User login
FDA approves Olumiant for treatment of rheumatoid arthritis
, an orally administered Janus kinase (JAK) inhibitor, to treat adults with moderate to severe rheumatoid arthritis (RA) who have responded inadequately or poorly to methotrexate, its manufacturer, Eli Lilly, announced June 1. The regulators voted against approval of the 4-mg dose because of concerns about the safety profile.
Olumiant is accompanied by a boxed warning about the risk of serious infections, malignancies, and thrombosis. Patients taking Olumiant also have experienced tuberculosis and opportunistic viral, fungal, and bacterial infections. These infections have led to hospitalization or death.
As part of the approval, Lilly and the original developer of baricitinib, Incyte, have agreed to conduct further randomized and controlled clinical trials to evaluate the long-term safety of Olumiant.
Lilly said in its announcement that it expects to launch Olumiant in the United States by the end of the second quarter of 2018 at a targeted price that is 60% less than “the leading TNF inhibitor.” Additionally, Lilly will offer patient support in the form of a patient support program called Olumiant Together. More information for the program can be obtained by calling 844-OLUMIANT.
, an orally administered Janus kinase (JAK) inhibitor, to treat adults with moderate to severe rheumatoid arthritis (RA) who have responded inadequately or poorly to methotrexate, its manufacturer, Eli Lilly, announced June 1. The regulators voted against approval of the 4-mg dose because of concerns about the safety profile.
Olumiant is accompanied by a boxed warning about the risk of serious infections, malignancies, and thrombosis. Patients taking Olumiant also have experienced tuberculosis and opportunistic viral, fungal, and bacterial infections. These infections have led to hospitalization or death.
As part of the approval, Lilly and the original developer of baricitinib, Incyte, have agreed to conduct further randomized and controlled clinical trials to evaluate the long-term safety of Olumiant.
Lilly said in its announcement that it expects to launch Olumiant in the United States by the end of the second quarter of 2018 at a targeted price that is 60% less than “the leading TNF inhibitor.” Additionally, Lilly will offer patient support in the form of a patient support program called Olumiant Together. More information for the program can be obtained by calling 844-OLUMIANT.
, an orally administered Janus kinase (JAK) inhibitor, to treat adults with moderate to severe rheumatoid arthritis (RA) who have responded inadequately or poorly to methotrexate, its manufacturer, Eli Lilly, announced June 1. The regulators voted against approval of the 4-mg dose because of concerns about the safety profile.
Olumiant is accompanied by a boxed warning about the risk of serious infections, malignancies, and thrombosis. Patients taking Olumiant also have experienced tuberculosis and opportunistic viral, fungal, and bacterial infections. These infections have led to hospitalization or death.
As part of the approval, Lilly and the original developer of baricitinib, Incyte, have agreed to conduct further randomized and controlled clinical trials to evaluate the long-term safety of Olumiant.
Lilly said in its announcement that it expects to launch Olumiant in the United States by the end of the second quarter of 2018 at a targeted price that is 60% less than “the leading TNF inhibitor.” Additionally, Lilly will offer patient support in the form of a patient support program called Olumiant Together. More information for the program can be obtained by calling 844-OLUMIANT.
Experimental Therapy Shows Promise in Early-Stage Huntington’s Disease
LOS ANGELES—IONIS-HTTRx, an antisense oligonucleotide (ASO), effectively reduced levels of the protein responsible for Huntington’s disease in early-stage patients, according to findings presented at the American Academy of Neurology’s 70th Annual Meeting.
“ASO technology has the potential to provide disease-modifying benefits to patients with neurodegenerative diseases,” said lead author Sarah Tabrizi, BSc, MBChB, PhD, Professor of Clinical Neurology at the University College London Institute of Neurology, and colleagues. “In this phase I/IIa trial in patients with early-stage Huntington’s disease, IONIS-HTTRx delivered via intrathecal injection was well tolerated with no study-drug-related adverse safety signals during the treatment or follow-up periods.” In addition, significant dose-dependent reductions in CSF mutant huntingtin protein (mHTT) were observed, suggesting that IONIS-HTTRx is a promising therapeutic for the treatment of Huntington’s disease.
Huntington’s disease is caused by CAG repeat expansion in the HTT gene resulting in polyglutamine expansion in the mHTT with a toxic gain-of-function disease mechanism. A comprehensive drug discovery effort, including extensive preclinical testing, was undertaken to design a well-tolerated ASO with high specificity to human HTT mRNA that could potently suppresses mHTT production.
In the present study—a first-in-human, multicenter, double-blind clinical trial—46 patients were randomized (3:1) to receive four doses of IONIS-HTTRx or placebo by monthly bolus intrathecal injection, followed by a four-month untreated period. Five ascending-dose cohorts were enrolled with independent Data Safety Monitoring Board review of safety, pharmacokinetics, and target engagement prior to dose escalation.
Dr. Tabrizi reported that IONIS-HTTRx was well tolerated at all doses tested. Adverse events were mostly mild and unrelated to the study drug. There were no adverse trends in laboratory parameters. No patients prematurely discontinued the treatment. ASO was measurable in CSF and plasma, and concentrations were generally aligned with predictions from a linked pharmacokinetic/pharmacodynamic preclinical model. Significant, dose-dependent reductions in CSF mHTT were observed. At the highest dose of the ASO, 40% to 60% reductions in CSF mHTT were observed. “When we looked more carefully at the data with exploratory analyses, we found a link between lowering of CSF mHTT and improvement in total motor scores and neurologic function,” Dr. Tabrizi said.
LOS ANGELES—IONIS-HTTRx, an antisense oligonucleotide (ASO), effectively reduced levels of the protein responsible for Huntington’s disease in early-stage patients, according to findings presented at the American Academy of Neurology’s 70th Annual Meeting.
“ASO technology has the potential to provide disease-modifying benefits to patients with neurodegenerative diseases,” said lead author Sarah Tabrizi, BSc, MBChB, PhD, Professor of Clinical Neurology at the University College London Institute of Neurology, and colleagues. “In this phase I/IIa trial in patients with early-stage Huntington’s disease, IONIS-HTTRx delivered via intrathecal injection was well tolerated with no study-drug-related adverse safety signals during the treatment or follow-up periods.” In addition, significant dose-dependent reductions in CSF mutant huntingtin protein (mHTT) were observed, suggesting that IONIS-HTTRx is a promising therapeutic for the treatment of Huntington’s disease.
Huntington’s disease is caused by CAG repeat expansion in the HTT gene resulting in polyglutamine expansion in the mHTT with a toxic gain-of-function disease mechanism. A comprehensive drug discovery effort, including extensive preclinical testing, was undertaken to design a well-tolerated ASO with high specificity to human HTT mRNA that could potently suppresses mHTT production.
In the present study—a first-in-human, multicenter, double-blind clinical trial—46 patients were randomized (3:1) to receive four doses of IONIS-HTTRx or placebo by monthly bolus intrathecal injection, followed by a four-month untreated period. Five ascending-dose cohorts were enrolled with independent Data Safety Monitoring Board review of safety, pharmacokinetics, and target engagement prior to dose escalation.
Dr. Tabrizi reported that IONIS-HTTRx was well tolerated at all doses tested. Adverse events were mostly mild and unrelated to the study drug. There were no adverse trends in laboratory parameters. No patients prematurely discontinued the treatment. ASO was measurable in CSF and plasma, and concentrations were generally aligned with predictions from a linked pharmacokinetic/pharmacodynamic preclinical model. Significant, dose-dependent reductions in CSF mHTT were observed. At the highest dose of the ASO, 40% to 60% reductions in CSF mHTT were observed. “When we looked more carefully at the data with exploratory analyses, we found a link between lowering of CSF mHTT and improvement in total motor scores and neurologic function,” Dr. Tabrizi said.
LOS ANGELES—IONIS-HTTRx, an antisense oligonucleotide (ASO), effectively reduced levels of the protein responsible for Huntington’s disease in early-stage patients, according to findings presented at the American Academy of Neurology’s 70th Annual Meeting.
“ASO technology has the potential to provide disease-modifying benefits to patients with neurodegenerative diseases,” said lead author Sarah Tabrizi, BSc, MBChB, PhD, Professor of Clinical Neurology at the University College London Institute of Neurology, and colleagues. “In this phase I/IIa trial in patients with early-stage Huntington’s disease, IONIS-HTTRx delivered via intrathecal injection was well tolerated with no study-drug-related adverse safety signals during the treatment or follow-up periods.” In addition, significant dose-dependent reductions in CSF mutant huntingtin protein (mHTT) were observed, suggesting that IONIS-HTTRx is a promising therapeutic for the treatment of Huntington’s disease.
Huntington’s disease is caused by CAG repeat expansion in the HTT gene resulting in polyglutamine expansion in the mHTT with a toxic gain-of-function disease mechanism. A comprehensive drug discovery effort, including extensive preclinical testing, was undertaken to design a well-tolerated ASO with high specificity to human HTT mRNA that could potently suppresses mHTT production.
In the present study—a first-in-human, multicenter, double-blind clinical trial—46 patients were randomized (3:1) to receive four doses of IONIS-HTTRx or placebo by monthly bolus intrathecal injection, followed by a four-month untreated period. Five ascending-dose cohorts were enrolled with independent Data Safety Monitoring Board review of safety, pharmacokinetics, and target engagement prior to dose escalation.
Dr. Tabrizi reported that IONIS-HTTRx was well tolerated at all doses tested. Adverse events were mostly mild and unrelated to the study drug. There were no adverse trends in laboratory parameters. No patients prematurely discontinued the treatment. ASO was measurable in CSF and plasma, and concentrations were generally aligned with predictions from a linked pharmacokinetic/pharmacodynamic preclinical model. Significant, dose-dependent reductions in CSF mHTT were observed. At the highest dose of the ASO, 40% to 60% reductions in CSF mHTT were observed. “When we looked more carefully at the data with exploratory analyses, we found a link between lowering of CSF mHTT and improvement in total motor scores and neurologic function,” Dr. Tabrizi said.
First antidepressant use not tied to first abortion
Women who have had an abortion are more likely to use antidepressants than women who have not had an abortion, but new research suggests that this association is a result of differences in risk factors for depression.
“Thus, policies based on the notion that abortion harms women’s mental health may be misinformed,” wrote Julia R. Steinberg, PhD, and her coauthors in JAMA Psychiatry.
Dr. Steinberg and her coauthors looked at a cohort of Danish women, intending to examine the association between first-time antidepressant use and either first-trimester abortion or first childbirth. “One shortcoming of many studies in the field is their reliance on self-report of both abortion and mental health problems, which is subject to both faulty memory and social desirability in reporting,” wrote Dr. Steinberg of the University of Maryland, College Park, and her coauthors. Using data on abortion, childbirth, and antidepressants from the Danish population registries, which were collected over time, avoided that limitation.
Of the 396,397 women, 17,294 (4.4%) had at least one first-trimester abortion and no children, 72,052 (18.2%) had no abortions and at least one childbirth, 13,540 (3.4%) had at least one abortion and at least one childbirth, and 293,511 (74.1%) had neither an abortion nor a childbirth.
Of 30,834 women who had an abortion and filled at least one antidepressant prescription, 5,705 (18.5%) initiated antidepressant use after a first abortion. Of 85,592 women who gave birth and filled at least one antidepressant prescription, 10,825 (12.7%) initiated antidepressant use after a first childbirth.
The researchers were limited to 2-month increments of incidence rates for patient confidentiality reasons.
