User login
PAOLA-1/ENGOT-ov25 trial: PARP inhibitor for maintenance improves PFS in advanced ovarian cancer
BARCELONA – Adding in the phase 3 PAOLA-1/ENGOT-ov25 trial.
The benefit was particularly pronounced in patients with a tumor BRCA mutation (tBRCAm), and in those with homologous recombination deficiency (HRD)–positive disease, Isabelle Ray-Coquard, MD, PhD, reported at the European Society for Medical Oncology Congress.
Investigator-assessed median PFS was 22.1 months in 537 patients randomized to receive the poly adenosine diphosphate–ribose polymerase (PARP) inhibitor olaparib plus bevacizumab maintenance, compared with 16.6 months in 269 patients who received placebo plus bevacizumab in the randomized, double-blind trial.
The difference was statistically significant (hazard ratio, 0.59; P = .0001), said Dr. Ray-Coquard, a medical oncologist at Centre Léon Bérard and a professor of medical oncology at the Université Claude Bernard, Lyon, France.
In patients with tBRCAm, the median PFS in the olaparib and placebo arms, respectively, was 37.2 vs. 21.7 months (HR, 0.31); in HRD-positive patients – inclusive of those with tBRCAm – median PFS was 37.2 vs. 17.7 months, respectively; and in HRD-positive patients without tBRCAm, median PFS was 28.1 and 16.6 months, respectively (HR, 0.43), she said.
“So PAOLA-1 met its primary endpoint of a statistically significant improvement in PFS in the [intent-to-treat] population, in favor of the olaparib arm,” Dr. Ray-Coquard said. “What does that mean? For advanced ovarian cancer ... a benefit of a median time of nearly 30 months without relapse.”
The findings of the subgroup analysis showing an even stronger association between combination olaparib and bevacizumab and improved PFS in patients with tBRCAm are similar to those reported from the SOLO1 trial of olaparib monotherapy vs. chemotherapy in newly diagnosed advanced ovarian cancer, she noted.
“It is interesting to note that our PFS in the control arm is longer than [that seen] in the SOLO1 trial, which is probably due to the use of bevacizumab” in PAOLA-1, she said.
Additionally, a “new population of patients was identified: HRD-positive [patients] without BRCA mutation,” who experienced a PFS improvement of almost 1 year with olaparib versus placebo, Dr. Ray-Coquard noted.
Patients enrolled in the international trial – the first randomized trial to explore the efficacy and safety of maintenance olaparib plus bevacizumab in this setting and in patients with or without tBRCAm – had newly diagnosed stage III-IV, high-grade serous or endometrioid ovarian cancer, fallopian tube cancer, or primary peritoneal cancer; had undergone upfront or interval surgery; had received platinum-taxane–based chemotherapy; and had received at least three cycles of bevacizumab. They were randomized 2:1 to receive maintenance therapy with 300-mg olaparib tablets given twice daily for up to 24 months along with bevacizumab at a dose of 15 mg/kg on day 1 every 3 weeks for 15 months (including when combined with platinum-based chemotherapy), or placebo plus bevacizumab.
The current standard of care for most patients with newly diagnosed advanced ovarian cancer is surgery and platinum-based chemotherapy combined with bevacizumab, followed by bevacizumab alone for maintenance.
Patient characteristics in the two arms were well balanced, Dr. Ray-Coquard said. “The PAOLA-1 population is representative of the majority of patients with advanced ovarian cancer, as the patient selection was not restricted by surgical outcome or BRCA mutation,” she commented, adding that the safety profile of the olaparib-bevacizumab combination was generally consistent with that seen in previous trials.
The addition of olaparib did not appear to affect bevacizumab tolerability or quality of life, she noted.
During a press briefing at the congress, Susan Banerjee, MBBS, PhD, who reported the SOLO1 results at the 2018 ESMO Congress, said that “PARP inhibitors have revolutionized the treatment landscape in ovarian cancer,” and have been approved in the recurrent ovarian cancer setting.
“But if we’re really going to have a chance to increase overall survival, and hopefully [have] more women cured, we need to bring these treatments into the first-line setting,” said Dr. Banerjee, consultant medical oncologist and research lead for the gynecology unit of the Royal Marsden Hospital, NHS Foundation Trust, London.
Given these and other recent findings, answers to the key question of how to improve outcomes in the first-line setting – and in patients beyond those with BRCA mutations – are emerging and showing that more women with ovarian cancer can benefit from PARP inhibitors.
“We know now ... that we can use PARP inhibitors in the first-line setting, beyond women with BRCA mutations,” Dr. Banerjee said. “The key question, really, is ‘What about patients that don’t have HRD deficiency? We do know that patients with BRCA mutations and who are HRD-positive have the greatest benefit with either a PARP inhibitor alone or, indeed, in combination with bevacizumab.”
In an ESMO press release about the PAOLA-1 and other related data presented at the congress, Ana Oaknin, MD, of Vall D’Hebron Institute of Oncology in Barcelona, stated that “the main goal in ovarian cancer is to avoid relapse after first-line therapy because otherwise the probability of cure is quite low.”
Combination olaparib and bevacizumab for first-line maintenance therapy “should become a new standard of care for patients with advanced ovarian cancer,” she said, noting that, while the PAOLA-1/ENGOT-ov25 trial did not include patients with no response to first-line chemotherapy (who comprise a small group of ovarian cancer patients), the trial is “a significant step forward in treatment for these women.”
In the context of other positive trial findings, including those from SOLO1 and other studies of PARP inhibition in this setting, the results represent a milestone, Dr. Oaknin said. “After decades studying different chemotherapy approaches, it is the first time we have meaningfully prolonged progression-free survival, and hopefully we will improve long-term outcome.”
As for the “next priority for research in this field, Dr. Oaknin said that strategies are needed to improve the current 45% 5-year overall survival rate for ovarian cancer. “I think the next approach is to incorporate immunotherapy as part of first-line therapy; ongoing trials are expected to report in 2-3 years,” she commented.
The PAOLA-1/ENGOT-ov25 trial was funded by ARCAGY Research, AstraZeneca, and Roche. Dr. Ray-Coquard reported relationships with AstraZeneca, Clovis Oncology, Tesaro, Pharma Mar, Roche, and Genmab, including receiving honoraria, travel/accommodations/expenses, and/or research grant funding, and/or serving as an advisor or consultant. Dr. Banerjee is a lecturer and/or advisory board member for AstraZeneca, Clovis, Gamamabs, Merck Serono, Pharmamar, Seattle Genetics, Roche, and Tesaro, and has received a travel grant from Nucana. Dr. Oaknin reported having no disclosures.
SOURCE: Ray-Coquard I et al. ESMO 2019, Abstract LBA2. Ann Oncol. 19;30:suppl 9.
BARCELONA – Adding in the phase 3 PAOLA-1/ENGOT-ov25 trial.
The benefit was particularly pronounced in patients with a tumor BRCA mutation (tBRCAm), and in those with homologous recombination deficiency (HRD)–positive disease, Isabelle Ray-Coquard, MD, PhD, reported at the European Society for Medical Oncology Congress.
Investigator-assessed median PFS was 22.1 months in 537 patients randomized to receive the poly adenosine diphosphate–ribose polymerase (PARP) inhibitor olaparib plus bevacizumab maintenance, compared with 16.6 months in 269 patients who received placebo plus bevacizumab in the randomized, double-blind trial.
The difference was statistically significant (hazard ratio, 0.59; P = .0001), said Dr. Ray-Coquard, a medical oncologist at Centre Léon Bérard and a professor of medical oncology at the Université Claude Bernard, Lyon, France.
In patients with tBRCAm, the median PFS in the olaparib and placebo arms, respectively, was 37.2 vs. 21.7 months (HR, 0.31); in HRD-positive patients – inclusive of those with tBRCAm – median PFS was 37.2 vs. 17.7 months, respectively; and in HRD-positive patients without tBRCAm, median PFS was 28.1 and 16.6 months, respectively (HR, 0.43), she said.
“So PAOLA-1 met its primary endpoint of a statistically significant improvement in PFS in the [intent-to-treat] population, in favor of the olaparib arm,” Dr. Ray-Coquard said. “What does that mean? For advanced ovarian cancer ... a benefit of a median time of nearly 30 months without relapse.”
The findings of the subgroup analysis showing an even stronger association between combination olaparib and bevacizumab and improved PFS in patients with tBRCAm are similar to those reported from the SOLO1 trial of olaparib monotherapy vs. chemotherapy in newly diagnosed advanced ovarian cancer, she noted.
“It is interesting to note that our PFS in the control arm is longer than [that seen] in the SOLO1 trial, which is probably due to the use of bevacizumab” in PAOLA-1, she said.
Additionally, a “new population of patients was identified: HRD-positive [patients] without BRCA mutation,” who experienced a PFS improvement of almost 1 year with olaparib versus placebo, Dr. Ray-Coquard noted.
Patients enrolled in the international trial – the first randomized trial to explore the efficacy and safety of maintenance olaparib plus bevacizumab in this setting and in patients with or without tBRCAm – had newly diagnosed stage III-IV, high-grade serous or endometrioid ovarian cancer, fallopian tube cancer, or primary peritoneal cancer; had undergone upfront or interval surgery; had received platinum-taxane–based chemotherapy; and had received at least three cycles of bevacizumab. They were randomized 2:1 to receive maintenance therapy with 300-mg olaparib tablets given twice daily for up to 24 months along with bevacizumab at a dose of 15 mg/kg on day 1 every 3 weeks for 15 months (including when combined with platinum-based chemotherapy), or placebo plus bevacizumab.
The current standard of care for most patients with newly diagnosed advanced ovarian cancer is surgery and platinum-based chemotherapy combined with bevacizumab, followed by bevacizumab alone for maintenance.
Patient characteristics in the two arms were well balanced, Dr. Ray-Coquard said. “The PAOLA-1 population is representative of the majority of patients with advanced ovarian cancer, as the patient selection was not restricted by surgical outcome or BRCA mutation,” she commented, adding that the safety profile of the olaparib-bevacizumab combination was generally consistent with that seen in previous trials.
The addition of olaparib did not appear to affect bevacizumab tolerability or quality of life, she noted.
During a press briefing at the congress, Susan Banerjee, MBBS, PhD, who reported the SOLO1 results at the 2018 ESMO Congress, said that “PARP inhibitors have revolutionized the treatment landscape in ovarian cancer,” and have been approved in the recurrent ovarian cancer setting.
“But if we’re really going to have a chance to increase overall survival, and hopefully [have] more women cured, we need to bring these treatments into the first-line setting,” said Dr. Banerjee, consultant medical oncologist and research lead for the gynecology unit of the Royal Marsden Hospital, NHS Foundation Trust, London.
Given these and other recent findings, answers to the key question of how to improve outcomes in the first-line setting – and in patients beyond those with BRCA mutations – are emerging and showing that more women with ovarian cancer can benefit from PARP inhibitors.
“We know now ... that we can use PARP inhibitors in the first-line setting, beyond women with BRCA mutations,” Dr. Banerjee said. “The key question, really, is ‘What about patients that don’t have HRD deficiency? We do know that patients with BRCA mutations and who are HRD-positive have the greatest benefit with either a PARP inhibitor alone or, indeed, in combination with bevacizumab.”
In an ESMO press release about the PAOLA-1 and other related data presented at the congress, Ana Oaknin, MD, of Vall D’Hebron Institute of Oncology in Barcelona, stated that “the main goal in ovarian cancer is to avoid relapse after first-line therapy because otherwise the probability of cure is quite low.”
Combination olaparib and bevacizumab for first-line maintenance therapy “should become a new standard of care for patients with advanced ovarian cancer,” she said, noting that, while the PAOLA-1/ENGOT-ov25 trial did not include patients with no response to first-line chemotherapy (who comprise a small group of ovarian cancer patients), the trial is “a significant step forward in treatment for these women.”
In the context of other positive trial findings, including those from SOLO1 and other studies of PARP inhibition in this setting, the results represent a milestone, Dr. Oaknin said. “After decades studying different chemotherapy approaches, it is the first time we have meaningfully prolonged progression-free survival, and hopefully we will improve long-term outcome.”
As for the “next priority for research in this field, Dr. Oaknin said that strategies are needed to improve the current 45% 5-year overall survival rate for ovarian cancer. “I think the next approach is to incorporate immunotherapy as part of first-line therapy; ongoing trials are expected to report in 2-3 years,” she commented.
The PAOLA-1/ENGOT-ov25 trial was funded by ARCAGY Research, AstraZeneca, and Roche. Dr. Ray-Coquard reported relationships with AstraZeneca, Clovis Oncology, Tesaro, Pharma Mar, Roche, and Genmab, including receiving honoraria, travel/accommodations/expenses, and/or research grant funding, and/or serving as an advisor or consultant. Dr. Banerjee is a lecturer and/or advisory board member for AstraZeneca, Clovis, Gamamabs, Merck Serono, Pharmamar, Seattle Genetics, Roche, and Tesaro, and has received a travel grant from Nucana. Dr. Oaknin reported having no disclosures.
SOURCE: Ray-Coquard I et al. ESMO 2019, Abstract LBA2. Ann Oncol. 19;30:suppl 9.
BARCELONA – Adding in the phase 3 PAOLA-1/ENGOT-ov25 trial.
The benefit was particularly pronounced in patients with a tumor BRCA mutation (tBRCAm), and in those with homologous recombination deficiency (HRD)–positive disease, Isabelle Ray-Coquard, MD, PhD, reported at the European Society for Medical Oncology Congress.
Investigator-assessed median PFS was 22.1 months in 537 patients randomized to receive the poly adenosine diphosphate–ribose polymerase (PARP) inhibitor olaparib plus bevacizumab maintenance, compared with 16.6 months in 269 patients who received placebo plus bevacizumab in the randomized, double-blind trial.
The difference was statistically significant (hazard ratio, 0.59; P = .0001), said Dr. Ray-Coquard, a medical oncologist at Centre Léon Bérard and a professor of medical oncology at the Université Claude Bernard, Lyon, France.
In patients with tBRCAm, the median PFS in the olaparib and placebo arms, respectively, was 37.2 vs. 21.7 months (HR, 0.31); in HRD-positive patients – inclusive of those with tBRCAm – median PFS was 37.2 vs. 17.7 months, respectively; and in HRD-positive patients without tBRCAm, median PFS was 28.1 and 16.6 months, respectively (HR, 0.43), she said.
“So PAOLA-1 met its primary endpoint of a statistically significant improvement in PFS in the [intent-to-treat] population, in favor of the olaparib arm,” Dr. Ray-Coquard said. “What does that mean? For advanced ovarian cancer ... a benefit of a median time of nearly 30 months without relapse.”
The findings of the subgroup analysis showing an even stronger association between combination olaparib and bevacizumab and improved PFS in patients with tBRCAm are similar to those reported from the SOLO1 trial of olaparib monotherapy vs. chemotherapy in newly diagnosed advanced ovarian cancer, she noted.
“It is interesting to note that our PFS in the control arm is longer than [that seen] in the SOLO1 trial, which is probably due to the use of bevacizumab” in PAOLA-1, she said.
Additionally, a “new population of patients was identified: HRD-positive [patients] without BRCA mutation,” who experienced a PFS improvement of almost 1 year with olaparib versus placebo, Dr. Ray-Coquard noted.
Patients enrolled in the international trial – the first randomized trial to explore the efficacy and safety of maintenance olaparib plus bevacizumab in this setting and in patients with or without tBRCAm – had newly diagnosed stage III-IV, high-grade serous or endometrioid ovarian cancer, fallopian tube cancer, or primary peritoneal cancer; had undergone upfront or interval surgery; had received platinum-taxane–based chemotherapy; and had received at least three cycles of bevacizumab. They were randomized 2:1 to receive maintenance therapy with 300-mg olaparib tablets given twice daily for up to 24 months along with bevacizumab at a dose of 15 mg/kg on day 1 every 3 weeks for 15 months (including when combined with platinum-based chemotherapy), or placebo plus bevacizumab.
The current standard of care for most patients with newly diagnosed advanced ovarian cancer is surgery and platinum-based chemotherapy combined with bevacizumab, followed by bevacizumab alone for maintenance.
Patient characteristics in the two arms were well balanced, Dr. Ray-Coquard said. “The PAOLA-1 population is representative of the majority of patients with advanced ovarian cancer, as the patient selection was not restricted by surgical outcome or BRCA mutation,” she commented, adding that the safety profile of the olaparib-bevacizumab combination was generally consistent with that seen in previous trials.
The addition of olaparib did not appear to affect bevacizumab tolerability or quality of life, she noted.
During a press briefing at the congress, Susan Banerjee, MBBS, PhD, who reported the SOLO1 results at the 2018 ESMO Congress, said that “PARP inhibitors have revolutionized the treatment landscape in ovarian cancer,” and have been approved in the recurrent ovarian cancer setting.
“But if we’re really going to have a chance to increase overall survival, and hopefully [have] more women cured, we need to bring these treatments into the first-line setting,” said Dr. Banerjee, consultant medical oncologist and research lead for the gynecology unit of the Royal Marsden Hospital, NHS Foundation Trust, London.
Given these and other recent findings, answers to the key question of how to improve outcomes in the first-line setting – and in patients beyond those with BRCA mutations – are emerging and showing that more women with ovarian cancer can benefit from PARP inhibitors.
