User login
Asthma attacks account for 1.8 million emergency department (ED) visits each year in the US and for 10 deaths daily.1 Management of asthma attacks includes administration of inhaled ß2 adrenergic agonists, inhaled anticholinergic agents, IV magnesium sulfate, and corticosteroids.2 Status asthmaticus is an intense acute exacerbation of asthma that does not respond to repeated treatments of bronchodilators and corticosteroids.3 It is a medical emergency requiring immediate recognition and treatment. The decision to intubate a patient with status asthmaticus is a clinical decision based on work of breathing, respiratory acidosis, and failure to respond to medical interventions.
In refractory cases of status asthmaticus, intubation and mechanical ventilation are undertaken to provide oxygenation and ventilation until the bronchospasm resolves. However, mechanical ventilation is associated with significant risks, including high end-inspiratory pressures, barotrauma, and volutrauma.4 Rescue therapies include muscle relaxation, infusion of ketamine (central acting nonopioid analgesic with bronchodilatory properties), heliox, and general anesthesia.2,4 We report a case of a patient with life-threatening asthma and status asthmaticus treated with sevoflurane general anesthesia.
Case Presentation
A 55-year-old woman whose medical history was notable for asthma, psoriasis, hypothyroidism, tobacco, and alcohol abuse, and posttraumatic stress disorder (PTSD) presented to the ED. The patient had rarely sought medical attention and had no prior ED visits or hospitalizations in the electronic health record. Her home regimen included an albuterol inhaler used as needed. Her family reported that they had found her in distress in bed in a tripod position, unable to speak and struggling to breath.
Emergency medical services found the patient cyanotic, apneic, and pulseless. She received cardiopulmonary resuscitation for 30 seconds and 1-mg IV epinephrine, and spontaneous circulation returned. The patient arrived in the ED with an oral airway in place receiving bag valve mask ventilation. The patient expelled the oral airway. She was unable to speak due to dyspnea, exhibited persistent cyanosis, fatigue due to work of breathing, and failed to respond to nebulized albuterol/ipratropium bromide, IV methylprednisolone, and magnesium sulfate. The patient met criteria for acute severe asthma, or status asthmaticus. Thus, the patient received rapid sequence induction with rocuronium and ketamine and was intubated.
According to her family, the patient had no previous intensive care unit (ICU) admissions or prior intubations. Her only asthma medication was an albuterol inhaler as needed. The patient worked as a supervisor at a window blind manufacturing company. She lived alone, smoked 2 packs of cigarettes a day for more than 30 years, had no pets, drank unknown quantities of beer, wine, and hard liquor daily, and had smoked marijuana for several years.
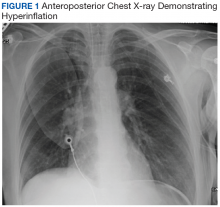
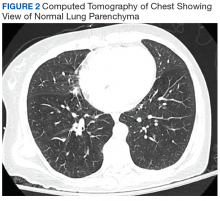
The patient’s physical examination was notable for diffuse expiratory wheezes. Laboratory analysis revealed white blood cell count of 13.7 k/mcL, sodium 140 mmol/L, potassium 4.9 mmol/L, chloride 105 mmol/L, CO2 17 mmol/L, creatinine 0.98 mg/dL, troponin 0.03 ng/mL, lactate 7.2 mmol/L. Her chest X-ray showed hyperinflation but no focal opacities, pneumothorax, or pulmonary edema. Her endotracheal tube was in good position (Figure 1). A computed tomography pulmonary angiogram showed no pulmonary embolus or emphysema. There were atelectatic changes in the dependent portion of the right lower lobe, central bronchial wall thickening, and no stigmata of air trapping (Figure 2). An echocardiogram revealed a left ventricular ejection fraction of 45%, normal right ventricle and right ventricular size and function with an estimated right ventricular systolic pressure of 40 mm Hg.
The patient was admitted to the ICU and started on continuous infusion cisatracurium for paralysis and deep sedation to improve ventilatory synchrony and decrease auto positive end-expiratory pressure (PEEP). Mechanical ventilation was initiated with volume-cycled assist control ventilation, 6 mL/kg/ideal body weight (IBW) at 5-cm H2O PEEP, and 1 minute ventilation of 10 liters. The patient had severe air trapping and high airway pressures. The dynamic PEEP was 22-cm H2O (normal PEEP of 5-cm H2O), peak airway pressure (PAP) 41-cm H2O, and plateau pressure 31-cm H2O. In addition, the arterial blood gas (ABG) showed severe hypercapnic respiratory acidosis without significant hypoxemia with pH 7.15, PaCO2 90 mm Hg, and PaO2 150 mm Hg.
