User login
Wuhan case review: COVID-19 characteristics differ in children vs. adults
Pediatric cases of COVID-19 infection are typically mild, but underlying coinfection may be more common in children than in adults, according to an analysis of clinical, laboratory, and chest CT features of pediatric inpatients in Wuhan, China.

The findings point toward a need for early chest CT with corresponding pathogen detection in children with suspected COVID-19 infection, Wei Xia, MD, of Huazhong University of Science and Technology, Wuhan, China, and colleagues reported in Pediatric Pulmonology.
The most common symptoms in 20 pediatric patients hospitalized between Jan. 23 and Feb. 8, 2020, with COVID-19 infection confirmed by the pharyngeal swab COVID-19 nucleic acid test were fever and cough, which occurred in 60% and 65% of patients, respectively. Coinfection was detected in eight patients (40%), they noted.
Clinical manifestations were similar to those seen in adults, but overall symptoms were relatively mild and overall prognosis was good. Of particular note, 7 of the 20 (35%) patients had a previously diagnosed congenital or acquired diseases, suggesting that children with underlying conditions may be more susceptible, Dr. Xia and colleagues wrote.
Laboratory findings also were notable in that 80% of the children had procalcitonin (PCT) elevations not typically seen in adults with COVID-19. PCT is a marker for bacterial infection and “[this finding] may suggest that routine antibacterial treatment should be considered in pediatric patients,” the investigators wrote.
As for imaging results, chest CT findings in children were similar to those in adults.“The typical manifestations were unilateral or bilateral subpleural ground-glass opacities, and consolidations with surrounding halo signs,” Dr. Xia and associates wrote, adding that consolidations with surrounding halo sign accounted for about half the pediatric cases and should be considered as “typical signs in pediatric patients.”
Pediatric cases were “rather rare” in the early days of the COVID-19 outbreak in Wuhan, where the first cases of infection were reported.
“As a pediatric group is usually susceptible to upper respiratory tract infection, because of their developing immune system, the delayed presence of pediatric patients is confusing,” the investigators wrote, noting that a low detection rate of pharyngeal swab COVID-19 nucleic acid test, distinguishing the virus from other common respiratory tract infectious pathogens in pediatric patients, “is still a problem.”
To better characterize the clinical and imaging features in children versus adults with COVID-19, Dr. Xia and associates reviewed these 20 pediatric cases, including 13 boys and 7 girls with ages ranging from less than 1 month to 14 years, 7 months (median 2 years, 1.5 months). Thirteen had an identified close contact with a COVID-19–diagnosed family member, and all were treated in an isolation ward. A total of 18 children were cured and discharged after an average stay of 13 days, and 2 neonates remained under observation because of positive swab results with negative CT findings. The investigators speculated that the different findings in neonates were perhaps caused by the influence of delivery on sampling or the specific CT manifestations for neonates, adding that more samples are needed for further clarification.
Based on these findings, “the CT imaging of COVID-19 infection should be differentiated with other virus pneumonias such as influenza virus, parainfluenza virus, respiratory syncytial virus, and adenovirus,” they concluded. It also should “be differentiated from bacterial pneumonia, mycoplasma pneumonia, and chlamydia pneumonia ... the density of pneumonia lesions caused by the latter pathogens is relatively higher.”
However, Dr. Xia and colleagues noted that chest CT manifestations of pneumonia caused by different pathogens overlap, and COVID-19 pneumonia “can be superimposed with serious and complex imaging manifestations, so epidemiological and etiological examinations should be combined.”
The investigators concluded that COVID-19 virus pneumonia in children is generally mild, and that the characteristic changes of subpleural ground-glass opacities and consolidations with surrounding halo on chest CT provide an “effective means for follow-up and evaluating the changes of lung lesions.”
“In the case that the positive rate of COVID-19 nucleic acid test from pharyngeal swab samples is not high, the early detection of lesions by CT is conducive to reasonable management and early treatment for pediatric patients. However, the diagnosis of COVID-19 pneumonia by CT imaging alone is not sufficient enough, especially in the case of coinfection with other pathogens,” Dr. Xia and associates wrote. “Therefore, early chest CT screening and timely follow-up, combined with corresponding pathogen detection, is a feasible clinical protocol in children.”
An early study
In a separate retrospective analysis described in a letter to the editor of the New England Journal of Medicine, Weiyong Liu, PhD, of Tongji Hospital of Huazhong University of Science and Technology and colleagues found that the most frequently detected pathogens in 366 children under the age of 16 years hospitalized with respiratory infections in Wuhan during Jan. 7-15, 2020, were influenza A virus (6.3% of cases) and influenza B virus (5.5% of cases), whereas COVID-19 was detected in 1.6% of cases.
The median age of the COVID-19 patients in that series was 3 years (range 1-7 years), and in contrast to the findings of Xia et al., all previously had been “completely healthy.” Common characteristics were high fever and cough in all six patients, and vomiting in four patients. Five had pneumonia as assessed by X-ray, and CTs showed typical viral pneumonia patterns.
One patient was admitted to a pediatric ICU. All patients received antiviral agents, antibiotic agents, and supportive therapies; all recovered after a median hospital stay of 7.5 days (median range, 5-13 days).
In contrast with the findings of Xia et al., the findings of Liu et al. showed COVID-19 caused moderate to severe respiratory illness in children, and that infections in children were occurring early in the epidemic.
Some perspective
In an interview regarding the findings by Xia et al., Stephen I. Pelton, MD, professor of pediatrics and epidemiology at Boston University, and director of pediatric infectious diseases at Boston Medical Center, noted the absence of fever in 40% of cases.
“This is important, as the criteria for testing by public health departments has been high fever, cough, and shortness of breath,” he said. “The absence of fever is not inconsistent with COVID-19 disease.”
Another important point regarding the findings by Xia et al. is that the highest attack rates appear to be in children under 1 year of age, he said, further noting that the finding of concurrent influenza A, influenza B, or respiratory syncytial virus underscores that “concurrent infection can occur, and the presence of another virus in diagnostic tests does not mean that COVID-19 is not causal.”
As for whether the finding of elevated procalcitonin levels in 80% of cases reflects COVID-19 disease or coinfection with bacteria, the answer is unclear. But none of the children in the study were proven to have bacterial disease, he said, adding that “this marker will need to be interpreted with caution in the setting of COVID-19 disease.”
Dr. Xia and colleagues reported having no disclosures. Dr. Liu and associates also reported having no disclosures. The study by Liu et al. was supported by the Ministry of Science and Technology of China, the National Mega Project on Major Infectious Disease Prevention, and the National Key Research and Development Program of China.
SOURCES: Xia W et al. Ped Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718; Liu W et al. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Pediatric cases of COVID-19 infection are typically mild, but underlying coinfection may be more common in children than in adults, according to an analysis of clinical, laboratory, and chest CT features of pediatric inpatients in Wuhan, China.

The findings point toward a need for early chest CT with corresponding pathogen detection in children with suspected COVID-19 infection, Wei Xia, MD, of Huazhong University of Science and Technology, Wuhan, China, and colleagues reported in Pediatric Pulmonology.
The most common symptoms in 20 pediatric patients hospitalized between Jan. 23 and Feb. 8, 2020, with COVID-19 infection confirmed by the pharyngeal swab COVID-19 nucleic acid test were fever and cough, which occurred in 60% and 65% of patients, respectively. Coinfection was detected in eight patients (40%), they noted.
Clinical manifestations were similar to those seen in adults, but overall symptoms were relatively mild and overall prognosis was good. Of particular note, 7 of the 20 (35%) patients had a previously diagnosed congenital or acquired diseases, suggesting that children with underlying conditions may be more susceptible, Dr. Xia and colleagues wrote.
Laboratory findings also were notable in that 80% of the children had procalcitonin (PCT) elevations not typically seen in adults with COVID-19. PCT is a marker for bacterial infection and “[this finding] may suggest that routine antibacterial treatment should be considered in pediatric patients,” the investigators wrote.
As for imaging results, chest CT findings in children were similar to those in adults.“The typical manifestations were unilateral or bilateral subpleural ground-glass opacities, and consolidations with surrounding halo signs,” Dr. Xia and associates wrote, adding that consolidations with surrounding halo sign accounted for about half the pediatric cases and should be considered as “typical signs in pediatric patients.”
Pediatric cases were “rather rare” in the early days of the COVID-19 outbreak in Wuhan, where the first cases of infection were reported.
“As a pediatric group is usually susceptible to upper respiratory tract infection, because of their developing immune system, the delayed presence of pediatric patients is confusing,” the investigators wrote, noting that a low detection rate of pharyngeal swab COVID-19 nucleic acid test, distinguishing the virus from other common respiratory tract infectious pathogens in pediatric patients, “is still a problem.”
To better characterize the clinical and imaging features in children versus adults with COVID-19, Dr. Xia and associates reviewed these 20 pediatric cases, including 13 boys and 7 girls with ages ranging from less than 1 month to 14 years, 7 months (median 2 years, 1.5 months). Thirteen had an identified close contact with a COVID-19–diagnosed family member, and all were treated in an isolation ward. A total of 18 children were cured and discharged after an average stay of 13 days, and 2 neonates remained under observation because of positive swab results with negative CT findings. The investigators speculated that the different findings in neonates were perhaps caused by the influence of delivery on sampling or the specific CT manifestations for neonates, adding that more samples are needed for further clarification.
Based on these findings, “the CT imaging of COVID-19 infection should be differentiated with other virus pneumonias such as influenza virus, parainfluenza virus, respiratory syncytial virus, and adenovirus,” they concluded. It also should “be differentiated from bacterial pneumonia, mycoplasma pneumonia, and chlamydia pneumonia ... the density of pneumonia lesions caused by the latter pathogens is relatively higher.”
However, Dr. Xia and colleagues noted that chest CT manifestations of pneumonia caused by different pathogens overlap, and COVID-19 pneumonia “can be superimposed with serious and complex imaging manifestations, so epidemiological and etiological examinations should be combined.”
The investigators concluded that COVID-19 virus pneumonia in children is generally mild, and that the characteristic changes of subpleural ground-glass opacities and consolidations with surrounding halo on chest CT provide an “effective means for follow-up and evaluating the changes of lung lesions.”
“In the case that the positive rate of COVID-19 nucleic acid test from pharyngeal swab samples is not high, the early detection of lesions by CT is conducive to reasonable management and early treatment for pediatric patients. However, the diagnosis of COVID-19 pneumonia by CT imaging alone is not sufficient enough, especially in the case of coinfection with other pathogens,” Dr. Xia and associates wrote. “Therefore, early chest CT screening and timely follow-up, combined with corresponding pathogen detection, is a feasible clinical protocol in children.”
An early study
In a separate retrospective analysis described in a letter to the editor of the New England Journal of Medicine, Weiyong Liu, PhD, of Tongji Hospital of Huazhong University of Science and Technology and colleagues found that the most frequently detected pathogens in 366 children under the age of 16 years hospitalized with respiratory infections in Wuhan during Jan. 7-15, 2020, were influenza A virus (6.3% of cases) and influenza B virus (5.5% of cases), whereas COVID-19 was detected in 1.6% of cases.
The median age of the COVID-19 patients in that series was 3 years (range 1-7 years), and in contrast to the findings of Xia et al., all previously had been “completely healthy.” Common characteristics were high fever and cough in all six patients, and vomiting in four patients. Five had pneumonia as assessed by X-ray, and CTs showed typical viral pneumonia patterns.
One patient was admitted to a pediatric ICU. All patients received antiviral agents, antibiotic agents, and supportive therapies; all recovered after a median hospital stay of 7.5 days (median range, 5-13 days).
In contrast with the findings of Xia et al., the findings of Liu et al. showed COVID-19 caused moderate to severe respiratory illness in children, and that infections in children were occurring early in the epidemic.
Some perspective
In an interview regarding the findings by Xia et al., Stephen I. Pelton, MD, professor of pediatrics and epidemiology at Boston University, and director of pediatric infectious diseases at Boston Medical Center, noted the absence of fever in 40% of cases.
“This is important, as the criteria for testing by public health departments has been high fever, cough, and shortness of breath,” he said. “The absence of fever is not inconsistent with COVID-19 disease.”
Another important point regarding the findings by Xia et al. is that the highest attack rates appear to be in children under 1 year of age, he said, further noting that the finding of concurrent influenza A, influenza B, or respiratory syncytial virus underscores that “concurrent infection can occur, and the presence of another virus in diagnostic tests does not mean that COVID-19 is not causal.”
As for whether the finding of elevated procalcitonin levels in 80% of cases reflects COVID-19 disease or coinfection with bacteria, the answer is unclear. But none of the children in the study were proven to have bacterial disease, he said, adding that “this marker will need to be interpreted with caution in the setting of COVID-19 disease.”
Dr. Xia and colleagues reported having no disclosures. Dr. Liu and associates also reported having no disclosures. The study by Liu et al. was supported by the Ministry of Science and Technology of China, the National Mega Project on Major Infectious Disease Prevention, and the National Key Research and Development Program of China.
