User login
Lump in breast
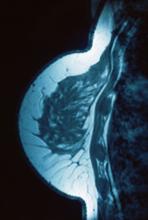
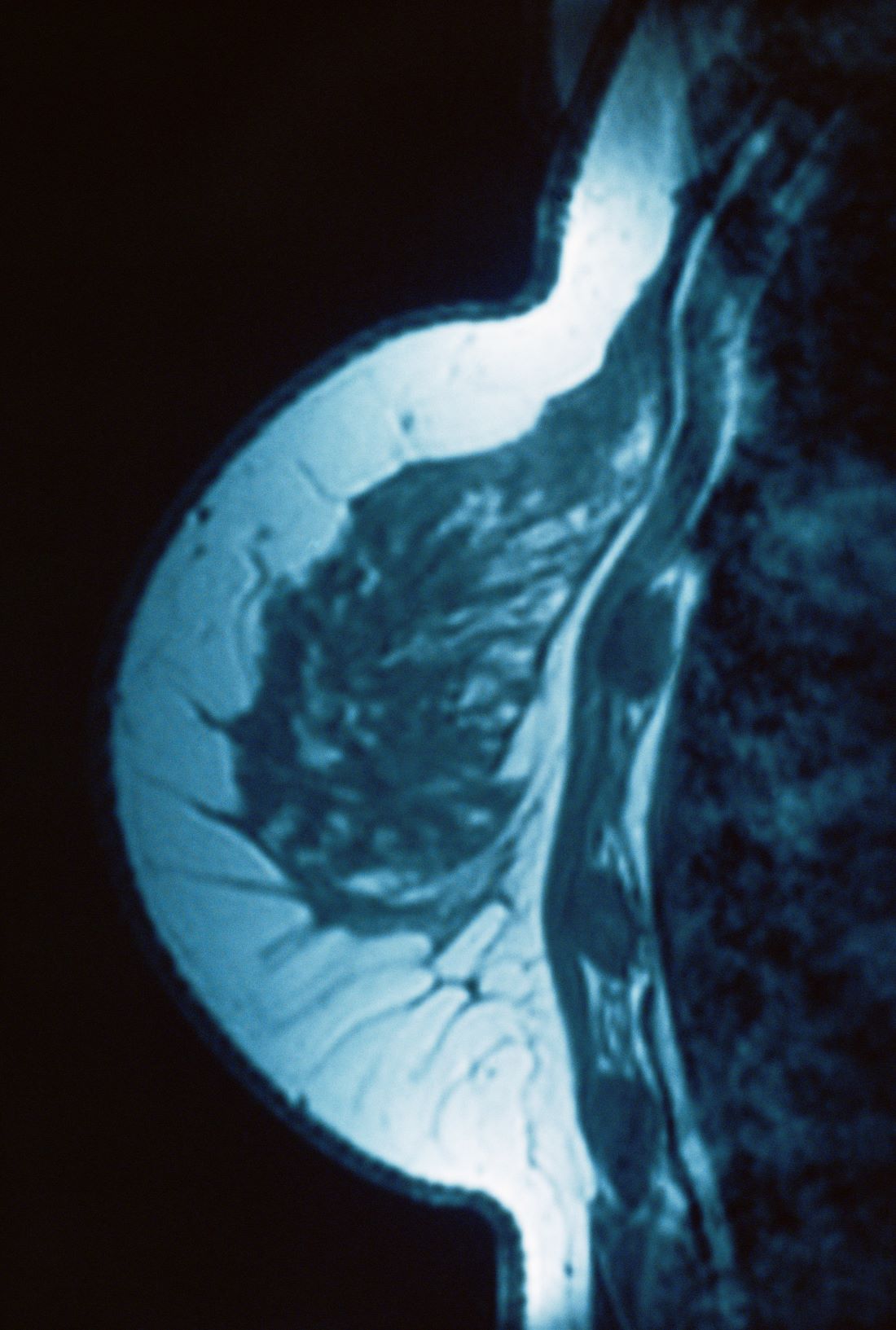
Given clinical and imaging outcomes, as well as results on IHC assay, this patient is diagnosed with triple-negative breast cancer and is referred for further consultation with a multidisciplinary care team.
Triple-negative breast cancer accounts for 15% of all female breast cancer cases. The term "triple-negative" refers to the absence of estrogen receptor (ER) and progesterone receptor (PR) targets and the HER2 target for treatment. Otherwise, triple-negative breast cancer is highly heterogeneous and includes multiple molecular subtypes. Typically, triple-negative breast cancer occurs in younger patients, has a higher grade, a greater tumor size, a higher clinical stage at diagnosis, and a poorer prognosis than non–triple-negative breast cancers.
Biopsy samples from patients with a new primary or newly metastatic breast cancer diagnosis should undergo HR testing, both ER and PR, as well as HER2 receptor testing. Results of ER and PR testing are negative if a validated IHC assay shows 0% to < 1% of nuclei stain, according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HR testing guideline. HER2 results are negative if a validated IHC assay shows weak to moderate complete membrane staining in < 10% of tumor cells, according to ASCO/CAP guidelines. If HER2 results of a primary tumor are negative and specific clinical criteria suggest further testing, the oncologist may choose to order another IHC assay or a validated dual probe in situ hybridization (ISH) assay to aid in clinical decision-making.
Pathologic classification of triple-negative breast cancers includes multiple subgroups determined by therapeutic possibilities: low-risk histologic types (salivary gland–like carcinomas, fibromatosis-like carcinoma, low-grade adenosquamous breast carcinoma), immune activation (tumor-infiltrating lymphocytes, CD8, programmed death–ligand 1, tumor mutational burden), HER2-low status (IHC score 1+/2+ nonamplified), associated germline BRCA mutations, and other targets (trophoblast cell-surface antigen 2, HER3).
Family history is the most common risk factor for developing breast cancer. If a person's mother or sister had breast cancer, the lifetime risk for that individual is four times greater than that of the general population. Risk for breast cancer grows even more if two or more first-degree relatives are diagnosed, or if one first-degree relative was diagnosed with breast cancer at ≤ 50 years of age. About 25% of triple-negative breast cancer cases have accompanying germline BRCA mutations. A recent study by Ahearn and colleagues found that 85 variants were linked to one or more tumor feature of ER-negative disease, and 32 of those variants were associated with triple-negative disease.
For a breast cancer diagnosis, the National Comprehensive Cancer Network recommends multidisciplinary care as well as the development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Because triple-negative breast cancer lacks HR and HER2 therapeutic targets, it can be difficult to treat. Standard therapy for high-risk triple-negative tumors includes chemotherapy with taxanes (paclitaxel or docetaxel) plus anthracycline-based treatment (cyclophosphamide plus doxorubicin). Neoadjuvant chemotherapy is recommended for patients with stage 2 and 3 tumors to reduce locoregional surgical extension and to allow for personalized treatment based on pathologic response. In 2018, two poly ADP ribose polymerase inhibitors, olaparib and talazoparib, were approved for use in select patients with triple-negative breast cancer, significantly changing the treatment paradigm for this disease. Other promising treatments have emerged recently in clinical trials for triple-negative disease, including immune checkpoint inhibitors, PI3K/AKT pathway inhibitors, and cytotoxin-conjugated antibodies.
Daniel S. Schwartz, MD, is Medical Director of Thoracic Oncology, St. Catherine of Siena Medical Center, Catholic Health Services, Smithtown, New York.
Dr. Schwartz serve(d) as a member of the following medical societies:
American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, Western Thoracic Surgical Association.
Disclosure: Daniel S. Schwartz, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given clinical and imaging outcomes, as well as results on IHC assay, this patient is diagnosed with triple-negative breast cancer and is referred for further consultation with a multidisciplinary care team.
Triple-negative breast cancer accounts for 15% of all female breast cancer cases. The term "triple-negative" refers to the absence of estrogen receptor (ER) and progesterone receptor (PR) targets and the HER2 target for treatment. Otherwise, triple-negative breast cancer is highly heterogeneous and includes multiple molecular subtypes. Typically, triple-negative breast cancer occurs in younger patients, has a higher grade, a greater tumor size, a higher clinical stage at diagnosis, and a poorer prognosis than non–triple-negative breast cancers.
Biopsy samples from patients with a new primary or newly metastatic breast cancer diagnosis should undergo HR testing, both ER and PR, as well as HER2 receptor testing. Results of ER and PR testing are negative if a validated IHC assay shows 0% to < 1% of nuclei stain, according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HR testing guideline. HER2 results are negative if a validated IHC assay shows weak to moderate complete membrane staining in < 10% of tumor cells, according to ASCO/CAP guidelines. If HER2 results of a primary tumor are negative and specific clinical criteria suggest further testing, the oncologist may choose to order another IHC assay or a validated dual probe in situ hybridization (ISH) assay to aid in clinical decision-making.
Pathologic classification of triple-negative breast cancers includes multiple subgroups determined by therapeutic possibilities: low-risk histologic types (salivary gland–like carcinomas, fibromatosis-like carcinoma, low-grade adenosquamous breast carcinoma), immune activation (tumor-infiltrating lymphocytes, CD8, programmed death–ligand 1, tumor mutational burden), HER2-low status (IHC score 1+/2+ nonamplified), associated germline BRCA mutations, and other targets (trophoblast cell-surface antigen 2, HER3).
Family history is the most common risk factor for developing breast cancer. If a person's mother or sister had breast cancer, the lifetime risk for that individual is four times greater than that of the general population. Risk for breast cancer grows even more if two or more first-degree relatives are diagnosed, or if one first-degree relative was diagnosed with breast cancer at ≤ 50 years of age. About 25% of triple-negative breast cancer cases have accompanying germline BRCA mutations. A recent study by Ahearn and colleagues found that 85 variants were linked to one or more tumor feature of ER-negative disease, and 32 of those variants were associated with triple-negative disease.
For a breast cancer diagnosis, the National Comprehensive Cancer Network recommends multidisciplinary care as well as the development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Because triple-negative breast cancer lacks HR and HER2 therapeutic targets, it can be difficult to treat. Standard therapy for high-risk triple-negative tumors includes chemotherapy with taxanes (paclitaxel or docetaxel) plus anthracycline-based treatment (cyclophosphamide plus doxorubicin). Neoadjuvant chemotherapy is recommended for patients with stage 2 and 3 tumors to reduce locoregional surgical extension and to allow for personalized treatment based on pathologic response. In 2018, two poly ADP ribose polymerase inhibitors, olaparib and talazoparib, were approved for use in select patients with triple-negative breast cancer, significantly changing the treatment paradigm for this disease. Other promising treatments have emerged recently in clinical trials for triple-negative disease, including immune checkpoint inhibitors, PI3K/AKT pathway inhibitors, and cytotoxin-conjugated antibodies.
Daniel S. Schwartz, MD, is Medical Director of Thoracic Oncology, St. Catherine of Siena Medical Center, Catholic Health Services, Smithtown, New York.
Dr. Schwartz serve(d) as a member of the following medical societies:
American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, Western Thoracic Surgical Association.
Disclosure: Daniel S. Schwartz, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given clinical and imaging outcomes, as well as results on IHC assay, this patient is diagnosed with triple-negative breast cancer and is referred for further consultation with a multidisciplinary care team.
Triple-negative breast cancer accounts for 15% of all female breast cancer cases. The term "triple-negative" refers to the absence of estrogen receptor (ER) and progesterone receptor (PR) targets and the HER2 target for treatment. Otherwise, triple-negative breast cancer is highly heterogeneous and includes multiple molecular subtypes. Typically, triple-negative breast cancer occurs in younger patients, has a higher grade, a greater tumor size, a higher clinical stage at diagnosis, and a poorer prognosis than non–triple-negative breast cancers.
Biopsy samples from patients with a new primary or newly metastatic breast cancer diagnosis should undergo HR testing, both ER and PR, as well as HER2 receptor testing. Results of ER and PR testing are negative if a validated IHC assay shows 0% to < 1% of nuclei stain, according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) HR testing guideline. HER2 results are negative if a validated IHC assay shows weak to moderate complete membrane staining in < 10% of tumor cells, according to ASCO/CAP guidelines. If HER2 results of a primary tumor are negative and specific clinical criteria suggest further testing, the oncologist may choose to order another IHC assay or a validated dual probe in situ hybridization (ISH) assay to aid in clinical decision-making.
Pathologic classification of triple-negative breast cancers includes multiple subgroups determined by therapeutic possibilities: low-risk histologic types (salivary gland–like carcinomas, fibromatosis-like carcinoma, low-grade adenosquamous breast carcinoma), immune activation (tumor-infiltrating lymphocytes, CD8, programmed death–ligand 1, tumor mutational burden), HER2-low status (IHC score 1+/2+ nonamplified), associated germline BRCA mutations, and other targets (trophoblast cell-surface antigen 2, HER3).
Family history is the most common risk factor for developing breast cancer. If a person's mother or sister had breast cancer, the lifetime risk for that individual is four times greater than that of the general population. Risk for breast cancer grows even more if two or more first-degree relatives are diagnosed, or if one first-degree relative was diagnosed with breast cancer at ≤ 50 years of age. About 25% of triple-negative breast cancer cases have accompanying germline BRCA mutations. A recent study by Ahearn and colleagues found that 85 variants were linked to one or more tumor feature of ER-negative disease, and 32 of those variants were associated with triple-negative disease.
For a breast cancer diagnosis, the National Comprehensive Cancer Network recommends multidisciplinary care as well as the development of a personalized survivorship treatment plan, which includes a customized summary of possible long-term treatment toxicities. In addition, multidisciplinary care coordination encourages close follow-up that helps patients adhere to their medications and stay current with ongoing screening.
Because triple-negative breast cancer lacks HR and HER2 therapeutic targets, it can be difficult to treat. Standard therapy for high-risk triple-negative tumors includes chemotherapy with taxanes (paclitaxel or docetaxel) plus anthracycline-based treatment (cyclophosphamide plus doxorubicin). Neoadjuvant chemotherapy is recommended for patients with stage 2 and 3 tumors to reduce locoregional surgical extension and to allow for personalized treatment based on pathologic response. In 2018, two poly ADP ribose polymerase inhibitors, olaparib and talazoparib, were approved for use in select patients with triple-negative breast cancer, significantly changing the treatment paradigm for this disease. Other promising treatments have emerged recently in clinical trials for triple-negative disease, including immune checkpoint inhibitors, PI3K/AKT pathway inhibitors, and cytotoxin-conjugated antibodies.
Daniel S. Schwartz, MD, is Medical Director of Thoracic Oncology, St. Catherine of Siena Medical Center, Catholic Health Services, Smithtown, New York.
Dr. Schwartz serve(d) as a member of the following medical societies:
American College of Chest Physicians, American College of Surgeons, Society of Thoracic Surgeons, Western Thoracic Surgical Association.
Disclosure: Daniel S. Schwartz, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 34-year-old woman visits her primary care physician after detecting a lump in her right breast. The physician conducts a physical exam and finds a hard, poorly mobile mass in the outer upper quadrant. Ultrasonography reveals an irregular hypoechoic mass of 22.1 x 15.1 mm with several abnormal axillary lymph nodes. MRI of the right breast shows an irregularly shaped and lobulated mass in the outer upper quadrant with enlarged axillary lymph nodes. On further radiologic examination, there is no detectable metastatic spread to the brain, chest, or upper abdomen. The patient undergoes a core needle biopsy of the tumor, which confirms ductal carcinoma and right axillary lymph node metastasis. Immunohistochemistry (IHC) assay of the sample is negative for hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2).
