User login
Review highlights shortage of data on elderly cancer patients
Phase 3 clinical trials for cancer are underreporting safety and efficacy data for elderly patients, according to a systematic review of 159 articles.
Roughly 40% of articles reporting efficacy data and 9% of articles reporting safety data had results stratified by age, Karlynn BrintzenhofeSzoc, PhD, of the University of Cincinnati, and colleagues noted in the Journal of Geriatric Oncology.
“Results of our systematic review suggest that there is inadequate reporting of treatment efficacy and adverse events as well as discrepancies as to how older age is defined, considered, and reported,” the investigators wrote. “This sparse and varied reporting critically limits the evidence base for treating older patients with cancer.”
This study was inspired by the American Society of Clinical Oncology, which turned a spotlight on the age-specific data shortage in 2015, when it published a statement that called for inclusion of more elderly patients in cancer trials (J Clin Oncol. 2015 Nov 10;33[32]:3826-33).
According to Tammy Hshieh, MD, a geriatrician at Dana-Farber Cancer Institute in Boston, data for elderly patients with cancer are needed more than ever.
“Cancer care has become, increasingly, a field where precision medicine is at its strongest,” Dr. Hshieh said in an interview. “[Oncologists] have a lot of data on patients that allow them to tailor their care to each individual patient’s profile, and so the fact that there is not a lot of evidence looking at toxicities and side effects for older patients makes it basically harder for oncologists to practice evidence-based medicine for this vulnerable but growing population.” This leads to poorer and more variable outcomes, Dr. Hshieh said. When data aren’t available, clinicians must rely on experience and recognize that patient age isn’t as simple as date of birth.
“Oncologists looking at older patients really have to trust their gestalt and their experience in determining how to provide the best care for their older patients,” she said. “They have to look at the chronological age of the patient and try to determine whether that actually matches more of what we’re saying is the physiological age of the patient and use that to guide their treatment.”
Study details
The study included phase 3 clinical trials of adult cancer patients that were conducted from mid-2016 through mid-2017. After identifying 929 manuscripts, the investigators removed duplicates and those that did not meet criteria. This left 159 articles published in 36 journals and covering 25 cancer types.
Of the 159 articles, 73.6% included age-specific medians (in addition to age means), and 47.2% had data stratified by age.
Efficacy was often reported (96.2%), but only 39.9% of articles specified age when describing effectiveness. Although most articles (84.9%) included safety data, only 8.9% had safety findings stratified by age.
In article discussion sections, age was mentioned infrequently in relation to treatment efficacy (13.8%) and rarely in relation to complications and adverse events (5.7%).Beyond underreporting of age-specific data, the investigators found that age categories themselves may be an area in need of improvement.“When outcomes pertaining to older adults were reported, the results were inconsistent as evidenced by the array of age distributions and varying categorization of ‘older adults,’” the investigators wrote. “There is a significant and timely need to design all clinical trials to include older adults and utilize a broad array of geriatric-specific outcomes.” Dr. Hshieh said these findings are concerning, but the study itself suggests the medical community is making efforts to correct the data shortage.“It was actually an important study, even though the results are a little discouraging,” Dr. Hshieh said. “What I’m hoping is that [these findings], combined with all the other literature that’s starting to come out about the need for more research in older patients with cancer, is going to be an impetus for us to do more research, and to be more open to treating older patients, and not to be afraid to confront this head on.”When asked about strategies for managing elderly patients, Dr. Hshieh first recommended the 2018 ASCO Guideline for Geriatric Oncology (J Clin Oncol. 2018 Aug 1;36[22]:2326-47).
“It’s very well written,” she said. “It is clear and user-friendly.”
Dr. Hshieh also offered some simple principles that may help guide clinical decision making.“I’m thinking of three things that an oncologist in the community would want to look at when they see an older patient and they’re trying to determine their treatment plan,” she said. “I would say [the oncologist] should look at [the patient’s] function; their psychosocial status, which includes mood and the support that they have in the community; and cognition.”
Dr. Hshieh and the study authors reported no conflicts of interest.
SOURCE: BrintzenhofeSzoc K et al. J Geriatr Oncol. 2020 Jan 10. pii: S1879-4068(19)30501-6.
Phase 3 clinical trials for cancer are underreporting safety and efficacy data for elderly patients, according to a systematic review of 159 articles.
Roughly 40% of articles reporting efficacy data and 9% of articles reporting safety data had results stratified by age, Karlynn BrintzenhofeSzoc, PhD, of the University of Cincinnati, and colleagues noted in the Journal of Geriatric Oncology.
“Results of our systematic review suggest that there is inadequate reporting of treatment efficacy and adverse events as well as discrepancies as to how older age is defined, considered, and reported,” the investigators wrote. “This sparse and varied reporting critically limits the evidence base for treating older patients with cancer.”
This study was inspired by the American Society of Clinical Oncology, which turned a spotlight on the age-specific data shortage in 2015, when it published a statement that called for inclusion of more elderly patients in cancer trials (J Clin Oncol. 2015 Nov 10;33[32]:3826-33).
According to Tammy Hshieh, MD, a geriatrician at Dana-Farber Cancer Institute in Boston, data for elderly patients with cancer are needed more than ever.
“Cancer care has become, increasingly, a field where precision medicine is at its strongest,” Dr. Hshieh said in an interview. “[Oncologists] have a lot of data on patients that allow them to tailor their care to each individual patient’s profile, and so the fact that there is not a lot of evidence looking at toxicities and side effects for older patients makes it basically harder for oncologists to practice evidence-based medicine for this vulnerable but growing population.” This leads to poorer and more variable outcomes, Dr. Hshieh said. When data aren’t available, clinicians must rely on experience and recognize that patient age isn’t as simple as date of birth.
“Oncologists looking at older patients really have to trust their gestalt and their experience in determining how to provide the best care for their older patients,” she said. “They have to look at the chronological age of the patient and try to determine whether that actually matches more of what we’re saying is the physiological age of the patient and use that to guide their treatment.”
Study details
The study included phase 3 clinical trials of adult cancer patients that were conducted from mid-2016 through mid-2017. After identifying 929 manuscripts, the investigators removed duplicates and those that did not meet criteria. This left 159 articles published in 36 journals and covering 25 cancer types.
Of the 159 articles, 73.6% included age-specific medians (in addition to age means), and 47.2% had data stratified by age.
Efficacy was often reported (96.2%), but only 39.9% of articles specified age when describing effectiveness. Although most articles (84.9%) included safety data, only 8.9% had safety findings stratified by age.
In article discussion sections, age was mentioned infrequently in relation to treatment efficacy (13.8%) and rarely in relation to complications and adverse events (5.7%).Beyond underreporting of age-specific data, the investigators found that age categories themselves may be an area in need of improvement.“When outcomes pertaining to older adults were reported, the results were inconsistent as evidenced by the array of age distributions and varying categorization of ‘older adults,’” the investigators wrote. “There is a significant and timely need to design all clinical trials to include older adults and utilize a broad array of geriatric-specific outcomes.” Dr. Hshieh said these findings are concerning, but the study itself suggests the medical community is making efforts to correct the data shortage.“It was actually an important study, even though the results are a little discouraging,” Dr. Hshieh said. “What I’m hoping is that [these findings], combined with all the other literature that’s starting to come out about the need for more research in older patients with cancer, is going to be an impetus for us to do more research, and to be more open to treating older patients, and not to be afraid to confront this head on.”When asked about strategies for managing elderly patients, Dr. Hshieh first recommended the 2018 ASCO Guideline for Geriatric Oncology (J Clin Oncol. 2018 Aug 1;36[22]:2326-47).
“It’s very well written,” she said. “It is clear and user-friendly.”
Dr. Hshieh also offered some simple principles that may help guide clinical decision making.“I’m thinking of three things that an oncologist in the community would want to look at when they see an older patient and they’re trying to determine their treatment plan,” she said. “I would say [the oncologist] should look at [the patient’s] function; their psychosocial status, which includes mood and the support that they have in the community; and cognition.”
Dr. Hshieh and the study authors reported no conflicts of interest.
SOURCE: BrintzenhofeSzoc K et al. J Geriatr Oncol. 2020 Jan 10. pii: S1879-4068(19)30501-6.
Phase 3 clinical trials for cancer are underreporting safety and efficacy data for elderly patients, according to a systematic review of 159 articles.
Roughly 40% of articles reporting efficacy data and 9% of articles reporting safety data had results stratified by age, Karlynn BrintzenhofeSzoc, PhD, of the University of Cincinnati, and colleagues noted in the Journal of Geriatric Oncology.
“Results of our systematic review suggest that there is inadequate reporting of treatment efficacy and adverse events as well as discrepancies as to how older age is defined, considered, and reported,” the investigators wrote. “This sparse and varied reporting critically limits the evidence base for treating older patients with cancer.”
This study was inspired by the American Society of Clinical Oncology, which turned a spotlight on the age-specific data shortage in 2015, when it published a statement that called for inclusion of more elderly patients in cancer trials (J Clin Oncol. 2015 Nov 10;33[32]:3826-33).
According to Tammy Hshieh, MD, a geriatrician at Dana-Farber Cancer Institute in Boston, data for elderly patients with cancer are needed more than ever.
“Cancer care has become, increasingly, a field where precision medicine is at its strongest,” Dr. Hshieh said in an interview. “[Oncologists] have a lot of data on patients that allow them to tailor their care to each individual patient’s profile, and so the fact that there is not a lot of evidence looking at toxicities and side effects for older patients makes it basically harder for oncologists to practice evidence-based medicine for this vulnerable but growing population.” This leads to poorer and more variable outcomes, Dr. Hshieh said. When data aren’t available, clinicians must rely on experience and recognize that patient age isn’t as simple as date of birth.
“Oncologists looking at older patients really have to trust their gestalt and their experience in determining how to provide the best care for their older patients,” she said. “They have to look at the chronological age of the patient and try to determine whether that actually matches more of what we’re saying is the physiological age of the patient and use that to guide their treatment.”
Study details
The study included phase 3 clinical trials of adult cancer patients that were conducted from mid-2016 through mid-2017. After identifying 929 manuscripts, the investigators removed duplicates and those that did not meet criteria. This left 159 articles published in 36 journals and covering 25 cancer types.
Of the 159 articles, 73.6% included age-specific medians (in addition to age means), and 47.2% had data stratified by age.
Efficacy was often reported (96.2%), but only 39.9% of articles specified age when describing effectiveness. Although most articles (84.9%) included safety data, only 8.9% had safety findings stratified by age.
In article discussion sections, age was mentioned infrequently in relation to treatment efficacy (13.8%) and rarely in relation to complications and adverse events (5.7%).Beyond underreporting of age-specific data, the investigators found that age categories themselves may be an area in need of improvement.“When outcomes pertaining to older adults were reported, the results were inconsistent as evidenced by the array of age distributions and varying categorization of ‘older adults,’” the investigators wrote. “There is a significant and timely need to design all clinical trials to include older adults and utilize a broad array of geriatric-specific outcomes.” Dr. Hshieh said these findings are concerning, but the study itself suggests the medical community is making efforts to correct the data shortage.“It was actually an important study, even though the results are a little discouraging,” Dr. Hshieh said. “What I’m hoping is that [these findings], combined with all the other literature that’s starting to come out about the need for more research in older patients with cancer, is going to be an impetus for us to do more research, and to be more open to treating older patients, and not to be afraid to confront this head on.”When asked about strategies for managing elderly patients, Dr. Hshieh first recommended the 2018 ASCO Guideline for Geriatric Oncology (J Clin Oncol. 2018 Aug 1;36[22]:2326-47).
“It’s very well written,” she said. “It is clear and user-friendly.”
Dr. Hshieh also offered some simple principles that may help guide clinical decision making.“I’m thinking of three things that an oncologist in the community would want to look at when they see an older patient and they’re trying to determine their treatment plan,” she said. “I would say [the oncologist] should look at [the patient’s] function; their psychosocial status, which includes mood and the support that they have in the community; and cognition.”
Dr. Hshieh and the study authors reported no conflicts of interest.
SOURCE: BrintzenhofeSzoc K et al. J Geriatr Oncol. 2020 Jan 10. pii: S1879-4068(19)30501-6.
