User login
Testosterone Replacement May Cause ... Fracture?
This transcript has been edited for clarity.
I am showing you a graph without any labels.
What could this line represent? The stock price of some company that made a big splash but failed to live up to expectations? An outbreak curve charting the introduction of a new infectious agent to a population? The performance of a viral tweet?
I’ll tell you what it is in a moment, but I wanted you to recognize that there is something inherently wistful in this shape, something that speaks of past glory and inevitable declines. It’s a graph that induces a feeling of resistance — no, do not go gently into that good night.
The graph actually represents (roughly) the normal level of serum testosterone in otherwise-healthy men as they age.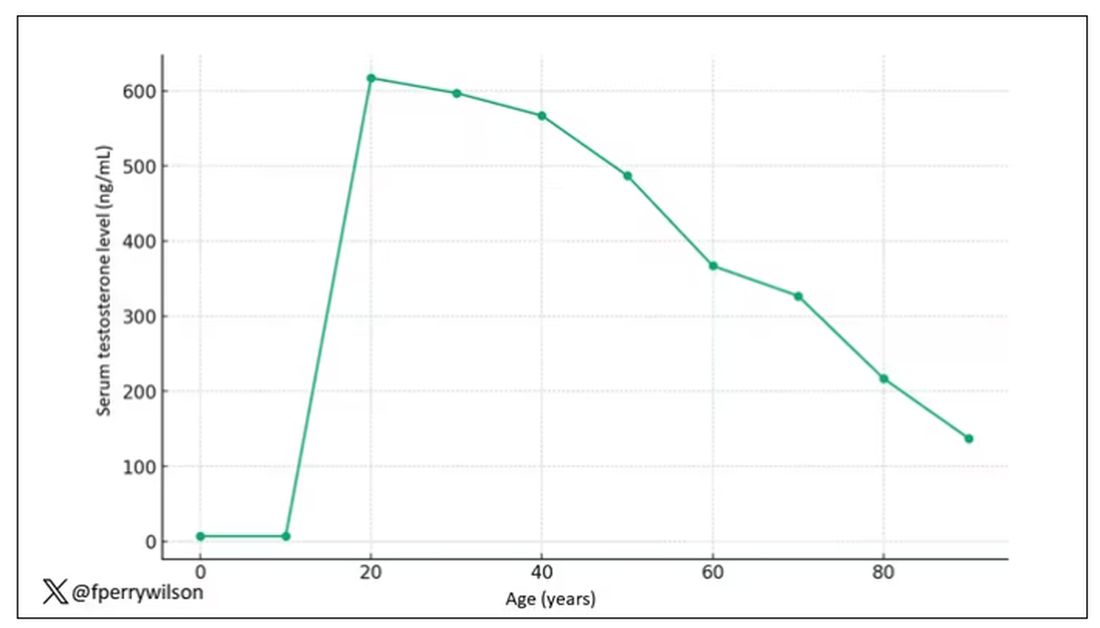
A caveat here: These numbers are not as well defined as I made them seem on this graph, particularly for those older than 65 years. But it is clear that testosterone levels decline with time, and the idea to supplement testosterone is hardly new. Like all treatments, testosterone supplementation has risks and benefits. Some risks are predictable, like exacerbating the symptoms of benign prostatic hyperplasia. Some risks seem to come completely out of left field. That’s what we have today, in a study suggesting that testosterone supplementation increases the risk for bone fractures.
Let me set the stage here by saying that nearly all prior research into the effects of testosterone supplementation has suggested that it is pretty good for bone health. It increases bone mineral density, bone strength, and improves bone architecture.
So if you were to do a randomized trial of testosterone supplementation and look at fracture risk in the testosterone group compared with the placebo group, you would expect the fracture risk would be much lower in those getting supplemented. Of course, this is why we actually do studies instead of assuming we know the answer already — because in this case, you’d be wrong.
I’m talking about this study, appearing in The New England Journal of Medicine.
It’s a prespecified secondary analysis of a randomized trial known as the TRAVERSE trial, which randomly assigned 5246 men with low testosterone levels to transdermal testosterone gel vs placebo. The primary goal of that trial was to assess the cardiovascular risk associated with testosterone supplementation, and the major take-home was that there was no difference in cardiovascular event rates between the testosterone and placebo groups.
This secondary analysis looked at fracture incidence. Researchers contacted participants multiple times in the first year of the study and yearly thereafter. Each time, they asked whether the participant had sustained a fracture. If they answered in the affirmative, a request for medical records was made and the researchers, still blinded to randomization status, adjudicated whether there was indeed a fracture or not, along with some details as to location, situation, and so on.
This was a big study, though, and that translates to just a 3.5% fracture rate in testosterone vs 2.5% in control, but the difference was statistically significant.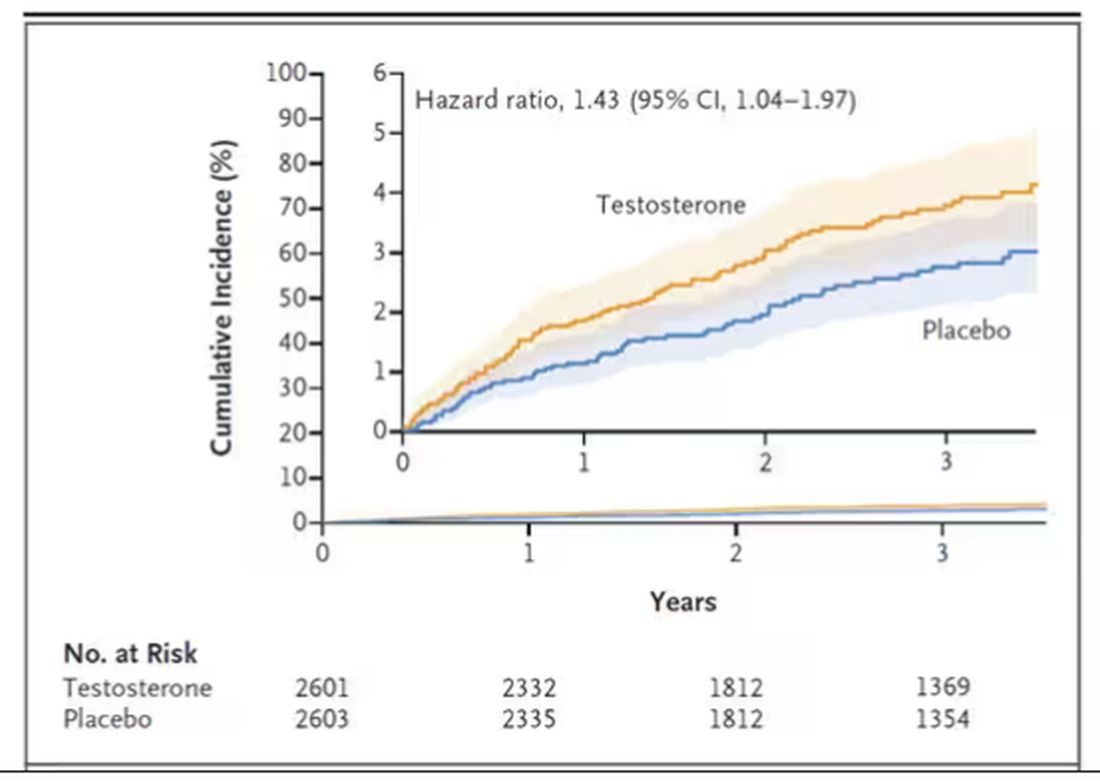
This difference persisted across various fracture types (non–high-impact fractures, for example) after excluding the small percentage of men taking osteoporosis medication.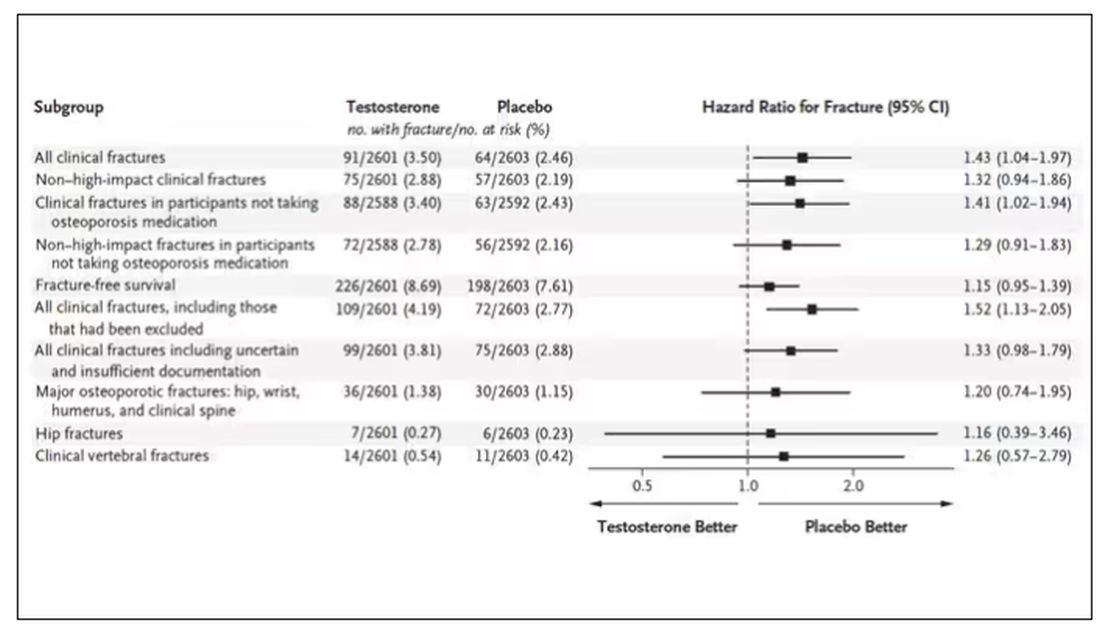
How does a drug that increases bone mineral density and bone strength increase the risk for fracture?
Well, one clue — and this was pointed out in a nice editorial by Matthis Grossman and Bradley Anawalt — is that the increased risk for fracture occurs quite soon after starting treatment, which is not consistent with direct bone effects. Rather, this might represent behavioral differences. Testosterone supplementation seems to increase energy levels; might it lead men to engage in activities that put them at higher risk for fracture?
Regardless of the cause, this adds to our knowledge about the rather complex mix of risks and benefits of testosterone supplementation and probably puts a bit more weight on the risks side. The truth is that testosterone levels do decline with age, as do many things, and it may not be appropriate to try to fight against that in all people. It’s worth noting that all of these studies use low levels of total serum testosterone as an entry criterion. But total testosterone is not what your body “sees.” It sees free testosterone, the portion not bound to sex hormone–binding globulin. And that binding protein is affected by lots of stuff — diabetes and obesity lower it, for example — making total testosterone levels seem low when free testosterone might be just fine.
In other words, testosterone supplementation is probably not terrible, but it is definitely not the cure for aging. In situations like this, we need better data to guide exactly who will benefit from the therapy and who will only be exposed to the risks.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I am showing you a graph without any labels.
What could this line represent? The stock price of some company that made a big splash but failed to live up to expectations? An outbreak curve charting the introduction of a new infectious agent to a population? The performance of a viral tweet?
I’ll tell you what it is in a moment, but I wanted you to recognize that there is something inherently wistful in this shape, something that speaks of past glory and inevitable declines. It’s a graph that induces a feeling of resistance — no, do not go gently into that good night.
The graph actually represents (roughly) the normal level of serum testosterone in otherwise-healthy men as they age.
A caveat here: These numbers are not as well defined as I made them seem on this graph, particularly for those older than 65 years. But it is clear that testosterone levels decline with time, and the idea to supplement testosterone is hardly new. Like all treatments, testosterone supplementation has risks and benefits. Some risks are predictable, like exacerbating the symptoms of benign prostatic hyperplasia. Some risks seem to come completely out of left field. That’s what we have today, in a study suggesting that testosterone supplementation increases the risk for bone fractures.
Let me set the stage here by saying that nearly all prior research into the effects of testosterone supplementation has suggested that it is pretty good for bone health. It increases bone mineral density, bone strength, and improves bone architecture.
So if you were to do a randomized trial of testosterone supplementation and look at fracture risk in the testosterone group compared with the placebo group, you would expect the fracture risk would be much lower in those getting supplemented. Of course, this is why we actually do studies instead of assuming we know the answer already — because in this case, you’d be wrong.
I’m talking about this study, appearing in The New England Journal of Medicine.
It’s a prespecified secondary analysis of a randomized trial known as the TRAVERSE trial, which randomly assigned 5246 men with low testosterone levels to transdermal testosterone gel vs placebo. The primary goal of that trial was to assess the cardiovascular risk associated with testosterone supplementation, and the major take-home was that there was no difference in cardiovascular event rates between the testosterone and placebo groups.
This secondary analysis looked at fracture incidence. Researchers contacted participants multiple times in the first year of the study and yearly thereafter. Each time, they asked whether the participant had sustained a fracture. If they answered in the affirmative, a request for medical records was made and the researchers, still blinded to randomization status, adjudicated whether there was indeed a fracture or not, along with some details as to location, situation, and so on.
This was a big study, though, and that translates to just a 3.5% fracture rate in testosterone vs 2.5% in control, but the difference was statistically significant.
This difference persisted across various fracture types (non–high-impact fractures, for example) after excluding the small percentage of men taking osteoporosis medication.
How does a drug that increases bone mineral density and bone strength increase the risk for fracture?
Well, one clue — and this was pointed out in a nice editorial by Matthis Grossman and Bradley Anawalt — is that the increased risk for fracture occurs quite soon after starting treatment, which is not consistent with direct bone effects. Rather, this might represent behavioral differences. Testosterone supplementation seems to increase energy levels; might it lead men to engage in activities that put them at higher risk for fracture?
Regardless of the cause, this adds to our knowledge about the rather complex mix of risks and benefits of testosterone supplementation and probably puts a bit more weight on the risks side. The truth is that testosterone levels do decline with age, as do many things, and it may not be appropriate to try to fight against that in all people. It’s worth noting that all of these studies use low levels of total serum testosterone as an entry criterion. But total testosterone is not what your body “sees.” It sees free testosterone, the portion not bound to sex hormone–binding globulin. And that binding protein is affected by lots of stuff — diabetes and obesity lower it, for example — making total testosterone levels seem low when free testosterone might be just fine.
In other words, testosterone supplementation is probably not terrible, but it is definitely not the cure for aging. In situations like this, we need better data to guide exactly who will benefit from the therapy and who will only be exposed to the risks.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I am showing you a graph without any labels.
What could this line represent? The stock price of some company that made a big splash but failed to live up to expectations? An outbreak curve charting the introduction of a new infectious agent to a population? The performance of a viral tweet?
I’ll tell you what it is in a moment, but I wanted you to recognize that there is something inherently wistful in this shape, something that speaks of past glory and inevitable declines. It’s a graph that induces a feeling of resistance — no, do not go gently into that good night.
The graph actually represents (roughly) the normal level of serum testosterone in otherwise-healthy men as they age.
A caveat here: These numbers are not as well defined as I made them seem on this graph, particularly for those older than 65 years. But it is clear that testosterone levels decline with time, and the idea to supplement testosterone is hardly new. Like all treatments, testosterone supplementation has risks and benefits. Some risks are predictable, like exacerbating the symptoms of benign prostatic hyperplasia. Some risks seem to come completely out of left field. That’s what we have today, in a study suggesting that testosterone supplementation increases the risk for bone fractures.
Let me set the stage here by saying that nearly all prior research into the effects of testosterone supplementation has suggested that it is pretty good for bone health. It increases bone mineral density, bone strength, and improves bone architecture.
So if you were to do a randomized trial of testosterone supplementation and look at fracture risk in the testosterone group compared with the placebo group, you would expect the fracture risk would be much lower in those getting supplemented. Of course, this is why we actually do studies instead of assuming we know the answer already — because in this case, you’d be wrong.
I’m talking about this study, appearing in The New England Journal of Medicine.
It’s a prespecified secondary analysis of a randomized trial known as the TRAVERSE trial, which randomly assigned 5246 men with low testosterone levels to transdermal testosterone gel vs placebo. The primary goal of that trial was to assess the cardiovascular risk associated with testosterone supplementation, and the major take-home was that there was no difference in cardiovascular event rates between the testosterone and placebo groups.
This secondary analysis looked at fracture incidence. Researchers contacted participants multiple times in the first year of the study and yearly thereafter. Each time, they asked whether the participant had sustained a fracture. If they answered in the affirmative, a request for medical records was made and the researchers, still blinded to randomization status, adjudicated whether there was indeed a fracture or not, along with some details as to location, situation, and so on.
This was a big study, though, and that translates to just a 3.5% fracture rate in testosterone vs 2.5% in control, but the difference was statistically significant.
This difference persisted across various fracture types (non–high-impact fractures, for example) after excluding the small percentage of men taking osteoporosis medication.
How does a drug that increases bone mineral density and bone strength increase the risk for fracture?
Well, one clue — and this was pointed out in a nice editorial by Matthis Grossman and Bradley Anawalt — is that the increased risk for fracture occurs quite soon after starting treatment, which is not consistent with direct bone effects. Rather, this might represent behavioral differences. Testosterone supplementation seems to increase energy levels; might it lead men to engage in activities that put them at higher risk for fracture?
Regardless of the cause, this adds to our knowledge about the rather complex mix of risks and benefits of testosterone supplementation and probably puts a bit more weight on the risks side. The truth is that testosterone levels do decline with age, as do many things, and it may not be appropriate to try to fight against that in all people. It’s worth noting that all of these studies use low levels of total serum testosterone as an entry criterion. But total testosterone is not what your body “sees.” It sees free testosterone, the portion not bound to sex hormone–binding globulin. And that binding protein is affected by lots of stuff — diabetes and obesity lower it, for example — making total testosterone levels seem low when free testosterone might be just fine.
In other words, testosterone supplementation is probably not terrible, but it is definitely not the cure for aging. In situations like this, we need better data to guide exactly who will benefit from the therapy and who will only be exposed to the risks.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Smoking Associated With Increased Risk for Hair Loss Among Men
, according to a new study.
In addition, the odds of developing AGA are higher among those who smoke at least 10 cigarettes per day than among those who smoke less, the study authors found.
“Men who smoke are more likely to develop and experience progression of male pattern hair loss,” lead author Aditya Gupta, MD, PhD, professor of medicine at the University of Toronto, Toronto, and director of clinical research at Mediprobe Research Inc., London, Ontario, Canada, told this news organization.
“Our patients with male pattern baldness need to be educated about the negative effects of smoking, given that this condition can have a profound negative psychological impact on those who suffer from it,” he said.
The study was published online in the Journal of Cosmetic Dermatology.
Analyzing Smoking’s Effects
Smoking generally has been accepted as a risk factor for the development and progression of AGA or the most common form of hair loss. The research evidence on this association has been inconsistent, however, the authors wrote.
The investigators conducted a review and meta-analysis of eight observational studies to understand the links between smoking and AGA. Ever-smokers were defined as current and former smokers.
Overall, based on six studies, men who have ever smoked are 1.8 times more likely (P < .05) to develop AGA.
Based on two studies, men who smoke 10 or more cigarettes daily are about twice as likely (P < .05) to develop AGA than those who smoke up to 10 cigarettes per day.