“We know now ... that we can use PARP inhibitors in the first-line setting, beyond women with BRCA mutations,” Dr. Banerjee said. “The key question, really, is ‘What about patients that don’t have HRD deficiency? We do know that patients with BRCA mutations and who are HRD-positive have the greatest benefit with either a PARP inhibitor alone or, indeed, in combination with bevacizumab.”
In an ESMO press release about the PAOLA-1 and other related data presented at the congress, Ana Oaknin, MD, of Vall D’Hebron Institute of Oncology in Barcelona, stated that “the main goal in ovarian cancer is to avoid relapse after first-line therapy because otherwise the probability of cure is quite low.”
Combination olaparib and bevacizumab for first-line maintenance therapy “should become a new standard of care for patients with advanced ovarian cancer,” she said, noting that, while the PAOLA-1/ENGOT-ov25 trial did not include patients with no response to first-line chemotherapy (who comprise a small group of ovarian cancer patients), the trial is “a significant step forward in treatment for these women.”
In the context of other positive trial findings, including those from SOLO1 and other studies of PARP inhibition in this setting, the results represent a milestone, Dr. Oaknin said. “After decades studying different chemotherapy approaches, it is the first time we have meaningfully prolonged progression-free survival, and hopefully we will improve long-term outcome.”
As for the “next priority for research in this field, Dr. Oaknin said that strategies are needed to improve the current 45% 5-year overall survival rate for ovarian cancer. “I think the next approach is to incorporate immunotherapy as part of first-line therapy; ongoing trials are expected to report in 2-3 years,” she commented.
The PAOLA-1/ENGOT-ov25 trial was funded by ARCAGY Research, AstraZeneca, and Roche. Dr. Ray-Coquard reported relationships with AstraZeneca, Clovis Oncology, Tesaro, Pharma Mar, Roche, and Genmab, including receiving honoraria, travel/accommodations/expenses, and/or research grant funding, and/or serving as an advisor or consultant. Dr. Banerjee is a lecturer and/or advisory board member for AstraZeneca, Clovis, Gamamabs, Merck Serono, Pharmamar, Seattle Genetics, Roche, and Tesaro, and has received a travel grant from Nucana. Dr. Oaknin reported having no disclosures.
SOURCE: Ray-Coquard I et al. ESMO 2019, Abstract LBA2. Ann Oncol. 19;30:suppl 9.
REPORTING FROM ESMO 2019
Cardiotoxicity after checkpoint inhibitor treatment seen early, linked to elevated biomarkers
PHILADELPHIA – , and occurred more frequently in patients with elevated biomarkers, in a retrospective cohort study reported at the annual scientific meeting of the Heart Failure Society of America.
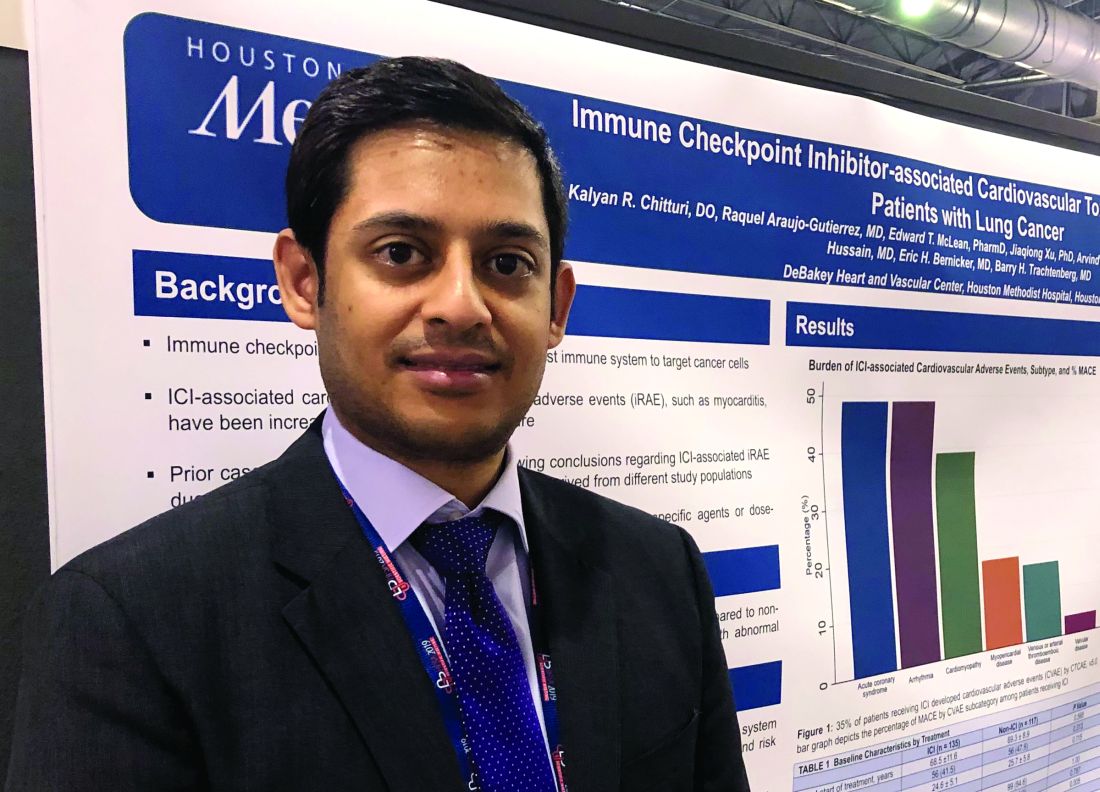
The findings support monitoring of cardiac biomarkers in the initial phase of checkpoint inhibitor treatment to identify patient at high cardiac risk, according to Kalyan R. Chitturi, DO, a resident physician with the DeBakey Heart and Vascular Center, Houston Methodist Hospital, who presented the results.
“It’s the early period that warrants the closest monitoring, as within the first 30-40 days, there’s higher risk,” Dr. Chitturi said in an interview. “When there was a biomarker elevation, it markedly increased the risk of MACE, warranting a closer vigilance during that time period.”
The retrospective study conducted by Dr. Chitturi and colleagues included a total of 252 patients with lung cancer who had been treated at one of seven different sites in Houston Methodist Cancer Center between Aug. 1, 2015, and Aug. 1, 2018.
Immune checkpoint inhibitors did not significantly increase the risk of MACE, compared with other lung cancer therapies, with incidences of 13.3% and 10.3%, respectively (P = .632), the investigators found.
However, MACE did occur earlier in the checkpoint inhibitor group, at a median time to event of 40 days, compared with 118 days in the patients not treated with checkpoint inhibitors, they found.
Risk of MACE with checkpoint inhibitor treatment was increased in patients with elevated troponin (hazard ratio, 2.48; 95% confidence interval, 1.18-5.21; P = .017) or elevated brain natriuretic peptide (HR, 5.77; 95% CI, 2.70-12.35; P less than .001), according to multivariate logistic regression analysis results.
These results suggest biomarkers such as cardiac troponin and brain natriuretic peptide are warranted to monitor patients in the early phase of checkpoint inhibitor treatment, according to Dr. Chitturi. “In the cost-benefit ratio of often-lethal MACE, it’s well worth it to collect these,” he said in the interview.
The results corroborate findings from some other recent studies, he noted. These include a recent study that linked elevated serum troponin to myocarditis in patients treated with immune checkpoint inhibitors (J Am Coll Cardiol. 2018 Apr 24;71[16]:1755-64).
Dr. Chitturi and coauthors reported no disclosures related to their presentation at the HFSA meeting.
SOURCE: Chitturi KR et al. HFSA 2019, Abstract 127.
PHILADELPHIA – , and occurred more frequently in patients with elevated biomarkers, in a retrospective cohort study reported at the annual scientific meeting of the Heart Failure Society of America.
The findings support monitoring of cardiac biomarkers in the initial phase of checkpoint inhibitor treatment to identify patient at high cardiac risk, according to Kalyan R. Chitturi, DO, a resident physician with the DeBakey Heart and Vascular Center, Houston Methodist Hospital, who presented the results.
“It’s the early period that warrants the closest monitoring, as within the first 30-40 days, there’s higher risk,” Dr. Chitturi said in an interview. “When there was a biomarker elevation, it markedly increased the risk of MACE, warranting a closer vigilance during that time period.”
The retrospective study conducted by Dr. Chitturi and colleagues included a total of 252 patients with lung cancer who had been treated at one of seven different sites in Houston Methodist Cancer Center between Aug. 1, 2015, and Aug. 1, 2018.
Immune checkpoint inhibitors did not significantly increase the risk of MACE, compared with other lung cancer therapies, with incidences of 13.3% and 10.3%, respectively (P = .632), the investigators found.
However, MACE did occur earlier in the checkpoint inhibitor group, at a median time to event of 40 days, compared with 118 days in the patients not treated with checkpoint inhibitors, they found.
Risk of MACE with checkpoint inhibitor treatment was increased in patients with elevated troponin (hazard ratio, 2.48; 95% confidence interval, 1.18-5.21; P = .017) or elevated brain natriuretic peptide (HR, 5.77; 95% CI, 2.70-12.35; P less than .001), according to multivariate logistic regression analysis results.
These results suggest biomarkers such as cardiac troponin and brain natriuretic peptide are warranted to monitor patients in the early phase of checkpoint inhibitor treatment, according to Dr. Chitturi. “In the cost-benefit ratio of often-lethal MACE, it’s well worth it to collect these,” he said in the interview.
The results corroborate findings from some other recent studies, he noted. These include a recent study that linked elevated serum troponin to myocarditis in patients treated with immune checkpoint inhibitors (J Am Coll Cardiol. 2018 Apr 24;71[16]:1755-64).
Dr. Chitturi and coauthors reported no disclosures related to their presentation at the HFSA meeting.
SOURCE: Chitturi KR et al. HFSA 2019, Abstract 127.
PHILADELPHIA – , and occurred more frequently in patients with elevated biomarkers, in a retrospective cohort study reported at the annual scientific meeting of the Heart Failure Society of America.
The findings support monitoring of cardiac biomarkers in the initial phase of checkpoint inhibitor treatment to identify patient at high cardiac risk, according to Kalyan R. Chitturi, DO, a resident physician with the DeBakey Heart and Vascular Center, Houston Methodist Hospital, who presented the results.
“It’s the early period that warrants the closest monitoring, as within the first 30-40 days, there’s higher risk,” Dr. Chitturi said in an interview. “When there was a biomarker elevation, it markedly increased the risk of MACE, warranting a closer vigilance during that time period.”
The retrospective study conducted by Dr. Chitturi and colleagues included a total of 252 patients with lung cancer who had been treated at one of seven different sites in Houston Methodist Cancer Center between Aug. 1, 2015, and Aug. 1, 2018.
Immune checkpoint inhibitors did not significantly increase the risk of MACE, compared with other lung cancer therapies, with incidences of 13.3% and 10.3%, respectively (P = .632), the investigators found.
However, MACE did occur earlier in the checkpoint inhibitor group, at a median time to event of 40 days, compared with 118 days in the patients not treated with checkpoint inhibitors, they found.
Risk of MACE with checkpoint inhibitor treatment was increased in patients with elevated troponin (hazard ratio, 2.48; 95% confidence interval, 1.18-5.21; P = .017) or elevated brain natriuretic peptide (HR, 5.77; 95% CI, 2.70-12.35; P less than .001), according to multivariate logistic regression analysis results.
These results suggest biomarkers such as cardiac troponin and brain natriuretic peptide are warranted to monitor patients in the early phase of checkpoint inhibitor treatment, according to Dr. Chitturi. “In the cost-benefit ratio of often-lethal MACE, it’s well worth it to collect these,” he said in the interview.
The results corroborate findings from some other recent studies, he noted. These include a recent study that linked elevated serum troponin to myocarditis in patients treated with immune checkpoint inhibitors (J Am Coll Cardiol. 2018 Apr 24;71[16]:1755-64).
Dr. Chitturi and coauthors reported no disclosures related to their presentation at the HFSA meeting.
SOURCE: Chitturi KR et al. HFSA 2019, Abstract 127.
REPORTING FROM HFSA 2019
MitraClip learning curve may top 200 cases
SAN FRANCISCO – It took in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry from November 2013 to March 2018.
“These findings demonstrate the key role of operator experience in optimizing outcomes” of transcatheter mitral valve repair (TMVr) with MitraClip (Abbott Structural), said investigators, led by Adnan Chhatriwalla, MD, an interventional cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“New operators may experience a ‘learning curve’ irrespective of the overall site experience or experience of other members of the Heart Team,” they wrote in the study (JACC Cardiovasc Interv. 2019 Sep 27. doi: 10.1016/j.jacc.2019.09.014).
“As TMVr becomes more prevalent in the U.S., it may be prudent for less experienced operators to be cognizant of where they sit on the ‘learning curve’ and to pay particular attention to case selection in their early experience, considering that more complex patients may be referred to more experienced centers for treatment when prudent,” they noted.
“The overall duration of the learning curve may exceed 200 cases,” Dr. Chhatriwalla said at the Transcatheter Cardiovascular Therapeutics annual meeting in a presentation that coincided with the study’s publication.
“This is a more complex procedure than [transcatheter aortic valve replacement], and the volume/outcome relationship is stronger. We are seeing issues that are related to early experience in low-volume programs. Public reporting so consumers can determine how many cases a center does is going to be critical,” said cardiothoracic surgeon Michael Mack, MD, director of the cardiovascular service line at a health system in Dallas, after the talk. He was one of the authors of the study.
The investigators compared outcomes among 549 operators who had done 1-25 MitraClip cases, 230 who had performed 26-50 cases, and 116 who had performed 50 or more.
Optimal procedural success – defined as less than or equal to 1+ residual mitral regurgitation (MR) without death or cardiac surgery – was 63.9%, 68.4%, and 75.1%, respectively, across the three groups (P less than .001). The “acceptable” procedural success rate – less than or equal to 2+ residual MR without death or cardiac surgery – was 91.4%, 92.4%, and 93.8% (P less than .001). No interaction was observed between the mechanism of mitral valve regurgitation and procedural outcomes.
Procedure time decreased as operators gained experience (145, 118, and 99 minutes), and atrial septal defect closure rates increased (0.9%, 1.4%, and 2.2%, respectively).
Composite complications rates also fell (9.7%, 8.1%, and 7.3%), driven mostly by less frequent cardiac perforation (1.0%, 1.1%, and 0.4%) and less frequent blood transfusion (9.6%, 8.6%, and 6.5%). The results were statistically significant.
“Adjusted learning curves for procedural success were visually evident after approximately 50 cases, and continued improvement in clinical outcomes was observed for the entire case sequence up to 200 cases,” the investigators wrote. The improvements could not be attributed to patient selection alone, they said.
More experienced operators were more likely to use more than one clip per case, and more frequently treated central and medial, as opposed to lateral, pathology. Operators with more than 50 cases were less likely to treat patients who had preexisting mitral stenosis or required home oxygen, and experienced operators were more likely to perform the procedure in unstable patients, when appropriate. The proportion of patients with functional MR – as opposed to degenerative disease – increased with increasing experience.
There were no statistically significant differences across the groups in stroke rates (P = .26), single-leaflet device attachments (P = .11), trans-septal complications (P = .25), urgent cardiac surgery (P = .42), or in-hospital mortality (P = .55).
Patients were a median of 81 years old, and most were white; 93% had 3+ or 4+ MR at baseline, and 86.3% had degenerative mitral disease. Two-thirds had atrial fibrillation/flutter.
The work was supported by the ACC/STS TVT Registry. Dr. Chhatriwalla is a proctor for Edwards Lifesciences and Medtronic, and is a speaker for Abbott, Edwards Lifesciences, and Medtronic. Dr. Mack has served as an investigator for Edwards Lifesciences and Abbott, and as a study chair for Medtronic. Other investigators reported similar industry disclosures.
The meeting is sponsored by the Cardiovascular Research Foundation.
SOURCE: Chhatriwalla A et. al. JACC Cardiovasc Interv. 2019 Sep 27. doi: 10.1016/j.jacc.2019.09.014.
SAN FRANCISCO – It took in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry from November 2013 to March 2018.
“These findings demonstrate the key role of operator experience in optimizing outcomes” of transcatheter mitral valve repair (TMVr) with MitraClip (Abbott Structural), said investigators, led by Adnan Chhatriwalla, MD, an interventional cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“New operators may experience a ‘learning curve’ irrespective of the overall site experience or experience of other members of the Heart Team,” they wrote in the study (JACC Cardiovasc Interv. 2019 Sep 27. doi: 10.1016/j.jacc.2019.09.014).
“As TMVr becomes more prevalent in the U.S., it may be prudent for less experienced operators to be cognizant of where they sit on the ‘learning curve’ and to pay particular attention to case selection in their early experience, considering that more complex patients may be referred to more experienced centers for treatment when prudent,” they noted.
“The overall duration of the learning curve may exceed 200 cases,” Dr. Chhatriwalla said at the Transcatheter Cardiovascular Therapeutics annual meeting in a presentation that coincided with the study’s publication.
“This is a more complex procedure than [transcatheter aortic valve replacement], and the volume/outcome relationship is stronger. We are seeing issues that are related to early experience in low-volume programs. Public reporting so consumers can determine how many cases a center does is going to be critical,” said cardiothoracic surgeon Michael Mack, MD, director of the cardiovascular service line at a health system in Dallas, after the talk. He was one of the authors of the study.
The investigators compared outcomes among 549 operators who had done 1-25 MitraClip cases, 230 who had performed 26-50 cases, and 116 who had performed 50 or more.
