User login
Could liraglutide stall the onset of type 2 diabetes in children?
LOS ANGELES – Until the recent approval of liraglutide for the treatment of children and adolescents with type 2 diabetes, investigators like Sonia Caprio, MD, were at their wits’ end watching the beta-cell function of their patients decline on metformin treatment.
“The kids were not doing well. It was like they were being treated with water,” Dr. Caprio, a pediatric endocrinologist at Yale University, New Haven, Conn., said at the annual World Congress on Insulin Resistance, Diabetes and Cardiovascular Disease.
For example, in the NIH-funded TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study that began enrollment in 2004, 699 patients aged between 10 and 17 years and with type 2 diabetes were treated with metformin (1,000 mg, twice daily) to attain a glycated hemoglobin level of less than 8% and were then randomly assigned to continued treatment with metformin alone or to metformin combined with rosiglitazone (4 mg, twice a day) or a lifestyle-intervention program that focused on weight loss through modifying eating and activity behaviors (N Engl J Med. 2012;366:2247-56).
Over the course of 11 months, the researchers found that 46% of the children were failing treatment. “The worst arm was the metformin arm,” said Dr. Caprio, who was involved with the study. “Kids were not responding to the drug at all. About 52% of children failed to do better using metformin – a classic drug that we all start kids on when we diagnose them with type 2 diabetes.”
Findings from a follow-up study, TODAY2, showed that these young patients were prone to serious diabetes-related events, such as heart attacks, chronic kidney disease, retinal disease, neuropathy, and complications in the offspring of pregnancies.
In addition, results from the RISE (Restoring Insulin Secretion) Pediatric Medication Study found that, in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes, neither 3 months of insulin glargine followed by 9 months of metformin nor 12 months of metformin alone halted the progressive deterioration of beta-cell function (Diabetes Care. 2018 Aug; 41[8]:1717-25).
“The uniqueness of RISE is that we employed very sophisticated techniques to measure insulin secretion and sensitivity while they were being treated with these usual drugs,” said Dr. Caprio, who was one of the study investigators. “The beta cell is unresponsive to metformin and other treatments. The question is, why?”
Despite these findings, 2018 consensus guidelines from the American Diabetes Association on the evaluation and management of youth-onset diabetes (Diabetes Care. 2018;41:2648-68) call for the administration of metformin twice daily in youth with new-onset diabetes who have a hemoglobin A1c (HbA1c) level of less than 8.5%. “I argue that is not the way. We need better ways to treat [these patients] because they are moving fast to having complications,” she said.
Enter the Ellipse Trial, a pivotal multicenter, randomized study that evaluated the effect of the glucagonlike peptide-1 receptor agonist liraglutide in children and adolescents with type 2 diabetes (N Engl J Med. 2019;381:637-46).
Researchers, led by William V. Tamborlane, MD, chief of Yale Medicine Pediatric Endocrinology, also in New Haven, randomized 135 patients to one of two arms: 66 to subcutaneous liraglutide (up to 1.8 mg/day) and 69 to placebo for a 26-week, double-blind period, followed by a 26-week open-label extension period. All patients received metformin during the trial. More than half of the study participants (62%) were female, the mean age was 15 years, 65% were white, the mean body mass index was 33.9 kg/m2, their mean fasting glucose was 8.4 mmol/L, and their mean HbA1c was 7.8%.
for an estimated treatment difference of −1.06 percentage points (P less than .001). By 52 weeks, the difference increased to −1.30 percentage points.
“There was also a significant drop in BMI z score in patients treated with liraglutide, which is important,” Dr. Caprio said. “This medication is having an impact on weight, which is a key driver of the onset of type 2 diabetes in youth. This is a remarkable achievement because weight loss is hard to achieve in obese adolescents, as we showed in the TODAY study.”
The number of adverse events reported by patients was similar in the treatment and placebo groups (85% and 81%, respectively), but the overall rates of adverse events and gastrointestinal adverse events were higher with liraglutide.
“I use liraglutide just for weight reduction because I mainly see a lot of kids with obesity. Many kids are not responding because of the GI effects of this drug. I think the weight loss could have been better had the investigators moved to a dose of 1.8 mg, which we use in adults.”
A fasting plasma glucose of 6.1 mmol/L was the primary reason for participants remaining on a lower dose of liraglutide, she said. At the same time, liraglutide concentration data indicated a high rate of noncompliance, which was expected in this population. “That’s a big problem we face with children,” Dr. Caprio said. “Some of them are not constantly taking the medication. They skip doses a lot. But that happens with patients in this age group.”
“Finally, we have something else to help children and teenagers to delay the complications we are seeing,” Dr. Caprio said. “To me, I think this is a new era. I have hope. It will be interesting to see whether liraglutide and perhaps SGLT2 [sodium-glucose transporter 2] inhibitors can delay the onset of type 2 diabetes in children. In my view, we will be doing this with drugs. I don’t think the weight loss [concerns are] going to go away without medication, unfortunately.”
Dr. Caprio reported having no financial disclosures.
LOS ANGELES – Until the recent approval of liraglutide for the treatment of children and adolescents with type 2 diabetes, investigators like Sonia Caprio, MD, were at their wits’ end watching the beta-cell function of their patients decline on metformin treatment.
“The kids were not doing well. It was like they were being treated with water,” Dr. Caprio, a pediatric endocrinologist at Yale University, New Haven, Conn., said at the annual World Congress on Insulin Resistance, Diabetes and Cardiovascular Disease.
For example, in the NIH-funded TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study that began enrollment in 2004, 699 patients aged between 10 and 17 years and with type 2 diabetes were treated with metformin (1,000 mg, twice daily) to attain a glycated hemoglobin level of less than 8% and were then randomly assigned to continued treatment with metformin alone or to metformin combined with rosiglitazone (4 mg, twice a day) or a lifestyle-intervention program that focused on weight loss through modifying eating and activity behaviors (N Engl J Med. 2012;366:2247-56).
Over the course of 11 months, the researchers found that 46% of the children were failing treatment. “The worst arm was the metformin arm,” said Dr. Caprio, who was involved with the study. “Kids were not responding to the drug at all. About 52% of children failed to do better using metformin – a classic drug that we all start kids on when we diagnose them with type 2 diabetes.”
Findings from a follow-up study, TODAY2, showed that these young patients were prone to serious diabetes-related events, such as heart attacks, chronic kidney disease, retinal disease, neuropathy, and complications in the offspring of pregnancies.
In addition, results from the RISE (Restoring Insulin Secretion) Pediatric Medication Study found that, in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes, neither 3 months of insulin glargine followed by 9 months of metformin nor 12 months of metformin alone halted the progressive deterioration of beta-cell function (Diabetes Care. 2018 Aug; 41[8]:1717-25).
“The uniqueness of RISE is that we employed very sophisticated techniques to measure insulin secretion and sensitivity while they were being treated with these usual drugs,” said Dr. Caprio, who was one of the study investigators. “The beta cell is unresponsive to metformin and other treatments. The question is, why?”
Despite these findings, 2018 consensus guidelines from the American Diabetes Association on the evaluation and management of youth-onset diabetes (Diabetes Care. 2018;41:2648-68) call for the administration of metformin twice daily in youth with new-onset diabetes who have a hemoglobin A1c (HbA1c) level of less than 8.5%. “I argue that is not the way. We need better ways to treat [these patients] because they are moving fast to having complications,” she said.
Enter the Ellipse Trial, a pivotal multicenter, randomized study that evaluated the effect of the glucagonlike peptide-1 receptor agonist liraglutide in children and adolescents with type 2 diabetes (N Engl J Med. 2019;381:637-46).
Researchers, led by William V. Tamborlane, MD, chief of Yale Medicine Pediatric Endocrinology, also in New Haven, randomized 135 patients to one of two arms: 66 to subcutaneous liraglutide (up to 1.8 mg/day) and 69 to placebo for a 26-week, double-blind period, followed by a 26-week open-label extension period. All patients received metformin during the trial. More than half of the study participants (62%) were female, the mean age was 15 years, 65% were white, the mean body mass index was 33.9 kg/m2, their mean fasting glucose was 8.4 mmol/L, and their mean HbA1c was 7.8%.
for an estimated treatment difference of −1.06 percentage points (P less than .001). By 52 weeks, the difference increased to −1.30 percentage points.
“There was also a significant drop in BMI z score in patients treated with liraglutide, which is important,” Dr. Caprio said. “This medication is having an impact on weight, which is a key driver of the onset of type 2 diabetes in youth. This is a remarkable achievement because weight loss is hard to achieve in obese adolescents, as we showed in the TODAY study.”
The number of adverse events reported by patients was similar in the treatment and placebo groups (85% and 81%, respectively), but the overall rates of adverse events and gastrointestinal adverse events were higher with liraglutide.
“I use liraglutide just for weight reduction because I mainly see a lot of kids with obesity. Many kids are not responding because of the GI effects of this drug. I think the weight loss could have been better had the investigators moved to a dose of 1.8 mg, which we use in adults.”
A fasting plasma glucose of 6.1 mmol/L was the primary reason for participants remaining on a lower dose of liraglutide, she said. At the same time, liraglutide concentration data indicated a high rate of noncompliance, which was expected in this population. “That’s a big problem we face with children,” Dr. Caprio said. “Some of them are not constantly taking the medication. They skip doses a lot. But that happens with patients in this age group.”
“Finally, we have something else to help children and teenagers to delay the complications we are seeing,” Dr. Caprio said. “To me, I think this is a new era. I have hope. It will be interesting to see whether liraglutide and perhaps SGLT2 [sodium-glucose transporter 2] inhibitors can delay the onset of type 2 diabetes in children. In my view, we will be doing this with drugs. I don’t think the weight loss [concerns are] going to go away without medication, unfortunately.”
Dr. Caprio reported having no financial disclosures.
LOS ANGELES – Until the recent approval of liraglutide for the treatment of children and adolescents with type 2 diabetes, investigators like Sonia Caprio, MD, were at their wits’ end watching the beta-cell function of their patients decline on metformin treatment.
“The kids were not doing well. It was like they were being treated with water,” Dr. Caprio, a pediatric endocrinologist at Yale University, New Haven, Conn., said at the annual World Congress on Insulin Resistance, Diabetes and Cardiovascular Disease.
For example, in the NIH-funded TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study that began enrollment in 2004, 699 patients aged between 10 and 17 years and with type 2 diabetes were treated with metformin (1,000 mg, twice daily) to attain a glycated hemoglobin level of less than 8% and were then randomly assigned to continued treatment with metformin alone or to metformin combined with rosiglitazone (4 mg, twice a day) or a lifestyle-intervention program that focused on weight loss through modifying eating and activity behaviors (N Engl J Med. 2012;366:2247-56).
Over the course of 11 months, the researchers found that 46% of the children were failing treatment. “The worst arm was the metformin arm,” said Dr. Caprio, who was involved with the study. “Kids were not responding to the drug at all. About 52% of children failed to do better using metformin – a classic drug that we all start kids on when we diagnose them with type 2 diabetes.”
Findings from a follow-up study, TODAY2, showed that these young patients were prone to serious diabetes-related events, such as heart attacks, chronic kidney disease, retinal disease, neuropathy, and complications in the offspring of pregnancies.
In addition, results from the RISE (Restoring Insulin Secretion) Pediatric Medication Study found that, in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes, neither 3 months of insulin glargine followed by 9 months of metformin nor 12 months of metformin alone halted the progressive deterioration of beta-cell function (Diabetes Care. 2018 Aug; 41[8]:1717-25).
“The uniqueness of RISE is that we employed very sophisticated techniques to measure insulin secretion and sensitivity while they were being treated with these usual drugs,” said Dr. Caprio, who was one of the study investigators. “The beta cell is unresponsive to metformin and other treatments. The question is, why?”
Despite these findings, 2018 consensus guidelines from the American Diabetes Association on the evaluation and management of youth-onset diabetes (Diabetes Care. 2018;41:2648-68) call for the administration of metformin twice daily in youth with new-onset diabetes who have a hemoglobin A1c (HbA1c) level of less than 8.5%. “I argue that is not the way. We need better ways to treat [these patients] because they are moving fast to having complications,” she said.
Enter the Ellipse Trial, a pivotal multicenter, randomized study that evaluated the effect of the glucagonlike peptide-1 receptor agonist liraglutide in children and adolescents with type 2 diabetes (N Engl J Med. 2019;381:637-46).
Researchers, led by William V. Tamborlane, MD, chief of Yale Medicine Pediatric Endocrinology, also in New Haven, randomized 135 patients to one of two arms: 66 to subcutaneous liraglutide (up to 1.8 mg/day) and 69 to placebo for a 26-week, double-blind period, followed by a 26-week open-label extension period. All patients received metformin during the trial. More than half of the study participants (62%) were female, the mean age was 15 years, 65% were white, the mean body mass index was 33.9 kg/m2, their mean fasting glucose was 8.4 mmol/L, and their mean HbA1c was 7.8%.
for an estimated treatment difference of −1.06 percentage points (P less than .001). By 52 weeks, the difference increased to −1.30 percentage points.
“There was also a significant drop in BMI z score in patients treated with liraglutide, which is important,” Dr. Caprio said. “This medication is having an impact on weight, which is a key driver of the onset of type 2 diabetes in youth. This is a remarkable achievement because weight loss is hard to achieve in obese adolescents, as we showed in the TODAY study.”
The number of adverse events reported by patients was similar in the treatment and placebo groups (85% and 81%, respectively), but the overall rates of adverse events and gastrointestinal adverse events were higher with liraglutide.
“I use liraglutide just for weight reduction because I mainly see a lot of kids with obesity. Many kids are not responding because of the GI effects of this drug. I think the weight loss could have been better had the investigators moved to a dose of 1.8 mg, which we use in adults.”
A fasting plasma glucose of 6.1 mmol/L was the primary reason for participants remaining on a lower dose of liraglutide, she said. At the same time, liraglutide concentration data indicated a high rate of noncompliance, which was expected in this population. “That’s a big problem we face with children,” Dr. Caprio said. “Some of them are not constantly taking the medication. They skip doses a lot. But that happens with patients in this age group.”
“Finally, we have something else to help children and teenagers to delay the complications we are seeing,” Dr. Caprio said. “To me, I think this is a new era. I have hope. It will be interesting to see whether liraglutide and perhaps SGLT2 [sodium-glucose transporter 2] inhibitors can delay the onset of type 2 diabetes in children. In my view, we will be doing this with drugs. I don’t think the weight loss [concerns are] going to go away without medication, unfortunately.”
Dr. Caprio reported having no financial disclosures.
EXPERT ANALYSIS FROM THE WCIRDC 2019
FB Support Groups Enable Rapid Access to Large Numbers of Patients With Rare Disease
Investigators conducted a survey study of 214 patients with dermatofibrosarcoma protuberans (DFSP) or their family members using in part existing Facebook patient support groups (FBSG) to recruit respondents. They found the approach provides a “powerful” tool to collect relevant disease information from large numbers of patients with rare diseases.
