User login
Scoring system quantified chances of HCV treatment benefit
A new scoring system predicted which patients with decompensated cirrhosis caused by hepatitis C virus (HCV) infection were most likely to experience meaningful benefits from direct-acting antiviral (DAA) therapy.
Dubbed BEA3, their scoring system assigns one point each for body mass index under 25 kg/m2, absence of encephalopathy, absence of ascites, ALT more than 1.5 times the upper limit of normal, and albumin above 3.5 g/dL. Patients who scored 4 or 5 were more than 50 times more likely to improve to Child-Pugh Turcotte (CPT) class A (compensated) cirrhosis with DAA therapy than were patients who scored 0 (hazard ratio, 52.3; 95% confidence interval, 15.2-179.7; P less than .001), wrote Omar El-Sherif, MB, BCh, of St. James’s Hospital, Dublin, together with his associates in the June issue of Gastroenterology.
Eradicating HCV does not necessarily improve the odds of transplant-free survival in the setting of decompensated cirrhosis, the researchers noted. Patients can end up in “MELD [Model for End-Stage Liver Disease] purgatory,” meaning they are still decompensated despite achieving sustained virologic response and improved MELD scores. Such patients can face longer waits for liver transplantation than if they had foregone DAA therapy. “There is an urgent need for data to refine our understanding of the reversibility of hepatic decompensation with viral eradication, and, ultimately, define the “point of no return,” the degree of liver dysfunction at which HCV therapy does not yield any meaningful clinical benefit, the researchers wrote.
Their study included 622 patients from the SOLAR-1, SOLAR-2, ASTRAL-4, and GS-US-334-0125 trials, which evaluated interferon-free sofosbuvir-based DAA therapy in patients with chronic hepatitis C virus infection and advanced liver disease. Patients received 12 or 24 weeks of therapy with ledipasvir, sofosbuvir, and ribavirin or velpatasvir, sofosbuvir, and/or ribavirin, or 48 weeks of treatment with sofosbuvir and ribavirin.
A total of 32% of patients with CPT class B cirrhosis improved to class A, as did 12% of patients with class C cirrhosis. Each factor in the scoring system independently affected the chances of reaching CPT class A cirrhosis, even after accounting for SVR.
Notably, patients with intermediate BEA3 scores of 1, 2, or 3 were significantly more likely to reach CPT class A cirrhosis than were patients with scores of 0, with hazard ratios ranging from 4.2 (for a score of 1) to 21.2 (for a score of 3). Most patients had scores of 0 (106 individuals), 1 (219 individuals), or 2 (180 individuals), and only 23 patients scored a 4 or a 5.
CPT score reflects prothrombin time, serum albumin and bilirubin, and the presence or severity of ascites. The investigators called the new scoring system “a tool that can enhance shared decision making at the point of care, quantifying the potential benefits of DAA therapy for patients with decompensated cirrhosis in the pretransplant setting.”Dr. El-Sherif disclosed ties to Gilead Sciences, Bristol-Myers Squibb, and the Health Research Board of Ireland. Four coinvestigators disclosed employment with Gilead, and several other coinvestigators disclosed ties to Gilead, BMS, AbbVie, and other companies.
SOURCE: El-Sherif O et al. Gastroenterology. 2018 Mar 10. doi: 10.1053/j.gastro.2018.03.022.
Patients with decompensated cirrhosis are now able to receive antiviral therapy without risk of worsening symptoms of decompensation. More clinics are able to offer DAA therapy to patients with hepatitis C, without the need for expertise in managing the side effects of interferon-based therapy.
The study by El-Sherif et al. summarizes well the benefits and potential pitfalls of treatment of hepatitis C in patients with decompensated cirrhosis. Their scoring system is largely intuitive and mirrors the traditional Child-Turcotte-Pugh score in that patients with low serum albumin, hepatic encephalopathy, and ascites are at risk of failing to improve clinically. Patients can have their hepatitis C successfully treated but can be trapped in “MELD purgatory,” a state of significant symptoms of liver disease, without the objective priority points necessary to be candidates for liver transplantation.
As experience is gained in the use of DAA medications for HCV, it is incumbent on physicians to gather knowledge that will further refine their understanding of which patients with signs of liver decompensation might benefit. It is also clear that patients with decompensated cirrhosis should be managed by clinicians who have experience in liver transplantation, to ensure that patients are counseled regarding not just the benefits, but potential risks of DAA therapy for hepatitis C.
Roman E. Perri, MD, is assistant professor of medicine, division of gastroenterology and hepatology, Medical Director for Liver Transplantion, Vanderbilt University, Nashville, Tenn. He has no conflicts of interest.
Patients with decompensated cirrhosis are now able to receive antiviral therapy without risk of worsening symptoms of decompensation. More clinics are able to offer DAA therapy to patients with hepatitis C, without the need for expertise in managing the side effects of interferon-based therapy.
The study by El-Sherif et al. summarizes well the benefits and potential pitfalls of treatment of hepatitis C in patients with decompensated cirrhosis. Their scoring system is largely intuitive and mirrors the traditional Child-Turcotte-Pugh score in that patients with low serum albumin, hepatic encephalopathy, and ascites are at risk of failing to improve clinically. Patients can have their hepatitis C successfully treated but can be trapped in “MELD purgatory,” a state of significant symptoms of liver disease, without the objective priority points necessary to be candidates for liver transplantation.
As experience is gained in the use of DAA medications for HCV, it is incumbent on physicians to gather knowledge that will further refine their understanding of which patients with signs of liver decompensation might benefit. It is also clear that patients with decompensated cirrhosis should be managed by clinicians who have experience in liver transplantation, to ensure that patients are counseled regarding not just the benefits, but potential risks of DAA therapy for hepatitis C.
Roman E. Perri, MD, is assistant professor of medicine, division of gastroenterology and hepatology, Medical Director for Liver Transplantion, Vanderbilt University, Nashville, Tenn. He has no conflicts of interest.
Patients with decompensated cirrhosis are now able to receive antiviral therapy without risk of worsening symptoms of decompensation. More clinics are able to offer DAA therapy to patients with hepatitis C, without the need for expertise in managing the side effects of interferon-based therapy.
The study by El-Sherif et al. summarizes well the benefits and potential pitfalls of treatment of hepatitis C in patients with decompensated cirrhosis. Their scoring system is largely intuitive and mirrors the traditional Child-Turcotte-Pugh score in that patients with low serum albumin, hepatic encephalopathy, and ascites are at risk of failing to improve clinically. Patients can have their hepatitis C successfully treated but can be trapped in “MELD purgatory,” a state of significant symptoms of liver disease, without the objective priority points necessary to be candidates for liver transplantation.
As experience is gained in the use of DAA medications for HCV, it is incumbent on physicians to gather knowledge that will further refine their understanding of which patients with signs of liver decompensation might benefit. It is also clear that patients with decompensated cirrhosis should be managed by clinicians who have experience in liver transplantation, to ensure that patients are counseled regarding not just the benefits, but potential risks of DAA therapy for hepatitis C.
Roman E. Perri, MD, is assistant professor of medicine, division of gastroenterology and hepatology, Medical Director for Liver Transplantion, Vanderbilt University, Nashville, Tenn. He has no conflicts of interest.
A new scoring system predicted which patients with decompensated cirrhosis caused by hepatitis C virus (HCV) infection were most likely to experience meaningful benefits from direct-acting antiviral (DAA) therapy.
Dubbed BEA3, their scoring system assigns one point each for body mass index under 25 kg/m2, absence of encephalopathy, absence of ascites, ALT more than 1.5 times the upper limit of normal, and albumin above 3.5 g/dL. Patients who scored 4 or 5 were more than 50 times more likely to improve to Child-Pugh Turcotte (CPT) class A (compensated) cirrhosis with DAA therapy than were patients who scored 0 (hazard ratio, 52.3; 95% confidence interval, 15.2-179.7; P less than .001), wrote Omar El-Sherif, MB, BCh, of St. James’s Hospital, Dublin, together with his associates in the June issue of Gastroenterology.
Eradicating HCV does not necessarily improve the odds of transplant-free survival in the setting of decompensated cirrhosis, the researchers noted. Patients can end up in “MELD [Model for End-Stage Liver Disease] purgatory,” meaning they are still decompensated despite achieving sustained virologic response and improved MELD scores. Such patients can face longer waits for liver transplantation than if they had foregone DAA therapy. “There is an urgent need for data to refine our understanding of the reversibility of hepatic decompensation with viral eradication, and, ultimately, define the “point of no return,” the degree of liver dysfunction at which HCV therapy does not yield any meaningful clinical benefit, the researchers wrote.
Their study included 622 patients from the SOLAR-1, SOLAR-2, ASTRAL-4, and GS-US-334-0125 trials, which evaluated interferon-free sofosbuvir-based DAA therapy in patients with chronic hepatitis C virus infection and advanced liver disease. Patients received 12 or 24 weeks of therapy with ledipasvir, sofosbuvir, and ribavirin or velpatasvir, sofosbuvir, and/or ribavirin, or 48 weeks of treatment with sofosbuvir and ribavirin.
A total of 32% of patients with CPT class B cirrhosis improved to class A, as did 12% of patients with class C cirrhosis. Each factor in the scoring system independently affected the chances of reaching CPT class A cirrhosis, even after accounting for SVR.
Notably, patients with intermediate BEA3 scores of 1, 2, or 3 were significantly more likely to reach CPT class A cirrhosis than were patients with scores of 0, with hazard ratios ranging from 4.2 (for a score of 1) to 21.2 (for a score of 3). Most patients had scores of 0 (106 individuals), 1 (219 individuals), or 2 (180 individuals), and only 23 patients scored a 4 or a 5.
CPT score reflects prothrombin time, serum albumin and bilirubin, and the presence or severity of ascites. The investigators called the new scoring system “a tool that can enhance shared decision making at the point of care, quantifying the potential benefits of DAA therapy for patients with decompensated cirrhosis in the pretransplant setting.”Dr. El-Sherif disclosed ties to Gilead Sciences, Bristol-Myers Squibb, and the Health Research Board of Ireland. Four coinvestigators disclosed employment with Gilead, and several other coinvestigators disclosed ties to Gilead, BMS, AbbVie, and other companies.
SOURCE: El-Sherif O et al. Gastroenterology. 2018 Mar 10. doi: 10.1053/j.gastro.2018.03.022.
A new scoring system predicted which patients with decompensated cirrhosis caused by hepatitis C virus (HCV) infection were most likely to experience meaningful benefits from direct-acting antiviral (DAA) therapy.
Dubbed BEA3, their scoring system assigns one point each for body mass index under 25 kg/m2, absence of encephalopathy, absence of ascites, ALT more than 1.5 times the upper limit of normal, and albumin above 3.5 g/dL. Patients who scored 4 or 5 were more than 50 times more likely to improve to Child-Pugh Turcotte (CPT) class A (compensated) cirrhosis with DAA therapy than were patients who scored 0 (hazard ratio, 52.3; 95% confidence interval, 15.2-179.7; P less than .001), wrote Omar El-Sherif, MB, BCh, of St. James’s Hospital, Dublin, together with his associates in the June issue of Gastroenterology.
Eradicating HCV does not necessarily improve the odds of transplant-free survival in the setting of decompensated cirrhosis, the researchers noted. Patients can end up in “MELD [Model for End-Stage Liver Disease] purgatory,” meaning they are still decompensated despite achieving sustained virologic response and improved MELD scores. Such patients can face longer waits for liver transplantation than if they had foregone DAA therapy. “There is an urgent need for data to refine our understanding of the reversibility of hepatic decompensation with viral eradication, and, ultimately, define the “point of no return,” the degree of liver dysfunction at which HCV therapy does not yield any meaningful clinical benefit, the researchers wrote.
Their study included 622 patients from the SOLAR-1, SOLAR-2, ASTRAL-4, and GS-US-334-0125 trials, which evaluated interferon-free sofosbuvir-based DAA therapy in patients with chronic hepatitis C virus infection and advanced liver disease. Patients received 12 or 24 weeks of therapy with ledipasvir, sofosbuvir, and ribavirin or velpatasvir, sofosbuvir, and/or ribavirin, or 48 weeks of treatment with sofosbuvir and ribavirin.
A total of 32% of patients with CPT class B cirrhosis improved to class A, as did 12% of patients with class C cirrhosis. Each factor in the scoring system independently affected the chances of reaching CPT class A cirrhosis, even after accounting for SVR.
Notably, patients with intermediate BEA3 scores of 1, 2, or 3 were significantly more likely to reach CPT class A cirrhosis than were patients with scores of 0, with hazard ratios ranging from 4.2 (for a score of 1) to 21.2 (for a score of 3). Most patients had scores of 0 (106 individuals), 1 (219 individuals), or 2 (180 individuals), and only 23 patients scored a 4 or a 5.
CPT score reflects prothrombin time, serum albumin and bilirubin, and the presence or severity of ascites. The investigators called the new scoring system “a tool that can enhance shared decision making at the point of care, quantifying the potential benefits of DAA therapy for patients with decompensated cirrhosis in the pretransplant setting.”Dr. El-Sherif disclosed ties to Gilead Sciences, Bristol-Myers Squibb, and the Health Research Board of Ireland. Four coinvestigators disclosed employment with Gilead, and several other coinvestigators disclosed ties to Gilead, BMS, AbbVie, and other companies.
SOURCE: El-Sherif O et al. Gastroenterology. 2018 Mar 10. doi: 10.1053/j.gastro.2018.03.022.
FROM GASTROENTEROLOGY
June 2018 Digital Edition
Click here to access the June 2018 Digital Edition.
Table of Contents
- Setting and Method of Measurement Affect Blood Pressure Readings
- Workforce Assessment of VA Home-Based Primary Care Pharmacists
- Using Simulation to Enhance Care of Older Veterans
- Effect of High-Dose Ergocalciferol on Rate of Falls
- Vitreous Hemorrhage in the Setting of a Vascular Loop
- Nephrogenic Systemic Fibrosis in a Patient With Multiple Inflammatory Disorders
- C difficile Colitis in a Patient With Abdominal Complications
- The VA Cannot Be Privatized

Click here to access the June 2018 Digital Edition.
Table of Contents
- Setting and Method of Measurement Affect Blood Pressure Readings
- Workforce Assessment of VA Home-Based Primary Care Pharmacists
- Using Simulation to Enhance Care of Older Veterans
- Effect of High-Dose Ergocalciferol on Rate of Falls
- Vitreous Hemorrhage in the Setting of a Vascular Loop
- Nephrogenic Systemic Fibrosis in a Patient With Multiple Inflammatory Disorders
- C difficile Colitis in a Patient With Abdominal Complications
- The VA Cannot Be Privatized

Click here to access the June 2018 Digital Edition.
Table of Contents
- Setting and Method of Measurement Affect Blood Pressure Readings
- Workforce Assessment of VA Home-Based Primary Care Pharmacists
- Using Simulation to Enhance Care of Older Veterans
- Effect of High-Dose Ergocalciferol on Rate of Falls
- Vitreous Hemorrhage in the Setting of a Vascular Loop
- Nephrogenic Systemic Fibrosis in a Patient With Multiple Inflammatory Disorders
- C difficile Colitis in a Patient With Abdominal Complications
- The VA Cannot Be Privatized

Liquid biopsies may be the future in managing MM patients
Scientists from the Dana-Farber Cancer Institute and the Broad Institute in Boston, Massachusetts, have shown that in multiple myeloma (MM), liquid biopsies provide similar genetic information as tumor biopsies.
Unlike tumor biopsies, liquid biopsies are noninvasive and, in the future, may offer a cost-effective way of following disease state, disease progression, and response to treatment.
The scientists used cell-free DNA (cfDNA) from 107 patients, circulating tumor cells (CTCs) from 56 patients, and compared their genomic profiling with bone marrow biopsies from 9 patients with MM.
The researchers used a two-step approach using the 2 kinds of liquid biopsies—CTCs and cfDNA. They first used “ultra-low pass” whole genome sequencing, which was a rapid and cost-effective method to identify blood samples with tumor DNA of at least 5%-10% (tumor fraction).
These samples were then subject to whole-exome sequencing (WES), which analyzes protein-coding sequence of the genome.
The researchers found genetic alterations in MM such as copy number alterations (1p, 13q deletions or 11q gain) and somatic single nucleotide variations (SSNVs), among others, which they observed in matched cfDNA, CTCs, and tumor DNA (tDNA) from tumor biopsies.
The liquid biopsies also captured the clonal heterogeneity characteristic of MM—99% of clonal mutations present in tDNA were also seen in cfDNA or CTCs, and 94% of mutations present in cfDNA or CTCs were seen in tDNA.
In addition, CTCs or cfDNA samples with higher tumor purity uncovered more mutations than were seen in tDNA.
“[The] combination of CTCs and cfDNA was able to detect almost all clonal mutations identified in the bone marrow biopsy sample and defined other subclones that were not identified in the bone marrow,” the scientists noted.
This increased sensitivity was “potentially due to the limitation of sampling site of the bone marrow,” they pointed out.
The scientists also showed that the tumor fraction in cfDNA and enriched CTCs correlated with disease progression from MGUS (monoclonal gammopathy of undetermined significance) and SMM (smoldering MM), to overt MM.
The scientists also evaluated liquid biopsies to determine response to treatment.
For example, a patient who responded to carfilzomib, lenalidomide, and dexamethasone, had a decreased cfDNA tumor fraction—from 22% to 2%.
Another patient who progressed on daratumumab over a period of 2 months showed an increase in tumor fraction—from 11% to 46%.
CTCs and cfDNA captured the mutational landscape of MM and provided a non-invasive profiling of tumor evolution. The liquid biopsy approach may be used as a novel biomarker for disease progression and response to therapy, the scientists suggest.
"Our discovery that cfDNA and CTC analyses agree with each other at the comprehensive level is an important finding,” said co-senior author Irene Ghobrial, MD, “because this means that routine genetic profiling of patient tumors from blood would be feasible."
"Our ultimate goal is to eventually use all the samples to monitor disease progression," added Jihye Park, PhD, researcher in the Ghobrial lab and co-first author on the paper.
Currently, bone marrow biopsies are the gold standard for diagnosing and monitoring the progression of MM.
However, due to their invasive nature and associated costs, they are not done at every visit. Liquid biopsies are an attractive way forward.
Nevertheless, before they can be used in the clinical management of patients with MM, these results need to be confirmed in larger prospective studies.
The investigators reported their findings in Nature Communications.
Scientists from the Dana-Farber Cancer Institute and the Broad Institute in Boston, Massachusetts, have shown that in multiple myeloma (MM), liquid biopsies provide similar genetic information as tumor biopsies.
Unlike tumor biopsies, liquid biopsies are noninvasive and, in the future, may offer a cost-effective way of following disease state, disease progression, and response to treatment.
The scientists used cell-free DNA (cfDNA) from 107 patients, circulating tumor cells (CTCs) from 56 patients, and compared their genomic profiling with bone marrow biopsies from 9 patients with MM.
The researchers used a two-step approach using the 2 kinds of liquid biopsies—CTCs and cfDNA. They first used “ultra-low pass” whole genome sequencing, which was a rapid and cost-effective method to identify blood samples with tumor DNA of at least 5%-10% (tumor fraction).
These samples were then subject to whole-exome sequencing (WES), which analyzes protein-coding sequence of the genome.
The researchers found genetic alterations in MM such as copy number alterations (1p, 13q deletions or 11q gain) and somatic single nucleotide variations (SSNVs), among others, which they observed in matched cfDNA, CTCs, and tumor DNA (tDNA) from tumor biopsies.
The liquid biopsies also captured the clonal heterogeneity characteristic of MM—99% of clonal mutations present in tDNA were also seen in cfDNA or CTCs, and 94% of mutations present in cfDNA or CTCs were seen in tDNA.
In addition, CTCs or cfDNA samples with higher tumor purity uncovered more mutations than were seen in tDNA.
“[The] combination of CTCs and cfDNA was able to detect almost all clonal mutations identified in the bone marrow biopsy sample and defined other subclones that were not identified in the bone marrow,” the scientists noted.
This increased sensitivity was “potentially due to the limitation of sampling site of the bone marrow,” they pointed out.
The scientists also showed that the tumor fraction in cfDNA and enriched CTCs correlated with disease progression from MGUS (monoclonal gammopathy of undetermined significance) and SMM (smoldering MM), to overt MM.
The scientists also evaluated liquid biopsies to determine response to treatment.
For example, a patient who responded to carfilzomib, lenalidomide, and dexamethasone, had a decreased cfDNA tumor fraction—from 22% to 2%.
Another patient who progressed on daratumumab over a period of 2 months showed an increase in tumor fraction—from 11% to 46%.
CTCs and cfDNA captured the mutational landscape of MM and provided a non-invasive profiling of tumor evolution. The liquid biopsy approach may be used as a novel biomarker for disease progression and response to therapy, the scientists suggest.
"Our discovery that cfDNA and CTC analyses agree with each other at the comprehensive level is an important finding,” said co-senior author Irene Ghobrial, MD, “because this means that routine genetic profiling of patient tumors from blood would be feasible."
"Our ultimate goal is to eventually use all the samples to monitor disease progression," added Jihye Park, PhD, researcher in the Ghobrial lab and co-first author on the paper.
Currently, bone marrow biopsies are the gold standard for diagnosing and monitoring the progression of MM.
However, due to their invasive nature and associated costs, they are not done at every visit. Liquid biopsies are an attractive way forward.
Nevertheless, before they can be used in the clinical management of patients with MM, these results need to be confirmed in larger prospective studies.
The investigators reported their findings in Nature Communications.
Scientists from the Dana-Farber Cancer Institute and the Broad Institute in Boston, Massachusetts, have shown that in multiple myeloma (MM), liquid biopsies provide similar genetic information as tumor biopsies.
Unlike tumor biopsies, liquid biopsies are noninvasive and, in the future, may offer a cost-effective way of following disease state, disease progression, and response to treatment.
The scientists used cell-free DNA (cfDNA) from 107 patients, circulating tumor cells (CTCs) from 56 patients, and compared their genomic profiling with bone marrow biopsies from 9 patients with MM.
The researchers used a two-step approach using the 2 kinds of liquid biopsies—CTCs and cfDNA. They first used “ultra-low pass” whole genome sequencing, which was a rapid and cost-effective method to identify blood samples with tumor DNA of at least 5%-10% (tumor fraction).
These samples were then subject to whole-exome sequencing (WES), which analyzes protein-coding sequence of the genome.
The researchers found genetic alterations in MM such as copy number alterations (1p, 13q deletions or 11q gain) and somatic single nucleotide variations (SSNVs), among others, which they observed in matched cfDNA, CTCs, and tumor DNA (tDNA) from tumor biopsies.
The liquid biopsies also captured the clonal heterogeneity characteristic of MM—99% of clonal mutations present in tDNA were also seen in cfDNA or CTCs, and 94% of mutations present in cfDNA or CTCs were seen in tDNA.
In addition, CTCs or cfDNA samples with higher tumor purity uncovered more mutations than were seen in tDNA.
“[The] combination of CTCs and cfDNA was able to detect almost all clonal mutations identified in the bone marrow biopsy sample and defined other subclones that were not identified in the bone marrow,” the scientists noted.
This increased sensitivity was “potentially due to the limitation of sampling site of the bone marrow,” they pointed out.
The scientists also showed that the tumor fraction in cfDNA and enriched CTCs correlated with disease progression from MGUS (monoclonal gammopathy of undetermined significance) and SMM (smoldering MM), to overt MM.
The scientists also evaluated liquid biopsies to determine response to treatment.
For example, a patient who responded to carfilzomib, lenalidomide, and dexamethasone, had a decreased cfDNA tumor fraction—from 22% to 2%.
Another patient who progressed on daratumumab over a period of 2 months showed an increase in tumor fraction—from 11% to 46%.
CTCs and cfDNA captured the mutational landscape of MM and provided a non-invasive profiling of tumor evolution. The liquid biopsy approach may be used as a novel biomarker for disease progression and response to therapy, the scientists suggest.
"Our discovery that cfDNA and CTC analyses agree with each other at the comprehensive level is an important finding,” said co-senior author Irene Ghobrial, MD, “because this means that routine genetic profiling of patient tumors from blood would be feasible."
"Our ultimate goal is to eventually use all the samples to monitor disease progression," added Jihye Park, PhD, researcher in the Ghobrial lab and co-first author on the paper.
Currently, bone marrow biopsies are the gold standard for diagnosing and monitoring the progression of MM.
However, due to their invasive nature and associated costs, they are not done at every visit. Liquid biopsies are an attractive way forward.
Nevertheless, before they can be used in the clinical management of patients with MM, these results need to be confirmed in larger prospective studies.
The investigators reported their findings in Nature Communications.
When the answer to vaccines is “No”
We all know how challenging and time-consuming it can be to convince vaccine-hesitant patients that vaccinations are what is best for them and their children. Patients are bombarded with misinformation through the news and social media that seeds or “confirms” their doubts about vaccines. And for our part, we have only a few minutes during an office visit to refute all of the false claims that are a mere click or scroll away.
To better prepare for this challenge, this article details a practical approach to discussing vaccines with your patients. Using the patient-friendly language and evidence described here, you will be well positioned to refute 13 common vaccine misconceptions and overcome the barriers that stand in the way of these lifesaving interventions.
A few important baseline concepts
In discussing vaccination with our patients, it is important to keep the following in mind:
Patients don’t refuse vaccinations just to make our lives difficult. They truly are trying to make the best decisions they can for themselves and their families. Recognizing this can significantly reduce frustration levels.
Time well spent. While educating patients about the value of vaccines takes time, the return is worth it. The more consistently we offer vaccines, along with the reasons they are important, the more likely patients are to give vaccines a second thought. In fact, studies show that provider recommendation is the most important factor in patients’ decisions to vaccinate.1
Approach matters. In all other aspects of medicine, we attempt to use a participatory approach, involving our patients in decisions regarding their health care. When discussing vaccines, however, a participatory approach (eg, “What do you want to do about vaccines today?”) can introduce doubt into patients’ minds. Studies show that a presumptive approach (eg, “Today we are going to provide the tetanus, human papillomavirus [HPV], and meningitis vaccines”) is a much more effective way to get patients to vaccinate.2
[polldaddy:10018427]
Continue to: Barriers to counseling
Barriers to counseling. Health care providers report a variety of barriers to effective vaccine counseling (limited time and resources, lack of confidence in addressing patients’ concerns, etc).3 In addition, providers sometimes worry that strong encouragement of vaccination will create an adversarial relationship with vaccine-hesitant patients. Developing a good rapport and trusting relationship, as well as using motivational interviewing approaches, can help communicate the importance of vaccines, while leaving patients with the sense that you have heard them and respect their intentions. (See “Facilitate vaccine discussions using these 2 approaches.” 4-7)
SIDEBAR
Facilitate vaccine discussions using these 2 approaches4-7
C.A.S.E.
Corroborate
Acknowledge concerns and find some point on which you can agree.
Example: "It sounds like we both want to keep your child healthy and safe."
About me
Describe what you have done to build your expertise on the subject.
Example: "I have been practicing medicine for 15 years and have spent a great deal of time researching the data on vaccinations."
Science
Review the data and science behind vaccines.
Example: "Vaccines are more rigorously studied and safer than almost any other intervention we have in medicine."
Explain/advise
Explain your recommendations, based on the science.
Example: "This is why I vaccinate my children, and this is why I recommend this vaccine for your child."
3As
Ask
Don't stop at a patient's first "No." Respectfully dig a bit deeper.
Example: "What questions do you have about the vaccines we are recommending today? Tell me what worries you about them."
Acknowledge
Acknowledge your patient's concerns.