Pressure controlled ventilation was attempted unsuccessfully due to high airway resistance. Ultimately, the patient was set on volume control with low tidal volume, 6 mL/kg/IBW, high flow 90 L/min, PEEP 0 cm of H2O, and a low respiratory rate of 10 to achieve an inspiratory to expiratory (I:E) ratio of 1:7. Managing the ventilator to avoid dynamic hyperinflation and auto-PEEP, she remained relatively stable and improved.
By day 4 the patient’s ventilator was set to volume assist control with respiratory rate of 16, tidal volume, 6 mL/kg/IBW, PEEP 5-cm H2O with auto PEEP of 3-cm H2O, and fraction of inspired ABG O2 (FiO2) 0.35 with PAP of 46-cm H2O and plateau pressure of 17-cm H2O. The ABG was pH 7.32, PaCO2 65 mm Hg, and PaO2 74 mm Hg. However, on hospital day 5, she developed worsening PAP 60 to 77-cm H2O, plateau pressures 17-cm H2O, and a dynamic PEEP 16-cm H2O and was unresponsive to ventilator maneuvers to lower airway pressures and improve ventilation.
The patient had been receiving continuous albuterol and ipratropium nebulizer treatments. Ketamine infusion was considered fraught with potential for a dissociative reaction due to the patient’s significant PTSD. The patient’s family requested avoidance of ketamine infusion since the patient was paralyzed and psychiatric effects could not be monitored. Heliox 80/20 mixture was considered; however, it is incompatible with the ventilator that was being used since it could not account for the density of the helium gas flow in the tidal volumes. Extracorporeal membrane oxygenation (ECMO) was not available at our facility, and the patient was not a candidate for the regional ECMO center.
On hospital day 8, the patient developed worsening respiratory acidosis. The patient’s PAP increased to > 77-cm H2O, and her ABG revealed pH 7.22, PaCO2 90 mm Hg, and PaO2 77 mm Hg with FiO2 0.4. A chest X-ray demonstrated a new left lower lobe infiltrate. Fiber optic bronchoscopy was notable for scattered thick secretions throughout both lungs without obstructing mucus plug. Removal of airway secretions did not improve airway pressures or dynamic hyperinflation.
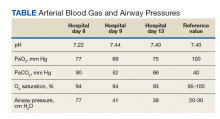
After consultation and discussion with the chief of anesthesia, the patient was placed on an anesthesia ventilator and started on sevoflurane 1.5% in the ICU. Anesthesiology was available 24 hours a day, and the anesthesiologist rounded with the intensivist frequently for this patient. The anesthesia technician worked closely with respiratory therapy regarding ventilator setting and changing the anesthesia gas scavenging charcoal canister. Within 4 hours, her gas exchange normalized (Table). The patient’s ABG was pH 7.44, PaCO2 52 mm Hg, and PaO2 69 mm Hg on FiO2 0.4. On volume cycled ventilation with a rate of 12, flow rate of 40 L/min, and tidal volume 6 mL/kg/IBW, the PAP decreased to 41-cm H2O.
Within 24 hours bronchospasm improved as evidenced by decreased airway pressures, resolution of wheezing, and decreased CO2 retention. The sevoflurane was easily weaned over the next 48 hours by decreasing the dose by 25% every 12-hour shift without rebound bronchospasm. Airway pressures and ABGs were frequently monitored during the weaning process. The patient resumed conventional mechanical ventilation, cisatracurium was discontinued, and she underwent a percutaneous tracheostomy for critical illness polymyopathy. Her respiratory muscle strength recovered more robustly than anticipated. Prior to discharge to a skilled nursing facility for continued rehabilitation, she was removed from mechanical ventilation and decannulated.