A team of medical practitioners and patients developed the multiple-choice survey, and after testing the survey twice, posted a survey announcement on FBSGs for DFSP. The survey was live for 3 weeks in 2015. The investigators rapidly collected disease statistics, including information on recurrence, metastasis, surgical outcomes, diagnostic delay, and more, suggesting that FBSGs are useful medical research tools.
One hundred ninety-nine respondents were patients and 15 were family members. The respondents reported a median of 4 years to receive a correct diagnosis after noticing a lesion, ranging from less than 1 year to 42 years. About half the patients (52.3%) believed they received a misdiagnosis at some point, either from a dermatologist, primary care clinician, or another type of physician. Patients first noticed DFSP at a median age of 29.6 years. Many of their lesions appeared initially as flat plaques that eventually became protuberant. Because of this disconnect between the disease name and its clinical presentation, the investigators proposed the alternative term, dermatofibrosarcoma, often protuberant, be adopted. The investigators concluded that “FBSGs appear to be powerful tools to synergize effective and rapid research collaborations with large numbers of international patients with rare disease.” TSJ
Investigators conducted a survey study of 214 patients with dermatofibrosarcoma protuberans (DFSP) or their family members using in part existing Facebook patient support groups (FBSG) to recruit respondents. They found the approach provides a “powerful” tool to collect relevant disease information from large numbers of patients with rare diseases.
A team of medical practitioners and patients developed the multiple-choice survey, and after testing the survey twice, posted a survey announcement on FBSGs for DFSP. The survey was live for 3 weeks in 2015. The investigators rapidly collected disease statistics, including information on recurrence, metastasis, surgical outcomes, diagnostic delay, and more, suggesting that FBSGs are useful medical research tools.
One hundred ninety-nine respondents were patients and 15 were family members. The respondents reported a median of 4 years to receive a correct diagnosis after noticing a lesion, ranging from less than 1 year to 42 years. About half the patients (52.3%) believed they received a misdiagnosis at some point, either from a dermatologist, primary care clinician, or another type of physician. Patients first noticed DFSP at a median age of 29.6 years. Many of their lesions appeared initially as flat plaques that eventually became protuberant. Because of this disconnect between the disease name and its clinical presentation, the investigators proposed the alternative term, dermatofibrosarcoma, often protuberant, be adopted. The investigators concluded that “FBSGs appear to be powerful tools to synergize effective and rapid research collaborations with large numbers of international patients with rare disease.” TSJ
Investigators conducted a survey study of 214 patients with dermatofibrosarcoma protuberans (DFSP) or their family members using in part existing Facebook patient support groups (FBSG) to recruit respondents. They found the approach provides a “powerful” tool to collect relevant disease information from large numbers of patients with rare diseases.
A team of medical practitioners and patients developed the multiple-choice survey, and after testing the survey twice, posted a survey announcement on FBSGs for DFSP. The survey was live for 3 weeks in 2015. The investigators rapidly collected disease statistics, including information on recurrence, metastasis, surgical outcomes, diagnostic delay, and more, suggesting that FBSGs are useful medical research tools.
One hundred ninety-nine respondents were patients and 15 were family members. The respondents reported a median of 4 years to receive a correct diagnosis after noticing a lesion, ranging from less than 1 year to 42 years. About half the patients (52.3%) believed they received a misdiagnosis at some point, either from a dermatologist, primary care clinician, or another type of physician. Patients first noticed DFSP at a median age of 29.6 years. Many of their lesions appeared initially as flat plaques that eventually became protuberant. Because of this disconnect between the disease name and its clinical presentation, the investigators proposed the alternative term, dermatofibrosarcoma, often protuberant, be adopted. The investigators concluded that “FBSGs appear to be powerful tools to synergize effective and rapid research collaborations with large numbers of international patients with rare disease.” TSJ
Pexidartinib Receives Category 1 Recommendation from NCCN
Pexidartinib, the newly approved agent to treat patients with tenosynovial giant cell tumor (TGCT), received a category 1 recommendation from the National Comprehensive Cancer Network (NCCN) in the recent update of its Clinical Practice Guidelines in Oncology, Soft Tissue Sarcoma (Version 4.2019). A category 1 recommendation is based on a high level of evidence with uniform consensus that the intervention is appropriate.
The NCCN based its recommendation on the randomized, placebo-controlled phase 3 ENLIVEN study (NCT02371369) published in The Lancet (Tap WD, Gelderblom H, Palmerini E, et al. Lancet. 2019;394:478-487). The placebo-controlled portion of the study showed that patients treated with pexidartinib achieved a significantly higher overall response than patients in the placebo arm, 39% compared to none, respectively. The investigators identified mixed or cholestatic hepatotoxicity to be a risk of systemic therapy with the agent. Nevertheless, the “robust tumour response,” they wrote, “with improved patient symptoms and functional outcomes” establish pexidartinib as a potential treatment for TGCT in cases not amenable to improvement with surgery.
Pexidartinib, the newly approved agent to treat patients with tenosynovial giant cell tumor (TGCT), received a category 1 recommendation from the National Comprehensive Cancer Network (NCCN) in the recent update of its Clinical Practice Guidelines in Oncology, Soft Tissue Sarcoma (Version 4.2019). A category 1 recommendation is based on a high level of evidence with uniform consensus that the intervention is appropriate.
The NCCN based its recommendation on the randomized, placebo-controlled phase 3 ENLIVEN study (NCT02371369) published in The Lancet (Tap WD, Gelderblom H, Palmerini E, et al. Lancet. 2019;394:478-487). The placebo-controlled portion of the study showed that patients treated with pexidartinib achieved a significantly higher overall response than patients in the placebo arm, 39% compared to none, respectively. The investigators identified mixed or cholestatic hepatotoxicity to be a risk of systemic therapy with the agent. Nevertheless, the “robust tumour response,” they wrote, “with improved patient symptoms and functional outcomes” establish pexidartinib as a potential treatment for TGCT in cases not amenable to improvement with surgery.
Pexidartinib, the newly approved agent to treat patients with tenosynovial giant cell tumor (TGCT), received a category 1 recommendation from the National Comprehensive Cancer Network (NCCN) in the recent update of its Clinical Practice Guidelines in Oncology, Soft Tissue Sarcoma (Version 4.2019). A category 1 recommendation is based on a high level of evidence with uniform consensus that the intervention is appropriate.
The NCCN based its recommendation on the randomized, placebo-controlled phase 3 ENLIVEN study (NCT02371369) published in The Lancet (Tap WD, Gelderblom H, Palmerini E, et al. Lancet. 2019;394:478-487). The placebo-controlled portion of the study showed that patients treated with pexidartinib achieved a significantly higher overall response than patients in the placebo arm, 39% compared to none, respectively. The investigators identified mixed or cholestatic hepatotoxicity to be a risk of systemic therapy with the agent. Nevertheless, the “robust tumour response,” they wrote, “with improved patient symptoms and functional outcomes” establish pexidartinib as a potential treatment for TGCT in cases not amenable to improvement with surgery.
Metastatic angiosarcoma arising in a patient with long-standing treatment-refractory hemangioma
Angiosarcomas are malignant tumors of the vascular endothelium and are typically idiopathic. These tumors comprise 2% of all soft tissue sarcomas and have an estimated incidence of 2 per million.1,2 Known causes of angiosarcoma include genetic syndromes—such as von Hippel- Lindau, Chuvash polycythemia, Bannayan- Riley-Ruvalcaba, Cowden, and hamartomatous polyposis syndromes— chronic lymphedema, and exposure to radiation.3 Vinyl chloride, arsenicals, and thorotrast are known to increase the incidence of liver angiosarcoma.4
Malignant transformation of hemangioma is rare. We describe metastatic angiosarcoma in a patient with a large, longterm treatment-resistant subcutaneous hemangioma, illustrating such a possibility. We review similar cases and discuss the value of determining pathogenesis in such patients.
Case Presentation and Summary
A 55-year-old female with a long-standing childhood hemangioma of the left lower extremity was referred to Ochsner Medical Center for tissue diagnosis of new pulmonary nodules. Her medical history included a 7 pack-year smoking history; she had quit 3 years prior. Her family history included a sister who died from breast cancer. The patient initially had a progressive, intermittently bleeding tumor in the left foot at age 7. She was diagnosed with hemangioma in her twenties. At that point, her tumor began to involve the posterior calf and femur, causing deformity. She had multiple surgical resections but reportedly all pathology demonstrated benign hemangioma. She received radiation for pain, a routine treatment at the time, but developed a focus of progression in the heel. Above-knee amputation was considered but could not be performed when hemangioma was discovered in the hip area. She was lost to follow-up between 2001 and 2015. Lower extremity magnetic resonance imaging in 2015 was stable with imaging prior to 2001. A repeat biopsy in 2016 demonstrated hemangioma. The patient then received radiation to a wider field, including the femur, with minimal response. She completed a course of steroids as well. Bevacizumab was started in 2017 and improved foot deformity. She also briefly trialed pazopanib for 4 weeks in 2018 in an attempt to switch to oral medications. Despite partial response, she discontinued both agents in July 2018 because of toxicity and the burden of recurrent infusions.
Four months later, she presented with 2 months of intermittent hemoptysis and 18 months of metallic odors. Additionally, she lost 25 pounds in 3 months, which she attributed to a diet plan. At this visit, her left lower extremity exhibited multiple subcutaneous tumors and nodules.
Computed tomography (CT) with contrast demonstrated innumerable pulmonary nodules, the largest measuring 2.2 cm in the right lower lobe superior segment. Positron emission tomography (PET)/CT revealed 2 nodules with mild hypermetabolic activity; the largest nodule had a maximum standardized uptake value of 2.7. Bronchoalveolar lavage studies showed intra-alveolar hemorrhage with hemosiderin-laden macrophages. No malignancy, granuloma, or dysplasia was found in transbronchial needle aspirate of the largest nodule. The patient had no lymphadenopathy.
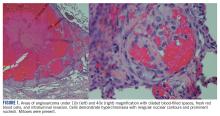
At this hospital, surgical resection by video-assisted thoracoscopic surgery confirmed multifocal malignant epithelioid neoplasm suspicious for angiosarcoma. Multiple areas showed proliferation of atypical epithelioid-to-spindle cells. There were prominent associated hemosiderin-laden macrophages, fresh red blood cells, and dilated blood-filled spaces. Cells demonstrated hyperchromasia with irregular nuclear contours, prominent nucleoli, and mitoses (FIGURE 1). Additionally, there were areas of focal organizing pneumonia. For atypical cells, staining was CD31-positive and CD34-negative. Staining was strongly positive for ERG. There was increased Ki-67 with retained INI expression and patchy weak reactivity for Fli-1.
Next-generation sequencing was performed. Specimen tumor content was 15%. Genomic findings included IDH1 p.R132C mutation, with variant allele frequency <10%. Testing was inconclusive for MSI and TMB mutations. PD-L1 assessment could not be performed. Unfortunately, the patient did not qualify for any clinical trials, as there were no matching alterations. This patient was lost to follow-up.
Discussion
Angiosarcoma accounts for 2% of soft tissue sarcomas.1 Cutaneous angiosarcomas most commonly occur in the face and scalp of the elderly, or in sites of chronic lymphedema. Angiosarcoma also develops following radiation therapy.5 For breast cancers and tumors of the head and neck, irradiation has <1% risk of inducing secondary malignancy, including angiosarcoma.6
This patient had a new diagnosis of angiosarcoma in the setting of long-standing benign hemangioma with history of radiation treatment. Thus, it is unclear whether this angiosarcoma was primary, radiation-induced, or secondary to transformation from the preexisting vascular tumor. Post-irradiation sarcoma carries a less favorable prognosis compared to de novo sarcoma; however, reports conflict on whether this holds for angiosarcoma subtypes.6 Determining etiology may benefit patients for prognostication and possibly inform future selection of treatment modalities.
The mutational signature in radiation- associated sarcomas differs from that of sporadic sarcomas. First, radiation- associated sarcomas demonstrate more frequent small deletions and balanced translocations. TP53 mutations are found in up to 1/3 of radiation-associated sarcomas and are more often due to small deletions than in sporadic sarcomas.7 High-level MYC amplification occurs in 54%-100% of secondary angiosarcomas, compared to 0-7% in sporadic angiosarcomas. Co-amplification of FLT4 occurs in 11%-25% of secondary angiosarcomas.8 Additionally, transcriptome analysis revealed differential expression of a 135-gene signature compared to non-radiation- induced sarcomas.7 Although this patient was not specifically analyzed for such alterations, such tests may differentiate post-irradiation angiosarcoma from sporadic etiologies.
In this patient, the R132C IDH1 mutation was identified and may be the first reported case in angiosarcoma. Typically, this mutation occurs in chondrosarcoma, myeloid neoplasms, gliomas, and cholangiocarcinomas. It is also found in spindle cell hemangiomas but not in other vascular tumors.9 The clinical significance of this mutation is uncertain at this time.
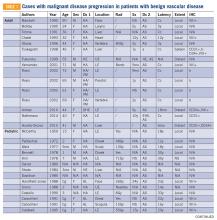
There are approximately 36 reported cases of malignant disease arising in patients with less aggressive vascular tumors (TABLE 1). Of these, 25 of 36 involve angiosarcoma arising in patients with hemangioma. Four cases of angiosarcoma were reported in patients with hemangioendothelioma, 1 case of hemangioendothelioma in a patient with hemangioma, 1 case of Dabska tumor in a patient with hemangioma, and 1 case of angiosarcoma in a patient with Dabska tumor. Fifteen cases involved initial disease with adult onset and 21 involved initial disease with pediatric onset, suggesting even distribution. Malignant disease mostly occurred in adulthood, in 26 out of 33 cases. Latency to malignancy ranged from concurrent discovery to 54 years. Mean latency, excluding cases with concurrent discovery, was shorter with adult-onset initial disease, at 4.2 years, compared to 16 years among patients with onset of initial disease in childhood. Longer latency in the pediatric-onset population correlated with longer latent periods for radiation-induced angiosarcoma following benign disease, which is reported to average 23 years.10 Thirteen of 19 cases with pediatric onset disease had a history of radiotherapy, while 2 of 13 cases with adult onset disease did. Sixteen cases involved tumor in the bone and soft tissue, as in this patient. Notably, 4 of these cases involved long-standing hemangioma for 10 years or more, as in this patient, suggesting a possible correlation between long-standing vascular tumors and malignant transformation. Angiosarcoma arising in non-irradiated patients suggests that malignant transformation and de novo transformation may compete with radiation-induced mutation in tumorigenesis. Further, 8 cases involved angiosarcoma growing within another vascular tumor, demonstrating the possibility of malignant transformation. Dehner and Ishak described a histological model for quantifying such a risk; a validated model may be particularly useful in patients with long-standing hemangioma.11
Etiology of tumorigenesis in cases of angiosarcoma arising in patients with a history of benign hemangioma may benefit prognostication and inform treatment selection in the future. Owing to long latent periods, radiation-associated angiosarcoma incidence may rise, as radiation therapy for benign hemangioma was recently routine. Future research may provide insight into disease progression and possibly predict the risk of angiosarcoma in patients with long-standing benign disease. TSJ
1. Tambe SA, Nayak CS. Metastatic angiosarcoma of lower extremity. Indian Dermatol Online J. 2018;9(3)177-181.
2. Cioffi A, Reichert S, Antonescu CR, Maki RG. Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am.2013;27(5):975-988.
