User login
Let low-risk moms eat during labor?
Illustrative Case
A 23-year-old nulliparous female at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the last 3 hours and says she’s hungry.
What type of diet should you order for this patient? Should you place any restrictions in the diet order?
Since the first reports of Mendelson Syndrome (aspiration during general anesthesia) in the early 1940s,2 many health care providers managing laboring women restrict their diets to clear liquids or less with little evidence to support the decision. In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a study of 172,334 patients ≥18 years of age in the general population undergoing a total of 215,488 emergency or elective surgeries with general anesthesia, the risk of aspiration was 1:895 and 1:3886, respectively.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less restrictive diet during labor.
Continue to: STUDY SUMMARY
STUDY SUMMARY
Meta-analysis finds not one case of aspiration
This meta-analysis of 10 RCTs, including 3982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥37 weeks, 2 studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes (95% confidence interval [CI], -25 to -7). Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal intensive care unit were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk=1.00; 95% CI, 0.81-1.23). None of the 3982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
Restricting diets during labor is outdated
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk of complications/surgery during labor are not justified based on current data.
Continue to: CAVEATS
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ciardulli A, Saccone G, Anastasio H, et al. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129:473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38:1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during perioperative period. Anesthesiology. 1993;78:56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14:171-175.
6. Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
Illustrative Case
A 23-year-old nulliparous female at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the last 3 hours and says she’s hungry.
What type of diet should you order for this patient? Should you place any restrictions in the diet order?
Since the first reports of Mendelson Syndrome (aspiration during general anesthesia) in the early 1940s,2 many health care providers managing laboring women restrict their diets to clear liquids or less with little evidence to support the decision. In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a study of 172,334 patients ≥18 years of age in the general population undergoing a total of 215,488 emergency or elective surgeries with general anesthesia, the risk of aspiration was 1:895 and 1:3886, respectively.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less restrictive diet during labor.
Continue to: STUDY SUMMARY
STUDY SUMMARY
Meta-analysis finds not one case of aspiration
This meta-analysis of 10 RCTs, including 3982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥37 weeks, 2 studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes (95% confidence interval [CI], -25 to -7). Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal intensive care unit were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk=1.00; 95% CI, 0.81-1.23). None of the 3982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
Restricting diets during labor is outdated
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk of complications/surgery during labor are not justified based on current data.
Continue to: CAVEATS
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Illustrative Case
A 23-year-old nulliparous female at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the last 3 hours and says she’s hungry.
What type of diet should you order for this patient? Should you place any restrictions in the diet order?
Since the first reports of Mendelson Syndrome (aspiration during general anesthesia) in the early 1940s,2 many health care providers managing laboring women restrict their diets to clear liquids or less with little evidence to support the decision. In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a study of 172,334 patients ≥18 years of age in the general population undergoing a total of 215,488 emergency or elective surgeries with general anesthesia, the risk of aspiration was 1:895 and 1:3886, respectively.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less restrictive diet during labor.
Continue to: STUDY SUMMARY
STUDY SUMMARY
Meta-analysis finds not one case of aspiration
This meta-analysis of 10 RCTs, including 3982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥37 weeks, 2 studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes (95% confidence interval [CI], -25 to -7). Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal intensive care unit were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk=1.00; 95% CI, 0.81-1.23). None of the 3982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
Restricting diets during labor is outdated
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk of complications/surgery during labor are not justified based on current data.
Continue to: CAVEATS
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ciardulli A, Saccone G, Anastasio H, et al. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129:473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38:1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during perioperative period. Anesthesiology. 1993;78:56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14:171-175.
6. Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
1. Ciardulli A, Saccone G, Anastasio H, et al. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129:473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38:1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during perioperative period. Anesthesiology. 1993;78:56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14:171-175.
6. Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
PRACTICE CHANGER
Allowing low-risk patients planning for a vaginal delivery less restrictive diets during labor does not seem to increase the risk of aspiration or other harms and may shorten labor.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of 10 randomized controlled trials (RCTs) in tertiary hospitals.
Ciardulli A, Saccone G, Anastasio H, et al. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129:473-480.
Enlarging nodule under the toenail • no history of trauma • unremarkable medical history • Dx?
THE CASE
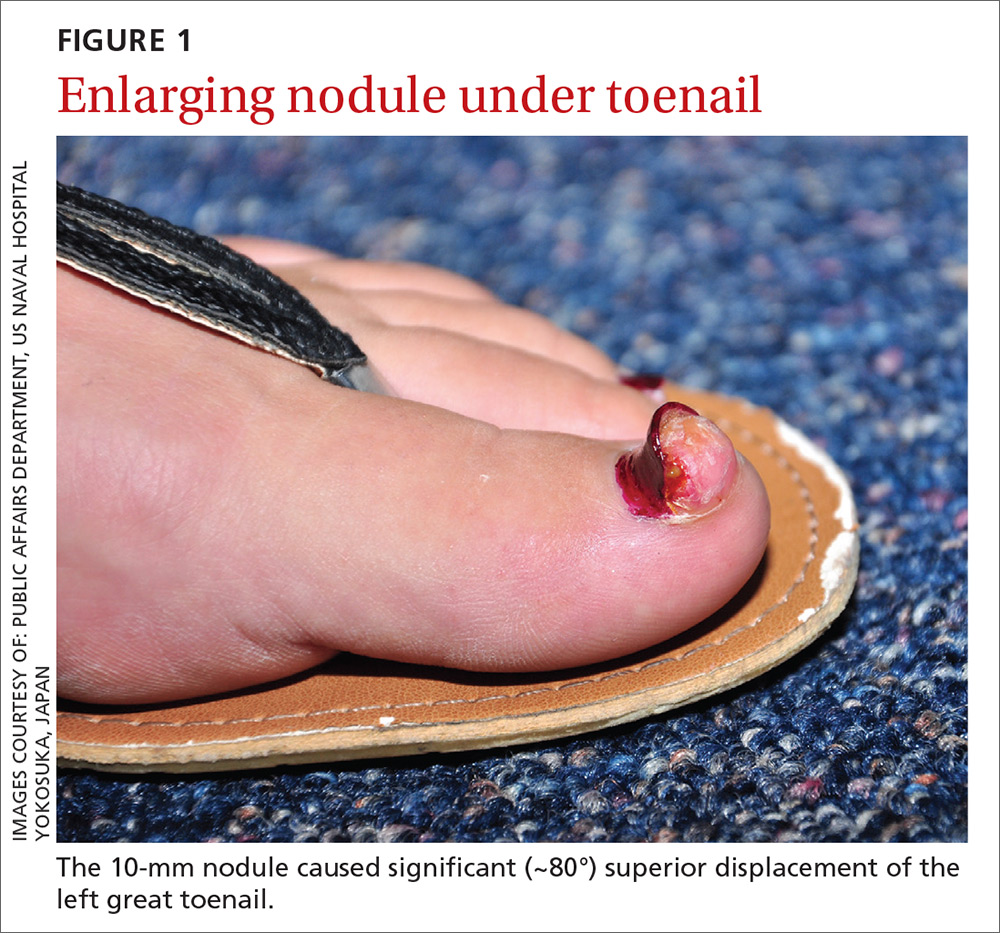
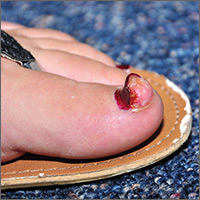
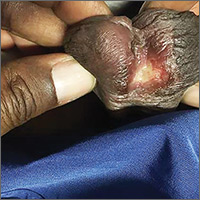
A 28-year-old woman with an unremarkable medical history presented with an enlarging nodule that had been growing under her left great toenail for 6 months. The patient monitored the nodule, hoping that it would resolve on its own, but found that it steadily increased in size and began to displace the nail, causing pain. At the time of presentation, the nodule measured approximately 10 mm in diameter, and there was significant (~80°) superior displacement of the nail (FIGURE 1).
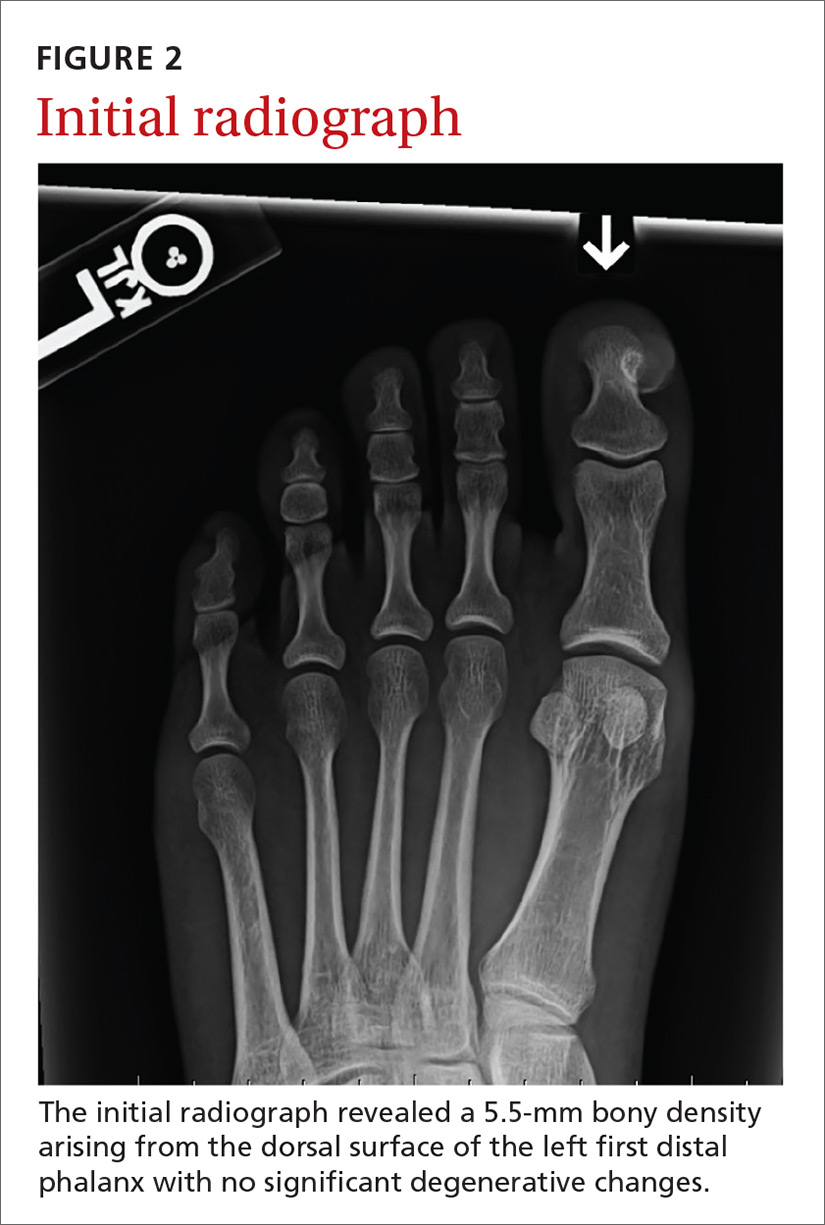
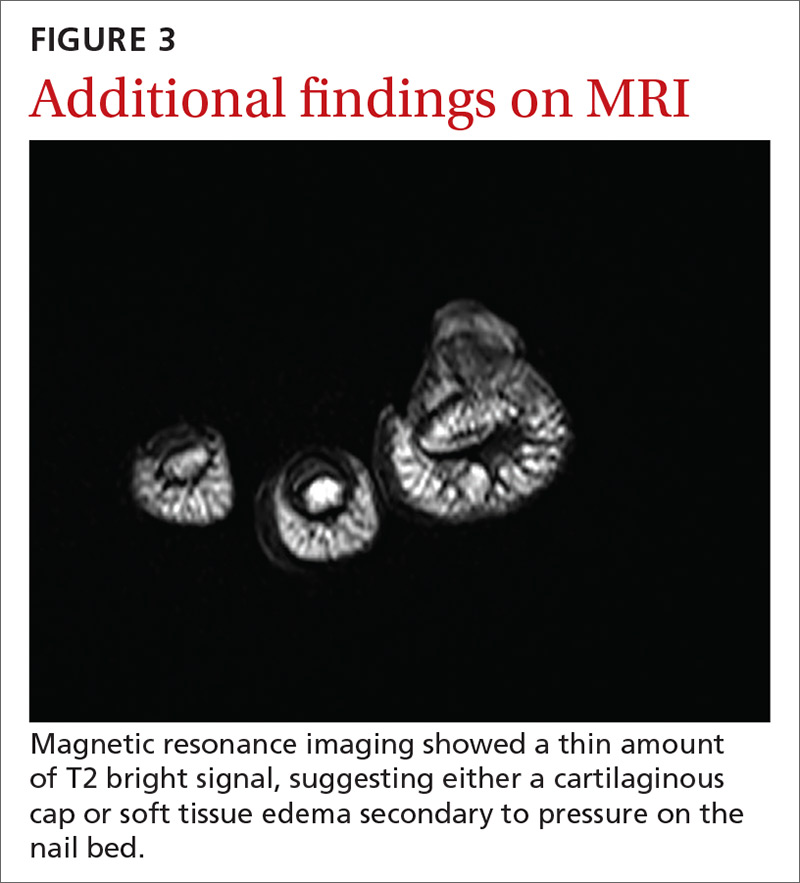
An initial radiograph identified a 5.5-mm bony density arising from the dorsal surface of the left first distal phalanx with no significant degenerative changes (FIGURE 2). A subsequent magnetic resonance image confirmed the bony excrescence and noted marrow continuity. A thin amount of T2 bright signal was also observed, suggesting either a cartilaginous cap or soft tissue edema secondary to pressure on the nail bed (FIGURE 3).
THE DIAGNOSIS
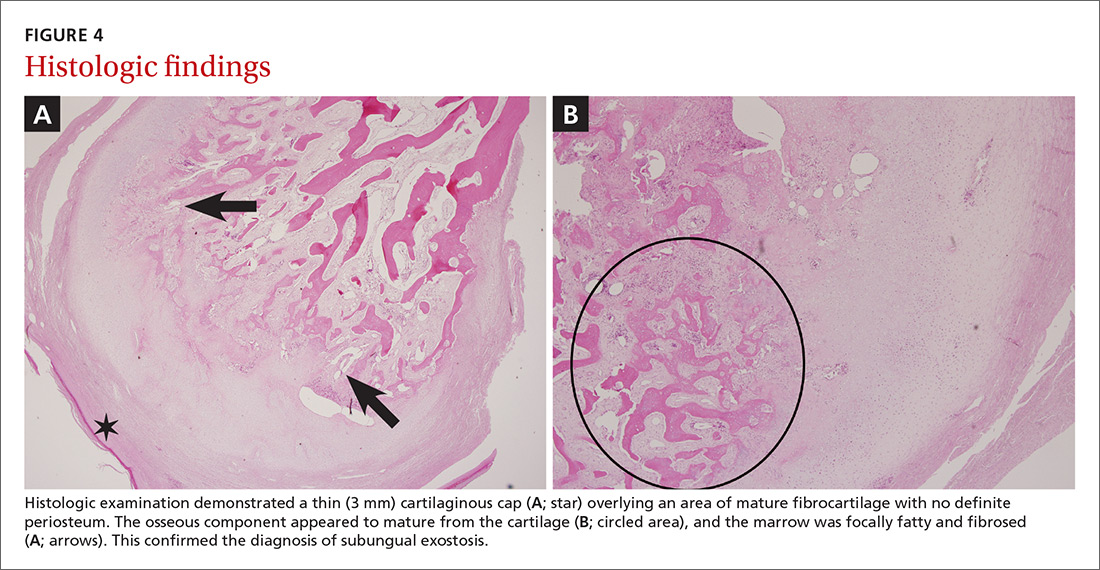
Histologic examination demonstrated a thin (3 mm) cartilaginous cap overlying an area of mature fibrocartilage with no definite periosteum. The osseous component appeared to mature from the cartilage, and the marrow was focally fatty and fibrosed (FIGURES 4A and 4B). Expert consultation with the Joint Pathology Center confirmed a benign osteochondromatous lesion.
The histologic differential diagnosis of this patient’s lesion included subungual exostosis and osteochondroma. Based on the patient’s age, location of the lesion, and histologic findings, the final diagnosis was subungual exostosis.
DISCUSSION
Subungual exostoses are benign osteocartilaginous tumors that most commonly affect children and young adults. They predominantly manifest on the dorsomedial aspect of the tip of the great toe (~80%), but can occur on other digits of the foot or hand.1 They are caused by a proliferation of fibrous tissue under the nail bed. The fibrocartilage cap then undergoes endochondral ossification to woven bone and lamellar bone trabeculae. As these lesions mature, they establish continuity with the underlying bone in the phalanx.2 Subungual exostoses were once thought to represent a proliferative response to trauma, but further research has identified a recurrent t(X;6) (q22;q13-14) translocation, suggesting a neoplastic origin.3
Osteochondromas are also common benign tumors formed by endochondral ossification, although secondary transformation into low-grade chondrosarcomas is well-documented.1 Osteochondromas commonly affect younger patients. They occur at epiphyseal areas of developing bone and have a hyaline matrix and chondrocyte pattern similar to that of a normal epiphyseal area, with confluence to the underlying trabecular and cortical bone. They are not caused by previous trauma and generally only become symptomatic after they have grown large enough to cause mechanical problems.1
Continue to: More diagnoses to consider
More diagnoses to consider
Other potential diagnoses for benign osteochondromatous lesions include bizarre parosteal osteochondromatous proliferations (BPOP) and digital mucous cysts.
BPOPs, also known as Nora’s lesions (crediting preliminary research performed by Nora and colleagues in 19834), are irregular formations of hypercellular cartilage, bone, and large chondrocytes. They predominantly occur in the small bones of the hands and feet, but may involve the skull and long bones.3 Unlike subungual exostoses and osteochondromas, BPOPs tend to occur in the third and fourth decades of life and generally do not alter, or have continuity with, the underlying bone.4
Histologically, BPOPs undergo irregular maturation, leaving a characteristic blue tint at the border of the newly formed trabecular bone. As with subungual exostoses, these lesions were traditionally believed to be reactive in nature. However, cytogenetic studies have identified variant translocations involving 1q32 (most commonly t[1;17] [q32;q21]) that are unique and common to these lesions.5
Digital mucous cysts are benign ganglion cysts that typically appear in the distal interphalangeal joints or at the proximal nail fold. They are believed to result from mucoid degeneration of connective tissue. Although generally associated with the hands, these cysts can also occur on the feet.6
Continue to: Our patient's outcome
Our patient’s outcome
After orthopedic consultation, the lesion and a 5 × 5-mm portion of the adherent germinal nail matrix were resected operatively through a medial excision. A small flap of the lateral nail matrix was rotated to cover the matrix defect, and the wound was closed. Postoperatively, the patient experienced slow wound healing (a total of 3 weeks), but there was no recurrence of the lesion at the 2-month follow-up.
THE TAKEAWAY
Osteocartilaginous tumors present as rapidly growing lesions on the distal tips of fingers and toes, but they may also occur on long bones and on the skull. Rarely malignant in nature, most of these lesions can be differentiated by location, histopathologic features, and patient age at onset. Consider surgical consultation and excision for relief of pain and/or cosmetic reasons. Recurrence is rare.
CORRESPONDENCE
Michael Barna, MD, Naval Hospital Camp Lejeune, Department of Family Medicine, 100 Brewster Blvd, Camp Lejeune, NC 28547; [email protected].
1. Miller-Breslow A, Dorfman HD. Dupuytren’s (subungual) exostosis. Am J Surg Pathol. 1988;12:368-378.
2. DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259.
3. Meneses MF, Unni KK, Swee RG. Bizarre parosteal osteochondromatous proliferation of bone (Nora’s lesion). Am J Surg Pathol. 1993;17:691-697.
4. Nora FE, Dahlin DC, Beabout JW. Bizarre parosteal osteochondromatous proliferations of the hand and feet. Am J Surg Pathol. 1983;7:245-250.
5. Zambrano E, Nosé V, Perez-Atayde AR, et al. Distinct chromosomal rearrangements in subungual (Dupuytren) exostosis and bizarre parosteal osteochondromatous proliferation (Nora lesion). Am J Surg Pathol. 2004;28:1033-1039.
6. Salerni G, Alonso C. Images in clinical medicine. Digital mucous cyst. N Engl J Med. 2012;366:1335.
THE CASE
A 28-year-old woman with an unremarkable medical history presented with an enlarging nodule that had been growing under her left great toenail for 6 months. The patient monitored the nodule, hoping that it would resolve on its own, but found that it steadily increased in size and began to displace the nail, causing pain. At the time of presentation, the nodule measured approximately 10 mm in diameter, and there was significant (~80°) superior displacement of the nail (FIGURE 1).
An initial radiograph identified a 5.5-mm bony density arising from the dorsal surface of the left first distal phalanx with no significant degenerative changes (FIGURE 2). A subsequent magnetic resonance image confirmed the bony excrescence and noted marrow continuity. A thin amount of T2 bright signal was also observed, suggesting either a cartilaginous cap or soft tissue edema secondary to pressure on the nail bed (FIGURE 3).
THE DIAGNOSIS
Histologic examination demonstrated a thin (3 mm) cartilaginous cap overlying an area of mature fibrocartilage with no definite periosteum. The osseous component appeared to mature from the cartilage, and the marrow was focally fatty and fibrosed (FIGURES 4A and 4B). Expert consultation with the Joint Pathology Center confirmed a benign osteochondromatous lesion.
The histologic differential diagnosis of this patient’s lesion included subungual exostosis and osteochondroma. Based on the patient’s age, location of the lesion, and histologic findings, the final diagnosis was subungual exostosis.
DISCUSSION
Subungual exostoses are benign osteocartilaginous tumors that most commonly affect children and young adults. They predominantly manifest on the dorsomedial aspect of the tip of the great toe (~80%), but can occur on other digits of the foot or hand.1 They are caused by a proliferation of fibrous tissue under the nail bed. The fibrocartilage cap then undergoes endochondral ossification to woven bone and lamellar bone trabeculae. As these lesions mature, they establish continuity with the underlying bone in the phalanx.2 Subungual exostoses were once thought to represent a proliferative response to trauma, but further research has identified a recurrent t(X;6) (q22;q13-14) translocation, suggesting a neoplastic origin.3
Osteochondromas are also common benign tumors formed by endochondral ossification, although secondary transformation into low-grade chondrosarcomas is well-documented.1 Osteochondromas commonly affect younger patients. They occur at epiphyseal areas of developing bone and have a hyaline matrix and chondrocyte pattern similar to that of a normal epiphyseal area, with confluence to the underlying trabecular and cortical bone. They are not caused by previous trauma and generally only become symptomatic after they have grown large enough to cause mechanical problems.1
Continue to: More diagnoses to consider
More diagnoses to consider
Other potential diagnoses for benign osteochondromatous lesions include bizarre parosteal osteochondromatous proliferations (BPOP) and digital mucous cysts.
BPOPs, also known as Nora’s lesions (crediting preliminary research performed by Nora and colleagues in 19834), are irregular formations of hypercellular cartilage, bone, and large chondrocytes. They predominantly occur in the small bones of the hands and feet, but may involve the skull and long bones.3 Unlike subungual exostoses and osteochondromas, BPOPs tend to occur in the third and fourth decades of life and generally do not alter, or have continuity with, the underlying bone.4
Histologically, BPOPs undergo irregular maturation, leaving a characteristic blue tint at the border of the newly formed trabecular bone. As with subungual exostoses, these lesions were traditionally believed to be reactive in nature. However, cytogenetic studies have identified variant translocations involving 1q32 (most commonly t[1;17] [q32;q21]) that are unique and common to these lesions.5
Digital mucous cysts are benign ganglion cysts that typically appear in the distal interphalangeal joints or at the proximal nail fold. They are believed to result from mucoid degeneration of connective tissue. Although generally associated with the hands, these cysts can also occur on the feet.6
Continue to: Our patient's outcome
Our patient’s outcome
After orthopedic consultation, the lesion and a 5 × 5-mm portion of the adherent germinal nail matrix were resected operatively through a medial excision. A small flap of the lateral nail matrix was rotated to cover the matrix defect, and the wound was closed. Postoperatively, the patient experienced slow wound healing (a total of 3 weeks), but there was no recurrence of the lesion at the 2-month follow-up.
THE TAKEAWAY
Osteocartilaginous tumors present as rapidly growing lesions on the distal tips of fingers and toes, but they may also occur on long bones and on the skull. Rarely malignant in nature, most of these lesions can be differentiated by location, histopathologic features, and patient age at onset. Consider surgical consultation and excision for relief of pain and/or cosmetic reasons. Recurrence is rare.
CORRESPONDENCE
Michael Barna, MD, Naval Hospital Camp Lejeune, Department of Family Medicine, 100 Brewster Blvd, Camp Lejeune, NC 28547; [email protected].