Ozempic is Appealing, but Not Cost-Effective, for Obesity Treatment
, according to a modeling study that compared the drugs with surgery and endoscopy.
Sleeve gastrectomy (SG) for moderate to severe (class II/III) obesity and the less invasive endoscopic sleeve gastroplasty (ESG) for mild (class I) obesity were both cost effective strategies to reduce obesity, the researchers report.
“SG should be offered as the first-line treatment for class II and class III obesity,” write Monica Saumoy, MD, of the Center for Digestive Health, Penn Medicine Princeton Medical Center, Plainsboro, N.J., and coauthors. “ESG is an effective and cost-effective nonsurgical treatment for class I, class II and class III obesity, and more efforts are needed to ensure that patients have access to this procedure.
“While semaglutide is highly effective for weight loss, and there is substantial patient interest, it is not currently cost-effective due to its high cost,” they add. “With methods to reduce semaglutide’s annual cost, it may provide an effective and cost-effective method to reduce the morbidity related to obesity.”
The study was published in Gut.
Cost Concerns
One in two Americans will likely be obese by 2030, according to current models, and nearly one in four adults will be severely obese.
Several weight-loss therapies exist to treat obesity. Evidence shows bariatric surgery is effective in reducing weight, metabolic comorbidities, and mortality in people with obesity compared with lifestyle intervention alone, but surgery has risks, adverse events, and poor national uptake. Patients are likely more interested in less invasive options, the authors write.
Recent trials have reported effective weight loss from less invasive options. A five-year follow-up of the randomized controlled MERIT trial found that ESG was associated with a 13.6% total body weight loss for people with mild to moderate obesity.
On the pharmaceutical front, other randomized controlled trials have shown that semaglutide is linked with as much as 17% total body weight loss at two years. Also, recent guidance from the American Gastroenterological Association (AGA) states that long-term treatment with a semaglutide is the preferred strategy for weight loss.
“However, concerns about the cost and the cost-effectiveness of these [less invasive] interventions have limited their usage in the USA,” the study authors write.
The aim of the study was to perform a cost-effectiveness analysis comparing SG, ESG, semaglutide, and lifestyle interventions (LI) for patients with obesity in class I (defined as BMI 30-34.9 kg/m2), class II (35-29.9 kg/m2), and class III (>40kg/m2) obesity.
Researchers used a state-transition, semi-Markov microsimulation model to analyze the effectiveness of ESG, SG, semaglutide, and LI in a simulated 40-year-old with three different base-case scenarios of class I, II, or III obesity. They then performed a detailed threshold and sensitivity analysis to change the cost of treatment modalities and the semaglutide adherence rate. Outcome measures included a willingness-to-pay threshold of US $100,000/quality-adjusted life years (QALY) and incremental cost-effectiveness ratios (ICERs).
Cost-Effectiveness of Treatments
When the treatment modalities were compared with each other, findings showed that for class I obesity, ESG was cost effective (US $4,105/QALY). For class II and III obesity, SG was cost-effective as well (US $5,883/QALY) and (US $7,821/QALY), respectively.
In all classes of obesity, SG and ESG were cost-effective compared with LI. Semaglutide was not cost-effective compared with LI for class I, II, and III obesity (ICER US $508,414/QALY, $420,483/QALY, and $350,637/QALY, respectively).
“For semaglutide to be cost-effective when compared with ESG, it would have to cost less than US $1,879 (class III), US $1,204 (class II), or US $297 (class I) annually,” the authors note.
The authors addressed recent guidelines to consider bariatric surgery in all obese patients. They recommend SG remain the standard of care for patients with severe obesity.
But national projections show that SG would address only 0.5% of life-years lost due to obesity.
“Barring a dramatic increase in patient adherence, bariatric surgery will not likely successfully mitigate the harm from the obesity epidemic,” they write.
“ESG may fill this gap and provide an additional option for patients with obesity as it demonstrated sustained weight loss at 2-5 years.” While insurance coverage is limited, they write, “our model demonstrates that payer coverage for ESG would provide an alternative tool to combat the obesity epidemic as part of a multidisciplinary approach.”
Semaglutide shows sustained weight loss in trials for up to two years but has a substantial annual cost, the authors note.
At lower prices, semaglutide can make a “major impact on the obesity pandemic as it can be prescribed in multiple healthcare settings and due to increased patient interested in non-invasive obesity treatment,” they write.
One limitation to the study is a lack of long-term data available for ESG and semaglutide. Authors were also not able to use a lifetime horizon because of a lack of long-term weight loss.
One study author reports financial relationships with BSC, Cook Medical, Surgical Intuitive, and Olympus America. Another author reports relationships with ACI, AGA-Varia, BSC, Dark Canyon Labs, Endiatx, Medtronic, Olympus, Virgo Systems; equity: AGA-Varia, Dark Canyon Labs, Endiatx, EndoSound, and Virgo Systems. The rest of the authors have no conflicts to disclose.
, according to a modeling study that compared the drugs with surgery and endoscopy.
Sleeve gastrectomy (SG) for moderate to severe (class II/III) obesity and the less invasive endoscopic sleeve gastroplasty (ESG) for mild (class I) obesity were both cost effective strategies to reduce obesity, the researchers report.
“SG should be offered as the first-line treatment for class II and class III obesity,” write Monica Saumoy, MD, of the Center for Digestive Health, Penn Medicine Princeton Medical Center, Plainsboro, N.J., and coauthors. “ESG is an effective and cost-effective nonsurgical treatment for class I, class II and class III obesity, and more efforts are needed to ensure that patients have access to this procedure.
“While semaglutide is highly effective for weight loss, and there is substantial patient interest, it is not currently cost-effective due to its high cost,” they add. “With methods to reduce semaglutide’s annual cost, it may provide an effective and cost-effective method to reduce the morbidity related to obesity.”
The study was published in Gut.
Cost Concerns
One in two Americans will likely be obese by 2030, according to current models, and nearly one in four adults will be severely obese.
Several weight-loss therapies exist to treat obesity. Evidence shows bariatric surgery is effective in reducing weight, metabolic comorbidities, and mortality in people with obesity compared with lifestyle intervention alone, but surgery has risks, adverse events, and poor national uptake. Patients are likely more interested in less invasive options, the authors write.
Recent trials have reported effective weight loss from less invasive options. A five-year follow-up of the randomized controlled MERIT trial found that ESG was associated with a 13.6% total body weight loss for people with mild to moderate obesity.
On the pharmaceutical front, other randomized controlled trials have shown that semaglutide is linked with as much as 17% total body weight loss at two years. Also, recent guidance from the American Gastroenterological Association (AGA) states that long-term treatment with a semaglutide is the preferred strategy for weight loss.
“However, concerns about the cost and the cost-effectiveness of these [less invasive] interventions have limited their usage in the USA,” the study authors write.
The aim of the study was to perform a cost-effectiveness analysis comparing SG, ESG, semaglutide, and lifestyle interventions (LI) for patients with obesity in class I (defined as BMI 30-34.9 kg/m2), class II (35-29.9 kg/m2), and class III (>40kg/m2) obesity.
Researchers used a state-transition, semi-Markov microsimulation model to analyze the effectiveness of ESG, SG, semaglutide, and LI in a simulated 40-year-old with three different base-case scenarios of class I, II, or III obesity. They then performed a detailed threshold and sensitivity analysis to change the cost of treatment modalities and the semaglutide adherence rate. Outcome measures included a willingness-to-pay threshold of US $100,000/quality-adjusted life years (QALY) and incremental cost-effectiveness ratios (ICERs).
Cost-Effectiveness of Treatments
When the treatment modalities were compared with each other, findings showed that for class I obesity, ESG was cost effective (US $4,105/QALY). For class II and III obesity, SG was cost-effective as well (US $5,883/QALY) and (US $7,821/QALY), respectively.
In all classes of obesity, SG and ESG were cost-effective compared with LI. Semaglutide was not cost-effective compared with LI for class I, II, and III obesity (ICER US $508,414/QALY, $420,483/QALY, and $350,637/QALY, respectively).
“For semaglutide to be cost-effective when compared with ESG, it would have to cost less than US $1,879 (class III), US $1,204 (class II), or US $297 (class I) annually,” the authors note.
The authors addressed recent guidelines to consider bariatric surgery in all obese patients. They recommend SG remain the standard of care for patients with severe obesity.
But national projections show that SG would address only 0.5% of life-years lost due to obesity.
“Barring a dramatic increase in patient adherence, bariatric surgery will not likely successfully mitigate the harm from the obesity epidemic,” they write.
“ESG may fill this gap and provide an additional option for patients with obesity as it demonstrated sustained weight loss at 2-5 years.” While insurance coverage is limited, they write, “our model demonstrates that payer coverage for ESG would provide an alternative tool to combat the obesity epidemic as part of a multidisciplinary approach.”
Semaglutide shows sustained weight loss in trials for up to two years but has a substantial annual cost, the authors note.
At lower prices, semaglutide can make a “major impact on the obesity pandemic as it can be prescribed in multiple healthcare settings and due to increased patient interested in non-invasive obesity treatment,” they write.
One limitation to the study is a lack of long-term data available for ESG and semaglutide. Authors were also not able to use a lifetime horizon because of a lack of long-term weight loss.
One study author reports financial relationships with BSC, Cook Medical, Surgical Intuitive, and Olympus America. Another author reports relationships with ACI, AGA-Varia, BSC, Dark Canyon Labs, Endiatx, Medtronic, Olympus, Virgo Systems; equity: AGA-Varia, Dark Canyon Labs, Endiatx, EndoSound, and Virgo Systems. The rest of the authors have no conflicts to disclose.
, according to a modeling study that compared the drugs with surgery and endoscopy.
Sleeve gastrectomy (SG) for moderate to severe (class II/III) obesity and the less invasive endoscopic sleeve gastroplasty (ESG) for mild (class I) obesity were both cost effective strategies to reduce obesity, the researchers report.
“SG should be offered as the first-line treatment for class II and class III obesity,” write Monica Saumoy, MD, of the Center for Digestive Health, Penn Medicine Princeton Medical Center, Plainsboro, N.J., and coauthors. “ESG is an effective and cost-effective nonsurgical treatment for class I, class II and class III obesity, and more efforts are needed to ensure that patients have access to this procedure.
“While semaglutide is highly effective for weight loss, and there is substantial patient interest, it is not currently cost-effective due to its high cost,” they add. “With methods to reduce semaglutide’s annual cost, it may provide an effective and cost-effective method to reduce the morbidity related to obesity.”
The study was published in Gut.
Cost Concerns
One in two Americans will likely be obese by 2030, according to current models, and nearly one in four adults will be severely obese.
Several weight-loss therapies exist to treat obesity. Evidence shows bariatric surgery is effective in reducing weight, metabolic comorbidities, and mortality in people with obesity compared with lifestyle intervention alone, but surgery has risks, adverse events, and poor national uptake. Patients are likely more interested in less invasive options, the authors write.
Recent trials have reported effective weight loss from less invasive options. A five-year follow-up of the randomized controlled MERIT trial found that ESG was associated with a 13.6% total body weight loss for people with mild to moderate obesity.
On the pharmaceutical front, other randomized controlled trials have shown that semaglutide is linked with as much as 17% total body weight loss at two years. Also, recent guidance from the American Gastroenterological Association (AGA) states that long-term treatment with a semaglutide is the preferred strategy for weight loss.
“However, concerns about the cost and the cost-effectiveness of these [less invasive] interventions have limited their usage in the USA,” the study authors write.
The aim of the study was to perform a cost-effectiveness analysis comparing SG, ESG, semaglutide, and lifestyle interventions (LI) for patients with obesity in class I (defined as BMI 30-34.9 kg/m2), class II (35-29.9 kg/m2), and class III (>40kg/m2) obesity.
Researchers used a state-transition, semi-Markov microsimulation model to analyze the effectiveness of ESG, SG, semaglutide, and LI in a simulated 40-year-old with three different base-case scenarios of class I, II, or III obesity. They then performed a detailed threshold and sensitivity analysis to change the cost of treatment modalities and the semaglutide adherence rate. Outcome measures included a willingness-to-pay threshold of US $100,000/quality-adjusted life years (QALY) and incremental cost-effectiveness ratios (ICERs).
Cost-Effectiveness of Treatments
When the treatment modalities were compared with each other, findings showed that for class I obesity, ESG was cost effective (US $4,105/QALY). For class II and III obesity, SG was cost-effective as well (US $5,883/QALY) and (US $7,821/QALY), respectively.
In all classes of obesity, SG and ESG were cost-effective compared with LI. Semaglutide was not cost-effective compared with LI for class I, II, and III obesity (ICER US $508,414/QALY, $420,483/QALY, and $350,637/QALY, respectively).
“For semaglutide to be cost-effective when compared with ESG, it would have to cost less than US $1,879 (class III), US $1,204 (class II), or US $297 (class I) annually,” the authors note.
The authors addressed recent guidelines to consider bariatric surgery in all obese patients. They recommend SG remain the standard of care for patients with severe obesity.
But national projections show that SG would address only 0.5% of life-years lost due to obesity.
“Barring a dramatic increase in patient adherence, bariatric surgery will not likely successfully mitigate the harm from the obesity epidemic,” they write.
“ESG may fill this gap and provide an additional option for patients with obesity as it demonstrated sustained weight loss at 2-5 years.” While insurance coverage is limited, they write, “our model demonstrates that payer coverage for ESG would provide an alternative tool to combat the obesity epidemic as part of a multidisciplinary approach.”
Semaglutide shows sustained weight loss in trials for up to two years but has a substantial annual cost, the authors note.
At lower prices, semaglutide can make a “major impact on the obesity pandemic as it can be prescribed in multiple healthcare settings and due to increased patient interested in non-invasive obesity treatment,” they write.
One limitation to the study is a lack of long-term data available for ESG and semaglutide. Authors were also not able to use a lifetime horizon because of a lack of long-term weight loss.
One study author reports financial relationships with BSC, Cook Medical, Surgical Intuitive, and Olympus America. Another author reports relationships with ACI, AGA-Varia, BSC, Dark Canyon Labs, Endiatx, Medtronic, Olympus, Virgo Systems; equity: AGA-Varia, Dark Canyon Labs, Endiatx, EndoSound, and Virgo Systems. The rest of the authors have no conflicts to disclose.
FROM GUT
Pet Peeves About the State of Primary Care – Part 2
I have received lots of notes from readers about other pet peeves they have about practicing primary care in our current environment and wanted to share some of them. I appreciate all the emails I received on this topic.
- The rapid increase in the number of hospital administrators in the last 50 years
This has increased health system costs without providing any relief for practicing physicians, and often has led to policies that have been harmful and detrimental. This would be a great place to start cutting back to get true savings without affecting quality of care.
- Emergency physicians and specialists who refer my patient elsewhere for a service we provide in our office
It is expensive for patients to go to a specialty provider for a simple procedure that can be easily done in a primary care practice, or to be referred to see a specialist for a problem that does not need specialty care. This creates further problems accessing specialists.
- Online reviews of practices, including reviews from people who have never been patients
I am concerned about the accuracy and intent of online reviews. If a patient is upset because they did not receive an antibiotic or narcotic, they can vent their frustration in a review, when what the medical professional was actually doing was good medicine. More concerning to me is that some organizations use these reviews to determine compensation, promotion, and support. These reviews are not evidence based or accurately collected.