3. Cohen SM, Storer RD, Criswell KA, et al. Hemangiosarcoma in rodents: mode-of-action evaluation and human relevance. Toxicol Sci. 2009;111(1):4-18.
4. Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol. 1978;92(2):349- 376.
5. Mark RJ, Bailet JW, Poen J, et al. Postirradiation sarcoma of the head and neck. Cancer. 1993;72(3):887-893.
6. Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20(4):1267-1274.
7. Mito JK, Mitra D, Doyle LA. Radiation-associated sarcomas: an update on clinical, histologic, and molecular features. Surg Pathol Clin. 2019;12(1):139-148.
8. Weidema ME, Versleijen-Jonkers YMH, Flucke UE, Desar IME, van der Graaf WTA. Targeting angiosarcomas of the soft tissues: A challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019;138:120-131.
9. Kurek KC, Pansuriya TC, van Ruler MAJH, et al. R132C IDH1 mutations are found in spindle cell hemangiomas and not in other vascular tumors or malformations. Am J Pathol. 2013;182(5):1494-1500.
10. Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
11. Dehner LP, Ishak KG. Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol. 1971;92(2):101-111.
Angiosarcomas are malignant tumors of the vascular endothelium and are typically idiopathic. These tumors comprise 2% of all soft tissue sarcomas and have an estimated incidence of 2 per million.1,2 Known causes of angiosarcoma include genetic syndromes—such as von Hippel- Lindau, Chuvash polycythemia, Bannayan- Riley-Ruvalcaba, Cowden, and hamartomatous polyposis syndromes— chronic lymphedema, and exposure to radiation.3 Vinyl chloride, arsenicals, and thorotrast are known to increase the incidence of liver angiosarcoma.4
Malignant transformation of hemangioma is rare. We describe metastatic angiosarcoma in a patient with a large, longterm treatment-resistant subcutaneous hemangioma, illustrating such a possibility. We review similar cases and discuss the value of determining pathogenesis in such patients.
Case Presentation and Summary
A 55-year-old female with a long-standing childhood hemangioma of the left lower extremity was referred to Ochsner Medical Center for tissue diagnosis of new pulmonary nodules. Her medical history included a 7 pack-year smoking history; she had quit 3 years prior. Her family history included a sister who died from breast cancer. The patient initially had a progressive, intermittently bleeding tumor in the left foot at age 7. She was diagnosed with hemangioma in her twenties. At that point, her tumor began to involve the posterior calf and femur, causing deformity. She had multiple surgical resections but reportedly all pathology demonstrated benign hemangioma. She received radiation for pain, a routine treatment at the time, but developed a focus of progression in the heel. Above-knee amputation was considered but could not be performed when hemangioma was discovered in the hip area. She was lost to follow-up between 2001 and 2015. Lower extremity magnetic resonance imaging in 2015 was stable with imaging prior to 2001. A repeat biopsy in 2016 demonstrated hemangioma. The patient then received radiation to a wider field, including the femur, with minimal response. She completed a course of steroids as well. Bevacizumab was started in 2017 and improved foot deformity. She also briefly trialed pazopanib for 4 weeks in 2018 in an attempt to switch to oral medications. Despite partial response, she discontinued both agents in July 2018 because of toxicity and the burden of recurrent infusions.
Four months later, she presented with 2 months of intermittent hemoptysis and 18 months of metallic odors. Additionally, she lost 25 pounds in 3 months, which she attributed to a diet plan. At this visit, her left lower extremity exhibited multiple subcutaneous tumors and nodules.
Computed tomography (CT) with contrast demonstrated innumerable pulmonary nodules, the largest measuring 2.2 cm in the right lower lobe superior segment. Positron emission tomography (PET)/CT revealed 2 nodules with mild hypermetabolic activity; the largest nodule had a maximum standardized uptake value of 2.7. Bronchoalveolar lavage studies showed intra-alveolar hemorrhage with hemosiderin-laden macrophages. No malignancy, granuloma, or dysplasia was found in transbronchial needle aspirate of the largest nodule. The patient had no lymphadenopathy.
At this hospital, surgical resection by video-assisted thoracoscopic surgery confirmed multifocal malignant epithelioid neoplasm suspicious for angiosarcoma. Multiple areas showed proliferation of atypical epithelioid-to-spindle cells. There were prominent associated hemosiderin-laden macrophages, fresh red blood cells, and dilated blood-filled spaces. Cells demonstrated hyperchromasia with irregular nuclear contours, prominent nucleoli, and mitoses (FIGURE 1). Additionally, there were areas of focal organizing pneumonia. For atypical cells, staining was CD31-positive and CD34-negative. Staining was strongly positive for ERG. There was increased Ki-67 with retained INI expression and patchy weak reactivity for Fli-1.
Next-generation sequencing was performed. Specimen tumor content was 15%. Genomic findings included IDH1 p.R132C mutation, with variant allele frequency <10%. Testing was inconclusive for MSI and TMB mutations. PD-L1 assessment could not be performed. Unfortunately, the patient did not qualify for any clinical trials, as there were no matching alterations. This patient was lost to follow-up.
Discussion
Angiosarcoma accounts for 2% of soft tissue sarcomas.1 Cutaneous angiosarcomas most commonly occur in the face and scalp of the elderly, or in sites of chronic lymphedema. Angiosarcoma also develops following radiation therapy.5 For breast cancers and tumors of the head and neck, irradiation has <1% risk of inducing secondary malignancy, including angiosarcoma.6
This patient had a new diagnosis of angiosarcoma in the setting of long-standing benign hemangioma with history of radiation treatment. Thus, it is unclear whether this angiosarcoma was primary, radiation-induced, or secondary to transformation from the preexisting vascular tumor. Post-irradiation sarcoma carries a less favorable prognosis compared to de novo sarcoma; however, reports conflict on whether this holds for angiosarcoma subtypes.6 Determining etiology may benefit patients for prognostication and possibly inform future selection of treatment modalities.
The mutational signature in radiation- associated sarcomas differs from that of sporadic sarcomas. First, radiation- associated sarcomas demonstrate more frequent small deletions and balanced translocations. TP53 mutations are found in up to 1/3 of radiation-associated sarcomas and are more often due to small deletions than in sporadic sarcomas.7 High-level MYC amplification occurs in 54%-100% of secondary angiosarcomas, compared to 0-7% in sporadic angiosarcomas. Co-amplification of FLT4 occurs in 11%-25% of secondary angiosarcomas.8 Additionally, transcriptome analysis revealed differential expression of a 135-gene signature compared to non-radiation- induced sarcomas.7 Although this patient was not specifically analyzed for such alterations, such tests may differentiate post-irradiation angiosarcoma from sporadic etiologies.
In this patient, the R132C IDH1 mutation was identified and may be the first reported case in angiosarcoma. Typically, this mutation occurs in chondrosarcoma, myeloid neoplasms, gliomas, and cholangiocarcinomas. It is also found in spindle cell hemangiomas but not in other vascular tumors.9 The clinical significance of this mutation is uncertain at this time.
There are approximately 36 reported cases of malignant disease arising in patients with less aggressive vascular tumors (TABLE 1). Of these, 25 of 36 involve angiosarcoma arising in patients with hemangioma. Four cases of angiosarcoma were reported in patients with hemangioendothelioma, 1 case of hemangioendothelioma in a patient with hemangioma, 1 case of Dabska tumor in a patient with hemangioma, and 1 case of angiosarcoma in a patient with Dabska tumor. Fifteen cases involved initial disease with adult onset and 21 involved initial disease with pediatric onset, suggesting even distribution. Malignant disease mostly occurred in adulthood, in 26 out of 33 cases. Latency to malignancy ranged from concurrent discovery to 54 years. Mean latency, excluding cases with concurrent discovery, was shorter with adult-onset initial disease, at 4.2 years, compared to 16 years among patients with onset of initial disease in childhood. Longer latency in the pediatric-onset population correlated with longer latent periods for radiation-induced angiosarcoma following benign disease, which is reported to average 23 years.10 Thirteen of 19 cases with pediatric onset disease had a history of radiotherapy, while 2 of 13 cases with adult onset disease did. Sixteen cases involved tumor in the bone and soft tissue, as in this patient. Notably, 4 of these cases involved long-standing hemangioma for 10 years or more, as in this patient, suggesting a possible correlation between long-standing vascular tumors and malignant transformation. Angiosarcoma arising in non-irradiated patients suggests that malignant transformation and de novo transformation may compete with radiation-induced mutation in tumorigenesis. Further, 8 cases involved angiosarcoma growing within another vascular tumor, demonstrating the possibility of malignant transformation. Dehner and Ishak described a histological model for quantifying such a risk; a validated model may be particularly useful in patients with long-standing hemangioma.11
Etiology of tumorigenesis in cases of angiosarcoma arising in patients with a history of benign hemangioma may benefit prognostication and inform treatment selection in the future. Owing to long latent periods, radiation-associated angiosarcoma incidence may rise, as radiation therapy for benign hemangioma was recently routine. Future research may provide insight into disease progression and possibly predict the risk of angiosarcoma in patients with long-standing benign disease. TSJ
Angiosarcomas are malignant tumors of the vascular endothelium and are typically idiopathic. These tumors comprise 2% of all soft tissue sarcomas and have an estimated incidence of 2 per million.1,2 Known causes of angiosarcoma include genetic syndromes—such as von Hippel- Lindau, Chuvash polycythemia, Bannayan- Riley-Ruvalcaba, Cowden, and hamartomatous polyposis syndromes— chronic lymphedema, and exposure to radiation.3 Vinyl chloride, arsenicals, and thorotrast are known to increase the incidence of liver angiosarcoma.4
Malignant transformation of hemangioma is rare. We describe metastatic angiosarcoma in a patient with a large, longterm treatment-resistant subcutaneous hemangioma, illustrating such a possibility. We review similar cases and discuss the value of determining pathogenesis in such patients.
Case Presentation and Summary
A 55-year-old female with a long-standing childhood hemangioma of the left lower extremity was referred to Ochsner Medical Center for tissue diagnosis of new pulmonary nodules. Her medical history included a 7 pack-year smoking history; she had quit 3 years prior. Her family history included a sister who died from breast cancer. The patient initially had a progressive, intermittently bleeding tumor in the left foot at age 7. She was diagnosed with hemangioma in her twenties. At that point, her tumor began to involve the posterior calf and femur, causing deformity. She had multiple surgical resections but reportedly all pathology demonstrated benign hemangioma. She received radiation for pain, a routine treatment at the time, but developed a focus of progression in the heel. Above-knee amputation was considered but could not be performed when hemangioma was discovered in the hip area. She was lost to follow-up between 2001 and 2015. Lower extremity magnetic resonance imaging in 2015 was stable with imaging prior to 2001. A repeat biopsy in 2016 demonstrated hemangioma. The patient then received radiation to a wider field, including the femur, with minimal response. She completed a course of steroids as well. Bevacizumab was started in 2017 and improved foot deformity. She also briefly trialed pazopanib for 4 weeks in 2018 in an attempt to switch to oral medications. Despite partial response, she discontinued both agents in July 2018 because of toxicity and the burden of recurrent infusions.
Four months later, she presented with 2 months of intermittent hemoptysis and 18 months of metallic odors. Additionally, she lost 25 pounds in 3 months, which she attributed to a diet plan. At this visit, her left lower extremity exhibited multiple subcutaneous tumors and nodules.
Computed tomography (CT) with contrast demonstrated innumerable pulmonary nodules, the largest measuring 2.2 cm in the right lower lobe superior segment. Positron emission tomography (PET)/CT revealed 2 nodules with mild hypermetabolic activity; the largest nodule had a maximum standardized uptake value of 2.7. Bronchoalveolar lavage studies showed intra-alveolar hemorrhage with hemosiderin-laden macrophages. No malignancy, granuloma, or dysplasia was found in transbronchial needle aspirate of the largest nodule. The patient had no lymphadenopathy.
At this hospital, surgical resection by video-assisted thoracoscopic surgery confirmed multifocal malignant epithelioid neoplasm suspicious for angiosarcoma. Multiple areas showed proliferation of atypical epithelioid-to-spindle cells. There were prominent associated hemosiderin-laden macrophages, fresh red blood cells, and dilated blood-filled spaces. Cells demonstrated hyperchromasia with irregular nuclear contours, prominent nucleoli, and mitoses (FIGURE 1). Additionally, there were areas of focal organizing pneumonia. For atypical cells, staining was CD31-positive and CD34-negative. Staining was strongly positive for ERG. There was increased Ki-67 with retained INI expression and patchy weak reactivity for Fli-1.
Next-generation sequencing was performed. Specimen tumor content was 15%. Genomic findings included IDH1 p.R132C mutation, with variant allele frequency <10%. Testing was inconclusive for MSI and TMB mutations. PD-L1 assessment could not be performed. Unfortunately, the patient did not qualify for any clinical trials, as there were no matching alterations. This patient was lost to follow-up.
Discussion
Angiosarcoma accounts for 2% of soft tissue sarcomas.1 Cutaneous angiosarcomas most commonly occur in the face and scalp of the elderly, or in sites of chronic lymphedema. Angiosarcoma also develops following radiation therapy.5 For breast cancers and tumors of the head and neck, irradiation has <1% risk of inducing secondary malignancy, including angiosarcoma.6
This patient had a new diagnosis of angiosarcoma in the setting of long-standing benign hemangioma with history of radiation treatment. Thus, it is unclear whether this angiosarcoma was primary, radiation-induced, or secondary to transformation from the preexisting vascular tumor. Post-irradiation sarcoma carries a less favorable prognosis compared to de novo sarcoma; however, reports conflict on whether this holds for angiosarcoma subtypes.6 Determining etiology may benefit patients for prognostication and possibly inform future selection of treatment modalities.
The mutational signature in radiation- associated sarcomas differs from that of sporadic sarcomas. First, radiation- associated sarcomas demonstrate more frequent small deletions and balanced translocations. TP53 mutations are found in up to 1/3 of radiation-associated sarcomas and are more often due to small deletions than in sporadic sarcomas.7 High-level MYC amplification occurs in 54%-100% of secondary angiosarcomas, compared to 0-7% in sporadic angiosarcomas. Co-amplification of FLT4 occurs in 11%-25% of secondary angiosarcomas.8 Additionally, transcriptome analysis revealed differential expression of a 135-gene signature compared to non-radiation- induced sarcomas.7 Although this patient was not specifically analyzed for such alterations, such tests may differentiate post-irradiation angiosarcoma from sporadic etiologies.
