User login
Prior authorizations for infusibles cause delays, toxicities
Rheumatologist Zachary S. Wallace, MD, knew just how prior authorization requirements were impacting his staff time and work flow when he embarked on a study several years ago. Managing authorizations for infusible medications alone was about to become a full-time job for one of the administrative assistants in the rheumatology unit at Massachusetts General Hospital in Boston.
His research questions concerned patients. “There’s a lot of talk about how much onus prior authorization requirements put on providers and the practice,” Dr. Wallace said. ”I was interested in understanding what impact [these requirements] have on patients themselves.”
Dr. Wallace led a review of the EHRs of 225 patients for whom an infusible medication such as rituximab and infliximab was ordered by 1 of the 16 physicians in the rheumatology unit between July 2016 and June 2018. The findings – that patients who needed prior authorizations for infusible medications had a significantly longer time to treatment initiation and higher prednisone-equivalent glucocorticoid exposure – were reported online in Arthritis Care & Research.
Among patients whose authorizations were initially denied, these differences were “pretty drastic,” Dr. Wallace said. The median time to receiving a first infusion was 50 days, compared with 27 days when permission was not required, and glucocorticoid exposure during the 3 months following the request was 605 mg versus 160 mg.
Among patients whose authorizations were not denied, the median time to first infusion was 31 days, compared with 27 days when authorization was not required, and the mean glucocorticoid exposure over 3 months was 364 mg versus 160 mg.
“I hope that our findings will help facilitate discussions with insurance providers, pharmacy benefit managers, and state and federal legislators about the need to address the impact that prior authorization requirements have on patients and providers,” said Dr. Wallace, also of the clinical epidemiology program in the division of rheumatology, allergy and immunology at Massachusetts General, and an assistant professor of medicine at Harvard Medical School, Boston.
Of the 225 patients for whom an infusible medication was ordered, 71% required preauthorization. Of these, 79% were approved and 21% were denied after the first request. And in a finding that Dr. Wallace called “somewhat surprising,” 82% of the authorizations originally denied were approved after appeal.
All told, prior authorizations for infusible medications were eventually approved in all but a small number of cases. “We go through all this effort to get these prior authorizations approved, and 96% of the time, they were ultimately approved,” he said in an interview.
Christopher Phillips, MD, a community rheumatologist in Paducah, Ky., who serves as chair of the insurance subcommittee of the American College of Rheumatology’s committee on rheumatologic care, said the findings “give further credence” to rheumatologists’ concerns. “We know [from our own experiences] that prior authorizations delay care, and we know that delays can cause harm to patients. We now have hard data backing up this assertion.”
Regarding the high number of authorization approvals, “there’s an argument to be made that for certain treatments and certain conditions where the success rate of appeals is high enough, you shouldn’t be subjecting these treatments to these [preauthorization] policies,” he said.
Calls for prior authorization reform
Most patients in the study (71%) had private insurance. But the findings also have implications for Medicare, Dr. Wallace said, as recent federal policies have expanded Medicare Advantage plans’ authority to use prior authorization in conjunction with step therapy for medications administered under Part B. Step therapy favors primary use of what insurers deem the most cost-effective therapies.
The ACR is one of almost 370 physician, patient, and health care organizations that are urging Congress to pass a bipartisan bill aimed at streamlining and standardizing prior authorization under the Medicare Advantage program. The legislation – Improving Seniors’ Timely Access to Care Act of 2019 (H.R. 3107) – was introduced by Reps. Suzan DelBene (D-Wash.), Mike Kelly (R-Pa.), Roger Marshall, MD (R-Kan.), and Ami Bera, MD (D-Calif.).
The bill calls for the creation of an electronic prior authorization program and a “real-time process for items and services that are routinely approved,” as well as greater Centers for Medicare & Medicaid Services oversight on how Medicare Advantage plans use prior authorization. Plans would be required to report to the CMS on the extent of their use of prior authorization and the rate of approvals or denials. They would also be held accountable for making timely prior authorization determinations and providing rationales for denials, according to a letter to Congress cosigned by the ACR.
In a press release about the legislation, Paula Marchetta, MD, president of the ACR, said that “the unregulated use of prior authorization has devolved into a time-consuming and obstructive process that often stalls or outright revokes patient access to medically necessary therapies.” She added that “many health care plans now use prior authorization indiscriminately.”
Cathryn Donaldson, director of communications for America’s Health Insurance Plans (AHIP), said in an email that prior authorization is used for less than 15% of covered services, and that, along with step therapy, it “helps ensure that patients receive care that is safe, effective, and necessary.” AHIP “knows that prior authorization can be improved,” she said, and is committed to streamlining the process.
A demonstration project on the automation of various parts of prior authorization is being coordinated with health information technology companies, plans, and providers, she noted.
The federal legislation is based at least partly on a consensus statement drafted by AHIP, the American Medical Association, and four other organizations representing hospitals, medical groups, and health plans on ways to improve the prior authorization process. Among the items mentioned in the statement is that “regular review” of services subject to prior authorization could help identify therapies that “no longer warrant” prior authorization because of low denial rates.
Outside of Medicare Advantage, the AMA is aware of at least 85 bills being introduced in states this year that address utilization management in commercial plans. Nearly all these bills attempt to reform prior authorization programs in some way, according to R. J. Mills, media relations coordinator for the AMA.
Rheumatologic patients hard hit
Off-label medication use was the most common reason (82%) for a prior authorization denial in the Massachusetts General study, even though 78% of the patients for whom infusible medications were prescribed had a condition with no Food and Drug Administration–approved treatment. Having such a condition was associated with 120% or 190% higher odds of having a denial in unadjusted and adjusted (for age and sex) analyses, Dr. Wallace and colleagues reported.
Moreover, nearly half (48%) of the patients with denials had already tried or were currently taking an oral disease-modifying antirheumatic drug, such as methotrexate.
The majority of denials were for the use of rituximab (70%), followed by infliximab (12%) and tocilizumab (12%). Most of the denials (79%) were appealed successfully through a peer-to-peer discussion. In five cases, the insurer’s preferred drug (for example, adalimumab) had to be used rather than the requested infusion (for example, infliximab).
Infused medications, many of which are biologics, are among the most expensive drugs prescribed for patients with rheumatic diseases. They were easiest for Dr. Wallace to study because of the way prior authorizations are handled in his unit, but prior authorization requirements are “widespread” in rheumatology practices across treatment types, he and Dr. Phillips said.
“Some of our relatively inexpensive treatments are subject to prior authorization requirements,” Dr. Phillips said. “We hear stories about prednisone needing a prior authorization sometimes.”
With respect to infusible medications, the insurance subcommittee is hearing from ACR members about seemingly increasing numbers of both clinical coverage reviews – for example, reviews of prior treatments – and site-of-care restrictions, Dr. Phillips noted. “Some carriers are insisting on infusions in non-hospital-based settings, for cost savings, or on home infusions, which are concerning because of [possible] infusion reactions and medical service availability.”
The application of step therapy to rheumatologic patients is troubling because of the “often unique medical circumstances of the patient,” Dr. Phillips said. “There are enough differences among the [tumor necrosis factor] antagonists, for instance, that make one more appropriate for a certain patient than another. Those differences are not brought into consideration with these policies.”
There are other ways in which prior authorization processes “are not well informed medically,” he said, recalling a case brought to the attention of the subcommittee in which a patient prescribed a biologic drug for psoriatic arthritis was denied authorization because “the documentation did not include a [disease activity measure] that is specific to RA and not used for psoriatic arthritis.”
It is not uncommon for authorizations for infusible medications to take 2 weeks or longer to secure – even when initially approved. In the AMA’s 2018 Prior Authorization Physician Survey, 65% reported waiting at least 1 business day for a decision and 26% reported waiting at least 3 business days for responses. “With infusibles, we’re absolutely dealing with a much longer time,” Dr. Phillips said.
In Dr. Wallace’s study, the finding that prior authorizations facilitated greater prednisone-equivalent glucocorticoid exposure is important, he and his colleagues wrote, because these medications may put patients at higher risk of infection, cardiovascular disease, and diabetes – even in low doses and with short-term use. Notably, the median delay to the initiation of treatment was 29 days, regardless of prior authorization requirements. Dr. Wallace said the delays “likely reflect a combination of factors” – including infusion center waiting lists and patient-level factors – and that his team is “thinking about how to facilitate better access [to their practice’s infusion center] for those who are approved for treatment.”
The most common conditions for which infused medication was ordered were inflammatory arthritis (32%), vasculitis (23%), and IgG4-related disease (17%). The 225 patients in the study had an average age of 53 years.
Dr. Wallace reported that he has no relevant financial disclosures.
SOURCE: Wallace ZS et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24062.
Rheumatologist Zachary S. Wallace, MD, knew just how prior authorization requirements were impacting his staff time and work flow when he embarked on a study several years ago. Managing authorizations for infusible medications alone was about to become a full-time job for one of the administrative assistants in the rheumatology unit at Massachusetts General Hospital in Boston.
His research questions concerned patients. “There’s a lot of talk about how much onus prior authorization requirements put on providers and the practice,” Dr. Wallace said. ”I was interested in understanding what impact [these requirements] have on patients themselves.”
Dr. Wallace led a review of the EHRs of 225 patients for whom an infusible medication such as rituximab and infliximab was ordered by 1 of the 16 physicians in the rheumatology unit between July 2016 and June 2018. The findings – that patients who needed prior authorizations for infusible medications had a significantly longer time to treatment initiation and higher prednisone-equivalent glucocorticoid exposure – were reported online in Arthritis Care & Research.
Among patients whose authorizations were initially denied, these differences were “pretty drastic,” Dr. Wallace said. The median time to receiving a first infusion was 50 days, compared with 27 days when permission was not required, and glucocorticoid exposure during the 3 months following the request was 605 mg versus 160 mg.
Among patients whose authorizations were not denied, the median time to first infusion was 31 days, compared with 27 days when authorization was not required, and the mean glucocorticoid exposure over 3 months was 364 mg versus 160 mg.
“I hope that our findings will help facilitate discussions with insurance providers, pharmacy benefit managers, and state and federal legislators about the need to address the impact that prior authorization requirements have on patients and providers,” said Dr. Wallace, also of the clinical epidemiology program in the division of rheumatology, allergy and immunology at Massachusetts General, and an assistant professor of medicine at Harvard Medical School, Boston.
Of the 225 patients for whom an infusible medication was ordered, 71% required preauthorization. Of these, 79% were approved and 21% were denied after the first request. And in a finding that Dr. Wallace called “somewhat surprising,” 82% of the authorizations originally denied were approved after appeal.
All told, prior authorizations for infusible medications were eventually approved in all but a small number of cases. “We go through all this effort to get these prior authorizations approved, and 96% of the time, they were ultimately approved,” he said in an interview.
Christopher Phillips, MD, a community rheumatologist in Paducah, Ky., who serves as chair of the insurance subcommittee of the American College of Rheumatology’s committee on rheumatologic care, said the findings “give further credence” to rheumatologists’ concerns. “We know [from our own experiences] that prior authorizations delay care, and we know that delays can cause harm to patients. We now have hard data backing up this assertion.”
Regarding the high number of authorization approvals, “there’s an argument to be made that for certain treatments and certain conditions where the success rate of appeals is high enough, you shouldn’t be subjecting these treatments to these [preauthorization] policies,” he said.
Calls for prior authorization reform
Most patients in the study (71%) had private insurance. But the findings also have implications for Medicare, Dr. Wallace said, as recent federal policies have expanded Medicare Advantage plans’ authority to use prior authorization in conjunction with step therapy for medications administered under Part B. Step therapy favors primary use of what insurers deem the most cost-effective therapies.
The ACR is one of almost 370 physician, patient, and health care organizations that are urging Congress to pass a bipartisan bill aimed at streamlining and standardizing prior authorization under the Medicare Advantage program. The legislation – Improving Seniors’ Timely Access to Care Act of 2019 (H.R. 3107) – was introduced by Reps. Suzan DelBene (D-Wash.), Mike Kelly (R-Pa.), Roger Marshall, MD (R-Kan.), and Ami Bera, MD (D-Calif.).
The bill calls for the creation of an electronic prior authorization program and a “real-time process for items and services that are routinely approved,” as well as greater Centers for Medicare & Medicaid Services oversight on how Medicare Advantage plans use prior authorization. Plans would be required to report to the CMS on the extent of their use of prior authorization and the rate of approvals or denials. They would also be held accountable for making timely prior authorization determinations and providing rationales for denials, according to a letter to Congress cosigned by the ACR.
In a press release about the legislation, Paula Marchetta, MD, president of the ACR, said that “the unregulated use of prior authorization has devolved into a time-consuming and obstructive process that often stalls or outright revokes patient access to medically necessary therapies.” She added that “many health care plans now use prior authorization indiscriminately.”
Cathryn Donaldson, director of communications for America’s Health Insurance Plans (AHIP), said in an email that prior authorization is used for less than 15% of covered services, and that, along with step therapy, it “helps ensure that patients receive care that is safe, effective, and necessary.” AHIP “knows that prior authorization can be improved,” she said, and is committed to streamlining the process.
A demonstration project on the automation of various parts of prior authorization is being coordinated with health information technology companies, plans, and providers, she noted.
