecular cysts: a rare yet potentially fatal cause of upper airway obstruction and failure to thrive in the newborn. J Otolaryngol Head Neck Surg. 2009;38:6-10.
11. Pinelli J, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database Syst Rev 2005. 2010;4:CD001071.
12. Cozzi F, Albani R, Cardi E. A common pathophysiology for sudden cot death and sleep apnoea. “the vacuum-glossoptosis syndrome.” Med Hypotheses. 1979;5:329-338.
THE CASE
A primiparous mother gave birth to a girl at 38 and 4/7 weeks via uncomplicated vaginal delivery. Prenatal labs were normal. Neonatal physical examination was normal and the child’s birth weight was in the 33rd percentile. APGAR scores were 8 and 9. The neonate was afebrile during hospitalization, with a heart rate of 120 to 150 beats/min and a respiratory rate of 30 to 48 breaths/min. Her preductal and postductal oxygen saturations were 100% and 98%, respectively. She was discharged on Day 2 of life, having lost only 3% of her birth weight.
The patient was seen in clinic on Day 6 of life for a well-child exam and was in the 17th percentile for weight. At another visit for a well-child exam on Day 14 of life, she had not fully regained her birth weight. At both visits, the mother reported no issues with breastfeeding and said she was supplementing with formula. The patient was seen again for follow-up on Days 16 and 21 of life and demonstrated no weight gain despite close follow-up with the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), which determined the newborn had some breastfeeding issues but seemed to be consuming adequate calories. However, WIC assessments revealed that during feeding, the child was expending too many calories and had nasal congestion. The patient was admitted to the hospital on Day 21 of life with a diagnosis of failure to thrive (FTT), at which point she was in the 12th percentile for weight.
THE DIAGNOSIS
Shortly after the infant was admitted, she showed signs of respiratory distress. On physical examination, the on-call resident noted intermittent retractions with inspiratory stridor, and the patient demonstrated intermittent severe oxygen desaturations into the 70s. She also was sucking her pacifier furiously, which appeared to provide some relief from the respiratory distress. The child’s parents noted that she had demonstrated intermittent periods of respiratory distress since shortly after birth that seemed to be increasing in frequency.
Upon careful examination, the on-call resident identified a cystic lesion at the base of the child’s tongue. The otolaryngologist on call was brought in for an urgent consultation but was unable to visualize the lesion on physical examination and did not recommend further intervention at that time. The patient continued to demonstrate respiratory distress with hypoxia and was transferred to the pediatric intensive care unit for close monitoring.
The next morning a second otolaryngology consultation was requested. A computed tomography scan of the neck demonstrated a 1.5-cm cystic-appearing mass at the base of the tongue that was obstructing the patient’s airway. Direct flexible bronchoscopy confirmed the radiographic findings. The patient underwent immediate surgical resection of the lesion using a laser. A clear and milky gray cystic fluid exuded from the cyst when the lesion was pierced. The otolaryngologist visualized a widely patent airway following excision of the lesion (FIGURE).

Pathology results revealed no evidence of malignancy. The final diagnosis was a simple base-of-tongue cyst.
DISCUSSION
Failure to thrive is common in neonates and occurs most often due to inadequate caloric intake; however, it also can be caused by systemic disease associated with inadequate gastrointestinal absorption or increased caloric expenditure, such as congenital heart disease, renal disease (eg, renal tubular acidosis), chronic pulmonary disease (eg, cystic fibrosis), laryngomalacia, malignancy, immunodeficiency, or thyroid disease.1
Continue to: Respiratory distress
Respiratory distress in neonates also is common but tends to occur shortly after birth.2 Conditions associated with respiratory distress in neonates include transient tachypnea of the newborn, respiratory distress syndrome, pneumothorax, persistent pulmonary hypertension of the newborn, pneumonia, and meconium aspiration syndrome.2 Interestingly, there are additional reports in the literature of FTT and respiratory distress in neonat
Base-of-tongue cysts are rare in infants. Fewer than 50 cases were reported prior to 2011, with many being described as asymptomatic nonpainful lesions.6 Given the anatomic location of base-of-tongue cysts, the differential diagnosis should also include mucoceles, thyroglossal duct cysts, dermoid cysts, epidermoid cysts, vallecular cysts, hemangiomas, cystic hygromas, lymphangiomas, thyroid remnant cysts, teratomas, and hamartomas.4,7,8 When tongue cysts are initially discovered, inspiratory stridor, FTT, swallowing deficits, oxygen desaturation, respiratory failure, and/or acute life-threatening events have been reported.6,9,10
One important clinical observation made in our case was the use of an external apparatus to relieve the neonate’s respiratory distress. During physical examination, the on-call resident noted the patient was furiously sucking her pacifier, which seemed to reduce the respiratory difficulty and desaturations. It is known that non-nutritive sucking (NNS) can provide provisions for stress relief, improve oxygenation, and provide proprioceptive positioning of key anatomical structures within the oral cavity.11 Without the use of an external apparatus like a pacifier during restful states, neonates may develop vacuum-glossoptosis syndrome, in which the dorsum of the tongue and the soft palate adhere to the posterior pharyngeal wall and obstruct the airway.12 Our patient may have used the pacifier as an NNS task to move the tongue forward and break the glossoptosis-pharyngeal seal by sucking hard and fast during periods of respiratory distress, which reduced the potential for a vacuum-glossoptosis phenomenon that was likely created by the cyst during restful states.
Our patient was seen in clinic for follow-up after surgery on Day 35 of life. She was thriving and her weight was in the 24th percentile. She was seen again on Day 67 of life for a well-child exam and was in the 43rd percentile for weight.
THE TAKEAWAY
There is a sizeable list of possible diagnoses to consider when a neonate presents with FTT and respiratory distress. It is important to consider mechanical obstruction as a possible diagnosis and one which, if identified early, may be lifesaving. Our case demonstrates a proposed mechanism by which a mechanical obstruction such as a base-of-tongue cyst can cause the vacuum-glossoptosis syndrome; it also highlights NNS as a potential means of overcoming this phenomenon.
CORRESPONDENCE
Benjamin P. Hansen, MD, Renown Medical Group, 4796 Caughlin Pkwy, Ste 108, Reno, NV 89519; [email protected]
THE CASE
A primiparous mother gave birth to a girl at 38 and 4/7 weeks via uncomplicated vaginal delivery. Prenatal labs were normal. Neonatal physical examination was normal and the child’s birth weight was in the 33rd percentile. APGAR scores were 8 and 9. The neonate was afebrile during hospitalization, with a heart rate of 120 to 150 beats/min and a respiratory rate of 30 to 48 breaths/min. Her preductal and postductal oxygen saturations were 100% and 98%, respectively. She was discharged on Day 2 of life, having lost only 3% of her birth weight.
The patient was seen in clinic on Day 6 of life for a well-child exam and was in the 17th percentile for weight. At another visit for a well-child exam on Day 14 of life, she had not fully regained her birth weight. At both visits, the mother reported no issues with breastfeeding and said she was supplementing with formula. The patient was seen again for follow-up on Days 16 and 21 of life and demonstrated no weight gain despite close follow-up with the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), which determined the newborn had some breastfeeding issues but seemed to be consuming adequate calories. However, WIC assessments revealed that during feeding, the child was expending too many calories and had nasal congestion. The patient was admitted to the hospital on Day 21 of life with a diagnosis of failure to thrive (FTT), at which point she was in the 12th percentile for weight.
THE DIAGNOSIS
Shortly after the infant was admitted, she showed signs of respiratory distress. On physical examination, the on-call resident noted intermittent retractions with inspiratory stridor, and the patient demonstrated intermittent severe oxygen desaturations into the 70s. She also was sucking her pacifier furiously, which appeared to provide some relief from the respiratory distress. The child’s parents noted that she had demonstrated intermittent periods of respiratory distress since shortly after birth that seemed to be increasing in frequency.
Upon careful examination, the on-call resident identified a cystic lesion at the base of the child’s tongue. The otolaryngologist on call was brought in for an urgent consultation but was unable to visualize the lesion on physical examination and did not recommend further intervention at that time. The patient continued to demonstrate respiratory distress with hypoxia and was transferred to the pediatric intensive care unit for close monitoring.
The next morning a second otolaryngology consultation was requested. A computed tomography scan of the neck demonstrated a 1.5-cm cystic-appearing mass at the base of the tongue that was obstructing the patient’s airway. Direct flexible bronchoscopy confirmed the radiographic findings. The patient underwent immediate surgical resection of the lesion using a laser. A clear and milky gray cystic fluid exuded from the cyst when the lesion was pierced. The otolaryngologist visualized a widely patent airway following excision of the lesion (FIGURE).

Pathology results revealed no evidence of malignancy. The final diagnosis was a simple base-of-tongue cyst.
DISCUSSION
Failure to thrive is common in neonates and occurs most often due to inadequate caloric intake; however, it also can be caused by systemic disease associated with inadequate gastrointestinal absorption or increased caloric expenditure, such as congenital heart disease, renal disease (eg, renal tubular acidosis), chronic pulmonary disease (eg, cystic fibrosis), laryngomalacia, malignancy, immunodeficiency, or thyroid disease.1
Continue to: Respiratory distress
Respiratory distress in neonates also is common but tends to occur shortly after birth.2 Conditions associated with respiratory distress in neonates include transient tachypnea of the newborn, respiratory distress syndrome, pneumothorax, persistent pulmonary hypertension of the newborn, pneumonia, and meconium aspiration syndrome.2 Interestingly, there are additional reports in the literature of FTT and respiratory distress in neonat
Base-of-tongue cysts are rare in infants. Fewer than 50 cases were reported prior to 2011, with many being described as asymptomatic nonpainful lesions.6 Given the anatomic location of base-of-tongue cysts, the differential diagnosis should also include mucoceles, thyroglossal duct cysts, dermoid cysts, epidermoid cysts, vallecular cysts, hemangiomas, cystic hygromas, lymphangiomas, thyroid remnant cysts, teratomas, and hamartomas.4,7,8 When tongue cysts are initially discovered, inspiratory stridor, FTT, swallowing deficits, oxygen desaturation, respiratory failure, and/or acute life-threatening events have been reported.6,9,10
One important clinical observation made in our case was the use of an external apparatus to relieve the neonate’s respiratory distress. During physical examination, the on-call resident noted the patient was furiously sucking her pacifier, which seemed to reduce the respiratory difficulty and desaturations. It is known that non-nutritive sucking (NNS) can provide provisions for stress relief, improve oxygenation, and provide proprioceptive positioning of key anatomical structures within the oral cavity.11 Without the use of an external apparatus like a pacifier during restful states, neonates may develop vacuum-glossoptosis syndrome, in which the dorsum of the tongue and the soft palate adhere to the posterior pharyngeal wall and obstruct the airway.12 Our patient may have used the pacifier as an NNS task to move the tongue forward and break the glossoptosis-pharyngeal seal by sucking hard and fast during periods of respiratory distress, which reduced the potential for a vacuum-glossoptosis phenomenon that was likely created by the cyst during restful states.
Our patient was seen in clinic for follow-up after surgery on Day 35 of life. She was thriving and her weight was in the 24th percentile. She was seen again on Day 67 of life for a well-child exam and was in the 43rd percentile for weight.
THE TAKEAWAY
There is a sizeable list of possible diagnoses to consider when a neonate presents with FTT and respiratory distress. It is important to consider mechanical obstruction as a possible diagnosis and one which, if identified early, may be lifesaving. Our case demonstrates a proposed mechanism by which a mechanical obstruction such as a base-of-tongue cyst can cause the vacuum-glossoptosis syndrome; it also highlights NNS as a potential means of overcoming this phenomenon.
CORRESPONDENCE
Benjamin P. Hansen, MD, Renown Medical Group, 4796 Caughlin Pkwy, Ste 108, Reno, NV 89519; [email protected]
1. Larson-Nath C, Biank VF. Clinical review of failure to thrive in pediatric patients. Pediatr Ann. 2016;45:e46-e49.
2. Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14:29-37.
3. Brennan T, Rastatter JC. Multilevel airway obstruction including rare tongue base mass presenting as severe croup in an infant. Int J Pediatr Otorhinolaryngol. 2013;77:128-129.
4. Gutiérrez JP, Berkowitz RG, Robertson CF. Vallecular cysts in newborns and young infants. Pediatr Pulmonol. 1999;27:282-285.
5. Wong KS, Huang YH, Wu CT. A vanishing tongue-base cyst. Turk J Pediatr. 2007;49:451-452.
6. Aubin A, Lescanne E, Pondaven S, et al. Stridor and lingual thyroglossal duct cyst in a newborn. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:321-323.
7. Hur JH, Byun JS, Kim JK, et al. Mucocele in the base of the tongue mimicking a thyroglossal duct cyst: a very rare location. Iran J Radiol. 2016;13:4-7.
8. Tárrega ER, Rojas SF, Portero RG, et al. Prenatal ultrasound diagnosis of a cyst of the oral cavity: an unusual case of thyroglossal duct cyst located on the tongue base [published online January 21, 2016]. 2016;2016:7816306.
9. Parelkar SV, Patel JL, Sanghvi BV, et al. An unusual presentation of vallecular cyst with near fatal respiratory distress and management using conventional laparoscopic instruments. J Surg Tech Case Rep. 2012;4:118-120.
10. Sands NB, Anand SM, Manoukian JJ. Series of congenital vallecular cysts: a rare yet potentially fatal cause of upper airway obstruction and failure to thrive in the newborn. J Otolaryngol Head Neck Surg. 2009;38:6-10.
11. Pinelli J, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database Syst Rev 2005. 2010;4:CD001071.
12. Cozzi F, Albani R, Cardi E. A common pathophysiology for sudden cot death and sleep apnoea. “the vacuum-glossoptosis syndrome.” Med Hypotheses. 1979;5:329-338.
1. Larson-Nath C, Biank VF. Clinical review of failure to thrive in pediatric patients. Pediatr Ann. 2016;45:e46-e49.
2. Edwards MO, Kotecha SJ, Kotecha S. Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14:29-37.
3. Brennan T, Rastatter JC. Multilevel airway obstruction including rare tongue base mass presenting as severe croup in an infant. Int J Pediatr Otorhinolaryngol. 2013;77:128-129.
4. Gutiérrez JP, Berkowitz RG, Robertson CF. Vallecular cysts in newborns and young infants. Pediatr Pulmonol. 1999;27:282-285.
5. Wong KS, Huang YH, Wu CT. A vanishing tongue-base cyst. Turk J Pediatr. 2007;49:451-452.
6. Aubin A, Lescanne E, Pondaven S, et al. Stridor and lingual thyroglossal duct cyst in a newborn. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:321-323.
7. Hur JH, Byun JS, Kim JK, et al. Mucocele in the base of the tongue mimicking a thyroglossal duct cyst: a very rare location. Iran J Radiol. 2016;13:4-7.
8. Tárrega ER, Rojas SF, Portero RG, et al. Prenatal ultrasound diagnosis of a cyst of the oral cavity: an unusual case of thyroglossal duct cyst located on the tongue base [published online January 21, 2016]. 2016;2016:7816306.
9. Parelkar SV, Patel JL, Sanghvi BV, et al. An unusual presentation of vallecular cyst with near fatal respiratory distress and management using conventional laparoscopic instruments. J Surg Tech Case Rep. 2012;4:118-120.
10. Sands NB, Anand SM, Manoukian JJ. Series of congenital vallecular cysts: a rare yet potentially fatal cause of upper airway obstruction and failure to thrive in the newborn. J Otolaryngol Head Neck Surg. 2009;38:6-10.
11. Pinelli J, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database Syst Rev 2005. 2010;4:CD001071.
12. Cozzi F, Albani R, Cardi E. A common pathophysiology for sudden cot death and sleep apnoea. “the vacuum-glossoptosis syndrome.” Med Hypotheses. 1979;5:329-338.
Antibiotic Overprescribing: Still a Major Concern

