User login
Colorectal cancer deaths projected for 2018
Approximately 50,630 deaths from colorectal cancer are predicted for the year in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics.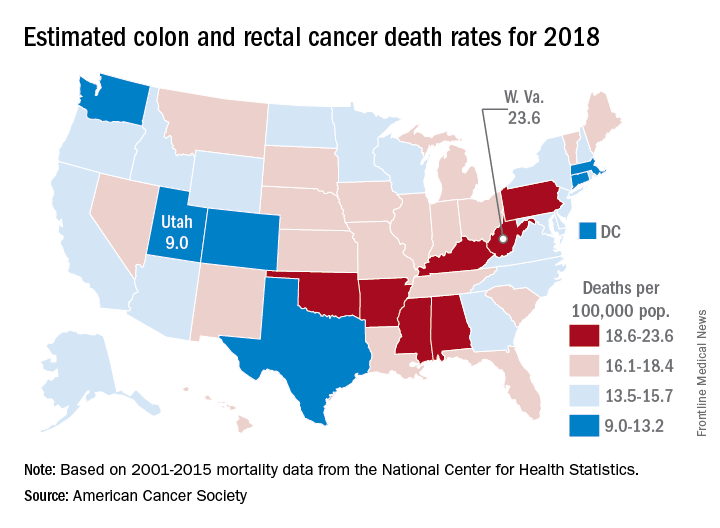
Nationally, the colorectal cancer death rate has been declining for decades, but hidden inside that long-term trend are a couple of competing ones: From 2006 to 2015, mortality dropped 2.9% a year for those aged 55 years and older but increased by 1% annually for adults aged 55 and under, the ACS said.
Incidence rates for colon cancer and rectal cancer showed a similar trend: From 2005 to 2015 they were down by 3.8% (colon) and 3.5% (rectal) a year for adults aged 55 and older but rose 1.4% and 2.4%, respectively, for adults younger than 55. Accurate statistics on colon and rectal cancer deaths are not available separately “because many deaths from rectal cancer are misclassified as colon cancer on death certificates,” the ACS said.
Approximately 50,630 deaths from colorectal cancer are predicted for the year in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics.
Nationally, the colorectal cancer death rate has been declining for decades, but hidden inside that long-term trend are a couple of competing ones: From 2006 to 2015, mortality dropped 2.9% a year for those aged 55 years and older but increased by 1% annually for adults aged 55 and under, the ACS said.
Incidence rates for colon cancer and rectal cancer showed a similar trend: From 2005 to 2015 they were down by 3.8% (colon) and 3.5% (rectal) a year for adults aged 55 and older but rose 1.4% and 2.4%, respectively, for adults younger than 55. Accurate statistics on colon and rectal cancer deaths are not available separately “because many deaths from rectal cancer are misclassified as colon cancer on death certificates,” the ACS said.
Approximately 50,630 deaths from colorectal cancer are predicted for the year in the United States by the American Cancer Society (ACS) in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics.
Nationally, the colorectal cancer death rate has been declining for decades, but hidden inside that long-term trend are a couple of competing ones: From 2006 to 2015, mortality dropped 2.9% a year for those aged 55 years and older but increased by 1% annually for adults aged 55 and under, the ACS said.
Incidence rates for colon cancer and rectal cancer showed a similar trend: From 2005 to 2015 they were down by 3.8% (colon) and 3.5% (rectal) a year for adults aged 55 and older but rose 1.4% and 2.4%, respectively, for adults younger than 55. Accurate statistics on colon and rectal cancer deaths are not available separately “because many deaths from rectal cancer are misclassified as colon cancer on death certificates,” the ACS said.
Therapeutic horseback riding may lower veterans’ PTSD symptoms
Veterans with posttraumatic stress disorder might benefit from therapeutic horseback riding, a small study suggests.
“Our findings provide empirical evidence that [therapeutic horseback riding] is effective at improving coping skills and in lessening one’s difficulty with emotional regulation, especially with longer riding interventions,” wrote Rebecca A. Johnson, PhD, of the University of Missouri, Columbia, and her associates.
Overall, 57 veterans were recruited and 28 enrolled in the randomized trial at baseline. Those individuals were randomized into two groups: a wait-list control group and a treatment group. Eventually, all of the veterans participated in the therapeutic riding program. Meanwhile, the riding center staff were not aware of which veterans had been assigned to either group. The Professional Association of Therapeutic Horsemanship, a nonprofit group that promotes equine-related activities for people with special needs, selected the horses that were used in the study. During the data collection periods, PTSD symptoms were measured via the PTSD Checklist–Military Version, or PCL-M. This self-report measure asks patients about problems in response to “stressful military experiences,” the researchers wrote. The Coping Self-Efficacy Scale and the Difficulties in Emotion Regulation Scale were among the other instruments used.
While riding, the results showed, participants had a statistically significant decrease in PTSD symptoms over the course of the 6-week program. “,” Dr. Johnson and her associates wrote. “Further detailed examination showed that participants had a 66.7% likelihood of having lower PTSD scores at 3 weeks, and an 87.5% likelihood at 6 weeks.”
Anecdotally, some of the veterans wanted to continue therapeutic riding after the end of the program, and they were able to do so.
“We conclude that [therapeutic horseback riding] shows promise as a beneficial intervention for veterans with PTSD, but did not measure functional ability,” they wrote.
Veterans with posttraumatic stress disorder might benefit from therapeutic horseback riding, a small study suggests.
“Our findings provide empirical evidence that [therapeutic horseback riding] is effective at improving coping skills and in lessening one’s difficulty with emotional regulation, especially with longer riding interventions,” wrote Rebecca A. Johnson, PhD, of the University of Missouri, Columbia, and her associates.
Overall, 57 veterans were recruited and 28 enrolled in the randomized trial at baseline. Those individuals were randomized into two groups: a wait-list control group and a treatment group. Eventually, all of the veterans participated in the therapeutic riding program. Meanwhile, the riding center staff were not aware of which veterans had been assigned to either group. The Professional Association of Therapeutic Horsemanship, a nonprofit group that promotes equine-related activities for people with special needs, selected the horses that were used in the study. During the data collection periods, PTSD symptoms were measured via the PTSD Checklist–Military Version, or PCL-M. This self-report measure asks patients about problems in response to “stressful military experiences,” the researchers wrote. The Coping Self-Efficacy Scale and the Difficulties in Emotion Regulation Scale were among the other instruments used.
While riding, the results showed, participants had a statistically significant decrease in PTSD symptoms over the course of the 6-week program. “,” Dr. Johnson and her associates wrote. “Further detailed examination showed that participants had a 66.7% likelihood of having lower PTSD scores at 3 weeks, and an 87.5% likelihood at 6 weeks.”
Anecdotally, some of the veterans wanted to continue therapeutic riding after the end of the program, and they were able to do so.
“We conclude that [therapeutic horseback riding] shows promise as a beneficial intervention for veterans with PTSD, but did not measure functional ability,” they wrote.
Veterans with posttraumatic stress disorder might benefit from therapeutic horseback riding, a small study suggests.
“Our findings provide empirical evidence that [therapeutic horseback riding] is effective at improving coping skills and in lessening one’s difficulty with emotional regulation, especially with longer riding interventions,” wrote Rebecca A. Johnson, PhD, of the University of Missouri, Columbia, and her associates.
Overall, 57 veterans were recruited and 28 enrolled in the randomized trial at baseline. Those individuals were randomized into two groups: a wait-list control group and a treatment group. Eventually, all of the veterans participated in the therapeutic riding program. Meanwhile, the riding center staff were not aware of which veterans had been assigned to either group. The Professional Association of Therapeutic Horsemanship, a nonprofit group that promotes equine-related activities for people with special needs, selected the horses that were used in the study. During the data collection periods, PTSD symptoms were measured via the PTSD Checklist–Military Version, or PCL-M. This self-report measure asks patients about problems in response to “stressful military experiences,” the researchers wrote. The Coping Self-Efficacy Scale and the Difficulties in Emotion Regulation Scale were among the other instruments used.
While riding, the results showed, participants had a statistically significant decrease in PTSD symptoms over the course of the 6-week program. “,” Dr. Johnson and her associates wrote. “Further detailed examination showed that participants had a 66.7% likelihood of having lower PTSD scores at 3 weeks, and an 87.5% likelihood at 6 weeks.”
Anecdotally, some of the veterans wanted to continue therapeutic riding after the end of the program, and they were able to do so.
“We conclude that [therapeutic horseback riding] shows promise as a beneficial intervention for veterans with PTSD, but did not measure functional ability,” they wrote.
