User login
Newer cholesterol-lowering agents: What you must know
Over the past 3 decades, age-adjusted mortality for cardiovascular disease (CVD) in the United States has dropped by more than 50%.1 While multiple factors have contributed to this remarkable decline, the introduction and widespread use of statin therapy is unquestionably one key factor. Despite nearly overwhelming evidence that statins effectively lower low-density lipoprotein cholesterol (LDL-C) and predictably reduce cardiovascular events, less than half of patients with clinical coronary heart disease receive high-intensity statin therapy, leaving this population at increased risk for future events.2
Statins: Latest evidence on risks and benefits
Statins aren’t perfect. Not every patient is able to achieve the desired LDL-C lowering with statin therapy, and some patients develop adverse effects such as myopathy, new-onset diabetes, and occasionally hemorrhagic stroke. A recent report puts the risks of statin therapy in perspective, estimating that the treatment of 10,000 patients for 5 years would cause one case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 hemorrhagic strokes.3 The same treatment would avert approximately 1000 CVD events among those with preexisting disease, and approximately 500 CVD events among those with elevated risk, but no preexisting disease.3
In blinded randomized controlled trials, statin therapy is associated with relatively few adverse events (AEs). In open-label observational studies, however, substantially more AEs are reported. During the blinded phase of one recent study, muscle-related AEs and erectile dysfunction were reported at a similar rate by participants randomly assigned to receive atorvastatin or placebo. During the nonblinded nonrandomized phase, complaints of muscle-related AEs were 41% more likely in participants taking statins compared with those who were not.4
Statin therapy offers predictable CVD risk reduction. The evidence report accompanying the 2016 US Preventive Services Task Force (USPSTF) guidelines on statins for the prevention of CVD states that the use of low- or moderate-dose statin therapy was associated with an approximately 30% relative risk reduction (RRR) in CVD events and in CVD deaths, and a 10% to 15% RRR in all-cause mortality.5 Those with greater baseline CVD risk will have greater absolute risk reduction (ARR) than those with low baseline risk.5
How effective are non-statin therapies?
Multiple studies have demonstrated that some drugs can favorably modify lipid levels but not improve patient outcomes—eg, niacin, fibrates, and omega-3 fatty acids. The therapies that do improve outcomes are those that act via upregulation of LDL-receptor expression: statins, ezetimibe, bile acid sequestrants, dietary interventions, and ileal bypass surgery.
A recent meta-analysis found that with a 38.7-mg/dL (1-mmol/L) reduction in LDL-C level, the relative risk for major vascular events was 0.77 (95% CI, 0.71-0.84) for statins and 0.75 (95% CI, 0.66-0.86) for monotherapy with non-statin interventions that upregulate LDL-receptor expression.6
Ezetimibe. Less impressive is the incremental benefit of adding some non-statin therapies to effective statin therapy. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) reported that adding ezetimibe to effective statin therapy in stable patients with previous acute coronary syndrome reduced the LDL-C level from 69.5 mg/dL to 53.7 mg/dL (TABLE7-13).8 After 7 years of treatment, relative risk of atherosclerotic cardiovascular disease (ASCVD) outcomes decreased by 5.8%; absolute decrease in risk was 2%: from 34.7% to 32.7% (number needed to treat [NNT]=50).8 Consider adding ezetimibe to maximally-tolerated statin therapy for patients not meeting LDL-C goals with a statin alone.
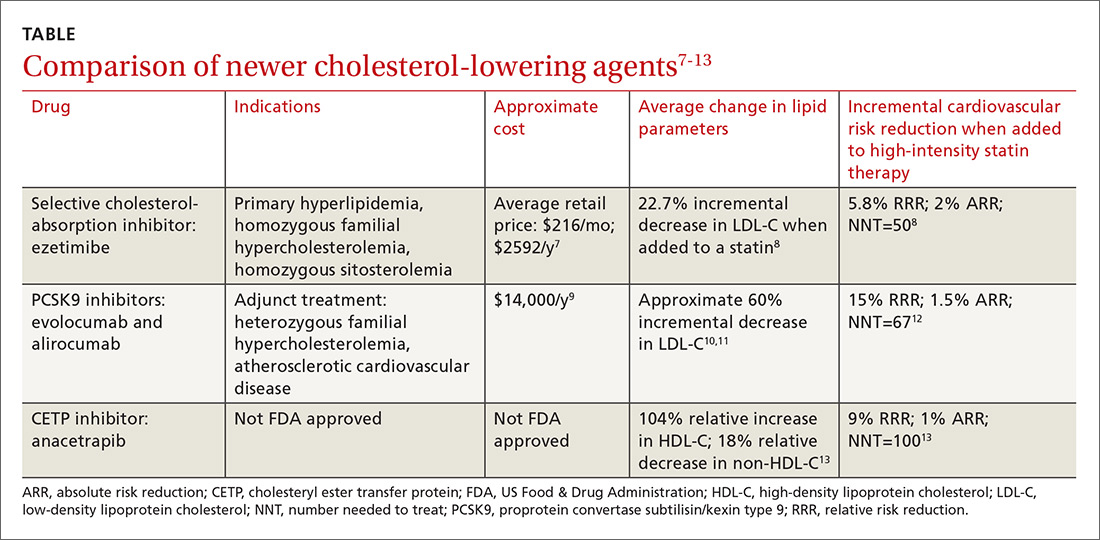
Continue to: A new class to lower LDL-C: PCSK9 inhibitors
A new class to lower LDL-C: PCSK9 inhibitors
It is clear that additional approaches to LDL-C reduction are needed. A new drug class that effectively lowers LDL-C levels is monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 activity is directly proportional to the circulating LDL-C level: gene mutations that increase PCSK9 function are one cause of elevated LDL-C and CVD risk in familial hypercholesterolemia (FH),14 whereas mutations that decrease PCSK9 activity are associated with a decrease in LDL-C levels and risk of ASCVD.15
Circulating PCSK9 initiates LDL-receptor clearance by binding to the LDL receptor; the complex is then taken into the hepatocyte, where it undergoes degradation, and the receptor is not recycled to the cell’s surface. The resultant decreased level of cholesterol within the hepatocyte upregulates HMG-CoA reductase (the enzyme that controls the rate-limiting step in cholesterol production and is targeted by statin therapy) and LDL-receptor activity to increase the available cholesterol in the hepatocyte. Unfortunately, statins promote the upregulation of both the LDL receptor and PCSK9, thereby limiting their LDL-C-lowering potency. Combined inhibition of HMG-CoA reductase with statins and PCSK9 with monoclonal antibodies exerts additive reductions in LDL-C.16
Evolocumab and alirocumab—monoclonal antibodies that prevent circulating PCSK9 from binding to the LDL receptor—have been approved by the US Food and Drug Administration (FDA) for use as adjuncts to diet and maximally-tolerated statin therapy in adults who have heterozygous familial hypercholesterolemia (HeFH) or clinical ASCVD and who must further lower LDL-C levels. The addition of a PCSK9 inhibitor to statin therapy consistently results in an incremental decrease in LDL-C of around 60%.10,11 Much of the data supporting the use of PCSK9 inhibitors are disease-oriented. Among patients with angiographic coronary disease treated with statins, the addition of evolocumab resulted in regression of atherosclerotic plaque measured by intravascular ultrasound after 18 months of treatment.10
Continue to: PCSK9 inhibitors reduce adverse CVD events when added to a statin
PCSK9 inhibitors reduce adverse CVD events when added to a statin. In a study designed to evaluate AEs and LDL-C lowering with evolocumab, a prespecified exploratory outcome was the incidence of adjudicated CVD events. After one year of therapy, the rate of events was reduced from 2.18% in the standard-therapy group to 0.95% in the evolocumab group—a relative decrease of 53%, but an absolute decrease of 1.23% (NNT=81).17
A similar reduction in the rate of major adverse CVD events was found in adding alirocumab to ongoing statin therapy. In a post hoc analysis of patients who received either adjunctive alirocumab or placebo, CVD events (death from coronary heart disease, nonfatal myocardial infarction [MI], fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) were 1.7% vs 3.3% (hazard ratio=0.52; 95% confidence interval, 0.31-0.90).11
FOURIER, the first major trial designed to evaluate cardiovascular outcomes with PCSK9 therapy, showed that adding evolocumab to effective statin therapy reduced the average LDL-C level from 92 mg/dL to 30 mg/dL.12 Evolocumab decreased the composite CVD outcome (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) over 2.2 years from 11.3% to 9.8%—a 15% RRR and a 1.5% ARR (NNT=67). Most of the participants were receiving high-intensity statin therapy at study entry. AEs were similar between the study groups.12
A prespecified analysis of FOURIER data found that evolocumab did not increase the risk of new-onset diabetes in patients without diabetes or prediabetes at baseline. Fasting plasma glucose and hemoglobin A1c levels in the evolocumab and placebo groups remained similar throughout the trial in patients with diabetes, prediabetes, or normoglycemia.18 Additionally, a randomized trial involving patients who received either evolocumab or placebo in addition to statin therapy found no significant difference in cognitive function between the groups over a median of 19 months.19
Continue to: Effective, but expensive
Effective, but expensive. At its current list price of approximately $14,000 per year,9 evolocumab, added to standard therapy in patients with ASCVD, exceeds the generally accepted cost-effectiveness threshold of $150,000 per quality-adjusted life year (QALY) achieved.20 Similar analysis in patients with HeFH estimated a cost of $503,000 per QALY achieved with evolocumab.21 The outcomes of cost-effectiveness analyses hinge on the event rate in the study population and the threshold for initiating therapy. For the FOURIER trial participants, with an annual event rate of 4.2 per 100 patient-years, a net annual price of approximately $6700 would be necessary to meet a $150,000 per QALY threshold.22
At 2015 prices, the addition of PCSK9 inhibitor therapy for all eligible patients would reduce cardiovascular care costs by an estimated $29 billion over 5 years but would also increase drug costs by an estimated $592 billion, representing a 38% increase over 2015 prescription drug expenditures.21 Treatment of less than 20 million US adults with evolocumab at the cost of this single drug would match the entire cost for all other prescription pharmaceuticals for all diseases in the United States combined.23
In 2012, 27.9% of US adults ages 40 years and older were taking prescribed lipid-lowering treatment; 23.2% were taking only statins.24 If the
Until the cost of PCSK9 inhibitors decreases to a justifiable level and outcomes of longer term studies are available, consider prescribing other adjunctive treatments for patients who have not achieved LDL-C goals with statin therapy alone. Generally, reserve use of PCSK9 inhibitors for the highest-risk adults: those with HeFH or clinical ASCVD who must further lower LDL-C levels. Some insurers, including Medicare, are covering PCSK9 inhibitors, but many patients have difficulty obtaining coverage.27
Continue to: CETP inhibitors: Not FDA approved
CETP inhibitors: Not FDA approved
In a recent trial of the cholesteryl ester transfer protein (CETP) inhibitor evacetrapib, the drug had favorable effects on lipid biomarkers but did not improve cardiovascular outcomes.28 More recently, the CETP inhibitor anacetrapib was shown to decrease the composite outcome of coronary death, MI, or coronary revascularization in adults with established ASCVD who were receiving high-intensity atorvastatin therapy.13 At the trial midpoint, mean high-density lipoprotein (HDL) cholesterol levels increased by 43 mg/dL in the anacetrapib group compared with that of the placebo group (a relative difference of 104%); mean non-HDL cholesterol decreased by 17 mg/dL, a relative difference of −18%. Over a median follow-up period of 4.1 years, the addition of anacetrapib was associated with a 9% RRR and a 1% absolute reduction in the composite outcome over a statin alone (NNT=100).13 At this point, the manufacturers of both agents have halted efforts to gain FDA approval.
Future directions
Newer strategies to inhibit PCSK9 function are under development. Small peptides that inhibit PCSK9 interaction with the LDL receptor offer the potential advantage of oral administration, as opposed to the currently available injectable anti-PCSK9 antibodies.29 A recent trial found that inhibition of PCSK9 messenger RNA (mRNA) synthesis with the small interfering RNA (siRNA) molecule inclisiran lowered LDL-C in patients with high cardiovascular risk and elevated LDL-C levels despite aggressive statin therapy.30 The effect of these strategies on cardiovascular outcomes remains unproven.
CORRESPONDENCE
Jonathon Firnhaber, MD, Department of Family Medicine, Brody School of Medicine, 101 Heart Drive, Mail Stop 654, Greenville, NC 27834; [email protected].
1. Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths — trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157.
2. Rodriguez F, Harrington RA. Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and the future of LDL-C lowering. JAMA. 2016;316:1967-1968.
3. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561.
4. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
5. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024.
6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289-1297.
7. GoodRx. Ezetimibe. Available at: https://www.goodrx.com/ezetimibe. Accessed May 2, 2018.
8. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
9. American Journal of Managed Care. Outcomes-based pricing for PCSK9 inhibitors. Available at: http://www.ajmc.com/contributor/inmaculada-hernandez-pharmd/2017/09/outcomes-based-pricing-for-pcsk9-inhibitors. Accessed May 2, 2018.
10. Nicholls S, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384.
11. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
12. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
13. HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-1227.
14. Hunt SC, Hopkins PN, Bulka K, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089-1093.
15. Cohen J, Pertsemlidis A, Kotowski I, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161-165.
16. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
17. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941-950.
19. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633-643.
20. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-2322.
21. Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743-753.
22. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069-1078.
23. Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420.
24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS data Brief. 2014;177:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Accessed May 2, 2018.
25. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
26. Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA. 2017;317:1563-1567.
27
28. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-1942.
29. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
30. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-1440.
Over the past 3 decades, age-adjusted mortality for cardiovascular disease (CVD) in the United States has dropped by more than 50%.1 While multiple factors have contributed to this remarkable decline, the introduction and widespread use of statin therapy is unquestionably one key factor. Despite nearly overwhelming evidence that statins effectively lower low-density lipoprotein cholesterol (LDL-C) and predictably reduce cardiovascular events, less than half of patients with clinical coronary heart disease receive high-intensity statin therapy, leaving this population at increased risk for future events.2
Statins: Latest evidence on risks and benefits
Statins aren’t perfect. Not every patient is able to achieve the desired LDL-C lowering with statin therapy, and some patients develop adverse effects such as myopathy, new-onset diabetes, and occasionally hemorrhagic stroke. A recent report puts the risks of statin therapy in perspective, estimating that the treatment of 10,000 patients for 5 years would cause one case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 hemorrhagic strokes.3 The same treatment would avert approximately 1000 CVD events among those with preexisting disease, and approximately 500 CVD events among those with elevated risk, but no preexisting disease.3
In blinded randomized controlled trials, statin therapy is associated with relatively few adverse events (AEs). In open-label observational studies, however, substantially more AEs are reported. During the blinded phase of one recent study, muscle-related AEs and erectile dysfunction were reported at a similar rate by participants randomly assigned to receive atorvastatin or placebo. During the nonblinded nonrandomized phase, complaints of muscle-related AEs were 41% more likely in participants taking statins compared with those who were not.4
Statin therapy offers predictable CVD risk reduction. The evidence report accompanying the 2016 US Preventive Services Task Force (USPSTF) guidelines on statins for the prevention of CVD states that the use of low- or moderate-dose statin therapy was associated with an approximately 30% relative risk reduction (RRR) in CVD events and in CVD deaths, and a 10% to 15% RRR in all-cause mortality.5 Those with greater baseline CVD risk will have greater absolute risk reduction (ARR) than those with low baseline risk.5
How effective are non-statin therapies?
Multiple studies have demonstrated that some drugs can favorably modify lipid levels but not improve patient outcomes—eg, niacin, fibrates, and omega-3 fatty acids. The therapies that do improve outcomes are those that act via upregulation of LDL-receptor expression: statins, ezetimibe, bile acid sequestrants, dietary interventions, and ileal bypass surgery.
A recent meta-analysis found that with a 38.7-mg/dL (1-mmol/L) reduction in LDL-C level, the relative risk for major vascular events was 0.77 (95% CI, 0.71-0.84) for statins and 0.75 (95% CI, 0.66-0.86) for monotherapy with non-statin interventions that upregulate LDL-receptor expression.6
Ezetimibe. Less impressive is the incremental benefit of adding some non-statin therapies to effective statin therapy. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) reported that adding ezetimibe to effective statin therapy in stable patients with previous acute coronary syndrome reduced the LDL-C level from 69.5 mg/dL to 53.7 mg/dL (TABLE7-13).8 After 7 years of treatment, relative risk of atherosclerotic cardiovascular disease (ASCVD) outcomes decreased by 5.8%; absolute decrease in risk was 2%: from 34.7% to 32.7% (number needed to treat [NNT]=50).8 Consider adding ezetimibe to maximally-tolerated statin therapy for patients not meeting LDL-C goals with a statin alone.

