User login
13 weeks' gestation • heart palpitations • chest tightness • Dx?
THE CASE
A 29-year-old G1P0 woman at 13 weeks’ gestation came in for a routine prenatal visit complaining of sudden-onset heart palpitations that were occurring about once a week. Each episode lasted between 15 and 60 minutes and was accompanied by chest tightness, with no identifiable cause. The patient could inconsistently terminate the episodes with Valsalva maneuvers. She reported having had 2 similar incidents of palpitations within the past year. Her family history was significant for sudden cardiac death of her father and paternal grandfather in their fifth decades of life.
A cardiovascular exam was normal; heart auscultation revealed a regular rate and rhythm without murmurs, rubs, or gallops, and the peripheral pulses were normal. A thyroid-stimulating hormone (TSH) level, basic metabolic panel (BMP), and complete blood count (CBC) were within normal limits. A transthoracic echocardiogram was negative for structural heart disease.
THE DIAGNOSIS
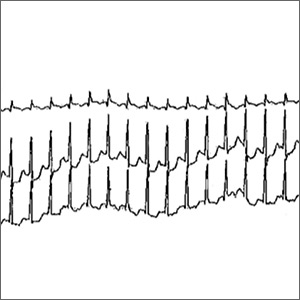
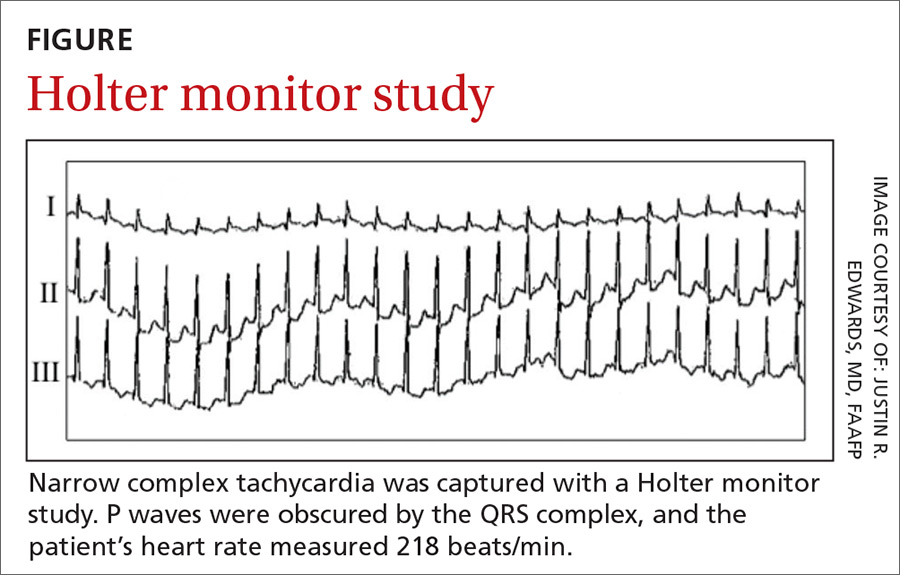
An initial Holter monitor study failed to capture an episode of her palpitations. The frequency of her palpitations increased as her pregnancy progressed, occurring almost daily by the second half of the third trimester, and a repeat Holter monitor study in the third trimester was significant for a 3-minute episode of supraventricular tachycardia (SVT) that correlated with patient-recorded symptoms (FIGURE).
Based on these results, we diagnosed the patient with an atrioventricular nodal reentry tachycardia (AVNRT). Although atrioventricular reciprocating tachycardia (AVRT) remained a remote possibility, it is far less common, and a 12-lead electrocardiogram (EKG) showed no evidence of pre-excitation.
DISCUSSION
AVNRT is the most common form of paroxysmal supraventricular tachycardia (PSVT). It occurs more frequently in women and typically manifests in the second to fourth decades of life.1 AVNRT is a narrow complex tachycardia characterized by a heart rate of 120 to >200 beats/min.
Hemodynamic changes in pregnancy can trigger arrhythmias
During pregnancy, hemodynamic changes (including increased blood volume and cardiac output) are thought to stimulate stretch-activated ion channels within the walls of the heart.2-4 Such changes may exacerbate previously existing cardiac arrhythmias or (less commonly) cause new-onset arrhythmias.3,4 A family history positive for arrhythmias or sudden cardiac death increases the likelihood of developing tachyarrhythmia during pregnancy.3 Women with a known history of PSVT might experience symptom exacerbation despite being on prophylactic therapy.4
Detection and diagnosis
While AVNRT is relatively benign in pregnancy, other cardiac arrhythmias (eg, atrial fibrillation/flutter, ventricular tachycardia) carry a greater risk for fetal and maternal complications, underscoring the need to correctly identify the type of arrhythmia.2,3
Continue to: Physical exam findings
Physical exam findings are often unremarkable unless the patient is actively experiencing SVT in the office, in which case prominent jugular pulsations may be seen due to simultaneous contraction of the atria and ventricles.
The initial evaluation of a pregnant patient presenting with tachycardia should include a BMP, TSH, 12-lead EKG, and transthoracic echocardiography.3,5 In most patients with AVNRT, the results of these tests will be normal. A Holter monitor can be used to document an arrhythmia if the episodes are relatively frequent or an event monitor can be used if the episodes are infrequent.5
EKG findings. When patients are actively experiencing SVT, EKG findings include a P wave obscured by the QRS complex, sometimes manifesting as a pseudo-R wave in the V1 lead and a pseudo-S wave in leads II, III, and AVF. The QRS complex is narrow and the R-R interval is regular.6
Types of treatment
Valsalva maneuvers. Treatment of AVNRT in pregnancy should first involve addressing any precipitating causes, including metabolic and endocrine abnormalities.3 As virtually all antiarrhythmic drugs cross the placenta and are traceable in breast milk,2,3 patients should be counseled to try to stop episodes using Valsalva maneuvers before moving to pharmacologic treatment.
Antiarrhythmics. First-line pharmacologic treatment for the prevention of AVNRT in pregnancy is metoprolol or verapamil.2,5 Neither drug has been associated with adverse outcomes in infants, although there is a large body of evidence suggesting that low levels of metoprolol are present in breast milk.7
Continue to: Acute episodes of SVT that are refractory to...
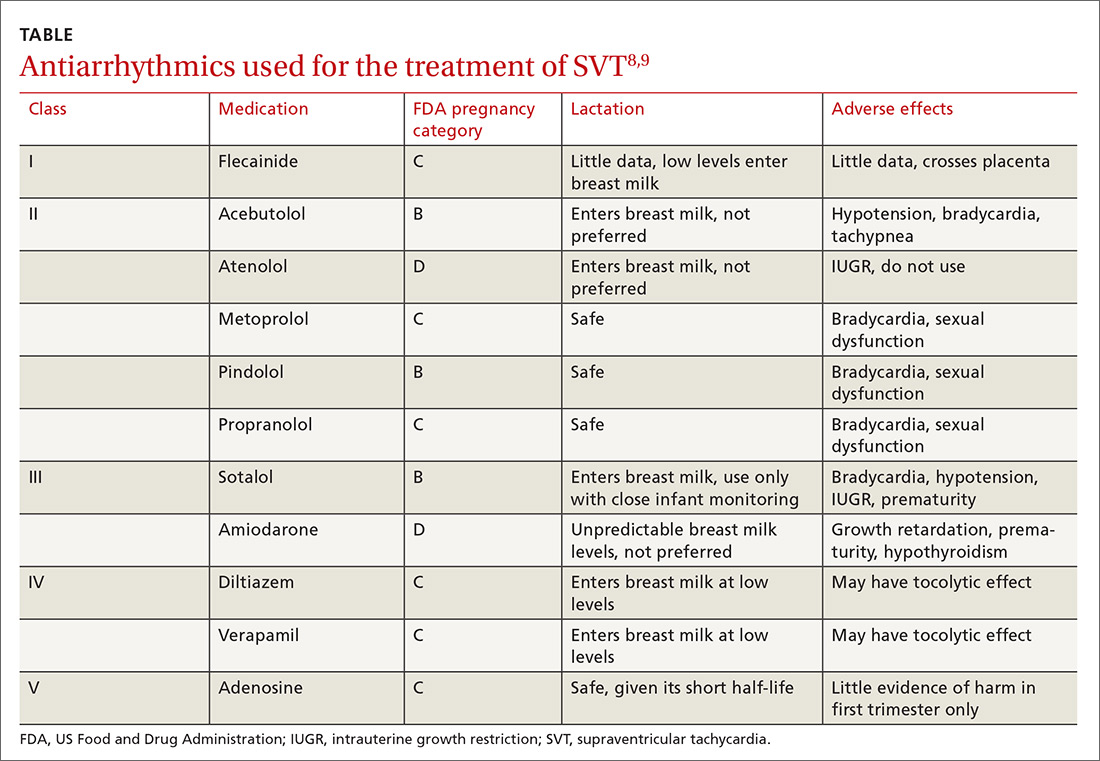
Acute episodes of SVT that are refractory to vagal maneuvers or occur despite medical management can be treated acutely in pregnancy with adenosine, which effectively stops episodes about 90% of the time.2 (See the TABLE8,9 for a list of antiarrhythmics that may be used to treat AVNRT.)
Catheter ablation is first-line treatment for AVNRT in nonpregnant patients.1,5 The risks of undergoing ablation during pregnancy include fetal exposure to radiation and anesthetic drugs.2,3 Therefore, this treatment should be used only when pharmacologic treatment is unsuccessful and risks to the mother and fetus due to the arrhythmia outweigh the risks of the procedure. Ablation can be offered postpartum as more definitive therapy.
Our patient was started on metoprolol tartrate 12.5 mg bid at 35 weeks’ gestation due to increasingly common and persistent palpitations. This helped control the episodes for 2 weeks, at which point they increased again in frequency. These were terminated using Valsalva maneuvers; increasing the metoprolol dosage was prohibitive due to patient intolerance.
Following an uncomplicated delivery, and discontinuation of metoprolol, the patient reported a decrease in both the number of episodes and the duration of SVT. Ultimately, she opted for a catheter ablation to prevent SVT exacerbation during subsequent pregnancies.
THE TAKEAWAY
AVNRT (and other tachyarrhythmias) may worsen or manifest with physiologic changes that occur during pregnancy. After establishing the diagnosis, effort should be made to manage the condition conservatively with Valsalva maneuvers and medication. Catheter ablation should be offered postpartum as a more definitive treatment option.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine Medical Director, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Kwaku KF, Josephson ME. Typical AVNRT—an update on mechanisms and therapy. Card Electrophysiol Rev. 2002;6:414-421.
2. Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961-967.
3. Knotts RJ, Garan H. Cardiac arrhythmias in pregnancy. Semin Perinatol. 2014;38:285-288.
4. Silversides CK, Harris L, Haberer K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tacharrhythmias and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206-1212.
5. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e471-e505.
6. Di Biase L, Gianni C, Bagliani G, et. al. Arrhythmias involving the atrioventricular junction. Card Electrophysiol Clin. 2017;9:435-452.
7. Fitzpatrick RB. LactMed: drugs and lactation database. J Electron Resour Med Libr. 2007;4:155.
8. Yaksh A, van der Does LJ, Lanters EA, et al. Pharmacological therapy of tachyarrhythmias during pregnancy. Arrhythm Electrophysiol Rev. 2016;5:41-44.
9. US National Library of Medicine. Drugs and lactation database (LactMed). Available at: toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed July 3, 2018.
THE CASE
A 29-year-old G1P0 woman at 13 weeks’ gestation came in for a routine prenatal visit complaining of sudden-onset heart palpitations that were occurring about once a week. Each episode lasted between 15 and 60 minutes and was accompanied by chest tightness, with no identifiable cause. The patient could inconsistently terminate the episodes with Valsalva maneuvers. She reported having had 2 similar incidents of palpitations within the past year. Her family history was significant for sudden cardiac death of her father and paternal grandfather in their fifth decades of life.
A cardiovascular exam was normal; heart auscultation revealed a regular rate and rhythm without murmurs, rubs, or gallops, and the peripheral pulses were normal. A thyroid-stimulating hormone (TSH) level, basic metabolic panel (BMP), and complete blood count (CBC) were within normal limits. A transthoracic echocardiogram was negative for structural heart disease.
THE DIAGNOSIS
An initial Holter monitor study failed to capture an episode of her palpitations. The frequency of her palpitations increased as her pregnancy progressed, occurring almost daily by the second half of the third trimester, and a repeat Holter monitor study in the third trimester was significant for a 3-minute episode of supraventricular tachycardia (SVT) that correlated with patient-recorded symptoms (FIGURE).

Based on these results, we diagnosed the patient with an atrioventricular nodal reentry tachycardia (AVNRT). Although atrioventricular reciprocating tachycardia (AVRT) remained a remote possibility, it is far less common, and a 12-lead electrocardiogram (EKG) showed no evidence of pre-excitation.
DISCUSSION
AVNRT is the most common form of paroxysmal supraventricular tachycardia (PSVT). It occurs more frequently in women and typically manifests in the second to fourth decades of life.1 AVNRT is a narrow complex tachycardia characterized by a heart rate of 120 to >200 beats/min.
Hemodynamic changes in pregnancy can trigger arrhythmias
During pregnancy, hemodynamic changes (including increased blood volume and cardiac output) are thought to stimulate stretch-activated ion channels within the walls of the heart.2-4 Such changes may exacerbate previously existing cardiac arrhythmias or (less commonly) cause new-onset arrhythmias.3,4 A family history positive for arrhythmias or sudden cardiac death increases the likelihood of developing tachyarrhythmia during pregnancy.3 Women with a known history of PSVT might experience symptom exacerbation despite being on prophylactic therapy.4
Detection and diagnosis
While AVNRT is relatively benign in pregnancy, other cardiac arrhythmias (eg, atrial fibrillation/flutter, ventricular tachycardia) carry a greater risk for fetal and maternal complications, underscoring the need to correctly identify the type of arrhythmia.2,3
Continue to: Physical exam findings
Physical exam findings are often unremarkable unless the patient is actively experiencing SVT in the office, in which case prominent jugular pulsations may be seen due to simultaneous contraction of the atria and ventricles.
The initial evaluation of a pregnant patient presenting with tachycardia should include a BMP, TSH, 12-lead EKG, and transthoracic echocardiography.3,5 In most patients with AVNRT, the results of these tests will be normal. A Holter monitor can be used to document an arrhythmia if the episodes are relatively frequent or an event monitor can be used if the episodes are infrequent.5
EKG findings. When patients are actively experiencing SVT, EKG findings include a P wave obscured by the QRS complex, sometimes manifesting as a pseudo-R wave in the V1 lead and a pseudo-S wave in leads II, III, and AVF. The QRS complex is narrow and the R-R interval is regular.6
Types of treatment
Valsalva maneuvers. Treatment of AVNRT in pregnancy should first involve addressing any precipitating causes, including metabolic and endocrine abnormalities.3 As virtually all antiarrhythmic drugs cross the placenta and are traceable in breast milk,2,3 patients should be counseled to try to stop episodes using Valsalva maneuvers before moving to pharmacologic treatment.
Antiarrhythmics. First-line pharmacologic treatment for the prevention of AVNRT in pregnancy is metoprolol or verapamil.2,5 Neither drug has been associated with adverse outcomes in infants, although there is a large body of evidence suggesting that low levels of metoprolol are present in breast milk.7
Continue to: Acute episodes of SVT that are refractory to...
Acute episodes of SVT that are refractory to vagal maneuvers or occur despite medical management can be treated acutely in pregnancy with adenosine, which effectively stops episodes about 90% of the time.2 (See the TABLE8,9 for a list of antiarrhythmics that may be used to treat AVNRT.)