FROM MILITARY MEDICAL RESEARCH
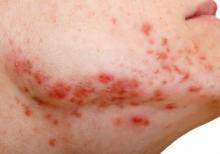
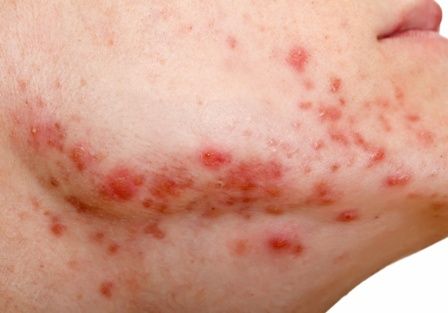
Debunking Acne Myths: Does Wearing Makeup Cause Acne?
Myth: Wearing makeup causes acne breakouts
Acne breakouts caused by makeup and other skin care products, known as acne cosmetica, typically resolve when patients stop using pore-clogging products; however, the overall impact of cosmetics on the development of acne lesions is considered to be negligible. Many cosmetics are not inherently comedogenic and can be used safely by patients in combination with proper skin care techniques.
Although dermatologists may be inclined to discourage makeup use during acne treatment or breakouts due to its potential to aggravate the patient’s condition, research has shown that treatment results and quality of life (QoL) scores associated with makeup use in acne patients may improve when patients receive instruction on how to use skin care products and cosmetics effectively. In one study of 50 female acne patients, 25 participants were instructed on how to use skin care products and cosmetics, and the other 25 participants received no specific instructions from dermatologists. After 4 weeks of treatment with conventional topical and/or oral acne medications, the investigators concluded that use of skin care products did not negatively impact acne treatment, and the group that received application instructions showed more notable improvements in QoL scores versus those who did not. In another study, the overall number of acne eruptions decreased over a 2- to 4-week period in female acne patients who were trained by a makeup artist to apply cosmetics while undergoing acne treatment. These results suggest that acne patients who wear makeup may benefit from a conversation with their dermatologist about what products and skin care techniques they can use to minimize exacerbation of or even improve their condition.
When choosing makeup that will not cause or exacerbate acne breakouts, patients should look for packaging that indicates the product will not clog pores and is oil-free, noncomedogenic, and/or nonacnegenic. Some makeup products are specifically formulated to help camouflage redness and pimples, which can help improve quality of life and self-esteem in acne patients who otherwise may be self-conscious about their appearance. Mineral-based cosmetics containing powdered formulas of silica, titanium dioxide, and zinc oxide can be used to absorb oil, camouflage redness, and prevent irritation. Anti-inflammatory ingredients and antioxidants also are used in some makeup products to reduce skin irritation and promote barrier repair. Additional cosmetic ingredients that can affect the mechanisms of acne pathogenesis and may contribute to a decrease in acne lesions include nicotinamide, lactic acid, triethyl acetate/ethyllineolate, and prebiotic plant extracts.
Makeup should be applied gently to avoid irritating the skin. It also is important to remind patients not to share their makeup brushes and applicators and to clean them weekly to ensure that bacteria, dead skin cells, and oil are not spread to the skin, which can lead to new breakouts. Although patients may be compelled to scrub the skin to remove makeup, a mild cleanser should be gently applied using the fingertips and rinsed off with lukewarm water to minimize skin irritation. Any makeup remaining on the skin after washing should be gently removed with an oil-free makeup remover.
Hayashi N, Imori M, Yanagisawa M, et al. Make-up improves the quality of life of acne patients without aggravating acne eruptions during treatments. Eur J Dermatol. 2005;15:284-287.
I have acne! is it okay to wear makeup? American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/makeup-with-acne. Accessed February 13, 2018.
Korting HC, Borelli C, Schöllmann C. Acne vulgaris. role of cosmetics [in German]. 2010;61:126-131.
Matsuoka Y, Yoneda K, Sadahira C, et al. Effects of skin care and makeup under instructions from dermatologists on the quality of life of female patients with acne vulgaris. J Dermatol. 2006;33:745-752.
Proper skin care lays the foundation for successful acne and rosacea treatment. American Academy of Dermatology website. https://www.aad.org/media/news-releases/proper-skin-care-lays-the-foundation-for-successful-acne-and-rosacea-treatment Published July 31, 2013. Accessed February 13, 2018.
Myth: Wearing makeup causes acne breakouts
Acne breakouts caused by makeup and other skin care products, known as acne cosmetica, typically resolve when patients stop using pore-clogging products; however, the overall impact of cosmetics on the development of acne lesions is considered to be negligible. Many cosmetics are not inherently comedogenic and can be used safely by patients in combination with proper skin care techniques.
Although dermatologists may be inclined to discourage makeup use during acne treatment or breakouts due to its potential to aggravate the patient’s condition, research has shown that treatment results and quality of life (QoL) scores associated with makeup use in acne patients may improve when patients receive instruction on how to use skin care products and cosmetics effectively. In one study of 50 female acne patients, 25 participants were instructed on how to use skin care products and cosmetics, and the other 25 participants received no specific instructions from dermatologists. After 4 weeks of treatment with conventional topical and/or oral acne medications, the investigators concluded that use of skin care products did not negatively impact acne treatment, and the group that received application instructions showed more notable improvements in QoL scores versus those who did not. In another study, the overall number of acne eruptions decreased over a 2- to 4-week period in female acne patients who were trained by a makeup artist to apply cosmetics while undergoing acne treatment. These results suggest that acne patients who wear makeup may benefit from a conversation with their dermatologist about what products and skin care techniques they can use to minimize exacerbation of or even improve their condition.
When choosing makeup that will not cause or exacerbate acne breakouts, patients should look for packaging that indicates the product will not clog pores and is oil-free, noncomedogenic, and/or nonacnegenic. Some makeup products are specifically formulated to help camouflage redness and pimples, which can help improve quality of life and self-esteem in acne patients who otherwise may be self-conscious about their appearance. Mineral-based cosmetics containing powdered formulas of silica, titanium dioxide, and zinc oxide can be used to absorb oil, camouflage redness, and prevent irritation. Anti-inflammatory ingredients and antioxidants also are used in some makeup products to reduce skin irritation and promote barrier repair. Additional cosmetic ingredients that can affect the mechanisms of acne pathogenesis and may contribute to a decrease in acne lesions include nicotinamide, lactic acid, triethyl acetate/ethyllineolate, and prebiotic plant extracts.
Makeup should be applied gently to avoid irritating the skin. It also is important to remind patients not to share their makeup brushes and applicators and to clean them weekly to ensure that bacteria, dead skin cells, and oil are not spread to the skin, which can lead to new breakouts. Although patients may be compelled to scrub the skin to remove makeup, a mild cleanser should be gently applied using the fingertips and rinsed off with lukewarm water to minimize skin irritation. Any makeup remaining on the skin after washing should be gently removed with an oil-free makeup remover.
Myth: Wearing makeup causes acne breakouts
Acne breakouts caused by makeup and other skin care products, known as acne cosmetica, typically resolve when patients stop using pore-clogging products; however, the overall impact of cosmetics on the development of acne lesions is considered to be negligible. Many cosmetics are not inherently comedogenic and can be used safely by patients in combination with proper skin care techniques.
Although dermatologists may be inclined to discourage makeup use during acne treatment or breakouts due to its potential to aggravate the patient’s condition, research has shown that treatment results and quality of life (QoL) scores associated with makeup use in acne patients may improve when patients receive instruction on how to use skin care products and cosmetics effectively. In one study of 50 female acne patients, 25 participants were instructed on how to use skin care products and cosmetics, and the other 25 participants received no specific instructions from dermatologists. After 4 weeks of treatment with conventional topical and/or oral acne medications, the investigators concluded that use of skin care products did not negatively impact acne treatment, and the group that received application instructions showed more notable improvements in QoL scores versus those who did not. In another study, the overall number of acne eruptions decreased over a 2- to 4-week period in female acne patients who were trained by a makeup artist to apply cosmetics while undergoing acne treatment. These results suggest that acne patients who wear makeup may benefit from a conversation with their dermatologist about what products and skin care techniques they can use to minimize exacerbation of or even improve their condition.
When choosing makeup that will not cause or exacerbate acne breakouts, patients should look for packaging that indicates the product will not clog pores and is oil-free, noncomedogenic, and/or nonacnegenic. Some makeup products are specifically formulated to help camouflage redness and pimples, which can help improve quality of life and self-esteem in acne patients who otherwise may be self-conscious about their appearance. Mineral-based cosmetics containing powdered formulas of silica, titanium dioxide, and zinc oxide can be used to absorb oil, camouflage redness, and prevent irritation. Anti-inflammatory ingredients and antioxidants also are used in some makeup products to reduce skin irritation and promote barrier repair. Additional cosmetic ingredients that can affect the mechanisms of acne pathogenesis and may contribute to a decrease in acne lesions include nicotinamide, lactic acid, triethyl acetate/ethyllineolate, and prebiotic plant extracts.