THE CASE
A 28-year-old woman with an unremarkable medical history presented with an enlarging nodule that had been growing under her left great toenail for 6 months. The patient monitored the nodule, hoping that it would resolve on its own, but found that it steadily increased in size and began to displace the nail, causing pain. At the time of presentation, the nodule measured approximately 10 mm in diameter, and there was significant (~80°) superior displacement of the nail (FIGURE 1).
An initial radiograph identified a 5.5-mm bony density arising from the dorsal surface of the left first distal phalanx with no significant degenerative changes (FIGURE 2). A subsequent magnetic resonance image confirmed the bony excrescence and noted marrow continuity. A thin amount of T2 bright signal was also observed, suggesting either a cartilaginous cap or soft tissue edema secondary to pressure on the nail bed (FIGURE 3).
THE DIAGNOSIS
Histologic examination demonstrated a thin (3 mm) cartilaginous cap overlying an area of mature fibrocartilage with no definite periosteum. The osseous component appeared to mature from the cartilage, and the marrow was focally fatty and fibrosed (FIGURES 4A and 4B). Expert consultation with the Joint Pathology Center confirmed a benign osteochondromatous lesion.
The histologic differential diagnosis of this patient’s lesion included subungual exostosis and osteochondroma. Based on the patient’s age, location of the lesion, and histologic findings, the final diagnosis was subungual exostosis.
DISCUSSION
Subungual exostoses are benign osteocartilaginous tumors that most commonly affect children and young adults. They predominantly manifest on the dorsomedial aspect of the tip of the great toe (~80%), but can occur on other digits of the foot or hand.1 They are caused by a proliferation of fibrous tissue under the nail bed. The fibrocartilage cap then undergoes endochondral ossification to woven bone and lamellar bone trabeculae. As these lesions mature, they establish continuity with the underlying bone in the phalanx.2 Subungual exostoses were once thought to represent a proliferative response to trauma, but further research has identified a recurrent t(X;6) (q22;q13-14) translocation, suggesting a neoplastic origin.3
Osteochondromas are also common benign tumors formed by endochondral ossification, although secondary transformation into low-grade chondrosarcomas is well-documented.1 Osteochondromas commonly affect younger patients. They occur at epiphyseal areas of developing bone and have a hyaline matrix and chondrocyte pattern similar to that of a normal epiphyseal area, with confluence to the underlying trabecular and cortical bone. They are not caused by previous trauma and generally only become symptomatic after they have grown large enough to cause mechanical problems.1
Continue to: More diagnoses to consider
More diagnoses to consider
Other potential diagnoses for benign osteochondromatous lesions include bizarre parosteal osteochondromatous proliferations (BPOP) and digital mucous cysts.
BPOPs, also known as Nora’s lesions (crediting preliminary research performed by Nora and colleagues in 19834), are irregular formations of hypercellular cartilage, bone, and large chondrocytes. They predominantly occur in the small bones of the hands and feet, but may involve the skull and long bones.3 Unlike subungual exostoses and osteochondromas, BPOPs tend to occur in the third and fourth decades of life and generally do not alter, or have continuity with, the underlying bone.4
Histologically, BPOPs undergo irregular maturation, leaving a characteristic blue tint at the border of the newly formed trabecular bone. As with subungual exostoses, these lesions were traditionally believed to be reactive in nature. However, cytogenetic studies have identified variant translocations involving 1q32 (most commonly t[1;17] [q32;q21]) that are unique and common to these lesions.5
Digital mucous cysts are benign ganglion cysts that typically appear in the distal interphalangeal joints or at the proximal nail fold. They are believed to result from mucoid degeneration of connective tissue. Although generally associated with the hands, these cysts can also occur on the feet.6
Continue to: Our patient's outcome
Our patient’s outcome
After orthopedic consultation, the lesion and a 5 × 5-mm portion of the adherent germinal nail matrix were resected operatively through a medial excision. A small flap of the lateral nail matrix was rotated to cover the matrix defect, and the wound was closed. Postoperatively, the patient experienced slow wound healing (a total of 3 weeks), but there was no recurrence of the lesion at the 2-month follow-up.
THE TAKEAWAY
Osteocartilaginous tumors present as rapidly growing lesions on the distal tips of fingers and toes, but they may also occur on long bones and on the skull. Rarely malignant in nature, most of these lesions can be differentiated by location, histopathologic features, and patient age at onset. Consider surgical consultation and excision for relief of pain and/or cosmetic reasons. Recurrence is rare.
CORRESPONDENCE
Michael Barna, MD, Naval Hospital Camp Lejeune, Department of Family Medicine, 100 Brewster Blvd, Camp Lejeune, NC 28547; [email protected].
1. Miller-Breslow A, Dorfman HD. Dupuytren’s (subungual) exostosis. Am J Surg Pathol. 1988;12:368-378.
2. DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259.
3. Meneses MF, Unni KK, Swee RG. Bizarre parosteal osteochondromatous proliferation of bone (Nora’s lesion). Am J Surg Pathol. 1993;17:691-697.
4. Nora FE, Dahlin DC, Beabout JW. Bizarre parosteal osteochondromatous proliferations of the hand and feet. Am J Surg Pathol. 1983;7:245-250.
5. Zambrano E, Nosé V, Perez-Atayde AR, et al. Distinct chromosomal rearrangements in subungual (Dupuytren) exostosis and bizarre parosteal osteochondromatous proliferation (Nora lesion). Am J Surg Pathol. 2004;28:1033-1039.
6. Salerni G, Alonso C. Images in clinical medicine. Digital mucous cyst. N Engl J Med. 2012;366:1335.
1. Miller-Breslow A, Dorfman HD. Dupuytren’s (subungual) exostosis. Am J Surg Pathol. 1988;12:368-378.
2. DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259.
3. Meneses MF, Unni KK, Swee RG. Bizarre parosteal osteochondromatous proliferation of bone (Nora’s lesion). Am J Surg Pathol. 1993;17:691-697.
4. Nora FE, Dahlin DC, Beabout JW. Bizarre parosteal osteochondromatous proliferations of the hand and feet. Am J Surg Pathol. 1983;7:245-250.
5. Zambrano E, Nosé V, Perez-Atayde AR, et al. Distinct chromosomal rearrangements in subungual (Dupuytren) exostosis and bizarre parosteal osteochondromatous proliferation (Nora lesion). Am J Surg Pathol. 2004;28:1033-1039.
6. Salerni G, Alonso C. Images in clinical medicine. Digital mucous cyst. N Engl J Med. 2012;366:1335.
Cognitive bias: Its influence on clinical diagnosis
CASE A patient with a history of drug-seeking behavior asks to be seen by you for lower back pain. Your impression upon entering the examination room is that the patient appears to be in minimal pain. A review of the patient’s chart leads you to suspect that the patient’s past behavior pattern is the reason for the visit. You find yourself downplaying his reports of weight loss, changed bowel habits, and lower extremity weakness—despite the fact that these complaints might have led you to consider more concerning causes of back pain in a different patient.
This situation is not uncommon. At one time or another, it’s likely that we have all placed an undue emphasis on a patient’s social background to reinforce a pre-existing opinion of the likely diagnosis. Doing so is an example of both anchoring and confirmation biases—just 2 of the many biases known to influence critical thinking in clinical practice (and which we’ll describe in a bit).
Reconsidering the diagnostic process. Previous attempts to address the issue of incorrect diagnosis and medical error have focused on systems-based approaches such as adopting electronic medical records to avert prescribing errors or eliminating confusing abbreviations in documentation.
Graber et al reviewed 100 errors involving internists and found that 46% of the errors resulted from a combination of systems-based and cognitive reasoning factors.2 More surprisingly, 28% of errors were attributable to reasoning failures alone.2 Singh et al showed that in one primary care network, most errors occurred during the patient-doctor encounter, with 56% involving errors in history taking and 47% involving oversights in the physical examination.3 Furthermore, most of the errors occurred in the context of common conditions such as pneumonia and congestive heart failure—rather than esoteric diseases—implying that the failures were due to errors in the diagnostic process rather than from a lack of knowledge.3
An understanding of the diagnostic process and the etiology of diagnostic error is of utmost importance in primary care. Family physicians who, on a daily basis, see a high volume of patients with predominantly low-acuity conditions, must be vigilant for the rare life-threatening condition that may mimic a more benign disease. It is in this setting that cognitive errors may abound, leading to both patient harm and emotional stress in physicians.3
This article reviews the current understanding of the cognitive pathways involved in diagnostic decision making, explains the factors that contribute to diagnostic errors, and summarizes the current research aimed at preventing these errors.
Continue to: The diagnostic process, as currently understood
The diagnostic process, as currently understood
Much of what is understood about the cognitive processes involved in diagnostic reasoning is built on research done in the field of behavioral science—specifically, the foundational work by psychologists Amos Tversky and Daniel Kahneman in the 1970s.4 Only relatively recently has the medical field begun to apply the findings of this research in its attempt to understand how clinicians diagnose.1 This work led to the description of 2 main cognitive pathways described by Croskerry and others.5
Type 1 processing, also referred to as the “intuitive” approach, uses a rapid, largely subconscious pattern-recognition method. Much in the same way one recognizes a familiar face, the clinician using a type 1 process rapidly comes to a conclusion by seeing a recognizable pattern among the patient’s signs and symptoms. For example, crushing chest pain radiating to the left arm instantly brings to mind a myocardial infarction without the clinician methodically formulating a differential diagnosis.4,5
Type 2 processing is an “analytic” approach in which the provider considers the salient characteristics of the case, generates a list of hypotheses, and proceeds to systematically test them and come to a more definitive conclusion.5 For example, an intern encountering a patient with a painfully swollen knee will consider the possibilities of septic arthritis, Lyme disease, and gout, and then carefully determine the likelihood of each disease based on the evidence available at the time.
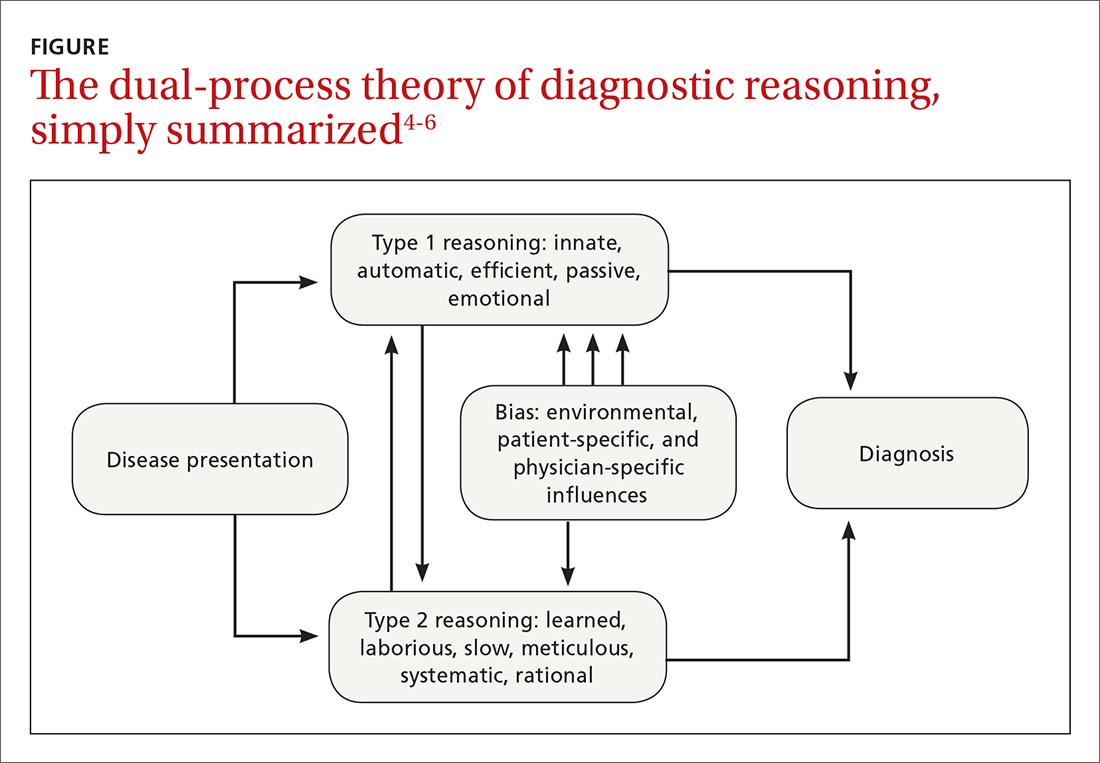
How the processes work in practice. While these 2 pathways are well studied within behavioral circles and are even supported by neurobiologic evidence, most clinical encounters incorporate both methodologies in a parallel system known as the “dual-process” theory (FIGURE).4-6
For example, during an initial visit for back pain, a patient may begin by relaying that the discomfort began after lifting a heavy object. Immediately the clinician, using a type 1 process, will suspect a simple lumbar strain. However, upon further questioning, the patient reveals that the pain occurs at rest and wakes him from sleep; these characteristics are atypical for a simple strain. At this point, the clinician may switch to a type 2 analytic approach and generate a broad differential that includes infection and malignancy.
Continue to: Heuristics: Indispensable, yet susceptible to bias
Heuristics: Indispensable, yet susceptible to bias
Heuristics are cognitive shortcuts often operating subconsciously to solve problems more quickly and efficiently than if the problem were analyzed and solved deductively.7 The act of driving a car, for instance, is a complex everyday task wherein the use of heuristics is not just efficient but essential. Deliberately analyzing and consciously considering every action required in daily living prior to execution would be impractical and even dangerous.
Heuristics also have a role in the practice of medicine. When presented with a large volume of low-acuity patients, the primary care provider would find it impractical to formulate an extensive differential and test each diagnosis before devising a plan of action. Using heuristics during clinical decision-making, however, does make the clinician more vulnerable to biases, which are described in the text that follows.
Biases
Bias is the psychological tendency to make a decision based on incomplete information and subjective factors rather than empirical evidence.4
Anchoring. One of the best-known biases, described in both behavioral science and medical literature, is anchoring. With this bias, the clinician fixates on a particular aspect of the patient’s initial presentation, excluding other more relevant clinical facts.8
A busy clinician, for example, may be notified by a medical assistant that the patient in Room One is complaining about fatigue and seems very depressed. The clinician then unduly anchors his thought process to this initial label of a depressed patient and, without much deliberation, prescribes an antidepressant medication. Had the physician inquired about skin and hair changes (unusual in depression), the more probable diagnosis of hypothyroidism would have come to mind.
Continue to: Premature closure...
Premature closure is another well-known bias associated with diagnostic errors.2,6 This is the tendency to cease inquiry once a possible solution for a problem is found. As the name implies, premature closure leads to an incomplete investigation of the problem and perhaps to incorrect conclusions.
If police arrested a potential suspect in a crime and halted the investigation, it’s possible the true culprit might not be found. In medicine, a classic example would be a junior clinician presented with a case of rectal bleeding in a 75-year-old man who has experienced weight loss and a change in bowel movements. The clinician observes a small nonfriable external hemorrhoid, incorrectly attributes the patient’s symptoms to that finding, and does not pursue the more appropriate investigation for gastrointestinal malignancy.
Interconnected biases. Often diagnostic errors are the result of multiple interconnected biases. For example, a busy emergency department physician is told that an unconscious patient smells of alcohol, so he is “probably drunk and just needs to sleep it off” (anchoring bias). The physician then examines the patient, who is barely arousable and indeed has a heavy odor of alcohol. The physician, therefore, decides not to order a basic laboratory work-up (premature closure). Because of this, the physician misses the correct and life-threatening diagnosis of a mental status change due to alcoholic ketoacidosis.6
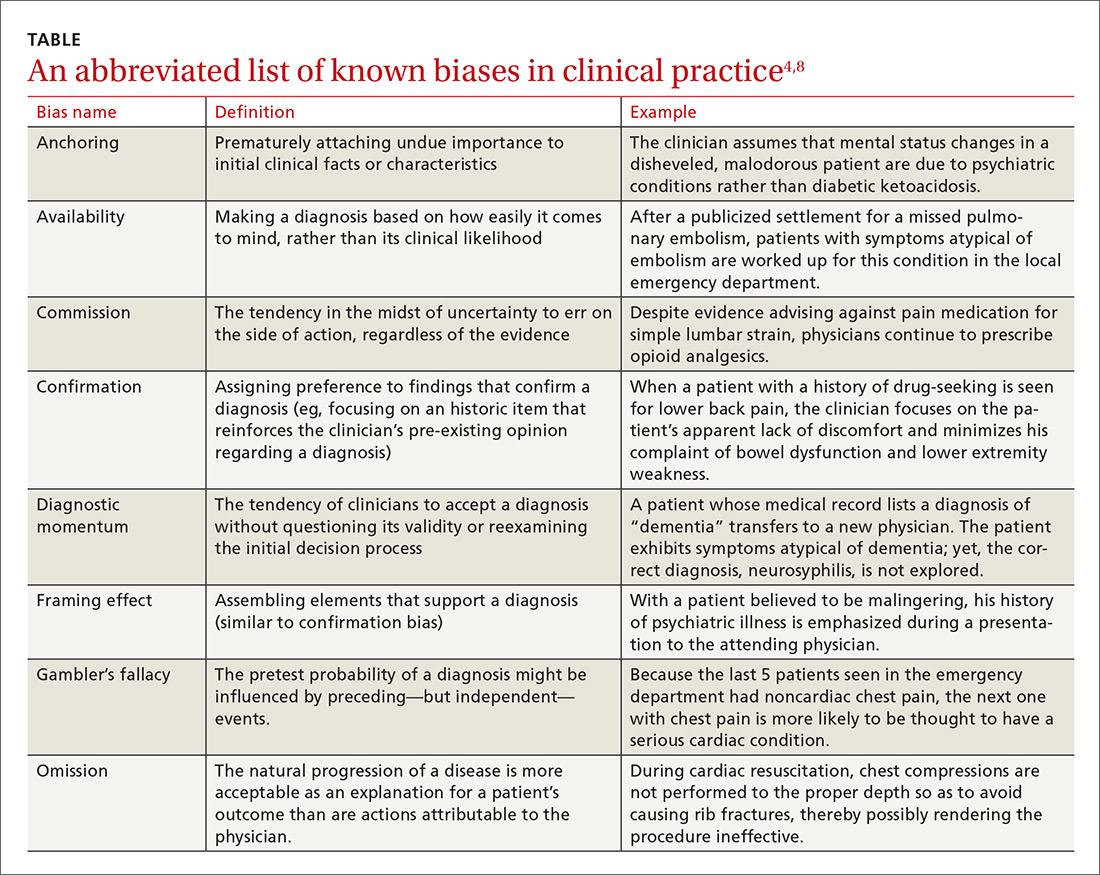
Numerous other biases have been identified and studied.4,8 While an in-depth examination of all biases is beyond the scope of this article, some of those most relevant to medical practice are listed and briefly defined in the TABLE.4,8
Multiple studies point to the central role biases play in diagnostic error. A systematic review by Saposnik et al found that physician cognitive biases were associated with diagnostic errors in 36.5% to 77% of case studies, and that 71% of the studies reviewed found an association between cognitive errors and therapeutic errors.6 In experimental studies, cognitive biases have also been shown to decrease accuracy in the interpretation of radiologic studies and electrocardiograms.9 In one case review, cognitive errors were identified in 74% of cases where an actual medical error had been committed.2
Continue to: The human component: When the patient is "difficult"
The human component: When the patient is “difficult”
Failures in reasoning are not solely responsible for diagnostic errors. One increasingly scrutinized cause of impaired clinical judgment is the physician-patient relationship, especially one involving a “difficult” patient. Additionally, the medical literature is beginning to highlight the strong correlation between clinician fatigue or burnout and diagnostic errors.10
Patient-specific factors clearly impact the likelihood of diagnostic error. One randomized controlled trial showed that patients with disruptive behaviors negatively influence the accuracy of clinicians’ diagnoses.11 In this study, family medicine residents made 42% more diagnostic errors when evaluating complex clinical presentations involving patients with negative interpersonal characteristics (demeaning, aggressive, or demanding communication styles). Even with simple clinical problems, difficult patient behaviors were associated with a 6% higher rate of error than when such behaviors were absent, although this finding did not reach statistical significance.11
Researchers have proposed the “resource depletion” theory as an explanation for this finding.11 A patient with difficult behaviors will require additional cognitive resources from the physician to manage those behaviors.11 This leaves less cognitive capacity for solving the diagnostic problem.11 Furthermore, Riskin et al demonstrated that pediatric intensive care teams committed increased rates of medical errors and experienced poorer team performance when exposed to simulated families displaying rude behavior.12 Clearly, the power of the patient-physician relationship cannot be overstated when discussing diagnostic error.
Continue to: Strategies for reducing errors in the diagnostic process
Strategies for reducing errors in the diagnostic process
Although the mental pathways involved in diagnostic reasoning have become better elucidated, there is still considerable controversy and uncertainty surrounding effective ways to counter errors. In their review of the literature, Norman et al concluded that diagnostic errors are multifactorial and that strategies that solely educate novice clinicians about biases are unlikely to lead to significant gains because of “limited transfer.”9 That is, in simply teaching the theory of cognitive errors before trainees have had time to accumulate real-world experience, they do not learn how to apply corrective solutions.
Graber et al argue that mental shortcuts are often a beneficial behavior, and it would be unrealistic and perhaps even detrimental to eliminate them completely from clinical judgment.13 Despite the controversy, several corrective methods have been proposed and have shown promise. Two such methods are medical education on cognitive error and the use of differential diagnosis generators.2
Medical education on cognitive error. If heuristics and biases are acquired subconscious patterns of thinking, then it would be logical to assume that the most effective way to prevent their intrusion into the clinical decision-making process would be to intervene when the art of diagnosis is taught. Graber et al reference several small studies that demonstrated a small improvement in diagnostic accuracy when learners were educated about cognitive biases and clinical judgment.13
Additionally, with medical students, Mamede et al describe how structured reflection during case-based learning enhanced diagnostic accuracy.14 However, none of these studies have proven that increased awareness of cognitive biases results in fewer delayed or missed diagnoses in clinical practice. Clearly, further research is needed to determine whether the skills gained in the classroom would be transferable to clinical practice and result in lower rates of delayed or missed diagnoses. Future studies could also investigate if these findings are replicable when applied to more experienced clinicians rather than medical students and residents.
Continue to: Differential diagnosis generators
Differential diagnosis generators.
However, few randomized controlled studies have investigated whether the use of a DDx generator reduces diagnostic error, and evidence is lacking to prove their usefulness in clinical practice. Furthermore, while an exhaustive list of possible diagnoses may be helpful, some proposed diagnoses may be irrelevant and may distract from timely attention being paid to more likely possibilities. Additionally, forming an extensive DDx list during every patient encounter would significantly add to the physician’s workload and could contribute to physician burnout.
Selective use? We believe that DDx generators would be best used selectively as a safeguard for the clinician who becomes aware of an increased risk of diagnostic error in a particular patient. As previously discussed, errors involving cognitive processes are more often errors of improper reasoning rather than of insufficient knowledge.3 The DDx generator then serves as a way of double-checking to ensure that additional diagnoses are being considered. This can be especially helpful when facing patients who display difficult behaviors or when the clinician’s cognitive reserve is depleted by other factors.
DDx generators may also help the physician expand his or her differential diagnosis when a patient is failing to improve despite appropriately treating the working diagnosis.
Another option worth studying? Future studies could also investigate whether discussing a case with another clinician is an effective way to reduce cognitive biases and diagnostic errors.
Continue to: Looking foward
Looking forward
More research will hopefully lead to corrective solutions. But it is also likely that solutions will require additional time and resources on the part of already overburdened providers. Thus, new challenges will arise in applying remedies to the current model of health care management and reimbursement.
Despite clinically useful advances in technology and science, family physicians are left with the unsettling conclusion that the most common source of error may also be the most difficult to change: physicians themselves. Fortunately, history has shown that the field of medicine can overcome even the most ingrained and harmful tendencies of the human mind, including prejudice and superstition.16,17 This next challenge will be no exception.
CORRESPONDENCE
Thomas Yuen, MD, Crozer Keystone Family Medicine Residency, 1260 East Woodland Avenue, Suite 200, Philadelphia, PA 19064; [email protected].
1. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
2. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165:1493-1499.
3. Singh H, Giardina TD, Meyer AN, et al. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med. 2013;173:418-425.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131.
5. Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009;84:1022-1028.
6. Saposnik G, Redelmeier D, Ruff CC, et al. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138.
7. Gigerenzer G, Gaissmaier W. Heuristic decision making. Annu Rev Psychol. 2011;62:451-482.
8. Wellbery C. Flaws in clinical reasoning: a common cause of diagnostic error. Am Fam Physician. 2011;84:1042-1048.
9. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30.
10. Lockley SW, Cronin JW, Evans EE, et al. Effect of reducing interns’ weekly work hours on sleep and attentional failures. NEJM. 2004;351:1829-1837.
