User login
Diagnosed with a chronic illness: Should you tell your patients?
Physicians are not immune to chronic illness. Those who choose to continue working after being diagnosed with a chronic illness need to decide whether or not to tell their patients. The idea of physicians being a “blank slate” to their patients would be challenged by such self-disclosure. But ignoring an obvious change in the therapeutic space could be detrimental to your patient’s therapy.1 Every patient has his or her own ideas or perceptions about their physician that contribute to how likely they are to continue to engage in therapy or take prescribed medications. Could letting your patients know you have a chronic illness threaten the image they have of you, and potentially jeopardize their treatment?
Physician factors
Once diagnosed with a chronic illness, a physician who previously defined his or her identity as a clinician now must also assume the role of a patient. This transition gives rise to anxiety. Patient encounters may give a physician the opportunity to feel safe to discuss such anxiety.2 However, patients often view their physicians as omnipotent. When their physician admits weakness and vulnerability, that perception may be damaged.3 This damage could manifest as medication nonadherence, missed appointments, or even termination of treatment. A fear of such abandonment may lead a physician to not disclose his or her illness. To avoid discussing this uncomfortable topic, a physician might be more defensive in his or her interactions with the patient.2
Patient factors
Every patient presents with unique characteristics that contribute to the patient–physician relationship. Receiving news that one’s physician has a chronic or severe illness will elicit different reactions in each patient. These reactions will vary depending upon the patient’s pathology, stage of treatment, and background.3 The previous work done between the patient and physician is crucial in predicting the treatment course after the physician discloses that he or she has a chronic illness. Also, patients may notice the physical changes of their physician’s illness. Deciding to disclose—or to not disclose—something that is obvious can elicit feelings of worry, anger, or even triumph in the patient.3
CASES
Two patients, two different responses
Dr. T recently was diagnosed with leukemia and has begun to receive treatment. He decides to continue working. Since receiving the diagnosis, he finds himself more anxious. Adding to his anxiety is the question of whether or not he should tell his patients about his diagnosis. He decides to tell 2 of his patients—Mr. G and Ms. N—and receives a drastically different response from each of them.
Mr. G, age 45, has been Dr. T’s patient for 2 years. He is married, has 2 children, and works at a car dealership. Mr. G initially presented for treatment of depressive symptoms after his mother died. Those symptoms were stabilized with medicatio
Dr. T discloses the news of his illness to Mr. G at their next appointment. Mr. G offers his condolences and speaks about how on one hand, he is sympathetic and wishes to be supportive, but on the other hand, he fears another loss in his life. Mr. G thanks Dr. T for disclosing this news and hopes they can begin to discuss this situation in therapy. He remains compliant with appointments.
Ms. N, age 59, has been Dr. T’s patient for 6 months. She was diagnosed with schizophrenia when she was in her early 20s. She is single, unemployed, lives alone, and lacks social support. Ms. N has a history of multiple hospitalizations. She has a pattern of presenting to an emergency department and asking to be admitted whenever she faces an acute stressor.
Continue to: Ms. N came to Dr. T through another psychiatrist...
Ms. N came to Dr. T through another psychiatrist and Dr. T continues to provide medication management. He has implemented a biweekly appointment schedule for supportive therapy to work on Ms. N’s personal goals to cook more, clean her house, and lose weight. They also address issues regarding her father and his absence in her life since she was age 18.
During their next appointment, Dr. T discloses the news of his illness to Ms. N. Ms. N asks, “Are you sure?” Dr. T confirms and asks her how she feels about this news. She replies, “It’s fine.” Soon after, she stops attending her biweekly appointments and is lost to follow-up.
Consider your patient’s ability to cope
Dr. T faced the challenge of whether to disclose his diagnosis to his patients. He understood the potential implications on his therapeutic work and his battles with his own anxiety. Ultimately, he decided to tell his patients, but he did not consider how they might have been able to handle such news.
Mr. G was receptive to the news and remained engaged in treatment after learning of Dr. T’s illness. His ability to do so likely was the result of many factors. Mr. G is a high-functioning individual who seems to have a secure attachment style. He is able to express his conflicts. He has had good relationships in his life, was able to work through his mother’s death, and is engaged in treatment to help him cope with the inevitable loss of his father. Mr. G can handle the potential loss of his physician because he has shown his ability to cope with such losses in his life.
Continue to: On the other hand...
On the other hand, although Ms. N stated that the news of Dr. T’s diagnosis was “fine,” she was soon lost to follow-up, which suggests she was unable to handle the news. This is supported by her history of unstable relationships. Her insecure attachment style likely contributed to her inability to handle stressors, as evidenced by her frequent requests for admission. Dr. T also should have considered the possibility of transference, given that Ms. N struggled with abandonment by her father. Dr. T’s potential departure could represent such abandonment. In a patient such as Ms. N, being upfront about having a chronic illness would be more harmful than beneficial.
Maintain a patient-focused view
Receiving a diagnosis of a severe or chronic illness can be extremely stressful for physicians. Adopting the new identity of patient in addition to that of physician can cause tremendous anxiety. If you decide to continue working with your patients, it is crucial to be mindful of this anxiety and its potential to influence your decision to disclose your diagnosis to your patients. Do not allow your anxiety to contaminate the therapeutic work. Maintaining a patient-focused view of treatment will allow you to determine each patient’s ability to process disclosure vs nondisclosure of your diagnosis. Ultimately, this will help determine which patients you should tell, and which ones you should not.
1. Abend SM. Serious illness in the analyst: countertransference considerations. J Am Psychoanal Assoc. 1982;30(2):365-379.
2. Dewald PA. Serious illness in the analyst: transference, countertransference, and reality responses. J Am Psychoanal Assoc. 1982;30(2):347-363.
3. Torrigiani MG, Marzi A. When the analyst is physically ill: vicissitudes in the analytical relationship. Int J Psychoanal. 2005;86(pt 5):1373-1389.
Physicians are not immune to chronic illness. Those who choose to continue working after being diagnosed with a chronic illness need to decide whether or not to tell their patients. The idea of physicians being a “blank slate” to their patients would be challenged by such self-disclosure. But ignoring an obvious change in the therapeutic space could be detrimental to your patient’s therapy.1 Every patient has his or her own ideas or perceptions about their physician that contribute to how likely they are to continue to engage in therapy or take prescribed medications. Could letting your patients know you have a chronic illness threaten the image they have of you, and potentially jeopardize their treatment?
Physician factors
Once diagnosed with a chronic illness, a physician who previously defined his or her identity as a clinician now must also assume the role of a patient. This transition gives rise to anxiety. Patient encounters may give a physician the opportunity to feel safe to discuss such anxiety.2 However, patients often view their physicians as omnipotent. When their physician admits weakness and vulnerability, that perception may be damaged.3 This damage could manifest as medication nonadherence, missed appointments, or even termination of treatment. A fear of such abandonment may lead a physician to not disclose his or her illness. To avoid discussing this uncomfortable topic, a physician might be more defensive in his or her interactions with the patient.2
Patient factors
Every patient presents with unique characteristics that contribute to the patient–physician relationship. Receiving news that one’s physician has a chronic or severe illness will elicit different reactions in each patient. These reactions will vary depending upon the patient’s pathology, stage of treatment, and background.3 The previous work done between the patient and physician is crucial in predicting the treatment course after the physician discloses that he or she has a chronic illness. Also, patients may notice the physical changes of their physician’s illness. Deciding to disclose—or to not disclose—something that is obvious can elicit feelings of worry, anger, or even triumph in the patient.3
CASES
Two patients, two different responses
Dr. T recently was diagnosed with leukemia and has begun to receive treatment. He decides to continue working. Since receiving the diagnosis, he finds himself more anxious. Adding to his anxiety is the question of whether or not he should tell his patients about his diagnosis. He decides to tell 2 of his patients—Mr. G and Ms. N—and receives a drastically different response from each of them.
Mr. G, age 45, has been Dr. T’s patient for 2 years. He is married, has 2 children, and works at a car dealership. Mr. G initially presented for treatment of depressive symptoms after his mother died. Those symptoms were stabilized with medicatio
Dr. T discloses the news of his illness to Mr. G at their next appointment. Mr. G offers his condolences and speaks about how on one hand, he is sympathetic and wishes to be supportive, but on the other hand, he fears another loss in his life. Mr. G thanks Dr. T for disclosing this news and hopes they can begin to discuss this situation in therapy. He remains compliant with appointments.
Ms. N, age 59, has been Dr. T’s patient for 6 months. She was diagnosed with schizophrenia when she was in her early 20s. She is single, unemployed, lives alone, and lacks social support. Ms. N has a history of multiple hospitalizations. She has a pattern of presenting to an emergency department and asking to be admitted whenever she faces an acute stressor.
Continue to: Ms. N came to Dr. T through another psychiatrist...
Ms. N came to Dr. T through another psychiatrist and Dr. T continues to provide medication management. He has implemented a biweekly appointment schedule for supportive therapy to work on Ms. N’s personal goals to cook more, clean her house, and lose weight. They also address issues regarding her father and his absence in her life since she was age 18.
During their next appointment, Dr. T discloses the news of his illness to Ms. N. Ms. N asks, “Are you sure?” Dr. T confirms and asks her how she feels about this news. She replies, “It’s fine.” Soon after, she stops attending her biweekly appointments and is lost to follow-up.
Consider your patient’s ability to cope
Dr. T faced the challenge of whether to disclose his diagnosis to his patients. He understood the potential implications on his therapeutic work and his battles with his own anxiety. Ultimately, he decided to tell his patients, but he did not consider how they might have been able to handle such news.
Mr. G was receptive to the news and remained engaged in treatment after learning of Dr. T’s illness. His ability to do so likely was the result of many factors. Mr. G is a high-functioning individual who seems to have a secure attachment style. He is able to express his conflicts. He has had good relationships in his life, was able to work through his mother’s death, and is engaged in treatment to help him cope with the inevitable loss of his father. Mr. G can handle the potential loss of his physician because he has shown his ability to cope with such losses in his life.
Continue to: On the other hand...
On the other hand, although Ms. N stated that the news of Dr. T’s diagnosis was “fine,” she was soon lost to follow-up, which suggests she was unable to handle the news. This is supported by her history of unstable relationships. Her insecure attachment style likely contributed to her inability to handle stressors, as evidenced by her frequent requests for admission. Dr. T also should have considered the possibility of transference, given that Ms. N struggled with abandonment by her father. Dr. T’s potential departure could represent such abandonment. In a patient such as Ms. N, being upfront about having a chronic illness would be more harmful than beneficial.
Maintain a patient-focused view
Receiving a diagnosis of a severe or chronic illness can be extremely stressful for physicians. Adopting the new identity of patient in addition to that of physician can cause tremendous anxiety. If you decide to continue working with your patients, it is crucial to be mindful of this anxiety and its potential to influence your decision to disclose your diagnosis to your patients. Do not allow your anxiety to contaminate the therapeutic work. Maintaining a patient-focused view of treatment will allow you to determine each patient’s ability to process disclosure vs nondisclosure of your diagnosis. Ultimately, this will help determine which patients you should tell, and which ones you should not.
Physicians are not immune to chronic illness. Those who choose to continue working after being diagnosed with a chronic illness need to decide whether or not to tell their patients. The idea of physicians being a “blank slate” to their patients would be challenged by such self-disclosure. But ignoring an obvious change in the therapeutic space could be detrimental to your patient’s therapy.1 Every patient has his or her own ideas or perceptions about their physician that contribute to how likely they are to continue to engage in therapy or take prescribed medications. Could letting your patients know you have a chronic illness threaten the image they have of you, and potentially jeopardize their treatment?
Physician factors
Once diagnosed with a chronic illness, a physician who previously defined his or her identity as a clinician now must also assume the role of a patient. This transition gives rise to anxiety. Patient encounters may give a physician the opportunity to feel safe to discuss such anxiety.2 However, patients often view their physicians as omnipotent. When their physician admits weakness and vulnerability, that perception may be damaged.3 This damage could manifest as medication nonadherence, missed appointments, or even termination of treatment. A fear of such abandonment may lead a physician to not disclose his or her illness. To avoid discussing this uncomfortable topic, a physician might be more defensive in his or her interactions with the patient.2
Patient factors
Every patient presents with unique characteristics that contribute to the patient–physician relationship. Receiving news that one’s physician has a chronic or severe illness will elicit different reactions in each patient. These reactions will vary depending upon the patient’s pathology, stage of treatment, and background.3 The previous work done between the patient and physician is crucial in predicting the treatment course after the physician discloses that he or she has a chronic illness. Also, patients may notice the physical changes of their physician’s illness. Deciding to disclose—or to not disclose—something that is obvious can elicit feelings of worry, anger, or even triumph in the patient.3
CASES
Two patients, two different responses
Dr. T recently was diagnosed with leukemia and has begun to receive treatment. He decides to continue working. Since receiving the diagnosis, he finds himself more anxious. Adding to his anxiety is the question of whether or not he should tell his patients about his diagnosis. He decides to tell 2 of his patients—Mr. G and Ms. N—and receives a drastically different response from each of them.
Mr. G, age 45, has been Dr. T’s patient for 2 years. He is married, has 2 children, and works at a car dealership. Mr. G initially presented for treatment of depressive symptoms after his mother died. Those symptoms were stabilized with medicatio
Dr. T discloses the news of his illness to Mr. G at their next appointment. Mr. G offers his condolences and speaks about how on one hand, he is sympathetic and wishes to be supportive, but on the other hand, he fears another loss in his life. Mr. G thanks Dr. T for disclosing this news and hopes they can begin to discuss this situation in therapy. He remains compliant with appointments.
Ms. N, age 59, has been Dr. T’s patient for 6 months. She was diagnosed with schizophrenia when she was in her early 20s. She is single, unemployed, lives alone, and lacks social support. Ms. N has a history of multiple hospitalizations. She has a pattern of presenting to an emergency department and asking to be admitted whenever she faces an acute stressor.
Continue to: Ms. N came to Dr. T through another psychiatrist...
Ms. N came to Dr. T through another psychiatrist and Dr. T continues to provide medication management. He has implemented a biweekly appointment schedule for supportive therapy to work on Ms. N’s personal goals to cook more, clean her house, and lose weight. They also address issues regarding her father and his absence in her life since she was age 18.
During their next appointment, Dr. T discloses the news of his illness to Ms. N. Ms. N asks, “Are you sure?” Dr. T confirms and asks her how she feels about this news. She replies, “It’s fine.” Soon after, she stops attending her biweekly appointments and is lost to follow-up.
Consider your patient’s ability to cope
Dr. T faced the challenge of whether to disclose his diagnosis to his patients. He understood the potential implications on his therapeutic work and his battles with his own anxiety. Ultimately, he decided to tell his patients, but he did not consider how they might have been able to handle such news.
Mr. G was receptive to the news and remained engaged in treatment after learning of Dr. T’s illness. His ability to do so likely was the result of many factors. Mr. G is a high-functioning individual who seems to have a secure attachment style. He is able to express his conflicts. He has had good relationships in his life, was able to work through his mother’s death, and is engaged in treatment to help him cope with the inevitable loss of his father. Mr. G can handle the potential loss of his physician because he has shown his ability to cope with such losses in his life.
Continue to: On the other hand...
On the other hand, although Ms. N stated that the news of Dr. T’s diagnosis was “fine,” she was soon lost to follow-up, which suggests she was unable to handle the news. This is supported by her history of unstable relationships. Her insecure attachment style likely contributed to her inability to handle stressors, as evidenced by her frequent requests for admission. Dr. T also should have considered the possibility of transference, given that Ms. N struggled with abandonment by her father. Dr. T’s potential departure could represent such abandonment. In a patient such as Ms. N, being upfront about having a chronic illness would be more harmful than beneficial.
Maintain a patient-focused view
Receiving a diagnosis of a severe or chronic illness can be extremely stressful for physicians. Adopting the new identity of patient in addition to that of physician can cause tremendous anxiety. If you decide to continue working with your patients, it is crucial to be mindful of this anxiety and its potential to influence your decision to disclose your diagnosis to your patients. Do not allow your anxiety to contaminate the therapeutic work. Maintaining a patient-focused view of treatment will allow you to determine each patient’s ability to process disclosure vs nondisclosure of your diagnosis. Ultimately, this will help determine which patients you should tell, and which ones you should not.
1. Abend SM. Serious illness in the analyst: countertransference considerations. J Am Psychoanal Assoc. 1982;30(2):365-379.
2. Dewald PA. Serious illness in the analyst: transference, countertransference, and reality responses. J Am Psychoanal Assoc. 1982;30(2):347-363.
3. Torrigiani MG, Marzi A. When the analyst is physically ill: vicissitudes in the analytical relationship. Int J Psychoanal. 2005;86(pt 5):1373-1389.
1. Abend SM. Serious illness in the analyst: countertransference considerations. J Am Psychoanal Assoc. 1982;30(2):365-379.
2. Dewald PA. Serious illness in the analyst: transference, countertransference, and reality responses. J Am Psychoanal Assoc. 1982;30(2):347-363.
3. Torrigiani MG, Marzi A. When the analyst is physically ill: vicissitudes in the analytical relationship. Int J Psychoanal. 2005;86(pt 5):1373-1389.
Working at a long-term psychiatric hospital? Consider your patient’s point of view
Working at a long-term psychiatric hospital can present challenges similar to those found in other institutions, such as correctional facilities1; however, in this setting, additional obstacles that could affect treatment may not readily come to mind. Following the 2 simple approaches described here can help you to understand your patient’s point of view and improve the treatment relationship.
Allow patients some control. Many patients in long-term psychiatric hospitals are prescribed medications that can result in metabolic complications such as weight gain or hyperlipidemia. To avoid these complications, we may need to institute dietary restrictions. Despite our explanations of why these restrictions are necessary, some patients may continue to insist on eating food that we believe will worsen their physical health; they may feel that they have little control in their lives and have nothing to look forward to except for what they can eat.2
For patients in long-term psychiatric hospitals, everyday life usually is structured from morning to evening. This includes when meals and snacks are served, as well as what they are allowed to eat. Food is a basic human necessity, and we often forget its psychological significance. Because most patients can control what they put in their mouths, food allows them to exert control in an environment where they may believe they have no influence. This may explain why patients insist on certain meals, purchase unhealthy food, or engage in a surreptitious snack distribution system with other patients. We usually can decide what and when we eat, but many of our hospitalized patients do not have that opportunity. Within reason, negotiating meals and snacks could provide patients with a sense of control, and might increase treatment compliance.2
Mind what you say. At the hospital, patients are acutely aware that we are there for a short period each day. For these patients, the hospital serves as their home. Many will live there for months to years; some will spend the remainder of their lives there. The way these patients view us can become adversely affected when they see that we occasionally bring a negative attitude toward having to spend the day in their living space, telling them how to behave and what to do. This daily temporary relationship between hospital staff and patients can greatly affect treatment.
Although the hospital can serve as a home, patients do not have input into how we should behave in their home. Be mindful of your actions and the comments you make while in the hospital. We would not appreciate someone making a negative comment about our homes, so it is likely that our patients do not want to hear us complain about the hospital. Furthermore, they likely do not enjoy hearing hospital staff discussing plans they have made in their personal lives. Many patients do not enjoy being in the hospital, and they could view such expressions as “rubbing it in,” which could adversely affect treatment.
1. Khajuria K. CORRECT: insights into working at correctional facilities. Current Psychiatry. 2017;16(2):54-55.
2. Joshi KG. Can I have cheese on my ham sandwich? BMJ. 2016;355:i6024. doi: 10.1136/bmj.i6024.
Working at a long-term psychiatric hospital can present challenges similar to those found in other institutions, such as correctional facilities1; however, in this setting, additional obstacles that could affect treatment may not readily come to mind. Following the 2 simple approaches described here can help you to understand your patient’s point of view and improve the treatment relationship.
Allow patients some control. Many patients in long-term psychiatric hospitals are prescribed medications that can result in metabolic complications such as weight gain or hyperlipidemia. To avoid these complications, we may need to institute dietary restrictions. Despite our explanations of why these restrictions are necessary, some patients may continue to insist on eating food that we believe will worsen their physical health; they may feel that they have little control in their lives and have nothing to look forward to except for what they can eat.2
For patients in long-term psychiatric hospitals, everyday life usually is structured from morning to evening. This includes when meals and snacks are served, as well as what they are allowed to eat. Food is a basic human necessity, and we often forget its psychological significance. Because most patients can control what they put in their mouths, food allows them to exert control in an environment where they may believe they have no influence. This may explain why patients insist on certain meals, purchase unhealthy food, or engage in a surreptitious snack distribution system with other patients. We usually can decide what and when we eat, but many of our hospitalized patients do not have that opportunity. Within reason, negotiating meals and snacks could provide patients with a sense of control, and might increase treatment compliance.2
Mind what you say. At the hospital, patients are acutely aware that we are there for a short period each day. For these patients, the hospital serves as their home. Many will live there for months to years; some will spend the remainder of their lives there. The way these patients view us can become adversely affected when they see that we occasionally bring a negative attitude toward having to spend the day in their living space, telling them how to behave and what to do. This daily temporary relationship between hospital staff and patients can greatly affect treatment.
Although the hospital can serve as a home, patients do not have input into how we should behave in their home. Be mindful of your actions and the comments you make while in the hospital. We would not appreciate someone making a negative comment about our homes, so it is likely that our patients do not want to hear us complain about the hospital. Furthermore, they likely do not enjoy hearing hospital staff discussing plans they have made in their personal lives. Many patients do not enjoy being in the hospital, and they could view such expressions as “rubbing it in,” which could adversely affect treatment.
Working at a long-term psychiatric hospital can present challenges similar to those found in other institutions, such as correctional facilities1; however, in this setting, additional obstacles that could affect treatment may not readily come to mind. Following the 2 simple approaches described here can help you to understand your patient’s point of view and improve the treatment relationship.
Allow patients some control. Many patients in long-term psychiatric hospitals are prescribed medications that can result in metabolic complications such as weight gain or hyperlipidemia. To avoid these complications, we may need to institute dietary restrictions. Despite our explanations of why these restrictions are necessary, some patients may continue to insist on eating food that we believe will worsen their physical health; they may feel that they have little control in their lives and have nothing to look forward to except for what they can eat.2
For patients in long-term psychiatric hospitals, everyday life usually is structured from morning to evening. This includes when meals and snacks are served, as well as what they are allowed to eat. Food is a basic human necessity, and we often forget its psychological significance. Because most patients can control what they put in their mouths, food allows them to exert control in an environment where they may believe they have no influence. This may explain why patients insist on certain meals, purchase unhealthy food, or engage in a surreptitious snack distribution system with other patients. We usually can decide what and when we eat, but many of our hospitalized patients do not have that opportunity. Within reason, negotiating meals and snacks could provide patients with a sense of control, and might increase treatment compliance.2
Mind what you say. At the hospital, patients are acutely aware that we are there for a short period each day. For these patients, the hospital serves as their home. Many will live there for months to years; some will spend the remainder of their lives there. The way these patients view us can become adversely affected when they see that we occasionally bring a negative attitude toward having to spend the day in their living space, telling them how to behave and what to do. This daily temporary relationship between hospital staff and patients can greatly affect treatment.
Although the hospital can serve as a home, patients do not have input into how we should behave in their home. Be mindful of your actions and the comments you make while in the hospital. We would not appreciate someone making a negative comment about our homes, so it is likely that our patients do not want to hear us complain about the hospital. Furthermore, they likely do not enjoy hearing hospital staff discussing plans they have made in their personal lives. Many patients do not enjoy being in the hospital, and they could view such expressions as “rubbing it in,” which could adversely affect treatment.
1. Khajuria K. CORRECT: insights into working at correctional facilities. Current Psychiatry. 2017;16(2):54-55.
2. Joshi KG. Can I have cheese on my ham sandwich? BMJ. 2016;355:i6024. doi: 10.1136/bmj.i6024.
1. Khajuria K. CORRECT: insights into working at correctional facilities. Current Psychiatry. 2017;16(2):54-55.
2. Joshi KG. Can I have cheese on my ham sandwich? BMJ. 2016;355:i6024. doi: 10.1136/bmj.i6024.
Helping patients quit smoking: Lessons from the EAGLES trial
Psychiatrists often fail to adequately address their patients’ smoking, and often underestimate the impact of ongoing tobacco use. Evidence suggests that heavy smoking is a risk factor for major depressive disorder; it also is associated with increased suicidal ideations and attempts.1,2 Tobacco use also has a mood-altering impact that can change the trajectory of mental illness, and alters the metabolism of most psychotropics.
Previously, psychiatrists may have been reluctant to prescribe the most effective interventions for smoking cessation—varenicline and bupropion—because these medications carried an FDA “black-box” warning of neuropsychiatric adverse effects, including increased aggression and suicidality. However,
The EAGLES trial was a large, multi-site global trial that included patients with and without mental illness. Its primary objective was to assess the risk of “clinically significant” adverse effects for individuals receiving varenicline, bupropion, nicotine replacement therapy (NRT), or placebo, and whether having a history of psychiatric conditions increased the risk of developing adverse effects when taking these therapies. Overall, 2% of smokers without mental illness experienced adverse effects, compared with 5% to 7% in the psychiatric cohort, regardless of treatment arm. The rate of neuropsychiatric events and scores on suicide severity scales were similar across treatment arms in both cohorts.3
We should take lessons from the EAGLES trial. We propose that clinicians ask themselves the following 6 questions when forming a treatment plan to address their patients’ tobacco use:
1. Does the patient meet DSM-5 criteria for nicotine use disorder and, if yes, what is the severity of his or her nicotine dependence? The Fagerstrom Test for Nicotine Dependence (FTND)5 is a 6-question instrument for evaluating the quantity of cigarette consumption, compulsion to use, and dependence. It provides clinicians with guidelines on preventing withdrawal by implementing NRTs, such as lozenges, an inhaler, patches, and/or gum. A score of 1 to 2 (low dependence) indicates that no NRT is needed; a score of 3 to 4 (low to moderate dependence) requires 1 NRT; and scores of 5 to 7 (moderate dependence) and ≥8 (high dependence) require a combination of NRTs.
In the EAGLES trial, all participants smoked at least 10 cigarettes per day, and had moderate dependence, with an average FTND score of 5 to 6.
2. What stage of change is the patient in, and how many times has he or she attempted to quit? Based on the answers, motivational interviewing may be appropriate.
Continue to: In the EAGLES trial...
In the EAGLES trial, the participants were motivated individuals who had on average 3 past quit attempts. Research suggests that even patients who have a serious mental illness can be motivated to quit (Box).6-9
Box
Mental illness and motivation to quit smoking
In the past, clinicians may have believed that many individuals with mental illness typically weren’t motivated to quit smoking. We now know this is not the case and that such patients’ motivation is similar to that of the general population, and the reasons driving their desire are the same—health concerns and social influences.6 Even individuals with serious mental illness such as schizophrenia who have a long history of tobacco use are highly motivated and persistent in their attempts to quit.7,8 The prevalence of future “readiness to quit” among individuals diagnosed with schizophrenia and depression ranges from 21% to 49%, which is similar to that among the general population (26% to 41%). Evidence also suggests that motivation translates into successful quitting, with quit rates of up to 22% for people with mental illness who use a combination of psychosocial and pharmacological interventions.9
3. What is the patient’s mental health status? What is the patient’s psychiatric diagnosis and how clinically stable is he or she? What is his or her suicide risk? Consider using the Columbia Suicide Severity Rating Scale (C-SSRS).10
In the EAGLES trial, the psychiatric cohort included only patients who had been clinically stable for the past 6 months and had received the same medication regimen for at least the past 3 months, with no expected changes for 12 weeks. Patients with certain diagnoses were excluded (eg, delusional disorder, schizophreniform disorder, impulse control disorders), and only 1% had a personality disorder, which increases mood lability and likelihood of suicidality behavior.
Continue to: Does the patient have another comorbid substance use disorder?
4. Does the patient have another comorbid substance use disorder? In the EAGLES trial, those who had active substance use in the past year or were receiving methadone or buprenorphine/naloxone were excluded.
5. Does the patient have any medical conditions? Does he or she have a history of seizures or eating disorders? It is important to determine if a patient has a seizure disorder or another medical condition that is a contraindication for using varenicline or bupropion.
I
6. Have you discussed smoking cessation and a treatment plan with the patient at every visit? In the EAGLES trial, participants received 10-minute cessation counseling at every outpatient visit.
Continue to: When it comes time to select a medication regimen...
When it comes time to select a medication regimen, for bupropion, consider starting the patient with 150 mg/d, and increasing the dose to 150 mg twice a day 4 days later. The target quit date should be 7 days after starting the medication. Monitor the patient for symptoms of anxiety and insomnia.
For varenicline, start the patient at 0.5 mg/d, and increase the dose to 0.5 mg twice a day 4 days later. After another 4 days, increase the dose to 1 mg twice a day. Set a target quit date for 7 days after starting medication. Monitor the patient for nausea, insomnia, and abnormal dreams.
1. Khaled SM, Bulloch AG, Williams JV, et al. Persistent heavy smoking as risk factor for major depression (MD) incidence--evidence from a longitudinal Canadian cohort of the National Population Health Survey. J Psychiatr Res. 2012;46(4):436-443.
2. Han B, Compton WM, Blanco C. Tobacco use and 12-month suicidality among adults in the United States. Nicotine Tob Res. 2017;19(1):39-48.
3. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520.
4. FDA Drug Safety Communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/Drugs/DrugSafety/ucm532221.htm. Accessed April 16, 2018.
5. Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström Test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127.
6. Moeller‐Saxone K. Cigarette smoking and interest in quitting among consumers at a psychiatric disability rehabilitation and support service in Victoria. Aust N Z J Public Health. 2008;32(5):479-481.
7. Evins AE, Cather C, Rigotti NA, et al. Two-year follow-up of a smoking cessation trial in patients with schizophrenia: increased rates of smoking cessation and reduction. J Clin Psychiatry. 2004;65(3):307-311; quiz 452-453.
8. Weiner E, Ahmed S. Smoking cessation in schizophrenia. Cur Psychiatry Rev. 2013;9(2):164-172.
9. Banham L, Gilbody S. Smoking cessation in severe mental illness: what works? Addiction. 2010;105(7):1176-1189.
10. Posner K, Brent D, Lucas C, et al. Columbia-Suicide Severity Rating Scale (C-SSRS). The Research Foundation for Mental Hygiene, Inc. http://cssrs.columbia.edu/wp-content/uploads/C-SSRS_Pediatric-SLC_11.14.16.pdf. Updated June 23, 2010. Accessed April 26, 2018.