FROM JOURNAL OF GERIATRIC ONCOLOGY
Society of Hospital Medicine cancels 2020 Annual Conference
The Society of Hospital Medicine (SHM) has canceled its annual conference, scheduled for mid-April, joining a growing list of events shuttered by coronavirus (COVID-19) concerns.
In a March 13 announcement, SHM said it would be impossible for the society to host the Hospital Medicine 2020 conference amid the escalating health concerns regarding the global COVID-19 outbreak. For more information about the cancellation and the society’s refund policies, see the SHM website for a list of frequently answered questions.
The Society of Hospital Medicine (SHM) has canceled its annual conference, scheduled for mid-April, joining a growing list of events shuttered by coronavirus (COVID-19) concerns.
In a March 13 announcement, SHM said it would be impossible for the society to host the Hospital Medicine 2020 conference amid the escalating health concerns regarding the global COVID-19 outbreak. For more information about the cancellation and the society’s refund policies, see the SHM website for a list of frequently answered questions.
The Society of Hospital Medicine (SHM) has canceled its annual conference, scheduled for mid-April, joining a growing list of events shuttered by coronavirus (COVID-19) concerns.
In a March 13 announcement, SHM said it would be impossible for the society to host the Hospital Medicine 2020 conference amid the escalating health concerns regarding the global COVID-19 outbreak. For more information about the cancellation and the society’s refund policies, see the SHM website for a list of frequently answered questions.
President declares national emergency for COVID-19, ramps up testing capability
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
President Donald Trump has declared a national emergency to allow for additional resources to combat the COVID-19 pandemic and announced increased testing capacity in partnership with private industry.
During a March 13 press conference, the president said the declaration would “open up access to up to $50 billion” for states and territories in combating the spread of the disease.
He also called on all states to “set up emergency operation centers, effective immediately” and for every hospital “to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere.”
Additionally, he said the declaration will confer broad new authority on the Department of Health & Human Services Secretary Alex Azar that will allow him to “immediately waive provisions of applicable laws and regulations to give doctors, all hospitals, and health care providers maximum flexibility to respond to the virus and care for patients.”
Some of the powers he highlighted included the ability to waive laws to enable telehealth; to waive certain federal license requirements to allow doctors licensed in one state to offer services in other states; the ability to waive limits on beds in critical access hospitals; and to waive rules that hinder hospitals from hiring additional physicians.
The president also announced that more testing capacity will be made available within the next week, in partnership with private industry.
“We want to make sure that those who need a test can get a test very safely, quickly, and conveniently, but we don’t want people to take a test if we feel that they shouldn’t be doing it,” he said.
To help make that determination, a website, developed with Google, is expected to be launched the weekend of March 13 to will allow individuals to input their symptoms and risk factors to help determine if they should be tested. If certain criteria are met, the website will provide locations for drive-through testing facilities. Individuals will be tested using a nasal swab and will receive results within 24-36 hours.
The testing is being done in partnership with retailers, including Target and Walmart (who are providing parking lot space for the pop-up testing facilities) and testing companies LabCorp and Quest Diagnostics.
The new test was developed by Roche and just received emergency use authorization from the Food and Drug Administration.
“We therefore expect up to a half-million additional tests will be available early next week,” President Trump said, adding that testing locations will “probably” be announced on Sunday, March 15.
A second application for a new test, submitted by Thermo Fisher, is currently under review at the FDA and is expected to be approved within the next 24 hours, he said. This would add an additional 1.4 million tests in the next week and 5 million within a month, according to the president.
Liver cancer risk reduced by aspirin in chronic viral hepatitis
The risk of liver cancer and liver-related death in patients with chronic viral hepatitis was substantially reduced with the use of low-dose aspirin, results from a nationwide study from Sweden suggest.
The risk of hepatocellular carcinoma (HCC) was reduced by 31% compared with no aspirin use, and liver-related mortality dropped by 27%, as long as aspirin use continued.
“We were excited to find for the first time in a nationwide Western population that low-dose aspirin use was associated with substantial reduction in risk of developing incident HCC,” lead author Tracey G. Simon, MD, MPH, of Massachusetts General Hospital and Harvard Medical School in Boston, told Medscape Medical News.
The study was published in the March 12 issue of the New England Journal of Medicine.
HCC is the fourth-leading cause of cancer mortality worldwide, and is driven mostly by viral hepatitis B (HBV) and viral hepatitis C (HCV) infection, noted Jennifer A. Flemming, MD, of Queen’s University, Kingston, Canada, an expert not involved with the study. HCC is also one of the only cancers to show a rising incidence over the past several decades, she added .
However, the results of this do not change clinical practice. “It is premature to prescribe low dose ASA [acetylsalicylic acid] in patients with viral hepatitis for the sole indication of HCC prevention in routine clinical practice without support from prospective randomized data,” she said.
“The results of this study make it clear that a prospective randomized study comparing ASA to placebo in patients with viral hepatitis without an indication for low-dose ASA is justified to evaluate the risk of incident HCC,” she told Medscape Medical News.
The study authors agree, and they also emphasize that the findings from this observational study “should not yet change clinical practice.”
More research is needed in populations with compensated and decompensated cirrhosis to determine the optimal timing of aspirin initiation — or cessation of therapy — that will maximize benefit and prevent adverse events, said Simon.
Study Details
Although several earlier studies have suggested a duration-dependent benefit of aspirin use in preventing HCC in smaller populations, this study is the first to confirm a duration-response relationship with low-dose aspirin use in an unselected European population with confirmed viral hepatitis, Simon pointed out.
For their study, Simon and colleagues used the Swedish Register for Surveillance of Communicable Diseases database to identify 50,275 adults diagnosed between 2005 and 2015 with acute and chronic HBV and HCV infection. Some 13,276 adults had HBV and 36,999 had HCV, and this included 14,205 low-dose (75 mg or 160 mg) aspirin users and 36,070 nonusers.
The analysis showed that in aspirin users, the 10-year cumulative incidence of HCC was 4% compared with 8.3% in nonusers. After multivariable adjustment, aspirin users had a risk of HCC that was 31% lower compared with nonusers (adjusted subhazard ratio, 0.69; 95% confidence interval [CI], 0.62 - 0.76).
Patients taking low-dose aspirin had a 10-year liver-related mortality of 11% compared with 17.9% among nonusers. The adjusted risk of liver-related mortality was 27% lower in aspirin users than in nonusers.
There was no significant difference in the 10-year risk of gastrointestinal bleeding between users and nonusers of aspirin (7.8% and 6.9%, respectively). In addition, the analysis showed that the risks of any gastrointestinal bleeding were similar among aspirin-users with compensated cirrhosis and those without cirrhosis (8.3% and 7.5%, respectively).
Notably, the risk of HCC was significantly lower after 3 to 5 years of aspirin use and after 5 or more years of use (adjusted hazard ratio [HR], 0.66, 0.57, respectively) compared with short-term use (3 months to <1 year; adjusted HR, 0.90) or with intermittent, discontinued, or no aspirin use. But when those with chronic viral hepatitis stopped taking aspirin, their risk of HCC rose to become 22% higher compared with peers who continued to use aspirin.
The risk of liver-related death also rose by 31% in aspirin users who stopped taking aspirin compared with those who did not stop (subhazard ratio, 1.31). Again, this relationship appeared to be duration-dependent, with the risk of incident HCC rising sharply among those who discontinued aspirin and increasing in magnitude over time.
The consistency of aspirin use also influenced risk. In individuals who had an on-again, off-again pattern of aspirin use, the incidence of HCC was 5.9% compared with 1.1% in those who used it consistently.
“Our results were consistent regardless of sex, cause of hepatitis, or underlying compensated cirrhosis,” the authors write. “The consistent duration-response associations lend further credence to a potential causal relationship.”
Limitations of the Study
The current study findings are not new, but this is the best-designed study to date, commented Flemming. Still, there were a number of limitations, she noted. Although cirrhosis is the strongest risk factor for HCC in patients with viral hepatitis, for instance, it was assessed only at cohort entry, and not during the median 8 years of follow-up. There was also a lack of information about sustained virologic response (SVR) rates.
Since less than 25% of patients with HCV received HCV therapy, this indicates they were likely treated with interferon-based therapy, Flemming suggested. Interferon-based therapy is associated with much lower SVR rates than direct acting antiviral (DAA) therapy, which can produce SVR in approximately 95% of patients, she pointed out.
“Therefore, a large proportion of the study patients were likely viremic and at a higher baseline risk of HCC than contemporary HCV populations.”
Evidence from a number of studies indicates that achieving SVR with DAA therapy is associated with a 70% risk reduction for incident HCC and liver-related events, Flemming said. “Whether the use of ASA in patients who have achieved SVR provides the same HCC risk reduction and decrease in hepatic outcomes is unknown.”
Also, the study did not provide information on the specific type of HBV therapy used in patients with HBV, Flemming noted. When considering the prevention of HCC in patients with chronic HBV infection, recent data support a differential protective effect of tenofovir disoproxil fumarate (multiple brands) compared with entecavir (Baraclude, Bristol-Myers Squibb), she pointed out. As previously reported by Medscape Medical News, these data also indicate that tenofovir may be more effective than entecavir in reducing the risk of liver failure and all-cause mortality.
This study was funded by the US National Institutes of Health, Nyckelfonden, Region Stockholm County, the American Association for the Study of Liver Diseases, Boston Nutrition Obesity Research Council, Region Örebro County, and Karolinska Institutet. Simon has disclosed no relevant financial relationships. A number of study coauthors disclosed having relationships with industry; the full list can be found with the original article. Flemming reported relationships with Gilead Sciences Canada, AbbVie, and Lupin Pharmaceuticals.
This article first appeared on Medscape.com.
N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMoa1912035.
The risk of liver cancer and liver-related death in patients with chronic viral hepatitis was substantially reduced with the use of low-dose aspirin, results from a nationwide study from Sweden suggest.
The risk of hepatocellular carcinoma (HCC) was reduced by 31% compared with no aspirin use, and liver-related mortality dropped by 27%, as long as aspirin use continued.
“We were excited to find for the first time in a nationwide Western population that low-dose aspirin use was associated with substantial reduction in risk of developing incident HCC,” lead author Tracey G. Simon, MD, MPH, of Massachusetts General Hospital and Harvard Medical School in Boston, told Medscape Medical News.
The study was published in the March 12 issue of the New England Journal of Medicine.
HCC is the fourth-leading cause of cancer mortality worldwide, and is driven mostly by viral hepatitis B (HBV) and viral hepatitis C (HCV) infection, noted Jennifer A. Flemming, MD, of Queen’s University, Kingston, Canada, an expert not involved with the study. HCC is also one of the only cancers to show a rising incidence over the past several decades, she added .
However, the results of this do not change clinical practice. “It is premature to prescribe low dose ASA [acetylsalicylic acid] in patients with viral hepatitis for the sole indication of HCC prevention in routine clinical practice without support from prospective randomized data,” she said.
“The results of this study make it clear that a prospective randomized study comparing ASA to placebo in patients with viral hepatitis without an indication for low-dose ASA is justified to evaluate the risk of incident HCC,” she told Medscape Medical News.
The study authors agree, and they also emphasize that the findings from this observational study “should not yet change clinical practice.”
More research is needed in populations with compensated and decompensated cirrhosis to determine the optimal timing of aspirin initiation — or cessation of therapy — that will maximize benefit and prevent adverse events, said Simon.
Study Details
Although several earlier studies have suggested a duration-dependent benefit of aspirin use in preventing HCC in smaller populations, this study is the first to confirm a duration-response relationship with low-dose aspirin use in an unselected European population with confirmed viral hepatitis, Simon pointed out.
For their study, Simon and colleagues used the Swedish Register for Surveillance of Communicable Diseases database to identify 50,275 adults diagnosed between 2005 and 2015 with acute and chronic HBV and HCV infection. Some 13,276 adults had HBV and 36,999 had HCV, and this included 14,205 low-dose (75 mg or 160 mg) aspirin users and 36,070 nonusers.
The analysis showed that in aspirin users, the 10-year cumulative incidence of HCC was 4% compared with 8.3% in nonusers. After multivariable adjustment, aspirin users had a risk of HCC that was 31% lower compared with nonusers (adjusted subhazard ratio, 0.69; 95% confidence interval [CI], 0.62 - 0.76).