11. Schmidt HG, Van Gog T, Schuit SC, et al. Do patients’ disruptive behaviours influence the accuracy of a doctor’s diagnosis? A randomised experiment. BMJ Qual Saf. 2017;26:19-23.
12. Riskin A, Erez A, Foulk TA, et al. Rudeness and medical team performance. Pediatrics. 2017;139:e20162305.
13. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77:981-992.
14. Mamede S, Van Gog T, Sampaio AM, et al. How can students’ diagnostic competence benefit most from practice with clinical cases? The effects of structured reflection on future diagnosis of the same and novel diseases. Acad Med. 2014;89:121-127.
15. Bond WF, Schwartz LM, Weaver KR, et al. Differential diagnosis generators: an evaluation of currently available computer programs. J Gen Intern Med. 2012;27:213-219.
16. Porter R. The Greatest Benefit to Mankind: A Medical History of Humanity. New York, NY: W.W. Norton and Company, Inc.;1999.
17. Lazarus BA. The practice of medicine and prejudice in a New England town: the founding of Mount Sinai Hospital, Hartford, Connecticut. J Am Ethn Hist. 1991;10:21-41.
CASE A patient with a history of drug-seeking behavior asks to be seen by you for lower back pain. Your impression upon entering the examination room is that the patient appears to be in minimal pain. A review of the patient’s chart leads you to suspect that the patient’s past behavior pattern is the reason for the visit. You find yourself downplaying his reports of weight loss, changed bowel habits, and lower extremity weakness—despite the fact that these complaints might have led you to consider more concerning causes of back pain in a different patient.
This situation is not uncommon. At one time or another, it’s likely that we have all placed an undue emphasis on a patient’s social background to reinforce a pre-existing opinion of the likely diagnosis. Doing so is an example of both anchoring and confirmation biases—just 2 of the many biases known to influence critical thinking in clinical practice (and which we’ll describe in a bit).
Reconsidering the diagnostic process. Previous attempts to address the issue of incorrect diagnosis and medical error have focused on systems-based approaches such as adopting electronic medical records to avert prescribing errors or eliminating confusing abbreviations in documentation.
Graber et al reviewed 100 errors involving internists and found that 46% of the errors resulted from a combination of systems-based and cognitive reasoning factors.2 More surprisingly, 28% of errors were attributable to reasoning failures alone.2 Singh et al showed that in one primary care network, most errors occurred during the patient-doctor encounter, with 56% involving errors in history taking and 47% involving oversights in the physical examination.3 Furthermore, most of the errors occurred in the context of common conditions such as pneumonia and congestive heart failure—rather than esoteric diseases—implying that the failures were due to errors in the diagnostic process rather than from a lack of knowledge.3
An understanding of the diagnostic process and the etiology of diagnostic error is of utmost importance in primary care. Family physicians who, on a daily basis, see a high volume of patients with predominantly low-acuity conditions, must be vigilant for the rare life-threatening condition that may mimic a more benign disease. It is in this setting that cognitive errors may abound, leading to both patient harm and emotional stress in physicians.3
This article reviews the current understanding of the cognitive pathways involved in diagnostic decision making, explains the factors that contribute to diagnostic errors, and summarizes the current research aimed at preventing these errors.
Continue to: The diagnostic process, as currently understood
The diagnostic process, as currently understood
Much of what is understood about the cognitive processes involved in diagnostic reasoning is built on research done in the field of behavioral science—specifically, the foundational work by psychologists Amos Tversky and Daniel Kahneman in the 1970s.4 Only relatively recently has the medical field begun to apply the findings of this research in its attempt to understand how clinicians diagnose.1 This work led to the description of 2 main cognitive pathways described by Croskerry and others.5
Type 1 processing, also referred to as the “intuitive” approach, uses a rapid, largely subconscious pattern-recognition method. Much in the same way one recognizes a familiar face, the clinician using a type 1 process rapidly comes to a conclusion by seeing a recognizable pattern among the patient’s signs and symptoms. For example, crushing chest pain radiating to the left arm instantly brings to mind a myocardial infarction without the clinician methodically formulating a differential diagnosis.4,5
Type 2 processing is an “analytic” approach in which the provider considers the salient characteristics of the case, generates a list of hypotheses, and proceeds to systematically test them and come to a more definitive conclusion.5 For example, an intern encountering a patient with a painfully swollen knee will consider the possibilities of septic arthritis, Lyme disease, and gout, and then carefully determine the likelihood of each disease based on the evidence available at the time.
How the processes work in practice. While these 2 pathways are well studied within behavioral circles and are even supported by neurobiologic evidence, most clinical encounters incorporate both methodologies in a parallel system known as the “dual-process” theory (FIGURE).4-6
For example, during an initial visit for back pain, a patient may begin by relaying that the discomfort began after lifting a heavy object. Immediately the clinician, using a type 1 process, will suspect a simple lumbar strain. However, upon further questioning, the patient reveals that the pain occurs at rest and wakes him from sleep; these characteristics are atypical for a simple strain. At this point, the clinician may switch to a type 2 analytic approach and generate a broad differential that includes infection and malignancy.
Continue to: Heuristics: Indispensable, yet susceptible to bias
Heuristics: Indispensable, yet susceptible to bias
Heuristics are cognitive shortcuts often operating subconsciously to solve problems more quickly and efficiently than if the problem were analyzed and solved deductively.7 The act of driving a car, for instance, is a complex everyday task wherein the use of heuristics is not just efficient but essential. Deliberately analyzing and consciously considering every action required in daily living prior to execution would be impractical and even dangerous.
Heuristics also have a role in the practice of medicine. When presented with a large volume of low-acuity patients, the primary care provider would find it impractical to formulate an extensive differential and test each diagnosis before devising a plan of action. Using heuristics during clinical decision-making, however, does make the clinician more vulnerable to biases, which are described in the text that follows.
Biases
Bias is the psychological tendency to make a decision based on incomplete information and subjective factors rather than empirical evidence.4
Anchoring. One of the best-known biases, described in both behavioral science and medical literature, is anchoring. With this bias, the clinician fixates on a particular aspect of the patient’s initial presentation, excluding other more relevant clinical facts.8
A busy clinician, for example, may be notified by a medical assistant that the patient in Room One is complaining about fatigue and seems very depressed. The clinician then unduly anchors his thought process to this initial label of a depressed patient and, without much deliberation, prescribes an antidepressant medication. Had the physician inquired about skin and hair changes (unusual in depression), the more probable diagnosis of hypothyroidism would have come to mind.
Continue to: Premature closure...
Premature closure is another well-known bias associated with diagnostic errors.2,6 This is the tendency to cease inquiry once a possible solution for a problem is found. As the name implies, premature closure leads to an incomplete investigation of the problem and perhaps to incorrect conclusions.
If police arrested a potential suspect in a crime and halted the investigation, it’s possible the true culprit might not be found. In medicine, a classic example would be a junior clinician presented with a case of rectal bleeding in a 75-year-old man who has experienced weight loss and a change in bowel movements. The clinician observes a small nonfriable external hemorrhoid, incorrectly attributes the patient’s symptoms to that finding, and does not pursue the more appropriate investigation for gastrointestinal malignancy.
Interconnected biases. Often diagnostic errors are the result of multiple interconnected biases. For example, a busy emergency department physician is told that an unconscious patient smells of alcohol, so he is “probably drunk and just needs to sleep it off” (anchoring bias). The physician then examines the patient, who is barely arousable and indeed has a heavy odor of alcohol. The physician, therefore, decides not to order a basic laboratory work-up (premature closure). Because of this, the physician misses the correct and life-threatening diagnosis of a mental status change due to alcoholic ketoacidosis.6
Numerous other biases have been identified and studied.4,8 While an in-depth examination of all biases is beyond the scope of this article, some of those most relevant to medical practice are listed and briefly defined in the TABLE.4,8
Multiple studies point to the central role biases play in diagnostic error. A systematic review by Saposnik et al found that physician cognitive biases were associated with diagnostic errors in 36.5% to 77% of case studies, and that 71% of the studies reviewed found an association between cognitive errors and therapeutic errors.6 In experimental studies, cognitive biases have also been shown to decrease accuracy in the interpretation of radiologic studies and electrocardiograms.9 In one case review, cognitive errors were identified in 74% of cases where an actual medical error had been committed.2
Continue to: The human component: When the patient is "difficult"
The human component: When the patient is “difficult”
Failures in reasoning are not solely responsible for diagnostic errors. One increasingly scrutinized cause of impaired clinical judgment is the physician-patient relationship, especially one involving a “difficult” patient. Additionally, the medical literature is beginning to highlight the strong correlation between clinician fatigue or burnout and diagnostic errors.10
Patient-specific factors clearly impact the likelihood of diagnostic error. One randomized controlled trial showed that patients with disruptive behaviors negatively influence the accuracy of clinicians’ diagnoses.11 In this study, family medicine residents made 42% more diagnostic errors when evaluating complex clinical presentations involving patients with negative interpersonal characteristics (demeaning, aggressive, or demanding communication styles). Even with simple clinical problems, difficult patient behaviors were associated with a 6% higher rate of error than when such behaviors were absent, although this finding did not reach statistical significance.11
Researchers have proposed the “resource depletion” theory as an explanation for this finding.11 A patient with difficult behaviors will require additional cognitive resources from the physician to manage those behaviors.11 This leaves less cognitive capacity for solving the diagnostic problem.11 Furthermore, Riskin et al demonstrated that pediatric intensive care teams committed increased rates of medical errors and experienced poorer team performance when exposed to simulated families displaying rude behavior.12 Clearly, the power of the patient-physician relationship cannot be overstated when discussing diagnostic error.
Continue to: Strategies for reducing errors in the diagnostic process
Strategies for reducing errors in the diagnostic process
Although the mental pathways involved in diagnostic reasoning have become better elucidated, there is still considerable controversy and uncertainty surrounding effective ways to counter errors. In their review of the literature, Norman et al concluded that diagnostic errors are multifactorial and that strategies that solely educate novice clinicians about biases are unlikely to lead to significant gains because of “limited transfer.”9 That is, in simply teaching the theory of cognitive errors before trainees have had time to accumulate real-world experience, they do not learn how to apply corrective solutions.
Graber et al argue that mental shortcuts are often a beneficial behavior, and it would be unrealistic and perhaps even detrimental to eliminate them completely from clinical judgment.13 Despite the controversy, several corrective methods have been proposed and have shown promise. Two such methods are medical education on cognitive error and the use of differential diagnosis generators.2
Medical education on cognitive error. If heuristics and biases are acquired subconscious patterns of thinking, then it would be logical to assume that the most effective way to prevent their intrusion into the clinical decision-making process would be to intervene when the art of diagnosis is taught. Graber et al reference several small studies that demonstrated a small improvement in diagnostic accuracy when learners were educated about cognitive biases and clinical judgment.13
Additionally, with medical students, Mamede et al describe how structured reflection during case-based learning enhanced diagnostic accuracy.14 However, none of these studies have proven that increased awareness of cognitive biases results in fewer delayed or missed diagnoses in clinical practice. Clearly, further research is needed to determine whether the skills gained in the classroom would be transferable to clinical practice and result in lower rates of delayed or missed diagnoses. Future studies could also investigate if these findings are replicable when applied to more experienced clinicians rather than medical students and residents.
Continue to: Differential diagnosis generators
Differential diagnosis generators.
However, few randomized controlled studies have investigated whether the use of a DDx generator reduces diagnostic error, and evidence is lacking to prove their usefulness in clinical practice. Furthermore, while an exhaustive list of possible diagnoses may be helpful, some proposed diagnoses may be irrelevant and may distract from timely attention being paid to more likely possibilities. Additionally, forming an extensive DDx list during every patient encounter would significantly add to the physician’s workload and could contribute to physician burnout.
Selective use? We believe that DDx generators would be best used selectively as a safeguard for the clinician who becomes aware of an increased risk of diagnostic error in a particular patient. As previously discussed, errors involving cognitive processes are more often errors of improper reasoning rather than of insufficient knowledge.3 The DDx generator then serves as a way of double-checking to ensure that additional diagnoses are being considered. This can be especially helpful when facing patients who display difficult behaviors or when the clinician’s cognitive reserve is depleted by other factors.
DDx generators may also help the physician expand his or her differential diagnosis when a patient is failing to improve despite appropriately treating the working diagnosis.
Another option worth studying? Future studies could also investigate whether discussing a case with another clinician is an effective way to reduce cognitive biases and diagnostic errors.
Continue to: Looking foward
Looking forward
More research will hopefully lead to corrective solutions. But it is also likely that solutions will require additional time and resources on the part of already overburdened providers. Thus, new challenges will arise in applying remedies to the current model of health care management and reimbursement.
Despite clinically useful advances in technology and science, family physicians are left with the unsettling conclusion that the most common source of error may also be the most difficult to change: physicians themselves. Fortunately, history has shown that the field of medicine can overcome even the most ingrained and harmful tendencies of the human mind, including prejudice and superstition.16,17 This next challenge will be no exception.
CORRESPONDENCE
Thomas Yuen, MD, Crozer Keystone Family Medicine Residency, 1260 East Woodland Avenue, Suite 200, Philadelphia, PA 19064; [email protected].
CASE A patient with a history of drug-seeking behavior asks to be seen by you for lower back pain. Your impression upon entering the examination room is that the patient appears to be in minimal pain. A review of the patient’s chart leads you to suspect that the patient’s past behavior pattern is the reason for the visit. You find yourself downplaying his reports of weight loss, changed bowel habits, and lower extremity weakness—despite the fact that these complaints might have led you to consider more concerning causes of back pain in a different patient.
This situation is not uncommon. At one time or another, it’s likely that we have all placed an undue emphasis on a patient’s social background to reinforce a pre-existing opinion of the likely diagnosis. Doing so is an example of both anchoring and confirmation biases—just 2 of the many biases known to influence critical thinking in clinical practice (and which we’ll describe in a bit).
Reconsidering the diagnostic process. Previous attempts to address the issue of incorrect diagnosis and medical error have focused on systems-based approaches such as adopting electronic medical records to avert prescribing errors or eliminating confusing abbreviations in documentation.
Graber et al reviewed 100 errors involving internists and found that 46% of the errors resulted from a combination of systems-based and cognitive reasoning factors.2 More surprisingly, 28% of errors were attributable to reasoning failures alone.2 Singh et al showed that in one primary care network, most errors occurred during the patient-doctor encounter, with 56% involving errors in history taking and 47% involving oversights in the physical examination.3 Furthermore, most of the errors occurred in the context of common conditions such as pneumonia and congestive heart failure—rather than esoteric diseases—implying that the failures were due to errors in the diagnostic process rather than from a lack of knowledge.3
An understanding of the diagnostic process and the etiology of diagnostic error is of utmost importance in primary care. Family physicians who, on a daily basis, see a high volume of patients with predominantly low-acuity conditions, must be vigilant for the rare life-threatening condition that may mimic a more benign disease. It is in this setting that cognitive errors may abound, leading to both patient harm and emotional stress in physicians.3
This article reviews the current understanding of the cognitive pathways involved in diagnostic decision making, explains the factors that contribute to diagnostic errors, and summarizes the current research aimed at preventing these errors.
Continue to: The diagnostic process, as currently understood
The diagnostic process, as currently understood
Much of what is understood about the cognitive processes involved in diagnostic reasoning is built on research done in the field of behavioral science—specifically, the foundational work by psychologists Amos Tversky and Daniel Kahneman in the 1970s.4 Only relatively recently has the medical field begun to apply the findings of this research in its attempt to understand how clinicians diagnose.1 This work led to the description of 2 main cognitive pathways described by Croskerry and others.5
Type 1 processing, also referred to as the “intuitive” approach, uses a rapid, largely subconscious pattern-recognition method. Much in the same way one recognizes a familiar face, the clinician using a type 1 process rapidly comes to a conclusion by seeing a recognizable pattern among the patient’s signs and symptoms. For example, crushing chest pain radiating to the left arm instantly brings to mind a myocardial infarction without the clinician methodically formulating a differential diagnosis.4,5
Type 2 processing is an “analytic” approach in which the provider considers the salient characteristics of the case, generates a list of hypotheses, and proceeds to systematically test them and come to a more definitive conclusion.5 For example, an intern encountering a patient with a painfully swollen knee will consider the possibilities of septic arthritis, Lyme disease, and gout, and then carefully determine the likelihood of each disease based on the evidence available at the time.
How the processes work in practice. While these 2 pathways are well studied within behavioral circles and are even supported by neurobiologic evidence, most clinical encounters incorporate both methodologies in a parallel system known as the “dual-process” theory (FIGURE).4-6
For example, during an initial visit for back pain, a patient may begin by relaying that the discomfort began after lifting a heavy object. Immediately the clinician, using a type 1 process, will suspect a simple lumbar strain. However, upon further questioning, the patient reveals that the pain occurs at rest and wakes him from sleep; these characteristics are atypical for a simple strain. At this point, the clinician may switch to a type 2 analytic approach and generate a broad differential that includes infection and malignancy.
Continue to: Heuristics: Indispensable, yet susceptible to bias
Heuristics: Indispensable, yet susceptible to bias
Heuristics are cognitive shortcuts often operating subconsciously to solve problems more quickly and efficiently than if the problem were analyzed and solved deductively.7 The act of driving a car, for instance, is a complex everyday task wherein the use of heuristics is not just efficient but essential. Deliberately analyzing and consciously considering every action required in daily living prior to execution would be impractical and even dangerous.
Heuristics also have a role in the practice of medicine. When presented with a large volume of low-acuity patients, the primary care provider would find it impractical to formulate an extensive differential and test each diagnosis before devising a plan of action. Using heuristics during clinical decision-making, however, does make the clinician more vulnerable to biases, which are described in the text that follows.
Biases
Bias is the psychological tendency to make a decision based on incomplete information and subjective factors rather than empirical evidence.4
Anchoring. One of the best-known biases, described in both behavioral science and medical literature, is anchoring. With this bias, the clinician fixates on a particular aspect of the patient’s initial presentation, excluding other more relevant clinical facts.8
A busy clinician, for example, may be notified by a medical assistant that the patient in Room One is complaining about fatigue and seems very depressed. The clinician then unduly anchors his thought process to this initial label of a depressed patient and, without much deliberation, prescribes an antidepressant medication. Had the physician inquired about skin and hair changes (unusual in depression), the more probable diagnosis of hypothyroidism would have come to mind.
Continue to: Premature closure...
Premature closure is another well-known bias associated with diagnostic errors.2,6 This is the tendency to cease inquiry once a possible solution for a problem is found. As the name implies, premature closure leads to an incomplete investigation of the problem and perhaps to incorrect conclusions.
If police arrested a potential suspect in a crime and halted the investigation, it’s possible the true culprit might not be found. In medicine, a classic example would be a junior clinician presented with a case of rectal bleeding in a 75-year-old man who has experienced weight loss and a change in bowel movements. The clinician observes a small nonfriable external hemorrhoid, incorrectly attributes the patient’s symptoms to that finding, and does not pursue the more appropriate investigation for gastrointestinal malignancy.
Interconnected biases. Often diagnostic errors are the result of multiple interconnected biases. For example, a busy emergency department physician is told that an unconscious patient smells of alcohol, so he is “probably drunk and just needs to sleep it off” (anchoring bias). The physician then examines the patient, who is barely arousable and indeed has a heavy odor of alcohol. The physician, therefore, decides not to order a basic laboratory work-up (premature closure). Because of this, the physician misses the correct and life-threatening diagnosis of a mental status change due to alcoholic ketoacidosis.6
Numerous other biases have been identified and studied.4,8 While an in-depth examination of all biases is beyond the scope of this article, some of those most relevant to medical practice are listed and briefly defined in the TABLE.4,8
Multiple studies point to the central role biases play in diagnostic error. A systematic review by Saposnik et al found that physician cognitive biases were associated with diagnostic errors in 36.5% to 77% of case studies, and that 71% of the studies reviewed found an association between cognitive errors and therapeutic errors.6 In experimental studies, cognitive biases have also been shown to decrease accuracy in the interpretation of radiologic studies and electrocardiograms.9 In one case review, cognitive errors were identified in 74% of cases where an actual medical error had been committed.2
Continue to: The human component: When the patient is "difficult"
The human component: When the patient is “difficult”
Failures in reasoning are not solely responsible for diagnostic errors. One increasingly scrutinized cause of impaired clinical judgment is the physician-patient relationship, especially one involving a “difficult” patient. Additionally, the medical literature is beginning to highlight the strong correlation between clinician fatigue or burnout and diagnostic errors.10
Patient-specific factors clearly impact the likelihood of diagnostic error. One randomized controlled trial showed that patients with disruptive behaviors negatively influence the accuracy of clinicians’ diagnoses.11 In this study, family medicine residents made 42% more diagnostic errors when evaluating complex clinical presentations involving patients with negative interpersonal characteristics (demeaning, aggressive, or demanding communication styles). Even with simple clinical problems, difficult patient behaviors were associated with a 6% higher rate of error than when such behaviors were absent, although this finding did not reach statistical significance.11
Researchers have proposed the “resource depletion” theory as an explanation for this finding.11 A patient with difficult behaviors will require additional cognitive resources from the physician to manage those behaviors.11 This leaves less cognitive capacity for solving the diagnostic problem.11 Furthermore, Riskin et al demonstrated that pediatric intensive care teams committed increased rates of medical errors and experienced poorer team performance when exposed to simulated families displaying rude behavior.12 Clearly, the power of the patient-physician relationship cannot be overstated when discussing diagnostic error.
Continue to: Strategies for reducing errors in the diagnostic process
Strategies for reducing errors in the diagnostic process
Although the mental pathways involved in diagnostic reasoning have become better elucidated, there is still considerable controversy and uncertainty surrounding effective ways to counter errors. In their review of the literature, Norman et al concluded that diagnostic errors are multifactorial and that strategies that solely educate novice clinicians about biases are unlikely to lead to significant gains because of “limited transfer.”9 That is, in simply teaching the theory of cognitive errors before trainees have had time to accumulate real-world experience, they do not learn how to apply corrective solutions.
Graber et al argue that mental shortcuts are often a beneficial behavior, and it would be unrealistic and perhaps even detrimental to eliminate them completely from clinical judgment.13 Despite the controversy, several corrective methods have been proposed and have shown promise. Two such methods are medical education on cognitive error and the use of differential diagnosis generators.2
Medical education on cognitive error. If heuristics and biases are acquired subconscious patterns of thinking, then it would be logical to assume that the most effective way to prevent their intrusion into the clinical decision-making process would be to intervene when the art of diagnosis is taught. Graber et al reference several small studies that demonstrated a small improvement in diagnostic accuracy when learners were educated about cognitive biases and clinical judgment.13
Additionally, with medical students, Mamede et al describe how structured reflection during case-based learning enhanced diagnostic accuracy.14 However, none of these studies have proven that increased awareness of cognitive biases results in fewer delayed or missed diagnoses in clinical practice. Clearly, further research is needed to determine whether the skills gained in the classroom would be transferable to clinical practice and result in lower rates of delayed or missed diagnoses. Future studies could also investigate if these findings are replicable when applied to more experienced clinicians rather than medical students and residents.
Continue to: Differential diagnosis generators
Differential diagnosis generators.
However, few randomized controlled studies have investigated whether the use of a DDx generator reduces diagnostic error, and evidence is lacking to prove their usefulness in clinical practice. Furthermore, while an exhaustive list of possible diagnoses may be helpful, some proposed diagnoses may be irrelevant and may distract from timely attention being paid to more likely possibilities. Additionally, forming an extensive DDx list during every patient encounter would significantly add to the physician’s workload and could contribute to physician burnout.
Selective use? We believe that DDx generators would be best used selectively as a safeguard for the clinician who becomes aware of an increased risk of diagnostic error in a particular patient. As previously discussed, errors involving cognitive processes are more often errors of improper reasoning rather than of insufficient knowledge.3 The DDx generator then serves as a way of double-checking to ensure that additional diagnoses are being considered. This can be especially helpful when facing patients who display difficult behaviors or when the clinician’s cognitive reserve is depleted by other factors.
DDx generators may also help the physician expand his or her differential diagnosis when a patient is failing to improve despite appropriately treating the working diagnosis.
Another option worth studying? Future studies could also investigate whether discussing a case with another clinician is an effective way to reduce cognitive biases and diagnostic errors.
Continue to: Looking foward
Looking forward
More research will hopefully lead to corrective solutions. But it is also likely that solutions will require additional time and resources on the part of already overburdened providers. Thus, new challenges will arise in applying remedies to the current model of health care management and reimbursement.
Despite clinically useful advances in technology and science, family physicians are left with the unsettling conclusion that the most common source of error may also be the most difficult to change: physicians themselves. Fortunately, history has shown that the field of medicine can overcome even the most ingrained and harmful tendencies of the human mind, including prejudice and superstition.16,17 This next challenge will be no exception.
CORRESPONDENCE
Thomas Yuen, MD, Crozer Keystone Family Medicine Residency, 1260 East Woodland Avenue, Suite 200, Philadelphia, PA 19064; [email protected].
1. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
2. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165:1493-1499.
3. Singh H, Giardina TD, Meyer AN, et al. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med. 2013;173:418-425.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131.
5. Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009;84:1022-1028.
6. Saposnik G, Redelmeier D, Ruff CC, et al. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138.
7. Gigerenzer G, Gaissmaier W. Heuristic decision making. Annu Rev Psychol. 2011;62:451-482.
8. Wellbery C. Flaws in clinical reasoning: a common cause of diagnostic error. Am Fam Physician. 2011;84:1042-1048.
9. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30.
10. Lockley SW, Cronin JW, Evans EE, et al. Effect of reducing interns’ weekly work hours on sleep and attentional failures. NEJM. 2004;351:1829-1837.
11. Schmidt HG, Van Gog T, Schuit SC, et al. Do patients’ disruptive behaviours influence the accuracy of a doctor’s diagnosis? A randomised experiment. BMJ Qual Saf. 2017;26:19-23.
12. Riskin A, Erez A, Foulk TA, et al. Rudeness and medical team performance. Pediatrics. 2017;139:e20162305.
13. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77:981-992.
14. Mamede S, Van Gog T, Sampaio AM, et al. How can students’ diagnostic competence benefit most from practice with clinical cases? The effects of structured reflection on future diagnosis of the same and novel diseases. Acad Med. 2014;89:121-127.
15. Bond WF, Schwartz LM, Weaver KR, et al. Differential diagnosis generators: an evaluation of currently available computer programs. J Gen Intern Med. 2012;27:213-219.
16. Porter R. The Greatest Benefit to Mankind: A Medical History of Humanity. New York, NY: W.W. Norton and Company, Inc.;1999.
17. Lazarus BA. The practice of medicine and prejudice in a New England town: the founding of Mount Sinai Hospital, Hartford, Connecticut. J Am Ethn Hist. 1991;10:21-41.
1. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
2. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165:1493-1499.
3. Singh H, Giardina TD, Meyer AN, et al. Types and origins of diagnostic errors in primary care settings. JAMA Intern Med. 2013;173:418-425.
4. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124-1131.
5. Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009;84:1022-1028.
6. Saposnik G, Redelmeier D, Ruff CC, et al. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16:138.
7. Gigerenzer G, Gaissmaier W. Heuristic decision making. Annu Rev Psychol. 2011;62:451-482.
8. Wellbery C. Flaws in clinical reasoning: a common cause of diagnostic error. Am Fam Physician. 2011;84:1042-1048.
9. Norman GR, Monteiro SD, Sherbino J, et al. The causes of errors in clinical reasoning: cognitive biases, knowledge deficits, and dual process thinking. Acad Med. 2017;92:23-30.
10. Lockley SW, Cronin JW, Evans EE, et al. Effect of reducing interns’ weekly work hours on sleep and attentional failures. NEJM. 2004;351:1829-1837.
11. Schmidt HG, Van Gog T, Schuit SC, et al. Do patients’ disruptive behaviours influence the accuracy of a doctor’s diagnosis? A randomised experiment. BMJ Qual Saf. 2017;26:19-23.
12. Riskin A, Erez A, Foulk TA, et al. Rudeness and medical team performance. Pediatrics. 2017;139:e20162305.
13. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77:981-992.
14. Mamede S, Van Gog T, Sampaio AM, et al. How can students’ diagnostic competence benefit most from practice with clinical cases? The effects of structured reflection on future diagnosis of the same and novel diseases. Acad Med. 2014;89:121-127.
15. Bond WF, Schwartz LM, Weaver KR, et al. Differential diagnosis generators: an evaluation of currently available computer programs. J Gen Intern Med. 2012;27:213-219.
16. Porter R. The Greatest Benefit to Mankind: A Medical History of Humanity. New York, NY: W.W. Norton and Company, Inc.;1999.
17. Lazarus BA. The practice of medicine and prejudice in a New England town: the founding of Mount Sinai Hospital, Hartford, Connecticut. J Am Ethn Hist. 1991;10:21-41.
PRACTICE RECOMMENDATIONS
› Acquire a basic understanding of key cognitive biases to better appreciate how they could interfere with your diagnostic reasoning. C
› Consider using a differential diagnosis generator as a safeguard if you suspect an increased risk of diagnostic error in a particular patient. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Painless penile ulcer and tender inguinal lymphadenopathy
A 38-year-old man presented to our emergency department with a 2-day history of fever, general malaise, and a painless genital ulcer. He denied having any abdominal pain, myalgias, arthralgias, or other rashes. He had been treated about a month earlier on an outpatient basis with penicillin for a presumed diagnosis of syphilis, but his symptoms did not resolve. His medical history included well-controlled human immunodeficiency virus (HIV), hepatitis B, hypertension, anxiety, and fibromyalgia for which he took lisinopril, emtricitabine/tenofovir, metoprolol, and darunavir/cobicistat. He smoked a half-pack of cigarettes a day and had unprotected sex with men.
On physical examination, the patient was febrile (103.1° F) with otherwise normal vital signs. A genital examination revealed a nontender, irregularly shaped 8-mm ulcer at the base of the glans penis (FIGURE). Tender unilateral inguinal lymphadenopathy was noted on the right side.
A chart review showed a normal CD4 count (obtained 2 months earlier). We were unable to access the results of his outpatient rapid plasma reagin test for syphilis. Due to the patient’s degree of pain from his lymphadenopathy, fever, and general malaise, he was admitted to the hospital for overnight observation.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lymphogranuloma venereum
Based on the patient’s history and clinical presentation, we suspected lymphogranuloma venereum (LGV). A positive nucleic acid amplification test (NAAT) for Chlamydia trachomatis confirmed the diagnosis.
LGV is an infection caused by C trachomatis—specifically serovars L1, L2, and L3—that is transmitted through unprotected sex.1-3 During intercourse, the serovars cross the epithelial cells through breaks in the skin and enter the lymphatic system, often resulting in painful lymphadenopathy. LGV is more commonly reported in men (primarily men who have sex with men [MSM]), but can occur in either gender.4 The true incidence and prevalence of LGV are difficult to ascertain as the disease primarily occurs in tropical areas, but outbreaks in the United States occur predominantly in patients infected with HIV.
The 3 stages of infection
Following an incubation period of 3 to 30 days, LGV progresses through 3 stages. The first stage involves a small, painless lesion at the inoculation site—usually the prepuce or glans of the penis or the vulva or vaginal wall. The lesion typically heals in about one week.1,2,4
Two to 6 weeks later, LGV enters the second phase, characterized by painful unilateral inguinal or femoral lymphadenopathy or proctitis. Inguinal and femoral lymphadenopathy is more common in men.1,4 The “groove sign”—lymphadenopathy occurring above and below the inguinal ligament—is seen in 10% to 20% of men with LGV.1,3 In women, lymphatic drainage occurs in the retroperitoneal or intra-abdominal nodes and may result in abdominal or back pain.1,4 Proctitis is reported primarily in women and in MSM.1,4
Tertiary LGV (aka genitoanorectal syndrome) is also more common in women and MSM, due to the location of the involved lymphatics. In this stage, chronic inflammation causes scarring and destruction of tissue.1,4 Left untreated, LGV can lead to lymphatic obstruction, deep tissue abscesses, chronic pain, strictures, or fistulas.1,3,4
Continue to: Other genital ulcers can mimic LGV
Other genital ulcers can mimic LGV
LGV can be confused with other causes of genital ulcers (such as syphilis, chancroid, herpes simplex virus, and Behçet’s disease). Identification of the causal bacteria is often needed to make a definitive diagnosis.
Syphilis, caused by the spirochete Treponema pallidum, initially manifests as a chancre (a single, well-demarcated, painless ulcer). It is less commonly associated with inguinal lymphadenopathy and can be diagnosed via serologic testing.5,6
Chancroid is caused by Haemophilus ducreyi and manifests as a painful ulcer with a friable base covered with a necrotic exudate. It can be associated with tender unilateral inguinal lymphadenopathy.5 Due to the widespread availability of culture media to test for H ducreyi, the diagnosis of chancroid is based on the clinical exam plus a handful of clinical criteria: painful genital ulcers, no evidence of T
HSV, the most common cause of genital ulcers in the United States,5 typically manifests with multiple vesicular painful lesions, with or without lymphadenopathy. Constitutional symptoms, including fever, headache, malaise, and myalgias, occur in 66% of females and 40% of males.5,7 Identification of HSV on culture or PCR can confirm the diagnosis.5
Behçet’s disease is a noninfectious syndrome associated with intermittent arthritis, recurrent painful oral and genital ulcers, uveitis, and skin lesions. While most symptoms of Behçet’s are self-limited, recurrent uveitis can result in blindness. A biopsy may be warranted to diagnose Behçet’s disease; the results may show diffuse arteritis with venulitis.5,8
Continue to: NAAT is recommended to confirm the diagnosis
NAAT is recommended to confirm the diagnosis
In the outpatient setting, diagnosis of LGV relies on physical exam, clinical presentation, confirmation of infection, and exclusion of other causes of genital ulcer, lymphadenopathy, and proctitis.3 Diagnostic tests include culture identification of C trachomatis, visualization of inclusion bodies on immunofluorescence of bubo aspirate, and positive serology for C trachomatis.1-3 (Serology to differentiate LGV from non-LGV C trachomatis serovars is difficult and not widely available.)
Recommendations regarding lab studies have shifted away from serologic testing and toward the use of NAAT. NAAT via PCR has a sensitivity and specificity comparable to invasive testing methods: 83% and 99.5% with urine samples and 86% and 99.6% with cervical samples, respectively.9 NAAT has a sensitivity and specificity that is superior to culture for detecting chlamydia in rectal specimens10 and is preferred by patients because it doesn’t require a pelvic exam or a urethral swab.
Treat with antibiotics
Oral antibiotics are the treatment of choice for LGV. Standard treatment includes doxycycline 100 mg bid for 21 days. Women who are pregnant or lactating may alternatively be treated with macrolides (eg, erythromycin).1,2,4 Buboes may be aspirated for pain relief and to prevent the development of ulcerations or fistulas.3,4
Our patient was started on oral doxycycline, which resolved his fever and reduced the size of his ulcer. He was discharged on oral doxycycline and continued on the full 21-day course. Two weeks later, the ulcer and lymphadenopathy had completely resolved. On follow-up in our office, the resident physician who treated the patient in the hospital discussed future use of safe sexual practices.
CORRESPONDENCE
Jeffrey Walden, MD, Cone Health Family Medicine Residency, 1125 North Church Street, Greensboro, NC 27401; [email protected].
1. Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect.
2. Roett MA, Mayor MT, Uduhiri KA. Diagnosis and management of genital ulcers. Am Fam Physician. 2012;85:254-262.
3. Stoner BP, Cohen SE. Lymphogranuloma venereum 2015: clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2015;61:S865-S873.
4. Ceovic R, Gulin SJ. Lymphogranuloma venereum: diagnostic and treatment challenges. Infect Drug Resist. 2015;8:39-47.
5. Roett MA, Mayor MT, Uduhiri KA. Diagnosis and management of genital ulcers. Am Fam Physician. 2012;85:254-262.
6. Mattei PL, Beachkofsky TM, Gilson RT, et al. Syphilis: a reemerging infection. Am Fam Physician. 2012;86:433-440.
7. Kimberlin DW, Rouse DJ. Genital herpes. N Engl J Med. 2004;350:1970-1977.
8. Sakane T, Takeno M, Suzuki N, et al. Behçet’s disease. N Engl J Med. 1999;341:1284-1291.
9. Cook RL, Hutchison SL, Østergaard L, et al. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhea. Ann Intern Med. 2005;142:914-925.
10. Geisler WM. Diagnosis and management of uncomplicated Chlamydia trachomatis infections in adolescents and adults: summary of evidence reviewed for the 2010 Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis. 2011;53 suppl 3:s92-s98.
A 38-year-old man presented to our emergency department with a 2-day history of fever, general malaise, and a painless genital ulcer. He denied having any abdominal pain, myalgias, arthralgias, or other rashes. He had been treated about a month earlier on an outpatient basis with penicillin for a presumed diagnosis of syphilis, but his symptoms did not resolve. His medical history included well-controlled human immunodeficiency virus (HIV), hepatitis B, hypertension, anxiety, and fibromyalgia for which he took lisinopril, emtricitabine/tenofovir, metoprolol, and darunavir/cobicistat. He smoked a half-pack of cigarettes a day and had unprotected sex with men.
On physical examination, the patient was febrile (103.1° F) with otherwise normal vital signs. A genital examination revealed a nontender, irregularly shaped 8-mm ulcer at the base of the glans penis (FIGURE). Tender unilateral inguinal lymphadenopathy was noted on the right side.
A chart review showed a normal CD4 count (obtained 2 months earlier). We were unable to access the results of his outpatient rapid plasma reagin test for syphilis. Due to the patient’s degree of pain from his lymphadenopathy, fever, and general malaise, he was admitted to the hospital for overnight observation.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lymphogranuloma venereum
Based on the patient’s history and clinical presentation, we suspected lymphogranuloma venereum (LGV). A positive nucleic acid amplification test (NAAT) for Chlamydia trachomatis confirmed the diagnosis.
LGV is an infection caused by C trachomatis—specifically serovars L1, L2, and L3—that is transmitted through unprotected sex.1-3 During intercourse, the serovars cross the epithelial cells through breaks in the skin and enter the lymphatic system, often resulting in painful lymphadenopathy. LGV is more commonly reported in men (primarily men who have sex with men [MSM]), but can occur in either gender.4 The true incidence and prevalence of LGV are difficult to ascertain as the disease primarily occurs in tropical areas, but outbreaks in the United States occur predominantly in patients infected with HIV.
The 3 stages of infection
Following an incubation period of 3 to 30 days, LGV progresses through 3 stages. The first stage involves a small, painless lesion at the inoculation site—usually the prepuce or glans of the penis or the vulva or vaginal wall. The lesion typically heals in about one week.1,2,4
Two to 6 weeks later, LGV enters the second phase, characterized by painful unilateral inguinal or femoral lymphadenopathy or proctitis. Inguinal and femoral lymphadenopathy is more common in men.1,4 The “groove sign”—lymphadenopathy occurring above and below the inguinal ligament—is seen in 10% to 20% of men with LGV.1,3 In women, lymphatic drainage occurs in the retroperitoneal or intra-abdominal nodes and may result in abdominal or back pain.1,4 Proctitis is reported primarily in women and in MSM.1,4
Tertiary LGV (aka genitoanorectal syndrome) is also more common in women and MSM, due to the location of the involved lymphatics. In this stage, chronic inflammation causes scarring and destruction of tissue.1,4 Left untreated, LGV can lead to lymphatic obstruction, deep tissue abscesses, chronic pain, strictures, or fistulas.1,3,4
Continue to: Other genital ulcers can mimic LGV
Other genital ulcers can mimic LGV
LGV can be confused with other causes of genital ulcers (such as syphilis, chancroid, herpes simplex virus, and Behçet’s disease). Identification of the causal bacteria is often needed to make a definitive diagnosis.
Syphilis, caused by the spirochete Treponema pallidum, initially manifests as a chancre (a single, well-demarcated, painless ulcer). It is less commonly associated with inguinal lymphadenopathy and can be diagnosed via serologic testing.5,6
Chancroid is caused by Haemophilus ducreyi and manifests as a painful ulcer with a friable base covered with a necrotic exudate. It can be associated with tender unilateral inguinal lymphadenopathy.5 Due to the widespread availability of culture media to test for H ducreyi, the diagnosis of chancroid is based on the clinical exam plus a handful of clinical criteria: painful genital ulcers, no evidence of T
HSV, the most common cause of genital ulcers in the United States,5 typically manifests with multiple vesicular painful lesions, with or without lymphadenopathy. Constitutional symptoms, including fever, headache, malaise, and myalgias, occur in 66% of females and 40% of males.5,7 Identification of HSV on culture or PCR can confirm the diagnosis.5
Behçet’s disease is a noninfectious syndrome associated with intermittent arthritis, recurrent painful oral and genital ulcers, uveitis, and skin lesions. While most symptoms of Behçet’s are self-limited, recurrent uveitis can result in blindness. A biopsy may be warranted to diagnose Behçet’s disease; the results may show diffuse arteritis with venulitis.5,8
Continue to: NAAT is recommended to confirm the diagnosis
NAAT is recommended to confirm the diagnosis
In the outpatient setting, diagnosis of LGV relies on physical exam, clinical presentation, confirmation of infection, and exclusion of other causes of genital ulcer, lymphadenopathy, and proctitis.3 Diagnostic tests include culture identification of C trachomatis, visualization of inclusion bodies on immunofluorescence of bubo aspirate, and positive serology for C trachomatis.1-3 (Serology to differentiate LGV from non-LGV C trachomatis serovars is difficult and not widely available.)
Recommendations regarding lab studies have shifted away from serologic testing and toward the use of NAAT. NAAT via PCR has a sensitivity and specificity comparable to invasive testing methods: 83% and 99.5% with urine samples and 86% and 99.6% with cervical samples, respectively.9 NAAT has a sensitivity and specificity that is superior to culture for detecting chlamydia in rectal specimens10 and is preferred by patients because it doesn’t require a pelvic exam or a urethral swab.
Treat with antibiotics
Oral antibiotics are the treatment of choice for LGV. Standard treatment includes doxycycline 100 mg bid for 21 days. Women who are pregnant or lactating may alternatively be treated with macrolides (eg, erythromycin).1,2,4 Buboes may be aspirated for pain relief and to prevent the development of ulcerations or fistulas.3,4
Our patient was started on oral doxycycline, which resolved his fever and reduced the size of his ulcer. He was discharged on oral doxycycline and continued on the full 21-day course. Two weeks later, the ulcer and lymphadenopathy had completely resolved. On follow-up in our office, the resident physician who treated the patient in the hospital discussed future use of safe sexual practices.
CORRESPONDENCE
Jeffrey Walden, MD, Cone Health Family Medicine Residency, 1125 North Church Street, Greensboro, NC 27401; [email protected].
A 38-year-old man presented to our emergency department with a 2-day history of fever, general malaise, and a painless genital ulcer. He denied having any abdominal pain, myalgias, arthralgias, or other rashes. He had been treated about a month earlier on an outpatient basis with penicillin for a presumed diagnosis of syphilis, but his symptoms did not resolve. His medical history included well-controlled human immunodeficiency virus (HIV), hepatitis B, hypertension, anxiety, and fibromyalgia for which he took lisinopril, emtricitabine/tenofovir, metoprolol, and darunavir/cobicistat. He smoked a half-pack of cigarettes a day and had unprotected sex with men.
On physical examination, the patient was febrile (103.1° F) with otherwise normal vital signs. A genital examination revealed a nontender, irregularly shaped 8-mm ulcer at the base of the glans penis (FIGURE). Tender unilateral inguinal lymphadenopathy was noted on the right side.
A chart review showed a normal CD4 count (obtained 2 months earlier). We were unable to access the results of his outpatient rapid plasma reagin test for syphilis. Due to the patient’s degree of pain from his lymphadenopathy, fever, and general malaise, he was admitted to the hospital for overnight observation.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Lymphogranuloma venereum
Based on the patient’s history and clinical presentation, we suspected lymphogranuloma venereum (LGV). A positive nucleic acid amplification test (NAAT) for Chlamydia trachomatis confirmed the diagnosis.
LGV is an infection caused by C trachomatis—specifically serovars L1, L2, and L3—that is transmitted through unprotected sex.1-3 During intercourse, the serovars cross the epithelial cells through breaks in the skin and enter the lymphatic system, often resulting in painful lymphadenopathy. LGV is more commonly reported in men (primarily men who have sex with men [MSM]), but can occur in either gender.4 The true incidence and prevalence of LGV are difficult to ascertain as the disease primarily occurs in tropical areas, but outbreaks in the United States occur predominantly in patients infected with HIV.
The 3 stages of infection
Following an incubation period of 3 to 30 days, LGV progresses through 3 stages. The first stage involves a small, painless lesion at the inoculation site—usually the prepuce or glans of the penis or the vulva or vaginal wall. The lesion typically heals in about one week.1,2,4
Two to 6 weeks later, LGV enters the second phase, characterized by painful unilateral inguinal or femoral lymphadenopathy or proctitis. Inguinal and femoral lymphadenopathy is more common in men.1,4 The “groove sign”—lymphadenopathy occurring above and below the inguinal ligament—is seen in 10% to 20% of men with LGV.1,3 In women, lymphatic drainage occurs in the retroperitoneal or intra-abdominal nodes and may result in abdominal or back pain.1,4 Proctitis is reported primarily in women and in MSM.1,4
Tertiary LGV (aka genitoanorectal syndrome) is also more common in women and MSM, due to the location of the involved lymphatics. In this stage, chronic inflammation causes scarring and destruction of tissue.1,4 Left untreated, LGV can lead to lymphatic obstruction, deep tissue abscesses, chronic pain, strictures, or fistulas.1,3,4
Continue to: Other genital ulcers can mimic LGV
Other genital ulcers can mimic LGV
LGV can be confused with other causes of genital ulcers (such as syphilis, chancroid, herpes simplex virus, and Behçet’s disease). Identification of the causal bacteria is often needed to make a definitive diagnosis.
Syphilis, caused by the spirochete Treponema pallidum, initially manifests as a chancre (a single, well-demarcated, painless ulcer). It is less commonly associated with inguinal lymphadenopathy and can be diagnosed via serologic testing.5,6
Chancroid is caused by Haemophilus ducreyi and manifests as a painful ulcer with a friable base covered with a necrotic exudate. It can be associated with tender unilateral inguinal lymphadenopathy.5 Due to the widespread availability of culture media to test for H ducreyi, the diagnosis of chancroid is based on the clinical exam plus a handful of clinical criteria: painful genital ulcers, no evidence of T
HSV, the most common cause of genital ulcers in the United States,5 typically manifests with multiple vesicular painful lesions, with or without lymphadenopathy. Constitutional symptoms, including fever, headache, malaise, and myalgias, occur in 66% of females and 40% of males.5,7 Identification of HSV on culture or PCR can confirm the diagnosis.5
Behçet’s disease is a noninfectious syndrome associated with intermittent arthritis, recurrent painful oral and genital ulcers, uveitis, and skin lesions. While most symptoms of Behçet’s are self-limited, recurrent uveitis can result in blindness. A biopsy may be warranted to diagnose Behçet’s disease; the results may show diffuse arteritis with venulitis.5,8
Continue to: NAAT is recommended to confirm the diagnosis
NAAT is recommended to confirm the diagnosis
In the outpatient setting, diagnosis of LGV relies on physical exam, clinical presentation, confirmation of infection, and exclusion of other causes of genital ulcer, lymphadenopathy, and proctitis.3 Diagnostic tests include culture identification of C trachomatis, visualization of inclusion bodies on immunofluorescence of bubo aspirate, and positive serology for C trachomatis.1-3 (Serology to differentiate LGV from non-LGV C trachomatis serovars is difficult and not widely available.)
Recommendations regarding lab studies have shifted away from serologic testing and toward the use of NAAT. NAAT via PCR has a sensitivity and specificity comparable to invasive testing methods: 83% and 99.5% with urine samples and 86% and 99.6% with cervical samples, respectively.9 NAAT has a sensitivity and specificity that is superior to culture for detecting chlamydia in rectal specimens10 and is preferred by patients because it doesn’t require a pelvic exam or a urethral swab.
Treat with antibiotics
Oral antibiotics are the treatment of choice for LGV. Standard treatment includes doxycycline 100 mg bid for 21 days. Women who are pregnant or lactating may alternatively be treated with macrolides (eg, erythromycin).1,2,4 Buboes may be aspirated for pain relief and to prevent the development of ulcerations or fistulas.3,4
Our patient was started on oral doxycycline, which resolved his fever and reduced the size of his ulcer. He was discharged on oral doxycycline and continued on the full 21-day course. Two weeks later, the ulcer and lymphadenopathy had completely resolved. On follow-up in our office, the resident physician who treated the patient in the hospital discussed future use of safe sexual practices.
CORRESPONDENCE
Jeffrey Walden, MD, Cone Health Family Medicine Residency, 1125 North Church Street, Greensboro, NC 27401; [email protected].
1. Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect.
2. Roett MA, Mayor MT, Uduhiri KA. Diagnosis and management of genital ulcers. Am Fam Physician. 2012;85:254-262.
3. Stoner BP, Cohen SE. Lymphogranuloma venereum 2015: clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2015;61:S865-S873.