Discussion
This case illustrates the successful treatment of a patient with extreme status asthmaticus given inhalational anesthesia as supportive care while the bronchospasm and status asthmaticus abated. This is an unusual treatment in an ominous situation. Inhalational anesthetics are potent bronchodilators and have been successfully used in the management of status asthmaticus refractory to conventional therapy.4 Inhalational anesthetics have been shown to decrease airway resistance, dynamic hyperinflation, and intrinsic PEEP.5 These agents result in rapid bronchodilation by relaxing the smooth muscle and are associated with early liberation from mechanical ventilation.5,6 Although there are no guidelines regarding which inhalational agent is best, specific dosing, duration, or titration, case reports in the literature regarding the successful use of inhalational agents in life-threatening status asthmaticus exist.2,5,7
Caveats regarding the use of inhalational anesthetics in status asthmaticus include proarrhythmias, severe hepatic and renal toxicity. Although isoflurane is less likely to cause arrhythmia, both isoflurane and sevoflurane can cause dose-dependent hypotension by peripheral vasodilatation.7 Ourpatient did not manifest any adverse effects.
Additional challenges regarding the use of inhalational anesthetics for status asthmaticus include differences in ventilators and occupational hazards.8 Anesthesia or operating room ventilators differ from ICU ventilators in flow and pressure capabilities.7 The anesthesia ventilator is not capable of generating inspiratory pressures sufficient to ventilate patients with severely elevated airway resistance. Thus, the decrease inspiratory flow that occurs with increasing airway pressure limits the tidal volume delivered and consequently the minute volume. Although newer anesthesia ventilators have increased flow capabilities, they require a fully trained staff.8
Potential occupational exposure to these volatile anesthetic gases occurs as patients being treated may exhale considerable amounts of volatile anesthetics.8 An anesthesia gas scavenging device, such as a charcoal canister, must be attached to the ventilator to capture the exhaled anesthetic gases and should be changed every 12 hours.8 Finally, there is a potential for rebound bronchospasm as the anesthetic agent is tapered.6,7,9-11
Conclusion
Inhalational anesthetics are an option as rescue therapy for severe life-threatening asthma when all other therapies have failed. Use of inhalational anesthetics in status asthmaticus consists of case reports of which half are in children.2,5,7 Our patient contributes to the literature of case reports regarding using sevoflurane in refractory status asthmaticus. A decision to choose them must be a collaborative team approach with anesthesiology, pulmonary/critical care medicine, respiratory therapy, and ICU nurses, and the risks and benefits should be discussed with decision-making family members. Since there are no specific guidelines for the use of inhalational agents in status asthmaticus, close attention to inspiratory flows, gas scavenging devices, and clinical response is required. Additionally, the team must be comfortable with the plan to use an anesthesia ventilator and trained on its limitations.
1. Centers for Disease Control and Prevention. Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Updated May 2019. Accessed September 5, 2019.
2. Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
3. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2012;33(suppl 1):47-50.
4. Mutlu GM, Factor P, Schwartz DE, Snajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia Crit Care Med. 2002;30(2):477-480.
5. Maltais, F, Sovilj M, Goldber P, Gottfried SB. Respiratory mechanism in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401-1406.
6. Parnass SM, Feld JM, Chamberlin WH, Segil LJ. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66(2):193-195.
7. Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698-701.
8. Meiser A, Laubenthal H. Inhalational anesthetics in the ICU: theory and practice of inhalational sedation in the ICU economics, risk-benefit. Best Pract Res Clin Anesthesiol. 2005;19(3):523-538.
9. Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
10. Nakao S, Hatano K, Sumi C, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010;110(3):775-779.
11. Stachnik J. Inhaled anesthetic agents. Am J Health-Syst Pharm. 2006;63(7):623-634.
Asthma attacks account for 1.8 million emergency department (ED) visits each year in the US and for 10 deaths daily.1 Management of asthma attacks includes administration of inhaled ß2 adrenergic agonists, inhaled anticholinergic agents, IV magnesium sulfate, and corticosteroids.2 Status asthmaticus is an intense acute exacerbation of asthma that does not respond to repeated treatments of bronchodilators and corticosteroids.3 It is a medical emergency requiring immediate recognition and treatment. The decision to intubate a patient with status asthmaticus is a clinical decision based on work of breathing, respiratory acidosis, and failure to respond to medical interventions.
In refractory cases of status asthmaticus, intubation and mechanical ventilation are undertaken to provide oxygenation and ventilation until the bronchospasm resolves. However, mechanical ventilation is associated with significant risks, including high end-inspiratory pressures, barotrauma, and volutrauma.4 Rescue therapies include muscle relaxation, infusion of ketamine (central acting nonopioid analgesic with bronchodilatory properties), heliox, and general anesthesia.2,4 We report a case of a patient with life-threatening asthma and status asthmaticus treated with sevoflurane general anesthesia.