Makeup should be applied gently to avoid irritating the skin. It also is important to remind patients not to share their makeup brushes and applicators and to clean them weekly to ensure that bacteria, dead skin cells, and oil are not spread to the skin, which can lead to new breakouts. Although patients may be compelled to scrub the skin to remove makeup, a mild cleanser should be gently applied using the fingertips and rinsed off with lukewarm water to minimize skin irritation. Any makeup remaining on the skin after washing should be gently removed with an oil-free makeup remover.
Hayashi N, Imori M, Yanagisawa M, et al. Make-up improves the quality of life of acne patients without aggravating acne eruptions during treatments. Eur J Dermatol. 2005;15:284-287.
I have acne! is it okay to wear makeup? American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/makeup-with-acne. Accessed February 13, 2018.
Korting HC, Borelli C, Schöllmann C. Acne vulgaris. role of cosmetics [in German]. 2010;61:126-131.
Matsuoka Y, Yoneda K, Sadahira C, et al. Effects of skin care and makeup under instructions from dermatologists on the quality of life of female patients with acne vulgaris. J Dermatol. 2006;33:745-752.
Proper skin care lays the foundation for successful acne and rosacea treatment. American Academy of Dermatology website. https://www.aad.org/media/news-releases/proper-skin-care-lays-the-foundation-for-successful-acne-and-rosacea-treatment Published July 31, 2013. Accessed February 13, 2018.
Hayashi N, Imori M, Yanagisawa M, et al. Make-up improves the quality of life of acne patients without aggravating acne eruptions during treatments. Eur J Dermatol. 2005;15:284-287.
I have acne! is it okay to wear makeup? American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/makeup-with-acne. Accessed February 13, 2018.
Korting HC, Borelli C, Schöllmann C. Acne vulgaris. role of cosmetics [in German]. 2010;61:126-131.
Matsuoka Y, Yoneda K, Sadahira C, et al. Effects of skin care and makeup under instructions from dermatologists on the quality of life of female patients with acne vulgaris. J Dermatol. 2006;33:745-752.
Proper skin care lays the foundation for successful acne and rosacea treatment. American Academy of Dermatology website. https://www.aad.org/media/news-releases/proper-skin-care-lays-the-foundation-for-successful-acne-and-rosacea-treatment Published July 31, 2013. Accessed February 13, 2018.
Acne is linked to higher chances of major depression
according to a retrospective cohort analysis published as a research letter.
“The onset of acne, when patients present for treatment because of active disease, is associated with a greater risk of developing depression” wrote Isabelle A. Vallerand, an MD/PhD student at the University of Calgary (Alta.), and her associates. “Although the severity of acne was not assessed directly in the current study, this finding suggests a potential dose/response relationship such that more active disease may lead to a greater risk of depression.”
In total, 134,437 acne patients and 1,731,608 patients without acne were identified from THIN. Over a 15-year follow-up, the probability of developing MDD was 18.5% among patients with acne, and 12% for those without acne. This risk was much higher within the first year after diagnosis (adjusted hazard ratio, 1.63; 95% confidence interval, 1.33-2), which subsequently decreased.
The researchers found that patients with acne tended to be younger (67.6% vs. 22.8%), female (58.6% vs. 48.6%), and of a higher socioeconomic status (24.4% vs. 22.1%) compared with patients without acne. Those with acne tended to smoke (58.4% vs. 48.6%) and to have comorbidities (17.2% vs. 13.8%). Conversely, acne patients were less likely to use alcohol (17% vs. 39%) and less likely to be obese (2.3% vs. 6.6%) (all P less than .001).
Although these results are promising, there are several limitations that could have influenced the study findings. The misclassification of patients with acne and MDD could have occurred if patients did not present themselves to a physician for treatment. Another limitation was that isotretinoin was the only acne treatment that was considered by the researchers. Considering that treatment has been shown to reduce depressive symptoms, the researchers believe that their estimates are conservative.
“Given the tremendous burden of MDD and its temporal association with active acne, it is critical that physicians monitor mood symptoms in patients with acne and initiate prompt MDD management or seek consultation from a psychiatrist when needed” wrote Ms. Vallerand and her colleagues.
Ms. Vallerand received funding for this study from the Alberta Innovates Health Solutions MD-PhD Studentship and from the Mach-Gaensslen Foundation of Canada. None of the other authors had disclosures to report.
SOURCE: Vallerand I et al. Br J Dermatol. 2018 Feb 7. doi: 10.1111/bjd.16099.
according to a retrospective cohort analysis published as a research letter.
“The onset of acne, when patients present for treatment because of active disease, is associated with a greater risk of developing depression” wrote Isabelle A. Vallerand, an MD/PhD student at the University of Calgary (Alta.), and her associates. “Although the severity of acne was not assessed directly in the current study, this finding suggests a potential dose/response relationship such that more active disease may lead to a greater risk of depression.”
In total, 134,437 acne patients and 1,731,608 patients without acne were identified from THIN. Over a 15-year follow-up, the probability of developing MDD was 18.5% among patients with acne, and 12% for those without acne. This risk was much higher within the first year after diagnosis (adjusted hazard ratio, 1.63; 95% confidence interval, 1.33-2), which subsequently decreased.
The researchers found that patients with acne tended to be younger (67.6% vs. 22.8%), female (58.6% vs. 48.6%), and of a higher socioeconomic status (24.4% vs. 22.1%) compared with patients without acne. Those with acne tended to smoke (58.4% vs. 48.6%) and to have comorbidities (17.2% vs. 13.8%). Conversely, acne patients were less likely to use alcohol (17% vs. 39%) and less likely to be obese (2.3% vs. 6.6%) (all P less than .001).
Although these results are promising, there are several limitations that could have influenced the study findings. The misclassification of patients with acne and MDD could have occurred if patients did not present themselves to a physician for treatment. Another limitation was that isotretinoin was the only acne treatment that was considered by the researchers. Considering that treatment has been shown to reduce depressive symptoms, the researchers believe that their estimates are conservative.
“Given the tremendous burden of MDD and its temporal association with active acne, it is critical that physicians monitor mood symptoms in patients with acne and initiate prompt MDD management or seek consultation from a psychiatrist when needed” wrote Ms. Vallerand and her colleagues.
Ms. Vallerand received funding for this study from the Alberta Innovates Health Solutions MD-PhD Studentship and from the Mach-Gaensslen Foundation of Canada. None of the other authors had disclosures to report.
SOURCE: Vallerand I et al. Br J Dermatol. 2018 Feb 7. doi: 10.1111/bjd.16099.
according to a retrospective cohort analysis published as a research letter.
“The onset of acne, when patients present for treatment because of active disease, is associated with a greater risk of developing depression” wrote Isabelle A. Vallerand, an MD/PhD student at the University of Calgary (Alta.), and her associates. “Although the severity of acne was not assessed directly in the current study, this finding suggests a potential dose/response relationship such that more active disease may lead to a greater risk of depression.”
In total, 134,437 acne patients and 1,731,608 patients without acne were identified from THIN. Over a 15-year follow-up, the probability of developing MDD was 18.5% among patients with acne, and 12% for those without acne. This risk was much higher within the first year after diagnosis (adjusted hazard ratio, 1.63; 95% confidence interval, 1.33-2), which subsequently decreased.
The researchers found that patients with acne tended to be younger (67.6% vs. 22.8%), female (58.6% vs. 48.6%), and of a higher socioeconomic status (24.4% vs. 22.1%) compared with patients without acne. Those with acne tended to smoke (58.4% vs. 48.6%) and to have comorbidities (17.2% vs. 13.8%). Conversely, acne patients were less likely to use alcohol (17% vs. 39%) and less likely to be obese (2.3% vs. 6.6%) (all P less than .001).
Although these results are promising, there are several limitations that could have influenced the study findings. The misclassification of patients with acne and MDD could have occurred if patients did not present themselves to a physician for treatment. Another limitation was that isotretinoin was the only acne treatment that was considered by the researchers. Considering that treatment has been shown to reduce depressive symptoms, the researchers believe that their estimates are conservative.
“Given the tremendous burden of MDD and its temporal association with active acne, it is critical that physicians monitor mood symptoms in patients with acne and initiate prompt MDD management or seek consultation from a psychiatrist when needed” wrote Ms. Vallerand and her colleagues.
