User login
Bevacizumab, etoposide, and cisplatin before WBRT benefits intractable breast cancer brain metastases
Key clinical point: Induction treatment with bevacizumab, etoposide, and cisplatin (BEEP) before whole-brain radiotherapy (WBRT) vs WBRT alone improved brain-specific progression-free survival (PFS) outcomes in patients with brain metastases from breast cancer (BMBC).
Major finding: Patients who received BEEP + WBRT vs WBRT alone had significantly longer median brain-specific PFS based on a predefined α level of ≤0.20 (8.1 vs 6.5 months; hazard ratio 0.71; P = .15) and higher 8-month brain-specific PFS rate (48.7% vs 26.3%; P = .03). Neutropenia, nausea, anemia, and leukopenia were the most common adverse events in the BEEP induction arm.
Study details: Findings are from the phase 2 A-PLUS trial that included 118 WBRT-naive patients with invasive breast cancer who had ≥1 metastatic brain tumors and were randomly assigned to receive BEEP induction followed by WBRT or only WBRT.
Disclosures: This study received investigational product support and grants funding from Roche Taiwan, Chugai Pharma Taiwan, and other sources. Four authors declared receiving personal fees, lecture fees, consulting or speakers’ bureau fees, travel support, or grants from Roche and other sources.
Source: Chen TW et al. Whole-brain radiotherapy alone vs preceded by bevacizumab, etoposide, and cisplatin for untreated brain metastases from breast cancer: A randomized clinical trial. JAMA Oncol. 2023 (Dec 21). doi: 10.1001/jamaoncol.2023.5456
Key clinical point: Induction treatment with bevacizumab, etoposide, and cisplatin (BEEP) before whole-brain radiotherapy (WBRT) vs WBRT alone improved brain-specific progression-free survival (PFS) outcomes in patients with brain metastases from breast cancer (BMBC).
Major finding: Patients who received BEEP + WBRT vs WBRT alone had significantly longer median brain-specific PFS based on a predefined α level of ≤0.20 (8.1 vs 6.5 months; hazard ratio 0.71; P = .15) and higher 8-month brain-specific PFS rate (48.7% vs 26.3%; P = .03). Neutropenia, nausea, anemia, and leukopenia were the most common adverse events in the BEEP induction arm.
Study details: Findings are from the phase 2 A-PLUS trial that included 118 WBRT-naive patients with invasive breast cancer who had ≥1 metastatic brain tumors and were randomly assigned to receive BEEP induction followed by WBRT or only WBRT.
Disclosures: This study received investigational product support and grants funding from Roche Taiwan, Chugai Pharma Taiwan, and other sources. Four authors declared receiving personal fees, lecture fees, consulting or speakers’ bureau fees, travel support, or grants from Roche and other sources.
Source: Chen TW et al. Whole-brain radiotherapy alone vs preceded by bevacizumab, etoposide, and cisplatin for untreated brain metastases from breast cancer: A randomized clinical trial. JAMA Oncol. 2023 (Dec 21). doi: 10.1001/jamaoncol.2023.5456
Key clinical point: Induction treatment with bevacizumab, etoposide, and cisplatin (BEEP) before whole-brain radiotherapy (WBRT) vs WBRT alone improved brain-specific progression-free survival (PFS) outcomes in patients with brain metastases from breast cancer (BMBC).
Major finding: Patients who received BEEP + WBRT vs WBRT alone had significantly longer median brain-specific PFS based on a predefined α level of ≤0.20 (8.1 vs 6.5 months; hazard ratio 0.71; P = .15) and higher 8-month brain-specific PFS rate (48.7% vs 26.3%; P = .03). Neutropenia, nausea, anemia, and leukopenia were the most common adverse events in the BEEP induction arm.
Study details: Findings are from the phase 2 A-PLUS trial that included 118 WBRT-naive patients with invasive breast cancer who had ≥1 metastatic brain tumors and were randomly assigned to receive BEEP induction followed by WBRT or only WBRT.
Disclosures: This study received investigational product support and grants funding from Roche Taiwan, Chugai Pharma Taiwan, and other sources. Four authors declared receiving personal fees, lecture fees, consulting or speakers’ bureau fees, travel support, or grants from Roche and other sources.
Source: Chen TW et al. Whole-brain radiotherapy alone vs preceded by bevacizumab, etoposide, and cisplatin for untreated brain metastases from breast cancer: A randomized clinical trial. JAMA Oncol. 2023 (Dec 21). doi: 10.1001/jamaoncol.2023.5456
Carboplatin + atezolizumab combo improves OS in metastatic TNBC
Key clinical point: Adding atezolizumab to carboplatin improved overall survival (OS) outcomes in patients with metastatic triple-negative breast cancer (TNBC).
Major finding: Compared with carboplatin alone, carboplatin + atezolizumab demonstrated significant improvement in OS outcomes (hazard ratio [HR] 0.60; log-rank P = .03) even though the improvement in the primary endpoint of progression-free survival was not statistically significant (HR 0.66; log-rank P = .05). The prevalence of grade 3/4 serious adverse events was higher with carboplatin + atezolizumab vs carboplatin alone (41% vs 8%).
Study details: Findings are from the phase 2 TBCRC 043 trial that included 106 patients with metastatic TNBC who were randomly assigned to receive either carboplatin alone or in combination with atezolizumab.
Disclosures: This study was supported by a US National Cancer Institute grant, Susan G. Komen grants, and other sources. Some authors declared receiving grants or personal fees from or having other ties with various sources, including the funding agencies.
Source: Lehmann BD et al. Atezolizumab in combination with carboplatin and survival outcomes in patients with metastatic triple-negative breast cancer: The TBCRC 043 phase 2 randomized clinical trial. JAMA Oncol. 2023 (Dec 14). doi: 10.1001/jamaoncol.2023.5424
Key clinical point: Adding atezolizumab to carboplatin improved overall survival (OS) outcomes in patients with metastatic triple-negative breast cancer (TNBC).
Major finding: Compared with carboplatin alone, carboplatin + atezolizumab demonstrated significant improvement in OS outcomes (hazard ratio [HR] 0.60; log-rank P = .03) even though the improvement in the primary endpoint of progression-free survival was not statistically significant (HR 0.66; log-rank P = .05). The prevalence of grade 3/4 serious adverse events was higher with carboplatin + atezolizumab vs carboplatin alone (41% vs 8%).
Study details: Findings are from the phase 2 TBCRC 043 trial that included 106 patients with metastatic TNBC who were randomly assigned to receive either carboplatin alone or in combination with atezolizumab.
Disclosures: This study was supported by a US National Cancer Institute grant, Susan G. Komen grants, and other sources. Some authors declared receiving grants or personal fees from or having other ties with various sources, including the funding agencies.
Source: Lehmann BD et al. Atezolizumab in combination with carboplatin and survival outcomes in patients with metastatic triple-negative breast cancer: The TBCRC 043 phase 2 randomized clinical trial. JAMA Oncol. 2023 (Dec 14). doi: 10.1001/jamaoncol.2023.5424
Key clinical point: Adding atezolizumab to carboplatin improved overall survival (OS) outcomes in patients with metastatic triple-negative breast cancer (TNBC).
Major finding: Compared with carboplatin alone, carboplatin + atezolizumab demonstrated significant improvement in OS outcomes (hazard ratio [HR] 0.60; log-rank P = .03) even though the improvement in the primary endpoint of progression-free survival was not statistically significant (HR 0.66; log-rank P = .05). The prevalence of grade 3/4 serious adverse events was higher with carboplatin + atezolizumab vs carboplatin alone (41% vs 8%).
Study details: Findings are from the phase 2 TBCRC 043 trial that included 106 patients with metastatic TNBC who were randomly assigned to receive either carboplatin alone or in combination with atezolizumab.
Disclosures: This study was supported by a US National Cancer Institute grant, Susan G. Komen grants, and other sources. Some authors declared receiving grants or personal fees from or having other ties with various sources, including the funding agencies.
Source: Lehmann BD et al. Atezolizumab in combination with carboplatin and survival outcomes in patients with metastatic triple-negative breast cancer: The TBCRC 043 phase 2 randomized clinical trial. JAMA Oncol. 2023 (Dec 14). doi: 10.1001/jamaoncol.2023.5424
Benign breast disease on percutaneous biopsy increases breast cancer risk
Key clinical point: Compared with the general population, the risk for overall breast cancer (BC) was nearly double in women with benign breast disease (BBD) diagnosed by percutaneous biopsies.
Major finding: Patients with BBD vs the general population were at a significantly higher risk for overall BC (standard incidence ratio [SIR] 1.95; 95% CI 1.76-2.17), including invasive BC (SIR 1.56; 95% CI 1.37-1.78) and ductal carcinoma in situ (SIR 3.10; 95% CI 2.54-3.77). The SIR for overall BC increased progressively with increasing BBD severity (nonproliferative 1.42; 95% CI 1.19-1.71; proliferative disease without atypia 2.19; 95% CI 1.88-2.54; atypical hyperplasia 3.91; 95% CI 2.97-5.14).
