User login
Improving sepsis-related outcomes
Early diagnosis a key goal
Sepsis is a leading cause of death and disease among patients in hospitals, and it’s the subject of a recent quality improvement study in the Journal for Healthcare Quality.
“The number of cases per year has been increasing in the U.S., and it is the most expensive condition treated in U.S. hospitals,” said lead author M. Courtney Hughes, PhD, of Northern Illinois University in DeKalb.
But early identification of symptoms can be complex and difficult for clinicians, meaning there’s a continuing need for research studies examining sepsis identification and prevention. “The purpose of this study was to examine a quality improvement project that consisted of clinical alerts, audit and feedback, and staff education at an integrated health care system in the Midwest,” she said.
In a retrospective analysis, the researchers examined data from three health systems to determine the impact of a 10-month sepsis quality improvement program that consisted of clinical alerts, audit and feedback, and staff education. The results showed that, compared with the control group, the intervention group significantly decreased length of stay and costs per stay.
“One way to improve sepsis health outcomes and decrease costs may be for hospitals to implement a sepsis quality improvement program,” Dr. Hughes said. “Also, providing sepsis performance data and education to hospital providers and administrators can arm staff with the knowledge and tools necessary for improving processes and performance related to sepsis.”
Dr. Hughes said that she hopes this work will encourage hospitalists to seek sepsis-related performance data and training. “By doing so, they may help achieve earlier diagnosis of sepsis cases and initiation of the Surviving Sepsis Campaign bundle.”
Reference
Hughes MC et al. A quality improvement project to improve sepsis-related outcomes at an integrated healthcare system. J Healthc Qual. Published online 2019 Mar 14. doi: 10.1097/JHQ.0000000000000193.
Early diagnosis a key goal
Early diagnosis a key goal
Sepsis is a leading cause of death and disease among patients in hospitals, and it’s the subject of a recent quality improvement study in the Journal for Healthcare Quality.
“The number of cases per year has been increasing in the U.S., and it is the most expensive condition treated in U.S. hospitals,” said lead author M. Courtney Hughes, PhD, of Northern Illinois University in DeKalb.
But early identification of symptoms can be complex and difficult for clinicians, meaning there’s a continuing need for research studies examining sepsis identification and prevention. “The purpose of this study was to examine a quality improvement project that consisted of clinical alerts, audit and feedback, and staff education at an integrated health care system in the Midwest,” she said.
In a retrospective analysis, the researchers examined data from three health systems to determine the impact of a 10-month sepsis quality improvement program that consisted of clinical alerts, audit and feedback, and staff education. The results showed that, compared with the control group, the intervention group significantly decreased length of stay and costs per stay.
“One way to improve sepsis health outcomes and decrease costs may be for hospitals to implement a sepsis quality improvement program,” Dr. Hughes said. “Also, providing sepsis performance data and education to hospital providers and administrators can arm staff with the knowledge and tools necessary for improving processes and performance related to sepsis.”
Dr. Hughes said that she hopes this work will encourage hospitalists to seek sepsis-related performance data and training. “By doing so, they may help achieve earlier diagnosis of sepsis cases and initiation of the Surviving Sepsis Campaign bundle.”
Reference
Hughes MC et al. A quality improvement project to improve sepsis-related outcomes at an integrated healthcare system. J Healthc Qual. Published online 2019 Mar 14. doi: 10.1097/JHQ.0000000000000193.
Sepsis is a leading cause of death and disease among patients in hospitals, and it’s the subject of a recent quality improvement study in the Journal for Healthcare Quality.
“The number of cases per year has been increasing in the U.S., and it is the most expensive condition treated in U.S. hospitals,” said lead author M. Courtney Hughes, PhD, of Northern Illinois University in DeKalb.
But early identification of symptoms can be complex and difficult for clinicians, meaning there’s a continuing need for research studies examining sepsis identification and prevention. “The purpose of this study was to examine a quality improvement project that consisted of clinical alerts, audit and feedback, and staff education at an integrated health care system in the Midwest,” she said.
In a retrospective analysis, the researchers examined data from three health systems to determine the impact of a 10-month sepsis quality improvement program that consisted of clinical alerts, audit and feedback, and staff education. The results showed that, compared with the control group, the intervention group significantly decreased length of stay and costs per stay.
“One way to improve sepsis health outcomes and decrease costs may be for hospitals to implement a sepsis quality improvement program,” Dr. Hughes said. “Also, providing sepsis performance data and education to hospital providers and administrators can arm staff with the knowledge and tools necessary for improving processes and performance related to sepsis.”
Dr. Hughes said that she hopes this work will encourage hospitalists to seek sepsis-related performance data and training. “By doing so, they may help achieve earlier diagnosis of sepsis cases and initiation of the Surviving Sepsis Campaign bundle.”
Reference
Hughes MC et al. A quality improvement project to improve sepsis-related outcomes at an integrated healthcare system. J Healthc Qual. Published online 2019 Mar 14. doi: 10.1097/JHQ.0000000000000193.
The Great Pretender
Susannah Cahalan’s new book challenges an experiment that changed psychiatry
As an undergraduate psychology major, I was taught about the Rosenhan study in several of my courses. My professors lectured about the shocking findings psychologist David Rosenhan, PhD, documented in a 1973 Science article, “On Being Sane in Insane Places” and these findings lent themselves to lecture hall drama. Eight people presented to hospitals and said they heard voices saying: “empty, hollow, thud.” These “pseudopatients” exhibited no other psychiatric symptoms but were admitted, diagnosed with schizophrenia, and observations of their behavior were made. The charts included notes such as, “Patient exhibits writing behavior,” my professors said. The pseudopatients were kept for an average of 19 days, and one for as long as 51 days. The decades have passed, and there are many things I learned in college that I have since forgotten, but I remember “empty, hollow, thud,” and the famous Rosenhan experiment.
I was eager to read Susannah Cahalan’s book, “The Great Pretender” (Grand Central Publishing, 2019), which puts both Dr. Rosenhan and his pseudopatient study under a microscope. Ms. Cahalan is the author of the page-turner, Brain on Fire: My Month of Madness (Free Press, 2012), where she recounted her own struggle with a psychotic episode. Ms. Cahalan, a young reporter in New York City, became psychotic and then catatonic; her condition perplexed the neurologists who were treating her on an inpatient unit, and they were on the verge of transferring her to psychiatry when a diagnosis of anti-NMDA receptor encephalitis was suspected and then confirmed with a brain biopsy. Ms. Cahalan made a full recovery after treatment with steroids, intravenous immunoglobulin, and plasmapheresis. While Ms. Cahalan’s symptoms were classic for a severe psychotic disorder, there was reason to believe that this was not a primary psychiatric disorder: She was having grand mal seizures. Her book was a bestseller, and she has spoken widely to make others aware of this rare illness that masquerades as psychosis. I heard her speak at the opening session of the American Psychiatric Association’s annual meeting in May of 2017.“My family, like many families before them, fought against the tyranny of the mental illness label,” Ms. Cahalan writes at the very beginning of “The Great Pretender.” She goes on to talk about how psychiatry differs from other medical fields: It’s the only specialty where people can be treated against their will; psychiatry casts judgments on the person; mental illness is poorly defined – perhaps there is no clear divide between normal and mad; and psychiatric disorders are less “real” than other illnesses. Throughout the book she refers to psychiatrists as smug and arrogant.
Ms. Cahalan takes on the task of documenting the horrors of psychiatry’s often sordid history, starting with journalist Nellie Bly’s 1887 journey into to a psychiatric facility to expose the abuses there. Certainly, psychiatry’s history is sordid. Ms. Cahalan talks about inhumane conditions in overcrowded psychiatric hospitals, about our sad chapter of lobotomies, about the influence of psychoanalysis on diagnosis and treatment, and about how homosexuality was once an illness and now is not. She includes “One Flew Over the Cuckoo’s Nest,” “The Myth of Mental Illness,” big pharma, and the Goldwater fiasco. In her recounting of the history, it’s all bad. She mentions Benjamin Rush, MD, only once, as the creator of “ ‘... the tranquilizing chair’ (a case of the worst false advertising ever), a terrifying sensory-deprivation apparatus in which patients were strapped down to a chair with a wooden box placed over their heads to block stimulation, restrict movement, and reduce blood to the brain.” Dr. Rush’s role as the father of American psychiatry who challenged the belief that mental illness was the result of demonic possession, gets no mention. Nor does Ms. Cahalan note that he founded Pennsylvania Hospital, where moral and occupational therapy revolutionized the treatment of those with mental illness.
So there’s her story, her rendition of the history of American psychiatry, and through this she weaves in the story of the Rosenhan experiment.
“ ‘It all started out as a dare,’ Dr. Rosenhan told a local newspaper, ‘I was teaching psychology at Swarthmore, and my students were saying that the course was too conceptual and abstract. So I said, ‘Okay, if you really want to know what mental patients are like, become mental patients.’ ”
Really? I read this and wondered how a psychologist could talk about people who had been hospitalized with psychiatric disorders as though they were aliens. Certainly, some of these students, their family members, or their friends must have been hospitalized at some point. Yet all through, there is this sense that the patients are other, and the discovery of the undercover operation is that the patients are actually human beings! Dr. Rosenhan, who was one of the pseudopatients, goes on to conclude that the label is everything, that once labeled they are treated differently by the nurses in “cages” and the doctors who walk by and avert their gaze. A second man Ms. Cahalan named, also one of the pseudopatients, had a similar experience. A third subject she located was not included in the study: His experience was counter to the findings of the study, his time in the hospital was a positive, he found it comforting, and the experiences he had there had a lasting positive influence on his life.
Ms. Cahalan talks about the publication of “On Being Sane in Insane Places” as a study that was finally scientific, one that changed all of psychiatry, and was the driving force for the creation of the DSM-III and the closure of state hospitals. I wondered if it was as influential as Ms. Cahalan claims, and I asked some psychiatrists who were practicing in 1973 when the article was published. I wanted to know if this study rocked their world.
“At first, with the great amount of publicity the study generated, it was added fodder for the antipsychiatrists, including the Scientologists and Szaszians,” Steven Sharfstein, MD, a former president of the American Psychiatric Association, told me. “But as young psychiatrists in the trenches, business continued to boom, and we continued to do the best job we could with diagnosis, assessment of risk, involuntary commitment, and treatment. And from what I recall, morale was high in the 1970s. We had some new medications and psychotherapies, and there was community activism. Faking symptoms to gain admission seemed to be a no-brainer, but keeping people for long stays was more problematic.”
E. Fuller Torrey, MD, the founder of the Treatment Advocacy Center who worked for many years treating patients at St. Elizabeth’s Hospital, replied: “It is important to remember that this study was published at the height of the deinstitutionalization movement and quite likely accelerated it. As I recall, at the time it seemed odd that all eight patients claimed to have had similar experiences while hospitalized. I think the main effect of the study was to provide ammunition for the antipsychiatrists.”
Ms. Cahalan has bought into the antipsychiatry movement full force. It’s not until the very end that there is any acknowledgment that psychiatry ever helped anyone, and even then, it’s a bit begrudging. Worse, she neglects to mention that people with psychiatric disorders suffer because of their psychic pain; one could get through this book and believe that people with mental illness struggle only because they are labeled and then mistreated, and for someone who has suffered herself, she misses the essence of how awful it is to be ill, and that people are often helped by psychiatric treatments. When she finally adds a paragraph talking about the usefulness of psychotropics, it’s with a caveat. “But I’m not here to rail against the drugs. There are plenty of places you can get that perspective. I see that these drugs help many people lead full and meaningful lives. It would be folly to discount their worth. We also can’t deny that the situation is complicated.”
There are moments in the manuscript where I found it difficult to know what were Dr. Rosenhan’s interpretations and what were Ms. Callahan’s interpretations of Dr. Rosenhan’s experiences. A lot of assumptions are made – particularly about the motivations of the hospital staff – and I wasn’t always sure they were correct. For example, on his second day in the hospital, Dr. Rosenhan asked a nurse for the newspaper. When she tells him it hasn’t come yet, he concludes that the staff is keeping the newspapers from the patients. And when a staff member is initially chatty then later shuns Dr. Rosenhan, he concludes that the man initially mistook him for a psychiatrist because he looks professorial. Both Ms. Cahalan and Dr. Rosenhan approach psychiatry with biases, and they don’t always question their assumptions.
, a professor who didn’t treat patients. This intermixing of the two fields felt contrived to me, and gave too much credence to the idea that no one really knows sane from disordered, and everyone was embracing the antipsychiatry dogma. Surely, someone during the those years must have liked their psychiatrist.
That said, Ms. Cahalan does a phenomenal job of infiltrating the world of the late Dr. Rosenhan. She starts out enamored by him and by his finding that psychiatrists can’t tell real illness from faked disorder. She meets with his friends, his son, his colleagues, his students, and she flies all over the country to meet with those who can help her understand him. She gains access to his personal files and to the book he started to write about the experiment, then abandoned, which eventually resulted in a lawsuit by Doubleday to have the book’s advance returned. At one point, she even hires a private detective.
What is Ms. Cahalan looking for so desperately? She’s looking for these anonymous pseudopatients, the people who were admitted to these unnamed state hospitals, who made observations and took notes, who were diagnosed with schizophrenia and then finally released. She’s looking for the truth, and while she identifies Dr. Rosenhan and two other pseudopatients as people who faked their way into the hospital, she finds a mass of contradictions. The one pseudopatient was excluded from the study – he is the one who felt comforted by his time in the hospital. The other six pseudopatients could not be found, despite Ms. Cahalan’s heroic attempts. Furthermore, she found many inconsistencies in what Dr. Rosenhan reported, and his hospital notes revealed more than a presentation for voices saying “empty, hollow, thud.” He reported it had been going on for months, that he had put copper over his ears to block the sound, and that he felt suicidal.
Ultimately, Ms. Cahalan was left to conclude that the Rosenhan experiment was a lie, that the pseudopatients likely never existed and the article was a fabrication. She brings up other studies that have been proven to be fraudulent, and by this point, our faith in all of science is pretty shaken.
Ms. Cahalan took a long journey to get us to this place, one that spent a lot of effort in bashing psychiatry, finally concluding that, as a result of this fraudulent experiment, too many hospitals have been shuttered – leaving our sickest patients to the streets and to the jails – and that there are not enough mental health professionals. As a psychiatrist – one who is often willing to question our practices – I was distracted by the flagrant antipsychiatry sentiments. Reading past that, Ms. Cahalan’s remarkable detective work and creative intermingling of the Rosenhan experiment layered on the history of psychiatry, further layered on her own experience with psychosis, makes for an amazing story. The Rosenhan study may have rocked the world of psychiatry; the fact that it was fabricated should rock us even more.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Susannah Cahalan’s new book challenges an experiment that changed psychiatry
Susannah Cahalan’s new book challenges an experiment that changed psychiatry
As an undergraduate psychology major, I was taught about the Rosenhan study in several of my courses. My professors lectured about the shocking findings psychologist David Rosenhan, PhD, documented in a 1973 Science article, “On Being Sane in Insane Places” and these findings lent themselves to lecture hall drama. Eight people presented to hospitals and said they heard voices saying: “empty, hollow, thud.” These “pseudopatients” exhibited no other psychiatric symptoms but were admitted, diagnosed with schizophrenia, and observations of their behavior were made. The charts included notes such as, “Patient exhibits writing behavior,” my professors said. The pseudopatients were kept for an average of 19 days, and one for as long as 51 days. The decades have passed, and there are many things I learned in college that I have since forgotten, but I remember “empty, hollow, thud,” and the famous Rosenhan experiment.
I was eager to read Susannah Cahalan’s book, “The Great Pretender” (Grand Central Publishing, 2019), which puts both Dr. Rosenhan and his pseudopatient study under a microscope. Ms. Cahalan is the author of the page-turner, Brain on Fire: My Month of Madness (Free Press, 2012), where she recounted her own struggle with a psychotic episode. Ms. Cahalan, a young reporter in New York City, became psychotic and then catatonic; her condition perplexed the neurologists who were treating her on an inpatient unit, and they were on the verge of transferring her to psychiatry when a diagnosis of anti-NMDA receptor encephalitis was suspected and then confirmed with a brain biopsy. Ms. Cahalan made a full recovery after treatment with steroids, intravenous immunoglobulin, and plasmapheresis. While Ms. Cahalan’s symptoms were classic for a severe psychotic disorder, there was reason to believe that this was not a primary psychiatric disorder: She was having grand mal seizures. Her book was a bestseller, and she has spoken widely to make others aware of this rare illness that masquerades as psychosis. I heard her speak at the opening session of the American Psychiatric Association’s annual meeting in May of 2017.“My family, like many families before them, fought against the tyranny of the mental illness label,” Ms. Cahalan writes at the very beginning of “The Great Pretender.” She goes on to talk about how psychiatry differs from other medical fields: It’s the only specialty where people can be treated against their will; psychiatry casts judgments on the person; mental illness is poorly defined – perhaps there is no clear divide between normal and mad; and psychiatric disorders are less “real” than other illnesses. Throughout the book she refers to psychiatrists as smug and arrogant.
Ms. Cahalan takes on the task of documenting the horrors of psychiatry’s often sordid history, starting with journalist Nellie Bly’s 1887 journey into to a psychiatric facility to expose the abuses there. Certainly, psychiatry’s history is sordid. Ms. Cahalan talks about inhumane conditions in overcrowded psychiatric hospitals, about our sad chapter of lobotomies, about the influence of psychoanalysis on diagnosis and treatment, and about how homosexuality was once an illness and now is not. She includes “One Flew Over the Cuckoo’s Nest,” “The Myth of Mental Illness,” big pharma, and the Goldwater fiasco. In her recounting of the history, it’s all bad. She mentions Benjamin Rush, MD, only once, as the creator of “ ‘... the tranquilizing chair’ (a case of the worst false advertising ever), a terrifying sensory-deprivation apparatus in which patients were strapped down to a chair with a wooden box placed over their heads to block stimulation, restrict movement, and reduce blood to the brain.” Dr. Rush’s role as the father of American psychiatry who challenged the belief that mental illness was the result of demonic possession, gets no mention. Nor does Ms. Cahalan note that he founded Pennsylvania Hospital, where moral and occupational therapy revolutionized the treatment of those with mental illness.
So there’s her story, her rendition of the history of American psychiatry, and through this she weaves in the story of the Rosenhan experiment.
“ ‘It all started out as a dare,’ Dr. Rosenhan told a local newspaper, ‘I was teaching psychology at Swarthmore, and my students were saying that the course was too conceptual and abstract. So I said, ‘Okay, if you really want to know what mental patients are like, become mental patients.’ ”
Really? I read this and wondered how a psychologist could talk about people who had been hospitalized with psychiatric disorders as though they were aliens. Certainly, some of these students, their family members, or their friends must have been hospitalized at some point. Yet all through, there is this sense that the patients are other, and the discovery of the undercover operation is that the patients are actually human beings! Dr. Rosenhan, who was one of the pseudopatients, goes on to conclude that the label is everything, that once labeled they are treated differently by the nurses in “cages” and the doctors who walk by and avert their gaze. A second man Ms. Cahalan named, also one of the pseudopatients, had a similar experience. A third subject she located was not included in the study: His experience was counter to the findings of the study, his time in the hospital was a positive, he found it comforting, and the experiences he had there had a lasting positive influence on his life.
