User login
TKI preserved lung function in patients with fibrosing interstitial disease
MADRID – according to findings from a phase 3, placebo-controlled trial presented at the annual congress of the European Respiratory Society.
The trial, called INBUILD, enrolled patients who had a progressive lung disease with a fibrosing phenotype, such as interstitial pneumonia with autoimmune features (IPAF) or noninterstitial pneumonia (NSIP), on the premise that these conditions might share a pathology responsive to a common therapy, explained Kevin R. Flaherty, MD, of National Jewish Health, Denver. The INBUILD trial was a randomized, double-blind, placebo-controlled, parallel-group trial conducted at 153 sites in 15 countries. A total of 663 patients underwent randomization and received at least one dose of nintedanib (332) or placebo (331).
Patients with fibrosing lung disease affecting more than 10% of lung volume were randomized to 150 mg twice daily of nintedanib, which inhibits intracellular growth factors implicated in fibrosis and is already indicated for IPF, or matching placebo.
On the primary endpoint of change in forced vital capacity (FVC) at 52 weeks, those in the nintedanib arm lost lung function at a rate that was less than half that of those randomized to placebo (–80.8 vs. –187.8 mL/year; P less than .001).
In a preplanned stratification, the protection from nintedanib against a decline in lung function was found to be at least as good in those with a usual interstitial pneumonia (UIP-like) pattern of fibrosis on baseline imaging (–82.9 vs. –211.1 mL/year), compared with those with other fibrotic patterns (–79.0 vs. –154.2 mL/year). The UIP-like subgroup represented about 60% of those enrolled.
“The relative protection from decline in lung function supports the hypothesis that progressive fibrosing interstitial lung diseases have a similar pathobiologic mechanism,” said Dr. Flaherty. Results from the INBUILD were published simultaneously with his ERS presentation (N Engl J Med. 2019 Sep 29. doi: 10.1056/NEJMoa1908681).
The curves documenting change of lung function in favor of nintedanib relative to placebo separated within 12 weeks of treatment initiation, according to Dr. Flaherty. The ERS-invited discussant, Martin Kolb, MD, PhD, professor of respirology, McMaster University, Hamilton, Ont., called the reductions in loss of lung function “profound” and “very impactful.”
However, despite these reductions, there was no significant difference in quality of life as measured with the King’s Brief Interstitial Lung Disease (KBILD) questionnaire, which was a secondary outcome. The problem was that there was little change in KBILD in either group at 52 weeks, limiting the ability to show differences.
The rates of death were numerically lower at 52 weeks in the nintedanib arm for the study overall (4.8% vs. 5.1%) and for the UIP-like subgroup (5.3% vs. 7.8%), but the differences did not reach statistical significance.
A suggestion of benefit was derived from a design feature of INBUILD that called for patients to remain on blinded therapy until all enrolled patients completed the trial. When the effect of nintedanib was evaluated in this extended analysis, the event curves for the combined endpoint of interstitial lung disease or death separated and approached significance.
In this extended analysis, which suggests that clinical benefit is likely to accrue after longer periods of treatment, “we saw similar trends when we looked at mortality as an independent outcome,” Dr. Flaherty reported.
More patients in the nintedanib group discontinued therapy because of adverse events (19.6% vs. 10.3%), but Dr. Flaherty characterized the rate of serious adverse events as “similar.” He made this statement even though several adverse events, particularly those involving the gastrointestinal tract, such as diarrhea (66.9% vs. 23.9%), nausea (28.9% vs. 9.4%), vomiting (18.4% vs. 5.1%), and abdominal pain (10.2% vs. 2.4%), were higher in the nintedanib arm.
The INBUILD trial demonstrates that nintedanib preserves lung function in fibrosing lung diseases other than IPF. In his review of this paper, Dr. Kolb pointed out that non-IPF etiologies represent about 75% of interstitial lung diseases. For these patients “we have no drugs, so there is a big medical need.”
Dr. Flaherty reports no potential conflicts of interest. The study was funded by Boehringer-Ingelheim, which produces nintedanib.
SOURCE: Flaherty KR et al. N Engl J Med. 2019 Sep 29. doi: 10.1056/NEJMoa1908681.
MADRID – according to findings from a phase 3, placebo-controlled trial presented at the annual congress of the European Respiratory Society.
The trial, called INBUILD, enrolled patients who had a progressive lung disease with a fibrosing phenotype, such as interstitial pneumonia with autoimmune features (IPAF) or noninterstitial pneumonia (NSIP), on the premise that these conditions might share a pathology responsive to a common therapy, explained Kevin R. Flaherty, MD, of National Jewish Health, Denver. The INBUILD trial was a randomized, double-blind, placebo-controlled, parallel-group trial conducted at 153 sites in 15 countries. A total of 663 patients underwent randomization and received at least one dose of nintedanib (332) or placebo (331).
Patients with fibrosing lung disease affecting more than 10% of lung volume were randomized to 150 mg twice daily of nintedanib, which inhibits intracellular growth factors implicated in fibrosis and is already indicated for IPF, or matching placebo.
On the primary endpoint of change in forced vital capacity (FVC) at 52 weeks, those in the nintedanib arm lost lung function at a rate that was less than half that of those randomized to placebo (–80.8 vs. –187.8 mL/year; P less than .001).
In a preplanned stratification, the protection from nintedanib against a decline in lung function was found to be at least as good in those with a usual interstitial pneumonia (UIP-like) pattern of fibrosis on baseline imaging (–82.9 vs. –211.1 mL/year), compared with those with other fibrotic patterns (–79.0 vs. –154.2 mL/year). The UIP-like subgroup represented about 60% of those enrolled.
“The relative protection from decline in lung function supports the hypothesis that progressive fibrosing interstitial lung diseases have a similar pathobiologic mechanism,” said Dr. Flaherty. Results from the INBUILD were published simultaneously with his ERS presentation (N Engl J Med. 2019 Sep 29. doi: 10.1056/NEJMoa1908681).
The curves documenting change of lung function in favor of nintedanib relative to placebo separated within 12 weeks of treatment initiation, according to Dr. Flaherty. The ERS-invited discussant, Martin Kolb, MD, PhD, professor of respirology, McMaster University, Hamilton, Ont., called the reductions in loss of lung function “profound” and “very impactful.”
However, despite these reductions, there was no significant difference in quality of life as measured with the King’s Brief Interstitial Lung Disease (KBILD) questionnaire, which was a secondary outcome. The problem was that there was little change in KBILD in either group at 52 weeks, limiting the ability to show differences.
The rates of death were numerically lower at 52 weeks in the nintedanib arm for the study overall (4.8% vs. 5.1%) and for the UIP-like subgroup (5.3% vs. 7.8%), but the differences did not reach statistical significance.
A suggestion of benefit was derived from a design feature of INBUILD that called for patients to remain on blinded therapy until all enrolled patients completed the trial. When the effect of nintedanib was evaluated in this extended analysis, the event curves for the combined endpoint of interstitial lung disease or death separated and approached significance.
In this extended analysis, which suggests that clinical benefit is likely to accrue after longer periods of treatment, “we saw similar trends when we looked at mortality as an independent outcome,” Dr. Flaherty reported.
More patients in the nintedanib group discontinued therapy because of adverse events (19.6% vs. 10.3%), but Dr. Flaherty characterized the rate of serious adverse events as “similar.” He made this statement even though several adverse events, particularly those involving the gastrointestinal tract, such as diarrhea (66.9% vs. 23.9%), nausea (28.9% vs. 9.4%), vomiting (18.4% vs. 5.1%), and abdominal pain (10.2% vs. 2.4%), were higher in the nintedanib arm.
The INBUILD trial demonstrates that nintedanib preserves lung function in fibrosing lung diseases other than IPF. In his review of this paper, Dr. Kolb pointed out that non-IPF etiologies represent about 75% of interstitial lung diseases. For these patients “we have no drugs, so there is a big medical need.”
Dr. Flaherty reports no potential conflicts of interest. The study was funded by Boehringer-Ingelheim, which produces nintedanib.
SOURCE: Flaherty KR et al. N Engl J Med. 2019 Sep 29. doi: 10.1056/NEJMoa1908681.
MADRID – according to findings from a phase 3, placebo-controlled trial presented at the annual congress of the European Respiratory Society.