Study details: Findings are from a retrospective cohort study including 4819 female patients who underwent a BBD biopsy, of whom 338 patients had incident BC.
Disclosures: This study was supported by a grant from the US National Institutes of Health (NIH). Four authors declared receiving grants, research support, or personal fees from NIH and other sources.
Source: Sherman ME et al. Benign breast disease and breast cancer risk in the percutaneous biopsy era. JAMA Surg. 2023 (Dec 13). doi: 10.1001/jamasurg.2023.6382
Key clinical point: Compared with the general population, the risk for overall breast cancer (BC) was nearly double in women with benign breast disease (BBD) diagnosed by percutaneous biopsies.
Major finding: Patients with BBD vs the general population were at a significantly higher risk for overall BC (standard incidence ratio [SIR] 1.95; 95% CI 1.76-2.17), including invasive BC (SIR 1.56; 95% CI 1.37-1.78) and ductal carcinoma in situ (SIR 3.10; 95% CI 2.54-3.77). The SIR for overall BC increased progressively with increasing BBD severity (nonproliferative 1.42; 95% CI 1.19-1.71; proliferative disease without atypia 2.19; 95% CI 1.88-2.54; atypical hyperplasia 3.91; 95% CI 2.97-5.14).
Study details: Findings are from a retrospective cohort study including 4819 female patients who underwent a BBD biopsy, of whom 338 patients had incident BC.
Disclosures: This study was supported by a grant from the US National Institutes of Health (NIH). Four authors declared receiving grants, research support, or personal fees from NIH and other sources.
Source: Sherman ME et al. Benign breast disease and breast cancer risk in the percutaneous biopsy era. JAMA Surg. 2023 (Dec 13). doi: 10.1001/jamasurg.2023.6382
Key clinical point: Compared with the general population, the risk for overall breast cancer (BC) was nearly double in women with benign breast disease (BBD) diagnosed by percutaneous biopsies.
Major finding: Patients with BBD vs the general population were at a significantly higher risk for overall BC (standard incidence ratio [SIR] 1.95; 95% CI 1.76-2.17), including invasive BC (SIR 1.56; 95% CI 1.37-1.78) and ductal carcinoma in situ (SIR 3.10; 95% CI 2.54-3.77). The SIR for overall BC increased progressively with increasing BBD severity (nonproliferative 1.42; 95% CI 1.19-1.71; proliferative disease without atypia 2.19; 95% CI 1.88-2.54; atypical hyperplasia 3.91; 95% CI 2.97-5.14).
Study details: Findings are from a retrospective cohort study including 4819 female patients who underwent a BBD biopsy, of whom 338 patients had incident BC.
Disclosures: This study was supported by a grant from the US National Institutes of Health (NIH). Four authors declared receiving grants, research support, or personal fees from NIH and other sources.
Source: Sherman ME et al. Benign breast disease and breast cancer risk in the percutaneous biopsy era. JAMA Surg. 2023 (Dec 13). doi: 10.1001/jamasurg.2023.6382
Coffee, COVID, and the Universal Antimicrobial
A recent article in Cell & Bioscience suggested that regular coffee consumption can reduce the risk of COVID infections.
The study does make some interesting points about the benefits of coffee’s different polyphenols and antioxidants and their effects on different COVID variants. Most of it is based on lab data, although one section, using serum from coffee versus water drinkers, did find that it was more effective at inhibiting the virions. Caffeinated versus decaffeinated didn’t matter.
I’m not saying coffee doesn’t impair the virus. The data are worth looking at. But the majority of adults in North America, Europe, and pretty much the entire planet drink coffee on a regular basis. A large number of them still caught COVID. Would they have had worse cases if they didn’t drink coffee? Maybe, maybe not.
The problem here is that, as always, preliminary data like this get pushed into mass media, making it sound like “COFFEE CURES COVID!!!” Never mind that that’s not what the article said, but it sure gets clicks and retweets and FaceBook “likes.”
Suddenly fringe groups are claiming the coffee cure was there all along, and hidden from them by the evil government-pharma-medical cartel. Others claim the research is flawed because of this or that. The signal gets drowned out by the noise.
Definitely, food can be a medicine. Look at all the benefits proven of the Mediterranean diet. Coffee may help, especially if we can identify and isolate the specific components that reduce COVID risk. But, as they always say at the end, the study is preliminary and further research is needed.
Once or twice a year, an adult with epilepsy comes in, waving a copy of the ketogenic diet around and upset that I never tried it on them — again proof of the evil government-pharma-medical cartel that I’m in league with. I calm them down and explain the diet in detail. Maybe 50% of them decide to go ahead with it. In 25 years of practice, my record for an otherwise normal adult sticking with it is 5 days.
You don’t have to go too far back to remember Linus Pauling, an absolutely brilliant scientist, but not the best of nutritionists. With two Nobel prizes behind him, he took a stab at medicine in the 1970s, arguing that megadoses of vitamin C worked for the common cold. While it may be good for us, and certainly most people like orange juice, but those claims about the common cold never panned out. In fact, we’re no closer to curing it now than we were then.
It might, but this doesn’t mean the “truth” is being maliciously hidden by an evil cartel. It just means we have (as always) more to learn.
I’ll still drink my single cup of coffee every weekday morning. I’m a creature of habit, and heaven knows I need the caffeine. If it also boosts my immune system, so much the better.
Besides, we still have that universal antimicrobial called chicken soup.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A recent article in Cell & Bioscience suggested that regular coffee consumption can reduce the risk of COVID infections.
The study does make some interesting points about the benefits of coffee’s different polyphenols and antioxidants and their effects on different COVID variants. Most of it is based on lab data, although one section, using serum from coffee versus water drinkers, did find that it was more effective at inhibiting the virions. Caffeinated versus decaffeinated didn’t matter.
I’m not saying coffee doesn’t impair the virus. The data are worth looking at. But the majority of adults in North America, Europe, and pretty much the entire planet drink coffee on a regular basis. A large number of them still caught COVID. Would they have had worse cases if they didn’t drink coffee? Maybe, maybe not.
The problem here is that, as always, preliminary data like this get pushed into mass media, making it sound like “COFFEE CURES COVID!!!” Never mind that that’s not what the article said, but it sure gets clicks and retweets and FaceBook “likes.”
Suddenly fringe groups are claiming the coffee cure was there all along, and hidden from them by the evil government-pharma-medical cartel. Others claim the research is flawed because of this or that. The signal gets drowned out by the noise.
Definitely, food can be a medicine. Look at all the benefits proven of the Mediterranean diet. Coffee may help, especially if we can identify and isolate the specific components that reduce COVID risk. But, as they always say at the end, the study is preliminary and further research is needed.
Once or twice a year, an adult with epilepsy comes in, waving a copy of the ketogenic diet around and upset that I never tried it on them — again proof of the evil government-pharma-medical cartel that I’m in league with. I calm them down and explain the diet in detail. Maybe 50% of them decide to go ahead with it. In 25 years of practice, my record for an otherwise normal adult sticking with it is 5 days.
You don’t have to go too far back to remember Linus Pauling, an absolutely brilliant scientist, but not the best of nutritionists. With two Nobel prizes behind him, he took a stab at medicine in the 1970s, arguing that megadoses of vitamin C worked for the common cold. While it may be good for us, and certainly most people like orange juice, but those claims about the common cold never panned out. In fact, we’re no closer to curing it now than we were then.
It might, but this doesn’t mean the “truth” is being maliciously hidden by an evil cartel. It just means we have (as always) more to learn.
I’ll still drink my single cup of coffee every weekday morning. I’m a creature of habit, and heaven knows I need the caffeine. If it also boosts my immune system, so much the better.
Besides, we still have that universal antimicrobial called chicken soup.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A recent article in Cell & Bioscience suggested that regular coffee consumption can reduce the risk of COVID infections.
The study does make some interesting points about the benefits of coffee’s different polyphenols and antioxidants and their effects on different COVID variants. Most of it is based on lab data, although one section, using serum from coffee versus water drinkers, did find that it was more effective at inhibiting the virions. Caffeinated versus decaffeinated didn’t matter.
I’m not saying coffee doesn’t impair the virus. The data are worth looking at. But the majority of adults in North America, Europe, and pretty much the entire planet drink coffee on a regular basis. A large number of them still caught COVID. Would they have had worse cases if they didn’t drink coffee? Maybe, maybe not.
The problem here is that, as always, preliminary data like this get pushed into mass media, making it sound like “COFFEE CURES COVID!!!” Never mind that that’s not what the article said, but it sure gets clicks and retweets and FaceBook “likes.”
Suddenly fringe groups are claiming the coffee cure was there all along, and hidden from them by the evil government-pharma-medical cartel. Others claim the research is flawed because of this or that. The signal gets drowned out by the noise.
Definitely, food can be a medicine. Look at all the benefits proven of the Mediterranean diet. Coffee may help, especially if we can identify and isolate the specific components that reduce COVID risk. But, as they always say at the end, the study is preliminary and further research is needed.