Despite universal agreement that antibiotic overprescribing is a problem, the practice continues to vex us. Antibiotic use—whether appropriate or not—has been linked to rising rates of antimicrobial resistance, disruption of the gut microbiome leading to Clostridium difficile infections (CDI), allergic reactions, and increased health care costs (see Table 1).1-6 And yet, clinicians continue to overprescribe this class of medication.

A 2016 report from the CDC estimates that at least 30% of antibiotics prescribed in US outpatient settings are unnecessary.7 Another report cites a slightly higher figure across a variety of health care settings.8 Pair these findings with the fact that there are currently few new drugs in development to target resistant bacteria, and you have the potential for a postantibiotic era in which common infections could become lethal.7
In 2003, the CDC launched its “Get Smart: Know When Antibiotics Work” program (now known as “Be Antibiotics Aware”), focused on decreasing inappropriate antibiotic use in the outpatient setting.9 In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria, with a goal of decreasing inappropriate outpatient antibiotic use by 50% and inappropriate inpatient use by 20% by 2020.10 And, on an international level, the World Health Organization (WHO) in 2015 developed a five-year strategic framework for implementing its Global Action Plan on Antimicrobial Resistance.11
Family practitioners are on the front lines of this battle. Here’s what we can do now.
WHEN AND WHERE ARE ANTIBIOTICS MOST OFTEN INAPPROPRIATELY PRESCRIBED?
The diagnosis leading to the most frequent inappropriate prescribing of antibiotics is acute respiratory tract infection (ARTI), which includes bronchitis, otitis media, pharyngitis, sinusitis, tonsillitis, the common cold, and pneumonia. Up to 40% of antibiotic prescriptions for these conditions are unnecessary.8,12 Bronchitis is the most common ARTI diagnosis associated with inappropriate antibiotic prescriptions, while sinusitis, suppurative otitis media, and pharyngitis are the diagnoses associated with the lion’s share of all (appropriate and inappropriate) antibiotic prescriptions within the ARTI category.8,9,12,13 Refer to national clinical guidelines, which delineate when antibiotic treatment is appropriate for these conditions.14-16
With respect to setting, there are conflicting findings as to whether antibiotic prescribing differs in office-based versus emergency department (ED) settings.
- One study found a higher rate of antibiotic prescribing during ED visits than office visits (21% vs 9%), even though, between 2007 and 2009, more antibiotic prescriptions were written for adults in primary care offices than in either outpatient hospital clinics or EDs.17
- In a cross-sectional study using data from 2005 to 2010 National Ambulatory Medical Care Surveys (NAMCS) and National Hospital Ambulatory Medical Care Surveys (NHAMCS), more than half of patients with uncomplicated acute rhinosinusitis received a prescription for antibiotics, but there was no overall difference in antibiotic prescriptions between primary care and ED presentation.18
- A retrospective analysis found that between 2006 and 2010, outpatient hospital practices (56%) and community-practice offices (60%) prescribed more antibiotics for ARTIs than EDs did (51%).12
STICK TO NARROW-SPECTRUM AGENTS WHEN POSSIBLE
Using broad-spectrum antibiotics, such as quinolones or imipenem, firstline, contributes more to the problem of antibiotic resistance than does prescribing narrow-spectrum antibiotics such as amoxicillin, cephalexin, or trimethoprim-sulfamethoxazole.7 Yet between 2007 and 2009, broad-spectrum agents were prescribed for 61% of outpatient adult visits in which patients received an antibiotic prescription.17 Quinolones (25%), macrolides (20%), and aminopenicillins (12%) were most commonly prescribed, and antibiotic prescriptions were most often written for respiratory conditions, such as bronchitis, for which we now know antibiotics are rarely indicated.17
Between 2006 and 2008, pediatric patients who received antibiotic prescriptions were given broad-spectrum agents 50% of the time, of which macrolides were the class most commonly prescribed.13
More recently, researchers examined the frequency with which clinicians prescribe narrow-spectrum, firstline antibiotics for otitis media, sinusitis, and pharyngitis using 2010 to 2011 NAMCS/NHAMCS data. They found that providers used firstline agents recommended by professional guidelines 52% of the time, although it was estimated that they would have been appropriate in 80% of cases; pediatric patients were more likely to receive appropriate firstline antibiotics than adult patients.19 Macrolides, especially azithromycin, were the most common non-firstline antibiotics prescribed.19,20 The bottom line is that when antibiotics are indicated for upper respiratory infections (otitis media, sinusitis, and pharyngitis), clinicians should prescribe a narrow-spectrum antibiotic first.
ANTIBIOTIC OVERPRESCIBING AFFECTS THE GUT AND BEYOND
The human intestinal microbiome is composed of a diverse array of bacteria, viruses, and parasites.21 The main functions of the gut microbiome include interacting with the immune system and participating in biochemical reactions in the gut, such as absorption of fat-soluble vitamins and the production of vitamin K.
As we know, antibiotics decrease the diversity of gut bacteria, which, in turn, can cause less efficient nutrient extraction, as well as vulnerability to enteric infections.21 It is well known, for example, that the bacterial gut microbiome can either inhibit or promote diarrheal illnesses such as those caused by CDI. CDI is now the most common health care-related infection, accounting for about a half-million health care facility infections per year.22 It extends hospital stays an average of almost 10 days and is estimated to cost the health care system $6.3 billion annually.23
Antibiotics can also eliminate antibiotic-susceptible organisms, allowing resistant organisms to proliferate.4 They also promote the transmission of genes for antibiotic resistance between gut bacteria.4
Beyond the gut
Less well known is that gut bacteria can promote or inhibit extraintestinal infections.
Gut bacteria and HIV. In early HIV infections, for example, gut populations of Lactobacillus and Bifidobacteria are reduced, and the gut barrier becomes compromised.24 Increasing translocation of bacterial products is associated with HIV disease progression. Preservation of Lactobacillus populations in the gut is associated with markers predictive of better HIV outcomes, including a higher CD4 count, a lower viral load, and less evidence of gut microbial translocation.24 This underscores the importance of maintaining healthy gut flora in patients with HIV, using such steps as avoiding unnecessary antibiotics.
Gut bacteria and stress, depression. Antibiotics directly induce the expression of key genes that affect the stress response.25 While causative studies are lacking, there is a growing body of evidence suggesting that the gut microbiome is involved in two-way communication with the brain and can affect, and be affected by, stress and depression.21,26-30 Diseases and conditions that seem to have a putative connection to a disordered microbiome (dysbiosis) include depression, anxiety, Crohn disease, type 2 diabetes, and obesity. (For a discussion of the relationship between the gut microbiome and diabetes, see Endocrine Consult: The Gut Microbiome in Type 2 Diabetes.)
Gut bacteria and childhood obesity. Repeated use of broader-spectrum antibiotics in children younger than 24 months of age increases the risk for childhood obesity.1,6 One theory for the association is that the effects of broad-spectrum antibiotics on the intestinal flora of young children may alter long-term energy homeostasis, resulting in a higher risk for obesity.1
Gut bacteria and asthma. Studies demonstrate differences in the gut microbiomes of asthmatic and nonasthmatic patients. These differences affect the activities of helper T-cell subsets (Th1 and Th2), which in turn affect the development of immune tolerance.31
Although additional studies are needed to confirm these findings, the evidence collected thus far should make us all pause before prescribing drugs that can alter our microbiome in complex and only partially understood ways.
WHAT CAN WE DO RIGHT NOW?
The issues created by the inappropriate prescribing of antibiotics have been known for decades, and multiple attempts have been made to find solutions and implement change. Although some small successes have occurred, little overall progress has been made in reducing antibiotic prescribing in the general population. A historical review of why clinicians prescribe antibiotics inappropriately and the interventions that have successfully reduced this prescribing may prove valuable as we continue to look for new, effective answers.
Why do we overprescribe antibiotics? A 2015 systematic literature review found that patient demand, pharmaceutical company marketing activities, limited up-to-date information sources, and fear of losing patients are major reasons providers cite for prescribing antibiotics.32
In a separate study that explored antibiotic prescribing habits for acute bronchitis, clinicians cited “patient demand” as the major reason for prescribing antibiotics. Respondents also reported that “other physicians were responsible for inappropriate antibiotic prescribing.”33
Strategies that work
Some early intervention programs directed at reducing antibiotic prescribing demonstrated success (see Table 2).34-36 
One example comes from a 1996-1998 study of four primary care practices.34 Researchers evaluated the impact of a multidimensional intervention effort targeted at clinicians and patients and aimed at lowering the use of antimicrobial agents for acute uncomplicated bronchitis in adults. It incorporated multiple elements, including office-based and household patient educational materials and a clinician intervention involving education, practice profiling, and academic detailing. Clinicians in this program reduced their rates of antibiotic prescribing for uncomplicated bronchitis from 74% to 48%.34
Employing EMRs. A more recent study focused on use of electronic medical records (EMRs) and communications to modify clinician antibiotic prescribing.35 By sending clinicians monthly emails comparing their prescribing patterns to those of peers and “typical top performers,” inappropriate antibiotic prescriptions for ARTIs went from 19.9% to 3.7%.35
In another effort, the same researchers modified providers’ EMRs to detect when potentially inappropriate antibiotics were prescribed. The system then prompted the clinician to provide an “antibiotic justification note,” which remained visible in the patient’s chart. This approach, which encouraged providers to follow prescribing guidelines by capitalizing on their concerns about their reputations, produced a 77% reduction in antibiotic prescribing.35
Focusing on the public. Studies have also examined the effectiveness of educating the public about when antibiotics are not likely to be helpful and of the harms of unnecessary antibiotics.
Studies conducted in Tennessee and Wisconsin that combined prescriber and community education about unnecessary antibiotics for children found that the intervention reduced antibiotic prescribing in both locations by about 19%, compared with about a 9% reduction in the control groups.36,37
DOES PRESCRIBING ANTIBIOTICS AFFECT PATIENT SATISFACTION?
The results are mixed as to whether prescribing antibiotics affects patient satisfaction. Two studies in the early 2000s found that both patients and parents reported higher satisfaction with clinicians who explained why antibiotics were not indicated versus those who simply prescribed them—and that such explanations do not need to take a lot of time (see Table 3 for patient care tips).37,38
A more recent study found that higher antibiotic prescribing practices in Britain were associated with modestly higher patient satisfaction ratings.39 The authors of this study noted, however, that reduced antibiotic prescribing may be a proxy for other practice patterns that affected satisfaction ratings.
REDUCING ANTIBIOTIC PRESCRIBING REDUCES RESISTANCE
There is also strong evidence that when clinicians decrease antibiotic prescribing, antimicrobial resistance follows suit. One of the earlier landmark studies to demonstrate this was a Finnish study published in 1997.40 The authors found that a reduction of macrolide antibiotic consumption in Finland led to a reduction in streptococci macrolide resistance from 16.5% to 8.6%.40
Multiple studies have since demonstrated similar results for both respiratory and urinary tract infections.41,42 A 2017 meta-analysis of 32 studies found that antibiotic stewardship programs reduced the incidence of infections and colonization with multidrug-resistant Gram-negative bacteria (by 51%), extended-spectrum beta-lactamase–producing Gram-negative bacteria (48%), and methicillin-resistant Staphylococcus aureus (37%). There was also a reduction in the incidence of CDI (32%).43
1. Bailey LC, Forrest CB, Zhang P, et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069.
2. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
3. Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000;21:53-60.
4. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
5. Logan AC, Jacka FN, Craig JM, et al. The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol Neurosci. 2016;14:131-147.
6. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010.
7. Harris AM, Hicks LA, Qaseem A; the High Value Care Task Force of the American College of Physicians and the CDC. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016; 164:425-434.
8. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315: 1864-1873.
9. CDC. Antibiotic prescribing and use. www.cdc.gov/antibiotic-use/index.html. Accessed January 16, 2018.
10. The White House. National action plan for combating antibiotic-resistant bacteria. March 2015:1-63. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed January 16, 2018.
11. World Health Organization. Global action plan on antimicrobial resistance (2015). www.who.int/antimicrobial-resistance/global-action-plan/en/. Accessed January 16, 2018.
12. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
13. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061.
14. Chow AW, Benninger MS, Brook I, et al. Executive summary: IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:1041-1045.
15. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
16. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
17. Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother. 2014;69:234-240.
18. Bergmark RW, Sedaghat AR. Antibiotic prescription for acute rhinosinusitis: emergency departments versus primary care providers. Laryngoscope. 2016;126:2439-2444.
19. Hersh AL, Fleming-Dutra KE, Shapiro DJ, et al. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870-1872.
20. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308-1316.
21. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39.
22. Lessa FC, Gould CV, McDonald CL. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(suppl 2):S65-S70.
23. Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447.
24. Pérez-Santiago J, Gianella S, Massanella M, et al. Gut lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
25. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50.
26. Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667-672.
27. Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258-1270.
28. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369-1378.
29. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312.
30. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014; 38:1-12.31. Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. 2015;11:35.
32. Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665-680.
33. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract . 2014;15:194.
34. Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice. JAMA . 1999;281:1512-1519.
35. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA . 2016;315:562-570.
36. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA . 2002;287:3103-3109.
37. Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics . 2001;108:575-583.
38. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155:800-806.
39. Ashworth M, White P, Jongsma H, et al. Antibiotic prescribing and patient satisfaction in primary care in England: cross-sectional analysis of national patient survey data and prescribing data. Br J Gen Pract . 2016;66:e40-e46.
40. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441-446.
41. Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin g–nonsusceptible Streptococcus pneumoniae . Clin Infect Dis. 2005;41:930-938.
42. Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007; 57:785-792.
43. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990-1001.