The trial, called INBUILD, enrolled patients who had a progressive lung disease with a fibrosing phenotype, such as interstitial pneumonia with autoimmune features (IPAF) or noninterstitial pneumonia (NSIP), on the premise that these conditions might share a pathology responsive to a common therapy, explained Kevin R. Flaherty, MD, of National Jewish Health, Denver. The INBUILD trial was a randomized, double-blind, placebo-controlled, parallel-group trial conducted at 153 sites in 15 countries. A total of 663 patients underwent randomization and received at least one dose of nintedanib (332) or placebo (331).
Patients with fibrosing lung disease affecting more than 10% of lung volume were randomized to 150 mg twice daily of nintedanib, which inhibits intracellular growth factors implicated in fibrosis and is already indicated for IPF, or matching placebo.
On the primary endpoint of change in forced vital capacity (FVC) at 52 weeks, those in the nintedanib arm lost lung function at a rate that was less than half that of those randomized to placebo (–80.8 vs. –187.8 mL/year; P less than .001).
In a preplanned stratification, the protection from nintedanib against a decline in lung function was found to be at least as good in those with a usual interstitial pneumonia (UIP-like) pattern of fibrosis on baseline imaging (–82.9 vs. –211.1 mL/year), compared with those with other fibrotic patterns (–79.0 vs. –154.2 mL/year). The UIP-like subgroup represented about 60% of those enrolled.
“The relative protection from decline in lung function supports the hypothesis that progressive fibrosing interstitial lung diseases have a similar pathobiologic mechanism,” said Dr. Flaherty. Results from the INBUILD were published simultaneously with his ERS presentation (N Engl J Med. 2019 Sep 29. doi: 10.1056/NEJMoa1908681).
The curves documenting change of lung function in favor of nintedanib relative to placebo separated within 12 weeks of treatment initiation, according to Dr. Flaherty. The ERS-invited discussant, Martin Kolb, MD, PhD, professor of respirology, McMaster University, Hamilton, Ont., called the reductions in loss of lung function “profound” and “very impactful.”
However, despite these reductions, there was no significant difference in quality of life as measured with the King’s Brief Interstitial Lung Disease (KBILD) questionnaire, which was a secondary outcome. The problem was that there was little change in KBILD in either group at 52 weeks, limiting the ability to show differences.
The rates of death were numerically lower at 52 weeks in the nintedanib arm for the study overall (4.8% vs. 5.1%) and for the UIP-like subgroup (5.3% vs. 7.8%), but the differences did not reach statistical significance.
A suggestion of benefit was derived from a design feature of INBUILD that called for patients to remain on blinded therapy until all enrolled patients completed the trial. When the effect of nintedanib was evaluated in this extended analysis, the event curves for the combined endpoint of interstitial lung disease or death separated and approached significance.
In this extended analysis, which suggests that clinical benefit is likely to accrue after longer periods of treatment, “we saw similar trends when we looked at mortality as an independent outcome,” Dr. Flaherty reported.
More patients in the nintedanib group discontinued therapy because of adverse events (19.6% vs. 10.3%), but Dr. Flaherty characterized the rate of serious adverse events as “similar.” He made this statement even though several adverse events, particularly those involving the gastrointestinal tract, such as diarrhea (66.9% vs. 23.9%), nausea (28.9% vs. 9.4%), vomiting (18.4% vs. 5.1%), and abdominal pain (10.2% vs. 2.4%), were higher in the nintedanib arm.
The INBUILD trial demonstrates that nintedanib preserves lung function in fibrosing lung diseases other than IPF. In his review of this paper, Dr. Kolb pointed out that non-IPF etiologies represent about 75% of interstitial lung diseases. For these patients “we have no drugs, so there is a big medical need.”
Dr. Flaherty reports no potential conflicts of interest. The study was funded by Boehringer-Ingelheim, which produces nintedanib.
SOURCE: Flaherty KR et al. N Engl J Med. 2019 Sep 29. doi: 10.1056/NEJMoa1908681.
REPORTING FROM ERS 2019
Direct-acting antiviral therapy boosts survival for infected HCC patients
Direct-acting antiviral (DAA) therapy significantly reduced the risk of death in patients with hepatitis C infections and a history of hepatocellular carcinoma, based on data from 797 individuals.
Previous studies have reported a benefit of direct-acting antiviral (DAA) therapy for reducing mortality in patients with hepatocellular carcinoma (HCC), but data on its impact in patients with complete responses to HCC therapy are limited, wrote Amit G. Singal, MD, of the University of Texas, Dallas, and colleagues.
In a study published in Gastroenterology, the researchers identified adult HCC patients who achieved complete treatment response between January 2013 and December 2017. The study included patients from 31 locations in the United States and Canada. Complete response to treatment was defined as “disappearance of arterial enhancement from all HCC lesions on contrast-enhanced cross-sectional imaging.”
A total of 383 (48.1%) of patients were randomized to DAA therapy, and 414 (51.9%) did not receive DAA treatment for their HCV infection after complete response to prior HCC therapy.
A total of 43 deaths occurred among DAA patients over 941 person-years of follow-up, compared with 103 deaths over 527 person-years of follow-up for the untreated controls. Overall, DAA therapy was associated with a significantly reduced risk of death (hazard ratio, 0.54), compared with no therapy. Of note, patients with a sustained virologic response showed a reduced risk of death (HR, 0.29), but those without a sustained virologic response to DAA therapy did not (HR, 1.13).
The findings support those from previous studies suggesting that DAA therapy may reduce mortality in patients with a history of HCC, the researchers said.
The study findings were limited by several factors, including potential confounding if DAA was given to patients with better prognoses, the researchers noted. Other limitations include the use of imaging in routine clinical care rather than centralized review, the loss of approximately 9% of the patients to follow-up, and the lack of data on hepatic decompensation during follow-up, the researchers said. However, the results were strengthened by the multicenter design, large cohort, and inclusion of untreated controls, and support the use of DAA therapies as “likely beneficial in HCV-infected patients with a history of HCC,” they concluded.
The study was funded in part by the National Cancer Institute and AbbVie. Dr. Singal disclosed relationships with companies including AbbVie, Gilead, Bayer, Eisai, Wako Diagnostics, Exact Sciences, Exelixis, Roche, Glycotest, and Bristol-Myers Squibb.
SOURCE: Singal AG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.07.040.
Direct-acting antiviral (DAA) therapy significantly reduced the risk of death in patients with hepatitis C infections and a history of hepatocellular carcinoma, based on data from 797 individuals.
Previous studies have reported a benefit of direct-acting antiviral (DAA) therapy for reducing mortality in patients with hepatocellular carcinoma (HCC), but data on its impact in patients with complete responses to HCC therapy are limited, wrote Amit G. Singal, MD, of the University of Texas, Dallas, and colleagues.
In a study published in Gastroenterology, the researchers identified adult HCC patients who achieved complete treatment response between January 2013 and December 2017. The study included patients from 31 locations in the United States and Canada. Complete response to treatment was defined as “disappearance of arterial enhancement from all HCC lesions on contrast-enhanced cross-sectional imaging.”
A total of 383 (48.1%) of patients were randomized to DAA therapy, and 414 (51.9%) did not receive DAA treatment for their HCV infection after complete response to prior HCC therapy.
A total of 43 deaths occurred among DAA patients over 941 person-years of follow-up, compared with 103 deaths over 527 person-years of follow-up for the untreated controls. Overall, DAA therapy was associated with a significantly reduced risk of death (hazard ratio, 0.54), compared with no therapy. Of note, patients with a sustained virologic response showed a reduced risk of death (HR, 0.29), but those without a sustained virologic response to DAA therapy did not (HR, 1.13).
The findings support those from previous studies suggesting that DAA therapy may reduce mortality in patients with a history of HCC, the researchers said.
The study findings were limited by several factors, including potential confounding if DAA was given to patients with better prognoses, the researchers noted. Other limitations include the use of imaging in routine clinical care rather than centralized review, the loss of approximately 9% of the patients to follow-up, and the lack of data on hepatic decompensation during follow-up, the researchers said. However, the results were strengthened by the multicenter design, large cohort, and inclusion of untreated controls, and support the use of DAA therapies as “likely beneficial in HCV-infected patients with a history of HCC,” they concluded.
The study was funded in part by the National Cancer Institute and AbbVie. Dr. Singal disclosed relationships with companies including AbbVie, Gilead, Bayer, Eisai, Wako Diagnostics, Exact Sciences, Exelixis, Roche, Glycotest, and Bristol-Myers Squibb.
SOURCE: Singal AG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.07.040.