Continue to: A new class to lower LDL-C: PCSK9 inhibitors
A new class to lower LDL-C: PCSK9 inhibitors
It is clear that additional approaches to LDL-C reduction are needed. A new drug class that effectively lowers LDL-C levels is monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 activity is directly proportional to the circulating LDL-C level: gene mutations that increase PCSK9 function are one cause of elevated LDL-C and CVD risk in familial hypercholesterolemia (FH),14 whereas mutations that decrease PCSK9 activity are associated with a decrease in LDL-C levels and risk of ASCVD.15
Circulating PCSK9 initiates LDL-receptor clearance by binding to the LDL receptor; the complex is then taken into the hepatocyte, where it undergoes degradation, and the receptor is not recycled to the cell’s surface. The resultant decreased level of cholesterol within the hepatocyte upregulates HMG-CoA reductase (the enzyme that controls the rate-limiting step in cholesterol production and is targeted by statin therapy) and LDL-receptor activity to increase the available cholesterol in the hepatocyte. Unfortunately, statins promote the upregulation of both the LDL receptor and PCSK9, thereby limiting their LDL-C-lowering potency. Combined inhibition of HMG-CoA reductase with statins and PCSK9 with monoclonal antibodies exerts additive reductions in LDL-C.16
Evolocumab and alirocumab—monoclonal antibodies that prevent circulating PCSK9 from binding to the LDL receptor—have been approved by the US Food and Drug Administration (FDA) for use as adjuncts to diet and maximally-tolerated statin therapy in adults who have heterozygous familial hypercholesterolemia (HeFH) or clinical ASCVD and who must further lower LDL-C levels. The addition of a PCSK9 inhibitor to statin therapy consistently results in an incremental decrease in LDL-C of around 60%.10,11 Much of the data supporting the use of PCSK9 inhibitors are disease-oriented. Among patients with angiographic coronary disease treated with statins, the addition of evolocumab resulted in regression of atherosclerotic plaque measured by intravascular ultrasound after 18 months of treatment.10
Continue to: PCSK9 inhibitors reduce adverse CVD events when added to a statin
PCSK9 inhibitors reduce adverse CVD events when added to a statin. In a study designed to evaluate AEs and LDL-C lowering with evolocumab, a prespecified exploratory outcome was the incidence of adjudicated CVD events. After one year of therapy, the rate of events was reduced from 2.18% in the standard-therapy group to 0.95% in the evolocumab group—a relative decrease of 53%, but an absolute decrease of 1.23% (NNT=81).17
A similar reduction in the rate of major adverse CVD events was found in adding alirocumab to ongoing statin therapy. In a post hoc analysis of patients who received either adjunctive alirocumab or placebo, CVD events (death from coronary heart disease, nonfatal myocardial infarction [MI], fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) were 1.7% vs 3.3% (hazard ratio=0.52; 95% confidence interval, 0.31-0.90).11
FOURIER, the first major trial designed to evaluate cardiovascular outcomes with PCSK9 therapy, showed that adding evolocumab to effective statin therapy reduced the average LDL-C level from 92 mg/dL to 30 mg/dL.12 Evolocumab decreased the composite CVD outcome (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) over 2.2 years from 11.3% to 9.8%—a 15% RRR and a 1.5% ARR (NNT=67). Most of the participants were receiving high-intensity statin therapy at study entry. AEs were similar between the study groups.12
A prespecified analysis of FOURIER data found that evolocumab did not increase the risk of new-onset diabetes in patients without diabetes or prediabetes at baseline. Fasting plasma glucose and hemoglobin A1c levels in the evolocumab and placebo groups remained similar throughout the trial in patients with diabetes, prediabetes, or normoglycemia.18 Additionally, a randomized trial involving patients who received either evolocumab or placebo in addition to statin therapy found no significant difference in cognitive function between the groups over a median of 19 months.19
Continue to: Effective, but expensive
Effective, but expensive. At its current list price of approximately $14,000 per year,9 evolocumab, added to standard therapy in patients with ASCVD, exceeds the generally accepted cost-effectiveness threshold of $150,000 per quality-adjusted life year (QALY) achieved.20 Similar analysis in patients with HeFH estimated a cost of $503,000 per QALY achieved with evolocumab.21 The outcomes of cost-effectiveness analyses hinge on the event rate in the study population and the threshold for initiating therapy. For the FOURIER trial participants, with an annual event rate of 4.2 per 100 patient-years, a net annual price of approximately $6700 would be necessary to meet a $150,000 per QALY threshold.22
At 2015 prices, the addition of PCSK9 inhibitor therapy for all eligible patients would reduce cardiovascular care costs by an estimated $29 billion over 5 years but would also increase drug costs by an estimated $592 billion, representing a 38% increase over 2015 prescription drug expenditures.21 Treatment of less than 20 million US adults with evolocumab at the cost of this single drug would match the entire cost for all other prescription pharmaceuticals for all diseases in the United States combined.23
In 2012, 27.9% of US adults ages 40 years and older were taking prescribed lipid-lowering treatment; 23.2% were taking only statins.24 If the
Until the cost of PCSK9 inhibitors decreases to a justifiable level and outcomes of longer term studies are available, consider prescribing other adjunctive treatments for patients who have not achieved LDL-C goals with statin therapy alone. Generally, reserve use of PCSK9 inhibitors for the highest-risk adults: those with HeFH or clinical ASCVD who must further lower LDL-C levels. Some insurers, including Medicare, are covering PCSK9 inhibitors, but many patients have difficulty obtaining coverage.27
Continue to: CETP inhibitors: Not FDA approved
CETP inhibitors: Not FDA approved
In a recent trial of the cholesteryl ester transfer protein (CETP) inhibitor evacetrapib, the drug had favorable effects on lipid biomarkers but did not improve cardiovascular outcomes.28 More recently, the CETP inhibitor anacetrapib was shown to decrease the composite outcome of coronary death, MI, or coronary revascularization in adults with established ASCVD who were receiving high-intensity atorvastatin therapy.13 At the trial midpoint, mean high-density lipoprotein (HDL) cholesterol levels increased by 43 mg/dL in the anacetrapib group compared with that of the placebo group (a relative difference of 104%); mean non-HDL cholesterol decreased by 17 mg/dL, a relative difference of −18%. Over a median follow-up period of 4.1 years, the addition of anacetrapib was associated with a 9% RRR and a 1% absolute reduction in the composite outcome over a statin alone (NNT=100).13 At this point, the manufacturers of both agents have halted efforts to gain FDA approval.
Future directions
Newer strategies to inhibit PCSK9 function are under development. Small peptides that inhibit PCSK9 interaction with the LDL receptor offer the potential advantage of oral administration, as opposed to the currently available injectable anti-PCSK9 antibodies.29 A recent trial found that inhibition of PCSK9 messenger RNA (mRNA) synthesis with the small interfering RNA (siRNA) molecule inclisiran lowered LDL-C in patients with high cardiovascular risk and elevated LDL-C levels despite aggressive statin therapy.30 The effect of these strategies on cardiovascular outcomes remains unproven.
CORRESPONDENCE
Jonathon Firnhaber, MD, Department of Family Medicine, Brody School of Medicine, 101 Heart Drive, Mail Stop 654, Greenville, NC 27834; [email protected].
Over the past 3 decades, age-adjusted mortality for cardiovascular disease (CVD) in the United States has dropped by more than 50%.1 While multiple factors have contributed to this remarkable decline, the introduction and widespread use of statin therapy is unquestionably one key factor. Despite nearly overwhelming evidence that statins effectively lower low-density lipoprotein cholesterol (LDL-C) and predictably reduce cardiovascular events, less than half of patients with clinical coronary heart disease receive high-intensity statin therapy, leaving this population at increased risk for future events.2
Statins: Latest evidence on risks and benefits
Statins aren’t perfect. Not every patient is able to achieve the desired LDL-C lowering with statin therapy, and some patients develop adverse effects such as myopathy, new-onset diabetes, and occasionally hemorrhagic stroke. A recent report puts the risks of statin therapy in perspective, estimating that the treatment of 10,000 patients for 5 years would cause one case of rhabdomyolysis, 5 cases of myopathy, 75 new cases of diabetes, and 7 hemorrhagic strokes.3 The same treatment would avert approximately 1000 CVD events among those with preexisting disease, and approximately 500 CVD events among those with elevated risk, but no preexisting disease.3
In blinded randomized controlled trials, statin therapy is associated with relatively few adverse events (AEs). In open-label observational studies, however, substantially more AEs are reported. During the blinded phase of one recent study, muscle-related AEs and erectile dysfunction were reported at a similar rate by participants randomly assigned to receive atorvastatin or placebo. During the nonblinded nonrandomized phase, complaints of muscle-related AEs were 41% more likely in participants taking statins compared with those who were not.4
Statin therapy offers predictable CVD risk reduction. The evidence report accompanying the 2016 US Preventive Services Task Force (USPSTF) guidelines on statins for the prevention of CVD states that the use of low- or moderate-dose statin therapy was associated with an approximately 30% relative risk reduction (RRR) in CVD events and in CVD deaths, and a 10% to 15% RRR in all-cause mortality.5 Those with greater baseline CVD risk will have greater absolute risk reduction (ARR) than those with low baseline risk.5
How effective are non-statin therapies?
Multiple studies have demonstrated that some drugs can favorably modify lipid levels but not improve patient outcomes—eg, niacin, fibrates, and omega-3 fatty acids. The therapies that do improve outcomes are those that act via upregulation of LDL-receptor expression: statins, ezetimibe, bile acid sequestrants, dietary interventions, and ileal bypass surgery.
A recent meta-analysis found that with a 38.7-mg/dL (1-mmol/L) reduction in LDL-C level, the relative risk for major vascular events was 0.77 (95% CI, 0.71-0.84) for statins and 0.75 (95% CI, 0.66-0.86) for monotherapy with non-statin interventions that upregulate LDL-receptor expression.6
Ezetimibe. Less impressive is the incremental benefit of adding some non-statin therapies to effective statin therapy. The IMProved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) reported that adding ezetimibe to effective statin therapy in stable patients with previous acute coronary syndrome reduced the LDL-C level from 69.5 mg/dL to 53.7 mg/dL (TABLE7-13).8 After 7 years of treatment, relative risk of atherosclerotic cardiovascular disease (ASCVD) outcomes decreased by 5.8%; absolute decrease in risk was 2%: from 34.7% to 32.7% (number needed to treat [NNT]=50).8 Consider adding ezetimibe to maximally-tolerated statin therapy for patients not meeting LDL-C goals with a statin alone.

Continue to: A new class to lower LDL-C: PCSK9 inhibitors
A new class to lower LDL-C: PCSK9 inhibitors
It is clear that additional approaches to LDL-C reduction are needed. A new drug class that effectively lowers LDL-C levels is monoclonal antibodies that inhibit PCSK9 (proprotein convertase subtilisin/kexin type 9). PCSK9 activity is directly proportional to the circulating LDL-C level: gene mutations that increase PCSK9 function are one cause of elevated LDL-C and CVD risk in familial hypercholesterolemia (FH),14 whereas mutations that decrease PCSK9 activity are associated with a decrease in LDL-C levels and risk of ASCVD.15
Circulating PCSK9 initiates LDL-receptor clearance by binding to the LDL receptor; the complex is then taken into the hepatocyte, where it undergoes degradation, and the receptor is not recycled to the cell’s surface. The resultant decreased level of cholesterol within the hepatocyte upregulates HMG-CoA reductase (the enzyme that controls the rate-limiting step in cholesterol production and is targeted by statin therapy) and LDL-receptor activity to increase the available cholesterol in the hepatocyte. Unfortunately, statins promote the upregulation of both the LDL receptor and PCSK9, thereby limiting their LDL-C-lowering potency. Combined inhibition of HMG-CoA reductase with statins and PCSK9 with monoclonal antibodies exerts additive reductions in LDL-C.16
Evolocumab and alirocumab—monoclonal antibodies that prevent circulating PCSK9 from binding to the LDL receptor—have been approved by the US Food and Drug Administration (FDA) for use as adjuncts to diet and maximally-tolerated statin therapy in adults who have heterozygous familial hypercholesterolemia (HeFH) or clinical ASCVD and who must further lower LDL-C levels. The addition of a PCSK9 inhibitor to statin therapy consistently results in an incremental decrease in LDL-C of around 60%.10,11 Much of the data supporting the use of PCSK9 inhibitors are disease-oriented. Among patients with angiographic coronary disease treated with statins, the addition of evolocumab resulted in regression of atherosclerotic plaque measured by intravascular ultrasound after 18 months of treatment.10
Continue to: PCSK9 inhibitors reduce adverse CVD events when added to a statin
PCSK9 inhibitors reduce adverse CVD events when added to a statin. In a study designed to evaluate AEs and LDL-C lowering with evolocumab, a prespecified exploratory outcome was the incidence of adjudicated CVD events. After one year of therapy, the rate of events was reduced from 2.18% in the standard-therapy group to 0.95% in the evolocumab group—a relative decrease of 53%, but an absolute decrease of 1.23% (NNT=81).17
A similar reduction in the rate of major adverse CVD events was found in adding alirocumab to ongoing statin therapy. In a post hoc analysis of patients who received either adjunctive alirocumab or placebo, CVD events (death from coronary heart disease, nonfatal myocardial infarction [MI], fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization) were 1.7% vs 3.3% (hazard ratio=0.52; 95% confidence interval, 0.31-0.90).11
FOURIER, the first major trial designed to evaluate cardiovascular outcomes with PCSK9 therapy, showed that adding evolocumab to effective statin therapy reduced the average LDL-C level from 92 mg/dL to 30 mg/dL.12 Evolocumab decreased the composite CVD outcome (cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization) over 2.2 years from 11.3% to 9.8%—a 15% RRR and a 1.5% ARR (NNT=67). Most of the participants were receiving high-intensity statin therapy at study entry. AEs were similar between the study groups.12
A prespecified analysis of FOURIER data found that evolocumab did not increase the risk of new-onset diabetes in patients without diabetes or prediabetes at baseline. Fasting plasma glucose and hemoglobin A1c levels in the evolocumab and placebo groups remained similar throughout the trial in patients with diabetes, prediabetes, or normoglycemia.18 Additionally, a randomized trial involving patients who received either evolocumab or placebo in addition to statin therapy found no significant difference in cognitive function between the groups over a median of 19 months.19
Continue to: Effective, but expensive
Effective, but expensive. At its current list price of approximately $14,000 per year,9 evolocumab, added to standard therapy in patients with ASCVD, exceeds the generally accepted cost-effectiveness threshold of $150,000 per quality-adjusted life year (QALY) achieved.20 Similar analysis in patients with HeFH estimated a cost of $503,000 per QALY achieved with evolocumab.21 The outcomes of cost-effectiveness analyses hinge on the event rate in the study population and the threshold for initiating therapy. For the FOURIER trial participants, with an annual event rate of 4.2 per 100 patient-years, a net annual price of approximately $6700 would be necessary to meet a $150,000 per QALY threshold.22
At 2015 prices, the addition of PCSK9 inhibitor therapy for all eligible patients would reduce cardiovascular care costs by an estimated $29 billion over 5 years but would also increase drug costs by an estimated $592 billion, representing a 38% increase over 2015 prescription drug expenditures.21 Treatment of less than 20 million US adults with evolocumab at the cost of this single drug would match the entire cost for all other prescription pharmaceuticals for all diseases in the United States combined.23
In 2012, 27.9% of US adults ages 40 years and older were taking prescribed lipid-lowering treatment; 23.2% were taking only statins.24 If the
Until the cost of PCSK9 inhibitors decreases to a justifiable level and outcomes of longer term studies are available, consider prescribing other adjunctive treatments for patients who have not achieved LDL-C goals with statin therapy alone. Generally, reserve use of PCSK9 inhibitors for the highest-risk adults: those with HeFH or clinical ASCVD who must further lower LDL-C levels. Some insurers, including Medicare, are covering PCSK9 inhibitors, but many patients have difficulty obtaining coverage.27
Continue to: CETP inhibitors: Not FDA approved
CETP inhibitors: Not FDA approved
In a recent trial of the cholesteryl ester transfer protein (CETP) inhibitor evacetrapib, the drug had favorable effects on lipid biomarkers but did not improve cardiovascular outcomes.28 More recently, the CETP inhibitor anacetrapib was shown to decrease the composite outcome of coronary death, MI, or coronary revascularization in adults with established ASCVD who were receiving high-intensity atorvastatin therapy.13 At the trial midpoint, mean high-density lipoprotein (HDL) cholesterol levels increased by 43 mg/dL in the anacetrapib group compared with that of the placebo group (a relative difference of 104%); mean non-HDL cholesterol decreased by 17 mg/dL, a relative difference of −18%. Over a median follow-up period of 4.1 years, the addition of anacetrapib was associated with a 9% RRR and a 1% absolute reduction in the composite outcome over a statin alone (NNT=100).13 At this point, the manufacturers of both agents have halted efforts to gain FDA approval.