Despite universal agreement that antibiotic overprescribing is a problem, the practice continues to vex us. Antibiotic use—whether appropriate or not—has been linked to rising rates of antimicrobial resistance, disruption of the gut microbiome leading to Clostridium difficile infections (CDI), allergic reactions, and increased health care costs (see Table 1).1-6 And yet, clinicians continue to overprescribe this class of medication.

A 2016 report from the CDC estimates that at least 30% of antibiotics prescribed in US outpatient settings are unnecessary.7 Another report cites a slightly higher figure across a variety of health care settings.8 Pair these findings with the fact that there are currently few new drugs in development to target resistant bacteria, and you have the potential for a postantibiotic era in which common infections could become lethal.7
In 2003, the CDC launched its “Get Smart: Know When Antibiotics Work” program (now known as “Be Antibiotics Aware”), focused on decreasing inappropriate antibiotic use in the outpatient setting.9 In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria, with a goal of decreasing inappropriate outpatient antibiotic use by 50% and inappropriate inpatient use by 20% by 2020.10 And, on an international level, the World Health Organization (WHO) in 2015 developed a five-year strategic framework for implementing its Global Action Plan on Antimicrobial Resistance.11
Family practitioners are on the front lines of this battle. Here’s what we can do now.
WHEN AND WHERE ARE ANTIBIOTICS MOST OFTEN INAPPROPRIATELY PRESCRIBED?
The diagnosis leading to the most frequent inappropriate prescribing of antibiotics is acute respiratory tract infection (ARTI), which includes bronchitis, otitis media, pharyngitis, sinusitis, tonsillitis, the common cold, and pneumonia. Up to 40% of antibiotic prescriptions for these conditions are unnecessary.8,12 Bronchitis is the most common ARTI diagnosis associated with inappropriate antibiotic prescriptions, while sinusitis, suppurative otitis media, and pharyngitis are the diagnoses associated with the lion’s share of all (appropriate and inappropriate) antibiotic prescriptions within the ARTI category.8,9,12,13 Refer to national clinical guidelines, which delineate when antibiotic treatment is appropriate for these conditions.14-16
With respect to setting, there are conflicting findings as to whether antibiotic prescribing differs in office-based versus emergency department (ED) settings.
- One study found a higher rate of antibiotic prescribing during ED visits than office visits (21% vs 9%), even though, between 2007 and 2009, more antibiotic prescriptions were written for adults in primary care offices than in either outpatient hospital clinics or EDs.17
- In a cross-sectional study using data from 2005 to 2010 National Ambulatory Medical Care Surveys (NAMCS) and National Hospital Ambulatory Medical Care Surveys (NHAMCS), more than half of patients with uncomplicated acute rhinosinusitis received a prescription for antibiotics, but there was no overall difference in antibiotic prescriptions between primary care and ED presentation.18
- A retrospective analysis found that between 2006 and 2010, outpatient hospital practices (56%) and community-practice offices (60%) prescribed more antibiotics for ARTIs than EDs did (51%).12
STICK TO NARROW-SPECTRUM AGENTS WHEN POSSIBLE
Using broad-spectrum antibiotics, such as quinolones or imipenem, firstline, contributes more to the problem of antibiotic resistance than does prescribing narrow-spectrum antibiotics such as amoxicillin, cephalexin, or trimethoprim-sulfamethoxazole.7 Yet between 2007 and 2009, broad-spectrum agents were prescribed for 61% of outpatient adult visits in which patients received an antibiotic prescription.17 Quinolones (25%), macrolides (20%), and aminopenicillins (12%) were most commonly prescribed, and antibiotic prescriptions were most often written for respiratory conditions, such as bronchitis, for which we now know antibiotics are rarely indicated.17
Between 2006 and 2008, pediatric patients who received antibiotic prescriptions were given broad-spectrum agents 50% of the time, of which macrolides were the class most commonly prescribed.13
More recently, researchers examined the frequency with which clinicians prescribe narrow-spectrum, firstline antibiotics for otitis media, sinusitis, and pharyngitis using 2010 to 2011 NAMCS/NHAMCS data. They found that providers used firstline agents recommended by professional guidelines 52% of the time, although it was estimated that they would have been appropriate in 80% of cases; pediatric patients were more likely to receive appropriate firstline antibiotics than adult patients.19 Macrolides, especially azithromycin, were the most common non-firstline antibiotics prescribed.19,20 The bottom line is that when antibiotics are indicated for upper respiratory infections (otitis media, sinusitis, and pharyngitis), clinicians should prescribe a narrow-spectrum antibiotic first.
ANTIBIOTIC OVERPRESCIBING AFFECTS THE GUT AND BEYOND
The human intestinal microbiome is composed of a diverse array of bacteria, viruses, and parasites.21 The main functions of the gut microbiome include interacting with the immune system and participating in biochemical reactions in the gut, such as absorption of fat-soluble vitamins and the production of vitamin K.
As we know, antibiotics decrease the diversity of gut bacteria, which, in turn, can cause less efficient nutrient extraction, as well as vulnerability to enteric infections.21 It is well known, for example, that the bacterial gut microbiome can either inhibit or promote diarrheal illnesses such as those caused by CDI. CDI is now the most common health care-related infection, accounting for about a half-million health care facility infections per year.22 It extends hospital stays an average of almost 10 days and is estimated to cost the health care system $6.3 billion annually.23
Antibiotics can also eliminate antibiotic-susceptible organisms, allowing resistant organisms to proliferate.4 They also promote the transmission of genes for antibiotic resistance between gut bacteria.4
Beyond the gut
Less well known is that gut bacteria can promote or inhibit extraintestinal infections.
Gut bacteria and HIV. In early HIV infections, for example, gut populations of Lactobacillus and Bifidobacteria are reduced, and the gut barrier becomes compromised.24 Increasing translocation of bacterial products is associated with HIV disease progression. Preservation of Lactobacillus populations in the gut is associated with markers predictive of better HIV outcomes, including a higher CD4 count, a lower viral load, and less evidence of gut microbial translocation.24 This underscores the importance of maintaining healthy gut flora in patients with HIV, using such steps as avoiding unnecessary antibiotics.
Gut bacteria and stress, depression. Antibiotics directly induce the expression of key genes that affect the stress response.25 While causative studies are lacking, there is a growing body of evidence suggesting that the gut microbiome is involved in two-way communication with the brain and can affect, and be affected by, stress and depression.21,26-30 Diseases and conditions that seem to have a putative connection to a disordered microbiome (dysbiosis) include depression, anxiety, Crohn disease, type 2 diabetes, and obesity. (For a discussion of the relationship between the gut microbiome and diabetes, see Endocrine Consult: The Gut Microbiome in Type 2 Diabetes.)
Gut bacteria and childhood obesity. Repeated use of broader-spectrum antibiotics in children younger than 24 months of age increases the risk for childhood obesity.1,6 One theory for the association is that the effects of broad-spectrum antibiotics on the intestinal flora of young children may alter long-term energy homeostasis, resulting in a higher risk for obesity.1
Gut bacteria and asthma. Studies demonstrate differences in the gut microbiomes of asthmatic and nonasthmatic patients. These differences affect the activities of helper T-cell subsets (Th1 and Th2), which in turn affect the development of immune tolerance.31
Although additional studies are needed to confirm these findings, the evidence collected thus far should make us all pause before prescribing drugs that can alter our microbiome in complex and only partially understood ways.
WHAT CAN WE DO RIGHT NOW?
The issues created by the inappropriate prescribing of antibiotics have been known for decades, and multiple attempts have been made to find solutions and implement change. Although some small successes have occurred, little overall progress has been made in reducing antibiotic prescribing in the general population. A historical review of why clinicians prescribe antibiotics inappropriately and the interventions that have successfully reduced this prescribing may prove valuable as we continue to look for new, effective answers.
Why do we overprescribe antibiotics? A 2015 systematic literature review found that patient demand, pharmaceutical company marketing activities, limited up-to-date information sources, and fear of losing patients are major reasons providers cite for prescribing antibiotics.32
In a separate study that explored antibiotic prescribing habits for acute bronchitis, clinicians cited “patient demand” as the major reason for prescribing antibiotics. Respondents also reported that “other physicians were responsible for inappropriate antibiotic prescribing.”33
Strategies that work
Some early intervention programs directed at reducing antibiotic prescribing demonstrated success (see Table 2).34-36 
One example comes from a 1996-1998 study of four primary care practices.34 Researchers evaluated the impact of a multidimensional intervention effort targeted at clinicians and patients and aimed at lowering the use of antimicrobial agents for acute uncomplicated bronchitis in adults. It incorporated multiple elements, including office-based and household patient educational materials and a clinician intervention involving education, practice profiling, and academic detailing. Clinicians in this program reduced their rates of antibiotic prescribing for uncomplicated bronchitis from 74% to 48%.34
Employing EMRs. A more recent study focused on use of electronic medical records (EMRs) and communications to modify clinician antibiotic prescribing.35 By sending clinicians monthly emails comparing their prescribing patterns to those of peers and “typical top performers,” inappropriate antibiotic prescriptions for ARTIs went from 19.9% to 3.7%.35
In another effort, the same researchers modified providers’ EMRs to detect when potentially inappropriate antibiotics were prescribed. The system then prompted the clinician to provide an “antibiotic justification note,” which remained visible in the patient’s chart. This approach, which encouraged providers to follow prescribing guidelines by capitalizing on their concerns about their reputations, produced a 77% reduction in antibiotic prescribing.35
Focusing on the public. Studies have also examined the effectiveness of educating the public about when antibiotics are not likely to be helpful and of the harms of unnecessary antibiotics.
Studies conducted in Tennessee and Wisconsin that combined prescriber and community education about unnecessary antibiotics for children found that the intervention reduced antibiotic prescribing in both locations by about 19%, compared with about a 9% reduction in the control groups.36,37
DOES PRESCRIBING ANTIBIOTICS AFFECT PATIENT SATISFACTION?
The results are mixed as to whether prescribing antibiotics affects patient satisfaction. Two studies in the early 2000s found that both patients and parents reported higher satisfaction with clinicians who explained why antibiotics were not indicated versus those who simply prescribed them—and that such explanations do not need to take a lot of time (see Table 3 for patient care tips).37,38
A more recent study found that higher antibiotic prescribing practices in Britain were associated with modestly higher patient satisfaction ratings.39 The authors of this study noted, however, that reduced antibiotic prescribing may be a proxy for other practice patterns that affected satisfaction ratings.
REDUCING ANTIBIOTIC PRESCRIBING REDUCES RESISTANCE
There is also strong evidence that when clinicians decrease antibiotic prescribing, antimicrobial resistance follows suit. One of the earlier landmark studies to demonstrate this was a Finnish study published in 1997.40 The authors found that a reduction of macrolide antibiotic consumption in Finland led to a reduction in streptococci macrolide resistance from 16.5% to 8.6%.40
Multiple studies have since demonstrated similar results for both respiratory and urinary tract infections.41,42 A 2017 meta-analysis of 32 studies found that antibiotic stewardship programs reduced the incidence of infections and colonization with multidrug-resistant Gram-negative bacteria (by 51%), extended-spectrum beta-lactamase–producing Gram-negative bacteria (48%), and methicillin-resistant Staphylococcus aureus (37%). There was also a reduction in the incidence of CDI (32%).43