Catheter ablation is first-line treatment for AVNRT in nonpregnant patients.1,5 The risks of undergoing ablation during pregnancy include fetal exposure to radiation and anesthetic drugs.2,3 Therefore, this treatment should be used only when pharmacologic treatment is unsuccessful and risks to the mother and fetus due to the arrhythmia outweigh the risks of the procedure. Ablation can be offered postpartum as more definitive therapy.
Our patient was started on metoprolol tartrate 12.5 mg bid at 35 weeks’ gestation due to increasingly common and persistent palpitations. This helped control the episodes for 2 weeks, at which point they increased again in frequency. These were terminated using Valsalva maneuvers; increasing the metoprolol dosage was prohibitive due to patient intolerance.
Following an uncomplicated delivery, and discontinuation of metoprolol, the patient reported a decrease in both the number of episodes and the duration of SVT. Ultimately, she opted for a catheter ablation to prevent SVT exacerbation during subsequent pregnancies.
THE TAKEAWAY
AVNRT (and other tachyarrhythmias) may worsen or manifest with physiologic changes that occur during pregnancy. After establishing the diagnosis, effort should be made to manage the condition conservatively with Valsalva maneuvers and medication. Catheter ablation should be offered postpartum as a more definitive treatment option.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine Medical Director, 101 Heart Drive, Greenville, NC 27834; [email protected].
THE CASE
A 29-year-old G1P0 woman at 13 weeks’ gestation came in for a routine prenatal visit complaining of sudden-onset heart palpitations that were occurring about once a week. Each episode lasted between 15 and 60 minutes and was accompanied by chest tightness, with no identifiable cause. The patient could inconsistently terminate the episodes with Valsalva maneuvers. She reported having had 2 similar incidents of palpitations within the past year. Her family history was significant for sudden cardiac death of her father and paternal grandfather in their fifth decades of life.
A cardiovascular exam was normal; heart auscultation revealed a regular rate and rhythm without murmurs, rubs, or gallops, and the peripheral pulses were normal. A thyroid-stimulating hormone (TSH) level, basic metabolic panel (BMP), and complete blood count (CBC) were within normal limits. A transthoracic echocardiogram was negative for structural heart disease.
THE DIAGNOSIS
An initial Holter monitor study failed to capture an episode of her palpitations. The frequency of her palpitations increased as her pregnancy progressed, occurring almost daily by the second half of the third trimester, and a repeat Holter monitor study in the third trimester was significant for a 3-minute episode of supraventricular tachycardia (SVT) that correlated with patient-recorded symptoms (FIGURE).

Based on these results, we diagnosed the patient with an atrioventricular nodal reentry tachycardia (AVNRT). Although atrioventricular reciprocating tachycardia (AVRT) remained a remote possibility, it is far less common, and a 12-lead electrocardiogram (EKG) showed no evidence of pre-excitation.
DISCUSSION
AVNRT is the most common form of paroxysmal supraventricular tachycardia (PSVT). It occurs more frequently in women and typically manifests in the second to fourth decades of life.1 AVNRT is a narrow complex tachycardia characterized by a heart rate of 120 to >200 beats/min.
Hemodynamic changes in pregnancy can trigger arrhythmias
During pregnancy, hemodynamic changes (including increased blood volume and cardiac output) are thought to stimulate stretch-activated ion channels within the walls of the heart.2-4 Such changes may exacerbate previously existing cardiac arrhythmias or (less commonly) cause new-onset arrhythmias.3,4 A family history positive for arrhythmias or sudden cardiac death increases the likelihood of developing tachyarrhythmia during pregnancy.3 Women with a known history of PSVT might experience symptom exacerbation despite being on prophylactic therapy.4
Detection and diagnosis
While AVNRT is relatively benign in pregnancy, other cardiac arrhythmias (eg, atrial fibrillation/flutter, ventricular tachycardia) carry a greater risk for fetal and maternal complications, underscoring the need to correctly identify the type of arrhythmia.2,3
Continue to: Physical exam findings
Physical exam findings are often unremarkable unless the patient is actively experiencing SVT in the office, in which case prominent jugular pulsations may be seen due to simultaneous contraction of the atria and ventricles.
The initial evaluation of a pregnant patient presenting with tachycardia should include a BMP, TSH, 12-lead EKG, and transthoracic echocardiography.3,5 In most patients with AVNRT, the results of these tests will be normal. A Holter monitor can be used to document an arrhythmia if the episodes are relatively frequent or an event monitor can be used if the episodes are infrequent.5
EKG findings. When patients are actively experiencing SVT, EKG findings include a P wave obscured by the QRS complex, sometimes manifesting as a pseudo-R wave in the V1 lead and a pseudo-S wave in leads II, III, and AVF. The QRS complex is narrow and the R-R interval is regular.6
Types of treatment
Valsalva maneuvers. Treatment of AVNRT in pregnancy should first involve addressing any precipitating causes, including metabolic and endocrine abnormalities.3 As virtually all antiarrhythmic drugs cross the placenta and are traceable in breast milk,2,3 patients should be counseled to try to stop episodes using Valsalva maneuvers before moving to pharmacologic treatment.
Antiarrhythmics. First-line pharmacologic treatment for the prevention of AVNRT in pregnancy is metoprolol or verapamil.2,5 Neither drug has been associated with adverse outcomes in infants, although there is a large body of evidence suggesting that low levels of metoprolol are present in breast milk.7
Continue to: Acute episodes of SVT that are refractory to...
Acute episodes of SVT that are refractory to vagal maneuvers or occur despite medical management can be treated acutely in pregnancy with adenosine, which effectively stops episodes about 90% of the time.2 (See the TABLE8,9 for a list of antiarrhythmics that may be used to treat AVNRT.)

Catheter ablation is first-line treatment for AVNRT in nonpregnant patients.1,5 The risks of undergoing ablation during pregnancy include fetal exposure to radiation and anesthetic drugs.2,3 Therefore, this treatment should be used only when pharmacologic treatment is unsuccessful and risks to the mother and fetus due to the arrhythmia outweigh the risks of the procedure. Ablation can be offered postpartum as more definitive therapy.
Our patient was started on metoprolol tartrate 12.5 mg bid at 35 weeks’ gestation due to increasingly common and persistent palpitations. This helped control the episodes for 2 weeks, at which point they increased again in frequency. These were terminated using Valsalva maneuvers; increasing the metoprolol dosage was prohibitive due to patient intolerance.
Following an uncomplicated delivery, and discontinuation of metoprolol, the patient reported a decrease in both the number of episodes and the duration of SVT. Ultimately, she opted for a catheter ablation to prevent SVT exacerbation during subsequent pregnancies.
THE TAKEAWAY
AVNRT (and other tachyarrhythmias) may worsen or manifest with physiologic changes that occur during pregnancy. After establishing the diagnosis, effort should be made to manage the condition conservatively with Valsalva maneuvers and medication. Catheter ablation should be offered postpartum as a more definitive treatment option.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine Medical Director, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Kwaku KF, Josephson ME. Typical AVNRT—an update on mechanisms and therapy. Card Electrophysiol Rev. 2002;6:414-421.
2. Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961-967.
3. Knotts RJ, Garan H. Cardiac arrhythmias in pregnancy. Semin Perinatol. 2014;38:285-288.
4. Silversides CK, Harris L, Haberer K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tacharrhythmias and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206-1212.
5. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e471-e505.
6. Di Biase L, Gianni C, Bagliani G, et. al. Arrhythmias involving the atrioventricular junction. Card Electrophysiol Clin. 2017;9:435-452.
7. Fitzpatrick RB. LactMed: drugs and lactation database. J Electron Resour Med Libr. 2007;4:155.
8. Yaksh A, van der Does LJ, Lanters EA, et al. Pharmacological therapy of tachyarrhythmias during pregnancy. Arrhythm Electrophysiol Rev. 2016;5:41-44.
9. US National Library of Medicine. Drugs and lactation database (LactMed). Available at: toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed July 3, 2018.
1. Kwaku KF, Josephson ME. Typical AVNRT—an update on mechanisms and therapy. Card Electrophysiol Rev. 2002;6:414-421.
2. Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961-967.
3. Knotts RJ, Garan H. Cardiac arrhythmias in pregnancy. Semin Perinatol. 2014;38:285-288.
4. Silversides CK, Harris L, Haberer K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tacharrhythmias and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206-1212.
5. Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2016;133:e471-e505.
6. Di Biase L, Gianni C, Bagliani G, et. al. Arrhythmias involving the atrioventricular junction. Card Electrophysiol Clin. 2017;9:435-452.
7. Fitzpatrick RB. LactMed: drugs and lactation database. J Electron Resour Med Libr. 2007;4:155.
8. Yaksh A, van der Does LJ, Lanters EA, et al. Pharmacological therapy of tachyarrhythmias during pregnancy. Arrhythm Electrophysiol Rev. 2016;5:41-44.
9. US National Library of Medicine. Drugs and lactation database (LactMed). Available at: toxnet.nlm.nih.gov/newtoxnet/lactmed.htm. Accessed July 3, 2018.
Is the "breast is best" mantra an oversimplification?
The benefits of breastfeeding for infants have long been touted as numerous and supported by overwhelming evidence. The World Health Organization (WHO), American College of Obstetricians and Gynecologists, American Academy of Pediatrics (AAP), and American Academy of Family Physicians all strongly recommend exclusive breastfeeding for the first 6 months of life, citing numerous health benefits for child and mother. These groups recommend that some breastfeeding be continued through the first 12 months of life, or longer, as desired (the WHO extends the recommendation to 2 years).1-4 In 2000, the Surgeon General of the United States released a strategic plan to increase rates of breastfeeding,5 setting goals (by 2010) of:
- 75% of mothers leaving the hospital breastfeeding
- 50% of babies breastfeeding at 6 months
- 25% of babies breastfeeding at 1 year.
Massive public health campaigns citing data for the many benefits of breastfeeding have been launched with the goal of increasing the breastfeeding rate. In 2014, statistics offered a testament to the success of these campaigns6:
- 82.5% of infants had been breastfed “ever”
- 55.3% were breastfed “some”
- 24.9% were breastfed exclusively through 6 months of age
- 33.7% were breastfed “some” at 12 months.
Breastfeeding advocacy has become clouded
In recent years, an increasing number of researchers, physicians, and authors have begun to question whether, in the United States, the benefits of breastfeeding children are exaggerated and the emphasis on breastfeeding might be leading to feelings of inadequacy, guilt, and anxiety among mothers.7-13 In 2016, the US Preventive Services Task Force (USPSTF) amended its recommendation to “promote and support breastfeeding” to simply “support breastfeeding”—a change that created substantial debate and prompted the Task Force to clarify its stance in changing the language: In its response to public comment, the USPSTF said that its position regarding promotion had not changed, but the language in the original statement had been revised to “ensure that the autonomy of women is respected.” 2,14-16
In contrast, others suggest counseling women on the risks of formula feeding rather than on the benefits of breastfeeding, citing substantial health outcome distinctions.17 Indeed, wide-ranging conclusions have been drawn from the same data on the topic, potentially creating uncertainty for physicians on how best to counsel women on their choice of how to feed their infant.
In this article, we address this uncertainty by utilizing the most recent and comprehensive data to examine infant health outcomes. When possible, the number needed to treat (NNT) for a given outcome has been calculated or approximated, allowing the reader to estimate the likelihood of benefit for an individual mother–infant dyad. Exercise caution when interpreting the NNT, however: The numbers suggest causality that cannot be definitively established using the observational data on which those numbers are based.
Continue to: Infectious disease
Infectious disease
Acute otitis media. Exclusive breastfeeding for 6 months is associated with a 43% reduction in the risk of acute otitis media (AOM) by 2 years of age (odds ratio [OR]=0.57; 95% confidence interval [CI], 0.44-0.75). Beyond 2 years of age, or when comparing “ever” and “never” breastfeeding, the effect disappears. All studies in this meta-analysis had serious limitations.18
Nearly half of children will have at least one case of AOM by one year of age; 80%, by 2 years.19,20 Since the introduction of the heptavalent pneumococcal conjugate vaccine, the rate of AOM at 2 years has fallen by as much as 20%.21 Assuming an incidence of 60% to 80% of AOM by 2 years, only 2 or 3 infants need to be exclusively breastfed for 6 months to prevent a single case of AOM.18 Prevention of AOM through breastfeeding may be related to head position during feeding, antibacterial effects of breast milk, protective oral microbiome in the breastfed infant pharynx, and/or prevention of primary viral upper respiratory infection (URI), which nearly always precedes AOM.18,19
Upper and lower respiratory tract infections. Infants who are exclusively breastfed for 4 months and partially breastfed after 4 months have a lower risk of URI (OR=0.65; 95% CI, 0.51-0.83) and of lower respiratory tract infection (LRTI; OR=0.50; 95% CI, 0.32-0.72).22
The effect is stronger for URI among infants exclusively breastfed for at least 6 months (OR=0.37; 95% CI, 0.18-0.74), but is no longer significant by that time for LRTI (OR=0.33; 95% CI, 0.08-1.40). Importantly, AOM was included in the URI group, and, as previously discussed, AOM has independently been shown to have an inverse relationship with breastfeeding duration.
At 7 to 12 months of age, no association was seen between breastfeeding and the incidence of URI. Curiously, an association with LRTI was again detected for infants breastfed exclusively for 4 months and partially thereafter, but was not detected with exclusive breastfeeding for at least 6 months (OR=0.46; 95% CI, 0.31-0.69). In this study, in the first 6 months of life, 40% of infants had a URI and 8% had an LRTI. The findings in this cohort suggest an NNT of 6 or 7 for prevention of URI and an NNT of 25 for prevention of LRTI in the first 6 months of life.22
Continue to: Children younger than 2 years are...
Children younger than 2 years are estimated to have approximately 6 bouts of the common cold a year, and essentially 100% have at least one bout—perhaps lowering the NNT for URI if applied widely. However, these data are not divided into 6-month intervals, making accurate extrapolation difficult.23
Gastrointestinal infection. The rate of diarrheal illness in the first year of life is lower in infants who are exclusively breastfed for at least 4 months and partially breastfed after.
Both the Promotion of Breastfeeding Intervention Trial (PROBIT; a clinical trial in which infants were randomized to a breastfeeding education intervention or standard care) and a 2010 prospective cohort study in the Netherlands of more than 3400 infants found a reduction in the risk of one or more gastrointestinal (GI) infections at a similar rate.22,24
- In PROBIT, 9.1% of infants in the intervention group, compared to 13.2% in the standard care group (OR=0.60; 95% CI, 0.40-0.91), had one or more GI infections at 12 months of age.24
- In the 2010 Netherlands cohort, 8% of infants had a GI infection by 6 months of age. Infants breastfed exclusively for at least 4 or 6 months had a decreased risk for GI infection (respectively: adjusted OR=0.41; 95% CI, 0.26-0.64 and adjusted OR=0.46; 95% CI, 0.14-1.59). No such association was found for any feeding group 7 to 12 months of age.22
These studies are notable for the low incidence of GI infection, which is frequently cited as 1.3 to 2.3 episodes per child per year in children younger than 3 years in the United States.25 However, that high incidence has likely declined significantly since the introduction of rotavirus vaccine in 2006. In the years following the introduction of the vaccine, infant visits for gastroenteritis decreased by >90% in all care settings in the South, Northeast, and Midwest regions of the United States and by 53% to 63% in the West region.26 Recent accurate epidemiologic information, in an era of significantly higher vaccination rates, is lacking.