Once or twice a year, an adult with epilepsy comes in, waving a copy of the ketogenic diet around and upset that I never tried it on them — again proof of the evil government-pharma-medical cartel that I’m in league with. I calm them down and explain the diet in detail. Maybe 50% of them decide to go ahead with it. In 25 years of practice, my record for an otherwise normal adult sticking with it is 5 days.
You don’t have to go too far back to remember Linus Pauling, an absolutely brilliant scientist, but not the best of nutritionists. With two Nobel prizes behind him, he took a stab at medicine in the 1970s, arguing that megadoses of vitamin C worked for the common cold. While it may be good for us, and certainly most people like orange juice, but those claims about the common cold never panned out. In fact, we’re no closer to curing it now than we were then.
It might, but this doesn’t mean the “truth” is being maliciously hidden by an evil cartel. It just means we have (as always) more to learn.
I’ll still drink my single cup of coffee every weekday morning. I’m a creature of habit, and heaven knows I need the caffeine. If it also boosts my immune system, so much the better.
Besides, we still have that universal antimicrobial called chicken soup.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).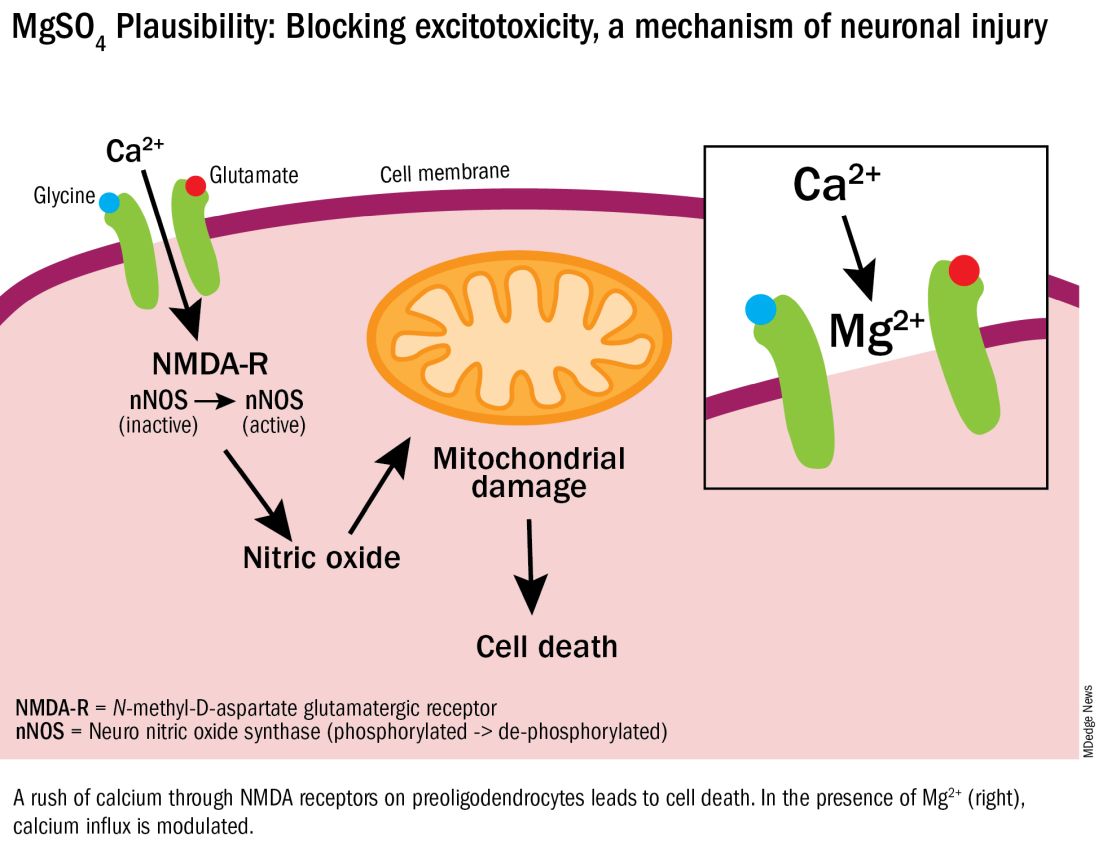
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.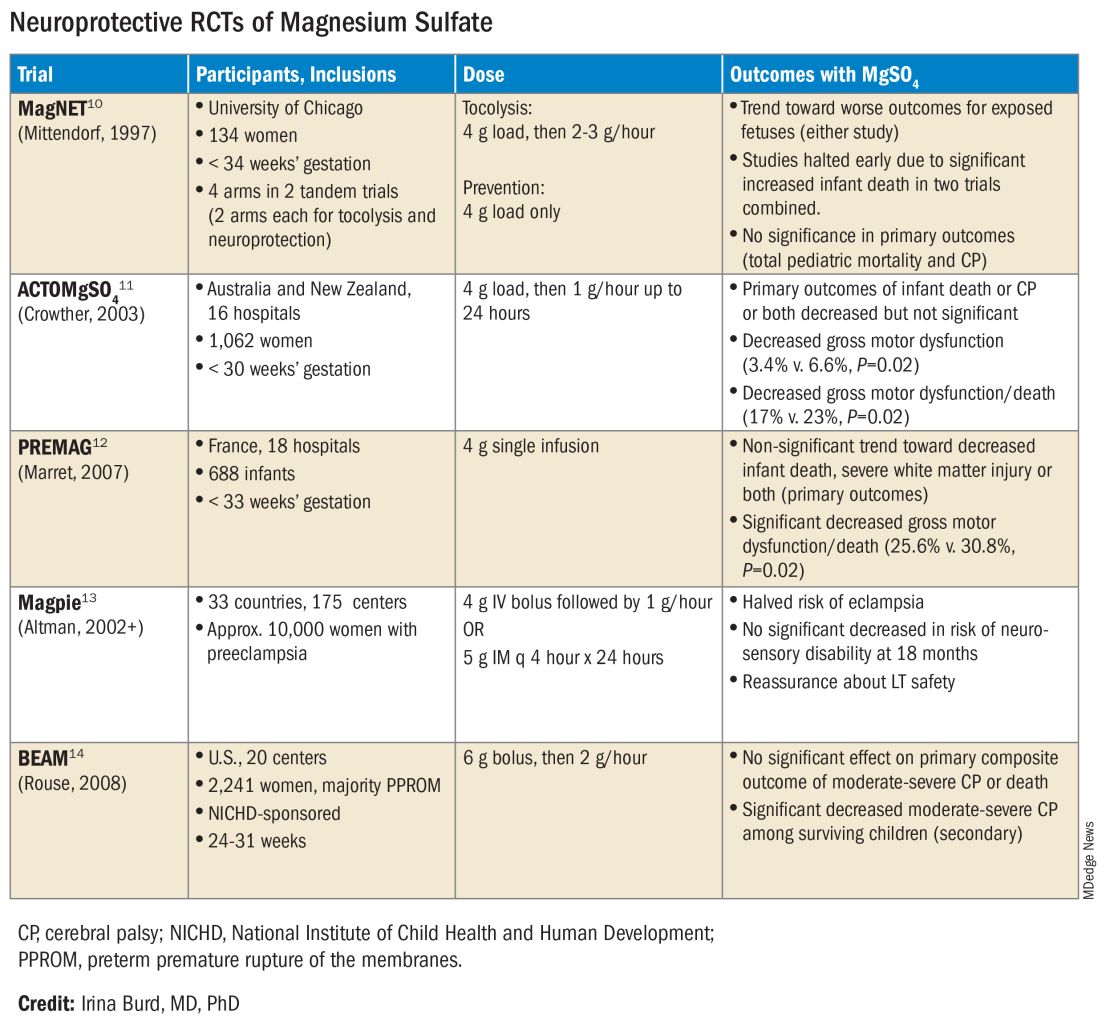
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Direct Measurement of T3 Is Likely Vital, Say Researchers
To assess thyroid function, clinicians typically determine blood levels of thyroid-stimulating hormone (TSH) and thyroxine (T4) and do not directly measure triiodothyronine (T3).
However, linking socioeconomic forces, human biology, and aging,” researchers reported.
The study, with lead author Ralph I. Lawton, an MD-PhD student at Harvard Medical School, Boston, was published online on January 4, 2024, in Proceedings of the National Academy of Sciences. In this national sample of healthy adults older than 20, free T3 was negatively related to household income. At age 40 and older, free T3 was significantly linked to being employed. Among adults from age 51 to 80, free T3 levels were inversely related to mortality.
“It is important to note that the more typically measured biomarkers for thyroid function (T4 and TSH) are poorly linked to free T3 levels,” Dr. Lawton and colleagues wrote.
The ratio of TSH:T4 explained only 1.7% of variation in T3 levels, they noted, which suggested that TSH and T4 may not be accurate surrogates of free T3. Thus, “direct measurement of free T3 is likely vital to properly stratify the effects of HPT-axis variation,” they maintained. “Improved methods for measurement [of T3] and further investigation of the role of free T3 in clinical conditions may be high yield,” according to the researchers.
Further studies of the relationship between the action of deiodinase in converting T4 to T3 and social forces and aging could elucidate critical biology. “New therapeutics targeting T3/T4 ratios,” they suggested, “may be more beneficial for a significant cohort of older individuals [with hypothyroidism] currently treated with levothyroxine [(LT4)].”