Ms. Vallerand received funding for this study from the Alberta Innovates Health Solutions MD-PhD Studentship and from the Mach-Gaensslen Foundation of Canada. None of the other authors had disclosures to report.
SOURCE: Vallerand I et al. Br J Dermatol. 2018 Feb 7. doi: 10.1111/bjd.16099.
FROM BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: Patients with acne have a much higher chance of developing major depressive disorder (MDD).
Major finding: The chance of developing MDD was 18.5% in patients with acne.
Study details: Analysis of retrospective cohort data obtained from the Health Improvement Network (THIN) between 1986 and 2012.
Disclosures: Ms. Vallerand received funding for this study from the Alberta Innovates Health Solutions MD-PhD Studentship and from the Mach-Gaensslen Foundation of Canada. None of the other authors had disclosures to report.
Source: Vallerand I et al. Br J Dermatol. 2018 Feb 7. doi: 10.1111/bjd.16099.
Are you as frustrated with medical care as we are?
How did the experience of office visits get to be so frustrating for both patients and doctors? Let’s put it under the microscope and examine it.
To medicine’s credit, it realized the value of looking for diseases before symptoms occurred, such as using mammograms to detect breast cancer and controlling blood pressure and blood sugar to avert comorbidities.
Today, a doctor looks at the computer screen and checks off when a mammogram was done and whether blood pressure and blood sugar are controlled. “Authorities” believe that good health is achieved by performing positive checkoffs to questions like this. This definition of quality care is, in reality, “quantity care” and can be tied to physician compensation. Physicians who did not adequately meet Physician Quality Reporting System requirements have received letters informing them that their Medicare Part B payments for 2018 will be reduced by 3%.
Many seasoned clinicians recognize that practicing good medicine involves more than following a computer printout of tests and treatments based on the patient’s symptoms, more than plugging into the diagnostic and prescription mills that are part of today’s managed care system. Making the correct diagnosis requires a carefully taken history, listening to the patients’ stories of their journeys into and through illness, and using a bio-psychosocial-spiritual approach.
Getting to know the patient as a person requires that the doctor and patient take a journey together. In that journey, when the doctor empathizes with the patient and understands what makes the patient tick, the doctor can empower the patient – giving the patient a fuller understanding of their medical conditions, greater participation in the diagnostic work-up and in treatments, and hope for success – all leading to better outcomes.
Doctors are frustrated with the current medical assembly-line system. A study has shown that physicians spend 2 hours on electronic health records and clerical work for every hour they provide direct patient care (Ann Intern Med. 2016;165[11]:753-60). Nearly half of physicians now report that they are “burned out” by the demand to achieve the quantitative requirements on the one hand and their inability to minister to the needs of their patients on the other hand. Patients are also frustrated by the system as they cope with health insurance and costs, with the short time allocated for office visits, and with a fragmented and impersonal medical system. Patients feel that they are little more than a source of information for boxes to be checked off by the physician whose eyes are forced to be on the computer and the clock.
How can we begin to integrate these measures of quality into “quantity medicine” and make the experience of medical visits less frustrating for doctors and patients? How can we reward the skills that recognize that the course of an illness is influenced by patients’ emotions and thoughts related to their problems, their supportive or stressful relationships with others, and the context within which they conceptualize their lives – particularly their religious and spiritual beliefs about life’s purpose and challenges and attitudes toward death?
Caring for patients requires a more sophisticated approach than seeing patients as computer checkoffs. Office visits need to focus on the patient who has the symptoms, not just the symptoms the patient has.
Isn’t it time to make patient-centered care a reality and not just a slogan? If this speaks to you, then what should you do? Even though solutions may not be simple, we should not be deterred from finding patient-centered systems since patients and doctors are unhappy with today’s system. Why not have patients grade their office visits?
While this approach has its shortcomings, and isn’t the only solution, it does place the patient at the center of the process, answering questions about whether the doctor listened to them, heard their concerns, and presented a reasonable plan to help them get better.
In addition, all those involved with medical care should be involved in the process to replace today’s deficient system. The nation’s main organizations representing physicians should propose solutions to support patient-centered care. Individual physicians should become involved, speaking up and sending articles and letters to medical journals and the lay press.
Patients should be empowered to open up a public discussion – in print and broadcast media – on how they want to improve their own medical experiences and the quality of their health care.
It’s worth it. It’s our health.
Dr. Banner is a practicing internist in Philadelphia and chair emeritus of the Albert Einstein Medical Center Medical Ethics Committee. Dr. Benor is a psychiatric psychotherapist in the United States and a wholistic psychotherapist in Canada. Dr. Reiser is adjunct professor, University of Texas School of Public Health, Austin, and the UT Austin Plan II Honors Program, and teaches medical history, medical ethics, and public policy. The authors are indebted to Benjamin Sharfman, PhD, and Jane Brown, PhD, for their important roles in creating this article.
How did the experience of office visits get to be so frustrating for both patients and doctors? Let’s put it under the microscope and examine it.
To medicine’s credit, it realized the value of looking for diseases before symptoms occurred, such as using mammograms to detect breast cancer and controlling blood pressure and blood sugar to avert comorbidities.
Today, a doctor looks at the computer screen and checks off when a mammogram was done and whether blood pressure and blood sugar are controlled. “Authorities” believe that good health is achieved by performing positive checkoffs to questions like this. This definition of quality care is, in reality, “quantity care” and can be tied to physician compensation. Physicians who did not adequately meet Physician Quality Reporting System requirements have received letters informing them that their Medicare Part B payments for 2018 will be reduced by 3%.
Many seasoned clinicians recognize that practicing good medicine involves more than following a computer printout of tests and treatments based on the patient’s symptoms, more than plugging into the diagnostic and prescription mills that are part of today’s managed care system. Making the correct diagnosis requires a carefully taken history, listening to the patients’ stories of their journeys into and through illness, and using a bio-psychosocial-spiritual approach.
Getting to know the patient as a person requires that the doctor and patient take a journey together. In that journey, when the doctor empathizes with the patient and understands what makes the patient tick, the doctor can empower the patient – giving the patient a fuller understanding of their medical conditions, greater participation in the diagnostic work-up and in treatments, and hope for success – all leading to better outcomes.
Doctors are frustrated with the current medical assembly-line system. A study has shown that physicians spend 2 hours on electronic health records and clerical work for every hour they provide direct patient care (Ann Intern Med. 2016;165[11]:753-60). Nearly half of physicians now report that they are “burned out” by the demand to achieve the quantitative requirements on the one hand and their inability to minister to the needs of their patients on the other hand. Patients are also frustrated by the system as they cope with health insurance and costs, with the short time allocated for office visits, and with a fragmented and impersonal medical system. Patients feel that they are little more than a source of information for boxes to be checked off by the physician whose eyes are forced to be on the computer and the clock.
How can we begin to integrate these measures of quality into “quantity medicine” and make the experience of medical visits less frustrating for doctors and patients? How can we reward the skills that recognize that the course of an illness is influenced by patients’ emotions and thoughts related to their problems, their supportive or stressful relationships with others, and the context within which they conceptualize their lives – particularly their religious and spiritual beliefs about life’s purpose and challenges and attitudes toward death?
Caring for patients requires a more sophisticated approach than seeing patients as computer checkoffs. Office visits need to focus on the patient who has the symptoms, not just the symptoms the patient has.
Isn’t it time to make patient-centered care a reality and not just a slogan? If this speaks to you, then what should you do? Even though solutions may not be simple, we should not be deterred from finding patient-centered systems since patients and doctors are unhappy with today’s system. Why not have patients grade their office visits?
While this approach has its shortcomings, and isn’t the only solution, it does place the patient at the center of the process, answering questions about whether the doctor listened to them, heard their concerns, and presented a reasonable plan to help them get better.
In addition, all those involved with medical care should be involved in the process to replace today’s deficient system. The nation’s main organizations representing physicians should propose solutions to support patient-centered care. Individual physicians should become involved, speaking up and sending articles and letters to medical journals and the lay press.
Patients should be empowered to open up a public discussion – in print and broadcast media – on how they want to improve their own medical experiences and the quality of their health care.
It’s worth it. It’s our health.
Dr. Banner is a practicing internist in Philadelphia and chair emeritus of the Albert Einstein Medical Center Medical Ethics Committee. Dr. Benor is a psychiatric psychotherapist in the United States and a wholistic psychotherapist in Canada. Dr. Reiser is adjunct professor, University of Texas School of Public Health, Austin, and the UT Austin Plan II Honors Program, and teaches medical history, medical ethics, and public policy. The authors are indebted to Benjamin Sharfman, PhD, and Jane Brown, PhD, for their important roles in creating this article.