While women who had an abortion were more likely than women who had not to obtain an antidepressant prescription, “the rate of new antidepressant use was the same in the year before and year after and decreased with increasing time after the abortion,” the researchers wrote. “ after the abortion.”
The strongest risk factors for initiating antidepressant use were indicators for earlier mental health problems, such as a previous psychiatric contact. The authors speculated that mental health problems may lead women to have unintended pregnancies and therefore abortions, as Kelli Stidham Hall, PhD, and associates discussed (Soc Sci Med. 2014 Jan;0:62-71).
The combination of the absence of a postprocedure increase in antidepressant use, the gradual decrease in their use over time, and the lack of statistical significance of the incidence rate ratios when adjusted for risk factors led Dr. Steinberg and her coauthors to conclude that “compared with women who do not have an abortion, women who have an abortion may be at higher risk of depression after undergoing the procedure because they were at higher risk to begin with.”
Dr. Steinberg disclosed that she has served as a scientific expert on the topic of abortion and mental health in legal cases and has consulted for the Center for Reproductive Rights and Planned Parenthood Federation of America. The study was supported by grants from the Society of Family Planning, the American Foundation for Suicide Prevention, and the Lundbeck Foundation Initiative for Integrative Psychiatric Research.
SOURCE: Steinberg JR et al. JAMA Psychiatry. 2018 May 30. doi: 10.1001/jamapsychiatry.2018.0849.
The study by Julia R. Steinberg, PhD, and her associates adds to the body of evidence showing that abortion is not associated with an increased risk of mental disorders, wrote Nada L. Stotland, MD, and Angela D. Shrestha, MD, in an editorial accompanying the article in JAMA Psychiatry (2018 May 30. doi: 10.1001/jamapsychiatry.2018.0838).
“The authors report that, compared with women who had not had an abortion, women who had a first abortion had a higher risk of first-time antidepressant prescription,” Dr. Stotland and Dr. Shrestha wrote. “However, the risk of first-time antidepressant prescription was similar to the year before and after an abortion. Giving birth was associated with a lower likelihood of a prescription, but this likelihood rose after childbirth and continued to rise for 5 years after delivery.”
Dr. Stotland and Dr. Shrestha also praised the methodology used by Dr. Steinberg and her associates, particularly the decision to make pregnant women who choose to remain pregnant a control group. However, women who opt to continue their pregnancies are more likely to have supportive and healthy circumstances that influence them to make that decision, Dr. Stotland and Dr. Shrestha said.
The decision to have an abortion is fraught with complexities. Ultimately, clinicians must recognize that abortions are indeed significant events for women, and offer them both accurate information and nonjudgmental support, they wrote.
Dr. Stotland is affiliated with Rush University in Chicago and the University of Illinois at Chicago. Dr. Shrestha also is affiliated with the University of Illinois at Chicago. They reported no conflicts of interest.
The study by Julia R. Steinberg, PhD, and her associates adds to the body of evidence showing that abortion is not associated with an increased risk of mental disorders, wrote Nada L. Stotland, MD, and Angela D. Shrestha, MD, in an editorial accompanying the article in JAMA Psychiatry (2018 May 30. doi: 10.1001/jamapsychiatry.2018.0838).
“The authors report that, compared with women who had not had an abortion, women who had a first abortion had a higher risk of first-time antidepressant prescription,” Dr. Stotland and Dr. Shrestha wrote. “However, the risk of first-time antidepressant prescription was similar to the year before and after an abortion. Giving birth was associated with a lower likelihood of a prescription, but this likelihood rose after childbirth and continued to rise for 5 years after delivery.”
Dr. Stotland and Dr. Shrestha also praised the methodology used by Dr. Steinberg and her associates, particularly the decision to make pregnant women who choose to remain pregnant a control group. However, women who opt to continue their pregnancies are more likely to have supportive and healthy circumstances that influence them to make that decision, Dr. Stotland and Dr. Shrestha said.
The decision to have an abortion is fraught with complexities. Ultimately, clinicians must recognize that abortions are indeed significant events for women, and offer them both accurate information and nonjudgmental support, they wrote.
Dr. Stotland is affiliated with Rush University in Chicago and the University of Illinois at Chicago. Dr. Shrestha also is affiliated with the University of Illinois at Chicago. They reported no conflicts of interest.
The study by Julia R. Steinberg, PhD, and her associates adds to the body of evidence showing that abortion is not associated with an increased risk of mental disorders, wrote Nada L. Stotland, MD, and Angela D. Shrestha, MD, in an editorial accompanying the article in JAMA Psychiatry (2018 May 30. doi: 10.1001/jamapsychiatry.2018.0838).
“The authors report that, compared with women who had not had an abortion, women who had a first abortion had a higher risk of first-time antidepressant prescription,” Dr. Stotland and Dr. Shrestha wrote. “However, the risk of first-time antidepressant prescription was similar to the year before and after an abortion. Giving birth was associated with a lower likelihood of a prescription, but this likelihood rose after childbirth and continued to rise for 5 years after delivery.”
Dr. Stotland and Dr. Shrestha also praised the methodology used by Dr. Steinberg and her associates, particularly the decision to make pregnant women who choose to remain pregnant a control group. However, women who opt to continue their pregnancies are more likely to have supportive and healthy circumstances that influence them to make that decision, Dr. Stotland and Dr. Shrestha said.
The decision to have an abortion is fraught with complexities. Ultimately, clinicians must recognize that abortions are indeed significant events for women, and offer them both accurate information and nonjudgmental support, they wrote.
Dr. Stotland is affiliated with Rush University in Chicago and the University of Illinois at Chicago. Dr. Shrestha also is affiliated with the University of Illinois at Chicago. They reported no conflicts of interest.
Women who have had an abortion are more likely to use antidepressants than women who have not had an abortion, but new research suggests that this association is a result of differences in risk factors for depression.
“Thus, policies based on the notion that abortion harms women’s mental health may be misinformed,” wrote Julia R. Steinberg, PhD, and her coauthors in JAMA Psychiatry.
Dr. Steinberg and her coauthors looked at a cohort of Danish women, intending to examine the association between first-time antidepressant use and either first-trimester abortion or first childbirth. “One shortcoming of many studies in the field is their reliance on self-report of both abortion and mental health problems, which is subject to both faulty memory and social desirability in reporting,” wrote Dr. Steinberg of the University of Maryland, College Park, and her coauthors. Using data on abortion, childbirth, and antidepressants from the Danish population registries, which were collected over time, avoided that limitation.
Of the 396,397 women, 17,294 (4.4%) had at least one first-trimester abortion and no children, 72,052 (18.2%) had no abortions and at least one childbirth, 13,540 (3.4%) had at least one abortion and at least one childbirth, and 293,511 (74.1%) had neither an abortion nor a childbirth.
Of 30,834 women who had an abortion and filled at least one antidepressant prescription, 5,705 (18.5%) initiated antidepressant use after a first abortion. Of 85,592 women who gave birth and filled at least one antidepressant prescription, 10,825 (12.7%) initiated antidepressant use after a first childbirth.
The researchers were limited to 2-month increments of incidence rates for patient confidentiality reasons.
While women who had an abortion were more likely than women who had not to obtain an antidepressant prescription, “the rate of new antidepressant use was the same in the year before and year after and decreased with increasing time after the abortion,” the researchers wrote. “ after the abortion.”
The strongest risk factors for initiating antidepressant use were indicators for earlier mental health problems, such as a previous psychiatric contact. The authors speculated that mental health problems may lead women to have unintended pregnancies and therefore abortions, as Kelli Stidham Hall, PhD, and associates discussed (Soc Sci Med. 2014 Jan;0:62-71).
The combination of the absence of a postprocedure increase in antidepressant use, the gradual decrease in their use over time, and the lack of statistical significance of the incidence rate ratios when adjusted for risk factors led Dr. Steinberg and her coauthors to conclude that “compared with women who do not have an abortion, women who have an abortion may be at higher risk of depression after undergoing the procedure because they were at higher risk to begin with.”
Dr. Steinberg disclosed that she has served as a scientific expert on the topic of abortion and mental health in legal cases and has consulted for the Center for Reproductive Rights and Planned Parenthood Federation of America. The study was supported by grants from the Society of Family Planning, the American Foundation for Suicide Prevention, and the Lundbeck Foundation Initiative for Integrative Psychiatric Research.
SOURCE: Steinberg JR et al. JAMA Psychiatry. 2018 May 30. doi: 10.1001/jamapsychiatry.2018.0849.
Women who have had an abortion are more likely to use antidepressants than women who have not had an abortion, but new research suggests that this association is a result of differences in risk factors for depression.
“Thus, policies based on the notion that abortion harms women’s mental health may be misinformed,” wrote Julia R. Steinberg, PhD, and her coauthors in JAMA Psychiatry.
Dr. Steinberg and her coauthors looked at a cohort of Danish women, intending to examine the association between first-time antidepressant use and either first-trimester abortion or first childbirth. “One shortcoming of many studies in the field is their reliance on self-report of both abortion and mental health problems, which is subject to both faulty memory and social desirability in reporting,” wrote Dr. Steinberg of the University of Maryland, College Park, and her coauthors. Using data on abortion, childbirth, and antidepressants from the Danish population registries, which were collected over time, avoided that limitation.
Of the 396,397 women, 17,294 (4.4%) had at least one first-trimester abortion and no children, 72,052 (18.2%) had no abortions and at least one childbirth, 13,540 (3.4%) had at least one abortion and at least one childbirth, and 293,511 (74.1%) had neither an abortion nor a childbirth.
Of 30,834 women who had an abortion and filled at least one antidepressant prescription, 5,705 (18.5%) initiated antidepressant use after a first abortion. Of 85,592 women who gave birth and filled at least one antidepressant prescription, 10,825 (12.7%) initiated antidepressant use after a first childbirth.
The researchers were limited to 2-month increments of incidence rates for patient confidentiality reasons.
While women who had an abortion were more likely than women who had not to obtain an antidepressant prescription, “the rate of new antidepressant use was the same in the year before and year after and decreased with increasing time after the abortion,” the researchers wrote. “ after the abortion.”
The strongest risk factors for initiating antidepressant use were indicators for earlier mental health problems, such as a previous psychiatric contact. The authors speculated that mental health problems may lead women to have unintended pregnancies and therefore abortions, as Kelli Stidham Hall, PhD, and associates discussed (Soc Sci Med. 2014 Jan;0:62-71).
The combination of the absence of a postprocedure increase in antidepressant use, the gradual decrease in their use over time, and the lack of statistical significance of the incidence rate ratios when adjusted for risk factors led Dr. Steinberg and her coauthors to conclude that “compared with women who do not have an abortion, women who have an abortion may be at higher risk of depression after undergoing the procedure because they were at higher risk to begin with.”
Dr. Steinberg disclosed that she has served as a scientific expert on the topic of abortion and mental health in legal cases and has consulted for the Center for Reproductive Rights and Planned Parenthood Federation of America. The study was supported by grants from the Society of Family Planning, the American Foundation for Suicide Prevention, and the Lundbeck Foundation Initiative for Integrative Psychiatric Research.
SOURCE: Steinberg JR et al. JAMA Psychiatry. 2018 May 30. doi: 10.1001/jamapsychiatry.2018.0849.