Future directions
Newer strategies to inhibit PCSK9 function are under development. Small peptides that inhibit PCSK9 interaction with the LDL receptor offer the potential advantage of oral administration, as opposed to the currently available injectable anti-PCSK9 antibodies.29 A recent trial found that inhibition of PCSK9 messenger RNA (mRNA) synthesis with the small interfering RNA (siRNA) molecule inclisiran lowered LDL-C in patients with high cardiovascular risk and elevated LDL-C levels despite aggressive statin therapy.30 The effect of these strategies on cardiovascular outcomes remains unproven.
CORRESPONDENCE
Jonathon Firnhaber, MD, Department of Family Medicine, Brody School of Medicine, 101 Heart Drive, Mail Stop 654, Greenville, NC 27834; [email protected].
1. Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths — trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157.
2. Rodriguez F, Harrington RA. Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and the future of LDL-C lowering. JAMA. 2016;316:1967-1968.
3. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561.
4. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
5. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024.
6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289-1297.
7. GoodRx. Ezetimibe. Available at: https://www.goodrx.com/ezetimibe. Accessed May 2, 2018.
8. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
9. American Journal of Managed Care. Outcomes-based pricing for PCSK9 inhibitors. Available at: http://www.ajmc.com/contributor/inmaculada-hernandez-pharmd/2017/09/outcomes-based-pricing-for-pcsk9-inhibitors. Accessed May 2, 2018.
10. Nicholls S, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384.
11. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
12. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
13. HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-1227.
14. Hunt SC, Hopkins PN, Bulka K, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089-1093.
15. Cohen J, Pertsemlidis A, Kotowski I, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161-165.
16. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
17. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941-950.
19. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633-643.
20. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-2322.
21. Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743-753.
22. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069-1078.
23. Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420.
24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS data Brief. 2014;177:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Accessed May 2, 2018.
25. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
26. Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA. 2017;317:1563-1567.
27
28. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-1942.
29. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
30. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-1440.
1. Weir HK, Anderson RN, Coleman King SM, et al. Heart disease and cancer deaths — trends and projections in the United States, 1969-2020. Prev Chronic Dis. 2016;13:E157.
2. Rodriguez F, Harrington RA. Cholesterol, cardiovascular risk, statins, PCSK9 inhibitors, and the future of LDL-C lowering. JAMA. 2016;316:1967-1968.
3. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532-2561.
4. Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473-2481.
5. Chou R, Dana T, Blazina I, et al. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;316:2008-2024.
6. Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289-1297.
7. GoodRx. Ezetimibe. Available at: https://www.goodrx.com/ezetimibe. Accessed May 2, 2018.
8. Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-2397.
9. American Journal of Managed Care. Outcomes-based pricing for PCSK9 inhibitors. Available at: http://www.ajmc.com/contributor/inmaculada-hernandez-pharmd/2017/09/outcomes-based-pricing-for-pcsk9-inhibitors. Accessed May 2, 2018.
10. Nicholls S, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373-2384.
11. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489-1499.
12. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722.
13. HPS3/TIMI55-REVEAL Collaborative Group. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377:1217-1227.
14. Hunt SC, Hopkins PN, Bulka K, et al. Genetic localization to chromosome 1p32 of the third locus for familial hypercholesterolemia in a Utah kindred. Arterioscler Thromb Vasc Biol. 2000;20:1089-1093.
15. Cohen J, Pertsemlidis A, Kotowski I, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161-165.
16. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
17. Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500-1509.
18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941-950.
19. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med. 2017;377:633-643.
20. Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304-2322.
21. Kazi DS, Moran AE, Coxson PG, et al. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316:743-753.
22. Fonarow GC, Keech AC, Pedersen TR, et al. Cost-effectiveness of evolocumab therapy for reducing cardiovascular events in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2017;2:1069-1078.
23. Ioannidis JPA. Inconsistent guideline recommendations for cardiovascular prevention and the debate about zeroing in on and zeroing LDL-C levels with PCSK9 inhibitors. JAMA. 2017;318:419-420.
24. Gu Q, Paulose-Ram R, Burt VL, et al. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003-2012. NCHS data Brief. 2014;177:1-8. Available at: https://www.cdc.gov/nchs/data/databriefs/db177.pdf. Accessed May 2, 2018.
25. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1-S45.
26. Pagidipati NJ, Navar AM, Mulder H, et al. Comparison of recommended eligibility for primary prevention statin therapy based on the US Preventive Services Task Force Recommendations vs the ACC/AHA Guidelines. JAMA. 2017;317:1563-1567.
27
28. Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376:1933-1942.
29. Dixon DL, Trankle C, Buckley L, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073-1080.
30. Ray KK, Landmesser U, Leiter LA, et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med. 2017;376:1430-1440.
From The Journal of Family Practice | 2018;67(6):339-341,344-345.
PRACTICE RECOMMENDATIONS
› Consider adding ezetimibe to maximally tolerated statin therapy for patients not meeting low-density lipoprotein cholesterol (LDL-C) goals with a statin alone. B
› Limit consideration of PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors to adults at highest risk: those with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease who must further lower LDL-C levels. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Letter from the Editor: A moment of grace
She was my 3:45 p.m. return visit. She had been a new patient with dyspepsia and I had found Helicobacter pylori–gastritis on endoscopy. She had marketplace-based coverage (silver, high-deductible plan), which was why she had to travel from her community to our university for care. That she did not look like me (older white male) was an understatement. She was so enthusiastic as she thanked me for helping her. Then she described how she, her husband (both hourly-wage, working adults) and their two young kids had gathered around the dinner table to discuss how they could reduce their food allowance for 2 months to have money for mom’s H. pylori medication. She said her entire family was grateful for the help to get her well. It was a moment of grace. She is why I keep fighting to make our health system great again.
Our cover articles this month discuss HCV treatment in patients with special risk factors or co-conditions. Fascinating information will emerge from studies like the article about artificial sweeteners. Scientists studied hunger-related enzyme levels and brain blood flow with functional MRI’s in obese and lean individuals after ingesting placebo and sucralose-containing solutions. Results might influence our weight loss advice to patients. Worried about malpractice? See our third front page article.
Additional articles discuss a reversal agent for one of the direct-acting oral anticoagulants and a hemospray for GI bleeding. Clinically impactful articles from the AGA journals are highlighted.
Finally, I would like to thank all of you who filled out the readership survey last fall. We are proud that you identified GI & Hepatology News as the best overall source for news information in gastroenterology.
John I. Allen, MD, MBA, AGAF
Editor in Chief
She was my 3:45 p.m. return visit. She had been a new patient with dyspepsia and I had found Helicobacter pylori–gastritis on endoscopy. She had marketplace-based coverage (silver, high-deductible plan), which was why she had to travel from her community to our university for care. That she did not look like me (older white male) was an understatement. She was so enthusiastic as she thanked me for helping her. Then she described how she, her husband (both hourly-wage, working adults) and their two young kids had gathered around the dinner table to discuss how they could reduce their food allowance for 2 months to have money for mom’s H. pylori medication. She said her entire family was grateful for the help to get her well. It was a moment of grace. She is why I keep fighting to make our health system great again.
Our cover articles this month discuss HCV treatment in patients with special risk factors or co-conditions. Fascinating information will emerge from studies like the article about artificial sweeteners. Scientists studied hunger-related enzyme levels and brain blood flow with functional MRI’s in obese and lean individuals after ingesting placebo and sucralose-containing solutions. Results might influence our weight loss advice to patients. Worried about malpractice? See our third front page article.
Additional articles discuss a reversal agent for one of the direct-acting oral anticoagulants and a hemospray for GI bleeding. Clinically impactful articles from the AGA journals are highlighted.
Finally, I would like to thank all of you who filled out the readership survey last fall. We are proud that you identified GI & Hepatology News as the best overall source for news information in gastroenterology.
John I. Allen, MD, MBA, AGAF
Editor in Chief
She was my 3:45 p.m. return visit. She had been a new patient with dyspepsia and I had found Helicobacter pylori–gastritis on endoscopy. She had marketplace-based coverage (silver, high-deductible plan), which was why she had to travel from her community to our university for care. That she did not look like me (older white male) was an understatement. She was so enthusiastic as she thanked me for helping her. Then she described how she, her husband (both hourly-wage, working adults) and their two young kids had gathered around the dinner table to discuss how they could reduce their food allowance for 2 months to have money for mom’s H. pylori medication. She said her entire family was grateful for the help to get her well. It was a moment of grace. She is why I keep fighting to make our health system great again.
Our cover articles this month discuss HCV treatment in patients with special risk factors or co-conditions. Fascinating information will emerge from studies like the article about artificial sweeteners. Scientists studied hunger-related enzyme levels and brain blood flow with functional MRI’s in obese and lean individuals after ingesting placebo and sucralose-containing solutions. Results might influence our weight loss advice to patients. Worried about malpractice? See our third front page article.
Additional articles discuss a reversal agent for one of the direct-acting oral anticoagulants and a hemospray for GI bleeding. Clinically impactful articles from the AGA journals are highlighted.
Finally, I would like to thank all of you who filled out the readership survey last fall. We are proud that you identified GI & Hepatology News as the best overall source for news information in gastroenterology.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Jump start immunizations in NICU
MALMO, SWEDEN – The neonatal intensive care unit often represents a lost opportunity to bring an infant fully up to date for recommended age-appropriate immunizations – but it needn’t be that way, Raymond C. Stetson, MD, declared at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We were able to find that within our unit a small number of quality improvement measures enabled us to drastically increase our vaccination rate in this population. I think this shows that other units ought to be auditing their immunization rates, and if they find similar root causes of low rates our experience could be generalized to those units as well,” Dr. Stetson said.
It’s well established that premature infants are at increased risk for underimmunization. Dr. Stetson and his coinvestigators deemed the baseline 56% on-time immunization rate in their NICU patients to be unacceptable, because underimmunized infants are more vulnerable to vaccine-preventable illnesses after discharge. So using the quality improvement methodology known as DMAIC – for Define, Measure, Analyze, Improve, Control – the investigators surveyed Mayo NICU physicians and nurses and identified three root causes of the quality gap: lack of staff knowledge of the routine immunization schedule, lack of awareness of when a NICU patient’s vaccines were actually due, and parental vaccine hesitancy.
Session chair Karina Butler, MD, was clearly impressed.
“You make it sound so easy to get such an increment. What were the barriers and obstacles you ran into?” asked Dr. Butler of Temple Street Children’s University Hospital, Dublin.
“Certain providers in our group were a bit more hesitant about giving vaccines,” Dr. Stetson replied. “There had to be a lot of provider education to get them to use the resources we’d created. And parental vaccine hesitancy was a barrier for us. Of that 6% of infants who weren’t fully up to date at discharge, the majority of those were due to parental vaccine hesitancy. I think that’s still a barrier that’s going to need more work.”
Dr. Stetson reported having no relevant financial disclosures.
MALMO, SWEDEN – The neonatal intensive care unit often represents a lost opportunity to bring an infant fully up to date for recommended age-appropriate immunizations – but it needn’t be that way, Raymond C. Stetson, MD, declared at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We were able to find that within our unit a small number of quality improvement measures enabled us to drastically increase our vaccination rate in this population. I think this shows that other units ought to be auditing their immunization rates, and if they find similar root causes of low rates our experience could be generalized to those units as well,” Dr. Stetson said.
It’s well established that premature infants are at increased risk for underimmunization. Dr. Stetson and his coinvestigators deemed the baseline 56% on-time immunization rate in their NICU patients to be unacceptable, because underimmunized infants are more vulnerable to vaccine-preventable illnesses after discharge. So using the quality improvement methodology known as DMAIC – for Define, Measure, Analyze, Improve, Control – the investigators surveyed Mayo NICU physicians and nurses and identified three root causes of the quality gap: lack of staff knowledge of the routine immunization schedule, lack of awareness of when a NICU patient’s vaccines were actually due, and parental vaccine hesitancy.
Session chair Karina Butler, MD, was clearly impressed.
“You make it sound so easy to get such an increment. What were the barriers and obstacles you ran into?” asked Dr. Butler of Temple Street Children’s University Hospital, Dublin.
“Certain providers in our group were a bit more hesitant about giving vaccines,” Dr. Stetson replied. “There had to be a lot of provider education to get them to use the resources we’d created. And parental vaccine hesitancy was a barrier for us. Of that 6% of infants who weren’t fully up to date at discharge, the majority of those were due to parental vaccine hesitancy. I think that’s still a barrier that’s going to need more work.”
Dr. Stetson reported having no relevant financial disclosures.
MALMO, SWEDEN – The neonatal intensive care unit often represents a lost opportunity to bring an infant fully up to date for recommended age-appropriate immunizations – but it needn’t be that way, Raymond C. Stetson, MD, declared at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We were able to find that within our unit a small number of quality improvement measures enabled us to drastically increase our vaccination rate in this population. I think this shows that other units ought to be auditing their immunization rates, and if they find similar root causes of low rates our experience could be generalized to those units as well,” Dr. Stetson said.
It’s well established that premature infants are at increased risk for underimmunization. Dr. Stetson and his coinvestigators deemed the baseline 56% on-time immunization rate in their NICU patients to be unacceptable, because underimmunized infants are more vulnerable to vaccine-preventable illnesses after discharge. So using the quality improvement methodology known as DMAIC – for Define, Measure, Analyze, Improve, Control – the investigators surveyed Mayo NICU physicians and nurses and identified three root causes of the quality gap: lack of staff knowledge of the routine immunization schedule, lack of awareness of when a NICU patient’s vaccines were actually due, and parental vaccine hesitancy.
Session chair Karina Butler, MD, was clearly impressed.
“You make it sound so easy to get such an increment. What were the barriers and obstacles you ran into?” asked Dr. Butler of Temple Street Children’s University Hospital, Dublin.
“Certain providers in our group were a bit more hesitant about giving vaccines,” Dr. Stetson replied. “There had to be a lot of provider education to get them to use the resources we’d created. And parental vaccine hesitancy was a barrier for us. Of that 6% of infants who weren’t fully up to date at discharge, the majority of those were due to parental vaccine hesitancy. I think that’s still a barrier that’s going to need more work.”
Dr. Stetson reported having no relevant financial disclosures.
REPORTING FROM ESPID 2018
Key clinical point:
Major finding: Only 56% of 754 NICU patients from 2015 through mid-2017 were up to date for the ACIP-recommended vaccinations at discharge or transfer. After an intervention, the on-time immunization rate rose to 94% in 155 patients discharged during the first 6 months.
Study details: A study comparing 754 NICU patients prior to intervention and 155 after intervention.
Disclosures: Dr. Stetson reported having no relevant financial disclosures.
Source: Stetson R. E-Poster Discussion Session 04.
Mesenteric adipose–derived stromal cell lactoferrin may mediate protective effects in Crohn’s disease
Inflammatory bowel disease (IBD) and Crohn’s disease (CD), in particular, are characterized by an unusual ectopic extension of mesenteric adipose tissue. This intra-abdominal fat, also known as “creeping fat,” which wraps around the intestine during the onset of CD, is associated with inflammation and ulceration of the small or large intestine. The role of this fat in the development of CD, and whether it is protective or harmful, however, is not clear.
The current study demonstrates that adipose-derived stromal cells (ADSCs), the precursor cell population of adipose tissue, promote colonocyte proliferation and exhibit a differential gene expression profile in a disease-dependent manner. according to Jill M. Hoffman, MD, and her colleagues at the University of California, Los Angeles. Increased expression and release of lactoferrin by ADSCs – an iron-binding glycoprotein and antimicrobial peptide usually found in large quantities in breast milk – was shown to be a likely mediator that could regulate inflammatory responses during CD. These results were published in Cellular and Molecular Gastroenterology and Hepatology (doi: 10.1016/j.jcmgh.2018.02.001).
Intestinal inflammation is primarily mediated by cytokine production, and targeted anticytokine therapy is the current standard for IBD treatment. The cytokine profile from CD patient–derived mesenteric ADSCs and fat tissue was significantly different from that of these patients’ disease-free counterparts. The authors hypothesized that mesenteric ADSCs release adipokines in response to disease-associated signals; this release of adipokines results from differential gene expression of mesenteric ADSCs in CD versus control patients. To test this hypothesis, conditioned media from CD patient–derived ADSCs was used to study gene expression in colonic intestinal epithelial cells in vitro and in mice with experimental colitis in vivo.