Example: "You are obviously a very devoted parent, and I know that you are trying to make the best decision you can for your child. With everything we see on the news and social media, it's not always easy to know what to believe about vaccines."
Advise
Advise patients/parents of the facts about vaccines and provide a strong recommendation to vaccinate.
Example: "Depending on the year, influenza kills 12,000 to 56,000 people annually; the vast majority of those who die did not receive the flu vaccine.7 My family and I get the flu shot every year, and I strongly encourage you and your children to get this lifesaving vaccine."
Continue to: If at first you don't succeed...
If at first you don’t succeed, try again because patients often have an experience that changes their mind. Perhaps a friend died of throat cancer or a family member developed a complication of the flu that required hospitalization. You never know when something will influence patients’ choices.
Don’t wait for scheduled well visits. Use every patient encounter as a means to catch patients up on missing vaccinations.
Common misconceptions and concerns and how to counter them
1. I’ve heard that vaccines can actually make you sick.
When patients raise this concern, start with an explanation of how vaccines work. Explain that our bodies protect us from foreign invaders (such as viruses and bacteria) by mounting an immune response when we are exposed to these proteins. Vaccinations work by exploiting this immune response; they expose the body to killed or weakened viral or bacterial proteins in a safe and controlled manner. In this way, our immune system will have already developed antibodies to these invaders by the time we are exposed to an active infection.
To use an analogy to war, instead of being subjected to a surprise attack where we suffer large losses in the battle, vaccination prepares us with weapons (antibodies) to defend ourselves so that our bodies are now able to successfully fight off that attack.
Because the majority of vaccines are killed virus vaccines, they cannot cause the illness against which they are meant to protect. Triggering the immune system may make some recipients feel a little “under the weather” for a day or 2, but they do not make us “sick.”
Live attenuated vaccines are similarly safe for those with a healthy immune system. We don’t administer them, however, to people who have a weakened immune system (eg, pregnant women, newborns, people with acquired immunodeficiency virus, or patients receiving chemotherapy or other types of immunosuppression) because these patients could develop the illness that we are trying to protect against.
Continue to: 2. Don't vaccines cause autism? Aren't they toxic to the nervous system?
2. Don’t vaccines cause autism? Aren’t they toxic to the nervous system?
The largest setback to vaccination efforts in recent history was a 1998 study by Andrew Wakefield that suggested that vaccination (specifically the mercury [in the form of thimerosal] present in the measles, mumps, rubella [MMR] vaccine) was linked to the development of autism.8 This research was subsequently debunked,9 and the author of the 1998 study was stripped of his medical license for falsifying data. However, the damage to vaccination efforts had already been done.
Aluminum. Thimerosal is not the only agent that patients may find concerning. Some also worry about the aluminum content of vaccines. Aluminum works as an additive to boost the body’s immune response to a vaccine. It is used only in killed virus vaccines—not in live attenuated ones. The Agency for Toxic Substances and Disease Registry monitors minimum risk levels (MRLs) of aluminum and other compounds in potentially hazardous substances. The amount of aluminum in vaccines is far below the MRL for aluminum, which is 1 mg/kg/d.10 (See “The facts about thimerosal and aluminum in vaccines.”11-16)
SIDEBAR
The facts about thimerosal and aluminum in vaccines
Thimerosal
Ethyl-mercury was used (in the form of thimerosal) as a preservative to prevent bacterial and fungal contamination of vaccines. Since 2001, however, thimerosal has been removed from all US-licensed vaccines—except multidose vials of influenza vaccine—as a precautionary measure (and not for any reproducible evidence of harm). The multidose flu vial contains <0.01% thimerosal.11
Ethyl-mercury is cleared from the body much more rapidly than methyl-mercury (the kind found in certain types of fish) and is less toxic.12
Since the removal of thimerosal from vaccines, the Centers for Disease Control and Prevention notes that the rates of autism have actually increased.13
Even Autism Speaks, the leading organization dedicated to advocacy for patients with autism and their families, denies a link between vaccines and autism.14
Aluminum
We are exposed to aluminum in products we use extensively every day, such as pots and pans, aluminum foil, seasonings, cereal, baby formula, paints, fuels, and antiperspirants.15
Infants are exposed to about 4.4 mg of aluminum in the vaccines typically administered in the first 6 months of life.16 However, infants typically ingest more than that during the first 6 months of life. Breast milk contains about 7 mg over 6 months; milk-based formulas contain about 38 mg over 6 months; and soy-based formulas contain about 117 mg over 6 months.16
Contine to: 3. I'm healthy. I never get sick. Why do I need vaccinations?
3. I’m healthy. I never get sick. Why do I need vaccinations?
A good way to counter this comment is to respond: “Saying you don’t need vaccinations because you never get sick is like saying you don’t need to wear a seat belt because you’ve never been in a car accident.” Advise patients that we seek to vaccinate all members of a community—not just those who are sick or at high risk—to protect ourselves and to provide “herd immunity.” It’s important to explain that herd immunity is resistance to the spread of a contagious disease that results if a sufficiently high number of people (depending on the illness, typically 80%-95%) are immune to the disease, especially through vaccination.17,18 If vaccination levels fall, we see a rise in cases of vaccine-preventable illness (as was seen during the 2017 measles outbreak in a community in Minnesota).19
Even though many of us may not suffer severe consequences of an infection, we can still pass that infection to others. While the whooping cough that a healthy 35-year-old gets may cause only prolonged annoyance or time off from work, it can kill the baby that is sitting next to that adult on the plane or bus.
4. Isn’t it true that we see fewer serious illnesses because of improved hygiene and sanitation, rather than vaccines?
Our current US sanitation standards were established under the Safe Drinking Water Act of 1974.20 While improvements in hygiene, sanitation, nutrition, and other public health measures have undoubtedly decreased the spread of disease and improved survival rates, there is no denying the significant drop in disease that occurs after the introduction of a vaccine for a particular illness or the increase in cases of that disease when vaccination rates drop off.
By the early 1990s, our current sanitation standards were already well established. Yet we didn’t see a significant decrease in the incidence of infections with Haemophilus influenzae type b (Hib) until after the conjugate Hib vaccines were introduced (dropping from about 20,000 cases/year to 1419 cases/year by 1993).21
In Britain, a drop in the rate of pertussis (whooping cough) vaccination in 1974 resulted in an epidemic of more than 100,000 cases and 36 deaths by 1978. There was no decrease in hygiene or sanitation standards to explain this rise.21
Continue to: 5. Vaccines are just another way for "big pharma" to make "big money."
5. Vaccines are just another way for “big pharma” to make “big money.”
Patients may benefit from knowing that in the earlier days of vaccines, pharmaceutical companies actually moved away from production of vaccines because they were not very profitable. These days, with worldwide distribution, drug companies are back in the swing of making vaccines and, as we would expect from all companies, are in business to make a profit.
That said, health care providers receive no payments from drug companies for offering vaccines or for offering one vaccine over another. The reason we recommend vaccination is because we know it is best for our patients’ health and the health of the community.

6. We don’t see polio anymore. Why do I need the vaccine?
One of the factors contributing to the rise in antivaccine sentiment is that we rarely see vaccine-preventable illnesses (such as polio, measles, and mumps). But the absence of these illnesses is precisely due to prior years’ vaccination efforts.
Smallpox, a deadly and disfiguring disease that killed many millions of people and contributed to the downfall of the Roman, Aztec, and Incan empires, was eradicated from the planet in 1979, thanks to focused vaccination efforts by the World Health Organization. Vaccination works, but we have to keep at it.
While we no longer see as many of these vaccine-preventable illnesses in the United States, they are still present in other parts of the world. Our world is much smaller than it used to be. International travel is common, and illnesses can be reintroduced into a community with relative ease. We must remain vigilant.
Continue to: 7. I heard that vaccines are made from aborted fetal tissue.
7. I heard that vaccines are made from aborted fetal tissue.
There are 5 vaccines (varicella, rubella, hepatitis A, shingles, and rabies vaccines) that were originally made using aborted fetal tissue. In 1960, tissue from 2 fetuses aborted by maternal choice (and not for the purpose of vaccine production) was used to propagate cell lines that are still used in vaccine development today.
Human cells provide advantages for vaccine production that other cells do not. Some viruses do not grow well in animal cells. Animal cells can introduce contamination by bacteria and viruses that are not carried in human cell lines. Vaccine production can be hindered or halted, resulting in a vaccine shortage, if animal products used in development are threatened (eg, if an illness strikes egg-producing chickens; eggs are used to make the influenza vaccine).22
Some patients, particularly those who are Catholic, may have concerns about these vaccines. The National Catholic Bioethics Center has prepared a statement regarding the use of these vaccines that may help settle any moral dilemmas.23 It reads:
“The cell lines under consideration were begun using cells taken from one or more fetuses aborted almost 40 years ago. Since that time, the cell lines have grown independently. It is important to note that descendent cells are not the cells of the aborted child.”
“One is morally free to use the vaccine regardless of its historical association with abortion. The reason is that the risk to public health, if one chooses not to vaccinate, outweighs the legitimate concern about the origins of the vaccine. This is especially important for parents, who have a moral obligation to protect the life and health of their children and those around them.”
Continue to: 8. Vaccines aren't studied—or monitored—thoroughly enough.
8. Vaccines aren’t studied—or monitored— thoroughly enough.
Patients would benefit from knowing that vaccines are some of the most thoroughly studied products brought to market. They undergo rigorous testing and oversight, from both public and private organizations, for 10 to 15 years before being released for distribution. Post-licensure monitoring is ongoing, and the manufacturer may voluntarily participate in Phase IV trials to continue to test the safety and efficacy of a vaccine after release to market.
Monitoring adverse effects. In addition, in 1990, the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration established the Vaccine Adverse Events Reporting System (VAERS) to “detect possible signals of adverse events associated with vaccines.”24 Most events reported are coincidental, but some common mild adverse events (like redness and swelling at the injection site) are often underreported.
Serious events are always thoroughly investigated and are often found unrelated. However, rare associations have been found. For example, an intestinal problem called intussusception, related to the original rotavirus vaccine, was discovered, and the vaccine causing it was removed from the market.25 A new, safer rotavirus vaccine option is now available. Patients need to know that we do have an effective system of checks and balances in which we can place our trust.
9. People can become paralyzed or stop breathing after receiving a vaccination. Why run those risks?
One of the most feared reactions to vaccination is Guillain-Barré syndrome (GBS), which can cause paralysis. The CDC estimates the risk for GBS associated with the flu vaccine, for example, to be 1 to 2 cases per 1 million people vaccinated.26 Another potential concern is the rate of anaphylaxis following vaccination. However, in a 2016 study in the Journal of Allergy and Clinical Immunology, the rate of anaphylaxis for all vaccines combined was only 1.31 per 1 million vaccines.27
The risk of developing severe complications from an illness is much greater than that of developing complications from the vaccine meant to protect a person against that illness. In the United States, the population-based risk for influenza-related hospitalization in children, for example, is as high as 150 in 100,000 with as many as 125 deaths annually.26
Continue to: 10. Isn't vaccination a personal choice? How does my health/illness impact the community?
10. Isn’t vaccination a personal choice? How does my health/illness impact the community?
Patients may not realize that most viruses are contagious from 1 to 2 days before symptoms appear, which means we can spread an illness before we even know we have it. Protecting oneself also protects those around us.
Economic concerns. There’s also the economic impact of these illnesses to consider. This includes the personal cost of being out of school or work for an extended period and the cost of a patient’s care, which can become astronomical if hospitalization is required and which can become the country’s problem if a person lacks sufficient health insurance coverage.
A study looking at the cost of 4 major adult vaccine-preventable illnesses (influenza, pneumococcal disease, shingles, and whooping cough) in the United States in 2013 estimated the annual cost for these illnesses in adults ≥50 years to be $26.5 billion.28 And that doesn’t include the cost of childhood vaccine-preventable diseases.
Countering 3 concerns about childhood vaccinations
1. I can’t afford vaccines for my child.
The Vaccines for Children program is a federally-funded program that covers the cost of all vaccines for children younger than 19 years of age who are Medicaid-eligible, American Indian, Alaskan Native, uninsured, or underinsured.29 Although there may be a small administration fee charged by the provider’s office, the vaccine is free.
2. Don’t all of the vaccines recommended for children overwhelm their immune systems?
Children are exposed to so many more proteins on a daily basis (by crawling around on the floor, putting their hands in their mouths, attending school or day care, etc) than they are ever exposed to in a series of vaccines.30 Exposure to these proteins in their environment and to those in vaccines only serves to boost their immunity and keep them healthier in the long run.
And thanks to advances in vaccine production, the immunologic load in vaccines is far less than it used to be. The 14 vaccines given today contain <200 bacterial and viral proteins or polysaccharides, compared with the >3000 of these immunologic components in the 7 vaccines administered in 1980.31
Continue to: Influenza vaccine: Patient-friendly talking points
SIDEBAR
Influenza vaccine: Patient-friendly talking points
- Some people think that getting the flu is no big deal. While it is true that the flu takes a greater toll on the very young and very old, the chronically ill, and the immune compromised, even healthy people can become seriously ill or die. The Centers for Disease Control and Prevention estimates that the flu is responsible for 140,000 to 720,000 hospitalizations and 12,000 to 56,000 deaths in the United States every year.7 Of those who die from the flu, approximately 80% did not receive a flu shot.36 Of children who died from the flu between 2004 and 2012, more than 40% had no risk factors for complications.37
- The flu shot is a killed virus vaccine, so it can't give you the flu. People sometimes feel under the weather (achy, low-grade fever) after a vaccine, but this is considered normal and evidence that your body's immune system is "revving up."
- It takes 2 weeks before the vaccine becomes effective so a person can still get the flu during that time. This is why it is so important to get the vaccine earlier in the fall, before the flu season takes hold.
- The "stomach flu" is not the flu. The flu vaccine does not protect against the "stomach flu" or other flu-like illnesses.
- The flu vaccine is not perfect. It is an educated guess as to which strains will be circulating that year. (At its best, the flu vaccine is about 60% effective.38) However, it makes the chance of getting the flu less likely and significantly decreases the odds of severe complications/death.
- Egg allergies are no longer a reason to avoid the flu vaccine. There is an egg-free vaccine called Flublok (for those ≥18 years of age). In 2016-17, the Advisory Committee on Immunization Practices changed the recommendations for flu vaccine in egg-allergic people. The recommendations say that if reactions are mild, or you can eat cooked eggs without a problem, you can receive a flu vaccine. If you have severe reactions, such as trouble breathing or recurrent vomiting, you can still receive the flu vaccine, but must be monitored by a health care provider who can recognize and respond to a severe allergic reaction.39
Continue to: 3. Why don't we adhere to Dr. Sears' vaccine schedule?
3. Why don’t we adhere to Dr. Sears’ vaccine schedule?
There are multiple ways in which Dr. Robert Sears’ book, The Vaccine Book: Making the Right Decision for your Child, published in 2007, misrepresents vaccine science and leads patients astray in making decisions regarding vaccinations.32 Most important to note is that Dr. Sears’ Alternative Vaccine Schedule, which seeks to make it so that children do not receive more than 2 vaccinations per office visit, would require visits to a health care provider at 2, 3, 4, 5, 6, 7, 9, 12, 15, 18, and 21 months, and at 2, 2.5, 3, 3.5, 4, 5, and 6 years of age. This significantly increases the number of office visits and needle sticks, and raises the age at which vaccines are given, increasing the risk of illness outbreaks and decreasing the likelihood that parents would return to the office to complete the full series.
Acceptance of influenza and HPV vaccines remains a challenge
We are significantly less successful at getting parents and patients to agree to influenza and HPV vaccines than to the other vaccines we offer. The influenza vaccine success rate in 2016 was 59% in children and 43.3% in adults.33 Compared to the Tdap vaccine (88%) and the meningococcal vaccine (82%), which are offered at the same age as the HPV vaccine, success rates for HPV vaccine are significantly lower. In 2016, only 60.4% of boys and girls were current on their first HPV injection and only 43.3% were up to date with the full series.34
Newness of vaccines a factor?
Perhaps it is because the recommendations for these 2 vaccines are relatively new, and people don’t yet grasp the seriousness and scope of the diseases. Until 2010, the flu shot was recommended only for the very young, the elderly, and the medically high risk.
Similarly, the HPV vaccine was originally introduced for girls in 2006 and wasn’t recommended for boys until 2011.
Continue to: Human papillomavirus vaccine: Patient-friendly talking points
SIDEBAR
Human papillomavirus vaccine: Patient-friendly talking points
- Human papillomavirus (HPV) causes genital warts and cancer of the cervix, vagina, vulva, anus, rectum, penis, and oropharynx.
- The HPV vaccine is a cancer prevention vaccine. The 9-valent vaccine is active against 2 genital wart-causing strains and 7 cancer-causing strains of HPV.
- HPV is highly prevalent; 79 million Americans are currently infected, nearly 14 million people become newly infected each year, and nearly all of us will be exposed at some point in our sexual lives.40
- There are often no outward signs of infection, so it is a difficult infection to avoid.
- It takes no high-risk sexual activity to be exposed to the HPV virus.
- The HPV vaccine is recommended for both boys and girls usually around age 11 to 12 years (but as early as 9 years and as late as 26 years is acceptable). If the first vaccine is administered before 15 years of age, only 2 injections are needed 6 to 12 months apart. If the first vaccine is administered after 15 years of age, 3 injections are needed at 0, 2 months, and 6 months.41
- Completing the series before sexual activity begins is the best way to protect our children because the vaccine is a preventive measure, not a treatment.
- The HPV vaccine is highly effective with >90% efficacy against high-risk cancer-causing strains.42
- The HPV vaccine offers long-term protection. The vaccine has been on the market since 2006, and immunity has not yet diminished. Further monitoring is ongoing.43
- The HPV vaccine is covered under the Vaccines For Children program until age 19 years. Then it is up to individual insurance plans to cover it.
- The HPV vaccine does not cause infertility.44 HPV infection, on the other hand, can lead to fertility problems if, for example, treatment for cervical precancer or cancer requires partial removal of the cervix or a hysterectomy.
- The HPV vaccine does not cause autoimmune diseases.45,46 Studies show no difference between vaccinated and unvaccinated groups in rates of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes mellitus, multiple sclerosis, Hashimoto's thyroiditis, Graves' disease, and others.
- The HPV vaccine does not encourage earlier sexual activity. There was no earlier incidence of outcomes related to sexual activity (pregnancy, sexually transmitted infection testing or diagnosis, or contraceptive counseling) in vaccinated vs unvaccinated patients studied.47
Continue to: A sensitive subject
A sensitive subject. Discussion of a vaccine related to a child’s sexual health makes some parents uncomfortable. Studies show that focusing on the cancer prevention aspects of the vaccine, rather than on sexual transmission of HPV, results in greater vaccine acceptance.35
However, if discussion of sexual transmission is unavoidable, remind parents to consider their own adolescence and whether they chose to share everything with their parents. Point out that there were probably things they did that they later looked back on and thought, “What was I thinking?” Their children, no matter how wonderful and levelheaded they are, will be no different. And, as much as parents don’t want to think about it, some kids will suffer unwanted sexual contact. Shouldn’t parents protect their children as best as they can?
A teen’s right to choose? Some states have passed a Mature Minor Doctrine, which provides for mature, unemancipated teens to make their own medical decisions regarding such issues as sexuality, mental health, and drug and alcohol use without their parents’ consent. In these states, teens may elect to receive the HPV vaccine without parental permission. (Check your state’s laws for specifics, and see the 2 boxes with patient-friendly talking points for influenza vaccine7,36-39 and human papillomavirus vaccine.40-47)
CORRESPONDENCE
Gretchen LaSalle, MD, MultiCare Rockwood Clinic, 2214 East 29th Avenue, Spokane, WA 99203; [email protected].
1. Paterson P, Meurice F, Stanberry LR, et al. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700-6706.
2. Opel DJ, Heritage J, Taylor J, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132:1037-1046.
3. Palmer J, Carrico C, Costanzo C. Identifying and overcoming perceived barriers of providers towards vaccination: a literature review. J Vaccines. 2015;1-7.
4. Autism Science Foundation. Making the CASE for vaccines: a new model for talking to patients about vaccines. Available at: http://autismsciencefoundation.org/wp-content/uploads/2015/12/Making-the-CASE-for-Vaccines-Guide_final.pdf. Accessed April 8, 2018.
5. Jacobson RM, Van Etta L, Bahta L. The C.A.S.E approach: guidance for talking to vaccine-hesitant patients. Minn Med. 2013;96:49-50.
6. Henrickson NB, Opel DJ, Grothaus L, et al. Physician communication training and parental vaccine hesitancy: a randomized trial. Pediatrics. 2015;136:70-79.
7. Centers for Disease Control and Prevention. Key facts about seasonal flu vaccine. Available at: https://www.cdc.gov/flu/protect/keyfacts.htm. Accessed April 8, 2018.
8. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641.
9. Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32:3623-3629.
10. Agency for Toxic Substances & Disease Registry. Minimal risk levels for hazardous substances. Available at: https://www.atsdr.cdc.gov/mrls/mrllist.asp#34tag. Accessed April 8, 2018.
11. US Food and Drug Administration. Thimerosal and vaccines. Available at: https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed April 8, 2018.
12. Hviid A, Stellfeld M, Wohlfahrt J, et al. Association between thimerosal-containing vaccine and autism. JAMA. 2003;290:1763-1766.
13. Centers for Disease Control and Prevention. Thimerosal in vaccines. Available at: https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed May 8, 2018.
14. Autism Speaks. Frequently asked questions. Available at: https://www.autismspeaks.org/what-autism/faq. Accessed April 8, 2018.
15. Agency for Toxic Substances & Disease Registry. Toxic substances portal-aluminum. Public Health Statement for Aluminum, CAS #7429-90-5. Available at: https://www.atsdr.cdc.gov/PHS/PHS.asp?id=1076&tid=34. Accessed April 8, 2018.
16. Children’s Hospital of Philadelphia. Vaccine ingredients-aluminum. Available at: www.chop.edu/centers-programs/vaccine-education-center/vaccine-ingredients/aluminum. Accessed April 8, 2018.
17. Orenstein W, Seib K. Mounting a good offense against measles. N Engl J Med. 2014;371:1661-1663.
18. Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med. 2012;55:72-77.
19. Hall V, Banerjee E, Kenyon C, et al. Measles outbreak – Minnesota April-May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:713-717.
20. The National Academies of Sciences Engineering Medicine. History of U.S. water and wastewater systems. Privatization of Water Services in the United States: an Assessment of Issues and Experience. Washington, DC: The National Academies Press; 2002:29-40. Available at: https://www.nap.edu/read/10135/chapter/4#35. Accessed May 7, 2018.
21. World Health Organization. Global vaccine safety. Six common misconceptions about immunization. Available at: http://www.who.int/vaccine_safety/initiative/detection/immunization_misconceptions/en/index1.html. Accessed May 7, 2018.
22. The history of vaccines. Human cell strains in vaccine development. Available at: https://www.historyofvaccines.org/content/articles/human-cell-strains-vaccine-development. Accessed April 8, 2018.
23. The National Catholic Bioethics Center. Frequently asked questions. Available at: https://www.ncbcenter.org/resources/frequently-asked-questions/use-vaccines/. Accessed April 8, 2018.
24. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33:4398-4405.
25. Foster S. Rotavirus vaccine and intussusception. J Pediatr Pharmacol Ther. 2007;12:4-7.
26. Mistry RD, Fischer JB, Prasad PA, et al. Severe complications of influenza-like illnesses. Pediatrics. 2014;134:e684-e690.
27. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
28. McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36:259-273.
29. Centers for Disease Control and Prevention. Vaccines for Children (VFC) Program. Available at: https://www.cdc.gov/features/vfcprogram/index.html. Accessed April 8, 2018.
30. Plotkin S, Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis. 2009;48:456-461.
31. Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics. 2002;109:124-129.
32. Offit PA, Moser CA. The problem with Dr. Bob’s alternative vaccine schedule. Pediatrics. 2009;123:e164-e169.
33. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. April 8. 2018.
34. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
35. Thomas TL. Cancer prevention: HPV vaccination. Semin Oncol Nurs. 2016:32:273-280.
36. Centers for Disease Control and Prevention. Estimating seasonal influenza-associated deaths in the United States. Available at: https://www.cdc.gov/flu/about/disease/US_flu-related_deaths.htm. Accessed May 8, 2018.
37. Wong KK, Jain S, Blanton L, et al. Influenza-associated pediatric deaths in the United States: 2004-2012. Pediatrics. 2013;132:796-804.
38. Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed April 8, 2018.
39. Centers for Disease Control and Prevention. Influenza (flu). Flu vaccine and people with egg allergies. Available at: https://www.cdc.gov/flu/protect/vaccine/egg-allergies.htm. Accessed April 8, 2018.
40. Centers for Disease Control and Prevention. For parents: vaccines for your children. HPV vaccine for preteens and teens. Available at: https://www.cdc.gov/vaccines/parents/diseases/teen/hpv.html. Accessed April 8, 2018.
41. Centers for Disease Control and Prevention. Vaccines and preventable diseases. HPV vaccine recommendations. Available at: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed May 7, 2018.
42. Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007;85:719-726.
43. De Vincenzo R, Conte C, Ricci C, et al. Long-term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999-1010.
44. McInerney KA, Hatch EE, Wesselink AK. The effect of vaccination against human papillomavirus on fecundability. Paedeatr Perinat Epidemiol. 2017;31:531-536.
45. Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193-203.
46. Vichnin M, Bonanni P, Klein NP, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006-2015. Ped Infect Dis J. 2015;34:983-991.
47. Bednarczyk RA, Davis R, Ault K, et al. Sexual activity-related outcomes after human papillomavirus vaccination of 11-to-12-year-olds. Pediatrics. 2012;130:798-805.
We all know how challenging and time-consuming it can be to convince vaccine-hesitant patients that vaccinations are what is best for them and their children. Patients are bombarded with misinformation through the news and social media that seeds or “confirms” their doubts about vaccines. And for our part, we have only a few minutes during an office visit to refute all of the false claims that are a mere click or scroll away.
To better prepare for this challenge, this article details a practical approach to discussing vaccines with your patients. Using the patient-friendly language and evidence described here, you will be well positioned to refute 13 common vaccine misconceptions and overcome the barriers that stand in the way of these lifesaving interventions.
A few important baseline concepts
In discussing vaccination with our patients, it is important to keep the following in mind:
Patients don’t refuse vaccinations just to make our lives difficult. They truly are trying to make the best decisions they can for themselves and their families. Recognizing this can significantly reduce frustration levels.
Time well spent. While educating patients about the value of vaccines takes time, the return is worth it. The more consistently we offer vaccines, along with the reasons they are important, the more likely patients are to give vaccines a second thought. In fact, studies show that provider recommendation is the most important factor in patients’ decisions to vaccinate.1
Approach matters. In all other aspects of medicine, we attempt to use a participatory approach, involving our patients in decisions regarding their health care. When discussing vaccines, however, a participatory approach (eg, “What do you want to do about vaccines today?”) can introduce doubt into patients’ minds. Studies show that a presumptive approach (eg, “Today we are going to provide the tetanus, human papillomavirus [HPV], and meningitis vaccines”) is a much more effective way to get patients to vaccinate.2
[polldaddy:10018427]
Continue to: Barriers to counseling
Barriers to counseling. Health care providers report a variety of barriers to effective vaccine counseling (limited time and resources, lack of confidence in addressing patients’ concerns, etc).3 In addition, providers sometimes worry that strong encouragement of vaccination will create an adversarial relationship with vaccine-hesitant patients. Developing a good rapport and trusting relationship, as well as using motivational interviewing approaches, can help communicate the importance of vaccines, while leaving patients with the sense that you have heard them and respect their intentions. (See “Facilitate vaccine discussions using these 2 approaches.” 4-7)
SIDEBAR
Facilitate vaccine discussions using these 2 approaches4-7
C.A.S.E.