FROM JAMA PSYCHIATRY
What to eat
Where did you learn about nutrition? Was it primarily at home supplemented by a few teachers as you navigated K through 12? Studies have shown that it probably wasn’t during medical school (“How much does your doctor actually know about nutrition?” American Heart Association News, April 30, 2018). A survey of one-third of medicals schools done in 1985 found “inadequate exposure to nutrition,” which prompted the National Academy of Sciences to recommend a minimum of 25 classroom hours. A more recent survey in 2013 discovered that 71% percent of medical schools fail to meet that benchmark.
I certainly don’t recall receiving any teaching in medical school that was specifically targeted at nutrition. And to be perfectly honest I never felt that I had missed anything. It’s not that I don’t believe nutrition is important. What we eat joins exercise and sleep at the core of a healthy lifestyle. The problem is that I was never confident that I or anyone else knew what a healthy diet should be. I learned what happened if child didn’t eat enough fruits and vegetables or consume enough vitamin D. But the tide seemed to keep going in and out on how much of each category of food was optimal. What was the perfect nutritional pyramid? And then there was the whole apparent flip-flop on eggs. For myself, I tried to follow the old dictum “everything in moderation ... including moderation.”
Don’t misunderstand me. I think dietitians have a critical role in health maintenance and disease management and should be on the forefront of our efforts to seek the causes of those medical conditions that have yet to be fully explained. It would be a mistake to recommend a low-salt diet to a patient without encouraging him or her (and the family) to consult with a dietitian. However, is having a medical students spend an afternoon in a kitchen preparing a low-salt diet a worthwhile investment of 4 precious hours of their educational time? It sounds cool, and at the end of the day, the student will certainly have a better understanding of how difficult his dietary recommendations will be to follow. But if the student ends up being a pediatrician, how often will he look back on the kitchen experience as a positive?
Giving specific and detailed instruction on how to shop for and prepare a medically prescribed diet can be very time consuming, and it can’t be done well without close follow-up that might even include a home visit or two. In some practices, the best option is to have a dietitian on the team.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Where did you learn about nutrition? Was it primarily at home supplemented by a few teachers as you navigated K through 12? Studies have shown that it probably wasn’t during medical school (“How much does your doctor actually know about nutrition?” American Heart Association News, April 30, 2018). A survey of one-third of medicals schools done in 1985 found “inadequate exposure to nutrition,” which prompted the National Academy of Sciences to recommend a minimum of 25 classroom hours. A more recent survey in 2013 discovered that 71% percent of medical schools fail to meet that benchmark.
I certainly don’t recall receiving any teaching in medical school that was specifically targeted at nutrition. And to be perfectly honest I never felt that I had missed anything. It’s not that I don’t believe nutrition is important. What we eat joins exercise and sleep at the core of a healthy lifestyle. The problem is that I was never confident that I or anyone else knew what a healthy diet should be. I learned what happened if child didn’t eat enough fruits and vegetables or consume enough vitamin D. But the tide seemed to keep going in and out on how much of each category of food was optimal. What was the perfect nutritional pyramid? And then there was the whole apparent flip-flop on eggs. For myself, I tried to follow the old dictum “everything in moderation ... including moderation.”
Don’t misunderstand me. I think dietitians have a critical role in health maintenance and disease management and should be on the forefront of our efforts to seek the causes of those medical conditions that have yet to be fully explained. It would be a mistake to recommend a low-salt diet to a patient without encouraging him or her (and the family) to consult with a dietitian. However, is having a medical students spend an afternoon in a kitchen preparing a low-salt diet a worthwhile investment of 4 precious hours of their educational time? It sounds cool, and at the end of the day, the student will certainly have a better understanding of how difficult his dietary recommendations will be to follow. But if the student ends up being a pediatrician, how often will he look back on the kitchen experience as a positive?
Giving specific and detailed instruction on how to shop for and prepare a medically prescribed diet can be very time consuming, and it can’t be done well without close follow-up that might even include a home visit or two. In some practices, the best option is to have a dietitian on the team.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Where did you learn about nutrition? Was it primarily at home supplemented by a few teachers as you navigated K through 12? Studies have shown that it probably wasn’t during medical school (“How much does your doctor actually know about nutrition?” American Heart Association News, April 30, 2018). A survey of one-third of medicals schools done in 1985 found “inadequate exposure to nutrition,” which prompted the National Academy of Sciences to recommend a minimum of 25 classroom hours. A more recent survey in 2013 discovered that 71% percent of medical schools fail to meet that benchmark.
I certainly don’t recall receiving any teaching in medical school that was specifically targeted at nutrition. And to be perfectly honest I never felt that I had missed anything. It’s not that I don’t believe nutrition is important. What we eat joins exercise and sleep at the core of a healthy lifestyle. The problem is that I was never confident that I or anyone else knew what a healthy diet should be. I learned what happened if child didn’t eat enough fruits and vegetables or consume enough vitamin D. But the tide seemed to keep going in and out on how much of each category of food was optimal. What was the perfect nutritional pyramid? And then there was the whole apparent flip-flop on eggs. For myself, I tried to follow the old dictum “everything in moderation ... including moderation.”
Don’t misunderstand me. I think dietitians have a critical role in health maintenance and disease management and should be on the forefront of our efforts to seek the causes of those medical conditions that have yet to be fully explained. It would be a mistake to recommend a low-salt diet to a patient without encouraging him or her (and the family) to consult with a dietitian. However, is having a medical students spend an afternoon in a kitchen preparing a low-salt diet a worthwhile investment of 4 precious hours of their educational time? It sounds cool, and at the end of the day, the student will certainly have a better understanding of how difficult his dietary recommendations will be to follow. But if the student ends up being a pediatrician, how often will he look back on the kitchen experience as a positive?
Giving specific and detailed instruction on how to shop for and prepare a medically prescribed diet can be very time consuming, and it can’t be done well without close follow-up that might even include a home visit or two. In some practices, the best option is to have a dietitian on the team.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
It’s not about time
Like most couples of retirement age, rituals dominate our breakfasts. I eat eggs. Marilyn leans toward baked goods. We each have a bowl of fruit and finish by working the New York Times mini-crossword on our electronic devices. Solving it usually takes somewhere between 40 seconds and 4 minutes. The challenge lies in how fast one can complete the puzzle. And, being who we are, Marilyn and I have ritualized this into a serious competition. She usually takes the first turn and then tries to psyche me out by announcing, “I did it in 2:34, but you should be able to solve it in less than 2 minutes.” This bit of gamesmanship often means that I am going to start the day with thin layer of nervous perspiration.
The claimed disabilities range from an anxiety disorder and ADHD to a problem with reading comprehension. The number of students requesting a test environment modification at Pomona College, Claremont, Calif., is 22% up from 5% in 2014. At Marlboro College in Vermont, one in three students asks for more time or a less distracting setting.
This phenomenon raises two obvious questions. First, what has happened to the bell-shaped curve? Was it too boring hanging out with all those people under the bell? Do folks feel safer and more secure in the tails? I guess we have to be happy that young people are less afraid to admit they are different. But it does make one wonder how we should go about defining a disability.
The second question is whether timed tests deserve a place in our educational toolbox? How often is processing speed important? I would like the woman piloting my flight to San Francisco to be quick-witted. But what about the research chemist working on a more durable tire compound? Is it a problem that it took him 30% longer than his classmates to successfully finish his college statistics final exam?
What about the lawyer who bills you $500 per hour to review the contract with your employer? It might have been helpful to know before you hired him that he routinely requested an extra hour and a half to complete his exams in law school. But I suspect that for the most part timed tests probably don’t produce better graduates. In the past they may have been used to thin oversubscribed disciplines, and certainly time limits have been the norm at every level of education I encountered. However, the best taught courses had exams with an abundance of time. Either you knew the information or you didn’t. An extra 2 hours wasn’t going to make a difference.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Like most couples of retirement age, rituals dominate our breakfasts. I eat eggs. Marilyn leans toward baked goods. We each have a bowl of fruit and finish by working the New York Times mini-crossword on our electronic devices. Solving it usually takes somewhere between 40 seconds and 4 minutes. The challenge lies in how fast one can complete the puzzle. And, being who we are, Marilyn and I have ritualized this into a serious competition. She usually takes the first turn and then tries to psyche me out by announcing, “I did it in 2:34, but you should be able to solve it in less than 2 minutes.” This bit of gamesmanship often means that I am going to start the day with thin layer of nervous perspiration.
The claimed disabilities range from an anxiety disorder and ADHD to a problem with reading comprehension. The number of students requesting a test environment modification at Pomona College, Claremont, Calif., is 22% up from 5% in 2014. At Marlboro College in Vermont, one in three students asks for more time or a less distracting setting.
This phenomenon raises two obvious questions. First, what has happened to the bell-shaped curve? Was it too boring hanging out with all those people under the bell? Do folks feel safer and more secure in the tails? I guess we have to be happy that young people are less afraid to admit they are different. But it does make one wonder how we should go about defining a disability.
The second question is whether timed tests deserve a place in our educational toolbox? How often is processing speed important? I would like the woman piloting my flight to San Francisco to be quick-witted. But what about the research chemist working on a more durable tire compound? Is it a problem that it took him 30% longer than his classmates to successfully finish his college statistics final exam?
What about the lawyer who bills you $500 per hour to review the contract with your employer? It might have been helpful to know before you hired him that he routinely requested an extra hour and a half to complete his exams in law school. But I suspect that for the most part timed tests probably don’t produce better graduates. In the past they may have been used to thin oversubscribed disciplines, and certainly time limits have been the norm at every level of education I encountered. However, the best taught courses had exams with an abundance of time. Either you knew the information or you didn’t. An extra 2 hours wasn’t going to make a difference.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Like most couples of retirement age, rituals dominate our breakfasts. I eat eggs. Marilyn leans toward baked goods. We each have a bowl of fruit and finish by working the New York Times mini-crossword on our electronic devices. Solving it usually takes somewhere between 40 seconds and 4 minutes. The challenge lies in how fast one can complete the puzzle. And, being who we are, Marilyn and I have ritualized this into a serious competition. She usually takes the first turn and then tries to psyche me out by announcing, “I did it in 2:34, but you should be able to solve it in less than 2 minutes.” This bit of gamesmanship often means that I am going to start the day with thin layer of nervous perspiration.
The claimed disabilities range from an anxiety disorder and ADHD to a problem with reading comprehension. The number of students requesting a test environment modification at Pomona College, Claremont, Calif., is 22% up from 5% in 2014. At Marlboro College in Vermont, one in three students asks for more time or a less distracting setting.
This phenomenon raises two obvious questions. First, what has happened to the bell-shaped curve? Was it too boring hanging out with all those people under the bell? Do folks feel safer and more secure in the tails? I guess we have to be happy that young people are less afraid to admit they are different. But it does make one wonder how we should go about defining a disability.
The second question is whether timed tests deserve a place in our educational toolbox? How often is processing speed important? I would like the woman piloting my flight to San Francisco to be quick-witted. But what about the research chemist working on a more durable tire compound? Is it a problem that it took him 30% longer than his classmates to successfully finish his college statistics final exam?
What about the lawyer who bills you $500 per hour to review the contract with your employer? It might have been helpful to know before you hired him that he routinely requested an extra hour and a half to complete his exams in law school. But I suspect that for the most part timed tests probably don’t produce better graduates. In the past they may have been used to thin oversubscribed disciplines, and certainly time limits have been the norm at every level of education I encountered. However, the best taught courses had exams with an abundance of time. Either you knew the information or you didn’t. An extra 2 hours wasn’t going to make a difference.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Pediatric Dermatology Consult - June 2018
The patient was diagnosed with granuloma annulare on the basis of history and clinical exam. A potassium hydroxide prep of skin scrapings was performed to rule out tinea corporis, and did not show evidence of fungal elements. The patient was treated with topical betamethasone with partial improvement.