Direct-acting antiviral (DAA) therapy significantly reduced the risk of death in patients with hepatitis C infections and a history of hepatocellular carcinoma, based on data from 797 individuals.
Previous studies have reported a benefit of direct-acting antiviral (DAA) therapy for reducing mortality in patients with hepatocellular carcinoma (HCC), but data on its impact in patients with complete responses to HCC therapy are limited, wrote Amit G. Singal, MD, of the University of Texas, Dallas, and colleagues.
In a study published in Gastroenterology, the researchers identified adult HCC patients who achieved complete treatment response between January 2013 and December 2017. The study included patients from 31 locations in the United States and Canada. Complete response to treatment was defined as “disappearance of arterial enhancement from all HCC lesions on contrast-enhanced cross-sectional imaging.”
A total of 383 (48.1%) of patients were randomized to DAA therapy, and 414 (51.9%) did not receive DAA treatment for their HCV infection after complete response to prior HCC therapy.
A total of 43 deaths occurred among DAA patients over 941 person-years of follow-up, compared with 103 deaths over 527 person-years of follow-up for the untreated controls. Overall, DAA therapy was associated with a significantly reduced risk of death (hazard ratio, 0.54), compared with no therapy. Of note, patients with a sustained virologic response showed a reduced risk of death (HR, 0.29), but those without a sustained virologic response to DAA therapy did not (HR, 1.13).
The findings support those from previous studies suggesting that DAA therapy may reduce mortality in patients with a history of HCC, the researchers said.
The study findings were limited by several factors, including potential confounding if DAA was given to patients with better prognoses, the researchers noted. Other limitations include the use of imaging in routine clinical care rather than centralized review, the loss of approximately 9% of the patients to follow-up, and the lack of data on hepatic decompensation during follow-up, the researchers said. However, the results were strengthened by the multicenter design, large cohort, and inclusion of untreated controls, and support the use of DAA therapies as “likely beneficial in HCV-infected patients with a history of HCC,” they concluded.
The study was funded in part by the National Cancer Institute and AbbVie. Dr. Singal disclosed relationships with companies including AbbVie, Gilead, Bayer, Eisai, Wako Diagnostics, Exact Sciences, Exelixis, Roche, Glycotest, and Bristol-Myers Squibb.
SOURCE: Singal AG et al. Gastroenterology. 2019. doi: 10.1053/j.gastro.2019.07.040.
FROM GASTROENTEROLOGY
NICUs admitting more normal-weight newborns
Almost half of the newborns admitted to U.S. neonatal intensive care units in 2017 were of normal birth weight, according to a new report from the Dartmouth Institute for Health Policy & Clinical Practice.
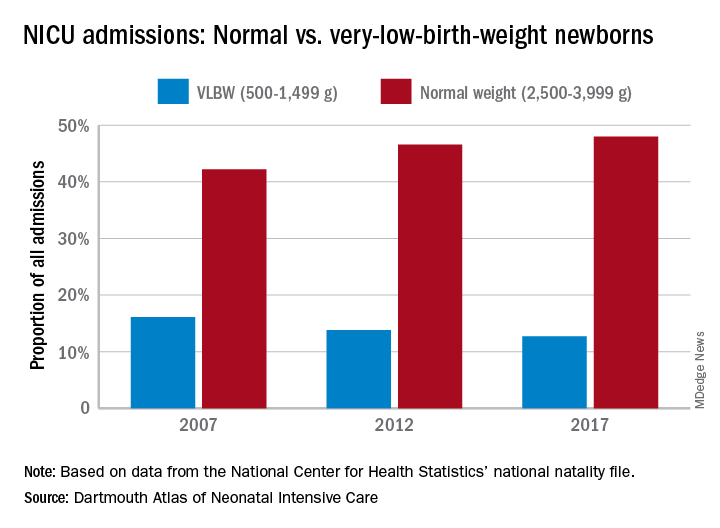
The proportion of NICU admissions involving normal-weight (2,500-3,999 g) newborns increased from 42% in 2007 to 48% in 2017, investigators said in the Dartmouth Atlas of Neonatal Intensive Care. Over that same period, admissions of very-low-birth-weight (500-1,499 g) babies dropped from 16% to 13% of the total.
Those changes were part of a larger, longer-term trend. “The expansion of NICUs and beds in recent decades has been associated with changes in the newborn population receiving NICU care,” the investigators said in the report.
The number of NICU beds increased by 65% from 1995 to 2013, and the number of neonatologists rose by 75% from 1996 to 2013. “At the same time, ” they said in a written statement.
The increases in NICU and neonatologist supply, however, did not always follow the need for such care. Areas of the country with high rates of newborn prematurity, or of risk factors such as low maternal education levels or high cesarean section rates, do not have higher supplies of NICU beds or neonatologists, the researchers noted.
“We should not spare a dollar in providing the best care for newborns. But spending more doesn’t help infants if they could receive the care they need in a maternity unit or home with their mothers. It is very troubling that such a valuable and expensive health care resource is not distributed where it is needed,” said principal author David C. Goodman, MD, of the Dartmouth Institute for Health Policy & Clinical Practice in Lebanon, N.H.
Almost half of the newborns admitted to U.S. neonatal intensive care units in 2017 were of normal birth weight, according to a new report from the Dartmouth Institute for Health Policy & Clinical Practice.

The proportion of NICU admissions involving normal-weight (2,500-3,999 g) newborns increased from 42% in 2007 to 48% in 2017, investigators said in the Dartmouth Atlas of Neonatal Intensive Care. Over that same period, admissions of very-low-birth-weight (500-1,499 g) babies dropped from 16% to 13% of the total.
Those changes were part of a larger, longer-term trend. “The expansion of NICUs and beds in recent decades has been associated with changes in the newborn population receiving NICU care,” the investigators said in the report.
The number of NICU beds increased by 65% from 1995 to 2013, and the number of neonatologists rose by 75% from 1996 to 2013. “At the same time, ” they said in a written statement.
The increases in NICU and neonatologist supply, however, did not always follow the need for such care. Areas of the country with high rates of newborn prematurity, or of risk factors such as low maternal education levels or high cesarean section rates, do not have higher supplies of NICU beds or neonatologists, the researchers noted.
“We should not spare a dollar in providing the best care for newborns. But spending more doesn’t help infants if they could receive the care they need in a maternity unit or home with their mothers. It is very troubling that such a valuable and expensive health care resource is not distributed where it is needed,” said principal author David C. Goodman, MD, of the Dartmouth Institute for Health Policy & Clinical Practice in Lebanon, N.H.
Almost half of the newborns admitted to U.S. neonatal intensive care units in 2017 were of normal birth weight, according to a new report from the Dartmouth Institute for Health Policy & Clinical Practice.

The proportion of NICU admissions involving normal-weight (2,500-3,999 g) newborns increased from 42% in 2007 to 48% in 2017, investigators said in the Dartmouth Atlas of Neonatal Intensive Care. Over that same period, admissions of very-low-birth-weight (500-1,499 g) babies dropped from 16% to 13% of the total.
Those changes were part of a larger, longer-term trend. “The expansion of NICUs and beds in recent decades has been associated with changes in the newborn population receiving NICU care,” the investigators said in the report.
The number of NICU beds increased by 65% from 1995 to 2013, and the number of neonatologists rose by 75% from 1996 to 2013. “At the same time, ” they said in a written statement.
The increases in NICU and neonatologist supply, however, did not always follow the need for such care. Areas of the country with high rates of newborn prematurity, or of risk factors such as low maternal education levels or high cesarean section rates, do not have higher supplies of NICU beds or neonatologists, the researchers noted.
“We should not spare a dollar in providing the best care for newborns. But spending more doesn’t help infants if they could receive the care they need in a maternity unit or home with their mothers. It is very troubling that such a valuable and expensive health care resource is not distributed where it is needed,” said principal author David C. Goodman, MD, of the Dartmouth Institute for Health Policy & Clinical Practice in Lebanon, N.H.
Caution with IVC filters in elderly
Background: Acute pulmonary embolism is a common cause of morbidity and mortality in older adults, and IVC filters have historically and frequently been used to prevent subsequent PE. Almost one in six elderly Medicare fee-for-service (FFS) beneficiaries with PE currently receives an IVC filter.
Study design: Retrospective, matched cohort study.
Setting: United States inpatients during 2011-2014.