Assuming the low incidence of GI infection reported in PROBIT and the Netherlands trials, about 25 to 30 infants need to be exclusively breastfed for 4 to 6 months to prevent a single GI infection during the first 6 to 12 months of life.22,24 Assuming a 60% incidence by age 12 months before introduction of the rotavirus vaccine, the NNT would be approximately 4.24 The true number is likely somewhere between those 2 NNTs.
Continue to: Hospitalization
Hospitalization
Risk of infection is decreased. A large cohort study in Scotland, involving more than 500,000 children, found an association between exclusive breastfeeding for 6 to 8 weeks and decreased risk of hospitalization within the first 6 months of life. Formula-fed and mixed-fed infants had an increased hazard ratio (HR) for hospitalization for common childhood illness (HR=1.40; 95% CI, 1.35-1.45 for formula-fed infants and HR=1.18; 95% CI, 1.11-1.25 for mixed-fed infants).27 The study also found increased rates of hospitalization for conditions for which other meta-analyses have failed to show a protective effect from breastfeeding—leading to suspicion of residual confounding in the study. Another United Kingdom cohort demonstrated lower rates of hospitalization for GI infection (NNT=171) and LRTI (NNT=115) among exclusively breastfed infants by 8 months of age.28
Risk of neonatal readmission is increased. Late preterm infants who are exclusively breastfed are nearly twice as likely to be hospitalized as breastfed term or non-breastfed preterm infants, primarily due to dehydration, failure to thrive, weight loss, and hyperbilirubinemia. In fact, exclusive breastfeeding at discharge from the hospital is likely the single greatest risk factor for hospital readmission in newborns.29,30 Term infants who are exclusively breastfed are more likely to be hospitalized compared to formula-fed or mixed-fed infants, due to hyperbilirubinemia, dehydration, hypernatremia, and weight loss (number needed to harm (NNH)=71).30-32 For weight loss >10% of birth weight with or without hospitalization, the NNH for breastfed infants is 13.32
Many of these hospitalizations and events could be avoided with appropriate monitoring and medically indicated supplementation; the likelihood of long-term harm is low. Formula supplementation is often avoided if possible in hospitals to promote exclusive breastfeeding; however, several small randomized clinical trials have demonstrated that limited formula supplementation in breastfed infants does not affect the breastfeeding continuation rate at 3 and 6 months, and, therefore, might be a way to decrease infant rehospitalization.33,34
Necrotizing enterocolitis
In preterm infants, breastfeeding has been associated with a lower rate of necrotizing enterocolitis. In the 2007 Agency for Healthcare Research and Quality report, the association was found to be only marginally statistically significant, and the authors warned that, first, evidence is old and heterogeneous and, second, present preterm formula is much different than the formula used in earlier studies of preterm infant nutrition and necrotizing enterocolitis.35 A 2012 Cochrane review included newer studies in its analysis but reached the same conclusion on the quality and heterogeneity of available evidence, with a NNT of 25.36
Continue to: Sudden infant death syndrome
Sudden infant death syndrome
There is a statistically significant association between sudden infant death syndrome (SIDS) and feeding method. Infants whose cause of death is SIDS are approximately one half as likely to have been breastfed as matched controls.35,37
In 2005, AAP did not recommend breastfeeding as a means to reduce the risk of SIDS because available evidence was mixed, and studies at the time were poorly controlled.38 Since that time, case-control meta-analyses have shed additional light on the association between SIDS and feeding method.35,37
The protective effect exists for any amount of breastfeeding and is stronger for exclusive breastfeeding, suggesting a protective role—not simply an association. Caution should be employed with this conclusion, however, because the studies included in the meta-analysis used univariate analysis primarily and did not control sufficiently for known confounders. In addition, the authors warn that publication bias might overestimate the association.38
Potential mechanisms of a protective role include decreased risk of infection and greater arousability from sleep in breastfed infants. Assuming a protective role, available data suggest that more than 3500 infants need to be breastfed to prevent one case of SIDS.39
Continue to: Allergic disease
Allergic disease
Asthma. There is evidence of a small protective effect of breastfeeding “ever” on asthma at 5 to 18 years of age in high-income countries (OR=0.90; 95% CI, 0.83-0.97). A family history of asthma or atopy did not affect this finding. The authors note there is some evidence of publication bias in this review, which is the largest and most comprehensive on the topic.40
With a lifetime prevalence of asthma in the United States of approximately 13.2%, this association would confer an NNT of roughly 76.41 Earlier, the literature demonstrated mixed and conflicting evidence, and some experts suggested an effect only when there is a family history of asthma or atopy.36
Eczema. For children younger than 2 years, there is low-grade- and very-low-grade-quality evidence that exclusive breastfeeding longer than 3 to 4 months is associated with a reduced risk of eczema (OR=0.74; 95% CI, 0.57-0.97).40
Previously, data suggested that this association existed only in children who had a family history of atopy.35 The protective association, however, exists regardless of family history and does not persist beyond 2 years of age. The authors noted evidence of publication bias, reverse causation, and misdiagnosis of early childhood rashes as eczema as limitations of their findings.40
Continue to: Reliable epidemiologic evidence...
Reliable epidemiologic evidence on the incidence of eczema in infants in the United States is limited, but the prevalence in the United States in children younger than 17 years is approximately 10.7% (with wide regional variation). Extrapolating these data generously, the NNT to prevent eczema in the first 2 years of life could be estimated at approximately 36.42
Allergic rhinitis. There is low-grade- and very-low-grade-quality evidence that more breastfeeding, compared to less breastfeeding, is associated with a lower risk of allergic rhinitis in children younger than 5 years (OR=0.79; 95% CI, 0.63-0.98). The association exists regardless of family history and disappears after 5 years of age. The differentiation of allergic rhinitis from rhinovirus infection (for which there is higher-quality evidence of a protective effect with breastfeeding) must be considered when interpreting these data.40
Reliable epidemiologic evidence on allergic rhinitis in children younger than 5 years is lacking, and incidence varies by region. A rough estimate, using data from 6- and 7-year-olds, indicates an NNT of 54 to 70.43
Food allergy. There is no evidence to suggest an association between breastfeeding and food allergy, either as protective or as a risk factor, and studies are limited.40 Interestingly, as data accumulate associating early exposure to foods with protection, some authors have proposed reexamining the recommendation from WHO and US health organizations for exclusive breastfeeding for the first 6 months of life.7,44
Continue to: Dental health
Dental health
Dental caries. There is consistent evidence that breastfeeding beyond 12 months of age is associated with the development of dental caries of deciduous teeth to 6 years of age (OR=2.90; 95% CI, 2.33-3.60). Many of the studies that showed this association did not control for the introduction of sugary foods and drinks, and there was a trend toward publication bias showing the association.45
Dental malocclusion. There is consistent evidence for approximately a two-thirds reduction in malocclusions in deciduous teeth in breastfed infants (OR=0.32; 95% CI, 0.25-0.40). Although the large majority of these data come from low-income and middle-income countries, the incidence of malocclusion is not thought to be associated with socioeconomic status, as so many other breastfeeding outcomes are.46
Childhood leukemia
In the largest meta-analysis available, a statistically significant inverse relationship between any breastfeeding for >6 months and childhood leukemia is evident in developed countries (OR=0.84; 95% CI, 0.78-0.91), although significant heterogeneity among studies and lack of control for confounding variables are significant limitations. In particular, an association has been demonstrated with acute lymphoblastic leukemia (ALL) but not with acute myelogenous leukemia.47 Given the rarity of childhood ALL, approximately 12,500 infants would need to be breastfed to prevent one case.48
Continue to: Long-term outcomes
Long-term outcomes
Cognitive development. Several studies conducted in developed countries have linked breastfeeding to positive cognitive outcomes in children, including higher intelligence quotient (IQ).35,49-52
These effects are conflicting, however, in studies that include sibling analysis and ones that control for maternal IQ.8,35,43,52-54 In the 2013 WHO meta-analysis, breastfeeding was associated with an increase of 2.2 points on normalized testing when only high-quality studies were included.51 A 2015 meta-analysis identified 4 high-quality studies with a large sample size and recall time <3 years, which demonstrated a mean difference of 1.76 points in IQ (95% CI, 0.25-3.26) in childhood and adolescence.52 Although statistically significant, this modest increase is of questionable clinical benefit and of unknown duration.
Obesity. The relationship between breastfeeding and obesity later in life is debatable. A large, systematic 2014 review of 15 cohort and 10 cross-sectional studies found a significantly reduced risk of childhood obesity among children who were breastfed (adjusted OR=0.78; 95% CI, 0.74-0.81).55 However, the review included studies that controlled for different confounders, and smaller effects were found in studies in which more confounders were taken into account.
The 2013 WHO meta-analysis found a small (approximately 10%) reduction in the prevalence of overweight or obese children, but cautioned that residual confounding and publication bias were likely.51 At 6.5 and 11.5 years of follow-up, PROBIT failed to demonstrate a protective effect for exclusively or “ever” breastfed infants.56 Sibling analysis similarly fails to demonstrate a statistically significant relationship.8
Continue to: A 2015 meta-analysis of 23 high-quality studies...
A 2015 meta-analysis of 23 high-quality studies with a sample size >1500 children and controlled for important confounders showed a pooled reduction in the prevalence of overweight or obesity of 13% (95% CI, 6-19).57 The protection in this meta-analysis showed a dilution of the effect as the participants aged and an inverse relationship of the effect with sample size.
Breastfeeding is, therefore, unlikely to play a significant, if any, role in combatting the obesity epidemic.
Hypertension. A meta-analysis of high-quality trials demonstrates a <1 mm Hg reduction in systolic blood pressure and no significant difference in diastolic pressure in breastfed infants.57 Similarly, no significant effect of breastfeeding on blood pressure has been demonstrated in trials of preterm infants.51
Type 2 diabetes. Available data are limited and heterogeneous for the association between breastfeeding and later development of type 2 diabetes. Only 2 high-quality trials were identified in the 2013 WHO meta-analysis, and their results conflict.51 A 2015 meta-analysis identified only 3 high-quality studies, without a statistically significant relationship.57
Dyslipidemia. Although earlier data suggested an association between breastfeeding and reduced cholesterol levels later in life, the 2013 WHO meta-analysis and a 2015 meta-analysis concluded that no association exists. The limited data available for preterm infants conflict.51,57
Growth. There is no evidence that feeding method has a short- or long-term effect on weight gain or length gain in preterm or term infants.35,36,58
Death. No clear association has been found between mortality and breastfeeding status in developed countries, except for the association with SIDS.35
Continue to: What issues frame and guide counseling on breastfeeding?
What issues frame and guide counseling on breastfeeding?
There is that “problem” with the evidence. The evidence for infant breastfeeding status and its association with health outcomes faces significant limitations; the great majority of those limitations tend to overestimate the benefits of breastfeeding. Nearly all evidence is based on observational studies, in which causality cannot be determined and self-selection bias, recall bias, and residual confounding limit the value or strength of the findings.
Breastfeeding rates are strongly socially patterned alongside socioeconomic status, race, and education level, all of which are simultaneously strongly tied to short- and long-term health outcomes.6 Other factors limiting the strength of the data set include varying definitions of infant feeding practices in different studies, varying definitions of outcomes and diseases, reverse causation, and evidence of publication bias in many meta-analyses. Given these shortcomings, the NNTs in this article probably represent a best-case scenario for breastfeeding outcomes for infants in the United States (TABLE 118,22-24,28,36,39-43,47,48).
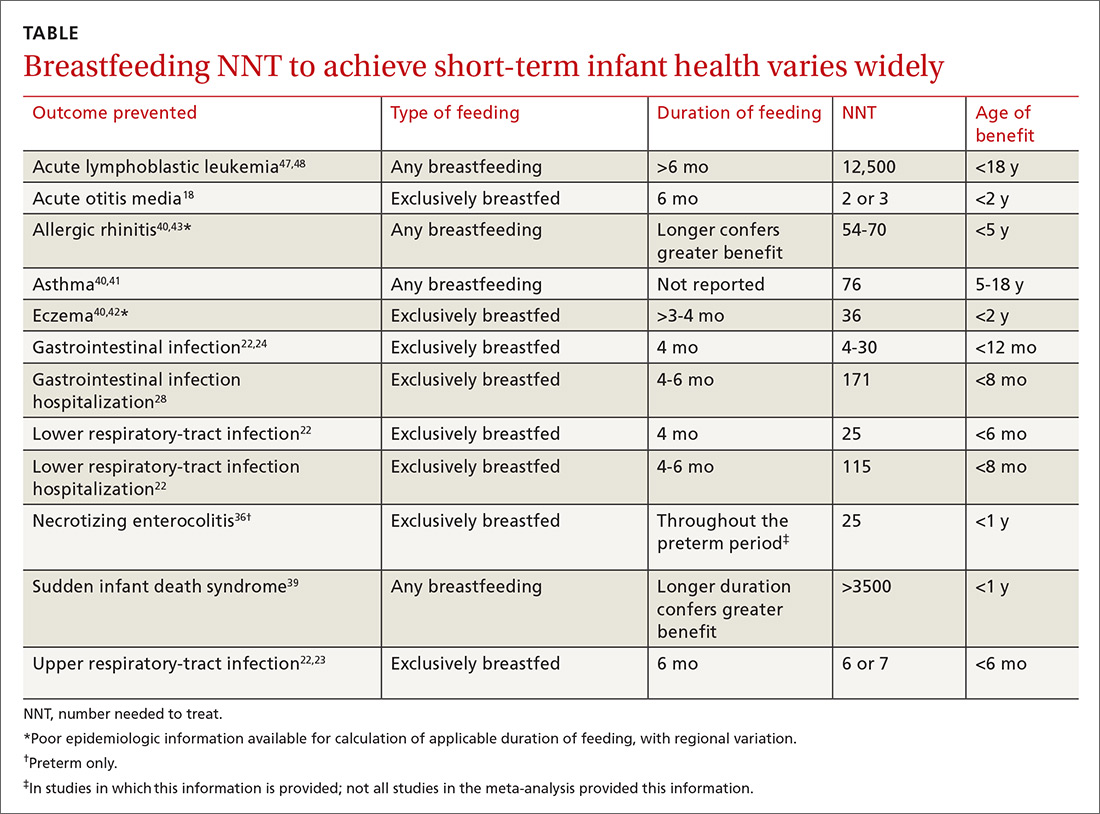
Data need to be put into context. The NNTs for many breastfeeding outcomes (TABLE) compare favorably with other recommended interventions, particularly for other preventive care measures. Two examples: 81 mg/d aspirin for a 50-year-old man has an NNT of 35 to 45 for preventing nonfatal myocardial infarction, and the number needed to invite to screen with mammography to prevent one breast cancer death for a 50-year-old woman is 1339.59,60
In both of these examples, >95% of patients will not benefit from the intervention, yet these preventive measures are routinely recommended and have a significant impact at the public health level. Notably, these outcomes are more serious than most breastfeeding outcomes; have a longer-lasting effect, better-quality data, and better data for potential harms; are causally linked to the intervention; and require much less effort and commitment of time than breastfeeding.
The question must be reckoned with: Can advocacy be harmful?
In recent years, a growing number of concerns have been raised about:
- the potential harms of breastfeeding advocacy
- exaggeration of the benefits of breastfeeding
- promotion of breastfeeding at the expense of evidence-based medicine.
The “Ten Steps to Successful Breastfeeding” program of the Baby-friendly Hospital Initiative (BFHI; launched by UNICEF and WHO) has come under scrutiny because of an increasing number of reports of sudden unexpected postnatal collapse; fall injuries; modeling and encouragement of unsafe sleep practices; an overly rigid resistance to the use of formula supplementation; and the ban on pacifier use.61,62 The BFHI, promoted by the Centers for Disease Control and Prevention, is increasingly being adopted by hospitals with the expressed goal of increasing the breastfeeding rate from birth to discharge.