Psychiatrists often fail to adequately address their patients’ smoking, and often underestimate the impact of ongoing tobacco use. Evidence suggests that heavy smoking is a risk factor for major depressive disorder; it also is associated with increased suicidal ideations and attempts.1,2 Tobacco use also has a mood-altering impact that can change the trajectory of mental illness, and alters the metabolism of most psychotropics.
Previously, psychiatrists may have been reluctant to prescribe the most effective interventions for smoking cessation—varenicline and bupropion—because these medications carried an FDA “black-box” warning of neuropsychiatric adverse effects, including increased aggression and suicidality. However,
The EAGLES trial was a large, multi-site global trial that included patients with and without mental illness. Its primary objective was to assess the risk of “clinically significant” adverse effects for individuals receiving varenicline, bupropion, nicotine replacement therapy (NRT), or placebo, and whether having a history of psychiatric conditions increased the risk of developing adverse effects when taking these therapies. Overall, 2% of smokers without mental illness experienced adverse effects, compared with 5% to 7% in the psychiatric cohort, regardless of treatment arm. The rate of neuropsychiatric events and scores on suicide severity scales were similar across treatment arms in both cohorts.3
We should take lessons from the EAGLES trial. We propose that clinicians ask themselves the following 6 questions when forming a treatment plan to address their patients’ tobacco use:
1. Does the patient meet DSM-5 criteria for nicotine use disorder and, if yes, what is the severity of his or her nicotine dependence? The Fagerstrom Test for Nicotine Dependence (FTND)5 is a 6-question instrument for evaluating the quantity of cigarette consumption, compulsion to use, and dependence. It provides clinicians with guidelines on preventing withdrawal by implementing NRTs, such as lozenges, an inhaler, patches, and/or gum. A score of 1 to 2 (low dependence) indicates that no NRT is needed; a score of 3 to 4 (low to moderate dependence) requires 1 NRT; and scores of 5 to 7 (moderate dependence) and ≥8 (high dependence) require a combination of NRTs.
In the EAGLES trial, all participants smoked at least 10 cigarettes per day, and had moderate dependence, with an average FTND score of 5 to 6.
2. What stage of change is the patient in, and how many times has he or she attempted to quit? Based on the answers, motivational interviewing may be appropriate.
Continue to: In the EAGLES trial...
In the EAGLES trial, the participants were motivated individuals who had on average 3 past quit attempts. Research suggests that even patients who have a serious mental illness can be motivated to quit (Box).6-9
Box
Mental illness and motivation to quit smoking
In the past, clinicians may have believed that many individuals with mental illness typically weren’t motivated to quit smoking. We now know this is not the case and that such patients’ motivation is similar to that of the general population, and the reasons driving their desire are the same—health concerns and social influences.6 Even individuals with serious mental illness such as schizophrenia who have a long history of tobacco use are highly motivated and persistent in their attempts to quit.7,8 The prevalence of future “readiness to quit” among individuals diagnosed with schizophrenia and depression ranges from 21% to 49%, which is similar to that among the general population (26% to 41%). Evidence also suggests that motivation translates into successful quitting, with quit rates of up to 22% for people with mental illness who use a combination of psychosocial and pharmacological interventions.9
3. What is the patient’s mental health status? What is the patient’s psychiatric diagnosis and how clinically stable is he or she? What is his or her suicide risk? Consider using the Columbia Suicide Severity Rating Scale (C-SSRS).10
In the EAGLES trial, the psychiatric cohort included only patients who had been clinically stable for the past 6 months and had received the same medication regimen for at least the past 3 months, with no expected changes for 12 weeks. Patients with certain diagnoses were excluded (eg, delusional disorder, schizophreniform disorder, impulse control disorders), and only 1% had a personality disorder, which increases mood lability and likelihood of suicidality behavior.
Continue to: Does the patient have another comorbid substance use disorder?
4. Does the patient have another comorbid substance use disorder? In the EAGLES trial, those who had active substance use in the past year or were receiving methadone or buprenorphine/naloxone were excluded.
5. Does the patient have any medical conditions? Does he or she have a history of seizures or eating disorders? It is important to determine if a patient has a seizure disorder or another medical condition that is a contraindication for using varenicline or bupropion.
I
6. Have you discussed smoking cessation and a treatment plan with the patient at every visit? In the EAGLES trial, participants received 10-minute cessation counseling at every outpatient visit.
Continue to: When it comes time to select a medication regimen...
When it comes time to select a medication regimen, for bupropion, consider starting the patient with 150 mg/d, and increasing the dose to 150 mg twice a day 4 days later. The target quit date should be 7 days after starting the medication. Monitor the patient for symptoms of anxiety and insomnia.
For varenicline, start the patient at 0.5 mg/d, and increase the dose to 0.5 mg twice a day 4 days later. After another 4 days, increase the dose to 1 mg twice a day. Set a target quit date for 7 days after starting medication. Monitor the patient for nausea, insomnia, and abnormal dreams.
Psychiatrists often fail to adequately address their patients’ smoking, and often underestimate the impact of ongoing tobacco use. Evidence suggests that heavy smoking is a risk factor for major depressive disorder; it also is associated with increased suicidal ideations and attempts.1,2 Tobacco use also has a mood-altering impact that can change the trajectory of mental illness, and alters the metabolism of most psychotropics.
Previously, psychiatrists may have been reluctant to prescribe the most effective interventions for smoking cessation—varenicline and bupropion—because these medications carried an FDA “black-box” warning of neuropsychiatric adverse effects, including increased aggression and suicidality. However,
The EAGLES trial was a large, multi-site global trial that included patients with and without mental illness. Its primary objective was to assess the risk of “clinically significant” adverse effects for individuals receiving varenicline, bupropion, nicotine replacement therapy (NRT), or placebo, and whether having a history of psychiatric conditions increased the risk of developing adverse effects when taking these therapies. Overall, 2% of smokers without mental illness experienced adverse effects, compared with 5% to 7% in the psychiatric cohort, regardless of treatment arm. The rate of neuropsychiatric events and scores on suicide severity scales were similar across treatment arms in both cohorts.3
We should take lessons from the EAGLES trial. We propose that clinicians ask themselves the following 6 questions when forming a treatment plan to address their patients’ tobacco use:
1. Does the patient meet DSM-5 criteria for nicotine use disorder and, if yes, what is the severity of his or her nicotine dependence? The Fagerstrom Test for Nicotine Dependence (FTND)5 is a 6-question instrument for evaluating the quantity of cigarette consumption, compulsion to use, and dependence. It provides clinicians with guidelines on preventing withdrawal by implementing NRTs, such as lozenges, an inhaler, patches, and/or gum. A score of 1 to 2 (low dependence) indicates that no NRT is needed; a score of 3 to 4 (low to moderate dependence) requires 1 NRT; and scores of 5 to 7 (moderate dependence) and ≥8 (high dependence) require a combination of NRTs.
In the EAGLES trial, all participants smoked at least 10 cigarettes per day, and had moderate dependence, with an average FTND score of 5 to 6.
2. What stage of change is the patient in, and how many times has he or she attempted to quit? Based on the answers, motivational interviewing may be appropriate.
Continue to: In the EAGLES trial...
In the EAGLES trial, the participants were motivated individuals who had on average 3 past quit attempts. Research suggests that even patients who have a serious mental illness can be motivated to quit (Box).6-9
Box
Mental illness and motivation to quit smoking
In the past, clinicians may have believed that many individuals with mental illness typically weren’t motivated to quit smoking. We now know this is not the case and that such patients’ motivation is similar to that of the general population, and the reasons driving their desire are the same—health concerns and social influences.6 Even individuals with serious mental illness such as schizophrenia who have a long history of tobacco use are highly motivated and persistent in their attempts to quit.7,8 The prevalence of future “readiness to quit” among individuals diagnosed with schizophrenia and depression ranges from 21% to 49%, which is similar to that among the general population (26% to 41%). Evidence also suggests that motivation translates into successful quitting, with quit rates of up to 22% for people with mental illness who use a combination of psychosocial and pharmacological interventions.9
3. What is the patient’s mental health status? What is the patient’s psychiatric diagnosis and how clinically stable is he or she? What is his or her suicide risk? Consider using the Columbia Suicide Severity Rating Scale (C-SSRS).10
In the EAGLES trial, the psychiatric cohort included only patients who had been clinically stable for the past 6 months and had received the same medication regimen for at least the past 3 months, with no expected changes for 12 weeks. Patients with certain diagnoses were excluded (eg, delusional disorder, schizophreniform disorder, impulse control disorders), and only 1% had a personality disorder, which increases mood lability and likelihood of suicidality behavior.
Continue to: Does the patient have another comorbid substance use disorder?
4. Does the patient have another comorbid substance use disorder? In the EAGLES trial, those who had active substance use in the past year or were receiving methadone or buprenorphine/naloxone were excluded.
5. Does the patient have any medical conditions? Does he or she have a history of seizures or eating disorders? It is important to determine if a patient has a seizure disorder or another medical condition that is a contraindication for using varenicline or bupropion.
I
6. Have you discussed smoking cessation and a treatment plan with the patient at every visit? In the EAGLES trial, participants received 10-minute cessation counseling at every outpatient visit.
Continue to: When it comes time to select a medication regimen...
When it comes time to select a medication regimen, for bupropion, consider starting the patient with 150 mg/d, and increasing the dose to 150 mg twice a day 4 days later. The target quit date should be 7 days after starting the medication. Monitor the patient for symptoms of anxiety and insomnia.
For varenicline, start the patient at 0.5 mg/d, and increase the dose to 0.5 mg twice a day 4 days later. After another 4 days, increase the dose to 1 mg twice a day. Set a target quit date for 7 days after starting medication. Monitor the patient for nausea, insomnia, and abnormal dreams.
1. Khaled SM, Bulloch AG, Williams JV, et al. Persistent heavy smoking as risk factor for major depression (MD) incidence--evidence from a longitudinal Canadian cohort of the National Population Health Survey. J Psychiatr Res. 2012;46(4):436-443.
2. Han B, Compton WM, Blanco C. Tobacco use and 12-month suicidality among adults in the United States. Nicotine Tob Res. 2017;19(1):39-48.
3. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520.
4. FDA Drug Safety Communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/Drugs/DrugSafety/ucm532221.htm. Accessed April 16, 2018.
5. Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström Test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127.
6. Moeller‐Saxone K. Cigarette smoking and interest in quitting among consumers at a psychiatric disability rehabilitation and support service in Victoria. Aust N Z J Public Health. 2008;32(5):479-481.
7. Evins AE, Cather C, Rigotti NA, et al. Two-year follow-up of a smoking cessation trial in patients with schizophrenia: increased rates of smoking cessation and reduction. J Clin Psychiatry. 2004;65(3):307-311; quiz 452-453.
8. Weiner E, Ahmed S. Smoking cessation in schizophrenia. Cur Psychiatry Rev. 2013;9(2):164-172.
9. Banham L, Gilbody S. Smoking cessation in severe mental illness: what works? Addiction. 2010;105(7):1176-1189.
10. Posner K, Brent D, Lucas C, et al. Columbia-Suicide Severity Rating Scale (C-SSRS). The Research Foundation for Mental Hygiene, Inc. http://cssrs.columbia.edu/wp-content/uploads/C-SSRS_Pediatric-SLC_11.14.16.pdf. Updated June 23, 2010. Accessed April 26, 2018.
1. Khaled SM, Bulloch AG, Williams JV, et al. Persistent heavy smoking as risk factor for major depression (MD) incidence--evidence from a longitudinal Canadian cohort of the National Population Health Survey. J Psychiatr Res. 2012;46(4):436-443.
2. Han B, Compton WM, Blanco C. Tobacco use and 12-month suicidality among adults in the United States. Nicotine Tob Res. 2017;19(1):39-48.
3. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520.
4. FDA Drug Safety Communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov/Drugs/DrugSafety/ucm532221.htm. Accessed April 16, 2018.
5. Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström Test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119-1127.
6. Moeller‐Saxone K. Cigarette smoking and interest in quitting among consumers at a psychiatric disability rehabilitation and support service in Victoria. Aust N Z J Public Health. 2008;32(5):479-481.
7. Evins AE, Cather C, Rigotti NA, et al. Two-year follow-up of a smoking cessation trial in patients with schizophrenia: increased rates of smoking cessation and reduction. J Clin Psychiatry. 2004;65(3):307-311; quiz 452-453.
8. Weiner E, Ahmed S. Smoking cessation in schizophrenia. Cur Psychiatry Rev. 2013;9(2):164-172.
9. Banham L, Gilbody S. Smoking cessation in severe mental illness: what works? Addiction. 2010;105(7):1176-1189.
10. Posner K, Brent D, Lucas C, et al. Columbia-Suicide Severity Rating Scale (C-SSRS). The Research Foundation for Mental Hygiene, Inc. http://cssrs.columbia.edu/wp-content/uploads/C-SSRS_Pediatric-SLC_11.14.16.pdf. Updated June 23, 2010. Accessed April 26, 2018.
Bipolar disorder: How to avoid overdiagnosis
Over the past decade, bipolar disorder (BD) has gained widespread recognition in mainstream culture and in the media,1 and awareness of this condition has increased substantially. As a result, patients commonly present with preconceived ideas about bipolarity that may or may not actually correspond with this diagnosis. In anticipation of seeing such patients, I offer 4 recommendations to help clinicians more accurately diagnose BD.
1. Screen for periods of manic or hypomanic mood. Effective screening questions include:
- “Have you ever had periods when you felt too happy, too angry, or on top of the world for several days in a row?”
- “Have you had periods when you would go several days without much sleep and still feel fine during the day?”
If the patient reports irritability rather than euphoria, try to better understand the phenomenology of his or her irritable mood. Among patients who experience mania, irritability often results from impatience, which in turn seems to be secondary to grandiosity, increased energy, and accelerated thought processes.2
2. Avoid using terms with low specificity, such as “mood swings” and “racing thoughts,” when you screen for manic symptoms. If the patient mentions these phrases, do not take them at face value; ask him or her to characterize them in detail. Differentiate chronic, quick fluctuations in affect—which are usually triggered by environmental factors and typically are reported by patients with personality disorders—from more persistent periods of mood polarization. Similarly, anxious patients commonly report having “racing thoughts.”
3. Distinguish patients who have a chronic, ongoing preoccupation with shopping from those who exhibit intermittent periods of excessive shopping and prodigality, which usually are associated with other manic symptoms.3 Spending money in excess is often cited as a classic symptom of mania or hypomania, but it may be an indicator of other conditions, such as compulsive buying.
4. Ask about any increases in goal-directed activity. This is a good way to identify true manic or hypomanic periods. Patients with anxiety or agitated depression may report an increase in psychomotor activity, but this is usually characterized more by restlessness and wandering, and not by a true increase in activity.
Consider a temporary diagnosis
When in doubt, it may be advisable to establish a temporary diagnosis of unspecified mood disorder, until you can learn more about the patient, obtain collateral information from family or friends, and request past medical records.
1. Ghouse AA, Sanches M, Zunta-Soares G, et al. Overdiagnosis of bipolar disorder: a critical analysis of the literature. Scientific World Journal. 2013;2013:297087. doi: 10.1155/2013/297087.
2. Carlat DJ. My favorite tips for sorting out diagnostic quandaries with bipolar disorder and adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2007;30(2):233-238.
3. Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6(1):14-18.
Over the past decade, bipolar disorder (BD) has gained widespread recognition in mainstream culture and in the media,1 and awareness of this condition has increased substantially. As a result, patients commonly present with preconceived ideas about bipolarity that may or may not actually correspond with this diagnosis. In anticipation of seeing such patients, I offer 4 recommendations to help clinicians more accurately diagnose BD.
1. Screen for periods of manic or hypomanic mood. Effective screening questions include:
- “Have you ever had periods when you felt too happy, too angry, or on top of the world for several days in a row?”
- “Have you had periods when you would go several days without much sleep and still feel fine during the day?”
If the patient reports irritability rather than euphoria, try to better understand the phenomenology of his or her irritable mood. Among patients who experience mania, irritability often results from impatience, which in turn seems to be secondary to grandiosity, increased energy, and accelerated thought processes.2
2. Avoid using terms with low specificity, such as “mood swings” and “racing thoughts,” when you screen for manic symptoms. If the patient mentions these phrases, do not take them at face value; ask him or her to characterize them in detail. Differentiate chronic, quick fluctuations in affect—which are usually triggered by environmental factors and typically are reported by patients with personality disorders—from more persistent periods of mood polarization. Similarly, anxious patients commonly report having “racing thoughts.”
3. Distinguish patients who have a chronic, ongoing preoccupation with shopping from those who exhibit intermittent periods of excessive shopping and prodigality, which usually are associated with other manic symptoms.3 Spending money in excess is often cited as a classic symptom of mania or hypomania, but it may be an indicator of other conditions, such as compulsive buying.
4. Ask about any increases in goal-directed activity. This is a good way to identify true manic or hypomanic periods. Patients with anxiety or agitated depression may report an increase in psychomotor activity, but this is usually characterized more by restlessness and wandering, and not by a true increase in activity.
Consider a temporary diagnosis
When in doubt, it may be advisable to establish a temporary diagnosis of unspecified mood disorder, until you can learn more about the patient, obtain collateral information from family or friends, and request past medical records.
Over the past decade, bipolar disorder (BD) has gained widespread recognition in mainstream culture and in the media,1 and awareness of this condition has increased substantially. As a result, patients commonly present with preconceived ideas about bipolarity that may or may not actually correspond with this diagnosis. In anticipation of seeing such patients, I offer 4 recommendations to help clinicians more accurately diagnose BD.
1. Screen for periods of manic or hypomanic mood. Effective screening questions include:
- “Have you ever had periods when you felt too happy, too angry, or on top of the world for several days in a row?”
- “Have you had periods when you would go several days without much sleep and still feel fine during the day?”
If the patient reports irritability rather than euphoria, try to better understand the phenomenology of his or her irritable mood. Among patients who experience mania, irritability often results from impatience, which in turn seems to be secondary to grandiosity, increased energy, and accelerated thought processes.2
2. Avoid using terms with low specificity, such as “mood swings” and “racing thoughts,” when you screen for manic symptoms. If the patient mentions these phrases, do not take them at face value; ask him or her to characterize them in detail. Differentiate chronic, quick fluctuations in affect—which are usually triggered by environmental factors and typically are reported by patients with personality disorders—from more persistent periods of mood polarization. Similarly, anxious patients commonly report having “racing thoughts.”
3. Distinguish patients who have a chronic, ongoing preoccupation with shopping from those who exhibit intermittent periods of excessive shopping and prodigality, which usually are associated with other manic symptoms.3 Spending money in excess is often cited as a classic symptom of mania or hypomania, but it may be an indicator of other conditions, such as compulsive buying.
4. Ask about any increases in goal-directed activity. This is a good way to identify true manic or hypomanic periods. Patients with anxiety or agitated depression may report an increase in psychomotor activity, but this is usually characterized more by restlessness and wandering, and not by a true increase in activity.
Consider a temporary diagnosis
When in doubt, it may be advisable to establish a temporary diagnosis of unspecified mood disorder, until you can learn more about the patient, obtain collateral information from family or friends, and request past medical records.
1. Ghouse AA, Sanches M, Zunta-Soares G, et al. Overdiagnosis of bipolar disorder: a critical analysis of the literature. Scientific World Journal. 2013;2013:297087. doi: 10.1155/2013/297087.
2. Carlat DJ. My favorite tips for sorting out diagnostic quandaries with bipolar disorder and adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2007;30(2):233-238.
3. Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6(1):14-18.
1. Ghouse AA, Sanches M, Zunta-Soares G, et al. Overdiagnosis of bipolar disorder: a critical analysis of the literature. Scientific World Journal. 2013;2013:297087. doi: 10.1155/2013/297087.
2. Carlat DJ. My favorite tips for sorting out diagnostic quandaries with bipolar disorder and adult attention-deficit hyperactivity disorder. Psychiatr Clin North Am. 2007;30(2):233-238.
3. Black DW. A review of compulsive buying disorder. World Psychiatry. 2007;6(1):14-18.
Sexual harassment and medicine
Sexual harassment hit a peak of cultural awareness over the past year. Will medicine be the next field to experience a reckoning?
In 2017, Time magazine’s Person of the Year Award went to the Silence Breakers who spoke out against sexual assault and harassment.1 The exposure of predatory behavior exhibited by once-celebrated movie producers, newscasters, and actors has given rise to a powerful change. The #MeToo movement has risen to support survivors and end sexual violence.
Just like show business, other industries have rich histories of discrimination and power. Think Wall Street, Silicon Valley, hospitality services, and the list goes on and on.2 But what about medicine? To answer this question, this article aims to:
- review the dilemma
- explore our duty to our patients and each other
- discuss solutions to address the problem.
Sexual harassment: A brief history
Decades ago, Anita Hill accused U.S. Supreme Court nominee Clarence Thomas, her boss at the U.S. Department of Education and the Equal Employment Opportunity Commission (EEOC), of sexual harassment.3
The year was 1991, and President George H. W. Bush had nominated Thomas, a federal Circuit Judge, to succeed retiring Associate Supreme Court Justice Thurgood Marshall. With Thomas’s good character presented as a primary qualification, he appeared to be a sure thing.
Continue to: That was until an FBI interview...
That was until an FBI interview of Hill was leaked to the press. Hill asserted that Thomas had sexually harassed her while he was her supervisor at the Department of Education and the EEOC.4 Heavily scrutinized for her choice to follow Thomas to a second job after he had already allegedly harassed her, Hill was in a conundrum shared by many women—putting up with abuse in exchange for a reputable position and the opportunity to fulfill a career ambition.
Hill is a trailblazer for women yearning to speak the truth, and she brought national attention to sexual harassment in the early 1990s. On December 16, 2017, the Commission on Sexual Harassment and Advancing Equality in the Workplace was formed. Hill was selected to lead the charge against sexual harassment in the entertainment industry.5
A forensic assessment of harassment
Hill’s courageous story is one of many touched upon in the 2016 book Because of Sex.6 Author Gillian Thomas, a senior staff attorney with the American Civil Liberties Union’s Women’s Rights Project, explores how Title VII of the Civil Rights Act of 1964 made it illegal to discriminate “because of sex.”
The field of forensic psychiatry has long been attentive to themes of sexual harassment and discrimination. The American Academy of Psychiatry and Law has a robust list of landmark cases thought to be especially important and significant for forensic psychiatry.7 This list includes cases brought forth by tenacious, yet ordinary women who used the law to advocate, and some have taken their fight all the way to the Supreme Court. Let’s consider 2 such cases:
Meritor Savings Bank, FSB v Vinson (1986).8 This was a U.S. labor law case. Michelle Vinson rose through the ranks at Meritor Savings Bank, only to be fired for excessive sick leave. She filed a Title VII suit against the bank. Vinson alleged that the bank was liable for sexual harassment perpetrated by its employee and vice president, Sidney Taylor. Vinson claimed that there had been 40 to 50 sexual encounters over 4 years, ranging from fondling to indecent exposure to rape. Vinson asserted that she never reported these events for fear of losing her job. The Supreme Court, in a 9-to-0 decision, recognized sexual harassment as a violation of Title VII of the Civil Rights Act of 1964.
Continue to: Harris v Forklift Systems, Inc. (1993)
Harris v Forklift Systems, Inc. (1993).9 Teresa Harris, a manager at Forklift Systems, Inc., claimed that the company’s president frequently directed offensive remarks at her that were sexual and discriminatory. The Supreme Court clarified the definition of a “hostile” or “abusive” work environment under Title VII of the Civil Rights Act of 1964. Associate Justice Sandra Day O’Connor was joined by a unanimous majority opinion in agreement with Harris.
Physicians are not immune
Clinicians are affected by sexual harassment, too. We have a duty to protect our patients, colleagues, and ourselves. Psychiatrists in particular often are on the frontlines of helping victims process their trauma.10
But will the field of medicine also face a reckoning when it comes to perpetrating harassment? It seems likely that the medical field would be ripe with harassment when you consider its history of male domination and a hierarchical structure with strong power differentials—not to mention the late nights, exhaustion, easy access to beds, and late-night encounters where inhibitions may be lowered.11
A shocking number of female doctors are sexually harassed. Thirty percent of the top female clinician-researchers have experienced blatant sexual harassment on the job, according to a survey of 573 men and 493 women who received career development awards from the National Institutes of Health in 2006 to 2009.12 In this survey, harassment covered the scope of sexist remarks or behavior, unwanted sexual advances, bribery, threats, and coercion. The majority of those affected said the experience undermined their confidence as professionals, and many said the harassment negatively affected their career advancement.12
Continue to: But what about the progress women have made...
But what about the progress women have made in medicine? Women are surpassing men in terms of admittance to medical school. Last year, for the first time, women accounted for more than half of the enrollees in U.S. medical schools, according to the Association of American Medical Colleges.13 Yet there has been a stalling in terms of change when it comes to harassment.12 Women may be more vulnerable to harassment, both when they’re perceived as weak and when they’re so strong that they challenge traditional hierarchies.
Perpetuating the problem is the trouble with reporting sexual harassment. Victims do not fare well in our society. Even in the #MeToo era, reporting such behavior is far from straightforward.11 Women fear that reporting any harassment will make them a target. Think of Anita Hill—her testimony against Clarence Thomas during his confirmation hearings for the Supreme Court showed that women who report sexual harassment experience marginalization, retaliation, stigmatization, and worse.
The result is that medical professionals tend to suppress the recognition of harassment. We make excuses for it, blame ourselves, or just take it on the chin and move on. There’s also confusion regarding what constitutes harassment. As doctors, especially psychiatrists, we hear harrowing stories. It’s reasonable to downplay our own experiences. Turning everyone into a victim of sexual harassment could detract from the stories of women who were raped, molested, and severely taken advantage of. There is a reasonable fear that diluting their message could be further damaging.14
Time for action
The field of medicine needs to do better in terms of education, support, anticipation, prevention, and reaction to harassment. We have the awareness. Now, we need action.
Continue to: One way to change any culture...
One way to change any culture of harassment or discrimination would be the advancement of more female physicians into leadership positions. The Association of American Medical Colleges has reported that fewer women than men hold faculty positions and full professorships.15,16 There’s also a striking imbalance among fields of medicine practiced by men and women, with more women seen in pediatrics, obstetrics, and gynecology as opposed to surgery. Advancement into policy-setting echelons of medicine is essential for change. Sexual harassment can be a silent problem that will be corrected only when institutions and leaders put it on the forefront of discussions.17
Another possible solution would be to shift problem-solving from punishment to prevention. Many institutions set expectations about intolerance of sexual harassment and conduct occasional lectures about it. However, enforcing protocols and safeguards that support and enforce policy are difficult on the ground level. In any event, punishment alone won’t change a culture.17
Working with students until they are comfortable disclosing details of incidents can be helpful. For example, the University of Wisconsin-Madison employs an ombuds to help with this process.18 All institutions should encourage reporting along confidential pathways and have multiple ways to report.17 Tracking complaints, even seemingly minor infractions, can help identify patterns of behavior and anticipate future incidents.
Some solutions seem obvious, such as informal and retaliation-free reporting that allows institutions to track perpetrators’ behavior; mandatory training that includes bystander training; and disciplining and monitoring transgressors and terminating their employment when appropriate—something along the lines of a zero-tolerance policy. There needs to be more research on the prevalence, severity, and outcomes of sexual harassment, and subsequent investigations, along with research into evidence-based prevention and intervention strategies.17
Continue to: Although this article focuses...
Although this article focuses on harassment of women, men are equally important to this conversation because they, too, can be victims. Men also can serve a pivotal role in mentoring and championing their female counterparts as they strive for advancement, equality, and respect.
The task ahead is large, and this discussion is not over.
1. Felsenthal E. TIME’s 2017 Person of the Year: the Silence Breakers. TIME. http://time.com/magazine/us/5055335/december-18th-2017-vol-190-no-25-u-s/. Published December 18, 2017. Accessed April 23, 2018.
2. Hiltzik M. Los Angeles Times. Will medicine be the next field to face a sexual harassment reckoning? http://www.latimes.com/business/hiltzik/la-fi-hiltzik-medicine-harassment-20180110-story.html. Published January 10, 2018. Accessed April 23, 2018.
3. Thompson K. For Anita Hill, the Clarence Thomas hearings haven’t really ended. The Washington Post. https://www.washingtonpost.com/politics/for-anita-hill-the-clarence-thomas-hearings-havent-really-ended/2011/10/05/gIQAy2b5QL_story.html. Published October 6, 2011. Accessed April 23, 2018.
4. Toobin J. Good versus evil. In: Toobin J. The nine: inside the secret world of the Supreme Court. New York, NY: Doubleday; 2007:30-32.
5. Barnes B. Motion picture academy finds no merit to accusations against its president. https://www.nytimes.com/2018/03/28/business/media/john-bailey-sexual-harassment-academy.html. The New York Times. Published March 28, 2018. Accessed April 23, 2018.
6. Thomas G. Because of sex: one law, ten cases, and fifty years that changed American women’s lives at work. New York, NY: Picador; 2016.
7. Landmark cases 2014. American Academy of Psychiatry and Law. http://www.aapl.org/landmark_list.htm. 2014. Accessed April 22, 2018.
8. Meritor Savings Bank v Vinson, 477 US 57 (1986).
9. Harris v Forklift Systems, Inc., 114 S Ct 367 (1993).
10. Okwerekwu JA. #MeToo: so many of my patients have a story. And absorbing them is taking its toll. STAT. https://www.scribd.com/article/367482959/Me-Too-So-Many-Of-My-Patients-Have-A-Story-And-Absorbing-Them-Is-Taking-Its-Toll. Published December 18, 2017. Accessed April 23, 2018.
11. Jagsi R. Sexual harassment in medicine—#MeToo. N Engl J Med. 2018;378:209-211.
12. Jagsi R, Griffith KA, Jones R. et al. Sexual harassment and discrimination experiences of academic medical faculty. JAMA. 2016;315(19):2120-2121.
13. AAMCNEWS. More women than men enrolled in U.S. medical schools in 2017. https://news.aamc.org/press-releases/article/applicant-enrollment-2017/. Published December 18, 2017. Accessed May 4, 2018.
14. Miller D. #MeToo: does it help? Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/150148/depression/metoo-does-it-help. Published October 24, 2017. Accessed April 23, 2018.
15. Chang S, Morahan PS, Magrane D, et al. Retaining faculty in academic medicine: the impact of career development programs for women. J Womens Health (Larchmt). 2016;25(7):687-696.
16. Lautenberger DM, Dandar, VM, Raezer CL, et al. The state of women in academic medicine: the pipeline and pathways to leadership, 2013-2014. AAMC. https://members.aamc.org/eweb/upload/The%20State%20of%20Women%20in%20Academic%20Medicine%202013-2014%20FINAL.pdf. Published 2014. Accessed May 4, 2018.