First described as a “ringed eruption of the fingers” by Thomas Colcott Fox in 1895, granuloma annulare (GA) is a relatively common, benign, and self-limited condition whose precise etiology remains unclear. It is characterized commonly by pink to violaceous aciform or annular plaques on clinical examination. In some cases of GA, annular lesions are not present, or may be formed of grouped papules.
GA is characterized histologically by patchy interstitial lymphocytes and histiocytes palisading around mucin. Deep GA, an unusual subtype observed only in children, features a fibrin rather than a mucin core. This granulomatous picture is consistent with a Th1-mediated inflammatory process, and indeed, macrophage tumor necrosis factor production, as well as interleukin-2 and interferon-gamma production have been observed in GA. The reason for this exaggerated Th1 response is unknown, although in susceptible individuals trauma3 (an example of the Koebner phenomenon), arthropod assault,4 and herpes simplex infection5 (an example of Wolf isotopic response) all have been observed to trigger localized and/or generalized GA. Generalized GA has been associated with hyperlipidemia and the human leukocyte antigen–BW35 allele. GA has been described as a paraneoplastic eruption; atypical features such as associated pain or appearance in an uncharacteristic location often are present in such cases.6,7
Diagnosis of typical GA is clinical. If unusual features make you suspect tinea, leprosy, mycosis fungoides, or other annular lesions, then biopsy showing features typical of GA can reveal the correct diagnosis. Biopsy also can help to distinguish papular GA from warts or molluscum contagiosum. If extensive GA are present, then serum lipid testing for hypercholesterolemia or hypertriglyceridemia should be considered.
Other annular and raised lesions are on the differential for GA, but careful attention to the patient’s history and examination can clarify the diagnosis. Urticaria multiforme, a variant of annular urticaria, presents with numerous annular and polycyclic wheals, sometimes with central darkening that may be mistaken for necrosis. This patient did not present with polycyclic wheals. Furthermore, the lesions in urticaria multiforme are typically transient, with individual lesions lasting less than 24 hours, which was not the case with this patient. Wells syndrome, also known as eosinophilic cellulitis, is a condition marked by recurrent episodes of pruritus followed by appearance of edematous, painful, indurated, or edematous papules or plaques, although bullae and vesicles also may be present. The face and extremities are frequently involved and spontaneous resolution typically occurs in 2 months. Annular lesions are possible but papules, plaques, and nodules are more common in Wells syndrome. Annular elastolytic giant cell granuloma (AEGCG), also known as actinic granuloma and Miescher granuloma of the face, is an entity characterized clinically by chronic, persistent, sun-distributed, annular plaques typically seen in older women with significant sun damage. AEGCG is considered by some to be a variant of GA, but if this is the case, it is a distinct subtype with different epidemiologic, clinical, and histopathologic characteristics from GA. Interstitial granulomatous dermatitis is histologically and clinically distinct from GA, presenting as subtly erythematous cords or extensive annular or serpiginous plaques in the axilla, groin, buttocks, or chest, typically in adult patients with rheumatoid arthritis, reactive arthritis, psoriatic arthritis, or ankylosing spondylitis. Tinea corporis, the clinical manifestation of cutaneous dermatophyte infection, may be mistaken for granuloma annulare. However, tinea corporis lesions are scaly, whereas GA does not scale. Histologic examination of tinea corporis reveals hyphae, which are not present in GA.
GA is a relatively common, idiopathic, benign skin disease with numerous annular and papular mimics. Absence of scale, pain, and significant pruritus are important clues to the diagnosis, and biopsy can be helpful when the diagnosis is unclear. Treatment, although not necessary, may be offered using any of a number of modalities. The most consistent and effective healer of GA, however, is time.
References
1. J Am Acad Dermatol. 1980 Sep;3(3):217-30.
2. J Am Acad Dermatol. 2016 Sep;75(3):457-65.
3. Dermatol Online J. 2013 Dec 16;19(12):20719.
4. Acta Derm Venereol. 2008;88(5):519-20.
5. J Cutan Med Surg. 2014 Nov;18(6):413-9.
6. South Med J. 1997 Oct;90(10):1056-9.
7. Am J Dermatopathol. 2003 Apr;25(2):113-6.
8. Am J Clin Dermatol. 2013 Aug;14(4):279-90.
9. Br J Dermatol. 1994 Apr;130(4):494-7.
The patient was diagnosed with granuloma annulare on the basis of history and clinical exam. A potassium hydroxide prep of skin scrapings was performed to rule out tinea corporis, and did not show evidence of fungal elements. The patient was treated with topical betamethasone with partial improvement.
First described as a “ringed eruption of the fingers” by Thomas Colcott Fox in 1895, granuloma annulare (GA) is a relatively common, benign, and self-limited condition whose precise etiology remains unclear. It is characterized commonly by pink to violaceous aciform or annular plaques on clinical examination. In some cases of GA, annular lesions are not present, or may be formed of grouped papules.
GA is characterized histologically by patchy interstitial lymphocytes and histiocytes palisading around mucin. Deep GA, an unusual subtype observed only in children, features a fibrin rather than a mucin core. This granulomatous picture is consistent with a Th1-mediated inflammatory process, and indeed, macrophage tumor necrosis factor production, as well as interleukin-2 and interferon-gamma production have been observed in GA. The reason for this exaggerated Th1 response is unknown, although in susceptible individuals trauma3 (an example of the Koebner phenomenon), arthropod assault,4 and herpes simplex infection5 (an example of Wolf isotopic response) all have been observed to trigger localized and/or generalized GA. Generalized GA has been associated with hyperlipidemia and the human leukocyte antigen–BW35 allele. GA has been described as a paraneoplastic eruption; atypical features such as associated pain or appearance in an uncharacteristic location often are present in such cases.6,7
Diagnosis of typical GA is clinical. If unusual features make you suspect tinea, leprosy, mycosis fungoides, or other annular lesions, then biopsy showing features typical of GA can reveal the correct diagnosis. Biopsy also can help to distinguish papular GA from warts or molluscum contagiosum. If extensive GA are present, then serum lipid testing for hypercholesterolemia or hypertriglyceridemia should be considered.
Other annular and raised lesions are on the differential for GA, but careful attention to the patient’s history and examination can clarify the diagnosis. Urticaria multiforme, a variant of annular urticaria, presents with numerous annular and polycyclic wheals, sometimes with central darkening that may be mistaken for necrosis. This patient did not present with polycyclic wheals. Furthermore, the lesions in urticaria multiforme are typically transient, with individual lesions lasting less than 24 hours, which was not the case with this patient. Wells syndrome, also known as eosinophilic cellulitis, is a condition marked by recurrent episodes of pruritus followed by appearance of edematous, painful, indurated, or edematous papules or plaques, although bullae and vesicles also may be present. The face and extremities are frequently involved and spontaneous resolution typically occurs in 2 months. Annular lesions are possible but papules, plaques, and nodules are more common in Wells syndrome. Annular elastolytic giant cell granuloma (AEGCG), also known as actinic granuloma and Miescher granuloma of the face, is an entity characterized clinically by chronic, persistent, sun-distributed, annular plaques typically seen in older women with significant sun damage. AEGCG is considered by some to be a variant of GA, but if this is the case, it is a distinct subtype with different epidemiologic, clinical, and histopathologic characteristics from GA. Interstitial granulomatous dermatitis is histologically and clinically distinct from GA, presenting as subtly erythematous cords or extensive annular or serpiginous plaques in the axilla, groin, buttocks, or chest, typically in adult patients with rheumatoid arthritis, reactive arthritis, psoriatic arthritis, or ankylosing spondylitis. Tinea corporis, the clinical manifestation of cutaneous dermatophyte infection, may be mistaken for granuloma annulare. However, tinea corporis lesions are scaly, whereas GA does not scale. Histologic examination of tinea corporis reveals hyphae, which are not present in GA.
GA is a relatively common, idiopathic, benign skin disease with numerous annular and papular mimics. Absence of scale, pain, and significant pruritus are important clues to the diagnosis, and biopsy can be helpful when the diagnosis is unclear. Treatment, although not necessary, may be offered using any of a number of modalities. The most consistent and effective healer of GA, however, is time.
References
1. J Am Acad Dermatol. 1980 Sep;3(3):217-30.
2. J Am Acad Dermatol. 2016 Sep;75(3):457-65.
3. Dermatol Online J. 2013 Dec 16;19(12):20719.
4. Acta Derm Venereol. 2008;88(5):519-20.
5. J Cutan Med Surg. 2014 Nov;18(6):413-9.
6. South Med J. 1997 Oct;90(10):1056-9.
7. Am J Dermatopathol. 2003 Apr;25(2):113-6.
8. Am J Clin Dermatol. 2013 Aug;14(4):279-90.
9. Br J Dermatol. 1994 Apr;130(4):494-7.
The patient was diagnosed with granuloma annulare on the basis of history and clinical exam. A potassium hydroxide prep of skin scrapings was performed to rule out tinea corporis, and did not show evidence of fungal elements. The patient was treated with topical betamethasone with partial improvement.
First described as a “ringed eruption of the fingers” by Thomas Colcott Fox in 1895, granuloma annulare (GA) is a relatively common, benign, and self-limited condition whose precise etiology remains unclear. It is characterized commonly by pink to violaceous aciform or annular plaques on clinical examination. In some cases of GA, annular lesions are not present, or may be formed of grouped papules.
GA is characterized histologically by patchy interstitial lymphocytes and histiocytes palisading around mucin. Deep GA, an unusual subtype observed only in children, features a fibrin rather than a mucin core. This granulomatous picture is consistent with a Th1-mediated inflammatory process, and indeed, macrophage tumor necrosis factor production, as well as interleukin-2 and interferon-gamma production have been observed in GA. The reason for this exaggerated Th1 response is unknown, although in susceptible individuals trauma3 (an example of the Koebner phenomenon), arthropod assault,4 and herpes simplex infection5 (an example of Wolf isotopic response) all have been observed to trigger localized and/or generalized GA. Generalized GA has been associated with hyperlipidemia and the human leukocyte antigen–BW35 allele. GA has been described as a paraneoplastic eruption; atypical features such as associated pain or appearance in an uncharacteristic location often are present in such cases.6,7
Diagnosis of typical GA is clinical. If unusual features make you suspect tinea, leprosy, mycosis fungoides, or other annular lesions, then biopsy showing features typical of GA can reveal the correct diagnosis. Biopsy also can help to distinguish papular GA from warts or molluscum contagiosum. If extensive GA are present, then serum lipid testing for hypercholesterolemia or hypertriglyceridemia should be considered.