Despite universal agreement that antibiotic overprescribing is a problem, the practice continues to vex us. Antibiotic use—whether appropriate or not—has been linked to rising rates of antimicrobial resistance, disruption of the gut microbiome leading to Clostridium difficile infections (CDI), allergic reactions, and increased health care costs (see Table 1).1-6 And yet, clinicians continue to overprescribe this class of medication.

A 2016 report from the CDC estimates that at least 30% of antibiotics prescribed in US outpatient settings are unnecessary.7 Another report cites a slightly higher figure across a variety of health care settings.8 Pair these findings with the fact that there are currently few new drugs in development to target resistant bacteria, and you have the potential for a postantibiotic era in which common infections could become lethal.7
In 2003, the CDC launched its “Get Smart: Know When Antibiotics Work” program (now known as “Be Antibiotics Aware”), focused on decreasing inappropriate antibiotic use in the outpatient setting.9 In 2015, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria, with a goal of decreasing inappropriate outpatient antibiotic use by 50% and inappropriate inpatient use by 20% by 2020.10 And, on an international level, the World Health Organization (WHO) in 2015 developed a five-year strategic framework for implementing its Global Action Plan on Antimicrobial Resistance.11
Family practitioners are on the front lines of this battle. Here’s what we can do now.
WHEN AND WHERE ARE ANTIBIOTICS MOST OFTEN INAPPROPRIATELY PRESCRIBED?
The diagnosis leading to the most frequent inappropriate prescribing of antibiotics is acute respiratory tract infection (ARTI), which includes bronchitis, otitis media, pharyngitis, sinusitis, tonsillitis, the common cold, and pneumonia. Up to 40% of antibiotic prescriptions for these conditions are unnecessary.8,12 Bronchitis is the most common ARTI diagnosis associated with inappropriate antibiotic prescriptions, while sinusitis, suppurative otitis media, and pharyngitis are the diagnoses associated with the lion’s share of all (appropriate and inappropriate) antibiotic prescriptions within the ARTI category.8,9,12,13 Refer to national clinical guidelines, which delineate when antibiotic treatment is appropriate for these conditions.14-16
With respect to setting, there are conflicting findings as to whether antibiotic prescribing differs in office-based versus emergency department (ED) settings.
- One study found a higher rate of antibiotic prescribing during ED visits than office visits (21% vs 9%), even though, between 2007 and 2009, more antibiotic prescriptions were written for adults in primary care offices than in either outpatient hospital clinics or EDs.17
- In a cross-sectional study using data from 2005 to 2010 National Ambulatory Medical Care Surveys (NAMCS) and National Hospital Ambulatory Medical Care Surveys (NHAMCS), more than half of patients with uncomplicated acute rhinosinusitis received a prescription for antibiotics, but there was no overall difference in antibiotic prescriptions between primary care and ED presentation.18
- A retrospective analysis found that between 2006 and 2010, outpatient hospital practices (56%) and community-practice offices (60%) prescribed more antibiotics for ARTIs than EDs did (51%).12
STICK TO NARROW-SPECTRUM AGENTS WHEN POSSIBLE
Using broad-spectrum antibiotics, such as quinolones or imipenem, firstline, contributes more to the problem of antibiotic resistance than does prescribing narrow-spectrum antibiotics such as amoxicillin, cephalexin, or trimethoprim-sulfamethoxazole.7 Yet between 2007 and 2009, broad-spectrum agents were prescribed for 61% of outpatient adult visits in which patients received an antibiotic prescription.17 Quinolones (25%), macrolides (20%), and aminopenicillins (12%) were most commonly prescribed, and antibiotic prescriptions were most often written for respiratory conditions, such as bronchitis, for which we now know antibiotics are rarely indicated.17
Between 2006 and 2008, pediatric patients who received antibiotic prescriptions were given broad-spectrum agents 50% of the time, of which macrolides were the class most commonly prescribed.13
More recently, researchers examined the frequency with which clinicians prescribe narrow-spectrum, firstline antibiotics for otitis media, sinusitis, and pharyngitis using 2010 to 2011 NAMCS/NHAMCS data. They found that providers used firstline agents recommended by professional guidelines 52% of the time, although it was estimated that they would have been appropriate in 80% of cases; pediatric patients were more likely to receive appropriate firstline antibiotics than adult patients.19 Macrolides, especially azithromycin, were the most common non-firstline antibiotics prescribed.19,20 The bottom line is that when antibiotics are indicated for upper respiratory infections (otitis media, sinusitis, and pharyngitis), clinicians should prescribe a narrow-spectrum antibiotic first.
ANTIBIOTIC OVERPRESCIBING AFFECTS THE GUT AND BEYOND
The human intestinal microbiome is composed of a diverse array of bacteria, viruses, and parasites.21 The main functions of the gut microbiome include interacting with the immune system and participating in biochemical reactions in the gut, such as absorption of fat-soluble vitamins and the production of vitamin K.
As we know, antibiotics decrease the diversity of gut bacteria, which, in turn, can cause less efficient nutrient extraction, as well as vulnerability to enteric infections.21 It is well known, for example, that the bacterial gut microbiome can either inhibit or promote diarrheal illnesses such as those caused by CDI. CDI is now the most common health care-related infection, accounting for about a half-million health care facility infections per year.22 It extends hospital stays an average of almost 10 days and is estimated to cost the health care system $6.3 billion annually.23
Antibiotics can also eliminate antibiotic-susceptible organisms, allowing resistant organisms to proliferate.4 They also promote the transmission of genes for antibiotic resistance between gut bacteria.4
Beyond the gut
Less well known is that gut bacteria can promote or inhibit extraintestinal infections.
Gut bacteria and HIV. In early HIV infections, for example, gut populations of Lactobacillus and Bifidobacteria are reduced, and the gut barrier becomes compromised.24 Increasing translocation of bacterial products is associated with HIV disease progression. Preservation of Lactobacillus populations in the gut is associated with markers predictive of better HIV outcomes, including a higher CD4 count, a lower viral load, and less evidence of gut microbial translocation.24 This underscores the importance of maintaining healthy gut flora in patients with HIV, using such steps as avoiding unnecessary antibiotics.
Gut bacteria and stress, depression. Antibiotics directly induce the expression of key genes that affect the stress response.25 While causative studies are lacking, there is a growing body of evidence suggesting that the gut microbiome is involved in two-way communication with the brain and can affect, and be affected by, stress and depression.21,26-30 Diseases and conditions that seem to have a putative connection to a disordered microbiome (dysbiosis) include depression, anxiety, Crohn disease, type 2 diabetes, and obesity. (For a discussion of the relationship between the gut microbiome and diabetes, see Endocrine Consult: The Gut Microbiome in Type 2 Diabetes.)
Gut bacteria and childhood obesity. Repeated use of broader-spectrum antibiotics in children younger than 24 months of age increases the risk for childhood obesity.1,6 One theory for the association is that the effects of broad-spectrum antibiotics on the intestinal flora of young children may alter long-term energy homeostasis, resulting in a higher risk for obesity.1
Gut bacteria and asthma. Studies demonstrate differences in the gut microbiomes of asthmatic and nonasthmatic patients. These differences affect the activities of helper T-cell subsets (Th1 and Th2), which in turn affect the development of immune tolerance.31
Although additional studies are needed to confirm these findings, the evidence collected thus far should make us all pause before prescribing drugs that can alter our microbiome in complex and only partially understood ways.
WHAT CAN WE DO RIGHT NOW?
The issues created by the inappropriate prescribing of antibiotics have been known for decades, and multiple attempts have been made to find solutions and implement change. Although some small successes have occurred, little overall progress has been made in reducing antibiotic prescribing in the general population. A historical review of why clinicians prescribe antibiotics inappropriately and the interventions that have successfully reduced this prescribing may prove valuable as we continue to look for new, effective answers.
Why do we overprescribe antibiotics? A 2015 systematic literature review found that patient demand, pharmaceutical company marketing activities, limited up-to-date information sources, and fear of losing patients are major reasons providers cite for prescribing antibiotics.32
In a separate study that explored antibiotic prescribing habits for acute bronchitis, clinicians cited “patient demand” as the major reason for prescribing antibiotics. Respondents also reported that “other physicians were responsible for inappropriate antibiotic prescribing.”33
Strategies that work
Some early intervention programs directed at reducing antibiotic prescribing demonstrated success (see Table 2).34-36 
One example comes from a 1996-1998 study of four primary care practices.34 Researchers evaluated the impact of a multidimensional intervention effort targeted at clinicians and patients and aimed at lowering the use of antimicrobial agents for acute uncomplicated bronchitis in adults. It incorporated multiple elements, including office-based and household patient educational materials and a clinician intervention involving education, practice profiling, and academic detailing. Clinicians in this program reduced their rates of antibiotic prescribing for uncomplicated bronchitis from 74% to 48%.34
Employing EMRs. A more recent study focused on use of electronic medical records (EMRs) and communications to modify clinician antibiotic prescribing.35 By sending clinicians monthly emails comparing their prescribing patterns to those of peers and “typical top performers,” inappropriate antibiotic prescriptions for ARTIs went from 19.9% to 3.7%.35
In another effort, the same researchers modified providers’ EMRs to detect when potentially inappropriate antibiotics were prescribed. The system then prompted the clinician to provide an “antibiotic justification note,” which remained visible in the patient’s chart. This approach, which encouraged providers to follow prescribing guidelines by capitalizing on their concerns about their reputations, produced a 77% reduction in antibiotic prescribing.35
Focusing on the public. Studies have also examined the effectiveness of educating the public about when antibiotics are not likely to be helpful and of the harms of unnecessary antibiotics.
Studies conducted in Tennessee and Wisconsin that combined prescriber and community education about unnecessary antibiotics for children found that the intervention reduced antibiotic prescribing in both locations by about 19%, compared with about a 9% reduction in the control groups.36,37
DOES PRESCRIBING ANTIBIOTICS AFFECT PATIENT SATISFACTION?
The results are mixed as to whether prescribing antibiotics affects patient satisfaction. Two studies in the early 2000s found that both patients and parents reported higher satisfaction with clinicians who explained why antibiotics were not indicated versus those who simply prescribed them—and that such explanations do not need to take a lot of time (see Table 3 for patient care tips).37,38
A more recent study found that higher antibiotic prescribing practices in Britain were associated with modestly higher patient satisfaction ratings.39 The authors of this study noted, however, that reduced antibiotic prescribing may be a proxy for other practice patterns that affected satisfaction ratings.
REDUCING ANTIBIOTIC PRESCRIBING REDUCES RESISTANCE
There is also strong evidence that when clinicians decrease antibiotic prescribing, antimicrobial resistance follows suit. One of the earlier landmark studies to demonstrate this was a Finnish study published in 1997.40 The authors found that a reduction of macrolide antibiotic consumption in Finland led to a reduction in streptococci macrolide resistance from 16.5% to 8.6%.40
Multiple studies have since demonstrated similar results for both respiratory and urinary tract infections.41,42 A 2017 meta-analysis of 32 studies found that antibiotic stewardship programs reduced the incidence of infections and colonization with multidrug-resistant Gram-negative bacteria (by 51%), extended-spectrum beta-lactamase–producing Gram-negative bacteria (48%), and methicillin-resistant Staphylococcus aureus (37%). There was also a reduction in the incidence of CDI (32%).43
1. Bailey LC, Forrest CB, Zhang P, et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069.
2. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
3. Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000;21:53-60.
4. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
5. Logan AC, Jacka FN, Craig JM, et al. The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol Neurosci. 2016;14:131-147.
6. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010.
7. Harris AM, Hicks LA, Qaseem A; the High Value Care Task Force of the American College of Physicians and the CDC. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016; 164:425-434.
8. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315: 1864-1873.
9. CDC. Antibiotic prescribing and use. www.cdc.gov/antibiotic-use/index.html. Accessed January 16, 2018.
10. The White House. National action plan for combating antibiotic-resistant bacteria. March 2015:1-63. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed January 16, 2018.
11. World Health Organization. Global action plan on antimicrobial resistance (2015). www.who.int/antimicrobial-resistance/global-action-plan/en/. Accessed January 16, 2018.
12. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
13. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061.
14. Chow AW, Benninger MS, Brook I, et al. Executive summary: IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:1041-1045.
15. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
16. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
17. Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother. 2014;69:234-240.