The federal legislation is based at least partly on a consensus statement drafted by AHIP, the American Medical Association, and four other organizations representing hospitals, medical groups, and health plans on ways to improve the prior authorization process. Among the items mentioned in the statement is that “regular review” of services subject to prior authorization could help identify therapies that “no longer warrant” prior authorization because of low denial rates.
Outside of Medicare Advantage, the AMA is aware of at least 85 bills being introduced in states this year that address utilization management in commercial plans. Nearly all these bills attempt to reform prior authorization programs in some way, according to R. J. Mills, media relations coordinator for the AMA.
Rheumatologic patients hard hit
Off-label medication use was the most common reason (82%) for a prior authorization denial in the Massachusetts General study, even though 78% of the patients for whom infusible medications were prescribed had a condition with no Food and Drug Administration–approved treatment. Having such a condition was associated with 120% or 190% higher odds of having a denial in unadjusted and adjusted (for age and sex) analyses, Dr. Wallace and colleagues reported.
Moreover, nearly half (48%) of the patients with denials had already tried or were currently taking an oral disease-modifying antirheumatic drug, such as methotrexate.
The majority of denials were for the use of rituximab (70%), followed by infliximab (12%) and tocilizumab (12%). Most of the denials (79%) were appealed successfully through a peer-to-peer discussion. In five cases, the insurer’s preferred drug (for example, adalimumab) had to be used rather than the requested infusion (for example, infliximab).
Infused medications, many of which are biologics, are among the most expensive drugs prescribed for patients with rheumatic diseases. They were easiest for Dr. Wallace to study because of the way prior authorizations are handled in his unit, but prior authorization requirements are “widespread” in rheumatology practices across treatment types, he and Dr. Phillips said.
“Some of our relatively inexpensive treatments are subject to prior authorization requirements,” Dr. Phillips said. “We hear stories about prednisone needing a prior authorization sometimes.”
With respect to infusible medications, the insurance subcommittee is hearing from ACR members about seemingly increasing numbers of both clinical coverage reviews – for example, reviews of prior treatments – and site-of-care restrictions, Dr. Phillips noted. “Some carriers are insisting on infusions in non-hospital-based settings, for cost savings, or on home infusions, which are concerning because of [possible] infusion reactions and medical service availability.”
The application of step therapy to rheumatologic patients is troubling because of the “often unique medical circumstances of the patient,” Dr. Phillips said. “There are enough differences among the [tumor necrosis factor] antagonists, for instance, that make one more appropriate for a certain patient than another. Those differences are not brought into consideration with these policies.”
There are other ways in which prior authorization processes “are not well informed medically,” he said, recalling a case brought to the attention of the subcommittee in which a patient prescribed a biologic drug for psoriatic arthritis was denied authorization because “the documentation did not include a [disease activity measure] that is specific to RA and not used for psoriatic arthritis.”
It is not uncommon for authorizations for infusible medications to take 2 weeks or longer to secure – even when initially approved. In the AMA’s 2018 Prior Authorization Physician Survey, 65% reported waiting at least 1 business day for a decision and 26% reported waiting at least 3 business days for responses. “With infusibles, we’re absolutely dealing with a much longer time,” Dr. Phillips said.
In Dr. Wallace’s study, the finding that prior authorizations facilitated greater prednisone-equivalent glucocorticoid exposure is important, he and his colleagues wrote, because these medications may put patients at higher risk of infection, cardiovascular disease, and diabetes – even in low doses and with short-term use. Notably, the median delay to the initiation of treatment was 29 days, regardless of prior authorization requirements. Dr. Wallace said the delays “likely reflect a combination of factors” – including infusion center waiting lists and patient-level factors – and that his team is “thinking about how to facilitate better access [to their practice’s infusion center] for those who are approved for treatment.”
The most common conditions for which infused medication was ordered were inflammatory arthritis (32%), vasculitis (23%), and IgG4-related disease (17%). The 225 patients in the study had an average age of 53 years.
Dr. Wallace reported that he has no relevant financial disclosures.
SOURCE: Wallace ZS et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24062.
Rheumatologist Zachary S. Wallace, MD, knew just how prior authorization requirements were impacting his staff time and work flow when he embarked on a study several years ago. Managing authorizations for infusible medications alone was about to become a full-time job for one of the administrative assistants in the rheumatology unit at Massachusetts General Hospital in Boston.
His research questions concerned patients. “There’s a lot of talk about how much onus prior authorization requirements put on providers and the practice,” Dr. Wallace said. ”I was interested in understanding what impact [these requirements] have on patients themselves.”
Dr. Wallace led a review of the EHRs of 225 patients for whom an infusible medication such as rituximab and infliximab was ordered by 1 of the 16 physicians in the rheumatology unit between July 2016 and June 2018. The findings – that patients who needed prior authorizations for infusible medications had a significantly longer time to treatment initiation and higher prednisone-equivalent glucocorticoid exposure – were reported online in Arthritis Care & Research.
Among patients whose authorizations were initially denied, these differences were “pretty drastic,” Dr. Wallace said. The median time to receiving a first infusion was 50 days, compared with 27 days when permission was not required, and glucocorticoid exposure during the 3 months following the request was 605 mg versus 160 mg.
Among patients whose authorizations were not denied, the median time to first infusion was 31 days, compared with 27 days when authorization was not required, and the mean glucocorticoid exposure over 3 months was 364 mg versus 160 mg.
“I hope that our findings will help facilitate discussions with insurance providers, pharmacy benefit managers, and state and federal legislators about the need to address the impact that prior authorization requirements have on patients and providers,” said Dr. Wallace, also of the clinical epidemiology program in the division of rheumatology, allergy and immunology at Massachusetts General, and an assistant professor of medicine at Harvard Medical School, Boston.
Of the 225 patients for whom an infusible medication was ordered, 71% required preauthorization. Of these, 79% were approved and 21% were denied after the first request. And in a finding that Dr. Wallace called “somewhat surprising,” 82% of the authorizations originally denied were approved after appeal.
All told, prior authorizations for infusible medications were eventually approved in all but a small number of cases. “We go through all this effort to get these prior authorizations approved, and 96% of the time, they were ultimately approved,” he said in an interview.
Christopher Phillips, MD, a community rheumatologist in Paducah, Ky., who serves as chair of the insurance subcommittee of the American College of Rheumatology’s committee on rheumatologic care, said the findings “give further credence” to rheumatologists’ concerns. “We know [from our own experiences] that prior authorizations delay care, and we know that delays can cause harm to patients. We now have hard data backing up this assertion.”
Regarding the high number of authorization approvals, “there’s an argument to be made that for certain treatments and certain conditions where the success rate of appeals is high enough, you shouldn’t be subjecting these treatments to these [preauthorization] policies,” he said.
Calls for prior authorization reform
Most patients in the study (71%) had private insurance. But the findings also have implications for Medicare, Dr. Wallace said, as recent federal policies have expanded Medicare Advantage plans’ authority to use prior authorization in conjunction with step therapy for medications administered under Part B. Step therapy favors primary use of what insurers deem the most cost-effective therapies.
The ACR is one of almost 370 physician, patient, and health care organizations that are urging Congress to pass a bipartisan bill aimed at streamlining and standardizing prior authorization under the Medicare Advantage program. The legislation – Improving Seniors’ Timely Access to Care Act of 2019 (H.R. 3107) – was introduced by Reps. Suzan DelBene (D-Wash.), Mike Kelly (R-Pa.), Roger Marshall, MD (R-Kan.), and Ami Bera, MD (D-Calif.).
The bill calls for the creation of an electronic prior authorization program and a “real-time process for items and services that are routinely approved,” as well as greater Centers for Medicare & Medicaid Services oversight on how Medicare Advantage plans use prior authorization. Plans would be required to report to the CMS on the extent of their use of prior authorization and the rate of approvals or denials. They would also be held accountable for making timely prior authorization determinations and providing rationales for denials, according to a letter to Congress cosigned by the ACR.
In a press release about the legislation, Paula Marchetta, MD, president of the ACR, said that “the unregulated use of prior authorization has devolved into a time-consuming and obstructive process that often stalls or outright revokes patient access to medically necessary therapies.” She added that “many health care plans now use prior authorization indiscriminately.”
Cathryn Donaldson, director of communications for America’s Health Insurance Plans (AHIP), said in an email that prior authorization is used for less than 15% of covered services, and that, along with step therapy, it “helps ensure that patients receive care that is safe, effective, and necessary.” AHIP “knows that prior authorization can be improved,” she said, and is committed to streamlining the process.
A demonstration project on the automation of various parts of prior authorization is being coordinated with health information technology companies, plans, and providers, she noted.
The federal legislation is based at least partly on a consensus statement drafted by AHIP, the American Medical Association, and four other organizations representing hospitals, medical groups, and health plans on ways to improve the prior authorization process. Among the items mentioned in the statement is that “regular review” of services subject to prior authorization could help identify therapies that “no longer warrant” prior authorization because of low denial rates.
Outside of Medicare Advantage, the AMA is aware of at least 85 bills being introduced in states this year that address utilization management in commercial plans. Nearly all these bills attempt to reform prior authorization programs in some way, according to R. J. Mills, media relations coordinator for the AMA.
Rheumatologic patients hard hit
Off-label medication use was the most common reason (82%) for a prior authorization denial in the Massachusetts General study, even though 78% of the patients for whom infusible medications were prescribed had a condition with no Food and Drug Administration–approved treatment. Having such a condition was associated with 120% or 190% higher odds of having a denial in unadjusted and adjusted (for age and sex) analyses, Dr. Wallace and colleagues reported.
Moreover, nearly half (48%) of the patients with denials had already tried or were currently taking an oral disease-modifying antirheumatic drug, such as methotrexate.
The majority of denials were for the use of rituximab (70%), followed by infliximab (12%) and tocilizumab (12%). Most of the denials (79%) were appealed successfully through a peer-to-peer discussion. In five cases, the insurer’s preferred drug (for example, adalimumab) had to be used rather than the requested infusion (for example, infliximab).
Infused medications, many of which are biologics, are among the most expensive drugs prescribed for patients with rheumatic diseases. They were easiest for Dr. Wallace to study because of the way prior authorizations are handled in his unit, but prior authorization requirements are “widespread” in rheumatology practices across treatment types, he and Dr. Phillips said.
“Some of our relatively inexpensive treatments are subject to prior authorization requirements,” Dr. Phillips said. “We hear stories about prednisone needing a prior authorization sometimes.”
With respect to infusible medications, the insurance subcommittee is hearing from ACR members about seemingly increasing numbers of both clinical coverage reviews – for example, reviews of prior treatments – and site-of-care restrictions, Dr. Phillips noted. “Some carriers are insisting on infusions in non-hospital-based settings, for cost savings, or on home infusions, which are concerning because of [possible] infusion reactions and medical service availability.”
The application of step therapy to rheumatologic patients is troubling because of the “often unique medical circumstances of the patient,” Dr. Phillips said. “There are enough differences among the [tumor necrosis factor] antagonists, for instance, that make one more appropriate for a certain patient than another. Those differences are not brought into consideration with these policies.”
There are other ways in which prior authorization processes “are not well informed medically,” he said, recalling a case brought to the attention of the subcommittee in which a patient prescribed a biologic drug for psoriatic arthritis was denied authorization because “the documentation did not include a [disease activity measure] that is specific to RA and not used for psoriatic arthritis.”
It is not uncommon for authorizations for infusible medications to take 2 weeks or longer to secure – even when initially approved. In the AMA’s 2018 Prior Authorization Physician Survey, 65% reported waiting at least 1 business day for a decision and 26% reported waiting at least 3 business days for responses. “With infusibles, we’re absolutely dealing with a much longer time,” Dr. Phillips said.
In Dr. Wallace’s study, the finding that prior authorizations facilitated greater prednisone-equivalent glucocorticoid exposure is important, he and his colleagues wrote, because these medications may put patients at higher risk of infection, cardiovascular disease, and diabetes – even in low doses and with short-term use. Notably, the median delay to the initiation of treatment was 29 days, regardless of prior authorization requirements. Dr. Wallace said the delays “likely reflect a combination of factors” – including infusion center waiting lists and patient-level factors – and that his team is “thinking about how to facilitate better access [to their practice’s infusion center] for those who are approved for treatment.”
The most common conditions for which infused medication was ordered were inflammatory arthritis (32%), vasculitis (23%), and IgG4-related disease (17%). The 225 patients in the study had an average age of 53 years.
Dr. Wallace reported that he has no relevant financial disclosures.
SOURCE: Wallace ZS et al. Arthritis Care Res. 2019 Sep 10. doi: 10.1002/acr.24062.
FROM ARTHRITIS CARE & RESEARCH
New 2020 Priorities: Expanding AVAHO Outreach and Influence
MINNEAPOLIS -- When William “Bill” Wachsman, MD, PhD, joined the executive board of the Association of VA Hematology/Oncology earlier this decade, the organization revolved around its annual meeting. Now, AVAHO is expanding its horizons, and Dr. Wachsman plans to push for a wider focus and greater impact as its new president.