SOURCES: Xia W et al. Ped Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718; Liu W et al. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Pediatric cases of COVID-19 infection are typically mild, but underlying coinfection may be more common in children than in adults, according to an analysis of clinical, laboratory, and chest CT features of pediatric inpatients in Wuhan, China.

The findings point toward a need for early chest CT with corresponding pathogen detection in children with suspected COVID-19 infection, Wei Xia, MD, of Huazhong University of Science and Technology, Wuhan, China, and colleagues reported in Pediatric Pulmonology.
The most common symptoms in 20 pediatric patients hospitalized between Jan. 23 and Feb. 8, 2020, with COVID-19 infection confirmed by the pharyngeal swab COVID-19 nucleic acid test were fever and cough, which occurred in 60% and 65% of patients, respectively. Coinfection was detected in eight patients (40%), they noted.
Clinical manifestations were similar to those seen in adults, but overall symptoms were relatively mild and overall prognosis was good. Of particular note, 7 of the 20 (35%) patients had a previously diagnosed congenital or acquired diseases, suggesting that children with underlying conditions may be more susceptible, Dr. Xia and colleagues wrote.
Laboratory findings also were notable in that 80% of the children had procalcitonin (PCT) elevations not typically seen in adults with COVID-19. PCT is a marker for bacterial infection and “[this finding] may suggest that routine antibacterial treatment should be considered in pediatric patients,” the investigators wrote.
As for imaging results, chest CT findings in children were similar to those in adults.“The typical manifestations were unilateral or bilateral subpleural ground-glass opacities, and consolidations with surrounding halo signs,” Dr. Xia and associates wrote, adding that consolidations with surrounding halo sign accounted for about half the pediatric cases and should be considered as “typical signs in pediatric patients.”
Pediatric cases were “rather rare” in the early days of the COVID-19 outbreak in Wuhan, where the first cases of infection were reported.
“As a pediatric group is usually susceptible to upper respiratory tract infection, because of their developing immune system, the delayed presence of pediatric patients is confusing,” the investigators wrote, noting that a low detection rate of pharyngeal swab COVID-19 nucleic acid test, distinguishing the virus from other common respiratory tract infectious pathogens in pediatric patients, “is still a problem.”
To better characterize the clinical and imaging features in children versus adults with COVID-19, Dr. Xia and associates reviewed these 20 pediatric cases, including 13 boys and 7 girls with ages ranging from less than 1 month to 14 years, 7 months (median 2 years, 1.5 months). Thirteen had an identified close contact with a COVID-19–diagnosed family member, and all were treated in an isolation ward. A total of 18 children were cured and discharged after an average stay of 13 days, and 2 neonates remained under observation because of positive swab results with negative CT findings. The investigators speculated that the different findings in neonates were perhaps caused by the influence of delivery on sampling or the specific CT manifestations for neonates, adding that more samples are needed for further clarification.
Based on these findings, “the CT imaging of COVID-19 infection should be differentiated with other virus pneumonias such as influenza virus, parainfluenza virus, respiratory syncytial virus, and adenovirus,” they concluded. It also should “be differentiated from bacterial pneumonia, mycoplasma pneumonia, and chlamydia pneumonia ... the density of pneumonia lesions caused by the latter pathogens is relatively higher.”
However, Dr. Xia and colleagues noted that chest CT manifestations of pneumonia caused by different pathogens overlap, and COVID-19 pneumonia “can be superimposed with serious and complex imaging manifestations, so epidemiological and etiological examinations should be combined.”
The investigators concluded that COVID-19 virus pneumonia in children is generally mild, and that the characteristic changes of subpleural ground-glass opacities and consolidations with surrounding halo on chest CT provide an “effective means for follow-up and evaluating the changes of lung lesions.”
“In the case that the positive rate of COVID-19 nucleic acid test from pharyngeal swab samples is not high, the early detection of lesions by CT is conducive to reasonable management and early treatment for pediatric patients. However, the diagnosis of COVID-19 pneumonia by CT imaging alone is not sufficient enough, especially in the case of coinfection with other pathogens,” Dr. Xia and associates wrote. “Therefore, early chest CT screening and timely follow-up, combined with corresponding pathogen detection, is a feasible clinical protocol in children.”
An early study
In a separate retrospective analysis described in a letter to the editor of the New England Journal of Medicine, Weiyong Liu, PhD, of Tongji Hospital of Huazhong University of Science and Technology and colleagues found that the most frequently detected pathogens in 366 children under the age of 16 years hospitalized with respiratory infections in Wuhan during Jan. 7-15, 2020, were influenza A virus (6.3% of cases) and influenza B virus (5.5% of cases), whereas COVID-19 was detected in 1.6% of cases.
The median age of the COVID-19 patients in that series was 3 years (range 1-7 years), and in contrast to the findings of Xia et al., all previously had been “completely healthy.” Common characteristics were high fever and cough in all six patients, and vomiting in four patients. Five had pneumonia as assessed by X-ray, and CTs showed typical viral pneumonia patterns.
One patient was admitted to a pediatric ICU. All patients received antiviral agents, antibiotic agents, and supportive therapies; all recovered after a median hospital stay of 7.5 days (median range, 5-13 days).
In contrast with the findings of Xia et al., the findings of Liu et al. showed COVID-19 caused moderate to severe respiratory illness in children, and that infections in children were occurring early in the epidemic.
Some perspective
In an interview regarding the findings by Xia et al., Stephen I. Pelton, MD, professor of pediatrics and epidemiology at Boston University, and director of pediatric infectious diseases at Boston Medical Center, noted the absence of fever in 40% of cases.
“This is important, as the criteria for testing by public health departments has been high fever, cough, and shortness of breath,” he said. “The absence of fever is not inconsistent with COVID-19 disease.”
Another important point regarding the findings by Xia et al. is that the highest attack rates appear to be in children under 1 year of age, he said, further noting that the finding of concurrent influenza A, influenza B, or respiratory syncytial virus underscores that “concurrent infection can occur, and the presence of another virus in diagnostic tests does not mean that COVID-19 is not causal.”
As for whether the finding of elevated procalcitonin levels in 80% of cases reflects COVID-19 disease or coinfection with bacteria, the answer is unclear. But none of the children in the study were proven to have bacterial disease, he said, adding that “this marker will need to be interpreted with caution in the setting of COVID-19 disease.”
Dr. Xia and colleagues reported having no disclosures. Dr. Liu and associates also reported having no disclosures. The study by Liu et al. was supported by the Ministry of Science and Technology of China, the National Mega Project on Major Infectious Disease Prevention, and the National Key Research and Development Program of China.
SOURCES: Xia W et al. Ped Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718; Liu W et al. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
FROM PEDIATRIC PULMONOLOGY
Sickle cell patients with vitamin D deficiency prone to more ED visits, longer stays
Patients with sickle cell disease (SCD) plus vitamin D deficiency were found to have more hospitalization outcomes, including number of emergency department (ED) visits, the number of hospital admissions for pain crisis, and the length of hospital admission, according to a study published online by researchers from New York-Presbyterian Brooklyn Methodist Hospital.
The researchers performed a retrospective chart review of all 134 pediatric patients with SCD (aged 1-21 years) from January 2015 to January 2016 in an urban-based hospital setting. Ninety patients with at least one reported vitamin D level who maintained follow-up during the time studied were enrolled. Hospitalization rates were compared between vitamin D deficiency (< 20 ng/mL) and sufficiency (> 20 ng/mL) patients.
When compared to patients with SCD and sufficient vitamin D levels, patients with both SCD and vitamin D deficiency were more likely to have at least one ED visit (P < .01), at least one admission for pain crisis (P < .01), and a longer length of admission (P < .0001), the researchers found.
“Screening and treatment for vitamin D deficiency is generally cost effective and readily available, potentially having a significant impact on the quality of life for those living with sickle cell disease,” the researchers concluded.
The authors reported that there was no study funding and that they had no conflicts of interest.
SOURCE: Brown B et al. Blood Cells Mol Dis. 2020. doi: 10.1016/j.bcmd.2020.102415.
Patients with sickle cell disease (SCD) plus vitamin D deficiency were found to have more hospitalization outcomes, including number of emergency department (ED) visits, the number of hospital admissions for pain crisis, and the length of hospital admission, according to a study published online by researchers from New York-Presbyterian Brooklyn Methodist Hospital.
The researchers performed a retrospective chart review of all 134 pediatric patients with SCD (aged 1-21 years) from January 2015 to January 2016 in an urban-based hospital setting. Ninety patients with at least one reported vitamin D level who maintained follow-up during the time studied were enrolled. Hospitalization rates were compared between vitamin D deficiency (< 20 ng/mL) and sufficiency (> 20 ng/mL) patients.
When compared to patients with SCD and sufficient vitamin D levels, patients with both SCD and vitamin D deficiency were more likely to have at least one ED visit (P < .01), at least one admission for pain crisis (P < .01), and a longer length of admission (P < .0001), the researchers found.
“Screening and treatment for vitamin D deficiency is generally cost effective and readily available, potentially having a significant impact on the quality of life for those living with sickle cell disease,” the researchers concluded.
The authors reported that there was no study funding and that they had no conflicts of interest.
SOURCE: Brown B et al. Blood Cells Mol Dis. 2020. doi: 10.1016/j.bcmd.2020.102415.
Patients with sickle cell disease (SCD) plus vitamin D deficiency were found to have more hospitalization outcomes, including number of emergency department (ED) visits, the number of hospital admissions for pain crisis, and the length of hospital admission, according to a study published online by researchers from New York-Presbyterian Brooklyn Methodist Hospital.
The researchers performed a retrospective chart review of all 134 pediatric patients with SCD (aged 1-21 years) from January 2015 to January 2016 in an urban-based hospital setting. Ninety patients with at least one reported vitamin D level who maintained follow-up during the time studied were enrolled. Hospitalization rates were compared between vitamin D deficiency (< 20 ng/mL) and sufficiency (> 20 ng/mL) patients.
When compared to patients with SCD and sufficient vitamin D levels, patients with both SCD and vitamin D deficiency were more likely to have at least one ED visit (P < .01), at least one admission for pain crisis (P < .01), and a longer length of admission (P < .0001), the researchers found.
“Screening and treatment for vitamin D deficiency is generally cost effective and readily available, potentially having a significant impact on the quality of life for those living with sickle cell disease,” the researchers concluded.
The authors reported that there was no study funding and that they had no conflicts of interest.
SOURCE: Brown B et al. Blood Cells Mol Dis. 2020. doi: 10.1016/j.bcmd.2020.102415.
FROM BLOOD CELLS, MOLECULES, AND DISEASES
Novel mitral valve device shows encouraging mortality data
NATIONAL HARBOR, MD. – In a pooled analysis of three trials conducted with the transcatheter Carillon Mitral Contour System for mitral valve repair, the 5-year survival is 56.2%, which is an encouraging outcome that justifies the ongoing multinational pivotal Carillon trial, according to the principal investigator of the analysis presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
In patients with functional mitral valve regurgitation (FMR), “the Carillon device shows extremely encouraging long-term mortality data from prospective controlled trials in comparison with guideline-directed medical therapy or with the COAPT results,” according to Janusz Lipiecki, MD, PhD, department of cardiology, University Hospital, Clermont-Ferrand, France.
The COAPT trial is an important benchmark, because it was the first large, randomized trial to show benefit for a percutaneous device in the treatment of heart failure patients with moderate to severe FMR (N Engl J Med. 2018;379:2307-18). The study associated the MitraClip with a nearly 40% reduction in all-cause mortality (29.1% vs. 46.1%) at 24 months, relative to guideline-directed medical therapy (GDMT).
The Carillon device, which is also delivered percutaneously, does not engage the valve leaflets to treat FMR. Rather, it is anchored in coronary sinus to reform the mitral annulus. This reduces FMR without damage to the mitral valve, thus preserving the potential for future valve repairs, according to Dr. Lipiecki, who reported that more than 1,100 devices have now been implanted, mostly in Europe.
The data so far are encouraging, said Dr. Lipiecki, who provided survival data in 74 patients followed for at least 5 years. Of these, 23 were drawn from the REDUCE FMR trial, which was blinded and sham controlled, and the remainder from the TITAN and TITAN II studies, which were prospective but not controlled.
In this series of 74 patients, there were no serious complications associated with the procedure, and all achieved a reduction in mitral regurgitation at 12 months, Dr. Lipiecki said. Furthermore, the reduction in FMR was associated with improvements in symptoms and “favorable remodeling” reflected in reduced left ventricular volume, he said.
In this series, the 5-year survival is 56%, which substantially exceeds what would be expected with GDMT, according to Dr. Lipiecki.
There were no baseline predictors of long-term survival, but improvements within the 6 months in functional heart class, 6-minute walk distance (6MWD), and mitral regurgitation were significantly associated with a greater likelihood of being alive at 5 years.