Other annular and raised lesions are on the differential for GA, but careful attention to the patient’s history and examination can clarify the diagnosis. Urticaria multiforme, a variant of annular urticaria, presents with numerous annular and polycyclic wheals, sometimes with central darkening that may be mistaken for necrosis. This patient did not present with polycyclic wheals. Furthermore, the lesions in urticaria multiforme are typically transient, with individual lesions lasting less than 24 hours, which was not the case with this patient. Wells syndrome, also known as eosinophilic cellulitis, is a condition marked by recurrent episodes of pruritus followed by appearance of edematous, painful, indurated, or edematous papules or plaques, although bullae and vesicles also may be present. The face and extremities are frequently involved and spontaneous resolution typically occurs in 2 months. Annular lesions are possible but papules, plaques, and nodules are more common in Wells syndrome. Annular elastolytic giant cell granuloma (AEGCG), also known as actinic granuloma and Miescher granuloma of the face, is an entity characterized clinically by chronic, persistent, sun-distributed, annular plaques typically seen in older women with significant sun damage. AEGCG is considered by some to be a variant of GA, but if this is the case, it is a distinct subtype with different epidemiologic, clinical, and histopathologic characteristics from GA. Interstitial granulomatous dermatitis is histologically and clinically distinct from GA, presenting as subtly erythematous cords or extensive annular or serpiginous plaques in the axilla, groin, buttocks, or chest, typically in adult patients with rheumatoid arthritis, reactive arthritis, psoriatic arthritis, or ankylosing spondylitis. Tinea corporis, the clinical manifestation of cutaneous dermatophyte infection, may be mistaken for granuloma annulare. However, tinea corporis lesions are scaly, whereas GA does not scale. Histologic examination of tinea corporis reveals hyphae, which are not present in GA.
GA is a relatively common, idiopathic, benign skin disease with numerous annular and papular mimics. Absence of scale, pain, and significant pruritus are important clues to the diagnosis, and biopsy can be helpful when the diagnosis is unclear. Treatment, although not necessary, may be offered using any of a number of modalities. The most consistent and effective healer of GA, however, is time.
References
1. J Am Acad Dermatol. 1980 Sep;3(3):217-30.
2. J Am Acad Dermatol. 2016 Sep;75(3):457-65.
3. Dermatol Online J. 2013 Dec 16;19(12):20719.
4. Acta Derm Venereol. 2008;88(5):519-20.
5. J Cutan Med Surg. 2014 Nov;18(6):413-9.
6. South Med J. 1997 Oct;90(10):1056-9.
7. Am J Dermatopathol. 2003 Apr;25(2):113-6.
8. Am J Clin Dermatol. 2013 Aug;14(4):279-90.
9. Br J Dermatol. 1994 Apr;130(4):494-7.
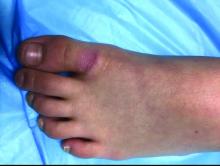
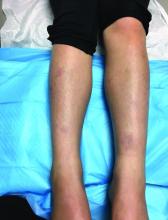
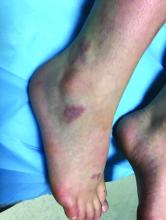
A 9-year-old girl presented to the dermatology clinic, referred by her pediatrician, for evaluation of asymptomatic lesions on her shins and feet for 2 months. She started developing one lesion over her right shin with other lesions appearing on the opposite leg a few weeks after, and treated the areas initially with an over-the-counter antifungal cream without improvement. She has been healthy and denied any recent fevers or upper respiratory infections, and said she had not taken any medications or vitamin supplements. She reported camping with her father occasionally but denied any bug or tick bites. No other family members were affected. There was no personal or family history of diabetes mellitus or high cholesterol, and there are no pets at home.
VIDEO: PML prevention is possible, even when treating patients with aggressive MS
NASHVILLE, TENN. – Armed with new statistics, neurologist Joseph R. Berger, MD, has a message for colleagues about the widely feared risk of progressive multifocal leukoencephalopathy (PML) in multiple sclerosis: It’s not as inevitable as you might think.
“You can actually prevent this disease from occurring because we have risk-limiting strategies in many circumstances,” said Dr. Berger of the University of Pennsylvania, Philadelphia, in a presentation on PML at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Unlike other conditions such as HIV, MS itself is not linked to a higher risk of PML, said Dr. Berger, a leading PML researcher. Instead, it’s the medications that spark the condition, he said, with at least three and possibly four drugs posing a risk to patients.
Natalizumab (Tysabri) is especially risky. “We know that the risk with natalizumab is incredibly high in the context of JC [John Cunningham] virus antibody positivity and prolonged therapy,” Dr. Berger said in an interview after his presentation.
Still, “you can safely give natalizumab for a short period of time when treating patients with aggressive MS,” he said. “I will frequently employ that strategy even in the context of JC virus antibody positivity.”
According to Dr. Berger, there’s no risk of PML when natalizumab is used for under 8 months (Mult Scler Relat Disord. 2017 Feb;12:59-63).
However, “if you leave people on the drug indefinitely, there is a substantial risk of developing PML,” he said. “Individuals who have been left on the drug for 2 years, who’ve seen prior immunosuppressant therapy, who are JC virus antibody positive – that group of individuals develops PML at rates of 1 in 50 to 1 in 100.”
These levels are “enormous,” he said, higher even than those in the HIV population before the rise of antiretroviral medications.
Overall, as of Nov. 30, 2017, 177,800 patients have received natalizumab in the postmarketing setting, and 756 cases of PML have been reported as of Dec. 7, 2017. All but three of those cases were in patients with MS, and the overall incidence was 4.19/1,000.
Dr. Berger recommends regular screening MRIs for PML in patients taking natalizumab, and he advised physicians to be on alert for signs of trouble like the appearance of new neurologic symptoms or a new or increasing JC virus antibody index.
Two other MS drugs, fingolimod (Gilenya) and dimethyl fumarate (Tecfidera), fall into the category of low risk, with just 19 and 5 reported cases, respectively, as of February 2018, Dr. Berger said. He added that two of the fingolimod patients had earlier exposure to natalizumab.
With dimethyl fumarate, the risk appears to disappear – although this isn’t confirmed – when JC antibody–positive patients are taken off the drug, and their lymphocyte counts fall below 500 per mcL, Dr. Berger said.
“Unfortunately for fingolimod, we don’t have a defined risk-mitigation strategy,” he said. However, researchers have noticed that the fingolimod cases have occurred more often in older people, possibly because of the aging of the immune system, he said.
Another three MS drugs – alemtuzumab (Lemtrada), ocrelizumab (Ocrevus; with rituximab as proxy), and teriflunomide (Aubagio; with leflunomide as proxy) have unknown risk, according to Dr. Berger. There have been three cases in ocrelizumab (rituximab as proxy) and one in teriflunomide (leflunomide as proxy), but all were carry-overs from natalizumab or fingolimod exposure or occurred after natalizumab exposure.
What can physicians do if a patient develops PML? Stopping the drug and restoring the immune system is crucial, he said.
While there’s evidence that plasma exchange clears natalizumab (Neurology. 2009 Feb 3;72[5]:402-9), “there’s no study that demonstrates it’s in the patient’s best interest,” Dr. Berger said during his presentation. He noted that a retrospective study found no improvement in morbidity or mortality (Neurology. 2017 Mar 21;88[12];1144-52).
Multiple strategies to treat PML – including immunizations and inhibitors of DNA replication – have failed to make an impact so far, Dr. Berger said. According to him, the reasons for the failure of PML treatment are a lack of hard evidence, apart from anecdotal, to support them, based on a history of failed clinical trials.
Dr. Berger disclosed serving as a consultant for numerous pharmaceutical companies.
NASHVILLE, TENN. – Armed with new statistics, neurologist Joseph R. Berger, MD, has a message for colleagues about the widely feared risk of progressive multifocal leukoencephalopathy (PML) in multiple sclerosis: It’s not as inevitable as you might think.
“You can actually prevent this disease from occurring because we have risk-limiting strategies in many circumstances,” said Dr. Berger of the University of Pennsylvania, Philadelphia, in a presentation on PML at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Unlike other conditions such as HIV, MS itself is not linked to a higher risk of PML, said Dr. Berger, a leading PML researcher. Instead, it’s the medications that spark the condition, he said, with at least three and possibly four drugs posing a risk to patients.
Natalizumab (Tysabri) is especially risky. “We know that the risk with natalizumab is incredibly high in the context of JC [John Cunningham] virus antibody positivity and prolonged therapy,” Dr. Berger said in an interview after his presentation.
Still, “you can safely give natalizumab for a short period of time when treating patients with aggressive MS,” he said. “I will frequently employ that strategy even in the context of JC virus antibody positivity.”
According to Dr. Berger, there’s no risk of PML when natalizumab is used for under 8 months (Mult Scler Relat Disord. 2017 Feb;12:59-63).
However, “if you leave people on the drug indefinitely, there is a substantial risk of developing PML,” he said. “Individuals who have been left on the drug for 2 years, who’ve seen prior immunosuppressant therapy, who are JC virus antibody positive – that group of individuals develops PML at rates of 1 in 50 to 1 in 100.”
These levels are “enormous,” he said, higher even than those in the HIV population before the rise of antiretroviral medications.
Overall, as of Nov. 30, 2017, 177,800 patients have received natalizumab in the postmarketing setting, and 756 cases of PML have been reported as of Dec. 7, 2017. All but three of those cases were in patients with MS, and the overall incidence was 4.19/1,000.
Dr. Berger recommends regular screening MRIs for PML in patients taking natalizumab, and he advised physicians to be on alert for signs of trouble like the appearance of new neurologic symptoms or a new or increasing JC virus antibody index.
Two other MS drugs, fingolimod (Gilenya) and dimethyl fumarate (Tecfidera), fall into the category of low risk, with just 19 and 5 reported cases, respectively, as of February 2018, Dr. Berger said. He added that two of the fingolimod patients had earlier exposure to natalizumab.
With dimethyl fumarate, the risk appears to disappear – although this isn’t confirmed – when JC antibody–positive patients are taken off the drug, and their lymphocyte counts fall below 500 per mcL, Dr. Berger said.
“Unfortunately for fingolimod, we don’t have a defined risk-mitigation strategy,” he said. However, researchers have noticed that the fingolimod cases have occurred more often in older people, possibly because of the aging of the immune system, he said.
Another three MS drugs – alemtuzumab (Lemtrada), ocrelizumab (Ocrevus; with rituximab as proxy), and teriflunomide (Aubagio; with leflunomide as proxy) have unknown risk, according to Dr. Berger. There have been three cases in ocrelizumab (rituximab as proxy) and one in teriflunomide (leflunomide as proxy), but all were carry-overs from natalizumab or fingolimod exposure or occurred after natalizumab exposure.
What can physicians do if a patient develops PML? Stopping the drug and restoring the immune system is crucial, he said.
While there’s evidence that plasma exchange clears natalizumab (Neurology. 2009 Feb 3;72[5]:402-9), “there’s no study that demonstrates it’s in the patient’s best interest,” Dr. Berger said during his presentation. He noted that a retrospective study found no improvement in morbidity or mortality (Neurology. 2017 Mar 21;88[12];1144-52).
Multiple strategies to treat PML – including immunizations and inhibitors of DNA replication – have failed to make an impact so far, Dr. Berger said. According to him, the reasons for the failure of PML treatment are a lack of hard evidence, apart from anecdotal, to support them, based on a history of failed clinical trials.
Dr. Berger disclosed serving as a consultant for numerous pharmaceutical companies.
NASHVILLE, TENN. – Armed with new statistics, neurologist Joseph R. Berger, MD, has a message for colleagues about the widely feared risk of progressive multifocal leukoencephalopathy (PML) in multiple sclerosis: It’s not as inevitable as you might think.
“You can actually prevent this disease from occurring because we have risk-limiting strategies in many circumstances,” said Dr. Berger of the University of Pennsylvania, Philadelphia, in a presentation on PML at the annual meeting of the Consortium of Multiple Sclerosis Centers.