‘Cherry on Top’
“I think this is a really important paper,” thyroidology expert Antonio Bianco, MD, PhD, professor of medicine, The University of Chicago, who was not involved with this research, told this news organization.
His research group and others “have slowly been providing evidence of the relevance of measuring T3 levels,” he said. “This paper is sort of a cherry on top. It is really the most recent strong evidence that looking at T3 levels in plasma is really important. We need to do a better job at developing a much more precise assay for T3,” he said, “because now we know T3 is a very important parameter that endocrinologists should be looking for. We know that about 20 million Americans have hypothyroidism, and are being treated with T4 (levothyroxine),” Dr. Bianco continued.
“As endocrinologists, we believe that if you treat someone with levothyroxine, you normalize T3 levels. However, many times, with standard of care with levothyroxine, T3 levels are not normalized.”
Larger Variations in T3
Thyroid hormones (TSH, free T4, and free T3) influence many aspects of human physiology and behavior, but it is not known how variations in these levels in a population free of thyroid disease are associated with mortality and socioeconomic factors.
The researchers analyzed data from 7626 adults over age 20 up to age 80 who participated in the National Health and Nutrition Examination Survey during 2007-2012 and had blood tests to determine TSH, free T4, and free T3 and were not being actively treated for thyroid conditions. Among the 3603 older adults (over age 50), there were 981 deaths up until the end of 2019. TSH levels were not linked to mortality, but increasing levels of free T3 and decreasing levels of free T4 were protective for mortality (hazard ratios, 0.88 and 1.26, respectively; P < .01).
There was a robust negative relationship between household income and employment and free T3 but no relationship between these factors and T4. There was a much larger variation across age in levels of free T3 than in levels of TSH or free T4. Free T4 and free T3 diverged at older ages, when free T4 increased and free T3 continually decreased.
Older adults with symptoms of hypothyroidism may have high free T4 levels despite low free T3, the researchers noted.
“In a nonclinical sample representative of the US population,” they summarized, “we find that free T3 is much more strongly related to all domains studied — age, sex, seasonality, household income, employment, and longevity — than the other molecules of the HPT axis.”
This work was supported by the Burroughs Wellcome Fund Career at Scientific Interface award, a Brain and Behavior Research Foundation young investigator award, the Howard Hughes Medical Institute, the National Institute of Aging, and the National Institute of General Medical Sciences. Dr. Bianco served as a consultant for AbbVie, Avion Pharmaceuticals, Synthonics, Synthion, and Thyron and recently published the book Rethinking Hypothyroidism: Why Treatment Must Change and What Patients Can Do.
A version of this article appeared on Medscape.com.
To assess thyroid function, clinicians typically determine blood levels of thyroid-stimulating hormone (TSH) and thyroxine (T4) and do not directly measure triiodothyronine (T3).
However, linking socioeconomic forces, human biology, and aging,” researchers reported.
The study, with lead author Ralph I. Lawton, an MD-PhD student at Harvard Medical School, Boston, was published online on January 4, 2024, in Proceedings of the National Academy of Sciences. In this national sample of healthy adults older than 20, free T3 was negatively related to household income. At age 40 and older, free T3 was significantly linked to being employed. Among adults from age 51 to 80, free T3 levels were inversely related to mortality.
“It is important to note that the more typically measured biomarkers for thyroid function (T4 and TSH) are poorly linked to free T3 levels,” Dr. Lawton and colleagues wrote.
The ratio of TSH:T4 explained only 1.7% of variation in T3 levels, they noted, which suggested that TSH and T4 may not be accurate surrogates of free T3. Thus, “direct measurement of free T3 is likely vital to properly stratify the effects of HPT-axis variation,” they maintained. “Improved methods for measurement [of T3] and further investigation of the role of free T3 in clinical conditions may be high yield,” according to the researchers.
Further studies of the relationship between the action of deiodinase in converting T4 to T3 and social forces and aging could elucidate critical biology. “New therapeutics targeting T3/T4 ratios,” they suggested, “may be more beneficial for a significant cohort of older individuals [with hypothyroidism] currently treated with levothyroxine [(LT4)].”
‘Cherry on Top’
“I think this is a really important paper,” thyroidology expert Antonio Bianco, MD, PhD, professor of medicine, The University of Chicago, who was not involved with this research, told this news organization.
His research group and others “have slowly been providing evidence of the relevance of measuring T3 levels,” he said. “This paper is sort of a cherry on top. It is really the most recent strong evidence that looking at T3 levels in plasma is really important. We need to do a better job at developing a much more precise assay for T3,” he said, “because now we know T3 is a very important parameter that endocrinologists should be looking for. We know that about 20 million Americans have hypothyroidism, and are being treated with T4 (levothyroxine),” Dr. Bianco continued.
“As endocrinologists, we believe that if you treat someone with levothyroxine, you normalize T3 levels. However, many times, with standard of care with levothyroxine, T3 levels are not normalized.”
Larger Variations in T3
Thyroid hormones (TSH, free T4, and free T3) influence many aspects of human physiology and behavior, but it is not known how variations in these levels in a population free of thyroid disease are associated with mortality and socioeconomic factors.
The researchers analyzed data from 7626 adults over age 20 up to age 80 who participated in the National Health and Nutrition Examination Survey during 2007-2012 and had blood tests to determine TSH, free T4, and free T3 and were not being actively treated for thyroid conditions. Among the 3603 older adults (over age 50), there were 981 deaths up until the end of 2019. TSH levels were not linked to mortality, but increasing levels of free T3 and decreasing levels of free T4 were protective for mortality (hazard ratios, 0.88 and 1.26, respectively; P < .01).
There was a robust negative relationship between household income and employment and free T3 but no relationship between these factors and T4. There was a much larger variation across age in levels of free T3 than in levels of TSH or free T4. Free T4 and free T3 diverged at older ages, when free T4 increased and free T3 continually decreased.
Older adults with symptoms of hypothyroidism may have high free T4 levels despite low free T3, the researchers noted.
“In a nonclinical sample representative of the US population,” they summarized, “we find that free T3 is much more strongly related to all domains studied — age, sex, seasonality, household income, employment, and longevity — than the other molecules of the HPT axis.”
This work was supported by the Burroughs Wellcome Fund Career at Scientific Interface award, a Brain and Behavior Research Foundation young investigator award, the Howard Hughes Medical Institute, the National Institute of Aging, and the National Institute of General Medical Sciences. Dr. Bianco served as a consultant for AbbVie, Avion Pharmaceuticals, Synthonics, Synthion, and Thyron and recently published the book Rethinking Hypothyroidism: Why Treatment Must Change and What Patients Can Do.
A version of this article appeared on Medscape.com.
To assess thyroid function, clinicians typically determine blood levels of thyroid-stimulating hormone (TSH) and thyroxine (T4) and do not directly measure triiodothyronine (T3).
However, linking socioeconomic forces, human biology, and aging,” researchers reported.
The study, with lead author Ralph I. Lawton, an MD-PhD student at Harvard Medical School, Boston, was published online on January 4, 2024, in Proceedings of the National Academy of Sciences. In this national sample of healthy adults older than 20, free T3 was negatively related to household income. At age 40 and older, free T3 was significantly linked to being employed. Among adults from age 51 to 80, free T3 levels were inversely related to mortality.
“It is important to note that the more typically measured biomarkers for thyroid function (T4 and TSH) are poorly linked to free T3 levels,” Dr. Lawton and colleagues wrote.
The ratio of TSH:T4 explained only 1.7% of variation in T3 levels, they noted, which suggested that TSH and T4 may not be accurate surrogates of free T3. Thus, “direct measurement of free T3 is likely vital to properly stratify the effects of HPT-axis variation,” they maintained. “Improved methods for measurement [of T3] and further investigation of the role of free T3 in clinical conditions may be high yield,” according to the researchers.
Further studies of the relationship between the action of deiodinase in converting T4 to T3 and social forces and aging could elucidate critical biology. “New therapeutics targeting T3/T4 ratios,” they suggested, “may be more beneficial for a significant cohort of older individuals [with hypothyroidism] currently treated with levothyroxine [(LT4)].”
‘Cherry on Top’
“I think this is a really important paper,” thyroidology expert Antonio Bianco, MD, PhD, professor of medicine, The University of Chicago, who was not involved with this research, told this news organization.
His research group and others “have slowly been providing evidence of the relevance of measuring T3 levels,” he said. “This paper is sort of a cherry on top. It is really the most recent strong evidence that looking at T3 levels in plasma is really important. We need to do a better job at developing a much more precise assay for T3,” he said, “because now we know T3 is a very important parameter that endocrinologists should be looking for. We know that about 20 million Americans have hypothyroidism, and are being treated with T4 (levothyroxine),” Dr. Bianco continued.
“As endocrinologists, we believe that if you treat someone with levothyroxine, you normalize T3 levels. However, many times, with standard of care with levothyroxine, T3 levels are not normalized.”