Using the Human LncRNA Expression Microarray V4.0, expression of 20,730 protein-coding mRNA targets was analysed, and 992 mRNA transcripts were found to be differentially (less than or equal to twofold change) expressed in CD patient–derived ADSCs, compared with control patient–derived ADSCs. Subsequent pathway analysis suggested activation of cellular growth and proliferation pathways with caspase 8 and p42/44 as top predicted networks that are differentially regulated in CD patient–derived ADSCs with respect to those of control patients.
The investigators treated intestinal epithelial cells – specifically, NCM460 – with conditioned 233 media from the same CD or control patient–derived ADSCs; subsequent microarray profiling using the GeneChip Human Gene ST Array showed increased expression of interleukin-17A, CCL23, and VEGFA. Ingenuity Pathway Analysis of mRNA expression indicated convergence in injury and inflammation pathways with the SERPINE1 gene, which suggests it’s the central regulator of the differential gene expression network.
In vivo, mice with active dextran sulfate sodium (DSS) colitis that were treated with daily injections of conditioned media from CD patients showed attenuation of colitis as compared with mice treated with vehicle or conditioned media from control patients. Furthermore, the mRNA expression of proinflammatory cytokines was reduced with increased proliferative response (as measured by Ki67 expression) in intestinal epithelial cells in the dextran sulfate sodium–treated mice receiving media from CD patients, compared with that in mice receiving media from control patients or vehicle-treated mice.
Cell proliferation was studied in real time (during a period of 120 hours) using the xCELLigence platform. The authors suggested that mesenteric adipose tissue–derived mediators may regulate proliferative responses in intestinal epithelial cells during intestinal inflammation, as observed by enhanced cell-doubling time in conditioned media from CD patient–derived ADSCs.
Levels of lactoferrin mRNA (validated by real time polymerase chain reaction; 92.70 ± 18.41 versus 28.98 ± 5.681; P less than .05) and protein (validated by ELISA; 142.2 ± 5.653 versus 120.1 ± 3.664; P less than .01) were increased in human mesenteric ADSCs and conditioned media from CD patients, respectively, compared with that from controls.
“Compared with mice receiving vehicle injections, mice receiving daily injections of lactoferrin had improved clinical scores (5.625 ± 0.565 versus 11.125 ± 0.743; n = 8) and colon length at day 7 (6.575 ± 0.1688 versus 5.613 ± 0.1445; n = 8). In addition, we found epithelial cell proliferation was increased in the colons of lactoferrin-treated mice with colitis, compared with vehicle-treated controls (3.548e7 ± 1.547e6 versus 1.184e7 ± 2.915e6; P less than .01),” said the authors.
Collectively, the presented data was suggestive of a protective role of mesenteric adipose tissue–derived mediators, such as lactoferrin, in the pathophysiology of CD.
The study was supported by the Broad Medical Research Program (IBD-0390), an NIDDK Q51856 Ruth L. Kirschstein National Research Service Award Postdoctoral Fellowship 1857 (F32 DK102322), the Neuroendocrine Assay and Models of Gastrointestinal Function and Disease Cores (P50 DK 64539), an AGA-1858 Broad Student Research Fellowship, the Blinder Center for Crohn’s 1859 Disease Research, the Eli and Edythe Broad Chair, and NIH/NIDDK grant DK047343.
The authors disclosed no conflicts of interest.
SOURCE: Hoffman J et al. Cell Molec Gastro Hepatol. doi: 10.1016/j.jcmgh.2018.02.001.
Inflammatory bowel disease (IBD), including Crohn’s disease, is a chronic inflammatory condition of the gastrointestinal tract that is often associated with changes in adipose tissue. However, the pathophysiological significance of fat wrapping in Crohn’s disease remains largely elusive. A correlation of IBD with obesity has been established by a number of studies, which report 15%-40% of adults with IBD are obese. Obesity is found to have a negative effect on disease activity and progression to surgery in patients with Crohn’s disease. In contrast, adipose-derived stromal or stem cells exhibit regenerative and anti-inflammatory function.
A recent study published in Cellular and Molecular Gastroenterology and Hepatology by Jill M. Hoffman and her colleagues highlighted the immune-modulatory function of adipose-derived stromal cells (ADSCs) in Crohn’s disease patients. They observed that patient-derived ADSCs promote colonocyte proliferation and exhibit distinct gene expression patterns, compared with healthy controls. The authors successfully identified ADSC-derived lactoferrin, an iron binding glycoprotein and an antimicrobial peptide, as a potential immunoregulatory molecule.
Amlan Biswas, PhD, is an instructor in pediatrics at Harvard Medical School, Boston, and is affiliated with Boston Children’s Hospital in the division of gastroenterology and nutrition. He has no conflicts of interest
Inflammatory bowel disease (IBD), including Crohn’s disease, is a chronic inflammatory condition of the gastrointestinal tract that is often associated with changes in adipose tissue. However, the pathophysiological significance of fat wrapping in Crohn’s disease remains largely elusive. A correlation of IBD with obesity has been established by a number of studies, which report 15%-40% of adults with IBD are obese. Obesity is found to have a negative effect on disease activity and progression to surgery in patients with Crohn’s disease. In contrast, adipose-derived stromal or stem cells exhibit regenerative and anti-inflammatory function.
A recent study published in Cellular and Molecular Gastroenterology and Hepatology by Jill M. Hoffman and her colleagues highlighted the immune-modulatory function of adipose-derived stromal cells (ADSCs) in Crohn’s disease patients. They observed that patient-derived ADSCs promote colonocyte proliferation and exhibit distinct gene expression patterns, compared with healthy controls. The authors successfully identified ADSC-derived lactoferrin, an iron binding glycoprotein and an antimicrobial peptide, as a potential immunoregulatory molecule.
Amlan Biswas, PhD, is an instructor in pediatrics at Harvard Medical School, Boston, and is affiliated with Boston Children’s Hospital in the division of gastroenterology and nutrition. He has no conflicts of interest
Inflammatory bowel disease (IBD), including Crohn’s disease, is a chronic inflammatory condition of the gastrointestinal tract that is often associated with changes in adipose tissue. However, the pathophysiological significance of fat wrapping in Crohn’s disease remains largely elusive. A correlation of IBD with obesity has been established by a number of studies, which report 15%-40% of adults with IBD are obese. Obesity is found to have a negative effect on disease activity and progression to surgery in patients with Crohn’s disease. In contrast, adipose-derived stromal or stem cells exhibit regenerative and anti-inflammatory function.
A recent study published in Cellular and Molecular Gastroenterology and Hepatology by Jill M. Hoffman and her colleagues highlighted the immune-modulatory function of adipose-derived stromal cells (ADSCs) in Crohn’s disease patients. They observed that patient-derived ADSCs promote colonocyte proliferation and exhibit distinct gene expression patterns, compared with healthy controls. The authors successfully identified ADSC-derived lactoferrin, an iron binding glycoprotein and an antimicrobial peptide, as a potential immunoregulatory molecule.
Amlan Biswas, PhD, is an instructor in pediatrics at Harvard Medical School, Boston, and is affiliated with Boston Children’s Hospital in the division of gastroenterology and nutrition. He has no conflicts of interest
Inflammatory bowel disease (IBD) and Crohn’s disease (CD), in particular, are characterized by an unusual ectopic extension of mesenteric adipose tissue. This intra-abdominal fat, also known as “creeping fat,” which wraps around the intestine during the onset of CD, is associated with inflammation and ulceration of the small or large intestine. The role of this fat in the development of CD, and whether it is protective or harmful, however, is not clear.
The current study demonstrates that adipose-derived stromal cells (ADSCs), the precursor cell population of adipose tissue, promote colonocyte proliferation and exhibit a differential gene expression profile in a disease-dependent manner. according to Jill M. Hoffman, MD, and her colleagues at the University of California, Los Angeles. Increased expression and release of lactoferrin by ADSCs – an iron-binding glycoprotein and antimicrobial peptide usually found in large quantities in breast milk – was shown to be a likely mediator that could regulate inflammatory responses during CD. These results were published in Cellular and Molecular Gastroenterology and Hepatology (doi: 10.1016/j.jcmgh.2018.02.001).
Intestinal inflammation is primarily mediated by cytokine production, and targeted anticytokine therapy is the current standard for IBD treatment. The cytokine profile from CD patient–derived mesenteric ADSCs and fat tissue was significantly different from that of these patients’ disease-free counterparts. The authors hypothesized that mesenteric ADSCs release adipokines in response to disease-associated signals; this release of adipokines results from differential gene expression of mesenteric ADSCs in CD versus control patients. To test this hypothesis, conditioned media from CD patient–derived ADSCs was used to study gene expression in colonic intestinal epithelial cells in vitro and in mice with experimental colitis in vivo.
Using the Human LncRNA Expression Microarray V4.0, expression of 20,730 protein-coding mRNA targets was analysed, and 992 mRNA transcripts were found to be differentially (less than or equal to twofold change) expressed in CD patient–derived ADSCs, compared with control patient–derived ADSCs. Subsequent pathway analysis suggested activation of cellular growth and proliferation pathways with caspase 8 and p42/44 as top predicted networks that are differentially regulated in CD patient–derived ADSCs with respect to those of control patients.
The investigators treated intestinal epithelial cells – specifically, NCM460 – with conditioned 233 media from the same CD or control patient–derived ADSCs; subsequent microarray profiling using the GeneChip Human Gene ST Array showed increased expression of interleukin-17A, CCL23, and VEGFA. Ingenuity Pathway Analysis of mRNA expression indicated convergence in injury and inflammation pathways with the SERPINE1 gene, which suggests it’s the central regulator of the differential gene expression network.
In vivo, mice with active dextran sulfate sodium (DSS) colitis that were treated with daily injections of conditioned media from CD patients showed attenuation of colitis as compared with mice treated with vehicle or conditioned media from control patients. Furthermore, the mRNA expression of proinflammatory cytokines was reduced with increased proliferative response (as measured by Ki67 expression) in intestinal epithelial cells in the dextran sulfate sodium–treated mice receiving media from CD patients, compared with that in mice receiving media from control patients or vehicle-treated mice.
Cell proliferation was studied in real time (during a period of 120 hours) using the xCELLigence platform. The authors suggested that mesenteric adipose tissue–derived mediators may regulate proliferative responses in intestinal epithelial cells during intestinal inflammation, as observed by enhanced cell-doubling time in conditioned media from CD patient–derived ADSCs.
Levels of lactoferrin mRNA (validated by real time polymerase chain reaction; 92.70 ± 18.41 versus 28.98 ± 5.681; P less than .05) and protein (validated by ELISA; 142.2 ± 5.653 versus 120.1 ± 3.664; P less than .01) were increased in human mesenteric ADSCs and conditioned media from CD patients, respectively, compared with that from controls.
“Compared with mice receiving vehicle injections, mice receiving daily injections of lactoferrin had improved clinical scores (5.625 ± 0.565 versus 11.125 ± 0.743; n = 8) and colon length at day 7 (6.575 ± 0.1688 versus 5.613 ± 0.1445; n = 8). In addition, we found epithelial cell proliferation was increased in the colons of lactoferrin-treated mice with colitis, compared with vehicle-treated controls (3.548e7 ± 1.547e6 versus 1.184e7 ± 2.915e6; P less than .01),” said the authors.
Collectively, the presented data was suggestive of a protective role of mesenteric adipose tissue–derived mediators, such as lactoferrin, in the pathophysiology of CD.
The study was supported by the Broad Medical Research Program (IBD-0390), an NIDDK Q51856 Ruth L. Kirschstein National Research Service Award Postdoctoral Fellowship 1857 (F32 DK102322), the Neuroendocrine Assay and Models of Gastrointestinal Function and Disease Cores (P50 DK 64539), an AGA-1858 Broad Student Research Fellowship, the Blinder Center for Crohn’s 1859 Disease Research, the Eli and Edythe Broad Chair, and NIH/NIDDK grant DK047343.
The authors disclosed no conflicts of interest.
SOURCE: Hoffman J et al. Cell Molec Gastro Hepatol. doi: 10.1016/j.jcmgh.2018.02.001.
Inflammatory bowel disease (IBD) and Crohn’s disease (CD), in particular, are characterized by an unusual ectopic extension of mesenteric adipose tissue. This intra-abdominal fat, also known as “creeping fat,” which wraps around the intestine during the onset of CD, is associated with inflammation and ulceration of the small or large intestine. The role of this fat in the development of CD, and whether it is protective or harmful, however, is not clear.
The current study demonstrates that adipose-derived stromal cells (ADSCs), the precursor cell population of adipose tissue, promote colonocyte proliferation and exhibit a differential gene expression profile in a disease-dependent manner. according to Jill M. Hoffman, MD, and her colleagues at the University of California, Los Angeles. Increased expression and release of lactoferrin by ADSCs – an iron-binding glycoprotein and antimicrobial peptide usually found in large quantities in breast milk – was shown to be a likely mediator that could regulate inflammatory responses during CD. These results were published in Cellular and Molecular Gastroenterology and Hepatology (doi: 10.1016/j.jcmgh.2018.02.001).
Intestinal inflammation is primarily mediated by cytokine production, and targeted anticytokine therapy is the current standard for IBD treatment. The cytokine profile from CD patient–derived mesenteric ADSCs and fat tissue was significantly different from that of these patients’ disease-free counterparts. The authors hypothesized that mesenteric ADSCs release adipokines in response to disease-associated signals; this release of adipokines results from differential gene expression of mesenteric ADSCs in CD versus control patients. To test this hypothesis, conditioned media from CD patient–derived ADSCs was used to study gene expression in colonic intestinal epithelial cells in vitro and in mice with experimental colitis in vivo.
Using the Human LncRNA Expression Microarray V4.0, expression of 20,730 protein-coding mRNA targets was analysed, and 992 mRNA transcripts were found to be differentially (less than or equal to twofold change) expressed in CD patient–derived ADSCs, compared with control patient–derived ADSCs. Subsequent pathway analysis suggested activation of cellular growth and proliferation pathways with caspase 8 and p42/44 as top predicted networks that are differentially regulated in CD patient–derived ADSCs with respect to those of control patients.
The investigators treated intestinal epithelial cells – specifically, NCM460 – with conditioned 233 media from the same CD or control patient–derived ADSCs; subsequent microarray profiling using the GeneChip Human Gene ST Array showed increased expression of interleukin-17A, CCL23, and VEGFA. Ingenuity Pathway Analysis of mRNA expression indicated convergence in injury and inflammation pathways with the SERPINE1 gene, which suggests it’s the central regulator of the differential gene expression network.
In vivo, mice with active dextran sulfate sodium (DSS) colitis that were treated with daily injections of conditioned media from CD patients showed attenuation of colitis as compared with mice treated with vehicle or conditioned media from control patients. Furthermore, the mRNA expression of proinflammatory cytokines was reduced with increased proliferative response (as measured by Ki67 expression) in intestinal epithelial cells in the dextran sulfate sodium–treated mice receiving media from CD patients, compared with that in mice receiving media from control patients or vehicle-treated mice.
Cell proliferation was studied in real time (during a period of 120 hours) using the xCELLigence platform. The authors suggested that mesenteric adipose tissue–derived mediators may regulate proliferative responses in intestinal epithelial cells during intestinal inflammation, as observed by enhanced cell-doubling time in conditioned media from CD patient–derived ADSCs.
Levels of lactoferrin mRNA (validated by real time polymerase chain reaction; 92.70 ± 18.41 versus 28.98 ± 5.681; P less than .05) and protein (validated by ELISA; 142.2 ± 5.653 versus 120.1 ± 3.664; P less than .01) were increased in human mesenteric ADSCs and conditioned media from CD patients, respectively, compared with that from controls.
“Compared with mice receiving vehicle injections, mice receiving daily injections of lactoferrin had improved clinical scores (5.625 ± 0.565 versus 11.125 ± 0.743; n = 8) and colon length at day 7 (6.575 ± 0.1688 versus 5.613 ± 0.1445; n = 8). In addition, we found epithelial cell proliferation was increased in the colons of lactoferrin-treated mice with colitis, compared with vehicle-treated controls (3.548e7 ± 1.547e6 versus 1.184e7 ± 2.915e6; P less than .01),” said the authors.
Collectively, the presented data was suggestive of a protective role of mesenteric adipose tissue–derived mediators, such as lactoferrin, in the pathophysiology of CD.
