User login
Klotho allele lowers APOE4-associated risk of Alzheimer’s
Cognitively normal carriers of the apolipoprotein E epsilon-4 (APOE4) allele aged 60 years and older who also showed heterozygosity for the Klotho-VS allele had a significantly reduced risk of developing Alzheimer’s disease, according to data from 22 research groups including more than 20,000 adults.
The transmembrane protein known as klotho (KL) is part of a functional haplotype known as KL-VS. “Specifically, heterozygosity for KL-VS (KL-VSHET+ status) has been shown to increase serum levels of KL and exert protective effects on healthy aging and longevity when compared with individuals who are homozygotes for the major or minor alleles (KL-VSHET−),” wrote Michael E. Belloy, PhD, of Stanford (Calif.) University and colleagues. However, the possible role of KL-VS in protecting against neurodegenerative disorders such as Alzheimer’s disease (AD) remains unclear, they said.
In a study published in JAMA Neurology, the researchers reviewed data from 20,928 participants in case-control studies, as well as 3,008 participants in conversion studies, 556 in amyloid-beta (a-beta) cerebrospinal fluid regression analyses, and 251 in brain amyloid PET regression analyses. The participants were aged 60-80 years, and of non-Hispanic northern European ancestry and were identified as cognitively normal or having mild cognitive impairment (MCI) or AD.
Overall, individuals with the APOE4 allele who were cognitively normal and heterozygous for KL-VS had a significantly reduced risk for developing AD (odds ratio, 0.75).
In addition, cognitively normal carriers of APOE4 with KL-VS heterozygosity had significantly lower risk of developing either MCI or AD (hazard ratio, 0.64). Also, those persons with APOE4 and positive KL-VS heterozygosity had higher a-beta in cerebrospinal fluid (P = .03) and lower a-beta on PET scans (P = .04). However, no association with cognitive outcomes were noted among APOE4 noncarriers, the researchers noted.
“This suggests that KL-VS interacts with aspects of AD pathology that are more pronounced in those who carry APOE4, such as a-beta accumulation during the presymptomatic phases of the disease,” they said.
The study findings were limited by the variable age and diagnoses across the multiple cohorts, but strengthened by the meta- and mega-analyses and sensitivity analyses that yielded consistent results, the researchers noted.
“Our work paves the way for biological validation studies to elucidate the molecular pathways by which KL-VS and APOE interact,” they said.
“The specificity of KL-VS benefits on AD in individuals who carry APOE4 is striking and suggests a yet-unstudied interaction between biological pathways of the klotho and APOE4 proteins,” wrote Dena B. Dubal, MD, and Jennifer S. Yokoyama, PhD, of the University of California, San Francisco, in an accompanying editorial. Despite limitations, the study findings have implications for clinical neurology, as well as clinical and translational research, they said.
“For personalized genomics, KL-VS status should integrate into knowledge that both lifestyle and genetics can negate or at least mitigate harmful influences of APOE4,” they noted. “In light of this, we might consider an individual’s KLOTHO genotype when counseling individuals who carry APOE4 about their prognosis for AD. In clinical trials using APOE4 for trial enrichment, further selection of individuals who carry APOE4 without KL-VS could define a population more likely to convert to AD and thus increase detection of a therapeutic benefit. In translational research, understanding how klotho itself or its biological pathways may counter APOE4 could lead to monumental progress in the future treatment of AD,” they added.
“Applying our growing knowledge of klotho to APOE4 and AD could ultimately pave the path to novel therapeutics for individuals who carry APOE4,” they concluded.
The study was supported by the Iqbal Farrukh & Asad Jamal Center for Cognitive Health in Aging, the South Palm Beach County Foundation, and the National Institutes of Health. The researchers had no financial conflicts to disclose. Dr. Dubal disclosed holding a patent for Methods for Improving Cognition that includes klotho, as well as consulting for Unity Biotechnology and receiving research funding from the National Institutes of Health, the American Federation for Aging Research, Glenn Medical Foundation, Unity Biotechnology, and other philanthropic support for translational research. Dr. Yokoyama disclosed research funding from the National Institutes of Health, the Department of Defense, and other foundations and philanthropic donors.
SOURCE: Belloy ME et al. JAMA Neurol. 2020 Apr 13. doi: 10.1001/jamaneurol.2020.0414.
Cognitively normal carriers of the apolipoprotein E epsilon-4 (APOE4) allele aged 60 years and older who also showed heterozygosity for the Klotho-VS allele had a significantly reduced risk of developing Alzheimer’s disease, according to data from 22 research groups including more than 20,000 adults.
The transmembrane protein known as klotho (KL) is part of a functional haplotype known as KL-VS. “Specifically, heterozygosity for KL-VS (KL-VSHET+ status) has been shown to increase serum levels of KL and exert protective effects on healthy aging and longevity when compared with individuals who are homozygotes for the major or minor alleles (KL-VSHET−),” wrote Michael E. Belloy, PhD, of Stanford (Calif.) University and colleagues. However, the possible role of KL-VS in protecting against neurodegenerative disorders such as Alzheimer’s disease (AD) remains unclear, they said.
In a study published in JAMA Neurology, the researchers reviewed data from 20,928 participants in case-control studies, as well as 3,008 participants in conversion studies, 556 in amyloid-beta (a-beta) cerebrospinal fluid regression analyses, and 251 in brain amyloid PET regression analyses. The participants were aged 60-80 years, and of non-Hispanic northern European ancestry and were identified as cognitively normal or having mild cognitive impairment (MCI) or AD.
Overall, individuals with the APOE4 allele who were cognitively normal and heterozygous for KL-VS had a significantly reduced risk for developing AD (odds ratio, 0.75).
In addition, cognitively normal carriers of APOE4 with KL-VS heterozygosity had significantly lower risk of developing either MCI or AD (hazard ratio, 0.64). Also, those persons with APOE4 and positive KL-VS heterozygosity had higher a-beta in cerebrospinal fluid (P = .03) and lower a-beta on PET scans (P = .04). However, no association with cognitive outcomes were noted among APOE4 noncarriers, the researchers noted.
“This suggests that KL-VS interacts with aspects of AD pathology that are more pronounced in those who carry APOE4, such as a-beta accumulation during the presymptomatic phases of the disease,” they said.
The study findings were limited by the variable age and diagnoses across the multiple cohorts, but strengthened by the meta- and mega-analyses and sensitivity analyses that yielded consistent results, the researchers noted.
“Our work paves the way for biological validation studies to elucidate the molecular pathways by which KL-VS and APOE interact,” they said.
“The specificity of KL-VS benefits on AD in individuals who carry APOE4 is striking and suggests a yet-unstudied interaction between biological pathways of the klotho and APOE4 proteins,” wrote Dena B. Dubal, MD, and Jennifer S. Yokoyama, PhD, of the University of California, San Francisco, in an accompanying editorial. Despite limitations, the study findings have implications for clinical neurology, as well as clinical and translational research, they said.
“For personalized genomics, KL-VS status should integrate into knowledge that both lifestyle and genetics can negate or at least mitigate harmful influences of APOE4,” they noted. “In light of this, we might consider an individual’s KLOTHO genotype when counseling individuals who carry APOE4 about their prognosis for AD. In clinical trials using APOE4 for trial enrichment, further selection of individuals who carry APOE4 without KL-VS could define a population more likely to convert to AD and thus increase detection of a therapeutic benefit. In translational research, understanding how klotho itself or its biological pathways may counter APOE4 could lead to monumental progress in the future treatment of AD,” they added.
“Applying our growing knowledge of klotho to APOE4 and AD could ultimately pave the path to novel therapeutics for individuals who carry APOE4,” they concluded.
The study was supported by the Iqbal Farrukh & Asad Jamal Center for Cognitive Health in Aging, the South Palm Beach County Foundation, and the National Institutes of Health. The researchers had no financial conflicts to disclose. Dr. Dubal disclosed holding a patent for Methods for Improving Cognition that includes klotho, as well as consulting for Unity Biotechnology and receiving research funding from the National Institutes of Health, the American Federation for Aging Research, Glenn Medical Foundation, Unity Biotechnology, and other philanthropic support for translational research. Dr. Yokoyama disclosed research funding from the National Institutes of Health, the Department of Defense, and other foundations and philanthropic donors.
SOURCE: Belloy ME et al. JAMA Neurol. 2020 Apr 13. doi: 10.1001/jamaneurol.2020.0414.
Cognitively normal carriers of the apolipoprotein E epsilon-4 (APOE4) allele aged 60 years and older who also showed heterozygosity for the Klotho-VS allele had a significantly reduced risk of developing Alzheimer’s disease, according to data from 22 research groups including more than 20,000 adults.
The transmembrane protein known as klotho (KL) is part of a functional haplotype known as KL-VS. “Specifically, heterozygosity for KL-VS (KL-VSHET+ status) has been shown to increase serum levels of KL and exert protective effects on healthy aging and longevity when compared with individuals who are homozygotes for the major or minor alleles (KL-VSHET−),” wrote Michael E. Belloy, PhD, of Stanford (Calif.) University and colleagues. However, the possible role of KL-VS in protecting against neurodegenerative disorders such as Alzheimer’s disease (AD) remains unclear, they said.
In a study published in JAMA Neurology, the researchers reviewed data from 20,928 participants in case-control studies, as well as 3,008 participants in conversion studies, 556 in amyloid-beta (a-beta) cerebrospinal fluid regression analyses, and 251 in brain amyloid PET regression analyses. The participants were aged 60-80 years, and of non-Hispanic northern European ancestry and were identified as cognitively normal or having mild cognitive impairment (MCI) or AD.
Overall, individuals with the APOE4 allele who were cognitively normal and heterozygous for KL-VS had a significantly reduced risk for developing AD (odds ratio, 0.75).
In addition, cognitively normal carriers of APOE4 with KL-VS heterozygosity had significantly lower risk of developing either MCI or AD (hazard ratio, 0.64). Also, those persons with APOE4 and positive KL-VS heterozygosity had higher a-beta in cerebrospinal fluid (P = .03) and lower a-beta on PET scans (P = .04). However, no association with cognitive outcomes were noted among APOE4 noncarriers, the researchers noted.
“This suggests that KL-VS interacts with aspects of AD pathology that are more pronounced in those who carry APOE4, such as a-beta accumulation during the presymptomatic phases of the disease,” they said.
The study findings were limited by the variable age and diagnoses across the multiple cohorts, but strengthened by the meta- and mega-analyses and sensitivity analyses that yielded consistent results, the researchers noted.
“Our work paves the way for biological validation studies to elucidate the molecular pathways by which KL-VS and APOE interact,” they said.
“The specificity of KL-VS benefits on AD in individuals who carry APOE4 is striking and suggests a yet-unstudied interaction between biological pathways of the klotho and APOE4 proteins,” wrote Dena B. Dubal, MD, and Jennifer S. Yokoyama, PhD, of the University of California, San Francisco, in an accompanying editorial. Despite limitations, the study findings have implications for clinical neurology, as well as clinical and translational research, they said.
“For personalized genomics, KL-VS status should integrate into knowledge that both lifestyle and genetics can negate or at least mitigate harmful influences of APOE4,” they noted. “In light of this, we might consider an individual’s KLOTHO genotype when counseling individuals who carry APOE4 about their prognosis for AD. In clinical trials using APOE4 for trial enrichment, further selection of individuals who carry APOE4 without KL-VS could define a population more likely to convert to AD and thus increase detection of a therapeutic benefit. In translational research, understanding how klotho itself or its biological pathways may counter APOE4 could lead to monumental progress in the future treatment of AD,” they added.
“Applying our growing knowledge of klotho to APOE4 and AD could ultimately pave the path to novel therapeutics for individuals who carry APOE4,” they concluded.
The study was supported by the Iqbal Farrukh & Asad Jamal Center for Cognitive Health in Aging, the South Palm Beach County Foundation, and the National Institutes of Health. The researchers had no financial conflicts to disclose. Dr. Dubal disclosed holding a patent for Methods for Improving Cognition that includes klotho, as well as consulting for Unity Biotechnology and receiving research funding from the National Institutes of Health, the American Federation for Aging Research, Glenn Medical Foundation, Unity Biotechnology, and other philanthropic support for translational research. Dr. Yokoyama disclosed research funding from the National Institutes of Health, the Department of Defense, and other foundations and philanthropic donors.
SOURCE: Belloy ME et al. JAMA Neurol. 2020 Apr 13. doi: 10.1001/jamaneurol.2020.0414.
FROM JAMA NEUROLOGY
Use of diabetes technology is lower among black, Hispanic patients
A retrospective study of patients at a minority-serving, safety-net hospital showed low uptake of diabetes technology among black patients with type 1 diabetes, compared with their white counterparts.
The researchers also found lower usage of the technology among Hispanic patients, but the difference, compared with their white counterparts, was not statistically significant after adjustments for language, insurance, age, and income. Patients who identified as “other” also were less likely than white patients to use the technology, which included continuous glucose monitors and continuous subcutaneous insulin infusion devices.
The data differes from other, similar studies of technology use in patients with type 1 diabetes, because the study population, drawn from the Boston University Medical Center, was more diverse than other studies, according to Kamonkiat Wirunsawanya, who is an endocrinology fellow at the medical center. The abstract had been slated for presentation at ENDO 2020, the Endocrine Society's annual meeting, which was canceled because of the COVID-19 pandemic.
Dr. Wirunsawanya and his colleagues are now using questionnaires to try to identify specific patient and physician factors that might explain the differences in technology use.
“Once we know which factors could be a barrier to using the technology, we’ll be able to implement a strategy to increase use in those patients,” Dr. Wirunsawanya said in an interview. The issue could be a two-way street, he noted, because some providers may be uncomfortable using the technology, or may perceive minorities as less adept at using technology.
The study included 227 adult patients who were seen at the medical center between October 2016 and September 2017. The mean age was 39 years, and 59% were men. The mean duration of type 1 diabetes was 21 years, and 30% of the patients were overweight, 22% were obese, 80% spoke English, and 50% were on government insurance. In all, 43% of the patients were white, 25% were black, 15% were Hispanic, 2% were Asian, and 15% identified as other.
Patients who used technology had lower mean levels of hemoglobin A1c, compared with nonusers (8.27% vs. 9.49%, respectively). Those with government health insurance were less likely than those with private insurance to use technology (odds ratio, 0.43; 95% confidential interval, 0.25-0.74).
Overall, 26% of the patients used continuous subcutaneous insulin infusion devices. Of those, 43% were white, 10% black, 14% Hispanic, none were Asian, and 18% identified as other.