17. Jablow M. Zero tolerance: combating sexual harassment in academic medicine. AAMCNews. https://news.aamc.org/diversity/article/combating-sexual-harassment-academic-medicine. Published April 4, 2017. Accessed April 23, 2018.
18. University of Wisconsin-Madison, the School of Medicine and Public Health. UW-Madison Policy on Sexual Harassment and Sexual Violence. https://compliance.wiscweb.wisc.edu/wp-content/uploads/sites/102/2018/01/UW-Madison-Policy-on-Sexual-Harassment-And-Sexual-Violence-January-2018.pdf. Published January 2018. Accessed April 22, 2018.
Sexual harassment hit a peak of cultural awareness over the past year. Will medicine be the next field to experience a reckoning?
In 2017, Time magazine’s Person of the Year Award went to the Silence Breakers who spoke out against sexual assault and harassment.1 The exposure of predatory behavior exhibited by once-celebrated movie producers, newscasters, and actors has given rise to a powerful change. The #MeToo movement has risen to support survivors and end sexual violence.
Just like show business, other industries have rich histories of discrimination and power. Think Wall Street, Silicon Valley, hospitality services, and the list goes on and on.2 But what about medicine? To answer this question, this article aims to:
- review the dilemma
- explore our duty to our patients and each other
- discuss solutions to address the problem.
Sexual harassment: A brief history
Decades ago, Anita Hill accused U.S. Supreme Court nominee Clarence Thomas, her boss at the U.S. Department of Education and the Equal Employment Opportunity Commission (EEOC), of sexual harassment.3
The year was 1991, and President George H. W. Bush had nominated Thomas, a federal Circuit Judge, to succeed retiring Associate Supreme Court Justice Thurgood Marshall. With Thomas’s good character presented as a primary qualification, he appeared to be a sure thing.
Continue to: That was until an FBI interview...
That was until an FBI interview of Hill was leaked to the press. Hill asserted that Thomas had sexually harassed her while he was her supervisor at the Department of Education and the EEOC.4 Heavily scrutinized for her choice to follow Thomas to a second job after he had already allegedly harassed her, Hill was in a conundrum shared by many women—putting up with abuse in exchange for a reputable position and the opportunity to fulfill a career ambition.
Hill is a trailblazer for women yearning to speak the truth, and she brought national attention to sexual harassment in the early 1990s. On December 16, 2017, the Commission on Sexual Harassment and Advancing Equality in the Workplace was formed. Hill was selected to lead the charge against sexual harassment in the entertainment industry.5
A forensic assessment of harassment
Hill’s courageous story is one of many touched upon in the 2016 book Because of Sex.6 Author Gillian Thomas, a senior staff attorney with the American Civil Liberties Union’s Women’s Rights Project, explores how Title VII of the Civil Rights Act of 1964 made it illegal to discriminate “because of sex.”
The field of forensic psychiatry has long been attentive to themes of sexual harassment and discrimination. The American Academy of Psychiatry and Law has a robust list of landmark cases thought to be especially important and significant for forensic psychiatry.7 This list includes cases brought forth by tenacious, yet ordinary women who used the law to advocate, and some have taken their fight all the way to the Supreme Court. Let’s consider 2 such cases:
Meritor Savings Bank, FSB v Vinson (1986).8 This was a U.S. labor law case. Michelle Vinson rose through the ranks at Meritor Savings Bank, only to be fired for excessive sick leave. She filed a Title VII suit against the bank. Vinson alleged that the bank was liable for sexual harassment perpetrated by its employee and vice president, Sidney Taylor. Vinson claimed that there had been 40 to 50 sexual encounters over 4 years, ranging from fondling to indecent exposure to rape. Vinson asserted that she never reported these events for fear of losing her job. The Supreme Court, in a 9-to-0 decision, recognized sexual harassment as a violation of Title VII of the Civil Rights Act of 1964.
Continue to: Harris v Forklift Systems, Inc. (1993)
Harris v Forklift Systems, Inc. (1993).9 Teresa Harris, a manager at Forklift Systems, Inc., claimed that the company’s president frequently directed offensive remarks at her that were sexual and discriminatory. The Supreme Court clarified the definition of a “hostile” or “abusive” work environment under Title VII of the Civil Rights Act of 1964. Associate Justice Sandra Day O’Connor was joined by a unanimous majority opinion in agreement with Harris.
Physicians are not immune
Clinicians are affected by sexual harassment, too. We have a duty to protect our patients, colleagues, and ourselves. Psychiatrists in particular often are on the frontlines of helping victims process their trauma.10
But will the field of medicine also face a reckoning when it comes to perpetrating harassment? It seems likely that the medical field would be ripe with harassment when you consider its history of male domination and a hierarchical structure with strong power differentials—not to mention the late nights, exhaustion, easy access to beds, and late-night encounters where inhibitions may be lowered.11
A shocking number of female doctors are sexually harassed. Thirty percent of the top female clinician-researchers have experienced blatant sexual harassment on the job, according to a survey of 573 men and 493 women who received career development awards from the National Institutes of Health in 2006 to 2009.12 In this survey, harassment covered the scope of sexist remarks or behavior, unwanted sexual advances, bribery, threats, and coercion. The majority of those affected said the experience undermined their confidence as professionals, and many said the harassment negatively affected their career advancement.12
Continue to: But what about the progress women have made...
But what about the progress women have made in medicine? Women are surpassing men in terms of admittance to medical school. Last year, for the first time, women accounted for more than half of the enrollees in U.S. medical schools, according to the Association of American Medical Colleges.13 Yet there has been a stalling in terms of change when it comes to harassment.12 Women may be more vulnerable to harassment, both when they’re perceived as weak and when they’re so strong that they challenge traditional hierarchies.
Perpetuating the problem is the trouble with reporting sexual harassment. Victims do not fare well in our society. Even in the #MeToo era, reporting such behavior is far from straightforward.11 Women fear that reporting any harassment will make them a target. Think of Anita Hill—her testimony against Clarence Thomas during his confirmation hearings for the Supreme Court showed that women who report sexual harassment experience marginalization, retaliation, stigmatization, and worse.
The result is that medical professionals tend to suppress the recognition of harassment. We make excuses for it, blame ourselves, or just take it on the chin and move on. There’s also confusion regarding what constitutes harassment. As doctors, especially psychiatrists, we hear harrowing stories. It’s reasonable to downplay our own experiences. Turning everyone into a victim of sexual harassment could detract from the stories of women who were raped, molested, and severely taken advantage of. There is a reasonable fear that diluting their message could be further damaging.14
Time for action
The field of medicine needs to do better in terms of education, support, anticipation, prevention, and reaction to harassment. We have the awareness. Now, we need action.
Continue to: One way to change any culture...
One way to change any culture of harassment or discrimination would be the advancement of more female physicians into leadership positions. The Association of American Medical Colleges has reported that fewer women than men hold faculty positions and full professorships.15,16 There’s also a striking imbalance among fields of medicine practiced by men and women, with more women seen in pediatrics, obstetrics, and gynecology as opposed to surgery. Advancement into policy-setting echelons of medicine is essential for change. Sexual harassment can be a silent problem that will be corrected only when institutions and leaders put it on the forefront of discussions.17
Another possible solution would be to shift problem-solving from punishment to prevention. Many institutions set expectations about intolerance of sexual harassment and conduct occasional lectures about it. However, enforcing protocols and safeguards that support and enforce policy are difficult on the ground level. In any event, punishment alone won’t change a culture.17
Working with students until they are comfortable disclosing details of incidents can be helpful. For example, the University of Wisconsin-Madison employs an ombuds to help with this process.18 All institutions should encourage reporting along confidential pathways and have multiple ways to report.17 Tracking complaints, even seemingly minor infractions, can help identify patterns of behavior and anticipate future incidents.
Some solutions seem obvious, such as informal and retaliation-free reporting that allows institutions to track perpetrators’ behavior; mandatory training that includes bystander training; and disciplining and monitoring transgressors and terminating their employment when appropriate—something along the lines of a zero-tolerance policy. There needs to be more research on the prevalence, severity, and outcomes of sexual harassment, and subsequent investigations, along with research into evidence-based prevention and intervention strategies.17
Continue to: Although this article focuses...
Although this article focuses on harassment of women, men are equally important to this conversation because they, too, can be victims. Men also can serve a pivotal role in mentoring and championing their female counterparts as they strive for advancement, equality, and respect.
The task ahead is large, and this discussion is not over.
Sexual harassment hit a peak of cultural awareness over the past year. Will medicine be the next field to experience a reckoning?
In 2017, Time magazine’s Person of the Year Award went to the Silence Breakers who spoke out against sexual assault and harassment.1 The exposure of predatory behavior exhibited by once-celebrated movie producers, newscasters, and actors has given rise to a powerful change. The #MeToo movement has risen to support survivors and end sexual violence.
Just like show business, other industries have rich histories of discrimination and power. Think Wall Street, Silicon Valley, hospitality services, and the list goes on and on.2 But what about medicine? To answer this question, this article aims to:
- review the dilemma
- explore our duty to our patients and each other
- discuss solutions to address the problem.
Sexual harassment: A brief history
Decades ago, Anita Hill accused U.S. Supreme Court nominee Clarence Thomas, her boss at the U.S. Department of Education and the Equal Employment Opportunity Commission (EEOC), of sexual harassment.3
The year was 1991, and President George H. W. Bush had nominated Thomas, a federal Circuit Judge, to succeed retiring Associate Supreme Court Justice Thurgood Marshall. With Thomas’s good character presented as a primary qualification, he appeared to be a sure thing.
Continue to: That was until an FBI interview...
That was until an FBI interview of Hill was leaked to the press. Hill asserted that Thomas had sexually harassed her while he was her supervisor at the Department of Education and the EEOC.4 Heavily scrutinized for her choice to follow Thomas to a second job after he had already allegedly harassed her, Hill was in a conundrum shared by many women—putting up with abuse in exchange for a reputable position and the opportunity to fulfill a career ambition.
Hill is a trailblazer for women yearning to speak the truth, and she brought national attention to sexual harassment in the early 1990s. On December 16, 2017, the Commission on Sexual Harassment and Advancing Equality in the Workplace was formed. Hill was selected to lead the charge against sexual harassment in the entertainment industry.5
A forensic assessment of harassment
Hill’s courageous story is one of many touched upon in the 2016 book Because of Sex.6 Author Gillian Thomas, a senior staff attorney with the American Civil Liberties Union’s Women’s Rights Project, explores how Title VII of the Civil Rights Act of 1964 made it illegal to discriminate “because of sex.”
The field of forensic psychiatry has long been attentive to themes of sexual harassment and discrimination. The American Academy of Psychiatry and Law has a robust list of landmark cases thought to be especially important and significant for forensic psychiatry.7 This list includes cases brought forth by tenacious, yet ordinary women who used the law to advocate, and some have taken their fight all the way to the Supreme Court. Let’s consider 2 such cases:
Meritor Savings Bank, FSB v Vinson (1986).8 This was a U.S. labor law case. Michelle Vinson rose through the ranks at Meritor Savings Bank, only to be fired for excessive sick leave. She filed a Title VII suit against the bank. Vinson alleged that the bank was liable for sexual harassment perpetrated by its employee and vice president, Sidney Taylor. Vinson claimed that there had been 40 to 50 sexual encounters over 4 years, ranging from fondling to indecent exposure to rape. Vinson asserted that she never reported these events for fear of losing her job. The Supreme Court, in a 9-to-0 decision, recognized sexual harassment as a violation of Title VII of the Civil Rights Act of 1964.
Continue to: Harris v Forklift Systems, Inc. (1993)
Harris v Forklift Systems, Inc. (1993).9 Teresa Harris, a manager at Forklift Systems, Inc., claimed that the company’s president frequently directed offensive remarks at her that were sexual and discriminatory. The Supreme Court clarified the definition of a “hostile” or “abusive” work environment under Title VII of the Civil Rights Act of 1964. Associate Justice Sandra Day O’Connor was joined by a unanimous majority opinion in agreement with Harris.
Physicians are not immune
Clinicians are affected by sexual harassment, too. We have a duty to protect our patients, colleagues, and ourselves. Psychiatrists in particular often are on the frontlines of helping victims process their trauma.10
But will the field of medicine also face a reckoning when it comes to perpetrating harassment? It seems likely that the medical field would be ripe with harassment when you consider its history of male domination and a hierarchical structure with strong power differentials—not to mention the late nights, exhaustion, easy access to beds, and late-night encounters where inhibitions may be lowered.11
A shocking number of female doctors are sexually harassed. Thirty percent of the top female clinician-researchers have experienced blatant sexual harassment on the job, according to a survey of 573 men and 493 women who received career development awards from the National Institutes of Health in 2006 to 2009.12 In this survey, harassment covered the scope of sexist remarks or behavior, unwanted sexual advances, bribery, threats, and coercion. The majority of those affected said the experience undermined their confidence as professionals, and many said the harassment negatively affected their career advancement.12
Continue to: But what about the progress women have made...
But what about the progress women have made in medicine? Women are surpassing men in terms of admittance to medical school. Last year, for the first time, women accounted for more than half of the enrollees in U.S. medical schools, according to the Association of American Medical Colleges.13 Yet there has been a stalling in terms of change when it comes to harassment.12 Women may be more vulnerable to harassment, both when they’re perceived as weak and when they’re so strong that they challenge traditional hierarchies.
Perpetuating the problem is the trouble with reporting sexual harassment. Victims do not fare well in our society. Even in the #MeToo era, reporting such behavior is far from straightforward.11 Women fear that reporting any harassment will make them a target. Think of Anita Hill—her testimony against Clarence Thomas during his confirmation hearings for the Supreme Court showed that women who report sexual harassment experience marginalization, retaliation, stigmatization, and worse.
The result is that medical professionals tend to suppress the recognition of harassment. We make excuses for it, blame ourselves, or just take it on the chin and move on. There’s also confusion regarding what constitutes harassment. As doctors, especially psychiatrists, we hear harrowing stories. It’s reasonable to downplay our own experiences. Turning everyone into a victim of sexual harassment could detract from the stories of women who were raped, molested, and severely taken advantage of. There is a reasonable fear that diluting their message could be further damaging.14
Time for action
The field of medicine needs to do better in terms of education, support, anticipation, prevention, and reaction to harassment. We have the awareness. Now, we need action.
Continue to: One way to change any culture...
One way to change any culture of harassment or discrimination would be the advancement of more female physicians into leadership positions. The Association of American Medical Colleges has reported that fewer women than men hold faculty positions and full professorships.15,16 There’s also a striking imbalance among fields of medicine practiced by men and women, with more women seen in pediatrics, obstetrics, and gynecology as opposed to surgery. Advancement into policy-setting echelons of medicine is essential for change. Sexual harassment can be a silent problem that will be corrected only when institutions and leaders put it on the forefront of discussions.17
Another possible solution would be to shift problem-solving from punishment to prevention. Many institutions set expectations about intolerance of sexual harassment and conduct occasional lectures about it. However, enforcing protocols and safeguards that support and enforce policy are difficult on the ground level. In any event, punishment alone won’t change a culture.17
Working with students until they are comfortable disclosing details of incidents can be helpful. For example, the University of Wisconsin-Madison employs an ombuds to help with this process.18 All institutions should encourage reporting along confidential pathways and have multiple ways to report.17 Tracking complaints, even seemingly minor infractions, can help identify patterns of behavior and anticipate future incidents.
Some solutions seem obvious, such as informal and retaliation-free reporting that allows institutions to track perpetrators’ behavior; mandatory training that includes bystander training; and disciplining and monitoring transgressors and terminating their employment when appropriate—something along the lines of a zero-tolerance policy. There needs to be more research on the prevalence, severity, and outcomes of sexual harassment, and subsequent investigations, along with research into evidence-based prevention and intervention strategies.17
Continue to: Although this article focuses...
Although this article focuses on harassment of women, men are equally important to this conversation because they, too, can be victims. Men also can serve a pivotal role in mentoring and championing their female counterparts as they strive for advancement, equality, and respect.
The task ahead is large, and this discussion is not over.
1. Felsenthal E. TIME’s 2017 Person of the Year: the Silence Breakers. TIME. http://time.com/magazine/us/5055335/december-18th-2017-vol-190-no-25-u-s/. Published December 18, 2017. Accessed April 23, 2018.
2. Hiltzik M. Los Angeles Times. Will medicine be the next field to face a sexual harassment reckoning? http://www.latimes.com/business/hiltzik/la-fi-hiltzik-medicine-harassment-20180110-story.html. Published January 10, 2018. Accessed April 23, 2018.
3. Thompson K. For Anita Hill, the Clarence Thomas hearings haven’t really ended. The Washington Post. https://www.washingtonpost.com/politics/for-anita-hill-the-clarence-thomas-hearings-havent-really-ended/2011/10/05/gIQAy2b5QL_story.html. Published October 6, 2011. Accessed April 23, 2018.
4. Toobin J. Good versus evil. In: Toobin J. The nine: inside the secret world of the Supreme Court. New York, NY: Doubleday; 2007:30-32.
5. Barnes B. Motion picture academy finds no merit to accusations against its president. https://www.nytimes.com/2018/03/28/business/media/john-bailey-sexual-harassment-academy.html. The New York Times. Published March 28, 2018. Accessed April 23, 2018.
6. Thomas G. Because of sex: one law, ten cases, and fifty years that changed American women’s lives at work. New York, NY: Picador; 2016.
7. Landmark cases 2014. American Academy of Psychiatry and Law. http://www.aapl.org/landmark_list.htm. 2014. Accessed April 22, 2018.
8. Meritor Savings Bank v Vinson, 477 US 57 (1986).
9. Harris v Forklift Systems, Inc., 114 S Ct 367 (1993).
10. Okwerekwu JA. #MeToo: so many of my patients have a story. And absorbing them is taking its toll. STAT. https://www.scribd.com/article/367482959/Me-Too-So-Many-Of-My-Patients-Have-A-Story-And-Absorbing-Them-Is-Taking-Its-Toll. Published December 18, 2017. Accessed April 23, 2018.
11. Jagsi R. Sexual harassment in medicine—#MeToo. N Engl J Med. 2018;378:209-211.
12. Jagsi R, Griffith KA, Jones R. et al. Sexual harassment and discrimination experiences of academic medical faculty. JAMA. 2016;315(19):2120-2121.
13. AAMCNEWS. More women than men enrolled in U.S. medical schools in 2017. https://news.aamc.org/press-releases/article/applicant-enrollment-2017/. Published December 18, 2017. Accessed May 4, 2018.
14. Miller D. #MeToo: does it help? Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/150148/depression/metoo-does-it-help. Published October 24, 2017. Accessed April 23, 2018.
15. Chang S, Morahan PS, Magrane D, et al. Retaining faculty in academic medicine: the impact of career development programs for women. J Womens Health (Larchmt). 2016;25(7):687-696.
16. Lautenberger DM, Dandar, VM, Raezer CL, et al. The state of women in academic medicine: the pipeline and pathways to leadership, 2013-2014. AAMC. https://members.aamc.org/eweb/upload/The%20State%20of%20Women%20in%20Academic%20Medicine%202013-2014%20FINAL.pdf. Published 2014. Accessed May 4, 2018.
17. Jablow M. Zero tolerance: combating sexual harassment in academic medicine. AAMCNews. https://news.aamc.org/diversity/article/combating-sexual-harassment-academic-medicine. Published April 4, 2017. Accessed April 23, 2018.
18. University of Wisconsin-Madison, the School of Medicine and Public Health. UW-Madison Policy on Sexual Harassment and Sexual Violence. https://compliance.wiscweb.wisc.edu/wp-content/uploads/sites/102/2018/01/UW-Madison-Policy-on-Sexual-Harassment-And-Sexual-Violence-January-2018.pdf. Published January 2018. Accessed April 22, 2018.
1. Felsenthal E. TIME’s 2017 Person of the Year: the Silence Breakers. TIME. http://time.com/magazine/us/5055335/december-18th-2017-vol-190-no-25-u-s/. Published December 18, 2017. Accessed April 23, 2018.
2. Hiltzik M. Los Angeles Times. Will medicine be the next field to face a sexual harassment reckoning? http://www.latimes.com/business/hiltzik/la-fi-hiltzik-medicine-harassment-20180110-story.html. Published January 10, 2018. Accessed April 23, 2018.
3. Thompson K. For Anita Hill, the Clarence Thomas hearings haven’t really ended. The Washington Post. https://www.washingtonpost.com/politics/for-anita-hill-the-clarence-thomas-hearings-havent-really-ended/2011/10/05/gIQAy2b5QL_story.html. Published October 6, 2011. Accessed April 23, 2018.
4. Toobin J. Good versus evil. In: Toobin J. The nine: inside the secret world of the Supreme Court. New York, NY: Doubleday; 2007:30-32.
5. Barnes B. Motion picture academy finds no merit to accusations against its president. https://www.nytimes.com/2018/03/28/business/media/john-bailey-sexual-harassment-academy.html. The New York Times. Published March 28, 2018. Accessed April 23, 2018.
6. Thomas G. Because of sex: one law, ten cases, and fifty years that changed American women’s lives at work. New York, NY: Picador; 2016.
7. Landmark cases 2014. American Academy of Psychiatry and Law. http://www.aapl.org/landmark_list.htm. 2014. Accessed April 22, 2018.
8. Meritor Savings Bank v Vinson, 477 US 57 (1986).
9. Harris v Forklift Systems, Inc., 114 S Ct 367 (1993).
10. Okwerekwu JA. #MeToo: so many of my patients have a story. And absorbing them is taking its toll. STAT. https://www.scribd.com/article/367482959/Me-Too-So-Many-Of-My-Patients-Have-A-Story-And-Absorbing-Them-Is-Taking-Its-Toll. Published December 18, 2017. Accessed April 23, 2018.
11. Jagsi R. Sexual harassment in medicine—#MeToo. N Engl J Med. 2018;378:209-211.
12. Jagsi R, Griffith KA, Jones R. et al. Sexual harassment and discrimination experiences of academic medical faculty. JAMA. 2016;315(19):2120-2121.
13. AAMCNEWS. More women than men enrolled in U.S. medical schools in 2017. https://news.aamc.org/press-releases/article/applicant-enrollment-2017/. Published December 18, 2017. Accessed May 4, 2018.
14. Miller D. #MeToo: does it help? Clinical Psychiatry News. https://www.mdedge.com/psychiatry/article/150148/depression/metoo-does-it-help. Published October 24, 2017. Accessed April 23, 2018.
15. Chang S, Morahan PS, Magrane D, et al. Retaining faculty in academic medicine: the impact of career development programs for women. J Womens Health (Larchmt). 2016;25(7):687-696.
16. Lautenberger DM, Dandar, VM, Raezer CL, et al. The state of women in academic medicine: the pipeline and pathways to leadership, 2013-2014. AAMC. https://members.aamc.org/eweb/upload/The%20State%20of%20Women%20in%20Academic%20Medicine%202013-2014%20FINAL.pdf. Published 2014. Accessed May 4, 2018.
17. Jablow M. Zero tolerance: combating sexual harassment in academic medicine. AAMCNews. https://news.aamc.org/diversity/article/combating-sexual-harassment-academic-medicine. Published April 4, 2017. Accessed April 23, 2018.
18. University of Wisconsin-Madison, the School of Medicine and Public Health. UW-Madison Policy on Sexual Harassment and Sexual Violence. https://compliance.wiscweb.wisc.edu/wp-content/uploads/sites/102/2018/01/UW-Madison-Policy-on-Sexual-Harassment-And-Sexual-Violence-January-2018.pdf. Published January 2018. Accessed April 22, 2018.
N-acetylcysteine: A potential treatment for substance use disorders
Pharmacologic treatment options for many substance use disorders (SUDs) are limited. This is especially true for cocaine use disorder and cannabis use disorder, for which there are no FDA-approved medications. FDA-approved medications for other SUDs often take the form of replacement or agonist therapies (eg, nicotine replacement therapy) that substitute the effects of the substance to aid in cessation. Other pharmacotherapies treat symptoms of withdrawal, reduce craving, or provide aversive counter-conditioning if the patient consumes the substance while on the medication (eg, disulfiram).
The over-the-counter (OTC) antioxidant N-acetylcysteine (NAC) may be a potential treatment for SUDs. Although NAC is not approved by the FDA for treating SUDs, its proposed mechanism of action differs from that of current FDA-approved medications for SUDs. NAC’s potential for broad applicability, favorable adverse-effect profile, accessibility, and low cost make it an intriguing option for patients with multiple comorbidities, and potentially for individuals with polysubstance use. This article reviews the current evidence supporting NAC for treating SUDs, to provide insight about which patients may benefit from NAC and under which circumstances they are most likely to benefit.
NAC may correct glutamate dysregulation
Approximately 85% of individuals with an SUD do not seek treatment for it, and those who do are older, have a longer history of use, have more severe dependence, and have sought treatment numerous times before.1 By the time most people seek treatment, years of chronic substance use have likely led to significant brain-related adaptations. Individuals with SUDs often indicate that their substance use began as a pleasurable activity—the effects of the drug were enjoyable and they were motivated to use it again. With repeated substance use, they may begin to develop a stronger urge to use the drug, driven not necessarily by a desire for pleasure, but by compulsion.2
Numerous neural adaptations underlie the transition from “liking” a substance to engaging in the compulsive use that is characteristic of an SUD.2 For example, repeated use of an addictive substance may result in excess glutamate in the nucleus accumbens,3,4 an area of the brain that plays a critical role in motivation and learning. As a result, it has been proposed that pharmacotherapies that help correct glutamate dysregulation may be effective in promoting abstinence or preventing relapse to a substance.5,6
NAC may reverse the neural dysfunction seen in SUDs. As an OTC antioxidant that impacts glutamatergic functioning in the brain, NAC has long been used to treat acetaminophen overdose; however, in recent years, researchers have begun to tap its potential for treating substance use and psychiatric disorders. NAC is thought to upregulate the glutamate transporter (GLT-1) that removes excess glutamate from the nucleus accumbens.6 Several published reviews provide more in-depth information about the neurobiology of NAC.6-10
The adverse-effect profile of NAC is relatively benign. Nausea, vomiting, diarrhea, and sleepiness are relatively infrequent and mild.11,12 The bioavailability of NAC is about 4% to 9%, with an approximate half-life of 6.25 hours when orally administered.13 Because NAC is classified as an OTC supplement, the potency and preparation may vary by supplier. To maximize consistency, NAC should be obtained from a supplier that meets United States Pharmacopeia (USP) standards.
NAC for SUDs: Emerging evidence
Several recent reviews have described the efficacy of NAC for SUDs and other psychiatric disorders. Here we summarize the current research examining the efficacy of NAC for stimulant (ie, cocaine and methamphetamine), cannabis, tobacco, and alcohol use disorders.
Continue to: Stimulant use disorders
Stimulant use disorders. The United Nations Office for Drugs and Crime estimates that worldwide, more than 18 million people use cocaine and more than 35 million use amphetamines.14 There are currently no FDA-approved treatments for stimulant use disorders, and clinicians treating patients with cocaine or amphetamine dependence often are at a loss for how best to promote abstinence. Recent studies suggest that NAC may decrease drug-seeking behavior and cravings in adults who seek treatment. The results of studies examining NAC for treating cocaine use and methamphetamine use are summarized in Table 115-17 and Table 2,18,19 respectively.
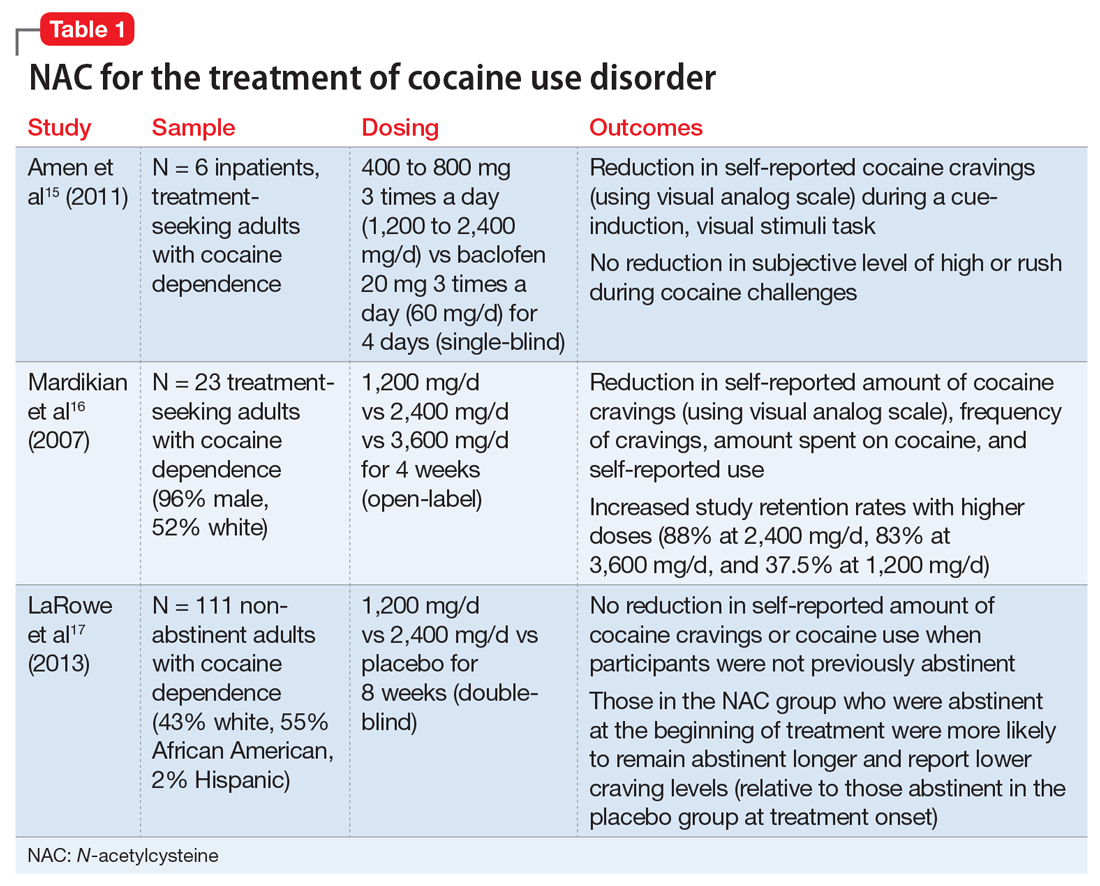
Cocaine cessation and relapse prevention. Several small pilot projects15,16 found that compared with placebo, various doses of NAC reduced craving (as measured with a visual analog scale). However, in a double-blind, placebo-controlled study, NAC did not decrease cravings or use after 8 weeks of treatment in individuals with cocaine use disorder who were still using cocaine (ie, they had not yet become abstinent). Interestingly, those who were abstinent when treatment began reported lower craving and remained abstinent longer if they received NAC (vs placebo), which suggests that NAC may be useful for preventing relapse.17
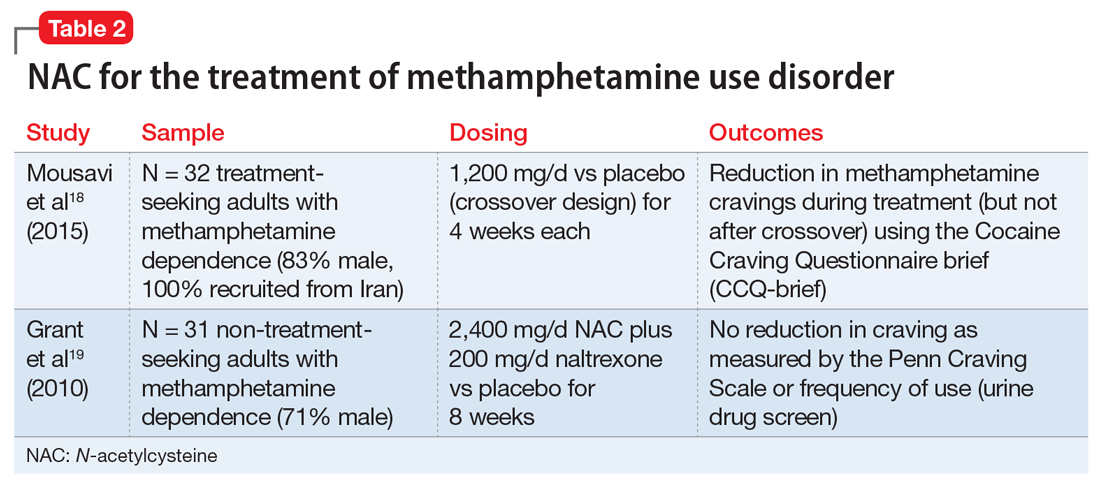
Methamphetamine cessation and relapse prevention. One study (N = 32) that evaluated the use of NAC, 1,200 mg/d for 4 weeks, vs placebo found reduced cravings among methamphetamine users who were seeking treatment.18 In contrast, a study of 31 methamphetamine users who were not seeking treatment evaluated the use of NAC, 2,400 mg/d, plus naltrexone, 200 mg/d, vs placebo for 8 weeks.19 It found no significant differences in craving or use patterns. Further research is needed to optimize the use of NAC for stimulant use disorders, and to better understand the role that abstinence plays.