Larger Variations in T3
Thyroid hormones (TSH, free T4, and free T3) influence many aspects of human physiology and behavior, but it is not known how variations in these levels in a population free of thyroid disease are associated with mortality and socioeconomic factors.
The researchers analyzed data from 7626 adults over age 20 up to age 80 who participated in the National Health and Nutrition Examination Survey during 2007-2012 and had blood tests to determine TSH, free T4, and free T3 and were not being actively treated for thyroid conditions. Among the 3603 older adults (over age 50), there were 981 deaths up until the end of 2019. TSH levels were not linked to mortality, but increasing levels of free T3 and decreasing levels of free T4 were protective for mortality (hazard ratios, 0.88 and 1.26, respectively; P < .01).
There was a robust negative relationship between household income and employment and free T3 but no relationship between these factors and T4. There was a much larger variation across age in levels of free T3 than in levels of TSH or free T4. Free T4 and free T3 diverged at older ages, when free T4 increased and free T3 continually decreased.
Older adults with symptoms of hypothyroidism may have high free T4 levels despite low free T3, the researchers noted.
“In a nonclinical sample representative of the US population,” they summarized, “we find that free T3 is much more strongly related to all domains studied — age, sex, seasonality, household income, employment, and longevity — than the other molecules of the HPT axis.”
This work was supported by the Burroughs Wellcome Fund Career at Scientific Interface award, a Brain and Behavior Research Foundation young investigator award, the Howard Hughes Medical Institute, the National Institute of Aging, and the National Institute of General Medical Sciences. Dr. Bianco served as a consultant for AbbVie, Avion Pharmaceuticals, Synthonics, Synthion, and Thyron and recently published the book Rethinking Hypothyroidism: Why Treatment Must Change and What Patients Can Do.
A version of this article appeared on Medscape.com.
Are You Unwittingly Aiding the Rise of Superfungi?
Unnecessary or incorrect use of topical antifungal medications is driving the spread of fungal infections like ringworm, which are becoming more difficult to treat, according to a January 11 study published in Morbidity and Mortality Weekly Report.
If a patient’s condition is not caused by a fungus but is treated as such, treatment will be ineffective.
such as clotrimazole or combinations of antifungals and corticosteroids. And because many topical treatments are also available over-the-counter, doctors should advise patients about how to use them correctly.
“In the last few years, there have been many antifungal resistant cases of tinea corporisand onychomycosisreported,” or ringworm and finger or toenail infections, respectively, said Shari Lipner, MD, PhD, a dermatologist at Weill Cornell Medicine in New York, and an author of the study.
Many of these cases originated in South Asia and have also been reported in Europe and Canada. In 2023, the first cases of a new strain of antifungal-resistant ringworm were reported in the United States. This species, Trichophyton indotineae, does not respond to topical medications, requiring oral treatment instead.
“It’s really a serious problem and a huge public health concern,” Dr. Lipner said.
For the new study, Dr. Lipner and colleagues examined prescription patterns from 2021 Medicare Part D claims of topical antifungals. They report that 6.5 million topical antifungal prescriptions were filled that year, some of which included steroids in the formulation. Primary care clinicians wrote 40% of these prescriptions, the most for any clinician group. The estimate is almost certainly an undercount of topical antifungal use because the database did not include over-the-counter purchases or data from other insurance payers.
The number of prescriptions equate to 1 in every 8 Medicare Part D beneficiary receiving an antifungal, the researchers reported.
“If I think about the patients that come into my office, I’m certainly not giving an antifungal to 1 in 8 of them, and I see a lot of fungal infections,” Dr. Lipner said. The findings suggest to Dr. Lipner that some clinicians are diagnosing ringworm by eyesight alone rather than confirming the diagnosis with techniques such as microscopy, fungal culture testing, or polymerase chain reaction testing.
Sometimes what looks like ringworm may actually be eczema, in which case, the topical antifungal would not be appropriate, according to Avrom Caplan, MD, a dermatologist at NYU Langone Health in New York.
“If you’re prescribing something to somebody that they don’t need, you’re basically exposing them to the side effects without the benefit,” Dr. Caplan, who was not part of the study, said.
Dr. Caplan, who reported the first cases of ringworm that only responded to oral medications in the United States, stressed that topical treatments work fine for many ringworm cases today. But if indiscriminate prescribing spurs the development of more resilient fungi, more situations may arise in which only oral medications work in the future, Dr. Caplan said. In addition, oral medications are inherently more demanding on a patient than something they can rub on their skin, Dr. Caplan added.
“We hope that physicians will really think hard about this study and change their practices if they’re not confirming the diagnosis,” Dr. Lipner said.
Dr. Lipner and Dr. Caplan report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Unnecessary or incorrect use of topical antifungal medications is driving the spread of fungal infections like ringworm, which are becoming more difficult to treat, according to a January 11 study published in Morbidity and Mortality Weekly Report.
If a patient’s condition is not caused by a fungus but is treated as such, treatment will be ineffective.
such as clotrimazole or combinations of antifungals and corticosteroids. And because many topical treatments are also available over-the-counter, doctors should advise patients about how to use them correctly.
“In the last few years, there have been many antifungal resistant cases of tinea corporisand onychomycosisreported,” or ringworm and finger or toenail infections, respectively, said Shari Lipner, MD, PhD, a dermatologist at Weill Cornell Medicine in New York, and an author of the study.
Many of these cases originated in South Asia and have also been reported in Europe and Canada. In 2023, the first cases of a new strain of antifungal-resistant ringworm were reported in the United States. This species, Trichophyton indotineae, does not respond to topical medications, requiring oral treatment instead.
“It’s really a serious problem and a huge public health concern,” Dr. Lipner said.
For the new study, Dr. Lipner and colleagues examined prescription patterns from 2021 Medicare Part D claims of topical antifungals. They report that 6.5 million topical antifungal prescriptions were filled that year, some of which included steroids in the formulation. Primary care clinicians wrote 40% of these prescriptions, the most for any clinician group. The estimate is almost certainly an undercount of topical antifungal use because the database did not include over-the-counter purchases or data from other insurance payers.
The number of prescriptions equate to 1 in every 8 Medicare Part D beneficiary receiving an antifungal, the researchers reported.
“If I think about the patients that come into my office, I’m certainly not giving an antifungal to 1 in 8 of them, and I see a lot of fungal infections,” Dr. Lipner said. The findings suggest to Dr. Lipner that some clinicians are diagnosing ringworm by eyesight alone rather than confirming the diagnosis with techniques such as microscopy, fungal culture testing, or polymerase chain reaction testing.
Sometimes what looks like ringworm may actually be eczema, in which case, the topical antifungal would not be appropriate, according to Avrom Caplan, MD, a dermatologist at NYU Langone Health in New York.
“If you’re prescribing something to somebody that they don’t need, you’re basically exposing them to the side effects without the benefit,” Dr. Caplan, who was not part of the study, said.
Dr. Caplan, who reported the first cases of ringworm that only responded to oral medications in the United States, stressed that topical treatments work fine for many ringworm cases today. But if indiscriminate prescribing spurs the development of more resilient fungi, more situations may arise in which only oral medications work in the future, Dr. Caplan said. In addition, oral medications are inherently more demanding on a patient than something they can rub on their skin, Dr. Caplan added.
“We hope that physicians will really think hard about this study and change their practices if they’re not confirming the diagnosis,” Dr. Lipner said.
Dr. Lipner and Dr. Caplan report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Unnecessary or incorrect use of topical antifungal medications is driving the spread of fungal infections like ringworm, which are becoming more difficult to treat, according to a January 11 study published in Morbidity and Mortality Weekly Report.
If a patient’s condition is not caused by a fungus but is treated as such, treatment will be ineffective.
such as clotrimazole or combinations of antifungals and corticosteroids. And because many topical treatments are also available over-the-counter, doctors should advise patients about how to use them correctly.
“In the last few years, there have been many antifungal resistant cases of tinea corporisand onychomycosisreported,” or ringworm and finger or toenail infections, respectively, said Shari Lipner, MD, PhD, a dermatologist at Weill Cornell Medicine in New York, and an author of the study.
Many of these cases originated in South Asia and have also been reported in Europe and Canada. In 2023, the first cases of a new strain of antifungal-resistant ringworm were reported in the United States. This species, Trichophyton indotineae, does not respond to topical medications, requiring oral treatment instead.
“It’s really a serious problem and a huge public health concern,” Dr. Lipner said.
For the new study, Dr. Lipner and colleagues examined prescription patterns from 2021 Medicare Part D claims of topical antifungals. They report that 6.5 million topical antifungal prescriptions were filled that year, some of which included steroids in the formulation. Primary care clinicians wrote 40% of these prescriptions, the most for any clinician group. The estimate is almost certainly an undercount of topical antifungal use because the database did not include over-the-counter purchases or data from other insurance payers.
The number of prescriptions equate to 1 in every 8 Medicare Part D beneficiary receiving an antifungal, the researchers reported.
