User login
Clinical Presentation of Rheumatoid Arthritis
Viral cause of acute flaccid myelitis eludes detection
A study of 305 cases of acute flaccid myelitis has found further evidence of a viral etiology but is yet to identify a single pathogen as the primary cause.
Writing in Pediatrics, researchers published an analysis of patients presenting with acute flaccid limb weakness from January 2015 to December 2017 across 43 states.
A total of 25 cases were judged as probable for acute flaccid myelitis (AFM) because they met clinical criteria and had a white blood cell count above 5 cells per mm3 in cerebrospinal fluid, while 193 were judged as confirmed cases based on the additional presence of spinal cord gray matter lesions on MRI.
Overall, 83% of patients had experienced fever, cough, runny nose, vomiting, and/or diarrhea for a median of 5 days before limb weakness began. Two-thirds of patients had experienced a respiratory illness, 62% had experienced a fever, and 29% had experienced gastrointestinal illness.
Overall, 47% of the 193 patients who had specimens tested at a Centers for Disease Control and Prevention or non-CDC laboratory had a pathogen found at any site, 10% had a pathogen detected from a sterile site such as cerebrospinal fluid or sera, and 42% had a pathogen detected from a nonsterile site.
Among 72 patients who had serum specimens tested at the CDC, 2 were positive for enteroviruses. Among the 90 patients who had upper respiratory specimens tested, 36% were positive for either enteroviruses or rhinoviruses.
A number of stool specimens were also tested; 15% were positive for enteroviruses or rhinoviruses and one was positive for parechovirus.
Cerebrospinal fluid was tested in 170 patients, of which 4 were positive for enteroviruses. The testing also found adenovirus, Epstein-Barr virus, human herpesvirus 6, and mycoplasma in six patients. Sera testing of 123 patients found 9 were positive for enteroviruses, West Nile virus, mycoplasma, and coxsackievirus B.
“In our summary of national AFM surveillance from 2015 to 2017, we demonstrate that cases were widely distributed across the United States, the majority of cases occurred in late summer or fall, children were predominantly affected, there is a spectrum of clinical severity, and no single pathogen was identified as the primary cause of AFM,” wrote Tracy Ayers, PhD, from the National Center for Immunization and Respiratory Diseases, and coauthors. “We conclude that symptoms of a viral syndrome within the week before limb weakness, detection of viral pathogens from sterile and nonsterile sites from almost half of patients, and seasonality of AFM incidence, particularly during the 2016 peak year, strongly suggest a viral etiology, including [enteroviruses].”
The authors of an accompanying editorial noted that the clinical syndrome of acute flaccid paralysis caused by myelitis in the gray matter of the spinal cord has previously been associated with a range of viruses, including poliovirus, enteroviruses, and flaviviruses, so a single etiology to explain all cases would not be expected.
“The central question remains: What is driving seasonal biennial nationwide outbreaks of AFM since 2014?” wrote Kevin Messaca, MD, and colleagues from the University of Colorado at Denver, Aurora.
Two authors declared consultancies, grants, and research contracts with the pharmaceutical sector. No other conflicts of interest were declared. One editorial author declared funding from the National Institute of Allergy and Infectious Diseases.
SOURCE: Ayers T et al. Pediatrics. 2019 Oct 7. doi: 10.1542/peds.2019-1619.
*Updated 10/14/2019.
A study of 305 cases of acute flaccid myelitis has found further evidence of a viral etiology but is yet to identify a single pathogen as the primary cause.
Writing in Pediatrics, researchers published an analysis of patients presenting with acute flaccid limb weakness from January 2015 to December 2017 across 43 states.
A total of 25 cases were judged as probable for acute flaccid myelitis (AFM) because they met clinical criteria and had a white blood cell count above 5 cells per mm3 in cerebrospinal fluid, while 193 were judged as confirmed cases based on the additional presence of spinal cord gray matter lesions on MRI.
Overall, 83% of patients had experienced fever, cough, runny nose, vomiting, and/or diarrhea for a median of 5 days before limb weakness began. Two-thirds of patients had experienced a respiratory illness, 62% had experienced a fever, and 29% had experienced gastrointestinal illness.
Overall, 47% of the 193 patients who had specimens tested at a Centers for Disease Control and Prevention or non-CDC laboratory had a pathogen found at any site, 10% had a pathogen detected from a sterile site such as cerebrospinal fluid or sera, and 42% had a pathogen detected from a nonsterile site.
Among 72 patients who had serum specimens tested at the CDC, 2 were positive for enteroviruses. Among the 90 patients who had upper respiratory specimens tested, 36% were positive for either enteroviruses or rhinoviruses.
A number of stool specimens were also tested; 15% were positive for enteroviruses or rhinoviruses and one was positive for parechovirus.
Cerebrospinal fluid was tested in 170 patients, of which 4 were positive for enteroviruses. The testing also found adenovirus, Epstein-Barr virus, human herpesvirus 6, and mycoplasma in six patients. Sera testing of 123 patients found 9 were positive for enteroviruses, West Nile virus, mycoplasma, and coxsackievirus B.
“In our summary of national AFM surveillance from 2015 to 2017, we demonstrate that cases were widely distributed across the United States, the majority of cases occurred in late summer or fall, children were predominantly affected, there is a spectrum of clinical severity, and no single pathogen was identified as the primary cause of AFM,” wrote Tracy Ayers, PhD, from the National Center for Immunization and Respiratory Diseases, and coauthors. “We conclude that symptoms of a viral syndrome within the week before limb weakness, detection of viral pathogens from sterile and nonsterile sites from almost half of patients, and seasonality of AFM incidence, particularly during the 2016 peak year, strongly suggest a viral etiology, including [enteroviruses].”
The authors of an accompanying editorial noted that the clinical syndrome of acute flaccid paralysis caused by myelitis in the gray matter of the spinal cord has previously been associated with a range of viruses, including poliovirus, enteroviruses, and flaviviruses, so a single etiology to explain all cases would not be expected.
“The central question remains: What is driving seasonal biennial nationwide outbreaks of AFM since 2014?” wrote Kevin Messaca, MD, and colleagues from the University of Colorado at Denver, Aurora.
Two authors declared consultancies, grants, and research contracts with the pharmaceutical sector. No other conflicts of interest were declared. One editorial author declared funding from the National Institute of Allergy and Infectious Diseases.
SOURCE: Ayers T et al. Pediatrics. 2019 Oct 7. doi: 10.1542/peds.2019-1619.
*Updated 10/14/2019.
A study of 305 cases of acute flaccid myelitis has found further evidence of a viral etiology but is yet to identify a single pathogen as the primary cause.
Writing in Pediatrics, researchers published an analysis of patients presenting with acute flaccid limb weakness from January 2015 to December 2017 across 43 states.
A total of 25 cases were judged as probable for acute flaccid myelitis (AFM) because they met clinical criteria and had a white blood cell count above 5 cells per mm3 in cerebrospinal fluid, while 193 were judged as confirmed cases based on the additional presence of spinal cord gray matter lesions on MRI.
Overall, 83% of patients had experienced fever, cough, runny nose, vomiting, and/or diarrhea for a median of 5 days before limb weakness began. Two-thirds of patients had experienced a respiratory illness, 62% had experienced a fever, and 29% had experienced gastrointestinal illness.
Overall, 47% of the 193 patients who had specimens tested at a Centers for Disease Control and Prevention or non-CDC laboratory had a pathogen found at any site, 10% had a pathogen detected from a sterile site such as cerebrospinal fluid or sera, and 42% had a pathogen detected from a nonsterile site.
Among 72 patients who had serum specimens tested at the CDC, 2 were positive for enteroviruses. Among the 90 patients who had upper respiratory specimens tested, 36% were positive for either enteroviruses or rhinoviruses.
A number of stool specimens were also tested; 15% were positive for enteroviruses or rhinoviruses and one was positive for parechovirus.
Cerebrospinal fluid was tested in 170 patients, of which 4 were positive for enteroviruses. The testing also found adenovirus, Epstein-Barr virus, human herpesvirus 6, and mycoplasma in six patients. Sera testing of 123 patients found 9 were positive for enteroviruses, West Nile virus, mycoplasma, and coxsackievirus B.
“In our summary of national AFM surveillance from 2015 to 2017, we demonstrate that cases were widely distributed across the United States, the majority of cases occurred in late summer or fall, children were predominantly affected, there is a spectrum of clinical severity, and no single pathogen was identified as the primary cause of AFM,” wrote Tracy Ayers, PhD, from the National Center for Immunization and Respiratory Diseases, and coauthors. “We conclude that symptoms of a viral syndrome within the week before limb weakness, detection of viral pathogens from sterile and nonsterile sites from almost half of patients, and seasonality of AFM incidence, particularly during the 2016 peak year, strongly suggest a viral etiology, including [enteroviruses].”
The authors of an accompanying editorial noted that the clinical syndrome of acute flaccid paralysis caused by myelitis in the gray matter of the spinal cord has previously been associated with a range of viruses, including poliovirus, enteroviruses, and flaviviruses, so a single etiology to explain all cases would not be expected.
“The central question remains: What is driving seasonal biennial nationwide outbreaks of AFM since 2014?” wrote Kevin Messaca, MD, and colleagues from the University of Colorado at Denver, Aurora.
Two authors declared consultancies, grants, and research contracts with the pharmaceutical sector. No other conflicts of interest were declared. One editorial author declared funding from the National Institute of Allergy and Infectious Diseases.
SOURCE: Ayers T et al. Pediatrics. 2019 Oct 7. doi: 10.1542/peds.2019-1619.
*Updated 10/14/2019.
FROM PEDIATRICS
Key clinical point: Acute flaccid myelitis shows a strong suggestion of viral etiology but a single causal virus is not identified.
Major finding: Patients with acute flaccid myelitis show infection with a range of viruses including enteroviruses.
Study details: A study of 305 cases of acute flaccid myelitis in the United States.
Disclosures: Two authors declared consultancies, grants, and research contracts with the pharmaceutical sector. No other conflicts of interest were declared.
Source: Ayers T et al. Pediatrics. 2019 Oct 7. doi: 10.1542/peds.2019-1619.
Enough Fuss; She Wants Lunch!
ANSWER
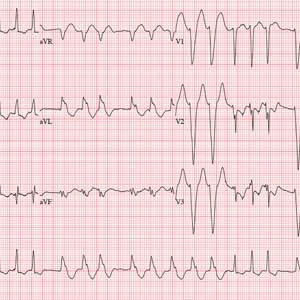
The correct interpretation is atrial fibrillation with aberrantly conducted complexes. The lead I rhythm strip at the bottom of the ECG shows the irregularly irregular rate. There are narrow complexes (see beats 3-7 and 16-18), indicating normal conduction through the atrioventricular node and His-Purkinje system. The remainder of the complexes are wide and aberrantly conducted and are in the same vector as the normally conducted (narrow) complexes.
An important take-away from this case
ANSWER
The correct interpretation is atrial fibrillation with aberrantly conducted complexes. The lead I rhythm strip at the bottom of the ECG shows the irregularly irregular rate. There are narrow complexes (see beats 3-7 and 16-18), indicating normal conduction through the atrioventricular node and His-Purkinje system. The remainder of the complexes are wide and aberrantly conducted and are in the same vector as the normally conducted (narrow) complexes.
An important take-away from this case
ANSWER
The correct interpretation is atrial fibrillation with aberrantly conducted complexes. The lead I rhythm strip at the bottom of the ECG shows the irregularly irregular rate. There are narrow complexes (see beats 3-7 and 16-18), indicating normal conduction through the atrioventricular node and His-Purkinje system. The remainder of the complexes are wide and aberrantly conducted and are in the same vector as the normally conducted (narrow) complexes.
An important take-away from this case
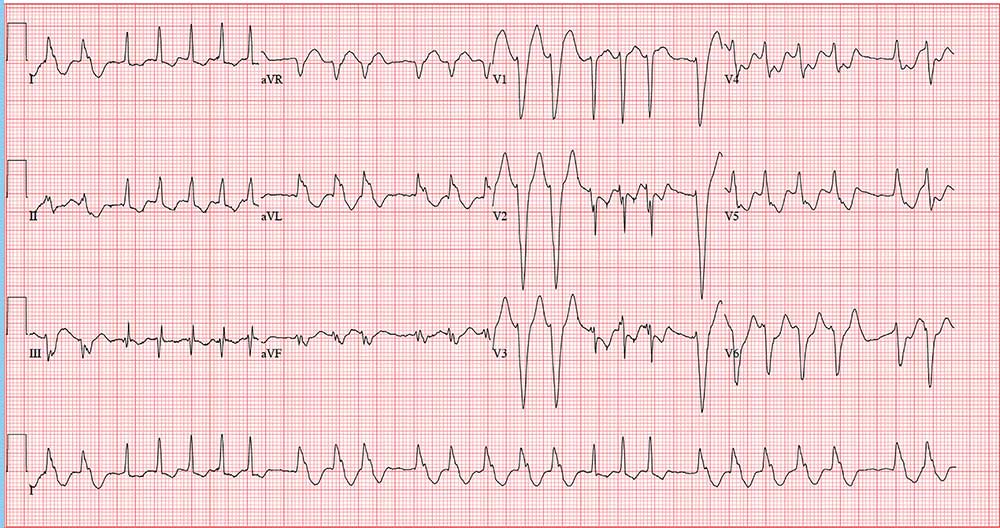
During morning rounds at a skilled nursing facility (SNF), a 74-year-old woman is found to have a rapid heart rate. She is placed on telemetry, which reveals a wide complex tachycardia. Concerned about possible ventricular tachycardia, the charge nurse contacts the on-call physician, who recommends calling 911. The patient is transferred via ACLS ambulance to your facility.
When you see her, she seems embarrassed by all the attention she’s receiving and expresses her desire to return to the SNF before she misses lunch. She is in no pain or discomfort, is not particularly short of breath, and does not feel dizzy or lightheaded. According to reports, she was friendly and conversive with both the nursing staff at the SNF and the paramedics during transport.
History is remarkable for several transient ischemic attacks with no residual sequelae, hypertension (under good control), and hypothyroidism (treatedwith medication). Surgical history includes a hyster-ectomy, a cholecystectomy, and an open reduction and metal plate fixation of a high (right) ankle break—all of which were performed more than 10 years ago.
Her medications include warfarin, hydrochlorothiazide, atorvastatin, and levothyroxine. She has no known drug allergies.
The patient is a retired junior high school principal. Her husband died of lung cancer 4 years ago. She has 3 adult children who are all in good health. She has never smoked but does enjoy a daily nightcap. She denies alcohol abuse or illicit drug use.
Family history reveals her parents died in a train accident and her paternal grandparents died of tuberculosis. She does not know her maternal grandparents’ medical history.
Review of systems is positive for chronic constipation and chronic hip and knee discomfort. Vital signs include a blood pressure of 124/88 mm Hg; pulse, 140 beats/min; respiratory rate, 14 breaths/min; and temperature, 97.6°F. Her weight is 158 lb, and her height is not measured.
Physical exam reveals a pleasant elderly woman in no distress. She is dressed appropriately, her hair is styled, and she is wearing makeup as she usually does. The HEENT exam reveals hearing aids and corrective lenses. Her neck has no jugular venous distention, carotid bruits, or thyromegaly.
Her lungs are clear in all fields. Her heart has a rapid and questionably irregular rhythm. There are no appreciable murmurs or rubs. Her abdominal exam is normal, with the exception of well-healed surgical scars. There is no peripheral edema, and all pulses are equal bilaterally in both upper and lower extremities. The neurologic exam is grossly normal with normal affect and mood.
An ECG reveals a ventricular rate of 152 beats/min; PR interval, 128 ms; QRS duration, 88 ms; QT/QTc interval, 280/445 ms; P axis, 27°; R axis, 23°; and T axis, 232°. What is your interpretation?
Aplastic Anemia: Diagnosis and Treatment
From the Oregon Health and Science University, Portland, OR.
Abstract
- Objective: To describe the current approach to diagnosis and treatment of aplastic anemia.
- Methods: Review of the literature.
- Results: Aplastic anemia can be acquired or associated with an inherited marrow failure syndrome (IMFS), and the treatment and prognosis vary dramatically between these 2 etiologies. Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to life-threatening neutropenic infections or bleeding. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia.
- Conclusion: Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marro
w transplant program to evaluate for early transplantation is the new standard of care for aplastic anemia.
Keywords: inherited marrow failure syndrome; Fanconi anemia; immunosuppression; transplant; stem cell.
Aplastic anemia is a clinical and pathological entity of bone marrow failure that causes progressive loss of hematopoietic progenitor stem cells (HPSC), resulting in pancytopenia.1 Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to having life-threatening neutropenic infections or bleeding. Aplastic anemia results from either inherited or acquired causes, and the pathophysiology and treatment approach vary significantly between these 2 causes. Therefore, recognition of inherited marrow failure diseases, such as Fanconi anemia and telomere biology disorders, is critical to establishing the management plan.
Epidemiology
Aplastic anemia is a rare disorder, with an incidence of approximately 1.5 to 7 cases per million individuals per year.2,3 A recent Scandinavian study reported that the incidence of aplastic anemia among the Swedish population is 2.3 cases per million individuals per year, with a median age at diagnosis of 60 years and a slight female predominance (52% versus 48%, respectively).2 This data is congruent with prior observations made in Barcelona, where the incidence was 2.34 cases per million individuals per year, albeit with a slightly higher incidence in males compared to females (2.54 versus 2.16, respectively).4 The incidence of aplastic anemia varies globally, with a disproportionate increase in incidence seen among Asian populations, with rates as high as 8.8 per million individuals per year.3-5 This variation in incidence in Asia versus other countries has not been well explained. There appears to be a bimodal distribution, with incidence peaks seen in young adults and in older adults.2,3,6
Pathophysiology
Acquired Aplastic Anemia
The leading hypothesis as to the cause of most cases of acquired aplastic anemia is that a dysregulated immune system destroys HPSCs. Inciting etiologies implicated in the development of acquired aplastic anemia include pregnancy, infection, medications, and exposure to certain chemicals, such as benzene.1,7 The historical understanding of acquired aplastic anemia implicates cytotoxic T-lymphocyte–mediated destruction of CD34+ hematopoietic stem cells.1,8,9 This hypothesis served as the basis for treatment of acquired aplastic anemia with immunosuppressive therapy, predominantly anti-thymocyte globulin (ATG) combined with cyclosporine A.1,8 More recent work has focused on cytokine interactions, particularly the suppressive role of interferon (IFN)-γ on hematopoietic stem cells independent of T-lymphocyte–mediated destruction, which has been demonstrated in a murine model.8 The interaction of IFN-γ with the hematopoietic stem cell pool is dynamic. IFN-γ levels are elevated during an acute inflammatory response, such as a viral infection, providing further basis for the immune-mediated nature of the acquired disease.10 Specifically, in vitro studies suggest the effects of IFN-γ on HPSC may be secondary to interruption of thrombopoietin and its respective signaling pathways, which play a key role in hematopoietic stem cell renewal.11 Eltrombopag, a thrombopoietin receptor antagonist, has shown promise in the treatment of refractory aplastic anemia, with studies indicating that its effectiveness is independent of IFN-γ levels.11,12
Inherited Aplastic Anemia
The inherited marrow failure syndromes (IMFSs) are a group of disorders characterized by cellular maintenance and repair defects, leading to cytopenias, increased cancer risk, structural defects, and risk of end organ damage, such as liver cirrhosis and pulmonary fibrosis.13-15 The most common diseases include Fanconi anemia, dyskeratosis congenita/telomere biology disorders, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, but with the advent of whole exome sequencing, new syndromes continue to be discovered. While classically these disorders present in children, adult presentations are now commonplace. Broadly, the pathophysiology of inherited aplastic anemia relates to the defective HPSCs and an accelerated decline of the hematopoietic stem cell compartment.
The most common IMFSs, Fanconi anemia and telomere biology disorders, are associated with numerous mutations in DNA damage repair pathways and telomere maintenance pathways. TERT, DKC, and TERC mutations are most commonly associated with dyskeratosis congenita, but may also be found infrequently in patients with aplastic anemia presenting at an older age in the absence of the classic phenotypical features.1,16,17 The recognition of an underlying genetic disorder or telomere biology disorder leading to constitutional aplastic anemia is significant, as these conditions are associated not only with marrow failure, but also with endocrinopathies, organ fibrosis, and and hematopoietic and solid organ malignancies.13-15 In particular, TERT and TERC gene mutations have been associated with dyskeratosis congenita as well as pulmonary fibrosis and cirrhosis.18,19 The implications of early diagnosis of an IMFS lie in the approach to treatment and prognosis.
Clonal Disorders and Secondary Malignancies
Myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML) are 2 clonal disorders that may arise from a background of aplastic anemia.9,20,21 Hypoplastic MDS can be difficult to differentiate from aplastic anemia at diagnosis based on morphology alone, although recent work has demonstrated that molecular testing for somatic mutations in ASXL1, DNMT3A, and BCOR can aid in differentiating a subset of aplastic anemia patients who are more likely to progress to MDS.21 Clonal populations of cells harboring 6p uniparental disomy are seen in more than 10% of patients with aplastic anemia on cytogenetic analysis, which can help differentiate the diseases.9 Yoshizato and colleagues found lower rates of ASXL1 and DNMT3A mutations in patients with aplastic anemia as compared with patients with MDS or AML. In this study, patients with aplastic anemia had higher rates of mutations in PIGA (reflecting the increased paroxysmal nocturnal hemoglobinuria [PNH] clonality seen in aplastic anemia) and BCOR.9 Mutations were also found in genes commonly mutated in MDS and AML, including TET2, RUNX1, TP53, and JAK2, albeit at lower frequencies.9 These mutations as a whole have not predicted response to therapy or prognosis. However, when performing survival analysis in patients with specific mutations, those commonly encountered in MDS/AML (ASXL1, DNMT3A, TP53, RUNX1, CSMD1) are associated with faster progression to overt MDS/AML and decreased overall survival (OS),20,21 suggesting these mutations may represent early clonality that can lead to clonal evolution and the development of secondary malignancies. Conversely, mutations in BCOR and BCORL appear to identify patients who may have a favorable outcome in response to immunosuppressive therapy and, similar to patients with PIGA mutations, improved OS.9
Paroxysmal Nocturnal Hemoglobinuria
In addition to having an increased risk of myelodysplasia and malignancy due to the development of a dominant pre-malignant clone, patients with aplastic anemia often harbor progenitor cell clones associated with PNH.1,17 PNH clones have been identified in more than 50% of patients with aplastic anemia.22,23 PNH represents a clonal disorder of hematopoiesis in which cells harbor X-linked somatic mutations in the PIGA gene; this gene encodes a protein responsible for the synthesis of glycosylphosphatidylinositol anchors on the cell surface.22,24 The lack of these cell surface proteins, specifically CD55 (also known as decay accelerating factor) and CD59 (also known as membrane inhibitor of reactive lysis), predisposes red cells to increased complement-mediated lysis.25 The exact mechanism for the development of these clones in patients with aplastic anemia is not fully understood. Current theories hypothesize that the clones are protected from the immune-mediated destruction of normal hematopoietic stem cells due to the absence of the cell surface proteins.1,20 The role of these clones over time in patients with aplastic anemia is less clear, though recent work demonstrated that despite differences in clonality over the disease course, aplastic anemia patients with small PNH clones are less likely to develop overt hemolysis and larger PNH clones compared to patients harboring larger (≥ 50%) PNH clones at diagnosis.23,26,27 Additionally, PNH clones in patients with aplastic anemia infrequently become clinically significant.27 It should be noted that these conditions exist along a continuum; that is, patients with aplastic anemia may develop PNH clones, while conversely patients with PNH may develop aplastic anemia.20 Patients with PNH clones should be followed via peripheral blood flow cytometry and complete blood count to track clonal stability and identify clinically significant PNH among aplastic anemia patients.28
Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying; pulmonary, renal, and liver disease; and blood disorders.
Patients with an IMFS (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, classic phenotypical findings may be lacking in up to 30% to 40% of patients with an IMFS.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered, including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7
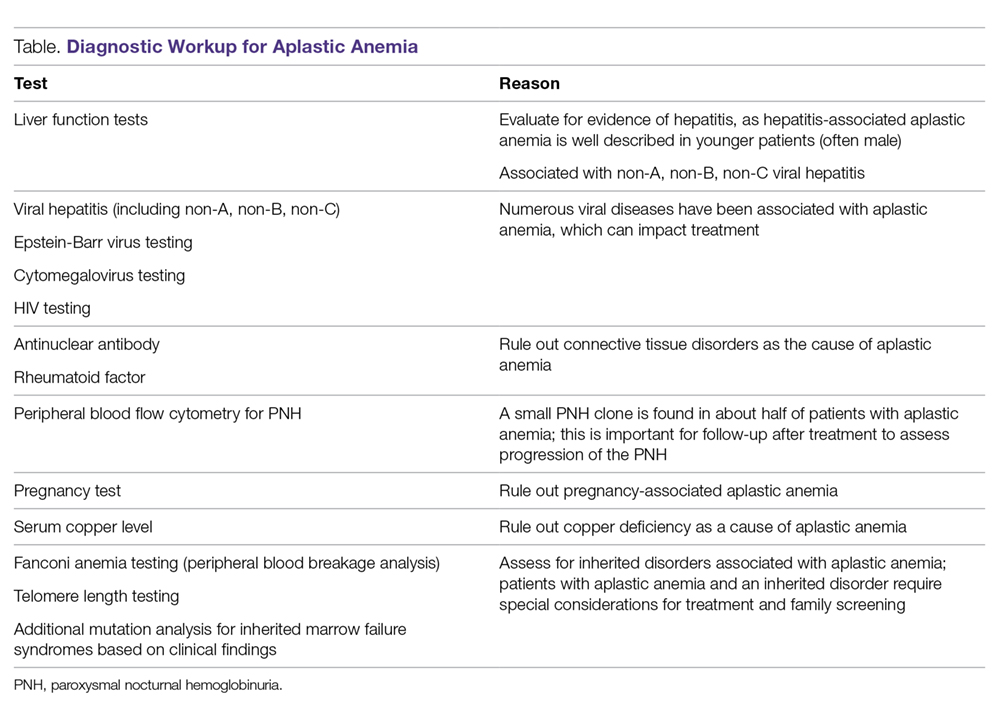
Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 Typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35
In addition to peripheral blood findings, bone marrow biopsy is essential for the diagnosis, and should demonstrate a markedly hypocellular marrow (cellularity < 25%), occasionally with an increase in T lymphocytes.7,39 Because marrow cellularity varies with age and can be challenging to assess, additional biopsies may be needed to confirm the diagnosis.29 A 1- to 2-cm core biopsy is necessary to confirm hypocellularity, as small areas of residual hematopoiesis may be present and obscure the diagnosis.35
Excluding Hypocellular MDS and IMFS
Excluding hypocellular MDS is challenging, especially in the older adult presenting with aplastic anemia, as patients with aplastic anemia may have some degree of erythroid dysplasia on bone marrow morphology.36 The presence of a PNH clone on flow cytometry can aid in diagnosing aplastic anemia and excluding MDS,34 although PNH clones can be present in refractory anemia MDS. Patients with aplastic anemia have a lower ratio of CD34+ cells compared to those with hypoplastic MDS, with 1 study demonstrating a mean CD34+ percentage of < 0.5% in aplastic anemia versus 3.7% in hypoplastic MDS.40 Cytogenetic and molecular testing can also aid in making this distinction by identifying mutations commonly implicated in MDS.7 The presence of monosomy 7 (-7) in aplastic anemia patients is associated with a poor overall prognosis.34,41
Peripheral blood screening using chromosome breakage analysis (done using either mitomycin C or diepoxybutane as in vitro DNA-crosslinking agents)42 and telomere length testing (of peripheral blood leukocytes) is necessary to exclude the main IMFSs, Fanconi anemia and telomere biology disorders, respectively. Ruling out these conditions is imperative, as the approach to treatment varies significantly between IMFS and aplastic anemia. Patients with shortened telomeres should undergo genetic screening for mutations in the telomere maintenance genes to evaluate the underlying defect leading to shortened telomeres. Patients with increased peripheral blood breakage should have genetic testing to detect mutations associated with Fanconi anemia.
Classification
Once the diagnosis of aplastic anemia has been made, the patient should be classified according to the severity of their disease. Disease severity is determined based on peripheral blood ANC: non-severe aplastic anemia (NSAA), ANC > 500 polymorphonuclear neutrophils (PMNs)/µL; severe aplastic anemia (SAA), 200–500 PMNs/µL; and very severe aplastic anemia (VSAA), 0–200 PMNs/µL.4,34 Disease classification is important, as VSAA is associated with a decreased OS compared to SAA.2 Disease classification may affect treatment decisions, as patients with NSAA may be observed for a short period of time, while, conversely, patients with SAA have a worse prognosis with delays in therapy.43-45
Treatment of Inherited Aplastic Anemia
First-line treatment options for patients with IMFS are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect. Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with multiple studies in IMFSs and SAA showing superior outcomes with a bone marrow product compared to peripheral blood stem cells.46-48 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.49,50 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival46,49,51 compared to cyclophosphamide conditioning, which was historically used in matched related donors.50,52 Adding fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.51,53 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data are limited.5
For patients presenting with AML or a high-risk MDS who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggest that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased OS, despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.54,55 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Treatment of Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan are being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.2
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.56,57 All blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.58 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an ANC < 500 cells/µL).59-62 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.63 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006). This difference was largely driven by a decrease in infectious episodes in patients with VSAA treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).63
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.64 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.62,65
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.61,62 This appears to be changing with time. Valdez et al demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.65 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.62 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with SAA or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).60,66 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.60 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues showed that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level of 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).67 Approximately 25% of patients in this trial had an increase in creatinine, with patients taking concomitant cyclosporine affected to a greater degree than those on chelation therapy alone. For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.58
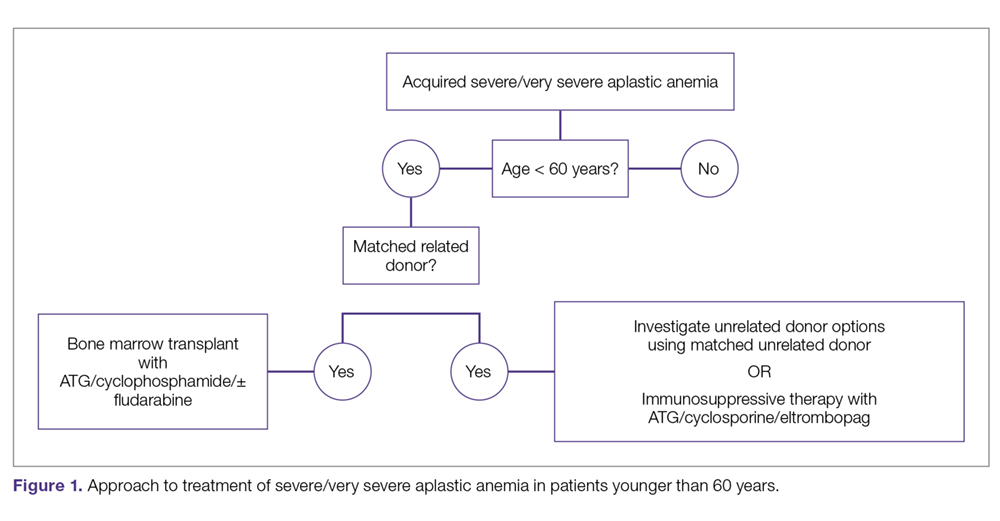
Approach to Therapy
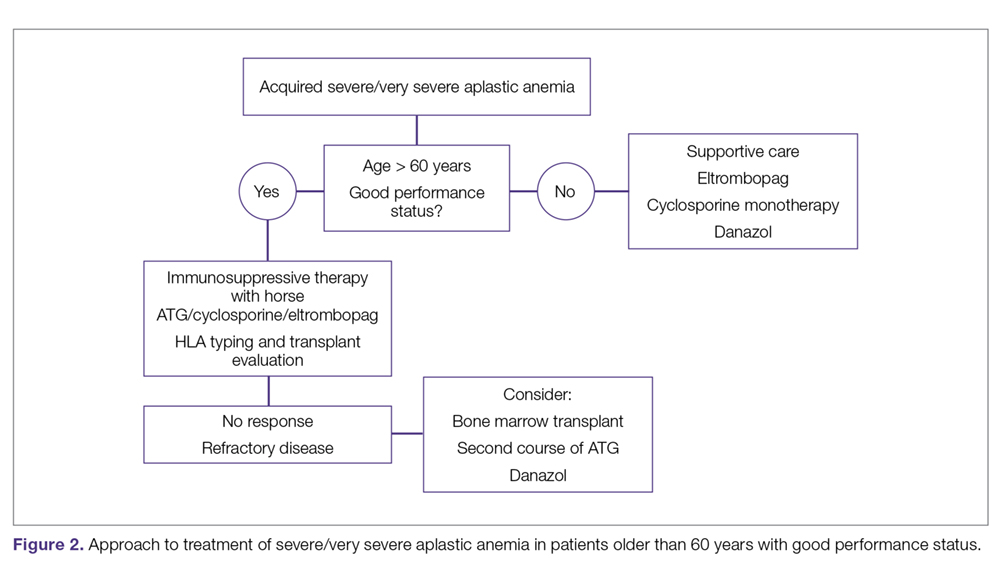
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.
Matched Sibling Donor Transplant. Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).43 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and MUD HSCT (38% and 65%).43 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.68
Current conditioning regimens typically use a combination of cyclophosphamide and ATG,69,70 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.53 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.71
Kim et al evaluated their experience with patients older than 40 years receiving matched related donors, finding comparable outcomes in those ages 41 to 50 years compared to younger patients. Outcomes declined in those over the age of 50 years.72 Long-term data for matched related donor transplant for aplastic anemia show excellent long-term outcomes, with minimal chronic GVHD and good performance status.73 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and MUD transplants for pediatric patients74,75 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.76,77
Immunosuppressive Therapy. For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.78 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.59,78,79
Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.80 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).59 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS, 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.81 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.81 The combination of ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.79 In this study, patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.82 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.83 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.84
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.34,84 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.84 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.85 When given at a dose of 150 mg daily in patients ages 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.45 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.12 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.85 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.86 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.48,65 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant. For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation (EBMT) analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-IV GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Factors associated with improved survival in this analysis include transplant under age 20 years (84% versus 72%), transplant within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and cytomegalovirus-negative donor and recipient as compared to other combinations (82% versus 76%).87 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.34,48 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).75 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.48
A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.75 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical PNH, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.88
With continued improvement of less toxic and more immunomodulating conditioning regimens,utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.89 However, there is still a large population of patients without matched sibling or unrelated donor options. Given the need to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant PNH, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.90
Summary
Aplastic anemia is a rare but potentially life-threatening disorder with pancytopenia and a marked reduction in the HSC compartment. It can be acquired or associated with an IMFS, and the treatment and prognosis vary dramatically between these 2 etiologies. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
Corresponding author: Gabrielle Meyers, MD, 3181 SW Sam Jackson Park Road, Mail Code UHN73C, Portland, OR 97239.
Financial disclosures: None.
1. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519.
2. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
3. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718-1721.
4. Montané E, Ibanez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica. 2008;93:518-523.
5. Ohta A, Nagai M, Nishina M, et al. Incidence of aplastic anemia in Japan: analysis of data from a nationwide registration system. Int J Epidemiol. 2015; 44(suppl_1):i178.
6. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
7. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
8. Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoiesis stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699-3708.
9. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35-47.
10. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479-2486.
11. Cheng H, Cheruku PS, Alvarado L, et al. Interferon-γ perturbs key signaling pathways induced by thrombopoietin, but not eltrombopag, in human hematopoietic stem/progenitor cells. Blood. 2016;128:3870.
12. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
13. Townsley DM, Dumitriu B, Young NS, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922-1931.
14. Feurstein S, Drazer MW, Godley LA. Genetic predisposition to leukemia and other hematologic malignancies. Semin Oncol. 2016;43:598-608.
15. Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775-2783.
16. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16:S119-S125.
17. Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446-4455.
18. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
19. Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J. 2016;48:1721-1731.
20. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337-347.
21. Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood. 2014;124:2698-2704.
22. Mukhina GL, Buckley JT, Barber JP, et al. Multilineage glycosylphosphatidylinositol anchor‐deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476-482.
23. Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45.
24. Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-5340.
25. Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190-198.
26. Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308-1314.
27. Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-1080.
28. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709.
29. Guinan EC. Diagnosis and management of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:76-81.
30. Giampietro PF, Verlander PC, Davis JG, Auerbach AD. Diagnosis of Fanconi anemia in patients without congenital malformations: an international Fanconi Anemia Registry Study. Am J Med Genetics. 1997;68:58-61.
31. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4-10.
32. Giampietro PF, Davis JG, Adler-Brecher B, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116-1120.
33. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
34. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
35. DeZern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol. 2011;4:221-230.
36. Tichelli A, Gratwohl A, Nissen C, et al. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood. 1992;80:337-345.
37. Camitta BM, Storb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645-652.
38. Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177-182.
39. Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136-2141.
40. Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458-462.
41. Maciejewski JP, Risitano AM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129-3135.
42. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
43. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-18.
44. Passweg JR, Socié G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858-864.
45. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95:2119-2125.
46. Peffault de Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122:4279-4286.
47. Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
48. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
49. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Bone Marrow Transplant. 2015;50:1168-1172.
50. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
51. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
52. Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
53. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
54. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-e200.
55. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for Fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
56. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
57. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
58. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
59. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430-438.
60. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
61. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol. 2009;46:269-276.
62. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
63. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
64. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
65. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
66. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
67. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2454.
68. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
69. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
70. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. Hematology Am Soc Hematol Educ Program. 2013;2013:82-86.
71. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
72. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
73. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
74. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
75. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br J Haematol. 2015;171:585-594.
76. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
77. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
78. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
79. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al; German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
80. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
81. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
82. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
83. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
84. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
85. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
86. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018;124:4192-4201.
87. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
88. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. Am J Hematol. 2019; 94:80-86.
89. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
90. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
From the Oregon Health and Science University, Portland, OR.
Abstract
- Objective: To describe the current approach to diagnosis and treatment of aplastic anemia.
- Methods: Review of the literature.
- Results: Aplastic anemia can be acquired or associated with an inherited marrow failure syndrome (IMFS), and the treatment and prognosis vary dramatically between these 2 etiologies. Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to life-threatening neutropenic infections or bleeding. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia.
- Conclusion: Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marro
w transplant program to evaluate for early transplantation is the new standard of care for aplastic anemia.
Keywords: inherited marrow failure syndrome; Fanconi anemia; immunosuppression; transplant; stem cell.
Aplastic anemia is a clinical and pathological entity of bone marrow failure that causes progressive loss of hematopoietic progenitor stem cells (HPSC), resulting in pancytopenia.1 Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to having life-threatening neutropenic infections or bleeding. Aplastic anemia results from either inherited or acquired causes, and the pathophysiology and treatment approach vary significantly between these 2 causes. Therefore, recognition of inherited marrow failure diseases, such as Fanconi anemia and telomere biology disorders, is critical to establishing the management plan.
Epidemiology
Aplastic anemia is a rare disorder, with an incidence of approximately 1.5 to 7 cases per million individuals per year.2,3 A recent Scandinavian study reported that the incidence of aplastic anemia among the Swedish population is 2.3 cases per million individuals per year, with a median age at diagnosis of 60 years and a slight female predominance (52% versus 48%, respectively).2 This data is congruent with prior observations made in Barcelona, where the incidence was 2.34 cases per million individuals per year, albeit with a slightly higher incidence in males compared to females (2.54 versus 2.16, respectively).4 The incidence of aplastic anemia varies globally, with a disproportionate increase in incidence seen among Asian populations, with rates as high as 8.8 per million individuals per year.3-5 This variation in incidence in Asia versus other countries has not been well explained. There appears to be a bimodal distribution, with incidence peaks seen in young adults and in older adults.2,3,6
Pathophysiology
Acquired Aplastic Anemia
The leading hypothesis as to the cause of most cases of acquired aplastic anemia is that a dysregulated immune system destroys HPSCs. Inciting etiologies implicated in the development of acquired aplastic anemia include pregnancy, infection, medications, and exposure to certain chemicals, such as benzene.1,7 The historical understanding of acquired aplastic anemia implicates cytotoxic T-lymphocyte–mediated destruction of CD34+ hematopoietic stem cells.1,8,9 This hypothesis served as the basis for treatment of acquired aplastic anemia with immunosuppressive therapy, predominantly anti-thymocyte globulin (ATG) combined with cyclosporine A.1,8 More recent work has focused on cytokine interactions, particularly the suppressive role of interferon (IFN)-γ on hematopoietic stem cells independent of T-lymphocyte–mediated destruction, which has been demonstrated in a murine model.8 The interaction of IFN-γ with the hematopoietic stem cell pool is dynamic. IFN-γ levels are elevated during an acute inflammatory response, such as a viral infection, providing further basis for the immune-mediated nature of the acquired disease.10 Specifically, in vitro studies suggest the effects of IFN-γ on HPSC may be secondary to interruption of thrombopoietin and its respective signaling pathways, which play a key role in hematopoietic stem cell renewal.11 Eltrombopag, a thrombopoietin receptor antagonist, has shown promise in the treatment of refractory aplastic anemia, with studies indicating that its effectiveness is independent of IFN-γ levels.11,12
Inherited Aplastic Anemia
The inherited marrow failure syndromes (IMFSs) are a group of disorders characterized by cellular maintenance and repair defects, leading to cytopenias, increased cancer risk, structural defects, and risk of end organ damage, such as liver cirrhosis and pulmonary fibrosis.13-15 The most common diseases include Fanconi anemia, dyskeratosis congenita/telomere biology disorders, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, but with the advent of whole exome sequencing, new syndromes continue to be discovered. While classically these disorders present in children, adult presentations are now commonplace. Broadly, the pathophysiology of inherited aplastic anemia relates to the defective HPSCs and an accelerated decline of the hematopoietic stem cell compartment.
The most common IMFSs, Fanconi anemia and telomere biology disorders, are associated with numerous mutations in DNA damage repair pathways and telomere maintenance pathways. TERT, DKC, and TERC mutations are most commonly associated with dyskeratosis congenita, but may also be found infrequently in patients with aplastic anemia presenting at an older age in the absence of the classic phenotypical features.1,16,17 The recognition of an underlying genetic disorder or telomere biology disorder leading to constitutional aplastic anemia is significant, as these conditions are associated not only with marrow failure, but also with endocrinopathies, organ fibrosis, and and hematopoietic and solid organ malignancies.13-15 In particular, TERT and TERC gene mutations have been associated with dyskeratosis congenita as well as pulmonary fibrosis and cirrhosis.18,19 The implications of early diagnosis of an IMFS lie in the approach to treatment and prognosis.