Corroborate
Acknowledge concerns and find some point on which you can agree.
Example: "It sounds like we both want to keep your child healthy and safe."
About me
Describe what you have done to build your expertise on the subject.
Example: "I have been practicing medicine for 15 years and have spent a great deal of time researching the data on vaccinations."
Science
Review the data and science behind vaccines.
Example: "Vaccines are more rigorously studied and safer than almost any other intervention we have in medicine."
Explain/advise
Explain your recommendations, based on the science.
Example: "This is why I vaccinate my children, and this is why I recommend this vaccine for your child."
3As
Ask
Don't stop at a patient's first "No." Respectfully dig a bit deeper.
Example: "What questions do you have about the vaccines we are recommending today? Tell me what worries you about them."
Acknowledge
Acknowledge your patient's concerns.
Example: "You are obviously a very devoted parent, and I know that you are trying to make the best decision you can for your child. With everything we see on the news and social media, it's not always easy to know what to believe about vaccines."
Advise
Advise patients/parents of the facts about vaccines and provide a strong recommendation to vaccinate.
Example: "Depending on the year, influenza kills 12,000 to 56,000 people annually; the vast majority of those who die did not receive the flu vaccine.7 My family and I get the flu shot every year, and I strongly encourage you and your children to get this lifesaving vaccine."
Continue to: If at first you don't succeed...
If at first you don’t succeed, try again because patients often have an experience that changes their mind. Perhaps a friend died of throat cancer or a family member developed a complication of the flu that required hospitalization. You never know when something will influence patients’ choices.
Don’t wait for scheduled well visits. Use every patient encounter as a means to catch patients up on missing vaccinations.
Common misconceptions and concerns and how to counter them
1. I’ve heard that vaccines can actually make you sick.
When patients raise this concern, start with an explanation of how vaccines work. Explain that our bodies protect us from foreign invaders (such as viruses and bacteria) by mounting an immune response when we are exposed to these proteins. Vaccinations work by exploiting this immune response; they expose the body to killed or weakened viral or bacterial proteins in a safe and controlled manner. In this way, our immune system will have already developed antibodies to these invaders by the time we are exposed to an active infection.
To use an analogy to war, instead of being subjected to a surprise attack where we suffer large losses in the battle, vaccination prepares us with weapons (antibodies) to defend ourselves so that our bodies are now able to successfully fight off that attack.
Because the majority of vaccines are killed virus vaccines, they cannot cause the illness against which they are meant to protect. Triggering the immune system may make some recipients feel a little “under the weather” for a day or 2, but they do not make us “sick.”
Live attenuated vaccines are similarly safe for those with a healthy immune system. We don’t administer them, however, to people who have a weakened immune system (eg, pregnant women, newborns, people with acquired immunodeficiency virus, or patients receiving chemotherapy or other types of immunosuppression) because these patients could develop the illness that we are trying to protect against.
Continue to: 2. Don't vaccines cause autism? Aren't they toxic to the nervous system?
2. Don’t vaccines cause autism? Aren’t they toxic to the nervous system?
The largest setback to vaccination efforts in recent history was a 1998 study by Andrew Wakefield that suggested that vaccination (specifically the mercury [in the form of thimerosal] present in the measles, mumps, rubella [MMR] vaccine) was linked to the development of autism.8 This research was subsequently debunked,9 and the author of the 1998 study was stripped of his medical license for falsifying data. However, the damage to vaccination efforts had already been done.
Aluminum. Thimerosal is not the only agent that patients may find concerning. Some also worry about the aluminum content of vaccines. Aluminum works as an additive to boost the body’s immune response to a vaccine. It is used only in killed virus vaccines—not in live attenuated ones. The Agency for Toxic Substances and Disease Registry monitors minimum risk levels (MRLs) of aluminum and other compounds in potentially hazardous substances. The amount of aluminum in vaccines is far below the MRL for aluminum, which is 1 mg/kg/d.10 (See “The facts about thimerosal and aluminum in vaccines.”11-16)
SIDEBAR
The facts about thimerosal and aluminum in vaccines
Thimerosal
Ethyl-mercury was used (in the form of thimerosal) as a preservative to prevent bacterial and fungal contamination of vaccines. Since 2001, however, thimerosal has been removed from all US-licensed vaccines—except multidose vials of influenza vaccine—as a precautionary measure (and not for any reproducible evidence of harm). The multidose flu vial contains <0.01% thimerosal.11
Ethyl-mercury is cleared from the body much more rapidly than methyl-mercury (the kind found in certain types of fish) and is less toxic.12
Since the removal of thimerosal from vaccines, the Centers for Disease Control and Prevention notes that the rates of autism have actually increased.13
Even Autism Speaks, the leading organization dedicated to advocacy for patients with autism and their families, denies a link between vaccines and autism.14
Aluminum
We are exposed to aluminum in products we use extensively every day, such as pots and pans, aluminum foil, seasonings, cereal, baby formula, paints, fuels, and antiperspirants.15
Infants are exposed to about 4.4 mg of aluminum in the vaccines typically administered in the first 6 months of life.16 However, infants typically ingest more than that during the first 6 months of life. Breast milk contains about 7 mg over 6 months; milk-based formulas contain about 38 mg over 6 months; and soy-based formulas contain about 117 mg over 6 months.16
Contine to: 3. I'm healthy. I never get sick. Why do I need vaccinations?
3. I’m healthy. I never get sick. Why do I need vaccinations?
A good way to counter this comment is to respond: “Saying you don’t need vaccinations because you never get sick is like saying you don’t need to wear a seat belt because you’ve never been in a car accident.” Advise patients that we seek to vaccinate all members of a community—not just those who are sick or at high risk—to protect ourselves and to provide “herd immunity.” It’s important to explain that herd immunity is resistance to the spread of a contagious disease that results if a sufficiently high number of people (depending on the illness, typically 80%-95%) are immune to the disease, especially through vaccination.17,18 If vaccination levels fall, we see a rise in cases of vaccine-preventable illness (as was seen during the 2017 measles outbreak in a community in Minnesota).19
Even though many of us may not suffer severe consequences of an infection, we can still pass that infection to others. While the whooping cough that a healthy 35-year-old gets may cause only prolonged annoyance or time off from work, it can kill the baby that is sitting next to that adult on the plane or bus.
4. Isn’t it true that we see fewer serious illnesses because of improved hygiene and sanitation, rather than vaccines?
Our current US sanitation standards were established under the Safe Drinking Water Act of 1974.20 While improvements in hygiene, sanitation, nutrition, and other public health measures have undoubtedly decreased the spread of disease and improved survival rates, there is no denying the significant drop in disease that occurs after the introduction of a vaccine for a particular illness or the increase in cases of that disease when vaccination rates drop off.
By the early 1990s, our current sanitation standards were already well established. Yet we didn’t see a significant decrease in the incidence of infections with Haemophilus influenzae type b (Hib) until after the conjugate Hib vaccines were introduced (dropping from about 20,000 cases/year to 1419 cases/year by 1993).21
In Britain, a drop in the rate of pertussis (whooping cough) vaccination in 1974 resulted in an epidemic of more than 100,000 cases and 36 deaths by 1978. There was no decrease in hygiene or sanitation standards to explain this rise.21
Continue to: 5. Vaccines are just another way for "big pharma" to make "big money."
5. Vaccines are just another way for “big pharma” to make “big money.”
Patients may benefit from knowing that in the earlier days of vaccines, pharmaceutical companies actually moved away from production of vaccines because they were not very profitable. These days, with worldwide distribution, drug companies are back in the swing of making vaccines and, as we would expect from all companies, are in business to make a profit.
That said, health care providers receive no payments from drug companies for offering vaccines or for offering one vaccine over another. The reason we recommend vaccination is because we know it is best for our patients’ health and the health of the community.

6. We don’t see polio anymore. Why do I need the vaccine?
One of the factors contributing to the rise in antivaccine sentiment is that we rarely see vaccine-preventable illnesses (such as polio, measles, and mumps). But the absence of these illnesses is precisely due to prior years’ vaccination efforts.
Smallpox, a deadly and disfiguring disease that killed many millions of people and contributed to the downfall of the Roman, Aztec, and Incan empires, was eradicated from the planet in 1979, thanks to focused vaccination efforts by the World Health Organization. Vaccination works, but we have to keep at it.
While we no longer see as many of these vaccine-preventable illnesses in the United States, they are still present in other parts of the world. Our world is much smaller than it used to be. International travel is common, and illnesses can be reintroduced into a community with relative ease. We must remain vigilant.
Continue to: 7. I heard that vaccines are made from aborted fetal tissue.
7. I heard that vaccines are made from aborted fetal tissue.
There are 5 vaccines (varicella, rubella, hepatitis A, shingles, and rabies vaccines) that were originally made using aborted fetal tissue. In 1960, tissue from 2 fetuses aborted by maternal choice (and not for the purpose of vaccine production) was used to propagate cell lines that are still used in vaccine development today.
Human cells provide advantages for vaccine production that other cells do not. Some viruses do not grow well in animal cells. Animal cells can introduce contamination by bacteria and viruses that are not carried in human cell lines. Vaccine production can be hindered or halted, resulting in a vaccine shortage, if animal products used in development are threatened (eg, if an illness strikes egg-producing chickens; eggs are used to make the influenza vaccine).22
Some patients, particularly those who are Catholic, may have concerns about these vaccines. The National Catholic Bioethics Center has prepared a statement regarding the use of these vaccines that may help settle any moral dilemmas.23 It reads:
“The cell lines under consideration were begun using cells taken from one or more fetuses aborted almost 40 years ago. Since that time, the cell lines have grown independently. It is important to note that descendent cells are not the cells of the aborted child.”
“One is morally free to use the vaccine regardless of its historical association with abortion. The reason is that the risk to public health, if one chooses not to vaccinate, outweighs the legitimate concern about the origins of the vaccine. This is especially important for parents, who have a moral obligation to protect the life and health of their children and those around them.”
Continue to: 8. Vaccines aren't studied—or monitored—thoroughly enough.
8. Vaccines aren’t studied—or monitored— thoroughly enough.
Patients would benefit from knowing that vaccines are some of the most thoroughly studied products brought to market. They undergo rigorous testing and oversight, from both public and private organizations, for 10 to 15 years before being released for distribution. Post-licensure monitoring is ongoing, and the manufacturer may voluntarily participate in Phase IV trials to continue to test the safety and efficacy of a vaccine after release to market.
Monitoring adverse effects. In addition, in 1990, the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration established the Vaccine Adverse Events Reporting System (VAERS) to “detect possible signals of adverse events associated with vaccines.”24 Most events reported are coincidental, but some common mild adverse events (like redness and swelling at the injection site) are often underreported.
Serious events are always thoroughly investigated and are often found unrelated. However, rare associations have been found. For example, an intestinal problem called intussusception, related to the original rotavirus vaccine, was discovered, and the vaccine causing it was removed from the market.25 A new, safer rotavirus vaccine option is now available. Patients need to know that we do have an effective system of checks and balances in which we can place our trust.
9. People can become paralyzed or stop breathing after receiving a vaccination. Why run those risks?
One of the most feared reactions to vaccination is Guillain-Barré syndrome (GBS), which can cause paralysis. The CDC estimates the risk for GBS associated with the flu vaccine, for example, to be 1 to 2 cases per 1 million people vaccinated.26 Another potential concern is the rate of anaphylaxis following vaccination. However, in a 2016 study in the Journal of Allergy and Clinical Immunology, the rate of anaphylaxis for all vaccines combined was only 1.31 per 1 million vaccines.27
The risk of developing severe complications from an illness is much greater than that of developing complications from the vaccine meant to protect a person against that illness. In the United States, the population-based risk for influenza-related hospitalization in children, for example, is as high as 150 in 100,000 with as many as 125 deaths annually.26
Continue to: 10. Isn't vaccination a personal choice? How does my health/illness impact the community?
10. Isn’t vaccination a personal choice? How does my health/illness impact the community?
Patients may not realize that most viruses are contagious from 1 to 2 days before symptoms appear, which means we can spread an illness before we even know we have it. Protecting oneself also protects those around us.
Economic concerns. There’s also the economic impact of these illnesses to consider. This includes the personal cost of being out of school or work for an extended period and the cost of a patient’s care, which can become astronomical if hospitalization is required and which can become the country’s problem if a person lacks sufficient health insurance coverage.
A study looking at the cost of 4 major adult vaccine-preventable illnesses (influenza, pneumococcal disease, shingles, and whooping cough) in the United States in 2013 estimated the annual cost for these illnesses in adults ≥50 years to be $26.5 billion.28 And that doesn’t include the cost of childhood vaccine-preventable diseases.
Countering 3 concerns about childhood vaccinations
1. I can’t afford vaccines for my child.
The Vaccines for Children program is a federally-funded program that covers the cost of all vaccines for children younger than 19 years of age who are Medicaid-eligible, American Indian, Alaskan Native, uninsured, or underinsured.29 Although there may be a small administration fee charged by the provider’s office, the vaccine is free.
2. Don’t all of the vaccines recommended for children overwhelm their immune systems?
Children are exposed to so many more proteins on a daily basis (by crawling around on the floor, putting their hands in their mouths, attending school or day care, etc) than they are ever exposed to in a series of vaccines.30 Exposure to these proteins in their environment and to those in vaccines only serves to boost their immunity and keep them healthier in the long run.
And thanks to advances in vaccine production, the immunologic load in vaccines is far less than it used to be. The 14 vaccines given today contain <200 bacterial and viral proteins or polysaccharides, compared with the >3000 of these immunologic components in the 7 vaccines administered in 1980.31
Continue to: Influenza vaccine: Patient-friendly talking points
SIDEBAR
Influenza vaccine: Patient-friendly talking points
- Some people think that getting the flu is no big deal. While it is true that the flu takes a greater toll on the very young and very old, the chronically ill, and the immune compromised, even healthy people can become seriously ill or die. The Centers for Disease Control and Prevention estimates that the flu is responsible for 140,000 to 720,000 hospitalizations and 12,000 to 56,000 deaths in the United States every year.7 Of those who die from the flu, approximately 80% did not receive a flu shot.36 Of children who died from the flu between 2004 and 2012, more than 40% had no risk factors for complications.37
- The flu shot is a killed virus vaccine, so it can't give you the flu. People sometimes feel under the weather (achy, low-grade fever) after a vaccine, but this is considered normal and evidence that your body's immune system is "revving up."
- It takes 2 weeks before the vaccine becomes effective so a person can still get the flu during that time. This is why it is so important to get the vaccine earlier in the fall, before the flu season takes hold.
- The "stomach flu" is not the flu. The flu vaccine does not protect against the "stomach flu" or other flu-like illnesses.
- The flu vaccine is not perfect. It is an educated guess as to which strains will be circulating that year. (At its best, the flu vaccine is about 60% effective.38) However, it makes the chance of getting the flu less likely and significantly decreases the odds of severe complications/death.
- Egg allergies are no longer a reason to avoid the flu vaccine. There is an egg-free vaccine called Flublok (for those ≥18 years of age). In 2016-17, the Advisory Committee on Immunization Practices changed the recommendations for flu vaccine in egg-allergic people. The recommendations say that if reactions are mild, or you can eat cooked eggs without a problem, you can receive a flu vaccine. If you have severe reactions, such as trouble breathing or recurrent vomiting, you can still receive the flu vaccine, but must be monitored by a health care provider who can recognize and respond to a severe allergic reaction.39
Continue to: 3. Why don't we adhere to Dr. Sears' vaccine schedule?
3. Why don’t we adhere to Dr. Sears’ vaccine schedule?
There are multiple ways in which Dr. Robert Sears’ book, The Vaccine Book: Making the Right Decision for your Child, published in 2007, misrepresents vaccine science and leads patients astray in making decisions regarding vaccinations.32 Most important to note is that Dr. Sears’ Alternative Vaccine Schedule, which seeks to make it so that children do not receive more than 2 vaccinations per office visit, would require visits to a health care provider at 2, 3, 4, 5, 6, 7, 9, 12, 15, 18, and 21 months, and at 2, 2.5, 3, 3.5, 4, 5, and 6 years of age. This significantly increases the number of office visits and needle sticks, and raises the age at which vaccines are given, increasing the risk of illness outbreaks and decreasing the likelihood that parents would return to the office to complete the full series.
Acceptance of influenza and HPV vaccines remains a challenge
We are significantly less successful at getting parents and patients to agree to influenza and HPV vaccines than to the other vaccines we offer. The influenza vaccine success rate in 2016 was 59% in children and 43.3% in adults.33 Compared to the Tdap vaccine (88%) and the meningococcal vaccine (82%), which are offered at the same age as the HPV vaccine, success rates for HPV vaccine are significantly lower. In 2016, only 60.4% of boys and girls were current on their first HPV injection and only 43.3% were up to date with the full series.34
Newness of vaccines a factor?
Perhaps it is because the recommendations for these 2 vaccines are relatively new, and people don’t yet grasp the seriousness and scope of the diseases. Until 2010, the flu shot was recommended only for the very young, the elderly, and the medically high risk.
Similarly, the HPV vaccine was originally introduced for girls in 2006 and wasn’t recommended for boys until 2011.
Continue to: Human papillomavirus vaccine: Patient-friendly talking points
SIDEBAR
Human papillomavirus vaccine: Patient-friendly talking points
- Human papillomavirus (HPV) causes genital warts and cancer of the cervix, vagina, vulva, anus, rectum, penis, and oropharynx.
- The HPV vaccine is a cancer prevention vaccine. The 9-valent vaccine is active against 2 genital wart-causing strains and 7 cancer-causing strains of HPV.
- HPV is highly prevalent; 79 million Americans are currently infected, nearly 14 million people become newly infected each year, and nearly all of us will be exposed at some point in our sexual lives.40
- There are often no outward signs of infection, so it is a difficult infection to avoid.
- It takes no high-risk sexual activity to be exposed to the HPV virus.
- The HPV vaccine is recommended for both boys and girls usually around age 11 to 12 years (but as early as 9 years and as late as 26 years is acceptable). If the first vaccine is administered before 15 years of age, only 2 injections are needed 6 to 12 months apart. If the first vaccine is administered after 15 years of age, 3 injections are needed at 0, 2 months, and 6 months.41
- Completing the series before sexual activity begins is the best way to protect our children because the vaccine is a preventive measure, not a treatment.
- The HPV vaccine is highly effective with >90% efficacy against high-risk cancer-causing strains.42
- The HPV vaccine offers long-term protection. The vaccine has been on the market since 2006, and immunity has not yet diminished. Further monitoring is ongoing.43
- The HPV vaccine is covered under the Vaccines For Children program until age 19 years. Then it is up to individual insurance plans to cover it.
- The HPV vaccine does not cause infertility.44 HPV infection, on the other hand, can lead to fertility problems if, for example, treatment for cervical precancer or cancer requires partial removal of the cervix or a hysterectomy.
- The HPV vaccine does not cause autoimmune diseases.45,46 Studies show no difference between vaccinated and unvaccinated groups in rates of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes mellitus, multiple sclerosis, Hashimoto's thyroiditis, Graves' disease, and others.
- The HPV vaccine does not encourage earlier sexual activity. There was no earlier incidence of outcomes related to sexual activity (pregnancy, sexually transmitted infection testing or diagnosis, or contraceptive counseling) in vaccinated vs unvaccinated patients studied.47
Continue to: A sensitive subject
A sensitive subject. Discussion of a vaccine related to a child’s sexual health makes some parents uncomfortable. Studies show that focusing on the cancer prevention aspects of the vaccine, rather than on sexual transmission of HPV, results in greater vaccine acceptance.35
However, if discussion of sexual transmission is unavoidable, remind parents to consider their own adolescence and whether they chose to share everything with their parents. Point out that there were probably things they did that they later looked back on and thought, “What was I thinking?” Their children, no matter how wonderful and levelheaded they are, will be no different. And, as much as parents don’t want to think about it, some kids will suffer unwanted sexual contact. Shouldn’t parents protect their children as best as they can?
A teen’s right to choose? Some states have passed a Mature Minor Doctrine, which provides for mature, unemancipated teens to make their own medical decisions regarding such issues as sexuality, mental health, and drug and alcohol use without their parents’ consent. In these states, teens may elect to receive the HPV vaccine without parental permission. (Check your state’s laws for specifics, and see the 2 boxes with patient-friendly talking points for influenza vaccine7,36-39 and human papillomavirus vaccine.40-47)
CORRESPONDENCE
Gretchen LaSalle, MD, MultiCare Rockwood Clinic, 2214 East 29th Avenue, Spokane, WA 99203; [email protected].
We all know how challenging and time-consuming it can be to convince vaccine-hesitant patients that vaccinations are what is best for them and their children. Patients are bombarded with misinformation through the news and social media that seeds or “confirms” their doubts about vaccines. And for our part, we have only a few minutes during an office visit to refute all of the false claims that are a mere click or scroll away.
To better prepare for this challenge, this article details a practical approach to discussing vaccines with your patients. Using the patient-friendly language and evidence described here, you will be well positioned to refute 13 common vaccine misconceptions and overcome the barriers that stand in the way of these lifesaving interventions.
A few important baseline concepts
In discussing vaccination with our patients, it is important to keep the following in mind:
Patients don’t refuse vaccinations just to make our lives difficult. They truly are trying to make the best decisions they can for themselves and their families. Recognizing this can significantly reduce frustration levels.
Time well spent. While educating patients about the value of vaccines takes time, the return is worth it. The more consistently we offer vaccines, along with the reasons they are important, the more likely patients are to give vaccines a second thought. In fact, studies show that provider recommendation is the most important factor in patients’ decisions to vaccinate.1
Approach matters. In all other aspects of medicine, we attempt to use a participatory approach, involving our patients in decisions regarding their health care. When discussing vaccines, however, a participatory approach (eg, “What do you want to do about vaccines today?”) can introduce doubt into patients’ minds. Studies show that a presumptive approach (eg, “Today we are going to provide the tetanus, human papillomavirus [HPV], and meningitis vaccines”) is a much more effective way to get patients to vaccinate.2
[polldaddy:10018427]
Continue to: Barriers to counseling
Barriers to counseling. Health care providers report a variety of barriers to effective vaccine counseling (limited time and resources, lack of confidence in addressing patients’ concerns, etc).3 In addition, providers sometimes worry that strong encouragement of vaccination will create an adversarial relationship with vaccine-hesitant patients. Developing a good rapport and trusting relationship, as well as using motivational interviewing approaches, can help communicate the importance of vaccines, while leaving patients with the sense that you have heard them and respect their intentions. (See “Facilitate vaccine discussions using these 2 approaches.” 4-7)
SIDEBAR
Facilitate vaccine discussions using these 2 approaches4-7
C.A.S.E.
Corroborate
Acknowledge concerns and find some point on which you can agree.
Example: "It sounds like we both want to keep your child healthy and safe."
About me
Describe what you have done to build your expertise on the subject.
Example: "I have been practicing medicine for 15 years and have spent a great deal of time researching the data on vaccinations."
Science
Review the data and science behind vaccines.
Example: "Vaccines are more rigorously studied and safer than almost any other intervention we have in medicine."
Explain/advise
Explain your recommendations, based on the science.
Example: "This is why I vaccinate my children, and this is why I recommend this vaccine for your child."
3As
Ask
Don't stop at a patient's first "No." Respectfully dig a bit deeper.
Example: "What questions do you have about the vaccines we are recommending today? Tell me what worries you about them."
Acknowledge
Acknowledge your patient's concerns.
Example: "You are obviously a very devoted parent, and I know that you are trying to make the best decision you can for your child. With everything we see on the news and social media, it's not always easy to know what to believe about vaccines."
Advise
Advise patients/parents of the facts about vaccines and provide a strong recommendation to vaccinate.
Example: "Depending on the year, influenza kills 12,000 to 56,000 people annually; the vast majority of those who die did not receive the flu vaccine.7 My family and I get the flu shot every year, and I strongly encourage you and your children to get this lifesaving vaccine."
Continue to: If at first you don't succeed...
If at first you don’t succeed, try again because patients often have an experience that changes their mind. Perhaps a friend died of throat cancer or a family member developed a complication of the flu that required hospitalization. You never know when something will influence patients’ choices.
Don’t wait for scheduled well visits. Use every patient encounter as a means to catch patients up on missing vaccinations.
Common misconceptions and concerns and how to counter them
1. I’ve heard that vaccines can actually make you sick.
When patients raise this concern, start with an explanation of how vaccines work. Explain that our bodies protect us from foreign invaders (such as viruses and bacteria) by mounting an immune response when we are exposed to these proteins. Vaccinations work by exploiting this immune response; they expose the body to killed or weakened viral or bacterial proteins in a safe and controlled manner. In this way, our immune system will have already developed antibodies to these invaders by the time we are exposed to an active infection.
To use an analogy to war, instead of being subjected to a surprise attack where we suffer large losses in the battle, vaccination prepares us with weapons (antibodies) to defend ourselves so that our bodies are now able to successfully fight off that attack.
Because the majority of vaccines are killed virus vaccines, they cannot cause the illness against which they are meant to protect. Triggering the immune system may make some recipients feel a little “under the weather” for a day or 2, but they do not make us “sick.”
Live attenuated vaccines are similarly safe for those with a healthy immune system. We don’t administer them, however, to people who have a weakened immune system (eg, pregnant women, newborns, people with acquired immunodeficiency virus, or patients receiving chemotherapy or other types of immunosuppression) because these patients could develop the illness that we are trying to protect against.
Continue to: 2. Don't vaccines cause autism? Aren't they toxic to the nervous system?
2. Don’t vaccines cause autism? Aren’t they toxic to the nervous system?
The largest setback to vaccination efforts in recent history was a 1998 study by Andrew Wakefield that suggested that vaccination (specifically the mercury [in the form of thimerosal] present in the measles, mumps, rubella [MMR] vaccine) was linked to the development of autism.8 This research was subsequently debunked,9 and the author of the 1998 study was stripped of his medical license for falsifying data. However, the damage to vaccination efforts had already been done.
Aluminum. Thimerosal is not the only agent that patients may find concerning. Some also worry about the aluminum content of vaccines. Aluminum works as an additive to boost the body’s immune response to a vaccine. It is used only in killed virus vaccines—not in live attenuated ones. The Agency for Toxic Substances and Disease Registry monitors minimum risk levels (MRLs) of aluminum and other compounds in potentially hazardous substances. The amount of aluminum in vaccines is far below the MRL for aluminum, which is 1 mg/kg/d.10 (See “The facts about thimerosal and aluminum in vaccines.”11-16)
SIDEBAR
The facts about thimerosal and aluminum in vaccines
Thimerosal
Ethyl-mercury was used (in the form of thimerosal) as a preservative to prevent bacterial and fungal contamination of vaccines. Since 2001, however, thimerosal has been removed from all US-licensed vaccines—except multidose vials of influenza vaccine—as a precautionary measure (and not for any reproducible evidence of harm). The multidose flu vial contains <0.01% thimerosal.11
Ethyl-mercury is cleared from the body much more rapidly than methyl-mercury (the kind found in certain types of fish) and is less toxic.12
Since the removal of thimerosal from vaccines, the Centers for Disease Control and Prevention notes that the rates of autism have actually increased.13
Even Autism Speaks, the leading organization dedicated to advocacy for patients with autism and their families, denies a link between vaccines and autism.14
Aluminum
We are exposed to aluminum in products we use extensively every day, such as pots and pans, aluminum foil, seasonings, cereal, baby formula, paints, fuels, and antiperspirants.15
Infants are exposed to about 4.4 mg of aluminum in the vaccines typically administered in the first 6 months of life.16 However, infants typically ingest more than that during the first 6 months of life. Breast milk contains about 7 mg over 6 months; milk-based formulas contain about 38 mg over 6 months; and soy-based formulas contain about 117 mg over 6 months.16
Contine to: 3. I'm healthy. I never get sick. Why do I need vaccinations?