4. Ceovic R, Gulin SJ. Lymphogranuloma venereum: diagnostic and treatment challenges. Infect Drug Resist. 2015;8:39-47.
5. Roett MA, Mayor MT, Uduhiri KA. Diagnosis and management of genital ulcers. Am Fam Physician. 2012;85:254-262.
6. Mattei PL, Beachkofsky TM, Gilson RT, et al. Syphilis: a reemerging infection. Am Fam Physician. 2012;86:433-440.
7. Kimberlin DW, Rouse DJ. Genital herpes. N Engl J Med. 2004;350:1970-1977.
8. Sakane T, Takeno M, Suzuki N, et al. Behçet’s disease. N Engl J Med. 1999;341:1284-1291.
9. Cook RL, Hutchison SL, Østergaard L, et al. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhea. Ann Intern Med. 2005;142:914-925.
10. Geisler WM. Diagnosis and management of uncomplicated Chlamydia trachomatis infections in adolescents and adults: summary of evidence reviewed for the 2010 Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis. 2011;53 suppl 3:s92-s98.
1. Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect.
2. Roett MA, Mayor MT, Uduhiri KA. Diagnosis and management of genital ulcers. Am Fam Physician. 2012;85:254-262.
3. Stoner BP, Cohen SE. Lymphogranuloma venereum 2015: clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2015;61:S865-S873.
4. Ceovic R, Gulin SJ. Lymphogranuloma venereum: diagnostic and treatment challenges. Infect Drug Resist. 2015;8:39-47.
5. Roett MA, Mayor MT, Uduhiri KA. Diagnosis and management of genital ulcers. Am Fam Physician. 2012;85:254-262.
6. Mattei PL, Beachkofsky TM, Gilson RT, et al. Syphilis: a reemerging infection. Am Fam Physician. 2012;86:433-440.
7. Kimberlin DW, Rouse DJ. Genital herpes. N Engl J Med. 2004;350:1970-1977.
8. Sakane T, Takeno M, Suzuki N, et al. Behçet’s disease. N Engl J Med. 1999;341:1284-1291.
9. Cook RL, Hutchison SL, Østergaard L, et al. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhea. Ann Intern Med. 2005;142:914-925.
10. Geisler WM. Diagnosis and management of uncomplicated Chlamydia trachomatis infections in adolescents and adults: summary of evidence reviewed for the 2010 Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis. 2011;53 suppl 3:s92-s98.
Does prophylactic azithromycin reduce the number of COPD exacerbations or hospitalizations?
EVIDENCE SUMMARY
A randomized, placebo-controlled trial including 1142 patients with COPD (forced expiratory volume in one second [FEV1] <70%, postbronchodilator FEV1 <80%) found that daily azithromycin 250 mg reduced acute exacerbations more than placebo over one year.1 Researchers recruited patients who were using supplemental oxygen, had required glucocorticoids, or had been hospitalized for an acute exacerbation in the last year. Patients with asthma, resting heart rate >100 beats/min, prolonged QTc interval (or on prolonging medications), or hearing impairment were excluded.
Azithromycin increased the median time to first exacerbation (defined as increase or new onset of cough, sputum, wheeze, and chest tightness for 3 days requiring antibiotics or systemic steroids) compared with the placebo group (266 days vs 174 days; P<.001) and reduced the risk of an acute exacerbation per patient year (hazard ratio [HR]=0.73; 95% confidence [CI], 0.63-0.84). It also reduced the rate of acute exacerbations per patient year (1.83 vs 1.43; P=.01; rate ratio=0.83; 95% CI, 0.72-0.95). The number needed to treat to prevent one exacerbation was 2.86.
No differences in death from any cause (3% vs 4%; P=.87), death from respiratory cause (2% vs 1%; P=.48), or death from cardiovascular cause (0.2% vs 0.2%; P=1.0) were found between azithromycin and placebo. Nor did rates of hospitalizations for acute exacerbations differ.
The groups also showed no significant difference in serious adverse events leading to discontinuation of medication. Notably, more patients in the azithromycin group had audiogram-confirmed hearing loss (25% vs 20%; P=.04), although the authors state that their criteria for hearing loss may have been too stringent because hearing improved on repeat testing whether or not the study drug was discontinued. In addition, more patients in the placebo group developed nasopharyngeal colonization with methicillin-resistant Staphylococcus aureus (31% vs 12%; P<.001).
Older ex-smokers on long-term O2 benefit most from the antibiotic
A retrospective subgroup analysis of the RCT identified patients who benefited most from daily azithromycin therapy.2 Compared with placebo, azithromycin decreased the time to first exacerbation in patients >65 years (542 patients; HR=0.59; 95% CI, 0.47-0.74), but not patients ≤65 years (571 patients; HR=0.84; 95% CI, 0.68-1.04).
The azithromycin group also demonstrated decreased time to first exacerbation in ex-smokers (867 patients; HR=0.65; 95% CI, 0.55-0.77) and patients on long-term oxygen (659 patients; HR=0.66; 95% CI, 0.55-0.80) but not current smokers (246 patients; HR=0.99; 95% CI, 0.71-1.38) or patients not using long-term oxygen (454 patients; HR=0.80; 95% CI, 0.62-1.03).
Azithromycin administration decreased exacerbations in patients with GOLD stages II (292 patients; HR=0.55; 95% CI, 0.40-0.75) and III (451 patients; HR=0.71; 95% CI, 0.56-0.90), but not stage IV (370 patients; HR=0.84; 95% CI, 0.65-1.08). The significance of the results is limited because the study was not originally powered for this level of subgroup analysis.
Continue to: Smaller study shows similar results
Smaller study shows similar results
A smaller RCT of 92 patients that evaluated exacerbation rates with azithromycin and placebo recruited patients with at least 3 acute COPD exacerbations in the previous year.3
Compared with placebo, oral azithromycin 500 mg 3 times a week (Monday, Wednesday, and Friday) increased the time between exacerbations over a 12-month period (59 days vs 130 days; P=.001). It also reduced the exacerbation rate per person per year (1.94 vs 3.22; risk ratio=0.60; 95% CI, 0.43-0.84) but didn’t change the hospitalization rate (odds ratio=1.34; 95% CI, 0.67-2.7).
No difference in serious adverse events was found between the azithromycin and placebo groups (3 patients vs 5 patients; P=NS), but an increase in diarrhea (9 patients vs 1 patient; P=.015) was noted.
RECOMMENDATIONS
An evidence-based guideline by the American College of Chest Physicians and Canadian Thoracic Society recommends long-term macrolide therapy to prevent acute exacerbations in patients >40 years with moderate or severe COPD and a history of ≥1 moderate or severe exacerbation in the previous year despite maximized inhaler therapy (Grade 2A, weak recommendation, high-quality evidence).4 The guideline also states that the duration and optimal dosages are unknown.
1. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
2. Han M, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Resp Crit Care. 2014;189:1503-1508.
3. Pomares X, Montón C, Espasa M, et al. Long-term azithromycin therapy in patients with severe COPD and repeated exacerbations. Int J Chron Obstruct Pulmon Dis. 2011;6:449-456.
4. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894-942.
EVIDENCE SUMMARY
A randomized, placebo-controlled trial including 1142 patients with COPD (forced expiratory volume in one second [FEV1] <70%, postbronchodilator FEV1 <80%) found that daily azithromycin 250 mg reduced acute exacerbations more than placebo over one year.1 Researchers recruited patients who were using supplemental oxygen, had required glucocorticoids, or had been hospitalized for an acute exacerbation in the last year. Patients with asthma, resting heart rate >100 beats/min, prolonged QTc interval (or on prolonging medications), or hearing impairment were excluded.
Azithromycin increased the median time to first exacerbation (defined as increase or new onset of cough, sputum, wheeze, and chest tightness for 3 days requiring antibiotics or systemic steroids) compared with the placebo group (266 days vs 174 days; P<.001) and reduced the risk of an acute exacerbation per patient year (hazard ratio [HR]=0.73; 95% confidence [CI], 0.63-0.84). It also reduced the rate of acute exacerbations per patient year (1.83 vs 1.43; P=.01; rate ratio=0.83; 95% CI, 0.72-0.95). The number needed to treat to prevent one exacerbation was 2.86.
No differences in death from any cause (3% vs 4%; P=.87), death from respiratory cause (2% vs 1%; P=.48), or death from cardiovascular cause (0.2% vs 0.2%; P=1.0) were found between azithromycin and placebo. Nor did rates of hospitalizations for acute exacerbations differ.
The groups also showed no significant difference in serious adverse events leading to discontinuation of medication. Notably, more patients in the azithromycin group had audiogram-confirmed hearing loss (25% vs 20%; P=.04), although the authors state that their criteria for hearing loss may have been too stringent because hearing improved on repeat testing whether or not the study drug was discontinued. In addition, more patients in the placebo group developed nasopharyngeal colonization with methicillin-resistant Staphylococcus aureus (31% vs 12%; P<.001).
Older ex-smokers on long-term O2 benefit most from the antibiotic
A retrospective subgroup analysis of the RCT identified patients who benefited most from daily azithromycin therapy.2 Compared with placebo, azithromycin decreased the time to first exacerbation in patients >65 years (542 patients; HR=0.59; 95% CI, 0.47-0.74), but not patients ≤65 years (571 patients; HR=0.84; 95% CI, 0.68-1.04).
The azithromycin group also demonstrated decreased time to first exacerbation in ex-smokers (867 patients; HR=0.65; 95% CI, 0.55-0.77) and patients on long-term oxygen (659 patients; HR=0.66; 95% CI, 0.55-0.80) but not current smokers (246 patients; HR=0.99; 95% CI, 0.71-1.38) or patients not using long-term oxygen (454 patients; HR=0.80; 95% CI, 0.62-1.03).
Azithromycin administration decreased exacerbations in patients with GOLD stages II (292 patients; HR=0.55; 95% CI, 0.40-0.75) and III (451 patients; HR=0.71; 95% CI, 0.56-0.90), but not stage IV (370 patients; HR=0.84; 95% CI, 0.65-1.08). The significance of the results is limited because the study was not originally powered for this level of subgroup analysis.
Continue to: Smaller study shows similar results
Smaller study shows similar results
A smaller RCT of 92 patients that evaluated exacerbation rates with azithromycin and placebo recruited patients with at least 3 acute COPD exacerbations in the previous year.3
Compared with placebo, oral azithromycin 500 mg 3 times a week (Monday, Wednesday, and Friday) increased the time between exacerbations over a 12-month period (59 days vs 130 days; P=.001). It also reduced the exacerbation rate per person per year (1.94 vs 3.22; risk ratio=0.60; 95% CI, 0.43-0.84) but didn’t change the hospitalization rate (odds ratio=1.34; 95% CI, 0.67-2.7).
No difference in serious adverse events was found between the azithromycin and placebo groups (3 patients vs 5 patients; P=NS), but an increase in diarrhea (9 patients vs 1 patient; P=.015) was noted.
RECOMMENDATIONS
An evidence-based guideline by the American College of Chest Physicians and Canadian Thoracic Society recommends long-term macrolide therapy to prevent acute exacerbations in patients >40 years with moderate or severe COPD and a history of ≥1 moderate or severe exacerbation in the previous year despite maximized inhaler therapy (Grade 2A, weak recommendation, high-quality evidence).4 The guideline also states that the duration and optimal dosages are unknown.
EVIDENCE SUMMARY
A randomized, placebo-controlled trial including 1142 patients with COPD (forced expiratory volume in one second [FEV1] <70%, postbronchodilator FEV1 <80%) found that daily azithromycin 250 mg reduced acute exacerbations more than placebo over one year.1 Researchers recruited patients who were using supplemental oxygen, had required glucocorticoids, or had been hospitalized for an acute exacerbation in the last year. Patients with asthma, resting heart rate >100 beats/min, prolonged QTc interval (or on prolonging medications), or hearing impairment were excluded.
Azithromycin increased the median time to first exacerbation (defined as increase or new onset of cough, sputum, wheeze, and chest tightness for 3 days requiring antibiotics or systemic steroids) compared with the placebo group (266 days vs 174 days; P<.001) and reduced the risk of an acute exacerbation per patient year (hazard ratio [HR]=0.73; 95% confidence [CI], 0.63-0.84). It also reduced the rate of acute exacerbations per patient year (1.83 vs 1.43; P=.01; rate ratio=0.83; 95% CI, 0.72-0.95). The number needed to treat to prevent one exacerbation was 2.86.
No differences in death from any cause (3% vs 4%; P=.87), death from respiratory cause (2% vs 1%; P=.48), or death from cardiovascular cause (0.2% vs 0.2%; P=1.0) were found between azithromycin and placebo. Nor did rates of hospitalizations for acute exacerbations differ.
The groups also showed no significant difference in serious adverse events leading to discontinuation of medication. Notably, more patients in the azithromycin group had audiogram-confirmed hearing loss (25% vs 20%; P=.04), although the authors state that their criteria for hearing loss may have been too stringent because hearing improved on repeat testing whether or not the study drug was discontinued. In addition, more patients in the placebo group developed nasopharyngeal colonization with methicillin-resistant Staphylococcus aureus (31% vs 12%; P<.001).
Older ex-smokers on long-term O2 benefit most from the antibiotic
A retrospective subgroup analysis of the RCT identified patients who benefited most from daily azithromycin therapy.2 Compared with placebo, azithromycin decreased the time to first exacerbation in patients >65 years (542 patients; HR=0.59; 95% CI, 0.47-0.74), but not patients ≤65 years (571 patients; HR=0.84; 95% CI, 0.68-1.04).
The azithromycin group also demonstrated decreased time to first exacerbation in ex-smokers (867 patients; HR=0.65; 95% CI, 0.55-0.77) and patients on long-term oxygen (659 patients; HR=0.66; 95% CI, 0.55-0.80) but not current smokers (246 patients; HR=0.99; 95% CI, 0.71-1.38) or patients not using long-term oxygen (454 patients; HR=0.80; 95% CI, 0.62-1.03).
Azithromycin administration decreased exacerbations in patients with GOLD stages II (292 patients; HR=0.55; 95% CI, 0.40-0.75) and III (451 patients; HR=0.71; 95% CI, 0.56-0.90), but not stage IV (370 patients; HR=0.84; 95% CI, 0.65-1.08). The significance of the results is limited because the study was not originally powered for this level of subgroup analysis.
Continue to: Smaller study shows similar results
Smaller study shows similar results
A smaller RCT of 92 patients that evaluated exacerbation rates with azithromycin and placebo recruited patients with at least 3 acute COPD exacerbations in the previous year.3
Compared with placebo, oral azithromycin 500 mg 3 times a week (Monday, Wednesday, and Friday) increased the time between exacerbations over a 12-month period (59 days vs 130 days; P=.001). It also reduced the exacerbation rate per person per year (1.94 vs 3.22; risk ratio=0.60; 95% CI, 0.43-0.84) but didn’t change the hospitalization rate (odds ratio=1.34; 95% CI, 0.67-2.7).
No difference in serious adverse events was found between the azithromycin and placebo groups (3 patients vs 5 patients; P=NS), but an increase in diarrhea (9 patients vs 1 patient; P=.015) was noted.
RECOMMENDATIONS
An evidence-based guideline by the American College of Chest Physicians and Canadian Thoracic Society recommends long-term macrolide therapy to prevent acute exacerbations in patients >40 years with moderate or severe COPD and a history of ≥1 moderate or severe exacerbation in the previous year despite maximized inhaler therapy (Grade 2A, weak recommendation, high-quality evidence).4 The guideline also states that the duration and optimal dosages are unknown.
1. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
2. Han M, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Resp Crit Care. 2014;189:1503-1508.
3. Pomares X, Montón C, Espasa M, et al. Long-term azithromycin therapy in patients with severe COPD and repeated exacerbations. Int J Chron Obstruct Pulmon Dis. 2011;6:449-456.
4. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894-942.
1. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
2. Han M, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Resp Crit Care. 2014;189:1503-1508.
3. Pomares X, Montón C, Espasa M, et al. Long-term azithromycin therapy in patients with severe COPD and repeated exacerbations. Int J Chron Obstruct Pulmon Dis. 2011;6:449-456.
4. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894-942.
EVIDENCE-BASED ANSWER:
Yes for exacerbations, no for hospitalizations. Prophylactic azithromycin reduces the number of exacerbations by about 25%. It also extends the time between exacerbations by approximately 90 days for patients with moderate-to-severe chronic obstructive pulmonary disease (COPD). Azithromycin benefits patients who are >65 years, patients with Global Initiative for Obstructive Lung Disease (GOLD) stage II or III COPD, former smokers, and patients using long-term oxygen; it doesn’t benefit patients ≤65 years, patients with GOLD stage IV COPD, current smokers, or patients not using oxygen (strength of recommendation [SOR]: B, randomized controlled trials [RCTs]).
Prophylactic azithromycin doesn’t reduce hospitalizations overall (SOR: B, single small RCT).
Asking patients about their sexuality
Current Psychiatry/AACP Psychiatry Update 2018
THURSDAY, MARCH 22, 2018
MORNING SESSION
Henry A. Nasrallah, MD, Saint Louis University, began the conference by presenting the latest information on several recent advances in schizophrenia, including genetic and nongenetic etiologies, abnormalities in the mitochondria and microglia activation leading to oxidative stress and inflammation, and emerging treatment and prevention strategies.
Next, Dr. Nasrallah described how to identify and treat the schizophrenia prodrome. He covered frequently used assessment tools, neuroimaging findings, and the consequences of delayed treatment.
Michael J. Gitlin, MD, University of California, Los Angeles, described current uses of stimulants for treating psychiatric disorders. He covered their use for attention-deficit/hyperactivity disorder (ADHD), unipolar depression, bipolar depression, and other conditions.
AFTERNOON SESSION
The afternoon began with Donald W. Black, MD, University of Iowa, providing an update on obsessive-compulsive disorder and related conditions. He reviewed the efficacy of several treatments, including pharmacologic and behavior therapies.
Next, Dr. Gitlin covered the potential relationships between depression and several comorbid disorders, including borderline personality disorder, schizophrenia, alcohol use disorders, and other mental illnesses.
Dr. Black presented an update on hoarding. He detailed the differential diagnosis, clinical characteristics, and clinical management strategies for addressing this challenging disorder.
Tracy D. Gunter, MD, Indiana University, identified common legal issues encountered by clinical psychiatrists and the challenges they pose. She described how to develop strategies for approaching common clinical problems while minimizing medicolegal risk.
Continue to: Friday sessions
FRIDAY, MARCH 23, 2018
MORNING SESSION
To start Day 2, Marlene P. Freeman, MD, Massachusetts General Hospital, focused on bipolar disorder in women. She described factors to consider when treating a pregnant woman with this illness.
Dr. Freeman continued with a unique session that covered topics attendees had requested in advance. These included the relationship between polycystic ovarian syndrome and depression, infertility and psychiatry, and nonpharmacologic treatments for depression in pregnant women.
Dr. Gunter presented a session on correctional psychiatry, including the major differences between office-based clinical psychiatry and on-site correctional psychiatry and some of the challenges of working in this setting.
AFTERNOON SESSION
Robert M. McCarron, DO, University of California, Irvine, described how to treat psychiatric patients who have chronic pain. He covered the responsible use of opioid analgesics and how to diagnose and treat somatic symptom disorders.
Anthony L. Rostain, MD, MS, University of Pennsylvania, discussed practical approaches to managing ADHD comorbidities. He outlined pharmacologic and psychosocial strategies for patients who have ADHD and comorbid anxiety, depression, substance use disorders, and certain medical conditions.
Dr. McCarron continued the afternoon with a session that focused on preventing endocrine and cardiovascular disorders. He provided prevention and diagnostic measures for metabolic syndrome and tips for preventing and diagnosing dyslipidemia and diabetes.
Dr. Rostain continued by presenting a session on autistic spectrum disorders in adults. He covered clinical features, common comorbidities, and medication management.
The day concluded with a special AACP Members’ Reception.
Continue to: Saturday session
SATURDAY, MARCH 24, 2018
MORNING SESSION
George T. Grossberg, MD, Saint Louis University, began the day by covering the neuroanatomic and neurochemical substrates of behavioral symptoms in Alzheimer’s disease and their implications for treatment. He described behavioral and pharmacologic treatments for agitation and behavioral symptoms in patients with dementia.
Next came a special keynote address by Mark A. Frye, MD, Mayo Clinic, Rochester, titled Ketamine Update: What Should Clinicians Know and Expect.
Stephen B. Levine
Dr. Grossberg presented on aging, traumatic brain injury, chronic traumatic encephalopathy, and Alzheimer’s disease. He covered the sequelae, prevention, and treatment of these conditions.
Dr. Levine concluded the conference by providing a clinical overview of transgender issues. He included a discussion of the role mental health professionals should play in gender affirmation.
Continue to: SPONSORS AND SUPPORTERS
SPONSORS AND SUPPORTERS
- Admera Health
- Alkermes
- Alpha Genomix Laboratories
- Alzheimer's Association
- Amita Health Alexian Brothers Behavioral Health Hospital
- Aurora Health Care
- Bellin Psychiatric Center
- GeneSight
- Genoa Telepsychiatry
- Howard Brown Health
- Jaymac Pharmaceuticals
- Legally Mine
- Professional Risk Management Services, Inc.
- Pine Rest Christian Mental Health Services
- Sunovion Pharmaceuticals, Inc.
- Supernus Pharmaceuticals, Inc.
- Takeda Pharmaceuticals U.S.A., Inc. / Lundbeck
- U.S. Army Medicine Civilian Corps
- Wexford Health Sources
- Wolters Kluwer
The meeting organizers acknowledge the support provided by our sponsors. Determination of educational content for this program and the selection of speakers are responsibilities of the program director and co-chairs. Sponsors and supporters did not have input in these areas.
THURSDAY, MARCH 22, 2018
MORNING SESSION
Henry A. Nasrallah, MD, Saint Louis University, began the conference by presenting the latest information on several recent advances in schizophrenia, including genetic and nongenetic etiologies, abnormalities in the mitochondria and microglia activation leading to oxidative stress and inflammation, and emerging treatment and prevention strategies.
Next, Dr. Nasrallah described how to identify and treat the schizophrenia prodrome. He covered frequently used assessment tools, neuroimaging findings, and the consequences of delayed treatment.
Michael J. Gitlin, MD, University of California, Los Angeles, described current uses of stimulants for treating psychiatric disorders. He covered their use for attention-deficit/hyperactivity disorder (ADHD), unipolar depression, bipolar depression, and other conditions.
AFTERNOON SESSION
The afternoon began with Donald W. Black, MD, University of Iowa, providing an update on obsessive-compulsive disorder and related conditions. He reviewed the efficacy of several treatments, including pharmacologic and behavior therapies.
Next, Dr. Gitlin covered the potential relationships between depression and several comorbid disorders, including borderline personality disorder, schizophrenia, alcohol use disorders, and other mental illnesses.
Dr. Black presented an update on hoarding. He detailed the differential diagnosis, clinical characteristics, and clinical management strategies for addressing this challenging disorder.
Tracy D. Gunter, MD, Indiana University, identified common legal issues encountered by clinical psychiatrists and the challenges they pose. She described how to develop strategies for approaching common clinical problems while minimizing medicolegal risk.
Continue to: Friday sessions
FRIDAY, MARCH 23, 2018
MORNING SESSION
To start Day 2, Marlene P. Freeman, MD, Massachusetts General Hospital, focused on bipolar disorder in women. She described factors to consider when treating a pregnant woman with this illness.
Dr. Freeman continued with a unique session that covered topics attendees had requested in advance. These included the relationship between polycystic ovarian syndrome and depression, infertility and psychiatry, and nonpharmacologic treatments for depression in pregnant women.
Dr. Gunter presented a session on correctional psychiatry, including the major differences between office-based clinical psychiatry and on-site correctional psychiatry and some of the challenges of working in this setting.
AFTERNOON SESSION
Robert M. McCarron, DO, University of California, Irvine, described how to treat psychiatric patients who have chronic pain. He covered the responsible use of opioid analgesics and how to diagnose and treat somatic symptom disorders.
Anthony L. Rostain, MD, MS, University of Pennsylvania, discussed practical approaches to managing ADHD comorbidities. He outlined pharmacologic and psychosocial strategies for patients who have ADHD and comorbid anxiety, depression, substance use disorders, and certain medical conditions.
Dr. McCarron continued the afternoon with a session that focused on preventing endocrine and cardiovascular disorders. He provided prevention and diagnostic measures for metabolic syndrome and tips for preventing and diagnosing dyslipidemia and diabetes.
Dr. Rostain continued by presenting a session on autistic spectrum disorders in adults. He covered clinical features, common comorbidities, and medication management.
The day concluded with a special AACP Members’ Reception.
Continue to: Saturday session
SATURDAY, MARCH 24, 2018
MORNING SESSION
George T. Grossberg, MD, Saint Louis University, began the day by covering the neuroanatomic and neurochemical substrates of behavioral symptoms in Alzheimer’s disease and their implications for treatment. He described behavioral and pharmacologic treatments for agitation and behavioral symptoms in patients with dementia.
Next came a special keynote address by Mark A. Frye, MD, Mayo Clinic, Rochester, titled Ketamine Update: What Should Clinicians Know and Expect.