Synopsis: Of 214,579 Medicare FFS patients aged 65 years or older who were hospitalized for acute PE, 13.4% received an IVC filter. Mortality was higher in those receiving an IVC filter (11.6%), compared with those who did not receive an IVC filter (9.3%), with an adjusted odds ratio of 30-day mortality of 1.02 (95% CI, 0.98-1.06). One-year mortality rates were 20.5% in the IVC filter group and 13.4% in the group with no IVC filter, with an adjusted OR of 1.35 (95% CI, 1.31-1.40).
In the 76,198 Medicare FFS patients hospitalized with acute PE in the matched cohort group, 18.2% received an IVC filter. The IVC-filter group had higher odds for 30-day mortality, compared with the no–IVC filter group (OR, 2.19; 95% CI, 2.06-2.33).
Bottom line: In patients aged 65 years or older, use caution when considering IVC filter placement for prevention of subsequent PE. Future studies across patient subgroups are needed to analyze the safety and value of IVC filters.
Citation: Bikdeli B et al. Association of inferior vena cava filter use with mortality rates in older adults with acute pulmonary embolism. JAMA Intern Med. 2019;179(2):263-5.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: Acute pulmonary embolism is a common cause of morbidity and mortality in older adults, and IVC filters have historically and frequently been used to prevent subsequent PE. Almost one in six elderly Medicare fee-for-service (FFS) beneficiaries with PE currently receives an IVC filter.
Study design: Retrospective, matched cohort study.
Setting: United States inpatients during 2011-2014.
Synopsis: Of 214,579 Medicare FFS patients aged 65 years or older who were hospitalized for acute PE, 13.4% received an IVC filter. Mortality was higher in those receiving an IVC filter (11.6%), compared with those who did not receive an IVC filter (9.3%), with an adjusted odds ratio of 30-day mortality of 1.02 (95% CI, 0.98-1.06). One-year mortality rates were 20.5% in the IVC filter group and 13.4% in the group with no IVC filter, with an adjusted OR of 1.35 (95% CI, 1.31-1.40).
In the 76,198 Medicare FFS patients hospitalized with acute PE in the matched cohort group, 18.2% received an IVC filter. The IVC-filter group had higher odds for 30-day mortality, compared with the no–IVC filter group (OR, 2.19; 95% CI, 2.06-2.33).
Bottom line: In patients aged 65 years or older, use caution when considering IVC filter placement for prevention of subsequent PE. Future studies across patient subgroups are needed to analyze the safety and value of IVC filters.
Citation: Bikdeli B et al. Association of inferior vena cava filter use with mortality rates in older adults with acute pulmonary embolism. JAMA Intern Med. 2019;179(2):263-5.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: Acute pulmonary embolism is a common cause of morbidity and mortality in older adults, and IVC filters have historically and frequently been used to prevent subsequent PE. Almost one in six elderly Medicare fee-for-service (FFS) beneficiaries with PE currently receives an IVC filter.
Study design: Retrospective, matched cohort study.
Setting: United States inpatients during 2011-2014.
Synopsis: Of 214,579 Medicare FFS patients aged 65 years or older who were hospitalized for acute PE, 13.4% received an IVC filter. Mortality was higher in those receiving an IVC filter (11.6%), compared with those who did not receive an IVC filter (9.3%), with an adjusted odds ratio of 30-day mortality of 1.02 (95% CI, 0.98-1.06). One-year mortality rates were 20.5% in the IVC filter group and 13.4% in the group with no IVC filter, with an adjusted OR of 1.35 (95% CI, 1.31-1.40).
In the 76,198 Medicare FFS patients hospitalized with acute PE in the matched cohort group, 18.2% received an IVC filter. The IVC-filter group had higher odds for 30-day mortality, compared with the no–IVC filter group (OR, 2.19; 95% CI, 2.06-2.33).
Bottom line: In patients aged 65 years or older, use caution when considering IVC filter placement for prevention of subsequent PE. Future studies across patient subgroups are needed to analyze the safety and value of IVC filters.
Citation: Bikdeli B et al. Association of inferior vena cava filter use with mortality rates in older adults with acute pulmonary embolism. JAMA Intern Med. 2019;179(2):263-5.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Using reimbursement to drive innovation in tumor biomarker tests
Implementing a tiered, value-based reimbursement policy for tumor biomarker tests could be an important part of improving innovation and the quality of this tests.
“Precision medicine in cancer care depends on on accurate tumor biomarker tests (TBTs) to determine prognosis and guide treatment selection,” wrote Michaela Dinan, PhD, of Duke University, Durham, N.C., and colleagues. Their report is in JCO Precision Oncology.
“The true clinical utility of a TBT is often difficult to determine, initiating a vicious cycle in which TBTs are often undervalued and poorly reimbursed because of a lack of high-level evidence of clinical utility,” they continued. “An inconsistent regulatory and reimbursement environment results in unfavorable return on investment in the research and development (R&D) needed to generate the high levels of evidence (LOEs) necessary to demonstrate the clinical utility of TBTs.”
To that end, the authors have developed a tiered, value-based reimbursement policy to help spur innovation and improve the clinical utility of TBTs.
The tiering involves three categories: Opt-In, Opt-Out, and Opt-Alt, with each of these terms referring to a decision that might be made with the results of the TBT compared with decisions that would be made based on standard of care (SOC) without TBT results.
“Often in oncology, the SOC is to treat all patients in a given context, knowing that all patients will be exposed to potential risks and costs but only a fraction will benefit,” Dr. Dinan and colleagues wrote. “Opt-Out TBTs have value and the potential to be directly cost saving by reducing the use of unnecessary or ineffective yet toxic and costly therapies, either by indicating that the patient does not need additional therapy (prognosis) or that the therapy under consideration is unlikely to work.”
And example of Opt-Out TBTs are tests used to determine whether adjuvant chemotherapy should be given to patients with estrogen receptor–positive, human epidermal growth factor receptor 2–negative, and node-negative breast cancer.
For the Opt-In tier, “the SOC is to not treat, and the TBT leads to treatment,” the authors noted. “In this case, the value of the TBT is generated by turning a minimally effective or unacceptable therapy into one that is indicated, and therefore acceptable, because of an altered risk-benefit that justifies or refines treatment. An Opt-In TBT will typically increase costs, but still has value because it may result in improved long-term clinical outcomes.”
An example of an Opt-In TBT is an analysis of germline susceptibility genes, such as BRCA1 or BRCA2, which could show increased odds of developing and dying as a result of breast and/or ovarian cancer, though the risk can be cut with prophylactic mastectomy and salpingo-oophorectomy, respectively.
“In this case, these otherwise unacceptable interventions become appropriate because of the increased chances of long-term survival as a result of the intervention in a high-risk population,” the authors wrote.
The Opt-Alt TBT is a subcategory of Opt-In, where the SOC is treatment A but a positive TBT results in the selection of a different treatment.
In each teir, the minimum reimbursement of the TBT increases with the analytic validity and clinical utility of the test. For example, the highest minimum reimbursement would come with tests that have been validated as a primary endpoint in prospective randomized trials, while the lowest would be TBTs with ad hoc analyses of convenience cohorts. In the middle would be those validated ad secondary endpoints in prospective trials.
“Our tiered approach provides a clear and achievable opportunity for companies to develop high-quality TBTs with a guarantee for a commensurate return on investment,” the authors state. “Although our system does not reward a TBT for having slightly better performance than another for a similar indication, it does so indirectly. We posit that by ensuring a fertile ground for multiple high-quality competing TBTs, market forces will be allowed to then appropriately drive the use of TBTs that perform best, given current and local practices.”
The authors make no economic estimates related to the implementation of this type of system.
SOURCE: Michaela Dinan et al. JCO Precis Oncol. 2019 Sep 18. doi: 10.1200/PO.19.00210.
Implementing a tiered, value-based reimbursement policy for tumor biomarker tests could be an important part of improving innovation and the quality of this tests.
“Precision medicine in cancer care depends on on accurate tumor biomarker tests (TBTs) to determine prognosis and guide treatment selection,” wrote Michaela Dinan, PhD, of Duke University, Durham, N.C., and colleagues. Their report is in JCO Precision Oncology.
“The true clinical utility of a TBT is often difficult to determine, initiating a vicious cycle in which TBTs are often undervalued and poorly reimbursed because of a lack of high-level evidence of clinical utility,” they continued. “An inconsistent regulatory and reimbursement environment results in unfavorable return on investment in the research and development (R&D) needed to generate the high levels of evidence (LOEs) necessary to demonstrate the clinical utility of TBTs.”