“If I think about the patients that come into my office, I’m certainly not giving an antifungal to 1 in 8 of them, and I see a lot of fungal infections,” Dr. Lipner said. The findings suggest to Dr. Lipner that some clinicians are diagnosing ringworm by eyesight alone rather than confirming the diagnosis with techniques such as microscopy, fungal culture testing, or polymerase chain reaction testing.
Sometimes what looks like ringworm may actually be eczema, in which case, the topical antifungal would not be appropriate, according to Avrom Caplan, MD, a dermatologist at NYU Langone Health in New York.
“If you’re prescribing something to somebody that they don’t need, you’re basically exposing them to the side effects without the benefit,” Dr. Caplan, who was not part of the study, said.
Dr. Caplan, who reported the first cases of ringworm that only responded to oral medications in the United States, stressed that topical treatments work fine for many ringworm cases today. But if indiscriminate prescribing spurs the development of more resilient fungi, more situations may arise in which only oral medications work in the future, Dr. Caplan said. In addition, oral medications are inherently more demanding on a patient than something they can rub on their skin, Dr. Caplan added.
“We hope that physicians will really think hard about this study and change their practices if they’re not confirming the diagnosis,” Dr. Lipner said.
Dr. Lipner and Dr. Caplan report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Chronic Fatigue Syndrome and Fibromyalgia: A Single Disease Entity?
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and fibromyalgia (FM) have overlapping neurologic symptoms — particularly profound fatigue. The similarity between these two conditions has led to the question of whether they are indeed distinct central nervous system (CNS) entities, or whether they exist along a spectrum and are actually two different manifestations of the same disease process.
A new study utilized a novel methodology — unbiased quantitative mass spectrometry-based proteomics — to investigate this question by analyzing cerebrospinal fluid (CSF) in a group of patients with ME/CFS and another group of patients diagnosed with both ME/CFS and FM.
Close to 2,100 proteins were identified, of which nearly 1,800 were common to both conditions.
“ME/CFS and fibromyalgia do not appear to be distinct entities, with respect to their cerebrospinal fluid proteins,” lead author Steven Schutzer, MD, professor of medicine, Rutgers New Jersey School of Medicine, told this news organization.
“Work is underway to solve the multiple mysteries of ME/CFS, fibromyalgia, and other neurologic-associated diseases,” he continued. “We have further affirmed that we have a precise objective discovery tool in our hands. Collectively studying multiple diseases brings clarity to each individual disease.”
The study was published in the December 2023 issue of Annals of Medicine.
Cutting-Edge Technology
“ME/CFS is characterized by disabling fatigue, and FM is an illness characterized by body-wide pain,” Dr. Schutzer said. These “medically unexplained” illnesses often coexist by current definitions, and the overlap between them has suggested that they may be part of the “same illness spectrum.”
But co-investigator Benjamin Natelson, MD, professor of neurology and director of the Pain and Fatigue Study Center, Mount Sinai, New York, and others found in previous research that there are distinct differences between the conditions, raising the possibility that there may be different pathophysiological processes.
“The physicians and scientists on our team have had longstanding interest in studying neurologic diseases with cutting-edge tools such as mass spectrometry applied to CSF,” Dr. Schutzer said. “We have had success using this message to distinguish diseases such as ME/CFS from post-treatment Lyme disease, multiple sclerosis, and healthy normal people.”
Dr. Schutzer explained that Dr. Natelson had acquired CSF samples from “well-characterized [ME/CFS] patients and controls.”
Since the cause of ME/CFS is “unknown,” it seemed “ripe to investigate it further with the discovery tool of mass spectrometry” by harnessing the “most advanced equipment in the country at the pacific Northwest National Laboratory, which is part of the US Department of Energy.”
Dr. Schutzer noted that it was the “merger of different clinical and laboratory expertise” that enabled them to address whether ME/CFS and FM are two distinct disease processes.
The choice of analyzing CSF is that it’s the fluid closest to the brain, he added. “A lot of people have studied ME/CFS peripherally because they don’t have access to spinal fluid or it’s easier to look peripherally in the blood, but that doesn’t mean that the blood is where the real ‘action’ is occurring.”
The researchers compared the CSF of 15 patients with ME/CFS only to 15 patients with ME/CFS+FM using mass spectrometry-based proteomics, which they had employed in previous research to see whether ME/CFS was distinct from persistent neurologic Lyme disease syndrome.
This technology has become the “method of choice and discovery tool to rapidly uncover protein biomarkers that can distinguish one disease from another,” the authors stated.
In particular, in unbiased quantitative mass spectrometry-based proteomics, the researchers do not have to know in advance what’s in a sample before studying it, Dr. Schutzer explained.
Shared Pathophysiology?
Both groups of patients were of similar age (41.3 ± 9.4 years and 40.1 ± 11.0 years, respectively), with no differences in gender or rates of current comorbid psychiatric diagnoses between the groups.
The researchers quantified a total of 2,083 proteins, including 1,789 that were specifically quantified in all of the CSF samples, regardless of the presence or absence of FM.
Several analyses (including an ANOVA analysis with adjusted P values, a Random Forest machine learning approach that looked at relative protein abundance changes between those with ME/CFS and ME/CFS+FM, and unsupervised hierarchical clustering analyses) did not find distinguishing differences between the groups.
the authors stated.
They noted that both conditions are “medically unexplained,” with core symptoms of pain, fatigue, sleep problems, and cognitive difficulty. The fact that these two syndromes coexist so often has led to the assumption that the “similarities between them outweigh the differences,” they wrote.
They pointed to some differences between the conditions, including an increase in substance P in the CSF of FM patients, but not in ME/CFS patients reported by others. There are also some immunological, physiological and genetic differences.
But if the conclusion that the two illnesses may share a similar pathophysiological basis is supported by other research that includes FM-only patients as comparators to those with ME/CFS, “this would support the notion that the two illnesses fall along a common illness spectrum and may be approached as a single entity — with implications for both diagnosis and the development of new treatment approaches,” they concluded.
‘Noncontributory’ Findings
Commenting on the research, Robert G. Lahita, MD, PhD, director of the Institute for Autoimmune and Rheumatic Diseases, St. Joseph Health, Wayne, New Jersey, stated that he does not regard these diseases as neurologic but rather as rheumatologic.
“Most neurologists don’t see these diseases, but as a rheumatologist, I see them every day,” said Dr. Lahita, professor of medicine at Hackensack (New Jersey) Meridian School of Medicine and a clinical professor of medicine at Rutgers New Jersey Medical School, New Brunswick. “ME/CFS isn’t as common in my practice, but we do deal with many post-COVID patients who are afflicted mostly with ME/CFS.”
He noted that an important reason for fatigue in FM is that patients generally don’t sleep, or their sleep is disrupted. This is different from the cause of fatigue in ME/CFS.
In addition, the small sample size and the lack of difference between males and females were both limitations of the current study, said Dr. Lahita, who was not involved in this research. “We know that FM disproportionately affects women — in my practice, for example, over 95% of the patients with FM are female — while ME/CFS affects both genders similarly.”
Using proteomics as a biomarker was also problematic, according to Dr. Lahita. “It would have been more valuable to investigate differences in cytokines, for example,” he suggested.
Ultimately, Dr. Lahita thinks that the study is “non-contributory to the field and, as complex as the analysis was, it does nothing to shed differentiate the two conditions or explain the syndromes themselves.”
He added that it would have been more valuable to compare ME/CFS not only to ME/CFS plus FM but also with FM without ME/CFS and to healthy controls, and perhaps to a group with an autoimmune condition, such as lupus or Hashimoto’s thyroiditis.
Dr. Schutzer acknowledged that a limitation of the current study is that his team was unable analyze the CSF of patients with only FM. He and his colleagues “combed the world’s labs” for existing CSF samples of patients with FM alone but were unable to obtain any. “We see this study as a ‘stepping stone’ and hope that future studies will include patients with FM who are willing to donate CSF samples that we can use for comparison,” he said.
The authors received support from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and National Institute of Neurological Disorders and Stroke. Dr. Schutzer, coauthors, and Dr. Lahita reported no relevant financial relationships.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and fibromyalgia (FM) have overlapping neurologic symptoms — particularly profound fatigue. The similarity between these two conditions has led to the question of whether they are indeed distinct central nervous system (CNS) entities, or whether they exist along a spectrum and are actually two different manifestations of the same disease process.
A new study utilized a novel methodology — unbiased quantitative mass spectrometry-based proteomics — to investigate this question by analyzing cerebrospinal fluid (CSF) in a group of patients with ME/CFS and another group of patients diagnosed with both ME/CFS and FM.
Close to 2,100 proteins were identified, of which nearly 1,800 were common to both conditions.
“ME/CFS and fibromyalgia do not appear to be distinct entities, with respect to their cerebrospinal fluid proteins,” lead author Steven Schutzer, MD, professor of medicine, Rutgers New Jersey School of Medicine, told this news organization.
“Work is underway to solve the multiple mysteries of ME/CFS, fibromyalgia, and other neurologic-associated diseases,” he continued. “We have further affirmed that we have a precise objective discovery tool in our hands. Collectively studying multiple diseases brings clarity to each individual disease.”
The study was published in the December 2023 issue of Annals of Medicine.