Unlike other conditions such as HIV, MS itself is not linked to a higher risk of PML, said Dr. Berger, a leading PML researcher. Instead, it’s the medications that spark the condition, he said, with at least three and possibly four drugs posing a risk to patients.
Natalizumab (Tysabri) is especially risky. “We know that the risk with natalizumab is incredibly high in the context of JC [John Cunningham] virus antibody positivity and prolonged therapy,” Dr. Berger said in an interview after his presentation.
Still, “you can safely give natalizumab for a short period of time when treating patients with aggressive MS,” he said. “I will frequently employ that strategy even in the context of JC virus antibody positivity.”
According to Dr. Berger, there’s no risk of PML when natalizumab is used for under 8 months (Mult Scler Relat Disord. 2017 Feb;12:59-63).
However, “if you leave people on the drug indefinitely, there is a substantial risk of developing PML,” he said. “Individuals who have been left on the drug for 2 years, who’ve seen prior immunosuppressant therapy, who are JC virus antibody positive – that group of individuals develops PML at rates of 1 in 50 to 1 in 100.”
These levels are “enormous,” he said, higher even than those in the HIV population before the rise of antiretroviral medications.
Overall, as of Nov. 30, 2017, 177,800 patients have received natalizumab in the postmarketing setting, and 756 cases of PML have been reported as of Dec. 7, 2017. All but three of those cases were in patients with MS, and the overall incidence was 4.19/1,000.
Dr. Berger recommends regular screening MRIs for PML in patients taking natalizumab, and he advised physicians to be on alert for signs of trouble like the appearance of new neurologic symptoms or a new or increasing JC virus antibody index.
Two other MS drugs, fingolimod (Gilenya) and dimethyl fumarate (Tecfidera), fall into the category of low risk, with just 19 and 5 reported cases, respectively, as of February 2018, Dr. Berger said. He added that two of the fingolimod patients had earlier exposure to natalizumab.
With dimethyl fumarate, the risk appears to disappear – although this isn’t confirmed – when JC antibody–positive patients are taken off the drug, and their lymphocyte counts fall below 500 per mcL, Dr. Berger said.
“Unfortunately for fingolimod, we don’t have a defined risk-mitigation strategy,” he said. However, researchers have noticed that the fingolimod cases have occurred more often in older people, possibly because of the aging of the immune system, he said.
Another three MS drugs – alemtuzumab (Lemtrada), ocrelizumab (Ocrevus; with rituximab as proxy), and teriflunomide (Aubagio; with leflunomide as proxy) have unknown risk, according to Dr. Berger. There have been three cases in ocrelizumab (rituximab as proxy) and one in teriflunomide (leflunomide as proxy), but all were carry-overs from natalizumab or fingolimod exposure or occurred after natalizumab exposure.
What can physicians do if a patient develops PML? Stopping the drug and restoring the immune system is crucial, he said.
While there’s evidence that plasma exchange clears natalizumab (Neurology. 2009 Feb 3;72[5]:402-9), “there’s no study that demonstrates it’s in the patient’s best interest,” Dr. Berger said during his presentation. He noted that a retrospective study found no improvement in morbidity or mortality (Neurology. 2017 Mar 21;88[12];1144-52).
Multiple strategies to treat PML – including immunizations and inhibitors of DNA replication – have failed to make an impact so far, Dr. Berger said. According to him, the reasons for the failure of PML treatment are a lack of hard evidence, apart from anecdotal, to support them, based on a history of failed clinical trials.
Dr. Berger disclosed serving as a consultant for numerous pharmaceutical companies.
REPORTING FROM THE CMSC ANNUAL MEETING
Prior knee injury osteoarthritis ‘distinct subgroup’
LIVERPOOL, ENGLAND – according to data from the Good Life with osteoArthritis in Denmark (GLA:D®) Registry.
Clear differences in the clinical presentation can be observed between patients with knee OA who have and have not had a previous knee injury, researcher Paetur Mikal Holm reported at the World Congress on Osteoarthritis.
“These differences include being younger, being a male, having a lower BMI [body mass index], and being more physically active; also, we see reduced quality of life, longer symptom duration, and widespread pain” said Mr. Holm, a PhD fellow in the department of sports science and clinical biomechanics, musculoskeletal function, and physiotherapy at the University of Southern Denmark in Odense. “The last two characteristics in particular – widespread pain and symptom duration – may have an important impact on treatment,” he added.
“People with a prior knee injury have a 25% higher probability of having widespread pain compared to people without a prior knee injury,” Mr. Holm and his associates said in their abstract. This could mean that specialized interventions are needed to address pain in these individuals, especially when there is a long duration of symptoms.
Disease characteristics and trajectories in knee osteoarthritis are highly variable, Mr. Holm observed, and there are multiple phenotypes already reported. Posttraumatic knee OA is one of these phenotypes, but, until now, their clinical characteristics were poorly understood.
A cross-sectional study was performed using baseline data on 5,477 individuals enrolled in the GLA:D® Registry, 91% of whom had radiographic knee OA. These patients were divided into two groups based on whether they did (n = 2,440) or did not (n = 3,037) self-report a prior knee injury. This is one of the limitations of the study, Mr. Holm acknowledged. There was no specific investigation of what the prior knee injury actually was.
However, results clearly showed a significant (P less than .05) difference between the two groups. For example, the mean age of those with a prior knee injury was 64 years, whereas those without were about 65 years old (odds ratio, 0.99; 95% confidence interval, 0.98-0.99). About 29% of those with a previous knee injury were men, versus roughly 25% of those without (OR, 1.28; 95% CI, 1.12-1.46). Widespread pain was present in about 45% and 38%, respectively, for those with and without a prior knee injury (OR, 1.25; 95% CI, 1.11-1.40), and the mean duration of symptoms was 60 versus 35 months (OR, 1.05; 95% CI, 1.04-1.06). Those who reported exercising 2-4 days a week had an OR of 1.30 (95% CI, 1.05-1.59).
“Being a clinician, to me it is a worry to see that these people are young and have had symptoms for a long time,” coauthor Ewa M. Roos, PT, PhD, said at the congress, which was sponsored by the Osteoarthritis Research Society International.
“Here is a group of people that we don’t necessarily think about as athletes, these are people with osteoarthritis. They are seen in primary care, but maybe we don’t think about them as osteoarthritis patients at a younger age, but this is a group that we need to be very careful to recognize,” added Dr. Roos, also of University of Southern Denmark. She cochaired the session where the findings were presented.
The GLA:D® Registry is funded by various sources including the Danish Rheumatism Association, Naestved-Slagelse-Ringsted Hospitals, Region Zealand, and the Danish Physiotherapy Association. Dr. Holm had no conflicts of interest.
SOURCE: Holm PM et al. Osteoarthr Cartil. 2018:26(1):S56.OARSI 2018 Abstract 84.
LIVERPOOL, ENGLAND – according to data from the Good Life with osteoArthritis in Denmark (GLA:D®) Registry.
Clear differences in the clinical presentation can be observed between patients with knee OA who have and have not had a previous knee injury, researcher Paetur Mikal Holm reported at the World Congress on Osteoarthritis.
“These differences include being younger, being a male, having a lower BMI [body mass index], and being more physically active; also, we see reduced quality of life, longer symptom duration, and widespread pain” said Mr. Holm, a PhD fellow in the department of sports science and clinical biomechanics, musculoskeletal function, and physiotherapy at the University of Southern Denmark in Odense. “The last two characteristics in particular – widespread pain and symptom duration – may have an important impact on treatment,” he added.
“People with a prior knee injury have a 25% higher probability of having widespread pain compared to people without a prior knee injury,” Mr. Holm and his associates said in their abstract. This could mean that specialized interventions are needed to address pain in these individuals, especially when there is a long duration of symptoms.
Disease characteristics and trajectories in knee osteoarthritis are highly variable, Mr. Holm observed, and there are multiple phenotypes already reported. Posttraumatic knee OA is one of these phenotypes, but, until now, their clinical characteristics were poorly understood.
A cross-sectional study was performed using baseline data on 5,477 individuals enrolled in the GLA:D® Registry, 91% of whom had radiographic knee OA. These patients were divided into two groups based on whether they did (n = 2,440) or did not (n = 3,037) self-report a prior knee injury. This is one of the limitations of the study, Mr. Holm acknowledged. There was no specific investigation of what the prior knee injury actually was.
However, results clearly showed a significant (P less than .05) difference between the two groups. For example, the mean age of those with a prior knee injury was 64 years, whereas those without were about 65 years old (odds ratio, 0.99; 95% confidence interval, 0.98-0.99). About 29% of those with a previous knee injury were men, versus roughly 25% of those without (OR, 1.28; 95% CI, 1.12-1.46). Widespread pain was present in about 45% and 38%, respectively, for those with and without a prior knee injury (OR, 1.25; 95% CI, 1.11-1.40), and the mean duration of symptoms was 60 versus 35 months (OR, 1.05; 95% CI, 1.04-1.06). Those who reported exercising 2-4 days a week had an OR of 1.30 (95% CI, 1.05-1.59).
“Being a clinician, to me it is a worry to see that these people are young and have had symptoms for a long time,” coauthor Ewa M. Roos, PT, PhD, said at the congress, which was sponsored by the Osteoarthritis Research Society International.
“Here is a group of people that we don’t necessarily think about as athletes, these are people with osteoarthritis. They are seen in primary care, but maybe we don’t think about them as osteoarthritis patients at a younger age, but this is a group that we need to be very careful to recognize,” added Dr. Roos, also of University of Southern Denmark. She cochaired the session where the findings were presented.
The GLA:D® Registry is funded by various sources including the Danish Rheumatism Association, Naestved-Slagelse-Ringsted Hospitals, Region Zealand, and the Danish Physiotherapy Association. Dr. Holm had no conflicts of interest.
SOURCE: Holm PM et al. Osteoarthr Cartil. 2018:26(1):S56.OARSI 2018 Abstract 84.
LIVERPOOL, ENGLAND – according to data from the Good Life with osteoArthritis in Denmark (GLA:D®) Registry.
Clear differences in the clinical presentation can be observed between patients with knee OA who have and have not had a previous knee injury, researcher Paetur Mikal Holm reported at the World Congress on Osteoarthritis.
“These differences include being younger, being a male, having a lower BMI [body mass index], and being more physically active; also, we see reduced quality of life, longer symptom duration, and widespread pain” said Mr. Holm, a PhD fellow in the department of sports science and clinical biomechanics, musculoskeletal function, and physiotherapy at the University of Southern Denmark in Odense. “The last two characteristics in particular – widespread pain and symptom duration – may have an important impact on treatment,” he added.
“People with a prior knee injury have a 25% higher probability of having widespread pain compared to people without a prior knee injury,” Mr. Holm and his associates said in their abstract. This could mean that specialized interventions are needed to address pain in these individuals, especially when there is a long duration of symptoms.
Disease characteristics and trajectories in knee osteoarthritis are highly variable, Mr. Holm observed, and there are multiple phenotypes already reported. Posttraumatic knee OA is one of these phenotypes, but, until now, their clinical characteristics were poorly understood.
A cross-sectional study was performed using baseline data on 5,477 individuals enrolled in the GLA:D® Registry, 91% of whom had radiographic knee OA. These patients were divided into two groups based on whether they did (n = 2,440) or did not (n = 3,037) self-report a prior knee injury. This is one of the limitations of the study, Mr. Holm acknowledged. There was no specific investigation of what the prior knee injury actually was.