“We’re a group of like-minded individuals who came together about 15 years ago and said we want to take better care of our patients, coordinate our services, and better educate ourselves,” said Dr. Wachsman, a hematologist/oncologist with US Department of Veterans Affairs (VA) San Diego Health Care System, University of California San Diego School of Medicine, and Moores Cancer Center. “We’re still dedicated to this mission. Moving forward, I want to improve educational opportunities, encourage our interest groups to develop initiatives, and utilize our foundation to support medical professionals and improve patient care within the VA.”
Dr. Wachsman took over as AVAHO’s president on the last day of the organization’s annual meeting in Minneapolis. He replaces immediate past president Mark Klein, MD, and will serve for 1 year.
According to Dr. Wachsman, AVAHO is unique among cancer/hematology associations because it’s not limited to physicians. “Everyone who’s involved with the care of patients with hematologic or oncologic disease can be involved. You don’t need to be an employee of the VA.”
Indeed, AVAHO’s approximately 800 members include medical oncologists and hematologists, surgical oncologists, radiation oncologists, pharmacists, nurses, nurse practitioners, advanced practice registered nurses, physician assistants, social workers, cancer registrars, and other allied health professionals.
AVAHO is also unique because it’s not a VA organization. “It’s an association of people are interested in better care for patients at the VA,” Dr. Wachsman said.
Over the next year, Dr. Wachsman hopes to form a community advisory board “that can not only give us advice, but reach out to other associations in the VA and in oncology to spread the word about what we’re doing.” Other forms of outreach can help AVAHO gain influence among policymakers, he said.
As for AVAHO’s foundation, he hopes to bring in funding through grants to support fellowship awards and to help VA sites around the nation develop infrastructure to support clinical trials.
On another national level, he said, AVAHO can improve its relationship with the VA with a goal of promoting honest and productive communication that goes both ways. “You have to get to know each other,” he said, “before you jump into the same pool and begin to swim.”
MINNEAPOLIS -- When William “Bill” Wachsman, MD, PhD, joined the executive board of the Association of VA Hematology/Oncology earlier this decade, the organization revolved around its annual meeting. Now, AVAHO is expanding its horizons, and Dr. Wachsman plans to push for a wider focus and greater impact as its new president.
“We’re a group of like-minded individuals who came together about 15 years ago and said we want to take better care of our patients, coordinate our services, and better educate ourselves,” said Dr. Wachsman, a hematologist/oncologist with US Department of Veterans Affairs (VA) San Diego Health Care System, University of California San Diego School of Medicine, and Moores Cancer Center. “We’re still dedicated to this mission. Moving forward, I want to improve educational opportunities, encourage our interest groups to develop initiatives, and utilize our foundation to support medical professionals and improve patient care within the VA.”
Dr. Wachsman took over as AVAHO’s president on the last day of the organization’s annual meeting in Minneapolis. He replaces immediate past president Mark Klein, MD, and will serve for 1 year.
According to Dr. Wachsman, AVAHO is unique among cancer/hematology associations because it’s not limited to physicians. “Everyone who’s involved with the care of patients with hematologic or oncologic disease can be involved. You don’t need to be an employee of the VA.”
Indeed, AVAHO’s approximately 800 members include medical oncologists and hematologists, surgical oncologists, radiation oncologists, pharmacists, nurses, nurse practitioners, advanced practice registered nurses, physician assistants, social workers, cancer registrars, and other allied health professionals.
AVAHO is also unique because it’s not a VA organization. “It’s an association of people are interested in better care for patients at the VA,” Dr. Wachsman said.
Over the next year, Dr. Wachsman hopes to form a community advisory board “that can not only give us advice, but reach out to other associations in the VA and in oncology to spread the word about what we’re doing.” Other forms of outreach can help AVAHO gain influence among policymakers, he said.
As for AVAHO’s foundation, he hopes to bring in funding through grants to support fellowship awards and to help VA sites around the nation develop infrastructure to support clinical trials.
On another national level, he said, AVAHO can improve its relationship with the VA with a goal of promoting honest and productive communication that goes both ways. “You have to get to know each other,” he said, “before you jump into the same pool and begin to swim.”
MINNEAPOLIS -- When William “Bill” Wachsman, MD, PhD, joined the executive board of the Association of VA Hematology/Oncology earlier this decade, the organization revolved around its annual meeting. Now, AVAHO is expanding its horizons, and Dr. Wachsman plans to push for a wider focus and greater impact as its new president.
“We’re a group of like-minded individuals who came together about 15 years ago and said we want to take better care of our patients, coordinate our services, and better educate ourselves,” said Dr. Wachsman, a hematologist/oncologist with US Department of Veterans Affairs (VA) San Diego Health Care System, University of California San Diego School of Medicine, and Moores Cancer Center. “We’re still dedicated to this mission. Moving forward, I want to improve educational opportunities, encourage our interest groups to develop initiatives, and utilize our foundation to support medical professionals and improve patient care within the VA.”
Dr. Wachsman took over as AVAHO’s president on the last day of the organization’s annual meeting in Minneapolis. He replaces immediate past president Mark Klein, MD, and will serve for 1 year.
According to Dr. Wachsman, AVAHO is unique among cancer/hematology associations because it’s not limited to physicians. “Everyone who’s involved with the care of patients with hematologic or oncologic disease can be involved. You don’t need to be an employee of the VA.”
Indeed, AVAHO’s approximately 800 members include medical oncologists and hematologists, surgical oncologists, radiation oncologists, pharmacists, nurses, nurse practitioners, advanced practice registered nurses, physician assistants, social workers, cancer registrars, and other allied health professionals.
AVAHO is also unique because it’s not a VA organization. “It’s an association of people are interested in better care for patients at the VA,” Dr. Wachsman said.
Over the next year, Dr. Wachsman hopes to form a community advisory board “that can not only give us advice, but reach out to other associations in the VA and in oncology to spread the word about what we’re doing.” Other forms of outreach can help AVAHO gain influence among policymakers, he said.
As for AVAHO’s foundation, he hopes to bring in funding through grants to support fellowship awards and to help VA sites around the nation develop infrastructure to support clinical trials.
On another national level, he said, AVAHO can improve its relationship with the VA with a goal of promoting honest and productive communication that goes both ways. “You have to get to know each other,” he said, “before you jump into the same pool and begin to swim.”
Click for Credit: Psoriasis relief; Stress & CV problems; more
Here are 5 articles from the October issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Bronchiolitis is a feared complication of connective tissue disease
To take the posttest, go to: https://bit.ly/2klWpRb
Expires April 8, 2020
2. Stress incontinence surgery improves sexual dysfunction
To take the posttest, go to: https://bit.ly/2m0wb71
Expires April 10, 2020
3. Survey finds psoriasis patients seek relief with alternative therapies
To take the posttest, go to: https://bit.ly/2lZZDtO
Expires April 10, 2020
4. New data further suggest that stress does a number on the CV system
To take the posttest, go to: https://bit.ly/2lR31ax
Expires April 11, 2020
5. Rate of objects ingested by young children increased over last two decades
To take the posttest, go to: https://bit.ly/2mmYptb
Expires April 12, 2020
Here are 5 articles from the October issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Bronchiolitis is a feared complication of connective tissue disease
To take the posttest, go to: https://bit.ly/2klWpRb
Expires April 8, 2020
2. Stress incontinence surgery improves sexual dysfunction
To take the posttest, go to: https://bit.ly/2m0wb71
Expires April 10, 2020
3. Survey finds psoriasis patients seek relief with alternative therapies
To take the posttest, go to: https://bit.ly/2lZZDtO
Expires April 10, 2020
4. New data further suggest that stress does a number on the CV system
To take the posttest, go to: https://bit.ly/2lR31ax
Expires April 11, 2020
5. Rate of objects ingested by young children increased over last two decades
To take the posttest, go to: https://bit.ly/2mmYptb
Expires April 12, 2020
Here are 5 articles from the October issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Bronchiolitis is a feared complication of connective tissue disease
To take the posttest, go to: https://bit.ly/2klWpRb
Expires April 8, 2020
2. Stress incontinence surgery improves sexual dysfunction
To take the posttest, go to: https://bit.ly/2m0wb71
Expires April 10, 2020
3. Survey finds psoriasis patients seek relief with alternative therapies
To take the posttest, go to: https://bit.ly/2lZZDtO
Expires April 10, 2020
4. New data further suggest that stress does a number on the CV system
To take the posttest, go to: https://bit.ly/2lR31ax
Expires April 11, 2020
5. Rate of objects ingested by young children increased over last two decades
To take the posttest, go to: https://bit.ly/2mmYptb
Expires April 12, 2020
In Informed Consent, Capacity Is Crucial
MINNEAPOLIS -- Picture this: A patient with cancer wants to get better and needs your help. But he or she refuses to hear the prognosis or understand the treatment options. The patient, in essence, has embraced a personal don’t-ask, don’t-tell policy.
What should you do as a medical professional? Get help from a psychologist and consider the ethics of the situation, advised a VA psychologist in a presentation at the September 2019 annual meeting of the Association of VA Hematology/Oncology.
Alyssa Ford, PhD, psychosocial oncology coordinator at VA Pittsburgh Healthcare System in Pennsylvania, said she has faced this situation. “We didn’t know the staging yet, but the veteran did not want to know about their prognosis or the treatment options,” she recalled. “They just wanted to fight this cancer.”
At issue in this case, she said, is this question: “What do we do when a patient opts out about receiving sufficient information to make an informed choice?”
As she explained, the key is to understand the person’s capacity—the ability to make an informed decision. “It can be assessed by any licensed health care provider who understands the components of capacity and is able to assess them.”
Ford evaluates a patient’s capacity by analyzing whether he or she can perform 4 tasks: Make decisions, live independently, manage finances, and grant power of attorney. “Often,” she said, “they have 1 but not all.”
Other components of capacity include the ability to understand one’s medical situation, an appreciation of the pros and cons of treatment options, the consistency of choices over time, and the ability to reason. “Can the patient consider the risks and benefits of each option and consider quality of life vs quantity of life in light of their own cultural identity and personal values?”
Keep in mind that levels of capacity can change over time, Ford said, and remember that these judgements are not arbitrary or punitive.
When someone doesn’t have capacity, she said, “it doesn’t necessarily tell us why or whether it will come back. But it does say they can’t provide informed consent.”
What happened to the determinedly reluctant patient who simply wanted to “fight” and not make decisions?
“The oncology provider chose to have the psychology provider in the room while staging information and prognosis was shared,” Ford said in an interview following her presentation. “And the psychology provider assisted with ensuring that the veteran received education in simple terms and in promoting active coping.”
In addition, she said, “the psychologist provider also spent several minutes after the visit giving the veteran an opportunity to discuss their feelings. And the provider physically escorted the veteran to the laboratory to ensure that the impact of receiving difficult news did not impair mood or cognition to the point that the veteran left the medical center instead of engaging in the next step of needed medical care.”
Ford reports no relevant disclosures.
MINNEAPOLIS -- Picture this: A patient with cancer wants to get better and needs your help. But he or she refuses to hear the prognosis or understand the treatment options. The patient, in essence, has embraced a personal don’t-ask, don’t-tell policy.
What should you do as a medical professional? Get help from a psychologist and consider the ethics of the situation, advised a VA psychologist in a presentation at the September 2019 annual meeting of the Association of VA Hematology/Oncology.
Alyssa Ford, PhD, psychosocial oncology coordinator at VA Pittsburgh Healthcare System in Pennsylvania, said she has faced this situation. “We didn’t know the staging yet, but the veteran did not want to know about their prognosis or the treatment options,” she recalled. “They just wanted to fight this cancer.”
At issue in this case, she said, is this question: “What do we do when a patient opts out about receiving sufficient information to make an informed choice?”
As she explained, the key is to understand the person’s capacity—the ability to make an informed decision. “It can be assessed by any licensed health care provider who understands the components of capacity and is able to assess them.”
Ford evaluates a patient’s capacity by analyzing whether he or she can perform 4 tasks: Make decisions, live independently, manage finances, and grant power of attorney. “Often,” she said, “they have 1 but not all.”
Other components of capacity include the ability to understand one’s medical situation, an appreciation of the pros and cons of treatment options, the consistency of choices over time, and the ability to reason. “Can the patient consider the risks and benefits of each option and consider quality of life vs quantity of life in light of their own cultural identity and personal values?”
Keep in mind that levels of capacity can change over time, Ford said, and remember that these judgements are not arbitrary or punitive.
When someone doesn’t have capacity, she said, “it doesn’t necessarily tell us why or whether it will come back. But it does say they can’t provide informed consent.”
What happened to the determinedly reluctant patient who simply wanted to “fight” and not make decisions?
“The oncology provider chose to have the psychology provider in the room while staging information and prognosis was shared,” Ford said in an interview following her presentation. “And the psychology provider assisted with ensuring that the veteran received education in simple terms and in promoting active coping.”
In addition, she said, “the psychologist provider also spent several minutes after the visit giving the veteran an opportunity to discuss their feelings. And the provider physically escorted the veteran to the laboratory to ensure that the impact of receiving difficult news did not impair mood or cognition to the point that the veteran left the medical center instead of engaging in the next step of needed medical care.”
Ford reports no relevant disclosures.