Clonal Disorders and Secondary Malignancies
Myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML) are 2 clonal disorders that may arise from a background of aplastic anemia.9,20,21 Hypoplastic MDS can be difficult to differentiate from aplastic anemia at diagnosis based on morphology alone, although recent work has demonstrated that molecular testing for somatic mutations in ASXL1, DNMT3A, and BCOR can aid in differentiating a subset of aplastic anemia patients who are more likely to progress to MDS.21 Clonal populations of cells harboring 6p uniparental disomy are seen in more than 10% of patients with aplastic anemia on cytogenetic analysis, which can help differentiate the diseases.9 Yoshizato and colleagues found lower rates of ASXL1 and DNMT3A mutations in patients with aplastic anemia as compared with patients with MDS or AML. In this study, patients with aplastic anemia had higher rates of mutations in PIGA (reflecting the increased paroxysmal nocturnal hemoglobinuria [PNH] clonality seen in aplastic anemia) and BCOR.9 Mutations were also found in genes commonly mutated in MDS and AML, including TET2, RUNX1, TP53, and JAK2, albeit at lower frequencies.9 These mutations as a whole have not predicted response to therapy or prognosis. However, when performing survival analysis in patients with specific mutations, those commonly encountered in MDS/AML (ASXL1, DNMT3A, TP53, RUNX1, CSMD1) are associated with faster progression to overt MDS/AML and decreased overall survival (OS),20,21 suggesting these mutations may represent early clonality that can lead to clonal evolution and the development of secondary malignancies. Conversely, mutations in BCOR and BCORL appear to identify patients who may have a favorable outcome in response to immunosuppressive therapy and, similar to patients with PIGA mutations, improved OS.9
Paroxysmal Nocturnal Hemoglobinuria
In addition to having an increased risk of myelodysplasia and malignancy due to the development of a dominant pre-malignant clone, patients with aplastic anemia often harbor progenitor cell clones associated with PNH.1,17 PNH clones have been identified in more than 50% of patients with aplastic anemia.22,23 PNH represents a clonal disorder of hematopoiesis in which cells harbor X-linked somatic mutations in the PIGA gene; this gene encodes a protein responsible for the synthesis of glycosylphosphatidylinositol anchors on the cell surface.22,24 The lack of these cell surface proteins, specifically CD55 (also known as decay accelerating factor) and CD59 (also known as membrane inhibitor of reactive lysis), predisposes red cells to increased complement-mediated lysis.25 The exact mechanism for the development of these clones in patients with aplastic anemia is not fully understood. Current theories hypothesize that the clones are protected from the immune-mediated destruction of normal hematopoietic stem cells due to the absence of the cell surface proteins.1,20 The role of these clones over time in patients with aplastic anemia is less clear, though recent work demonstrated that despite differences in clonality over the disease course, aplastic anemia patients with small PNH clones are less likely to develop overt hemolysis and larger PNH clones compared to patients harboring larger (≥ 50%) PNH clones at diagnosis.23,26,27 Additionally, PNH clones in patients with aplastic anemia infrequently become clinically significant.27 It should be noted that these conditions exist along a continuum; that is, patients with aplastic anemia may develop PNH clones, while conversely patients with PNH may develop aplastic anemia.20 Patients with PNH clones should be followed via peripheral blood flow cytometry and complete blood count to track clonal stability and identify clinically significant PNH among aplastic anemia patients.28
Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying; pulmonary, renal, and liver disease; and blood disorders.
Patients with an IMFS (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, classic phenotypical findings may be lacking in up to 30% to 40% of patients with an IMFS.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered, including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7

Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 Typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35
In addition to peripheral blood findings, bone marrow biopsy is essential for the diagnosis, and should demonstrate a markedly hypocellular marrow (cellularity < 25%), occasionally with an increase in T lymphocytes.7,39 Because marrow cellularity varies with age and can be challenging to assess, additional biopsies may be needed to confirm the diagnosis.29 A 1- to 2-cm core biopsy is necessary to confirm hypocellularity, as small areas of residual hematopoiesis may be present and obscure the diagnosis.35
Excluding Hypocellular MDS and IMFS
Excluding hypocellular MDS is challenging, especially in the older adult presenting with aplastic anemia, as patients with aplastic anemia may have some degree of erythroid dysplasia on bone marrow morphology.36 The presence of a PNH clone on flow cytometry can aid in diagnosing aplastic anemia and excluding MDS,34 although PNH clones can be present in refractory anemia MDS. Patients with aplastic anemia have a lower ratio of CD34+ cells compared to those with hypoplastic MDS, with 1 study demonstrating a mean CD34+ percentage of < 0.5% in aplastic anemia versus 3.7% in hypoplastic MDS.40 Cytogenetic and molecular testing can also aid in making this distinction by identifying mutations commonly implicated in MDS.7 The presence of monosomy 7 (-7) in aplastic anemia patients is associated with a poor overall prognosis.34,41
Peripheral blood screening using chromosome breakage analysis (done using either mitomycin C or diepoxybutane as in vitro DNA-crosslinking agents)42 and telomere length testing (of peripheral blood leukocytes) is necessary to exclude the main IMFSs, Fanconi anemia and telomere biology disorders, respectively. Ruling out these conditions is imperative, as the approach to treatment varies significantly between IMFS and aplastic anemia. Patients with shortened telomeres should undergo genetic screening for mutations in the telomere maintenance genes to evaluate the underlying defect leading to shortened telomeres. Patients with increased peripheral blood breakage should have genetic testing to detect mutations associated with Fanconi anemia.
Classification
Once the diagnosis of aplastic anemia has been made, the patient should be classified according to the severity of their disease. Disease severity is determined based on peripheral blood ANC: non-severe aplastic anemia (NSAA), ANC > 500 polymorphonuclear neutrophils (PMNs)/µL; severe aplastic anemia (SAA), 200–500 PMNs/µL; and very severe aplastic anemia (VSAA), 0–200 PMNs/µL.4,34 Disease classification is important, as VSAA is associated with a decreased OS compared to SAA.2 Disease classification may affect treatment decisions, as patients with NSAA may be observed for a short period of time, while, conversely, patients with SAA have a worse prognosis with delays in therapy.43-45
Treatment of Inherited Aplastic Anemia
First-line treatment options for patients with IMFS are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect. Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with multiple studies in IMFSs and SAA showing superior outcomes with a bone marrow product compared to peripheral blood stem cells.46-48 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.49,50 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival46,49,51 compared to cyclophosphamide conditioning, which was historically used in matched related donors.50,52 Adding fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.51,53 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data are limited.5
For patients presenting with AML or a high-risk MDS who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggest that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased OS, despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.54,55 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Treatment of Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan are being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.2
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.56,57 All blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.58 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an ANC < 500 cells/µL).59-62 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.63 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006). This difference was largely driven by a decrease in infectious episodes in patients with VSAA treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).63
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.64 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.62,65
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.61,62 This appears to be changing with time. Valdez et al demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.65 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.62 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with SAA or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).60,66 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.60 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues showed that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level of 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).67 Approximately 25% of patients in this trial had an increase in creatinine, with patients taking concomitant cyclosporine affected to a greater degree than those on chelation therapy alone. For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.58
Approach to Therapy
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.

Matched Sibling Donor Transplant. Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).43 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and MUD HSCT (38% and 65%).43 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.68

Current conditioning regimens typically use a combination of cyclophosphamide and ATG,69,70 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.53 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.71
Kim et al evaluated their experience with patients older than 40 years receiving matched related donors, finding comparable outcomes in those ages 41 to 50 years compared to younger patients. Outcomes declined in those over the age of 50 years.72 Long-term data for matched related donor transplant for aplastic anemia show excellent long-term outcomes, with minimal chronic GVHD and good performance status.73 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and MUD transplants for pediatric patients74,75 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.76,77
Immunosuppressive Therapy. For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.78 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.59,78,79
Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.80 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).59 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS, 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.81 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.81 The combination of ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.79 In this study, patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.82 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.83 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.84
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.34,84 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.84 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.85 When given at a dose of 150 mg daily in patients ages 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.45 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.12 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.85 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.86 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.48,65 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant. For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation (EBMT) analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-IV GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Factors associated with improved survival in this analysis include transplant under age 20 years (84% versus 72%), transplant within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and cytomegalovirus-negative donor and recipient as compared to other combinations (82% versus 76%).87 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.34,48 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).75 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.48
A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.75 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical PNH, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.88
With continued improvement of less toxic and more immunomodulating conditioning regimens,utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.89 However, there is still a large population of patients without matched sibling or unrelated donor options. Given the need to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant PNH, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.90
Summary
Aplastic anemia is a rare but potentially life-threatening disorder with pancytopenia and a marked reduction in the HSC compartment. It can be acquired or associated with an IMFS, and the treatment and prognosis vary dramatically between these 2 etiologies. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
Corresponding author: Gabrielle Meyers, MD, 3181 SW Sam Jackson Park Road, Mail Code UHN73C, Portland, OR 97239.
Financial disclosures: None.
From the Oregon Health and Science University, Portland, OR.
Abstract
- Objective: To describe the current approach to diagnosis and treatment of aplastic anemia.
- Methods: Review of the literature.
- Results: Aplastic anemia can be acquired or associated with an inherited marrow failure syndrome (IMFS), and the treatment and prognosis vary dramatically between these 2 etiologies. Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to life-threatening neutropenic infections or bleeding. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia.
- Conclusion: Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marro
w transplant program to evaluate for early transplantation is the new standard of care for aplastic anemia.
Keywords: inherited marrow failure syndrome; Fanconi anemia; immunosuppression; transplant; stem cell.
Aplastic anemia is a clinical and pathological entity of bone marrow failure that causes progressive loss of hematopoietic progenitor stem cells (HPSC), resulting in pancytopenia.1 Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to having life-threatening neutropenic infections or bleeding. Aplastic anemia results from either inherited or acquired causes, and the pathophysiology and treatment approach vary significantly between these 2 causes. Therefore, recognition of inherited marrow failure diseases, such as Fanconi anemia and telomere biology disorders, is critical to establishing the management plan.
Epidemiology
Aplastic anemia is a rare disorder, with an incidence of approximately 1.5 to 7 cases per million individuals per year.2,3 A recent Scandinavian study reported that the incidence of aplastic anemia among the Swedish population is 2.3 cases per million individuals per year, with a median age at diagnosis of 60 years and a slight female predominance (52% versus 48%, respectively).2 This data is congruent with prior observations made in Barcelona, where the incidence was 2.34 cases per million individuals per year, albeit with a slightly higher incidence in males compared to females (2.54 versus 2.16, respectively).4 The incidence of aplastic anemia varies globally, with a disproportionate increase in incidence seen among Asian populations, with rates as high as 8.8 per million individuals per year.3-5 This variation in incidence in Asia versus other countries has not been well explained. There appears to be a bimodal distribution, with incidence peaks seen in young adults and in older adults.2,3,6
Pathophysiology
Acquired Aplastic Anemia
The leading hypothesis as to the cause of most cases of acquired aplastic anemia is that a dysregulated immune system destroys HPSCs. Inciting etiologies implicated in the development of acquired aplastic anemia include pregnancy, infection, medications, and exposure to certain chemicals, such as benzene.1,7 The historical understanding of acquired aplastic anemia implicates cytotoxic T-lymphocyte–mediated destruction of CD34+ hematopoietic stem cells.1,8,9 This hypothesis served as the basis for treatment of acquired aplastic anemia with immunosuppressive therapy, predominantly anti-thymocyte globulin (ATG) combined with cyclosporine A.1,8 More recent work has focused on cytokine interactions, particularly the suppressive role of interferon (IFN)-γ on hematopoietic stem cells independent of T-lymphocyte–mediated destruction, which has been demonstrated in a murine model.8 The interaction of IFN-γ with the hematopoietic stem cell pool is dynamic. IFN-γ levels are elevated during an acute inflammatory response, such as a viral infection, providing further basis for the immune-mediated nature of the acquired disease.10 Specifically, in vitro studies suggest the effects of IFN-γ on HPSC may be secondary to interruption of thrombopoietin and its respective signaling pathways, which play a key role in hematopoietic stem cell renewal.11 Eltrombopag, a thrombopoietin receptor antagonist, has shown promise in the treatment of refractory aplastic anemia, with studies indicating that its effectiveness is independent of IFN-γ levels.11,12
Inherited Aplastic Anemia
The inherited marrow failure syndromes (IMFSs) are a group of disorders characterized by cellular maintenance and repair defects, leading to cytopenias, increased cancer risk, structural defects, and risk of end organ damage, such as liver cirrhosis and pulmonary fibrosis.13-15 The most common diseases include Fanconi anemia, dyskeratosis congenita/telomere biology disorders, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, but with the advent of whole exome sequencing, new syndromes continue to be discovered. While classically these disorders present in children, adult presentations are now commonplace. Broadly, the pathophysiology of inherited aplastic anemia relates to the defective HPSCs and an accelerated decline of the hematopoietic stem cell compartment.
The most common IMFSs, Fanconi anemia and telomere biology disorders, are associated with numerous mutations in DNA damage repair pathways and telomere maintenance pathways. TERT, DKC, and TERC mutations are most commonly associated with dyskeratosis congenita, but may also be found infrequently in patients with aplastic anemia presenting at an older age in the absence of the classic phenotypical features.1,16,17 The recognition of an underlying genetic disorder or telomere biology disorder leading to constitutional aplastic anemia is significant, as these conditions are associated not only with marrow failure, but also with endocrinopathies, organ fibrosis, and and hematopoietic and solid organ malignancies.13-15 In particular, TERT and TERC gene mutations have been associated with dyskeratosis congenita as well as pulmonary fibrosis and cirrhosis.18,19 The implications of early diagnosis of an IMFS lie in the approach to treatment and prognosis.
Clonal Disorders and Secondary Malignancies
Myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML) are 2 clonal disorders that may arise from a background of aplastic anemia.9,20,21 Hypoplastic MDS can be difficult to differentiate from aplastic anemia at diagnosis based on morphology alone, although recent work has demonstrated that molecular testing for somatic mutations in ASXL1, DNMT3A, and BCOR can aid in differentiating a subset of aplastic anemia patients who are more likely to progress to MDS.21 Clonal populations of cells harboring 6p uniparental disomy are seen in more than 10% of patients with aplastic anemia on cytogenetic analysis, which can help differentiate the diseases.9 Yoshizato and colleagues found lower rates of ASXL1 and DNMT3A mutations in patients with aplastic anemia as compared with patients with MDS or AML. In this study, patients with aplastic anemia had higher rates of mutations in PIGA (reflecting the increased paroxysmal nocturnal hemoglobinuria [PNH] clonality seen in aplastic anemia) and BCOR.9 Mutations were also found in genes commonly mutated in MDS and AML, including TET2, RUNX1, TP53, and JAK2, albeit at lower frequencies.9 These mutations as a whole have not predicted response to therapy or prognosis. However, when performing survival analysis in patients with specific mutations, those commonly encountered in MDS/AML (ASXL1, DNMT3A, TP53, RUNX1, CSMD1) are associated with faster progression to overt MDS/AML and decreased overall survival (OS),20,21 suggesting these mutations may represent early clonality that can lead to clonal evolution and the development of secondary malignancies. Conversely, mutations in BCOR and BCORL appear to identify patients who may have a favorable outcome in response to immunosuppressive therapy and, similar to patients with PIGA mutations, improved OS.9
Paroxysmal Nocturnal Hemoglobinuria
In addition to having an increased risk of myelodysplasia and malignancy due to the development of a dominant pre-malignant clone, patients with aplastic anemia often harbor progenitor cell clones associated with PNH.1,17 PNH clones have been identified in more than 50% of patients with aplastic anemia.22,23 PNH represents a clonal disorder of hematopoiesis in which cells harbor X-linked somatic mutations in the PIGA gene; this gene encodes a protein responsible for the synthesis of glycosylphosphatidylinositol anchors on the cell surface.22,24 The lack of these cell surface proteins, specifically CD55 (also known as decay accelerating factor) and CD59 (also known as membrane inhibitor of reactive lysis), predisposes red cells to increased complement-mediated lysis.25 The exact mechanism for the development of these clones in patients with aplastic anemia is not fully understood. Current theories hypothesize that the clones are protected from the immune-mediated destruction of normal hematopoietic stem cells due to the absence of the cell surface proteins.1,20 The role of these clones over time in patients with aplastic anemia is less clear, though recent work demonstrated that despite differences in clonality over the disease course, aplastic anemia patients with small PNH clones are less likely to develop overt hemolysis and larger PNH clones compared to patients harboring larger (≥ 50%) PNH clones at diagnosis.23,26,27 Additionally, PNH clones in patients with aplastic anemia infrequently become clinically significant.27 It should be noted that these conditions exist along a continuum; that is, patients with aplastic anemia may develop PNH clones, while conversely patients with PNH may develop aplastic anemia.20 Patients with PNH clones should be followed via peripheral blood flow cytometry and complete blood count to track clonal stability and identify clinically significant PNH among aplastic anemia patients.28
Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying; pulmonary, renal, and liver disease; and blood disorders.
Patients with an IMFS (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, classic phenotypical findings may be lacking in up to 30% to 40% of patients with an IMFS.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered, including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7

Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 Typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35
In addition to peripheral blood findings, bone marrow biopsy is essential for the diagnosis, and should demonstrate a markedly hypocellular marrow (cellularity < 25%), occasionally with an increase in T lymphocytes.7,39 Because marrow cellularity varies with age and can be challenging to assess, additional biopsies may be needed to confirm the diagnosis.29 A 1- to 2-cm core biopsy is necessary to confirm hypocellularity, as small areas of residual hematopoiesis may be present and obscure the diagnosis.35
Excluding Hypocellular MDS and IMFS
Excluding hypocellular MDS is challenging, especially in the older adult presenting with aplastic anemia, as patients with aplastic anemia may have some degree of erythroid dysplasia on bone marrow morphology.36 The presence of a PNH clone on flow cytometry can aid in diagnosing aplastic anemia and excluding MDS,34 although PNH clones can be present in refractory anemia MDS. Patients with aplastic anemia have a lower ratio of CD34+ cells compared to those with hypoplastic MDS, with 1 study demonstrating a mean CD34+ percentage of < 0.5% in aplastic anemia versus 3.7% in hypoplastic MDS.40 Cytogenetic and molecular testing can also aid in making this distinction by identifying mutations commonly implicated in MDS.7 The presence of monosomy 7 (-7) in aplastic anemia patients is associated with a poor overall prognosis.34,41
Peripheral blood screening using chromosome breakage analysis (done using either mitomycin C or diepoxybutane as in vitro DNA-crosslinking agents)42 and telomere length testing (of peripheral blood leukocytes) is necessary to exclude the main IMFSs, Fanconi anemia and telomere biology disorders, respectively. Ruling out these conditions is imperative, as the approach to treatment varies significantly between IMFS and aplastic anemia. Patients with shortened telomeres should undergo genetic screening for mutations in the telomere maintenance genes to evaluate the underlying defect leading to shortened telomeres. Patients with increased peripheral blood breakage should have genetic testing to detect mutations associated with Fanconi anemia.
Classification
Once the diagnosis of aplastic anemia has been made, the patient should be classified according to the severity of their disease. Disease severity is determined based on peripheral blood ANC: non-severe aplastic anemia (NSAA), ANC > 500 polymorphonuclear neutrophils (PMNs)/µL; severe aplastic anemia (SAA), 200–500 PMNs/µL; and very severe aplastic anemia (VSAA), 0–200 PMNs/µL.4,34 Disease classification is important, as VSAA is associated with a decreased OS compared to SAA.2 Disease classification may affect treatment decisions, as patients with NSAA may be observed for a short period of time, while, conversely, patients with SAA have a worse prognosis with delays in therapy.43-45
Treatment of Inherited Aplastic Anemia
First-line treatment options for patients with IMFS are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect. Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with multiple studies in IMFSs and SAA showing superior outcomes with a bone marrow product compared to peripheral blood stem cells.46-48 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.49,50 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival46,49,51 compared to cyclophosphamide conditioning, which was historically used in matched related donors.50,52 Adding fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.51,53 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data are limited.5
For patients presenting with AML or a high-risk MDS who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggest that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased OS, despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.54,55 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Treatment of Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan are being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.2
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.56,57 All blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.58 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an ANC < 500 cells/µL).59-62 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.63 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006). This difference was largely driven by a decrease in infectious episodes in patients with VSAA treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).63
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.64 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.62,65
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.61,62 This appears to be changing with time. Valdez et al demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.65 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.62 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with SAA or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).60,66 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.60 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues showed that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level of 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).67 Approximately 25% of patients in this trial had an increase in creatinine, with patients taking concomitant cyclosporine affected to a greater degree than those on chelation therapy alone. For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.58
Approach to Therapy
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.

Matched Sibling Donor Transplant. Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).43 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and MUD HSCT (38% and 65%).43 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.68

Current conditioning regimens typically use a combination of cyclophosphamide and ATG,69,70 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.53 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.71
Kim et al evaluated their experience with patients older than 40 years receiving matched related donors, finding comparable outcomes in those ages 41 to 50 years compared to younger patients. Outcomes declined in those over the age of 50 years.72 Long-term data for matched related donor transplant for aplastic anemia show excellent long-term outcomes, with minimal chronic GVHD and good performance status.73 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and MUD transplants for pediatric patients74,75 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.76,77
Immunosuppressive Therapy. For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.78 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.59,78,79
Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.80 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).59 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS, 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.81 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.81 The combination of ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.79 In this study, patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.82 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.83 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.84
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.34,84 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.84 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.85 When given at a dose of 150 mg daily in patients ages 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.45 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.12 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.85 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.86 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.48,65 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant. For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation (EBMT) analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-IV GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Factors associated with improved survival in this analysis include transplant under age 20 years (84% versus 72%), transplant within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and cytomegalovirus-negative donor and recipient as compared to other combinations (82% versus 76%).87 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.34,48 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).75 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.48
A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.75 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical PNH, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.88
With continued improvement of less toxic and more immunomodulating conditioning regimens,utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.89 However, there is still a large population of patients without matched sibling or unrelated donor options. Given the need to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant PNH, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.90
Summary
Aplastic anemia is a rare but potentially life-threatening disorder with pancytopenia and a marked reduction in the HSC compartment. It can be acquired or associated with an IMFS, and the treatment and prognosis vary dramatically between these 2 etiologies. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
Corresponding author: Gabrielle Meyers, MD, 3181 SW Sam Jackson Park Road, Mail Code UHN73C, Portland, OR 97239.
Financial disclosures: None.
1. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519.
2. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
3. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718-1721.
4. Montané E, Ibanez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica. 2008;93:518-523.
5. Ohta A, Nagai M, Nishina M, et al. Incidence of aplastic anemia in Japan: analysis of data from a nationwide registration system. Int J Epidemiol. 2015; 44(suppl_1):i178.
6. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
7. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
8. Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoiesis stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699-3708.
9. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35-47.
10. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479-2486.
11. Cheng H, Cheruku PS, Alvarado L, et al. Interferon-γ perturbs key signaling pathways induced by thrombopoietin, but not eltrombopag, in human hematopoietic stem/progenitor cells. Blood. 2016;128:3870.
12. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
13. Townsley DM, Dumitriu B, Young NS, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922-1931.
14. Feurstein S, Drazer MW, Godley LA. Genetic predisposition to leukemia and other hematologic malignancies. Semin Oncol. 2016;43:598-608.
15. Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775-2783.
16. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16:S119-S125.
17. Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446-4455.
18. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
19. Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J. 2016;48:1721-1731.
20. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337-347.
21. Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood. 2014;124:2698-2704.
22. Mukhina GL, Buckley JT, Barber JP, et al. Multilineage glycosylphosphatidylinositol anchor‐deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476-482.
23. Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45.
24. Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-5340.
25. Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190-198.
26. Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308-1314.
27. Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-1080.
28. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709.
29. Guinan EC. Diagnosis and management of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:76-81.
30. Giampietro PF, Verlander PC, Davis JG, Auerbach AD. Diagnosis of Fanconi anemia in patients without congenital malformations: an international Fanconi Anemia Registry Study. Am J Med Genetics. 1997;68:58-61.
31. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4-10.
32. Giampietro PF, Davis JG, Adler-Brecher B, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116-1120.
33. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
34. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
35. DeZern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol. 2011;4:221-230.
36. Tichelli A, Gratwohl A, Nissen C, et al. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood. 1992;80:337-345.
37. Camitta BM, Storb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645-652.
38. Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177-182.
39. Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136-2141.
40. Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458-462.
41. Maciejewski JP, Risitano AM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129-3135.
42. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
43. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-18.
44. Passweg JR, Socié G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858-864.
45. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95:2119-2125.
46. Peffault de Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122:4279-4286.
47. Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
48. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
49. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Bone Marrow Transplant. 2015;50:1168-1172.
50. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
51. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
52. Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
53. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
54. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-e200.
55. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for Fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
56. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
57. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
58. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
59. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430-438.
60. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
61. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol. 2009;46:269-276.
62. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
63. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
64. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
65. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
66. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
67. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2454.
68. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
69. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
70. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. Hematology Am Soc Hematol Educ Program. 2013;2013:82-86.
71. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
72. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
73. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
74. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
75. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br J Haematol. 2015;171:585-594.
76. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
77. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
78. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
79. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al; German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
80. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
81. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
82. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
83. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
84. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
85. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
86. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018;124:4192-4201.
87. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
88. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. Am J Hematol. 2019; 94:80-86.
89. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
90. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
1. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519.
2. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
3. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718-1721.
4. Montané E, Ibanez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica. 2008;93:518-523.
5. Ohta A, Nagai M, Nishina M, et al. Incidence of aplastic anemia in Japan: analysis of data from a nationwide registration system. Int J Epidemiol. 2015; 44(suppl_1):i178.
6. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
7. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
8. Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoiesis stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699-3708.
9. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35-47.
10. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479-2486.
11. Cheng H, Cheruku PS, Alvarado L, et al. Interferon-γ perturbs key signaling pathways induced by thrombopoietin, but not eltrombopag, in human hematopoietic stem/progenitor cells. Blood. 2016;128:3870.
12. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
13. Townsley DM, Dumitriu B, Young NS, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922-1931.
14. Feurstein S, Drazer MW, Godley LA. Genetic predisposition to leukemia and other hematologic malignancies. Semin Oncol. 2016;43:598-608.
15. Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775-2783.
16. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16:S119-S125.
17. Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446-4455.
18. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
19. Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J. 2016;48:1721-1731.
20. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337-347.
21. Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood. 2014;124:2698-2704.
22. Mukhina GL, Buckley JT, Barber JP, et al. Multilineage glycosylphosphatidylinositol anchor‐deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476-482.
23. Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45.
24. Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-5340.
25. Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190-198.
26. Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308-1314.
27. Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-1080.
28. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709.
29. Guinan EC. Diagnosis and management of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:76-81.
30. Giampietro PF, Verlander PC, Davis JG, Auerbach AD. Diagnosis of Fanconi anemia in patients without congenital malformations: an international Fanconi Anemia Registry Study. Am J Med Genetics. 1997;68:58-61.
31. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4-10.
32. Giampietro PF, Davis JG, Adler-Brecher B, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116-1120.
33. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
34. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
35. DeZern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol. 2011;4:221-230.
36. Tichelli A, Gratwohl A, Nissen C, et al. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood. 1992;80:337-345.
37. Camitta BM, Storb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645-652.
38. Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177-182.
39. Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136-2141.
40. Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458-462.
41. Maciejewski JP, Risitano AM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129-3135.
42. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
43. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-18.
44. Passweg JR, Socié G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858-864.
45. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95:2119-2125.
46. Peffault de Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122:4279-4286.
47. Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
48. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
49. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Bone Marrow Transplant. 2015;50:1168-1172.
50. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
51. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
52. Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
53. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
54. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-e200.
55. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for Fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
56. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
57. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
58. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
59. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430-438.
60. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
61. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol. 2009;46:269-276.
62. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
63. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
64. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
65. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
66. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
67. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2454.
68. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
69. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
70. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. Hematology Am Soc Hematol Educ Program. 2013;2013:82-86.
71. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
72. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
73. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
74. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
75. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br J Haematol. 2015;171:585-594.
76. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
77. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
78. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
79. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al; German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
80. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
81. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
82. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
83. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
84. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
85. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
86. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018;124:4192-4201.
87. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
88. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. Am J Hematol. 2019; 94:80-86.
89. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
90. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
Role of Yoga Across the Cancer Care Continuum: From Diagnosis Through Survivorship
From the University of Texas MD Anderson Cancer Center, Houston, TX (Drs. Narayanan, Lopez, Chaoul, Liu, Milbury, and Cohen, and Ms. Mallaiah); the University of Texas Health Science Center at Tyler (Dr. Meegada); and Texas Tech University Health Sciences Center, Lubbock, TX (Ms. Francisco).
Abstract
- Objective: To review the effects of yoga as an adjunct supportive care modality alongside conventional cancer treatment on quality of life (QOL), physical and mental health outcomes, and physiological and biological measures of cancer survivors.
- Methods: Nonsystematic review of the literature.
- Results: Yoga therapy, one of the most frequently used mind-body modalities, has been studied extensively in cancer survivors (from the time of diagnosis through long-term recovery). Yoga affects human physiology on multiple levels, including psychological outcomes, immune and endocrine function, and cardiovascular parameters, as well as multiple areas of QOL. It has been found to reduce psychological stress and fatigue and improve QOL in cancer patients and survivors. Yoga has also been used to manage symptoms such as arthralgia, fatigue, and insomnia. In addition, yoga offers benefits not only for cancer survivors but also for their caregivers.
- Conclusion: As part of an integrative, evidence-informed approach to cancer care, yoga may provide benefits that support the health of cancer survivors and caregivers.
Keywords: fatigue; cancer; proinflammatory cytokines; integrative; mind-body practices; meditation; DNA damage; stress; psychoneuro-immunoendocrine axis; lymphedema; insomnia.
A diagnosis of cancer and adverse effects related to its treatment may have negative effects on quality of life (QOL), contributing to emotional and physical distress in patients and caregivers. Many patients express an interest in pursuing nonpharmacological options, alone or as an adjunct to conventional therapy, to help manage symptoms. The use of complementary medicine approaches to health, including nonpharmacological approaches to symptom management, is highest among individuals with cancer.1 According to a published expert consensus, integrative oncology is defined as a “patient-centered, evidence-informed field of cancer care that utilizes mind and body practices, natural products, and/or lifestyle modifications from different traditions alongside conventional cancer treatments. Integrative oncology aims to optimize health, QOL, and clinical outcomes across the cancer care continuum and to empower people to prevent cancer and become active participants before, during, and beyond cancer treatment.”2 A key component of this definition, often misunderstood in the field of oncology, is that these modalities and treatments are used alongside conventional cancer treatments and not as an alternative. In an attempt to meet patients’ needs and appropriately use these approaches, integrative oncology programs are now part of most cancer centers in the United States.3-6
Because of their overall safety, mind-body therapies are commonly used by patients and recommended by clinicians. Mind-body therapies include yoga, tai chi, qigong, meditation, and relaxation. Expressive arts such as journaling and music, art, and dance therapies also fall in the mind-body category.7 Yoga is a movement-based mind-body practice that focuses on synchronizing body, breath, and mind. Yoga has been increasingly used by patients for health benefits,8 and numerous studies have evaluated yoga as a complementary intervention for individuals with cancer.9-14 Here, we review the physiological basis of yoga in oncology and the effects of yoga on biological processes, QOL, and symptoms during and after cancer treatment.
Physiological Basis
Many patients may use mind-body programs such as yoga to help manage the psychological and physiological consequences of unmanaged chronic stress and improve their overall QOL. The central nervous system, endocrine system, and immune system influence and interact with each other in a complex manner in response to chronic stress.15,16 In a stressful situation, the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS) are activated. HPA axis stimulation leads to adrenocorticotrophic hormone production by the pituitary gland, which releases glucocorticoid hormones. SNS axis stimulation leads to epinephrine and norepinephrine production by the adrenal gland.17,18 Recently, studies have explored modulation of signal transduction between the nervous and immune systems and how that may impact tumor growth and metastasis.19 Multiple studies, controlled for prognosis, disease stage, and other factors, have shown that patients experiencing more distress or higher levels of depressive symptoms do not live as long as their counterparts with low distress or depression levels.20 Both the meditative and physical components of yoga can lead to enhanced relaxation, reduced SNS activation, and greater parasympathetic tone, countering the negative physiological effects of chronic stress. The effects of yoga on the HPA axis and SNS, proinflammatory cytokines, immune function, and DNA damage are discussed below.
Biological Processes
Nervous System
The effects of yoga and other forms of meditation on brain functions have been established through several studies. Yoga seems to influence basal ganglia function by improving circuits that are involved in complex cognitive functions, motor coordination, and somatosensory and emotional processes.21,22 Additionally, changes in neurotransmitter levels have been observed after yoga practice. For instance, in a 12-week yoga intervention in healthy subjects, increased levels of thalamic gamma-aminobutyric acid (GABA) in the yoga group were reported to have a positive correlation with improved mood and decreased anxiety compared with a group who did metabolically matched walking exercise.23 Levels of GABA, an inhibitory neurotransmitter, are decreased in conditions such as anxiety, depression, and epilepsy.24 Yoga therapy has been shown to improve symptoms of mood disorders and epilepsy, which leads to the hypothesis that the mechanism driving the benefits of yoga may work through stimulation of vagal efferents and an increase in GABA-mediated cortical-inhibitory tone.24,25
HPA Axis
Stress activates the HPA/SNS axis, which releases hormones such as cortisol and norepinephrine. These hormones may play a role in angiogenesis, inflammation, immune suppression, and other physiological functions, and may even reduce the effect of chemotherapeutic agents.26,27 Regular yoga practice has been shown to reduce SNS and HPA axis activity, most likely by increasing parasympathetic dominance through vagal stimulation, as demonstrated through increases in heart rate variability.28 One indicator of HPA axis dysregulation, diurnal salivary cortisol rhythm, was shown to predict survival in patients with advanced breast and renal cancer.29-33 Yoga has been shown to lead to less cortisol dysregulation due to radiotherapy and to reductions in mean cortisol levels and early morning cortisol levels in breast cancer patients undergoing radiotherapy.34 This lends support to the hypothesis that yoga helps restore HPA axis balance.
Proinflammatory Cytokines
Cancer patients tend to have increased levels of inflammatory markers such as interleukin (IL)-4, IL-10, tumor necrosis factor (TNF), interferon-γ, and C-reactive protein. This increase in inflammation is associated with worse outcomes in cancer.35 This association becomes highly relevant because the effect of inflammation on host cells in the tumor microenvironment is connected to disease progression.26 Inflammatory cytokines are also implicated in cancer-related symptoms such as fatigue, cognitive dysfunction, peripheral neuropathy, and sleep disturbances.36
Yoga is known to reduce stress and may directly or indirectly decrease inflammatory cytokines. A randomized clinical trial of a 12-week hatha yoga intervention among breast cancer survivors demonstrated decreases in IL-6, IL-1β, TNF, corticotropin-releasing factor, and cognitive complaints in the yoga group compared with those in the standard care group after 3 months.37,38 Furthermore, Carlson et al showed that, after mindfulness-based stress reduction involving a combination of gentle yoga, meditation techniques, and relaxation exercises, breast and prostate cancer patients had reduced levels of proinflammatory cytokines and cortisol.39 These reductions translated into patients reporting decreased stress levels and enhanced QOL.
Immune Function
The effects of yoga practice on the immune system have been studied in both healthy individuals and individuals with cancer. The effects on T and B lymphocytes, natural killer (NK) cells, and other immune effector cells demonstrate that meditation and yoga have beneficial effects on immune activity.40 Hormones such as catecholamines and glucocorticoids are thought to influence the availability and function of NK cells, and, as noted above, yoga has been shown to modulate stress hormones and lead to reduced immune suppression in patients with early-stage breast cancer undergoing chemotherapy.41 Additional evidence supports the ability of yoga to reduce immune suppression in the postsurgical setting, with no observed decrease in NK cell percentage after surgery for those in a yoga group compared with a control group.42 This finding is relevant to patients undergoing surgical management of their cancer and highlights the impact of yoga on the immune system.
DNA Damage
Radiation damages DNA in the peripheral blood lymphocytes of patients undergoing treatment.43,44 This damage is significant in breast cancer patients undergoing radiotherapy.45 Stress additionally causes DNA damage46 and is correlated to impaired DNA repair capacity.47,48 In a study conducted by Banerjee et al, breast cancer patients were randomly assigned to a yoga group or a supportive therapy group for 6 weeks during radiotherapy.49 Prior to the intervention, patients in the study had significant genomic instability. After treatment, patients in the yoga group experienced not only a significant reduction in anxiety and depression levels, but also a reduction in DNA damage due to radiotherapy.
Yoga in Quality of Life and Symptom Management
There is evidence showing that yoga therapy improves multiple aspects of QOL, including physical functioning, emotional health outcomes, and the symptoms cancer patients may experience, such as sleep disturbances, fatigue, and pain. Danhauer et al systematically reviewed both nonrandomized trials and randomized controlled trials involving yoga during cancer treatment.50 They found that yoga improved depression and anxiety as well as sleep and fatigue. Benefits of yoga in cancer based on randomized controlled trials are summarized in the Table. The role of yoga in improving QOL and managing symptoms patients experience during and after treatment is discussed in the following sections.
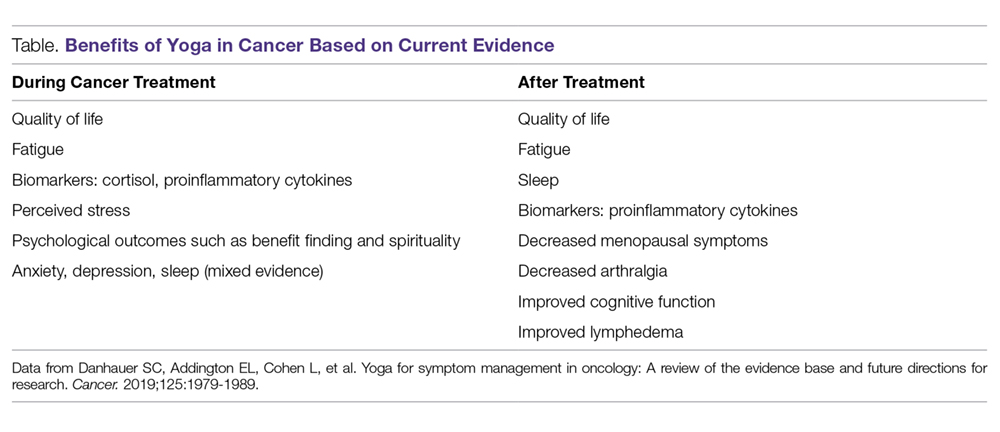
Quality of Life
Danhauer et al’s systematic review of trials involving yoga during cancer treatment found that yoga improved multiple aspects of QOL.50 For example, yoga has been shown to improve QOL in breast cancer patients undergoing radiotherapy. In a study by Chandwani et al, yoga (60-minute sessions twice a week for 6 weeks) was associated with better general health perception and physical functioning scores as well as greater benefit finding, or finding meaning in their experience, after radiotherapy compared with a wait-list group.51 The yoga group had an increase in intrusive thoughts, believed to be due to a more thorough processing of the cancer experience, which helps to improve patients’ outlook on life.52 The benefits of yoga extend beyond psychological measures during radiation treatment. Yoga was found to increase physical functioning compared with stretching in breast cancer patients undergoing radiotherapy.53
Cognitive Function
Cancer-related cognitive impairment commonly occurs during cancer treatments (eg, chemotherapy, radiotherapy, surgery, hormone therapy) and persists for months or years in survivors.54 Impairment of memory, executive function, attention, and concentration are commonly reported. In a trial of a combined hatha and restorative yoga program called Yoga for Cancer Survivors (YOCAS), which was designed by researchers at the University of Rochester, patients in the yoga arm had less memory difficulty than did patients in the standard care arm.55 However, the primary aim of the trial was to treat insomnia, so this secondary outcome needs to be interpreted with caution. Deficits in attention, memory, and executive function are often seen in cancer-related cognitive impairment, and the meditative aspect of yoga may have behavioral and neurophysical benefits that could improve cognitive functions.56 More evidence is needed to understand the role of yoga in improving cognitive functioning.
Emotional Health
Psychosocial stress is high among breast cancer patients and survivors.57,58 This causes circadian rhythm and cortisol regulation abnormalities, which are reported in women with breast cancer.59-64 Yoga is known to help stress and psychosocial and physical functioning in patients with cancer.65 Yoga was also shown to be equivalent to cognitive behavioral therapy in stress management in a population of patients without cancer.66 Daily yoga sessions lasting 60 minutes were shown to reduce reactive anxiety and trait anxiety in early-stage breast cancer patients undergoing conventional radiotherapy and chemotherapy compared with patients receiving supportive therapy, highlighting the role of yoga in managing anxiety related to treatment.67 In a study done by Culos-Reed et al, 20 cancer survivors who did 75 minutes of yoga per week for 7 weeks were compared with 18 cancer survivors who served as a control group.68 The intervention group reported significant improvement in emotional well-being, depression, concentration, and mood disturbances. In a longitudinal study by Mackenzie et al, 66 cancer survivors completed a 7-week yoga program and were assessed at baseline, immediately after the final yoga session, and at 3 and 6 months after the final session.69 Participants had significantly improved energy levels and affect. They also had moderate improvement in mindfulness and a moderate decrease in stress. Breast cancer patients who underwent restorative yoga sessions found improvements in mental health, depression, positive affect, and spirituality (peace/meaning).70 This was more pronounced in women with higher negative affect and lower emotional well-being at baseline. In a study of patients with ovarian cancer receiving chemotherapy, patients were instructed to perform up to 15-minute sessions including awareness, body movement, and breathing.71 Even with just 1 session of yoga intervention, patients experienced decreased anxiety.
Fatigue
Studies on yoga show improvement in fatigue both during and after treatment. In breast cancer patients undergoing chemotherapy, yoga was shown to benefit cognitive fatigue.72 Older cancer survivors also seem to benefit from yoga interventions.73 In a trial of a DVD-based yoga program, the benefits of yoga were similar to those of strengthening exercises, and both interventions helped decrease fatigue and improve QOL during the first year after diagnosis in early-stage breast cancer patients with cancer-related fatigue.74 Bower et al also showed that, for breast cancer survivors experiencing persistent chronic fatigue, a targeted yoga intervention led to significant improvements in fatigue and vigor over a 3-month follow-up compared with controls.75 Fatigue is commonly seen in breast cancer patients who are receiving adjuvant chemotherapy. In a study by Taso et al, women with breast cancer receiving chemotherapy were assigned to 60-minute yoga sessions incorporating Anusara yoga, gentle stretching, and relaxation twice a week for 8 weeks.76 By week 4, patients with low pretest fatigue in the yoga group experienced a reduction in fatigue. By week 8, all patients in the yoga group experienced a reduction in fatigue. Four weeks after the yoga intervention, patients in the group maintained the reduction in fatigue. This study shows the feasibility of an 8-week yoga program for women undergoing breast cancer therapy by improving fatigue. Yoga recently was added to National Comprehensive Cancer Network (NCCN) guidelines for management of cancer-related fatigue (level 1 evidence).77 However, the evidence was based on studies in women with breast cancer and survivors; therefore, more studies are needed in men and women with other cancers.
Surgical Setting/Postoperative Distress
Distress surrounding surgery in patients with breast cancer can impact postoperative outcomes. Yoga interventions, including breathing exercises, regulated breathing, and yogic relaxation techniques, improved several postsurgical measures such as length of hospital stay, drain retention, and suture removal.78 In this study, patients who practiced yoga also experienced a decrease in plasma TNF and better wound healing. Symptoms of anxiety and distress that occur preoperatively can lead to impaired immune function in addition to decreased QOL. In a study of yoga in early-stage breast cancer patients undergoing surgery, the benefit of yoga was seen not only with stress reduction but also with immune enhancement.42
Yoga has been shown to help alleviate acute pain and distress in women undergoing major surgery for gynecological cancer. A regimen of 3 15-minute sessions of yoga, including awareness meditation, coordination of breath with movement, and relaxation breathing, was shown to reduce acute pain and distress in such patients in an inpatient setting.79
Menopausal Symptoms
Breast cancer survivors have more severe menopausal symptoms compared with women without cancer.80,81 Hot flashes cause sleep disturbances and worsen fatigue and QOL.82 Tamoxifen and aromatase inhibitors significantly worsen menopausal symptoms such as hot flashes.81 Carson et al conducted a study of yoga that included postures, breathing techniques, didactic presentations, and group discussions.83 The yoga awareness regimen consisted of 8 weekly 120-minute group classes. Patients in the yoga arm had statistically significant improvements in the frequency, severity, and number of hot flashes. There were also improvements in arthralgia (joint pain), fatigue, sleep disturbance, vigor, and acceptance.