How did the experience of office visits get to be so frustrating for both patients and doctors? Let’s put it under the microscope and examine it.
To medicine’s credit, it realized the value of looking for diseases before symptoms occurred, such as using mammograms to detect breast cancer and controlling blood pressure and blood sugar to avert comorbidities.
Today, a doctor looks at the computer screen and checks off when a mammogram was done and whether blood pressure and blood sugar are controlled. “Authorities” believe that good health is achieved by performing positive checkoffs to questions like this. This definition of quality care is, in reality, “quantity care” and can be tied to physician compensation. Physicians who did not adequately meet Physician Quality Reporting System requirements have received letters informing them that their Medicare Part B payments for 2018 will be reduced by 3%.
Many seasoned clinicians recognize that practicing good medicine involves more than following a computer printout of tests and treatments based on the patient’s symptoms, more than plugging into the diagnostic and prescription mills that are part of today’s managed care system. Making the correct diagnosis requires a carefully taken history, listening to the patients’ stories of their journeys into and through illness, and using a bio-psychosocial-spiritual approach.
Getting to know the patient as a person requires that the doctor and patient take a journey together. In that journey, when the doctor empathizes with the patient and understands what makes the patient tick, the doctor can empower the patient – giving the patient a fuller understanding of their medical conditions, greater participation in the diagnostic work-up and in treatments, and hope for success – all leading to better outcomes.
Doctors are frustrated with the current medical assembly-line system. A study has shown that physicians spend 2 hours on electronic health records and clerical work for every hour they provide direct patient care (Ann Intern Med. 2016;165[11]:753-60). Nearly half of physicians now report that they are “burned out” by the demand to achieve the quantitative requirements on the one hand and their inability to minister to the needs of their patients on the other hand. Patients are also frustrated by the system as they cope with health insurance and costs, with the short time allocated for office visits, and with a fragmented and impersonal medical system. Patients feel that they are little more than a source of information for boxes to be checked off by the physician whose eyes are forced to be on the computer and the clock.
How can we begin to integrate these measures of quality into “quantity medicine” and make the experience of medical visits less frustrating for doctors and patients? How can we reward the skills that recognize that the course of an illness is influenced by patients’ emotions and thoughts related to their problems, their supportive or stressful relationships with others, and the context within which they conceptualize their lives – particularly their religious and spiritual beliefs about life’s purpose and challenges and attitudes toward death?
Caring for patients requires a more sophisticated approach than seeing patients as computer checkoffs. Office visits need to focus on the patient who has the symptoms, not just the symptoms the patient has.
Isn’t it time to make patient-centered care a reality and not just a slogan? If this speaks to you, then what should you do? Even though solutions may not be simple, we should not be deterred from finding patient-centered systems since patients and doctors are unhappy with today’s system. Why not have patients grade their office visits?
While this approach has its shortcomings, and isn’t the only solution, it does place the patient at the center of the process, answering questions about whether the doctor listened to them, heard their concerns, and presented a reasonable plan to help them get better.
In addition, all those involved with medical care should be involved in the process to replace today’s deficient system. The nation’s main organizations representing physicians should propose solutions to support patient-centered care. Individual physicians should become involved, speaking up and sending articles and letters to medical journals and the lay press.
Patients should be empowered to open up a public discussion – in print and broadcast media – on how they want to improve their own medical experiences and the quality of their health care.
It’s worth it. It’s our health.
Dr. Banner is a practicing internist in Philadelphia and chair emeritus of the Albert Einstein Medical Center Medical Ethics Committee. Dr. Benor is a psychiatric psychotherapist in the United States and a wholistic psychotherapist in Canada. Dr. Reiser is adjunct professor, University of Texas School of Public Health, Austin, and the UT Austin Plan II Honors Program, and teaches medical history, medical ethics, and public policy. The authors are indebted to Benjamin Sharfman, PhD, and Jane Brown, PhD, for their important roles in creating this article.
MDedge Daily News: Sleep apnea protects hearts?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Does obstructive sleep apnea protect the heart? Just say no to routine ovarian cancer screening, why MS patients miss out in primary care, and how malpractice claims mark middle age.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Does obstructive sleep apnea protect the heart? Just say no to routine ovarian cancer screening, why MS patients miss out in primary care, and how malpractice claims mark middle age.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Does obstructive sleep apnea protect the heart? Just say no to routine ovarian cancer screening, why MS patients miss out in primary care, and how malpractice claims mark middle age.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Early Intervention for Mental Health Pays Off Later
The facts are dire: In 2014, people diagnosed with schizophrenia or mood disorders made 10.8 million visits to emergency departments (EDs). Between 2006 and 2014, the rate of ED visits related to mental health/substance abuse jumped 44%. The suicide rate among people with serious emotional disturbances (SEDs) is 25 times higher than that in the general population. Two million people with serious mental illness (SMI) are jailed annually, but only about 1 in 3 is currently receiving any treatment.
However, early intervention for SMI can help many people stay out of EDs and jails. That is the focus of The Way Forward: Federal Action for a System That Works for All People Living With SMI and SED and Their Families and Caregivers, a report recently released by the Substance Abuse and Mental Health Services Administration (SAMHSA).
“The emergency room is not a place for people that are experiencing exacerbations of mental health conditions,” says Elinore McCance-Katz, MD, PhD, assistant secretary for mental health and substance use at SAMHSA and chair of the Interdepartmental Serious Mental Illness Coordinating Committee, which produced the report.
In the report, the committee cited the 2003 President’s New Freedom Commission on Mental Health, which concluded that America’s mental health service delivery system was “in shambles,” with “fragmented, disconnected and often inadequate” mental health services and supports. Yet a number of the commission’s recommendations still have not been implemented or only “partially realized,” the committee notes.
In an interview with MedPageToday.com, McCance-Katz says the solution is a “national system of crisis intervention services”—a continuum of care with outpatient services as alternatives to inpatient care. Most states report insufficient psychiatric crisis response capacity, as well as insufficient numbers of inpatient psychiatric hospital beds. If the right system, one that includes community interventions and adequate resources, were in place, McCance-Katz says, “we might not need so many beds.”
The facts are dire: In 2014, people diagnosed with schizophrenia or mood disorders made 10.8 million visits to emergency departments (EDs). Between 2006 and 2014, the rate of ED visits related to mental health/substance abuse jumped 44%. The suicide rate among people with serious emotional disturbances (SEDs) is 25 times higher than that in the general population. Two million people with serious mental illness (SMI) are jailed annually, but only about 1 in 3 is currently receiving any treatment.
However, early intervention for SMI can help many people stay out of EDs and jails. That is the focus of The Way Forward: Federal Action for a System That Works for All People Living With SMI and SED and Their Families and Caregivers, a report recently released by the Substance Abuse and Mental Health Services Administration (SAMHSA).
“The emergency room is not a place for people that are experiencing exacerbations of mental health conditions,” says Elinore McCance-Katz, MD, PhD, assistant secretary for mental health and substance use at SAMHSA and chair of the Interdepartmental Serious Mental Illness Coordinating Committee, which produced the report.
In the report, the committee cited the 2003 President’s New Freedom Commission on Mental Health, which concluded that America’s mental health service delivery system was “in shambles,” with “fragmented, disconnected and often inadequate” mental health services and supports. Yet a number of the commission’s recommendations still have not been implemented or only “partially realized,” the committee notes.
In an interview with MedPageToday.com, McCance-Katz says the solution is a “national system of crisis intervention services”—a continuum of care with outpatient services as alternatives to inpatient care. Most states report insufficient psychiatric crisis response capacity, as well as insufficient numbers of inpatient psychiatric hospital beds. If the right system, one that includes community interventions and adequate resources, were in place, McCance-Katz says, “we might not need so many beds.”
The facts are dire: In 2014, people diagnosed with schizophrenia or mood disorders made 10.8 million visits to emergency departments (EDs). Between 2006 and 2014, the rate of ED visits related to mental health/substance abuse jumped 44%. The suicide rate among people with serious emotional disturbances (SEDs) is 25 times higher than that in the general population. Two million people with serious mental illness (SMI) are jailed annually, but only about 1 in 3 is currently receiving any treatment.
However, early intervention for SMI can help many people stay out of EDs and jails. That is the focus of The Way Forward: Federal Action for a System That Works for All People Living With SMI and SED and Their Families and Caregivers, a report recently released by the Substance Abuse and Mental Health Services Administration (SAMHSA).