Appropriate populations. The most support for use of NAC has been as an anti-relapse agent in treatment-seeking adults.
Continue to: Safety and dosing
Safety and dosing. Suggested dosages for the treatment of cocaine use disorder range from 1,200 to 3,600 mg/d (typically 600 to 1,800 mg twice daily, due to NAC’s short half-life), with higher retention rates noted in individuals who received 2,400 mg/d and 3,600 mg/d.16
Clinical implications. NAC is thought to act as an anti-relapse agent, rather than an agent that can help someone who is actively using stimulants to stop. Consequently, NAC will likely be most helpful for patients who are motivated to quit and are abstinent when they start taking NAC; however, this hypothesis needs further testing.
Cannabis use disorder
There are no FDA-approved treatments for cannabis use disorder. Individuals who use marijuana or other forms of cannabis may be less likely to report negative consequences or seek treatment compared with those who use other substances. Approximately 9% of individuals who use marijuana develop cannabis use disorder20; those who begin using marijuana earlier in adolescence are at increased risk.21 Commonly reported reasons for wanting to stop using marijuana include being concerned about health consequences, regaining or demonstrating self-control, saving money, avoiding legal consequences, obtaining or keeping employment, and reducing interpersonal conflict.22,23 Table 324-27 summarizes initial evidence that suggests NAC may be particularly useful in reducing marijuana use among adolescents (age 15 to 21).24,25
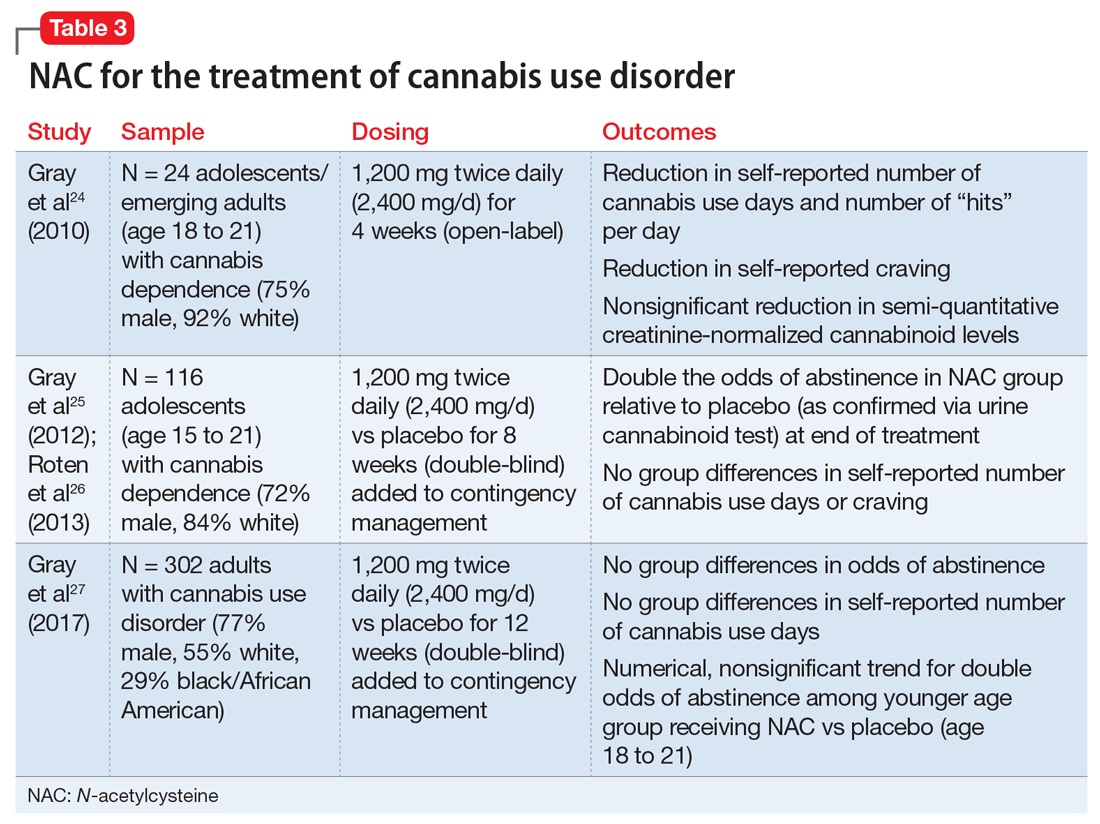
Cessation. An open-label, pilot clinical trial found significant reductions in self-reported marijuana use and craving—but not in biomarkers of use—among 24 adolescents after 4 weeks of NAC, 1,200 mg twice daily.24 In an 8-week, double-blind, randomized controlled trial of 116 adolescents, NAC, 1,200 mg twice daily, plus contingency management doubled the odds of abstinence, but had no effect on self-reported craving or use.25,26 In a sample of 302 adults, a 12-week trial of NAC, 1,200 mg twice daily, plus contingency management was no more effective than contingency management alone in promoting abstinence.27
Continue to: Appropriate populations
Appropriate populations. Evidence is stronger for use of NAC among adolescents (age 15 to 21) than for individuals older than age 21.25,27 Further research is needed to explore potential reasons for age-specific effects.
Safety and dosing. A safe and potentially efficacious dosage for the treatment of cannabis use disorder is 2,400 mg/d (1,200 mg twice daily).24,25,27
Clinical implications. Combined with contingency management, NAC might be efficacious for adolescents with cannabis use disorder, with treatment gains evident by the fourth week of treatment.24,25 To date, no clinical trials have examined the efficacy of NAC for treating cannabis use disorder without adjunctive contingency management, and research is needed to isolate the clinical effect of NAC among adolescents.
Tobacco use disorder
Cigarette smoking remains a leading cause of preventable death in the United States,28 and nearly 70% of people who start using tobacco become dependent.20 Existing FDA-approved treatments include nicotine replacement products, varenicline, and bupropion. Even though efficacious treatments exist, successful and sustained quit attempts are infrequent.29 NAC may exert a complementary effect to existing tobacco cessation interventions, such as varenicline.30 While these medications promote abstinence, NAC may be particularly beneficial in preventing relapse after abstinence has been achieved (Table 430-36).
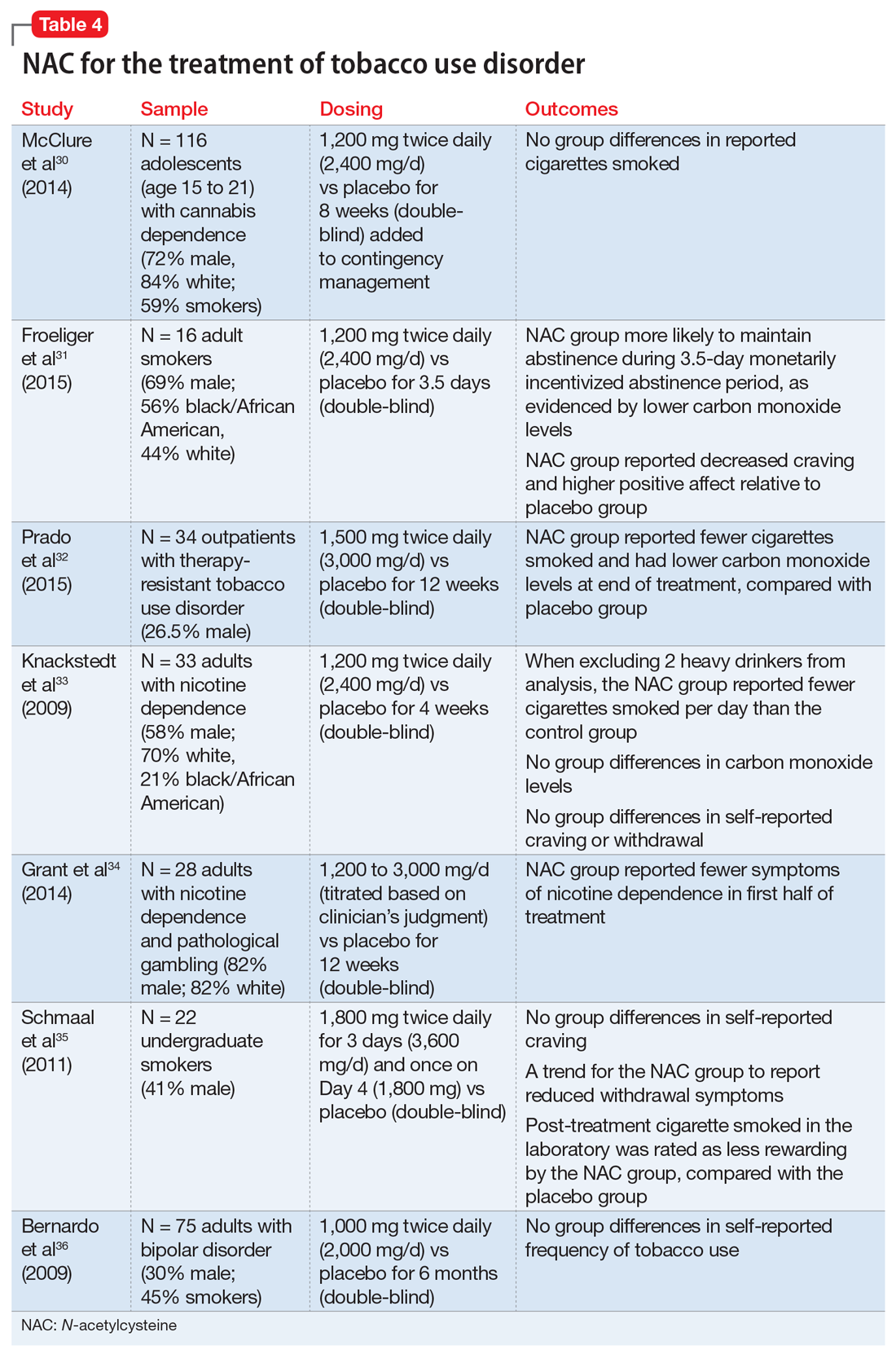
Continue to: Cessation and relapse prevention
Cessation and relapse prevention. Several pilot studies found that adult smokers who received NAC (alone or in combination with another treatment) had lower carbon monoxide levels,31,32 smoked fewer cigarettes,32,33 and had fewer self-reported symptoms of nicotine dependence34 and/or less craving for cigarettes.31 However, one study of 33 smokers did not find a reduction in craving or carbon monoxide for NAC compared with placebo.33 Another pilot study of 22 young adult smokers found that those who received NAC rated their first cigarette after treatment (smoked in the laboratory) as less rewarding, relative to smokers who received a placebo.35
Secondary analyses of adults with bipolar disorder36 and adolescents with cannabis use disorder37 found no decreases in tobacco use among those who received NAC compared with placebo. However, the studies in these analyses did not specifically recruit tobacco users, and participants who were tobacco users were not necessarily interested in quitting. This may partially explain discrepant findings.
Appropriate populations. NAC has been studied mostly in adult cigarette smokers.
Safety and dosing. Suggested dosages for treating tobacco use disorder range from 1,200 to 3,600 mg/d (600 to 1,800 mg twice daily).
Continue to: Clinical implications
Clinical implications. Data on NAC’s efficacy for tobacco use disorder come from small, pilot trials. Although initial evidence is promising, it is premature to suggest NAC for smoking cessation until a fully powered, randomized clinical trial provides evidence of efficacy.
Alcohol use disorder
Alcohol use disorders are widely prevalent; 13.9% of U.S. adults met criteria in the past year, and 29.1% of U.S. adults meet criteria in their lifetime.38 Alcohol use disorders can result in significant negative consequences, including relationship problems, violent behavior, medical problems, and death. Existing FDA-approved medications for alcohol use disorder include naltrexone, acamprosate, and disulfiram.
Due to the severe potential health consequences of alcohol, NAC has been examined as a possible aid in preventing relapse. However, most studies have been conducted using animals. Three studies have examined alcohol use in humans (Table 536,39,40). One was a pilot study,39 and the other 2 were secondary data analyses.36,40 None of them specifically focused on alcohol use disorders. A pilot study of 35 veterans with co-occurring posttraumautic stress disorder (PTSD) and SUDs (82% of whom had an alcohol use disorder) found that compared with placebo, NAC significantly decreased PTSD symptoms, craving, and depression.39 In a study of 75 adults with bipolar disorder, secondary alcohol use was not significantly reduced.36 However, one study suggested that NAC may decrease adolescent alcohol and marijuana co-use.40 Future work is needed to examine the potential clinical utility of NAC in individuals with alcohol use disorders.
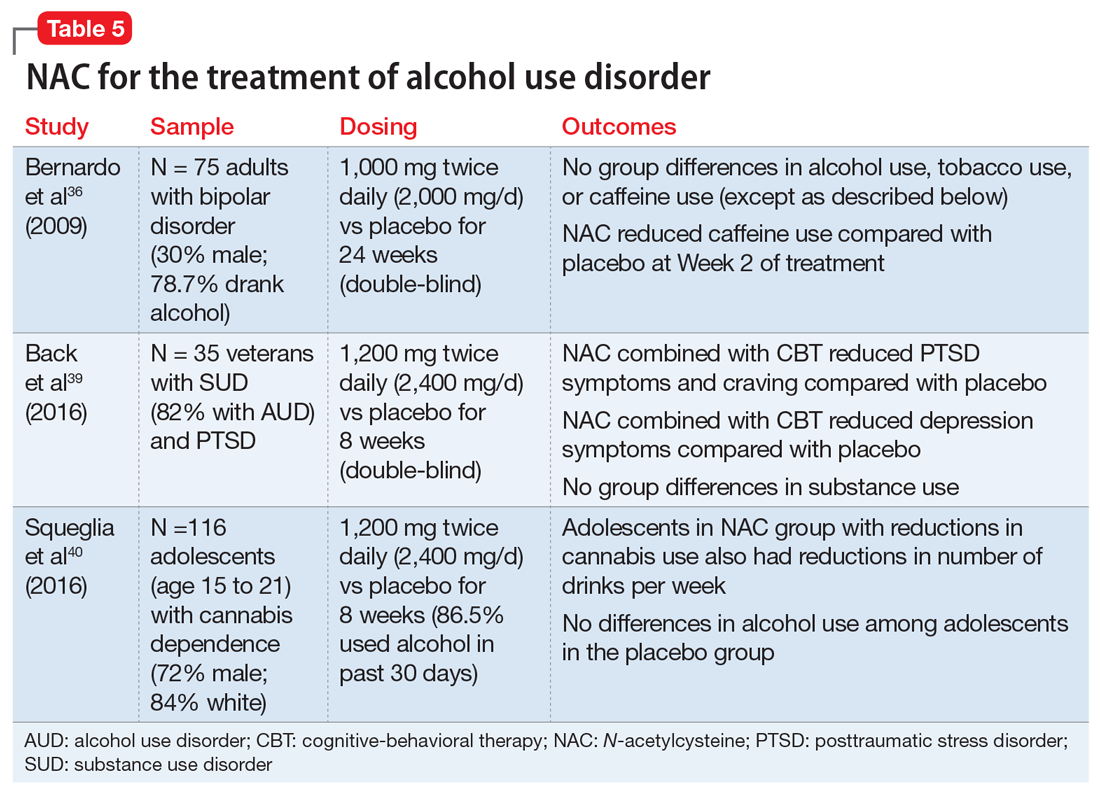
Findings from animal studies indicate that NAC may:
- reduce alcohol-seeking41
- reduce withdrawal symptoms42
- reduce the teratogenic effects of alcohol43
- prevent alcohol toxicity44
- reduce health-related consequences of alcohol (eg, myocardial oxidative stress45 and alcohol-related steatohepatitis46).
Continue to: Appropriate populations
Appropriate populations. Pilot studies have suggested that appropriate populations may include veterans with SUD and PTSD39 and adolescents with marijuana dependence who use alcohol.40
Safety and dosing. Suggested dosages for the treatment of alcohol use disorder based on these studies range from 1,000 to 2,400 mg/d (500 to 1,200 mg twice daily).
Clinical implications. Future work is needed to determine if NAC is effective for treating alcohol use disorders. Ongoing randomized clinical trials are examining the efficacy of NAC in reducing alcohol use among individuals with alcohol use disorder. It is premature to recommend NAC for treatment of alcohol use disorders.
Other psychiatric uses
Although we have highlighted NAC’s effect on glutamatergic transmission, evidence suggests that NAC may have multiple mechanisms of action that could impact psychiatric functioning. For example, NAC may also reverse oxidative stress, which is frequently observed in psychiatric disorders such as schizophrenia and bipolar disorder.10,12 NAC also has anti-inflammatory properties. When inflammatory pathways of the CNS are dysregulated, production of neurotransmitters may be impaired, resulting in depression-like symptoms.10,12,47 Preliminary evidence suggests that NAC may be effective in treating mood-related symptoms (eg, irritability, depression) in individuals with psychiatric disorders (eg, bipolar and depressive disorders, PTSD, and SUDs) and general symptoms of schizophrenia, obsessive-compulsive disorder, and trichotillomania, although mixed findings in controlled studies suggest a need for further research.12,39
Continue to: NAC: A promising candidate
NAC: A promising candidate
Initial evidence suggests NAC may be helpful for treating patients with SUDs. A patient seeking SUD treatment who is treated with NAC may experience a decreased drive, craving, or compulsion to use. Notably, NAC may be particularly useful in preventing relapse after an individual has achieved abstinence. Evidence suggests that NAC may be useful in the treatment of adults with cocaine use disorders who have achieved abstinence, and adolescents with cannabis use disorders. Preliminary results for adult tobacco use disorder are also promising. Human data examining the efficacy of NAC for alcohol use disorder is limited. Researchers’ ongoing challenge is to identify which patients with which SUDs are most likely to benefit from NAC, and to create clear clinical guidelines for the provider.
Bottom Line
N-acetylcysteine is likely to have modest effects for some patients who have a substance use disorder, particularly adults who use cocaine and adolescents who use marijuana. It may be useful in preventing relapse to substance use after an individual has achieved abstinence.
Related Resources
- Deepmala, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294-321.
- Roberts-Wolfe D, Kalivas P. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14(6):745-756.
- National Institute on Drug Abuse. Treatment approaches for drug addiction. https://www.drugabuse.gov/publications/drugfacts/treatment-approaches-drug-addiction.
Drug Brand Names
Acamprosate • Campral
Acetaminophen • Tylenol
Baclofen • Lioresal
Bupropion • Zyban
Disulfiram • Antabuse
Naltrexone • Revia,Vivitrol
Varenicline • Chantix
1. Grella CE, Karno MP, Warda US, et al. Perceptions of need and help received for substance dependence in a national probability survey. Psychiatr Serv. 2009;60(8):1068-1074.
2. Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23-50.
3. McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531-3537.
4. LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170-3177.
5. Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974-986.
6. Roberts-Wolfe D, Kalivas PW. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14(6):745-756.
7. Berk M, Malhi GS, Gray LJ, et al. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34(3):167-177.
8. McClure EA, Gipson CD, Malcolm RJ, et al. Potential role of N-acetylcysteine in the management of substance use disorders. CNS drugs. 2014;28(2):95-106.
9. Deepmala, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294-321.
10. Minarini A, Ferrari S, Galletti M, et al. N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin Drug Metab Toxicol. 2017;13(3):279-292.
11. Grandjean EM, Berthet P, Ruffman R, et al. Efficacy of oral long-term N‑acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther. 2000;22(2):209‑221.
12. Rhodes K, Braakhuis A. Performance and side effects of supplementation with N-acetylcysteine: a systematic review and meta-analysis. Sports Med. 2017;47(8):1619-1636.
13. Olsson B, Johansson M, Gabrielsson J, et al. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34(1):77-82.
14. United Nations Office on Drugs and Crime. World Drug Report 2016 (United Nations publication, Sales No. E.16.XI.7). https://www.unodc.org/doc/wdr2016/WORLD_DRUG_REPORT_2016_web.pdf. Published May 2016. Accessed April 26, 2018.
15. Amen SL, Piacentine LB, Ahmad ME, et al. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36(4):871-878.
16. Mardikian PN, LaRowe SD, Hedden S, et al. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):389-394.
17. LaRowe SD, Kalivas PW, Nicholas JS, et al. A double‐blind placebo‐controlled trial of N‐acetylcysteine in the treatment of cocaine dependence. Am J Addict. 2013;22(5):443-452.
18. Mousavi SG, Sharbafchi MR, Salehi M, et al. The efficacy of N-acetylcysteine in the treatment of methamphetamine dependence: a double-blind controlled, crossover study. Arch Iran Med. 2015;18(1):28-33.
19. Grant JE, Odlaug BL, Kim SW. A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. Eur Neuropsychopharmacol. 2010;20(11):823-828.
20. Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011;115(1-2):120-130.
21. Chen CY, O’Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000-2001. Drug Alcohol Depend. 2005;79(1):11-22.
22. Copersino ML, Boyd SJ, Tashkin DP, et al. Quitting among non-treatment-seeking marijuana users: reasons and changes in other substance use. Am J Addict. 2006;15(4):297-302.
23. Weiner MD, Sussman S, McCuller WJ, et al. Factors in marijuana cessation among high-risk youth. J Drug Educ. 1999;29(4):337-357.
24. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
25. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
26. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791.
27. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 2017;177:249-257.
28. Rostron B. Mortality risks associated with environmental tobacco smoke exposure in the United States. Nicotine Tob Res. 2013;15(10):1722-1728.
29. Centers for Disease Control and Prevention. Quitting smoking among adults – United States, 2001–2010. MMWR. 2011;60(44):1513-1519.
30. McClure EA, Baker NL, Gipson CD, et al. An open-label pilot trial of N-acetylcysteine and varenicline in adult cigarette smokers. Am J Drug Alcohol Abuse. 2015;41(1):52-56.
31. Froeliger B, McConnell P, Stankeviciute N, et al. The effects of N-acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: a double-blind, placebo-controlled fMRI pilot study. Drug Alcohol Depend. 2015;156:234-242.
32. Prado E, Maes M, Piccoli LG, et al. N-acetylcysteine for therapy-resistant tobacco use disorder: a pilot study. Redox Rep. 2015;20(5):215-222.
33. Knackstedt LA, LaRowe S, Mardikian P, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841-845.
34. Grant JE, Odlaug BL, Chamberlain SR, et al. A randomized, placebo-controlled trial of N-acetylcysteine plus imaginal desensitization for nicotine-dependent pathological gamblers. J Clin Psychiatry. 2014;75(1):39-45.
35. Schmaal L, Berk L, Hulstijn KP, et al. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. Eur Addiction Res. 2011;17(4):211-216.
36. Bernardo M, Dodd S, Gama CS, et al. Effects of N‐acetylcysteine on substance use in bipolar disorder: a randomised placebo‐controlled clinical trial. Acta Neuropsychiatr. 2009;21(5):239-245.
37. McClure EA, Baker NL, Gray KM. Cigarette smoking during an N-acetylcysteine-assisted cannabis cessation trial in adolescents. Am J Drug Alcohol Abuse. 2014;40(4):285-291.
38. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
39. Back SE, McCauley JL, Korte KJ, et al. A double-blind randomized controlled pilot trial of N-acetylcysteine in veterans with PTSD and substance use disorders. J Clin Psychiatry. 2016;77(11):e1439-e1446.
40. Squeglia LM, Baker NL, McClure EA, et al. Alcohol use during a trial of N-acetylcysteine for adolescent marijuana cessation. Addict Behav. 2016;63:172-177.
41. Lebourgeois S, González-Marín MC, Jeanblanc J, et al. Effect of N-acetylcysteine on motivation, seeking and relapse to ethanol self-administration. Addict Biol. 2018;23(2):643-652.
42. Schneider R Jr, Santos CF, Clarimundo V, et al. N-acetylcysteine prevents behavioral and biochemical changes induced by alcohol cessation in rats. Alcohol. 2015;49(3):259-263.
43. Parnell SE, Sulik KK, Dehart DB, et al. Reduction of ethanol-induced ocular abnormalities in mice via dietary administration of N-acetylcysteine. Alcohol. 2010;44(7-8):699-705.
44. Ozkol H, Bulut G, Balahoroglu R, et al. Protective effects of Selenium, N-acetylcysteine and Vitamin E against acute ethanol intoxication in rats. Biol Trace Elem Res. 2017;175(1):177-185.
45. Seiva FR, Amauchi JF, Rocha KK, et al. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol. 2009;43(8):649-656.
46. Setshedi M, Longato L, Petersen DR, et al. Limited therapeutic effect of N‐acetylcysteine on hepatic insulin resistance in an experimental model of alcohol‐induced steatohepatitis. Alcohol Clin Exp Res. 2011;35(12):2139-2151.
47. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732-741.
Pharmacologic treatment options for many substance use disorders (SUDs) are limited. This is especially true for cocaine use disorder and cannabis use disorder, for which there are no FDA-approved medications. FDA-approved medications for other SUDs often take the form of replacement or agonist therapies (eg, nicotine replacement therapy) that substitute the effects of the substance to aid in cessation. Other pharmacotherapies treat symptoms of withdrawal, reduce craving, or provide aversive counter-conditioning if the patient consumes the substance while on the medication (eg, disulfiram).
The over-the-counter (OTC) antioxidant N-acetylcysteine (NAC) may be a potential treatment for SUDs. Although NAC is not approved by the FDA for treating SUDs, its proposed mechanism of action differs from that of current FDA-approved medications for SUDs. NAC’s potential for broad applicability, favorable adverse-effect profile, accessibility, and low cost make it an intriguing option for patients with multiple comorbidities, and potentially for individuals with polysubstance use. This article reviews the current evidence supporting NAC for treating SUDs, to provide insight about which patients may benefit from NAC and under which circumstances they are most likely to benefit.
NAC may correct glutamate dysregulation
Approximately 85% of individuals with an SUD do not seek treatment for it, and those who do are older, have a longer history of use, have more severe dependence, and have sought treatment numerous times before.1 By the time most people seek treatment, years of chronic substance use have likely led to significant brain-related adaptations. Individuals with SUDs often indicate that their substance use began as a pleasurable activity—the effects of the drug were enjoyable and they were motivated to use it again. With repeated substance use, they may begin to develop a stronger urge to use the drug, driven not necessarily by a desire for pleasure, but by compulsion.2
Numerous neural adaptations underlie the transition from “liking” a substance to engaging in the compulsive use that is characteristic of an SUD.2 For example, repeated use of an addictive substance may result in excess glutamate in the nucleus accumbens,3,4 an area of the brain that plays a critical role in motivation and learning. As a result, it has been proposed that pharmacotherapies that help correct glutamate dysregulation may be effective in promoting abstinence or preventing relapse to a substance.5,6
NAC may reverse the neural dysfunction seen in SUDs. As an OTC antioxidant that impacts glutamatergic functioning in the brain, NAC has long been used to treat acetaminophen overdose; however, in recent years, researchers have begun to tap its potential for treating substance use and psychiatric disorders. NAC is thought to upregulate the glutamate transporter (GLT-1) that removes excess glutamate from the nucleus accumbens.6 Several published reviews provide more in-depth information about the neurobiology of NAC.6-10
The adverse-effect profile of NAC is relatively benign. Nausea, vomiting, diarrhea, and sleepiness are relatively infrequent and mild.11,12 The bioavailability of NAC is about 4% to 9%, with an approximate half-life of 6.25 hours when orally administered.13 Because NAC is classified as an OTC supplement, the potency and preparation may vary by supplier. To maximize consistency, NAC should be obtained from a supplier that meets United States Pharmacopeia (USP) standards.
NAC for SUDs: Emerging evidence
Several recent reviews have described the efficacy of NAC for SUDs and other psychiatric disorders. Here we summarize the current research examining the efficacy of NAC for stimulant (ie, cocaine and methamphetamine), cannabis, tobacco, and alcohol use disorders.