18. Bergmark RW, Sedaghat AR. Antibiotic prescription for acute rhinosinusitis: emergency departments versus primary care providers. Laryngoscope. 2016;126:2439-2444.
19. Hersh AL, Fleming-Dutra KE, Shapiro DJ, et al. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870-1872.
20. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308-1316.
21. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39.
22. Lessa FC, Gould CV, McDonald CL. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(suppl 2):S65-S70.
23. Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447.
24. Pérez-Santiago J, Gianella S, Massanella M, et al. Gut lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
25. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50.
26. Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667-672.
27. Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258-1270.
28. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369-1378.
29. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312.
30. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014; 38:1-12.31. Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. 2015;11:35.
32. Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665-680.
33. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract . 2014;15:194.
34. Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice. JAMA . 1999;281:1512-1519.
35. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA . 2016;315:562-570.
36. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA . 2002;287:3103-3109.
37. Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics . 2001;108:575-583.
38. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155:800-806.
39. Ashworth M, White P, Jongsma H, et al. Antibiotic prescribing and patient satisfaction in primary care in England: cross-sectional analysis of national patient survey data and prescribing data. Br J Gen Pract . 2016;66:e40-e46.
40. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441-446.
41. Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin g–nonsusceptible Streptococcus pneumoniae . Clin Infect Dis. 2005;41:930-938.
42. Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007; 57:785-792.
43. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990-1001.
1. Bailey LC, Forrest CB, Zhang P, et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069.
2. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
3. Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000;21:53-60.
4. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
5. Logan AC, Jacka FN, Craig JM, et al. The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol Neurosci. 2016;14:131-147.
6. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010.
7. Harris AM, Hicks LA, Qaseem A; the High Value Care Task Force of the American College of Physicians and the CDC. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016; 164:425-434.
8. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315: 1864-1873.
9. CDC. Antibiotic prescribing and use. www.cdc.gov/antibiotic-use/index.html. Accessed January 16, 2018.
10. The White House. National action plan for combating antibiotic-resistant bacteria. March 2015:1-63. https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed January 16, 2018.
11. World Health Organization. Global action plan on antimicrobial resistance (2015). www.who.int/antimicrobial-resistance/global-action-plan/en/. Accessed January 16, 2018.
12. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
13. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061.
14. Chow AW, Benninger MS, Brook I, et al. Executive summary: IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:1041-1045.
15. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 suppl):S1-S39.
16. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
17. Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother. 2014;69:234-240.
18. Bergmark RW, Sedaghat AR. Antibiotic prescription for acute rhinosinusitis: emergency departments versus primary care providers. Laryngoscope. 2016;126:2439-2444.
19. Hersh AL, Fleming-Dutra KE, Shapiro DJ, et al. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870-1872.
20. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308-1316.
21. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39.
22. Lessa FC, Gould CV, McDonald CL. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(suppl 2):S65-S70.
23. Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447.
24. Pérez-Santiago J, Gianella S, Massanella M, et al. Gut lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
25. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50.
26. Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667-672.
27. Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258-1270.
28. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369-1378.
29. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312.
30. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014; 38:1-12.31. Riiser A. The human microbiome, asthma, and allergy. Allergy Asthma Clin Immunol. 2015;11:35.
32. Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665-680.
33. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract . 2014;15:194.
34. Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice. JAMA . 1999;281:1512-1519.
35. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA . 2016;315:562-570.
36. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA . 2002;287:3103-3109.
37. Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics . 2001;108:575-583.
38. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155:800-806.
39. Ashworth M, White P, Jongsma H, et al. Antibiotic prescribing and patient satisfaction in primary care in England: cross-sectional analysis of national patient survey data and prescribing data. Br J Gen Pract . 2016;66:e40-e46.
40. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441-446.
41. Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin g–nonsusceptible Streptococcus pneumoniae . Clin Infect Dis. 2005;41:930-938.
42. Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007; 57:785-792.
43. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990-1001.
Antibiotic overprescribing: Still a major concern
Despite universal agreement that antibiotic overprescribing is a problem, the practice continues to vex us. Antibiotic use—whether appropriate or not—has been linked to rising rates of antimicrobial resistance, disruption of the gut microbiome leading to Clostridium difficile infections, allergic reactions, and increased health care costs (TABLE 11-6). And yet, physicians continue to overprescribe this class of medication.
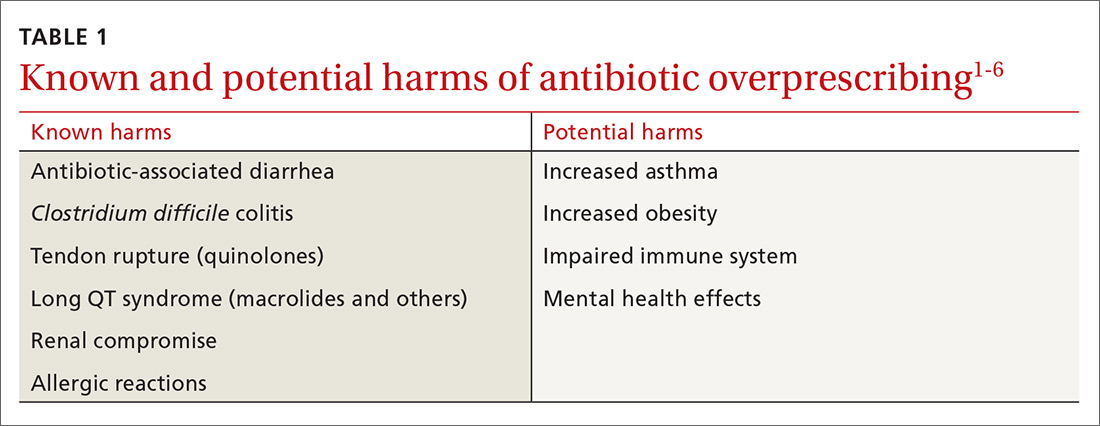
A 2016 Centers for Disease Control and Prevention (CDC) report estimates that at least 30% of antibiotics prescribed in US outpatient settings are unnecessary.7 Another report cites a slightly higher figure across a variety of health care settings.8 Pair these findings with the fact that there are currently few new drugs in development to target resistant bacteria, and you have the potential for a post-antibiotic era in which common infections could become lethal.7
In 2003, the CDC launched its “Get Smart: Know When Antibiotics Work” program, focused on decreasing inappropriate antibiotic use in the outpatient setting.9 In 2014, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria with a goal of decreasing inappropriate outpatient antibiotic use by 50% and inappropriate inpatient use by 20% by 2020.10 And, on an international level, the World Health Organization (WHO) developed a 5-year strategic framework in 2015 for implementing its Global Action Plan on Antimicrobial Resistance.11
Family practitioners are on the front lines of this battle. Here’s what we can do now.
[polldaddy:9885811]
When and where are antibiotics most often inappropriately prescribed?
The diagnosis leading to the most frequent inappropriate prescribing of antibiotics is acute respiratory tract infection (ARTI), which includes bronchitis, otitis media, pharyngitis, sinusitis, tonsillitis, the common cold, and pneumonia. Up to 40% of antibiotic prescriptions for these conditions are unnecessary.8,12 Bronchitis is the most common ARTI diagnosis associated with inappropriate antibiotic prescriptions, while sinusitis, suppurative otitis media, and pharyngitis are the diagnoses associated with the lion’s share of all (appropriate and inappropriate) antibiotic prescriptions within the ARTI category.8,9,12,13 There are national clinical guidelines delineating when antibiotic treatment is appropriate for these conditions.14-16
With respect to setting, studies have presented conflicting results as to whether there is a difference between antibiotic prescribing in office-based vs emergency department (ED) settings. Here is a sample of some of the literature to date:
- One study found a higher rate of antibiotic prescribing during ED visits (21%) than office visits (9%), despite the fact that between 2007 and 2009, more antibiotic prescriptions were written for adults in primary care offices than in either outpatient hospital clinics or EDs.17
- A cross-sectional study focused on the frequency with which antibiotics were prescribed for uncomplicated acute rhinosinusitis. Researchers analyzed data from 2005 to 2010 National Ambulatory Medical Care Surveys (NAMCS) and National Hospital Ambulatory Medical Care Surveys (NHAMCS) and found that more than half of the patients received prescriptions for antibiotics, but that there was no overall difference in antibiotic prescriptions between primary care and ED presentation.18
- A retrospective analysis that examined antibiotic prescribing found that between 2006 and 2010, outpatient hospital practices (56%) and community-practice offices (60%) prescribed more antibiotics for ARTIs than EDs (51%).12
Stick to narrow-spectrum agents when possible
Using broad-spectrum antibiotics, such as quinolones or imipenem, first line, contributes more to the problem of antibiotic resistance than does prescribing narrow-spectrum antibiotics such as amoxicillin, cephalexin, or trimethoprim-sulfamethoxazole.7 Yet between 2007 and 2009, broad-spectrum agents were prescribed for 61% of outpatient adult visits in which patients received an antibiotic prescription.17 Quinolones (25%), macrolides (20%), and aminopenicillins (12%) were most commonly prescribed, and antibiotic prescriptions were most often written for respiratory conditions, such as bronchitis, for which we now know antibiotics are rarely indicated.17
Between 2006 and 2008, pediatric patients who received antibiotic prescriptions were given broad-spectrum agents 50% of the time, of which macrolides were the class most commonly prescribed.13
More recently, researchers examined the frequency with which physicians prescribe narrow-spectrum, first-line antibiotics for otitis media, sinusitis, and pharyngitis using 2010 to 2011 NAMCS/NHAMCS data. They found that physicians used first-line agents recommended by professional guidelines 52% of the time, although it was estimated that they would have been appropriate in 80% of cases; pediatric patients were more likely to receive appropriate first-line antibiotics than adult patients.19 Macrolides, especially azithromycin, were the most common non–first-line antibiotics prescribed.19,20 The bottom line is that when antibiotics are indicated for upper respiratory infections (otitis media, sinusitis, and pharyngitis), physicians should prescribe a narrow-spectrum antibiotic first.
Antibiotic overprescribing affects the gut and beyond
The human intestinal microbiome is composed of a diverse array of bacteria, viruses, and parasites.21 The main functions of the gut microbiome include interacting with the immune system and participating in biochemical reactions in the gut, such as absorption of fat-soluble vitamins and the production of vitamin K.
As we know, antibiotics decrease the diversity of gut bacteria, which, in turn, can cause less efficient nutrient extraction, as well as a vulnerability to enteric infections.21 It is well known, for example, that the bacterial gut microbiome can either inhibit or promote diarrheal illnesses such as those caused by C. difficile. C. difficile infection (CDI) is now the most common health care-related infection, accounting for approximately a half million health care facility infections a year.22 CDI extends hospital stays an average of almost 10 days and is estimated to cost the health care system $6.3 billion annually.23
Antibiotics can also eliminate antibiotic-susceptible organisms, allowing resistant organisms to proliferate.4 They also promote the transmission of genes for antibiotic resistance between gut bacteria.4
Beyond the gut
Less well known is that gut bacteria can promote or inhibit extraintestinal infections.
Gut bacteria and HIV. In early human immunodeficiency virus (HIV) infections, for example, gut populations of Lactobacillus and Bifidobacteria are reduced, and the gut barrier becomes compromised.24 Increasing translocation of bacterial products is associated with HIV disease progression. Preservation of Lactobacillus populations in the gut is associated with markers predictive of better HIV outcomes, including a higher CD4 count, a lower viral load, and less evidence of gut microbial translocation.24 This underscores the importance of maintaining a healthy gut flora in patients with HIV, using such steps as avoiding unnecessary antibiotics.
Gut bacteria and stress, depression. Antibiotics directly induce the expression of key genes that affect the stress response.25 While causative studies are lacking, there is a growing body of evidence suggesting that the gut microbiome is involved in 2-way communication with the brain and can affect, and be affected by, stress and depression.21,26-30 Diseases and conditions that seem to have a putative connection to a disordered microbiome (dysbiosis) include depression, anxiety, Crohn’s disease, type 2 diabetes, and obesity.
Gut bacteria and childhood obesity. Repeated use of broader-spectrum antibiotics in children <24 months of age increases the risk of developing childhood obesity.1,6 One theory for the association is that the effects of broad-spectrum antibiotics on the intestinal flora of young children may alter long-term energy homeostasis resulting in a higher risk for obesity.1
Gut bacteria and asthma. Studies demonstrate differences in the gut microbiome of asthmatic and nonasthmatic patients. These differences affect the activities of helper T-cell subsets (Th1 and Th2), which in turn affect the development of immune tolerance.31
Although additional studies are needed to confirm these findings, the evidence collected thus far should make us all pause before prescribing drugs that can alter our microbiome in complex and only partially understood ways.