Case Presentation
A 55-year-old woman whose medical history was notable for asthma, psoriasis, hypothyroidism, tobacco, and alcohol abuse, and posttraumatic stress disorder (PTSD) presented to the ED. The patient had rarely sought medical attention and had no prior ED visits or hospitalizations in the electronic health record. Her home regimen included an albuterol inhaler used as needed. Her family reported that they had found her in distress in bed in a tripod position, unable to speak and struggling to breath.
Emergency medical services found the patient cyanotic, apneic, and pulseless. She received cardiopulmonary resuscitation for 30 seconds and 1-mg IV epinephrine, and spontaneous circulation returned. The patient arrived in the ED with an oral airway in place receiving bag valve mask ventilation. The patient expelled the oral airway. She was unable to speak due to dyspnea, exhibited persistent cyanosis, fatigue due to work of breathing, and failed to respond to nebulized albuterol/ipratropium bromide, IV methylprednisolone, and magnesium sulfate. The patient met criteria for acute severe asthma, or status asthmaticus. Thus, the patient received rapid sequence induction with rocuronium and ketamine and was intubated.
According to her family, the patient had no previous intensive care unit (ICU) admissions or prior intubations. Her only asthma medication was an albuterol inhaler as needed. The patient worked as a supervisor at a window blind manufacturing company. She lived alone, smoked 2 packs of cigarettes a day for more than 30 years, had no pets, drank unknown quantities of beer, wine, and hard liquor daily, and had smoked marijuana for several years.
The patient’s physical examination was notable for diffuse expiratory wheezes. Laboratory analysis revealed white blood cell count of 13.7 k/mcL, sodium 140 mmol/L, potassium 4.9 mmol/L, chloride 105 mmol/L, CO2 17 mmol/L, creatinine 0.98 mg/dL, troponin 0.03 ng/mL, lactate 7.2 mmol/L. Her chest X-ray showed hyperinflation but no focal opacities, pneumothorax, or pulmonary edema. Her endotracheal tube was in good position (Figure 1). A computed tomography pulmonary angiogram showed no pulmonary embolus or emphysema. There were atelectatic changes in the dependent portion of the right lower lobe, central bronchial wall thickening, and no stigmata of air trapping (Figure 2). An echocardiogram revealed a left ventricular ejection fraction of 45%, normal right ventricle and right ventricular size and function with an estimated right ventricular systolic pressure of 40 mm Hg.
The patient was admitted to the ICU and started on continuous infusion cisatracurium for paralysis and deep sedation to improve ventilatory synchrony and decrease auto positive end-expiratory pressure (PEEP). Mechanical ventilation was initiated with volume-cycled assist control ventilation, 6 mL/kg/ideal body weight (IBW) at 5-cm H2O PEEP, and 1 minute ventilation of 10 liters. The patient had severe air trapping and high airway pressures. The dynamic PEEP was 22-cm H2O (normal PEEP of 5-cm H2O), peak airway pressure (PAP) 41-cm H2O, and plateau pressure 31-cm H2O. In addition, the arterial blood gas (ABG) showed severe hypercapnic respiratory acidosis without significant hypoxemia with pH 7.15, PaCO2 90 mm Hg, and PaO2 150 mm Hg.
Pressure controlled ventilation was attempted unsuccessfully due to high airway resistance. Ultimately, the patient was set on volume control with low tidal volume, 6 mL/kg/IBW, high flow 90 L/min, PEEP 0 cm of H2O, and a low respiratory rate of 10 to achieve an inspiratory to expiratory (I:E) ratio of 1:7. Managing the ventilator to avoid dynamic hyperinflation and auto-PEEP, she remained relatively stable and improved.
By day 4 the patient’s ventilator was set to volume assist control with respiratory rate of 16, tidal volume, 6 mL/kg/IBW, PEEP 5-cm H2O with auto PEEP of 3-cm H2O, and fraction of inspired ABG O2 (FiO2) 0.35 with PAP of 46-cm H2O and plateau pressure of 17-cm H2O. The ABG was pH 7.32, PaCO2 65 mm Hg, and PaO2 74 mm Hg. However, on hospital day 5, she developed worsening PAP 60 to 77-cm H2O, plateau pressures 17-cm H2O, and a dynamic PEEP 16-cm H2O and was unresponsive to ventilator maneuvers to lower airway pressures and improve ventilation.