Arthralgia
Joint pain can be a major side effect that interferes with daily functions and activities in postmenopausal breast cancer survivors who receive aromatase inhibitor therapy.84 Arthralgia is reported in up to 50% of patients treated with aromatase inhibitors.84,85 It can affect functional status and lead to discontinuation of aromatase inhibitor therapy, jeopardizing clinical outcomes.86 Yoga as a complementary therapy has been shown to improve conditions such as low back pain87 and knee osteoarthritis88 in patients who do not have cancer. In a single-arm pilot trial by Galantino et al, breast cancer patients with aromatase inhibitor–related joint pain were provided with twice-weekly yoga sessions for 8 weeks. There were statistically significant improvements in balance, flexibility, pain severity, and health-related QOL.89 As noted above, improvement in arthralgia was also found in the study conducted by Carson et al.83
Insomnia
Insomnia is common among cancer patients and survivors90,91 and leads to increased fatigue and depression, decreased adherence to cancer treatments, and poor physical function and QOL.90-92 Management of insomnia consists of pharmacologic therapies such as benzodiazepines93,94 and nonpharmacologic options such as cognitive behavioral therapy.95
The first study of yoga found to improve sleep quality was conducted at MD Anderson Cancer Center in lymphoma patients.96 The effects of Tibetan yoga practices incorporating controlled breathing and visualization, mindfulness techniques, and low-impact postures were studied. Patients in the Tibetan yoga group had better subjective sleep quality, faster sleep latency, longer sleep duration, and less use of sleep medications. Mustian et al conducted a large yoga study in cancer survivors in which patients reporting chronic sleep disturbances were randomly assigned to the YOCAS program, which consisted of pranayama (breath control), 16 gentle hatha and restorative yoga postures, and meditation, or to usual care.92 The study reported improvements in global sleep quality, subjective sleep quality, actigraphy measures (wake after sleep onset, sleep efficiency), daytime dysfunction, and use of sleep medication after the yoga intervention compared with participants who received standard care.
Yoga to Address Other Symptoms
There is preliminary evidence supporting yoga as an integrative therapy for other symptoms unique to cancer survivors. For example, in head and neck cancer survivors, soft tissue damage involving the jaw, neck, shoulders, and chest results in swallowing issues, trismus, and aspiration, which are more pronounced in patients treated with conventional radiotherapy than in those treated with intensity-modulated radiotherapy.97 Some late effects of radiotherapy for head and neck cancer—such as pain, anxiety, and impaired shoulder function—were shown to be improved through the practice of hatha yoga in 1 study.98 Similarly, in a randomized controlled pilot study of patients with stage I to III breast cancer 6 months after treatment, participants in an 8-week yoga program experienced a reduction in arm induration and improvement in a QOL subscale of lymphedema symptoms. However, more evidence is needed to support the use of yoga as a therapeutic measure for breast cancer lymphedema.99,100
Yoga for Caregivers
Along with cancer patients, caregivers face psychological and physical burdens as well as deterioration in their QOL. Caregivers tend to report clinical levels of anxiety, depression, sleep disturbance, and fatigue and have similar or in fact higher levels than those of the patients for whom they are caring.101,102 Yoga has been found to help caregivers of patients with cancer. Recently, MD Anderson researchers conducted a trial in patients with high-grade glioma and their caregivers as dyads.103,104 Each dyad attended 2 or 3 60-minute weekly Vivekananda yoga sessions involving breathing exercises, physical exercises, relaxation, and meditation. The researchers found that the yoga program was safe, feasible, acceptable, and subjectively useful for patients with high-grade glioma and their caregivers. Preliminary evidence of QOL improvement for both patients and caregivers was noted. An improvement in QOL was also demonstrated in another preliminary study of yoga in patients undergoing thoracic radiotherapy and their caregivers.105
Another study by the group at MD Anderson evaluated a couple-based Tibetan yoga program that emphasized breathing exercises, gentle movements, guided visualizations, and emotional connectedness during radiotherapy for lung cancer.106 This study included 10 patient‐caregiver dyads and found the program to be feasible, safe, and acceptable. The researchers also found preliminary evidence of improved QOL by the end of radiotherapy relative to baseline—specifically in the areas of spiritual well‐being for patients, fatigue for caregivers, and sleep disturbances and mental health issues such as anxiety and depressive symptoms for both patients and caregivers. This is noteworthy, as QOL typically deteriorates during the course of radiotherapy, and the yoga program was able to buffer these changes.
Conclusion
Yoga therapy has been used successfully as an adjunct modality to improve QOL and cancer-related symptoms. As a part of an integrative medicine approach, yoga is commonly recommended for patients undergoing cancer treatment. Danhauer et al reviewed randomized controlled trials during and after treatment and concluded that the evidence is clearly positive for QOL, fatigue, and perceived stress.107 Results are less consistent but supportive for psychosocial outcomes such as benefit finding and spirituality. Evidence is mixed for sleep, anxiety, and depression. Post-treatment studies demonstrate improvements in fatigue, sleep, and multiple QOL domains. Yoga has been included in NCCN guidelines for fatigue management. Yoga, if approved by a physician, is also included among the behavioral therapies for anticipatory emesis and prevention and treatment of nausea in the recent update of the NCCN guidelines.108 The Society for Integrative Oncology guidelines include yoga for anxiety/stress reduction as a part of integrative treatment in breast cancer patients during and after therapy, which was endorsed by the American Society of Clinical Oncology.109
Because of the strong evidence for its benefits and a low side-effect profile, yoga is offered in group-class settings for patients during and after treatment and/or for caregivers in our institution. We often prescribe yoga as a therapeutic modality for selected groups of patients in our clinical practice. However, some patients may have restrictions after surgery that must be considered. In general, yoga has an excellent safety profile, the evidence base is strong, and we recommend that yoga therapy should be part of the standard of care as an integrative approach for patients with cancer undergoing active treatment as well as for cancer survivors and caregivers.
Acknowledgement: The authors thank Bryan Tutt for providing editorial assistance.
Corresponding author: Santhosshi Narayanan, MD, Department of Palliative, Rehabilitation, and Integrative Medicine, Unit 1414, The University of Texas MD Anderson Cancer Center, 1400 Pressler St., Houston, TX 77030; [email protected].
Financial disclosures: None.
1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Reports. 2008;(12):1-23.
2. Witt CM, Balneaves LG, Cardoso MJ, et al. A comprehensive definition for integrative oncology. NCI Monographs. 2017;2017(52):3-8.
3. Brauer JA, El Sehamy A, Metz JM, Mao JJ. Complementary and alternative medicine and supportive care at leading cancer centers: a systematic analysis of websites. J Altern Complement Med. 2010;16:183-186.
4. Lopez G, McQuade J, Cohen L, et al. Integrative oncology physician consultations at a comprehensive cancer center: analysis of demographic, clinical and patient reported outcomes. J Cancer. 2017;8:395-402.
5. Latte-Naor S, Mao JJ. Putting integrative oncology into practice: concepts and approaches. J Oncol Pract. 2019;15:7-14.
6. Richardson MA, Sanders T, Palmer JL, et al. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18:2505-2514.
7. Chaoul A, Milbury K, Sood AK, et al. Mind-body practices in cancer care. Curr Oncol Rep. 2014;16:417.
8. Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10:44-49.
9. Smith KB, Pukall CF. An evidence-based review of yoga as a complementary intervention for patients with cancer. Psychooncology. 2009;18:465-475.
10. Buffart LM, van Uffelen JG, Riphagen II, et al. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2012;12:559.
11. Cramer H, Lange S, Klose P, et al. Yoga for breast cancer patients and survivors: a systematic review and meta-analysis. BMC Cancer. 2012;12:412.
12. Felbel S, Meerpohl JJ, Monsef I, et al. Yoga in addition to standard care for patients with haematological malignancies. Cochrane Database Syst Rev. 2014;(6):CD010146.
13. Greenlee H, Balneaves LG, Carlson LE, et al. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. NCI Monographs. 2014;2014:346-358.
14. Sharma M, Haider T, Knowlden AP. Yoga as an alternative and complementary treatment for cancer: a systematic review. J Altern Complement Med. 2013;19:870-875.
15. Kordon C, Bihoreau C. Integrated communication between the nervous, endocrine and immune systems. Horm Res. 1989;31:100-104.
16. Gonzalez-Diaz SN, Arias-Cruz A, Elizondo-Villarreal B, Monge-Ortega OP. Psychoneuroimmunoendocrinology: clinical implications. World Allergy Organ J. 2017;10:19.
17. Godoy LD, Rossignoli MT, Delfino-Pereira P, et al. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci. 2018;12:127.
18. Goldstein DS. Adrenal responses to stress. Cell Mol Neurobiol. 2010;30:1433-1440.
19. Cole SW, Nagaraja AS, Lutgendorf SK, et al. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563-567.
20. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349-5361.
21. Arsalidou M, Duerden EG, Taylor MJ. The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum Brain Mapp. 2013;34:3031-3054.
22. Gard T, Taquet M, Dixit R, et al. Greater widespread functional connectivity of the caudate in older adults who practice kripalu yoga and vipassana meditation than in controls. Front Hum Neurosci. 2015;9:137.
23. Streeter CC, Gerbarg PL, Saper RB, et al. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78:571-579.
24. Brambilla P, Perez J, Barale F, et al. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721-737.
25. Streeter CC, Gerbarg PL, Saper RB, et al. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78:571-579.
26. Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: physiological pathways and mechanisms. Psychosom Med. 2011;73:724-730.
27. Armaiz-Pena GN, Cole SW, Lutgendorf SK, Sood AK. Neuroendocrine influences on cancer progression. Brain Behav Immun. 2013;30(suppl):S19-S25.
28. Tyagi A, Cohen M. Yoga and heart rate variability: A comprehensive review of the literature. Int J Yoga. 2016;9:97-113.
29. Sephton SE, Lush E, Dedert EA, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30 (suppl):S163-S170.
30. Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994-1000.
31. Arafah BM, Nishiyama FJ, Tlaygeh H, Hejal R. Measurement of salivary cortisol concentration in the assessment of adrenal function in critically ill subjects: a surrogate marker of the circulating free cortisol. J Clin Endocrinol Metab. 2007;92:2965-2971.
32. Cohen L, de Moor C, Devine D, et al. Endocrine levels at the start of treatment are associated with subsequent psychological adjustment in cancer patients with metastatic disease. Psychosom Med. 2001;63:951-958.
33. Cohen L, Cole SW, Sood AK, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PloS One. 2012;7:e42324.
34. Vadiraja HS, Raghavendra RM, Nagarathna R, et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Integr Cancer Ther. 2009;8:37-46.
35. Wu Y, Antony S, Meitzler JL, Doroshow JH. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014;345:164-173.
36. Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(suppl):S48-S57.
37. Derry HM, Jaremka LM, Bennett JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24:958-966.
38. Kiecolt-Glaser JK, Christian L, Preston H, et al. Stress, inflammation, and yoga practice. Psychosom Med. 2010;72:113-121.
39. Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21:1038-1049.
40. Infante JR, Peran F, Rayo JI, et al. Levels of immune cells in transcendental meditation practitioners. Int J Yoga. 2014;7:147-151.
41. Rao RM, Telles S, Nagendra HR, et al. Effects of yoga on natural killer cell counts in early breast cancer patients undergoing conventional treatment. Comment to: recreational music-making modulates natural killer cell activity, cytokines, and mood states in corporate employees Masatada Wachi, Masahiro Koyama, Masanori Utsuyama, Barry B. Bittman, Masanobu Kitagawa, Katsuiku Hirokawa Med Sci Monit, 2007; 13(2): CR57-70. Med Sci Monit. 2008;14:LE3-4.
42. Rao RM, Nagendra HR, Raghuram N, et al. Influence of yoga on mood states, distress, quality of life and immune outcomes in early stage breast cancer patients undergoing surgery. Int J Yoga. 2008;1:11-20.
43. Mozdarani H, Mansouri Z, Haeri SA. Cytogenetic radiosensitivity of g0-lymphocytes of breast and esophageal cancer patients as determined by micronucleus assay. J Radiat Res. 2005;46:111-116.
44. Scott D, Barber JB, Levine EL, et al. Radiation-induced micronucleus induction in lymphocytes identifies a high frequency of radiosensitive cases among breast cancer patients: a test for predisposition? Br J Cancer. 1998;77:614-620.
45. Banerjee B, Sharma S, Hegde S, Hande MP. Analysis of telomere damage by fluorescence in situ hybridisation on micronuclei in lymphocytes of breast carcinoma patients after radiotherapy. Breast Cancer Res Treat. 2008;107:25-31.
46. Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312-17315.
47. Glaser R, Thorn BE, Tarr KL, et al. Effects of stress on methyltransferase synthesis: an important DNA repair enzyme. Health Psychol. 1985;4:403-412.
48. Kiecolt-Glaser JK, Stephens RE, Lipetz PD, et al. Distress and DNA repair in human lymphocytes. J Behav Med. 1985;8:311-320.
49. Banerjee B, Vadiraj HS, Ram A, et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007;6:242-250.
50. Danhauer SC, Addington EL, Sohl SJ, et al. Review of yoga therapy during cancer treatment. Support Care Cancer. 2017;25:1357-1372.
51. Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8:43-55.
52. Ratcliff CG, Milbury K, Chandwani KD, et al. Examining mediators and moderators of yoga for women with breast cancer undergoing radiotherapy. Integr Cancer Ther. 2016;15:250-262.
53. Chandwani KD, Perkins G, Nagendra HR, et al. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol. 2014;32:1058-1065.
54. Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102-113.
55. Janelsins MC, Peppone LJ, Heckler CE, et al. YOCAS(c)(R) yoga reduces self-reported memory difficulty in cancer survivors in a nationwide randomized clinical trial: investigating relationships between memory and sleep. Integr Cancer Ther. 2016;15:263-271.
56. Biegler KA, Chaoul MA, Cohen L. Cancer, cognitive impairment, and meditation. Acta Oncol. 2009;48:18-26.
57. Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90:2297-2304.
58. Herschbach P, Keller M, Knight L, et al. Psychological problems of cancer patients: a cancer distress screening with a cancer-specific questionnaire. Br J Cancer. 2004;91:504-511.
59. Abercrombie HC, Giese-Davis J, Sephton S, et al. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082-1092.
60. Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med. 2005;67:277-280.
61. Bower JE, Ganz PA, Dickerson SS, et al. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92-100.
62. Giese-Davis J, Sephton SE, Abercrombie HC, et al. Repression and high anxiety are associated with aberrant diurnal cortisol rhythms in women with metastatic breast cancer. Health Psychol. 2004;23:645-650.
63. Giese-Davis J, DiMiceli S, Sephton S, Spiegel D. Emotional expression and diurnal cortisol slope in women with metastatic breast cancer in supportive-expressive group therapy: a preliminary study. Biol Psychol. 2006;73:190-198.
64. Stone AA, Schwartz JE, Smyth J, et al. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26:295-306.
65. Bower JE, Woolery A, Sternlieb B, Garet D. Yoga for cancer patients and survivors. Cancer Control. 2005;12:165-171.
66. Granath J, Ingvarsson S, von Thiele U, Lundberg U. Stress management: a randomized study of cognitive behavioural therapy and yoga. Cogn Behav Therap. 2006;35:3-10.
67. Rao MR, Raghuram N, Nagendra HR, et al. Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: a randomized controlled trial. Complement Ther Med. 2009;17:1-8.
68. Culos-Reed SN, Carlson LE, Daroux LM, Hately-Aldous S. A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psychooncology. 2006;15:891-897.
69. Mackenzie MJ, Carlson LE, Ekkekakis P, et al. Affect and mindfulness as predictors of change in mood disturbance, stress symptoms, and quality of life in a community-based yoga program for cancer survivors. Evid Based Complement Alternat Med. 2013;2013:419496.
70. Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psycho-oncology. 2009;18:360-368.
71. Sohl SJ, Danhauer SC, Schnur JB, et al. Feasibility of a brief yoga intervention during chemotherapy for persistent or recurrent ovarian cancer. Explore (NY). 2012;8:197-198.
72. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer. 2016;24:4005-4015.
73. Sprod LK, Fernandez ID, Janelsins MC, et al. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. J Geriatr Oncol. 2015;6:8-14.
74. Wang G, Wang S, Jiang P, Zeng C. Effect of yoga on cancer related fatigue in breast cancer patients with chemotherapy [in Chinese]. Zhong Nan Da Xue Bao Yi Xue Ban. 2014;39:1077-1082.
75. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118:3766-3775.
76. Taso CJ, Lin HS, Lin WL, et al. The effect of yoga exercise on improving depression, anxiety, and fatigue in women with breast cancer: a randomized controlled trial. J Nurs Res. 2014;22:155-164.
77. Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-related fatigue, Version 2.2015. J Natl Compr Canc Netw. 2015;13:1012-1039.
78. Rao RM, Nagendra HR, Raghuram N, et al. Influence of yoga on postoperative outcomes and wound healing in early operable breast cancer patients undergoing surgery. Int J Yoga. 2008;1:33-41.
79. Sohl SJ, Avis NE, Stanbery K, et al. Feasibility of a brief yoga intervention for improving acute pain and distress post gynecologic surgery. Int J Yoga Therap. 2016;26:43-47.
80. Gupta P, Sturdee DW, Palin SL, et al. Menopausal symptoms in women treated for breast cancer: the prevalence and severity of symptoms and their perceived effects on quality of life. Climacteric. 2006;9:49-58.
81. Canney PA, Hatton MQ. The prevalence of menopausal symptoms in patients treated for breast cancer. Clin Oncol (R Coll Radiol). 1994;6:297-299.
82. Carpenter JS, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum. 2002;29:E16-25.
83. Carson JW, Carson KM, Porter LS, et al. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer. 2009;17:1301-1309.
84. Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16:223-234.
85. Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115:3631-3639.
86. Presant CA, Bosserman L, Young T, et al. Aromatase inhibitor-associated arthralgia and/or bone pain: frequency and characterization in non-clinical trial patients. Clin Breast Cancer. 2007;7:775-778.
87. Saper RB, Sherman KJ, Cullum-Dugan D, et al. Yoga for chronic low back pain in a predominantly minority population: a pilot randomized controlled trial. Altern Ther Health Med. 2009;15:18-27.
88. Kolasinski SL, Garfinkel M, Tsai AG, et al. Iyengar yoga for treating symptoms of osteoarthritis of the knees: a pilot study. J Altern Complement Med. 2005;11:689-693.
89. Galantino ML, Desai K, Greene L, et al. Impact of yoga on functional outcomes in breast cancer survivors with aromatase inhibitor-associated arthralgias. Integr Cancer Ther. 2012;11:313-320.
90. Ancoli-Israel S. Recognition and treatment of sleep disturbances in cancer. J Clin Oncol. 2009;27:5864-5866.
91. Savard J, Ivers H, Villa J, et al. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol. 2011;29:3580-3586.
92. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31:3233-3241.
93. Moore TA, Berger AM, Dizona P. Sleep aid use during and following breast cancer adjuvant chemotherapy. Psychooncology. 2011;20:321-325.
94. Omvik S, Pallesen S, Bjorvatn B, et al. Patient characteristics and predictors of sleep medication use. Int Clin Psychopharmacol. 2010;25:91-100.
95. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133.
96. Cohen L, Warneke C, Fouladi RT, et al. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253-2260.
97. Kraaijenga SA, Oskam IM, van der Molen L, et al. Evaluation of long term (10-years+) dysphagia and trismus in patients treated with concurrent chemo-radiotherapy for advanced head and neck cancer. Oral Oncol. 2015;51:787-794.
98. Adair M, Murphy B, Yarlagadda S, et al. Feasibility and preliminary efficacy of tailored yoga in survivors of head and neck cancer: a pilot study. Integr Cancer Ther. 2018;17:774-784.
99. Loudon A, Barnett T, Williams A. Yoga, breast cancer-related lymphoedema and well-being: A descriptive report of women’s participation in a clinical trial. J Clin Nurs. 2017;26:4685-4695.
100. Loudon A, Barnett T, Piller N, et al. The effects of yoga on shoulder and spinal actions for women with breast cancer-related lymphoedema of the arm: A randomised controlled pilot study. BMC Complement Altern Med. 2016;16:343.
101. Petruzzi A, Finocchiaro CY, Lamperti E, Salmaggi A. Living with a brain tumor: reaction profiles in patients and their caregivers. Support Care Cancer. 2013;21:1105-1111.
102. Pawl JD, Lee SY, Clark PC, Sherwood PR. Sleep characteristics of family caregivers of individuals with a primary malignant brain tumor. Oncol Nurs Forum. 2013;40:171-179.
103. Milbury K, Mallaiah S, Mahajan A, et al. Yoga program for high-grade glioma patients undergoing radiotherapy and their family caregivers. Integr Cancer Ther. 2018;17:332-336.
104. Milbury K, Li J, Weathers S-P, et al. Pilot randomized controlled trial of a dyadic yoga program for glioma patients undergoing radiotherapy and their family caregivers. Neurooncol Pract. 2019;6:311-320.
105. Milbury K, Liao Z, Shannon V, et al. Dyadic yoga program for patients undergoing thoracic radiotherapy and their family caregivers: Results of a pilot randomized controlled trial. Psychooncology. 2019;28:615-621.
106. Milbury K, Chaoul A, Engle R, et al. Couple-based Tibetan yoga program for lung cancer patients and their caregivers. Psychooncology. 2015;24:117-120.
107. Danhauer SC, Addington EL, Cohen L, et al. Yoga for symptom management in oncology: A review of the evidence base and future directions for research. Cancer. 2019;125:1979-1989.
108. National Comprehensive Cancer Center. Flash Update: NCCN Guidelines® and NCCN Compendium® for Antiemesis. NCCN website. Accessed August 29, 2019.
109. Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36:2647-2655.
From the University of Texas MD Anderson Cancer Center, Houston, TX (Drs. Narayanan, Lopez, Chaoul, Liu, Milbury, and Cohen, and Ms. Mallaiah); the University of Texas Health Science Center at Tyler (Dr. Meegada); and Texas Tech University Health Sciences Center, Lubbock, TX (Ms. Francisco).
Abstract
- Objective: To review the effects of yoga as an adjunct supportive care modality alongside conventional cancer treatment on quality of life (QOL), physical and mental health outcomes, and physiological and biological measures of cancer survivors.
- Methods: Nonsystematic review of the literature.
- Results: Yoga therapy, one of the most frequently used mind-body modalities, has been studied extensively in cancer survivors (from the time of diagnosis through long-term recovery). Yoga affects human physiology on multiple levels, including psychological outcomes, immune and endocrine function, and cardiovascular parameters, as well as multiple areas of QOL. It has been found to reduce psychological stress and fatigue and improve QOL in cancer patients and survivors. Yoga has also been used to manage symptoms such as arthralgia, fatigue, and insomnia. In addition, yoga offers benefits not only for cancer survivors but also for their caregivers.
- Conclusion: As part of an integrative, evidence-informed approach to cancer care, yoga may provide benefits that support the health of cancer survivors and caregivers.
Keywords: fatigue; cancer; proinflammatory cytokines; integrative; mind-body practices; meditation; DNA damage; stress; psychoneuro-immunoendocrine axis; lymphedema; insomnia.
A diagnosis of cancer and adverse effects related to its treatment may have negative effects on quality of life (QOL), contributing to emotional and physical distress in patients and caregivers. Many patients express an interest in pursuing nonpharmacological options, alone or as an adjunct to conventional therapy, to help manage symptoms. The use of complementary medicine approaches to health, including nonpharmacological approaches to symptom management, is highest among individuals with cancer.1 According to a published expert consensus, integrative oncology is defined as a “patient-centered, evidence-informed field of cancer care that utilizes mind and body practices, natural products, and/or lifestyle modifications from different traditions alongside conventional cancer treatments. Integrative oncology aims to optimize health, QOL, and clinical outcomes across the cancer care continuum and to empower people to prevent cancer and become active participants before, during, and beyond cancer treatment.”2 A key component of this definition, often misunderstood in the field of oncology, is that these modalities and treatments are used alongside conventional cancer treatments and not as an alternative. In an attempt to meet patients’ needs and appropriately use these approaches, integrative oncology programs are now part of most cancer centers in the United States.3-6
Because of their overall safety, mind-body therapies are commonly used by patients and recommended by clinicians. Mind-body therapies include yoga, tai chi, qigong, meditation, and relaxation. Expressive arts such as journaling and music, art, and dance therapies also fall in the mind-body category.7 Yoga is a movement-based mind-body practice that focuses on synchronizing body, breath, and mind. Yoga has been increasingly used by patients for health benefits,8 and numerous studies have evaluated yoga as a complementary intervention for individuals with cancer.9-14 Here, we review the physiological basis of yoga in oncology and the effects of yoga on biological processes, QOL, and symptoms during and after cancer treatment.
Physiological Basis
Many patients may use mind-body programs such as yoga to help manage the psychological and physiological consequences of unmanaged chronic stress and improve their overall QOL. The central nervous system, endocrine system, and immune system influence and interact with each other in a complex manner in response to chronic stress.15,16 In a stressful situation, the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS) are activated. HPA axis stimulation leads to adrenocorticotrophic hormone production by the pituitary gland, which releases glucocorticoid hormones. SNS axis stimulation leads to epinephrine and norepinephrine production by the adrenal gland.17,18 Recently, studies have explored modulation of signal transduction between the nervous and immune systems and how that may impact tumor growth and metastasis.19 Multiple studies, controlled for prognosis, disease stage, and other factors, have shown that patients experiencing more distress or higher levels of depressive symptoms do not live as long as their counterparts with low distress or depression levels.20 Both the meditative and physical components of yoga can lead to enhanced relaxation, reduced SNS activation, and greater parasympathetic tone, countering the negative physiological effects of chronic stress. The effects of yoga on the HPA axis and SNS, proinflammatory cytokines, immune function, and DNA damage are discussed below.
Biological Processes
Nervous System
The effects of yoga and other forms of meditation on brain functions have been established through several studies. Yoga seems to influence basal ganglia function by improving circuits that are involved in complex cognitive functions, motor coordination, and somatosensory and emotional processes.21,22 Additionally, changes in neurotransmitter levels have been observed after yoga practice. For instance, in a 12-week yoga intervention in healthy subjects, increased levels of thalamic gamma-aminobutyric acid (GABA) in the yoga group were reported to have a positive correlation with improved mood and decreased anxiety compared with a group who did metabolically matched walking exercise.23 Levels of GABA, an inhibitory neurotransmitter, are decreased in conditions such as anxiety, depression, and epilepsy.24 Yoga therapy has been shown to improve symptoms of mood disorders and epilepsy, which leads to the hypothesis that the mechanism driving the benefits of yoga may work through stimulation of vagal efferents and an increase in GABA-mediated cortical-inhibitory tone.24,25
HPA Axis
Stress activates the HPA/SNS axis, which releases hormones such as cortisol and norepinephrine. These hormones may play a role in angiogenesis, inflammation, immune suppression, and other physiological functions, and may even reduce the effect of chemotherapeutic agents.26,27 Regular yoga practice has been shown to reduce SNS and HPA axis activity, most likely by increasing parasympathetic dominance through vagal stimulation, as demonstrated through increases in heart rate variability.28 One indicator of HPA axis dysregulation, diurnal salivary cortisol rhythm, was shown to predict survival in patients with advanced breast and renal cancer.29-33 Yoga has been shown to lead to less cortisol dysregulation due to radiotherapy and to reductions in mean cortisol levels and early morning cortisol levels in breast cancer patients undergoing radiotherapy.34 This lends support to the hypothesis that yoga helps restore HPA axis balance.
Proinflammatory Cytokines
Cancer patients tend to have increased levels of inflammatory markers such as interleukin (IL)-4, IL-10, tumor necrosis factor (TNF), interferon-γ, and C-reactive protein. This increase in inflammation is associated with worse outcomes in cancer.35 This association becomes highly relevant because the effect of inflammation on host cells in the tumor microenvironment is connected to disease progression.26 Inflammatory cytokines are also implicated in cancer-related symptoms such as fatigue, cognitive dysfunction, peripheral neuropathy, and sleep disturbances.36
Yoga is known to reduce stress and may directly or indirectly decrease inflammatory cytokines. A randomized clinical trial of a 12-week hatha yoga intervention among breast cancer survivors demonstrated decreases in IL-6, IL-1β, TNF, corticotropin-releasing factor, and cognitive complaints in the yoga group compared with those in the standard care group after 3 months.37,38 Furthermore, Carlson et al showed that, after mindfulness-based stress reduction involving a combination of gentle yoga, meditation techniques, and relaxation exercises, breast and prostate cancer patients had reduced levels of proinflammatory cytokines and cortisol.39 These reductions translated into patients reporting decreased stress levels and enhanced QOL.
Immune Function
The effects of yoga practice on the immune system have been studied in both healthy individuals and individuals with cancer. The effects on T and B lymphocytes, natural killer (NK) cells, and other immune effector cells demonstrate that meditation and yoga have beneficial effects on immune activity.40 Hormones such as catecholamines and glucocorticoids are thought to influence the availability and function of NK cells, and, as noted above, yoga has been shown to modulate stress hormones and lead to reduced immune suppression in patients with early-stage breast cancer undergoing chemotherapy.41 Additional evidence supports the ability of yoga to reduce immune suppression in the postsurgical setting, with no observed decrease in NK cell percentage after surgery for those in a yoga group compared with a control group.42 This finding is relevant to patients undergoing surgical management of their cancer and highlights the impact of yoga on the immune system.
DNA Damage
Radiation damages DNA in the peripheral blood lymphocytes of patients undergoing treatment.43,44 This damage is significant in breast cancer patients undergoing radiotherapy.45 Stress additionally causes DNA damage46 and is correlated to impaired DNA repair capacity.47,48 In a study conducted by Banerjee et al, breast cancer patients were randomly assigned to a yoga group or a supportive therapy group for 6 weeks during radiotherapy.49 Prior to the intervention, patients in the study had significant genomic instability. After treatment, patients in the yoga group experienced not only a significant reduction in anxiety and depression levels, but also a reduction in DNA damage due to radiotherapy.
Yoga in Quality of Life and Symptom Management
There is evidence showing that yoga therapy improves multiple aspects of QOL, including physical functioning, emotional health outcomes, and the symptoms cancer patients may experience, such as sleep disturbances, fatigue, and pain. Danhauer et al systematically reviewed both nonrandomized trials and randomized controlled trials involving yoga during cancer treatment.50 They found that yoga improved depression and anxiety as well as sleep and fatigue. Benefits of yoga in cancer based on randomized controlled trials are summarized in the Table. The role of yoga in improving QOL and managing symptoms patients experience during and after treatment is discussed in the following sections.

Quality of Life
Danhauer et al’s systematic review of trials involving yoga during cancer treatment found that yoga improved multiple aspects of QOL.50 For example, yoga has been shown to improve QOL in breast cancer patients undergoing radiotherapy. In a study by Chandwani et al, yoga (60-minute sessions twice a week for 6 weeks) was associated with better general health perception and physical functioning scores as well as greater benefit finding, or finding meaning in their experience, after radiotherapy compared with a wait-list group.51 The yoga group had an increase in intrusive thoughts, believed to be due to a more thorough processing of the cancer experience, which helps to improve patients’ outlook on life.52 The benefits of yoga extend beyond psychological measures during radiation treatment. Yoga was found to increase physical functioning compared with stretching in breast cancer patients undergoing radiotherapy.53
Cognitive Function
Cancer-related cognitive impairment commonly occurs during cancer treatments (eg, chemotherapy, radiotherapy, surgery, hormone therapy) and persists for months or years in survivors.54 Impairment of memory, executive function, attention, and concentration are commonly reported. In a trial of a combined hatha and restorative yoga program called Yoga for Cancer Survivors (YOCAS), which was designed by researchers at the University of Rochester, patients in the yoga arm had less memory difficulty than did patients in the standard care arm.55 However, the primary aim of the trial was to treat insomnia, so this secondary outcome needs to be interpreted with caution. Deficits in attention, memory, and executive function are often seen in cancer-related cognitive impairment, and the meditative aspect of yoga may have behavioral and neurophysical benefits that could improve cognitive functions.56 More evidence is needed to understand the role of yoga in improving cognitive functioning.
Emotional Health
Psychosocial stress is high among breast cancer patients and survivors.57,58 This causes circadian rhythm and cortisol regulation abnormalities, which are reported in women with breast cancer.59-64 Yoga is known to help stress and psychosocial and physical functioning in patients with cancer.65 Yoga was also shown to be equivalent to cognitive behavioral therapy in stress management in a population of patients without cancer.66 Daily yoga sessions lasting 60 minutes were shown to reduce reactive anxiety and trait anxiety in early-stage breast cancer patients undergoing conventional radiotherapy and chemotherapy compared with patients receiving supportive therapy, highlighting the role of yoga in managing anxiety related to treatment.67 In a study done by Culos-Reed et al, 20 cancer survivors who did 75 minutes of yoga per week for 7 weeks were compared with 18 cancer survivors who served as a control group.68 The intervention group reported significant improvement in emotional well-being, depression, concentration, and mood disturbances. In a longitudinal study by Mackenzie et al, 66 cancer survivors completed a 7-week yoga program and were assessed at baseline, immediately after the final yoga session, and at 3 and 6 months after the final session.69 Participants had significantly improved energy levels and affect. They also had moderate improvement in mindfulness and a moderate decrease in stress. Breast cancer patients who underwent restorative yoga sessions found improvements in mental health, depression, positive affect, and spirituality (peace/meaning).70 This was more pronounced in women with higher negative affect and lower emotional well-being at baseline. In a study of patients with ovarian cancer receiving chemotherapy, patients were instructed to perform up to 15-minute sessions including awareness, body movement, and breathing.71 Even with just 1 session of yoga intervention, patients experienced decreased anxiety.
Fatigue
Studies on yoga show improvement in fatigue both during and after treatment. In breast cancer patients undergoing chemotherapy, yoga was shown to benefit cognitive fatigue.72 Older cancer survivors also seem to benefit from yoga interventions.73 In a trial of a DVD-based yoga program, the benefits of yoga were similar to those of strengthening exercises, and both interventions helped decrease fatigue and improve QOL during the first year after diagnosis in early-stage breast cancer patients with cancer-related fatigue.74 Bower et al also showed that, for breast cancer survivors experiencing persistent chronic fatigue, a targeted yoga intervention led to significant improvements in fatigue and vigor over a 3-month follow-up compared with controls.75 Fatigue is commonly seen in breast cancer patients who are receiving adjuvant chemotherapy. In a study by Taso et al, women with breast cancer receiving chemotherapy were assigned to 60-minute yoga sessions incorporating Anusara yoga, gentle stretching, and relaxation twice a week for 8 weeks.76 By week 4, patients with low pretest fatigue in the yoga group experienced a reduction in fatigue. By week 8, all patients in the yoga group experienced a reduction in fatigue. Four weeks after the yoga intervention, patients in the group maintained the reduction in fatigue. This study shows the feasibility of an 8-week yoga program for women undergoing breast cancer therapy by improving fatigue. Yoga recently was added to National Comprehensive Cancer Network (NCCN) guidelines for management of cancer-related fatigue (level 1 evidence).77 However, the evidence was based on studies in women with breast cancer and survivors; therefore, more studies are needed in men and women with other cancers.
Surgical Setting/Postoperative Distress
Distress surrounding surgery in patients with breast cancer can impact postoperative outcomes. Yoga interventions, including breathing exercises, regulated breathing, and yogic relaxation techniques, improved several postsurgical measures such as length of hospital stay, drain retention, and suture removal.78 In this study, patients who practiced yoga also experienced a decrease in plasma TNF and better wound healing. Symptoms of anxiety and distress that occur preoperatively can lead to impaired immune function in addition to decreased QOL. In a study of yoga in early-stage breast cancer patients undergoing surgery, the benefit of yoga was seen not only with stress reduction but also with immune enhancement.42
Yoga has been shown to help alleviate acute pain and distress in women undergoing major surgery for gynecological cancer. A regimen of 3 15-minute sessions of yoga, including awareness meditation, coordination of breath with movement, and relaxation breathing, was shown to reduce acute pain and distress in such patients in an inpatient setting.79
Menopausal Symptoms
Breast cancer survivors have more severe menopausal symptoms compared with women without cancer.80,81 Hot flashes cause sleep disturbances and worsen fatigue and QOL.82 Tamoxifen and aromatase inhibitors significantly worsen menopausal symptoms such as hot flashes.81 Carson et al conducted a study of yoga that included postures, breathing techniques, didactic presentations, and group discussions.83 The yoga awareness regimen consisted of 8 weekly 120-minute group classes. Patients in the yoga arm had statistically significant improvements in the frequency, severity, and number of hot flashes. There were also improvements in arthralgia (joint pain), fatigue, sleep disturbance, vigor, and acceptance.
Arthralgia
Joint pain can be a major side effect that interferes with daily functions and activities in postmenopausal breast cancer survivors who receive aromatase inhibitor therapy.84 Arthralgia is reported in up to 50% of patients treated with aromatase inhibitors.84,85 It can affect functional status and lead to discontinuation of aromatase inhibitor therapy, jeopardizing clinical outcomes.86 Yoga as a complementary therapy has been shown to improve conditions such as low back pain87 and knee osteoarthritis88 in patients who do not have cancer. In a single-arm pilot trial by Galantino et al, breast cancer patients with aromatase inhibitor–related joint pain were provided with twice-weekly yoga sessions for 8 weeks. There were statistically significant improvements in balance, flexibility, pain severity, and health-related QOL.89 As noted above, improvement in arthralgia was also found in the study conducted by Carson et al.83
Insomnia
Insomnia is common among cancer patients and survivors90,91 and leads to increased fatigue and depression, decreased adherence to cancer treatments, and poor physical function and QOL.90-92 Management of insomnia consists of pharmacologic therapies such as benzodiazepines93,94 and nonpharmacologic options such as cognitive behavioral therapy.95
The first study of yoga found to improve sleep quality was conducted at MD Anderson Cancer Center in lymphoma patients.96 The effects of Tibetan yoga practices incorporating controlled breathing and visualization, mindfulness techniques, and low-impact postures were studied. Patients in the Tibetan yoga group had better subjective sleep quality, faster sleep latency, longer sleep duration, and less use of sleep medications. Mustian et al conducted a large yoga study in cancer survivors in which patients reporting chronic sleep disturbances were randomly assigned to the YOCAS program, which consisted of pranayama (breath control), 16 gentle hatha and restorative yoga postures, and meditation, or to usual care.92 The study reported improvements in global sleep quality, subjective sleep quality, actigraphy measures (wake after sleep onset, sleep efficiency), daytime dysfunction, and use of sleep medication after the yoga intervention compared with participants who received standard care.
Yoga to Address Other Symptoms
There is preliminary evidence supporting yoga as an integrative therapy for other symptoms unique to cancer survivors. For example, in head and neck cancer survivors, soft tissue damage involving the jaw, neck, shoulders, and chest results in swallowing issues, trismus, and aspiration, which are more pronounced in patients treated with conventional radiotherapy than in those treated with intensity-modulated radiotherapy.97 Some late effects of radiotherapy for head and neck cancer—such as pain, anxiety, and impaired shoulder function—were shown to be improved through the practice of hatha yoga in 1 study.98 Similarly, in a randomized controlled pilot study of patients with stage I to III breast cancer 6 months after treatment, participants in an 8-week yoga program experienced a reduction in arm induration and improvement in a QOL subscale of lymphedema symptoms. However, more evidence is needed to support the use of yoga as a therapeutic measure for breast cancer lymphedema.99,100
Yoga for Caregivers
Along with cancer patients, caregivers face psychological and physical burdens as well as deterioration in their QOL. Caregivers tend to report clinical levels of anxiety, depression, sleep disturbance, and fatigue and have similar or in fact higher levels than those of the patients for whom they are caring.101,102 Yoga has been found to help caregivers of patients with cancer. Recently, MD Anderson researchers conducted a trial in patients with high-grade glioma and their caregivers as dyads.103,104 Each dyad attended 2 or 3 60-minute weekly Vivekananda yoga sessions involving breathing exercises, physical exercises, relaxation, and meditation. The researchers found that the yoga program was safe, feasible, acceptable, and subjectively useful for patients with high-grade glioma and their caregivers. Preliminary evidence of QOL improvement for both patients and caregivers was noted. An improvement in QOL was also demonstrated in another preliminary study of yoga in patients undergoing thoracic radiotherapy and their caregivers.105
Another study by the group at MD Anderson evaluated a couple-based Tibetan yoga program that emphasized breathing exercises, gentle movements, guided visualizations, and emotional connectedness during radiotherapy for lung cancer.106 This study included 10 patient‐caregiver dyads and found the program to be feasible, safe, and acceptable. The researchers also found preliminary evidence of improved QOL by the end of radiotherapy relative to baseline—specifically in the areas of spiritual well‐being for patients, fatigue for caregivers, and sleep disturbances and mental health issues such as anxiety and depressive symptoms for both patients and caregivers. This is noteworthy, as QOL typically deteriorates during the course of radiotherapy, and the yoga program was able to buffer these changes.
Conclusion
Yoga therapy has been used successfully as an adjunct modality to improve QOL and cancer-related symptoms. As a part of an integrative medicine approach, yoga is commonly recommended for patients undergoing cancer treatment. Danhauer et al reviewed randomized controlled trials during and after treatment and concluded that the evidence is clearly positive for QOL, fatigue, and perceived stress.107 Results are less consistent but supportive for psychosocial outcomes such as benefit finding and spirituality. Evidence is mixed for sleep, anxiety, and depression. Post-treatment studies demonstrate improvements in fatigue, sleep, and multiple QOL domains. Yoga has been included in NCCN guidelines for fatigue management. Yoga, if approved by a physician, is also included among the behavioral therapies for anticipatory emesis and prevention and treatment of nausea in the recent update of the NCCN guidelines.108 The Society for Integrative Oncology guidelines include yoga for anxiety/stress reduction as a part of integrative treatment in breast cancer patients during and after therapy, which was endorsed by the American Society of Clinical Oncology.109
Because of the strong evidence for its benefits and a low side-effect profile, yoga is offered in group-class settings for patients during and after treatment and/or for caregivers in our institution. We often prescribe yoga as a therapeutic modality for selected groups of patients in our clinical practice. However, some patients may have restrictions after surgery that must be considered. In general, yoga has an excellent safety profile, the evidence base is strong, and we recommend that yoga therapy should be part of the standard of care as an integrative approach for patients with cancer undergoing active treatment as well as for cancer survivors and caregivers.
Acknowledgement: The authors thank Bryan Tutt for providing editorial assistance.
Corresponding author: Santhosshi Narayanan, MD, Department of Palliative, Rehabilitation, and Integrative Medicine, Unit 1414, The University of Texas MD Anderson Cancer Center, 1400 Pressler St., Houston, TX 77030; [email protected].
Financial disclosures: None.
From the University of Texas MD Anderson Cancer Center, Houston, TX (Drs. Narayanan, Lopez, Chaoul, Liu, Milbury, and Cohen, and Ms. Mallaiah); the University of Texas Health Science Center at Tyler (Dr. Meegada); and Texas Tech University Health Sciences Center, Lubbock, TX (Ms. Francisco).
Abstract
- Objective: To review the effects of yoga as an adjunct supportive care modality alongside conventional cancer treatment on quality of life (QOL), physical and mental health outcomes, and physiological and biological measures of cancer survivors.
- Methods: Nonsystematic review of the literature.
- Results: Yoga therapy, one of the most frequently used mind-body modalities, has been studied extensively in cancer survivors (from the time of diagnosis through long-term recovery). Yoga affects human physiology on multiple levels, including psychological outcomes, immune and endocrine function, and cardiovascular parameters, as well as multiple areas of QOL. It has been found to reduce psychological stress and fatigue and improve QOL in cancer patients and survivors. Yoga has also been used to manage symptoms such as arthralgia, fatigue, and insomnia. In addition, yoga offers benefits not only for cancer survivors but also for their caregivers.
- Conclusion: As part of an integrative, evidence-informed approach to cancer care, yoga may provide benefits that support the health of cancer survivors and caregivers.
Keywords: fatigue; cancer; proinflammatory cytokines; integrative; mind-body practices; meditation; DNA damage; stress; psychoneuro-immunoendocrine axis; lymphedema; insomnia.