However, results clearly showed a significant (P less than .05) difference between the two groups. For example, the mean age of those with a prior knee injury was 64 years, whereas those without were about 65 years old (odds ratio, 0.99; 95% confidence interval, 0.98-0.99). About 29% of those with a previous knee injury were men, versus roughly 25% of those without (OR, 1.28; 95% CI, 1.12-1.46). Widespread pain was present in about 45% and 38%, respectively, for those with and without a prior knee injury (OR, 1.25; 95% CI, 1.11-1.40), and the mean duration of symptoms was 60 versus 35 months (OR, 1.05; 95% CI, 1.04-1.06). Those who reported exercising 2-4 days a week had an OR of 1.30 (95% CI, 1.05-1.59).
“Being a clinician, to me it is a worry to see that these people are young and have had symptoms for a long time,” coauthor Ewa M. Roos, PT, PhD, said at the congress, which was sponsored by the Osteoarthritis Research Society International.
“Here is a group of people that we don’t necessarily think about as athletes, these are people with osteoarthritis. They are seen in primary care, but maybe we don’t think about them as osteoarthritis patients at a younger age, but this is a group that we need to be very careful to recognize,” added Dr. Roos, also of University of Southern Denmark. She cochaired the session where the findings were presented.
The GLA:D® Registry is funded by various sources including the Danish Rheumatism Association, Naestved-Slagelse-Ringsted Hospitals, Region Zealand, and the Danish Physiotherapy Association. Dr. Holm had no conflicts of interest.
SOURCE: Holm PM et al. Osteoarthr Cartil. 2018:26(1):S56.OARSI 2018 Abstract 84.
REPORTING FROM OARSI 2018
Key clinical point: People with posttraumatic knee osteoarthritis are a distinct subgroup of patients that might be underrecognized.
Major finding: Widespread pain was present in about 45% and 38%, respectively, of those with and without a prior knee injury (OR, 1.25, 95% CI, 1.11-1.40).
Study details: Cross-sectional study of 5,477 patients enrolled in the Good Life with osteoArthritis in Denmark (GLA:D®) Registry.
Disclosures: The GLA:D® Registry is funded by various sources including the Danish Rheumatism Association, Naestved-Slagelse-Ringsted Hospitals, Region Zealand, and the Danish Physiotherapy Association. The presenting author, Dr. Paetur Mikal Holm, had no conflicts of interest.
Source: Holm PM et al. Osteoarthr Cartil. 2018:26(1):S56. Abstract 84.
Neonatal deaths lower in high-volume hospitals
AUSTIN, TEX. – A first look at the timing of neonatal deaths showed an association with weekend deliveries in one Texas county. However, birth weight and ethnicity attenuated the association, according to a recent study. Higher hospital volumes were associated with lower risk of neonatal deaths.
The retrospective, population-based cohort study, presented during the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, used data from birth certificates and infant death certificates in the state of Texas. The investigators, said Elizabeth Restrepo, PhD, chose to examine data from Tarrant County, Tex., which has historically had persistently high infant mortality rates; in 2013, she said, the infant mortality rate in that county was 7.11/1,000 births – the highest in the state for that year.
The first question Dr. Restrepo and her colleagues at Texas Women’s University, Denton, wanted to answer was whether there was an association between the risk of neonatal mortality and the day of the week of the birth. For this and the study’s other research questions, she and her colleagues looked at 2012 data, matching 32,140 birth certificate records with 92 infant death certificates.
The investigators found an independent association between the risk of neonatal death and whether the birth happened on a weekday (Monday at 7:00 a.m. through Friday at 6:59 p.m.), or on a weekend (Friday at 7:00 p.m. through Monday at 6:59 a.m.). However, once birth weight and ethnicity were controlled in the statistical analysis, the association was not statistically significant despite an odds ratio of 1.44 (95% confidence interval, 0.911-2.27; P = .119).
“Births in the 12 hospitals studied appear to have been organized to take place more frequently on the working weekday rather than weekend days,” wrote Dr. Restrepo and her colleagues in the poster accompanying the presentation. Although the study wasn’t designed to answer this particular question, Dr. Restrepo said in discussion during the poster session that planned deliveries, such as inductions and cesarean deliveries, are likely to happen during the week, while the case mix is wider on weekends. Patient characteristics, as well as staffing patterns, may come into play.
The researchers also asked whether birth volume at a given institution increases the odds of neonatal death on weekends. Here, they found a significant inverse relationship between hospital birth volume and neonatal deaths (r = –0.021; P less than .001). With each additional increase of 1% in the weekday birth rate, the odds of neonatal death dropped by approximately 7.4%.
Examining the Tarrant County data further, Dr. Restrepo and her colleagues found that the hospitals with higher birth volumes had a more even distribution of births across the days of the week, with resulting lower concentrations of births during the week (r = –.394; P less than .001).
To classify infant deaths, the investigators included only ICD-10 diagnoses classified as P-codes to capture deaths occurring in the first 28 days after birth, but excluding congenital problems that are incompatible with life or that usually cause early death.
The researchers reported that they had no conflicts of interest; the study was funded by a research enhancement program award from the Texas Women’s University Office of Research and Sponsored Programs.
SOURCE: Restrepo E et al. ACOG 2018, Abstract 22R.
AUSTIN, TEX. – A first look at the timing of neonatal deaths showed an association with weekend deliveries in one Texas county. However, birth weight and ethnicity attenuated the association, according to a recent study. Higher hospital volumes were associated with lower risk of neonatal deaths.
The retrospective, population-based cohort study, presented during the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, used data from birth certificates and infant death certificates in the state of Texas. The investigators, said Elizabeth Restrepo, PhD, chose to examine data from Tarrant County, Tex., which has historically had persistently high infant mortality rates; in 2013, she said, the infant mortality rate in that county was 7.11/1,000 births – the highest in the state for that year.
The first question Dr. Restrepo and her colleagues at Texas Women’s University, Denton, wanted to answer was whether there was an association between the risk of neonatal mortality and the day of the week of the birth. For this and the study’s other research questions, she and her colleagues looked at 2012 data, matching 32,140 birth certificate records with 92 infant death certificates.
The investigators found an independent association between the risk of neonatal death and whether the birth happened on a weekday (Monday at 7:00 a.m. through Friday at 6:59 p.m.), or on a weekend (Friday at 7:00 p.m. through Monday at 6:59 a.m.). However, once birth weight and ethnicity were controlled in the statistical analysis, the association was not statistically significant despite an odds ratio of 1.44 (95% confidence interval, 0.911-2.27; P = .119).
“Births in the 12 hospitals studied appear to have been organized to take place more frequently on the working weekday rather than weekend days,” wrote Dr. Restrepo and her colleagues in the poster accompanying the presentation. Although the study wasn’t designed to answer this particular question, Dr. Restrepo said in discussion during the poster session that planned deliveries, such as inductions and cesarean deliveries, are likely to happen during the week, while the case mix is wider on weekends. Patient characteristics, as well as staffing patterns, may come into play.
The researchers also asked whether birth volume at a given institution increases the odds of neonatal death on weekends. Here, they found a significant inverse relationship between hospital birth volume and neonatal deaths (r = –0.021; P less than .001). With each additional increase of 1% in the weekday birth rate, the odds of neonatal death dropped by approximately 7.4%.
Examining the Tarrant County data further, Dr. Restrepo and her colleagues found that the hospitals with higher birth volumes had a more even distribution of births across the days of the week, with resulting lower concentrations of births during the week (r = –.394; P less than .001).
To classify infant deaths, the investigators included only ICD-10 diagnoses classified as P-codes to capture deaths occurring in the first 28 days after birth, but excluding congenital problems that are incompatible with life or that usually cause early death.
The researchers reported that they had no conflicts of interest; the study was funded by a research enhancement program award from the Texas Women’s University Office of Research and Sponsored Programs.
SOURCE: Restrepo E et al. ACOG 2018, Abstract 22R.
AUSTIN, TEX. – A first look at the timing of neonatal deaths showed an association with weekend deliveries in one Texas county. However, birth weight and ethnicity attenuated the association, according to a recent study. Higher hospital volumes were associated with lower risk of neonatal deaths.
The retrospective, population-based cohort study, presented during the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, used data from birth certificates and infant death certificates in the state of Texas. The investigators, said Elizabeth Restrepo, PhD, chose to examine data from Tarrant County, Tex., which has historically had persistently high infant mortality rates; in 2013, she said, the infant mortality rate in that county was 7.11/1,000 births – the highest in the state for that year.
The first question Dr. Restrepo and her colleagues at Texas Women’s University, Denton, wanted to answer was whether there was an association between the risk of neonatal mortality and the day of the week of the birth. For this and the study’s other research questions, she and her colleagues looked at 2012 data, matching 32,140 birth certificate records with 92 infant death certificates.
The investigators found an independent association between the risk of neonatal death and whether the birth happened on a weekday (Monday at 7:00 a.m. through Friday at 6:59 p.m.), or on a weekend (Friday at 7:00 p.m. through Monday at 6:59 a.m.). However, once birth weight and ethnicity were controlled in the statistical analysis, the association was not statistically significant despite an odds ratio of 1.44 (95% confidence interval, 0.911-2.27; P = .119).
“Births in the 12 hospitals studied appear to have been organized to take place more frequently on the working weekday rather than weekend days,” wrote Dr. Restrepo and her colleagues in the poster accompanying the presentation. Although the study wasn’t designed to answer this particular question, Dr. Restrepo said in discussion during the poster session that planned deliveries, such as inductions and cesarean deliveries, are likely to happen during the week, while the case mix is wider on weekends. Patient characteristics, as well as staffing patterns, may come into play.
The researchers also asked whether birth volume at a given institution increases the odds of neonatal death on weekends. Here, they found a significant inverse relationship between hospital birth volume and neonatal deaths (r = –0.021; P less than .001). With each additional increase of 1% in the weekday birth rate, the odds of neonatal death dropped by approximately 7.4%.
Examining the Tarrant County data further, Dr. Restrepo and her colleagues found that the hospitals with higher birth volumes had a more even distribution of births across the days of the week, with resulting lower concentrations of births during the week (r = –.394; P less than .001).
To classify infant deaths, the investigators included only ICD-10 diagnoses classified as P-codes to capture deaths occurring in the first 28 days after birth, but excluding congenital problems that are incompatible with life or that usually cause early death.
The researchers reported that they had no conflicts of interest; the study was funded by a research enhancement program award from the Texas Women’s University Office of Research and Sponsored Programs.
SOURCE: Restrepo E et al. ACOG 2018, Abstract 22R.
REPORTING FROM ACOG 2018
Key clinical point: Neonatal deaths were lower in hospitals with higher delivery volumes.
Major finding: Higher weekday birth volumes were associated with lower risk of neonatal death (P = .002).
Study details: Retrospective cohort study of 92 neonatal deaths in a single Texas county in 2012.
Disclosures: The study was funded by Texas Women’s University. The authors reported that they had no relevant disclosures.
Source: Restrepo E et al. ACOG 2018, Abstract 22R.
Esketamine nasal spray prevails in two phase 3 trials
The combination of an esketamine nasal spray and an oral antidepressant may provide additional benefits for patients with treatment-resistant major depressive disorder, new research suggested.
Two posters at a meeting of the American Society of Clinical Psychopharmacology, formerly known as the New Clinical Drug Evaluation Unit meeting, presented data from two phase 3 studies on the safety and efficacy of an esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression.
The first study – a double-blind, randomized withdrawal study – enrolled 705 patients with recurrent or single episode major depressive disorder who were either enrolled directly or after completing the double-blind phase of an acute, short-term study.