Although there is no comparable follow-up with other devices, including the MitraClip, Dr. Lipiecki did compare the 67.9% survival at 3 years in this series to that of the COAPT trial. He restricted the comparison to those treated for grade 3+ or 4+ mitral regurgitation. At 36 months, survival rates were 57.2% and 44.5% for those treated with MitraClip and GDMT, respectively.
“COAPT patients might not be comparable for a variety of reasons, including the anatomic restrictions important to the use of either of these devices,” Dr. Lipiecki acknowledged, but he said the long-term data with the Carillon device provide support for the pivotal Carillon trial now enrolling.
In this blinded trial, more than 350 patients at 75 sites are being randomized to placement of the Carillon device, which has been available in Europe since 2011, or a sham procedure. The trial is scheduled for completion in 2025.
NATIONAL HARBOR, MD. – In a pooled analysis of three trials conducted with the transcatheter Carillon Mitral Contour System for mitral valve repair, the 5-year survival is 56.2%, which is an encouraging outcome that justifies the ongoing multinational pivotal Carillon trial, according to the principal investigator of the analysis presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
In patients with functional mitral valve regurgitation (FMR), “the Carillon device shows extremely encouraging long-term mortality data from prospective controlled trials in comparison with guideline-directed medical therapy or with the COAPT results,” according to Janusz Lipiecki, MD, PhD, department of cardiology, University Hospital, Clermont-Ferrand, France.
The COAPT trial is an important benchmark, because it was the first large, randomized trial to show benefit for a percutaneous device in the treatment of heart failure patients with moderate to severe FMR (N Engl J Med. 2018;379:2307-18). The study associated the MitraClip with a nearly 40% reduction in all-cause mortality (29.1% vs. 46.1%) at 24 months, relative to guideline-directed medical therapy (GDMT).
The Carillon device, which is also delivered percutaneously, does not engage the valve leaflets to treat FMR. Rather, it is anchored in coronary sinus to reform the mitral annulus. This reduces FMR without damage to the mitral valve, thus preserving the potential for future valve repairs, according to Dr. Lipiecki, who reported that more than 1,100 devices have now been implanted, mostly in Europe.
The data so far are encouraging, said Dr. Lipiecki, who provided survival data in 74 patients followed for at least 5 years. Of these, 23 were drawn from the REDUCE FMR trial, which was blinded and sham controlled, and the remainder from the TITAN and TITAN II studies, which were prospective but not controlled.
In this series of 74 patients, there were no serious complications associated with the procedure, and all achieved a reduction in mitral regurgitation at 12 months, Dr. Lipiecki said. Furthermore, the reduction in FMR was associated with improvements in symptoms and “favorable remodeling” reflected in reduced left ventricular volume, he said.
In this series, the 5-year survival is 56%, which substantially exceeds what would be expected with GDMT, according to Dr. Lipiecki.
There were no baseline predictors of long-term survival, but improvements within the 6 months in functional heart class, 6-minute walk distance (6MWD), and mitral regurgitation were significantly associated with a greater likelihood of being alive at 5 years.
Although there is no comparable follow-up with other devices, including the MitraClip, Dr. Lipiecki did compare the 67.9% survival at 3 years in this series to that of the COAPT trial. He restricted the comparison to those treated for grade 3+ or 4+ mitral regurgitation. At 36 months, survival rates were 57.2% and 44.5% for those treated with MitraClip and GDMT, respectively.
“COAPT patients might not be comparable for a variety of reasons, including the anatomic restrictions important to the use of either of these devices,” Dr. Lipiecki acknowledged, but he said the long-term data with the Carillon device provide support for the pivotal Carillon trial now enrolling.
In this blinded trial, more than 350 patients at 75 sites are being randomized to placement of the Carillon device, which has been available in Europe since 2011, or a sham procedure. The trial is scheduled for completion in 2025.
NATIONAL HARBOR, MD. – In a pooled analysis of three trials conducted with the transcatheter Carillon Mitral Contour System for mitral valve repair, the 5-year survival is 56.2%, which is an encouraging outcome that justifies the ongoing multinational pivotal Carillon trial, according to the principal investigator of the analysis presented at CRT 2020 sponsored by MedStar Heart & Vascular Institute.
In patients with functional mitral valve regurgitation (FMR), “the Carillon device shows extremely encouraging long-term mortality data from prospective controlled trials in comparison with guideline-directed medical therapy or with the COAPT results,” according to Janusz Lipiecki, MD, PhD, department of cardiology, University Hospital, Clermont-Ferrand, France.
The COAPT trial is an important benchmark, because it was the first large, randomized trial to show benefit for a percutaneous device in the treatment of heart failure patients with moderate to severe FMR (N Engl J Med. 2018;379:2307-18). The study associated the MitraClip with a nearly 40% reduction in all-cause mortality (29.1% vs. 46.1%) at 24 months, relative to guideline-directed medical therapy (GDMT).
The Carillon device, which is also delivered percutaneously, does not engage the valve leaflets to treat FMR. Rather, it is anchored in coronary sinus to reform the mitral annulus. This reduces FMR without damage to the mitral valve, thus preserving the potential for future valve repairs, according to Dr. Lipiecki, who reported that more than 1,100 devices have now been implanted, mostly in Europe.
The data so far are encouraging, said Dr. Lipiecki, who provided survival data in 74 patients followed for at least 5 years. Of these, 23 were drawn from the REDUCE FMR trial, which was blinded and sham controlled, and the remainder from the TITAN and TITAN II studies, which were prospective but not controlled.
In this series of 74 patients, there were no serious complications associated with the procedure, and all achieved a reduction in mitral regurgitation at 12 months, Dr. Lipiecki said. Furthermore, the reduction in FMR was associated with improvements in symptoms and “favorable remodeling” reflected in reduced left ventricular volume, he said.
In this series, the 5-year survival is 56%, which substantially exceeds what would be expected with GDMT, according to Dr. Lipiecki.
There were no baseline predictors of long-term survival, but improvements within the 6 months in functional heart class, 6-minute walk distance (6MWD), and mitral regurgitation were significantly associated with a greater likelihood of being alive at 5 years.
Although there is no comparable follow-up with other devices, including the MitraClip, Dr. Lipiecki did compare the 67.9% survival at 3 years in this series to that of the COAPT trial. He restricted the comparison to those treated for grade 3+ or 4+ mitral regurgitation. At 36 months, survival rates were 57.2% and 44.5% for those treated with MitraClip and GDMT, respectively.
“COAPT patients might not be comparable for a variety of reasons, including the anatomic restrictions important to the use of either of these devices,” Dr. Lipiecki acknowledged, but he said the long-term data with the Carillon device provide support for the pivotal Carillon trial now enrolling.
In this blinded trial, more than 350 patients at 75 sites are being randomized to placement of the Carillon device, which has been available in Europe since 2011, or a sham procedure. The trial is scheduled for completion in 2025.
REPORTING FROM CRT 2020
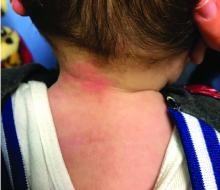
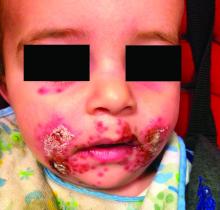
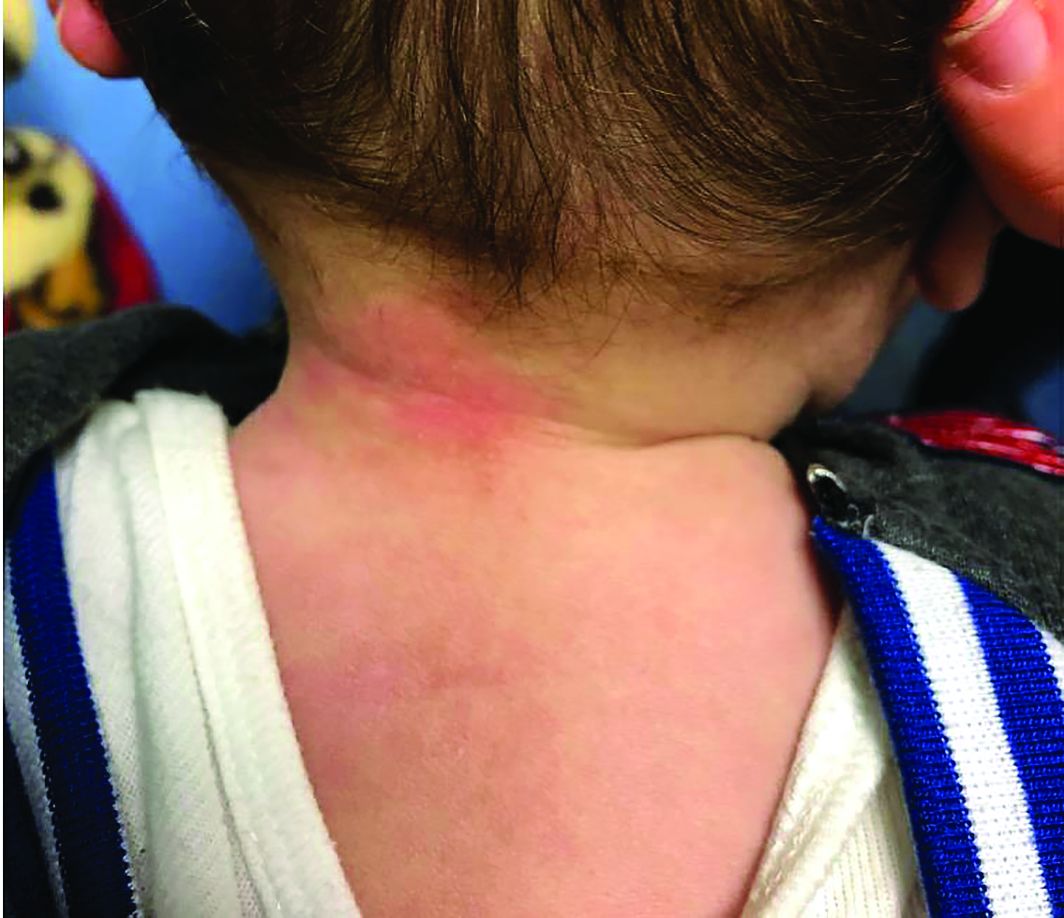
A 7-month-old male presents with perioral rash and fever
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at [email protected].
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at [email protected].
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Patients with atopic dermatitis are at risk for developing the herpes simplex virus (HSV)–related skin complication “eczema herpeticum,” also known as Kaposi’s varicelliform eruption. Eczema herpeticum is characterized by cutaneous pain and vesicular skin lesions, most commonly secondary to infection with HSV-1. The condition may affect individuals with atopic dermatitis or other inflammatory skin disorders. Eczema herpeticum develops when the virus infects large areas of skin, rather than being confined to a small area as in the common cold sore. Eczema herpeticum often appears on the face and neck, although it can appear anywhere on the body. In some cases, the rash may be difficult to distinguish from a patient’s baseline eczema if the latter is poorly controlled. Skin symptoms of eczema herpeticum include clusters of small blisters that are itchy and painful; vesicles that appear red, purple, or black; purulent blisters; or crusting. Classically, the morphology of vesicles or crusted lesions shows a “cluster of grapes” appearance. Eczema herpeticum may present with a high fever, chills, and swollen lymph glands.
While a clinical diagnosis based on the history, physical findings, and morphologic appearance of the rash is reasonable, testing may confirm the diagnosis. The most sensitive and specific tests are polymerase chain reaction sequencing for HSV, direct fluorescent antibody stain, and/or viral culture, while Tzanck smear may show characteristic histologic changes. Treatment is with oral antiviral therapy and treatment of the eczema.
Hand, foot, and mouth disease (HFMD) is a common viral illness usually affecting infants and children. The infection often involves the hands, feet, mouth, and sometimes, the genitals and buttocks. The viral exanthem is most commonly caused by the coxsackievirus, of the enterovirus family. Coxsackievirus A16 and enterovirus A71 are the serotypes that are most commonly implicated as the causative agents. HFMD initially presents with a low-grade fever, reduced appetite, and general malaise. About 1-2 days later, the child may develop painful mouth sores with an exanthem that involves the dorsum of the hands, soles of the feet, buttocks, legs, and arms. The exanthem consists of vesicles surrounded by a thin halo of erythema, eventually rupturing and forming superficial ulcers with a gray-yellow base and erythematous rim. The exanthem is itchy, and can be macular, papular, or vesicular. The lesions are nonpruritic, and typically not painful. The diagnosis of HFMD usually is made clinically, although a physician can swab the mouth or get a stool sample for polymerase chain reaction, which will show the virus; treatment is supportive. In children with atopic dermatitis, lesions also can tend to concentrate in areas previously or currently affected by the dermatitis, similar to eczema herpeticum, and the terms eczema coxsackium or atypical HFMD are applicable. In young adults, the disease may present with erythematous papulovesicular lesions on the face, oral mucosa, extensor surfaces of the upper and lower extremities, and palms and soles; confluent, hemorrhagic, and crusted lesions also can be seen on the extremities. Systemic symptoms usually subside in a few days; the skin lesions resolve without scarring in days to weeks.