MINNEAPOLIS -- Picture this: A patient with cancer wants to get better and needs your help. But he or she refuses to hear the prognosis or understand the treatment options. The patient, in essence, has embraced a personal don’t-ask, don’t-tell policy.
What should you do as a medical professional? Get help from a psychologist and consider the ethics of the situation, advised a VA psychologist in a presentation at the September 2019 annual meeting of the Association of VA Hematology/Oncology.
Alyssa Ford, PhD, psychosocial oncology coordinator at VA Pittsburgh Healthcare System in Pennsylvania, said she has faced this situation. “We didn’t know the staging yet, but the veteran did not want to know about their prognosis or the treatment options,” she recalled. “They just wanted to fight this cancer.”
At issue in this case, she said, is this question: “What do we do when a patient opts out about receiving sufficient information to make an informed choice?”
As she explained, the key is to understand the person’s capacity—the ability to make an informed decision. “It can be assessed by any licensed health care provider who understands the components of capacity and is able to assess them.”
Ford evaluates a patient’s capacity by analyzing whether he or she can perform 4 tasks: Make decisions, live independently, manage finances, and grant power of attorney. “Often,” she said, “they have 1 but not all.”
Other components of capacity include the ability to understand one’s medical situation, an appreciation of the pros and cons of treatment options, the consistency of choices over time, and the ability to reason. “Can the patient consider the risks and benefits of each option and consider quality of life vs quantity of life in light of their own cultural identity and personal values?”
Keep in mind that levels of capacity can change over time, Ford said, and remember that these judgements are not arbitrary or punitive.
When someone doesn’t have capacity, she said, “it doesn’t necessarily tell us why or whether it will come back. But it does say they can’t provide informed consent.”
What happened to the determinedly reluctant patient who simply wanted to “fight” and not make decisions?
“The oncology provider chose to have the psychology provider in the room while staging information and prognosis was shared,” Ford said in an interview following her presentation. “And the psychology provider assisted with ensuring that the veteran received education in simple terms and in promoting active coping.”
In addition, she said, “the psychologist provider also spent several minutes after the visit giving the veteran an opportunity to discuss their feelings. And the provider physically escorted the veteran to the laboratory to ensure that the impact of receiving difficult news did not impair mood or cognition to the point that the veteran left the medical center instead of engaging in the next step of needed medical care.”
Ford reports no relevant disclosures.
Prevalence of developmental disabilities up significantly since 2009
The significant increase in developmental disability prevalence in U.S. children from 2009 to 2017 may have been driven by factors such as better identification and availability of services, according to analysis of National Health Interview Survey (NHIS) data.
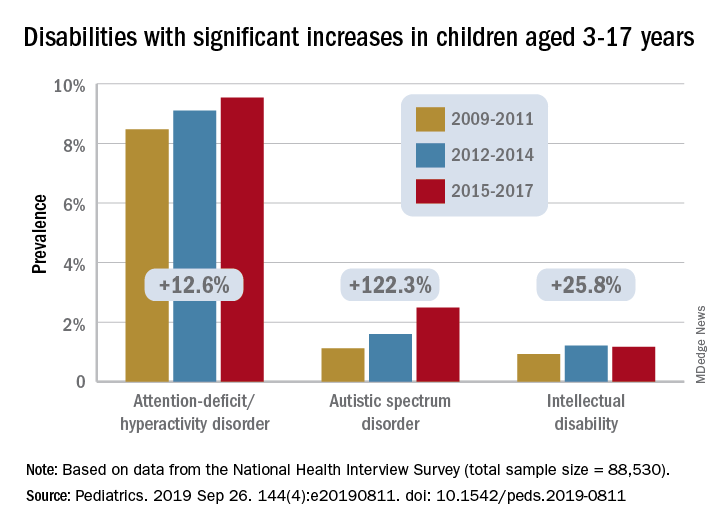
The prevalence of any developmental disability rose from 16% in 2009 to 18% in 2017 in children aged 3-17 years, for an increase of 9.5%, Benjamin Zablotsky, PhD, of the National Center for Health Statistics, Hyattsville, Md., and associates reported in Pediatrics.
Changes among the various conditions were not uniform, however, and most of the increase came from ADHD, autism spectrum disorder (ASD), and intellectual disability, which were up by 12.6%, 122.3%, and 25.8%, respectively, they said, based on data for 88,530 children from the NHIS.
Because other studies have shown that the “prevalence of ADHD symptoms and impairment has remained steady over time,” the increase according to NHIS data “could be driven by better identification of children who meet criteria for ADHD, as current estimates of diagnosed prevalence are in line with community-based studies,” Dr. Zablotsky and associates wrote.
Improved identification, “related to increasing parental awareness and changing provider practices” also may account for much of the rise in ASD prevalence, they said.
It also may be related to changes in the survey itself, as “an increase of about 80% was seen in the 2014 NHIS following changes to the wording and ordering of the question capturing ASD.”
The investigators offered a similar explanation for the increase in intellectual disability prevalence, which increased by 72% from 2011 to 2013 when the phrasing of the NHIS question was changed from “mental retardation” to “intellectual disability, also known as mental retardation.”
The other specific conditions – blindness, cerebral palsy, hearing loss, learning disability, seizures, and stuttering/stammering – all saw nonsignificant changes during the study period, with one exception. “Other developmental delay” dropped by a significant 13%. “It is possible that parents have become less likely to select this category because their children have increasingly been diagnosed with another specified condition on the survey,” Dr. Zablotsky and associates said.
“These findings have major implications for pediatric training and workforce needs and more broadly for public health policies and resources to meet the complex medical and educational needs of the rising number of children with disabilities and their families,” Maureen S. Durkin, PhD, DrPH, said in an accompanying editorial.
The trends reported by Dr. Zablotsky and associates, which have been seen in other countries, are the result of improved survival among children, so, “in this sense, a rise in the prevalence of developmental disabilities may be seen as a global indicator of progress in children’s health and pediatric care,” said Dr. Durkin, a epidemiologist in Madison, Wis.
Dr. Zablotsky and coauthors said that there was no external funding for the study and that they had no relevant financial relationships to disclose. Dr. Durkin said that she had no potential conflicts of interest.
SOURCES: Zablotsky B et al. Pediatrics. 2019 Sep 26. 144(4):e20190811. doi: 10.1542/peds.2019-0811; Durkin MS. Pediatrics. 2019 Sep 26. 144[4]:e20192005. doi: 10.1542/peds.2019-2005.
The significant increase in developmental disability prevalence in U.S. children from 2009 to 2017 may have been driven by factors such as better identification and availability of services, according to analysis of National Health Interview Survey (NHIS) data.

The prevalence of any developmental disability rose from 16% in 2009 to 18% in 2017 in children aged 3-17 years, for an increase of 9.5%, Benjamin Zablotsky, PhD, of the National Center for Health Statistics, Hyattsville, Md., and associates reported in Pediatrics.
Changes among the various conditions were not uniform, however, and most of the increase came from ADHD, autism spectrum disorder (ASD), and intellectual disability, which were up by 12.6%, 122.3%, and 25.8%, respectively, they said, based on data for 88,530 children from the NHIS.
Because other studies have shown that the “prevalence of ADHD symptoms and impairment has remained steady over time,” the increase according to NHIS data “could be driven by better identification of children who meet criteria for ADHD, as current estimates of diagnosed prevalence are in line with community-based studies,” Dr. Zablotsky and associates wrote.
Improved identification, “related to increasing parental awareness and changing provider practices” also may account for much of the rise in ASD prevalence, they said.
It also may be related to changes in the survey itself, as “an increase of about 80% was seen in the 2014 NHIS following changes to the wording and ordering of the question capturing ASD.”
The investigators offered a similar explanation for the increase in intellectual disability prevalence, which increased by 72% from 2011 to 2013 when the phrasing of the NHIS question was changed from “mental retardation” to “intellectual disability, also known as mental retardation.”
The other specific conditions – blindness, cerebral palsy, hearing loss, learning disability, seizures, and stuttering/stammering – all saw nonsignificant changes during the study period, with one exception. “Other developmental delay” dropped by a significant 13%. “It is possible that parents have become less likely to select this category because their children have increasingly been diagnosed with another specified condition on the survey,” Dr. Zablotsky and associates said.
“These findings have major implications for pediatric training and workforce needs and more broadly for public health policies and resources to meet the complex medical and educational needs of the rising number of children with disabilities and their families,” Maureen S. Durkin, PhD, DrPH, said in an accompanying editorial.
The trends reported by Dr. Zablotsky and associates, which have been seen in other countries, are the result of improved survival among children, so, “in this sense, a rise in the prevalence of developmental disabilities may be seen as a global indicator of progress in children’s health and pediatric care,” said Dr. Durkin, a epidemiologist in Madison, Wis.
Dr. Zablotsky and coauthors said that there was no external funding for the study and that they had no relevant financial relationships to disclose. Dr. Durkin said that she had no potential conflicts of interest.
SOURCES: Zablotsky B et al. Pediatrics. 2019 Sep 26. 144(4):e20190811. doi: 10.1542/peds.2019-0811; Durkin MS. Pediatrics. 2019 Sep 26. 144[4]:e20192005. doi: 10.1542/peds.2019-2005.
The significant increase in developmental disability prevalence in U.S. children from 2009 to 2017 may have been driven by factors such as better identification and availability of services, according to analysis of National Health Interview Survey (NHIS) data.

The prevalence of any developmental disability rose from 16% in 2009 to 18% in 2017 in children aged 3-17 years, for an increase of 9.5%, Benjamin Zablotsky, PhD, of the National Center for Health Statistics, Hyattsville, Md., and associates reported in Pediatrics.
Changes among the various conditions were not uniform, however, and most of the increase came from ADHD, autism spectrum disorder (ASD), and intellectual disability, which were up by 12.6%, 122.3%, and 25.8%, respectively, they said, based on data for 88,530 children from the NHIS.
Because other studies have shown that the “prevalence of ADHD symptoms and impairment has remained steady over time,” the increase according to NHIS data “could be driven by better identification of children who meet criteria for ADHD, as current estimates of diagnosed prevalence are in line with community-based studies,” Dr. Zablotsky and associates wrote.
Improved identification, “related to increasing parental awareness and changing provider practices” also may account for much of the rise in ASD prevalence, they said.
It also may be related to changes in the survey itself, as “an increase of about 80% was seen in the 2014 NHIS following changes to the wording and ordering of the question capturing ASD.”
The investigators offered a similar explanation for the increase in intellectual disability prevalence, which increased by 72% from 2011 to 2013 when the phrasing of the NHIS question was changed from “mental retardation” to “intellectual disability, also known as mental retardation.”
The other specific conditions – blindness, cerebral palsy, hearing loss, learning disability, seizures, and stuttering/stammering – all saw nonsignificant changes during the study period, with one exception. “Other developmental delay” dropped by a significant 13%. “It is possible that parents have become less likely to select this category because their children have increasingly been diagnosed with another specified condition on the survey,” Dr. Zablotsky and associates said.
“These findings have major implications for pediatric training and workforce needs and more broadly for public health policies and resources to meet the complex medical and educational needs of the rising number of children with disabilities and their families,” Maureen S. Durkin, PhD, DrPH, said in an accompanying editorial.
The trends reported by Dr. Zablotsky and associates, which have been seen in other countries, are the result of improved survival among children, so, “in this sense, a rise in the prevalence of developmental disabilities may be seen as a global indicator of progress in children’s health and pediatric care,” said Dr. Durkin, a epidemiologist in Madison, Wis.
Dr. Zablotsky and coauthors said that there was no external funding for the study and that they had no relevant financial relationships to disclose. Dr. Durkin said that she had no potential conflicts of interest.
SOURCES: Zablotsky B et al. Pediatrics. 2019 Sep 26. 144(4):e20190811. doi: 10.1542/peds.2019-0811; Durkin MS. Pediatrics. 2019 Sep 26. 144[4]:e20192005. doi: 10.1542/peds.2019-2005.
FROM PEDIATRICS
Key clinical point:
Major finding: Among U.S. children aged 3-17 years, prevalence of developmental disabilities increased by 9.5% from 2009 to 2017.
Study details: The sample from the National Health Interview Survey included 88,530 children.
Disclosures: The investigators said that there was no external funding for the study and that they had no relevant financial relationships to disclose.
Source: Zablotsky B et al. Pediatrics. 2019 Sep 26. 144(4):e20190811. doi: 10.1542/peds.2019-0811.
Blips on Lips
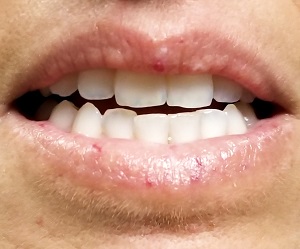
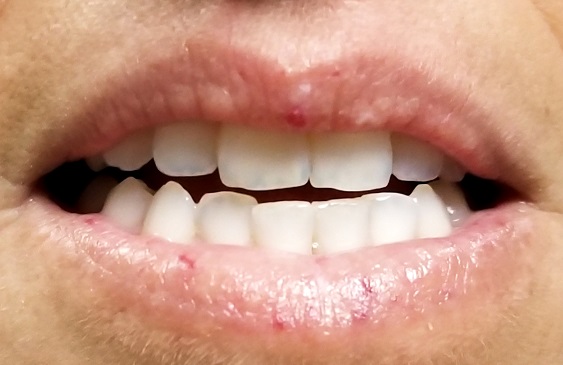
A 39-year-old woman presents with red vascular streaks on her upper and lower lips. They have slowly multiplied and become more prominent since she first noticed them several years ago. Although the lesions are asymptomatic, their effect on her appearance bothers the patient.