Patients taking low-dose aspirin had a 10-year liver-related mortality of 11% compared with 17.9% among nonusers. The adjusted risk of liver-related mortality was 27% lower in aspirin users than in nonusers.
There was no significant difference in the 10-year risk of gastrointestinal bleeding between users and nonusers of aspirin (7.8% and 6.9%, respectively). In addition, the analysis showed that the risks of any gastrointestinal bleeding were similar among aspirin-users with compensated cirrhosis and those without cirrhosis (8.3% and 7.5%, respectively).
Notably, the risk of HCC was significantly lower after 3 to 5 years of aspirin use and after 5 or more years of use (adjusted hazard ratio [HR], 0.66, 0.57, respectively) compared with short-term use (3 months to <1 year; adjusted HR, 0.90) or with intermittent, discontinued, or no aspirin use. But when those with chronic viral hepatitis stopped taking aspirin, their risk of HCC rose to become 22% higher compared with peers who continued to use aspirin.
The risk of liver-related death also rose by 31% in aspirin users who stopped taking aspirin compared with those who did not stop (subhazard ratio, 1.31). Again, this relationship appeared to be duration-dependent, with the risk of incident HCC rising sharply among those who discontinued aspirin and increasing in magnitude over time.
The consistency of aspirin use also influenced risk. In individuals who had an on-again, off-again pattern of aspirin use, the incidence of HCC was 5.9% compared with 1.1% in those who used it consistently.
“Our results were consistent regardless of sex, cause of hepatitis, or underlying compensated cirrhosis,” the authors write. “The consistent duration-response associations lend further credence to a potential causal relationship.”
Limitations of the Study
The current study findings are not new, but this is the best-designed study to date, commented Flemming. Still, there were a number of limitations, she noted. Although cirrhosis is the strongest risk factor for HCC in patients with viral hepatitis, for instance, it was assessed only at cohort entry, and not during the median 8 years of follow-up. There was also a lack of information about sustained virologic response (SVR) rates.
Since less than 25% of patients with HCV received HCV therapy, this indicates they were likely treated with interferon-based therapy, Flemming suggested. Interferon-based therapy is associated with much lower SVR rates than direct acting antiviral (DAA) therapy, which can produce SVR in approximately 95% of patients, she pointed out.
“Therefore, a large proportion of the study patients were likely viremic and at a higher baseline risk of HCC than contemporary HCV populations.”
Evidence from a number of studies indicates that achieving SVR with DAA therapy is associated with a 70% risk reduction for incident HCC and liver-related events, Flemming said. “Whether the use of ASA in patients who have achieved SVR provides the same HCC risk reduction and decrease in hepatic outcomes is unknown.”
Also, the study did not provide information on the specific type of HBV therapy used in patients with HBV, Flemming noted. When considering the prevention of HCC in patients with chronic HBV infection, recent data support a differential protective effect of tenofovir disoproxil fumarate (multiple brands) compared with entecavir (Baraclude, Bristol-Myers Squibb), she pointed out. As previously reported by Medscape Medical News, these data also indicate that tenofovir may be more effective than entecavir in reducing the risk of liver failure and all-cause mortality.
This study was funded by the US National Institutes of Health, Nyckelfonden, Region Stockholm County, the American Association for the Study of Liver Diseases, Boston Nutrition Obesity Research Council, Region Örebro County, and Karolinska Institutet. Simon has disclosed no relevant financial relationships. A number of study coauthors disclosed having relationships with industry; the full list can be found with the original article. Flemming reported relationships with Gilead Sciences Canada, AbbVie, and Lupin Pharmaceuticals.
This article first appeared on Medscape.com.
N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMoa1912035.
The risk of liver cancer and liver-related death in patients with chronic viral hepatitis was substantially reduced with the use of low-dose aspirin, results from a nationwide study from Sweden suggest.
The risk of hepatocellular carcinoma (HCC) was reduced by 31% compared with no aspirin use, and liver-related mortality dropped by 27%, as long as aspirin use continued.
“We were excited to find for the first time in a nationwide Western population that low-dose aspirin use was associated with substantial reduction in risk of developing incident HCC,” lead author Tracey G. Simon, MD, MPH, of Massachusetts General Hospital and Harvard Medical School in Boston, told Medscape Medical News.
The study was published in the March 12 issue of the New England Journal of Medicine.
HCC is the fourth-leading cause of cancer mortality worldwide, and is driven mostly by viral hepatitis B (HBV) and viral hepatitis C (HCV) infection, noted Jennifer A. Flemming, MD, of Queen’s University, Kingston, Canada, an expert not involved with the study. HCC is also one of the only cancers to show a rising incidence over the past several decades, she added .
However, the results of this do not change clinical practice. “It is premature to prescribe low dose ASA [acetylsalicylic acid] in patients with viral hepatitis for the sole indication of HCC prevention in routine clinical practice without support from prospective randomized data,” she said.
“The results of this study make it clear that a prospective randomized study comparing ASA to placebo in patients with viral hepatitis without an indication for low-dose ASA is justified to evaluate the risk of incident HCC,” she told Medscape Medical News.
The study authors agree, and they also emphasize that the findings from this observational study “should not yet change clinical practice.”
More research is needed in populations with compensated and decompensated cirrhosis to determine the optimal timing of aspirin initiation — or cessation of therapy — that will maximize benefit and prevent adverse events, said Simon.
Study Details
Although several earlier studies have suggested a duration-dependent benefit of aspirin use in preventing HCC in smaller populations, this study is the first to confirm a duration-response relationship with low-dose aspirin use in an unselected European population with confirmed viral hepatitis, Simon pointed out.
For their study, Simon and colleagues used the Swedish Register for Surveillance of Communicable Diseases database to identify 50,275 adults diagnosed between 2005 and 2015 with acute and chronic HBV and HCV infection. Some 13,276 adults had HBV and 36,999 had HCV, and this included 14,205 low-dose (75 mg or 160 mg) aspirin users and 36,070 nonusers.
The analysis showed that in aspirin users, the 10-year cumulative incidence of HCC was 4% compared with 8.3% in nonusers. After multivariable adjustment, aspirin users had a risk of HCC that was 31% lower compared with nonusers (adjusted subhazard ratio, 0.69; 95% confidence interval [CI], 0.62 - 0.76).
Patients taking low-dose aspirin had a 10-year liver-related mortality of 11% compared with 17.9% among nonusers. The adjusted risk of liver-related mortality was 27% lower in aspirin users than in nonusers.
There was no significant difference in the 10-year risk of gastrointestinal bleeding between users and nonusers of aspirin (7.8% and 6.9%, respectively). In addition, the analysis showed that the risks of any gastrointestinal bleeding were similar among aspirin-users with compensated cirrhosis and those without cirrhosis (8.3% and 7.5%, respectively).
Notably, the risk of HCC was significantly lower after 3 to 5 years of aspirin use and after 5 or more years of use (adjusted hazard ratio [HR], 0.66, 0.57, respectively) compared with short-term use (3 months to <1 year; adjusted HR, 0.90) or with intermittent, discontinued, or no aspirin use. But when those with chronic viral hepatitis stopped taking aspirin, their risk of HCC rose to become 22% higher compared with peers who continued to use aspirin.
The risk of liver-related death also rose by 31% in aspirin users who stopped taking aspirin compared with those who did not stop (subhazard ratio, 1.31). Again, this relationship appeared to be duration-dependent, with the risk of incident HCC rising sharply among those who discontinued aspirin and increasing in magnitude over time.
The consistency of aspirin use also influenced risk. In individuals who had an on-again, off-again pattern of aspirin use, the incidence of HCC was 5.9% compared with 1.1% in those who used it consistently.
“Our results were consistent regardless of sex, cause of hepatitis, or underlying compensated cirrhosis,” the authors write. “The consistent duration-response associations lend further credence to a potential causal relationship.”
Limitations of the Study
The current study findings are not new, but this is the best-designed study to date, commented Flemming. Still, there were a number of limitations, she noted. Although cirrhosis is the strongest risk factor for HCC in patients with viral hepatitis, for instance, it was assessed only at cohort entry, and not during the median 8 years of follow-up. There was also a lack of information about sustained virologic response (SVR) rates.
Since less than 25% of patients with HCV received HCV therapy, this indicates they were likely treated with interferon-based therapy, Flemming suggested. Interferon-based therapy is associated with much lower SVR rates than direct acting antiviral (DAA) therapy, which can produce SVR in approximately 95% of patients, she pointed out.
“Therefore, a large proportion of the study patients were likely viremic and at a higher baseline risk of HCC than contemporary HCV populations.”
Evidence from a number of studies indicates that achieving SVR with DAA therapy is associated with a 70% risk reduction for incident HCC and liver-related events, Flemming said. “Whether the use of ASA in patients who have achieved SVR provides the same HCC risk reduction and decrease in hepatic outcomes is unknown.”
Also, the study did not provide information on the specific type of HBV therapy used in patients with HBV, Flemming noted. When considering the prevention of HCC in patients with chronic HBV infection, recent data support a differential protective effect of tenofovir disoproxil fumarate (multiple brands) compared with entecavir (Baraclude, Bristol-Myers Squibb), she pointed out. As previously reported by Medscape Medical News, these data also indicate that tenofovir may be more effective than entecavir in reducing the risk of liver failure and all-cause mortality.
This study was funded by the US National Institutes of Health, Nyckelfonden, Region Stockholm County, the American Association for the Study of Liver Diseases, Boston Nutrition Obesity Research Council, Region Örebro County, and Karolinska Institutet. Simon has disclosed no relevant financial relationships. A number of study coauthors disclosed having relationships with industry; the full list can be found with the original article. Flemming reported relationships with Gilead Sciences Canada, AbbVie, and Lupin Pharmaceuticals.
This article first appeared on Medscape.com.
N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMoa1912035.
Coronavirus on the inpatient unit: A new challenge for psychiatry
For weeks now, the coronavirus epidemic has monopolized the media. As cases of COVID-19 have crossed borders and then oceans, the pandemic has caused fear and anxiety as Americans struggle with the uncertain nature of this highly contagious disease. Those exposed may be infectious before they are symptomatic, and the expression of the virus varies greatly: Some people have a mild illness and others quickly progress to severe pulmonary disease with a bilateral interstitial pneumonia that requires intubation and respiratory support. So far, the number of people infected and the absolute mortality is a fraction of what we have seen with this year’s seasonal flu, but in countries where the virus has spread quickly, medical systems have not been able to keep up with demand for high-intensity care and mortality rates have been many times higher than that of the flu. Italy, in particular, has not been able to halt the spread, even with the entire country on lockdown, and the medical system has been overwhelmed, resulting in rationing of care and many deaths.
COVID-19 represents a new challenge for the inpatient psychiatry unit. Some patients on an acute psychiatric unit may be agitated, uncooperative, or even violent, and it’s not hard to imagine the distress of anyone who has a patient spit on them as we’re all trying to remember not to shake hands. Inevitably, there will be patients who present for psychiatric admission with no respiratory symptoms, who are admitted and then become ill and are diagnosed with COVID-19. In the meantime, the potential is there for contagion to other patients on the unit, the hospital staff, and visitors to the unit.
While many hospital units treat infectious patients, the issues with psychiatry are different; psychiatry units are not set up to have aggressive infection control, staff and patients don’t typically wear protective gear, and people with psychiatric illnesses are ambulatory and interactive. The treatment of psychiatric illnesses involves more – not less – social interaction and patients attend groups and occupational therapy sessions; they dine in communal areas and watch television together in day rooms. Cell phones are typically not permitted for issues of privacy, and patients may use communal telephones. Patients who are very ill with psychiatric disorders may resist hygiene measures, and they may intrude on the personal space of others.
Patients with known COVID-19 can be isolated or transferred to another unit if more intensive medical care is necessary, but by that time, others have been exposed and potentially infected. How to contain this potential risk has been a topic of concern for psychiatric units everywhere. Following a potential or confirmed exposure, it’s not completely clear who should be sent home for self-quarantine: Do the staff who have had contact with the patient leave for 2 weeks, and if so, is there enough staff to replace them? Do they continue to work with protective equipment and leave only if they become symptomatic and test positive? Do staff remain at the hospital, or do they go home at night, potentially infecting those on public transportation and their family members? Presumably new patients would not be admitted to the unit, but our psychiatric system is taxed already with a lack of available beds.