Secondary bacterial infection is not uncommon in eczema herpeticum patients, reflecting common Staphylococcus aureus infection in atopic dermatitis patients. Streptococcus also may be seen as a concurrent infection. Treatment of secondary bacterial infection may be considered based on clinic context and culture.
Impetiginized eczema also is in the differential diagnosis of eczema herpeticum. S. aureus and Streptococci are the most important causative organisms. Lesions can manifest as a single red papule or macule that quickly becomes vesicular or eroded. Subsequently, the content dries, forming honey-colored crusts. Impetigo may resolve spontaneously, although in the context of infected eczema both topical anti-inflammatory agents (e.g. topical corticosteroids) along with systemic antibiotics may be a reasonable treatment option. Although our patient had honey-colored crusting, the wound culture showed normal bacterial flora.
Primary varicella infection causes acute fever and rash, with an initial exanthem of disseminated pruritic erythematous macules that progress beyond the papular stage, forming clear, fluid-filled vesicles (like dewdrops on a rose petal). In children, the rash presents on the stomach, back, and face, and then spreads to other parts of the body. Blisters also can arise inside the mouth.
In this patient, perioral HSV PCR 1 was positive, and wound culture showed normal oral flora with no organisms or white blood cells seen. The patient responded well to oral acyclovir, and treatment of his underlying atopic dermatitis with low-potency topical corticosteroids.
Dr. Bhatti is a research fellow in pediatric dermatology at Rady Children’s Hospital and the University of California, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Neither of the physicians had relevant financial disclosures. Email them at [email protected].
Sources
Can Fam Physician. 2012 Dec;58(12):1358-61.
William L Weston, MD., William Howe, MD. UpToDate. Treatment of atopic dermatitis (eczema).
Christine Johnson, MD, Anna Wald, MD, MPH. UpToDate. Epidemiology, clinical manifestations, and diagnosis of herpes simplex virus type 1 infection.
Robert Sidbury, MD, MPH. UpToDate. Atypical exanthems in children.
National Eczema Association. Eczema herpeticum.
Centers for Disease Control and Prevention. Symptoms and diagnosis of hand, foot, and mouth disease (HFMD).
Accelerated fetal growth in boys associated with development of AML
Accelerated fetal growth was associated with acute myeloid leukemia (AML), especially in infant boys and those with minimally differentiated leukemia, according to researchers from the Childhood Leukemia International Consortium (CLIC).
They assessed data from 22 studies involving a total of 3,564 cases to determine if there was an association between fetal growth and AML. The researchers also examined whether this association might vary by age, sex and disease subtype, according to their report published in the European Journal of Cancer.
The researchers calculated pooled estimates by age, sex and overall for harmonized fetal growth markers in association with AML. They used data from 17 International Fetal and Newborn Growth Consortium for the 21st Century Project studies and performed meta-analyses on 5 more studies. They also did subanalyses based on AML subtype.
They found a nearly 50% increased risk of AML among large-for-gestational-age infant boys (odds ratio [OR]: 1.49, 95% confidence interval [CI]: 1.03-2.14), reduced to 34% in boys aged less than 2 years (OR: 1.34, 95% CI: 1.05-1.71) and 25% in boys aged 0-14 years (OR: 1.25, 95% CI: 1.06-1.46). The association of large for gestational age was stronger in boys with the M0/M1 subtype (OR: 1.80, 95% CI: 1.15-2.83). In addition, large birth length for gestational age was also positively associated with AML (OR: 1.38, 95% CI: 1.00-1.92) in boys. By contrast, there, none of these factors were associated with AML in girls, nor were there associates for girls with respect to decelerated fetal growth markers.
“Although the absolute risk seems to be low at a population level, given the rarity of childhood AML, it would be worth exploring whether modifiable factors leading to macrosomia may also affect AML risk to stimulate future monitoring and preventive interventions before and during pregnancy,” the researchers suggested.
The authors reported that they had no conflicts of interest.
SOURCE: Karalexi MA et al. Eur J Canc. 2020;130:1-11.
Accelerated fetal growth was associated with acute myeloid leukemia (AML), especially in infant boys and those with minimally differentiated leukemia, according to researchers from the Childhood Leukemia International Consortium (CLIC).
They assessed data from 22 studies involving a total of 3,564 cases to determine if there was an association between fetal growth and AML. The researchers also examined whether this association might vary by age, sex and disease subtype, according to their report published in the European Journal of Cancer.
The researchers calculated pooled estimates by age, sex and overall for harmonized fetal growth markers in association with AML. They used data from 17 International Fetal and Newborn Growth Consortium for the 21st Century Project studies and performed meta-analyses on 5 more studies. They also did subanalyses based on AML subtype.
They found a nearly 50% increased risk of AML among large-for-gestational-age infant boys (odds ratio [OR]: 1.49, 95% confidence interval [CI]: 1.03-2.14), reduced to 34% in boys aged less than 2 years (OR: 1.34, 95% CI: 1.05-1.71) and 25% in boys aged 0-14 years (OR: 1.25, 95% CI: 1.06-1.46). The association of large for gestational age was stronger in boys with the M0/M1 subtype (OR: 1.80, 95% CI: 1.15-2.83). In addition, large birth length for gestational age was also positively associated with AML (OR: 1.38, 95% CI: 1.00-1.92) in boys. By contrast, there, none of these factors were associated with AML in girls, nor were there associates for girls with respect to decelerated fetal growth markers.
“Although the absolute risk seems to be low at a population level, given the rarity of childhood AML, it would be worth exploring whether modifiable factors leading to macrosomia may also affect AML risk to stimulate future monitoring and preventive interventions before and during pregnancy,” the researchers suggested.
The authors reported that they had no conflicts of interest.
SOURCE: Karalexi MA et al. Eur J Canc. 2020;130:1-11.
Accelerated fetal growth was associated with acute myeloid leukemia (AML), especially in infant boys and those with minimally differentiated leukemia, according to researchers from the Childhood Leukemia International Consortium (CLIC).
They assessed data from 22 studies involving a total of 3,564 cases to determine if there was an association between fetal growth and AML. The researchers also examined whether this association might vary by age, sex and disease subtype, according to their report published in the European Journal of Cancer.
The researchers calculated pooled estimates by age, sex and overall for harmonized fetal growth markers in association with AML. They used data from 17 International Fetal and Newborn Growth Consortium for the 21st Century Project studies and performed meta-analyses on 5 more studies. They also did subanalyses based on AML subtype.
They found a nearly 50% increased risk of AML among large-for-gestational-age infant boys (odds ratio [OR]: 1.49, 95% confidence interval [CI]: 1.03-2.14), reduced to 34% in boys aged less than 2 years (OR: 1.34, 95% CI: 1.05-1.71) and 25% in boys aged 0-14 years (OR: 1.25, 95% CI: 1.06-1.46). The association of large for gestational age was stronger in boys with the M0/M1 subtype (OR: 1.80, 95% CI: 1.15-2.83). In addition, large birth length for gestational age was also positively associated with AML (OR: 1.38, 95% CI: 1.00-1.92) in boys. By contrast, there, none of these factors were associated with AML in girls, nor were there associates for girls with respect to decelerated fetal growth markers.
“Although the absolute risk seems to be low at a population level, given the rarity of childhood AML, it would be worth exploring whether modifiable factors leading to macrosomia may also affect AML risk to stimulate future monitoring and preventive interventions before and during pregnancy,” the researchers suggested.
The authors reported that they had no conflicts of interest.
SOURCE: Karalexi MA et al. Eur J Canc. 2020;130:1-11.
FROM THE EUROPEAN JOURNAL OF CANCER
The pros and cons of pathology lab ownership: What early career GI doctors need to know
From colonoscopies to endoscopic ultrasound, gastroenterology is fundamentally a procedure-based specialty. Given that reality, making a decision to have as much control as possible over the entire process just makes sense for many GI practices.
Back in 2008, I was in charge of the process to develop a pathology lab at Arizona Digestive Health, a physician group with 26 locations throughout the state, as part of our decision to form a supergroup with eight ambulatory surgery centers. For us, having ambulatory surgery centers had been a game changer. We learned we could double our efficiency with procedures when we controlled the process from start to finish. We began to consider other processes – in this case pathology – that we could improve.
Prior to running our own pathology lab, doctors who read our slides were general pathologists who did not always understand the language of gastroenterology. We had results that came back by fax that were often cumbersome to read and did not always give us the information we needed in the way we needed it. Consistency was a problem. We knew we needed a change.
I cannot lie – setting up and running your own pathology lab is not always easy. But with the right factors in place, here are some benefits to consider when you are making a decision about joining a practice.
Quality, efficiency can lead to opportunity
Our lab has three GI fellowship–trained pathologists reading our slides. That means they are highly specialized and know exactly what we are looking for in a pathology report. We have a 24-hour turnaround for results. A courier service delivers biopsy specimens from our endoscopy centers to our path lab every day, and each morning our gastropathologists have a stack of pathology slides waiting for them. It’s added predictability and stability to the process, and we get the level of quality, specificity, and uniformity we need in a report.
The efficiencies are beneficial and it has given us more leverage in our negotiations with payers. We know what our costs are and have great quality metrics as well as read rates that we can provide. This signals to health plans that quality is a top priority for us.
We have also gained a reputational benefit with patients. Although much of the work is happening behind the scenes for our patients, they get results faster and a consistency with costs. It also allows us to easily access the slides of patients we have been seeing for years, giving us a richer data set and more confidence in our diagnosis.
Now that we have our own lab, we can look at our pathology data and conduct studies that will benefit all patients. For example, a few of our GI fellows were able to work with our pathologists to conduct a study on adenoma detection rates, exhausting a tissue block when no adenoma was found on initial review. We found a significant increase in adenoma detection using this method; we plan to publish results soon. The ability to conduct this kind of research is worth considering when early career gastroenterologists are selecting a practice to join.
And last but not least, having our own pathology lab acts as a unifying force for our group, which is spread out across 26 offices. When diagnoses are available and we get a call from our pathologist, we know to pick up the phone immediately.
What to consider before jumping in
Setting up our own pathology lab from the ground up was a learning process. We had enough patient volume for the move to make sense, so it is possible that smaller practices might not be able to make the investment if they have lower patient volume or cannot control their specimen flow. One option is to set up a technical lab and contract out for the slide reading. We felt it was important for our pathologists to also be our practice partners, and time has proven this to be a good decision for us.
We designed a lab with our work flow in mind, and it helped to have a pathologist on board from the beginning who knows gastroenterology. We even created our own lab information system with the help of a software engineer. It took a little bit over a year from conception to a functioning comprehensive lab.
Of course, there are regulatory factors to consider – the federal physician self-referral (Stark) law and the federal Anti-Markup Rule – come to mind. But we made sure to get a legal opinion that allows us to comply with the law. That’s something anyone who wants to make a move in this direction should do.
Looking back over the experience, I would not do anything differently. Yes, there are startup costs and a learning curve. But the quality we get from having our own pathology lab dedicated to GI and the efficiencies we have gained are well worth it.
Dr. Berggreen is the president of Arizona Digestive Health and chief strategy officer of the GI Alliance. He is also a board member of the Digestive Health Physicians Association. He received his doctorate at Louisiana State University, New Orleans. He is the former site director of the Good Samaritan GI Fellowship Program and named one of Phoenix Magazine’s “Top Docs.”
From colonoscopies to endoscopic ultrasound, gastroenterology is fundamentally a procedure-based specialty. Given that reality, making a decision to have as much control as possible over the entire process just makes sense for many GI practices.
Back in 2008, I was in charge of the process to develop a pathology lab at Arizona Digestive Health, a physician group with 26 locations throughout the state, as part of our decision to form a supergroup with eight ambulatory surgery centers. For us, having ambulatory surgery centers had been a game changer. We learned we could double our efficiency with procedures when we controlled the process from start to finish. We began to consider other processes – in this case pathology – that we could improve.
Prior to running our own pathology lab, doctors who read our slides were general pathologists who did not always understand the language of gastroenterology. We had results that came back by fax that were often cumbersome to read and did not always give us the information we needed in the way we needed it. Consistency was a problem. We knew we needed a change.
I cannot lie – setting up and running your own pathology lab is not always easy. But with the right factors in place, here are some benefits to consider when you are making a decision about joining a practice.
Quality, efficiency can lead to opportunity
Our lab has three GI fellowship–trained pathologists reading our slides. That means they are highly specialized and know exactly what we are looking for in a pathology report. We have a 24-hour turnaround for results. A courier service delivers biopsy specimens from our endoscopy centers to our path lab every day, and each morning our gastropathologists have a stack of pathology slides waiting for them. It’s added predictability and stability to the process, and we get the level of quality, specificity, and uniformity we need in a report.