Optimal procedural success – defined as less than or equal to 1+ residual mitral regurgitation (MR) without death or cardiac surgery – was 63.9%, 68.4%, and 75.1%, respectively, across the three groups (P less than .001). The “acceptable” procedural success rate – less than or equal to 2+ residual MR without death or cardiac surgery – was 91.4%, 92.4%, and 93.8% (P less than .001). No interaction was observed between the mechanism of mitral valve regurgitation and procedural outcomes.
Procedure time decreased as operators gained experience (145, 118, and 99 minutes), and atrial septal defect closure rates increased (0.9%, 1.4%, and 2.2%, respectively).
Composite complications rates also fell (9.7%, 8.1%, and 7.3%), driven mostly by less frequent cardiac perforation (1.0%, 1.1%, and 0.4%) and less frequent blood transfusion (9.6%, 8.6%, and 6.5%). The results were statistically significant.
“Adjusted learning curves for procedural success were visually evident after approximately 50 cases, and continued improvement in clinical outcomes was observed for the entire case sequence up to 200 cases,” the investigators wrote. The improvements could not be attributed to patient selection alone, they said.
More experienced operators were more likely to use more than one clip per case, and more frequently treated central and medial, as opposed to lateral, pathology. Operators with more than 50 cases were less likely to treat patients who had preexisting mitral stenosis or required home oxygen, and experienced operators were more likely to perform the procedure in unstable patients, when appropriate. The proportion of patients with functional MR – as opposed to degenerative disease – increased with increasing experience.
There were no statistically significant differences across the groups in stroke rates (P = .26), single-leaflet device attachments (P = .11), trans-septal complications (P = .25), urgent cardiac surgery (P = .42), or in-hospital mortality (P = .55).
Patients were a median of 81 years old, and most were white; 93% had 3+ or 4+ MR at baseline, and 86.3% had degenerative mitral disease. Two-thirds had atrial fibrillation/flutter.
The work was supported by the ACC/STS TVT Registry. Dr. Chhatriwalla is a proctor for Edwards Lifesciences and Medtronic, and is a speaker for Abbott, Edwards Lifesciences, and Medtronic. Dr. Mack has served as an investigator for Edwards Lifesciences and Abbott, and as a study chair for Medtronic. Other investigators reported similar industry disclosures.
The meeting is sponsored by the Cardiovascular Research Foundation.
SOURCE: Chhatriwalla A et. al. JACC Cardiovasc Interv. 2019 Sep 27. doi: 10.1016/j.jacc.2019.09.014.
SAN FRANCISCO – It took in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry from November 2013 to March 2018.
“These findings demonstrate the key role of operator experience in optimizing outcomes” of transcatheter mitral valve repair (TMVr) with MitraClip (Abbott Structural), said investigators, led by Adnan Chhatriwalla, MD, an interventional cardiologist at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“New operators may experience a ‘learning curve’ irrespective of the overall site experience or experience of other members of the Heart Team,” they wrote in the study (JACC Cardiovasc Interv. 2019 Sep 27. doi: 10.1016/j.jacc.2019.09.014).
“As TMVr becomes more prevalent in the U.S., it may be prudent for less experienced operators to be cognizant of where they sit on the ‘learning curve’ and to pay particular attention to case selection in their early experience, considering that more complex patients may be referred to more experienced centers for treatment when prudent,” they noted.
“The overall duration of the learning curve may exceed 200 cases,” Dr. Chhatriwalla said at the Transcatheter Cardiovascular Therapeutics annual meeting in a presentation that coincided with the study’s publication.
“This is a more complex procedure than [transcatheter aortic valve replacement], and the volume/outcome relationship is stronger. We are seeing issues that are related to early experience in low-volume programs. Public reporting so consumers can determine how many cases a center does is going to be critical,” said cardiothoracic surgeon Michael Mack, MD, director of the cardiovascular service line at a health system in Dallas, after the talk. He was one of the authors of the study.
The investigators compared outcomes among 549 operators who had done 1-25 MitraClip cases, 230 who had performed 26-50 cases, and 116 who had performed 50 or more.
Optimal procedural success – defined as less than or equal to 1+ residual mitral regurgitation (MR) without death or cardiac surgery – was 63.9%, 68.4%, and 75.1%, respectively, across the three groups (P less than .001). The “acceptable” procedural success rate – less than or equal to 2+ residual MR without death or cardiac surgery – was 91.4%, 92.4%, and 93.8% (P less than .001). No interaction was observed between the mechanism of mitral valve regurgitation and procedural outcomes.
Procedure time decreased as operators gained experience (145, 118, and 99 minutes), and atrial septal defect closure rates increased (0.9%, 1.4%, and 2.2%, respectively).
Composite complications rates also fell (9.7%, 8.1%, and 7.3%), driven mostly by less frequent cardiac perforation (1.0%, 1.1%, and 0.4%) and less frequent blood transfusion (9.6%, 8.6%, and 6.5%). The results were statistically significant.
“Adjusted learning curves for procedural success were visually evident after approximately 50 cases, and continued improvement in clinical outcomes was observed for the entire case sequence up to 200 cases,” the investigators wrote. The improvements could not be attributed to patient selection alone, they said.
More experienced operators were more likely to use more than one clip per case, and more frequently treated central and medial, as opposed to lateral, pathology. Operators with more than 50 cases were less likely to treat patients who had preexisting mitral stenosis or required home oxygen, and experienced operators were more likely to perform the procedure in unstable patients, when appropriate. The proportion of patients with functional MR – as opposed to degenerative disease – increased with increasing experience.
There were no statistically significant differences across the groups in stroke rates (P = .26), single-leaflet device attachments (P = .11), trans-septal complications (P = .25), urgent cardiac surgery (P = .42), or in-hospital mortality (P = .55).
Patients were a median of 81 years old, and most were white; 93% had 3+ or 4+ MR at baseline, and 86.3% had degenerative mitral disease. Two-thirds had atrial fibrillation/flutter.
The work was supported by the ACC/STS TVT Registry. Dr. Chhatriwalla is a proctor for Edwards Lifesciences and Medtronic, and is a speaker for Abbott, Edwards Lifesciences, and Medtronic. Dr. Mack has served as an investigator for Edwards Lifesciences and Abbott, and as a study chair for Medtronic. Other investigators reported similar industry disclosures.
The meeting is sponsored by the Cardiovascular Research Foundation.
SOURCE: Chhatriwalla A et. al. JACC Cardiovasc Interv. 2019 Sep 27. doi: 10.1016/j.jacc.2019.09.014.
REPORTING FROM TCT 2019
Ticagrelor monotherapy tops DAPT for high-risk PCI patients
SAN FRANCISCO – After 3 months of ticagrelor (Brilinta) plus aspirin following cardiac stenting, stopping the aspirin but continuing the ticagrelor resulted in less bleeding with no increase in ischemic events in a randomized trial with more than 7,000 drug-eluting stent patients at high risk for both.
“This was a superior therapy” to staying on both drugs, the more usual approach, said lead investigator Roxana Mehran, MD, director of interventional cardiovascular research and clinical trials at the Icahn School of Medicine at Mount Sinai, New York.
“We can’t say this is for all comers, but for patients whose physician felt comfortable putting them on aspirin and ticagrelor,” who tolerated it well for the first 3 months, and who had clinical and angiographic indications of risk, “I think these patients can be peeled away” from aspirin, she said in a presentation at the Transcatheter Cardiovascular Therapeutics annual meeting that coincided with publication of the trial, dubbed TWILIGHT (Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention).
Interventional cardiologists have long sought the sweet spot for dual-antiplatelet therapy (DAPT) after stenting; the idea is to maximize thrombosis prevention while minimizing bleeding risk. The trial supports the trend in recent years towards shorter DAPT. Often, however, it’s the P2Y12 inhibitor – ticagrelor, clopidogrel (Plavix), or prasugrel (Effient) – that goes first, not the aspirin.
Responding to an audience question about why the trial didn’t include an aspirin monotherapy arm, Dr. Mehran said that aspirin alone wouldn’t have been sufficient in high-risk patients “in whom you have almost 70% acute coronary syndrome.” She added that her team has data showing that aspirin itself doesn’t have much effect on blood thrombogenicity.
The 7,119 patients in TWILIGHT were on ticagrelor 90 mg twice daily and aspirin 81-100 mg daily for 3 months, then evenly randomized to continued treatment or ticagrelor plus an aspirin placebo for a year.
Subjects had to have at least one clinical and angiographic finding that put them at high risk for bleeding or an ischemic event, such as chronic kidney disease, acute coronary syndrome, diabetes, or a bifurcated target lesion treated with two stents.
One year after randomization, 4% in the ticagrelor monotherapy group versus 7.1% in the ticagrelor plus aspirin arm reached the primary end point, actionable (type 2), severe (type 3), or fatal (type 5) bleeding on the Bleeding Academic Research Consortium scale (hazard ratio, 0.56; 95% confidence interval, 0.45 - 0.68, P less than .001).
The incidence of death from any cause, nonfatal myocardial infarction, or nonfatal stroke was 3.9% in both groups (HR, 0.99; 95% CI, 0.78-1.25; P less than .001 for noninferiority).
There were more ischemic strokes in the ticagrelor monotherapy arm (0.5% versus 0.2%). All-cause mortality (1.3% versus 1%) and stent thrombosis (0.6% versus 0.4%) were more frequent in the ticagrelor/aspirin group, but the differences were not statistically significant.
The two groups were well balanced. The mean age was 65 years, 23.8% of the patients were female, 37% had diabetes, and 65% had percutaneous coronary intervention for an acute coronary syndrome. Almost two-thirds had multivessel disease. Mean stent length was about 40 mm. The trial excluded patients with prior strokes.
Almost 2,000 patients originally enrolled in the trial never made it to randomization because they had a major bleeding or ischemic event in the 3-month run up, or dyspnea or some other reaction to ticagrelor.
The recent STOPDAPT-2 trial had a similar outcome – less bleeding with no increase in ischemic events – with clopidogrel monotherapy after a month-long run in of dual therapy with aspirin, versus continued treatment with both, in patients at low risk for ischemic events after stenting (JAMA. 2019 Jun 25;321[24]:2414-27).
Another recent study, GLOBAL LEADERS, concluded that 1 month of DAPT followed by ticagrelor monotherapy for 23 months was not superior to 12 months of DAPT followed by a year of aspirin. There was a numerical advantage for solo ticagrelor on death, myocardial infarction, and bleeding, but it did not reach statistical significance (Lancet. 2018 Sep 15;392[10151]:940-9).
The work was funded by ticagrelor’s maker, AstraZeneca. Dr. Mehran reported consulting and other relationships with Abbott, Janssen, and other companies.
SOURCE: Mehran A et al. N Engl J Med. 2019 Sep 26. doi: 10.1056/NEJMoa1908419.
SAN FRANCISCO – After 3 months of ticagrelor (Brilinta) plus aspirin following cardiac stenting, stopping the aspirin but continuing the ticagrelor resulted in less bleeding with no increase in ischemic events in a randomized trial with more than 7,000 drug-eluting stent patients at high risk for both.
“This was a superior therapy” to staying on both drugs, the more usual approach, said lead investigator Roxana Mehran, MD, director of interventional cardiovascular research and clinical trials at the Icahn School of Medicine at Mount Sinai, New York.
“We can’t say this is for all comers, but for patients whose physician felt comfortable putting them on aspirin and ticagrelor,” who tolerated it well for the first 3 months, and who had clinical and angiographic indications of risk, “I think these patients can be peeled away” from aspirin, she said in a presentation at the Transcatheter Cardiovascular Therapeutics annual meeting that coincided with publication of the trial, dubbed TWILIGHT (Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention).
Interventional cardiologists have long sought the sweet spot for dual-antiplatelet therapy (DAPT) after stenting; the idea is to maximize thrombosis prevention while minimizing bleeding risk. The trial supports the trend in recent years towards shorter DAPT. Often, however, it’s the P2Y12 inhibitor – ticagrelor, clopidogrel (Plavix), or prasugrel (Effient) – that goes first, not the aspirin.
Responding to an audience question about why the trial didn’t include an aspirin monotherapy arm, Dr. Mehran said that aspirin alone wouldn’t have been sufficient in high-risk patients “in whom you have almost 70% acute coronary syndrome.” She added that her team has data showing that aspirin itself doesn’t have much effect on blood thrombogenicity.
The 7,119 patients in TWILIGHT were on ticagrelor 90 mg twice daily and aspirin 81-100 mg daily for 3 months, then evenly randomized to continued treatment or ticagrelor plus an aspirin placebo for a year.
Subjects had to have at least one clinical and angiographic finding that put them at high risk for bleeding or an ischemic event, such as chronic kidney disease, acute coronary syndrome, diabetes, or a bifurcated target lesion treated with two stents.
One year after randomization, 4% in the ticagrelor monotherapy group versus 7.1% in the ticagrelor plus aspirin arm reached the primary end point, actionable (type 2), severe (type 3), or fatal (type 5) bleeding on the Bleeding Academic Research Consortium scale (hazard ratio, 0.56; 95% confidence interval, 0.45 - 0.68, P less than .001).
The incidence of death from any cause, nonfatal myocardial infarction, or nonfatal stroke was 3.9% in both groups (HR, 0.99; 95% CI, 0.78-1.25; P less than .001 for noninferiority).
There were more ischemic strokes in the ticagrelor monotherapy arm (0.5% versus 0.2%). All-cause mortality (1.3% versus 1%) and stent thrombosis (0.6% versus 0.4%) were more frequent in the ticagrelor/aspirin group, but the differences were not statistically significant.
The two groups were well balanced. The mean age was 65 years, 23.8% of the patients were female, 37% had diabetes, and 65% had percutaneous coronary intervention for an acute coronary syndrome. Almost two-thirds had multivessel disease. Mean stent length was about 40 mm. The trial excluded patients with prior strokes.
Almost 2,000 patients originally enrolled in the trial never made it to randomization because they had a major bleeding or ischemic event in the 3-month run up, or dyspnea or some other reaction to ticagrelor.
The recent STOPDAPT-2 trial had a similar outcome – less bleeding with no increase in ischemic events – with clopidogrel monotherapy after a month-long run in of dual therapy with aspirin, versus continued treatment with both, in patients at low risk for ischemic events after stenting (JAMA. 2019 Jun 25;321[24]:2414-27).
Another recent study, GLOBAL LEADERS, concluded that 1 month of DAPT followed by ticagrelor monotherapy for 23 months was not superior to 12 months of DAPT followed by a year of aspirin. There was a numerical advantage for solo ticagrelor on death, myocardial infarction, and bleeding, but it did not reach statistical significance (Lancet. 2018 Sep 15;392[10151]:940-9).
The work was funded by ticagrelor’s maker, AstraZeneca. Dr. Mehran reported consulting and other relationships with Abbott, Janssen, and other companies.
SOURCE: Mehran A et al. N Engl J Med. 2019 Sep 26. doi: 10.1056/NEJMoa1908419.
SAN FRANCISCO – After 3 months of ticagrelor (Brilinta) plus aspirin following cardiac stenting, stopping the aspirin but continuing the ticagrelor resulted in less bleeding with no increase in ischemic events in a randomized trial with more than 7,000 drug-eluting stent patients at high risk for both.
“This was a superior therapy” to staying on both drugs, the more usual approach, said lead investigator Roxana Mehran, MD, director of interventional cardiovascular research and clinical trials at the Icahn School of Medicine at Mount Sinai, New York.
“We can’t say this is for all comers, but for patients whose physician felt comfortable putting them on aspirin and ticagrelor,” who tolerated it well for the first 3 months, and who had clinical and angiographic indications of risk, “I think these patients can be peeled away” from aspirin, she said in a presentation at the Transcatheter Cardiovascular Therapeutics annual meeting that coincided with publication of the trial, dubbed TWILIGHT (Ticagrelor with Aspirin or Alone in High-Risk Patients after Coronary Intervention).
Interventional cardiologists have long sought the sweet spot for dual-antiplatelet therapy (DAPT) after stenting; the idea is to maximize thrombosis prevention while minimizing bleeding risk. The trial supports the trend in recent years towards shorter DAPT. Often, however, it’s the P2Y12 inhibitor – ticagrelor, clopidogrel (Plavix), or prasugrel (Effient) – that goes first, not the aspirin.
Responding to an audience question about why the trial didn’t include an aspirin monotherapy arm, Dr. Mehran said that aspirin alone wouldn’t have been sufficient in high-risk patients “in whom you have almost 70% acute coronary syndrome.” She added that her team has data showing that aspirin itself doesn’t have much effect on blood thrombogenicity.
The 7,119 patients in TWILIGHT were on ticagrelor 90 mg twice daily and aspirin 81-100 mg daily for 3 months, then evenly randomized to continued treatment or ticagrelor plus an aspirin placebo for a year.
Subjects had to have at least one clinical and angiographic finding that put them at high risk for bleeding or an ischemic event, such as chronic kidney disease, acute coronary syndrome, diabetes, or a bifurcated target lesion treated with two stents.
One year after randomization, 4% in the ticagrelor monotherapy group versus 7.1% in the ticagrelor plus aspirin arm reached the primary end point, actionable (type 2), severe (type 3), or fatal (type 5) bleeding on the Bleeding Academic Research Consortium scale (hazard ratio, 0.56; 95% confidence interval, 0.45 - 0.68, P less than .001).
