User login
Building Blocks: AVAHO Past President Looks Back
MINNEAPOLIS -- Oncologist Mark Klein, MD, may have just stepped down as president of the Association of VA Hematology/Oncology (AVAHO), but his main legacy—a foundation that AVAHO can call its own—is set in stone.
Over the past year, Klein has guided AVAHO as it leveraged its remarkable growth in recent years into the landmark creation of a foundation devoted to research. “We want to provide funds to researchers and support access to clinical trials for patients within the VA,” said Klein in an interview after he stepped down as association president at the 2019 AVAHO annual meeting.
Dr. Klein, who works for the Minneapolis VA Healthcare System and University of Minnesota in Minneapolis said the foundation is being seeded with $250,000. One goal is to use the foundation to support unique research projects that may not otherwise draw funding, he said.
For example, he said, the foundation could fund a research project by dietitians into severe weight loss in cancer. Or it could support a study by speech pathologists into swallowing in cancer patients.
In addition, he said, the foundation will focus on providing grants to support junior faculty, including researchers who aren’t MDs. And its funds will be used to boost access to clinical trials in cancer.
Klein said he has also focused on strategic planning and developing partnerships with industry and the leadership of both the VA and the National Cancer Institute. “We’re working to come up with unique ways to get people thinking about the barriers to clinical trials and providing better access for veterans.”
He is especially proud of AVAHO’s partnership with National Association of Veterans’ Research and Education Foundations, which includes partial support of a program manager position.
On the corporate front, he said, “we’re going to start offering corporate memberships so that we can form more industry relationships. That’s another new change and a step in our growth as we work to help more veterans and make a bigger difference.”
MINNEAPOLIS -- Oncologist Mark Klein, MD, may have just stepped down as president of the Association of VA Hematology/Oncology (AVAHO), but his main legacy—a foundation that AVAHO can call its own—is set in stone.
Over the past year, Klein has guided AVAHO as it leveraged its remarkable growth in recent years into the landmark creation of a foundation devoted to research. “We want to provide funds to researchers and support access to clinical trials for patients within the VA,” said Klein in an interview after he stepped down as association president at the 2019 AVAHO annual meeting.
Dr. Klein, who works for the Minneapolis VA Healthcare System and University of Minnesota in Minneapolis said the foundation is being seeded with $250,000. One goal is to use the foundation to support unique research projects that may not otherwise draw funding, he said.
For example, he said, the foundation could fund a research project by dietitians into severe weight loss in cancer. Or it could support a study by speech pathologists into swallowing in cancer patients.
In addition, he said, the foundation will focus on providing grants to support junior faculty, including researchers who aren’t MDs. And its funds will be used to boost access to clinical trials in cancer.
Klein said he has also focused on strategic planning and developing partnerships with industry and the leadership of both the VA and the National Cancer Institute. “We’re working to come up with unique ways to get people thinking about the barriers to clinical trials and providing better access for veterans.”
He is especially proud of AVAHO’s partnership with National Association of Veterans’ Research and Education Foundations, which includes partial support of a program manager position.
On the corporate front, he said, “we’re going to start offering corporate memberships so that we can form more industry relationships. That’s another new change and a step in our growth as we work to help more veterans and make a bigger difference.”
MINNEAPOLIS -- Oncologist Mark Klein, MD, may have just stepped down as president of the Association of VA Hematology/Oncology (AVAHO), but his main legacy—a foundation that AVAHO can call its own—is set in stone.
Over the past year, Klein has guided AVAHO as it leveraged its remarkable growth in recent years into the landmark creation of a foundation devoted to research. “We want to provide funds to researchers and support access to clinical trials for patients within the VA,” said Klein in an interview after he stepped down as association president at the 2019 AVAHO annual meeting.
Dr. Klein, who works for the Minneapolis VA Healthcare System and University of Minnesota in Minneapolis said the foundation is being seeded with $250,000. One goal is to use the foundation to support unique research projects that may not otherwise draw funding, he said.
For example, he said, the foundation could fund a research project by dietitians into severe weight loss in cancer. Or it could support a study by speech pathologists into swallowing in cancer patients.
In addition, he said, the foundation will focus on providing grants to support junior faculty, including researchers who aren’t MDs. And its funds will be used to boost access to clinical trials in cancer.
Klein said he has also focused on strategic planning and developing partnerships with industry and the leadership of both the VA and the National Cancer Institute. “We’re working to come up with unique ways to get people thinking about the barriers to clinical trials and providing better access for veterans.”
He is especially proud of AVAHO’s partnership with National Association of Veterans’ Research and Education Foundations, which includes partial support of a program manager position.
On the corporate front, he said, “we’re going to start offering corporate memberships so that we can form more industry relationships. That’s another new change and a step in our growth as we work to help more veterans and make a bigger difference.”
FDA expands Dysport’s upper-limb spasticity indication to children
The Food and Drug Administration has expanded the indication of abobotulinumtoxinA (Dysport) for upper-limb spasticity to include patients aged 2 years and older, according to a release from Ipsen. This botulinum toxin product received approval for this indication in adults in 2015 and approval for lower-limb spasticity in patients aged 2 years and older in 2016. Notably, Orphan Drug Exclusivity prevents it from being indicated for patients with cerebral palsy because another botulinum toxin product, onabotulinumtoxinA (Botox), already was approved for the indication in June 2019.
Spasticity affects the muscles and joints of extremities, especially in growing children, and is usually caused by nerve damage, such as head trauma or spinal cord injury. The degree of spasticity can vary from mild muscle stiffness to severe, painful, and uncontrollable muscle spasms.
AbobotulinumtoxinA was evaluated for upper-limb spasticity in a phase 3, randomized, double-blind, low-dose controlled, multicenter study; the study enrolled 210 children aged 2-17 years with the condition and a Modified Ashworth Scale grade 2 or greater for elbow and wrist flexors. The children were randomized 1:1:1 to injections of either 8 units/kg, 16 units/kg, or 2 units/kg into the elbow flexors and wrist flexors. At 6 weeks, there were statistically significant improvements in Modified Ashworth Scale grade, the primary endpoint, with least-square mean changes from baseline of –2.0, –2.3, and –1.6, respectively.
AbobotulinumtoxinA and all other botulinum toxin products carry a boxed warning, the most serious warning the FDA issues. This warning refers to risk of botulism-like symptoms caused by the botulinum toxin spreading away from the injection area; these symptoms can included sometimes life-threatening difficulty swallowing or breathing. AbobotulinumtoxinA is contraindicated in patients with known hypersensitivity to any botulinum toxin or any of the components, those with presence of infection at proposed injection site(s), and those with known allergy to cow’s milk protein. It is also important to note that botulinum toxin preparations are not interchangeable; the potency units of one are not the same as those of another. Full prescribing information can be found on the Ipsen website.
The Food and Drug Administration has expanded the indication of abobotulinumtoxinA (Dysport) for upper-limb spasticity to include patients aged 2 years and older, according to a release from Ipsen. This botulinum toxin product received approval for this indication in adults in 2015 and approval for lower-limb spasticity in patients aged 2 years and older in 2016. Notably, Orphan Drug Exclusivity prevents it from being indicated for patients with cerebral palsy because another botulinum toxin product, onabotulinumtoxinA (Botox), already was approved for the indication in June 2019.
Spasticity affects the muscles and joints of extremities, especially in growing children, and is usually caused by nerve damage, such as head trauma or spinal cord injury. The degree of spasticity can vary from mild muscle stiffness to severe, painful, and uncontrollable muscle spasms.
AbobotulinumtoxinA was evaluated for upper-limb spasticity in a phase 3, randomized, double-blind, low-dose controlled, multicenter study; the study enrolled 210 children aged 2-17 years with the condition and a Modified Ashworth Scale grade 2 or greater for elbow and wrist flexors. The children were randomized 1:1:1 to injections of either 8 units/kg, 16 units/kg, or 2 units/kg into the elbow flexors and wrist flexors. At 6 weeks, there were statistically significant improvements in Modified Ashworth Scale grade, the primary endpoint, with least-square mean changes from baseline of –2.0, –2.3, and –1.6, respectively.
AbobotulinumtoxinA and all other botulinum toxin products carry a boxed warning, the most serious warning the FDA issues. This warning refers to risk of botulism-like symptoms caused by the botulinum toxin spreading away from the injection area; these symptoms can included sometimes life-threatening difficulty swallowing or breathing. AbobotulinumtoxinA is contraindicated in patients with known hypersensitivity to any botulinum toxin or any of the components, those with presence of infection at proposed injection site(s), and those with known allergy to cow’s milk protein. It is also important to note that botulinum toxin preparations are not interchangeable; the potency units of one are not the same as those of another. Full prescribing information can be found on the Ipsen website.
The Food and Drug Administration has expanded the indication of abobotulinumtoxinA (Dysport) for upper-limb spasticity to include patients aged 2 years and older, according to a release from Ipsen. This botulinum toxin product received approval for this indication in adults in 2015 and approval for lower-limb spasticity in patients aged 2 years and older in 2016. Notably, Orphan Drug Exclusivity prevents it from being indicated for patients with cerebral palsy because another botulinum toxin product, onabotulinumtoxinA (Botox), already was approved for the indication in June 2019.
Spasticity affects the muscles and joints of extremities, especially in growing children, and is usually caused by nerve damage, such as head trauma or spinal cord injury. The degree of spasticity can vary from mild muscle stiffness to severe, painful, and uncontrollable muscle spasms.
AbobotulinumtoxinA was evaluated for upper-limb spasticity in a phase 3, randomized, double-blind, low-dose controlled, multicenter study; the study enrolled 210 children aged 2-17 years with the condition and a Modified Ashworth Scale grade 2 or greater for elbow and wrist flexors. The children were randomized 1:1:1 to injections of either 8 units/kg, 16 units/kg, or 2 units/kg into the elbow flexors and wrist flexors. At 6 weeks, there were statistically significant improvements in Modified Ashworth Scale grade, the primary endpoint, with least-square mean changes from baseline of –2.0, –2.3, and –1.6, respectively.
AbobotulinumtoxinA and all other botulinum toxin products carry a boxed warning, the most serious warning the FDA issues. This warning refers to risk of botulism-like symptoms caused by the botulinum toxin spreading away from the injection area; these symptoms can included sometimes life-threatening difficulty swallowing or breathing. AbobotulinumtoxinA is contraindicated in patients with known hypersensitivity to any botulinum toxin or any of the components, those with presence of infection at proposed injection site(s), and those with known allergy to cow’s milk protein. It is also important to note that botulinum toxin preparations are not interchangeable; the potency units of one are not the same as those of another. Full prescribing information can be found on the Ipsen website.
Patient-reported outcomes are here to stay
WASHINGTON – The federal official who helps oversee Medicare’s use of quality measures predicted a continued emphasis on patient-reported outcomes in the assessments of physician performance.
Reena Duseja, MD, chief medical officer for quality measurement at the Centers for Medicare & Medicaid Services, said she has seen “more emphasis” in her 2 years with the agency in collecting outcome measures, including ones reported by patients. In doing this, CMS officials are also looking to identify the core elements that willl be part of patient-reported outcomes (PROs).
“We really have to get better at standardization,” Dr. Duseja said during a policy summit sponsored by the National Comprehensive Cancer Network (NCCN). “There is room for improvement there. We’re continuing to think of ways that we can support that.”
She also said the CMS is working, in general, to try to give physicians feedback sooner on how they are faring on measurements.
“The commitment of our agency is trying to think about how we collect data in a way that shortens the cycle of measure development” and speeds the delivery of this data back to providers, Dr. Duseja said.