To that end, the authors have developed a tiered, value-based reimbursement policy to help spur innovation and improve the clinical utility of TBTs.
The tiering involves three categories: Opt-In, Opt-Out, and Opt-Alt, with each of these terms referring to a decision that might be made with the results of the TBT compared with decisions that would be made based on standard of care (SOC) without TBT results.
“Often in oncology, the SOC is to treat all patients in a given context, knowing that all patients will be exposed to potential risks and costs but only a fraction will benefit,” Dr. Dinan and colleagues wrote. “Opt-Out TBTs have value and the potential to be directly cost saving by reducing the use of unnecessary or ineffective yet toxic and costly therapies, either by indicating that the patient does not need additional therapy (prognosis) or that the therapy under consideration is unlikely to work.”
And example of Opt-Out TBTs are tests used to determine whether adjuvant chemotherapy should be given to patients with estrogen receptor–positive, human epidermal growth factor receptor 2–negative, and node-negative breast cancer.
For the Opt-In tier, “the SOC is to not treat, and the TBT leads to treatment,” the authors noted. “In this case, the value of the TBT is generated by turning a minimally effective or unacceptable therapy into one that is indicated, and therefore acceptable, because of an altered risk-benefit that justifies or refines treatment. An Opt-In TBT will typically increase costs, but still has value because it may result in improved long-term clinical outcomes.”
An example of an Opt-In TBT is an analysis of germline susceptibility genes, such as BRCA1 or BRCA2, which could show increased odds of developing and dying as a result of breast and/or ovarian cancer, though the risk can be cut with prophylactic mastectomy and salpingo-oophorectomy, respectively.
“In this case, these otherwise unacceptable interventions become appropriate because of the increased chances of long-term survival as a result of the intervention in a high-risk population,” the authors wrote.
The Opt-Alt TBT is a subcategory of Opt-In, where the SOC is treatment A but a positive TBT results in the selection of a different treatment.
In each teir, the minimum reimbursement of the TBT increases with the analytic validity and clinical utility of the test. For example, the highest minimum reimbursement would come with tests that have been validated as a primary endpoint in prospective randomized trials, while the lowest would be TBTs with ad hoc analyses of convenience cohorts. In the middle would be those validated ad secondary endpoints in prospective trials.
“Our tiered approach provides a clear and achievable opportunity for companies to develop high-quality TBTs with a guarantee for a commensurate return on investment,” the authors state. “Although our system does not reward a TBT for having slightly better performance than another for a similar indication, it does so indirectly. We posit that by ensuring a fertile ground for multiple high-quality competing TBTs, market forces will be allowed to then appropriately drive the use of TBTs that perform best, given current and local practices.”
The authors make no economic estimates related to the implementation of this type of system.
SOURCE: Michaela Dinan et al. JCO Precis Oncol. 2019 Sep 18. doi: 10.1200/PO.19.00210.
Implementing a tiered, value-based reimbursement policy for tumor biomarker tests could be an important part of improving innovation and the quality of this tests.
“Precision medicine in cancer care depends on on accurate tumor biomarker tests (TBTs) to determine prognosis and guide treatment selection,” wrote Michaela Dinan, PhD, of Duke University, Durham, N.C., and colleagues. Their report is in JCO Precision Oncology.
“The true clinical utility of a TBT is often difficult to determine, initiating a vicious cycle in which TBTs are often undervalued and poorly reimbursed because of a lack of high-level evidence of clinical utility,” they continued. “An inconsistent regulatory and reimbursement environment results in unfavorable return on investment in the research and development (R&D) needed to generate the high levels of evidence (LOEs) necessary to demonstrate the clinical utility of TBTs.”
To that end, the authors have developed a tiered, value-based reimbursement policy to help spur innovation and improve the clinical utility of TBTs.
The tiering involves three categories: Opt-In, Opt-Out, and Opt-Alt, with each of these terms referring to a decision that might be made with the results of the TBT compared with decisions that would be made based on standard of care (SOC) without TBT results.
“Often in oncology, the SOC is to treat all patients in a given context, knowing that all patients will be exposed to potential risks and costs but only a fraction will benefit,” Dr. Dinan and colleagues wrote. “Opt-Out TBTs have value and the potential to be directly cost saving by reducing the use of unnecessary or ineffective yet toxic and costly therapies, either by indicating that the patient does not need additional therapy (prognosis) or that the therapy under consideration is unlikely to work.”
And example of Opt-Out TBTs are tests used to determine whether adjuvant chemotherapy should be given to patients with estrogen receptor–positive, human epidermal growth factor receptor 2–negative, and node-negative breast cancer.
For the Opt-In tier, “the SOC is to not treat, and the TBT leads to treatment,” the authors noted. “In this case, the value of the TBT is generated by turning a minimally effective or unacceptable therapy into one that is indicated, and therefore acceptable, because of an altered risk-benefit that justifies or refines treatment. An Opt-In TBT will typically increase costs, but still has value because it may result in improved long-term clinical outcomes.”
An example of an Opt-In TBT is an analysis of germline susceptibility genes, such as BRCA1 or BRCA2, which could show increased odds of developing and dying as a result of breast and/or ovarian cancer, though the risk can be cut with prophylactic mastectomy and salpingo-oophorectomy, respectively.
“In this case, these otherwise unacceptable interventions become appropriate because of the increased chances of long-term survival as a result of the intervention in a high-risk population,” the authors wrote.
The Opt-Alt TBT is a subcategory of Opt-In, where the SOC is treatment A but a positive TBT results in the selection of a different treatment.
In each teir, the minimum reimbursement of the TBT increases with the analytic validity and clinical utility of the test. For example, the highest minimum reimbursement would come with tests that have been validated as a primary endpoint in prospective randomized trials, while the lowest would be TBTs with ad hoc analyses of convenience cohorts. In the middle would be those validated ad secondary endpoints in prospective trials.
“Our tiered approach provides a clear and achievable opportunity for companies to develop high-quality TBTs with a guarantee for a commensurate return on investment,” the authors state. “Although our system does not reward a TBT for having slightly better performance than another for a similar indication, it does so indirectly. We posit that by ensuring a fertile ground for multiple high-quality competing TBTs, market forces will be allowed to then appropriately drive the use of TBTs that perform best, given current and local practices.”
The authors make no economic estimates related to the implementation of this type of system.
SOURCE: Michaela Dinan et al. JCO Precis Oncol. 2019 Sep 18. doi: 10.1200/PO.19.00210.