Her primary care provider tried to resolve the problem with cryotherapy. But the treatment attempt was unsuccessful.
During history-taking, the patient divulges other existing health problems. She has been diagnosed with Raynaud syndrome, which flares several times a year, especially in cold weather or times of exceptional stress. She also has permanent thickening of the skin on her distal fingers, a result of sudden-onset swelling of all 10 fingers several years ago.
She denies any problems with eating, such as heartburn or difficulty swallowing. She also denies any respiratory problems or chronic fatigue.
EXAMINATION
Seven very slender telangiectasias, most aligned vertically, are seen on the upper and lower vermillion surfaces. They range from a pinpoint to 6 mm in length. There are no similar lesions are on the rest of the oral mucosae, the face, or the chest.
The patient’s fingers, from the metacarpals to the tips, are decidedly edematous and firm but not tender. Several fingertips are scarred from past Raynaud episodes.
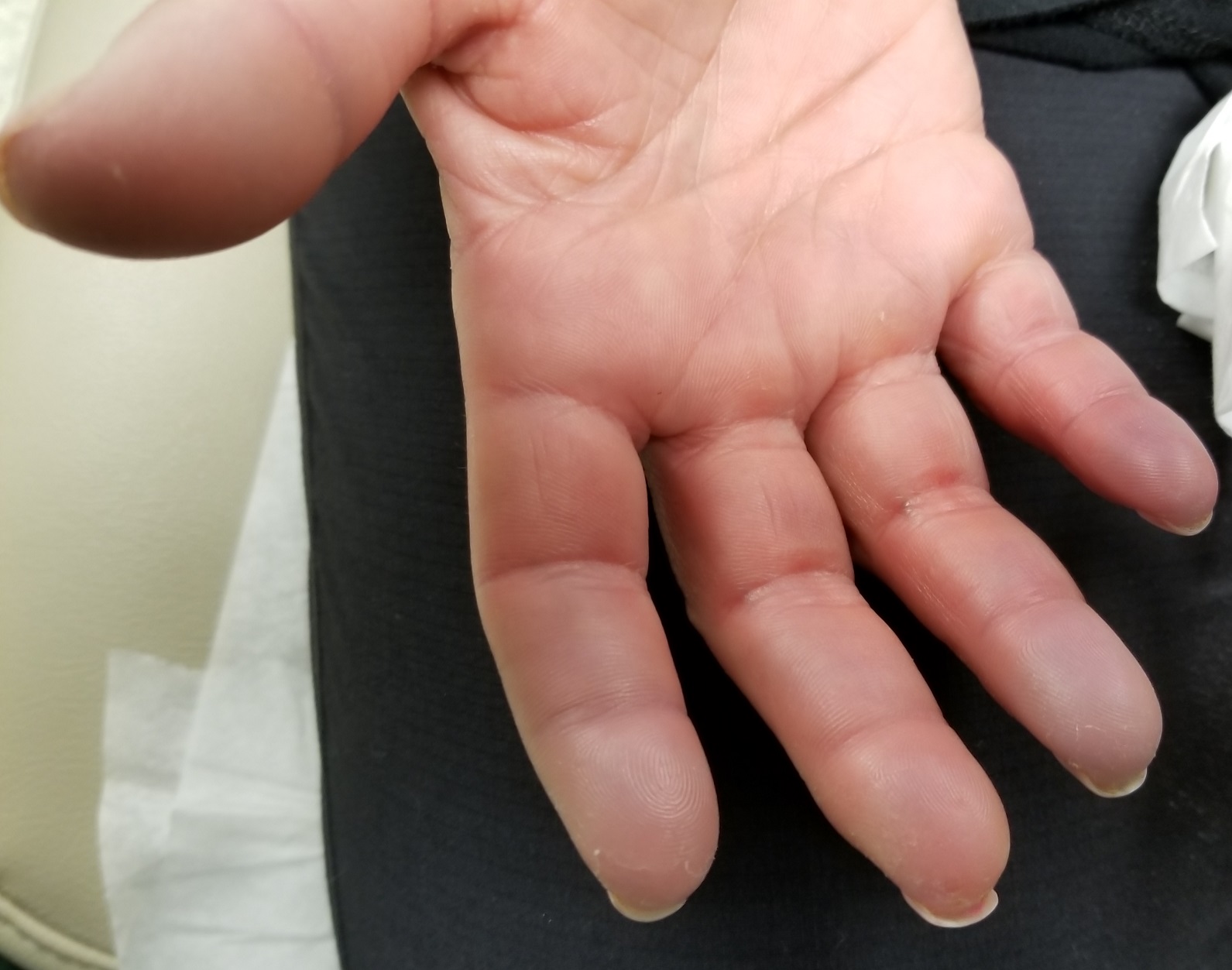
The patient looks her stated age and is in no distress.
What’s the diagnosis?
DISCUSSION
This patient almost certainly has CREST syndrome, a limited form of systemic sclerosis (or scleroderma). Both represent an autoimmune process in which antibodies attack cell DNA and centromeres (a component of the cell nucleus).
CREST can be difficult to diagnose because it can involve diverse organ systems. The name of the syndrome is an acronym for the symptoms it causes:
Calcinosis is a deposition of calcium triggered by inflammation. It manifests as small subcutaneous nodules, which are usually felt on the hands or seen on radiographs of the hands.
Raynaud syndrome causes intense vasoconstriction of blood vessels, usually in fingertips or toes, which first turn white, then red. The phenomenon is accompanied by pain and can be triggered by cold or stress.
Esophageal dysmotility occurs when atrophy and/or fibrosis of the esophageal lining leads to difficulty swallowing food.
Sclerodactyly is the term for tightening and thickening of the skin on the hands and fingers.
Telangiectasias are dilated capillaries that can worsen with time; they are common on lips, mucosal surfaces, the face, and the chest. They are often associated with vascular disease (eg, pulmonary hypertension).
Any of these signs can be seen with full-blown systemic sclerosis, but CREST is usually much less aggressive and seldom leads to renal or congestive heart failure—the 2 leading causes of death in systemic sclerosis.
Diagnosis is based on recognition of the clinical signs and symptoms, as well as blood work (ie, antinuclear antibody and anti-centromere antibody tests). A skin biopsy is often performed to confirm the diagnosis.
Since there is no effective treatment for the overarching disease, the specific components of CREST are treated separately. For this patient, her lip lesions were treated with light electrodessication. Another option would have been laser treatment.
TAKE-HOME LEARNING POINTS
- CREST syndrome, a limited form of systemic sclerosis, is a rare autoimmune condition caused by antibodies to cellular DNA and nuclear centromeres.
- CREST is an acronym for the symptomatic manifestations of the condition: calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasias.
- Diagnosis is based on connecting the clinical dots and taking a thorough history, as well as lab results (for antinuclear antibodies and anti-centromere antibodies) and a skin biopsy.
- CREST is usually treated symptomatically, one system at a time, since no definitive treatment exists for the disease itself.
A 39-year-old woman presents with red vascular streaks on her upper and lower lips. They have slowly multiplied and become more prominent since she first noticed them several years ago. Although the lesions are asymptomatic, their effect on her appearance bothers the patient.
Her primary care provider tried to resolve the problem with cryotherapy. But the treatment attempt was unsuccessful.
During history-taking, the patient divulges other existing health problems. She has been diagnosed with Raynaud syndrome, which flares several times a year, especially in cold weather or times of exceptional stress. She also has permanent thickening of the skin on her distal fingers, a result of sudden-onset swelling of all 10 fingers several years ago.
She denies any problems with eating, such as heartburn or difficulty swallowing. She also denies any respiratory problems or chronic fatigue.

EXAMINATION
Seven very slender telangiectasias, most aligned vertically, are seen on the upper and lower vermillion surfaces. They range from a pinpoint to 6 mm in length. There are no similar lesions are on the rest of the oral mucosae, the face, or the chest.
The patient’s fingers, from the metacarpals to the tips, are decidedly edematous and firm but not tender. Several fingertips are scarred from past Raynaud episodes.

The patient looks her stated age and is in no distress.
What’s the diagnosis?
DISCUSSION
This patient almost certainly has CREST syndrome, a limited form of systemic sclerosis (or scleroderma). Both represent an autoimmune process in which antibodies attack cell DNA and centromeres (a component of the cell nucleus).
CREST can be difficult to diagnose because it can involve diverse organ systems. The name of the syndrome is an acronym for the symptoms it causes:
Calcinosis is a deposition of calcium triggered by inflammation. It manifests as small subcutaneous nodules, which are usually felt on the hands or seen on radiographs of the hands.
Raynaud syndrome causes intense vasoconstriction of blood vessels, usually in fingertips or toes, which first turn white, then red. The phenomenon is accompanied by pain and can be triggered by cold or stress.
Esophageal dysmotility occurs when atrophy and/or fibrosis of the esophageal lining leads to difficulty swallowing food.
Sclerodactyly is the term for tightening and thickening of the skin on the hands and fingers.
Telangiectasias are dilated capillaries that can worsen with time; they are common on lips, mucosal surfaces, the face, and the chest. They are often associated with vascular disease (eg, pulmonary hypertension).
Any of these signs can be seen with full-blown systemic sclerosis, but CREST is usually much less aggressive and seldom leads to renal or congestive heart failure—the 2 leading causes of death in systemic sclerosis.
Diagnosis is based on recognition of the clinical signs and symptoms, as well as blood work (ie, antinuclear antibody and anti-centromere antibody tests). A skin biopsy is often performed to confirm the diagnosis.
Since there is no effective treatment for the overarching disease, the specific components of CREST are treated separately. For this patient, her lip lesions were treated with light electrodessication. Another option would have been laser treatment.
TAKE-HOME LEARNING POINTS
- CREST syndrome, a limited form of systemic sclerosis, is a rare autoimmune condition caused by antibodies to cellular DNA and nuclear centromeres.
- CREST is an acronym for the symptomatic manifestations of the condition: calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasias.
- Diagnosis is based on connecting the clinical dots and taking a thorough history, as well as lab results (for antinuclear antibodies and anti-centromere antibodies) and a skin biopsy.
- CREST is usually treated symptomatically, one system at a time, since no definitive treatment exists for the disease itself.
A 39-year-old woman presents with red vascular streaks on her upper and lower lips. They have slowly multiplied and become more prominent since she first noticed them several years ago. Although the lesions are asymptomatic, their effect on her appearance bothers the patient.
Her primary care provider tried to resolve the problem with cryotherapy. But the treatment attempt was unsuccessful.
During history-taking, the patient divulges other existing health problems. She has been diagnosed with Raynaud syndrome, which flares several times a year, especially in cold weather or times of exceptional stress. She also has permanent thickening of the skin on her distal fingers, a result of sudden-onset swelling of all 10 fingers several years ago.
She denies any problems with eating, such as heartburn or difficulty swallowing. She also denies any respiratory problems or chronic fatigue.

EXAMINATION
Seven very slender telangiectasias, most aligned vertically, are seen on the upper and lower vermillion surfaces. They range from a pinpoint to 6 mm in length. There are no similar lesions are on the rest of the oral mucosae, the face, or the chest.
The patient’s fingers, from the metacarpals to the tips, are decidedly edematous and firm but not tender. Several fingertips are scarred from past Raynaud episodes.

The patient looks her stated age and is in no distress.
What’s the diagnosis?
DISCUSSION
This patient almost certainly has CREST syndrome, a limited form of systemic sclerosis (or scleroderma). Both represent an autoimmune process in which antibodies attack cell DNA and centromeres (a component of the cell nucleus).
CREST can be difficult to diagnose because it can involve diverse organ systems. The name of the syndrome is an acronym for the symptoms it causes:
Calcinosis is a deposition of calcium triggered by inflammation. It manifests as small subcutaneous nodules, which are usually felt on the hands or seen on radiographs of the hands.
Raynaud syndrome causes intense vasoconstriction of blood vessels, usually in fingertips or toes, which first turn white, then red. The phenomenon is accompanied by pain and can be triggered by cold or stress.
Esophageal dysmotility occurs when atrophy and/or fibrosis of the esophageal lining leads to difficulty swallowing food.
Sclerodactyly is the term for tightening and thickening of the skin on the hands and fingers.
Telangiectasias are dilated capillaries that can worsen with time; they are common on lips, mucosal surfaces, the face, and the chest. They are often associated with vascular disease (eg, pulmonary hypertension).
Any of these signs can be seen with full-blown systemic sclerosis, but CREST is usually much less aggressive and seldom leads to renal or congestive heart failure—the 2 leading causes of death in systemic sclerosis.
Diagnosis is based on recognition of the clinical signs and symptoms, as well as blood work (ie, antinuclear antibody and anti-centromere antibody tests). A skin biopsy is often performed to confirm the diagnosis.
Since there is no effective treatment for the overarching disease, the specific components of CREST are treated separately. For this patient, her lip lesions were treated with light electrodessication. Another option would have been laser treatment.