Her fellow panelists discussed the difficulties in designing PRO measures, including the need to account for special challenges for patients living in or near poverty. Avoiding emergency department visits and hospitalizations, for example, may be a key priority for people who are paid hourly wages, said Kashyap Patel, MD, managing partner of Carolina Blood and Cancer Care in Rock Hill, S.C. These patients will not only face the inconvenience and cost of a hospital stay, but will also lose wages from missed work. He urged policymakers to take these factors into consideration in designing quality measures, and not to forget that “there is a human being behind this.”
Ronald S. Walters, MD, associate head of the Institute for Cancer Care Innovation at the University of Texas MD Anderson Cancer Center, and chair of NCCN’s board of directors, cautioned against continued attempts to devise a “Nirvana” list of outcome measures that can be universally applied. Instead, payers may be better off with a “mix and match” approach. Certain measures may be used across the board, such as pain and quality of life metrics, while other measures could be more tailored.
Dr. Walters also called out a missed opportunity to tie PROs to Medicare payment in the area of chimeric antigen receptor (CAR) T-cell therapy.
In 2018, the CMS indicated it was considering the use of PROs in connection with CAR T-cell payment. The CMS asked its Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) to consider the role of PROs in connection with payment for CAR T-cell therapy. At an August 2018 meeting, MEDCAC panelists generally expressed confidence in PROs in a series of votes about the use of this approach to quality measurement in cancer trials.
But drugmakers and physician groups raised strong objections at the MEDCAC meeting. In its national coverage decision on CAR T, issued in August 2019, the CMS said it had received many comments on PROs “ranging from support of their collection to recommendations for additional assessment tools to request to remove PRO requirements.” The CMS opted at this time to encourage participation in the Center for International Blood and Marrow Transplantation Research (CIBMTR) database “that currently collects health outcomes (and aims to collect patient reported outcomes in the future) on patients who have received CAR T-cell treatments.”
For Dr. Walters, this setback for the use of PROs in CAR T therapy payment is telling, as the treatment is known to produce serious side effects and is administered in well-controlled circumstances.
“If you can’t organize collecting patient-reported outcomes after CAR T cell, that kind of tells you the state of where we are on collecting them on everybody,” Dr. Walters said.
WASHINGTON – The federal official who helps oversee Medicare’s use of quality measures predicted a continued emphasis on patient-reported outcomes in the assessments of physician performance.
Reena Duseja, MD, chief medical officer for quality measurement at the Centers for Medicare & Medicaid Services, said she has seen “more emphasis” in her 2 years with the agency in collecting outcome measures, including ones reported by patients. In doing this, CMS officials are also looking to identify the core elements that willl be part of patient-reported outcomes (PROs).
“We really have to get better at standardization,” Dr. Duseja said during a policy summit sponsored by the National Comprehensive Cancer Network (NCCN). “There is room for improvement there. We’re continuing to think of ways that we can support that.”
She also said the CMS is working, in general, to try to give physicians feedback sooner on how they are faring on measurements.
“The commitment of our agency is trying to think about how we collect data in a way that shortens the cycle of measure development” and speeds the delivery of this data back to providers, Dr. Duseja said.
Her fellow panelists discussed the difficulties in designing PRO measures, including the need to account for special challenges for patients living in or near poverty. Avoiding emergency department visits and hospitalizations, for example, may be a key priority for people who are paid hourly wages, said Kashyap Patel, MD, managing partner of Carolina Blood and Cancer Care in Rock Hill, S.C. These patients will not only face the inconvenience and cost of a hospital stay, but will also lose wages from missed work. He urged policymakers to take these factors into consideration in designing quality measures, and not to forget that “there is a human being behind this.”
Ronald S. Walters, MD, associate head of the Institute for Cancer Care Innovation at the University of Texas MD Anderson Cancer Center, and chair of NCCN’s board of directors, cautioned against continued attempts to devise a “Nirvana” list of outcome measures that can be universally applied. Instead, payers may be better off with a “mix and match” approach. Certain measures may be used across the board, such as pain and quality of life metrics, while other measures could be more tailored.
Dr. Walters also called out a missed opportunity to tie PROs to Medicare payment in the area of chimeric antigen receptor (CAR) T-cell therapy.
In 2018, the CMS indicated it was considering the use of PROs in connection with CAR T-cell payment. The CMS asked its Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) to consider the role of PROs in connection with payment for CAR T-cell therapy. At an August 2018 meeting, MEDCAC panelists generally expressed confidence in PROs in a series of votes about the use of this approach to quality measurement in cancer trials.
But drugmakers and physician groups raised strong objections at the MEDCAC meeting. In its national coverage decision on CAR T, issued in August 2019, the CMS said it had received many comments on PROs “ranging from support of their collection to recommendations for additional assessment tools to request to remove PRO requirements.” The CMS opted at this time to encourage participation in the Center for International Blood and Marrow Transplantation Research (CIBMTR) database “that currently collects health outcomes (and aims to collect patient reported outcomes in the future) on patients who have received CAR T-cell treatments.”
For Dr. Walters, this setback for the use of PROs in CAR T therapy payment is telling, as the treatment is known to produce serious side effects and is administered in well-controlled circumstances.
“If you can’t organize collecting patient-reported outcomes after CAR T cell, that kind of tells you the state of where we are on collecting them on everybody,” Dr. Walters said.
WASHINGTON – The federal official who helps oversee Medicare’s use of quality measures predicted a continued emphasis on patient-reported outcomes in the assessments of physician performance.
Reena Duseja, MD, chief medical officer for quality measurement at the Centers for Medicare & Medicaid Services, said she has seen “more emphasis” in her 2 years with the agency in collecting outcome measures, including ones reported by patients. In doing this, CMS officials are also looking to identify the core elements that willl be part of patient-reported outcomes (PROs).
“We really have to get better at standardization,” Dr. Duseja said during a policy summit sponsored by the National Comprehensive Cancer Network (NCCN). “There is room for improvement there. We’re continuing to think of ways that we can support that.”
She also said the CMS is working, in general, to try to give physicians feedback sooner on how they are faring on measurements.
“The commitment of our agency is trying to think about how we collect data in a way that shortens the cycle of measure development” and speeds the delivery of this data back to providers, Dr. Duseja said.
Her fellow panelists discussed the difficulties in designing PRO measures, including the need to account for special challenges for patients living in or near poverty. Avoiding emergency department visits and hospitalizations, for example, may be a key priority for people who are paid hourly wages, said Kashyap Patel, MD, managing partner of Carolina Blood and Cancer Care in Rock Hill, S.C. These patients will not only face the inconvenience and cost of a hospital stay, but will also lose wages from missed work. He urged policymakers to take these factors into consideration in designing quality measures, and not to forget that “there is a human being behind this.”
Ronald S. Walters, MD, associate head of the Institute for Cancer Care Innovation at the University of Texas MD Anderson Cancer Center, and chair of NCCN’s board of directors, cautioned against continued attempts to devise a “Nirvana” list of outcome measures that can be universally applied. Instead, payers may be better off with a “mix and match” approach. Certain measures may be used across the board, such as pain and quality of life metrics, while other measures could be more tailored.
Dr. Walters also called out a missed opportunity to tie PROs to Medicare payment in the area of chimeric antigen receptor (CAR) T-cell therapy.
In 2018, the CMS indicated it was considering the use of PROs in connection with CAR T-cell payment. The CMS asked its Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) to consider the role of PROs in connection with payment for CAR T-cell therapy. At an August 2018 meeting, MEDCAC panelists generally expressed confidence in PROs in a series of votes about the use of this approach to quality measurement in cancer trials.
But drugmakers and physician groups raised strong objections at the MEDCAC meeting. In its national coverage decision on CAR T, issued in August 2019, the CMS said it had received many comments on PROs “ranging from support of their collection to recommendations for additional assessment tools to request to remove PRO requirements.” The CMS opted at this time to encourage participation in the Center for International Blood and Marrow Transplantation Research (CIBMTR) database “that currently collects health outcomes (and aims to collect patient reported outcomes in the future) on patients who have received CAR T-cell treatments.”
For Dr. Walters, this setback for the use of PROs in CAR T therapy payment is telling, as the treatment is known to produce serious side effects and is administered in well-controlled circumstances.
“If you can’t organize collecting patient-reported outcomes after CAR T cell, that kind of tells you the state of where we are on collecting them on everybody,” Dr. Walters said.
REPORTING FROM NCCN POLICY SUMMIT 2019
Legislators disagree on new drug-pricing proposal
Legislators sparred about the best way to lower drug prices during a Sept. 25 hearing, bickering along partisan lines over new legislation by U.S. House Speaker Nancy Pelosi, (D-Calif.) that would require Medicare to negotiate drug prices with manufacturers.
The more than 4-hour hearing by the House Committee on Energy & Commerce Subcommittee on Health centered on HR 3, the “Lower Drug Costs Now Act of 2019,” introduced by Speaker Pelosi on Sept. 24, which would compel the Centers for Medicare & Medicaid Services to make deals with manufacturers on the maximum, reasonable price for the top 250 highest-cost drugs. If a drug maker refused to participate in the negotiation, the company would face a steep noncompliance fee, according to the bill, while manufacturers that overcharged Medicare or failed to offer the negotiated price would be subject to a civil penalty equal to 10 times the cost difference. The legislation includes a $2,000 out-of-pocket limit for Medicare patients.
If enacted, the legislation would transform the pricing landscape of medications and allow more families to access needed treatments, Speaker Pelosi said during the hearing.
“What it does, as you know; it ends the ban – imagine, there’s a ban on negotiating for lower drug prices,” she said. “So, it ends the ban for the [U.S. Department of Health and Human Services] Secretary now to have the opportunity to negotiate for lower prices. But, the even better news is that these drug prices will be lower not just for Medicare recipients, which was one of the original proposals, but for everyone. It will stop companies from ripping us off by charging five times, four times, three times what is charged in other countries.”
However, Republican legislators spent much of the hearing criticizing the bill, claiming the legislation was crafted behind closed doors by Democrats who made no efforts to gain Republican feedback.
“There is no debate that Republicans and Democrats want to work together to lower drug cost for consumers,” Ranking Member Greg Walden (R-Ore.) said during the hearing. “Madam chair, I have to strongly express my great frustration about the decision to sabotage both the tradition of this committee and the bipartisan work that you know was well underway to tackle high-cost drugs. ... We’ve worked in a bipartisan way up until now. I thought we were headed in a good faith down that same path until the speaker’s office dropped this partisan plan on our [progress]. This is partisan politics at its worst, and it’s an avoidable failure.”
Amid the partisan squabbling, legislators heard differing views from economists about the logistics of the legislation and whether the pricing negotiation model makes sense for the United States.
Gerard F. Anderson, PhD, a professor at Johns Hopkins University and director of the Johns Hopkins Center for Hospital Finance and Management in Baltimore told congressional leaders that negotiation between the government and drug makers is both possible and results in lower prices, even for drugs without therapeutic competition. He noted that the Medicaid program currently negotiates prices for supplemental rebates and that the Veterans Administration and the Department of Defense routinely negotiate discounts greater than the federal supply schedule. Dr. Anderson estimated that the Veterans Administration and the Department of Defense pay an average of 30%-40% less than Medicare prescription drug plans for the same medications.
“Allowing the federal government to negotiate prices for expensive drugs without competition in both the public and private sectors will be more effective in lowering drug prices for everyone,” Dr. Anderson said during testimony. “ Imposing financial penalties for drug companies will bring them to the table. International prices can be used to determine if the drug company is negotiating in good faith.”
But Benedic Ippolito, PhD, a research fellow for the American Enterprise Institute, Washington, raised concerns the legislation may cause reduced innovation. He said the United States accounts for about 60% of drug spending in the developed world, and that, because the market is so large, changes in spending will have first-order implications for the types of future drugs available, Dr. Ippolito said during testimony.