- Offices and organizations being dropped by insurance carriers
Insurance companies are running amok. They make their own rules, which can devastate practices and patients. They can change fees paid unilaterally, and drop practices without explanation or valid reasons. Patients suffer terribly because they now cannot see their long-time physicians or they have to pay much more to see them as they are suddenly “out of network.”
- The lack of appreciation by organizations as well as the general public of the enormous cost savings primary care professionals contribute to the healthcare system
There are many studies showing that patients who see a primary care physician save the system money and have better health outcomes. US adults who regularly see a primary care physician have 33% lower healthcare costs and 19% lower odds of dying prematurely than those who see only a specialist.1
In one study, for every $1 invested in primary care, there was $13 in savings in healthcare costs.2 I had a patient a few years ago complain about the “enormous” bill she received for a visit where I had done an annual exam, cryotherapy for three actinic keratoses, and a steroid injection for her ailing knee. The cost savings was well over $700 (the new patient cost for two specialty visits). There is no doubt that patients who have stable primary care save money themselves and for the whole medical system.
- The stress of being witness to a dysfunctional system
It is really hard to see the hurt and difficulty our patients go through on a daily basis while trying to navigate a broken system. We bear witness to them and listen to all the stories when things have gone wrong. This also takes its toll on us, as we are part of the system, and our patients’ frustrations sometimes boil over. We are also the ones who care for the whole patient, so every bad experience with a specialty clinic is shared with us.
Many thanks extended to those who wrote to share their ideas (Drs. Sylvia Androne, Bhawna Bahethi, Pierre Ghassibi, Richard Katz, Louis Kasunic, Rebecca Keenan, David Kosnosky, Gregory Miller, and James Wilkens).
Dr. Paauw is professor of medicine in the Division of General Internal Medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington, Seattle. Contact Dr. Paauw at [email protected].
References
1. Forbes.com. Why Primary Care Matters, and What We Can Do To Increase It. 2023 Nov 27.
2. Washingtonpost.com. A Health Care Solution We Can’t Afford to Ignore: Primary Care.
I have received lots of notes from readers about other pet peeves they have about practicing primary care in our current environment and wanted to share some of them. I appreciate all the emails I received on this topic.
- The rapid increase in the number of hospital administrators in the last 50 years
This has increased health system costs without providing any relief for practicing physicians, and often has led to policies that have been harmful and detrimental. This would be a great place to start cutting back to get true savings without affecting quality of care.
- Emergency physicians and specialists who refer my patient elsewhere for a service we provide in our office
It is expensive for patients to go to a specialty provider for a simple procedure that can be easily done in a primary care practice, or to be referred to see a specialist for a problem that does not need specialty care. This creates further problems accessing specialists.
- Online reviews of practices, including reviews from people who have never been patients
I am concerned about the accuracy and intent of online reviews. If a patient is upset because they did not receive an antibiotic or narcotic, they can vent their frustration in a review, when what the medical professional was actually doing was good medicine. More concerning to me is that some organizations use these reviews to determine compensation, promotion, and support. These reviews are not evidence based or accurately collected.
- Offices and organizations being dropped by insurance carriers
Insurance companies are running amok. They make their own rules, which can devastate practices and patients. They can change fees paid unilaterally, and drop practices without explanation or valid reasons. Patients suffer terribly because they now cannot see their long-time physicians or they have to pay much more to see them as they are suddenly “out of network.”
- The lack of appreciation by organizations as well as the general public of the enormous cost savings primary care professionals contribute to the healthcare system
There are many studies showing that patients who see a primary care physician save the system money and have better health outcomes. US adults who regularly see a primary care physician have 33% lower healthcare costs and 19% lower odds of dying prematurely than those who see only a specialist.1
In one study, for every $1 invested in primary care, there was $13 in savings in healthcare costs.2 I had a patient a few years ago complain about the “enormous” bill she received for a visit where I had done an annual exam, cryotherapy for three actinic keratoses, and a steroid injection for her ailing knee. The cost savings was well over $700 (the new patient cost for two specialty visits). There is no doubt that patients who have stable primary care save money themselves and for the whole medical system.
- The stress of being witness to a dysfunctional system
It is really hard to see the hurt and difficulty our patients go through on a daily basis while trying to navigate a broken system. We bear witness to them and listen to all the stories when things have gone wrong. This also takes its toll on us, as we are part of the system, and our patients’ frustrations sometimes boil over. We are also the ones who care for the whole patient, so every bad experience with a specialty clinic is shared with us.
Many thanks extended to those who wrote to share their ideas (Drs. Sylvia Androne, Bhawna Bahethi, Pierre Ghassibi, Richard Katz, Louis Kasunic, Rebecca Keenan, David Kosnosky, Gregory Miller, and James Wilkens).
Dr. Paauw is professor of medicine in the Division of General Internal Medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington, Seattle. Contact Dr. Paauw at [email protected].
References
1. Forbes.com. Why Primary Care Matters, and What We Can Do To Increase It. 2023 Nov 27.
2. Washingtonpost.com. A Health Care Solution We Can’t Afford to Ignore: Primary Care.
I have received lots of notes from readers about other pet peeves they have about practicing primary care in our current environment and wanted to share some of them. I appreciate all the emails I received on this topic.
- The rapid increase in the number of hospital administrators in the last 50 years
This has increased health system costs without providing any relief for practicing physicians, and often has led to policies that have been harmful and detrimental. This would be a great place to start cutting back to get true savings without affecting quality of care.
- Emergency physicians and specialists who refer my patient elsewhere for a service we provide in our office
It is expensive for patients to go to a specialty provider for a simple procedure that can be easily done in a primary care practice, or to be referred to see a specialist for a problem that does not need specialty care. This creates further problems accessing specialists.
- Online reviews of practices, including reviews from people who have never been patients
I am concerned about the accuracy and intent of online reviews. If a patient is upset because they did not receive an antibiotic or narcotic, they can vent their frustration in a review, when what the medical professional was actually doing was good medicine. More concerning to me is that some organizations use these reviews to determine compensation, promotion, and support. These reviews are not evidence based or accurately collected.
- Offices and organizations being dropped by insurance carriers
Insurance companies are running amok. They make their own rules, which can devastate practices and patients. They can change fees paid unilaterally, and drop practices without explanation or valid reasons. Patients suffer terribly because they now cannot see their long-time physicians or they have to pay much more to see them as they are suddenly “out of network.”
- The lack of appreciation by organizations as well as the general public of the enormous cost savings primary care professionals contribute to the healthcare system
There are many studies showing that patients who see a primary care physician save the system money and have better health outcomes. US adults who regularly see a primary care physician have 33% lower healthcare costs and 19% lower odds of dying prematurely than those who see only a specialist.1
In one study, for every $1 invested in primary care, there was $13 in savings in healthcare costs.2 I had a patient a few years ago complain about the “enormous” bill she received for a visit where I had done an annual exam, cryotherapy for three actinic keratoses, and a steroid injection for her ailing knee. The cost savings was well over $700 (the new patient cost for two specialty visits). There is no doubt that patients who have stable primary care save money themselves and for the whole medical system.
- The stress of being witness to a dysfunctional system
It is really hard to see the hurt and difficulty our patients go through on a daily basis while trying to navigate a broken system. We bear witness to them and listen to all the stories when things have gone wrong. This also takes its toll on us, as we are part of the system, and our patients’ frustrations sometimes boil over. We are also the ones who care for the whole patient, so every bad experience with a specialty clinic is shared with us.
Many thanks extended to those who wrote to share their ideas (Drs. Sylvia Androne, Bhawna Bahethi, Pierre Ghassibi, Richard Katz, Louis Kasunic, Rebecca Keenan, David Kosnosky, Gregory Miller, and James Wilkens).
Dr. Paauw is professor of medicine in the Division of General Internal Medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington, Seattle. Contact Dr. Paauw at [email protected].
References
1. Forbes.com. Why Primary Care Matters, and What We Can Do To Increase It. 2023 Nov 27.
2. Washingtonpost.com. A Health Care Solution We Can’t Afford to Ignore: Primary Care.
HPV Vax Tied to Lower Odds of Cervical Lesion Progression
TOPLINE:
Among women with cervical intraepithelial neoplasia grade 2 (CIN2), vaccination against human papillomavirus (HPV) before age 20 is associated with lower odds of progression.
METHODOLOGY:
- Researchers analyzed data from 7904 women in Denmark who were undergoing active surveillance for CIN2 between 2007 and 2020.
- CIN2 lesions on their own. Removing them can increase the risk for during subsequent pregnancies, the researchers noted.
- Nearly half of the women had received at least one dose of an HPV vaccine at least 1 year before the diagnosis of cervical dysplasia.
TAKEAWAY:
- During 28 months of follow-up, the risk for progression was 22.9% for women vaccinated before age 15, 31.5% for women vaccinated between ages 15 and 20, and 37.6% for women who were not vaccinated.
- Women vaccinated before age 15 had a 35% lower risk for progression than unvaccinated women, after adjusting for cytology, income, and education (adjusted relative risk, 0.65; 95% CI, 0.57-0.75).
- Cervical cancer developed in 0.37% of the unvaccinated women and 0.13% of the vaccinated women.
- All cases of cervical cancer in the vaccinated group occurred in women who received the vaccine after age 20.
IN PRACTICE:
“These findings suggest that HPV vaccination status may be used to identify women at higher risk for progression, thereby enabling risk stratification at the time of CIN2 diagnosis,” the researchers wrote.
SOURCE:
Louise Krog, BscMed, with Aarhus University, Aarhus, Denmark, was the corresponding author of the study. The research was published online in the American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study authors had limited information about potential confounders such as smoking, immunosuppressive conditions, and the age at which patients became sexually active.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Carpenter Axel Kastrup-Nielsen’s Memorial Fund, and the Dagmar Marshall’s Fund. Co-authors disclosed ties to AstraZeneca, Roche, and Hologic.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women with cervical intraepithelial neoplasia grade 2 (CIN2), vaccination against human papillomavirus (HPV) before age 20 is associated with lower odds of progression.
METHODOLOGY:
- Researchers analyzed data from 7904 women in Denmark who were undergoing active surveillance for CIN2 between 2007 and 2020.
- CIN2 lesions on their own. Removing them can increase the risk for during subsequent pregnancies, the researchers noted.
- Nearly half of the women had received at least one dose of an HPV vaccine at least 1 year before the diagnosis of cervical dysplasia.
TAKEAWAY:
- During 28 months of follow-up, the risk for progression was 22.9% for women vaccinated before age 15, 31.5% for women vaccinated between ages 15 and 20, and 37.6% for women who were not vaccinated.
- Women vaccinated before age 15 had a 35% lower risk for progression than unvaccinated women, after adjusting for cytology, income, and education (adjusted relative risk, 0.65; 95% CI, 0.57-0.75).
- Cervical cancer developed in 0.37% of the unvaccinated women and 0.13% of the vaccinated women.
- All cases of cervical cancer in the vaccinated group occurred in women who received the vaccine after age 20.
IN PRACTICE:
“These findings suggest that HPV vaccination status may be used to identify women at higher risk for progression, thereby enabling risk stratification at the time of CIN2 diagnosis,” the researchers wrote.
SOURCE:
Louise Krog, BscMed, with Aarhus University, Aarhus, Denmark, was the corresponding author of the study. The research was published online in the American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study authors had limited information about potential confounders such as smoking, immunosuppressive conditions, and the age at which patients became sexually active.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Carpenter Axel Kastrup-Nielsen’s Memorial Fund, and the Dagmar Marshall’s Fund. Co-authors disclosed ties to AstraZeneca, Roche, and Hologic.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women with cervical intraepithelial neoplasia grade 2 (CIN2), vaccination against human papillomavirus (HPV) before age 20 is associated with lower odds of progression.
METHODOLOGY:
- Researchers analyzed data from 7904 women in Denmark who were undergoing active surveillance for CIN2 between 2007 and 2020.
- CIN2 lesions on their own. Removing them can increase the risk for during subsequent pregnancies, the researchers noted.
- Nearly half of the women had received at least one dose of an HPV vaccine at least 1 year before the diagnosis of cervical dysplasia.
TAKEAWAY:
- During 28 months of follow-up, the risk for progression was 22.9% for women vaccinated before age 15, 31.5% for women vaccinated between ages 15 and 20, and 37.6% for women who were not vaccinated.
- Women vaccinated before age 15 had a 35% lower risk for progression than unvaccinated women, after adjusting for cytology, income, and education (adjusted relative risk, 0.65; 95% CI, 0.57-0.75).
- Cervical cancer developed in 0.37% of the unvaccinated women and 0.13% of the vaccinated women.
- All cases of cervical cancer in the vaccinated group occurred in women who received the vaccine after age 20.
IN PRACTICE:
“These findings suggest that HPV vaccination status may be used to identify women at higher risk for progression, thereby enabling risk stratification at the time of CIN2 diagnosis,” the researchers wrote.
SOURCE:
Louise Krog, BscMed, with Aarhus University, Aarhus, Denmark, was the corresponding author of the study. The research was published online in the American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study authors had limited information about potential confounders such as smoking, immunosuppressive conditions, and the age at which patients became sexually active.
DISCLOSURES:
The study was funded by the Danish Cancer Society, the Carpenter Axel Kastrup-Nielsen’s Memorial Fund, and the Dagmar Marshall’s Fund. Co-authors disclosed ties to AstraZeneca, Roche, and Hologic.
A version of this article appeared on Medscape.com.
Severely Irregular Sleep Patterns and OSA Prompt Increased Odds of Hypertension
TOPLINE:
Severe sleep irregularity often occurs with obstructive sleep apnea (OSA), and this combination approximately doubled the odds of hypertension in middle-aged individuals.
METHODOLOGY:
- OSA has demonstrated an association with hypertension, but data on the impact of sleep irregularity on this relationship are lacking.
- The researchers used the recently developed sleep regularity index (SRI) to determine sleep patterns using a scale of 0-100 (with higher numbers indicating greater regularity) to assess relationships between OSA, sleep patterns, and hypertension in 602 adults with a mean age of 57 years.
- The study’s goal was an assessment of the associations between sleep regularity, OSA, and hypertension in a community sample of adults with normal circadian patterns.
TAKEAWAY:
- The odds of OSA were significantly greater for individuals with mildly irregular or severely irregular sleep than for regular sleepers (odds ratios, 1.97 and 2.06, respectively).
- Individuals with OSA and severely irregular sleep had the highest odds of hypertension compared with individuals with no OSA and regular sleep (OR, 2.34).
- However, participants with OSA and regular sleep or mildly irregular sleep had no significant increase in hypertension risk.
IN PRACTICE:
“Irregular sleep may be an important marker of OSA-related sleep disruption and may be an important modifiable health target,” the researchers wrote.
SOURCE:
The study was led by Kelly Sansom, a PhD candidate at the Centre for Sleep Science at the University of Western Australia, Albany. The study was published online in the journal Sleep.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality, and the SRI is a nonspecific measure that may capture a range of phenotypes with one score; other limitations included the small sample sizes of sleep regularity groups and the use of actigraphy to collect sleep times.
DISCLOSURES:
The study was supported by an Australian Government Research Training Program Scholarship and the Raine Study PhD Top-up Scholarship; the Raine Study Scholarship is supported by the NHMRC, the Centre for Sleep Science, School of Anatomy, Physiology & Human Biology of the University of Western Australia, and the Lions Eye Institute. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Severe sleep irregularity often occurs with obstructive sleep apnea (OSA), and this combination approximately doubled the odds of hypertension in middle-aged individuals.