In South Korea, patients and staff at the Daenam hospital reportedly faced this exact scenario. The hospital was locked down and 101 patients in the psychiatric facility developed COVID-19; 7 of those patients died, an outcome we hope to never see again. As of this writing, there are two patients on a 22-bed geropsychiatric unit at the UW Medical Center – Northwest in Seattle who developed COVID-19 after they were admitted to the unit. They have been isolated, and the rest of the patients on the unit have been quarantined. The staff are now wearing masks, gowns, and gloves.
“We started precautions for all 22 patients. ... We instituted our protocols for every room around, donning and doffing personal protective equipment (PPE). We had conversations with their family members,” said Santiago Neme, MD, MPH, an infectious disease physician at UW Medical Center – Northwest, in a press conference released by the university. “The patients were transferred and both remain stable. All patients on the unit were tested even though there were no concerning symptoms.”
These measures are necessary for infection control, but they are not helpful for the treatment of psychiatric disorders. Treatment consists, in part, of getting patients out of their rooms and involved in therapeutic activities in a milieu that removes them from the usual stresses of their daily lives. How insurance companies will respond to any need for extra days is one more concern to throw into the mix.
Paul Summergrad, MD, chairman of psychiatry at Tufts University in Boston, has been very interested in what facilities around the world have been doing. “In Washington state, after the nursing home infections, they sent home over a hundred staff members who had been exposed. In Hong Kong, the psychiatric hospitals have limited how patients circulate on the units even if no one is infected; this is not something that would go over well in the U.S.,” he said in an interview. Dr. Summergrad believes that higher death rates are seen in countries with higher smoking rates, and patients with psychiatric disorders are more likely to smoke than the general public, possibly placing them at higher risk for more severe morbidity and mortality.
Patrick Triplett, MD, clinical director of the department of psychiatry at Johns Hopkins University in Baltimore, communicated with me about their plans to manage a scenario in which a patient becomes ill on a psychiatry unit.
“If we think a patient might be infected, we will isolate them in a room with a closed door (We would need to account for their psychiatric needs as well during this period, say constant observation, for example.) and call the centralized command center, where the Hospital Epidemiology and Infection Control (HEIC) team gets involved. They will determine if the patient should be tested for COVID-19. If it’s determined that the patient is infected, they will likely be transferred to a floor with negative pressure rooms. We would coordinate psychiatric nursing needs with the receiving unit, based on the patient’s clinical needs.”
Dr. Triplett elaborated on the exposure of staff and visitors. “We take our lead on postexposure management from [HEIC] and Occupational Health. There are procedures in place for environmental cleaning, waste, linens, etc. The [Centers for Disease Control and Prevention] has guidelines on work restrictions for staff who have cared for patients with confirmed COVID-19, and HEIC helps determine the provider’s risk category. We would also involve them in determining risk levels and management for other patients on the floor and visitors. But prior to any known exposure, we are already limiting visitors for patients per the governor’s mandate of only one adult visitor per patient.”
The next couple of weeks will be telling, and as we readjust to a life of social distancing, it is certain to be a challenge to keep ourselves and our patients safe, healthy, and emotionally strong.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
For weeks now, the coronavirus epidemic has monopolized the media. As cases of COVID-19 have crossed borders and then oceans, the pandemic has caused fear and anxiety as Americans struggle with the uncertain nature of this highly contagious disease. Those exposed may be infectious before they are symptomatic, and the expression of the virus varies greatly: Some people have a mild illness and others quickly progress to severe pulmonary disease with a bilateral interstitial pneumonia that requires intubation and respiratory support. So far, the number of people infected and the absolute mortality is a fraction of what we have seen with this year’s seasonal flu, but in countries where the virus has spread quickly, medical systems have not been able to keep up with demand for high-intensity care and mortality rates have been many times higher than that of the flu. Italy, in particular, has not been able to halt the spread, even with the entire country on lockdown, and the medical system has been overwhelmed, resulting in rationing of care and many deaths.
COVID-19 represents a new challenge for the inpatient psychiatry unit. Some patients on an acute psychiatric unit may be agitated, uncooperative, or even violent, and it’s not hard to imagine the distress of anyone who has a patient spit on them as we’re all trying to remember not to shake hands. Inevitably, there will be patients who present for psychiatric admission with no respiratory symptoms, who are admitted and then become ill and are diagnosed with COVID-19. In the meantime, the potential is there for contagion to other patients on the unit, the hospital staff, and visitors to the unit.
While many hospital units treat infectious patients, the issues with psychiatry are different; psychiatry units are not set up to have aggressive infection control, staff and patients don’t typically wear protective gear, and people with psychiatric illnesses are ambulatory and interactive. The treatment of psychiatric illnesses involves more – not less – social interaction and patients attend groups and occupational therapy sessions; they dine in communal areas and watch television together in day rooms. Cell phones are typically not permitted for issues of privacy, and patients may use communal telephones. Patients who are very ill with psychiatric disorders may resist hygiene measures, and they may intrude on the personal space of others.
Patients with known COVID-19 can be isolated or transferred to another unit if more intensive medical care is necessary, but by that time, others have been exposed and potentially infected. How to contain this potential risk has been a topic of concern for psychiatric units everywhere. Following a potential or confirmed exposure, it’s not completely clear who should be sent home for self-quarantine: Do the staff who have had contact with the patient leave for 2 weeks, and if so, is there enough staff to replace them? Do they continue to work with protective equipment and leave only if they become symptomatic and test positive? Do staff remain at the hospital, or do they go home at night, potentially infecting those on public transportation and their family members? Presumably new patients would not be admitted to the unit, but our psychiatric system is taxed already with a lack of available beds.
In South Korea, patients and staff at the Daenam hospital reportedly faced this exact scenario. The hospital was locked down and 101 patients in the psychiatric facility developed COVID-19; 7 of those patients died, an outcome we hope to never see again. As of this writing, there are two patients on a 22-bed geropsychiatric unit at the UW Medical Center – Northwest in Seattle who developed COVID-19 after they were admitted to the unit. They have been isolated, and the rest of the patients on the unit have been quarantined. The staff are now wearing masks, gowns, and gloves.
“We started precautions for all 22 patients. ... We instituted our protocols for every room around, donning and doffing personal protective equipment (PPE). We had conversations with their family members,” said Santiago Neme, MD, MPH, an infectious disease physician at UW Medical Center – Northwest, in a press conference released by the university. “The patients were transferred and both remain stable. All patients on the unit were tested even though there were no concerning symptoms.”
These measures are necessary for infection control, but they are not helpful for the treatment of psychiatric disorders. Treatment consists, in part, of getting patients out of their rooms and involved in therapeutic activities in a milieu that removes them from the usual stresses of their daily lives. How insurance companies will respond to any need for extra days is one more concern to throw into the mix.
Paul Summergrad, MD, chairman of psychiatry at Tufts University in Boston, has been very interested in what facilities around the world have been doing. “In Washington state, after the nursing home infections, they sent home over a hundred staff members who had been exposed. In Hong Kong, the psychiatric hospitals have limited how patients circulate on the units even if no one is infected; this is not something that would go over well in the U.S.,” he said in an interview. Dr. Summergrad believes that higher death rates are seen in countries with higher smoking rates, and patients with psychiatric disorders are more likely to smoke than the general public, possibly placing them at higher risk for more severe morbidity and mortality.
Patrick Triplett, MD, clinical director of the department of psychiatry at Johns Hopkins University in Baltimore, communicated with me about their plans to manage a scenario in which a patient becomes ill on a psychiatry unit.
“If we think a patient might be infected, we will isolate them in a room with a closed door (We would need to account for their psychiatric needs as well during this period, say constant observation, for example.) and call the centralized command center, where the Hospital Epidemiology and Infection Control (HEIC) team gets involved. They will determine if the patient should be tested for COVID-19. If it’s determined that the patient is infected, they will likely be transferred to a floor with negative pressure rooms. We would coordinate psychiatric nursing needs with the receiving unit, based on the patient’s clinical needs.”
Dr. Triplett elaborated on the exposure of staff and visitors. “We take our lead on postexposure management from [HEIC] and Occupational Health. There are procedures in place for environmental cleaning, waste, linens, etc. The [Centers for Disease Control and Prevention] has guidelines on work restrictions for staff who have cared for patients with confirmed COVID-19, and HEIC helps determine the provider’s risk category. We would also involve them in determining risk levels and management for other patients on the floor and visitors. But prior to any known exposure, we are already limiting visitors for patients per the governor’s mandate of only one adult visitor per patient.”
The next couple of weeks will be telling, and as we readjust to a life of social distancing, it is certain to be a challenge to keep ourselves and our patients safe, healthy, and emotionally strong.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
For weeks now, the coronavirus epidemic has monopolized the media. As cases of COVID-19 have crossed borders and then oceans, the pandemic has caused fear and anxiety as Americans struggle with the uncertain nature of this highly contagious disease. Those exposed may be infectious before they are symptomatic, and the expression of the virus varies greatly: Some people have a mild illness and others quickly progress to severe pulmonary disease with a bilateral interstitial pneumonia that requires intubation and respiratory support. So far, the number of people infected and the absolute mortality is a fraction of what we have seen with this year’s seasonal flu, but in countries where the virus has spread quickly, medical systems have not been able to keep up with demand for high-intensity care and mortality rates have been many times higher than that of the flu. Italy, in particular, has not been able to halt the spread, even with the entire country on lockdown, and the medical system has been overwhelmed, resulting in rationing of care and many deaths.
COVID-19 represents a new challenge for the inpatient psychiatry unit. Some patients on an acute psychiatric unit may be agitated, uncooperative, or even violent, and it’s not hard to imagine the distress of anyone who has a patient spit on them as we’re all trying to remember not to shake hands. Inevitably, there will be patients who present for psychiatric admission with no respiratory symptoms, who are admitted and then become ill and are diagnosed with COVID-19. In the meantime, the potential is there for contagion to other patients on the unit, the hospital staff, and visitors to the unit.
While many hospital units treat infectious patients, the issues with psychiatry are different; psychiatry units are not set up to have aggressive infection control, staff and patients don’t typically wear protective gear, and people with psychiatric illnesses are ambulatory and interactive. The treatment of psychiatric illnesses involves more – not less – social interaction and patients attend groups and occupational therapy sessions; they dine in communal areas and watch television together in day rooms. Cell phones are typically not permitted for issues of privacy, and patients may use communal telephones. Patients who are very ill with psychiatric disorders may resist hygiene measures, and they may intrude on the personal space of others.
Patients with known COVID-19 can be isolated or transferred to another unit if more intensive medical care is necessary, but by that time, others have been exposed and potentially infected. How to contain this potential risk has been a topic of concern for psychiatric units everywhere. Following a potential or confirmed exposure, it’s not completely clear who should be sent home for self-quarantine: Do the staff who have had contact with the patient leave for 2 weeks, and if so, is there enough staff to replace them? Do they continue to work with protective equipment and leave only if they become symptomatic and test positive? Do staff remain at the hospital, or do they go home at night, potentially infecting those on public transportation and their family members? Presumably new patients would not be admitted to the unit, but our psychiatric system is taxed already with a lack of available beds.
In South Korea, patients and staff at the Daenam hospital reportedly faced this exact scenario. The hospital was locked down and 101 patients in the psychiatric facility developed COVID-19; 7 of those patients died, an outcome we hope to never see again. As of this writing, there are two patients on a 22-bed geropsychiatric unit at the UW Medical Center – Northwest in Seattle who developed COVID-19 after they were admitted to the unit. They have been isolated, and the rest of the patients on the unit have been quarantined. The staff are now wearing masks, gowns, and gloves.
“We started precautions for all 22 patients. ... We instituted our protocols for every room around, donning and doffing personal protective equipment (PPE). We had conversations with their family members,” said Santiago Neme, MD, MPH, an infectious disease physician at UW Medical Center – Northwest, in a press conference released by the university. “The patients were transferred and both remain stable. All patients on the unit were tested even though there were no concerning symptoms.”