Based on four studies, ever smoking is associated with 1.3 times higher odds of AGA progressing from mild (ie, Norwood-Hamilton stages I-III) to more severe (stages IV-VII) than among those who have never smoked.
Based on two studies, there’s no association between AGA progression and smoking intensity (as defined as smoking up to 20 cigarettes daily vs smoking 20 or more cigarettes per day).
“Though our pooled analysis found no significant association between smoking intensity and severity of male AGA, a positive correlation may exist and be detected through an analysis that is statistically better powered,” said Dr. Gupta.
The investigators noted the limitations of their analysis, such as its reliance on observational studies and its lack of data about nicotine levels, smoking intensity, and smoking cessation among study participants.
Additional studies are needed to better understand the links between smoking and hair loss, said Dr. Gupta, as well as the effects of smoking cessation.
Improving Practice and Research
Commenting on the findings for this news organization, Arash Babadjouni, MD, a dermatologist at Midwestern University, Glendale, Arizona, said, “Smoking is not only a preventable cause of significant systemic disease but also affects the follicular growth cycle and fiber pigmentation. The prevalence of hair loss and premature hair graying is higher in smokers than nonsmokers.”
Dr. Babadjouni, who wasn’t involved with this study, has researched the associations between smoking and hair loss and premature hair graying.
“Evidence of this association can be used to clinically promote smoking cessation and emphasize the consequences of smoking on hair,” he said. “Smoking status should be assessed in patients who are presenting to their dermatologist and physicians alike for evaluation of alopecia and premature hair graying.”
The study was conducted without outside funding, and the authors declared no conflicts of interest. Dr. Babadjouni reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a new study.
In addition, the odds of developing AGA are higher among those who smoke at least 10 cigarettes per day than among those who smoke less, the study authors found.
“Men who smoke are more likely to develop and experience progression of male pattern hair loss,” lead author Aditya Gupta, MD, PhD, professor of medicine at the University of Toronto, Toronto, and director of clinical research at Mediprobe Research Inc., London, Ontario, Canada, told this news organization.
“Our patients with male pattern baldness need to be educated about the negative effects of smoking, given that this condition can have a profound negative psychological impact on those who suffer from it,” he said.
The study was published online in the Journal of Cosmetic Dermatology.
Analyzing Smoking’s Effects
Smoking generally has been accepted as a risk factor for the development and progression of AGA or the most common form of hair loss. The research evidence on this association has been inconsistent, however, the authors wrote.
The investigators conducted a review and meta-analysis of eight observational studies to understand the links between smoking and AGA. Ever-smokers were defined as current and former smokers.
Overall, based on six studies, men who have ever smoked are 1.8 times more likely (P < .05) to develop AGA.
Based on two studies, men who smoke 10 or more cigarettes daily are about twice as likely (P < .05) to develop AGA than those who smoke up to 10 cigarettes per day.
Based on four studies, ever smoking is associated with 1.3 times higher odds of AGA progressing from mild (ie, Norwood-Hamilton stages I-III) to more severe (stages IV-VII) than among those who have never smoked.
Based on two studies, there’s no association between AGA progression and smoking intensity (as defined as smoking up to 20 cigarettes daily vs smoking 20 or more cigarettes per day).
“Though our pooled analysis found no significant association between smoking intensity and severity of male AGA, a positive correlation may exist and be detected through an analysis that is statistically better powered,” said Dr. Gupta.
The investigators noted the limitations of their analysis, such as its reliance on observational studies and its lack of data about nicotine levels, smoking intensity, and smoking cessation among study participants.
Additional studies are needed to better understand the links between smoking and hair loss, said Dr. Gupta, as well as the effects of smoking cessation.
Improving Practice and Research
Commenting on the findings for this news organization, Arash Babadjouni, MD, a dermatologist at Midwestern University, Glendale, Arizona, said, “Smoking is not only a preventable cause of significant systemic disease but also affects the follicular growth cycle and fiber pigmentation. The prevalence of hair loss and premature hair graying is higher in smokers than nonsmokers.”
Dr. Babadjouni, who wasn’t involved with this study, has researched the associations between smoking and hair loss and premature hair graying.
“Evidence of this association can be used to clinically promote smoking cessation and emphasize the consequences of smoking on hair,” he said. “Smoking status should be assessed in patients who are presenting to their dermatologist and physicians alike for evaluation of alopecia and premature hair graying.”
The study was conducted without outside funding, and the authors declared no conflicts of interest. Dr. Babadjouni reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to a new study.
In addition, the odds of developing AGA are higher among those who smoke at least 10 cigarettes per day than among those who smoke less, the study authors found.
“Men who smoke are more likely to develop and experience progression of male pattern hair loss,” lead author Aditya Gupta, MD, PhD, professor of medicine at the University of Toronto, Toronto, and director of clinical research at Mediprobe Research Inc., London, Ontario, Canada, told this news organization.
“Our patients with male pattern baldness need to be educated about the negative effects of smoking, given that this condition can have a profound negative psychological impact on those who suffer from it,” he said.
The study was published online in the Journal of Cosmetic Dermatology.
Analyzing Smoking’s Effects
Smoking generally has been accepted as a risk factor for the development and progression of AGA or the most common form of hair loss. The research evidence on this association has been inconsistent, however, the authors wrote.
The investigators conducted a review and meta-analysis of eight observational studies to understand the links between smoking and AGA. Ever-smokers were defined as current and former smokers.
Overall, based on six studies, men who have ever smoked are 1.8 times more likely (P < .05) to develop AGA.
Based on two studies, men who smoke 10 or more cigarettes daily are about twice as likely (P < .05) to develop AGA than those who smoke up to 10 cigarettes per day.
Based on four studies, ever smoking is associated with 1.3 times higher odds of AGA progressing from mild (ie, Norwood-Hamilton stages I-III) to more severe (stages IV-VII) than among those who have never smoked.
Based on two studies, there’s no association between AGA progression and smoking intensity (as defined as smoking up to 20 cigarettes daily vs smoking 20 or more cigarettes per day).
“Though our pooled analysis found no significant association between smoking intensity and severity of male AGA, a positive correlation may exist and be detected through an analysis that is statistically better powered,” said Dr. Gupta.
The investigators noted the limitations of their analysis, such as its reliance on observational studies and its lack of data about nicotine levels, smoking intensity, and smoking cessation among study participants.
Additional studies are needed to better understand the links between smoking and hair loss, said Dr. Gupta, as well as the effects of smoking cessation.
Improving Practice and Research
Commenting on the findings for this news organization, Arash Babadjouni, MD, a dermatologist at Midwestern University, Glendale, Arizona, said, “Smoking is not only a preventable cause of significant systemic disease but also affects the follicular growth cycle and fiber pigmentation. The prevalence of hair loss and premature hair graying is higher in smokers than nonsmokers.”
Dr. Babadjouni, who wasn’t involved with this study, has researched the associations between smoking and hair loss and premature hair graying.
“Evidence of this association can be used to clinically promote smoking cessation and emphasize the consequences of smoking on hair,” he said. “Smoking status should be assessed in patients who are presenting to their dermatologist and physicians alike for evaluation of alopecia and premature hair graying.”
The study was conducted without outside funding, and the authors declared no conflicts of interest. Dr. Babadjouni reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM THE JOURNAL OF COSMETIC DERMATOLOGY
Spotting Varicocele: A Common Cause of Male Infertility
The report from the 2023 annual conference of French Urology Association (AFU), which was dedicated to male fertility, focused heavily on the diagnosis and treatment of varicocele, the most common reversible cause of infertility in men. Long a controversial subject, it has now been determined that treating this condition improves sperm analysis results, spontaneous fertility, and outcomes from medically assisted reproduction techniques. The AFU’s conference was a chance to present an overall snapshot of the issue.
Between 1973 and 2018, male sperm concentration dropped by 51.6% around the world, whereas total sperm counts dropped by 62.3%, according to Charlotte Methorst, MD, urological surgeon in Saint Cloud, France, president of the French Language Andrology Society, and coordinator of the AFU 2023 Report on Male Fertility. What’s more,
Declining Fertility
Nowadays, around 15% of couples experience infertility (60,000 new cases/year in France). About 20% of these are strictly attributed to male infertility and 40% are mixed, implying a male factor. Consequently, Dr. Methorst explained that “we must routinely assess male fertility within the context of a medically assisted reproduction (MAR) pathway, without limiting testing to semen analysis. Varicocele, one of the causes of infertility that men should be screened for and defined as an abnormal and/or tortuous enlargement of the pampiniform plexus veins, is present in 15%-20% of the overall male population, 35% of men with primary infertility, and more than 70% of those experiencing secondary infertility.”
In infertile men, varicocele is mostly unilateral, occurring on the left side (85%-90% of cases). A link has been established between varicocele and insufficiency at the saphenofemoral junction, as well as venous insufficiency of the lower limbs (odds ratio, 2.34; P < .0001), suggesting a predisposition towards a vascular network favoring the presence of varicoceles.
Varicocele Underdiagnosed
Analysis of a large multicenter database recently suggested the underdiagnosis of varicocele in men being assessed for infertility. Diagnosis is primarily based on physical examination, with physicians finding a soft, serpiginous swelling in the upper and posterior part of the affected side of the scrotum. Testicular Doppler ultrasonography provides confirmation of a varicocele, taking into account aspects such as size and reflux duration during the Valsalva maneuver. Generally, this is enough to make a differential diagnosis, but MRI may be considered, especially in cases where a millimeter-sized nodule is also found.
Reversal Improves Fertility
A Cochrane meta-analysis, despite containing significant bias, raised doubts about the efficacy of varicocele treatment. Nevertheless, over the past 10 years, a randomized trial and several meta-analyses have put an end to the controversy, confirming that treatment for varicocele significantly improves natural pregnancy rates.
Microscopic subinguinal varicocelectomy is the gold-standard option for cases of clinical varicocele and for those in which sperm analysis findings are abnormal. This approach is associated with superior efficacy outcomes, such as improvement in sperm analysis results and pregnancy rates while leading to lower rates of recurrence (< 4%) and a favorable outcome in terms of complications. It should be noted that treating subclinical varicocele is not recommended.
Specifically, microscopic subinguinal varicocelectomy improves live birth and pregnancy rates, both naturally and via in vitro fertilization. What’s more, it has a positive impact on sperm count, total and progressive motility, morphology, and DNA fragmentation levels. Overall, this surgery changes the MAR approach used in around one in two cases. The grade and unilateral or bilateral nature of varicocele are important predictive factors of improvement in sperm analysis findings and pregnancy rates associated with this interventional procedure. Treating clinical varicocele (grades 1-3) leads to improved sperm analysis results, observed in 60%-70% of cases. According to a meta-analysis, the mean increase is said to be a concentration of 12 million spermatozoids per milliliter, as well as a mean improvement of 11% in sperm motility.
Notably, embolization can be considered as an alternative to surgery. This minimally invasive X-ray-guided procedure performed by an interventional radiologist attempts to block the dilated testicular vein.
Guidelines for clinical practice in treating varicocele were recently published by the AFU’s Andrology committee.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
The report from the 2023 annual conference of French Urology Association (AFU), which was dedicated to male fertility, focused heavily on the diagnosis and treatment of varicocele, the most common reversible cause of infertility in men. Long a controversial subject, it has now been determined that treating this condition improves sperm analysis results, spontaneous fertility, and outcomes from medically assisted reproduction techniques. The AFU’s conference was a chance to present an overall snapshot of the issue.
Between 1973 and 2018, male sperm concentration dropped by 51.6% around the world, whereas total sperm counts dropped by 62.3%, according to Charlotte Methorst, MD, urological surgeon in Saint Cloud, France, president of the French Language Andrology Society, and coordinator of the AFU 2023 Report on Male Fertility. What’s more,
Declining Fertility
Nowadays, around 15% of couples experience infertility (60,000 new cases/year in France). About 20% of these are strictly attributed to male infertility and 40% are mixed, implying a male factor. Consequently, Dr. Methorst explained that “we must routinely assess male fertility within the context of a medically assisted reproduction (MAR) pathway, without limiting testing to semen analysis. Varicocele, one of the causes of infertility that men should be screened for and defined as an abnormal and/or tortuous enlargement of the pampiniform plexus veins, is present in 15%-20% of the overall male population, 35% of men with primary infertility, and more than 70% of those experiencing secondary infertility.”
In infertile men, varicocele is mostly unilateral, occurring on the left side (85%-90% of cases). A link has been established between varicocele and insufficiency at the saphenofemoral junction, as well as venous insufficiency of the lower limbs (odds ratio, 2.34; P < .0001), suggesting a predisposition towards a vascular network favoring the presence of varicoceles.
Varicocele Underdiagnosed
Analysis of a large multicenter database recently suggested the underdiagnosis of varicocele in men being assessed for infertility. Diagnosis is primarily based on physical examination, with physicians finding a soft, serpiginous swelling in the upper and posterior part of the affected side of the scrotum. Testicular Doppler ultrasonography provides confirmation of a varicocele, taking into account aspects such as size and reflux duration during the Valsalva maneuver. Generally, this is enough to make a differential diagnosis, but MRI may be considered, especially in cases where a millimeter-sized nodule is also found.
Reversal Improves Fertility
A Cochrane meta-analysis, despite containing significant bias, raised doubts about the efficacy of varicocele treatment. Nevertheless, over the past 10 years, a randomized trial and several meta-analyses have put an end to the controversy, confirming that treatment for varicocele significantly improves natural pregnancy rates.
Microscopic subinguinal varicocelectomy is the gold-standard option for cases of clinical varicocele and for those in which sperm analysis findings are abnormal. This approach is associated with superior efficacy outcomes, such as improvement in sperm analysis results and pregnancy rates while leading to lower rates of recurrence (< 4%) and a favorable outcome in terms of complications. It should be noted that treating subclinical varicocele is not recommended.
Specifically, microscopic subinguinal varicocelectomy improves live birth and pregnancy rates, both naturally and via in vitro fertilization. What’s more, it has a positive impact on sperm count, total and progressive motility, morphology, and DNA fragmentation levels. Overall, this surgery changes the MAR approach used in around one in two cases. The grade and unilateral or bilateral nature of varicocele are important predictive factors of improvement in sperm analysis findings and pregnancy rates associated with this interventional procedure. Treating clinical varicocele (grades 1-3) leads to improved sperm analysis results, observed in 60%-70% of cases. According to a meta-analysis, the mean increase is said to be a concentration of 12 million spermatozoids per milliliter, as well as a mean improvement of 11% in sperm motility.
Notably, embolization can be considered as an alternative to surgery. This minimally invasive X-ray-guided procedure performed by an interventional radiologist attempts to block the dilated testicular vein.
Guidelines for clinical practice in treating varicocele were recently published by the AFU’s Andrology committee.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
The report from the 2023 annual conference of French Urology Association (AFU), which was dedicated to male fertility, focused heavily on the diagnosis and treatment of varicocele, the most common reversible cause of infertility in men. Long a controversial subject, it has now been determined that treating this condition improves sperm analysis results, spontaneous fertility, and outcomes from medically assisted reproduction techniques. The AFU’s conference was a chance to present an overall snapshot of the issue.
Between 1973 and 2018, male sperm concentration dropped by 51.6% around the world, whereas total sperm counts dropped by 62.3%, according to Charlotte Methorst, MD, urological surgeon in Saint Cloud, France, president of the French Language Andrology Society, and coordinator of the AFU 2023 Report on Male Fertility. What’s more,
Declining Fertility
Nowadays, around 15% of couples experience infertility (60,000 new cases/year in France). About 20% of these are strictly attributed to male infertility and 40% are mixed, implying a male factor. Consequently, Dr. Methorst explained that “we must routinely assess male fertility within the context of a medically assisted reproduction (MAR) pathway, without limiting testing to semen analysis. Varicocele, one of the causes of infertility that men should be screened for and defined as an abnormal and/or tortuous enlargement of the pampiniform plexus veins, is present in 15%-20% of the overall male population, 35% of men with primary infertility, and more than 70% of those experiencing secondary infertility.”
In infertile men, varicocele is mostly unilateral, occurring on the left side (85%-90% of cases). A link has been established between varicocele and insufficiency at the saphenofemoral junction, as well as venous insufficiency of the lower limbs (odds ratio, 2.34; P < .0001), suggesting a predisposition towards a vascular network favoring the presence of varicoceles.
Varicocele Underdiagnosed
Analysis of a large multicenter database recently suggested the underdiagnosis of varicocele in men being assessed for infertility. Diagnosis is primarily based on physical examination, with physicians finding a soft, serpiginous swelling in the upper and posterior part of the affected side of the scrotum. Testicular Doppler ultrasonography provides confirmation of a varicocele, taking into account aspects such as size and reflux duration during the Valsalva maneuver. Generally, this is enough to make a differential diagnosis, but MRI may be considered, especially in cases where a millimeter-sized nodule is also found.
Reversal Improves Fertility
A Cochrane meta-analysis, despite containing significant bias, raised doubts about the efficacy of varicocele treatment. Nevertheless, over the past 10 years, a randomized trial and several meta-analyses have put an end to the controversy, confirming that treatment for varicocele significantly improves natural pregnancy rates.
Microscopic subinguinal varicocelectomy is the gold-standard option for cases of clinical varicocele and for those in which sperm analysis findings are abnormal. This approach is associated with superior efficacy outcomes, such as improvement in sperm analysis results and pregnancy rates while leading to lower rates of recurrence (< 4%) and a favorable outcome in terms of complications. It should be noted that treating subclinical varicocele is not recommended.
Specifically, microscopic subinguinal varicocelectomy improves live birth and pregnancy rates, both naturally and via in vitro fertilization. What’s more, it has a positive impact on sperm count, total and progressive motility, morphology, and DNA fragmentation levels. Overall, this surgery changes the MAR approach used in around one in two cases. The grade and unilateral or bilateral nature of varicocele are important predictive factors of improvement in sperm analysis findings and pregnancy rates associated with this interventional procedure. Treating clinical varicocele (grades 1-3) leads to improved sperm analysis results, observed in 60%-70% of cases. According to a meta-analysis, the mean increase is said to be a concentration of 12 million spermatozoids per milliliter, as well as a mean improvement of 11% in sperm motility.
Notably, embolization can be considered as an alternative to surgery. This minimally invasive X-ray-guided procedure performed by an interventional radiologist attempts to block the dilated testicular vein.
Guidelines for clinical practice in treating varicocele were recently published by the AFU’s Andrology committee.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Traumatic Brain Injury and CVD: What’s the Link?
The long-term impact of traumatic brain injury (TBI) on neurologic and psychiatric function is well-established, but a growing body of research is pointing to unexpected medical sequalae, including cardiovascular disease (CVD).
A recent review looked at the investigation to date into this surprising connection, not only summarizing study findings but also suggesting potential mechanisms that might account for the association.
“; consequently, they should undergo regular monitoring,” senior author Ross Zafonte, DO, president of Spaulding Rehabilitation Network, Boston, and lead author Saef Izzy, MD, MBChB, a neurologist at the Stroke and Cerebrovascular Center of Brigham and Women’s Hospital, Boston, Massachusetts, told this news organization.
“This holds significant importance for healthcare practitioners, as there exist several strategies to mitigate cardiovascular disease risk — including weight management, adopting a healthy diet, engaging in regular physical activity, and quitting smoking,” they stated.