“Consider the incentives associated with the [bill’s] negotiation process,” he said. “Drugs that have no competitors would be subject to aggressive rate regulation by the [HHS] Secretary. The same is not true of drugs with at least one such competitor. It is entirely possible that being a second market entrant could prove substantially more profitable than bringing a novel therapeutic to market. Thus, the proposal could substantially depress incentives to pursue path-breaking drugs.”
Outside the hearing, Speaker Pelosi’s proposed bill is being praised by some physician groups, including the American College of Physicians. In a statement, ACP President Robert McLean, MD, said the college was pleased that the bill focuses on keeping drugs affordable for patients and includes provisions that would support research into new therapies and treatment options.
“ACP specifically supports the provisions that would allow Medicare to negotiate prices with manufacturers,” Dr. McLean said in the statement. “The College has longstanding policy supporting the ability of Medicare to leverage its purchasing power and directly negotiate with manufacturers for drug prices. Although ACP does not have policy on several of the specific provisions in the bill, we are supportive of its overall goals and direction, particularly the emphasis on negotiation and transparency in drug pricing.
Meanwhile, Senate Finance Chairman Charles E. Grassley, (R-Iowa) and Ranking Member Ron Wyden (D-Ore.) released the statutory text of their own drug-pricing legislation on Sept. 25, titled “the Prescription Drug Pricing Reduction Act of 2019 (S-2543).” The bill calls for a number of changes to the Medicare Part D program, such as reduced beneficiary cost sharing and linking drug price increases to the rate of inflation. The legislation would also make more information available regarding pharmacy benefit manager practices and change how Medicare calculates Part B prescription drug payment amounts to lower spending and out-of-pocket costs for patients.
“President Trump has called on Congress to work together on a bipartisan bill to lower prescription drug prices, [and] there’s only one bipartisan bill in Congress to lower prescription drug prices that’s passed the committee process,” Chairman Grassley said in a statement. “This is it. Making prescription drugs more affordable consistently ranks as a top issue for Americans from every corner of the country. I encourage my colleagues on both sides of the aisle to come to the table and work with us to get a bill passed and signed into law.”
Legislators sparred about the best way to lower drug prices during a Sept. 25 hearing, bickering along partisan lines over new legislation by U.S. House Speaker Nancy Pelosi, (D-Calif.) that would require Medicare to negotiate drug prices with manufacturers.
The more than 4-hour hearing by the House Committee on Energy & Commerce Subcommittee on Health centered on HR 3, the “Lower Drug Costs Now Act of 2019,” introduced by Speaker Pelosi on Sept. 24, which would compel the Centers for Medicare & Medicaid Services to make deals with manufacturers on the maximum, reasonable price for the top 250 highest-cost drugs. If a drug maker refused to participate in the negotiation, the company would face a steep noncompliance fee, according to the bill, while manufacturers that overcharged Medicare or failed to offer the negotiated price would be subject to a civil penalty equal to 10 times the cost difference. The legislation includes a $2,000 out-of-pocket limit for Medicare patients.
If enacted, the legislation would transform the pricing landscape of medications and allow more families to access needed treatments, Speaker Pelosi said during the hearing.
“What it does, as you know; it ends the ban – imagine, there’s a ban on negotiating for lower drug prices,” she said. “So, it ends the ban for the [U.S. Department of Health and Human Services] Secretary now to have the opportunity to negotiate for lower prices. But, the even better news is that these drug prices will be lower not just for Medicare recipients, which was one of the original proposals, but for everyone. It will stop companies from ripping us off by charging five times, four times, three times what is charged in other countries.”
However, Republican legislators spent much of the hearing criticizing the bill, claiming the legislation was crafted behind closed doors by Democrats who made no efforts to gain Republican feedback.
“There is no debate that Republicans and Democrats want to work together to lower drug cost for consumers,” Ranking Member Greg Walden (R-Ore.) said during the hearing. “Madam chair, I have to strongly express my great frustration about the decision to sabotage both the tradition of this committee and the bipartisan work that you know was well underway to tackle high-cost drugs. ... We’ve worked in a bipartisan way up until now. I thought we were headed in a good faith down that same path until the speaker’s office dropped this partisan plan on our [progress]. This is partisan politics at its worst, and it’s an avoidable failure.”
Amid the partisan squabbling, legislators heard differing views from economists about the logistics of the legislation and whether the pricing negotiation model makes sense for the United States.
Gerard F. Anderson, PhD, a professor at Johns Hopkins University and director of the Johns Hopkins Center for Hospital Finance and Management in Baltimore told congressional leaders that negotiation between the government and drug makers is both possible and results in lower prices, even for drugs without therapeutic competition. He noted that the Medicaid program currently negotiates prices for supplemental rebates and that the Veterans Administration and the Department of Defense routinely negotiate discounts greater than the federal supply schedule. Dr. Anderson estimated that the Veterans Administration and the Department of Defense pay an average of 30%-40% less than Medicare prescription drug plans for the same medications.
“Allowing the federal government to negotiate prices for expensive drugs without competition in both the public and private sectors will be more effective in lowering drug prices for everyone,” Dr. Anderson said during testimony. “ Imposing financial penalties for drug companies will bring them to the table. International prices can be used to determine if the drug company is negotiating in good faith.”
But Benedic Ippolito, PhD, a research fellow for the American Enterprise Institute, Washington, raised concerns the legislation may cause reduced innovation. He said the United States accounts for about 60% of drug spending in the developed world, and that, because the market is so large, changes in spending will have first-order implications for the types of future drugs available, Dr. Ippolito said during testimony.
“Consider the incentives associated with the [bill’s] negotiation process,” he said. “Drugs that have no competitors would be subject to aggressive rate regulation by the [HHS] Secretary. The same is not true of drugs with at least one such competitor. It is entirely possible that being a second market entrant could prove substantially more profitable than bringing a novel therapeutic to market. Thus, the proposal could substantially depress incentives to pursue path-breaking drugs.”
Outside the hearing, Speaker Pelosi’s proposed bill is being praised by some physician groups, including the American College of Physicians. In a statement, ACP President Robert McLean, MD, said the college was pleased that the bill focuses on keeping drugs affordable for patients and includes provisions that would support research into new therapies and treatment options.
“ACP specifically supports the provisions that would allow Medicare to negotiate prices with manufacturers,” Dr. McLean said in the statement. “The College has longstanding policy supporting the ability of Medicare to leverage its purchasing power and directly negotiate with manufacturers for drug prices. Although ACP does not have policy on several of the specific provisions in the bill, we are supportive of its overall goals and direction, particularly the emphasis on negotiation and transparency in drug pricing.
Meanwhile, Senate Finance Chairman Charles E. Grassley, (R-Iowa) and Ranking Member Ron Wyden (D-Ore.) released the statutory text of their own drug-pricing legislation on Sept. 25, titled “the Prescription Drug Pricing Reduction Act of 2019 (S-2543).” The bill calls for a number of changes to the Medicare Part D program, such as reduced beneficiary cost sharing and linking drug price increases to the rate of inflation. The legislation would also make more information available regarding pharmacy benefit manager practices and change how Medicare calculates Part B prescription drug payment amounts to lower spending and out-of-pocket costs for patients.
“President Trump has called on Congress to work together on a bipartisan bill to lower prescription drug prices, [and] there’s only one bipartisan bill in Congress to lower prescription drug prices that’s passed the committee process,” Chairman Grassley said in a statement. “This is it. Making prescription drugs more affordable consistently ranks as a top issue for Americans from every corner of the country. I encourage my colleagues on both sides of the aisle to come to the table and work with us to get a bill passed and signed into law.”
Legislators sparred about the best way to lower drug prices during a Sept. 25 hearing, bickering along partisan lines over new legislation by U.S. House Speaker Nancy Pelosi, (D-Calif.) that would require Medicare to negotiate drug prices with manufacturers.
The more than 4-hour hearing by the House Committee on Energy & Commerce Subcommittee on Health centered on HR 3, the “Lower Drug Costs Now Act of 2019,” introduced by Speaker Pelosi on Sept. 24, which would compel the Centers for Medicare & Medicaid Services to make deals with manufacturers on the maximum, reasonable price for the top 250 highest-cost drugs. If a drug maker refused to participate in the negotiation, the company would face a steep noncompliance fee, according to the bill, while manufacturers that overcharged Medicare or failed to offer the negotiated price would be subject to a civil penalty equal to 10 times the cost difference. The legislation includes a $2,000 out-of-pocket limit for Medicare patients.
If enacted, the legislation would transform the pricing landscape of medications and allow more families to access needed treatments, Speaker Pelosi said during the hearing.
“What it does, as you know; it ends the ban – imagine, there’s a ban on negotiating for lower drug prices,” she said. “So, it ends the ban for the [U.S. Department of Health and Human Services] Secretary now to have the opportunity to negotiate for lower prices. But, the even better news is that these drug prices will be lower not just for Medicare recipients, which was one of the original proposals, but for everyone. It will stop companies from ripping us off by charging five times, four times, three times what is charged in other countries.”
However, Republican legislators spent much of the hearing criticizing the bill, claiming the legislation was crafted behind closed doors by Democrats who made no efforts to gain Republican feedback.
“There is no debate that Republicans and Democrats want to work together to lower drug cost for consumers,” Ranking Member Greg Walden (R-Ore.) said during the hearing. “Madam chair, I have to strongly express my great frustration about the decision to sabotage both the tradition of this committee and the bipartisan work that you know was well underway to tackle high-cost drugs. ... We’ve worked in a bipartisan way up until now. I thought we were headed in a good faith down that same path until the speaker’s office dropped this partisan plan on our [progress]. This is partisan politics at its worst, and it’s an avoidable failure.”
Amid the partisan squabbling, legislators heard differing views from economists about the logistics of the legislation and whether the pricing negotiation model makes sense for the United States.
Gerard F. Anderson, PhD, a professor at Johns Hopkins University and director of the Johns Hopkins Center for Hospital Finance and Management in Baltimore told congressional leaders that negotiation between the government and drug makers is both possible and results in lower prices, even for drugs without therapeutic competition. He noted that the Medicaid program currently negotiates prices for supplemental rebates and that the Veterans Administration and the Department of Defense routinely negotiate discounts greater than the federal supply schedule. Dr. Anderson estimated that the Veterans Administration and the Department of Defense pay an average of 30%-40% less than Medicare prescription drug plans for the same medications.
“Allowing the federal government to negotiate prices for expensive drugs without competition in both the public and private sectors will be more effective in lowering drug prices for everyone,” Dr. Anderson said during testimony. “ Imposing financial penalties for drug companies will bring them to the table. International prices can be used to determine if the drug company is negotiating in good faith.”
But Benedic Ippolito, PhD, a research fellow for the American Enterprise Institute, Washington, raised concerns the legislation may cause reduced innovation. He said the United States accounts for about 60% of drug spending in the developed world, and that, because the market is so large, changes in spending will have first-order implications for the types of future drugs available, Dr. Ippolito said during testimony.
“Consider the incentives associated with the [bill’s] negotiation process,” he said. “Drugs that have no competitors would be subject to aggressive rate regulation by the [HHS] Secretary. The same is not true of drugs with at least one such competitor. It is entirely possible that being a second market entrant could prove substantially more profitable than bringing a novel therapeutic to market. Thus, the proposal could substantially depress incentives to pursue path-breaking drugs.”
Outside the hearing, Speaker Pelosi’s proposed bill is being praised by some physician groups, including the American College of Physicians. In a statement, ACP President Robert McLean, MD, said the college was pleased that the bill focuses on keeping drugs affordable for patients and includes provisions that would support research into new therapies and treatment options.
“ACP specifically supports the provisions that would allow Medicare to negotiate prices with manufacturers,” Dr. McLean said in the statement. “The College has longstanding policy supporting the ability of Medicare to leverage its purchasing power and directly negotiate with manufacturers for drug prices. Although ACP does not have policy on several of the specific provisions in the bill, we are supportive of its overall goals and direction, particularly the emphasis on negotiation and transparency in drug pricing.