These measures are necessary for infection control, but they are not helpful for the treatment of psychiatric disorders. Treatment consists, in part, of getting patients out of their rooms and involved in therapeutic activities in a milieu that removes them from the usual stresses of their daily lives. How insurance companies will respond to any need for extra days is one more concern to throw into the mix.
Paul Summergrad, MD, chairman of psychiatry at Tufts University in Boston, has been very interested in what facilities around the world have been doing. “In Washington state, after the nursing home infections, they sent home over a hundred staff members who had been exposed. In Hong Kong, the psychiatric hospitals have limited how patients circulate on the units even if no one is infected; this is not something that would go over well in the U.S.,” he said in an interview. Dr. Summergrad believes that higher death rates are seen in countries with higher smoking rates, and patients with psychiatric disorders are more likely to smoke than the general public, possibly placing them at higher risk for more severe morbidity and mortality.
Patrick Triplett, MD, clinical director of the department of psychiatry at Johns Hopkins University in Baltimore, communicated with me about their plans to manage a scenario in which a patient becomes ill on a psychiatry unit.
“If we think a patient might be infected, we will isolate them in a room with a closed door (We would need to account for their psychiatric needs as well during this period, say constant observation, for example.) and call the centralized command center, where the Hospital Epidemiology and Infection Control (HEIC) team gets involved. They will determine if the patient should be tested for COVID-19. If it’s determined that the patient is infected, they will likely be transferred to a floor with negative pressure rooms. We would coordinate psychiatric nursing needs with the receiving unit, based on the patient’s clinical needs.”
Dr. Triplett elaborated on the exposure of staff and visitors. “We take our lead on postexposure management from [HEIC] and Occupational Health. There are procedures in place for environmental cleaning, waste, linens, etc. The [Centers for Disease Control and Prevention] has guidelines on work restrictions for staff who have cared for patients with confirmed COVID-19, and HEIC helps determine the provider’s risk category. We would also involve them in determining risk levels and management for other patients on the floor and visitors. But prior to any known exposure, we are already limiting visitors for patients per the governor’s mandate of only one adult visitor per patient.”
The next couple of weeks will be telling, and as we readjust to a life of social distancing, it is certain to be a challenge to keep ourselves and our patients safe, healthy, and emotionally strong.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
After weeks of decline, influenza activity increases slightly
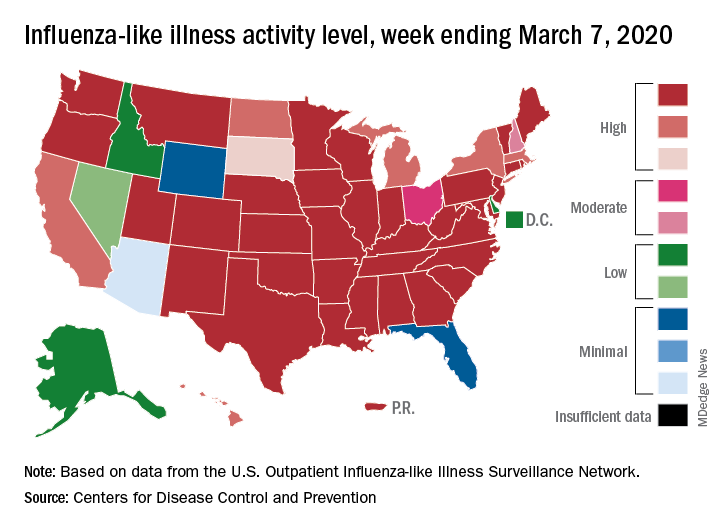
The two leading measures of influenza activity – the percentage of respiratory specimens testing positive for influenza and the proportion of visits to health care providers for influenza-like illness (ILI) – had been following a similar downward path since mid-February. But during the week ending March 7, their paths diverged, according to the Centers for Disease Control and Prevention.
The percentage of respiratory specimens testing positive for influenza dropped for the fourth consecutive week, falling from 26.1% to 21.5%, while the proportion of visits to health care providers for ILI increased from 5.1% to 5.2%, the CDC’s influenza division reported.
One possible explanation for that rise: “The largest increases in ILI activity occurred in areas of the country where COVID-19 is most prevalent. More people may be seeking care for respiratory illness than usual at this time,” the influenza division said March 13 in its weekly Fluview report.
This week’s map puts 34 states and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity, one more state than the week before, and 43 jurisdictions in the “high” range of 8-10, compared with 42 the previous week, the CDC said.
Rates of hospitalizations associated with influenza “remain moderate compared to recent seasons, but rates for children 0-4 years and adults 18-49 years are now the highest CDC has on record for these age groups, surpassing rates reported during the 2009 H1N1 pandemic,” the Fluview report said. Rates for children aged 5-17 years “are higher than any recent regular season but remain lower than rates experienced by this age group during the pandemic.”
The number of pediatric deaths this season is now up to 144, equaling the total for all of the 2018-2019 season. This year’s count led the CDC to invoke 2009 again, since it “is higher for the same time period than in every season since reporting began in 2004-2005, except for the 2009 pandemic.”
For the 2019-2020 season so far there have been 36 million flu illnesses, 370,000 hospitalizations, and 22,000 deaths from flu and pneumonia, the CDC estimated.

The two leading measures of influenza activity – the percentage of respiratory specimens testing positive for influenza and the proportion of visits to health care providers for influenza-like illness (ILI) – had been following a similar downward path since mid-February. But during the week ending March 7, their paths diverged, according to the Centers for Disease Control and Prevention.
The percentage of respiratory specimens testing positive for influenza dropped for the fourth consecutive week, falling from 26.1% to 21.5%, while the proportion of visits to health care providers for ILI increased from 5.1% to 5.2%, the CDC’s influenza division reported.
One possible explanation for that rise: “The largest increases in ILI activity occurred in areas of the country where COVID-19 is most prevalent. More people may be seeking care for respiratory illness than usual at this time,” the influenza division said March 13 in its weekly Fluview report.
This week’s map puts 34 states and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity, one more state than the week before, and 43 jurisdictions in the “high” range of 8-10, compared with 42 the previous week, the CDC said.
Rates of hospitalizations associated with influenza “remain moderate compared to recent seasons, but rates for children 0-4 years and adults 18-49 years are now the highest CDC has on record for these age groups, surpassing rates reported during the 2009 H1N1 pandemic,” the Fluview report said. Rates for children aged 5-17 years “are higher than any recent regular season but remain lower than rates experienced by this age group during the pandemic.”
The number of pediatric deaths this season is now up to 144, equaling the total for all of the 2018-2019 season. This year’s count led the CDC to invoke 2009 again, since it “is higher for the same time period than in every season since reporting began in 2004-2005, except for the 2009 pandemic.”
For the 2019-2020 season so far there have been 36 million flu illnesses, 370,000 hospitalizations, and 22,000 deaths from flu and pneumonia, the CDC estimated.

The two leading measures of influenza activity – the percentage of respiratory specimens testing positive for influenza and the proportion of visits to health care providers for influenza-like illness (ILI) – had been following a similar downward path since mid-February. But during the week ending March 7, their paths diverged, according to the Centers for Disease Control and Prevention.
The percentage of respiratory specimens testing positive for influenza dropped for the fourth consecutive week, falling from 26.1% to 21.5%, while the proportion of visits to health care providers for ILI increased from 5.1% to 5.2%, the CDC’s influenza division reported.
One possible explanation for that rise: “The largest increases in ILI activity occurred in areas of the country where COVID-19 is most prevalent. More people may be seeking care for respiratory illness than usual at this time,” the influenza division said March 13 in its weekly Fluview report.
This week’s map puts 34 states and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity, one more state than the week before, and 43 jurisdictions in the “high” range of 8-10, compared with 42 the previous week, the CDC said.
Rates of hospitalizations associated with influenza “remain moderate compared to recent seasons, but rates for children 0-4 years and adults 18-49 years are now the highest CDC has on record for these age groups, surpassing rates reported during the 2009 H1N1 pandemic,” the Fluview report said. Rates for children aged 5-17 years “are higher than any recent regular season but remain lower than rates experienced by this age group during the pandemic.”
The number of pediatric deaths this season is now up to 144, equaling the total for all of the 2018-2019 season. This year’s count led the CDC to invoke 2009 again, since it “is higher for the same time period than in every season since reporting began in 2004-2005, except for the 2009 pandemic.”
For the 2019-2020 season so far there have been 36 million flu illnesses, 370,000 hospitalizations, and 22,000 deaths from flu and pneumonia, the CDC estimated.
Microbiome studies may require correction for PPI use
Microbiome studies should be correcting statistics to account for proton pump inhibitor (PPI) use, according to a leading expert.
After antibiotics, PPIs are the leading cause of microbiome variance in both research and general populations, and these alterations could have a range of consequences, reported Rinse K. Weersma, MD, PhD, of the University of Groningen (the Netherlands).
About 20% of people are taking a PPI, Dr. Weersma said at the annual Gut Microbiota for Health World Summit, noting that, in countries such as the United States and the United Kingdom, this figure may be higher.
“There’s chronic use of proton pump inhibitors in the population on a massive scale,” Dr. Weersma said.
To complicate matters, estimates suggest that 25%-70% of people who are taking PPIs have no appropriate indication. While this issue is partly because of increasing over-the-counter usage, physicians are also contributing to the problem by prescribing PPIs without adequate follow-up.
“The number of people using proton pump inhibitors is steadily increasing,” Dr. Weersma said. “The number of people getting them prescribed is relatively stable. The problem is, we never stop.”
According to Dr. Weersma, a growing body of research shows that PPI use may increase the risk of developing other conditions. Although many of these relationships are correlative, some are now widely accepted as causal. Most notable and clinically relevant, Dr. Weersma said, are enteric infections. Clostridioides difficile–associated diarrhea, for instance, is 65% more common among PPI users.
While the mechanisms behind this susceptibility to infection are uncertain, Dr. Weersma suggested that the most likely cause is “oralization” of the gut microbiome caused by loss of the acid barrier, which introduces upper gastrointestinal bacteria, or oral bacteria, into the lower intestines.
Perhaps more relevant to clinical trials, PPIs may also influence the safety and efficacy of drugs.
“There is a lot of interaction between the gut microbiome and a lot of drugs,” Dr. Weersma said at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility. “We really don’t know a lot about this at the moment.”
He went on to explain that bidirectional interactions between drugs and the microbiome may actually present clinical opportunities.
“This is a field that people currently call pharmacomicrobiomics,” Dr. Weersma said. “This is very intriguing, of course, because everyone knows about pharmacogenomics ... which lets you stratify your patients, but you cannot intervene; you cannot change your genetic background to increase efficacy or avoid toxicity. But in fact, with the microbiome, we could modulate the microbiome and improve bioavailability, for example.”
Conversely, Dr. Weersma pointed out that PPI use may be interfering with drug efficacy to a life-altering degree.
He cited a recent study by Chalabi and colleagues, which found that PPI use affected responses to immune checkpoint inhibitors (Ann Oncol. 2020 Jan 16. doi: 10.1016/j.annonc.2020.01.006). Among 169 patients with lung cancer who were treated with atezolizumab, overall survival was significantly lower in PPI users (9.6 vs. 14.5 months; P = .001).
A number of other clinical implications are also possible, Dr. Weersma said, although these require further investigation. For example, a 2019 study by Stark and colleagues suggested that childhood use of PPIs may increase obesity risk.
“[There are] no microbiome data here,” Dr. Weersma said, “but it makes you think.”
While considering the downsides of PPIs, Dr. Weersma also emphasized their importance in clinical practice. “[Proton pump inhibitors] are very great drugs. They are cheap, they are safe, they are very effective. So if you have evidence-based indications to use proton pump inhibitors, you should definitely use them and not stop them.”
Dr. Weersma called for responsible use of PPIs, and suggested that clinicians need to prepare for pushback from patients, who, after stopping PPIs, may experience a temporary resurgence of symptoms because of acid rebound.
“You have to make them aware [of acid rebound],” Dr. Weersma said. “Say: ‘Wait 2 or 3 weeks and this rebound is gone.’ We should say that way, way, way more often.”
But clinicians shouldn’t bear the burden of responsible usage alone, Dr. Weersma said.