In this patient, the R132C IDH1 mutation was identified and may be the first reported case in angiosarcoma. Typically, this mutation occurs in chondrosarcoma, myeloid neoplasms, gliomas, and cholangiocarcinomas. It is also found in spindle cell hemangiomas but not in other vascular tumors.9 The clinical significance of this mutation is uncertain at this time.
There are approximately 36 reported cases of malignant disease arising in patients with less aggressive vascular tumors (TABLE 1). Of these, 25 of 36 involve angiosarcoma arising in patients with hemangioma. Four cases of angiosarcoma were reported in patients with hemangioendothelioma, 1 case of hemangioendothelioma in a patient with hemangioma, 1 case of Dabska tumor in a patient with hemangioma, and 1 case of angiosarcoma in a patient with Dabska tumor. Fifteen cases involved initial disease with adult onset and 21 involved initial disease with pediatric onset, suggesting even distribution. Malignant disease mostly occurred in adulthood, in 26 out of 33 cases. Latency to malignancy ranged from concurrent discovery to 54 years. Mean latency, excluding cases with concurrent discovery, was shorter with adult-onset initial disease, at 4.2 years, compared to 16 years among patients with onset of initial disease in childhood. Longer latency in the pediatric-onset population correlated with longer latent periods for radiation-induced angiosarcoma following benign disease, which is reported to average 23 years.10 Thirteen of 19 cases with pediatric onset disease had a history of radiotherapy, while 2 of 13 cases with adult onset disease did. Sixteen cases involved tumor in the bone and soft tissue, as in this patient. Notably, 4 of these cases involved long-standing hemangioma for 10 years or more, as in this patient, suggesting a possible correlation between long-standing vascular tumors and malignant transformation. Angiosarcoma arising in non-irradiated patients suggests that malignant transformation and de novo transformation may compete with radiation-induced mutation in tumorigenesis. Further, 8 cases involved angiosarcoma growing within another vascular tumor, demonstrating the possibility of malignant transformation. Dehner and Ishak described a histological model for quantifying such a risk; a validated model may be particularly useful in patients with long-standing hemangioma.11
Etiology of tumorigenesis in cases of angiosarcoma arising in patients with a history of benign hemangioma may benefit prognostication and inform treatment selection in the future. Owing to long latent periods, radiation-associated angiosarcoma incidence may rise, as radiation therapy for benign hemangioma was recently routine. Future research may provide insight into disease progression and possibly predict the risk of angiosarcoma in patients with long-standing benign disease. TSJ
1. Tambe SA, Nayak CS. Metastatic angiosarcoma of lower extremity. Indian Dermatol Online J. 2018;9(3)177-181.
2. Cioffi A, Reichert S, Antonescu CR, Maki RG. Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am.2013;27(5):975-988.
3. Cohen SM, Storer RD, Criswell KA, et al. Hemangiosarcoma in rodents: mode-of-action evaluation and human relevance. Toxicol Sci. 2009;111(1):4-18.
4. Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol. 1978;92(2):349- 376.
5. Mark RJ, Bailet JW, Poen J, et al. Postirradiation sarcoma of the head and neck. Cancer. 1993;72(3):887-893.
6. Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20(4):1267-1274.
7. Mito JK, Mitra D, Doyle LA. Radiation-associated sarcomas: an update on clinical, histologic, and molecular features. Surg Pathol Clin. 2019;12(1):139-148.
8. Weidema ME, Versleijen-Jonkers YMH, Flucke UE, Desar IME, van der Graaf WTA. Targeting angiosarcomas of the soft tissues: A challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019;138:120-131.
9. Kurek KC, Pansuriya TC, van Ruler MAJH, et al. R132C IDH1 mutations are found in spindle cell hemangiomas and not in other vascular tumors or malformations. Am J Pathol. 2013;182(5):1494-1500.
10. Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
11. Dehner LP, Ishak KG. Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol. 1971;92(2):101-111.
1. Tambe SA, Nayak CS. Metastatic angiosarcoma of lower extremity. Indian Dermatol Online J. 2018;9(3)177-181.
2. Cioffi A, Reichert S, Antonescu CR, Maki RG. Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am.2013;27(5):975-988.
3. Cohen SM, Storer RD, Criswell KA, et al. Hemangiosarcoma in rodents: mode-of-action evaluation and human relevance. Toxicol Sci. 2009;111(1):4-18.
4. Popper H, Thomas LB, Telles NC, Falk H, Selikoff IJ. Development of hepatic angiosarcoma in man induced by vinyl chloride, thorotrast, and arsenic. Comparison with cases of unknown etiology. Am J Pathol. 1978;92(2):349- 376.
5. Mark RJ, Bailet JW, Poen J, et al. Postirradiation sarcoma of the head and neck. Cancer. 1993;72(3):887-893.
6. Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013;20(4):1267-1274.
7. Mito JK, Mitra D, Doyle LA. Radiation-associated sarcomas: an update on clinical, histologic, and molecular features. Surg Pathol Clin. 2019;12(1):139-148.
8. Weidema ME, Versleijen-Jonkers YMH, Flucke UE, Desar IME, van der Graaf WTA. Targeting angiosarcomas of the soft tissues: A challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019;138:120-131.
9. Kurek KC, Pansuriya TC, van Ruler MAJH, et al. R132C IDH1 mutations are found in spindle cell hemangiomas and not in other vascular tumors or malformations. Am J Pathol. 2013;182(5):1494-1500.
10. Goette DK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12(5 pt 2):922-926.
11. Dehner LP, Ishak KG. Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol. 1971;92(2):101-111.
The branching tree of hospital medicine
Diversity of training backgrounds
You’ve probably heard of a “nocturnist,” but have you ever heard of a “weekendist?”
The field of hospital medicine (HM) has evolved dramatically since the term “hospitalist” was introduced in the literature in 1996.1 There is a saying in HM that “if you know one HM program, you know one HM program,” alluding to the fact that every HM program is unique. The diversity of individual HM programs combined with the overall evolution of the field has expanded the range of jobs available in HM.
The nomenclature of adding an -ist to the end of the specific roles (e.g., nocturnist, weekendist) has become commonplace. These roles have developed with the increasing need for day and night staffing at many hospitals secondary to increased and more complex patients, less availability of residents because of work hour restrictions, and the Accreditation Council for Graduate Medical Education (ACGME) rules that require overnight supervision of residents
Additionally, the field of HM increasingly includes physicians trained in internal medicine, family medicine, pediatrics, and medicine-pediatrics (med-peds). In this article, we describe the variety of roles available to trainees joining HM and the multitude of different training backgrounds hospitalists come from.
Nocturnists
The 2018 State of Hospital Medicine Report notes that 76.1% of adult-only HM groups have nocturnists, hospitalists who work primarily at night to admit and to provide coverage for admitted patients.2 Nocturnists often provide benefit to the rest of their hospitalist group by allowing fewer required night shifts for those that prefer to work during the day.
Nocturnists may choose a nighttime schedule for several reasons, including the ability to be home more during the day. They also have the potential to work fewer total hours or shifts while still earning a similar or increased income, compared with predominantly daytime hospitalists, increasing their flexibility to pursue other interests. These nocturnists become experts in navigating the admission process and responding to inpatient emergencies often with less support when compared with daytime hospitalists.
In addition to career nocturnist work, nocturnist jobs can be a great fit for those residency graduates who are undecided about fellowship and enjoy the acuity of inpatient medicine. It provides an opportunity to hone their clinical skill set prior to specialized training while earning an attending salary, and offers flexible hours which may allow for research or other endeavors. In academic centers, nocturnist educational roles take on a different character as well and may involve more 1:1 educational experiences. The role of nocturnists as educators is expanding as ACGME rules call for more oversight and educational opportunities for residents who are working at night.
However, challenges exist for nocturnists, including keeping abreast of new changes in their HM groups and hospital systems and engaging in quality initiatives, given that most meetings occur during the day. Additionally, nocturnists must adapt to sleeping during the day, potentially getting less sleep then they would otherwise and being “off cycle” with family and friends. For nocturnists raising children, being off cycle may be advantageous as it can allow them to be home with their children after school.
Weekendists
Another common hospitalist role is the weekendist, hospitalists who spend much of their clinical time preferentially working weekends. Similar to nocturnists, weekendists provide benefit to their hospitalist group by allowing others to have more weekends off.
Weekendists may prefer working weekends because of fewer total shifts or hours and/or higher compensation per shift. Additionally, weekendists have the flexibility to do other work on weekdays, such as research or another hospitalist job. For those that do nonclinical work during the week, a weekendist position may allow them to keep their clinical skills up to date. However, weekendists may face intense clinical days with a higher census because of fewer hospitalists rounding on the weekends.
Weekendists must balance having more potential time available during the weekdays but less time on the weekends to devote to family and friends. Furthermore, weekendists may feel less engaged with nonclinical opportunities, including quality improvement, educational offerings, and teaching opportunities.
SNFists
With increasing emphasis on transitions of care and the desire to avoid readmission penalties, some hospitalists have transitioned to work partly or primarily in skilled nursing facilities (SNF) and have been referred to as “SNFists.” Some of these hospitalists may split their clinical time between SNFs and acute care hospitals, while others may work exclusively at SNFs.
SNFists have the potential to be invaluable in improving transitions of care after discharge to post–acute care facilities because of increased provider presence in these facilities, comfort with medically complex patients, and appreciation of government regulations.4 SNFists may face potential challenges of needing to staff more than one post–acute care hospital and of having less resources available, compared with an acute care hospital.
Specific specialty hospitalists
For a variety of reasons including clinical interest, many hospitalists have become specialized with regards to their primary inpatient population. Some hospitalists spend the majority of their clinical time on a specific service in the hospital, often working closely with the subspecialist caring for that patient. These hospitalists may focus on hematology, oncology, bone-marrow transplant, neurology, cardiology, surgery services, or critical care, among others. Hospitalists focused on a specific service often become knowledge experts in that specialty. Conversely, by focusing on a specific service, certain pathologies may be less commonly seen, which may narrow the breadth of the hospital medicine job.
Hospitalist training
Internal medicine hospitalists may be the most common hospitalists encountered in many hospitals and at each Society of Hospital Medicine annual conference, but there has also been rapid growth in hospitalists from other specialties and backgrounds.
Family medicine hospitalists are a part of 64.9% of HM groups and about 9% of family medicine graduates are choosing HM as a career path.2,3 Most family medicine hospitalists work in adult HM groups, but some, particularly in rural or academic settings, care for pediatric, newborn, and/or maternity patients. Similarly, pediatric hospitalists have become entrenched at many hospitals where children are admitted. These pediatric hospitalists, like adult hospitalists, may work in a variety of different clinical roles including in EDs, newborn nurseries, and inpatient wards or ICUs; they may also provide consult, sedation, or procedural services.
Med-peds hospitalists that split time between internal medicine and pediatrics are becoming more commonplace in the field. Many work at academic centers where they often work on each side separately, doing the same work as their internal medicine or pediatrics colleagues, and then switching to the other side after a period of time. Some centers offer unique roles for med-peds hospitalists including working on adult consult teams in children’s hospitals, where they provide consult care to older patients that may still receive their care at a children’s hospital. There are also nonacademic hospitals that primarily staff med-peds hospitalists, where they can provide the full spectrum of care from the newborn nursery to the inpatient pediatric and adult wards.
Hospital medicine is a young field that is constantly changing with new and developing roles for hospitalists from a wide variety of backgrounds. Stick around to see which “-ist” will come next in HM.
Dr. Hall is a med-peds hospitalist and assistant professor at the University of Kentucky, Lexington. Dr. Sanyal-Dey is an academic hospitalist at Zuckerberg San Francisco General Hospital and Trauma Center and the University of California, San Francisco, where she is the director of clinical operations, and director of the faculty inpatient service. Dr. Chang is associate professor and interprofessional education thread director (MD curriculum) at Washington University, St. Louis. Dr. Kwan is a hospitalist at the Veterans Affairs San Diego Healthcare System and associate professor at the University of California, San Diego. He is the chair of SHM’s Physicians in Training committee. Dr. Seymour is family medicine hospitalist education director at the University of Massachusetts Memorial Medical Center, Worcester, and associate professor at the University of Massachusetts.
References
1. Wachter RM, Goldman L. The Emerging Role of “Hospitalists” in the American Health Care System. N Engl J Med. 1996;335(7):514-7.
2. 2018 State of Hospital Medicine Report. Philadelphia: Society of Hospital Medicine, 2018.
3. Weaver SP, Hill J. Academician Attitudes and Beliefs Regarding the Use of Hospitalists: A CERA Study. Fam Med. 2015;47(5):357-61.
4. Teno JM et al. Temporal Trends in the Numbers of Skilled Nursing Facility Specialists From 2007 Through 2014. JAMA Intern Med. 2017;177(9):1376-8.
Diversity of training backgrounds
Diversity of training backgrounds
You’ve probably heard of a “nocturnist,” but have you ever heard of a “weekendist?”
The field of hospital medicine (HM) has evolved dramatically since the term “hospitalist” was introduced in the literature in 1996.1 There is a saying in HM that “if you know one HM program, you know one HM program,” alluding to the fact that every HM program is unique. The diversity of individual HM programs combined with the overall evolution of the field has expanded the range of jobs available in HM.
The nomenclature of adding an -ist to the end of the specific roles (e.g., nocturnist, weekendist) has become commonplace. These roles have developed with the increasing need for day and night staffing at many hospitals secondary to increased and more complex patients, less availability of residents because of work hour restrictions, and the Accreditation Council for Graduate Medical Education (ACGME) rules that require overnight supervision of residents
Additionally, the field of HM increasingly includes physicians trained in internal medicine, family medicine, pediatrics, and medicine-pediatrics (med-peds). In this article, we describe the variety of roles available to trainees joining HM and the multitude of different training backgrounds hospitalists come from.
Nocturnists
The 2018 State of Hospital Medicine Report notes that 76.1% of adult-only HM groups have nocturnists, hospitalists who work primarily at night to admit and to provide coverage for admitted patients.2 Nocturnists often provide benefit to the rest of their hospitalist group by allowing fewer required night shifts for those that prefer to work during the day.
Nocturnists may choose a nighttime schedule for several reasons, including the ability to be home more during the day. They also have the potential to work fewer total hours or shifts while still earning a similar or increased income, compared with predominantly daytime hospitalists, increasing their flexibility to pursue other interests. These nocturnists become experts in navigating the admission process and responding to inpatient emergencies often with less support when compared with daytime hospitalists.
In addition to career nocturnist work, nocturnist jobs can be a great fit for those residency graduates who are undecided about fellowship and enjoy the acuity of inpatient medicine. It provides an opportunity to hone their clinical skill set prior to specialized training while earning an attending salary, and offers flexible hours which may allow for research or other endeavors. In academic centers, nocturnist educational roles take on a different character as well and may involve more 1:1 educational experiences. The role of nocturnists as educators is expanding as ACGME rules call for more oversight and educational opportunities for residents who are working at night.