TAKE-HOME LEARNING POINTS
- CREST syndrome, a limited form of systemic sclerosis, is a rare autoimmune condition caused by antibodies to cellular DNA and nuclear centromeres.
- CREST is an acronym for the symptomatic manifestations of the condition: calcinosis, Raynaud disease, esophageal dysmotility, sclerodactyly, and telangiectasias.
- Diagnosis is based on connecting the clinical dots and taking a thorough history, as well as lab results (for antinuclear antibodies and anti-centromere antibodies) and a skin biopsy.
- CREST is usually treated symptomatically, one system at a time, since no definitive treatment exists for the disease itself.
Chronic Subjective Tinnitus
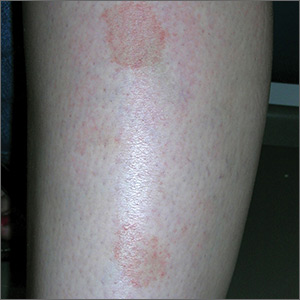
Marks on lower leg

The FP suspected that this was lichen aureus (a type of capillaritis that causes a pigmented purpuric dermatosis). Capillaritis is characterized by extravasation of erythrocytes in the skin with marked hemosiderin deposition. It is not palpable. Lichen aureus is a localized and often well circumscribed pigmented purpuric dermatosis that is seen in younger patients. It often occurs on the leg(s) but can be seen on other parts of the body.
Dermoscopy can help to visualize the red or pink dots with a brown background that represent inflamed capillaries with surrounding hemosiderin deposits.
In this case, the FP used his dermatoscope and could see pink dots with a brown background. He explained that this was sufficient to make the diagnosis of lichen aureus but gave the patient a choice to get a biopsy to confirm the clinical impression. The patient preferred to avoid the biopsy and accepted the diagnosis.
There is no proven beneficial therapy for lichen aureus or other types of capillaritis. Fortunately, it is benign and carries no associated health risks. The FP offered triamcinolone cream 0.1% to be applied once to twice daily, but did not promise that it would make the spots go away. The patient wanted to try something, so she accepted the prescription. No follow-up appointment was needed, but the FP did let the patient know that if the condition worsened, further evaluation, including a biopsy, could be performed in the future. The patient was seen a year later for a well woman exam and stated that the rash resolved about 6 months after she’d sought treatment for it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine, R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP suspected that this was lichen aureus (a type of capillaritis that causes a pigmented purpuric dermatosis). Capillaritis is characterized by extravasation of erythrocytes in the skin with marked hemosiderin deposition. It is not palpable. Lichen aureus is a localized and often well circumscribed pigmented purpuric dermatosis that is seen in younger patients. It often occurs on the leg(s) but can be seen on other parts of the body.
Dermoscopy can help to visualize the red or pink dots with a brown background that represent inflamed capillaries with surrounding hemosiderin deposits.
In this case, the FP used his dermatoscope and could see pink dots with a brown background. He explained that this was sufficient to make the diagnosis of lichen aureus but gave the patient a choice to get a biopsy to confirm the clinical impression. The patient preferred to avoid the biopsy and accepted the diagnosis.
There is no proven beneficial therapy for lichen aureus or other types of capillaritis. Fortunately, it is benign and carries no associated health risks. The FP offered triamcinolone cream 0.1% to be applied once to twice daily, but did not promise that it would make the spots go away. The patient wanted to try something, so she accepted the prescription. No follow-up appointment was needed, but the FP did let the patient know that if the condition worsened, further evaluation, including a biopsy, could be performed in the future. The patient was seen a year later for a well woman exam and stated that the rash resolved about 6 months after she’d sought treatment for it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine, R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/

The FP suspected that this was lichen aureus (a type of capillaritis that causes a pigmented purpuric dermatosis). Capillaritis is characterized by extravasation of erythrocytes in the skin with marked hemosiderin deposition. It is not palpable. Lichen aureus is a localized and often well circumscribed pigmented purpuric dermatosis that is seen in younger patients. It often occurs on the leg(s) but can be seen on other parts of the body.
Dermoscopy can help to visualize the red or pink dots with a brown background that represent inflamed capillaries with surrounding hemosiderin deposits.
In this case, the FP used his dermatoscope and could see pink dots with a brown background. He explained that this was sufficient to make the diagnosis of lichen aureus but gave the patient a choice to get a biopsy to confirm the clinical impression. The patient preferred to avoid the biopsy and accepted the diagnosis.
There is no proven beneficial therapy for lichen aureus or other types of capillaritis. Fortunately, it is benign and carries no associated health risks. The FP offered triamcinolone cream 0.1% to be applied once to twice daily, but did not promise that it would make the spots go away. The patient wanted to try something, so she accepted the prescription. No follow-up appointment was needed, but the FP did let the patient know that if the condition worsened, further evaluation, including a biopsy, could be performed in the future. The patient was seen a year later for a well woman exam and stated that the rash resolved about 6 months after she’d sought treatment for it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Mayeaux EJ, Usatine, R, Martin N, et al. Vasculitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill; 2019:1169-1173.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the 3rd edition of the Color Atlas and Synopsis of Family Medicine as an app by clicking on this link: https://usatinemedia.com/app/color-atlas-of-family-medicine/
Auto-brewery syndrome and hangovers as ‘illnesses’
Food for thought/fermentation
The earliest known alcoholic beverage in the world was a fermented drink of rice, honey, and hawthorn fruit and/or grape that was brewed about 9,000 years ago in China’s Yellow River Valley.
Now there’s another candidate. Meet Klebsiella pneumonia, a common type of gut bacteria that just happens to make its own alcohol and appears to be the cause of a rare condition known as auto-brewery syndrome, which causes those affected to become drunk after eating alcohol-free and high-sugar food.
A group of Chinese investigators had a patient with auto-brewery syndrome and nonalcoholic fatty liver disease (NAFLD) and found he had several strains of K. pneumonia in his gut that produced high levels of alcohol. Then they sampled the gut microbiota from 43 NAFLD patients and 48 healthy people: 60% of the NAFLD patients had high- and medium-alcohol–producing K. pneumonia in their gut, compared with 6% of the controls.
When the team gave mice with NAFLD that had the alcohol-producing bacteria an antibiotic that killed K. pneumonia, their condition was reversed.
“These bacteria damage your liver just like alcohol, except you don’t have a choice,” lead author Jing Yuan said in a written statement.
That got us wondering: What if you do have a choice? Would a diet high in Cabernet and Merlot grapes give K. pneumonia the makings of Château Lafite Rothschild? Would you get Grey Goose if you ate enough French wheat? Would consumption of Optic or Belgravia malts give you Glenfiddich?
Why Ah-nold is more pumped than you
If you’ve watched “Pumping Iron” or “The Terminator,” you know the star of those films, Arnold Schwarzenegger, is driven to achieve his goals. Such as remorselessly squeezing the bodybuilding dreams of fellow “Pumping Iron” star Lou Ferrigno like a tube of toothpaste. Or finding Sarah Connor.
Given that quality, it probably shouldn’t be surprising that the seven-time Mr. Olympia with the 50-pound Austrian accent would somehow become California’s governator.
But why? Because Ah-nold is clearly a human bursting at his cyborglike biceps with “planfulness.”
Planfulness is the personality trait possessed by those who develop a clear plan when they have a goal that’s important to them. To find out how planfulness interacts with achieving long-term goals, University of Oregon researchers looked at the gym attendance of 282 people looking to get pumped up at a campus rec center.
Using a Planfulness Scale, the investigators tracked their study participants’ progress toward pumpitude. The ones who rated themselves as strong on planfulness were more likely to hit the gym consistently than were those with scrawny scores. In fact, a one-point increase on the five-point Planfulness Scale meant more than 14 extra gym visits over the course of two semesters.
Perhaps Ms. Connor should blame Hollywood’s planfulness for box-office profit, not Ah-nold’s, for the relentless pursuit of her in multiple “Terminator” movies.
A six-pack of illness juice, please
College students, rejoice: Your flimsy excuse to your professor that you’re sick and can’t go to class (when in reality you were out drinking – fruit juice for those with auto-brewery syndrome – all night and have a raging hangover) just got a lot stronger. That hangover is now classified as an illness.
Well, in Germany at least.
A court in Frankfurt has recently ruled against the manufacturer of a supposed “antihangover” cure, a product that contained antioxidants, electrolytes, and vitamins meant to combat the headaches, nausea, and tiredness associated with hangovers.
According to the German court, this is false advertising. Hangovers, by their definition, represent a small (clearly they’ve never dealt with a big hangover headache) and temporary change to the body’s normal state that is cured over time, which falls under the classification of an illness. And, in Germany, it is illegal for food and drink products to claim that they can cure illnesses.
Of course, the Germans have nailed the timing of this groundbreaking decision perfectly, as Oktoberfest is underway in Munich.
We still doubt your professor will believe your “Oh, I’m very sick today, I can’t come in” email, but at least you’ll be technically correct. And that’s the best sort of correct.

Food for thought/fermentation
The earliest known alcoholic beverage in the world was a fermented drink of rice, honey, and hawthorn fruit and/or grape that was brewed about 9,000 years ago in China’s Yellow River Valley.
Now there’s another candidate. Meet Klebsiella pneumonia, a common type of gut bacteria that just happens to make its own alcohol and appears to be the cause of a rare condition known as auto-brewery syndrome, which causes those affected to become drunk after eating alcohol-free and high-sugar food.
A group of Chinese investigators had a patient with auto-brewery syndrome and nonalcoholic fatty liver disease (NAFLD) and found he had several strains of K. pneumonia in his gut that produced high levels of alcohol. Then they sampled the gut microbiota from 43 NAFLD patients and 48 healthy people: 60% of the NAFLD patients had high- and medium-alcohol–producing K. pneumonia in their gut, compared with 6% of the controls.
When the team gave mice with NAFLD that had the alcohol-producing bacteria an antibiotic that killed K. pneumonia, their condition was reversed.
“These bacteria damage your liver just like alcohol, except you don’t have a choice,” lead author Jing Yuan said in a written statement.
That got us wondering: What if you do have a choice? Would a diet high in Cabernet and Merlot grapes give K. pneumonia the makings of Château Lafite Rothschild? Would you get Grey Goose if you ate enough French wheat? Would consumption of Optic or Belgravia malts give you Glenfiddich?
Why Ah-nold is more pumped than you
If you’ve watched “Pumping Iron” or “The Terminator,” you know the star of those films, Arnold Schwarzenegger, is driven to achieve his goals. Such as remorselessly squeezing the bodybuilding dreams of fellow “Pumping Iron” star Lou Ferrigno like a tube of toothpaste. Or finding Sarah Connor.
Given that quality, it probably shouldn’t be surprising that the seven-time Mr. Olympia with the 50-pound Austrian accent would somehow become California’s governator.
But why? Because Ah-nold is clearly a human bursting at his cyborglike biceps with “planfulness.”
Planfulness is the personality trait possessed by those who develop a clear plan when they have a goal that’s important to them. To find out how planfulness interacts with achieving long-term goals, University of Oregon researchers looked at the gym attendance of 282 people looking to get pumped up at a campus rec center.
Using a Planfulness Scale, the investigators tracked their study participants’ progress toward pumpitude. The ones who rated themselves as strong on planfulness were more likely to hit the gym consistently than were those with scrawny scores. In fact, a one-point increase on the five-point Planfulness Scale meant more than 14 extra gym visits over the course of two semesters.
Perhaps Ms. Connor should blame Hollywood’s planfulness for box-office profit, not Ah-nold’s, for the relentless pursuit of her in multiple “Terminator” movies.
A six-pack of illness juice, please
College students, rejoice: Your flimsy excuse to your professor that you’re sick and can’t go to class (when in reality you were out drinking – fruit juice for those with auto-brewery syndrome – all night and have a raging hangover) just got a lot stronger. That hangover is now classified as an illness.
Well, in Germany at least.
A court in Frankfurt has recently ruled against the manufacturer of a supposed “antihangover” cure, a product that contained antioxidants, electrolytes, and vitamins meant to combat the headaches, nausea, and tiredness associated with hangovers.
According to the German court, this is false advertising. Hangovers, by their definition, represent a small (clearly they’ve never dealt with a big hangover headache) and temporary change to the body’s normal state that is cured over time, which falls under the classification of an illness. And, in Germany, it is illegal for food and drink products to claim that they can cure illnesses.
Of course, the Germans have nailed the timing of this groundbreaking decision perfectly, as Oktoberfest is underway in Munich.
We still doubt your professor will believe your “Oh, I’m very sick today, I can’t come in” email, but at least you’ll be technically correct. And that’s the best sort of correct.

Food for thought/fermentation
The earliest known alcoholic beverage in the world was a fermented drink of rice, honey, and hawthorn fruit and/or grape that was brewed about 9,000 years ago in China’s Yellow River Valley.
Now there’s another candidate. Meet Klebsiella pneumonia, a common type of gut bacteria that just happens to make its own alcohol and appears to be the cause of a rare condition known as auto-brewery syndrome, which causes those affected to become drunk after eating alcohol-free and high-sugar food.