A diagnosis of cancer and adverse effects related to its treatment may have negative effects on quality of life (QOL), contributing to emotional and physical distress in patients and caregivers. Many patients express an interest in pursuing nonpharmacological options, alone or as an adjunct to conventional therapy, to help manage symptoms. The use of complementary medicine approaches to health, including nonpharmacological approaches to symptom management, is highest among individuals with cancer.1 According to a published expert consensus, integrative oncology is defined as a “patient-centered, evidence-informed field of cancer care that utilizes mind and body practices, natural products, and/or lifestyle modifications from different traditions alongside conventional cancer treatments. Integrative oncology aims to optimize health, QOL, and clinical outcomes across the cancer care continuum and to empower people to prevent cancer and become active participants before, during, and beyond cancer treatment.”2 A key component of this definition, often misunderstood in the field of oncology, is that these modalities and treatments are used alongside conventional cancer treatments and not as an alternative. In an attempt to meet patients’ needs and appropriately use these approaches, integrative oncology programs are now part of most cancer centers in the United States.3-6
Because of their overall safety, mind-body therapies are commonly used by patients and recommended by clinicians. Mind-body therapies include yoga, tai chi, qigong, meditation, and relaxation. Expressive arts such as journaling and music, art, and dance therapies also fall in the mind-body category.7 Yoga is a movement-based mind-body practice that focuses on synchronizing body, breath, and mind. Yoga has been increasingly used by patients for health benefits,8 and numerous studies have evaluated yoga as a complementary intervention for individuals with cancer.9-14 Here, we review the physiological basis of yoga in oncology and the effects of yoga on biological processes, QOL, and symptoms during and after cancer treatment.
Physiological Basis
Many patients may use mind-body programs such as yoga to help manage the psychological and physiological consequences of unmanaged chronic stress and improve their overall QOL. The central nervous system, endocrine system, and immune system influence and interact with each other in a complex manner in response to chronic stress.15,16 In a stressful situation, the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic nervous system (SNS) are activated. HPA axis stimulation leads to adrenocorticotrophic hormone production by the pituitary gland, which releases glucocorticoid hormones. SNS axis stimulation leads to epinephrine and norepinephrine production by the adrenal gland.17,18 Recently, studies have explored modulation of signal transduction between the nervous and immune systems and how that may impact tumor growth and metastasis.19 Multiple studies, controlled for prognosis, disease stage, and other factors, have shown that patients experiencing more distress or higher levels of depressive symptoms do not live as long as their counterparts with low distress or depression levels.20 Both the meditative and physical components of yoga can lead to enhanced relaxation, reduced SNS activation, and greater parasympathetic tone, countering the negative physiological effects of chronic stress. The effects of yoga on the HPA axis and SNS, proinflammatory cytokines, immune function, and DNA damage are discussed below.
Biological Processes
Nervous System
The effects of yoga and other forms of meditation on brain functions have been established through several studies. Yoga seems to influence basal ganglia function by improving circuits that are involved in complex cognitive functions, motor coordination, and somatosensory and emotional processes.21,22 Additionally, changes in neurotransmitter levels have been observed after yoga practice. For instance, in a 12-week yoga intervention in healthy subjects, increased levels of thalamic gamma-aminobutyric acid (GABA) in the yoga group were reported to have a positive correlation with improved mood and decreased anxiety compared with a group who did metabolically matched walking exercise.23 Levels of GABA, an inhibitory neurotransmitter, are decreased in conditions such as anxiety, depression, and epilepsy.24 Yoga therapy has been shown to improve symptoms of mood disorders and epilepsy, which leads to the hypothesis that the mechanism driving the benefits of yoga may work through stimulation of vagal efferents and an increase in GABA-mediated cortical-inhibitory tone.24,25
HPA Axis
Stress activates the HPA/SNS axis, which releases hormones such as cortisol and norepinephrine. These hormones may play a role in angiogenesis, inflammation, immune suppression, and other physiological functions, and may even reduce the effect of chemotherapeutic agents.26,27 Regular yoga practice has been shown to reduce SNS and HPA axis activity, most likely by increasing parasympathetic dominance through vagal stimulation, as demonstrated through increases in heart rate variability.28 One indicator of HPA axis dysregulation, diurnal salivary cortisol rhythm, was shown to predict survival in patients with advanced breast and renal cancer.29-33 Yoga has been shown to lead to less cortisol dysregulation due to radiotherapy and to reductions in mean cortisol levels and early morning cortisol levels in breast cancer patients undergoing radiotherapy.34 This lends support to the hypothesis that yoga helps restore HPA axis balance.
Proinflammatory Cytokines
Cancer patients tend to have increased levels of inflammatory markers such as interleukin (IL)-4, IL-10, tumor necrosis factor (TNF), interferon-γ, and C-reactive protein. This increase in inflammation is associated with worse outcomes in cancer.35 This association becomes highly relevant because the effect of inflammation on host cells in the tumor microenvironment is connected to disease progression.26 Inflammatory cytokines are also implicated in cancer-related symptoms such as fatigue, cognitive dysfunction, peripheral neuropathy, and sleep disturbances.36
Yoga is known to reduce stress and may directly or indirectly decrease inflammatory cytokines. A randomized clinical trial of a 12-week hatha yoga intervention among breast cancer survivors demonstrated decreases in IL-6, IL-1β, TNF, corticotropin-releasing factor, and cognitive complaints in the yoga group compared with those in the standard care group after 3 months.37,38 Furthermore, Carlson et al showed that, after mindfulness-based stress reduction involving a combination of gentle yoga, meditation techniques, and relaxation exercises, breast and prostate cancer patients had reduced levels of proinflammatory cytokines and cortisol.39 These reductions translated into patients reporting decreased stress levels and enhanced QOL.
Immune Function
The effects of yoga practice on the immune system have been studied in both healthy individuals and individuals with cancer. The effects on T and B lymphocytes, natural killer (NK) cells, and other immune effector cells demonstrate that meditation and yoga have beneficial effects on immune activity.40 Hormones such as catecholamines and glucocorticoids are thought to influence the availability and function of NK cells, and, as noted above, yoga has been shown to modulate stress hormones and lead to reduced immune suppression in patients with early-stage breast cancer undergoing chemotherapy.41 Additional evidence supports the ability of yoga to reduce immune suppression in the postsurgical setting, with no observed decrease in NK cell percentage after surgery for those in a yoga group compared with a control group.42 This finding is relevant to patients undergoing surgical management of their cancer and highlights the impact of yoga on the immune system.
DNA Damage
Radiation damages DNA in the peripheral blood lymphocytes of patients undergoing treatment.43,44 This damage is significant in breast cancer patients undergoing radiotherapy.45 Stress additionally causes DNA damage46 and is correlated to impaired DNA repair capacity.47,48 In a study conducted by Banerjee et al, breast cancer patients were randomly assigned to a yoga group or a supportive therapy group for 6 weeks during radiotherapy.49 Prior to the intervention, patients in the study had significant genomic instability. After treatment, patients in the yoga group experienced not only a significant reduction in anxiety and depression levels, but also a reduction in DNA damage due to radiotherapy.
Yoga in Quality of Life and Symptom Management
There is evidence showing that yoga therapy improves multiple aspects of QOL, including physical functioning, emotional health outcomes, and the symptoms cancer patients may experience, such as sleep disturbances, fatigue, and pain. Danhauer et al systematically reviewed both nonrandomized trials and randomized controlled trials involving yoga during cancer treatment.50 They found that yoga improved depression and anxiety as well as sleep and fatigue. Benefits of yoga in cancer based on randomized controlled trials are summarized in the Table. The role of yoga in improving QOL and managing symptoms patients experience during and after treatment is discussed in the following sections.

Quality of Life
Danhauer et al’s systematic review of trials involving yoga during cancer treatment found that yoga improved multiple aspects of QOL.50 For example, yoga has been shown to improve QOL in breast cancer patients undergoing radiotherapy. In a study by Chandwani et al, yoga (60-minute sessions twice a week for 6 weeks) was associated with better general health perception and physical functioning scores as well as greater benefit finding, or finding meaning in their experience, after radiotherapy compared with a wait-list group.51 The yoga group had an increase in intrusive thoughts, believed to be due to a more thorough processing of the cancer experience, which helps to improve patients’ outlook on life.52 The benefits of yoga extend beyond psychological measures during radiation treatment. Yoga was found to increase physical functioning compared with stretching in breast cancer patients undergoing radiotherapy.53
Cognitive Function
Cancer-related cognitive impairment commonly occurs during cancer treatments (eg, chemotherapy, radiotherapy, surgery, hormone therapy) and persists for months or years in survivors.54 Impairment of memory, executive function, attention, and concentration are commonly reported. In a trial of a combined hatha and restorative yoga program called Yoga for Cancer Survivors (YOCAS), which was designed by researchers at the University of Rochester, patients in the yoga arm had less memory difficulty than did patients in the standard care arm.55 However, the primary aim of the trial was to treat insomnia, so this secondary outcome needs to be interpreted with caution. Deficits in attention, memory, and executive function are often seen in cancer-related cognitive impairment, and the meditative aspect of yoga may have behavioral and neurophysical benefits that could improve cognitive functions.56 More evidence is needed to understand the role of yoga in improving cognitive functioning.
Emotional Health
Psychosocial stress is high among breast cancer patients and survivors.57,58 This causes circadian rhythm and cortisol regulation abnormalities, which are reported in women with breast cancer.59-64 Yoga is known to help stress and psychosocial and physical functioning in patients with cancer.65 Yoga was also shown to be equivalent to cognitive behavioral therapy in stress management in a population of patients without cancer.66 Daily yoga sessions lasting 60 minutes were shown to reduce reactive anxiety and trait anxiety in early-stage breast cancer patients undergoing conventional radiotherapy and chemotherapy compared with patients receiving supportive therapy, highlighting the role of yoga in managing anxiety related to treatment.67 In a study done by Culos-Reed et al, 20 cancer survivors who did 75 minutes of yoga per week for 7 weeks were compared with 18 cancer survivors who served as a control group.68 The intervention group reported significant improvement in emotional well-being, depression, concentration, and mood disturbances. In a longitudinal study by Mackenzie et al, 66 cancer survivors completed a 7-week yoga program and were assessed at baseline, immediately after the final yoga session, and at 3 and 6 months after the final session.69 Participants had significantly improved energy levels and affect. They also had moderate improvement in mindfulness and a moderate decrease in stress. Breast cancer patients who underwent restorative yoga sessions found improvements in mental health, depression, positive affect, and spirituality (peace/meaning).70 This was more pronounced in women with higher negative affect and lower emotional well-being at baseline. In a study of patients with ovarian cancer receiving chemotherapy, patients were instructed to perform up to 15-minute sessions including awareness, body movement, and breathing.71 Even with just 1 session of yoga intervention, patients experienced decreased anxiety.
Fatigue
Studies on yoga show improvement in fatigue both during and after treatment. In breast cancer patients undergoing chemotherapy, yoga was shown to benefit cognitive fatigue.72 Older cancer survivors also seem to benefit from yoga interventions.73 In a trial of a DVD-based yoga program, the benefits of yoga were similar to those of strengthening exercises, and both interventions helped decrease fatigue and improve QOL during the first year after diagnosis in early-stage breast cancer patients with cancer-related fatigue.74 Bower et al also showed that, for breast cancer survivors experiencing persistent chronic fatigue, a targeted yoga intervention led to significant improvements in fatigue and vigor over a 3-month follow-up compared with controls.75 Fatigue is commonly seen in breast cancer patients who are receiving adjuvant chemotherapy. In a study by Taso et al, women with breast cancer receiving chemotherapy were assigned to 60-minute yoga sessions incorporating Anusara yoga, gentle stretching, and relaxation twice a week for 8 weeks.76 By week 4, patients with low pretest fatigue in the yoga group experienced a reduction in fatigue. By week 8, all patients in the yoga group experienced a reduction in fatigue. Four weeks after the yoga intervention, patients in the group maintained the reduction in fatigue. This study shows the feasibility of an 8-week yoga program for women undergoing breast cancer therapy by improving fatigue. Yoga recently was added to National Comprehensive Cancer Network (NCCN) guidelines for management of cancer-related fatigue (level 1 evidence).77 However, the evidence was based on studies in women with breast cancer and survivors; therefore, more studies are needed in men and women with other cancers.
Surgical Setting/Postoperative Distress
Distress surrounding surgery in patients with breast cancer can impact postoperative outcomes. Yoga interventions, including breathing exercises, regulated breathing, and yogic relaxation techniques, improved several postsurgical measures such as length of hospital stay, drain retention, and suture removal.78 In this study, patients who practiced yoga also experienced a decrease in plasma TNF and better wound healing. Symptoms of anxiety and distress that occur preoperatively can lead to impaired immune function in addition to decreased QOL. In a study of yoga in early-stage breast cancer patients undergoing surgery, the benefit of yoga was seen not only with stress reduction but also with immune enhancement.42
Yoga has been shown to help alleviate acute pain and distress in women undergoing major surgery for gynecological cancer. A regimen of 3 15-minute sessions of yoga, including awareness meditation, coordination of breath with movement, and relaxation breathing, was shown to reduce acute pain and distress in such patients in an inpatient setting.79
Menopausal Symptoms
Breast cancer survivors have more severe menopausal symptoms compared with women without cancer.80,81 Hot flashes cause sleep disturbances and worsen fatigue and QOL.82 Tamoxifen and aromatase inhibitors significantly worsen menopausal symptoms such as hot flashes.81 Carson et al conducted a study of yoga that included postures, breathing techniques, didactic presentations, and group discussions.83 The yoga awareness regimen consisted of 8 weekly 120-minute group classes. Patients in the yoga arm had statistically significant improvements in the frequency, severity, and number of hot flashes. There were also improvements in arthralgia (joint pain), fatigue, sleep disturbance, vigor, and acceptance.
Arthralgia
Joint pain can be a major side effect that interferes with daily functions and activities in postmenopausal breast cancer survivors who receive aromatase inhibitor therapy.84 Arthralgia is reported in up to 50% of patients treated with aromatase inhibitors.84,85 It can affect functional status and lead to discontinuation of aromatase inhibitor therapy, jeopardizing clinical outcomes.86 Yoga as a complementary therapy has been shown to improve conditions such as low back pain87 and knee osteoarthritis88 in patients who do not have cancer. In a single-arm pilot trial by Galantino et al, breast cancer patients with aromatase inhibitor–related joint pain were provided with twice-weekly yoga sessions for 8 weeks. There were statistically significant improvements in balance, flexibility, pain severity, and health-related QOL.89 As noted above, improvement in arthralgia was also found in the study conducted by Carson et al.83
Insomnia
Insomnia is common among cancer patients and survivors90,91 and leads to increased fatigue and depression, decreased adherence to cancer treatments, and poor physical function and QOL.90-92 Management of insomnia consists of pharmacologic therapies such as benzodiazepines93,94 and nonpharmacologic options such as cognitive behavioral therapy.95
The first study of yoga found to improve sleep quality was conducted at MD Anderson Cancer Center in lymphoma patients.96 The effects of Tibetan yoga practices incorporating controlled breathing and visualization, mindfulness techniques, and low-impact postures were studied. Patients in the Tibetan yoga group had better subjective sleep quality, faster sleep latency, longer sleep duration, and less use of sleep medications. Mustian et al conducted a large yoga study in cancer survivors in which patients reporting chronic sleep disturbances were randomly assigned to the YOCAS program, which consisted of pranayama (breath control), 16 gentle hatha and restorative yoga postures, and meditation, or to usual care.92 The study reported improvements in global sleep quality, subjective sleep quality, actigraphy measures (wake after sleep onset, sleep efficiency), daytime dysfunction, and use of sleep medication after the yoga intervention compared with participants who received standard care.
Yoga to Address Other Symptoms
There is preliminary evidence supporting yoga as an integrative therapy for other symptoms unique to cancer survivors. For example, in head and neck cancer survivors, soft tissue damage involving the jaw, neck, shoulders, and chest results in swallowing issues, trismus, and aspiration, which are more pronounced in patients treated with conventional radiotherapy than in those treated with intensity-modulated radiotherapy.97 Some late effects of radiotherapy for head and neck cancer—such as pain, anxiety, and impaired shoulder function—were shown to be improved through the practice of hatha yoga in 1 study.98 Similarly, in a randomized controlled pilot study of patients with stage I to III breast cancer 6 months after treatment, participants in an 8-week yoga program experienced a reduction in arm induration and improvement in a QOL subscale of lymphedema symptoms. However, more evidence is needed to support the use of yoga as a therapeutic measure for breast cancer lymphedema.99,100
Yoga for Caregivers
Along with cancer patients, caregivers face psychological and physical burdens as well as deterioration in their QOL. Caregivers tend to report clinical levels of anxiety, depression, sleep disturbance, and fatigue and have similar or in fact higher levels than those of the patients for whom they are caring.101,102 Yoga has been found to help caregivers of patients with cancer. Recently, MD Anderson researchers conducted a trial in patients with high-grade glioma and their caregivers as dyads.103,104 Each dyad attended 2 or 3 60-minute weekly Vivekananda yoga sessions involving breathing exercises, physical exercises, relaxation, and meditation. The researchers found that the yoga program was safe, feasible, acceptable, and subjectively useful for patients with high-grade glioma and their caregivers. Preliminary evidence of QOL improvement for both patients and caregivers was noted. An improvement in QOL was also demonstrated in another preliminary study of yoga in patients undergoing thoracic radiotherapy and their caregivers.105
Another study by the group at MD Anderson evaluated a couple-based Tibetan yoga program that emphasized breathing exercises, gentle movements, guided visualizations, and emotional connectedness during radiotherapy for lung cancer.106 This study included 10 patient‐caregiver dyads and found the program to be feasible, safe, and acceptable. The researchers also found preliminary evidence of improved QOL by the end of radiotherapy relative to baseline—specifically in the areas of spiritual well‐being for patients, fatigue for caregivers, and sleep disturbances and mental health issues such as anxiety and depressive symptoms for both patients and caregivers. This is noteworthy, as QOL typically deteriorates during the course of radiotherapy, and the yoga program was able to buffer these changes.
Conclusion
Yoga therapy has been used successfully as an adjunct modality to improve QOL and cancer-related symptoms. As a part of an integrative medicine approach, yoga is commonly recommended for patients undergoing cancer treatment. Danhauer et al reviewed randomized controlled trials during and after treatment and concluded that the evidence is clearly positive for QOL, fatigue, and perceived stress.107 Results are less consistent but supportive for psychosocial outcomes such as benefit finding and spirituality. Evidence is mixed for sleep, anxiety, and depression. Post-treatment studies demonstrate improvements in fatigue, sleep, and multiple QOL domains. Yoga has been included in NCCN guidelines for fatigue management. Yoga, if approved by a physician, is also included among the behavioral therapies for anticipatory emesis and prevention and treatment of nausea in the recent update of the NCCN guidelines.108 The Society for Integrative Oncology guidelines include yoga for anxiety/stress reduction as a part of integrative treatment in breast cancer patients during and after therapy, which was endorsed by the American Society of Clinical Oncology.109
Because of the strong evidence for its benefits and a low side-effect profile, yoga is offered in group-class settings for patients during and after treatment and/or for caregivers in our institution. We often prescribe yoga as a therapeutic modality for selected groups of patients in our clinical practice. However, some patients may have restrictions after surgery that must be considered. In general, yoga has an excellent safety profile, the evidence base is strong, and we recommend that yoga therapy should be part of the standard of care as an integrative approach for patients with cancer undergoing active treatment as well as for cancer survivors and caregivers.
Acknowledgement: The authors thank Bryan Tutt for providing editorial assistance.
Corresponding author: Santhosshi Narayanan, MD, Department of Palliative, Rehabilitation, and Integrative Medicine, Unit 1414, The University of Texas MD Anderson Cancer Center, 1400 Pressler St., Houston, TX 77030; [email protected].
Financial disclosures: None.
1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Reports. 2008;(12):1-23.
2. Witt CM, Balneaves LG, Cardoso MJ, et al. A comprehensive definition for integrative oncology. NCI Monographs. 2017;2017(52):3-8.
3. Brauer JA, El Sehamy A, Metz JM, Mao JJ. Complementary and alternative medicine and supportive care at leading cancer centers: a systematic analysis of websites. J Altern Complement Med. 2010;16:183-186.
4. Lopez G, McQuade J, Cohen L, et al. Integrative oncology physician consultations at a comprehensive cancer center: analysis of demographic, clinical and patient reported outcomes. J Cancer. 2017;8:395-402.
5. Latte-Naor S, Mao JJ. Putting integrative oncology into practice: concepts and approaches. J Oncol Pract. 2019;15:7-14.
6. Richardson MA, Sanders T, Palmer JL, et al. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18:2505-2514.
7. Chaoul A, Milbury K, Sood AK, et al. Mind-body practices in cancer care. Curr Oncol Rep. 2014;16:417.
8. Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10:44-49.
9. Smith KB, Pukall CF. An evidence-based review of yoga as a complementary intervention for patients with cancer. Psychooncology. 2009;18:465-475.
10. Buffart LM, van Uffelen JG, Riphagen II, et al. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2012;12:559.
11. Cramer H, Lange S, Klose P, et al. Yoga for breast cancer patients and survivors: a systematic review and meta-analysis. BMC Cancer. 2012;12:412.
12. Felbel S, Meerpohl JJ, Monsef I, et al. Yoga in addition to standard care for patients with haematological malignancies. Cochrane Database Syst Rev. 2014;(6):CD010146.
13. Greenlee H, Balneaves LG, Carlson LE, et al. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. NCI Monographs. 2014;2014:346-358.
14. Sharma M, Haider T, Knowlden AP. Yoga as an alternative and complementary treatment for cancer: a systematic review. J Altern Complement Med. 2013;19:870-875.
15. Kordon C, Bihoreau C. Integrated communication between the nervous, endocrine and immune systems. Horm Res. 1989;31:100-104.
16. Gonzalez-Diaz SN, Arias-Cruz A, Elizondo-Villarreal B, Monge-Ortega OP. Psychoneuroimmunoendocrinology: clinical implications. World Allergy Organ J. 2017;10:19.
17. Godoy LD, Rossignoli MT, Delfino-Pereira P, et al. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci. 2018;12:127.
18. Goldstein DS. Adrenal responses to stress. Cell Mol Neurobiol. 2010;30:1433-1440.
19. Cole SW, Nagaraja AS, Lutgendorf SK, et al. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563-567.
20. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349-5361.
21. Arsalidou M, Duerden EG, Taylor MJ. The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum Brain Mapp. 2013;34:3031-3054.
22. Gard T, Taquet M, Dixit R, et al. Greater widespread functional connectivity of the caudate in older adults who practice kripalu yoga and vipassana meditation than in controls. Front Hum Neurosci. 2015;9:137.
23. Streeter CC, Gerbarg PL, Saper RB, et al. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78:571-579.
24. Brambilla P, Perez J, Barale F, et al. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721-737.
25. Streeter CC, Gerbarg PL, Saper RB, et al. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78:571-579.
26. Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: physiological pathways and mechanisms. Psychosom Med. 2011;73:724-730.
27. Armaiz-Pena GN, Cole SW, Lutgendorf SK, Sood AK. Neuroendocrine influences on cancer progression. Brain Behav Immun. 2013;30(suppl):S19-S25.
28. Tyagi A, Cohen M. Yoga and heart rate variability: A comprehensive review of the literature. Int J Yoga. 2016;9:97-113.
29. Sephton SE, Lush E, Dedert EA, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30 (suppl):S163-S170.
30. Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994-1000.
31. Arafah BM, Nishiyama FJ, Tlaygeh H, Hejal R. Measurement of salivary cortisol concentration in the assessment of adrenal function in critically ill subjects: a surrogate marker of the circulating free cortisol. J Clin Endocrinol Metab. 2007;92:2965-2971.
32. Cohen L, de Moor C, Devine D, et al. Endocrine levels at the start of treatment are associated with subsequent psychological adjustment in cancer patients with metastatic disease. Psychosom Med. 2001;63:951-958.
33. Cohen L, Cole SW, Sood AK, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PloS One. 2012;7:e42324.
34. Vadiraja HS, Raghavendra RM, Nagarathna R, et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Integr Cancer Ther. 2009;8:37-46.
35. Wu Y, Antony S, Meitzler JL, Doroshow JH. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014;345:164-173.
36. Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(suppl):S48-S57.
37. Derry HM, Jaremka LM, Bennett JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24:958-966.
38. Kiecolt-Glaser JK, Christian L, Preston H, et al. Stress, inflammation, and yoga practice. Psychosom Med. 2010;72:113-121.
39. Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21:1038-1049.
40. Infante JR, Peran F, Rayo JI, et al. Levels of immune cells in transcendental meditation practitioners. Int J Yoga. 2014;7:147-151.
41. Rao RM, Telles S, Nagendra HR, et al. Effects of yoga on natural killer cell counts in early breast cancer patients undergoing conventional treatment. Comment to: recreational music-making modulates natural killer cell activity, cytokines, and mood states in corporate employees Masatada Wachi, Masahiro Koyama, Masanori Utsuyama, Barry B. Bittman, Masanobu Kitagawa, Katsuiku Hirokawa Med Sci Monit, 2007; 13(2): CR57-70. Med Sci Monit. 2008;14:LE3-4.
42. Rao RM, Nagendra HR, Raghuram N, et al. Influence of yoga on mood states, distress, quality of life and immune outcomes in early stage breast cancer patients undergoing surgery. Int J Yoga. 2008;1:11-20.
43. Mozdarani H, Mansouri Z, Haeri SA. Cytogenetic radiosensitivity of g0-lymphocytes of breast and esophageal cancer patients as determined by micronucleus assay. J Radiat Res. 2005;46:111-116.
44. Scott D, Barber JB, Levine EL, et al. Radiation-induced micronucleus induction in lymphocytes identifies a high frequency of radiosensitive cases among breast cancer patients: a test for predisposition? Br J Cancer. 1998;77:614-620.
45. Banerjee B, Sharma S, Hegde S, Hande MP. Analysis of telomere damage by fluorescence in situ hybridisation on micronuclei in lymphocytes of breast carcinoma patients after radiotherapy. Breast Cancer Res Treat. 2008;107:25-31.
46. Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312-17315.
47. Glaser R, Thorn BE, Tarr KL, et al. Effects of stress on methyltransferase synthesis: an important DNA repair enzyme. Health Psychol. 1985;4:403-412.
48. Kiecolt-Glaser JK, Stephens RE, Lipetz PD, et al. Distress and DNA repair in human lymphocytes. J Behav Med. 1985;8:311-320.
49. Banerjee B, Vadiraj HS, Ram A, et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007;6:242-250.
50. Danhauer SC, Addington EL, Sohl SJ, et al. Review of yoga therapy during cancer treatment. Support Care Cancer. 2017;25:1357-1372.
51. Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8:43-55.
52. Ratcliff CG, Milbury K, Chandwani KD, et al. Examining mediators and moderators of yoga for women with breast cancer undergoing radiotherapy. Integr Cancer Ther. 2016;15:250-262.
53. Chandwani KD, Perkins G, Nagendra HR, et al. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol. 2014;32:1058-1065.
54. Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102-113.
55. Janelsins MC, Peppone LJ, Heckler CE, et al. YOCAS(c)(R) yoga reduces self-reported memory difficulty in cancer survivors in a nationwide randomized clinical trial: investigating relationships between memory and sleep. Integr Cancer Ther. 2016;15:263-271.
56. Biegler KA, Chaoul MA, Cohen L. Cancer, cognitive impairment, and meditation. Acta Oncol. 2009;48:18-26.
57. Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90:2297-2304.
58. Herschbach P, Keller M, Knight L, et al. Psychological problems of cancer patients: a cancer distress screening with a cancer-specific questionnaire. Br J Cancer. 2004;91:504-511.
59. Abercrombie HC, Giese-Davis J, Sephton S, et al. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082-1092.
60. Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med. 2005;67:277-280.
61. Bower JE, Ganz PA, Dickerson SS, et al. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92-100.
62. Giese-Davis J, Sephton SE, Abercrombie HC, et al. Repression and high anxiety are associated with aberrant diurnal cortisol rhythms in women with metastatic breast cancer. Health Psychol. 2004;23:645-650.
63. Giese-Davis J, DiMiceli S, Sephton S, Spiegel D. Emotional expression and diurnal cortisol slope in women with metastatic breast cancer in supportive-expressive group therapy: a preliminary study. Biol Psychol. 2006;73:190-198.
64. Stone AA, Schwartz JE, Smyth J, et al. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26:295-306.
65. Bower JE, Woolery A, Sternlieb B, Garet D. Yoga for cancer patients and survivors. Cancer Control. 2005;12:165-171.
66. Granath J, Ingvarsson S, von Thiele U, Lundberg U. Stress management: a randomized study of cognitive behavioural therapy and yoga. Cogn Behav Therap. 2006;35:3-10.
67. Rao MR, Raghuram N, Nagendra HR, et al. Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: a randomized controlled trial. Complement Ther Med. 2009;17:1-8.
68. Culos-Reed SN, Carlson LE, Daroux LM, Hately-Aldous S. A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psychooncology. 2006;15:891-897.
69. Mackenzie MJ, Carlson LE, Ekkekakis P, et al. Affect and mindfulness as predictors of change in mood disturbance, stress symptoms, and quality of life in a community-based yoga program for cancer survivors. Evid Based Complement Alternat Med. 2013;2013:419496.
70. Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psycho-oncology. 2009;18:360-368.
71. Sohl SJ, Danhauer SC, Schnur JB, et al. Feasibility of a brief yoga intervention during chemotherapy for persistent or recurrent ovarian cancer. Explore (NY). 2012;8:197-198.
72. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer. 2016;24:4005-4015.
73. Sprod LK, Fernandez ID, Janelsins MC, et al. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. J Geriatr Oncol. 2015;6:8-14.
74. Wang G, Wang S, Jiang P, Zeng C. Effect of yoga on cancer related fatigue in breast cancer patients with chemotherapy [in Chinese]. Zhong Nan Da Xue Bao Yi Xue Ban. 2014;39:1077-1082.
75. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118:3766-3775.
76. Taso CJ, Lin HS, Lin WL, et al. The effect of yoga exercise on improving depression, anxiety, and fatigue in women with breast cancer: a randomized controlled trial. J Nurs Res. 2014;22:155-164.
77. Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-related fatigue, Version 2.2015. J Natl Compr Canc Netw. 2015;13:1012-1039.
78. Rao RM, Nagendra HR, Raghuram N, et al. Influence of yoga on postoperative outcomes and wound healing in early operable breast cancer patients undergoing surgery. Int J Yoga. 2008;1:33-41.
79. Sohl SJ, Avis NE, Stanbery K, et al. Feasibility of a brief yoga intervention for improving acute pain and distress post gynecologic surgery. Int J Yoga Therap. 2016;26:43-47.
80. Gupta P, Sturdee DW, Palin SL, et al. Menopausal symptoms in women treated for breast cancer: the prevalence and severity of symptoms and their perceived effects on quality of life. Climacteric. 2006;9:49-58.
81. Canney PA, Hatton MQ. The prevalence of menopausal symptoms in patients treated for breast cancer. Clin Oncol (R Coll Radiol). 1994;6:297-299.
82. Carpenter JS, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum. 2002;29:E16-25.
83. Carson JW, Carson KM, Porter LS, et al. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer. 2009;17:1301-1309.
84. Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16:223-234.
85. Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115:3631-3639.
86. Presant CA, Bosserman L, Young T, et al. Aromatase inhibitor-associated arthralgia and/or bone pain: frequency and characterization in non-clinical trial patients. Clin Breast Cancer. 2007;7:775-778.
87. Saper RB, Sherman KJ, Cullum-Dugan D, et al. Yoga for chronic low back pain in a predominantly minority population: a pilot randomized controlled trial. Altern Ther Health Med. 2009;15:18-27.
88. Kolasinski SL, Garfinkel M, Tsai AG, et al. Iyengar yoga for treating symptoms of osteoarthritis of the knees: a pilot study. J Altern Complement Med. 2005;11:689-693.
89. Galantino ML, Desai K, Greene L, et al. Impact of yoga on functional outcomes in breast cancer survivors with aromatase inhibitor-associated arthralgias. Integr Cancer Ther. 2012;11:313-320.
90. Ancoli-Israel S. Recognition and treatment of sleep disturbances in cancer. J Clin Oncol. 2009;27:5864-5866.
91. Savard J, Ivers H, Villa J, et al. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol. 2011;29:3580-3586.
92. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31:3233-3241.
93. Moore TA, Berger AM, Dizona P. Sleep aid use during and following breast cancer adjuvant chemotherapy. Psychooncology. 2011;20:321-325.
94. Omvik S, Pallesen S, Bjorvatn B, et al. Patient characteristics and predictors of sleep medication use. Int Clin Psychopharmacol. 2010;25:91-100.
95. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133.
96. Cohen L, Warneke C, Fouladi RT, et al. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253-2260.
97. Kraaijenga SA, Oskam IM, van der Molen L, et al. Evaluation of long term (10-years+) dysphagia and trismus in patients treated with concurrent chemo-radiotherapy for advanced head and neck cancer. Oral Oncol. 2015;51:787-794.
98. Adair M, Murphy B, Yarlagadda S, et al. Feasibility and preliminary efficacy of tailored yoga in survivors of head and neck cancer: a pilot study. Integr Cancer Ther. 2018;17:774-784.
99. Loudon A, Barnett T, Williams A. Yoga, breast cancer-related lymphoedema and well-being: A descriptive report of women’s participation in a clinical trial. J Clin Nurs. 2017;26:4685-4695.
100. Loudon A, Barnett T, Piller N, et al. The effects of yoga on shoulder and spinal actions for women with breast cancer-related lymphoedema of the arm: A randomised controlled pilot study. BMC Complement Altern Med. 2016;16:343.
101. Petruzzi A, Finocchiaro CY, Lamperti E, Salmaggi A. Living with a brain tumor: reaction profiles in patients and their caregivers. Support Care Cancer. 2013;21:1105-1111.
102. Pawl JD, Lee SY, Clark PC, Sherwood PR. Sleep characteristics of family caregivers of individuals with a primary malignant brain tumor. Oncol Nurs Forum. 2013;40:171-179.
103. Milbury K, Mallaiah S, Mahajan A, et al. Yoga program for high-grade glioma patients undergoing radiotherapy and their family caregivers. Integr Cancer Ther. 2018;17:332-336.
104. Milbury K, Li J, Weathers S-P, et al. Pilot randomized controlled trial of a dyadic yoga program for glioma patients undergoing radiotherapy and their family caregivers. Neurooncol Pract. 2019;6:311-320.
105. Milbury K, Liao Z, Shannon V, et al. Dyadic yoga program for patients undergoing thoracic radiotherapy and their family caregivers: Results of a pilot randomized controlled trial. Psychooncology. 2019;28:615-621.
106. Milbury K, Chaoul A, Engle R, et al. Couple-based Tibetan yoga program for lung cancer patients and their caregivers. Psychooncology. 2015;24:117-120.
107. Danhauer SC, Addington EL, Cohen L, et al. Yoga for symptom management in oncology: A review of the evidence base and future directions for research. Cancer. 2019;125:1979-1989.
108. National Comprehensive Cancer Center. Flash Update: NCCN Guidelines® and NCCN Compendium® for Antiemesis. NCCN website. Accessed August 29, 2019.
109. Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36:2647-2655.
1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Reports. 2008;(12):1-23.
2. Witt CM, Balneaves LG, Cardoso MJ, et al. A comprehensive definition for integrative oncology. NCI Monographs. 2017;2017(52):3-8.
3. Brauer JA, El Sehamy A, Metz JM, Mao JJ. Complementary and alternative medicine and supportive care at leading cancer centers: a systematic analysis of websites. J Altern Complement Med. 2010;16:183-186.
4. Lopez G, McQuade J, Cohen L, et al. Integrative oncology physician consultations at a comprehensive cancer center: analysis of demographic, clinical and patient reported outcomes. J Cancer. 2017;8:395-402.
5. Latte-Naor S, Mao JJ. Putting integrative oncology into practice: concepts and approaches. J Oncol Pract. 2019;15:7-14.
6. Richardson MA, Sanders T, Palmer JL, et al. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18:2505-2514.
7. Chaoul A, Milbury K, Sood AK, et al. Mind-body practices in cancer care. Curr Oncol Rep. 2014;16:417.
8. Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10:44-49.
9. Smith KB, Pukall CF. An evidence-based review of yoga as a complementary intervention for patients with cancer. Psychooncology. 2009;18:465-475.
10. Buffart LM, van Uffelen JG, Riphagen II, et al. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2012;12:559.
11. Cramer H, Lange S, Klose P, et al. Yoga for breast cancer patients and survivors: a systematic review and meta-analysis. BMC Cancer. 2012;12:412.
12. Felbel S, Meerpohl JJ, Monsef I, et al. Yoga in addition to standard care for patients with haematological malignancies. Cochrane Database Syst Rev. 2014;(6):CD010146.
13. Greenlee H, Balneaves LG, Carlson LE, et al. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. NCI Monographs. 2014;2014:346-358.
14. Sharma M, Haider T, Knowlden AP. Yoga as an alternative and complementary treatment for cancer: a systematic review. J Altern Complement Med. 2013;19:870-875.
15. Kordon C, Bihoreau C. Integrated communication between the nervous, endocrine and immune systems. Horm Res. 1989;31:100-104.
16. Gonzalez-Diaz SN, Arias-Cruz A, Elizondo-Villarreal B, Monge-Ortega OP. Psychoneuroimmunoendocrinology: clinical implications. World Allergy Organ J. 2017;10:19.
17. Godoy LD, Rossignoli MT, Delfino-Pereira P, et al. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci. 2018;12:127.
18. Goldstein DS. Adrenal responses to stress. Cell Mol Neurobiol. 2010;30:1433-1440.
19. Cole SW, Nagaraja AS, Lutgendorf SK, et al. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563-567.
20. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349-5361.
21. Arsalidou M, Duerden EG, Taylor MJ. The centre of the brain: topographical model of motor, cognitive, affective, and somatosensory functions of the basal ganglia. Hum Brain Mapp. 2013;34:3031-3054.
22. Gard T, Taquet M, Dixit R, et al. Greater widespread functional connectivity of the caudate in older adults who practice kripalu yoga and vipassana meditation than in controls. Front Hum Neurosci. 2015;9:137.
23. Streeter CC, Gerbarg PL, Saper RB, et al. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78:571-579.
24. Brambilla P, Perez J, Barale F, et al. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721-737.
25. Streeter CC, Gerbarg PL, Saper RB, et al. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78:571-579.
26. Lutgendorf SK, Sood AK. Biobehavioral factors and cancer progression: physiological pathways and mechanisms. Psychosom Med. 2011;73:724-730.
27. Armaiz-Pena GN, Cole SW, Lutgendorf SK, Sood AK. Neuroendocrine influences on cancer progression. Brain Behav Immun. 2013;30(suppl):S19-S25.
28. Tyagi A, Cohen M. Yoga and heart rate variability: A comprehensive review of the literature. Int J Yoga. 2016;9:97-113.
29. Sephton SE, Lush E, Dedert EA, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30 (suppl):S163-S170.
30. Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994-1000.
31. Arafah BM, Nishiyama FJ, Tlaygeh H, Hejal R. Measurement of salivary cortisol concentration in the assessment of adrenal function in critically ill subjects: a surrogate marker of the circulating free cortisol. J Clin Endocrinol Metab. 2007;92:2965-2971.
32. Cohen L, de Moor C, Devine D, et al. Endocrine levels at the start of treatment are associated with subsequent psychological adjustment in cancer patients with metastatic disease. Psychosom Med. 2001;63:951-958.
33. Cohen L, Cole SW, Sood AK, et al. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PloS One. 2012;7:e42324.
34. Vadiraja HS, Raghavendra RM, Nagarathna R, et al. Effects of a yoga program on cortisol rhythm and mood states in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Integr Cancer Ther. 2009;8:37-46.
35. Wu Y, Antony S, Meitzler JL, Doroshow JH. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014;345:164-173.
36. Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(suppl):S48-S57.
37. Derry HM, Jaremka LM, Bennett JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24:958-966.
38. Kiecolt-Glaser JK, Christian L, Preston H, et al. Stress, inflammation, and yoga practice. Psychosom Med. 2010;72:113-121.
39. Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21:1038-1049.
40. Infante JR, Peran F, Rayo JI, et al. Levels of immune cells in transcendental meditation practitioners. Int J Yoga. 2014;7:147-151.
41. Rao RM, Telles S, Nagendra HR, et al. Effects of yoga on natural killer cell counts in early breast cancer patients undergoing conventional treatment. Comment to: recreational music-making modulates natural killer cell activity, cytokines, and mood states in corporate employees Masatada Wachi, Masahiro Koyama, Masanori Utsuyama, Barry B. Bittman, Masanobu Kitagawa, Katsuiku Hirokawa Med Sci Monit, 2007; 13(2): CR57-70. Med Sci Monit. 2008;14:LE3-4.
42. Rao RM, Nagendra HR, Raghuram N, et al. Influence of yoga on mood states, distress, quality of life and immune outcomes in early stage breast cancer patients undergoing surgery. Int J Yoga. 2008;1:11-20.
43. Mozdarani H, Mansouri Z, Haeri SA. Cytogenetic radiosensitivity of g0-lymphocytes of breast and esophageal cancer patients as determined by micronucleus assay. J Radiat Res. 2005;46:111-116.
44. Scott D, Barber JB, Levine EL, et al. Radiation-induced micronucleus induction in lymphocytes identifies a high frequency of radiosensitive cases among breast cancer patients: a test for predisposition? Br J Cancer. 1998;77:614-620.
45. Banerjee B, Sharma S, Hegde S, Hande MP. Analysis of telomere damage by fluorescence in situ hybridisation on micronuclei in lymphocytes of breast carcinoma patients after radiotherapy. Breast Cancer Res Treat. 2008;107:25-31.
46. Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312-17315.
47. Glaser R, Thorn BE, Tarr KL, et al. Effects of stress on methyltransferase synthesis: an important DNA repair enzyme. Health Psychol. 1985;4:403-412.
48. Kiecolt-Glaser JK, Stephens RE, Lipetz PD, et al. Distress and DNA repair in human lymphocytes. J Behav Med. 1985;8:311-320.
49. Banerjee B, Vadiraj HS, Ram A, et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther. 2007;6:242-250.
50. Danhauer SC, Addington EL, Sohl SJ, et al. Review of yoga therapy during cancer treatment. Support Care Cancer. 2017;25:1357-1372.
51. Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8:43-55.
52. Ratcliff CG, Milbury K, Chandwani KD, et al. Examining mediators and moderators of yoga for women with breast cancer undergoing radiotherapy. Integr Cancer Ther. 2016;15:250-262.
53. Chandwani KD, Perkins G, Nagendra HR, et al. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol. 2014;32:1058-1065.
54. Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102-113.
55. Janelsins MC, Peppone LJ, Heckler CE, et al. YOCAS(c)(R) yoga reduces self-reported memory difficulty in cancer survivors in a nationwide randomized clinical trial: investigating relationships between memory and sleep. Integr Cancer Ther. 2016;15:263-271.
56. Biegler KA, Chaoul MA, Cohen L. Cancer, cognitive impairment, and meditation. Acta Oncol. 2009;48:18-26.
57. Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90:2297-2304.
58. Herschbach P, Keller M, Knight L, et al. Psychological problems of cancer patients: a cancer distress screening with a cancer-specific questionnaire. Br J Cancer. 2004;91:504-511.
59. Abercrombie HC, Giese-Davis J, Sephton S, et al. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082-1092.
60. Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med. 2005;67:277-280.
61. Bower JE, Ganz PA, Dickerson SS, et al. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92-100.
62. Giese-Davis J, Sephton SE, Abercrombie HC, et al. Repression and high anxiety are associated with aberrant diurnal cortisol rhythms in women with metastatic breast cancer. Health Psychol. 2004;23:645-650.
63. Giese-Davis J, DiMiceli S, Sephton S, Spiegel D. Emotional expression and diurnal cortisol slope in women with metastatic breast cancer in supportive-expressive group therapy: a preliminary study. Biol Psychol. 2006;73:190-198.
64. Stone AA, Schwartz JE, Smyth J, et al. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26:295-306.
65. Bower JE, Woolery A, Sternlieb B, Garet D. Yoga for cancer patients and survivors. Cancer Control. 2005;12:165-171.
66. Granath J, Ingvarsson S, von Thiele U, Lundberg U. Stress management: a randomized study of cognitive behavioural therapy and yoga. Cogn Behav Therap. 2006;35:3-10.
67. Rao MR, Raghuram N, Nagendra HR, et al. Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: a randomized controlled trial. Complement Ther Med. 2009;17:1-8.
68. Culos-Reed SN, Carlson LE, Daroux LM, Hately-Aldous S. A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psychooncology. 2006;15:891-897.