The incidence of death from any cause, nonfatal myocardial infarction, or nonfatal stroke was 3.9% in both groups (HR, 0.99; 95% CI, 0.78-1.25; P less than .001 for noninferiority).
There were more ischemic strokes in the ticagrelor monotherapy arm (0.5% versus 0.2%). All-cause mortality (1.3% versus 1%) and stent thrombosis (0.6% versus 0.4%) were more frequent in the ticagrelor/aspirin group, but the differences were not statistically significant.
The two groups were well balanced. The mean age was 65 years, 23.8% of the patients were female, 37% had diabetes, and 65% had percutaneous coronary intervention for an acute coronary syndrome. Almost two-thirds had multivessel disease. Mean stent length was about 40 mm. The trial excluded patients with prior strokes.
Almost 2,000 patients originally enrolled in the trial never made it to randomization because they had a major bleeding or ischemic event in the 3-month run up, or dyspnea or some other reaction to ticagrelor.
The recent STOPDAPT-2 trial had a similar outcome – less bleeding with no increase in ischemic events – with clopidogrel monotherapy after a month-long run in of dual therapy with aspirin, versus continued treatment with both, in patients at low risk for ischemic events after stenting (JAMA. 2019 Jun 25;321[24]:2414-27).
Another recent study, GLOBAL LEADERS, concluded that 1 month of DAPT followed by ticagrelor monotherapy for 23 months was not superior to 12 months of DAPT followed by a year of aspirin. There was a numerical advantage for solo ticagrelor on death, myocardial infarction, and bleeding, but it did not reach statistical significance (Lancet. 2018 Sep 15;392[10151]:940-9).
The work was funded by ticagrelor’s maker, AstraZeneca. Dr. Mehran reported consulting and other relationships with Abbott, Janssen, and other companies.
SOURCE: Mehran A et al. N Engl J Med. 2019 Sep 26. doi: 10.1056/NEJMoa1908419.
REPORTING FROM TCT 2019
Osimertinib improves survival in advanced NSCLC
BARCELONA – In patients with , compared with other agents targeted against NSCLC with epidermal growth factor–receptor (EGFR) mutations, investigators for the FLAURA trial reported.
After median follow-up ranging from 27 to 35.8 months, the median overall survival was 38.6 months for patients randomized to osimertinib, compared with 31.8 months for patients assigned to either of two comparator tyrosine kinase inhibitors (TKIs), gefitinib (Iressa) or erlotinib (Tarceva).
The hazard ratio for death with osimertinib was 0.799 (P = .0462), reported Suresh Ramalingam, MD, director of the lung cancer program at Winship Cancer Institute of Emory University, Atlanta.
“I’m excited that the new milestone accomplished with osimertinib in this trial will serve as the platform to build on in our efforts to improve the lives of patients with lung cancer,” he said at the European Society for Medical Oncology Congress.
Osimertinib is the first TKI to show improvement in overall survival over another TKI in the treatment of advanced stage cancers, he noted.
Overall survival was a secondary endpoint of the FLAURA trial. As previously reported, FLAURA met its primary endpoint of improvement in progression-free survival (PFS) in an interim analysis presented at ESMO 2017. In that analysis, osimertinib cut the risk of disease progression by 54%, compared with gefitinib or erlotinib.
Among 279 patients with EGFR-mutated locally advanced or metastatic NSCLC treated with osimertinib, the median PFS was 18.9 months, compared with 10.2 months for 277 patients treated with the standard of care, which translated into a HR of 0.46 (P less than .0001).
The FLAURA results supported Food and Drug Administration approval of osimertinib in April 2018 for first-line treatment of patients with metastatic NSCLC with EGFR mutations as detected by an FDA-approved test.
The current overall survival analysis, although not powered to show differences among patient subgroups, showed trends favoring osimertinib over a comparator TKI among both men and women, older and younger patients, patients with central nervous system metastases at trial entry, and patients with the EGFR exon 19 deletion at randomization.
The 31.8 month median overall survival for the control (comparator-TKI) arm is among the highest reported for patients with EGFR-mutated NSCLC, Dr. Ramalingam noted.
“That is because a lot of patients crossed over from the control group to receive osimertinib on progression,” he said, adding that the magnitude of benefit from osimertinib was greater among non-Asian patients, compared with Asians.
FLAURA details
In the phase 3 FLAURA trial, investigators stratified patients with previously untreated NSCLC positive for EGFR resistance mutations according to mutation status (exon 19 deletion or the L858R amino acid substitution in exon 21) and race (Asian or non-Asian).
Patients were randomly assigned to treatment with either oral osimertinib 80 mg daily or an EGFR TKI, either oral gefitinib 250 mg or erlotinib 150 mg daily.
The patients were assessed by Response Evaluation Criteria in Solid Tumors (RECIST) every 6 weeks until objective disease progression.
Patients assigned to the standard-of-care arm who had central confirmation of progression and T790M positivity were allowed to cross over to open-label osimertinib.
PFS, the primary endpoint, was also significantly better with osimertinib than with either of the comparator TKIs in patients with and without central nervous system metastases at study entry (HR, 0.47; P = .0009 for patients with CNS metastases; HR, 0.46; P less than .0001 for patients with no CNS metastases).
“For clinicians, for patients, and also for our health authorities, the results in terms of overall survival are really relevant, and this is why this study is so important, knowing this secondary endpoint from a statistical point of view. The study is statistically significant and clinically relevant,” commented Pilar Garrido, MD, from the department of medical oncology, Hospital Universitario Ramón y Cajal in Madrid, the invited discussant at a briefing where Dr. Ramalingam outlined the study findings prior to his presentation of the data in a symposium.
“What’s the future of EGFR mutant lung cancer? Well, I think we should be done with single-agent EGFR-TKI comparisons: We have a clear agent that’s associated with an improvement in survival. I think our focus needs to shift to building on or adding to osimertinib,” commented Pasi A Jänne, MD, PhD, director of the Lowe Center for Thoracic Oncology at Dana-Farber Cancer Institute in Boston, the invited discussant at the symposium.
He said that the challenge for clinicians will be to identify high- and low-risk EGFR-mutant NSCLC, and to determine which patients could be treated with a single agent, and which may require a combination therapy approach.
FLAURA was sponsored by AstraZeneca. Dr. Ramalingam disclosed honoraria, an advisory or consulting role, and research funding from that company and others. Dr. Garrido disclosed a speaker and advisory role for AstraZeneca and others. Dr. Jänne disclosed prior consulting for AstraZeneca.
SOURCE: Ramalingam S et al. ESMO 2019. Abstract LBA5_PR.
BARCELONA – In patients with , compared with other agents targeted against NSCLC with epidermal growth factor–receptor (EGFR) mutations, investigators for the FLAURA trial reported.
After median follow-up ranging from 27 to 35.8 months, the median overall survival was 38.6 months for patients randomized to osimertinib, compared with 31.8 months for patients assigned to either of two comparator tyrosine kinase inhibitors (TKIs), gefitinib (Iressa) or erlotinib (Tarceva).
The hazard ratio for death with osimertinib was 0.799 (P = .0462), reported Suresh Ramalingam, MD, director of the lung cancer program at Winship Cancer Institute of Emory University, Atlanta.
“I’m excited that the new milestone accomplished with osimertinib in this trial will serve as the platform to build on in our efforts to improve the lives of patients with lung cancer,” he said at the European Society for Medical Oncology Congress.
Osimertinib is the first TKI to show improvement in overall survival over another TKI in the treatment of advanced stage cancers, he noted.
Overall survival was a secondary endpoint of the FLAURA trial. As previously reported, FLAURA met its primary endpoint of improvement in progression-free survival (PFS) in an interim analysis presented at ESMO 2017. In that analysis, osimertinib cut the risk of disease progression by 54%, compared with gefitinib or erlotinib.
Among 279 patients with EGFR-mutated locally advanced or metastatic NSCLC treated with osimertinib, the median PFS was 18.9 months, compared with 10.2 months for 277 patients treated with the standard of care, which translated into a HR of 0.46 (P less than .0001).
The FLAURA results supported Food and Drug Administration approval of osimertinib in April 2018 for first-line treatment of patients with metastatic NSCLC with EGFR mutations as detected by an FDA-approved test.
The current overall survival analysis, although not powered to show differences among patient subgroups, showed trends favoring osimertinib over a comparator TKI among both men and women, older and younger patients, patients with central nervous system metastases at trial entry, and patients with the EGFR exon 19 deletion at randomization.
The 31.8 month median overall survival for the control (comparator-TKI) arm is among the highest reported for patients with EGFR-mutated NSCLC, Dr. Ramalingam noted.
“That is because a lot of patients crossed over from the control group to receive osimertinib on progression,” he said, adding that the magnitude of benefit from osimertinib was greater among non-Asian patients, compared with Asians.
FLAURA details
In the phase 3 FLAURA trial, investigators stratified patients with previously untreated NSCLC positive for EGFR resistance mutations according to mutation status (exon 19 deletion or the L858R amino acid substitution in exon 21) and race (Asian or non-Asian).
Patients were randomly assigned to treatment with either oral osimertinib 80 mg daily or an EGFR TKI, either oral gefitinib 250 mg or erlotinib 150 mg daily.
The patients were assessed by Response Evaluation Criteria in Solid Tumors (RECIST) every 6 weeks until objective disease progression.
Patients assigned to the standard-of-care arm who had central confirmation of progression and T790M positivity were allowed to cross over to open-label osimertinib.
PFS, the primary endpoint, was also significantly better with osimertinib than with either of the comparator TKIs in patients with and without central nervous system metastases at study entry (HR, 0.47; P = .0009 for patients with CNS metastases; HR, 0.46; P less than .0001 for patients with no CNS metastases).
“For clinicians, for patients, and also for our health authorities, the results in terms of overall survival are really relevant, and this is why this study is so important, knowing this secondary endpoint from a statistical point of view. The study is statistically significant and clinically relevant,” commented Pilar Garrido, MD, from the department of medical oncology, Hospital Universitario Ramón y Cajal in Madrid, the invited discussant at a briefing where Dr. Ramalingam outlined the study findings prior to his presentation of the data in a symposium.
“What’s the future of EGFR mutant lung cancer? Well, I think we should be done with single-agent EGFR-TKI comparisons: We have a clear agent that’s associated with an improvement in survival. I think our focus needs to shift to building on or adding to osimertinib,” commented Pasi A Jänne, MD, PhD, director of the Lowe Center for Thoracic Oncology at Dana-Farber Cancer Institute in Boston, the invited discussant at the symposium.
He said that the challenge for clinicians will be to identify high- and low-risk EGFR-mutant NSCLC, and to determine which patients could be treated with a single agent, and which may require a combination therapy approach.
FLAURA was sponsored by AstraZeneca. Dr. Ramalingam disclosed honoraria, an advisory or consulting role, and research funding from that company and others. Dr. Garrido disclosed a speaker and advisory role for AstraZeneca and others. Dr. Jänne disclosed prior consulting for AstraZeneca.
SOURCE: Ramalingam S et al. ESMO 2019. Abstract LBA5_PR.
BARCELONA – In patients with , compared with other agents targeted against NSCLC with epidermal growth factor–receptor (EGFR) mutations, investigators for the FLAURA trial reported.
After median follow-up ranging from 27 to 35.8 months, the median overall survival was 38.6 months for patients randomized to osimertinib, compared with 31.8 months for patients assigned to either of two comparator tyrosine kinase inhibitors (TKIs), gefitinib (Iressa) or erlotinib (Tarceva).
The hazard ratio for death with osimertinib was 0.799 (P = .0462), reported Suresh Ramalingam, MD, director of the lung cancer program at Winship Cancer Institute of Emory University, Atlanta.
“I’m excited that the new milestone accomplished with osimertinib in this trial will serve as the platform to build on in our efforts to improve the lives of patients with lung cancer,” he said at the European Society for Medical Oncology Congress.
Osimertinib is the first TKI to show improvement in overall survival over another TKI in the treatment of advanced stage cancers, he noted.
Overall survival was a secondary endpoint of the FLAURA trial. As previously reported, FLAURA met its primary endpoint of improvement in progression-free survival (PFS) in an interim analysis presented at ESMO 2017. In that analysis, osimertinib cut the risk of disease progression by 54%, compared with gefitinib or erlotinib.
Among 279 patients with EGFR-mutated locally advanced or metastatic NSCLC treated with osimertinib, the median PFS was 18.9 months, compared with 10.2 months for 277 patients treated with the standard of care, which translated into a HR of 0.46 (P less than .0001).
The FLAURA results supported Food and Drug Administration approval of osimertinib in April 2018 for first-line treatment of patients with metastatic NSCLC with EGFR mutations as detected by an FDA-approved test.
The current overall survival analysis, although not powered to show differences among patient subgroups, showed trends favoring osimertinib over a comparator TKI among both men and women, older and younger patients, patients with central nervous system metastases at trial entry, and patients with the EGFR exon 19 deletion at randomization.
The 31.8 month median overall survival for the control (comparator-TKI) arm is among the highest reported for patients with EGFR-mutated NSCLC, Dr. Ramalingam noted.
“That is because a lot of patients crossed over from the control group to receive osimertinib on progression,” he said, adding that the magnitude of benefit from osimertinib was greater among non-Asian patients, compared with Asians.
FLAURA details
In the phase 3 FLAURA trial, investigators stratified patients with previously untreated NSCLC positive for EGFR resistance mutations according to mutation status (exon 19 deletion or the L858R amino acid substitution in exon 21) and race (Asian or non-Asian).
Patients were randomly assigned to treatment with either oral osimertinib 80 mg daily or an EGFR TKI, either oral gefitinib 250 mg or erlotinib 150 mg daily.
The patients were assessed by Response Evaluation Criteria in Solid Tumors (RECIST) every 6 weeks until objective disease progression.
Patients assigned to the standard-of-care arm who had central confirmation of progression and T790M positivity were allowed to cross over to open-label osimertinib.
PFS, the primary endpoint, was also significantly better with osimertinib than with either of the comparator TKIs in patients with and without central nervous system metastases at study entry (HR, 0.47; P = .0009 for patients with CNS metastases; HR, 0.46; P less than .0001 for patients with no CNS metastases).
“For clinicians, for patients, and also for our health authorities, the results in terms of overall survival are really relevant, and this is why this study is so important, knowing this secondary endpoint from a statistical point of view. The study is statistically significant and clinically relevant,” commented Pilar Garrido, MD, from the department of medical oncology, Hospital Universitario Ramón y Cajal in Madrid, the invited discussant at a briefing where Dr. Ramalingam outlined the study findings prior to his presentation of the data in a symposium.
“What’s the future of EGFR mutant lung cancer? Well, I think we should be done with single-agent EGFR-TKI comparisons: We have a clear agent that’s associated with an improvement in survival. I think our focus needs to shift to building on or adding to osimertinib,” commented Pasi A Jänne, MD, PhD, director of the Lowe Center for Thoracic Oncology at Dana-Farber Cancer Institute in Boston, the invited discussant at the symposium.
He said that the challenge for clinicians will be to identify high- and low-risk EGFR-mutant NSCLC, and to determine which patients could be treated with a single agent, and which may require a combination therapy approach.
FLAURA was sponsored by AstraZeneca. Dr. Ramalingam disclosed honoraria, an advisory or consulting role, and research funding from that company and others. Dr. Garrido disclosed a speaker and advisory role for AstraZeneca and others. Dr. Jänne disclosed prior consulting for AstraZeneca.
SOURCE: Ramalingam S et al. ESMO 2019. Abstract LBA5_PR.
REPORTING FROM ESMO 2019
Hypoattenuated leaflet thickening often present in bioprosthetic valves
SAN FRANCISCO – The was 10% at 30 days and increased to 24% at 1 year, results from a PARTNER 3 substudy demonstrated.
However, the lack of a clear association with serious clinical events such as death, MI, and stroke “does not justify the routine prophylactic use of anticoagulation [following TAVR] in all patients,” lead study investigator Raj R. Makkar, MD, said during a press briefing at the Transcatheter Cardiovascular Therapeutics annual meeting.
“Subclinical leaflet thrombosis characterized by hypoattenuated leaflet thickening and reduced leaflet motion has been frequently observed in transcatheter and surgical aortic bioprosthetic valves,” said Dr. Makkar, director of the interventional cardiology division, Cedars-Sinai Medical Center, Los Angeles. “Thrombus on bioprosthetic valves can present as a spectrum: HALT with relatively normal leaflet motion, HALT with reduced leaflet motion but normal gradients, and clinical valve thrombosis with elevated gradients.”
The primary objective of the current Food and Drug Administration–mandated study, known as the PARTNER 3 Low-Risk Computed Tomography Sub-study, was to evaluate HALT and reduced leaflet motion in terms of differences in transcatheter and surgical bioprosthetic aortic valves among patients enrolled in the randomized PARTNER 3 cohort, to understand the natural history of HALT and reduced leaflet motion in the absence of anticoagulation, and to understand its impact on valve hemodynamics and clinical outcomes. Patients underwent specialized serial CTs at 30 days and at 1 year post TAVR or surgical aortic valve replacement (SAVR). All scans were analyzed by a CT core lab blinded to patient information or time of the scans, and the treating investigators were blinded to the results of the 30-day and 1-year CT scans. A clinical events committee adjudicated key clinical events.