The efficiencies are beneficial and it has given us more leverage in our negotiations with payers. We know what our costs are and have great quality metrics as well as read rates that we can provide. This signals to health plans that quality is a top priority for us.
We have also gained a reputational benefit with patients. Although much of the work is happening behind the scenes for our patients, they get results faster and a consistency with costs. It also allows us to easily access the slides of patients we have been seeing for years, giving us a richer data set and more confidence in our diagnosis.
Now that we have our own lab, we can look at our pathology data and conduct studies that will benefit all patients. For example, a few of our GI fellows were able to work with our pathologists to conduct a study on adenoma detection rates, exhausting a tissue block when no adenoma was found on initial review. We found a significant increase in adenoma detection using this method; we plan to publish results soon. The ability to conduct this kind of research is worth considering when early career gastroenterologists are selecting a practice to join.
And last but not least, having our own pathology lab acts as a unifying force for our group, which is spread out across 26 offices. When diagnoses are available and we get a call from our pathologist, we know to pick up the phone immediately.
What to consider before jumping in
Setting up our own pathology lab from the ground up was a learning process. We had enough patient volume for the move to make sense, so it is possible that smaller practices might not be able to make the investment if they have lower patient volume or cannot control their specimen flow. One option is to set up a technical lab and contract out for the slide reading. We felt it was important for our pathologists to also be our practice partners, and time has proven this to be a good decision for us.
We designed a lab with our work flow in mind, and it helped to have a pathologist on board from the beginning who knows gastroenterology. We even created our own lab information system with the help of a software engineer. It took a little bit over a year from conception to a functioning comprehensive lab.
Of course, there are regulatory factors to consider – the federal physician self-referral (Stark) law and the federal Anti-Markup Rule – come to mind. But we made sure to get a legal opinion that allows us to comply with the law. That’s something anyone who wants to make a move in this direction should do.
Looking back over the experience, I would not do anything differently. Yes, there are startup costs and a learning curve. But the quality we get from having our own pathology lab dedicated to GI and the efficiencies we have gained are well worth it.
Dr. Berggreen is the president of Arizona Digestive Health and chief strategy officer of the GI Alliance. He is also a board member of the Digestive Health Physicians Association. He received his doctorate at Louisiana State University, New Orleans. He is the former site director of the Good Samaritan GI Fellowship Program and named one of Phoenix Magazine’s “Top Docs.”
From colonoscopies to endoscopic ultrasound, gastroenterology is fundamentally a procedure-based specialty. Given that reality, making a decision to have as much control as possible over the entire process just makes sense for many GI practices.
Back in 2008, I was in charge of the process to develop a pathology lab at Arizona Digestive Health, a physician group with 26 locations throughout the state, as part of our decision to form a supergroup with eight ambulatory surgery centers. For us, having ambulatory surgery centers had been a game changer. We learned we could double our efficiency with procedures when we controlled the process from start to finish. We began to consider other processes – in this case pathology – that we could improve.
Prior to running our own pathology lab, doctors who read our slides were general pathologists who did not always understand the language of gastroenterology. We had results that came back by fax that were often cumbersome to read and did not always give us the information we needed in the way we needed it. Consistency was a problem. We knew we needed a change.
I cannot lie – setting up and running your own pathology lab is not always easy. But with the right factors in place, here are some benefits to consider when you are making a decision about joining a practice.
Quality, efficiency can lead to opportunity
Our lab has three GI fellowship–trained pathologists reading our slides. That means they are highly specialized and know exactly what we are looking for in a pathology report. We have a 24-hour turnaround for results. A courier service delivers biopsy specimens from our endoscopy centers to our path lab every day, and each morning our gastropathologists have a stack of pathology slides waiting for them. It’s added predictability and stability to the process, and we get the level of quality, specificity, and uniformity we need in a report.
The efficiencies are beneficial and it has given us more leverage in our negotiations with payers. We know what our costs are and have great quality metrics as well as read rates that we can provide. This signals to health plans that quality is a top priority for us.
We have also gained a reputational benefit with patients. Although much of the work is happening behind the scenes for our patients, they get results faster and a consistency with costs. It also allows us to easily access the slides of patients we have been seeing for years, giving us a richer data set and more confidence in our diagnosis.
Now that we have our own lab, we can look at our pathology data and conduct studies that will benefit all patients. For example, a few of our GI fellows were able to work with our pathologists to conduct a study on adenoma detection rates, exhausting a tissue block when no adenoma was found on initial review. We found a significant increase in adenoma detection using this method; we plan to publish results soon. The ability to conduct this kind of research is worth considering when early career gastroenterologists are selecting a practice to join.
And last but not least, having our own pathology lab acts as a unifying force for our group, which is spread out across 26 offices. When diagnoses are available and we get a call from our pathologist, we know to pick up the phone immediately.
What to consider before jumping in
Setting up our own pathology lab from the ground up was a learning process. We had enough patient volume for the move to make sense, so it is possible that smaller practices might not be able to make the investment if they have lower patient volume or cannot control their specimen flow. One option is to set up a technical lab and contract out for the slide reading. We felt it was important for our pathologists to also be our practice partners, and time has proven this to be a good decision for us.
We designed a lab with our work flow in mind, and it helped to have a pathologist on board from the beginning who knows gastroenterology. We even created our own lab information system with the help of a software engineer. It took a little bit over a year from conception to a functioning comprehensive lab.
Of course, there are regulatory factors to consider – the federal physician self-referral (Stark) law and the federal Anti-Markup Rule – come to mind. But we made sure to get a legal opinion that allows us to comply with the law. That’s something anyone who wants to make a move in this direction should do.
Looking back over the experience, I would not do anything differently. Yes, there are startup costs and a learning curve. But the quality we get from having our own pathology lab dedicated to GI and the efficiencies we have gained are well worth it.
Dr. Berggreen is the president of Arizona Digestive Health and chief strategy officer of the GI Alliance. He is also a board member of the Digestive Health Physicians Association. He received his doctorate at Louisiana State University, New Orleans. He is the former site director of the Good Samaritan GI Fellowship Program and named one of Phoenix Magazine’s “Top Docs.”
Marijuana allergies on the rise
“Cannabis sativa is a weed and it causes reactions just like any other pollen allergy,” said William Silvers, MD, from the University of Colorado School of Medicine in Aurora.
Silvers’ clinic began to see people with allergic reactions to the plant after the increase in direct exposure that accompanied the legalization of recreational marijuana in Colorado. For people with allergic tendencies, first- and second-hand exposure to C. sativa will increase “classic responses,” such as allergic rhinitis, sneezing, wheezing, itching, and asthma, he told Medscape Medical News.
Smoking the weed, direct exposure to the plant, contact with others who have touched plants, and breathing air in a grow operation “can all cause reactions,” he said. “And the more exposure they had, the greater the reaction, especially those who have allergic tendency,” he said.
The type of exposure to C. sativa is also a factor. Smoking the plant can induce typical allergic responses, the ingestion of hemp seed has been known to induce anaphylaxis, and “working with the plant can lead to dermatitis or contact urticaria,” he explained.
Edibles made with C. sativa have led to overdoses because dosing is difficult to determine. “It takes an hour or so to have an effect, so you don›t have as much control as inhaling it,” Silvers explained.
Stoned Fruit, Stoned Patient
A 2018 case report describes a 24-year-old daily marijuana smoker who experienced anaphylaxis after ingesting hemp seed. He had a history of allergies to stoned fruits, nuts, crustaceans, and aeroallergens. It was his first known exposure to hemp seed.
The patient developed urticaria on his arms after contact with C. sativa leaves and flowers, but had no reaction when smoking marijuana. This case indicates how important mode of exposure is.
“There are only a few cases of anaphylaxis known from ingestion of hemp seed,” Silvers said, “but the ‘stoned fruit, stoned patients’ cross-reactivity looks to be a real thing.”
People allergic to ragweed and sage are more likely than others to have a reaction to cantaloupe and other fruits in the melon family, he explained. There is a common antigen in the C. sativa pollen and in certain foods with cross-reacting proteins, such as tomato, peach, and hazelnut. “We see a pollen and food cross-reactivity via nonspecific lipid transfer proteins.”
A 2017 review of C. sativa allergy points out that few reports of IgE-dependent allergic reactions have been published because of the illegal status of cannabis. However, it is becoming more prevalent as a potential allergen. For example, in Nebraska, C. sativa pollen accounts for 36% of the total pollen count.
People with IgE-mediated cannabis allergy can have a sensitization to the nonspecific lipid transfer protein of C. sativa, Can s 3, which might explain the secondary plant-derived food allergies seen in European patients with a cannabis allergy, according to the review. Can s 3 cross-reacts with various plant homologues.
“This is the sort of information that allergists need to have,” Silvers said.
Stigma Limits Discussion
The fact that federal law prohibits cannabis use in the United States has made research difficult.
A strain distributed by the University of Mississippi can be used for research, “but its potency is very low, at 5% or 7%,” Silvers explained. At medical marijuana dispensaries, the potency of the flower can be as high as 25%, and in other forms, the THC content can be above 80%.
The legal status makes cannabis allergy difficult to diagnose and impossible to treat. Immunotherapy is out of the question. “With federal illegality, we need to stay out of trouble in that regard,” said Silvers, adding that, currently, avoidance is advised.
But research is emerging from Canada, where medicinal and recreational marijuana use is legal.
Stigma around cannabis is still high. “Nobody wants to be seen as a ‘pot doctor’,” said Silvers. But after it became legal in Colorado in 2015, he was asked to give a talk and decided to speak up.
“I have never written a medical prescription for marijuana,” he said, explaining that he is involved with the Center for Bioethics and Humanities at the University of Colorado. “I try to take a societal as well as a medical perspective, looking at the value and concerns for abuse and misuse.”
“As it becomes more available, more legalized, patients are having more reactions,” he said. “Allergists need to get in the game.”
Attitudes need to change. Physicians and allergists need to understand what’s happening in the population “and be open-minded about it so they know what to do,” he added.
Patients Don’t Want to Be Told to Stop
Users of medical marijuana can become dependent, said Ellen Burnham, MD, also from the University of Colorado.
“Patients want a blessing from care providers that it’s okay to use,” she told Medscape Medical News. “We’re in a state where people are really interested in holistic approaches to health, and cannabis is a natural product, but it may exacerbate allergies.”
Some components of cannabis might have bronchodilator properties but there are so many unknowns at this time. “I don’t think allergists should be recommending or condoning cannabis as part of a patient’s therapy,” she said. “It’s not okay for everybody.”
As business flourishes for operators in the cannabis industry and for the legal profession, Burnham said she worries that there isn’t enough protection for workers. “Do workers exposed to plant material on a daily basis have adequate workplace protection,” such as masks and gowns? “There’s a downstream effect that impacts people that nobody has really thought about,” she pointed out.
If the cannabis industry becomes driven by money, with a lobby like the tobacco industry, there will be no way to keep people who are vulnerable from using cannabis.
Is an occasional joint, much like an occasional glass of wine, okay? “We don’t know,” said Burnham. “We just don’t have enough information about it.”
Research is needed to develop medicinal strains of cannabidiol, cannabigerol, and cannabinol, which offer “medicinal and anti-inflammatory relief without the psychologic affects,” Silvers added.
This article first appeared on Medscape.com.
“Cannabis sativa is a weed and it causes reactions just like any other pollen allergy,” said William Silvers, MD, from the University of Colorado School of Medicine in Aurora.
Silvers’ clinic began to see people with allergic reactions to the plant after the increase in direct exposure that accompanied the legalization of recreational marijuana in Colorado. For people with allergic tendencies, first- and second-hand exposure to C. sativa will increase “classic responses,” such as allergic rhinitis, sneezing, wheezing, itching, and asthma, he told Medscape Medical News.
Smoking the weed, direct exposure to the plant, contact with others who have touched plants, and breathing air in a grow operation “can all cause reactions,” he said. “And the more exposure they had, the greater the reaction, especially those who have allergic tendency,” he said.
The type of exposure to C. sativa is also a factor. Smoking the plant can induce typical allergic responses, the ingestion of hemp seed has been known to induce anaphylaxis, and “working with the plant can lead to dermatitis or contact urticaria,” he explained.
Edibles made with C. sativa have led to overdoses because dosing is difficult to determine. “It takes an hour or so to have an effect, so you don›t have as much control as inhaling it,” Silvers explained.
Stoned Fruit, Stoned Patient
A 2018 case report describes a 24-year-old daily marijuana smoker who experienced anaphylaxis after ingesting hemp seed. He had a history of allergies to stoned fruits, nuts, crustaceans, and aeroallergens. It was his first known exposure to hemp seed.
The patient developed urticaria on his arms after contact with C. sativa leaves and flowers, but had no reaction when smoking marijuana. This case indicates how important mode of exposure is.
“There are only a few cases of anaphylaxis known from ingestion of hemp seed,” Silvers said, “but the ‘stoned fruit, stoned patients’ cross-reactivity looks to be a real thing.”