Cutting-Edge Technology
“ME/CFS is characterized by disabling fatigue, and FM is an illness characterized by body-wide pain,” Dr. Schutzer said. These “medically unexplained” illnesses often coexist by current definitions, and the overlap between them has suggested that they may be part of the “same illness spectrum.”
But co-investigator Benjamin Natelson, MD, professor of neurology and director of the Pain and Fatigue Study Center, Mount Sinai, New York, and others found in previous research that there are distinct differences between the conditions, raising the possibility that there may be different pathophysiological processes.
“The physicians and scientists on our team have had longstanding interest in studying neurologic diseases with cutting-edge tools such as mass spectrometry applied to CSF,” Dr. Schutzer said. “We have had success using this message to distinguish diseases such as ME/CFS from post-treatment Lyme disease, multiple sclerosis, and healthy normal people.”
Dr. Schutzer explained that Dr. Natelson had acquired CSF samples from “well-characterized [ME/CFS] patients and controls.”
Since the cause of ME/CFS is “unknown,” it seemed “ripe to investigate it further with the discovery tool of mass spectrometry” by harnessing the “most advanced equipment in the country at the pacific Northwest National Laboratory, which is part of the US Department of Energy.”
Dr. Schutzer noted that it was the “merger of different clinical and laboratory expertise” that enabled them to address whether ME/CFS and FM are two distinct disease processes.
The choice of analyzing CSF is that it’s the fluid closest to the brain, he added. “A lot of people have studied ME/CFS peripherally because they don’t have access to spinal fluid or it’s easier to look peripherally in the blood, but that doesn’t mean that the blood is where the real ‘action’ is occurring.”
The researchers compared the CSF of 15 patients with ME/CFS only to 15 patients with ME/CFS+FM using mass spectrometry-based proteomics, which they had employed in previous research to see whether ME/CFS was distinct from persistent neurologic Lyme disease syndrome.
This technology has become the “method of choice and discovery tool to rapidly uncover protein biomarkers that can distinguish one disease from another,” the authors stated.
In particular, in unbiased quantitative mass spectrometry-based proteomics, the researchers do not have to know in advance what’s in a sample before studying it, Dr. Schutzer explained.
Shared Pathophysiology?
Both groups of patients were of similar age (41.3 ± 9.4 years and 40.1 ± 11.0 years, respectively), with no differences in gender or rates of current comorbid psychiatric diagnoses between the groups.
The researchers quantified a total of 2,083 proteins, including 1,789 that were specifically quantified in all of the CSF samples, regardless of the presence or absence of FM.
Several analyses (including an ANOVA analysis with adjusted P values, a Random Forest machine learning approach that looked at relative protein abundance changes between those with ME/CFS and ME/CFS+FM, and unsupervised hierarchical clustering analyses) did not find distinguishing differences between the groups.
the authors stated.
They noted that both conditions are “medically unexplained,” with core symptoms of pain, fatigue, sleep problems, and cognitive difficulty. The fact that these two syndromes coexist so often has led to the assumption that the “similarities between them outweigh the differences,” they wrote.
They pointed to some differences between the conditions, including an increase in substance P in the CSF of FM patients, but not in ME/CFS patients reported by others. There are also some immunological, physiological and genetic differences.
But if the conclusion that the two illnesses may share a similar pathophysiological basis is supported by other research that includes FM-only patients as comparators to those with ME/CFS, “this would support the notion that the two illnesses fall along a common illness spectrum and may be approached as a single entity — with implications for both diagnosis and the development of new treatment approaches,” they concluded.
‘Noncontributory’ Findings
Commenting on the research, Robert G. Lahita, MD, PhD, director of the Institute for Autoimmune and Rheumatic Diseases, St. Joseph Health, Wayne, New Jersey, stated that he does not regard these diseases as neurologic but rather as rheumatologic.
“Most neurologists don’t see these diseases, but as a rheumatologist, I see them every day,” said Dr. Lahita, professor of medicine at Hackensack (New Jersey) Meridian School of Medicine and a clinical professor of medicine at Rutgers New Jersey Medical School, New Brunswick. “ME/CFS isn’t as common in my practice, but we do deal with many post-COVID patients who are afflicted mostly with ME/CFS.”
He noted that an important reason for fatigue in FM is that patients generally don’t sleep, or their sleep is disrupted. This is different from the cause of fatigue in ME/CFS.
In addition, the small sample size and the lack of difference between males and females were both limitations of the current study, said Dr. Lahita, who was not involved in this research. “We know that FM disproportionately affects women — in my practice, for example, over 95% of the patients with FM are female — while ME/CFS affects both genders similarly.”
Using proteomics as a biomarker was also problematic, according to Dr. Lahita. “It would have been more valuable to investigate differences in cytokines, for example,” he suggested.
Ultimately, Dr. Lahita thinks that the study is “non-contributory to the field and, as complex as the analysis was, it does nothing to shed differentiate the two conditions or explain the syndromes themselves.”
He added that it would have been more valuable to compare ME/CFS not only to ME/CFS plus FM but also with FM without ME/CFS and to healthy controls, and perhaps to a group with an autoimmune condition, such as lupus or Hashimoto’s thyroiditis.
Dr. Schutzer acknowledged that a limitation of the current study is that his team was unable analyze the CSF of patients with only FM. He and his colleagues “combed the world’s labs” for existing CSF samples of patients with FM alone but were unable to obtain any. “We see this study as a ‘stepping stone’ and hope that future studies will include patients with FM who are willing to donate CSF samples that we can use for comparison,” he said.
The authors received support from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and National Institute of Neurological Disorders and Stroke. Dr. Schutzer, coauthors, and Dr. Lahita reported no relevant financial relationships.
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and fibromyalgia (FM) have overlapping neurologic symptoms — particularly profound fatigue. The similarity between these two conditions has led to the question of whether they are indeed distinct central nervous system (CNS) entities, or whether they exist along a spectrum and are actually two different manifestations of the same disease process.
A new study utilized a novel methodology — unbiased quantitative mass spectrometry-based proteomics — to investigate this question by analyzing cerebrospinal fluid (CSF) in a group of patients with ME/CFS and another group of patients diagnosed with both ME/CFS and FM.
Close to 2,100 proteins were identified, of which nearly 1,800 were common to both conditions.
“ME/CFS and fibromyalgia do not appear to be distinct entities, with respect to their cerebrospinal fluid proteins,” lead author Steven Schutzer, MD, professor of medicine, Rutgers New Jersey School of Medicine, told this news organization.
“Work is underway to solve the multiple mysteries of ME/CFS, fibromyalgia, and other neurologic-associated diseases,” he continued. “We have further affirmed that we have a precise objective discovery tool in our hands. Collectively studying multiple diseases brings clarity to each individual disease.”
The study was published in the December 2023 issue of Annals of Medicine.
Cutting-Edge Technology
“ME/CFS is characterized by disabling fatigue, and FM is an illness characterized by body-wide pain,” Dr. Schutzer said. These “medically unexplained” illnesses often coexist by current definitions, and the overlap between them has suggested that they may be part of the “same illness spectrum.”
But co-investigator Benjamin Natelson, MD, professor of neurology and director of the Pain and Fatigue Study Center, Mount Sinai, New York, and others found in previous research that there are distinct differences between the conditions, raising the possibility that there may be different pathophysiological processes.
“The physicians and scientists on our team have had longstanding interest in studying neurologic diseases with cutting-edge tools such as mass spectrometry applied to CSF,” Dr. Schutzer said. “We have had success using this message to distinguish diseases such as ME/CFS from post-treatment Lyme disease, multiple sclerosis, and healthy normal people.”
Dr. Schutzer explained that Dr. Natelson had acquired CSF samples from “well-characterized [ME/CFS] patients and controls.”
Since the cause of ME/CFS is “unknown,” it seemed “ripe to investigate it further with the discovery tool of mass spectrometry” by harnessing the “most advanced equipment in the country at the pacific Northwest National Laboratory, which is part of the US Department of Energy.”
Dr. Schutzer noted that it was the “merger of different clinical and laboratory expertise” that enabled them to address whether ME/CFS and FM are two distinct disease processes.
The choice of analyzing CSF is that it’s the fluid closest to the brain, he added. “A lot of people have studied ME/CFS peripherally because they don’t have access to spinal fluid or it’s easier to look peripherally in the blood, but that doesn’t mean that the blood is where the real ‘action’ is occurring.”
The researchers compared the CSF of 15 patients with ME/CFS only to 15 patients with ME/CFS+FM using mass spectrometry-based proteomics, which they had employed in previous research to see whether ME/CFS was distinct from persistent neurologic Lyme disease syndrome.
This technology has become the “method of choice and discovery tool to rapidly uncover protein biomarkers that can distinguish one disease from another,” the authors stated.
In particular, in unbiased quantitative mass spectrometry-based proteomics, the researchers do not have to know in advance what’s in a sample before studying it, Dr. Schutzer explained.
Shared Pathophysiology?
Both groups of patients were of similar age (41.3 ± 9.4 years and 40.1 ± 11.0 years, respectively), with no differences in gender or rates of current comorbid psychiatric diagnoses between the groups.