However, challenges exist for nocturnists, including keeping abreast of new changes in their HM groups and hospital systems and engaging in quality initiatives, given that most meetings occur during the day. Additionally, nocturnists must adapt to sleeping during the day, potentially getting less sleep then they would otherwise and being “off cycle” with family and friends. For nocturnists raising children, being off cycle may be advantageous as it can allow them to be home with their children after school.
Weekendists
Another common hospitalist role is the weekendist, hospitalists who spend much of their clinical time preferentially working weekends. Similar to nocturnists, weekendists provide benefit to their hospitalist group by allowing others to have more weekends off.
Weekendists may prefer working weekends because of fewer total shifts or hours and/or higher compensation per shift. Additionally, weekendists have the flexibility to do other work on weekdays, such as research or another hospitalist job. For those that do nonclinical work during the week, a weekendist position may allow them to keep their clinical skills up to date. However, weekendists may face intense clinical days with a higher census because of fewer hospitalists rounding on the weekends.
Weekendists must balance having more potential time available during the weekdays but less time on the weekends to devote to family and friends. Furthermore, weekendists may feel less engaged with nonclinical opportunities, including quality improvement, educational offerings, and teaching opportunities.
SNFists
With increasing emphasis on transitions of care and the desire to avoid readmission penalties, some hospitalists have transitioned to work partly or primarily in skilled nursing facilities (SNF) and have been referred to as “SNFists.” Some of these hospitalists may split their clinical time between SNFs and acute care hospitals, while others may work exclusively at SNFs.
SNFists have the potential to be invaluable in improving transitions of care after discharge to post–acute care facilities because of increased provider presence in these facilities, comfort with medically complex patients, and appreciation of government regulations.4 SNFists may face potential challenges of needing to staff more than one post–acute care hospital and of having less resources available, compared with an acute care hospital.
Specific specialty hospitalists
For a variety of reasons including clinical interest, many hospitalists have become specialized with regards to their primary inpatient population. Some hospitalists spend the majority of their clinical time on a specific service in the hospital, often working closely with the subspecialist caring for that patient. These hospitalists may focus on hematology, oncology, bone-marrow transplant, neurology, cardiology, surgery services, or critical care, among others. Hospitalists focused on a specific service often become knowledge experts in that specialty. Conversely, by focusing on a specific service, certain pathologies may be less commonly seen, which may narrow the breadth of the hospital medicine job.
Hospitalist training
Internal medicine hospitalists may be the most common hospitalists encountered in many hospitals and at each Society of Hospital Medicine annual conference, but there has also been rapid growth in hospitalists from other specialties and backgrounds.
Family medicine hospitalists are a part of 64.9% of HM groups and about 9% of family medicine graduates are choosing HM as a career path.2,3 Most family medicine hospitalists work in adult HM groups, but some, particularly in rural or academic settings, care for pediatric, newborn, and/or maternity patients. Similarly, pediatric hospitalists have become entrenched at many hospitals where children are admitted. These pediatric hospitalists, like adult hospitalists, may work in a variety of different clinical roles including in EDs, newborn nurseries, and inpatient wards or ICUs; they may also provide consult, sedation, or procedural services.
Med-peds hospitalists that split time between internal medicine and pediatrics are becoming more commonplace in the field. Many work at academic centers where they often work on each side separately, doing the same work as their internal medicine or pediatrics colleagues, and then switching to the other side after a period of time. Some centers offer unique roles for med-peds hospitalists including working on adult consult teams in children’s hospitals, where they provide consult care to older patients that may still receive their care at a children’s hospital. There are also nonacademic hospitals that primarily staff med-peds hospitalists, where they can provide the full spectrum of care from the newborn nursery to the inpatient pediatric and adult wards.
Hospital medicine is a young field that is constantly changing with new and developing roles for hospitalists from a wide variety of backgrounds. Stick around to see which “-ist” will come next in HM.
Dr. Hall is a med-peds hospitalist and assistant professor at the University of Kentucky, Lexington. Dr. Sanyal-Dey is an academic hospitalist at Zuckerberg San Francisco General Hospital and Trauma Center and the University of California, San Francisco, where she is the director of clinical operations, and director of the faculty inpatient service. Dr. Chang is associate professor and interprofessional education thread director (MD curriculum) at Washington University, St. Louis. Dr. Kwan is a hospitalist at the Veterans Affairs San Diego Healthcare System and associate professor at the University of California, San Diego. He is the chair of SHM’s Physicians in Training committee. Dr. Seymour is family medicine hospitalist education director at the University of Massachusetts Memorial Medical Center, Worcester, and associate professor at the University of Massachusetts.
References
1. Wachter RM, Goldman L. The Emerging Role of “Hospitalists” in the American Health Care System. N Engl J Med. 1996;335(7):514-7.
2. 2018 State of Hospital Medicine Report. Philadelphia: Society of Hospital Medicine, 2018.
3. Weaver SP, Hill J. Academician Attitudes and Beliefs Regarding the Use of Hospitalists: A CERA Study. Fam Med. 2015;47(5):357-61.
4. Teno JM et al. Temporal Trends in the Numbers of Skilled Nursing Facility Specialists From 2007 Through 2014. JAMA Intern Med. 2017;177(9):1376-8.
You’ve probably heard of a “nocturnist,” but have you ever heard of a “weekendist?”
The field of hospital medicine (HM) has evolved dramatically since the term “hospitalist” was introduced in the literature in 1996.1 There is a saying in HM that “if you know one HM program, you know one HM program,” alluding to the fact that every HM program is unique. The diversity of individual HM programs combined with the overall evolution of the field has expanded the range of jobs available in HM.
The nomenclature of adding an -ist to the end of the specific roles (e.g., nocturnist, weekendist) has become commonplace. These roles have developed with the increasing need for day and night staffing at many hospitals secondary to increased and more complex patients, less availability of residents because of work hour restrictions, and the Accreditation Council for Graduate Medical Education (ACGME) rules that require overnight supervision of residents
Additionally, the field of HM increasingly includes physicians trained in internal medicine, family medicine, pediatrics, and medicine-pediatrics (med-peds). In this article, we describe the variety of roles available to trainees joining HM and the multitude of different training backgrounds hospitalists come from.
Nocturnists
The 2018 State of Hospital Medicine Report notes that 76.1% of adult-only HM groups have nocturnists, hospitalists who work primarily at night to admit and to provide coverage for admitted patients.2 Nocturnists often provide benefit to the rest of their hospitalist group by allowing fewer required night shifts for those that prefer to work during the day.
Nocturnists may choose a nighttime schedule for several reasons, including the ability to be home more during the day. They also have the potential to work fewer total hours or shifts while still earning a similar or increased income, compared with predominantly daytime hospitalists, increasing their flexibility to pursue other interests. These nocturnists become experts in navigating the admission process and responding to inpatient emergencies often with less support when compared with daytime hospitalists.
In addition to career nocturnist work, nocturnist jobs can be a great fit for those residency graduates who are undecided about fellowship and enjoy the acuity of inpatient medicine. It provides an opportunity to hone their clinical skill set prior to specialized training while earning an attending salary, and offers flexible hours which may allow for research or other endeavors. In academic centers, nocturnist educational roles take on a different character as well and may involve more 1:1 educational experiences. The role of nocturnists as educators is expanding as ACGME rules call for more oversight and educational opportunities for residents who are working at night.
However, challenges exist for nocturnists, including keeping abreast of new changes in their HM groups and hospital systems and engaging in quality initiatives, given that most meetings occur during the day. Additionally, nocturnists must adapt to sleeping during the day, potentially getting less sleep then they would otherwise and being “off cycle” with family and friends. For nocturnists raising children, being off cycle may be advantageous as it can allow them to be home with their children after school.
Weekendists
Another common hospitalist role is the weekendist, hospitalists who spend much of their clinical time preferentially working weekends. Similar to nocturnists, weekendists provide benefit to their hospitalist group by allowing others to have more weekends off.
Weekendists may prefer working weekends because of fewer total shifts or hours and/or higher compensation per shift. Additionally, weekendists have the flexibility to do other work on weekdays, such as research or another hospitalist job. For those that do nonclinical work during the week, a weekendist position may allow them to keep their clinical skills up to date. However, weekendists may face intense clinical days with a higher census because of fewer hospitalists rounding on the weekends.
Weekendists must balance having more potential time available during the weekdays but less time on the weekends to devote to family and friends. Furthermore, weekendists may feel less engaged with nonclinical opportunities, including quality improvement, educational offerings, and teaching opportunities.
SNFists
With increasing emphasis on transitions of care and the desire to avoid readmission penalties, some hospitalists have transitioned to work partly or primarily in skilled nursing facilities (SNF) and have been referred to as “SNFists.” Some of these hospitalists may split their clinical time between SNFs and acute care hospitals, while others may work exclusively at SNFs.
SNFists have the potential to be invaluable in improving transitions of care after discharge to post–acute care facilities because of increased provider presence in these facilities, comfort with medically complex patients, and appreciation of government regulations.4 SNFists may face potential challenges of needing to staff more than one post–acute care hospital and of having less resources available, compared with an acute care hospital.
Specific specialty hospitalists
For a variety of reasons including clinical interest, many hospitalists have become specialized with regards to their primary inpatient population. Some hospitalists spend the majority of their clinical time on a specific service in the hospital, often working closely with the subspecialist caring for that patient. These hospitalists may focus on hematology, oncology, bone-marrow transplant, neurology, cardiology, surgery services, or critical care, among others. Hospitalists focused on a specific service often become knowledge experts in that specialty. Conversely, by focusing on a specific service, certain pathologies may be less commonly seen, which may narrow the breadth of the hospital medicine job.
Hospitalist training
Internal medicine hospitalists may be the most common hospitalists encountered in many hospitals and at each Society of Hospital Medicine annual conference, but there has also been rapid growth in hospitalists from other specialties and backgrounds.
Family medicine hospitalists are a part of 64.9% of HM groups and about 9% of family medicine graduates are choosing HM as a career path.2,3 Most family medicine hospitalists work in adult HM groups, but some, particularly in rural or academic settings, care for pediatric, newborn, and/or maternity patients. Similarly, pediatric hospitalists have become entrenched at many hospitals where children are admitted. These pediatric hospitalists, like adult hospitalists, may work in a variety of different clinical roles including in EDs, newborn nurseries, and inpatient wards or ICUs; they may also provide consult, sedation, or procedural services.
Med-peds hospitalists that split time between internal medicine and pediatrics are becoming more commonplace in the field. Many work at academic centers where they often work on each side separately, doing the same work as their internal medicine or pediatrics colleagues, and then switching to the other side after a period of time. Some centers offer unique roles for med-peds hospitalists including working on adult consult teams in children’s hospitals, where they provide consult care to older patients that may still receive their care at a children’s hospital. There are also nonacademic hospitals that primarily staff med-peds hospitalists, where they can provide the full spectrum of care from the newborn nursery to the inpatient pediatric and adult wards.
Hospital medicine is a young field that is constantly changing with new and developing roles for hospitalists from a wide variety of backgrounds. Stick around to see which “-ist” will come next in HM.
Dr. Hall is a med-peds hospitalist and assistant professor at the University of Kentucky, Lexington. Dr. Sanyal-Dey is an academic hospitalist at Zuckerberg San Francisco General Hospital and Trauma Center and the University of California, San Francisco, where she is the director of clinical operations, and director of the faculty inpatient service. Dr. Chang is associate professor and interprofessional education thread director (MD curriculum) at Washington University, St. Louis. Dr. Kwan is a hospitalist at the Veterans Affairs San Diego Healthcare System and associate professor at the University of California, San Diego. He is the chair of SHM’s Physicians in Training committee. Dr. Seymour is family medicine hospitalist education director at the University of Massachusetts Memorial Medical Center, Worcester, and associate professor at the University of Massachusetts.
References
1. Wachter RM, Goldman L. The Emerging Role of “Hospitalists” in the American Health Care System. N Engl J Med. 1996;335(7):514-7.
2. 2018 State of Hospital Medicine Report. Philadelphia: Society of Hospital Medicine, 2018.
3. Weaver SP, Hill J. Academician Attitudes and Beliefs Regarding the Use of Hospitalists: A CERA Study. Fam Med. 2015;47(5):357-61.
4. Teno JM et al. Temporal Trends in the Numbers of Skilled Nursing Facility Specialists From 2007 Through 2014. JAMA Intern Med. 2017;177(9):1376-8.
TNBC: Adding capecitabine boosts disease-free survival in prospective trial
SAN ANTONIO – For patients with early triple-negative breast cancer (TNBC), adding capecitabine to standard adjuvant chemotherapy may extend disease-free survival (DFS), based results of a phase 3 trial conducted in China.
Although capecitabine boosted DFS by approximately 6%, overall survival remained unchanged, reported lead author Junjie Li, MD, of Fudan University in Shanghai, China.
In a presentation at the San Antonio Breast Cancer Symposium, Dr. Li explained that these findings provide much-needed support for the concomitant use of capecitabine in breast cancer.
“Capecitabine has been proven [effective] in advanced breast cancer, while in earlier stage disease, available data are still inconsistent,” Dr. Li said.
To help resolve some of this uncertainty, Dr. Li and colleagues conducted an open-label, phase 3 trial involving 585 patients with TNBC who were either node positive or negative and had tumors more than 1 cm in diameter. Patients were randomized in a 1:1 ratio to receive either standard therapy with three cycles of docetaxel followed by three cycles of cyclophosphamide, epirubicin, and fluorouracil; or three cycles of capecitabine and docetaxel followed by three cycles of cyclophosphamide, epirubicin, and capecitabine.
After a median follow-up of 67 months, 288 patients had received the capecitabine regimen and 273 had undergone standard therapy. The 5-year DFS was significantly longer in the capecitabine group, at 86.26%, than the control group, at 80.23% (hazard ratio, 0.66; P = .038). This benefit extended across subgroups, Dr. Li noted. While a slight numerical difference in overall survival was seen between capecitabine and control arms, this was not statistically significant (93.27% vs. 90.55%).
The investigators described safety profiles between treatment arms as “generally comparable.” In the capecitabine group, more than one-third of patients (38.89%) had dose reductions, while 8.42% reported grade 3-4 hand-foot syndrome. Grade 3-4 toxicities were more common in the capecitabine group, including neutropenia (45.79% vs. 41.32%) and febrile neutropenia (16.5% vs. 15.97%).
“Capecitabine concomitant use with docetaxel and epirubicin may significantly improve disease-free survival in early triple-negative patients,” Dr. Li concluded. He suggested that the study regimen be viewed as an alternative adjuvant regimen, as it provided clinically meaningful improvements in survival while maintaining tolerability.
Conference attendee Mohamed El-Naghy, MD, PhD, a practicing oncologist in Oneida, N.Y., expressed concern for one aspect of the trial methodology during the question and answer session following Dr. Li’s presentation: Although all patients in the trial were considered triple negative, 10 patients had estrogen receptor/progesterone receptor (ER/PR) expression ranging from 1 to 9%, which conflicts with the definition of TNBCr provided by current guidelines, according to Dr. Li.