Leslie Croll, MD, American Heart Association volunteer and assistant professor of clinical neurology at the Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, told this news organization that it’s “extremely important to learn more about the interplay between TBI, neurologic disease, psychiatric complications, and the cardiovascular system.”
Hopefully, she added, “future research will help us understand what kind of cardiovascular disease monitoring and prevention measures stand to give TBI patients the most benefit.”
Chronic Condition
TBI is “a major cause of long-term disability and premature death,” and is “highly prevalent among contact sports players, military personnel (eg, due to injuries sustained during conflict), and the general population (eg, due to falls and road traffic incidents),” the authors wrote.
Most studies pertaining to TBI have “primarily focused on establishing connections between single TBI, repetitive TBI, and their acute and chronic neurological and psychiatric consequences, such as Parkinson’s disease, Alzheimer’s disease, and chronic traumatic encephalopathy (CTE),” Drs. Zafonte and Izzy noted. By contrast, there has been a “notable lack of research attention given to non-neurological conditions associated with TBI.”
They pointed out that recent insights into TBI — particularly the acknowledgment of TBI as an “emerging chronic condition rather than merely an acute aftermath of brain injury” — have come to light through epidemiologic and pathologic investigations involving military veterans, professional American-style football players, and the civilian population. “This recognition opens up an opportunity to broaden our perspective and delve into the medical aspects of health that may be influenced by TBI.”
To broaden the investigation, the researchers reviewed literature published between January 1, 2001, and June 18, 2023. Of 26,335 articles, they narrowed their review down to 15 studies that investigated CVD, CVD risk factors, and cerebrovascular disease in the chronic phase of TBI, including community, military, or sport-related brain trauma, regardless of the timing of disease occurrence with respect to brain injury via TBI or repetitive head impact.
New Cardiovascular Risk
Studies that used national or local registries tended to be retrospective and predominantly conducted in people with preexisting cardiovascular conditions. In these studies, TBI was found to be an independent risk factor for myocardial dysfunction. However, although these studies do provide evidence of elevated cardiovascular risk subsequent to a single TBI, including individuals with preexisting medical comorbidities “makes it difficult to determine the timing of incident cardiovascular disease and cardiovascular risk factors subsequent to brain injury,” they wrote.
However, some studies showed that even individuals with TBI but without preexisting myocardial dysfunction at baseline had a significantly higher risk for CVD than those without a history of TBI.
In fact, several studies included populations without preexisting medical and cardiovascular comorbidities to “better refine the order and timing of CVD and other risk factors in individuals with TBI.”
For example, one study of concussion survivors without preexisting diagnoses showed that cardiovascular, endocrinological, and neuropsychiatric comorbidities occurred at a “significantly higher incidence within 5 years after concussive TBI compared with healthy individuals who were matched in terms of age, race, and sex and didn’t have a TBI exposure.” Other studies yielded similar findings.
Because cardiovascular risk factors and events become more common with age, it’s important to account for age in evaluating the effects of TBI. Although many studies of TBI and subsequent CVD didn’t stratify individuals by age, one 10-year study of people without any known cardiovascular or neuropsychiatric conditions who sustained TBI found that people as young as 18-40 years were more likely to develop hypertension, hyperlipidemia, obesity, and diabetes within 3-5 years following brain injury than matched individuals in the control group.
“Individuals who have encountered TBI, surprisingly even those who are young and in good health with no prior comorbid conditions, face an increased risk of adverse cardiovascular outcomes for an extended duration after the initial event,” Drs. Zafonte and Izzy summarized. “Therefore, it’s imperative that they receive regular and long-term screenings for CVD and associated risk factors.”
Bidirectional Relationship
Brain injury has been associated with acute cardiovascular dysfunction, including autonomic heart-brain axis dysregulation, imbalances between the sympathetic and parasympathetic nervous systems, and excessive catecholamine release, the authors noted.
Drs. Zafonte and Izzy suggested several plausible links between TBI and cardiovascular dysfunction, noting that they are “likely multifaceted, potentially encompassing risk factors that span the pre-injury, injury, and post-injury phases of the condition.”
TBI may induce alterations in neurobiological processes, which have been reported to be associated with an increased risk for CVD (eg, chronic dysfunction of the autonomic system, systemic inflammation, and modifications in the brain-gut connection).
Patients with TBI might develop additional risk factors following the injury, including conditions like posttraumatic stress disorder, depression, and other psychiatric illnesses, which are “known to augment the risk of CVD.”
TBI can lead to subsequent behavioral and lifestyle changes that place patients at an elevated risk for both cardiovascular and cognitive dysfunction when compared to the general population of TBI survivors.
There may be additional as yet undefined risks.
They believe there’s a bidirectional relationship between TBI and CVD. “On one hand, TBI has been associated with an elevated risk of CVD,” they said. “Conversely, cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, and sleep disturbances that have been demonstrated to negatively influence cognitive function and heighten the risk of dementia. Consequently, this interplay can further compound the long-term consequences of the injury.”
Their work aims to try and disentangle this “complex series of relationships.”
They recommend screening to identify diseases in their earliest and “most manageable phases” because TBI has been “unveiled as an underappreciated risk factor for CVD within contact sports, military, and community setting.”
An effective screening program “should rely on quantifiable and dependable biomarkers such as blood pressure, BMI, waist circumference, blood lipid levels, and glucose. Additionally, it should take into account other factors like smoking habits, physical activity, and dietary choices,” they recommended.
Heart-Brain Connection
Dr. Croll noted that TBI is “associated with many poorly understood physiologic changes and complications, so it’s exciting to see research aimed at clarifying this chronic disease process.”
In recent years, “we have seen a greater appreciation and understanding of the heart-brain connection,” she said. “Moving forward, more research, including TBI research, will target that connection.”
She added that there are probably “multiple mechanisms” at play underlying the connection between TBI and CVD.
Most importantly, “we are increasingly learning that TBI is not only a discrete event that requires immediate treatment but also a chronic disease process,” and when we “think about the substantial long-term morbidity associated with TBI, we should keep increased risk for CVD on top of mind,” said Dr. Croll.
The review received no funding. Izzy reported receiving grants from the US National Institutes of Health (NIH) and 2023 Stepping Strong Innovator Award. Dr. Zafonte reported receiving grants from the NIH and royalties from Springer and Demos publishing for serving as a coeditor of Brain Injury Medicine. Dr. Zafonte has also served as an adviser to Myomo, Oncare.ai, Nanodiagnostics, and Kisbee. He reported evaluating patients in the Massachusetts General Hospital Brain and Body–TRUST Program, which is funded by the NFL Players Association. The other authors’ disclosures are listed on the original paper. Dr. Croll declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
The long-term impact of traumatic brain injury (TBI) on neurologic and psychiatric function is well-established, but a growing body of research is pointing to unexpected medical sequalae, including cardiovascular disease (CVD).
A recent review looked at the investigation to date into this surprising connection, not only summarizing study findings but also suggesting potential mechanisms that might account for the association.
“; consequently, they should undergo regular monitoring,” senior author Ross Zafonte, DO, president of Spaulding Rehabilitation Network, Boston, and lead author Saef Izzy, MD, MBChB, a neurologist at the Stroke and Cerebrovascular Center of Brigham and Women’s Hospital, Boston, Massachusetts, told this news organization.
“This holds significant importance for healthcare practitioners, as there exist several strategies to mitigate cardiovascular disease risk — including weight management, adopting a healthy diet, engaging in regular physical activity, and quitting smoking,” they stated.
Leslie Croll, MD, American Heart Association volunteer and assistant professor of clinical neurology at the Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, told this news organization that it’s “extremely important to learn more about the interplay between TBI, neurologic disease, psychiatric complications, and the cardiovascular system.”
Hopefully, she added, “future research will help us understand what kind of cardiovascular disease monitoring and prevention measures stand to give TBI patients the most benefit.”
Chronic Condition
TBI is “a major cause of long-term disability and premature death,” and is “highly prevalent among contact sports players, military personnel (eg, due to injuries sustained during conflict), and the general population (eg, due to falls and road traffic incidents),” the authors wrote.
Most studies pertaining to TBI have “primarily focused on establishing connections between single TBI, repetitive TBI, and their acute and chronic neurological and psychiatric consequences, such as Parkinson’s disease, Alzheimer’s disease, and chronic traumatic encephalopathy (CTE),” Drs. Zafonte and Izzy noted. By contrast, there has been a “notable lack of research attention given to non-neurological conditions associated with TBI.”
They pointed out that recent insights into TBI — particularly the acknowledgment of TBI as an “emerging chronic condition rather than merely an acute aftermath of brain injury” — have come to light through epidemiologic and pathologic investigations involving military veterans, professional American-style football players, and the civilian population. “This recognition opens up an opportunity to broaden our perspective and delve into the medical aspects of health that may be influenced by TBI.”
To broaden the investigation, the researchers reviewed literature published between January 1, 2001, and June 18, 2023. Of 26,335 articles, they narrowed their review down to 15 studies that investigated CVD, CVD risk factors, and cerebrovascular disease in the chronic phase of TBI, including community, military, or sport-related brain trauma, regardless of the timing of disease occurrence with respect to brain injury via TBI or repetitive head impact.
New Cardiovascular Risk
Studies that used national or local registries tended to be retrospective and predominantly conducted in people with preexisting cardiovascular conditions. In these studies, TBI was found to be an independent risk factor for myocardial dysfunction. However, although these studies do provide evidence of elevated cardiovascular risk subsequent to a single TBI, including individuals with preexisting medical comorbidities “makes it difficult to determine the timing of incident cardiovascular disease and cardiovascular risk factors subsequent to brain injury,” they wrote.
However, some studies showed that even individuals with TBI but without preexisting myocardial dysfunction at baseline had a significantly higher risk for CVD than those without a history of TBI.
In fact, several studies included populations without preexisting medical and cardiovascular comorbidities to “better refine the order and timing of CVD and other risk factors in individuals with TBI.”
For example, one study of concussion survivors without preexisting diagnoses showed that cardiovascular, endocrinological, and neuropsychiatric comorbidities occurred at a “significantly higher incidence within 5 years after concussive TBI compared with healthy individuals who were matched in terms of age, race, and sex and didn’t have a TBI exposure.” Other studies yielded similar findings.
Because cardiovascular risk factors and events become more common with age, it’s important to account for age in evaluating the effects of TBI. Although many studies of TBI and subsequent CVD didn’t stratify individuals by age, one 10-year study of people without any known cardiovascular or neuropsychiatric conditions who sustained TBI found that people as young as 18-40 years were more likely to develop hypertension, hyperlipidemia, obesity, and diabetes within 3-5 years following brain injury than matched individuals in the control group.
“Individuals who have encountered TBI, surprisingly even those who are young and in good health with no prior comorbid conditions, face an increased risk of adverse cardiovascular outcomes for an extended duration after the initial event,” Drs. Zafonte and Izzy summarized. “Therefore, it’s imperative that they receive regular and long-term screenings for CVD and associated risk factors.”
Bidirectional Relationship
Brain injury has been associated with acute cardiovascular dysfunction, including autonomic heart-brain axis dysregulation, imbalances between the sympathetic and parasympathetic nervous systems, and excessive catecholamine release, the authors noted.
Drs. Zafonte and Izzy suggested several plausible links between TBI and cardiovascular dysfunction, noting that they are “likely multifaceted, potentially encompassing risk factors that span the pre-injury, injury, and post-injury phases of the condition.”
TBI may induce alterations in neurobiological processes, which have been reported to be associated with an increased risk for CVD (eg, chronic dysfunction of the autonomic system, systemic inflammation, and modifications in the brain-gut connection).
Patients with TBI might develop additional risk factors following the injury, including conditions like posttraumatic stress disorder, depression, and other psychiatric illnesses, which are “known to augment the risk of CVD.”
TBI can lead to subsequent behavioral and lifestyle changes that place patients at an elevated risk for both cardiovascular and cognitive dysfunction when compared to the general population of TBI survivors.
There may be additional as yet undefined risks.
They believe there’s a bidirectional relationship between TBI and CVD. “On one hand, TBI has been associated with an elevated risk of CVD,” they said. “Conversely, cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, and sleep disturbances that have been demonstrated to negatively influence cognitive function and heighten the risk of dementia. Consequently, this interplay can further compound the long-term consequences of the injury.”
Their work aims to try and disentangle this “complex series of relationships.”
They recommend screening to identify diseases in their earliest and “most manageable phases” because TBI has been “unveiled as an underappreciated risk factor for CVD within contact sports, military, and community setting.”
An effective screening program “should rely on quantifiable and dependable biomarkers such as blood pressure, BMI, waist circumference, blood lipid levels, and glucose. Additionally, it should take into account other factors like smoking habits, physical activity, and dietary choices,” they recommended.
Heart-Brain Connection
Dr. Croll noted that TBI is “associated with many poorly understood physiologic changes and complications, so it’s exciting to see research aimed at clarifying this chronic disease process.”
In recent years, “we have seen a greater appreciation and understanding of the heart-brain connection,” she said. “Moving forward, more research, including TBI research, will target that connection.”
She added that there are probably “multiple mechanisms” at play underlying the connection between TBI and CVD.
Most importantly, “we are increasingly learning that TBI is not only a discrete event that requires immediate treatment but also a chronic disease process,” and when we “think about the substantial long-term morbidity associated with TBI, we should keep increased risk for CVD on top of mind,” said Dr. Croll.
The review received no funding. Izzy reported receiving grants from the US National Institutes of Health (NIH) and 2023 Stepping Strong Innovator Award. Dr. Zafonte reported receiving grants from the NIH and royalties from Springer and Demos publishing for serving as a coeditor of Brain Injury Medicine. Dr. Zafonte has also served as an adviser to Myomo, Oncare.ai, Nanodiagnostics, and Kisbee. He reported evaluating patients in the Massachusetts General Hospital Brain and Body–TRUST Program, which is funded by the NFL Players Association. The other authors’ disclosures are listed on the original paper. Dr. Croll declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
The long-term impact of traumatic brain injury (TBI) on neurologic and psychiatric function is well-established, but a growing body of research is pointing to unexpected medical sequalae, including cardiovascular disease (CVD).
A recent review looked at the investigation to date into this surprising connection, not only summarizing study findings but also suggesting potential mechanisms that might account for the association.
“; consequently, they should undergo regular monitoring,” senior author Ross Zafonte, DO, president of Spaulding Rehabilitation Network, Boston, and lead author Saef Izzy, MD, MBChB, a neurologist at the Stroke and Cerebrovascular Center of Brigham and Women’s Hospital, Boston, Massachusetts, told this news organization.
“This holds significant importance for healthcare practitioners, as there exist several strategies to mitigate cardiovascular disease risk — including weight management, adopting a healthy diet, engaging in regular physical activity, and quitting smoking,” they stated.
Leslie Croll, MD, American Heart Association volunteer and assistant professor of clinical neurology at the Temple University Lewis Katz School of Medicine, Philadelphia, Pennsylvania, told this news organization that it’s “extremely important to learn more about the interplay between TBI, neurologic disease, psychiatric complications, and the cardiovascular system.”
Hopefully, she added, “future research will help us understand what kind of cardiovascular disease monitoring and prevention measures stand to give TBI patients the most benefit.”
Chronic Condition
TBI is “a major cause of long-term disability and premature death,” and is “highly prevalent among contact sports players, military personnel (eg, due to injuries sustained during conflict), and the general population (eg, due to falls and road traffic incidents),” the authors wrote.
Most studies pertaining to TBI have “primarily focused on establishing connections between single TBI, repetitive TBI, and their acute and chronic neurological and psychiatric consequences, such as Parkinson’s disease, Alzheimer’s disease, and chronic traumatic encephalopathy (CTE),” Drs. Zafonte and Izzy noted. By contrast, there has been a “notable lack of research attention given to non-neurological conditions associated with TBI.”
They pointed out that recent insights into TBI — particularly the acknowledgment of TBI as an “emerging chronic condition rather than merely an acute aftermath of brain injury” — have come to light through epidemiologic and pathologic investigations involving military veterans, professional American-style football players, and the civilian population. “This recognition opens up an opportunity to broaden our perspective and delve into the medical aspects of health that may be influenced by TBI.”
To broaden the investigation, the researchers reviewed literature published between January 1, 2001, and June 18, 2023. Of 26,335 articles, they narrowed their review down to 15 studies that investigated CVD, CVD risk factors, and cerebrovascular disease in the chronic phase of TBI, including community, military, or sport-related brain trauma, regardless of the timing of disease occurrence with respect to brain injury via TBI or repetitive head impact.
New Cardiovascular Risk
Studies that used national or local registries tended to be retrospective and predominantly conducted in people with preexisting cardiovascular conditions. In these studies, TBI was found to be an independent risk factor for myocardial dysfunction. However, although these studies do provide evidence of elevated cardiovascular risk subsequent to a single TBI, including individuals with preexisting medical comorbidities “makes it difficult to determine the timing of incident cardiovascular disease and cardiovascular risk factors subsequent to brain injury,” they wrote.
However, some studies showed that even individuals with TBI but without preexisting myocardial dysfunction at baseline had a significantly higher risk for CVD than those without a history of TBI.
In fact, several studies included populations without preexisting medical and cardiovascular comorbidities to “better refine the order and timing of CVD and other risk factors in individuals with TBI.”
For example, one study of concussion survivors without preexisting diagnoses showed that cardiovascular, endocrinological, and neuropsychiatric comorbidities occurred at a “significantly higher incidence within 5 years after concussive TBI compared with healthy individuals who were matched in terms of age, race, and sex and didn’t have a TBI exposure.” Other studies yielded similar findings.
Because cardiovascular risk factors and events become more common with age, it’s important to account for age in evaluating the effects of TBI. Although many studies of TBI and subsequent CVD didn’t stratify individuals by age, one 10-year study of people without any known cardiovascular or neuropsychiatric conditions who sustained TBI found that people as young as 18-40 years were more likely to develop hypertension, hyperlipidemia, obesity, and diabetes within 3-5 years following brain injury than matched individuals in the control group.
“Individuals who have encountered TBI, surprisingly even those who are young and in good health with no prior comorbid conditions, face an increased risk of adverse cardiovascular outcomes for an extended duration after the initial event,” Drs. Zafonte and Izzy summarized. “Therefore, it’s imperative that they receive regular and long-term screenings for CVD and associated risk factors.”
Bidirectional Relationship
Brain injury has been associated with acute cardiovascular dysfunction, including autonomic heart-brain axis dysregulation, imbalances between the sympathetic and parasympathetic nervous systems, and excessive catecholamine release, the authors noted.
Drs. Zafonte and Izzy suggested several plausible links between TBI and cardiovascular dysfunction, noting that they are “likely multifaceted, potentially encompassing risk factors that span the pre-injury, injury, and post-injury phases of the condition.”
TBI may induce alterations in neurobiological processes, which have been reported to be associated with an increased risk for CVD (eg, chronic dysfunction of the autonomic system, systemic inflammation, and modifications in the brain-gut connection).
Patients with TBI might develop additional risk factors following the injury, including conditions like posttraumatic stress disorder, depression, and other psychiatric illnesses, which are “known to augment the risk of CVD.”
TBI can lead to subsequent behavioral and lifestyle changes that place patients at an elevated risk for both cardiovascular and cognitive dysfunction when compared to the general population of TBI survivors.