Dr. Makkar reported outcomes from 408 patients: 213 who underwent TAVR and 195 who underwent surgery. There were 348 evaluable serial CT scans at 30 days and 312 at 1 year. The incidence of HALT at 30 days was 13.3% in the TAVR group and 5% in the surgery group, a difference that reached statistical significance (P = .03). At 1 year, however, the difference was not significant (27.5% vs. 20.2%, respectively; P = .19). In the overall cohort, he said, the incidence of HALT was 10% at 30 days and increased to 24% at 1 year.
The researchers also found that HALT was dynamic and spontaneously resolved in 56% of patients in the absence of anticoagulation at 30 days, while new HALT appeared in 21% of patients at 1 year.
“In terms of its impact on valve gradient, the impact was minimal,” Dr. Makkar said. “There was an increase of 1-2 mm Hg in patients who had HALT and in patients who had reduced leaflet motion.”
As for impact on clinical outcomes, the researchers observed no deaths or any myocardial infarction at any time point in patients who had HALT. “There were four cases of valve thrombosis, three of which occurred in patients who had HALT,” Dr. Makkar said at the meeting, sponsored by the Cardiovascular Research Foundation. “One stroke occurred in each group. TIA [transient ischemic attack] occurred in 1 patient out of 35 in the HALT group and 3 out of 311 in the no-HALT group. There was one case of retinal artery embolism in each group.”
In a pooled analysis of clinical events, he and his colleagues observed a numerical increase in death, stroke, TIA, and thrombotic events in patients who had HALT at 30 days, compared with those who did not (8.6% vs. 2.9%, respectively), but the difference did not reach statistical significance (P = .11). “However, given the low total number of events, the data are inconclusive and only hypothesis generating,” he said. “A longer-term follow-up and [a] larger data set will further clarify the impact on clinical outcomes.”
Dr. Makkar emphasized that routine post–TAVR/SAVR CT scans outside of research protocols are not indicated. “CTs should be prompted by increased gradients or thromboembolic events,” he said.
One of the discussants at the briefing, Michael J. Mack, MD, chair of the cardiovascular service line at Baylor Scott and White Health in Dallas and primary author of the PARTNER 3 study, said that prior to the substudy results, “I’ve always thought that the incidence of valve thrombosis would be higher with TAVR than with surgery. So the fact that it was higher at 30 days didn’t surprise me. One of the reasons is that you lose the backwashing effect by changing flow dynamics in the aortic route. What did surprise me is the percent that resolved without anticoagulation.”
He added, “The impact of all this is that we are not justified recommending routine anticoagulation [after bioprosthetic aortic valve replacement surgery]. I think it does call into question the guidelines for surgical valves, because we did that based on smaller observational studies. Now that we have routine surveillance of surgical valves, I think it calls into question the class IIa recommendation for 3 months of anticoagulation. It’s what we’ve always done, and we’ll probably stop doing it on the basis of this. The other shoe that hasn’t dropped is its effect on long-term structural valve deterioration. I do think that early HALT does explain premature structural valve deterioration.”
The trial was sponsored by Edwards Lifesciences. Dr. Makkar disclosed that he is a consultant for and has received research grants from Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific. Dr. Mack is a consultant to Gore and an investigator for Abbott, Edwards Lifesciences, and Medtronic.
SAN FRANCISCO – The was 10% at 30 days and increased to 24% at 1 year, results from a PARTNER 3 substudy demonstrated.
However, the lack of a clear association with serious clinical events such as death, MI, and stroke “does not justify the routine prophylactic use of anticoagulation [following TAVR] in all patients,” lead study investigator Raj R. Makkar, MD, said during a press briefing at the Transcatheter Cardiovascular Therapeutics annual meeting.
“Subclinical leaflet thrombosis characterized by hypoattenuated leaflet thickening and reduced leaflet motion has been frequently observed in transcatheter and surgical aortic bioprosthetic valves,” said Dr. Makkar, director of the interventional cardiology division, Cedars-Sinai Medical Center, Los Angeles. “Thrombus on bioprosthetic valves can present as a spectrum: HALT with relatively normal leaflet motion, HALT with reduced leaflet motion but normal gradients, and clinical valve thrombosis with elevated gradients.”
The primary objective of the current Food and Drug Administration–mandated study, known as the PARTNER 3 Low-Risk Computed Tomography Sub-study, was to evaluate HALT and reduced leaflet motion in terms of differences in transcatheter and surgical bioprosthetic aortic valves among patients enrolled in the randomized PARTNER 3 cohort, to understand the natural history of HALT and reduced leaflet motion in the absence of anticoagulation, and to understand its impact on valve hemodynamics and clinical outcomes. Patients underwent specialized serial CTs at 30 days and at 1 year post TAVR or surgical aortic valve replacement (SAVR). All scans were analyzed by a CT core lab blinded to patient information or time of the scans, and the treating investigators were blinded to the results of the 30-day and 1-year CT scans. A clinical events committee adjudicated key clinical events.
Dr. Makkar reported outcomes from 408 patients: 213 who underwent TAVR and 195 who underwent surgery. There were 348 evaluable serial CT scans at 30 days and 312 at 1 year. The incidence of HALT at 30 days was 13.3% in the TAVR group and 5% in the surgery group, a difference that reached statistical significance (P = .03). At 1 year, however, the difference was not significant (27.5% vs. 20.2%, respectively; P = .19). In the overall cohort, he said, the incidence of HALT was 10% at 30 days and increased to 24% at 1 year.
The researchers also found that HALT was dynamic and spontaneously resolved in 56% of patients in the absence of anticoagulation at 30 days, while new HALT appeared in 21% of patients at 1 year.
“In terms of its impact on valve gradient, the impact was minimal,” Dr. Makkar said. “There was an increase of 1-2 mm Hg in patients who had HALT and in patients who had reduced leaflet motion.”
As for impact on clinical outcomes, the researchers observed no deaths or any myocardial infarction at any time point in patients who had HALT. “There were four cases of valve thrombosis, three of which occurred in patients who had HALT,” Dr. Makkar said at the meeting, sponsored by the Cardiovascular Research Foundation. “One stroke occurred in each group. TIA [transient ischemic attack] occurred in 1 patient out of 35 in the HALT group and 3 out of 311 in the no-HALT group. There was one case of retinal artery embolism in each group.”
In a pooled analysis of clinical events, he and his colleagues observed a numerical increase in death, stroke, TIA, and thrombotic events in patients who had HALT at 30 days, compared with those who did not (8.6% vs. 2.9%, respectively), but the difference did not reach statistical significance (P = .11). “However, given the low total number of events, the data are inconclusive and only hypothesis generating,” he said. “A longer-term follow-up and [a] larger data set will further clarify the impact on clinical outcomes.”
Dr. Makkar emphasized that routine post–TAVR/SAVR CT scans outside of research protocols are not indicated. “CTs should be prompted by increased gradients or thromboembolic events,” he said.
One of the discussants at the briefing, Michael J. Mack, MD, chair of the cardiovascular service line at Baylor Scott and White Health in Dallas and primary author of the PARTNER 3 study, said that prior to the substudy results, “I’ve always thought that the incidence of valve thrombosis would be higher with TAVR than with surgery. So the fact that it was higher at 30 days didn’t surprise me. One of the reasons is that you lose the backwashing effect by changing flow dynamics in the aortic route. What did surprise me is the percent that resolved without anticoagulation.”
He added, “The impact of all this is that we are not justified recommending routine anticoagulation [after bioprosthetic aortic valve replacement surgery]. I think it does call into question the guidelines for surgical valves, because we did that based on smaller observational studies. Now that we have routine surveillance of surgical valves, I think it calls into question the class IIa recommendation for 3 months of anticoagulation. It’s what we’ve always done, and we’ll probably stop doing it on the basis of this. The other shoe that hasn’t dropped is its effect on long-term structural valve deterioration. I do think that early HALT does explain premature structural valve deterioration.”
The trial was sponsored by Edwards Lifesciences. Dr. Makkar disclosed that he is a consultant for and has received research grants from Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific. Dr. Mack is a consultant to Gore and an investigator for Abbott, Edwards Lifesciences, and Medtronic.
SAN FRANCISCO – The was 10% at 30 days and increased to 24% at 1 year, results from a PARTNER 3 substudy demonstrated.
However, the lack of a clear association with serious clinical events such as death, MI, and stroke “does not justify the routine prophylactic use of anticoagulation [following TAVR] in all patients,” lead study investigator Raj R. Makkar, MD, said during a press briefing at the Transcatheter Cardiovascular Therapeutics annual meeting.
“Subclinical leaflet thrombosis characterized by hypoattenuated leaflet thickening and reduced leaflet motion has been frequently observed in transcatheter and surgical aortic bioprosthetic valves,” said Dr. Makkar, director of the interventional cardiology division, Cedars-Sinai Medical Center, Los Angeles. “Thrombus on bioprosthetic valves can present as a spectrum: HALT with relatively normal leaflet motion, HALT with reduced leaflet motion but normal gradients, and clinical valve thrombosis with elevated gradients.”
The primary objective of the current Food and Drug Administration–mandated study, known as the PARTNER 3 Low-Risk Computed Tomography Sub-study, was to evaluate HALT and reduced leaflet motion in terms of differences in transcatheter and surgical bioprosthetic aortic valves among patients enrolled in the randomized PARTNER 3 cohort, to understand the natural history of HALT and reduced leaflet motion in the absence of anticoagulation, and to understand its impact on valve hemodynamics and clinical outcomes. Patients underwent specialized serial CTs at 30 days and at 1 year post TAVR or surgical aortic valve replacement (SAVR). All scans were analyzed by a CT core lab blinded to patient information or time of the scans, and the treating investigators were blinded to the results of the 30-day and 1-year CT scans. A clinical events committee adjudicated key clinical events.
Dr. Makkar reported outcomes from 408 patients: 213 who underwent TAVR and 195 who underwent surgery. There were 348 evaluable serial CT scans at 30 days and 312 at 1 year. The incidence of HALT at 30 days was 13.3% in the TAVR group and 5% in the surgery group, a difference that reached statistical significance (P = .03). At 1 year, however, the difference was not significant (27.5% vs. 20.2%, respectively; P = .19). In the overall cohort, he said, the incidence of HALT was 10% at 30 days and increased to 24% at 1 year.
The researchers also found that HALT was dynamic and spontaneously resolved in 56% of patients in the absence of anticoagulation at 30 days, while new HALT appeared in 21% of patients at 1 year.
“In terms of its impact on valve gradient, the impact was minimal,” Dr. Makkar said. “There was an increase of 1-2 mm Hg in patients who had HALT and in patients who had reduced leaflet motion.”
As for impact on clinical outcomes, the researchers observed no deaths or any myocardial infarction at any time point in patients who had HALT. “There were four cases of valve thrombosis, three of which occurred in patients who had HALT,” Dr. Makkar said at the meeting, sponsored by the Cardiovascular Research Foundation. “One stroke occurred in each group. TIA [transient ischemic attack] occurred in 1 patient out of 35 in the HALT group and 3 out of 311 in the no-HALT group. There was one case of retinal artery embolism in each group.”
In a pooled analysis of clinical events, he and his colleagues observed a numerical increase in death, stroke, TIA, and thrombotic events in patients who had HALT at 30 days, compared with those who did not (8.6% vs. 2.9%, respectively), but the difference did not reach statistical significance (P = .11). “However, given the low total number of events, the data are inconclusive and only hypothesis generating,” he said. “A longer-term follow-up and [a] larger data set will further clarify the impact on clinical outcomes.”
Dr. Makkar emphasized that routine post–TAVR/SAVR CT scans outside of research protocols are not indicated. “CTs should be prompted by increased gradients or thromboembolic events,” he said.
One of the discussants at the briefing, Michael J. Mack, MD, chair of the cardiovascular service line at Baylor Scott and White Health in Dallas and primary author of the PARTNER 3 study, said that prior to the substudy results, “I’ve always thought that the incidence of valve thrombosis would be higher with TAVR than with surgery. So the fact that it was higher at 30 days didn’t surprise me. One of the reasons is that you lose the backwashing effect by changing flow dynamics in the aortic route. What did surprise me is the percent that resolved without anticoagulation.”
He added, “The impact of all this is that we are not justified recommending routine anticoagulation [after bioprosthetic aortic valve replacement surgery]. I think it does call into question the guidelines for surgical valves, because we did that based on smaller observational studies. Now that we have routine surveillance of surgical valves, I think it calls into question the class IIa recommendation for 3 months of anticoagulation. It’s what we’ve always done, and we’ll probably stop doing it on the basis of this. The other shoe that hasn’t dropped is its effect on long-term structural valve deterioration. I do think that early HALT does explain premature structural valve deterioration.”
The trial was sponsored by Edwards Lifesciences. Dr. Makkar disclosed that he is a consultant for and has received research grants from Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific. Dr. Mack is a consultant to Gore and an investigator for Abbott, Edwards Lifesciences, and Medtronic.
REPORTING FROM TCT 2019
Key clinical point: Hypo-attenuated leaflet thickening (HALT) and reduced leaflet motion resulted in a minimal increase in valve gradients, which can be considered clinically insignificant.
Major finding: The incidence of HALT at 30 days was 13.3% in the TAVR group and 5% in the surgery group, a difference that reached statistical significance (P = .03). At 1 year, however, the difference did not differ significantly (27.5% vs. 20.2%, respectively; P = .19).
Study details: An analysis of 408 patients in the PARTNER 3 Low-Risk Computed Tomography Sub-study.
Disclosures: The trial was sponsored by Edwards Lifesciences. Dr. Makkar disclosed that he is a consultant for and has received research grants from Edwards Lifesciences, Abbott, Medtronic, and Boston Scientific. Dr. Mack is a consultant to Gore and an investigator for Abbott, Edwards Lifesciences, and Medtronic.
Source: Makkar R et al. TCT 2019.
European postmarket trial confirms findings of Disrupt CAD I
SAN FRANCISCO – A postmarket analysis of the coronary intravascular lithotripsy (IVL) system validated the safety and utility of the procedure first described in the Disrupt CAD I study.
“We now have an efficient technology to treat calcium that is safe to use, simple to learn, and able to achieve 100% success in delivering stents, with low final residual stenosis (7.8%),” Carlo Di Mario, MD, said in an interview in advance of the Transcatheter Cardiovascular Therapeutics annual meeting. “This was accomplished with a low rate of complications.”
Developed by Shockwave Medical, coronary IVL is an innovative lesion preparation tool designed to fracture challenging calcium using sonic pressure waves in order to facilitate stent delivery, deployment, and optimal expansion. In a feasibility study known as DISRUPT CAD I, Dr. Di Mario, director of structural interventional cardiology at Careggi University Hospital in Florence, Italy, and his colleagues performed the procedure in 60 patients (Circulation 2019;139:834-6). Clinical success, defined as residual stenosis of less than 50% post PCI with no evidence of in-hospital major adverse cardiac events (MACE), was 95%, while device success, defined as successful delivery and IVL treatment at target lesion, reached 98.3%.
In an effort to ensure that results of DISRUPT CAD 1 were generalizable to a broader population, Dr. Di Mario and his colleagues enrolled 120 subjects at 15 sites in nine European countries into DISRUPT CAD II, a postmarket, single-arm study. They underwent vessel preparation for stent implantation with IVL, and the primary endpoint was in-hospital MACE, defined as cardiac death, myocardial infarction, or target vessel revascularization. The researchers also performed an optical coherence tomography (OCT) substudy to evaluate the mechanism of action of IVL and to quantify coronary artery calcium characteristics and calcium plaque fracture.
The mean age of the 120 patients was 72 years, 78% were male, 80% had hypertension, 72% had hyperlipidemia, 32% had diabetes, and 65% had class I or II angina. Most of the patients (94%) had severe calcification, with a mean lesion length of about 26 mm. The lesions were concentric in 70% of cases, and 30% had side-branch involvement.
Dr. Di Mario and his associates reported that IVL was delivered successfully in all cases. It also delivered stents successfully in all cases, with a high acute luminal gain (a mean of 1.7 mm2), and low residual stenosis (7.8%). The primary endpoint occurred in 5.8% of patients, consisting of seven non–Q-wave myocardial infarctions. The IVL mechanism of action was shown to be intraplaque calcium fracture, which occurred in about 80% of lesions analyzed by OCT.
“Based on my previous experience with IVL, I was confident that it could modify the calcium, but the results of the OCT substudy of the Disrupt CAD II utilizing OCT to evaluate the mechanism of action was clearly more positive than expected,” said Dr. Di Mario, who was a coprincipal investigator for the trial. “It demonstrated that IVL creates visible calcium fractures in the majority of cases and confirmed that full stent expansion secondary to the circumferential calcium modification is achievable, despite that nearly all the patients (94%) had severe coronary artery calcification. We know from previous work that full stent expansion is required to minimize complications and improve clinical outcomes, which was not always achievable before IVL.”
He acknowledged certain limitations of the study, including the fact that it lacked a concurrent control group, “but it was run very carefully with complete monitoring of events and core lab and CEC [clinical endpoint committee] adjudication,” he said. “Also, the study was used to confirm short-term safety, and as such, did not include long term follow-up.”
In an interview at the meeting, Ajay J. Kirtane, MD, an interventional cardiologist at Columbia University Medical Center, New York, called the findings “reassuring,” but said that he looks forward to results from the trial of the system currently under way in the United States known as the DISRUPT CAD III IDE Study. “The technology is accessible to many physicians because it’s a balloon-based technology,” he said. “Yet in terms of performance, we need to not only evaluate short-term outcomes, we need to see long-term outcomes as well, to make sure there are no untoward effects.”