People allergic to ragweed and sage are more likely than others to have a reaction to cantaloupe and other fruits in the melon family, he explained. There is a common antigen in the C. sativa pollen and in certain foods with cross-reacting proteins, such as tomato, peach, and hazelnut. “We see a pollen and food cross-reactivity via nonspecific lipid transfer proteins.”
A 2017 review of C. sativa allergy points out that few reports of IgE-dependent allergic reactions have been published because of the illegal status of cannabis. However, it is becoming more prevalent as a potential allergen. For example, in Nebraska, C. sativa pollen accounts for 36% of the total pollen count.
People with IgE-mediated cannabis allergy can have a sensitization to the nonspecific lipid transfer protein of C. sativa, Can s 3, which might explain the secondary plant-derived food allergies seen in European patients with a cannabis allergy, according to the review. Can s 3 cross-reacts with various plant homologues.
“This is the sort of information that allergists need to have,” Silvers said.
Stigma Limits Discussion
The fact that federal law prohibits cannabis use in the United States has made research difficult.
A strain distributed by the University of Mississippi can be used for research, “but its potency is very low, at 5% or 7%,” Silvers explained. At medical marijuana dispensaries, the potency of the flower can be as high as 25%, and in other forms, the THC content can be above 80%.
The legal status makes cannabis allergy difficult to diagnose and impossible to treat. Immunotherapy is out of the question. “With federal illegality, we need to stay out of trouble in that regard,” said Silvers, adding that, currently, avoidance is advised.
But research is emerging from Canada, where medicinal and recreational marijuana use is legal.
Stigma around cannabis is still high. “Nobody wants to be seen as a ‘pot doctor’,” said Silvers. But after it became legal in Colorado in 2015, he was asked to give a talk and decided to speak up.
“I have never written a medical prescription for marijuana,” he said, explaining that he is involved with the Center for Bioethics and Humanities at the University of Colorado. “I try to take a societal as well as a medical perspective, looking at the value and concerns for abuse and misuse.”
“As it becomes more available, more legalized, patients are having more reactions,” he said. “Allergists need to get in the game.”
Attitudes need to change. Physicians and allergists need to understand what’s happening in the population “and be open-minded about it so they know what to do,” he added.
Patients Don’t Want to Be Told to Stop
Users of medical marijuana can become dependent, said Ellen Burnham, MD, also from the University of Colorado.
“Patients want a blessing from care providers that it’s okay to use,” she told Medscape Medical News. “We’re in a state where people are really interested in holistic approaches to health, and cannabis is a natural product, but it may exacerbate allergies.”
Some components of cannabis might have bronchodilator properties but there are so many unknowns at this time. “I don’t think allergists should be recommending or condoning cannabis as part of a patient’s therapy,” she said. “It’s not okay for everybody.”
As business flourishes for operators in the cannabis industry and for the legal profession, Burnham said she worries that there isn’t enough protection for workers. “Do workers exposed to plant material on a daily basis have adequate workplace protection,” such as masks and gowns? “There’s a downstream effect that impacts people that nobody has really thought about,” she pointed out.
If the cannabis industry becomes driven by money, with a lobby like the tobacco industry, there will be no way to keep people who are vulnerable from using cannabis.
Is an occasional joint, much like an occasional glass of wine, okay? “We don’t know,” said Burnham. “We just don’t have enough information about it.”
Research is needed to develop medicinal strains of cannabidiol, cannabigerol, and cannabinol, which offer “medicinal and anti-inflammatory relief without the psychologic affects,” Silvers added.
This article first appeared on Medscape.com.
“Cannabis sativa is a weed and it causes reactions just like any other pollen allergy,” said William Silvers, MD, from the University of Colorado School of Medicine in Aurora.
Silvers’ clinic began to see people with allergic reactions to the plant after the increase in direct exposure that accompanied the legalization of recreational marijuana in Colorado. For people with allergic tendencies, first- and second-hand exposure to C. sativa will increase “classic responses,” such as allergic rhinitis, sneezing, wheezing, itching, and asthma, he told Medscape Medical News.
Smoking the weed, direct exposure to the plant, contact with others who have touched plants, and breathing air in a grow operation “can all cause reactions,” he said. “And the more exposure they had, the greater the reaction, especially those who have allergic tendency,” he said.
The type of exposure to C. sativa is also a factor. Smoking the plant can induce typical allergic responses, the ingestion of hemp seed has been known to induce anaphylaxis, and “working with the plant can lead to dermatitis or contact urticaria,” he explained.
Edibles made with C. sativa have led to overdoses because dosing is difficult to determine. “It takes an hour or so to have an effect, so you don›t have as much control as inhaling it,” Silvers explained.
Stoned Fruit, Stoned Patient
A 2018 case report describes a 24-year-old daily marijuana smoker who experienced anaphylaxis after ingesting hemp seed. He had a history of allergies to stoned fruits, nuts, crustaceans, and aeroallergens. It was his first known exposure to hemp seed.
The patient developed urticaria on his arms after contact with C. sativa leaves and flowers, but had no reaction when smoking marijuana. This case indicates how important mode of exposure is.
“There are only a few cases of anaphylaxis known from ingestion of hemp seed,” Silvers said, “but the ‘stoned fruit, stoned patients’ cross-reactivity looks to be a real thing.”
People allergic to ragweed and sage are more likely than others to have a reaction to cantaloupe and other fruits in the melon family, he explained. There is a common antigen in the C. sativa pollen and in certain foods with cross-reacting proteins, such as tomato, peach, and hazelnut. “We see a pollen and food cross-reactivity via nonspecific lipid transfer proteins.”
A 2017 review of C. sativa allergy points out that few reports of IgE-dependent allergic reactions have been published because of the illegal status of cannabis. However, it is becoming more prevalent as a potential allergen. For example, in Nebraska, C. sativa pollen accounts for 36% of the total pollen count.
People with IgE-mediated cannabis allergy can have a sensitization to the nonspecific lipid transfer protein of C. sativa, Can s 3, which might explain the secondary plant-derived food allergies seen in European patients with a cannabis allergy, according to the review. Can s 3 cross-reacts with various plant homologues.
“This is the sort of information that allergists need to have,” Silvers said.
Stigma Limits Discussion
The fact that federal law prohibits cannabis use in the United States has made research difficult.
A strain distributed by the University of Mississippi can be used for research, “but its potency is very low, at 5% or 7%,” Silvers explained. At medical marijuana dispensaries, the potency of the flower can be as high as 25%, and in other forms, the THC content can be above 80%.
The legal status makes cannabis allergy difficult to diagnose and impossible to treat. Immunotherapy is out of the question. “With federal illegality, we need to stay out of trouble in that regard,” said Silvers, adding that, currently, avoidance is advised.
But research is emerging from Canada, where medicinal and recreational marijuana use is legal.
Stigma around cannabis is still high. “Nobody wants to be seen as a ‘pot doctor’,” said Silvers. But after it became legal in Colorado in 2015, he was asked to give a talk and decided to speak up.
“I have never written a medical prescription for marijuana,” he said, explaining that he is involved with the Center for Bioethics and Humanities at the University of Colorado. “I try to take a societal as well as a medical perspective, looking at the value and concerns for abuse and misuse.”
“As it becomes more available, more legalized, patients are having more reactions,” he said. “Allergists need to get in the game.”
Attitudes need to change. Physicians and allergists need to understand what’s happening in the population “and be open-minded about it so they know what to do,” he added.
Patients Don’t Want to Be Told to Stop
Users of medical marijuana can become dependent, said Ellen Burnham, MD, also from the University of Colorado.
“Patients want a blessing from care providers that it’s okay to use,” she told Medscape Medical News. “We’re in a state where people are really interested in holistic approaches to health, and cannabis is a natural product, but it may exacerbate allergies.”
Some components of cannabis might have bronchodilator properties but there are so many unknowns at this time. “I don’t think allergists should be recommending or condoning cannabis as part of a patient’s therapy,” she said. “It’s not okay for everybody.”
As business flourishes for operators in the cannabis industry and for the legal profession, Burnham said she worries that there isn’t enough protection for workers. “Do workers exposed to plant material on a daily basis have adequate workplace protection,” such as masks and gowns? “There’s a downstream effect that impacts people that nobody has really thought about,” she pointed out.
If the cannabis industry becomes driven by money, with a lobby like the tobacco industry, there will be no way to keep people who are vulnerable from using cannabis.
Is an occasional joint, much like an occasional glass of wine, okay? “We don’t know,” said Burnham. “We just don’t have enough information about it.”
Research is needed to develop medicinal strains of cannabidiol, cannabigerol, and cannabinol, which offer “medicinal and anti-inflammatory relief without the psychologic affects,” Silvers added.
This article first appeared on Medscape.com.
The role of medication in autism spectrum disorder
Efforts toward early identification and treatment are an important facet of the public health work in autism spectrum disorder (ASD).
The prevalence of ASD is rising. With the most recent estimate from the Centers from Disease Control and Prevention of 1 in 59* children aged 8 years,1 it is important for pediatric health care providers to have an understanding of current recommendations for treatment so they can counsel and guide affected families. ASD is a heterogeneous condition, so this article seeks to touch on broad principles, recognizing that clinicians must take into account the full clinical picture of each individual and family.
It is important to acknowledge that while there is no cure for ASD, there are treatment modalities that have an evidence base for addressing specific areas that may be impaired in children with autism. While it is beyond the scope of this article to review all of the potential areas of intervention in children with ASD, it is important to be keep in mind a few important principles.
1. The best evidenced treatment for addressing challenging and problematic behavior as well as improving a host of outcomes in children with ASD is itself behavioral in nature. These treatments are based on the principles of applied behavioral analysis,2 an educational and therapeutic approach which involves looking at antecedents and consequences of behaviors. This approach also looks to shape, motivate, and reinforce functional behaviors while discouraging harmful and disruptive ones.
2. Because communication often is impaired in children with ASD, providers always should investigate for possible medical causes of pain or discomfort that might explain sudden behavior change, as well as environmental changes that could be involved.
3. – because children with ASD often are particularly sensitive to medication side effects.
Irritability/aggression/extreme mood lability
There are only two medications with Food and Drug Administration labeling for an autism specific condition, and those are aripiprazole and risperidone, two second-generation antipsychotic agents approved for irritability associated with ASD on the basis of randomized controlled trials (RCTs) demonstrating their efficacy.3,4 Included under the umbrella of irritability are aggression, deliberate self-injurious behavior, extreme temper tantrums, and quick and extreme mood changes. For aripiprazole the approved ages are 6-17 years; a dosing range of 2-15 mg/day is recommended. For risperidone, the approved age range is 5-17 years; the recommended dosing range is 0.25-4 mg/day. Prior to starting either of these medications, a cardiac history should be obtained, and baseline laboratory values, particularly lipid levels and hemoglobin A1c (HbA1c) are recommended. All second-generation antipsychotics carry the risk of tardive dyskinesia (a movement disorder), as well as risk of weight gain and metabolic effects. Baseline weight prior to medication initiation with routine follow-up measurement is encouraged. In light of the burden of potential side effects, these medications tend to be reserved by clinicians for circumstances where there is a significant impact on functioning. Both medications are available in liquid form for children with difficulty swallowing pills.
ADHD
There are positive RCTs of methylphenidate in co-occurring ASD and ADHD,5 making it the preferred first line agent for treatment. Amphetamine salt based stimulant preparations do not have any RCTs in co-occurring ASD, but theoretically should be similarly effective. Again, the principle of starting low and going slow is applicable. Second line are the alpha 2 adrenergic agonists guanfacine and clonidine, both of whose long-acting formulations are approved for treatment of ADHD in children and adolescents without ASD, as well as atomoxetine, a selective norepinephrine reuptake inhibitor approved for ADHD. Guanfacine and atomoxetine have the stronger evidence base in the co-occurring condition. None of the second-line medications come in liquid preparation, although the immediate-release forms of guanfacine and clonidine both can be crushed and are used in clinical practice when the extended-release forms are not practicable.
Anxiety disorders and depression
Repetitive behaviors and insistence on sameness are broad headings that can be thought of as similar to obsessive compulsive disorder in children without ASD. However, controlled studies of SSRIs and clomipramine (a tricyclic antidepressant) have not shown a clear benefit in these behaviors in children with autism. There are no RCTs looking specifically at treatment of anxiety disorders in children with ASD, but expert consensus is that pharmacologic treatment is similar to that of children without ASD, with the SSRIs fluoxetine and sertraline the first-line agents due to the robust evidence for these two medications in treatment of anxiety disorders in children.6 Especially for kids with higher functioning ASD, cognitive behavioral therapy (CBT) should be considered and has some evidence for the co-occurring condition. Similarly, there are no RCTs for co-occurring depression in ASD, and clinical practice is to treat it as you would depression in the non-ASD population. Be aware that the studies of SSRIs in children with ASD reported higher than typical rates of behavioral activation on these medications, and again the principle of starting low and going slow is emphasized. Fluoxetine and sertraline both come in liquid form.