A group of Chinese investigators had a patient with auto-brewery syndrome and nonalcoholic fatty liver disease (NAFLD) and found he had several strains of K. pneumonia in his gut that produced high levels of alcohol. Then they sampled the gut microbiota from 43 NAFLD patients and 48 healthy people: 60% of the NAFLD patients had high- and medium-alcohol–producing K. pneumonia in their gut, compared with 6% of the controls.
When the team gave mice with NAFLD that had the alcohol-producing bacteria an antibiotic that killed K. pneumonia, their condition was reversed.
“These bacteria damage your liver just like alcohol, except you don’t have a choice,” lead author Jing Yuan said in a written statement.
That got us wondering: What if you do have a choice? Would a diet high in Cabernet and Merlot grapes give K. pneumonia the makings of Château Lafite Rothschild? Would you get Grey Goose if you ate enough French wheat? Would consumption of Optic or Belgravia malts give you Glenfiddich?
Why Ah-nold is more pumped than you
If you’ve watched “Pumping Iron” or “The Terminator,” you know the star of those films, Arnold Schwarzenegger, is driven to achieve his goals. Such as remorselessly squeezing the bodybuilding dreams of fellow “Pumping Iron” star Lou Ferrigno like a tube of toothpaste. Or finding Sarah Connor.
Given that quality, it probably shouldn’t be surprising that the seven-time Mr. Olympia with the 50-pound Austrian accent would somehow become California’s governator.
But why? Because Ah-nold is clearly a human bursting at his cyborglike biceps with “planfulness.”
Planfulness is the personality trait possessed by those who develop a clear plan when they have a goal that’s important to them. To find out how planfulness interacts with achieving long-term goals, University of Oregon researchers looked at the gym attendance of 282 people looking to get pumped up at a campus rec center.
Using a Planfulness Scale, the investigators tracked their study participants’ progress toward pumpitude. The ones who rated themselves as strong on planfulness were more likely to hit the gym consistently than were those with scrawny scores. In fact, a one-point increase on the five-point Planfulness Scale meant more than 14 extra gym visits over the course of two semesters.
Perhaps Ms. Connor should blame Hollywood’s planfulness for box-office profit, not Ah-nold’s, for the relentless pursuit of her in multiple “Terminator” movies.
A six-pack of illness juice, please
College students, rejoice: Your flimsy excuse to your professor that you’re sick and can’t go to class (when in reality you were out drinking – fruit juice for those with auto-brewery syndrome – all night and have a raging hangover) just got a lot stronger. That hangover is now classified as an illness.
Well, in Germany at least.
A court in Frankfurt has recently ruled against the manufacturer of a supposed “antihangover” cure, a product that contained antioxidants, electrolytes, and vitamins meant to combat the headaches, nausea, and tiredness associated with hangovers.
According to the German court, this is false advertising. Hangovers, by their definition, represent a small (clearly they’ve never dealt with a big hangover headache) and temporary change to the body’s normal state that is cured over time, which falls under the classification of an illness. And, in Germany, it is illegal for food and drink products to claim that they can cure illnesses.
Of course, the Germans have nailed the timing of this groundbreaking decision perfectly, as Oktoberfest is underway in Munich.
We still doubt your professor will believe your “Oh, I’m very sick today, I can’t come in” email, but at least you’ll be technically correct. And that’s the best sort of correct.

Biologics beyond anti-TNF therapies show promise for ulcerative colitis
In one of two such trials published in The New England Journal of Medicine on Sept. 26, 2019, researchers led by Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, and associates evaluated ustekinumab as an 8-week induction therapy and a 44-week maintenance therapy in patients with moderate to severe ulcerative colitis (N Engl J Med 2019 Sep 25 doi: 10/1056/NEJMoa1900750). For the phase 3 trial, known as UNIFI, researchers randomly assigned 961 patients to receive an intravenous induction dose of ustekinumab over the course of 8 weeks (320 to a dose of 130 mg and 322 to a weight-range–based dose that approximated 6 mg per kilogram of body weight), while the remaining 319 received placebo. Patients who responded to induction therapy were randomly assigned to a 44-week maintenance phase in which they received subcutaneous maintenance injections of 90 mg of ustekinumab (172 to injections every 12 weeks, 176 to injections every 8 weeks, and 175 to placebo). The primary endpoint for both phases of the trial was clinical remission, defined as a total score of 2 or lower on the Mayo scale, and no subscore greater than 1 on any of the four Mayo scale components.
Dr. Sands and his colleagues found that at week 8, clinical remission was achieved in 15.6% of patients in the 130-mg ustekinumab infusion group, compared with 15.5% in the 6-mg per kg of body weight group, and 5.3% of those in the placebo group. “The percentages of patients who met major secondary endpoints or had histo-endoscopic mucosal healing were significantly higher in both ustekinumab groups than in the placebo group,” the researchers wrote. “Through week 8, the median changes from baseline in the IBDQ [Inflammatory Bowel Disease Questionnaire] score were significantly greater in both ustekinumab groups than in the placebo group.”
Meanwhile, at week 44, clinical remission was achieved in 38.4% of patients in the group receiving 90-mg subcutaneous ustekinumab every 12 weeks, compared with 43.8% of those in the group receiving 90 mg every 8 weeks, and 24% of those in the placebo group. “The percentages of patients with maintenance of clinical response through week 44, endoscopic improvement at week 44, or corticosteroid-free clinical remission (with either definition of clinical remission) at week 44 were significantly higher in both ustekinumab groups than in the placebo group,” the researchers wrote.
When they evaluated other endpoints, Dr. Sands and his colleagues observed that improvements in partial Mayo scores and reductions in serum and fecal concentrations of inflammatory biomarkers that occurred with induction were sustained through week 44. “Although our findings suggest that ustekinumab was effective in patients with or without previous treatment failure with biologics for both induction and maintenance therapy, the percentages of patients in whom each endpoint was achieved were lower across groups with previous treatment failure with biologics,” they wrote.
In the second study, known as VARSITY, researchers led by Dr. Sands conducted a randomized, phase 3b, head-to-head trial comparing vedolizumab with adalimumab in 769 adults with moderate to severe ulcerative colitis, in an effort to determine if vedolizumab is superior after 52 weeks of treatment (N Engl J Med 2019 Sep 25. doi: 10/1056/NEJMoa1905725). They assigned patients to receive IV infusions of 300 mg of vedolizumab on day 1 and at weeks 2, 6, 14, 22, 30, 38, and 46 (plus injections of placebo) or subcutaneous injections of 40 mg of adalimumab, with a total dose of 160 mg at week 1, 80 mg at week 2, and 40 mg every 2 weeks thereafter until week 50 (plus infusions of placebo). The primary endpoint was clinical remission, defined as a total score of 2 or lower on the Mayo scale, and no subscore greater than 1 on any of the four Mayo scale components.
At week 52, the researchers found that 31.3% of patients in the vedolizumab group achieved clinical remission, compared with 22.5% of those in the adalimumab group, while a higher percentage of patients in the vedolizumab group demonstrated endoscopic involvement (the first secondary outcome), compared with their counterparts in the adalimumab group (39.7% vs. 27.7%, respectively). Treatment effects were more pronounced in patients who had not previously used a TNF inhibitor.
In contrast, Dr. Sands and colleagues reported that at week 52, corticosteroid-free clinical remission was observed in 12.6% of patients in the vedolizumab group, compared with 21.8% of their counterparts in the adalimumab group. “It is difficult to explain the inconsistency of the results between this secondary remission outcome and the primary remission outcome,” they wrote. “The trial did not require a specific schedule for corticosteroid tapering, which can vary among practitioners. However, this limitation should not have resulted in differential effects in the two treatment groups.”
They noted that dosing regimens used in VARSITY were based on a conservative approach and use according to U.S. labels. “Real-world studies have shown improved efficacy outcomes after dose intensification in both adalimumab and vedolizumab therapies,” they wrote. “Data from ongoing trials of adalimumab (NCT02065622) and vedolizumab (NCT03029143) may further characterize the effect of higher doses on efficacy outcomes.”
UNIFI was funded by Janssen Research and Development. Dr. Sands disclosed that the Icahn School of Medicine received an institutional grant from Janssen to conduct the study. VARSITY was funded with support from Takeda. Dr. Sands reported that he received grant support and consulting fees from Takeda. Dr. Sands and coauthors reported having financial ties to many other companies in the pharmaceutical and biotechnology industries.
SOURCE: Sands B et al. N Engl J Med. 2019 Sep 25 doi: 10/1056/NEJMoa1900750; N Engl J Med. 2019 Sep 25 doi:.10/1056/NEJMoa1905725.
Long-term rates of colectomy for ulcerative colitis have not declined over a 10-year period, a fact that highlights the need for new biologic therapies and strategies.
Although the VARSITY trial presents a head-to-head comparison of biologics for inflammatory bowel disease and aims to determine the first-line biologic therapy for ulcerative colitis, any clinical superiority of vedolizumab should be balanced against the significant cost advantages of a subcutaneous regimen of adalimumab. In many respects, the ideal trial to assess whether vedolizumab should supplant anti-TNF therapies would involve a head-to-head comparison of infliximab infusions with vedolizumab infusions in patients who have not previously received anti-TNF therapies.
The UNIFI trial assessed the combination of a single induction infusion followed by a maintenance subcutaneous regimen in patients with ulcerative colitis and may lead to the assessment of similar regimens in future trials of biologics in an effort to reduce our dependence on expensive, completely infusion-based biologic regimens, not to mention to relieve pressure on our increasingly busy infusion units. Indeed, the landscape of biologic therapies for ulcerative colitis has changed so dramatically over the past decade with the widespread introduction of less-expensive infliximab and adalimumab biosimilars, as well as vedolizumab, oral Janus kinase inhibitors (tofacitinib), and now ustekinumab, that biologics rather than hospitalization or colectomy are now the main driver of health care costs in the management of inflammatory bowel disease.
The findings in both these trials by Sands et al. highlight the importance of alternative biologic treatments and regimens for ulcerative colitis in patients who are not able to receive anti-TNF therapies because of unacceptable side effects or who have disease that is refractory to anti-TNF therapies. The cost-effectiveness of all biologics will have to come into sharper focus in future trials and longitudinal studies of biologics to help determine not only their eventual place in the treatment algorithm for moderate to severe ulcerative colitis but also the true effect of existing and newer biologics on disease course and rates of colectomy.
This text was extracted from an editorial by Richard J. Farrell, MD, that appeared online Sep. 25, 2019, in The New England Journal of Medicine. Dr. Farrell is with Connolly Hospital and Royal College of Surgeons in Dublin, Ireland.
Long-term rates of colectomy for ulcerative colitis have not declined over a 10-year period, a fact that highlights the need for new biologic therapies and strategies.
Although the VARSITY trial presents a head-to-head comparison of biologics for inflammatory bowel disease and aims to determine the first-line biologic therapy for ulcerative colitis, any clinical superiority of vedolizumab should be balanced against the significant cost advantages of a subcutaneous regimen of adalimumab. In many respects, the ideal trial to assess whether vedolizumab should supplant anti-TNF therapies would involve a head-to-head comparison of infliximab infusions with vedolizumab infusions in patients who have not previously received anti-TNF therapies.
The UNIFI trial assessed the combination of a single induction infusion followed by a maintenance subcutaneous regimen in patients with ulcerative colitis and may lead to the assessment of similar regimens in future trials of biologics in an effort to reduce our dependence on expensive, completely infusion-based biologic regimens, not to mention to relieve pressure on our increasingly busy infusion units. Indeed, the landscape of biologic therapies for ulcerative colitis has changed so dramatically over the past decade with the widespread introduction of less-expensive infliximab and adalimumab biosimilars, as well as vedolizumab, oral Janus kinase inhibitors (tofacitinib), and now ustekinumab, that biologics rather than hospitalization or colectomy are now the main driver of health care costs in the management of inflammatory bowel disease.
The findings in both these trials by Sands et al. highlight the importance of alternative biologic treatments and regimens for ulcerative colitis in patients who are not able to receive anti-TNF therapies because of unacceptable side effects or who have disease that is refractory to anti-TNF therapies. The cost-effectiveness of all biologics will have to come into sharper focus in future trials and longitudinal studies of biologics to help determine not only their eventual place in the treatment algorithm for moderate to severe ulcerative colitis but also the true effect of existing and newer biologics on disease course and rates of colectomy.
This text was extracted from an editorial by Richard J. Farrell, MD, that appeared online Sep. 25, 2019, in The New England Journal of Medicine. Dr. Farrell is with Connolly Hospital and Royal College of Surgeons in Dublin, Ireland.
Long-term rates of colectomy for ulcerative colitis have not declined over a 10-year period, a fact that highlights the need for new biologic therapies and strategies.
Although the VARSITY trial presents a head-to-head comparison of biologics for inflammatory bowel disease and aims to determine the first-line biologic therapy for ulcerative colitis, any clinical superiority of vedolizumab should be balanced against the significant cost advantages of a subcutaneous regimen of adalimumab. In many respects, the ideal trial to assess whether vedolizumab should supplant anti-TNF therapies would involve a head-to-head comparison of infliximab infusions with vedolizumab infusions in patients who have not previously received anti-TNF therapies.