Continue to: Stimulant use disorders
Stimulant use disorders. The United Nations Office for Drugs and Crime estimates that worldwide, more than 18 million people use cocaine and more than 35 million use amphetamines.14 There are currently no FDA-approved treatments for stimulant use disorders, and clinicians treating patients with cocaine or amphetamine dependence often are at a loss for how best to promote abstinence. Recent studies suggest that NAC may decrease drug-seeking behavior and cravings in adults who seek treatment. The results of studies examining NAC for treating cocaine use and methamphetamine use are summarized in Table 115-17 and Table 2,18,19 respectively.

Cocaine cessation and relapse prevention. Several small pilot projects15,16 found that compared with placebo, various doses of NAC reduced craving (as measured with a visual analog scale). However, in a double-blind, placebo-controlled study, NAC did not decrease cravings or use after 8 weeks of treatment in individuals with cocaine use disorder who were still using cocaine (ie, they had not yet become abstinent). Interestingly, those who were abstinent when treatment began reported lower craving and remained abstinent longer if they received NAC (vs placebo), which suggests that NAC may be useful for preventing relapse.17

Methamphetamine cessation and relapse prevention. One study (N = 32) that evaluated the use of NAC, 1,200 mg/d for 4 weeks, vs placebo found reduced cravings among methamphetamine users who were seeking treatment.18 In contrast, a study of 31 methamphetamine users who were not seeking treatment evaluated the use of NAC, 2,400 mg/d, plus naltrexone, 200 mg/d, vs placebo for 8 weeks.19 It found no significant differences in craving or use patterns. Further research is needed to optimize the use of NAC for stimulant use disorders, and to better understand the role that abstinence plays.
Appropriate populations. The most support for use of NAC has been as an anti-relapse agent in treatment-seeking adults.
Continue to: Safety and dosing
Safety and dosing. Suggested dosages for the treatment of cocaine use disorder range from 1,200 to 3,600 mg/d (typically 600 to 1,800 mg twice daily, due to NAC’s short half-life), with higher retention rates noted in individuals who received 2,400 mg/d and 3,600 mg/d.16
Clinical implications. NAC is thought to act as an anti-relapse agent, rather than an agent that can help someone who is actively using stimulants to stop. Consequently, NAC will likely be most helpful for patients who are motivated to quit and are abstinent when they start taking NAC; however, this hypothesis needs further testing.
Cannabis use disorder
There are no FDA-approved treatments for cannabis use disorder. Individuals who use marijuana or other forms of cannabis may be less likely to report negative consequences or seek treatment compared with those who use other substances. Approximately 9% of individuals who use marijuana develop cannabis use disorder20; those who begin using marijuana earlier in adolescence are at increased risk.21 Commonly reported reasons for wanting to stop using marijuana include being concerned about health consequences, regaining or demonstrating self-control, saving money, avoiding legal consequences, obtaining or keeping employment, and reducing interpersonal conflict.22,23 Table 324-27 summarizes initial evidence that suggests NAC may be particularly useful in reducing marijuana use among adolescents (age 15 to 21).24,25

Cessation. An open-label, pilot clinical trial found significant reductions in self-reported marijuana use and craving—but not in biomarkers of use—among 24 adolescents after 4 weeks of NAC, 1,200 mg twice daily.24 In an 8-week, double-blind, randomized controlled trial of 116 adolescents, NAC, 1,200 mg twice daily, plus contingency management doubled the odds of abstinence, but had no effect on self-reported craving or use.25,26 In a sample of 302 adults, a 12-week trial of NAC, 1,200 mg twice daily, plus contingency management was no more effective than contingency management alone in promoting abstinence.27
Continue to: Appropriate populations
Appropriate populations. Evidence is stronger for use of NAC among adolescents (age 15 to 21) than for individuals older than age 21.25,27 Further research is needed to explore potential reasons for age-specific effects.
Safety and dosing. A safe and potentially efficacious dosage for the treatment of cannabis use disorder is 2,400 mg/d (1,200 mg twice daily).24,25,27
Clinical implications. Combined with contingency management, NAC might be efficacious for adolescents with cannabis use disorder, with treatment gains evident by the fourth week of treatment.24,25 To date, no clinical trials have examined the efficacy of NAC for treating cannabis use disorder without adjunctive contingency management, and research is needed to isolate the clinical effect of NAC among adolescents.
Tobacco use disorder
Cigarette smoking remains a leading cause of preventable death in the United States,28 and nearly 70% of people who start using tobacco become dependent.20 Existing FDA-approved treatments include nicotine replacement products, varenicline, and bupropion. Even though efficacious treatments exist, successful and sustained quit attempts are infrequent.29 NAC may exert a complementary effect to existing tobacco cessation interventions, such as varenicline.30 While these medications promote abstinence, NAC may be particularly beneficial in preventing relapse after abstinence has been achieved (Table 430-36).

Continue to: Cessation and relapse prevention
Cessation and relapse prevention. Several pilot studies found that adult smokers who received NAC (alone or in combination with another treatment) had lower carbon monoxide levels,31,32 smoked fewer cigarettes,32,33 and had fewer self-reported symptoms of nicotine dependence34 and/or less craving for cigarettes.31 However, one study of 33 smokers did not find a reduction in craving or carbon monoxide for NAC compared with placebo.33 Another pilot study of 22 young adult smokers found that those who received NAC rated their first cigarette after treatment (smoked in the laboratory) as less rewarding, relative to smokers who received a placebo.35
Secondary analyses of adults with bipolar disorder36 and adolescents with cannabis use disorder37 found no decreases in tobacco use among those who received NAC compared with placebo. However, the studies in these analyses did not specifically recruit tobacco users, and participants who were tobacco users were not necessarily interested in quitting. This may partially explain discrepant findings.
Appropriate populations. NAC has been studied mostly in adult cigarette smokers.
Safety and dosing. Suggested dosages for treating tobacco use disorder range from 1,200 to 3,600 mg/d (600 to 1,800 mg twice daily).
Continue to: Clinical implications
Clinical implications. Data on NAC’s efficacy for tobacco use disorder come from small, pilot trials. Although initial evidence is promising, it is premature to suggest NAC for smoking cessation until a fully powered, randomized clinical trial provides evidence of efficacy.
Alcohol use disorder
Alcohol use disorders are widely prevalent; 13.9% of U.S. adults met criteria in the past year, and 29.1% of U.S. adults meet criteria in their lifetime.38 Alcohol use disorders can result in significant negative consequences, including relationship problems, violent behavior, medical problems, and death. Existing FDA-approved medications for alcohol use disorder include naltrexone, acamprosate, and disulfiram.
Due to the severe potential health consequences of alcohol, NAC has been examined as a possible aid in preventing relapse. However, most studies have been conducted using animals. Three studies have examined alcohol use in humans (Table 536,39,40). One was a pilot study,39 and the other 2 were secondary data analyses.36,40 None of them specifically focused on alcohol use disorders. A pilot study of 35 veterans with co-occurring posttraumautic stress disorder (PTSD) and SUDs (82% of whom had an alcohol use disorder) found that compared with placebo, NAC significantly decreased PTSD symptoms, craving, and depression.39 In a study of 75 adults with bipolar disorder, secondary alcohol use was not significantly reduced.36 However, one study suggested that NAC may decrease adolescent alcohol and marijuana co-use.40 Future work is needed to examine the potential clinical utility of NAC in individuals with alcohol use disorders.

Findings from animal studies indicate that NAC may:
- reduce alcohol-seeking41
- reduce withdrawal symptoms42
- reduce the teratogenic effects of alcohol43
- prevent alcohol toxicity44
- reduce health-related consequences of alcohol (eg, myocardial oxidative stress45 and alcohol-related steatohepatitis46).
Continue to: Appropriate populations
Appropriate populations. Pilot studies have suggested that appropriate populations may include veterans with SUD and PTSD39 and adolescents with marijuana dependence who use alcohol.40
Safety and dosing. Suggested dosages for the treatment of alcohol use disorder based on these studies range from 1,000 to 2,400 mg/d (500 to 1,200 mg twice daily).
Clinical implications. Future work is needed to determine if NAC is effective for treating alcohol use disorders. Ongoing randomized clinical trials are examining the efficacy of NAC in reducing alcohol use among individuals with alcohol use disorder. It is premature to recommend NAC for treatment of alcohol use disorders.
Other psychiatric uses
Although we have highlighted NAC’s effect on glutamatergic transmission, evidence suggests that NAC may have multiple mechanisms of action that could impact psychiatric functioning. For example, NAC may also reverse oxidative stress, which is frequently observed in psychiatric disorders such as schizophrenia and bipolar disorder.10,12 NAC also has anti-inflammatory properties. When inflammatory pathways of the CNS are dysregulated, production of neurotransmitters may be impaired, resulting in depression-like symptoms.10,12,47 Preliminary evidence suggests that NAC may be effective in treating mood-related symptoms (eg, irritability, depression) in individuals with psychiatric disorders (eg, bipolar and depressive disorders, PTSD, and SUDs) and general symptoms of schizophrenia, obsessive-compulsive disorder, and trichotillomania, although mixed findings in controlled studies suggest a need for further research.12,39
Continue to: NAC: A promising candidate
NAC: A promising candidate
Initial evidence suggests NAC may be helpful for treating patients with SUDs. A patient seeking SUD treatment who is treated with NAC may experience a decreased drive, craving, or compulsion to use. Notably, NAC may be particularly useful in preventing relapse after an individual has achieved abstinence. Evidence suggests that NAC may be useful in the treatment of adults with cocaine use disorders who have achieved abstinence, and adolescents with cannabis use disorders. Preliminary results for adult tobacco use disorder are also promising. Human data examining the efficacy of NAC for alcohol use disorder is limited. Researchers’ ongoing challenge is to identify which patients with which SUDs are most likely to benefit from NAC, and to create clear clinical guidelines for the provider.
Bottom Line
N-acetylcysteine is likely to have modest effects for some patients who have a substance use disorder, particularly adults who use cocaine and adolescents who use marijuana. It may be useful in preventing relapse to substance use after an individual has achieved abstinence.
Related Resources
- Deepmala, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294-321.
- Roberts-Wolfe D, Kalivas P. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14(6):745-756.
- National Institute on Drug Abuse. Treatment approaches for drug addiction. https://www.drugabuse.gov/publications/drugfacts/treatment-approaches-drug-addiction.
Drug Brand Names
Acamprosate • Campral
Acetaminophen • Tylenol
Baclofen • Lioresal
Bupropion • Zyban
Disulfiram • Antabuse
Naltrexone • Revia,Vivitrol
Varenicline • Chantix
Pharmacologic treatment options for many substance use disorders (SUDs) are limited. This is especially true for cocaine use disorder and cannabis use disorder, for which there are no FDA-approved medications. FDA-approved medications for other SUDs often take the form of replacement or agonist therapies (eg, nicotine replacement therapy) that substitute the effects of the substance to aid in cessation. Other pharmacotherapies treat symptoms of withdrawal, reduce craving, or provide aversive counter-conditioning if the patient consumes the substance while on the medication (eg, disulfiram).
The over-the-counter (OTC) antioxidant N-acetylcysteine (NAC) may be a potential treatment for SUDs. Although NAC is not approved by the FDA for treating SUDs, its proposed mechanism of action differs from that of current FDA-approved medications for SUDs. NAC’s potential for broad applicability, favorable adverse-effect profile, accessibility, and low cost make it an intriguing option for patients with multiple comorbidities, and potentially for individuals with polysubstance use. This article reviews the current evidence supporting NAC for treating SUDs, to provide insight about which patients may benefit from NAC and under which circumstances they are most likely to benefit.
NAC may correct glutamate dysregulation
Approximately 85% of individuals with an SUD do not seek treatment for it, and those who do are older, have a longer history of use, have more severe dependence, and have sought treatment numerous times before.1 By the time most people seek treatment, years of chronic substance use have likely led to significant brain-related adaptations. Individuals with SUDs often indicate that their substance use began as a pleasurable activity—the effects of the drug were enjoyable and they were motivated to use it again. With repeated substance use, they may begin to develop a stronger urge to use the drug, driven not necessarily by a desire for pleasure, but by compulsion.2
Numerous neural adaptations underlie the transition from “liking” a substance to engaging in the compulsive use that is characteristic of an SUD.2 For example, repeated use of an addictive substance may result in excess glutamate in the nucleus accumbens,3,4 an area of the brain that plays a critical role in motivation and learning. As a result, it has been proposed that pharmacotherapies that help correct glutamate dysregulation may be effective in promoting abstinence or preventing relapse to a substance.5,6
NAC may reverse the neural dysfunction seen in SUDs. As an OTC antioxidant that impacts glutamatergic functioning in the brain, NAC has long been used to treat acetaminophen overdose; however, in recent years, researchers have begun to tap its potential for treating substance use and psychiatric disorders. NAC is thought to upregulate the glutamate transporter (GLT-1) that removes excess glutamate from the nucleus accumbens.6 Several published reviews provide more in-depth information about the neurobiology of NAC.6-10
The adverse-effect profile of NAC is relatively benign. Nausea, vomiting, diarrhea, and sleepiness are relatively infrequent and mild.11,12 The bioavailability of NAC is about 4% to 9%, with an approximate half-life of 6.25 hours when orally administered.13 Because NAC is classified as an OTC supplement, the potency and preparation may vary by supplier. To maximize consistency, NAC should be obtained from a supplier that meets United States Pharmacopeia (USP) standards.
NAC for SUDs: Emerging evidence
Several recent reviews have described the efficacy of NAC for SUDs and other psychiatric disorders. Here we summarize the current research examining the efficacy of NAC for stimulant (ie, cocaine and methamphetamine), cannabis, tobacco, and alcohol use disorders.
Continue to: Stimulant use disorders
Stimulant use disorders. The United Nations Office for Drugs and Crime estimates that worldwide, more than 18 million people use cocaine and more than 35 million use amphetamines.14 There are currently no FDA-approved treatments for stimulant use disorders, and clinicians treating patients with cocaine or amphetamine dependence often are at a loss for how best to promote abstinence. Recent studies suggest that NAC may decrease drug-seeking behavior and cravings in adults who seek treatment. The results of studies examining NAC for treating cocaine use and methamphetamine use are summarized in Table 115-17 and Table 2,18,19 respectively.

Cocaine cessation and relapse prevention. Several small pilot projects15,16 found that compared with placebo, various doses of NAC reduced craving (as measured with a visual analog scale). However, in a double-blind, placebo-controlled study, NAC did not decrease cravings or use after 8 weeks of treatment in individuals with cocaine use disorder who were still using cocaine (ie, they had not yet become abstinent). Interestingly, those who were abstinent when treatment began reported lower craving and remained abstinent longer if they received NAC (vs placebo), which suggests that NAC may be useful for preventing relapse.17

Methamphetamine cessation and relapse prevention. One study (N = 32) that evaluated the use of NAC, 1,200 mg/d for 4 weeks, vs placebo found reduced cravings among methamphetamine users who were seeking treatment.18 In contrast, a study of 31 methamphetamine users who were not seeking treatment evaluated the use of NAC, 2,400 mg/d, plus naltrexone, 200 mg/d, vs placebo for 8 weeks.19 It found no significant differences in craving or use patterns. Further research is needed to optimize the use of NAC for stimulant use disorders, and to better understand the role that abstinence plays.
Appropriate populations. The most support for use of NAC has been as an anti-relapse agent in treatment-seeking adults.
Continue to: Safety and dosing
Safety and dosing. Suggested dosages for the treatment of cocaine use disorder range from 1,200 to 3,600 mg/d (typically 600 to 1,800 mg twice daily, due to NAC’s short half-life), with higher retention rates noted in individuals who received 2,400 mg/d and 3,600 mg/d.16
Clinical implications. NAC is thought to act as an anti-relapse agent, rather than an agent that can help someone who is actively using stimulants to stop. Consequently, NAC will likely be most helpful for patients who are motivated to quit and are abstinent when they start taking NAC; however, this hypothesis needs further testing.
Cannabis use disorder
There are no FDA-approved treatments for cannabis use disorder. Individuals who use marijuana or other forms of cannabis may be less likely to report negative consequences or seek treatment compared with those who use other substances. Approximately 9% of individuals who use marijuana develop cannabis use disorder20; those who begin using marijuana earlier in adolescence are at increased risk.21 Commonly reported reasons for wanting to stop using marijuana include being concerned about health consequences, regaining or demonstrating self-control, saving money, avoiding legal consequences, obtaining or keeping employment, and reducing interpersonal conflict.22,23 Table 324-27 summarizes initial evidence that suggests NAC may be particularly useful in reducing marijuana use among adolescents (age 15 to 21).24,25

Cessation. An open-label, pilot clinical trial found significant reductions in self-reported marijuana use and craving—but not in biomarkers of use—among 24 adolescents after 4 weeks of NAC, 1,200 mg twice daily.24 In an 8-week, double-blind, randomized controlled trial of 116 adolescents, NAC, 1,200 mg twice daily, plus contingency management doubled the odds of abstinence, but had no effect on self-reported craving or use.25,26 In a sample of 302 adults, a 12-week trial of NAC, 1,200 mg twice daily, plus contingency management was no more effective than contingency management alone in promoting abstinence.27
Continue to: Appropriate populations
Appropriate populations. Evidence is stronger for use of NAC among adolescents (age 15 to 21) than for individuals older than age 21.25,27 Further research is needed to explore potential reasons for age-specific effects.
Safety and dosing. A safe and potentially efficacious dosage for the treatment of cannabis use disorder is 2,400 mg/d (1,200 mg twice daily).24,25,27
Clinical implications. Combined with contingency management, NAC might be efficacious for adolescents with cannabis use disorder, with treatment gains evident by the fourth week of treatment.24,25 To date, no clinical trials have examined the efficacy of NAC for treating cannabis use disorder without adjunctive contingency management, and research is needed to isolate the clinical effect of NAC among adolescents.
Tobacco use disorder
Cigarette smoking remains a leading cause of preventable death in the United States,28 and nearly 70% of people who start using tobacco become dependent.20 Existing FDA-approved treatments include nicotine replacement products, varenicline, and bupropion. Even though efficacious treatments exist, successful and sustained quit attempts are infrequent.29 NAC may exert a complementary effect to existing tobacco cessation interventions, such as varenicline.30 While these medications promote abstinence, NAC may be particularly beneficial in preventing relapse after abstinence has been achieved (Table 430-36).

Continue to: Cessation and relapse prevention
Cessation and relapse prevention. Several pilot studies found that adult smokers who received NAC (alone or in combination with another treatment) had lower carbon monoxide levels,31,32 smoked fewer cigarettes,32,33 and had fewer self-reported symptoms of nicotine dependence34 and/or less craving for cigarettes.31 However, one study of 33 smokers did not find a reduction in craving or carbon monoxide for NAC compared with placebo.33 Another pilot study of 22 young adult smokers found that those who received NAC rated their first cigarette after treatment (smoked in the laboratory) as less rewarding, relative to smokers who received a placebo.35
Secondary analyses of adults with bipolar disorder36 and adolescents with cannabis use disorder37 found no decreases in tobacco use among those who received NAC compared with placebo. However, the studies in these analyses did not specifically recruit tobacco users, and participants who were tobacco users were not necessarily interested in quitting. This may partially explain discrepant findings.
Appropriate populations. NAC has been studied mostly in adult cigarette smokers.
Safety and dosing. Suggested dosages for treating tobacco use disorder range from 1,200 to 3,600 mg/d (600 to 1,800 mg twice daily).
Continue to: Clinical implications
Clinical implications. Data on NAC’s efficacy for tobacco use disorder come from small, pilot trials. Although initial evidence is promising, it is premature to suggest NAC for smoking cessation until a fully powered, randomized clinical trial provides evidence of efficacy.
Alcohol use disorder
Alcohol use disorders are widely prevalent; 13.9% of U.S. adults met criteria in the past year, and 29.1% of U.S. adults meet criteria in their lifetime.38 Alcohol use disorders can result in significant negative consequences, including relationship problems, violent behavior, medical problems, and death. Existing FDA-approved medications for alcohol use disorder include naltrexone, acamprosate, and disulfiram.
Due to the severe potential health consequences of alcohol, NAC has been examined as a possible aid in preventing relapse. However, most studies have been conducted using animals. Three studies have examined alcohol use in humans (Table 536,39,40). One was a pilot study,39 and the other 2 were secondary data analyses.36,40 None of them specifically focused on alcohol use disorders. A pilot study of 35 veterans with co-occurring posttraumautic stress disorder (PTSD) and SUDs (82% of whom had an alcohol use disorder) found that compared with placebo, NAC significantly decreased PTSD symptoms, craving, and depression.39 In a study of 75 adults with bipolar disorder, secondary alcohol use was not significantly reduced.36 However, one study suggested that NAC may decrease adolescent alcohol and marijuana co-use.40 Future work is needed to examine the potential clinical utility of NAC in individuals with alcohol use disorders.

Findings from animal studies indicate that NAC may:
- reduce alcohol-seeking41
- reduce withdrawal symptoms42
- reduce the teratogenic effects of alcohol43
- prevent alcohol toxicity44
- reduce health-related consequences of alcohol (eg, myocardial oxidative stress45 and alcohol-related steatohepatitis46).
Continue to: Appropriate populations
Appropriate populations. Pilot studies have suggested that appropriate populations may include veterans with SUD and PTSD39 and adolescents with marijuana dependence who use alcohol.40
Safety and dosing. Suggested dosages for the treatment of alcohol use disorder based on these studies range from 1,000 to 2,400 mg/d (500 to 1,200 mg twice daily).
Clinical implications. Future work is needed to determine if NAC is effective for treating alcohol use disorders. Ongoing randomized clinical trials are examining the efficacy of NAC in reducing alcohol use among individuals with alcohol use disorder. It is premature to recommend NAC for treatment of alcohol use disorders.
Other psychiatric uses
Although we have highlighted NAC’s effect on glutamatergic transmission, evidence suggests that NAC may have multiple mechanisms of action that could impact psychiatric functioning. For example, NAC may also reverse oxidative stress, which is frequently observed in psychiatric disorders such as schizophrenia and bipolar disorder.10,12 NAC also has anti-inflammatory properties. When inflammatory pathways of the CNS are dysregulated, production of neurotransmitters may be impaired, resulting in depression-like symptoms.10,12,47 Preliminary evidence suggests that NAC may be effective in treating mood-related symptoms (eg, irritability, depression) in individuals with psychiatric disorders (eg, bipolar and depressive disorders, PTSD, and SUDs) and general symptoms of schizophrenia, obsessive-compulsive disorder, and trichotillomania, although mixed findings in controlled studies suggest a need for further research.12,39
Continue to: NAC: A promising candidate
NAC: A promising candidate
Initial evidence suggests NAC may be helpful for treating patients with SUDs. A patient seeking SUD treatment who is treated with NAC may experience a decreased drive, craving, or compulsion to use. Notably, NAC may be particularly useful in preventing relapse after an individual has achieved abstinence. Evidence suggests that NAC may be useful in the treatment of adults with cocaine use disorders who have achieved abstinence, and adolescents with cannabis use disorders. Preliminary results for adult tobacco use disorder are also promising. Human data examining the efficacy of NAC for alcohol use disorder is limited. Researchers’ ongoing challenge is to identify which patients with which SUDs are most likely to benefit from NAC, and to create clear clinical guidelines for the provider.
Bottom Line
N-acetylcysteine is likely to have modest effects for some patients who have a substance use disorder, particularly adults who use cocaine and adolescents who use marijuana. It may be useful in preventing relapse to substance use after an individual has achieved abstinence.
Related Resources
- Deepmala, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294-321.
- Roberts-Wolfe D, Kalivas P. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14(6):745-756.
- National Institute on Drug Abuse. Treatment approaches for drug addiction. https://www.drugabuse.gov/publications/drugfacts/treatment-approaches-drug-addiction.
Drug Brand Names
Acamprosate • Campral
Acetaminophen • Tylenol
Baclofen • Lioresal
Bupropion • Zyban
Disulfiram • Antabuse
Naltrexone • Revia,Vivitrol
Varenicline • Chantix
1. Grella CE, Karno MP, Warda US, et al. Perceptions of need and help received for substance dependence in a national probability survey. Psychiatr Serv. 2009;60(8):1068-1074.
2. Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23-50.
3. McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531-3537.
4. LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170-3177.
5. Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974-986.
6. Roberts-Wolfe D, Kalivas PW. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14(6):745-756.
7. Berk M, Malhi GS, Gray LJ, et al. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34(3):167-177.
8. McClure EA, Gipson CD, Malcolm RJ, et al. Potential role of N-acetylcysteine in the management of substance use disorders. CNS drugs. 2014;28(2):95-106.
9. Deepmala, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294-321.
10. Minarini A, Ferrari S, Galletti M, et al. N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin Drug Metab Toxicol. 2017;13(3):279-292.
11. Grandjean EM, Berthet P, Ruffman R, et al. Efficacy of oral long-term N‑acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther. 2000;22(2):209‑221.
12. Rhodes K, Braakhuis A. Performance and side effects of supplementation with N-acetylcysteine: a systematic review and meta-analysis. Sports Med. 2017;47(8):1619-1636.
13. Olsson B, Johansson M, Gabrielsson J, et al. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34(1):77-82.
14. United Nations Office on Drugs and Crime. World Drug Report 2016 (United Nations publication, Sales No. E.16.XI.7). https://www.unodc.org/doc/wdr2016/WORLD_DRUG_REPORT_2016_web.pdf. Published May 2016. Accessed April 26, 2018.
15. Amen SL, Piacentine LB, Ahmad ME, et al. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36(4):871-878.
16. Mardikian PN, LaRowe SD, Hedden S, et al. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):389-394.
17. LaRowe SD, Kalivas PW, Nicholas JS, et al. A double‐blind placebo‐controlled trial of N‐acetylcysteine in the treatment of cocaine dependence. Am J Addict. 2013;22(5):443-452.
18. Mousavi SG, Sharbafchi MR, Salehi M, et al. The efficacy of N-acetylcysteine in the treatment of methamphetamine dependence: a double-blind controlled, crossover study. Arch Iran Med. 2015;18(1):28-33.
19. Grant JE, Odlaug BL, Kim SW. A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. Eur Neuropsychopharmacol. 2010;20(11):823-828.
20. Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011;115(1-2):120-130.
21. Chen CY, O’Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000-2001. Drug Alcohol Depend. 2005;79(1):11-22.
22. Copersino ML, Boyd SJ, Tashkin DP, et al. Quitting among non-treatment-seeking marijuana users: reasons and changes in other substance use. Am J Addict. 2006;15(4):297-302.
23. Weiner MD, Sussman S, McCuller WJ, et al. Factors in marijuana cessation among high-risk youth. J Drug Educ. 1999;29(4):337-357.
24. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
25. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
26. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791.
27. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 2017;177:249-257.
28. Rostron B. Mortality risks associated with environmental tobacco smoke exposure in the United States. Nicotine Tob Res. 2013;15(10):1722-1728.
29. Centers for Disease Control and Prevention. Quitting smoking among adults – United States, 2001–2010. MMWR. 2011;60(44):1513-1519.
30. McClure EA, Baker NL, Gipson CD, et al. An open-label pilot trial of N-acetylcysteine and varenicline in adult cigarette smokers. Am J Drug Alcohol Abuse. 2015;41(1):52-56.
31. Froeliger B, McConnell P, Stankeviciute N, et al. The effects of N-acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: a double-blind, placebo-controlled fMRI pilot study. Drug Alcohol Depend. 2015;156:234-242.
32. Prado E, Maes M, Piccoli LG, et al. N-acetylcysteine for therapy-resistant tobacco use disorder: a pilot study. Redox Rep. 2015;20(5):215-222.
33. Knackstedt LA, LaRowe S, Mardikian P, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841-845.
34. Grant JE, Odlaug BL, Chamberlain SR, et al. A randomized, placebo-controlled trial of N-acetylcysteine plus imaginal desensitization for nicotine-dependent pathological gamblers. J Clin Psychiatry. 2014;75(1):39-45.
35. Schmaal L, Berk L, Hulstijn KP, et al. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. Eur Addiction Res. 2011;17(4):211-216.
36. Bernardo M, Dodd S, Gama CS, et al. Effects of N‐acetylcysteine on substance use in bipolar disorder: a randomised placebo‐controlled clinical trial. Acta Neuropsychiatr. 2009;21(5):239-245.
37. McClure EA, Baker NL, Gray KM. Cigarette smoking during an N-acetylcysteine-assisted cannabis cessation trial in adolescents. Am J Drug Alcohol Abuse. 2014;40(4):285-291.
38. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
39. Back SE, McCauley JL, Korte KJ, et al. A double-blind randomized controlled pilot trial of N-acetylcysteine in veterans with PTSD and substance use disorders. J Clin Psychiatry. 2016;77(11):e1439-e1446.
40. Squeglia LM, Baker NL, McClure EA, et al. Alcohol use during a trial of N-acetylcysteine for adolescent marijuana cessation. Addict Behav. 2016;63:172-177.
41. Lebourgeois S, González-Marín MC, Jeanblanc J, et al. Effect of N-acetylcysteine on motivation, seeking and relapse to ethanol self-administration. Addict Biol. 2018;23(2):643-652.
42. Schneider R Jr, Santos CF, Clarimundo V, et al. N-acetylcysteine prevents behavioral and biochemical changes induced by alcohol cessation in rats. Alcohol. 2015;49(3):259-263.
43. Parnell SE, Sulik KK, Dehart DB, et al. Reduction of ethanol-induced ocular abnormalities in mice via dietary administration of N-acetylcysteine. Alcohol. 2010;44(7-8):699-705.
44. Ozkol H, Bulut G, Balahoroglu R, et al. Protective effects of Selenium, N-acetylcysteine and Vitamin E against acute ethanol intoxication in rats. Biol Trace Elem Res. 2017;175(1):177-185.
45. Seiva FR, Amauchi JF, Rocha KK, et al. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol. 2009;43(8):649-656.
46. Setshedi M, Longato L, Petersen DR, et al. Limited therapeutic effect of N‐acetylcysteine on hepatic insulin resistance in an experimental model of alcohol‐induced steatohepatitis. Alcohol Clin Exp Res. 2011;35(12):2139-2151.
47. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732-741.
1. Grella CE, Karno MP, Warda US, et al. Perceptions of need and help received for substance dependence in a national probability survey. Psychiatr Serv. 2009;60(8):1068-1074.
2. Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23-50.
3. McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23(8):3531-3537.
4. LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28(12):3170-3177.
5. Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974-986.
6. Roberts-Wolfe D, Kalivas PW. Glutamate transporter GLT-1 as a therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets. 2015;14(6):745-756.
7. Berk M, Malhi GS, Gray LJ, et al. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34(3):167-177.
8. McClure EA, Gipson CD, Malcolm RJ, et al. Potential role of N-acetylcysteine in the management of substance use disorders. CNS drugs. 2014;28(2):95-106.