In addition, 30% of the patients used continuous glucose monitors; of those, 47% were white, 14% black, 23% Hispanic, 25% Asian, and none identified as other.
After adjustments for insurance and language, the researchers found that black patients were less likely to use technology than were the white patients (OR, 0.25; 95% CI, 0.11-0.53). The same was found for those who identified as other (OR, 0.33; 95% CI, 0.12-0.89). There was no significant differences in technology use between white and Asian patients. After adjustments, the researchers showed that fewer Hispanic patients used technology, compared with their white counterparts, but the difference was not statistically significant.
In a multivariable logistic regression model that adjusted for insurance and language, black patients had lower odds of using technology, compared with white patients (OR, 0.25; 95% CI, 0.11-0.53), as did those identifying as other (OR, 0.33; 95% CI, 0.12-0.89).
In an interview, Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, said that the study highlights a common problem with introducing technology to underserved populations. “These study [findings are] not at all surprising. It’s something that is a puzzle for those of us who work in the field of diabetes management in patients from underserved communities. Even if you can get access to the technology, even when I get them tools and native Spanish-speaking educators and people who should be able to teach them how to use the technology, the adoption of the technology has been really much less than would be expected,” said Dr. Peters, who is also a professor of medicine at USC and was not involved in the research.
Part of the problem may be lack of contact with health care services, she said. White children with type 1 diabetes are treated from an early age and learn how to manage daily blood sugar levels. They often grow up to embrace technology, even enthusiastically. But in some minority communities, diabetes is viewed as something to be hidden away. That cultural difference is also a frustrating barrier.
“What happens in more affluent groups is that people learn from their peers, and they see that [managing their blood sugar] is possible. One of my big beliefs is that the answer has to come from [the community]. ... We need to get champions, people from the community who use these tools, to encourage others, and that’s hard to do because type 1 diabetes is such a small subset of the people with diabetes who are [black] or Latino,” said Dr. Peters.
Ultimately, the solution will require a shift in messaging by finding a way to help communities look differently at diabetes and its treatment. “There’s something that must come educationally and culturally that I’ve not figured out [yet]. I can get resources and fight for them, but we have to figure out how to make [technology] part of that culture, and I don’t know that we’ve done that,” she said.
Dr. Wirunsawanya reported no financial conflicts of interest. Dr. Peters has been on an advisory panel for Abbott Diabetes Care and has received research funding from Dexcom.
Dr. Wirunsawanya and colleagues’ research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Wirunsawanya K et al. ENDO 2020, Abstract OR30-03.
This article was updated on 4/17/2020.
A retrospective study of patients at a minority-serving, safety-net hospital showed low uptake of diabetes technology among black patients with type 1 diabetes, compared with their white counterparts.
The researchers also found lower usage of the technology among Hispanic patients, but the difference, compared with their white counterparts, was not statistically significant after adjustments for language, insurance, age, and income. Patients who identified as “other” also were less likely than white patients to use the technology, which included continuous glucose monitors and continuous subcutaneous insulin infusion devices.
The data differes from other, similar studies of technology use in patients with type 1 diabetes, because the study population, drawn from the Boston University Medical Center, was more diverse than other studies, according to Kamonkiat Wirunsawanya, who is an endocrinology fellow at the medical center. The abstract had been slated for presentation at ENDO 2020, the Endocrine Society's annual meeting, which was canceled because of the COVID-19 pandemic.
Dr. Wirunsawanya and his colleagues are now using questionnaires to try to identify specific patient and physician factors that might explain the differences in technology use.
“Once we know which factors could be a barrier to using the technology, we’ll be able to implement a strategy to increase use in those patients,” Dr. Wirunsawanya said in an interview. The issue could be a two-way street, he noted, because some providers may be uncomfortable using the technology, or may perceive minorities as less adept at using technology.
The study included 227 adult patients who were seen at the medical center between October 2016 and September 2017. The mean age was 39 years, and 59% were men. The mean duration of type 1 diabetes was 21 years, and 30% of the patients were overweight, 22% were obese, 80% spoke English, and 50% were on government insurance. In all, 43% of the patients were white, 25% were black, 15% were Hispanic, 2% were Asian, and 15% identified as other.
Patients who used technology had lower mean levels of hemoglobin A1c, compared with nonusers (8.27% vs. 9.49%, respectively). Those with government health insurance were less likely than those with private insurance to use technology (odds ratio, 0.43; 95% confidential interval, 0.25-0.74).
Overall, 26% of the patients used continuous subcutaneous insulin infusion devices. Of those, 43% were white, 10% black, 14% Hispanic, none were Asian, and 18% identified as other.
In addition, 30% of the patients used continuous glucose monitors; of those, 47% were white, 14% black, 23% Hispanic, 25% Asian, and none identified as other.
After adjustments for insurance and language, the researchers found that black patients were less likely to use technology than were the white patients (OR, 0.25; 95% CI, 0.11-0.53). The same was found for those who identified as other (OR, 0.33; 95% CI, 0.12-0.89). There was no significant differences in technology use between white and Asian patients. After adjustments, the researchers showed that fewer Hispanic patients used technology, compared with their white counterparts, but the difference was not statistically significant.
In a multivariable logistic regression model that adjusted for insurance and language, black patients had lower odds of using technology, compared with white patients (OR, 0.25; 95% CI, 0.11-0.53), as did those identifying as other (OR, 0.33; 95% CI, 0.12-0.89).
In an interview, Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, said that the study highlights a common problem with introducing technology to underserved populations. “These study [findings are] not at all surprising. It’s something that is a puzzle for those of us who work in the field of diabetes management in patients from underserved communities. Even if you can get access to the technology, even when I get them tools and native Spanish-speaking educators and people who should be able to teach them how to use the technology, the adoption of the technology has been really much less than would be expected,” said Dr. Peters, who is also a professor of medicine at USC and was not involved in the research.
Part of the problem may be lack of contact with health care services, she said. White children with type 1 diabetes are treated from an early age and learn how to manage daily blood sugar levels. They often grow up to embrace technology, even enthusiastically. But in some minority communities, diabetes is viewed as something to be hidden away. That cultural difference is also a frustrating barrier.
“What happens in more affluent groups is that people learn from their peers, and they see that [managing their blood sugar] is possible. One of my big beliefs is that the answer has to come from [the community]. ... We need to get champions, people from the community who use these tools, to encourage others, and that’s hard to do because type 1 diabetes is such a small subset of the people with diabetes who are [black] or Latino,” said Dr. Peters.
Ultimately, the solution will require a shift in messaging by finding a way to help communities look differently at diabetes and its treatment. “There’s something that must come educationally and culturally that I’ve not figured out [yet]. I can get resources and fight for them, but we have to figure out how to make [technology] part of that culture, and I don’t know that we’ve done that,” she said.
Dr. Wirunsawanya reported no financial conflicts of interest. Dr. Peters has been on an advisory panel for Abbott Diabetes Care and has received research funding from Dexcom.
Dr. Wirunsawanya and colleagues’ research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Wirunsawanya K et al. ENDO 2020, Abstract OR30-03.
This article was updated on 4/17/2020.
A retrospective study of patients at a minority-serving, safety-net hospital showed low uptake of diabetes technology among black patients with type 1 diabetes, compared with their white counterparts.
The researchers also found lower usage of the technology among Hispanic patients, but the difference, compared with their white counterparts, was not statistically significant after adjustments for language, insurance, age, and income. Patients who identified as “other” also were less likely than white patients to use the technology, which included continuous glucose monitors and continuous subcutaneous insulin infusion devices.
The data differes from other, similar studies of technology use in patients with type 1 diabetes, because the study population, drawn from the Boston University Medical Center, was more diverse than other studies, according to Kamonkiat Wirunsawanya, who is an endocrinology fellow at the medical center. The abstract had been slated for presentation at ENDO 2020, the Endocrine Society's annual meeting, which was canceled because of the COVID-19 pandemic.
Dr. Wirunsawanya and his colleagues are now using questionnaires to try to identify specific patient and physician factors that might explain the differences in technology use.
“Once we know which factors could be a barrier to using the technology, we’ll be able to implement a strategy to increase use in those patients,” Dr. Wirunsawanya said in an interview. The issue could be a two-way street, he noted, because some providers may be uncomfortable using the technology, or may perceive minorities as less adept at using technology.
The study included 227 adult patients who were seen at the medical center between October 2016 and September 2017. The mean age was 39 years, and 59% were men. The mean duration of type 1 diabetes was 21 years, and 30% of the patients were overweight, 22% were obese, 80% spoke English, and 50% were on government insurance. In all, 43% of the patients were white, 25% were black, 15% were Hispanic, 2% were Asian, and 15% identified as other.
Patients who used technology had lower mean levels of hemoglobin A1c, compared with nonusers (8.27% vs. 9.49%, respectively). Those with government health insurance were less likely than those with private insurance to use technology (odds ratio, 0.43; 95% confidential interval, 0.25-0.74).
Overall, 26% of the patients used continuous subcutaneous insulin infusion devices. Of those, 43% were white, 10% black, 14% Hispanic, none were Asian, and 18% identified as other.
In addition, 30% of the patients used continuous glucose monitors; of those, 47% were white, 14% black, 23% Hispanic, 25% Asian, and none identified as other.
After adjustments for insurance and language, the researchers found that black patients were less likely to use technology than were the white patients (OR, 0.25; 95% CI, 0.11-0.53). The same was found for those who identified as other (OR, 0.33; 95% CI, 0.12-0.89). There was no significant differences in technology use between white and Asian patients. After adjustments, the researchers showed that fewer Hispanic patients used technology, compared with their white counterparts, but the difference was not statistically significant.
In a multivariable logistic regression model that adjusted for insurance and language, black patients had lower odds of using technology, compared with white patients (OR, 0.25; 95% CI, 0.11-0.53), as did those identifying as other (OR, 0.33; 95% CI, 0.12-0.89).
In an interview, Anne Peters, MD, director of clinical diabetes programs at the University of Southern California, Los Angeles, said that the study highlights a common problem with introducing technology to underserved populations. “These study [findings are] not at all surprising. It’s something that is a puzzle for those of us who work in the field of diabetes management in patients from underserved communities. Even if you can get access to the technology, even when I get them tools and native Spanish-speaking educators and people who should be able to teach them how to use the technology, the adoption of the technology has been really much less than would be expected,” said Dr. Peters, who is also a professor of medicine at USC and was not involved in the research.
Part of the problem may be lack of contact with health care services, she said. White children with type 1 diabetes are treated from an early age and learn how to manage daily blood sugar levels. They often grow up to embrace technology, even enthusiastically. But in some minority communities, diabetes is viewed as something to be hidden away. That cultural difference is also a frustrating barrier.
“What happens in more affluent groups is that people learn from their peers, and they see that [managing their blood sugar] is possible. One of my big beliefs is that the answer has to come from [the community]. ... We need to get champions, people from the community who use these tools, to encourage others, and that’s hard to do because type 1 diabetes is such a small subset of the people with diabetes who are [black] or Latino,” said Dr. Peters.
Ultimately, the solution will require a shift in messaging by finding a way to help communities look differently at diabetes and its treatment. “There’s something that must come educationally and culturally that I’ve not figured out [yet]. I can get resources and fight for them, but we have to figure out how to make [technology] part of that culture, and I don’t know that we’ve done that,” she said.
Dr. Wirunsawanya reported no financial conflicts of interest. Dr. Peters has been on an advisory panel for Abbott Diabetes Care and has received research funding from Dexcom.
Dr. Wirunsawanya and colleagues’ research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
SOURCE: Wirunsawanya K et al. ENDO 2020, Abstract OR30-03.
This article was updated on 4/17/2020.
FROM ENDO 2020
Doing things right vs. doing the right things
A framework for a COVID-19 Person Under Investigation unit
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
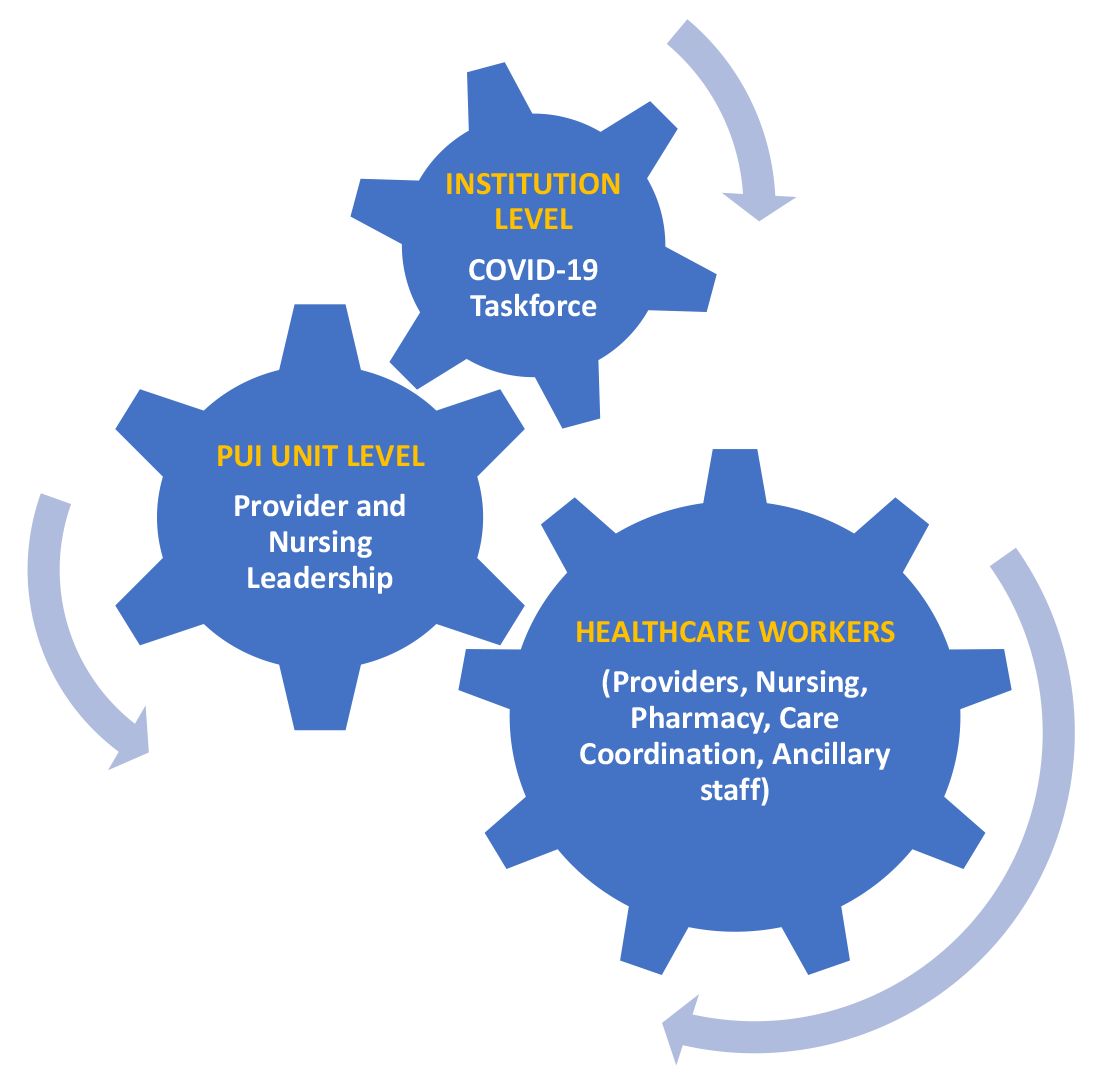
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.)
Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris ([email protected]) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang ([email protected]) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
A framework for a COVID-19 Person Under Investigation unit
A framework for a COVID-19 Person Under Investigation unit
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.)

Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris ([email protected]) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang ([email protected]) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
The current coronavirus disease 2019 (COVID-19) pandemic shocked the world with its rapid spread despite stringent containment efforts, and it continues to wreak havoc. The surrounding uncertainty due to the novelty of this virus has prompted significant investigation to determine proper containment, treatment, and eradication efforts.1,2 In addition, health care facilities are facing surge capacity issues and a shortage of resources resulting in lower quality care for patients and putting health care workers (HCWs) at risk for infection.3,4
While there is a lot of emerging clinical and basic science research in this area, there has been inconsistent guidance in regard to the containment and prevention of spread in health care systems. An initiative to minimize HCW exposure risk and to provide the highest quality care to patients was implemented by the Section of Hospital Medicine at our large academic medical center. We used a hospital medicine medical-surgical unit and converted it into a Person Under Investigation (PUI) unit for patients suspected of COVID-19.
Unit goals
- Deliver dedicated, comprehensive, and high-quality care to our PUI patients suspected of COVID-19.
- Minimize cross contamination with healthy patients on other hospital units.
- Provide clear and direct communications with our HCWs.
- Educate HCWs on optimal donning and doffing techniques.
- Minimize our HCW exposure risk.
- Efficiently use our personal protective equipment (PPE) supply.
Unit and team characteristics
We used a preexisting 24-bed hospital medicine medical-surgical unit with a dyad rounding model of an attending physician and advanced practice provider (APP). Other team members include a designated care coordinator (social worker/case manager), pharmacist, respiratory therapist, physical/occupational therapist, speech language pathologist, unit medical director, and nurse manager. A daily multidisciplinary huddle with all the team members was held to discuss the care of the PUI patients.
Administrative leadership
A COVID-19 task force composed of the medical director of clinical operations from the Section of Hospital Medicine, infectious disease, infection prevention, and several other important stakeholders conducted a daily conference call. This call allowed for the dissemination of information, including any treatment updates based on literature review or care processes. This information was then relayed to the HCWs following the meeting through the PUI unit medical director and nurse manager, who also facilitated feedback from the HCWs to the COVID-19 task force during the daily conference call. (See Figure 1.)

Patient flow
Hospital medicine was designated as the default service for all PUI patients suspected of COVID-19 and confirmed COVID-19 cases requiring hospitalization. These patients were admitted to this PUI unit directly from the emergency department (ED), or as transfers from outside institutions with assistance from our patient placement specialist team. Those patients admitted from our ED were tested for COVID-19 prior to arriving on the unit. Other suspected COVID-19 patients arriving as transfers from outside institutions were screened by the patient placement specialist team asking the following questions about the patient:
- “Has the patient had a fever or cough and been in contact with a laboratory-confirmed COVID-19 patient?”
- “Has the patient had a fever and cough?”
If the answer to either screening question was “yes,” then the patient was accepted to the PUI unit and tested upon arrival. Lastly, patients who were found to be COVID-19 positive at the outside institution, but who required transfer for other clinical reasons, were placed on this PUI unit as well.
Mechanisms to efficiently utilize PPE and mitigate HCW exposure risk
Our objectives are reducing the number of HCWs encountering PUI patients, reducing the number of encounters the HCWs have with PUI patients, and reducing the amount of time HCWs spent with PUI patients.
First, we maintained a log outside each patient’s room to track the details of staff encounters. Second, there was only one medical provider (either the attending physician or APP) assigned to each patient to limit personnel exposure. Third, we removed all learners (e.g. residents and students) from this unit. Fourth, we limited the number of entries into patient rooms to only critical staff directly involved in patient care (e.g. dietary and other ancillary staff were not allowed to enter the rooms) and provided updates to the patients by calling into the rooms. In addition, care coordination, pharmacy, and other staff members also utilized the same approach of calling into the room to speak with the patient regarding updates to minimize the duration of time spent in the room. Furthermore, our medical providers – with the help of the pharmacist and nursing – timed a patient’s medications to help reduce the number of entries into the room.
The medical providers also eliminated any unnecessary blood draws, imaging, and other procedures to minimize the number of encounters our HCWs had with the PUI. Lastly, the medical providers also avoided using any nebulizer treatments and noninvasive positive pressure ventilation to reduce any aerosol transmission of the virus. These measures not only helped to minimize our HCWs exposures, but also helped with the preservation of PPE.
Other efforts involved collaboration with infection prevention. They assisted with the training of our HCWs on proper PPE donning and doffing skills. This included watching a video and having an infection prevention specialist guide the HCWs throughout the entire process. We felt this was vital given the high amount of active failures with PPE use (up to 87%) reported in the literature.5 Furthermore, to ensure adequate mastery of these skills, infection prevention performed daily direct observation checks and provided real-time feedback to our HCWs.
Other things to consider for your PUI unit
There are several ideas that were not implemented in our PUI unit, but something to consider for your PUI unit, including:
- The use of elongated intravenous (IV) tubing, such that the IV poles and pumps were stationed outside the patient’s room, would be useful in reducing the amount of PPE required as well as HCW exposure to the patient.
- Having designated chest radiography, computed tomography, and magnetic resonance imaging scanners for these PUI patients to help minimize contamination with our non-PUI patients and to standardize the cleaning process.
- Supply our HCWs with designated scrubs at the beginning of their shifts, such that they can discard them at the end of their shifts for decontamination/sterilization purposes. This would help reduce HCWs fear of potentially exposing their families at home.
- Supply our HCWs with a designated place to stay, such as a hotel or other living quarters, to reduce HCWs fear of potentially exposing their families at home.
- Although we encouraged providers and staff to utilize designated phones to conduct patient history and review of systems information-gathering, to decrease the time spent in the room, the availability of more sophisticated audiovisual equipment could also improve the quality of the interview.
Conclusions
The increasing incidence in suspected COVID-19 patients has led to significant strain on health care systems of the world along with the associated economic and social crisis. Some health care facilities are facing surge capacity issues and inadequate resources, while others are facing a humanitarian crisis. Overall, we are all being affected by this pandemic, but are most concerned about its effects on our HCWs and our patients.
To address the concerns of low-quality care to our patients and anxiety levels among HCWs, we created this dedicated PUI unit in an effort to provide high-quality care for these suspected (and confirmed) COVID-19 patients and to maintain clear direct and constant communication with our HCWs.
Dr. Sunkara ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine, Winston-Salem, N.C. He is the medical director for Hospital Medicine Units and the newly established PUI Unit, and is the corresponding author for this article. Dr. Lippert ([email protected]) is assistant professor of internal medicine at Wake Forest School of Medicine. Dr. Morris ([email protected]) is a PGY-3 internal medicine resident at Wake Forest School of Medicine. Dr. Huang ([email protected]) is associate professor of internal medicine at Wake Forest School of Medicine.
References
1. Food and Drug Administration. Recommendations for investigational COVID-19 convalescent plasma. 2020 Apr 8.
2. Fauci AS et al. Covid-19 – Navigating the uncharted. N Engl J Med. 2020 Feb 28. doi: 10.1056/NEJMe2002387. 3. Emanuel EJ et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020 Mar 23. doi: 10.1056/NEJMsb2005114.
4. Li Ran et al. Risk factors of healthcare workers with corona virus disease 2019: A retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 Mar 17. doi: 10.1093/cid/ciaa287.
5. Krein SL et al. Identification and characterization of failures in infectious agent transmission precaution practices in hospitals: A qualitative study. JAMA Intern Med. 2018;178(8):1016-57. doi: 10.1001/jamainternmed.2018.1898.
Coronavirus tests are being fast-tracked by the FDA, but it’s unclear how accurate they are
Kendra Boroff believes she contracted the coronavirus on her 71st birthday, Feb. 20, when her family went out for a celebratory dinner, perhaps from their waiter, who was coughing into his elbow. Four days later, she developed a fever and a raging sore throat.
“You feel like you’re suffocating,” recalled Boroff, a real estate agent in Maineville, Ohio. “You cough and breathe with the top fourth or maybe less of your chest, because everything else is in a vise.”
Over the course of the next 3 weeks, as Boroff started getting chills and nausea, a series of doctors would suggest that it could be the common cold, bronchitis or pneumonia. She tested negative for the flu, and her chest x-rays showed signs of lung damage, including white patches called “ground-glass opacities” that are common in COVID-19 cases. By March 7, she was pretty sure it was COVID-19, but she couldn’t get a test until she arrived at the emergency room at the University of Cincinnati Health Center on March 19. She had a 103 degree fever and her oxygen levels were plummeting, so the doctors admitted her immediately.
Nearly a week later, as Boroff’s condition was stabilizing, the test results came back: negative.
Boroff was flummoxed, but her physician was clear that she had the virus, no matter what her test said.
“ ‘This is my diagnosis,’ ” she recalled him saying. “ ‘There is no other explanation.’ ”
Tests turning up negative even when all signs point to COVID-19 has been a common experience in American hospitals over the past month, public health experts have told ProPublica. It’s unclear what proportion of these negative results are inaccurate – known as “false negatives” – and whether that’s due to some external factor, like bad sample collection, or because of an issue inherent in the tests’ design.
Neither the major test manufacturers, the U.S. Food and Drug Administration, or the U.S. Centers for Disease Control and Prevention would say how common false negatives are. While the FDA requires test makers to report any known instances of false negatives as a condition of granting them provisional approval, known as emergency use authorizations, no such reports are visible in a database the agency maintains for that purpose.
Without much data on how COVID-19 tests are performing in the real world, concerns are mounting that a lack of accurate testing will make it more difficult for America to relax social distancing, as the ability to track and trace new infections will be critical for any strategy to reopen the country.
Those warnings have reached Capitol Hill, where Texas Democratic Rep. Lloyd Doggett had heard from a doctor in his district about the accuracy of the tests. On Thursday, he and Rep. Rosa DeLauro of Connecticut sent a letter to the FDA demanding more data about the prevalence of false negatives in both the diagnostic tests currently in widespread use, as well as inaccuracies in the coming wave of rapid blood tests that detect immunity once the infection has passed.
“I’m very concerned about it,” Doggett told ProPublica. Too many false results, he worries, could lead to a new surge of infections when people go back to work or are allowed to gather in bars, sports arenas, and restaurants. “They have to monitor this very closely to ensure that we’re not creating false expectations, and in the process ending up with an epidemic that is even worse than the one we have now.”
Lowering the bar in an emergency
In the early days of the pandemic, the FDA, which regulates diagnostic tests, was criticized for not moving quickly enough to make testing widely available. For much of February, the only available test was the CDC’s, which initially had flaws when it was sent out to public health labs. Only on Feb. 29 did the FDA announce a new policy that made it easier for private labs and academic medical centers to make tests available as well.
Since then, the ongoing need for even more testing capacity across the United States has pushed the agency to loosen its typical requirements for manufacturers to prove that their tests are accurate before allowing them onto the market.
Normally, to get FDA approval, diagnostic makers need to run trials to gather evidence on their tests’ performance, a process that can take months or even years. The agency is currently skipping a lot of those steps by issuing emergency use authorizations.
Manufacturers are now required to run their COVID-19 tests on a minimum of 30 positive samples and 30 negative samples. They must demonstrate to the agency that the test has at least a 95% sensitivity, meaning it must correctly identify at least 95% of the positive samples as having the coronavirus, and 100% specificity, meaning that it must accurately identify all the negative samples as not having the coronavirus.
But the manufacturers are demonstrating their diagnostics’ performance with what’s known as “contrived samples,” which are not taken from actual patients. A contrived sample is made by taking coronavirus RNA made in a lab and putting it into a medium that mimics nasal mucus.
“This is supposed to represent a swab specimen, but it’s not a positive sample from a real patient, and that does make a real difference,” said Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care.
It’s not clear if the concentrations of virus on the simulated samples are representative of the full range of material taken from patients’ bodies in the real world. Pinksy says that it’s reasonable for the FDA to allow the use of contrived samples, because it makes it much faster for a manufacturer to run validation studies, and the need for speed has been pressing.
“But then we need to have studies to compare these assays and see how they perform with real-world samples, and whether some are more or less sensitive and whether some are more or less specific,” Pinsky said. “We don’t know the answer to these questions at this point.”
To compensate for the lower standard up front, experts say the FDA should track data on accuracy to make sure the tests are performing as expected, but this is easier said than done.
“In diagnostic tests in particular, it’s very difficult to know if something is failing,” said Alberto Gutierrez, former director of the FDA’s Office of In Vitro Diagnostics and Radiological Health. “When are you getting more erroneous results than you should? It’s not always easy to figure out.”
Swiss manufacturer Roche, whose test was authorized by the FDA on March 12, told ProPublica it couldn’t give specific numbers about its test’s actual rate of false negatives and false positives, though it said studies have demonstrated its test could detect very low levels of the coronavirus.
“Clinical studies, which take months to run and would be part of a regular (nonemergency) test approval process, are needed to give us an exact percentage of false negatives and false positives,” Roche spokesman Mike Weist wrote in an email. “We will continue to work with the FDA on ongoing studies post-EUA that will allow us to potentially say more in the future.”
Abbott, which makes a rapid COVID-19 test, also said that “performance characteristics, including accuracy data, will continue to be collected in the field.”