There may be additional as yet undefined risks.
They believe there’s a bidirectional relationship between TBI and CVD. “On one hand, TBI has been associated with an elevated risk of CVD,” they said. “Conversely, cardiovascular risk factors such as diabetes, hypertension, hyperlipidemia, and sleep disturbances that have been demonstrated to negatively influence cognitive function and heighten the risk of dementia. Consequently, this interplay can further compound the long-term consequences of the injury.”
Their work aims to try and disentangle this “complex series of relationships.”
They recommend screening to identify diseases in their earliest and “most manageable phases” because TBI has been “unveiled as an underappreciated risk factor for CVD within contact sports, military, and community setting.”
An effective screening program “should rely on quantifiable and dependable biomarkers such as blood pressure, BMI, waist circumference, blood lipid levels, and glucose. Additionally, it should take into account other factors like smoking habits, physical activity, and dietary choices,” they recommended.
Heart-Brain Connection
Dr. Croll noted that TBI is “associated with many poorly understood physiologic changes and complications, so it’s exciting to see research aimed at clarifying this chronic disease process.”
In recent years, “we have seen a greater appreciation and understanding of the heart-brain connection,” she said. “Moving forward, more research, including TBI research, will target that connection.”
She added that there are probably “multiple mechanisms” at play underlying the connection between TBI and CVD.
Most importantly, “we are increasingly learning that TBI is not only a discrete event that requires immediate treatment but also a chronic disease process,” and when we “think about the substantial long-term morbidity associated with TBI, we should keep increased risk for CVD on top of mind,” said Dr. Croll.
The review received no funding. Izzy reported receiving grants from the US National Institutes of Health (NIH) and 2023 Stepping Strong Innovator Award. Dr. Zafonte reported receiving grants from the NIH and royalties from Springer and Demos publishing for serving as a coeditor of Brain Injury Medicine. Dr. Zafonte has also served as an adviser to Myomo, Oncare.ai, Nanodiagnostics, and Kisbee. He reported evaluating patients in the Massachusetts General Hospital Brain and Body–TRUST Program, which is funded by the NFL Players Association. The other authors’ disclosures are listed on the original paper. Dr. Croll declared no relevant financial relationships.
A version of this article appeared on Medscape.com.
Testosterone Supplements: Overcoming Current Misconceptions
Underdiagnosis, reluctant doctors, patient preconceptions: Treating low testosterone levels is a tricky business in France despite the proven benefits of replacement therapy. About 20% of patients with symptomatic low testosterone levels are treated for the deficiency, said Eric Huygue, MD, PhD, urologic surgeon at Toulouse University Hospital in France, at the 117th annual conference of the French Urology Association (AFU).
, said Dr. Huygue, who was involved in drawing up the first French recommendations on treating low testosterone in 2021.
“We must keep up communication efforts to make patients and doctors aware” of the benefits of supplementation, he said.
Testosterone Levels
Testosterone deficiency mostly affects men older than 40 years. A drop in androgen levels, which varies by individual, can lead to sexual problems (such as erectile dysfunction and low libido), physical symptoms (fatigue, hot flashes, loss of muscle mass, and osteoporosis), and mental disorders (anxiety, irritability, and depression).
There are an estimated 340,000 men with symptomatic testosterone deficiency in France. Just 70,000 of these are receiving replacement therapy (see box), which accounts for only 20% of those affected. For Dr. Huygue, this low treatment rate is due to underdiagnosis, as well as reluctance on the part of doctors and patients.
Although routine screening of low testosterone in the general population is not recommended, some individuals are particularly at risk, noted the urologist.
This is especially true for patients with metabolic disorders associated with insulin resistance (such as obesity and type 2 diabetes), cardiovascular diseases (hypertension, heart failure, and atrial fibrillation), or other chronic conditions (chronic obstructive pulmonary disease, cancer, and depression). Some medications (corticosteroids, antipsychotics, chemotherapy drugs, and antiretroviral therapies) can also lead to low testosterone.
Per the French recommendations for managing low testosterone, diagnosis must be based on free or bioavailable testosterone and not total testosterone levels, which can give a skewed result. Levels must be tested twice, 1 month apart, in the morning and while fasting. The reference range is determined by taking the lower threshold level of young men as measured in the laboratory.
Threshold Values
The current practice of using the reference range associated with the patient’s age group undoubtedly contributes to the underdiagnosis of low testosterone, said Dr. Huygue. According to a survey of AFU members in 2021, the year in which the recommendations were published, 77% of urologists interviewed reported referring to reference ranges for patients of the same age.
In their defense, “this method has long been in use, but it has eventually become apparent that symptomatic patients with an undiagnosed deficiency could be in the reference patients’ group,” Dr. Huygue explained.
Once a deficiency has been diagnosed, doctors may be reluctant to prescribe replacement therapy due to the perceived risk of developing prostate cancer. Several international studies have shown that “the risk of prostate cancer is the single biggest reason for doctors refusing to prescribe testosterone,” said Dr. Huygue.
Despite this reluctance, numerous studies have clearly shown that there is no link between a high testosterone level and the risk of developing prostate cancer. It even seems that a low testosterone level might expose a person to an increased risk for an aggressive form of cancer.
“This is a time of many surprising discoveries concerning the link between the prostate and testosterone, which go against what we have thought up to now. It has been observed that men with low testosterone develop more serious types of cancer,” said Dr. Huygue at a previous meeting of the AFU, during which he announced the publication of the French recommendations.
Prostate Cancer Recurrence
Urologists are also wary of testosterone supplementation in patients with a previous history of prostate cancer. According to the AFU’s survey, 40% of urologists questioned think that testosterone is contraindicated in this population. One in two urologists prescribe testosterone after radical prostatectomy for low or intermediate risk and most commonly after 3 years of undetectable prostate-specific antigen (PSA) levels.
Nevertheless, “several retrospective studies show the safety of testosterone replacement therapy in men who have undergone radical prostatectomy or radiotherapy or who are under active monitoring,” said Dr. Huygue. Testosterone “does not appear to increase the risk of relapse” after treatment of prostate cancer.
Dr. Huygue invited prescribing physicians to refer to the French recommendations, which specify that 1 year of undetectable PSA after prostatectomy is sufficient before prescribing replacement therapy. “This is clearly indicated in the recommendations for patients with a previous history of prostate cancer.”
Neither prostate cancer nor benign prostatic hyperplasia is a contraindication. According to the recommendations, the only contraindications to testosterone prescription are the following:
- Hematocrit > 54%
- Current breast or prostate cancer
- Cardiovascular event less than 3-6 months prior
- Trying to conceive
Cardiovascular Benefits
Another more commonly used argument by general practitioners and endocrinologists to justify their reluctance to prescribe testosterone is the risk to cardiovascular health. In early 2010, a series of American studies alerted clinicians to this risk when taking testosterone. Since then, other studies have had reassuring findings.
In response to the alert issued by the United States, the European Medicines Agency specified that “the data are not sufficient for a warning,” before the American Heart Association colleagues concluded that testosterone should only be avoided in the first 6 months following a severe cardiovascular event.
Conversely, in 2021, the European Society of Cardiology put forward the benefits of testosterone in an article in favor of replacement therapy to prevent cardiovascular risk. In particular, the hormone is thought to have a beneficial effect on arterial stiffness, the appearance of calcified plaques, and coronary artery dilatation.
The final hurdle to overcome before a testosterone prescription is filled relates to patients themselves, who often regard such treatment unfavorably. Many wrongly believe that androgens are hormones that “increase the risk of cancer, make you aggressive, cause weight gain, lead to hair loss, and cause body hair growth,” said Dr. Huygue.
Finally, breaks in the supply chain for Androtardyl, the only injectable form available for reimbursement by French social security schemes, were reported in the country in 2023, said Dr. Huygue. This situation only complicates further the prescription and use of testosterone replacement therapy.
Which Supplement?
Testosterone replacement therapies are available on the market in the following formulations:
Via transcutaneous administration: Testosterone-based gels, not covered by the French social security system (Androgel and Fortigel), to be applied daily. Users must be careful to avoid any potential transfer of the product to women or children in case of contact with the site after application.
Via an injection: Androtardyl (testosterone enanthate), covered by French social security, to be administered intramuscularly once a month. Nebido (testosterone undecanoate), not covered by French social security, with a more beneficial bioavailability profile, to be administered once every 3 months.
Pantestone (testosterone undecanoate), administered orally, is not marketed since 2021. It had the major disadvantage of requiring a high-fat diet to ensure optimal absorption.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Underdiagnosis, reluctant doctors, patient preconceptions: Treating low testosterone levels is a tricky business in France despite the proven benefits of replacement therapy. About 20% of patients with symptomatic low testosterone levels are treated for the deficiency, said Eric Huygue, MD, PhD, urologic surgeon at Toulouse University Hospital in France, at the 117th annual conference of the French Urology Association (AFU).
, said Dr. Huygue, who was involved in drawing up the first French recommendations on treating low testosterone in 2021.
“We must keep up communication efforts to make patients and doctors aware” of the benefits of supplementation, he said.
Testosterone Levels
Testosterone deficiency mostly affects men older than 40 years. A drop in androgen levels, which varies by individual, can lead to sexual problems (such as erectile dysfunction and low libido), physical symptoms (fatigue, hot flashes, loss of muscle mass, and osteoporosis), and mental disorders (anxiety, irritability, and depression).
There are an estimated 340,000 men with symptomatic testosterone deficiency in France. Just 70,000 of these are receiving replacement therapy (see box), which accounts for only 20% of those affected. For Dr. Huygue, this low treatment rate is due to underdiagnosis, as well as reluctance on the part of doctors and patients.
Although routine screening of low testosterone in the general population is not recommended, some individuals are particularly at risk, noted the urologist.
This is especially true for patients with metabolic disorders associated with insulin resistance (such as obesity and type 2 diabetes), cardiovascular diseases (hypertension, heart failure, and atrial fibrillation), or other chronic conditions (chronic obstructive pulmonary disease, cancer, and depression). Some medications (corticosteroids, antipsychotics, chemotherapy drugs, and antiretroviral therapies) can also lead to low testosterone.
Per the French recommendations for managing low testosterone, diagnosis must be based on free or bioavailable testosterone and not total testosterone levels, which can give a skewed result. Levels must be tested twice, 1 month apart, in the morning and while fasting. The reference range is determined by taking the lower threshold level of young men as measured in the laboratory.
Threshold Values
The current practice of using the reference range associated with the patient’s age group undoubtedly contributes to the underdiagnosis of low testosterone, said Dr. Huygue. According to a survey of AFU members in 2021, the year in which the recommendations were published, 77% of urologists interviewed reported referring to reference ranges for patients of the same age.
In their defense, “this method has long been in use, but it has eventually become apparent that symptomatic patients with an undiagnosed deficiency could be in the reference patients’ group,” Dr. Huygue explained.
Once a deficiency has been diagnosed, doctors may be reluctant to prescribe replacement therapy due to the perceived risk of developing prostate cancer. Several international studies have shown that “the risk of prostate cancer is the single biggest reason for doctors refusing to prescribe testosterone,” said Dr. Huygue.
Despite this reluctance, numerous studies have clearly shown that there is no link between a high testosterone level and the risk of developing prostate cancer. It even seems that a low testosterone level might expose a person to an increased risk for an aggressive form of cancer.
“This is a time of many surprising discoveries concerning the link between the prostate and testosterone, which go against what we have thought up to now. It has been observed that men with low testosterone develop more serious types of cancer,” said Dr. Huygue at a previous meeting of the AFU, during which he announced the publication of the French recommendations.
Prostate Cancer Recurrence
Urologists are also wary of testosterone supplementation in patients with a previous history of prostate cancer. According to the AFU’s survey, 40% of urologists questioned think that testosterone is contraindicated in this population. One in two urologists prescribe testosterone after radical prostatectomy for low or intermediate risk and most commonly after 3 years of undetectable prostate-specific antigen (PSA) levels.
Nevertheless, “several retrospective studies show the safety of testosterone replacement therapy in men who have undergone radical prostatectomy or radiotherapy or who are under active monitoring,” said Dr. Huygue. Testosterone “does not appear to increase the risk of relapse” after treatment of prostate cancer.
Dr. Huygue invited prescribing physicians to refer to the French recommendations, which specify that 1 year of undetectable PSA after prostatectomy is sufficient before prescribing replacement therapy. “This is clearly indicated in the recommendations for patients with a previous history of prostate cancer.”
Neither prostate cancer nor benign prostatic hyperplasia is a contraindication. According to the recommendations, the only contraindications to testosterone prescription are the following:
- Hematocrit > 54%
- Current breast or prostate cancer
- Cardiovascular event less than 3-6 months prior
- Trying to conceive
Cardiovascular Benefits
Another more commonly used argument by general practitioners and endocrinologists to justify their reluctance to prescribe testosterone is the risk to cardiovascular health. In early 2010, a series of American studies alerted clinicians to this risk when taking testosterone. Since then, other studies have had reassuring findings.
In response to the alert issued by the United States, the European Medicines Agency specified that “the data are not sufficient for a warning,” before the American Heart Association colleagues concluded that testosterone should only be avoided in the first 6 months following a severe cardiovascular event.
Conversely, in 2021, the European Society of Cardiology put forward the benefits of testosterone in an article in favor of replacement therapy to prevent cardiovascular risk. In particular, the hormone is thought to have a beneficial effect on arterial stiffness, the appearance of calcified plaques, and coronary artery dilatation.
The final hurdle to overcome before a testosterone prescription is filled relates to patients themselves, who often regard such treatment unfavorably. Many wrongly believe that androgens are hormones that “increase the risk of cancer, make you aggressive, cause weight gain, lead to hair loss, and cause body hair growth,” said Dr. Huygue.
Finally, breaks in the supply chain for Androtardyl, the only injectable form available for reimbursement by French social security schemes, were reported in the country in 2023, said Dr. Huygue. This situation only complicates further the prescription and use of testosterone replacement therapy.
Which Supplement?
Testosterone replacement therapies are available on the market in the following formulations:
Via transcutaneous administration: Testosterone-based gels, not covered by the French social security system (Androgel and Fortigel), to be applied daily. Users must be careful to avoid any potential transfer of the product to women or children in case of contact with the site after application.
Via an injection: Androtardyl (testosterone enanthate), covered by French social security, to be administered intramuscularly once a month. Nebido (testosterone undecanoate), not covered by French social security, with a more beneficial bioavailability profile, to be administered once every 3 months.
Pantestone (testosterone undecanoate), administered orally, is not marketed since 2021. It had the major disadvantage of requiring a high-fat diet to ensure optimal absorption.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
Underdiagnosis, reluctant doctors, patient preconceptions: Treating low testosterone levels is a tricky business in France despite the proven benefits of replacement therapy. About 20% of patients with symptomatic low testosterone levels are treated for the deficiency, said Eric Huygue, MD, PhD, urologic surgeon at Toulouse University Hospital in France, at the 117th annual conference of the French Urology Association (AFU).
, said Dr. Huygue, who was involved in drawing up the first French recommendations on treating low testosterone in 2021.
“We must keep up communication efforts to make patients and doctors aware” of the benefits of supplementation, he said.
Testosterone Levels
Testosterone deficiency mostly affects men older than 40 years. A drop in androgen levels, which varies by individual, can lead to sexual problems (such as erectile dysfunction and low libido), physical symptoms (fatigue, hot flashes, loss of muscle mass, and osteoporosis), and mental disorders (anxiety, irritability, and depression).
There are an estimated 340,000 men with symptomatic testosterone deficiency in France. Just 70,000 of these are receiving replacement therapy (see box), which accounts for only 20% of those affected. For Dr. Huygue, this low treatment rate is due to underdiagnosis, as well as reluctance on the part of doctors and patients.
Although routine screening of low testosterone in the general population is not recommended, some individuals are particularly at risk, noted the urologist.
This is especially true for patients with metabolic disorders associated with insulin resistance (such as obesity and type 2 diabetes), cardiovascular diseases (hypertension, heart failure, and atrial fibrillation), or other chronic conditions (chronic obstructive pulmonary disease, cancer, and depression). Some medications (corticosteroids, antipsychotics, chemotherapy drugs, and antiretroviral therapies) can also lead to low testosterone.
Per the French recommendations for managing low testosterone, diagnosis must be based on free or bioavailable testosterone and not total testosterone levels, which can give a skewed result. Levels must be tested twice, 1 month apart, in the morning and while fasting. The reference range is determined by taking the lower threshold level of young men as measured in the laboratory.
Threshold Values
The current practice of using the reference range associated with the patient’s age group undoubtedly contributes to the underdiagnosis of low testosterone, said Dr. Huygue. According to a survey of AFU members in 2021, the year in which the recommendations were published, 77% of urologists interviewed reported referring to reference ranges for patients of the same age.
In their defense, “this method has long been in use, but it has eventually become apparent that symptomatic patients with an undiagnosed deficiency could be in the reference patients’ group,” Dr. Huygue explained.
Once a deficiency has been diagnosed, doctors may be reluctant to prescribe replacement therapy due to the perceived risk of developing prostate cancer. Several international studies have shown that “the risk of prostate cancer is the single biggest reason for doctors refusing to prescribe testosterone,” said Dr. Huygue.
Despite this reluctance, numerous studies have clearly shown that there is no link between a high testosterone level and the risk of developing prostate cancer. It even seems that a low testosterone level might expose a person to an increased risk for an aggressive form of cancer.
“This is a time of many surprising discoveries concerning the link between the prostate and testosterone, which go against what we have thought up to now. It has been observed that men with low testosterone develop more serious types of cancer,” said Dr. Huygue at a previous meeting of the AFU, during which he announced the publication of the French recommendations.
Prostate Cancer Recurrence
Urologists are also wary of testosterone supplementation in patients with a previous history of prostate cancer. According to the AFU’s survey, 40% of urologists questioned think that testosterone is contraindicated in this population. One in two urologists prescribe testosterone after radical prostatectomy for low or intermediate risk and most commonly after 3 years of undetectable prostate-specific antigen (PSA) levels.
Nevertheless, “several retrospective studies show the safety of testosterone replacement therapy in men who have undergone radical prostatectomy or radiotherapy or who are under active monitoring,” said Dr. Huygue. Testosterone “does not appear to increase the risk of relapse” after treatment of prostate cancer.
Dr. Huygue invited prescribing physicians to refer to the French recommendations, which specify that 1 year of undetectable PSA after prostatectomy is sufficient before prescribing replacement therapy. “This is clearly indicated in the recommendations for patients with a previous history of prostate cancer.”
Neither prostate cancer nor benign prostatic hyperplasia is a contraindication. According to the recommendations, the only contraindications to testosterone prescription are the following:
- Hematocrit > 54%
- Current breast or prostate cancer
- Cardiovascular event less than 3-6 months prior
- Trying to conceive
Cardiovascular Benefits
Another more commonly used argument by general practitioners and endocrinologists to justify their reluctance to prescribe testosterone is the risk to cardiovascular health. In early 2010, a series of American studies alerted clinicians to this risk when taking testosterone. Since then, other studies have had reassuring findings.
In response to the alert issued by the United States, the European Medicines Agency specified that “the data are not sufficient for a warning,” before the American Heart Association colleagues concluded that testosterone should only be avoided in the first 6 months following a severe cardiovascular event.