The patient had been receiving continuous albuterol and ipratropium nebulizer treatments. Ketamine infusion was considered fraught with potential for a dissociative reaction due to the patient’s significant PTSD. The patient’s family requested avoidance of ketamine infusion since the patient was paralyzed and psychiatric effects could not be monitored. Heliox 80/20 mixture was considered; however, it is incompatible with the ventilator that was being used since it could not account for the density of the helium gas flow in the tidal volumes. Extracorporeal membrane oxygenation (ECMO) was not available at our facility, and the patient was not a candidate for the regional ECMO center.
On hospital day 8, the patient developed worsening respiratory acidosis. The patient’s PAP increased to > 77-cm H2O, and her ABG revealed pH 7.22, PaCO2 90 mm Hg, and PaO2 77 mm Hg with FiO2 0.4. A chest X-ray demonstrated a new left lower lobe infiltrate. Fiber optic bronchoscopy was notable for scattered thick secretions throughout both lungs without obstructing mucus plug. Removal of airway secretions did not improve airway pressures or dynamic hyperinflation.
After consultation and discussion with the chief of anesthesia, the patient was placed on an anesthesia ventilator and started on sevoflurane 1.5% in the ICU. Anesthesiology was available 24 hours a day, and the anesthesiologist rounded with the intensivist frequently for this patient. The anesthesia technician worked closely with respiratory therapy regarding ventilator setting and changing the anesthesia gas scavenging charcoal canister. Within 4 hours, her gas exchange normalized (Table). The patient’s ABG was pH 7.44, PaCO2 52 mm Hg, and PaO2 69 mm Hg on FiO2 0.4. On volume cycled ventilation with a rate of 12, flow rate of 40 L/min, and tidal volume 6 mL/kg/IBW, the PAP decreased to 41-cm H2O.
Within 24 hours bronchospasm improved as evidenced by decreased airway pressures, resolution of wheezing, and decreased CO2 retention. The sevoflurane was easily weaned over the next 48 hours by decreasing the dose by 25% every 12-hour shift without rebound bronchospasm. Airway pressures and ABGs were frequently monitored during the weaning process. The patient resumed conventional mechanical ventilation, cisatracurium was discontinued, and she underwent a percutaneous tracheostomy for critical illness polymyopathy. Her respiratory muscle strength recovered more robustly than anticipated. Prior to discharge to a skilled nursing facility for continued rehabilitation, she was removed from mechanical ventilation and decannulated.
Discussion
This case illustrates the successful treatment of a patient with extreme status asthmaticus given inhalational anesthesia as supportive care while the bronchospasm and status asthmaticus abated. This is an unusual treatment in an ominous situation. Inhalational anesthetics are potent bronchodilators and have been successfully used in the management of status asthmaticus refractory to conventional therapy.4 Inhalational anesthetics have been shown to decrease airway resistance, dynamic hyperinflation, and intrinsic PEEP.5 These agents result in rapid bronchodilation by relaxing the smooth muscle and are associated with early liberation from mechanical ventilation.5,6 Although there are no guidelines regarding which inhalational agent is best, specific dosing, duration, or titration, case reports in the literature regarding the successful use of inhalational agents in life-threatening status asthmaticus exist.2,5,7
Caveats regarding the use of inhalational anesthetics in status asthmaticus include proarrhythmias, severe hepatic and renal toxicity. Although isoflurane is less likely to cause arrhythmia, both isoflurane and sevoflurane can cause dose-dependent hypotension by peripheral vasodilatation.7 Ourpatient did not manifest any adverse effects.
Additional challenges regarding the use of inhalational anesthetics for status asthmaticus include differences in ventilators and occupational hazards.8 Anesthesia or operating room ventilators differ from ICU ventilators in flow and pressure capabilities.7 The anesthesia ventilator is not capable of generating inspiratory pressures sufficient to ventilate patients with severely elevated airway resistance. Thus, the decrease inspiratory flow that occurs with increasing airway pressure limits the tidal volume delivered and consequently the minute volume. Although newer anesthesia ventilators have increased flow capabilities, they require a fully trained staff.8
Potential occupational exposure to these volatile anesthetic gases occurs as patients being treated may exhale considerable amounts of volatile anesthetics.8 An anesthesia gas scavenging device, such as a charcoal canister, must be attached to the ventilator to capture the exhaled anesthetic gases and should be changed every 12 hours.8 Finally, there is a potential for rebound bronchospasm as the anesthetic agent is tapered.6,7,9-11
Conclusion
Inhalational anesthetics are an option as rescue therapy for severe life-threatening asthma when all other therapies have failed. Use of inhalational anesthetics in status asthmaticus consists of case reports of which half are in children.2,5,7 Our patient contributes to the literature of case reports regarding using sevoflurane in refractory status asthmaticus. A decision to choose them must be a collaborative team approach with anesthesiology, pulmonary/critical care medicine, respiratory therapy, and ICU nurses, and the risks and benefits should be discussed with decision-making family members. Since there are no specific guidelines for the use of inhalational agents in status asthmaticus, close attention to inspiratory flows, gas scavenging devices, and clinical response is required. Additionally, the team must be comfortable with the plan to use an anesthesia ventilator and trained on its limitations.