“It is always important to have common language,” Dr. El-Naghy said. “And if you define [TNBC by] ER/PR negativity, and still you have 10% [expression], that is unacceptable. The whole point is, I looked at your numbers – 10 patients in the whole study – maybe you say they are triple negative, but these [patients] are contamination to your sample.”
Responding to this comment, Dr. Li said that the data from these patients had a minimal impact on the study results.
“We did another analysis to exclude these 10 patients, but still the result is quite similar, because they are just 10 patients – 5 in each group,” Dr. Li said.
Invited discussant Priyanka Sharma, MD, of the University of Kansas Medical Center, Kansas City, offered some additional viewpoints on the trial.
“A large proportion of patients had non–grade III disease, and one could say that the dose of docetaxel monotherapy in the control arm was not the traditional 100 mg/m2, and that could have negatively impacted the outcomes in the control arm,” Dr. Sharma said. “In addition, the trial is representative of patients only from China, and whether these findings would hold true in other ethnic and racial groups is not known. Furthermore, there seemed to be limited efficacy in patients with N0 disease, which comprised two-thirds of the trial.”
Despite these potential limitations, based on the trial results and other evidence, Dr. Sharma suggested that adding capecitabine to standard systemic therapy may be considered a viable option for patients with early TNBC.
The investigators reported no disclosures.
SOURCE: Li J et al. SABCS 2019, Abstract GS1-08.
SAN ANTONIO – For patients with early triple-negative breast cancer (TNBC), adding capecitabine to standard adjuvant chemotherapy may extend disease-free survival (DFS), based results of a phase 3 trial conducted in China.
Although capecitabine boosted DFS by approximately 6%, overall survival remained unchanged, reported lead author Junjie Li, MD, of Fudan University in Shanghai, China.
In a presentation at the San Antonio Breast Cancer Symposium, Dr. Li explained that these findings provide much-needed support for the concomitant use of capecitabine in breast cancer.
“Capecitabine has been proven [effective] in advanced breast cancer, while in earlier stage disease, available data are still inconsistent,” Dr. Li said.
To help resolve some of this uncertainty, Dr. Li and colleagues conducted an open-label, phase 3 trial involving 585 patients with TNBC who were either node positive or negative and had tumors more than 1 cm in diameter. Patients were randomized in a 1:1 ratio to receive either standard therapy with three cycles of docetaxel followed by three cycles of cyclophosphamide, epirubicin, and fluorouracil; or three cycles of capecitabine and docetaxel followed by three cycles of cyclophosphamide, epirubicin, and capecitabine.
After a median follow-up of 67 months, 288 patients had received the capecitabine regimen and 273 had undergone standard therapy. The 5-year DFS was significantly longer in the capecitabine group, at 86.26%, than the control group, at 80.23% (hazard ratio, 0.66; P = .038). This benefit extended across subgroups, Dr. Li noted. While a slight numerical difference in overall survival was seen between capecitabine and control arms, this was not statistically significant (93.27% vs. 90.55%).
The investigators described safety profiles between treatment arms as “generally comparable.” In the capecitabine group, more than one-third of patients (38.89%) had dose reductions, while 8.42% reported grade 3-4 hand-foot syndrome. Grade 3-4 toxicities were more common in the capecitabine group, including neutropenia (45.79% vs. 41.32%) and febrile neutropenia (16.5% vs. 15.97%).
“Capecitabine concomitant use with docetaxel and epirubicin may significantly improve disease-free survival in early triple-negative patients,” Dr. Li concluded. He suggested that the study regimen be viewed as an alternative adjuvant regimen, as it provided clinically meaningful improvements in survival while maintaining tolerability.
Conference attendee Mohamed El-Naghy, MD, PhD, a practicing oncologist in Oneida, N.Y., expressed concern for one aspect of the trial methodology during the question and answer session following Dr. Li’s presentation: Although all patients in the trial were considered triple negative, 10 patients had estrogen receptor/progesterone receptor (ER/PR) expression ranging from 1 to 9%, which conflicts with the definition of TNBCr provided by current guidelines, according to Dr. Li.
“It is always important to have common language,” Dr. El-Naghy said. “And if you define [TNBC by] ER/PR negativity, and still you have 10% [expression], that is unacceptable. The whole point is, I looked at your numbers – 10 patients in the whole study – maybe you say they are triple negative, but these [patients] are contamination to your sample.”
Responding to this comment, Dr. Li said that the data from these patients had a minimal impact on the study results.
“We did another analysis to exclude these 10 patients, but still the result is quite similar, because they are just 10 patients – 5 in each group,” Dr. Li said.
Invited discussant Priyanka Sharma, MD, of the University of Kansas Medical Center, Kansas City, offered some additional viewpoints on the trial.
“A large proportion of patients had non–grade III disease, and one could say that the dose of docetaxel monotherapy in the control arm was not the traditional 100 mg/m2, and that could have negatively impacted the outcomes in the control arm,” Dr. Sharma said. “In addition, the trial is representative of patients only from China, and whether these findings would hold true in other ethnic and racial groups is not known. Furthermore, there seemed to be limited efficacy in patients with N0 disease, which comprised two-thirds of the trial.”
Despite these potential limitations, based on the trial results and other evidence, Dr. Sharma suggested that adding capecitabine to standard systemic therapy may be considered a viable option for patients with early TNBC.
The investigators reported no disclosures.
SOURCE: Li J et al. SABCS 2019, Abstract GS1-08.
SAN ANTONIO – For patients with early triple-negative breast cancer (TNBC), adding capecitabine to standard adjuvant chemotherapy may extend disease-free survival (DFS), based results of a phase 3 trial conducted in China.
Although capecitabine boosted DFS by approximately 6%, overall survival remained unchanged, reported lead author Junjie Li, MD, of Fudan University in Shanghai, China.
In a presentation at the San Antonio Breast Cancer Symposium, Dr. Li explained that these findings provide much-needed support for the concomitant use of capecitabine in breast cancer.
“Capecitabine has been proven [effective] in advanced breast cancer, while in earlier stage disease, available data are still inconsistent,” Dr. Li said.
To help resolve some of this uncertainty, Dr. Li and colleagues conducted an open-label, phase 3 trial involving 585 patients with TNBC who were either node positive or negative and had tumors more than 1 cm in diameter. Patients were randomized in a 1:1 ratio to receive either standard therapy with three cycles of docetaxel followed by three cycles of cyclophosphamide, epirubicin, and fluorouracil; or three cycles of capecitabine and docetaxel followed by three cycles of cyclophosphamide, epirubicin, and capecitabine.
After a median follow-up of 67 months, 288 patients had received the capecitabine regimen and 273 had undergone standard therapy. The 5-year DFS was significantly longer in the capecitabine group, at 86.26%, than the control group, at 80.23% (hazard ratio, 0.66; P = .038). This benefit extended across subgroups, Dr. Li noted. While a slight numerical difference in overall survival was seen between capecitabine and control arms, this was not statistically significant (93.27% vs. 90.55%).
The investigators described safety profiles between treatment arms as “generally comparable.” In the capecitabine group, more than one-third of patients (38.89%) had dose reductions, while 8.42% reported grade 3-4 hand-foot syndrome. Grade 3-4 toxicities were more common in the capecitabine group, including neutropenia (45.79% vs. 41.32%) and febrile neutropenia (16.5% vs. 15.97%).
“Capecitabine concomitant use with docetaxel and epirubicin may significantly improve disease-free survival in early triple-negative patients,” Dr. Li concluded. He suggested that the study regimen be viewed as an alternative adjuvant regimen, as it provided clinically meaningful improvements in survival while maintaining tolerability.
Conference attendee Mohamed El-Naghy, MD, PhD, a practicing oncologist in Oneida, N.Y., expressed concern for one aspect of the trial methodology during the question and answer session following Dr. Li’s presentation: Although all patients in the trial were considered triple negative, 10 patients had estrogen receptor/progesterone receptor (ER/PR) expression ranging from 1 to 9%, which conflicts with the definition of TNBCr provided by current guidelines, according to Dr. Li.
“It is always important to have common language,” Dr. El-Naghy said. “And if you define [TNBC by] ER/PR negativity, and still you have 10% [expression], that is unacceptable. The whole point is, I looked at your numbers – 10 patients in the whole study – maybe you say they are triple negative, but these [patients] are contamination to your sample.”
Responding to this comment, Dr. Li said that the data from these patients had a minimal impact on the study results.
“We did another analysis to exclude these 10 patients, but still the result is quite similar, because they are just 10 patients – 5 in each group,” Dr. Li said.
Invited discussant Priyanka Sharma, MD, of the University of Kansas Medical Center, Kansas City, offered some additional viewpoints on the trial.
“A large proportion of patients had non–grade III disease, and one could say that the dose of docetaxel monotherapy in the control arm was not the traditional 100 mg/m2, and that could have negatively impacted the outcomes in the control arm,” Dr. Sharma said. “In addition, the trial is representative of patients only from China, and whether these findings would hold true in other ethnic and racial groups is not known. Furthermore, there seemed to be limited efficacy in patients with N0 disease, which comprised two-thirds of the trial.”
Despite these potential limitations, based on the trial results and other evidence, Dr. Sharma suggested that adding capecitabine to standard systemic therapy may be considered a viable option for patients with early TNBC.
The investigators reported no disclosures.
SOURCE: Li J et al. SABCS 2019, Abstract GS1-08.
REPORTING FROM SABCS 2019
2020 open enrollment: HealthCare.gov heats up in week 6
HealthCare.gov just had its busiest week of the 2020 open enrollment season, but the plan count for all consumers continues to run below last year’s total through 6 weeks, according to the Centers for Medicare & Medicaid Services.
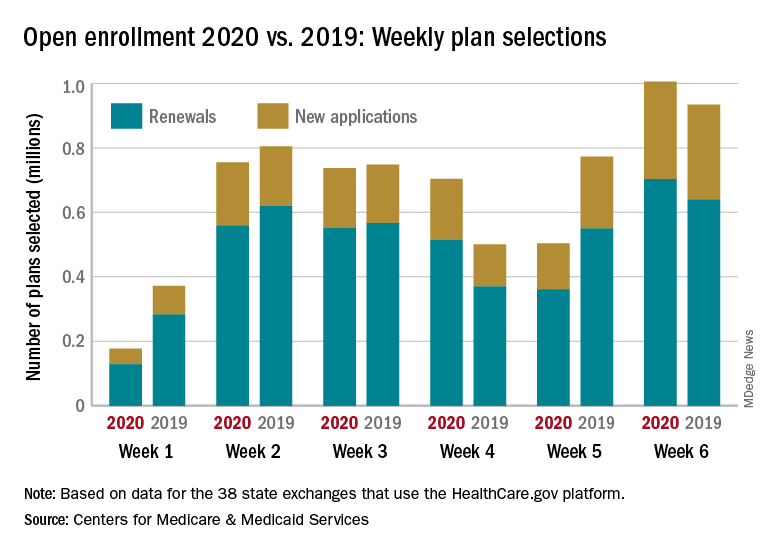
Just over 1 million plans – 302,000 new consumers and 703,000 renewals – were selected during the week of Dec. 1-7, eclipsing the season high of 755,000 from week 2 of open enrollment for the 38 states using the HealthCare.gov platform for the 2020 coverage year, CMS reported Dec. 12.
That brings the cumulative count for the 2020 open enrollment to almost 3.9 million plans through 6 weeks, compared with over 4.1 million selections last year for 2019 coverage, CMS said. If recent history is any indication, though, the cumulative total for 2020 can be expected to approximately double in the final week, as has occurred in the previous 2 years.
Last year, the cumulative total went from 4.1 million after 6 weeks to a final tally of 8.4 million after 7 weeks, and in 2017 it rose from 4.7 million through 6 weeks to a season-ending 8.8 million. Comparisons with seasons before that are not as relevant, because the open-enrollment period was about twice as long, extending through the end of January.
The 2020 open enrollment started Nov. 1 and will end Dec. 15, and week 7 actually ends Dec. 14, so there will be a 1-day week 8 after that.
HealthCare.gov just had its busiest week of the 2020 open enrollment season, but the plan count for all consumers continues to run below last year’s total through 6 weeks, according to the Centers for Medicare & Medicaid Services.

Just over 1 million plans – 302,000 new consumers and 703,000 renewals – were selected during the week of Dec. 1-7, eclipsing the season high of 755,000 from week 2 of open enrollment for the 38 states using the HealthCare.gov platform for the 2020 coverage year, CMS reported Dec. 12.
That brings the cumulative count for the 2020 open enrollment to almost 3.9 million plans through 6 weeks, compared with over 4.1 million selections last year for 2019 coverage, CMS said. If recent history is any indication, though, the cumulative total for 2020 can be expected to approximately double in the final week, as has occurred in the previous 2 years.
Last year, the cumulative total went from 4.1 million after 6 weeks to a final tally of 8.4 million after 7 weeks, and in 2017 it rose from 4.7 million through 6 weeks to a season-ending 8.8 million. Comparisons with seasons before that are not as relevant, because the open-enrollment period was about twice as long, extending through the end of January.
The 2020 open enrollment started Nov. 1 and will end Dec. 15, and week 7 actually ends Dec. 14, so there will be a 1-day week 8 after that.
HealthCare.gov just had its busiest week of the 2020 open enrollment season, but the plan count for all consumers continues to run below last year’s total through 6 weeks, according to the Centers for Medicare & Medicaid Services.

Just over 1 million plans – 302,000 new consumers and 703,000 renewals – were selected during the week of Dec. 1-7, eclipsing the season high of 755,000 from week 2 of open enrollment for the 38 states using the HealthCare.gov platform for the 2020 coverage year, CMS reported Dec. 12.
That brings the cumulative count for the 2020 open enrollment to almost 3.9 million plans through 6 weeks, compared with over 4.1 million selections last year for 2019 coverage, CMS said. If recent history is any indication, though, the cumulative total for 2020 can be expected to approximately double in the final week, as has occurred in the previous 2 years.
Last year, the cumulative total went from 4.1 million after 6 weeks to a final tally of 8.4 million after 7 weeks, and in 2017 it rose from 4.7 million through 6 weeks to a season-ending 8.8 million. Comparisons with seasons before that are not as relevant, because the open-enrollment period was about twice as long, extending through the end of January.
The 2020 open enrollment started Nov. 1 and will end Dec. 15, and week 7 actually ends Dec. 14, so there will be a 1-day week 8 after that.
Becoming the paradigm for clinical trial enrollment
The previous issue of The Sarcoma Journal focused on findings from numerous clinical trials in sarcomas of various histologies presented at ASCO’s annual meeting. This issue features a study on enrollment issues that surround clinical trials in sarcoma and sheds light on patient perceptions on clinical trial enrollment.