Continue to: Some of the "Ten Steps"...
Some of the “Ten Steps,” such as the call for skin-to-skin care and 24-hour rooming-in, have well-established benefit yet, when performed without supervision, can have the rare but serious unintended consequences of sudden unexpected postnatal collapse (the incidence of which may be higher than that of SIDS) and unsafe sleeping practices.62,63
Furthermore, despite evidence that early formula supplementation, when medically necessary, does not adversely impact the breastfeeding rate, the “Ten Steps” program advises that giving formula before breast milk comes in might “lead to failure to breastfeed.”33,34,61,63
Similarly, the ban on pacifiers is contrary to available evidence. The use of pacifiers before last sleep is more protective against SIDS than breastfeeding (NNT=2733), and there is evidence at one hospital that BFHI-inspired pacifier restriction is associated with a decrease in the rate of breastfeeding.64,65
Other harms of advocacy are even more poorly studied. Most of the evidence for harm comes from the psychology and social science literature—not the medical literature, perhaps because the prevailing opinion in the medical community is that breastfeeding has overwhelming evidence for benefit. In fact, in the USPSTF’s 2008 recommendation, the evidence review of breastfeeding promotion practices in primary care did not identify a single study that measured harm; in the 2016 update of that recommendation, only 2 such studies were identified.15,66
The literature that does investigate harm consistently finds that women who have difficulty breastfeeding or choose formula feeding report feelings of inadequacy, guilt, loss of agency, anxiety, and physical pain during breastfeeding that interferes with 1) their ability to bond or otherwise care for their infant and 2) competing work obligations.11-13,67-69 Given the lack of attention paid to these variables in the medical literature, it is the individual mother who is best positioned to weigh these factors against the benefits of breastfeeding.
Continue to: Shared decision-making is best—for mother and baby
Shared decision-making is best—for mother and baby
Breastfeeding might prevent certain infections in as many as 50% of infants, but a mother unable to breastfeed can take solace in the fact that >95% of breastfed infants will not realize any benefit from the preventive potential of breastfeeding in regard to hospitalization or allergic disease, and >99% will not realize benefit from either the prevention of SIDS or ALL, or from improvement in long-term health measures (except for, perhaps, a slightly higher IQ). The “breast is best” mantra is likely true at a public-health level; for the individual mother–infant dyad, however, where there is a need to balance personal, social, family, and financial factors, that mantra is an oversimplification.
Regrettably, there is a paucity of data on the risks of breastfeeding promotion—an area that deserves more study. Balancing the abundant, but often limited-quality, data on the benefits of breastfeeding and the sheer lack of data regarding the risks of advocacy represents a clinical and an ethical challenge for physicians. It is a challenge that can only be resolved through individualization of care and shared decision-making, in which the physician is expert on the benefits of breastfeeding, and the mother is expert on the personal circumstances to be weighed against those benefits.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization, and New York, NY: UNICEF; 2003. Available at: www.who.int/maternal_child_adolescent/documents/9241562218/en/. Accessed April 4, 2018.
2. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Breastfeeding Expert Work Group. Committee Opinion No. 658: Optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2016;127:e86-e92.
3. Gartner LM, Morton J, Lawrence RA, et al; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
4. Breastfeeding (policy statement). Leawood, KS: American Academy of Family Physicians; 2007. Available at: https://www.aafp.org/about/policies/all/breastfeeding.html. Accessed April 3, 2018.
5. Office of the Surgeon General (US); Centers for Disease Control and Prevention (US); Office on Women’s Health (US). The Surgeon General’s call to action to support breastfeeding. Rockville, MD: US Department of Health and Human Services; 2011. Available at: www.surgeongeneral.gov/library/calls/breastfeeding/index.html. Updated August 12, 2014. Accessed April 4, 2018.
6. Breastfeeding: data & statistics. Atlanta, GA: Centers for Disease Control and Prevention; December 11, 2017. Available at: http://www.cdc.gov/breastfeeding/data/. Accessed May 17, 2018.
7. Fewtrell M, Wilson DC, Booth I, et al. A. Six months of exclusive breast feeding: how good is the evidence? BMJ. 2010;342:c5955.
8. Colen CG, Ramey DM. Is breast truly best? Estimating the effect of breastfeeding on long-term child wellbeing in the United States using sibling comparisons. Soc Sci Med. 2014;109:55-65.
9. Wolf J. Is Breast Best? Taking on the Breastfeeding Experts and the New High Stakes of Motherhood. New York, NY: NYU Press; 2010.
10. Tuteur A. Push Back: Guilt in the Age of Natural Parenting. New York, NY: HarperCollins Publishers; 2016.
11. Lee E. Health, morality, and infant feeding: British mothers’ experiences of formula milk use in the early weeks. Sociol Health Illn. 2007;29:1075-1090.
12. Williams K, Donaghue N, Kurz T. “Giving guilt the flick”?: an investigation of mothers’ talk about guilt in relation to infant feeding. Psychol Women Q. 2013;37:97-112.
13. Fahlquist JN, Roeser S. Ethical problems with information on infant feeding in developed countries. J Health Polit Policy Law. 2012;37:155-160.
14. U.S. Preventive Services Task Force. Final Recommendation Statement. Breastfeeding: Counseling. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breastfeeding-counseling. Accessed April 4, 2018.
15. US Preventive Services Task Force. Primary Care Interventions to Support Breastfeeding: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1688-1693.
16. Zahn CM, Hanley LE. Concerns over USPSTF draft recommendation on breastfeeding interventions [letter]. Washington, DC: The American College of Obstetricians and Gynecologists; May 18, 2016. Available at: https://www.acog.org/-/media/Departments/Breastfeeding/Breast-Feeding-ACOG-USPSTF.pdf?dmc=1&ts=20180518T1850056558. Accessed May 22, 2018.
17. Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222-231.
18. Bowatte G, Tham R, Allen KJ, et al. Breastfeeding and childhood acute otitis media: a systematic review and meta-analysis. Acta Paediatr. 2015;104:85-95.
19. Chonmaitree T, Trujillo R, Jennings K, et al. Acute otitis media and other complications of viral respiratory infection. Pediatrics. 2016;137:e20153555.
20. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
21. Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118:865-873.
22. Duijts L, Jaddoe VW, Hofman A, et al. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126:e18-e25.
23. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186:190-199.
24. Kramer MS, Chalmers B, Hodnett ED, et al; PROBIT Study Group (Promotion of Breastfeeding Intervention Trial). Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413-420.
25. Dennehy PH. Acute diarrheal disease in children: epidemiology, prevention, and treatment. Infect Dis Clin North Am. 2005;19:585-602.
26. Cortese MM, Tate JE, Simonsen L, et al. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatric Infect Dis J. 2010;29:489-494.
27. Ajetunmobi OM, Whyte B, Chalmers J, et al. Breastfeeding is associated with reduced childhood hospitalization: evidence from a Scottish birth cohort (1997-2009). J Pediatr. 2015;166:620-625.
28. Quigley MA, Kelly YJ, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007;119:e837-e842.
29. Radtke JV. The paradox of breastfeeding-associated morbidity among late preterm infants. J Obstet Gynecol Neonatal Nurs. 2011;40:9-24.
30. Escobar GJ, Gonzales VM, Armstrong M, et al. Rehospitalization for neonatal dehydration: a nested case-control study. Arch Pediatr Adolesc Med. 2002;156:155-161.
31. Salas AA, Salazar J, Burgoa CV, et al. Significant weight loss in breastfed term infants readmitted for hyperbilirubinemia. BMC Pediatr. 2009;9:82.
32. Tarcan A, Tiker F, Vatandaş NS, et al. Weight loss and hypernatremia in breast-fed babies: frequency in neonates with non-hemolytic jaundice. J Paediatr Child Health. 2005;41:484-487.
33. Flaherman VJ, Aby J, Burgos AE, et al. Effect of early limited formula on duration and exclusivity of breastfeeding in at-risk infants: an RCT. Pediatrics. 2013;131:1059-1065.
34. Straňák Z, Feyereislova S, Černá M, et al. J. Limited amount of formula may facilitate breastfeeding: randomized, controlled trial to compare standard clinical practice versus limited supplemental feeding. Denning PW, ed. PLoS One. 2016;11:e0150053.
35. Ip S, Chung M, Raman G, et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Rockville, MD: Agency for Healthcare Research and Quality (US); 2007. Evidence Reports/Technology Assessments, No. 153. Available at: www.ncbi.nlm.nih.gov/books/NBK38337/. Accessed April 3, 2018.
36. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;(4):CD002971.
37. Hauck FR, Thompson JM, Tanabe KO, et al. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128:103-110.
38. American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245-1255.
39. Moon RY, Fu L. Sudden infant death syndrome: an update. Pediatr Rev. 2012;33:314-320.
40. Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38-53.
41. Brim SN, Rudd RA, Funk RH, et al. Asthma prevalence among US children in underrepresented minority populations: American Indian/Alaska Native, Chinese, Filipino, and Asian Indian. Pediatrics. 2008;122:e217-e222.
42. Shaw TF, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
43. Mallol J, Crane J, von Mutius E, et al. The international study of asthma and allergies in childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73-85.
44. Flohr C, Nagel G, Weinmayr G, et al. Lack of evidence for a protective effect of prolonged breastfeeding on childhood eczema: lessons from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br J Dermatol. 2011;165:1280-1289.
45. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
46. Peres KG, Cascaes AM, Nascimento GG, et al. Effect of breastfeeding on malocclusions: a systematic review and meta-analysis. Acta Paediatr. 2015;104:54-61.
47. Amitya EL, Keinan-Boker L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 2015;169:e151025.
48. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943-1955.
49. Guxens M, Mendez MA, Moltó-Puigmartí C, et al. Breastfeeding, long-chain polyunsaturated fatty acids in colostrum, and infant mental development.
2012;129:1134-1140.
51. Horta BL, Victora CG. Long-term effects of breastfeeding: a systematic review. Geneva, Switzerland: World Health Organization; 2013. Available at: http://apps.who.int/iris/bitstream/10665/79198/1/9789241505307_eng.pdf. Accessed August 16, 2016.
52. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104:14-19.
53. Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333:945.
54. Sajjad A, Tharner A, Kiefte-de Jong JC, et al. Breastfeeding duration and non-verbal IQ in children. J Epidemiol Community Health 2015;69:775-781.
55. Yan J, Liu L, Zhu Y, et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267.
56. Martin RM, Patel R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
57. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure, and type 2 diabetes: systematic review and meta-analysis. Acta Paediatr. 2015;104:30-37.
58. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Syst Rev. 2012;15:CD003517.
59. U.S. Preventive Services Task Force. Final recommendation statement: aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Published September 2017. Accessed April 3, 2018.
60. U.S. Preventive Services Task Force. Screening for breast cancer. Available at: www.uspreventiveservicestaskforce.org/Page/SupportingDoc/breast-cancer-screening/final-evidence-summary9. Published November 2009. Accessed April 2, 2018.
61. Bass JL, Gartley T, Kleinman R. Unintended consequences of current breastfeeding initiatives. JAMA Pediatr. 2016;170:923-924.
62. Feldman-Winter L, Goldsmith JP; Committee on Fetus and Newborn; Task Force on Sudden Infant Death Syndrome. Safe sleep and skin-to-skin care in the neonatal period for healthy term newborns. Pediatrics. 2016;138:e20161889.
63. The Mother and Child Health and Education Trust. Ten steps to successful breastfeeding. Available at: www.tensteps.org. Published November 8, 2017. Accessed April 3, 2018.
64. Hauck FR, Omojokun OO, Siadaty MS. Do pacifiers reduce the risk of sudden infant death syndrome? A meta-analysis. Pediatrics. 2005;116:e716-e723.
65. Kair LR, Kenron D, Etheredge K, et al. Pacifier restriction and exclusive breastfeeding. Pediatrics. 2013;131:e1101-e1107.
66. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
67. Wolf JB. Is breast really best? Risk and total motherhood in the National Breastfeeding Awareness Campaign. J Health Polit Policy Law. 2007;32:595-636.
68. Marshall JL, Godfrey M, Renfrew MJ. Being a ‘good mother’: managing breastfeeding and merging identities. Soc Sci Med. 2007;65:2147-2159.
69. Kelleher CM. The physical challenges of early breastfeeding. Soc Sci Med. 2006;63:2727-2738.
The benefits of breastfeeding for infants have long been touted as numerous and supported by overwhelming evidence. The World Health Organization (WHO), American College of Obstetricians and Gynecologists, American Academy of Pediatrics (AAP), and American Academy of Family Physicians all strongly recommend exclusive breastfeeding for the first 6 months of life, citing numerous health benefits for child and mother. These groups recommend that some breastfeeding be continued through the first 12 months of life, or longer, as desired (the WHO extends the recommendation to 2 years).1-4 In 2000, the Surgeon General of the United States released a strategic plan to increase rates of breastfeeding,5 setting goals (by 2010) of:
- 75% of mothers leaving the hospital breastfeeding
- 50% of babies breastfeeding at 6 months
- 25% of babies breastfeeding at 1 year.
Massive public health campaigns citing data for the many benefits of breastfeeding have been launched with the goal of increasing the breastfeeding rate. In 2014, statistics offered a testament to the success of these campaigns6:
- 82.5% of infants had been breastfed “ever”
- 55.3% were breastfed “some”
- 24.9% were breastfed exclusively through 6 months of age
- 33.7% were breastfed “some” at 12 months.
Breastfeeding advocacy has become clouded
In recent years, an increasing number of researchers, physicians, and authors have begun to question whether, in the United States, the benefits of breastfeeding children are exaggerated and the emphasis on breastfeeding might be leading to feelings of inadequacy, guilt, and anxiety among mothers.7-13 In 2016, the US Preventive Services Task Force (USPSTF) amended its recommendation to “promote and support breastfeeding” to simply “support breastfeeding”—a change that created substantial debate and prompted the Task Force to clarify its stance in changing the language: In its response to public comment, the USPSTF said that its position regarding promotion had not changed, but the language in the original statement had been revised to “ensure that the autonomy of women is respected.” 2,14-16
In contrast, others suggest counseling women on the risks of formula feeding rather than on the benefits of breastfeeding, citing substantial health outcome distinctions.17 Indeed, wide-ranging conclusions have been drawn from the same data on the topic, potentially creating uncertainty for physicians on how best to counsel women on their choice of how to feed their infant.
In this article, we address this uncertainty by utilizing the most recent and comprehensive data to examine infant health outcomes. When possible, the number needed to treat (NNT) for a given outcome has been calculated or approximated, allowing the reader to estimate the likelihood of benefit for an individual mother–infant dyad. Exercise caution when interpreting the NNT, however: The numbers suggest causality that cannot be definitively established using the observational data on which those numbers are based.