What can we do right now?
The issues created by the inappropriate prescribing of antibiotics have been known for decades, and multiple attempts have been made to find solutions and implement change. Although some small successes have occurred, little overall progress has been made in reducing antibiotic prescribing in the general population. A historical review of why physicians prescribe antibiotics inappropriately and the interventions that have successfully reduced this prescribing may prove valuable as we continue to look for new, effective answers.
Why do we overprescribe antibiotics? A 2015 systematic literature review found that patient demand, pharmaceutical company marketing activities, limited up-to-date information sources, and physician fear of losing their patients are major reasons physicians cite for prescribing antibiotics.32
In a separate study that explored antibiotic prescribing habits for acute bronchitis,33 clinicians cited “patient demand” as the major reason for prescribing antibiotics. Respondents also reported that “other physicians were responsible for inappropriate antibiotic prescribing.”33
Strategies that work
Some early intervention programs directed at reducing antibiotic prescribing demonstrated success (TABLE 2).34-36 One example comes from a 1996 to 1998 study of 4 primary care practices.34 Researchers evaluated the impact of a multidimensional intervention effort targeted at clinicians and patients and aimed at lowering the use of antimicrobial agents for acute uncomplicated bronchitis in adults. It incorporated a number of elements, including office-based and household patient educational materials, and a clinician intervention involving education, practice profiling, and academic detailing. Physicians in this program reduced their rates of antibiotic prescribing for uncomplicated bronchitis from 74% to 48%.34
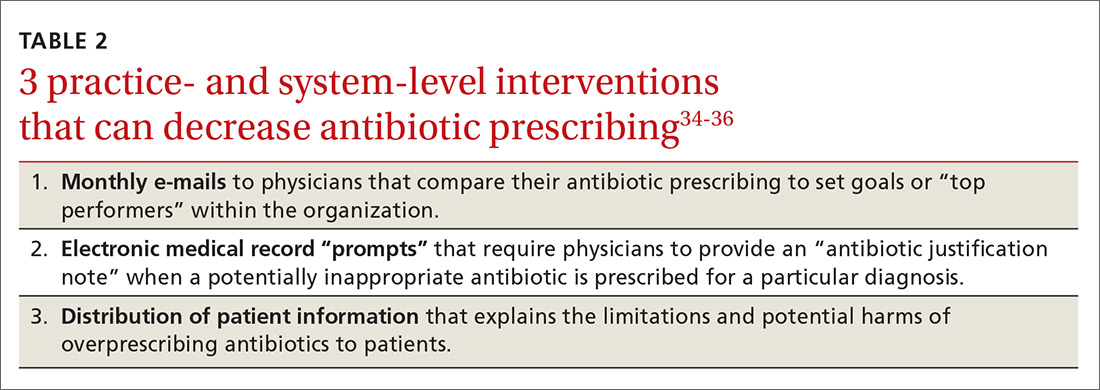
Employing EMRs. A more recent study focused on using electronic medical records (EMRs) and communications to modify physician antibiotic prescribing.35 By sending physicians monthly emails comparing their prescribing patterns to peers and “typical top performers,” inappropriate antibiotic prescriptions for ARTIs went from 19.9% to 3.7%.35
In another effort, the same researchers modified physicians’ EMRs to detect when potentially inappropriate antibiotics were prescribed. The system then prompted the physician to provide an “antibiotic justification note,” which remained visible in the patient’s chart. This approach, which encouraged physicians to follow prescribing guidelines by taking advantage of their concerns about their reputations, produced a 77% reduction in antibiotic prescribing.35
Focusing on the public. Studies have also examined the effectiveness of educating the public about when antibiotics are not likely to be helpful and of the harms of unnecessary antibiotics. Studies conducted in Tennessee and Wisconsin that combined prescriber and community education about unnecessary antibiotics for children found that the intervention reduced antibiotic prescribing in both locations by about 19% compared with about a 9% reduction in the control groups.36,37
Does prescribing antibiotics affect patient satisfaction?
The results are mixed as to whether prescribing antibiotics affects patient satisfaction. Two studies in the early 2000s found that both patients and parents reported higher satisfaction with physicians who explained why antibiotics were not indicated vs physicians who simply prescribed them, and that such explanations do not need to take a lot of time.37,38 (See TABLE 39,37,38 for patient care tips.)
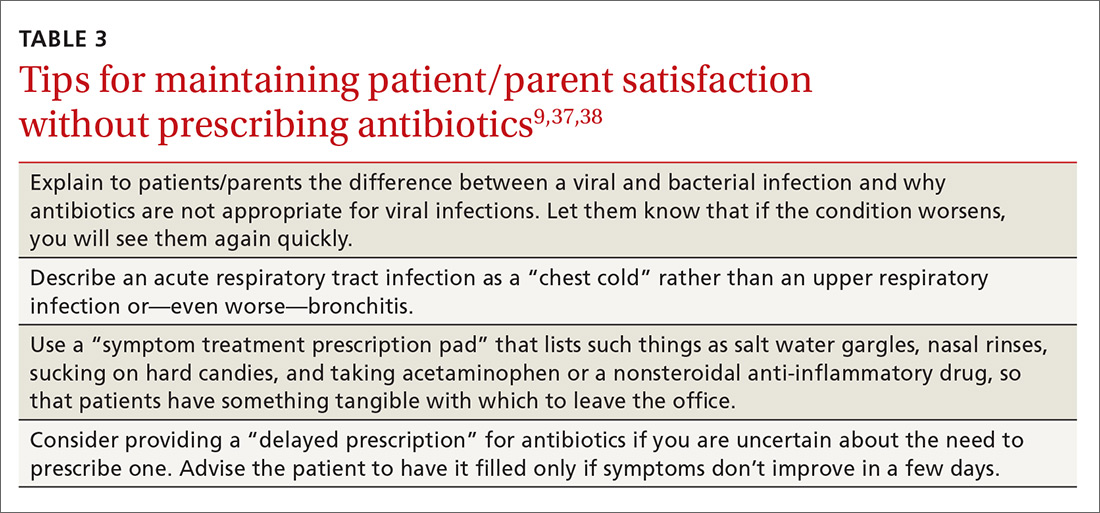
A more recent study found that higher antibiotic prescribing practices in Britain were associated with modestly higher patient satisfaction ratings.39 The authors of this study noted, however, that reduced antibiotic prescribing may be a proxy for other practice patterns that affected satisfaction ratings.
Reducing antibiotic prescribing reduces resistance
There is also strong evidence that when physicians decrease antibiotic prescribing, antimicrobial resistance follows suit. One of the earlier landmark studies to demonstrate this was a Finnish study published in 1997.40 The authors found that a reduction of macrolide antibiotic consumption in Finland led to a reduction in streptococci macrolide resistance from 16.5% to 8.6%.40
Since then, multiple studies have demonstrated similar results for both respiratory and urinary tract infections.41,42 A 2017 meta-analysis analyzing 32 studies found that antibiotic stewardship programs reduced the incidence of infections and colonization with multidrug-resistant Gram-negative bacteria (51% reduction), extended-spectrum beta-lactamase–producing Gram-negative bacteria (48%), and methicillin-resistant Staphylococcus aureus (37%). There was also a reduction in the incidence of C. difficile infections (32%).43
CORRESPONDENCE
David C. Fiore, MD, Department of Family and Community Medicine, University of Nevada, Reno School of Medicine, Brigham Bldg, MS 316, Reno, NV 89557; [email protected].
1. Bailey LC, Forrest CB, Zhang P, et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069.
2. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
3. Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000;21:53-60.
4. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
5. Logan AC, Jacka FN, Craig JM, et al. The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol Neurosci. 2016;14:131-147.
6. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010.
7. Harris AM, Hicks LA, Qaseem A, for the High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
8. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
9. Centers for Disease Control and Prevention. Antibiotic prescribing and use. Available at: http://www.cdc.gov/getsmart/. Accessed October 23, 2017.
10. The White House. National action plan for combating antibiotic-resistant bacteria. March 2015:1-63. Available at: https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed October 23, 2017.
11. World Health Organization. Global action plan on antimicrobial resistance. 2015. Available at: http://www.who.int/drugresistance/global_action_plan/en/. Accessed October 23, 2017.
12. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
13. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061.
14. Chow AW, Benninger MS, Brook I, et al. Executive summary: IDSA Clinical Practice Guideline for Acute Bacterial Rhinosinusitis in Children and Adults. Clin Infect Dis. 2012;54:1041-1045.
15. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 Suppl):S1-S39.
16. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
17. Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother. 2014;69:234-240.
18. Bergmark RW, Sedaghat AR. Antibiotic prescription for acute rhinosinusitis: emergency departments versus primary care providers. Laryngoscope. 2016;(November):1-6.
19. Hersh AL, Fleming-Dutra KE, Shapiro DJ, et al. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870-1872.
20. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308-1316.
21. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39.
22. Lessa FC, Gould CV, McDonald CL. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65-S70.
23. Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447.
24. Pérez-Santiago J, Gianella S, Massanella M, et al. Gut lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
25. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50.
26. Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667-672.
27. Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: An integrative view. Cell. 2012;148:1258-1270.
28. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369-1378.
29. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312.
30. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1-12.
31. Riiser A. The human microbiome, asthma, and allergy. Allergy, Asthma, and Clinical Immunology. 2015;11:35.
32. Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665-680.
33. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract. 2014;15:194.
34. Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice. JAMA. 1999;281:1512-1519.
35. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315:562-570.
36. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287:3103-3109.
37. Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108:575-583.
38. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155:800-806.
39. Ashworth M, White P, Jongsma H,et al. Antibiotic prescribing and patient satisfaction in primary care in England: cross-sectional analysis of national patient survey data and prescribing data. Br J Gen Pract. 2016;66:e40-e46.
40. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441-446.
41. Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin g–nonsusceptible Streptococcus pneumoniae. Clin Infect Dis. 2005;41:930-938.
42. Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007;57:785-792.
43. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990-1001.
Despite universal agreement that antibiotic overprescribing is a problem, the practice continues to vex us. Antibiotic use—whether appropriate or not—has been linked to rising rates of antimicrobial resistance, disruption of the gut microbiome leading to Clostridium difficile infections, allergic reactions, and increased health care costs (TABLE 11-6). And yet, physicians continue to overprescribe this class of medication.