9. Deepmala, Slattery J, Kumar N, et al. Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev. 2015;55:294-321.
10. Minarini A, Ferrari S, Galletti M, et al. N-acetylcysteine in the treatment of psychiatric disorders: current status and future prospects. Expert Opin Drug Metab Toxicol. 2017;13(3):279-292.
11. Grandjean EM, Berthet P, Ruffman R, et al. Efficacy of oral long-term N‑acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther. 2000;22(2):209‑221.
12. Rhodes K, Braakhuis A. Performance and side effects of supplementation with N-acetylcysteine: a systematic review and meta-analysis. Sports Med. 2017;47(8):1619-1636.
13. Olsson B, Johansson M, Gabrielsson J, et al. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34(1):77-82.
14. United Nations Office on Drugs and Crime. World Drug Report 2016 (United Nations publication, Sales No. E.16.XI.7). https://www.unodc.org/doc/wdr2016/WORLD_DRUG_REPORT_2016_web.pdf. Published May 2016. Accessed April 26, 2018.
15. Amen SL, Piacentine LB, Ahmad ME, et al. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology. 2011;36(4):871-878.
16. Mardikian PN, LaRowe SD, Hedden S, et al. An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):389-394.
17. LaRowe SD, Kalivas PW, Nicholas JS, et al. A double‐blind placebo‐controlled trial of N‐acetylcysteine in the treatment of cocaine dependence. Am J Addict. 2013;22(5):443-452.
18. Mousavi SG, Sharbafchi MR, Salehi M, et al. The efficacy of N-acetylcysteine in the treatment of methamphetamine dependence: a double-blind controlled, crossover study. Arch Iran Med. 2015;18(1):28-33.
19. Grant JE, Odlaug BL, Kim SW. A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. Eur Neuropsychopharmacol. 2010;20(11):823-828.
20. Lopez-Quintero C, Pérez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011;115(1-2):120-130.
21. Chen CY, O’Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000-2001. Drug Alcohol Depend. 2005;79(1):11-22.
22. Copersino ML, Boyd SJ, Tashkin DP, et al. Quitting among non-treatment-seeking marijuana users: reasons and changes in other substance use. Am J Addict. 2006;15(4):297-302.
23. Weiner MD, Sussman S, McCuller WJ, et al. Factors in marijuana cessation among high-risk youth. J Drug Educ. 1999;29(4):337-357.
24. Gray KM, Watson NL, Carpenter MJ, et al. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict. 2010;19(2):187-189.
25. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812.
26. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791.
27. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Depend. 2017;177:249-257.
28. Rostron B. Mortality risks associated with environmental tobacco smoke exposure in the United States. Nicotine Tob Res. 2013;15(10):1722-1728.
29. Centers for Disease Control and Prevention. Quitting smoking among adults – United States, 2001–2010. MMWR. 2011;60(44):1513-1519.
30. McClure EA, Baker NL, Gipson CD, et al. An open-label pilot trial of N-acetylcysteine and varenicline in adult cigarette smokers. Am J Drug Alcohol Abuse. 2015;41(1):52-56.
31. Froeliger B, McConnell P, Stankeviciute N, et al. The effects of N-acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: a double-blind, placebo-controlled fMRI pilot study. Drug Alcohol Depend. 2015;156:234-242.
32. Prado E, Maes M, Piccoli LG, et al. N-acetylcysteine for therapy-resistant tobacco use disorder: a pilot study. Redox Rep. 2015;20(5):215-222.
33. Knackstedt LA, LaRowe S, Mardikian P, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65(10):841-845.
34. Grant JE, Odlaug BL, Chamberlain SR, et al. A randomized, placebo-controlled trial of N-acetylcysteine plus imaginal desensitization for nicotine-dependent pathological gamblers. J Clin Psychiatry. 2014;75(1):39-45.
35. Schmaal L, Berk L, Hulstijn KP, et al. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. Eur Addiction Res. 2011;17(4):211-216.
36. Bernardo M, Dodd S, Gama CS, et al. Effects of N‐acetylcysteine on substance use in bipolar disorder: a randomised placebo‐controlled clinical trial. Acta Neuropsychiatr. 2009;21(5):239-245.
37. McClure EA, Baker NL, Gray KM. Cigarette smoking during an N-acetylcysteine-assisted cannabis cessation trial in adolescents. Am J Drug Alcohol Abuse. 2014;40(4):285-291.
38. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
39. Back SE, McCauley JL, Korte KJ, et al. A double-blind randomized controlled pilot trial of N-acetylcysteine in veterans with PTSD and substance use disorders. J Clin Psychiatry. 2016;77(11):e1439-e1446.
40. Squeglia LM, Baker NL, McClure EA, et al. Alcohol use during a trial of N-acetylcysteine for adolescent marijuana cessation. Addict Behav. 2016;63:172-177.
41. Lebourgeois S, González-Marín MC, Jeanblanc J, et al. Effect of N-acetylcysteine on motivation, seeking and relapse to ethanol self-administration. Addict Biol. 2018;23(2):643-652.
42. Schneider R Jr, Santos CF, Clarimundo V, et al. N-acetylcysteine prevents behavioral and biochemical changes induced by alcohol cessation in rats. Alcohol. 2015;49(3):259-263.
43. Parnell SE, Sulik KK, Dehart DB, et al. Reduction of ethanol-induced ocular abnormalities in mice via dietary administration of N-acetylcysteine. Alcohol. 2010;44(7-8):699-705.
44. Ozkol H, Bulut G, Balahoroglu R, et al. Protective effects of Selenium, N-acetylcysteine and Vitamin E against acute ethanol intoxication in rats. Biol Trace Elem Res. 2017;175(1):177-185.
45. Seiva FR, Amauchi JF, Rocha KK, et al. Alcoholism and alcohol abstinence: N-acetylcysteine to improve energy expenditure, myocardial oxidative stress, and energy metabolism in alcoholic heart disease. Alcohol. 2009;43(8):649-656.
46. Setshedi M, Longato L, Petersen DR, et al. Limited therapeutic effect of N‐acetylcysteine on hepatic insulin resistance in an experimental model of alcohol‐induced steatohepatitis. Alcohol Clin Exp Res. 2011;35(12):2139-2151.
47. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732-741.
Repetitive transcranial magnetic stimulation for tic disorders
Tourette syndrome (TS) is a chronic neuropsychiatric disorder occurring in early childhood or adolescence that’s characterized by multiple motor and vocal tics that are usually preceded by premonitory urges.1,2 Usually, the tics are repetitive, sudden, stereotypical, non-rhythmic movements and/or vocalizations.3,4 Individuals with TS and other tic disorders often experience impulsivity, aggression, obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder, and various mood and anxiety disorders.3 Psychosocial issues may include having low self-esteem, increased family conflict, and poor social skills. Males are affected 3 to 5 times more often than females.3
There is no definitive treatment for TS. Commonly used interventions are pharmacotherapy and/or behavioral therapy, which includes supportive psychotherapy, habit reversal training, exposure with response prevention, relaxation therapy, cognitive-behavioral therapy, and self-monitoring. Pharmacotherapy for TS and other tic disorders consists mainly of antipsychotics such as haloperidol, pimozide, and aripiprazole, and alpha-2 agonists (guanfacine and clonidine).4,8-10 Unfortunately, not all children respond to these medications, and these agents are associated with multiple adverse effects.11 Therefore, there is a need for additional treatment options for patients with TS and other tic disorders, especially those who are not helped by conventional treatments.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive therapeutic technique in which high-intensity magnetic impulses are delivered through an electromagnetic coil placed on the patient’s scalp to stimulate cortical neurons. The effect is determined by various parameters, including the intensity, frequency, pulse number, duration, coil location, and type of coil.3,8
rTMS is FDA-approved for treating depression, and has been used to treat anxiety disorders, Parkinson’s disease, chronic pain syndromes, and dystonia.12,13 Researchers have begun to evaluate the usefulness of rTMS for patients with TS or other tic disorders. In this article, we review the findings of 11 studies—9 clinical trials and 2 case studies—that evaluated rTMS as a treatment option for patients with tic disorders.
A proposed mechanism of action
TS is believed to be caused by multiple factors, including neurotransmitter imbalances and genetic, environmental, and psychosocial factors.14 Evidence strongly suggests the involvement of the motor cortex, basal ganglia, and reticular activating system in the expression of TS.2,15-17
Researchers have consistently identified networks of regions in the brain, including the supplementary motor area (SMA), that are active in the seconds before tics occur in patients with these disorders.6,18-22 The SMA modulates the way information is channeled between motor circuits, the limbic system, and the cognitive processes.3,23-26 The SMA can be used as a target for focal brain stimulation to modulate activity in those circuits and improve symptoms in resistant patients. Recent rTMS studies that targeted the SMA have found that stimulation to this area may be an effective way to treat TS.19,20,23,27
Continue to: rTMS for tics: Mixed evidence
rTMS for tics: Mixed evidence
We reviewed the results of 11 studies that described the use of rTMS for TS and other tic disorders (Table 11,24-26,28,29 and Table 23,8,23,27,30,31). They included:
- 2 double-blind, randomized controlled trials28,29
- 2 single-blind trials24-26
- 1 double-blind trial with an open-label extension1
- 4 open-label studies3,8,23,30
- 1 case series27 and 1 case report.31
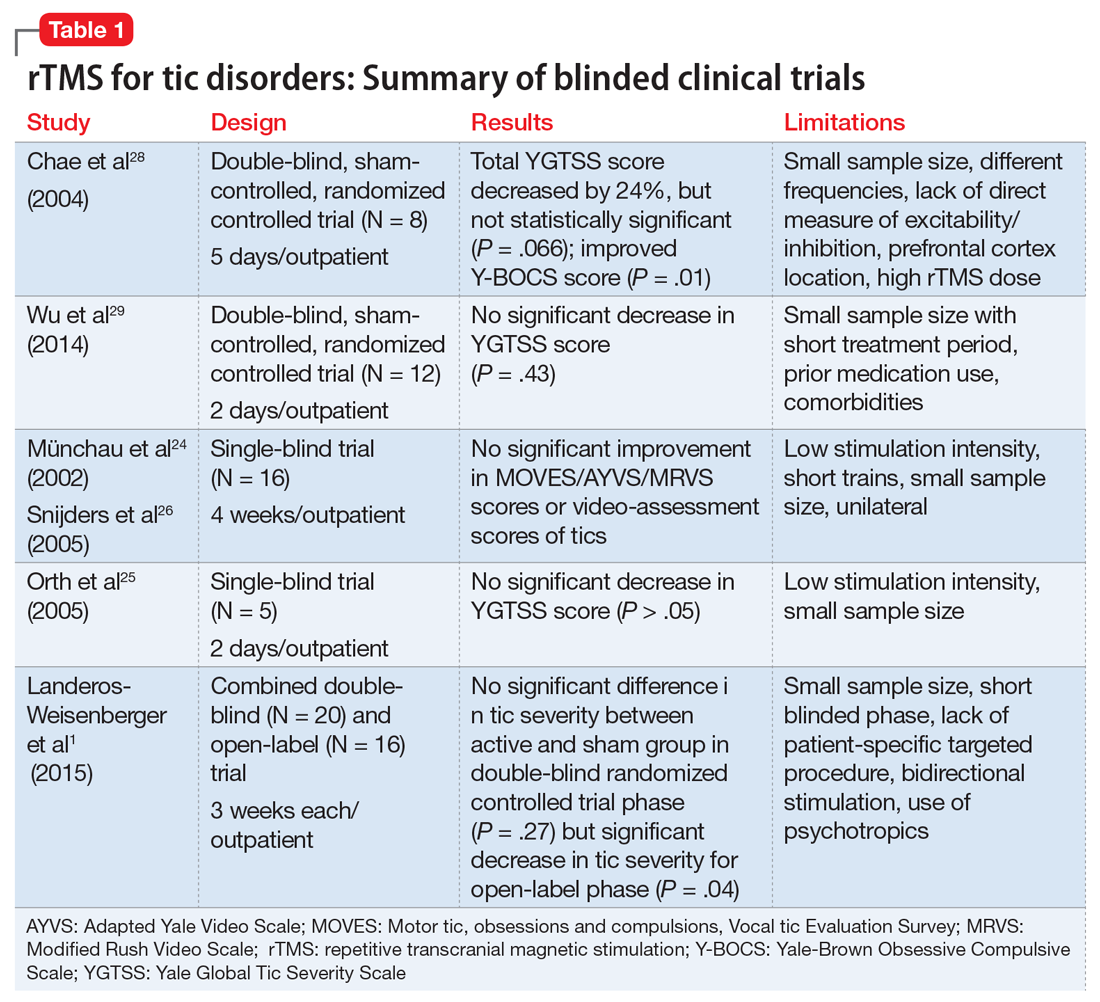
Study characteristics. In the 11 studies we reviewed, the duration of rTMS treatment varied from 2 days to 4 weeks. The pulses used were 900, 1,200, 1,800, and 2,400 per day, and the frequencies were 1 Hz, 4 Hz, 15 Hz and 30 Hz. Seven studies did not use placebo- or sham-controlled arms.1,3,8,23,27,30,31
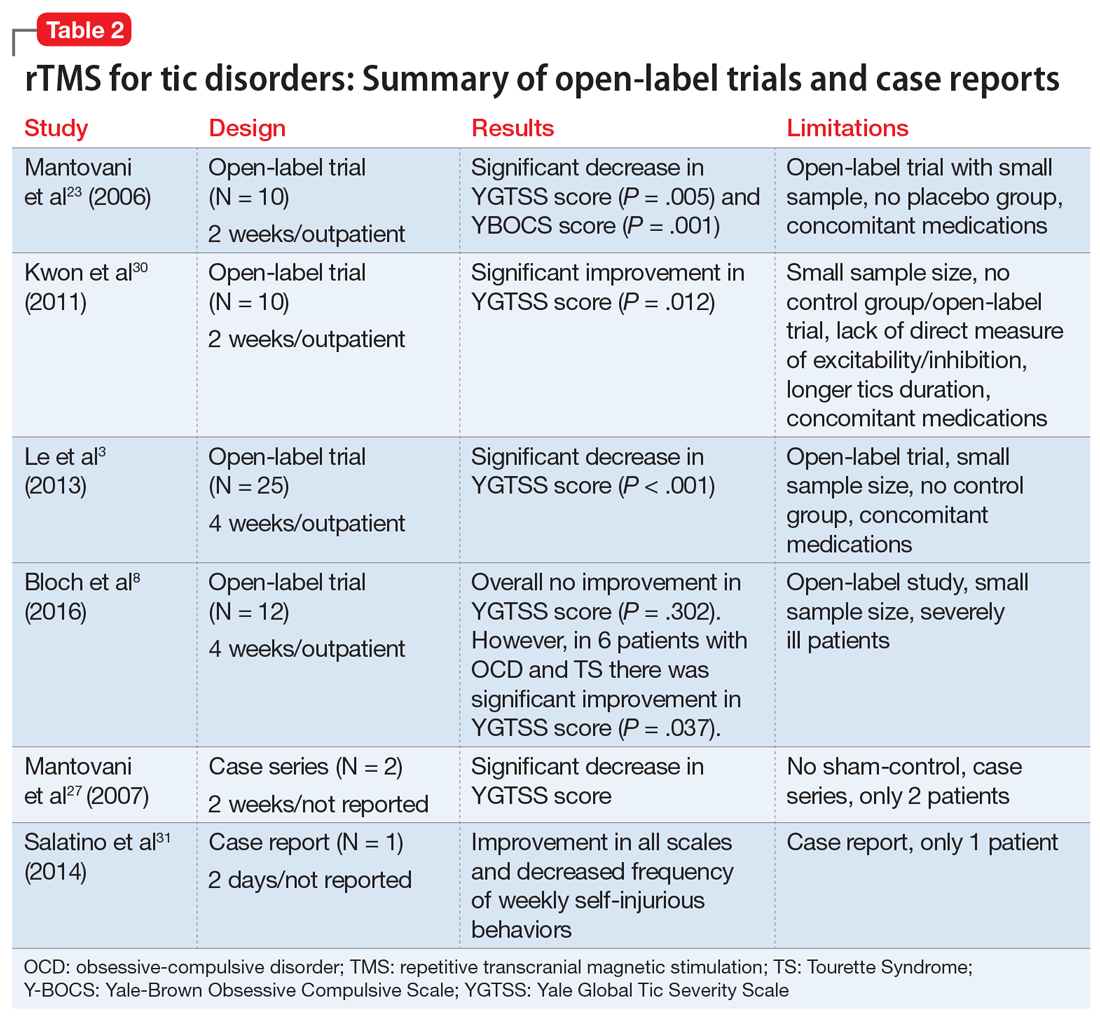
Efficacy. Two double-blind trials28,29 found no significant improvement in tic severity in patients treated with rTMS (P = .066 and P = .43, respectively). In addition, the 2 single-blind studies showed no beneficial effects of rTMS for patients with tics (P > .05).24-26 However, 3 of the 4 open-label studies found a significant improvement in tics.3,23,30 In one of the double-blind trials, researchers added an open-label extension phase.1 They found no significant results in the double-blind phase of the study (P = .27), but in the open-label phase, patients experienced a significant improvement in tic severity (P = .04).1 Lastly, the case series and case report found an improvement in tic severity and improvement in TS symptoms, respectively, with rTMS treatment.
rTMS might also improve symptoms of OCD that may co-occur with TS.8,23,28 Two studies found significant improvement in tic severity in a subgroup of patients suffering from comorbid OCD.8,28
Continue to: Safety profile and adverse effects
Safety profile and adverse effects. In the studies we reviewed, the adverse effects associated with rTMS included headache (45%),1,8,24,26,28,29 scalp pain (18%),8,30 self-injurious crisis (9%),31 abdominal pain (9%),29 red eyes (9%),29 neck pain (9%),1 muscle sprain (9%),1 tiredness (9%),24,26 and increase in motor excitability (9%).28 There were no severe adverse effects reported in any of the studies. The self-injurious crisis reported by a patient early in one study as a seizure was later ruled out after careful clinical and electroencephalographic evaluation. This patient demonstrated self-injurious behaviors prior to the treatment, and overall there was a reduction in frequency and intensity of self-injurious behavior as well as an improvement in tics.31
Dissimilar studies
There was great heterogeneity among the 11 studies we reviewed. One case series27 and one case report31 found significant improvement in tics, but these studies did not have control groups. Both studies employed rTMS with a frequency of 1 Hz and between 900 to 1,200 pulses per day. Three open-label studies that found significant improvement in tic severity used the same frequency of stimulation (1 Hz with 1,200 pulses per day).3,23,30 All studies we analyzed differed in the total number of rTMS sessions and number of trains per stimulation.
The studies also differed in terms of the age of the participants. Some studies focused primarily on pediatric patients,3,30 but many of them also included adults. The main limitations of the 11 studies included a small sample size,1,3,8,23-25,28-30 no placebo or controlled arm,1,3,8,23,27,30,31 concomitant psychiatric comorbidities8,28,29 or medications,1,3,23,29,30 low stimulation intensity,24-26 and use of short trains24,26 or unilateral cerebral stimulation.24,26 Among the blinded studies, limitations included a small sample size, prior medications used, comorbidities, low stimulation intensity, and high rTMS dose.1,24-26,28,29
A possible option for treatment-resistant tics
We cannot offer a definitive conclusion on the safety and effectiveness of rTMS for the treatment of TS and other tic disorders because of the inconsistent results, heterogeneity, and small sample sizes of the studies we analyzed. Higher-quality studies failed to find evidence supporting the use of rTMS for treating TS and other tics disorders, but open-label studies and case reports found significant improvements. In light of this evidence and the treatment’s relatively favorable adverse-effects profile, rTMS might be an option for certain patients with treatment-resistant tics, particularly those with comorbid OCD symptoms.
Continue to: Bottom Line
Bottom Line
The evidence for using repetitive transcranial stimulation (rTMS) to treat patients with Tourette syndrome and other tic disorders is mixed. Higher-quality studies have found no significant improvements, whereas open-label studies and case studies have. Although not recommended for the routine treatment of tic disorders, rTMS may be an option for patients with treatment-resistant tics, particularly those with comorbid obsessive-compulsive symptoms.
Related Resources
- Tourette Association of America. https://www.tourette.org/.
- Harris E. Children with tic disorders: How to match treatment with symptoms. Current Psychiatry. 2010;9(3):29-36.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres, Duraclon
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Pimozide • Orap
1. Landeros-Weisenberger A, Mantovani A, Motlagh MG, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe Tourette syndrome. Brain Stimulat. 2015;8(3):574-581.
2. Kamble N, Netravathi M, Pal PK. Therapeutic applications of repetitive transcranial magnetic stimulation (rTMS) in movement disorders: a review. Parkinsonism Relat Disord. 2014;20(7):695-707.
3. Le K, Liu L, Sun M, et al. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20(2):257-262.
4. Cavanna AE, Seri S. Tourette’s syndrome. BMJ. 2013;347:f4964. doi:10.1136/bmj.f4964.
5. Leckman JF, Bloch MH, Scahill L, et al. Tourette syndrome: the self under siege. J Child Neurol. 2006;21(8):642-649.
6. Bloch MH, Peterson BS, Scahill L, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):65-69.
7. Bloch M, State M, Pittenger C. Recent advances in Tourette syndrome. Curr Opin Neurol. 2011;24(2):119-125.
8. Bloch Y, Arad S, Levkovitz Y. Deep TMS add-on treatment for intractable Tourette syndrome: a feasibility study. World J Biol Psychiatry. 2016;17(7):557-561.
9. Robertson MM. The Gilles de la Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed. 2012;97(5):166-175.
10. Párraga HC, Harris KM, Párraga KL, et al. An overview of the treatment of Tourette’s disorder and tics. J Child Adolesc Psychopharmacol. 2010;20(4):249-262.
11. Du JC, Chiu TF, Lee KM, et al. Tourette syndrome in children: an updated review. Pediatr Neonatol. 2010;51(5):255-264.
12. Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13(4):372-378.
13. Di Lazzaro V, Oliviero A, Berardelli A, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002;144(4):549-553.
14. Olson LL, Singer HS, Goodman WK, et al. Tourette syndrome: diagnosis, strategies, therapies, pathogenesis, and future research directions. J Child Neurol. 2006;21(8):630-641.
15. Gerard E, Peterson BS. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res. 2003;55(1):13-22.
16. Kurlan R. Hypothesis II: Tourette’s syndrome is part of a clinical spectrum that includes normal brain development. Arch Neurol. 1994;51(11):1145-1150.
17. Peterson BS. Neuroimaging in child and adolescent neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 1995;34(12):1560-1576.
18. Sheppard DM, Bradshaw JL, Purcell R, et al. Tourette’s and comorbid syndromes: obsessive compulsive and attention deficit hyperactivity disorder. A common etiology? Clin Psychol Rev. 1999;19(5):531-552.
19. Bohlhalter S, Goldfine A, Matteson S, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(pt 8):2029-2037.
20. Hampson M, Tokoglu F, King RA, et al. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65(7):594-599.
21. Eichele H, Plessen KJ. Neural plasticity in functional and anatomical MRI studies of children with Tourette syndrome. Behav Neurol. 2013;27(1):33-45.
22. Neuner I, Schneider F, Shah NJ. Functional neuroanatomy of tics. Int Rev Neurobiol. 2013;112:35-71.
23. Mantovani A, Lisanby SH, Pieraccini F, et al. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS). Int J Neuropsychopharmacol. 2006;9(1):95-100.
24. Münchau A, Bloem BR, Thilo KV, et al. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology. 2002;59(11):1789-1791.
25. Orth M, Kirby R, Richardson MP, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patients with Gilles de la Tourette syndrome. Clin Neurophysiol. 2005;116(4):764-768.
26. Snijders AH, Bloem BR, Orth M, et al. Video assessment of rTMS for Tourette syndrome. J Neurol Neurosurg Psychiatry. 2005;76(12):1743-1744.
27. Mantovani A, Leckman JF, Grantz H, et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: report of two cases. Clin Neurophysiol. 2007;118(10):2314-2315.
28. Chae JH, Nahas Z, Wassermann E, et al. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette’s syndrome. Cogn Behav Neurol. 2004;17(2):109-117.
29. Wu SW, Maloney T, Gilbert DL, et al. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul. 2014;7(2):212-218.
30. Kwon HJ, Lim WS, Lim MH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492(1):1-4.
31. Salatino A, Momo E, Nobili M, et al. Awareness of symptoms amelioration following low-frequency repetitive transcranial magnetic stimulation in a patient with Tourette syndrome and comorbid obsessive-compulsive disorder. Brain Stimulat. 2014;7(2):341-343.
Tourette syndrome (TS) is a chronic neuropsychiatric disorder occurring in early childhood or adolescence that’s characterized by multiple motor and vocal tics that are usually preceded by premonitory urges.1,2 Usually, the tics are repetitive, sudden, stereotypical, non-rhythmic movements and/or vocalizations.3,4 Individuals with TS and other tic disorders often experience impulsivity, aggression, obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder, and various mood and anxiety disorders.3 Psychosocial issues may include having low self-esteem, increased family conflict, and poor social skills. Males are affected 3 to 5 times more often than females.3
There is no definitive treatment for TS. Commonly used interventions are pharmacotherapy and/or behavioral therapy, which includes supportive psychotherapy, habit reversal training, exposure with response prevention, relaxation therapy, cognitive-behavioral therapy, and self-monitoring. Pharmacotherapy for TS and other tic disorders consists mainly of antipsychotics such as haloperidol, pimozide, and aripiprazole, and alpha-2 agonists (guanfacine and clonidine).4,8-10 Unfortunately, not all children respond to these medications, and these agents are associated with multiple adverse effects.11 Therefore, there is a need for additional treatment options for patients with TS and other tic disorders, especially those who are not helped by conventional treatments.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive therapeutic technique in which high-intensity magnetic impulses are delivered through an electromagnetic coil placed on the patient’s scalp to stimulate cortical neurons. The effect is determined by various parameters, including the intensity, frequency, pulse number, duration, coil location, and type of coil.3,8
rTMS is FDA-approved for treating depression, and has been used to treat anxiety disorders, Parkinson’s disease, chronic pain syndromes, and dystonia.12,13 Researchers have begun to evaluate the usefulness of rTMS for patients with TS or other tic disorders. In this article, we review the findings of 11 studies—9 clinical trials and 2 case studies—that evaluated rTMS as a treatment option for patients with tic disorders.
A proposed mechanism of action
TS is believed to be caused by multiple factors, including neurotransmitter imbalances and genetic, environmental, and psychosocial factors.14 Evidence strongly suggests the involvement of the motor cortex, basal ganglia, and reticular activating system in the expression of TS.2,15-17
Researchers have consistently identified networks of regions in the brain, including the supplementary motor area (SMA), that are active in the seconds before tics occur in patients with these disorders.6,18-22 The SMA modulates the way information is channeled between motor circuits, the limbic system, and the cognitive processes.3,23-26 The SMA can be used as a target for focal brain stimulation to modulate activity in those circuits and improve symptoms in resistant patients. Recent rTMS studies that targeted the SMA have found that stimulation to this area may be an effective way to treat TS.19,20,23,27
Continue to: rTMS for tics: Mixed evidence
rTMS for tics: Mixed evidence
We reviewed the results of 11 studies that described the use of rTMS for TS and other tic disorders (Table 11,24-26,28,29 and Table 23,8,23,27,30,31). They included:
- 2 double-blind, randomized controlled trials28,29
- 2 single-blind trials24-26
- 1 double-blind trial with an open-label extension1
- 4 open-label studies3,8,23,30
- 1 case series27 and 1 case report.31

Study characteristics. In the 11 studies we reviewed, the duration of rTMS treatment varied from 2 days to 4 weeks. The pulses used were 900, 1,200, 1,800, and 2,400 per day, and the frequencies were 1 Hz, 4 Hz, 15 Hz and 30 Hz. Seven studies did not use placebo- or sham-controlled arms.1,3,8,23,27,30,31

Efficacy. Two double-blind trials28,29 found no significant improvement in tic severity in patients treated with rTMS (P = .066 and P = .43, respectively). In addition, the 2 single-blind studies showed no beneficial effects of rTMS for patients with tics (P > .05).24-26 However, 3 of the 4 open-label studies found a significant improvement in tics.3,23,30 In one of the double-blind trials, researchers added an open-label extension phase.1 They found no significant results in the double-blind phase of the study (P = .27), but in the open-label phase, patients experienced a significant improvement in tic severity (P = .04).1 Lastly, the case series and case report found an improvement in tic severity and improvement in TS symptoms, respectively, with rTMS treatment.
rTMS might also improve symptoms of OCD that may co-occur with TS.8,23,28 Two studies found significant improvement in tic severity in a subgroup of patients suffering from comorbid OCD.8,28
Continue to: Safety profile and adverse effects
Safety profile and adverse effects. In the studies we reviewed, the adverse effects associated with rTMS included headache (45%),1,8,24,26,28,29 scalp pain (18%),8,30 self-injurious crisis (9%),31 abdominal pain (9%),29 red eyes (9%),29 neck pain (9%),1 muscle sprain (9%),1 tiredness (9%),24,26 and increase in motor excitability (9%).28 There were no severe adverse effects reported in any of the studies. The self-injurious crisis reported by a patient early in one study as a seizure was later ruled out after careful clinical and electroencephalographic evaluation. This patient demonstrated self-injurious behaviors prior to the treatment, and overall there was a reduction in frequency and intensity of self-injurious behavior as well as an improvement in tics.31
Dissimilar studies
There was great heterogeneity among the 11 studies we reviewed. One case series27 and one case report31 found significant improvement in tics, but these studies did not have control groups. Both studies employed rTMS with a frequency of 1 Hz and between 900 to 1,200 pulses per day. Three open-label studies that found significant improvement in tic severity used the same frequency of stimulation (1 Hz with 1,200 pulses per day).3,23,30 All studies we analyzed differed in the total number of rTMS sessions and number of trains per stimulation.
The studies also differed in terms of the age of the participants. Some studies focused primarily on pediatric patients,3,30 but many of them also included adults. The main limitations of the 11 studies included a small sample size,1,3,8,23-25,28-30 no placebo or controlled arm,1,3,8,23,27,30,31 concomitant psychiatric comorbidities8,28,29 or medications,1,3,23,29,30 low stimulation intensity,24-26 and use of short trains24,26 or unilateral cerebral stimulation.24,26 Among the blinded studies, limitations included a small sample size, prior medications used, comorbidities, low stimulation intensity, and high rTMS dose.1,24-26,28,29
A possible option for treatment-resistant tics
We cannot offer a definitive conclusion on the safety and effectiveness of rTMS for the treatment of TS and other tic disorders because of the inconsistent results, heterogeneity, and small sample sizes of the studies we analyzed. Higher-quality studies failed to find evidence supporting the use of rTMS for treating TS and other tics disorders, but open-label studies and case reports found significant improvements. In light of this evidence and the treatment’s relatively favorable adverse-effects profile, rTMS might be an option for certain patients with treatment-resistant tics, particularly those with comorbid OCD symptoms.