3. I’m healthy. I never get sick. Why do I need vaccinations?
A good way to counter this comment is to respond: “Saying you don’t need vaccinations because you never get sick is like saying you don’t need to wear a seat belt because you’ve never been in a car accident.” Advise patients that we seek to vaccinate all members of a community—not just those who are sick or at high risk—to protect ourselves and to provide “herd immunity.” It’s important to explain that herd immunity is resistance to the spread of a contagious disease that results if a sufficiently high number of people (depending on the illness, typically 80%-95%) are immune to the disease, especially through vaccination.17,18 If vaccination levels fall, we see a rise in cases of vaccine-preventable illness (as was seen during the 2017 measles outbreak in a community in Minnesota).19
Even though many of us may not suffer severe consequences of an infection, we can still pass that infection to others. While the whooping cough that a healthy 35-year-old gets may cause only prolonged annoyance or time off from work, it can kill the baby that is sitting next to that adult on the plane or bus.
4. Isn’t it true that we see fewer serious illnesses because of improved hygiene and sanitation, rather than vaccines?
Our current US sanitation standards were established under the Safe Drinking Water Act of 1974.20 While improvements in hygiene, sanitation, nutrition, and other public health measures have undoubtedly decreased the spread of disease and improved survival rates, there is no denying the significant drop in disease that occurs after the introduction of a vaccine for a particular illness or the increase in cases of that disease when vaccination rates drop off.
By the early 1990s, our current sanitation standards were already well established. Yet we didn’t see a significant decrease in the incidence of infections with Haemophilus influenzae type b (Hib) until after the conjugate Hib vaccines were introduced (dropping from about 20,000 cases/year to 1419 cases/year by 1993).21
In Britain, a drop in the rate of pertussis (whooping cough) vaccination in 1974 resulted in an epidemic of more than 100,000 cases and 36 deaths by 1978. There was no decrease in hygiene or sanitation standards to explain this rise.21
Continue to: 5. Vaccines are just another way for "big pharma" to make "big money."
5. Vaccines are just another way for “big pharma” to make “big money.”
Patients may benefit from knowing that in the earlier days of vaccines, pharmaceutical companies actually moved away from production of vaccines because they were not very profitable. These days, with worldwide distribution, drug companies are back in the swing of making vaccines and, as we would expect from all companies, are in business to make a profit.
That said, health care providers receive no payments from drug companies for offering vaccines or for offering one vaccine over another. The reason we recommend vaccination is because we know it is best for our patients’ health and the health of the community.

6. We don’t see polio anymore. Why do I need the vaccine?
One of the factors contributing to the rise in antivaccine sentiment is that we rarely see vaccine-preventable illnesses (such as polio, measles, and mumps). But the absence of these illnesses is precisely due to prior years’ vaccination efforts.
Smallpox, a deadly and disfiguring disease that killed many millions of people and contributed to the downfall of the Roman, Aztec, and Incan empires, was eradicated from the planet in 1979, thanks to focused vaccination efforts by the World Health Organization. Vaccination works, but we have to keep at it.
While we no longer see as many of these vaccine-preventable illnesses in the United States, they are still present in other parts of the world. Our world is much smaller than it used to be. International travel is common, and illnesses can be reintroduced into a community with relative ease. We must remain vigilant.
Continue to: 7. I heard that vaccines are made from aborted fetal tissue.
7. I heard that vaccines are made from aborted fetal tissue.
There are 5 vaccines (varicella, rubella, hepatitis A, shingles, and rabies vaccines) that were originally made using aborted fetal tissue. In 1960, tissue from 2 fetuses aborted by maternal choice (and not for the purpose of vaccine production) was used to propagate cell lines that are still used in vaccine development today.
Human cells provide advantages for vaccine production that other cells do not. Some viruses do not grow well in animal cells. Animal cells can introduce contamination by bacteria and viruses that are not carried in human cell lines. Vaccine production can be hindered or halted, resulting in a vaccine shortage, if animal products used in development are threatened (eg, if an illness strikes egg-producing chickens; eggs are used to make the influenza vaccine).22
Some patients, particularly those who are Catholic, may have concerns about these vaccines. The National Catholic Bioethics Center has prepared a statement regarding the use of these vaccines that may help settle any moral dilemmas.23 It reads:
“The cell lines under consideration were begun using cells taken from one or more fetuses aborted almost 40 years ago. Since that time, the cell lines have grown independently. It is important to note that descendent cells are not the cells of the aborted child.”
“One is morally free to use the vaccine regardless of its historical association with abortion. The reason is that the risk to public health, if one chooses not to vaccinate, outweighs the legitimate concern about the origins of the vaccine. This is especially important for parents, who have a moral obligation to protect the life and health of their children and those around them.”
Continue to: 8. Vaccines aren't studied—or monitored—thoroughly enough.
8. Vaccines aren’t studied—or monitored— thoroughly enough.
Patients would benefit from knowing that vaccines are some of the most thoroughly studied products brought to market. They undergo rigorous testing and oversight, from both public and private organizations, for 10 to 15 years before being released for distribution. Post-licensure monitoring is ongoing, and the manufacturer may voluntarily participate in Phase IV trials to continue to test the safety and efficacy of a vaccine after release to market.
Monitoring adverse effects. In addition, in 1990, the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration established the Vaccine Adverse Events Reporting System (VAERS) to “detect possible signals of adverse events associated with vaccines.”24 Most events reported are coincidental, but some common mild adverse events (like redness and swelling at the injection site) are often underreported.
Serious events are always thoroughly investigated and are often found unrelated. However, rare associations have been found. For example, an intestinal problem called intussusception, related to the original rotavirus vaccine, was discovered, and the vaccine causing it was removed from the market.25 A new, safer rotavirus vaccine option is now available. Patients need to know that we do have an effective system of checks and balances in which we can place our trust.
9. People can become paralyzed or stop breathing after receiving a vaccination. Why run those risks?
One of the most feared reactions to vaccination is Guillain-Barré syndrome (GBS), which can cause paralysis. The CDC estimates the risk for GBS associated with the flu vaccine, for example, to be 1 to 2 cases per 1 million people vaccinated.26 Another potential concern is the rate of anaphylaxis following vaccination. However, in a 2016 study in the Journal of Allergy and Clinical Immunology, the rate of anaphylaxis for all vaccines combined was only 1.31 per 1 million vaccines.27
The risk of developing severe complications from an illness is much greater than that of developing complications from the vaccine meant to protect a person against that illness. In the United States, the population-based risk for influenza-related hospitalization in children, for example, is as high as 150 in 100,000 with as many as 125 deaths annually.26
Continue to: 10. Isn't vaccination a personal choice? How does my health/illness impact the community?
10. Isn’t vaccination a personal choice? How does my health/illness impact the community?
Patients may not realize that most viruses are contagious from 1 to 2 days before symptoms appear, which means we can spread an illness before we even know we have it. Protecting oneself also protects those around us.
Economic concerns. There’s also the economic impact of these illnesses to consider. This includes the personal cost of being out of school or work for an extended period and the cost of a patient’s care, which can become astronomical if hospitalization is required and which can become the country’s problem if a person lacks sufficient health insurance coverage.
A study looking at the cost of 4 major adult vaccine-preventable illnesses (influenza, pneumococcal disease, shingles, and whooping cough) in the United States in 2013 estimated the annual cost for these illnesses in adults ≥50 years to be $26.5 billion.28 And that doesn’t include the cost of childhood vaccine-preventable diseases.
Countering 3 concerns about childhood vaccinations
1. I can’t afford vaccines for my child.
The Vaccines for Children program is a federally-funded program that covers the cost of all vaccines for children younger than 19 years of age who are Medicaid-eligible, American Indian, Alaskan Native, uninsured, or underinsured.29 Although there may be a small administration fee charged by the provider’s office, the vaccine is free.
2. Don’t all of the vaccines recommended for children overwhelm their immune systems?
Children are exposed to so many more proteins on a daily basis (by crawling around on the floor, putting their hands in their mouths, attending school or day care, etc) than they are ever exposed to in a series of vaccines.30 Exposure to these proteins in their environment and to those in vaccines only serves to boost their immunity and keep them healthier in the long run.
And thanks to advances in vaccine production, the immunologic load in vaccines is far less than it used to be. The 14 vaccines given today contain <200 bacterial and viral proteins or polysaccharides, compared with the >3000 of these immunologic components in the 7 vaccines administered in 1980.31
Continue to: Influenza vaccine: Patient-friendly talking points
SIDEBAR
Influenza vaccine: Patient-friendly talking points
- Some people think that getting the flu is no big deal. While it is true that the flu takes a greater toll on the very young and very old, the chronically ill, and the immune compromised, even healthy people can become seriously ill or die. The Centers for Disease Control and Prevention estimates that the flu is responsible for 140,000 to 720,000 hospitalizations and 12,000 to 56,000 deaths in the United States every year.7 Of those who die from the flu, approximately 80% did not receive a flu shot.36 Of children who died from the flu between 2004 and 2012, more than 40% had no risk factors for complications.37
- The flu shot is a killed virus vaccine, so it can't give you the flu. People sometimes feel under the weather (achy, low-grade fever) after a vaccine, but this is considered normal and evidence that your body's immune system is "revving up."
- It takes 2 weeks before the vaccine becomes effective so a person can still get the flu during that time. This is why it is so important to get the vaccine earlier in the fall, before the flu season takes hold.
- The "stomach flu" is not the flu. The flu vaccine does not protect against the "stomach flu" or other flu-like illnesses.
- The flu vaccine is not perfect. It is an educated guess as to which strains will be circulating that year. (At its best, the flu vaccine is about 60% effective.38) However, it makes the chance of getting the flu less likely and significantly decreases the odds of severe complications/death.
- Egg allergies are no longer a reason to avoid the flu vaccine. There is an egg-free vaccine called Flublok (for those ≥18 years of age). In 2016-17, the Advisory Committee on Immunization Practices changed the recommendations for flu vaccine in egg-allergic people. The recommendations say that if reactions are mild, or you can eat cooked eggs without a problem, you can receive a flu vaccine. If you have severe reactions, such as trouble breathing or recurrent vomiting, you can still receive the flu vaccine, but must be monitored by a health care provider who can recognize and respond to a severe allergic reaction.39
Continue to: 3. Why don't we adhere to Dr. Sears' vaccine schedule?
3. Why don’t we adhere to Dr. Sears’ vaccine schedule?
There are multiple ways in which Dr. Robert Sears’ book, The Vaccine Book: Making the Right Decision for your Child, published in 2007, misrepresents vaccine science and leads patients astray in making decisions regarding vaccinations.32 Most important to note is that Dr. Sears’ Alternative Vaccine Schedule, which seeks to make it so that children do not receive more than 2 vaccinations per office visit, would require visits to a health care provider at 2, 3, 4, 5, 6, 7, 9, 12, 15, 18, and 21 months, and at 2, 2.5, 3, 3.5, 4, 5, and 6 years of age. This significantly increases the number of office visits and needle sticks, and raises the age at which vaccines are given, increasing the risk of illness outbreaks and decreasing the likelihood that parents would return to the office to complete the full series.
Acceptance of influenza and HPV vaccines remains a challenge
We are significantly less successful at getting parents and patients to agree to influenza and HPV vaccines than to the other vaccines we offer. The influenza vaccine success rate in 2016 was 59% in children and 43.3% in adults.33 Compared to the Tdap vaccine (88%) and the meningococcal vaccine (82%), which are offered at the same age as the HPV vaccine, success rates for HPV vaccine are significantly lower. In 2016, only 60.4% of boys and girls were current on their first HPV injection and only 43.3% were up to date with the full series.34
Newness of vaccines a factor?
Perhaps it is because the recommendations for these 2 vaccines are relatively new, and people don’t yet grasp the seriousness and scope of the diseases. Until 2010, the flu shot was recommended only for the very young, the elderly, and the medically high risk.
Similarly, the HPV vaccine was originally introduced for girls in 2006 and wasn’t recommended for boys until 2011.
Continue to: Human papillomavirus vaccine: Patient-friendly talking points
SIDEBAR
Human papillomavirus vaccine: Patient-friendly talking points
- Human papillomavirus (HPV) causes genital warts and cancer of the cervix, vagina, vulva, anus, rectum, penis, and oropharynx.
- The HPV vaccine is a cancer prevention vaccine. The 9-valent vaccine is active against 2 genital wart-causing strains and 7 cancer-causing strains of HPV.
- HPV is highly prevalent; 79 million Americans are currently infected, nearly 14 million people become newly infected each year, and nearly all of us will be exposed at some point in our sexual lives.40
- There are often no outward signs of infection, so it is a difficult infection to avoid.
- It takes no high-risk sexual activity to be exposed to the HPV virus.
- The HPV vaccine is recommended for both boys and girls usually around age 11 to 12 years (but as early as 9 years and as late as 26 years is acceptable). If the first vaccine is administered before 15 years of age, only 2 injections are needed 6 to 12 months apart. If the first vaccine is administered after 15 years of age, 3 injections are needed at 0, 2 months, and 6 months.41
- Completing the series before sexual activity begins is the best way to protect our children because the vaccine is a preventive measure, not a treatment.
- The HPV vaccine is highly effective with >90% efficacy against high-risk cancer-causing strains.42
- The HPV vaccine offers long-term protection. The vaccine has been on the market since 2006, and immunity has not yet diminished. Further monitoring is ongoing.43
- The HPV vaccine is covered under the Vaccines For Children program until age 19 years. Then it is up to individual insurance plans to cover it.
- The HPV vaccine does not cause infertility.44 HPV infection, on the other hand, can lead to fertility problems if, for example, treatment for cervical precancer or cancer requires partial removal of the cervix or a hysterectomy.
- The HPV vaccine does not cause autoimmune diseases.45,46 Studies show no difference between vaccinated and unvaccinated groups in rates of autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, type 1 diabetes mellitus, multiple sclerosis, Hashimoto's thyroiditis, Graves' disease, and others.
- The HPV vaccine does not encourage earlier sexual activity. There was no earlier incidence of outcomes related to sexual activity (pregnancy, sexually transmitted infection testing or diagnosis, or contraceptive counseling) in vaccinated vs unvaccinated patients studied.47
Continue to: A sensitive subject
A sensitive subject. Discussion of a vaccine related to a child’s sexual health makes some parents uncomfortable. Studies show that focusing on the cancer prevention aspects of the vaccine, rather than on sexual transmission of HPV, results in greater vaccine acceptance.35
However, if discussion of sexual transmission is unavoidable, remind parents to consider their own adolescence and whether they chose to share everything with their parents. Point out that there were probably things they did that they later looked back on and thought, “What was I thinking?” Their children, no matter how wonderful and levelheaded they are, will be no different. And, as much as parents don’t want to think about it, some kids will suffer unwanted sexual contact. Shouldn’t parents protect their children as best as they can?
A teen’s right to choose? Some states have passed a Mature Minor Doctrine, which provides for mature, unemancipated teens to make their own medical decisions regarding such issues as sexuality, mental health, and drug and alcohol use without their parents’ consent. In these states, teens may elect to receive the HPV vaccine without parental permission. (Check your state’s laws for specifics, and see the 2 boxes with patient-friendly talking points for influenza vaccine7,36-39 and human papillomavirus vaccine.40-47)
CORRESPONDENCE
Gretchen LaSalle, MD, MultiCare Rockwood Clinic, 2214 East 29th Avenue, Spokane, WA 99203; [email protected].
1. Paterson P, Meurice F, Stanberry LR, et al. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700-6706.
2. Opel DJ, Heritage J, Taylor J, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132:1037-1046.
3. Palmer J, Carrico C, Costanzo C. Identifying and overcoming perceived barriers of providers towards vaccination: a literature review. J Vaccines. 2015;1-7.
4. Autism Science Foundation. Making the CASE for vaccines: a new model for talking to patients about vaccines. Available at: http://autismsciencefoundation.org/wp-content/uploads/2015/12/Making-the-CASE-for-Vaccines-Guide_final.pdf. Accessed April 8, 2018.
5. Jacobson RM, Van Etta L, Bahta L. The C.A.S.E approach: guidance for talking to vaccine-hesitant patients. Minn Med. 2013;96:49-50.
6. Henrickson NB, Opel DJ, Grothaus L, et al. Physician communication training and parental vaccine hesitancy: a randomized trial. Pediatrics. 2015;136:70-79.
7. Centers for Disease Control and Prevention. Key facts about seasonal flu vaccine. Available at: https://www.cdc.gov/flu/protect/keyfacts.htm. Accessed April 8, 2018.
8. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641.
9. Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32:3623-3629.
10. Agency for Toxic Substances & Disease Registry. Minimal risk levels for hazardous substances. Available at: https://www.atsdr.cdc.gov/mrls/mrllist.asp#34tag. Accessed April 8, 2018.
11. US Food and Drug Administration. Thimerosal and vaccines. Available at: https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed April 8, 2018.
12. Hviid A, Stellfeld M, Wohlfahrt J, et al. Association between thimerosal-containing vaccine and autism. JAMA. 2003;290:1763-1766.
13. Centers for Disease Control and Prevention. Thimerosal in vaccines. Available at: https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed May 8, 2018.
14. Autism Speaks. Frequently asked questions. Available at: https://www.autismspeaks.org/what-autism/faq. Accessed April 8, 2018.
15. Agency for Toxic Substances & Disease Registry. Toxic substances portal-aluminum. Public Health Statement for Aluminum, CAS #7429-90-5. Available at: https://www.atsdr.cdc.gov/PHS/PHS.asp?id=1076&tid=34. Accessed April 8, 2018.
16. Children’s Hospital of Philadelphia. Vaccine ingredients-aluminum. Available at: www.chop.edu/centers-programs/vaccine-education-center/vaccine-ingredients/aluminum. Accessed April 8, 2018.
17. Orenstein W, Seib K. Mounting a good offense against measles. N Engl J Med. 2014;371:1661-1663.
18. Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med. 2012;55:72-77.
19. Hall V, Banerjee E, Kenyon C, et al. Measles outbreak – Minnesota April-May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:713-717.
20. The National Academies of Sciences Engineering Medicine. History of U.S. water and wastewater systems. Privatization of Water Services in the United States: an Assessment of Issues and Experience. Washington, DC: The National Academies Press; 2002:29-40. Available at: https://www.nap.edu/read/10135/chapter/4#35. Accessed May 7, 2018.
21. World Health Organization. Global vaccine safety. Six common misconceptions about immunization. Available at: http://www.who.int/vaccine_safety/initiative/detection/immunization_misconceptions/en/index1.html. Accessed May 7, 2018.
22. The history of vaccines. Human cell strains in vaccine development. Available at: https://www.historyofvaccines.org/content/articles/human-cell-strains-vaccine-development. Accessed April 8, 2018.
23. The National Catholic Bioethics Center. Frequently asked questions. Available at: https://www.ncbcenter.org/resources/frequently-asked-questions/use-vaccines/. Accessed April 8, 2018.
24. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33:4398-4405.
25. Foster S. Rotavirus vaccine and intussusception. J Pediatr Pharmacol Ther. 2007;12:4-7.
26. Mistry RD, Fischer JB, Prasad PA, et al. Severe complications of influenza-like illnesses. Pediatrics. 2014;134:e684-e690.
27. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
28. McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36:259-273.
29. Centers for Disease Control and Prevention. Vaccines for Children (VFC) Program. Available at: https://www.cdc.gov/features/vfcprogram/index.html. Accessed April 8, 2018.
30. Plotkin S, Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis. 2009;48:456-461.
31. Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics. 2002;109:124-129.
32. Offit PA, Moser CA. The problem with Dr. Bob’s alternative vaccine schedule. Pediatrics. 2009;123:e164-e169.
33. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. April 8. 2018.
34. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
35. Thomas TL. Cancer prevention: HPV vaccination. Semin Oncol Nurs. 2016:32:273-280.
36. Centers for Disease Control and Prevention. Estimating seasonal influenza-associated deaths in the United States. Available at: https://www.cdc.gov/flu/about/disease/US_flu-related_deaths.htm. Accessed May 8, 2018.
37. Wong KK, Jain S, Blanton L, et al. Influenza-associated pediatric deaths in the United States: 2004-2012. Pediatrics. 2013;132:796-804.
38. Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed April 8, 2018.
39. Centers for Disease Control and Prevention. Influenza (flu). Flu vaccine and people with egg allergies. Available at: https://www.cdc.gov/flu/protect/vaccine/egg-allergies.htm. Accessed April 8, 2018.
40. Centers for Disease Control and Prevention. For parents: vaccines for your children. HPV vaccine for preteens and teens. Available at: https://www.cdc.gov/vaccines/parents/diseases/teen/hpv.html. Accessed April 8, 2018.
41. Centers for Disease Control and Prevention. Vaccines and preventable diseases. HPV vaccine recommendations. Available at: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed May 7, 2018.
42. Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007;85:719-726.
43. De Vincenzo R, Conte C, Ricci C, et al. Long-term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999-1010.
44. McInerney KA, Hatch EE, Wesselink AK. The effect of vaccination against human papillomavirus on fecundability. Paedeatr Perinat Epidemiol. 2017;31:531-536.
45. Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193-203.
46. Vichnin M, Bonanni P, Klein NP, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006-2015. Ped Infect Dis J. 2015;34:983-991.
47. Bednarczyk RA, Davis R, Ault K, et al. Sexual activity-related outcomes after human papillomavirus vaccination of 11-to-12-year-olds. Pediatrics. 2012;130:798-805.
1. Paterson P, Meurice F, Stanberry LR, et al. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700-6706.
2. Opel DJ, Heritage J, Taylor J, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132:1037-1046.
3. Palmer J, Carrico C, Costanzo C. Identifying and overcoming perceived barriers of providers towards vaccination: a literature review. J Vaccines. 2015;1-7.
4. Autism Science Foundation. Making the CASE for vaccines: a new model for talking to patients about vaccines. Available at: http://autismsciencefoundation.org/wp-content/uploads/2015/12/Making-the-CASE-for-Vaccines-Guide_final.pdf. Accessed April 8, 2018.
5. Jacobson RM, Van Etta L, Bahta L. The C.A.S.E approach: guidance for talking to vaccine-hesitant patients. Minn Med. 2013;96:49-50.
6. Henrickson NB, Opel DJ, Grothaus L, et al. Physician communication training and parental vaccine hesitancy: a randomized trial. Pediatrics. 2015;136:70-79.
7. Centers for Disease Control and Prevention. Key facts about seasonal flu vaccine. Available at: https://www.cdc.gov/flu/protect/keyfacts.htm. Accessed April 8, 2018.
8. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641.
9. Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014;32:3623-3629.
10. Agency for Toxic Substances & Disease Registry. Minimal risk levels for hazardous substances. Available at: https://www.atsdr.cdc.gov/mrls/mrllist.asp#34tag. Accessed April 8, 2018.
11. US Food and Drug Administration. Thimerosal and vaccines. Available at: https://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228. Accessed April 8, 2018.
12. Hviid A, Stellfeld M, Wohlfahrt J, et al. Association between thimerosal-containing vaccine and autism. JAMA. 2003;290:1763-1766.
13. Centers for Disease Control and Prevention. Thimerosal in vaccines. Available at: https://www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed May 8, 2018.
14. Autism Speaks. Frequently asked questions. Available at: https://www.autismspeaks.org/what-autism/faq. Accessed April 8, 2018.
15. Agency for Toxic Substances & Disease Registry. Toxic substances portal-aluminum. Public Health Statement for Aluminum, CAS #7429-90-5. Available at: https://www.atsdr.cdc.gov/PHS/PHS.asp?id=1076&tid=34. Accessed April 8, 2018.
16. Children’s Hospital of Philadelphia. Vaccine ingredients-aluminum. Available at: www.chop.edu/centers-programs/vaccine-education-center/vaccine-ingredients/aluminum. Accessed April 8, 2018.
17. Orenstein W, Seib K. Mounting a good offense against measles. N Engl J Med. 2014;371:1661-1663.
18. Plans-Rubió P. The vaccination coverage required to establish herd immunity against influenza viruses. Prev Med. 2012;55:72-77.
19. Hall V, Banerjee E, Kenyon C, et al. Measles outbreak – Minnesota April-May 2017. MMWR Morb Mortal Wkly Rep. 2017;66:713-717.
20. The National Academies of Sciences Engineering Medicine. History of U.S. water and wastewater systems. Privatization of Water Services in the United States: an Assessment of Issues and Experience. Washington, DC: The National Academies Press; 2002:29-40. Available at: https://www.nap.edu/read/10135/chapter/4#35. Accessed May 7, 2018.
21. World Health Organization. Global vaccine safety. Six common misconceptions about immunization. Available at: http://www.who.int/vaccine_safety/initiative/detection/immunization_misconceptions/en/index1.html. Accessed May 7, 2018.
22. The history of vaccines. Human cell strains in vaccine development. Available at: https://www.historyofvaccines.org/content/articles/human-cell-strains-vaccine-development. Accessed April 8, 2018.
23. The National Catholic Bioethics Center. Frequently asked questions. Available at: https://www.ncbcenter.org/resources/frequently-asked-questions/use-vaccines/. Accessed April 8, 2018.
24. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. 2015;33:4398-4405.
25. Foster S. Rotavirus vaccine and intussusception. J Pediatr Pharmacol Ther. 2007;12:4-7.
26. Mistry RD, Fischer JB, Prasad PA, et al. Severe complications of influenza-like illnesses. Pediatrics. 2014;134:e684-e690.
27. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
28. McLaughlin JM, McGinnis JJ, Tan L, et al. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36:259-273.
29. Centers for Disease Control and Prevention. Vaccines for Children (VFC) Program. Available at: https://www.cdc.gov/features/vfcprogram/index.html. Accessed April 8, 2018.
30. Plotkin S, Gerber JS, Offit PA. Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis. 2009;48:456-461.
31. Offit PA, Quarles J, Gerber MA, et al. Addressing parents’ concerns: do multiple vaccines overwhelm or weaken the infant’s immune system? Pediatrics. 2002;109:124-129.
32. Offit PA, Moser CA. The problem with Dr. Bob’s alternative vaccine schedule. Pediatrics. 2009;123:e164-e169.
33. Centers for Disease Control and Prevention. Flu vaccination coverage, United States, 2016-17 influenza season. Available at: https://www.cdc.gov/flu/fluvaxview/coverage-1617estimates.htm. April 8. 2018.
34. Walker TY, Elam-Evans LD, Singleton JA, et al. National, regional, state and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:874-882.
35. Thomas TL. Cancer prevention: HPV vaccination. Semin Oncol Nurs. 2016:32:273-280.
36. Centers for Disease Control and Prevention. Estimating seasonal influenza-associated deaths in the United States. Available at: https://www.cdc.gov/flu/about/disease/US_flu-related_deaths.htm. Accessed May 8, 2018.
37. Wong KK, Jain S, Blanton L, et al. Influenza-associated pediatric deaths in the United States: 2004-2012. Pediatrics. 2013;132:796-804.
38. Centers for Disease Control and Prevention. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed April 8, 2018.
39. Centers for Disease Control and Prevention. Influenza (flu). Flu vaccine and people with egg allergies. Available at: https://www.cdc.gov/flu/protect/vaccine/egg-allergies.htm. Accessed April 8, 2018.