A 2016 Centers for Disease Control and Prevention (CDC) report estimates that at least 30% of antibiotics prescribed in US outpatient settings are unnecessary.7 Another report cites a slightly higher figure across a variety of health care settings.8 Pair these findings with the fact that there are currently few new drugs in development to target resistant bacteria, and you have the potential for a post-antibiotic era in which common infections could become lethal.7
In 2003, the CDC launched its “Get Smart: Know When Antibiotics Work” program, focused on decreasing inappropriate antibiotic use in the outpatient setting.9 In 2014, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria with a goal of decreasing inappropriate outpatient antibiotic use by 50% and inappropriate inpatient use by 20% by 2020.10 And, on an international level, the World Health Organization (WHO) developed a 5-year strategic framework in 2015 for implementing its Global Action Plan on Antimicrobial Resistance.11
Family practitioners are on the front lines of this battle. Here’s what we can do now.
[polldaddy:9885811]
When and where are antibiotics most often inappropriately prescribed?
The diagnosis leading to the most frequent inappropriate prescribing of antibiotics is acute respiratory tract infection (ARTI), which includes bronchitis, otitis media, pharyngitis, sinusitis, tonsillitis, the common cold, and pneumonia. Up to 40% of antibiotic prescriptions for these conditions are unnecessary.8,12 Bronchitis is the most common ARTI diagnosis associated with inappropriate antibiotic prescriptions, while sinusitis, suppurative otitis media, and pharyngitis are the diagnoses associated with the lion’s share of all (appropriate and inappropriate) antibiotic prescriptions within the ARTI category.8,9,12,13 There are national clinical guidelines delineating when antibiotic treatment is appropriate for these conditions.14-16
With respect to setting, studies have presented conflicting results as to whether there is a difference between antibiotic prescribing in office-based vs emergency department (ED) settings. Here is a sample of some of the literature to date:
- One study found a higher rate of antibiotic prescribing during ED visits (21%) than office visits (9%), despite the fact that between 2007 and 2009, more antibiotic prescriptions were written for adults in primary care offices than in either outpatient hospital clinics or EDs.17
- A cross-sectional study focused on the frequency with which antibiotics were prescribed for uncomplicated acute rhinosinusitis. Researchers analyzed data from 2005 to 2010 National Ambulatory Medical Care Surveys (NAMCS) and National Hospital Ambulatory Medical Care Surveys (NHAMCS) and found that more than half of the patients received prescriptions for antibiotics, but that there was no overall difference in antibiotic prescriptions between primary care and ED presentation.18
- A retrospective analysis that examined antibiotic prescribing found that between 2006 and 2010, outpatient hospital practices (56%) and community-practice offices (60%) prescribed more antibiotics for ARTIs than EDs (51%).12
Stick to narrow-spectrum agents when possible
Using broad-spectrum antibiotics, such as quinolones or imipenem, first line, contributes more to the problem of antibiotic resistance than does prescribing narrow-spectrum antibiotics such as amoxicillin, cephalexin, or trimethoprim-sulfamethoxazole.7 Yet between 2007 and 2009, broad-spectrum agents were prescribed for 61% of outpatient adult visits in which patients received an antibiotic prescription.17 Quinolones (25%), macrolides (20%), and aminopenicillins (12%) were most commonly prescribed, and antibiotic prescriptions were most often written for respiratory conditions, such as bronchitis, for which we now know antibiotics are rarely indicated.17
Between 2006 and 2008, pediatric patients who received antibiotic prescriptions were given broad-spectrum agents 50% of the time, of which macrolides were the class most commonly prescribed.13
More recently, researchers examined the frequency with which physicians prescribe narrow-spectrum, first-line antibiotics for otitis media, sinusitis, and pharyngitis using 2010 to 2011 NAMCS/NHAMCS data. They found that physicians used first-line agents recommended by professional guidelines 52% of the time, although it was estimated that they would have been appropriate in 80% of cases; pediatric patients were more likely to receive appropriate first-line antibiotics than adult patients.19 Macrolides, especially azithromycin, were the most common non–first-line antibiotics prescribed.19,20 The bottom line is that when antibiotics are indicated for upper respiratory infections (otitis media, sinusitis, and pharyngitis), physicians should prescribe a narrow-spectrum antibiotic first.
Antibiotic overprescribing affects the gut and beyond
The human intestinal microbiome is composed of a diverse array of bacteria, viruses, and parasites.21 The main functions of the gut microbiome include interacting with the immune system and participating in biochemical reactions in the gut, such as absorption of fat-soluble vitamins and the production of vitamin K.
As we know, antibiotics decrease the diversity of gut bacteria, which, in turn, can cause less efficient nutrient extraction, as well as a vulnerability to enteric infections.21 It is well known, for example, that the bacterial gut microbiome can either inhibit or promote diarrheal illnesses such as those caused by C. difficile. C. difficile infection (CDI) is now the most common health care-related infection, accounting for approximately a half million health care facility infections a year.22 CDI extends hospital stays an average of almost 10 days and is estimated to cost the health care system $6.3 billion annually.23
Antibiotics can also eliminate antibiotic-susceptible organisms, allowing resistant organisms to proliferate.4 They also promote the transmission of genes for antibiotic resistance between gut bacteria.4
Beyond the gut
Less well known is that gut bacteria can promote or inhibit extraintestinal infections.
Gut bacteria and HIV. In early human immunodeficiency virus (HIV) infections, for example, gut populations of Lactobacillus and Bifidobacteria are reduced, and the gut barrier becomes compromised.24 Increasing translocation of bacterial products is associated with HIV disease progression. Preservation of Lactobacillus populations in the gut is associated with markers predictive of better HIV outcomes, including a higher CD4 count, a lower viral load, and less evidence of gut microbial translocation.24 This underscores the importance of maintaining a healthy gut flora in patients with HIV, using such steps as avoiding unnecessary antibiotics.
Gut bacteria and stress, depression. Antibiotics directly induce the expression of key genes that affect the stress response.25 While causative studies are lacking, there is a growing body of evidence suggesting that the gut microbiome is involved in 2-way communication with the brain and can affect, and be affected by, stress and depression.21,26-30 Diseases and conditions that seem to have a putative connection to a disordered microbiome (dysbiosis) include depression, anxiety, Crohn’s disease, type 2 diabetes, and obesity.
Gut bacteria and childhood obesity. Repeated use of broader-spectrum antibiotics in children <24 months of age increases the risk of developing childhood obesity.1,6 One theory for the association is that the effects of broad-spectrum antibiotics on the intestinal flora of young children may alter long-term energy homeostasis resulting in a higher risk for obesity.1
Gut bacteria and asthma. Studies demonstrate differences in the gut microbiome of asthmatic and nonasthmatic patients. These differences affect the activities of helper T-cell subsets (Th1 and Th2), which in turn affect the development of immune tolerance.31
Although additional studies are needed to confirm these findings, the evidence collected thus far should make us all pause before prescribing drugs that can alter our microbiome in complex and only partially understood ways.

What can we do right now?
The issues created by the inappropriate prescribing of antibiotics have been known for decades, and multiple attempts have been made to find solutions and implement change. Although some small successes have occurred, little overall progress has been made in reducing antibiotic prescribing in the general population. A historical review of why physicians prescribe antibiotics inappropriately and the interventions that have successfully reduced this prescribing may prove valuable as we continue to look for new, effective answers.
Why do we overprescribe antibiotics? A 2015 systematic literature review found that patient demand, pharmaceutical company marketing activities, limited up-to-date information sources, and physician fear of losing their patients are major reasons physicians cite for prescribing antibiotics.32
In a separate study that explored antibiotic prescribing habits for acute bronchitis,33 clinicians cited “patient demand” as the major reason for prescribing antibiotics. Respondents also reported that “other physicians were responsible for inappropriate antibiotic prescribing.”33
Strategies that work
Some early intervention programs directed at reducing antibiotic prescribing demonstrated success (TABLE 2).34-36 One example comes from a 1996 to 1998 study of 4 primary care practices.34 Researchers evaluated the impact of a multidimensional intervention effort targeted at clinicians and patients and aimed at lowering the use of antimicrobial agents for acute uncomplicated bronchitis in adults. It incorporated a number of elements, including office-based and household patient educational materials, and a clinician intervention involving education, practice profiling, and academic detailing. Physicians in this program reduced their rates of antibiotic prescribing for uncomplicated bronchitis from 74% to 48%.34

Employing EMRs. A more recent study focused on using electronic medical records (EMRs) and communications to modify physician antibiotic prescribing.35 By sending physicians monthly emails comparing their prescribing patterns to peers and “typical top performers,” inappropriate antibiotic prescriptions for ARTIs went from 19.9% to 3.7%.35
In another effort, the same researchers modified physicians’ EMRs to detect when potentially inappropriate antibiotics were prescribed. The system then prompted the physician to provide an “antibiotic justification note,” which remained visible in the patient’s chart. This approach, which encouraged physicians to follow prescribing guidelines by taking advantage of their concerns about their reputations, produced a 77% reduction in antibiotic prescribing.35
Focusing on the public. Studies have also examined the effectiveness of educating the public about when antibiotics are not likely to be helpful and of the harms of unnecessary antibiotics. Studies conducted in Tennessee and Wisconsin that combined prescriber and community education about unnecessary antibiotics for children found that the intervention reduced antibiotic prescribing in both locations by about 19% compared with about a 9% reduction in the control groups.36,37
Does prescribing antibiotics affect patient satisfaction?
The results are mixed as to whether prescribing antibiotics affects patient satisfaction. Two studies in the early 2000s found that both patients and parents reported higher satisfaction with physicians who explained why antibiotics were not indicated vs physicians who simply prescribed them, and that such explanations do not need to take a lot of time.37,38 (See TABLE 39,37,38 for patient care tips.)

A more recent study found that higher antibiotic prescribing practices in Britain were associated with modestly higher patient satisfaction ratings.39 The authors of this study noted, however, that reduced antibiotic prescribing may be a proxy for other practice patterns that affected satisfaction ratings.
Reducing antibiotic prescribing reduces resistance
There is also strong evidence that when physicians decrease antibiotic prescribing, antimicrobial resistance follows suit. One of the earlier landmark studies to demonstrate this was a Finnish study published in 1997.40 The authors found that a reduction of macrolide antibiotic consumption in Finland led to a reduction in streptococci macrolide resistance from 16.5% to 8.6%.40
Since then, multiple studies have demonstrated similar results for both respiratory and urinary tract infections.41,42 A 2017 meta-analysis analyzing 32 studies found that antibiotic stewardship programs reduced the incidence of infections and colonization with multidrug-resistant Gram-negative bacteria (51% reduction), extended-spectrum beta-lactamase–producing Gram-negative bacteria (48%), and methicillin-resistant Staphylococcus aureus (37%). There was also a reduction in the incidence of C. difficile infections (32%).43
CORRESPONDENCE
David C. Fiore, MD, Department of Family and Community Medicine, University of Nevada, Reno School of Medicine, Brigham Bldg, MS 316, Reno, NV 89557; [email protected].
Despite universal agreement that antibiotic overprescribing is a problem, the practice continues to vex us. Antibiotic use—whether appropriate or not—has been linked to rising rates of antimicrobial resistance, disruption of the gut microbiome leading to Clostridium difficile infections, allergic reactions, and increased health care costs (TABLE 11-6). And yet, physicians continue to overprescribe this class of medication.