Abbott and the testing firms LabCorp and Quest Diagnostics all told ProPublica that tests should be used by physicians along with other information to form a diagnosis.
Even good tests can give inaccurate results
Clinicians and researchers said that a number of factors could cause inaccurate results on COVID-19 tests, and many of them have nothing to do with the test’s design.
For starters, the timing of when a patient receives the test matters. “If you’re far out from the initial exposure, the more days you are after onset, viral load goes down,” explained Stanford’s Pinsky. Viral load refers to the amount of virus that is being emitted from an infected person’s cells, and if that drops too low, even a person who still has an active infection may test negative.
Another issue is where the virus is in a person’s body. As the disease progresses, scientists think the virus tends to move down into a patient’s lungs, so the window of time when a nose swab will return a positive result may be limited.
“One of the issues with this stupid virus is that, if it’s down in your lungs, and we’re putting a swab up your nose, that’s not the best way to measure what’s in your lungs,” said Alex Greninger, assistant director of the clinical virology lab at the University of Washington Medical Center.
While it is possible to stick a scope down a patient’s airway to collect a sample from the bottom of the lungs, this is a much more complex procedure that requires sedating the patient. Technicians can ask a patient to cough up phlegm, known as sputum, but doing so substantially raises the risk of infecting health care workers. Even with a nasopharyngeal sample collected with a nose swab, one needs to collect it properly, which involves sticking the swab quite far up a patient’s nose.
Daniel Brook, a freelance journalist and historian in New Orleans, says he thinks his test result may have been a false negative because he was incorrectly swabbed.
During Mardi Gras, he hung out with a friend who was visiting from Manhattan. A few days later, as he started to get night sweats and chills, Brook’s friend texted to say that he had tested positive for COVID-19. Brook has asthma, so when he started to have trouble breathing, he went to an urgent care center, which said it didn’t have enough tests to give him one.
Four days later, as Brook found himself even more winded going up stairs, he and his girlfriend, who also had symptoms, received a letter from an emergency room doctor that would get them a test at a drive-through center. They first were tested for the flu and then finally for COVID-19.
“This flu test was way the hell in there. It was almost like you ate too much hot pepper,” Brook said. “And then we had this COVID test, and it was barely in the nose at all, which may be one of the issues.” Nine days later, they received their results: Both were negative.
Brook was confused. He had been trying to tell all of the people he had been in contact with, like his barber, that they might have been exposed, and he shared the good news with many of them. But his doctor told him that clinically, he had all the symptoms of COVID-19, and that his diagnosis would not change based on his test result.
Even if the sample is taken correctly, mishandling of the swab can also invalidate the result. RNA is similar to DNA but due to chemical differences is a much more fragile material and degrades more readily. This coronavirus is an RNA virus, essentially a string of RNA encased in a membrane “envelope.”
Abbott, one of the test makers, said that it recommends that samples be kept for no more than 8 hours at about 60-85 degrees Fahrenheit, or refrigerated for 72 hours. “People should make sure it is tested in a timely fashion,” Abbott said in its statement to ProPublica.
None of this bodes well for the numerous labs that have reported backlogs of tens of thousands of samples that are waiting to be tested.
A technician at an academic laboratory, who asked for anonymity because he is not authorized to speak on behalf of his university, described seeing basic mishandling of samples that is probably ruining dozens of patients’ test results.
“I don’t know why, but with COVID, we’ve just been awash with problems,” he told ProPublica. “Even simple things like caps not screwed on tightly – we’ll get a bag of samples, and two or three of them will be leaking, so you have this media completely soaking the inside of the bag. If one of those leaking samples is positive, you’ll have droplets all over the bag.”
Those samples, the technician said, often can’t be processed at all. His experience isn’t unique: In Alabama in late March, hundreds of samples were ruined in transit to a lab in Montgomery.
The dangers of inaccuracy
In the absence of data, physicians and public health officials are left to guess how many false negatives may be occurring – which could have serious consequences both for individuals and for combating the spread of the disease.
“You want to be right every time, because you miss somebody, and tell them that they’re negative, then you’re infecting people,” said Gutierrez, the former FDA official. “Let’s say you consider Amazon essential, and at the warehouse they’re testing people, even if they miss 1 or 5 people out of 100, that can be problematic.”
In addition, false negatives can make it more difficult to track spread of the virus, since those patients are not reported as confirmed cases and people who die without a positive test result won’t be counted in COVID-19 mortality statistics.
False positives also present problems. If you mistakenly think a patient has COVID-19, “then you have the potential to clog up the health care system and waste personal protective equipment and the time and effort of health care workers who think they are caring for individuals with COVID-19,” Stanford’s Pinsky said. “In addition, you’re producing a lot of anxiety for the patient.”
Pinsky says he hopes that real-world data will be gathered on the tests’ performance, especially as more and more come on the market: “If physicians have this information, they could move on to a different, better performing test and use that instead.”
Dr. Yukari Manabe, associate director of Global Health Research and Innovation at Johns Hopkins Medicine, estimates that 10%-25% of test results are false negatives. That’s not based on any data, she cautions, since hard evidence isn’t available. But she has been noticing many patients in the Hopkins system being tested more than once, when the first result doesn’t match their clinical symptoms.
Like others, Manabe acknowledges that the FDA has needed to greenlight tests quickly in order to get them out into the public. But she laments that companies weren’t encouraged to develop diagnostics earlier, which might have allowed the agency to keep the bar for approval higher, and also churn out more tests sooner.
“If people had seen the writing on the wall back in December, someone should’ve paid these companies what they needed to develop these tests on platforms that could’ve been rapidly ramped up to millions of tests,” Manabe said.
Instead, a test shortage caused doctors to limit tests to only the sickest patients, at a time when the virus had probably moved out of the back of the nasal cavity and into their lungs. A larger supply would have allowed for testing more people as soon as they started showing symptoms. That would have resulted in a lower rate of false negatives, Manabe said, since nose swabs are more likely to detect the virus soon after it’s been contracted.
The next wave of tests may be even less accurate
The questions swirling around the accuracy of the COVID-19 diagnostic tests are likely to persist as the next set of tests – antibody blood tests – start hitting the market. Already, the FDA has authorized the first of these tests, which search for molecules in a patient’s blood that can indicate if the immune system did battle with the coronavirus. Unlike the swab-based tests, which look for the viral RNA that indicate active infection, antibody tests are used to seek evidence of a past encounter with the virus.
Antibody tests are already seen as a critical tool in lifting lock-down measures, because they could potentially be used to figure out who has immunity to the coronavirus. In this case, false positives would be the greater concern, because it could be dangerous to tell someone that they have antibodies and are safe to go back to work when that is a false signal.
There are issues that need to be figured out before rushing to rely on these tests, Stanford’s Pinsky warned. What level of antibodies are needed to mean that someone is protected? And if you are protected, how long are you protected? The answers to these basic questions are still unknown, he said. This week, the World Health Organization put out guidance recommending against using antibody tests for clinical decision making.
The FDA, meanwhile, is lowering the bar even further. On March 16, it issued new guidance allowing manufacturers to distribute tests even before receiving emergency use authorization, for a “reasonable period of time” – about 15 days – after a diagnostic maker had validated the test internally and while preparing its request to the agency for an EUA.
Local governments are desperate enough for tests that they’ll buy them without assurances of accuracy at all. Chicago recently ordered 11,000 antibody tests made in South Korea that had not been reviewed by the FDA but are legal to distribute as long as they include several disclaimers including a recommendation that any negative result be confirmed with a diagnostic test.
“There’s no time really to put the effort into saying, ’Where’s the problem here?’ ” said Catherine Troisi, an epidemiologist at the University of Texas Health Science Center. “I’m not saying the test is bad. But what good is a test if you don’t know it’s giving you reliable results? We just don’t know.”
Correction, April 10, 2020: This story originally said incorrectly that Kendra Boroff was admitted to an intensive care unit.
This article was first published on ProPublica.com.
Kendra Boroff believes she contracted the coronavirus on her 71st birthday, Feb. 20, when her family went out for a celebratory dinner, perhaps from their waiter, who was coughing into his elbow. Four days later, she developed a fever and a raging sore throat.
“You feel like you’re suffocating,” recalled Boroff, a real estate agent in Maineville, Ohio. “You cough and breathe with the top fourth or maybe less of your chest, because everything else is in a vise.”
Over the course of the next 3 weeks, as Boroff started getting chills and nausea, a series of doctors would suggest that it could be the common cold, bronchitis or pneumonia. She tested negative for the flu, and her chest x-rays showed signs of lung damage, including white patches called “ground-glass opacities” that are common in COVID-19 cases. By March 7, she was pretty sure it was COVID-19, but she couldn’t get a test until she arrived at the emergency room at the University of Cincinnati Health Center on March 19. She had a 103 degree fever and her oxygen levels were plummeting, so the doctors admitted her immediately.
Nearly a week later, as Boroff’s condition was stabilizing, the test results came back: negative.
Boroff was flummoxed, but her physician was clear that she had the virus, no matter what her test said.
“ ‘This is my diagnosis,’ ” she recalled him saying. “ ‘There is no other explanation.’ ”
Tests turning up negative even when all signs point to COVID-19 has been a common experience in American hospitals over the past month, public health experts have told ProPublica. It’s unclear what proportion of these negative results are inaccurate – known as “false negatives” – and whether that’s due to some external factor, like bad sample collection, or because of an issue inherent in the tests’ design.
Neither the major test manufacturers, the U.S. Food and Drug Administration, or the U.S. Centers for Disease Control and Prevention would say how common false negatives are. While the FDA requires test makers to report any known instances of false negatives as a condition of granting them provisional approval, known as emergency use authorizations, no such reports are visible in a database the agency maintains for that purpose.
Without much data on how COVID-19 tests are performing in the real world, concerns are mounting that a lack of accurate testing will make it more difficult for America to relax social distancing, as the ability to track and trace new infections will be critical for any strategy to reopen the country.
Those warnings have reached Capitol Hill, where Texas Democratic Rep. Lloyd Doggett had heard from a doctor in his district about the accuracy of the tests. On Thursday, he and Rep. Rosa DeLauro of Connecticut sent a letter to the FDA demanding more data about the prevalence of false negatives in both the diagnostic tests currently in widespread use, as well as inaccuracies in the coming wave of rapid blood tests that detect immunity once the infection has passed.
“I’m very concerned about it,” Doggett told ProPublica. Too many false results, he worries, could lead to a new surge of infections when people go back to work or are allowed to gather in bars, sports arenas, and restaurants. “They have to monitor this very closely to ensure that we’re not creating false expectations, and in the process ending up with an epidemic that is even worse than the one we have now.”
Lowering the bar in an emergency
In the early days of the pandemic, the FDA, which regulates diagnostic tests, was criticized for not moving quickly enough to make testing widely available. For much of February, the only available test was the CDC’s, which initially had flaws when it was sent out to public health labs. Only on Feb. 29 did the FDA announce a new policy that made it easier for private labs and academic medical centers to make tests available as well.
Since then, the ongoing need for even more testing capacity across the United States has pushed the agency to loosen its typical requirements for manufacturers to prove that their tests are accurate before allowing them onto the market.
Normally, to get FDA approval, diagnostic makers need to run trials to gather evidence on their tests’ performance, a process that can take months or even years. The agency is currently skipping a lot of those steps by issuing emergency use authorizations.
Manufacturers are now required to run their COVID-19 tests on a minimum of 30 positive samples and 30 negative samples. They must demonstrate to the agency that the test has at least a 95% sensitivity, meaning it must correctly identify at least 95% of the positive samples as having the coronavirus, and 100% specificity, meaning that it must accurately identify all the negative samples as not having the coronavirus.
But the manufacturers are demonstrating their diagnostics’ performance with what’s known as “contrived samples,” which are not taken from actual patients. A contrived sample is made by taking coronavirus RNA made in a lab and putting it into a medium that mimics nasal mucus.
“This is supposed to represent a swab specimen, but it’s not a positive sample from a real patient, and that does make a real difference,” said Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care.
It’s not clear if the concentrations of virus on the simulated samples are representative of the full range of material taken from patients’ bodies in the real world. Pinksy says that it’s reasonable for the FDA to allow the use of contrived samples, because it makes it much faster for a manufacturer to run validation studies, and the need for speed has been pressing.
“But then we need to have studies to compare these assays and see how they perform with real-world samples, and whether some are more or less sensitive and whether some are more or less specific,” Pinsky said. “We don’t know the answer to these questions at this point.”
To compensate for the lower standard up front, experts say the FDA should track data on accuracy to make sure the tests are performing as expected, but this is easier said than done.
“In diagnostic tests in particular, it’s very difficult to know if something is failing,” said Alberto Gutierrez, former director of the FDA’s Office of In Vitro Diagnostics and Radiological Health. “When are you getting more erroneous results than you should? It’s not always easy to figure out.”
Swiss manufacturer Roche, whose test was authorized by the FDA on March 12, told ProPublica it couldn’t give specific numbers about its test’s actual rate of false negatives and false positives, though it said studies have demonstrated its test could detect very low levels of the coronavirus.
“Clinical studies, which take months to run and would be part of a regular (nonemergency) test approval process, are needed to give us an exact percentage of false negatives and false positives,” Roche spokesman Mike Weist wrote in an email. “We will continue to work with the FDA on ongoing studies post-EUA that will allow us to potentially say more in the future.”
Abbott, which makes a rapid COVID-19 test, also said that “performance characteristics, including accuracy data, will continue to be collected in the field.”
Abbott and the testing firms LabCorp and Quest Diagnostics all told ProPublica that tests should be used by physicians along with other information to form a diagnosis.
Even good tests can give inaccurate results
Clinicians and researchers said that a number of factors could cause inaccurate results on COVID-19 tests, and many of them have nothing to do with the test’s design.
For starters, the timing of when a patient receives the test matters. “If you’re far out from the initial exposure, the more days you are after onset, viral load goes down,” explained Stanford’s Pinsky. Viral load refers to the amount of virus that is being emitted from an infected person’s cells, and if that drops too low, even a person who still has an active infection may test negative.
Another issue is where the virus is in a person’s body. As the disease progresses, scientists think the virus tends to move down into a patient’s lungs, so the window of time when a nose swab will return a positive result may be limited.
“One of the issues with this stupid virus is that, if it’s down in your lungs, and we’re putting a swab up your nose, that’s not the best way to measure what’s in your lungs,” said Alex Greninger, assistant director of the clinical virology lab at the University of Washington Medical Center.