69. Mackenzie MJ, Carlson LE, Ekkekakis P, et al. Affect and mindfulness as predictors of change in mood disturbance, stress symptoms, and quality of life in a community-based yoga program for cancer survivors. Evid Based Complement Alternat Med. 2013;2013:419496.
70. Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psycho-oncology. 2009;18:360-368.
71. Sohl SJ, Danhauer SC, Schnur JB, et al. Feasibility of a brief yoga intervention during chemotherapy for persistent or recurrent ovarian cancer. Explore (NY). 2012;8:197-198.
72. Stan DL, Croghan KA, Croghan IT, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer. 2016;24:4005-4015.
73. Sprod LK, Fernandez ID, Janelsins MC, et al. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. J Geriatr Oncol. 2015;6:8-14.
74. Wang G, Wang S, Jiang P, Zeng C. Effect of yoga on cancer related fatigue in breast cancer patients with chemotherapy [in Chinese]. Zhong Nan Da Xue Bao Yi Xue Ban. 2014;39:1077-1082.
75. Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118:3766-3775.
76. Taso CJ, Lin HS, Lin WL, et al. The effect of yoga exercise on improving depression, anxiety, and fatigue in women with breast cancer: a randomized controlled trial. J Nurs Res. 2014;22:155-164.
77. Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-related fatigue, Version 2.2015. J Natl Compr Canc Netw. 2015;13:1012-1039.
78. Rao RM, Nagendra HR, Raghuram N, et al. Influence of yoga on postoperative outcomes and wound healing in early operable breast cancer patients undergoing surgery. Int J Yoga. 2008;1:33-41.
79. Sohl SJ, Avis NE, Stanbery K, et al. Feasibility of a brief yoga intervention for improving acute pain and distress post gynecologic surgery. Int J Yoga Therap. 2016;26:43-47.
80. Gupta P, Sturdee DW, Palin SL, et al. Menopausal symptoms in women treated for breast cancer: the prevalence and severity of symptoms and their perceived effects on quality of life. Climacteric. 2006;9:49-58.
81. Canney PA, Hatton MQ. The prevalence of menopausal symptoms in patients treated for breast cancer. Clin Oncol (R Coll Radiol). 1994;6:297-299.
82. Carpenter JS, Johnson D, Wagner L, Andrykowski M. Hot flashes and related outcomes in breast cancer survivors and matched comparison women. Oncol Nurs Forum. 2002;29:E16-25.
83. Carson JW, Carson KM, Porter LS, et al. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Support Care Cancer. 2009;17:1301-1309.
84. Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16:223-234.
85. Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor-related arthralgia among breast cancer survivors. Cancer. 2009;115:3631-3639.
86. Presant CA, Bosserman L, Young T, et al. Aromatase inhibitor-associated arthralgia and/or bone pain: frequency and characterization in non-clinical trial patients. Clin Breast Cancer. 2007;7:775-778.
87. Saper RB, Sherman KJ, Cullum-Dugan D, et al. Yoga for chronic low back pain in a predominantly minority population: a pilot randomized controlled trial. Altern Ther Health Med. 2009;15:18-27.
88. Kolasinski SL, Garfinkel M, Tsai AG, et al. Iyengar yoga for treating symptoms of osteoarthritis of the knees: a pilot study. J Altern Complement Med. 2005;11:689-693.
89. Galantino ML, Desai K, Greene L, et al. Impact of yoga on functional outcomes in breast cancer survivors with aromatase inhibitor-associated arthralgias. Integr Cancer Ther. 2012;11:313-320.
90. Ancoli-Israel S. Recognition and treatment of sleep disturbances in cancer. J Clin Oncol. 2009;27:5864-5866.
91. Savard J, Ivers H, Villa J, et al. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol. 2011;29:3580-3586.
92. Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31:3233-3241.
93. Moore TA, Berger AM, Dizona P. Sleep aid use during and following breast cancer adjuvant chemotherapy. Psychooncology. 2011;20:321-325.
94. Omvik S, Pallesen S, Bjorvatn B, et al. Patient characteristics and predictors of sleep medication use. Int Clin Psychopharmacol. 2010;25:91-100.
95. Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133.
96. Cohen L, Warneke C, Fouladi RT, et al. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253-2260.
97. Kraaijenga SA, Oskam IM, van der Molen L, et al. Evaluation of long term (10-years+) dysphagia and trismus in patients treated with concurrent chemo-radiotherapy for advanced head and neck cancer. Oral Oncol. 2015;51:787-794.
98. Adair M, Murphy B, Yarlagadda S, et al. Feasibility and preliminary efficacy of tailored yoga in survivors of head and neck cancer: a pilot study. Integr Cancer Ther. 2018;17:774-784.
99. Loudon A, Barnett T, Williams A. Yoga, breast cancer-related lymphoedema and well-being: A descriptive report of women’s participation in a clinical trial. J Clin Nurs. 2017;26:4685-4695.
100. Loudon A, Barnett T, Piller N, et al. The effects of yoga on shoulder and spinal actions for women with breast cancer-related lymphoedema of the arm: A randomised controlled pilot study. BMC Complement Altern Med. 2016;16:343.
101. Petruzzi A, Finocchiaro CY, Lamperti E, Salmaggi A. Living with a brain tumor: reaction profiles in patients and their caregivers. Support Care Cancer. 2013;21:1105-1111.
102. Pawl JD, Lee SY, Clark PC, Sherwood PR. Sleep characteristics of family caregivers of individuals with a primary malignant brain tumor. Oncol Nurs Forum. 2013;40:171-179.
103. Milbury K, Mallaiah S, Mahajan A, et al. Yoga program for high-grade glioma patients undergoing radiotherapy and their family caregivers. Integr Cancer Ther. 2018;17:332-336.
104. Milbury K, Li J, Weathers S-P, et al. Pilot randomized controlled trial of a dyadic yoga program for glioma patients undergoing radiotherapy and their family caregivers. Neurooncol Pract. 2019;6:311-320.
105. Milbury K, Liao Z, Shannon V, et al. Dyadic yoga program for patients undergoing thoracic radiotherapy and their family caregivers: Results of a pilot randomized controlled trial. Psychooncology. 2019;28:615-621.
106. Milbury K, Chaoul A, Engle R, et al. Couple-based Tibetan yoga program for lung cancer patients and their caregivers. Psychooncology. 2015;24:117-120.
107. Danhauer SC, Addington EL, Cohen L, et al. Yoga for symptom management in oncology: A review of the evidence base and future directions for research. Cancer. 2019;125:1979-1989.
108. National Comprehensive Cancer Center. Flash Update: NCCN Guidelines® and NCCN Compendium® for Antiemesis. NCCN website. Accessed August 29, 2019.
109. Lyman GH, Greenlee H, Bohlke K, et al. Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol. 2018;36:2647-2655.
Disclosure After Adverse Medical Outcomes: A Multidimensional Challenge
From The Communication in Healthcare Group, Seattle, WA.
Abstract
- Objective: To review established approaches to disclosure and resolution following adverse medical outcomes and highlight barriers that may hinder universal implementation of effective disclosure/resolution practices.
- Methods: An overview of established approaches to disclosure and resolution of adverse medical outcomes is presented.
- Results: Clinicians must be equipped to manage situations where adverse medical outcomes occur even though the care provided was reasonable, within the standard, as well as in situations where preventable problems in the care provided were likely the cause of patient harm. Established approaches that have proven useful for investigating, disclosing, and resolving situations, captured in the acronyms AIDR, ALEE, and TEAM, can assist clinicians in the disclosure and ultimate resolution of these 2 types of situations.
- Conclusion: Health care organizations with a solid commitment and a reliable structure for ensuring adherence to full disclosure and fair resolution of adverse outcomes have demonstrated sustainable progress in ethically and effectively resolving situations where patients are harmed by medical care.
Keywords: safety; medical error; adverse outcomes; resolution; communication.
Much has been learned over the 20 years since the Institute of Medicine’s (IOM) report To Err Is Human1 was published. At the time it was published, the IOM report made it clear that only a minority of preventable patient harms were being acknowledged, investigated, and reported. In the face of adverse outcomes “dissemble, deny, and defend” was a common strategy of many clinicians, institutions, and liability carriers.2 The health care system appeared to place a priority on protecting itself from reputational and financial harm over the rights of injured patients to be given an accurate understanding of what had happened in their care and to pursue restitution, if appropriate.3-5
The emerging quality improvement movement was accompanied by calls for increased patient advocacy. This included the goal of greater transparency and more timely and equitable resolutions with patients who have been harmed by problems in care. Health care systems pressed for confidentiality protections in exchange for increased focus on quality improvement.6 Applying medical ethics of autonomy, no-maleficence, beneficence, and justice initially took a backseat, as risk management was given priority.7 Insurance carriers have no ethical obligation, and a clear disincentive, to assure that harmed patients are fully informed and offered restitution. Some self-insured health systems, however, began experimenting with more proactive and transparent approaches to disclosure and resolution. In contrast to the often-reported fear of a liability explosion, they reported reduced claims and suits, shorter time to resolution, and reduced overall financial cost,8-10 providing some evidence that perhaps greater openness could work after all.
But for providers and staff to allow transparency and candor to become the norm, institutions needed to create a more “just culture” for managing errors. Individual impairment or willful disregard of safe practice would need to be handled differently from the slips and lapses that more often contributed to preventable harm.11 For example, the nurse who was inadequately oriented to the equipment on an unfamiliar unit where she was asked to work a double shift due to a staffing shortage should not be held as accountable as an employee who knowingly violated agreed upon safe practices, even though patient harm resulted in each situation. It became clear that patient harm was usually the result of multiple factors involving individuals, communication, procedures, systems, and equipment. Blaming and disciplining individuals at the sharp end would not reliably reduce adverse outcomes.
Since the 1999 IOM report, we have developed general agreement on best practices for investigating, disclosing, and resolving situations where patients are harmed by medical care.12,13 This article reviews the perspectives and practices that appear necessary for effective disclosure and resolution after an adverse outcome and highlights barriers to reliably enacting them in practice.
Elements of Effective Disclosure
Effective disclosure to patients and families hinges on determining and providing an accurate understanding of what happened in the patient’s care. It should be the care providers’ and their institution’s responsibility to determine causation and disclose it. This should not require only the most upset patients and families initiating a legal process taking 3 years or more to complete. The most consequential question must be answered, “Was the care provided reasonable?” That is, was everything done within the standard, as would have been expected by similarly trained clinicians with the information and resources available at that time? It follows that if care was reasonable, then the adverse outcome could not normally have been prevented, no correction in care processes is called for, and no financial compensation is required. If the care review reveals deficiencies in care that were linked to patient harm, then achieving a satisfying resolution would be more complex and difficult.13 First, individuals would have to accept that they have contributed to patient harm, itself an often-contentious process and psychologically devastating realization. Then they must have this difficult conversation with patient and family, creating liability risk for themselves in the process. They must commit to correcting the problems that contributed to the harm. They must facilitate, rather than obstruct, a path to a restitution that addresses the medical, practical, and financial harms that have resulted. Given the challenges inherent to disclosure and resolution, it is no wonder that dissembling, denying, and defending was the common practice for the preceding decades.14
Disclosure and Resolution Pathways
I was the co-developer of an approach to disclosure and resolution which is now widely accepted and that has been taught across the United States and Canada to more than 50,000 health care providers and administrators over 18 years.15,16 We learned that resolving adverse medical outcomes is a 4-part process (anticipate, investigate, disclose, resolve [AIDR]). Most adverse or simply disappointing outcomes occur despite reasonable care (eg, due to biological variability, the imprecision of the science and limitations and risks of the procedures). The minority of harms are associated with deficiencies in the care (ie, unreasonable care). We need to equip ourselves to manage both situations effectively. The approach we developed can be captured in 3 acronyms: AIDR, ALEE, and TEAM,
AIDR
This acronym encapsulates the overview guidance for clinicians after an adverse event or outcome, regardless of the cause.
Anticipate the thoughts and feelings of the harmed/disappointed patient and family and reach out immediately with an expression of sympathy.
Investigate sufficiently to address questions about most likely causation and do not conjecture prior to investigation. Ask for patience—“You deserve more than a guess”—and keep in regular contact to reinforce the promise that there will be a full reporting when the review is complete.
Disclose (in a planned and coordinated manner) what has been learned in the investigation.
Resolve the situation with the patient and family consistent with our ethical principles.
If our failure caused the harm (care unreasonable/breached the standard), then working toward a fair restitution and taking corrective actions are appropriate. If the care was found to have been reasonable, then compensation would not be offered and corrective action is unwarranted. The organization would defend reasonable care if a claim was still pursued.
This process involves ethical clarity, emotional intelligence, and discipline. Clinicians must first acknowledge that a disappointing outcome or event has occurred. Clinicians involved in the care, usually led by the attending provider, then immediately reach out to the patient and family with sympathy, a plan of care to address the medical issues, and the promise to investigate and follow-up with the patient and family when the harm and its causes are more clearly determined. To disclose simply means to provide an accurate understanding (ie, the understanding determined by the investigation we conducted) of what happened, its causes, and consequences. Depending on the extent of the harm and the complexity and time needed for the investigation, a “coach” or “disclosure coordinator” who has advanced training in managing these situations is brought in to guide the process. The disclosure coach/coordinator provides a consistent and steady hand throughout the process of investigation, disclosure, and ultimately resolution with patient and family. Patients and families often move across settings during the time of the AIDR process, and it is easy to lose track of them unless someone is following the entire process until resolved.
ALEE
When the investigation of an adverse/disappointing outcome determines the care was reasonable and therefore the adverse outcome could not have been prevented, we use the ALEE pathway to guide the disclosure conversation (Step 3 in AIDR) with the patient and family:
Anticipate. What are the questions, thoughts, and feelings we would expect the patient and family will have? On this track, there is nothing to apologize for since the care was reasonable, yet expressing compassion and sympathy for the patient’s experience is essential. “I/we really sympathize with how differently this has turned out than we had hoped.”
Listen. Invite and listen for their questions and concerns, how they are seeing the situation, and where and what they are finding most upsetting and in need of explanation.
Empathize. There are 2 kinds of empathy required here. Cognitive empathy means showing that we understand their thinking from their perspective, separate from whether we fully agree. Emotional empathy involves demonstrating that their emotions are understandable given the situation, even if those emotions are painful for clinicians to experience. Listening in step 2 is how we learned their perspective and emotions. Now we can show accurate empathy: I/we can understand how upsetting it is to be facing another set of procedures to treat the unfortunate complications from your last surgery.
Explain. Even when care is reasonable, questions and perhaps suspicions are to be expected. Listening and empathizing sets us up to focus our explanations on the patient’s and family’s key questions with a level of thoughtfulness and transparency that conveys credibility. We should not assume, however, that they have accepted our explanation. Instead, solicit their reactions and unresolved questions as part of the disclosure discussion. It is normal for additional concerns to emerge in the days after the disclosure discussion, and we should be ready to address these concerns until resolved. In some instances, the patient and family will not be satisfied and it may be helpful to offer an independent review of the care. If the unresolved patient and family engages an attorney, that will be the first step taken anyway. Proactively offering an independent review signals confidence in your objectivity and sensitivity to the importance of fairness for the patient and family: Your questions and concerns are completely normal in light of the disappointing experience you have had. Let me see if I/we can address those now to your satisfaction.
TEAM
If the investigation determines that aspects of the care were unreasonable (breached the standard) and the adverse outcome/harm was related to the deficiencies in the care, then we use the TEAM pathway to disclose and resolve the situation with the patient and family
Truthful and Transparent and Teamwork. We should be offering our most accurate understanding of how the adverse outcome occurred, with sufficient depth and clarity that the patient and family can see how we reached that conclusion. In straightforward situations involving minor harm (eg, an allergic reaction to a medication that the clinician overlooked and that resulted in an urgent care center visit), a very limited investigation may clarify the situation sufficiently that the prescribing provider, accompanied by an office or staff nurse as support and witness, may be able to complete an effective disclosure in a single discussion, and simply writing off a bill or arranging to reimburse the urgent care center visit cost may satisfy the affected patient.
In more complex situations involving greater harm, a number of people must be involved to accomplish TEAM tasks: to offer an explanation, to answer questions, to make apologies, to explain changes intended to reduce the chance of harm to others in the future, and to work through any restitution that may be appropriate. Appointing a disclosure coach/coordinator/facilitator who has had extended training in the disclosure process can help guide these more complex situations. Risk management, insurance carriers, and legal counsel should be aware and advising throughout the process and participating directly in meetings with the patient and family, as appropriate. Since on the TEAM track we are admitting liability, offering a path to financial restitution may be warranted and the disclosure process may trigger reporting requirements with regulatory as well as human resource implications.
The patient and family may want to include other people on their “team” as well. Since complex disclosure meetings need to be carefully planned in advance, we should clarify who will be attending from the health care side and who the family intends to involve. We should anticipate potential requests and questions such as: Would it be OK to record this meeting? Can we ask our attorney to attend? Who are all these people and why are they in this meeting? (We should introduce all team members and clarify how their involvement is necessary to help reach the most satisfying resolution for all involved.)
Empathize. Admitting that deficient aspects in the care contributed to the harm will trigger thoughts, emotions, and expectations for the patient and family. Empathizing involves seeing the whole situation from their perspective and acknowledging their emotions as understandable. Empathizing is not the same as fully agreeing with the patient’s and family’s perspective, but we will not be able to effectively address concerns and expectations that we have not understood. Organizations should have supports in place for staff who are involved in these difficult situations. Nonetheless, we must prioritize the patient’s and family‘s feelings in a disclosure meeting.
Apologize and be Accountable. This calls for both expressions of sympathy as well as a genuine apology for having caused harm by failure in some aspect of care: We are very sorry you are going through this difficult situation. We are especially sorry to tell you that we now recognize that problems in the care we provided are the most likely cause of this harm. Would this be a good time to explain what we learned?
Having the responsible clinicians present increases the chances of achieving the most complete resolution in a single planned and well facilitated meeting. The tasks for that meeting include: offering an explanation that reveals the problems in care that contributed to the adverse outcome, making sincere apologies, and explaining changes to reduce chance of harm to others. The disclosure coach can work with individuals to help them understand how and why their involvement can be important and to help staff members become ready to participate constructively in the disclosure meeting. When individuals appear unable or unwilling to contribute constructively, a plan is needed for how their part can be replaced (eg, a charge nurse or department chair might need to step in to explain and apologize for the care of a subordinate). Managers/administrators can explain contributory factors for what may at first appear to have been simply individual negligence. Administrators can describe the actions that the organization is taking to correct problems that contributed to the patient harm: As nursing executive, it is my responsibility to see that all our staff have been adequately trained on the equipment we are asking them to use. We now recognize that the nurse’s lack of familiarity with that equipment contributed to the harm you experienced and I am very sorry for that. It is my responsibility to get that problem corrected, and we are already taking steps to assure that. Patients and families often have ideas for improving care processes and appreciate being invited to share these ideas as a service to future patients.
Manage until resolved. On the “care unreasonable” track, we must signal openness to helping with the patient’s and family’s immediate and longer-term needs, as well as their expectations about financial and other forms of restitution. Someone should be in the meeting who can describe the next steps in working towards a fair restitution and how that process will take place following the conclusion of the disclosure meeting. The close involvement of risk and claims professionals throughout the process of investigation through to the disclosure discussion itself will assure a more satisfactory handoff to questions about around financial compensation
Psychological Barriers to Implementation of Disclosure Pathways
Many organizations and researchers agree that disclosure and resolution pathways as just described are the most ethical and effective ways for all parties to resolve these painful situations. So why isn’t this approach universally practiced? In concluding this article, it may be helpful to point out some of the human dynamics that make resolution more difficult and how they might be addressed.
A key issue is the “urge to self-preservation.” Health care organizations have often been accused of disclosing only what they cannot hide. We have repeatedly observed how individuals and organizations are often initially motivated to do whatever is needed to protect themselves, even when those behaviors are frankly deceptive. This is almost to be expected. By age 4 children have learned to use deception as a defensive strategy when confronted with misbehavior. Research shows that children and adults continue the strategy to escape censure or punishment and simply get better at hiding their tracks.18 Because people want to preserve their image as ethical individuals, they have also learned to rationalize/justify this deception as necessary for self-preservation (“My dad would have killed me,” “I will lose my license,” “It is not fair that I take the blame when others have done the same thing and gotten away with it.”). Imagining the most extreme, and therefore “unfair” consequences, helps justify the individual’s use of dissembling and frank deception in order to avoid them. Clinicians and organizations may convince themselves that they are the victims entitled to protection rather than the injured patient. Patients and families often accept explanations that are less than candid, as doctors and nurses remain among the most trusted of professionals. Sufficiently understanding the complexities of the care is beyond the capability of most lay people. Successfully challenging the clinician’s or institution’s exculpatory explanation for an adverse outcome is very difficult, even though many clinicians believe that the tort system is stacked against them.
As a result, even the most sensible of best practices, toolkits, and trainings will not make full disclosure and fair resolution of adverse outcomes more likely without a counterweight of solid ethical commitment and a reliable structure for ensuring adherence. Sustainable progress has been demonstrated in those institutions8,10,17 where: (1) institutional values and ethics around disclosure were elevated above self-protection, (2) efficient processes for recognizing and objectively reviewing care involving an adverse outcome were developed and followed, (3) salaried and institutionally insured staff and providers were required to participate in and accept a fair path to resolution in the context of a just culture, and (4) the institution was able to deliver on any commitments (eg, financial, corrective actions) it has made. Conversely, disclosure and resolution programs have struggled in the following situations: where values and ethics are not clarified and made primary; where the processes for reviewing adverse outcomes are slow, inconsistent, and open to political interference; where independent providers have latitude to insist on self-protective behaviors; and where liability carriers who place highest priority on avoiding financial exposure are involved.
Conclusion
The challenge of effectively disclosing and resolving adverse medical outcomes will continue to be most formidable for health care systems with independent medical staffs with separate liability carriers. Can these systems get a firm consensus on the ethics that are paramount in disclosure situations? Can they create care review systems that are efficient and objective and reach conclusions that are binding on those involved? Are they willing to provide explanations to patients and families regardless of the consequences to themselves? Can they coordinate an efficient path to financial and other forms of restitution in those situations where problems in the care contributed to the patient being harmed? And can they enforce these practices despite the self-concerns of all the involved parties? The good news is we now know how to disclose and resolve adverse medical outcomes with patients and families in a way that is fair to providers, staff, and institutions and will not break the bank. For health care organizations, implementing effective disclosure and resolution practices starts with a commitment to both build consensus for this process and consistently enforce it.
Corresponding author: Daniel O’Connell, PhD, 2212 Queen Anne Ave. N. #810, Seattle, WA 98109; [email protected].
Financial disclosures: None.
1. Kohn L, Corrigan J, Donaldson M, eds. To Err Is Human: Building a Safer Health System. Washington, DC: Committee on Quality of Health Care in America, Institute of Medicine. National Academies Press; 1999.
2. Gibson R, Singh JP. Wall of Silence: The Untold Story of the Medical Mistakes That Kill and Injure Millions of Americans. Washington, DC: Lifeline Press; 2003.
3. Rathert C, Phillips W. Medical error disclosure training: evidence for values-based ethical environments. J Bus Ethics. 2010;97:491-503.
4. Wu AW, Cavanaugh TA, McPhee SJ, et al. To tell the truth: ethical and practical issues in disclosing medical mistakes to patients. J Gen Intern Med. 1997;12:770-775.
5. Gallagher TH, Waterman AD, Ebers AG, et al. Patients’ and doctors’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289:1001-1007.
6. The Patient Safety and Quality Improvement Act of 2005 (PSQIA); Public Law 109-41, 119 Stat. 424-434, which amended the Public Health Service Act.
7. Banja J. Moral courage in medicine—disclosing medical error. Bioethics Forum. 2001;17:7-115
8. Boothman R, Imhoff SJ, Campbell DA. Nurturing a culture of patient safety and achieving lower malpractice risk through disclosure: Lessons learned and future directions. Front Health Serv Manage. 2012;28:13-27.
9. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
10. Mello MM, Boothman RC, McDonald T, et al. Communication and resolution programs: the challenges and lessons learned from six early adopters. Health Affairs. 2014;33:20-29.
11. Marx D. Patient Safety and the Just Culture: A Primer for Health Care Executives. New York, NY: Trustees of Columbia University; 2001.
12. AHRQ Communication and Optimal Resolution (CANDOR) Toolkit. Rockville, MD: Agency for Healthcare Research and Quality; May 2016.
13. O’Connell D, White MK, Platt F. Disclosing unanticipated outcomes and medical errors. J Clin Outcomes Manag. 2003;10:25-29.
14. Berlinger N. After Harm: Medical Error and the Ethics of Forgiveness. Baltimore, MD: Johns Hopkins University Press; 2005.
15. O’Connell D, Reifsteck SW Disclosing unexpected outcomes and medical error. J Med Prac Manag. 2004;19:317-323.
16. Robson R, and Pelletier E. Giving back the pen: Disclosure, apology and early compensation discussions after harm in the healthcare setting. Healthc Q. 2008;11(3 Spec No.)85-90.
17. Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
18. Ding XP, Wellman HM, WangY, et al. Theory-of-mind training causes honest young children to lie. Psychol Sci. 2015;26:1812-1821.
From The Communication in Healthcare Group, Seattle, WA.
Abstract
- Objective: To review established approaches to disclosure and resolution following adverse medical outcomes and highlight barriers that may hinder universal implementation of effective disclosure/resolution practices.
- Methods: An overview of established approaches to disclosure and resolution of adverse medical outcomes is presented.
- Results: Clinicians must be equipped to manage situations where adverse medical outcomes occur even though the care provided was reasonable, within the standard, as well as in situations where preventable problems in the care provided were likely the cause of patient harm. Established approaches that have proven useful for investigating, disclosing, and resolving situations, captured in the acronyms AIDR, ALEE, and TEAM, can assist clinicians in the disclosure and ultimate resolution of these 2 types of situations.
- Conclusion: Health care organizations with a solid commitment and a reliable structure for ensuring adherence to full disclosure and fair resolution of adverse outcomes have demonstrated sustainable progress in ethically and effectively resolving situations where patients are harmed by medical care.
Keywords: safety; medical error; adverse outcomes; resolution; communication.
Much has been learned over the 20 years since the Institute of Medicine’s (IOM) report To Err Is Human1 was published. At the time it was published, the IOM report made it clear that only a minority of preventable patient harms were being acknowledged, investigated, and reported. In the face of adverse outcomes “dissemble, deny, and defend” was a common strategy of many clinicians, institutions, and liability carriers.2 The health care system appeared to place a priority on protecting itself from reputational and financial harm over the rights of injured patients to be given an accurate understanding of what had happened in their care and to pursue restitution, if appropriate.3-5
The emerging quality improvement movement was accompanied by calls for increased patient advocacy. This included the goal of greater transparency and more timely and equitable resolutions with patients who have been harmed by problems in care. Health care systems pressed for confidentiality protections in exchange for increased focus on quality improvement.6 Applying medical ethics of autonomy, no-maleficence, beneficence, and justice initially took a backseat, as risk management was given priority.7 Insurance carriers have no ethical obligation, and a clear disincentive, to assure that harmed patients are fully informed and offered restitution. Some self-insured health systems, however, began experimenting with more proactive and transparent approaches to disclosure and resolution. In contrast to the often-reported fear of a liability explosion, they reported reduced claims and suits, shorter time to resolution, and reduced overall financial cost,8-10 providing some evidence that perhaps greater openness could work after all.
But for providers and staff to allow transparency and candor to become the norm, institutions needed to create a more “just culture” for managing errors. Individual impairment or willful disregard of safe practice would need to be handled differently from the slips and lapses that more often contributed to preventable harm.11 For example, the nurse who was inadequately oriented to the equipment on an unfamiliar unit where she was asked to work a double shift due to a staffing shortage should not be held as accountable as an employee who knowingly violated agreed upon safe practices, even though patient harm resulted in each situation. It became clear that patient harm was usually the result of multiple factors involving individuals, communication, procedures, systems, and equipment. Blaming and disciplining individuals at the sharp end would not reliably reduce adverse outcomes.
Since the 1999 IOM report, we have developed general agreement on best practices for investigating, disclosing, and resolving situations where patients are harmed by medical care.12,13 This article reviews the perspectives and practices that appear necessary for effective disclosure and resolution after an adverse outcome and highlights barriers to reliably enacting them in practice.
Elements of Effective Disclosure
Effective disclosure to patients and families hinges on determining and providing an accurate understanding of what happened in the patient’s care. It should be the care providers’ and their institution’s responsibility to determine causation and disclose it. This should not require only the most upset patients and families initiating a legal process taking 3 years or more to complete. The most consequential question must be answered, “Was the care provided reasonable?” That is, was everything done within the standard, as would have been expected by similarly trained clinicians with the information and resources available at that time? It follows that if care was reasonable, then the adverse outcome could not normally have been prevented, no correction in care processes is called for, and no financial compensation is required. If the care review reveals deficiencies in care that were linked to patient harm, then achieving a satisfying resolution would be more complex and difficult.13 First, individuals would have to accept that they have contributed to patient harm, itself an often-contentious process and psychologically devastating realization. Then they must have this difficult conversation with patient and family, creating liability risk for themselves in the process. They must commit to correcting the problems that contributed to the harm. They must facilitate, rather than obstruct, a path to a restitution that addresses the medical, practical, and financial harms that have resulted. Given the challenges inherent to disclosure and resolution, it is no wonder that dissembling, denying, and defending was the common practice for the preceding decades.14
Disclosure and Resolution Pathways
I was the co-developer of an approach to disclosure and resolution which is now widely accepted and that has been taught across the United States and Canada to more than 50,000 health care providers and administrators over 18 years.15,16 We learned that resolving adverse medical outcomes is a 4-part process (anticipate, investigate, disclose, resolve [AIDR]). Most adverse or simply disappointing outcomes occur despite reasonable care (eg, due to biological variability, the imprecision of the science and limitations and risks of the procedures). The minority of harms are associated with deficiencies in the care (ie, unreasonable care). We need to equip ourselves to manage both situations effectively. The approach we developed can be captured in 3 acronyms: AIDR, ALEE, and TEAM,
AIDR
This acronym encapsulates the overview guidance for clinicians after an adverse event or outcome, regardless of the cause.
Anticipate the thoughts and feelings of the harmed/disappointed patient and family and reach out immediately with an expression of sympathy.
Investigate sufficiently to address questions about most likely causation and do not conjecture prior to investigation. Ask for patience—“You deserve more than a guess”—and keep in regular contact to reinforce the promise that there will be a full reporting when the review is complete.
Disclose (in a planned and coordinated manner) what has been learned in the investigation.
Resolve the situation with the patient and family consistent with our ethical principles.
If our failure caused the harm (care unreasonable/breached the standard), then working toward a fair restitution and taking corrective actions are appropriate. If the care was found to have been reasonable, then compensation would not be offered and corrective action is unwarranted. The organization would defend reasonable care if a claim was still pursued.
This process involves ethical clarity, emotional intelligence, and discipline. Clinicians must first acknowledge that a disappointing outcome or event has occurred. Clinicians involved in the care, usually led by the attending provider, then immediately reach out to the patient and family with sympathy, a plan of care to address the medical issues, and the promise to investigate and follow-up with the patient and family when the harm and its causes are more clearly determined. To disclose simply means to provide an accurate understanding (ie, the understanding determined by the investigation we conducted) of what happened, its causes, and consequences. Depending on the extent of the harm and the complexity and time needed for the investigation, a “coach” or “disclosure coordinator” who has advanced training in managing these situations is brought in to guide the process. The disclosure coach/coordinator provides a consistent and steady hand throughout the process of investigation, disclosure, and ultimately resolution with patient and family. Patients and families often move across settings during the time of the AIDR process, and it is easy to lose track of them unless someone is following the entire process until resolved.
ALEE
When the investigation of an adverse/disappointing outcome determines the care was reasonable and therefore the adverse outcome could not have been prevented, we use the ALEE pathway to guide the disclosure conversation (Step 3 in AIDR) with the patient and family:
Anticipate. What are the questions, thoughts, and feelings we would expect the patient and family will have? On this track, there is nothing to apologize for since the care was reasonable, yet expressing compassion and sympathy for the patient’s experience is essential. “I/we really sympathize with how differently this has turned out than we had hoped.”
Listen. Invite and listen for their questions and concerns, how they are seeing the situation, and where and what they are finding most upsetting and in need of explanation.
Empathize. There are 2 kinds of empathy required here. Cognitive empathy means showing that we understand their thinking from their perspective, separate from whether we fully agree. Emotional empathy involves demonstrating that their emotions are understandable given the situation, even if those emotions are painful for clinicians to experience. Listening in step 2 is how we learned their perspective and emotions. Now we can show accurate empathy: I/we can understand how upsetting it is to be facing another set of procedures to treat the unfortunate complications from your last surgery.
Explain. Even when care is reasonable, questions and perhaps suspicions are to be expected. Listening and empathizing sets us up to focus our explanations on the patient’s and family’s key questions with a level of thoughtfulness and transparency that conveys credibility. We should not assume, however, that they have accepted our explanation. Instead, solicit their reactions and unresolved questions as part of the disclosure discussion. It is normal for additional concerns to emerge in the days after the disclosure discussion, and we should be ready to address these concerns until resolved. In some instances, the patient and family will not be satisfied and it may be helpful to offer an independent review of the care. If the unresolved patient and family engages an attorney, that will be the first step taken anyway. Proactively offering an independent review signals confidence in your objectivity and sensitivity to the importance of fairness for the patient and family: Your questions and concerns are completely normal in light of the disappointing experience you have had. Let me see if I/we can address those now to your satisfaction.
TEAM
If the investigation determines that aspects of the care were unreasonable (breached the standard) and the adverse outcome/harm was related to the deficiencies in the care, then we use the TEAM pathway to disclose and resolve the situation with the patient and family
Truthful and Transparent and Teamwork. We should be offering our most accurate understanding of how the adverse outcome occurred, with sufficient depth and clarity that the patient and family can see how we reached that conclusion. In straightforward situations involving minor harm (eg, an allergic reaction to a medication that the clinician overlooked and that resulted in an urgent care center visit), a very limited investigation may clarify the situation sufficiently that the prescribing provider, accompanied by an office or staff nurse as support and witness, may be able to complete an effective disclosure in a single discussion, and simply writing off a bill or arranging to reimburse the urgent care center visit cost may satisfy the affected patient.
In more complex situations involving greater harm, a number of people must be involved to accomplish TEAM tasks: to offer an explanation, to answer questions, to make apologies, to explain changes intended to reduce the chance of harm to others in the future, and to work through any restitution that may be appropriate. Appointing a disclosure coach/coordinator/facilitator who has had extended training in the disclosure process can help guide these more complex situations. Risk management, insurance carriers, and legal counsel should be aware and advising throughout the process and participating directly in meetings with the patient and family, as appropriate. Since on the TEAM track we are admitting liability, offering a path to financial restitution may be warranted and the disclosure process may trigger reporting requirements with regulatory as well as human resource implications.
The patient and family may want to include other people on their “team” as well. Since complex disclosure meetings need to be carefully planned in advance, we should clarify who will be attending from the health care side and who the family intends to involve. We should anticipate potential requests and questions such as: Would it be OK to record this meeting? Can we ask our attorney to attend? Who are all these people and why are they in this meeting? (We should introduce all team members and clarify how their involvement is necessary to help reach the most satisfying resolution for all involved.)
Empathize. Admitting that deficient aspects in the care contributed to the harm will trigger thoughts, emotions, and expectations for the patient and family. Empathizing involves seeing the whole situation from their perspective and acknowledging their emotions as understandable. Empathizing is not the same as fully agreeing with the patient’s and family’s perspective, but we will not be able to effectively address concerns and expectations that we have not understood. Organizations should have supports in place for staff who are involved in these difficult situations. Nonetheless, we must prioritize the patient’s and family‘s feelings in a disclosure meeting.
Apologize and be Accountable. This calls for both expressions of sympathy as well as a genuine apology for having caused harm by failure in some aspect of care: We are very sorry you are going through this difficult situation. We are especially sorry to tell you that we now recognize that problems in the care we provided are the most likely cause of this harm. Would this be a good time to explain what we learned?
Having the responsible clinicians present increases the chances of achieving the most complete resolution in a single planned and well facilitated meeting. The tasks for that meeting include: offering an explanation that reveals the problems in care that contributed to the adverse outcome, making sincere apologies, and explaining changes to reduce chance of harm to others. The disclosure coach can work with individuals to help them understand how and why their involvement can be important and to help staff members become ready to participate constructively in the disclosure meeting. When individuals appear unable or unwilling to contribute constructively, a plan is needed for how their part can be replaced (eg, a charge nurse or department chair might need to step in to explain and apologize for the care of a subordinate). Managers/administrators can explain contributory factors for what may at first appear to have been simply individual negligence. Administrators can describe the actions that the organization is taking to correct problems that contributed to the patient harm: As nursing executive, it is my responsibility to see that all our staff have been adequately trained on the equipment we are asking them to use. We now recognize that the nurse’s lack of familiarity with that equipment contributed to the harm you experienced and I am very sorry for that. It is my responsibility to get that problem corrected, and we are already taking steps to assure that. Patients and families often have ideas for improving care processes and appreciate being invited to share these ideas as a service to future patients.
Manage until resolved. On the “care unreasonable” track, we must signal openness to helping with the patient’s and family’s immediate and longer-term needs, as well as their expectations about financial and other forms of restitution. Someone should be in the meeting who can describe the next steps in working towards a fair restitution and how that process will take place following the conclusion of the disclosure meeting. The close involvement of risk and claims professionals throughout the process of investigation through to the disclosure discussion itself will assure a more satisfactory handoff to questions about around financial compensation
Psychological Barriers to Implementation of Disclosure Pathways
Many organizations and researchers agree that disclosure and resolution pathways as just described are the most ethical and effective ways for all parties to resolve these painful situations. So why isn’t this approach universally practiced? In concluding this article, it may be helpful to point out some of the human dynamics that make resolution more difficult and how they might be addressed.
A key issue is the “urge to self-preservation.” Health care organizations have often been accused of disclosing only what they cannot hide. We have repeatedly observed how individuals and organizations are often initially motivated to do whatever is needed to protect themselves, even when those behaviors are frankly deceptive. This is almost to be expected. By age 4 children have learned to use deception as a defensive strategy when confronted with misbehavior. Research shows that children and adults continue the strategy to escape censure or punishment and simply get better at hiding their tracks.18 Because people want to preserve their image as ethical individuals, they have also learned to rationalize/justify this deception as necessary for self-preservation (“My dad would have killed me,” “I will lose my license,” “It is not fair that I take the blame when others have done the same thing and gotten away with it.”). Imagining the most extreme, and therefore “unfair” consequences, helps justify the individual’s use of dissembling and frank deception in order to avoid them. Clinicians and organizations may convince themselves that they are the victims entitled to protection rather than the injured patient. Patients and families often accept explanations that are less than candid, as doctors and nurses remain among the most trusted of professionals. Sufficiently understanding the complexities of the care is beyond the capability of most lay people. Successfully challenging the clinician’s or institution’s exculpatory explanation for an adverse outcome is very difficult, even though many clinicians believe that the tort system is stacked against them.
As a result, even the most sensible of best practices, toolkits, and trainings will not make full disclosure and fair resolution of adverse outcomes more likely without a counterweight of solid ethical commitment and a reliable structure for ensuring adherence. Sustainable progress has been demonstrated in those institutions8,10,17 where: (1) institutional values and ethics around disclosure were elevated above self-protection, (2) efficient processes for recognizing and objectively reviewing care involving an adverse outcome were developed and followed, (3) salaried and institutionally insured staff and providers were required to participate in and accept a fair path to resolution in the context of a just culture, and (4) the institution was able to deliver on any commitments (eg, financial, corrective actions) it has made. Conversely, disclosure and resolution programs have struggled in the following situations: where values and ethics are not clarified and made primary; where the processes for reviewing adverse outcomes are slow, inconsistent, and open to political interference; where independent providers have latitude to insist on self-protective behaviors; and where liability carriers who place highest priority on avoiding financial exposure are involved.
Conclusion
The challenge of effectively disclosing and resolving adverse medical outcomes will continue to be most formidable for health care systems with independent medical staffs with separate liability carriers. Can these systems get a firm consensus on the ethics that are paramount in disclosure situations? Can they create care review systems that are efficient and objective and reach conclusions that are binding on those involved? Are they willing to provide explanations to patients and families regardless of the consequences to themselves? Can they coordinate an efficient path to financial and other forms of restitution in those situations where problems in the care contributed to the patient being harmed? And can they enforce these practices despite the self-concerns of all the involved parties? The good news is we now know how to disclose and resolve adverse medical outcomes with patients and families in a way that is fair to providers, staff, and institutions and will not break the bank. For health care organizations, implementing effective disclosure and resolution practices starts with a commitment to both build consensus for this process and consistently enforce it.
Corresponding author: Daniel O’Connell, PhD, 2212 Queen Anne Ave. N. #810, Seattle, WA 98109; [email protected].
Financial disclosures: None.
From The Communication in Healthcare Group, Seattle, WA.
Abstract
- Objective: To review established approaches to disclosure and resolution following adverse medical outcomes and highlight barriers that may hinder universal implementation of effective disclosure/resolution practices.
- Methods: An overview of established approaches to disclosure and resolution of adverse medical outcomes is presented.
- Results: Clinicians must be equipped to manage situations where adverse medical outcomes occur even though the care provided was reasonable, within the standard, as well as in situations where preventable problems in the care provided were likely the cause of patient harm. Established approaches that have proven useful for investigating, disclosing, and resolving situations, captured in the acronyms AIDR, ALEE, and TEAM, can assist clinicians in the disclosure and ultimate resolution of these 2 types of situations.
- Conclusion: Health care organizations with a solid commitment and a reliable structure for ensuring adherence to full disclosure and fair resolution of adverse outcomes have demonstrated sustainable progress in ethically and effectively resolving situations where patients are harmed by medical care.
Keywords: safety; medical error; adverse outcomes; resolution; communication.
Much has been learned over the 20 years since the Institute of Medicine’s (IOM) report To Err Is Human1 was published. At the time it was published, the IOM report made it clear that only a minority of preventable patient harms were being acknowledged, investigated, and reported. In the face of adverse outcomes “dissemble, deny, and defend” was a common strategy of many clinicians, institutions, and liability carriers.2 The health care system appeared to place a priority on protecting itself from reputational and financial harm over the rights of injured patients to be given an accurate understanding of what had happened in their care and to pursue restitution, if appropriate.3-5
The emerging quality improvement movement was accompanied by calls for increased patient advocacy. This included the goal of greater transparency and more timely and equitable resolutions with patients who have been harmed by problems in care. Health care systems pressed for confidentiality protections in exchange for increased focus on quality improvement.6 Applying medical ethics of autonomy, no-maleficence, beneficence, and justice initially took a backseat, as risk management was given priority.7 Insurance carriers have no ethical obligation, and a clear disincentive, to assure that harmed patients are fully informed and offered restitution. Some self-insured health systems, however, began experimenting with more proactive and transparent approaches to disclosure and resolution. In contrast to the often-reported fear of a liability explosion, they reported reduced claims and suits, shorter time to resolution, and reduced overall financial cost,8-10 providing some evidence that perhaps greater openness could work after all.
But for providers and staff to allow transparency and candor to become the norm, institutions needed to create a more “just culture” for managing errors. Individual impairment or willful disregard of safe practice would need to be handled differently from the slips and lapses that more often contributed to preventable harm.11 For example, the nurse who was inadequately oriented to the equipment on an unfamiliar unit where she was asked to work a double shift due to a staffing shortage should not be held as accountable as an employee who knowingly violated agreed upon safe practices, even though patient harm resulted in each situation. It became clear that patient harm was usually the result of multiple factors involving individuals, communication, procedures, systems, and equipment. Blaming and disciplining individuals at the sharp end would not reliably reduce adverse outcomes.
Since the 1999 IOM report, we have developed general agreement on best practices for investigating, disclosing, and resolving situations where patients are harmed by medical care.12,13 This article reviews the perspectives and practices that appear necessary for effective disclosure and resolution after an adverse outcome and highlights barriers to reliably enacting them in practice.