Insomnia
Insomnia is a common occurrence in children with ASD, and studies suggest melatonin can be effective, with immediate release clonidine a consideration with some limited evidence, if melatonin is not successful.
Finally I would be remiss in not mentioning that there is preliminary evidence from review7 and meta-analysis8 articles to suggest that regular exercise for individuals with ASD has a positive effect on multiple symptom domains, suggesting that this is an important additional treatment recommendation for children and families.
In conclusion, identification and treatment of ASD and co-occurring syndromes is often challenging, and while specialty referral often will be necessary, it is hoped that this overview provides a helpful frame of reference for primary care providers who encounter these conditions in clinical practice.
For further reading on this important subject, I recommend the American Academy of Child and Adolescent Psychiatry Practice Parameter for the Assessment and Treatment of Children and Adolescents with ASD and the Parents Medication Guide for Autism Spectrum Disorders.
Dr. Hoffnung is a pediatric psychiatrist at the University of Vermont Children’s Hospital and an assistant professor of psychiatry at the Robert Larner, M.D. College of Medicine at the University of Vermont, both in Burlington. He has no relevant financial disclosures. Email him at [email protected].
References
1. MMWR Surveill Summ 2018;67(No. SS-6):1–23*
2. National Standards Project, Phase 2. National Autism Center 2015.
3. N Engl J Med. 2002 Aug 1;347(5):314-21.
4. J Am Acad Child Adolesc Psychiatry. 2009 Nov;48(11):1110-9.
5. Arch Gen Psychiatry. 2005 Nov;62(11):1266-74.
6. Pediatrics. 2016 Feb;137(Supplement 2):S115-S123.
7. Research in Autism Spectrum Disorders. 2010 Dec;4(4):565-76.
8. Research in Autism Spectrum Disorders. 2012;6(1):46-57.
*This article was updated 4/2/2020.
Efforts toward early identification and treatment are an important facet of the public health work in autism spectrum disorder (ASD).
The prevalence of ASD is rising. With the most recent estimate from the Centers from Disease Control and Prevention of 1 in 59* children aged 8 years,1 it is important for pediatric health care providers to have an understanding of current recommendations for treatment so they can counsel and guide affected families. ASD is a heterogeneous condition, so this article seeks to touch on broad principles, recognizing that clinicians must take into account the full clinical picture of each individual and family.
It is important to acknowledge that while there is no cure for ASD, there are treatment modalities that have an evidence base for addressing specific areas that may be impaired in children with autism. While it is beyond the scope of this article to review all of the potential areas of intervention in children with ASD, it is important to be keep in mind a few important principles.
1. The best evidenced treatment for addressing challenging and problematic behavior as well as improving a host of outcomes in children with ASD is itself behavioral in nature. These treatments are based on the principles of applied behavioral analysis,2 an educational and therapeutic approach which involves looking at antecedents and consequences of behaviors. This approach also looks to shape, motivate, and reinforce functional behaviors while discouraging harmful and disruptive ones.
2. Because communication often is impaired in children with ASD, providers always should investigate for possible medical causes of pain or discomfort that might explain sudden behavior change, as well as environmental changes that could be involved.
3. – because children with ASD often are particularly sensitive to medication side effects.
Irritability/aggression/extreme mood lability
There are only two medications with Food and Drug Administration labeling for an autism specific condition, and those are aripiprazole and risperidone, two second-generation antipsychotic agents approved for irritability associated with ASD on the basis of randomized controlled trials (RCTs) demonstrating their efficacy.3,4 Included under the umbrella of irritability are aggression, deliberate self-injurious behavior, extreme temper tantrums, and quick and extreme mood changes. For aripiprazole the approved ages are 6-17 years; a dosing range of 2-15 mg/day is recommended. For risperidone, the approved age range is 5-17 years; the recommended dosing range is 0.25-4 mg/day. Prior to starting either of these medications, a cardiac history should be obtained, and baseline laboratory values, particularly lipid levels and hemoglobin A1c (HbA1c) are recommended. All second-generation antipsychotics carry the risk of tardive dyskinesia (a movement disorder), as well as risk of weight gain and metabolic effects. Baseline weight prior to medication initiation with routine follow-up measurement is encouraged. In light of the burden of potential side effects, these medications tend to be reserved by clinicians for circumstances where there is a significant impact on functioning. Both medications are available in liquid form for children with difficulty swallowing pills.
ADHD
There are positive RCTs of methylphenidate in co-occurring ASD and ADHD,5 making it the preferred first line agent for treatment. Amphetamine salt based stimulant preparations do not have any RCTs in co-occurring ASD, but theoretically should be similarly effective. Again, the principle of starting low and going slow is applicable. Second line are the alpha 2 adrenergic agonists guanfacine and clonidine, both of whose long-acting formulations are approved for treatment of ADHD in children and adolescents without ASD, as well as atomoxetine, a selective norepinephrine reuptake inhibitor approved for ADHD. Guanfacine and atomoxetine have the stronger evidence base in the co-occurring condition. None of the second-line medications come in liquid preparation, although the immediate-release forms of guanfacine and clonidine both can be crushed and are used in clinical practice when the extended-release forms are not practicable.
Anxiety disorders and depression
Repetitive behaviors and insistence on sameness are broad headings that can be thought of as similar to obsessive compulsive disorder in children without ASD. However, controlled studies of SSRIs and clomipramine (a tricyclic antidepressant) have not shown a clear benefit in these behaviors in children with autism. There are no RCTs looking specifically at treatment of anxiety disorders in children with ASD, but expert consensus is that pharmacologic treatment is similar to that of children without ASD, with the SSRIs fluoxetine and sertraline the first-line agents due to the robust evidence for these two medications in treatment of anxiety disorders in children.6 Especially for kids with higher functioning ASD, cognitive behavioral therapy (CBT) should be considered and has some evidence for the co-occurring condition. Similarly, there are no RCTs for co-occurring depression in ASD, and clinical practice is to treat it as you would depression in the non-ASD population. Be aware that the studies of SSRIs in children with ASD reported higher than typical rates of behavioral activation on these medications, and again the principle of starting low and going slow is emphasized. Fluoxetine and sertraline both come in liquid form.
Insomnia
Insomnia is a common occurrence in children with ASD, and studies suggest melatonin can be effective, with immediate release clonidine a consideration with some limited evidence, if melatonin is not successful.
Finally I would be remiss in not mentioning that there is preliminary evidence from review7 and meta-analysis8 articles to suggest that regular exercise for individuals with ASD has a positive effect on multiple symptom domains, suggesting that this is an important additional treatment recommendation for children and families.
In conclusion, identification and treatment of ASD and co-occurring syndromes is often challenging, and while specialty referral often will be necessary, it is hoped that this overview provides a helpful frame of reference for primary care providers who encounter these conditions in clinical practice.
For further reading on this important subject, I recommend the American Academy of Child and Adolescent Psychiatry Practice Parameter for the Assessment and Treatment of Children and Adolescents with ASD and the Parents Medication Guide for Autism Spectrum Disorders.
Dr. Hoffnung is a pediatric psychiatrist at the University of Vermont Children’s Hospital and an assistant professor of psychiatry at the Robert Larner, M.D. College of Medicine at the University of Vermont, both in Burlington. He has no relevant financial disclosures. Email him at [email protected].
References
1. MMWR Surveill Summ 2018;67(No. SS-6):1–23*
2. National Standards Project, Phase 2. National Autism Center 2015.
3. N Engl J Med. 2002 Aug 1;347(5):314-21.
4. J Am Acad Child Adolesc Psychiatry. 2009 Nov;48(11):1110-9.
5. Arch Gen Psychiatry. 2005 Nov;62(11):1266-74.
6. Pediatrics. 2016 Feb;137(Supplement 2):S115-S123.
7. Research in Autism Spectrum Disorders. 2010 Dec;4(4):565-76.
8. Research in Autism Spectrum Disorders. 2012;6(1):46-57.
*This article was updated 4/2/2020.
Efforts toward early identification and treatment are an important facet of the public health work in autism spectrum disorder (ASD).
The prevalence of ASD is rising. With the most recent estimate from the Centers from Disease Control and Prevention of 1 in 59* children aged 8 years,1 it is important for pediatric health care providers to have an understanding of current recommendations for treatment so they can counsel and guide affected families. ASD is a heterogeneous condition, so this article seeks to touch on broad principles, recognizing that clinicians must take into account the full clinical picture of each individual and family.
It is important to acknowledge that while there is no cure for ASD, there are treatment modalities that have an evidence base for addressing specific areas that may be impaired in children with autism. While it is beyond the scope of this article to review all of the potential areas of intervention in children with ASD, it is important to be keep in mind a few important principles.
1. The best evidenced treatment for addressing challenging and problematic behavior as well as improving a host of outcomes in children with ASD is itself behavioral in nature. These treatments are based on the principles of applied behavioral analysis,2 an educational and therapeutic approach which involves looking at antecedents and consequences of behaviors. This approach also looks to shape, motivate, and reinforce functional behaviors while discouraging harmful and disruptive ones.
2. Because communication often is impaired in children with ASD, providers always should investigate for possible medical causes of pain or discomfort that might explain sudden behavior change, as well as environmental changes that could be involved.
3. – because children with ASD often are particularly sensitive to medication side effects.
Irritability/aggression/extreme mood lability
There are only two medications with Food and Drug Administration labeling for an autism specific condition, and those are aripiprazole and risperidone, two second-generation antipsychotic agents approved for irritability associated with ASD on the basis of randomized controlled trials (RCTs) demonstrating their efficacy.3,4 Included under the umbrella of irritability are aggression, deliberate self-injurious behavior, extreme temper tantrums, and quick and extreme mood changes. For aripiprazole the approved ages are 6-17 years; a dosing range of 2-15 mg/day is recommended. For risperidone, the approved age range is 5-17 years; the recommended dosing range is 0.25-4 mg/day. Prior to starting either of these medications, a cardiac history should be obtained, and baseline laboratory values, particularly lipid levels and hemoglobin A1c (HbA1c) are recommended. All second-generation antipsychotics carry the risk of tardive dyskinesia (a movement disorder), as well as risk of weight gain and metabolic effects. Baseline weight prior to medication initiation with routine follow-up measurement is encouraged. In light of the burden of potential side effects, these medications tend to be reserved by clinicians for circumstances where there is a significant impact on functioning. Both medications are available in liquid form for children with difficulty swallowing pills.
ADHD
There are positive RCTs of methylphenidate in co-occurring ASD and ADHD,5 making it the preferred first line agent for treatment. Amphetamine salt based stimulant preparations do not have any RCTs in co-occurring ASD, but theoretically should be similarly effective. Again, the principle of starting low and going slow is applicable. Second line are the alpha 2 adrenergic agonists guanfacine and clonidine, both of whose long-acting formulations are approved for treatment of ADHD in children and adolescents without ASD, as well as atomoxetine, a selective norepinephrine reuptake inhibitor approved for ADHD. Guanfacine and atomoxetine have the stronger evidence base in the co-occurring condition. None of the second-line medications come in liquid preparation, although the immediate-release forms of guanfacine and clonidine both can be crushed and are used in clinical practice when the extended-release forms are not practicable.
Anxiety disorders and depression
Repetitive behaviors and insistence on sameness are broad headings that can be thought of as similar to obsessive compulsive disorder in children without ASD. However, controlled studies of SSRIs and clomipramine (a tricyclic antidepressant) have not shown a clear benefit in these behaviors in children with autism. There are no RCTs looking specifically at treatment of anxiety disorders in children with ASD, but expert consensus is that pharmacologic treatment is similar to that of children without ASD, with the SSRIs fluoxetine and sertraline the first-line agents due to the robust evidence for these two medications in treatment of anxiety disorders in children.6 Especially for kids with higher functioning ASD, cognitive behavioral therapy (CBT) should be considered and has some evidence for the co-occurring condition. Similarly, there are no RCTs for co-occurring depression in ASD, and clinical practice is to treat it as you would depression in the non-ASD population. Be aware that the studies of SSRIs in children with ASD reported higher than typical rates of behavioral activation on these medications, and again the principle of starting low and going slow is emphasized. Fluoxetine and sertraline both come in liquid form.
Insomnia
Insomnia is a common occurrence in children with ASD, and studies suggest melatonin can be effective, with immediate release clonidine a consideration with some limited evidence, if melatonin is not successful.
Finally I would be remiss in not mentioning that there is preliminary evidence from review7 and meta-analysis8 articles to suggest that regular exercise for individuals with ASD has a positive effect on multiple symptom domains, suggesting that this is an important additional treatment recommendation for children and families.
In conclusion, identification and treatment of ASD and co-occurring syndromes is often challenging, and while specialty referral often will be necessary, it is hoped that this overview provides a helpful frame of reference for primary care providers who encounter these conditions in clinical practice.
For further reading on this important subject, I recommend the American Academy of Child and Adolescent Psychiatry Practice Parameter for the Assessment and Treatment of Children and Adolescents with ASD and the Parents Medication Guide for Autism Spectrum Disorders.