Meanwhile, Senate Finance Chairman Charles E. Grassley, (R-Iowa) and Ranking Member Ron Wyden (D-Ore.) released the statutory text of their own drug-pricing legislation on Sept. 25, titled “the Prescription Drug Pricing Reduction Act of 2019 (S-2543).” The bill calls for a number of changes to the Medicare Part D program, such as reduced beneficiary cost sharing and linking drug price increases to the rate of inflation. The legislation would also make more information available regarding pharmacy benefit manager practices and change how Medicare calculates Part B prescription drug payment amounts to lower spending and out-of-pocket costs for patients.
“President Trump has called on Congress to work together on a bipartisan bill to lower prescription drug prices, [and] there’s only one bipartisan bill in Congress to lower prescription drug prices that’s passed the committee process,” Chairman Grassley said in a statement. “This is it. Making prescription drugs more affordable consistently ranks as a top issue for Americans from every corner of the country. I encourage my colleagues on both sides of the aisle to come to the table and work with us to get a bill passed and signed into law.”
Palliative care programs continue growth in U.S. hospitals
Growth continues among palliative care programs in the United States, although access often depends “more upon accidents of geography than it does upon the needs of patients,” according to the Center to Advance Palliative Care and the National Palliative Care Research Center.
“As is true for many aspects of health care, geography is destiny. Where you live determines your access to the best quality of life and highest quality of care during a serious illness,” said Diane E. Meier, MD, director of the Center to Advance Palliative Care, in a written statement.
the two organizations said in their 2019 report card on palliative care access. What hasn’t changed since 2015, however, is the country’s overall grade, which remains a B.
Delaware, New Hampshire, Rhode Island, and Vermont have a palliative care program in all of their hospitals with 50 or more beds and each earned a grade of A (palliative care rate of greater than 80%), along with 17 other states. The lowest-performing states – Alabama, Mississippi, New Mexico, Oklahoma, and Wyoming – all received Ds for having a rate below 40%, the CAPC said.
The urban/rural divide also is prominent in palliative care: “90% of hospitals with palliative care are in urban areas. Only 17% of rural hospitals with fifty or more beds report palliative care programs,” the report said.
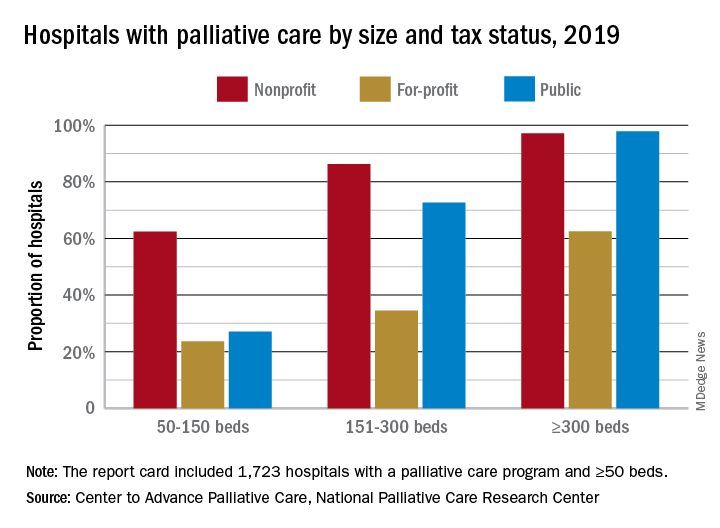
Hospital type is another source of disparity. Small, nonprofit hospitals are much more likely to offer access to palliative care than either for-profit or public facilities of the same size, but the gap closes as size increases, at least between nonprofit and public hospitals. For the largest institutions, the public hospitals pull into the lead, 98% versus 97%, over the nonprofits, with the for-profit facilities well behind at 63%.
“High quality palliative care has been shown to improve patient and family quality of life, improve patients’ and families’ health care experiences, and in certain diseases, prolong life. Palliative care has also been shown to improve hospital efficiency and reduce unnecessary spending,” said R. Sean Morrison, MD, director of the National Palliative Care Research Center.
The report card is based on data from the American Hospital Association’s Annual Survey Database, with additional data from the National Palliative Care Registry and Center to Advance Palliative Care’s Mapping Community Palliative Care initiative. The final sample included 2,409 hospitals with 50 or more beds.
Growth continues among palliative care programs in the United States, although access often depends “more upon accidents of geography than it does upon the needs of patients,” according to the Center to Advance Palliative Care and the National Palliative Care Research Center.

“As is true for many aspects of health care, geography is destiny. Where you live determines your access to the best quality of life and highest quality of care during a serious illness,” said Diane E. Meier, MD, director of the Center to Advance Palliative Care, in a written statement.
the two organizations said in their 2019 report card on palliative care access. What hasn’t changed since 2015, however, is the country’s overall grade, which remains a B.
Delaware, New Hampshire, Rhode Island, and Vermont have a palliative care program in all of their hospitals with 50 or more beds and each earned a grade of A (palliative care rate of greater than 80%), along with 17 other states. The lowest-performing states – Alabama, Mississippi, New Mexico, Oklahoma, and Wyoming – all received Ds for having a rate below 40%, the CAPC said.
The urban/rural divide also is prominent in palliative care: “90% of hospitals with palliative care are in urban areas. Only 17% of rural hospitals with fifty or more beds report palliative care programs,” the report said.
Hospital type is another source of disparity. Small, nonprofit hospitals are much more likely to offer access to palliative care than either for-profit or public facilities of the same size, but the gap closes as size increases, at least between nonprofit and public hospitals. For the largest institutions, the public hospitals pull into the lead, 98% versus 97%, over the nonprofits, with the for-profit facilities well behind at 63%.
“High quality palliative care has been shown to improve patient and family quality of life, improve patients’ and families’ health care experiences, and in certain diseases, prolong life. Palliative care has also been shown to improve hospital efficiency and reduce unnecessary spending,” said R. Sean Morrison, MD, director of the National Palliative Care Research Center.
The report card is based on data from the American Hospital Association’s Annual Survey Database, with additional data from the National Palliative Care Registry and Center to Advance Palliative Care’s Mapping Community Palliative Care initiative. The final sample included 2,409 hospitals with 50 or more beds.
Growth continues among palliative care programs in the United States, although access often depends “more upon accidents of geography than it does upon the needs of patients,” according to the Center to Advance Palliative Care and the National Palliative Care Research Center.

“As is true for many aspects of health care, geography is destiny. Where you live determines your access to the best quality of life and highest quality of care during a serious illness,” said Diane E. Meier, MD, director of the Center to Advance Palliative Care, in a written statement.
the two organizations said in their 2019 report card on palliative care access. What hasn’t changed since 2015, however, is the country’s overall grade, which remains a B.
Delaware, New Hampshire, Rhode Island, and Vermont have a palliative care program in all of their hospitals with 50 or more beds and each earned a grade of A (palliative care rate of greater than 80%), along with 17 other states. The lowest-performing states – Alabama, Mississippi, New Mexico, Oklahoma, and Wyoming – all received Ds for having a rate below 40%, the CAPC said.
The urban/rural divide also is prominent in palliative care: “90% of hospitals with palliative care are in urban areas. Only 17% of rural hospitals with fifty or more beds report palliative care programs,” the report said.
Hospital type is another source of disparity. Small, nonprofit hospitals are much more likely to offer access to palliative care than either for-profit or public facilities of the same size, but the gap closes as size increases, at least between nonprofit and public hospitals. For the largest institutions, the public hospitals pull into the lead, 98% versus 97%, over the nonprofits, with the for-profit facilities well behind at 63%.
“High quality palliative care has been shown to improve patient and family quality of life, improve patients’ and families’ health care experiences, and in certain diseases, prolong life. Palliative care has also been shown to improve hospital efficiency and reduce unnecessary spending,” said R. Sean Morrison, MD, director of the National Palliative Care Research Center.
The report card is based on data from the American Hospital Association’s Annual Survey Database, with additional data from the National Palliative Care Registry and Center to Advance Palliative Care’s Mapping Community Palliative Care initiative. The final sample included 2,409 hospitals with 50 or more beds.
CMS weighs extension of Oncology Care Model
WASHINGTON – Federal officials are considering how they might extend a test of payment approaches meant to spur more coordinated cancer care beyond the program’s current 2021 end date.
Alexandra Chong, PhD, the team lead for the Oncology Care Model at the Center for Medicare and Medicaid Innovation (CMMI), said her agency found strong support for sustaining this kind of effort at “practice transformation.” The Oncology Care Model, which kicked off in 2016, involves about 175 practices and 10 insurers.
“As we look forward, we certainly recognize and appreciate the importance of the impact that [the Oncology Care Model] made and the desire for a continuation, either in an iteration of the current model or a different model,” Dr. Chong said during a panel discussion at a policy summit sponsored by the National Comprehensive Cancer Network (NCCN).
Dr. Chong said the Centers for Medicare & Medicaid Services is aware that there is “a lot of interest” in the future of the program. “We have heard from our practices that this model has been an opportunity for them to provide the care that they really want to provide,” Dr. Chong said.
A fellow panelist at the NCCN event, Kerin Adelson, MD, chief quality officer of Yale’s Smilow Cancer Hospital, New Haven, Conn., pressed for continuation of this kind of a payment test. Smilow has used revenue from the Oncology Care Model program to develop dashboards to measure individual clinicians’ patterns of care and share data with them, Dr. Adelson said.
The changes have been “transformative,” including allowing for hiring of additional support staff, Dr. Adelson said.
“I am terrified about what’s going to happen at the end of the program if there is not another model to transition to,” Dr. Adelson said. “We will potentially be looking at layoffs of these people who are so incredible and have done so much for the care we are giving.”
In May 2019, some of Dr. Chong’s CMMI colleagues published a report in the Journal of the National Cancer Institute about the experiences of clinics participating in the Oncology Care Model. For instance, the U.S. Oncology Network used Monthly Enhanced Oncology Services [MEOS] payments through the program for new hires such as navigators, social workers, additional advanced practice providers, “and even data analysts,” according to the report.
Still, there have been “growing pains” with the start-up of the model, Dr. Chong said. Fellow participants on the NCCN panel cited the introduction of costly new drugs as one wrinkle in the early days of the Oncology Care Model. Another is that physicians in practices signed up for the program sometimes are unaware of it.
The American Society of Clinical Oncology also is advocating for a continuation of a model along the lines of the Oncology Care Model.
“It would be good to see it continue. We’re certainly supportive of continuing to work on this model and refine it and see where it goes,” Stephen S. Grubbs, MD, ASCO’s vice president for clinical affairs, said in an interview.
Dr. Grubbs said that CMS’s Oncology Care Model could not be greatly expanded in its current form, as it imposes a steep administrative burden for both practices who participate, as well as for CMMI. The current model also does not serve smaller and rural practices well, he said.
ASCO is refining its own proposal for an alternative payment approach. The group plans to present its Patient-Centered Oncology Payment (PCOP) model, first published in 2015, before an influential federal advisory group, the Physician-Focused Payment Model Technical Advisory Committee (PTAC), at a meeting in 2020.
WASHINGTON – Federal officials are considering how they might extend a test of payment approaches meant to spur more coordinated cancer care beyond the program’s current 2021 end date.
Alexandra Chong, PhD, the team lead for the Oncology Care Model at the Center for Medicare and Medicaid Innovation (CMMI), said her agency found strong support for sustaining this kind of effort at “practice transformation.” The Oncology Care Model, which kicked off in 2016, involves about 175 practices and 10 insurers.