Elements of Effective Disclosure
Effective disclosure to patients and families hinges on determining and providing an accurate understanding of what happened in the patient’s care. It should be the care providers’ and their institution’s responsibility to determine causation and disclose it. This should not require only the most upset patients and families initiating a legal process taking 3 years or more to complete. The most consequential question must be answered, “Was the care provided reasonable?” That is, was everything done within the standard, as would have been expected by similarly trained clinicians with the information and resources available at that time? It follows that if care was reasonable, then the adverse outcome could not normally have been prevented, no correction in care processes is called for, and no financial compensation is required. If the care review reveals deficiencies in care that were linked to patient harm, then achieving a satisfying resolution would be more complex and difficult.13 First, individuals would have to accept that they have contributed to patient harm, itself an often-contentious process and psychologically devastating realization. Then they must have this difficult conversation with patient and family, creating liability risk for themselves in the process. They must commit to correcting the problems that contributed to the harm. They must facilitate, rather than obstruct, a path to a restitution that addresses the medical, practical, and financial harms that have resulted. Given the challenges inherent to disclosure and resolution, it is no wonder that dissembling, denying, and defending was the common practice for the preceding decades.14
Disclosure and Resolution Pathways
I was the co-developer of an approach to disclosure and resolution which is now widely accepted and that has been taught across the United States and Canada to more than 50,000 health care providers and administrators over 18 years.15,16 We learned that resolving adverse medical outcomes is a 4-part process (anticipate, investigate, disclose, resolve [AIDR]). Most adverse or simply disappointing outcomes occur despite reasonable care (eg, due to biological variability, the imprecision of the science and limitations and risks of the procedures). The minority of harms are associated with deficiencies in the care (ie, unreasonable care). We need to equip ourselves to manage both situations effectively. The approach we developed can be captured in 3 acronyms: AIDR, ALEE, and TEAM,
AIDR
This acronym encapsulates the overview guidance for clinicians after an adverse event or outcome, regardless of the cause.
Anticipate the thoughts and feelings of the harmed/disappointed patient and family and reach out immediately with an expression of sympathy.
Investigate sufficiently to address questions about most likely causation and do not conjecture prior to investigation. Ask for patience—“You deserve more than a guess”—and keep in regular contact to reinforce the promise that there will be a full reporting when the review is complete.
Disclose (in a planned and coordinated manner) what has been learned in the investigation.
Resolve the situation with the patient and family consistent with our ethical principles.
If our failure caused the harm (care unreasonable/breached the standard), then working toward a fair restitution and taking corrective actions are appropriate. If the care was found to have been reasonable, then compensation would not be offered and corrective action is unwarranted. The organization would defend reasonable care if a claim was still pursued.
This process involves ethical clarity, emotional intelligence, and discipline. Clinicians must first acknowledge that a disappointing outcome or event has occurred. Clinicians involved in the care, usually led by the attending provider, then immediately reach out to the patient and family with sympathy, a plan of care to address the medical issues, and the promise to investigate and follow-up with the patient and family when the harm and its causes are more clearly determined. To disclose simply means to provide an accurate understanding (ie, the understanding determined by the investigation we conducted) of what happened, its causes, and consequences. Depending on the extent of the harm and the complexity and time needed for the investigation, a “coach” or “disclosure coordinator” who has advanced training in managing these situations is brought in to guide the process. The disclosure coach/coordinator provides a consistent and steady hand throughout the process of investigation, disclosure, and ultimately resolution with patient and family. Patients and families often move across settings during the time of the AIDR process, and it is easy to lose track of them unless someone is following the entire process until resolved.
ALEE
When the investigation of an adverse/disappointing outcome determines the care was reasonable and therefore the adverse outcome could not have been prevented, we use the ALEE pathway to guide the disclosure conversation (Step 3 in AIDR) with the patient and family:
Anticipate. What are the questions, thoughts, and feelings we would expect the patient and family will have? On this track, there is nothing to apologize for since the care was reasonable, yet expressing compassion and sympathy for the patient’s experience is essential. “I/we really sympathize with how differently this has turned out than we had hoped.”
Listen. Invite and listen for their questions and concerns, how they are seeing the situation, and where and what they are finding most upsetting and in need of explanation.
Empathize. There are 2 kinds of empathy required here. Cognitive empathy means showing that we understand their thinking from their perspective, separate from whether we fully agree. Emotional empathy involves demonstrating that their emotions are understandable given the situation, even if those emotions are painful for clinicians to experience. Listening in step 2 is how we learned their perspective and emotions. Now we can show accurate empathy: I/we can understand how upsetting it is to be facing another set of procedures to treat the unfortunate complications from your last surgery.
Explain. Even when care is reasonable, questions and perhaps suspicions are to be expected. Listening and empathizing sets us up to focus our explanations on the patient’s and family’s key questions with a level of thoughtfulness and transparency that conveys credibility. We should not assume, however, that they have accepted our explanation. Instead, solicit their reactions and unresolved questions as part of the disclosure discussion. It is normal for additional concerns to emerge in the days after the disclosure discussion, and we should be ready to address these concerns until resolved. In some instances, the patient and family will not be satisfied and it may be helpful to offer an independent review of the care. If the unresolved patient and family engages an attorney, that will be the first step taken anyway. Proactively offering an independent review signals confidence in your objectivity and sensitivity to the importance of fairness for the patient and family: Your questions and concerns are completely normal in light of the disappointing experience you have had. Let me see if I/we can address those now to your satisfaction.
TEAM
If the investigation determines that aspects of the care were unreasonable (breached the standard) and the adverse outcome/harm was related to the deficiencies in the care, then we use the TEAM pathway to disclose and resolve the situation with the patient and family
Truthful and Transparent and Teamwork. We should be offering our most accurate understanding of how the adverse outcome occurred, with sufficient depth and clarity that the patient and family can see how we reached that conclusion. In straightforward situations involving minor harm (eg, an allergic reaction to a medication that the clinician overlooked and that resulted in an urgent care center visit), a very limited investigation may clarify the situation sufficiently that the prescribing provider, accompanied by an office or staff nurse as support and witness, may be able to complete an effective disclosure in a single discussion, and simply writing off a bill or arranging to reimburse the urgent care center visit cost may satisfy the affected patient.
In more complex situations involving greater harm, a number of people must be involved to accomplish TEAM tasks: to offer an explanation, to answer questions, to make apologies, to explain changes intended to reduce the chance of harm to others in the future, and to work through any restitution that may be appropriate. Appointing a disclosure coach/coordinator/facilitator who has had extended training in the disclosure process can help guide these more complex situations. Risk management, insurance carriers, and legal counsel should be aware and advising throughout the process and participating directly in meetings with the patient and family, as appropriate. Since on the TEAM track we are admitting liability, offering a path to financial restitution may be warranted and the disclosure process may trigger reporting requirements with regulatory as well as human resource implications.
The patient and family may want to include other people on their “team” as well. Since complex disclosure meetings need to be carefully planned in advance, we should clarify who will be attending from the health care side and who the family intends to involve. We should anticipate potential requests and questions such as: Would it be OK to record this meeting? Can we ask our attorney to attend? Who are all these people and why are they in this meeting? (We should introduce all team members and clarify how their involvement is necessary to help reach the most satisfying resolution for all involved.)
Empathize. Admitting that deficient aspects in the care contributed to the harm will trigger thoughts, emotions, and expectations for the patient and family. Empathizing involves seeing the whole situation from their perspective and acknowledging their emotions as understandable. Empathizing is not the same as fully agreeing with the patient’s and family’s perspective, but we will not be able to effectively address concerns and expectations that we have not understood. Organizations should have supports in place for staff who are involved in these difficult situations. Nonetheless, we must prioritize the patient’s and family‘s feelings in a disclosure meeting.
Apologize and be Accountable. This calls for both expressions of sympathy as well as a genuine apology for having caused harm by failure in some aspect of care: We are very sorry you are going through this difficult situation. We are especially sorry to tell you that we now recognize that problems in the care we provided are the most likely cause of this harm. Would this be a good time to explain what we learned?
Having the responsible clinicians present increases the chances of achieving the most complete resolution in a single planned and well facilitated meeting. The tasks for that meeting include: offering an explanation that reveals the problems in care that contributed to the adverse outcome, making sincere apologies, and explaining changes to reduce chance of harm to others. The disclosure coach can work with individuals to help them understand how and why their involvement can be important and to help staff members become ready to participate constructively in the disclosure meeting. When individuals appear unable or unwilling to contribute constructively, a plan is needed for how their part can be replaced (eg, a charge nurse or department chair might need to step in to explain and apologize for the care of a subordinate). Managers/administrators can explain contributory factors for what may at first appear to have been simply individual negligence. Administrators can describe the actions that the organization is taking to correct problems that contributed to the patient harm: As nursing executive, it is my responsibility to see that all our staff have been adequately trained on the equipment we are asking them to use. We now recognize that the nurse’s lack of familiarity with that equipment contributed to the harm you experienced and I am very sorry for that. It is my responsibility to get that problem corrected, and we are already taking steps to assure that. Patients and families often have ideas for improving care processes and appreciate being invited to share these ideas as a service to future patients.
Manage until resolved. On the “care unreasonable” track, we must signal openness to helping with the patient’s and family’s immediate and longer-term needs, as well as their expectations about financial and other forms of restitution. Someone should be in the meeting who can describe the next steps in working towards a fair restitution and how that process will take place following the conclusion of the disclosure meeting. The close involvement of risk and claims professionals throughout the process of investigation through to the disclosure discussion itself will assure a more satisfactory handoff to questions about around financial compensation
Psychological Barriers to Implementation of Disclosure Pathways
Many organizations and researchers agree that disclosure and resolution pathways as just described are the most ethical and effective ways for all parties to resolve these painful situations. So why isn’t this approach universally practiced? In concluding this article, it may be helpful to point out some of the human dynamics that make resolution more difficult and how they might be addressed.
A key issue is the “urge to self-preservation.” Health care organizations have often been accused of disclosing only what they cannot hide. We have repeatedly observed how individuals and organizations are often initially motivated to do whatever is needed to protect themselves, even when those behaviors are frankly deceptive. This is almost to be expected. By age 4 children have learned to use deception as a defensive strategy when confronted with misbehavior. Research shows that children and adults continue the strategy to escape censure or punishment and simply get better at hiding their tracks.18 Because people want to preserve their image as ethical individuals, they have also learned to rationalize/justify this deception as necessary for self-preservation (“My dad would have killed me,” “I will lose my license,” “It is not fair that I take the blame when others have done the same thing and gotten away with it.”). Imagining the most extreme, and therefore “unfair” consequences, helps justify the individual’s use of dissembling and frank deception in order to avoid them. Clinicians and organizations may convince themselves that they are the victims entitled to protection rather than the injured patient. Patients and families often accept explanations that are less than candid, as doctors and nurses remain among the most trusted of professionals. Sufficiently understanding the complexities of the care is beyond the capability of most lay people. Successfully challenging the clinician’s or institution’s exculpatory explanation for an adverse outcome is very difficult, even though many clinicians believe that the tort system is stacked against them.
As a result, even the most sensible of best practices, toolkits, and trainings will not make full disclosure and fair resolution of adverse outcomes more likely without a counterweight of solid ethical commitment and a reliable structure for ensuring adherence. Sustainable progress has been demonstrated in those institutions8,10,17 where: (1) institutional values and ethics around disclosure were elevated above self-protection, (2) efficient processes for recognizing and objectively reviewing care involving an adverse outcome were developed and followed, (3) salaried and institutionally insured staff and providers were required to participate in and accept a fair path to resolution in the context of a just culture, and (4) the institution was able to deliver on any commitments (eg, financial, corrective actions) it has made. Conversely, disclosure and resolution programs have struggled in the following situations: where values and ethics are not clarified and made primary; where the processes for reviewing adverse outcomes are slow, inconsistent, and open to political interference; where independent providers have latitude to insist on self-protective behaviors; and where liability carriers who place highest priority on avoiding financial exposure are involved.
Conclusion
The challenge of effectively disclosing and resolving adverse medical outcomes will continue to be most formidable for health care systems with independent medical staffs with separate liability carriers. Can these systems get a firm consensus on the ethics that are paramount in disclosure situations? Can they create care review systems that are efficient and objective and reach conclusions that are binding on those involved? Are they willing to provide explanations to patients and families regardless of the consequences to themselves? Can they coordinate an efficient path to financial and other forms of restitution in those situations where problems in the care contributed to the patient being harmed? And can they enforce these practices despite the self-concerns of all the involved parties? The good news is we now know how to disclose and resolve adverse medical outcomes with patients and families in a way that is fair to providers, staff, and institutions and will not break the bank. For health care organizations, implementing effective disclosure and resolution practices starts with a commitment to both build consensus for this process and consistently enforce it.
Corresponding author: Daniel O’Connell, PhD, 2212 Queen Anne Ave. N. #810, Seattle, WA 98109; [email protected].
Financial disclosures: None.
1. Kohn L, Corrigan J, Donaldson M, eds. To Err Is Human: Building a Safer Health System. Washington, DC: Committee on Quality of Health Care in America, Institute of Medicine. National Academies Press; 1999.
2. Gibson R, Singh JP. Wall of Silence: The Untold Story of the Medical Mistakes That Kill and Injure Millions of Americans. Washington, DC: Lifeline Press; 2003.
3. Rathert C, Phillips W. Medical error disclosure training: evidence for values-based ethical environments. J Bus Ethics. 2010;97:491-503.
4. Wu AW, Cavanaugh TA, McPhee SJ, et al. To tell the truth: ethical and practical issues in disclosing medical mistakes to patients. J Gen Intern Med. 1997;12:770-775.
5. Gallagher TH, Waterman AD, Ebers AG, et al. Patients’ and doctors’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289:1001-1007.
6. The Patient Safety and Quality Improvement Act of 2005 (PSQIA); Public Law 109-41, 119 Stat. 424-434, which amended the Public Health Service Act.
7. Banja J. Moral courage in medicine—disclosing medical error. Bioethics Forum. 2001;17:7-115
8. Boothman R, Imhoff SJ, Campbell DA. Nurturing a culture of patient safety and achieving lower malpractice risk through disclosure: Lessons learned and future directions. Front Health Serv Manage. 2012;28:13-27.
9. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
10. Mello MM, Boothman RC, McDonald T, et al. Communication and resolution programs: the challenges and lessons learned from six early adopters. Health Affairs. 2014;33:20-29.
11. Marx D. Patient Safety and the Just Culture: A Primer for Health Care Executives. New York, NY: Trustees of Columbia University; 2001.
12. AHRQ Communication and Optimal Resolution (CANDOR) Toolkit. Rockville, MD: Agency for Healthcare Research and Quality; May 2016.
13. O’Connell D, White MK, Platt F. Disclosing unanticipated outcomes and medical errors. J Clin Outcomes Manag. 2003;10:25-29.
14. Berlinger N. After Harm: Medical Error and the Ethics of Forgiveness. Baltimore, MD: Johns Hopkins University Press; 2005.
15. O’Connell D, Reifsteck SW Disclosing unexpected outcomes and medical error. J Med Prac Manag. 2004;19:317-323.
16. Robson R, and Pelletier E. Giving back the pen: Disclosure, apology and early compensation discussions after harm in the healthcare setting. Healthc Q. 2008;11(3 Spec No.)85-90.
17. Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
18. Ding XP, Wellman HM, WangY, et al. Theory-of-mind training causes honest young children to lie. Psychol Sci. 2015;26:1812-1821.
1. Kohn L, Corrigan J, Donaldson M, eds. To Err Is Human: Building a Safer Health System. Washington, DC: Committee on Quality of Health Care in America, Institute of Medicine. National Academies Press; 1999.
2. Gibson R, Singh JP. Wall of Silence: The Untold Story of the Medical Mistakes That Kill and Injure Millions of Americans. Washington, DC: Lifeline Press; 2003.
3. Rathert C, Phillips W. Medical error disclosure training: evidence for values-based ethical environments. J Bus Ethics. 2010;97:491-503.
4. Wu AW, Cavanaugh TA, McPhee SJ, et al. To tell the truth: ethical and practical issues in disclosing medical mistakes to patients. J Gen Intern Med. 1997;12:770-775.
5. Gallagher TH, Waterman AD, Ebers AG, et al. Patients’ and doctors’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289:1001-1007.
6. The Patient Safety and Quality Improvement Act of 2005 (PSQIA); Public Law 109-41, 119 Stat. 424-434, which amended the Public Health Service Act.
7. Banja J. Moral courage in medicine—disclosing medical error. Bioethics Forum. 2001;17:7-115
8. Boothman R, Imhoff SJ, Campbell DA. Nurturing a culture of patient safety and achieving lower malpractice risk through disclosure: Lessons learned and future directions. Front Health Serv Manage. 2012;28:13-27.
9. Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221.
10. Mello MM, Boothman RC, McDonald T, et al. Communication and resolution programs: the challenges and lessons learned from six early adopters. Health Affairs. 2014;33:20-29.
11. Marx D. Patient Safety and the Just Culture: A Primer for Health Care Executives. New York, NY: Trustees of Columbia University; 2001.
12. AHRQ Communication and Optimal Resolution (CANDOR) Toolkit. Rockville, MD: Agency for Healthcare Research and Quality; May 2016.
13. O’Connell D, White MK, Platt F. Disclosing unanticipated outcomes and medical errors. J Clin Outcomes Manag. 2003;10:25-29.
14. Berlinger N. After Harm: Medical Error and the Ethics of Forgiveness. Baltimore, MD: Johns Hopkins University Press; 2005.
15. O’Connell D, Reifsteck SW Disclosing unexpected outcomes and medical error. J Med Prac Manag. 2004;19:317-323.
16. Robson R, and Pelletier E. Giving back the pen: Disclosure, apology and early compensation discussions after harm in the healthcare setting. Healthc Q. 2008;11(3 Spec No.)85-90.
17. Kraman SS, Hamm G. Risk management: extreme honesty may be the best policy. Ann Intern Med. 1999;131:963-967.
18. Ding XP, Wellman HM, WangY, et al. Theory-of-mind training causes honest young children to lie. Psychol Sci. 2015;26:1812-1821.
Optimizing a Total Joint Replacement Care Pathway to Reduce Skilled Nursing Facility Utilization
From Grant Medical Center (Dr. Wasielewski, Dr. Polonia, Ms. Barca, Ms. Cebriak, Ms. Lucki), and OhioHealth Group (Mr. Rogers, Ms. St. John, Dr. Gascon), Columbus, OH.
Abstract
- Background: Organizations participating in the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative enter into payment arrangements that include financial and performance accountability for episodes of care. There is growing evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care (eg, skilled nursing facilities [SNF], long-term care facilities, and inpatient rehabilitation facilities).
- Objective: To describe a pilot program designed to reduce the utilization of institutional post-acute care for total joint replacement surgical patients.
- Methods: A multidisciplinary intervention team optimized scheduling, preadmission testing, patient communication, and patient education along a total joint replacement care pathway.
- Results: Among Medicare patients, total discharges to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period. The risk of SNF utilization among patients in the intervention period was nearly half (0.45; 95% confidence interval, 0.314-0.639) that of patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001). The readmission rate was substantially lower than national norms.
- Conclusion: Optimizing patient care throughout the care pathway using the concerted efforts of a multidisciplinary team is possible given a common vision, shared goals, and clearly communicated expectations.
Keywords: arthroplasty; readmissions; Medicare; post-acute care; Bundled Payment for Care Improvement.
Quality improvement in health care is partially dependent upon processes that standardize episodes of care. This is especially true in the post–acute care setting, where efforts to increase patient engagement and care coordination can improve a patient’s recovery process. One framework for optimizing patient care across an episode of care is the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative, which links payments for the multiple services patients receive during an episode of care. Organizations participating in the BPCI initiative enter into payment arrangements that include financial and performance accountability for episodes of care. The BPCI initiative provides a framework for episodes of care across multiple types of facilities and clinicians over periods of time (30-day, 60-day, and 90-day episodes).1-5 Evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality is accumulating. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care, such as skilled nursing facilities (SNF), long-term care facilities, and inpatient rehabilitation facilities.1
Our hospital, the Grant Medical Center, which is part of the OhioHealth system, had agreed to participate in the CMS BPCI–Advanced Model for major joint replacement of the lower extremity, with a start date of October 1, 2018. Prior to adopting this bundled payment and service delivery model for major joint replacement through the BPCI program, we implemented strategic interventions to improve the efficiency of care delivery and reduce post-acute utilization. Although the BPCI program applied only to Medicare patients, the interventions that were developed, implemented, and evaluated in this quality improvement project, which we describe in this article, were provided to all patients who underwent lower-extremity joint replacement, regardless of payer.
Gap Analysis
Prior to developing and implementing the intervention, a gap analysis was performed to determine differences between Grant Medical Center’s care pathway/processes and evidence-based best practice. A steering committee comprised of physician champions, rehabilitation services, senior leadership from the Bone and Joint Center, and care management was gathered. The gap analysis examined the care paths in the preoperative phase, index admission phase, and the post-acute phase of care. Findings of the gap analysis included an underutilized joint education class, overutilization of SNF placement, and lack of key resources to assess the needs of patients prior to surgery.
The Grant Bone and Joint Center offered a comprehensive joint education class in person, but also gave patients the option of utilizing an online learning source. While both were successful, staff believed the in-person class had a greater impact than the online version. Patients who attended the class completed a preoperative assessment by hand, which included a social assessment for identifying potential challenges prior to admission. However, because class attendance was low (< 10%), this assessment tool was not utilized until the patient’s admission in most cases. The gap analysis also identified that the educational content of the class lacked key points to encourage a home-going plan.
The baseline SNF utilization rate (using data from July 1, 2015–June 30, 2016) within the Medicare population was 39.5% (70/177), with an overall (all payers) rate of 22.9% (192/838). This SNF utilization rate was higher than the national benchmark, underscoring the need to focus on a preoperative “plan for home” message. In addition, review of Medicare fee-for-service data from 2014 to 2016 revealed that Grant Medical Center utilized 83 different SNFs over the course of approximately 2 years. In this context, staff saw an opportunity to develop stronger relationships with fewer SNF partners, as well as OhioHealth Home Care, in order to improve care by standardizing functional recovery in post-acute care management.
In sum, the gap analysis found that a lack of standardization, follow through, and engagement in daily multidisciplinary rounds often led to a plan for SNF discharge instead of home. As such, it was determined that the pilot program would target the preoperative and post-acute phases of care and that its primary endpoint would be the reduction of the SNF discharge rate among Medicare fee-for-service patients.
Literature Review
Targeting the SNF discharge rate as the endpoint was also supported by a recent study that showed that most of the reduction in spending for total joint replacement within BPCI hospitals was linked to reduced use of institutional post-acute care.1 Other studies in the joint replacement literature have delineated the specific aspects of care redesign that allow hospitals to provide more efficient and effective care delivery during an episode of care.6-8 A review of these and similar studies of joint replacement quality improvement interventions yielded a number of actionable findings:
- Engaging and educating patients and families is critical. Families and other caregivers must be identified preoperatively, actively engaged, and committed to helping the patient recover.
- Providers must build the expectation in patients that they will return home as soon as it is safe to do so. This includes working to restore physiologic function, managing pain with oral medication, and restoring the physical capability of adapting to a home environment.
- Relationships must be established with post-acute care providers who are able to demonstrate best practices and be willing partners on performance outcomes.
- Providers need to invest in personnel to coordinate care transitions initiated preoperatively that continue through the post-acute phase of care.
- Providers must promote processes that allow patients to more fully own their recovery through coaching and improved communication.
A total joint replacement initiative undertaken at another hospital within the OhioHealth system, Riverside Methodist Hospital, that incorporated these aspects of care redesign demonstrated a significant reduction in SNF utilization. That success informed the development of the initiative at Grant Medical Center.
Methods
Setting
This pilot project was conducted from July 2017 through July 2018 at the Grant Bone and Joint Center in the Grant Medical Center, an urban hospital in Columbus, Ohio. The Bone and Joint Center performs more than 7400 surgeries each year, of which 12% are total joint replacements. Grant Medical Center is a Level I Trauma Center that has received a fourth designation in Magnet Nursing status and has attained The Joint Commission Disease-Specific Care Certification for its total knee and hip arthroplasty, total shoulder, and hip fracture programs.
Intervention
The findings from the gap analysis were incorporated into a standardized preoperative care pathway at the Center. Standardization of the care pathway required developing relationships between office staff, schedulers, inpatient work teams, and preadmission testing, as well as physician group realignment with the larger organization.
During this time, the steering committee identified the need for a designated full-time pre-habilitation case manager to support patients undergoing hip and knee replacements at the Center. With the addition of a new role, prehab case manager, attention was directed to patient social assessment and communication. After the surgery was scheduled but prior to the preoperative education class, each patient received a screening phone call from the prehab case manager that included an initial social assessment. The electronic health record (EHR) was utilized to communicate the results of the assessment, including barriers to a home-going plan. The phone call allowed the case manager to reinforce the importance of class attendance for the patient and primary caregiver, and also allowed any specific concerns identified to be addressed and resolved prior to surgery.
A work group consisting of the prehab case manager, office staff, and the bone and joint leadership team focused on improving the core preoperative education content, same-day preadmission clearance, and class attendance. To support these efforts, the work group (1) created a scheduling document to align preadmission testing and the preoperative education class so they occurred on the same day, (2) improved prior authorization of the surgery to support the post-surgical care team, (3) clarified expectations and roles among office staff and care management staff to optimize the discharge process, and (4) engaged the physician team to encourage a culture change towards setting patient expectations to be discharged to home or a preferred SNF location. Regarding prior authorization communications, prior to the intervention, communication was insufficient, paper driven, and lacking in continuity. Utilizing the EHR, an EPIC platform, a designated documentation location was created, which increased communication among the office schedulers, preadmission testing, and surgery schedulers. The consistent location for recording prior authorization avoided canceled surgeries and claim denials due to missing pieces of information. In addition to these process changes, the surgical offices were geographically realigned to a new single location, a change that brought the staff together and in turn allowed for role clarification and team building and also allowed the staff to identify opportunities for improvement.
The core content of the education class was redesigned to support patients’ understanding of the procedure and what to expect during the hospital stay and discharge for home. Staff worked first to align all preadmission patient requirements with the content of the class. In pilot studies, patients were asked to provide feedback, and this feedback was incorporated into subsequent revisions. Staff developed multilingual handouts, including a surgery instruction sheet with a preoperative social assessment, in addition to creating opportunities for 1:1 education when it was medically or socially appropriate.
The work group brought the physicians in the care pathway together for a focused conversation and education regarding the need for including class attendance as part of their practice. They worked with the physicians’ support team to coordinate standardized follow-up care in the post-acute setting and encouraged the physicians to create a “home-going” culture and reset expectations for length of stay. Prior to the start of this project, the rehabilitation department began using the 6-Clicks Inpatient Basic Mobility Short Form, a standardized instrument comprised of short forms created from the Activity Measure for Post-Acute Care (AM-PAC) instrument. The AMPAC score is used to help establish the patient’s functional level; patients scoring 18 or higher have a higher probability of a successful return home (rather than an institutional discharge). Physical therapists at the Center adminster the 6-Clicks tool for each therapy patient daily, and document the AM-PAC score in the appropriate flowsheet in the EHR in a manner consistent with best practices.9-11 The group utilized the AMPAC score to determine functional status upon discharge, offering guidance for the correct post-acute discharge plan.
The workgroup also established a relationship with the lead home health care service to develop a standard notification process to set volume and service expectations. Finally, they worked with the lead SNFs to explain the surgical procedure and set expectations for patient recovery at the facilities. These changes are summarized in the Figure.
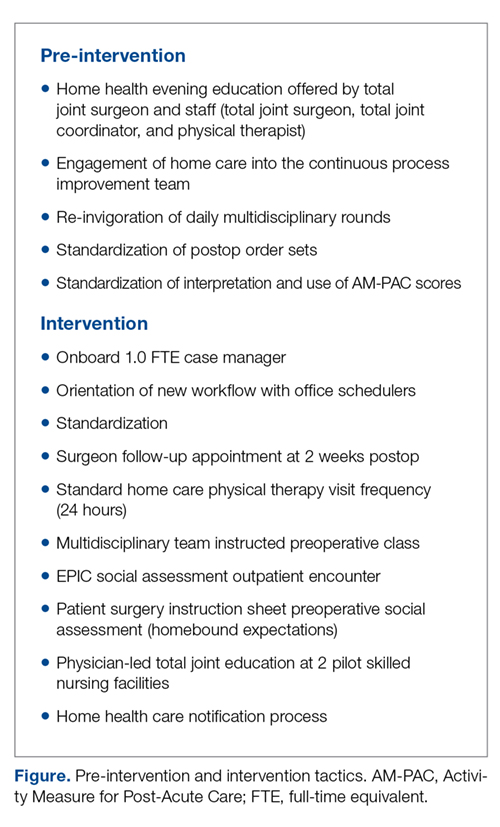
Analysis
Descriptive statistics were used to describe patients and metrics in the baseline and performance periods. The difference in the proportion of patients discharged to a SNF in the baseline and performance periods was examined by a Z-test of proportion and change in relative risk. A Z-test of proportion was also used examine differences in the 90-day all-cause readmission rate and in the average length of stay between the 2 periods.
Results
Differences between Medicare fee-for-service patients in the baseline and performance periods are reported in Table 1. While age, sex, diagnoses, and surgical types were similar, AM-PAC scores and the proportion of patients married or living with someone were higher in the performance period. The AM-PAC score in Table 1 represents the last documented score prior to discharge.
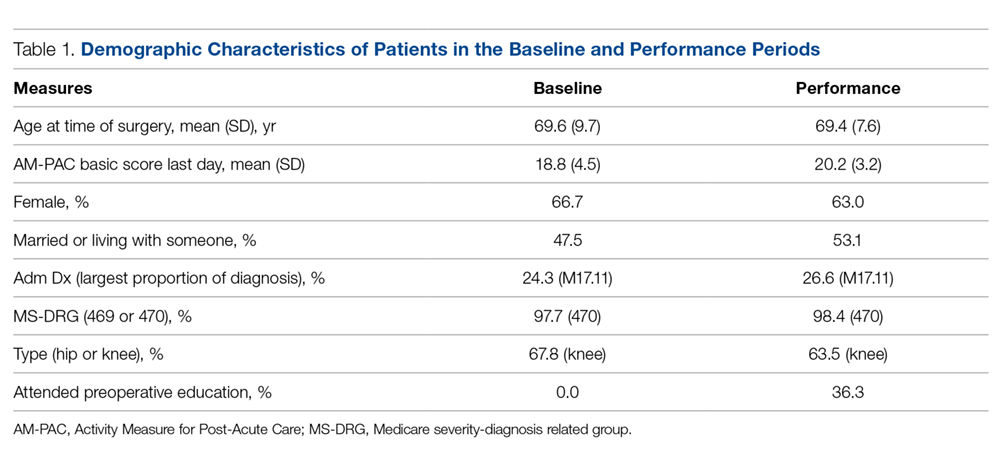
The proportion of Medicare patients discharged to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period (Table 2). The 21.9% difference was significant at the 0.05 level (Z = 4.6586, P = 0.0001). Medicare patients in the intervention period had nearly half (0.45) the risk (95% confidence interval, 0.314-0.639) of SNF utilization compared with patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001).
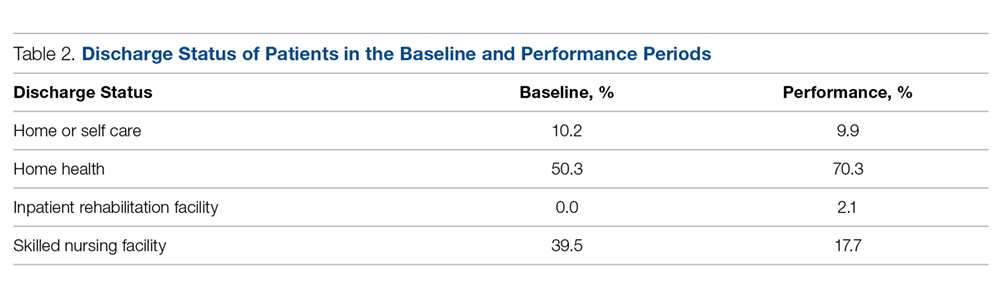
Concomitantly, the 90-day all-cause readmission rate among Medicare patients rose from 2.8% (5/177) to 4.7% (9/192), but the difference in proportions was not statistically significant (Z = –0.9356, P = 0.3495). Similarly, the average length of stay for Medicare patients was 2.9 days in both the baseline and performance periods.
Discussion
The intervention was associated with a statistically significant decrease in the SNF discharge rate following total joint replacement. The readmission rate and average length of stay were statistically unchanged. The lack of a statistically significant change in readmissions is important, as previous research has found that total joint replacement patients discharged to a SNF have higher odds of hospital readmission within 90 days than those discharged home.12 Moreover, the readmission rate in the performance period (4.7%) was still substantially lower than the national estimate of 90-day readmission rates associated with total hip arthroplasty (7.7%) and total knee arthroplasty (9.7%).13 For patients, these quality improvements are associated with improved outcomes and lower costs of care.
These outcomes were achieved after a substantial effort at the facility level to onboard new staff; orient them and their colleagues in each step along the care pathway, from scheduling through post-acute care; and build trust among all team members. The critical difference was changing expectations for post-acute recovery and rehabilitation throughout both the new clinical coordination workflow and processes and the existing processes. Orientation of the clinical coordination role was necessary to establish relationships with inpatient team members who were not as intimately involved with the position and expectations. To accomplish this, competing priorities had to be addressed and resolved through standardization efforts developed and implemented by the multidisciplinary team. The team first reviewed reports of such efforts in the initial review of the BPCI literature.1-5 Subsequent analyses of the most germane study3 and related research14 confirmed that a team-based approach to standardization could be successful. The former study used physician and affiliated care teams to build a care pathway that minimized variation in practices across the episode of care, and the latter used a multidisciplinary team approach to develop rapid recovery protocols. Subsequent research has validated the findings that hospital-based multidisciplinary teams have long been associated with improved patient safety, shorter length of stay, and fewer complications.15
Limitations
This quality improvement project was limited to a single facility. As such, adapting the improvements made to Grant Medical Center’s care pathway for implementation throughout the OhioHealth system will take time due to variation of care provided at each campus; scale-up efforts are ongoing. In the Grant facility, the project uncovered instances of unstandardized provider communication pathways, clinical staff workflows, and expectations for patients. Standardization in all 3 instances improved the patient experience. Additionally, data collection requirements for rigorous research and evaluation efforts that had to be done by hand during the project are now being integrated into the EHR.
The improvements described here, which were implemented in anticipation of adopting the CMS BPCI bundled payment model for joint replacement of the lower extremity, were provided to every patient regardless of payer. Patient outcomes varied across payer, as did preoperative education rates and other variables (Table 1). These differences are being tracked and analyzed following the Center’s entry into the CMS BPCI model on October 1, 2018.
Corresponding author: Gregg M. Gascon, PhD, CHDA, OhioHealth Group, 155 E. Broad Street, Suite 1700, Columbus, Ohio 43215; [email protected].
Financial disclosures: None.
1. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316:1267-1278.
2. Urdapilleta O, Weinberg D, Pedersen S, et al. Evaluation of the Medicare Acute Care Episodes (ACE) demonstration: final evaluation report. Centers for Medicare & Medicaid Services. May 31, 2013. http://downloads.cms.gov/files/cmmi/ACE-EvaluationReport-Final-5-2-14.pdf.
3. Froemke C, Wang L, DeHart ML, et al. Standardizing care and improving quality under a bundled payment initiative for total joint arthroplasty. J Arthroplasty. 2015;30:1676-1682.
4. US Department of Health and Human Services. (2015 Better, smarter, healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. New Release. January 26, 2015.
5. Tsai TC, Joynt KE, Wild RC, et al. Medicare’s Bundled Payment Initiatives: most hospitals are focused on a few high-volume conditions. Health Aff (Millwood). 2015;34;371-380.
6. Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011,365:777-779.
7. Mallinson TR, Bateman J, Tseng HY, et al. A comparison of discharge functional status after rehabilitation in skilled nursing, home health, and medical rehabilitation settings for patients after lower-extremity joint replacement surgery. Arch Phys Med Rehabil. 2011:92:712-720.
8. Froimson M, Deadwiller M, Schill M, Cousineau K. Early results of a total joint bundled payment program: the BPCI initiative. Poster No. 2026. Poster presented at Orthopaedic Research Society Annual Meeting; March 15-18, 2014; New Orleans, LA.
9. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):S49-S61.
10. Jette DU, Stilphen M, Ranganathan VK, et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94;379-391.
11. Jette DU, Stilphen M, Ranganathan VK, et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94:1252-1261.
12. Bini SA, Fithian DC, Paxton LW, et al. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25:114-117.
13. Ramkumar PN, Chu CT, Harris JD, et al. Causes and rates of unplanned readmissions after elective primary total joint arthroplasty: a systematic review and meta-analysis. Am J Orthop. 2015;44:397-405.
14. Klingenstein GG, Schoifet SD, Jain RK, et al. Rapid discharge to home after total knee arthroplasty is safe in eligible Medicare patients. J Arthroplasty. 2017;32:3308-3313.
15. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-S303.
From Grant Medical Center (Dr. Wasielewski, Dr. Polonia, Ms. Barca, Ms. Cebriak, Ms. Lucki), and OhioHealth Group (Mr. Rogers, Ms. St. John, Dr. Gascon), Columbus, OH.
Abstract
- Background: Organizations participating in the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative enter into payment arrangements that include financial and performance accountability for episodes of care. There is growing evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care (eg, skilled nursing facilities [SNF], long-term care facilities, and inpatient rehabilitation facilities).
- Objective: To describe a pilot program designed to reduce the utilization of institutional post-acute care for total joint replacement surgical patients.
- Methods: A multidisciplinary intervention team optimized scheduling, preadmission testing, patient communication, and patient education along a total joint replacement care pathway.
- Results: Among Medicare patients, total discharges to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period. The risk of SNF utilization among patients in the intervention period was nearly half (0.45; 95% confidence interval, 0.314-0.639) that of patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001). The readmission rate was substantially lower than national norms.
- Conclusion: Optimizing patient care throughout the care pathway using the concerted efforts of a multidisciplinary team is possible given a common vision, shared goals, and clearly communicated expectations.
Keywords: arthroplasty; readmissions; Medicare; post-acute care; Bundled Payment for Care Improvement.
Quality improvement in health care is partially dependent upon processes that standardize episodes of care. This is especially true in the post–acute care setting, where efforts to increase patient engagement and care coordination can improve a patient’s recovery process. One framework for optimizing patient care across an episode of care is the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative, which links payments for the multiple services patients receive during an episode of care. Organizations participating in the BPCI initiative enter into payment arrangements that include financial and performance accountability for episodes of care. The BPCI initiative provides a framework for episodes of care across multiple types of facilities and clinicians over periods of time (30-day, 60-day, and 90-day episodes).1-5 Evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality is accumulating. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care, such as skilled nursing facilities (SNF), long-term care facilities, and inpatient rehabilitation facilities.1
Our hospital, the Grant Medical Center, which is part of the OhioHealth system, had agreed to participate in the CMS BPCI–Advanced Model for major joint replacement of the lower extremity, with a start date of October 1, 2018. Prior to adopting this bundled payment and service delivery model for major joint replacement through the BPCI program, we implemented strategic interventions to improve the efficiency of care delivery and reduce post-acute utilization. Although the BPCI program applied only to Medicare patients, the interventions that were developed, implemented, and evaluated in this quality improvement project, which we describe in this article, were provided to all patients who underwent lower-extremity joint replacement, regardless of payer.
Gap Analysis
Prior to developing and implementing the intervention, a gap analysis was performed to determine differences between Grant Medical Center’s care pathway/processes and evidence-based best practice. A steering committee comprised of physician champions, rehabilitation services, senior leadership from the Bone and Joint Center, and care management was gathered. The gap analysis examined the care paths in the preoperative phase, index admission phase, and the post-acute phase of care. Findings of the gap analysis included an underutilized joint education class, overutilization of SNF placement, and lack of key resources to assess the needs of patients prior to surgery.
The Grant Bone and Joint Center offered a comprehensive joint education class in person, but also gave patients the option of utilizing an online learning source. While both were successful, staff believed the in-person class had a greater impact than the online version. Patients who attended the class completed a preoperative assessment by hand, which included a social assessment for identifying potential challenges prior to admission. However, because class attendance was low (< 10%), this assessment tool was not utilized until the patient’s admission in most cases. The gap analysis also identified that the educational content of the class lacked key points to encourage a home-going plan.
The baseline SNF utilization rate (using data from July 1, 2015–June 30, 2016) within the Medicare population was 39.5% (70/177), with an overall (all payers) rate of 22.9% (192/838). This SNF utilization rate was higher than the national benchmark, underscoring the need to focus on a preoperative “plan for home” message. In addition, review of Medicare fee-for-service data from 2014 to 2016 revealed that Grant Medical Center utilized 83 different SNFs over the course of approximately 2 years. In this context, staff saw an opportunity to develop stronger relationships with fewer SNF partners, as well as OhioHealth Home Care, in order to improve care by standardizing functional recovery in post-acute care management.
In sum, the gap analysis found that a lack of standardization, follow through, and engagement in daily multidisciplinary rounds often led to a plan for SNF discharge instead of home. As such, it was determined that the pilot program would target the preoperative and post-acute phases of care and that its primary endpoint would be the reduction of the SNF discharge rate among Medicare fee-for-service patients.
Literature Review
Targeting the SNF discharge rate as the endpoint was also supported by a recent study that showed that most of the reduction in spending for total joint replacement within BPCI hospitals was linked to reduced use of institutional post-acute care.1 Other studies in the joint replacement literature have delineated the specific aspects of care redesign that allow hospitals to provide more efficient and effective care delivery during an episode of care.6-8 A review of these and similar studies of joint replacement quality improvement interventions yielded a number of actionable findings:
- Engaging and educating patients and families is critical. Families and other caregivers must be identified preoperatively, actively engaged, and committed to helping the patient recover.
- Providers must build the expectation in patients that they will return home as soon as it is safe to do so. This includes working to restore physiologic function, managing pain with oral medication, and restoring the physical capability of adapting to a home environment.
- Relationships must be established with post-acute care providers who are able to demonstrate best practices and be willing partners on performance outcomes.
- Providers need to invest in personnel to coordinate care transitions initiated preoperatively that continue through the post-acute phase of care.
- Providers must promote processes that allow patients to more fully own their recovery through coaching and improved communication.
A total joint replacement initiative undertaken at another hospital within the OhioHealth system, Riverside Methodist Hospital, that incorporated these aspects of care redesign demonstrated a significant reduction in SNF utilization. That success informed the development of the initiative at Grant Medical Center.
Methods
Setting
This pilot project was conducted from July 2017 through July 2018 at the Grant Bone and Joint Center in the Grant Medical Center, an urban hospital in Columbus, Ohio. The Bone and Joint Center performs more than 7400 surgeries each year, of which 12% are total joint replacements. Grant Medical Center is a Level I Trauma Center that has received a fourth designation in Magnet Nursing status and has attained The Joint Commission Disease-Specific Care Certification for its total knee and hip arthroplasty, total shoulder, and hip fracture programs.
Intervention
The findings from the gap analysis were incorporated into a standardized preoperative care pathway at the Center. Standardization of the care pathway required developing relationships between office staff, schedulers, inpatient work teams, and preadmission testing, as well as physician group realignment with the larger organization.
During this time, the steering committee identified the need for a designated full-time pre-habilitation case manager to support patients undergoing hip and knee replacements at the Center. With the addition of a new role, prehab case manager, attention was directed to patient social assessment and communication. After the surgery was scheduled but prior to the preoperative education class, each patient received a screening phone call from the prehab case manager that included an initial social assessment. The electronic health record (EHR) was utilized to communicate the results of the assessment, including barriers to a home-going plan. The phone call allowed the case manager to reinforce the importance of class attendance for the patient and primary caregiver, and also allowed any specific concerns identified to be addressed and resolved prior to surgery.