Ms. Cahalan talks about the publication of “On Being Sane in Insane Places” as a study that was finally scientific, one that changed all of psychiatry, and was the driving force for the creation of the DSM-III and the closure of state hospitals. I wondered if it was as influential as Ms. Cahalan claims, and I asked some psychiatrists who were practicing in 1973 when the article was published. I wanted to know if this study rocked their world.
“At first, with the great amount of publicity the study generated, it was added fodder for the antipsychiatrists, including the Scientologists and Szaszians,” Steven Sharfstein, MD, a former president of the American Psychiatric Association, told me. “But as young psychiatrists in the trenches, business continued to boom, and we continued to do the best job we could with diagnosis, assessment of risk, involuntary commitment, and treatment. And from what I recall, morale was high in the 1970s. We had some new medications and psychotherapies, and there was community activism. Faking symptoms to gain admission seemed to be a no-brainer, but keeping people for long stays was more problematic.”
E. Fuller Torrey, MD, the founder of the Treatment Advocacy Center who worked for many years treating patients at St. Elizabeth’s Hospital, replied: “It is important to remember that this study was published at the height of the deinstitutionalization movement and quite likely accelerated it. As I recall, at the time it seemed odd that all eight patients claimed to have had similar experiences while hospitalized. I think the main effect of the study was to provide ammunition for the antipsychiatrists.”
Ms. Cahalan has bought into the antipsychiatry movement full force. It’s not until the very end that there is any acknowledgment that psychiatry ever helped anyone, and even then, it’s a bit begrudging. Worse, she neglects to mention that people with psychiatric disorders suffer because of their psychic pain; one could get through this book and believe that people with mental illness struggle only because they are labeled and then mistreated, and for someone who has suffered herself, she misses the essence of how awful it is to be ill, and that people are often helped by psychiatric treatments. When she finally adds a paragraph talking about the usefulness of psychotropics, it’s with a caveat. “But I’m not here to rail against the drugs. There are plenty of places you can get that perspective. I see that these drugs help many people lead full and meaningful lives. It would be folly to discount their worth. We also can’t deny that the situation is complicated.”
There are moments in the manuscript where I found it difficult to know what were Dr. Rosenhan’s interpretations and what were Ms. Callahan’s interpretations of Dr. Rosenhan’s experiences. A lot of assumptions are made – particularly about the motivations of the hospital staff – and I wasn’t always sure they were correct. For example, on his second day in the hospital, Dr. Rosenhan asked a nurse for the newspaper. When she tells him it hasn’t come yet, he concludes that the staff is keeping the newspapers from the patients. And when a staff member is initially chatty then later shuns Dr. Rosenhan, he concludes that the man initially mistook him for a psychiatrist because he looks professorial. Both Ms. Cahalan and Dr. Rosenhan approach psychiatry with biases, and they don’t always question their assumptions.
, a professor who didn’t treat patients. This intermixing of the two fields felt contrived to me, and gave too much credence to the idea that no one really knows sane from disordered, and everyone was embracing the antipsychiatry dogma. Surely, someone during the those years must have liked their psychiatrist.
That said, Ms. Cahalan does a phenomenal job of infiltrating the world of the late Dr. Rosenhan. She starts out enamored by him and by his finding that psychiatrists can’t tell real illness from faked disorder. She meets with his friends, his son, his colleagues, his students, and she flies all over the country to meet with those who can help her understand him. She gains access to his personal files and to the book he started to write about the experiment, then abandoned, which eventually resulted in a lawsuit by Doubleday to have the book’s advance returned. At one point, she even hires a private detective.
What is Ms. Cahalan looking for so desperately? She’s looking for these anonymous pseudopatients, the people who were admitted to these unnamed state hospitals, who made observations and took notes, who were diagnosed with schizophrenia and then finally released. She’s looking for the truth, and while she identifies Dr. Rosenhan and two other pseudopatients as people who faked their way into the hospital, she finds a mass of contradictions. The one pseudopatient was excluded from the study – he is the one who felt comforted by his time in the hospital. The other six pseudopatients could not be found, despite Ms. Cahalan’s heroic attempts. Furthermore, she found many inconsistencies in what Dr. Rosenhan reported, and his hospital notes revealed more than a presentation for voices saying “empty, hollow, thud.” He reported it had been going on for months, that he had put copper over his ears to block the sound, and that he felt suicidal.
Ultimately, Ms. Cahalan was left to conclude that the Rosenhan experiment was a lie, that the pseudopatients likely never existed and the article was a fabrication. She brings up other studies that have been proven to be fraudulent, and by this point, our faith in all of science is pretty shaken.
Ms. Cahalan took a long journey to get us to this place, one that spent a lot of effort in bashing psychiatry, finally concluding that, as a result of this fraudulent experiment, too many hospitals have been shuttered – leaving our sickest patients to the streets and to the jails – and that there are not enough mental health professionals. As a psychiatrist – one who is often willing to question our practices – I was distracted by the flagrant antipsychiatry sentiments. Reading past that, Ms. Cahalan’s remarkable detective work and creative intermingling of the Rosenhan experiment layered on the history of psychiatry, further layered on her own experience with psychosis, makes for an amazing story. The Rosenhan study may have rocked the world of psychiatry; the fact that it was fabricated should rock us even more.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
As an undergraduate psychology major, I was taught about the Rosenhan study in several of my courses. My professors lectured about the shocking findings psychologist David Rosenhan, PhD, documented in a 1973 Science article, “On Being Sane in Insane Places” and these findings lent themselves to lecture hall drama. Eight people presented to hospitals and said they heard voices saying: “empty, hollow, thud.” These “pseudopatients” exhibited no other psychiatric symptoms but were admitted, diagnosed with schizophrenia, and observations of their behavior were made. The charts included notes such as, “Patient exhibits writing behavior,” my professors said. The pseudopatients were kept for an average of 19 days, and one for as long as 51 days. The decades have passed, and there are many things I learned in college that I have since forgotten, but I remember “empty, hollow, thud,” and the famous Rosenhan experiment.
I was eager to read Susannah Cahalan’s book, “The Great Pretender” (Grand Central Publishing, 2019), which puts both Dr. Rosenhan and his pseudopatient study under a microscope. Ms. Cahalan is the author of the page-turner, Brain on Fire: My Month of Madness (Free Press, 2012), where she recounted her own struggle with a psychotic episode. Ms. Cahalan, a young reporter in New York City, became psychotic and then catatonic; her condition perplexed the neurologists who were treating her on an inpatient unit, and they were on the verge of transferring her to psychiatry when a diagnosis of anti-NMDA receptor encephalitis was suspected and then confirmed with a brain biopsy. Ms. Cahalan made a full recovery after treatment with steroids, intravenous immunoglobulin, and plasmapheresis. While Ms. Cahalan’s symptoms were classic for a severe psychotic disorder, there was reason to believe that this was not a primary psychiatric disorder: She was having grand mal seizures. Her book was a bestseller, and she has spoken widely to make others aware of this rare illness that masquerades as psychosis. I heard her speak at the opening session of the American Psychiatric Association’s annual meeting in May of 2017.“My family, like many families before them, fought against the tyranny of the mental illness label,” Ms. Cahalan writes at the very beginning of “The Great Pretender.” She goes on to talk about how psychiatry differs from other medical fields: It’s the only specialty where people can be treated against their will; psychiatry casts judgments on the person; mental illness is poorly defined – perhaps there is no clear divide between normal and mad; and psychiatric disorders are less “real” than other illnesses. Throughout the book she refers to psychiatrists as smug and arrogant.
Ms. Cahalan takes on the task of documenting the horrors of psychiatry’s often sordid history, starting with journalist Nellie Bly’s 1887 journey into to a psychiatric facility to expose the abuses there. Certainly, psychiatry’s history is sordid. Ms. Cahalan talks about inhumane conditions in overcrowded psychiatric hospitals, about our sad chapter of lobotomies, about the influence of psychoanalysis on diagnosis and treatment, and about how homosexuality was once an illness and now is not. She includes “One Flew Over the Cuckoo’s Nest,” “The Myth of Mental Illness,” big pharma, and the Goldwater fiasco. In her recounting of the history, it’s all bad. She mentions Benjamin Rush, MD, only once, as the creator of “ ‘... the tranquilizing chair’ (a case of the worst false advertising ever), a terrifying sensory-deprivation apparatus in which patients were strapped down to a chair with a wooden box placed over their heads to block stimulation, restrict movement, and reduce blood to the brain.” Dr. Rush’s role as the father of American psychiatry who challenged the belief that mental illness was the result of demonic possession, gets no mention. Nor does Ms. Cahalan note that he founded Pennsylvania Hospital, where moral and occupational therapy revolutionized the treatment of those with mental illness.
So there’s her story, her rendition of the history of American psychiatry, and through this she weaves in the story of the Rosenhan experiment.
“ ‘It all started out as a dare,’ Dr. Rosenhan told a local newspaper, ‘I was teaching psychology at Swarthmore, and my students were saying that the course was too conceptual and abstract. So I said, ‘Okay, if you really want to know what mental patients are like, become mental patients.’ ”
Really? I read this and wondered how a psychologist could talk about people who had been hospitalized with psychiatric disorders as though they were aliens. Certainly, some of these students, their family members, or their friends must have been hospitalized at some point. Yet all through, there is this sense that the patients are other, and the discovery of the undercover operation is that the patients are actually human beings! Dr. Rosenhan, who was one of the pseudopatients, goes on to conclude that the label is everything, that once labeled they are treated differently by the nurses in “cages” and the doctors who walk by and avert their gaze. A second man Ms. Cahalan named, also one of the pseudopatients, had a similar experience. A third subject she located was not included in the study: His experience was counter to the findings of the study, his time in the hospital was a positive, he found it comforting, and the experiences he had there had a lasting positive influence on his life.
Ms. Cahalan talks about the publication of “On Being Sane in Insane Places” as a study that was finally scientific, one that changed all of psychiatry, and was the driving force for the creation of the DSM-III and the closure of state hospitals. I wondered if it was as influential as Ms. Cahalan claims, and I asked some psychiatrists who were practicing in 1973 when the article was published. I wanted to know if this study rocked their world.
“At first, with the great amount of publicity the study generated, it was added fodder for the antipsychiatrists, including the Scientologists and Szaszians,” Steven Sharfstein, MD, a former president of the American Psychiatric Association, told me. “But as young psychiatrists in the trenches, business continued to boom, and we continued to do the best job we could with diagnosis, assessment of risk, involuntary commitment, and treatment. And from what I recall, morale was high in the 1970s. We had some new medications and psychotherapies, and there was community activism. Faking symptoms to gain admission seemed to be a no-brainer, but keeping people for long stays was more problematic.”
E. Fuller Torrey, MD, the founder of the Treatment Advocacy Center who worked for many years treating patients at St. Elizabeth’s Hospital, replied: “It is important to remember that this study was published at the height of the deinstitutionalization movement and quite likely accelerated it. As I recall, at the time it seemed odd that all eight patients claimed to have had similar experiences while hospitalized. I think the main effect of the study was to provide ammunition for the antipsychiatrists.”
Ms. Cahalan has bought into the antipsychiatry movement full force. It’s not until the very end that there is any acknowledgment that psychiatry ever helped anyone, and even then, it’s a bit begrudging. Worse, she neglects to mention that people with psychiatric disorders suffer because of their psychic pain; one could get through this book and believe that people with mental illness struggle only because they are labeled and then mistreated, and for someone who has suffered herself, she misses the essence of how awful it is to be ill, and that people are often helped by psychiatric treatments. When she finally adds a paragraph talking about the usefulness of psychotropics, it’s with a caveat. “But I’m not here to rail against the drugs. There are plenty of places you can get that perspective. I see that these drugs help many people lead full and meaningful lives. It would be folly to discount their worth. We also can’t deny that the situation is complicated.”
There are moments in the manuscript where I found it difficult to know what were Dr. Rosenhan’s interpretations and what were Ms. Callahan’s interpretations of Dr. Rosenhan’s experiences. A lot of assumptions are made – particularly about the motivations of the hospital staff – and I wasn’t always sure they were correct. For example, on his second day in the hospital, Dr. Rosenhan asked a nurse for the newspaper. When she tells him it hasn’t come yet, he concludes that the staff is keeping the newspapers from the patients. And when a staff member is initially chatty then later shuns Dr. Rosenhan, he concludes that the man initially mistook him for a psychiatrist because he looks professorial. Both Ms. Cahalan and Dr. Rosenhan approach psychiatry with biases, and they don’t always question their assumptions.
, a professor who didn’t treat patients. This intermixing of the two fields felt contrived to me, and gave too much credence to the idea that no one really knows sane from disordered, and everyone was embracing the antipsychiatry dogma. Surely, someone during the those years must have liked their psychiatrist.
That said, Ms. Cahalan does a phenomenal job of infiltrating the world of the late Dr. Rosenhan. She starts out enamored by him and by his finding that psychiatrists can’t tell real illness from faked disorder. She meets with his friends, his son, his colleagues, his students, and she flies all over the country to meet with those who can help her understand him. She gains access to his personal files and to the book he started to write about the experiment, then abandoned, which eventually resulted in a lawsuit by Doubleday to have the book’s advance returned. At one point, she even hires a private detective.
What is Ms. Cahalan looking for so desperately? She’s looking for these anonymous pseudopatients, the people who were admitted to these unnamed state hospitals, who made observations and took notes, who were diagnosed with schizophrenia and then finally released. She’s looking for the truth, and while she identifies Dr. Rosenhan and two other pseudopatients as people who faked their way into the hospital, she finds a mass of contradictions. The one pseudopatient was excluded from the study – he is the one who felt comforted by his time in the hospital. The other six pseudopatients could not be found, despite Ms. Cahalan’s heroic attempts. Furthermore, she found many inconsistencies in what Dr. Rosenhan reported, and his hospital notes revealed more than a presentation for voices saying “empty, hollow, thud.” He reported it had been going on for months, that he had put copper over his ears to block the sound, and that he felt suicidal.
Ultimately, Ms. Cahalan was left to conclude that the Rosenhan experiment was a lie, that the pseudopatients likely never existed and the article was a fabrication. She brings up other studies that have been proven to be fraudulent, and by this point, our faith in all of science is pretty shaken.
Ms. Cahalan took a long journey to get us to this place, one that spent a lot of effort in bashing psychiatry, finally concluding that, as a result of this fraudulent experiment, too many hospitals have been shuttered – leaving our sickest patients to the streets and to the jails – and that there are not enough mental health professionals. As a psychiatrist – one who is often willing to question our practices – I was distracted by the flagrant antipsychiatry sentiments. Reading past that, Ms. Cahalan’s remarkable detective work and creative intermingling of the Rosenhan experiment layered on the history of psychiatry, further layered on her own experience with psychosis, makes for an amazing story. The Rosenhan study may have rocked the world of psychiatry; the fact that it was fabricated should rock us even more.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Patients need physicians who see – and feel – beyond the EMR
CHICAGO – Speaking to a rapt audience of radiologists, an infectious disease physician who writes and teaches about the importance of human touch in medicine held sway at the opening session of the annual meeting of the Radiological Society of North America.
It wasn’t hard for Abraham Verghese, MD, to find points of commonality between those who sit in dark reading rooms and those who roam the wards.
The EMR, Dr. Verghese said, is a “system of epic disaster. It was not designed for ease of use; it was designed for billing. ... Frankly, we are the highest-paid clerical workers in the hospital, and that has to change. The Stone Age didn’t end because we ran out of stone; it ended because we had better ideas.”
The daily EMR click count for physicians has been estimated at 4,000, and it’s but part of the problem, said Dr. Verghese, professor of medicine at Stanford (Calif.) University. “For every hour of cumulative patient care, physicians spend 1½ hours on the computer, and another hour of our personal time at home dealing with our inbox,” he said. EMR systems may dominate clinical life for physicians, “but they were not built for our ease.”
Dr. Verghese is a practicing physician and medical educator, and is also the author of a body of fiction and nonfiction literature that delineates the physician-patient relationship. His TED-style talk followed opening remarks from Valerie Jackson, MD, the president of the Radiological Society of North America, who encouraged radiologists to reach out for a more direct connection with patients and with nonradiologist colleagues.
The patient connection – the human factor that leads many into the practice of medicine – can be eroded for myriad reasons, but health care systems that don’t elevate the physician-patient relationship do so at the peril of serious physician burnout, said Dr. Verghese. By some measures, and in some specialties, half of physicians score high on validated burnout indices – and a burned-out physician is at high risk for leaving the profession.
Dr. Verghese quoted the poet Anatole Broyard, who was treated for prostate cancer and wrote extensively about his experiences.
Wishing for a more personal connection with his physician, Mr. Broyard wrote: “I just wish he would brood on my situation for perhaps 5 minutes, that he would give me his whole mind just once, be bonded with me for a brief space, survey my soul as well as my flesh, to get at my illness, for each man is ill in his own way.”
It’s this opportunity for connection and contemplation that is sacrificed when, as Dr. Verghese said, “the patient in the bed has become a mere icon for the ‘real’ patient in the computer.”
Dr. Jackson, executive director of the American Board of Radiology, and Dr. Verghese both acknowledged that authentic patient connections can make practice more rewarding and reduce the risk of burnout.
Dr. Verghese also discussed other areas of risk when patients and their physicians are separated by an electronic divide.
“We are all getting distracted by our peripheral brains,” and patients may suffer when medical errors result from inattention and a reluctance to “trust what our eyes are showing us,” he said. He and his colleagues solicited and reported 208 vignettes of medical error. In 63% of the cases, the root cause of the error was failure to perform a physical examination (Am J Med. 2015 Dec;128[12]:1322-4.e3). “Patients have a front side – and a back side!” he said, to appreciative laughter. A careful physical exam, he said, involves inspecting – and palpating – both sides.
The act of putting hands on an unclothed patient for a physical exam would violate many societal norms, said Dr. Verghese, were it not for the special rules conferred on the physician-patient relationship.
“One individual in this dyad disrobes and allows touch. In any other context in this society, this is assault,” he said. “The very great privilege of our profession ... is that we are privileged to examine [patients’] bodies, and to touch.”
The gift of this ritual is not to be squandered, he said, adding that patients understand the special rhythm of the physical examination. “If you come in and do a half-assed probe of their belly and stick your stethoscope on top of their paper gown, they are on to you.”
Describing his own method for the physical exam, Dr. Verghese said that there’s something that feels commandeering and intrusive about beginning directly at the head, as one is taught. Instead, he offers an outstretched hand and begins with a handshake, noting grip strength, any tremor, hydration, and condition of skin and nails. Then, he caps the handshake with his other hand and slides two fingers over to the radial pulse, where he gathers more information, all the while strengthening his bond with his patient. His exam, he said, is his own, with its own rhythms and order which have not varied in decades.
Whatever the method, “this skill has to be passed on, and there is no easy way to do it. ... But when you examine well, you are preserving the ‘person-ality,’ the embodied identity of the patient.”
From the time of William Osler – and perhaps before – the physical examination has been a “symbolic centering on the body as a locus of personhood and disease,” said Dr. Verghese.
Dr. Jackson encouraged her radiologist peers to come out from the reading room to greet and connect with patients in the imaging suite. Similarly, Dr. Verghese said, technology can be used to “connect the image, or the biopsy report, or the lab test, to the personhood” of the patient. Bringing a tablet with imaging results or a laboratory readout to the bedside or the exam table and helping the patient place the findings on or within her own body marries the best of old and new.
He shared with the audience his practice for examining patients presenting with chronic fatigue – a condition that can be challenging to diagnose and manage.