Stephen B. Levine
Dr. Grossberg presented on aging, traumatic brain injury, chronic traumatic encephalopathy, and Alzheimer’s disease. He covered the sequelae, prevention, and treatment of these conditions.
Dr. Levine concluded the conference by providing a clinical overview of transgender issues. He included a discussion of the role mental health professionals should play in gender affirmation.
Continue to: SPONSORS AND SUPPORTERS
SPONSORS AND SUPPORTERS
- Admera Health
- Alkermes
- Alpha Genomix Laboratories
- Alzheimer's Association
- Amita Health Alexian Brothers Behavioral Health Hospital
- Aurora Health Care
- Bellin Psychiatric Center
- GeneSight
- Genoa Telepsychiatry
- Howard Brown Health
- Jaymac Pharmaceuticals
- Legally Mine
- Professional Risk Management Services, Inc.
- Pine Rest Christian Mental Health Services
- Sunovion Pharmaceuticals, Inc.
- Supernus Pharmaceuticals, Inc.
- Takeda Pharmaceuticals U.S.A., Inc. / Lundbeck
- U.S. Army Medicine Civilian Corps
- Wexford Health Sources
- Wolters Kluwer
The meeting organizers acknowledge the support provided by our sponsors. Determination of educational content for this program and the selection of speakers are responsibilities of the program director and co-chairs. Sponsors and supporters did not have input in these areas.
THURSDAY, MARCH 22, 2018
MORNING SESSION
Henry A. Nasrallah, MD, Saint Louis University, began the conference by presenting the latest information on several recent advances in schizophrenia, including genetic and nongenetic etiologies, abnormalities in the mitochondria and microglia activation leading to oxidative stress and inflammation, and emerging treatment and prevention strategies.
Next, Dr. Nasrallah described how to identify and treat the schizophrenia prodrome. He covered frequently used assessment tools, neuroimaging findings, and the consequences of delayed treatment.
Michael J. Gitlin, MD, University of California, Los Angeles, described current uses of stimulants for treating psychiatric disorders. He covered their use for attention-deficit/hyperactivity disorder (ADHD), unipolar depression, bipolar depression, and other conditions.
AFTERNOON SESSION
The afternoon began with Donald W. Black, MD, University of Iowa, providing an update on obsessive-compulsive disorder and related conditions. He reviewed the efficacy of several treatments, including pharmacologic and behavior therapies.
Next, Dr. Gitlin covered the potential relationships between depression and several comorbid disorders, including borderline personality disorder, schizophrenia, alcohol use disorders, and other mental illnesses.
Dr. Black presented an update on hoarding. He detailed the differential diagnosis, clinical characteristics, and clinical management strategies for addressing this challenging disorder.
Tracy D. Gunter, MD, Indiana University, identified common legal issues encountered by clinical psychiatrists and the challenges they pose. She described how to develop strategies for approaching common clinical problems while minimizing medicolegal risk.
Continue to: Friday sessions
FRIDAY, MARCH 23, 2018
MORNING SESSION
To start Day 2, Marlene P. Freeman, MD, Massachusetts General Hospital, focused on bipolar disorder in women. She described factors to consider when treating a pregnant woman with this illness.
Dr. Freeman continued with a unique session that covered topics attendees had requested in advance. These included the relationship between polycystic ovarian syndrome and depression, infertility and psychiatry, and nonpharmacologic treatments for depression in pregnant women.
Dr. Gunter presented a session on correctional psychiatry, including the major differences between office-based clinical psychiatry and on-site correctional psychiatry and some of the challenges of working in this setting.
AFTERNOON SESSION
Robert M. McCarron, DO, University of California, Irvine, described how to treat psychiatric patients who have chronic pain. He covered the responsible use of opioid analgesics and how to diagnose and treat somatic symptom disorders.
Anthony L. Rostain, MD, MS, University of Pennsylvania, discussed practical approaches to managing ADHD comorbidities. He outlined pharmacologic and psychosocial strategies for patients who have ADHD and comorbid anxiety, depression, substance use disorders, and certain medical conditions.
Dr. McCarron continued the afternoon with a session that focused on preventing endocrine and cardiovascular disorders. He provided prevention and diagnostic measures for metabolic syndrome and tips for preventing and diagnosing dyslipidemia and diabetes.
Dr. Rostain continued by presenting a session on autistic spectrum disorders in adults. He covered clinical features, common comorbidities, and medication management.
The day concluded with a special AACP Members’ Reception.
Continue to: Saturday session
SATURDAY, MARCH 24, 2018
MORNING SESSION
George T. Grossberg, MD, Saint Louis University, began the day by covering the neuroanatomic and neurochemical substrates of behavioral symptoms in Alzheimer’s disease and their implications for treatment. He described behavioral and pharmacologic treatments for agitation and behavioral symptoms in patients with dementia.
Next came a special keynote address by Mark A. Frye, MD, Mayo Clinic, Rochester, titled Ketamine Update: What Should Clinicians Know and Expect.
Stephen B. Levine
Dr. Grossberg presented on aging, traumatic brain injury, chronic traumatic encephalopathy, and Alzheimer’s disease. He covered the sequelae, prevention, and treatment of these conditions.
Dr. Levine concluded the conference by providing a clinical overview of transgender issues. He included a discussion of the role mental health professionals should play in gender affirmation.
Continue to: SPONSORS AND SUPPORTERS
SPONSORS AND SUPPORTERS
- Admera Health
- Alkermes
- Alpha Genomix Laboratories
- Alzheimer's Association
- Amita Health Alexian Brothers Behavioral Health Hospital
- Aurora Health Care
- Bellin Psychiatric Center
- GeneSight
- Genoa Telepsychiatry
- Howard Brown Health
- Jaymac Pharmaceuticals
- Legally Mine
- Professional Risk Management Services, Inc.
- Pine Rest Christian Mental Health Services
- Sunovion Pharmaceuticals, Inc.
- Supernus Pharmaceuticals, Inc.
- Takeda Pharmaceuticals U.S.A., Inc. / Lundbeck
- U.S. Army Medicine Civilian Corps
- Wexford Health Sources
- Wolters Kluwer
The meeting organizers acknowledge the support provided by our sponsors. Determination of educational content for this program and the selection of speakers are responsibilities of the program director and co-chairs. Sponsors and supporters did not have input in these areas.
Sudden-onset memory problems, visual hallucinations, and odd behaviors
CASE A rapid decline
Ms. D, age 62, presents to a psychiatric emergency room (ER) after experiencing visual hallucinations, exhibiting odd behaviors, and having memory problems. On interview, she is disoriented, distractible, tearful, and tangential. She plays with her shirt and glasses, and occasionally shouts. She perseverates on “the aerialists,” acrobatic children she has been seeing in her apartment. She becomes distressed and shouts, “I would love to just get them!”
Ms. D is unable to provide an account of her history. Collateral information is obtained from her daughter, who has brought Ms. D to the ER for evaluation. She reports that her mother has no relevant medical or psychiatric history, and does not take any medications, except a mixture of Chinese herbs that she brews into a tea.
Ms. D’s daughter says that her mother began to deteriorate 5 months ago, after she traveled to California to care for her sister, who was seriously ill and passed away. After Ms. D returned, she would cry frequently. She also appeared “spaced out,” complained of feeling dizzy, and frequently misplaced belongings. Three months before presenting to the ER, she began to experience weakness, fatigue, and difficulty walking. Her daughter became more worried 2 months ago, when Ms. D began sleeping with her purse and hiding her belongings around their house. When asked about these odd behaviors, Ms. D claimed that “the aerialists” were climbing through her windows at night and stealing her things.
A week before seeking treatment at the ER, Ms. D’s daughter had taken her to a neurologist at another facility for clinical evaluation. An MRI of the brain showed minimal dilation in the subarachnoid space and a focal 1 cm lipoma in the anterior falx cerebri, but was otherwise unremarkable. However, Ms. D’s symptoms continued to worsen, and began to interfere with her ability to care for herself.
The team in the psychiatric ER attributes Ms. D’s symptoms to a severe, psychotic depressive episode. They admit her to the psychiatric inpatient unit for further evaluation.
[polldaddy:10012742]
Continue to: The authors' observations
The authors’ observations
Ms. D was plagued by several mood and psychotic symptoms. Such symptoms can arise from many different psychiatric or organic etiologies. In Ms. D’s case, several aspects of her presentation suggest that her illness was psychiatric. The severe illness of a beloved family member is a significant stressor that could cause a great deal of grief and devastation, possibly leading to depression. Indeed, Ms. D’s daughter noticed that her mother was crying frequently, which is consistent with grief or depression.
Memory problems, which might manifest as misplacing belongings, can also indicate a depressive illness, especially in older patients. Moreover, impaired concentration, which can cause one to appear “spaced out” or distractible, is a core symptom of major depressive disorder. Sadness and grief also can be appropriate during bereavement and in response to significant losses. Therefore, in Ms. D’s case, it is possible her frequent crying, “spaced out” appearance, and other mood symptoms she experienced immediately after caring for her sister were an appropriate response to her sister’s illness and death.
However, other aspects of Ms. D’s presentation suggested an organic etiology. Her rapid deterioration and symptom onset relatively late in life were consistent with dementia and malignancy. Her complaint of feeling dizzy suggested a neurologic process was affecting her vestibular system. Finally, while psychiatric disorders can certainly cause visual hallucinations, they occur in only a small percentage of cases.1 Visual hallucinations are commonly associated with delirium, intoxication, and neurologic illness.
Continue to: EVALUATION Severe impairment
EVALUATION Severe impairment
On the psychiatric inpatient unit, Ms. D remains unable to give a coherent account of her illness or recent events. During interviews, she abruptly shifts from laughing to crying for no apparent reason. While answering questions, her responses trail off and she appears to forget what she had been saying. However, she continues to speak at length about “the aerialists,” stating that she sees them living in her wardrobe and jumping from rooftop to rooftop in her neighborhood.
A mental status examination finds evidence of severe cognitive impairment. Ms. D is unable to identify the correct date, time, or place, and appears surprised when told she is in a hospital. She can repeat the names of 3 objects but cannot recall them a few minutes later. Finally, she scores a 14 on the Mini-Mental State Examination (MMSE) and a 5 on the Montreal Cognitive Assessment (MoCA), indicating severe impairment.
On the unit, Ms. D cannot remember the location of her room or bathroom, and even when given directions, she needs to be escorted to her destination. Her gait is unsteady and wide-spaced, and she walks on her toes at times. When food is placed before her, she needs to be shown how to take the lids off containers, pick up utensils, and start eating.
All laboratory results are unremarkable, including a complete blood count, basic metabolic panel, liver function tests, gamma-glutamyl transpeptidase, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, methylmalonic acid, homocysteine, folate, erythrocyte sedimentation rate, C-reactive protein, antinuclear antibodies, rapid plasma reagin, human immunodeficiency virus, and Lyme titers. The team also considers Ms. D’s history of herbal medicine use, because herbal mixtures can contain heavy metals and other contaminants. However, all toxicology results are normal, including arsenic, mercury, lead, copper, and zinc.
To address her symptoms, Ms. D is given risperidone, 0.5 mg twice a day, and donepezil, 5 mg/d.
[polldaddy:10012743]
Continue to: The authors' observations
The authors’ observations
Despite her persistent psychiatric symptoms, Ms. D had several neurologic symptoms that warranted further investigation. Her abrupt shifts from laughter to tears for no apparent reason were consistent with pseudobulbar affect. Her inability to remember how to use utensils during meals was consistent with apraxia. Finally, her abnormal gait raised concern for a process affecting her motor system.
OUTCOME A rare disorder
Given the psychiatry team’s suspicions for a neurologic etiology of Ms. D’s symptoms, an MRI of her brain is repeated. The results are notable for abnormal restricted diffusion in the caudate and putamen bilaterally, which is consistent with Creutzfeldt-Jakob disease (CJD). EEG shows moderate diffuse cerebral dysfunction, frontal intermittent delta activity, and diffuse cortical hyperexcitability, consistent with early- to mid-onset prion disease. Upon evaluation by the neurology team, Ms. D appears fearful, suspicious, and disorganized, but her examination does not reveal additional significant sensorimotor findings.
Ms. D is transferred to the neurology service for further workup and management. A lumbar puncture is positive for real-time quaking-induced conversion (RT-QuIC) and 14-3-3 protein with elevated tau proteins; these findings also are consistent with CJD. She develops transaminitis, with an alanine transaminase (ALT) of 127 and aspartate transaminase (AST) of 355, and a malignancy is suspected. However, CT scans of the chest, abdomen, and pelvis show no evidence of malignancy, and an extensive gastrointestinal workup is unremarkable, including anti-smooth muscle antibodies, anti-liver-kidney microsomal antibody, antimitochondrial antibodies, gliadin antibody, alpha-1 antitrypsin, liver/kidney microsomal antibody, and hepatitis serologies. While on the neurology service, risperidone and donepezil are discontinued because
After discontinuing these medications, she is evaluated by the psychiatry consult team for mood lability. The psychiatry consult team recommends quetiapine, which is later started at 25 mg nightly at bedtime.
Clinically, Ms. D’s mental status continues to deteriorate. She becomes nonverbal and minimally able to follow commands. She is ultimately discharged to an inpatient hospice for end-of-life care and the team recommends that she continue with quetiapine once there.
Continue to: The authors' observations
The authors’ observations
CJD is a rare, rapidly progressive, fatal form of dementia. In the United States, the incidence is approximately 1 to 1.5 cases per 1 million people each year.2 There are various forms of the disease. Sporadic CJD is the most common, representing 85% of cases.3 Sporadic CJD typically occurs in patients in their 60s and quickly leads to death—50% of patients die within 5 months, and 90% of patients die within 1 year.2,3 The illness is hypothesized to arise from the production of misfolded prion proteins, ultimately leading to vacuolation, neuronal loss, and the spongiform appearance characteristic of CJD.3,4
Psychiatric symptoms have long been acknowledged as a feature of CJD. Recent data indicates that psychiatric symptoms occur in 90% to 92% of cases.5,6 Sleep disturbances and depressive symptoms, including vegetative symptoms, anhedonia, and tearfulness, appear to be most common.5 Psychotic symptoms occur in approximately 42% of cases and may include persecutory and paranoid delusions, as well as an array of vivid auditory, visual, and tactile hallucinations.5,7
There is also evidence that psychiatric symptoms may be an early marker of CJD.5,8 A Mayo Clinic study found that psychiatric symptoms occurred within the prodromal phase of CJD in 26% of cases, and psychiatric symptoms occurred within the first 100 days of illness in 86% of cases.5
Case reports have described patients with CJD who initially presented with depression, psychosis, and other psychiatric symptoms.9-11 Interestingly, there have been cases with only psychiatric symptoms, and no neurologic symptoms until relatively late in the illness.10,11 Several patients with CJD have been evaluated in psychiatric ERs, admitted to psychiatric hospitals, and treated with psychiatric medications and ECT.5,9 In one study, 44% of CJD cases were misdiagnosed as “psychiatric patients” due to the prominence of their psychiatric symptomatology.8
Continue to: Making the diagnosis in psychiatric settings
Making the diagnosis in psychiatric settings. Often, the most difficult aspect of CJD is making the diagnosis.3,12 Sporadic CJD in particular can vary widely in its clinical presentation.3 The core clinical feature of CJD is rapidly progressive dementia, so suspect CJD in these patients. However, CJD can be difficult to distinguish from other rapidly progressive dementias, such as autoimmune and paraneoplastic encephalopathies.2,3 The presence of neurologic features, specifically myoclonus, akinetic mutism, and visual, cerebellar, and extrapyramidal symptoms, should also be considered a red flag for the disorder3 (Table).
Finally, positive findings on MRI, EEG, or CSF assay can indicate a probable diagnosis of CJD.13 MRI, particularly diffusion weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR), is recognized as the most studied, sensitive, and overall useful neuroimaging modality for detecting CJD.2,3,12 Although the appearance of CJD on MRI can vary widely, asymmetric hyperintensities in ≥3 cortical gyri, particularly in the frontal and parietal lobes, provide strong evidence of CJD and are observed in 80% to 81% of cases.4,12 Asymmetric hyperintensities in the basal ganglia, particularly the caudate and rostral putamen, are observed in 69% to 70% of cases.4,12,13
EEG and CSF assay also can be useful for making the diagnosis. While diffuse slowing and frontal rhythmic delta activity appear early in the course of CJD, periodic sharp wave complexes emerge later in the illness.4 However, EEG findings are not diagnostic, because periodic sharp wave complexes are seen in only two-thirds of CJD cases and also occur in other neurologic illnesses.3,4 In recent years, lumbar puncture with subsequent CSF testing has become increasingly useful in detecting the illness. The presence of the 14-3-3 protein and tau protein is highly sensitive, although not specific, for CJD.3 A definite diagnosis of CJD requires discovery of the misfolded prion proteins, such as by RT-QuIC or brain biopsy.2,3,13
Management of CJD in psychiatric patients. CJD is an invariably fatal disease for which there is no effective cure or disease modifying treatment.2 Therefore, supportive therapies are the mainstay of care. Psychotropic medications can be used to provide symptom relief. While the sleep disturbances, anxiety, and agitation/hallucinations associated with CJD appear to respond well to hypnotic, anxiolytic, and antipsychotic medications, respectively, antidepressants and mood-stabilizing medications appear to have little benefit for patients with CJD.5 During the final stages of the disease, patients may suffer from akinetic mutism and inability to swallow, which often leads to aspiration pneumonia.14 Patients should also be offered end-of-life counseling, planning, and care, and provided with other comfort measures wherever possible (Figure).
Continue to: Bottom Line
Bottom Line
Patients with Creutzfeldt-Jakob disease (CJD) may present to psychiatric settings, particularly to a psychiatric emergency room. Consider CJD as a possible etiology in patients with rapidly progressive dementia, depression, and psychosis. CJD is invariably fatal and there is no effective disease-modifying treatment. Supportive therapies are the mainstay of care.
Related Resources
- National Institute of Neurological Disorders and Stroke. Creutzfeldt-Jakob disease fact sheet. http://www.ninds.nih.gov/disorders/cjd/detail_cjd.htm.
- Centers for Disease Control and Prevention. Creutzfeldt-Jakob disease, classic (CJD). http://www.cdc.gov/prions/cjd.
Drug Brand Names
Donepezil • Aricept
Risperidone • Risperdal
Quetiapine • Seroquel
1. Resnick PJ. The detection of malingered psychosis. Psychiatr Clin North Am. 1999;22(1):159-172.
2. Bucelli RC, Ances BM. Diagnosis and evaluation of a patient with rapidly progressive dementia. Mo Med. 2013;110(5):422-428.
3. Manix M, Kalakoti P, Henry M, et al. Creutzfeldt-Jakob disease: updated diagnostic criteria, treatment algorithm, and the utility of brain biopsy. Neurosurg Focus. 2015;39(5):E2.
4. Puoti G, Bizzi A, Forloni G, et al. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. 2012;11(7):618-628.
5. Wall CA, Rummans TA, Aksamit AJ, et al. Psychiatric manifestations of Creutzfeldt-Jakob disease: a 25-year analysis. J Neuropsychiatry Clin Neurosci. 2005;17(4):489-495.
6. Krasnianski A, Bohling GT, Harden M, et al. Psychiatric symptoms in patients with sporadic Creutzfeldt-Jakob disease in Germany. J Clin Psychiatry. 2015;76(9):1209-1215.
7. Javed Q, Alam F, Krishna S, et al. An unusual case of sporadic Creutzfeldt-Jakob disease (CJD). BMJ Case Rep. 2010;pii: bcr1220092576. doi:10.1136/bcr.12.2009.2576.
8. Abudy A, Juven-Wetzler A, Zohar J. The different faces of Creutzfeldt-Jacob disease CJD in psychiatry. Gen Hosp Psychiatry. 2014;36(3):245-248.
9. Jiang TT, Moses H, Gordon H, et al. Sporadic Creutzfeldt-Jakob disease presenting as major depression. South Med J. 1999;92(8):807-808.
10. Ali R, Baborie A, Larner AJ et al. Psychiatric presentation of sporadic Creutzfeldt-Jakob disease: a challenge to current diagnostic criteria. J Neuropsychiatry Clin Neurosci. 2013;25(4):335-338.
11. Gençer AG, Pelin Z, Küçükali CI., et al. Creutzfeldt-Jakob disease. Psychogeriatrics. 2011;11(2):119-124.
12. Caobelli F, Cobelli M, Pizzocaro C, et al. The role of neuroimaging in evaluating patients affected by Creutzfeldt-Jakob disease: a systematic review of the literature. J Neuroimaging. 2015;25(1):2-13.
13. Centers for Disease Control and Prevention. CDC's diagnostic criteria for Creutzfeldt-Jakob disease, 2010. http://www.cdc.gov/prions/cjd/diagnostic-criteria.html. Updated February 11, 2015. Accessed August 2, 2016.
14. Martindale JL, Geschwind MD, Miller BL. Psychiatric and neuroimaging findings in Creutzfeldt-Jakob disease. Curr Psychiatry Rep. 2003;5(1):43-46.
CASE A rapid decline
Ms. D, age 62, presents to a psychiatric emergency room (ER) after experiencing visual hallucinations, exhibiting odd behaviors, and having memory problems. On interview, she is disoriented, distractible, tearful, and tangential. She plays with her shirt and glasses, and occasionally shouts. She perseverates on “the aerialists,” acrobatic children she has been seeing in her apartment. She becomes distressed and shouts, “I would love to just get them!”
Ms. D is unable to provide an account of her history. Collateral information is obtained from her daughter, who has brought Ms. D to the ER for evaluation. She reports that her mother has no relevant medical or psychiatric history, and does not take any medications, except a mixture of Chinese herbs that she brews into a tea.
Ms. D’s daughter says that her mother began to deteriorate 5 months ago, after she traveled to California to care for her sister, who was seriously ill and passed away. After Ms. D returned, she would cry frequently. She also appeared “spaced out,” complained of feeling dizzy, and frequently misplaced belongings. Three months before presenting to the ER, she began to experience weakness, fatigue, and difficulty walking. Her daughter became more worried 2 months ago, when Ms. D began sleeping with her purse and hiding her belongings around their house. When asked about these odd behaviors, Ms. D claimed that “the aerialists” were climbing through her windows at night and stealing her things.
A week before seeking treatment at the ER, Ms. D’s daughter had taken her to a neurologist at another facility for clinical evaluation. An MRI of the brain showed minimal dilation in the subarachnoid space and a focal 1 cm lipoma in the anterior falx cerebri, but was otherwise unremarkable. However, Ms. D’s symptoms continued to worsen, and began to interfere with her ability to care for herself.
The team in the psychiatric ER attributes Ms. D’s symptoms to a severe, psychotic depressive episode. They admit her to the psychiatric inpatient unit for further evaluation.
[polldaddy:10012742]
Continue to: The authors' observations
The authors’ observations
Ms. D was plagued by several mood and psychotic symptoms. Such symptoms can arise from many different psychiatric or organic etiologies. In Ms. D’s case, several aspects of her presentation suggest that her illness was psychiatric. The severe illness of a beloved family member is a significant stressor that could cause a great deal of grief and devastation, possibly leading to depression. Indeed, Ms. D’s daughter noticed that her mother was crying frequently, which is consistent with grief or depression.
Memory problems, which might manifest as misplacing belongings, can also indicate a depressive illness, especially in older patients. Moreover, impaired concentration, which can cause one to appear “spaced out” or distractible, is a core symptom of major depressive disorder. Sadness and grief also can be appropriate during bereavement and in response to significant losses. Therefore, in Ms. D’s case, it is possible her frequent crying, “spaced out” appearance, and other mood symptoms she experienced immediately after caring for her sister were an appropriate response to her sister’s illness and death.
However, other aspects of Ms. D’s presentation suggested an organic etiology. Her rapid deterioration and symptom onset relatively late in life were consistent with dementia and malignancy. Her complaint of feeling dizzy suggested a neurologic process was affecting her vestibular system. Finally, while psychiatric disorders can certainly cause visual hallucinations, they occur in only a small percentage of cases.1 Visual hallucinations are commonly associated with delirium, intoxication, and neurologic illness.
Continue to: EVALUATION Severe impairment
EVALUATION Severe impairment
On the psychiatric inpatient unit, Ms. D remains unable to give a coherent account of her illness or recent events. During interviews, she abruptly shifts from laughing to crying for no apparent reason. While answering questions, her responses trail off and she appears to forget what she had been saying. However, she continues to speak at length about “the aerialists,” stating that she sees them living in her wardrobe and jumping from rooftop to rooftop in her neighborhood.
A mental status examination finds evidence of severe cognitive impairment. Ms. D is unable to identify the correct date, time, or place, and appears surprised when told she is in a hospital. She can repeat the names of 3 objects but cannot recall them a few minutes later. Finally, she scores a 14 on the Mini-Mental State Examination (MMSE) and a 5 on the Montreal Cognitive Assessment (MoCA), indicating severe impairment.