A work group consisting of the prehab case manager, office staff, and the bone and joint leadership team focused on improving the core preoperative education content, same-day preadmission clearance, and class attendance. To support these efforts, the work group (1) created a scheduling document to align preadmission testing and the preoperative education class so they occurred on the same day, (2) improved prior authorization of the surgery to support the post-surgical care team, (3) clarified expectations and roles among office staff and care management staff to optimize the discharge process, and (4) engaged the physician team to encourage a culture change towards setting patient expectations to be discharged to home or a preferred SNF location. Regarding prior authorization communications, prior to the intervention, communication was insufficient, paper driven, and lacking in continuity. Utilizing the EHR, an EPIC platform, a designated documentation location was created, which increased communication among the office schedulers, preadmission testing, and surgery schedulers. The consistent location for recording prior authorization avoided canceled surgeries and claim denials due to missing pieces of information. In addition to these process changes, the surgical offices were geographically realigned to a new single location, a change that brought the staff together and in turn allowed for role clarification and team building and also allowed the staff to identify opportunities for improvement.
The core content of the education class was redesigned to support patients’ understanding of the procedure and what to expect during the hospital stay and discharge for home. Staff worked first to align all preadmission patient requirements with the content of the class. In pilot studies, patients were asked to provide feedback, and this feedback was incorporated into subsequent revisions. Staff developed multilingual handouts, including a surgery instruction sheet with a preoperative social assessment, in addition to creating opportunities for 1:1 education when it was medically or socially appropriate.
The work group brought the physicians in the care pathway together for a focused conversation and education regarding the need for including class attendance as part of their practice. They worked with the physicians’ support team to coordinate standardized follow-up care in the post-acute setting and encouraged the physicians to create a “home-going” culture and reset expectations for length of stay. Prior to the start of this project, the rehabilitation department began using the 6-Clicks Inpatient Basic Mobility Short Form, a standardized instrument comprised of short forms created from the Activity Measure for Post-Acute Care (AM-PAC) instrument. The AMPAC score is used to help establish the patient’s functional level; patients scoring 18 or higher have a higher probability of a successful return home (rather than an institutional discharge). Physical therapists at the Center adminster the 6-Clicks tool for each therapy patient daily, and document the AM-PAC score in the appropriate flowsheet in the EHR in a manner consistent with best practices.9-11 The group utilized the AMPAC score to determine functional status upon discharge, offering guidance for the correct post-acute discharge plan.
The workgroup also established a relationship with the lead home health care service to develop a standard notification process to set volume and service expectations. Finally, they worked with the lead SNFs to explain the surgical procedure and set expectations for patient recovery at the facilities. These changes are summarized in the Figure.

Analysis
Descriptive statistics were used to describe patients and metrics in the baseline and performance periods. The difference in the proportion of patients discharged to a SNF in the baseline and performance periods was examined by a Z-test of proportion and change in relative risk. A Z-test of proportion was also used examine differences in the 90-day all-cause readmission rate and in the average length of stay between the 2 periods.
Results
Differences between Medicare fee-for-service patients in the baseline and performance periods are reported in Table 1. While age, sex, diagnoses, and surgical types were similar, AM-PAC scores and the proportion of patients married or living with someone were higher in the performance period. The AM-PAC score in Table 1 represents the last documented score prior to discharge.

The proportion of Medicare patients discharged to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period (Table 2). The 21.9% difference was significant at the 0.05 level (Z = 4.6586, P = 0.0001). Medicare patients in the intervention period had nearly half (0.45) the risk (95% confidence interval, 0.314-0.639) of SNF utilization compared with patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001).

Concomitantly, the 90-day all-cause readmission rate among Medicare patients rose from 2.8% (5/177) to 4.7% (9/192), but the difference in proportions was not statistically significant (Z = –0.9356, P = 0.3495). Similarly, the average length of stay for Medicare patients was 2.9 days in both the baseline and performance periods.
Discussion
The intervention was associated with a statistically significant decrease in the SNF discharge rate following total joint replacement. The readmission rate and average length of stay were statistically unchanged. The lack of a statistically significant change in readmissions is important, as previous research has found that total joint replacement patients discharged to a SNF have higher odds of hospital readmission within 90 days than those discharged home.12 Moreover, the readmission rate in the performance period (4.7%) was still substantially lower than the national estimate of 90-day readmission rates associated with total hip arthroplasty (7.7%) and total knee arthroplasty (9.7%).13 For patients, these quality improvements are associated with improved outcomes and lower costs of care.
These outcomes were achieved after a substantial effort at the facility level to onboard new staff; orient them and their colleagues in each step along the care pathway, from scheduling through post-acute care; and build trust among all team members. The critical difference was changing expectations for post-acute recovery and rehabilitation throughout both the new clinical coordination workflow and processes and the existing processes. Orientation of the clinical coordination role was necessary to establish relationships with inpatient team members who were not as intimately involved with the position and expectations. To accomplish this, competing priorities had to be addressed and resolved through standardization efforts developed and implemented by the multidisciplinary team. The team first reviewed reports of such efforts in the initial review of the BPCI literature.1-5 Subsequent analyses of the most germane study3 and related research14 confirmed that a team-based approach to standardization could be successful. The former study used physician and affiliated care teams to build a care pathway that minimized variation in practices across the episode of care, and the latter used a multidisciplinary team approach to develop rapid recovery protocols. Subsequent research has validated the findings that hospital-based multidisciplinary teams have long been associated with improved patient safety, shorter length of stay, and fewer complications.15
Limitations
This quality improvement project was limited to a single facility. As such, adapting the improvements made to Grant Medical Center’s care pathway for implementation throughout the OhioHealth system will take time due to variation of care provided at each campus; scale-up efforts are ongoing. In the Grant facility, the project uncovered instances of unstandardized provider communication pathways, clinical staff workflows, and expectations for patients. Standardization in all 3 instances improved the patient experience. Additionally, data collection requirements for rigorous research and evaluation efforts that had to be done by hand during the project are now being integrated into the EHR.
The improvements described here, which were implemented in anticipation of adopting the CMS BPCI bundled payment model for joint replacement of the lower extremity, were provided to every patient regardless of payer. Patient outcomes varied across payer, as did preoperative education rates and other variables (Table 1). These differences are being tracked and analyzed following the Center’s entry into the CMS BPCI model on October 1, 2018.
Corresponding author: Gregg M. Gascon, PhD, CHDA, OhioHealth Group, 155 E. Broad Street, Suite 1700, Columbus, Ohio 43215; [email protected].
Financial disclosures: None.
From Grant Medical Center (Dr. Wasielewski, Dr. Polonia, Ms. Barca, Ms. Cebriak, Ms. Lucki), and OhioHealth Group (Mr. Rogers, Ms. St. John, Dr. Gascon), Columbus, OH.
Abstract
- Background: Organizations participating in the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative enter into payment arrangements that include financial and performance accountability for episodes of care. There is growing evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care (eg, skilled nursing facilities [SNF], long-term care facilities, and inpatient rehabilitation facilities).
- Objective: To describe a pilot program designed to reduce the utilization of institutional post-acute care for total joint replacement surgical patients.
- Methods: A multidisciplinary intervention team optimized scheduling, preadmission testing, patient communication, and patient education along a total joint replacement care pathway.
- Results: Among Medicare patients, total discharges to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period. The risk of SNF utilization among patients in the intervention period was nearly half (0.45; 95% confidence interval, 0.314-0.639) that of patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001). The readmission rate was substantially lower than national norms.
- Conclusion: Optimizing patient care throughout the care pathway using the concerted efforts of a multidisciplinary team is possible given a common vision, shared goals, and clearly communicated expectations.
Keywords: arthroplasty; readmissions; Medicare; post-acute care; Bundled Payment for Care Improvement.
Quality improvement in health care is partially dependent upon processes that standardize episodes of care. This is especially true in the post–acute care setting, where efforts to increase patient engagement and care coordination can improve a patient’s recovery process. One framework for optimizing patient care across an episode of care is the Centers for Medicare and Medicaid Services (CMS) Bundled Payment for Care Improvement (BPCI) initiative, which links payments for the multiple services patients receive during an episode of care. Organizations participating in the BPCI initiative enter into payment arrangements that include financial and performance accountability for episodes of care. The BPCI initiative provides a framework for episodes of care across multiple types of facilities and clinicians over periods of time (30-day, 60-day, and 90-day episodes).1-5 Evidence that the use of these payment incentives reduces spending for episodes of care while maintaining or improving quality is accumulating. A recent study of BPCI and quality outcomes in joint replacement episodes found that nearly all the reduction in spending within BPCI hospitals was generated from the reduced use of institutional post-acute care, such as skilled nursing facilities (SNF), long-term care facilities, and inpatient rehabilitation facilities.1
Our hospital, the Grant Medical Center, which is part of the OhioHealth system, had agreed to participate in the CMS BPCI–Advanced Model for major joint replacement of the lower extremity, with a start date of October 1, 2018. Prior to adopting this bundled payment and service delivery model for major joint replacement through the BPCI program, we implemented strategic interventions to improve the efficiency of care delivery and reduce post-acute utilization. Although the BPCI program applied only to Medicare patients, the interventions that were developed, implemented, and evaluated in this quality improvement project, which we describe in this article, were provided to all patients who underwent lower-extremity joint replacement, regardless of payer.
Gap Analysis
Prior to developing and implementing the intervention, a gap analysis was performed to determine differences between Grant Medical Center’s care pathway/processes and evidence-based best practice. A steering committee comprised of physician champions, rehabilitation services, senior leadership from the Bone and Joint Center, and care management was gathered. The gap analysis examined the care paths in the preoperative phase, index admission phase, and the post-acute phase of care. Findings of the gap analysis included an underutilized joint education class, overutilization of SNF placement, and lack of key resources to assess the needs of patients prior to surgery.
The Grant Bone and Joint Center offered a comprehensive joint education class in person, but also gave patients the option of utilizing an online learning source. While both were successful, staff believed the in-person class had a greater impact than the online version. Patients who attended the class completed a preoperative assessment by hand, which included a social assessment for identifying potential challenges prior to admission. However, because class attendance was low (< 10%), this assessment tool was not utilized until the patient’s admission in most cases. The gap analysis also identified that the educational content of the class lacked key points to encourage a home-going plan.
The baseline SNF utilization rate (using data from July 1, 2015–June 30, 2016) within the Medicare population was 39.5% (70/177), with an overall (all payers) rate of 22.9% (192/838). This SNF utilization rate was higher than the national benchmark, underscoring the need to focus on a preoperative “plan for home” message. In addition, review of Medicare fee-for-service data from 2014 to 2016 revealed that Grant Medical Center utilized 83 different SNFs over the course of approximately 2 years. In this context, staff saw an opportunity to develop stronger relationships with fewer SNF partners, as well as OhioHealth Home Care, in order to improve care by standardizing functional recovery in post-acute care management.
In sum, the gap analysis found that a lack of standardization, follow through, and engagement in daily multidisciplinary rounds often led to a plan for SNF discharge instead of home. As such, it was determined that the pilot program would target the preoperative and post-acute phases of care and that its primary endpoint would be the reduction of the SNF discharge rate among Medicare fee-for-service patients.
Literature Review
Targeting the SNF discharge rate as the endpoint was also supported by a recent study that showed that most of the reduction in spending for total joint replacement within BPCI hospitals was linked to reduced use of institutional post-acute care.1 Other studies in the joint replacement literature have delineated the specific aspects of care redesign that allow hospitals to provide more efficient and effective care delivery during an episode of care.6-8 A review of these and similar studies of joint replacement quality improvement interventions yielded a number of actionable findings:
- Engaging and educating patients and families is critical. Families and other caregivers must be identified preoperatively, actively engaged, and committed to helping the patient recover.
- Providers must build the expectation in patients that they will return home as soon as it is safe to do so. This includes working to restore physiologic function, managing pain with oral medication, and restoring the physical capability of adapting to a home environment.
- Relationships must be established with post-acute care providers who are able to demonstrate best practices and be willing partners on performance outcomes.
- Providers need to invest in personnel to coordinate care transitions initiated preoperatively that continue through the post-acute phase of care.
- Providers must promote processes that allow patients to more fully own their recovery through coaching and improved communication.
A total joint replacement initiative undertaken at another hospital within the OhioHealth system, Riverside Methodist Hospital, that incorporated these aspects of care redesign demonstrated a significant reduction in SNF utilization. That success informed the development of the initiative at Grant Medical Center.
Methods
Setting
This pilot project was conducted from July 2017 through July 2018 at the Grant Bone and Joint Center in the Grant Medical Center, an urban hospital in Columbus, Ohio. The Bone and Joint Center performs more than 7400 surgeries each year, of which 12% are total joint replacements. Grant Medical Center is a Level I Trauma Center that has received a fourth designation in Magnet Nursing status and has attained The Joint Commission Disease-Specific Care Certification for its total knee and hip arthroplasty, total shoulder, and hip fracture programs.
Intervention
The findings from the gap analysis were incorporated into a standardized preoperative care pathway at the Center. Standardization of the care pathway required developing relationships between office staff, schedulers, inpatient work teams, and preadmission testing, as well as physician group realignment with the larger organization.
During this time, the steering committee identified the need for a designated full-time pre-habilitation case manager to support patients undergoing hip and knee replacements at the Center. With the addition of a new role, prehab case manager, attention was directed to patient social assessment and communication. After the surgery was scheduled but prior to the preoperative education class, each patient received a screening phone call from the prehab case manager that included an initial social assessment. The electronic health record (EHR) was utilized to communicate the results of the assessment, including barriers to a home-going plan. The phone call allowed the case manager to reinforce the importance of class attendance for the patient and primary caregiver, and also allowed any specific concerns identified to be addressed and resolved prior to surgery.
A work group consisting of the prehab case manager, office staff, and the bone and joint leadership team focused on improving the core preoperative education content, same-day preadmission clearance, and class attendance. To support these efforts, the work group (1) created a scheduling document to align preadmission testing and the preoperative education class so they occurred on the same day, (2) improved prior authorization of the surgery to support the post-surgical care team, (3) clarified expectations and roles among office staff and care management staff to optimize the discharge process, and (4) engaged the physician team to encourage a culture change towards setting patient expectations to be discharged to home or a preferred SNF location. Regarding prior authorization communications, prior to the intervention, communication was insufficient, paper driven, and lacking in continuity. Utilizing the EHR, an EPIC platform, a designated documentation location was created, which increased communication among the office schedulers, preadmission testing, and surgery schedulers. The consistent location for recording prior authorization avoided canceled surgeries and claim denials due to missing pieces of information. In addition to these process changes, the surgical offices were geographically realigned to a new single location, a change that brought the staff together and in turn allowed for role clarification and team building and also allowed the staff to identify opportunities for improvement.
The core content of the education class was redesigned to support patients’ understanding of the procedure and what to expect during the hospital stay and discharge for home. Staff worked first to align all preadmission patient requirements with the content of the class. In pilot studies, patients were asked to provide feedback, and this feedback was incorporated into subsequent revisions. Staff developed multilingual handouts, including a surgery instruction sheet with a preoperative social assessment, in addition to creating opportunities for 1:1 education when it was medically or socially appropriate.
The work group brought the physicians in the care pathway together for a focused conversation and education regarding the need for including class attendance as part of their practice. They worked with the physicians’ support team to coordinate standardized follow-up care in the post-acute setting and encouraged the physicians to create a “home-going” culture and reset expectations for length of stay. Prior to the start of this project, the rehabilitation department began using the 6-Clicks Inpatient Basic Mobility Short Form, a standardized instrument comprised of short forms created from the Activity Measure for Post-Acute Care (AM-PAC) instrument. The AMPAC score is used to help establish the patient’s functional level; patients scoring 18 or higher have a higher probability of a successful return home (rather than an institutional discharge). Physical therapists at the Center adminster the 6-Clicks tool for each therapy patient daily, and document the AM-PAC score in the appropriate flowsheet in the EHR in a manner consistent with best practices.9-11 The group utilized the AMPAC score to determine functional status upon discharge, offering guidance for the correct post-acute discharge plan.
The workgroup also established a relationship with the lead home health care service to develop a standard notification process to set volume and service expectations. Finally, they worked with the lead SNFs to explain the surgical procedure and set expectations for patient recovery at the facilities. These changes are summarized in the Figure.

Analysis
Descriptive statistics were used to describe patients and metrics in the baseline and performance periods. The difference in the proportion of patients discharged to a SNF in the baseline and performance periods was examined by a Z-test of proportion and change in relative risk. A Z-test of proportion was also used examine differences in the 90-day all-cause readmission rate and in the average length of stay between the 2 periods.
Results
Differences between Medicare fee-for-service patients in the baseline and performance periods are reported in Table 1. While age, sex, diagnoses, and surgical types were similar, AM-PAC scores and the proportion of patients married or living with someone were higher in the performance period. The AM-PAC score in Table 1 represents the last documented score prior to discharge.

The proportion of Medicare patients discharged to a SNF fell from 39.5% (70/177) in the baseline period to 17.7% (34/192) in the performance period (Table 2). The 21.9% difference was significant at the 0.05 level (Z = 4.6586, P = 0.0001). Medicare patients in the intervention period had nearly half (0.45) the risk (95% confidence interval, 0.314-0.639) of SNF utilization compared with patients in the baseline period. Using Fisher’s exact test and a 2-tailed test, this reduction was found to be significant (P < 0.0001).

Concomitantly, the 90-day all-cause readmission rate among Medicare patients rose from 2.8% (5/177) to 4.7% (9/192), but the difference in proportions was not statistically significant (Z = –0.9356, P = 0.3495). Similarly, the average length of stay for Medicare patients was 2.9 days in both the baseline and performance periods.
Discussion
The intervention was associated with a statistically significant decrease in the SNF discharge rate following total joint replacement. The readmission rate and average length of stay were statistically unchanged. The lack of a statistically significant change in readmissions is important, as previous research has found that total joint replacement patients discharged to a SNF have higher odds of hospital readmission within 90 days than those discharged home.12 Moreover, the readmission rate in the performance period (4.7%) was still substantially lower than the national estimate of 90-day readmission rates associated with total hip arthroplasty (7.7%) and total knee arthroplasty (9.7%).13 For patients, these quality improvements are associated with improved outcomes and lower costs of care.
These outcomes were achieved after a substantial effort at the facility level to onboard new staff; orient them and their colleagues in each step along the care pathway, from scheduling through post-acute care; and build trust among all team members. The critical difference was changing expectations for post-acute recovery and rehabilitation throughout both the new clinical coordination workflow and processes and the existing processes. Orientation of the clinical coordination role was necessary to establish relationships with inpatient team members who were not as intimately involved with the position and expectations. To accomplish this, competing priorities had to be addressed and resolved through standardization efforts developed and implemented by the multidisciplinary team. The team first reviewed reports of such efforts in the initial review of the BPCI literature.1-5 Subsequent analyses of the most germane study3 and related research14 confirmed that a team-based approach to standardization could be successful. The former study used physician and affiliated care teams to build a care pathway that minimized variation in practices across the episode of care, and the latter used a multidisciplinary team approach to develop rapid recovery protocols. Subsequent research has validated the findings that hospital-based multidisciplinary teams have long been associated with improved patient safety, shorter length of stay, and fewer complications.15
Limitations
This quality improvement project was limited to a single facility. As such, adapting the improvements made to Grant Medical Center’s care pathway for implementation throughout the OhioHealth system will take time due to variation of care provided at each campus; scale-up efforts are ongoing. In the Grant facility, the project uncovered instances of unstandardized provider communication pathways, clinical staff workflows, and expectations for patients. Standardization in all 3 instances improved the patient experience. Additionally, data collection requirements for rigorous research and evaluation efforts that had to be done by hand during the project are now being integrated into the EHR.
The improvements described here, which were implemented in anticipation of adopting the CMS BPCI bundled payment model for joint replacement of the lower extremity, were provided to every patient regardless of payer. Patient outcomes varied across payer, as did preoperative education rates and other variables (Table 1). These differences are being tracked and analyzed following the Center’s entry into the CMS BPCI model on October 1, 2018.
Corresponding author: Gregg M. Gascon, PhD, CHDA, OhioHealth Group, 155 E. Broad Street, Suite 1700, Columbus, Ohio 43215; [email protected].
Financial disclosures: None.
1. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316:1267-1278.
2. Urdapilleta O, Weinberg D, Pedersen S, et al. Evaluation of the Medicare Acute Care Episodes (ACE) demonstration: final evaluation report. Centers for Medicare & Medicaid Services. May 31, 2013. http://downloads.cms.gov/files/cmmi/ACE-EvaluationReport-Final-5-2-14.pdf.
3. Froemke C, Wang L, DeHart ML, et al. Standardizing care and improving quality under a bundled payment initiative for total joint arthroplasty. J Arthroplasty. 2015;30:1676-1682.
4. US Department of Health and Human Services. (2015 Better, smarter, healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. New Release. January 26, 2015.
5. Tsai TC, Joynt KE, Wild RC, et al. Medicare’s Bundled Payment Initiatives: most hospitals are focused on a few high-volume conditions. Health Aff (Millwood). 2015;34;371-380.
6. Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011,365:777-779.
7. Mallinson TR, Bateman J, Tseng HY, et al. A comparison of discharge functional status after rehabilitation in skilled nursing, home health, and medical rehabilitation settings for patients after lower-extremity joint replacement surgery. Arch Phys Med Rehabil. 2011:92:712-720.
8. Froimson M, Deadwiller M, Schill M, Cousineau K. Early results of a total joint bundled payment program: the BPCI initiative. Poster No. 2026. Poster presented at Orthopaedic Research Society Annual Meeting; March 15-18, 2014; New Orleans, LA.
9. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):S49-S61.
10. Jette DU, Stilphen M, Ranganathan VK, et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94;379-391.
11. Jette DU, Stilphen M, Ranganathan VK, et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94:1252-1261.
12. Bini SA, Fithian DC, Paxton LW, et al. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25:114-117.
13. Ramkumar PN, Chu CT, Harris JD, et al. Causes and rates of unplanned readmissions after elective primary total joint arthroplasty: a systematic review and meta-analysis. Am J Orthop. 2015;44:397-405.
14. Klingenstein GG, Schoifet SD, Jain RK, et al. Rapid discharge to home after total knee arthroplasty is safe in eligible Medicare patients. J Arthroplasty. 2017;32:3308-3313.
15. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-S303.
1. Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. JAMA. 2016;316:1267-1278.
2. Urdapilleta O, Weinberg D, Pedersen S, et al. Evaluation of the Medicare Acute Care Episodes (ACE) demonstration: final evaluation report. Centers for Medicare & Medicaid Services. May 31, 2013. http://downloads.cms.gov/files/cmmi/ACE-EvaluationReport-Final-5-2-14.pdf.
3. Froemke C, Wang L, DeHart ML, et al. Standardizing care and improving quality under a bundled payment initiative for total joint arthroplasty. J Arthroplasty. 2015;30:1676-1682.
4. US Department of Health and Human Services. (2015 Better, smarter, healthier: In historic announcement, HHS sets clear goals and timeline for shifting Medicare reimbursements from volume to value. New Release. January 26, 2015.
5. Tsai TC, Joynt KE, Wild RC, et al. Medicare’s Bundled Payment Initiatives: most hospitals are focused on a few high-volume conditions. Health Aff (Millwood). 2015;34;371-380.
6. Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011,365:777-779.
7. Mallinson TR, Bateman J, Tseng HY, et al. A comparison of discharge functional status after rehabilitation in skilled nursing, home health, and medical rehabilitation settings for patients after lower-extremity joint replacement surgery. Arch Phys Med Rehabil. 2011:92:712-720.
8. Froimson M, Deadwiller M, Schill M, Cousineau K. Early results of a total joint bundled payment program: the BPCI initiative. Poster No. 2026. Poster presented at Orthopaedic Research Society Annual Meeting; March 15-18, 2014; New Orleans, LA.
9. Haley SM, Coster WJ, Andres PL, et al. Activity outcome measurement for postacute care. Med Care. 2004;42(1 Suppl):S49-S61.
10. Jette DU, Stilphen M, Ranganathan VK, et al. Validity of the AM-PAC “6-Clicks” inpatient daily activity and basic mobility short forms. Phys Ther. 2014;94;379-391.
11. Jette DU, Stilphen M, Ranganathan VK, et al. AM-PAC “6-Clicks” functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94:1252-1261.
12. Bini SA, Fithian DC, Paxton LW, et al. Does discharge disposition after primary total joint arthroplasty affect readmission rates? J Arthroplasty. 2010;25:114-117.
13. Ramkumar PN, Chu CT, Harris JD, et al. Causes and rates of unplanned readmissions after elective primary total joint arthroplasty: a systematic review and meta-analysis. Am J Orthop. 2015;44:397-405.
14. Klingenstein GG, Schoifet SD, Jain RK, et al. Rapid discharge to home after total knee arthroplasty is safe in eligible Medicare patients. J Arthroplasty. 2017;32:3308-3313.
15. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-S303.
Higher Step Volume Is Associated with Lower Mortality in Older Women
Study Overview
Objective. To evaluate the association of number of steps taken per day and stepping intensity with all-cause mortality in older women.
Design. This was a prospective cohort study of US women participating in the Women’s Health Study (WHS). Participants wore an accelerometer device (ActiGraph GT3X+, ActiGraph Corp, Pensacola, FL) on the hip during waking hours for 7 consecutive days between 2011 and 2015. The accelerator data were collected at 30 Hz and aggregated into 60-second, time-stamped epochs. Data from participants who were adherent with wearing devices (defined as ≥ 10 hours/day of wear on ≥ 4 days) were used in an analysis that was conducted between 2018 and 2019. The exposure variables were defined as steps taken per day and measures of stepping intensity (ie, peak 1-minute cadence; peak 30-minute cadence; maximum 5-minute cadence; and time spent at a stepping rate of ≥ 40 steps/minute, reflecting purposeful steps).
Setting and participants. In total, 18,289 women participated in this study. Of these, 17,708 wore and returned their accelerometer devices, and data were downloaded successfully from 17,466 devices. Compliant wearers of the device (≥ 10 hours/day of wear on ≥4 days) included 16,741 participants (96% compliance rate of all downloaded device data).
Main outcome measure. All-cause mortality as ascertained through the National Death Index or confirmed by medical records and death certificates.
Main results. In this cohort of 16,741 women, average age at baseline was 72.0 ± 5.7 years (range, 62 to 101 years) and the mean step count was 5499 per day (median, 5094 steps/day) during the 7-day data capture period between 2011 and 2015. Not taking steps (0 steps/minute) accounted for 51.4% of the recorded time, incidental steps (1 to 39 steps/minute) accounted for 45.5%, and purposeful steps (≥ 40 steps/minute) accounted for 3.1%. The mean follow-up period was 4.3 years; during this time, 504 participants died. The median steps per day across quartiles were 2718 (lowest), 4363, 5905, and 8442 (highest). The corresponding quartile hazard ratios (HRs) associated with mortality adjusted for confounders were 1.00 (reference; lowest quartile), 0.59 (95% confidence interval [CI], 0.47-0.75), 0.54 (95% CI, 0.41-0.72), and 0.42 (95% CI, 0.30-0.60; highest quartile), respectively (P < 0.01). A higher mean step count per day, up to approximately 7500 steps/day, corresponded with progressive and steady decline in mortality HRs using spline analyses. Similar results were observed using sensitivity analyses that minimized reverse causation bias. While the adjusted analysis of measures of stepping intensity showed an inverse association with mortality rates, these associations were no longer significant after accounting for steps per day. Specifically, adjusted HRs comparing highest to lowest quartile were 0.87 (95% CI, 0.68-1.11) for peak 1-minute cadence; 0.86 (95% CI, 0.65-1.13) for peak 30-minute cadence; 0.80 (95% CI, 0.62-1.05) for maximum 5-minute cadence; and 1.27 (95% CI, 0.96-1.68) for time spent at a stepping rate of ≥ 40 steps/minute.
Conclusion. Older women who took approximately 4400 steps per day had lower all-cause mortality rates during a follow-up period of 4.3 years compared to those who took approximately 2700 steps each day. Progressive reduction in mortality rates was associated with increased steps per day before leveling at about 7500 steps/day. Stepping intensity, when accounting for number of steps taken per day, was not associated with reduction in mortality rates in older women.
Commentary
The health and mortality benefits of exercise are well recognized. The 2018 Department of Health and Human Services Physical Activity Guidelines (DHHS-PAG) recommend that adults should do at least 150 to 300 minutes of moderate-intensity aerobic physical activity per week, or 75 to 150 minutes of vigorous-intensity aerobic physical activity per week, in addition to doing muscle-strengthening activities on 2 or more days a week.1 Importantly, the guidelines emphasize that moving more and sitting less benefit nearly everyone, and note that measures of steps as a metric of ambulation can further promote translation of research into public health recommendations for exercise interventions. Despite this recognition, there is limited information centering on the number of daily steps (step volume) and the intensity of stepping that are needed to achieve optimal health outcomes in older adults. The study reported by Lee and colleagues adds new knowledge regarding the relationship between step volume and intensity and mortality in older women.
To date, only a handful of studies conducted outside of the United States have investigated the association between mortality and objectively measured step volume as determined by pedometer or accelerometer.2-4 While these studies observed that higher step counts are associated with lower mortality rates during follow-up periods of 5 to 10 years, their sample sizes were smaller and the study populations were different from those included in the study reported by Lee and colleagues. For example, the cohort from the United Kingdom included only men,2 and the participants in the Australian study were considerably younger, with a mean age of 59 years.4 In the current study, the largest of its kind thus far, it was observed that older women in the United States who take about 4400 steps a day have a lower mortality rate compared to those who take about 2700 steps a day. Moreover, the benefit of increased step volume on mortality progressively increases until plateauing at about 7500 steps per day. On the other hand, stepping intensity does not appear to lower mortality when step volume is accounted for. These results are important in that they add novel evidence that in older women, a patient population that tends to be sedentary, increased step volume (steps per day) but not stepping intensity (how quickly steps are taken) is associated with a reduction in mortality. Thus, these findings help to better characterize steps as a metric of ambulation in sedentary older adults per DHHS-PAG and add to the evidence necessary to translate this line of research into public health recommendations and programs.
While the health benefit of regular physical activity is well known and has been brought to the foreground with DDHA-PAG, only a small percentage of older adults engage in the recommended amounts and types of exercises. In other words, finding motivation to exercise is hard. Thus, identifying practical methods to facilitate behavioral change that increase and sustain physical activity in sedentary older adults would be essential to promoting health in this population. The use of wearable technologies such as fitness trackers and smartphone apps, devices that are now widely used, has shown promise for measuring and encouraging physical activity. The study by Lee and colleagues adds to this notion and further highlights the potential significance of step volume and mortality benefits in older women. Thus, future research in fitness technology should aim to integrate behavior change techniques (such as goal setting, feedback rewards, and action planning) and physical activity levels in order to improve health outcomes in older adults.5
In this study, the large sample size (> 16,000 participants), high compliance rate of accelerometer use (96% compliance rate), and reliable and continuous data capture (a built-in device feature) provide a large and complete dataset. This dataset, a major strength of the study, allowed the investigators to adequately control for potential confounders of physical activity, such as history of smoking, alcohol use, diet, and self-rated health, and therefore statistically minimize biases that are common in observational studies. However, some limitations inherent to the observational design are noted in this study. For instance, the observed association between step volume and mortality is correlational rather than causal, and a one-time assessment of steps taken over 7 consecutive days (ie, exposure) may not accurately reflect step volume and intensity of study participants over the span of 4.3 years of follow-up. Also, participants of WHS are predominately white, have higher socioeconomic status, and are more physically active than a national sample in the United States; therefore, caution should be exercised when making inferences to the general population.
Applications for Clinical Practice
Increased steps taken each day, up to about 7500 steps per day, is associated with lower mortality in older women. This finding can help inform the discussion when clinicians offer physical activity recommendations to older sedentary patients.
—Fred Ko, MD
1. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020-2028.
2. Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53:1013-1020.
3. Yamamoto N, Miyazaki H, Shimada M, et al. Daily step count and all-cause mortality in a sample of Japanese elderly people: a cohort study. BMC Public Health. 2018;18:540.
4. Dwyer T, Pezic A, Sun C, et al. Objectively measured daily steps and subsequent long term all-cause mortality: the Tasped prospective cohort study. PLoS One. 2015;10:e0141274.
5. Sullivan AN, Lachman ME. Behavior change with fitness technology in sedentary adults: a review of the evidence for increasing physical activity. Front Public Health. 2016;4:289.
Study Overview
Objective. To evaluate the association of number of steps taken per day and stepping intensity with all-cause mortality in older women.
Design. This was a prospective cohort study of US women participating in the Women’s Health Study (WHS). Participants wore an accelerometer device (ActiGraph GT3X+, ActiGraph Corp, Pensacola, FL) on the hip during waking hours for 7 consecutive days between 2011 and 2015. The accelerator data were collected at 30 Hz and aggregated into 60-second, time-stamped epochs. Data from participants who were adherent with wearing devices (defined as ≥ 10 hours/day of wear on ≥ 4 days) were used in an analysis that was conducted between 2018 and 2019. The exposure variables were defined as steps taken per day and measures of stepping intensity (ie, peak 1-minute cadence; peak 30-minute cadence; maximum 5-minute cadence; and time spent at a stepping rate of ≥ 40 steps/minute, reflecting purposeful steps).
Setting and participants. In total, 18,289 women participated in this study. Of these, 17,708 wore and returned their accelerometer devices, and data were downloaded successfully from 17,466 devices. Compliant wearers of the device (≥ 10 hours/day of wear on ≥4 days) included 16,741 participants (96% compliance rate of all downloaded device data).
Main outcome measure. All-cause mortality as ascertained through the National Death Index or confirmed by medical records and death certificates.
Main results. In this cohort of 16,741 women, average age at baseline was 72.0 ± 5.7 years (range, 62 to 101 years) and the mean step count was 5499 per day (median, 5094 steps/day) during the 7-day data capture period between 2011 and 2015. Not taking steps (0 steps/minute) accounted for 51.4% of the recorded time, incidental steps (1 to 39 steps/minute) accounted for 45.5%, and purposeful steps (≥ 40 steps/minute) accounted for 3.1%. The mean follow-up period was 4.3 years; during this time, 504 participants died. The median steps per day across quartiles were 2718 (lowest), 4363, 5905, and 8442 (highest). The corresponding quartile hazard ratios (HRs) associated with mortality adjusted for confounders were 1.00 (reference; lowest quartile), 0.59 (95% confidence interval [CI], 0.47-0.75), 0.54 (95% CI, 0.41-0.72), and 0.42 (95% CI, 0.30-0.60; highest quartile), respectively (P < 0.01). A higher mean step count per day, up to approximately 7500 steps/day, corresponded with progressive and steady decline in mortality HRs using spline analyses. Similar results were observed using sensitivity analyses that minimized reverse causation bias. While the adjusted analysis of measures of stepping intensity showed an inverse association with mortality rates, these associations were no longer significant after accounting for steps per day. Specifically, adjusted HRs comparing highest to lowest quartile were 0.87 (95% CI, 0.68-1.11) for peak 1-minute cadence; 0.86 (95% CI, 0.65-1.13) for peak 30-minute cadence; 0.80 (95% CI, 0.62-1.05) for maximum 5-minute cadence; and 1.27 (95% CI, 0.96-1.68) for time spent at a stepping rate of ≥ 40 steps/minute.
Conclusion. Older women who took approximately 4400 steps per day had lower all-cause mortality rates during a follow-up period of 4.3 years compared to those who took approximately 2700 steps each day. Progressive reduction in mortality rates was associated with increased steps per day before leveling at about 7500 steps/day. Stepping intensity, when accounting for number of steps taken per day, was not associated with reduction in mortality rates in older women.
Commentary
The health and mortality benefits of exercise are well recognized. The 2018 Department of Health and Human Services Physical Activity Guidelines (DHHS-PAG) recommend that adults should do at least 150 to 300 minutes of moderate-intensity aerobic physical activity per week, or 75 to 150 minutes of vigorous-intensity aerobic physical activity per week, in addition to doing muscle-strengthening activities on 2 or more days a week.1 Importantly, the guidelines emphasize that moving more and sitting less benefit nearly everyone, and note that measures of steps as a metric of ambulation can further promote translation of research into public health recommendations for exercise interventions. Despite this recognition, there is limited information centering on the number of daily steps (step volume) and the intensity of stepping that are needed to achieve optimal health outcomes in older adults. The study reported by Lee and colleagues adds new knowledge regarding the relationship between step volume and intensity and mortality in older women.
To date, only a handful of studies conducted outside of the United States have investigated the association between mortality and objectively measured step volume as determined by pedometer or accelerometer.2-4 While these studies observed that higher step counts are associated with lower mortality rates during follow-up periods of 5 to 10 years, their sample sizes were smaller and the study populations were different from those included in the study reported by Lee and colleagues. For example, the cohort from the United Kingdom included only men,2 and the participants in the Australian study were considerably younger, with a mean age of 59 years.4 In the current study, the largest of its kind thus far, it was observed that older women in the United States who take about 4400 steps a day have a lower mortality rate compared to those who take about 2700 steps a day. Moreover, the benefit of increased step volume on mortality progressively increases until plateauing at about 7500 steps per day. On the other hand, stepping intensity does not appear to lower mortality when step volume is accounted for. These results are important in that they add novel evidence that in older women, a patient population that tends to be sedentary, increased step volume (steps per day) but not stepping intensity (how quickly steps are taken) is associated with a reduction in mortality. Thus, these findings help to better characterize steps as a metric of ambulation in sedentary older adults per DHHS-PAG and add to the evidence necessary to translate this line of research into public health recommendations and programs.
While the health benefit of regular physical activity is well known and has been brought to the foreground with DDHA-PAG, only a small percentage of older adults engage in the recommended amounts and types of exercises. In other words, finding motivation to exercise is hard. Thus, identifying practical methods to facilitate behavioral change that increase and sustain physical activity in sedentary older adults would be essential to promoting health in this population. The use of wearable technologies such as fitness trackers and smartphone apps, devices that are now widely used, has shown promise for measuring and encouraging physical activity. The study by Lee and colleagues adds to this notion and further highlights the potential significance of step volume and mortality benefits in older women. Thus, future research in fitness technology should aim to integrate behavior change techniques (such as goal setting, feedback rewards, and action planning) and physical activity levels in order to improve health outcomes in older adults.5
In this study, the large sample size (> 16,000 participants), high compliance rate of accelerometer use (96% compliance rate), and reliable and continuous data capture (a built-in device feature) provide a large and complete dataset. This dataset, a major strength of the study, allowed the investigators to adequately control for potential confounders of physical activity, such as history of smoking, alcohol use, diet, and self-rated health, and therefore statistically minimize biases that are common in observational studies. However, some limitations inherent to the observational design are noted in this study. For instance, the observed association between step volume and mortality is correlational rather than causal, and a one-time assessment of steps taken over 7 consecutive days (ie, exposure) may not accurately reflect step volume and intensity of study participants over the span of 4.3 years of follow-up. Also, participants of WHS are predominately white, have higher socioeconomic status, and are more physically active than a national sample in the United States; therefore, caution should be exercised when making inferences to the general population.
Applications for Clinical Practice
Increased steps taken each day, up to about 7500 steps per day, is associated with lower mortality in older women. This finding can help inform the discussion when clinicians offer physical activity recommendations to older sedentary patients.
—Fred Ko, MD
Study Overview
Objective. To evaluate the association of number of steps taken per day and stepping intensity with all-cause mortality in older women.
Design. This was a prospective cohort study of US women participating in the Women’s Health Study (WHS). Participants wore an accelerometer device (ActiGraph GT3X+, ActiGraph Corp, Pensacola, FL) on the hip during waking hours for 7 consecutive days between 2011 and 2015. The accelerator data were collected at 30 Hz and aggregated into 60-second, time-stamped epochs. Data from participants who were adherent with wearing devices (defined as ≥ 10 hours/day of wear on ≥ 4 days) were used in an analysis that was conducted between 2018 and 2019. The exposure variables were defined as steps taken per day and measures of stepping intensity (ie, peak 1-minute cadence; peak 30-minute cadence; maximum 5-minute cadence; and time spent at a stepping rate of ≥ 40 steps/minute, reflecting purposeful steps).
Setting and participants. In total, 18,289 women participated in this study. Of these, 17,708 wore and returned their accelerometer devices, and data were downloaded successfully from 17,466 devices. Compliant wearers of the device (≥ 10 hours/day of wear on ≥4 days) included 16,741 participants (96% compliance rate of all downloaded device data).
Main outcome measure. All-cause mortality as ascertained through the National Death Index or confirmed by medical records and death certificates.
Main results. In this cohort of 16,741 women, average age at baseline was 72.0 ± 5.7 years (range, 62 to 101 years) and the mean step count was 5499 per day (median, 5094 steps/day) during the 7-day data capture period between 2011 and 2015. Not taking steps (0 steps/minute) accounted for 51.4% of the recorded time, incidental steps (1 to 39 steps/minute) accounted for 45.5%, and purposeful steps (≥ 40 steps/minute) accounted for 3.1%. The mean follow-up period was 4.3 years; during this time, 504 participants died. The median steps per day across quartiles were 2718 (lowest), 4363, 5905, and 8442 (highest). The corresponding quartile hazard ratios (HRs) associated with mortality adjusted for confounders were 1.00 (reference; lowest quartile), 0.59 (95% confidence interval [CI], 0.47-0.75), 0.54 (95% CI, 0.41-0.72), and 0.42 (95% CI, 0.30-0.60; highest quartile), respectively (P < 0.01). A higher mean step count per day, up to approximately 7500 steps/day, corresponded with progressive and steady decline in mortality HRs using spline analyses. Similar results were observed using sensitivity analyses that minimized reverse causation bias. While the adjusted analysis of measures of stepping intensity showed an inverse association with mortality rates, these associations were no longer significant after accounting for steps per day. Specifically, adjusted HRs comparing highest to lowest quartile were 0.87 (95% CI, 0.68-1.11) for peak 1-minute cadence; 0.86 (95% CI, 0.65-1.13) for peak 30-minute cadence; 0.80 (95% CI, 0.62-1.05) for maximum 5-minute cadence; and 1.27 (95% CI, 0.96-1.68) for time spent at a stepping rate of ≥ 40 steps/minute.
Conclusion. Older women who took approximately 4400 steps per day had lower all-cause mortality rates during a follow-up period of 4.3 years compared to those who took approximately 2700 steps each day. Progressive reduction in mortality rates was associated with increased steps per day before leveling at about 7500 steps/day. Stepping intensity, when accounting for number of steps taken per day, was not associated with reduction in mortality rates in older women.
Commentary
The health and mortality benefits of exercise are well recognized. The 2018 Department of Health and Human Services Physical Activity Guidelines (DHHS-PAG) recommend that adults should do at least 150 to 300 minutes of moderate-intensity aerobic physical activity per week, or 75 to 150 minutes of vigorous-intensity aerobic physical activity per week, in addition to doing muscle-strengthening activities on 2 or more days a week.1 Importantly, the guidelines emphasize that moving more and sitting less benefit nearly everyone, and note that measures of steps as a metric of ambulation can further promote translation of research into public health recommendations for exercise interventions. Despite this recognition, there is limited information centering on the number of daily steps (step volume) and the intensity of stepping that are needed to achieve optimal health outcomes in older adults. The study reported by Lee and colleagues adds new knowledge regarding the relationship between step volume and intensity and mortality in older women.
To date, only a handful of studies conducted outside of the United States have investigated the association between mortality and objectively measured step volume as determined by pedometer or accelerometer.2-4 While these studies observed that higher step counts are associated with lower mortality rates during follow-up periods of 5 to 10 years, their sample sizes were smaller and the study populations were different from those included in the study reported by Lee and colleagues. For example, the cohort from the United Kingdom included only men,2 and the participants in the Australian study were considerably younger, with a mean age of 59 years.4 In the current study, the largest of its kind thus far, it was observed that older women in the United States who take about 4400 steps a day have a lower mortality rate compared to those who take about 2700 steps a day. Moreover, the benefit of increased step volume on mortality progressively increases until plateauing at about 7500 steps per day. On the other hand, stepping intensity does not appear to lower mortality when step volume is accounted for. These results are important in that they add novel evidence that in older women, a patient population that tends to be sedentary, increased step volume (steps per day) but not stepping intensity (how quickly steps are taken) is associated with a reduction in mortality. Thus, these findings help to better characterize steps as a metric of ambulation in sedentary older adults per DHHS-PAG and add to the evidence necessary to translate this line of research into public health recommendations and programs.