Continue to: Infectious disease
Infectious disease
Acute otitis media. Exclusive breastfeeding for 6 months is associated with a 43% reduction in the risk of acute otitis media (AOM) by 2 years of age (odds ratio [OR]=0.57; 95% confidence interval [CI], 0.44-0.75). Beyond 2 years of age, or when comparing “ever” and “never” breastfeeding, the effect disappears. All studies in this meta-analysis had serious limitations.18
Nearly half of children will have at least one case of AOM by one year of age; 80%, by 2 years.19,20 Since the introduction of the heptavalent pneumococcal conjugate vaccine, the rate of AOM at 2 years has fallen by as much as 20%.21 Assuming an incidence of 60% to 80% of AOM by 2 years, only 2 or 3 infants need to be exclusively breastfed for 6 months to prevent a single case of AOM.18 Prevention of AOM through breastfeeding may be related to head position during feeding, antibacterial effects of breast milk, protective oral microbiome in the breastfed infant pharynx, and/or prevention of primary viral upper respiratory infection (URI), which nearly always precedes AOM.18,19
Upper and lower respiratory tract infections. Infants who are exclusively breastfed for 4 months and partially breastfed after 4 months have a lower risk of URI (OR=0.65; 95% CI, 0.51-0.83) and of lower respiratory tract infection (LRTI; OR=0.50; 95% CI, 0.32-0.72).22
The effect is stronger for URI among infants exclusively breastfed for at least 6 months (OR=0.37; 95% CI, 0.18-0.74), but is no longer significant by that time for LRTI (OR=0.33; 95% CI, 0.08-1.40). Importantly, AOM was included in the URI group, and, as previously discussed, AOM has independently been shown to have an inverse relationship with breastfeeding duration.
At 7 to 12 months of age, no association was seen between breastfeeding and the incidence of URI. Curiously, an association with LRTI was again detected for infants breastfed exclusively for 4 months and partially thereafter, but was not detected with exclusive breastfeeding for at least 6 months (OR=0.46; 95% CI, 0.31-0.69). In this study, in the first 6 months of life, 40% of infants had a URI and 8% had an LRTI. The findings in this cohort suggest an NNT of 6 or 7 for prevention of URI and an NNT of 25 for prevention of LRTI in the first 6 months of life.22
Continue to: Children younger than 2 years are...
Children younger than 2 years are estimated to have approximately 6 bouts of the common cold a year, and essentially 100% have at least one bout—perhaps lowering the NNT for URI if applied widely. However, these data are not divided into 6-month intervals, making accurate extrapolation difficult.23
Gastrointestinal infection. The rate of diarrheal illness in the first year of life is lower in infants who are exclusively breastfed for at least 4 months and partially breastfed after.
Both the Promotion of Breastfeeding Intervention Trial (PROBIT; a clinical trial in which infants were randomized to a breastfeeding education intervention or standard care) and a 2010 prospective cohort study in the Netherlands of more than 3400 infants found a reduction in the risk of one or more gastrointestinal (GI) infections at a similar rate.22,24
- In PROBIT, 9.1% of infants in the intervention group, compared to 13.2% in the standard care group (OR=0.60; 95% CI, 0.40-0.91), had one or more GI infections at 12 months of age.24
- In the 2010 Netherlands cohort, 8% of infants had a GI infection by 6 months of age. Infants breastfed exclusively for at least 4 or 6 months had a decreased risk for GI infection (respectively: adjusted OR=0.41; 95% CI, 0.26-0.64 and adjusted OR=0.46; 95% CI, 0.14-1.59). No such association was found for any feeding group 7 to 12 months of age.22
These studies are notable for the low incidence of GI infection, which is frequently cited as 1.3 to 2.3 episodes per child per year in children younger than 3 years in the United States.25 However, that high incidence has likely declined significantly since the introduction of rotavirus vaccine in 2006. In the years following the introduction of the vaccine, infant visits for gastroenteritis decreased by >90% in all care settings in the South, Northeast, and Midwest regions of the United States and by 53% to 63% in the West region.26 Recent accurate epidemiologic information, in an era of significantly higher vaccination rates, is lacking.
Assuming the low incidence of GI infection reported in PROBIT and the Netherlands trials, about 25 to 30 infants need to be exclusively breastfed for 4 to 6 months to prevent a single GI infection during the first 6 to 12 months of life.22,24 Assuming a 60% incidence by age 12 months before introduction of the rotavirus vaccine, the NNT would be approximately 4.24 The true number is likely somewhere between those 2 NNTs.
Continue to: Hospitalization
Hospitalization
Risk of infection is decreased. A large cohort study in Scotland, involving more than 500,000 children, found an association between exclusive breastfeeding for 6 to 8 weeks and decreased risk of hospitalization within the first 6 months of life. Formula-fed and mixed-fed infants had an increased hazard ratio (HR) for hospitalization for common childhood illness (HR=1.40; 95% CI, 1.35-1.45 for formula-fed infants and HR=1.18; 95% CI, 1.11-1.25 for mixed-fed infants).27 The study also found increased rates of hospitalization for conditions for which other meta-analyses have failed to show a protective effect from breastfeeding—leading to suspicion of residual confounding in the study. Another United Kingdom cohort demonstrated lower rates of hospitalization for GI infection (NNT=171) and LRTI (NNT=115) among exclusively breastfed infants by 8 months of age.28
Risk of neonatal readmission is increased. Late preterm infants who are exclusively breastfed are nearly twice as likely to be hospitalized as breastfed term or non-breastfed preterm infants, primarily due to dehydration, failure to thrive, weight loss, and hyperbilirubinemia. In fact, exclusive breastfeeding at discharge from the hospital is likely the single greatest risk factor for hospital readmission in newborns.29,30 Term infants who are exclusively breastfed are more likely to be hospitalized compared to formula-fed or mixed-fed infants, due to hyperbilirubinemia, dehydration, hypernatremia, and weight loss (number needed to harm (NNH)=71).30-32 For weight loss >10% of birth weight with or without hospitalization, the NNH for breastfed infants is 13.32
Many of these hospitalizations and events could be avoided with appropriate monitoring and medically indicated supplementation; the likelihood of long-term harm is low. Formula supplementation is often avoided if possible in hospitals to promote exclusive breastfeeding; however, several small randomized clinical trials have demonstrated that limited formula supplementation in breastfed infants does not affect the breastfeeding continuation rate at 3 and 6 months, and, therefore, might be a way to decrease infant rehospitalization.33,34
Necrotizing enterocolitis
In preterm infants, breastfeeding has been associated with a lower rate of necrotizing enterocolitis. In the 2007 Agency for Healthcare Research and Quality report, the association was found to be only marginally statistically significant, and the authors warned that, first, evidence is old and heterogeneous and, second, present preterm formula is much different than the formula used in earlier studies of preterm infant nutrition and necrotizing enterocolitis.35 A 2012 Cochrane review included newer studies in its analysis but reached the same conclusion on the quality and heterogeneity of available evidence, with a NNT of 25.36
Continue to: Sudden infant death syndrome
Sudden infant death syndrome
There is a statistically significant association between sudden infant death syndrome (SIDS) and feeding method. Infants whose cause of death is SIDS are approximately one half as likely to have been breastfed as matched controls.35,37
In 2005, AAP did not recommend breastfeeding as a means to reduce the risk of SIDS because available evidence was mixed, and studies at the time were poorly controlled.38 Since that time, case-control meta-analyses have shed additional light on the association between SIDS and feeding method.35,37
The protective effect exists for any amount of breastfeeding and is stronger for exclusive breastfeeding, suggesting a protective role—not simply an association. Caution should be employed with this conclusion, however, because the studies included in the meta-analysis used univariate analysis primarily and did not control sufficiently for known confounders. In addition, the authors warn that publication bias might overestimate the association.38
Potential mechanisms of a protective role include decreased risk of infection and greater arousability from sleep in breastfed infants. Assuming a protective role, available data suggest that more than 3500 infants need to be breastfed to prevent one case of SIDS.39
Continue to: Allergic disease
Allergic disease
Asthma. There is evidence of a small protective effect of breastfeeding “ever” on asthma at 5 to 18 years of age in high-income countries (OR=0.90; 95% CI, 0.83-0.97). A family history of asthma or atopy did not affect this finding. The authors note there is some evidence of publication bias in this review, which is the largest and most comprehensive on the topic.40
With a lifetime prevalence of asthma in the United States of approximately 13.2%, this association would confer an NNT of roughly 76.41 Earlier, the literature demonstrated mixed and conflicting evidence, and some experts suggested an effect only when there is a family history of asthma or atopy.36
Eczema. For children younger than 2 years, there is low-grade- and very-low-grade-quality evidence that exclusive breastfeeding longer than 3 to 4 months is associated with a reduced risk of eczema (OR=0.74; 95% CI, 0.57-0.97).40
Previously, data suggested that this association existed only in children who had a family history of atopy.35 The protective association, however, exists regardless of family history and does not persist beyond 2 years of age. The authors noted evidence of publication bias, reverse causation, and misdiagnosis of early childhood rashes as eczema as limitations of their findings.40
Continue to: Reliable epidemiologic evidence...
Reliable epidemiologic evidence on the incidence of eczema in infants in the United States is limited, but the prevalence in the United States in children younger than 17 years is approximately 10.7% (with wide regional variation). Extrapolating these data generously, the NNT to prevent eczema in the first 2 years of life could be estimated at approximately 36.42
Allergic rhinitis. There is low-grade- and very-low-grade-quality evidence that more breastfeeding, compared to less breastfeeding, is associated with a lower risk of allergic rhinitis in children younger than 5 years (OR=0.79; 95% CI, 0.63-0.98). The association exists regardless of family history and disappears after 5 years of age. The differentiation of allergic rhinitis from rhinovirus infection (for which there is higher-quality evidence of a protective effect with breastfeeding) must be considered when interpreting these data.40
Reliable epidemiologic evidence on allergic rhinitis in children younger than 5 years is lacking, and incidence varies by region. A rough estimate, using data from 6- and 7-year-olds, indicates an NNT of 54 to 70.43
Food allergy. There is no evidence to suggest an association between breastfeeding and food allergy, either as protective or as a risk factor, and studies are limited.40 Interestingly, as data accumulate associating early exposure to foods with protection, some authors have proposed reexamining the recommendation from WHO and US health organizations for exclusive breastfeeding for the first 6 months of life.7,44
Continue to: Dental health
Dental health
Dental caries. There is consistent evidence that breastfeeding beyond 12 months of age is associated with the development of dental caries of deciduous teeth to 6 years of age (OR=2.90; 95% CI, 2.33-3.60). Many of the studies that showed this association did not control for the introduction of sugary foods and drinks, and there was a trend toward publication bias showing the association.45
Dental malocclusion. There is consistent evidence for approximately a two-thirds reduction in malocclusions in deciduous teeth in breastfed infants (OR=0.32; 95% CI, 0.25-0.40). Although the large majority of these data come from low-income and middle-income countries, the incidence of malocclusion is not thought to be associated with socioeconomic status, as so many other breastfeeding outcomes are.46
Childhood leukemia
In the largest meta-analysis available, a statistically significant inverse relationship between any breastfeeding for >6 months and childhood leukemia is evident in developed countries (OR=0.84; 95% CI, 0.78-0.91), although significant heterogeneity among studies and lack of control for confounding variables are significant limitations. In particular, an association has been demonstrated with acute lymphoblastic leukemia (ALL) but not with acute myelogenous leukemia.47 Given the rarity of childhood ALL, approximately 12,500 infants would need to be breastfed to prevent one case.48
Continue to: Long-term outcomes
Long-term outcomes
Cognitive development. Several studies conducted in developed countries have linked breastfeeding to positive cognitive outcomes in children, including higher intelligence quotient (IQ).35,49-52
These effects are conflicting, however, in studies that include sibling analysis and ones that control for maternal IQ.8,35,43,52-54 In the 2013 WHO meta-analysis, breastfeeding was associated with an increase of 2.2 points on normalized testing when only high-quality studies were included.51 A 2015 meta-analysis identified 4 high-quality studies with a large sample size and recall time <3 years, which demonstrated a mean difference of 1.76 points in IQ (95% CI, 0.25-3.26) in childhood and adolescence.52 Although statistically significant, this modest increase is of questionable clinical benefit and of unknown duration.
Obesity. The relationship between breastfeeding and obesity later in life is debatable. A large, systematic 2014 review of 15 cohort and 10 cross-sectional studies found a significantly reduced risk of childhood obesity among children who were breastfed (adjusted OR=0.78; 95% CI, 0.74-0.81).55 However, the review included studies that controlled for different confounders, and smaller effects were found in studies in which more confounders were taken into account.
The 2013 WHO meta-analysis found a small (approximately 10%) reduction in the prevalence of overweight or obese children, but cautioned that residual confounding and publication bias were likely.51 At 6.5 and 11.5 years of follow-up, PROBIT failed to demonstrate a protective effect for exclusively or “ever” breastfed infants.56 Sibling analysis similarly fails to demonstrate a statistically significant relationship.8
Continue to: A 2015 meta-analysis of 23 high-quality studies...
A 2015 meta-analysis of 23 high-quality studies with a sample size >1500 children and controlled for important confounders showed a pooled reduction in the prevalence of overweight or obesity of 13% (95% CI, 6-19).57 The protection in this meta-analysis showed a dilution of the effect as the participants aged and an inverse relationship of the effect with sample size.
Breastfeeding is, therefore, unlikely to play a significant, if any, role in combatting the obesity epidemic.
Hypertension. A meta-analysis of high-quality trials demonstrates a <1 mm Hg reduction in systolic blood pressure and no significant difference in diastolic pressure in breastfed infants.57 Similarly, no significant effect of breastfeeding on blood pressure has been demonstrated in trials of preterm infants.51
Type 2 diabetes. Available data are limited and heterogeneous for the association between breastfeeding and later development of type 2 diabetes. Only 2 high-quality trials were identified in the 2013 WHO meta-analysis, and their results conflict.51 A 2015 meta-analysis identified only 3 high-quality studies, without a statistically significant relationship.57
Dyslipidemia. Although earlier data suggested an association between breastfeeding and reduced cholesterol levels later in life, the 2013 WHO meta-analysis and a 2015 meta-analysis concluded that no association exists. The limited data available for preterm infants conflict.51,57
Growth. There is no evidence that feeding method has a short- or long-term effect on weight gain or length gain in preterm or term infants.35,36,58
Death. No clear association has been found between mortality and breastfeeding status in developed countries, except for the association with SIDS.35
Continue to: What issues frame and guide counseling on breastfeeding?
What issues frame and guide counseling on breastfeeding?
There is that “problem” with the evidence. The evidence for infant breastfeeding status and its association with health outcomes faces significant limitations; the great majority of those limitations tend to overestimate the benefits of breastfeeding. Nearly all evidence is based on observational studies, in which causality cannot be determined and self-selection bias, recall bias, and residual confounding limit the value or strength of the findings.
Breastfeeding rates are strongly socially patterned alongside socioeconomic status, race, and education level, all of which are simultaneously strongly tied to short- and long-term health outcomes.6 Other factors limiting the strength of the data set include varying definitions of infant feeding practices in different studies, varying definitions of outcomes and diseases, reverse causation, and evidence of publication bias in many meta-analyses. Given these shortcomings, the NNTs in this article probably represent a best-case scenario for breastfeeding outcomes for infants in the United States (TABLE 118,22-24,28,36,39-43,47,48).

Data need to be put into context. The NNTs for many breastfeeding outcomes (TABLE) compare favorably with other recommended interventions, particularly for other preventive care measures. Two examples: 81 mg/d aspirin for a 50-year-old man has an NNT of 35 to 45 for preventing nonfatal myocardial infarction, and the number needed to invite to screen with mammography to prevent one breast cancer death for a 50-year-old woman is 1339.59,60
In both of these examples, >95% of patients will not benefit from the intervention, yet these preventive measures are routinely recommended and have a significant impact at the public health level. Notably, these outcomes are more serious than most breastfeeding outcomes; have a longer-lasting effect, better-quality data, and better data for potential harms; are causally linked to the intervention; and require much less effort and commitment of time than breastfeeding.
The question must be reckoned with: Can advocacy be harmful?