While it is possible to stick a scope down a patient’s airway to collect a sample from the bottom of the lungs, this is a much more complex procedure that requires sedating the patient. Technicians can ask a patient to cough up phlegm, known as sputum, but doing so substantially raises the risk of infecting health care workers. Even with a nasopharyngeal sample collected with a nose swab, one needs to collect it properly, which involves sticking the swab quite far up a patient’s nose.
Daniel Brook, a freelance journalist and historian in New Orleans, says he thinks his test result may have been a false negative because he was incorrectly swabbed.
During Mardi Gras, he hung out with a friend who was visiting from Manhattan. A few days later, as he started to get night sweats and chills, Brook’s friend texted to say that he had tested positive for COVID-19. Brook has asthma, so when he started to have trouble breathing, he went to an urgent care center, which said it didn’t have enough tests to give him one.
Four days later, as Brook found himself even more winded going up stairs, he and his girlfriend, who also had symptoms, received a letter from an emergency room doctor that would get them a test at a drive-through center. They first were tested for the flu and then finally for COVID-19.
“This flu test was way the hell in there. It was almost like you ate too much hot pepper,” Brook said. “And then we had this COVID test, and it was barely in the nose at all, which may be one of the issues.” Nine days later, they received their results: Both were negative.
Brook was confused. He had been trying to tell all of the people he had been in contact with, like his barber, that they might have been exposed, and he shared the good news with many of them. But his doctor told him that clinically, he had all the symptoms of COVID-19, and that his diagnosis would not change based on his test result.
Even if the sample is taken correctly, mishandling of the swab can also invalidate the result. RNA is similar to DNA but due to chemical differences is a much more fragile material and degrades more readily. This coronavirus is an RNA virus, essentially a string of RNA encased in a membrane “envelope.”
Abbott, one of the test makers, said that it recommends that samples be kept for no more than 8 hours at about 60-85 degrees Fahrenheit, or refrigerated for 72 hours. “People should make sure it is tested in a timely fashion,” Abbott said in its statement to ProPublica.
None of this bodes well for the numerous labs that have reported backlogs of tens of thousands of samples that are waiting to be tested.
A technician at an academic laboratory, who asked for anonymity because he is not authorized to speak on behalf of his university, described seeing basic mishandling of samples that is probably ruining dozens of patients’ test results.
“I don’t know why, but with COVID, we’ve just been awash with problems,” he told ProPublica. “Even simple things like caps not screwed on tightly – we’ll get a bag of samples, and two or three of them will be leaking, so you have this media completely soaking the inside of the bag. If one of those leaking samples is positive, you’ll have droplets all over the bag.”
Those samples, the technician said, often can’t be processed at all. His experience isn’t unique: In Alabama in late March, hundreds of samples were ruined in transit to a lab in Montgomery.
The dangers of inaccuracy
In the absence of data, physicians and public health officials are left to guess how many false negatives may be occurring – which could have serious consequences both for individuals and for combating the spread of the disease.
“You want to be right every time, because you miss somebody, and tell them that they’re negative, then you’re infecting people,” said Gutierrez, the former FDA official. “Let’s say you consider Amazon essential, and at the warehouse they’re testing people, even if they miss 1 or 5 people out of 100, that can be problematic.”
In addition, false negatives can make it more difficult to track spread of the virus, since those patients are not reported as confirmed cases and people who die without a positive test result won’t be counted in COVID-19 mortality statistics.
False positives also present problems. If you mistakenly think a patient has COVID-19, “then you have the potential to clog up the health care system and waste personal protective equipment and the time and effort of health care workers who think they are caring for individuals with COVID-19,” Stanford’s Pinsky said. “In addition, you’re producing a lot of anxiety for the patient.”
Pinsky says he hopes that real-world data will be gathered on the tests’ performance, especially as more and more come on the market: “If physicians have this information, they could move on to a different, better performing test and use that instead.”
Dr. Yukari Manabe, associate director of Global Health Research and Innovation at Johns Hopkins Medicine, estimates that 10%-25% of test results are false negatives. That’s not based on any data, she cautions, since hard evidence isn’t available. But she has been noticing many patients in the Hopkins system being tested more than once, when the first result doesn’t match their clinical symptoms.
Like others, Manabe acknowledges that the FDA has needed to greenlight tests quickly in order to get them out into the public. But she laments that companies weren’t encouraged to develop diagnostics earlier, which might have allowed the agency to keep the bar for approval higher, and also churn out more tests sooner.
“If people had seen the writing on the wall back in December, someone should’ve paid these companies what they needed to develop these tests on platforms that could’ve been rapidly ramped up to millions of tests,” Manabe said.
Instead, a test shortage caused doctors to limit tests to only the sickest patients, at a time when the virus had probably moved out of the back of the nasal cavity and into their lungs. A larger supply would have allowed for testing more people as soon as they started showing symptoms. That would have resulted in a lower rate of false negatives, Manabe said, since nose swabs are more likely to detect the virus soon after it’s been contracted.
The next wave of tests may be even less accurate
The questions swirling around the accuracy of the COVID-19 diagnostic tests are likely to persist as the next set of tests – antibody blood tests – start hitting the market. Already, the FDA has authorized the first of these tests, which search for molecules in a patient’s blood that can indicate if the immune system did battle with the coronavirus. Unlike the swab-based tests, which look for the viral RNA that indicate active infection, antibody tests are used to seek evidence of a past encounter with the virus.
Antibody tests are already seen as a critical tool in lifting lock-down measures, because they could potentially be used to figure out who has immunity to the coronavirus. In this case, false positives would be the greater concern, because it could be dangerous to tell someone that they have antibodies and are safe to go back to work when that is a false signal.
There are issues that need to be figured out before rushing to rely on these tests, Stanford’s Pinsky warned. What level of antibodies are needed to mean that someone is protected? And if you are protected, how long are you protected? The answers to these basic questions are still unknown, he said. This week, the World Health Organization put out guidance recommending against using antibody tests for clinical decision making.
The FDA, meanwhile, is lowering the bar even further. On March 16, it issued new guidance allowing manufacturers to distribute tests even before receiving emergency use authorization, for a “reasonable period of time” – about 15 days – after a diagnostic maker had validated the test internally and while preparing its request to the agency for an EUA.
Local governments are desperate enough for tests that they’ll buy them without assurances of accuracy at all. Chicago recently ordered 11,000 antibody tests made in South Korea that had not been reviewed by the FDA but are legal to distribute as long as they include several disclaimers including a recommendation that any negative result be confirmed with a diagnostic test.
“There’s no time really to put the effort into saying, ’Where’s the problem here?’ ” said Catherine Troisi, an epidemiologist at the University of Texas Health Science Center. “I’m not saying the test is bad. But what good is a test if you don’t know it’s giving you reliable results? We just don’t know.”
Correction, April 10, 2020: This story originally said incorrectly that Kendra Boroff was admitted to an intensive care unit.
This article was first published on ProPublica.com.
Kendra Boroff believes she contracted the coronavirus on her 71st birthday, Feb. 20, when her family went out for a celebratory dinner, perhaps from their waiter, who was coughing into his elbow. Four days later, she developed a fever and a raging sore throat.
“You feel like you’re suffocating,” recalled Boroff, a real estate agent in Maineville, Ohio. “You cough and breathe with the top fourth or maybe less of your chest, because everything else is in a vise.”
Over the course of the next 3 weeks, as Boroff started getting chills and nausea, a series of doctors would suggest that it could be the common cold, bronchitis or pneumonia. She tested negative for the flu, and her chest x-rays showed signs of lung damage, including white patches called “ground-glass opacities” that are common in COVID-19 cases. By March 7, she was pretty sure it was COVID-19, but she couldn’t get a test until she arrived at the emergency room at the University of Cincinnati Health Center on March 19. She had a 103 degree fever and her oxygen levels were plummeting, so the doctors admitted her immediately.
Nearly a week later, as Boroff’s condition was stabilizing, the test results came back: negative.
Boroff was flummoxed, but her physician was clear that she had the virus, no matter what her test said.
“ ‘This is my diagnosis,’ ” she recalled him saying. “ ‘There is no other explanation.’ ”
Tests turning up negative even when all signs point to COVID-19 has been a common experience in American hospitals over the past month, public health experts have told ProPublica. It’s unclear what proportion of these negative results are inaccurate – known as “false negatives” – and whether that’s due to some external factor, like bad sample collection, or because of an issue inherent in the tests’ design.
Neither the major test manufacturers, the U.S. Food and Drug Administration, or the U.S. Centers for Disease Control and Prevention would say how common false negatives are. While the FDA requires test makers to report any known instances of false negatives as a condition of granting them provisional approval, known as emergency use authorizations, no such reports are visible in a database the agency maintains for that purpose.
Without much data on how COVID-19 tests are performing in the real world, concerns are mounting that a lack of accurate testing will make it more difficult for America to relax social distancing, as the ability to track and trace new infections will be critical for any strategy to reopen the country.
Those warnings have reached Capitol Hill, where Texas Democratic Rep. Lloyd Doggett had heard from a doctor in his district about the accuracy of the tests. On Thursday, he and Rep. Rosa DeLauro of Connecticut sent a letter to the FDA demanding more data about the prevalence of false negatives in both the diagnostic tests currently in widespread use, as well as inaccuracies in the coming wave of rapid blood tests that detect immunity once the infection has passed.
“I’m very concerned about it,” Doggett told ProPublica. Too many false results, he worries, could lead to a new surge of infections when people go back to work or are allowed to gather in bars, sports arenas, and restaurants. “They have to monitor this very closely to ensure that we’re not creating false expectations, and in the process ending up with an epidemic that is even worse than the one we have now.”
Lowering the bar in an emergency
In the early days of the pandemic, the FDA, which regulates diagnostic tests, was criticized for not moving quickly enough to make testing widely available. For much of February, the only available test was the CDC’s, which initially had flaws when it was sent out to public health labs. Only on Feb. 29 did the FDA announce a new policy that made it easier for private labs and academic medical centers to make tests available as well.
Since then, the ongoing need for even more testing capacity across the United States has pushed the agency to loosen its typical requirements for manufacturers to prove that their tests are accurate before allowing them onto the market.
Normally, to get FDA approval, diagnostic makers need to run trials to gather evidence on their tests’ performance, a process that can take months or even years. The agency is currently skipping a lot of those steps by issuing emergency use authorizations.
Manufacturers are now required to run their COVID-19 tests on a minimum of 30 positive samples and 30 negative samples. They must demonstrate to the agency that the test has at least a 95% sensitivity, meaning it must correctly identify at least 95% of the positive samples as having the coronavirus, and 100% specificity, meaning that it must accurately identify all the negative samples as not having the coronavirus.
But the manufacturers are demonstrating their diagnostics’ performance with what’s known as “contrived samples,” which are not taken from actual patients. A contrived sample is made by taking coronavirus RNA made in a lab and putting it into a medium that mimics nasal mucus.
“This is supposed to represent a swab specimen, but it’s not a positive sample from a real patient, and that does make a real difference,” said Benjamin Pinsky, medical director of the Clinical Virology Laboratory for Stanford Health Care.
It’s not clear if the concentrations of virus on the simulated samples are representative of the full range of material taken from patients’ bodies in the real world. Pinksy says that it’s reasonable for the FDA to allow the use of contrived samples, because it makes it much faster for a manufacturer to run validation studies, and the need for speed has been pressing.
“But then we need to have studies to compare these assays and see how they perform with real-world samples, and whether some are more or less sensitive and whether some are more or less specific,” Pinsky said. “We don’t know the answer to these questions at this point.”
To compensate for the lower standard up front, experts say the FDA should track data on accuracy to make sure the tests are performing as expected, but this is easier said than done.
“In diagnostic tests in particular, it’s very difficult to know if something is failing,” said Alberto Gutierrez, former director of the FDA’s Office of In Vitro Diagnostics and Radiological Health. “When are you getting more erroneous results than you should? It’s not always easy to figure out.”
Swiss manufacturer Roche, whose test was authorized by the FDA on March 12, told ProPublica it couldn’t give specific numbers about its test’s actual rate of false negatives and false positives, though it said studies have demonstrated its test could detect very low levels of the coronavirus.
“Clinical studies, which take months to run and would be part of a regular (nonemergency) test approval process, are needed to give us an exact percentage of false negatives and false positives,” Roche spokesman Mike Weist wrote in an email. “We will continue to work with the FDA on ongoing studies post-EUA that will allow us to potentially say more in the future.”
Abbott, which makes a rapid COVID-19 test, also said that “performance characteristics, including accuracy data, will continue to be collected in the field.”
Abbott and the testing firms LabCorp and Quest Diagnostics all told ProPublica that tests should be used by physicians along with other information to form a diagnosis.
Even good tests can give inaccurate results
Clinicians and researchers said that a number of factors could cause inaccurate results on COVID-19 tests, and many of them have nothing to do with the test’s design.
For starters, the timing of when a patient receives the test matters. “If you’re far out from the initial exposure, the more days you are after onset, viral load goes down,” explained Stanford’s Pinsky. Viral load refers to the amount of virus that is being emitted from an infected person’s cells, and if that drops too low, even a person who still has an active infection may test negative.
Another issue is where the virus is in a person’s body. As the disease progresses, scientists think the virus tends to move down into a patient’s lungs, so the window of time when a nose swab will return a positive result may be limited.
“One of the issues with this stupid virus is that, if it’s down in your lungs, and we’re putting a swab up your nose, that’s not the best way to measure what’s in your lungs,” said Alex Greninger, assistant director of the clinical virology lab at the University of Washington Medical Center.
While it is possible to stick a scope down a patient’s airway to collect a sample from the bottom of the lungs, this is a much more complex procedure that requires sedating the patient. Technicians can ask a patient to cough up phlegm, known as sputum, but doing so substantially raises the risk of infecting health care workers. Even with a nasopharyngeal sample collected with a nose swab, one needs to collect it properly, which involves sticking the swab quite far up a patient’s nose.
Daniel Brook, a freelance journalist and historian in New Orleans, says he thinks his test result may have been a false negative because he was incorrectly swabbed.
During Mardi Gras, he hung out with a friend who was visiting from Manhattan. A few days later, as he started to get night sweats and chills, Brook’s friend texted to say that he had tested positive for COVID-19. Brook has asthma, so when he started to have trouble breathing, he went to an urgent care center, which said it didn’t have enough tests to give him one.