METHODOLOGY:
- OSA has demonstrated an association with hypertension, but data on the impact of sleep irregularity on this relationship are lacking.
- The researchers used the recently developed sleep regularity index (SRI) to determine sleep patterns using a scale of 0-100 (with higher numbers indicating greater regularity) to assess relationships between OSA, sleep patterns, and hypertension in 602 adults with a mean age of 57 years.
- The study’s goal was an assessment of the associations between sleep regularity, OSA, and hypertension in a community sample of adults with normal circadian patterns.
TAKEAWAY:
- The odds of OSA were significantly greater for individuals with mildly irregular or severely irregular sleep than for regular sleepers (odds ratios, 1.97 and 2.06, respectively).
- Individuals with OSA and severely irregular sleep had the highest odds of hypertension compared with individuals with no OSA and regular sleep (OR, 2.34).
- However, participants with OSA and regular sleep or mildly irregular sleep had no significant increase in hypertension risk.
IN PRACTICE:
“Irregular sleep may be an important marker of OSA-related sleep disruption and may be an important modifiable health target,” the researchers wrote.
SOURCE:
The study was led by Kelly Sansom, a PhD candidate at the Centre for Sleep Science at the University of Western Australia, Albany. The study was published online in the journal Sleep.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality, and the SRI is a nonspecific measure that may capture a range of phenotypes with one score; other limitations included the small sample sizes of sleep regularity groups and the use of actigraphy to collect sleep times.
DISCLOSURES:
The study was supported by an Australian Government Research Training Program Scholarship and the Raine Study PhD Top-up Scholarship; the Raine Study Scholarship is supported by the NHMRC, the Centre for Sleep Science, School of Anatomy, Physiology & Human Biology of the University of Western Australia, and the Lions Eye Institute. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Severe sleep irregularity often occurs with obstructive sleep apnea (OSA), and this combination approximately doubled the odds of hypertension in middle-aged individuals.
METHODOLOGY:
- OSA has demonstrated an association with hypertension, but data on the impact of sleep irregularity on this relationship are lacking.
- The researchers used the recently developed sleep regularity index (SRI) to determine sleep patterns using a scale of 0-100 (with higher numbers indicating greater regularity) to assess relationships between OSA, sleep patterns, and hypertension in 602 adults with a mean age of 57 years.
- The study’s goal was an assessment of the associations between sleep regularity, OSA, and hypertension in a community sample of adults with normal circadian patterns.
TAKEAWAY:
- The odds of OSA were significantly greater for individuals with mildly irregular or severely irregular sleep than for regular sleepers (odds ratios, 1.97 and 2.06, respectively).
- Individuals with OSA and severely irregular sleep had the highest odds of hypertension compared with individuals with no OSA and regular sleep (OR, 2.34).
- However, participants with OSA and regular sleep or mildly irregular sleep had no significant increase in hypertension risk.
IN PRACTICE:
“Irregular sleep may be an important marker of OSA-related sleep disruption and may be an important modifiable health target,” the researchers wrote.
SOURCE:
The study was led by Kelly Sansom, a PhD candidate at the Centre for Sleep Science at the University of Western Australia, Albany. The study was published online in the journal Sleep.
LIMITATIONS:
The cross-sectional design prevented conclusions of causality, and the SRI is a nonspecific measure that may capture a range of phenotypes with one score; other limitations included the small sample sizes of sleep regularity groups and the use of actigraphy to collect sleep times.
DISCLOSURES:
The study was supported by an Australian Government Research Training Program Scholarship and the Raine Study PhD Top-up Scholarship; the Raine Study Scholarship is supported by the NHMRC, the Centre for Sleep Science, School of Anatomy, Physiology & Human Biology of the University of Western Australia, and the Lions Eye Institute. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Strict Glycemic Control for Renal Benefit May Come With Risk
TOPLINE:
Patients with type 2 diabetes (T2D) at an elevated risk for kidney failure may stand to gain the most renal benefit with intensive glycemic control, but they also face the highest overall risk for death and hypoglycemic events.
METHODOLOGY:
- Studies show the primary benefit of intensive glycemic control in T2D is microvascular outcomes, mostly in the kidney, but no clear criteria exist to identify patients who may benefit most.
- Researchers conducted a post hoc analysis of the ACCORD trial, including 9777 patients with diabetes and cardiovascular disease or two or more cardiovascular risk factors.
- The 5-year kidney failure risk was estimated using the validated kidney failure risk equation (KFRE).
- The patients were randomly assigned to receive intensive glycemic control (A1c, < 6.0%) or standard glycemic control (A1c, 7.0%-7.9%).
- The primary outcomes were kidney microvascular events and all-cause mortality.
TAKEAWAY:
- Over a 7-year period, intensive vs standard glycemic control delayed the onset of kidney microvascular outcomes by 48.4 days (corresponding hazard ratio [HR], 0.75; 95% CI, 0.65-0.86) but reduced the time to death by 23.6 days (HR, 1.20; 95% CI, 1.04-1.40).
- Patients in the highest quartile of 5-year kidney failure risk according to KFRE benefited the most with intensive vs standard glycemic control and reported the longest delay in the onset of kidney microvascular outcomes (114.8 days; 95% CI, 58.1-176.4).
- Although renal outcomes improved, the time to death was shortened by 56.7 days in patients with elevated risk for kidney failure receiving intensive glycemic control.
IN PRACTICE:
“The observed effect of intensive glycemic control on kidney microvascular outcomes in ACCORD is almost entirely driven by a subset of patients representing one quarter of the trial eligible population at elevated risk for kidney failure at baseline,” the authors wrote.
SOURCE:
Vivek Charu of Stanford University School of Medicine, Stanford, California, led this study, which was published online on December 11, 2023, in the Journal of the American Society of Nephrology
LIMITATIONS:
The ACCORD study enrolled participants with a low risk for kidney disease. Therefore, this study lacks relevant data that might be needed to analyze the risks and benefits of intensive glycemic control in a population at high risk for kidney disease. Treatment options and monitoring approaches to glycemic control have evolved in the nearly 20 years since the ACCORD trial, which used insulin and sulfonylurea agents for glycemic control.
DISCLOSURES:
This work was supported by several grants secured by the authors. Some authors declared serving in advisory or leadership roles, receiving honoraria and research funding, and other ties with several sources.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients with type 2 diabetes (T2D) at an elevated risk for kidney failure may stand to gain the most renal benefit with intensive glycemic control, but they also face the highest overall risk for death and hypoglycemic events.
METHODOLOGY:
- Studies show the primary benefit of intensive glycemic control in T2D is microvascular outcomes, mostly in the kidney, but no clear criteria exist to identify patients who may benefit most.
- Researchers conducted a post hoc analysis of the ACCORD trial, including 9777 patients with diabetes and cardiovascular disease or two or more cardiovascular risk factors.
- The 5-year kidney failure risk was estimated using the validated kidney failure risk equation (KFRE).
- The patients were randomly assigned to receive intensive glycemic control (A1c, < 6.0%) or standard glycemic control (A1c, 7.0%-7.9%).
- The primary outcomes were kidney microvascular events and all-cause mortality.
TAKEAWAY:
- Over a 7-year period, intensive vs standard glycemic control delayed the onset of kidney microvascular outcomes by 48.4 days (corresponding hazard ratio [HR], 0.75; 95% CI, 0.65-0.86) but reduced the time to death by 23.6 days (HR, 1.20; 95% CI, 1.04-1.40).
- Patients in the highest quartile of 5-year kidney failure risk according to KFRE benefited the most with intensive vs standard glycemic control and reported the longest delay in the onset of kidney microvascular outcomes (114.8 days; 95% CI, 58.1-176.4).
- Although renal outcomes improved, the time to death was shortened by 56.7 days in patients with elevated risk for kidney failure receiving intensive glycemic control.
IN PRACTICE:
“The observed effect of intensive glycemic control on kidney microvascular outcomes in ACCORD is almost entirely driven by a subset of patients representing one quarter of the trial eligible population at elevated risk for kidney failure at baseline,” the authors wrote.
SOURCE:
Vivek Charu of Stanford University School of Medicine, Stanford, California, led this study, which was published online on December 11, 2023, in the Journal of the American Society of Nephrology
LIMITATIONS:
The ACCORD study enrolled participants with a low risk for kidney disease. Therefore, this study lacks relevant data that might be needed to analyze the risks and benefits of intensive glycemic control in a population at high risk for kidney disease. Treatment options and monitoring approaches to glycemic control have evolved in the nearly 20 years since the ACCORD trial, which used insulin and sulfonylurea agents for glycemic control.
DISCLOSURES:
This work was supported by several grants secured by the authors. Some authors declared serving in advisory or leadership roles, receiving honoraria and research funding, and other ties with several sources.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients with type 2 diabetes (T2D) at an elevated risk for kidney failure may stand to gain the most renal benefit with intensive glycemic control, but they also face the highest overall risk for death and hypoglycemic events.
METHODOLOGY:
- Studies show the primary benefit of intensive glycemic control in T2D is microvascular outcomes, mostly in the kidney, but no clear criteria exist to identify patients who may benefit most.
- Researchers conducted a post hoc analysis of the ACCORD trial, including 9777 patients with diabetes and cardiovascular disease or two or more cardiovascular risk factors.
- The 5-year kidney failure risk was estimated using the validated kidney failure risk equation (KFRE).
- The patients were randomly assigned to receive intensive glycemic control (A1c, < 6.0%) or standard glycemic control (A1c, 7.0%-7.9%).
- The primary outcomes were kidney microvascular events and all-cause mortality.
TAKEAWAY:
- Over a 7-year period, intensive vs standard glycemic control delayed the onset of kidney microvascular outcomes by 48.4 days (corresponding hazard ratio [HR], 0.75; 95% CI, 0.65-0.86) but reduced the time to death by 23.6 days (HR, 1.20; 95% CI, 1.04-1.40).
- Patients in the highest quartile of 5-year kidney failure risk according to KFRE benefited the most with intensive vs standard glycemic control and reported the longest delay in the onset of kidney microvascular outcomes (114.8 days; 95% CI, 58.1-176.4).
- Although renal outcomes improved, the time to death was shortened by 56.7 days in patients with elevated risk for kidney failure receiving intensive glycemic control.
IN PRACTICE:
“The observed effect of intensive glycemic control on kidney microvascular outcomes in ACCORD is almost entirely driven by a subset of patients representing one quarter of the trial eligible population at elevated risk for kidney failure at baseline,” the authors wrote.
SOURCE:
Vivek Charu of Stanford University School of Medicine, Stanford, California, led this study, which was published online on December 11, 2023, in the Journal of the American Society of Nephrology
LIMITATIONS:
The ACCORD study enrolled participants with a low risk for kidney disease. Therefore, this study lacks relevant data that might be needed to analyze the risks and benefits of intensive glycemic control in a population at high risk for kidney disease. Treatment options and monitoring approaches to glycemic control have evolved in the nearly 20 years since the ACCORD trial, which used insulin and sulfonylurea agents for glycemic control.
DISCLOSURES:
This work was supported by several grants secured by the authors. Some authors declared serving in advisory or leadership roles, receiving honoraria and research funding, and other ties with several sources.
A version of this article appeared on Medscape.com.
BC axillary dissection may be unnecessary for isolated tumor cells after NAC
SAN ANTONIO — Axillary lymph node dissection is the current standard of care in breast cancer when metastases are found in sentinel lymph nodes after neoadjuvant chemotherapy.
However, what to do when isolated tumor cells instead of outright metastases are found in sentinel nodes is an open question. Some surgeons opt for a full axillary dissection while others do not, and there is no standard of care, explained Giacomo Montagna, MD, a breast cancer surgeon at Memorial Sloan Kettering Cancer Center, New York City.
The study led and presented by Dr. Montagna at the San Antonio Breast Cancer Symposium brings some much-needed clarity to the issue.
The findings argue strongly against “routine axillary lymph node dissection” — with its considerable morbidities — “in patients with residual isolated tumor cells after neoadjuvant chemotherapy,” Dr. Montagna said.
Study discussant Elizabeth Mittendorf, MD, PhD, a breast cancer surgeon at Brigham and Women’s Hospital, Boston, agreed.
“It appears that the presence of isolated tumor cells in the sentinel nodes does not negatively impact oncologic outcomes. These additional data allow us to debunk some of the surgical dogma we grew up with, specifically that lymph node dissection is required for either survival or local control,” Dr. Mittendorf said.
However, there was concern among audience members that the information gleaned from a full dissection might still be needed to guide follow-on adjuvant therapy decisions.
Dr. Mittendorf didn’t think so. Although additional positive lymph nodes were found in almost a third of women who had axillary dissections in the review, the majority of involved nodes simply had more isolated tumor cells; macrometastases were found in just 5% of cases.
So, for most patients, additional information from axillary dissections is “unlikely needed to inform adjuvant therapy, and in fact,” based on the 5% figure, “we are thinking we would have to do well over a hundred lymph node dissections in such patients to inform treatment recommendations for fewer than five. This comes at the cost of fair morbidity,” she said.
Study details
The retrospective study, dubbed OPBC05/EUBREAST-14R/ICARO, included 583 women with cT1-4 N0-3 breast cancer treated at 62 centers in 18 countries. The majority of subjects were from the United States and Europe.
Every patient was found to have isolated tumor cells (ITCs) in their sentinel lymph nodes after neoadjuvant chemotherapy (NAC), which generally included anthracycline and taxane-based regimens. The majority of patients did not have a pathologic complete response to NAC.
Overall, 182 patients (31%) had a subsequent axillary lymph node dissection; the rest did not.
Dissections were more common in the presence of lymphovascular invasion and N2/N3 disease as well as when fewer lymph nodes were removed and when ITCs were found during surgery on frozen section, which was the case in a quarter of patients.
Additional positive nodes were found in 30% of patients in the dissection group and consisted of more nodes with ITCs in 18%, micrometastases in 7%, and macrometastases in 5%. Receptor status and nodal status at presentation did not have an impact on the likelihood of finding macrometastases.
The main finding of the study was that there were no statistically significant differences in recurrence outcomes between the two groups.
The 5-year rate of isolated axillary recurrence was 1.7% with axillary lymph node dissection (ALND) versus 1.1% without it. The 5-year rate of any invasive recurrence was 16% in the ALND arm and 19% in the no-dissection group.
The median age in the study was 48 years. The majority of patients (57%) had clinical T2 tumors. Most were HR positive and either HER2 negative (41%) or HER2 positive (28%).
Regional nodal radiation was more common in the ALND group, 82% versus 75%. The dissection arm had a mean of 2.8 sentinel lymph nodes removed versus 3.5 in the no-dissection group.
“The likelihood of finding additional positive lymph nodes in patients with residual ITCs after NAC is lower than in patients with residual micro- and macrometastases. In the majority of cases, they contain ITCs. Nodal recurrence after omission of ALND is rare in this population,” the investigators concluded in their abstract.
The work was funded by EUBREAST. Dr. Montagna doesn’t have any disclosures. Dr. Mittendorf has several industry ties, including being an advisor for Roche, AstraZeneca, and Moderna and a speaker for Merck.
SAN ANTONIO — Axillary lymph node dissection is the current standard of care in breast cancer when metastases are found in sentinel lymph nodes after neoadjuvant chemotherapy.
However, what to do when isolated tumor cells instead of outright metastases are found in sentinel nodes is an open question. Some surgeons opt for a full axillary dissection while others do not, and there is no standard of care, explained Giacomo Montagna, MD, a breast cancer surgeon at Memorial Sloan Kettering Cancer Center, New York City.