Shockwave C2 Coronary IVL catheters are commercially available for the treatment of de novo coronary artery disease in Europe and other select countries; in the United States they are limited to investigational use within the DISRUPT CAD III IDE Study.
At the meeting, Ziad A. Ali, MD, an interventional cardiologist at Columbia University/New York–Presbyterian Hospital, presented results from Disrupt CAD II, and the content was published online at the time of presentation. The meeting was sponsored by the Cardiovascular Research Foundation. Dr. Di Mario disclosed that Shockwave Medical provided a grant for the study to Careggi University Hospital. Dr. Ali reported having personal equity and fees from Shockwave Medical, as well as grants from other companies outside the scope of the study.
SOURCE: Di Mario C et al. Circ Cardiovasc Interv. 2019 Sep 25 doi: 10.1161/CIRCINTERVENTIONS.119.008434.
SAN FRANCISCO – A postmarket analysis of the coronary intravascular lithotripsy (IVL) system validated the safety and utility of the procedure first described in the Disrupt CAD I study.
“We now have an efficient technology to treat calcium that is safe to use, simple to learn, and able to achieve 100% success in delivering stents, with low final residual stenosis (7.8%),” Carlo Di Mario, MD, said in an interview in advance of the Transcatheter Cardiovascular Therapeutics annual meeting. “This was accomplished with a low rate of complications.”
Developed by Shockwave Medical, coronary IVL is an innovative lesion preparation tool designed to fracture challenging calcium using sonic pressure waves in order to facilitate stent delivery, deployment, and optimal expansion. In a feasibility study known as DISRUPT CAD I, Dr. Di Mario, director of structural interventional cardiology at Careggi University Hospital in Florence, Italy, and his colleagues performed the procedure in 60 patients (Circulation 2019;139:834-6). Clinical success, defined as residual stenosis of less than 50% post PCI with no evidence of in-hospital major adverse cardiac events (MACE), was 95%, while device success, defined as successful delivery and IVL treatment at target lesion, reached 98.3%.
In an effort to ensure that results of DISRUPT CAD 1 were generalizable to a broader population, Dr. Di Mario and his colleagues enrolled 120 subjects at 15 sites in nine European countries into DISRUPT CAD II, a postmarket, single-arm study. They underwent vessel preparation for stent implantation with IVL, and the primary endpoint was in-hospital MACE, defined as cardiac death, myocardial infarction, or target vessel revascularization. The researchers also performed an optical coherence tomography (OCT) substudy to evaluate the mechanism of action of IVL and to quantify coronary artery calcium characteristics and calcium plaque fracture.
The mean age of the 120 patients was 72 years, 78% were male, 80% had hypertension, 72% had hyperlipidemia, 32% had diabetes, and 65% had class I or II angina. Most of the patients (94%) had severe calcification, with a mean lesion length of about 26 mm. The lesions were concentric in 70% of cases, and 30% had side-branch involvement.
Dr. Di Mario and his associates reported that IVL was delivered successfully in all cases. It also delivered stents successfully in all cases, with a high acute luminal gain (a mean of 1.7 mm2), and low residual stenosis (7.8%). The primary endpoint occurred in 5.8% of patients, consisting of seven non–Q-wave myocardial infarctions. The IVL mechanism of action was shown to be intraplaque calcium fracture, which occurred in about 80% of lesions analyzed by OCT.
“Based on my previous experience with IVL, I was confident that it could modify the calcium, but the results of the OCT substudy of the Disrupt CAD II utilizing OCT to evaluate the mechanism of action was clearly more positive than expected,” said Dr. Di Mario, who was a coprincipal investigator for the trial. “It demonstrated that IVL creates visible calcium fractures in the majority of cases and confirmed that full stent expansion secondary to the circumferential calcium modification is achievable, despite that nearly all the patients (94%) had severe coronary artery calcification. We know from previous work that full stent expansion is required to minimize complications and improve clinical outcomes, which was not always achievable before IVL.”
He acknowledged certain limitations of the study, including the fact that it lacked a concurrent control group, “but it was run very carefully with complete monitoring of events and core lab and CEC [clinical endpoint committee] adjudication,” he said. “Also, the study was used to confirm short-term safety, and as such, did not include long term follow-up.”
In an interview at the meeting, Ajay J. Kirtane, MD, an interventional cardiologist at Columbia University Medical Center, New York, called the findings “reassuring,” but said that he looks forward to results from the trial of the system currently under way in the United States known as the DISRUPT CAD III IDE Study. “The technology is accessible to many physicians because it’s a balloon-based technology,” he said. “Yet in terms of performance, we need to not only evaluate short-term outcomes, we need to see long-term outcomes as well, to make sure there are no untoward effects.”
Shockwave C2 Coronary IVL catheters are commercially available for the treatment of de novo coronary artery disease in Europe and other select countries; in the United States they are limited to investigational use within the DISRUPT CAD III IDE Study.
At the meeting, Ziad A. Ali, MD, an interventional cardiologist at Columbia University/New York–Presbyterian Hospital, presented results from Disrupt CAD II, and the content was published online at the time of presentation. The meeting was sponsored by the Cardiovascular Research Foundation. Dr. Di Mario disclosed that Shockwave Medical provided a grant for the study to Careggi University Hospital. Dr. Ali reported having personal equity and fees from Shockwave Medical, as well as grants from other companies outside the scope of the study.
SOURCE: Di Mario C et al. Circ Cardiovasc Interv. 2019 Sep 25 doi: 10.1161/CIRCINTERVENTIONS.119.008434.
SAN FRANCISCO – A postmarket analysis of the coronary intravascular lithotripsy (IVL) system validated the safety and utility of the procedure first described in the Disrupt CAD I study.
“We now have an efficient technology to treat calcium that is safe to use, simple to learn, and able to achieve 100% success in delivering stents, with low final residual stenosis (7.8%),” Carlo Di Mario, MD, said in an interview in advance of the Transcatheter Cardiovascular Therapeutics annual meeting. “This was accomplished with a low rate of complications.”
Developed by Shockwave Medical, coronary IVL is an innovative lesion preparation tool designed to fracture challenging calcium using sonic pressure waves in order to facilitate stent delivery, deployment, and optimal expansion. In a feasibility study known as DISRUPT CAD I, Dr. Di Mario, director of structural interventional cardiology at Careggi University Hospital in Florence, Italy, and his colleagues performed the procedure in 60 patients (Circulation 2019;139:834-6). Clinical success, defined as residual stenosis of less than 50% post PCI with no evidence of in-hospital major adverse cardiac events (MACE), was 95%, while device success, defined as successful delivery and IVL treatment at target lesion, reached 98.3%.
In an effort to ensure that results of DISRUPT CAD 1 were generalizable to a broader population, Dr. Di Mario and his colleagues enrolled 120 subjects at 15 sites in nine European countries into DISRUPT CAD II, a postmarket, single-arm study. They underwent vessel preparation for stent implantation with IVL, and the primary endpoint was in-hospital MACE, defined as cardiac death, myocardial infarction, or target vessel revascularization. The researchers also performed an optical coherence tomography (OCT) substudy to evaluate the mechanism of action of IVL and to quantify coronary artery calcium characteristics and calcium plaque fracture.
The mean age of the 120 patients was 72 years, 78% were male, 80% had hypertension, 72% had hyperlipidemia, 32% had diabetes, and 65% had class I or II angina. Most of the patients (94%) had severe calcification, with a mean lesion length of about 26 mm. The lesions were concentric in 70% of cases, and 30% had side-branch involvement.
Dr. Di Mario and his associates reported that IVL was delivered successfully in all cases. It also delivered stents successfully in all cases, with a high acute luminal gain (a mean of 1.7 mm2), and low residual stenosis (7.8%). The primary endpoint occurred in 5.8% of patients, consisting of seven non–Q-wave myocardial infarctions. The IVL mechanism of action was shown to be intraplaque calcium fracture, which occurred in about 80% of lesions analyzed by OCT.
“Based on my previous experience with IVL, I was confident that it could modify the calcium, but the results of the OCT substudy of the Disrupt CAD II utilizing OCT to evaluate the mechanism of action was clearly more positive than expected,” said Dr. Di Mario, who was a coprincipal investigator for the trial. “It demonstrated that IVL creates visible calcium fractures in the majority of cases and confirmed that full stent expansion secondary to the circumferential calcium modification is achievable, despite that nearly all the patients (94%) had severe coronary artery calcification. We know from previous work that full stent expansion is required to minimize complications and improve clinical outcomes, which was not always achievable before IVL.”
He acknowledged certain limitations of the study, including the fact that it lacked a concurrent control group, “but it was run very carefully with complete monitoring of events and core lab and CEC [clinical endpoint committee] adjudication,” he said. “Also, the study was used to confirm short-term safety, and as such, did not include long term follow-up.”
In an interview at the meeting, Ajay J. Kirtane, MD, an interventional cardiologist at Columbia University Medical Center, New York, called the findings “reassuring,” but said that he looks forward to results from the trial of the system currently under way in the United States known as the DISRUPT CAD III IDE Study. “The technology is accessible to many physicians because it’s a balloon-based technology,” he said. “Yet in terms of performance, we need to not only evaluate short-term outcomes, we need to see long-term outcomes as well, to make sure there are no untoward effects.”
Shockwave C2 Coronary IVL catheters are commercially available for the treatment of de novo coronary artery disease in Europe and other select countries; in the United States they are limited to investigational use within the DISRUPT CAD III IDE Study.
At the meeting, Ziad A. Ali, MD, an interventional cardiologist at Columbia University/New York–Presbyterian Hospital, presented results from Disrupt CAD II, and the content was published online at the time of presentation. The meeting was sponsored by the Cardiovascular Research Foundation. Dr. Di Mario disclosed that Shockwave Medical provided a grant for the study to Careggi University Hospital. Dr. Ali reported having personal equity and fees from Shockwave Medical, as well as grants from other companies outside the scope of the study.
SOURCE: Di Mario C et al. Circ Cardiovasc Interv. 2019 Sep 25 doi: 10.1161/CIRCINTERVENTIONS.119.008434.
REPORTING FROM TCT 2019
BAROCCO study: Cediranib-olaparib combination shows promise in PROC
BARCELONA – (Best Approach in Recurrent Ovarian Cancer With Cediranib-Olaparib) study.
The continuous treatment approach was also associated with a trend toward improved progression-free survival (PFS) versus weekly paclitaxel in heavily pretreated patients with PROC, and this was particularly true in patients with germline BRCA wild-type (gBRCAwt), with a hazard ratio for PFS of 0.63, Nicolleta Colombo, MD, reported at the European Society of Medical Oncology Congress.
Median investigator-assessed PFS was 3.1, 5.7, and 3.8 months, respectively, in 123 patients with PROC and any gBRCA status who were enrolled from multiple centers in Italy and randomized 1:1:1 to receive 80 mg/m2 of weekly paclitaxel for up to 24 weeks, continuous combination therapy with 20 mg of cediranib daily and 300 mg of olaparib twice daily, or intermittent dosing with 20 mg of cediranib given 5 days per week until progression, said Dr. Colombo, director of gynecologic cancer medical treatments at Istituto Europeo di Oncologia, Milan.
The hazard ratios for PFS with continuous therapy and intermittent therapy versus paclitaxel were 0.76 and 1.08, respectively, but the former comparison was not proportional and the difference in the area under the PFS curves was assessed and found to be 1.25 months in favor of continuous therapy with cediranib and olaparib, she explained.
“Surprisingly, the intermittent regimen did not perform as well,” she noted.
Subgroup analyses showed that in patients with gBRCAwt or unknown BRCA status, median PFS was 2.1, 5.8, and 3.8 months in the paclitaxel, continuous, and intermittent groups, respectively, demonstrating a trend toward greater benefit with continuous combination therapy versus the other arms (HR, 0.63; P = .13). The area under the PFS curves showed a difference of 1.32 months in favor of continuous therapy, she said.
Similarly, a trend toward greater benefit was seen in patients who had received up to two prior lines of therapy (HR, 0.47; P = .28).
As for the secondary study endpoint of response rates, complete responses occurred in 2 of 24 evaluable paclitaxel patients, and none of 39 and 35 patients in the continuous and intermittent groups. Partial responses occurred in 6, 7, and 4 patients in the groups, respectively; stable disease occurred in 5, 26, and 18, respectively; and progressive disease occurred in 11, 6, and 13 patients, respectively.
“The [rate of] progression was much higher with paclitaxel than in the continuous and intermittent arms,” Dr. Colombo said. “So the clinical benefit was very good; it was 84.6% for the continuous arm, and 62.8% for the intermittent arm.”
Clinical benefit in the paclitaxel arm was 54.1%, and duration of response was 4.4, 6.2, and 2.7 months in the arms, respectively.
The findings are of note, as the BAROCCO study includes a difficult-to-treat population with a high unmet need, she said, explaining that 59% of patients had received three or more prior lines of therapy, and the median platinum-free interval was only 1.8 months.
“Median progression-free survival for these patients is only about 3-4 months, even after weekly paclitaxel, which is recognized as the most effective chemotherapy regimen in this patient population,” she said, noting that new therapeutic options in this setting are of great clinical interest.
Additionally, BAROCCO is the first trial of combined cediranib-olaparib in PROC to include a control arm, she noted.
Single-agent olaparib was approved by the Food and Drug Administration in 2017 for the treatment of patients with germline BRCA mutated (gBRCAm) relapsed ovarian cancer after 3 or more lines of chemotherapy, but the efficacy of the poly adenosine diphosphate–ribose polymerase (PARP) inhibitor in gBRCAwt PROC is limited.
Findings from the CLIO trial presented in June at American Society of Clinical Oncology annual meeting showed an overall response rate (ORR) of 13% and PFS of 2.9 months in gBRCAwt patients with PROC treated with olaparib, and in the QUADRA study, the ORR in gBRCAwt PROC was just 3%
“On the other hand, olaparib activity was observed beyond BRCA-mutated tumors in the platinum-sensitive relapsed ovarian cancer patients, and was increased when combined with an antiangiogenic agent,” Dr. Colombo said. “In fact, both the trials with cediranib and olaparib, and with bevacizumab and niraparib, showed an improvement in progression-free survival, compared with a single agent.
“So there is a strong rationale for the combination and the synergistic effect of cediranib and olaparib, because molecular pharmacologic studies suggest that cediranib induces downregulation of some genes that are involved in homologous recombination, thus producing a sort of functional ‘BRCAness’ that favors the selective activity of the PARP inhibitors,” she explained.
Preclinical evidence suggests this may be related to the antiangiogenic effect of cediranib, or possibly to the inhibition of the platinum-derived growth factor signaling by cediranib.
BAROCCO was designed to assess whether the combination would provide superior PFS, compared with weekly paclitaxel, in the platinum-resistant population, and if an intermittent schedule might improve gastrointestinal tolerability, as treatment has been associated with severe diarrhea in previous trials, she said.
However, the toxicity profile of the study arms was as expected, and similar between experimental arms, with 11%, 18%, and 7% of patients in the paclitaxel, continuous, and intermittent arms discontinuing treatment because of adverse events, she noted.
Five serious adverse drug reactions occurred and two were fatal, including one in the control arm and one in the continuous arm.
Although not statistically significant, the continuous administration regimen was well tolerated, and showed “a promising trend for improved PFS,” particularly in gBRCAwt patients.
Notably, only 5% of patients in that arm experienced severe diarrhea, Dr. Colombo said, adding that “the continuous administration of cediranib and olaparib is active in PROC patients, with clinical benefit observed in 85% of cases.”
“We believe that [this combination] represents an active, feasible oral regimen, which deserves further investigation, and these results support ongoing trials investigating the same combination in platinum-resistant ovarian cancer,” she concluded.
Invited discussant Antonio Gonzalez Martin, MD, said that some of the drug-related adverse events reported in the study – which occurred in 70%-78% of patients in the three arms and included diarrhea, nausea, fatigue, and others – could have an impact on quality of life.
“It’s something that we need to study; patient-reported outcomes should be integrated in these types of trials for this very important population,” said Dr. Gonzalez-Martin, head of medical oncology at Clinica Universidad de Navarra, Madrid.
He added that the future of antiangiogenic and PARP inhibitor combinations remains to be defined in the first-line, platinum-sensitive, and platinum-resistant ovarian cancer settings.
The BAROCCO Trial was funded by AstraZeneca. Dr. Colombo has received honoraria from or been an advisor or consultant to Roche/Genentech, PharmaMar; AstraZeneca, Tesaro, Clovis Oncology, Pfizer, MSD Oncology, BioCad, and Takeda. Dr. Gonzalez-Martin has received fees, honoraria, and/or grants from Roche, AstraZeneca, Tesaro, GSK, Clovis, MSD, Pfizer, Novartis, PharmaMar, and Imugene.
SOURCE: Colombo N et al. ESMO 2019, Abstract LBA58. Ann Oncol. 2019;30:suppl 5.
BARCELONA – (Best Approach in Recurrent Ovarian Cancer With Cediranib-Olaparib) study.
The continuous treatment approach was also associated with a trend toward improved progression-free survival (PFS) versus weekly paclitaxel in heavily pretreated patients with PROC, and this was particularly true in patients with germline BRCA wild-type (gBRCAwt), with a hazard ratio for PFS of 0.63, Nicolleta Colombo, MD, reported at the European Society of Medical Oncology Congress.