Four days later, as Brook found himself even more winded going up stairs, he and his girlfriend, who also had symptoms, received a letter from an emergency room doctor that would get them a test at a drive-through center. They first were tested for the flu and then finally for COVID-19.
“This flu test was way the hell in there. It was almost like you ate too much hot pepper,” Brook said. “And then we had this COVID test, and it was barely in the nose at all, which may be one of the issues.” Nine days later, they received their results: Both were negative.
Brook was confused. He had been trying to tell all of the people he had been in contact with, like his barber, that they might have been exposed, and he shared the good news with many of them. But his doctor told him that clinically, he had all the symptoms of COVID-19, and that his diagnosis would not change based on his test result.
Even if the sample is taken correctly, mishandling of the swab can also invalidate the result. RNA is similar to DNA but due to chemical differences is a much more fragile material and degrades more readily. This coronavirus is an RNA virus, essentially a string of RNA encased in a membrane “envelope.”
Abbott, one of the test makers, said that it recommends that samples be kept for no more than 8 hours at about 60-85 degrees Fahrenheit, or refrigerated for 72 hours. “People should make sure it is tested in a timely fashion,” Abbott said in its statement to ProPublica.
None of this bodes well for the numerous labs that have reported backlogs of tens of thousands of samples that are waiting to be tested.
A technician at an academic laboratory, who asked for anonymity because he is not authorized to speak on behalf of his university, described seeing basic mishandling of samples that is probably ruining dozens of patients’ test results.
“I don’t know why, but with COVID, we’ve just been awash with problems,” he told ProPublica. “Even simple things like caps not screwed on tightly – we’ll get a bag of samples, and two or three of them will be leaking, so you have this media completely soaking the inside of the bag. If one of those leaking samples is positive, you’ll have droplets all over the bag.”
Those samples, the technician said, often can’t be processed at all. His experience isn’t unique: In Alabama in late March, hundreds of samples were ruined in transit to a lab in Montgomery.
The dangers of inaccuracy
In the absence of data, physicians and public health officials are left to guess how many false negatives may be occurring – which could have serious consequences both for individuals and for combating the spread of the disease.
“You want to be right every time, because you miss somebody, and tell them that they’re negative, then you’re infecting people,” said Gutierrez, the former FDA official. “Let’s say you consider Amazon essential, and at the warehouse they’re testing people, even if they miss 1 or 5 people out of 100, that can be problematic.”
In addition, false negatives can make it more difficult to track spread of the virus, since those patients are not reported as confirmed cases and people who die without a positive test result won’t be counted in COVID-19 mortality statistics.
False positives also present problems. If you mistakenly think a patient has COVID-19, “then you have the potential to clog up the health care system and waste personal protective equipment and the time and effort of health care workers who think they are caring for individuals with COVID-19,” Stanford’s Pinsky said. “In addition, you’re producing a lot of anxiety for the patient.”
Pinsky says he hopes that real-world data will be gathered on the tests’ performance, especially as more and more come on the market: “If physicians have this information, they could move on to a different, better performing test and use that instead.”
Dr. Yukari Manabe, associate director of Global Health Research and Innovation at Johns Hopkins Medicine, estimates that 10%-25% of test results are false negatives. That’s not based on any data, she cautions, since hard evidence isn’t available. But she has been noticing many patients in the Hopkins system being tested more than once, when the first result doesn’t match their clinical symptoms.
Like others, Manabe acknowledges that the FDA has needed to greenlight tests quickly in order to get them out into the public. But she laments that companies weren’t encouraged to develop diagnostics earlier, which might have allowed the agency to keep the bar for approval higher, and also churn out more tests sooner.
“If people had seen the writing on the wall back in December, someone should’ve paid these companies what they needed to develop these tests on platforms that could’ve been rapidly ramped up to millions of tests,” Manabe said.
Instead, a test shortage caused doctors to limit tests to only the sickest patients, at a time when the virus had probably moved out of the back of the nasal cavity and into their lungs. A larger supply would have allowed for testing more people as soon as they started showing symptoms. That would have resulted in a lower rate of false negatives, Manabe said, since nose swabs are more likely to detect the virus soon after it’s been contracted.
The next wave of tests may be even less accurate
The questions swirling around the accuracy of the COVID-19 diagnostic tests are likely to persist as the next set of tests – antibody blood tests – start hitting the market. Already, the FDA has authorized the first of these tests, which search for molecules in a patient’s blood that can indicate if the immune system did battle with the coronavirus. Unlike the swab-based tests, which look for the viral RNA that indicate active infection, antibody tests are used to seek evidence of a past encounter with the virus.
Antibody tests are already seen as a critical tool in lifting lock-down measures, because they could potentially be used to figure out who has immunity to the coronavirus. In this case, false positives would be the greater concern, because it could be dangerous to tell someone that they have antibodies and are safe to go back to work when that is a false signal.
There are issues that need to be figured out before rushing to rely on these tests, Stanford’s Pinsky warned. What level of antibodies are needed to mean that someone is protected? And if you are protected, how long are you protected? The answers to these basic questions are still unknown, he said. This week, the World Health Organization put out guidance recommending against using antibody tests for clinical decision making.
The FDA, meanwhile, is lowering the bar even further. On March 16, it issued new guidance allowing manufacturers to distribute tests even before receiving emergency use authorization, for a “reasonable period of time” – about 15 days – after a diagnostic maker had validated the test internally and while preparing its request to the agency for an EUA.
Local governments are desperate enough for tests that they’ll buy them without assurances of accuracy at all. Chicago recently ordered 11,000 antibody tests made in South Korea that had not been reviewed by the FDA but are legal to distribute as long as they include several disclaimers including a recommendation that any negative result be confirmed with a diagnostic test.
“There’s no time really to put the effort into saying, ’Where’s the problem here?’ ” said Catherine Troisi, an epidemiologist at the University of Texas Health Science Center. “I’m not saying the test is bad. But what good is a test if you don’t know it’s giving you reliable results? We just don’t know.”
Correction, April 10, 2020: This story originally said incorrectly that Kendra Boroff was admitted to an intensive care unit.
This article was first published on ProPublica.com.
Can diabetes be cured?
In his Guest Editorial “How to help patients become successful diabetes self-managers” (J Fam Pract. 2020;69:8-9), Dr. Unger makes several very good points. I especially liked his recommendation to ask patients why they are concerned about having diabetes; this question alone can kick-start the behavior modification process leading to improved diabetes control.
However, I disagree with Dr. Unger’s assertion that “diabetes cannot be cured.” Based on multiple case studies, clinical trials, results from lifestyle intervention programs, and my own experience as a family physician, it is clear that diabetes can be reversed with lifestyle changes designed to counteract the modifiable factors (eg, diet, lack of exercise) that usually cause this condition.
Rather than merely considering the diabetes measure of success to be blood glucose controlled by prescribed medication, it is important to offer a more collaborative approach to patients willing to make lifestyle changes. We can show many—if not most— that they can achieve the goal of blood glucose control without medication.
Allan Olson MD
Diplomate, American Board of Family Medicine and American Board of Lifestyle Medicine
Kewaunee, WI
In his Guest Editorial “How to help patients become successful diabetes self-managers” (J Fam Pract. 2020;69:8-9), Dr. Unger makes several very good points. I especially liked his recommendation to ask patients why they are concerned about having diabetes; this question alone can kick-start the behavior modification process leading to improved diabetes control.
However, I disagree with Dr. Unger’s assertion that “diabetes cannot be cured.” Based on multiple case studies, clinical trials, results from lifestyle intervention programs, and my own experience as a family physician, it is clear that diabetes can be reversed with lifestyle changes designed to counteract the modifiable factors (eg, diet, lack of exercise) that usually cause this condition.
Rather than merely considering the diabetes measure of success to be blood glucose controlled by prescribed medication, it is important to offer a more collaborative approach to patients willing to make lifestyle changes. We can show many—if not most— that they can achieve the goal of blood glucose control without medication.
Allan Olson MD
Diplomate, American Board of Family Medicine and American Board of Lifestyle Medicine
Kewaunee, WI
In his Guest Editorial “How to help patients become successful diabetes self-managers” (J Fam Pract. 2020;69:8-9), Dr. Unger makes several very good points. I especially liked his recommendation to ask patients why they are concerned about having diabetes; this question alone can kick-start the behavior modification process leading to improved diabetes control.
However, I disagree with Dr. Unger’s assertion that “diabetes cannot be cured.” Based on multiple case studies, clinical trials, results from lifestyle intervention programs, and my own experience as a family physician, it is clear that diabetes can be reversed with lifestyle changes designed to counteract the modifiable factors (eg, diet, lack of exercise) that usually cause this condition.
Rather than merely considering the diabetes measure of success to be blood glucose controlled by prescribed medication, it is important to offer a more collaborative approach to patients willing to make lifestyle changes. We can show many—if not most— that they can achieve the goal of blood glucose control without medication.
Allan Olson MD
Diplomate, American Board of Family Medicine and American Board of Lifestyle Medicine
Kewaunee, WI
There’s one less weight-loss drug on the market
I would like to provide an update to my article, “10 proven strategies to help patients maintain weight loss” (J Fam Pract. 2020;69:20-25), which mentioned lorcaserin as one of several medications approved by the US Food and Drug Administration (FDA) for long-term use in weight maintenance. On February 13, 2020, the FDA requested that the manufacturer of Belviq and Belviq XR (lorcaserin), Eisai Inc., voluntarily withdraw the weight-loss drug from the US market because a safety clinical trial demonstrated an increased occurrence of cancer. Eisai Inc. has submitted a request to voluntarily withdraw the drug. The FDA has advised patients to stop taking lorcaserin and talk to their health care provider about alternative weight-loss medicines and weight management programs.
Marijane Hynes, MD
Weight Management Program
The George Washington University
Medical Faculty Associates
Washington, DC
I would like to provide an update to my article, “10 proven strategies to help patients maintain weight loss” (J Fam Pract. 2020;69:20-25), which mentioned lorcaserin as one of several medications approved by the US Food and Drug Administration (FDA) for long-term use in weight maintenance. On February 13, 2020, the FDA requested that the manufacturer of Belviq and Belviq XR (lorcaserin), Eisai Inc., voluntarily withdraw the weight-loss drug from the US market because a safety clinical trial demonstrated an increased occurrence of cancer. Eisai Inc. has submitted a request to voluntarily withdraw the drug. The FDA has advised patients to stop taking lorcaserin and talk to their health care provider about alternative weight-loss medicines and weight management programs.
Marijane Hynes, MD
Weight Management Program
The George Washington University
Medical Faculty Associates
Washington, DC
I would like to provide an update to my article, “10 proven strategies to help patients maintain weight loss” (J Fam Pract. 2020;69:20-25), which mentioned lorcaserin as one of several medications approved by the US Food and Drug Administration (FDA) for long-term use in weight maintenance. On February 13, 2020, the FDA requested that the manufacturer of Belviq and Belviq XR (lorcaserin), Eisai Inc., voluntarily withdraw the weight-loss drug from the US market because a safety clinical trial demonstrated an increased occurrence of cancer. Eisai Inc. has submitted a request to voluntarily withdraw the drug. The FDA has advised patients to stop taking lorcaserin and talk to their health care provider about alternative weight-loss medicines and weight management programs.
Marijane Hynes, MD
Weight Management Program
The George Washington University
Medical Faculty Associates
Washington, DC
Cancer prevalence among COVID-19 patients may be higher than previously reported
An early report pegged the prevalence of cancer among COVID-19 patients at 1%, but authors of a recent meta-analysis found an overall prevalence of 2% and up to 3% depending on the subset of data they reviewed.
However, those findings are limited by the retrospective nature of the studies published to date, according to the authors of the meta-analysis, led by Aakash Desai, MBBS, of the University of Connecticut, Farmington.
Nevertheless, the results do confirm that cancer patients and survivors are an important at-risk population for COVID-19, according to Dr. Desai and colleagues.
“We hope that additional data from China and Italy will provide information on the characteristics of patients with cancer at risk, types of cancer that confer higher risk, and systemic regimens that may increase COVID-19 infection complications,” the authors wrote in JCO Global Oncology.
More than 15 million individuals with cancer and many more cancer survivors are at increased risk of COVID-19 because of compromised immune systems, according to the authors.
Exactly how many individuals with cancer are among the COVID-19 cases remains unclear, though a previous report suggested the prevalence of cancer was 1% (95% confidence interval, 0.61%-1.65%) among COVID-19 patients in China (Lancet Oncol. 2020 Mar;21[3]:335-7). This “seems to be higher” than the 0.29% prevalence of cancer in the overall Chinese population, the investigators noted at the time.
That study revealed 18 cancer patients among 1,590 COVID-19 cases, though it was “hypothesis generating,” according to Dr. Desai and colleagues, who rolled that data into their meta-analysis of 11 reports including 3,661 COVID-19 cases.
Overall, Dr. Desai and colleagues found the pooled prevalence of cancer was 2.0% (95% CI, 2.0%-3.0%) in that population. In a subgroup analysis of five studies with sample sizes of less than 100 COVID-19 patients, the researchers found a “slightly higher” prevalence of 3.0% (95% CI, 1.0%-6.0%).
However, even that data wasn’t robust enough for Dr. Desai and colleagues to make any pronouncements on cancer prevalence. “Overall, current evidence on the association between cancer and COVID-19 remains inconclusive,” they wrote.
Though inconclusive, the findings raise questions about whether treatments or interventions might need to be postponed in certain patients, whether cancer patients and survivors need stronger personal protection, and how to deal with potential delays in cancer clinical trials, according to Dr. Desai and colleagues.
“As the evidence continues to rise, we must strive to answer the unanswered clinical questions,” the authors wrote.
Dr. Desai and colleagues reported no potential conflicts of interest related to the study.
SOURCE: Desai A et al. JCO Glob Oncol. 2020 Apr 6. doi: 10.1200/GO.20.00097.
An early report pegged the prevalence of cancer among COVID-19 patients at 1%, but authors of a recent meta-analysis found an overall prevalence of 2% and up to 3% depending on the subset of data they reviewed.
However, those findings are limited by the retrospective nature of the studies published to date, according to the authors of the meta-analysis, led by Aakash Desai, MBBS, of the University of Connecticut, Farmington.
Nevertheless, the results do confirm that cancer patients and survivors are an important at-risk population for COVID-19, according to Dr. Desai and colleagues.
“We hope that additional data from China and Italy will provide information on the characteristics of patients with cancer at risk, types of cancer that confer higher risk, and systemic regimens that may increase COVID-19 infection complications,” the authors wrote in JCO Global Oncology.