“As we look forward, we certainly recognize and appreciate the importance of the impact that [the Oncology Care Model] made and the desire for a continuation, either in an iteration of the current model or a different model,” Dr. Chong said during a panel discussion at a policy summit sponsored by the National Comprehensive Cancer Network (NCCN).
Dr. Chong said the Centers for Medicare & Medicaid Services is aware that there is “a lot of interest” in the future of the program. “We have heard from our practices that this model has been an opportunity for them to provide the care that they really want to provide,” Dr. Chong said.
A fellow panelist at the NCCN event, Kerin Adelson, MD, chief quality officer of Yale’s Smilow Cancer Hospital, New Haven, Conn., pressed for continuation of this kind of a payment test. Smilow has used revenue from the Oncology Care Model program to develop dashboards to measure individual clinicians’ patterns of care and share data with them, Dr. Adelson said.
The changes have been “transformative,” including allowing for hiring of additional support staff, Dr. Adelson said.
“I am terrified about what’s going to happen at the end of the program if there is not another model to transition to,” Dr. Adelson said. “We will potentially be looking at layoffs of these people who are so incredible and have done so much for the care we are giving.”
In May 2019, some of Dr. Chong’s CMMI colleagues published a report in the Journal of the National Cancer Institute about the experiences of clinics participating in the Oncology Care Model. For instance, the U.S. Oncology Network used Monthly Enhanced Oncology Services [MEOS] payments through the program for new hires such as navigators, social workers, additional advanced practice providers, “and even data analysts,” according to the report.
Still, there have been “growing pains” with the start-up of the model, Dr. Chong said. Fellow participants on the NCCN panel cited the introduction of costly new drugs as one wrinkle in the early days of the Oncology Care Model. Another is that physicians in practices signed up for the program sometimes are unaware of it.
The American Society of Clinical Oncology also is advocating for a continuation of a model along the lines of the Oncology Care Model.
“It would be good to see it continue. We’re certainly supportive of continuing to work on this model and refine it and see where it goes,” Stephen S. Grubbs, MD, ASCO’s vice president for clinical affairs, said in an interview.
Dr. Grubbs said that CMS’s Oncology Care Model could not be greatly expanded in its current form, as it imposes a steep administrative burden for both practices who participate, as well as for CMMI. The current model also does not serve smaller and rural practices well, he said.
ASCO is refining its own proposal for an alternative payment approach. The group plans to present its Patient-Centered Oncology Payment (PCOP) model, first published in 2015, before an influential federal advisory group, the Physician-Focused Payment Model Technical Advisory Committee (PTAC), at a meeting in 2020.
WASHINGTON – Federal officials are considering how they might extend a test of payment approaches meant to spur more coordinated cancer care beyond the program’s current 2021 end date.
Alexandra Chong, PhD, the team lead for the Oncology Care Model at the Center for Medicare and Medicaid Innovation (CMMI), said her agency found strong support for sustaining this kind of effort at “practice transformation.” The Oncology Care Model, which kicked off in 2016, involves about 175 practices and 10 insurers.
“As we look forward, we certainly recognize and appreciate the importance of the impact that [the Oncology Care Model] made and the desire for a continuation, either in an iteration of the current model or a different model,” Dr. Chong said during a panel discussion at a policy summit sponsored by the National Comprehensive Cancer Network (NCCN).
Dr. Chong said the Centers for Medicare & Medicaid Services is aware that there is “a lot of interest” in the future of the program. “We have heard from our practices that this model has been an opportunity for them to provide the care that they really want to provide,” Dr. Chong said.
A fellow panelist at the NCCN event, Kerin Adelson, MD, chief quality officer of Yale’s Smilow Cancer Hospital, New Haven, Conn., pressed for continuation of this kind of a payment test. Smilow has used revenue from the Oncology Care Model program to develop dashboards to measure individual clinicians’ patterns of care and share data with them, Dr. Adelson said.
The changes have been “transformative,” including allowing for hiring of additional support staff, Dr. Adelson said.
“I am terrified about what’s going to happen at the end of the program if there is not another model to transition to,” Dr. Adelson said. “We will potentially be looking at layoffs of these people who are so incredible and have done so much for the care we are giving.”
In May 2019, some of Dr. Chong’s CMMI colleagues published a report in the Journal of the National Cancer Institute about the experiences of clinics participating in the Oncology Care Model. For instance, the U.S. Oncology Network used Monthly Enhanced Oncology Services [MEOS] payments through the program for new hires such as navigators, social workers, additional advanced practice providers, “and even data analysts,” according to the report.
Still, there have been “growing pains” with the start-up of the model, Dr. Chong said. Fellow participants on the NCCN panel cited the introduction of costly new drugs as one wrinkle in the early days of the Oncology Care Model. Another is that physicians in practices signed up for the program sometimes are unaware of it.
The American Society of Clinical Oncology also is advocating for a continuation of a model along the lines of the Oncology Care Model.
“It would be good to see it continue. We’re certainly supportive of continuing to work on this model and refine it and see where it goes,” Stephen S. Grubbs, MD, ASCO’s vice president for clinical affairs, said in an interview.
Dr. Grubbs said that CMS’s Oncology Care Model could not be greatly expanded in its current form, as it imposes a steep administrative burden for both practices who participate, as well as for CMMI. The current model also does not serve smaller and rural practices well, he said.
ASCO is refining its own proposal for an alternative payment approach. The group plans to present its Patient-Centered Oncology Payment (PCOP) model, first published in 2015, before an influential federal advisory group, the Physician-Focused Payment Model Technical Advisory Committee (PTAC), at a meeting in 2020.
REPORTING FROM NCCN POLICY SUMMIT 2019
Zostavax proves safe, effective in patients with nonactive SLE
A live-attenuated herpes zoster vaccine can be used in individuals with systemic lupus erythematosus (SLE) if they are not intensively immunosuppressed and their condition is dormant, research suggests.
A paper published in Annals of the Rheumatic Diseases reported the outcomes of a randomized, placebo-controlled trial of the Zostavax herpes zoster vaccine in 90 adults with clinically stable SLE. Participants had to have been on a stable dose of immunosuppressive agents for at least 6 months and have a history of chicken pox or herpes zoster infection.
Chi Chiu Mok, MD, of the Tuen Mun Hospital in Hong Kong and coauthors wrote that herpes zoster reactivation has been reported to occur in 6.4 to 91.4 individuals with SLE per 1,000 patient-years, with consequences including postherpetic neuralgia and even death from disseminated infection. But because Zostavax is live-attenuated, it has not been widely used in immunocompromised people.
After a single subcutaneous dose of either the vaccine or placebo, researchers saw a significant increase in anti–varicella zoster virus (VZV) IgG antibodies in vaccinated individuals over 6 weeks. The magnitude of the increase in anti-VZV IgG seen in vaccinated individuals was on par with that previously seen in vaccinated healthy controls, although the authors noted that the absolute increase in values was lower.
“While the reason is not apparent, one contributing factor is the high rate of previous exposure to VZV infection in most participants, which could have led to a higher baseline anti-IgG anti-VZV value that limited its rise after vaccination,” the authors wrote.
In contrast, IgG reactivity declined in those who received the placebo injection, and the difference between the two groups was statistically significant after adjustment for baseline antibody titers.
The study also looked at the cell-mediated immune response to the vaccine and found the number of interferon-gamma secreting CD4+ T-cell spots increased in the vaccinated patients but decreased in the placebo arm, and by week 6 it was significantly higher in the treated group. The increase in the vaccine-treated patients was again similar to that previously seen in healthy controls.
However, prednisolone use at baseline may have attenuated the vaccine response. Vaccinated patients who were treated with prednisolone at baseline had a lower increase in T-cell spots and lower anti-VZV IgG reactivity after the vaccination than did those not taking prednisolone, although the difference between the two groups was not statistically significant. The study did not see any effect of age, sex, baseline lymphocyte count, disease activity scores, and other factors on response to the vaccine.
None of the patients who received the vaccine withdrew from the study because of serious adverse events. The most common adverse events reported were injection-site redness and pain, which were more common in the vaccine-treated group than in the placebo group. However these symptoms were mild and resolved by themselves after a few days. Two patients in the vaccine group and one in the placebo group experienced mild or moderate SLE flares.
The authors commented that this was the first randomized, controlled trial examining the safety and immune response of a live-attenuated herpes zoster vaccine in individuals with SLE and this trial showed it was safe and well tolerated in those with stable disease who were not on intensive immunosuppressive therapy.
“Despite the increased risk of HZ [herpes zoster] infection, SLE had the lowest HZ vaccination rates among age-eligible subjects, probably because of the concern of vaccine safety, the principle of contraindication to live-attenuated vaccines in immunocompromised hosts, as well as the current ambiguous guidelines for HZ vaccination in SLE,” they wrote.
But they also stressed that their results did not apply to patients with active disease or on more intensive immunosuppression and that longer-term data on the persistence of vaccine immunogenicity was still being collected.
The study was funded by the Hong Kong Research Fund Secretariat. No conflicts of interest were declared.
SOURCE: Mok CC et al. Ann Rheum Dis. 2019 Sep 17. doi: 10.1136/annrheumdis-2019-215925
Probably like me you have seen a bit of zoster in our patients with SLE, and rarely we get severe outbreaks in multiple dermatomes or in the eyes or other vulnerable areas in patients on immune suppression. So I think of Zostavax the way I think of shingles per se: The more immune compromised you are, the higher the risk of something bad happening … maybe. But we do know with Zostavax the risk is small.
Shingrix is a lot more effective than Zostavax and does not have the same issue of potentially causing the thing it prevents. But the most likely reason it works so well is that it has an adjuvant. We are generally a lot more concerned about injecting adjuvants in autoimmune patients here in the United States than they are in Europe where they have more experience with that, but this one is apparently a new adjuvant and has never been used in autoimmune patients, who were excluded from the trials of Shingrix. And a fair number of nonautoimmune patients get autoimmune-like symptoms in the Shingrix trials such as myalgias and fevers. I don’t think we have full confidence yet until we figure out just how worried we ought to be about that. In other words, if Shingrix only causes mild/moderate transient flares, then our patients might rationally consider that a fair trade for lifelong protection.
I think in some patients this is an easier decision than others. If somebody is 50 years old and healthy, hasn’t had nephritis or anything bad before (or not in the last 10 years), and is on no immune suppressant or just using stable, modest doses of such therapies, you would probably recommend doing something to avoid getting zoster. And here you can explain the choice to the patient: Zostavax provides good protection but less than Shingrix, is unlikely to make the patient flare, has very low risk of live vaccine causing much trouble in a generally healthy person; Shingrix is more effective overall, has caused some autoimmune symptoms in healthy people, and has unclear risk for a flare in a patient with a diagnosis (but that can be monitored).
For the sicker patients, we just have to weigh the risk of a natural zoster outbreak against the risk of a flare and the risk of disseminated zoster from the Zostavax, which is a pretty small risk but it is there. It’s a discussion you need to have in advance with each patient. Maybe with some patients, it is best to wait for an optimal time for either choice, when there’s not too much disease and not too much immune-compromising medication.
An unsolved issue for herpes zoster vaccination is age. Greater knowledge about how to best vaccinate would go a long way toward bolstering confidence in using the vaccines in patients a bit younger than 50 years given that zoster does occur in lupus patients at that age.
Joan Merrill, MD, is OMRF Professor of Medicine at the University of Oklahoma Health Sciences Center and a member of the Arthritis & Clinical Immunology Research Program at the Oklahoma Medical Research Foundation, both in Oklahoma City. She is a member of the editorial advisory board of Rheumatology News.
Probably like me you have seen a bit of zoster in our patients with SLE, and rarely we get severe outbreaks in multiple dermatomes or in the eyes or other vulnerable areas in patients on immune suppression. So I think of Zostavax the way I think of shingles per se: The more immune compromised you are, the higher the risk of something bad happening … maybe. But we do know with Zostavax the risk is small.