In recent years, a growing number of concerns have been raised about:
- the potential harms of breastfeeding advocacy
- exaggeration of the benefits of breastfeeding
- promotion of breastfeeding at the expense of evidence-based medicine.
The “Ten Steps to Successful Breastfeeding” program of the Baby-friendly Hospital Initiative (BFHI; launched by UNICEF and WHO) has come under scrutiny because of an increasing number of reports of sudden unexpected postnatal collapse; fall injuries; modeling and encouragement of unsafe sleep practices; an overly rigid resistance to the use of formula supplementation; and the ban on pacifier use.61,62 The BFHI, promoted by the Centers for Disease Control and Prevention, is increasingly being adopted by hospitals with the expressed goal of increasing the breastfeeding rate from birth to discharge.
Continue to: Some of the "Ten Steps"...
Some of the “Ten Steps,” such as the call for skin-to-skin care and 24-hour rooming-in, have well-established benefit yet, when performed without supervision, can have the rare but serious unintended consequences of sudden unexpected postnatal collapse (the incidence of which may be higher than that of SIDS) and unsafe sleeping practices.62,63
Furthermore, despite evidence that early formula supplementation, when medically necessary, does not adversely impact the breastfeeding rate, the “Ten Steps” program advises that giving formula before breast milk comes in might “lead to failure to breastfeed.”33,34,61,63
Similarly, the ban on pacifiers is contrary to available evidence. The use of pacifiers before last sleep is more protective against SIDS than breastfeeding (NNT=2733), and there is evidence at one hospital that BFHI-inspired pacifier restriction is associated with a decrease in the rate of breastfeeding.64,65
Other harms of advocacy are even more poorly studied. Most of the evidence for harm comes from the psychology and social science literature—not the medical literature, perhaps because the prevailing opinion in the medical community is that breastfeeding has overwhelming evidence for benefit. In fact, in the USPSTF’s 2008 recommendation, the evidence review of breastfeeding promotion practices in primary care did not identify a single study that measured harm; in the 2016 update of that recommendation, only 2 such studies were identified.15,66
The literature that does investigate harm consistently finds that women who have difficulty breastfeeding or choose formula feeding report feelings of inadequacy, guilt, loss of agency, anxiety, and physical pain during breastfeeding that interferes with 1) their ability to bond or otherwise care for their infant and 2) competing work obligations.11-13,67-69 Given the lack of attention paid to these variables in the medical literature, it is the individual mother who is best positioned to weigh these factors against the benefits of breastfeeding.
Continue to: Shared decision-making is best—for mother and baby
Shared decision-making is best—for mother and baby
Breastfeeding might prevent certain infections in as many as 50% of infants, but a mother unable to breastfeed can take solace in the fact that >95% of breastfed infants will not realize any benefit from the preventive potential of breastfeeding in regard to hospitalization or allergic disease, and >99% will not realize benefit from either the prevention of SIDS or ALL, or from improvement in long-term health measures (except for, perhaps, a slightly higher IQ). The “breast is best” mantra is likely true at a public-health level; for the individual mother–infant dyad, however, where there is a need to balance personal, social, family, and financial factors, that mantra is an oversimplification.
Regrettably, there is a paucity of data on the risks of breastfeeding promotion—an area that deserves more study. Balancing the abundant, but often limited-quality, data on the benefits of breastfeeding and the sheer lack of data regarding the risks of advocacy represents a clinical and an ethical challenge for physicians. It is a challenge that can only be resolved through individualization of care and shared decision-making, in which the physician is expert on the benefits of breastfeeding, and the mother is expert on the personal circumstances to be weighed against those benefits.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine, 101 Heart Drive, Greenville, NC 27834; [email protected].
The benefits of breastfeeding for infants have long been touted as numerous and supported by overwhelming evidence. The World Health Organization (WHO), American College of Obstetricians and Gynecologists, American Academy of Pediatrics (AAP), and American Academy of Family Physicians all strongly recommend exclusive breastfeeding for the first 6 months of life, citing numerous health benefits for child and mother. These groups recommend that some breastfeeding be continued through the first 12 months of life, or longer, as desired (the WHO extends the recommendation to 2 years).1-4 In 2000, the Surgeon General of the United States released a strategic plan to increase rates of breastfeeding,5 setting goals (by 2010) of:
- 75% of mothers leaving the hospital breastfeeding
- 50% of babies breastfeeding at 6 months
- 25% of babies breastfeeding at 1 year.
Massive public health campaigns citing data for the many benefits of breastfeeding have been launched with the goal of increasing the breastfeeding rate. In 2014, statistics offered a testament to the success of these campaigns6:
- 82.5% of infants had been breastfed “ever”
- 55.3% were breastfed “some”
- 24.9% were breastfed exclusively through 6 months of age
- 33.7% were breastfed “some” at 12 months.
Breastfeeding advocacy has become clouded
In recent years, an increasing number of researchers, physicians, and authors have begun to question whether, in the United States, the benefits of breastfeeding children are exaggerated and the emphasis on breastfeeding might be leading to feelings of inadequacy, guilt, and anxiety among mothers.7-13 In 2016, the US Preventive Services Task Force (USPSTF) amended its recommendation to “promote and support breastfeeding” to simply “support breastfeeding”—a change that created substantial debate and prompted the Task Force to clarify its stance in changing the language: In its response to public comment, the USPSTF said that its position regarding promotion had not changed, but the language in the original statement had been revised to “ensure that the autonomy of women is respected.” 2,14-16
In contrast, others suggest counseling women on the risks of formula feeding rather than on the benefits of breastfeeding, citing substantial health outcome distinctions.17 Indeed, wide-ranging conclusions have been drawn from the same data on the topic, potentially creating uncertainty for physicians on how best to counsel women on their choice of how to feed their infant.
In this article, we address this uncertainty by utilizing the most recent and comprehensive data to examine infant health outcomes. When possible, the number needed to treat (NNT) for a given outcome has been calculated or approximated, allowing the reader to estimate the likelihood of benefit for an individual mother–infant dyad. Exercise caution when interpreting the NNT, however: The numbers suggest causality that cannot be definitively established using the observational data on which those numbers are based.
Continue to: Infectious disease
Infectious disease
Acute otitis media. Exclusive breastfeeding for 6 months is associated with a 43% reduction in the risk of acute otitis media (AOM) by 2 years of age (odds ratio [OR]=0.57; 95% confidence interval [CI], 0.44-0.75). Beyond 2 years of age, or when comparing “ever” and “never” breastfeeding, the effect disappears. All studies in this meta-analysis had serious limitations.18
Nearly half of children will have at least one case of AOM by one year of age; 80%, by 2 years.19,20 Since the introduction of the heptavalent pneumococcal conjugate vaccine, the rate of AOM at 2 years has fallen by as much as 20%.21 Assuming an incidence of 60% to 80% of AOM by 2 years, only 2 or 3 infants need to be exclusively breastfed for 6 months to prevent a single case of AOM.18 Prevention of AOM through breastfeeding may be related to head position during feeding, antibacterial effects of breast milk, protective oral microbiome in the breastfed infant pharynx, and/or prevention of primary viral upper respiratory infection (URI), which nearly always precedes AOM.18,19
Upper and lower respiratory tract infections. Infants who are exclusively breastfed for 4 months and partially breastfed after 4 months have a lower risk of URI (OR=0.65; 95% CI, 0.51-0.83) and of lower respiratory tract infection (LRTI; OR=0.50; 95% CI, 0.32-0.72).22
The effect is stronger for URI among infants exclusively breastfed for at least 6 months (OR=0.37; 95% CI, 0.18-0.74), but is no longer significant by that time for LRTI (OR=0.33; 95% CI, 0.08-1.40). Importantly, AOM was included in the URI group, and, as previously discussed, AOM has independently been shown to have an inverse relationship with breastfeeding duration.
At 7 to 12 months of age, no association was seen between breastfeeding and the incidence of URI. Curiously, an association with LRTI was again detected for infants breastfed exclusively for 4 months and partially thereafter, but was not detected with exclusive breastfeeding for at least 6 months (OR=0.46; 95% CI, 0.31-0.69). In this study, in the first 6 months of life, 40% of infants had a URI and 8% had an LRTI. The findings in this cohort suggest an NNT of 6 or 7 for prevention of URI and an NNT of 25 for prevention of LRTI in the first 6 months of life.22
Continue to: Children younger than 2 years are...
Children younger than 2 years are estimated to have approximately 6 bouts of the common cold a year, and essentially 100% have at least one bout—perhaps lowering the NNT for URI if applied widely. However, these data are not divided into 6-month intervals, making accurate extrapolation difficult.23
Gastrointestinal infection. The rate of diarrheal illness in the first year of life is lower in infants who are exclusively breastfed for at least 4 months and partially breastfed after.
Both the Promotion of Breastfeeding Intervention Trial (PROBIT; a clinical trial in which infants were randomized to a breastfeeding education intervention or standard care) and a 2010 prospective cohort study in the Netherlands of more than 3400 infants found a reduction in the risk of one or more gastrointestinal (GI) infections at a similar rate.22,24
- In PROBIT, 9.1% of infants in the intervention group, compared to 13.2% in the standard care group (OR=0.60; 95% CI, 0.40-0.91), had one or more GI infections at 12 months of age.24
- In the 2010 Netherlands cohort, 8% of infants had a GI infection by 6 months of age. Infants breastfed exclusively for at least 4 or 6 months had a decreased risk for GI infection (respectively: adjusted OR=0.41; 95% CI, 0.26-0.64 and adjusted OR=0.46; 95% CI, 0.14-1.59). No such association was found for any feeding group 7 to 12 months of age.22
These studies are notable for the low incidence of GI infection, which is frequently cited as 1.3 to 2.3 episodes per child per year in children younger than 3 years in the United States.25 However, that high incidence has likely declined significantly since the introduction of rotavirus vaccine in 2006. In the years following the introduction of the vaccine, infant visits for gastroenteritis decreased by >90% in all care settings in the South, Northeast, and Midwest regions of the United States and by 53% to 63% in the West region.26 Recent accurate epidemiologic information, in an era of significantly higher vaccination rates, is lacking.
Assuming the low incidence of GI infection reported in PROBIT and the Netherlands trials, about 25 to 30 infants need to be exclusively breastfed for 4 to 6 months to prevent a single GI infection during the first 6 to 12 months of life.22,24 Assuming a 60% incidence by age 12 months before introduction of the rotavirus vaccine, the NNT would be approximately 4.24 The true number is likely somewhere between those 2 NNTs.
Continue to: Hospitalization
Hospitalization
Risk of infection is decreased. A large cohort study in Scotland, involving more than 500,000 children, found an association between exclusive breastfeeding for 6 to 8 weeks and decreased risk of hospitalization within the first 6 months of life. Formula-fed and mixed-fed infants had an increased hazard ratio (HR) for hospitalization for common childhood illness (HR=1.40; 95% CI, 1.35-1.45 for formula-fed infants and HR=1.18; 95% CI, 1.11-1.25 for mixed-fed infants).27 The study also found increased rates of hospitalization for conditions for which other meta-analyses have failed to show a protective effect from breastfeeding—leading to suspicion of residual confounding in the study. Another United Kingdom cohort demonstrated lower rates of hospitalization for GI infection (NNT=171) and LRTI (NNT=115) among exclusively breastfed infants by 8 months of age.28
Risk of neonatal readmission is increased. Late preterm infants who are exclusively breastfed are nearly twice as likely to be hospitalized as breastfed term or non-breastfed preterm infants, primarily due to dehydration, failure to thrive, weight loss, and hyperbilirubinemia. In fact, exclusive breastfeeding at discharge from the hospital is likely the single greatest risk factor for hospital readmission in newborns.29,30 Term infants who are exclusively breastfed are more likely to be hospitalized compared to formula-fed or mixed-fed infants, due to hyperbilirubinemia, dehydration, hypernatremia, and weight loss (number needed to harm (NNH)=71).30-32 For weight loss >10% of birth weight with or without hospitalization, the NNH for breastfed infants is 13.32
Many of these hospitalizations and events could be avoided with appropriate monitoring and medically indicated supplementation; the likelihood of long-term harm is low. Formula supplementation is often avoided if possible in hospitals to promote exclusive breastfeeding; however, several small randomized clinical trials have demonstrated that limited formula supplementation in breastfed infants does not affect the breastfeeding continuation rate at 3 and 6 months, and, therefore, might be a way to decrease infant rehospitalization.33,34
Necrotizing enterocolitis
In preterm infants, breastfeeding has been associated with a lower rate of necrotizing enterocolitis. In the 2007 Agency for Healthcare Research and Quality report, the association was found to be only marginally statistically significant, and the authors warned that, first, evidence is old and heterogeneous and, second, present preterm formula is much different than the formula used in earlier studies of preterm infant nutrition and necrotizing enterocolitis.35 A 2012 Cochrane review included newer studies in its analysis but reached the same conclusion on the quality and heterogeneity of available evidence, with a NNT of 25.36
Continue to: Sudden infant death syndrome
Sudden infant death syndrome
There is a statistically significant association between sudden infant death syndrome (SIDS) and feeding method. Infants whose cause of death is SIDS are approximately one half as likely to have been breastfed as matched controls.35,37
In 2005, AAP did not recommend breastfeeding as a means to reduce the risk of SIDS because available evidence was mixed, and studies at the time were poorly controlled.38 Since that time, case-control meta-analyses have shed additional light on the association between SIDS and feeding method.35,37
The protective effect exists for any amount of breastfeeding and is stronger for exclusive breastfeeding, suggesting a protective role—not simply an association. Caution should be employed with this conclusion, however, because the studies included in the meta-analysis used univariate analysis primarily and did not control sufficiently for known confounders. In addition, the authors warn that publication bias might overestimate the association.38
Potential mechanisms of a protective role include decreased risk of infection and greater arousability from sleep in breastfed infants. Assuming a protective role, available data suggest that more than 3500 infants need to be breastfed to prevent one case of SIDS.39
Continue to: Allergic disease
Allergic disease
Asthma. There is evidence of a small protective effect of breastfeeding “ever” on asthma at 5 to 18 years of age in high-income countries (OR=0.90; 95% CI, 0.83-0.97). A family history of asthma or atopy did not affect this finding. The authors note there is some evidence of publication bias in this review, which is the largest and most comprehensive on the topic.40
With a lifetime prevalence of asthma in the United States of approximately 13.2%, this association would confer an NNT of roughly 76.41 Earlier, the literature demonstrated mixed and conflicting evidence, and some experts suggested an effect only when there is a family history of asthma or atopy.36
Eczema. For children younger than 2 years, there is low-grade- and very-low-grade-quality evidence that exclusive breastfeeding longer than 3 to 4 months is associated with a reduced risk of eczema (OR=0.74; 95% CI, 0.57-0.97).40
Previously, data suggested that this association existed only in children who had a family history of atopy.35 The protective association, however, exists regardless of family history and does not persist beyond 2 years of age. The authors noted evidence of publication bias, reverse causation, and misdiagnosis of early childhood rashes as eczema as limitations of their findings.40
Continue to: Reliable epidemiologic evidence...