These patients “come to you ready for you to join the long line of physicians who have disappointed them,” said Dr. Verghese, who at one time saw many such patients. He said that he developed a strategy of first listening, and then examining. “A very interesting thing happened – the voluble patient began to quiet down” under his examiner’s hands. If patients could, through his approach, relinquish their ceaseless quest for a definitive diagnosis “and instead begin a partnership toward wellness,” he felt he’d reached success. “It was because something magical had transpired in that encounter.”
Neither Dr. Verghese nor Dr. Jackson reported any conflicts of interest relevant to their presentations.
CHICAGO – Speaking to a rapt audience of radiologists, an infectious disease physician who writes and teaches about the importance of human touch in medicine held sway at the opening session of the annual meeting of the Radiological Society of North America.
It wasn’t hard for Abraham Verghese, MD, to find points of commonality between those who sit in dark reading rooms and those who roam the wards.
The EMR, Dr. Verghese said, is a “system of epic disaster. It was not designed for ease of use; it was designed for billing. ... Frankly, we are the highest-paid clerical workers in the hospital, and that has to change. The Stone Age didn’t end because we ran out of stone; it ended because we had better ideas.”
The daily EMR click count for physicians has been estimated at 4,000, and it’s but part of the problem, said Dr. Verghese, professor of medicine at Stanford (Calif.) University. “For every hour of cumulative patient care, physicians spend 1½ hours on the computer, and another hour of our personal time at home dealing with our inbox,” he said. EMR systems may dominate clinical life for physicians, “but they were not built for our ease.”
Dr. Verghese is a practicing physician and medical educator, and is also the author of a body of fiction and nonfiction literature that delineates the physician-patient relationship. His TED-style talk followed opening remarks from Valerie Jackson, MD, the president of the Radiological Society of North America, who encouraged radiologists to reach out for a more direct connection with patients and with nonradiologist colleagues.
The patient connection – the human factor that leads many into the practice of medicine – can be eroded for myriad reasons, but health care systems that don’t elevate the physician-patient relationship do so at the peril of serious physician burnout, said Dr. Verghese. By some measures, and in some specialties, half of physicians score high on validated burnout indices – and a burned-out physician is at high risk for leaving the profession.
Dr. Verghese quoted the poet Anatole Broyard, who was treated for prostate cancer and wrote extensively about his experiences.
Wishing for a more personal connection with his physician, Mr. Broyard wrote: “I just wish he would brood on my situation for perhaps 5 minutes, that he would give me his whole mind just once, be bonded with me for a brief space, survey my soul as well as my flesh, to get at my illness, for each man is ill in his own way.”
It’s this opportunity for connection and contemplation that is sacrificed when, as Dr. Verghese said, “the patient in the bed has become a mere icon for the ‘real’ patient in the computer.”
Dr. Jackson, executive director of the American Board of Radiology, and Dr. Verghese both acknowledged that authentic patient connections can make practice more rewarding and reduce the risk of burnout.
Dr. Verghese also discussed other areas of risk when patients and their physicians are separated by an electronic divide.
“We are all getting distracted by our peripheral brains,” and patients may suffer when medical errors result from inattention and a reluctance to “trust what our eyes are showing us,” he said. He and his colleagues solicited and reported 208 vignettes of medical error. In 63% of the cases, the root cause of the error was failure to perform a physical examination (Am J Med. 2015 Dec;128[12]:1322-4.e3). “Patients have a front side – and a back side!” he said, to appreciative laughter. A careful physical exam, he said, involves inspecting – and palpating – both sides.
The act of putting hands on an unclothed patient for a physical exam would violate many societal norms, said Dr. Verghese, were it not for the special rules conferred on the physician-patient relationship.
“One individual in this dyad disrobes and allows touch. In any other context in this society, this is assault,” he said. “The very great privilege of our profession ... is that we are privileged to examine [patients’] bodies, and to touch.”
The gift of this ritual is not to be squandered, he said, adding that patients understand the special rhythm of the physical examination. “If you come in and do a half-assed probe of their belly and stick your stethoscope on top of their paper gown, they are on to you.”
Describing his own method for the physical exam, Dr. Verghese said that there’s something that feels commandeering and intrusive about beginning directly at the head, as one is taught. Instead, he offers an outstretched hand and begins with a handshake, noting grip strength, any tremor, hydration, and condition of skin and nails. Then, he caps the handshake with his other hand and slides two fingers over to the radial pulse, where he gathers more information, all the while strengthening his bond with his patient. His exam, he said, is his own, with its own rhythms and order which have not varied in decades.
Whatever the method, “this skill has to be passed on, and there is no easy way to do it. ... But when you examine well, you are preserving the ‘person-ality,’ the embodied identity of the patient.”
From the time of William Osler – and perhaps before – the physical examination has been a “symbolic centering on the body as a locus of personhood and disease,” said Dr. Verghese.
Dr. Jackson encouraged her radiologist peers to come out from the reading room to greet and connect with patients in the imaging suite. Similarly, Dr. Verghese said, technology can be used to “connect the image, or the biopsy report, or the lab test, to the personhood” of the patient. Bringing a tablet with imaging results or a laboratory readout to the bedside or the exam table and helping the patient place the findings on or within her own body marries the best of old and new.
He shared with the audience his practice for examining patients presenting with chronic fatigue – a condition that can be challenging to diagnose and manage.
These patients “come to you ready for you to join the long line of physicians who have disappointed them,” said Dr. Verghese, who at one time saw many such patients. He said that he developed a strategy of first listening, and then examining. “A very interesting thing happened – the voluble patient began to quiet down” under his examiner’s hands. If patients could, through his approach, relinquish their ceaseless quest for a definitive diagnosis “and instead begin a partnership toward wellness,” he felt he’d reached success. “It was because something magical had transpired in that encounter.”
Neither Dr. Verghese nor Dr. Jackson reported any conflicts of interest relevant to their presentations.
CHICAGO – Speaking to a rapt audience of radiologists, an infectious disease physician who writes and teaches about the importance of human touch in medicine held sway at the opening session of the annual meeting of the Radiological Society of North America.
It wasn’t hard for Abraham Verghese, MD, to find points of commonality between those who sit in dark reading rooms and those who roam the wards.
The EMR, Dr. Verghese said, is a “system of epic disaster. It was not designed for ease of use; it was designed for billing. ... Frankly, we are the highest-paid clerical workers in the hospital, and that has to change. The Stone Age didn’t end because we ran out of stone; it ended because we had better ideas.”
The daily EMR click count for physicians has been estimated at 4,000, and it’s but part of the problem, said Dr. Verghese, professor of medicine at Stanford (Calif.) University. “For every hour of cumulative patient care, physicians spend 1½ hours on the computer, and another hour of our personal time at home dealing with our inbox,” he said. EMR systems may dominate clinical life for physicians, “but they were not built for our ease.”
Dr. Verghese is a practicing physician and medical educator, and is also the author of a body of fiction and nonfiction literature that delineates the physician-patient relationship. His TED-style talk followed opening remarks from Valerie Jackson, MD, the president of the Radiological Society of North America, who encouraged radiologists to reach out for a more direct connection with patients and with nonradiologist colleagues.
The patient connection – the human factor that leads many into the practice of medicine – can be eroded for myriad reasons, but health care systems that don’t elevate the physician-patient relationship do so at the peril of serious physician burnout, said Dr. Verghese. By some measures, and in some specialties, half of physicians score high on validated burnout indices – and a burned-out physician is at high risk for leaving the profession.
Dr. Verghese quoted the poet Anatole Broyard, who was treated for prostate cancer and wrote extensively about his experiences.
Wishing for a more personal connection with his physician, Mr. Broyard wrote: “I just wish he would brood on my situation for perhaps 5 minutes, that he would give me his whole mind just once, be bonded with me for a brief space, survey my soul as well as my flesh, to get at my illness, for each man is ill in his own way.”
It’s this opportunity for connection and contemplation that is sacrificed when, as Dr. Verghese said, “the patient in the bed has become a mere icon for the ‘real’ patient in the computer.”
Dr. Jackson, executive director of the American Board of Radiology, and Dr. Verghese both acknowledged that authentic patient connections can make practice more rewarding and reduce the risk of burnout.
Dr. Verghese also discussed other areas of risk when patients and their physicians are separated by an electronic divide.
“We are all getting distracted by our peripheral brains,” and patients may suffer when medical errors result from inattention and a reluctance to “trust what our eyes are showing us,” he said. He and his colleagues solicited and reported 208 vignettes of medical error. In 63% of the cases, the root cause of the error was failure to perform a physical examination (Am J Med. 2015 Dec;128[12]:1322-4.e3). “Patients have a front side – and a back side!” he said, to appreciative laughter. A careful physical exam, he said, involves inspecting – and palpating – both sides.
The act of putting hands on an unclothed patient for a physical exam would violate many societal norms, said Dr. Verghese, were it not for the special rules conferred on the physician-patient relationship.
“One individual in this dyad disrobes and allows touch. In any other context in this society, this is assault,” he said. “The very great privilege of our profession ... is that we are privileged to examine [patients’] bodies, and to touch.”
The gift of this ritual is not to be squandered, he said, adding that patients understand the special rhythm of the physical examination. “If you come in and do a half-assed probe of their belly and stick your stethoscope on top of their paper gown, they are on to you.”
Describing his own method for the physical exam, Dr. Verghese said that there’s something that feels commandeering and intrusive about beginning directly at the head, as one is taught. Instead, he offers an outstretched hand and begins with a handshake, noting grip strength, any tremor, hydration, and condition of skin and nails. Then, he caps the handshake with his other hand and slides two fingers over to the radial pulse, where he gathers more information, all the while strengthening his bond with his patient. His exam, he said, is his own, with its own rhythms and order which have not varied in decades.
Whatever the method, “this skill has to be passed on, and there is no easy way to do it. ... But when you examine well, you are preserving the ‘person-ality,’ the embodied identity of the patient.”
From the time of William Osler – and perhaps before – the physical examination has been a “symbolic centering on the body as a locus of personhood and disease,” said Dr. Verghese.
Dr. Jackson encouraged her radiologist peers to come out from the reading room to greet and connect with patients in the imaging suite. Similarly, Dr. Verghese said, technology can be used to “connect the image, or the biopsy report, or the lab test, to the personhood” of the patient. Bringing a tablet with imaging results or a laboratory readout to the bedside or the exam table and helping the patient place the findings on or within her own body marries the best of old and new.
He shared with the audience his practice for examining patients presenting with chronic fatigue – a condition that can be challenging to diagnose and manage.
These patients “come to you ready for you to join the long line of physicians who have disappointed them,” said Dr. Verghese, who at one time saw many such patients. He said that he developed a strategy of first listening, and then examining. “A very interesting thing happened – the voluble patient began to quiet down” under his examiner’s hands. If patients could, through his approach, relinquish their ceaseless quest for a definitive diagnosis “and instead begin a partnership toward wellness,” he felt he’d reached success. “It was because something magical had transpired in that encounter.”
Neither Dr. Verghese nor Dr. Jackson reported any conflicts of interest relevant to their presentations.
EXPERT ANALYSIS FROM RSNA 2019
Capecitabine extends survival in triple-negative breast cancer
SAN ANTONIO – For patients with early-stage triple-negative breast cancer, adding capecitabine to systemic treatment may extend overall survival, according to a meta-analysis involving more than 15,000 patients.
Although a variety of trials have tested capecitabine therapy for early-stage breast cancer, this study is the first to evaluate individual patient data across trials, reported lead author Marion van Mackelenbergh, MD, of University Medical Center Schleswig-Holstein in Kiel, Germany, and colleagues.
According to Dr. Mackelenbergh, who presented findings at the San Antonio Breast Cancer Symposium, the two previous literature-based meta-analyses of capecitabine for patients with early-stage breast cancer reported conflicting results; the first study suggested that capecitabine had no benefit as a neoadjuvant therapy, whereas the second study concluded that capecitabine could improve disease-free survival (DFS).
To build upon these findings, the primary objective of the present meta-analysis was to determine how treatment with capecitabine impacts DFS, Dr. Mackelenbergh said. A variety of secondary objectives were also evaluated, including overall survival and a possible interaction between capecitabine-specific toxicities and treatment effects.
The analysis involved 15,457 patients from 12 randomized prospective clinical trials, of whom about half (n = 7,477) were treated in control arms. Slightly more than half (55.9%) of the patients had stage II tumors and about three-fourths (74.0%) presented with nodal involvement. About two-thirds of patients (66.0%) had estrogen receptor–positive disease, about half (56.9%) were progesterone receptor–positive, and 15.1% were human epidermal growth factor receptor 2–positive. Most of the patients (81.8%) were treated with chemotherapy in the adjuvant setting, whereas the remainder (18.2%) received neoadjuvant therapy.
Cox regression analysis involving all patients in the dataset showed that capecitabine was not associated with a significant improvement in DFS, nor was a significant improvement seen in trials that compared capecitabine against other treatment options. In contrast, adding capecitabine to systemic treatment supported a modest but significant improvement in DFS (hazard ratio, 0.888; P = .0005).
Across all patients, capecitabine was associated with an overall survival advantage, although this benefit was relatively small, with a hazard ratio of 0.892. The overall survival benefit became more pronounced when capecitabine was added to systemic treatment (HR, 0.837).
Of clinical importance, biological subtype analysis showed that only patients with triple-negative breast cancer were deriving survival benefit from capecitabine, particularly when capecitabine was added to systemic treatment. Among patients with triple-negative disease, a 17% overall survival benefit was associated with capecitabine (HR, 0.828). When capecitabine was added to systemic treatment, this survival advantage improved to 22% (HR, 0.778).
No relationship was found between capecitabine-specific toxicity (mucositis, hand-foot syndrome, diarrhea) and patient outcome.
“It can be concluded that the addition of capecitabine to other systemic treatment may be recommended for triple-negative breast cancer patients,” Dr. Mackelenbergh said.
Invited discussant Priyanka Sharma, MD, of the University of Kansas Medical Center in Kansas City agreed with this conclusion.
“Routine use of capecitabine as a component of neoadjuvant or adjuvant regimens in unselected patients cannot be endorsed,” Dr. Sharma said. “However, capecitabine should be considered in patients with triple-negative breast cancer, especially if response to neoadjuvant chemotherapy is utilized as a way to identify eligible patients so as to limit exposure and toxicity to those at the highest risk.”
In terms of the future, Dr. Sharma suggested that research is needed to determine the potential for adjuvant capecitabine among patients with triple-negative breast cancer who have received platinum-based chemotherapy and/or immune checkpoint inhibitor therapy as part of their neoadjuvant regimen. In addition, investigators should evaluate capecitabine efficacy in terms of residual disease and identify relevant predictive biomarkers, Dr. Sharma said.
The investigators reported relationships with Amgen, Lilly, Pfizer, and others.
SOURCE: van Mackelenbergh M et al. SABCS 2019, Abstract GS1-07.
SAN ANTONIO – For patients with early-stage triple-negative breast cancer, adding capecitabine to systemic treatment may extend overall survival, according to a meta-analysis involving more than 15,000 patients.
Although a variety of trials have tested capecitabine therapy for early-stage breast cancer, this study is the first to evaluate individual patient data across trials, reported lead author Marion van Mackelenbergh, MD, of University Medical Center Schleswig-Holstein in Kiel, Germany, and colleagues.
According to Dr. Mackelenbergh, who presented findings at the San Antonio Breast Cancer Symposium, the two previous literature-based meta-analyses of capecitabine for patients with early-stage breast cancer reported conflicting results; the first study suggested that capecitabine had no benefit as a neoadjuvant therapy, whereas the second study concluded that capecitabine could improve disease-free survival (DFS).
To build upon these findings, the primary objective of the present meta-analysis was to determine how treatment with capecitabine impacts DFS, Dr. Mackelenbergh said. A variety of secondary objectives were also evaluated, including overall survival and a possible interaction between capecitabine-specific toxicities and treatment effects.
The analysis involved 15,457 patients from 12 randomized prospective clinical trials, of whom about half (n = 7,477) were treated in control arms. Slightly more than half (55.9%) of the patients had stage II tumors and about three-fourths (74.0%) presented with nodal involvement. About two-thirds of patients (66.0%) had estrogen receptor–positive disease, about half (56.9%) were progesterone receptor–positive, and 15.1% were human epidermal growth factor receptor 2–positive. Most of the patients (81.8%) were treated with chemotherapy in the adjuvant setting, whereas the remainder (18.2%) received neoadjuvant therapy.
Cox regression analysis involving all patients in the dataset showed that capecitabine was not associated with a significant improvement in DFS, nor was a significant improvement seen in trials that compared capecitabine against other treatment options. In contrast, adding capecitabine to systemic treatment supported a modest but significant improvement in DFS (hazard ratio, 0.888; P = .0005).
Across all patients, capecitabine was associated with an overall survival advantage, although this benefit was relatively small, with a hazard ratio of 0.892. The overall survival benefit became more pronounced when capecitabine was added to systemic treatment (HR, 0.837).
Of clinical importance, biological subtype analysis showed that only patients with triple-negative breast cancer were deriving survival benefit from capecitabine, particularly when capecitabine was added to systemic treatment. Among patients with triple-negative disease, a 17% overall survival benefit was associated with capecitabine (HR, 0.828). When capecitabine was added to systemic treatment, this survival advantage improved to 22% (HR, 0.778).
No relationship was found between capecitabine-specific toxicity (mucositis, hand-foot syndrome, diarrhea) and patient outcome.
“It can be concluded that the addition of capecitabine to other systemic treatment may be recommended for triple-negative breast cancer patients,” Dr. Mackelenbergh said.
Invited discussant Priyanka Sharma, MD, of the University of Kansas Medical Center in Kansas City agreed with this conclusion.
“Routine use of capecitabine as a component of neoadjuvant or adjuvant regimens in unselected patients cannot be endorsed,” Dr. Sharma said. “However, capecitabine should be considered in patients with triple-negative breast cancer, especially if response to neoadjuvant chemotherapy is utilized as a way to identify eligible patients so as to limit exposure and toxicity to those at the highest risk.”
In terms of the future, Dr. Sharma suggested that research is needed to determine the potential for adjuvant capecitabine among patients with triple-negative breast cancer who have received platinum-based chemotherapy and/or immune checkpoint inhibitor therapy as part of their neoadjuvant regimen. In addition, investigators should evaluate capecitabine efficacy in terms of residual disease and identify relevant predictive biomarkers, Dr. Sharma said.
The investigators reported relationships with Amgen, Lilly, Pfizer, and others.
SOURCE: van Mackelenbergh M et al. SABCS 2019, Abstract GS1-07.
SAN ANTONIO – For patients with early-stage triple-negative breast cancer, adding capecitabine to systemic treatment may extend overall survival, according to a meta-analysis involving more than 15,000 patients.
Although a variety of trials have tested capecitabine therapy for early-stage breast cancer, this study is the first to evaluate individual patient data across trials, reported lead author Marion van Mackelenbergh, MD, of University Medical Center Schleswig-Holstein in Kiel, Germany, and colleagues.
According to Dr. Mackelenbergh, who presented findings at the San Antonio Breast Cancer Symposium, the two previous literature-based meta-analyses of capecitabine for patients with early-stage breast cancer reported conflicting results; the first study suggested that capecitabine had no benefit as a neoadjuvant therapy, whereas the second study concluded that capecitabine could improve disease-free survival (DFS).
To build upon these findings, the primary objective of the present meta-analysis was to determine how treatment with capecitabine impacts DFS, Dr. Mackelenbergh said. A variety of secondary objectives were also evaluated, including overall survival and a possible interaction between capecitabine-specific toxicities and treatment effects.
The analysis involved 15,457 patients from 12 randomized prospective clinical trials, of whom about half (n = 7,477) were treated in control arms. Slightly more than half (55.9%) of the patients had stage II tumors and about three-fourths (74.0%) presented with nodal involvement. About two-thirds of patients (66.0%) had estrogen receptor–positive disease, about half (56.9%) were progesterone receptor–positive, and 15.1% were human epidermal growth factor receptor 2–positive. Most of the patients (81.8%) were treated with chemotherapy in the adjuvant setting, whereas the remainder (18.2%) received neoadjuvant therapy.
Cox regression analysis involving all patients in the dataset showed that capecitabine was not associated with a significant improvement in DFS, nor was a significant improvement seen in trials that compared capecitabine against other treatment options. In contrast, adding capecitabine to systemic treatment supported a modest but significant improvement in DFS (hazard ratio, 0.888; P = .0005).
Across all patients, capecitabine was associated with an overall survival advantage, although this benefit was relatively small, with a hazard ratio of 0.892. The overall survival benefit became more pronounced when capecitabine was added to systemic treatment (HR, 0.837).
Of clinical importance, biological subtype analysis showed that only patients with triple-negative breast cancer were deriving survival benefit from capecitabine, particularly when capecitabine was added to systemic treatment. Among patients with triple-negative disease, a 17% overall survival benefit was associated with capecitabine (HR, 0.828). When capecitabine was added to systemic treatment, this survival advantage improved to 22% (HR, 0.778).
No relationship was found between capecitabine-specific toxicity (mucositis, hand-foot syndrome, diarrhea) and patient outcome.
“It can be concluded that the addition of capecitabine to other systemic treatment may be recommended for triple-negative breast cancer patients,” Dr. Mackelenbergh said.
Invited discussant Priyanka Sharma, MD, of the University of Kansas Medical Center in Kansas City agreed with this conclusion.
“Routine use of capecitabine as a component of neoadjuvant or adjuvant regimens in unselected patients cannot be endorsed,” Dr. Sharma said. “However, capecitabine should be considered in patients with triple-negative breast cancer, especially if response to neoadjuvant chemotherapy is utilized as a way to identify eligible patients so as to limit exposure and toxicity to those at the highest risk.”
In terms of the future, Dr. Sharma suggested that research is needed to determine the potential for adjuvant capecitabine among patients with triple-negative breast cancer who have received platinum-based chemotherapy and/or immune checkpoint inhibitor therapy as part of their neoadjuvant regimen. In addition, investigators should evaluate capecitabine efficacy in terms of residual disease and identify relevant predictive biomarkers, Dr. Sharma said.
The investigators reported relationships with Amgen, Lilly, Pfizer, and others.
SOURCE: van Mackelenbergh M et al. SABCS 2019, Abstract GS1-07.
REPORTING FROM SABCS 2019
Evidence builds for effects of obesity, low physical activity on development of psoriatic arthritis
A new Norwegian study has identified a high body mass index and lower levels of physical activity as modifiable risk factors for developing psoriatic arthritis (PsA).
“Our study adds to the growing evidence that the risk of PsA is modifiable and highlights the importance of preventive work against obesity as well as encouraging physical activity in order to reduce the incidence of PsA,” wrote Ruth S. Thomsen, MD, of the Norwegian University of Science and Technology, and coauthors. The study was published in Arthritis Care & Research.
To determine the impact that adiposity and body fat distribution have on developing PsA, the researchers analyzed data from 36,626 women and men who participated in two surveys from the longitudinal, population-based Norwegian HUNT Study. All participants did not have diagnosed PsA at baseline in 1995-1997. Variables used in statistical analysis included calculated baseline BMI, waist circumference, waist-hip ratio, and level of physical activity.
Between baseline and follow-up in 2012, 185 new cases of PsA were reported. One standard deviation increase in BMI (4.2 kg/m2 for women, 3.5 kg/m2 for men) and waist circumstance (10.8 cm for women, 8.6 cm for men) was associated with adjusted hazard ratios of 1.40 (95% confidence interval, 1.24-1.58) and 1.48 (95% CI, 1.31-1.68), accordingly. Obese individuals – defined as BMI of 30 kg/m2 or higher – had an adjusted HR of 2.46 (95% CI, 1.65-3.68) when compared with individuals at normal weight.
Compared to individuals with BMI less than 25 kg/m2 and a high level of physical activity, individuals with BMI at 25 kg/m2 or higher and any lower level of physical activity had an adjusted HR of 2.06 (95% CI, 1.18-3.58). In addition, individuals with a high waist circumference – defined as 81 cm or more in women and 95 cm or more in men – and low physical activity had an adjusted HR of 2.22 (95% CI, 1.37-3.58) in comparison to those with a high waist circumference and high physical activity (adjusted HR, 1.84; 95% CI, 0.97-3.47). Physical activity level was considered low with less than 3 hours of light physical activity and no hard physical activity per week and high with any amount of light activity plus 1 or more hours of hard physical activity.
The authors acknowledged the study’s potential limitations, including the requirement for participants to complete the final two HUNT study surveys and the use of stricter criteria than usual in validating PsA diagnoses.
The study was partially funded by a grant Dr. Thomsen received from the Norwegian Extra Foundation for Health and Rehabilitation. The authors reported no conflicts of interest.
SOURCE: Thomsen RS et al. Arthritis Care Res. 2019 Dec 7. doi: 10.1002/acr.24121.
A new Norwegian study has identified a high body mass index and lower levels of physical activity as modifiable risk factors for developing psoriatic arthritis (PsA).
“Our study adds to the growing evidence that the risk of PsA is modifiable and highlights the importance of preventive work against obesity as well as encouraging physical activity in order to reduce the incidence of PsA,” wrote Ruth S. Thomsen, MD, of the Norwegian University of Science and Technology, and coauthors. The study was published in Arthritis Care & Research.
To determine the impact that adiposity and body fat distribution have on developing PsA, the researchers analyzed data from 36,626 women and men who participated in two surveys from the longitudinal, population-based Norwegian HUNT Study. All participants did not have diagnosed PsA at baseline in 1995-1997. Variables used in statistical analysis included calculated baseline BMI, waist circumference, waist-hip ratio, and level of physical activity.
Between baseline and follow-up in 2012, 185 new cases of PsA were reported. One standard deviation increase in BMI (4.2 kg/m2 for women, 3.5 kg/m2 for men) and waist circumstance (10.8 cm for women, 8.6 cm for men) was associated with adjusted hazard ratios of 1.40 (95% confidence interval, 1.24-1.58) and 1.48 (95% CI, 1.31-1.68), accordingly. Obese individuals – defined as BMI of 30 kg/m2 or higher – had an adjusted HR of 2.46 (95% CI, 1.65-3.68) when compared with individuals at normal weight.
Compared to individuals with BMI less than 25 kg/m2 and a high level of physical activity, individuals with BMI at 25 kg/m2 or higher and any lower level of physical activity had an adjusted HR of 2.06 (95% CI, 1.18-3.58). In addition, individuals with a high waist circumference – defined as 81 cm or more in women and 95 cm or more in men – and low physical activity had an adjusted HR of 2.22 (95% CI, 1.37-3.58) in comparison to those with a high waist circumference and high physical activity (adjusted HR, 1.84; 95% CI, 0.97-3.47). Physical activity level was considered low with less than 3 hours of light physical activity and no hard physical activity per week and high with any amount of light activity plus 1 or more hours of hard physical activity.
The authors acknowledged the study’s potential limitations, including the requirement for participants to complete the final two HUNT study surveys and the use of stricter criteria than usual in validating PsA diagnoses.
The study was partially funded by a grant Dr. Thomsen received from the Norwegian Extra Foundation for Health and Rehabilitation. The authors reported no conflicts of interest.
SOURCE: Thomsen RS et al. Arthritis Care Res. 2019 Dec 7. doi: 10.1002/acr.24121.
A new Norwegian study has identified a high body mass index and lower levels of physical activity as modifiable risk factors for developing psoriatic arthritis (PsA).
“Our study adds to the growing evidence that the risk of PsA is modifiable and highlights the importance of preventive work against obesity as well as encouraging physical activity in order to reduce the incidence of PsA,” wrote Ruth S. Thomsen, MD, of the Norwegian University of Science and Technology, and coauthors. The study was published in Arthritis Care & Research.
To determine the impact that adiposity and body fat distribution have on developing PsA, the researchers analyzed data from 36,626 women and men who participated in two surveys from the longitudinal, population-based Norwegian HUNT Study. All participants did not have diagnosed PsA at baseline in 1995-1997. Variables used in statistical analysis included calculated baseline BMI, waist circumference, waist-hip ratio, and level of physical activity.
Between baseline and follow-up in 2012, 185 new cases of PsA were reported. One standard deviation increase in BMI (4.2 kg/m2 for women, 3.5 kg/m2 for men) and waist circumstance (10.8 cm for women, 8.6 cm for men) was associated with adjusted hazard ratios of 1.40 (95% confidence interval, 1.24-1.58) and 1.48 (95% CI, 1.31-1.68), accordingly. Obese individuals – defined as BMI of 30 kg/m2 or higher – had an adjusted HR of 2.46 (95% CI, 1.65-3.68) when compared with individuals at normal weight.
Compared to individuals with BMI less than 25 kg/m2 and a high level of physical activity, individuals with BMI at 25 kg/m2 or higher and any lower level of physical activity had an adjusted HR of 2.06 (95% CI, 1.18-3.58). In addition, individuals with a high waist circumference – defined as 81 cm or more in women and 95 cm or more in men – and low physical activity had an adjusted HR of 2.22 (95% CI, 1.37-3.58) in comparison to those with a high waist circumference and high physical activity (adjusted HR, 1.84; 95% CI, 0.97-3.47). Physical activity level was considered low with less than 3 hours of light physical activity and no hard physical activity per week and high with any amount of light activity plus 1 or more hours of hard physical activity.
The authors acknowledged the study’s potential limitations, including the requirement for participants to complete the final two HUNT study surveys and the use of stricter criteria than usual in validating PsA diagnoses.
The study was partially funded by a grant Dr. Thomsen received from the Norwegian Extra Foundation for Health and Rehabilitation. The authors reported no conflicts of interest.
SOURCE: Thomsen RS et al. Arthritis Care Res. 2019 Dec 7. doi: 10.1002/acr.24121.
FROM ARTHRITIS CARE & RESEARCH
Phase 2 studies show potential of FcRn blockade in primary ITP
ORLANDO – Treatments targeted to the neonatal Fc receptor are showing promise in phase 2 studies in primary immune thrombocytopenia, investigators reported at the annual meeting of the American Society of Hematology.
Encouraging outcomes support the continued phase 3 development of these agents, which are designed to block the neonatal Fc receptor (FcRn) in patients with this IgG-mediated disease.
Blocking FcRN is intended to prevent recycling of IgG, resulting in IgG degradation, according to the authors of studies evaluating rozanolixizumab, a subcutaneously administered monoclonal antibody, and efgartigimod, an intravenously administered antibody fragment, in primary immune thrombocytopenia (ITP).
Rozanolixizumab
Results of the phase 2 study of rozanolixizumab demonstrated that this agent reduced IgG levels and improved platelet counts at all doses tested, according to the investigators, led by Tadeusz Robak, MD, of the department of hematology at the Medical University of Lodz (Poland).
Efficacy endpoints were seen more quickly – by day 8 of treatment – with single subcutaneous infusions at higher doses, according to the researchers.
Headaches of mild to moderate severity were noted at higher doses, and no patients left the study because of adverse events, they reported.
“These safety, tolerability, and efficacy data support phase 3 development of rozanolixizumab in patients with primary ITP,” wrote Dr. Robak and coauthors in the abstract for their study.
A total of 66 adult patients with primary ITP were enrolled and treated with single or multiple subcutaneous doses of rozanolixizumab administered at 1-week intervals.
Baseline characteristics suggested a “difficult-to-treat” patient cohort that had a median ITP duration of nearly 6 years and a median of four prior therapies, including thrombopoietin receptor agonists in about one-third of patients, according to the investigators.
Platelet counts of at least 50 x 109/L were achieved by day 8 in more than half of patients who received single doses of rozanolixizumab at higher dose levels of 15 mg/kg (58.3%) and 20 mg/kg (54.5%), Dr. Robak and colleagues reported.
Mild to moderate headaches were seen in about 40% of patients over an 8-week observation period. There were no serious infections and, of four serious adverse events occurring during the study, none were deemed to be treatment related, according to the investigators.
“People who have primary ITP may experience low platelet count that can put them at risk for severe bleeding, and there are limited options that provide a rapid increase in platelet count to reduce this risk,” Dr. Robak said in an interview. “These data build on the growing body of evidence that suggest targeting the FcRn pathway could have the potential to transform the treatment experience for people with rare IgG autoantibody–mediated diseases such as primary ITP.”
Efgartigimod
Substantial reductions in IgG levels and clinically relevant increases in platelet counts were seen following a 3-week treatment cycle with efgartigimod in patients with treatment-refractory ITP, according to investigator Adrian C. Newland, MB, BCh, of the Royal London Hospital and coinvestigators.
The human IgG1 antibody Fc-fragment, a natural ligand of FcRN, is engineered to have increased affinity to FcRn, while preserving its pH‐dependent binding, according to the investigators.
Efgartigimod treatment was well tolerated and reduced the proportion of patients with bleeding in the phase 2 study presented at ASH 2019.
“This suggests that targeted IgG reduction with efgartigimod is a potential new treatment modality in primary ITP, and warrants evaluation of longer-term treatment in a larger phase 3 study,” the investigators reported in the abstract for their study.
The report described 38 patients randomized to four weekly intravenous infusions of placebo or efgartigimod at one of two dosing levels. Patients had long-standing ITP, with a median 4.8 years disease duration, and all had either failed splenectomy or had inadequate response to prior treatment.
Efgartigimod treatment rapidly reduced total IgG in all patients who received it, with a mean change from baseline of up to 63.7%, according to investigators.
Platelet counts favored the investigational treatment over placebo by several measures. Platelet counts of at least 50 x 109/L on two or more occasions were seen in 46% of efgartigimod-treated patients and 25% of the placebo group; that platelet count was achieved for 10 or more days in 38% and 0% of the efgartigimod and placebo groups, respectively.
Treatment was well tolerated, according to the investigators, who said there were “no dose-related safety observations.” Full results of the phase 2 investigation were published in the American Journal of Hematology, concurrent with the meeting (2019 Dec 10. doi.org/10.1002/ajh.25680).
The study of rozanolixizumab was supported by UCB; Dr. Robak reported disclosures related to UCB (honoraria, research funding), as well as Takeda, Janssen, Amgen, Roche, AbbVie, Gilead, BeiGene, Acerta, and MorphoSys. The study of efgartigimod was supported by argenx; Dr. Newland reported disclosures related to argenx, Novartis, Angle, Amgen, Ono Pharmaceutical, Shionogi, Rigel, and Dova Pharmaceuticals.
SOURCEs: Robak T et al. ASH 2019, Abstract 897; Newland AC et al. ASH 2019, Abstract 895.
ORLANDO – Treatments targeted to the neonatal Fc receptor are showing promise in phase 2 studies in primary immune thrombocytopenia, investigators reported at the annual meeting of the American Society of Hematology.
Encouraging outcomes support the continued phase 3 development of these agents, which are designed to block the neonatal Fc receptor (FcRn) in patients with this IgG-mediated disease.
Blocking FcRN is intended to prevent recycling of IgG, resulting in IgG degradation, according to the authors of studies evaluating rozanolixizumab, a subcutaneously administered monoclonal antibody, and efgartigimod, an intravenously administered antibody fragment, in primary immune thrombocytopenia (ITP).
Rozanolixizumab
Results of the phase 2 study of rozanolixizumab demonstrated that this agent reduced IgG levels and improved platelet counts at all doses tested, according to the investigators, led by Tadeusz Robak, MD, of the department of hematology at the Medical University of Lodz (Poland).
Efficacy endpoints were seen more quickly – by day 8 of treatment – with single subcutaneous infusions at higher doses, according to the researchers.
Headaches of mild to moderate severity were noted at higher doses, and no patients left the study because of adverse events, they reported.
“These safety, tolerability, and efficacy data support phase 3 development of rozanolixizumab in patients with primary ITP,” wrote Dr. Robak and coauthors in the abstract for their study.
A total of 66 adult patients with primary ITP were enrolled and treated with single or multiple subcutaneous doses of rozanolixizumab administered at 1-week intervals.
Baseline characteristics suggested a “difficult-to-treat” patient cohort that had a median ITP duration of nearly 6 years and a median of four prior therapies, including thrombopoietin receptor agonists in about one-third of patients, according to the investigators.
Platelet counts of at least 50 x 109/L were achieved by day 8 in more than half of patients who received single doses of rozanolixizumab at higher dose levels of 15 mg/kg (58.3%) and 20 mg/kg (54.5%), Dr. Robak and colleagues reported.
Mild to moderate headaches were seen in about 40% of patients over an 8-week observation period. There were no serious infections and, of four serious adverse events occurring during the study, none were deemed to be treatment related, according to the investigators.
“People who have primary ITP may experience low platelet count that can put them at risk for severe bleeding, and there are limited options that provide a rapid increase in platelet count to reduce this risk,” Dr. Robak said in an interview. “These data build on the growing body of evidence that suggest targeting the FcRn pathway could have the potential to transform the treatment experience for people with rare IgG autoantibody–mediated diseases such as primary ITP.”
Efgartigimod
Substantial reductions in IgG levels and clinically relevant increases in platelet counts were seen following a 3-week treatment cycle with efgartigimod in patients with treatment-refractory ITP, according to investigator Adrian C. Newland, MB, BCh, of the Royal London Hospital and coinvestigators.
The human IgG1 antibody Fc-fragment, a natural ligand of FcRN, is engineered to have increased affinity to FcRn, while preserving its pH‐dependent binding, according to the investigators.
Efgartigimod treatment was well tolerated and reduced the proportion of patients with bleeding in the phase 2 study presented at ASH 2019.
“This suggests that targeted IgG reduction with efgartigimod is a potential new treatment modality in primary ITP, and warrants evaluation of longer-term treatment in a larger phase 3 study,” the investigators reported in the abstract for their study.
The report described 38 patients randomized to four weekly intravenous infusions of placebo or efgartigimod at one of two dosing levels. Patients had long-standing ITP, with a median 4.8 years disease duration, and all had either failed splenectomy or had inadequate response to prior treatment.
Efgartigimod treatment rapidly reduced total IgG in all patients who received it, with a mean change from baseline of up to 63.7%, according to investigators.
Platelet counts favored the investigational treatment over placebo by several measures. Platelet counts of at least 50 x 109/L on two or more occasions were seen in 46% of efgartigimod-treated patients and 25% of the placebo group; that platelet count was achieved for 10 or more days in 38% and 0% of the efgartigimod and placebo groups, respectively.
Treatment was well tolerated, according to the investigators, who said there were “no dose-related safety observations.” Full results of the phase 2 investigation were published in the American Journal of Hematology, concurrent with the meeting (2019 Dec 10. doi.org/10.1002/ajh.25680).
The study of rozanolixizumab was supported by UCB; Dr. Robak reported disclosures related to UCB (honoraria, research funding), as well as Takeda, Janssen, Amgen, Roche, AbbVie, Gilead, BeiGene, Acerta, and MorphoSys. The study of efgartigimod was supported by argenx; Dr. Newland reported disclosures related to argenx, Novartis, Angle, Amgen, Ono Pharmaceutical, Shionogi, Rigel, and Dova Pharmaceuticals.
SOURCEs: Robak T et al. ASH 2019, Abstract 897; Newland AC et al. ASH 2019, Abstract 895.
ORLANDO – Treatments targeted to the neonatal Fc receptor are showing promise in phase 2 studies in primary immune thrombocytopenia, investigators reported at the annual meeting of the American Society of Hematology.
Encouraging outcomes support the continued phase 3 development of these agents, which are designed to block the neonatal Fc receptor (FcRn) in patients with this IgG-mediated disease.
Blocking FcRN is intended to prevent recycling of IgG, resulting in IgG degradation, according to the authors of studies evaluating rozanolixizumab, a subcutaneously administered monoclonal antibody, and efgartigimod, an intravenously administered antibody fragment, in primary immune thrombocytopenia (ITP).
Rozanolixizumab
Results of the phase 2 study of rozanolixizumab demonstrated that this agent reduced IgG levels and improved platelet counts at all doses tested, according to the investigators, led by Tadeusz Robak, MD, of the department of hematology at the Medical University of Lodz (Poland).
Efficacy endpoints were seen more quickly – by day 8 of treatment – with single subcutaneous infusions at higher doses, according to the researchers.
Headaches of mild to moderate severity were noted at higher doses, and no patients left the study because of adverse events, they reported.
“These safety, tolerability, and efficacy data support phase 3 development of rozanolixizumab in patients with primary ITP,” wrote Dr. Robak and coauthors in the abstract for their study.
A total of 66 adult patients with primary ITP were enrolled and treated with single or multiple subcutaneous doses of rozanolixizumab administered at 1-week intervals.
Baseline characteristics suggested a “difficult-to-treat” patient cohort that had a median ITP duration of nearly 6 years and a median of four prior therapies, including thrombopoietin receptor agonists in about one-third of patients, according to the investigators.
Platelet counts of at least 50 x 109/L were achieved by day 8 in more than half of patients who received single doses of rozanolixizumab at higher dose levels of 15 mg/kg (58.3%) and 20 mg/kg (54.5%), Dr. Robak and colleagues reported.
Mild to moderate headaches were seen in about 40% of patients over an 8-week observation period. There were no serious infections and, of four serious adverse events occurring during the study, none were deemed to be treatment related, according to the investigators.
“People who have primary ITP may experience low platelet count that can put them at risk for severe bleeding, and there are limited options that provide a rapid increase in platelet count to reduce this risk,” Dr. Robak said in an interview. “These data build on the growing body of evidence that suggest targeting the FcRn pathway could have the potential to transform the treatment experience for people with rare IgG autoantibody–mediated diseases such as primary ITP.”
Efgartigimod
Substantial reductions in IgG levels and clinically relevant increases in platelet counts were seen following a 3-week treatment cycle with efgartigimod in patients with treatment-refractory ITP, according to investigator Adrian C. Newland, MB, BCh, of the Royal London Hospital and coinvestigators.
The human IgG1 antibody Fc-fragment, a natural ligand of FcRN, is engineered to have increased affinity to FcRn, while preserving its pH‐dependent binding, according to the investigators.
Efgartigimod treatment was well tolerated and reduced the proportion of patients with bleeding in the phase 2 study presented at ASH 2019.
“This suggests that targeted IgG reduction with efgartigimod is a potential new treatment modality in primary ITP, and warrants evaluation of longer-term treatment in a larger phase 3 study,” the investigators reported in the abstract for their study.
The report described 38 patients randomized to four weekly intravenous infusions of placebo or efgartigimod at one of two dosing levels. Patients had long-standing ITP, with a median 4.8 years disease duration, and all had either failed splenectomy or had inadequate response to prior treatment.
Efgartigimod treatment rapidly reduced total IgG in all patients who received it, with a mean change from baseline of up to 63.7%, according to investigators.
Platelet counts favored the investigational treatment over placebo by several measures. Platelet counts of at least 50 x 109/L on two or more occasions were seen in 46% of efgartigimod-treated patients and 25% of the placebo group; that platelet count was achieved for 10 or more days in 38% and 0% of the efgartigimod and placebo groups, respectively.
Treatment was well tolerated, according to the investigators, who said there were “no dose-related safety observations.” Full results of the phase 2 investigation were published in the American Journal of Hematology, concurrent with the meeting (2019 Dec 10. doi.org/10.1002/ajh.25680).
The study of rozanolixizumab was supported by UCB; Dr. Robak reported disclosures related to UCB (honoraria, research funding), as well as Takeda, Janssen, Amgen, Roche, AbbVie, Gilead, BeiGene, Acerta, and MorphoSys. The study of efgartigimod was supported by argenx; Dr. Newland reported disclosures related to argenx, Novartis, Angle, Amgen, Ono Pharmaceutical, Shionogi, Rigel, and Dova Pharmaceuticals.
SOURCEs: Robak T et al. ASH 2019, Abstract 897; Newland AC et al. ASH 2019, Abstract 895.
REPORTING FROM ASH 2019
Poor sleep due to ADHD or ADHD due to poor sleep?
The day wouldn’t be so bad if he would just go to sleep at night! How many times have you heard this plea from parents of your patients with ADHD? Sleep is important for everyone, but getting enough is both more important and more difficult for children with ADHD. About three-quarters of children with ADHD have significant problems with sleep, most even before any medication treatment. And inadequate sleep can exacerbate or even cause ADHD symptoms!
Solving sleep problems for children with ADHD is not always simple. The kinds of sleep issues that are more common in children (and adults) with ADHD, compared with typical children, include behavioral bedtime resistance, circadian rhythm sleep disorder (CRSD), insomnia, morning sleepiness, night waking, periodic limb movement disorder (PLMD), restless leg syndrome (RLS), and sleep disordered breathing (SDB). Such a broad differential means a careful history and sometimes even lab studies may be needed.
Both initial and follow-up visits for ADHD should include a sleep history or, ideally, a tool such as BEARS sleep screening tool or Children’s Sleep Habits Questionnaire and a 2-week sleep diary (http://www.sleepfoundation.org/). These are good ways to collect signs of allergies or apnea (for SDB), limb movements or limb pain (for RLS or PLMD), mouth breathing, night waking, and snoring.
You also need to ask about alcohol, drugs, caffeine, and nicotine; asthma; comorbid conditions such as mental health disorders or their treatments; and enuresis (alone or part of nocturnal seizures).
Do I need to remind you to find out about electronics activating the child before bedtime – hidden under the covers, or signaling messages from friends in the middle of the night – and to encourage limits on these? Some sleep disorders warrant polysomnography in a sleep lab or from MyZeo.com (for PLMD and some SDB) or ferritin less than 50 mg/L (for RLS) for diagnosis and to guide treatment. Nasal steroids, antihistamines, or montelukast may help SDB when there are enlarged tonsils or adenoids, but adenotonsillectomy is usually curative.
The first line and most effective treatment for sleep problems in children with or without ADHD is improving sleep hygiene. The key component is establishing habits for the entire sleep cycle: a steady pattern of reduced stimulation in the hour before bedtime (sans electronics); a friendly rather than irritated bedtime routine; and the same bedtime and wake up time, ideally 7 days per week. Bedtime stories read to the child can soothe at any age, not just toddlers! Of course, both children and families want fun and special occasions. For most, varying bedtime by up to 1 hour won’t mess up their biological clock, but for some even this should be avoided. Sleeping alone in a cool, dark, quiet room, nightly in the same bed (not used for other activities), is considered the ideal. Earplugs, white noise generators, and eye masks may be helpful. If sleeping with siblings is a necessity, bedtimes can be staggered to put the child to bed earlier or after others are asleep.
Struggles postponing bedtime may be part of a pattern of oppositionality common in ADHD, but the child may not be tired due to being off schedule (from CRSD), napping on the bus or after school, sleeping in mornings, or unrealistic parent expectations for sleep duration. Parents may want their hyperactive children to give them a break and go to bed at 8 p.m., but children aged 6-10 years need only 10-11 hours and those aged 10-17 years need 8.5-9.25 hours of sleep.
Not tired may instead be “wired” from lingering stimulant effects or even lack of such medication leaving the child overactive or rebounding from earlier medications. Lower afternoon doses or shorter-acting medication may solve lasting medication issues, but sometimes an additional low dose of stimulants actually will help a child with ADHD settle at bedtime. All stimulant medications can prolong sleep onset, often by 30 minutes, but this varies by individual and tends to resolve on its own a few weeks after a new or changed medicine. Switching medication category may allow a child to fall asleep faster. Atomoxetine and alpha agonists are less likely to delay sleep than methylphenidate (MPH).
What if sleep hygiene, behavioral methods, and adjusting ADHD medications is not enough? If sleep issues are causing significant problems, medication for sleep is worth a try. Controlled-release melatonin 1-2 hours before bedtime has data for effectiveness. There is no defined dose, so the lowest effective dose should be used, but 3-6 mg may be needed. Because many families with a child with ADHD are not organized enough to give medicine on this schedule, sublingual melatonin that acts in 15-20 minutes is a good alternative or even first choice. Clonidine 0.05-0.2 mg 1 hour before bedtime speeds sleep onset, lasts 3 hours, and does not carry over to sedation the next day. Stronger psychopharmaceuticals can assist sleep onset, including low dose mirtazapine or trazodone, but have the side effect of daytime sleepiness.
Management of waking in the middle of the night can be more difficult to treat as sleep drive has been dissipated. First, consider whether trips out of bed reflect a sleep association that has not been extinguished. Daytime atomoxetine or, better yet, MPH may improve night waking, and sometimes even a low-dose evening, long-acting medication, such as osmotic release oral system (OROS) extended release methylphenidate HCL (OROS MPH), helps. Short-acting clonidine or melatonin in the middle of the night or bedtime mirtazapine or trazodone also may be worth a try.
When dealing with sleep, keep in mind that 50% or more of children with ADHD have a coexisting mental health disorder. Anxiety, separation anxiety, depression, and dysthymia all often affect sleep onset, night waking, and sometimes early morning waking. The child or teen may need extra reassurance or company at bedtime (siblings or pets may suffice). Reading positive stories or playing soft music may be better at setting a positive mood and sense of safety for sleep, certainly more so than social media, which should be avoided.
Keep in mind that substance use is more common in ADHD as well as with those other mental health conditions and can interfere with restful sleep and make RLS worse. Bipolar disorder can be mistaken for ADHD as it often presents with hyperactivity but also can be comorbid. Sleep problems are increased sixfold when both are present. Prolonged periods awake at night and diminished need for sleep are signs that help differentiate bipolar from ADHD. Medication management for the bipolar disorder with atypicals can reduce sleep latency and reduce REM sleep, but also causes morning fatigue. Medications to treat other mental health problems can help sleep onset (for example, anticonvulsants, atypicals), or prolong it (SSRIs), change REM states (atypicals), and even exacerbate RLS (SSRIs). You can make changes or work with the child’s mental health specialist if medications are causing significant sleep problems.
When we help improve sleep for children with ADHD, it can lessen not only ADHD symptoms but also some symptoms of other mental health disorders, improve learning and behavior, and greatly improve family quality of life!
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
The day wouldn’t be so bad if he would just go to sleep at night! How many times have you heard this plea from parents of your patients with ADHD? Sleep is important for everyone, but getting enough is both more important and more difficult for children with ADHD. About three-quarters of children with ADHD have significant problems with sleep, most even before any medication treatment. And inadequate sleep can exacerbate or even cause ADHD symptoms!
Solving sleep problems for children with ADHD is not always simple. The kinds of sleep issues that are more common in children (and adults) with ADHD, compared with typical children, include behavioral bedtime resistance, circadian rhythm sleep disorder (CRSD), insomnia, morning sleepiness, night waking, periodic limb movement disorder (PLMD), restless leg syndrome (RLS), and sleep disordered breathing (SDB). Such a broad differential means a careful history and sometimes even lab studies may be needed.
Both initial and follow-up visits for ADHD should include a sleep history or, ideally, a tool such as BEARS sleep screening tool or Children’s Sleep Habits Questionnaire and a 2-week sleep diary (http://www.sleepfoundation.org/). These are good ways to collect signs of allergies or apnea (for SDB), limb movements or limb pain (for RLS or PLMD), mouth breathing, night waking, and snoring.
You also need to ask about alcohol, drugs, caffeine, and nicotine; asthma; comorbid conditions such as mental health disorders or their treatments; and enuresis (alone or part of nocturnal seizures).
Do I need to remind you to find out about electronics activating the child before bedtime – hidden under the covers, or signaling messages from friends in the middle of the night – and to encourage limits on these? Some sleep disorders warrant polysomnography in a sleep lab or from MyZeo.com (for PLMD and some SDB) or ferritin less than 50 mg/L (for RLS) for diagnosis and to guide treatment. Nasal steroids, antihistamines, or montelukast may help SDB when there are enlarged tonsils or adenoids, but adenotonsillectomy is usually curative.
The first line and most effective treatment for sleep problems in children with or without ADHD is improving sleep hygiene. The key component is establishing habits for the entire sleep cycle: a steady pattern of reduced stimulation in the hour before bedtime (sans electronics); a friendly rather than irritated bedtime routine; and the same bedtime and wake up time, ideally 7 days per week. Bedtime stories read to the child can soothe at any age, not just toddlers! Of course, both children and families want fun and special occasions. For most, varying bedtime by up to 1 hour won’t mess up their biological clock, but for some even this should be avoided. Sleeping alone in a cool, dark, quiet room, nightly in the same bed (not used for other activities), is considered the ideal. Earplugs, white noise generators, and eye masks may be helpful. If sleeping with siblings is a necessity, bedtimes can be staggered to put the child to bed earlier or after others are asleep.
Struggles postponing bedtime may be part of a pattern of oppositionality common in ADHD, but the child may not be tired due to being off schedule (from CRSD), napping on the bus or after school, sleeping in mornings, or unrealistic parent expectations for sleep duration. Parents may want their hyperactive children to give them a break and go to bed at 8 p.m., but children aged 6-10 years need only 10-11 hours and those aged 10-17 years need 8.5-9.25 hours of sleep.
Not tired may instead be “wired” from lingering stimulant effects or even lack of such medication leaving the child overactive or rebounding from earlier medications. Lower afternoon doses or shorter-acting medication may solve lasting medication issues, but sometimes an additional low dose of stimulants actually will help a child with ADHD settle at bedtime. All stimulant medications can prolong sleep onset, often by 30 minutes, but this varies by individual and tends to resolve on its own a few weeks after a new or changed medicine. Switching medication category may allow a child to fall asleep faster. Atomoxetine and alpha agonists are less likely to delay sleep than methylphenidate (MPH).
What if sleep hygiene, behavioral methods, and adjusting ADHD medications is not enough? If sleep issues are causing significant problems, medication for sleep is worth a try. Controlled-release melatonin 1-2 hours before bedtime has data for effectiveness. There is no defined dose, so the lowest effective dose should be used, but 3-6 mg may be needed. Because many families with a child with ADHD are not organized enough to give medicine on this schedule, sublingual melatonin that acts in 15-20 minutes is a good alternative or even first choice. Clonidine 0.05-0.2 mg 1 hour before bedtime speeds sleep onset, lasts 3 hours, and does not carry over to sedation the next day. Stronger psychopharmaceuticals can assist sleep onset, including low dose mirtazapine or trazodone, but have the side effect of daytime sleepiness.
Management of waking in the middle of the night can be more difficult to treat as sleep drive has been dissipated. First, consider whether trips out of bed reflect a sleep association that has not been extinguished. Daytime atomoxetine or, better yet, MPH may improve night waking, and sometimes even a low-dose evening, long-acting medication, such as osmotic release oral system (OROS) extended release methylphenidate HCL (OROS MPH), helps. Short-acting clonidine or melatonin in the middle of the night or bedtime mirtazapine or trazodone also may be worth a try.
When dealing with sleep, keep in mind that 50% or more of children with ADHD have a coexisting mental health disorder. Anxiety, separation anxiety, depression, and dysthymia all often affect sleep onset, night waking, and sometimes early morning waking. The child or teen may need extra reassurance or company at bedtime (siblings or pets may suffice). Reading positive stories or playing soft music may be better at setting a positive mood and sense of safety for sleep, certainly more so than social media, which should be avoided.
Keep in mind that substance use is more common in ADHD as well as with those other mental health conditions and can interfere with restful sleep and make RLS worse. Bipolar disorder can be mistaken for ADHD as it often presents with hyperactivity but also can be comorbid. Sleep problems are increased sixfold when both are present. Prolonged periods awake at night and diminished need for sleep are signs that help differentiate bipolar from ADHD. Medication management for the bipolar disorder with atypicals can reduce sleep latency and reduce REM sleep, but also causes morning fatigue. Medications to treat other mental health problems can help sleep onset (for example, anticonvulsants, atypicals), or prolong it (SSRIs), change REM states (atypicals), and even exacerbate RLS (SSRIs). You can make changes or work with the child’s mental health specialist if medications are causing significant sleep problems.
When we help improve sleep for children with ADHD, it can lessen not only ADHD symptoms but also some symptoms of other mental health disorders, improve learning and behavior, and greatly improve family quality of life!
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
The day wouldn’t be so bad if he would just go to sleep at night! How many times have you heard this plea from parents of your patients with ADHD? Sleep is important for everyone, but getting enough is both more important and more difficult for children with ADHD. About three-quarters of children with ADHD have significant problems with sleep, most even before any medication treatment. And inadequate sleep can exacerbate or even cause ADHD symptoms!
Solving sleep problems for children with ADHD is not always simple. The kinds of sleep issues that are more common in children (and adults) with ADHD, compared with typical children, include behavioral bedtime resistance, circadian rhythm sleep disorder (CRSD), insomnia, morning sleepiness, night waking, periodic limb movement disorder (PLMD), restless leg syndrome (RLS), and sleep disordered breathing (SDB). Such a broad differential means a careful history and sometimes even lab studies may be needed.
Both initial and follow-up visits for ADHD should include a sleep history or, ideally, a tool such as BEARS sleep screening tool or Children’s Sleep Habits Questionnaire and a 2-week sleep diary (http://www.sleepfoundation.org/). These are good ways to collect signs of allergies or apnea (for SDB), limb movements or limb pain (for RLS or PLMD), mouth breathing, night waking, and snoring.
You also need to ask about alcohol, drugs, caffeine, and nicotine; asthma; comorbid conditions such as mental health disorders or their treatments; and enuresis (alone or part of nocturnal seizures).
Do I need to remind you to find out about electronics activating the child before bedtime – hidden under the covers, or signaling messages from friends in the middle of the night – and to encourage limits on these? Some sleep disorders warrant polysomnography in a sleep lab or from MyZeo.com (for PLMD and some SDB) or ferritin less than 50 mg/L (for RLS) for diagnosis and to guide treatment. Nasal steroids, antihistamines, or montelukast may help SDB when there are enlarged tonsils or adenoids, but adenotonsillectomy is usually curative.
The first line and most effective treatment for sleep problems in children with or without ADHD is improving sleep hygiene. The key component is establishing habits for the entire sleep cycle: a steady pattern of reduced stimulation in the hour before bedtime (sans electronics); a friendly rather than irritated bedtime routine; and the same bedtime and wake up time, ideally 7 days per week. Bedtime stories read to the child can soothe at any age, not just toddlers! Of course, both children and families want fun and special occasions. For most, varying bedtime by up to 1 hour won’t mess up their biological clock, but for some even this should be avoided. Sleeping alone in a cool, dark, quiet room, nightly in the same bed (not used for other activities), is considered the ideal. Earplugs, white noise generators, and eye masks may be helpful. If sleeping with siblings is a necessity, bedtimes can be staggered to put the child to bed earlier or after others are asleep.
Struggles postponing bedtime may be part of a pattern of oppositionality common in ADHD, but the child may not be tired due to being off schedule (from CRSD), napping on the bus or after school, sleeping in mornings, or unrealistic parent expectations for sleep duration. Parents may want their hyperactive children to give them a break and go to bed at 8 p.m., but children aged 6-10 years need only 10-11 hours and those aged 10-17 years need 8.5-9.25 hours of sleep.
Not tired may instead be “wired” from lingering stimulant effects or even lack of such medication leaving the child overactive or rebounding from earlier medications. Lower afternoon doses or shorter-acting medication may solve lasting medication issues, but sometimes an additional low dose of stimulants actually will help a child with ADHD settle at bedtime. All stimulant medications can prolong sleep onset, often by 30 minutes, but this varies by individual and tends to resolve on its own a few weeks after a new or changed medicine. Switching medication category may allow a child to fall asleep faster. Atomoxetine and alpha agonists are less likely to delay sleep than methylphenidate (MPH).
What if sleep hygiene, behavioral methods, and adjusting ADHD medications is not enough? If sleep issues are causing significant problems, medication for sleep is worth a try. Controlled-release melatonin 1-2 hours before bedtime has data for effectiveness. There is no defined dose, so the lowest effective dose should be used, but 3-6 mg may be needed. Because many families with a child with ADHD are not organized enough to give medicine on this schedule, sublingual melatonin that acts in 15-20 minutes is a good alternative or even first choice. Clonidine 0.05-0.2 mg 1 hour before bedtime speeds sleep onset, lasts 3 hours, and does not carry over to sedation the next day. Stronger psychopharmaceuticals can assist sleep onset, including low dose mirtazapine or trazodone, but have the side effect of daytime sleepiness.
Management of waking in the middle of the night can be more difficult to treat as sleep drive has been dissipated. First, consider whether trips out of bed reflect a sleep association that has not been extinguished. Daytime atomoxetine or, better yet, MPH may improve night waking, and sometimes even a low-dose evening, long-acting medication, such as osmotic release oral system (OROS) extended release methylphenidate HCL (OROS MPH), helps. Short-acting clonidine or melatonin in the middle of the night or bedtime mirtazapine or trazodone also may be worth a try.
When dealing with sleep, keep in mind that 50% or more of children with ADHD have a coexisting mental health disorder. Anxiety, separation anxiety, depression, and dysthymia all often affect sleep onset, night waking, and sometimes early morning waking. The child or teen may need extra reassurance or company at bedtime (siblings or pets may suffice). Reading positive stories or playing soft music may be better at setting a positive mood and sense of safety for sleep, certainly more so than social media, which should be avoided.
Keep in mind that substance use is more common in ADHD as well as with those other mental health conditions and can interfere with restful sleep and make RLS worse. Bipolar disorder can be mistaken for ADHD as it often presents with hyperactivity but also can be comorbid. Sleep problems are increased sixfold when both are present. Prolonged periods awake at night and diminished need for sleep are signs that help differentiate bipolar from ADHD. Medication management for the bipolar disorder with atypicals can reduce sleep latency and reduce REM sleep, but also causes morning fatigue. Medications to treat other mental health problems can help sleep onset (for example, anticonvulsants, atypicals), or prolong it (SSRIs), change REM states (atypicals), and even exacerbate RLS (SSRIs). You can make changes or work with the child’s mental health specialist if medications are causing significant sleep problems.
When we help improve sleep for children with ADHD, it can lessen not only ADHD symptoms but also some symptoms of other mental health disorders, improve learning and behavior, and greatly improve family quality of life!
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Sutimlimab boosts hemoglobin, quality of life in cold agglutinin disease
ORLANDO – An investigational selective inhibitor of the complement pathway, sutimlimab, induced rapid and sustained benefits in patients with cold agglutinin disease, a rare autoimmune hemolytic anemia with no currently approved effective therapies.
Among 24 patients with cold agglutinin disease who received at least one dose of sutimlimab in a phase 3 trial, 20 had a mean increase in hemoglobin of at least 1 g/dL, and 17 remained transfusion free from weeks 5 to 26 following sutimlimab infusion.
“Sutimlimab has the potential to change treatment practices for patients with this disease,” said lead author Alexander Röth, MD, from the University of Duisburg-Essen (Germany), at a late-breaking abstract session at the annual meeting of the American Society of Hematology.
Mean total bilirubin rapidly normalized within 1-3 weeks of infusion of sutimlimab, and patients had a mean improvement of 11 points on the Functional Assessment of Chronic Illness Therapy–Fatigue scale (FACIT-F), indicating a substantial improvement in their quality of life, Dr. Röth said.
Cold agglutinin disease is an acquired hemolytic anemia with an underlying lymphoproliferative disorder. The estimated prevalence of the disease is 16 per 1 million persons. The disease is characterized by hemolysis driven by activation of the complement pathway, leading to opsonization of erythrocytes (coating of erythrocytes with particles that facilitate phagocytosis and other immune reactions), extravascular hemolysis (primarily in the liver), intravascular hemolysis, and anemia.
Patients experience severe fatigue and poor quality of life, as well as increased risk for thrombosis and mortality, compared with matched cohorts.
Sutimlimab is a humanized monoclonal antibody that blocks the C1s component of the classical complement pathway, thereby stopping pathway activation while leaving alternative lectin pathways intact.
Dr. Röth presented results of the phase 3, open-label Cardinal study. Patients with cold agglutinin disease with baseline hemoglobin of 10 g/dL or less, active hemolysis signaled by total bilirubin levels above normal, and at least one blood transfusion within the past 6 months were eligible for the study. Patients with secondary cold agglutinin syndrome or rituximab therapy within the last 3 months or combination therapies within the last 6 months were excluded.
Sutimlimab was delivered intravenously at a dose of 6.5 g for patients under 75 kg in weight and 7.5 g for those 75 kg and over at day 0 and 7, then every 2 weeks thereafter.
A total of 24 patients with a mean age of 71 years were enrolled. Of the 24 patients, 15 (62.5%) were women.
The patients had received a mean of 3.2 transfusions (range 1-19) in the previous 6 months, and 15 had received one or more prior targeted therapies for the disease within the last 5 years. The mean baseline hemoglobin level was 8.6 (range 4.9-11.1) g/dL.
Hemoglobin levels increased rapidly after the first infusion, with a mean increase of 1.2 g/dL at the end of week 1, and 2.3 g/dL after week 3.
The estimated mean increase at treatment assessment (an average of weeks 23, 25, and 26) – the primary endpoint – was 2.6 g/dL, exceeding the prespecified increase of at least 2 g/dL. Normalization of hemoglobin to 12 g/dL or greater was an alternative primary endpoint. The trial met the primary endpoint, with 13 of 24 patients (54.2%) achieving either of the two prespecified events.
The mean overall hemoglobin level was maintained above 11 g/dL after week 3. Of the 24 patients, 20 had hemoglobin increases of 1 g/dL or greater.
Mean total bilirubin, a marker of hemolysis, dropped markedly within hours of infusion and was normalized by week 3.
As noted before, patient quality of life, as measured by the FACIT-F scale, improved by a mean of 11 points from a mean baseline of 32 out of 52 points.
All but two patients had one or more treatment-emergent adverse events, and seven of these patients had a serious treatment-related event, although none of the serious events were thought to be related to sutimlimab. One patient with liver cancer died from causes deemed unrelated to the drug. There were no meningococcal infections.
All 22 patients who completed the 26 weeks of therapy continued on an extended safety phase of the study.
The study results demonstrate that targeting the complement pathways is an novel and effective approach to managing cold agglutinin disease, Dr. Röth concluded.
In a press briefing the day before the presentation, moderator Robert Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins School, Baltimore, who treats patients with cold agglutinin disease, said that the results “are very exciting.”
“These patients are very difficult to treat and there really is no approved drug,” he said. “Right now, we usually use [rituximab] first line, but only half of those patients respond, and usually it only lasts for 6 months or so, so this is a welcome addition.”
Sutimlimab was granted Breakthrough Therapy designation by the Food and Drug Administration, and Orphan Drug status by the FDA, European Medicines Agency, and the Pharmaceuticals and Medical Devices Agency in Japan.
The study was supported by Sanofi. Dr. Röth reported financial relationships with Sanofi and other companies.
SOURCE: Röth A et al. ASH 2019, Abstract LBA-2.
ORLANDO – An investigational selective inhibitor of the complement pathway, sutimlimab, induced rapid and sustained benefits in patients with cold agglutinin disease, a rare autoimmune hemolytic anemia with no currently approved effective therapies.
Among 24 patients with cold agglutinin disease who received at least one dose of sutimlimab in a phase 3 trial, 20 had a mean increase in hemoglobin of at least 1 g/dL, and 17 remained transfusion free from weeks 5 to 26 following sutimlimab infusion.
“Sutimlimab has the potential to change treatment practices for patients with this disease,” said lead author Alexander Röth, MD, from the University of Duisburg-Essen (Germany), at a late-breaking abstract session at the annual meeting of the American Society of Hematology.
Mean total bilirubin rapidly normalized within 1-3 weeks of infusion of sutimlimab, and patients had a mean improvement of 11 points on the Functional Assessment of Chronic Illness Therapy–Fatigue scale (FACIT-F), indicating a substantial improvement in their quality of life, Dr. Röth said.
Cold agglutinin disease is an acquired hemolytic anemia with an underlying lymphoproliferative disorder. The estimated prevalence of the disease is 16 per 1 million persons. The disease is characterized by hemolysis driven by activation of the complement pathway, leading to opsonization of erythrocytes (coating of erythrocytes with particles that facilitate phagocytosis and other immune reactions), extravascular hemolysis (primarily in the liver), intravascular hemolysis, and anemia.
Patients experience severe fatigue and poor quality of life, as well as increased risk for thrombosis and mortality, compared with matched cohorts.
Sutimlimab is a humanized monoclonal antibody that blocks the C1s component of the classical complement pathway, thereby stopping pathway activation while leaving alternative lectin pathways intact.
Dr. Röth presented results of the phase 3, open-label Cardinal study. Patients with cold agglutinin disease with baseline hemoglobin of 10 g/dL or less, active hemolysis signaled by total bilirubin levels above normal, and at least one blood transfusion within the past 6 months were eligible for the study. Patients with secondary cold agglutinin syndrome or rituximab therapy within the last 3 months or combination therapies within the last 6 months were excluded.
Sutimlimab was delivered intravenously at a dose of 6.5 g for patients under 75 kg in weight and 7.5 g for those 75 kg and over at day 0 and 7, then every 2 weeks thereafter.
A total of 24 patients with a mean age of 71 years were enrolled. Of the 24 patients, 15 (62.5%) were women.
The patients had received a mean of 3.2 transfusions (range 1-19) in the previous 6 months, and 15 had received one or more prior targeted therapies for the disease within the last 5 years. The mean baseline hemoglobin level was 8.6 (range 4.9-11.1) g/dL.
Hemoglobin levels increased rapidly after the first infusion, with a mean increase of 1.2 g/dL at the end of week 1, and 2.3 g/dL after week 3.
The estimated mean increase at treatment assessment (an average of weeks 23, 25, and 26) – the primary endpoint – was 2.6 g/dL, exceeding the prespecified increase of at least 2 g/dL. Normalization of hemoglobin to 12 g/dL or greater was an alternative primary endpoint. The trial met the primary endpoint, with 13 of 24 patients (54.2%) achieving either of the two prespecified events.
The mean overall hemoglobin level was maintained above 11 g/dL after week 3. Of the 24 patients, 20 had hemoglobin increases of 1 g/dL or greater.
Mean total bilirubin, a marker of hemolysis, dropped markedly within hours of infusion and was normalized by week 3.
As noted before, patient quality of life, as measured by the FACIT-F scale, improved by a mean of 11 points from a mean baseline of 32 out of 52 points.
All but two patients had one or more treatment-emergent adverse events, and seven of these patients had a serious treatment-related event, although none of the serious events were thought to be related to sutimlimab. One patient with liver cancer died from causes deemed unrelated to the drug. There were no meningococcal infections.
All 22 patients who completed the 26 weeks of therapy continued on an extended safety phase of the study.
The study results demonstrate that targeting the complement pathways is an novel and effective approach to managing cold agglutinin disease, Dr. Röth concluded.
In a press briefing the day before the presentation, moderator Robert Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins School, Baltimore, who treats patients with cold agglutinin disease, said that the results “are very exciting.”
“These patients are very difficult to treat and there really is no approved drug,” he said. “Right now, we usually use [rituximab] first line, but only half of those patients respond, and usually it only lasts for 6 months or so, so this is a welcome addition.”
Sutimlimab was granted Breakthrough Therapy designation by the Food and Drug Administration, and Orphan Drug status by the FDA, European Medicines Agency, and the Pharmaceuticals and Medical Devices Agency in Japan.
The study was supported by Sanofi. Dr. Röth reported financial relationships with Sanofi and other companies.
SOURCE: Röth A et al. ASH 2019, Abstract LBA-2.
ORLANDO – An investigational selective inhibitor of the complement pathway, sutimlimab, induced rapid and sustained benefits in patients with cold agglutinin disease, a rare autoimmune hemolytic anemia with no currently approved effective therapies.
Among 24 patients with cold agglutinin disease who received at least one dose of sutimlimab in a phase 3 trial, 20 had a mean increase in hemoglobin of at least 1 g/dL, and 17 remained transfusion free from weeks 5 to 26 following sutimlimab infusion.
“Sutimlimab has the potential to change treatment practices for patients with this disease,” said lead author Alexander Röth, MD, from the University of Duisburg-Essen (Germany), at a late-breaking abstract session at the annual meeting of the American Society of Hematology.
Mean total bilirubin rapidly normalized within 1-3 weeks of infusion of sutimlimab, and patients had a mean improvement of 11 points on the Functional Assessment of Chronic Illness Therapy–Fatigue scale (FACIT-F), indicating a substantial improvement in their quality of life, Dr. Röth said.
Cold agglutinin disease is an acquired hemolytic anemia with an underlying lymphoproliferative disorder. The estimated prevalence of the disease is 16 per 1 million persons. The disease is characterized by hemolysis driven by activation of the complement pathway, leading to opsonization of erythrocytes (coating of erythrocytes with particles that facilitate phagocytosis and other immune reactions), extravascular hemolysis (primarily in the liver), intravascular hemolysis, and anemia.
Patients experience severe fatigue and poor quality of life, as well as increased risk for thrombosis and mortality, compared with matched cohorts.
Sutimlimab is a humanized monoclonal antibody that blocks the C1s component of the classical complement pathway, thereby stopping pathway activation while leaving alternative lectin pathways intact.
Dr. Röth presented results of the phase 3, open-label Cardinal study. Patients with cold agglutinin disease with baseline hemoglobin of 10 g/dL or less, active hemolysis signaled by total bilirubin levels above normal, and at least one blood transfusion within the past 6 months were eligible for the study. Patients with secondary cold agglutinin syndrome or rituximab therapy within the last 3 months or combination therapies within the last 6 months were excluded.
Sutimlimab was delivered intravenously at a dose of 6.5 g for patients under 75 kg in weight and 7.5 g for those 75 kg and over at day 0 and 7, then every 2 weeks thereafter.
A total of 24 patients with a mean age of 71 years were enrolled. Of the 24 patients, 15 (62.5%) were women.
The patients had received a mean of 3.2 transfusions (range 1-19) in the previous 6 months, and 15 had received one or more prior targeted therapies for the disease within the last 5 years. The mean baseline hemoglobin level was 8.6 (range 4.9-11.1) g/dL.
Hemoglobin levels increased rapidly after the first infusion, with a mean increase of 1.2 g/dL at the end of week 1, and 2.3 g/dL after week 3.
The estimated mean increase at treatment assessment (an average of weeks 23, 25, and 26) – the primary endpoint – was 2.6 g/dL, exceeding the prespecified increase of at least 2 g/dL. Normalization of hemoglobin to 12 g/dL or greater was an alternative primary endpoint. The trial met the primary endpoint, with 13 of 24 patients (54.2%) achieving either of the two prespecified events.
The mean overall hemoglobin level was maintained above 11 g/dL after week 3. Of the 24 patients, 20 had hemoglobin increases of 1 g/dL or greater.
Mean total bilirubin, a marker of hemolysis, dropped markedly within hours of infusion and was normalized by week 3.
As noted before, patient quality of life, as measured by the FACIT-F scale, improved by a mean of 11 points from a mean baseline of 32 out of 52 points.
All but two patients had one or more treatment-emergent adverse events, and seven of these patients had a serious treatment-related event, although none of the serious events were thought to be related to sutimlimab. One patient with liver cancer died from causes deemed unrelated to the drug. There were no meningococcal infections.
All 22 patients who completed the 26 weeks of therapy continued on an extended safety phase of the study.
The study results demonstrate that targeting the complement pathways is an novel and effective approach to managing cold agglutinin disease, Dr. Röth concluded.
In a press briefing the day before the presentation, moderator Robert Brodsky, MD, professor of medicine and director of the division of hematology at Johns Hopkins School, Baltimore, who treats patients with cold agglutinin disease, said that the results “are very exciting.”
“These patients are very difficult to treat and there really is no approved drug,” he said. “Right now, we usually use [rituximab] first line, but only half of those patients respond, and usually it only lasts for 6 months or so, so this is a welcome addition.”
Sutimlimab was granted Breakthrough Therapy designation by the Food and Drug Administration, and Orphan Drug status by the FDA, European Medicines Agency, and the Pharmaceuticals and Medical Devices Agency in Japan.
The study was supported by Sanofi. Dr. Röth reported financial relationships with Sanofi and other companies.
SOURCE: Röth A et al. ASH 2019, Abstract LBA-2.
REPORTING FROM ASH 2019
Persistent Pruritic Papules on the Buttocks
The Diagnosis: Dermatitis Herpetiformis
Dermatitis herpetiformis (DH), also known as Duhring disease, is a rare autoimmune disease. It is the cutaneous manifestation of gluten sensitivity with antibodies targeting epidermal transglutaminase.1 Symptoms of DH generally arise in the fourth decade of life, and children are less commonly affected,2 though the diagnosis should be considered at any age, as our patient was aged 19 years at the time of presentation. Dermatitis herpetiformis predominantly affects white individuals of Northern European heritage, and males more often are affected than females.2 There is a strong association with HLA-B8, HLA-DR3, and HLA-DQw2.3 It also is associated with other autoimmune disorders, including autoimmune thyroid disease, type 1 diabetes mellitus, and pernicious anemia.2
Clinically, DH is characterized by groups of intensely pruritic papulovesicles that are symmetrically located on the extensor surfaces of the upper and lower extremities, scalp, nuchal area, back, and buttocks (quiz image). Less often, the groin also may be involved, as it was in our patient (Figure 1).4 Lesions can be papulovesicular or bullous, though they often are excoriated, and primary lesions may be difficult to identify.2 The disease may have spontaneous remissions with frequent relapses. Most patients with DH have an asymptomatic gluten-sensitive enteropathy.3

A punch biopsy of a representative nonexcoriated lesion from our patient showed the characteristic collections of neutrophils and fibrin at the tips of dermal papillae on hematoxylin and eosin staining (Figure 2). These findings are suggestive of DH.1 However, other bullous diseases, such as linear IgA dermatosis and epidermolysis bullosa acquisita, may have similar appearance on histology.1 Direct immunofluorescence (DIF) of perilesional skin is the gold standard for diagnosing DH, with a sensitivity and specificity close to 100%.1 Deposits of IgA generally are concentrated in previously involved skin or noninflamed perilesional skin; DIF of erythematous or lesional skin may be false negative.5 In our patient, DIF of perilesional uninvolved skin showed granular deposits of IgA at the dermoepidermal junction with accentuation in the dermal papillae (Figure 3), further suggestive of the diagnosis of DH.6
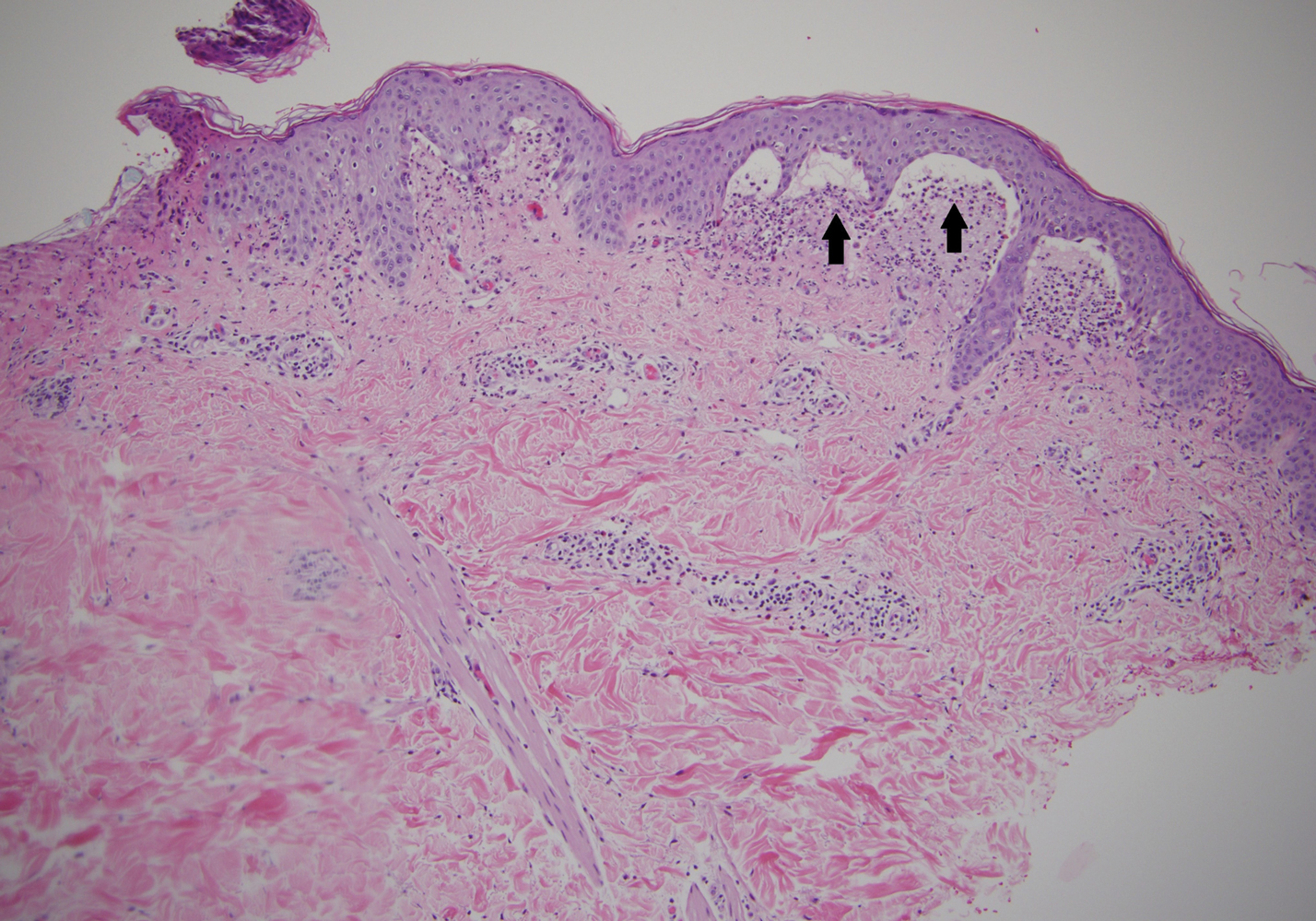

Patients with DH frequently will have specific IgA antibodies including anti-tissue transglutaminase (tTG), anti-epidermal transglutaminase, antiendomysial antibodies, and anti-synthetic deamidated gliadin peptides.1 Only some of these tests are widely available, and the anti-tTG enzyme-linked immunosorbent assay is the least expensive and easiest to perform. A positive anti-tTG antibody test has a sensitivity ranging from 47% to 95% and a specificity greater than 90%.1 Our patient tested positive for anti-tTG and anti-deamidated synthetic gliadin-derived peptides. A diagnostic algorithm based on current evidence suggests that in patients with clinical evidence of DH, typical DIF findings combined with positive anti-tTG antibodies confirms the diagnosis of DH, as was seen in our patient.1,7 In situations where histopathology and/or antibody testing are inconclusive, additional testing to include HLA antigen typing, duodenal biopsy, and supplemental skin biopsies may be performed to help confirm or exclude a diagnosis of DH. It is unnecessary to perform a duodenal biopsy in patients with a confirmed diagnosis of DH, as DH is considered the specific cutaneous manifestation of celiac disease, and the diagnosis of DH is a diagnosis of celiac disease.1
Although pruritic papules may be found in several conditions, clinical, histopathologic, and DIF findings can help to confirm the diagnosis. Allergic contact dermatitis is a type IV hypersensitivity reaction causing eruptions of varying morphology, generally manifesting as pruritic scaly plaques that can occur anywhere on the body exposed to the offending allergen. Key histopathologic features of allergic contact dermatitis are eosinophilic spongiosis and exocytosis of eosinophils and lymphocytes.8 Papular urticaria is a hypersensitivity disorder to insect bites that consists of pruritic papules on exposed areas of skin, typically in children younger than 10 years. Genital and axillary areas usually are spared. The diagnosis is clinical.9 Recurrent herpes simplex virus infection is a short-lived outbreak that generally improves within 10 days. Herpes simplex virus infections usually are comprised of small vesicular or ulcerative lesions. Tzanck smear, skin biopsy, direct fluorescent antibody, viral culture, and polymerase chain reaction are diagnostic methods for herpes simplex virus.10 Scabies lesions typically are pruritic, erythematous, often excoriated papules and burrows located most commonly in the webs of fingers, wrists, axillae, areolae, waist, and genitalia. Diagnosis can be confirmed with scabies preparation (skin scraping showing mites, eggs, or feces), dermoscopy showing mites and burrows in vivo, or biopsy.11
First-line treatment in patients with DH and celiac disease is a strict gluten-free diet (GFD).1 A GFD will resolve the cutaneous and gastrointestinal manifestations and is the only thing that will reduce the risk for lymphoma and other diseases associated with gluten-induced enteropathy.1,2 A GFD alone will provide symptomatic relief over several months; dapsone and sulfapyridine can provide rapid relief of the pruritus and skin manifestations and usually can be weaned or discontinued after several months of following a strict GFD.12 Patients on sulfone therapy require regular follow-up and monitoring due to the risk for hemolytic anemia and other adverse effects as well as to determine the appropriate time to discontinue the medication.12 Although some patients are able to tolerate reintroduction of gluten into their diets after a period of remission, most will experience recurrent dermatologic manifestations if they continue to consume gluten.3
Because of our patient's impending move out of the area, no oral medications were started, and he was instructed to follow a GFD and seek medical care at his new location. The patient was contacted 6 months later and reported resolution of all skin lesions with just a GFD. The patient continued to follow-up with a dermatologist and gastroenterologist at his new location.
- Antiga E, Caproni M. The diagnosis and treatment of dermatitis herpetiformis. Clin Cosmet Investig Dermatol. 2015;8:257-265.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatitis herpetiformis and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Vol 1. 4th ed. Philadelphia, PA: Elsevier; 2017:527-537.
- James WD, Elston DM, Treat JR, et al. Chronic blistering dermatoses. In: James WD, Elston DM, Treat JR, et al, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 13th ed. Philadelphia, PA: Elsevier; 2020:453-474.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. part 1. epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol. 2011;64:1017-1024.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Lever WF, Elder DE. Lever's Histopathology of the Skin. Vol 1. Philadelphia, PA: Wolters Kluwer Health/Lippincott; 2009.
- Hull C. Dermatitis herpetiformis. https://www.uptodate.com/contents/dermatitis-herpetiformis. Published October 14, 2016. Updated September 25, 2019. Accessed December 10, 2019.
Yiannias J. Clinical features and diagnosis of allergic contact dermatitis. UpToDate. https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-allergic-contact-dermatitissearch=allergic%20contact%20dermatitis&source=search_result&selectedTitle=2~150
&usage_type=default&display_rank=2#H27385290. Updated May 17, 2019. Accessed December 11, 2019.Goddard J, Stewart PH. Insect and other arthropod bites. UpToDate. https://www.uptodate.com/contents/insect-and-other-arthropod-bites?search=papular%2urticaria§ionRank=1&usage_type=default&anchor=H4&source=machineLearning&selectedTitle=1~24&display_rank=1#
H4. Updated October 31, 2019. Accessed December 11, 2019.Christine J, Wald A. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection. UpToDate. https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection?search=herpes%20simplex&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated July 23, 2019. Accessed December 11, 2019.
- Goldstein B, Goldstein A. Scabies: epidemiology, clinical features, and diagnosis. UpToDate. https://www.uptodate.com/contents/scabies-epidemiology-clinical-features-and-diagnosis?search=scabies&source=search_result&selectedTitle=1~92&usagetype=default&display_rank=1. Updated August 2, 2019. Accessed December 11, 2019.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. part 2. diagnosis, management, and prognosis. J Am Acad Dermatol. 2011;64:1027-1033.
The Diagnosis: Dermatitis Herpetiformis
Dermatitis herpetiformis (DH), also known as Duhring disease, is a rare autoimmune disease. It is the cutaneous manifestation of gluten sensitivity with antibodies targeting epidermal transglutaminase.1 Symptoms of DH generally arise in the fourth decade of life, and children are less commonly affected,2 though the diagnosis should be considered at any age, as our patient was aged 19 years at the time of presentation. Dermatitis herpetiformis predominantly affects white individuals of Northern European heritage, and males more often are affected than females.2 There is a strong association with HLA-B8, HLA-DR3, and HLA-DQw2.3 It also is associated with other autoimmune disorders, including autoimmune thyroid disease, type 1 diabetes mellitus, and pernicious anemia.2
Clinically, DH is characterized by groups of intensely pruritic papulovesicles that are symmetrically located on the extensor surfaces of the upper and lower extremities, scalp, nuchal area, back, and buttocks (quiz image). Less often, the groin also may be involved, as it was in our patient (Figure 1).4 Lesions can be papulovesicular or bullous, though they often are excoriated, and primary lesions may be difficult to identify.2 The disease may have spontaneous remissions with frequent relapses. Most patients with DH have an asymptomatic gluten-sensitive enteropathy.3

A punch biopsy of a representative nonexcoriated lesion from our patient showed the characteristic collections of neutrophils and fibrin at the tips of dermal papillae on hematoxylin and eosin staining (Figure 2). These findings are suggestive of DH.1 However, other bullous diseases, such as linear IgA dermatosis and epidermolysis bullosa acquisita, may have similar appearance on histology.1 Direct immunofluorescence (DIF) of perilesional skin is the gold standard for diagnosing DH, with a sensitivity and specificity close to 100%.1 Deposits of IgA generally are concentrated in previously involved skin or noninflamed perilesional skin; DIF of erythematous or lesional skin may be false negative.5 In our patient, DIF of perilesional uninvolved skin showed granular deposits of IgA at the dermoepidermal junction with accentuation in the dermal papillae (Figure 3), further suggestive of the diagnosis of DH.6


Patients with DH frequently will have specific IgA antibodies including anti-tissue transglutaminase (tTG), anti-epidermal transglutaminase, antiendomysial antibodies, and anti-synthetic deamidated gliadin peptides.1 Only some of these tests are widely available, and the anti-tTG enzyme-linked immunosorbent assay is the least expensive and easiest to perform. A positive anti-tTG antibody test has a sensitivity ranging from 47% to 95% and a specificity greater than 90%.1 Our patient tested positive for anti-tTG and anti-deamidated synthetic gliadin-derived peptides. A diagnostic algorithm based on current evidence suggests that in patients with clinical evidence of DH, typical DIF findings combined with positive anti-tTG antibodies confirms the diagnosis of DH, as was seen in our patient.1,7 In situations where histopathology and/or antibody testing are inconclusive, additional testing to include HLA antigen typing, duodenal biopsy, and supplemental skin biopsies may be performed to help confirm or exclude a diagnosis of DH. It is unnecessary to perform a duodenal biopsy in patients with a confirmed diagnosis of DH, as DH is considered the specific cutaneous manifestation of celiac disease, and the diagnosis of DH is a diagnosis of celiac disease.1
Although pruritic papules may be found in several conditions, clinical, histopathologic, and DIF findings can help to confirm the diagnosis. Allergic contact dermatitis is a type IV hypersensitivity reaction causing eruptions of varying morphology, generally manifesting as pruritic scaly plaques that can occur anywhere on the body exposed to the offending allergen. Key histopathologic features of allergic contact dermatitis are eosinophilic spongiosis and exocytosis of eosinophils and lymphocytes.8 Papular urticaria is a hypersensitivity disorder to insect bites that consists of pruritic papules on exposed areas of skin, typically in children younger than 10 years. Genital and axillary areas usually are spared. The diagnosis is clinical.9 Recurrent herpes simplex virus infection is a short-lived outbreak that generally improves within 10 days. Herpes simplex virus infections usually are comprised of small vesicular or ulcerative lesions. Tzanck smear, skin biopsy, direct fluorescent antibody, viral culture, and polymerase chain reaction are diagnostic methods for herpes simplex virus.10 Scabies lesions typically are pruritic, erythematous, often excoriated papules and burrows located most commonly in the webs of fingers, wrists, axillae, areolae, waist, and genitalia. Diagnosis can be confirmed with scabies preparation (skin scraping showing mites, eggs, or feces), dermoscopy showing mites and burrows in vivo, or biopsy.11
First-line treatment in patients with DH and celiac disease is a strict gluten-free diet (GFD).1 A GFD will resolve the cutaneous and gastrointestinal manifestations and is the only thing that will reduce the risk for lymphoma and other diseases associated with gluten-induced enteropathy.1,2 A GFD alone will provide symptomatic relief over several months; dapsone and sulfapyridine can provide rapid relief of the pruritus and skin manifestations and usually can be weaned or discontinued after several months of following a strict GFD.12 Patients on sulfone therapy require regular follow-up and monitoring due to the risk for hemolytic anemia and other adverse effects as well as to determine the appropriate time to discontinue the medication.12 Although some patients are able to tolerate reintroduction of gluten into their diets after a period of remission, most will experience recurrent dermatologic manifestations if they continue to consume gluten.3
Because of our patient's impending move out of the area, no oral medications were started, and he was instructed to follow a GFD and seek medical care at his new location. The patient was contacted 6 months later and reported resolution of all skin lesions with just a GFD. The patient continued to follow-up with a dermatologist and gastroenterologist at his new location.
The Diagnosis: Dermatitis Herpetiformis
Dermatitis herpetiformis (DH), also known as Duhring disease, is a rare autoimmune disease. It is the cutaneous manifestation of gluten sensitivity with antibodies targeting epidermal transglutaminase.1 Symptoms of DH generally arise in the fourth decade of life, and children are less commonly affected,2 though the diagnosis should be considered at any age, as our patient was aged 19 years at the time of presentation. Dermatitis herpetiformis predominantly affects white individuals of Northern European heritage, and males more often are affected than females.2 There is a strong association with HLA-B8, HLA-DR3, and HLA-DQw2.3 It also is associated with other autoimmune disorders, including autoimmune thyroid disease, type 1 diabetes mellitus, and pernicious anemia.2
Clinically, DH is characterized by groups of intensely pruritic papulovesicles that are symmetrically located on the extensor surfaces of the upper and lower extremities, scalp, nuchal area, back, and buttocks (quiz image). Less often, the groin also may be involved, as it was in our patient (Figure 1).4 Lesions can be papulovesicular or bullous, though they often are excoriated, and primary lesions may be difficult to identify.2 The disease may have spontaneous remissions with frequent relapses. Most patients with DH have an asymptomatic gluten-sensitive enteropathy.3

A punch biopsy of a representative nonexcoriated lesion from our patient showed the characteristic collections of neutrophils and fibrin at the tips of dermal papillae on hematoxylin and eosin staining (Figure 2). These findings are suggestive of DH.1 However, other bullous diseases, such as linear IgA dermatosis and epidermolysis bullosa acquisita, may have similar appearance on histology.1 Direct immunofluorescence (DIF) of perilesional skin is the gold standard for diagnosing DH, with a sensitivity and specificity close to 100%.1 Deposits of IgA generally are concentrated in previously involved skin or noninflamed perilesional skin; DIF of erythematous or lesional skin may be false negative.5 In our patient, DIF of perilesional uninvolved skin showed granular deposits of IgA at the dermoepidermal junction with accentuation in the dermal papillae (Figure 3), further suggestive of the diagnosis of DH.6


Patients with DH frequently will have specific IgA antibodies including anti-tissue transglutaminase (tTG), anti-epidermal transglutaminase, antiendomysial antibodies, and anti-synthetic deamidated gliadin peptides.1 Only some of these tests are widely available, and the anti-tTG enzyme-linked immunosorbent assay is the least expensive and easiest to perform. A positive anti-tTG antibody test has a sensitivity ranging from 47% to 95% and a specificity greater than 90%.1 Our patient tested positive for anti-tTG and anti-deamidated synthetic gliadin-derived peptides. A diagnostic algorithm based on current evidence suggests that in patients with clinical evidence of DH, typical DIF findings combined with positive anti-tTG antibodies confirms the diagnosis of DH, as was seen in our patient.1,7 In situations where histopathology and/or antibody testing are inconclusive, additional testing to include HLA antigen typing, duodenal biopsy, and supplemental skin biopsies may be performed to help confirm or exclude a diagnosis of DH. It is unnecessary to perform a duodenal biopsy in patients with a confirmed diagnosis of DH, as DH is considered the specific cutaneous manifestation of celiac disease, and the diagnosis of DH is a diagnosis of celiac disease.1
Although pruritic papules may be found in several conditions, clinical, histopathologic, and DIF findings can help to confirm the diagnosis. Allergic contact dermatitis is a type IV hypersensitivity reaction causing eruptions of varying morphology, generally manifesting as pruritic scaly plaques that can occur anywhere on the body exposed to the offending allergen. Key histopathologic features of allergic contact dermatitis are eosinophilic spongiosis and exocytosis of eosinophils and lymphocytes.8 Papular urticaria is a hypersensitivity disorder to insect bites that consists of pruritic papules on exposed areas of skin, typically in children younger than 10 years. Genital and axillary areas usually are spared. The diagnosis is clinical.9 Recurrent herpes simplex virus infection is a short-lived outbreak that generally improves within 10 days. Herpes simplex virus infections usually are comprised of small vesicular or ulcerative lesions. Tzanck smear, skin biopsy, direct fluorescent antibody, viral culture, and polymerase chain reaction are diagnostic methods for herpes simplex virus.10 Scabies lesions typically are pruritic, erythematous, often excoriated papules and burrows located most commonly in the webs of fingers, wrists, axillae, areolae, waist, and genitalia. Diagnosis can be confirmed with scabies preparation (skin scraping showing mites, eggs, or feces), dermoscopy showing mites and burrows in vivo, or biopsy.11
First-line treatment in patients with DH and celiac disease is a strict gluten-free diet (GFD).1 A GFD will resolve the cutaneous and gastrointestinal manifestations and is the only thing that will reduce the risk for lymphoma and other diseases associated with gluten-induced enteropathy.1,2 A GFD alone will provide symptomatic relief over several months; dapsone and sulfapyridine can provide rapid relief of the pruritus and skin manifestations and usually can be weaned or discontinued after several months of following a strict GFD.12 Patients on sulfone therapy require regular follow-up and monitoring due to the risk for hemolytic anemia and other adverse effects as well as to determine the appropriate time to discontinue the medication.12 Although some patients are able to tolerate reintroduction of gluten into their diets after a period of remission, most will experience recurrent dermatologic manifestations if they continue to consume gluten.3
Because of our patient's impending move out of the area, no oral medications were started, and he was instructed to follow a GFD and seek medical care at his new location. The patient was contacted 6 months later and reported resolution of all skin lesions with just a GFD. The patient continued to follow-up with a dermatologist and gastroenterologist at his new location.
- Antiga E, Caproni M. The diagnosis and treatment of dermatitis herpetiformis. Clin Cosmet Investig Dermatol. 2015;8:257-265.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatitis herpetiformis and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Vol 1. 4th ed. Philadelphia, PA: Elsevier; 2017:527-537.
- James WD, Elston DM, Treat JR, et al. Chronic blistering dermatoses. In: James WD, Elston DM, Treat JR, et al, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 13th ed. Philadelphia, PA: Elsevier; 2020:453-474.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. part 1. epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol. 2011;64:1017-1024.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Lever WF, Elder DE. Lever's Histopathology of the Skin. Vol 1. Philadelphia, PA: Wolters Kluwer Health/Lippincott; 2009.
- Hull C. Dermatitis herpetiformis. https://www.uptodate.com/contents/dermatitis-herpetiformis. Published October 14, 2016. Updated September 25, 2019. Accessed December 10, 2019.
Yiannias J. Clinical features and diagnosis of allergic contact dermatitis. UpToDate. https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-allergic-contact-dermatitissearch=allergic%20contact%20dermatitis&source=search_result&selectedTitle=2~150
&usage_type=default&display_rank=2#H27385290. Updated May 17, 2019. Accessed December 11, 2019.Goddard J, Stewart PH. Insect and other arthropod bites. UpToDate. https://www.uptodate.com/contents/insect-and-other-arthropod-bites?search=papular%2urticaria§ionRank=1&usage_type=default&anchor=H4&source=machineLearning&selectedTitle=1~24&display_rank=1#
H4. Updated October 31, 2019. Accessed December 11, 2019.Christine J, Wald A. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection. UpToDate. https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection?search=herpes%20simplex&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated July 23, 2019. Accessed December 11, 2019.
- Goldstein B, Goldstein A. Scabies: epidemiology, clinical features, and diagnosis. UpToDate. https://www.uptodate.com/contents/scabies-epidemiology-clinical-features-and-diagnosis?search=scabies&source=search_result&selectedTitle=1~92&usagetype=default&display_rank=1. Updated August 2, 2019. Accessed December 11, 2019.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. part 2. diagnosis, management, and prognosis. J Am Acad Dermatol. 2011;64:1027-1033.
- Antiga E, Caproni M. The diagnosis and treatment of dermatitis herpetiformis. Clin Cosmet Investig Dermatol. 2015;8:257-265.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatitis herpetiformis and linear IgA bullous dermatosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Vol 1. 4th ed. Philadelphia, PA: Elsevier; 2017:527-537.
- James WD, Elston DM, Treat JR, et al. Chronic blistering dermatoses. In: James WD, Elston DM, Treat JR, et al, eds. Andrews' Diseases of the Skin: Clinical Dermatology. 13th ed. Philadelphia, PA: Elsevier; 2020:453-474.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. part 1. epidemiology, pathogenesis, and clinical presentation. J Am Acad Dermatol. 2011;64:1017-1024.
- Zone JJ, Meyer LJ, Petersen MJ. Deposition of granular IgA relative to clinical lesions in dermatitis herpetiformis. Arch Dermatol. 1996;132:912-918.
- Lever WF, Elder DE. Lever's Histopathology of the Skin. Vol 1. Philadelphia, PA: Wolters Kluwer Health/Lippincott; 2009.
- Hull C. Dermatitis herpetiformis. https://www.uptodate.com/contents/dermatitis-herpetiformis. Published October 14, 2016. Updated September 25, 2019. Accessed December 10, 2019.
Yiannias J. Clinical features and diagnosis of allergic contact dermatitis. UpToDate. https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-allergic-contact-dermatitissearch=allergic%20contact%20dermatitis&source=search_result&selectedTitle=2~150
&usage_type=default&display_rank=2#H27385290. Updated May 17, 2019. Accessed December 11, 2019.Goddard J, Stewart PH. Insect and other arthropod bites. UpToDate. https://www.uptodate.com/contents/insect-and-other-arthropod-bites?search=papular%2urticaria§ionRank=1&usage_type=default&anchor=H4&source=machineLearning&selectedTitle=1~24&display_rank=1#
H4. Updated October 31, 2019. Accessed December 11, 2019.Christine J, Wald A. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection. UpToDate. https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection?search=herpes%20simplex&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1. Updated July 23, 2019. Accessed December 11, 2019.
- Goldstein B, Goldstein A. Scabies: epidemiology, clinical features, and diagnosis. UpToDate. https://www.uptodate.com/contents/scabies-epidemiology-clinical-features-and-diagnosis?search=scabies&source=search_result&selectedTitle=1~92&usagetype=default&display_rank=1. Updated August 2, 2019. Accessed December 11, 2019.
- Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. part 2. diagnosis, management, and prognosis. J Am Acad Dermatol. 2011;64:1027-1033.
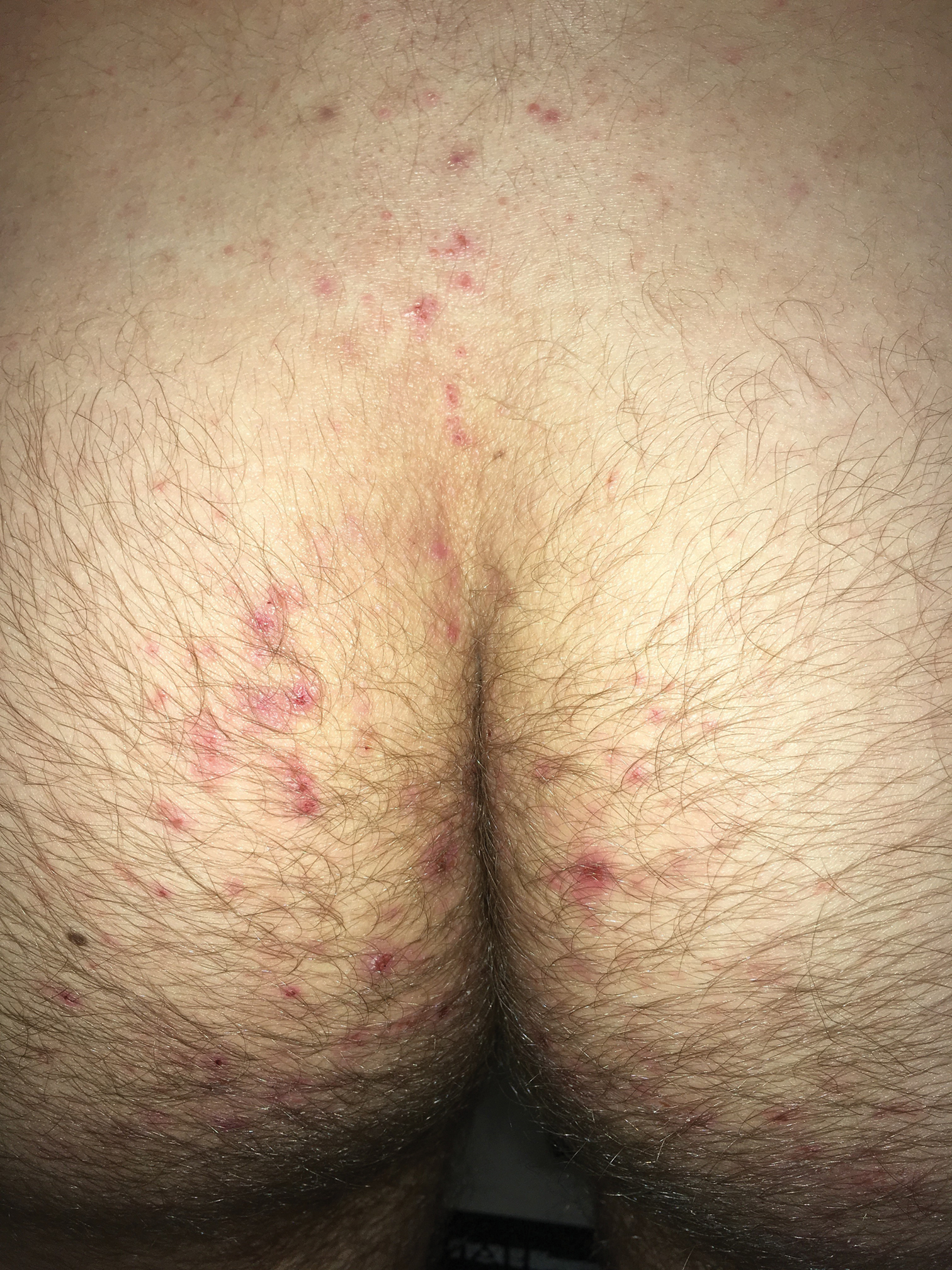
A 19-year-old man presented to the dermatology clinic with intermittent pruritic lesions that began on the bilateral buttocks when he was living in Reserve Officers' Training Corps dormitories several months prior. The eruption then spread to involve the penis, suprapubic area, periumbilical area, and flanks. The patient attempted to treat the lesions with topical antifungals prior to evaluation in the emergency department where he was treated with permethrin 5% on 2 separate occasions without any improvement. A medical history was normal, and he denied recent travel, animal contacts, or new medications. Physical examination revealed several 2- to 4-mm erythematous papules and superficial erosions with an ill-defined erythematous background most notable on the penis, suprapubic area, periumbilical area, flanks, and buttocks.
Culture Change Needed Around Addiction Treatment in the Military
More work is needed to address how addiction is handled both within the military and for veterans after they serve. Removing the stigma around addiction treatment is key to addressing the issue, Anthony Dekker, DO, Northern Arizona VA Healthcare System, said at the AMSUS annual meeting on December 4, 2019.
“When we are talking about treating addiction in the military and the VA, those are 2 different issues,” Dr. Dekker said. “In the VA, it should be understood this is a service that needs to be provided. In the military, it is highly dependent on command. Some commands are in favor of treatment. Some commands are in favor of separation.”
But that separation comes with a cost. Dr. Dekker noted that the military spends about $200,000 to get a person from recruitment to an E-5 pay grade and about $400,000 to an O-5 pay grade. That can be a huge investment loss considering the cost of treatment for someone who is suffering from a use disorder.
“Treatment is going to cost about $44,000,” he said. “That will be treatment in a residential center. That was a person who is serious enough to come into a residential center, follow up with a partial hospital program and continue with ongoing [treatment] that would last a year.”
He touted some of the successes addiction treatment programs have had, recalling data from 2008-2009 from 110 military active-duty service members treated across 5 residential treatment centers in the Washington, DC, area. Within a year, 91% had separated from the military either because of a command decision before treatment or because of loss of recover.
“We know what works in addiction,” he said, noting that program changes that involved using a combination of medication-assisted treatments with regular substance use screenings and medical practitioner follow-up has helped to reduce the rate of lost recovery to 12%. He also noted that in 2013-2014, 41 active military members who received treatment in the same centers were able to be redeployed to Afghanistan and had no relapses during that deployment.
“We try to take the stigma away,” Dr. Dekker said. “So if you have leadership who has a stigma against addiction treatment, you are going to have a steep incline to work against, whereas if you have leadership that were, I would say, endowed with a different sense of knowledge and experience,” there is a much greater chance for helping both military service members and veterans alike.
He called on the US Department of Veterans Affairs (VA) to look more closely at how it is prescribing opioids. While acknowledging the opioid addiction epidemic, he noted that simply cutting back on prescribing may not be the right solution because it is having a ripple effect and causing other problems, namely that although the VA has written 60% fewer opioid prescriptions from 2012 to 2019, overdose rates have doubled as military members and veterans are seeking opioids from other sources outside of a controlled, safer medical environment.
It can be especially problematic for those who have a legitimate medical need for opioids but have a disqualifying event that causes the pain medication to be cut off.
“We need to have a different answer to this because termination of opioids because a patient is positive for marijuana or even positive for cocaine doesn’t mean you take services away. You ramp services up,” Dr. Dekker said. “If I have a patient who has a chronic pain syndrome, the only thing that is going to push him out of my system is if they threaten my staff.…The loss from recovery is not a reason to lose treatment. That is another concept that needs to be addressed and we need to really look at that.”
He also called on VA leadership to be more encouraging in prescribing buprenorphine, noting that many doctors and nurses have waivers to prescribe it, but there is a lot of reluctance to do so, even though there is a lot of success with that treatment.
More work is needed to address how addiction is handled both within the military and for veterans after they serve. Removing the stigma around addiction treatment is key to addressing the issue, Anthony Dekker, DO, Northern Arizona VA Healthcare System, said at the AMSUS annual meeting on December 4, 2019.
“When we are talking about treating addiction in the military and the VA, those are 2 different issues,” Dr. Dekker said. “In the VA, it should be understood this is a service that needs to be provided. In the military, it is highly dependent on command. Some commands are in favor of treatment. Some commands are in favor of separation.”
But that separation comes with a cost. Dr. Dekker noted that the military spends about $200,000 to get a person from recruitment to an E-5 pay grade and about $400,000 to an O-5 pay grade. That can be a huge investment loss considering the cost of treatment for someone who is suffering from a use disorder.
“Treatment is going to cost about $44,000,” he said. “That will be treatment in a residential center. That was a person who is serious enough to come into a residential center, follow up with a partial hospital program and continue with ongoing [treatment] that would last a year.”
He touted some of the successes addiction treatment programs have had, recalling data from 2008-2009 from 110 military active-duty service members treated across 5 residential treatment centers in the Washington, DC, area. Within a year, 91% had separated from the military either because of a command decision before treatment or because of loss of recover.
“We know what works in addiction,” he said, noting that program changes that involved using a combination of medication-assisted treatments with regular substance use screenings and medical practitioner follow-up has helped to reduce the rate of lost recovery to 12%. He also noted that in 2013-2014, 41 active military members who received treatment in the same centers were able to be redeployed to Afghanistan and had no relapses during that deployment.
“We try to take the stigma away,” Dr. Dekker said. “So if you have leadership who has a stigma against addiction treatment, you are going to have a steep incline to work against, whereas if you have leadership that were, I would say, endowed with a different sense of knowledge and experience,” there is a much greater chance for helping both military service members and veterans alike.
He called on the US Department of Veterans Affairs (VA) to look more closely at how it is prescribing opioids. While acknowledging the opioid addiction epidemic, he noted that simply cutting back on prescribing may not be the right solution because it is having a ripple effect and causing other problems, namely that although the VA has written 60% fewer opioid prescriptions from 2012 to 2019, overdose rates have doubled as military members and veterans are seeking opioids from other sources outside of a controlled, safer medical environment.
It can be especially problematic for those who have a legitimate medical need for opioids but have a disqualifying event that causes the pain medication to be cut off.
“We need to have a different answer to this because termination of opioids because a patient is positive for marijuana or even positive for cocaine doesn’t mean you take services away. You ramp services up,” Dr. Dekker said. “If I have a patient who has a chronic pain syndrome, the only thing that is going to push him out of my system is if they threaten my staff.…The loss from recovery is not a reason to lose treatment. That is another concept that needs to be addressed and we need to really look at that.”
He also called on VA leadership to be more encouraging in prescribing buprenorphine, noting that many doctors and nurses have waivers to prescribe it, but there is a lot of reluctance to do so, even though there is a lot of success with that treatment.
More work is needed to address how addiction is handled both within the military and for veterans after they serve. Removing the stigma around addiction treatment is key to addressing the issue, Anthony Dekker, DO, Northern Arizona VA Healthcare System, said at the AMSUS annual meeting on December 4, 2019.
“When we are talking about treating addiction in the military and the VA, those are 2 different issues,” Dr. Dekker said. “In the VA, it should be understood this is a service that needs to be provided. In the military, it is highly dependent on command. Some commands are in favor of treatment. Some commands are in favor of separation.”
But that separation comes with a cost. Dr. Dekker noted that the military spends about $200,000 to get a person from recruitment to an E-5 pay grade and about $400,000 to an O-5 pay grade. That can be a huge investment loss considering the cost of treatment for someone who is suffering from a use disorder.
“Treatment is going to cost about $44,000,” he said. “That will be treatment in a residential center. That was a person who is serious enough to come into a residential center, follow up with a partial hospital program and continue with ongoing [treatment] that would last a year.”
He touted some of the successes addiction treatment programs have had, recalling data from 2008-2009 from 110 military active-duty service members treated across 5 residential treatment centers in the Washington, DC, area. Within a year, 91% had separated from the military either because of a command decision before treatment or because of loss of recover.
“We know what works in addiction,” he said, noting that program changes that involved using a combination of medication-assisted treatments with regular substance use screenings and medical practitioner follow-up has helped to reduce the rate of lost recovery to 12%. He also noted that in 2013-2014, 41 active military members who received treatment in the same centers were able to be redeployed to Afghanistan and had no relapses during that deployment.
“We try to take the stigma away,” Dr. Dekker said. “So if you have leadership who has a stigma against addiction treatment, you are going to have a steep incline to work against, whereas if you have leadership that were, I would say, endowed with a different sense of knowledge and experience,” there is a much greater chance for helping both military service members and veterans alike.
He called on the US Department of Veterans Affairs (VA) to look more closely at how it is prescribing opioids. While acknowledging the opioid addiction epidemic, he noted that simply cutting back on prescribing may not be the right solution because it is having a ripple effect and causing other problems, namely that although the VA has written 60% fewer opioid prescriptions from 2012 to 2019, overdose rates have doubled as military members and veterans are seeking opioids from other sources outside of a controlled, safer medical environment.
It can be especially problematic for those who have a legitimate medical need for opioids but have a disqualifying event that causes the pain medication to be cut off.
“We need to have a different answer to this because termination of opioids because a patient is positive for marijuana or even positive for cocaine doesn’t mean you take services away. You ramp services up,” Dr. Dekker said. “If I have a patient who has a chronic pain syndrome, the only thing that is going to push him out of my system is if they threaten my staff.…The loss from recovery is not a reason to lose treatment. That is another concept that needs to be addressed and we need to really look at that.”
He also called on VA leadership to be more encouraging in prescribing buprenorphine, noting that many doctors and nurses have waivers to prescribe it, but there is a lot of reluctance to do so, even though there is a lot of success with that treatment.