On the unit, Ms. D cannot remember the location of her room or bathroom, and even when given directions, she needs to be escorted to her destination. Her gait is unsteady and wide-spaced, and she walks on her toes at times. When food is placed before her, she needs to be shown how to take the lids off containers, pick up utensils, and start eating.
All laboratory results are unremarkable, including a complete blood count, basic metabolic panel, liver function tests, gamma-glutamyl transpeptidase, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, methylmalonic acid, homocysteine, folate, erythrocyte sedimentation rate, C-reactive protein, antinuclear antibodies, rapid plasma reagin, human immunodeficiency virus, and Lyme titers. The team also considers Ms. D’s history of herbal medicine use, because herbal mixtures can contain heavy metals and other contaminants. However, all toxicology results are normal, including arsenic, mercury, lead, copper, and zinc.
To address her symptoms, Ms. D is given risperidone, 0.5 mg twice a day, and donepezil, 5 mg/d.
[polldaddy:10012743]
Continue to: The authors' observations
The authors’ observations
Despite her persistent psychiatric symptoms, Ms. D had several neurologic symptoms that warranted further investigation. Her abrupt shifts from laughter to tears for no apparent reason were consistent with pseudobulbar affect. Her inability to remember how to use utensils during meals was consistent with apraxia. Finally, her abnormal gait raised concern for a process affecting her motor system.
OUTCOME A rare disorder
Given the psychiatry team’s suspicions for a neurologic etiology of Ms. D’s symptoms, an MRI of her brain is repeated. The results are notable for abnormal restricted diffusion in the caudate and putamen bilaterally, which is consistent with Creutzfeldt-Jakob disease (CJD). EEG shows moderate diffuse cerebral dysfunction, frontal intermittent delta activity, and diffuse cortical hyperexcitability, consistent with early- to mid-onset prion disease. Upon evaluation by the neurology team, Ms. D appears fearful, suspicious, and disorganized, but her examination does not reveal additional significant sensorimotor findings.
Ms. D is transferred to the neurology service for further workup and management. A lumbar puncture is positive for real-time quaking-induced conversion (RT-QuIC) and 14-3-3 protein with elevated tau proteins; these findings also are consistent with CJD. She develops transaminitis, with an alanine transaminase (ALT) of 127 and aspartate transaminase (AST) of 355, and a malignancy is suspected. However, CT scans of the chest, abdomen, and pelvis show no evidence of malignancy, and an extensive gastrointestinal workup is unremarkable, including anti-smooth muscle antibodies, anti-liver-kidney microsomal antibody, antimitochondrial antibodies, gliadin antibody, alpha-1 antitrypsin, liver/kidney microsomal antibody, and hepatitis serologies. While on the neurology service, risperidone and donepezil are discontinued because
After discontinuing these medications, she is evaluated by the psychiatry consult team for mood lability. The psychiatry consult team recommends quetiapine, which is later started at 25 mg nightly at bedtime.
Clinically, Ms. D’s mental status continues to deteriorate. She becomes nonverbal and minimally able to follow commands. She is ultimately discharged to an inpatient hospice for end-of-life care and the team recommends that she continue with quetiapine once there.
Continue to: The authors' observations
The authors’ observations
CJD is a rare, rapidly progressive, fatal form of dementia. In the United States, the incidence is approximately 1 to 1.5 cases per 1 million people each year.2 There are various forms of the disease. Sporadic CJD is the most common, representing 85% of cases.3 Sporadic CJD typically occurs in patients in their 60s and quickly leads to death—50% of patients die within 5 months, and 90% of patients die within 1 year.2,3 The illness is hypothesized to arise from the production of misfolded prion proteins, ultimately leading to vacuolation, neuronal loss, and the spongiform appearance characteristic of CJD.3,4
Psychiatric symptoms have long been acknowledged as a feature of CJD. Recent data indicates that psychiatric symptoms occur in 90% to 92% of cases.5,6 Sleep disturbances and depressive symptoms, including vegetative symptoms, anhedonia, and tearfulness, appear to be most common.5 Psychotic symptoms occur in approximately 42% of cases and may include persecutory and paranoid delusions, as well as an array of vivid auditory, visual, and tactile hallucinations.5,7
There is also evidence that psychiatric symptoms may be an early marker of CJD.5,8 A Mayo Clinic study found that psychiatric symptoms occurred within the prodromal phase of CJD in 26% of cases, and psychiatric symptoms occurred within the first 100 days of illness in 86% of cases.5
Case reports have described patients with CJD who initially presented with depression, psychosis, and other psychiatric symptoms.9-11 Interestingly, there have been cases with only psychiatric symptoms, and no neurologic symptoms until relatively late in the illness.10,11 Several patients with CJD have been evaluated in psychiatric ERs, admitted to psychiatric hospitals, and treated with psychiatric medications and ECT.5,9 In one study, 44% of CJD cases were misdiagnosed as “psychiatric patients” due to the prominence of their psychiatric symptomatology.8
Continue to: Making the diagnosis in psychiatric settings
Making the diagnosis in psychiatric settings. Often, the most difficult aspect of CJD is making the diagnosis.3,12 Sporadic CJD in particular can vary widely in its clinical presentation.3 The core clinical feature of CJD is rapidly progressive dementia, so suspect CJD in these patients. However, CJD can be difficult to distinguish from other rapidly progressive dementias, such as autoimmune and paraneoplastic encephalopathies.2,3 The presence of neurologic features, specifically myoclonus, akinetic mutism, and visual, cerebellar, and extrapyramidal symptoms, should also be considered a red flag for the disorder3 (Table).
Finally, positive findings on MRI, EEG, or CSF assay can indicate a probable diagnosis of CJD.13 MRI, particularly diffusion weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR), is recognized as the most studied, sensitive, and overall useful neuroimaging modality for detecting CJD.2,3,12 Although the appearance of CJD on MRI can vary widely, asymmetric hyperintensities in ≥3 cortical gyri, particularly in the frontal and parietal lobes, provide strong evidence of CJD and are observed in 80% to 81% of cases.4,12 Asymmetric hyperintensities in the basal ganglia, particularly the caudate and rostral putamen, are observed in 69% to 70% of cases.4,12,13
EEG and CSF assay also can be useful for making the diagnosis. While diffuse slowing and frontal rhythmic delta activity appear early in the course of CJD, periodic sharp wave complexes emerge later in the illness.4 However, EEG findings are not diagnostic, because periodic sharp wave complexes are seen in only two-thirds of CJD cases and also occur in other neurologic illnesses.3,4 In recent years, lumbar puncture with subsequent CSF testing has become increasingly useful in detecting the illness. The presence of the 14-3-3 protein and tau protein is highly sensitive, although not specific, for CJD.3 A definite diagnosis of CJD requires discovery of the misfolded prion proteins, such as by RT-QuIC or brain biopsy.2,3,13
Management of CJD in psychiatric patients. CJD is an invariably fatal disease for which there is no effective cure or disease modifying treatment.2 Therefore, supportive therapies are the mainstay of care. Psychotropic medications can be used to provide symptom relief. While the sleep disturbances, anxiety, and agitation/hallucinations associated with CJD appear to respond well to hypnotic, anxiolytic, and antipsychotic medications, respectively, antidepressants and mood-stabilizing medications appear to have little benefit for patients with CJD.5 During the final stages of the disease, patients may suffer from akinetic mutism and inability to swallow, which often leads to aspiration pneumonia.14 Patients should also be offered end-of-life counseling, planning, and care, and provided with other comfort measures wherever possible (Figure).
Continue to: Bottom Line
Bottom Line
Patients with Creutzfeldt-Jakob disease (CJD) may present to psychiatric settings, particularly to a psychiatric emergency room. Consider CJD as a possible etiology in patients with rapidly progressive dementia, depression, and psychosis. CJD is invariably fatal and there is no effective disease-modifying treatment. Supportive therapies are the mainstay of care.
Related Resources
- National Institute of Neurological Disorders and Stroke. Creutzfeldt-Jakob disease fact sheet. http://www.ninds.nih.gov/disorders/cjd/detail_cjd.htm.
- Centers for Disease Control and Prevention. Creutzfeldt-Jakob disease, classic (CJD). http://www.cdc.gov/prions/cjd.
Drug Brand Names
Donepezil • Aricept
Risperidone • Risperdal
Quetiapine • Seroquel
CASE A rapid decline
Ms. D, age 62, presents to a psychiatric emergency room (ER) after experiencing visual hallucinations, exhibiting odd behaviors, and having memory problems. On interview, she is disoriented, distractible, tearful, and tangential. She plays with her shirt and glasses, and occasionally shouts. She perseverates on “the aerialists,” acrobatic children she has been seeing in her apartment. She becomes distressed and shouts, “I would love to just get them!”
Ms. D is unable to provide an account of her history. Collateral information is obtained from her daughter, who has brought Ms. D to the ER for evaluation. She reports that her mother has no relevant medical or psychiatric history, and does not take any medications, except a mixture of Chinese herbs that she brews into a tea.
Ms. D’s daughter says that her mother began to deteriorate 5 months ago, after she traveled to California to care for her sister, who was seriously ill and passed away. After Ms. D returned, she would cry frequently. She also appeared “spaced out,” complained of feeling dizzy, and frequently misplaced belongings. Three months before presenting to the ER, she began to experience weakness, fatigue, and difficulty walking. Her daughter became more worried 2 months ago, when Ms. D began sleeping with her purse and hiding her belongings around their house. When asked about these odd behaviors, Ms. D claimed that “the aerialists” were climbing through her windows at night and stealing her things.
A week before seeking treatment at the ER, Ms. D’s daughter had taken her to a neurologist at another facility for clinical evaluation. An MRI of the brain showed minimal dilation in the subarachnoid space and a focal 1 cm lipoma in the anterior falx cerebri, but was otherwise unremarkable. However, Ms. D’s symptoms continued to worsen, and began to interfere with her ability to care for herself.
The team in the psychiatric ER attributes Ms. D’s symptoms to a severe, psychotic depressive episode. They admit her to the psychiatric inpatient unit for further evaluation.
[polldaddy:10012742]
Continue to: The authors' observations
The authors’ observations
Ms. D was plagued by several mood and psychotic symptoms. Such symptoms can arise from many different psychiatric or organic etiologies. In Ms. D’s case, several aspects of her presentation suggest that her illness was psychiatric. The severe illness of a beloved family member is a significant stressor that could cause a great deal of grief and devastation, possibly leading to depression. Indeed, Ms. D’s daughter noticed that her mother was crying frequently, which is consistent with grief or depression.
Memory problems, which might manifest as misplacing belongings, can also indicate a depressive illness, especially in older patients. Moreover, impaired concentration, which can cause one to appear “spaced out” or distractible, is a core symptom of major depressive disorder. Sadness and grief also can be appropriate during bereavement and in response to significant losses. Therefore, in Ms. D’s case, it is possible her frequent crying, “spaced out” appearance, and other mood symptoms she experienced immediately after caring for her sister were an appropriate response to her sister’s illness and death.
However, other aspects of Ms. D’s presentation suggested an organic etiology. Her rapid deterioration and symptom onset relatively late in life were consistent with dementia and malignancy. Her complaint of feeling dizzy suggested a neurologic process was affecting her vestibular system. Finally, while psychiatric disorders can certainly cause visual hallucinations, they occur in only a small percentage of cases.1 Visual hallucinations are commonly associated with delirium, intoxication, and neurologic illness.
Continue to: EVALUATION Severe impairment
EVALUATION Severe impairment
On the psychiatric inpatient unit, Ms. D remains unable to give a coherent account of her illness or recent events. During interviews, she abruptly shifts from laughing to crying for no apparent reason. While answering questions, her responses trail off and she appears to forget what she had been saying. However, she continues to speak at length about “the aerialists,” stating that she sees them living in her wardrobe and jumping from rooftop to rooftop in her neighborhood.
A mental status examination finds evidence of severe cognitive impairment. Ms. D is unable to identify the correct date, time, or place, and appears surprised when told she is in a hospital. She can repeat the names of 3 objects but cannot recall them a few minutes later. Finally, she scores a 14 on the Mini-Mental State Examination (MMSE) and a 5 on the Montreal Cognitive Assessment (MoCA), indicating severe impairment.
On the unit, Ms. D cannot remember the location of her room or bathroom, and even when given directions, she needs to be escorted to her destination. Her gait is unsteady and wide-spaced, and she walks on her toes at times. When food is placed before her, she needs to be shown how to take the lids off containers, pick up utensils, and start eating.
All laboratory results are unremarkable, including a complete blood count, basic metabolic panel, liver function tests, gamma-glutamyl transpeptidase, magnesium, phosphate, thyroid-stimulating hormone, vitamin B12, methylmalonic acid, homocysteine, folate, erythrocyte sedimentation rate, C-reactive protein, antinuclear antibodies, rapid plasma reagin, human immunodeficiency virus, and Lyme titers. The team also considers Ms. D’s history of herbal medicine use, because herbal mixtures can contain heavy metals and other contaminants. However, all toxicology results are normal, including arsenic, mercury, lead, copper, and zinc.
To address her symptoms, Ms. D is given risperidone, 0.5 mg twice a day, and donepezil, 5 mg/d.
[polldaddy:10012743]
Continue to: The authors' observations
The authors’ observations
Despite her persistent psychiatric symptoms, Ms. D had several neurologic symptoms that warranted further investigation. Her abrupt shifts from laughter to tears for no apparent reason were consistent with pseudobulbar affect. Her inability to remember how to use utensils during meals was consistent with apraxia. Finally, her abnormal gait raised concern for a process affecting her motor system.
OUTCOME A rare disorder
Given the psychiatry team’s suspicions for a neurologic etiology of Ms. D’s symptoms, an MRI of her brain is repeated. The results are notable for abnormal restricted diffusion in the caudate and putamen bilaterally, which is consistent with Creutzfeldt-Jakob disease (CJD). EEG shows moderate diffuse cerebral dysfunction, frontal intermittent delta activity, and diffuse cortical hyperexcitability, consistent with early- to mid-onset prion disease. Upon evaluation by the neurology team, Ms. D appears fearful, suspicious, and disorganized, but her examination does not reveal additional significant sensorimotor findings.
Ms. D is transferred to the neurology service for further workup and management. A lumbar puncture is positive for real-time quaking-induced conversion (RT-QuIC) and 14-3-3 protein with elevated tau proteins; these findings also are consistent with CJD. She develops transaminitis, with an alanine transaminase (ALT) of 127 and aspartate transaminase (AST) of 355, and a malignancy is suspected. However, CT scans of the chest, abdomen, and pelvis show no evidence of malignancy, and an extensive gastrointestinal workup is unremarkable, including anti-smooth muscle antibodies, anti-liver-kidney microsomal antibody, antimitochondrial antibodies, gliadin antibody, alpha-1 antitrypsin, liver/kidney microsomal antibody, and hepatitis serologies. While on the neurology service, risperidone and donepezil are discontinued because
After discontinuing these medications, she is evaluated by the psychiatry consult team for mood lability. The psychiatry consult team recommends quetiapine, which is later started at 25 mg nightly at bedtime.
Clinically, Ms. D’s mental status continues to deteriorate. She becomes nonverbal and minimally able to follow commands. She is ultimately discharged to an inpatient hospice for end-of-life care and the team recommends that she continue with quetiapine once there.
Continue to: The authors' observations
The authors’ observations
CJD is a rare, rapidly progressive, fatal form of dementia. In the United States, the incidence is approximately 1 to 1.5 cases per 1 million people each year.2 There are various forms of the disease. Sporadic CJD is the most common, representing 85% of cases.3 Sporadic CJD typically occurs in patients in their 60s and quickly leads to death—50% of patients die within 5 months, and 90% of patients die within 1 year.2,3 The illness is hypothesized to arise from the production of misfolded prion proteins, ultimately leading to vacuolation, neuronal loss, and the spongiform appearance characteristic of CJD.3,4
Psychiatric symptoms have long been acknowledged as a feature of CJD. Recent data indicates that psychiatric symptoms occur in 90% to 92% of cases.5,6 Sleep disturbances and depressive symptoms, including vegetative symptoms, anhedonia, and tearfulness, appear to be most common.5 Psychotic symptoms occur in approximately 42% of cases and may include persecutory and paranoid delusions, as well as an array of vivid auditory, visual, and tactile hallucinations.5,7
There is also evidence that psychiatric symptoms may be an early marker of CJD.5,8 A Mayo Clinic study found that psychiatric symptoms occurred within the prodromal phase of CJD in 26% of cases, and psychiatric symptoms occurred within the first 100 days of illness in 86% of cases.5
Case reports have described patients with CJD who initially presented with depression, psychosis, and other psychiatric symptoms.9-11 Interestingly, there have been cases with only psychiatric symptoms, and no neurologic symptoms until relatively late in the illness.10,11 Several patients with CJD have been evaluated in psychiatric ERs, admitted to psychiatric hospitals, and treated with psychiatric medications and ECT.5,9 In one study, 44% of CJD cases were misdiagnosed as “psychiatric patients” due to the prominence of their psychiatric symptomatology.8
Continue to: Making the diagnosis in psychiatric settings
Making the diagnosis in psychiatric settings. Often, the most difficult aspect of CJD is making the diagnosis.3,12 Sporadic CJD in particular can vary widely in its clinical presentation.3 The core clinical feature of CJD is rapidly progressive dementia, so suspect CJD in these patients. However, CJD can be difficult to distinguish from other rapidly progressive dementias, such as autoimmune and paraneoplastic encephalopathies.2,3 The presence of neurologic features, specifically myoclonus, akinetic mutism, and visual, cerebellar, and extrapyramidal symptoms, should also be considered a red flag for the disorder3 (Table).
Finally, positive findings on MRI, EEG, or CSF assay can indicate a probable diagnosis of CJD.13 MRI, particularly diffusion weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR), is recognized as the most studied, sensitive, and overall useful neuroimaging modality for detecting CJD.2,3,12 Although the appearance of CJD on MRI can vary widely, asymmetric hyperintensities in ≥3 cortical gyri, particularly in the frontal and parietal lobes, provide strong evidence of CJD and are observed in 80% to 81% of cases.4,12 Asymmetric hyperintensities in the basal ganglia, particularly the caudate and rostral putamen, are observed in 69% to 70% of cases.4,12,13
EEG and CSF assay also can be useful for making the diagnosis. While diffuse slowing and frontal rhythmic delta activity appear early in the course of CJD, periodic sharp wave complexes emerge later in the illness.4 However, EEG findings are not diagnostic, because periodic sharp wave complexes are seen in only two-thirds of CJD cases and also occur in other neurologic illnesses.3,4 In recent years, lumbar puncture with subsequent CSF testing has become increasingly useful in detecting the illness. The presence of the 14-3-3 protein and tau protein is highly sensitive, although not specific, for CJD.3 A definite diagnosis of CJD requires discovery of the misfolded prion proteins, such as by RT-QuIC or brain biopsy.2,3,13
Management of CJD in psychiatric patients. CJD is an invariably fatal disease for which there is no effective cure or disease modifying treatment.2 Therefore, supportive therapies are the mainstay of care. Psychotropic medications can be used to provide symptom relief. While the sleep disturbances, anxiety, and agitation/hallucinations associated with CJD appear to respond well to hypnotic, anxiolytic, and antipsychotic medications, respectively, antidepressants and mood-stabilizing medications appear to have little benefit for patients with CJD.5 During the final stages of the disease, patients may suffer from akinetic mutism and inability to swallow, which often leads to aspiration pneumonia.14 Patients should also be offered end-of-life counseling, planning, and care, and provided with other comfort measures wherever possible (Figure).
Continue to: Bottom Line
Bottom Line
Patients with Creutzfeldt-Jakob disease (CJD) may present to psychiatric settings, particularly to a psychiatric emergency room. Consider CJD as a possible etiology in patients with rapidly progressive dementia, depression, and psychosis. CJD is invariably fatal and there is no effective disease-modifying treatment. Supportive therapies are the mainstay of care.
Related Resources
- National Institute of Neurological Disorders and Stroke. Creutzfeldt-Jakob disease fact sheet. http://www.ninds.nih.gov/disorders/cjd/detail_cjd.htm.
- Centers for Disease Control and Prevention. Creutzfeldt-Jakob disease, classic (CJD). http://www.cdc.gov/prions/cjd.
Drug Brand Names
Donepezil • Aricept
Risperidone • Risperdal
Quetiapine • Seroquel
1. Resnick PJ. The detection of malingered psychosis. Psychiatr Clin North Am. 1999;22(1):159-172.
2. Bucelli RC, Ances BM. Diagnosis and evaluation of a patient with rapidly progressive dementia. Mo Med. 2013;110(5):422-428.
3. Manix M, Kalakoti P, Henry M, et al. Creutzfeldt-Jakob disease: updated diagnostic criteria, treatment algorithm, and the utility of brain biopsy. Neurosurg Focus. 2015;39(5):E2.
4. Puoti G, Bizzi A, Forloni G, et al. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. 2012;11(7):618-628.
5. Wall CA, Rummans TA, Aksamit AJ, et al. Psychiatric manifestations of Creutzfeldt-Jakob disease: a 25-year analysis. J Neuropsychiatry Clin Neurosci. 2005;17(4):489-495.
6. Krasnianski A, Bohling GT, Harden M, et al. Psychiatric symptoms in patients with sporadic Creutzfeldt-Jakob disease in Germany. J Clin Psychiatry. 2015;76(9):1209-1215.
7. Javed Q, Alam F, Krishna S, et al. An unusual case of sporadic Creutzfeldt-Jakob disease (CJD). BMJ Case Rep. 2010;pii: bcr1220092576. doi:10.1136/bcr.12.2009.2576.
8. Abudy A, Juven-Wetzler A, Zohar J. The different faces of Creutzfeldt-Jacob disease CJD in psychiatry. Gen Hosp Psychiatry. 2014;36(3):245-248.
9. Jiang TT, Moses H, Gordon H, et al. Sporadic Creutzfeldt-Jakob disease presenting as major depression. South Med J. 1999;92(8):807-808.
10. Ali R, Baborie A, Larner AJ et al. Psychiatric presentation of sporadic Creutzfeldt-Jakob disease: a challenge to current diagnostic criteria. J Neuropsychiatry Clin Neurosci. 2013;25(4):335-338.
11. Gençer AG, Pelin Z, Küçükali CI., et al. Creutzfeldt-Jakob disease. Psychogeriatrics. 2011;11(2):119-124.
12. Caobelli F, Cobelli M, Pizzocaro C, et al. The role of neuroimaging in evaluating patients affected by Creutzfeldt-Jakob disease: a systematic review of the literature. J Neuroimaging. 2015;25(1):2-13.
13. Centers for Disease Control and Prevention. CDC's diagnostic criteria for Creutzfeldt-Jakob disease, 2010. http://www.cdc.gov/prions/cjd/diagnostic-criteria.html. Updated February 11, 2015. Accessed August 2, 2016.
14. Martindale JL, Geschwind MD, Miller BL. Psychiatric and neuroimaging findings in Creutzfeldt-Jakob disease. Curr Psychiatry Rep. 2003;5(1):43-46.
1. Resnick PJ. The detection of malingered psychosis. Psychiatr Clin North Am. 1999;22(1):159-172.
2. Bucelli RC, Ances BM. Diagnosis and evaluation of a patient with rapidly progressive dementia. Mo Med. 2013;110(5):422-428.
3. Manix M, Kalakoti P, Henry M, et al. Creutzfeldt-Jakob disease: updated diagnostic criteria, treatment algorithm, and the utility of brain biopsy. Neurosurg Focus. 2015;39(5):E2.
4. Puoti G, Bizzi A, Forloni G, et al. Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol. 2012;11(7):618-628.
5. Wall CA, Rummans TA, Aksamit AJ, et al. Psychiatric manifestations of Creutzfeldt-Jakob disease: a 25-year analysis. J Neuropsychiatry Clin Neurosci. 2005;17(4):489-495.
6. Krasnianski A, Bohling GT, Harden M, et al. Psychiatric symptoms in patients with sporadic Creutzfeldt-Jakob disease in Germany. J Clin Psychiatry. 2015;76(9):1209-1215.
7. Javed Q, Alam F, Krishna S, et al. An unusual case of sporadic Creutzfeldt-Jakob disease (CJD). BMJ Case Rep. 2010;pii: bcr1220092576. doi:10.1136/bcr.12.2009.2576.
8. Abudy A, Juven-Wetzler A, Zohar J. The different faces of Creutzfeldt-Jacob disease CJD in psychiatry. Gen Hosp Psychiatry. 2014;36(3):245-248.
9. Jiang TT, Moses H, Gordon H, et al. Sporadic Creutzfeldt-Jakob disease presenting as major depression. South Med J. 1999;92(8):807-808.
10. Ali R, Baborie A, Larner AJ et al. Psychiatric presentation of sporadic Creutzfeldt-Jakob disease: a challenge to current diagnostic criteria. J Neuropsychiatry Clin Neurosci. 2013;25(4):335-338.