Conversely, in 2021, the European Society of Cardiology put forward the benefits of testosterone in an article in favor of replacement therapy to prevent cardiovascular risk. In particular, the hormone is thought to have a beneficial effect on arterial stiffness, the appearance of calcified plaques, and coronary artery dilatation.
The final hurdle to overcome before a testosterone prescription is filled relates to patients themselves, who often regard such treatment unfavorably. Many wrongly believe that androgens are hormones that “increase the risk of cancer, make you aggressive, cause weight gain, lead to hair loss, and cause body hair growth,” said Dr. Huygue.
Finally, breaks in the supply chain for Androtardyl, the only injectable form available for reimbursement by French social security schemes, were reported in the country in 2023, said Dr. Huygue. This situation only complicates further the prescription and use of testosterone replacement therapy.
Which Supplement?
Testosterone replacement therapies are available on the market in the following formulations:
Via transcutaneous administration: Testosterone-based gels, not covered by the French social security system (Androgel and Fortigel), to be applied daily. Users must be careful to avoid any potential transfer of the product to women or children in case of contact with the site after application.
Via an injection: Androtardyl (testosterone enanthate), covered by French social security, to be administered intramuscularly once a month. Nebido (testosterone undecanoate), not covered by French social security, with a more beneficial bioavailability profile, to be administered once every 3 months.
Pantestone (testosterone undecanoate), administered orally, is not marketed since 2021. It had the major disadvantage of requiring a high-fat diet to ensure optimal absorption.
This article was translated from the Medscape French edition. A version of this article appeared on Medscape.com.
How much would you bet on a diagnosis?
“You have psoriasis,” I say all the time. I mean it when I say it, of course. But I don’t always to the same degree. Sometimes I’m trying to say, “You probably have psoriasis.” Other times I mean, “You most definitely have psoriasis.” I rarely use those terms though.
One 36-year-old man with a flaky scalp and scaly elbows wasn’t satisfied with my assessment. His dad has psoriasis. So does his older brother. He was in to see me to find out if he had psoriasis too. “Probably” was what I gave him. He pushed back, “What percent chance?” That’s a good question — must be an engineer. I’m unsure.
With the exception of the poker players, our species is notoriously bad at probabilities. We’re wired to notice the significance of events, but terrible at understanding their likelihood. This is salient in lottery ticket holders and some NFL offensive coordinators who persist despite very long odds of things working out. It’s also reflected in the language we use. Rarely do we say, there’s a sixty percent chance something will happen. Rather, we say, “it’s likely.” There are two problems here. One, we often misjudge the actual probability of something occurring and two, the terms we use are subjective and differences in interpretation can lead to misunderstandings.
Let’s take a look. A 55-year-old man with a chronic eczematous rash on his trunk and extremities is getting worse despite dupilumab. He recently had night sweats. Do you think he has atopic dermatitis or cutaneous T-cell lymphoma? If you had to place a $100 bet, would you change your answer? Immanuel Kant thinks you would. In his “Critique of Pure Reason,” the German philosopher proposes that betting helps clarify the mind, an antidote to brashness. The example Kant uses is of a physician who observes a patient and concludes he has phthisis (tuberculosis), but we really don’t know if the physician is confident. Kant proposes that if he had to bet on his conclusion, then we’d have insight into just how convinced he is of phthisis. So, what’s your bet?
If you’re a bad poker player, then you might bet he has cutaneous T-cell lymphoma. However, not having any additional information, the smart call is atopic dermatitis, which has a base rate 1000-fold higher than CTCL. It is therefore more probable to be eczema even in a case that worsens despite dupilumab or with recent night sweats, both of which could be a result of common variables such as weather and COVID. Failure to account for the base rate is a mistake we physicians sometimes make. Economists rarely do. Try to think like one before answering a likelihood question.
If you think about it, “probably” means something different even to me, depending on the situation. I might say I’ll probably go to Montana this summer and I’ll probably retire at 65. The actual likelihoods might be 95% and 70%. That’s a big difference. What about between probably and likely? Or possibly and maybe? Do they mean the same to you as to the person you’re speaking with? For much of the work we do, precise likelihoods aren’t critical. Yet, it can be important in decision making and in discussing probabilities, such as the risk of hepatitis on terbinafine or of melanoma recurrence after Mohs.
I told my patient “I say about a 70% chance you have psoriasis. I could do a biopsy today to confirm.” He thought for a second and asked, “What is the chance it’s psoriasis if the biopsy shows it?” “Eighty six percent,” I replied.
Seemed like a good bet to me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
“You have psoriasis,” I say all the time. I mean it when I say it, of course. But I don’t always to the same degree. Sometimes I’m trying to say, “You probably have psoriasis.” Other times I mean, “You most definitely have psoriasis.” I rarely use those terms though.
One 36-year-old man with a flaky scalp and scaly elbows wasn’t satisfied with my assessment. His dad has psoriasis. So does his older brother. He was in to see me to find out if he had psoriasis too. “Probably” was what I gave him. He pushed back, “What percent chance?” That’s a good question — must be an engineer. I’m unsure.
With the exception of the poker players, our species is notoriously bad at probabilities. We’re wired to notice the significance of events, but terrible at understanding their likelihood. This is salient in lottery ticket holders and some NFL offensive coordinators who persist despite very long odds of things working out. It’s also reflected in the language we use. Rarely do we say, there’s a sixty percent chance something will happen. Rather, we say, “it’s likely.” There are two problems here. One, we often misjudge the actual probability of something occurring and two, the terms we use are subjective and differences in interpretation can lead to misunderstandings.
Let’s take a look. A 55-year-old man with a chronic eczematous rash on his trunk and extremities is getting worse despite dupilumab. He recently had night sweats. Do you think he has atopic dermatitis or cutaneous T-cell lymphoma? If you had to place a $100 bet, would you change your answer? Immanuel Kant thinks you would. In his “Critique of Pure Reason,” the German philosopher proposes that betting helps clarify the mind, an antidote to brashness. The example Kant uses is of a physician who observes a patient and concludes he has phthisis (tuberculosis), but we really don’t know if the physician is confident. Kant proposes that if he had to bet on his conclusion, then we’d have insight into just how convinced he is of phthisis. So, what’s your bet?
If you’re a bad poker player, then you might bet he has cutaneous T-cell lymphoma. However, not having any additional information, the smart call is atopic dermatitis, which has a base rate 1000-fold higher than CTCL. It is therefore more probable to be eczema even in a case that worsens despite dupilumab or with recent night sweats, both of which could be a result of common variables such as weather and COVID. Failure to account for the base rate is a mistake we physicians sometimes make. Economists rarely do. Try to think like one before answering a likelihood question.
If you think about it, “probably” means something different even to me, depending on the situation. I might say I’ll probably go to Montana this summer and I’ll probably retire at 65. The actual likelihoods might be 95% and 70%. That’s a big difference. What about between probably and likely? Or possibly and maybe? Do they mean the same to you as to the person you’re speaking with? For much of the work we do, precise likelihoods aren’t critical. Yet, it can be important in decision making and in discussing probabilities, such as the risk of hepatitis on terbinafine or of melanoma recurrence after Mohs.
I told my patient “I say about a 70% chance you have psoriasis. I could do a biopsy today to confirm.” He thought for a second and asked, “What is the chance it’s psoriasis if the biopsy shows it?” “Eighty six percent,” I replied.
Seemed like a good bet to me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
“You have psoriasis,” I say all the time. I mean it when I say it, of course. But I don’t always to the same degree. Sometimes I’m trying to say, “You probably have psoriasis.” Other times I mean, “You most definitely have psoriasis.” I rarely use those terms though.
One 36-year-old man with a flaky scalp and scaly elbows wasn’t satisfied with my assessment. His dad has psoriasis. So does his older brother. He was in to see me to find out if he had psoriasis too. “Probably” was what I gave him. He pushed back, “What percent chance?” That’s a good question — must be an engineer. I’m unsure.
With the exception of the poker players, our species is notoriously bad at probabilities. We’re wired to notice the significance of events, but terrible at understanding their likelihood. This is salient in lottery ticket holders and some NFL offensive coordinators who persist despite very long odds of things working out. It’s also reflected in the language we use. Rarely do we say, there’s a sixty percent chance something will happen. Rather, we say, “it’s likely.” There are two problems here. One, we often misjudge the actual probability of something occurring and two, the terms we use are subjective and differences in interpretation can lead to misunderstandings.
Let’s take a look. A 55-year-old man with a chronic eczematous rash on his trunk and extremities is getting worse despite dupilumab. He recently had night sweats. Do you think he has atopic dermatitis or cutaneous T-cell lymphoma? If you had to place a $100 bet, would you change your answer? Immanuel Kant thinks you would. In his “Critique of Pure Reason,” the German philosopher proposes that betting helps clarify the mind, an antidote to brashness. The example Kant uses is of a physician who observes a patient and concludes he has phthisis (tuberculosis), but we really don’t know if the physician is confident. Kant proposes that if he had to bet on his conclusion, then we’d have insight into just how convinced he is of phthisis. So, what’s your bet?
If you’re a bad poker player, then you might bet he has cutaneous T-cell lymphoma. However, not having any additional information, the smart call is atopic dermatitis, which has a base rate 1000-fold higher than CTCL. It is therefore more probable to be eczema even in a case that worsens despite dupilumab or with recent night sweats, both of which could be a result of common variables such as weather and COVID. Failure to account for the base rate is a mistake we physicians sometimes make. Economists rarely do. Try to think like one before answering a likelihood question.
If you think about it, “probably” means something different even to me, depending on the situation. I might say I’ll probably go to Montana this summer and I’ll probably retire at 65. The actual likelihoods might be 95% and 70%. That’s a big difference. What about between probably and likely? Or possibly and maybe? Do they mean the same to you as to the person you’re speaking with? For much of the work we do, precise likelihoods aren’t critical. Yet, it can be important in decision making and in discussing probabilities, such as the risk of hepatitis on terbinafine or of melanoma recurrence after Mohs.
I told my patient “I say about a 70% chance you have psoriasis. I could do a biopsy today to confirm.” He thought for a second and asked, “What is the chance it’s psoriasis if the biopsy shows it?” “Eighty six percent,” I replied.
Seemed like a good bet to me.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
How a Simple Urine Test Could Reveal Early-Stage Lung Cancer
Lung cancer is the deadliest cancer in the world, largely because so many patients are diagnosed late.
Screening more patients could help, yet screening rates remain critically low. In the United States, only about 6% of eligible people get screened , according to the American Lung Association. Contrast that with screening rates for breast, cervical, and colorectal cancer, which all top 70%.
But what if lung cancer detection was as simple as taking a puff on an inhaler and following up with a urine test?
, according to research published this month in Science Advances. If the sensors spot these proteins, they produce a signal in the urine that can be detected with a paper test strip.
“It’s a more complex version of a pregnancy test, but it’s very simple to use,” said Qian Zhong, PhD, an MIT researcher and co-lead author of the study.
Currently, the only recommended screening test for lung cancer is low-dose CT. But not everyone has easy access to screening facilities, said the other co-lead author Edward Tan, PhD, a former MIT postdoc and currently a scientist at the biotech company Prime Medicine, Cambridge, Massachusetts.
“Our focus is to provide an alternative for the early detection of lung cancer that does not rely on resource-intensive infrastructure,” said Dr. Tan. “Most developing countries don’t have such resources” — and residents in some parts of the United States don’t have easy access, either, he said.
How It Works
The sensors are polymer nanoparticles coated in DNA barcodes, short DNA sequences that are unique and easy to identify. The researchers engineered the particles to be targeted by protease enzymes linked to stage I lung adenocarcinoma. Upon contact, the proteases cleave off the barcodes, which make their way into the bloodstream and are excreted in urine. A test strip can detect them, revealing results about 20 minutes from the time it’s dipped.
The researchers tested this system in mice genetically engineered to develop human-like lung tumors. Using aerosol nebulizers, they delivered 20 sensors to mice with the equivalent of stage I or II cancer. Using a machine learning algorithm, they identified the four most accurate sensors. With 100% specificity, those four sensors exhibited sensitivity of 84.6%.
“One advantage of using inhalation is that it’s noninvasive, and another advantage is that it distributes across the lung quite homogeneously,” said Dr. Tan. The time from inhalation to detection is also relatively fast — in mice, the whole process took about 2 hours, and Dr. Zhong speculated that it would not be much longer in humans.
Other Applications and Challenges
An injectable version of this technology, also developed at MIT, has already been tested in a phase 1 clinical trial for diagnosing liver cancer and nonalcoholic steatohepatitis. The injection also works in tandem with a urine test, the researchers showed in 2021. According to Tan, his research group (led by Sangeeta Bhatia, MD, PhD) was the first to describe this type of technology to screen for diseases.
The lab is also working toward using inhalable sensors to distinguish between viral, bacterial, and fungal pneumonia. And the technology could also be used to diagnose other lung conditions like asthma and chronic obstructive pulmonary disease, Dr. Tan said.
The tech is certainly “innovative,” remarked Gaetano Rocco, MD, a thoracic surgeon and lung cancer researcher at Memorial Sloan Kettering Cancer Center, Basking Ridge, New Jersey, who was not involved in the study.
Still, challenges may arise when applying it to people. Many factors are involved in regulating fluid volume, potentially interfering with the ability to detect the compounds in the urine, Rocco said. Diet, hydration, drug interference, renal function, and some chronic diseases could all limit effectiveness.
Another challenge: Human cancer can be more heterogeneous (containing different kinds of cancer cells), so four sensors may not be enough, Zhong said. He and colleagues are beginning to analyze human biopsy samples to see whether the same sensors that worked in mice would also work in humans. If all goes well, they hope to do studies on humans or nonhuman primates.
A version of this article appeared on Medscape.com.
Lung cancer is the deadliest cancer in the world, largely because so many patients are diagnosed late.
Screening more patients could help, yet screening rates remain critically low. In the United States, only about 6% of eligible people get screened , according to the American Lung Association. Contrast that with screening rates for breast, cervical, and colorectal cancer, which all top 70%.
But what if lung cancer detection was as simple as taking a puff on an inhaler and following up with a urine test?
, according to research published this month in Science Advances. If the sensors spot these proteins, they produce a signal in the urine that can be detected with a paper test strip.
“It’s a more complex version of a pregnancy test, but it’s very simple to use,” said Qian Zhong, PhD, an MIT researcher and co-lead author of the study.
Currently, the only recommended screening test for lung cancer is low-dose CT. But not everyone has easy access to screening facilities, said the other co-lead author Edward Tan, PhD, a former MIT postdoc and currently a scientist at the biotech company Prime Medicine, Cambridge, Massachusetts.
“Our focus is to provide an alternative for the early detection of lung cancer that does not rely on resource-intensive infrastructure,” said Dr. Tan. “Most developing countries don’t have such resources” — and residents in some parts of the United States don’t have easy access, either, he said.
How It Works
The sensors are polymer nanoparticles coated in DNA barcodes, short DNA sequences that are unique and easy to identify. The researchers engineered the particles to be targeted by protease enzymes linked to stage I lung adenocarcinoma. Upon contact, the proteases cleave off the barcodes, which make their way into the bloodstream and are excreted in urine. A test strip can detect them, revealing results about 20 minutes from the time it’s dipped.
The researchers tested this system in mice genetically engineered to develop human-like lung tumors. Using aerosol nebulizers, they delivered 20 sensors to mice with the equivalent of stage I or II cancer. Using a machine learning algorithm, they identified the four most accurate sensors. With 100% specificity, those four sensors exhibited sensitivity of 84.6%.
“One advantage of using inhalation is that it’s noninvasive, and another advantage is that it distributes across the lung quite homogeneously,” said Dr. Tan. The time from inhalation to detection is also relatively fast — in mice, the whole process took about 2 hours, and Dr. Zhong speculated that it would not be much longer in humans.
Other Applications and Challenges
An injectable version of this technology, also developed at MIT, has already been tested in a phase 1 clinical trial for diagnosing liver cancer and nonalcoholic steatohepatitis. The injection also works in tandem with a urine test, the researchers showed in 2021. According to Tan, his research group (led by Sangeeta Bhatia, MD, PhD) was the first to describe this type of technology to screen for diseases.
The lab is also working toward using inhalable sensors to distinguish between viral, bacterial, and fungal pneumonia. And the technology could also be used to diagnose other lung conditions like asthma and chronic obstructive pulmonary disease, Dr. Tan said.
The tech is certainly “innovative,” remarked Gaetano Rocco, MD, a thoracic surgeon and lung cancer researcher at Memorial Sloan Kettering Cancer Center, Basking Ridge, New Jersey, who was not involved in the study.
Still, challenges may arise when applying it to people. Many factors are involved in regulating fluid volume, potentially interfering with the ability to detect the compounds in the urine, Rocco said. Diet, hydration, drug interference, renal function, and some chronic diseases could all limit effectiveness.
Another challenge: Human cancer can be more heterogeneous (containing different kinds of cancer cells), so four sensors may not be enough, Zhong said. He and colleagues are beginning to analyze human biopsy samples to see whether the same sensors that worked in mice would also work in humans. If all goes well, they hope to do studies on humans or nonhuman primates.
A version of this article appeared on Medscape.com.
Lung cancer is the deadliest cancer in the world, largely because so many patients are diagnosed late.
Screening more patients could help, yet screening rates remain critically low. In the United States, only about 6% of eligible people get screened , according to the American Lung Association. Contrast that with screening rates for breast, cervical, and colorectal cancer, which all top 70%.
But what if lung cancer detection was as simple as taking a puff on an inhaler and following up with a urine test?
, according to research published this month in Science Advances. If the sensors spot these proteins, they produce a signal in the urine that can be detected with a paper test strip.
“It’s a more complex version of a pregnancy test, but it’s very simple to use,” said Qian Zhong, PhD, an MIT researcher and co-lead author of the study.
Currently, the only recommended screening test for lung cancer is low-dose CT. But not everyone has easy access to screening facilities, said the other co-lead author Edward Tan, PhD, a former MIT postdoc and currently a scientist at the biotech company Prime Medicine, Cambridge, Massachusetts.
“Our focus is to provide an alternative for the early detection of lung cancer that does not rely on resource-intensive infrastructure,” said Dr. Tan. “Most developing countries don’t have such resources” — and residents in some parts of the United States don’t have easy access, either, he said.
How It Works
The sensors are polymer nanoparticles coated in DNA barcodes, short DNA sequences that are unique and easy to identify. The researchers engineered the particles to be targeted by protease enzymes linked to stage I lung adenocarcinoma. Upon contact, the proteases cleave off the barcodes, which make their way into the bloodstream and are excreted in urine. A test strip can detect them, revealing results about 20 minutes from the time it’s dipped.
The researchers tested this system in mice genetically engineered to develop human-like lung tumors. Using aerosol nebulizers, they delivered 20 sensors to mice with the equivalent of stage I or II cancer. Using a machine learning algorithm, they identified the four most accurate sensors. With 100% specificity, those four sensors exhibited sensitivity of 84.6%.
“One advantage of using inhalation is that it’s noninvasive, and another advantage is that it distributes across the lung quite homogeneously,” said Dr. Tan. The time from inhalation to detection is also relatively fast — in mice, the whole process took about 2 hours, and Dr. Zhong speculated that it would not be much longer in humans.