“The emergency room is not a place for people that are experiencing exacerbations of mental health conditions,” says Elinore McCance-Katz, MD, PhD, assistant secretary for mental health and substance use at SAMHSA and chair of the Interdepartmental Serious Mental Illness Coordinating Committee, which produced the report.
In the report, the committee cited the 2003 President’s New Freedom Commission on Mental Health, which concluded that America’s mental health service delivery system was “in shambles,” with “fragmented, disconnected and often inadequate” mental health services and supports. Yet a number of the commission’s recommendations still have not been implemented or only “partially realized,” the committee notes.
In an interview with MedPageToday.com, McCance-Katz says the solution is a “national system of crisis intervention services”—a continuum of care with outpatient services as alternatives to inpatient care. Most states report insufficient psychiatric crisis response capacity, as well as insufficient numbers of inpatient psychiatric hospital beds. If the right system, one that includes community interventions and adequate resources, were in place, McCance-Katz says, “we might not need so many beds.”
Young kids with SCA not receiving recommended prophylaxis
Many young children with sickle cell anemia (SCA) may not be taking the recommended antibiotics to prevent invasive pneumococcal disease (IPD), according to research published in Pediatrics.
Results of a previous study indicated that daily treatment with penicillin could reduce the risk of IPD by 84% in young children with SCA.
In the current study, only 18% of young SCA patients received daily penicillin or an equivalent antibiotic as IPD prophylaxis.
“Most children with sickle cell anemia are not getting the antibiotics they should be to adequately protect against potentially deadly infections,” said study author Sarah Reeves, PhD, of the University of Michigan Medical School in Ann Arbor.
“Long-standing recommendations say children with sickle cell anemia should take antibiotics daily for their first 5 years of life. It can be life-saving.”
For this study, Dr Reeves and her colleagues analyzed data on 2821 SCA patients, ages 3 months to 5 years, living in Florida, Illinois, Louisiana, Michigan, South Carolina, and Texas.
The patients were continuously enrolled in the Medicaid program for at least 1 calendar year between 2005 and 2012. The researchers evaluated the receipt of antibiotics through the insurance claims for filled prescriptions.
The team found that, overall, 18% of patients received at least 300 days of antibiotics.
Sixteen percent of patients received at least 300 days of penicillin; 16% received at least 300 days of penicillin or erythromycin; 18% received at least 300 days of penicillin, erythromycin, or amoxicillin; and 22% received at least 300 days of any antibiotic to prevent Streptococcus pneumoniae.
On average, patients received 162 days of penicillin; 164 days of penicillin or erythromycin; 178 days of penicillin, erythromycin, or amoxicillin; and 193 days of any antibiotic to prevent S pneumoniae.
Multivariable analysis suggested that medical visits and a patient’s state of residence were associated with receiving at least 300 days of antibiotics.
The researchers said that each additional SCA-related outpatient visit and well-child visit was associated with incrementally increased odds of receiving at least 300 days of antibiotics. The odds ratio (OR) was 1.01 for SCA-related outpatient visits and 1.08 for well-child visits (P<0.05 for both).
Patients in Florida (OR=0.51, P<0.05), Louisiana (OR=0.57, P<0.05), Michigan (OR=0.60, P<0.05), and South Carolina (OR=0.62, P<0.05) had lower odds of receiving at least 300 days of antibiotics than patients in Illinois (OR=1.00) or Texas (OR=1.01).
The researchers did not investigate why children were not receiving recommended antibiotics, but Dr Reeves identified possible barriers to compliance. She noted that caregiver challenges include picking up prescriptions every 2 weeks from a pharmacy as well as remembering to administer an antibiotic to a young, healthy-appearing child twice a day.
“The types of challenges involved in making sure children get the recommended dose of antibiotics is exacerbated by the substantial burden of care already experienced by families to help control the symptoms of this disease,” Dr Reeves said.
She added that future studies should more deeply explore barriers preventing families from getting antibiotics and potential interventions to improve the rate of children receiving recommended prescriptions.
“Interventions to improve the receipt of antibiotics among children with sickle cell anemia should include enhanced collaboration between healthcare providers, pharmacists, and families,” Dr Reeves said.
“Doctors need to repeatedly discuss the importance of taking antibiotics with families of children with sickle cell anemia. Social factors that may impact receiving filled prescriptions should also be considered, such as the availability of transportation and time to travel to pharmacies to pick up the prescriptions.” ![]()
Many young children with sickle cell anemia (SCA) may not be taking the recommended antibiotics to prevent invasive pneumococcal disease (IPD), according to research published in Pediatrics.
Results of a previous study indicated that daily treatment with penicillin could reduce the risk of IPD by 84% in young children with SCA.
In the current study, only 18% of young SCA patients received daily penicillin or an equivalent antibiotic as IPD prophylaxis.
“Most children with sickle cell anemia are not getting the antibiotics they should be to adequately protect against potentially deadly infections,” said study author Sarah Reeves, PhD, of the University of Michigan Medical School in Ann Arbor.
“Long-standing recommendations say children with sickle cell anemia should take antibiotics daily for their first 5 years of life. It can be life-saving.”
For this study, Dr Reeves and her colleagues analyzed data on 2821 SCA patients, ages 3 months to 5 years, living in Florida, Illinois, Louisiana, Michigan, South Carolina, and Texas.
The patients were continuously enrolled in the Medicaid program for at least 1 calendar year between 2005 and 2012. The researchers evaluated the receipt of antibiotics through the insurance claims for filled prescriptions.
The team found that, overall, 18% of patients received at least 300 days of antibiotics.
Sixteen percent of patients received at least 300 days of penicillin; 16% received at least 300 days of penicillin or erythromycin; 18% received at least 300 days of penicillin, erythromycin, or amoxicillin; and 22% received at least 300 days of any antibiotic to prevent Streptococcus pneumoniae.
On average, patients received 162 days of penicillin; 164 days of penicillin or erythromycin; 178 days of penicillin, erythromycin, or amoxicillin; and 193 days of any antibiotic to prevent S pneumoniae.
Multivariable analysis suggested that medical visits and a patient’s state of residence were associated with receiving at least 300 days of antibiotics.
The researchers said that each additional SCA-related outpatient visit and well-child visit was associated with incrementally increased odds of receiving at least 300 days of antibiotics. The odds ratio (OR) was 1.01 for SCA-related outpatient visits and 1.08 for well-child visits (P<0.05 for both).
Patients in Florida (OR=0.51, P<0.05), Louisiana (OR=0.57, P<0.05), Michigan (OR=0.60, P<0.05), and South Carolina (OR=0.62, P<0.05) had lower odds of receiving at least 300 days of antibiotics than patients in Illinois (OR=1.00) or Texas (OR=1.01).
The researchers did not investigate why children were not receiving recommended antibiotics, but Dr Reeves identified possible barriers to compliance. She noted that caregiver challenges include picking up prescriptions every 2 weeks from a pharmacy as well as remembering to administer an antibiotic to a young, healthy-appearing child twice a day.
“The types of challenges involved in making sure children get the recommended dose of antibiotics is exacerbated by the substantial burden of care already experienced by families to help control the symptoms of this disease,” Dr Reeves said.
She added that future studies should more deeply explore barriers preventing families from getting antibiotics and potential interventions to improve the rate of children receiving recommended prescriptions.
“Interventions to improve the receipt of antibiotics among children with sickle cell anemia should include enhanced collaboration between healthcare providers, pharmacists, and families,” Dr Reeves said.
“Doctors need to repeatedly discuss the importance of taking antibiotics with families of children with sickle cell anemia. Social factors that may impact receiving filled prescriptions should also be considered, such as the availability of transportation and time to travel to pharmacies to pick up the prescriptions.” ![]()
Many young children with sickle cell anemia (SCA) may not be taking the recommended antibiotics to prevent invasive pneumococcal disease (IPD), according to research published in Pediatrics.
Results of a previous study indicated that daily treatment with penicillin could reduce the risk of IPD by 84% in young children with SCA.
In the current study, only 18% of young SCA patients received daily penicillin or an equivalent antibiotic as IPD prophylaxis.
“Most children with sickle cell anemia are not getting the antibiotics they should be to adequately protect against potentially deadly infections,” said study author Sarah Reeves, PhD, of the University of Michigan Medical School in Ann Arbor.
“Long-standing recommendations say children with sickle cell anemia should take antibiotics daily for their first 5 years of life. It can be life-saving.”
For this study, Dr Reeves and her colleagues analyzed data on 2821 SCA patients, ages 3 months to 5 years, living in Florida, Illinois, Louisiana, Michigan, South Carolina, and Texas.
The patients were continuously enrolled in the Medicaid program for at least 1 calendar year between 2005 and 2012. The researchers evaluated the receipt of antibiotics through the insurance claims for filled prescriptions.