The study led and presented by Dr. Montagna at the San Antonio Breast Cancer Symposium brings some much-needed clarity to the issue.
The findings argue strongly against “routine axillary lymph node dissection” — with its considerable morbidities — “in patients with residual isolated tumor cells after neoadjuvant chemotherapy,” Dr. Montagna said.
Study discussant Elizabeth Mittendorf, MD, PhD, a breast cancer surgeon at Brigham and Women’s Hospital, Boston, agreed.
“It appears that the presence of isolated tumor cells in the sentinel nodes does not negatively impact oncologic outcomes. These additional data allow us to debunk some of the surgical dogma we grew up with, specifically that lymph node dissection is required for either survival or local control,” Dr. Mittendorf said.
However, there was concern among audience members that the information gleaned from a full dissection might still be needed to guide follow-on adjuvant therapy decisions.
Dr. Mittendorf didn’t think so. Although additional positive lymph nodes were found in almost a third of women who had axillary dissections in the review, the majority of involved nodes simply had more isolated tumor cells; macrometastases were found in just 5% of cases.
So, for most patients, additional information from axillary dissections is “unlikely needed to inform adjuvant therapy, and in fact,” based on the 5% figure, “we are thinking we would have to do well over a hundred lymph node dissections in such patients to inform treatment recommendations for fewer than five. This comes at the cost of fair morbidity,” she said.
Study details
The retrospective study, dubbed OPBC05/EUBREAST-14R/ICARO, included 583 women with cT1-4 N0-3 breast cancer treated at 62 centers in 18 countries. The majority of subjects were from the United States and Europe.
Every patient was found to have isolated tumor cells (ITCs) in their sentinel lymph nodes after neoadjuvant chemotherapy (NAC), which generally included anthracycline and taxane-based regimens. The majority of patients did not have a pathologic complete response to NAC.
Overall, 182 patients (31%) had a subsequent axillary lymph node dissection; the rest did not.
Dissections were more common in the presence of lymphovascular invasion and N2/N3 disease as well as when fewer lymph nodes were removed and when ITCs were found during surgery on frozen section, which was the case in a quarter of patients.
Additional positive nodes were found in 30% of patients in the dissection group and consisted of more nodes with ITCs in 18%, micrometastases in 7%, and macrometastases in 5%. Receptor status and nodal status at presentation did not have an impact on the likelihood of finding macrometastases.
The main finding of the study was that there were no statistically significant differences in recurrence outcomes between the two groups.
The 5-year rate of isolated axillary recurrence was 1.7% with axillary lymph node dissection (ALND) versus 1.1% without it. The 5-year rate of any invasive recurrence was 16% in the ALND arm and 19% in the no-dissection group.
The median age in the study was 48 years. The majority of patients (57%) had clinical T2 tumors. Most were HR positive and either HER2 negative (41%) or HER2 positive (28%).
Regional nodal radiation was more common in the ALND group, 82% versus 75%. The dissection arm had a mean of 2.8 sentinel lymph nodes removed versus 3.5 in the no-dissection group.
“The likelihood of finding additional positive lymph nodes in patients with residual ITCs after NAC is lower than in patients with residual micro- and macrometastases. In the majority of cases, they contain ITCs. Nodal recurrence after omission of ALND is rare in this population,” the investigators concluded in their abstract.
The work was funded by EUBREAST. Dr. Montagna doesn’t have any disclosures. Dr. Mittendorf has several industry ties, including being an advisor for Roche, AstraZeneca, and Moderna and a speaker for Merck.
SAN ANTONIO — Axillary lymph node dissection is the current standard of care in breast cancer when metastases are found in sentinel lymph nodes after neoadjuvant chemotherapy.
However, what to do when isolated tumor cells instead of outright metastases are found in sentinel nodes is an open question. Some surgeons opt for a full axillary dissection while others do not, and there is no standard of care, explained Giacomo Montagna, MD, a breast cancer surgeon at Memorial Sloan Kettering Cancer Center, New York City.
The study led and presented by Dr. Montagna at the San Antonio Breast Cancer Symposium brings some much-needed clarity to the issue.
The findings argue strongly against “routine axillary lymph node dissection” — with its considerable morbidities — “in patients with residual isolated tumor cells after neoadjuvant chemotherapy,” Dr. Montagna said.
Study discussant Elizabeth Mittendorf, MD, PhD, a breast cancer surgeon at Brigham and Women’s Hospital, Boston, agreed.
“It appears that the presence of isolated tumor cells in the sentinel nodes does not negatively impact oncologic outcomes. These additional data allow us to debunk some of the surgical dogma we grew up with, specifically that lymph node dissection is required for either survival or local control,” Dr. Mittendorf said.
However, there was concern among audience members that the information gleaned from a full dissection might still be needed to guide follow-on adjuvant therapy decisions.
Dr. Mittendorf didn’t think so. Although additional positive lymph nodes were found in almost a third of women who had axillary dissections in the review, the majority of involved nodes simply had more isolated tumor cells; macrometastases were found in just 5% of cases.
So, for most patients, additional information from axillary dissections is “unlikely needed to inform adjuvant therapy, and in fact,” based on the 5% figure, “we are thinking we would have to do well over a hundred lymph node dissections in such patients to inform treatment recommendations for fewer than five. This comes at the cost of fair morbidity,” she said.
Study details
The retrospective study, dubbed OPBC05/EUBREAST-14R/ICARO, included 583 women with cT1-4 N0-3 breast cancer treated at 62 centers in 18 countries. The majority of subjects were from the United States and Europe.
Every patient was found to have isolated tumor cells (ITCs) in their sentinel lymph nodes after neoadjuvant chemotherapy (NAC), which generally included anthracycline and taxane-based regimens. The majority of patients did not have a pathologic complete response to NAC.
Overall, 182 patients (31%) had a subsequent axillary lymph node dissection; the rest did not.
Dissections were more common in the presence of lymphovascular invasion and N2/N3 disease as well as when fewer lymph nodes were removed and when ITCs were found during surgery on frozen section, which was the case in a quarter of patients.
Additional positive nodes were found in 30% of patients in the dissection group and consisted of more nodes with ITCs in 18%, micrometastases in 7%, and macrometastases in 5%. Receptor status and nodal status at presentation did not have an impact on the likelihood of finding macrometastases.
The main finding of the study was that there were no statistically significant differences in recurrence outcomes between the two groups.
The 5-year rate of isolated axillary recurrence was 1.7% with axillary lymph node dissection (ALND) versus 1.1% without it. The 5-year rate of any invasive recurrence was 16% in the ALND arm and 19% in the no-dissection group.
The median age in the study was 48 years. The majority of patients (57%) had clinical T2 tumors. Most were HR positive and either HER2 negative (41%) or HER2 positive (28%).
Regional nodal radiation was more common in the ALND group, 82% versus 75%. The dissection arm had a mean of 2.8 sentinel lymph nodes removed versus 3.5 in the no-dissection group.
“The likelihood of finding additional positive lymph nodes in patients with residual ITCs after NAC is lower than in patients with residual micro- and macrometastases. In the majority of cases, they contain ITCs. Nodal recurrence after omission of ALND is rare in this population,” the investigators concluded in their abstract.
The work was funded by EUBREAST. Dr. Montagna doesn’t have any disclosures. Dr. Mittendorf has several industry ties, including being an advisor for Roche, AstraZeneca, and Moderna and a speaker for Merck.
AT SABCS 2023
Genetic Testing Is Recommended for Adult Patients With Epilepsy
ORLANDO — The epilepsy community has yet to come to a consensus on genetic testing. During a session at the annual meeting of the American Epilepsy Society (AES), researchers and clinicians convened to share their insights on genetic testing of adult patients with epilepsy.
Colin Ellis, MD, assistant professor of neurology at the Hospital of the University of Pennsylvania in Philadelphia, shared his clinical experience to explain the importance of genetic testing in adults patients despite access challenges, limited information on certain variants, and physician reticence.
“There’s a false misconception that genetic testing should only apply to children,” Dr. Ellis told the audience. “The earlier the onset of seizures, the more likely you are to find a genetic cause.”
Guidelines Differ
The International League Against Epilepsy Task Force for Clinical Genetic Testing, Development and Epileptic Encephalopathies (DEE) recommends conducting genetic testing in patients who have focal or generalized epilepsies to whom the following circumstances apply: autism or dysmorphism, familial history, or drug-resistant epilepsy.
However, the National Society of Genetic Counselors’ guidelines recommends genetic testing for patients who have any unexplained or idiopathic epilepsies.
Guidelines identify the patients who should get tested regardless of their age.
Personal Experience
Dr. Ellis, who has spent nearly 5 years running tests on patients with epilepsy, recently tested the 300th patient at his clinic. According to him, the yield is higher in focal epilepsy than in general epilepsy — an occurrence that counters what many believe.
“Focal epilepsies are more common than monogenic epilepsies but not intuitive to many people in the industry, despite being stated in the literature,” he said. “The absence of family history shouldn’t preclude you from genetic testing because it’s still possible to have a de novo variant not inherited from either parent.”
Genetic testing can be conducted by interrogating either the exome or the genome. However, cost remains a major barrier to access.
Dr. Ellis made several arguments supporting the use of genetic testing. First, genetic testing allows for a higher diagnostic yield (i.e., 24% versus 19% in panels and 9% in microarrays). Genetic testing provides a more comprehensive overview of a patient’s genetic landscape, and it can enhance the ability to identify certain epileptic conditions, such as those caused by monogenic epilepsy — a condition associated with 926 different genes.
“You’re also less likely to find variants of uncertain significance (VUS),” Dr. Ellis said. “Regardless, you should provide the lab with phenotype information because it will help them help you.”
Variants of Uncertain Significance
The National Human Genome Research Institute defines VUS as a variant found in a patient’s genome for which it remains unclear as to whether a health condition is causing the variant. Oftentimes, such variants have very little information available due to their rarity.
In order to resolve VUS, Dr. Ellis recommended family segregation. “If the VUS appears to be de novo, you should test the parent because if they carry the gene, then it’s probably not the cause,” he said.
Dr. Ellis outlined several steps in resolving VUS.
For starters, clinicians should determine the phenotypic fit and run some ancillary tests. For example, in the case of Glu 1 abnormalities, one should consider conducting a spinal tap to determine whether the patient has cerebral spinal fluid before taking additional action.
In addition, Dr. Ellis recommends defining variant characteristics, as it becomes important in determining whether it is appropriate to take action because the majority of variances are benign.
“The take-home point is that you should not act clinically on a VUS unless you know what you’re doing,” he said. “I also disagree with the belief that VUS are rare — it’s just that they cause so much anxiety because we’re uncomfortable with this kind of testing.”
ORLANDO — The epilepsy community has yet to come to a consensus on genetic testing. During a session at the annual meeting of the American Epilepsy Society (AES), researchers and clinicians convened to share their insights on genetic testing of adult patients with epilepsy.
Colin Ellis, MD, assistant professor of neurology at the Hospital of the University of Pennsylvania in Philadelphia, shared his clinical experience to explain the importance of genetic testing in adults patients despite access challenges, limited information on certain variants, and physician reticence.
“There’s a false misconception that genetic testing should only apply to children,” Dr. Ellis told the audience. “The earlier the onset of seizures, the more likely you are to find a genetic cause.”
Guidelines Differ
The International League Against Epilepsy Task Force for Clinical Genetic Testing, Development and Epileptic Encephalopathies (DEE) recommends conducting genetic testing in patients who have focal or generalized epilepsies to whom the following circumstances apply: autism or dysmorphism, familial history, or drug-resistant epilepsy.
However, the National Society of Genetic Counselors’ guidelines recommends genetic testing for patients who have any unexplained or idiopathic epilepsies.
Guidelines identify the patients who should get tested regardless of their age.
Personal Experience
Dr. Ellis, who has spent nearly 5 years running tests on patients with epilepsy, recently tested the 300th patient at his clinic. According to him, the yield is higher in focal epilepsy than in general epilepsy — an occurrence that counters what many believe.
“Focal epilepsies are more common than monogenic epilepsies but not intuitive to many people in the industry, despite being stated in the literature,” he said. “The absence of family history shouldn’t preclude you from genetic testing because it’s still possible to have a de novo variant not inherited from either parent.”
Genetic testing can be conducted by interrogating either the exome or the genome. However, cost remains a major barrier to access.
Dr. Ellis made several arguments supporting the use of genetic testing. First, genetic testing allows for a higher diagnostic yield (i.e., 24% versus 19% in panels and 9% in microarrays). Genetic testing provides a more comprehensive overview of a patient’s genetic landscape, and it can enhance the ability to identify certain epileptic conditions, such as those caused by monogenic epilepsy — a condition associated with 926 different genes.
“You’re also less likely to find variants of uncertain significance (VUS),” Dr. Ellis said. “Regardless, you should provide the lab with phenotype information because it will help them help you.”
Variants of Uncertain Significance
The National Human Genome Research Institute defines VUS as a variant found in a patient’s genome for which it remains unclear as to whether a health condition is causing the variant. Oftentimes, such variants have very little information available due to their rarity.
In order to resolve VUS, Dr. Ellis recommended family segregation. “If the VUS appears to be de novo, you should test the parent because if they carry the gene, then it’s probably not the cause,” he said.
Dr. Ellis outlined several steps in resolving VUS.
For starters, clinicians should determine the phenotypic fit and run some ancillary tests. For example, in the case of Glu 1 abnormalities, one should consider conducting a spinal tap to determine whether the patient has cerebral spinal fluid before taking additional action.
In addition, Dr. Ellis recommends defining variant characteristics, as it becomes important in determining whether it is appropriate to take action because the majority of variances are benign.
“The take-home point is that you should not act clinically on a VUS unless you know what you’re doing,” he said. “I also disagree with the belief that VUS are rare — it’s just that they cause so much anxiety because we’re uncomfortable with this kind of testing.”
ORLANDO — The epilepsy community has yet to come to a consensus on genetic testing. During a session at the annual meeting of the American Epilepsy Society (AES), researchers and clinicians convened to share their insights on genetic testing of adult patients with epilepsy.
Colin Ellis, MD, assistant professor of neurology at the Hospital of the University of Pennsylvania in Philadelphia, shared his clinical experience to explain the importance of genetic testing in adults patients despite access challenges, limited information on certain variants, and physician reticence.
“There’s a false misconception that genetic testing should only apply to children,” Dr. Ellis told the audience. “The earlier the onset of seizures, the more likely you are to find a genetic cause.”
Guidelines Differ
The International League Against Epilepsy Task Force for Clinical Genetic Testing, Development and Epileptic Encephalopathies (DEE) recommends conducting genetic testing in patients who have focal or generalized epilepsies to whom the following circumstances apply: autism or dysmorphism, familial history, or drug-resistant epilepsy.
However, the National Society of Genetic Counselors’ guidelines recommends genetic testing for patients who have any unexplained or idiopathic epilepsies.
Guidelines identify the patients who should get tested regardless of their age.
Personal Experience
Dr. Ellis, who has spent nearly 5 years running tests on patients with epilepsy, recently tested the 300th patient at his clinic. According to him, the yield is higher in focal epilepsy than in general epilepsy — an occurrence that counters what many believe.
“Focal epilepsies are more common than monogenic epilepsies but not intuitive to many people in the industry, despite being stated in the literature,” he said. “The absence of family history shouldn’t preclude you from genetic testing because it’s still possible to have a de novo variant not inherited from either parent.”