Median investigator-assessed PFS was 3.1, 5.7, and 3.8 months, respectively, in 123 patients with PROC and any gBRCA status who were enrolled from multiple centers in Italy and randomized 1:1:1 to receive 80 mg/m2 of weekly paclitaxel for up to 24 weeks, continuous combination therapy with 20 mg of cediranib daily and 300 mg of olaparib twice daily, or intermittent dosing with 20 mg of cediranib given 5 days per week until progression, said Dr. Colombo, director of gynecologic cancer medical treatments at Istituto Europeo di Oncologia, Milan.
The hazard ratios for PFS with continuous therapy and intermittent therapy versus paclitaxel were 0.76 and 1.08, respectively, but the former comparison was not proportional and the difference in the area under the PFS curves was assessed and found to be 1.25 months in favor of continuous therapy with cediranib and olaparib, she explained.
“Surprisingly, the intermittent regimen did not perform as well,” she noted.
Subgroup analyses showed that in patients with gBRCAwt or unknown BRCA status, median PFS was 2.1, 5.8, and 3.8 months in the paclitaxel, continuous, and intermittent groups, respectively, demonstrating a trend toward greater benefit with continuous combination therapy versus the other arms (HR, 0.63; P = .13). The area under the PFS curves showed a difference of 1.32 months in favor of continuous therapy, she said.
Similarly, a trend toward greater benefit was seen in patients who had received up to two prior lines of therapy (HR, 0.47; P = .28).
As for the secondary study endpoint of response rates, complete responses occurred in 2 of 24 evaluable paclitaxel patients, and none of 39 and 35 patients in the continuous and intermittent groups. Partial responses occurred in 6, 7, and 4 patients in the groups, respectively; stable disease occurred in 5, 26, and 18, respectively; and progressive disease occurred in 11, 6, and 13 patients, respectively.
“The [rate of] progression was much higher with paclitaxel than in the continuous and intermittent arms,” Dr. Colombo said. “So the clinical benefit was very good; it was 84.6% for the continuous arm, and 62.8% for the intermittent arm.”
Clinical benefit in the paclitaxel arm was 54.1%, and duration of response was 4.4, 6.2, and 2.7 months in the arms, respectively.
The findings are of note, as the BAROCCO study includes a difficult-to-treat population with a high unmet need, she said, explaining that 59% of patients had received three or more prior lines of therapy, and the median platinum-free interval was only 1.8 months.
“Median progression-free survival for these patients is only about 3-4 months, even after weekly paclitaxel, which is recognized as the most effective chemotherapy regimen in this patient population,” she said, noting that new therapeutic options in this setting are of great clinical interest.
Additionally, BAROCCO is the first trial of combined cediranib-olaparib in PROC to include a control arm, she noted.
Single-agent olaparib was approved by the Food and Drug Administration in 2017 for the treatment of patients with germline BRCA mutated (gBRCAm) relapsed ovarian cancer after 3 or more lines of chemotherapy, but the efficacy of the poly adenosine diphosphate–ribose polymerase (PARP) inhibitor in gBRCAwt PROC is limited.
Findings from the CLIO trial presented in June at American Society of Clinical Oncology annual meeting showed an overall response rate (ORR) of 13% and PFS of 2.9 months in gBRCAwt patients with PROC treated with olaparib, and in the QUADRA study, the ORR in gBRCAwt PROC was just 3%
“On the other hand, olaparib activity was observed beyond BRCA-mutated tumors in the platinum-sensitive relapsed ovarian cancer patients, and was increased when combined with an antiangiogenic agent,” Dr. Colombo said. “In fact, both the trials with cediranib and olaparib, and with bevacizumab and niraparib, showed an improvement in progression-free survival, compared with a single agent.
“So there is a strong rationale for the combination and the synergistic effect of cediranib and olaparib, because molecular pharmacologic studies suggest that cediranib induces downregulation of some genes that are involved in homologous recombination, thus producing a sort of functional ‘BRCAness’ that favors the selective activity of the PARP inhibitors,” she explained.
Preclinical evidence suggests this may be related to the antiangiogenic effect of cediranib, or possibly to the inhibition of the platinum-derived growth factor signaling by cediranib.
BAROCCO was designed to assess whether the combination would provide superior PFS, compared with weekly paclitaxel, in the platinum-resistant population, and if an intermittent schedule might improve gastrointestinal tolerability, as treatment has been associated with severe diarrhea in previous trials, she said.
However, the toxicity profile of the study arms was as expected, and similar between experimental arms, with 11%, 18%, and 7% of patients in the paclitaxel, continuous, and intermittent arms discontinuing treatment because of adverse events, she noted.
Five serious adverse drug reactions occurred and two were fatal, including one in the control arm and one in the continuous arm.
Although not statistically significant, the continuous administration regimen was well tolerated, and showed “a promising trend for improved PFS,” particularly in gBRCAwt patients.
Notably, only 5% of patients in that arm experienced severe diarrhea, Dr. Colombo said, adding that “the continuous administration of cediranib and olaparib is active in PROC patients, with clinical benefit observed in 85% of cases.”
“We believe that [this combination] represents an active, feasible oral regimen, which deserves further investigation, and these results support ongoing trials investigating the same combination in platinum-resistant ovarian cancer,” she concluded.
Invited discussant Antonio Gonzalez Martin, MD, said that some of the drug-related adverse events reported in the study – which occurred in 70%-78% of patients in the three arms and included diarrhea, nausea, fatigue, and others – could have an impact on quality of life.
“It’s something that we need to study; patient-reported outcomes should be integrated in these types of trials for this very important population,” said Dr. Gonzalez-Martin, head of medical oncology at Clinica Universidad de Navarra, Madrid.
He added that the future of antiangiogenic and PARP inhibitor combinations remains to be defined in the first-line, platinum-sensitive, and platinum-resistant ovarian cancer settings.
The BAROCCO Trial was funded by AstraZeneca. Dr. Colombo has received honoraria from or been an advisor or consultant to Roche/Genentech, PharmaMar; AstraZeneca, Tesaro, Clovis Oncology, Pfizer, MSD Oncology, BioCad, and Takeda. Dr. Gonzalez-Martin has received fees, honoraria, and/or grants from Roche, AstraZeneca, Tesaro, GSK, Clovis, MSD, Pfizer, Novartis, PharmaMar, and Imugene.
SOURCE: Colombo N et al. ESMO 2019, Abstract LBA58. Ann Oncol. 2019;30:suppl 5.
BARCELONA – (Best Approach in Recurrent Ovarian Cancer With Cediranib-Olaparib) study.
The continuous treatment approach was also associated with a trend toward improved progression-free survival (PFS) versus weekly paclitaxel in heavily pretreated patients with PROC, and this was particularly true in patients with germline BRCA wild-type (gBRCAwt), with a hazard ratio for PFS of 0.63, Nicolleta Colombo, MD, reported at the European Society of Medical Oncology Congress.
Median investigator-assessed PFS was 3.1, 5.7, and 3.8 months, respectively, in 123 patients with PROC and any gBRCA status who were enrolled from multiple centers in Italy and randomized 1:1:1 to receive 80 mg/m2 of weekly paclitaxel for up to 24 weeks, continuous combination therapy with 20 mg of cediranib daily and 300 mg of olaparib twice daily, or intermittent dosing with 20 mg of cediranib given 5 days per week until progression, said Dr. Colombo, director of gynecologic cancer medical treatments at Istituto Europeo di Oncologia, Milan.
The hazard ratios for PFS with continuous therapy and intermittent therapy versus paclitaxel were 0.76 and 1.08, respectively, but the former comparison was not proportional and the difference in the area under the PFS curves was assessed and found to be 1.25 months in favor of continuous therapy with cediranib and olaparib, she explained.
“Surprisingly, the intermittent regimen did not perform as well,” she noted.
Subgroup analyses showed that in patients with gBRCAwt or unknown BRCA status, median PFS was 2.1, 5.8, and 3.8 months in the paclitaxel, continuous, and intermittent groups, respectively, demonstrating a trend toward greater benefit with continuous combination therapy versus the other arms (HR, 0.63; P = .13). The area under the PFS curves showed a difference of 1.32 months in favor of continuous therapy, she said.
Similarly, a trend toward greater benefit was seen in patients who had received up to two prior lines of therapy (HR, 0.47; P = .28).
As for the secondary study endpoint of response rates, complete responses occurred in 2 of 24 evaluable paclitaxel patients, and none of 39 and 35 patients in the continuous and intermittent groups. Partial responses occurred in 6, 7, and 4 patients in the groups, respectively; stable disease occurred in 5, 26, and 18, respectively; and progressive disease occurred in 11, 6, and 13 patients, respectively.
“The [rate of] progression was much higher with paclitaxel than in the continuous and intermittent arms,” Dr. Colombo said. “So the clinical benefit was very good; it was 84.6% for the continuous arm, and 62.8% for the intermittent arm.”
Clinical benefit in the paclitaxel arm was 54.1%, and duration of response was 4.4, 6.2, and 2.7 months in the arms, respectively.
The findings are of note, as the BAROCCO study includes a difficult-to-treat population with a high unmet need, she said, explaining that 59% of patients had received three or more prior lines of therapy, and the median platinum-free interval was only 1.8 months.
“Median progression-free survival for these patients is only about 3-4 months, even after weekly paclitaxel, which is recognized as the most effective chemotherapy regimen in this patient population,” she said, noting that new therapeutic options in this setting are of great clinical interest.
Additionally, BAROCCO is the first trial of combined cediranib-olaparib in PROC to include a control arm, she noted.
Single-agent olaparib was approved by the Food and Drug Administration in 2017 for the treatment of patients with germline BRCA mutated (gBRCAm) relapsed ovarian cancer after 3 or more lines of chemotherapy, but the efficacy of the poly adenosine diphosphate–ribose polymerase (PARP) inhibitor in gBRCAwt PROC is limited.
Findings from the CLIO trial presented in June at American Society of Clinical Oncology annual meeting showed an overall response rate (ORR) of 13% and PFS of 2.9 months in gBRCAwt patients with PROC treated with olaparib, and in the QUADRA study, the ORR in gBRCAwt PROC was just 3%
“On the other hand, olaparib activity was observed beyond BRCA-mutated tumors in the platinum-sensitive relapsed ovarian cancer patients, and was increased when combined with an antiangiogenic agent,” Dr. Colombo said. “In fact, both the trials with cediranib and olaparib, and with bevacizumab and niraparib, showed an improvement in progression-free survival, compared with a single agent.
“So there is a strong rationale for the combination and the synergistic effect of cediranib and olaparib, because molecular pharmacologic studies suggest that cediranib induces downregulation of some genes that are involved in homologous recombination, thus producing a sort of functional ‘BRCAness’ that favors the selective activity of the PARP inhibitors,” she explained.
Preclinical evidence suggests this may be related to the antiangiogenic effect of cediranib, or possibly to the inhibition of the platinum-derived growth factor signaling by cediranib.
BAROCCO was designed to assess whether the combination would provide superior PFS, compared with weekly paclitaxel, in the platinum-resistant population, and if an intermittent schedule might improve gastrointestinal tolerability, as treatment has been associated with severe diarrhea in previous trials, she said.
However, the toxicity profile of the study arms was as expected, and similar between experimental arms, with 11%, 18%, and 7% of patients in the paclitaxel, continuous, and intermittent arms discontinuing treatment because of adverse events, she noted.
Five serious adverse drug reactions occurred and two were fatal, including one in the control arm and one in the continuous arm.
Although not statistically significant, the continuous administration regimen was well tolerated, and showed “a promising trend for improved PFS,” particularly in gBRCAwt patients.
Notably, only 5% of patients in that arm experienced severe diarrhea, Dr. Colombo said, adding that “the continuous administration of cediranib and olaparib is active in PROC patients, with clinical benefit observed in 85% of cases.”
“We believe that [this combination] represents an active, feasible oral regimen, which deserves further investigation, and these results support ongoing trials investigating the same combination in platinum-resistant ovarian cancer,” she concluded.
Invited discussant Antonio Gonzalez Martin, MD, said that some of the drug-related adverse events reported in the study – which occurred in 70%-78% of patients in the three arms and included diarrhea, nausea, fatigue, and others – could have an impact on quality of life.
“It’s something that we need to study; patient-reported outcomes should be integrated in these types of trials for this very important population,” said Dr. Gonzalez-Martin, head of medical oncology at Clinica Universidad de Navarra, Madrid.
He added that the future of antiangiogenic and PARP inhibitor combinations remains to be defined in the first-line, platinum-sensitive, and platinum-resistant ovarian cancer settings.
The BAROCCO Trial was funded by AstraZeneca. Dr. Colombo has received honoraria from or been an advisor or consultant to Roche/Genentech, PharmaMar; AstraZeneca, Tesaro, Clovis Oncology, Pfizer, MSD Oncology, BioCad, and Takeda. Dr. Gonzalez-Martin has received fees, honoraria, and/or grants from Roche, AstraZeneca, Tesaro, GSK, Clovis, MSD, Pfizer, Novartis, PharmaMar, and Imugene.
SOURCE: Colombo N et al. ESMO 2019, Abstract LBA58. Ann Oncol. 2019;30:suppl 5.
REPORTING FROM ESMO 2019
Distinct mood, apathy profiles found in bipolar disorder patients
Neuroimaging
called parallel independent component analysis, or pICA, reported Wenhau Jiang and his associates.
pICA is a technique that enables researchers to analyze several modalities and the interconnections between them. The approach, which is considered fairly new, has been applied most often to neuropsychiatric disorders (Front Genet. 2015;6:276). The pICA involves using structural MRI and the Positive and Negative Syndrome Scale (PANSS) scores to assess the relationship between gray matter concentration in different areas of the brain and bipolar mood characteristics.
In the current study, published in NeuroImage: Clinical, Mr. Jiang and his associates used data from 110 patients with bipolar I and bipolar II from a large study conducted at the Norwegian Center for Mental Disorders Research in Oslo.
All patients were aged 18-65 years and had an IQ of over 70, and none had a history of severe head trauma. Most of the patients were women, about half had at least one psychotic episode, and all provided PANSS scores, reported Mr. Jiang of the department of psychology at Georgia State University in Atlanta and his associates.
After the patients were scanned, pICA was used to examine the preprocessed structural images and the PANSS item scores. The pICA showed two distinct profiles. One group showed preserved gray matter concentration in the right middle/superior temporal gyrus on the rMRI. These participants had more anxiety, and guilty feelings on the PANSS. Overall, participants with higher preserved gray matter concentration in bilateral, frontal, and parietal and left temporal regions show milder severity of these characteristics.
In the second pICA profile, participants with higher preserved gray matter concentration in bilateral front, parietal, and left temporal regions showed milder severity of several characteristics including blunted affect, emotional withdrawal, and passive/apathetic social withdrawal.
The investigators noted: “The mood profile was correlated with reductions in the right temporal gyrus, while the apathy/asocial profile correlated with a more widespread network including frontal, temporal, and parietal regions. It implicated the GM [gray matter] deficits in regional temporal lobe and frontal-temporal-parietal circuits that were separately related to clinical profiles as mood and apathy.”
The study was supported by the National Institutes of Health, the Research Council of Norway, the South-East Norway Health Authority, and the European Community’s Seventh Framework Programme. No author disclosures were stated.
SOURCE: Jiang W et al. Neuroimage Clin. 2019 Aug 19. doi: 10.1016/j.nicl.2019.101989.
Neuroimaging
Neuroimaging
called parallel independent component analysis, or pICA, reported Wenhau Jiang and his associates.
pICA is a technique that enables researchers to analyze several modalities and the interconnections between them. The approach, which is considered fairly new, has been applied most often to neuropsychiatric disorders (Front Genet. 2015;6:276). The pICA involves using structural MRI and the Positive and Negative Syndrome Scale (PANSS) scores to assess the relationship between gray matter concentration in different areas of the brain and bipolar mood characteristics.
In the current study, published in NeuroImage: Clinical, Mr. Jiang and his associates used data from 110 patients with bipolar I and bipolar II from a large study conducted at the Norwegian Center for Mental Disorders Research in Oslo.
All patients were aged 18-65 years and had an IQ of over 70, and none had a history of severe head trauma. Most of the patients were women, about half had at least one psychotic episode, and all provided PANSS scores, reported Mr. Jiang of the department of psychology at Georgia State University in Atlanta and his associates.
After the patients were scanned, pICA was used to examine the preprocessed structural images and the PANSS item scores. The pICA showed two distinct profiles. One group showed preserved gray matter concentration in the right middle/superior temporal gyrus on the rMRI. These participants had more anxiety, and guilty feelings on the PANSS. Overall, participants with higher preserved gray matter concentration in bilateral, frontal, and parietal and left temporal regions show milder severity of these characteristics.
In the second pICA profile, participants with higher preserved gray matter concentration in bilateral front, parietal, and left temporal regions showed milder severity of several characteristics including blunted affect, emotional withdrawal, and passive/apathetic social withdrawal.
The investigators noted: “The mood profile was correlated with reductions in the right temporal gyrus, while the apathy/asocial profile correlated with a more widespread network including frontal, temporal, and parietal regions. It implicated the GM [gray matter] deficits in regional temporal lobe and frontal-temporal-parietal circuits that were separately related to clinical profiles as mood and apathy.”
The study was supported by the National Institutes of Health, the Research Council of Norway, the South-East Norway Health Authority, and the European Community’s Seventh Framework Programme. No author disclosures were stated.