The study was supported by the Broad Medical Research Program (IBD-0390), an NIDDK Q51856 Ruth L. Kirschstein National Research Service Award Postdoctoral Fellowship 1857 (F32 DK102322), the Neuroendocrine Assay and Models of Gastrointestinal Function and Disease Cores (P50 DK 64539), an AGA-1858 Broad Student Research Fellowship, the Blinder Center for Crohn’s 1859 Disease Research, the Eli and Edythe Broad Chair, and NIH/NIDDK grant DK047343.
The authors disclosed no conflicts of interest.
SOURCE: Hoffman J et al. Cell Molec Gastro Hepatol. doi: 10.1016/j.jcmgh.2018.02.001.
FROM CMGH
Pediatric MS gets a win with fingolimod
NASHVILLE, TENN. – Pediatric multiple sclerosis is a confirmed clinical entity, which virtually always presents as relapsing-remitting disease, Brenda Banwell, MD, said at the at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“MS in children is the same disease as MS in adults,” said Dr. Banwell, chief of neurology at Children’s Hospital of Philadelphia and the Grace R. Loeb Endowed Chair in Neurosciences there. And although she is unaware of a single case of childhood MS presenting as primary progressive disease, the impact of relapsing-remitting childhood MS may ultimately be progressive damage.
Pediatric MS is also quite rare, a characteristic that makes therapeutic progress challenging. Recruiting sufficient patients for a definitive phase 3 trial is incredibly difficult, especially when multiple trials are commencing simultaneously, not only in the United States but around the world.
Nevertheless, there has been excellent news, Dr. Banwell said in a video interview. Fingolimod (Gilenya), the immunomodulator approved for adult relapsing-remitting MS, gained a pediatric approval under the breakthrough therapy designation on May 11, 2018, on the basis of the successful phase 3 PARADIGMS study.
Compared with intramuscular interferon beta-1a, fingolimod cut the annualized relapse rate by 82%. It also was associated with a 53% annualized reduction in new or newly enlarged T2 lesions and 66% decrease in gadolinium-enhancing T1 lesions.
This big win is prompting researchers and clinicians to rethink the pediatric MS treatment paradigm, Dr. Banwell said. Traditional thinking falls along a dose-escalation pattern that follows relapses. However, “we may take a cue from our rheumatology colleagues, who have seen the benefit of starting with more aggressive treatment and higher doses, getting disease control, and then slowly tapering off.”
Whether this option could actually modify disease progression, as it seems to do in some other inflammatory disorders, is an intriguing – but unproven – possibility, she said.
Dr. Banwell disclosed that she received financial remuneration as a central MRI reviewer for the Novartis-sponsored PARADIGMS study.
NASHVILLE, TENN. – Pediatric multiple sclerosis is a confirmed clinical entity, which virtually always presents as relapsing-remitting disease, Brenda Banwell, MD, said at the at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“MS in children is the same disease as MS in adults,” said Dr. Banwell, chief of neurology at Children’s Hospital of Philadelphia and the Grace R. Loeb Endowed Chair in Neurosciences there. And although she is unaware of a single case of childhood MS presenting as primary progressive disease, the impact of relapsing-remitting childhood MS may ultimately be progressive damage.
Pediatric MS is also quite rare, a characteristic that makes therapeutic progress challenging. Recruiting sufficient patients for a definitive phase 3 trial is incredibly difficult, especially when multiple trials are commencing simultaneously, not only in the United States but around the world.
Nevertheless, there has been excellent news, Dr. Banwell said in a video interview. Fingolimod (Gilenya), the immunomodulator approved for adult relapsing-remitting MS, gained a pediatric approval under the breakthrough therapy designation on May 11, 2018, on the basis of the successful phase 3 PARADIGMS study.
Compared with intramuscular interferon beta-1a, fingolimod cut the annualized relapse rate by 82%. It also was associated with a 53% annualized reduction in new or newly enlarged T2 lesions and 66% decrease in gadolinium-enhancing T1 lesions.
This big win is prompting researchers and clinicians to rethink the pediatric MS treatment paradigm, Dr. Banwell said. Traditional thinking falls along a dose-escalation pattern that follows relapses. However, “we may take a cue from our rheumatology colleagues, who have seen the benefit of starting with more aggressive treatment and higher doses, getting disease control, and then slowly tapering off.”
Whether this option could actually modify disease progression, as it seems to do in some other inflammatory disorders, is an intriguing – but unproven – possibility, she said.
Dr. Banwell disclosed that she received financial remuneration as a central MRI reviewer for the Novartis-sponsored PARADIGMS study.
NASHVILLE, TENN. – Pediatric multiple sclerosis is a confirmed clinical entity, which virtually always presents as relapsing-remitting disease, Brenda Banwell, MD, said at the at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“MS in children is the same disease as MS in adults,” said Dr. Banwell, chief of neurology at Children’s Hospital of Philadelphia and the Grace R. Loeb Endowed Chair in Neurosciences there. And although she is unaware of a single case of childhood MS presenting as primary progressive disease, the impact of relapsing-remitting childhood MS may ultimately be progressive damage.
Pediatric MS is also quite rare, a characteristic that makes therapeutic progress challenging. Recruiting sufficient patients for a definitive phase 3 trial is incredibly difficult, especially when multiple trials are commencing simultaneously, not only in the United States but around the world.
Nevertheless, there has been excellent news, Dr. Banwell said in a video interview. Fingolimod (Gilenya), the immunomodulator approved for adult relapsing-remitting MS, gained a pediatric approval under the breakthrough therapy designation on May 11, 2018, on the basis of the successful phase 3 PARADIGMS study.
Compared with intramuscular interferon beta-1a, fingolimod cut the annualized relapse rate by 82%. It also was associated with a 53% annualized reduction in new or newly enlarged T2 lesions and 66% decrease in gadolinium-enhancing T1 lesions.
This big win is prompting researchers and clinicians to rethink the pediatric MS treatment paradigm, Dr. Banwell said. Traditional thinking falls along a dose-escalation pattern that follows relapses. However, “we may take a cue from our rheumatology colleagues, who have seen the benefit of starting with more aggressive treatment and higher doses, getting disease control, and then slowly tapering off.”
Whether this option could actually modify disease progression, as it seems to do in some other inflammatory disorders, is an intriguing – but unproven – possibility, she said.
Dr. Banwell disclosed that she received financial remuneration as a central MRI reviewer for the Novartis-sponsored PARADIGMS study.
REPORTING FROM THE CMSC ANNUAL MEETING
New and Noteworthy Information—June 2018
CSF Predicts Progression of MS
CSF can predict the future progression of multiple sclerosis (MS), according to a study published online ahead of print May 9 in the Journal of Neurology, Neurosurgery & Psychiatry. CSF and peripheral blood were obtained from patients with clinically isolated syndrome, relapsing-remitting MS, primary progressive MS, or other inflammatory or noninflammatory neurologic diseases who underwent elective diagnostic lumbar puncture. Patients without MS served as controls. CSF samples were analyzed for free and immunoglobulin-associated light chains on B cells and plasmablasts. Clinical follow-up duration was five years. There was an increased median CSF κ:λ free light chain in all groups except controls. This ratio predicted Expanded Disability Status Scale (EDSS) score progression at five years. Median EDSS score was lower among patients with high CSF κ:λ free light chain.
Rathbone E, Durant L, Kinsella J, et al. Cerebrospinal fluid immunoglobulin light chain ratios predict disease progression in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2018 May 9 [Epub ahead of print].
First Anti-CGRP Monoclonal Antibody Gains FDA Approval
The FDA approved Aimovig (erenumab-aooe) for the preventive treatment of migraine in adults. Aimovig is the first FDA-approved preventive migraine treatment in a new class of drugs that blocks the activity of calcitonin gene-related peptide (CGRP). The treatment is given by once-monthly self-injections. Aimovig’s effectiveness was evaluated in three placebo-controlled clinical trials. The first included 955 patients with episodic migraine. Over six months, treated patients had, on average, one to two fewer monthly migraine days than controls. The second study included 577 patients with episodic migraine. Over three months, treated patients had, on average, one fewer migraine day per month than controls. The third study evaluated 667 patients with chronic migraine. Over three months, treated patients had, on average, 2.5 fewer monthly migraine days than controls. Aimovig is marketed by Amgen.
APOE Plays a Greater Role in Women Than in Men
There is a stronger association between APOE-ε4 and CSF tau levels among women than among men, according to a study published online ahead of print May 7 in JAMA Neurology. Investigators selected data from 10 longitudinal cohort studies of normal aging and Alzheimer’s disease. Biomarker analyses included CSF levels of β-amyloid 42, total tau, and phosphorylated tau. Of the 1,798 patients in the CSF biomarker cohort, 862 were women, 226 had Alzheimer’s disease, 1,690 were white, and the mean age was 70. Of the 5,109 patients in the autopsy cohort, 2,813 were women, 4,953 were white, and the mean age was 84. After correcting for multiple comparisons using the Bonferroni procedure, investigators observed a statistically significant interaction between APOE-ε4 and sex on CSF total tau and phosphorylated tau.
Hohman TJ, Dumitrescu L, Barnes LL. Sex-specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018 May 7 [Epub ahead of print].
FDA Issues Warning About Lamictal
The FDA recently warned that Lamictal (lamotrigine), frequently used for treating seizures and bipolar disorder, can cause a rare but serious immune system reaction called hemophagocytic lymphohistiocytosis (HLH), which can be life-threatening. HLH typically presents as a persistent fever, usually greater than 101° F, and can lead to severe problems with blood cells and vital organs. Health care professionals should be aware that prompt recognition and early treatment are important for improving HLH outcomes and decreasing mortality. Diagnosis is often complicated because early signs and symptoms, such as rash and fever, are not specific. HLH also may be confused with other serious immune-related adverse reactions such as drug reaction with eosinophilia and systemic symptoms (DRESS). The FDA is requiring a change to the drug’s prescribing information and drug labeling.
Is Tenecteplase Better Than Alteplase Before Thrombectomy?
When administered before thrombectomy, tenecteplase is associated with a higher incidence of reperfusion and better functional outcome than alteplase among patients with ischemic stroke treated within 4.5 hours after symptom onset, according to a study published April 26 in the New England Journal of Medicine. Researchers randomly assigned patients with ischemic stroke and occlusion of the internal carotid, basilar, or middle cerebral artery who were eligible for thrombectomy to receive tenecteplase or alteplase within 4.5 hours after symptom onset. The primary outcome was reperfusion of greater than 50% of the involved ischemic territory or an absence of retrievable thrombus at the initial angiographic assessment. Of 202 patients enrolled, 101 were assigned to receive tenecteplase. The primary outcome occurred in 22% of the tenecteplase group and 10% of the alteplase group.
Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582.
FDA Approves Gilenya for Pediatric Use
The FDA has approved Gilenya (fingolimod) for the treatment of children and adolescents between the ages of 10 and 18 with relapsing forms of multiple sclerosis (MS), making it the first disease-modifying therapy indicated for these young patients. The approval extends the age range for the drug, which was previously approved for patients age 18 and older with relapsing MS. Gilenya was granted Breakthrough Therapy status in December 2017 for this pediatric indication. The approval was supported by PARADIGMS, a double-blind, randomized, multicenter phase III safety and efficacy study of Gilenya versus interferon beta-1a. In this study, oral Gilenya reduced the annualized relapse rate by approximately 82% for as long as two years, compared with interferon beta-1a intramuscular injections in adolescents with relapsing MS. Gilenya is marketed by Novartis.
Lifestyle Factors at Midlife Could Influence Dementia Risk
Demographic and lifestyle factors assessed in midlife could potentially modify the risk of dementia in late adulthood, according to a study published in the March issue of Journal of Alzheimer’s Disease. Researchers used data collected from 1979 until 1983 in the Framingham Heart Study Offspring cohort to examine associations between lifestyle factors in midlife and late-life dementia. They examined the data with decision tree classifier and random forests analysis. Investigators then evaluated model performance by computing area under receiver operating characteristic (ROC) curve. Age was strongly associated with dementia. The analysis also identified widowed status, lower BMI, and less sleep at midlife as risk factors for dementia. The areas under the ROC curves were 0.79 for the decision tree and 0.89 for the random forest model.
Li J, Ogrodnik M, Kolachalama VB, et al. Assessment of the mid-life demographic and lifestyle risk factors of dementia using data from the Framingham Heart Study Offspring Cohort. J Alzheimers Dis. 2018;63(3):1119-1127.
DBS Device Approved for Refractory Epilepsy
The FDA granted premarket approval for Medtronic’s deep brain stimulation (DBS) therapy as adjunctive treatment for reducing the frequency of partial-onset seizures in patients age 18 or older who are refractory to three or more antiepileptic drugs. The approval is based on the blinded phase and seven-year follow-up data from the SANTE trial, which included 110 patients. The median total seizure frequency reduction from baseline was 40.4% in implanted patients versus 14.5% for the placebo group at three months and 75% at seven years with ongoing open-label therapy. Twenty subjects (18%) had at least one six-month seizure-free period between implant and year seven, including eight subjects (7%) who were seizure-free for the preceding two years. Seizure severity and quality-of-life scales showed statistically significant improvement from baseline to year seven. No significant cognitive declines or worsening of depression were noted.
Epilepsy Does Not Affect Women’s Fertility
In women without a history of infertility or related disorders, the likelihood of conceiving and having a live birth is no different between individuals with or without epilepsy, according to a study published online ahead of print April 30 in JAMA Neurology. Researchers examined data from the Women With Epilepsy: Pregnancy Outcomes and Deliveries study to compare fertility rates between women with and without epilepsy. The primary outcome was the proportion of women who achieved pregnancy within 12 months after enrollment. Of the 197 participants, 142 were white. Mean age was 31.9 among the 89 women with epilepsy and 31.1 among the 108 control women. Amon
Pennell PB, French JA, Harden CL, et al. Fertility and birth outcomes in women with epilepsy seeking pregnancy. JAMA Neurol. 2018 Apr 30 [Epub ahead of print].
FDA Approves Treatment for CIDP
The FDA has approved Hizentra (immune globulin subcutaneous [human] 20% liquid) as the first subcutaneous immunoglobulin (SCIg) for the treatment of chronic inflammatory demyelinating polyneuropathy (CIDP) as maintenance therapy to prevent relapse of neuromuscular disability and impairment. The approval was based on the phase III PATH study, which was the largest controlled clinical study of patients with CIDP to date. The percentage of patients experiencing CIDP relapse or withdrawal for any other reason during SCIg treatment was significantly lower with Hizentra (38.6% on low-dose Hizentra [0.2 g/kg weekly], 32.8% on high-dose Hizentra [0.4 g/kg weekly]) than with placebo (63.2%). Treated patients reported fewer systemic adverse reactions per infusion, compared with IVIg treatment (2.7% vs 9.8%, respectively). Approximately 93% of infusions caused no adverse reactions. Hizentra is marketed by CSL Behring.
Sauna Bathing Reduces Stroke Risk
Frequent sauna bathing is associated with a reduced risk of stroke, according to a study published online ahead of print May 2 in Neurology. Researchers assessed baseline habits of sauna bathing in 1,628 adults between ages 53 and 74 (mean age, 62.7) without a known history of stroke. The following sauna bathing frequency groups were defined: once per week, two to three times per week, and four to seven times per week. During a median follow-up of 14.9 years, 155 incident stroke events were recorded. Compared with people who took one sauna bath per week, the risk of stroke was 14% lower among people with two to three sessions and 61% lower among people with four to seven sessions. Controlling for stroke risk factors did not alter the association.
Kunutsor SK, Khan H, Zaccardi F, et al. Sauna bathing reduces the risk of stroke in Finnish men and women: a prospective cohort study. Neurology. 2018 May 2 [Epub ahead of print].
—Kimberly Williams
and Glenn Williams
CSF Predicts Progression of MS
CSF can predict the future progression of multiple sclerosis (MS), according to a study published online ahead of print May 9 in the Journal of Neurology, Neurosurgery & Psychiatry. CSF and peripheral blood were obtained from patients with clinically isolated syndrome, relapsing-remitting MS, primary progressive MS, or other inflammatory or noninflammatory neurologic diseases who underwent elective diagnostic lumbar puncture. Patients without MS served as controls. CSF samples were analyzed for free and immunoglobulin-associated light chains on B cells and plasmablasts. Clinical follow-up duration was five years. There was an increased median CSF κ:λ free light chain in all groups except controls. This ratio predicted Expanded Disability Status Scale (EDSS) score progression at five years. Median EDSS score was lower among patients with high CSF κ:λ free light chain.
Rathbone E, Durant L, Kinsella J, et al. Cerebrospinal fluid immunoglobulin light chain ratios predict disease progression in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2018 May 9 [Epub ahead of print].