Asthma attacks account for 1.8 million emergency department (ED) visits each year in the US and for 10 deaths daily.1 Management of asthma attacks includes administration of inhaled ß2 adrenergic agonists, inhaled anticholinergic agents, IV magnesium sulfate, and corticosteroids.2 Status asthmaticus is an intense acute exacerbation of asthma that does not respond to repeated treatments of bronchodilators and corticosteroids.3 It is a medical emergency requiring immediate recognition and treatment. The decision to intubate a patient with status asthmaticus is a clinical decision based on work of breathing, respiratory acidosis, and failure to respond to medical interventions.
In refractory cases of status asthmaticus, intubation and mechanical ventilation are undertaken to provide oxygenation and ventilation until the bronchospasm resolves. However, mechanical ventilation is associated with significant risks, including high end-inspiratory pressures, barotrauma, and volutrauma.4 Rescue therapies include muscle relaxation, infusion of ketamine (central acting nonopioid analgesic with bronchodilatory properties), heliox, and general anesthesia.2,4 We report a case of a patient with life-threatening asthma and status asthmaticus treated with sevoflurane general anesthesia.
Case Presentation
A 55-year-old woman whose medical history was notable for asthma, psoriasis, hypothyroidism, tobacco, and alcohol abuse, and posttraumatic stress disorder (PTSD) presented to the ED. The patient had rarely sought medical attention and had no prior ED visits or hospitalizations in the electronic health record. Her home regimen included an albuterol inhaler used as needed. Her family reported that they had found her in distress in bed in a tripod position, unable to speak and struggling to breath.
Emergency medical services found the patient cyanotic, apneic, and pulseless. She received cardiopulmonary resuscitation for 30 seconds and 1-mg IV epinephrine, and spontaneous circulation returned. The patient arrived in the ED with an oral airway in place receiving bag valve mask ventilation. The patient expelled the oral airway. She was unable to speak due to dyspnea, exhibited persistent cyanosis, fatigue due to work of breathing, and failed to respond to nebulized albuterol/ipratropium bromide, IV methylprednisolone, and magnesium sulfate. The patient met criteria for acute severe asthma, or status asthmaticus. Thus, the patient received rapid sequence induction with rocuronium and ketamine and was intubated.
According to her family, the patient had no previous intensive care unit (ICU) admissions or prior intubations. Her only asthma medication was an albuterol inhaler as needed. The patient worked as a supervisor at a window blind manufacturing company. She lived alone, smoked 2 packs of cigarettes a day for more than 30 years, had no pets, drank unknown quantities of beer, wine, and hard liquor daily, and had smoked marijuana for several years.
The patient’s physical examination was notable for diffuse expiratory wheezes. Laboratory analysis revealed white blood cell count of 13.7 k/mcL, sodium 140 mmol/L, potassium 4.9 mmol/L, chloride 105 mmol/L, CO2 17 mmol/L, creatinine 0.98 mg/dL, troponin 0.03 ng/mL, lactate 7.2 mmol/L. Her chest X-ray showed hyperinflation but no focal opacities, pneumothorax, or pulmonary edema. Her endotracheal tube was in good position (Figure 1). A computed tomography pulmonary angiogram showed no pulmonary embolus or emphysema. There were atelectatic changes in the dependent portion of the right lower lobe, central bronchial wall thickening, and no stigmata of air trapping (Figure 2). An echocardiogram revealed a left ventricular ejection fraction of 45%, normal right ventricle and right ventricular size and function with an estimated right ventricular systolic pressure of 40 mm Hg.