40. Centers for Disease Control and Prevention. For parents: vaccines for your children. HPV vaccine for preteens and teens. Available at: https://www.cdc.gov/vaccines/parents/diseases/teen/hpv.html. Accessed April 8, 2018.
41. Centers for Disease Control and Prevention. Vaccines and preventable diseases. HPV vaccine recommendations. Available at: https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html. Accessed May 7, 2018.
42. Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ. 2007;85:719-726.
43. De Vincenzo R, Conte C, Ricci C, et al. Long-term efficacy and safety of human papillomavirus vaccination. Int J Womens Health. 2014;6:999-1010.
44. McInerney KA, Hatch EE, Wesselink AK. The effect of vaccination against human papillomavirus on fecundability. Paedeatr Perinat Epidemiol. 2017;31:531-536.
45. Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271:193-203.
46. Vichnin M, Bonanni P, Klein NP, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006-2015. Ped Infect Dis J. 2015;34:983-991.
47. Bednarczyk RA, Davis R, Ault K, et al. Sexual activity-related outcomes after human papillomavirus vaccination of 11-to-12-year-olds. Pediatrics. 2012;130:798-805.
From The Journal of Family Practice | 2018;67(6):348-351,359-364.
PRACTICE RECOMMENDATIONS
› Use a presumptive approach when discussing vaccines with patients/parents. A
› Offer vaccines at every opportunity; provider recommendation is the most important factor in getting patients to vaccinate. A
› Focus on the cancer prevention aspect of the human papillomavirus vaccine to improve rates of vaccine acceptance. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
FDA places CTX001 for SCD on clinical hold
The US Food and Drug Administration (FDA) has placed a clinical hold on the investigational new drug application (IND) for CTX001. The agent is being developed for the treatment of sickle cell disease (SCD) and β-thalassemia.
The IND was submitted to the FDA in April to support the initiation of a phase 1/2 trial in the US in adult patients with SCD. The hold will be in place pending the resolution of questions as part of the FDA review.
The phase 1/2 trial in Europe in adult patients with transfusion-dependent β-thalassemia is expected to proceed according to schedule. Trial initiation is planned for the second half of 2018.
CTX001 is being co-developed and co-commercialized by CRISPR Therapeutics and Vertex Pharmaceuticals Incorporated.
The agent is an investigational ex vivo CRISPR gene edited therapy for patients with β-thalassemia or sickle cell disease.
The patient’s hematopoietic stem cells are engineered to produce high levels of fetal hemoglobin (HbF; hemoglobin F) in red blood cells.
The increase in HbF levels by CTX001 may potentially alleviate transfusion requirements for β-thalassemia patients and sickle crises for SCD patients.
The US Food and Drug Administration (FDA) has placed a clinical hold on the investigational new drug application (IND) for CTX001. The agent is being developed for the treatment of sickle cell disease (SCD) and β-thalassemia.
The IND was submitted to the FDA in April to support the initiation of a phase 1/2 trial in the US in adult patients with SCD. The hold will be in place pending the resolution of questions as part of the FDA review.
The phase 1/2 trial in Europe in adult patients with transfusion-dependent β-thalassemia is expected to proceed according to schedule. Trial initiation is planned for the second half of 2018.
CTX001 is being co-developed and co-commercialized by CRISPR Therapeutics and Vertex Pharmaceuticals Incorporated.
The agent is an investigational ex vivo CRISPR gene edited therapy for patients with β-thalassemia or sickle cell disease.
The patient’s hematopoietic stem cells are engineered to produce high levels of fetal hemoglobin (HbF; hemoglobin F) in red blood cells.
The increase in HbF levels by CTX001 may potentially alleviate transfusion requirements for β-thalassemia patients and sickle crises for SCD patients.
The US Food and Drug Administration (FDA) has placed a clinical hold on the investigational new drug application (IND) for CTX001. The agent is being developed for the treatment of sickle cell disease (SCD) and β-thalassemia.
The IND was submitted to the FDA in April to support the initiation of a phase 1/2 trial in the US in adult patients with SCD. The hold will be in place pending the resolution of questions as part of the FDA review.
The phase 1/2 trial in Europe in adult patients with transfusion-dependent β-thalassemia is expected to proceed according to schedule. Trial initiation is planned for the second half of 2018.
CTX001 is being co-developed and co-commercialized by CRISPR Therapeutics and Vertex Pharmaceuticals Incorporated.
The agent is an investigational ex vivo CRISPR gene edited therapy for patients with β-thalassemia or sickle cell disease.
The patient’s hematopoietic stem cells are engineered to produce high levels of fetal hemoglobin (HbF; hemoglobin F) in red blood cells.
The increase in HbF levels by CTX001 may potentially alleviate transfusion requirements for β-thalassemia patients and sickle crises for SCD patients.
USPSTF takes another stab at PSA screening recs
Resources
US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
Carter HB. Prostate-specific antigen (PSA) screening for prostate cancer: revisiting the evidence. JAMA. 2018;319:1866-1868.
US Preventive Services Task Force. Prostate cancer screening final recommendation. Available at: https://screeningforprostatecancer.org/. Accessed May 18, 2018.
US Preventive Services Task Force. Prostate cancer: screening, 2008. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening-2008. Accessed May 15, 2018.
US Preventive Services Task Force. Prostate cancer: screening. May 2012. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed May 15, 2018.
Resources
US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
Carter HB. Prostate-specific antigen (PSA) screening for prostate cancer: revisiting the evidence. JAMA. 2018;319:1866-1868.
US Preventive Services Task Force. Prostate cancer screening final recommendation. Available at: https://screeningforprostatecancer.org/. Accessed May 18, 2018.
US Preventive Services Task Force. Prostate cancer: screening, 2008. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening-2008. Accessed May 15, 2018.
US Preventive Services Task Force. Prostate cancer: screening. May 2012. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed May 15, 2018.
Resources
US Preventive Services Task Force. Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319:1901-1913.
Carter HB. Prostate-specific antigen (PSA) screening for prostate cancer: revisiting the evidence. JAMA. 2018;319:1866-1868.
US Preventive Services Task Force. Prostate cancer screening final recommendation. Available at: https://screeningforprostatecancer.org/. Accessed May 18, 2018.
US Preventive Services Task Force. Prostate cancer: screening, 2008. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/prostate-cancer-screening-2008. Accessed May 15, 2018.
US Preventive Services Task Force. Prostate cancer: screening. May 2012. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/prostate-cancer-screening. Accessed May 15, 2018.
USPSTF offers 3 recommendations for preventing falls in older adults
Resources
US Preventive Services Task Force. Final recommendation statement: Falls prevention in community-dwelling older adults: interventions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/falls-prevention-in-older-adults-interventions1. Accessed May 8, 2018.
US Preventive Services Task Force. Interventions to prevent falls in community-dwelling older adults. US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704.
Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults. Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716.
Resources
US Preventive Services Task Force. Final recommendation statement: Falls prevention in community-dwelling older adults: interventions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/falls-prevention-in-older-adults-interventions1. Accessed May 8, 2018.
US Preventive Services Task Force. Interventions to prevent falls in community-dwelling older adults. US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704.
Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults. Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716.
Resources
US Preventive Services Task Force. Final recommendation statement: Falls prevention in community-dwelling older adults: interventions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/falls-prevention-in-older-adults-interventions1. Accessed May 8, 2018.
US Preventive Services Task Force. Interventions to prevent falls in community-dwelling older adults. US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1696-1704.
Guirguis-Blake JM, Michael YL, Perdue LA, et al. Interventions to prevent falls in older adults. Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018;319:1705-1716.
Is the "breast is best" mantra an oversimplification?
The benefits of breastfeeding for infants have long been touted as numerous and supported by overwhelming evidence. The World Health Organization (WHO), American College of Obstetricians and Gynecologists, American Academy of Pediatrics (AAP), and American Academy of Family Physicians all strongly recommend exclusive breastfeeding for the first 6 months of life, citing numerous health benefits for child and mother. These groups recommend that some breastfeeding be continued through the first 12 months of life, or longer, as desired (the WHO extends the recommendation to 2 years).1-4 In 2000, the Surgeon General of the United States released a strategic plan to increase rates of breastfeeding,5 setting goals (by 2010) of:
- 75% of mothers leaving the hospital breastfeeding
- 50% of babies breastfeeding at 6 months
- 25% of babies breastfeeding at 1 year.
Massive public health campaigns citing data for the many benefits of breastfeeding have been launched with the goal of increasing the breastfeeding rate. In 2014, statistics offered a testament to the success of these campaigns6:
- 82.5% of infants had been breastfed “ever”
- 55.3% were breastfed “some”
- 24.9% were breastfed exclusively through 6 months of age
- 33.7% were breastfed “some” at 12 months.
Breastfeeding advocacy has become clouded
In recent years, an increasing number of researchers, physicians, and authors have begun to question whether, in the United States, the benefits of breastfeeding children are exaggerated and the emphasis on breastfeeding might be leading to feelings of inadequacy, guilt, and anxiety among mothers.7-13 In 2016, the US Preventive Services Task Force (USPSTF) amended its recommendation to “promote and support breastfeeding” to simply “support breastfeeding”—a change that created substantial debate and prompted the Task Force to clarify its stance in changing the language: In its response to public comment, the USPSTF said that its position regarding promotion had not changed, but the language in the original statement had been revised to “ensure that the autonomy of women is respected.” 2,14-16
In contrast, others suggest counseling women on the risks of formula feeding rather than on the benefits of breastfeeding, citing substantial health outcome distinctions.17 Indeed, wide-ranging conclusions have been drawn from the same data on the topic, potentially creating uncertainty for physicians on how best to counsel women on their choice of how to feed their infant.
In this article, we address this uncertainty by utilizing the most recent and comprehensive data to examine infant health outcomes. When possible, the number needed to treat (NNT) for a given outcome has been calculated or approximated, allowing the reader to estimate the likelihood of benefit for an individual mother–infant dyad. Exercise caution when interpreting the NNT, however: The numbers suggest causality that cannot be definitively established using the observational data on which those numbers are based.
Continue to: Infectious disease
Infectious disease
Acute otitis media. Exclusive breastfeeding for 6 months is associated with a 43% reduction in the risk of acute otitis media (AOM) by 2 years of age (odds ratio [OR]=0.57; 95% confidence interval [CI], 0.44-0.75). Beyond 2 years of age, or when comparing “ever” and “never” breastfeeding, the effect disappears. All studies in this meta-analysis had serious limitations.18
Nearly half of children will have at least one case of AOM by one year of age; 80%, by 2 years.19,20 Since the introduction of the heptavalent pneumococcal conjugate vaccine, the rate of AOM at 2 years has fallen by as much as 20%.21 Assuming an incidence of 60% to 80% of AOM by 2 years, only 2 or 3 infants need to be exclusively breastfed for 6 months to prevent a single case of AOM.18 Prevention of AOM through breastfeeding may be related to head position during feeding, antibacterial effects of breast milk, protective oral microbiome in the breastfed infant pharynx, and/or prevention of primary viral upper respiratory infection (URI), which nearly always precedes AOM.18,19
Upper and lower respiratory tract infections. Infants who are exclusively breastfed for 4 months and partially breastfed after 4 months have a lower risk of URI (OR=0.65; 95% CI, 0.51-0.83) and of lower respiratory tract infection (LRTI; OR=0.50; 95% CI, 0.32-0.72).22
The effect is stronger for URI among infants exclusively breastfed for at least 6 months (OR=0.37; 95% CI, 0.18-0.74), but is no longer significant by that time for LRTI (OR=0.33; 95% CI, 0.08-1.40). Importantly, AOM was included in the URI group, and, as previously discussed, AOM has independently been shown to have an inverse relationship with breastfeeding duration.
At 7 to 12 months of age, no association was seen between breastfeeding and the incidence of URI. Curiously, an association with LRTI was again detected for infants breastfed exclusively for 4 months and partially thereafter, but was not detected with exclusive breastfeeding for at least 6 months (OR=0.46; 95% CI, 0.31-0.69). In this study, in the first 6 months of life, 40% of infants had a URI and 8% had an LRTI. The findings in this cohort suggest an NNT of 6 or 7 for prevention of URI and an NNT of 25 for prevention of LRTI in the first 6 months of life.22
Continue to: Children younger than 2 years are...
Children younger than 2 years are estimated to have approximately 6 bouts of the common cold a year, and essentially 100% have at least one bout—perhaps lowering the NNT for URI if applied widely. However, these data are not divided into 6-month intervals, making accurate extrapolation difficult.23
Gastrointestinal infection. The rate of diarrheal illness in the first year of life is lower in infants who are exclusively breastfed for at least 4 months and partially breastfed after.
Both the Promotion of Breastfeeding Intervention Trial (PROBIT; a clinical trial in which infants were randomized to a breastfeeding education intervention or standard care) and a 2010 prospective cohort study in the Netherlands of more than 3400 infants found a reduction in the risk of one or more gastrointestinal (GI) infections at a similar rate.22,24
- In PROBIT, 9.1% of infants in the intervention group, compared to 13.2% in the standard care group (OR=0.60; 95% CI, 0.40-0.91), had one or more GI infections at 12 months of age.24
- In the 2010 Netherlands cohort, 8% of infants had a GI infection by 6 months of age. Infants breastfed exclusively for at least 4 or 6 months had a decreased risk for GI infection (respectively: adjusted OR=0.41; 95% CI, 0.26-0.64 and adjusted OR=0.46; 95% CI, 0.14-1.59). No such association was found for any feeding group 7 to 12 months of age.22
These studies are notable for the low incidence of GI infection, which is frequently cited as 1.3 to 2.3 episodes per child per year in children younger than 3 years in the United States.25 However, that high incidence has likely declined significantly since the introduction of rotavirus vaccine in 2006. In the years following the introduction of the vaccine, infant visits for gastroenteritis decreased by >90% in all care settings in the South, Northeast, and Midwest regions of the United States and by 53% to 63% in the West region.26 Recent accurate epidemiologic information, in an era of significantly higher vaccination rates, is lacking.
Assuming the low incidence of GI infection reported in PROBIT and the Netherlands trials, about 25 to 30 infants need to be exclusively breastfed for 4 to 6 months to prevent a single GI infection during the first 6 to 12 months of life.22,24 Assuming a 60% incidence by age 12 months before introduction of the rotavirus vaccine, the NNT would be approximately 4.24 The true number is likely somewhere between those 2 NNTs.
Continue to: Hospitalization
Hospitalization
Risk of infection is decreased. A large cohort study in Scotland, involving more than 500,000 children, found an association between exclusive breastfeeding for 6 to 8 weeks and decreased risk of hospitalization within the first 6 months of life. Formula-fed and mixed-fed infants had an increased hazard ratio (HR) for hospitalization for common childhood illness (HR=1.40; 95% CI, 1.35-1.45 for formula-fed infants and HR=1.18; 95% CI, 1.11-1.25 for mixed-fed infants).27 The study also found increased rates of hospitalization for conditions for which other meta-analyses have failed to show a protective effect from breastfeeding—leading to suspicion of residual confounding in the study. Another United Kingdom cohort demonstrated lower rates of hospitalization for GI infection (NNT=171) and LRTI (NNT=115) among exclusively breastfed infants by 8 months of age.28
Risk of neonatal readmission is increased. Late preterm infants who are exclusively breastfed are nearly twice as likely to be hospitalized as breastfed term or non-breastfed preterm infants, primarily due to dehydration, failure to thrive, weight loss, and hyperbilirubinemia. In fact, exclusive breastfeeding at discharge from the hospital is likely the single greatest risk factor for hospital readmission in newborns.29,30 Term infants who are exclusively breastfed are more likely to be hospitalized compared to formula-fed or mixed-fed infants, due to hyperbilirubinemia, dehydration, hypernatremia, and weight loss (number needed to harm (NNH)=71).30-32 For weight loss >10% of birth weight with or without hospitalization, the NNH for breastfed infants is 13.32
Many of these hospitalizations and events could be avoided with appropriate monitoring and medically indicated supplementation; the likelihood of long-term harm is low. Formula supplementation is often avoided if possible in hospitals to promote exclusive breastfeeding; however, several small randomized clinical trials have demonstrated that limited formula supplementation in breastfed infants does not affect the breastfeeding continuation rate at 3 and 6 months, and, therefore, might be a way to decrease infant rehospitalization.33,34
Necrotizing enterocolitis
In preterm infants, breastfeeding has been associated with a lower rate of necrotizing enterocolitis. In the 2007 Agency for Healthcare Research and Quality report, the association was found to be only marginally statistically significant, and the authors warned that, first, evidence is old and heterogeneous and, second, present preterm formula is much different than the formula used in earlier studies of preterm infant nutrition and necrotizing enterocolitis.35 A 2012 Cochrane review included newer studies in its analysis but reached the same conclusion on the quality and heterogeneity of available evidence, with a NNT of 25.36
Continue to: Sudden infant death syndrome
Sudden infant death syndrome
There is a statistically significant association between sudden infant death syndrome (SIDS) and feeding method. Infants whose cause of death is SIDS are approximately one half as likely to have been breastfed as matched controls.35,37
In 2005, AAP did not recommend breastfeeding as a means to reduce the risk of SIDS because available evidence was mixed, and studies at the time were poorly controlled.38 Since that time, case-control meta-analyses have shed additional light on the association between SIDS and feeding method.35,37
The protective effect exists for any amount of breastfeeding and is stronger for exclusive breastfeeding, suggesting a protective role—not simply an association. Caution should be employed with this conclusion, however, because the studies included in the meta-analysis used univariate analysis primarily and did not control sufficiently for known confounders. In addition, the authors warn that publication bias might overestimate the association.38
Potential mechanisms of a protective role include decreased risk of infection and greater arousability from sleep in breastfed infants. Assuming a protective role, available data suggest that more than 3500 infants need to be breastfed to prevent one case of SIDS.39
Continue to: Allergic disease
Allergic disease
Asthma. There is evidence of a small protective effect of breastfeeding “ever” on asthma at 5 to 18 years of age in high-income countries (OR=0.90; 95% CI, 0.83-0.97). A family history of asthma or atopy did not affect this finding. The authors note there is some evidence of publication bias in this review, which is the largest and most comprehensive on the topic.40
With a lifetime prevalence of asthma in the United States of approximately 13.2%, this association would confer an NNT of roughly 76.41 Earlier, the literature demonstrated mixed and conflicting evidence, and some experts suggested an effect only when there is a family history of asthma or atopy.36
Eczema. For children younger than 2 years, there is low-grade- and very-low-grade-quality evidence that exclusive breastfeeding longer than 3 to 4 months is associated with a reduced risk of eczema (OR=0.74; 95% CI, 0.57-0.97).40
Previously, data suggested that this association existed only in children who had a family history of atopy.35 The protective association, however, exists regardless of family history and does not persist beyond 2 years of age. The authors noted evidence of publication bias, reverse causation, and misdiagnosis of early childhood rashes as eczema as limitations of their findings.40
Continue to: Reliable epidemiologic evidence...
Reliable epidemiologic evidence on the incidence of eczema in infants in the United States is limited, but the prevalence in the United States in children younger than 17 years is approximately 10.7% (with wide regional variation). Extrapolating these data generously, the NNT to prevent eczema in the first 2 years of life could be estimated at approximately 36.42
Allergic rhinitis. There is low-grade- and very-low-grade-quality evidence that more breastfeeding, compared to less breastfeeding, is associated with a lower risk of allergic rhinitis in children younger than 5 years (OR=0.79; 95% CI, 0.63-0.98). The association exists regardless of family history and disappears after 5 years of age. The differentiation of allergic rhinitis from rhinovirus infection (for which there is higher-quality evidence of a protective effect with breastfeeding) must be considered when interpreting these data.40
Reliable epidemiologic evidence on allergic rhinitis in children younger than 5 years is lacking, and incidence varies by region. A rough estimate, using data from 6- and 7-year-olds, indicates an NNT of 54 to 70.43
Food allergy. There is no evidence to suggest an association between breastfeeding and food allergy, either as protective or as a risk factor, and studies are limited.40 Interestingly, as data accumulate associating early exposure to foods with protection, some authors have proposed reexamining the recommendation from WHO and US health organizations for exclusive breastfeeding for the first 6 months of life.7,44
Continue to: Dental health
Dental health
Dental caries. There is consistent evidence that breastfeeding beyond 12 months of age is associated with the development of dental caries of deciduous teeth to 6 years of age (OR=2.90; 95% CI, 2.33-3.60). Many of the studies that showed this association did not control for the introduction of sugary foods and drinks, and there was a trend toward publication bias showing the association.45
Dental malocclusion. There is consistent evidence for approximately a two-thirds reduction in malocclusions in deciduous teeth in breastfed infants (OR=0.32; 95% CI, 0.25-0.40). Although the large majority of these data come from low-income and middle-income countries, the incidence of malocclusion is not thought to be associated with socioeconomic status, as so many other breastfeeding outcomes are.46
Childhood leukemia
In the largest meta-analysis available, a statistically significant inverse relationship between any breastfeeding for >6 months and childhood leukemia is evident in developed countries (OR=0.84; 95% CI, 0.78-0.91), although significant heterogeneity among studies and lack of control for confounding variables are significant limitations. In particular, an association has been demonstrated with acute lymphoblastic leukemia (ALL) but not with acute myelogenous leukemia.47 Given the rarity of childhood ALL, approximately 12,500 infants would need to be breastfed to prevent one case.48
Continue to: Long-term outcomes
Long-term outcomes
Cognitive development. Several studies conducted in developed countries have linked breastfeeding to positive cognitive outcomes in children, including higher intelligence quotient (IQ).35,49-52
These effects are conflicting, however, in studies that include sibling analysis and ones that control for maternal IQ.8,35,43,52-54 In the 2013 WHO meta-analysis, breastfeeding was associated with an increase of 2.2 points on normalized testing when only high-quality studies were included.51 A 2015 meta-analysis identified 4 high-quality studies with a large sample size and recall time <3 years, which demonstrated a mean difference of 1.76 points in IQ (95% CI, 0.25-3.26) in childhood and adolescence.52 Although statistically significant, this modest increase is of questionable clinical benefit and of unknown duration.
Obesity. The relationship between breastfeeding and obesity later in life is debatable. A large, systematic 2014 review of 15 cohort and 10 cross-sectional studies found a significantly reduced risk of childhood obesity among children who were breastfed (adjusted OR=0.78; 95% CI, 0.74-0.81).55 However, the review included studies that controlled for different confounders, and smaller effects were found in studies in which more confounders were taken into account.
The 2013 WHO meta-analysis found a small (approximately 10%) reduction in the prevalence of overweight or obese children, but cautioned that residual confounding and publication bias were likely.51 At 6.5 and 11.5 years of follow-up, PROBIT failed to demonstrate a protective effect for exclusively or “ever” breastfed infants.56 Sibling analysis similarly fails to demonstrate a statistically significant relationship.8
Continue to: A 2015 meta-analysis of 23 high-quality studies...
A 2015 meta-analysis of 23 high-quality studies with a sample size >1500 children and controlled for important confounders showed a pooled reduction in the prevalence of overweight or obesity of 13% (95% CI, 6-19).57 The protection in this meta-analysis showed a dilution of the effect as the participants aged and an inverse relationship of the effect with sample size.
Breastfeeding is, therefore, unlikely to play a significant, if any, role in combatting the obesity epidemic.
Hypertension. A meta-analysis of high-quality trials demonstrates a <1 mm Hg reduction in systolic blood pressure and no significant difference in diastolic pressure in breastfed infants.57 Similarly, no significant effect of breastfeeding on blood pressure has been demonstrated in trials of preterm infants.51
Type 2 diabetes. Available data are limited and heterogeneous for the association between breastfeeding and later development of type 2 diabetes. Only 2 high-quality trials were identified in the 2013 WHO meta-analysis, and their results conflict.51 A 2015 meta-analysis identified only 3 high-quality studies, without a statistically significant relationship.57
Dyslipidemia. Although earlier data suggested an association between breastfeeding and reduced cholesterol levels later in life, the 2013 WHO meta-analysis and a 2015 meta-analysis concluded that no association exists. The limited data available for preterm infants conflict.51,57
Growth. There is no evidence that feeding method has a short- or long-term effect on weight gain or length gain in preterm or term infants.35,36,58
Death. No clear association has been found between mortality and breastfeeding status in developed countries, except for the association with SIDS.35
Continue to: What issues frame and guide counseling on breastfeeding?
What issues frame and guide counseling on breastfeeding?
There is that “problem” with the evidence. The evidence for infant breastfeeding status and its association with health outcomes faces significant limitations; the great majority of those limitations tend to overestimate the benefits of breastfeeding. Nearly all evidence is based on observational studies, in which causality cannot be determined and self-selection bias, recall bias, and residual confounding limit the value or strength of the findings.
Breastfeeding rates are strongly socially patterned alongside socioeconomic status, race, and education level, all of which are simultaneously strongly tied to short- and long-term health outcomes.6 Other factors limiting the strength of the data set include varying definitions of infant feeding practices in different studies, varying definitions of outcomes and diseases, reverse causation, and evidence of publication bias in many meta-analyses. Given these shortcomings, the NNTs in this article probably represent a best-case scenario for breastfeeding outcomes for infants in the United States (TABLE 118,22-24,28,36,39-43,47,48).
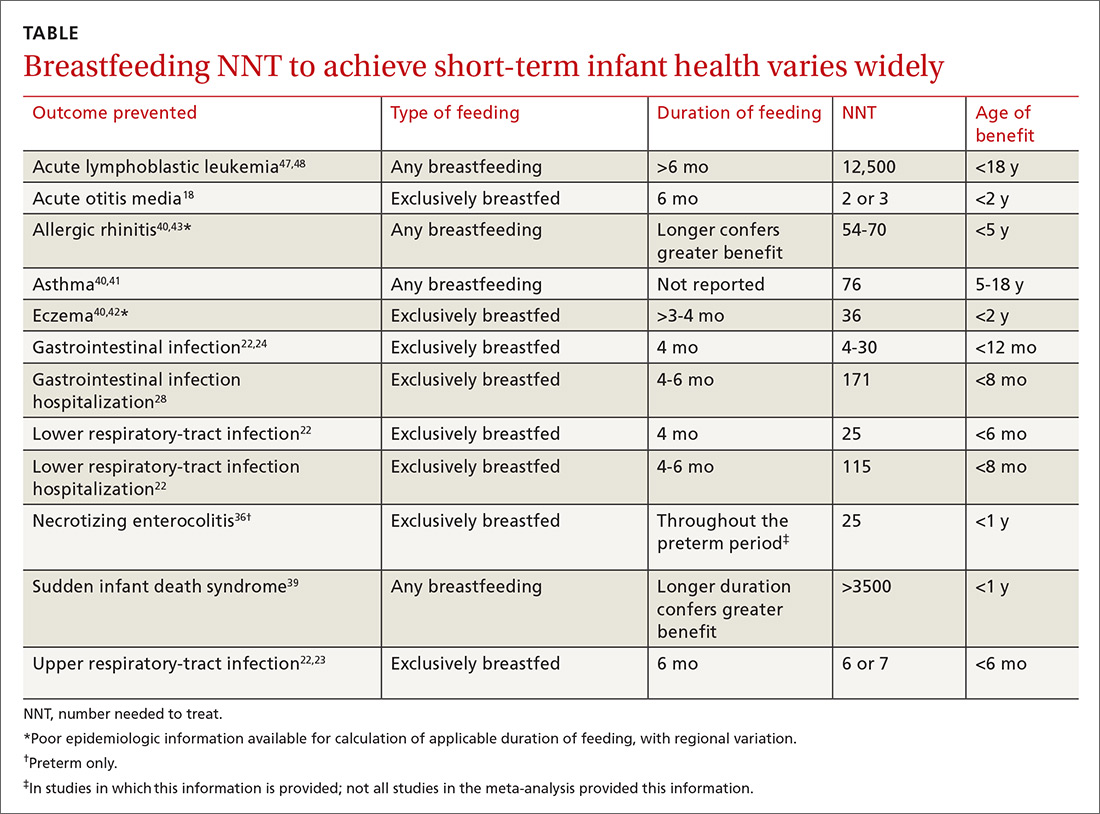
Data need to be put into context. The NNTs for many breastfeeding outcomes (TABLE) compare favorably with other recommended interventions, particularly for other preventive care measures. Two examples: 81 mg/d aspirin for a 50-year-old man has an NNT of 35 to 45 for preventing nonfatal myocardial infarction, and the number needed to invite to screen with mammography to prevent one breast cancer death for a 50-year-old woman is 1339.59,60
In both of these examples, >95% of patients will not benefit from the intervention, yet these preventive measures are routinely recommended and have a significant impact at the public health level. Notably, these outcomes are more serious than most breastfeeding outcomes; have a longer-lasting effect, better-quality data, and better data for potential harms; are causally linked to the intervention; and require much less effort and commitment of time than breastfeeding.