First Anti-CGRP Monoclonal Antibody Gains FDA Approval
The FDA approved Aimovig (erenumab-aooe) for the preventive treatment of migraine in adults. Aimovig is the first FDA-approved preventive migraine treatment in a new class of drugs that blocks the activity of calcitonin gene-related peptide (CGRP). The treatment is given by once-monthly self-injections. Aimovig’s effectiveness was evaluated in three placebo-controlled clinical trials. The first included 955 patients with episodic migraine. Over six months, treated patients had, on average, one to two fewer monthly migraine days than controls. The second study included 577 patients with episodic migraine. Over three months, treated patients had, on average, one fewer migraine day per month than controls. The third study evaluated 667 patients with chronic migraine. Over three months, treated patients had, on average, 2.5 fewer monthly migraine days than controls. Aimovig is marketed by Amgen.
APOE Plays a Greater Role in Women Than in Men
There is a stronger association between APOE-ε4 and CSF tau levels among women than among men, according to a study published online ahead of print May 7 in JAMA Neurology. Investigators selected data from 10 longitudinal cohort studies of normal aging and Alzheimer’s disease. Biomarker analyses included CSF levels of β-amyloid 42, total tau, and phosphorylated tau. Of the 1,798 patients in the CSF biomarker cohort, 862 were women, 226 had Alzheimer’s disease, 1,690 were white, and the mean age was 70. Of the 5,109 patients in the autopsy cohort, 2,813 were women, 4,953 were white, and the mean age was 84. After correcting for multiple comparisons using the Bonferroni procedure, investigators observed a statistically significant interaction between APOE-ε4 and sex on CSF total tau and phosphorylated tau.
Hohman TJ, Dumitrescu L, Barnes LL. Sex-specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018 May 7 [Epub ahead of print].
FDA Issues Warning About Lamictal
The FDA recently warned that Lamictal (lamotrigine), frequently used for treating seizures and bipolar disorder, can cause a rare but serious immune system reaction called hemophagocytic lymphohistiocytosis (HLH), which can be life-threatening. HLH typically presents as a persistent fever, usually greater than 101° F, and can lead to severe problems with blood cells and vital organs. Health care professionals should be aware that prompt recognition and early treatment are important for improving HLH outcomes and decreasing mortality. Diagnosis is often complicated because early signs and symptoms, such as rash and fever, are not specific. HLH also may be confused with other serious immune-related adverse reactions such as drug reaction with eosinophilia and systemic symptoms (DRESS). The FDA is requiring a change to the drug’s prescribing information and drug labeling.
Is Tenecteplase Better Than Alteplase Before Thrombectomy?
When administered before thrombectomy, tenecteplase is associated with a higher incidence of reperfusion and better functional outcome than alteplase among patients with ischemic stroke treated within 4.5 hours after symptom onset, according to a study published April 26 in the New England Journal of Medicine. Researchers randomly assigned patients with ischemic stroke and occlusion of the internal carotid, basilar, or middle cerebral artery who were eligible for thrombectomy to receive tenecteplase or alteplase within 4.5 hours after symptom onset. The primary outcome was reperfusion of greater than 50% of the involved ischemic territory or an absence of retrievable thrombus at the initial angiographic assessment. Of 202 patients enrolled, 101 were assigned to receive tenecteplase. The primary outcome occurred in 22% of the tenecteplase group and 10% of the alteplase group.
Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582.
FDA Approves Gilenya for Pediatric Use
The FDA has approved Gilenya (fingolimod) for the treatment of children and adolescents between the ages of 10 and 18 with relapsing forms of multiple sclerosis (MS), making it the first disease-modifying therapy indicated for these young patients. The approval extends the age range for the drug, which was previously approved for patients age 18 and older with relapsing MS. Gilenya was granted Breakthrough Therapy status in December 2017 for this pediatric indication. The approval was supported by PARADIGMS, a double-blind, randomized, multicenter phase III safety and efficacy study of Gilenya versus interferon beta-1a. In this study, oral Gilenya reduced the annualized relapse rate by approximately 82% for as long as two years, compared with interferon beta-1a intramuscular injections in adolescents with relapsing MS. Gilenya is marketed by Novartis.
Lifestyle Factors at Midlife Could Influence Dementia Risk
Demographic and lifestyle factors assessed in midlife could potentially modify the risk of dementia in late adulthood, according to a study published in the March issue of Journal of Alzheimer’s Disease. Researchers used data collected from 1979 until 1983 in the Framingham Heart Study Offspring cohort to examine associations between lifestyle factors in midlife and late-life dementia. They examined the data with decision tree classifier and random forests analysis. Investigators then evaluated model performance by computing area under receiver operating characteristic (ROC) curve. Age was strongly associated with dementia. The analysis also identified widowed status, lower BMI, and less sleep at midlife as risk factors for dementia. The areas under the ROC curves were 0.79 for the decision tree and 0.89 for the random forest model.
Li J, Ogrodnik M, Kolachalama VB, et al. Assessment of the mid-life demographic and lifestyle risk factors of dementia using data from the Framingham Heart Study Offspring Cohort. J Alzheimers Dis. 2018;63(3):1119-1127.
DBS Device Approved for Refractory Epilepsy
The FDA granted premarket approval for Medtronic’s deep brain stimulation (DBS) therapy as adjunctive treatment for reducing the frequency of partial-onset seizures in patients age 18 or older who are refractory to three or more antiepileptic drugs. The approval is based on the blinded phase and seven-year follow-up data from the SANTE trial, which included 110 patients. The median total seizure frequency reduction from baseline was 40.4% in implanted patients versus 14.5% for the placebo group at three months and 75% at seven years with ongoing open-label therapy. Twenty subjects (18%) had at least one six-month seizure-free period between implant and year seven, including eight subjects (7%) who were seizure-free for the preceding two years. Seizure severity and quality-of-life scales showed statistically significant improvement from baseline to year seven. No significant cognitive declines or worsening of depression were noted.
Epilepsy Does Not Affect Women’s Fertility
In women without a history of infertility or related disorders, the likelihood of conceiving and having a live birth is no different between individuals with or without epilepsy, according to a study published online ahead of print April 30 in JAMA Neurology. Researchers examined data from the Women With Epilepsy: Pregnancy Outcomes and Deliveries study to compare fertility rates between women with and without epilepsy. The primary outcome was the proportion of women who achieved pregnancy within 12 months after enrollment. Of the 197 participants, 142 were white. Mean age was 31.9 among the 89 women with epilepsy and 31.1 among the 108 control women. Amon
Pennell PB, French JA, Harden CL, et al. Fertility and birth outcomes in women with epilepsy seeking pregnancy. JAMA Neurol. 2018 Apr 30 [Epub ahead of print].
FDA Approves Treatment for CIDP
The FDA has approved Hizentra (immune globulin subcutaneous [human] 20% liquid) as the first subcutaneous immunoglobulin (SCIg) for the treatment of chronic inflammatory demyelinating polyneuropathy (CIDP) as maintenance therapy to prevent relapse of neuromuscular disability and impairment. The approval was based on the phase III PATH study, which was the largest controlled clinical study of patients with CIDP to date. The percentage of patients experiencing CIDP relapse or withdrawal for any other reason during SCIg treatment was significantly lower with Hizentra (38.6% on low-dose Hizentra [0.2 g/kg weekly], 32.8% on high-dose Hizentra [0.4 g/kg weekly]) than with placebo (63.2%). Treated patients reported fewer systemic adverse reactions per infusion, compared with IVIg treatment (2.7% vs 9.8%, respectively). Approximately 93% of infusions caused no adverse reactions. Hizentra is marketed by CSL Behring.
Sauna Bathing Reduces Stroke Risk
Frequent sauna bathing is associated with a reduced risk of stroke, according to a study published online ahead of print May 2 in Neurology. Researchers assessed baseline habits of sauna bathing in 1,628 adults between ages 53 and 74 (mean age, 62.7) without a known history of stroke. The following sauna bathing frequency groups were defined: once per week, two to three times per week, and four to seven times per week. During a median follow-up of 14.9 years, 155 incident stroke events were recorded. Compared with people who took one sauna bath per week, the risk of stroke was 14% lower among people with two to three sessions and 61% lower among people with four to seven sessions. Controlling for stroke risk factors did not alter the association.
Kunutsor SK, Khan H, Zaccardi F, et al. Sauna bathing reduces the risk of stroke in Finnish men and women: a prospective cohort study. Neurology. 2018 May 2 [Epub ahead of print].
—Kimberly Williams
and Glenn Williams
CSF Predicts Progression of MS
CSF can predict the future progression of multiple sclerosis (MS), according to a study published online ahead of print May 9 in the Journal of Neurology, Neurosurgery & Psychiatry. CSF and peripheral blood were obtained from patients with clinically isolated syndrome, relapsing-remitting MS, primary progressive MS, or other inflammatory or noninflammatory neurologic diseases who underwent elective diagnostic lumbar puncture. Patients without MS served as controls. CSF samples were analyzed for free and immunoglobulin-associated light chains on B cells and plasmablasts. Clinical follow-up duration was five years. There was an increased median CSF κ:λ free light chain in all groups except controls. This ratio predicted Expanded Disability Status Scale (EDSS) score progression at five years. Median EDSS score was lower among patients with high CSF κ:λ free light chain.
Rathbone E, Durant L, Kinsella J, et al. Cerebrospinal fluid immunoglobulin light chain ratios predict disease progression in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2018 May 9 [Epub ahead of print].
First Anti-CGRP Monoclonal Antibody Gains FDA Approval
The FDA approved Aimovig (erenumab-aooe) for the preventive treatment of migraine in adults. Aimovig is the first FDA-approved preventive migraine treatment in a new class of drugs that blocks the activity of calcitonin gene-related peptide (CGRP). The treatment is given by once-monthly self-injections. Aimovig’s effectiveness was evaluated in three placebo-controlled clinical trials. The first included 955 patients with episodic migraine. Over six months, treated patients had, on average, one to two fewer monthly migraine days than controls. The second study included 577 patients with episodic migraine. Over three months, treated patients had, on average, one fewer migraine day per month than controls. The third study evaluated 667 patients with chronic migraine. Over three months, treated patients had, on average, 2.5 fewer monthly migraine days than controls. Aimovig is marketed by Amgen.
APOE Plays a Greater Role in Women Than in Men
There is a stronger association between APOE-ε4 and CSF tau levels among women than among men, according to a study published online ahead of print May 7 in JAMA Neurology. Investigators selected data from 10 longitudinal cohort studies of normal aging and Alzheimer’s disease. Biomarker analyses included CSF levels of β-amyloid 42, total tau, and phosphorylated tau. Of the 1,798 patients in the CSF biomarker cohort, 862 were women, 226 had Alzheimer’s disease, 1,690 were white, and the mean age was 70. Of the 5,109 patients in the autopsy cohort, 2,813 were women, 4,953 were white, and the mean age was 84. After correcting for multiple comparisons using the Bonferroni procedure, investigators observed a statistically significant interaction between APOE-ε4 and sex on CSF total tau and phosphorylated tau.
Hohman TJ, Dumitrescu L, Barnes LL. Sex-specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018 May 7 [Epub ahead of print].
FDA Issues Warning About Lamictal
The FDA recently warned that Lamictal (lamotrigine), frequently used for treating seizures and bipolar disorder, can cause a rare but serious immune system reaction called hemophagocytic lymphohistiocytosis (HLH), which can be life-threatening. HLH typically presents as a persistent fever, usually greater than 101° F, and can lead to severe problems with blood cells and vital organs. Health care professionals should be aware that prompt recognition and early treatment are important for improving HLH outcomes and decreasing mortality. Diagnosis is often complicated because early signs and symptoms, such as rash and fever, are not specific. HLH also may be confused with other serious immune-related adverse reactions such as drug reaction with eosinophilia and systemic symptoms (DRESS). The FDA is requiring a change to the drug’s prescribing information and drug labeling.
Is Tenecteplase Better Than Alteplase Before Thrombectomy?
When administered before thrombectomy, tenecteplase is associated with a higher incidence of reperfusion and better functional outcome than alteplase among patients with ischemic stroke treated within 4.5 hours after symptom onset, according to a study published April 26 in the New England Journal of Medicine. Researchers randomly assigned patients with ischemic stroke and occlusion of the internal carotid, basilar, or middle cerebral artery who were eligible for thrombectomy to receive tenecteplase or alteplase within 4.5 hours after symptom onset. The primary outcome was reperfusion of greater than 50% of the involved ischemic territory or an absence of retrievable thrombus at the initial angiographic assessment. Of 202 patients enrolled, 101 were assigned to receive tenecteplase. The primary outcome occurred in 22% of the tenecteplase group and 10% of the alteplase group.
Campbell BCV, Mitchell PJ, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582.
FDA Approves Gilenya for Pediatric Use
The FDA has approved Gilenya (fingolimod) for the treatment of children and adolescents between the ages of 10 and 18 with relapsing forms of multiple sclerosis (MS), making it the first disease-modifying therapy indicated for these young patients. The approval extends the age range for the drug, which was previously approved for patients age 18 and older with relapsing MS. Gilenya was granted Breakthrough Therapy status in December 2017 for this pediatric indication. The approval was supported by PARADIGMS, a double-blind, randomized, multicenter phase III safety and efficacy study of Gilenya versus interferon beta-1a. In this study, oral Gilenya reduced the annualized relapse rate by approximately 82% for as long as two years, compared with interferon beta-1a intramuscular injections in adolescents with relapsing MS. Gilenya is marketed by Novartis.
Lifestyle Factors at Midlife Could Influence Dementia Risk
Demographic and lifestyle factors assessed in midlife could potentially modify the risk of dementia in late adulthood, according to a study published in the March issue of Journal of Alzheimer’s Disease. Researchers used data collected from 1979 until 1983 in the Framingham Heart Study Offspring cohort to examine associations between lifestyle factors in midlife and late-life dementia. They examined the data with decision tree classifier and random forests analysis. Investigators then evaluated model performance by computing area under receiver operating characteristic (ROC) curve. Age was strongly associated with dementia. The analysis also identified widowed status, lower BMI, and less sleep at midlife as risk factors for dementia. The areas under the ROC curves were 0.79 for the decision tree and 0.89 for the random forest model.
Li J, Ogrodnik M, Kolachalama VB, et al. Assessment of the mid-life demographic and lifestyle risk factors of dementia using data from the Framingham Heart Study Offspring Cohort. J Alzheimers Dis. 2018;63(3):1119-1127.
DBS Device Approved for Refractory Epilepsy
The FDA granted premarket approval for Medtronic’s deep brain stimulation (DBS) therapy as adjunctive treatment for reducing the frequency of partial-onset seizures in patients age 18 or older who are refractory to three or more antiepileptic drugs. The approval is based on the blinded phase and seven-year follow-up data from the SANTE trial, which included 110 patients. The median total seizure frequency reduction from baseline was 40.4% in implanted patients versus 14.5% for the placebo group at three months and 75% at seven years with ongoing open-label therapy. Twenty subjects (18%) had at least one six-month seizure-free period between implant and year seven, including eight subjects (7%) who were seizure-free for the preceding two years. Seizure severity and quality-of-life scales showed statistically significant improvement from baseline to year seven. No significant cognitive declines or worsening of depression were noted.
Epilepsy Does Not Affect Women’s Fertility
In women without a history of infertility or related disorders, the likelihood of conceiving and having a live birth is no different between individuals with or without epilepsy, according to a study published online ahead of print April 30 in JAMA Neurology. Researchers examined data from the Women With Epilepsy: Pregnancy Outcomes and Deliveries study to compare fertility rates between women with and without epilepsy. The primary outcome was the proportion of women who achieved pregnancy within 12 months after enrollment. Of the 197 participants, 142 were white. Mean age was 31.9 among the 89 women with epilepsy and 31.1 among the 108 control women. Amon
Pennell PB, French JA, Harden CL, et al. Fertility and birth outcomes in women with epilepsy seeking pregnancy. JAMA Neurol. 2018 Apr 30 [Epub ahead of print].
FDA Approves Treatment for CIDP
The FDA has approved Hizentra (immune globulin subcutaneous [human] 20% liquid) as the first subcutaneous immunoglobulin (SCIg) for the treatment of chronic inflammatory demyelinating polyneuropathy (CIDP) as maintenance therapy to prevent relapse of neuromuscular disability and impairment. The approval was based on the phase III PATH study, which was the largest controlled clinical study of patients with CIDP to date. The percentage of patients experiencing CIDP relapse or withdrawal for any other reason during SCIg treatment was significantly lower with Hizentra (38.6% on low-dose Hizentra [0.2 g/kg weekly], 32.8% on high-dose Hizentra [0.4 g/kg weekly]) than with placebo (63.2%). Treated patients reported fewer systemic adverse reactions per infusion, compared with IVIg treatment (2.7% vs 9.8%, respectively). Approximately 93% of infusions caused no adverse reactions. Hizentra is marketed by CSL Behring.