More than 15 million individuals with cancer and many more cancer survivors are at increased risk of COVID-19 because of compromised immune systems, according to the authors.
Exactly how many individuals with cancer are among the COVID-19 cases remains unclear, though a previous report suggested the prevalence of cancer was 1% (95% confidence interval, 0.61%-1.65%) among COVID-19 patients in China (Lancet Oncol. 2020 Mar;21[3]:335-7). This “seems to be higher” than the 0.29% prevalence of cancer in the overall Chinese population, the investigators noted at the time.
That study revealed 18 cancer patients among 1,590 COVID-19 cases, though it was “hypothesis generating,” according to Dr. Desai and colleagues, who rolled that data into their meta-analysis of 11 reports including 3,661 COVID-19 cases.
Overall, Dr. Desai and colleagues found the pooled prevalence of cancer was 2.0% (95% CI, 2.0%-3.0%) in that population. In a subgroup analysis of five studies with sample sizes of less than 100 COVID-19 patients, the researchers found a “slightly higher” prevalence of 3.0% (95% CI, 1.0%-6.0%).
However, even that data wasn’t robust enough for Dr. Desai and colleagues to make any pronouncements on cancer prevalence. “Overall, current evidence on the association between cancer and COVID-19 remains inconclusive,” they wrote.
Though inconclusive, the findings raise questions about whether treatments or interventions might need to be postponed in certain patients, whether cancer patients and survivors need stronger personal protection, and how to deal with potential delays in cancer clinical trials, according to Dr. Desai and colleagues.
“As the evidence continues to rise, we must strive to answer the unanswered clinical questions,” the authors wrote.
Dr. Desai and colleagues reported no potential conflicts of interest related to the study.
SOURCE: Desai A et al. JCO Glob Oncol. 2020 Apr 6. doi: 10.1200/GO.20.00097.
An early report pegged the prevalence of cancer among COVID-19 patients at 1%, but authors of a recent meta-analysis found an overall prevalence of 2% and up to 3% depending on the subset of data they reviewed.
However, those findings are limited by the retrospective nature of the studies published to date, according to the authors of the meta-analysis, led by Aakash Desai, MBBS, of the University of Connecticut, Farmington.
Nevertheless, the results do confirm that cancer patients and survivors are an important at-risk population for COVID-19, according to Dr. Desai and colleagues.
“We hope that additional data from China and Italy will provide information on the characteristics of patients with cancer at risk, types of cancer that confer higher risk, and systemic regimens that may increase COVID-19 infection complications,” the authors wrote in JCO Global Oncology.
More than 15 million individuals with cancer and many more cancer survivors are at increased risk of COVID-19 because of compromised immune systems, according to the authors.
Exactly how many individuals with cancer are among the COVID-19 cases remains unclear, though a previous report suggested the prevalence of cancer was 1% (95% confidence interval, 0.61%-1.65%) among COVID-19 patients in China (Lancet Oncol. 2020 Mar;21[3]:335-7). This “seems to be higher” than the 0.29% prevalence of cancer in the overall Chinese population, the investigators noted at the time.
That study revealed 18 cancer patients among 1,590 COVID-19 cases, though it was “hypothesis generating,” according to Dr. Desai and colleagues, who rolled that data into their meta-analysis of 11 reports including 3,661 COVID-19 cases.
Overall, Dr. Desai and colleagues found the pooled prevalence of cancer was 2.0% (95% CI, 2.0%-3.0%) in that population. In a subgroup analysis of five studies with sample sizes of less than 100 COVID-19 patients, the researchers found a “slightly higher” prevalence of 3.0% (95% CI, 1.0%-6.0%).
However, even that data wasn’t robust enough for Dr. Desai and colleagues to make any pronouncements on cancer prevalence. “Overall, current evidence on the association between cancer and COVID-19 remains inconclusive,” they wrote.
Though inconclusive, the findings raise questions about whether treatments or interventions might need to be postponed in certain patients, whether cancer patients and survivors need stronger personal protection, and how to deal with potential delays in cancer clinical trials, according to Dr. Desai and colleagues.
“As the evidence continues to rise, we must strive to answer the unanswered clinical questions,” the authors wrote.
Dr. Desai and colleagues reported no potential conflicts of interest related to the study.
SOURCE: Desai A et al. JCO Glob Oncol. 2020 Apr 6. doi: 10.1200/GO.20.00097.
FROM JCO GLOBAL ONCOLOGY
Do prophylactic antipyretics reduce vaccination-associated symptoms in children?
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
EVIDENCE-BASED ANSWER:
Yes for acetaminophen, not so much for ibuprofen. Prophylactic acetaminophen reduces the odds of febrile reactions in the first 48 hours after vaccination by 40% to 65% and pain of all grades by 36% to 43%. In contrast, prophylactic ibuprofen reduces pain of all grades by 34% only after primary vaccination and doesn’t alter pain after boosters. Nor does it alter early febrile reactions (strength of recommendation [SOR]: B, meta-analysis of randomized clinical trials [RCTs] with moderate-to-high risk of bias).
Prophylactic administration of acetaminophen or ibuprofen is associated with a reduction in antibody response to the primary vaccine series and to influenza vaccine, but antibody responses still achieve seroprotective levels (SOR: C, bench research).
Hypertension goes unmedicated in 40% of adults
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.
There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.

There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
Roughly 30% of adults in the United States had hypertension in 2017, and just under 60% of those adults reported using antihypertensive medication, according to the Centers for Disease Control and Prevention.

There is, however, quite a bit of variation from those age-standardized national figures when state-level data are considered.
In Alabama and West Virginia, the prevalence of hypertension in 2017 was 38.6%, the highest in the country, with Arkansas (38.5%) and Mississippi (38.2%) not far behind. Meanwhile, Minnesota came in with a lowest-in-the-nation rate of 24.3%, which was nearly matched by Colorado at 24.8%, Claudine M. Samanic, PhD, and associates wrote in the MMWR.
There was also a considerable gap between the states in hypertensive adults’ self-reported use of antihypertensive drugs, which was generally higher in the states with a greater prevalence of disease, they noted.
Adults in Mississippi were the most likely (71.2%) to be taking medication, along with those in Alabama (70.5%) and Arkansas (69.3%). Idaho occupied the other end of the scale with a rate of 50.2%, while Montana and Vermont were slightly better at 51.7%, based on survey data from the Behavioral Risk Factor Surveillance System.
“Prevalence of antihypertensive medication use was higher in older age groups, highest among blacks, and higher among women [64.0%] than men [56.7%]. This overall gender difference has been reported previously, but the reasons are unclear,” wrote Dr. Samanic and associates at the CDC’s National Center for Chronic Disease Prevention and Health Promotion.
The BRFSS data for 2017 are based on based on interviews with 450,016 adults. Respondents were asked, “Have you ever been told by a doctor, nurse, or other health professional that you have high blood pressure?” and were considered to have hypertension if they answered yes.
SOURCE: Samanic CM et al. MMWR. 2020 Apr 10;69(14):393-8.
FROM THE MMWR
AHA updates management when CAD and T2DM coincide
Patients with stable coronary artery disease and type 2 diabetes mellitus could benefit from a “plethora of newly available risk-reduction strategies,” but their “adoption into clinical practice has been slow” and inconsistent, prompting an expert panel organized by the American Heart Association to collate the range of treatment recommendations now applicable to this patient population in a scientific statement released on April 13.
“There are a number of things to consider when treating patients with stable coronary artery disease [CAD] and type 2 diabetes mellitus [T2DM], with new medications and trials and data emerging. It’s difficult to keep up with all of the complexities,” which was why the Association’s Councils on Lifestyle and Cardiometabolic Health and on Clinical Cardiology put together a writing group to summarize and prioritize the range of lifestyle, medical, and interventional options that now require consideration and potential use on patients managed in routine practice, explained Suzanne V. Arnold, MD, chair of the writing group, in an interview.
The new scientific statement (Circulation. 2020 Apr 13; doi: 10.1161/CIR.0000000000000766), aimed primarily at cardiologists but also intended to inform primary care physicians, endocrinologists, and all other clinicians who deal with these patients, pulls together “everything someone needs to think about if they care for patients with CAD and T2DM,” said Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and vice chair of the statement-writing panel in an interview. “There is a lot to know,” he added.
The statement covers antithrombotic therapies; blood pressure control, with a discussion of both the appropriate pressure goal and the best drug types used to reach it; lipid management; glycemic control; lifestyle modification; weight management, including the role of bariatric surgery; and approaches to managing stable angina, both medically and with revascularization.
“The goal was to give clinicians a good sense of what new treatments they should consider” for these patients, said Dr. Bhatt, who is also director of interventional cardiovascular programs at Brigham and Women’s Hospital, also in Boston. Because of the tight associations between T2DM and cardiovascular disease in general including CAD, “cardiologists are increasingly involved in managing patients with T2DM,” he noted. The statement gives a comprehensive overview and critical assessment of the management of these patients as of the end of 2019 as a consensus from a panel of 11 experts .
The statement also stressed that “substantial portions of patients with T2DM and CAD, including those after an acute coronary syndrome, do not receive therapies with proven cardiovascular benefit, such as high-intensity statins, dual-antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and glucose-lowering agents with proven cardiovascular benefits.
“These gaps in care highlight a critical opportunity for cardiovascular specialists to assume a more active role in the collaborative care of patients with T2DM and CAD,” the statement said. This includes “encouraging cardiologists to become more active in the selection of glucose-lowering medications” for these patients because it could “really move the needle,” said Dr. Arnold, a cardiologist with Saint Luke’s Health System in Kansas City, Mo. She was referring specifically to broader reliance on both the SGTL2 (sodium-glucose cotransporter 2) inhibitors and the GLP-1 (glucagonlike peptide-1) receptor agonists as top choices for controlling hyperglycemia. Based on recent evidence drugs in these two classes “could be considered first line for patients with T2DM and CAD, and would likely be preferred over metformin,” Dr. Arnold said in an interview. Although the statement identified the SGLT2 inhibitors as “the first drug class [for glycemic control] to show clear benefits on cardiovascular outcomes,” it does not explicitly label the class first-line and it also skirts that designation for the GLP-1 receptor agonist class, while noting that metformin “remains the drug most frequently recommended as first-line therapy in treatment guidelines.”
“I wouldn’t disagree with someone who said that SGLT2 inhibitors and GLP-1 receptor agonists are first line,” but prescribing patterns also depend on familiarity, cost, and access, noted Dr. Bhatt, which can all be issues with agents from these classes compared with metformin, a widely available generic with decades of use. “Metformin is safe and cheap, so we did not want to discount it,” said Dr. Arnold. Dr. Bhatt recently coauthored an editorial that gave an enthusiastic endorsement to using SGLT2 inhibitors in patients with diabetes (Cell Metab. 2019 Nov 5;30[5]:47-9).
Another notable feature of the statement is the potential it assigns to bariatric surgery as a management tool with documented safety and efficacy for improving cardiovascular risk factors. However, the statement also notes that randomized trials “have thus far been inadequately powered to assess cardiovascular events and mortality, although observational studies have consistently shown cardiovascular risk reduction with such procedures.” The statement continues that despite potential cardiovascular benefits “bariatric surgery remains underused among eligible patients,” and said that surgery performed as Roux-en-Y bypass or sleeve gastrectomy “may be another effective tool for cardiovascular risk reduction in the subset of patients with obesity,” particularly patients with a body mass index of at least 35 kg/m2.
“While the percentage of patients who are optimal for bariatric surgery is not known, the most recent NHANES [National Health and Nutrition Examination Study] study showed that less than 0.5% of eligible patients underwent bariatric surgery,” Dr. Arnold noted. Bariatric surgery is “certainly not a recommendation for everyone, or even a majority of patients, but bariatric surgery should be on our radar,” for patients with CAD and T2DM, she said.
Right now, “few cardiologists think about bariatric surgery,” as a treatment option, but study results have shown that “in carefully selected patients treated by skilled surgeons at high-volume centers, patients will do better with bariatric surgery than with best medical therapy for improvements in multiple risk factors, including glycemic control,” Dr. Bhatt said in the interview. “It’s not first-line treatment, but it’s an option to consider,” he added, while also noting that bariatric surgery is most beneficial to patients relatively early in the course of T2DM, when its been in place for just a few years rather than a couple of decades.
The statement also notably included a “first-line” call out for icosapent ethyl (Vascepa), a novel agent approved in December 2019 for routine use in U.S. patients, including those with CAD and T2DM as long as their blood triglyceride level was at least 150 mg/dL. Dr. Bhatt, who led the REDUCE-IT study that was pivotal for proving the safety and efficacy of icosapent ethyl (N Engl J Med. 2019 Jan 3;380[1]:11-22), estimated that anywhere from 15% to as many as half the patients with CAD and T2DM might have a triglyceride level that would allow them to receive icosapent ethyl. One population-based study in Canada of nearly 200,000 people with atherosclerotic cardiovascular disease found a 25% prevalence of the triglyceride level needed to qualify to receive icosapent ethyl under current labeling, he noted (Eur Heart J. 2020 Jan 1;41[1]:86-94). However, the FDA label does not specify that triglycerides be measured when fasting, and a nonfasting level of about 150 mg/dL will likely appear for patients with fasting levels that fall as low as about 100 mg/dL, Dr. Bhatt said. He hoped that future studies will assess the efficacy of icosapent ethyl in patients with even lower triglyceride levels.
Other sections of the statement also recommend that clinicians: Target long-term dual-antiplatelet therapy to CAD and T2DM patients with additional high-risk markers such as prior MI, younger age, and tobacco use; prescribe a low-dose oral anticoagulant along with an antiplatelet drug such as aspirin for secondary-prevention patients; promote a blood pressure target of less than 140/90 mm Hg for all CAD and T2DM patients and apply a goal of less than 130/80 mm Hg in higher-risk patients such as blacks, Asians, and those with cerebrovascular disease; and reassure patients that “despite a modest increase in blood sugars, the risk-benefit ratio is clearly in favor of administering statins to people with T2DM and CAD.”
Dr. Arnold had no disclosures. Dr. Bhatt has been an adviser to Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio; PLx Pharma, and Regado Biosciences, and he has received research funding from numerous companies including Amarin, the company that markets icosapent ethyl.
Patients with stable coronary artery disease and type 2 diabetes mellitus could benefit from a “plethora of newly available risk-reduction strategies,” but their “adoption into clinical practice has been slow” and inconsistent, prompting an expert panel organized by the American Heart Association to collate the range of treatment recommendations now applicable to this patient population in a scientific statement released on April 13.