Other Applications and Challenges
An injectable version of this technology, also developed at MIT, has already been tested in a phase 1 clinical trial for diagnosing liver cancer and nonalcoholic steatohepatitis. The injection also works in tandem with a urine test, the researchers showed in 2021. According to Tan, his research group (led by Sangeeta Bhatia, MD, PhD) was the first to describe this type of technology to screen for diseases.
The lab is also working toward using inhalable sensors to distinguish between viral, bacterial, and fungal pneumonia. And the technology could also be used to diagnose other lung conditions like asthma and chronic obstructive pulmonary disease, Dr. Tan said.
The tech is certainly “innovative,” remarked Gaetano Rocco, MD, a thoracic surgeon and lung cancer researcher at Memorial Sloan Kettering Cancer Center, Basking Ridge, New Jersey, who was not involved in the study.
Still, challenges may arise when applying it to people. Many factors are involved in regulating fluid volume, potentially interfering with the ability to detect the compounds in the urine, Rocco said. Diet, hydration, drug interference, renal function, and some chronic diseases could all limit effectiveness.
Another challenge: Human cancer can be more heterogeneous (containing different kinds of cancer cells), so four sensors may not be enough, Zhong said. He and colleagues are beginning to analyze human biopsy samples to see whether the same sensors that worked in mice would also work in humans. If all goes well, they hope to do studies on humans or nonhuman primates.
A version of this article appeared on Medscape.com.
10 Weight-Loss Strategies to Help Patients With Obesity
This transcript has been edited for clarity.
According to the Centers for Disease Control and Prevention, the obesity prevalence in America was 41.9% between 2017 and 2020. Just 10 years ago, no state had an obesity prevalence above 35%.
Over the past 3 years, many patients gained weight during the COVID-19 pandemic as a result of adopting more sedentary lifestyles, staying at home, avoiding the gym owing to the potential for respiratory spread, and working remotely. For a long time, patients were avoiding attending social events and, as a result, were walking much less.
and other physicians to help patients with obesity realize their goal of achieving weight loss.
1. Embracing the GLP-1 Revolution, With Some Caveats
Glucagon-like peptide-1 (GLP-1) receptor agonists have become a popular treatment for type 2 diabetes and weight loss. These medications, which are given as an injection either weekly or daily depending on the type, have helped patients achieve weight loss with tremendous success.
They work by stimulating the body to produce insulin, which in turn lowers blood sugar. GLP-1 receptor agonists also slow peristalsis and the movement of food from the stomach into the small bowel, which allows patients to eat less by feeling fuller for longer and decreasing hunger.
Two GLP-1 receptor agonists are approved by the US Food and Drug Administration (FDA) for weight loss in patients without diabetes: liraglutide (Saxenda) and semaglutide (Wegovy). There are also lower-dose versions of these active ingredients with the trade names Ozempic and Victoza, designed to help patients with diabetes achieve better glucose and A1c control. In November 2023, the FDA approved a new medication called tirzepatide (Zepbound), which is a glucose-dependent insulinotropic polypeptide (GIP) plus GLP-1 receptor agonist.
This is a very exciting time for the management of type 2 diabetes and weight loss. Gastroenterologists can work with endocrinologists and primary care physicians to help patients choose appropriate weight loss medications.
However, gastroenterologists should also be aware of common GLP-1 receptor agonist side effects, including nausea, vomiting, diarrhea, and — in severe cases — hypoglycemia. These medications can also cause pancreatitis, acute kidney injury, worsening diabetes-related retinopathy, tachycardia, headaches, indigestion, gastroparesis, bowel obstruction, or ileus. We don’t use these medications in patients with a family or personal history of medullary thyroid cancer or multiple endocrine neoplasia. Consider avoiding their use as well in patients with a personal history of pancreatitis.
Recently, the American Society of Anesthesiologists (ASA) suggested holding off on the use of GLP-1 receptor agonists prior to elective endoscopy procedures owing to case reports of aspiration. Gastroenterologists and anesthesiologists are working together to make esophagogastroduodenoscopy (EGD) and colonoscopy as safe as possible in patients taking these treatments.
According to the ASA recommendations, GLP-1 receptor agonists that are given at a daily dose should be held on the day of their procedure. Weekly-dose versions are supposed to be held for 1 week prior to colonoscopy or EGD. During EGD procedures, I also recommend keeping the head of the bed at a 45° angle to help prevent aspiration even further.
Gastroenterologists are eagerly awaiting additional studies to determine whether holding GLP-1 receptor agonists prior to endoscopy is really necessary. But for now, we recommend following the ASA guidelines.
2. Substituting Out Sugary Drinks
Gastroenterologists and primary care physicians constantly advise their patients to avoid consuming sugary drinks, such as soda, fruit juices, calorie-laden coffee drinks, sweetened tea, hot chocolate, and, of course, alcohol. Many of our patients drink three to six of these sugary drinks a day.
As a gastroenterologist, it’s important to counsel our overweight patients and obtain an accurate history about their daily and weekly consumption of excess calories.
Recommend substituting sugary drinks with water, unsweetened tea (either hot or cold), and coffee.
To prevent constipation, encourage patients to drink at least eight 8-ounce glasses of fluid per day. Drinking water, tea, and coffee can also help keep patients feeling fuller for longer and avoid those tempting snacks.
3. Adopting the Right Diet
Every day, I encourage my patients to avoid eating fried fatty foods and processed meats. We also advise patients to avoid junk food filled with carbohydrates and salt.
Instead, patients should try to eat a piece of fruit or some vegetables with every single meal, which keeps patients feeling fuller for longer, prevents diverticulitis from forming, and can even help prevent colon cancer.
Making small dietary changes can dramatically reduce daily calorie consumption, which adds up over time and can help patients lose weight in a safe way.
Meal prepping for the week ahead, perhaps on a Sunday, is a very simple way to eat more nutritious foods instead of constantly getting takeout and fast food.
Many of our patients have also successfully lost weight through intermittent fasting, although I recommend working with a nutritionist on this one.
A Mediterranean diet is also a great option.
4. Getting Active
I encourage patients to take daily walks, swim, play sports, take fitness classes, do yoga or Pilates, and use weights at a gym.
Exercise burns calories, which is great for our hearts, prevents hepatic steatosis, and helps relieve stress. Exercise also stimulates peristalsis, which can help our constipated patients achieve more regular bowel movements.
There are a few other things to keep in mind in this area. Try to avoid strenuous exercise right after eating, because this will help prevent both heartburn and gastroesophageal reflux disease (GERD).
5. Reducing Stomach Volume With a Gastric Balloon
A gastric balloon procedure is a temporary obesity treatment that helps patients lose weight by reducing the volume of the stomach so that they feel full more easily. This can be accomplished endoscopically through the mouth without the need for surgery.
Basically, a deflated balloon is placed through the mouth using an endoscope and advanced into the stomach by a gastroenterologist or surgeon. The balloon is inflated with salt water and can remain in the stomach for 6 months before it is removed.
This procedure can help patients feel full and consequently eat less, thereby leading to gradual and safe weight loss.
6. Using the Accordion Procedure
An endoscopic sleeve gastroplasty procedure, sometimes called an accordion procedure, is used for patients with a body mass index ≥ 30 when diet and exercise alone have failed. An EGD tube is equipped with small stitching instruments that are used to reduce the size of the stomach.
This procedure has less complications than open or laparoscopic surgery and can be reversed.
7. Injecting Botulinum Toxin
Another technique is having the gastroenterologist inject botulinum toxin into the stomach wall. This works by relaxing the stomach propulsion muscles, which delays gastric emptying so that patients feel fuller longer and more easily.
This approach is good for achieving moderate weight loss of approximately 5%-10% of body weight. It works best in combination with a good diet and exercise. The effects of the botulinum toxin can last for 3 months, and the procedure can be repeated every 6 months.
8. Adjusting Certain Lifestyle Factors
Gastroenterologists should also counsel our patients about exercise, stress management, and the importance of sleep to prevent overeating. Self-care is extremely important for patients. Walk, swim, lift weights, and play sports; I personally love basketball and tennis.
I also recommend allocating enough time for sleep each night. At least 7-9 hours of sleep is ideal. Good sleep hygiene can help keep a stable schedule. Create a comfortable bedroom that is free of disruptions like TV watching or playing on your phone or computer.
Gastroenterologists can provide simple instructions to their patients on how to achieve this. For example, unplug from electronics 30-60 minutes prior to sleep. Try also to avoid eating late at night, which will help patients prevent GERD and heartburn symptoms too.
9. Considering Orlistat as an Option
Orlistat is an oral over-the-counter lipase inhibitor that inhibits fat absorption in the intestines. This drug can interfere with the absorption of fat-soluble vitamins A, D, E, and K. Therefore, it’s important to take a multivitamin 2 hours before or 2 hours after taking orlistat.
However, orlistat can cause steatorrhea, so it’s often not our first choice.
10. Working With Dietitians
I highly recommend that gastroenterologists regularly refer patients to a registered dietitian for medical nutrition therapy. Dietitians help patients establish nutritional goals with calorie limits. I find that many of my patients like the nutritional counseling the dietitians provide, and this can even be done via telemedicine.
A dietitian will examine a patient’s eating habits and help them set weight loss goals that are both realistic and achievable. Having a dietitian motivate a patient through several clinic visits is important for success. A dietitian can plan how many calories a patient should consume in a day while maintaining food, protein, and vitamin intake.
With this therapy, many patients are able to lose approximately 1-1.5 pounds each week. A dietitian can help keep patients accountable for their weight loss goals. I encourage my patients to use their dietitian as a weight loss teacher and a coach who can personalize a diet plan that tastes great.
Some of our patients also have overlapping gastrointestinal issues, such as celiac disease or irritable bowel syndrome. Dietitians can also formulate diets that are great for these other diagnoses too.
There are also apps available on our phones to help with diet and weight loss.
Having a Difficult Conversation to Prevent Long-Term Disease
It’s important for gastroenterologists to work with patients to achieve weight loss. Addressing obesity is sometimes a difficult topic to bring up with patients, but it’s nonetheless very important.
Together, we can help treat obesity plus improve and prevent hepatic steatosis, metabolic dysfunction–associated steatotic liver disease (MASLD), and metabolic dysfunction–associated steatohepatitis (MASH). The estimated global prevalence of MASLD is 32% in adults, so gastroenterologists and hepatologists are working together to try to treat obesity and to prevent long-term liver disease.
Dr. Levy is a gastroenterologist at the University of Chicago. In 2017, Levy, a previous Fulbright Fellow in France, also started a gastroenterology clinic for refugees resettling in Chicago. His clinical projects focus on the development of colorectal cancer screening campaigns. Levy, who recently gave a TEDx Talk about building health education campaigns using music and concerts, organizes Tune It Up: A Concert To Raise Colorectal Cancer Awareness with the American College of Gastroenterology (ACG). He frequently publishes on a variety of gastroenterology topics and serves on ACG’s Public Relations Committee and FDA-Related Matters Committee. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
According to the Centers for Disease Control and Prevention, the obesity prevalence in America was 41.9% between 2017 and 2020. Just 10 years ago, no state had an obesity prevalence above 35%.
Over the past 3 years, many patients gained weight during the COVID-19 pandemic as a result of adopting more sedentary lifestyles, staying at home, avoiding the gym owing to the potential for respiratory spread, and working remotely. For a long time, patients were avoiding attending social events and, as a result, were walking much less.
and other physicians to help patients with obesity realize their goal of achieving weight loss.
1. Embracing the GLP-1 Revolution, With Some Caveats
Glucagon-like peptide-1 (GLP-1) receptor agonists have become a popular treatment for type 2 diabetes and weight loss. These medications, which are given as an injection either weekly or daily depending on the type, have helped patients achieve weight loss with tremendous success.
They work by stimulating the body to produce insulin, which in turn lowers blood sugar. GLP-1 receptor agonists also slow peristalsis and the movement of food from the stomach into the small bowel, which allows patients to eat less by feeling fuller for longer and decreasing hunger.
Two GLP-1 receptor agonists are approved by the US Food and Drug Administration (FDA) for weight loss in patients without diabetes: liraglutide (Saxenda) and semaglutide (Wegovy). There are also lower-dose versions of these active ingredients with the trade names Ozempic and Victoza, designed to help patients with diabetes achieve better glucose and A1c control. In November 2023, the FDA approved a new medication called tirzepatide (Zepbound), which is a glucose-dependent insulinotropic polypeptide (GIP) plus GLP-1 receptor agonist.
This is a very exciting time for the management of type 2 diabetes and weight loss. Gastroenterologists can work with endocrinologists and primary care physicians to help patients choose appropriate weight loss medications.
However, gastroenterologists should also be aware of common GLP-1 receptor agonist side effects, including nausea, vomiting, diarrhea, and — in severe cases — hypoglycemia. These medications can also cause pancreatitis, acute kidney injury, worsening diabetes-related retinopathy, tachycardia, headaches, indigestion, gastroparesis, bowel obstruction, or ileus. We don’t use these medications in patients with a family or personal history of medullary thyroid cancer or multiple endocrine neoplasia. Consider avoiding their use as well in patients with a personal history of pancreatitis.
Recently, the American Society of Anesthesiologists (ASA) suggested holding off on the use of GLP-1 receptor agonists prior to elective endoscopy procedures owing to case reports of aspiration. Gastroenterologists and anesthesiologists are working together to make esophagogastroduodenoscopy (EGD) and colonoscopy as safe as possible in patients taking these treatments.
According to the ASA recommendations, GLP-1 receptor agonists that are given at a daily dose should be held on the day of their procedure. Weekly-dose versions are supposed to be held for 1 week prior to colonoscopy or EGD. During EGD procedures, I also recommend keeping the head of the bed at a 45° angle to help prevent aspiration even further.
Gastroenterologists are eagerly awaiting additional studies to determine whether holding GLP-1 receptor agonists prior to endoscopy is really necessary. But for now, we recommend following the ASA guidelines.
2. Substituting Out Sugary Drinks
Gastroenterologists and primary care physicians constantly advise their patients to avoid consuming sugary drinks, such as soda, fruit juices, calorie-laden coffee drinks, sweetened tea, hot chocolate, and, of course, alcohol. Many of our patients drink three to six of these sugary drinks a day.
As a gastroenterologist, it’s important to counsel our overweight patients and obtain an accurate history about their daily and weekly consumption of excess calories.
Recommend substituting sugary drinks with water, unsweetened tea (either hot or cold), and coffee.
To prevent constipation, encourage patients to drink at least eight 8-ounce glasses of fluid per day. Drinking water, tea, and coffee can also help keep patients feeling fuller for longer and avoid those tempting snacks.
3. Adopting the Right Diet
Every day, I encourage my patients to avoid eating fried fatty foods and processed meats. We also advise patients to avoid junk food filled with carbohydrates and salt.
Instead, patients should try to eat a piece of fruit or some vegetables with every single meal, which keeps patients feeling fuller for longer, prevents diverticulitis from forming, and can even help prevent colon cancer.
Making small dietary changes can dramatically reduce daily calorie consumption, which adds up over time and can help patients lose weight in a safe way.
Meal prepping for the week ahead, perhaps on a Sunday, is a very simple way to eat more nutritious foods instead of constantly getting takeout and fast food.
Many of our patients have also successfully lost weight through intermittent fasting, although I recommend working with a nutritionist on this one.
A Mediterranean diet is also a great option.
4. Getting Active
I encourage patients to take daily walks, swim, play sports, take fitness classes, do yoga or Pilates, and use weights at a gym.
Exercise burns calories, which is great for our hearts, prevents hepatic steatosis, and helps relieve stress. Exercise also stimulates peristalsis, which can help our constipated patients achieve more regular bowel movements.
There are a few other things to keep in mind in this area. Try to avoid strenuous exercise right after eating, because this will help prevent both heartburn and gastroesophageal reflux disease (GERD).
5. Reducing Stomach Volume With a Gastric Balloon
A gastric balloon procedure is a temporary obesity treatment that helps patients lose weight by reducing the volume of the stomach so that they feel full more easily. This can be accomplished endoscopically through the mouth without the need for surgery.
Basically, a deflated balloon is placed through the mouth using an endoscope and advanced into the stomach by a gastroenterologist or surgeon. The balloon is inflated with salt water and can remain in the stomach for 6 months before it is removed.
This procedure can help patients feel full and consequently eat less, thereby leading to gradual and safe weight loss.
6. Using the Accordion Procedure
An endoscopic sleeve gastroplasty procedure, sometimes called an accordion procedure, is used for patients with a body mass index ≥ 30 when diet and exercise alone have failed. An EGD tube is equipped with small stitching instruments that are used to reduce the size of the stomach.
This procedure has less complications than open or laparoscopic surgery and can be reversed.
7. Injecting Botulinum Toxin
Another technique is having the gastroenterologist inject botulinum toxin into the stomach wall. This works by relaxing the stomach propulsion muscles, which delays gastric emptying so that patients feel fuller longer and more easily.
This approach is good for achieving moderate weight loss of approximately 5%-10% of body weight. It works best in combination with a good diet and exercise. The effects of the botulinum toxin can last for 3 months, and the procedure can be repeated every 6 months.
8. Adjusting Certain Lifestyle Factors
Gastroenterologists should also counsel our patients about exercise, stress management, and the importance of sleep to prevent overeating. Self-care is extremely important for patients. Walk, swim, lift weights, and play sports; I personally love basketball and tennis.
I also recommend allocating enough time for sleep each night. At least 7-9 hours of sleep is ideal. Good sleep hygiene can help keep a stable schedule. Create a comfortable bedroom that is free of disruptions like TV watching or playing on your phone or computer.
Gastroenterologists can provide simple instructions to their patients on how to achieve this. For example, unplug from electronics 30-60 minutes prior to sleep. Try also to avoid eating late at night, which will help patients prevent GERD and heartburn symptoms too.
9. Considering Orlistat as an Option
Orlistat is an oral over-the-counter lipase inhibitor that inhibits fat absorption in the intestines. This drug can interfere with the absorption of fat-soluble vitamins A, D, E, and K. Therefore, it’s important to take a multivitamin 2 hours before or 2 hours after taking orlistat.
However, orlistat can cause steatorrhea, so it’s often not our first choice.
10. Working With Dietitians
I highly recommend that gastroenterologists regularly refer patients to a registered dietitian for medical nutrition therapy. Dietitians help patients establish nutritional goals with calorie limits. I find that many of my patients like the nutritional counseling the dietitians provide, and this can even be done via telemedicine.
A dietitian will examine a patient’s eating habits and help them set weight loss goals that are both realistic and achievable. Having a dietitian motivate a patient through several clinic visits is important for success. A dietitian can plan how many calories a patient should consume in a day while maintaining food, protein, and vitamin intake.
With this therapy, many patients are able to lose approximately 1-1.5 pounds each week. A dietitian can help keep patients accountable for their weight loss goals. I encourage my patients to use their dietitian as a weight loss teacher and a coach who can personalize a diet plan that tastes great.
Some of our patients also have overlapping gastrointestinal issues, such as celiac disease or irritable bowel syndrome. Dietitians can also formulate diets that are great for these other diagnoses too.
There are also apps available on our phones to help with diet and weight loss.
Having a Difficult Conversation to Prevent Long-Term Disease
It’s important for gastroenterologists to work with patients to achieve weight loss. Addressing obesity is sometimes a difficult topic to bring up with patients, but it’s nonetheless very important.