Reliable epidemiologic evidence on the incidence of eczema in infants in the United States is limited, but the prevalence in the United States in children younger than 17 years is approximately 10.7% (with wide regional variation). Extrapolating these data generously, the NNT to prevent eczema in the first 2 years of life could be estimated at approximately 36.42
Allergic rhinitis. There is low-grade- and very-low-grade-quality evidence that more breastfeeding, compared to less breastfeeding, is associated with a lower risk of allergic rhinitis in children younger than 5 years (OR=0.79; 95% CI, 0.63-0.98). The association exists regardless of family history and disappears after 5 years of age. The differentiation of allergic rhinitis from rhinovirus infection (for which there is higher-quality evidence of a protective effect with breastfeeding) must be considered when interpreting these data.40
Reliable epidemiologic evidence on allergic rhinitis in children younger than 5 years is lacking, and incidence varies by region. A rough estimate, using data from 6- and 7-year-olds, indicates an NNT of 54 to 70.43
Food allergy. There is no evidence to suggest an association between breastfeeding and food allergy, either as protective or as a risk factor, and studies are limited.40 Interestingly, as data accumulate associating early exposure to foods with protection, some authors have proposed reexamining the recommendation from WHO and US health organizations for exclusive breastfeeding for the first 6 months of life.7,44
Continue to: Dental health
Dental health
Dental caries. There is consistent evidence that breastfeeding beyond 12 months of age is associated with the development of dental caries of deciduous teeth to 6 years of age (OR=2.90; 95% CI, 2.33-3.60). Many of the studies that showed this association did not control for the introduction of sugary foods and drinks, and there was a trend toward publication bias showing the association.45
Dental malocclusion. There is consistent evidence for approximately a two-thirds reduction in malocclusions in deciduous teeth in breastfed infants (OR=0.32; 95% CI, 0.25-0.40). Although the large majority of these data come from low-income and middle-income countries, the incidence of malocclusion is not thought to be associated with socioeconomic status, as so many other breastfeeding outcomes are.46
Childhood leukemia
In the largest meta-analysis available, a statistically significant inverse relationship between any breastfeeding for >6 months and childhood leukemia is evident in developed countries (OR=0.84; 95% CI, 0.78-0.91), although significant heterogeneity among studies and lack of control for confounding variables are significant limitations. In particular, an association has been demonstrated with acute lymphoblastic leukemia (ALL) but not with acute myelogenous leukemia.47 Given the rarity of childhood ALL, approximately 12,500 infants would need to be breastfed to prevent one case.48
Continue to: Long-term outcomes
Long-term outcomes
Cognitive development. Several studies conducted in developed countries have linked breastfeeding to positive cognitive outcomes in children, including higher intelligence quotient (IQ).35,49-52
These effects are conflicting, however, in studies that include sibling analysis and ones that control for maternal IQ.8,35,43,52-54 In the 2013 WHO meta-analysis, breastfeeding was associated with an increase of 2.2 points on normalized testing when only high-quality studies were included.51 A 2015 meta-analysis identified 4 high-quality studies with a large sample size and recall time <3 years, which demonstrated a mean difference of 1.76 points in IQ (95% CI, 0.25-3.26) in childhood and adolescence.52 Although statistically significant, this modest increase is of questionable clinical benefit and of unknown duration.
Obesity. The relationship between breastfeeding and obesity later in life is debatable. A large, systematic 2014 review of 15 cohort and 10 cross-sectional studies found a significantly reduced risk of childhood obesity among children who were breastfed (adjusted OR=0.78; 95% CI, 0.74-0.81).55 However, the review included studies that controlled for different confounders, and smaller effects were found in studies in which more confounders were taken into account.
The 2013 WHO meta-analysis found a small (approximately 10%) reduction in the prevalence of overweight or obese children, but cautioned that residual confounding and publication bias were likely.51 At 6.5 and 11.5 years of follow-up, PROBIT failed to demonstrate a protective effect for exclusively or “ever” breastfed infants.56 Sibling analysis similarly fails to demonstrate a statistically significant relationship.8
Continue to: A 2015 meta-analysis of 23 high-quality studies...
A 2015 meta-analysis of 23 high-quality studies with a sample size >1500 children and controlled for important confounders showed a pooled reduction in the prevalence of overweight or obesity of 13% (95% CI, 6-19).57 The protection in this meta-analysis showed a dilution of the effect as the participants aged and an inverse relationship of the effect with sample size.
Breastfeeding is, therefore, unlikely to play a significant, if any, role in combatting the obesity epidemic.
Hypertension. A meta-analysis of high-quality trials demonstrates a <1 mm Hg reduction in systolic blood pressure and no significant difference in diastolic pressure in breastfed infants.57 Similarly, no significant effect of breastfeeding on blood pressure has been demonstrated in trials of preterm infants.51
Type 2 diabetes. Available data are limited and heterogeneous for the association between breastfeeding and later development of type 2 diabetes. Only 2 high-quality trials were identified in the 2013 WHO meta-analysis, and their results conflict.51 A 2015 meta-analysis identified only 3 high-quality studies, without a statistically significant relationship.57
Dyslipidemia. Although earlier data suggested an association between breastfeeding and reduced cholesterol levels later in life, the 2013 WHO meta-analysis and a 2015 meta-analysis concluded that no association exists. The limited data available for preterm infants conflict.51,57
Growth. There is no evidence that feeding method has a short- or long-term effect on weight gain or length gain in preterm or term infants.35,36,58
Death. No clear association has been found between mortality and breastfeeding status in developed countries, except for the association with SIDS.35
Continue to: What issues frame and guide counseling on breastfeeding?
What issues frame and guide counseling on breastfeeding?
There is that “problem” with the evidence. The evidence for infant breastfeeding status and its association with health outcomes faces significant limitations; the great majority of those limitations tend to overestimate the benefits of breastfeeding. Nearly all evidence is based on observational studies, in which causality cannot be determined and self-selection bias, recall bias, and residual confounding limit the value or strength of the findings.
Breastfeeding rates are strongly socially patterned alongside socioeconomic status, race, and education level, all of which are simultaneously strongly tied to short- and long-term health outcomes.6 Other factors limiting the strength of the data set include varying definitions of infant feeding practices in different studies, varying definitions of outcomes and diseases, reverse causation, and evidence of publication bias in many meta-analyses. Given these shortcomings, the NNTs in this article probably represent a best-case scenario for breastfeeding outcomes for infants in the United States (TABLE 118,22-24,28,36,39-43,47,48).

Data need to be put into context. The NNTs for many breastfeeding outcomes (TABLE) compare favorably with other recommended interventions, particularly for other preventive care measures. Two examples: 81 mg/d aspirin for a 50-year-old man has an NNT of 35 to 45 for preventing nonfatal myocardial infarction, and the number needed to invite to screen with mammography to prevent one breast cancer death for a 50-year-old woman is 1339.59,60
In both of these examples, >95% of patients will not benefit from the intervention, yet these preventive measures are routinely recommended and have a significant impact at the public health level. Notably, these outcomes are more serious than most breastfeeding outcomes; have a longer-lasting effect, better-quality data, and better data for potential harms; are causally linked to the intervention; and require much less effort and commitment of time than breastfeeding.
The question must be reckoned with: Can advocacy be harmful?
In recent years, a growing number of concerns have been raised about:
- the potential harms of breastfeeding advocacy
- exaggeration of the benefits of breastfeeding
- promotion of breastfeeding at the expense of evidence-based medicine.
The “Ten Steps to Successful Breastfeeding” program of the Baby-friendly Hospital Initiative (BFHI; launched by UNICEF and WHO) has come under scrutiny because of an increasing number of reports of sudden unexpected postnatal collapse; fall injuries; modeling and encouragement of unsafe sleep practices; an overly rigid resistance to the use of formula supplementation; and the ban on pacifier use.61,62 The BFHI, promoted by the Centers for Disease Control and Prevention, is increasingly being adopted by hospitals with the expressed goal of increasing the breastfeeding rate from birth to discharge.
Continue to: Some of the "Ten Steps"...
Some of the “Ten Steps,” such as the call for skin-to-skin care and 24-hour rooming-in, have well-established benefit yet, when performed without supervision, can have the rare but serious unintended consequences of sudden unexpected postnatal collapse (the incidence of which may be higher than that of SIDS) and unsafe sleeping practices.62,63
Furthermore, despite evidence that early formula supplementation, when medically necessary, does not adversely impact the breastfeeding rate, the “Ten Steps” program advises that giving formula before breast milk comes in might “lead to failure to breastfeed.”33,34,61,63
Similarly, the ban on pacifiers is contrary to available evidence. The use of pacifiers before last sleep is more protective against SIDS than breastfeeding (NNT=2733), and there is evidence at one hospital that BFHI-inspired pacifier restriction is associated with a decrease in the rate of breastfeeding.64,65
Other harms of advocacy are even more poorly studied. Most of the evidence for harm comes from the psychology and social science literature—not the medical literature, perhaps because the prevailing opinion in the medical community is that breastfeeding has overwhelming evidence for benefit. In fact, in the USPSTF’s 2008 recommendation, the evidence review of breastfeeding promotion practices in primary care did not identify a single study that measured harm; in the 2016 update of that recommendation, only 2 such studies were identified.15,66
The literature that does investigate harm consistently finds that women who have difficulty breastfeeding or choose formula feeding report feelings of inadequacy, guilt, loss of agency, anxiety, and physical pain during breastfeeding that interferes with 1) their ability to bond or otherwise care for their infant and 2) competing work obligations.11-13,67-69 Given the lack of attention paid to these variables in the medical literature, it is the individual mother who is best positioned to weigh these factors against the benefits of breastfeeding.
Continue to: Shared decision-making is best—for mother and baby
Shared decision-making is best—for mother and baby
Breastfeeding might prevent certain infections in as many as 50% of infants, but a mother unable to breastfeed can take solace in the fact that >95% of breastfed infants will not realize any benefit from the preventive potential of breastfeeding in regard to hospitalization or allergic disease, and >99% will not realize benefit from either the prevention of SIDS or ALL, or from improvement in long-term health measures (except for, perhaps, a slightly higher IQ). The “breast is best” mantra is likely true at a public-health level; for the individual mother–infant dyad, however, where there is a need to balance personal, social, family, and financial factors, that mantra is an oversimplification.
Regrettably, there is a paucity of data on the risks of breastfeeding promotion—an area that deserves more study. Balancing the abundant, but often limited-quality, data on the benefits of breastfeeding and the sheer lack of data regarding the risks of advocacy represents a clinical and an ethical challenge for physicians. It is a challenge that can only be resolved through individualization of care and shared decision-making, in which the physician is expert on the benefits of breastfeeding, and the mother is expert on the personal circumstances to be weighed against those benefits.
CORRESPONDENCE
Joseph Lane Wilson, MD, ECU Brody School of Medicine, Department of Family Medicine, 101 Heart Drive, Greenville, NC 27834; [email protected].
1. Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization, and New York, NY: UNICEF; 2003. Available at: www.who.int/maternal_child_adolescent/documents/9241562218/en/. Accessed April 4, 2018.
2. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Breastfeeding Expert Work Group. Committee Opinion No. 658: Optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2016;127:e86-e92.
3. Gartner LM, Morton J, Lawrence RA, et al; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
4. Breastfeeding (policy statement). Leawood, KS: American Academy of Family Physicians; 2007. Available at: https://www.aafp.org/about/policies/all/breastfeeding.html. Accessed April 3, 2018.
5. Office of the Surgeon General (US); Centers for Disease Control and Prevention (US); Office on Women’s Health (US). The Surgeon General’s call to action to support breastfeeding. Rockville, MD: US Department of Health and Human Services; 2011. Available at: www.surgeongeneral.gov/library/calls/breastfeeding/index.html. Updated August 12, 2014. Accessed April 4, 2018.
6. Breastfeeding: data & statistics. Atlanta, GA: Centers for Disease Control and Prevention; December 11, 2017. Available at: http://www.cdc.gov/breastfeeding/data/. Accessed May 17, 2018.
7. Fewtrell M, Wilson DC, Booth I, et al. A. Six months of exclusive breast feeding: how good is the evidence? BMJ. 2010;342:c5955.
8. Colen CG, Ramey DM. Is breast truly best? Estimating the effect of breastfeeding on long-term child wellbeing in the United States using sibling comparisons. Soc Sci Med. 2014;109:55-65.
9. Wolf J. Is Breast Best? Taking on the Breastfeeding Experts and the New High Stakes of Motherhood. New York, NY: NYU Press; 2010.
10. Tuteur A. Push Back: Guilt in the Age of Natural Parenting. New York, NY: HarperCollins Publishers; 2016.
11. Lee E. Health, morality, and infant feeding: British mothers’ experiences of formula milk use in the early weeks. Sociol Health Illn. 2007;29:1075-1090.
12. Williams K, Donaghue N, Kurz T. “Giving guilt the flick”?: an investigation of mothers’ talk about guilt in relation to infant feeding. Psychol Women Q. 2013;37:97-112.
13. Fahlquist JN, Roeser S. Ethical problems with information on infant feeding in developed countries. J Health Polit Policy Law. 2012;37:155-160.
14. U.S. Preventive Services Task Force. Final Recommendation Statement. Breastfeeding: Counseling. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breastfeeding-counseling. Accessed April 4, 2018.
15. US Preventive Services Task Force. Primary Care Interventions to Support Breastfeeding: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1688-1693.
16. Zahn CM, Hanley LE. Concerns over USPSTF draft recommendation on breastfeeding interventions [letter]. Washington, DC: The American College of Obstetricians and Gynecologists; May 18, 2016. Available at: https://www.acog.org/-/media/Departments/Breastfeeding/Breast-Feeding-ACOG-USPSTF.pdf?dmc=1&ts=20180518T1850056558. Accessed May 22, 2018.
17. Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222-231.
18. Bowatte G, Tham R, Allen KJ, et al. Breastfeeding and childhood acute otitis media: a systematic review and meta-analysis. Acta Paediatr. 2015;104:85-95.
19. Chonmaitree T, Trujillo R, Jennings K, et al. Acute otitis media and other complications of viral respiratory infection. Pediatrics. 2016;137:e20153555.
20. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
21. Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118:865-873.
22. Duijts L, Jaddoe VW, Hofman A, et al. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126:e18-e25.
23. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186:190-199.
24. Kramer MS, Chalmers B, Hodnett ED, et al; PROBIT Study Group (Promotion of Breastfeeding Intervention Trial). Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413-420.
25. Dennehy PH. Acute diarrheal disease in children: epidemiology, prevention, and treatment. Infect Dis Clin North Am. 2005;19:585-602.
26. Cortese MM, Tate JE, Simonsen L, et al. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatric Infect Dis J. 2010;29:489-494.
27. Ajetunmobi OM, Whyte B, Chalmers J, et al. Breastfeeding is associated with reduced childhood hospitalization: evidence from a Scottish birth cohort (1997-2009). J Pediatr. 2015;166:620-625.
28. Quigley MA, Kelly YJ, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007;119:e837-e842.
29. Radtke JV. The paradox of breastfeeding-associated morbidity among late preterm infants. J Obstet Gynecol Neonatal Nurs. 2011;40:9-24.
30. Escobar GJ, Gonzales VM, Armstrong M, et al. Rehospitalization for neonatal dehydration: a nested case-control study. Arch Pediatr Adolesc Med. 2002;156:155-161.
31. Salas AA, Salazar J, Burgoa CV, et al. Significant weight loss in breastfed term infants readmitted for hyperbilirubinemia. BMC Pediatr. 2009;9:82.
32. Tarcan A, Tiker F, Vatandaş NS, et al. Weight loss and hypernatremia in breast-fed babies: frequency in neonates with non-hemolytic jaundice. J Paediatr Child Health. 2005;41:484-487.
33. Flaherman VJ, Aby J, Burgos AE, et al. Effect of early limited formula on duration and exclusivity of breastfeeding in at-risk infants: an RCT. Pediatrics. 2013;131:1059-1065.
34. Straňák Z, Feyereislova S, Černá M, et al. J. Limited amount of formula may facilitate breastfeeding: randomized, controlled trial to compare standard clinical practice versus limited supplemental feeding. Denning PW, ed. PLoS One. 2016;11:e0150053.