The question must be reckoned with: Can advocacy be harmful?
In recent years, a growing number of concerns have been raised about:
- the potential harms of breastfeeding advocacy
- exaggeration of the benefits of breastfeeding
- promotion of breastfeeding at the expense of evidence-based medicine.
The “Ten Steps to Successful Breastfeeding” program of the Baby-friendly Hospital Initiative (BFHI; launched by UNICEF and WHO) has come under scrutiny because of an increasing number of reports of sudden unexpected postnatal collapse; fall injuries; modeling and encouragement of unsafe sleep practices; an overly rigid resistance to the use of formula supplementation; and the ban on pacifier use.61,62 The BFHI, promoted by the Centers for Disease Control and Prevention, is increasingly being adopted by hospitals with the expressed goal of increasing the breastfeeding rate from birth to discharge.
Continue to: Some of the "Ten Steps"...
Some of the “Ten Steps,” such as the call for skin-to-skin care and 24-hour rooming-in, have well-established benefit yet, when performed without supervision, can have the rare but serious unintended consequences of sudden unexpected postnatal collapse (the incidence of which may be higher than that of SIDS) and unsafe sleeping practices.62,63
Furthermore, despite evidence that early formula supplementation, when medically necessary, does not adversely impact the breastfeeding rate, the “Ten Steps” program advises that giving formula before breast milk comes in might “lead to failure to breastfeed.”33,34,61,63
Similarly, the ban on pacifiers is contrary to available evidence. The use of pacifiers before last sleep is more protective against SIDS than breastfeeding (NNT=2733), and there is evidence at one hospital that BFHI-inspired pacifier restriction is associated with a decrease in the rate of breastfeeding.64,65
Other harms of advocacy are even more poorly studied. Most of the evidence for harm comes from the psychology and social science literature—not the medical literature, perhaps because the prevailing opinion in the medical community is that breastfeeding has overwhelming evidence for benefit. In fact, in the USPSTF’s 2008 recommendation, the evidence review of breastfeeding promotion practices in primary care did not identify a single study that measured harm; in the 2016 update of that recommendation, only 2 such studies were identified.15,66
The literature that does investigate harm consistently finds that women who have difficulty breastfeeding or choose formula feeding report feelings of inadequacy, guilt, loss of agency, anxiety, and physical pain during breastfeeding that interferes with 1) their ability to bond or otherwise care for their infant and 2) competing work obligations.11-13,67-69 Given the lack of attention paid to these variables in the medical literature, it is the individual mother who is best positioned to weigh these factors against the benefits of breastfeeding.
Continue to: Shared decision-making is best—for mother and baby
Shared decision-making is best—for mother and baby
Breastfeeding might prevent certain infections in as many as 50% of infants, but a mother unable to breastfeed can take solace in the fact that >95% of breastfed infants will not realize any benefit from the preventive potential of breastfeeding in regard to hospitalization or allergic disease, and >99% will not realize benefit from either the prevention of SIDS or ALL, or from improvement in long-term health measures (except for, perhaps, a slightly higher IQ). The “breast is best” mantra is likely true at a public-health level; for the individual mother–infant dyad, however, where there is a need to balance personal, social, family, and financial factors, that mantra is an oversimplification.
Regrettably, there is a paucity of data on the risks of breastfeeding promotion—an area that deserves more study. Balancing the abundant, but often limited-quality, data on the benefits of breastfeeding and the sheer lack of data regarding the risks of advocacy represents a clinical and an ethical challenge for physicians. It is a challenge that can only be resolved through individualization of care and shared decision-making, in which the physician is expert on the benefits of breastfeeding, and the mother is expert on the personal circumstances to be weighed against those benefits.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization, and New York, NY: UNICEF; 2003. Available at: www.who.int/maternal_child_adolescent/documents/9241562218/en/. Accessed April 4, 2018.
2. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Breastfeeding Expert Work Group. Committee Opinion No. 658: Optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2016;127:e86-e92.
3. Gartner LM, Morton J, Lawrence RA, et al; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
4. Breastfeeding (policy statement). Leawood, KS: American Academy of Family Physicians; 2007. Available at: https://www.aafp.org/about/policies/all/breastfeeding.html. Accessed April 3, 2018.
5. Office of the Surgeon General (US); Centers for Disease Control and Prevention (US); Office on Women’s Health (US). The Surgeon General’s call to action to support breastfeeding. Rockville, MD: US Department of Health and Human Services; 2011. Available at: www.surgeongeneral.gov/library/calls/breastfeeding/index.html. Updated August 12, 2014. Accessed April 4, 2018.
6. Breastfeeding: data & statistics. Atlanta, GA: Centers for Disease Control and Prevention; December 11, 2017. Available at: http://www.cdc.gov/breastfeeding/data/. Accessed May 17, 2018.
7. Fewtrell M, Wilson DC, Booth I, et al. A. Six months of exclusive breast feeding: how good is the evidence? BMJ. 2010;342:c5955.
8. Colen CG, Ramey DM. Is breast truly best? Estimating the effect of breastfeeding on long-term child wellbeing in the United States using sibling comparisons. Soc Sci Med. 2014;109:55-65.
9. Wolf J. Is Breast Best? Taking on the Breastfeeding Experts and the New High Stakes of Motherhood. New York, NY: NYU Press; 2010.
10. Tuteur A. Push Back: Guilt in the Age of Natural Parenting. New York, NY: HarperCollins Publishers; 2016.
11. Lee E. Health, morality, and infant feeding: British mothers’ experiences of formula milk use in the early weeks. Sociol Health Illn. 2007;29:1075-1090.
12. Williams K, Donaghue N, Kurz T. “Giving guilt the flick”?: an investigation of mothers’ talk about guilt in relation to infant feeding. Psychol Women Q. 2013;37:97-112.
13. Fahlquist JN, Roeser S. Ethical problems with information on infant feeding in developed countries. J Health Polit Policy Law. 2012;37:155-160.
14. U.S. Preventive Services Task Force. Final Recommendation Statement. Breastfeeding: Counseling. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breastfeeding-counseling. Accessed April 4, 2018.
15. US Preventive Services Task Force. Primary Care Interventions to Support Breastfeeding: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1688-1693.
16. Zahn CM, Hanley LE. Concerns over USPSTF draft recommendation on breastfeeding interventions [letter]. Washington, DC: The American College of Obstetricians and Gynecologists; May 18, 2016. Available at: https://www.acog.org/-/media/Departments/Breastfeeding/Breast-Feeding-ACOG-USPSTF.pdf?dmc=1&ts=20180518T1850056558. Accessed May 22, 2018.
17. Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222-231.
18. Bowatte G, Tham R, Allen KJ, et al. Breastfeeding and childhood acute otitis media: a systematic review and meta-analysis. Acta Paediatr. 2015;104:85-95.
19. Chonmaitree T, Trujillo R, Jennings K, et al. Acute otitis media and other complications of viral respiratory infection. Pediatrics. 2016;137:e20153555.
20. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
21. Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118:865-873.
22. Duijts L, Jaddoe VW, Hofman A, et al. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126:e18-e25.
23. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186:190-199.
24. Kramer MS, Chalmers B, Hodnett ED, et al; PROBIT Study Group (Promotion of Breastfeeding Intervention Trial). Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413-420.
25. Dennehy PH. Acute diarrheal disease in children: epidemiology, prevention, and treatment. Infect Dis Clin North Am. 2005;19:585-602.
26. Cortese MM, Tate JE, Simonsen L, et al. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatric Infect Dis J. 2010;29:489-494.
27. Ajetunmobi OM, Whyte B, Chalmers J, et al. Breastfeeding is associated with reduced childhood hospitalization: evidence from a Scottish birth cohort (1997-2009). J Pediatr. 2015;166:620-625.
28. Quigley MA, Kelly YJ, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007;119:e837-e842.
29. Radtke JV. The paradox of breastfeeding-associated morbidity among late preterm infants. J Obstet Gynecol Neonatal Nurs. 2011;40:9-24.
30. Escobar GJ, Gonzales VM, Armstrong M, et al. Rehospitalization for neonatal dehydration: a nested case-control study. Arch Pediatr Adolesc Med. 2002;156:155-161.
31. Salas AA, Salazar J, Burgoa CV, et al. Significant weight loss in breastfed term infants readmitted for hyperbilirubinemia. BMC Pediatr. 2009;9:82.
32. Tarcan A, Tiker F, Vatandaş NS, et al. Weight loss and hypernatremia in breast-fed babies: frequency in neonates with non-hemolytic jaundice. J Paediatr Child Health. 2005;41:484-487.
33. Flaherman VJ, Aby J, Burgos AE, et al. Effect of early limited formula on duration and exclusivity of breastfeeding in at-risk infants: an RCT. Pediatrics. 2013;131:1059-1065.
34. Straňák Z, Feyereislova S, Černá M, et al. J. Limited amount of formula may facilitate breastfeeding: randomized, controlled trial to compare standard clinical practice versus limited supplemental feeding. Denning PW, ed. PLoS One. 2016;11:e0150053.
35. Ip S, Chung M, Raman G, et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Rockville, MD: Agency for Healthcare Research and Quality (US); 2007. Evidence Reports/Technology Assessments, No. 153. Available at: www.ncbi.nlm.nih.gov/books/NBK38337/. Accessed April 3, 2018.
36. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;(4):CD002971.
37. Hauck FR, Thompson JM, Tanabe KO, et al. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128:103-110.
38. American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245-1255.
39. Moon RY, Fu L. Sudden infant death syndrome: an update. Pediatr Rev. 2012;33:314-320.
40. Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38-53.
41. Brim SN, Rudd RA, Funk RH, et al. Asthma prevalence among US children in underrepresented minority populations: American Indian/Alaska Native, Chinese, Filipino, and Asian Indian. Pediatrics. 2008;122:e217-e222.
42. Shaw TF, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
43. Mallol J, Crane J, von Mutius E, et al. The international study of asthma and allergies in childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73-85.
44. Flohr C, Nagel G, Weinmayr G, et al. Lack of evidence for a protective effect of prolonged breastfeeding on childhood eczema: lessons from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br J Dermatol. 2011;165:1280-1289.
45. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
46. Peres KG, Cascaes AM, Nascimento GG, et al. Effect of breastfeeding on malocclusions: a systematic review and meta-analysis. Acta Paediatr. 2015;104:54-61.
47. Amitya EL, Keinan-Boker L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 2015;169:e151025.
48. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943-1955.
49. Guxens M, Mendez MA, Moltó-Puigmartí C, et al. Breastfeeding, long-chain polyunsaturated fatty acids in colostrum, and infant mental development.
2012;129:1134-1140.
51. Horta BL, Victora CG. Long-term effects of breastfeeding: a systematic review. Geneva, Switzerland: World Health Organization; 2013. Available at: http://apps.who.int/iris/bitstream/10665/79198/1/9789241505307_eng.pdf. Accessed August 16, 2016.
52. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104:14-19.
53. Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333:945.
54. Sajjad A, Tharner A, Kiefte-de Jong JC, et al. Breastfeeding duration and non-verbal IQ in children. J Epidemiol Community Health 2015;69:775-781.
55. Yan J, Liu L, Zhu Y, et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267.
56. Martin RM, Patel R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
57. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure, and type 2 diabetes: systematic review and meta-analysis. Acta Paediatr. 2015;104:30-37.
58. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Syst Rev. 2012;15:CD003517.
59. U.S. Preventive Services Task Force. Final recommendation statement: aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Published September 2017. Accessed April 3, 2018.
60. U.S. Preventive Services Task Force. Screening for breast cancer. Available at: www.uspreventiveservicestaskforce.org/Page/SupportingDoc/breast-cancer-screening/final-evidence-summary9. Published November 2009. Accessed April 2, 2018.
61. Bass JL, Gartley T, Kleinman R. Unintended consequences of current breastfeeding initiatives. JAMA Pediatr. 2016;170:923-924.
62. Feldman-Winter L, Goldsmith JP; Committee on Fetus and Newborn; Task Force on Sudden Infant Death Syndrome. Safe sleep and skin-to-skin care in the neonatal period for healthy term newborns. Pediatrics. 2016;138:e20161889.
63. The Mother and Child Health and Education Trust. Ten steps to successful breastfeeding. Available at: www.tensteps.org. Published November 8, 2017. Accessed April 3, 2018.
64. Hauck FR, Omojokun OO, Siadaty MS. Do pacifiers reduce the risk of sudden infant death syndrome? A meta-analysis. Pediatrics. 2005;116:e716-e723.
65. Kair LR, Kenron D, Etheredge K, et al. Pacifier restriction and exclusive breastfeeding. Pediatrics. 2013;131:e1101-e1107.
66. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
67. Wolf JB. Is breast really best? Risk and total motherhood in the National Breastfeeding Awareness Campaign. J Health Polit Policy Law. 2007;32:595-636.
68. Marshall JL, Godfrey M, Renfrew MJ. Being a ‘good mother’: managing breastfeeding and merging identities. Soc Sci Med. 2007;65:2147-2159.
69. Kelleher CM. The physical challenges of early breastfeeding. Soc Sci Med. 2006;63:2727-2738.
The benefits of breastfeeding for infants have long been touted as numerous and supported by overwhelming evidence. The World Health Organization (WHO), American College of Obstetricians and Gynecologists, American Academy of Pediatrics (AAP), and American Academy of Family Physicians all strongly recommend exclusive breastfeeding for the first 6 months of life, citing numerous health benefits for child and mother. These groups recommend that some breastfeeding be continued through the first 12 months of life, or longer, as desired (the WHO extends the recommendation to 2 years).1-4 In 2000, the Surgeon General of the United States released a strategic plan to increase rates of breastfeeding,5 setting goals (by 2010) of:
- 75% of mothers leaving the hospital breastfeeding
- 50% of babies breastfeeding at 6 months
- 25% of babies breastfeeding at 1 year.
Massive public health campaigns citing data for the many benefits of breastfeeding have been launched with the goal of increasing the breastfeeding rate. In 2014, statistics offered a testament to the success of these campaigns6:
- 82.5% of infants had been breastfed “ever”
- 55.3% were breastfed “some”
- 24.9% were breastfed exclusively through 6 months of age
- 33.7% were breastfed “some” at 12 months.
Breastfeeding advocacy has become clouded
In recent years, an increasing number of researchers, physicians, and authors have begun to question whether, in the United States, the benefits of breastfeeding children are exaggerated and the emphasis on breastfeeding might be leading to feelings of inadequacy, guilt, and anxiety among mothers.7-13 In 2016, the US Preventive Services Task Force (USPSTF) amended its recommendation to “promote and support breastfeeding” to simply “support breastfeeding”—a change that created substantial debate and prompted the Task Force to clarify its stance in changing the language: In its response to public comment, the USPSTF said that its position regarding promotion had not changed, but the language in the original statement had been revised to “ensure that the autonomy of women is respected.” 2,14-16
In contrast, others suggest counseling women on the risks of formula feeding rather than on the benefits of breastfeeding, citing substantial health outcome distinctions.17 Indeed, wide-ranging conclusions have been drawn from the same data on the topic, potentially creating uncertainty for physicians on how best to counsel women on their choice of how to feed their infant.
In this article, we address this uncertainty by utilizing the most recent and comprehensive data to examine infant health outcomes. When possible, the number needed to treat (NNT) for a given outcome has been calculated or approximated, allowing the reader to estimate the likelihood of benefit for an individual mother–infant dyad. Exercise caution when interpreting the NNT, however: The numbers suggest causality that cannot be definitively established using the observational data on which those numbers are based.
Continue to: Infectious disease
Infectious disease
Acute otitis media. Exclusive breastfeeding for 6 months is associated with a 43% reduction in the risk of acute otitis media (AOM) by 2 years of age (odds ratio [OR]=0.57; 95% confidence interval [CI], 0.44-0.75). Beyond 2 years of age, or when comparing “ever” and “never” breastfeeding, the effect disappears. All studies in this meta-analysis had serious limitations.18
Nearly half of children will have at least one case of AOM by one year of age; 80%, by 2 years.19,20 Since the introduction of the heptavalent pneumococcal conjugate vaccine, the rate of AOM at 2 years has fallen by as much as 20%.21 Assuming an incidence of 60% to 80% of AOM by 2 years, only 2 or 3 infants need to be exclusively breastfed for 6 months to prevent a single case of AOM.18 Prevention of AOM through breastfeeding may be related to head position during feeding, antibacterial effects of breast milk, protective oral microbiome in the breastfed infant pharynx, and/or prevention of primary viral upper respiratory infection (URI), which nearly always precedes AOM.18,19
Upper and lower respiratory tract infections. Infants who are exclusively breastfed for 4 months and partially breastfed after 4 months have a lower risk of URI (OR=0.65; 95% CI, 0.51-0.83) and of lower respiratory tract infection (LRTI; OR=0.50; 95% CI, 0.32-0.72).22
The effect is stronger for URI among infants exclusively breastfed for at least 6 months (OR=0.37; 95% CI, 0.18-0.74), but is no longer significant by that time for LRTI (OR=0.33; 95% CI, 0.08-1.40). Importantly, AOM was included in the URI group, and, as previously discussed, AOM has independently been shown to have an inverse relationship with breastfeeding duration.
At 7 to 12 months of age, no association was seen between breastfeeding and the incidence of URI. Curiously, an association with LRTI was again detected for infants breastfed exclusively for 4 months and partially thereafter, but was not detected with exclusive breastfeeding for at least 6 months (OR=0.46; 95% CI, 0.31-0.69). In this study, in the first 6 months of life, 40% of infants had a URI and 8% had an LRTI. The findings in this cohort suggest an NNT of 6 or 7 for prevention of URI and an NNT of 25 for prevention of LRTI in the first 6 months of life.22
Continue to: Children younger than 2 years are...
Children younger than 2 years are estimated to have approximately 6 bouts of the common cold a year, and essentially 100% have at least one bout—perhaps lowering the NNT for URI if applied widely. However, these data are not divided into 6-month intervals, making accurate extrapolation difficult.23
Gastrointestinal infection. The rate of diarrheal illness in the first year of life is lower in infants who are exclusively breastfed for at least 4 months and partially breastfed after.
Both the Promotion of Breastfeeding Intervention Trial (PROBIT; a clinical trial in which infants were randomized to a breastfeeding education intervention or standard care) and a 2010 prospective cohort study in the Netherlands of more than 3400 infants found a reduction in the risk of one or more gastrointestinal (GI) infections at a similar rate.22,24
- In PROBIT, 9.1% of infants in the intervention group, compared to 13.2% in the standard care group (OR=0.60; 95% CI, 0.40-0.91), had one or more GI infections at 12 months of age.24
- In the 2010 Netherlands cohort, 8% of infants had a GI infection by 6 months of age. Infants breastfed exclusively for at least 4 or 6 months had a decreased risk for GI infection (respectively: adjusted OR=0.41; 95% CI, 0.26-0.64 and adjusted OR=0.46; 95% CI, 0.14-1.59). No such association was found for any feeding group 7 to 12 months of age.22
These studies are notable for the low incidence of GI infection, which is frequently cited as 1.3 to 2.3 episodes per child per year in children younger than 3 years in the United States.25 However, that high incidence has likely declined significantly since the introduction of rotavirus vaccine in 2006. In the years following the introduction of the vaccine, infant visits for gastroenteritis decreased by >90% in all care settings in the South, Northeast, and Midwest regions of the United States and by 53% to 63% in the West region.26 Recent accurate epidemiologic information, in an era of significantly higher vaccination rates, is lacking.
Assuming the low incidence of GI infection reported in PROBIT and the Netherlands trials, about 25 to 30 infants need to be exclusively breastfed for 4 to 6 months to prevent a single GI infection during the first 6 to 12 months of life.22,24 Assuming a 60% incidence by age 12 months before introduction of the rotavirus vaccine, the NNT would be approximately 4.24 The true number is likely somewhere between those 2 NNTs.
Continue to: Hospitalization
Hospitalization
Risk of infection is decreased. A large cohort study in Scotland, involving more than 500,000 children, found an association between exclusive breastfeeding for 6 to 8 weeks and decreased risk of hospitalization within the first 6 months of life. Formula-fed and mixed-fed infants had an increased hazard ratio (HR) for hospitalization for common childhood illness (HR=1.40; 95% CI, 1.35-1.45 for formula-fed infants and HR=1.18; 95% CI, 1.11-1.25 for mixed-fed infants).27 The study also found increased rates of hospitalization for conditions for which other meta-analyses have failed to show a protective effect from breastfeeding—leading to suspicion of residual confounding in the study. Another United Kingdom cohort demonstrated lower rates of hospitalization for GI infection (NNT=171) and LRTI (NNT=115) among exclusively breastfed infants by 8 months of age.28
Risk of neonatal readmission is increased. Late preterm infants who are exclusively breastfed are nearly twice as likely to be hospitalized as breastfed term or non-breastfed preterm infants, primarily due to dehydration, failure to thrive, weight loss, and hyperbilirubinemia. In fact, exclusive breastfeeding at discharge from the hospital is likely the single greatest risk factor for hospital readmission in newborns.29,30 Term infants who are exclusively breastfed are more likely to be hospitalized compared to formula-fed or mixed-fed infants, due to hyperbilirubinemia, dehydration, hypernatremia, and weight loss (number needed to harm (NNH)=71).30-32 For weight loss >10% of birth weight with or without hospitalization, the NNH for breastfed infants is 13.32
Many of these hospitalizations and events could be avoided with appropriate monitoring and medically indicated supplementation; the likelihood of long-term harm is low. Formula supplementation is often avoided if possible in hospitals to promote exclusive breastfeeding; however, several small randomized clinical trials have demonstrated that limited formula supplementation in breastfed infants does not affect the breastfeeding continuation rate at 3 and 6 months, and, therefore, might be a way to decrease infant rehospitalization.33,34
Necrotizing enterocolitis
In preterm infants, breastfeeding has been associated with a lower rate of necrotizing enterocolitis. In the 2007 Agency for Healthcare Research and Quality report, the association was found to be only marginally statistically significant, and the authors warned that, first, evidence is old and heterogeneous and, second, present preterm formula is much different than the formula used in earlier studies of preterm infant nutrition and necrotizing enterocolitis.35 A 2012 Cochrane review included newer studies in its analysis but reached the same conclusion on the quality and heterogeneity of available evidence, with a NNT of 25.36
Continue to: Sudden infant death syndrome
Sudden infant death syndrome
There is a statistically significant association between sudden infant death syndrome (SIDS) and feeding method. Infants whose cause of death is SIDS are approximately one half as likely to have been breastfed as matched controls.35,37
In 2005, AAP did not recommend breastfeeding as a means to reduce the risk of SIDS because available evidence was mixed, and studies at the time were poorly controlled.38 Since that time, case-control meta-analyses have shed additional light on the association between SIDS and feeding method.35,37
The protective effect exists for any amount of breastfeeding and is stronger for exclusive breastfeeding, suggesting a protective role—not simply an association. Caution should be employed with this conclusion, however, because the studies included in the meta-analysis used univariate analysis primarily and did not control sufficiently for known confounders. In addition, the authors warn that publication bias might overestimate the association.38
Potential mechanisms of a protective role include decreased risk of infection and greater arousability from sleep in breastfed infants. Assuming a protective role, available data suggest that more than 3500 infants need to be breastfed to prevent one case of SIDS.39
Continue to: Allergic disease
Allergic disease
Asthma. There is evidence of a small protective effect of breastfeeding “ever” on asthma at 5 to 18 years of age in high-income countries (OR=0.90; 95% CI, 0.83-0.97). A family history of asthma or atopy did not affect this finding. The authors note there is some evidence of publication bias in this review, which is the largest and most comprehensive on the topic.40
With a lifetime prevalence of asthma in the United States of approximately 13.2%, this association would confer an NNT of roughly 76.41 Earlier, the literature demonstrated mixed and conflicting evidence, and some experts suggested an effect only when there is a family history of asthma or atopy.36
Eczema. For children younger than 2 years, there is low-grade- and very-low-grade-quality evidence that exclusive breastfeeding longer than 3 to 4 months is associated with a reduced risk of eczema (OR=0.74; 95% CI, 0.57-0.97).40
Previously, data suggested that this association existed only in children who had a family history of atopy.35 The protective association, however, exists regardless of family history and does not persist beyond 2 years of age. The authors noted evidence of publication bias, reverse causation, and misdiagnosis of early childhood rashes as eczema as limitations of their findings.40
Continue to: Reliable epidemiologic evidence...
Reliable epidemiologic evidence on the incidence of eczema in infants in the United States is limited, but the prevalence in the United States in children younger than 17 years is approximately 10.7% (with wide regional variation). Extrapolating these data generously, the NNT to prevent eczema in the first 2 years of life could be estimated at approximately 36.42
Allergic rhinitis. There is low-grade- and very-low-grade-quality evidence that more breastfeeding, compared to less breastfeeding, is associated with a lower risk of allergic rhinitis in children younger than 5 years (OR=0.79; 95% CI, 0.63-0.98). The association exists regardless of family history and disappears after 5 years of age. The differentiation of allergic rhinitis from rhinovirus infection (for which there is higher-quality evidence of a protective effect with breastfeeding) must be considered when interpreting these data.40
Reliable epidemiologic evidence on allergic rhinitis in children younger than 5 years is lacking, and incidence varies by region. A rough estimate, using data from 6- and 7-year-olds, indicates an NNT of 54 to 70.43
Food allergy. There is no evidence to suggest an association between breastfeeding and food allergy, either as protective or as a risk factor, and studies are limited.40 Interestingly, as data accumulate associating early exposure to foods with protection, some authors have proposed reexamining the recommendation from WHO and US health organizations for exclusive breastfeeding for the first 6 months of life.7,44
Continue to: Dental health
Dental health
Dental caries. There is consistent evidence that breastfeeding beyond 12 months of age is associated with the development of dental caries of deciduous teeth to 6 years of age (OR=2.90; 95% CI, 2.33-3.60). Many of the studies that showed this association did not control for the introduction of sugary foods and drinks, and there was a trend toward publication bias showing the association.45
Dental malocclusion. There is consistent evidence for approximately a two-thirds reduction in malocclusions in deciduous teeth in breastfed infants (OR=0.32; 95% CI, 0.25-0.40). Although the large majority of these data come from low-income and middle-income countries, the incidence of malocclusion is not thought to be associated with socioeconomic status, as so many other breastfeeding outcomes are.46
Childhood leukemia
In the largest meta-analysis available, a statistically significant inverse relationship between any breastfeeding for >6 months and childhood leukemia is evident in developed countries (OR=0.84; 95% CI, 0.78-0.91), although significant heterogeneity among studies and lack of control for confounding variables are significant limitations. In particular, an association has been demonstrated with acute lymphoblastic leukemia (ALL) but not with acute myelogenous leukemia.47 Given the rarity of childhood ALL, approximately 12,500 infants would need to be breastfed to prevent one case.48
Continue to: Long-term outcomes
Long-term outcomes
Cognitive development. Several studies conducted in developed countries have linked breastfeeding to positive cognitive outcomes in children, including higher intelligence quotient (IQ).35,49-52
These effects are conflicting, however, in studies that include sibling analysis and ones that control for maternal IQ.8,35,43,52-54 In the 2013 WHO meta-analysis, breastfeeding was associated with an increase of 2.2 points on normalized testing when only high-quality studies were included.51 A 2015 meta-analysis identified 4 high-quality studies with a large sample size and recall time <3 years, which demonstrated a mean difference of 1.76 points in IQ (95% CI, 0.25-3.26) in childhood and adolescence.52 Although statistically significant, this modest increase is of questionable clinical benefit and of unknown duration.