“There’s a role for clinicians, patients, and regulatory bodies also, to think about the massive use of proton pump inhibitors now and in the future.”
In the discussion that followed the presentation, a summit attendee brought up the realities of clinical practice before PPIs, when patients frequently had gastrointestinal bleeding secondary to nonsteroidal anti-inflammatory use. In response, Dr. Weersma again emphasized that PPIs play a critical role for many patients. After once more encouraging responsible use, Dr. Weersma expressed concern about the risks involved in conveying his message; not only to the medical community, but also to the general public.
“This is a very difficult message [to deliver],” Dr. Weersma said. “In the Netherlands this was taken up by the media and the news, so my email inbox exploded. It’s difficult to get this nuance right.”
Dr. Weersma disclosed relationships with Takeda, Johnson & Johnson, Ferring, and others.
Microbiome studies should be correcting statistics to account for proton pump inhibitor (PPI) use, according to a leading expert.
After antibiotics, PPIs are the leading cause of microbiome variance in both research and general populations, and these alterations could have a range of consequences, reported Rinse K. Weersma, MD, PhD, of the University of Groningen (the Netherlands).
About 20% of people are taking a PPI, Dr. Weersma said at the annual Gut Microbiota for Health World Summit, noting that, in countries such as the United States and the United Kingdom, this figure may be higher.
“There’s chronic use of proton pump inhibitors in the population on a massive scale,” Dr. Weersma said.
To complicate matters, estimates suggest that 25%-70% of people who are taking PPIs have no appropriate indication. While this issue is partly because of increasing over-the-counter usage, physicians are also contributing to the problem by prescribing PPIs without adequate follow-up.
“The number of people using proton pump inhibitors is steadily increasing,” Dr. Weersma said. “The number of people getting them prescribed is relatively stable. The problem is, we never stop.”
According to Dr. Weersma, a growing body of research shows that PPI use may increase the risk of developing other conditions. Although many of these relationships are correlative, some are now widely accepted as causal. Most notable and clinically relevant, Dr. Weersma said, are enteric infections. Clostridioides difficile–associated diarrhea, for instance, is 65% more common among PPI users.
While the mechanisms behind this susceptibility to infection are uncertain, Dr. Weersma suggested that the most likely cause is “oralization” of the gut microbiome caused by loss of the acid barrier, which introduces upper gastrointestinal bacteria, or oral bacteria, into the lower intestines.
Perhaps more relevant to clinical trials, PPIs may also influence the safety and efficacy of drugs.
“There is a lot of interaction between the gut microbiome and a lot of drugs,” Dr. Weersma said at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility. “We really don’t know a lot about this at the moment.”
He went on to explain that bidirectional interactions between drugs and the microbiome may actually present clinical opportunities.
“This is a field that people currently call pharmacomicrobiomics,” Dr. Weersma said. “This is very intriguing, of course, because everyone knows about pharmacogenomics ... which lets you stratify your patients, but you cannot intervene; you cannot change your genetic background to increase efficacy or avoid toxicity. But in fact, with the microbiome, we could modulate the microbiome and improve bioavailability, for example.”
Conversely, Dr. Weersma pointed out that PPI use may be interfering with drug efficacy to a life-altering degree.
He cited a recent study by Chalabi and colleagues, which found that PPI use affected responses to immune checkpoint inhibitors (Ann Oncol. 2020 Jan 16. doi: 10.1016/j.annonc.2020.01.006). Among 169 patients with lung cancer who were treated with atezolizumab, overall survival was significantly lower in PPI users (9.6 vs. 14.5 months; P = .001).
A number of other clinical implications are also possible, Dr. Weersma said, although these require further investigation. For example, a 2019 study by Stark and colleagues suggested that childhood use of PPIs may increase obesity risk.
“[There are] no microbiome data here,” Dr. Weersma said, “but it makes you think.”
While considering the downsides of PPIs, Dr. Weersma also emphasized their importance in clinical practice. “[Proton pump inhibitors] are very great drugs. They are cheap, they are safe, they are very effective. So if you have evidence-based indications to use proton pump inhibitors, you should definitely use them and not stop them.”
Dr. Weersma called for responsible use of PPIs, and suggested that clinicians need to prepare for pushback from patients, who, after stopping PPIs, may experience a temporary resurgence of symptoms because of acid rebound.
“You have to make them aware [of acid rebound],” Dr. Weersma said. “Say: ‘Wait 2 or 3 weeks and this rebound is gone.’ We should say that way, way, way more often.”
But clinicians shouldn’t bear the burden of responsible usage alone, Dr. Weersma said.
“There’s a role for clinicians, patients, and regulatory bodies also, to think about the massive use of proton pump inhibitors now and in the future.”
In the discussion that followed the presentation, a summit attendee brought up the realities of clinical practice before PPIs, when patients frequently had gastrointestinal bleeding secondary to nonsteroidal anti-inflammatory use. In response, Dr. Weersma again emphasized that PPIs play a critical role for many patients. After once more encouraging responsible use, Dr. Weersma expressed concern about the risks involved in conveying his message; not only to the medical community, but also to the general public.
“This is a very difficult message [to deliver],” Dr. Weersma said. “In the Netherlands this was taken up by the media and the news, so my email inbox exploded. It’s difficult to get this nuance right.”
Dr. Weersma disclosed relationships with Takeda, Johnson & Johnson, Ferring, and others.
Microbiome studies should be correcting statistics to account for proton pump inhibitor (PPI) use, according to a leading expert.
After antibiotics, PPIs are the leading cause of microbiome variance in both research and general populations, and these alterations could have a range of consequences, reported Rinse K. Weersma, MD, PhD, of the University of Groningen (the Netherlands).
About 20% of people are taking a PPI, Dr. Weersma said at the annual Gut Microbiota for Health World Summit, noting that, in countries such as the United States and the United Kingdom, this figure may be higher.
“There’s chronic use of proton pump inhibitors in the population on a massive scale,” Dr. Weersma said.
To complicate matters, estimates suggest that 25%-70% of people who are taking PPIs have no appropriate indication. While this issue is partly because of increasing over-the-counter usage, physicians are also contributing to the problem by prescribing PPIs without adequate follow-up.
“The number of people using proton pump inhibitors is steadily increasing,” Dr. Weersma said. “The number of people getting them prescribed is relatively stable. The problem is, we never stop.”
According to Dr. Weersma, a growing body of research shows that PPI use may increase the risk of developing other conditions. Although many of these relationships are correlative, some are now widely accepted as causal. Most notable and clinically relevant, Dr. Weersma said, are enteric infections. Clostridioides difficile–associated diarrhea, for instance, is 65% more common among PPI users.
While the mechanisms behind this susceptibility to infection are uncertain, Dr. Weersma suggested that the most likely cause is “oralization” of the gut microbiome caused by loss of the acid barrier, which introduces upper gastrointestinal bacteria, or oral bacteria, into the lower intestines.
Perhaps more relevant to clinical trials, PPIs may also influence the safety and efficacy of drugs.
“There is a lot of interaction between the gut microbiome and a lot of drugs,” Dr. Weersma said at the meeting sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility. “We really don’t know a lot about this at the moment.”
He went on to explain that bidirectional interactions between drugs and the microbiome may actually present clinical opportunities.
“This is a field that people currently call pharmacomicrobiomics,” Dr. Weersma said. “This is very intriguing, of course, because everyone knows about pharmacogenomics ... which lets you stratify your patients, but you cannot intervene; you cannot change your genetic background to increase efficacy or avoid toxicity. But in fact, with the microbiome, we could modulate the microbiome and improve bioavailability, for example.”
Conversely, Dr. Weersma pointed out that PPI use may be interfering with drug efficacy to a life-altering degree.
He cited a recent study by Chalabi and colleagues, which found that PPI use affected responses to immune checkpoint inhibitors (Ann Oncol. 2020 Jan 16. doi: 10.1016/j.annonc.2020.01.006). Among 169 patients with lung cancer who were treated with atezolizumab, overall survival was significantly lower in PPI users (9.6 vs. 14.5 months; P = .001).
A number of other clinical implications are also possible, Dr. Weersma said, although these require further investigation. For example, a 2019 study by Stark and colleagues suggested that childhood use of PPIs may increase obesity risk.
“[There are] no microbiome data here,” Dr. Weersma said, “but it makes you think.”
While considering the downsides of PPIs, Dr. Weersma also emphasized their importance in clinical practice. “[Proton pump inhibitors] are very great drugs. They are cheap, they are safe, they are very effective. So if you have evidence-based indications to use proton pump inhibitors, you should definitely use them and not stop them.”
Dr. Weersma called for responsible use of PPIs, and suggested that clinicians need to prepare for pushback from patients, who, after stopping PPIs, may experience a temporary resurgence of symptoms because of acid rebound.
“You have to make them aware [of acid rebound],” Dr. Weersma said. “Say: ‘Wait 2 or 3 weeks and this rebound is gone.’ We should say that way, way, way more often.”
But clinicians shouldn’t bear the burden of responsible usage alone, Dr. Weersma said.
“There’s a role for clinicians, patients, and regulatory bodies also, to think about the massive use of proton pump inhibitors now and in the future.”
In the discussion that followed the presentation, a summit attendee brought up the realities of clinical practice before PPIs, when patients frequently had gastrointestinal bleeding secondary to nonsteroidal anti-inflammatory use. In response, Dr. Weersma again emphasized that PPIs play a critical role for many patients. After once more encouraging responsible use, Dr. Weersma expressed concern about the risks involved in conveying his message; not only to the medical community, but also to the general public.
“This is a very difficult message [to deliver],” Dr. Weersma said. “In the Netherlands this was taken up by the media and the news, so my email inbox exploded. It’s difficult to get this nuance right.”
Dr. Weersma disclosed relationships with Takeda, Johnson & Johnson, Ferring, and others.
EXPERT ANALYSIS FROM GMFH 2020
Smoking, hypoglycemia, kidney function tied to vision loss in type 2 diabetes
according to new findings published in the Journal of Diabetes and its Complications.
“Smoking cessation strategies and optimal cardiometabolic risk factor management, including blood glucose lowering regimens that minimize hypoglycemia, appear important in preventing the loss of vision associated with type 2 diabetes,” wrote Jocelyn J. Drinkwater of the University of Western Australia, Perth, and coauthors, noting that all three noted risk factors were “potentially modifiable.”
To investigate the impact of type 2 diabetes and associated risk factors on vision, the researchers recruited 1,732 participants for the Fremantle Diabetes Study Phase II, of whom 1,551 patients had type 2 diabetes and underwent face-to-face and visual acuity assessments at baseline and at 2 and 4 years. Visual acuity was measured via the Bailey Lovie chart at a distance of 3 m in a well-lit room. Normal or near-normal vision was classified as a visual acuity of equal to or less than 6/19; visual impairment, a visual acuity of greater than 6/19 and equal to or less than 6/48; and blindness, a visual acuity of greater than 6/48. A change in vision was classified as a difference in visual acuity of more than 10 letters from baseline measurement.
Of the initial 1,551 participants, 31 were excluded because of missing baseline data for visual acuity. The remaining group comprised 52.2% men, the mean age was 65.6 years, and the median diabetes duration was 8.5 years (interquartile range, 2.9-15.8). At baseline, the prevalence of visual impairment was 1.8% (28 patients), and prevalence of blindness was 0.7% (11 patients), so those 39 patients were also excluded from further analysis.
After 4 years, 599 patients (39%) were excluded because of attrition or missing data; among them, 138 (23%) died before the follow-up.
The remaining 882 participants (58%) had their visual acuity measured. Among these patients, 62.2% were men, with a mean age of 65.1 years and an initial median diabetes duration of 7 years (IQR, 2.0-15.0). Their cumulative incidence of visual impairment was 0.9% (eight patients), and no patients with normal or near-normal vision had developed blindness. Cumulative incidence of vision loss was 2.9% (26), and 1.9% (17) had improved visual acuity.
After multivariable logistic regression to determine predictors for vision loss, the researchers found that participants who smoked at baseline were more than three times more likely to lose their vision (odds ratio, 3.17; 95% confidence interval, 1.15-8.76; P = .026). Although smoking was noted as a “well-recognized risk factor for ocular disease,” the authors added that ex-smokers did not have significantly higher odds of vision loss, compared with nonsmokers, suggesting that the “ocular damage caused by smoking may not be permanent.”