Shingrix is a lot more effective than Zostavax and does not have the same issue of potentially causing the thing it prevents. But the most likely reason it works so well is that it has an adjuvant. We are generally a lot more concerned about injecting adjuvants in autoimmune patients here in the United States than they are in Europe where they have more experience with that, but this one is apparently a new adjuvant and has never been used in autoimmune patients, who were excluded from the trials of Shingrix. And a fair number of nonautoimmune patients get autoimmune-like symptoms in the Shingrix trials such as myalgias and fevers. I don’t think we have full confidence yet until we figure out just how worried we ought to be about that. In other words, if Shingrix only causes mild/moderate transient flares, then our patients might rationally consider that a fair trade for lifelong protection.
I think in some patients this is an easier decision than others. If somebody is 50 years old and healthy, hasn’t had nephritis or anything bad before (or not in the last 10 years), and is on no immune suppressant or just using stable, modest doses of such therapies, you would probably recommend doing something to avoid getting zoster. And here you can explain the choice to the patient: Zostavax provides good protection but less than Shingrix, is unlikely to make the patient flare, has very low risk of live vaccine causing much trouble in a generally healthy person; Shingrix is more effective overall, has caused some autoimmune symptoms in healthy people, and has unclear risk for a flare in a patient with a diagnosis (but that can be monitored).
For the sicker patients, we just have to weigh the risk of a natural zoster outbreak against the risk of a flare and the risk of disseminated zoster from the Zostavax, which is a pretty small risk but it is there. It’s a discussion you need to have in advance with each patient. Maybe with some patients, it is best to wait for an optimal time for either choice, when there’s not too much disease and not too much immune-compromising medication.
An unsolved issue for herpes zoster vaccination is age. Greater knowledge about how to best vaccinate would go a long way toward bolstering confidence in using the vaccines in patients a bit younger than 50 years given that zoster does occur in lupus patients at that age.
Joan Merrill, MD, is OMRF Professor of Medicine at the University of Oklahoma Health Sciences Center and a member of the Arthritis & Clinical Immunology Research Program at the Oklahoma Medical Research Foundation, both in Oklahoma City. She is a member of the editorial advisory board of Rheumatology News.
Probably like me you have seen a bit of zoster in our patients with SLE, and rarely we get severe outbreaks in multiple dermatomes or in the eyes or other vulnerable areas in patients on immune suppression. So I think of Zostavax the way I think of shingles per se: The more immune compromised you are, the higher the risk of something bad happening … maybe. But we do know with Zostavax the risk is small.
Shingrix is a lot more effective than Zostavax and does not have the same issue of potentially causing the thing it prevents. But the most likely reason it works so well is that it has an adjuvant. We are generally a lot more concerned about injecting adjuvants in autoimmune patients here in the United States than they are in Europe where they have more experience with that, but this one is apparently a new adjuvant and has never been used in autoimmune patients, who were excluded from the trials of Shingrix. And a fair number of nonautoimmune patients get autoimmune-like symptoms in the Shingrix trials such as myalgias and fevers. I don’t think we have full confidence yet until we figure out just how worried we ought to be about that. In other words, if Shingrix only causes mild/moderate transient flares, then our patients might rationally consider that a fair trade for lifelong protection.
I think in some patients this is an easier decision than others. If somebody is 50 years old and healthy, hasn’t had nephritis or anything bad before (or not in the last 10 years), and is on no immune suppressant or just using stable, modest doses of such therapies, you would probably recommend doing something to avoid getting zoster. And here you can explain the choice to the patient: Zostavax provides good protection but less than Shingrix, is unlikely to make the patient flare, has very low risk of live vaccine causing much trouble in a generally healthy person; Shingrix is more effective overall, has caused some autoimmune symptoms in healthy people, and has unclear risk for a flare in a patient with a diagnosis (but that can be monitored).
For the sicker patients, we just have to weigh the risk of a natural zoster outbreak against the risk of a flare and the risk of disseminated zoster from the Zostavax, which is a pretty small risk but it is there. It’s a discussion you need to have in advance with each patient. Maybe with some patients, it is best to wait for an optimal time for either choice, when there’s not too much disease and not too much immune-compromising medication.
An unsolved issue for herpes zoster vaccination is age. Greater knowledge about how to best vaccinate would go a long way toward bolstering confidence in using the vaccines in patients a bit younger than 50 years given that zoster does occur in lupus patients at that age.
Joan Merrill, MD, is OMRF Professor of Medicine at the University of Oklahoma Health Sciences Center and a member of the Arthritis & Clinical Immunology Research Program at the Oklahoma Medical Research Foundation, both in Oklahoma City. She is a member of the editorial advisory board of Rheumatology News.
A live-attenuated herpes zoster vaccine can be used in individuals with systemic lupus erythematosus (SLE) if they are not intensively immunosuppressed and their condition is dormant, research suggests.
A paper published in Annals of the Rheumatic Diseases reported the outcomes of a randomized, placebo-controlled trial of the Zostavax herpes zoster vaccine in 90 adults with clinically stable SLE. Participants had to have been on a stable dose of immunosuppressive agents for at least 6 months and have a history of chicken pox or herpes zoster infection.
Chi Chiu Mok, MD, of the Tuen Mun Hospital in Hong Kong and coauthors wrote that herpes zoster reactivation has been reported to occur in 6.4 to 91.4 individuals with SLE per 1,000 patient-years, with consequences including postherpetic neuralgia and even death from disseminated infection. But because Zostavax is live-attenuated, it has not been widely used in immunocompromised people.
After a single subcutaneous dose of either the vaccine or placebo, researchers saw a significant increase in anti–varicella zoster virus (VZV) IgG antibodies in vaccinated individuals over 6 weeks. The magnitude of the increase in anti-VZV IgG seen in vaccinated individuals was on par with that previously seen in vaccinated healthy controls, although the authors noted that the absolute increase in values was lower.
“While the reason is not apparent, one contributing factor is the high rate of previous exposure to VZV infection in most participants, which could have led to a higher baseline anti-IgG anti-VZV value that limited its rise after vaccination,” the authors wrote.
In contrast, IgG reactivity declined in those who received the placebo injection, and the difference between the two groups was statistically significant after adjustment for baseline antibody titers.
The study also looked at the cell-mediated immune response to the vaccine and found the number of interferon-gamma secreting CD4+ T-cell spots increased in the vaccinated patients but decreased in the placebo arm, and by week 6 it was significantly higher in the treated group. The increase in the vaccine-treated patients was again similar to that previously seen in healthy controls.
However, prednisolone use at baseline may have attenuated the vaccine response. Vaccinated patients who were treated with prednisolone at baseline had a lower increase in T-cell spots and lower anti-VZV IgG reactivity after the vaccination than did those not taking prednisolone, although the difference between the two groups was not statistically significant. The study did not see any effect of age, sex, baseline lymphocyte count, disease activity scores, and other factors on response to the vaccine.
None of the patients who received the vaccine withdrew from the study because of serious adverse events. The most common adverse events reported were injection-site redness and pain, which were more common in the vaccine-treated group than in the placebo group. However these symptoms were mild and resolved by themselves after a few days. Two patients in the vaccine group and one in the placebo group experienced mild or moderate SLE flares.
The authors commented that this was the first randomized, controlled trial examining the safety and immune response of a live-attenuated herpes zoster vaccine in individuals with SLE and this trial showed it was safe and well tolerated in those with stable disease who were not on intensive immunosuppressive therapy.
“Despite the increased risk of HZ [herpes zoster] infection, SLE had the lowest HZ vaccination rates among age-eligible subjects, probably because of the concern of vaccine safety, the principle of contraindication to live-attenuated vaccines in immunocompromised hosts, as well as the current ambiguous guidelines for HZ vaccination in SLE,” they wrote.
But they also stressed that their results did not apply to patients with active disease or on more intensive immunosuppression and that longer-term data on the persistence of vaccine immunogenicity was still being collected.
The study was funded by the Hong Kong Research Fund Secretariat. No conflicts of interest were declared.
SOURCE: Mok CC et al. Ann Rheum Dis. 2019 Sep 17. doi: 10.1136/annrheumdis-2019-215925
A live-attenuated herpes zoster vaccine can be used in individuals with systemic lupus erythematosus (SLE) if they are not intensively immunosuppressed and their condition is dormant, research suggests.
A paper published in Annals of the Rheumatic Diseases reported the outcomes of a randomized, placebo-controlled trial of the Zostavax herpes zoster vaccine in 90 adults with clinically stable SLE. Participants had to have been on a stable dose of immunosuppressive agents for at least 6 months and have a history of chicken pox or herpes zoster infection.
Chi Chiu Mok, MD, of the Tuen Mun Hospital in Hong Kong and coauthors wrote that herpes zoster reactivation has been reported to occur in 6.4 to 91.4 individuals with SLE per 1,000 patient-years, with consequences including postherpetic neuralgia and even death from disseminated infection. But because Zostavax is live-attenuated, it has not been widely used in immunocompromised people.
After a single subcutaneous dose of either the vaccine or placebo, researchers saw a significant increase in anti–varicella zoster virus (VZV) IgG antibodies in vaccinated individuals over 6 weeks. The magnitude of the increase in anti-VZV IgG seen in vaccinated individuals was on par with that previously seen in vaccinated healthy controls, although the authors noted that the absolute increase in values was lower.
“While the reason is not apparent, one contributing factor is the high rate of previous exposure to VZV infection in most participants, which could have led to a higher baseline anti-IgG anti-VZV value that limited its rise after vaccination,” the authors wrote.
In contrast, IgG reactivity declined in those who received the placebo injection, and the difference between the two groups was statistically significant after adjustment for baseline antibody titers.
The study also looked at the cell-mediated immune response to the vaccine and found the number of interferon-gamma secreting CD4+ T-cell spots increased in the vaccinated patients but decreased in the placebo arm, and by week 6 it was significantly higher in the treated group. The increase in the vaccine-treated patients was again similar to that previously seen in healthy controls.
However, prednisolone use at baseline may have attenuated the vaccine response. Vaccinated patients who were treated with prednisolone at baseline had a lower increase in T-cell spots and lower anti-VZV IgG reactivity after the vaccination than did those not taking prednisolone, although the difference between the two groups was not statistically significant. The study did not see any effect of age, sex, baseline lymphocyte count, disease activity scores, and other factors on response to the vaccine.
None of the patients who received the vaccine withdrew from the study because of serious adverse events. The most common adverse events reported were injection-site redness and pain, which were more common in the vaccine-treated group than in the placebo group. However these symptoms were mild and resolved by themselves after a few days. Two patients in the vaccine group and one in the placebo group experienced mild or moderate SLE flares.
The authors commented that this was the first randomized, controlled trial examining the safety and immune response of a live-attenuated herpes zoster vaccine in individuals with SLE and this trial showed it was safe and well tolerated in those with stable disease who were not on intensive immunosuppressive therapy.
“Despite the increased risk of HZ [herpes zoster] infection, SLE had the lowest HZ vaccination rates among age-eligible subjects, probably because of the concern of vaccine safety, the principle of contraindication to live-attenuated vaccines in immunocompromised hosts, as well as the current ambiguous guidelines for HZ vaccination in SLE,” they wrote.
But they also stressed that their results did not apply to patients with active disease or on more intensive immunosuppression and that longer-term data on the persistence of vaccine immunogenicity was still being collected.
The study was funded by the Hong Kong Research Fund Secretariat. No conflicts of interest were declared.
SOURCE: Mok CC et al. Ann Rheum Dis. 2019 Sep 17. doi: 10.1136/annrheumdis-2019-215925
FROM ANNALS OF THE RHEUMATIC DISEASES
German CLLM1 study: 4-year data raise concerns about lenalidomide maintenance
EDINBURGH – Lenalidomide maintenance therapy after chemoimmunotherapy in high-risk chronic lymphocytic leukemia (CLL) improved progression- and event-free survival, but not overall survival, and was associated with three unexpected cases of B-cell acute lymphoblastic leukemia (B-ALL), according to 4-year follow-up in the German, phase 3 CLLM1 study.