35. Ip S, Chung M, Raman G, et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Rockville, MD: Agency for Healthcare Research and Quality (US); 2007. Evidence Reports/Technology Assessments, No. 153. Available at: www.ncbi.nlm.nih.gov/books/NBK38337/. Accessed April 3, 2018.
36. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;(4):CD002971.
37. Hauck FR, Thompson JM, Tanabe KO, et al. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128:103-110.
38. American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245-1255.
39. Moon RY, Fu L. Sudden infant death syndrome: an update. Pediatr Rev. 2012;33:314-320.
40. Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38-53.
41. Brim SN, Rudd RA, Funk RH, et al. Asthma prevalence among US children in underrepresented minority populations: American Indian/Alaska Native, Chinese, Filipino, and Asian Indian. Pediatrics. 2008;122:e217-e222.
42. Shaw TF, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
43. Mallol J, Crane J, von Mutius E, et al. The international study of asthma and allergies in childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73-85.
44. Flohr C, Nagel G, Weinmayr G, et al. Lack of evidence for a protective effect of prolonged breastfeeding on childhood eczema: lessons from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br J Dermatol. 2011;165:1280-1289.
45. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
46. Peres KG, Cascaes AM, Nascimento GG, et al. Effect of breastfeeding on malocclusions: a systematic review and meta-analysis. Acta Paediatr. 2015;104:54-61.
47. Amitya EL, Keinan-Boker L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 2015;169:e151025.
48. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943-1955.
49. Guxens M, Mendez MA, Moltó-Puigmartí C, et al. Breastfeeding, long-chain polyunsaturated fatty acids in colostrum, and infant mental development.
2012;129:1134-1140.
51. Horta BL, Victora CG. Long-term effects of breastfeeding: a systematic review. Geneva, Switzerland: World Health Organization; 2013. Available at: http://apps.who.int/iris/bitstream/10665/79198/1/9789241505307_eng.pdf. Accessed August 16, 2016.
52. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104:14-19.
53. Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333:945.
54. Sajjad A, Tharner A, Kiefte-de Jong JC, et al. Breastfeeding duration and non-verbal IQ in children. J Epidemiol Community Health 2015;69:775-781.
55. Yan J, Liu L, Zhu Y, et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267.
56. Martin RM, Patel R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
57. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure, and type 2 diabetes: systematic review and meta-analysis. Acta Paediatr. 2015;104:30-37.
58. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Syst Rev. 2012;15:CD003517.
59. U.S. Preventive Services Task Force. Final recommendation statement: aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Published September 2017. Accessed April 3, 2018.
60. U.S. Preventive Services Task Force. Screening for breast cancer. Available at: www.uspreventiveservicestaskforce.org/Page/SupportingDoc/breast-cancer-screening/final-evidence-summary9. Published November 2009. Accessed April 2, 2018.
61. Bass JL, Gartley T, Kleinman R. Unintended consequences of current breastfeeding initiatives. JAMA Pediatr. 2016;170:923-924.
62. Feldman-Winter L, Goldsmith JP; Committee on Fetus and Newborn; Task Force on Sudden Infant Death Syndrome. Safe sleep and skin-to-skin care in the neonatal period for healthy term newborns. Pediatrics. 2016;138:e20161889.
63. The Mother and Child Health and Education Trust. Ten steps to successful breastfeeding. Available at: www.tensteps.org. Published November 8, 2017. Accessed April 3, 2018.
64. Hauck FR, Omojokun OO, Siadaty MS. Do pacifiers reduce the risk of sudden infant death syndrome? A meta-analysis. Pediatrics. 2005;116:e716-e723.
65. Kair LR, Kenron D, Etheredge K, et al. Pacifier restriction and exclusive breastfeeding. Pediatrics. 2013;131:e1101-e1107.
66. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
67. Wolf JB. Is breast really best? Risk and total motherhood in the National Breastfeeding Awareness Campaign. J Health Polit Policy Law. 2007;32:595-636.
68. Marshall JL, Godfrey M, Renfrew MJ. Being a ‘good mother’: managing breastfeeding and merging identities. Soc Sci Med. 2007;65:2147-2159.
69. Kelleher CM. The physical challenges of early breastfeeding. Soc Sci Med. 2006;63:2727-2738.
1. Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization, and New York, NY: UNICEF; 2003. Available at: www.who.int/maternal_child_adolescent/documents/9241562218/en/. Accessed April 4, 2018.
2. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice; Breastfeeding Expert Work Group. Committee Opinion No. 658: Optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2016;127:e86-e92.
3. Gartner LM, Morton J, Lawrence RA, et al; American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496-506.
4. Breastfeeding (policy statement). Leawood, KS: American Academy of Family Physicians; 2007. Available at: https://www.aafp.org/about/policies/all/breastfeeding.html. Accessed April 3, 2018.
5. Office of the Surgeon General (US); Centers for Disease Control and Prevention (US); Office on Women’s Health (US). The Surgeon General’s call to action to support breastfeeding. Rockville, MD: US Department of Health and Human Services; 2011. Available at: www.surgeongeneral.gov/library/calls/breastfeeding/index.html. Updated August 12, 2014. Accessed April 4, 2018.
6. Breastfeeding: data & statistics. Atlanta, GA: Centers for Disease Control and Prevention; December 11, 2017. Available at: http://www.cdc.gov/breastfeeding/data/. Accessed May 17, 2018.
7. Fewtrell M, Wilson DC, Booth I, et al. A. Six months of exclusive breast feeding: how good is the evidence? BMJ. 2010;342:c5955.
8. Colen CG, Ramey DM. Is breast truly best? Estimating the effect of breastfeeding on long-term child wellbeing in the United States using sibling comparisons. Soc Sci Med. 2014;109:55-65.
9. Wolf J. Is Breast Best? Taking on the Breastfeeding Experts and the New High Stakes of Motherhood. New York, NY: NYU Press; 2010.
10. Tuteur A. Push Back: Guilt in the Age of Natural Parenting. New York, NY: HarperCollins Publishers; 2016.
11. Lee E. Health, morality, and infant feeding: British mothers’ experiences of formula milk use in the early weeks. Sociol Health Illn. 2007;29:1075-1090.
12. Williams K, Donaghue N, Kurz T. “Giving guilt the flick”?: an investigation of mothers’ talk about guilt in relation to infant feeding. Psychol Women Q. 2013;37:97-112.
13. Fahlquist JN, Roeser S. Ethical problems with information on infant feeding in developed countries. J Health Polit Policy Law. 2012;37:155-160.
14. U.S. Preventive Services Task Force. Final Recommendation Statement. Breastfeeding: Counseling. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breastfeeding-counseling. Accessed April 4, 2018.
15. US Preventive Services Task Force. Primary Care Interventions to Support Breastfeeding: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:1688-1693.
16. Zahn CM, Hanley LE. Concerns over USPSTF draft recommendation on breastfeeding interventions [letter]. Washington, DC: The American College of Obstetricians and Gynecologists; May 18, 2016. Available at: https://www.acog.org/-/media/Departments/Breastfeeding/Breast-Feeding-ACOG-USPSTF.pdf?dmc=1&ts=20180518T1850056558. Accessed May 22, 2018.
17. Stuebe A. The risks of not breastfeeding for mothers and infants. Rev Obstet Gynecol. 2009;2:222-231.
18. Bowatte G, Tham R, Allen KJ, et al. Breastfeeding and childhood acute otitis media: a systematic review and meta-analysis. Acta Paediatr. 2015;104:85-95.
19. Chonmaitree T, Trujillo R, Jennings K, et al. Acute otitis media and other complications of viral respiratory infection. Pediatrics. 2016;137:e20153555.
20. Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83-94.
21. Grijalva CG, Poehling KA, Nuorti JP, et al. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118:865-873.
22. Duijts L, Jaddoe VW, Hofman A, et al. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126:e18-e25.
23. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186:190-199.
24. Kramer MS, Chalmers B, Hodnett ED, et al; PROBIT Study Group (Promotion of Breastfeeding Intervention Trial). Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413-420.
25. Dennehy PH. Acute diarrheal disease in children: epidemiology, prevention, and treatment. Infect Dis Clin North Am. 2005;19:585-602.
26. Cortese MM, Tate JE, Simonsen L, et al. Reduction in gastroenteritis in United States children and correlation with early rotavirus vaccine uptake from national medical claims databases. Pediatric Infect Dis J. 2010;29:489-494.
27. Ajetunmobi OM, Whyte B, Chalmers J, et al. Breastfeeding is associated with reduced childhood hospitalization: evidence from a Scottish birth cohort (1997-2009). J Pediatr. 2015;166:620-625.
28. Quigley MA, Kelly YJ, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007;119:e837-e842.
29. Radtke JV. The paradox of breastfeeding-associated morbidity among late preterm infants. J Obstet Gynecol Neonatal Nurs. 2011;40:9-24.
30. Escobar GJ, Gonzales VM, Armstrong M, et al. Rehospitalization for neonatal dehydration: a nested case-control study. Arch Pediatr Adolesc Med. 2002;156:155-161.
31. Salas AA, Salazar J, Burgoa CV, et al. Significant weight loss in breastfed term infants readmitted for hyperbilirubinemia. BMC Pediatr. 2009;9:82.
32. Tarcan A, Tiker F, Vatandaş NS, et al. Weight loss and hypernatremia in breast-fed babies: frequency in neonates with non-hemolytic jaundice. J Paediatr Child Health. 2005;41:484-487.
33. Flaherman VJ, Aby J, Burgos AE, et al. Effect of early limited formula on duration and exclusivity of breastfeeding in at-risk infants: an RCT. Pediatrics. 2013;131:1059-1065.
34. Straňák Z, Feyereislova S, Černá M, et al. J. Limited amount of formula may facilitate breastfeeding: randomized, controlled trial to compare standard clinical practice versus limited supplemental feeding. Denning PW, ed. PLoS One. 2016;11:e0150053.
35. Ip S, Chung M, Raman G, et al. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Rockville, MD: Agency for Healthcare Research and Quality (US); 2007. Evidence Reports/Technology Assessments, No. 153. Available at: www.ncbi.nlm.nih.gov/books/NBK38337/. Accessed April 3, 2018.
36. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;(4):CD002971.
37. Hauck FR, Thompson JM, Tanabe KO, et al. Breastfeeding and reduced risk of sudden infant death syndrome: a meta-analysis. Pediatrics. 2011;128:103-110.
38. American Academy of Pediatrics Task Force on Sudden Infant Death Syndrome. The changing concept of sudden infant death syndrome: diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245-1255.
39. Moon RY, Fu L. Sudden infant death syndrome: an update. Pediatr Rev. 2012;33:314-320.
40. Lodge CJ, Tan DJ, Lau MX, et al. Breastfeeding and asthma and allergies: a systematic review and meta-analysis. Acta Paediatr. 2015;104:38-53.
41. Brim SN, Rudd RA, Funk RH, et al. Asthma prevalence among US children in underrepresented minority populations: American Indian/Alaska Native, Chinese, Filipino, and Asian Indian. Pediatrics. 2008;122:e217-e222.
42. Shaw TF, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
43. Mallol J, Crane J, von Mutius E, et al. The international study of asthma and allergies in childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr). 2013;41:73-85.
44. Flohr C, Nagel G, Weinmayr G, et al. Lack of evidence for a protective effect of prolonged breastfeeding on childhood eczema: lessons from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Two. Br J Dermatol. 2011;165:1280-1289.
45. Tham R, Bowatte G, Dharmage SC, et al. Breastfeeding and the risk of dental caries: a systematic review and meta-analysis. Acta Paediatr. 2015;104:62-84.
46. Peres KG, Cascaes AM, Nascimento GG, et al. Effect of breastfeeding on malocclusions: a systematic review and meta-analysis. Acta Paediatr. 2015;104:54-61.
47. Amitya EL, Keinan-Boker L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 2015;169:e151025.
48. Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381:1943-1955.
49. Guxens M, Mendez MA, Moltó-Puigmartí C, et al. Breastfeeding, long-chain polyunsaturated fatty acids in colostrum, and infant mental development.
2012;129:1134-1140.
51. Horta BL, Victora CG. Long-term effects of breastfeeding: a systematic review. Geneva, Switzerland: World Health Organization; 2013. Available at: http://apps.who.int/iris/bitstream/10665/79198/1/9789241505307_eng.pdf. Accessed August 16, 2016.
52. Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104:14-19.
53. Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta-analysis. BMJ. 2006;333:945.
54. Sajjad A, Tharner A, Kiefte-de Jong JC, et al. Breastfeeding duration and non-verbal IQ in children. J Epidemiol Community Health 2015;69:775-781.
55. Yan J, Liu L, Zhu Y, et al. The association between breastfeeding and childhood obesity: a meta-analysis. BMC Public Health. 2014;14:1267.
56. Martin RM, Patel R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
57. Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure, and type 2 diabetes: systematic review and meta-analysis. Acta Paediatr. 2015;104:30-37.
58. Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database of Syst Rev. 2012;15:CD003517.
59. U.S. Preventive Services Task Force. Final recommendation statement: aspirin use to prevent cardiovascular disease and colorectal cancer: preventive medication. Available at: www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/aspirin-to-prevent-cardiovascular-disease-and-cancer. Published September 2017. Accessed April 3, 2018.
60. U.S. Preventive Services Task Force. Screening for breast cancer. Available at: www.uspreventiveservicestaskforce.org/Page/SupportingDoc/breast-cancer-screening/final-evidence-summary9. Published November 2009. Accessed April 2, 2018.
61. Bass JL, Gartley T, Kleinman R. Unintended consequences of current breastfeeding initiatives. JAMA Pediatr. 2016;170:923-924.
62. Feldman-Winter L, Goldsmith JP; Committee on Fetus and Newborn; Task Force on Sudden Infant Death Syndrome. Safe sleep and skin-to-skin care in the neonatal period for healthy term newborns. Pediatrics. 2016;138:e20161889.
63. The Mother and Child Health and Education Trust. Ten steps to successful breastfeeding. Available at: www.tensteps.org. Published November 8, 2017. Accessed April 3, 2018.
64. Hauck FR, Omojokun OO, Siadaty MS. Do pacifiers reduce the risk of sudden infant death syndrome? A meta-analysis. Pediatrics. 2005;116:e716-e723.
65. Kair LR, Kenron D, Etheredge K, et al. Pacifier restriction and exclusive breastfeeding. Pediatrics. 2013;131:e1101-e1107.
66. Chung M, Raman G, Trikalinos T, et al. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565-582.
67. Wolf JB. Is breast really best? Risk and total motherhood in the National Breastfeeding Awareness Campaign. J Health Polit Policy Law. 2007;32:595-636.
68. Marshall JL, Godfrey M, Renfrew MJ. Being a ‘good mother’: managing breastfeeding and merging identities. Soc Sci Med. 2007;65:2147-2159.
69. Kelleher CM. The physical challenges of early breastfeeding. Soc Sci Med. 2006;63:2727-2738.
From The Journal of Family Practice | 2018;67(6):E1-E9.
PRACTICE RECOMMENDATIONS
› Encourage breastfeeding for its potential to reduce the risk of acute otitis media, upper- and lower-respiratory infections, gastrointestinal infection, and dental malocclusion. A
› Promote breastfeeding for its potential to make a small difference in intelligence quotient and the incidence of overweight and obesity—but not for any other significant impact on long-term health. B
› Consider the needs and preferences of the individual when advocating breastfeeding so as to avoid potentially engendering maternal feelings of guilt and inadequacy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series