FROM JCO PRECISION ONCOLOGY
Transcervical ablation of symptomatic uterine fibroids under US guidance
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
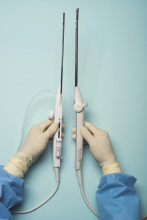
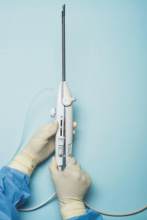
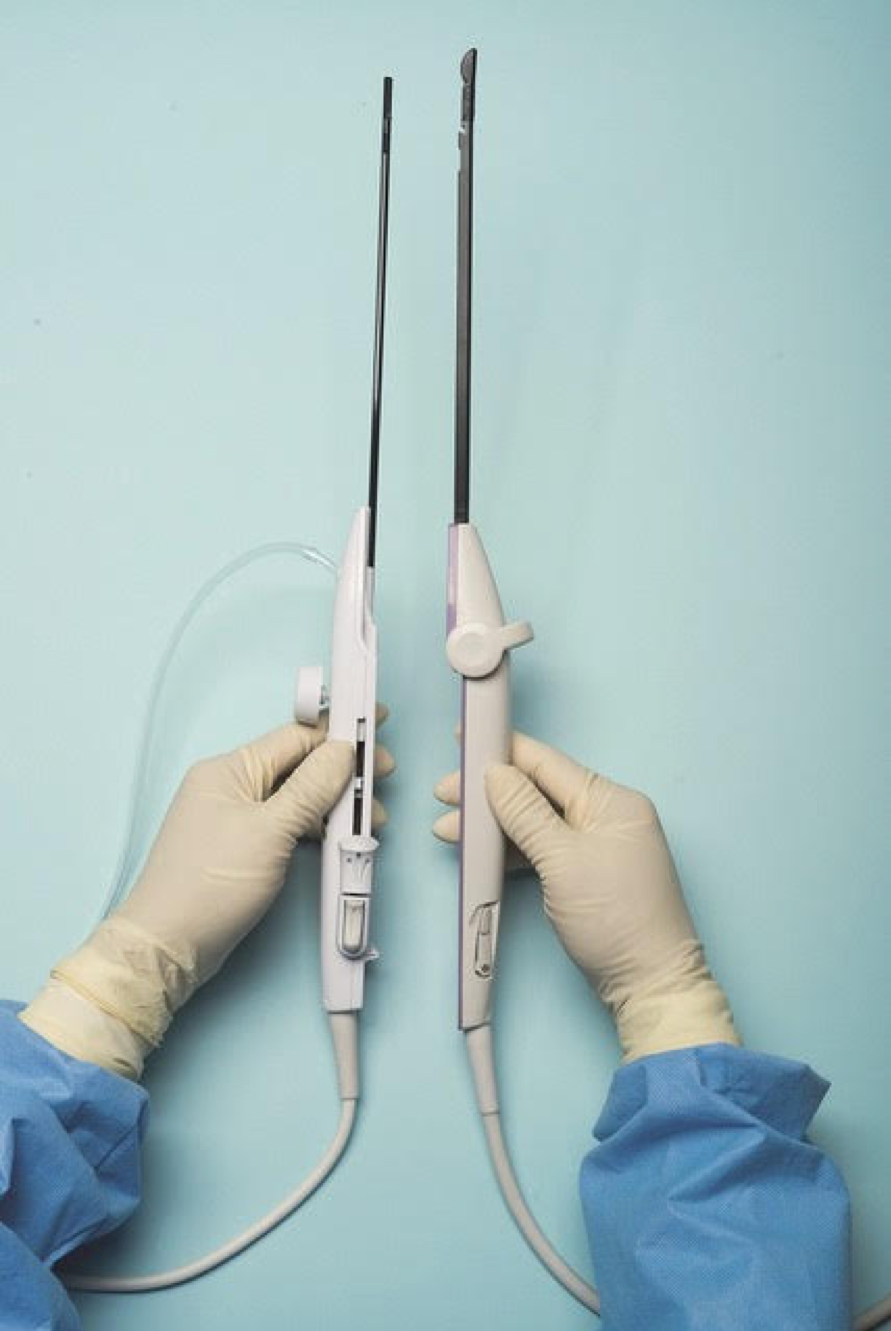
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
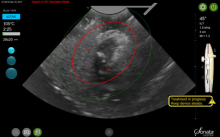
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
Treating uterine fibroids
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at [email protected].
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at [email protected].
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Uterine fibroids are the most common benign tumor in women originating from the smooth muscles of the myometrium. While some women are asymptomatic, others experience pelvic pain, pressure, and abnormal uterine bleeding. Uterine fibroids also are associated with gastrointestinal disturbances; urinary problems; infertility; and obstetrical complications including miscarriages, preterm delivery, and cesarean sections.
The first successful abdominal myomectomy was described in 1845 but the procedure quickly fell out of favor because of unacceptably high mortality rates. Myomectomies require special skills and, at times, are associated with bleeding resulting in massive transfusions or sometimes unwanted hysterectomies. In 1922, Victor Bonney developed a uterine artery clamp which significantly decreased bleeding associated with morbidity and mortality.1
The latter part of the 20th century belonged to the minimally invasive surgery (MIS) evolution. Currently, video- or robotic-assisted laparoscopic myomectomies are increasingly employed in fertility-sparing surgery. In 2014, electromechanical morcellators came under scrutiny with concerns about iatrogenic dissemination of both benign and malignant tissues. A media storm ensued, resulting in the 2014 Food and Drug Administration black-box warning, and electromechanical morcellators were pulled from shelves. Data are being collected to quantify and understand these risks more clearly.
While exposing patients to even a small risk of dissemination of an occult uterine malignancy is unwise, MIS should not be abandoned altogether given its advantages to patients.2 Most recently, the American College of Obstetricians and Gynecologists concluded that, although abdominal hysterectomy or myomectomy may reduce the chance of spreading undiagnosed leiomyosarcoma cells, it is associated with increased morbidity, compared with noninvasive approaches, and ob.gyns. should engage in open decision-making processes and explain nonsurgical options with patients.3
The author of this Master Class, Dr. Charles Miller, a world-renowned MIS surgeon, will enlighten readers on the latest development in noninvasive treatment of symptomatic patients. The Sonata system, a promising transcervical (and thus incisionless) treatment modality utilizing intrauterine sonography–guided radiofrequency ablation for uterine fibroids which does not require general anesthesia or hospitalization. He believes that Sonata “will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.”
Dr. Miller is on the editorial advisory boards of numerous academic journals and serves as the editor of the award-winning Master Class in Gynecologic Surgery column. For this installment, he has stepped into the role of guest author. Dr. Miller has received numerous awards for his educational contributions and was recently granted the distinct honor of taking the lead in the March 28, 2020 Worldwide EndoMarch–Chicago. It is my pleasure to take part in this introduction.
Dr. Nezhat is director of minimally invasive surgery and robotics as well as the medical director of training and education at Northside Hospital, both in Atlanta. He is fellowship director at Atlanta Center for Special Minimally Invasive Surgery & Reproductive Medicine. Dr. Nezhat also is an adjunct professor of gynecology and obstetrics at Emory University, Atlanta, and is past president of the Society of Reproductive Surgeons and the AAGL. He reported that he has no disclosures relevant to this Master Class. Email him at [email protected].
References
1. BJOG. 2018 Apr;125(5):586.
2. JAMA Oncol. 2015;1(1):78-9.
3. Obstet Gynecol. 2019 Mar;133(3):e238-48.
Cannabis-using MS patients improve cognition with 28 days of abstinence
STOCKHOLM – The good news about cognitive impairment in patients with multiple sclerosis who’ve been using cannabis heavily for symptom relief – even for many years – is that their memory, executive function, and information processing speed will improve significantly once they’ve been off the drug for just 28 days, according to the results of a randomized trial presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“It’s good for neurologists to know that, if they prescribe cannabis or their patient is self-medicating and chooses to stop, their cognition will improve considerably,” observed Cecilia Meza, a coinvestigator in the study led by Anthony Feinstein, MD, professor of psychiatry at the University of Toronto.
But there’s a surprise twist to this study, she explained in an interview: “We showed patients their results, and they also felt that their cognition was doing a lot better, but despite that, they would rather be using cannabis to feel better than to have their memory intact. The pain was that bad,” said Ms. Meza, a research coordinator at the university’s Sunnybrook Research Institute.
It’s known that cognitive impairment in healthy long-term cannabis users, provided they started as adults, is fully reversed after 28 days of abstinence. But disease-related cognitive dysfunction affects 40%-80% of patients with MS, and cannabis use may compound this impairment.
The study included 40 MS patients with global impairment of cognition, none of whom were cannabis users prior to their diagnosis. They typically started using it for MS symptom relief 2-3 years after receiving their diagnosis. By the time they were approached for study participation, they had been using cannabis four to five times per day or more for an average of 7 years for relief of symptoms, including incontinence, spasticity, poor sleep, headaches, and difficulties in eating.
All participants were willing to try 28 days of abstinence; half were randomized to do so, while the others stayed the course. Study endpoints included change from baseline to day 28 in the Brief Repeatable Neuropsychological Battery, functional MRI done while taking the Symbol Digit Modalities Test, and urine testing to assure compliance with abstinence.
By day 28, the abstinence group – and with one exception, urine testing confirmed they were bona fide cannabis quitters for the study duration – performed significantly better on the neuropsychological test battery than at baseline, with an associated significant increase in brain activation in the bilateral inferior frontal gyri, as well as the caudate and declive cerebellum while executing the Symbol Digit Modalities Test. The control group who kept on using cannabis showed no such improvements.
The full study details were published in conjunction with Ms. Meza’s presentation (Brain. 2019 Sep 1;142[9]:2800-12).
She reported having no financial conflicts regarding the study, funded by the Multiple Sclerosis Society of Canada.
SOURCE: Meza C. ECTRIMS 2019, Abstract P542.
STOCKHOLM – The good news about cognitive impairment in patients with multiple sclerosis who’ve been using cannabis heavily for symptom relief – even for many years – is that their memory, executive function, and information processing speed will improve significantly once they’ve been off the drug for just 28 days, according to the results of a randomized trial presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“It’s good for neurologists to know that, if they prescribe cannabis or their patient is self-medicating and chooses to stop, their cognition will improve considerably,” observed Cecilia Meza, a coinvestigator in the study led by Anthony Feinstein, MD, professor of psychiatry at the University of Toronto.