Participants who had suffered a severe hypoglycemic event before the study were five times more likely to lose their vision (OR, 5.59; 95% CI, 1.32-23.61; P = .019). The authors emphasized that severe hypoglycemia can worsen existing ischemic tissue damage or contribute to a long duration of poorly controlled diabetes, each of which could “increase the risk of ocular complications leading to impaired vision.”
The final notable risk factor was compromised kidney function, which is identified as a urinary albumin-creatinine ratio (uACR). The authors noted that the uACR has been associated with other ocular pathologies, such as retinopathy and macular edema, and that uACR may be a “surrogate marker of a variety of ocular diseases with shared risk factors, such as poor metabolic control, which have implications for vision.”
In regard to the possible limitations of the study, they authors noted that they had not used the “gold standard” Early Treatment Diabetic Retinopathy Study chart to assess visual acuity. In addition, although they had details on retinopathy, cataracts, and glaucoma status, they did not also consider less common ophthalmic conditions. Finally, as a survivor cohort, they acknowledged that they may have “underestimated the cumulative incidence” of vision issues in the participants.
The study was supported by the National Health and Medical Research Council of Australia. The authors reported no conflicts of interest.
SOURCE: Drinkwater JJ et al. J Diabetes Complications. 2020 Feb 20. doi: 10.1016/j.jdiacomp.2020.107560.
according to new findings published in the Journal of Diabetes and its Complications.
“Smoking cessation strategies and optimal cardiometabolic risk factor management, including blood glucose lowering regimens that minimize hypoglycemia, appear important in preventing the loss of vision associated with type 2 diabetes,” wrote Jocelyn J. Drinkwater of the University of Western Australia, Perth, and coauthors, noting that all three noted risk factors were “potentially modifiable.”
To investigate the impact of type 2 diabetes and associated risk factors on vision, the researchers recruited 1,732 participants for the Fremantle Diabetes Study Phase II, of whom 1,551 patients had type 2 diabetes and underwent face-to-face and visual acuity assessments at baseline and at 2 and 4 years. Visual acuity was measured via the Bailey Lovie chart at a distance of 3 m in a well-lit room. Normal or near-normal vision was classified as a visual acuity of equal to or less than 6/19; visual impairment, a visual acuity of greater than 6/19 and equal to or less than 6/48; and blindness, a visual acuity of greater than 6/48. A change in vision was classified as a difference in visual acuity of more than 10 letters from baseline measurement.
Of the initial 1,551 participants, 31 were excluded because of missing baseline data for visual acuity. The remaining group comprised 52.2% men, the mean age was 65.6 years, and the median diabetes duration was 8.5 years (interquartile range, 2.9-15.8). At baseline, the prevalence of visual impairment was 1.8% (28 patients), and prevalence of blindness was 0.7% (11 patients), so those 39 patients were also excluded from further analysis.
After 4 years, 599 patients (39%) were excluded because of attrition or missing data; among them, 138 (23%) died before the follow-up.
The remaining 882 participants (58%) had their visual acuity measured. Among these patients, 62.2% were men, with a mean age of 65.1 years and an initial median diabetes duration of 7 years (IQR, 2.0-15.0). Their cumulative incidence of visual impairment was 0.9% (eight patients), and no patients with normal or near-normal vision had developed blindness. Cumulative incidence of vision loss was 2.9% (26), and 1.9% (17) had improved visual acuity.
After multivariable logistic regression to determine predictors for vision loss, the researchers found that participants who smoked at baseline were more than three times more likely to lose their vision (odds ratio, 3.17; 95% confidence interval, 1.15-8.76; P = .026). Although smoking was noted as a “well-recognized risk factor for ocular disease,” the authors added that ex-smokers did not have significantly higher odds of vision loss, compared with nonsmokers, suggesting that the “ocular damage caused by smoking may not be permanent.”
Participants who had suffered a severe hypoglycemic event before the study were five times more likely to lose their vision (OR, 5.59; 95% CI, 1.32-23.61; P = .019). The authors emphasized that severe hypoglycemia can worsen existing ischemic tissue damage or contribute to a long duration of poorly controlled diabetes, each of which could “increase the risk of ocular complications leading to impaired vision.”
The final notable risk factor was compromised kidney function, which is identified as a urinary albumin-creatinine ratio (uACR). The authors noted that the uACR has been associated with other ocular pathologies, such as retinopathy and macular edema, and that uACR may be a “surrogate marker of a variety of ocular diseases with shared risk factors, such as poor metabolic control, which have implications for vision.”
In regard to the possible limitations of the study, they authors noted that they had not used the “gold standard” Early Treatment Diabetic Retinopathy Study chart to assess visual acuity. In addition, although they had details on retinopathy, cataracts, and glaucoma status, they did not also consider less common ophthalmic conditions. Finally, as a survivor cohort, they acknowledged that they may have “underestimated the cumulative incidence” of vision issues in the participants.
The study was supported by the National Health and Medical Research Council of Australia. The authors reported no conflicts of interest.
SOURCE: Drinkwater JJ et al. J Diabetes Complications. 2020 Feb 20. doi: 10.1016/j.jdiacomp.2020.107560.
according to new findings published in the Journal of Diabetes and its Complications.
“Smoking cessation strategies and optimal cardiometabolic risk factor management, including blood glucose lowering regimens that minimize hypoglycemia, appear important in preventing the loss of vision associated with type 2 diabetes,” wrote Jocelyn J. Drinkwater of the University of Western Australia, Perth, and coauthors, noting that all three noted risk factors were “potentially modifiable.”
To investigate the impact of type 2 diabetes and associated risk factors on vision, the researchers recruited 1,732 participants for the Fremantle Diabetes Study Phase II, of whom 1,551 patients had type 2 diabetes and underwent face-to-face and visual acuity assessments at baseline and at 2 and 4 years. Visual acuity was measured via the Bailey Lovie chart at a distance of 3 m in a well-lit room. Normal or near-normal vision was classified as a visual acuity of equal to or less than 6/19; visual impairment, a visual acuity of greater than 6/19 and equal to or less than 6/48; and blindness, a visual acuity of greater than 6/48. A change in vision was classified as a difference in visual acuity of more than 10 letters from baseline measurement.
Of the initial 1,551 participants, 31 were excluded because of missing baseline data for visual acuity. The remaining group comprised 52.2% men, the mean age was 65.6 years, and the median diabetes duration was 8.5 years (interquartile range, 2.9-15.8). At baseline, the prevalence of visual impairment was 1.8% (28 patients), and prevalence of blindness was 0.7% (11 patients), so those 39 patients were also excluded from further analysis.
After 4 years, 599 patients (39%) were excluded because of attrition or missing data; among them, 138 (23%) died before the follow-up.
The remaining 882 participants (58%) had their visual acuity measured. Among these patients, 62.2% were men, with a mean age of 65.1 years and an initial median diabetes duration of 7 years (IQR, 2.0-15.0). Their cumulative incidence of visual impairment was 0.9% (eight patients), and no patients with normal or near-normal vision had developed blindness. Cumulative incidence of vision loss was 2.9% (26), and 1.9% (17) had improved visual acuity.
After multivariable logistic regression to determine predictors for vision loss, the researchers found that participants who smoked at baseline were more than three times more likely to lose their vision (odds ratio, 3.17; 95% confidence interval, 1.15-8.76; P = .026). Although smoking was noted as a “well-recognized risk factor for ocular disease,” the authors added that ex-smokers did not have significantly higher odds of vision loss, compared with nonsmokers, suggesting that the “ocular damage caused by smoking may not be permanent.”
Participants who had suffered a severe hypoglycemic event before the study were five times more likely to lose their vision (OR, 5.59; 95% CI, 1.32-23.61; P = .019). The authors emphasized that severe hypoglycemia can worsen existing ischemic tissue damage or contribute to a long duration of poorly controlled diabetes, each of which could “increase the risk of ocular complications leading to impaired vision.”
The final notable risk factor was compromised kidney function, which is identified as a urinary albumin-creatinine ratio (uACR). The authors noted that the uACR has been associated with other ocular pathologies, such as retinopathy and macular edema, and that uACR may be a “surrogate marker of a variety of ocular diseases with shared risk factors, such as poor metabolic control, which have implications for vision.”
In regard to the possible limitations of the study, they authors noted that they had not used the “gold standard” Early Treatment Diabetic Retinopathy Study chart to assess visual acuity. In addition, although they had details on retinopathy, cataracts, and glaucoma status, they did not also consider less common ophthalmic conditions. Finally, as a survivor cohort, they acknowledged that they may have “underestimated the cumulative incidence” of vision issues in the participants.
The study was supported by the National Health and Medical Research Council of Australia. The authors reported no conflicts of interest.
SOURCE: Drinkwater JJ et al. J Diabetes Complications. 2020 Feb 20. doi: 10.1016/j.jdiacomp.2020.107560.
FROM THE JOURNAL OF DIABETES AND ITS COMPLICATIONS
American Academy of Neurology cancels annual meeting amid COVID-19 pandemic
“Protecting the health, safety, and well-being of our members, attendees, and ultimately our neurology patients is paramount, and serves as the reason for our decision to cancel the AAN annual meeting for the first time in our 72-year history,” AAN President James Stevens, MD, said in a statement. “Put simply, canceling the AAN annual meeting is the right thing to do during this historic time.”
Dr. Stevens added that it is “important to keep our members in their communities – where you stand by to help patients during this time of uncertainty. We also have a professional responsibility to model social distancing and not contribute to the spread of the virus through a large public gathering.”
AAN said it is currently processing full registration fee refunds for those who had registered to attend. Information for exhibitors and sponsors will be forthcoming.
As for missed CME opportunities related to attending the annual meeting, AAN will provide different educational opportunities throughout the remainder of 2020.
Further questions should be directed via email to [email protected]. Additional information related to the cancellation will be posted to the AAN website and via social media.
“Protecting the health, safety, and well-being of our members, attendees, and ultimately our neurology patients is paramount, and serves as the reason for our decision to cancel the AAN annual meeting for the first time in our 72-year history,” AAN President James Stevens, MD, said in a statement. “Put simply, canceling the AAN annual meeting is the right thing to do during this historic time.”
Dr. Stevens added that it is “important to keep our members in their communities – where you stand by to help patients during this time of uncertainty. We also have a professional responsibility to model social distancing and not contribute to the spread of the virus through a large public gathering.”
AAN said it is currently processing full registration fee refunds for those who had registered to attend. Information for exhibitors and sponsors will be forthcoming.
As for missed CME opportunities related to attending the annual meeting, AAN will provide different educational opportunities throughout the remainder of 2020.
Further questions should be directed via email to [email protected]. Additional information related to the cancellation will be posted to the AAN website and via social media.
“Protecting the health, safety, and well-being of our members, attendees, and ultimately our neurology patients is paramount, and serves as the reason for our decision to cancel the AAN annual meeting for the first time in our 72-year history,” AAN President James Stevens, MD, said in a statement. “Put simply, canceling the AAN annual meeting is the right thing to do during this historic time.”
Dr. Stevens added that it is “important to keep our members in their communities – where you stand by to help patients during this time of uncertainty. We also have a professional responsibility to model social distancing and not contribute to the spread of the virus through a large public gathering.”
AAN said it is currently processing full registration fee refunds for those who had registered to attend. Information for exhibitors and sponsors will be forthcoming.
As for missed CME opportunities related to attending the annual meeting, AAN will provide different educational opportunities throughout the remainder of 2020.
Further questions should be directed via email to [email protected]. Additional information related to the cancellation will be posted to the AAN website and via social media.
Expert says progress in gut-brain research requires an open mind
A growing body of research links the gut with the brain and behavior, but compartmentalization within the medical community may be slowing investigation of the gut-brain axis, according to a leading expert.
Studies have shown that the microbiome may influence a diverse range of behavioral and neurological processes, from acute and chronic stress responses to development of Parkinson’s and Alzheimer’s disease, reported John F. Cryan, PhD, of University College Cork, Ireland.