Obesity. The relationship between breastfeeding and obesity later in life is debatable. A large, systematic 2014 review of 15 cohort and 10 cross-sectional studies found a significantly reduced risk of childhood obesity among children who were breastfed (adjusted OR=0.78; 95% CI, 0.74-0.81).55 However, the review included studies that controlled for different confounders, and smaller effects were found in studies in which more confounders were taken into account.
The 2013 WHO meta-analysis found a small (approximately 10%) reduction in the prevalence of overweight or obese children, but cautioned that residual confounding and publication bias were likely.51 At 6.5 and 11.5 years of follow-up, PROBIT failed to demonstrate a protective effect for exclusively or “ever” breastfed infants.56 Sibling analysis similarly fails to demonstrate a statistically significant relationship.8
Continue to: A 2015 meta-analysis of 23 high-quality studies...
A 2015 meta-analysis of 23 high-quality studies with a sample size >1500 children and controlled for important confounders showed a pooled reduction in the prevalence of overweight or obesity of 13% (95% CI, 6-19).57 The protection in this meta-analysis showed a dilution of the effect as the participants aged and an inverse relationship of the effect with sample size.
Breastfeeding is, therefore, unlikely to play a significant, if any, role in combatting the obesity epidemic.
Hypertension. A meta-analysis of high-quality trials demonstrates a <1 mm Hg reduction in systolic blood pressure and no significant difference in diastolic pressure in breastfed infants.57 Similarly, no significant effect of breastfeeding on blood pressure has been demonstrated in trials of preterm infants.51
Type 2 diabetes. Available data are limited and heterogeneous for the association between breastfeeding and later development of type 2 diabetes. Only 2 high-quality trials were identified in the 2013 WHO meta-analysis, and their results conflict.51 A 2015 meta-analysis identified only 3 high-quality studies, without a statistically significant relationship.57
Dyslipidemia. Although earlier data suggested an association between breastfeeding and reduced cholesterol levels later in life, the 2013 WHO meta-analysis and a 2015 meta-analysis concluded that no association exists. The limited data available for preterm infants conflict.51,57
Growth. There is no evidence that feeding method has a short- or long-term effect on weight gain or length gain in preterm or term infants.35,36,58
Death. No clear association has been found between mortality and breastfeeding status in developed countries, except for the association with SIDS.35
Continue to: What issues frame and guide counseling on breastfeeding?
What issues frame and guide counseling on breastfeeding?
There is that “problem” with the evidence. The evidence for infant breastfeeding status and its association with health outcomes faces significant limitations; the great majority of those limitations tend to overestimate the benefits of breastfeeding. Nearly all evidence is based on observational studies, in which causality cannot be determined and self-selection bias, recall bias, and residual confounding limit the value or strength of the findings.
Breastfeeding rates are strongly socially patterned alongside socioeconomic status, race, and education level, all of which are simultaneously strongly tied to short- and long-term health outcomes.6 Other factors limiting the strength of the data set include varying definitions of infant feeding practices in different studies, varying definitions of outcomes and diseases, reverse causation, and evidence of publication bias in many meta-analyses. Given these shortcomings, the NNTs in this article probably represent a best-case scenario for breastfeeding outcomes for infants in the United States (TABLE 118,22-24,28,36,39-43,47,48).

Data need to be put into context. The NNTs for many breastfeeding outcomes (TABLE) compare favorably with other recommended interventions, particularly for other preventive care measures. Two examples: 81 mg/d aspirin for a 50-year-old man has an NNT of 35 to 45 for preventing nonfatal myocardial infarction, and the number needed to invite to screen with mammography to prevent one breast cancer death for a 50-year-old woman is 1339.59,60
In both of these examples, >95% of patients will not benefit from the intervention, yet these preventive measures are routinely recommended and have a significant impact at the public health level. Notably, these outcomes are more serious than most breastfeeding outcomes; have a longer-lasting effect, better-quality data, and better data for potential harms; are causally linked to the intervention; and require much less effort and commitment of time than breastfeeding.
The question must be reckoned with: Can advocacy be harmful?
In recent years, a growing number of concerns have been raised about:
- the potential harms of breastfeeding advocacy
- exaggeration of the benefits of breastfeeding
- promotion of breastfeeding at the expense of evidence-based medicine.
The “Ten Steps to Successful Breastfeeding” program of the Baby-friendly Hospital Initiative (BFHI; launched by UNICEF and WHO) has come under scrutiny because of an increasing number of reports of sudden unexpected postnatal collapse; fall injuries; modeling and encouragement of unsafe sleep practices; an overly rigid resistance to the use of formula supplementation; and the ban on pacifier use.61,62 The BFHI, promoted by the Centers for Disease Control and Prevention, is increasingly being adopted by hospitals with the expressed goal of increasing the breastfeeding rate from birth to discharge.
Continue to: Some of the "Ten Steps"...
Some of the “Ten Steps,” such as the call for skin-to-skin care and 24-hour rooming-in, have well-established benefit yet, when performed without supervision, can have the rare but serious unintended consequences of sudden unexpected postnatal collapse (the incidence of which may be higher than that of SIDS) and unsafe sleeping practices.62,63
Furthermore, despite evidence that early formula supplementation, when medically necessary, does not adversely impact the breastfeeding rate, the “Ten Steps” program advises that giving formula before breast milk comes in might “lead to failure to breastfeed.”33,34,61,63
Similarly, the ban on pacifiers is contrary to available evidence. The use of pacifiers before last sleep is more protective against SIDS than breastfeeding (NNT=2733), and there is evidence at one hospital that BFHI-inspired pacifier restriction is associated with a decrease in the rate of breastfeeding.64,65
Other harms of advocacy are even more poorly studied. Most of the evidence for harm comes from the psychology and social science literature—not the medical literature, perhaps because the prevailing opinion in the medical community is that breastfeeding has overwhelming evidence for benefit. In fact, in the USPSTF’s 2008 recommendation, the evidence review of breastfeeding promotion practices in primary care did not identify a single study that measured harm; in the 2016 update of that recommendation, only 2 such studies were identified.15,66
The literature that does investigate harm consistently finds that women who have difficulty breastfeeding or choose formula feeding report feelings of inadequacy, guilt, loss of agency, anxiety, and physical pain during breastfeeding that interferes with 1) their ability to bond or otherwise care for their infant and 2) competing work obligations.11-13,67-69 Given the lack of attention paid to these variables in the medical literature, it is the individual mother who is best positioned to weigh these factors against the benefits of breastfeeding.
Continue to: Shared decision-making is best—for mother and baby
Shared decision-making is best—for mother and baby
Breastfeeding might prevent certain infections in as many as 50% of infants, but a mother unable to breastfeed can take solace in the fact that >95% of breastfed infants will not realize any benefit from the preventive potential of breastfeeding in regard to hospitalization or allergic disease, and >99% will not realize benefit from either the prevention of SIDS or ALL, or from improvement in long-term health measures (except for, perhaps, a slightly higher IQ). The “breast is best” mantra is likely true at a public-health level; for the individual mother–infant dyad, however, where there is a need to balance personal, social, family, and financial factors, that mantra is an oversimplification.
Regrettably, there is a paucity of data on the risks of breastfeeding promotion—an area that deserves more study. Balancing the abundant, but often limited-quality, data on the benefits of breastfeeding and the sheer lack of data regarding the risks of advocacy represents a clinical and an ethical challenge for physicians. It is a challenge that can only be resolved through individualization of care and shared decision-making, in which the physician is expert on the benefits of breastfeeding, and the mother is expert on the personal circumstances to be weighed against those benefits.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine, 101 Heart Drive, Greenville, NC 27834; [email protected].
The benefits of breastfeeding for infants have long been touted as numerous and supported by overwhelming evidence. The World Health Organization (WHO), American College of Obstetricians and Gynecologists, American Academy of Pediatrics (AAP), and American Academy of Family Physicians all strongly recommend exclusive breastfeeding for the first 6 months of life, citing numerous health benefits for child and mother. These groups recommend that some breastfeeding be continued through the first 12 months of life, or longer, as desired (the WHO extends the recommendation to 2 years).1-4 In 2000, the Surgeon General of the United States released a strategic plan to increase rates of breastfeeding,5 setting goals (by 2010) of:
- 75% of mothers leaving the hospital breastfeeding
- 50% of babies breastfeeding at 6 months
- 25% of babies breastfeeding at 1 year.
Massive public health campaigns citing data for the many benefits of breastfeeding have been launched with the goal of increasing the breastfeeding rate. In 2014, statistics offered a testament to the success of these campaigns6:
- 82.5% of infants had been breastfed “ever”
- 55.3% were breastfed “some”
- 24.9% were breastfed exclusively through 6 months of age
- 33.7% were breastfed “some” at 12 months.
Breastfeeding advocacy has become clouded
In recent years, an increasing number of researchers, physicians, and authors have begun to question whether, in the United States, the benefits of breastfeeding children are exaggerated and the emphasis on breastfeeding might be leading to feelings of inadequacy, guilt, and anxiety among mothers.7-13 In 2016, the US Preventive Services Task Force (USPSTF) amended its recommendation to “promote and support breastfeeding” to simply “support breastfeeding”—a change that created substantial debate and prompted the Task Force to clarify its stance in changing the language: In its response to public comment, the USPSTF said that its position regarding promotion had not changed, but the language in the original statement had been revised to “ensure that the autonomy of women is respected.” 2,14-16
In contrast, others suggest counseling women on the risks of formula feeding rather than on the benefits of breastfeeding, citing substantial health outcome distinctions.17 Indeed, wide-ranging conclusions have been drawn from the same data on the topic, potentially creating uncertainty for physicians on how best to counsel women on their choice of how to feed their infant.
In this article, we address this uncertainty by utilizing the most recent and comprehensive data to examine infant health outcomes. When possible, the number needed to treat (NNT) for a given outcome has been calculated or approximated, allowing the reader to estimate the likelihood of benefit for an individual mother–infant dyad. Exercise caution when interpreting the NNT, however: The numbers suggest causality that cannot be definitively established using the observational data on which those numbers are based.
Continue to: Infectious disease
Infectious disease
Acute otitis media. Exclusive breastfeeding for 6 months is associated with a 43% reduction in the risk of acute otitis media (AOM) by 2 years of age (odds ratio [OR]=0.57; 95% confidence interval [CI], 0.44-0.75). Beyond 2 years of age, or when comparing “ever” and “never” breastfeeding, the effect disappears. All studies in this meta-analysis had serious limitations.18
Nearly half of children will have at least one case of AOM by one year of age; 80%, by 2 years.19,20 Since the introduction of the heptavalent pneumococcal conjugate vaccine, the rate of AOM at 2 years has fallen by as much as 20%.21 Assuming an incidence of 60% to 80% of AOM by 2 years, only 2 or 3 infants need to be exclusively breastfed for 6 months to prevent a single case of AOM.18 Prevention of AOM through breastfeeding may be related to head position during feeding, antibacterial effects of breast milk, protective oral microbiome in the breastfed infant pharynx, and/or prevention of primary viral upper respiratory infection (URI), which nearly always precedes AOM.18,19
Upper and lower respiratory tract infections. Infants who are exclusively breastfed for 4 months and partially breastfed after 4 months have a lower risk of URI (OR=0.65; 95% CI, 0.51-0.83) and of lower respiratory tract infection (LRTI; OR=0.50; 95% CI, 0.32-0.72).22
The effect is stronger for URI among infants exclusively breastfed for at least 6 months (OR=0.37; 95% CI, 0.18-0.74), but is no longer significant by that time for LRTI (OR=0.33; 95% CI, 0.08-1.40). Importantly, AOM was included in the URI group, and, as previously discussed, AOM has independently been shown to have an inverse relationship with breastfeeding duration.
At 7 to 12 months of age, no association was seen between breastfeeding and the incidence of URI. Curiously, an association with LRTI was again detected for infants breastfed exclusively for 4 months and partially thereafter, but was not detected with exclusive breastfeeding for at least 6 months (OR=0.46; 95% CI, 0.31-0.69). In this study, in the first 6 months of life, 40% of infants had a URI and 8% had an LRTI. The findings in this cohort suggest an NNT of 6 or 7 for prevention of URI and an NNT of 25 for prevention of LRTI in the first 6 months of life.22
Continue to: Children younger than 2 years are...
Children younger than 2 years are estimated to have approximately 6 bouts of the common cold a year, and essentially 100% have at least one bout—perhaps lowering the NNT for URI if applied widely. However, these data are not divided into 6-month intervals, making accurate extrapolation difficult.23
Gastrointestinal infection. The rate of diarrheal illness in the first year of life is lower in infants who are exclusively breastfed for at least 4 months and partially breastfed after.
Both the Promotion of Breastfeeding Intervention Trial (PROBIT; a clinical trial in which infants were randomized to a breastfeeding education intervention or standard care) and a 2010 prospective cohort study in the Netherlands of more than 3400 infants found a reduction in the risk of one or more gastrointestinal (GI) infections at a similar rate.22,24
- In PROBIT, 9.1% of infants in the intervention group, compared to 13.2% in the standard care group (OR=0.60; 95% CI, 0.40-0.91), had one or more GI infections at 12 months of age.24
- In the 2010 Netherlands cohort, 8% of infants had a GI infection by 6 months of age. Infants breastfed exclusively for at least 4 or 6 months had a decreased risk for GI infection (respectively: adjusted OR=0.41; 95% CI, 0.26-0.64 and adjusted OR=0.46; 95% CI, 0.14-1.59). No such association was found for any feeding group 7 to 12 months of age.22
These studies are notable for the low incidence of GI infection, which is frequently cited as 1.3 to 2.3 episodes per child per year in children younger than 3 years in the United States.25 However, that high incidence has likely declined significantly since the introduction of rotavirus vaccine in 2006. In the years following the introduction of the vaccine, infant visits for gastroenteritis decreased by >90% in all care settings in the South, Northeast, and Midwest regions of the United States and by 53% to 63% in the West region.26 Recent accurate epidemiologic information, in an era of significantly higher vaccination rates, is lacking.
Assuming the low incidence of GI infection reported in PROBIT and the Netherlands trials, about 25 to 30 infants need to be exclusively breastfed for 4 to 6 months to prevent a single GI infection during the first 6 to 12 months of life.22,24 Assuming a 60% incidence by age 12 months before introduction of the rotavirus vaccine, the NNT would be approximately 4.24 The true number is likely somewhere between those 2 NNTs.
Continue to: Hospitalization
Hospitalization
Risk of infection is decreased. A large cohort study in Scotland, involving more than 500,000 children, found an association between exclusive breastfeeding for 6 to 8 weeks and decreased risk of hospitalization within the first 6 months of life. Formula-fed and mixed-fed infants had an increased hazard ratio (HR) for hospitalization for common childhood illness (HR=1.40; 95% CI, 1.35-1.45 for formula-fed infants and HR=1.18; 95% CI, 1.11-1.25 for mixed-fed infants).27 The study also found increased rates of hospitalization for conditions for which other meta-analyses have failed to show a protective effect from breastfeeding—leading to suspicion of residual confounding in the study. Another United Kingdom cohort demonstrated lower rates of hospitalization for GI infection (NNT=171) and LRTI (NNT=115) among exclusively breastfed infants by 8 months of age.28
Risk of neonatal readmission is increased. Late preterm infants who are exclusively breastfed are nearly twice as likely to be hospitalized as breastfed term or non-breastfed preterm infants, primarily due to dehydration, failure to thrive, weight loss, and hyperbilirubinemia. In fact, exclusive breastfeeding at discharge from the hospital is likely the single greatest risk factor for hospital readmission in newborns.29,30 Term infants who are exclusively breastfed are more likely to be hospitalized compared to formula-fed or mixed-fed infants, due to hyperbilirubinemia, dehydration, hypernatremia, and weight loss (number needed to harm (NNH)=71).30-32 For weight loss >10% of birth weight with or without hospitalization, the NNH for breastfed infants is 13.32
Many of these hospitalizations and events could be avoided with appropriate monitoring and medically indicated supplementation; the likelihood of long-term harm is low. Formula supplementation is often avoided if possible in hospitals to promote exclusive breastfeeding; however, several small randomized clinical trials have demonstrated that limited formula supplementation in breastfed infants does not affect the breastfeeding continuation rate at 3 and 6 months, and, therefore, might be a way to decrease infant rehospitalization.33,34
Necrotizing enterocolitis
In preterm infants, breastfeeding has been associated with a lower rate of necrotizing enterocolitis. In the 2007 Agency for Healthcare Research and Quality report, the association was found to be only marginally statistically significant, and the authors warned that, first, evidence is old and heterogeneous and, second, present preterm formula is much different than the formula used in earlier studies of preterm infant nutrition and necrotizing enterocolitis.35 A 2012 Cochrane review included newer studies in its analysis but reached the same conclusion on the quality and heterogeneity of available evidence, with a NNT of 25.36
Continue to: Sudden infant death syndrome
Sudden infant death syndrome
There is a statistically significant association between sudden infant death syndrome (SIDS) and feeding method. Infants whose cause of death is SIDS are approximately one half as likely to have been breastfed as matched controls.35,37
In 2005, AAP did not recommend breastfeeding as a means to reduce the risk of SIDS because available evidence was mixed, and studies at the time were poorly controlled.38 Since that time, case-control meta-analyses have shed additional light on the association between SIDS and feeding method.35,37
The protective effect exists for any amount of breastfeeding and is stronger for exclusive breastfeeding, suggesting a protective role—not simply an association. Caution should be employed with this conclusion, however, because the studies included in the meta-analysis used univariate analysis primarily and did not control sufficiently for known confounders. In addition, the authors warn that publication bias might overestimate the association.38
Potential mechanisms of a protective role include decreased risk of infection and greater arousability from sleep in breastfed infants. Assuming a protective role, available data suggest that more than 3500 infants need to be breastfed to prevent one case of SIDS.39
Continue to: Allergic disease
Allergic disease
Asthma. There is evidence of a small protective effect of breastfeeding “ever” on asthma at 5 to 18 years of age in high-income countries (OR=0.90; 95% CI, 0.83-0.97). A family history of asthma or atopy did not affect this finding. The authors note there is some evidence of publication bias in this review, which is the largest and most comprehensive on the topic.40
With a lifetime prevalence of asthma in the United States of approximately 13.2%, this association would confer an NNT of roughly 76.41 Earlier, the literature demonstrated mixed and conflicting evidence, and some experts suggested an effect only when there is a family history of asthma or atopy.36
Eczema. For children younger than 2 years, there is low-grade- and very-low-grade-quality evidence that exclusive breastfeeding longer than 3 to 4 months is associated with a reduced risk of eczema (OR=0.74; 95% CI, 0.57-0.97).40
Previously, data suggested that this association existed only in children who had a family history of atopy.35 The protective association, however, exists regardless of family history and does not persist beyond 2 years of age. The authors noted evidence of publication bias, reverse causation, and misdiagnosis of early childhood rashes as eczema as limitations of their findings.40
Continue to: Reliable epidemiologic evidence...
Reliable epidemiologic evidence on the incidence of eczema in infants in the United States is limited, but the prevalence in the United States in children younger than 17 years is approximately 10.7% (with wide regional variation). Extrapolating these data generously, the NNT to prevent eczema in the first 2 years of life could be estimated at approximately 36.42
Allergic rhinitis. There is low-grade- and very-low-grade-quality evidence that more breastfeeding, compared to less breastfeeding, is associated with a lower risk of allergic rhinitis in children younger than 5 years (OR=0.79; 95% CI, 0.63-0.98). The association exists regardless of family history and disappears after 5 years of age. The differentiation of allergic rhinitis from rhinovirus infection (for which there is higher-quality evidence of a protective effect with breastfeeding) must be considered when interpreting these data.40
Reliable epidemiologic evidence on allergic rhinitis in children younger than 5 years is lacking, and incidence varies by region. A rough estimate, using data from 6- and 7-year-olds, indicates an NNT of 54 to 70.43
Food allergy. There is no evidence to suggest an association between breastfeeding and food allergy, either as protective or as a risk factor, and studies are limited.40 Interestingly, as data accumulate associating early exposure to foods with protection, some authors have proposed reexamining the recommendation from WHO and US health organizations for exclusive breastfeeding for the first 6 months of life.7,44
Continue to: Dental health
Dental health
Dental caries. There is consistent evidence that breastfeeding beyond 12 months of age is associated with the development of dental caries of deciduous teeth to 6 years of age (OR=2.90; 95% CI, 2.33-3.60). Many of the studies that showed this association did not control for the introduction of sugary foods and drinks, and there was a trend toward publication bias showing the association.45
Dental malocclusion. There is consistent evidence for approximately a two-thirds reduction in malocclusions in deciduous teeth in breastfed infants (OR=0.32; 95% CI, 0.25-0.40). Although the large majority of these data come from low-income and middle-income countries, the incidence of malocclusion is not thought to be associated with socioeconomic status, as so many other breastfeeding outcomes are.46
Childhood leukemia
In the largest meta-analysis available, a statistically significant inverse relationship between any breastfeeding for >6 months and childhood leukemia is evident in developed countries (OR=0.84; 95% CI, 0.78-0.91), although significant heterogeneity among studies and lack of control for confounding variables are significant limitations. In particular, an association has been demonstrated with acute lymphoblastic leukemia (ALL) but not with acute myelogenous leukemia.47 Given the rarity of childhood ALL, approximately 12,500 infants would need to be breastfed to prevent one case.48
Continue to: Long-term outcomes
Long-term outcomes
Cognitive development. Several studies conducted in developed countries have linked breastfeeding to positive cognitive outcomes in children, including higher intelligence quotient (IQ).35,49-52
These effects are conflicting, however, in studies that include sibling analysis and ones that control for maternal IQ.8,35,43,52-54 In the 2013 WHO meta-analysis, breastfeeding was associated with an increase of 2.2 points on normalized testing when only high-quality studies were included.51 A 2015 meta-analysis identified 4 high-quality studies with a large sample size and recall time <3 years, which demonstrated a mean difference of 1.76 points in IQ (95% CI, 0.25-3.26) in childhood and adolescence.52 Although statistically significant, this modest increase is of questionable clinical benefit and of unknown duration.
Obesity. The relationship between breastfeeding and obesity later in life is debatable. A large, systematic 2014 review of 15 cohort and 10 cross-sectional studies found a significantly reduced risk of childhood obesity among children who were breastfed (adjusted OR=0.78; 95% CI, 0.74-0.81).55 However, the review included studies that controlled for different confounders, and smaller effects were found in studies in which more confounders were taken into account.
The 2013 WHO meta-analysis found a small (approximately 10%) reduction in the prevalence of overweight or obese children, but cautioned that residual confounding and publication bias were likely.51 At 6.5 and 11.5 years of follow-up, PROBIT failed to demonstrate a protective effect for exclusively or “ever” breastfed infants.56 Sibling analysis similarly fails to demonstrate a statistically significant relationship.8
Continue to: A 2015 meta-analysis of 23 high-quality studies...
A 2015 meta-analysis of 23 high-quality studies with a sample size >1500 children and controlled for important confounders showed a pooled reduction in the prevalence of overweight or obesity of 13% (95% CI, 6-19).57 The protection in this meta-analysis showed a dilution of the effect as the participants aged and an inverse relationship of the effect with sample size.
Breastfeeding is, therefore, unlikely to play a significant, if any, role in combatting the obesity epidemic.
Hypertension. A meta-analysis of high-quality trials demonstrates a <1 mm Hg reduction in systolic blood pressure and no significant difference in diastolic pressure in breastfed infants.57 Similarly, no significant effect of breastfeeding on blood pressure has been demonstrated in trials of preterm infants.51
Type 2 diabetes. Available data are limited and heterogeneous for the association between breastfeeding and later development of type 2 diabetes. Only 2 high-quality trials were identified in the 2013 WHO meta-analysis, and their results conflict.51 A 2015 meta-analysis identified only 3 high-quality studies, without a statistically significant relationship.57
Dyslipidemia. Although earlier data suggested an association between breastfeeding and reduced cholesterol levels later in life, the 2013 WHO meta-analysis and a 2015 meta-analysis concluded that no association exists. The limited data available for preterm infants conflict.51,57
Growth. There is no evidence that feeding method has a short- or long-term effect on weight gain or length gain in preterm or term infants.35,36,58
Death. No clear association has been found between mortality and breastfeeding status in developed countries, except for the association with SIDS.35
Continue to: What issues frame and guide counseling on breastfeeding?
What issues frame and guide counseling on breastfeeding?
There is that “problem” with the evidence. The evidence for infant breastfeeding status and its association with health outcomes faces significant limitations; the great majority of those limitations tend to overestimate the benefits of breastfeeding. Nearly all evidence is based on observational studies, in which causality cannot be determined and self-selection bias, recall bias, and residual confounding limit the value or strength of the findings.
Breastfeeding rates are strongly socially patterned alongside socioeconomic status, race, and education level, all of which are simultaneously strongly tied to short- and long-term health outcomes.6 Other factors limiting the strength of the data set include varying definitions of infant feeding practices in different studies, varying definitions of outcomes and diseases, reverse causation, and evidence of publication bias in many meta-analyses. Given these shortcomings, the NNTs in this article probably represent a best-case scenario for breastfeeding outcomes for infants in the United States (TABLE 118,22-24,28,36,39-43,47,48).

Data need to be put into context. The NNTs for many breastfeeding outcomes (TABLE) compare favorably with other recommended interventions, particularly for other preventive care measures. Two examples: 81 mg/d aspirin for a 50-year-old man has an NNT of 35 to 45 for preventing nonfatal myocardial infarction, and the number needed to invite to screen with mammography to prevent one breast cancer death for a 50-year-old woman is 1339.59,60
In both of these examples, >95% of patients will not benefit from the intervention, yet these preventive measures are routinely recommended and have a significant impact at the public health level. Notably, these outcomes are more serious than most breastfeeding outcomes; have a longer-lasting effect, better-quality data, and better data for potential harms; are causally linked to the intervention; and require much less effort and commitment of time than breastfeeding.
The question must be reckoned with: Can advocacy be harmful?
In recent years, a growing number of concerns have been raised about:
- the potential harms of breastfeeding advocacy
- exaggeration of the benefits of breastfeeding
- promotion of breastfeeding at the expense of evidence-based medicine.
The “Ten Steps to Successful Breastfeeding” program of the Baby-friendly Hospital Initiative (BFHI; launched by UNICEF and WHO) has come under scrutiny because of an increasing number of reports of sudden unexpected postnatal collapse; fall injuries; modeling and encouragement of unsafe sleep practices; an overly rigid resistance to the use of formula supplementation; and the ban on pacifier use.61,62 The BFHI, promoted by the Centers for Disease Control and Prevention, is increasingly being adopted by hospitals with the expressed goal of increasing the breastfeeding rate from birth to discharge.