Clinical trials and their investigators are frequently impacted by enrollment issues, such as the limited number of eligible patients and the wide variations in time it can take to reach complete enrollment. For example, the phase 3 ANNOUNCE trial of olaratumab in soft tissue sarcoma completed its accrual of 509 patients in a record 10 months, while the trial of temozolomide by the European Pediatric Soft Tissue Sarcoma Study Group took 6 years to enroll 120 patients. Recruitment difficulties may even hamper the investigators’ and sponsors’ ability to bring a trial to a meaningful conclusion.
An interesting finding from the study published in this issue is the correlation between knowledge about trials and the positive attitude towards participating in them. People who had participated in clinical trials had higher levels of knowledge and developed more favorable attitudes towards clinical trials. One of the goals of the Sarcoma Foundation of America (curesarcoma.org) is to increase awareness of the numbers and types of ongoing clinical trials in sarcoma, benefitting patients and investigators alike. The SFA operates the Clinical Trial Navigating Service, which offers patients, caregivers, and health care professionals up-to-date information about sarcoma clinical trials throughout the United States and Canada. The service, provided in collaboration with EmergingMed, helps patients search for clinical trial options that match their specific diagnosis and treatment history.
The paper published in this issue suggests that, through patient education and careful trial design, sarcoma could become a paradigm for trial enrollment in other therapeutic areas. Together—as physicians, investigators, patients, trial sponsors, and anyone interested in curing sarcoma—we may be able to accomplish this. It’s certainly worth a try.
William D. Tap, MD
Editor-in-Chief
The previous issue of The Sarcoma Journal focused on findings from numerous clinical trials in sarcomas of various histologies presented at ASCO’s annual meeting. This issue features a study on enrollment issues that surround clinical trials in sarcoma and sheds light on patient perceptions on clinical trial enrollment.
Clinical trials and their investigators are frequently impacted by enrollment issues, such as the limited number of eligible patients and the wide variations in time it can take to reach complete enrollment. For example, the phase 3 ANNOUNCE trial of olaratumab in soft tissue sarcoma completed its accrual of 509 patients in a record 10 months, while the trial of temozolomide by the European Pediatric Soft Tissue Sarcoma Study Group took 6 years to enroll 120 patients. Recruitment difficulties may even hamper the investigators’ and sponsors’ ability to bring a trial to a meaningful conclusion.
An interesting finding from the study published in this issue is the correlation between knowledge about trials and the positive attitude towards participating in them. People who had participated in clinical trials had higher levels of knowledge and developed more favorable attitudes towards clinical trials. One of the goals of the Sarcoma Foundation of America (curesarcoma.org) is to increase awareness of the numbers and types of ongoing clinical trials in sarcoma, benefitting patients and investigators alike. The SFA operates the Clinical Trial Navigating Service, which offers patients, caregivers, and health care professionals up-to-date information about sarcoma clinical trials throughout the United States and Canada. The service, provided in collaboration with EmergingMed, helps patients search for clinical trial options that match their specific diagnosis and treatment history.
The paper published in this issue suggests that, through patient education and careful trial design, sarcoma could become a paradigm for trial enrollment in other therapeutic areas. Together—as physicians, investigators, patients, trial sponsors, and anyone interested in curing sarcoma—we may be able to accomplish this. It’s certainly worth a try.
William D. Tap, MD
Editor-in-Chief
The previous issue of The Sarcoma Journal focused on findings from numerous clinical trials in sarcomas of various histologies presented at ASCO’s annual meeting. This issue features a study on enrollment issues that surround clinical trials in sarcoma and sheds light on patient perceptions on clinical trial enrollment.
Clinical trials and their investigators are frequently impacted by enrollment issues, such as the limited number of eligible patients and the wide variations in time it can take to reach complete enrollment. For example, the phase 3 ANNOUNCE trial of olaratumab in soft tissue sarcoma completed its accrual of 509 patients in a record 10 months, while the trial of temozolomide by the European Pediatric Soft Tissue Sarcoma Study Group took 6 years to enroll 120 patients. Recruitment difficulties may even hamper the investigators’ and sponsors’ ability to bring a trial to a meaningful conclusion.
An interesting finding from the study published in this issue is the correlation between knowledge about trials and the positive attitude towards participating in them. People who had participated in clinical trials had higher levels of knowledge and developed more favorable attitudes towards clinical trials. One of the goals of the Sarcoma Foundation of America (curesarcoma.org) is to increase awareness of the numbers and types of ongoing clinical trials in sarcoma, benefitting patients and investigators alike. The SFA operates the Clinical Trial Navigating Service, which offers patients, caregivers, and health care professionals up-to-date information about sarcoma clinical trials throughout the United States and Canada. The service, provided in collaboration with EmergingMed, helps patients search for clinical trial options that match their specific diagnosis and treatment history.
The paper published in this issue suggests that, through patient education and careful trial design, sarcoma could become a paradigm for trial enrollment in other therapeutic areas. Together—as physicians, investigators, patients, trial sponsors, and anyone interested in curing sarcoma—we may be able to accomplish this. It’s certainly worth a try.
William D. Tap, MD
Editor-in-Chief
Pembrolizumab plus chemo boosts pCR rate in TNBC
SAN ANTONIO – Adding pembrolizumab to chemotherapy in the neoadjuvant setting increased the likelihood that women with stage III or early node-positive triple-negative breast cancer (TNBC) would have a pathologic complete response and sustained clinical benefit, results of the phase 3 KEYNOTE-522 study showed.
Among 602 patients evaluable in a definitive pathological complete response (pCR) analysis, the pCR rate was 64.8% for those treated with chemotherapy plus pembrolizumab (Keytruda), compared with 51.2% for patients treated with chemotherapy plus placebo, reported Peter Schmid, MD, PhD, from Barts Cancer Institute in London.
“The addition of neoadjuvant pembrolizumab to chemotherapy provided a significant increase in the path CR rate in all patients, but also a larger magnitude of path CR benefit versus chemotherapy alone in patients with higher-risk disease, such as stage III disease or node-positive early triple-negative breast cancer,” he said at the annual San Antonio Breast Cancer Symposium.
The overall pCR results were originally reported at the 2019 annual meeting of the European Society for Medical Oncology. At SABCS 2019, he reported pCR results for specific subgroups in KEYNOTE-522.
Investigators enrolled patients aged 18 years or older with newly diagnosed TNBC of either stage T1cN1-2, or T2-4N0-2 and an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients also had to have at least two separate tumor cores from the primary tumor for assessment of programmed death–ligand 1 (PD-L1).
After stratification for nodal status, tumor size, and carboplatin schedule (once weekly or every 3 weeks), patients were randomized to receive either pembrolizumab 200 mg every 3 weeks or placebo plus neoadjuvant carboplatin and paclitaxel for four 3-week cycles, followed by four cycles of chemotherapy with either doxorubicin or epirubicin plus cyclophosphamide (AC or EC). Patients went on to surgery, then received adjuvant therapy for nine cycles with either pembrolizumab at the neoadjuvant dose and schedule or placebo.
At the first preplanned interim analysis for event-free survival (EFS) based on 1,174 patients followed for a median of 15.5 months, events had occurred in 7.4% of patients on pembro/chemo, compared with 11.8% on placebo/chemo, but this difference did not meet the prespecified P value boundary of .000051 for significance, Dr. Schmid acknowledged.
When the investigators looked at pCR by disease stage, however, they saw the following benefits across all stages in the study:
- Stage IIA: 73.1% with pembrolizumab versus 62.1% with placebo, difference 11%.
- Stage IIB: 56.2% versus 48.4%, difference 7.8%.
- Stage IIIA: 66.7% versus 42.1%, difference 24.6%.
- Stage IIIB: 48.6% versus 23.1%, difference 25.6%.
The greatest benefit for the addition of pembrolizumab to chemotherapy appeared to be in the higher disease stages, Dr. Schmid said.
There was also a benefit from pembrolizumab for patients with both node-negative disease (pCR, 64.9% vs. 58.6% in the placebo arm) and node-positive disease (64.8% vs. 44.1%, respectively).
pCR rates were also superior with pembrolizumab versus placebo in patients who were PD-L1 negative, defined as a combined positive score (CPS) less than 1 (45.3% vs. 30.3%), as well as PD-L1–positive patients at each of three cutoff values: CPS 1 or greater (68.9% vs. 54.9%), CPS 10 or greater (77.9% vs. 59.8%), and CPS 20 or greater (81.7% vs. 62.5%).
Interestingly, adding pembrolizumab boosted pCR rates both in patients with exposure to a full planned course of chemotherapy (69.7% vs. 55.3% with placebo) and in those who received less than the full course (51.1% vs. 35.7%).
The most common immune-mediated adverse events with the largest between-group differences involved the thyroid, including hypothyroidism (in 14.9% of patients on pembrolizumab and 5.7% of those on placebo), hyperthyroidism (5.1% vs. 1.8%), and thyroiditis (1.7% vs. 1.0%).
“Immune-mediated adverse events are consistent with the known profiles of each regimen, and there’s no new safety signal, no new safety concern at this point in time,” Dr. Schmid said.
Further follow-up will be needed to determine EFS benefit and long-term safety. Investigators plan to perform additional biomarker analyses, including tumor-infiltrating lymphocytes and BRCA, he added.
“Will the KEYNOTE-522 regimen be the new standard of care if approved?” asked invited discussant Kevin Kalinsky, MD, MS, from Columbia University Irving Medical Center in New York. “These are exciting data, both in pCR and early event-free survival. But there’s a risk: a risk of overtreatment, as well as potentially [serious] toxicity in patients with curable disease.”
“The take-home is that this regimen will likely be practice changing in some patients, and with the absence of having a predictor, the benefit may outweigh the risk most in patients with high clinical risk,” he added.
The study was funded by Merck Sharp & Dohme. Dr. Schmid reported advising/consulting for and receiving honoraria from Merck. Dr. Kalinsky disclosed has disclosed that he receives salary from Array Biopharma, has received fees from various companies (not including Merck), and has contracted research with multiple companies, not including Merck.
SOURCE: Schmid P et al. SABCS 2019, Abstract GS3-03.
SAN ANTONIO – Adding pembrolizumab to chemotherapy in the neoadjuvant setting increased the likelihood that women with stage III or early node-positive triple-negative breast cancer (TNBC) would have a pathologic complete response and sustained clinical benefit, results of the phase 3 KEYNOTE-522 study showed.
Among 602 patients evaluable in a definitive pathological complete response (pCR) analysis, the pCR rate was 64.8% for those treated with chemotherapy plus pembrolizumab (Keytruda), compared with 51.2% for patients treated with chemotherapy plus placebo, reported Peter Schmid, MD, PhD, from Barts Cancer Institute in London.
“The addition of neoadjuvant pembrolizumab to chemotherapy provided a significant increase in the path CR rate in all patients, but also a larger magnitude of path CR benefit versus chemotherapy alone in patients with higher-risk disease, such as stage III disease or node-positive early triple-negative breast cancer,” he said at the annual San Antonio Breast Cancer Symposium.
The overall pCR results were originally reported at the 2019 annual meeting of the European Society for Medical Oncology. At SABCS 2019, he reported pCR results for specific subgroups in KEYNOTE-522.
Investigators enrolled patients aged 18 years or older with newly diagnosed TNBC of either stage T1cN1-2, or T2-4N0-2 and an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients also had to have at least two separate tumor cores from the primary tumor for assessment of programmed death–ligand 1 (PD-L1).
After stratification for nodal status, tumor size, and carboplatin schedule (once weekly or every 3 weeks), patients were randomized to receive either pembrolizumab 200 mg every 3 weeks or placebo plus neoadjuvant carboplatin and paclitaxel for four 3-week cycles, followed by four cycles of chemotherapy with either doxorubicin or epirubicin plus cyclophosphamide (AC or EC). Patients went on to surgery, then received adjuvant therapy for nine cycles with either pembrolizumab at the neoadjuvant dose and schedule or placebo.
At the first preplanned interim analysis for event-free survival (EFS) based on 1,174 patients followed for a median of 15.5 months, events had occurred in 7.4% of patients on pembro/chemo, compared with 11.8% on placebo/chemo, but this difference did not meet the prespecified P value boundary of .000051 for significance, Dr. Schmid acknowledged.
When the investigators looked at pCR by disease stage, however, they saw the following benefits across all stages in the study:
- Stage IIA: 73.1% with pembrolizumab versus 62.1% with placebo, difference 11%.
- Stage IIB: 56.2% versus 48.4%, difference 7.8%.
- Stage IIIA: 66.7% versus 42.1%, difference 24.6%.
- Stage IIIB: 48.6% versus 23.1%, difference 25.6%.
The greatest benefit for the addition of pembrolizumab to chemotherapy appeared to be in the higher disease stages, Dr. Schmid said.
There was also a benefit from pembrolizumab for patients with both node-negative disease (pCR, 64.9% vs. 58.6% in the placebo arm) and node-positive disease (64.8% vs. 44.1%, respectively).
pCR rates were also superior with pembrolizumab versus placebo in patients who were PD-L1 negative, defined as a combined positive score (CPS) less than 1 (45.3% vs. 30.3%), as well as PD-L1–positive patients at each of three cutoff values: CPS 1 or greater (68.9% vs. 54.9%), CPS 10 or greater (77.9% vs. 59.8%), and CPS 20 or greater (81.7% vs. 62.5%).
Interestingly, adding pembrolizumab boosted pCR rates both in patients with exposure to a full planned course of chemotherapy (69.7% vs. 55.3% with placebo) and in those who received less than the full course (51.1% vs. 35.7%).
The most common immune-mediated adverse events with the largest between-group differences involved the thyroid, including hypothyroidism (in 14.9% of patients on pembrolizumab and 5.7% of those on placebo), hyperthyroidism (5.1% vs. 1.8%), and thyroiditis (1.7% vs. 1.0%).
“Immune-mediated adverse events are consistent with the known profiles of each regimen, and there’s no new safety signal, no new safety concern at this point in time,” Dr. Schmid said.
Further follow-up will be needed to determine EFS benefit and long-term safety. Investigators plan to perform additional biomarker analyses, including tumor-infiltrating lymphocytes and BRCA, he added.
“Will the KEYNOTE-522 regimen be the new standard of care if approved?” asked invited discussant Kevin Kalinsky, MD, MS, from Columbia University Irving Medical Center in New York. “These are exciting data, both in pCR and early event-free survival. But there’s a risk: a risk of overtreatment, as well as potentially [serious] toxicity in patients with curable disease.”
“The take-home is that this regimen will likely be practice changing in some patients, and with the absence of having a predictor, the benefit may outweigh the risk most in patients with high clinical risk,” he added.
The study was funded by Merck Sharp & Dohme. Dr. Schmid reported advising/consulting for and receiving honoraria from Merck. Dr. Kalinsky disclosed has disclosed that he receives salary from Array Biopharma, has received fees from various companies (not including Merck), and has contracted research with multiple companies, not including Merck.
SOURCE: Schmid P et al. SABCS 2019, Abstract GS3-03.