Together, we can help treat obesity plus improve and prevent hepatic steatosis, metabolic dysfunction–associated steatotic liver disease (MASLD), and metabolic dysfunction–associated steatohepatitis (MASH). The estimated global prevalence of MASLD is 32% in adults, so gastroenterologists and hepatologists are working together to try to treat obesity and to prevent long-term liver disease.
Dr. Levy is a gastroenterologist at the University of Chicago. In 2017, Levy, a previous Fulbright Fellow in France, also started a gastroenterology clinic for refugees resettling in Chicago. His clinical projects focus on the development of colorectal cancer screening campaigns. Levy, who recently gave a TEDx Talk about building health education campaigns using music and concerts, organizes Tune It Up: A Concert To Raise Colorectal Cancer Awareness with the American College of Gastroenterology (ACG). He frequently publishes on a variety of gastroenterology topics and serves on ACG’s Public Relations Committee and FDA-Related Matters Committee. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
According to the Centers for Disease Control and Prevention, the obesity prevalence in America was 41.9% between 2017 and 2020. Just 10 years ago, no state had an obesity prevalence above 35%.
Over the past 3 years, many patients gained weight during the COVID-19 pandemic as a result of adopting more sedentary lifestyles, staying at home, avoiding the gym owing to the potential for respiratory spread, and working remotely. For a long time, patients were avoiding attending social events and, as a result, were walking much less.
and other physicians to help patients with obesity realize their goal of achieving weight loss.
1. Embracing the GLP-1 Revolution, With Some Caveats
Glucagon-like peptide-1 (GLP-1) receptor agonists have become a popular treatment for type 2 diabetes and weight loss. These medications, which are given as an injection either weekly or daily depending on the type, have helped patients achieve weight loss with tremendous success.
They work by stimulating the body to produce insulin, which in turn lowers blood sugar. GLP-1 receptor agonists also slow peristalsis and the movement of food from the stomach into the small bowel, which allows patients to eat less by feeling fuller for longer and decreasing hunger.
Two GLP-1 receptor agonists are approved by the US Food and Drug Administration (FDA) for weight loss in patients without diabetes: liraglutide (Saxenda) and semaglutide (Wegovy). There are also lower-dose versions of these active ingredients with the trade names Ozempic and Victoza, designed to help patients with diabetes achieve better glucose and A1c control. In November 2023, the FDA approved a new medication called tirzepatide (Zepbound), which is a glucose-dependent insulinotropic polypeptide (GIP) plus GLP-1 receptor agonist.
This is a very exciting time for the management of type 2 diabetes and weight loss. Gastroenterologists can work with endocrinologists and primary care physicians to help patients choose appropriate weight loss medications.
However, gastroenterologists should also be aware of common GLP-1 receptor agonist side effects, including nausea, vomiting, diarrhea, and — in severe cases — hypoglycemia. These medications can also cause pancreatitis, acute kidney injury, worsening diabetes-related retinopathy, tachycardia, headaches, indigestion, gastroparesis, bowel obstruction, or ileus. We don’t use these medications in patients with a family or personal history of medullary thyroid cancer or multiple endocrine neoplasia. Consider avoiding their use as well in patients with a personal history of pancreatitis.
Recently, the American Society of Anesthesiologists (ASA) suggested holding off on the use of GLP-1 receptor agonists prior to elective endoscopy procedures owing to case reports of aspiration. Gastroenterologists and anesthesiologists are working together to make esophagogastroduodenoscopy (EGD) and colonoscopy as safe as possible in patients taking these treatments.
According to the ASA recommendations, GLP-1 receptor agonists that are given at a daily dose should be held on the day of their procedure. Weekly-dose versions are supposed to be held for 1 week prior to colonoscopy or EGD. During EGD procedures, I also recommend keeping the head of the bed at a 45° angle to help prevent aspiration even further.
Gastroenterologists are eagerly awaiting additional studies to determine whether holding GLP-1 receptor agonists prior to endoscopy is really necessary. But for now, we recommend following the ASA guidelines.
2. Substituting Out Sugary Drinks
Gastroenterologists and primary care physicians constantly advise their patients to avoid consuming sugary drinks, such as soda, fruit juices, calorie-laden coffee drinks, sweetened tea, hot chocolate, and, of course, alcohol. Many of our patients drink three to six of these sugary drinks a day.
As a gastroenterologist, it’s important to counsel our overweight patients and obtain an accurate history about their daily and weekly consumption of excess calories.
Recommend substituting sugary drinks with water, unsweetened tea (either hot or cold), and coffee.
To prevent constipation, encourage patients to drink at least eight 8-ounce glasses of fluid per day. Drinking water, tea, and coffee can also help keep patients feeling fuller for longer and avoid those tempting snacks.
3. Adopting the Right Diet
Every day, I encourage my patients to avoid eating fried fatty foods and processed meats. We also advise patients to avoid junk food filled with carbohydrates and salt.
Instead, patients should try to eat a piece of fruit or some vegetables with every single meal, which keeps patients feeling fuller for longer, prevents diverticulitis from forming, and can even help prevent colon cancer.
Making small dietary changes can dramatically reduce daily calorie consumption, which adds up over time and can help patients lose weight in a safe way.
Meal prepping for the week ahead, perhaps on a Sunday, is a very simple way to eat more nutritious foods instead of constantly getting takeout and fast food.
Many of our patients have also successfully lost weight through intermittent fasting, although I recommend working with a nutritionist on this one.
A Mediterranean diet is also a great option.
4. Getting Active
I encourage patients to take daily walks, swim, play sports, take fitness classes, do yoga or Pilates, and use weights at a gym.
Exercise burns calories, which is great for our hearts, prevents hepatic steatosis, and helps relieve stress. Exercise also stimulates peristalsis, which can help our constipated patients achieve more regular bowel movements.
There are a few other things to keep in mind in this area. Try to avoid strenuous exercise right after eating, because this will help prevent both heartburn and gastroesophageal reflux disease (GERD).
5. Reducing Stomach Volume With a Gastric Balloon
A gastric balloon procedure is a temporary obesity treatment that helps patients lose weight by reducing the volume of the stomach so that they feel full more easily. This can be accomplished endoscopically through the mouth without the need for surgery.
Basically, a deflated balloon is placed through the mouth using an endoscope and advanced into the stomach by a gastroenterologist or surgeon. The balloon is inflated with salt water and can remain in the stomach for 6 months before it is removed.
This procedure can help patients feel full and consequently eat less, thereby leading to gradual and safe weight loss.
6. Using the Accordion Procedure
An endoscopic sleeve gastroplasty procedure, sometimes called an accordion procedure, is used for patients with a body mass index ≥ 30 when diet and exercise alone have failed. An EGD tube is equipped with small stitching instruments that are used to reduce the size of the stomach.
This procedure has less complications than open or laparoscopic surgery and can be reversed.
7. Injecting Botulinum Toxin
Another technique is having the gastroenterologist inject botulinum toxin into the stomach wall. This works by relaxing the stomach propulsion muscles, which delays gastric emptying so that patients feel fuller longer and more easily.
This approach is good for achieving moderate weight loss of approximately 5%-10% of body weight. It works best in combination with a good diet and exercise. The effects of the botulinum toxin can last for 3 months, and the procedure can be repeated every 6 months.
8. Adjusting Certain Lifestyle Factors
Gastroenterologists should also counsel our patients about exercise, stress management, and the importance of sleep to prevent overeating. Self-care is extremely important for patients. Walk, swim, lift weights, and play sports; I personally love basketball and tennis.
I also recommend allocating enough time for sleep each night. At least 7-9 hours of sleep is ideal. Good sleep hygiene can help keep a stable schedule. Create a comfortable bedroom that is free of disruptions like TV watching or playing on your phone or computer.
Gastroenterologists can provide simple instructions to their patients on how to achieve this. For example, unplug from electronics 30-60 minutes prior to sleep. Try also to avoid eating late at night, which will help patients prevent GERD and heartburn symptoms too.
9. Considering Orlistat as an Option
Orlistat is an oral over-the-counter lipase inhibitor that inhibits fat absorption in the intestines. This drug can interfere with the absorption of fat-soluble vitamins A, D, E, and K. Therefore, it’s important to take a multivitamin 2 hours before or 2 hours after taking orlistat.
However, orlistat can cause steatorrhea, so it’s often not our first choice.
10. Working With Dietitians
I highly recommend that gastroenterologists regularly refer patients to a registered dietitian for medical nutrition therapy. Dietitians help patients establish nutritional goals with calorie limits. I find that many of my patients like the nutritional counseling the dietitians provide, and this can even be done via telemedicine.
A dietitian will examine a patient’s eating habits and help them set weight loss goals that are both realistic and achievable. Having a dietitian motivate a patient through several clinic visits is important for success. A dietitian can plan how many calories a patient should consume in a day while maintaining food, protein, and vitamin intake.
With this therapy, many patients are able to lose approximately 1-1.5 pounds each week. A dietitian can help keep patients accountable for their weight loss goals. I encourage my patients to use their dietitian as a weight loss teacher and a coach who can personalize a diet plan that tastes great.
Some of our patients also have overlapping gastrointestinal issues, such as celiac disease or irritable bowel syndrome. Dietitians can also formulate diets that are great for these other diagnoses too.
There are also apps available on our phones to help with diet and weight loss.
Having a Difficult Conversation to Prevent Long-Term Disease
It’s important for gastroenterologists to work with patients to achieve weight loss. Addressing obesity is sometimes a difficult topic to bring up with patients, but it’s nonetheless very important.
Together, we can help treat obesity plus improve and prevent hepatic steatosis, metabolic dysfunction–associated steatotic liver disease (MASLD), and metabolic dysfunction–associated steatohepatitis (MASH). The estimated global prevalence of MASLD is 32% in adults, so gastroenterologists and hepatologists are working together to try to treat obesity and to prevent long-term liver disease.
Dr. Levy is a gastroenterologist at the University of Chicago. In 2017, Levy, a previous Fulbright Fellow in France, also started a gastroenterology clinic for refugees resettling in Chicago. His clinical projects focus on the development of colorectal cancer screening campaigns. Levy, who recently gave a TEDx Talk about building health education campaigns using music and concerts, organizes Tune It Up: A Concert To Raise Colorectal Cancer Awareness with the American College of Gastroenterology (ACG). He frequently publishes on a variety of gastroenterology topics and serves on ACG’s Public Relations Committee and FDA-Related Matters Committee. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Cutting Across the Bias
On a recent rainy afternoon I was speed skimming through the pile of publications sitting on the floor next to my Grampy’s chair. A bright patch of color jumped off the gray background of the printed page forcing me to pause and consider the content.
In the right upper corner was a photograph of an attractive Black woman nursing her baby. Her bare arms suggested she might be slightly overweight. She wore a simple off-white head wrap and smiled broadly as she played with her infant’s fingers. The image was a reproduction of a WIC poster encouraging women to take advantage of the program’s breastfeeding support services. The accompanying article from American Academy of Pediatrics offered ten strategies for achieving breastfeeding equity.
I must admit that I tend to shy away from discussions of equity because I’ve seldom found them very informative. However, the engaging image of this Black woman breastfeeding led me to read beyond the title.
The first of the strategies listed was “Check you biases.” I will certainly admit to having biases. We all have biases and see and interpret the world through lenses ground and tinted by our experiences and the environment we have inhabited. In the case of breastfeeding, I wasn’t sure where my biases lay. Maybe one of mine is reflected in a hesitancy to actively promote exclusive breastfeeding for the first 6 months. I prefer a more nuanced approach adjusted to the unique needs and limitations of each family. But I decided to chase down the Implicit Association Test (IAT) suggested in the article. I couldn’t make that link work, but found a long list of subjects on the Harvard Implicit Association Test website. None dealt with breastfeeding, so I chose the one described as Black/White.
If, like me, you have never had your implicit biases assessed by taking an IAT, you might find it interesting. Probably took me about 15 minutes using my laptop. There are a lot of demographic questions then some rapid-fire exercises in which you must provide your first response to a barrage of photos of faces and words. At times I sensed that the test makers were trying to trick me into making associations that I didn’t want to make by the order in which the exercises were presented. At the end I was told that I was a little slow in associating Black faces with positive words.
I’m not sure what this means. After doing a little internet searching I learned that one of the criticisms of the IAT is that, while it may hint at a bias, it is really more important whether you cut with or across that bias. If I acknowledge that where and how I grew up may have left me with some implicit biases, it is more important that I make a strong and honest effort to act independently of those biases.
In full disclosure I must tell you that there was one Black girl in my high school of a thousand students. I have lived and practiced in Maine for 50 years. At less than 2%, we are sixth from the bottom in Black population among other states. However, in the last 5 or 6 years here in Brunswick we have welcomed a large infusion of asylum seekers who come predominantly from Black African countries.
Skimming through the rest of the article, I found it hard to argue with the remaining nine recommendations for promoting breastfeeding, although most of them we not terribly applicable to small community practices. The photo of the Black woman nursing her baby at the top of the page remains as the primary message. The fact that I was drawn to that image is a testament to several of my biases and another example of a picture being worth far more than a thousand words.
I suspect that I’m not alone in appreciating the uniqueness of that image. Until recently, the standard photos of a mother breastfeeding have used trim White women as their models. I suspect and hope this poster will be effective in encouraging Black women to nurse. I urge you all to hang it in your office as a reminder to you and your staff of your biases and assumptions. Don’t bother to take the Implicit Association Test unless you’re retired and have 15 minutes to burn on a rainy afternoon.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
On a recent rainy afternoon I was speed skimming through the pile of publications sitting on the floor next to my Grampy’s chair. A bright patch of color jumped off the gray background of the printed page forcing me to pause and consider the content.
In the right upper corner was a photograph of an attractive Black woman nursing her baby. Her bare arms suggested she might be slightly overweight. She wore a simple off-white head wrap and smiled broadly as she played with her infant’s fingers. The image was a reproduction of a WIC poster encouraging women to take advantage of the program’s breastfeeding support services. The accompanying article from American Academy of Pediatrics offered ten strategies for achieving breastfeeding equity.
I must admit that I tend to shy away from discussions of equity because I’ve seldom found them very informative. However, the engaging image of this Black woman breastfeeding led me to read beyond the title.
The first of the strategies listed was “Check you biases.” I will certainly admit to having biases. We all have biases and see and interpret the world through lenses ground and tinted by our experiences and the environment we have inhabited. In the case of breastfeeding, I wasn’t sure where my biases lay. Maybe one of mine is reflected in a hesitancy to actively promote exclusive breastfeeding for the first 6 months. I prefer a more nuanced approach adjusted to the unique needs and limitations of each family. But I decided to chase down the Implicit Association Test (IAT) suggested in the article. I couldn’t make that link work, but found a long list of subjects on the Harvard Implicit Association Test website. None dealt with breastfeeding, so I chose the one described as Black/White.
If, like me, you have never had your implicit biases assessed by taking an IAT, you might find it interesting. Probably took me about 15 minutes using my laptop. There are a lot of demographic questions then some rapid-fire exercises in which you must provide your first response to a barrage of photos of faces and words. At times I sensed that the test makers were trying to trick me into making associations that I didn’t want to make by the order in which the exercises were presented. At the end I was told that I was a little slow in associating Black faces with positive words.
I’m not sure what this means. After doing a little internet searching I learned that one of the criticisms of the IAT is that, while it may hint at a bias, it is really more important whether you cut with or across that bias. If I acknowledge that where and how I grew up may have left me with some implicit biases, it is more important that I make a strong and honest effort to act independently of those biases.
In full disclosure I must tell you that there was one Black girl in my high school of a thousand students. I have lived and practiced in Maine for 50 years. At less than 2%, we are sixth from the bottom in Black population among other states. However, in the last 5 or 6 years here in Brunswick we have welcomed a large infusion of asylum seekers who come predominantly from Black African countries.
Skimming through the rest of the article, I found it hard to argue with the remaining nine recommendations for promoting breastfeeding, although most of them we not terribly applicable to small community practices. The photo of the Black woman nursing her baby at the top of the page remains as the primary message. The fact that I was drawn to that image is a testament to several of my biases and another example of a picture being worth far more than a thousand words.
I suspect that I’m not alone in appreciating the uniqueness of that image. Until recently, the standard photos of a mother breastfeeding have used trim White women as their models. I suspect and hope this poster will be effective in encouraging Black women to nurse. I urge you all to hang it in your office as a reminder to you and your staff of your biases and assumptions. Don’t bother to take the Implicit Association Test unless you’re retired and have 15 minutes to burn on a rainy afternoon.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
On a recent rainy afternoon I was speed skimming through the pile of publications sitting on the floor next to my Grampy’s chair. A bright patch of color jumped off the gray background of the printed page forcing me to pause and consider the content.
In the right upper corner was a photograph of an attractive Black woman nursing her baby. Her bare arms suggested she might be slightly overweight. She wore a simple off-white head wrap and smiled broadly as she played with her infant’s fingers. The image was a reproduction of a WIC poster encouraging women to take advantage of the program’s breastfeeding support services. The accompanying article from American Academy of Pediatrics offered ten strategies for achieving breastfeeding equity.
I must admit that I tend to shy away from discussions of equity because I’ve seldom found them very informative. However, the engaging image of this Black woman breastfeeding led me to read beyond the title.
The first of the strategies listed was “Check you biases.” I will certainly admit to having biases. We all have biases and see and interpret the world through lenses ground and tinted by our experiences and the environment we have inhabited. In the case of breastfeeding, I wasn’t sure where my biases lay. Maybe one of mine is reflected in a hesitancy to actively promote exclusive breastfeeding for the first 6 months. I prefer a more nuanced approach adjusted to the unique needs and limitations of each family. But I decided to chase down the Implicit Association Test (IAT) suggested in the article. I couldn’t make that link work, but found a long list of subjects on the Harvard Implicit Association Test website. None dealt with breastfeeding, so I chose the one described as Black/White.
If, like me, you have never had your implicit biases assessed by taking an IAT, you might find it interesting. Probably took me about 15 minutes using my laptop. There are a lot of demographic questions then some rapid-fire exercises in which you must provide your first response to a barrage of photos of faces and words. At times I sensed that the test makers were trying to trick me into making associations that I didn’t want to make by the order in which the exercises were presented. At the end I was told that I was a little slow in associating Black faces with positive words.
I’m not sure what this means. After doing a little internet searching I learned that one of the criticisms of the IAT is that, while it may hint at a bias, it is really more important whether you cut with or across that bias. If I acknowledge that where and how I grew up may have left me with some implicit biases, it is more important that I make a strong and honest effort to act independently of those biases.
In full disclosure I must tell you that there was one Black girl in my high school of a thousand students. I have lived and practiced in Maine for 50 years. At less than 2%, we are sixth from the bottom in Black population among other states. However, in the last 5 or 6 years here in Brunswick we have welcomed a large infusion of asylum seekers who come predominantly from Black African countries.
Skimming through the rest of the article, I found it hard to argue with the remaining nine recommendations for promoting breastfeeding, although most of them we not terribly applicable to small community practices. The photo of the Black woman nursing her baby at the top of the page remains as the primary message. The fact that I was drawn to that image is a testament to several of my biases and another example of a picture being worth far more than a thousand words.