Dr. Cryan began his presentation at the annual Gut Microbiota for Health World Summit by citing Hippocrates, who is thought to have stated that all diseases begin in the gut.
“That can be quite strange when I talk to my neurology or psychiatry colleagues,” Dr. Cryan said. “They sometimes look at me like I have two heads. Because in medicine we compartmentalize, and if you are studying neurology or psychiatry or [you are] in clinical practice, you are focusing on everything from the neck upwards.”
For more than a decade, Dr. Cryan and colleagues have been investigating the gut-brain axis, predominantly in mouse models, but also across animal species and in humans.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Cryan reviewed a variety of representative studies.
For instance, in both mice and humans, research has shown that C-section, which is associated with poorer microbiome diversity than vaginal delivery, has also been linked with social deficits and elevated stress responses. And in the case of mice, coprophagia, in which cesarean-delivered mice eat the feces of vaginally born mice, has been shown to ameliorate these psychiatric effects.
Dr. Cryan likened this process to an “artificial fecal transplant.”
“You know, co-housing and eating each other’s poo is not the translational approach that we were advocating by any means,” Dr. Cryan said. “But at least it tells us – in a proof-of-concept way – that if we change the microbiome, then we can reverse what’s going on.”
While the mechanisms behind the gut-brain axis remain incompletely understood, Dr. Cryan noted that the vagus nerve, which travels from the gut to the brain, plays a central role, and that transecting this nerve in mice stops the microbiome from affecting the brain.
“What happens in vagus doesn’t just stay in vagus, but will actually affect our emotions in different ways,” Dr. Cryan said.
He emphasized that communication travels both ways along the gut-brain axis, and went on to describe how this phenomenon has been demonstrated across a wide array of animals.
“From insects all the way through to primates, if you start to interfere with social behavior, you change the microbiome,” Dr. Cryan said. “But the opposite is also true; if you start to change the microbiome you can start to have widespread effects on social behavior.”
In humans, manipulating the microbiome could open up new psychiatric frontiers, Dr. Cryan said.
“[In the past 30 years], there really have been no real advances in how we manage mental health,” he said. “That’s very sobering when we are having such a mental health problem across all ages right now. And so perhaps it’s time for what we’ve coined the ‘psychobiotic revolution’ – time for a new way of thinking about mental health.”
According to Dr. Cryan, psychobiotics are interventions that target the microbiome for mental health purposes, including fermented foods, probiotics, prebiotics, synbiotics, parabiotics, and postbiotics.
Among these, probiotics have been a focal point of interventional research. Although results have been mixed, Dr. Cryan suggested that negative probiotic studies are more likely due to bacterial strain than a failure of the concept as a whole.
“Most strains of bacteria will do absolutely nothing,” Dr. Cryan said. “Strain is really important.”
In demonstration of this concept, he recounted a 2017 study conducted at University College Cork in which 22 healthy volunteers were given Bifidobacterium longum 1714, and then subjected to a social stress test. The results, published in Translational Psychiatry, showed that the probiotic, compared with placebo, was associated with attenuated stress responses, reduced daily stress, and enhanced visuospatial memory.
In contrast, a similar study by Dr. Cryan and colleagues, which tested Lactobacillus rhamnosus (JB-1), fell short.
“You [could not have gotten] more negative data into one paper if you tried,” Dr. Cryan said, referring to the study. “It did absolutely nothing.”
To find out which psychobiotics may have an impact, and how, Dr. Cryan called for more research.
“It’s still early days,” he said. “We probably have more meta-analyses and systematic reviews of the field than we have primary research papers.
Dr. Cryan concluded his presentation on an optimistic note.
“Neurology is waking up ... to understand that the microbiome could be playing a key role in many, many other disorders. ... Overall, what we’re beginning to see is that our state of gut markedly affects our state of mind.”
Dr. Cryan disclosed relationships with Abbott Nutrition, Roche Pharma, Nutricia, and others.
A growing body of research links the gut with the brain and behavior, but compartmentalization within the medical community may be slowing investigation of the gut-brain axis, according to a leading expert.
Studies have shown that the microbiome may influence a diverse range of behavioral and neurological processes, from acute and chronic stress responses to development of Parkinson’s and Alzheimer’s disease, reported John F. Cryan, PhD, of University College Cork, Ireland.
Dr. Cryan began his presentation at the annual Gut Microbiota for Health World Summit by citing Hippocrates, who is thought to have stated that all diseases begin in the gut.
“That can be quite strange when I talk to my neurology or psychiatry colleagues,” Dr. Cryan said. “They sometimes look at me like I have two heads. Because in medicine we compartmentalize, and if you are studying neurology or psychiatry or [you are] in clinical practice, you are focusing on everything from the neck upwards.”
For more than a decade, Dr. Cryan and colleagues have been investigating the gut-brain axis, predominantly in mouse models, but also across animal species and in humans.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Cryan reviewed a variety of representative studies.
For instance, in both mice and humans, research has shown that C-section, which is associated with poorer microbiome diversity than vaginal delivery, has also been linked with social deficits and elevated stress responses. And in the case of mice, coprophagia, in which cesarean-delivered mice eat the feces of vaginally born mice, has been shown to ameliorate these psychiatric effects.
Dr. Cryan likened this process to an “artificial fecal transplant.”
“You know, co-housing and eating each other’s poo is not the translational approach that we were advocating by any means,” Dr. Cryan said. “But at least it tells us – in a proof-of-concept way – that if we change the microbiome, then we can reverse what’s going on.”
While the mechanisms behind the gut-brain axis remain incompletely understood, Dr. Cryan noted that the vagus nerve, which travels from the gut to the brain, plays a central role, and that transecting this nerve in mice stops the microbiome from affecting the brain.
“What happens in vagus doesn’t just stay in vagus, but will actually affect our emotions in different ways,” Dr. Cryan said.
He emphasized that communication travels both ways along the gut-brain axis, and went on to describe how this phenomenon has been demonstrated across a wide array of animals.
“From insects all the way through to primates, if you start to interfere with social behavior, you change the microbiome,” Dr. Cryan said. “But the opposite is also true; if you start to change the microbiome you can start to have widespread effects on social behavior.”
In humans, manipulating the microbiome could open up new psychiatric frontiers, Dr. Cryan said.
“[In the past 30 years], there really have been no real advances in how we manage mental health,” he said. “That’s very sobering when we are having such a mental health problem across all ages right now. And so perhaps it’s time for what we’ve coined the ‘psychobiotic revolution’ – time for a new way of thinking about mental health.”
According to Dr. Cryan, psychobiotics are interventions that target the microbiome for mental health purposes, including fermented foods, probiotics, prebiotics, synbiotics, parabiotics, and postbiotics.
Among these, probiotics have been a focal point of interventional research. Although results have been mixed, Dr. Cryan suggested that negative probiotic studies are more likely due to bacterial strain than a failure of the concept as a whole.
“Most strains of bacteria will do absolutely nothing,” Dr. Cryan said. “Strain is really important.”
In demonstration of this concept, he recounted a 2017 study conducted at University College Cork in which 22 healthy volunteers were given Bifidobacterium longum 1714, and then subjected to a social stress test. The results, published in Translational Psychiatry, showed that the probiotic, compared with placebo, was associated with attenuated stress responses, reduced daily stress, and enhanced visuospatial memory.
In contrast, a similar study by Dr. Cryan and colleagues, which tested Lactobacillus rhamnosus (JB-1), fell short.
“You [could not have gotten] more negative data into one paper if you tried,” Dr. Cryan said, referring to the study. “It did absolutely nothing.”
To find out which psychobiotics may have an impact, and how, Dr. Cryan called for more research.
“It’s still early days,” he said. “We probably have more meta-analyses and systematic reviews of the field than we have primary research papers.
Dr. Cryan concluded his presentation on an optimistic note.
“Neurology is waking up ... to understand that the microbiome could be playing a key role in many, many other disorders. ... Overall, what we’re beginning to see is that our state of gut markedly affects our state of mind.”
Dr. Cryan disclosed relationships with Abbott Nutrition, Roche Pharma, Nutricia, and others.
A growing body of research links the gut with the brain and behavior, but compartmentalization within the medical community may be slowing investigation of the gut-brain axis, according to a leading expert.
Studies have shown that the microbiome may influence a diverse range of behavioral and neurological processes, from acute and chronic stress responses to development of Parkinson’s and Alzheimer’s disease, reported John F. Cryan, PhD, of University College Cork, Ireland.
Dr. Cryan began his presentation at the annual Gut Microbiota for Health World Summit by citing Hippocrates, who is thought to have stated that all diseases begin in the gut.
“That can be quite strange when I talk to my neurology or psychiatry colleagues,” Dr. Cryan said. “They sometimes look at me like I have two heads. Because in medicine we compartmentalize, and if you are studying neurology or psychiatry or [you are] in clinical practice, you are focusing on everything from the neck upwards.”
For more than a decade, Dr. Cryan and colleagues have been investigating the gut-brain axis, predominantly in mouse models, but also across animal species and in humans.
At the meeting, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility, Dr. Cryan reviewed a variety of representative studies.
For instance, in both mice and humans, research has shown that C-section, which is associated with poorer microbiome diversity than vaginal delivery, has also been linked with social deficits and elevated stress responses. And in the case of mice, coprophagia, in which cesarean-delivered mice eat the feces of vaginally born mice, has been shown to ameliorate these psychiatric effects.
Dr. Cryan likened this process to an “artificial fecal transplant.”
“You know, co-housing and eating each other’s poo is not the translational approach that we were advocating by any means,” Dr. Cryan said. “But at least it tells us – in a proof-of-concept way – that if we change the microbiome, then we can reverse what’s going on.”
While the mechanisms behind the gut-brain axis remain incompletely understood, Dr. Cryan noted that the vagus nerve, which travels from the gut to the brain, plays a central role, and that transecting this nerve in mice stops the microbiome from affecting the brain.
“What happens in vagus doesn’t just stay in vagus, but will actually affect our emotions in different ways,” Dr. Cryan said.
He emphasized that communication travels both ways along the gut-brain axis, and went on to describe how this phenomenon has been demonstrated across a wide array of animals.
“From insects all the way through to primates, if you start to interfere with social behavior, you change the microbiome,” Dr. Cryan said. “But the opposite is also true; if you start to change the microbiome you can start to have widespread effects on social behavior.”
In humans, manipulating the microbiome could open up new psychiatric frontiers, Dr. Cryan said.
“[In the past 30 years], there really have been no real advances in how we manage mental health,” he said. “That’s very sobering when we are having such a mental health problem across all ages right now. And so perhaps it’s time for what we’ve coined the ‘psychobiotic revolution’ – time for a new way of thinking about mental health.”
According to Dr. Cryan, psychobiotics are interventions that target the microbiome for mental health purposes, including fermented foods, probiotics, prebiotics, synbiotics, parabiotics, and postbiotics.
Among these, probiotics have been a focal point of interventional research. Although results have been mixed, Dr. Cryan suggested that negative probiotic studies are more likely due to bacterial strain than a failure of the concept as a whole.
“Most strains of bacteria will do absolutely nothing,” Dr. Cryan said. “Strain is really important.”
In demonstration of this concept, he recounted a 2017 study conducted at University College Cork in which 22 healthy volunteers were given Bifidobacterium longum 1714, and then subjected to a social stress test. The results, published in Translational Psychiatry, showed that the probiotic, compared with placebo, was associated with attenuated stress responses, reduced daily stress, and enhanced visuospatial memory.
In contrast, a similar study by Dr. Cryan and colleagues, which tested Lactobacillus rhamnosus (JB-1), fell short.
“You [could not have gotten] more negative data into one paper if you tried,” Dr. Cryan said, referring to the study. “It did absolutely nothing.”
To find out which psychobiotics may have an impact, and how, Dr. Cryan called for more research.
“It’s still early days,” he said. “We probably have more meta-analyses and systematic reviews of the field than we have primary research papers.
Dr. Cryan concluded his presentation on an optimistic note.
“Neurology is waking up ... to understand that the microbiome could be playing a key role in many, many other disorders. ... Overall, what we’re beginning to see is that our state of gut markedly affects our state of mind.”
Dr. Cryan disclosed relationships with Abbott Nutrition, Roche Pharma, Nutricia, and others.
FROM GMFH 2020