The UNIFI trial assessed the combination of a single induction infusion followed by a maintenance subcutaneous regimen in patients with ulcerative colitis and may lead to the assessment of similar regimens in future trials of biologics in an effort to reduce our dependence on expensive, completely infusion-based biologic regimens, not to mention to relieve pressure on our increasingly busy infusion units. Indeed, the landscape of biologic therapies for ulcerative colitis has changed so dramatically over the past decade with the widespread introduction of less-expensive infliximab and adalimumab biosimilars, as well as vedolizumab, oral Janus kinase inhibitors (tofacitinib), and now ustekinumab, that biologics rather than hospitalization or colectomy are now the main driver of health care costs in the management of inflammatory bowel disease.
The findings in both these trials by Sands et al. highlight the importance of alternative biologic treatments and regimens for ulcerative colitis in patients who are not able to receive anti-TNF therapies because of unacceptable side effects or who have disease that is refractory to anti-TNF therapies. The cost-effectiveness of all biologics will have to come into sharper focus in future trials and longitudinal studies of biologics to help determine not only their eventual place in the treatment algorithm for moderate to severe ulcerative colitis but also the true effect of existing and newer biologics on disease course and rates of colectomy.
This text was extracted from an editorial by Richard J. Farrell, MD, that appeared online Sep. 25, 2019, in The New England Journal of Medicine. Dr. Farrell is with Connolly Hospital and Royal College of Surgeons in Dublin, Ireland.
In one of two such trials published in The New England Journal of Medicine on Sept. 26, 2019, researchers led by Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, and associates evaluated ustekinumab as an 8-week induction therapy and a 44-week maintenance therapy in patients with moderate to severe ulcerative colitis (N Engl J Med 2019 Sep 25 doi: 10/1056/NEJMoa1900750). For the phase 3 trial, known as UNIFI, researchers randomly assigned 961 patients to receive an intravenous induction dose of ustekinumab over the course of 8 weeks (320 to a dose of 130 mg and 322 to a weight-range–based dose that approximated 6 mg per kilogram of body weight), while the remaining 319 received placebo. Patients who responded to induction therapy were randomly assigned to a 44-week maintenance phase in which they received subcutaneous maintenance injections of 90 mg of ustekinumab (172 to injections every 12 weeks, 176 to injections every 8 weeks, and 175 to placebo). The primary endpoint for both phases of the trial was clinical remission, defined as a total score of 2 or lower on the Mayo scale, and no subscore greater than 1 on any of the four Mayo scale components.
Dr. Sands and his colleagues found that at week 8, clinical remission was achieved in 15.6% of patients in the 130-mg ustekinumab infusion group, compared with 15.5% in the 6-mg per kg of body weight group, and 5.3% of those in the placebo group. “The percentages of patients who met major secondary endpoints or had histo-endoscopic mucosal healing were significantly higher in both ustekinumab groups than in the placebo group,” the researchers wrote. “Through week 8, the median changes from baseline in the IBDQ [Inflammatory Bowel Disease Questionnaire] score were significantly greater in both ustekinumab groups than in the placebo group.”
Meanwhile, at week 44, clinical remission was achieved in 38.4% of patients in the group receiving 90-mg subcutaneous ustekinumab every 12 weeks, compared with 43.8% of those in the group receiving 90 mg every 8 weeks, and 24% of those in the placebo group. “The percentages of patients with maintenance of clinical response through week 44, endoscopic improvement at week 44, or corticosteroid-free clinical remission (with either definition of clinical remission) at week 44 were significantly higher in both ustekinumab groups than in the placebo group,” the researchers wrote.
When they evaluated other endpoints, Dr. Sands and his colleagues observed that improvements in partial Mayo scores and reductions in serum and fecal concentrations of inflammatory biomarkers that occurred with induction were sustained through week 44. “Although our findings suggest that ustekinumab was effective in patients with or without previous treatment failure with biologics for both induction and maintenance therapy, the percentages of patients in whom each endpoint was achieved were lower across groups with previous treatment failure with biologics,” they wrote.
In the second study, known as VARSITY, researchers led by Dr. Sands conducted a randomized, phase 3b, head-to-head trial comparing vedolizumab with adalimumab in 769 adults with moderate to severe ulcerative colitis, in an effort to determine if vedolizumab is superior after 52 weeks of treatment (N Engl J Med 2019 Sep 25. doi: 10/1056/NEJMoa1905725). They assigned patients to receive IV infusions of 300 mg of vedolizumab on day 1 and at weeks 2, 6, 14, 22, 30, 38, and 46 (plus injections of placebo) or subcutaneous injections of 40 mg of adalimumab, with a total dose of 160 mg at week 1, 80 mg at week 2, and 40 mg every 2 weeks thereafter until week 50 (plus infusions of placebo). The primary endpoint was clinical remission, defined as a total score of 2 or lower on the Mayo scale, and no subscore greater than 1 on any of the four Mayo scale components.
At week 52, the researchers found that 31.3% of patients in the vedolizumab group achieved clinical remission, compared with 22.5% of those in the adalimumab group, while a higher percentage of patients in the vedolizumab group demonstrated endoscopic involvement (the first secondary outcome), compared with their counterparts in the adalimumab group (39.7% vs. 27.7%, respectively). Treatment effects were more pronounced in patients who had not previously used a TNF inhibitor.
In contrast, Dr. Sands and colleagues reported that at week 52, corticosteroid-free clinical remission was observed in 12.6% of patients in the vedolizumab group, compared with 21.8% of their counterparts in the adalimumab group. “It is difficult to explain the inconsistency of the results between this secondary remission outcome and the primary remission outcome,” they wrote. “The trial did not require a specific schedule for corticosteroid tapering, which can vary among practitioners. However, this limitation should not have resulted in differential effects in the two treatment groups.”
They noted that dosing regimens used in VARSITY were based on a conservative approach and use according to U.S. labels. “Real-world studies have shown improved efficacy outcomes after dose intensification in both adalimumab and vedolizumab therapies,” they wrote. “Data from ongoing trials of adalimumab (NCT02065622) and vedolizumab (NCT03029143) may further characterize the effect of higher doses on efficacy outcomes.”
UNIFI was funded by Janssen Research and Development. Dr. Sands disclosed that the Icahn School of Medicine received an institutional grant from Janssen to conduct the study. VARSITY was funded with support from Takeda. Dr. Sands reported that he received grant support and consulting fees from Takeda. Dr. Sands and coauthors reported having financial ties to many other companies in the pharmaceutical and biotechnology industries.
SOURCE: Sands B et al. N Engl J Med. 2019 Sep 25 doi: 10/1056/NEJMoa1900750; N Engl J Med. 2019 Sep 25 doi:.10/1056/NEJMoa1905725.
In one of two such trials published in The New England Journal of Medicine on Sept. 26, 2019, researchers led by Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, and associates evaluated ustekinumab as an 8-week induction therapy and a 44-week maintenance therapy in patients with moderate to severe ulcerative colitis (N Engl J Med 2019 Sep 25 doi: 10/1056/NEJMoa1900750). For the phase 3 trial, known as UNIFI, researchers randomly assigned 961 patients to receive an intravenous induction dose of ustekinumab over the course of 8 weeks (320 to a dose of 130 mg and 322 to a weight-range–based dose that approximated 6 mg per kilogram of body weight), while the remaining 319 received placebo. Patients who responded to induction therapy were randomly assigned to a 44-week maintenance phase in which they received subcutaneous maintenance injections of 90 mg of ustekinumab (172 to injections every 12 weeks, 176 to injections every 8 weeks, and 175 to placebo). The primary endpoint for both phases of the trial was clinical remission, defined as a total score of 2 or lower on the Mayo scale, and no subscore greater than 1 on any of the four Mayo scale components.
Dr. Sands and his colleagues found that at week 8, clinical remission was achieved in 15.6% of patients in the 130-mg ustekinumab infusion group, compared with 15.5% in the 6-mg per kg of body weight group, and 5.3% of those in the placebo group. “The percentages of patients who met major secondary endpoints or had histo-endoscopic mucosal healing were significantly higher in both ustekinumab groups than in the placebo group,” the researchers wrote. “Through week 8, the median changes from baseline in the IBDQ [Inflammatory Bowel Disease Questionnaire] score were significantly greater in both ustekinumab groups than in the placebo group.”
Meanwhile, at week 44, clinical remission was achieved in 38.4% of patients in the group receiving 90-mg subcutaneous ustekinumab every 12 weeks, compared with 43.8% of those in the group receiving 90 mg every 8 weeks, and 24% of those in the placebo group. “The percentages of patients with maintenance of clinical response through week 44, endoscopic improvement at week 44, or corticosteroid-free clinical remission (with either definition of clinical remission) at week 44 were significantly higher in both ustekinumab groups than in the placebo group,” the researchers wrote.
When they evaluated other endpoints, Dr. Sands and his colleagues observed that improvements in partial Mayo scores and reductions in serum and fecal concentrations of inflammatory biomarkers that occurred with induction were sustained through week 44. “Although our findings suggest that ustekinumab was effective in patients with or without previous treatment failure with biologics for both induction and maintenance therapy, the percentages of patients in whom each endpoint was achieved were lower across groups with previous treatment failure with biologics,” they wrote.
In the second study, known as VARSITY, researchers led by Dr. Sands conducted a randomized, phase 3b, head-to-head trial comparing vedolizumab with adalimumab in 769 adults with moderate to severe ulcerative colitis, in an effort to determine if vedolizumab is superior after 52 weeks of treatment (N Engl J Med 2019 Sep 25. doi: 10/1056/NEJMoa1905725). They assigned patients to receive IV infusions of 300 mg of vedolizumab on day 1 and at weeks 2, 6, 14, 22, 30, 38, and 46 (plus injections of placebo) or subcutaneous injections of 40 mg of adalimumab, with a total dose of 160 mg at week 1, 80 mg at week 2, and 40 mg every 2 weeks thereafter until week 50 (plus infusions of placebo). The primary endpoint was clinical remission, defined as a total score of 2 or lower on the Mayo scale, and no subscore greater than 1 on any of the four Mayo scale components.
At week 52, the researchers found that 31.3% of patients in the vedolizumab group achieved clinical remission, compared with 22.5% of those in the adalimumab group, while a higher percentage of patients in the vedolizumab group demonstrated endoscopic involvement (the first secondary outcome), compared with their counterparts in the adalimumab group (39.7% vs. 27.7%, respectively). Treatment effects were more pronounced in patients who had not previously used a TNF inhibitor.
In contrast, Dr. Sands and colleagues reported that at week 52, corticosteroid-free clinical remission was observed in 12.6% of patients in the vedolizumab group, compared with 21.8% of their counterparts in the adalimumab group. “It is difficult to explain the inconsistency of the results between this secondary remission outcome and the primary remission outcome,” they wrote. “The trial did not require a specific schedule for corticosteroid tapering, which can vary among practitioners. However, this limitation should not have resulted in differential effects in the two treatment groups.”
They noted that dosing regimens used in VARSITY were based on a conservative approach and use according to U.S. labels. “Real-world studies have shown improved efficacy outcomes after dose intensification in both adalimumab and vedolizumab therapies,” they wrote. “Data from ongoing trials of adalimumab (NCT02065622) and vedolizumab (NCT03029143) may further characterize the effect of higher doses on efficacy outcomes.”
UNIFI was funded by Janssen Research and Development. Dr. Sands disclosed that the Icahn School of Medicine received an institutional grant from Janssen to conduct the study. VARSITY was funded with support from Takeda. Dr. Sands reported that he received grant support and consulting fees from Takeda. Dr. Sands and coauthors reported having financial ties to many other companies in the pharmaceutical and biotechnology industries.
SOURCE: Sands B et al. N Engl J Med. 2019 Sep 25 doi: 10/1056/NEJMoa1900750; N Engl J Med. 2019 Sep 25 doi:.10/1056/NEJMoa1905725.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: New biologic therapies and strategies for ulcerative colitis patients continue to evolve.
Major finding: In UNIFI, a higher percentage of patients who received a single infusion of ustekinumab at 130 mg or 6 mg per kg of body weight achieved clinical remission at 8 weeks, compared with placebo (15.6% and 15.5%, vs. 5.3%, respectively. In VARSITY, a higher percentage of patients who received vedolizumab achieved clinical remission at 52 weeks, compared with those who received adalimumab (31.3% vs. 22.5%, respectively).
Study details: UNIFI, a phase 3 trial, included an 8-week randomized induction trial of 961 patients and a 44-week randomized-withdrawal maintenance trial. VARSITY was a randomized, phase 3b, head-to-head trial comparing vedolizumab with adalimumab in 769 adults with moderate to severe ulcerative colitis.
Disclosures: UNIFI was funded by Janssen Research and Development. Dr. Sands disclosed that the Icahn School of Medicine received an institutional grant from Janssen to conduct the study. VARSITY was funded with support from Takeda. Dr. Sands reported that he received grant support and consulting fees from Takeda. Dr. Sands and coauthors reported having financial ties to many other companies in the pharmaceutical and biotechnology industries.
Sources: Sands B et al. N Engl J Med. 2019 Sep 25. doi: 10/1056/NEJMoa1900750; N Engl J Med. 2019 Sep 25. doi: 10/1056/NEJMoa1905725.