“There are a number of things to consider when treating patients with stable coronary artery disease [CAD] and type 2 diabetes mellitus [T2DM], with new medications and trials and data emerging. It’s difficult to keep up with all of the complexities,” which was why the Association’s Councils on Lifestyle and Cardiometabolic Health and on Clinical Cardiology put together a writing group to summarize and prioritize the range of lifestyle, medical, and interventional options that now require consideration and potential use on patients managed in routine practice, explained Suzanne V. Arnold, MD, chair of the writing group, in an interview.
The new scientific statement (Circulation. 2020 Apr 13; doi: 10.1161/CIR.0000000000000766), aimed primarily at cardiologists but also intended to inform primary care physicians, endocrinologists, and all other clinicians who deal with these patients, pulls together “everything someone needs to think about if they care for patients with CAD and T2DM,” said Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and vice chair of the statement-writing panel in an interview. “There is a lot to know,” he added.
The statement covers antithrombotic therapies; blood pressure control, with a discussion of both the appropriate pressure goal and the best drug types used to reach it; lipid management; glycemic control; lifestyle modification; weight management, including the role of bariatric surgery; and approaches to managing stable angina, both medically and with revascularization.
“The goal was to give clinicians a good sense of what new treatments they should consider” for these patients, said Dr. Bhatt, who is also director of interventional cardiovascular programs at Brigham and Women’s Hospital, also in Boston. Because of the tight associations between T2DM and cardiovascular disease in general including CAD, “cardiologists are increasingly involved in managing patients with T2DM,” he noted. The statement gives a comprehensive overview and critical assessment of the management of these patients as of the end of 2019 as a consensus from a panel of 11 experts .
The statement also stressed that “substantial portions of patients with T2DM and CAD, including those after an acute coronary syndrome, do not receive therapies with proven cardiovascular benefit, such as high-intensity statins, dual-antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and glucose-lowering agents with proven cardiovascular benefits.
“These gaps in care highlight a critical opportunity for cardiovascular specialists to assume a more active role in the collaborative care of patients with T2DM and CAD,” the statement said. This includes “encouraging cardiologists to become more active in the selection of glucose-lowering medications” for these patients because it could “really move the needle,” said Dr. Arnold, a cardiologist with Saint Luke’s Health System in Kansas City, Mo. She was referring specifically to broader reliance on both the SGTL2 (sodium-glucose cotransporter 2) inhibitors and the GLP-1 (glucagonlike peptide-1) receptor agonists as top choices for controlling hyperglycemia. Based on recent evidence drugs in these two classes “could be considered first line for patients with T2DM and CAD, and would likely be preferred over metformin,” Dr. Arnold said in an interview. Although the statement identified the SGLT2 inhibitors as “the first drug class [for glycemic control] to show clear benefits on cardiovascular outcomes,” it does not explicitly label the class first-line and it also skirts that designation for the GLP-1 receptor agonist class, while noting that metformin “remains the drug most frequently recommended as first-line therapy in treatment guidelines.”
“I wouldn’t disagree with someone who said that SGLT2 inhibitors and GLP-1 receptor agonists are first line,” but prescribing patterns also depend on familiarity, cost, and access, noted Dr. Bhatt, which can all be issues with agents from these classes compared with metformin, a widely available generic with decades of use. “Metformin is safe and cheap, so we did not want to discount it,” said Dr. Arnold. Dr. Bhatt recently coauthored an editorial that gave an enthusiastic endorsement to using SGLT2 inhibitors in patients with diabetes (Cell Metab. 2019 Nov 5;30[5]:47-9).
Another notable feature of the statement is the potential it assigns to bariatric surgery as a management tool with documented safety and efficacy for improving cardiovascular risk factors. However, the statement also notes that randomized trials “have thus far been inadequately powered to assess cardiovascular events and mortality, although observational studies have consistently shown cardiovascular risk reduction with such procedures.” The statement continues that despite potential cardiovascular benefits “bariatric surgery remains underused among eligible patients,” and said that surgery performed as Roux-en-Y bypass or sleeve gastrectomy “may be another effective tool for cardiovascular risk reduction in the subset of patients with obesity,” particularly patients with a body mass index of at least 35 kg/m2.
“While the percentage of patients who are optimal for bariatric surgery is not known, the most recent NHANES [National Health and Nutrition Examination Study] study showed that less than 0.5% of eligible patients underwent bariatric surgery,” Dr. Arnold noted. Bariatric surgery is “certainly not a recommendation for everyone, or even a majority of patients, but bariatric surgery should be on our radar,” for patients with CAD and T2DM, she said.
Right now, “few cardiologists think about bariatric surgery,” as a treatment option, but study results have shown that “in carefully selected patients treated by skilled surgeons at high-volume centers, patients will do better with bariatric surgery than with best medical therapy for improvements in multiple risk factors, including glycemic control,” Dr. Bhatt said in the interview. “It’s not first-line treatment, but it’s an option to consider,” he added, while also noting that bariatric surgery is most beneficial to patients relatively early in the course of T2DM, when its been in place for just a few years rather than a couple of decades.
The statement also notably included a “first-line” call out for icosapent ethyl (Vascepa), a novel agent approved in December 2019 for routine use in U.S. patients, including those with CAD and T2DM as long as their blood triglyceride level was at least 150 mg/dL. Dr. Bhatt, who led the REDUCE-IT study that was pivotal for proving the safety and efficacy of icosapent ethyl (N Engl J Med. 2019 Jan 3;380[1]:11-22), estimated that anywhere from 15% to as many as half the patients with CAD and T2DM might have a triglyceride level that would allow them to receive icosapent ethyl. One population-based study in Canada of nearly 200,000 people with atherosclerotic cardiovascular disease found a 25% prevalence of the triglyceride level needed to qualify to receive icosapent ethyl under current labeling, he noted (Eur Heart J. 2020 Jan 1;41[1]:86-94). However, the FDA label does not specify that triglycerides be measured when fasting, and a nonfasting level of about 150 mg/dL will likely appear for patients with fasting levels that fall as low as about 100 mg/dL, Dr. Bhatt said. He hoped that future studies will assess the efficacy of icosapent ethyl in patients with even lower triglyceride levels.
Other sections of the statement also recommend that clinicians: Target long-term dual-antiplatelet therapy to CAD and T2DM patients with additional high-risk markers such as prior MI, younger age, and tobacco use; prescribe a low-dose oral anticoagulant along with an antiplatelet drug such as aspirin for secondary-prevention patients; promote a blood pressure target of less than 140/90 mm Hg for all CAD and T2DM patients and apply a goal of less than 130/80 mm Hg in higher-risk patients such as blacks, Asians, and those with cerebrovascular disease; and reassure patients that “despite a modest increase in blood sugars, the risk-benefit ratio is clearly in favor of administering statins to people with T2DM and CAD.”
Dr. Arnold had no disclosures. Dr. Bhatt has been an adviser to Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio; PLx Pharma, and Regado Biosciences, and he has received research funding from numerous companies including Amarin, the company that markets icosapent ethyl.
Patients with stable coronary artery disease and type 2 diabetes mellitus could benefit from a “plethora of newly available risk-reduction strategies,” but their “adoption into clinical practice has been slow” and inconsistent, prompting an expert panel organized by the American Heart Association to collate the range of treatment recommendations now applicable to this patient population in a scientific statement released on April 13.
“There are a number of things to consider when treating patients with stable coronary artery disease [CAD] and type 2 diabetes mellitus [T2DM], with new medications and trials and data emerging. It’s difficult to keep up with all of the complexities,” which was why the Association’s Councils on Lifestyle and Cardiometabolic Health and on Clinical Cardiology put together a writing group to summarize and prioritize the range of lifestyle, medical, and interventional options that now require consideration and potential use on patients managed in routine practice, explained Suzanne V. Arnold, MD, chair of the writing group, in an interview.
The new scientific statement (Circulation. 2020 Apr 13; doi: 10.1161/CIR.0000000000000766), aimed primarily at cardiologists but also intended to inform primary care physicians, endocrinologists, and all other clinicians who deal with these patients, pulls together “everything someone needs to think about if they care for patients with CAD and T2DM,” said Deepak L. Bhatt, MD, professor of medicine at Harvard Medical School in Boston and vice chair of the statement-writing panel in an interview. “There is a lot to know,” he added.
The statement covers antithrombotic therapies; blood pressure control, with a discussion of both the appropriate pressure goal and the best drug types used to reach it; lipid management; glycemic control; lifestyle modification; weight management, including the role of bariatric surgery; and approaches to managing stable angina, both medically and with revascularization.
“The goal was to give clinicians a good sense of what new treatments they should consider” for these patients, said Dr. Bhatt, who is also director of interventional cardiovascular programs at Brigham and Women’s Hospital, also in Boston. Because of the tight associations between T2DM and cardiovascular disease in general including CAD, “cardiologists are increasingly involved in managing patients with T2DM,” he noted. The statement gives a comprehensive overview and critical assessment of the management of these patients as of the end of 2019 as a consensus from a panel of 11 experts .
The statement also stressed that “substantial portions of patients with T2DM and CAD, including those after an acute coronary syndrome, do not receive therapies with proven cardiovascular benefit, such as high-intensity statins, dual-antiplatelet therapy, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, and glucose-lowering agents with proven cardiovascular benefits.
“These gaps in care highlight a critical opportunity for cardiovascular specialists to assume a more active role in the collaborative care of patients with T2DM and CAD,” the statement said. This includes “encouraging cardiologists to become more active in the selection of glucose-lowering medications” for these patients because it could “really move the needle,” said Dr. Arnold, a cardiologist with Saint Luke’s Health System in Kansas City, Mo. She was referring specifically to broader reliance on both the SGTL2 (sodium-glucose cotransporter 2) inhibitors and the GLP-1 (glucagonlike peptide-1) receptor agonists as top choices for controlling hyperglycemia. Based on recent evidence drugs in these two classes “could be considered first line for patients with T2DM and CAD, and would likely be preferred over metformin,” Dr. Arnold said in an interview. Although the statement identified the SGLT2 inhibitors as “the first drug class [for glycemic control] to show clear benefits on cardiovascular outcomes,” it does not explicitly label the class first-line and it also skirts that designation for the GLP-1 receptor agonist class, while noting that metformin “remains the drug most frequently recommended as first-line therapy in treatment guidelines.”
“I wouldn’t disagree with someone who said that SGLT2 inhibitors and GLP-1 receptor agonists are first line,” but prescribing patterns also depend on familiarity, cost, and access, noted Dr. Bhatt, which can all be issues with agents from these classes compared with metformin, a widely available generic with decades of use. “Metformin is safe and cheap, so we did not want to discount it,” said Dr. Arnold. Dr. Bhatt recently coauthored an editorial that gave an enthusiastic endorsement to using SGLT2 inhibitors in patients with diabetes (Cell Metab. 2019 Nov 5;30[5]:47-9).
Another notable feature of the statement is the potential it assigns to bariatric surgery as a management tool with documented safety and efficacy for improving cardiovascular risk factors. However, the statement also notes that randomized trials “have thus far been inadequately powered to assess cardiovascular events and mortality, although observational studies have consistently shown cardiovascular risk reduction with such procedures.” The statement continues that despite potential cardiovascular benefits “bariatric surgery remains underused among eligible patients,” and said that surgery performed as Roux-en-Y bypass or sleeve gastrectomy “may be another effective tool for cardiovascular risk reduction in the subset of patients with obesity,” particularly patients with a body mass index of at least 35 kg/m2.
“While the percentage of patients who are optimal for bariatric surgery is not known, the most recent NHANES [National Health and Nutrition Examination Study] study showed that less than 0.5% of eligible patients underwent bariatric surgery,” Dr. Arnold noted. Bariatric surgery is “certainly not a recommendation for everyone, or even a majority of patients, but bariatric surgery should be on our radar,” for patients with CAD and T2DM, she said.
Right now, “few cardiologists think about bariatric surgery,” as a treatment option, but study results have shown that “in carefully selected patients treated by skilled surgeons at high-volume centers, patients will do better with bariatric surgery than with best medical therapy for improvements in multiple risk factors, including glycemic control,” Dr. Bhatt said in the interview. “It’s not first-line treatment, but it’s an option to consider,” he added, while also noting that bariatric surgery is most beneficial to patients relatively early in the course of T2DM, when its been in place for just a few years rather than a couple of decades.
The statement also notably included a “first-line” call out for icosapent ethyl (Vascepa), a novel agent approved in December 2019 for routine use in U.S. patients, including those with CAD and T2DM as long as their blood triglyceride level was at least 150 mg/dL. Dr. Bhatt, who led the REDUCE-IT study that was pivotal for proving the safety and efficacy of icosapent ethyl (N Engl J Med. 2019 Jan 3;380[1]:11-22), estimated that anywhere from 15% to as many as half the patients with CAD and T2DM might have a triglyceride level that would allow them to receive icosapent ethyl. One population-based study in Canada of nearly 200,000 people with atherosclerotic cardiovascular disease found a 25% prevalence of the triglyceride level needed to qualify to receive icosapent ethyl under current labeling, he noted (Eur Heart J. 2020 Jan 1;41[1]:86-94). However, the FDA label does not specify that triglycerides be measured when fasting, and a nonfasting level of about 150 mg/dL will likely appear for patients with fasting levels that fall as low as about 100 mg/dL, Dr. Bhatt said. He hoped that future studies will assess the efficacy of icosapent ethyl in patients with even lower triglyceride levels.
Other sections of the statement also recommend that clinicians: Target long-term dual-antiplatelet therapy to CAD and T2DM patients with additional high-risk markers such as prior MI, younger age, and tobacco use; prescribe a low-dose oral anticoagulant along with an antiplatelet drug such as aspirin for secondary-prevention patients; promote a blood pressure target of less than 140/90 mm Hg for all CAD and T2DM patients and apply a goal of less than 130/80 mm Hg in higher-risk patients such as blacks, Asians, and those with cerebrovascular disease; and reassure patients that “despite a modest increase in blood sugars, the risk-benefit ratio is clearly in favor of administering statins to people with T2DM and CAD.”
Dr. Arnold had no disclosures. Dr. Bhatt has been an adviser to Cardax, Cereno Scientific, Medscape Cardiology, PhaseBio; PLx Pharma, and Regado Biosciences, and he has received research funding from numerous companies including Amarin, the company that markets icosapent ethyl.
FROM CIRCULATION