The patient was admitted to the ICU and started on continuous infusion cisatracurium for paralysis and deep sedation to improve ventilatory synchrony and decrease auto positive end-expiratory pressure (PEEP). Mechanical ventilation was initiated with volume-cycled assist control ventilation, 6 mL/kg/ideal body weight (IBW) at 5-cm H2O PEEP, and 1 minute ventilation of 10 liters. The patient had severe air trapping and high airway pressures. The dynamic PEEP was 22-cm H2O (normal PEEP of 5-cm H2O), peak airway pressure (PAP) 41-cm H2O, and plateau pressure 31-cm H2O. In addition, the arterial blood gas (ABG) showed severe hypercapnic respiratory acidosis without significant hypoxemia with pH 7.15, PaCO2 90 mm Hg, and PaO2 150 mm Hg.
Pressure controlled ventilation was attempted unsuccessfully due to high airway resistance. Ultimately, the patient was set on volume control with low tidal volume, 6 mL/kg/IBW, high flow 90 L/min, PEEP 0 cm of H2O, and a low respiratory rate of 10 to achieve an inspiratory to expiratory (I:E) ratio of 1:7. Managing the ventilator to avoid dynamic hyperinflation and auto-PEEP, she remained relatively stable and improved.
By day 4 the patient’s ventilator was set to volume assist control with respiratory rate of 16, tidal volume, 6 mL/kg/IBW, PEEP 5-cm H2O with auto PEEP of 3-cm H2O, and fraction of inspired ABG O2 (FiO2) 0.35 with PAP of 46-cm H2O and plateau pressure of 17-cm H2O. The ABG was pH 7.32, PaCO2 65 mm Hg, and PaO2 74 mm Hg. However, on hospital day 5, she developed worsening PAP 60 to 77-cm H2O, plateau pressures 17-cm H2O, and a dynamic PEEP 16-cm H2O and was unresponsive to ventilator maneuvers to lower airway pressures and improve ventilation.
The patient had been receiving continuous albuterol and ipratropium nebulizer treatments. Ketamine infusion was considered fraught with potential for a dissociative reaction due to the patient’s significant PTSD. The patient’s family requested avoidance of ketamine infusion since the patient was paralyzed and psychiatric effects could not be monitored. Heliox 80/20 mixture was considered; however, it is incompatible with the ventilator that was being used since it could not account for the density of the helium gas flow in the tidal volumes. Extracorporeal membrane oxygenation (ECMO) was not available at our facility, and the patient was not a candidate for the regional ECMO center.
On hospital day 8, the patient developed worsening respiratory acidosis. The patient’s PAP increased to > 77-cm H2O, and her ABG revealed pH 7.22, PaCO2 90 mm Hg, and PaO2 77 mm Hg with FiO2 0.4. A chest X-ray demonstrated a new left lower lobe infiltrate. Fiber optic bronchoscopy was notable for scattered thick secretions throughout both lungs without obstructing mucus plug. Removal of airway secretions did not improve airway pressures or dynamic hyperinflation.
After consultation and discussion with the chief of anesthesia, the patient was placed on an anesthesia ventilator and started on sevoflurane 1.5% in the ICU. Anesthesiology was available 24 hours a day, and the anesthesiologist rounded with the intensivist frequently for this patient. The anesthesia technician worked closely with respiratory therapy regarding ventilator setting and changing the anesthesia gas scavenging charcoal canister. Within 4 hours, her gas exchange normalized (Table). The patient’s ABG was pH 7.44, PaCO2 52 mm Hg, and PaO2 69 mm Hg on FiO2 0.4. On volume cycled ventilation with a rate of 12, flow rate of 40 L/min, and tidal volume 6 mL/kg/IBW, the PAP decreased to 41-cm H2O.
Within 24 hours bronchospasm improved as evidenced by decreased airway pressures, resolution of wheezing, and decreased CO2 retention. The sevoflurane was easily weaned over the next 48 hours by decreasing the dose by 25% every 12-hour shift without rebound bronchospasm. Airway pressures and ABGs were frequently monitored during the weaning process. The patient resumed conventional mechanical ventilation, cisatracurium was discontinued, and she underwent a percutaneous tracheostomy for critical illness polymyopathy. Her respiratory muscle strength recovered more robustly than anticipated. Prior to discharge to a skilled nursing facility for continued rehabilitation, she was removed from mechanical ventilation and decannulated.