A 2016 Centers for Disease Control and Prevention (CDC) report estimates that at least 30% of antibiotics prescribed in US outpatient settings are unnecessary.7 Another report cites a slightly higher figure across a variety of health care settings.8 Pair these findings with the fact that there are currently few new drugs in development to target resistant bacteria, and you have the potential for a post-antibiotic era in which common infections could become lethal.7
In 2003, the CDC launched its “Get Smart: Know When Antibiotics Work” program, focused on decreasing inappropriate antibiotic use in the outpatient setting.9 In 2014, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria with a goal of decreasing inappropriate outpatient antibiotic use by 50% and inappropriate inpatient use by 20% by 2020.10 And, on an international level, the World Health Organization (WHO) developed a 5-year strategic framework in 2015 for implementing its Global Action Plan on Antimicrobial Resistance.11
Family practitioners are on the front lines of this battle. Here’s what we can do now.
[polldaddy:9885811]
When and where are antibiotics most often inappropriately prescribed?
The diagnosis leading to the most frequent inappropriate prescribing of antibiotics is acute respiratory tract infection (ARTI), which includes bronchitis, otitis media, pharyngitis, sinusitis, tonsillitis, the common cold, and pneumonia. Up to 40% of antibiotic prescriptions for these conditions are unnecessary.8,12 Bronchitis is the most common ARTI diagnosis associated with inappropriate antibiotic prescriptions, while sinusitis, suppurative otitis media, and pharyngitis are the diagnoses associated with the lion’s share of all (appropriate and inappropriate) antibiotic prescriptions within the ARTI category.8,9,12,13 There are national clinical guidelines delineating when antibiotic treatment is appropriate for these conditions.14-16
With respect to setting, studies have presented conflicting results as to whether there is a difference between antibiotic prescribing in office-based vs emergency department (ED) settings. Here is a sample of some of the literature to date:
- One study found a higher rate of antibiotic prescribing during ED visits (21%) than office visits (9%), despite the fact that between 2007 and 2009, more antibiotic prescriptions were written for adults in primary care offices than in either outpatient hospital clinics or EDs.17
- A cross-sectional study focused on the frequency with which antibiotics were prescribed for uncomplicated acute rhinosinusitis. Researchers analyzed data from 2005 to 2010 National Ambulatory Medical Care Surveys (NAMCS) and National Hospital Ambulatory Medical Care Surveys (NHAMCS) and found that more than half of the patients received prescriptions for antibiotics, but that there was no overall difference in antibiotic prescriptions between primary care and ED presentation.18
- A retrospective analysis that examined antibiotic prescribing found that between 2006 and 2010, outpatient hospital practices (56%) and community-practice offices (60%) prescribed more antibiotics for ARTIs than EDs (51%).12
Stick to narrow-spectrum agents when possible
Using broad-spectrum antibiotics, such as quinolones or imipenem, first line, contributes more to the problem of antibiotic resistance than does prescribing narrow-spectrum antibiotics such as amoxicillin, cephalexin, or trimethoprim-sulfamethoxazole.7 Yet between 2007 and 2009, broad-spectrum agents were prescribed for 61% of outpatient adult visits in which patients received an antibiotic prescription.17 Quinolones (25%), macrolides (20%), and aminopenicillins (12%) were most commonly prescribed, and antibiotic prescriptions were most often written for respiratory conditions, such as bronchitis, for which we now know antibiotics are rarely indicated.17
Between 2006 and 2008, pediatric patients who received antibiotic prescriptions were given broad-spectrum agents 50% of the time, of which macrolides were the class most commonly prescribed.13
More recently, researchers examined the frequency with which physicians prescribe narrow-spectrum, first-line antibiotics for otitis media, sinusitis, and pharyngitis using 2010 to 2011 NAMCS/NHAMCS data. They found that physicians used first-line agents recommended by professional guidelines 52% of the time, although it was estimated that they would have been appropriate in 80% of cases; pediatric patients were more likely to receive appropriate first-line antibiotics than adult patients.19 Macrolides, especially azithromycin, were the most common non–first-line antibiotics prescribed.19,20 The bottom line is that when antibiotics are indicated for upper respiratory infections (otitis media, sinusitis, and pharyngitis), physicians should prescribe a narrow-spectrum antibiotic first.
Antibiotic overprescribing affects the gut and beyond
The human intestinal microbiome is composed of a diverse array of bacteria, viruses, and parasites.21 The main functions of the gut microbiome include interacting with the immune system and participating in biochemical reactions in the gut, such as absorption of fat-soluble vitamins and the production of vitamin K.
As we know, antibiotics decrease the diversity of gut bacteria, which, in turn, can cause less efficient nutrient extraction, as well as a vulnerability to enteric infections.21 It is well known, for example, that the bacterial gut microbiome can either inhibit or promote diarrheal illnesses such as those caused by C. difficile. C. difficile infection (CDI) is now the most common health care-related infection, accounting for approximately a half million health care facility infections a year.22 CDI extends hospital stays an average of almost 10 days and is estimated to cost the health care system $6.3 billion annually.23
Antibiotics can also eliminate antibiotic-susceptible organisms, allowing resistant organisms to proliferate.4 They also promote the transmission of genes for antibiotic resistance between gut bacteria.4
Beyond the gut
Less well known is that gut bacteria can promote or inhibit extraintestinal infections.
Gut bacteria and HIV. In early human immunodeficiency virus (HIV) infections, for example, gut populations of Lactobacillus and Bifidobacteria are reduced, and the gut barrier becomes compromised.24 Increasing translocation of bacterial products is associated with HIV disease progression. Preservation of Lactobacillus populations in the gut is associated with markers predictive of better HIV outcomes, including a higher CD4 count, a lower viral load, and less evidence of gut microbial translocation.24 This underscores the importance of maintaining a healthy gut flora in patients with HIV, using such steps as avoiding unnecessary antibiotics.
Gut bacteria and stress, depression. Antibiotics directly induce the expression of key genes that affect the stress response.25 While causative studies are lacking, there is a growing body of evidence suggesting that the gut microbiome is involved in 2-way communication with the brain and can affect, and be affected by, stress and depression.21,26-30 Diseases and conditions that seem to have a putative connection to a disordered microbiome (dysbiosis) include depression, anxiety, Crohn’s disease, type 2 diabetes, and obesity.
Gut bacteria and childhood obesity. Repeated use of broader-spectrum antibiotics in children <24 months of age increases the risk of developing childhood obesity.1,6 One theory for the association is that the effects of broad-spectrum antibiotics on the intestinal flora of young children may alter long-term energy homeostasis resulting in a higher risk for obesity.1
Gut bacteria and asthma. Studies demonstrate differences in the gut microbiome of asthmatic and nonasthmatic patients. These differences affect the activities of helper T-cell subsets (Th1 and Th2), which in turn affect the development of immune tolerance.31
Although additional studies are needed to confirm these findings, the evidence collected thus far should make us all pause before prescribing drugs that can alter our microbiome in complex and only partially understood ways.

What can we do right now?
The issues created by the inappropriate prescribing of antibiotics have been known for decades, and multiple attempts have been made to find solutions and implement change. Although some small successes have occurred, little overall progress has been made in reducing antibiotic prescribing in the general population. A historical review of why physicians prescribe antibiotics inappropriately and the interventions that have successfully reduced this prescribing may prove valuable as we continue to look for new, effective answers.
Why do we overprescribe antibiotics? A 2015 systematic literature review found that patient demand, pharmaceutical company marketing activities, limited up-to-date information sources, and physician fear of losing their patients are major reasons physicians cite for prescribing antibiotics.32
In a separate study that explored antibiotic prescribing habits for acute bronchitis,33 clinicians cited “patient demand” as the major reason for prescribing antibiotics. Respondents also reported that “other physicians were responsible for inappropriate antibiotic prescribing.”33
Strategies that work
Some early intervention programs directed at reducing antibiotic prescribing demonstrated success (TABLE 2).34-36 One example comes from a 1996 to 1998 study of 4 primary care practices.34 Researchers evaluated the impact of a multidimensional intervention effort targeted at clinicians and patients and aimed at lowering the use of antimicrobial agents for acute uncomplicated bronchitis in adults. It incorporated a number of elements, including office-based and household patient educational materials, and a clinician intervention involving education, practice profiling, and academic detailing. Physicians in this program reduced their rates of antibiotic prescribing for uncomplicated bronchitis from 74% to 48%.34

Employing EMRs. A more recent study focused on using electronic medical records (EMRs) and communications to modify physician antibiotic prescribing.35 By sending physicians monthly emails comparing their prescribing patterns to peers and “typical top performers,” inappropriate antibiotic prescriptions for ARTIs went from 19.9% to 3.7%.35
In another effort, the same researchers modified physicians’ EMRs to detect when potentially inappropriate antibiotics were prescribed. The system then prompted the physician to provide an “antibiotic justification note,” which remained visible in the patient’s chart. This approach, which encouraged physicians to follow prescribing guidelines by taking advantage of their concerns about their reputations, produced a 77% reduction in antibiotic prescribing.35
Focusing on the public. Studies have also examined the effectiveness of educating the public about when antibiotics are not likely to be helpful and of the harms of unnecessary antibiotics. Studies conducted in Tennessee and Wisconsin that combined prescriber and community education about unnecessary antibiotics for children found that the intervention reduced antibiotic prescribing in both locations by about 19% compared with about a 9% reduction in the control groups.36,37
Does prescribing antibiotics affect patient satisfaction?
The results are mixed as to whether prescribing antibiotics affects patient satisfaction. Two studies in the early 2000s found that both patients and parents reported higher satisfaction with physicians who explained why antibiotics were not indicated vs physicians who simply prescribed them, and that such explanations do not need to take a lot of time.37,38 (See TABLE 39,37,38 for patient care tips.)

A more recent study found that higher antibiotic prescribing practices in Britain were associated with modestly higher patient satisfaction ratings.39 The authors of this study noted, however, that reduced antibiotic prescribing may be a proxy for other practice patterns that affected satisfaction ratings.
Reducing antibiotic prescribing reduces resistance
There is also strong evidence that when physicians decrease antibiotic prescribing, antimicrobial resistance follows suit. One of the earlier landmark studies to demonstrate this was a Finnish study published in 1997.40 The authors found that a reduction of macrolide antibiotic consumption in Finland led to a reduction in streptococci macrolide resistance from 16.5% to 8.6%.40
Since then, multiple studies have demonstrated similar results for both respiratory and urinary tract infections.41,42 A 2017 meta-analysis analyzing 32 studies found that antibiotic stewardship programs reduced the incidence of infections and colonization with multidrug-resistant Gram-negative bacteria (51% reduction), extended-spectrum beta-lactamase–producing Gram-negative bacteria (48%), and methicillin-resistant Staphylococcus aureus (37%). There was also a reduction in the incidence of C. difficile infections (32%).43
CORRESPONDENCE
David C. Fiore, MD, Department of Family and Community Medicine, University of Nevada, Reno School of Medicine, Brigham Bldg, MS 316, Reno, NV 89557; [email protected].
1. Bailey LC, Forrest CB, Zhang P, et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069.
2. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
3. Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000;21:53-60.
4. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
5. Logan AC, Jacka FN, Craig JM, et al. The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol Neurosci. 2016;14:131-147.
6. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010.
7. Harris AM, Hicks LA, Qaseem A, for the High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
8. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
9. Centers for Disease Control and Prevention. Antibiotic prescribing and use. Available at: http://www.cdc.gov/getsmart/. Accessed October 23, 2017.
10. The White House. National action plan for combating antibiotic-resistant bacteria. March 2015:1-63. Available at: https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed October 23, 2017.
11. World Health Organization. Global action plan on antimicrobial resistance. 2015. Available at: http://www.who.int/drugresistance/global_action_plan/en/. Accessed October 23, 2017.
12. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
13. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061.
14. Chow AW, Benninger MS, Brook I, et al. Executive summary: IDSA Clinical Practice Guideline for Acute Bacterial Rhinosinusitis in Children and Adults. Clin Infect Dis. 2012;54:1041-1045.
15. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 Suppl):S1-S39.
16. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
17. Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother. 2014;69:234-240.
18. Bergmark RW, Sedaghat AR. Antibiotic prescription for acute rhinosinusitis: emergency departments versus primary care providers. Laryngoscope. 2016;(November):1-6.
19. Hersh AL, Fleming-Dutra KE, Shapiro DJ, et al. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870-1872.
20. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308-1316.
21. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39.
22. Lessa FC, Gould CV, McDonald CL. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65-S70.
23. Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447.
24. Pérez-Santiago J, Gianella S, Massanella M, et al. Gut lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
25. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50.
26. Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667-672.
27. Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: An integrative view. Cell. 2012;148:1258-1270.
28. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369-1378.
29. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312.
30. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1-12.
31. Riiser A. The human microbiome, asthma, and allergy. Allergy, Asthma, and Clinical Immunology. 2015;11:35.
32. Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665-680.
33. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract. 2014;15:194.
34. Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice. JAMA. 1999;281:1512-1519.
35. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315:562-570.
36. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287:3103-3109.
37. Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108:575-583.
38. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155:800-806.
39. Ashworth M, White P, Jongsma H,et al. Antibiotic prescribing and patient satisfaction in primary care in England: cross-sectional analysis of national patient survey data and prescribing data. Br J Gen Pract. 2016;66:e40-e46.
40. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441-446.
41. Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin g–nonsusceptible Streptococcus pneumoniae. Clin Infect Dis. 2005;41:930-938.
42. Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007;57:785-792.
43. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990-1001.
1. Bailey LC, Forrest CB, Zhang P, et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069.
2. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
3. Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000;21:53-60.
4. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
5. Logan AC, Jacka FN, Craig JM, et al. The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol Neurosci. 2016;14:131-147.
6. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010.
7. Harris AM, Hicks LA, Qaseem A, for the High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
8. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
9. Centers for Disease Control and Prevention. Antibiotic prescribing and use. Available at: http://www.cdc.gov/getsmart/. Accessed October 23, 2017.
10. The White House. National action plan for combating antibiotic-resistant bacteria. March 2015:1-63. Available at: https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed October 23, 2017.
11. World Health Organization. Global action plan on antimicrobial resistance. 2015. Available at: http://www.who.int/drugresistance/global_action_plan/en/. Accessed October 23, 2017.
12. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
13. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061.
14. Chow AW, Benninger MS, Brook I, et al. Executive summary: IDSA Clinical Practice Guideline for Acute Bacterial Rhinosinusitis in Children and Adults. Clin Infect Dis. 2012;54:1041-1045.
15. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 Suppl):S1-S39.
16. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
17. Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother. 2014;69:234-240.
18. Bergmark RW, Sedaghat AR. Antibiotic prescription for acute rhinosinusitis: emergency departments versus primary care providers. Laryngoscope. 2016;(November):1-6.
19. Hersh AL, Fleming-Dutra KE, Shapiro DJ, et al. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870-1872.
20. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308-1316.
21. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39.
22. Lessa FC, Gould CV, McDonald CL. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65-S70.
23. Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447.
24. Pérez-Santiago J, Gianella S, Massanella M, et al. Gut lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
25. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50.
26. Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667-672.
27. Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: An integrative view. Cell. 2012;148:1258-1270.
28. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369-1378.
29. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312.
30. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1-12.
31. Riiser A. The human microbiome, asthma, and allergy. Allergy, Asthma, and Clinical Immunology. 2015;11:35.
32. Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665-680.
33. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract. 2014;15:194.
34. Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice. JAMA. 1999;281:1512-1519.
35. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315:562-570.
36. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287:3103-3109.
37. Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108:575-583.
38. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155:800-806.
39. Ashworth M, White P, Jongsma H,et al. Antibiotic prescribing and patient satisfaction in primary care in England: cross-sectional analysis of national patient survey data and prescribing data. Br J Gen Pract. 2016;66:e40-e46.
40. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441-446.
41. Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin g–nonsusceptible Streptococcus pneumoniae. Clin Infect Dis. 2005;41:930-938.
42. Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007;57:785-792.
43. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990-1001.
PRACTICE RECOMMENDATIONS
› Explain to patients the rationale for not prescribing antibiotics when they are not indicated. A
› Advocate for health care system electronic medical record systems designed to limit antibiotic prescribing to only appropriate cases. A
› Provide patients and your community with educational materials to increase understanding of the risks of antibiotic overprescribing. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series