Continue to: Some of the "Ten Steps"...
Some of the “Ten Steps,” such as the call for skin-to-skin care and 24-hour rooming-in, have well-established benefit yet, when performed without supervision, can have the rare but serious unintended consequences of sudden unexpected postnatal collapse (the incidence of which may be higher than that of SIDS) and unsafe sleeping practices.62,63
Furthermore, despite evidence that early formula supplementation, when medically necessary, does not adversely impact the breastfeeding rate, the “Ten Steps” program advises that giving formula before breast milk comes in might “lead to failure to breastfeed.”33,34,61,63
Similarly, the ban on pacifiers is contrary to available evidence. The use of pacifiers before last sleep is more protective against SIDS than breastfeeding (NNT=2733), and there is evidence at one hospital that BFHI-inspired pacifier restriction is associated with a decrease in the rate of breastfeeding.64,65
Other harms of advocacy are even more poorly studied. Most of the evidence for harm comes from the psychology and social science literature—not the medical literature, perhaps because the prevailing opinion in the medical community is that breastfeeding has overwhelming evidence for benefit. In fact, in the USPSTF’s 2008 recommendation, the evidence review of breastfeeding promotion practices in primary care did not identify a single study that measured harm; in the 2016 update of that recommendation, only 2 such studies were identified.15,66
The literature that does investigate harm consistently finds that women who have difficulty breastfeeding or choose formula feeding report feelings of inadequacy, guilt, loss of agency, anxiety, and physical pain during breastfeeding that interferes with 1) their ability to bond or otherwise care for their infant and 2) competing work obligations.11-13,67-69 Given the lack of attention paid to these variables in the medical literature, it is the individual mother who is best positioned to weigh these factors against the benefits of breastfeeding.
Continue to: Shared decision-making is best—for mother and baby
Shared decision-making is best—for mother and baby
Breastfeeding might prevent certain infections in as many as 50% of infants, but a mother unable to breastfeed can take solace in the fact that >95% of breastfed infants will not realize any benefit from the preventive potential of breastfeeding in regard to hospitalization or allergic disease, and >99% will not realize benefit from either the prevention of SIDS or ALL, or from improvement in long-term health measures (except for, perhaps, a slightly higher IQ). The “breast is best” mantra is likely true at a public-health level; for the individual mother–infant dyad, however, where there is a need to balance personal, social, family, and financial factors, that mantra is an oversimplification.
Regrettably, there is a paucity of data on the risks of breastfeeding promotion—an area that deserves more study. Balancing the abundant, but often limited-quality, data on the benefits of breastfeeding and the sheer lack of data regarding the risks of advocacy represents a clinical and an ethical challenge for physicians. It is a challenge that can only be resolved through individualization of care and shared decision-making, in which the physician is expert on the benefits of breastfeeding, and the mother is expert on the personal circumstances to be weighed against those benefits.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization, and New York, NY: UNICEF; 2003. Available at: www.who.int/maternal_child_adolescent/documents/9241562218/en/. Accessed April 4, 2018.
2. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Breastfeeding Expert Work Group. Committee Opinion No. 658: Optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2016;127:e86-e92.
3. Gartner LM, Morton J, Lawrence RA, et al; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
4. Breastfeeding (policy statement). Leawood, KS: American Academy of Family Physicians; 2007. Available at: https://www.aafp.org/about/policies/all/breastfeeding.html. Accessed April 3, 2018.
5. Office of the Surgeon General (US); Centers for Disease Control and Prevention (US); Office on Women’s Health (US). The Surgeon General’s call to action to support breastfeeding. Rockville, MD: US Department of Health and Human Services; 2011. Available at: www.surgeongeneral.gov/library/calls/breastfeeding/index.html. Updated August 12, 2014. Accessed April 4, 2018.
6. Breastfeeding: data & statistics. Atlanta, GA: Centers for Disease Control and Prevention; December 11, 2017. Available at: http://www.cdc.gov/breastfeeding/data/. Accessed May 17, 2018.
7. Fewtrell M, Wilson DC, Booth I, et al. A. Six months of exclusive breast feeding: how good is the evidence? BMJ. 2010;342:c5955.
8. Colen CG, Ramey DM. Is breast truly best? Estimating the effect of breastfeeding on long-term child wellbeing in the United States using sibling comparisons. Soc Sci Med. 2014;109:55-65.
9. Wolf J. Is Breast Best? Taking on the Breastfeeding Experts and the New High Stakes of Motherhood. New York, NY: NYU Press; 2010.
10. Tuteur A. Push Back: Guilt in the Age of Natural Parenting. New York, NY: HarperCollins Publishers; 2016.
11. Lee E. Health, morality, and infant feeding: British mothers’ experiences of formula milk use in the early weeks. Sociol Health Illn. 2007;29:1075-1090.
12. Williams K, Donaghue N, Kurz T. “Giving guilt the flick”?: an investigation of mothers’ talk about guilt in relation to infant feeding. Psychol Women Q. 2013;37:97-112.
13. Fahlquist JN, Roeser S. Ethical problems with information on infant feeding in developed countries. J Health Polit Policy Law. 2012;37:155-160.
14. U.S. Preventive Services Task Force. Final Recommendation Statement. Breastfeeding: Counseling. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breastfeeding-counseling. Accessed April 4, 2018.
15. US Preventive Services Task Force. Primary Care Interventions to Support Breastfeeding: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1688-1693.
16. Zahn CM, Hanley LE. Concerns over USPSTF draft recommendation on breastfeeding interventions [letter]. Washington, DC: The American College of Obstetricians and Gynecologists; May 18, 2016. Available at: https://www.acog.org/-/media/Departments/Breastfeeding/Breast-Feeding-ACOG-USPSTF.pdf?dmc=1&ts=20180518T1850056558. Accessed May 22, 2018.
17. Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222-231.
18. Bowatte G, Tham R, Allen KJ, et al. Breastfeeding and childhood acute otitis media: a systematic review and meta-analysis. Acta Paediatr. 2015;104:85-95.
19. Chonmaitree T, Trujillo R, Jennings K, et al. Acute otitis media and other complications of viral respiratory infection. Pediatrics. 2016;137:e20153555.
20. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
21. Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118:865-873.
22. Duijts L, Jaddoe VW, Hofman A, et al. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126:e18-e25.
23. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186:190-199.
24. Kramer MS, Chalmers B, Hodnett ED, et al; PROBIT Study Group (Promotion of Breastfeeding Intervention Trial). Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413-420.
25. Dennehy PH. Acute diarrheal disease in children: epidemiology, prevention, and treatment. Infect Dis Clin North Am. 2005;19:585-602.
26. Cortese MM, Tate JE, Simonsen L, et al. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatric Infect Dis J. 2010;29:489-494.
27. Ajetunmobi OM, Whyte B, Chalmers J, et al. Breastfeeding is associated with reduced childhood hospitalization: evidence from a Scottish birth cohort (1997-2009). J Pediatr. 2015;166:620-625.
28. Quigley MA, Kelly YJ, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007;119:e837-e842.
29. Radtke JV. The paradox of breastfeeding-associated morbidity among late preterm infants. J Obstet Gynecol Neonatal Nurs. 2011;40:9-24.
30. Escobar GJ, Gonzales VM, Armstrong M, et al. Rehospitalization for neonatal dehydration: a nested case-control study. Arch Pediatr Adolesc Med. 2002;156:155-161.
31. Salas AA, Salazar J, Burgoa CV, et al. Significant weight loss in breastfed term infants readmitted for hyperbilirubinemia. BMC Pediatr. 2009;9:82.
32. Tarcan A, Tiker F, Vatandaş NS, et al. Weight loss and hypernatremia in breast-fed babies: frequency in neonates with non-hemolytic jaundice. J Paediatr Child Health. 2005;41:484-487.
33. Flaherman VJ, Aby J, Burgos AE, et al. Effect of early limited formula on duration and exclusivity of breastfeeding in at-risk infants: an RCT. Pediatrics. 2013;131:1059-1065.
34. Straňák Z, Feyereislova S, Černá M, et al. J. Limited amount of formula may facilitate breastfeeding: randomized, controlled trial to compare standard clinical practice versus limited supplemental feeding. Denning PW, ed. PLoS One. 2016;11:e0150053.
35. Ip S, Chung M, Raman G, et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Rockville, MD: Agency for Healthcare Research and Quality (US); 2007. Evidence Reports/Technology Assessments, No. 153. Available at: www.ncbi.nlm.nih.gov/books/NBK38337/. Accessed April 3, 2018.
36. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;(4):CD002971.
37. Hauck FR, Thompson JM, Tanabe KO, et al. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128:103-110.
38. American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245-1255.
39. Moon RY, Fu L. Sudden infant death syndrome: an update. Pediatr Rev. 2012;33:314-320.
40. Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38-53.
41. Brim SN, Rudd RA, Funk RH, et al. Asthma prevalence among US children in underrepresented minority populations: American Indian/Alaska Native, Chinese, Filipino, and Asian Indian. Pediatrics. 2008;122:e217-e222.
42. Shaw TF, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
43. Mallol J, Crane J, von Mutius E, et al. The international study of asthma and allergies in childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73-85.
44. Flohr C, Nagel G, Weinmayr G, et al. Lack of evidence for a protective effect of prolonged breastfeeding on childhood eczema: lessons from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br J Dermatol. 2011;165:1280-1289.
45. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
46. Peres KG, Cascaes AM, Nascimento GG, et al. Effect of breastfeeding on malocclusions: a systematic review and meta-analysis. Acta Paediatr. 2015;104:54-61.
47. Amitya EL, Keinan-Boker L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 2015;169:e151025.
48. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943-1955.
49. Guxens M, Mendez MA, Moltó-Puigmartí C, et al. Breastfeeding, long-chain polyunsaturated fatty acids in colostrum, and infant mental development.
2012;129:1134-1140.
51. Horta BL, Victora CG. Long-term effects of breastfeeding: a systematic review. Geneva, Switzerland: World Health Organization; 2013. Available at: http://apps.who.int/iris/bitstream/10665/79198/1/9789241505307_eng.pdf. Accessed August 16, 2016.
52. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104:14-19.
53. Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333:945.
54. Sajjad A, Tharner A, Kiefte-de Jong JC, et al. Breastfeeding duration and non-verbal IQ in children. J Epidemiol Community Health 2015;69:775-781.
55. Yan J, Liu L, Zhu Y, et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267.
56. Martin RM, Patel R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
57. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure, and type 2 diabetes: systematic review and meta-analysis. Acta Paediatr. 2015;104:30-37.
58. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Syst Rev. 2012;15:CD003517.
59. U.S. Preventive Services Task Force. Final recommendation statement: aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Published September 2017. Accessed April 3, 2018.
60. U.S. Preventive Services Task Force. Screening for breast cancer. Available at: www.uspreventiveservicestaskforce.org/Page/SupportingDoc/breast-cancer-screening/final-evidence-summary9. Published November 2009. Accessed April 2, 2018.
61. Bass JL, Gartley T, Kleinman R. Unintended consequences of current breastfeeding initiatives. JAMA Pediatr. 2016;170:923-924.
62. Feldman-Winter L, Goldsmith JP; Committee on Fetus and Newborn; Task Force on Sudden Infant Death Syndrome. Safe sleep and skin-to-skin care in the neonatal period for healthy term newborns. Pediatrics. 2016;138:e20161889.
63. The Mother and Child Health and Education Trust. Ten steps to successful breastfeeding. Available at: www.tensteps.org. Published November 8, 2017. Accessed April 3, 2018.
64. Hauck FR, Omojokun OO, Siadaty MS. Do pacifiers reduce the risk of sudden infant death syndrome? A meta-analysis. Pediatrics. 2005;116:e716-e723.
65. Kair LR, Kenron D, Etheredge K, et al. Pacifier restriction and exclusive breastfeeding. Pediatrics. 2013;131:e1101-e1107.
66. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
67. Wolf JB. Is breast really best? Risk and total motherhood in the National Breastfeeding Awareness Campaign. J Health Polit Policy Law. 2007;32:595-636.
68. Marshall JL, Godfrey M, Renfrew MJ. Being a ‘good mother’: managing breastfeeding and merging identities. Soc Sci Med. 2007;65:2147-2159.
69. Kelleher CM. The physical challenges of early breastfeeding. Soc Sci Med. 2006;63:2727-2738.
1. Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization, and New York, NY: UNICEF; 2003. Available at: www.who.int/maternal_child_adolescent/documents/9241562218/en/. Accessed April 4, 2018.
2. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Breastfeeding Expert Work Group. Committee Opinion No. 658: Optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2016;127:e86-e92.
3. Gartner LM, Morton J, Lawrence RA, et al; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
4. Breastfeeding (policy statement). Leawood, KS: American Academy of Family Physicians; 2007. Available at: https://www.aafp.org/about/policies/all/breastfeeding.html. Accessed April 3, 2018.
5. Office of the Surgeon General (US); Centers for Disease Control and Prevention (US); Office on Women’s Health (US). The Surgeon General’s call to action to support breastfeeding. Rockville, MD: US Department of Health and Human Services; 2011. Available at: www.surgeongeneral.gov/library/calls/breastfeeding/index.html. Updated August 12, 2014. Accessed April 4, 2018.
6. Breastfeeding: data & statistics. Atlanta, GA: Centers for Disease Control and Prevention; December 11, 2017. Available at: http://www.cdc.gov/breastfeeding/data/. Accessed May 17, 2018.
7. Fewtrell M, Wilson DC, Booth I, et al. A. Six months of exclusive breast feeding: how good is the evidence? BMJ. 2010;342:c5955.
8. Colen CG, Ramey DM. Is breast truly best? Estimating the effect of breastfeeding on long-term child wellbeing in the United States using sibling comparisons. Soc Sci Med. 2014;109:55-65.
9. Wolf J. Is Breast Best? Taking on the Breastfeeding Experts and the New High Stakes of Motherhood. New York, NY: NYU Press; 2010.
10. Tuteur A. Push Back: Guilt in the Age of Natural Parenting. New York, NY: HarperCollins Publishers; 2016.
11. Lee E. Health, morality, and infant feeding: British mothers’ experiences of formula milk use in the early weeks. Sociol Health Illn. 2007;29:1075-1090.
12. Williams K, Donaghue N, Kurz T. “Giving guilt the flick”?: an investigation of mothers’ talk about guilt in relation to infant feeding. Psychol Women Q. 2013;37:97-112.
13. Fahlquist JN, Roeser S. Ethical problems with information on infant feeding in developed countries. J Health Polit Policy Law. 2012;37:155-160.
14. U.S. Preventive Services Task Force. Final Recommendation Statement. Breastfeeding: Counseling. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breastfeeding-counseling. Accessed April 4, 2018.
15. US Preventive Services Task Force. Primary Care Interventions to Support Breastfeeding: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1688-1693.
16. Zahn CM, Hanley LE. Concerns over USPSTF draft recommendation on breastfeeding interventions [letter]. Washington, DC: The American College of Obstetricians and Gynecologists; May 18, 2016. Available at: https://www.acog.org/-/media/Departments/Breastfeeding/Breast-Feeding-ACOG-USPSTF.pdf?dmc=1&ts=20180518T1850056558. Accessed May 22, 2018.
17. Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222-231.
18. Bowatte G, Tham R, Allen KJ, et al. Breastfeeding and childhood acute otitis media: a systematic review and meta-analysis. Acta Paediatr. 2015;104:85-95.
19. Chonmaitree T, Trujillo R, Jennings K, et al. Acute otitis media and other complications of viral respiratory infection. Pediatrics. 2016;137:e20153555.
20. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
21. Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118:865-873.
22. Duijts L, Jaddoe VW, Hofman A, et al. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126:e18-e25.
23. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186:190-199.
24. Kramer MS, Chalmers B, Hodnett ED, et al; PROBIT Study Group (Promotion of Breastfeeding Intervention Trial). Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413-420.
25. Dennehy PH. Acute diarrheal disease in children: epidemiology, prevention, and treatment. Infect Dis Clin North Am. 2005;19:585-602.
26. Cortese MM, Tate JE, Simonsen L, et al. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatric Infect Dis J. 2010;29:489-494.
27. Ajetunmobi OM, Whyte B, Chalmers J, et al. Breastfeeding is associated with reduced childhood hospitalization: evidence from a Scottish birth cohort (1997-2009). J Pediatr. 2015;166:620-625.
28. Quigley MA, Kelly YJ, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007;119:e837-e842.
29. Radtke JV. The paradox of breastfeeding-associated morbidity among late preterm infants. J Obstet Gynecol Neonatal Nurs. 2011;40:9-24.
30. Escobar GJ, Gonzales VM, Armstrong M, et al. Rehospitalization for neonatal dehydration: a nested case-control study. Arch Pediatr Adolesc Med. 2002;156:155-161.
31. Salas AA, Salazar J, Burgoa CV, et al. Significant weight loss in breastfed term infants readmitted for hyperbilirubinemia. BMC Pediatr. 2009;9:82.
32. Tarcan A, Tiker F, Vatandaş NS, et al. Weight loss and hypernatremia in breast-fed babies: frequency in neonates with non-hemolytic jaundice. J Paediatr Child Health. 2005;41:484-487.
33. Flaherman VJ, Aby J, Burgos AE, et al. Effect of early limited formula on duration and exclusivity of breastfeeding in at-risk infants: an RCT. Pediatrics. 2013;131:1059-1065.
34. Straňák Z, Feyereislova S, Černá M, et al. J. Limited amount of formula may facilitate breastfeeding: randomized, controlled trial to compare standard clinical practice versus limited supplemental feeding. Denning PW, ed. PLoS One. 2016;11:e0150053.
35. Ip S, Chung M, Raman G, et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Rockville, MD: Agency for Healthcare Research and Quality (US); 2007. Evidence Reports/Technology Assessments, No. 153. Available at: www.ncbi.nlm.nih.gov/books/NBK38337/. Accessed April 3, 2018.
36. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;(4):CD002971.
37. Hauck FR, Thompson JM, Tanabe KO, et al. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128:103-110.
38. American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245-1255.
39. Moon RY, Fu L. Sudden infant death syndrome: an update. Pediatr Rev. 2012;33:314-320.
40. Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38-53.
41. Brim SN, Rudd RA, Funk RH, et al. Asthma prevalence among US children in underrepresented minority populations: American Indian/Alaska Native, Chinese, Filipino, and Asian Indian. Pediatrics. 2008;122:e217-e222.
42. Shaw TF, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
43. Mallol J, Crane J, von Mutius E, et al. The international study of asthma and allergies in childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73-85.
44. Flohr C, Nagel G, Weinmayr G, et al. Lack of evidence for a protective effect of prolonged breastfeeding on childhood eczema: lessons from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br J Dermatol. 2011;165:1280-1289.
45. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
46. Peres KG, Cascaes AM, Nascimento GG, et al. Effect of breastfeeding on malocclusions: a systematic review and meta-analysis. Acta Paediatr. 2015;104:54-61.
47. Amitya EL, Keinan-Boker L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 2015;169:e151025.
48. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943-1955.
49. Guxens M, Mendez MA, Moltó-Puigmartí C, et al. Breastfeeding, long-chain polyunsaturated fatty acids in colostrum, and infant mental development.
2012;129:1134-1140.
51. Horta BL, Victora CG. Long-term effects of breastfeeding: a systematic review. Geneva, Switzerland: World Health Organization; 2013. Available at: http://apps.who.int/iris/bitstream/10665/79198/1/9789241505307_eng.pdf. Accessed August 16, 2016.
52. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104:14-19.
53. Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333:945.
54. Sajjad A, Tharner A, Kiefte-de Jong JC, et al. Breastfeeding duration and non-verbal IQ in children. J Epidemiol Community Health 2015;69:775-781.
55. Yan J, Liu L, Zhu Y, et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267.
56. Martin RM, Patel R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
57. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure, and type 2 diabetes: systematic review and meta-analysis. Acta Paediatr. 2015;104:30-37.
58. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Syst Rev. 2012;15:CD003517.
59. U.S. Preventive Services Task Force. Final recommendation statement: aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Published September 2017. Accessed April 3, 2018.
60. U.S. Preventive Services Task Force. Screening for breast cancer. Available at: www.uspreventiveservicestaskforce.org/Page/SupportingDoc/breast-cancer-screening/final-evidence-summary9. Published November 2009. Accessed April 2, 2018.
61. Bass JL, Gartley T, Kleinman R. Unintended consequences of current breastfeeding initiatives. JAMA Pediatr. 2016;170:923-924.
62. Feldman-Winter L, Goldsmith JP; Committee on Fetus and Newborn; Task Force on Sudden Infant Death Syndrome. Safe sleep and skin-to-skin care in the neonatal period for healthy term newborns. Pediatrics. 2016;138:e20161889.
63. The Mother and Child Health and Education Trust. Ten steps to successful breastfeeding. Available at: www.tensteps.org. Published November 8, 2017. Accessed April 3, 2018.
64. Hauck FR, Omojokun OO, Siadaty MS. Do pacifiers reduce the risk of sudden infant death syndrome? A meta-analysis. Pediatrics. 2005;116:e716-e723.
65. Kair LR, Kenron D, Etheredge K, et al. Pacifier restriction and exclusive breastfeeding. Pediatrics. 2013;131:e1101-e1107.
66. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
67. Wolf JB. Is breast really best? Risk and total motherhood in the National Breastfeeding Awareness Campaign. J Health Polit Policy Law. 2007;32:595-636.
68. Marshall JL, Godfrey M, Renfrew MJ. Being a ‘good mother’: managing breastfeeding and merging identities. Soc Sci Med. 2007;65:2147-2159.
69. Kelleher CM. The physical challenges of early breastfeeding. Soc Sci Med. 2006;63:2727-2738.
From The Journal of Family Practice | 2018;67(6):E1-E9.
PRACTICE RECOMMENDATIONS
› Encourage breastfeeding for its potential to reduce the risk of acute otitis media, upper- and lower-respiratory infections, gastrointestinal infection, and dental malocclusion. A
› Promote breastfeeding for its potential to make a small difference in intelligence quotient and the incidence of overweight and obesity—but not for any other significant impact on long-term health. B
› Consider the needs and preferences of the individual when advocating breastfeeding so as to avoid potentially engendering maternal feelings of guilt and inadequacy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
June 2018: Click for Credit
Here are 4 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Dermatology Practice Gaps: Missed Diagnoses
To take the posttest, go to: https://bit.ly/2IhaxSm
Expires February 11, 2019
2. When to Worry About Congenital Melanocytic Nevi
To take the posttest, go to: https://bit.ly/2IhMOBC
Expires March 2, 2019
3. Unscheduled Visits for Pain After Hernia Surgery Common, Costly
To take the posttest, go to: https://bit.ly/2GbfWIV
Expires February 15, 2019
4. Melanoma Incidence Increased in Older Non-Hispanic Whites
To take the posttest, go to: https://bit.ly/2wOkVzZ
Expires February 9, 2019
Here are 4 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Dermatology Practice Gaps: Missed Diagnoses
To take the posttest, go to: https://bit.ly/2IhaxSm
Expires February 11, 2019
2. When to Worry About Congenital Melanocytic Nevi
To take the posttest, go to: https://bit.ly/2IhMOBC
Expires March 2, 2019
3. Unscheduled Visits for Pain After Hernia Surgery Common, Costly
To take the posttest, go to: https://bit.ly/2GbfWIV
Expires February 15, 2019
4. Melanoma Incidence Increased in Older Non-Hispanic Whites
To take the posttest, go to: https://bit.ly/2wOkVzZ
Expires February 9, 2019
Here are 4 articles from the June issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Dermatology Practice Gaps: Missed Diagnoses
To take the posttest, go to: https://bit.ly/2IhaxSm
Expires February 11, 2019
2. When to Worry About Congenital Melanocytic Nevi
To take the posttest, go to: https://bit.ly/2IhMOBC
Expires March 2, 2019
3. Unscheduled Visits for Pain After Hernia Surgery Common, Costly
To take the posttest, go to: https://bit.ly/2GbfWIV
Expires February 15, 2019
4. Melanoma Incidence Increased in Older Non-Hispanic Whites
To take the posttest, go to: https://bit.ly/2wOkVzZ
Expires February 9, 2019
Don’t overlook these uses of point-of-care ultrasound
In the article, “Point-of-care ultrasound: Coming soon to primary care?” (J Fam Pract. 2018;67:70-79), Bornemann et al outline potential uses for point-of-care ultrasound (POCUS), describing in detail its role in cardiovascular and pulmonary exams, screening for abdominal aortic aneurysms, and diagnosing deep vein thrombosis. The American Academy of Family Physicians, in the Recommended Curriculum Guidelines for Family Medicine Residents (available at: https://www.aafp.org/medical-school-residency/program-directors/curriculum.html), also discusses obstetric and gynecologic uses for POCUS, such as determining fetal presentation and distinguishing viable pregnancy from miscarriage.
In my practice, I most often use POCUS for gynecologic and pregnancy-related issues, such as to ensure proper placement of an intrauterine device (IUD) when the strings are not visible, to determine gestational age in patients with uncertain last menstrual periods, and to confirm pregnancy location when patients have risk factors for, or symptoms suggestive of, ectopic pregnancy.
The breadth of care provided in family medicine is what makes it special. We must make sure that as we expand our care with new technologies, we do not trade tried and true uses of those technologies for newer ones.
Zoey Thill, MD, MPP
Bronx, NY
In the article, “Point-of-care ultrasound: Coming soon to primary care?” (J Fam Pract. 2018;67:70-79), Bornemann et al outline potential uses for point-of-care ultrasound (POCUS), describing in detail its role in cardiovascular and pulmonary exams, screening for abdominal aortic aneurysms, and diagnosing deep vein thrombosis. The American Academy of Family Physicians, in the Recommended Curriculum Guidelines for Family Medicine Residents (available at: https://www.aafp.org/medical-school-residency/program-directors/curriculum.html), also discusses obstetric and gynecologic uses for POCUS, such as determining fetal presentation and distinguishing viable pregnancy from miscarriage.
In my practice, I most often use POCUS for gynecologic and pregnancy-related issues, such as to ensure proper placement of an intrauterine device (IUD) when the strings are not visible, to determine gestational age in patients with uncertain last menstrual periods, and to confirm pregnancy location when patients have risk factors for, or symptoms suggestive of, ectopic pregnancy.
The breadth of care provided in family medicine is what makes it special. We must make sure that as we expand our care with new technologies, we do not trade tried and true uses of those technologies for newer ones.
Zoey Thill, MD, MPP
Bronx, NY
In the article, “Point-of-care ultrasound: Coming soon to primary care?” (J Fam Pract. 2018;67:70-79), Bornemann et al outline potential uses for point-of-care ultrasound (POCUS), describing in detail its role in cardiovascular and pulmonary exams, screening for abdominal aortic aneurysms, and diagnosing deep vein thrombosis. The American Academy of Family Physicians, in the Recommended Curriculum Guidelines for Family Medicine Residents (available at: https://www.aafp.org/medical-school-residency/program-directors/curriculum.html), also discusses obstetric and gynecologic uses for POCUS, such as determining fetal presentation and distinguishing viable pregnancy from miscarriage.
In my practice, I most often use POCUS for gynecologic and pregnancy-related issues, such as to ensure proper placement of an intrauterine device (IUD) when the strings are not visible, to determine gestational age in patients with uncertain last menstrual periods, and to confirm pregnancy location when patients have risk factors for, or symptoms suggestive of, ectopic pregnancy.
The breadth of care provided in family medicine is what makes it special. We must make sure that as we expand our care with new technologies, we do not trade tried and true uses of those technologies for newer ones.
Zoey Thill, MD, MPP
Bronx, NY