Genetic testing can be conducted by interrogating either the exome or the genome. However, cost remains a major barrier to access.
Dr. Ellis made several arguments supporting the use of genetic testing. First, genetic testing allows for a higher diagnostic yield (i.e., 24% versus 19% in panels and 9% in microarrays). Genetic testing provides a more comprehensive overview of a patient’s genetic landscape, and it can enhance the ability to identify certain epileptic conditions, such as those caused by monogenic epilepsy — a condition associated with 926 different genes.
“You’re also less likely to find variants of uncertain significance (VUS),” Dr. Ellis said. “Regardless, you should provide the lab with phenotype information because it will help them help you.”
Variants of Uncertain Significance
The National Human Genome Research Institute defines VUS as a variant found in a patient’s genome for which it remains unclear as to whether a health condition is causing the variant. Oftentimes, such variants have very little information available due to their rarity.
In order to resolve VUS, Dr. Ellis recommended family segregation. “If the VUS appears to be de novo, you should test the parent because if they carry the gene, then it’s probably not the cause,” he said.
Dr. Ellis outlined several steps in resolving VUS.
For starters, clinicians should determine the phenotypic fit and run some ancillary tests. For example, in the case of Glu 1 abnormalities, one should consider conducting a spinal tap to determine whether the patient has cerebral spinal fluid before taking additional action.
In addition, Dr. Ellis recommends defining variant characteristics, as it becomes important in determining whether it is appropriate to take action because the majority of variances are benign.
“The take-home point is that you should not act clinically on a VUS unless you know what you’re doing,” he said. “I also disagree with the belief that VUS are rare — it’s just that they cause so much anxiety because we’re uncomfortable with this kind of testing.”
FROM AES 2023
Gestational Diabetes Treatment Moves Forward With Uncertainty And Hope
FAIRFAX, VIRGINIA — , but researchers at the biennial meeting of the Diabetes in Pregnancy Study Group of North America expressed hope for more clarity in the near future and the ability to someday individualize treatment to account for what is increasingly viewed as a heterogeneous condition.
Until studies in 2015 and 2018 cast doubt on glyburide, “we used to have 80% [of our GDM patients] on glyburide, and 20% on insulin,” Maisa Feghali, MD, of the University of Pittsburgh, said during a discussion period. “Now we have 95% on insulin and 5% on oral hypoglycemics. I rely on insulin because I don’t have a better option, and I rely on research efforts [underway to provide better options]” in the future.
The American College of Obstetricians and Gynecologists recommends insulin as the preferred first-line pharmacologic therapy for GDM when pharmacologic therapy is needed, with metformin as an option when patients decline or cannot safely use insulin. Glyburide, ACOG said in its 2018 practice bulletin on GDM (Obstet Gynecol. 2018;131[2]:e49-64), should not be recommended as a first-line pharmacologic therapy.
The Society of Maternal-Fetal Medicine, on the other hand, has accepted metformin as a “reasonable and safe” first-line alternative to insulin — while recognizing that half of women will still require insulin to achieve glycemic control — and does not rule out consideration of glyburide. In its 2018 statement on the pharmacologic treatment of GDM, the society said that the evidence of benefit of one oral agent over another remains limited.
“When you have dueling guidelines, it means the data are not that clear,” George Saade, MD, professor and chair of obstetrics and gynecology at the Eastern Virginia School of Medicine, Norfolk, said in a presentation on GDM. An upcoming $12 million multicenter study to be led by the Ohio State University College of Medicine — coined the DECIDE trial — should provide clarity, he said.
The trial, funded by the Patient-Centered Outcomes Research Institute, which funds comparative clinical effectiveness research designed to be broadly applicable to practice, will enroll and randomize over 1500 pregnant individuals with GDM to either oral metformin or insulin and will follow mothers and children until 2 years after delivery.
The study’s primary and secondary hypotheses, respectively, are that metformin is not inferior to insulin in reducing a composite adverse neonatal outcome (large for gestational age, neonatal hypoglycemia and/or hyperbilirubemia) and that metformin does not result in increased child body mass index at 2 years, compared with insulin. It will also look at patient-reported factors associated with metformin use compared to insulin use — factors that “are important ... to enable clinical implementation of study findings,” said Dr. Saade, who played a role in designing the study over the past several years.
The study will take a pragmatic, real-world approach by ensuring racial and ethnic, socioeconomic, urban and rural, and geographic diversity at both large academic and community-based sites across the United States.
The trial, to be led by Mark Landon, MD, and Kartik Venkatesh, MD, PhD, of Ohio State University, will be the first large trial in the United States to both directly compare the ability of oral hypoglycemics and insulin to prevent GDM-associated pregnancy complications, and to follow children for 2 years, Dr. Saade said. “Prior research was either outside the United States, not randomized, not adequately powered, or had no long-term child follow-up,” he added after the meeting.
The State Of Knowledge About Oral Hypoglycemics
The trial was envisioned several years ago as a three-arm comparative trial including the sulfonylurea glyburide, but data published in recent years has increasingly “not favored” glyburide, and many providers “have stopped using it,” Dr. Saade said during and after the meeting. At this point, “it would not be useful to include it” in a pragmatic trial, he said.
Glyburide became the number one agent after a seminal trial published in 2000 (N Engl J Med. 2000;343:1134-8) showed equivalent glycemic control in about 400 women with GDM who were randomized to receive insulin or glyburide. While the trial was not powered to evaluate other outcomes, there were no significant differences in neonatal complications.
In 2015, a large retrospective population-based study (JAMA Pediatr. 2015;169[5]:452-8) of more than 9,000 women with GDM showed higher risks of neonatal intensive care admission, neonatal hypoglycemia, and large-for-gestational age with glyburide compared with insulin. “It prompted a pause in thinking,” Dr. Saade recalled at the DPSG meeting. After that, several meta-analyses/systematic reviews compared the two treatments, showing varying and sometimes conflicting degrees of difference in neonatal outcomes.
In 2018, a French noninferiority randomized controlled trial (JAMA 2018;319[17]:1773-80) did not show that glyburide is not inferior to insulin in the prevention of perinatal outcomes (macrosomia, neonatal hypoglycemia, and hyperbilirubinemia). “If you add this trial to the systematic reviews, it would probably would shift more in favor of insulin,” Dr. Saade said, noting that the trial’s supplementary data included a higher rate of maternal hypoglycemia with glyburide. “I feel personally now, with all the data, that glyburide is inferior to insulin.”
A 2021 network meta-analysis (BMC Endocr Disord. 2021;21:199) that looked at glycemic control and neonatal outcomes in GDM treated with glyburide, metformin, or insulin, also offers valuable insight, Dr. Saade said. The meta-analysis used a Bayesian framework and presents results as a ranking estimated probability of a treatment being the best or worst — or in between — for different outcomes (glycemic control and neonatal outcomes), which “is one of the best ways to look at data these days,” he said.
“It tells us how likely [it is for one agent] to be better than others. Will it work most of the time? More than 60% of the time?” Dr. Saade explained. For example, the analysis “tell us that for large for gestational age, glyburide has a 94% chance of being the worst, metformin has an 80% change of being the best, and insulin a 76% chance of being in between.”
Overall, the 2021 analysis suggests that “glyburide is the most likely to be worst in most outcomes and that there is equipoise between metformin and insulin,” he said.
Meta-analyses of pharmacologic treatment of GDM have been challenged, he said, by inconsistent reporting in trials of GDM diagnostic criteria, severity of hyperglycemia, and small sample sizes (and wide confidence intervals). Criteria for supplemental insulin are also often “unclear” in trials, Dr. Saade said, as is involvement of social determinants of health and the “care package” enveloping pharmacologic interventions.
Dr. Saade, Dr. Landon, and other researchers have also lamented over the years that there is limited long-term follow-up of exposed offspring.
The Challenge of Heterogeneity
In another presentation on GDM, Maisa Feghali, MD, MS, emphasized that GDM is a heterogeneous condition, with clinical hyperglycemia not capturing individual variation in underlying physiologic processes. A 2016 study (Diabetes Care. 2016;39[6]:1052-5) assessing insulin sensitivity and secretion in 800-plus women at 24-30 weeks’ gestation found that about 50% of those with GDM had predominant insulin resistance, 30% had predominant insulin secretion deficit, and 20% were mixed.
Those with predominant insulin resistance had higher BMI, higher fasting glucose, larger infants, and greater risk of GDM-associated adverse outcomes, “suggesting that the risk is not universal or equivalent,” said Dr. Feghali, assistant professor in the department of obstetrics, gynecology and reproductive sciences at the University of Pittsburgh and the UPCM Magee-Women’s Hospital.
A 2019 multicenter European study (Diabetologia. 2019;62[11]:2118-28) found an even higher proportion of GDM involving predominant insulin resistance and, similarly, a greater risk of adverse pregnancy outcomes in these women than in insulin-sensitive women with GDM, “again suggesting that there’s probably some benefit to looking deeper at physiology to understand individual risk,” she said.
Research published decades ago showed that insulin sensitivity decreases by over 50% during pregnancy, and “what we’ve come to recognize is there [can be] insulin secretion deficiency that’s not able to surmount or overcome the insulin resistance that develops during advanced gestation,” she said. “We need to think not at the population level but at the individual level.”
Dr. Feghali is leading the MATCh-GDM (Metabolic Analysis for Treatment Choice in GDM) study, which has been randomizing women to receive either usual, unmatched treatment or treatment matched to GDM mechanism — metformin for predominant insulin resistance, glyburide, or insulin for predominant insulin secretion defects, and one of the three for combined mechanisms. Data are not available yet.
There is still more to be learned about the pharmacologic effects of oral hypoglycemics, she noted, pointing to a 2020 study (Clin Pharmacol Ther. 2020;107[6]:1362-72) that randomized women to glyburide, metformin, or glyburide/metformin combination therapy and measured insulin sensitivity, beta-cell responsivity, and disposition index. (The latter describes the overall metabolic state and is a product of insulin sensitivity and total beta-cell responsivity.)
“Somewhat surprisingly, they found metformin performed better than glyburide,” shifting the overall disposition index closer to normal, Dr. Feghali said. “But not surprisingly, they found the combination worked best.”
Total beta-cell responsivity occurred in 56% of the glyburide group and 74% of the combination group. Improvements in insulin sensitivity occurred in 84% of the metformin group and 74% of the combination group. Surprisingly, there was “a decrease in first-phase insulin secretion” with glyburide, noted Dr. Feghali — a finding that means “the glyburide story has turned out to be a little more complicated.” With metformin, there was a positive change in insulin secretion as well as insulin sensitivity.
The authors’ conclusion, she noted, “is that there’s potential in thinking about metformin first, as the primary treatment, and then adding glyburide after that.”
Future Use Of Incretin Mimetics, and Intensive Targets in Overweight/Obesity
Dr. Feghali wonders whether incretin hormone mimetics — such as glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) — could play a future role in GDM treatment, helping to increase insulin secretion.
She is currently recruiting for a pilot study on the pharmacokinetics and pharmacodynamics in GDM of exenatide, a FDA-approved GLP-1 agonist that has been shown not to cross the placenta and that should, research suggests, lower the risk of maternal hypoglycemia and limit the risk of excessive fetal growth, “overcoming some of the concerns we have with glyburide,” Dr. Feghali said.
A recent study of the gut-generated incretin response during an oral glucose tolerance test in pregnant women with and without GDM showed that post-load GLP-1 and GIP were higher in women with GDM, and that the GLP-1 secretion was associated with insulin secretion only in those with GDM (J Clin Endocrinol Metab. 2022;107(6):e2425-30). “In those with normal OGTT, insulin secretion was independent of GLP-1,” she said. “This study suggests there’s a potential role for incretin mimetics in GDM.”
Also regarding the individualization of GDM treatment, patients who are overweight or obese in the prepregnancy setting and have gestational diabetes represent a different phenotype, she noted, with higher fasting and postprandial blood glucose compared to normal-weight counterparts despite higher doses of medication.
“After controlling for gestational weight gain and glycemic control, we see there’s an independent effect of prepregnancy obesity specifically for an increased risk of macrosomia, preterm birth, and hypertensive disorders of pregnancy,” said Dr. Feghali, referring to a 2015 retrospective study of GDM and obesity (Obstet Gynecol. 2015;126:316-25). “It suggests that we might think about redrawing the line, not on diagnosis and screening but on treatment.”
The randomized, controlled Intensive Glycemic Targets in Overweight and Obese Women with Gestational Diabetes Mellitus (iGDM) trial, is now recruiting at multiple centers, including at Dr. Feghali’s University of Pittsburgh, and will investigate the effect of intensive glycemic targets (fasting < 90 mg/dL, 1-hour postprandial < 120 mg/dL) versus standard glycemic targets (fasting < 95 mg/dL, 1-hour postprandial < 140 mg/dL), she said.
In another presentation on GDM, Monica Longo, MD, PhD, of the Inova Health System in Fairfax, Va., said researchers are also looking at whether nutritional supplements such as myo-inositol can reduce the risk of adverse pregnancy outcomes in GDM, and whether probiotics can improve insulin sensitivity in some patients.
Data on newer insulin analogs in pregnancy are lacking, she noted. “Preliminary data has shown no malformations in infants, but there is some increase in hypoglycemia-related admissions to the NICU,” she said. “It’s worth it [to research more].”
FAIRFAX, VIRGINIA — , but researchers at the biennial meeting of the Diabetes in Pregnancy Study Group of North America expressed hope for more clarity in the near future and the ability to someday individualize treatment to account for what is increasingly viewed as a heterogeneous condition.
Until studies in 2015 and 2018 cast doubt on glyburide, “we used to have 80% [of our GDM patients] on glyburide, and 20% on insulin,” Maisa Feghali, MD, of the University of Pittsburgh, said during a discussion period. “Now we have 95% on insulin and 5% on oral hypoglycemics. I rely on insulin because I don’t have a better option, and I rely on research efforts [underway to provide better options]” in the future.
The American College of Obstetricians and Gynecologists recommends insulin as the preferred first-line pharmacologic therapy for GDM when pharmacologic therapy is needed, with metformin as an option when patients decline or cannot safely use insulin. Glyburide, ACOG said in its 2018 practice bulletin on GDM (Obstet Gynecol. 2018;131[2]:e49-64), should not be recommended as a first-line pharmacologic therapy.
The Society of Maternal-Fetal Medicine, on the other hand, has accepted metformin as a “reasonable and safe” first-line alternative to insulin — while recognizing that half of women will still require insulin to achieve glycemic control — and does not rule out consideration of glyburide. In its 2018 statement on the pharmacologic treatment of GDM, the society said that the evidence of benefit of one oral agent over another remains limited.
“When you have dueling guidelines, it means the data are not that clear,” George Saade, MD, professor and chair of obstetrics and gynecology at the Eastern Virginia School of Medicine, Norfolk, said in a presentation on GDM. An upcoming $12 million multicenter study to be led by the Ohio State University College of Medicine — coined the DECIDE trial — should provide clarity, he said.
The trial, funded by the Patient-Centered Outcomes Research Institute, which funds comparative clinical effectiveness research designed to be broadly applicable to practice, will enroll and randomize over 1500 pregnant individuals with GDM to either oral metformin or insulin and will follow mothers and children until 2 years after delivery.
The study’s primary and secondary hypotheses, respectively, are that metformin is not inferior to insulin in reducing a composite adverse neonatal outcome (large for gestational age, neonatal hypoglycemia and/or hyperbilirubemia) and that metformin does not result in increased child body mass index at 2 years, compared with insulin. It will also look at patient-reported factors associated with metformin use compared to insulin use — factors that “are important ... to enable clinical implementation of study findings,” said Dr. Saade, who played a role in designing the study over the past several years.