While the health benefit of regular physical activity is well known and has been brought to the foreground with DDHA-PAG, only a small percentage of older adults engage in the recommended amounts and types of exercises. In other words, finding motivation to exercise is hard. Thus, identifying practical methods to facilitate behavioral change that increase and sustain physical activity in sedentary older adults would be essential to promoting health in this population. The use of wearable technologies such as fitness trackers and smartphone apps, devices that are now widely used, has shown promise for measuring and encouraging physical activity. The study by Lee and colleagues adds to this notion and further highlights the potential significance of step volume and mortality benefits in older women. Thus, future research in fitness technology should aim to integrate behavior change techniques (such as goal setting, feedback rewards, and action planning) and physical activity levels in order to improve health outcomes in older adults.5
In this study, the large sample size (> 16,000 participants), high compliance rate of accelerometer use (96% compliance rate), and reliable and continuous data capture (a built-in device feature) provide a large and complete dataset. This dataset, a major strength of the study, allowed the investigators to adequately control for potential confounders of physical activity, such as history of smoking, alcohol use, diet, and self-rated health, and therefore statistically minimize biases that are common in observational studies. However, some limitations inherent to the observational design are noted in this study. For instance, the observed association between step volume and mortality is correlational rather than causal, and a one-time assessment of steps taken over 7 consecutive days (ie, exposure) may not accurately reflect step volume and intensity of study participants over the span of 4.3 years of follow-up. Also, participants of WHS are predominately white, have higher socioeconomic status, and are more physically active than a national sample in the United States; therefore, caution should be exercised when making inferences to the general population.
Applications for Clinical Practice
Increased steps taken each day, up to about 7500 steps per day, is associated with lower mortality in older women. This finding can help inform the discussion when clinicians offer physical activity recommendations to older sedentary patients.
—Fred Ko, MD
1. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020-2028.
2. Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53:1013-1020.
3. Yamamoto N, Miyazaki H, Shimada M, et al. Daily step count and all-cause mortality in a sample of Japanese elderly people: a cohort study. BMC Public Health. 2018;18:540.
4. Dwyer T, Pezic A, Sun C, et al. Objectively measured daily steps and subsequent long term all-cause mortality: the Tasped prospective cohort study. PLoS One. 2015;10:e0141274.
5. Sullivan AN, Lachman ME. Behavior change with fitness technology in sedentary adults: a review of the evidence for increasing physical activity. Front Public Health. 2016;4:289.
1. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320:2020-2028.
2. Jefferis BJ, Parsons TJ, Sartini C, et al. Objectively measured physical activity, sedentary behaviour and all-cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53:1013-1020.
3. Yamamoto N, Miyazaki H, Shimada M, et al. Daily step count and all-cause mortality in a sample of Japanese elderly people: a cohort study. BMC Public Health. 2018;18:540.
4. Dwyer T, Pezic A, Sun C, et al. Objectively measured daily steps and subsequent long term all-cause mortality: the Tasped prospective cohort study. PLoS One. 2015;10:e0141274.
5. Sullivan AN, Lachman ME. Behavior change with fitness technology in sedentary adults: a review of the evidence for increasing physical activity. Front Public Health. 2016;4:289.
AUGMENT: Lenalidomide/Rituximab vs Placebo/Rituximab in Relapsed or Refractory Indolent Lymphoma
Study Overview
Objective. To compare the efficacy and safety of lenalidomide in combination with rituximab (known as the R2 regimen) to rituximab plus placebo in patients with relapsed or refractory follicular lymphoma or marginal zone lymphoma (MZL).
Design. Phase 3, multicenter, international, placebo controlled randomized trial.
Setting and participants. 358 patients with rituximab-sensitive relapsed or refractory grade 1-3a follicular lymphoma or MZL.
Intervention. Patients were randomly assigned 1:1 to receive lenalidomide or placebo for 12 cycles plus rituximab once per week for 4 weeks in cycle 1 and day 1 of cycles 2 through 5.
Main outcome measures. The primary endpoint was progression-free survival (PFS) as determined by independent radiology reviewers using intent-to-treat analysis. Secondary end points included overall response rate, complete response rate, duration of response, overall survival, event-free survival, and time to next anti-lymphoma therapy. Time to next chemotherapy treatment and histologic transformation were exploratory endpoints. Responses were assessed by participating investigators and independent reviewers. Computed tomography or magnetic resonance imaging was used to obtain tumor measurements. Positron emission tomography was not used. Complete remissions were confirmed by bone marrow biopsy, as bone marrow involvement is exceedingly common in these lymphomas. Gastrointestinal endoscopy was performed to obtain disease status if there was involvement by lymphoma initially.
Improvement in primary and secondary endpoints as well as extrapolatory endpoints were reported in the R2 group. Primary efficacy analyses were conducted in the intention-to-treat population primary endpoint of PFS at 1-sided α = 0.025 level.
Main results. PFS was significantly improved for patients treated with the R2 regimen compared to those who recieved placebo plus rituximab, with a hazard ratio of 0.46 (95% confidence interval [CI], 0.34-0.62; P < 0.001). Median duration of PFS in the R2 group was 39.4 months (95% CI, 22.9 months to not reached) versus 14.1 months (95% CI, 11.4 to 16.7 months) in the rituximab/placebo group. Overall response in the R2 group was 78% (95% CI, 71%-83%) versus 53% (95% CI, 46%-61%; P < 0.0001) in the rituximab/placebo group, with 34% (95% CI, 27%-41%) versus 18% (95% CI, 13%-25%) of patients achieving complete remission (P = 0.001). There were 15 deaths in the R2 group versus 26 deaths in the rituximab/placebo group. Overall survival data is not mature yet.
Conclusion. The R2 regimen was superior to rituximab and placebo in relapsed or recurrent follicular lymphomas. The regimen’s safety profile was acceptable, with higher events of usual and expected but manageable toxicities in the R2 regimen compared to rituximab/placebo.
Commentary
Nearly half of non-Hodgkins lymphomas (NHLs) diagnosed in the United States are classified as indolent B-cell lymphomas.1 Follicular lymphomas constitute about 50% of all indolent NHLs, while MZLs comprise less than 15%.1 These slowly progressive B-cell lymphomas are currently considered treatable but have very low cure rates. Cure is primarily limited to early stage I/II disease and may be possible in less than half of patients by applying involved-field radiation therapy with curative intent.
More than two thirds of indolent lymphomas present in advanced stages (III-IV). Despite an advanced stage at presentation, initial chemoimmunotherapy can induce complete remission in nearly 60% of patients. Unfortunately, nearly all patients relapse over the next 10 years.2 The wait-and-watch approach is a common strategy, and most patients are administered initial therapy or subsequent lines of therapy if they are symptomatic.2 As such, for the majority of these patients, the goal of therapy is to minimize toxicities, preserve quality of life, treat symptoms, and achieve a long PFS without an attempt to cure. Following each line of therapy, patients often revert to watchful surveillance, sometimes for more than a decade. With additional subsequent lines of therapy, lymphoma tends to get more refractory to treatment.
A median survival of nearly 2 decades has been achieved in advanced follicular lymphomas2,3 and MZL.4 However, wide variation in overall response, duration of response, and survival is reported based on the individual risk profile.
The drug of interest in the present study by Leonard and colleagues, lenalidomide, has immunomodulatory properties and antiproliferative effects, possibly related to its binding of the E3 ligase protein cereblon and subsequent ubiquitination of the transcription factors Aiolos and Ikaros.5 The benefits of combination lenalidomide/rituximab against follicular lymphoma in preclinical settings have been attributed to mechanisms mediated by tumor-infiltrating lymphocytes, natural killer cells, monocytes, and antibody-dependent cell-mediated toxicity.5 The combination has now been studied in first-line and subsequent lines of therapy for follicular lymphoma and MZL.6
RELEVANCE, a phase 3 trial, compared the R2 regimen in the upfront setting in advanced follicular lymphoma with rituximab and chemotherapy combination (including CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone], CVP [cyclophosphamide, vincristine, prednisone], and bendamustine).7 Efficacy outcomes were similar between the comparators and R2 was noninferior. MAGNIFY, a phase 3b trial involving rituximab-sensitive and rituximab-refractory patients with previously treated follicular lymphoma and MZL, demonstrated an overall response rate of 73%, complete response rate of 45%, and median PFS of 36 months in patients who received the R2 regimen and who entered a plan to receive maintenance with rituximab.8
The AUGMENT trial was conducted at 97 centers in the United States and 14 Asian and European countries; it enrolled 358 patients, 82% of whom had a follicular lymphoma, between February 13, 2014 and January 26, 2017. The study was well conducted. The R2 regimen was compared to the often used second-line therapy of rituximab alone, and 1:1 randomization was done with stratification factors of prior rituximab use, marginal versus follicular histology, and time lapse of less than or greater than 2 years since last therapy. A limitation of this study is that it selected individuals with a better prognosis, as the study patients were not rituximab refractory and 57% had received only a single prior therapy.
As observed in other R2 regimen trials in follicular or marginal zone lymphomas, the most common adverse reactions (occurring in at least 20% of patients) were neutropenia, fatigue, and constipation. These were manageable with dose adjustments and interruptions, and, in the opinion of authors, did not take away from the overall benefits seen.
The authors acknowledge that a limitation of this study was a lower assessment of median PFS in both arms by investigators than by independent reviewers. The independent review committee assessed PFS for R2 at 39.4 months, whereas investigators assessed it at 25.4 months. The median PFS benefit remained at 14.1 months by both methods of assessment. This may highlight the differences of radiographic measurements in a central setting versus at individual centers.
Histologic transformation to a higher-grade aggressive lymphoma occurred in 2 patients in the R2 arm and 10 patients in the placebo/rituximab arm. After transformation, 1 patient in the R2 arm and 6 in the placebo plus rituximab arm died. A plausible mechanism for this variation has not been provided. If confirmed across a wider population, this may be one of the most significant benefits of the R2 regimen.
Applications for Clinical Practice
Therapy for relapsed and refractory indolent B-cell lymphomas continues to evolve. While chemotherapy remains an effective option, immunomodulation using non-chemotherapeutic intervention has emerged as an attractive strategy. The AUGMENT trial further solidifies adoption of the non-chemotherapy doublet option of rituximab/lenalidomide based on the premise of immunomodulation. Both the agents have been commercially available for more than a decade and are being used for other indications beyond the study population for this trial.
Based on the AUGMENT and MAGNIFY trials, lenalidomide combined with rituximab was approved by the Food and Drug Administration for use in relapsed and refractory follicular or marginal zone lymphomas soon after the AUGMENT study results were published. The recommended lenalidomide dose for both lymphomas is 20 mg once daily orally on days 1 to 21 of repeated 28-day cycles for up to 12 cycles.
The evidence from this trial has yielded what is likely to be a practice changing regimen, with R2 replacing single-agent rituximab for treating follicular lymphoma in the second line or beyond. The response rates and PFS periods were slightly lower in MZL. R2 offers advantages associated with a chemotherapy-free regimen and improved PFS. Also, in the AUGEMENT trial the secondary and exploratory endpoints of time to next therapy, overall response rates, and overall survival rates were improved in patients treated with R2.
Practitioners may choose lenalidomide plus rituximab over rituximab alone based on the AUGMENT study. When considering this regimen, several points should be kept in mind. A very careful selection of patients would be prudent, considering that the study’s follow-up of less than 4 years is short for a disease with long overall survival rates. The study was not powered to compare overall survival benefit. Also, practitioners are reminded to limit the use of lenalidomide to a maximum of 12 months, with planned interruptions and 8 doses of rituximab, replicating the trial schema. Additionally, as per the clinical trial design, the regimen is not intended for rituximab-refractory patients. Patients with MZL constituted only 18% of the study, and conclusions of superiority in this subgroup were not statistically significant. Lenalidomide is not approved for other indolent B cell lymphoproliferative malignancies, such as small lymphocytic lymphoma and chronic lymphocytic leukemia. The conclusion of the published study abstract suggests acceptable use in recurrent indolent lymphomas, but no such conclusion can be made due to lack of inclusion of all indolent lymphoma subtypes in this study.
Longer-term use of lenalidomide has been associated with a marginally increased risk of secondary hematologic malignancies in patients with multiple myeloma who were prescribed lenalidomide maintenance therapy for up to 2 years following high-dose chemotherapy and autologous hematopoietic stem cell transplant.9 Interestingly, in the AUGMENT study and other trials using lenalidomide/rituximab, no significant increase in secondary hematologic malignancies has been reported. The absence of prior myeloablative chemotherapy and a shorter duration of use (1 year) in this group of patients may be factors in why no additional risk of secondary hematologic malignancies was observed. Longer-term follow-up may be needed to evaluate this risk.
In the R2 arm of this study, 55% patients experienced grades 3 and 4 neutropenia. With a median age of presentation for both follicular lymphoma and MZL of over 60 years, oncologists should remain aware of this potentially fatal complication, especially in the frail, the elderly, and previously treated individuals who may have a high risk of myelosuppression. Clinicians should be prepared to rapidly adopt strategies of dose interruption, dose reduction, and growth factor use, as implemented in the trial. Of note, despite the high rates of severe neutropenia, only 3% of the participants experienced febrile neutropenia, and 71% patients in R2 group and 61% in rituximab group completed planned protocol therapy. Growth factor use was high at 36% in the R2 group, which may have been responsible for a lower incidence of febrile neutropenia.
Increased toxicities of tumor flare, rash, and constipation were observed in the R2 arm. Patients with greater than grade 1 neuropathy were excluded. For those at risk of thromboembolism, prophylactic anticoagulation or antiplatelet therapy was recommended in the trial. Lenalidomide dose was reduced to 10 mg for those with creatinine clearance of 30 to 59 mL/min.
The cost-effectiveness of lenalidomide/rituximab combination has not been fully studied against a sequential approach of using rituximab and lenalidomide for a limited number of cycles. The cost of a Revlimid 10-mg pill may be over $700.10 Costs associated with supportive care due to additional toxicities have not been quantified. For those with cost concerns or lack of insurance coverage, the R2 regimen may be cost prohibitive without financial assistance from charities.
Indolent NHL remains mostly incurable. The R2 approach is still not a curative one, and resources should be directed to investigate a cure for this population. Whenever feasible, participation in a clinical trial should be encouraged. Parameters have not been reported based on prognostic groups, and the study did not identify any biomarkers that may correlate with improved outcome. Perhaps a biomarker-based trial design may be most suitable in explaining the heterogeneity in follicular and marginal zone lymphomas.
—Rakesh Gaur, MD, MPH, FACP, Cancer and Blood Center at Kansas Institute of Medicine, Lenexa, KS
1. Perry AM, Diebold J, Nathwani BN, et al. Classification of non-Hodgkin lymphoma in seven geographic regions around the world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101:1244-1250.
2. Armitage JO, Longo DL. Is watch and wait still acceptable for patients with low-grade follicular lymphoma? Blood. 2016;127:2804-2808.
3. Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: The Stanford University experience. Blood. 2013;122:981-987.
4. Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2013;119:629-638.
5. Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164:811-821.
6. Leonard JP, Jung SH, Johnson J, et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). J Clin Oncol. 2015;33:3635-3640.
7. Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med. 2018;379:934-947.
8. Andorsky DJ, Coleman M, Yacoubeman A, et al. MAGNIFY: Phase IIIb interim analysis of induction R2 followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37 (suppl; abstr 7513).
9. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279-3289.
10. Revlimid prices, coupons and patient assistance programs. www.drugs.com/price-guide/revlimid. Accessed August 27, 2019.
Study Overview
Objective. To compare the efficacy and safety of lenalidomide in combination with rituximab (known as the R2 regimen) to rituximab plus placebo in patients with relapsed or refractory follicular lymphoma or marginal zone lymphoma (MZL).
Design. Phase 3, multicenter, international, placebo controlled randomized trial.
Setting and participants. 358 patients with rituximab-sensitive relapsed or refractory grade 1-3a follicular lymphoma or MZL.
Intervention. Patients were randomly assigned 1:1 to receive lenalidomide or placebo for 12 cycles plus rituximab once per week for 4 weeks in cycle 1 and day 1 of cycles 2 through 5.
Main outcome measures. The primary endpoint was progression-free survival (PFS) as determined by independent radiology reviewers using intent-to-treat analysis. Secondary end points included overall response rate, complete response rate, duration of response, overall survival, event-free survival, and time to next anti-lymphoma therapy. Time to next chemotherapy treatment and histologic transformation were exploratory endpoints. Responses were assessed by participating investigators and independent reviewers. Computed tomography or magnetic resonance imaging was used to obtain tumor measurements. Positron emission tomography was not used. Complete remissions were confirmed by bone marrow biopsy, as bone marrow involvement is exceedingly common in these lymphomas. Gastrointestinal endoscopy was performed to obtain disease status if there was involvement by lymphoma initially.
Improvement in primary and secondary endpoints as well as extrapolatory endpoints were reported in the R2 group. Primary efficacy analyses were conducted in the intention-to-treat population primary endpoint of PFS at 1-sided α = 0.025 level.
Main results. PFS was significantly improved for patients treated with the R2 regimen compared to those who recieved placebo plus rituximab, with a hazard ratio of 0.46 (95% confidence interval [CI], 0.34-0.62; P < 0.001). Median duration of PFS in the R2 group was 39.4 months (95% CI, 22.9 months to not reached) versus 14.1 months (95% CI, 11.4 to 16.7 months) in the rituximab/placebo group. Overall response in the R2 group was 78% (95% CI, 71%-83%) versus 53% (95% CI, 46%-61%; P < 0.0001) in the rituximab/placebo group, with 34% (95% CI, 27%-41%) versus 18% (95% CI, 13%-25%) of patients achieving complete remission (P = 0.001). There were 15 deaths in the R2 group versus 26 deaths in the rituximab/placebo group. Overall survival data is not mature yet.
Conclusion. The R2 regimen was superior to rituximab and placebo in relapsed or recurrent follicular lymphomas. The regimen’s safety profile was acceptable, with higher events of usual and expected but manageable toxicities in the R2 regimen compared to rituximab/placebo.
Commentary
Nearly half of non-Hodgkins lymphomas (NHLs) diagnosed in the United States are classified as indolent B-cell lymphomas.1 Follicular lymphomas constitute about 50% of all indolent NHLs, while MZLs comprise less than 15%.1 These slowly progressive B-cell lymphomas are currently considered treatable but have very low cure rates. Cure is primarily limited to early stage I/II disease and may be possible in less than half of patients by applying involved-field radiation therapy with curative intent.
More than two thirds of indolent lymphomas present in advanced stages (III-IV). Despite an advanced stage at presentation, initial chemoimmunotherapy can induce complete remission in nearly 60% of patients. Unfortunately, nearly all patients relapse over the next 10 years.2 The wait-and-watch approach is a common strategy, and most patients are administered initial therapy or subsequent lines of therapy if they are symptomatic.2 As such, for the majority of these patients, the goal of therapy is to minimize toxicities, preserve quality of life, treat symptoms, and achieve a long PFS without an attempt to cure. Following each line of therapy, patients often revert to watchful surveillance, sometimes for more than a decade. With additional subsequent lines of therapy, lymphoma tends to get more refractory to treatment.
A median survival of nearly 2 decades has been achieved in advanced follicular lymphomas2,3 and MZL.4 However, wide variation in overall response, duration of response, and survival is reported based on the individual risk profile.
The drug of interest in the present study by Leonard and colleagues, lenalidomide, has immunomodulatory properties and antiproliferative effects, possibly related to its binding of the E3 ligase protein cereblon and subsequent ubiquitination of the transcription factors Aiolos and Ikaros.5 The benefits of combination lenalidomide/rituximab against follicular lymphoma in preclinical settings have been attributed to mechanisms mediated by tumor-infiltrating lymphocytes, natural killer cells, monocytes, and antibody-dependent cell-mediated toxicity.5 The combination has now been studied in first-line and subsequent lines of therapy for follicular lymphoma and MZL.6
RELEVANCE, a phase 3 trial, compared the R2 regimen in the upfront setting in advanced follicular lymphoma with rituximab and chemotherapy combination (including CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone], CVP [cyclophosphamide, vincristine, prednisone], and bendamustine).7 Efficacy outcomes were similar between the comparators and R2 was noninferior. MAGNIFY, a phase 3b trial involving rituximab-sensitive and rituximab-refractory patients with previously treated follicular lymphoma and MZL, demonstrated an overall response rate of 73%, complete response rate of 45%, and median PFS of 36 months in patients who received the R2 regimen and who entered a plan to receive maintenance with rituximab.8
The AUGMENT trial was conducted at 97 centers in the United States and 14 Asian and European countries; it enrolled 358 patients, 82% of whom had a follicular lymphoma, between February 13, 2014 and January 26, 2017. The study was well conducted. The R2 regimen was compared to the often used second-line therapy of rituximab alone, and 1:1 randomization was done with stratification factors of prior rituximab use, marginal versus follicular histology, and time lapse of less than or greater than 2 years since last therapy. A limitation of this study is that it selected individuals with a better prognosis, as the study patients were not rituximab refractory and 57% had received only a single prior therapy.
As observed in other R2 regimen trials in follicular or marginal zone lymphomas, the most common adverse reactions (occurring in at least 20% of patients) were neutropenia, fatigue, and constipation. These were manageable with dose adjustments and interruptions, and, in the opinion of authors, did not take away from the overall benefits seen.
The authors acknowledge that a limitation of this study was a lower assessment of median PFS in both arms by investigators than by independent reviewers. The independent review committee assessed PFS for R2 at 39.4 months, whereas investigators assessed it at 25.4 months. The median PFS benefit remained at 14.1 months by both methods of assessment. This may highlight the differences of radiographic measurements in a central setting versus at individual centers.
Histologic transformation to a higher-grade aggressive lymphoma occurred in 2 patients in the R2 arm and 10 patients in the placebo/rituximab arm. After transformation, 1 patient in the R2 arm and 6 in the placebo plus rituximab arm died. A plausible mechanism for this variation has not been provided. If confirmed across a wider population, this may be one of the most significant benefits of the R2 regimen.
Applications for Clinical Practice
Therapy for relapsed and refractory indolent B-cell lymphomas continues to evolve. While chemotherapy remains an effective option, immunomodulation using non-chemotherapeutic intervention has emerged as an attractive strategy. The AUGMENT trial further solidifies adoption of the non-chemotherapy doublet option of rituximab/lenalidomide based on the premise of immunomodulation. Both the agents have been commercially available for more than a decade and are being used for other indications beyond the study population for this trial.
Based on the AUGMENT and MAGNIFY trials, lenalidomide combined with rituximab was approved by the Food and Drug Administration for use in relapsed and refractory follicular or marginal zone lymphomas soon after the AUGMENT study results were published. The recommended lenalidomide dose for both lymphomas is 20 mg once daily orally on days 1 to 21 of repeated 28-day cycles for up to 12 cycles.
The evidence from this trial has yielded what is likely to be a practice changing regimen, with R2 replacing single-agent rituximab for treating follicular lymphoma in the second line or beyond. The response rates and PFS periods were slightly lower in MZL. R2 offers advantages associated with a chemotherapy-free regimen and improved PFS. Also, in the AUGEMENT trial the secondary and exploratory endpoints of time to next therapy, overall response rates, and overall survival rates were improved in patients treated with R2.
Practitioners may choose lenalidomide plus rituximab over rituximab alone based on the AUGMENT study. When considering this regimen, several points should be kept in mind. A very careful selection of patients would be prudent, considering that the study’s follow-up of less than 4 years is short for a disease with long overall survival rates. The study was not powered to compare overall survival benefit. Also, practitioners are reminded to limit the use of lenalidomide to a maximum of 12 months, with planned interruptions and 8 doses of rituximab, replicating the trial schema. Additionally, as per the clinical trial design, the regimen is not intended for rituximab-refractory patients. Patients with MZL constituted only 18% of the study, and conclusions of superiority in this subgroup were not statistically significant. Lenalidomide is not approved for other indolent B cell lymphoproliferative malignancies, such as small lymphocytic lymphoma and chronic lymphocytic leukemia. The conclusion of the published study abstract suggests acceptable use in recurrent indolent lymphomas, but no such conclusion can be made due to lack of inclusion of all indolent lymphoma subtypes in this study.
Longer-term use of lenalidomide has been associated with a marginally increased risk of secondary hematologic malignancies in patients with multiple myeloma who were prescribed lenalidomide maintenance therapy for up to 2 years following high-dose chemotherapy and autologous hematopoietic stem cell transplant.9 Interestingly, in the AUGMENT study and other trials using lenalidomide/rituximab, no significant increase in secondary hematologic malignancies has been reported. The absence of prior myeloablative chemotherapy and a shorter duration of use (1 year) in this group of patients may be factors in why no additional risk of secondary hematologic malignancies was observed. Longer-term follow-up may be needed to evaluate this risk.
In the R2 arm of this study, 55% patients experienced grades 3 and 4 neutropenia. With a median age of presentation for both follicular lymphoma and MZL of over 60 years, oncologists should remain aware of this potentially fatal complication, especially in the frail, the elderly, and previously treated individuals who may have a high risk of myelosuppression. Clinicians should be prepared to rapidly adopt strategies of dose interruption, dose reduction, and growth factor use, as implemented in the trial. Of note, despite the high rates of severe neutropenia, only 3% of the participants experienced febrile neutropenia, and 71% patients in R2 group and 61% in rituximab group completed planned protocol therapy. Growth factor use was high at 36% in the R2 group, which may have been responsible for a lower incidence of febrile neutropenia.
Increased toxicities of tumor flare, rash, and constipation were observed in the R2 arm. Patients with greater than grade 1 neuropathy were excluded. For those at risk of thromboembolism, prophylactic anticoagulation or antiplatelet therapy was recommended in the trial. Lenalidomide dose was reduced to 10 mg for those with creatinine clearance of 30 to 59 mL/min.
The cost-effectiveness of lenalidomide/rituximab combination has not been fully studied against a sequential approach of using rituximab and lenalidomide for a limited number of cycles. The cost of a Revlimid 10-mg pill may be over $700.10 Costs associated with supportive care due to additional toxicities have not been quantified. For those with cost concerns or lack of insurance coverage, the R2 regimen may be cost prohibitive without financial assistance from charities.
Indolent NHL remains mostly incurable. The R2 approach is still not a curative one, and resources should be directed to investigate a cure for this population. Whenever feasible, participation in a clinical trial should be encouraged. Parameters have not been reported based on prognostic groups, and the study did not identify any biomarkers that may correlate with improved outcome. Perhaps a biomarker-based trial design may be most suitable in explaining the heterogeneity in follicular and marginal zone lymphomas.
—Rakesh Gaur, MD, MPH, FACP, Cancer and Blood Center at Kansas Institute of Medicine, Lenexa, KS
Study Overview
Objective. To compare the efficacy and safety of lenalidomide in combination with rituximab (known as the R2 regimen) to rituximab plus placebo in patients with relapsed or refractory follicular lymphoma or marginal zone lymphoma (MZL).
Design. Phase 3, multicenter, international, placebo controlled randomized trial.
Setting and participants. 358 patients with rituximab-sensitive relapsed or refractory grade 1-3a follicular lymphoma or MZL.
Intervention. Patients were randomly assigned 1:1 to receive lenalidomide or placebo for 12 cycles plus rituximab once per week for 4 weeks in cycle 1 and day 1 of cycles 2 through 5.
Main outcome measures. The primary endpoint was progression-free survival (PFS) as determined by independent radiology reviewers using intent-to-treat analysis. Secondary end points included overall response rate, complete response rate, duration of response, overall survival, event-free survival, and time to next anti-lymphoma therapy. Time to next chemotherapy treatment and histologic transformation were exploratory endpoints. Responses were assessed by participating investigators and independent reviewers. Computed tomography or magnetic resonance imaging was used to obtain tumor measurements. Positron emission tomography was not used. Complete remissions were confirmed by bone marrow biopsy, as bone marrow involvement is exceedingly common in these lymphomas. Gastrointestinal endoscopy was performed to obtain disease status if there was involvement by lymphoma initially.
Improvement in primary and secondary endpoints as well as extrapolatory endpoints were reported in the R2 group. Primary efficacy analyses were conducted in the intention-to-treat population primary endpoint of PFS at 1-sided α = 0.025 level.
Main results. PFS was significantly improved for patients treated with the R2 regimen compared to those who recieved placebo plus rituximab, with a hazard ratio of 0.46 (95% confidence interval [CI], 0.34-0.62; P < 0.001). Median duration of PFS in the R2 group was 39.4 months (95% CI, 22.9 months to not reached) versus 14.1 months (95% CI, 11.4 to 16.7 months) in the rituximab/placebo group. Overall response in the R2 group was 78% (95% CI, 71%-83%) versus 53% (95% CI, 46%-61%; P < 0.0001) in the rituximab/placebo group, with 34% (95% CI, 27%-41%) versus 18% (95% CI, 13%-25%) of patients achieving complete remission (P = 0.001). There were 15 deaths in the R2 group versus 26 deaths in the rituximab/placebo group. Overall survival data is not mature yet.
Conclusion. The R2 regimen was superior to rituximab and placebo in relapsed or recurrent follicular lymphomas. The regimen’s safety profile was acceptable, with higher events of usual and expected but manageable toxicities in the R2 regimen compared to rituximab/placebo.
Commentary
Nearly half of non-Hodgkins lymphomas (NHLs) diagnosed in the United States are classified as indolent B-cell lymphomas.1 Follicular lymphomas constitute about 50% of all indolent NHLs, while MZLs comprise less than 15%.1 These slowly progressive B-cell lymphomas are currently considered treatable but have very low cure rates. Cure is primarily limited to early stage I/II disease and may be possible in less than half of patients by applying involved-field radiation therapy with curative intent.
More than two thirds of indolent lymphomas present in advanced stages (III-IV). Despite an advanced stage at presentation, initial chemoimmunotherapy can induce complete remission in nearly 60% of patients. Unfortunately, nearly all patients relapse over the next 10 years.2 The wait-and-watch approach is a common strategy, and most patients are administered initial therapy or subsequent lines of therapy if they are symptomatic.2 As such, for the majority of these patients, the goal of therapy is to minimize toxicities, preserve quality of life, treat symptoms, and achieve a long PFS without an attempt to cure. Following each line of therapy, patients often revert to watchful surveillance, sometimes for more than a decade. With additional subsequent lines of therapy, lymphoma tends to get more refractory to treatment.
A median survival of nearly 2 decades has been achieved in advanced follicular lymphomas2,3 and MZL.4 However, wide variation in overall response, duration of response, and survival is reported based on the individual risk profile.
The drug of interest in the present study by Leonard and colleagues, lenalidomide, has immunomodulatory properties and antiproliferative effects, possibly related to its binding of the E3 ligase protein cereblon and subsequent ubiquitination of the transcription factors Aiolos and Ikaros.5 The benefits of combination lenalidomide/rituximab against follicular lymphoma in preclinical settings have been attributed to mechanisms mediated by tumor-infiltrating lymphocytes, natural killer cells, monocytes, and antibody-dependent cell-mediated toxicity.5 The combination has now been studied in first-line and subsequent lines of therapy for follicular lymphoma and MZL.6
RELEVANCE, a phase 3 trial, compared the R2 regimen in the upfront setting in advanced follicular lymphoma with rituximab and chemotherapy combination (including CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone], CVP [cyclophosphamide, vincristine, prednisone], and bendamustine).7 Efficacy outcomes were similar between the comparators and R2 was noninferior. MAGNIFY, a phase 3b trial involving rituximab-sensitive and rituximab-refractory patients with previously treated follicular lymphoma and MZL, demonstrated an overall response rate of 73%, complete response rate of 45%, and median PFS of 36 months in patients who received the R2 regimen and who entered a plan to receive maintenance with rituximab.8
The AUGMENT trial was conducted at 97 centers in the United States and 14 Asian and European countries; it enrolled 358 patients, 82% of whom had a follicular lymphoma, between February 13, 2014 and January 26, 2017. The study was well conducted. The R2 regimen was compared to the often used second-line therapy of rituximab alone, and 1:1 randomization was done with stratification factors of prior rituximab use, marginal versus follicular histology, and time lapse of less than or greater than 2 years since last therapy. A limitation of this study is that it selected individuals with a better prognosis, as the study patients were not rituximab refractory and 57% had received only a single prior therapy.
As observed in other R2 regimen trials in follicular or marginal zone lymphomas, the most common adverse reactions (occurring in at least 20% of patients) were neutropenia, fatigue, and constipation. These were manageable with dose adjustments and interruptions, and, in the opinion of authors, did not take away from the overall benefits seen.
The authors acknowledge that a limitation of this study was a lower assessment of median PFS in both arms by investigators than by independent reviewers. The independent review committee assessed PFS for R2 at 39.4 months, whereas investigators assessed it at 25.4 months. The median PFS benefit remained at 14.1 months by both methods of assessment. This may highlight the differences of radiographic measurements in a central setting versus at individual centers.
Histologic transformation to a higher-grade aggressive lymphoma occurred in 2 patients in the R2 arm and 10 patients in the placebo/rituximab arm. After transformation, 1 patient in the R2 arm and 6 in the placebo plus rituximab arm died. A plausible mechanism for this variation has not been provided. If confirmed across a wider population, this may be one of the most significant benefits of the R2 regimen.
Applications for Clinical Practice
Therapy for relapsed and refractory indolent B-cell lymphomas continues to evolve. While chemotherapy remains an effective option, immunomodulation using non-chemotherapeutic intervention has emerged as an attractive strategy. The AUGMENT trial further solidifies adoption of the non-chemotherapy doublet option of rituximab/lenalidomide based on the premise of immunomodulation. Both the agents have been commercially available for more than a decade and are being used for other indications beyond the study population for this trial.
Based on the AUGMENT and MAGNIFY trials, lenalidomide combined with rituximab was approved by the Food and Drug Administration for use in relapsed and refractory follicular or marginal zone lymphomas soon after the AUGMENT study results were published. The recommended lenalidomide dose for both lymphomas is 20 mg once daily orally on days 1 to 21 of repeated 28-day cycles for up to 12 cycles.
The evidence from this trial has yielded what is likely to be a practice changing regimen, with R2 replacing single-agent rituximab for treating follicular lymphoma in the second line or beyond. The response rates and PFS periods were slightly lower in MZL. R2 offers advantages associated with a chemotherapy-free regimen and improved PFS. Also, in the AUGEMENT trial the secondary and exploratory endpoints of time to next therapy, overall response rates, and overall survival rates were improved in patients treated with R2.
Practitioners may choose lenalidomide plus rituximab over rituximab alone based on the AUGMENT study. When considering this regimen, several points should be kept in mind. A very careful selection of patients would be prudent, considering that the study’s follow-up of less than 4 years is short for a disease with long overall survival rates. The study was not powered to compare overall survival benefit. Also, practitioners are reminded to limit the use of lenalidomide to a maximum of 12 months, with planned interruptions and 8 doses of rituximab, replicating the trial schema. Additionally, as per the clinical trial design, the regimen is not intended for rituximab-refractory patients. Patients with MZL constituted only 18% of the study, and conclusions of superiority in this subgroup were not statistically significant. Lenalidomide is not approved for other indolent B cell lymphoproliferative malignancies, such as small lymphocytic lymphoma and chronic lymphocytic leukemia. The conclusion of the published study abstract suggests acceptable use in recurrent indolent lymphomas, but no such conclusion can be made due to lack of inclusion of all indolent lymphoma subtypes in this study.
Longer-term use of lenalidomide has been associated with a marginally increased risk of secondary hematologic malignancies in patients with multiple myeloma who were prescribed lenalidomide maintenance therapy for up to 2 years following high-dose chemotherapy and autologous hematopoietic stem cell transplant.9 Interestingly, in the AUGMENT study and other trials using lenalidomide/rituximab, no significant increase in secondary hematologic malignancies has been reported. The absence of prior myeloablative chemotherapy and a shorter duration of use (1 year) in this group of patients may be factors in why no additional risk of secondary hematologic malignancies was observed. Longer-term follow-up may be needed to evaluate this risk.
In the R2 arm of this study, 55% patients experienced grades 3 and 4 neutropenia. With a median age of presentation for both follicular lymphoma and MZL of over 60 years, oncologists should remain aware of this potentially fatal complication, especially in the frail, the elderly, and previously treated individuals who may have a high risk of myelosuppression. Clinicians should be prepared to rapidly adopt strategies of dose interruption, dose reduction, and growth factor use, as implemented in the trial. Of note, despite the high rates of severe neutropenia, only 3% of the participants experienced febrile neutropenia, and 71% patients in R2 group and 61% in rituximab group completed planned protocol therapy. Growth factor use was high at 36% in the R2 group, which may have been responsible for a lower incidence of febrile neutropenia.
Increased toxicities of tumor flare, rash, and constipation were observed in the R2 arm. Patients with greater than grade 1 neuropathy were excluded. For those at risk of thromboembolism, prophylactic anticoagulation or antiplatelet therapy was recommended in the trial. Lenalidomide dose was reduced to 10 mg for those with creatinine clearance of 30 to 59 mL/min.
The cost-effectiveness of lenalidomide/rituximab combination has not been fully studied against a sequential approach of using rituximab and lenalidomide for a limited number of cycles. The cost of a Revlimid 10-mg pill may be over $700.10 Costs associated with supportive care due to additional toxicities have not been quantified. For those with cost concerns or lack of insurance coverage, the R2 regimen may be cost prohibitive without financial assistance from charities.
Indolent NHL remains mostly incurable. The R2 approach is still not a curative one, and resources should be directed to investigate a cure for this population. Whenever feasible, participation in a clinical trial should be encouraged. Parameters have not been reported based on prognostic groups, and the study did not identify any biomarkers that may correlate with improved outcome. Perhaps a biomarker-based trial design may be most suitable in explaining the heterogeneity in follicular and marginal zone lymphomas.
—Rakesh Gaur, MD, MPH, FACP, Cancer and Blood Center at Kansas Institute of Medicine, Lenexa, KS
1. Perry AM, Diebold J, Nathwani BN, et al. Classification of non-Hodgkin lymphoma in seven geographic regions around the world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101:1244-1250.
2. Armitage JO, Longo DL. Is watch and wait still acceptable for patients with low-grade follicular lymphoma? Blood. 2016;127:2804-2808.
3. Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: The Stanford University experience. Blood. 2013;122:981-987.
4. Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2013;119:629-638.
5. Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164:811-821.
6. Leonard JP, Jung SH, Johnson J, et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). J Clin Oncol. 2015;33:3635-3640.
7. Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med. 2018;379:934-947.
8. Andorsky DJ, Coleman M, Yacoubeman A, et al. MAGNIFY: Phase IIIb interim analysis of induction R2 followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37 (suppl; abstr 7513).
9. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279-3289.
10. Revlimid prices, coupons and patient assistance programs. www.drugs.com/price-guide/revlimid. Accessed August 27, 2019.
1. Perry AM, Diebold J, Nathwani BN, et al. Classification of non-Hodgkin lymphoma in seven geographic regions around the world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101:1244-1250.
2. Armitage JO, Longo DL. Is watch and wait still acceptable for patients with low-grade follicular lymphoma? Blood. 2016;127:2804-2808.
3. Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: The Stanford University experience. Blood. 2013;122:981-987.
4. Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2013;119:629-638.
5. Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164:811-821.
6. Leonard JP, Jung SH, Johnson J, et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). J Clin Oncol. 2015;33:3635-3640.
7. Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med. 2018;379:934-947.
8. Andorsky DJ, Coleman M, Yacoubeman A, et al. MAGNIFY: Phase IIIb interim analysis of induction R2 followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37 (suppl; abstr 7513).
9. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279-3289.
10. Revlimid prices, coupons and patient assistance programs. www.drugs.com/price-guide/revlimid. Accessed August 27, 2019.
Malpractice risk: Focus on care, documentation
SAN DIEGO – Mental health professionals can lower the risk of legal exposure from patients filing malpractice suits through proper assessment and proper documentation, a psychiatrist said at the annual Psych Congress.
“You don’t have to have a perfect performance, but you do have to gather relevant data. And you’ll be judged on whether you took reasonable precautions once you identified the risk,” said Phillip J. Resnick, MD, professor of psychiatry at Case Western Reserve University, Cleveland.
Research suggests that 35% of malpractice claims involve incorrect treatment and 19% involve medication problems. More than half of all U.S. physicians reportedly have been sued, and psychiatrists are named in lawsuits less often than other medical specialists.
What’s “reasonable” in terms of assessment and precautions? In the past, courts may have held to a community standard: Did you provide the usual care that others in your region would provide? This approach allowed physicians to avoid responsibility if “you could prove that the majority of [local] doctors do what you did,” Dr. Resnick said, even if they’re “sloppy and bad.”
A newer, “reasonably prudent practitioner” standard expects medical professionals to do the right thing no matter where they live. who has consulted on numerous well-known cases, including that of Jeffrey Dahmer, Susan Smith, Timothy McVey, and Theodore Kaczynski.
Psychiatrists can be vulnerable legally if they refuse to respond to concerns from family members, Dr. Resnick said. The best approach is to listen. “You don’t need permission to listen – only to release information. You can always listen.”
Dr. Resnick is a former president of the American Academy of Psychiatry and the Law. He has no disclosures.
SAN DIEGO – Mental health professionals can lower the risk of legal exposure from patients filing malpractice suits through proper assessment and proper documentation, a psychiatrist said at the annual Psych Congress.
“You don’t have to have a perfect performance, but you do have to gather relevant data. And you’ll be judged on whether you took reasonable precautions once you identified the risk,” said Phillip J. Resnick, MD, professor of psychiatry at Case Western Reserve University, Cleveland.
Research suggests that 35% of malpractice claims involve incorrect treatment and 19% involve medication problems. More than half of all U.S. physicians reportedly have been sued, and psychiatrists are named in lawsuits less often than other medical specialists.
What’s “reasonable” in terms of assessment and precautions? In the past, courts may have held to a community standard: Did you provide the usual care that others in your region would provide? This approach allowed physicians to avoid responsibility if “you could prove that the majority of [local] doctors do what you did,” Dr. Resnick said, even if they’re “sloppy and bad.”
A newer, “reasonably prudent practitioner” standard expects medical professionals to do the right thing no matter where they live. who has consulted on numerous well-known cases, including that of Jeffrey Dahmer, Susan Smith, Timothy McVey, and Theodore Kaczynski.
Psychiatrists can be vulnerable legally if they refuse to respond to concerns from family members, Dr. Resnick said. The best approach is to listen. “You don’t need permission to listen – only to release information. You can always listen.”
Dr. Resnick is a former president of the American Academy of Psychiatry and the Law. He has no disclosures.
SAN DIEGO – Mental health professionals can lower the risk of legal exposure from patients filing malpractice suits through proper assessment and proper documentation, a psychiatrist said at the annual Psych Congress.
“You don’t have to have a perfect performance, but you do have to gather relevant data. And you’ll be judged on whether you took reasonable precautions once you identified the risk,” said Phillip J. Resnick, MD, professor of psychiatry at Case Western Reserve University, Cleveland.
Research suggests that 35% of malpractice claims involve incorrect treatment and 19% involve medication problems. More than half of all U.S. physicians reportedly have been sued, and psychiatrists are named in lawsuits less often than other medical specialists.
What’s “reasonable” in terms of assessment and precautions? In the past, courts may have held to a community standard: Did you provide the usual care that others in your region would provide? This approach allowed physicians to avoid responsibility if “you could prove that the majority of [local] doctors do what you did,” Dr. Resnick said, even if they’re “sloppy and bad.”
A newer, “reasonably prudent practitioner” standard expects medical professionals to do the right thing no matter where they live. who has consulted on numerous well-known cases, including that of Jeffrey Dahmer, Susan Smith, Timothy McVey, and Theodore Kaczynski.
Psychiatrists can be vulnerable legally if they refuse to respond to concerns from family members, Dr. Resnick said. The best approach is to listen. “You don’t need permission to listen – only to release information. You can always listen.”
Dr. Resnick is a former president of the American Academy of Psychiatry and the Law. He has no disclosures.
REPORTING FROM PSYCH CONGRESS 2019