I suspect that I’m not alone in appreciating the uniqueness of that image. Until recently, the standard photos of a mother breastfeeding have used trim White women as their models. I suspect and hope this poster will be effective in encouraging Black women to nurse. I urge you all to hang it in your office as a reminder to you and your staff of your biases and assumptions. Don’t bother to take the Implicit Association Test unless you’re retired and have 15 minutes to burn on a rainy afternoon.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Rosemary, Part 1
A member of the Lamiaceae family, Salvia rosmarinus (rosemary),* an aromatic plant native to the Mediterranean region and now cultivated globally, has been used for centuries in cuisine and medicine, with several well-established biological activities.1-3 Thought to contribute to preventing hair loss, rosemary oil was also used for hundreds of years in hair rinses in the Mediterranean area.4 In traditional Iranian medicine, rosemary essential oil has been topically applied as an analgesic, anti-inflammatory, and anti-acne remedy.5 Rosemary is known to absorb UV light well and to impart antibacterial and antifungal activity, as well as help maintain skin homeostasis.3 It is also used and under further study for its anti-inflammatory, antioxidant, anti-infective, and anticancer activity.2,6-9 The health benefits of rosemary are typically ascribed to its constituent carnosol/carnosic and ursolic acids.7 .
Chemical Constituents
The key chemical components of S. rosmarinus include bitter principle, resin, tannic acid, flavonoids, and volatile oils (made up of borneol, bornyl acetate, camphene, cineol, pinene, and camphor).10 Other important constituents of rosemary oil, in particular, include p-Cymene, linalool, gamma-terpinene, thymol, beta-pinene, alpha-pinene, eucalyptol, and carnosic acid.9 Volatile oils of rosemary have been used in various oils and lotions to treat wounds and with the intention of stimulating hair growth.10
Wound Healing
In a 2022 study in 60 adult male rats, Bulhões and colleagues found that the use of rosemary leaf essential oil-based ointments on skin lesions spurred wound healing, decreased inflammation, and enhanced angiogenesis as well as collagen fiber density.11
Three years earlier, Labib and colleagues studied the wound healing capacity of three chitosan-based topical formulations containing either tea tree essential oil, rosemary essential oil, or a mixture of both oils in an excision wound model in rats.
The combination preparation was found to be the most effective in fostering various stages of wound healing, with significant increases in wound contraction percentage observed in the combination group compared with either group treated using individual essential oils or the untreated animals.12
A 2010 in vivo study by Abu-Al-Basal using BALB/c mice with diabetes revealed that the topical application of rosemary essential oil for three days reduced inflammation, enhanced wound contraction and re-epithelialization, and promoted angiogenesis, granulation tissue regeneration, and collagen deposition.13
Anticancer Activity
Using a 7,12-dimethlybenz(a)anthracene (DMBA)-initiated and croton oil-promoted model in 2006, Sancheti and Goyal determined that rosemary extract administered orally at a dose rate of 500 mg/kg body weight/mouse significantly inhibited two-stage skin tumorigenesis in mice.14 Nearly a decade later, Cattaneo and colleagues determined that a rosemary hydroalcoholic extract displayed antiproliferative effects on the human melanoma A375 cell line.8
The polyphenols carnosic acid and rosmarinic acid are most often cited as the sources of the reputed anticancer effects of rosemary.15
Hair Health
Early in 2023, Begum and colleagues developed a 1% hair lotion including a methanolic extract of the aerial part of S. rosmarinus that they assessed for potential hair growth activity in C57BL/6 mice. Using water as a control and 2% minoxidil hair lotion as standard, the investigators determined that their rosemary hair lotion demonstrated significant hair growth promotion, exceeding that seen in the mice treated with the drug standard.1
In a randomized controlled study in C57BL/6NCrSlc mice a decade earlier, Murata and colleagues evaluated the anti-androgenic activity and hair growth potential imparted by topical rosemary oil compared with finasteride and minoxidil. Rosemary oil leaf extract, with 12-O-methylcarnosic acid as its most active component, robustly suppressed 5alpha-reductase and stimulated hair growth in vivo in both the androgenetic alopecia/testosterone-treated mouse model, as well as the hair growth activating mouse model as compared with minoxidil. Further, the inhibitory activity of rosemary was 82.4% and 94.6% at 200 mcg/mL and 500 mcg/mL, respectively, whereas finasteride demonstrated 81.9% at 250 nM.16
A human study two years later was even more encouraging. Panahi and colleagues conducted a randomized comparative trial with 100 patients to investigate the effects of rosemary oil as opposed to minoxidil 2% for the treatment of androgenetic alopecia over 6 months. By 6 months, significantly greater hair counts were observed in both groups compared with baseline and 3-month readings, but no significant variations between groups. No differences were found in the frequency of dryness, greasiness, or dandruff at any time point or between groups. Scalp itching was significantly greater at the 3- and 6-month points in both groups, particularly in the minoxidil group at both of those time points. The investigators concluded that rosemary oil compared well with minoxidil as androgenetic alopecia therapy.17
Conclusion
Rosemary has been used in traditional medicine for hundreds of years and it has been a common ingredient in cosmetic and cosmeceutical formulations for more than 20 years. Recent findings suggest a broad array of applications in modern medicine, particularly dermatology. The next column will focus on the most recent studies pertaining to the antioxidant and anti-aging activity of this aromatic shrub.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. Begum A et al. Adv Biomed Res. 2023 Mar 21;12:60.
2. de Oliveira JR et al. J Biomed Sci. 2019 Jan 9;26(1):5.
3. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
4. Dinkins J et al. Int J Dermatol. 2023 Aug;62(8):980-5.
5. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
6. Allegra A et al. Nutrients. 2020 Jun 10;12(6):1739.
7. de Macedo LM et al. Plants (Basel). 2020 May 21;9(5):651.
8. Cattaneo L et al. PLoS One. 2015 Jul 15;10(7):e0132439.
9. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
10. Begum A et al. Acta Sci Pol Technol Aliment. 2013 Jan-Mar;12(1):61-73.
11. Bulhões AAVC et al. Acta Cir Bras. 2022 Apr 8;37(1):e370104.
12. Labib RM et al. PLoS One. 2019 Sep 16;14(9):e0219561.
13. Abu-Al-Basal MA. J Ethnopharmacol. 2010 Sep 15;131(2):443-50.
14. Sancheti G and Goyal PK. Phytother Res. 2006 Nov;20(11):981-6.
15. Moore J et al. Nutrients. 2016 Nov 17;8(11):731.
16. Murata K et al. Phytother Res. 2013 Feb;27(2):212-7.
17. Panahi Y et al. Skinmed. 2015 Jan-Feb;13(1):15-21.
*Correction, 2/27: This column was updated with the more recent name for rosemary, Salvia rosmarinus.
A member of the Lamiaceae family, Salvia rosmarinus (rosemary),* an aromatic plant native to the Mediterranean region and now cultivated globally, has been used for centuries in cuisine and medicine, with several well-established biological activities.1-3 Thought to contribute to preventing hair loss, rosemary oil was also used for hundreds of years in hair rinses in the Mediterranean area.4 In traditional Iranian medicine, rosemary essential oil has been topically applied as an analgesic, anti-inflammatory, and anti-acne remedy.5 Rosemary is known to absorb UV light well and to impart antibacterial and antifungal activity, as well as help maintain skin homeostasis.3 It is also used and under further study for its anti-inflammatory, antioxidant, anti-infective, and anticancer activity.2,6-9 The health benefits of rosemary are typically ascribed to its constituent carnosol/carnosic and ursolic acids.7 .
Chemical Constituents
The key chemical components of S. rosmarinus include bitter principle, resin, tannic acid, flavonoids, and volatile oils (made up of borneol, bornyl acetate, camphene, cineol, pinene, and camphor).10 Other important constituents of rosemary oil, in particular, include p-Cymene, linalool, gamma-terpinene, thymol, beta-pinene, alpha-pinene, eucalyptol, and carnosic acid.9 Volatile oils of rosemary have been used in various oils and lotions to treat wounds and with the intention of stimulating hair growth.10
Wound Healing
In a 2022 study in 60 adult male rats, Bulhões and colleagues found that the use of rosemary leaf essential oil-based ointments on skin lesions spurred wound healing, decreased inflammation, and enhanced angiogenesis as well as collagen fiber density.11
Three years earlier, Labib and colleagues studied the wound healing capacity of three chitosan-based topical formulations containing either tea tree essential oil, rosemary essential oil, or a mixture of both oils in an excision wound model in rats.
The combination preparation was found to be the most effective in fostering various stages of wound healing, with significant increases in wound contraction percentage observed in the combination group compared with either group treated using individual essential oils or the untreated animals.12
A 2010 in vivo study by Abu-Al-Basal using BALB/c mice with diabetes revealed that the topical application of rosemary essential oil for three days reduced inflammation, enhanced wound contraction and re-epithelialization, and promoted angiogenesis, granulation tissue regeneration, and collagen deposition.13
Anticancer Activity
Using a 7,12-dimethlybenz(a)anthracene (DMBA)-initiated and croton oil-promoted model in 2006, Sancheti and Goyal determined that rosemary extract administered orally at a dose rate of 500 mg/kg body weight/mouse significantly inhibited two-stage skin tumorigenesis in mice.14 Nearly a decade later, Cattaneo and colleagues determined that a rosemary hydroalcoholic extract displayed antiproliferative effects on the human melanoma A375 cell line.8
The polyphenols carnosic acid and rosmarinic acid are most often cited as the sources of the reputed anticancer effects of rosemary.15
Hair Health
Early in 2023, Begum and colleagues developed a 1% hair lotion including a methanolic extract of the aerial part of S. rosmarinus that they assessed for potential hair growth activity in C57BL/6 mice. Using water as a control and 2% minoxidil hair lotion as standard, the investigators determined that their rosemary hair lotion demonstrated significant hair growth promotion, exceeding that seen in the mice treated with the drug standard.1
In a randomized controlled study in C57BL/6NCrSlc mice a decade earlier, Murata and colleagues evaluated the anti-androgenic activity and hair growth potential imparted by topical rosemary oil compared with finasteride and minoxidil. Rosemary oil leaf extract, with 12-O-methylcarnosic acid as its most active component, robustly suppressed 5alpha-reductase and stimulated hair growth in vivo in both the androgenetic alopecia/testosterone-treated mouse model, as well as the hair growth activating mouse model as compared with minoxidil. Further, the inhibitory activity of rosemary was 82.4% and 94.6% at 200 mcg/mL and 500 mcg/mL, respectively, whereas finasteride demonstrated 81.9% at 250 nM.16
A human study two years later was even more encouraging. Panahi and colleagues conducted a randomized comparative trial with 100 patients to investigate the effects of rosemary oil as opposed to minoxidil 2% for the treatment of androgenetic alopecia over 6 months. By 6 months, significantly greater hair counts were observed in both groups compared with baseline and 3-month readings, but no significant variations between groups. No differences were found in the frequency of dryness, greasiness, or dandruff at any time point or between groups. Scalp itching was significantly greater at the 3- and 6-month points in both groups, particularly in the minoxidil group at both of those time points. The investigators concluded that rosemary oil compared well with minoxidil as androgenetic alopecia therapy.17
Conclusion
Rosemary has been used in traditional medicine for hundreds of years and it has been a common ingredient in cosmetic and cosmeceutical formulations for more than 20 years. Recent findings suggest a broad array of applications in modern medicine, particularly dermatology. The next column will focus on the most recent studies pertaining to the antioxidant and anti-aging activity of this aromatic shrub.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. Begum A et al. Adv Biomed Res. 2023 Mar 21;12:60.
2. de Oliveira JR et al. J Biomed Sci. 2019 Jan 9;26(1):5.
3. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
4. Dinkins J et al. Int J Dermatol. 2023 Aug;62(8):980-5.
5. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
6. Allegra A et al. Nutrients. 2020 Jun 10;12(6):1739.
7. de Macedo LM et al. Plants (Basel). 2020 May 21;9(5):651.
8. Cattaneo L et al. PLoS One. 2015 Jul 15;10(7):e0132439.
9. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
10. Begum A et al. Acta Sci Pol Technol Aliment. 2013 Jan-Mar;12(1):61-73.
11. Bulhões AAVC et al. Acta Cir Bras. 2022 Apr 8;37(1):e370104.
12. Labib RM et al. PLoS One. 2019 Sep 16;14(9):e0219561.
13. Abu-Al-Basal MA. J Ethnopharmacol. 2010 Sep 15;131(2):443-50.
14. Sancheti G and Goyal PK. Phytother Res. 2006 Nov;20(11):981-6.
15. Moore J et al. Nutrients. 2016 Nov 17;8(11):731.
16. Murata K et al. Phytother Res. 2013 Feb;27(2):212-7.
17. Panahi Y et al. Skinmed. 2015 Jan-Feb;13(1):15-21.
*Correction, 2/27: This column was updated with the more recent name for rosemary, Salvia rosmarinus.
A member of the Lamiaceae family, Salvia rosmarinus (rosemary),* an aromatic plant native to the Mediterranean region and now cultivated globally, has been used for centuries in cuisine and medicine, with several well-established biological activities.1-3 Thought to contribute to preventing hair loss, rosemary oil was also used for hundreds of years in hair rinses in the Mediterranean area.4 In traditional Iranian medicine, rosemary essential oil has been topically applied as an analgesic, anti-inflammatory, and anti-acne remedy.5 Rosemary is known to absorb UV light well and to impart antibacterial and antifungal activity, as well as help maintain skin homeostasis.3 It is also used and under further study for its anti-inflammatory, antioxidant, anti-infective, and anticancer activity.2,6-9 The health benefits of rosemary are typically ascribed to its constituent carnosol/carnosic and ursolic acids.7 .
Chemical Constituents
The key chemical components of S. rosmarinus include bitter principle, resin, tannic acid, flavonoids, and volatile oils (made up of borneol, bornyl acetate, camphene, cineol, pinene, and camphor).10 Other important constituents of rosemary oil, in particular, include p-Cymene, linalool, gamma-terpinene, thymol, beta-pinene, alpha-pinene, eucalyptol, and carnosic acid.9 Volatile oils of rosemary have been used in various oils and lotions to treat wounds and with the intention of stimulating hair growth.10
Wound Healing
In a 2022 study in 60 adult male rats, Bulhões and colleagues found that the use of rosemary leaf essential oil-based ointments on skin lesions spurred wound healing, decreased inflammation, and enhanced angiogenesis as well as collagen fiber density.11
Three years earlier, Labib and colleagues studied the wound healing capacity of three chitosan-based topical formulations containing either tea tree essential oil, rosemary essential oil, or a mixture of both oils in an excision wound model in rats.
The combination preparation was found to be the most effective in fostering various stages of wound healing, with significant increases in wound contraction percentage observed in the combination group compared with either group treated using individual essential oils or the untreated animals.12
A 2010 in vivo study by Abu-Al-Basal using BALB/c mice with diabetes revealed that the topical application of rosemary essential oil for three days reduced inflammation, enhanced wound contraction and re-epithelialization, and promoted angiogenesis, granulation tissue regeneration, and collagen deposition.13
Anticancer Activity
Using a 7,12-dimethlybenz(a)anthracene (DMBA)-initiated and croton oil-promoted model in 2006, Sancheti and Goyal determined that rosemary extract administered orally at a dose rate of 500 mg/kg body weight/mouse significantly inhibited two-stage skin tumorigenesis in mice.14 Nearly a decade later, Cattaneo and colleagues determined that a rosemary hydroalcoholic extract displayed antiproliferative effects on the human melanoma A375 cell line.8
The polyphenols carnosic acid and rosmarinic acid are most often cited as the sources of the reputed anticancer effects of rosemary.15
Hair Health
Early in 2023, Begum and colleagues developed a 1% hair lotion including a methanolic extract of the aerial part of S. rosmarinus that they assessed for potential hair growth activity in C57BL/6 mice. Using water as a control and 2% minoxidil hair lotion as standard, the investigators determined that their rosemary hair lotion demonstrated significant hair growth promotion, exceeding that seen in the mice treated with the drug standard.1
In a randomized controlled study in C57BL/6NCrSlc mice a decade earlier, Murata and colleagues evaluated the anti-androgenic activity and hair growth potential imparted by topical rosemary oil compared with finasteride and minoxidil. Rosemary oil leaf extract, with 12-O-methylcarnosic acid as its most active component, robustly suppressed 5alpha-reductase and stimulated hair growth in vivo in both the androgenetic alopecia/testosterone-treated mouse model, as well as the hair growth activating mouse model as compared with minoxidil. Further, the inhibitory activity of rosemary was 82.4% and 94.6% at 200 mcg/mL and 500 mcg/mL, respectively, whereas finasteride demonstrated 81.9% at 250 nM.16
A human study two years later was even more encouraging. Panahi and colleagues conducted a randomized comparative trial with 100 patients to investigate the effects of rosemary oil as opposed to minoxidil 2% for the treatment of androgenetic alopecia over 6 months. By 6 months, significantly greater hair counts were observed in both groups compared with baseline and 3-month readings, but no significant variations between groups. No differences were found in the frequency of dryness, greasiness, or dandruff at any time point or between groups. Scalp itching was significantly greater at the 3- and 6-month points in both groups, particularly in the minoxidil group at both of those time points. The investigators concluded that rosemary oil compared well with minoxidil as androgenetic alopecia therapy.17
Conclusion
Rosemary has been used in traditional medicine for hundreds of years and it has been a common ingredient in cosmetic and cosmeceutical formulations for more than 20 years. Recent findings suggest a broad array of applications in modern medicine, particularly dermatology. The next column will focus on the most recent studies pertaining to the antioxidant and anti-aging activity of this aromatic shrub.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. Begum A et al. Adv Biomed Res. 2023 Mar 21;12:60.
2. de Oliveira JR et al. J Biomed Sci. 2019 Jan 9;26(1):5.
3. González-Minero FJ et al. Cosmetics. 2020 Oct 3;7(4):77.
4. Dinkins J et al. Int J Dermatol. 2023 Aug;62(8):980-5.
5. Akbari J et al. Pharm Biol. 2015;53(10):1442-7.
6. Allegra A et al. Nutrients. 2020 Jun 10;12(6):1739.
7. de Macedo LM et al. Plants (Basel). 2020 May 21;9(5):651.
8. Cattaneo L et al. PLoS One. 2015 Jul 15;10(7):e0132439.
9. Borges RS et al. J Ethnopharmacol. 2019 Jan 30;229:29-45.
10. Begum A et al. Acta Sci Pol Technol Aliment. 2013 Jan-Mar;12(1):61-73.
11. Bulhões AAVC et al. Acta Cir Bras. 2022 Apr 8;37(1):e370104.
12. Labib RM et al. PLoS One. 2019 Sep 16;14(9):e0219561.
13. Abu-Al-Basal MA. J Ethnopharmacol. 2010 Sep 15;131(2):443-50.
14. Sancheti G and Goyal PK. Phytother Res. 2006 Nov;20(11):981-6.
15. Moore J et al. Nutrients. 2016 Nov 17;8(11):731.
16. Murata K et al. Phytother Res. 2013 Feb;27(2):212-7.
17. Panahi Y et al. Skinmed. 2015 Jan-Feb;13(1):15-21.
*Correction, 2/27: This column was updated with the more recent name for rosemary, Salvia rosmarinus.