The team found that, overall, 18% of patients received at least 300 days of antibiotics.
Sixteen percent of patients received at least 300 days of penicillin; 16% received at least 300 days of penicillin or erythromycin; 18% received at least 300 days of penicillin, erythromycin, or amoxicillin; and 22% received at least 300 days of any antibiotic to prevent Streptococcus pneumoniae.
On average, patients received 162 days of penicillin; 164 days of penicillin or erythromycin; 178 days of penicillin, erythromycin, or amoxicillin; and 193 days of any antibiotic to prevent S pneumoniae.
Multivariable analysis suggested that medical visits and a patient’s state of residence were associated with receiving at least 300 days of antibiotics.
The researchers said that each additional SCA-related outpatient visit and well-child visit was associated with incrementally increased odds of receiving at least 300 days of antibiotics. The odds ratio (OR) was 1.01 for SCA-related outpatient visits and 1.08 for well-child visits (P<0.05 for both).
Patients in Florida (OR=0.51, P<0.05), Louisiana (OR=0.57, P<0.05), Michigan (OR=0.60, P<0.05), and South Carolina (OR=0.62, P<0.05) had lower odds of receiving at least 300 days of antibiotics than patients in Illinois (OR=1.00) or Texas (OR=1.01).
The researchers did not investigate why children were not receiving recommended antibiotics, but Dr Reeves identified possible barriers to compliance. She noted that caregiver challenges include picking up prescriptions every 2 weeks from a pharmacy as well as remembering to administer an antibiotic to a young, healthy-appearing child twice a day.
“The types of challenges involved in making sure children get the recommended dose of antibiotics is exacerbated by the substantial burden of care already experienced by families to help control the symptoms of this disease,” Dr Reeves said.
She added that future studies should more deeply explore barriers preventing families from getting antibiotics and potential interventions to improve the rate of children receiving recommended prescriptions.
“Interventions to improve the receipt of antibiotics among children with sickle cell anemia should include enhanced collaboration between healthcare providers, pharmacists, and families,” Dr Reeves said.
“Doctors need to repeatedly discuss the importance of taking antibiotics with families of children with sickle cell anemia. Social factors that may impact receiving filled prescriptions should also be considered, such as the availability of transportation and time to travel to pharmacies to pick up the prescriptions.” ![]()
JAK2 inhibition could reduce risk of GVHD
Preclinical research suggests that targeting JAK2 can reduce the risk of graft-versus-host disease (GVHD) in transplant recipients.
Researchers found that genetic ablation of JAK2 on donor T cells or inhibition of JAK2 via treatment with pacritinib reduced GVHD in mice without compromising graft-versus-leukemia (GVL) activity.
“An effort to identify selective immune suppression whereby GVHD is reduced and the antitumor activity of the graft is preserved is key to improving the success of blood and marrow transplantation,” said Brian C. Betts, MD, of Moffitt Cancer Center in Tampa, Florida.
Dr Betts and his colleagues conducted this research and recounted their findings in PNAS.
In experiments with mice, the researchers found that donor T cells with JAK2 deletion were less likely than wild-type donor T cells to induce GVHD. However, JAK2 deletion did not impair the GVL effect.
Mice that received JAK2−/− T cells had longer survival, higher body weights, and less GVHD than control mice.
The researchers said targeting JAK2 may reduce GVHD, in part, by limiting Th1 differentiation and the migratory capacity of alloreactive T cells. However, targeting JAK2 promotes beneficial regulatory-T-cell and Th2 differentiation as well.
The researchers also tested the effects of JAK2 inhibition with pacritinib. Mice received allografts and were treated with pacritinib or vehicle control for 3 weeks.
As before, JAK2 inhibition reduced acute GVHD mortality while preserving the GVL effect.
The team also discovered that pacritinib could protect mice from tissue graft rejection, suggesting the drug could be used to prevent kidney or liver transplant rejection.
Now, the researchers are working on a phase 1/2 trial designed to determine if pacritinib and standard immune suppression can prevent acute GVHD after hematopoietic stem cell transplant (NCT02891603). ![]()
Preclinical research suggests that targeting JAK2 can reduce the risk of graft-versus-host disease (GVHD) in transplant recipients.
Researchers found that genetic ablation of JAK2 on donor T cells or inhibition of JAK2 via treatment with pacritinib reduced GVHD in mice without compromising graft-versus-leukemia (GVL) activity.
“An effort to identify selective immune suppression whereby GVHD is reduced and the antitumor activity of the graft is preserved is key to improving the success of blood and marrow transplantation,” said Brian C. Betts, MD, of Moffitt Cancer Center in Tampa, Florida.
Dr Betts and his colleagues conducted this research and recounted their findings in PNAS.
In experiments with mice, the researchers found that donor T cells with JAK2 deletion were less likely than wild-type donor T cells to induce GVHD. However, JAK2 deletion did not impair the GVL effect.
Mice that received JAK2−/− T cells had longer survival, higher body weights, and less GVHD than control mice.
The researchers said targeting JAK2 may reduce GVHD, in part, by limiting Th1 differentiation and the migratory capacity of alloreactive T cells. However, targeting JAK2 promotes beneficial regulatory-T-cell and Th2 differentiation as well.
The researchers also tested the effects of JAK2 inhibition with pacritinib. Mice received allografts and were treated with pacritinib or vehicle control for 3 weeks.
As before, JAK2 inhibition reduced acute GVHD mortality while preserving the GVL effect.
The team also discovered that pacritinib could protect mice from tissue graft rejection, suggesting the drug could be used to prevent kidney or liver transplant rejection.
Now, the researchers are working on a phase 1/2 trial designed to determine if pacritinib and standard immune suppression can prevent acute GVHD after hematopoietic stem cell transplant (NCT02891603). ![]()
Preclinical research suggests that targeting JAK2 can reduce the risk of graft-versus-host disease (GVHD) in transplant recipients.
Researchers found that genetic ablation of JAK2 on donor T cells or inhibition of JAK2 via treatment with pacritinib reduced GVHD in mice without compromising graft-versus-leukemia (GVL) activity.
“An effort to identify selective immune suppression whereby GVHD is reduced and the antitumor activity of the graft is preserved is key to improving the success of blood and marrow transplantation,” said Brian C. Betts, MD, of Moffitt Cancer Center in Tampa, Florida.
Dr Betts and his colleagues conducted this research and recounted their findings in PNAS.
In experiments with mice, the researchers found that donor T cells with JAK2 deletion were less likely than wild-type donor T cells to induce GVHD. However, JAK2 deletion did not impair the GVL effect.
Mice that received JAK2−/− T cells had longer survival, higher body weights, and less GVHD than control mice.
The researchers said targeting JAK2 may reduce GVHD, in part, by limiting Th1 differentiation and the migratory capacity of alloreactive T cells. However, targeting JAK2 promotes beneficial regulatory-T-cell and Th2 differentiation as well.
The researchers also tested the effects of JAK2 inhibition with pacritinib. Mice received allografts and were treated with pacritinib or vehicle control for 3 weeks.
As before, JAK2 inhibition reduced acute GVHD mortality while preserving the GVL effect.
The team also discovered that pacritinib could protect mice from tissue graft rejection, suggesting the drug could be used to prevent kidney or liver transplant rejection.
Now, the researchers are working on a phase 1/2 trial designed to determine if pacritinib and standard immune suppression can prevent acute GVHD after hematopoietic stem cell transplant (NCT02891603). ![]()
Child’s cancer diagnosis can affect mother’s income long-term
A study conducted in Sweden revealed that social benefits can ease financial burdens for parents of children recently diagnosed with cancer.
However, the study also showed that mothers experienced persistently lower income after benefits diminished.
Ayako Hiyoshi, PhD, of Örebro University in Örebro, Sweden, and her colleagues detailed these findings in Cancer.
The researchers gathered information from Swedish national registers and examined the trajectories of parents’ income from different sources.
Parents of children with cancer diagnosed between 2004 and 2009 were identified and matched with parents of children without cancer (reference parents).
In total, 20,091 families were followed from the year before cancer diagnosis to a maximum of 8 years.
The researchers noted that, around the time of a child’s cancer diagnosis, total income (from all sources) was, on average, higher in mothers of children with cancer than in reference mothers.
The ratio of mean total income for mothers of children with cancer, compared to reference mothers, was 1.032 at 1 year prior to the child’s diagnosis and 1.064 the year of diagnosis.
For fathers of children with cancer, total income was slightly lower than reference fathers’ income. The ratios were 0.987 at 1 year prior to diagnosis and 0.995 the year of diagnosis.