The study will take a pragmatic, real-world approach by ensuring racial and ethnic, socioeconomic, urban and rural, and geographic diversity at both large academic and community-based sites across the United States.
The trial, to be led by Mark Landon, MD, and Kartik Venkatesh, MD, PhD, of Ohio State University, will be the first large trial in the United States to both directly compare the ability of oral hypoglycemics and insulin to prevent GDM-associated pregnancy complications, and to follow children for 2 years, Dr. Saade said. “Prior research was either outside the United States, not randomized, not adequately powered, or had no long-term child follow-up,” he added after the meeting.
The State Of Knowledge About Oral Hypoglycemics
The trial was envisioned several years ago as a three-arm comparative trial including the sulfonylurea glyburide, but data published in recent years has increasingly “not favored” glyburide, and many providers “have stopped using it,” Dr. Saade said during and after the meeting. At this point, “it would not be useful to include it” in a pragmatic trial, he said.
Glyburide became the number one agent after a seminal trial published in 2000 (N Engl J Med. 2000;343:1134-8) showed equivalent glycemic control in about 400 women with GDM who were randomized to receive insulin or glyburide. While the trial was not powered to evaluate other outcomes, there were no significant differences in neonatal complications.
In 2015, a large retrospective population-based study (JAMA Pediatr. 2015;169[5]:452-8) of more than 9,000 women with GDM showed higher risks of neonatal intensive care admission, neonatal hypoglycemia, and large-for-gestational age with glyburide compared with insulin. “It prompted a pause in thinking,” Dr. Saade recalled at the DPSG meeting. After that, several meta-analyses/systematic reviews compared the two treatments, showing varying and sometimes conflicting degrees of difference in neonatal outcomes.
In 2018, a French noninferiority randomized controlled trial (JAMA 2018;319[17]:1773-80) did not show that glyburide is not inferior to insulin in the prevention of perinatal outcomes (macrosomia, neonatal hypoglycemia, and hyperbilirubinemia). “If you add this trial to the systematic reviews, it would probably would shift more in favor of insulin,” Dr. Saade said, noting that the trial’s supplementary data included a higher rate of maternal hypoglycemia with glyburide. “I feel personally now, with all the data, that glyburide is inferior to insulin.”
A 2021 network meta-analysis (BMC Endocr Disord. 2021;21:199) that looked at glycemic control and neonatal outcomes in GDM treated with glyburide, metformin, or insulin, also offers valuable insight, Dr. Saade said. The meta-analysis used a Bayesian framework and presents results as a ranking estimated probability of a treatment being the best or worst — or in between — for different outcomes (glycemic control and neonatal outcomes), which “is one of the best ways to look at data these days,” he said.
“It tells us how likely [it is for one agent] to be better than others. Will it work most of the time? More than 60% of the time?” Dr. Saade explained. For example, the analysis “tell us that for large for gestational age, glyburide has a 94% chance of being the worst, metformin has an 80% change of being the best, and insulin a 76% chance of being in between.”
Overall, the 2021 analysis suggests that “glyburide is the most likely to be worst in most outcomes and that there is equipoise between metformin and insulin,” he said.
Meta-analyses of pharmacologic treatment of GDM have been challenged, he said, by inconsistent reporting in trials of GDM diagnostic criteria, severity of hyperglycemia, and small sample sizes (and wide confidence intervals). Criteria for supplemental insulin are also often “unclear” in trials, Dr. Saade said, as is involvement of social determinants of health and the “care package” enveloping pharmacologic interventions.
Dr. Saade, Dr. Landon, and other researchers have also lamented over the years that there is limited long-term follow-up of exposed offspring.
The Challenge of Heterogeneity
In another presentation on GDM, Maisa Feghali, MD, MS, emphasized that GDM is a heterogeneous condition, with clinical hyperglycemia not capturing individual variation in underlying physiologic processes. A 2016 study (Diabetes Care. 2016;39[6]:1052-5) assessing insulin sensitivity and secretion in 800-plus women at 24-30 weeks’ gestation found that about 50% of those with GDM had predominant insulin resistance, 30% had predominant insulin secretion deficit, and 20% were mixed.
Those with predominant insulin resistance had higher BMI, higher fasting glucose, larger infants, and greater risk of GDM-associated adverse outcomes, “suggesting that the risk is not universal or equivalent,” said Dr. Feghali, assistant professor in the department of obstetrics, gynecology and reproductive sciences at the University of Pittsburgh and the UPCM Magee-Women’s Hospital.
A 2019 multicenter European study (Diabetologia. 2019;62[11]:2118-28) found an even higher proportion of GDM involving predominant insulin resistance and, similarly, a greater risk of adverse pregnancy outcomes in these women than in insulin-sensitive women with GDM, “again suggesting that there’s probably some benefit to looking deeper at physiology to understand individual risk,” she said.
Research published decades ago showed that insulin sensitivity decreases by over 50% during pregnancy, and “what we’ve come to recognize is there [can be] insulin secretion deficiency that’s not able to surmount or overcome the insulin resistance that develops during advanced gestation,” she said. “We need to think not at the population level but at the individual level.”
Dr. Feghali is leading the MATCh-GDM (Metabolic Analysis for Treatment Choice in GDM) study, which has been randomizing women to receive either usual, unmatched treatment or treatment matched to GDM mechanism — metformin for predominant insulin resistance, glyburide, or insulin for predominant insulin secretion defects, and one of the three for combined mechanisms. Data are not available yet.
There is still more to be learned about the pharmacologic effects of oral hypoglycemics, she noted, pointing to a 2020 study (Clin Pharmacol Ther. 2020;107[6]:1362-72) that randomized women to glyburide, metformin, or glyburide/metformin combination therapy and measured insulin sensitivity, beta-cell responsivity, and disposition index. (The latter describes the overall metabolic state and is a product of insulin sensitivity and total beta-cell responsivity.)
“Somewhat surprisingly, they found metformin performed better than glyburide,” shifting the overall disposition index closer to normal, Dr. Feghali said. “But not surprisingly, they found the combination worked best.”
Total beta-cell responsivity occurred in 56% of the glyburide group and 74% of the combination group. Improvements in insulin sensitivity occurred in 84% of the metformin group and 74% of the combination group. Surprisingly, there was “a decrease in first-phase insulin secretion” with glyburide, noted Dr. Feghali — a finding that means “the glyburide story has turned out to be a little more complicated.” With metformin, there was a positive change in insulin secretion as well as insulin sensitivity.
The authors’ conclusion, she noted, “is that there’s potential in thinking about metformin first, as the primary treatment, and then adding glyburide after that.”
Future Use Of Incretin Mimetics, and Intensive Targets in Overweight/Obesity
Dr. Feghali wonders whether incretin hormone mimetics — such as glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) — could play a future role in GDM treatment, helping to increase insulin secretion.
She is currently recruiting for a pilot study on the pharmacokinetics and pharmacodynamics in GDM of exenatide, a FDA-approved GLP-1 agonist that has been shown not to cross the placenta and that should, research suggests, lower the risk of maternal hypoglycemia and limit the risk of excessive fetal growth, “overcoming some of the concerns we have with glyburide,” Dr. Feghali said.
A recent study of the gut-generated incretin response during an oral glucose tolerance test in pregnant women with and without GDM showed that post-load GLP-1 and GIP were higher in women with GDM, and that the GLP-1 secretion was associated with insulin secretion only in those with GDM (J Clin Endocrinol Metab. 2022;107(6):e2425-30). “In those with normal OGTT, insulin secretion was independent of GLP-1,” she said. “This study suggests there’s a potential role for incretin mimetics in GDM.”
Also regarding the individualization of GDM treatment, patients who are overweight or obese in the prepregnancy setting and have gestational diabetes represent a different phenotype, she noted, with higher fasting and postprandial blood glucose compared to normal-weight counterparts despite higher doses of medication.
“After controlling for gestational weight gain and glycemic control, we see there’s an independent effect of prepregnancy obesity specifically for an increased risk of macrosomia, preterm birth, and hypertensive disorders of pregnancy,” said Dr. Feghali, referring to a 2015 retrospective study of GDM and obesity (Obstet Gynecol. 2015;126:316-25). “It suggests that we might think about redrawing the line, not on diagnosis and screening but on treatment.”
The randomized, controlled Intensive Glycemic Targets in Overweight and Obese Women with Gestational Diabetes Mellitus (iGDM) trial, is now recruiting at multiple centers, including at Dr. Feghali’s University of Pittsburgh, and will investigate the effect of intensive glycemic targets (fasting < 90 mg/dL, 1-hour postprandial < 120 mg/dL) versus standard glycemic targets (fasting < 95 mg/dL, 1-hour postprandial < 140 mg/dL), she said.
In another presentation on GDM, Monica Longo, MD, PhD, of the Inova Health System in Fairfax, Va., said researchers are also looking at whether nutritional supplements such as myo-inositol can reduce the risk of adverse pregnancy outcomes in GDM, and whether probiotics can improve insulin sensitivity in some patients.
Data on newer insulin analogs in pregnancy are lacking, she noted. “Preliminary data has shown no malformations in infants, but there is some increase in hypoglycemia-related admissions to the NICU,” she said. “It’s worth it [to research more].”
FAIRFAX, VIRGINIA — , but researchers at the biennial meeting of the Diabetes in Pregnancy Study Group of North America expressed hope for more clarity in the near future and the ability to someday individualize treatment to account for what is increasingly viewed as a heterogeneous condition.
Until studies in 2015 and 2018 cast doubt on glyburide, “we used to have 80% [of our GDM patients] on glyburide, and 20% on insulin,” Maisa Feghali, MD, of the University of Pittsburgh, said during a discussion period. “Now we have 95% on insulin and 5% on oral hypoglycemics. I rely on insulin because I don’t have a better option, and I rely on research efforts [underway to provide better options]” in the future.
The American College of Obstetricians and Gynecologists recommends insulin as the preferred first-line pharmacologic therapy for GDM when pharmacologic therapy is needed, with metformin as an option when patients decline or cannot safely use insulin. Glyburide, ACOG said in its 2018 practice bulletin on GDM (Obstet Gynecol. 2018;131[2]:e49-64), should not be recommended as a first-line pharmacologic therapy.
The Society of Maternal-Fetal Medicine, on the other hand, has accepted metformin as a “reasonable and safe” first-line alternative to insulin — while recognizing that half of women will still require insulin to achieve glycemic control — and does not rule out consideration of glyburide. In its 2018 statement on the pharmacologic treatment of GDM, the society said that the evidence of benefit of one oral agent over another remains limited.
“When you have dueling guidelines, it means the data are not that clear,” George Saade, MD, professor and chair of obstetrics and gynecology at the Eastern Virginia School of Medicine, Norfolk, said in a presentation on GDM. An upcoming $12 million multicenter study to be led by the Ohio State University College of Medicine — coined the DECIDE trial — should provide clarity, he said.
The trial, funded by the Patient-Centered Outcomes Research Institute, which funds comparative clinical effectiveness research designed to be broadly applicable to practice, will enroll and randomize over 1500 pregnant individuals with GDM to either oral metformin or insulin and will follow mothers and children until 2 years after delivery.
The study’s primary and secondary hypotheses, respectively, are that metformin is not inferior to insulin in reducing a composite adverse neonatal outcome (large for gestational age, neonatal hypoglycemia and/or hyperbilirubemia) and that metformin does not result in increased child body mass index at 2 years, compared with insulin. It will also look at patient-reported factors associated with metformin use compared to insulin use — factors that “are important ... to enable clinical implementation of study findings,” said Dr. Saade, who played a role in designing the study over the past several years.
The study will take a pragmatic, real-world approach by ensuring racial and ethnic, socioeconomic, urban and rural, and geographic diversity at both large academic and community-based sites across the United States.
The trial, to be led by Mark Landon, MD, and Kartik Venkatesh, MD, PhD, of Ohio State University, will be the first large trial in the United States to both directly compare the ability of oral hypoglycemics and insulin to prevent GDM-associated pregnancy complications, and to follow children for 2 years, Dr. Saade said. “Prior research was either outside the United States, not randomized, not adequately powered, or had no long-term child follow-up,” he added after the meeting.
The State Of Knowledge About Oral Hypoglycemics
The trial was envisioned several years ago as a three-arm comparative trial including the sulfonylurea glyburide, but data published in recent years has increasingly “not favored” glyburide, and many providers “have stopped using it,” Dr. Saade said during and after the meeting. At this point, “it would not be useful to include it” in a pragmatic trial, he said.
Glyburide became the number one agent after a seminal trial published in 2000 (N Engl J Med. 2000;343:1134-8) showed equivalent glycemic control in about 400 women with GDM who were randomized to receive insulin or glyburide. While the trial was not powered to evaluate other outcomes, there were no significant differences in neonatal complications.
In 2015, a large retrospective population-based study (JAMA Pediatr. 2015;169[5]:452-8) of more than 9,000 women with GDM showed higher risks of neonatal intensive care admission, neonatal hypoglycemia, and large-for-gestational age with glyburide compared with insulin. “It prompted a pause in thinking,” Dr. Saade recalled at the DPSG meeting. After that, several meta-analyses/systematic reviews compared the two treatments, showing varying and sometimes conflicting degrees of difference in neonatal outcomes.
In 2018, a French noninferiority randomized controlled trial (JAMA 2018;319[17]:1773-80) did not show that glyburide is not inferior to insulin in the prevention of perinatal outcomes (macrosomia, neonatal hypoglycemia, and hyperbilirubinemia). “If you add this trial to the systematic reviews, it would probably would shift more in favor of insulin,” Dr. Saade said, noting that the trial’s supplementary data included a higher rate of maternal hypoglycemia with glyburide. “I feel personally now, with all the data, that glyburide is inferior to insulin.”
A 2021 network meta-analysis (BMC Endocr Disord. 2021;21:199) that looked at glycemic control and neonatal outcomes in GDM treated with glyburide, metformin, or insulin, also offers valuable insight, Dr. Saade said. The meta-analysis used a Bayesian framework and presents results as a ranking estimated probability of a treatment being the best or worst — or in between — for different outcomes (glycemic control and neonatal outcomes), which “is one of the best ways to look at data these days,” he said.
“It tells us how likely [it is for one agent] to be better than others. Will it work most of the time? More than 60% of the time?” Dr. Saade explained. For example, the analysis “tell us that for large for gestational age, glyburide has a 94% chance of being the worst, metformin has an 80% change of being the best, and insulin a 76% chance of being in between.”
Overall, the 2021 analysis suggests that “glyburide is the most likely to be worst in most outcomes and that there is equipoise between metformin and insulin,” he said.
Meta-analyses of pharmacologic treatment of GDM have been challenged, he said, by inconsistent reporting in trials of GDM diagnostic criteria, severity of hyperglycemia, and small sample sizes (and wide confidence intervals). Criteria for supplemental insulin are also often “unclear” in trials, Dr. Saade said, as is involvement of social determinants of health and the “care package” enveloping pharmacologic interventions.
Dr. Saade, Dr. Landon, and other researchers have also lamented over the years that there is limited long-term follow-up of exposed offspring.