Patients began with a 16-week induction phase of esketamine nasal spray and oral antidepressant, then patients were randomized either to a placebo nasal spray or esketamine nasal spray – plus oral antidepressant – for the maintenance phase.
Researchers saw a significantly higher rate of relapse among patients randomized to the placebo nasal spray, compared with those randomized to the esketamine nasal spray. In patients classified as stable remitters at the start of the maintenance period, 26.7% of those who received the esketamine nasal spray experienced a relapse, compared with 45.3% of those who received the placebo nasal spray.
Among the stable responders, 25.8% of those who received esketamine experienced a relapse, compared with 57.6% of those who received the placebo nasal spray.
This represented a 51% lower risk of relapse with esketamine among stable remitters and a 70% lower risk of relapse among stable responders, compared with placebo. Treatment with esketamine also significantly delayed relapse, compared with placebo.
Most adverse events were mild to moderate, but six patients experienced serious adverse events that were possibly related to the study drug, including disorientation, hypothermia, lacunar stroke, sedation, and suicidal ideation, during the induction phase of the study. However on review, the study sponsor argued that the lacunar stroke and hypothermia were unlikely to be linked to the study medication.
Four patients in the esketamine group and three patients in the placebo group discontinued the nasal spray during the maintenance phase.
“The study provides support for a positive benefit-risk evaluation for treatment with esketamine plus an oral antidepressant and provides further safety data regarding longer-term, intermittent dosing frequency treatment,” wrote Ella J. Daly, MD, from Janssen Research & Development – which also funded the study – and coauthors.
The researchers also noted that, overall, cognitive performance remained stable or improved after long-term, intermittent treatment with the esketamine nasal spray plus antidepressant.
The second study – an open-label, international study – involved 802 patients with major depressive disorder who had all failed to respond to at least two oral antidepressants. They were treated with an esketamine nasal spray (28 mg, 56 mg, or 84 mg) in combination with a new oral antidepressant.
The study involved a screening phase, induction phase, and optimization/maintenance phase, but only 24.9% of patients completed the optimization/maintenance phase. However, this was enough to meet a predefined total patient exposure, and the study was terminated by the sponsor.
More than three-quarters of patients responded during the induction phase, and by the end of the induction phase, 47.2% of patients had achieved remission. By the end of the optimization/maintenance phase, 58.2% of patients who entered that phase achieved remission.
More than 90% of patients experienced at least one adverse event, although most were mild to moderate. There were two deaths during the optimization/maintenance phase – one from acute respiratory and cardiac failure and one from suicide – but neither was considered as being related to the esketamine.
Five serious adverse events – anxiety, delusion, delirium, suicidal ideation, and suicidal attempt – were judged by the investigator to be related to the esketamine.
Researchers also saw no declines in performance on multiple cognitive domains, including visual learning and memory, across the entire study.
“The adverse event profile following an up to 1 year of exposure was consistent with previous observations of esketamine in the completed short-term phase 2 and 3 studies, and no unexpected safety findings were reported,” wrote Ewa Wajs, MD, also with Janssen, and coauthors.
Both studies were funded by Janssen Research & Development. Of the authors in the first study, 12 were employees of Janssen, and 10 declared funding from the pharmaceutical industry, including Janssen. Of the authors in the second study, 11 were employees of Janssen, and four declared a range of funding from the pharmaceutical industry.
SOURCE: Daly EJ et al. ASCP 2018, Poster W68. Wajs E et al. ASCP 2018, Poster T67.
The combination of an esketamine nasal spray and an oral antidepressant may provide additional benefits for patients with treatment-resistant major depressive disorder, new research suggested.
Two posters at a meeting of the American Society of Clinical Psychopharmacology, formerly known as the New Clinical Drug Evaluation Unit meeting, presented data from two phase 3 studies on the safety and efficacy of an esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression.
The first study – a double-blind, randomized withdrawal study – enrolled 705 patients with recurrent or single episode major depressive disorder who were either enrolled directly or after completing the double-blind phase of an acute, short-term study.
Patients began with a 16-week induction phase of esketamine nasal spray and oral antidepressant, then patients were randomized either to a placebo nasal spray or esketamine nasal spray – plus oral antidepressant – for the maintenance phase.
Researchers saw a significantly higher rate of relapse among patients randomized to the placebo nasal spray, compared with those randomized to the esketamine nasal spray. In patients classified as stable remitters at the start of the maintenance period, 26.7% of those who received the esketamine nasal spray experienced a relapse, compared with 45.3% of those who received the placebo nasal spray.
Among the stable responders, 25.8% of those who received esketamine experienced a relapse, compared with 57.6% of those who received the placebo nasal spray.
This represented a 51% lower risk of relapse with esketamine among stable remitters and a 70% lower risk of relapse among stable responders, compared with placebo. Treatment with esketamine also significantly delayed relapse, compared with placebo.
Most adverse events were mild to moderate, but six patients experienced serious adverse events that were possibly related to the study drug, including disorientation, hypothermia, lacunar stroke, sedation, and suicidal ideation, during the induction phase of the study. However on review, the study sponsor argued that the lacunar stroke and hypothermia were unlikely to be linked to the study medication.
Four patients in the esketamine group and three patients in the placebo group discontinued the nasal spray during the maintenance phase.
“The study provides support for a positive benefit-risk evaluation for treatment with esketamine plus an oral antidepressant and provides further safety data regarding longer-term, intermittent dosing frequency treatment,” wrote Ella J. Daly, MD, from Janssen Research & Development – which also funded the study – and coauthors.
The researchers also noted that, overall, cognitive performance remained stable or improved after long-term, intermittent treatment with the esketamine nasal spray plus antidepressant.
The second study – an open-label, international study – involved 802 patients with major depressive disorder who had all failed to respond to at least two oral antidepressants. They were treated with an esketamine nasal spray (28 mg, 56 mg, or 84 mg) in combination with a new oral antidepressant.
The study involved a screening phase, induction phase, and optimization/maintenance phase, but only 24.9% of patients completed the optimization/maintenance phase. However, this was enough to meet a predefined total patient exposure, and the study was terminated by the sponsor.
More than three-quarters of patients responded during the induction phase, and by the end of the induction phase, 47.2% of patients had achieved remission. By the end of the optimization/maintenance phase, 58.2% of patients who entered that phase achieved remission.
More than 90% of patients experienced at least one adverse event, although most were mild to moderate. There were two deaths during the optimization/maintenance phase – one from acute respiratory and cardiac failure and one from suicide – but neither was considered as being related to the esketamine.
Five serious adverse events – anxiety, delusion, delirium, suicidal ideation, and suicidal attempt – were judged by the investigator to be related to the esketamine.
Researchers also saw no declines in performance on multiple cognitive domains, including visual learning and memory, across the entire study.
“The adverse event profile following an up to 1 year of exposure was consistent with previous observations of esketamine in the completed short-term phase 2 and 3 studies, and no unexpected safety findings were reported,” wrote Ewa Wajs, MD, also with Janssen, and coauthors.
Both studies were funded by Janssen Research & Development. Of the authors in the first study, 12 were employees of Janssen, and 10 declared funding from the pharmaceutical industry, including Janssen. Of the authors in the second study, 11 were employees of Janssen, and four declared a range of funding from the pharmaceutical industry.
SOURCE: Daly EJ et al. ASCP 2018, Poster W68. Wajs E et al. ASCP 2018, Poster T67.
The combination of an esketamine nasal spray and an oral antidepressant may provide additional benefits for patients with treatment-resistant major depressive disorder, new research suggested.
Two posters at a meeting of the American Society of Clinical Psychopharmacology, formerly known as the New Clinical Drug Evaluation Unit meeting, presented data from two phase 3 studies on the safety and efficacy of an esketamine nasal spray plus oral antidepressant in patients with treatment-resistant depression.
The first study – a double-blind, randomized withdrawal study – enrolled 705 patients with recurrent or single episode major depressive disorder who were either enrolled directly or after completing the double-blind phase of an acute, short-term study.
Patients began with a 16-week induction phase of esketamine nasal spray and oral antidepressant, then patients were randomized either to a placebo nasal spray or esketamine nasal spray – plus oral antidepressant – for the maintenance phase.
Researchers saw a significantly higher rate of relapse among patients randomized to the placebo nasal spray, compared with those randomized to the esketamine nasal spray. In patients classified as stable remitters at the start of the maintenance period, 26.7% of those who received the esketamine nasal spray experienced a relapse, compared with 45.3% of those who received the placebo nasal spray.
Among the stable responders, 25.8% of those who received esketamine experienced a relapse, compared with 57.6% of those who received the placebo nasal spray.
This represented a 51% lower risk of relapse with esketamine among stable remitters and a 70% lower risk of relapse among stable responders, compared with placebo. Treatment with esketamine also significantly delayed relapse, compared with placebo.
Most adverse events were mild to moderate, but six patients experienced serious adverse events that were possibly related to the study drug, including disorientation, hypothermia, lacunar stroke, sedation, and suicidal ideation, during the induction phase of the study. However on review, the study sponsor argued that the lacunar stroke and hypothermia were unlikely to be linked to the study medication.
Four patients in the esketamine group and three patients in the placebo group discontinued the nasal spray during the maintenance phase.
“The study provides support for a positive benefit-risk evaluation for treatment with esketamine plus an oral antidepressant and provides further safety data regarding longer-term, intermittent dosing frequency treatment,” wrote Ella J. Daly, MD, from Janssen Research & Development – which also funded the study – and coauthors.
The researchers also noted that, overall, cognitive performance remained stable or improved after long-term, intermittent treatment with the esketamine nasal spray plus antidepressant.
The second study – an open-label, international study – involved 802 patients with major depressive disorder who had all failed to respond to at least two oral antidepressants. They were treated with an esketamine nasal spray (28 mg, 56 mg, or 84 mg) in combination with a new oral antidepressant.
The study involved a screening phase, induction phase, and optimization/maintenance phase, but only 24.9% of patients completed the optimization/maintenance phase. However, this was enough to meet a predefined total patient exposure, and the study was terminated by the sponsor.
More than three-quarters of patients responded during the induction phase, and by the end of the induction phase, 47.2% of patients had achieved remission. By the end of the optimization/maintenance phase, 58.2% of patients who entered that phase achieved remission.
More than 90% of patients experienced at least one adverse event, although most were mild to moderate. There were two deaths during the optimization/maintenance phase – one from acute respiratory and cardiac failure and one from suicide – but neither was considered as being related to the esketamine.
Five serious adverse events – anxiety, delusion, delirium, suicidal ideation, and suicidal attempt – were judged by the investigator to be related to the esketamine.
Researchers also saw no declines in performance on multiple cognitive domains, including visual learning and memory, across the entire study.
“The adverse event profile following an up to 1 year of exposure was consistent with previous observations of esketamine in the completed short-term phase 2 and 3 studies, and no unexpected safety findings were reported,” wrote Ewa Wajs, MD, also with Janssen, and coauthors.
Both studies were funded by Janssen Research & Development. Of the authors in the first study, 12 were employees of Janssen, and 10 declared funding from the pharmaceutical industry, including Janssen. Of the authors in the second study, 11 were employees of Janssen, and four declared a range of funding from the pharmaceutical industry.
SOURCE: Daly EJ et al. ASCP 2018, Poster W68. Wajs E et al. ASCP 2018, Poster T67.
FROM THE ASCP ANNUAL MEETING