SAN ANTONIO – Adding pembrolizumab to chemotherapy in the neoadjuvant setting increased the likelihood that women with stage III or early node-positive triple-negative breast cancer (TNBC) would have a pathologic complete response and sustained clinical benefit, results of the phase 3 KEYNOTE-522 study showed.
Among 602 patients evaluable in a definitive pathological complete response (pCR) analysis, the pCR rate was 64.8% for those treated with chemotherapy plus pembrolizumab (Keytruda), compared with 51.2% for patients treated with chemotherapy plus placebo, reported Peter Schmid, MD, PhD, from Barts Cancer Institute in London.
“The addition of neoadjuvant pembrolizumab to chemotherapy provided a significant increase in the path CR rate in all patients, but also a larger magnitude of path CR benefit versus chemotherapy alone in patients with higher-risk disease, such as stage III disease or node-positive early triple-negative breast cancer,” he said at the annual San Antonio Breast Cancer Symposium.
The overall pCR results were originally reported at the 2019 annual meeting of the European Society for Medical Oncology. At SABCS 2019, he reported pCR results for specific subgroups in KEYNOTE-522.
Investigators enrolled patients aged 18 years or older with newly diagnosed TNBC of either stage T1cN1-2, or T2-4N0-2 and an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients also had to have at least two separate tumor cores from the primary tumor for assessment of programmed death–ligand 1 (PD-L1).
After stratification for nodal status, tumor size, and carboplatin schedule (once weekly or every 3 weeks), patients were randomized to receive either pembrolizumab 200 mg every 3 weeks or placebo plus neoadjuvant carboplatin and paclitaxel for four 3-week cycles, followed by four cycles of chemotherapy with either doxorubicin or epirubicin plus cyclophosphamide (AC or EC). Patients went on to surgery, then received adjuvant therapy for nine cycles with either pembrolizumab at the neoadjuvant dose and schedule or placebo.
At the first preplanned interim analysis for event-free survival (EFS) based on 1,174 patients followed for a median of 15.5 months, events had occurred in 7.4% of patients on pembro/chemo, compared with 11.8% on placebo/chemo, but this difference did not meet the prespecified P value boundary of .000051 for significance, Dr. Schmid acknowledged.
When the investigators looked at pCR by disease stage, however, they saw the following benefits across all stages in the study:
- Stage IIA: 73.1% with pembrolizumab versus 62.1% with placebo, difference 11%.
- Stage IIB: 56.2% versus 48.4%, difference 7.8%.
- Stage IIIA: 66.7% versus 42.1%, difference 24.6%.
- Stage IIIB: 48.6% versus 23.1%, difference 25.6%.
The greatest benefit for the addition of pembrolizumab to chemotherapy appeared to be in the higher disease stages, Dr. Schmid said.
There was also a benefit from pembrolizumab for patients with both node-negative disease (pCR, 64.9% vs. 58.6% in the placebo arm) and node-positive disease (64.8% vs. 44.1%, respectively).
pCR rates were also superior with pembrolizumab versus placebo in patients who were PD-L1 negative, defined as a combined positive score (CPS) less than 1 (45.3% vs. 30.3%), as well as PD-L1–positive patients at each of three cutoff values: CPS 1 or greater (68.9% vs. 54.9%), CPS 10 or greater (77.9% vs. 59.8%), and CPS 20 or greater (81.7% vs. 62.5%).
Interestingly, adding pembrolizumab boosted pCR rates both in patients with exposure to a full planned course of chemotherapy (69.7% vs. 55.3% with placebo) and in those who received less than the full course (51.1% vs. 35.7%).
The most common immune-mediated adverse events with the largest between-group differences involved the thyroid, including hypothyroidism (in 14.9% of patients on pembrolizumab and 5.7% of those on placebo), hyperthyroidism (5.1% vs. 1.8%), and thyroiditis (1.7% vs. 1.0%).
“Immune-mediated adverse events are consistent with the known profiles of each regimen, and there’s no new safety signal, no new safety concern at this point in time,” Dr. Schmid said.
Further follow-up will be needed to determine EFS benefit and long-term safety. Investigators plan to perform additional biomarker analyses, including tumor-infiltrating lymphocytes and BRCA, he added.
“Will the KEYNOTE-522 regimen be the new standard of care if approved?” asked invited discussant Kevin Kalinsky, MD, MS, from Columbia University Irving Medical Center in New York. “These are exciting data, both in pCR and early event-free survival. But there’s a risk: a risk of overtreatment, as well as potentially [serious] toxicity in patients with curable disease.”
“The take-home is that this regimen will likely be practice changing in some patients, and with the absence of having a predictor, the benefit may outweigh the risk most in patients with high clinical risk,” he added.
The study was funded by Merck Sharp & Dohme. Dr. Schmid reported advising/consulting for and receiving honoraria from Merck. Dr. Kalinsky disclosed has disclosed that he receives salary from Array Biopharma, has received fees from various companies (not including Merck), and has contracted research with multiple companies, not including Merck.
SOURCE: Schmid P et al. SABCS 2019, Abstract GS3-03.
REPORTING FROM SABCS 2019
Postextubation laryngeal injury has lasting effects
(ALgI), and the injury is associated with worse breathing and speaking at 10 weeks, according to a study published in Critical Care Medicine (2019 Dec;47[12]:1669-1706). The researchers, led by Alexander Gelbard, MD, of Vanderbilt Medical Center, Nashville, Tenn., found that higher body mass index, diabetes, and larger endotracheal tube (ETT) size were all associated with heightened risk.
The investigators assert that comparatively scarce data are available about how patients fare after receiving mechanical ventilation, and how adverse effects might interfere with recovery and return to daily activity. The larynx is rarely examined after extubation, and laryngeal injury may initially appear to be minor. Restricted glottic mobility therefore tends to be diagnosed after discharge, leaving critical care specialists unaware of the long-term impact.
The findings of the study should be a wake-up call for the development of guidelines for recognition and management of laryngeal injuries, according to John Robert Gowardman, MD, of Royal Brisbane (Australia) and Women’s Hospital, who wrote an accompanying editorial (Crit Care Med, 2019 Dec;47[12]:1802-04).
In addition, findings that ETT size, diabetes, and BMI represent risk factors for injury should help identify patients at risk, and the “practice of ‘putting in the biggest ETT just in case’ needs to be balanced against the dangers of an undersized ETT ... we should ask, ‘can my patient be safely managed with a smaller ETT?’ ” wrote Dr. Gowardman.
The researchers followed 100 consecutive adult patients who were examined with nasolaryngoscopy following an intubation of greater than 12 hours at Vanderbilt University Medical Center. They recorded baseline comorbidities and other factors. Fifty seven patients had an ALgI, defined as having glottic mucosal ulceration/granulation or subglottic granulation tissue/stenosis at the time of endoscopy. Nineteen patients had granulation tissue, 48 had posterior glottic ulceration, and 8 had subglottic mucosal ulceration.
Ten weeks after extubation, all patients were contacted by phone and asked to answer the Voice Handicap Index (VHI)-10 and the Clinical Chronic Obstructive Pulmonary Disease Questionnaire (CCQ). The questioner did not know the results of the patient’s endoscopy. Patients with ALgI were heavier on average (mean difference, 14 kg; BMI difference, 3.8 kg/m2), were more likely to have type 2 diabetes (46% versus 21%), and had more severe illness (median Charlson Comorbidity Index, 3.00 versus 2.00).
Sixty-seven patients completed the 10-week questionnaires, including 40 patients with ALgI and 27 without ALgI. Injury was associated with reports of worse breathing (median CCQ, 1.05 versus 0.20; P less than .001), as well as worse patient-reported voice outcomes (median VHI, 2 versus 0; P = .005).
ETT size appeared to be an important factor, according to multivariate analyses. Use of a 7.0 ETT was associated with lower frequency of injury than 7.5 (adjusted odds ratio, 0.04; P = .004) and 8.0 (OR, 0.03; P = .004). There was no significant difference between the 7.5 and 8.0 sizes.
The presence of type 2 diabetes altered the risk associated with BMI (P = .003 for interaction). Among patients who did not have type 2 diabetes, ALgI went up as a function of increasing BMI. Still, injury risk was higher in the presence of type 2 diabetes across all BMI ranges.
The Vanderbilt Institute for Clinical and Translational Research funded the study. Dr. Gowardman has no relevant disclosures.
SOURCE: Shinn JR et al. Crit Care Med;2019 Dec;47(12):1669-1706 .
(ALgI), and the injury is associated with worse breathing and speaking at 10 weeks, according to a study published in Critical Care Medicine (2019 Dec;47[12]:1669-1706). The researchers, led by Alexander Gelbard, MD, of Vanderbilt Medical Center, Nashville, Tenn., found that higher body mass index, diabetes, and larger endotracheal tube (ETT) size were all associated with heightened risk.
The investigators assert that comparatively scarce data are available about how patients fare after receiving mechanical ventilation, and how adverse effects might interfere with recovery and return to daily activity. The larynx is rarely examined after extubation, and laryngeal injury may initially appear to be minor. Restricted glottic mobility therefore tends to be diagnosed after discharge, leaving critical care specialists unaware of the long-term impact.
The findings of the study should be a wake-up call for the development of guidelines for recognition and management of laryngeal injuries, according to John Robert Gowardman, MD, of Royal Brisbane (Australia) and Women’s Hospital, who wrote an accompanying editorial (Crit Care Med, 2019 Dec;47[12]:1802-04).
In addition, findings that ETT size, diabetes, and BMI represent risk factors for injury should help identify patients at risk, and the “practice of ‘putting in the biggest ETT just in case’ needs to be balanced against the dangers of an undersized ETT ... we should ask, ‘can my patient be safely managed with a smaller ETT?’ ” wrote Dr. Gowardman.
The researchers followed 100 consecutive adult patients who were examined with nasolaryngoscopy following an intubation of greater than 12 hours at Vanderbilt University Medical Center. They recorded baseline comorbidities and other factors. Fifty seven patients had an ALgI, defined as having glottic mucosal ulceration/granulation or subglottic granulation tissue/stenosis at the time of endoscopy. Nineteen patients had granulation tissue, 48 had posterior glottic ulceration, and 8 had subglottic mucosal ulceration.
Ten weeks after extubation, all patients were contacted by phone and asked to answer the Voice Handicap Index (VHI)-10 and the Clinical Chronic Obstructive Pulmonary Disease Questionnaire (CCQ). The questioner did not know the results of the patient’s endoscopy. Patients with ALgI were heavier on average (mean difference, 14 kg; BMI difference, 3.8 kg/m2), were more likely to have type 2 diabetes (46% versus 21%), and had more severe illness (median Charlson Comorbidity Index, 3.00 versus 2.00).
Sixty-seven patients completed the 10-week questionnaires, including 40 patients with ALgI and 27 without ALgI. Injury was associated with reports of worse breathing (median CCQ, 1.05 versus 0.20; P less than .001), as well as worse patient-reported voice outcomes (median VHI, 2 versus 0; P = .005).
ETT size appeared to be an important factor, according to multivariate analyses. Use of a 7.0 ETT was associated with lower frequency of injury than 7.5 (adjusted odds ratio, 0.04; P = .004) and 8.0 (OR, 0.03; P = .004). There was no significant difference between the 7.5 and 8.0 sizes.
The presence of type 2 diabetes altered the risk associated with BMI (P = .003 for interaction). Among patients who did not have type 2 diabetes, ALgI went up as a function of increasing BMI. Still, injury risk was higher in the presence of type 2 diabetes across all BMI ranges.
The Vanderbilt Institute for Clinical and Translational Research funded the study. Dr. Gowardman has no relevant disclosures.
SOURCE: Shinn JR et al. Crit Care Med;2019 Dec;47(12):1669-1706 .
(ALgI), and the injury is associated with worse breathing and speaking at 10 weeks, according to a study published in Critical Care Medicine (2019 Dec;47[12]:1669-1706). The researchers, led by Alexander Gelbard, MD, of Vanderbilt Medical Center, Nashville, Tenn., found that higher body mass index, diabetes, and larger endotracheal tube (ETT) size were all associated with heightened risk.
The investigators assert that comparatively scarce data are available about how patients fare after receiving mechanical ventilation, and how adverse effects might interfere with recovery and return to daily activity. The larynx is rarely examined after extubation, and laryngeal injury may initially appear to be minor. Restricted glottic mobility therefore tends to be diagnosed after discharge, leaving critical care specialists unaware of the long-term impact.
The findings of the study should be a wake-up call for the development of guidelines for recognition and management of laryngeal injuries, according to John Robert Gowardman, MD, of Royal Brisbane (Australia) and Women’s Hospital, who wrote an accompanying editorial (Crit Care Med, 2019 Dec;47[12]:1802-04).
In addition, findings that ETT size, diabetes, and BMI represent risk factors for injury should help identify patients at risk, and the “practice of ‘putting in the biggest ETT just in case’ needs to be balanced against the dangers of an undersized ETT ... we should ask, ‘can my patient be safely managed with a smaller ETT?’ ” wrote Dr. Gowardman.
The researchers followed 100 consecutive adult patients who were examined with nasolaryngoscopy following an intubation of greater than 12 hours at Vanderbilt University Medical Center. They recorded baseline comorbidities and other factors. Fifty seven patients had an ALgI, defined as having glottic mucosal ulceration/granulation or subglottic granulation tissue/stenosis at the time of endoscopy. Nineteen patients had granulation tissue, 48 had posterior glottic ulceration, and 8 had subglottic mucosal ulceration.
Ten weeks after extubation, all patients were contacted by phone and asked to answer the Voice Handicap Index (VHI)-10 and the Clinical Chronic Obstructive Pulmonary Disease Questionnaire (CCQ). The questioner did not know the results of the patient’s endoscopy. Patients with ALgI were heavier on average (mean difference, 14 kg; BMI difference, 3.8 kg/m2), were more likely to have type 2 diabetes (46% versus 21%), and had more severe illness (median Charlson Comorbidity Index, 3.00 versus 2.00).
Sixty-seven patients completed the 10-week questionnaires, including 40 patients with ALgI and 27 without ALgI. Injury was associated with reports of worse breathing (median CCQ, 1.05 versus 0.20; P less than .001), as well as worse patient-reported voice outcomes (median VHI, 2 versus 0; P = .005).
ETT size appeared to be an important factor, according to multivariate analyses. Use of a 7.0 ETT was associated with lower frequency of injury than 7.5 (adjusted odds ratio, 0.04; P = .004) and 8.0 (OR, 0.03; P = .004). There was no significant difference between the 7.5 and 8.0 sizes.
The presence of type 2 diabetes altered the risk associated with BMI (P = .003 for interaction). Among patients who did not have type 2 diabetes, ALgI went up as a function of increasing BMI. Still, injury risk was higher in the presence of type 2 diabetes across all BMI ranges.
The Vanderbilt Institute for Clinical and Translational Research funded the study. Dr. Gowardman has no relevant disclosures.
SOURCE: Shinn JR et al. Crit Care Med;2019 Dec;47(12):1669-1706 .
FROM CRITICAL CARE MEDICINE