The Challenge of Heterogeneity
In another presentation on GDM, Maisa Feghali, MD, MS, emphasized that GDM is a heterogeneous condition, with clinical hyperglycemia not capturing individual variation in underlying physiologic processes. A 2016 study (Diabetes Care. 2016;39[6]:1052-5) assessing insulin sensitivity and secretion in 800-plus women at 24-30 weeks’ gestation found that about 50% of those with GDM had predominant insulin resistance, 30% had predominant insulin secretion deficit, and 20% were mixed.
Those with predominant insulin resistance had higher BMI, higher fasting glucose, larger infants, and greater risk of GDM-associated adverse outcomes, “suggesting that the risk is not universal or equivalent,” said Dr. Feghali, assistant professor in the department of obstetrics, gynecology and reproductive sciences at the University of Pittsburgh and the UPCM Magee-Women’s Hospital.
A 2019 multicenter European study (Diabetologia. 2019;62[11]:2118-28) found an even higher proportion of GDM involving predominant insulin resistance and, similarly, a greater risk of adverse pregnancy outcomes in these women than in insulin-sensitive women with GDM, “again suggesting that there’s probably some benefit to looking deeper at physiology to understand individual risk,” she said.
Research published decades ago showed that insulin sensitivity decreases by over 50% during pregnancy, and “what we’ve come to recognize is there [can be] insulin secretion deficiency that’s not able to surmount or overcome the insulin resistance that develops during advanced gestation,” she said. “We need to think not at the population level but at the individual level.”
Dr. Feghali is leading the MATCh-GDM (Metabolic Analysis for Treatment Choice in GDM) study, which has been randomizing women to receive either usual, unmatched treatment or treatment matched to GDM mechanism — metformin for predominant insulin resistance, glyburide, or insulin for predominant insulin secretion defects, and one of the three for combined mechanisms. Data are not available yet.
There is still more to be learned about the pharmacologic effects of oral hypoglycemics, she noted, pointing to a 2020 study (Clin Pharmacol Ther. 2020;107[6]:1362-72) that randomized women to glyburide, metformin, or glyburide/metformin combination therapy and measured insulin sensitivity, beta-cell responsivity, and disposition index. (The latter describes the overall metabolic state and is a product of insulin sensitivity and total beta-cell responsivity.)
“Somewhat surprisingly, they found metformin performed better than glyburide,” shifting the overall disposition index closer to normal, Dr. Feghali said. “But not surprisingly, they found the combination worked best.”
Total beta-cell responsivity occurred in 56% of the glyburide group and 74% of the combination group. Improvements in insulin sensitivity occurred in 84% of the metformin group and 74% of the combination group. Surprisingly, there was “a decrease in first-phase insulin secretion” with glyburide, noted Dr. Feghali — a finding that means “the glyburide story has turned out to be a little more complicated.” With metformin, there was a positive change in insulin secretion as well as insulin sensitivity.
The authors’ conclusion, she noted, “is that there’s potential in thinking about metformin first, as the primary treatment, and then adding glyburide after that.”
Future Use Of Incretin Mimetics, and Intensive Targets in Overweight/Obesity
Dr. Feghali wonders whether incretin hormone mimetics — such as glucagonlike peptide–1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) — could play a future role in GDM treatment, helping to increase insulin secretion.
She is currently recruiting for a pilot study on the pharmacokinetics and pharmacodynamics in GDM of exenatide, a FDA-approved GLP-1 agonist that has been shown not to cross the placenta and that should, research suggests, lower the risk of maternal hypoglycemia and limit the risk of excessive fetal growth, “overcoming some of the concerns we have with glyburide,” Dr. Feghali said.
A recent study of the gut-generated incretin response during an oral glucose tolerance test in pregnant women with and without GDM showed that post-load GLP-1 and GIP were higher in women with GDM, and that the GLP-1 secretion was associated with insulin secretion only in those with GDM (J Clin Endocrinol Metab. 2022;107(6):e2425-30). “In those with normal OGTT, insulin secretion was independent of GLP-1,” she said. “This study suggests there’s a potential role for incretin mimetics in GDM.”
Also regarding the individualization of GDM treatment, patients who are overweight or obese in the prepregnancy setting and have gestational diabetes represent a different phenotype, she noted, with higher fasting and postprandial blood glucose compared to normal-weight counterparts despite higher doses of medication.
“After controlling for gestational weight gain and glycemic control, we see there’s an independent effect of prepregnancy obesity specifically for an increased risk of macrosomia, preterm birth, and hypertensive disorders of pregnancy,” said Dr. Feghali, referring to a 2015 retrospective study of GDM and obesity (Obstet Gynecol. 2015;126:316-25). “It suggests that we might think about redrawing the line, not on diagnosis and screening but on treatment.”
The randomized, controlled Intensive Glycemic Targets in Overweight and Obese Women with Gestational Diabetes Mellitus (iGDM) trial, is now recruiting at multiple centers, including at Dr. Feghali’s University of Pittsburgh, and will investigate the effect of intensive glycemic targets (fasting < 90 mg/dL, 1-hour postprandial < 120 mg/dL) versus standard glycemic targets (fasting < 95 mg/dL, 1-hour postprandial < 140 mg/dL), she said.
In another presentation on GDM, Monica Longo, MD, PhD, of the Inova Health System in Fairfax, Va., said researchers are also looking at whether nutritional supplements such as myo-inositol can reduce the risk of adverse pregnancy outcomes in GDM, and whether probiotics can improve insulin sensitivity in some patients.
Data on newer insulin analogs in pregnancy are lacking, she noted. “Preliminary data has shown no malformations in infants, but there is some increase in hypoglycemia-related admissions to the NICU,” she said. “It’s worth it [to research more].”
FROM DPSG-NA 2023
Temporary Higher Stroke Rate After TAVR
TOPLINE:
Patients undergoing transcatheter aortic valve replacement (TAVR) have a higher risk for stroke for up to 2 years compared with an age- and sex-matched population, after which their risks are comparable, results of a large Swiss registry study suggest.
METHODOLOGY:
- The study included 11,957 patients from the prospective SwissTAVI Registry, an ongoing mandatory cohort study enrolling consecutive patients undergoing TAVR in Switzerland.
- The study population, which had a mean age of 81.8 years and mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) of 4.62, with 11.8% having a history of cerebrovascular accident (CVA) and 32.3% a history of atrial fibrillation, underwent TAVR at 15 centers between February 2011 and June 2021.
- The primary outcome was the incidence of stroke, with secondary outcomes including the incidence of CVA, a composite of stroke and transient ischemic attack (TIA).
- Researchers calculated standardized stroke ratios (SSRs) and compared stroke trends in patients undergoing TAVR with those of an age- and sex-matched general population in Switzerland derived from the 2019 Global Burden of Disease (GBD) study.
TAKEAWAY:
- , accounting for 69% of these events.
- After excluding 30-day events, the 1-year incidence rates of CVA and stroke were 1.7% and 1.4%, respectively, followed by an annual stroke incidence of 1.2%, 0.8%, 0.9%, and 0.7% in the second, third, fourth, and fifth years post TAVR, respectively.
- Only increased age and moderate/severe paravalvular leakage (PVL) at discharge were associated with an increased risk for early stroke (up to 30 days post TAVR), whereas dyslipidemia and history of atrial fibrillation and of CVA were associated with an increased risk for late stroke (30 days to 5 years after TAVR).
- SSR in the study population returned to a level comparable to that expected in the general Swiss population after 2 years and through to 5 years post-TAVR.
IN PRACTICE:
Although the study results “are reassuring” with respect to stroke risk beyond 2 years post TAVR, “our findings underscore the continued efforts of stroke-prevention measures” early and longer term, wrote the authors.
In an accompanying editorial, Lauge Østergaard, MD, PhD, Department of Cardiology, University of Copenhagen, Denmark, noted the study suggests reduced PVL could lower the risk for early stroke following TAVR and “highlights how assessment of usual risk factors (dyslipidemia and atrial fibrillation) could help reduce the burden of stroke in the long term.”
SOURCE:
The study was carried out by Taishi Okuno, MD, Department of Cardiology, Bern University Hospital, University of Bern, Switzerland, and colleagues. It was published online in the Journal of the American College of Cardiology (JACC): Cardiovascular Interventions.
LIMITATIONS:
The study couldn’t investigate the association between antithrombotic regimens and the risk for CVA. Definitions of CVA in the SwissTAVI Registry might differ from those used in the GBD study from which the matched population data were derived. The general population wasn’t matched on comorbidities usually associated with elevated stroke risk, which may have led to underestimation of stroke. As the mean age in the study was 82 years, results may not be extrapolated to a younger population.
DISCLOSURES:
The SwissTAVI registry is supported by the Swiss Heart Foundation, Swiss Working Group of Interventional Cardiology and Acute Coronary Syndromes, Medtronic, Edwards Lifesciences, Boston Scientific/Symetis, JenaValve, and St. Jude Medical. Dr. Okuno has no relevant conflicts of interest; see paper for disclosures of other study authors. Dr. Østergaard has received an independent research grant from the Novo Nordisk Foundation.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients undergoing transcatheter aortic valve replacement (TAVR) have a higher risk for stroke for up to 2 years compared with an age- and sex-matched population, after which their risks are comparable, results of a large Swiss registry study suggest.
METHODOLOGY:
- The study included 11,957 patients from the prospective SwissTAVI Registry, an ongoing mandatory cohort study enrolling consecutive patients undergoing TAVR in Switzerland.
- The study population, which had a mean age of 81.8 years and mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) of 4.62, with 11.8% having a history of cerebrovascular accident (CVA) and 32.3% a history of atrial fibrillation, underwent TAVR at 15 centers between February 2011 and June 2021.
- The primary outcome was the incidence of stroke, with secondary outcomes including the incidence of CVA, a composite of stroke and transient ischemic attack (TIA).
- Researchers calculated standardized stroke ratios (SSRs) and compared stroke trends in patients undergoing TAVR with those of an age- and sex-matched general population in Switzerland derived from the 2019 Global Burden of Disease (GBD) study.
TAKEAWAY:
- , accounting for 69% of these events.
- After excluding 30-day events, the 1-year incidence rates of CVA and stroke were 1.7% and 1.4%, respectively, followed by an annual stroke incidence of 1.2%, 0.8%, 0.9%, and 0.7% in the second, third, fourth, and fifth years post TAVR, respectively.
- Only increased age and moderate/severe paravalvular leakage (PVL) at discharge were associated with an increased risk for early stroke (up to 30 days post TAVR), whereas dyslipidemia and history of atrial fibrillation and of CVA were associated with an increased risk for late stroke (30 days to 5 years after TAVR).
- SSR in the study population returned to a level comparable to that expected in the general Swiss population after 2 years and through to 5 years post-TAVR.
IN PRACTICE:
Although the study results “are reassuring” with respect to stroke risk beyond 2 years post TAVR, “our findings underscore the continued efforts of stroke-prevention measures” early and longer term, wrote the authors.
In an accompanying editorial, Lauge Østergaard, MD, PhD, Department of Cardiology, University of Copenhagen, Denmark, noted the study suggests reduced PVL could lower the risk for early stroke following TAVR and “highlights how assessment of usual risk factors (dyslipidemia and atrial fibrillation) could help reduce the burden of stroke in the long term.”
SOURCE:
The study was carried out by Taishi Okuno, MD, Department of Cardiology, Bern University Hospital, University of Bern, Switzerland, and colleagues. It was published online in the Journal of the American College of Cardiology (JACC): Cardiovascular Interventions.
LIMITATIONS:
The study couldn’t investigate the association between antithrombotic regimens and the risk for CVA. Definitions of CVA in the SwissTAVI Registry might differ from those used in the GBD study from which the matched population data were derived. The general population wasn’t matched on comorbidities usually associated with elevated stroke risk, which may have led to underestimation of stroke. As the mean age in the study was 82 years, results may not be extrapolated to a younger population.
DISCLOSURES:
The SwissTAVI registry is supported by the Swiss Heart Foundation, Swiss Working Group of Interventional Cardiology and Acute Coronary Syndromes, Medtronic, Edwards Lifesciences, Boston Scientific/Symetis, JenaValve, and St. Jude Medical. Dr. Okuno has no relevant conflicts of interest; see paper for disclosures of other study authors. Dr. Østergaard has received an independent research grant from the Novo Nordisk Foundation.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients undergoing transcatheter aortic valve replacement (TAVR) have a higher risk for stroke for up to 2 years compared with an age- and sex-matched population, after which their risks are comparable, results of a large Swiss registry study suggest.
METHODOLOGY:
- The study included 11,957 patients from the prospective SwissTAVI Registry, an ongoing mandatory cohort study enrolling consecutive patients undergoing TAVR in Switzerland.
- The study population, which had a mean age of 81.8 years and mean Society of Thoracic Surgeons Predicted Risk of Mortality (STS PROM) of 4.62, with 11.8% having a history of cerebrovascular accident (CVA) and 32.3% a history of atrial fibrillation, underwent TAVR at 15 centers between February 2011 and June 2021.
- The primary outcome was the incidence of stroke, with secondary outcomes including the incidence of CVA, a composite of stroke and transient ischemic attack (TIA).
- Researchers calculated standardized stroke ratios (SSRs) and compared stroke trends in patients undergoing TAVR with those of an age- and sex-matched general population in Switzerland derived from the 2019 Global Burden of Disease (GBD) study.
TAKEAWAY:
- , accounting for 69% of these events.
- After excluding 30-day events, the 1-year incidence rates of CVA and stroke were 1.7% and 1.4%, respectively, followed by an annual stroke incidence of 1.2%, 0.8%, 0.9%, and 0.7% in the second, third, fourth, and fifth years post TAVR, respectively.
- Only increased age and moderate/severe paravalvular leakage (PVL) at discharge were associated with an increased risk for early stroke (up to 30 days post TAVR), whereas dyslipidemia and history of atrial fibrillation and of CVA were associated with an increased risk for late stroke (30 days to 5 years after TAVR).
- SSR in the study population returned to a level comparable to that expected in the general Swiss population after 2 years and through to 5 years post-TAVR.
IN PRACTICE:
Although the study results “are reassuring” with respect to stroke risk beyond 2 years post TAVR, “our findings underscore the continued efforts of stroke-prevention measures” early and longer term, wrote the authors.
In an accompanying editorial, Lauge Østergaard, MD, PhD, Department of Cardiology, University of Copenhagen, Denmark, noted the study suggests reduced PVL could lower the risk for early stroke following TAVR and “highlights how assessment of usual risk factors (dyslipidemia and atrial fibrillation) could help reduce the burden of stroke in the long term.”
SOURCE:
The study was carried out by Taishi Okuno, MD, Department of Cardiology, Bern University Hospital, University of Bern, Switzerland, and colleagues. It was published online in the Journal of the American College of Cardiology (JACC): Cardiovascular Interventions.
LIMITATIONS:
The study couldn’t investigate the association between antithrombotic regimens and the risk for CVA. Definitions of CVA in the SwissTAVI Registry might differ from those used in the GBD study from which the matched population data were derived. The general population wasn’t matched on comorbidities usually associated with elevated stroke risk, which may have led to underestimation of stroke. As the mean age in the study was 82 years, results may not be extrapolated to a younger population.
DISCLOSURES:
The SwissTAVI registry is supported by the Swiss Heart Foundation, Swiss Working Group of Interventional Cardiology and Acute Coronary Syndromes, Medtronic, Edwards Lifesciences, Boston Scientific/Symetis, JenaValve, and St. Jude Medical. Dr. Okuno has no relevant conflicts of interest; see paper for disclosures of other study authors. Dr. Østergaard has received an independent research grant from the Novo Nordisk Foundation.
A version of this article appeared on Medscape.com.