Sauna Bathing Reduces Stroke Risk
Frequent sauna bathing is associated with a reduced risk of stroke, according to a study published online ahead of print May 2 in Neurology. Researchers assessed baseline habits of sauna bathing in 1,628 adults between ages 53 and 74 (mean age, 62.7) without a known history of stroke. The following sauna bathing frequency groups were defined: once per week, two to three times per week, and four to seven times per week. During a median follow-up of 14.9 years, 155 incident stroke events were recorded. Compared with people who took one sauna bath per week, the risk of stroke was 14% lower among people with two to three sessions and 61% lower among people with four to seven sessions. Controlling for stroke risk factors did not alter the association.
Kunutsor SK, Khan H, Zaccardi F, et al. Sauna bathing reduces the risk of stroke in Finnish men and women: a prospective cohort study. Neurology. 2018 May 2 [Epub ahead of print].
—Kimberly Williams
and Glenn Williams
FDA issues Ebola preparedness statement
In response to the Ebola outbreak in the Democratic Republic of Congo (DRC), the Food and Drug Administration has announced steps the agency is taking to make diagnostic and medical products available as part of critical response efforts.
In addition to providing scientific and regulatory advice to medical product developers, the FDA is using its authorities to ensure Merck’s investigational Ebola Zaire vaccine is made available appropriately to vaccinate high-risk populations in the DRC. Additionally, the FDA also is committed to facilitating the development of investigational drugs for the treatment of Ebola virus and supporting access to these products under appropriate regulatory pathways, FDA Commissioner Scott Gottlieb, MD, said in a statement.
Clinical trials that are adaptive to the circumstances of an outbreak are essential, he added. “During the 2014-2015 Ebola outbreak, the FDA recognized that some of the medical products that initially appeared to show great promise sometimes, when subjected to objective testing, were not effective or may have done more harm than good.”
Further, as there are no approved treatments or vaccines for Ebola, the agency will be monitoring for false product claims to protect consumers from fraudulent products claiming to prevent, treat, or cure the disease.
“The FDA knows that it takes a sustained, robust, and globally coordinated effort to best protect our nation from various infectious disease threats,” Dr. Gottlieb wrote. “We’re committed to supporting the people of the DRC and preventing a worsening circumstance during the current outbreak. And we remain highly engaged in the international response efforts.”
In response to the Ebola outbreak in the Democratic Republic of Congo (DRC), the Food and Drug Administration has announced steps the agency is taking to make diagnostic and medical products available as part of critical response efforts.
In addition to providing scientific and regulatory advice to medical product developers, the FDA is using its authorities to ensure Merck’s investigational Ebola Zaire vaccine is made available appropriately to vaccinate high-risk populations in the DRC. Additionally, the FDA also is committed to facilitating the development of investigational drugs for the treatment of Ebola virus and supporting access to these products under appropriate regulatory pathways, FDA Commissioner Scott Gottlieb, MD, said in a statement.
Clinical trials that are adaptive to the circumstances of an outbreak are essential, he added. “During the 2014-2015 Ebola outbreak, the FDA recognized that some of the medical products that initially appeared to show great promise sometimes, when subjected to objective testing, were not effective or may have done more harm than good.”
Further, as there are no approved treatments or vaccines for Ebola, the agency will be monitoring for false product claims to protect consumers from fraudulent products claiming to prevent, treat, or cure the disease.
“The FDA knows that it takes a sustained, robust, and globally coordinated effort to best protect our nation from various infectious disease threats,” Dr. Gottlieb wrote. “We’re committed to supporting the people of the DRC and preventing a worsening circumstance during the current outbreak. And we remain highly engaged in the international response efforts.”
In response to the Ebola outbreak in the Democratic Republic of Congo (DRC), the Food and Drug Administration has announced steps the agency is taking to make diagnostic and medical products available as part of critical response efforts.
In addition to providing scientific and regulatory advice to medical product developers, the FDA is using its authorities to ensure Merck’s investigational Ebola Zaire vaccine is made available appropriately to vaccinate high-risk populations in the DRC. Additionally, the FDA also is committed to facilitating the development of investigational drugs for the treatment of Ebola virus and supporting access to these products under appropriate regulatory pathways, FDA Commissioner Scott Gottlieb, MD, said in a statement.
Clinical trials that are adaptive to the circumstances of an outbreak are essential, he added. “During the 2014-2015 Ebola outbreak, the FDA recognized that some of the medical products that initially appeared to show great promise sometimes, when subjected to objective testing, were not effective or may have done more harm than good.”
Further, as there are no approved treatments or vaccines for Ebola, the agency will be monitoring for false product claims to protect consumers from fraudulent products claiming to prevent, treat, or cure the disease.
“The FDA knows that it takes a sustained, robust, and globally coordinated effort to best protect our nation from various infectious disease threats,” Dr. Gottlieb wrote. “We’re committed to supporting the people of the DRC and preventing a worsening circumstance during the current outbreak. And we remain highly engaged in the international response efforts.”
Americans back from captivity need decompression period
The recent release of three Americans from North Korea has raised the question of how to bring back those who have been incarcerated abroad.
Jason Rezaian is the Iranian-American journalist who was detained in Iran for a year and a half. He recently discussed how beneficial it was for him to have time away from the spotlight after his release. He also expressed concern that the current administration’s tendency to parade former hostages before cameras right after their release could interfere with their ability to heal from their ordeal.
“I was so thankful that I had the opportunity to spend some time alone and with my family before that happened to me,” he said.
When I was in the Army, I was involved in planning for the release of an American pilot shot down over North Korea. Later, I talked on the Larry King Live about American soldiers taken prisoner in the beginning of the Iraq War who were being returned to Fort Bliss, Tex. And now, another American has been released from captivity, this time from Venezuela; by evening that same day, he was meeting with the president – and the press – at the White House.
In planning for repatriation, the military has built on the experience of former prisoners of war (POWs); in doing so, it has learned the best way to bring home those who have been captured. This experience builds on lessons learned from the return of POWs from the Korean war, the Vietnam war, the Gulf War, and other hostilities, according to the Borden Institute, an agency of the U.S. Army Medical Department Center and School now based in Fort Sam in Houston, Tex.
Optimal repatriation usually involves a decompression period, now often at the Army hospital in Landstuhl, Germany. About 3 days are allotted for medical and psychiatric exams, debriefing with intelligence agencies, and reunions with family members. Returning service members also catch up on sleep and nutrition; “three hots and a cot” is the Army mantra for dealing with combat stress, and it also applies here.
The returning service members also are prepared for the glare of media publicity which will follow. They learn how to avoid comments that might embarrass them later. If they have been held captive for a long period of time, they are brought up to date on recent events and news.
To quote the Borden Institute, “Most repatriated POWs, including those from the Persian Gulf War, have had little experience dealing with the media. The media are a substantial stressor that can have lifelong effects if a later, ‘Wish-I-had-never-said,’ statement is broadcast around the world. It is very important to both shield the POW and his family from early intrusive media coverage and to offer training in the management of media requests. This was done routinely for the POWs of the Persian Gulf War. Reminding POWs and their families that it is perfectly acceptable for them to say, ‘No,’ can be a most important intervention.”
So I would urge the current administration to take those lessons into account as it plans for the return of American hostages and returnees.
Dr. Ritchie, a forensic psychiatrist with expertise in military and veteran’s issues, is chief of psychiatry at MedStar Washington Hospital Center.
The recent release of three Americans from North Korea has raised the question of how to bring back those who have been incarcerated abroad.
Jason Rezaian is the Iranian-American journalist who was detained in Iran for a year and a half. He recently discussed how beneficial it was for him to have time away from the spotlight after his release. He also expressed concern that the current administration’s tendency to parade former hostages before cameras right after their release could interfere with their ability to heal from their ordeal.
“I was so thankful that I had the opportunity to spend some time alone and with my family before that happened to me,” he said.
When I was in the Army, I was involved in planning for the release of an American pilot shot down over North Korea. Later, I talked on the Larry King Live about American soldiers taken prisoner in the beginning of the Iraq War who were being returned to Fort Bliss, Tex. And now, another American has been released from captivity, this time from Venezuela; by evening that same day, he was meeting with the president – and the press – at the White House.
In planning for repatriation, the military has built on the experience of former prisoners of war (POWs); in doing so, it has learned the best way to bring home those who have been captured. This experience builds on lessons learned from the return of POWs from the Korean war, the Vietnam war, the Gulf War, and other hostilities, according to the Borden Institute, an agency of the U.S. Army Medical Department Center and School now based in Fort Sam in Houston, Tex.
Optimal repatriation usually involves a decompression period, now often at the Army hospital in Landstuhl, Germany. About 3 days are allotted for medical and psychiatric exams, debriefing with intelligence agencies, and reunions with family members. Returning service members also catch up on sleep and nutrition; “three hots and a cot” is the Army mantra for dealing with combat stress, and it also applies here.
The returning service members also are prepared for the glare of media publicity which will follow. They learn how to avoid comments that might embarrass them later. If they have been held captive for a long period of time, they are brought up to date on recent events and news.
To quote the Borden Institute, “Most repatriated POWs, including those from the Persian Gulf War, have had little experience dealing with the media. The media are a substantial stressor that can have lifelong effects if a later, ‘Wish-I-had-never-said,’ statement is broadcast around the world. It is very important to both shield the POW and his family from early intrusive media coverage and to offer training in the management of media requests. This was done routinely for the POWs of the Persian Gulf War. Reminding POWs and their families that it is perfectly acceptable for them to say, ‘No,’ can be a most important intervention.”
So I would urge the current administration to take those lessons into account as it plans for the return of American hostages and returnees.
Dr. Ritchie, a forensic psychiatrist with expertise in military and veteran’s issues, is chief of psychiatry at MedStar Washington Hospital Center.
The recent release of three Americans from North Korea has raised the question of how to bring back those who have been incarcerated abroad.
Jason Rezaian is the Iranian-American journalist who was detained in Iran for a year and a half. He recently discussed how beneficial it was for him to have time away from the spotlight after his release. He also expressed concern that the current administration’s tendency to parade former hostages before cameras right after their release could interfere with their ability to heal from their ordeal.
“I was so thankful that I had the opportunity to spend some time alone and with my family before that happened to me,” he said.
When I was in the Army, I was involved in planning for the release of an American pilot shot down over North Korea. Later, I talked on the Larry King Live about American soldiers taken prisoner in the beginning of the Iraq War who were being returned to Fort Bliss, Tex. And now, another American has been released from captivity, this time from Venezuela; by evening that same day, he was meeting with the president – and the press – at the White House.
In planning for repatriation, the military has built on the experience of former prisoners of war (POWs); in doing so, it has learned the best way to bring home those who have been captured. This experience builds on lessons learned from the return of POWs from the Korean war, the Vietnam war, the Gulf War, and other hostilities, according to the Borden Institute, an agency of the U.S. Army Medical Department Center and School now based in Fort Sam in Houston, Tex.
Optimal repatriation usually involves a decompression period, now often at the Army hospital in Landstuhl, Germany. About 3 days are allotted for medical and psychiatric exams, debriefing with intelligence agencies, and reunions with family members. Returning service members also catch up on sleep and nutrition; “three hots and a cot” is the Army mantra for dealing with combat stress, and it also applies here.
The returning service members also are prepared for the glare of media publicity which will follow. They learn how to avoid comments that might embarrass them later. If they have been held captive for a long period of time, they are brought up to date on recent events and news.
To quote the Borden Institute, “Most repatriated POWs, including those from the Persian Gulf War, have had little experience dealing with the media. The media are a substantial stressor that can have lifelong effects if a later, ‘Wish-I-had-never-said,’ statement is broadcast around the world. It is very important to both shield the POW and his family from early intrusive media coverage and to offer training in the management of media requests. This was done routinely for the POWs of the Persian Gulf War. Reminding POWs and their families that it is perfectly acceptable for them to say, ‘No,’ can be a most important intervention.”
So I would urge the current administration to take those lessons into account as it plans for the return of American hostages and returnees.
Dr. Ritchie, a forensic psychiatrist with expertise in military and veteran’s issues, is chief of psychiatry at MedStar Washington Hospital Center.
Meta-analysis supports endoscopic surveillance of Barrett’s esophagus
Endoscopic surveillance also was associated with modest improvements in all-cause and cancer-specific mortality, said Don C. Codipilly, MD, of the Mayo Clinic in Rochester, Minn., with his associates. The Barrett’s esophagus community “eagerly” await results from the multicenter, randomized BOSS trial (Barrett’s Oesophagus Surveillance versus endoscopy at need Study), the reviewers wrote in the June issue of Gastroenterology.
Guidelines recommend endoscopic surveillance of patients with Barrett’s esophagus, but it is unclear whether this practice improves survival. Hence, the reviewers searched databases such as Ovid MEDLINE, Embase, PubMed, and Scopus for studies published since 1996 that evaluated outcomes of endoscopic surveillance in Barrett’s esophagus. Eligible studies included a case-control study of a large hospital database in northern California, 6 retrospective cohort studies of endoscopic Barrett’s esophagus surveillance, and 11 prospective and retrospective studies comparing patients with esophageal adenocarcinoma (EAC) with and without a history of Barrett’s esophagus.
The case-control study found no link between endoscopic surveillance and improved survival in Barrett’s esophagus, said the reviewers. However, the retrospective cohort studies linked regular Barrett’s esophagus endoscopic surveillance with a 40% lower risk of death from EAC, compared with incomplete or no endoscopic surveillance, which was statistically significant (risk ratio, 0.60; 95% confidence interval, 0.50-0.71). Individual results of these studies also were consistent with the results of their meta-analysis. A separate meta-analysis of the remaining studies also linked endoscopic surveillance with a significantly lower risk of EAC-related mortality (RR, 0.73; 95% CI, 0.57-0.94), but these studies had substantial heterogeneity, the investigators said.
Meta-analyses also supported endoscopic surveillance for earlier detection of EAC. Unadjusted data from four studies indicated that Barrett’s esophagus surveillance helped detect EAC while it was still early stage (stage 0 or 1) rather than later stage (RR, 2.1; 95% CI, 1.1-4.1). Similarly, patients who had already been diagnosed with Barrett’s esophagus were significantly more likely to present with early-stage EAC (RR, 5.5; 95% CI, 3.7-8.2). In contrast, enrollment in a Barrett’s esophagus surveillance program did not appear to affect the likelihood of esophagectomy.
Additional meta-analyses suggested that endoscopic surveillance of Barrett’s esophagus might confer a “potentially small” overall survival benefit, the reviewers said. A meta-analysis of adjusted data from three studies linked surveillance with a 25% reduction in risk of all-cause mortality, compared with no surveillance (hazard ratio, 0.75; 95% CI, 0.58-0.94). Having a prior Barrett’s esophagus diagnosis also was associated with a 52% decrease in all-cause mortality, compared with having symptomatic cancer (RR, 0.48; 95% CI, 0.37-0.63) in a meta-analysis of unadjusted data from 12 studies. Five studies with adjusted data linked a prior Barrett’s esophagus diagnosis with a 41% lower risk of all-cause mortality (HR, 0.59; 95% CI, 0.45-0.76). However, individual findings varied substantially, and adjusting for lead time “almost eliminated this overall survival benefit,” the reviewers said.
The National Institutes of Health and a Public Health Service Award supported the study. Dr. Codipilly reported having no conflicts of interest. Five coinvestigators disclosed ties to Exact Sciences, C2 Therapeutics, and other companies.
SOURCE: Codipilly DC et al. Gastroenterology. 2018 Feb 17. doi: 10.1053/j.gastro.2018.02.022.
Endoscopic surveillance also was associated with modest improvements in all-cause and cancer-specific mortality, said Don C. Codipilly, MD, of the Mayo Clinic in Rochester, Minn., with his associates. The Barrett’s esophagus community “eagerly” await results from the multicenter, randomized BOSS trial (Barrett’s Oesophagus Surveillance versus endoscopy at need Study), the reviewers wrote in the June issue of Gastroenterology.
Guidelines recommend endoscopic surveillance of patients with Barrett’s esophagus, but it is unclear whether this practice improves survival. Hence, the reviewers searched databases such as Ovid MEDLINE, Embase, PubMed, and Scopus for studies published since 1996 that evaluated outcomes of endoscopic surveillance in Barrett’s esophagus. Eligible studies included a case-control study of a large hospital database in northern California, 6 retrospective cohort studies of endoscopic Barrett’s esophagus surveillance, and 11 prospective and retrospective studies comparing patients with esophageal adenocarcinoma (EAC) with and without a history of Barrett’s esophagus.