Discussion
This case illustrates the successful treatment of a patient with extreme status asthmaticus given inhalational anesthesia as supportive care while the bronchospasm and status asthmaticus abated. This is an unusual treatment in an ominous situation. Inhalational anesthetics are potent bronchodilators and have been successfully used in the management of status asthmaticus refractory to conventional therapy.4 Inhalational anesthetics have been shown to decrease airway resistance, dynamic hyperinflation, and intrinsic PEEP.5 These agents result in rapid bronchodilation by relaxing the smooth muscle and are associated with early liberation from mechanical ventilation.5,6 Although there are no guidelines regarding which inhalational agent is best, specific dosing, duration, or titration, case reports in the literature regarding the successful use of inhalational agents in life-threatening status asthmaticus exist.2,5,7
Caveats regarding the use of inhalational anesthetics in status asthmaticus include proarrhythmias, severe hepatic and renal toxicity. Although isoflurane is less likely to cause arrhythmia, both isoflurane and sevoflurane can cause dose-dependent hypotension by peripheral vasodilatation.7 Ourpatient did not manifest any adverse effects.
Additional challenges regarding the use of inhalational anesthetics for status asthmaticus include differences in ventilators and occupational hazards.8 Anesthesia or operating room ventilators differ from ICU ventilators in flow and pressure capabilities.7 The anesthesia ventilator is not capable of generating inspiratory pressures sufficient to ventilate patients with severely elevated airway resistance. Thus, the decrease inspiratory flow that occurs with increasing airway pressure limits the tidal volume delivered and consequently the minute volume. Although newer anesthesia ventilators have increased flow capabilities, they require a fully trained staff.8
Potential occupational exposure to these volatile anesthetic gases occurs as patients being treated may exhale considerable amounts of volatile anesthetics.8 An anesthesia gas scavenging device, such as a charcoal canister, must be attached to the ventilator to capture the exhaled anesthetic gases and should be changed every 12 hours.8 Finally, there is a potential for rebound bronchospasm as the anesthetic agent is tapered.6,7,9-11
Conclusion
Inhalational anesthetics are an option as rescue therapy for severe life-threatening asthma when all other therapies have failed. Use of inhalational anesthetics in status asthmaticus consists of case reports of which half are in children.2,5,7 Our patient contributes to the literature of case reports regarding using sevoflurane in refractory status asthmaticus. A decision to choose them must be a collaborative team approach with anesthesiology, pulmonary/critical care medicine, respiratory therapy, and ICU nurses, and the risks and benefits should be discussed with decision-making family members. Since there are no specific guidelines for the use of inhalational agents in status asthmaticus, close attention to inspiratory flows, gas scavenging devices, and clinical response is required. Additionally, the team must be comfortable with the plan to use an anesthesia ventilator and trained on its limitations.
1. Centers for Disease Control and Prevention. Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Updated May 2019. Accessed September 5, 2019.
2. Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
3. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2012;33(suppl 1):47-50.
4. Mutlu GM, Factor P, Schwartz DE, Snajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia Crit Care Med. 2002;30(2):477-480.
5. Maltais, F, Sovilj M, Goldber P, Gottfried SB. Respiratory mechanism in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401-1406.
6. Parnass SM, Feld JM, Chamberlin WH, Segil LJ. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66(2):193-195.
7. Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698-701.
8. Meiser A, Laubenthal H. Inhalational anesthetics in the ICU: theory and practice of inhalational sedation in the ICU economics, risk-benefit. Best Pract Res Clin Anesthesiol. 2005;19(3):523-538.
9. Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
10. Nakao S, Hatano K, Sumi C, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010;110(3):775-779.
11. Stachnik J. Inhaled anesthetic agents. Am J Health-Syst Pharm. 2006;63(7):623-634.
1. Centers for Disease Control and Prevention. Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Updated May 2019. Accessed September 5, 2019.
2. Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
3. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2012;33(suppl 1):47-50.
4. Mutlu GM, Factor P, Schwartz DE, Snajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia Crit Care Med. 2002;30(2):477-480.
5. Maltais, F, Sovilj M, Goldber P, Gottfried SB. Respiratory mechanism in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401-1406.
6. Parnass SM, Feld JM, Chamberlin WH, Segil LJ. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66(2):193-195.
7. Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698-701.
8. Meiser A, Laubenthal H. Inhalational anesthetics in the ICU: theory and practice of inhalational sedation in the ICU economics, risk-benefit. Best Pract Res Clin Anesthesiol. 2005;19(3):523-538.
9. Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
10. Nakao S, Hatano K, Sumi C, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010;110(3):775-779.
11. Stachnik J. Inhaled anesthetic agents. Am J Health-Syst Pharm. 2006;63(7):623-634.