The researchers quantified a total of 2,083 proteins, including 1,789 that were specifically quantified in all of the CSF samples, regardless of the presence or absence of FM.
Several analyses (including an ANOVA analysis with adjusted P values, a Random Forest machine learning approach that looked at relative protein abundance changes between those with ME/CFS and ME/CFS+FM, and unsupervised hierarchical clustering analyses) did not find distinguishing differences between the groups.
the authors stated.
They noted that both conditions are “medically unexplained,” with core symptoms of pain, fatigue, sleep problems, and cognitive difficulty. The fact that these two syndromes coexist so often has led to the assumption that the “similarities between them outweigh the differences,” they wrote.
They pointed to some differences between the conditions, including an increase in substance P in the CSF of FM patients, but not in ME/CFS patients reported by others. There are also some immunological, physiological and genetic differences.
But if the conclusion that the two illnesses may share a similar pathophysiological basis is supported by other research that includes FM-only patients as comparators to those with ME/CFS, “this would support the notion that the two illnesses fall along a common illness spectrum and may be approached as a single entity — with implications for both diagnosis and the development of new treatment approaches,” they concluded.
‘Noncontributory’ Findings
Commenting on the research, Robert G. Lahita, MD, PhD, director of the Institute for Autoimmune and Rheumatic Diseases, St. Joseph Health, Wayne, New Jersey, stated that he does not regard these diseases as neurologic but rather as rheumatologic.
“Most neurologists don’t see these diseases, but as a rheumatologist, I see them every day,” said Dr. Lahita, professor of medicine at Hackensack (New Jersey) Meridian School of Medicine and a clinical professor of medicine at Rutgers New Jersey Medical School, New Brunswick. “ME/CFS isn’t as common in my practice, but we do deal with many post-COVID patients who are afflicted mostly with ME/CFS.”
He noted that an important reason for fatigue in FM is that patients generally don’t sleep, or their sleep is disrupted. This is different from the cause of fatigue in ME/CFS.
In addition, the small sample size and the lack of difference between males and females were both limitations of the current study, said Dr. Lahita, who was not involved in this research. “We know that FM disproportionately affects women — in my practice, for example, over 95% of the patients with FM are female — while ME/CFS affects both genders similarly.”
Using proteomics as a biomarker was also problematic, according to Dr. Lahita. “It would have been more valuable to investigate differences in cytokines, for example,” he suggested.
Ultimately, Dr. Lahita thinks that the study is “non-contributory to the field and, as complex as the analysis was, it does nothing to shed differentiate the two conditions or explain the syndromes themselves.”
He added that it would have been more valuable to compare ME/CFS not only to ME/CFS plus FM but also with FM without ME/CFS and to healthy controls, and perhaps to a group with an autoimmune condition, such as lupus or Hashimoto’s thyroiditis.
Dr. Schutzer acknowledged that a limitation of the current study is that his team was unable analyze the CSF of patients with only FM. He and his colleagues “combed the world’s labs” for existing CSF samples of patients with FM alone but were unable to obtain any. “We see this study as a ‘stepping stone’ and hope that future studies will include patients with FM who are willing to donate CSF samples that we can use for comparison,” he said.
The authors received support from the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and National Institute of Neurological Disorders and Stroke. Dr. Schutzer, coauthors, and Dr. Lahita reported no relevant financial relationships.
Coming Soon: The First mRNA Vaccine for Melanoma?
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Moderna and Merck have presented promising results from their phase 2b clinical trial that investigated a combination of a messenger RNA (mRNA) vaccine and a cancer drug for the treatment of melanoma.
Is mRNA set to shake up the world of cancer treatment? This is certainly what Moderna seems to think; the pharmaceutical company has published the results of a phase 2b trial combining its mRNA vaccine (mRNA-4157 [V940]) with Merck’s cancer drug KEYTRUDA. While these are not the final results but rather mid-term data from the 3-year follow-up, they are somewhat promising. The randomized KEYNOTE-942/mRNA-4157-P201 clinical trial involves patients with high-risk (stage III/IV) melanoma following complete resection.
Relapse Risk Halved
Treatment with mRNA-4157 (V940) in combination with pembrolizumab led to a clinically meaningful improvement in recurrence-free survival, reducing the risk for recurrence or death by 49%, compared with pembrolizumab alone. T, reducing the risk of developing distant metastasis or death by 62%. “The KEYNOTE-942/mRNA-4157-P201 study was the first demonstration of efficacy for an investigational mRNA cancer treatment in a randomized clinical trial and the first combination therapy to show a significant benefit over pembrolizumab alone in adjuvant melanoma,” said Kyle Holen, MD, Moderna’s senior vice president, after presenting these results.
Side Effects
The combined treatment also did not demonstrate more significant side effects than pembrolizumab alone. The number of patients reporting treatment-related adverse events of grade 3 or greater was similar between the arms (25% for mRNA-4157 [V940] with pembrolizumab vs 20% for KEYTRUDA alone). The most common adverse events of any grade attributed to mRNA-4157 (V940) were fatigue (60.6%), injection site pain (56.7%), and chills (49%). Based on data from the phase 2b KEYNOTE-942/mRNA-4157-P201 study, the US Food and Drug Administration and European Medicines Agency granted breakthrough therapy designation and recognition under the the Priority Medicines scheme, respectively, for mRNA-4157 (V940) in combination with KEYTRUDA for the adjuvant treatment of patients with high-risk melanoma.
Phase 3 Trial
In July, Moderna and Merck announced the launch of a phase 3 trial, assessing “mRNA-4157 [V940] in combination with pembrolizumab as adjuvant treatment in patients with high-risk resected melanoma [stages IIB-IV].” Stéphane Bancel, Moderna’s director general, believes that an mRNA vaccine for melanoma could be available in 2025.
Other Cancer Vaccines
Moderna is not the only laboratory to set its sights on developing a vaccine for cancer. In May, BioNTech, in partnership with Roche, proposed a phase 1 clinical trial of a vaccine targeting pancreatic cancer in Nature. In June, at the American Society of Clinical Oncology›s conference, Transgene presented its conclusions concerning its viral vector vaccines against ENT and papillomavirus-linked cancers. And in September, Ose Immunotherapeutics made headlines with its vaccine for advanced lung cancer.
This article was translated from Univadis France, which is part of the Medscape Professional Network.
Efficacy of Topical Clascoterone for Acne Increased Over Time, Analysis Shows
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- A 1% cream formulation of clascoterone, a topical androgen receptor inhibitor, is approved for the treatment of acne vulgaris in patients aged 12 years and older based on results from two identical phase 3 12-week trials, NCT02608450 and NCT02608476, and a long-term extension (LTE) study.
- The purpose of the current study was to evaluate the integrated efficacy of clascoterone cream 1% (Winlevi) in the intention-to-treat population of patients from all three trials.
- In the pivotal trials, investigators randomized patients with acne 1:1 to receive clascoterone cream 1% or vehicle twice daily for 12 weeks. Participants were eligible to enter the LTE study, in which patients applied clascoterone to the face, and if they wanted to, the trunk for up to 9 more months.
- To assess combined efficacy, researchers evaluated the proportion of patients who achieved an Investigator’s Global Assessment (IGA) of 0 or 1.
TAKEAWAY:
- Of the 1143 patients from the pivotal trials who completed 12 weeks of treatment, 576 were in the clascoterone group and 567 were in the vehicle group. Of the 600 patients who entered the LTE study, 311 were in the clascoterone group and 289 were in the vehicle group. Of these, 343 completed the LTE study.
- At week 12, the proportion of patients who achieved treatment success was higher in the clascoterone group than in the vehicle group (19.9% vs 7.7%, respectively; P < .0001).
- In the LTE study, the proportion of patients previously treated with clascoterone who achieved a facial IGA of 0/1 increased from 13.5% at extension day 0 to 29.9% at extension day 274, while the proportion of patients previously treated with vehicle and switched to clascoterone who achieved a facial IGA of 0/1 increased from 6.2% at extension day 0 to 30.4% at extension day 274.
- Similarly, the proportion of patients in the LTE study with a truncal IGA of 0/1 increased from 4.9% at extension day 0 to 31.7% on extension day 274.
IN PRACTICE:
“Clinicians may consider counseling patients that treatment persistence is required to maximize the efficacy of clascoterone treatment,” the authors concluded.
SOURCE:
Lawrence F. Eichenfield, MD, of the departments of dermatology and pediatrics at the University of California and Rady Children’s Hospital, San Diego, California, led the research. The study was published in the January 2024 issue of the Journal of Drugs in Dermatology.
LIMITATIONS:
There was a high patient discontinuation rate before and during the LET study. Also, no assessment was made as to how clascoterone affected patients’ quality of life.
DISCLOSURES:
Clascoterone manufacturer Cassiopea funded the studies. Dr. Eichenfield and fellow investigators Adelaide A. Hebert, MD, and Linda Stein Gold, MD, received compensation from Cassiopea as advisers and disclosed ties to many other pharmaceutical companies.
A version of this article appeared on Medscape.com.