The case-control study found no link between endoscopic surveillance and improved survival in Barrett’s esophagus, said the reviewers. However, the retrospective cohort studies linked regular Barrett’s esophagus endoscopic surveillance with a 40% lower risk of death from EAC, compared with incomplete or no endoscopic surveillance, which was statistically significant (risk ratio, 0.60; 95% confidence interval, 0.50-0.71). Individual results of these studies also were consistent with the results of their meta-analysis. A separate meta-analysis of the remaining studies also linked endoscopic surveillance with a significantly lower risk of EAC-related mortality (RR, 0.73; 95% CI, 0.57-0.94), but these studies had substantial heterogeneity, the investigators said.
Meta-analyses also supported endoscopic surveillance for earlier detection of EAC. Unadjusted data from four studies indicated that Barrett’s esophagus surveillance helped detect EAC while it was still early stage (stage 0 or 1) rather than later stage (RR, 2.1; 95% CI, 1.1-4.1). Similarly, patients who had already been diagnosed with Barrett’s esophagus were significantly more likely to present with early-stage EAC (RR, 5.5; 95% CI, 3.7-8.2). In contrast, enrollment in a Barrett’s esophagus surveillance program did not appear to affect the likelihood of esophagectomy.
Additional meta-analyses suggested that endoscopic surveillance of Barrett’s esophagus might confer a “potentially small” overall survival benefit, the reviewers said. A meta-analysis of adjusted data from three studies linked surveillance with a 25% reduction in risk of all-cause mortality, compared with no surveillance (hazard ratio, 0.75; 95% CI, 0.58-0.94). Having a prior Barrett’s esophagus diagnosis also was associated with a 52% decrease in all-cause mortality, compared with having symptomatic cancer (RR, 0.48; 95% CI, 0.37-0.63) in a meta-analysis of unadjusted data from 12 studies. Five studies with adjusted data linked a prior Barrett’s esophagus diagnosis with a 41% lower risk of all-cause mortality (HR, 0.59; 95% CI, 0.45-0.76). However, individual findings varied substantially, and adjusting for lead time “almost eliminated this overall survival benefit,” the reviewers said.
The National Institutes of Health and a Public Health Service Award supported the study. Dr. Codipilly reported having no conflicts of interest. Five coinvestigators disclosed ties to Exact Sciences, C2 Therapeutics, and other companies.
SOURCE: Codipilly DC et al. Gastroenterology. 2018 Feb 17. doi: 10.1053/j.gastro.2018.02.022.
Endoscopic surveillance also was associated with modest improvements in all-cause and cancer-specific mortality, said Don C. Codipilly, MD, of the Mayo Clinic in Rochester, Minn., with his associates. The Barrett’s esophagus community “eagerly” await results from the multicenter, randomized BOSS trial (Barrett’s Oesophagus Surveillance versus endoscopy at need Study), the reviewers wrote in the June issue of Gastroenterology.
Guidelines recommend endoscopic surveillance of patients with Barrett’s esophagus, but it is unclear whether this practice improves survival. Hence, the reviewers searched databases such as Ovid MEDLINE, Embase, PubMed, and Scopus for studies published since 1996 that evaluated outcomes of endoscopic surveillance in Barrett’s esophagus. Eligible studies included a case-control study of a large hospital database in northern California, 6 retrospective cohort studies of endoscopic Barrett’s esophagus surveillance, and 11 prospective and retrospective studies comparing patients with esophageal adenocarcinoma (EAC) with and without a history of Barrett’s esophagus.
The case-control study found no link between endoscopic surveillance and improved survival in Barrett’s esophagus, said the reviewers. However, the retrospective cohort studies linked regular Barrett’s esophagus endoscopic surveillance with a 40% lower risk of death from EAC, compared with incomplete or no endoscopic surveillance, which was statistically significant (risk ratio, 0.60; 95% confidence interval, 0.50-0.71). Individual results of these studies also were consistent with the results of their meta-analysis. A separate meta-analysis of the remaining studies also linked endoscopic surveillance with a significantly lower risk of EAC-related mortality (RR, 0.73; 95% CI, 0.57-0.94), but these studies had substantial heterogeneity, the investigators said.
Meta-analyses also supported endoscopic surveillance for earlier detection of EAC. Unadjusted data from four studies indicated that Barrett’s esophagus surveillance helped detect EAC while it was still early stage (stage 0 or 1) rather than later stage (RR, 2.1; 95% CI, 1.1-4.1). Similarly, patients who had already been diagnosed with Barrett’s esophagus were significantly more likely to present with early-stage EAC (RR, 5.5; 95% CI, 3.7-8.2). In contrast, enrollment in a Barrett’s esophagus surveillance program did not appear to affect the likelihood of esophagectomy.
Additional meta-analyses suggested that endoscopic surveillance of Barrett’s esophagus might confer a “potentially small” overall survival benefit, the reviewers said. A meta-analysis of adjusted data from three studies linked surveillance with a 25% reduction in risk of all-cause mortality, compared with no surveillance (hazard ratio, 0.75; 95% CI, 0.58-0.94). Having a prior Barrett’s esophagus diagnosis also was associated with a 52% decrease in all-cause mortality, compared with having symptomatic cancer (RR, 0.48; 95% CI, 0.37-0.63) in a meta-analysis of unadjusted data from 12 studies. Five studies with adjusted data linked a prior Barrett’s esophagus diagnosis with a 41% lower risk of all-cause mortality (HR, 0.59; 95% CI, 0.45-0.76). However, individual findings varied substantially, and adjusting for lead time “almost eliminated this overall survival benefit,” the reviewers said.
The National Institutes of Health and a Public Health Service Award supported the study. Dr. Codipilly reported having no conflicts of interest. Five coinvestigators disclosed ties to Exact Sciences, C2 Therapeutics, and other companies.
SOURCE: Codipilly DC et al. Gastroenterology. 2018 Feb 17. doi: 10.1053/j.gastro.2018.02.022.
FROM GASTROENTEROLOGY
Key clinical point: Endoscopic surveillance of Barrett’s esophagus was associated with significantly earlier cancer detection and conferred a small survival benefit.
Major finding: Risk ratios for esophageal adenocarcinoma–specific mortality ranged from 0.60 to 0.73 and reached statistical significance.
Study details: A systematic review and meta-analysis of 18 studies.
Disclosures: The National Institutes of Health and a Public Health Service Award supported the study. Dr. Codipilly reported having no conflicts of interest. Five coinvestigators disclosed ties to Exact Sciences, C2 Therapeutics, and other companies.
Source: Codipilly DC et al. Gastroenterology. 2018 Feb 17. doi: 10.1053/j.gastro.2018.02.022.
Detached parents: How to help
While most of the parents you will see in your office will be thoughtful, engaged, and even anxious, occasionally you will encounter parents who seem detached from their children. Spending just a few extra minutes to understand this detachment could mean an enormous difference in the psychological health and developmental well-being of your patient.
It is striking when you see it: Flat affect, little eye contact, no questions or comments. If the parents seem unconnected to their children, it is worth being curious about it. Whether they are constantly on their phones or just disengaged, you should start by directing a question straight to them. Perhaps a question about the child’s sleep, appetite, or school performance is a good place to start. You would like to assess their knowledge of the child’s development, performance at school, friendships, and interests, to ensure that they are up to date and paying attention.
Once alone, you might start by offering your impression of how the child is doing, then observe that you noticed that they (the parents) seem quiet or distant. The following questions should help you better understand the nature of their detachment.
Depression
Ask about how they are sleeping. If the child is very young or the parents have a difficult work schedule, they might simply be sleep deprived, which you might help them address. Difficulty sleeping also can be a symptom of depression. Have they noticed any changes in their appetite or energy? Is it harder to concentrate? Have they felt more tearful, sad, or irritable? Have they noticed that they don’t get as much pleasure from things that usually bring them joy? Do they worry about being a burden to others? Major depressive disorder is relatively common, affecting as many as one in five women in the postpartum period and one in ten women generally.
Men experience depression at about half that rate, according the Centers for Disease Control and Prevention. It is a condition that can cause feelings of guilt and shame, so many suffer silently, missing the chance for treatment and raising the risk for suicide. Infants of depressed mothers often appear listless and may be fussier about sleeping and eating, which can exacerbate poor attachment with their parents, and lead to problems in later years, including anxiety, mood, or behavioral problems. A parent’s depression, particularly in the postpartum period, represents a serious threat to the child’s healthy development and well-being.
Tell the parents that what they have described to you sounds like it could be a depressive episode and that depression has a serious impact on the whole family. Offer that their primary care physicians can evaluate and treat depression, or learn about other resources in your community that you can refer them to for accessible care.
Overwhelming stress
Is the newborn or special needs child feeling like more responsibility than the parents can handle? Where do the parents find support? Has there been a recent job loss or has there been a financial setback for the family? Is a spouse ill, or are they also caring for an aging parent? Has there been a separation from the spouse? Many adults face multiple significant stresses at the same time. It is not uncommon for working parents to be in “soldiering on” mode, just surviving. But this takes a toll on being present and engaged with the children, and puts them at risk for depression and substance abuse.
Traumatic stress
Have they recently experienced a crime or accident, the unexpected loss of a loved one, or threats or abuse at home? They may be grieving, or may be experiencing symptoms of posttraumatic stress disorder that lead them to appear distant and detached. Grief will improve with time, but they may benefit from extra support or from assistance with their responsibilities (a leave from work or more child care). If they have experienced a traumatic stress, they will need a clinical evaluation for potential treatment options. If they are being threatened or abused at home, you need to find out if their children have witnessed the abuse or may be victims as well. You can offer these parents resources for survivors of domestic abuse and speak with them about your obligation to file with your state’s agency responsible for children’s welfare. It is critical that you listen to any concerns they may have about this filing, from losing their children to enraging their abusers.
A detached parent may make a child feel worried, worthless, or guilty. There is no substitute for a loving, engaged parent, and your brief interventions to help a parent reconnect with the child will be invaluable.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected]
While most of the parents you will see in your office will be thoughtful, engaged, and even anxious, occasionally you will encounter parents who seem detached from their children. Spending just a few extra minutes to understand this detachment could mean an enormous difference in the psychological health and developmental well-being of your patient.
It is striking when you see it: Flat affect, little eye contact, no questions or comments. If the parents seem unconnected to their children, it is worth being curious about it. Whether they are constantly on their phones or just disengaged, you should start by directing a question straight to them. Perhaps a question about the child’s sleep, appetite, or school performance is a good place to start. You would like to assess their knowledge of the child’s development, performance at school, friendships, and interests, to ensure that they are up to date and paying attention.
Once alone, you might start by offering your impression of how the child is doing, then observe that you noticed that they (the parents) seem quiet or distant. The following questions should help you better understand the nature of their detachment.
Depression
Ask about how they are sleeping. If the child is very young or the parents have a difficult work schedule, they might simply be sleep deprived, which you might help them address. Difficulty sleeping also can be a symptom of depression. Have they noticed any changes in their appetite or energy? Is it harder to concentrate? Have they felt more tearful, sad, or irritable? Have they noticed that they don’t get as much pleasure from things that usually bring them joy? Do they worry about being a burden to others? Major depressive disorder is relatively common, affecting as many as one in five women in the postpartum period and one in ten women generally.
Men experience depression at about half that rate, according the Centers for Disease Control and Prevention. It is a condition that can cause feelings of guilt and shame, so many suffer silently, missing the chance for treatment and raising the risk for suicide. Infants of depressed mothers often appear listless and may be fussier about sleeping and eating, which can exacerbate poor attachment with their parents, and lead to problems in later years, including anxiety, mood, or behavioral problems. A parent’s depression, particularly in the postpartum period, represents a serious threat to the child’s healthy development and well-being.
Tell the parents that what they have described to you sounds like it could be a depressive episode and that depression has a serious impact on the whole family. Offer that their primary care physicians can evaluate and treat depression, or learn about other resources in your community that you can refer them to for accessible care.
Overwhelming stress
Is the newborn or special needs child feeling like more responsibility than the parents can handle? Where do the parents find support? Has there been a recent job loss or has there been a financial setback for the family? Is a spouse ill, or are they also caring for an aging parent? Has there been a separation from the spouse? Many adults face multiple significant stresses at the same time. It is not uncommon for working parents to be in “soldiering on” mode, just surviving. But this takes a toll on being present and engaged with the children, and puts them at risk for depression and substance abuse.
Traumatic stress
Have they recently experienced a crime or accident, the unexpected loss of a loved one, or threats or abuse at home? They may be grieving, or may be experiencing symptoms of posttraumatic stress disorder that lead them to appear distant and detached. Grief will improve with time, but they may benefit from extra support or from assistance with their responsibilities (a leave from work or more child care). If they have experienced a traumatic stress, they will need a clinical evaluation for potential treatment options. If they are being threatened or abused at home, you need to find out if their children have witnessed the abuse or may be victims as well. You can offer these parents resources for survivors of domestic abuse and speak with them about your obligation to file with your state’s agency responsible for children’s welfare. It is critical that you listen to any concerns they may have about this filing, from losing their children to enraging their abusers.
A detached parent may make a child feel worried, worthless, or guilty. There is no substitute for a loving, engaged parent, and your brief interventions to help a parent reconnect with the child will be invaluable.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected]
While most of the parents you will see in your office will be thoughtful, engaged, and even anxious, occasionally you will encounter parents who seem detached from their children. Spending just a few extra minutes to understand this detachment could mean an enormous difference in the psychological health and developmental well-being of your patient.
It is striking when you see it: Flat affect, little eye contact, no questions or comments. If the parents seem unconnected to their children, it is worth being curious about it. Whether they are constantly on their phones or just disengaged, you should start by directing a question straight to them. Perhaps a question about the child’s sleep, appetite, or school performance is a good place to start. You would like to assess their knowledge of the child’s development, performance at school, friendships, and interests, to ensure that they are up to date and paying attention.
Once alone, you might start by offering your impression of how the child is doing, then observe that you noticed that they (the parents) seem quiet or distant. The following questions should help you better understand the nature of their detachment.
Depression
Ask about how they are sleeping. If the child is very young or the parents have a difficult work schedule, they might simply be sleep deprived, which you might help them address. Difficulty sleeping also can be a symptom of depression. Have they noticed any changes in their appetite or energy? Is it harder to concentrate? Have they felt more tearful, sad, or irritable? Have they noticed that they don’t get as much pleasure from things that usually bring them joy? Do they worry about being a burden to others? Major depressive disorder is relatively common, affecting as many as one in five women in the postpartum period and one in ten women generally.
Men experience depression at about half that rate, according the Centers for Disease Control and Prevention. It is a condition that can cause feelings of guilt and shame, so many suffer silently, missing the chance for treatment and raising the risk for suicide. Infants of depressed mothers often appear listless and may be fussier about sleeping and eating, which can exacerbate poor attachment with their parents, and lead to problems in later years, including anxiety, mood, or behavioral problems. A parent’s depression, particularly in the postpartum period, represents a serious threat to the child’s healthy development and well-being.
Tell the parents that what they have described to you sounds like it could be a depressive episode and that depression has a serious impact on the whole family. Offer that their primary care physicians can evaluate and treat depression, or learn about other resources in your community that you can refer them to for accessible care.
Overwhelming stress
Is the newborn or special needs child feeling like more responsibility than the parents can handle? Where do the parents find support? Has there been a recent job loss or has there been a financial setback for the family? Is a spouse ill, or are they also caring for an aging parent? Has there been a separation from the spouse? Many adults face multiple significant stresses at the same time. It is not uncommon for working parents to be in “soldiering on” mode, just surviving. But this takes a toll on being present and engaged with the children, and puts them at risk for depression and substance abuse.
Traumatic stress
Have they recently experienced a crime or accident, the unexpected loss of a loved one, or threats or abuse at home? They may be grieving, or may be experiencing symptoms of posttraumatic stress disorder that lead them to appear distant and detached. Grief will improve with time, but they may benefit from extra support or from assistance with their responsibilities (a leave from work or more child care). If they have experienced a traumatic stress, they will need a clinical evaluation for potential treatment options. If they are being threatened or abused at home, you need to find out if their children have witnessed the abuse or may be victims as well. You can offer these parents resources for survivors of domestic abuse and speak with them about your obligation to file with your state’s agency responsible for children’s welfare. It is critical that you listen to any concerns they may have about this filing, from losing their children to enraging their abusers.
A detached parent may make a child feel worried, worthless, or guilty. There is no substitute for a loving, engaged parent, and your brief interventions to help a parent reconnect with the child will be invaluable.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected]