User login
Uncertain generalizability limits AFib ablation in HFrEF
Despite several reports of dramatic efficacy and reasonable safety using catheter ablation of atrial fibrillation (AFib) in patients with heart failure, many clinicians, including many heart failure specialists, remain skeptical about whether existing evidence supports using ablation routinely in selected heart failure patients.
Though concerns vary, one core stumbling block is inadequate confidence that the ablation outcomes reported from studies represent the benefit that the average American heart failure patient might expect to receive from ablation done outside of a study. A related issue is whether atrial fibrillation ablation in patients with heart failure is cost effective, especially at sites that did not participate in the published studies.
The first part of this article discussed the building evidence that radiofrequency catheter ablation of atrial fibrillation (AFib) can produce striking reductions in all-cause mortality of nearly 50%, and a greater than one-third cut in cardiovascular hospitalizations in patients with heart failure with reduced ejection fraction (HFrEF), according to one recent meta-analysis (Circ Arrhythm Electrophysiol. 2019 Sep;12[9]:e007414). A key question about the implications of these findings is their generalizability.
“Experience is an issue, and I agree that not every operator should do it. A common perception is that ablation doesn’t work, but that mindset is changing,” said Luigi Di Biase, MD, director of arrhythmia services at Montefiore Medical Center and professor of medicine at Albert Einstein College of Medicine in New York. He noted that some apparent ablations failures happen because the treatment is used too late. “Ablation will fail if it is done too late. Think about using ablation earlier,” he advised. “The earlier you ablate, the earlier you reduce the AFib burden and the sooner the patient benefits. Ablation is a cost-effective, first-line strategy for younger patients with paroxysmal AFib. The unanswered question is whether it is cost effective for patients who have both AFib and heart failure. It may be, because in addition to the mortality benefit, there are likely savings from a lower rate of hospitalizations. A clearer picture should emerge from the cost-effectiveness analysis of CASTLE-AF.”
CASTLE-AF (Catheter Ablation Versus Standard Conventional Therapy in Patients With Left Ventricular Dysfunction and Atrial Fibrillation), which randomized patients with heart failure and AFib to ablation or medical management (N Engl J Med. 2018 Feb 1;378[5]:417-27), is one of the highest-profile studies reported so far showing AFib ablation’s efficacy in patients with heart failure. However, it has drawn skepticism over its generalizability because of its long enrollment period of 8 years despite running at 33 worldwide sites, and by its winnowing of 3,013 patients assessed down to the 398 actually enrolled and 363 randomized and included in the efficacy analysis.
“CASTLE-HF showed a remarkable benefit. The problem was that it took years and years to enroll the patients,” commented Mariell Jessup, MD, a heart failure specialist and chief science and medical officer of the American Heart Association in Dallas.
At the annual congress of the European Society of Cardiology in September 2019, French researchers reported data that supported the generalizability of the CASTLE-AF findings. The study used data collected from 252,395 patients in the French national hospital-discharge database during 2010-2018 who had diagnoses of both heart failure and AFib. Among these patients, 1,384 underwent catheter ablation and the remaining 251,011 were managed without ablation.
During a median follow-up of 537 days (about 1.5 years), the incidence of both all-cause death and heart failure hospitalization were both significantly lower in the ablated patients. The ablated patients were also much younger and were more often men, but both groups had several prevalent comorbidities at roughly similar rates. To better match the groups, the French researchers ran both a multivariate analysis, and then an even more intensively adjusting propensity-score analysis that compared the ablated patients with 1,384 closely matched patients from the nonablated group. Both analyses showed substantial incremental benefit from ablation. In the propensity score–matched analysis, ablation was linked with a relative 66% cut in all-cause death, and a relative 71% reduction in heart failure hospitalizations, compared with the patients who did not undergo ablation, reported Arnaud Bisson, MD, a cardiologist at the University of Tours (France).
Another recent assessment of the generalizability of the AFib ablation trial findings used data from nearly 184,000 U.S. patients treated for AFib during 2009-2016 in an administrative database, including more than 12,000 treated with ablation. This analysis did not take into account the coexistence of heart failure. After propensity-score matching of the ablated patients with a similar subgroup of those managed medically, the results showed a 25% relative cut in the combined primary endpoint used in the CABANA (Catheter Ablation vs. Anti-Arrhythmic Drug Therapy for Atrial Fibrillation Trial) study (JAMA. 2019 Mar 15;321[134]:1261-74). Among the 74% of ablated patients who met the enrollment criteria for CABANA, the primary endpoint reduction was even greater, a 30% drop relative to matched patients who did not undergo ablation (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
“Professional societies are working to clarify best practices for procedural volume, outcomes, etc.,” said Jonathan P. Piccini, MD, a cardiac electrophysiologist at Duke University, Durham, N.C., and a CABANA coinvestigator. “There are some data on ablation cost effectiveness, and they generally favor” positive cost efficacy, with more analyses now in progress,” he noted in an interview.
Many unanswered questions remain about AFib in heart failure patients and how aggressively to use ablation to treat it. Most of the data so far have come from patients with HFrEF, and so most experts consider AFib ablation in patients with heart failure with preserved ejection fraction (HFpEF) a big unknown, although nearly 80% of the heart failure patients enrolled in CABANA (the largest randomized trial of AFib ablation with more than 2,200 patients) had left ventricular ejection fraction of 50% or greater, which translates into HFpEF. Another gray area is how to think about asymptomatic (also called subclinical) AFib and whether that warrants ablation in heart failure patients. The presence or absence of symptoms is a major consideration because the traditional indication for ablation has been to reduce or eliminate symptoms like palpitations, a step that can substantially improve patients’ quality of life as well as their left ventricular function. The indication to ablate asymptomatic AFib for the purpose of improving survival and reducing hospitalizations is the new and controversial concept. Yet it has been embraced by some heart failure physicians.
“Whether or not AFib is symptomatic doesn’t matter” in a heart failure patient, said Maria Rosa Costanzo, MD, a heart failure physician at Edward Heart Hospital in Naperville, Ill. “A patient with AFib doesn’t get the atrial contribution to cardiac output. When we look deeper, a patient with ‘asymptomatic’ AFib often has symptoms, such as new fatigue or obstructive sleep apnea, so when you see a patient with asymptomatic AFib look for sleep apnea, a trigger for AFib,” Dr. Costanzo advised. “Sleep apnea, AFib, and heart failure form a triad” that often clusters in patients, and the three conditions interact in a vicious circle of reinforcing comorbidities, she said in an interview.
The cardiac electrophysiology and arrhythmia community clearly realizes that catheter ablation of AFib, in patients with or without heart failure, has many unaddressed questions about who should administer it and who should undergo it. In March 2019, the National Heart, Lung, and Blood Institute held a workshop on AFib ablation. “Numerous knowledge gaps remain” about the best way to use ablation, said a summary of the workshop (Circulation. 2019 Nov 20;doi: 10.1161/CIRCULATIONAHA.119.042706). Among the research needs highlighted by the workshop was “more definitive studies ... to delineate the impact of AFib ablation on outcomes in patients with heart failure with preserved ejection fraction.” The workshop recommended establishing a national U.S. registry for AFib ablations with a reliable source of funding, as well as “establishing the cause-effect relationship between ventricular dysfunction and AFib, and the potential moderating role of atrial structure and function.” The workshop also raised the possibility of sham-controlled assessments of AFib ablation, while conceding that enrollment into such trials would probably be very challenging.
The upshot is that, even while ablation advocates agree on the need for more study, clinicians are using AFib ablation on a growing number of heart failure patients (as well as on growing numbers of patients with AFib but without heart failure), with a focus on treating those who “have refractory symptoms or evidence of tachycardia-induced cardiomyopathy,” said Dr. Piccini. Extending that to a first-line, class I indication for heart failure patients seems to need more data, and also needs clinicians to collectively raise their comfort level with the ablation concept. If results from additional studies now underway support the dramatic efficacy and reasonable safety that’s already been seen with ablation, then increased comfort should follow.
CABANA received funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. CASTLE-AF was funded by Biotronik. Dr. Di Biase, Dr. Jessup, and Dr. Bisson had no disclosures. Dr. Piccini has been a consultant to Allergan, Biotronik, Medtronic, Phillips, and Sanofi Aventis; he has received research funding from Abbott, ARCA, Boston Scientific, Gilead, and Johnson & Johnson; and he had a financial relationship with GlaxoSmithKline. Dr. Costanzo has been a consultant to Abbott.
This is part 2 of a 2-part story. See part 1 here.
Despite several reports of dramatic efficacy and reasonable safety using catheter ablation of atrial fibrillation (AFib) in patients with heart failure, many clinicians, including many heart failure specialists, remain skeptical about whether existing evidence supports using ablation routinely in selected heart failure patients.
Though concerns vary, one core stumbling block is inadequate confidence that the ablation outcomes reported from studies represent the benefit that the average American heart failure patient might expect to receive from ablation done outside of a study. A related issue is whether atrial fibrillation ablation in patients with heart failure is cost effective, especially at sites that did not participate in the published studies.
The first part of this article discussed the building evidence that radiofrequency catheter ablation of atrial fibrillation (AFib) can produce striking reductions in all-cause mortality of nearly 50%, and a greater than one-third cut in cardiovascular hospitalizations in patients with heart failure with reduced ejection fraction (HFrEF), according to one recent meta-analysis (Circ Arrhythm Electrophysiol. 2019 Sep;12[9]:e007414). A key question about the implications of these findings is their generalizability.
“Experience is an issue, and I agree that not every operator should do it. A common perception is that ablation doesn’t work, but that mindset is changing,” said Luigi Di Biase, MD, director of arrhythmia services at Montefiore Medical Center and professor of medicine at Albert Einstein College of Medicine in New York. He noted that some apparent ablations failures happen because the treatment is used too late. “Ablation will fail if it is done too late. Think about using ablation earlier,” he advised. “The earlier you ablate, the earlier you reduce the AFib burden and the sooner the patient benefits. Ablation is a cost-effective, first-line strategy for younger patients with paroxysmal AFib. The unanswered question is whether it is cost effective for patients who have both AFib and heart failure. It may be, because in addition to the mortality benefit, there are likely savings from a lower rate of hospitalizations. A clearer picture should emerge from the cost-effectiveness analysis of CASTLE-AF.”
CASTLE-AF (Catheter Ablation Versus Standard Conventional Therapy in Patients With Left Ventricular Dysfunction and Atrial Fibrillation), which randomized patients with heart failure and AFib to ablation or medical management (N Engl J Med. 2018 Feb 1;378[5]:417-27), is one of the highest-profile studies reported so far showing AFib ablation’s efficacy in patients with heart failure. However, it has drawn skepticism over its generalizability because of its long enrollment period of 8 years despite running at 33 worldwide sites, and by its winnowing of 3,013 patients assessed down to the 398 actually enrolled and 363 randomized and included in the efficacy analysis.
“CASTLE-HF showed a remarkable benefit. The problem was that it took years and years to enroll the patients,” commented Mariell Jessup, MD, a heart failure specialist and chief science and medical officer of the American Heart Association in Dallas.
At the annual congress of the European Society of Cardiology in September 2019, French researchers reported data that supported the generalizability of the CASTLE-AF findings. The study used data collected from 252,395 patients in the French national hospital-discharge database during 2010-2018 who had diagnoses of both heart failure and AFib. Among these patients, 1,384 underwent catheter ablation and the remaining 251,011 were managed without ablation.
During a median follow-up of 537 days (about 1.5 years), the incidence of both all-cause death and heart failure hospitalization were both significantly lower in the ablated patients. The ablated patients were also much younger and were more often men, but both groups had several prevalent comorbidities at roughly similar rates. To better match the groups, the French researchers ran both a multivariate analysis, and then an even more intensively adjusting propensity-score analysis that compared the ablated patients with 1,384 closely matched patients from the nonablated group. Both analyses showed substantial incremental benefit from ablation. In the propensity score–matched analysis, ablation was linked with a relative 66% cut in all-cause death, and a relative 71% reduction in heart failure hospitalizations, compared with the patients who did not undergo ablation, reported Arnaud Bisson, MD, a cardiologist at the University of Tours (France).
Another recent assessment of the generalizability of the AFib ablation trial findings used data from nearly 184,000 U.S. patients treated for AFib during 2009-2016 in an administrative database, including more than 12,000 treated with ablation. This analysis did not take into account the coexistence of heart failure. After propensity-score matching of the ablated patients with a similar subgroup of those managed medically, the results showed a 25% relative cut in the combined primary endpoint used in the CABANA (Catheter Ablation vs. Anti-Arrhythmic Drug Therapy for Atrial Fibrillation Trial) study (JAMA. 2019 Mar 15;321[134]:1261-74). Among the 74% of ablated patients who met the enrollment criteria for CABANA, the primary endpoint reduction was even greater, a 30% drop relative to matched patients who did not undergo ablation (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
“Professional societies are working to clarify best practices for procedural volume, outcomes, etc.,” said Jonathan P. Piccini, MD, a cardiac electrophysiologist at Duke University, Durham, N.C., and a CABANA coinvestigator. “There are some data on ablation cost effectiveness, and they generally favor” positive cost efficacy, with more analyses now in progress,” he noted in an interview.
Many unanswered questions remain about AFib in heart failure patients and how aggressively to use ablation to treat it. Most of the data so far have come from patients with HFrEF, and so most experts consider AFib ablation in patients with heart failure with preserved ejection fraction (HFpEF) a big unknown, although nearly 80% of the heart failure patients enrolled in CABANA (the largest randomized trial of AFib ablation with more than 2,200 patients) had left ventricular ejection fraction of 50% or greater, which translates into HFpEF. Another gray area is how to think about asymptomatic (also called subclinical) AFib and whether that warrants ablation in heart failure patients. The presence or absence of symptoms is a major consideration because the traditional indication for ablation has been to reduce or eliminate symptoms like palpitations, a step that can substantially improve patients’ quality of life as well as their left ventricular function. The indication to ablate asymptomatic AFib for the purpose of improving survival and reducing hospitalizations is the new and controversial concept. Yet it has been embraced by some heart failure physicians.
“Whether or not AFib is symptomatic doesn’t matter” in a heart failure patient, said Maria Rosa Costanzo, MD, a heart failure physician at Edward Heart Hospital in Naperville, Ill. “A patient with AFib doesn’t get the atrial contribution to cardiac output. When we look deeper, a patient with ‘asymptomatic’ AFib often has symptoms, such as new fatigue or obstructive sleep apnea, so when you see a patient with asymptomatic AFib look for sleep apnea, a trigger for AFib,” Dr. Costanzo advised. “Sleep apnea, AFib, and heart failure form a triad” that often clusters in patients, and the three conditions interact in a vicious circle of reinforcing comorbidities, she said in an interview.
The cardiac electrophysiology and arrhythmia community clearly realizes that catheter ablation of AFib, in patients with or without heart failure, has many unaddressed questions about who should administer it and who should undergo it. In March 2019, the National Heart, Lung, and Blood Institute held a workshop on AFib ablation. “Numerous knowledge gaps remain” about the best way to use ablation, said a summary of the workshop (Circulation. 2019 Nov 20;doi: 10.1161/CIRCULATIONAHA.119.042706). Among the research needs highlighted by the workshop was “more definitive studies ... to delineate the impact of AFib ablation on outcomes in patients with heart failure with preserved ejection fraction.” The workshop recommended establishing a national U.S. registry for AFib ablations with a reliable source of funding, as well as “establishing the cause-effect relationship between ventricular dysfunction and AFib, and the potential moderating role of atrial structure and function.” The workshop also raised the possibility of sham-controlled assessments of AFib ablation, while conceding that enrollment into such trials would probably be very challenging.
The upshot is that, even while ablation advocates agree on the need for more study, clinicians are using AFib ablation on a growing number of heart failure patients (as well as on growing numbers of patients with AFib but without heart failure), with a focus on treating those who “have refractory symptoms or evidence of tachycardia-induced cardiomyopathy,” said Dr. Piccini. Extending that to a first-line, class I indication for heart failure patients seems to need more data, and also needs clinicians to collectively raise their comfort level with the ablation concept. If results from additional studies now underway support the dramatic efficacy and reasonable safety that’s already been seen with ablation, then increased comfort should follow.
CABANA received funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. CASTLE-AF was funded by Biotronik. Dr. Di Biase, Dr. Jessup, and Dr. Bisson had no disclosures. Dr. Piccini has been a consultant to Allergan, Biotronik, Medtronic, Phillips, and Sanofi Aventis; he has received research funding from Abbott, ARCA, Boston Scientific, Gilead, and Johnson & Johnson; and he had a financial relationship with GlaxoSmithKline. Dr. Costanzo has been a consultant to Abbott.
This is part 2 of a 2-part story. See part 1 here.
Despite several reports of dramatic efficacy and reasonable safety using catheter ablation of atrial fibrillation (AFib) in patients with heart failure, many clinicians, including many heart failure specialists, remain skeptical about whether existing evidence supports using ablation routinely in selected heart failure patients.
Though concerns vary, one core stumbling block is inadequate confidence that the ablation outcomes reported from studies represent the benefit that the average American heart failure patient might expect to receive from ablation done outside of a study. A related issue is whether atrial fibrillation ablation in patients with heart failure is cost effective, especially at sites that did not participate in the published studies.
The first part of this article discussed the building evidence that radiofrequency catheter ablation of atrial fibrillation (AFib) can produce striking reductions in all-cause mortality of nearly 50%, and a greater than one-third cut in cardiovascular hospitalizations in patients with heart failure with reduced ejection fraction (HFrEF), according to one recent meta-analysis (Circ Arrhythm Electrophysiol. 2019 Sep;12[9]:e007414). A key question about the implications of these findings is their generalizability.
“Experience is an issue, and I agree that not every operator should do it. A common perception is that ablation doesn’t work, but that mindset is changing,” said Luigi Di Biase, MD, director of arrhythmia services at Montefiore Medical Center and professor of medicine at Albert Einstein College of Medicine in New York. He noted that some apparent ablations failures happen because the treatment is used too late. “Ablation will fail if it is done too late. Think about using ablation earlier,” he advised. “The earlier you ablate, the earlier you reduce the AFib burden and the sooner the patient benefits. Ablation is a cost-effective, first-line strategy for younger patients with paroxysmal AFib. The unanswered question is whether it is cost effective for patients who have both AFib and heart failure. It may be, because in addition to the mortality benefit, there are likely savings from a lower rate of hospitalizations. A clearer picture should emerge from the cost-effectiveness analysis of CASTLE-AF.”
CASTLE-AF (Catheter Ablation Versus Standard Conventional Therapy in Patients With Left Ventricular Dysfunction and Atrial Fibrillation), which randomized patients with heart failure and AFib to ablation or medical management (N Engl J Med. 2018 Feb 1;378[5]:417-27), is one of the highest-profile studies reported so far showing AFib ablation’s efficacy in patients with heart failure. However, it has drawn skepticism over its generalizability because of its long enrollment period of 8 years despite running at 33 worldwide sites, and by its winnowing of 3,013 patients assessed down to the 398 actually enrolled and 363 randomized and included in the efficacy analysis.
“CASTLE-HF showed a remarkable benefit. The problem was that it took years and years to enroll the patients,” commented Mariell Jessup, MD, a heart failure specialist and chief science and medical officer of the American Heart Association in Dallas.
At the annual congress of the European Society of Cardiology in September 2019, French researchers reported data that supported the generalizability of the CASTLE-AF findings. The study used data collected from 252,395 patients in the French national hospital-discharge database during 2010-2018 who had diagnoses of both heart failure and AFib. Among these patients, 1,384 underwent catheter ablation and the remaining 251,011 were managed without ablation.
During a median follow-up of 537 days (about 1.5 years), the incidence of both all-cause death and heart failure hospitalization were both significantly lower in the ablated patients. The ablated patients were also much younger and were more often men, but both groups had several prevalent comorbidities at roughly similar rates. To better match the groups, the French researchers ran both a multivariate analysis, and then an even more intensively adjusting propensity-score analysis that compared the ablated patients with 1,384 closely matched patients from the nonablated group. Both analyses showed substantial incremental benefit from ablation. In the propensity score–matched analysis, ablation was linked with a relative 66% cut in all-cause death, and a relative 71% reduction in heart failure hospitalizations, compared with the patients who did not undergo ablation, reported Arnaud Bisson, MD, a cardiologist at the University of Tours (France).
Another recent assessment of the generalizability of the AFib ablation trial findings used data from nearly 184,000 U.S. patients treated for AFib during 2009-2016 in an administrative database, including more than 12,000 treated with ablation. This analysis did not take into account the coexistence of heart failure. After propensity-score matching of the ablated patients with a similar subgroup of those managed medically, the results showed a 25% relative cut in the combined primary endpoint used in the CABANA (Catheter Ablation vs. Anti-Arrhythmic Drug Therapy for Atrial Fibrillation Trial) study (JAMA. 2019 Mar 15;321[134]:1261-74). Among the 74% of ablated patients who met the enrollment criteria for CABANA, the primary endpoint reduction was even greater, a 30% drop relative to matched patients who did not undergo ablation (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
“Professional societies are working to clarify best practices for procedural volume, outcomes, etc.,” said Jonathan P. Piccini, MD, a cardiac electrophysiologist at Duke University, Durham, N.C., and a CABANA coinvestigator. “There are some data on ablation cost effectiveness, and they generally favor” positive cost efficacy, with more analyses now in progress,” he noted in an interview.
Many unanswered questions remain about AFib in heart failure patients and how aggressively to use ablation to treat it. Most of the data so far have come from patients with HFrEF, and so most experts consider AFib ablation in patients with heart failure with preserved ejection fraction (HFpEF) a big unknown, although nearly 80% of the heart failure patients enrolled in CABANA (the largest randomized trial of AFib ablation with more than 2,200 patients) had left ventricular ejection fraction of 50% or greater, which translates into HFpEF. Another gray area is how to think about asymptomatic (also called subclinical) AFib and whether that warrants ablation in heart failure patients. The presence or absence of symptoms is a major consideration because the traditional indication for ablation has been to reduce or eliminate symptoms like palpitations, a step that can substantially improve patients’ quality of life as well as their left ventricular function. The indication to ablate asymptomatic AFib for the purpose of improving survival and reducing hospitalizations is the new and controversial concept. Yet it has been embraced by some heart failure physicians.
“Whether or not AFib is symptomatic doesn’t matter” in a heart failure patient, said Maria Rosa Costanzo, MD, a heart failure physician at Edward Heart Hospital in Naperville, Ill. “A patient with AFib doesn’t get the atrial contribution to cardiac output. When we look deeper, a patient with ‘asymptomatic’ AFib often has symptoms, such as new fatigue or obstructive sleep apnea, so when you see a patient with asymptomatic AFib look for sleep apnea, a trigger for AFib,” Dr. Costanzo advised. “Sleep apnea, AFib, and heart failure form a triad” that often clusters in patients, and the three conditions interact in a vicious circle of reinforcing comorbidities, she said in an interview.
The cardiac electrophysiology and arrhythmia community clearly realizes that catheter ablation of AFib, in patients with or without heart failure, has many unaddressed questions about who should administer it and who should undergo it. In March 2019, the National Heart, Lung, and Blood Institute held a workshop on AFib ablation. “Numerous knowledge gaps remain” about the best way to use ablation, said a summary of the workshop (Circulation. 2019 Nov 20;doi: 10.1161/CIRCULATIONAHA.119.042706). Among the research needs highlighted by the workshop was “more definitive studies ... to delineate the impact of AFib ablation on outcomes in patients with heart failure with preserved ejection fraction.” The workshop recommended establishing a national U.S. registry for AFib ablations with a reliable source of funding, as well as “establishing the cause-effect relationship between ventricular dysfunction and AFib, and the potential moderating role of atrial structure and function.” The workshop also raised the possibility of sham-controlled assessments of AFib ablation, while conceding that enrollment into such trials would probably be very challenging.
The upshot is that, even while ablation advocates agree on the need for more study, clinicians are using AFib ablation on a growing number of heart failure patients (as well as on growing numbers of patients with AFib but without heart failure), with a focus on treating those who “have refractory symptoms or evidence of tachycardia-induced cardiomyopathy,” said Dr. Piccini. Extending that to a first-line, class I indication for heart failure patients seems to need more data, and also needs clinicians to collectively raise their comfort level with the ablation concept. If results from additional studies now underway support the dramatic efficacy and reasonable safety that’s already been seen with ablation, then increased comfort should follow.
CABANA received funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. CASTLE-AF was funded by Biotronik. Dr. Di Biase, Dr. Jessup, and Dr. Bisson had no disclosures. Dr. Piccini has been a consultant to Allergan, Biotronik, Medtronic, Phillips, and Sanofi Aventis; he has received research funding from Abbott, ARCA, Boston Scientific, Gilead, and Johnson & Johnson; and he had a financial relationship with GlaxoSmithKline. Dr. Costanzo has been a consultant to Abbott.
This is part 2 of a 2-part story. See part 1 here.
New heart failure trial data presage guideline revisions
PHILADELPHIA – The definition and treatment of heart failure with reduced ejection fraction should change based on recent findings and analyses from major trials, said a key heart failure leader at the American Heart Association scientific sessions.
The people charged with writing U.S. guidelines for heart failure management already have enough evidence to change the recommended way of using sacubitril/valsartan (Entresto) in patients with heart failure with reduced ejection fraction (HFrEF), said Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University, Chicago. Accumulated evidence from studies and more than 5 years of experience in routine practice with the angiotensin receptor neprilysin inhibitor (ARNI) combination sacubitril/valsartan for treating HFrEF patients justifies striking the existing recommendation to first start patients on an ACE inhibitor or angiotensin receptor blocker and only after that switching to sacubitril/valsartan, a sequence that has rankled some clinicians as an unnecessary delay and barrier to starting patients on the ARNI regimen.
U.S. guidelines should now suggest that ARNI treatment start immediately, suggested Dr. Yancy, who chaired the AHA/American College of Cardiology panel that updated U.S. guidelines for heart failure management in 2013 (Circulation. 2013 Oct 15;128[16]:e240-327), 2016 (J Am Coll Cardiol. 2016 Sep;68[13]:1476-88), and 2017 (Circulation. 2017 Aug 8; 136[6]:e137-61).
Expanding the heart failure group for sacubitril/valsartan
Dr. Yancy also proposed a second major and immediate change to the existing heart failure guideline based on a new appreciation of a heart failure population that could benefit from ARNI treatment: patients with “mid-range” heart failure, defined by a left ventricular ejection fraction (LVEF) of 41%-49% that places them between patients with HFrEF with an ejection fraction of 40% or less, and those with heart failure with preserved ejection fraction (HFpEF) of 50% or more. As yet unchanged in the 2013 AHA/ACC heart failure guideline is the proposition that patients with heart failure and an ejection fraction of 41%-49% have “borderline” heart failure with characteristics, treatment patterns, and outcomes “similar to patients with HFpEF.”
That premise should now go out the window, urged Dr. Yancy, based on a new analysis of data collected from both the recent PARAGON-HF trial of sacubitril/valsartan in patients with HFpEF and ejection fractions of 45% or higher (N Engl J Med. 2019 Oct 24;381[17]:1609-20) and the landmark PARADIGM-HF trial that established sacubitril/valsartan as a treatment for patients with HFrEF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). A combined analysis of the more than 13,000 total patients in both studies suggested that “patients with ejection fraction lower than normal, which includes those with so-called heart failure with mid-range ejection fraction or borderline ejection fraction, would likely benefit from sacubitril/valsartan, compared with RAS inhibition,” concluded the authors of the new analysis (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044586).
Dr. Yancy argued that, based on this new analysis, a further revision to the 2013 guideline should say that patients with heart failure with a LVEF of 41%-49% have characteristics, treatment responses, and outcomes that “appear similar to those of patient with HFrEF,” a sharp departure from the existing text that lumps these patients with the HFpEF subgroup. “There appears to be a signal that extends the benefit of ARNI to patients with ejection fractions above the current threshold for HFrEF but below what is typically HFpEF,” he said.
Bringing SGLT2 inhibitors into heart failure management
Dr. Yancy also cited recently reported data from another landmark trial, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), as an impetus for both another immediate change to the guideline and for a potential second change pending a report of confirmatory evidence that may arrive in 2020.
The DAPA-HF results showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) was just as effective for preventing all-cause death and heart failure hospitalizations and urgent visits in patients without type 2 diabetes as it is in patients with type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008), a remarkable finding for an agent that came onto the U.S. market as a diabetes drug specifically aimed at reducing levels of glycosylated hemoglobin.
Dr. Yancy proposed an immediate guideline change to acknowledge the proven protection against incident heart failure that treatment with a SGLT2 inhibitor gives patients with type 2 diabetes. There is now “a strong opportunity to use an SGLT2 inhibitor in patients with type 2 diabetes to reduce the incidence of heart failure,” he said.
And he added that, if results from EMPEROR REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), studying the SGLT2 inhibitor empagliflozin (Jardiance) in HFrEF patients with and without type 2 diabetes, can confirm the efficacy of a second drug from this class in preventing heart failure events in patients with HFrEF but without diabetes, then the time will have arrived for another guideline change to establish the SGLT2 inhibitors as a new “foundational” drug for the management of all HFrEF patients, regardless of their level of glycemic control. The SGLT2 inhibitors are a particularly attractive additional drug because they are taken once daily orally with no need for dosage adjustment, so far they have shown excellent safety in patients without diabetes with no episodes of hypoglycemia or ketoacidosis, and they have even shown evidence for heart failure benefit in patients older than 75 years, Dr. Yancy noted.
Dr. Yancy had no relevant disclosures.
PHILADELPHIA – The definition and treatment of heart failure with reduced ejection fraction should change based on recent findings and analyses from major trials, said a key heart failure leader at the American Heart Association scientific sessions.
The people charged with writing U.S. guidelines for heart failure management already have enough evidence to change the recommended way of using sacubitril/valsartan (Entresto) in patients with heart failure with reduced ejection fraction (HFrEF), said Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University, Chicago. Accumulated evidence from studies and more than 5 years of experience in routine practice with the angiotensin receptor neprilysin inhibitor (ARNI) combination sacubitril/valsartan for treating HFrEF patients justifies striking the existing recommendation to first start patients on an ACE inhibitor or angiotensin receptor blocker and only after that switching to sacubitril/valsartan, a sequence that has rankled some clinicians as an unnecessary delay and barrier to starting patients on the ARNI regimen.
U.S. guidelines should now suggest that ARNI treatment start immediately, suggested Dr. Yancy, who chaired the AHA/American College of Cardiology panel that updated U.S. guidelines for heart failure management in 2013 (Circulation. 2013 Oct 15;128[16]:e240-327), 2016 (J Am Coll Cardiol. 2016 Sep;68[13]:1476-88), and 2017 (Circulation. 2017 Aug 8; 136[6]:e137-61).
Expanding the heart failure group for sacubitril/valsartan
Dr. Yancy also proposed a second major and immediate change to the existing heart failure guideline based on a new appreciation of a heart failure population that could benefit from ARNI treatment: patients with “mid-range” heart failure, defined by a left ventricular ejection fraction (LVEF) of 41%-49% that places them between patients with HFrEF with an ejection fraction of 40% or less, and those with heart failure with preserved ejection fraction (HFpEF) of 50% or more. As yet unchanged in the 2013 AHA/ACC heart failure guideline is the proposition that patients with heart failure and an ejection fraction of 41%-49% have “borderline” heart failure with characteristics, treatment patterns, and outcomes “similar to patients with HFpEF.”
That premise should now go out the window, urged Dr. Yancy, based on a new analysis of data collected from both the recent PARAGON-HF trial of sacubitril/valsartan in patients with HFpEF and ejection fractions of 45% or higher (N Engl J Med. 2019 Oct 24;381[17]:1609-20) and the landmark PARADIGM-HF trial that established sacubitril/valsartan as a treatment for patients with HFrEF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). A combined analysis of the more than 13,000 total patients in both studies suggested that “patients with ejection fraction lower than normal, which includes those with so-called heart failure with mid-range ejection fraction or borderline ejection fraction, would likely benefit from sacubitril/valsartan, compared with RAS inhibition,” concluded the authors of the new analysis (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044586).
Dr. Yancy argued that, based on this new analysis, a further revision to the 2013 guideline should say that patients with heart failure with a LVEF of 41%-49% have characteristics, treatment responses, and outcomes that “appear similar to those of patient with HFrEF,” a sharp departure from the existing text that lumps these patients with the HFpEF subgroup. “There appears to be a signal that extends the benefit of ARNI to patients with ejection fractions above the current threshold for HFrEF but below what is typically HFpEF,” he said.
Bringing SGLT2 inhibitors into heart failure management
Dr. Yancy also cited recently reported data from another landmark trial, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), as an impetus for both another immediate change to the guideline and for a potential second change pending a report of confirmatory evidence that may arrive in 2020.
The DAPA-HF results showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) was just as effective for preventing all-cause death and heart failure hospitalizations and urgent visits in patients without type 2 diabetes as it is in patients with type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008), a remarkable finding for an agent that came onto the U.S. market as a diabetes drug specifically aimed at reducing levels of glycosylated hemoglobin.
Dr. Yancy proposed an immediate guideline change to acknowledge the proven protection against incident heart failure that treatment with a SGLT2 inhibitor gives patients with type 2 diabetes. There is now “a strong opportunity to use an SGLT2 inhibitor in patients with type 2 diabetes to reduce the incidence of heart failure,” he said.
And he added that, if results from EMPEROR REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), studying the SGLT2 inhibitor empagliflozin (Jardiance) in HFrEF patients with and without type 2 diabetes, can confirm the efficacy of a second drug from this class in preventing heart failure events in patients with HFrEF but without diabetes, then the time will have arrived for another guideline change to establish the SGLT2 inhibitors as a new “foundational” drug for the management of all HFrEF patients, regardless of their level of glycemic control. The SGLT2 inhibitors are a particularly attractive additional drug because they are taken once daily orally with no need for dosage adjustment, so far they have shown excellent safety in patients without diabetes with no episodes of hypoglycemia or ketoacidosis, and they have even shown evidence for heart failure benefit in patients older than 75 years, Dr. Yancy noted.
Dr. Yancy had no relevant disclosures.
PHILADELPHIA – The definition and treatment of heart failure with reduced ejection fraction should change based on recent findings and analyses from major trials, said a key heart failure leader at the American Heart Association scientific sessions.
The people charged with writing U.S. guidelines for heart failure management already have enough evidence to change the recommended way of using sacubitril/valsartan (Entresto) in patients with heart failure with reduced ejection fraction (HFrEF), said Clyde W. Yancy, MD, professor of medicine and chief of cardiology at Northwestern University, Chicago. Accumulated evidence from studies and more than 5 years of experience in routine practice with the angiotensin receptor neprilysin inhibitor (ARNI) combination sacubitril/valsartan for treating HFrEF patients justifies striking the existing recommendation to first start patients on an ACE inhibitor or angiotensin receptor blocker and only after that switching to sacubitril/valsartan, a sequence that has rankled some clinicians as an unnecessary delay and barrier to starting patients on the ARNI regimen.
U.S. guidelines should now suggest that ARNI treatment start immediately, suggested Dr. Yancy, who chaired the AHA/American College of Cardiology panel that updated U.S. guidelines for heart failure management in 2013 (Circulation. 2013 Oct 15;128[16]:e240-327), 2016 (J Am Coll Cardiol. 2016 Sep;68[13]:1476-88), and 2017 (Circulation. 2017 Aug 8; 136[6]:e137-61).
Expanding the heart failure group for sacubitril/valsartan
Dr. Yancy also proposed a second major and immediate change to the existing heart failure guideline based on a new appreciation of a heart failure population that could benefit from ARNI treatment: patients with “mid-range” heart failure, defined by a left ventricular ejection fraction (LVEF) of 41%-49% that places them between patients with HFrEF with an ejection fraction of 40% or less, and those with heart failure with preserved ejection fraction (HFpEF) of 50% or more. As yet unchanged in the 2013 AHA/ACC heart failure guideline is the proposition that patients with heart failure and an ejection fraction of 41%-49% have “borderline” heart failure with characteristics, treatment patterns, and outcomes “similar to patients with HFpEF.”
That premise should now go out the window, urged Dr. Yancy, based on a new analysis of data collected from both the recent PARAGON-HF trial of sacubitril/valsartan in patients with HFpEF and ejection fractions of 45% or higher (N Engl J Med. 2019 Oct 24;381[17]:1609-20) and the landmark PARADIGM-HF trial that established sacubitril/valsartan as a treatment for patients with HFrEF (N Engl J Med. 2014 Sep 11;371[11]:993-1004). A combined analysis of the more than 13,000 total patients in both studies suggested that “patients with ejection fraction lower than normal, which includes those with so-called heart failure with mid-range ejection fraction or borderline ejection fraction, would likely benefit from sacubitril/valsartan, compared with RAS inhibition,” concluded the authors of the new analysis (Circulation. 2019 Nov 17. doi: 10.1161/CIRCULATIONAHA.119.044586).
Dr. Yancy argued that, based on this new analysis, a further revision to the 2013 guideline should say that patients with heart failure with a LVEF of 41%-49% have characteristics, treatment responses, and outcomes that “appear similar to those of patient with HFrEF,” a sharp departure from the existing text that lumps these patients with the HFpEF subgroup. “There appears to be a signal that extends the benefit of ARNI to patients with ejection fractions above the current threshold for HFrEF but below what is typically HFpEF,” he said.
Bringing SGLT2 inhibitors into heart failure management
Dr. Yancy also cited recently reported data from another landmark trial, DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure), as an impetus for both another immediate change to the guideline and for a potential second change pending a report of confirmatory evidence that may arrive in 2020.
The DAPA-HF results showed that the sodium-glucose cotransporter 2 (SGLT2) inhibitor dapagliflozin (Farxiga) was just as effective for preventing all-cause death and heart failure hospitalizations and urgent visits in patients without type 2 diabetes as it is in patients with type 2 diabetes (N Engl J Med. 2019 Nov 21;381[21]:1995-2008), a remarkable finding for an agent that came onto the U.S. market as a diabetes drug specifically aimed at reducing levels of glycosylated hemoglobin.
Dr. Yancy proposed an immediate guideline change to acknowledge the proven protection against incident heart failure that treatment with a SGLT2 inhibitor gives patients with type 2 diabetes. There is now “a strong opportunity to use an SGLT2 inhibitor in patients with type 2 diabetes to reduce the incidence of heart failure,” he said.
And he added that, if results from EMPEROR REDUCED (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction), studying the SGLT2 inhibitor empagliflozin (Jardiance) in HFrEF patients with and without type 2 diabetes, can confirm the efficacy of a second drug from this class in preventing heart failure events in patients with HFrEF but without diabetes, then the time will have arrived for another guideline change to establish the SGLT2 inhibitors as a new “foundational” drug for the management of all HFrEF patients, regardless of their level of glycemic control. The SGLT2 inhibitors are a particularly attractive additional drug because they are taken once daily orally with no need for dosage adjustment, so far they have shown excellent safety in patients without diabetes with no episodes of hypoglycemia or ketoacidosis, and they have even shown evidence for heart failure benefit in patients older than 75 years, Dr. Yancy noted.
Dr. Yancy had no relevant disclosures.
EXPERT ANALYSIS FROM AHA 2019
Updated gout guidelines: Don’t let kidney function dictate allopurinol dosing
ATLANTA – Soon-to-be-published gout guidelines from the American College of Rheumatology will recommend dosing allopurinol above 300 mg/day to get serum urate below 6 mg/dL, even in people with renal impairment.
It’s the same strong treat-to-target recommendation the group made in its last outing in 2012, but “we now have more evidence to support it,” said co–lead author, rheumatologist, and epidemiologist Tuhina Neogi, MD, PhD, a professor of medicine at Boston University.
She gave a sneak preview of the new guidelines, which will be published in 2020, at the ACR annual meeting. They are under review, but she said the “major recommendations will remain the same.”
“There will still be controversy that we have not yet proven that a threshold of 6 mg/dL is better than a threshold of 7 mg/dL, but we know that” at physiologic pH and temperature, monosodium urate starts to crystallize out at 6.8 mg/dL. “Serum urate is not a perfect measure or total body urate, so we need to get urate to below at least 6 mg/dL,” she said, and perhaps lower in some.
A popular alternative in primary care – where most gout is managed – is to treat to avoid symptoms. It “has no evidence,” and people “end up getting tophaceous gout with joint destruction. Suppressive colchicine therapy does not manage underlying hyperuricemia,” Dr. Neogi said.
With the symptom approach, “patients are often [profoundly] dismayed” when they find out they have large tophi and joint damage because they weren’t managed properly. “Primary care physicians [don’t often] see that because those patients don’t go back to them,” she said.
Dr. Neogi suspects that, for rheumatologists, the biggest surprise in the new guidelines will be a deemphasis on lifestyle and dietary factors. They can be triggers, but “gout is increasingly recognized as largely genetically determined,” and the impact of other factors on serum urate is low. Plus, “patients are embarrassed” by gout, and even less comfortable being honest with physicians “if they think we are blaming them,” she said.
The new document will recommend allopurinol as the definitive first-line option for hyperuricemia. Febuxostat (Uloric) was put on pretty much equal footing in 2012, but now “we acknowledge” that allopurinol dosing in head-to-head trials – 300 mg/day or 200 mg/day with renal impairment – was too low for most people, “so to say febuxostat is equivalent or superior isn’t really fair.” The substantially higher cost of febuxostat was also taken into consideration, she said.
The ACR will broaden the indications for urate lowering beyond frequent flares, tophi, and radiologic joint damage to include conditional, shared decision-making recommendations for people who have less than two flares per year, those with kidney stones, and people with a first flare if they are particularly susceptible to a second – namely those with serum urate at or above 9 mg/dL and people with stage 3 or worse chronic kidney disease, who are less able to tolerate NSAIDs and colchicine for symptom treatment.
The group will also relax its advice against treating asymptomatic hyperuricemia. Febuxostat trials have shown a reduction in incident gout, but the number needed to treat was large, so the ACR will recommend shared decision making.
Inadequate allopurinol dosing, meanwhile, has been the bête noire of rheumatology for years, but there is still reluctance among many to go above 300 mg/day. Dr. Neogi said it’s because of a decades-old concern, “unsupported by any evidence, that higher doses may be detrimental in people with renal insufficiency.” It’s frustrating, she said, because “there is good data supporting the safety of increasing the dose above 300 mg/day even in those with renal impairment,” and not doing so opens the door to entirely preventable complications.
As for allopurinol hypersensitivity – another reason people shy away from higher dosing, especially in the renally impaired – the trick is to start low and slowly titrate allopurinol up to the target urate range. Asian and black people, especially, should be screened beforehand for the HLA-B*58:01 genetic variant that increases the risk of severe reactions. Both will be strong recommendations in the new guidelines.
Dr. Neogi didn’t have any relevant industry disclosures.
ATLANTA – Soon-to-be-published gout guidelines from the American College of Rheumatology will recommend dosing allopurinol above 300 mg/day to get serum urate below 6 mg/dL, even in people with renal impairment.
It’s the same strong treat-to-target recommendation the group made in its last outing in 2012, but “we now have more evidence to support it,” said co–lead author, rheumatologist, and epidemiologist Tuhina Neogi, MD, PhD, a professor of medicine at Boston University.
She gave a sneak preview of the new guidelines, which will be published in 2020, at the ACR annual meeting. They are under review, but she said the “major recommendations will remain the same.”
“There will still be controversy that we have not yet proven that a threshold of 6 mg/dL is better than a threshold of 7 mg/dL, but we know that” at physiologic pH and temperature, monosodium urate starts to crystallize out at 6.8 mg/dL. “Serum urate is not a perfect measure or total body urate, so we need to get urate to below at least 6 mg/dL,” she said, and perhaps lower in some.
A popular alternative in primary care – where most gout is managed – is to treat to avoid symptoms. It “has no evidence,” and people “end up getting tophaceous gout with joint destruction. Suppressive colchicine therapy does not manage underlying hyperuricemia,” Dr. Neogi said.
With the symptom approach, “patients are often [profoundly] dismayed” when they find out they have large tophi and joint damage because they weren’t managed properly. “Primary care physicians [don’t often] see that because those patients don’t go back to them,” she said.
Dr. Neogi suspects that, for rheumatologists, the biggest surprise in the new guidelines will be a deemphasis on lifestyle and dietary factors. They can be triggers, but “gout is increasingly recognized as largely genetically determined,” and the impact of other factors on serum urate is low. Plus, “patients are embarrassed” by gout, and even less comfortable being honest with physicians “if they think we are blaming them,” she said.
The new document will recommend allopurinol as the definitive first-line option for hyperuricemia. Febuxostat (Uloric) was put on pretty much equal footing in 2012, but now “we acknowledge” that allopurinol dosing in head-to-head trials – 300 mg/day or 200 mg/day with renal impairment – was too low for most people, “so to say febuxostat is equivalent or superior isn’t really fair.” The substantially higher cost of febuxostat was also taken into consideration, she said.
The ACR will broaden the indications for urate lowering beyond frequent flares, tophi, and radiologic joint damage to include conditional, shared decision-making recommendations for people who have less than two flares per year, those with kidney stones, and people with a first flare if they are particularly susceptible to a second – namely those with serum urate at or above 9 mg/dL and people with stage 3 or worse chronic kidney disease, who are less able to tolerate NSAIDs and colchicine for symptom treatment.
The group will also relax its advice against treating asymptomatic hyperuricemia. Febuxostat trials have shown a reduction in incident gout, but the number needed to treat was large, so the ACR will recommend shared decision making.
Inadequate allopurinol dosing, meanwhile, has been the bête noire of rheumatology for years, but there is still reluctance among many to go above 300 mg/day. Dr. Neogi said it’s because of a decades-old concern, “unsupported by any evidence, that higher doses may be detrimental in people with renal insufficiency.” It’s frustrating, she said, because “there is good data supporting the safety of increasing the dose above 300 mg/day even in those with renal impairment,” and not doing so opens the door to entirely preventable complications.
As for allopurinol hypersensitivity – another reason people shy away from higher dosing, especially in the renally impaired – the trick is to start low and slowly titrate allopurinol up to the target urate range. Asian and black people, especially, should be screened beforehand for the HLA-B*58:01 genetic variant that increases the risk of severe reactions. Both will be strong recommendations in the new guidelines.
Dr. Neogi didn’t have any relevant industry disclosures.
ATLANTA – Soon-to-be-published gout guidelines from the American College of Rheumatology will recommend dosing allopurinol above 300 mg/day to get serum urate below 6 mg/dL, even in people with renal impairment.
It’s the same strong treat-to-target recommendation the group made in its last outing in 2012, but “we now have more evidence to support it,” said co–lead author, rheumatologist, and epidemiologist Tuhina Neogi, MD, PhD, a professor of medicine at Boston University.
She gave a sneak preview of the new guidelines, which will be published in 2020, at the ACR annual meeting. They are under review, but she said the “major recommendations will remain the same.”
“There will still be controversy that we have not yet proven that a threshold of 6 mg/dL is better than a threshold of 7 mg/dL, but we know that” at physiologic pH and temperature, monosodium urate starts to crystallize out at 6.8 mg/dL. “Serum urate is not a perfect measure or total body urate, so we need to get urate to below at least 6 mg/dL,” she said, and perhaps lower in some.
A popular alternative in primary care – where most gout is managed – is to treat to avoid symptoms. It “has no evidence,” and people “end up getting tophaceous gout with joint destruction. Suppressive colchicine therapy does not manage underlying hyperuricemia,” Dr. Neogi said.
With the symptom approach, “patients are often [profoundly] dismayed” when they find out they have large tophi and joint damage because they weren’t managed properly. “Primary care physicians [don’t often] see that because those patients don’t go back to them,” she said.
Dr. Neogi suspects that, for rheumatologists, the biggest surprise in the new guidelines will be a deemphasis on lifestyle and dietary factors. They can be triggers, but “gout is increasingly recognized as largely genetically determined,” and the impact of other factors on serum urate is low. Plus, “patients are embarrassed” by gout, and even less comfortable being honest with physicians “if they think we are blaming them,” she said.
The new document will recommend allopurinol as the definitive first-line option for hyperuricemia. Febuxostat (Uloric) was put on pretty much equal footing in 2012, but now “we acknowledge” that allopurinol dosing in head-to-head trials – 300 mg/day or 200 mg/day with renal impairment – was too low for most people, “so to say febuxostat is equivalent or superior isn’t really fair.” The substantially higher cost of febuxostat was also taken into consideration, she said.
The ACR will broaden the indications for urate lowering beyond frequent flares, tophi, and radiologic joint damage to include conditional, shared decision-making recommendations for people who have less than two flares per year, those with kidney stones, and people with a first flare if they are particularly susceptible to a second – namely those with serum urate at or above 9 mg/dL and people with stage 3 or worse chronic kidney disease, who are less able to tolerate NSAIDs and colchicine for symptom treatment.
The group will also relax its advice against treating asymptomatic hyperuricemia. Febuxostat trials have shown a reduction in incident gout, but the number needed to treat was large, so the ACR will recommend shared decision making.
Inadequate allopurinol dosing, meanwhile, has been the bête noire of rheumatology for years, but there is still reluctance among many to go above 300 mg/day. Dr. Neogi said it’s because of a decades-old concern, “unsupported by any evidence, that higher doses may be detrimental in people with renal insufficiency.” It’s frustrating, she said, because “there is good data supporting the safety of increasing the dose above 300 mg/day even in those with renal impairment,” and not doing so opens the door to entirely preventable complications.
As for allopurinol hypersensitivity – another reason people shy away from higher dosing, especially in the renally impaired – the trick is to start low and slowly titrate allopurinol up to the target urate range. Asian and black people, especially, should be screened beforehand for the HLA-B*58:01 genetic variant that increases the risk of severe reactions. Both will be strong recommendations in the new guidelines.
Dr. Neogi didn’t have any relevant industry disclosures.
REPORTING FROM ACR 2019
Scalp EEG predicts temporal lobe resection success
BALTIMORE – In a review of 43 temporal lobe epilepsy patients at Yale University in New Haven, Conn., anteromedial temporal resection (AMTR) failed in every case in which initial ictal rhythm on scalp EEG spread beyond the medial temporal lobe to other brain regions within 10 seconds.
Among the 33 patients who had no spread on preoperative scalp EEG or who spread in 10 or more seconds, 31 (94%) had a good outcome, meaning they were seizure free or had only auras after AMTR. The findings could mean that scalp EEG can predict surgery outcome.
AMTR works in the majority of patients with refractory temporal lobe epilepsy, but about 10-20% continue to have seizures. Senior investigator Pue Farooque, DO, from Yale University wanted to find a way to identify patients likely to fail surgery beforehand to help counsel patients on what to expect and also to know when other treatment options might be a better bet.
“If you see seizures are spreading quickly to another area, like the frontal lobe or the temporal neocortex, you could implant RNS [responsive neurostimulation]” instead of doing an ATMR, “and that might improve your outcomes,” she said at the American Epilepsy Society’s annual meeting.
The findings are essentially the same as when the group used intracranial EEG to detect fast spread in a previous report, but scalp EEG is noninvasive and allows for easy preoperative assessment (JAMA Neurol. 2019 Apr 1;76[4]:462-9).
The team also found in their new study that diffuse hypometabolism in the entire temporal lobe on quantitative PET also predicted poor ATMR outcomes (P less than .001), but Dr. Farooque said more work is needed to quantify the finding. The investigators also plan to assess the predictive value of resting functional MRI.
The take home, she said, is that “we can do better” with epilepsy surgery, and “there are noninvasive markers we can use to help guide us.”
It’s unclear why more rapid seizure spread would predict AMTR failure. In the earlier study with intracranial EEG, the investigators said “the results are best explained by attributing epileptogenic potential to sites of early seizure spread that were not included in resection. This mechanism of failure implies that a distributed epileptogenic network rather than a single epileptogenic focus may underlie surgically refractory epilepsy.”
Patients in the new report had epilepsy for a mean of 24.4 years, and 25 (58%) were women; 30 cases (69%) were lesional, and follow-up was at least a year. The contralateral or lateralized seizure spread ranged from 1 to 63 seconds, with a mean of 18.5 seconds. Among patients who failed AMTR, seizure spread occurred at a mean of 7.1 seconds.
Electrographic pattern at onset and location of interictal epileptiform discharges did not predict outcome
There was no industry funding, and Dr. Farooque didn’t have any relevant disclosures.
SOURCE: Chiari J et al. AES 2019, Abstract 1.36.
BALTIMORE – In a review of 43 temporal lobe epilepsy patients at Yale University in New Haven, Conn., anteromedial temporal resection (AMTR) failed in every case in which initial ictal rhythm on scalp EEG spread beyond the medial temporal lobe to other brain regions within 10 seconds.
Among the 33 patients who had no spread on preoperative scalp EEG or who spread in 10 or more seconds, 31 (94%) had a good outcome, meaning they were seizure free or had only auras after AMTR. The findings could mean that scalp EEG can predict surgery outcome.
AMTR works in the majority of patients with refractory temporal lobe epilepsy, but about 10-20% continue to have seizures. Senior investigator Pue Farooque, DO, from Yale University wanted to find a way to identify patients likely to fail surgery beforehand to help counsel patients on what to expect and also to know when other treatment options might be a better bet.
“If you see seizures are spreading quickly to another area, like the frontal lobe or the temporal neocortex, you could implant RNS [responsive neurostimulation]” instead of doing an ATMR, “and that might improve your outcomes,” she said at the American Epilepsy Society’s annual meeting.
The findings are essentially the same as when the group used intracranial EEG to detect fast spread in a previous report, but scalp EEG is noninvasive and allows for easy preoperative assessment (JAMA Neurol. 2019 Apr 1;76[4]:462-9).
The team also found in their new study that diffuse hypometabolism in the entire temporal lobe on quantitative PET also predicted poor ATMR outcomes (P less than .001), but Dr. Farooque said more work is needed to quantify the finding. The investigators also plan to assess the predictive value of resting functional MRI.
The take home, she said, is that “we can do better” with epilepsy surgery, and “there are noninvasive markers we can use to help guide us.”
It’s unclear why more rapid seizure spread would predict AMTR failure. In the earlier study with intracranial EEG, the investigators said “the results are best explained by attributing epileptogenic potential to sites of early seizure spread that were not included in resection. This mechanism of failure implies that a distributed epileptogenic network rather than a single epileptogenic focus may underlie surgically refractory epilepsy.”
Patients in the new report had epilepsy for a mean of 24.4 years, and 25 (58%) were women; 30 cases (69%) were lesional, and follow-up was at least a year. The contralateral or lateralized seizure spread ranged from 1 to 63 seconds, with a mean of 18.5 seconds. Among patients who failed AMTR, seizure spread occurred at a mean of 7.1 seconds.
Electrographic pattern at onset and location of interictal epileptiform discharges did not predict outcome
There was no industry funding, and Dr. Farooque didn’t have any relevant disclosures.
SOURCE: Chiari J et al. AES 2019, Abstract 1.36.
BALTIMORE – In a review of 43 temporal lobe epilepsy patients at Yale University in New Haven, Conn., anteromedial temporal resection (AMTR) failed in every case in which initial ictal rhythm on scalp EEG spread beyond the medial temporal lobe to other brain regions within 10 seconds.
Among the 33 patients who had no spread on preoperative scalp EEG or who spread in 10 or more seconds, 31 (94%) had a good outcome, meaning they were seizure free or had only auras after AMTR. The findings could mean that scalp EEG can predict surgery outcome.
AMTR works in the majority of patients with refractory temporal lobe epilepsy, but about 10-20% continue to have seizures. Senior investigator Pue Farooque, DO, from Yale University wanted to find a way to identify patients likely to fail surgery beforehand to help counsel patients on what to expect and also to know when other treatment options might be a better bet.
“If you see seizures are spreading quickly to another area, like the frontal lobe or the temporal neocortex, you could implant RNS [responsive neurostimulation]” instead of doing an ATMR, “and that might improve your outcomes,” she said at the American Epilepsy Society’s annual meeting.
The findings are essentially the same as when the group used intracranial EEG to detect fast spread in a previous report, but scalp EEG is noninvasive and allows for easy preoperative assessment (JAMA Neurol. 2019 Apr 1;76[4]:462-9).
The team also found in their new study that diffuse hypometabolism in the entire temporal lobe on quantitative PET also predicted poor ATMR outcomes (P less than .001), but Dr. Farooque said more work is needed to quantify the finding. The investigators also plan to assess the predictive value of resting functional MRI.
The take home, she said, is that “we can do better” with epilepsy surgery, and “there are noninvasive markers we can use to help guide us.”
It’s unclear why more rapid seizure spread would predict AMTR failure. In the earlier study with intracranial EEG, the investigators said “the results are best explained by attributing epileptogenic potential to sites of early seizure spread that were not included in resection. This mechanism of failure implies that a distributed epileptogenic network rather than a single epileptogenic focus may underlie surgically refractory epilepsy.”
Patients in the new report had epilepsy for a mean of 24.4 years, and 25 (58%) were women; 30 cases (69%) were lesional, and follow-up was at least a year. The contralateral or lateralized seizure spread ranged from 1 to 63 seconds, with a mean of 18.5 seconds. Among patients who failed AMTR, seizure spread occurred at a mean of 7.1 seconds.
Electrographic pattern at onset and location of interictal epileptiform discharges did not predict outcome
There was no industry funding, and Dr. Farooque didn’t have any relevant disclosures.
SOURCE: Chiari J et al. AES 2019, Abstract 1.36.
REPORTING FROM AES 2019
Single-fraction spine stereotactic radiosurgery is cost-efficient
A comparative cost analysis suggests single-fraction spine stereotactic radiosurgery is associated with lower total resource utilization among other radiation therapy (RT) options, according to recent research.
“We quantified institutional costs associated with RT for spinal metastases, using a time-driven activity-based costing model,” wrote David Boyce-Fappiano, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. Their report is in the Journal of Oncology Practice.
The researchers compared resource utilization across four common RT regimens: single-fraction spine stereotactic radiosurgery (to 18 Gy), 3-fraction spine stereotactic radiosurgery (to 27 Gy), 10-fraction three-dimensional RT (3D-RT) (to 30 Gy), and 10-fraction intensity-modulated RT (IMRT) (to 30 Gy).
The analysis framework involved the creation of both process maps and process times, which included a detailed outline to map the complete clinical care process, while expert panel interviews were used to establish process times.
Other measures, such as the capacity cost rate, were calculated for each resource, and subsequently used to estimate total costs.
After analysis, the researchers found that across the four RT regimens, full-cycle care costs for single-fraction spine stereotactic radiosurgery were 17% less and 17% more than IMRT and 3D-RT, respectively. However, technical costs for IMRT were 50% and 77% more than 3-fraction and single-fraction SSRS, respectively.
Overall, the analysis “supports the institutional resource efficiency of single-fraction stereotactic radiosurgery for spinal metastases,” Dr. Boyce-Fappiano and associates said.
One key limitation of the analysis was the single-center design of the study. As a result, the findings may not be applicable to all clinical settings.
“Additional research can incorporate these data alongside toxicity and retreatment rates to evaluate the long-term cost effectiveness of spine stereotactic radiosurgery over a full cycle of care,” they concluded.
No funding sources were reported in the manuscript. The authors reported financial affiliations with AbbVie, AstraZeneca, Boston Scientific, Bristol-Myers Squibb BTG, Coleman Consulting, US Oncology, Oscar Health, RefleXion Medical, and several others.
SOURCE: Boyce-Fappiano D et al. J Oncol Pract. 2019 Nov 25. doi: 10.1200/JOP.19.00480.
A comparative cost analysis suggests single-fraction spine stereotactic radiosurgery is associated with lower total resource utilization among other radiation therapy (RT) options, according to recent research.
“We quantified institutional costs associated with RT for spinal metastases, using a time-driven activity-based costing model,” wrote David Boyce-Fappiano, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. Their report is in the Journal of Oncology Practice.
The researchers compared resource utilization across four common RT regimens: single-fraction spine stereotactic radiosurgery (to 18 Gy), 3-fraction spine stereotactic radiosurgery (to 27 Gy), 10-fraction three-dimensional RT (3D-RT) (to 30 Gy), and 10-fraction intensity-modulated RT (IMRT) (to 30 Gy).
The analysis framework involved the creation of both process maps and process times, which included a detailed outline to map the complete clinical care process, while expert panel interviews were used to establish process times.
Other measures, such as the capacity cost rate, were calculated for each resource, and subsequently used to estimate total costs.
After analysis, the researchers found that across the four RT regimens, full-cycle care costs for single-fraction spine stereotactic radiosurgery were 17% less and 17% more than IMRT and 3D-RT, respectively. However, technical costs for IMRT were 50% and 77% more than 3-fraction and single-fraction SSRS, respectively.
Overall, the analysis “supports the institutional resource efficiency of single-fraction stereotactic radiosurgery for spinal metastases,” Dr. Boyce-Fappiano and associates said.
One key limitation of the analysis was the single-center design of the study. As a result, the findings may not be applicable to all clinical settings.
“Additional research can incorporate these data alongside toxicity and retreatment rates to evaluate the long-term cost effectiveness of spine stereotactic radiosurgery over a full cycle of care,” they concluded.
No funding sources were reported in the manuscript. The authors reported financial affiliations with AbbVie, AstraZeneca, Boston Scientific, Bristol-Myers Squibb BTG, Coleman Consulting, US Oncology, Oscar Health, RefleXion Medical, and several others.
SOURCE: Boyce-Fappiano D et al. J Oncol Pract. 2019 Nov 25. doi: 10.1200/JOP.19.00480.
A comparative cost analysis suggests single-fraction spine stereotactic radiosurgery is associated with lower total resource utilization among other radiation therapy (RT) options, according to recent research.
“We quantified institutional costs associated with RT for spinal metastases, using a time-driven activity-based costing model,” wrote David Boyce-Fappiano, MD, of the University of Texas MD Anderson Cancer Center, Houston, and colleagues. Their report is in the Journal of Oncology Practice.
The researchers compared resource utilization across four common RT regimens: single-fraction spine stereotactic radiosurgery (to 18 Gy), 3-fraction spine stereotactic radiosurgery (to 27 Gy), 10-fraction three-dimensional RT (3D-RT) (to 30 Gy), and 10-fraction intensity-modulated RT (IMRT) (to 30 Gy).
The analysis framework involved the creation of both process maps and process times, which included a detailed outline to map the complete clinical care process, while expert panel interviews were used to establish process times.
Other measures, such as the capacity cost rate, were calculated for each resource, and subsequently used to estimate total costs.
After analysis, the researchers found that across the four RT regimens, full-cycle care costs for single-fraction spine stereotactic radiosurgery were 17% less and 17% more than IMRT and 3D-RT, respectively. However, technical costs for IMRT were 50% and 77% more than 3-fraction and single-fraction SSRS, respectively.
Overall, the analysis “supports the institutional resource efficiency of single-fraction stereotactic radiosurgery for spinal metastases,” Dr. Boyce-Fappiano and associates said.
One key limitation of the analysis was the single-center design of the study. As a result, the findings may not be applicable to all clinical settings.
“Additional research can incorporate these data alongside toxicity and retreatment rates to evaluate the long-term cost effectiveness of spine stereotactic radiosurgery over a full cycle of care,” they concluded.
No funding sources were reported in the manuscript. The authors reported financial affiliations with AbbVie, AstraZeneca, Boston Scientific, Bristol-Myers Squibb BTG, Coleman Consulting, US Oncology, Oscar Health, RefleXion Medical, and several others.
SOURCE: Boyce-Fappiano D et al. J Oncol Pract. 2019 Nov 25. doi: 10.1200/JOP.19.00480.
FROM THE JOURNAL OF ONCOLOGY PRACTICE
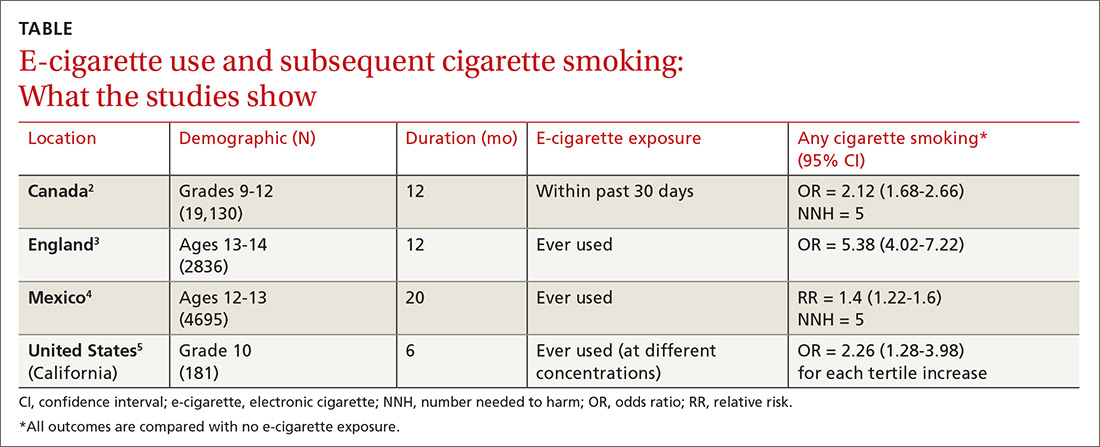
Does using e-cigarettes increase cigarette smoking in adolescents?
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5
RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5
RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5
RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
EVIDENCE-BASED ANSWER:
Probably. Electronic cigarette (e-cigarette) use by adolescents is associated with a 2- to 4-fold increase in cigarette smoking over the next year (strength of recommendation: A, meta-analysis and subsequent prospective cohort studies).
An erythematous facial rash
A 59-year-old woman presented to our clinic with a large asymptomatic facial rash that had developed several months earlier. The rash had been slowly growing but did not change day to day. Her past medical history was significant for hypertension, hyperlipidemia, and cutaneous lymphoma, which was localized to her arms. She denied the use of any new products, including hair or facial products, nail polish, or any new medications.
Initially, she was presumed (by an outside provider) to have rosacea, and she received treatment with doxycycline 100 mg/d for 2 months. However, the rash did not improve.
Physical examination revealed a large erythematous rash involving her cheeks, nose, and periocular area with no other significant findings (FIGURE).
A biopsy of her right cheek was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Mycosis fungoides
Following the biopsy of her right cheek, a histopathologic analysis demonstrated an atypical lymphocytic infiltrate positive for CD3 and CD4. These histopathologic features led to a diagnosis of recurrent mycosis fungoides (MF), a type of cutaneous lymphoma. (Our patient’s cutaneous lymphoma had been in remission for a year following local radiotherapy.)
MF is the most common type of cutaneous lymphoma, with an incidence of 6.4 to 9.6 cases per million people in the United States.1 There are also 2 rare subtypes of MF: the psoriasiform and palmoplantar forms. Psoriasiform MF presents with psoriasis-like plaques, while palmoplantar MF initially presents on the palms and soles.
Patients with classic MF typically present with patches and plaques—with the late evolution of tumors—on non–sun-exposed areas.1 Our patient’s clinical presentation was atypical because the rash manifested on a sun-exposed area of her body.
MF and other cutaneous lymphomas should always be part of the differential diagnosis for an unexplained persistent rash, especially in a patient with a history of MF. The development of lymphomas is thought to be a stepwise process through which chronic antigenic stimulation results in an accumulation of genetic mutations that then cause cells to undergo clonal expansion and, ultimately, malignant transformation. Genetic, environmental, and immunologic factors that contribute to the disease pathogenesis have been identified.2
Once clinical features point toward MF, the diagnosis can be further differentiated from other benign inflammatory mimics with a biopsy demonstrating cerebriform lymphocytes homing toward the epidermis, monoclonal expansion of T cells, and defective apoptosis.3
Continue to: Differential includes rosacea and seborrheic dermatitis
Differential includes rosacea and seborrheic dermatitis
The diagnosis of MF can be difficult as it often imitates other benign inflammatory conditions.
Rosacea manifests as an erythematous facial rash but usually spares the nasolabial folds and eyelids. There are several forms, including ocular (featuring swollen and irritated conjunctiva), erythematotelangiectatic (with visible blood vessels), and papulopustular (with acneic lesions). Over time, the skin may develop a thickened, bumpy texture, referred to as phymatous rosacea.4 A history of acute worsening with exposure to certain hot or spicy foods, alcohol, or ultraviolet light suggests a diagnosis of rosacea.
Seborrheic dermatitis classically presents as yellow scaling on a mildly erythematous base and often involves nasolabial folds and eyebrows. Seborrheic dermatitis can be associated with human immunodeficiency virus, Parkinson’s disease, and other chronic medical conditions.
Allergic contact dermatitis can look identical to MF, but in our case, there was no new allergen in the history. A thorough history regarding new medications, creams, and household supplies is integral to differentiating this diagnosis.
Misdiagnosis can lead to advanced-stage disease
This case of persistent facial erythema, originally treated as rosacea, highlights the importance of having a low threshold of suspicion of MF, especially in a patient with a prior history of MF. A recent study by Kelati et al3 indicated that certain subtypes of MF are easily misdiagnosed and treated as psoriasis or eczema respectively for an average of 10.5 years.3 These years of misdiagnosis are significantly correlated with the development of advanced-stage MF, which is more difficult to treat.3
Continue to: Treatment with topical desonide and mechlorethamine
Treatment with topical desonide and mechlorethamine
There are multiple treatment options for MF, depending on the stage, starting with topical therapies and advancing to systemic therapies in more advanced stages. Topical treatments include steroids, nitrogen mustard, and retinoids.5 Our patient was referred to a multidisciplinary lymphoma clinic, where topical treatment was initiated with desonide cream .05% and mechlorethamine gel .016%. Our patient experienced a 50% improvement in skin involvement at 3 months.
As MF progresses to more advanced stages, treatment often combines skin-directed therapies with systemic immunomodulators, biologics, radiation, and total skin electron beam therapy.6 TSEBT is a low-dose full-body radiation treatment that targets the skin surface and therefore effectively treats cutaneous lymphoma. Although TSEBT is usually well tolerated, there have been documented acute and chronic adverse effects, including dermatitis, alopecia, peripheral edema, cutaneous malignancies, and infertility in men.7
While the use of topical desonide and mechlorethamine was initially favored over radiation due to eyelid involvement, our patient developed new patches on her legs 11 months after her initial visit. When biopsies indicated MF with large cell transformation, she received 1 course of low-dose TSEBT (12 Gy), with complete response noted at the 2 month follow-up.
CORRESPONDENCE
Lucia Seminario-Vidal, MD, PhD, Department of Dermatology and Cutaneous Surgery, 13330 USF Laurel Drive, Tampa, FL 33612; [email protected]
1. Jawed S, Myskowski P, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-e16.
2. Wohl Y, Tur E. Environmental risk factors for mycosis fungoides. Curr Probl Dermatol. 2007;35:52-64.
3. Kelati A, Gallouj S, Tahiri L, et al. Defining the mimics and clinico-histological diagnosis criteria for mycosis fungoides to minimize misdiagnosis. Int J Womens Dermatol. 2017;3:100-106.
4. Two AM, Wu W, Gallo RL, et al. Rosacea. part I. Introduction, categorization, histology, pathogenesis, and risk factors. J AM Acad Dermatol. 2015;72:749-758.
5. Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
6. Jawed S, Myskowski P, Horwitz S, et al. Continuing medical education: Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-e17.
7. De Moraes FY, Carvalho Hde A, Hanna SA, et al. Literature review of clinical results of total skin electron irradiation (TSEBT) of mycosis fungoides in adults. Rep Pract Oncol Radiother. 2014;19:92-98.
A 59-year-old woman presented to our clinic with a large asymptomatic facial rash that had developed several months earlier. The rash had been slowly growing but did not change day to day. Her past medical history was significant for hypertension, hyperlipidemia, and cutaneous lymphoma, which was localized to her arms. She denied the use of any new products, including hair or facial products, nail polish, or any new medications.
Initially, she was presumed (by an outside provider) to have rosacea, and she received treatment with doxycycline 100 mg/d for 2 months. However, the rash did not improve.
Physical examination revealed a large erythematous rash involving her cheeks, nose, and periocular area with no other significant findings (FIGURE).
A biopsy of her right cheek was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Mycosis fungoides
Following the biopsy of her right cheek, a histopathologic analysis demonstrated an atypical lymphocytic infiltrate positive for CD3 and CD4. These histopathologic features led to a diagnosis of recurrent mycosis fungoides (MF), a type of cutaneous lymphoma. (Our patient’s cutaneous lymphoma had been in remission for a year following local radiotherapy.)
MF is the most common type of cutaneous lymphoma, with an incidence of 6.4 to 9.6 cases per million people in the United States.1 There are also 2 rare subtypes of MF: the psoriasiform and palmoplantar forms. Psoriasiform MF presents with psoriasis-like plaques, while palmoplantar MF initially presents on the palms and soles.
Patients with classic MF typically present with patches and plaques—with the late evolution of tumors—on non–sun-exposed areas.1 Our patient’s clinical presentation was atypical because the rash manifested on a sun-exposed area of her body.
MF and other cutaneous lymphomas should always be part of the differential diagnosis for an unexplained persistent rash, especially in a patient with a history of MF. The development of lymphomas is thought to be a stepwise process through which chronic antigenic stimulation results in an accumulation of genetic mutations that then cause cells to undergo clonal expansion and, ultimately, malignant transformation. Genetic, environmental, and immunologic factors that contribute to the disease pathogenesis have been identified.2
Once clinical features point toward MF, the diagnosis can be further differentiated from other benign inflammatory mimics with a biopsy demonstrating cerebriform lymphocytes homing toward the epidermis, monoclonal expansion of T cells, and defective apoptosis.3
Continue to: Differential includes rosacea and seborrheic dermatitis
Differential includes rosacea and seborrheic dermatitis
The diagnosis of MF can be difficult as it often imitates other benign inflammatory conditions.
Rosacea manifests as an erythematous facial rash but usually spares the nasolabial folds and eyelids. There are several forms, including ocular (featuring swollen and irritated conjunctiva), erythematotelangiectatic (with visible blood vessels), and papulopustular (with acneic lesions). Over time, the skin may develop a thickened, bumpy texture, referred to as phymatous rosacea.4 A history of acute worsening with exposure to certain hot or spicy foods, alcohol, or ultraviolet light suggests a diagnosis of rosacea.
Seborrheic dermatitis classically presents as yellow scaling on a mildly erythematous base and often involves nasolabial folds and eyebrows. Seborrheic dermatitis can be associated with human immunodeficiency virus, Parkinson’s disease, and other chronic medical conditions.
Allergic contact dermatitis can look identical to MF, but in our case, there was no new allergen in the history. A thorough history regarding new medications, creams, and household supplies is integral to differentiating this diagnosis.
Misdiagnosis can lead to advanced-stage disease
This case of persistent facial erythema, originally treated as rosacea, highlights the importance of having a low threshold of suspicion of MF, especially in a patient with a prior history of MF. A recent study by Kelati et al3 indicated that certain subtypes of MF are easily misdiagnosed and treated as psoriasis or eczema respectively for an average of 10.5 years.3 These years of misdiagnosis are significantly correlated with the development of advanced-stage MF, which is more difficult to treat.3
Continue to: Treatment with topical desonide and mechlorethamine
Treatment with topical desonide and mechlorethamine
There are multiple treatment options for MF, depending on the stage, starting with topical therapies and advancing to systemic therapies in more advanced stages. Topical treatments include steroids, nitrogen mustard, and retinoids.5 Our patient was referred to a multidisciplinary lymphoma clinic, where topical treatment was initiated with desonide cream .05% and mechlorethamine gel .016%. Our patient experienced a 50% improvement in skin involvement at 3 months.
As MF progresses to more advanced stages, treatment often combines skin-directed therapies with systemic immunomodulators, biologics, radiation, and total skin electron beam therapy.6 TSEBT is a low-dose full-body radiation treatment that targets the skin surface and therefore effectively treats cutaneous lymphoma. Although TSEBT is usually well tolerated, there have been documented acute and chronic adverse effects, including dermatitis, alopecia, peripheral edema, cutaneous malignancies, and infertility in men.7
While the use of topical desonide and mechlorethamine was initially favored over radiation due to eyelid involvement, our patient developed new patches on her legs 11 months after her initial visit. When biopsies indicated MF with large cell transformation, she received 1 course of low-dose TSEBT (12 Gy), with complete response noted at the 2 month follow-up.
CORRESPONDENCE
Lucia Seminario-Vidal, MD, PhD, Department of Dermatology and Cutaneous Surgery, 13330 USF Laurel Drive, Tampa, FL 33612; [email protected]
A 59-year-old woman presented to our clinic with a large asymptomatic facial rash that had developed several months earlier. The rash had been slowly growing but did not change day to day. Her past medical history was significant for hypertension, hyperlipidemia, and cutaneous lymphoma, which was localized to her arms. She denied the use of any new products, including hair or facial products, nail polish, or any new medications.
Initially, she was presumed (by an outside provider) to have rosacea, and she received treatment with doxycycline 100 mg/d for 2 months. However, the rash did not improve.
Physical examination revealed a large erythematous rash involving her cheeks, nose, and periocular area with no other significant findings (FIGURE).
A biopsy of her right cheek was performed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Mycosis fungoides
Following the biopsy of her right cheek, a histopathologic analysis demonstrated an atypical lymphocytic infiltrate positive for CD3 and CD4. These histopathologic features led to a diagnosis of recurrent mycosis fungoides (MF), a type of cutaneous lymphoma. (Our patient’s cutaneous lymphoma had been in remission for a year following local radiotherapy.)
MF is the most common type of cutaneous lymphoma, with an incidence of 6.4 to 9.6 cases per million people in the United States.1 There are also 2 rare subtypes of MF: the psoriasiform and palmoplantar forms. Psoriasiform MF presents with psoriasis-like plaques, while palmoplantar MF initially presents on the palms and soles.
Patients with classic MF typically present with patches and plaques—with the late evolution of tumors—on non–sun-exposed areas.1 Our patient’s clinical presentation was atypical because the rash manifested on a sun-exposed area of her body.
MF and other cutaneous lymphomas should always be part of the differential diagnosis for an unexplained persistent rash, especially in a patient with a history of MF. The development of lymphomas is thought to be a stepwise process through which chronic antigenic stimulation results in an accumulation of genetic mutations that then cause cells to undergo clonal expansion and, ultimately, malignant transformation. Genetic, environmental, and immunologic factors that contribute to the disease pathogenesis have been identified.2
Once clinical features point toward MF, the diagnosis can be further differentiated from other benign inflammatory mimics with a biopsy demonstrating cerebriform lymphocytes homing toward the epidermis, monoclonal expansion of T cells, and defective apoptosis.3
Continue to: Differential includes rosacea and seborrheic dermatitis
Differential includes rosacea and seborrheic dermatitis
The diagnosis of MF can be difficult as it often imitates other benign inflammatory conditions.
Rosacea manifests as an erythematous facial rash but usually spares the nasolabial folds and eyelids. There are several forms, including ocular (featuring swollen and irritated conjunctiva), erythematotelangiectatic (with visible blood vessels), and papulopustular (with acneic lesions). Over time, the skin may develop a thickened, bumpy texture, referred to as phymatous rosacea.4 A history of acute worsening with exposure to certain hot or spicy foods, alcohol, or ultraviolet light suggests a diagnosis of rosacea.
Seborrheic dermatitis classically presents as yellow scaling on a mildly erythematous base and often involves nasolabial folds and eyebrows. Seborrheic dermatitis can be associated with human immunodeficiency virus, Parkinson’s disease, and other chronic medical conditions.
Allergic contact dermatitis can look identical to MF, but in our case, there was no new allergen in the history. A thorough history regarding new medications, creams, and household supplies is integral to differentiating this diagnosis.
Misdiagnosis can lead to advanced-stage disease
This case of persistent facial erythema, originally treated as rosacea, highlights the importance of having a low threshold of suspicion of MF, especially in a patient with a prior history of MF. A recent study by Kelati et al3 indicated that certain subtypes of MF are easily misdiagnosed and treated as psoriasis or eczema respectively for an average of 10.5 years.3 These years of misdiagnosis are significantly correlated with the development of advanced-stage MF, which is more difficult to treat.3
Continue to: Treatment with topical desonide and mechlorethamine
Treatment with topical desonide and mechlorethamine
There are multiple treatment options for MF, depending on the stage, starting with topical therapies and advancing to systemic therapies in more advanced stages. Topical treatments include steroids, nitrogen mustard, and retinoids.5 Our patient was referred to a multidisciplinary lymphoma clinic, where topical treatment was initiated with desonide cream .05% and mechlorethamine gel .016%. Our patient experienced a 50% improvement in skin involvement at 3 months.
As MF progresses to more advanced stages, treatment often combines skin-directed therapies with systemic immunomodulators, biologics, radiation, and total skin electron beam therapy.6 TSEBT is a low-dose full-body radiation treatment that targets the skin surface and therefore effectively treats cutaneous lymphoma. Although TSEBT is usually well tolerated, there have been documented acute and chronic adverse effects, including dermatitis, alopecia, peripheral edema, cutaneous malignancies, and infertility in men.7
While the use of topical desonide and mechlorethamine was initially favored over radiation due to eyelid involvement, our patient developed new patches on her legs 11 months after her initial visit. When biopsies indicated MF with large cell transformation, she received 1 course of low-dose TSEBT (12 Gy), with complete response noted at the 2 month follow-up.
CORRESPONDENCE
Lucia Seminario-Vidal, MD, PhD, Department of Dermatology and Cutaneous Surgery, 13330 USF Laurel Drive, Tampa, FL 33612; [email protected]
1. Jawed S, Myskowski P, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-e16.
2. Wohl Y, Tur E. Environmental risk factors for mycosis fungoides. Curr Probl Dermatol. 2007;35:52-64.
3. Kelati A, Gallouj S, Tahiri L, et al. Defining the mimics and clinico-histological diagnosis criteria for mycosis fungoides to minimize misdiagnosis. Int J Womens Dermatol. 2017;3:100-106.
4. Two AM, Wu W, Gallo RL, et al. Rosacea. part I. Introduction, categorization, histology, pathogenesis, and risk factors. J AM Acad Dermatol. 2015;72:749-758.
5. Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
6. Jawed S, Myskowski P, Horwitz S, et al. Continuing medical education: Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-e17.
7. De Moraes FY, Carvalho Hde A, Hanna SA, et al. Literature review of clinical results of total skin electron irradiation (TSEBT) of mycosis fungoides in adults. Rep Pract Oncol Radiother. 2014;19:92-98.
1. Jawed S, Myskowski P, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Part I. Diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-e16.
2. Wohl Y, Tur E. Environmental risk factors for mycosis fungoides. Curr Probl Dermatol. 2007;35:52-64.
3. Kelati A, Gallouj S, Tahiri L, et al. Defining the mimics and clinico-histological diagnosis criteria for mycosis fungoides to minimize misdiagnosis. Int J Womens Dermatol. 2017;3:100-106.
4. Two AM, Wu W, Gallo RL, et al. Rosacea. part I. Introduction, categorization, histology, pathogenesis, and risk factors. J AM Acad Dermatol. 2015;72:749-758.
5. Lessin SR, Duvic M, Guitart J, et al. Topical chemotherapy in cutaneous T-cell lymphoma positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149:25-32.
6. Jawed S, Myskowski P, Horwitz S, et al. Continuing medical education: Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-e17.
7. De Moraes FY, Carvalho Hde A, Hanna SA, et al. Literature review of clinical results of total skin electron irradiation (TSEBT) of mycosis fungoides in adults. Rep Pract Oncol Radiother. 2014;19:92-98.
What to do when the evidence is not conclusive
Family physicians try to base treatment decisions on the very best available evidence from randomized trials and other high-quality studies. Very often, however, the evidence is not conclusive. Family physicians are confronted with questions about a wide variety of treatments that may or may not be effective. The classic example for me is the use of chondroitin sulfate/glucosamine for knee osteoarthritis. The preponderance of evidence tells us it is not effective, but one long-term clinical trial did find some benefit.1 And some patients swear by it!
In this issue of JFP, we have 2 articles that fall into this category: 1 by Hahn about the treatment of asthma with macrolides and the other by Sorsby et al about use of positive airway pressure (PAP) for obstructive sleep apnea (OSA).
The article by Hahn is an extensive literature review regarding the effectiveness of macrolides for asthma. Despite 2 meta-analyses and many clinical trials, the results are not conclusive; but they are highly suggestive that macrolides may benefit patients with new-onset asthma and severe asthma that does not respond completely to mainstream treatments. Why don't we have conclusive evidence? Because the right studies have not been done. Most studies of macrolides for asthma have not focused on these 2 groups, so any treatment effect may have been diluted by including patients not likely to respond.
The issue with PAP, also known as CPAP (or continuous positive airway pressure), for the treatment of OSA is different. In this case, the question is: What conditions and outcomes are improved by use of PAP? Studies strongly support that PAP is effective in reducing daytime sleepiness and motor vehicle accidents associated with OSA. Most of us had high hopes that PAP also would reduce the adverse cardiovascular outcomes associated with OSA. But the results of large randomized trials have not found a protective effective.
Enthusiasts argue that the studies have not been of sufficient duration and that the participants did not use their PAP devices long enough each night. Some follow-up studies have suggested a protective effective when the device is used for many years, but those studies have the major flaw of volunteer bias, meaning those who adhere to any treatment have better health outcomes than those who do not adhere.
What should you do when there is uncertainty regarding effectiveness? Use shared decision making: What does the patient want to do after you have explained the possible benefits and harms?
1. Reginster JY, Deroisy R, Rovati LC, et. al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256.
Family physicians try to base treatment decisions on the very best available evidence from randomized trials and other high-quality studies. Very often, however, the evidence is not conclusive. Family physicians are confronted with questions about a wide variety of treatments that may or may not be effective. The classic example for me is the use of chondroitin sulfate/glucosamine for knee osteoarthritis. The preponderance of evidence tells us it is not effective, but one long-term clinical trial did find some benefit.1 And some patients swear by it!
In this issue of JFP, we have 2 articles that fall into this category: 1 by Hahn about the treatment of asthma with macrolides and the other by Sorsby et al about use of positive airway pressure (PAP) for obstructive sleep apnea (OSA).
The article by Hahn is an extensive literature review regarding the effectiveness of macrolides for asthma. Despite 2 meta-analyses and many clinical trials, the results are not conclusive; but they are highly suggestive that macrolides may benefit patients with new-onset asthma and severe asthma that does not respond completely to mainstream treatments. Why don't we have conclusive evidence? Because the right studies have not been done. Most studies of macrolides for asthma have not focused on these 2 groups, so any treatment effect may have been diluted by including patients not likely to respond.
The issue with PAP, also known as CPAP (or continuous positive airway pressure), for the treatment of OSA is different. In this case, the question is: What conditions and outcomes are improved by use of PAP? Studies strongly support that PAP is effective in reducing daytime sleepiness and motor vehicle accidents associated with OSA. Most of us had high hopes that PAP also would reduce the adverse cardiovascular outcomes associated with OSA. But the results of large randomized trials have not found a protective effective.
Enthusiasts argue that the studies have not been of sufficient duration and that the participants did not use their PAP devices long enough each night. Some follow-up studies have suggested a protective effective when the device is used for many years, but those studies have the major flaw of volunteer bias, meaning those who adhere to any treatment have better health outcomes than those who do not adhere.
What should you do when there is uncertainty regarding effectiveness? Use shared decision making: What does the patient want to do after you have explained the possible benefits and harms?
Family physicians try to base treatment decisions on the very best available evidence from randomized trials and other high-quality studies. Very often, however, the evidence is not conclusive. Family physicians are confronted with questions about a wide variety of treatments that may or may not be effective. The classic example for me is the use of chondroitin sulfate/glucosamine for knee osteoarthritis. The preponderance of evidence tells us it is not effective, but one long-term clinical trial did find some benefit.1 And some patients swear by it!
In this issue of JFP, we have 2 articles that fall into this category: 1 by Hahn about the treatment of asthma with macrolides and the other by Sorsby et al about use of positive airway pressure (PAP) for obstructive sleep apnea (OSA).
The article by Hahn is an extensive literature review regarding the effectiveness of macrolides for asthma. Despite 2 meta-analyses and many clinical trials, the results are not conclusive; but they are highly suggestive that macrolides may benefit patients with new-onset asthma and severe asthma that does not respond completely to mainstream treatments. Why don't we have conclusive evidence? Because the right studies have not been done. Most studies of macrolides for asthma have not focused on these 2 groups, so any treatment effect may have been diluted by including patients not likely to respond.
The issue with PAP, also known as CPAP (or continuous positive airway pressure), for the treatment of OSA is different. In this case, the question is: What conditions and outcomes are improved by use of PAP? Studies strongly support that PAP is effective in reducing daytime sleepiness and motor vehicle accidents associated with OSA. Most of us had high hopes that PAP also would reduce the adverse cardiovascular outcomes associated with OSA. But the results of large randomized trials have not found a protective effective.
Enthusiasts argue that the studies have not been of sufficient duration and that the participants did not use their PAP devices long enough each night. Some follow-up studies have suggested a protective effective when the device is used for many years, but those studies have the major flaw of volunteer bias, meaning those who adhere to any treatment have better health outcomes than those who do not adhere.
What should you do when there is uncertainty regarding effectiveness? Use shared decision making: What does the patient want to do after you have explained the possible benefits and harms?
1. Reginster JY, Deroisy R, Rovati LC, et. al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256.
1. Reginster JY, Deroisy R, Rovati LC, et. al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256.
Infant with bilious emesis
A 4-week-old term boy presented to the emergency department (ED) with recurrent bilious emesis. He had a history of neonatal abstinence syndrome, related to his mother’s use of Subutex (a form of suboxone that is considered safer during pregnancy) for her opioid addiction, and a Ladd procedure at Day 7 of life for intestinal malrotation with volvulus. He had been discharged from the hospital 4 days earlier, after recovery from surgery.
He had been doing well until the prior evening, when he developed “yellow-green” emesis and appeared to have intermittent abdominal pain. His parents said that he was refusing to take formula and he’d had frequent bilious emesis. They also noted he’d had 1 wet diaper in the past 12 hours and appeared “sleepier” than usual.
In the ED, the patient was listless, with thin and tremulous extremities. His fontanelle was flat, and his pupils were equal, round, and reactive. His mucous membranes were dry, skin was mottled, and capillary refill was delayed. His cardiopulmonary exam was normal. His abdomen was soft, mildly distended, and diffusely tender to palpation, with well-healing laparotomy scars. His reflexes were normal, with slightly increased tone. No bruising was noted.
An acute abdominal series, including an AP view chest x-ray (FIGURE 1), was obtained to rule out recurrent volvulus, free air, or small bowel obstruction.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Nonaccidental trauma
The chest x-ray (FIGURE 1) showed multiple bilateral posterior rib fractures concerning for nonaccidental trauma (NAT). The remaining acute abdominal series films (not shown) revealed the reason for his bilious emesis: a partial bowel obstruction related to his surgical procedure. Review of x-rays obtained for peripherally inserted central catheter line confirmation during his previous admission (FIGURE 2) revealed that the rib fractures had been present at that time but had been overlooked.
This case illustrates the importance of considering NAT in the differential diagnosis of any sick infant. There are an estimated 700,000 cases of child abuse and neglect and 600 fatalities per year in the United States.1,2 The differential diagnosis for fracture or bruising in infants includes accidental trauma, bony abnormalities (eg, osteogenesis imperfecta), bleeding disorders, and trauma from medical procedures such as CPR or surgery.1
Ask these questions, look beyond that single bruise
When evaluating for NAT, the history and physical exam are crucial. It is essential to ask if there were any witnesses, establish who was caring for the child, and investigate any delays in seeking medical evaluation.1 During the exam, undress the child and examine every inch of skin, looking for bruising or abrasions, especially on the face, ear, neck, and oral cavity.
Any bruising in a nonambulatory infant should raise suspicion for NAT. One study showed that more than half of infants with a single bruise had additional injuries identified upon further work-up.3 Fundoscopic exam with photographs should be completed to evaluate for retinal hemorrhage.
Additional work-up should include a skeletal survey for all children younger than 24 months2 in addition to computed tomography (CT) or magnetic resonance imaging of the head, complete blood count, and a coagulation panel. If there is concern for abdominal trauma, a complete metabolic panel and lipase test may be useful.4 If liver function tests show elevated liver enzymes (> 80 IU/L), abdominal CT with contrast is indicated.4
Continue to: Research has underscored...
Research has underscored the importance of screening siblings and other contacts of abused children. In particular, the twin of an abused child has a much higher risk for abuse.5 A skeletal survey should be obtained in contacts (< 24 months) of abused children—regardless of their physical exam findings.5
Management depends on injury type
The management of children with NAT depends on the injuries. Once these injuries are addressed, the next step is to determine the safest place for the infant/child to be discharged. The involvement of local social workers and Child Protective Services (CPS) is pivotal for this determination.2
Our patient. To treat the partial small bowel obstruction noted on an abdominal CT, the patient received intravenous fluids and nasogastric tube decompression. However, due to ongoing distension and high nasogastric tube output, the patient was taken to the operating room for an exploratory laparotomy. An adhesive band in the right lower quadrant was found to be causing the obstruction and was lysed.
We consulted CPS and social workers about the rib fractures identified on x-ray. We considered osteogenesis imperfecta as a possible cause, but genetic testing was negative. The ophthalmology exam was negative for retinal hemorrhages. A bone scan confirmed posterior rib fractures with no other injuries. CPS was unable to confirm that the fractures had not been sustained while the child was an inpatient, so it was ultimately determined that the patient should be discharged home with his parents with supervision.
CORRESPONDENCE
Anne Huyler, MD, Maine Medical Center, 22 Bramhall Street, Portland, ME 04102; [email protected]
1. Berkowitz CD. Physical abuse of children. N Engl J Med. 2017;376:1659-1666.
2. Lindberg DM, Berger RP, Reynolds MS, et al. Yield of skeletal survey by age in children referred to abuse specialists. J Pediatr. 2014;164:1268-1273.e1.
3. Harper NS, Feldman KW, Sugar NF, et al. Additional injuries in young infants with concern for abuse and apparently isolated bruises. J Pediatr. 2014;165:383-388.e1.
4. Lindberg DM, Shapiro RA, Blood EA, et al. Utility of hepatic transaminases in children with concern for abuse. Pediatrics. 2013;131:268-275.
5. Lindberg DM, Shapiro RA, Laskey AL, et al. Prevalence of abusive injuries in siblings and household contacts of physically abused children. Pediatrics. 2012;130:193-201.
A 4-week-old term boy presented to the emergency department (ED) with recurrent bilious emesis. He had a history of neonatal abstinence syndrome, related to his mother’s use of Subutex (a form of suboxone that is considered safer during pregnancy) for her opioid addiction, and a Ladd procedure at Day 7 of life for intestinal malrotation with volvulus. He had been discharged from the hospital 4 days earlier, after recovery from surgery.
He had been doing well until the prior evening, when he developed “yellow-green” emesis and appeared to have intermittent abdominal pain. His parents said that he was refusing to take formula and he’d had frequent bilious emesis. They also noted he’d had 1 wet diaper in the past 12 hours and appeared “sleepier” than usual.
In the ED, the patient was listless, with thin and tremulous extremities. His fontanelle was flat, and his pupils were equal, round, and reactive. His mucous membranes were dry, skin was mottled, and capillary refill was delayed. His cardiopulmonary exam was normal. His abdomen was soft, mildly distended, and diffusely tender to palpation, with well-healing laparotomy scars. His reflexes were normal, with slightly increased tone. No bruising was noted.
An acute abdominal series, including an AP view chest x-ray (FIGURE 1), was obtained to rule out recurrent volvulus, free air, or small bowel obstruction.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Nonaccidental trauma
The chest x-ray (FIGURE 1) showed multiple bilateral posterior rib fractures concerning for nonaccidental trauma (NAT). The remaining acute abdominal series films (not shown) revealed the reason for his bilious emesis: a partial bowel obstruction related to his surgical procedure. Review of x-rays obtained for peripherally inserted central catheter line confirmation during his previous admission (FIGURE 2) revealed that the rib fractures had been present at that time but had been overlooked.
This case illustrates the importance of considering NAT in the differential diagnosis of any sick infant. There are an estimated 700,000 cases of child abuse and neglect and 600 fatalities per year in the United States.1,2 The differential diagnosis for fracture or bruising in infants includes accidental trauma, bony abnormalities (eg, osteogenesis imperfecta), bleeding disorders, and trauma from medical procedures such as CPR or surgery.1
Ask these questions, look beyond that single bruise
When evaluating for NAT, the history and physical exam are crucial. It is essential to ask if there were any witnesses, establish who was caring for the child, and investigate any delays in seeking medical evaluation.1 During the exam, undress the child and examine every inch of skin, looking for bruising or abrasions, especially on the face, ear, neck, and oral cavity.
Any bruising in a nonambulatory infant should raise suspicion for NAT. One study showed that more than half of infants with a single bruise had additional injuries identified upon further work-up.3 Fundoscopic exam with photographs should be completed to evaluate for retinal hemorrhage.
Additional work-up should include a skeletal survey for all children younger than 24 months2 in addition to computed tomography (CT) or magnetic resonance imaging of the head, complete blood count, and a coagulation panel. If there is concern for abdominal trauma, a complete metabolic panel and lipase test may be useful.4 If liver function tests show elevated liver enzymes (> 80 IU/L), abdominal CT with contrast is indicated.4
Continue to: Research has underscored...
Research has underscored the importance of screening siblings and other contacts of abused children. In particular, the twin of an abused child has a much higher risk for abuse.5 A skeletal survey should be obtained in contacts (< 24 months) of abused children—regardless of their physical exam findings.5
Management depends on injury type
The management of children with NAT depends on the injuries. Once these injuries are addressed, the next step is to determine the safest place for the infant/child to be discharged. The involvement of local social workers and Child Protective Services (CPS) is pivotal for this determination.2
Our patient. To treat the partial small bowel obstruction noted on an abdominal CT, the patient received intravenous fluids and nasogastric tube decompression. However, due to ongoing distension and high nasogastric tube output, the patient was taken to the operating room for an exploratory laparotomy. An adhesive band in the right lower quadrant was found to be causing the obstruction and was lysed.
We consulted CPS and social workers about the rib fractures identified on x-ray. We considered osteogenesis imperfecta as a possible cause, but genetic testing was negative. The ophthalmology exam was negative for retinal hemorrhages. A bone scan confirmed posterior rib fractures with no other injuries. CPS was unable to confirm that the fractures had not been sustained while the child was an inpatient, so it was ultimately determined that the patient should be discharged home with his parents with supervision.
CORRESPONDENCE
Anne Huyler, MD, Maine Medical Center, 22 Bramhall Street, Portland, ME 04102; [email protected]
A 4-week-old term boy presented to the emergency department (ED) with recurrent bilious emesis. He had a history of neonatal abstinence syndrome, related to his mother’s use of Subutex (a form of suboxone that is considered safer during pregnancy) for her opioid addiction, and a Ladd procedure at Day 7 of life for intestinal malrotation with volvulus. He had been discharged from the hospital 4 days earlier, after recovery from surgery.
He had been doing well until the prior evening, when he developed “yellow-green” emesis and appeared to have intermittent abdominal pain. His parents said that he was refusing to take formula and he’d had frequent bilious emesis. They also noted he’d had 1 wet diaper in the past 12 hours and appeared “sleepier” than usual.
In the ED, the patient was listless, with thin and tremulous extremities. His fontanelle was flat, and his pupils were equal, round, and reactive. His mucous membranes were dry, skin was mottled, and capillary refill was delayed. His cardiopulmonary exam was normal. His abdomen was soft, mildly distended, and diffusely tender to palpation, with well-healing laparotomy scars. His reflexes were normal, with slightly increased tone. No bruising was noted.
An acute abdominal series, including an AP view chest x-ray (FIGURE 1), was obtained to rule out recurrent volvulus, free air, or small bowel obstruction.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Nonaccidental trauma
The chest x-ray (FIGURE 1) showed multiple bilateral posterior rib fractures concerning for nonaccidental trauma (NAT). The remaining acute abdominal series films (not shown) revealed the reason for his bilious emesis: a partial bowel obstruction related to his surgical procedure. Review of x-rays obtained for peripherally inserted central catheter line confirmation during his previous admission (FIGURE 2) revealed that the rib fractures had been present at that time but had been overlooked.
This case illustrates the importance of considering NAT in the differential diagnosis of any sick infant. There are an estimated 700,000 cases of child abuse and neglect and 600 fatalities per year in the United States.1,2 The differential diagnosis for fracture or bruising in infants includes accidental trauma, bony abnormalities (eg, osteogenesis imperfecta), bleeding disorders, and trauma from medical procedures such as CPR or surgery.1
Ask these questions, look beyond that single bruise
When evaluating for NAT, the history and physical exam are crucial. It is essential to ask if there were any witnesses, establish who was caring for the child, and investigate any delays in seeking medical evaluation.1 During the exam, undress the child and examine every inch of skin, looking for bruising or abrasions, especially on the face, ear, neck, and oral cavity.
Any bruising in a nonambulatory infant should raise suspicion for NAT. One study showed that more than half of infants with a single bruise had additional injuries identified upon further work-up.3 Fundoscopic exam with photographs should be completed to evaluate for retinal hemorrhage.
Additional work-up should include a skeletal survey for all children younger than 24 months2 in addition to computed tomography (CT) or magnetic resonance imaging of the head, complete blood count, and a coagulation panel. If there is concern for abdominal trauma, a complete metabolic panel and lipase test may be useful.4 If liver function tests show elevated liver enzymes (> 80 IU/L), abdominal CT with contrast is indicated.4
Continue to: Research has underscored...
Research has underscored the importance of screening siblings and other contacts of abused children. In particular, the twin of an abused child has a much higher risk for abuse.5 A skeletal survey should be obtained in contacts (< 24 months) of abused children—regardless of their physical exam findings.5
Management depends on injury type
The management of children with NAT depends on the injuries. Once these injuries are addressed, the next step is to determine the safest place for the infant/child to be discharged. The involvement of local social workers and Child Protective Services (CPS) is pivotal for this determination.2
Our patient. To treat the partial small bowel obstruction noted on an abdominal CT, the patient received intravenous fluids and nasogastric tube decompression. However, due to ongoing distension and high nasogastric tube output, the patient was taken to the operating room for an exploratory laparotomy. An adhesive band in the right lower quadrant was found to be causing the obstruction and was lysed.
We consulted CPS and social workers about the rib fractures identified on x-ray. We considered osteogenesis imperfecta as a possible cause, but genetic testing was negative. The ophthalmology exam was negative for retinal hemorrhages. A bone scan confirmed posterior rib fractures with no other injuries. CPS was unable to confirm that the fractures had not been sustained while the child was an inpatient, so it was ultimately determined that the patient should be discharged home with his parents with supervision.
CORRESPONDENCE
Anne Huyler, MD, Maine Medical Center, 22 Bramhall Street, Portland, ME 04102; [email protected]
1. Berkowitz CD. Physical abuse of children. N Engl J Med. 2017;376:1659-1666.
2. Lindberg DM, Berger RP, Reynolds MS, et al. Yield of skeletal survey by age in children referred to abuse specialists. J Pediatr. 2014;164:1268-1273.e1.
3. Harper NS, Feldman KW, Sugar NF, et al. Additional injuries in young infants with concern for abuse and apparently isolated bruises. J Pediatr. 2014;165:383-388.e1.
4. Lindberg DM, Shapiro RA, Blood EA, et al. Utility of hepatic transaminases in children with concern for abuse. Pediatrics. 2013;131:268-275.
5. Lindberg DM, Shapiro RA, Laskey AL, et al. Prevalence of abusive injuries in siblings and household contacts of physically abused children. Pediatrics. 2012;130:193-201.
1. Berkowitz CD. Physical abuse of children. N Engl J Med. 2017;376:1659-1666.
2. Lindberg DM, Berger RP, Reynolds MS, et al. Yield of skeletal survey by age in children referred to abuse specialists. J Pediatr. 2014;164:1268-1273.e1.
3. Harper NS, Feldman KW, Sugar NF, et al. Additional injuries in young infants with concern for abuse and apparently isolated bruises. J Pediatr. 2014;165:383-388.e1.
4. Lindberg DM, Shapiro RA, Blood EA, et al. Utility of hepatic transaminases in children with concern for abuse. Pediatrics. 2013;131:268-275.
5. Lindberg DM, Shapiro RA, Laskey AL, et al. Prevalence of abusive injuries in siblings and household contacts of physically abused children. Pediatrics. 2012;130:193-201.
Was this patient's transdermal Tx making her dog sick?
THE CASE
A 56-year-old postmenopausal woman with a history of anxiety, depression, alcohol abuse, fatigue, insomnia, and mental fogginess presented to the family medicine clinic with concerns about her companion animal because of symptoms possibly associated with the patient’s medication. Of note, the patient’s physical exam was unremarkable.
The patient noticed that her 5-year-old, 4.5-lb spayed female Chihuahua dog was exhibiting peculiar behaviors, including excessive licking of the abdomen, nipples, and vulvar areas and straining with urination. The dog’s symptoms had started 1 week after the patient began using estradiol transdermal spray (Evamist) for her menopause symptoms. The patient’s menopause symptoms included hot flushes, insomnia, and mental fogginess.