Continue to: Bottom Line
Bottom Line
The evidence for using repetitive transcranial stimulation (rTMS) to treat patients with Tourette syndrome and other tic disorders is mixed. Higher-quality studies have found no significant improvements, whereas open-label studies and case studies have. Although not recommended for the routine treatment of tic disorders, rTMS may be an option for patients with treatment-resistant tics, particularly those with comorbid obsessive-compulsive symptoms.
Related Resources
- Tourette Association of America. https://www.tourette.org/.
- Harris E. Children with tic disorders: How to match treatment with symptoms. Current Psychiatry. 2010;9(3):29-36.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres, Duraclon
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Pimozide • Orap
Tourette syndrome (TS) is a chronic neuropsychiatric disorder occurring in early childhood or adolescence that’s characterized by multiple motor and vocal tics that are usually preceded by premonitory urges.1,2 Usually, the tics are repetitive, sudden, stereotypical, non-rhythmic movements and/or vocalizations.3,4 Individuals with TS and other tic disorders often experience impulsivity, aggression, obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder, and various mood and anxiety disorders.3 Psychosocial issues may include having low self-esteem, increased family conflict, and poor social skills. Males are affected 3 to 5 times more often than females.3
There is no definitive treatment for TS. Commonly used interventions are pharmacotherapy and/or behavioral therapy, which includes supportive psychotherapy, habit reversal training, exposure with response prevention, relaxation therapy, cognitive-behavioral therapy, and self-monitoring. Pharmacotherapy for TS and other tic disorders consists mainly of antipsychotics such as haloperidol, pimozide, and aripiprazole, and alpha-2 agonists (guanfacine and clonidine).4,8-10 Unfortunately, not all children respond to these medications, and these agents are associated with multiple adverse effects.11 Therefore, there is a need for additional treatment options for patients with TS and other tic disorders, especially those who are not helped by conventional treatments.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive therapeutic technique in which high-intensity magnetic impulses are delivered through an electromagnetic coil placed on the patient’s scalp to stimulate cortical neurons. The effect is determined by various parameters, including the intensity, frequency, pulse number, duration, coil location, and type of coil.3,8
rTMS is FDA-approved for treating depression, and has been used to treat anxiety disorders, Parkinson’s disease, chronic pain syndromes, and dystonia.12,13 Researchers have begun to evaluate the usefulness of rTMS for patients with TS or other tic disorders. In this article, we review the findings of 11 studies—9 clinical trials and 2 case studies—that evaluated rTMS as a treatment option for patients with tic disorders.
A proposed mechanism of action
TS is believed to be caused by multiple factors, including neurotransmitter imbalances and genetic, environmental, and psychosocial factors.14 Evidence strongly suggests the involvement of the motor cortex, basal ganglia, and reticular activating system in the expression of TS.2,15-17
Researchers have consistently identified networks of regions in the brain, including the supplementary motor area (SMA), that are active in the seconds before tics occur in patients with these disorders.6,18-22 The SMA modulates the way information is channeled between motor circuits, the limbic system, and the cognitive processes.3,23-26 The SMA can be used as a target for focal brain stimulation to modulate activity in those circuits and improve symptoms in resistant patients. Recent rTMS studies that targeted the SMA have found that stimulation to this area may be an effective way to treat TS.19,20,23,27
Continue to: rTMS for tics: Mixed evidence
rTMS for tics: Mixed evidence
We reviewed the results of 11 studies that described the use of rTMS for TS and other tic disorders (Table 11,24-26,28,29 and Table 23,8,23,27,30,31). They included:
- 2 double-blind, randomized controlled trials28,29
- 2 single-blind trials24-26
- 1 double-blind trial with an open-label extension1
- 4 open-label studies3,8,23,30
- 1 case series27 and 1 case report.31

Study characteristics. In the 11 studies we reviewed, the duration of rTMS treatment varied from 2 days to 4 weeks. The pulses used were 900, 1,200, 1,800, and 2,400 per day, and the frequencies were 1 Hz, 4 Hz, 15 Hz and 30 Hz. Seven studies did not use placebo- or sham-controlled arms.1,3,8,23,27,30,31

Efficacy. Two double-blind trials28,29 found no significant improvement in tic severity in patients treated with rTMS (P = .066 and P = .43, respectively). In addition, the 2 single-blind studies showed no beneficial effects of rTMS for patients with tics (P > .05).24-26 However, 3 of the 4 open-label studies found a significant improvement in tics.3,23,30 In one of the double-blind trials, researchers added an open-label extension phase.1 They found no significant results in the double-blind phase of the study (P = .27), but in the open-label phase, patients experienced a significant improvement in tic severity (P = .04).1 Lastly, the case series and case report found an improvement in tic severity and improvement in TS symptoms, respectively, with rTMS treatment.
rTMS might also improve symptoms of OCD that may co-occur with TS.8,23,28 Two studies found significant improvement in tic severity in a subgroup of patients suffering from comorbid OCD.8,28
Continue to: Safety profile and adverse effects
Safety profile and adverse effects. In the studies we reviewed, the adverse effects associated with rTMS included headache (45%),1,8,24,26,28,29 scalp pain (18%),8,30 self-injurious crisis (9%),31 abdominal pain (9%),29 red eyes (9%),29 neck pain (9%),1 muscle sprain (9%),1 tiredness (9%),24,26 and increase in motor excitability (9%).28 There were no severe adverse effects reported in any of the studies. The self-injurious crisis reported by a patient early in one study as a seizure was later ruled out after careful clinical and electroencephalographic evaluation. This patient demonstrated self-injurious behaviors prior to the treatment, and overall there was a reduction in frequency and intensity of self-injurious behavior as well as an improvement in tics.31
Dissimilar studies
There was great heterogeneity among the 11 studies we reviewed. One case series27 and one case report31 found significant improvement in tics, but these studies did not have control groups. Both studies employed rTMS with a frequency of 1 Hz and between 900 to 1,200 pulses per day. Three open-label studies that found significant improvement in tic severity used the same frequency of stimulation (1 Hz with 1,200 pulses per day).3,23,30 All studies we analyzed differed in the total number of rTMS sessions and number of trains per stimulation.
The studies also differed in terms of the age of the participants. Some studies focused primarily on pediatric patients,3,30 but many of them also included adults. The main limitations of the 11 studies included a small sample size,1,3,8,23-25,28-30 no placebo or controlled arm,1,3,8,23,27,30,31 concomitant psychiatric comorbidities8,28,29 or medications,1,3,23,29,30 low stimulation intensity,24-26 and use of short trains24,26 or unilateral cerebral stimulation.24,26 Among the blinded studies, limitations included a small sample size, prior medications used, comorbidities, low stimulation intensity, and high rTMS dose.1,24-26,28,29
A possible option for treatment-resistant tics
We cannot offer a definitive conclusion on the safety and effectiveness of rTMS for the treatment of TS and other tic disorders because of the inconsistent results, heterogeneity, and small sample sizes of the studies we analyzed. Higher-quality studies failed to find evidence supporting the use of rTMS for treating TS and other tics disorders, but open-label studies and case reports found significant improvements. In light of this evidence and the treatment’s relatively favorable adverse-effects profile, rTMS might be an option for certain patients with treatment-resistant tics, particularly those with comorbid OCD symptoms.
Continue to: Bottom Line
Bottom Line
The evidence for using repetitive transcranial stimulation (rTMS) to treat patients with Tourette syndrome and other tic disorders is mixed. Higher-quality studies have found no significant improvements, whereas open-label studies and case studies have. Although not recommended for the routine treatment of tic disorders, rTMS may be an option for patients with treatment-resistant tics, particularly those with comorbid obsessive-compulsive symptoms.
Related Resources
- Tourette Association of America. https://www.tourette.org/.
- Harris E. Children with tic disorders: How to match treatment with symptoms. Current Psychiatry. 2010;9(3):29-36.
Drug Brand Names
Aripiprazole • Abilify
Clonidine • Catapres, Duraclon
Guanfacine • Intuniv, Tenex
Haloperidol • Haldol
Pimozide • Orap
1. Landeros-Weisenberger A, Mantovani A, Motlagh MG, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe Tourette syndrome. Brain Stimulat. 2015;8(3):574-581.
2. Kamble N, Netravathi M, Pal PK. Therapeutic applications of repetitive transcranial magnetic stimulation (rTMS) in movement disorders: a review. Parkinsonism Relat Disord. 2014;20(7):695-707.
3. Le K, Liu L, Sun M, et al. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20(2):257-262.
4. Cavanna AE, Seri S. Tourette’s syndrome. BMJ. 2013;347:f4964. doi:10.1136/bmj.f4964.
5. Leckman JF, Bloch MH, Scahill L, et al. Tourette syndrome: the self under siege. J Child Neurol. 2006;21(8):642-649.
6. Bloch MH, Peterson BS, Scahill L, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):65-69.
7. Bloch M, State M, Pittenger C. Recent advances in Tourette syndrome. Curr Opin Neurol. 2011;24(2):119-125.
8. Bloch Y, Arad S, Levkovitz Y. Deep TMS add-on treatment for intractable Tourette syndrome: a feasibility study. World J Biol Psychiatry. 2016;17(7):557-561.
9. Robertson MM. The Gilles de la Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed. 2012;97(5):166-175.
10. Párraga HC, Harris KM, Párraga KL, et al. An overview of the treatment of Tourette’s disorder and tics. J Child Adolesc Psychopharmacol. 2010;20(4):249-262.
11. Du JC, Chiu TF, Lee KM, et al. Tourette syndrome in children: an updated review. Pediatr Neonatol. 2010;51(5):255-264.
12. Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13(4):372-378.
13. Di Lazzaro V, Oliviero A, Berardelli A, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002;144(4):549-553.
14. Olson LL, Singer HS, Goodman WK, et al. Tourette syndrome: diagnosis, strategies, therapies, pathogenesis, and future research directions. J Child Neurol. 2006;21(8):630-641.
15. Gerard E, Peterson BS. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res. 2003;55(1):13-22.
16. Kurlan R. Hypothesis II: Tourette’s syndrome is part of a clinical spectrum that includes normal brain development. Arch Neurol. 1994;51(11):1145-1150.
17. Peterson BS. Neuroimaging in child and adolescent neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 1995;34(12):1560-1576.
18. Sheppard DM, Bradshaw JL, Purcell R, et al. Tourette’s and comorbid syndromes: obsessive compulsive and attention deficit hyperactivity disorder. A common etiology? Clin Psychol Rev. 1999;19(5):531-552.
19. Bohlhalter S, Goldfine A, Matteson S, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(pt 8):2029-2037.
20. Hampson M, Tokoglu F, King RA, et al. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65(7):594-599.
21. Eichele H, Plessen KJ. Neural plasticity in functional and anatomical MRI studies of children with Tourette syndrome. Behav Neurol. 2013;27(1):33-45.
22. Neuner I, Schneider F, Shah NJ. Functional neuroanatomy of tics. Int Rev Neurobiol. 2013;112:35-71.
23. Mantovani A, Lisanby SH, Pieraccini F, et al. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS). Int J Neuropsychopharmacol. 2006;9(1):95-100.
24. Münchau A, Bloem BR, Thilo KV, et al. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology. 2002;59(11):1789-1791.
25. Orth M, Kirby R, Richardson MP, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patients with Gilles de la Tourette syndrome. Clin Neurophysiol. 2005;116(4):764-768.
26. Snijders AH, Bloem BR, Orth M, et al. Video assessment of rTMS for Tourette syndrome. J Neurol Neurosurg Psychiatry. 2005;76(12):1743-1744.
27. Mantovani A, Leckman JF, Grantz H, et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: report of two cases. Clin Neurophysiol. 2007;118(10):2314-2315.
28. Chae JH, Nahas Z, Wassermann E, et al. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette’s syndrome. Cogn Behav Neurol. 2004;17(2):109-117.
29. Wu SW, Maloney T, Gilbert DL, et al. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul. 2014;7(2):212-218.
30. Kwon HJ, Lim WS, Lim MH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492(1):1-4.
31. Salatino A, Momo E, Nobili M, et al. Awareness of symptoms amelioration following low-frequency repetitive transcranial magnetic stimulation in a patient with Tourette syndrome and comorbid obsessive-compulsive disorder. Brain Stimulat. 2014;7(2):341-343.
1. Landeros-Weisenberger A, Mantovani A, Motlagh MG, et al. Randomized sham controlled double-blind trial of repetitive transcranial magnetic stimulation for adults with severe Tourette syndrome. Brain Stimulat. 2015;8(3):574-581.
2. Kamble N, Netravathi M, Pal PK. Therapeutic applications of repetitive transcranial magnetic stimulation (rTMS) in movement disorders: a review. Parkinsonism Relat Disord. 2014;20(7):695-707.
3. Le K, Liu L, Sun M, et al. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20(2):257-262.
4. Cavanna AE, Seri S. Tourette’s syndrome. BMJ. 2013;347:f4964. doi:10.1136/bmj.f4964.
5. Leckman JF, Bloch MH, Scahill L, et al. Tourette syndrome: the self under siege. J Child Neurol. 2006;21(8):642-649.
6. Bloch MH, Peterson BS, Scahill L, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160(1):65-69.
7. Bloch M, State M, Pittenger C. Recent advances in Tourette syndrome. Curr Opin Neurol. 2011;24(2):119-125.
8. Bloch Y, Arad S, Levkovitz Y. Deep TMS add-on treatment for intractable Tourette syndrome: a feasibility study. World J Biol Psychiatry. 2016;17(7):557-561.
9. Robertson MM. The Gilles de la Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed. 2012;97(5):166-175.
10. Párraga HC, Harris KM, Párraga KL, et al. An overview of the treatment of Tourette’s disorder and tics. J Child Adolesc Psychopharmacol. 2010;20(4):249-262.
11. Du JC, Chiu TF, Lee KM, et al. Tourette syndrome in children: an updated review. Pediatr Neonatol. 2010;51(5):255-264.
12. Malizia AL. What do brain imaging studies tell us about anxiety disorders? J Psychopharmacol. 1999;13(4):372-378.
13. Di Lazzaro V, Oliviero A, Berardelli A, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002;144(4):549-553.
14. Olson LL, Singer HS, Goodman WK, et al. Tourette syndrome: diagnosis, strategies, therapies, pathogenesis, and future research directions. J Child Neurol. 2006;21(8):630-641.
15. Gerard E, Peterson BS. Developmental processes and brain imaging studies in Tourette syndrome. J Psychosom Res. 2003;55(1):13-22.
16. Kurlan R. Hypothesis II: Tourette’s syndrome is part of a clinical spectrum that includes normal brain development. Arch Neurol. 1994;51(11):1145-1150.
17. Peterson BS. Neuroimaging in child and adolescent neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 1995;34(12):1560-1576.
18. Sheppard DM, Bradshaw JL, Purcell R, et al. Tourette’s and comorbid syndromes: obsessive compulsive and attention deficit hyperactivity disorder. A common etiology? Clin Psychol Rev. 1999;19(5):531-552.
19. Bohlhalter S, Goldfine A, Matteson S, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129(pt 8):2029-2037.
20. Hampson M, Tokoglu F, King RA, et al. Brain areas coactivating with motor cortex during chronic motor tics and intentional movements. Biol Psychiatry. 2009;65(7):594-599.
21. Eichele H, Plessen KJ. Neural plasticity in functional and anatomical MRI studies of children with Tourette syndrome. Behav Neurol. 2013;27(1):33-45.
22. Neuner I, Schneider F, Shah NJ. Functional neuroanatomy of tics. Int Rev Neurobiol. 2013;112:35-71.
23. Mantovani A, Lisanby SH, Pieraccini F, et al. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS). Int J Neuropsychopharmacol. 2006;9(1):95-100.
24. Münchau A, Bloem BR, Thilo KV, et al. Repetitive transcranial magnetic stimulation for Tourette syndrome. Neurology. 2002;59(11):1789-1791.
25. Orth M, Kirby R, Richardson MP, et al. Subthreshold rTMS over pre-motor cortex has no effect on tics in patients with Gilles de la Tourette syndrome. Clin Neurophysiol. 2005;116(4):764-768.
26. Snijders AH, Bloem BR, Orth M, et al. Video assessment of rTMS for Tourette syndrome. J Neurol Neurosurg Psychiatry. 2005;76(12):1743-1744.
27. Mantovani A, Leckman JF, Grantz H, et al. Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: report of two cases. Clin Neurophysiol. 2007;118(10):2314-2315.
28. Chae JH, Nahas Z, Wassermann E, et al. A pilot safety study of repetitive transcranial magnetic stimulation (rTMS) in Tourette’s syndrome. Cogn Behav Neurol. 2004;17(2):109-117.
29. Wu SW, Maloney T, Gilbert DL, et al. Functional MRI-navigated repetitive transcranial magnetic stimulation over supplementary motor area in chronic tic disorders. Brain Stimul. 2014;7(2):212-218.
30. Kwon HJ, Lim WS, Lim MH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492(1):1-4.
31. Salatino A, Momo E, Nobili M, et al. Awareness of symptoms amelioration following low-frequency repetitive transcranial magnetic stimulation in a patient with Tourette syndrome and comorbid obsessive-compulsive disorder. Brain Stimulat. 2014;7(2):341-343.
Is anatomy destiny? Not according to GxE!
The long-held dogma that “anatomy is destiny” is fraying at the edges. The traditional nature vs nurture debate has also undergone a major transformation into a gene-by-environment interaction, abbreviated as GxE in the medical literature.1,2 This is as true for psychiatric brain disorders as for any other medical illness.
The pessimistic determinism of “anatomy is destiny” has given way to a much more optimistic perspective, especially for the most plastic of all organs, the human brain. While genes are essential to construct one’s anatomy, environmental factors can significantly modulate gene expression. A person’s life experiences, good or bad, can wield a lasting influence on one’s brain structure and function, often transcending what is coded by the genome. For the mind, its thoughts, emotions, and cognition, the neurogenetic “tyranny” can be curbed or modified by one’s experiences. This epigenetic process is alive and well and known to be mediated by DNA methylation and histone modifications.
Consider the following examples of how genes are not the sole determinants of one’s mental health:
- A landmark study conducted in New Zealand3 followed a cohort of 847 individuals from age 3 to 26. Researchers recorded stressful life events for each participant, including romantic breakups, grief, medical illness, or employment problems, between age 21 and 26. Participants were evaluated for depressive episodes and hospitalizations and their genes tested for whether each individual carried the short (S) or long (L) allele of the serotonin transporter (5-HTT) gene. They found that when life stresses occurred, the probability of depression was much higher among the subgroup who were SS homozygous than among the LL homozygous subgroup. Thus, the genetic vulnerability to depression did not manifest itself unless adverse environmental events occurred. This is a classic example of GxE interaction, where genes alone are insufficient to produce a psychiatric disorder without environmental events interacting with them and triggering the psychopathology.
- In the same cohort described above, investigators showed that some children who were abused at an early age developed antisocial behavior as adults, while others did not.4 They discovered that a high expression of a polymorphism in the gene that codes for monoamine oxidase A had a protective effect that decreased the likelihood of developing antisocial traits in children who experienced trauma. In this case, the life experience failed to worsen a child’s behavior in the presence of elevated levels of a genetically determined protective enzyme.
- Schizophrenia is a heterogeneous neurodevelopmental syndrome caused by numerous genetic factors (risk genes, copy number variants, and de novo mutations) and a wide variety of perinatal complications. Concordance for schizophrenia in monozygotic twins who have identical genes is only 50%, not 100% as would be expected.5 Obviously, nongenetic factors during fetal life must play a role in disrupting the neurodevelopment of the affected twin, but not in the healthy twin. Examples of such factors may include differential distribution of blood during fetal life, leading to low birthweight and hypoplastic brain volume in the affected twin. It may also be due to labor complications, where one twin has an uneventful vaginal delivery while the other experiences hypoxia, a brain insult, due to a complicated breech delivery. Thus, despite having the same genes, the postnatal outcome in a discordant monozygotic twin pair diverges dramatically.
- A recent study6 identified somatic mutations in monozygotic twins discordant for psychiatric disorders, including schizophrenia and delusional disorder. Such somatic mutations have also been found in Van der Woude syndrome, which includes cleft palate. However, skillful surgeons can repair the cleft palate and allow the affected twin to have a normal facial appearance and oral functions, offsetting the abnormal genetic code.
- A monozygotic twin pair (one of whom was a patient of mine) born to a mother with bipolar disorder and adopted at birth by different families developed bipolar disorder due to genetic transmission, but eventually had very different outcomes. One twin was promptly and successfully treated with lithium at the first manic episode and became a successful teacher and author, while his twin did not receive treatment, became addicted to drugs, was repeatedly incarcerated for assaultive behavior, and later completed suicide at a young age. The appropriate environment and experiences of a person who inherits a psychiatric disorder can dramatically alter the prognosis for the better.
The GxE neurobiological equation is a central feature in many of our patients. As clinicians, we can modulate the patient’s environment by providing timely therapeutic biopsychosocial interventions to our patient to catalyze the GxE equation and veer it towards health, resilience, and wellness. Psychiatric practice can effectively help our patients overcome their genetically and neurobiologically driven maladaptive behavior and enable them to recover from the ravages of neuropsychiatric illness. Thus, psychiatric care represents the ultimate “E” that can interact with and modulate the “G” and effectively demonstrate that anatomy is not destiny.
1. Ridley M. Nature via nurture: genes, experience and what makes us human. New York, NY: Harper Collins; 2003.
2. Rutter M. Genes and behavior: nature–nurture interplay explained. Malden, MA: Blackwell Publishing; 2006.
3. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386-389.
4. Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851-854.
5. Stabenau JR, Pollin W. Heredity and environment in schizophrenia, revisited. The contribution of twin and high-risk studies. J Nerv Ment Dis. 1993;181(5):290-297.
6. Nishioka M, Bundo M, Ueda J, et al. Identification of somatic mutations in monozygotic twins discordant for psychiatric disorders. NPJ Schizophr. 2018;4(1):7.
The long-held dogma that “anatomy is destiny” is fraying at the edges. The traditional nature vs nurture debate has also undergone a major transformation into a gene-by-environment interaction, abbreviated as GxE in the medical literature.1,2 This is as true for psychiatric brain disorders as for any other medical illness.
The pessimistic determinism of “anatomy is destiny” has given way to a much more optimistic perspective, especially for the most plastic of all organs, the human brain. While genes are essential to construct one’s anatomy, environmental factors can significantly modulate gene expression. A person’s life experiences, good or bad, can wield a lasting influence on one’s brain structure and function, often transcending what is coded by the genome. For the mind, its thoughts, emotions, and cognition, the neurogenetic “tyranny” can be curbed or modified by one’s experiences. This epigenetic process is alive and well and known to be mediated by DNA methylation and histone modifications.
Consider the following examples of how genes are not the sole determinants of one’s mental health:
- A landmark study conducted in New Zealand3 followed a cohort of 847 individuals from age 3 to 26. Researchers recorded stressful life events for each participant, including romantic breakups, grief, medical illness, or employment problems, between age 21 and 26. Participants were evaluated for depressive episodes and hospitalizations and their genes tested for whether each individual carried the short (S) or long (L) allele of the serotonin transporter (5-HTT) gene. They found that when life stresses occurred, the probability of depression was much higher among the subgroup who were SS homozygous than among the LL homozygous subgroup. Thus, the genetic vulnerability to depression did not manifest itself unless adverse environmental events occurred. This is a classic example of GxE interaction, where genes alone are insufficient to produce a psychiatric disorder without environmental events interacting with them and triggering the psychopathology.
- In the same cohort described above, investigators showed that some children who were abused at an early age developed antisocial behavior as adults, while others did not.4 They discovered that a high expression of a polymorphism in the gene that codes for monoamine oxidase A had a protective effect that decreased the likelihood of developing antisocial traits in children who experienced trauma. In this case, the life experience failed to worsen a child’s behavior in the presence of elevated levels of a genetically determined protective enzyme.
- Schizophrenia is a heterogeneous neurodevelopmental syndrome caused by numerous genetic factors (risk genes, copy number variants, and de novo mutations) and a wide variety of perinatal complications. Concordance for schizophrenia in monozygotic twins who have identical genes is only 50%, not 100% as would be expected.5 Obviously, nongenetic factors during fetal life must play a role in disrupting the neurodevelopment of the affected twin, but not in the healthy twin. Examples of such factors may include differential distribution of blood during fetal life, leading to low birthweight and hypoplastic brain volume in the affected twin. It may also be due to labor complications, where one twin has an uneventful vaginal delivery while the other experiences hypoxia, a brain insult, due to a complicated breech delivery. Thus, despite having the same genes, the postnatal outcome in a discordant monozygotic twin pair diverges dramatically.
- A recent study6 identified somatic mutations in monozygotic twins discordant for psychiatric disorders, including schizophrenia and delusional disorder. Such somatic mutations have also been found in Van der Woude syndrome, which includes cleft palate. However, skillful surgeons can repair the cleft palate and allow the affected twin to have a normal facial appearance and oral functions, offsetting the abnormal genetic code.
- A monozygotic twin pair (one of whom was a patient of mine) born to a mother with bipolar disorder and adopted at birth by different families developed bipolar disorder due to genetic transmission, but eventually had very different outcomes. One twin was promptly and successfully treated with lithium at the first manic episode and became a successful teacher and author, while his twin did not receive treatment, became addicted to drugs, was repeatedly incarcerated for assaultive behavior, and later completed suicide at a young age. The appropriate environment and experiences of a person who inherits a psychiatric disorder can dramatically alter the prognosis for the better.
The GxE neurobiological equation is a central feature in many of our patients. As clinicians, we can modulate the patient’s environment by providing timely therapeutic biopsychosocial interventions to our patient to catalyze the GxE equation and veer it towards health, resilience, and wellness. Psychiatric practice can effectively help our patients overcome their genetically and neurobiologically driven maladaptive behavior and enable them to recover from the ravages of neuropsychiatric illness. Thus, psychiatric care represents the ultimate “E” that can interact with and modulate the “G” and effectively demonstrate that anatomy is not destiny.
The long-held dogma that “anatomy is destiny” is fraying at the edges. The traditional nature vs nurture debate has also undergone a major transformation into a gene-by-environment interaction, abbreviated as GxE in the medical literature.1,2 This is as true for psychiatric brain disorders as for any other medical illness.
The pessimistic determinism of “anatomy is destiny” has given way to a much more optimistic perspective, especially for the most plastic of all organs, the human brain. While genes are essential to construct one’s anatomy, environmental factors can significantly modulate gene expression. A person’s life experiences, good or bad, can wield a lasting influence on one’s brain structure and function, often transcending what is coded by the genome. For the mind, its thoughts, emotions, and cognition, the neurogenetic “tyranny” can be curbed or modified by one’s experiences. This epigenetic process is alive and well and known to be mediated by DNA methylation and histone modifications.
Consider the following examples of how genes are not the sole determinants of one’s mental health:
- A landmark study conducted in New Zealand3 followed a cohort of 847 individuals from age 3 to 26. Researchers recorded stressful life events for each participant, including romantic breakups, grief, medical illness, or employment problems, between age 21 and 26. Participants were evaluated for depressive episodes and hospitalizations and their genes tested for whether each individual carried the short (S) or long (L) allele of the serotonin transporter (5-HTT) gene. They found that when life stresses occurred, the probability of depression was much higher among the subgroup who were SS homozygous than among the LL homozygous subgroup. Thus, the genetic vulnerability to depression did not manifest itself unless adverse environmental events occurred. This is a classic example of GxE interaction, where genes alone are insufficient to produce a psychiatric disorder without environmental events interacting with them and triggering the psychopathology.
- In the same cohort described above, investigators showed that some children who were abused at an early age developed antisocial behavior as adults, while others did not.4 They discovered that a high expression of a polymorphism in the gene that codes for monoamine oxidase A had a protective effect that decreased the likelihood of developing antisocial traits in children who experienced trauma. In this case, the life experience failed to worsen a child’s behavior in the presence of elevated levels of a genetically determined protective enzyme.
- Schizophrenia is a heterogeneous neurodevelopmental syndrome caused by numerous genetic factors (risk genes, copy number variants, and de novo mutations) and a wide variety of perinatal complications. Concordance for schizophrenia in monozygotic twins who have identical genes is only 50%, not 100% as would be expected.5 Obviously, nongenetic factors during fetal life must play a role in disrupting the neurodevelopment of the affected twin, but not in the healthy twin. Examples of such factors may include differential distribution of blood during fetal life, leading to low birthweight and hypoplastic brain volume in the affected twin. It may also be due to labor complications, where one twin has an uneventful vaginal delivery while the other experiences hypoxia, a brain insult, due to a complicated breech delivery. Thus, despite having the same genes, the postnatal outcome in a discordant monozygotic twin pair diverges dramatically.
- A recent study6 identified somatic mutations in monozygotic twins discordant for psychiatric disorders, including schizophrenia and delusional disorder. Such somatic mutations have also been found in Van der Woude syndrome, which includes cleft palate. However, skillful surgeons can repair the cleft palate and allow the affected twin to have a normal facial appearance and oral functions, offsetting the abnormal genetic code.
- A monozygotic twin pair (one of whom was a patient of mine) born to a mother with bipolar disorder and adopted at birth by different families developed bipolar disorder due to genetic transmission, but eventually had very different outcomes. One twin was promptly and successfully treated with lithium at the first manic episode and became a successful teacher and author, while his twin did not receive treatment, became addicted to drugs, was repeatedly incarcerated for assaultive behavior, and later completed suicide at a young age. The appropriate environment and experiences of a person who inherits a psychiatric disorder can dramatically alter the prognosis for the better.
The GxE neurobiological equation is a central feature in many of our patients. As clinicians, we can modulate the patient’s environment by providing timely therapeutic biopsychosocial interventions to our patient to catalyze the GxE equation and veer it towards health, resilience, and wellness. Psychiatric practice can effectively help our patients overcome their genetically and neurobiologically driven maladaptive behavior and enable them to recover from the ravages of neuropsychiatric illness. Thus, psychiatric care represents the ultimate “E” that can interact with and modulate the “G” and effectively demonstrate that anatomy is not destiny.