The researchers also noted that parents’ income from work was at its lowest around the time of a child’s cancer diagnosis but increased with time.
At cancer diagnosis, the ratio of mean income from work was 0.642 for mothers and 0.858 for fathers. One year later, the ratios were 0.786 and 0.956, respectively. At 3 years, the ratios were 0.876 and 0.986, respectively. At 6 years, the ratios were 0.856 and 1.058, respectively.
The researchers pointed out that sickness and childcare-related benefits, which compensated for income loss, were greater for parents of children with cancer than for reference parents. However, social benefits diminished over time.
One year prior to cancer diagnosis, the ratio of sickness benefits was 3.495 for mothers and 5.213 for fathers. The year of diagnosis, the ratios were 4.785 and 5.795, respectively. At 3 years, the ratios were 1.404 and 1.339, respectively. And at 6 years, the ratios were 0.931 and 1.421, respectively.
One year prior to cancer diagnosis, the ratio of childcare-related benefits was 2.830 for mothers and 3.514 for fathers. The year of diagnosis, the ratios were 4.553 and 4.930, respectively. At 3 years, the ratios were 2.225 and 1.948, respectively. And at 6 years, the ratios were 1.272 and 1.095, respectively.
The decline of social benefits over time meant that cancer mothers’ total income became lower than that of reference mothers, and this difference persisted over the period studied. This was not the case for cancer fathers, however.
The ratio of income from all sources for cancer mothers compared to reference mothers was 1.064 the year of diagnosis, 0.985 at 2 years, 0.966 at 4 years, and 0.934 at 6 years.
The ratio of income from all sources for cancer fathers compared to reference fathers was 0.995 the year of diagnosis, 0.993 at 2 years, 0.998 at 4 years, and 1.029 at 6 years.
“A significant and unexpected finding was that, although income from employment stayed lower for several years for mothers, total income was higher for mothers of children with cancer around the time of the child’s cancer diagnosis when the compensation from social benefits were included,” Dr Hiyoshi said.
“The persistently lower income from employment for mothers of children with cancer compared with mothers of cancer-free children implies potential long-term consequences for the mothers of children with cancer, including their career and future pension in old age.” ![]()
A study conducted in Sweden revealed that social benefits can ease financial burdens for parents of children recently diagnosed with cancer.
However, the study also showed that mothers experienced persistently lower income after benefits diminished.
Ayako Hiyoshi, PhD, of Örebro University in Örebro, Sweden, and her colleagues detailed these findings in Cancer.
The researchers gathered information from Swedish national registers and examined the trajectories of parents’ income from different sources.
Parents of children with cancer diagnosed between 2004 and 2009 were identified and matched with parents of children without cancer (reference parents).
In total, 20,091 families were followed from the year before cancer diagnosis to a maximum of 8 years.
The researchers noted that, around the time of a child’s cancer diagnosis, total income (from all sources) was, on average, higher in mothers of children with cancer than in reference mothers.
The ratio of mean total income for mothers of children with cancer, compared to reference mothers, was 1.032 at 1 year prior to the child’s diagnosis and 1.064 the year of diagnosis.
For fathers of children with cancer, total income was slightly lower than reference fathers’ income. The ratios were 0.987 at 1 year prior to diagnosis and 0.995 the year of diagnosis.
The researchers also noted that parents’ income from work was at its lowest around the time of a child’s cancer diagnosis but increased with time.
At cancer diagnosis, the ratio of mean income from work was 0.642 for mothers and 0.858 for fathers. One year later, the ratios were 0.786 and 0.956, respectively. At 3 years, the ratios were 0.876 and 0.986, respectively. At 6 years, the ratios were 0.856 and 1.058, respectively.
The researchers pointed out that sickness and childcare-related benefits, which compensated for income loss, were greater for parents of children with cancer than for reference parents. However, social benefits diminished over time.
One year prior to cancer diagnosis, the ratio of sickness benefits was 3.495 for mothers and 5.213 for fathers. The year of diagnosis, the ratios were 4.785 and 5.795, respectively. At 3 years, the ratios were 1.404 and 1.339, respectively. And at 6 years, the ratios were 0.931 and 1.421, respectively.
One year prior to cancer diagnosis, the ratio of childcare-related benefits was 2.830 for mothers and 3.514 for fathers. The year of diagnosis, the ratios were 4.553 and 4.930, respectively. At 3 years, the ratios were 2.225 and 1.948, respectively. And at 6 years, the ratios were 1.272 and 1.095, respectively.
The decline of social benefits over time meant that cancer mothers’ total income became lower than that of reference mothers, and this difference persisted over the period studied. This was not the case for cancer fathers, however.
The ratio of income from all sources for cancer mothers compared to reference mothers was 1.064 the year of diagnosis, 0.985 at 2 years, 0.966 at 4 years, and 0.934 at 6 years.
The ratio of income from all sources for cancer fathers compared to reference fathers was 0.995 the year of diagnosis, 0.993 at 2 years, 0.998 at 4 years, and 1.029 at 6 years.
“A significant and unexpected finding was that, although income from employment stayed lower for several years for mothers, total income was higher for mothers of children with cancer around the time of the child’s cancer diagnosis when the compensation from social benefits were included,” Dr Hiyoshi said.
“The persistently lower income from employment for mothers of children with cancer compared with mothers of cancer-free children implies potential long-term consequences for the mothers of children with cancer, including their career and future pension in old age.” ![]()
A study conducted in Sweden revealed that social benefits can ease financial burdens for parents of children recently diagnosed with cancer.
However, the study also showed that mothers experienced persistently lower income after benefits diminished.
Ayako Hiyoshi, PhD, of Örebro University in Örebro, Sweden, and her colleagues detailed these findings in Cancer.
The researchers gathered information from Swedish national registers and examined the trajectories of parents’ income from different sources.
Parents of children with cancer diagnosed between 2004 and 2009 were identified and matched with parents of children without cancer (reference parents).
In total, 20,091 families were followed from the year before cancer diagnosis to a maximum of 8 years.
The researchers noted that, around the time of a child’s cancer diagnosis, total income (from all sources) was, on average, higher in mothers of children with cancer than in reference mothers.
The ratio of mean total income for mothers of children with cancer, compared to reference mothers, was 1.032 at 1 year prior to the child’s diagnosis and 1.064 the year of diagnosis.
For fathers of children with cancer, total income was slightly lower than reference fathers’ income. The ratios were 0.987 at 1 year prior to diagnosis and 0.995 the year of diagnosis.
The researchers also noted that parents’ income from work was at its lowest around the time of a child’s cancer diagnosis but increased with time.
At cancer diagnosis, the ratio of mean income from work was 0.642 for mothers and 0.858 for fathers. One year later, the ratios were 0.786 and 0.956, respectively. At 3 years, the ratios were 0.876 and 0.986, respectively. At 6 years, the ratios were 0.856 and 1.058, respectively.
The researchers pointed out that sickness and childcare-related benefits, which compensated for income loss, were greater for parents of children with cancer than for reference parents. However, social benefits diminished over time.
One year prior to cancer diagnosis, the ratio of sickness benefits was 3.495 for mothers and 5.213 for fathers. The year of diagnosis, the ratios were 4.785 and 5.795, respectively. At 3 years, the ratios were 1.404 and 1.339, respectively. And at 6 years, the ratios were 0.931 and 1.421, respectively.
One year prior to cancer diagnosis, the ratio of childcare-related benefits was 2.830 for mothers and 3.514 for fathers. The year of diagnosis, the ratios were 4.553 and 4.930, respectively. At 3 years, the ratios were 2.225 and 1.948, respectively. And at 6 years, the ratios were 1.272 and 1.095, respectively.
The decline of social benefits over time meant that cancer mothers’ total income became lower than that of reference mothers, and this difference persisted over the period studied. This was not the case for cancer fathers, however.
The ratio of income from all sources for cancer mothers compared to reference mothers was 1.064 the year of diagnosis, 0.985 at 2 years, 0.966 at 4 years, and 0.934 at 6 years.
The ratio of income from all sources for cancer fathers compared to reference fathers was 0.995 the year of diagnosis, 0.993 at 2 years, 0.998 at 4 years, and 1.029 at 6 years.
“A significant and unexpected finding was that, although income from employment stayed lower for several years for mothers, total income was higher for mothers of children with cancer around the time of the child’s cancer diagnosis when the compensation from social benefits were included,” Dr Hiyoshi said.
“The persistently lower income from employment for mothers of children with cancer compared with mothers of cancer-free children implies potential long-term consequences for the mothers of children with cancer, including their career and future pension in old age.” ![]()