The patient had been applying the estradiol transdermal spray on her inner forearm twice daily, in the morning and at bedtime. She would let the applied medication dry for approximately 2 hours before allowing her arm to come in contact with other items. She worried that some of the hormone may have wiped off onto her couch, pillows, blankets, and other surfaces. In addition, she often cradled the dog in her arms, which allowed the canine’s back to come in contact with her inner forearms. To her knowledge, the dog did not lick or ingest the medication.
The patient had taken the dog to her veterinarian. On physical exam, the veterinarian noted that the dog had nipple and vulvar enlargement but no vaginal discharge, vaginal bleeding, skin changes, or urine abnormalities.
THE (PET’S) DIAGNOSIS, THE PATIENT’S Rx
The veterinarian diagnosed the Chihuahua with vaginal hyperplasia and vulvar enlargement secondary to hyperestrogenism. The animal’s symptoms were likely caused by exposure to the owner’s hormone replacement therapy (HRT) medication—the estradiol spray. The veterinarian advised the woman to return to her family physician to discuss her use of the topical estrogen.
The patient asked her physician (SS) to change her HRT formulation. She was given a prescription for an estradiol 0.05 mg/24-hour transdermal patch to be placed on her abdomen twice weekly. After 2 weeks of using the patch therapy, the patient’s menopausal symptoms were reported to be well controlled. In addition, the companion animal’s breast and vulvar changes resolved, as did the dog’s licking behavior.
DISCUSSION
Estrogen therapy, with or without progesterone, is the most effective treatment for postmenopausal vasomotor symptoms.1 Given the concerns raised in the Women’s Health Initiative (WHI) and other clinical trials regarding hormone therapy and cardiovascular and breast cancer findings, many clinicians look to alternative, nonoral dosage forms to improve the safety profile.
Continue to: Safety of nonroal estrogen therapy
Safety of nonoral estrogen therapy. Administration of nonoral estrogen is associated with avoidance of hepatic first-pass metabolism and a resulting lower impact on hepatic proteins. Thus, data indicate a potentially lower risk for venous thromboembolic events with transdermal estrogen compared to oral estrogen.1 Since the publication of the results of the WHI trials, prescribing patterns in the United States indicate a general decline in the proportion of oral hormones, while transdermal prescription volume has remained steady, and the use of vaginal formulations has increased.2
Topical estrogen formulations. Transdermal or topical delivery of estrogen can be achieved through various formulations, including patches, gels, and a spray. While patches are simple to use, some women display hypersensitivity to the adhesive. Use of gel and spray formulations avoids exposure to adhesives, but these pose a risk of transfer of hormonal ingredients that are not covered by a patch. This risk is amplified by the relative accessibility of the product-specific application sites, which include the arms or thighs. Each manufacturer recommends careful handwashing after handling the product, a specific drying time before the user covers the site with clothing, and avoidance of contact with the application site for a prescribed period of time, usually at least 1 to 2 hours.3-6
Our patient. This case illustrates the importance of discussing the risk of medication transfer to both humans and animals when prescribing individualized hormone therapy. While the Evamist prescribing information specifically addresses the risk of unintentional medication transfer to children, it does not discuss other contact risks.6 In the literature, there have been a limited number of reports on the adverse effects from transdermal or topical human medication transfer to pets. Notably, the American Pet Products Association estimates that in the United States, approximately 90 million dogs and 94 million cats are owned as a pet in 67% of households.7
THE TAKEAWAY
Use of HRT, including transdermal or topical estrogen formulations, is common. Given the large number of companion animals in the United States, physicians should consider that all members of a patient’s household—including pets—may be subject to unintentional secondary exposure to topical estrogen formulations and that they may experience adverse effects. This presents an opportunity for patient education, which can have a larger impact on all occupants of the home.
CORRESPONDENCE
Shannon Scott, DO, FACOFP, Clinical Associate Professor, Arizona College of Osteopathic Medicine, 19389 North 59th Avenue, Glendale, AZ 85308; [email protected].
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Steinkellner AR, Denison SE, Eldridge SL, et al. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women’s Health Initiative. Menopause. 2012;19:616-621.
3. Divigel [package insert]. Bridgewater, NJ: Vertical Pharmaceuticals, LLC; 2014.
4. Elestrin [package insert]. Somerset, NJ: Meda Pharmaceuticals; 2014.
5. Estrogel [package insert]. Herndon, VA: Ascend Therapeutics; 2018.
6. Evamist [package insert]. Minneapolis, MN: Perrigo; 2017.
7. American Pet Products Association. Pet Industry Market Size & Ownership Statistics. www.americanpetproducts.org/press_industrytrends.asp. Accessed November 1, 2019.
THE CASE
A 56-year-old postmenopausal woman with a history of anxiety, depression, alcohol abuse, fatigue, insomnia, and mental fogginess presented to the family medicine clinic with concerns about her companion animal because of symptoms possibly associated with the patient’s medication. Of note, the patient’s physical exam was unremarkable.
The patient noticed that her 5-year-old, 4.5-lb spayed female Chihuahua dog was exhibiting peculiar behaviors, including excessive licking of the abdomen, nipples, and vulvar areas and straining with urination. The dog’s symptoms had started 1 week after the patient began using estradiol transdermal spray (Evamist) for her menopause symptoms. The patient’s menopause symptoms included hot flushes, insomnia, and mental fogginess.
The patient had been applying the estradiol transdermal spray on her inner forearm twice daily, in the morning and at bedtime. She would let the applied medication dry for approximately 2 hours before allowing her arm to come in contact with other items. She worried that some of the hormone may have wiped off onto her couch, pillows, blankets, and other surfaces. In addition, she often cradled the dog in her arms, which allowed the canine’s back to come in contact with her inner forearms. To her knowledge, the dog did not lick or ingest the medication.
The patient had taken the dog to her veterinarian. On physical exam, the veterinarian noted that the dog had nipple and vulvar enlargement but no vaginal discharge, vaginal bleeding, skin changes, or urine abnormalities.
THE (PET’S) DIAGNOSIS, THE PATIENT’S Rx
The veterinarian diagnosed the Chihuahua with vaginal hyperplasia and vulvar enlargement secondary to hyperestrogenism. The animal’s symptoms were likely caused by exposure to the owner’s hormone replacement therapy (HRT) medication—the estradiol spray. The veterinarian advised the woman to return to her family physician to discuss her use of the topical estrogen.
The patient asked her physician (SS) to change her HRT formulation. She was given a prescription for an estradiol 0.05 mg/24-hour transdermal patch to be placed on her abdomen twice weekly. After 2 weeks of using the patch therapy, the patient’s menopausal symptoms were reported to be well controlled. In addition, the companion animal’s breast and vulvar changes resolved, as did the dog’s licking behavior.
DISCUSSION
Estrogen therapy, with or without progesterone, is the most effective treatment for postmenopausal vasomotor symptoms.1 Given the concerns raised in the Women’s Health Initiative (WHI) and other clinical trials regarding hormone therapy and cardiovascular and breast cancer findings, many clinicians look to alternative, nonoral dosage forms to improve the safety profile.
Continue to: Safety of nonroal estrogen therapy
Safety of nonoral estrogen therapy. Administration of nonoral estrogen is associated with avoidance of hepatic first-pass metabolism and a resulting lower impact on hepatic proteins. Thus, data indicate a potentially lower risk for venous thromboembolic events with transdermal estrogen compared to oral estrogen.1 Since the publication of the results of the WHI trials, prescribing patterns in the United States indicate a general decline in the proportion of oral hormones, while transdermal prescription volume has remained steady, and the use of vaginal formulations has increased.2
Topical estrogen formulations. Transdermal or topical delivery of estrogen can be achieved through various formulations, including patches, gels, and a spray. While patches are simple to use, some women display hypersensitivity to the adhesive. Use of gel and spray formulations avoids exposure to adhesives, but these pose a risk of transfer of hormonal ingredients that are not covered by a patch. This risk is amplified by the relative accessibility of the product-specific application sites, which include the arms or thighs. Each manufacturer recommends careful handwashing after handling the product, a specific drying time before the user covers the site with clothing, and avoidance of contact with the application site for a prescribed period of time, usually at least 1 to 2 hours.3-6
Our patient. This case illustrates the importance of discussing the risk of medication transfer to both humans and animals when prescribing individualized hormone therapy. While the Evamist prescribing information specifically addresses the risk of unintentional medication transfer to children, it does not discuss other contact risks.6 In the literature, there have been a limited number of reports on the adverse effects from transdermal or topical human medication transfer to pets. Notably, the American Pet Products Association estimates that in the United States, approximately 90 million dogs and 94 million cats are owned as a pet in 67% of households.7
THE TAKEAWAY
Use of HRT, including transdermal or topical estrogen formulations, is common. Given the large number of companion animals in the United States, physicians should consider that all members of a patient’s household—including pets—may be subject to unintentional secondary exposure to topical estrogen formulations and that they may experience adverse effects. This presents an opportunity for patient education, which can have a larger impact on all occupants of the home.
CORRESPONDENCE
Shannon Scott, DO, FACOFP, Clinical Associate Professor, Arizona College of Osteopathic Medicine, 19389 North 59th Avenue, Glendale, AZ 85308; [email protected].
THE CASE
A 56-year-old postmenopausal woman with a history of anxiety, depression, alcohol abuse, fatigue, insomnia, and mental fogginess presented to the family medicine clinic with concerns about her companion animal because of symptoms possibly associated with the patient’s medication. Of note, the patient’s physical exam was unremarkable.
The patient noticed that her 5-year-old, 4.5-lb spayed female Chihuahua dog was exhibiting peculiar behaviors, including excessive licking of the abdomen, nipples, and vulvar areas and straining with urination. The dog’s symptoms had started 1 week after the patient began using estradiol transdermal spray (Evamist) for her menopause symptoms. The patient’s menopause symptoms included hot flushes, insomnia, and mental fogginess.
The patient had been applying the estradiol transdermal spray on her inner forearm twice daily, in the morning and at bedtime. She would let the applied medication dry for approximately 2 hours before allowing her arm to come in contact with other items. She worried that some of the hormone may have wiped off onto her couch, pillows, blankets, and other surfaces. In addition, she often cradled the dog in her arms, which allowed the canine’s back to come in contact with her inner forearms. To her knowledge, the dog did not lick or ingest the medication.
The patient had taken the dog to her veterinarian. On physical exam, the veterinarian noted that the dog had nipple and vulvar enlargement but no vaginal discharge, vaginal bleeding, skin changes, or urine abnormalities.
THE (PET’S) DIAGNOSIS, THE PATIENT’S Rx
The veterinarian diagnosed the Chihuahua with vaginal hyperplasia and vulvar enlargement secondary to hyperestrogenism. The animal’s symptoms were likely caused by exposure to the owner’s hormone replacement therapy (HRT) medication—the estradiol spray. The veterinarian advised the woman to return to her family physician to discuss her use of the topical estrogen.
The patient asked her physician (SS) to change her HRT formulation. She was given a prescription for an estradiol 0.05 mg/24-hour transdermal patch to be placed on her abdomen twice weekly. After 2 weeks of using the patch therapy, the patient’s menopausal symptoms were reported to be well controlled. In addition, the companion animal’s breast and vulvar changes resolved, as did the dog’s licking behavior.
DISCUSSION
Estrogen therapy, with or without progesterone, is the most effective treatment for postmenopausal vasomotor symptoms.1 Given the concerns raised in the Women’s Health Initiative (WHI) and other clinical trials regarding hormone therapy and cardiovascular and breast cancer findings, many clinicians look to alternative, nonoral dosage forms to improve the safety profile.
Continue to: Safety of nonroal estrogen therapy
Safety of nonoral estrogen therapy. Administration of nonoral estrogen is associated with avoidance of hepatic first-pass metabolism and a resulting lower impact on hepatic proteins. Thus, data indicate a potentially lower risk for venous thromboembolic events with transdermal estrogen compared to oral estrogen.1 Since the publication of the results of the WHI trials, prescribing patterns in the United States indicate a general decline in the proportion of oral hormones, while transdermal prescription volume has remained steady, and the use of vaginal formulations has increased.2
Topical estrogen formulations. Transdermal or topical delivery of estrogen can be achieved through various formulations, including patches, gels, and a spray. While patches are simple to use, some women display hypersensitivity to the adhesive. Use of gel and spray formulations avoids exposure to adhesives, but these pose a risk of transfer of hormonal ingredients that are not covered by a patch. This risk is amplified by the relative accessibility of the product-specific application sites, which include the arms or thighs. Each manufacturer recommends careful handwashing after handling the product, a specific drying time before the user covers the site with clothing, and avoidance of contact with the application site for a prescribed period of time, usually at least 1 to 2 hours.3-6
Our patient. This case illustrates the importance of discussing the risk of medication transfer to both humans and animals when prescribing individualized hormone therapy. While the Evamist prescribing information specifically addresses the risk of unintentional medication transfer to children, it does not discuss other contact risks.6 In the literature, there have been a limited number of reports on the adverse effects from transdermal or topical human medication transfer to pets. Notably, the American Pet Products Association estimates that in the United States, approximately 90 million dogs and 94 million cats are owned as a pet in 67% of households.7
THE TAKEAWAY
Use of HRT, including transdermal or topical estrogen formulations, is common. Given the large number of companion animals in the United States, physicians should consider that all members of a patient’s household—including pets—may be subject to unintentional secondary exposure to topical estrogen formulations and that they may experience adverse effects. This presents an opportunity for patient education, which can have a larger impact on all occupants of the home.
CORRESPONDENCE
Shannon Scott, DO, FACOFP, Clinical Associate Professor, Arizona College of Osteopathic Medicine, 19389 North 59th Avenue, Glendale, AZ 85308; [email protected].
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Steinkellner AR, Denison SE, Eldridge SL, et al. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women’s Health Initiative. Menopause. 2012;19:616-621.
3. Divigel [package insert]. Bridgewater, NJ: Vertical Pharmaceuticals, LLC; 2014.
4. Elestrin [package insert]. Somerset, NJ: Meda Pharmaceuticals; 2014.
5. Estrogel [package insert]. Herndon, VA: Ascend Therapeutics; 2018.
6. Evamist [package insert]. Minneapolis, MN: Perrigo; 2017.
7. American Pet Products Association. Pet Industry Market Size & Ownership Statistics. www.americanpetproducts.org/press_industrytrends.asp. Accessed November 1, 2019.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Steinkellner AR, Denison SE, Eldridge SL, et al. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women’s Health Initiative. Menopause. 2012;19:616-621.
3. Divigel [package insert]. Bridgewater, NJ: Vertical Pharmaceuticals, LLC; 2014.
4. Elestrin [package insert]. Somerset, NJ: Meda Pharmaceuticals; 2014.
5. Estrogel [package insert]. Herndon, VA: Ascend Therapeutics; 2018.
6. Evamist [package insert]. Minneapolis, MN: Perrigo; 2017.
7. American Pet Products Association. Pet Industry Market Size & Ownership Statistics. www.americanpetproducts.org/press_industrytrends.asp. Accessed November 1, 2019.