1. Ridley M. Nature via nurture: genes, experience and what makes us human. New York, NY: Harper Collins; 2003.
2. Rutter M. Genes and behavior: nature–nurture interplay explained. Malden, MA: Blackwell Publishing; 2006.
3. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386-389.
4. Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851-854.
5. Stabenau JR, Pollin W. Heredity and environment in schizophrenia, revisited. The contribution of twin and high-risk studies. J Nerv Ment Dis. 1993;181(5):290-297.
6. Nishioka M, Bundo M, Ueda J, et al. Identification of somatic mutations in monozygotic twins discordant for psychiatric disorders. NPJ Schizophr. 2018;4(1):7.
1. Ridley M. Nature via nurture: genes, experience and what makes us human. New York, NY: Harper Collins; 2003.
2. Rutter M. Genes and behavior: nature–nurture interplay explained. Malden, MA: Blackwell Publishing; 2006.
3. Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386-389.
4. Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851-854.
5. Stabenau JR, Pollin W. Heredity and environment in schizophrenia, revisited. The contribution of twin and high-risk studies. J Nerv Ment Dis. 1993;181(5):290-297.
6. Nishioka M, Bundo M, Ueda J, et al. Identification of somatic mutations in monozygotic twins discordant for psychiatric disorders. NPJ Schizophr. 2018;4(1):7.
How effective and safe is fecal microbial transplant in preventing C difficile recurrence?
EVIDENCE SUMMARY
An open-label RCT enrolled 41 immunocompetent older adults who had relapsed CDI after at least one course of antibiotic therapy.1 Investigators randomized patients to 3 treatment groups:
- vancomycin therapy, bowel lavage (with 4 L nasogastric polyethylene glycol solution), and nasogastric-infused fresh donor feces;
- vancomycin with nasogastric bowel lavage without donor feces; or
- vancomycin alone.
Researchers defined cure as the absence of diarrhea or 3 negative stool samples (if patients continued to have persistent diarrhea) at 10 weeks without relapse.
Thirteen of 16 patients (81%) in the donor feces infusion group were cured with the first infusion. Two of the 3 remaining patients were cured after a second donor transplant. FMT produced higher total cure rates than those of vancomycin (94% vs 27%; P<.001; number needed to treat [NNT]=2). Bowel lavage had no effect on outcome.
FMT cures more patients than vancomycin alone
An open-label RCT of 39 patients compared healthy-donor, fresh FMT given via colonoscopy with vancomycin alone for recurrent CDIs.2 Researchers recruited immunocompetent adults who had recurrent CDIs after at least one course of vancomycin or metronidazole.
Patients in the treatment group received a short course of vancomycin, followed by bowel cleansing and fecal transplant via colonoscopy. Clinicians repeated the fecal transplant every 3 days until resolution for patients with pseudomembranous colitis. Patients in the control group were treated with vancomycin for at least 3 weeks. Researchers defined cure as the absence of diarrhea or 2 negative stool samples (if patients continued to have diarrhea) at 10 weeks without relapse.
Thirteen of 20 patients in the FMT group (65%) achieved cure after the first fecal infusion. The 7 remaining patients received multiple infusions; 5 were cured. Overall, FMT cured more patients than vancomycin alone (90% vs 26%; odds ratio=25.2; 99.9% confidence interval [CI], 1.26-502; NNT=2).
Continue to: Fresh and frozen stool are equally effective
Fresh and frozen stool are equally effective
A randomized, double-blind noninferiority trial compared the effectiveness of frozen and thawed FMT with that of fresh FMT in 219 patients ≥18 years of age with recurrent or refractory CDIs.3 Researchers prescribed suppressive antibiotics, which were discontinued within 24 to 48 hours of FMT, and then administered 50 mL of either fresh or frozen stool by enema. They repeated the FMT with the same donor stool if symptoms didn’t improve within 4 days. Any patient still unresponsive was offered repeat FMT or antibiotic therapy.
Researchers defined a 15% difference in outcome as a clinically important effect. Intention-to-treat analysis yielded no significant difference in clinical resolution between groups (75% frozen vs 70.3% fresh; P=.01 for noninferiority).
Nasogastric delivery works as well as colonoscopy
An open-label RCT (not included in the reviews described previously) evaluated the effectiveness of colonoscopically administered FMT compared with that of nasogastric administration in 20 patients with recurrent or refractory CDIs.4 Patients had experienced either a minimum of 3 episodes of mild-to-moderate CDI with a failed 6- to 8-week taper of vancomycin or 2 episodes of severe CDI resulting in hospitalization. Researchers offered patients from both groups a second FMT if symptoms didn’t improve with the initial administration.
Eight patients in the colonoscopy group and 6 in the nasogastric group were cured (all symptoms resolved) after the first FMT. One patient in the nasogastric group refused subsequent administration. All 5 remaining participants chose to have subsequent nasogastric administration (80% cure rate). Both methods of administering FMT produced comparable cure rates (80% in the initial nasogastric group vs 100% in the initial colonoscopy group; P=.53).
Continue to: A third of patients suffer adverse effects, but serious harms are rare
A third of patients suffer adverse effects, but serious harms are rare
A systematic review analyzed 50 trials (16 case series, 9 case reports, 4 RCTs, 21 unreported type; 1089 FMT-treated patients) for adverse effects of FMT.5 Most patients (831) had CDIs, 235 had inflammatory bowel disease, and 106 had both conditions. Donor screening tests for FMT included viral screenings (hepatitis A, B, and C; Epstein-Barr virus; human immunodeficiency virus; Treponema pallidum; and cytomegalovirus), stool tests for C difficile toxin, and routine bacterial culture for enteric pathogens (Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter), ova, and parasites.
Overall, 28.5% of patients receiving FMT experienced adverse events. Upper gastrointestinal (GI) administration resulted in more total adverse events than did lower GI delivery (43.6% vs 20.6%; P value not given), mostly abdominal discomfort. However, upper GI delivery was associated with fewer serious adverse events than was lower GI delivery (2% vs 6%; P value not given). FMT possibly or probably produced serious infections in 0.7% of patients, and there was one colonoscopy-associated death caused by aspiration (0.1% mortality).
RECOMMENDATIONS
Guidelines published by the American College of Gastroenterology in 2013 listed FMT as a treatment option for patients who have had 3 episodes of CDI and vancomycin therapy (based on moderate quality evidence).6
1. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415.
2. Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835-843.
3. Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection. JAMA. 2016;315:142-149.
4. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515-1522.
5. Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11:e0161174.
6. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
EVIDENCE SUMMARY
An open-label RCT enrolled 41 immunocompetent older adults who had relapsed CDI after at least one course of antibiotic therapy.1 Investigators randomized patients to 3 treatment groups:
- vancomycin therapy, bowel lavage (with 4 L nasogastric polyethylene glycol solution), and nasogastric-infused fresh donor feces;
- vancomycin with nasogastric bowel lavage without donor feces; or
- vancomycin alone.
Researchers defined cure as the absence of diarrhea or 3 negative stool samples (if patients continued to have persistent diarrhea) at 10 weeks without relapse.
Thirteen of 16 patients (81%) in the donor feces infusion group were cured with the first infusion. Two of the 3 remaining patients were cured after a second donor transplant. FMT produced higher total cure rates than those of vancomycin (94% vs 27%; P<.001; number needed to treat [NNT]=2). Bowel lavage had no effect on outcome.
FMT cures more patients than vancomycin alone
An open-label RCT of 39 patients compared healthy-donor, fresh FMT given via colonoscopy with vancomycin alone for recurrent CDIs.2 Researchers recruited immunocompetent adults who had recurrent CDIs after at least one course of vancomycin or metronidazole.
Patients in the treatment group received a short course of vancomycin, followed by bowel cleansing and fecal transplant via colonoscopy. Clinicians repeated the fecal transplant every 3 days until resolution for patients with pseudomembranous colitis. Patients in the control group were treated with vancomycin for at least 3 weeks. Researchers defined cure as the absence of diarrhea or 2 negative stool samples (if patients continued to have diarrhea) at 10 weeks without relapse.
Thirteen of 20 patients in the FMT group (65%) achieved cure after the first fecal infusion. The 7 remaining patients received multiple infusions; 5 were cured. Overall, FMT cured more patients than vancomycin alone (90% vs 26%; odds ratio=25.2; 99.9% confidence interval [CI], 1.26-502; NNT=2).
Continue to: Fresh and frozen stool are equally effective
Fresh and frozen stool are equally effective
A randomized, double-blind noninferiority trial compared the effectiveness of frozen and thawed FMT with that of fresh FMT in 219 patients ≥18 years of age with recurrent or refractory CDIs.3 Researchers prescribed suppressive antibiotics, which were discontinued within 24 to 48 hours of FMT, and then administered 50 mL of either fresh or frozen stool by enema. They repeated the FMT with the same donor stool if symptoms didn’t improve within 4 days. Any patient still unresponsive was offered repeat FMT or antibiotic therapy.
Researchers defined a 15% difference in outcome as a clinically important effect. Intention-to-treat analysis yielded no significant difference in clinical resolution between groups (75% frozen vs 70.3% fresh; P=.01 for noninferiority).
Nasogastric delivery works as well as colonoscopy
An open-label RCT (not included in the reviews described previously) evaluated the effectiveness of colonoscopically administered FMT compared with that of nasogastric administration in 20 patients with recurrent or refractory CDIs.4 Patients had experienced either a minimum of 3 episodes of mild-to-moderate CDI with a failed 6- to 8-week taper of vancomycin or 2 episodes of severe CDI resulting in hospitalization. Researchers offered patients from both groups a second FMT if symptoms didn’t improve with the initial administration.
Eight patients in the colonoscopy group and 6 in the nasogastric group were cured (all symptoms resolved) after the first FMT. One patient in the nasogastric group refused subsequent administration. All 5 remaining participants chose to have subsequent nasogastric administration (80% cure rate). Both methods of administering FMT produced comparable cure rates (80% in the initial nasogastric group vs 100% in the initial colonoscopy group; P=.53).
Continue to: A third of patients suffer adverse effects, but serious harms are rare
A third of patients suffer adverse effects, but serious harms are rare
A systematic review analyzed 50 trials (16 case series, 9 case reports, 4 RCTs, 21 unreported type; 1089 FMT-treated patients) for adverse effects of FMT.5 Most patients (831) had CDIs, 235 had inflammatory bowel disease, and 106 had both conditions. Donor screening tests for FMT included viral screenings (hepatitis A, B, and C; Epstein-Barr virus; human immunodeficiency virus; Treponema pallidum; and cytomegalovirus), stool tests for C difficile toxin, and routine bacterial culture for enteric pathogens (Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter), ova, and parasites.
Overall, 28.5% of patients receiving FMT experienced adverse events. Upper gastrointestinal (GI) administration resulted in more total adverse events than did lower GI delivery (43.6% vs 20.6%; P value not given), mostly abdominal discomfort. However, upper GI delivery was associated with fewer serious adverse events than was lower GI delivery (2% vs 6%; P value not given). FMT possibly or probably produced serious infections in 0.7% of patients, and there was one colonoscopy-associated death caused by aspiration (0.1% mortality).
RECOMMENDATIONS
Guidelines published by the American College of Gastroenterology in 2013 listed FMT as a treatment option for patients who have had 3 episodes of CDI and vancomycin therapy (based on moderate quality evidence).6
EVIDENCE SUMMARY
An open-label RCT enrolled 41 immunocompetent older adults who had relapsed CDI after at least one course of antibiotic therapy.1 Investigators randomized patients to 3 treatment groups:
- vancomycin therapy, bowel lavage (with 4 L nasogastric polyethylene glycol solution), and nasogastric-infused fresh donor feces;
- vancomycin with nasogastric bowel lavage without donor feces; or
- vancomycin alone.
Researchers defined cure as the absence of diarrhea or 3 negative stool samples (if patients continued to have persistent diarrhea) at 10 weeks without relapse.
Thirteen of 16 patients (81%) in the donor feces infusion group were cured with the first infusion. Two of the 3 remaining patients were cured after a second donor transplant. FMT produced higher total cure rates than those of vancomycin (94% vs 27%; P<.001; number needed to treat [NNT]=2). Bowel lavage had no effect on outcome.
FMT cures more patients than vancomycin alone
An open-label RCT of 39 patients compared healthy-donor, fresh FMT given via colonoscopy with vancomycin alone for recurrent CDIs.2 Researchers recruited immunocompetent adults who had recurrent CDIs after at least one course of vancomycin or metronidazole.
Patients in the treatment group received a short course of vancomycin, followed by bowel cleansing and fecal transplant via colonoscopy. Clinicians repeated the fecal transplant every 3 days until resolution for patients with pseudomembranous colitis. Patients in the control group were treated with vancomycin for at least 3 weeks. Researchers defined cure as the absence of diarrhea or 2 negative stool samples (if patients continued to have diarrhea) at 10 weeks without relapse.
Thirteen of 20 patients in the FMT group (65%) achieved cure after the first fecal infusion. The 7 remaining patients received multiple infusions; 5 were cured. Overall, FMT cured more patients than vancomycin alone (90% vs 26%; odds ratio=25.2; 99.9% confidence interval [CI], 1.26-502; NNT=2).
Continue to: Fresh and frozen stool are equally effective
Fresh and frozen stool are equally effective
A randomized, double-blind noninferiority trial compared the effectiveness of frozen and thawed FMT with that of fresh FMT in 219 patients ≥18 years of age with recurrent or refractory CDIs.3 Researchers prescribed suppressive antibiotics, which were discontinued within 24 to 48 hours of FMT, and then administered 50 mL of either fresh or frozen stool by enema. They repeated the FMT with the same donor stool if symptoms didn’t improve within 4 days. Any patient still unresponsive was offered repeat FMT or antibiotic therapy.
Researchers defined a 15% difference in outcome as a clinically important effect. Intention-to-treat analysis yielded no significant difference in clinical resolution between groups (75% frozen vs 70.3% fresh; P=.01 for noninferiority).
Nasogastric delivery works as well as colonoscopy
An open-label RCT (not included in the reviews described previously) evaluated the effectiveness of colonoscopically administered FMT compared with that of nasogastric administration in 20 patients with recurrent or refractory CDIs.4 Patients had experienced either a minimum of 3 episodes of mild-to-moderate CDI with a failed 6- to 8-week taper of vancomycin or 2 episodes of severe CDI resulting in hospitalization. Researchers offered patients from both groups a second FMT if symptoms didn’t improve with the initial administration.
Eight patients in the colonoscopy group and 6 in the nasogastric group were cured (all symptoms resolved) after the first FMT. One patient in the nasogastric group refused subsequent administration. All 5 remaining participants chose to have subsequent nasogastric administration (80% cure rate). Both methods of administering FMT produced comparable cure rates (80% in the initial nasogastric group vs 100% in the initial colonoscopy group; P=.53).
Continue to: A third of patients suffer adverse effects, but serious harms are rare
A third of patients suffer adverse effects, but serious harms are rare
A systematic review analyzed 50 trials (16 case series, 9 case reports, 4 RCTs, 21 unreported type; 1089 FMT-treated patients) for adverse effects of FMT.5 Most patients (831) had CDIs, 235 had inflammatory bowel disease, and 106 had both conditions. Donor screening tests for FMT included viral screenings (hepatitis A, B, and C; Epstein-Barr virus; human immunodeficiency virus; Treponema pallidum; and cytomegalovirus), stool tests for C difficile toxin, and routine bacterial culture for enteric pathogens (Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter), ova, and parasites.
Overall, 28.5% of patients receiving FMT experienced adverse events. Upper gastrointestinal (GI) administration resulted in more total adverse events than did lower GI delivery (43.6% vs 20.6%; P value not given), mostly abdominal discomfort. However, upper GI delivery was associated with fewer serious adverse events than was lower GI delivery (2% vs 6%; P value not given). FMT possibly or probably produced serious infections in 0.7% of patients, and there was one colonoscopy-associated death caused by aspiration (0.1% mortality).
RECOMMENDATIONS
Guidelines published by the American College of Gastroenterology in 2013 listed FMT as a treatment option for patients who have had 3 episodes of CDI and vancomycin therapy (based on moderate quality evidence).6
1. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415.
2. Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835-843.
3. Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection. JAMA. 2016;315:142-149.
4. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515-1522.
5. Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11:e0161174.
6. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
1. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415.
2. Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835-843.
3. Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection. JAMA. 2016;315:142-149.
4. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515-1522.
5. Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11:e0161174.
6. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
EVIDENCE-BASED ANSWER:
Fecal microbial transplant (FMT) is reasonably safe and effective. In patients who have had multiple Clostridium difficile infections (CDIs), FMT results in a 65% to 80% cure rate with one treatment and 90% to 95% cure rate with repeated treatments compared with a 25% to 27% cure rate for antibiotics (strength of recommendation [SOR]: B, small open-label randomized controlled trials [RCTs]).
Fresh and frozen donor feces, administered by either nasogastric tube or colonoscope, produce equal results (SOR B, RCTs).
FMT has an overall adverse event rate of 30%, primarily involving abdominal discomfort, but also, rarely, severe infections (0.7%) and death (0.1%) (SOR: B, systematic review not limited to RCTs).
History of posttraumatic stress disorder • priapism • Dx?
THE CASE
A 35-year-old African-American man, who was an active duty service member, presented to the Troop Medical Clinic with a 4-hour history of priapism. He had been taking sertraline 100 mg and prazosin 10 mg nightly for 4 months to treat his posttraumatic stress disorder (PTSD) with no reported adverse effects. These doses were titrated 2 months prior to presentation. The patient reported that he took his usual medication doses before bed and awoke at 3 am with a penile erection. At 7 am, he presented to the clinic because of pain from the continued erection.
THE DIAGNOSIS
A penile erection was present on physical exam. All medications were reviewed for adverse effects. A work-up for anemia, sickle cell disease, thalassemia, and platelet abnormalities was negative. A blood gas analysis performed on blood aspirated from the corpus cavernosum showed hypoxemia, hypercarbia, and acidosis, confirming a diagnosis of ischemic priapism.
DISCUSSION
Priapism is a prolonged erection of the penis that is usually not associated with sexual activity or stimulation. It is considered a urologic emergency and requires prompt treatment to prevent long-term complications, such as permanent erectile dysfunction.
Priapism is classified as one of 2 types: ischemic (“low flow”) or nonischemic (“high flow”).
Ischemic priapism is the most common type. It is caused by dysfunctional cavernosal smooth muscle, which creates a compartment-like syndrome in the cavernous tissue that leads to hypoxia and acidosis.1 Nonischemic priapism is often caused by a fistula between the cavernosal artery and corpus cavernosum and is common with traumatic injuries. Nonischemic priapism has a lower risk for long-term complications (due to the blood being well-oxygenated) and often resolves spontaneously without treatment.2,3
Certain medications can cause priapism
Our patient’s ischemic priapism was most likely caused by the combined antagonistic properties of prazosin and sertraline on alpha-1 adrenergic receptors.3,4 Adrenergic alpha-blockers block the sympathetic system, which can in turn inhibit penile detumescence and cause priapism.4
An increasingly common Tx combination. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are considered first-line treatment for the symptoms of PTSD, and prazosin has been found to be effective in the treatment of nightmares associated with PTSD. (Treatment of PTSD-related nightmares with prazosin is an off-label but frequent use of the medication.) This combination of medications is becoming increasingly common for the treatment of PTSD and its associated symptoms.5-7
Continue to: Cases to date provide interesting insight into this adverse effect
Cases to date provide interesting insight into this adverse effect
In our literature review, no documented cases of priapism were attributed to prazosin when it was used for the treatment of nightmares, but there are multiple case reports of priapism linked to the drug’s use for hypertension.
In the majority of these case reports, the dosage exceeded 10 mg/d and was much higher than the dosage typically used to treat nightmares.7 Many of the affected patients also had associated comorbidities such as diabetes or chronic kidney disease.4
Sertraline has been associated with priapism when used as monotherapy and in combination therapy with antipsychotics. All SSRIs have antagonistic properties to alpha-1 adrenergic receptors, but sertraline appears to have more than a 10-fold increase in affinity when compared to other SSRIs.3
Treatment: An injection and aspiration
Our patient was treated with phenylephrine injection and aspiration, which resolved the priapism. Prazosin was stopped, and the patient was weaned off of sertraline. He continued to follow up closely with Behavioral Health for further management of his PTSD and associated symptoms.
Continue to: THE TAKEAWAY
THE TAKEAWAY
PTSD is being diagnosed more frequently, especially in active duty soldiers, veterans, members of the National Guard, and reservists.8 Because nightmares are a common symptom of PTSD and SSRIs are first-line treatment for PTSD, the combination of prazosin and an SSRI for the treatment of PTSD is frequently encountered.5-7 Providers who prescribe and/or care for patients treated with these medications need to counsel patients on the risk of priapism and the risks associated with a delay in seeking medical care.
If a patient who is taking these medications presents with priapism, contact Urology immediately for acute management. Both medications must be stopped to prevent future episodes; prazosin can be stopped immediately, but patients must be weaned off of sertraline to avoid experiencing withdrawal symptoms. Patients will need to follow up with a behavioral health team for continued management of their PTSD symptoms.
CORRESPONDENCE
Caleb Dickison, DO, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116-120.
2. Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
3. Choua, R, Lee HC, Castro J, et al. Priapism associated with multiple psychotropics: a case report and review of the literature. 2007. Available at: http://primarypsychiatry.com/priapism-associated-with-multiple-psychotropics-a-case-report-and-review-of-the-literature/. Accessed on May 7, 2018.
4. Spagnul SJ, Cabral PH, Verndl DO, et al. Adrenergic alpha-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23:95-98.
5. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;CD002795.
6. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma PTSD: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
8. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
THE CASE
A 35-year-old African-American man, who was an active duty service member, presented to the Troop Medical Clinic with a 4-hour history of priapism. He had been taking sertraline 100 mg and prazosin 10 mg nightly for 4 months to treat his posttraumatic stress disorder (PTSD) with no reported adverse effects. These doses were titrated 2 months prior to presentation. The patient reported that he took his usual medication doses before bed and awoke at 3 am with a penile erection. At 7 am, he presented to the clinic because of pain from the continued erection.
THE DIAGNOSIS
A penile erection was present on physical exam. All medications were reviewed for adverse effects. A work-up for anemia, sickle cell disease, thalassemia, and platelet abnormalities was negative. A blood gas analysis performed on blood aspirated from the corpus cavernosum showed hypoxemia, hypercarbia, and acidosis, confirming a diagnosis of ischemic priapism.
DISCUSSION
Priapism is a prolonged erection of the penis that is usually not associated with sexual activity or stimulation. It is considered a urologic emergency and requires prompt treatment to prevent long-term complications, such as permanent erectile dysfunction.
Priapism is classified as one of 2 types: ischemic (“low flow”) or nonischemic (“high flow”).
Ischemic priapism is the most common type. It is caused by dysfunctional cavernosal smooth muscle, which creates a compartment-like syndrome in the cavernous tissue that leads to hypoxia and acidosis.1 Nonischemic priapism is often caused by a fistula between the cavernosal artery and corpus cavernosum and is common with traumatic injuries. Nonischemic priapism has a lower risk for long-term complications (due to the blood being well-oxygenated) and often resolves spontaneously without treatment.2,3
Certain medications can cause priapism
Our patient’s ischemic priapism was most likely caused by the combined antagonistic properties of prazosin and sertraline on alpha-1 adrenergic receptors.3,4 Adrenergic alpha-blockers block the sympathetic system, which can in turn inhibit penile detumescence and cause priapism.4
An increasingly common Tx combination. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are considered first-line treatment for the symptoms of PTSD, and prazosin has been found to be effective in the treatment of nightmares associated with PTSD. (Treatment of PTSD-related nightmares with prazosin is an off-label but frequent use of the medication.) This combination of medications is becoming increasingly common for the treatment of PTSD and its associated symptoms.5-7
Continue to: Cases to date provide interesting insight into this adverse effect
Cases to date provide interesting insight into this adverse effect
In our literature review, no documented cases of priapism were attributed to prazosin when it was used for the treatment of nightmares, but there are multiple case reports of priapism linked to the drug’s use for hypertension.
In the majority of these case reports, the dosage exceeded 10 mg/d and was much higher than the dosage typically used to treat nightmares.7 Many of the affected patients also had associated comorbidities such as diabetes or chronic kidney disease.4
Sertraline has been associated with priapism when used as monotherapy and in combination therapy with antipsychotics. All SSRIs have antagonistic properties to alpha-1 adrenergic receptors, but sertraline appears to have more than a 10-fold increase in affinity when compared to other SSRIs.3
Treatment: An injection and aspiration
Our patient was treated with phenylephrine injection and aspiration, which resolved the priapism. Prazosin was stopped, and the patient was weaned off of sertraline. He continued to follow up closely with Behavioral Health for further management of his PTSD and associated symptoms.
Continue to: THE TAKEAWAY
THE TAKEAWAY
PTSD is being diagnosed more frequently, especially in active duty soldiers, veterans, members of the National Guard, and reservists.8 Because nightmares are a common symptom of PTSD and SSRIs are first-line treatment for PTSD, the combination of prazosin and an SSRI for the treatment of PTSD is frequently encountered.5-7 Providers who prescribe and/or care for patients treated with these medications need to counsel patients on the risk of priapism and the risks associated with a delay in seeking medical care.
If a patient who is taking these medications presents with priapism, contact Urology immediately for acute management. Both medications must be stopped to prevent future episodes; prazosin can be stopped immediately, but patients must be weaned off of sertraline to avoid experiencing withdrawal symptoms. Patients will need to follow up with a behavioral health team for continued management of their PTSD symptoms.
CORRESPONDENCE
Caleb Dickison, DO, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
THE CASE
A 35-year-old African-American man, who was an active duty service member, presented to the Troop Medical Clinic with a 4-hour history of priapism. He had been taking sertraline 100 mg and prazosin 10 mg nightly for 4 months to treat his posttraumatic stress disorder (PTSD) with no reported adverse effects. These doses were titrated 2 months prior to presentation. The patient reported that he took his usual medication doses before bed and awoke at 3 am with a penile erection. At 7 am, he presented to the clinic because of pain from the continued erection.
THE DIAGNOSIS
A penile erection was present on physical exam. All medications were reviewed for adverse effects. A work-up for anemia, sickle cell disease, thalassemia, and platelet abnormalities was negative. A blood gas analysis performed on blood aspirated from the corpus cavernosum showed hypoxemia, hypercarbia, and acidosis, confirming a diagnosis of ischemic priapism.
DISCUSSION
Priapism is a prolonged erection of the penis that is usually not associated with sexual activity or stimulation. It is considered a urologic emergency and requires prompt treatment to prevent long-term complications, such as permanent erectile dysfunction.
Priapism is classified as one of 2 types: ischemic (“low flow”) or nonischemic (“high flow”).
Ischemic priapism is the most common type. It is caused by dysfunctional cavernosal smooth muscle, which creates a compartment-like syndrome in the cavernous tissue that leads to hypoxia and acidosis.1 Nonischemic priapism is often caused by a fistula between the cavernosal artery and corpus cavernosum and is common with traumatic injuries. Nonischemic priapism has a lower risk for long-term complications (due to the blood being well-oxygenated) and often resolves spontaneously without treatment.2,3
Certain medications can cause priapism
Our patient’s ischemic priapism was most likely caused by the combined antagonistic properties of prazosin and sertraline on alpha-1 adrenergic receptors.3,4 Adrenergic alpha-blockers block the sympathetic system, which can in turn inhibit penile detumescence and cause priapism.4
An increasingly common Tx combination. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are considered first-line treatment for the symptoms of PTSD, and prazosin has been found to be effective in the treatment of nightmares associated with PTSD. (Treatment of PTSD-related nightmares with prazosin is an off-label but frequent use of the medication.) This combination of medications is becoming increasingly common for the treatment of PTSD and its associated symptoms.5-7
Continue to: Cases to date provide interesting insight into this adverse effect
Cases to date provide interesting insight into this adverse effect
In our literature review, no documented cases of priapism were attributed to prazosin when it was used for the treatment of nightmares, but there are multiple case reports of priapism linked to the drug’s use for hypertension.
In the majority of these case reports, the dosage exceeded 10 mg/d and was much higher than the dosage typically used to treat nightmares.7 Many of the affected patients also had associated comorbidities such as diabetes or chronic kidney disease.4
Sertraline has been associated with priapism when used as monotherapy and in combination therapy with antipsychotics. All SSRIs have antagonistic properties to alpha-1 adrenergic receptors, but sertraline appears to have more than a 10-fold increase in affinity when compared to other SSRIs.3
Treatment: An injection and aspiration
Our patient was treated with phenylephrine injection and aspiration, which resolved the priapism. Prazosin was stopped, and the patient was weaned off of sertraline. He continued to follow up closely with Behavioral Health for further management of his PTSD and associated symptoms.
Continue to: THE TAKEAWAY
THE TAKEAWAY
PTSD is being diagnosed more frequently, especially in active duty soldiers, veterans, members of the National Guard, and reservists.8 Because nightmares are a common symptom of PTSD and SSRIs are first-line treatment for PTSD, the combination of prazosin and an SSRI for the treatment of PTSD is frequently encountered.5-7 Providers who prescribe and/or care for patients treated with these medications need to counsel patients on the risk of priapism and the risks associated with a delay in seeking medical care.
If a patient who is taking these medications presents with priapism, contact Urology immediately for acute management. Both medications must be stopped to prevent future episodes; prazosin can be stopped immediately, but patients must be weaned off of sertraline to avoid experiencing withdrawal symptoms. Patients will need to follow up with a behavioral health team for continued management of their PTSD symptoms.
CORRESPONDENCE
Caleb Dickison, DO, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116-120.
2. Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
3. Choua, R, Lee HC, Castro J, et al. Priapism associated with multiple psychotropics: a case report and review of the literature. 2007. Available at: http://primarypsychiatry.com/priapism-associated-with-multiple-psychotropics-a-case-report-and-review-of-the-literature/. Accessed on May 7, 2018.
4. Spagnul SJ, Cabral PH, Verndl DO, et al. Adrenergic alpha-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23:95-98.
5. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;CD002795.
6. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma PTSD: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
8. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116-120.
2. Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
3. Choua, R, Lee HC, Castro J, et al. Priapism associated with multiple psychotropics: a case report and review of the literature. 2007. Available at: http://primarypsychiatry.com/priapism-associated-with-multiple-psychotropics-a-case-report-and-review-of-the-literature/. Accessed on May 7, 2018.
4. Spagnul SJ, Cabral PH, Verndl DO, et al. Adrenergic alpha-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23:95-98.
5. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;CD002795.
6. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma PTSD: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
8. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.