But there’s a surprise twist to this study, she explained in an interview: “We showed patients their results, and they also felt that their cognition was doing a lot better, but despite that, they would rather be using cannabis to feel better than to have their memory intact. The pain was that bad,” said Ms. Meza, a research coordinator at the university’s Sunnybrook Research Institute.
It’s known that cognitive impairment in healthy long-term cannabis users, provided they started as adults, is fully reversed after 28 days of abstinence. But disease-related cognitive dysfunction affects 40%-80% of patients with MS, and cannabis use may compound this impairment.
The study included 40 MS patients with global impairment of cognition, none of whom were cannabis users prior to their diagnosis. They typically started using it for MS symptom relief 2-3 years after receiving their diagnosis. By the time they were approached for study participation, they had been using cannabis four to five times per day or more for an average of 7 years for relief of symptoms, including incontinence, spasticity, poor sleep, headaches, and difficulties in eating.
All participants were willing to try 28 days of abstinence; half were randomized to do so, while the others stayed the course. Study endpoints included change from baseline to day 28 in the Brief Repeatable Neuropsychological Battery, functional MRI done while taking the Symbol Digit Modalities Test, and urine testing to assure compliance with abstinence.
By day 28, the abstinence group – and with one exception, urine testing confirmed they were bona fide cannabis quitters for the study duration – performed significantly better on the neuropsychological test battery than at baseline, with an associated significant increase in brain activation in the bilateral inferior frontal gyri, as well as the caudate and declive cerebellum while executing the Symbol Digit Modalities Test. The control group who kept on using cannabis showed no such improvements.
The full study details were published in conjunction with Ms. Meza’s presentation (Brain. 2019 Sep 1;142[9]:2800-12).
She reported having no financial conflicts regarding the study, funded by the Multiple Sclerosis Society of Canada.
SOURCE: Meza C. ECTRIMS 2019, Abstract P542.
STOCKHOLM – The good news about cognitive impairment in patients with multiple sclerosis who’ve been using cannabis heavily for symptom relief – even for many years – is that their memory, executive function, and information processing speed will improve significantly once they’ve been off the drug for just 28 days, according to the results of a randomized trial presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis.
“It’s good for neurologists to know that, if they prescribe cannabis or their patient is self-medicating and chooses to stop, their cognition will improve considerably,” observed Cecilia Meza, a coinvestigator in the study led by Anthony Feinstein, MD, professor of psychiatry at the University of Toronto.
But there’s a surprise twist to this study, she explained in an interview: “We showed patients their results, and they also felt that their cognition was doing a lot better, but despite that, they would rather be using cannabis to feel better than to have their memory intact. The pain was that bad,” said Ms. Meza, a research coordinator at the university’s Sunnybrook Research Institute.
It’s known that cognitive impairment in healthy long-term cannabis users, provided they started as adults, is fully reversed after 28 days of abstinence. But disease-related cognitive dysfunction affects 40%-80% of patients with MS, and cannabis use may compound this impairment.
The study included 40 MS patients with global impairment of cognition, none of whom were cannabis users prior to their diagnosis. They typically started using it for MS symptom relief 2-3 years after receiving their diagnosis. By the time they were approached for study participation, they had been using cannabis four to five times per day or more for an average of 7 years for relief of symptoms, including incontinence, spasticity, poor sleep, headaches, and difficulties in eating.
All participants were willing to try 28 days of abstinence; half were randomized to do so, while the others stayed the course. Study endpoints included change from baseline to day 28 in the Brief Repeatable Neuropsychological Battery, functional MRI done while taking the Symbol Digit Modalities Test, and urine testing to assure compliance with abstinence.
By day 28, the abstinence group – and with one exception, urine testing confirmed they were bona fide cannabis quitters for the study duration – performed significantly better on the neuropsychological test battery than at baseline, with an associated significant increase in brain activation in the bilateral inferior frontal gyri, as well as the caudate and declive cerebellum while executing the Symbol Digit Modalities Test. The control group who kept on using cannabis showed no such improvements.
The full study details were published in conjunction with Ms. Meza’s presentation (Brain. 2019 Sep 1;142[9]:2800-12).
She reported having no financial conflicts regarding the study, funded by the Multiple Sclerosis Society of Canada.
SOURCE: Meza C. ECTRIMS 2019, Abstract P542.
REPORTING FROM ECTRIMS 2019
Preemptive pacifier promotion
I recently encountered an article aimed at parents who were struggling with what to do about their child’s persistent attachment to his pacifier (“How to Ditch the Pacifier,” by Anna Nowogrodski, New York Times, 2019 Sept. 16). For the most part, the author presented a sampling of sound advice from pediatricians and other health experts.
Most children will abandon their pacifiers at a time that is consistent with their developmental stage. Pacifiers seldom do any permanent damage, although they aren’t terribly appealing to look at when hanging out of a toddling toddler’s mouth. Parents were urged to be patient and consistent and were told that allowing the gooey thing to self-destruct often works, as does accelerating the process with a razor blade. Enlisting the aid of the Pacifier Fairy was suggested, but I’m not so sure that would work terribly well.
As I finished perusing the article, I couldn’t help think of how this vexing issue of pacifier removal can be avoided if parents follow a simple rule when they first introduced a pacifier to their child. If experienced parents think back to when they first resorted to using the pacifier, it wasn’t because the plastic and rubber gadget was a family heirloom that had been passed down from generation to generation like an engraved silver spoon. It wasn’t because the dentist told them that children who use pacifiers are less likely to need braces on their teeth. Nor was it a rumor filtered down from speech therapists that pacifiers improve articulation.
Parents reach for a pacifier in hopes that it will help their child will fall asleep. I think most parents of older children agree that at the beginning the pacifier was first and foremost a sleep aid. But here is where the critical oversight occurs: If you give your children pacifiers when you want them to go to sleep, why not simply add the stipulation of where you would like them to go to sleep as well?
Most parents prefer that their children sleep in their own space. We can argue of whether that should be in a side sleeper or their own crib, but most parents don’t want their 3-year-olds sleeping in their bed. Nor do they want their children sleeping on the couch in the living room with them while they watch a movie at 10:30 at night. And as pediatricians, we prefer that children not sleep with their necks flexed in a car seat or baby rocker, particularly if they’re a preemie.
Augmenting the primary association between sleep and the pacifier by adding a place has several important advantages. It gives parents more control of where their children will sleep or, more importantly, where they won’t be sleeping. It helps transitions to nonhome sleeping places like day care and long trips to grandma’s house go more smoothly.
Even more importantly, the crib/pacifier association helps parents who have had trouble reading their children’s cues. If they want a pacifier, it means they are tired and want to go to where the pacifier lives: bed
Finally, maintaining the link between sleeping and the pacifier promotes a more natural weaning process than going cold turkey or hiring the Pacifier Fairy. As naps disappear, the pacifier gradually become a less obvious accessory in the child’s life. However, it may linger in the background as a reminder of when the child needs some restorative sleep.
Of course, helping parents to think clearly enough to create and enforce a simple rule long enough to forge a healthy association when they are sleep deprived themselves is just another one of those challenges we must accept as concerned primary care pediatricians.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Is My Child Overtired? The Sleep Solution for Raising Happier, Healthier Children.” Email him at [email protected].
I recently encountered an article aimed at parents who were struggling with what to do about their child’s persistent attachment to his pacifier (“How to Ditch the Pacifier,” by Anna Nowogrodski, New York Times, 2019 Sept. 16). For the most part, the author presented a sampling of sound advice from pediatricians and other health experts.
Most children will abandon their pacifiers at a time that is consistent with their developmental stage. Pacifiers seldom do any permanent damage, although they aren’t terribly appealing to look at when hanging out of a toddling toddler’s mouth. Parents were urged to be patient and consistent and were told that allowing the gooey thing to self-destruct often works, as does accelerating the process with a razor blade. Enlisting the aid of the Pacifier Fairy was suggested, but I’m not so sure that would work terribly well.
As I finished perusing the article, I couldn’t help think of how this vexing issue of pacifier removal can be avoided if parents follow a simple rule when they first introduced a pacifier to their child. If experienced parents think back to when they first resorted to using the pacifier, it wasn’t because the plastic and rubber gadget was a family heirloom that had been passed down from generation to generation like an engraved silver spoon. It wasn’t because the dentist told them that children who use pacifiers are less likely to need braces on their teeth. Nor was it a rumor filtered down from speech therapists that pacifiers improve articulation.