SOURCE: Jiang W et al. Neuroimage Clin. 2019 Aug 19. doi: 10.1016/j.nicl.2019.101989.
called parallel independent component analysis, or pICA, reported Wenhau Jiang and his associates.
pICA is a technique that enables researchers to analyze several modalities and the interconnections between them. The approach, which is considered fairly new, has been applied most often to neuropsychiatric disorders (Front Genet. 2015;6:276). The pICA involves using structural MRI and the Positive and Negative Syndrome Scale (PANSS) scores to assess the relationship between gray matter concentration in different areas of the brain and bipolar mood characteristics.
In the current study, published in NeuroImage: Clinical, Mr. Jiang and his associates used data from 110 patients with bipolar I and bipolar II from a large study conducted at the Norwegian Center for Mental Disorders Research in Oslo.
All patients were aged 18-65 years and had an IQ of over 70, and none had a history of severe head trauma. Most of the patients were women, about half had at least one psychotic episode, and all provided PANSS scores, reported Mr. Jiang of the department of psychology at Georgia State University in Atlanta and his associates.
After the patients were scanned, pICA was used to examine the preprocessed structural images and the PANSS item scores. The pICA showed two distinct profiles. One group showed preserved gray matter concentration in the right middle/superior temporal gyrus on the rMRI. These participants had more anxiety, and guilty feelings on the PANSS. Overall, participants with higher preserved gray matter concentration in bilateral, frontal, and parietal and left temporal regions show milder severity of these characteristics.
In the second pICA profile, participants with higher preserved gray matter concentration in bilateral front, parietal, and left temporal regions showed milder severity of several characteristics including blunted affect, emotional withdrawal, and passive/apathetic social withdrawal.
The investigators noted: “The mood profile was correlated with reductions in the right temporal gyrus, while the apathy/asocial profile correlated with a more widespread network including frontal, temporal, and parietal regions. It implicated the GM [gray matter] deficits in regional temporal lobe and frontal-temporal-parietal circuits that were separately related to clinical profiles as mood and apathy.”
The study was supported by the National Institutes of Health, the Research Council of Norway, the South-East Norway Health Authority, and the European Community’s Seventh Framework Programme. No author disclosures were stated.
SOURCE: Jiang W et al. Neuroimage Clin. 2019 Aug 19. doi: 10.1016/j.nicl.2019.101989.
FROM NEUROIMAGE: CLINICAL
Adjuvant radiotherapy no better than salvage post prostatectomy
BARCELONA – , results of a large randomized trial and separate meta-analysis indicate.
Among nearly 1,400 men with postoperative prostate-specific antigen (PSA) levels below 0.2 ng/mL and one or more risk factors, followed for a median of 5 years, there were no significant differences in any of the secondary outcomes between men randomized to radiotherapy and observation alone, reported Chris Parker, MD, from the Royal Marsden Hospital in London.
“In comparison with a policy of early salvage radiotherapy, adjuvant radiotherapy did not improve biochemical progression-free survival and did not delay the further use of hormone therapy,” he said at the European Society for Medical Oncology Congress, on behalf of colleagues in the RADICALS-RT trial.
Results of the RADICAL-RT trial were also pooled with results from two other large trials in a collaborative series of meta-analyses of long-term prostate cancer outcomes – dubbed ARTISTIC – which found no significant differences in event-free survival (EFS) for men randomized to either adjuvant or salvage radiotherapy.
“We don’t see any evidence from the ARTISTIC results that adjuvant radiotherapy improves event-free survival, compared to early salvage radiotherapy, and our best estimate is of a small, 1% difference in event-free survival at 5 years,” said Claire Vale, PhD, a research fellow at University College, London.
RADICALS RT details
Dr. Parker presented results from an early analysis of the secondary endpoint of biochemical progression-free survival (PFS), conducted in cooperation with the ARTISTIC investigators.
In RADICALS RT, investigators in the United Kingdom, Denmark, Canada, and Ireland enrolled 1,369 men following radical prostatectomy and after stratification by Gleason score, margin status, treatment center, and radiotherapy schedule (52.5 Gy delivered in 20 fractions, or 66 Gy delivered), randomly assigned them to either postoperative radiotherapy or observation with radiotherapy.
The patients enrolled had postoperative PSAs less than 2 ng/mL and one or more risk factors, either pathologic stage 3/4, Gleason score 7-10, positive surgical margins, or preoperative PSA of 10 ng/mL or greater.
The trigger for radiotherapy in men assigned to observation was PSA failure, defined as a PSA level of at least 0.1 ng/mL or 3 consecutive PSA rises.
At the median 5-year follow-up, PFS rates were 85% for patients assigned to adjuvant radiotherapy, and 88% for those assigned to observation, translating to a hazard ratio of 1.10, which was not statistically significant.
However, there were significant differences between the groups in both self-reported urinary incontinence (5.3% of patients in the radiotherapy group vs. 2.7% in the observation group, P = .008) and grade 3 or 4 urethral stricture at any time (8% vs. 5%, respectively, P = .03).
Results of the primary outcome, freedom from distant metastases will require longer follow-up, Dr. Parker said.
ARTISTIC meta-analysis
Dr. Vale presented results of the ARTISTIC collaborative meta-analysis, which included data from three randomized trials, including RADICALS, GETUG-AFU 17, and RAVES.
The meta-analysis was designed to include a consistent definition of PSA-driven EFS, prior to the unblinding of trial results.
The definition of EFS used in the trial was time from randomization until the first evidence of either PSA of 0.4 ng/mL or greater and rising after completion of radiotherapy, clinical/radiological progression, initiation on nontrial treatment, death from prostate cancer following completion of radiotherapy, or PSA level of 2.0 ng/mL or greater any time after randomization.
They analyzed data on a total of 1,074 men assigned to adjuvant radiotherapy and 1,077 assigned to salvage radiotherapy.
In each of the three trials included in the meta-analysis, there was a nonsignificant trend toward better PSA-driven EFS with salvage radiotherapy vs. adjuvant radiotherapy. The overall hazard ratio was 1.12, with the 95% confidence interval crossing 1, indicating a nonsignificant result.
Dr. Vale noted that early salvage radiotherapy spares many men from potentially unnecessary radiation and its negative consequences. Of the men included in the trials, more than 60% of those randomized to observation with salvage radiotherapy have yet to start radiotherapy.
She noted that investigators still need to assess long-term definitive outcomes such as metastases and survival, and whether some patients may derive benefit from adjuvant radiotherapy.
Invited discussant Gert De Meerleer, MD, PhD, a radiation oncologist at University Hospitals Leuven in Belgium, acknowledged that he agreed with most of the findings of the RADICALS-RT and ARTISTIC investigators.
An important take-home message, he said, is that “cure is the aim, and radiotherapy the keystone. If you have PSA relapse after prostatectomy, you can say, ‘I don’t treat the patient’ – fair enough. But if you treat the patient, only giving systemic therapy is the wrong way to go, you have to add radiotherapy,” he said.
He also agreed that “salvage is probably the modality of choice, and it is also in a lot of European countries, provided it is early.”
The RADICALS-RT trial is supported by Cancer Research UK and the Medical Research Council UK. Dr. Parker said he had no disclosures relevant to the trial. ARTISTIC is funded by the Medical Research Council. Dr. Vale reported having no disclosures. Dr. De Meerleer reported speaker fees, advisory board activities, and scientific grants not related to the studies.
SOURCE: Parker C et al. and Vale C et al. ESMO 2019. Abstracts LBA49_PR and LBA48_PR.
BARCELONA – , results of a large randomized trial and separate meta-analysis indicate.
Among nearly 1,400 men with postoperative prostate-specific antigen (PSA) levels below 0.2 ng/mL and one or more risk factors, followed for a median of 5 years, there were no significant differences in any of the secondary outcomes between men randomized to radiotherapy and observation alone, reported Chris Parker, MD, from the Royal Marsden Hospital in London.
“In comparison with a policy of early salvage radiotherapy, adjuvant radiotherapy did not improve biochemical progression-free survival and did not delay the further use of hormone therapy,” he said at the European Society for Medical Oncology Congress, on behalf of colleagues in the RADICALS-RT trial.
Results of the RADICAL-RT trial were also pooled with results from two other large trials in a collaborative series of meta-analyses of long-term prostate cancer outcomes – dubbed ARTISTIC – which found no significant differences in event-free survival (EFS) for men randomized to either adjuvant or salvage radiotherapy.
“We don’t see any evidence from the ARTISTIC results that adjuvant radiotherapy improves event-free survival, compared to early salvage radiotherapy, and our best estimate is of a small, 1% difference in event-free survival at 5 years,” said Claire Vale, PhD, a research fellow at University College, London.
RADICALS RT details
Dr. Parker presented results from an early analysis of the secondary endpoint of biochemical progression-free survival (PFS), conducted in cooperation with the ARTISTIC investigators.
In RADICALS RT, investigators in the United Kingdom, Denmark, Canada, and Ireland enrolled 1,369 men following radical prostatectomy and after stratification by Gleason score, margin status, treatment center, and radiotherapy schedule (52.5 Gy delivered in 20 fractions, or 66 Gy delivered), randomly assigned them to either postoperative radiotherapy or observation with radiotherapy.
The patients enrolled had postoperative PSAs less than 2 ng/mL and one or more risk factors, either pathologic stage 3/4, Gleason score 7-10, positive surgical margins, or preoperative PSA of 10 ng/mL or greater.
The trigger for radiotherapy in men assigned to observation was PSA failure, defined as a PSA level of at least 0.1 ng/mL or 3 consecutive PSA rises.
At the median 5-year follow-up, PFS rates were 85% for patients assigned to adjuvant radiotherapy, and 88% for those assigned to observation, translating to a hazard ratio of 1.10, which was not statistically significant.
However, there were significant differences between the groups in both self-reported urinary incontinence (5.3% of patients in the radiotherapy group vs. 2.7% in the observation group, P = .008) and grade 3 or 4 urethral stricture at any time (8% vs. 5%, respectively, P = .03).
Results of the primary outcome, freedom from distant metastases will require longer follow-up, Dr. Parker said.
ARTISTIC meta-analysis
Dr. Vale presented results of the ARTISTIC collaborative meta-analysis, which included data from three randomized trials, including RADICALS, GETUG-AFU 17, and RAVES.
The meta-analysis was designed to include a consistent definition of PSA-driven EFS, prior to the unblinding of trial results.
The definition of EFS used in the trial was time from randomization until the first evidence of either PSA of 0.4 ng/mL or greater and rising after completion of radiotherapy, clinical/radiological progression, initiation on nontrial treatment, death from prostate cancer following completion of radiotherapy, or PSA level of 2.0 ng/mL or greater any time after randomization.
They analyzed data on a total of 1,074 men assigned to adjuvant radiotherapy and 1,077 assigned to salvage radiotherapy.
In each of the three trials included in the meta-analysis, there was a nonsignificant trend toward better PSA-driven EFS with salvage radiotherapy vs. adjuvant radiotherapy. The overall hazard ratio was 1.12, with the 95% confidence interval crossing 1, indicating a nonsignificant result.
Dr. Vale noted that early salvage radiotherapy spares many men from potentially unnecessary radiation and its negative consequences. Of the men included in the trials, more than 60% of those randomized to observation with salvage radiotherapy have yet to start radiotherapy.
She noted that investigators still need to assess long-term definitive outcomes such as metastases and survival, and whether some patients may derive benefit from adjuvant radiotherapy.
Invited discussant Gert De Meerleer, MD, PhD, a radiation oncologist at University Hospitals Leuven in Belgium, acknowledged that he agreed with most of the findings of the RADICALS-RT and ARTISTIC investigators.
An important take-home message, he said, is that “cure is the aim, and radiotherapy the keystone. If you have PSA relapse after prostatectomy, you can say, ‘I don’t treat the patient’ – fair enough. But if you treat the patient, only giving systemic therapy is the wrong way to go, you have to add radiotherapy,” he said.
He also agreed that “salvage is probably the modality of choice, and it is also in a lot of European countries, provided it is early.”
The RADICALS-RT trial is supported by Cancer Research UK and the Medical Research Council UK. Dr. Parker said he had no disclosures relevant to the trial. ARTISTIC is funded by the Medical Research Council. Dr. Vale reported having no disclosures. Dr. De Meerleer reported speaker fees, advisory board activities, and scientific grants not related to the studies.
SOURCE: Parker C et al. and Vale C et al. ESMO 2019. Abstracts LBA49_PR and LBA48_PR.
BARCELONA – , results of a large randomized trial and separate meta-analysis indicate.
Among nearly 1,400 men with postoperative prostate-specific antigen (PSA) levels below 0.2 ng/mL and one or more risk factors, followed for a median of 5 years, there were no significant differences in any of the secondary outcomes between men randomized to radiotherapy and observation alone, reported Chris Parker, MD, from the Royal Marsden Hospital in London.
“In comparison with a policy of early salvage radiotherapy, adjuvant radiotherapy did not improve biochemical progression-free survival and did not delay the further use of hormone therapy,” he said at the European Society for Medical Oncology Congress, on behalf of colleagues in the RADICALS-RT trial.
Results of the RADICAL-RT trial were also pooled with results from two other large trials in a collaborative series of meta-analyses of long-term prostate cancer outcomes – dubbed ARTISTIC – which found no significant differences in event-free survival (EFS) for men randomized to either adjuvant or salvage radiotherapy.
“We don’t see any evidence from the ARTISTIC results that adjuvant radiotherapy improves event-free survival, compared to early salvage radiotherapy, and our best estimate is of a small, 1% difference in event-free survival at 5 years,” said Claire Vale, PhD, a research fellow at University College, London.
RADICALS RT details
Dr. Parker presented results from an early analysis of the secondary endpoint of biochemical progression-free survival (PFS), conducted in cooperation with the ARTISTIC investigators.
In RADICALS RT, investigators in the United Kingdom, Denmark, Canada, and Ireland enrolled 1,369 men following radical prostatectomy and after stratification by Gleason score, margin status, treatment center, and radiotherapy schedule (52.5 Gy delivered in 20 fractions, or 66 Gy delivered), randomly assigned them to either postoperative radiotherapy or observation with radiotherapy.
The patients enrolled had postoperative PSAs less than 2 ng/mL and one or more risk factors, either pathologic stage 3/4, Gleason score 7-10, positive surgical margins, or preoperative PSA of 10 ng/mL or greater.
The trigger for radiotherapy in men assigned to observation was PSA failure, defined as a PSA level of at least 0.1 ng/mL or 3 consecutive PSA rises.
At the median 5-year follow-up, PFS rates were 85% for patients assigned to adjuvant radiotherapy, and 88% for those assigned to observation, translating to a hazard ratio of 1.10, which was not statistically significant.
However, there were significant differences between the groups in both self-reported urinary incontinence (5.3% of patients in the radiotherapy group vs. 2.7% in the observation group, P = .008) and grade 3 or 4 urethral stricture at any time (8% vs. 5%, respectively, P = .03).
Results of the primary outcome, freedom from distant metastases will require longer follow-up, Dr. Parker said.
ARTISTIC meta-analysis
Dr. Vale presented results of the ARTISTIC collaborative meta-analysis, which included data from three randomized trials, including RADICALS, GETUG-AFU 17, and RAVES.
The meta-analysis was designed to include a consistent definition of PSA-driven EFS, prior to the unblinding of trial results.
The definition of EFS used in the trial was time from randomization until the first evidence of either PSA of 0.4 ng/mL or greater and rising after completion of radiotherapy, clinical/radiological progression, initiation on nontrial treatment, death from prostate cancer following completion of radiotherapy, or PSA level of 2.0 ng/mL or greater any time after randomization.
They analyzed data on a total of 1,074 men assigned to adjuvant radiotherapy and 1,077 assigned to salvage radiotherapy.
In each of the three trials included in the meta-analysis, there was a nonsignificant trend toward better PSA-driven EFS with salvage radiotherapy vs. adjuvant radiotherapy. The overall hazard ratio was 1.12, with the 95% confidence interval crossing 1, indicating a nonsignificant result.
Dr. Vale noted that early salvage radiotherapy spares many men from potentially unnecessary radiation and its negative consequences. Of the men included in the trials, more than 60% of those randomized to observation with salvage radiotherapy have yet to start radiotherapy.
She noted that investigators still need to assess long-term definitive outcomes such as metastases and survival, and whether some patients may derive benefit from adjuvant radiotherapy.
Invited discussant Gert De Meerleer, MD, PhD, a radiation oncologist at University Hospitals Leuven in Belgium, acknowledged that he agreed with most of the findings of the RADICALS-RT and ARTISTIC investigators.
An important take-home message, he said, is that “cure is the aim, and radiotherapy the keystone. If you have PSA relapse after prostatectomy, you can say, ‘I don’t treat the patient’ – fair enough. But if you treat the patient, only giving systemic therapy is the wrong way to go, you have to add radiotherapy,” he said.
He also agreed that “salvage is probably the modality of choice, and it is also in a lot of European countries, provided it is early.”
The RADICALS-RT trial is supported by Cancer Research UK and the Medical Research Council UK. Dr. Parker said he had no disclosures relevant to the trial. ARTISTIC is funded by the Medical Research Council. Dr. Vale reported having no disclosures. Dr. De Meerleer reported speaker fees, advisory board activities, and scientific grants not related to the studies.
SOURCE: Parker C et al. and Vale C et al. ESMO 2019. Abstracts LBA49_PR and LBA48_PR.
REPORTING FROM ESMO 2019