Given these findings, and in particular the B-ALL cases, lenalidomide cannot be generally recommended as maintenance therapy in high-risk CLL, Moritz Fürstenau, MD, of the University of Cologne, reported in a poster at the International Workshop on Chronic Lymphocytic Leukemia.
At a median follow-up of 47.6 months, median progression-free survival (PFS) by investigator assessment was 54.7 months in 60 patients randomized to receive lenalidomide maintenance therapy, compared with 23.2 months for 29 who received placebo (hazard ratio, 0.22), and median event-free survival (EFS) was 46.2 months vs. 14.6 months in the groups, respectively (hazard ratio, 0.24), Dr. Fürstenau said during an oral poster presentation at the conference.
“So ... after 4 years of observation, we still see improvement in PFS, EFS, and time to next treatment,” he said, also noting that minimal residual disease (MRD) negativity was achieved by eight patients in the lenalidomide group, and in none of the patients in the placebo group.
However, overall survival was 79% and 87% in the lenalidomide and placebo groups, respectively (HR, 1.53). In total, 12 patients died, including 9 in the lenalidomide group from fatal infections, concomitant disease, CLL progression, or unknown causes. Three patients in the placebo group died from CLL progression or fatal infection.
In the lenalidomide group, hematological and solid tumor second primary malignancies were reported in three and four patients, respectively (5% and 7%), compared with zero and two patients, respectively (0% and 7%), in the placebo group.
The CLLM1 study of the German CLL Study Group evaluated maintenance with lenalidomide vs. placebo in patients with high risk of progression after first-line chemoimmunotherapy. Previously reported results also favored lenalidomide maintenance for PFS, but not OS, Dr. Fürstenau said, adding that the study was unblinded at a median follow-up of 17.9 months, and in November 2017 treatment was stopped when two cases of B-ALL were observed. A third case was reported in 2018.
The current analysis includes data available through December 2018, and the findings warrant further investigation to analyze the unexpectedly high incidence of B-ALL, he said.
The CLLM1 study was funded by Celgene.
[email protected]
EDINBURGH – Lenalidomide maintenance therapy after chemoimmunotherapy in high-risk chronic lymphocytic leukemia (CLL) improved progression- and event-free survival, but not overall survival, and was associated with three unexpected cases of B-cell acute lymphoblastic leukemia (B-ALL), according to 4-year follow-up in the German, phase 3 CLLM1 study.
Given these findings, and in particular the B-ALL cases, lenalidomide cannot be generally recommended as maintenance therapy in high-risk CLL, Moritz Fürstenau, MD, of the University of Cologne, reported in a poster at the International Workshop on Chronic Lymphocytic Leukemia.
At a median follow-up of 47.6 months, median progression-free survival (PFS) by investigator assessment was 54.7 months in 60 patients randomized to receive lenalidomide maintenance therapy, compared with 23.2 months for 29 who received placebo (hazard ratio, 0.22), and median event-free survival (EFS) was 46.2 months vs. 14.6 months in the groups, respectively (hazard ratio, 0.24), Dr. Fürstenau said during an oral poster presentation at the conference.
“So ... after 4 years of observation, we still see improvement in PFS, EFS, and time to next treatment,” he said, also noting that minimal residual disease (MRD) negativity was achieved by eight patients in the lenalidomide group, and in none of the patients in the placebo group.
However, overall survival was 79% and 87% in the lenalidomide and placebo groups, respectively (HR, 1.53). In total, 12 patients died, including 9 in the lenalidomide group from fatal infections, concomitant disease, CLL progression, or unknown causes. Three patients in the placebo group died from CLL progression or fatal infection.
In the lenalidomide group, hematological and solid tumor second primary malignancies were reported in three and four patients, respectively (5% and 7%), compared with zero and two patients, respectively (0% and 7%), in the placebo group.
The CLLM1 study of the German CLL Study Group evaluated maintenance with lenalidomide vs. placebo in patients with high risk of progression after first-line chemoimmunotherapy. Previously reported results also favored lenalidomide maintenance for PFS, but not OS, Dr. Fürstenau said, adding that the study was unblinded at a median follow-up of 17.9 months, and in November 2017 treatment was stopped when two cases of B-ALL were observed. A third case was reported in 2018.
The current analysis includes data available through December 2018, and the findings warrant further investigation to analyze the unexpectedly high incidence of B-ALL, he said.
The CLLM1 study was funded by Celgene.
[email protected]
EDINBURGH – Lenalidomide maintenance therapy after chemoimmunotherapy in high-risk chronic lymphocytic leukemia (CLL) improved progression- and event-free survival, but not overall survival, and was associated with three unexpected cases of B-cell acute lymphoblastic leukemia (B-ALL), according to 4-year follow-up in the German, phase 3 CLLM1 study.
Given these findings, and in particular the B-ALL cases, lenalidomide cannot be generally recommended as maintenance therapy in high-risk CLL, Moritz Fürstenau, MD, of the University of Cologne, reported in a poster at the International Workshop on Chronic Lymphocytic Leukemia.
At a median follow-up of 47.6 months, median progression-free survival (PFS) by investigator assessment was 54.7 months in 60 patients randomized to receive lenalidomide maintenance therapy, compared with 23.2 months for 29 who received placebo (hazard ratio, 0.22), and median event-free survival (EFS) was 46.2 months vs. 14.6 months in the groups, respectively (hazard ratio, 0.24), Dr. Fürstenau said during an oral poster presentation at the conference.
“So ... after 4 years of observation, we still see improvement in PFS, EFS, and time to next treatment,” he said, also noting that minimal residual disease (MRD) negativity was achieved by eight patients in the lenalidomide group, and in none of the patients in the placebo group.
However, overall survival was 79% and 87% in the lenalidomide and placebo groups, respectively (HR, 1.53). In total, 12 patients died, including 9 in the lenalidomide group from fatal infections, concomitant disease, CLL progression, or unknown causes. Three patients in the placebo group died from CLL progression or fatal infection.
In the lenalidomide group, hematological and solid tumor second primary malignancies were reported in three and four patients, respectively (5% and 7%), compared with zero and two patients, respectively (0% and 7%), in the placebo group.
The CLLM1 study of the German CLL Study Group evaluated maintenance with lenalidomide vs. placebo in patients with high risk of progression after first-line chemoimmunotherapy. Previously reported results also favored lenalidomide maintenance for PFS, but not OS, Dr. Fürstenau said, adding that the study was unblinded at a median follow-up of 17.9 months, and in November 2017 treatment was stopped when two cases of B-ALL were observed. A third case was reported in 2018.
The current analysis includes data available through December 2018, and the findings warrant further investigation to analyze the unexpectedly high incidence of B-ALL, he said.
The CLLM1 study was funded by Celgene.
[email protected]
REPORTING FROM iwCLL 2019
Apixaban prevents clots with cancer
Background: Active cancer places patients at increased risk for VTE. Ambulatory patients can be risk stratified using the validated Khorana score to assess risk for VTE, a complication resulting in significant morbidity, mortality, and health care costs.
Study design: Randomized, placebo-controlled, double-blind clinical trial.
Setting: Ambulatory; Canada.
Synopsis: A total of 1,809 patients were assessed for eligibility, 1,235 were excluded, and 574 with a Khorana score of 2 or higher were randomized to apixaban 2.5 mg twice daily or placebo. Treatment or placebo was given within 24 hours after the initiation of chemotherapy and continued for 180 days. The primary efficacy outcome – first episode of major VTE within 180 days of randomization – occurred in 4.2% of the apixaban group and in 10.2% of the placebo group (hazard ratio, 0.41; 95% confidence interval, 0.26-0.65; P less than .001). Major bleeding in the modified intention-to-treat analysis occurred in 3.5% in the apixaban group and 1.8% in the placebo group (HR, 2.00; 95% CI, 1.01-3.95; P = .046).
There was no significant difference in overall survival, with 87% of deaths were related to cancer or cancer progression.
Bottom line: VTE is significantly lower with the use of apixaban, compared with placebo, in intermediate- to high-risk ambulatory patients with active cancer who are initiating chemotherapy.
Citation: Carrier M et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: Active cancer places patients at increased risk for VTE. Ambulatory patients can be risk stratified using the validated Khorana score to assess risk for VTE, a complication resulting in significant morbidity, mortality, and health care costs.
Study design: Randomized, placebo-controlled, double-blind clinical trial.
Setting: Ambulatory; Canada.
Synopsis: A total of 1,809 patients were assessed for eligibility, 1,235 were excluded, and 574 with a Khorana score of 2 or higher were randomized to apixaban 2.5 mg twice daily or placebo. Treatment or placebo was given within 24 hours after the initiation of chemotherapy and continued for 180 days. The primary efficacy outcome – first episode of major VTE within 180 days of randomization – occurred in 4.2% of the apixaban group and in 10.2% of the placebo group (hazard ratio, 0.41; 95% confidence interval, 0.26-0.65; P less than .001). Major bleeding in the modified intention-to-treat analysis occurred in 3.5% in the apixaban group and 1.8% in the placebo group (HR, 2.00; 95% CI, 1.01-3.95; P = .046).
There was no significant difference in overall survival, with 87% of deaths were related to cancer or cancer progression.
Bottom line: VTE is significantly lower with the use of apixaban, compared with placebo, in intermediate- to high-risk ambulatory patients with active cancer who are initiating chemotherapy.
Citation: Carrier M et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
Background: Active cancer places patients at increased risk for VTE. Ambulatory patients can be risk stratified using the validated Khorana score to assess risk for VTE, a complication resulting in significant morbidity, mortality, and health care costs.
Study design: Randomized, placebo-controlled, double-blind clinical trial.
Setting: Ambulatory; Canada.
Synopsis: A total of 1,809 patients were assessed for eligibility, 1,235 were excluded, and 574 with a Khorana score of 2 or higher were randomized to apixaban 2.5 mg twice daily or placebo. Treatment or placebo was given within 24 hours after the initiation of chemotherapy and continued for 180 days. The primary efficacy outcome – first episode of major VTE within 180 days of randomization – occurred in 4.2% of the apixaban group and in 10.2% of the placebo group (hazard ratio, 0.41; 95% confidence interval, 0.26-0.65; P less than .001). Major bleeding in the modified intention-to-treat analysis occurred in 3.5% in the apixaban group and 1.8% in the placebo group (HR, 2.00; 95% CI, 1.01-3.95; P = .046).
There was no significant difference in overall survival, with 87% of deaths were related to cancer or cancer progression.
Bottom line: VTE is significantly lower with the use of apixaban, compared with placebo, in intermediate- to high-risk ambulatory patients with active cancer who are initiating chemotherapy.
Citation: Carrier M et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2018 Dec 4. doi: 10.1056/NEJMoa1814468.
Dr. Trammell Velasquez is an associate professor of medicine in the division of general and hospital medicine at UT Health San Antonio and a hospitalist at South Texas Veterans Health Care System.
CheckMate 817: Nivo+ipi shows safety, efficacy across stage IV NSCLC subgroups
BARCELONA – First-line flat-dose nivolumab plus weight-based ipilimumab was safe and showed encouraging clinical activity both in general and in patients with poor performance status and comorbidities in the multicenter CheckMate 817 study of patients with advanced non–small cell lung cancer.
The treatment-related adverse event (TRAE) rate in 139 patients with Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 2, for example, was 63%, compared with 77% in 391 patients with good ECOG PS (score of 0-1), and the rates of grade 3-4 AEs in the groups, respectively, were 26% and 35%, Fabrice Barlesi, MD, reported at the World Conference on Lung Cancer.