Parents reach for a pacifier in hopes that it will help their child will fall asleep. I think most parents of older children agree that at the beginning the pacifier was first and foremost a sleep aid. But here is where the critical oversight occurs: If you give your children pacifiers when you want them to go to sleep, why not simply add the stipulation of where you would like them to go to sleep as well?
Most parents prefer that their children sleep in their own space. We can argue of whether that should be in a side sleeper or their own crib, but most parents don’t want their 3-year-olds sleeping in their bed. Nor do they want their children sleeping on the couch in the living room with them while they watch a movie at 10:30 at night. And as pediatricians, we prefer that children not sleep with their necks flexed in a car seat or baby rocker, particularly if they’re a preemie.
Augmenting the primary association between sleep and the pacifier by adding a place has several important advantages. It gives parents more control of where their children will sleep or, more importantly, where they won’t be sleeping. It helps transitions to nonhome sleeping places like day care and long trips to grandma’s house go more smoothly.
Even more importantly, the crib/pacifier association helps parents who have had trouble reading their children’s cues. If they want a pacifier, it means they are tired and want to go to where the pacifier lives: bed
Finally, maintaining the link between sleeping and the pacifier promotes a more natural weaning process than going cold turkey or hiring the Pacifier Fairy. As naps disappear, the pacifier gradually become a less obvious accessory in the child’s life. However, it may linger in the background as a reminder of when the child needs some restorative sleep.
Of course, helping parents to think clearly enough to create and enforce a simple rule long enough to forge a healthy association when they are sleep deprived themselves is just another one of those challenges we must accept as concerned primary care pediatricians.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Is My Child Overtired? The Sleep Solution for Raising Happier, Healthier Children.” Email him at [email protected].
I recently encountered an article aimed at parents who were struggling with what to do about their child’s persistent attachment to his pacifier (“How to Ditch the Pacifier,” by Anna Nowogrodski, New York Times, 2019 Sept. 16). For the most part, the author presented a sampling of sound advice from pediatricians and other health experts.
Most children will abandon their pacifiers at a time that is consistent with their developmental stage. Pacifiers seldom do any permanent damage, although they aren’t terribly appealing to look at when hanging out of a toddling toddler’s mouth. Parents were urged to be patient and consistent and were told that allowing the gooey thing to self-destruct often works, as does accelerating the process with a razor blade. Enlisting the aid of the Pacifier Fairy was suggested, but I’m not so sure that would work terribly well.
As I finished perusing the article, I couldn’t help think of how this vexing issue of pacifier removal can be avoided if parents follow a simple rule when they first introduced a pacifier to their child. If experienced parents think back to when they first resorted to using the pacifier, it wasn’t because the plastic and rubber gadget was a family heirloom that had been passed down from generation to generation like an engraved silver spoon. It wasn’t because the dentist told them that children who use pacifiers are less likely to need braces on their teeth. Nor was it a rumor filtered down from speech therapists that pacifiers improve articulation.
Parents reach for a pacifier in hopes that it will help their child will fall asleep. I think most parents of older children agree that at the beginning the pacifier was first and foremost a sleep aid. But here is where the critical oversight occurs: If you give your children pacifiers when you want them to go to sleep, why not simply add the stipulation of where you would like them to go to sleep as well?
Most parents prefer that their children sleep in their own space. We can argue of whether that should be in a side sleeper or their own crib, but most parents don’t want their 3-year-olds sleeping in their bed. Nor do they want their children sleeping on the couch in the living room with them while they watch a movie at 10:30 at night. And as pediatricians, we prefer that children not sleep with their necks flexed in a car seat or baby rocker, particularly if they’re a preemie.
Augmenting the primary association between sleep and the pacifier by adding a place has several important advantages. It gives parents more control of where their children will sleep or, more importantly, where they won’t be sleeping. It helps transitions to nonhome sleeping places like day care and long trips to grandma’s house go more smoothly.
Even more importantly, the crib/pacifier association helps parents who have had trouble reading their children’s cues. If they want a pacifier, it means they are tired and want to go to where the pacifier lives: bed
Finally, maintaining the link between sleeping and the pacifier promotes a more natural weaning process than going cold turkey or hiring the Pacifier Fairy. As naps disappear, the pacifier gradually become a less obvious accessory in the child’s life. However, it may linger in the background as a reminder of when the child needs some restorative sleep.
Of course, helping parents to think clearly enough to create and enforce a simple rule long enough to forge a healthy association when they are sleep deprived themselves is just another one of those challenges we must accept as concerned primary care pediatricians.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “Is My Child Overtired? The Sleep Solution for Raising Happier, Healthier Children.” Email him at [email protected].
FDA approves rituximab to treat children with rare vasculitis
The Food and Drug Administration approved rituximab (Rituxan) by injection to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in children 2 years of age and older in combination with glucocorticoid treatment, according to an FDA news release.
These rare forms of vasculitis damage small blood vessels through inflammation and can lead to serious organ failure, including lungs and kidneys.
The Genentech drug received priority review and an orphan drug designation based on the results of a pediatric clinical trial of 25 patients aged 6-17 years with active GPA or MPA who were treated with rituximab in an international multicenter, open-label, uncontrolled study. Patients in the trial were also given methylprednisolone prior to starting treatment.
The trial consisted of a 6-month remission induction phase where patients were treated only with rituximab and glucocorticoids. In addition, patients who had not achieved remission could receive additional treatment, including other therapies, at the discretion of the investigator, according to the FDA. By 6 months, 14 of the patients were in remission, and after 18 months, all 25 patients were in remission.
Rituximab contains a boxed warning regarding increased risks of fatal infusion reactions, potentially fatal severe skin and mouth reactions, hepatitis B virus reactivation that may cause serious or lethal liver problems, and progressive multifocal leukoencephalopathy, a rare, potentially lethal brain infection.
The trial was conducted and sponsored by F. Hoffmann-La Roche, which owns Genentech.
The Food and Drug Administration approved rituximab (Rituxan) by injection to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in children 2 years of age and older in combination with glucocorticoid treatment, according to an FDA news release.
These rare forms of vasculitis damage small blood vessels through inflammation and can lead to serious organ failure, including lungs and kidneys.
The Genentech drug received priority review and an orphan drug designation based on the results of a pediatric clinical trial of 25 patients aged 6-17 years with active GPA or MPA who were treated with rituximab in an international multicenter, open-label, uncontrolled study. Patients in the trial were also given methylprednisolone prior to starting treatment.
The trial consisted of a 6-month remission induction phase where patients were treated only with rituximab and glucocorticoids. In addition, patients who had not achieved remission could receive additional treatment, including other therapies, at the discretion of the investigator, according to the FDA. By 6 months, 14 of the patients were in remission, and after 18 months, all 25 patients were in remission.
Rituximab contains a boxed warning regarding increased risks of fatal infusion reactions, potentially fatal severe skin and mouth reactions, hepatitis B virus reactivation that may cause serious or lethal liver problems, and progressive multifocal leukoencephalopathy, a rare, potentially lethal brain infection.
The trial was conducted and sponsored by F. Hoffmann-La Roche, which owns Genentech.
The Food and Drug Administration approved rituximab (Rituxan) by injection to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in children 2 years of age and older in combination with glucocorticoid treatment, according to an FDA news release.
These rare forms of vasculitis damage small blood vessels through inflammation and can lead to serious organ failure, including lungs and kidneys.
The Genentech drug received priority review and an orphan drug designation based on the results of a pediatric clinical trial of 25 patients aged 6-17 years with active GPA or MPA who were treated with rituximab in an international multicenter, open-label, uncontrolled study. Patients in the trial were also given methylprednisolone prior to starting treatment.
The trial consisted of a 6-month remission induction phase where patients were treated only with rituximab and glucocorticoids. In addition, patients who had not achieved remission could receive additional treatment, including other therapies, at the discretion of the investigator, according to the FDA. By 6 months, 14 of the patients were in remission, and after 18 months, all 25 patients were in remission.
Rituximab contains a boxed warning regarding increased risks of fatal infusion reactions, potentially fatal severe skin and mouth reactions, hepatitis B virus reactivation that may cause serious or lethal liver problems, and progressive multifocal leukoencephalopathy, a rare, potentially lethal brain infection.
The trial was conducted and sponsored by F. Hoffmann-La Roche, which owns Genentech.