Dr. Hoffnung is a pediatric psychiatrist at the University of Vermont Children’s Hospital and an assistant professor of psychiatry at the Robert Larner, M.D. College of Medicine at the University of Vermont, both in Burlington. He has no relevant financial disclosures. Email him at [email protected].
References
1. MMWR Surveill Summ 2018;67(No. SS-6):1–23*
2. National Standards Project, Phase 2. National Autism Center 2015.
3. N Engl J Med. 2002 Aug 1;347(5):314-21.
4. J Am Acad Child Adolesc Psychiatry. 2009 Nov;48(11):1110-9.
5. Arch Gen Psychiatry. 2005 Nov;62(11):1266-74.
6. Pediatrics. 2016 Feb;137(Supplement 2):S115-S123.
7. Research in Autism Spectrum Disorders. 2010 Dec;4(4):565-76.
8. Research in Autism Spectrum Disorders. 2012;6(1):46-57.
*This article was updated 4/2/2020.
Teriflunomide increases the likelihood of achieving NEDA in relapsing-remitting MS
WEST PALM BEACH, FLA. – , according to an analysis presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Teriflunomide reduces the risks of relapse, relapse resulting in hospital admission, and relapse resulting in prolonged hospitalization, compared with placebo.
Teriflunomide modulates the immune system and is an approved treatment for relapsing-remitting MS and clinically isolated syndrome. The phase 3 TEMSO study provided evidence that established the treatment’s safety and efficacy. In that study, significantly more patients who received a 14-mg dose of teriflunomide achieved NEDA, compared with patients who received placebo. Researchers generally weight all components of NEDA (i.e., confirmed disability worsening [CDW], relapse, and unique active MRI lesions) equally, but this approach could limit the interpretation of how each endpoint contributes to the effectiveness of a disease-modifying therapy.
A new analysis of TEMSO data
Keith R. Edwards, MD, director of the MS Center of Northeastern New York in Latham and colleagues conducted a win ratio matched-pairs analysis of TEMSO data to evaluate the efficacy of teriflunomide in enabling patients to achieve NEDA. In this analysis, the components of NEDA were assessed in order of priority, rather than as factors of equal weight.
In TEMSO, patients with relapsing-remitting MS received placebo or 14 mg of teriflunomide for 108 weeks. Dr. Edwards and colleagues matched active and control patients according to baseline characteristics. They compared the occurrence of disease activity events between the members of each pair. If a patient receiving teriflunomide had an event later than a control did, or did not have the event at all, teriflunomide was considered to “win.” If neither patient in a pair had a given event, the researchers omitted the pair from their analysis. Dr. Edwards and colleagues counted wins and summarized them as ratios. They conducted a second win ratio analysis of all relapses and relapses resulting in deaths, life-threatening events, prolonged hospitalizations, and hospital admissions.
NEDA components were ranked and assessed in the following order of decreasing priority: CDW, relapse, unique active MRI lesions. In a sensitivity analysis, the investigators ranked and assessed these components in the reverse order.
Sensitivity analysis supported primary analysis
Dr. Edwards and colleagues included 363 participants who received placebo and 358 who received teriflunomide in their analysis. Baseline characteristics did not differ significantly between the two groups. The population’s mean age was approximately 38 years, and about 73% of participants were female. The population’s mean baseline Expanded Disability Status Scale score was 2.7. Overall, about 72% of participants completed the study.
The researchers created 321 risk-matched pairs of participants. The win ratio analysis indicated that patients who received teriflunomide were significantly more likely to achieve NEDA, compared with controls (win ratio, 1.33). When the investigators analyzed the data by prioritizing the NEDA components in the reverse order, they found similar results (win ratio, 1.41).
When Dr. Edwards and colleagues analyzed relapse severity, they found that no relapses resulting in death or life-threatening events occurred in the active or control groups. Compared with placebo, teriflunomide significantly reduced the risk of relapse, relapses resulting in hospital admissions, and relapses resulting in prolonged hospitalizations (win ratio, 1.37).
The TEMSO study was funded by Sanofi. Dr. Edwards received grant or research support from Biogen, Genentech, Genzyme, and Novartis. Several authors received funding from Sanofi.
SOURCE: Edwards KR et al. ACTRIMS 2020, Abstract P036.
WEST PALM BEACH, FLA. – , according to an analysis presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Teriflunomide reduces the risks of relapse, relapse resulting in hospital admission, and relapse resulting in prolonged hospitalization, compared with placebo.
Teriflunomide modulates the immune system and is an approved treatment for relapsing-remitting MS and clinically isolated syndrome. The phase 3 TEMSO study provided evidence that established the treatment’s safety and efficacy. In that study, significantly more patients who received a 14-mg dose of teriflunomide achieved NEDA, compared with patients who received placebo. Researchers generally weight all components of NEDA (i.e., confirmed disability worsening [CDW], relapse, and unique active MRI lesions) equally, but this approach could limit the interpretation of how each endpoint contributes to the effectiveness of a disease-modifying therapy.
A new analysis of TEMSO data
Keith R. Edwards, MD, director of the MS Center of Northeastern New York in Latham and colleagues conducted a win ratio matched-pairs analysis of TEMSO data to evaluate the efficacy of teriflunomide in enabling patients to achieve NEDA. In this analysis, the components of NEDA were assessed in order of priority, rather than as factors of equal weight.
In TEMSO, patients with relapsing-remitting MS received placebo or 14 mg of teriflunomide for 108 weeks. Dr. Edwards and colleagues matched active and control patients according to baseline characteristics. They compared the occurrence of disease activity events between the members of each pair. If a patient receiving teriflunomide had an event later than a control did, or did not have the event at all, teriflunomide was considered to “win.” If neither patient in a pair had a given event, the researchers omitted the pair from their analysis. Dr. Edwards and colleagues counted wins and summarized them as ratios. They conducted a second win ratio analysis of all relapses and relapses resulting in deaths, life-threatening events, prolonged hospitalizations, and hospital admissions.
NEDA components were ranked and assessed in the following order of decreasing priority: CDW, relapse, unique active MRI lesions. In a sensitivity analysis, the investigators ranked and assessed these components in the reverse order.
Sensitivity analysis supported primary analysis
Dr. Edwards and colleagues included 363 participants who received placebo and 358 who received teriflunomide in their analysis. Baseline characteristics did not differ significantly between the two groups. The population’s mean age was approximately 38 years, and about 73% of participants were female. The population’s mean baseline Expanded Disability Status Scale score was 2.7. Overall, about 72% of participants completed the study.
The researchers created 321 risk-matched pairs of participants. The win ratio analysis indicated that patients who received teriflunomide were significantly more likely to achieve NEDA, compared with controls (win ratio, 1.33). When the investigators analyzed the data by prioritizing the NEDA components in the reverse order, they found similar results (win ratio, 1.41).
When Dr. Edwards and colleagues analyzed relapse severity, they found that no relapses resulting in death or life-threatening events occurred in the active or control groups. Compared with placebo, teriflunomide significantly reduced the risk of relapse, relapses resulting in hospital admissions, and relapses resulting in prolonged hospitalizations (win ratio, 1.37).
The TEMSO study was funded by Sanofi. Dr. Edwards received grant or research support from Biogen, Genentech, Genzyme, and Novartis. Several authors received funding from Sanofi.
SOURCE: Edwards KR et al. ACTRIMS 2020, Abstract P036.
WEST PALM BEACH, FLA. – , according to an analysis presented at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis. Teriflunomide reduces the risks of relapse, relapse resulting in hospital admission, and relapse resulting in prolonged hospitalization, compared with placebo.
Teriflunomide modulates the immune system and is an approved treatment for relapsing-remitting MS and clinically isolated syndrome. The phase 3 TEMSO study provided evidence that established the treatment’s safety and efficacy. In that study, significantly more patients who received a 14-mg dose of teriflunomide achieved NEDA, compared with patients who received placebo. Researchers generally weight all components of NEDA (i.e., confirmed disability worsening [CDW], relapse, and unique active MRI lesions) equally, but this approach could limit the interpretation of how each endpoint contributes to the effectiveness of a disease-modifying therapy.
A new analysis of TEMSO data
Keith R. Edwards, MD, director of the MS Center of Northeastern New York in Latham and colleagues conducted a win ratio matched-pairs analysis of TEMSO data to evaluate the efficacy of teriflunomide in enabling patients to achieve NEDA. In this analysis, the components of NEDA were assessed in order of priority, rather than as factors of equal weight.
In TEMSO, patients with relapsing-remitting MS received placebo or 14 mg of teriflunomide for 108 weeks. Dr. Edwards and colleagues matched active and control patients according to baseline characteristics. They compared the occurrence of disease activity events between the members of each pair. If a patient receiving teriflunomide had an event later than a control did, or did not have the event at all, teriflunomide was considered to “win.” If neither patient in a pair had a given event, the researchers omitted the pair from their analysis. Dr. Edwards and colleagues counted wins and summarized them as ratios. They conducted a second win ratio analysis of all relapses and relapses resulting in deaths, life-threatening events, prolonged hospitalizations, and hospital admissions.
NEDA components were ranked and assessed in the following order of decreasing priority: CDW, relapse, unique active MRI lesions. In a sensitivity analysis, the investigators ranked and assessed these components in the reverse order.
Sensitivity analysis supported primary analysis
Dr. Edwards and colleagues included 363 participants who received placebo and 358 who received teriflunomide in their analysis. Baseline characteristics did not differ significantly between the two groups. The population’s mean age was approximately 38 years, and about 73% of participants were female. The population’s mean baseline Expanded Disability Status Scale score was 2.7. Overall, about 72% of participants completed the study.
The researchers created 321 risk-matched pairs of participants. The win ratio analysis indicated that patients who received teriflunomide were significantly more likely to achieve NEDA, compared with controls (win ratio, 1.33). When the investigators analyzed the data by prioritizing the NEDA components in the reverse order, they found similar results (win ratio, 1.41).
When Dr. Edwards and colleagues analyzed relapse severity, they found that no relapses resulting in death or life-threatening events occurred in the active or control groups. Compared with placebo, teriflunomide significantly reduced the risk of relapse, relapses resulting in hospital admissions, and relapses resulting in prolonged hospitalizations (win ratio, 1.37).
The TEMSO study was funded by Sanofi. Dr. Edwards received grant or research support from Biogen, Genentech, Genzyme, and Novartis. Several authors received funding from Sanofi.
SOURCE: Edwards KR et al. ACTRIMS 2020, Abstract P036.
REPORTING FROM ACTRIMS Forum 2020
FDA, FTC uniting to promote biosimilars
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also sponsored a public workshop on March 9 to discuss competition for biologics.
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also sponsored a public workshop on March 9 to discuss competition for biologics.
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.
The Food and Drug Administration is collaborating with the Federal Trade Commission (FTC) to expand the biosimilars market.
The two agencies signed a joint statement on Feb. 3, 2020, outlining four sets of goals aimed at creating meaningful competition from biosimilars against their reference biologic products.
“Competition is key for helping American patients have access to affordable medicines,” FDA Commissioner Stephen Hahn, MD, said in a statement. “Strengthening efforts to curtail and discourage anticompetitive behavior is key for facilitating robust competition for patients in the biologics marketplace, including through biosimilars, bringing down the costs of these crucial products for patients.”
The statement highlighted four goals. First is that the agencies will coordinate to promote greater competition in the biologic market, including the development of materials to educate the market about biosimilars. The FDA and FTC also sponsored a public workshop on March 9 to discuss competition for biologics.
The second goal has the FDA and FTC working together “to deter behavior that impedes access to samples needed for the development of biologics, including biosimilars,” the joint statement notes.
Third, the agencies will crack down on “false or misleading communications about biologics, including biosimilars, within their respective authorities,” according to the joint statement.
“FDA and FTC, as authorized by their respective statutes, will work together to address false or misleading communications about biologics, including biosimilars,” the statement continues. “In particular, if a communication makes a false or misleading comparison between a reference product and a biosimilar in a manner that misrepresents the safety or efficacy of biosimilars, deceives consumers, or deters competition, FDA and FTC intend to take appropriate action within their respective authorities. FDA intends to take appropriate action to address such communications where those communications have the potential to impact public health.”
Finally, the FTC committed to review patent settlement agreements involving biologics, including biosimilars, for antitrust violations.
Separately, the FDA issued a draft guidance document for comment on manufacturers seeking licensure of biosimilar products that do not cover all the approved uses of the reference product, as well as how to add uses over time that were not part of the initial license of the biosimilar product. The draft guidance covers licensure of products, labeling of biosimilars with fewer indications than the reference product, supplemental applications for indications not on the initial biosimilar application but covered by the reference product, and the timing of applications.
The FDA notes in the draft guidance that this is needed to cover situations such as when some indications on the reference product are covered by exclusivity, although it does encourage a biosimilar manufacturer to seek licensure for all indications that the reference product does have.