11. Gençer AG, Pelin Z, Küçükali CI., et al. Creutzfeldt-Jakob disease. Psychogeriatrics. 2011;11(2):119-124.
12. Caobelli F, Cobelli M, Pizzocaro C, et al. The role of neuroimaging in evaluating patients affected by Creutzfeldt-Jakob disease: a systematic review of the literature. J Neuroimaging. 2015;25(1):2-13.
13. Centers for Disease Control and Prevention. CDC's diagnostic criteria for Creutzfeldt-Jakob disease, 2010. http://www.cdc.gov/prions/cjd/diagnostic-criteria.html. Updated February 11, 2015. Accessed August 2, 2016.
14. Martindale JL, Geschwind MD, Miller BL. Psychiatric and neuroimaging findings in Creutzfeldt-Jakob disease. Curr Psychiatry Rep. 2003;5(1):43-46.
Diagnosed with a chronic illness: Should you tell your patients?
Physicians are not immune to chronic illness. Those who choose to continue working after being diagnosed with a chronic illness need to decide whether or not to tell their patients. The idea of physicians being a “blank slate” to their patients would be challenged by such self-disclosure. But ignoring an obvious change in the therapeutic space could be detrimental to your patient’s therapy.1 Every patient has his or her own ideas or perceptions about their physician that contribute to how likely they are to continue to engage in therapy or take prescribed medications. Could letting your patients know you have a chronic illness threaten the image they have of you, and potentially jeopardize their treatment?
Physician factors
Once diagnosed with a chronic illness, a physician who previously defined his or her identity as a clinician now must also assume the role of a patient. This transition gives rise to anxiety. Patient encounters may give a physician the opportunity to feel safe to discuss such anxiety.2 However, patients often view their physicians as omnipotent. When their physician admits weakness and vulnerability, that perception may be damaged.3 This damage could manifest as medication nonadherence, missed appointments, or even termination of treatment. A fear of such abandonment may lead a physician to not disclose his or her illness. To avoid discussing this uncomfortable topic, a physician might be more defensive in his or her interactions with the patient.2
Patient factors
Every patient presents with unique characteristics that contribute to the patient–physician relationship. Receiving news that one’s physician has a chronic or severe illness will elicit different reactions in each patient. These reactions will vary depending upon the patient’s pathology, stage of treatment, and background.3 The previous work done between the patient and physician is crucial in predicting the treatment course after the physician discloses that he or she has a chronic illness. Also, patients may notice the physical changes of their physician’s illness. Deciding to disclose—or to not disclose—something that is obvious can elicit feelings of worry, anger, or even triumph in the patient.3
CASES
Two patients, two different responses
Dr. T recently was diagnosed with leukemia and has begun to receive treatment. He decides to continue working. Since receiving the diagnosis, he finds himself more anxious. Adding to his anxiety is the question of whether or not he should tell his patients about his diagnosis. He decides to tell 2 of his patients—Mr. G and Ms. N—and receives a drastically different response from each of them.
Mr. G, age 45, has been Dr. T’s patient for 2 years. He is married, has 2 children, and works at a car dealership. Mr. G initially presented for treatment of depressive symptoms after his mother died. Those symptoms were stabilized with medicatio
Dr. T discloses the news of his illness to Mr. G at their next appointment. Mr. G offers his condolences and speaks about how on one hand, he is sympathetic and wishes to be supportive, but on the other hand, he fears another loss in his life. Mr. G thanks Dr. T for disclosing this news and hopes they can begin to discuss this situation in therapy. He remains compliant with appointments.
Ms. N, age 59, has been Dr. T’s patient for 6 months. She was diagnosed with schizophrenia when she was in her early 20s. She is single, unemployed, lives alone, and lacks social support. Ms. N has a history of multiple hospitalizations. She has a pattern of presenting to an emergency department and asking to be admitted whenever she faces an acute stressor.
Continue to: Ms. N came to Dr. T through another psychiatrist...
Ms. N came to Dr. T through another psychiatrist and Dr. T continues to provide medication management. He has implemented a biweekly appointment schedule for supportive therapy to work on Ms. N’s personal goals to cook more, clean her house, and lose weight. They also address issues regarding her father and his absence in her life since she was age 18.
During their next appointment, Dr. T discloses the news of his illness to Ms. N. Ms. N asks, “Are you sure?” Dr. T confirms and asks her how she feels about this news. She replies, “It’s fine.” Soon after, she stops attending her biweekly appointments and is lost to follow-up.
Consider your patient’s ability to cope
Dr. T faced the challenge of whether to disclose his diagnosis to his patients. He understood the potential implications on his therapeutic work and his battles with his own anxiety. Ultimately, he decided to tell his patients, but he did not consider how they might have been able to handle such news.
Mr. G was receptive to the news and remained engaged in treatment after learning of Dr. T’s illness. His ability to do so likely was the result of many factors. Mr. G is a high-functioning individual who seems to have a secure attachment style. He is able to express his conflicts. He has had good relationships in his life, was able to work through his mother’s death, and is engaged in treatment to help him cope with the inevitable loss of his father. Mr. G can handle the potential loss of his physician because he has shown his ability to cope with such losses in his life.
Continue to: On the other hand...
On the other hand, although Ms. N stated that the news of Dr. T’s diagnosis was “fine,” she was soon lost to follow-up, which suggests she was unable to handle the news. This is supported by her history of unstable relationships. Her insecure attachment style likely contributed to her inability to handle stressors, as evidenced by her frequent requests for admission. Dr. T also should have considered the possibility of transference, given that Ms. N struggled with abandonment by her father. Dr. T’s potential departure could represent such abandonment. In a patient such as Ms. N, being upfront about having a chronic illness would be more harmful than beneficial.
Maintain a patient-focused view
Receiving a diagnosis of a severe or chronic illness can be extremely stressful for physicians. Adopting the new identity of patient in addition to that of physician can cause tremendous anxiety. If you decide to continue working with your patients, it is crucial to be mindful of this anxiety and its potential to influence your decision to disclose your diagnosis to your patients. Do not allow your anxiety to contaminate the therapeutic work. Maintaining a patient-focused view of treatment will allow you to determine each patient’s ability to process disclosure vs nondisclosure of your diagnosis. Ultimately, this will help determine which patients you should tell, and which ones you should not.
1. Abend SM. Serious illness in the analyst: countertransference considerations. J Am Psychoanal Assoc. 1982;30(2):365-379.
2. Dewald PA. Serious illness in the analyst: transference, countertransference, and reality responses. J Am Psychoanal Assoc. 1982;30(2):347-363.
3. Torrigiani MG, Marzi A. When the analyst is physically ill: vicissitudes in the analytical relationship. Int J Psychoanal. 2005;86(pt 5):1373-1389.
Physicians are not immune to chronic illness. Those who choose to continue working after being diagnosed with a chronic illness need to decide whether or not to tell their patients. The idea of physicians being a “blank slate” to their patients would be challenged by such self-disclosure. But ignoring an obvious change in the therapeutic space could be detrimental to your patient’s therapy.1 Every patient has his or her own ideas or perceptions about their physician that contribute to how likely they are to continue to engage in therapy or take prescribed medications. Could letting your patients know you have a chronic illness threaten the image they have of you, and potentially jeopardize their treatment?
Physician factors
Once diagnosed with a chronic illness, a physician who previously defined his or her identity as a clinician now must also assume the role of a patient. This transition gives rise to anxiety. Patient encounters may give a physician the opportunity to feel safe to discuss such anxiety.2 However, patients often view their physicians as omnipotent. When their physician admits weakness and vulnerability, that perception may be damaged.3 This damage could manifest as medication nonadherence, missed appointments, or even termination of treatment. A fear of such abandonment may lead a physician to not disclose his or her illness. To avoid discussing this uncomfortable topic, a physician might be more defensive in his or her interactions with the patient.2
Patient factors
Every patient presents with unique characteristics that contribute to the patient–physician relationship. Receiving news that one’s physician has a chronic or severe illness will elicit different reactions in each patient. These reactions will vary depending upon the patient’s pathology, stage of treatment, and background.3 The previous work done between the patient and physician is crucial in predicting the treatment course after the physician discloses that he or she has a chronic illness. Also, patients may notice the physical changes of their physician’s illness. Deciding to disclose—or to not disclose—something that is obvious can elicit feelings of worry, anger, or even triumph in the patient.3
CASES
Two patients, two different responses
Dr. T recently was diagnosed with leukemia and has begun to receive treatment. He decides to continue working. Since receiving the diagnosis, he finds himself more anxious. Adding to his anxiety is the question of whether or not he should tell his patients about his diagnosis. He decides to tell 2 of his patients—Mr. G and Ms. N—and receives a drastically different response from each of them.
Mr. G, age 45, has been Dr. T’s patient for 2 years. He is married, has 2 children, and works at a car dealership. Mr. G initially presented for treatment of depressive symptoms after his mother died. Those symptoms were stabilized with medicatio
Dr. T discloses the news of his illness to Mr. G at their next appointment. Mr. G offers his condolences and speaks about how on one hand, he is sympathetic and wishes to be supportive, but on the other hand, he fears another loss in his life. Mr. G thanks Dr. T for disclosing this news and hopes they can begin to discuss this situation in therapy. He remains compliant with appointments.
Ms. N, age 59, has been Dr. T’s patient for 6 months. She was diagnosed with schizophrenia when she was in her early 20s. She is single, unemployed, lives alone, and lacks social support. Ms. N has a history of multiple hospitalizations. She has a pattern of presenting to an emergency department and asking to be admitted whenever she faces an acute stressor.
Continue to: Ms. N came to Dr. T through another psychiatrist...
Ms. N came to Dr. T through another psychiatrist and Dr. T continues to provide medication management. He has implemented a biweekly appointment schedule for supportive therapy to work on Ms. N’s personal goals to cook more, clean her house, and lose weight. They also address issues regarding her father and his absence in her life since she was age 18.
During their next appointment, Dr. T discloses the news of his illness to Ms. N. Ms. N asks, “Are you sure?” Dr. T confirms and asks her how she feels about this news. She replies, “It’s fine.” Soon after, she stops attending her biweekly appointments and is lost to follow-up.
Consider your patient’s ability to cope
Dr. T faced the challenge of whether to disclose his diagnosis to his patients. He understood the potential implications on his therapeutic work and his battles with his own anxiety. Ultimately, he decided to tell his patients, but he did not consider how they might have been able to handle such news.
Mr. G was receptive to the news and remained engaged in treatment after learning of Dr. T’s illness. His ability to do so likely was the result of many factors. Mr. G is a high-functioning individual who seems to have a secure attachment style. He is able to express his conflicts. He has had good relationships in his life, was able to work through his mother’s death, and is engaged in treatment to help him cope with the inevitable loss of his father. Mr. G can handle the potential loss of his physician because he has shown his ability to cope with such losses in his life.
Continue to: On the other hand...
On the other hand, although Ms. N stated that the news of Dr. T’s diagnosis was “fine,” she was soon lost to follow-up, which suggests she was unable to handle the news. This is supported by her history of unstable relationships. Her insecure attachment style likely contributed to her inability to handle stressors, as evidenced by her frequent requests for admission. Dr. T also should have considered the possibility of transference, given that Ms. N struggled with abandonment by her father. Dr. T’s potential departure could represent such abandonment. In a patient such as Ms. N, being upfront about having a chronic illness would be more harmful than beneficial.
Maintain a patient-focused view
Receiving a diagnosis of a severe or chronic illness can be extremely stressful for physicians. Adopting the new identity of patient in addition to that of physician can cause tremendous anxiety. If you decide to continue working with your patients, it is crucial to be mindful of this anxiety and its potential to influence your decision to disclose your diagnosis to your patients. Do not allow your anxiety to contaminate the therapeutic work. Maintaining a patient-focused view of treatment will allow you to determine each patient’s ability to process disclosure vs nondisclosure of your diagnosis. Ultimately, this will help determine which patients you should tell, and which ones you should not.
Physicians are not immune to chronic illness. Those who choose to continue working after being diagnosed with a chronic illness need to decide whether or not to tell their patients. The idea of physicians being a “blank slate” to their patients would be challenged by such self-disclosure. But ignoring an obvious change in the therapeutic space could be detrimental to your patient’s therapy.1 Every patient has his or her own ideas or perceptions about their physician that contribute to how likely they are to continue to engage in therapy or take prescribed medications. Could letting your patients know you have a chronic illness threaten the image they have of you, and potentially jeopardize their treatment?
Physician factors
Once diagnosed with a chronic illness, a physician who previously defined his or her identity as a clinician now must also assume the role of a patient. This transition gives rise to anxiety. Patient encounters may give a physician the opportunity to feel safe to discuss such anxiety.2 However, patients often view their physicians as omnipotent. When their physician admits weakness and vulnerability, that perception may be damaged.3 This damage could manifest as medication nonadherence, missed appointments, or even termination of treatment. A fear of such abandonment may lead a physician to not disclose his or her illness. To avoid discussing this uncomfortable topic, a physician might be more defensive in his or her interactions with the patient.2
Patient factors
Every patient presents with unique characteristics that contribute to the patient–physician relationship. Receiving news that one’s physician has a chronic or severe illness will elicit different reactions in each patient. These reactions will vary depending upon the patient’s pathology, stage of treatment, and background.3 The previous work done between the patient and physician is crucial in predicting the treatment course after the physician discloses that he or she has a chronic illness. Also, patients may notice the physical changes of their physician’s illness. Deciding to disclose—or to not disclose—something that is obvious can elicit feelings of worry, anger, or even triumph in the patient.3
CASES
Two patients, two different responses
Dr. T recently was diagnosed with leukemia and has begun to receive treatment. He decides to continue working. Since receiving the diagnosis, he finds himself more anxious. Adding to his anxiety is the question of whether or not he should tell his patients about his diagnosis. He decides to tell 2 of his patients—Mr. G and Ms. N—and receives a drastically different response from each of them.
Mr. G, age 45, has been Dr. T’s patient for 2 years. He is married, has 2 children, and works at a car dealership. Mr. G initially presented for treatment of depressive symptoms after his mother died. Those symptoms were stabilized with medicatio
Dr. T discloses the news of his illness to Mr. G at their next appointment. Mr. G offers his condolences and speaks about how on one hand, he is sympathetic and wishes to be supportive, but on the other hand, he fears another loss in his life. Mr. G thanks Dr. T for disclosing this news and hopes they can begin to discuss this situation in therapy. He remains compliant with appointments.
Ms. N, age 59, has been Dr. T’s patient for 6 months. She was diagnosed with schizophrenia when she was in her early 20s. She is single, unemployed, lives alone, and lacks social support. Ms. N has a history of multiple hospitalizations. She has a pattern of presenting to an emergency department and asking to be admitted whenever she faces an acute stressor.
Continue to: Ms. N came to Dr. T through another psychiatrist...
Ms. N came to Dr. T through another psychiatrist and Dr. T continues to provide medication management. He has implemented a biweekly appointment schedule for supportive therapy to work on Ms. N’s personal goals to cook more, clean her house, and lose weight. They also address issues regarding her father and his absence in her life since she was age 18.
During their next appointment, Dr. T discloses the news of his illness to Ms. N. Ms. N asks, “Are you sure?” Dr. T confirms and asks her how she feels about this news. She replies, “It’s fine.” Soon after, she stops attending her biweekly appointments and is lost to follow-up.
Consider your patient’s ability to cope
Dr. T faced the challenge of whether to disclose his diagnosis to his patients. He understood the potential implications on his therapeutic work and his battles with his own anxiety. Ultimately, he decided to tell his patients, but he did not consider how they might have been able to handle such news.
Mr. G was receptive to the news and remained engaged in treatment after learning of Dr. T’s illness. His ability to do so likely was the result of many factors. Mr. G is a high-functioning individual who seems to have a secure attachment style. He is able to express his conflicts. He has had good relationships in his life, was able to work through his mother’s death, and is engaged in treatment to help him cope with the inevitable loss of his father. Mr. G can handle the potential loss of his physician because he has shown his ability to cope with such losses in his life.
Continue to: On the other hand...
On the other hand, although Ms. N stated that the news of Dr. T’s diagnosis was “fine,” she was soon lost to follow-up, which suggests she was unable to handle the news. This is supported by her history of unstable relationships. Her insecure attachment style likely contributed to her inability to handle stressors, as evidenced by her frequent requests for admission. Dr. T also should have considered the possibility of transference, given that Ms. N struggled with abandonment by her father. Dr. T’s potential departure could represent such abandonment. In a patient such as Ms. N, being upfront about having a chronic illness would be more harmful than beneficial.
Maintain a patient-focused view
Receiving a diagnosis of a severe or chronic illness can be extremely stressful for physicians. Adopting the new identity of patient in addition to that of physician can cause tremendous anxiety. If you decide to continue working with your patients, it is crucial to be mindful of this anxiety and its potential to influence your decision to disclose your diagnosis to your patients. Do not allow your anxiety to contaminate the therapeutic work. Maintaining a patient-focused view of treatment will allow you to determine each patient’s ability to process disclosure vs nondisclosure of your diagnosis. Ultimately, this will help determine which patients you should tell, and which ones you should not.
1. Abend SM. Serious illness in the analyst: countertransference considerations. J Am Psychoanal Assoc. 1982;30(2):365-379.
2. Dewald PA. Serious illness in the analyst: transference, countertransference, and reality responses. J Am Psychoanal Assoc. 1982;30(2):347-363.
3. Torrigiani MG, Marzi A. When the analyst is physically ill: vicissitudes in the analytical relationship. Int J Psychoanal. 2005;86(pt 5):1373-1389.
1. Abend SM. Serious illness in the analyst: countertransference considerations. J Am Psychoanal Assoc. 1982;30(2):365-379.
2. Dewald PA. Serious illness in the analyst: transference, countertransference, and reality responses. J Am Psychoanal Assoc. 1982;30(2):347-363.
3. Torrigiani MG, Marzi A. When the analyst is physically ill: vicissitudes in the analytical relationship. Int J Psychoanal. 2005;86(pt 5):1373-1389.
Working at a long-term psychiatric hospital? Consider your patient’s point of view
Working at a long-term psychiatric hospital can present challenges similar to those found in other institutions, such as correctional facilities1; however, in this setting, additional obstacles that could affect treatment may not readily come to mind. Following the 2 simple approaches described here can help you to understand your patient’s point of view and improve the treatment relationship.
Allow patients some control. Many patients in long-term psychiatric hospitals are prescribed medications that can result in metabolic complications such as weight gain or hyperlipidemia. To avoid these complications, we may need to institute dietary restrictions. Despite our explanations of why these restrictions are necessary, some patients may continue to insist on eating food that we believe will worsen their physical health; they may feel that they have little control in their lives and have nothing to look forward to except for what they can eat.2
For patients in long-term psychiatric hospitals, everyday life usually is structured from morning to evening. This includes when meals and snacks are served, as well as what they are allowed to eat. Food is a basic human necessity, and we often forget its psychological significance. Because most patients can control what they put in their mouths, food allows them to exert control in an environment where they may believe they have no influence. This may explain why patients insist on certain meals, purchase unhealthy food, or engage in a surreptitious snack distribution system with other patients. We usually can decide what and when we eat, but many of our hospitalized patients do not have that opportunity. Within reason, negotiating meals and snacks could provide patients with a sense of control, and might increase treatment compliance.2
Mind what you say. At the hospital, patients are acutely aware that we are there for a short period each day. For these patients, the hospital serves as their home. Many will live there for months to years; some will spend the remainder of their lives there. The way these patients view us can become adversely affected when they see that we occasionally bring a negative attitude toward having to spend the day in their living space, telling them how to behave and what to do. This daily temporary relationship between hospital staff and patients can greatly affect treatment.
Although the hospital can serve as a home, patients do not have input into how we should behave in their home. Be mindful of your actions and the comments you make while in the hospital. We would not appreciate someone making a negative comment about our homes, so it is likely that our patients do not want to hear us complain about the hospital. Furthermore, they likely do not enjoy hearing hospital staff discussing plans they have made in their personal lives. Many patients do not enjoy being in the hospital, and they could view such expressions as “rubbing it in,” which could adversely affect treatment.
1. Khajuria K. CORRECT: insights into working at correctional facilities. Current Psychiatry. 2017;16(2):54-55.
2. Joshi KG. Can I have cheese on my ham sandwich? BMJ. 2016;355:i6024. doi: 10.1136/bmj.i6024.
Working at a long-term psychiatric hospital can present challenges similar to those found in other institutions, such as correctional facilities1; however, in this setting, additional obstacles that could affect treatment may not readily come to mind. Following the 2 simple approaches described here can help you to understand your patient’s point of view and improve the treatment relationship.
Allow patients some control. Many patients in long-term psychiatric hospitals are prescribed medications that can result in metabolic complications such as weight gain or hyperlipidemia. To avoid these complications, we may need to institute dietary restrictions. Despite our explanations of why these restrictions are necessary, some patients may continue to insist on eating food that we believe will worsen their physical health; they may feel that they have little control in their lives and have nothing to look forward to except for what they can eat.2
For patients in long-term psychiatric hospitals, everyday life usually is structured from morning to evening. This includes when meals and snacks are served, as well as what they are allowed to eat. Food is a basic human necessity, and we often forget its psychological significance. Because most patients can control what they put in their mouths, food allows them to exert control in an environment where they may believe they have no influence. This may explain why patients insist on certain meals, purchase unhealthy food, or engage in a surreptitious snack distribution system with other patients. We usually can decide what and when we eat, but many of our hospitalized patients do not have that opportunity. Within reason, negotiating meals and snacks could provide patients with a sense of control, and might increase treatment compliance.2
Mind what you say. At the hospital, patients are acutely aware that we are there for a short period each day. For these patients, the hospital serves as their home. Many will live there for months to years; some will spend the remainder of their lives there. The way these patients view us can become adversely affected when they see that we occasionally bring a negative attitude toward having to spend the day in their living space, telling them how to behave and what to do. This daily temporary relationship between hospital staff and patients can greatly affect treatment.
Although the hospital can serve as a home, patients do not have input into how we should behave in their home. Be mindful of your actions and the comments you make while in the hospital. We would not appreciate someone making a negative comment about our homes, so it is likely that our patients do not want to hear us complain about the hospital. Furthermore, they likely do not enjoy hearing hospital staff discussing plans they have made in their personal lives. Many patients do not enjoy being in the hospital, and they could view such expressions as “rubbing it in,” which could adversely affect treatment.
Working at a long-term psychiatric hospital can present challenges similar to those found in other institutions, such as correctional facilities1; however, in this setting, additional obstacles that could affect treatment may not readily come to mind. Following the 2 simple approaches described here can help you to understand your patient’s point of view and improve the treatment relationship.
Allow patients some control. Many patients in long-term psychiatric hospitals are prescribed medications that can result in metabolic complications such as weight gain or hyperlipidemia. To avoid these complications, we may need to institute dietary restrictions. Despite our explanations of why these restrictions are necessary, some patients may continue to insist on eating food that we believe will worsen their physical health; they may feel that they have little control in their lives and have nothing to look forward to except for what they can eat.2
For patients in long-term psychiatric hospitals, everyday life usually is structured from morning to evening. This includes when meals and snacks are served, as well as what they are allowed to eat. Food is a basic human necessity, and we often forget its psychological significance. Because most patients can control what they put in their mouths, food allows them to exert control in an environment where they may believe they have no influence. This may explain why patients insist on certain meals, purchase unhealthy food, or engage in a surreptitious snack distribution system with other patients. We usually can decide what and when we eat, but many of our hospitalized patients do not have that opportunity. Within reason, negotiating meals and snacks could provide patients with a sense of control, and might increase treatment compliance.2
Mind what you say. At the hospital, patients are acutely aware that we are there for a short period each day. For these patients, the hospital serves as their home. Many will live there for months to years; some will spend the remainder of their lives there. The way these patients view us can become adversely affected when they see that we occasionally bring a negative attitude toward having to spend the day in their living space, telling them how to behave and what to do. This daily temporary relationship between hospital staff and patients can greatly affect treatment.
Although the hospital can serve as a home, patients do not have input into how we should behave in their home. Be mindful of your actions and the comments you make while in the hospital. We would not appreciate someone making a negative comment about our homes, so it is likely that our patients do not want to hear us complain about the hospital. Furthermore, they likely do not enjoy hearing hospital staff discussing plans they have made in their personal lives. Many patients do not enjoy being in the hospital, and they could view such expressions as “rubbing it in,” which could adversely affect treatment.
1. Khajuria K. CORRECT: insights into working at correctional facilities. Current Psychiatry. 2017;16(2):54-55.
2. Joshi KG. Can I have cheese on my ham sandwich? BMJ. 2016;355:i6024. doi: 10.1136/bmj.i6024.
1. Khajuria K. CORRECT: insights into working at correctional facilities. Current Psychiatry. 2017;16(2):54-55.
2. Joshi KG. Can I have cheese on my ham sandwich? BMJ. 2016;355:i6024. doi: 10.1136/bmj.i6024.