In 59 patients with ECOG PS of 0-1 plus either asymptomatic untreated brain metastases, hepatic or renal impairment, or HIV infection, the overall TRAE and grade 3-4 TRAE rates were 78% and 34%.
The combined TRAE and grade 3-4 TRAE rates in the two “special populations” cohorts were 67% and 28%, respectively, Dr. Barlesi, of Aix-Marseille Universite and Assistance Publique Hôpitaux de Marseille, France, said at the conference, which was sponsored by the International Association for the Study of Lung Cancer.
The overall response rate (ORR) in the ECOG PS-2 and PS-01+comorbidity groups was 19% and 37%, respectively, compared with 36% in the good PS cohort, and median duration of response in the three groups was 14.2 months, 9.7 months, and at least 18 months (median not reached), respectively.
The 1-year PFS rates were 25%, 27%, and 35% respectively, and median PFS was 3.6, 4.2, and 5.8, he said.
Among the 198 special population patients, those with PD-L1 expression of 50% or greater, 1% or greater, or less than 1% had 1-yr PFS rates of 46%, 24%, and 29% (median, 9.6, 3.2, and 3.9 months), and in those with 10 or greater mut/Mb and less than 10 mut/Mb, they were 42% and 17% (median, 8.3 and 2.8 months), respectively.
The single-arm, nonrandomized CheckMate 817 study evaluated the programmed death-ligand 1 (PD-L1) inhibitor nivolumab at a flat dose of 240 mg given intravenously every 2 weeks plus the CTLA-4 inhibitor ipilimumab at 1 mg/kg IV every 6 weeks, with treatment until disease progression or unacceptable toxicity for up to 2 years. Participants had stage IV non–small cell lung cancer (NSCLC), had received no prior systemic therapy, and had no known sensitizing EGFR or ALK alterations.
“Nivolumab and ipilimumab are immune checkpoint inhibitors with distinct ... but complementary mechanisms of action,” Dr. Barlesi said, adding that in combination they have demonstrated clinical benefit vs. chemotherapy in the first-line treatment of NSCLC.
However, data are limited on safety and efficacy of immunotherapy in patients with advanced NSCLC with other comorbidities such as brain metastases, kidney and renal disease, and HIV, as such patients – despite comprising the majority of NSCLC patients at presentation – are typically ineligible for trial registration, he explained, adding that CheckMate 817 is a multicohort, nonrandomized, phase 3b study evaluating the safety and efficacy of nivolumab plus ipilimumab in such patients.
The findings show a safety profile “clearly comparable” to that observed in prior studies using weight-based nivolumab, he noted.
“Nivolumab plus ipilimumab showed clearly encouraging clinical activity in this special population, with an overall response rate of 24%,” he said. “As expected, unfortunately, the outcomes in these special populations were affected by poor performance status, however, despite the poor performance status or comorbidities, those patients were shown to achieve durable responses ... with [an overall] duration of response at 1 year, of 57%.”
CheckMate 817 was sponsored by Bristol-Myers Squibb. Dr. Barlesi disclosed financial relationships with Abbvie, ACEA, Amgen, Astra-Zeneca, Bayer, Bristol-Myers Squibb, Boehringer–Ingelheim, Eisai, Eli Lilly Oncology, F. Hoffmann–La Roche Ltd, Genentech, Ipsen, Ignyta, Innate Pharma, Loxo, Novartis, Medimmune, Merck, MSD, Pierre Fabre, Pfizer, Sanofi-Aventis, and Takeda.
SOURCE: Barlesi F et al. WCLC 2019: Abstract OA04.02.
BARCELONA – First-line flat-dose nivolumab plus weight-based ipilimumab was safe and showed encouraging clinical activity both in general and in patients with poor performance status and comorbidities in the multicenter CheckMate 817 study of patients with advanced non–small cell lung cancer.
The treatment-related adverse event (TRAE) rate in 139 patients with Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 2, for example, was 63%, compared with 77% in 391 patients with good ECOG PS (score of 0-1), and the rates of grade 3-4 AEs in the groups, respectively, were 26% and 35%, Fabrice Barlesi, MD, reported at the World Conference on Lung Cancer.
In 59 patients with ECOG PS of 0-1 plus either asymptomatic untreated brain metastases, hepatic or renal impairment, or HIV infection, the overall TRAE and grade 3-4 TRAE rates were 78% and 34%.
The combined TRAE and grade 3-4 TRAE rates in the two “special populations” cohorts were 67% and 28%, respectively, Dr. Barlesi, of Aix-Marseille Universite and Assistance Publique Hôpitaux de Marseille, France, said at the conference, which was sponsored by the International Association for the Study of Lung Cancer.
The overall response rate (ORR) in the ECOG PS-2 and PS-01+comorbidity groups was 19% and 37%, respectively, compared with 36% in the good PS cohort, and median duration of response in the three groups was 14.2 months, 9.7 months, and at least 18 months (median not reached), respectively.
The 1-year PFS rates were 25%, 27%, and 35% respectively, and median PFS was 3.6, 4.2, and 5.8, he said.
Among the 198 special population patients, those with PD-L1 expression of 50% or greater, 1% or greater, or less than 1% had 1-yr PFS rates of 46%, 24%, and 29% (median, 9.6, 3.2, and 3.9 months), and in those with 10 or greater mut/Mb and less than 10 mut/Mb, they were 42% and 17% (median, 8.3 and 2.8 months), respectively.
The single-arm, nonrandomized CheckMate 817 study evaluated the programmed death-ligand 1 (PD-L1) inhibitor nivolumab at a flat dose of 240 mg given intravenously every 2 weeks plus the CTLA-4 inhibitor ipilimumab at 1 mg/kg IV every 6 weeks, with treatment until disease progression or unacceptable toxicity for up to 2 years. Participants had stage IV non–small cell lung cancer (NSCLC), had received no prior systemic therapy, and had no known sensitizing EGFR or ALK alterations.
“Nivolumab and ipilimumab are immune checkpoint inhibitors with distinct ... but complementary mechanisms of action,” Dr. Barlesi said, adding that in combination they have demonstrated clinical benefit vs. chemotherapy in the first-line treatment of NSCLC.
However, data are limited on safety and efficacy of immunotherapy in patients with advanced NSCLC with other comorbidities such as brain metastases, kidney and renal disease, and HIV, as such patients – despite comprising the majority of NSCLC patients at presentation – are typically ineligible for trial registration, he explained, adding that CheckMate 817 is a multicohort, nonrandomized, phase 3b study evaluating the safety and efficacy of nivolumab plus ipilimumab in such patients.
The findings show a safety profile “clearly comparable” to that observed in prior studies using weight-based nivolumab, he noted.
“Nivolumab plus ipilimumab showed clearly encouraging clinical activity in this special population, with an overall response rate of 24%,” he said. “As expected, unfortunately, the outcomes in these special populations were affected by poor performance status, however, despite the poor performance status or comorbidities, those patients were shown to achieve durable responses ... with [an overall] duration of response at 1 year, of 57%.”
CheckMate 817 was sponsored by Bristol-Myers Squibb. Dr. Barlesi disclosed financial relationships with Abbvie, ACEA, Amgen, Astra-Zeneca, Bayer, Bristol-Myers Squibb, Boehringer–Ingelheim, Eisai, Eli Lilly Oncology, F. Hoffmann–La Roche Ltd, Genentech, Ipsen, Ignyta, Innate Pharma, Loxo, Novartis, Medimmune, Merck, MSD, Pierre Fabre, Pfizer, Sanofi-Aventis, and Takeda.
SOURCE: Barlesi F et al. WCLC 2019: Abstract OA04.02.
BARCELONA – First-line flat-dose nivolumab plus weight-based ipilimumab was safe and showed encouraging clinical activity both in general and in patients with poor performance status and comorbidities in the multicenter CheckMate 817 study of patients with advanced non–small cell lung cancer.
The treatment-related adverse event (TRAE) rate in 139 patients with Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 2, for example, was 63%, compared with 77% in 391 patients with good ECOG PS (score of 0-1), and the rates of grade 3-4 AEs in the groups, respectively, were 26% and 35%, Fabrice Barlesi, MD, reported at the World Conference on Lung Cancer.
In 59 patients with ECOG PS of 0-1 plus either asymptomatic untreated brain metastases, hepatic or renal impairment, or HIV infection, the overall TRAE and grade 3-4 TRAE rates were 78% and 34%.
The combined TRAE and grade 3-4 TRAE rates in the two “special populations” cohorts were 67% and 28%, respectively, Dr. Barlesi, of Aix-Marseille Universite and Assistance Publique Hôpitaux de Marseille, France, said at the conference, which was sponsored by the International Association for the Study of Lung Cancer.
The overall response rate (ORR) in the ECOG PS-2 and PS-01+comorbidity groups was 19% and 37%, respectively, compared with 36% in the good PS cohort, and median duration of response in the three groups was 14.2 months, 9.7 months, and at least 18 months (median not reached), respectively.
The 1-year PFS rates were 25%, 27%, and 35% respectively, and median PFS was 3.6, 4.2, and 5.8, he said.
Among the 198 special population patients, those with PD-L1 expression of 50% or greater, 1% or greater, or less than 1% had 1-yr PFS rates of 46%, 24%, and 29% (median, 9.6, 3.2, and 3.9 months), and in those with 10 or greater mut/Mb and less than 10 mut/Mb, they were 42% and 17% (median, 8.3 and 2.8 months), respectively.
The single-arm, nonrandomized CheckMate 817 study evaluated the programmed death-ligand 1 (PD-L1) inhibitor nivolumab at a flat dose of 240 mg given intravenously every 2 weeks plus the CTLA-4 inhibitor ipilimumab at 1 mg/kg IV every 6 weeks, with treatment until disease progression or unacceptable toxicity for up to 2 years. Participants had stage IV non–small cell lung cancer (NSCLC), had received no prior systemic therapy, and had no known sensitizing EGFR or ALK alterations.
“Nivolumab and ipilimumab are immune checkpoint inhibitors with distinct ... but complementary mechanisms of action,” Dr. Barlesi said, adding that in combination they have demonstrated clinical benefit vs. chemotherapy in the first-line treatment of NSCLC.
However, data are limited on safety and efficacy of immunotherapy in patients with advanced NSCLC with other comorbidities such as brain metastases, kidney and renal disease, and HIV, as such patients – despite comprising the majority of NSCLC patients at presentation – are typically ineligible for trial registration, he explained, adding that CheckMate 817 is a multicohort, nonrandomized, phase 3b study evaluating the safety and efficacy of nivolumab plus ipilimumab in such patients.
The findings show a safety profile “clearly comparable” to that observed in prior studies using weight-based nivolumab, he noted.
“Nivolumab plus ipilimumab showed clearly encouraging clinical activity in this special population, with an overall response rate of 24%,” he said. “As expected, unfortunately, the outcomes in these special populations were affected by poor performance status, however, despite the poor performance status or comorbidities, those patients were shown to achieve durable responses ... with [an overall] duration of response at 1 year, of 57%.”
CheckMate 817 was sponsored by Bristol-Myers Squibb. Dr. Barlesi disclosed financial relationships with Abbvie, ACEA, Amgen, Astra-Zeneca, Bayer, Bristol-Myers Squibb, Boehringer–Ingelheim, Eisai, Eli Lilly Oncology, F. Hoffmann–La Roche Ltd, Genentech, Ipsen, Ignyta, Innate Pharma, Loxo, Novartis, Medimmune, Merck, MSD, Pierre Fabre, Pfizer, Sanofi-Aventis, and Takeda.
SOURCE: Barlesi F et al. WCLC 2019: Abstract OA04.02.
REPORTING FROM WCLC 2019















