User login
Pruritic Eruption With Skinfold Sparing
The Diagnosis: Papuloerythroderma of Ofuji
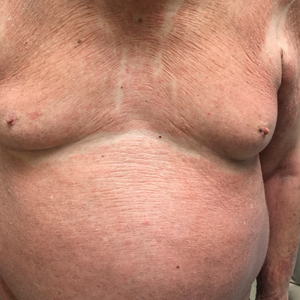
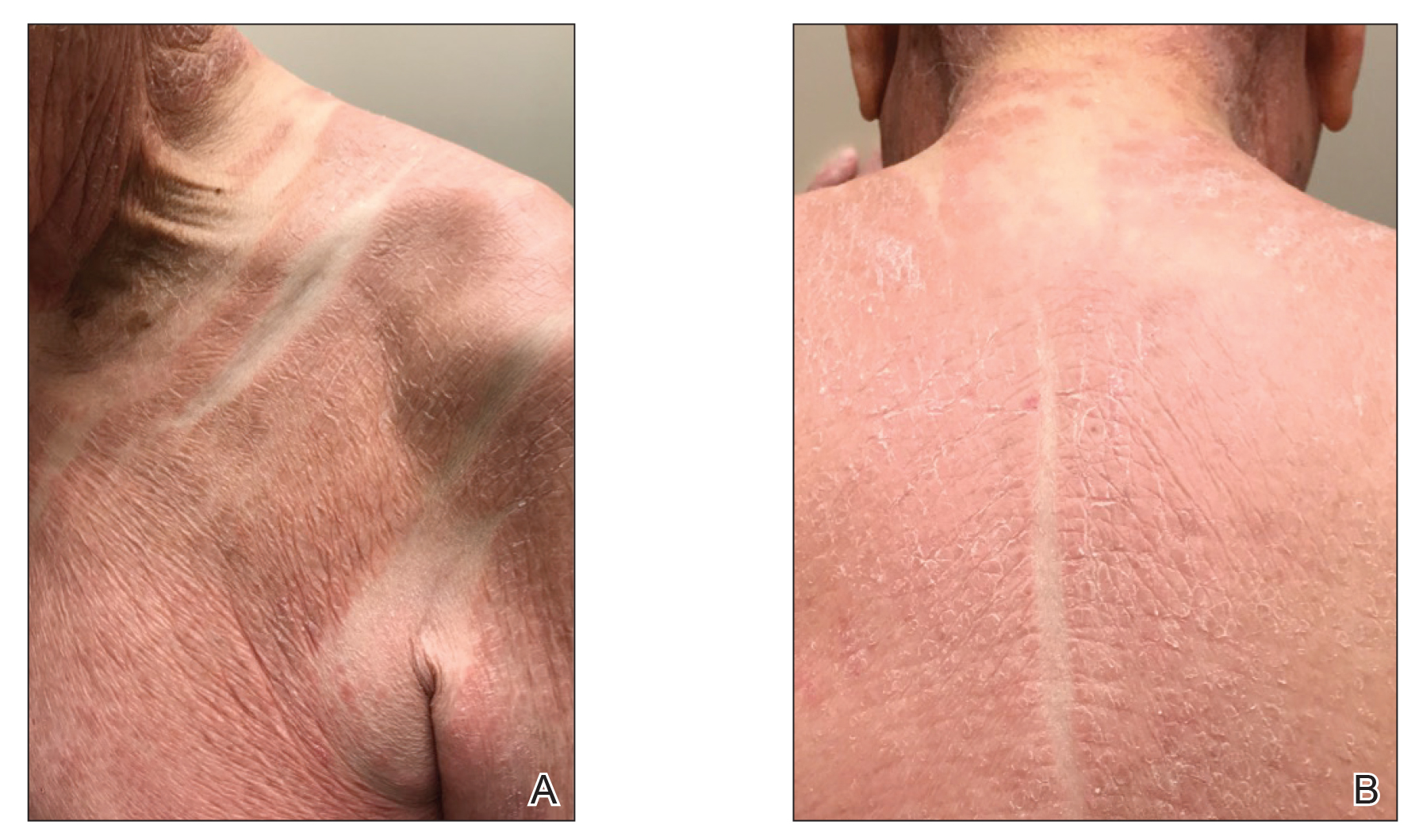
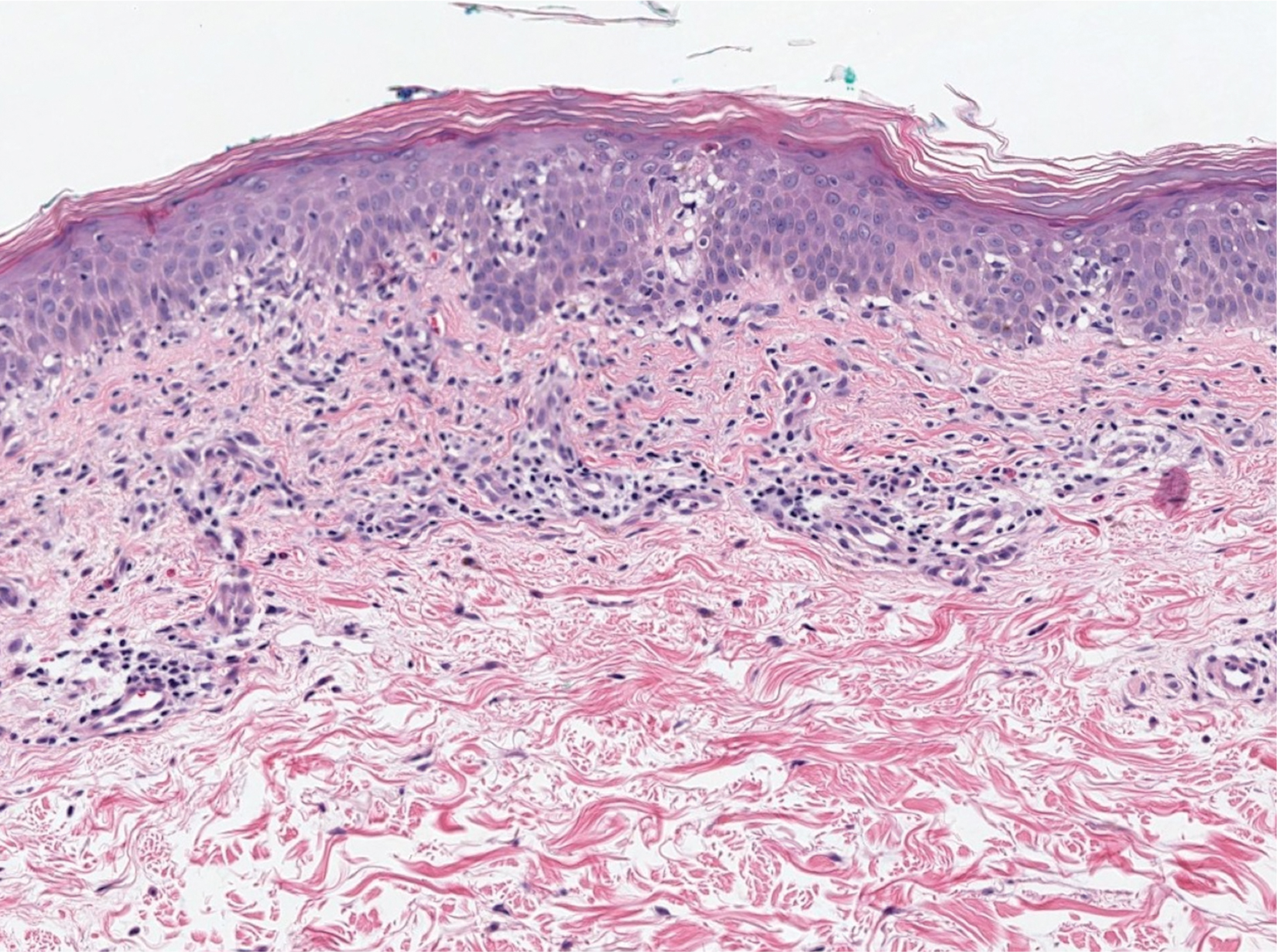
The patient presented with a characteristic finding of skinfold sparing, known as the "deck-chair sign" (Figure 1).1 A repeat biopsy at our institution revealed a dermal perivascular and bandlike infiltrate with lymphocytes and occasional eosinophils (Figure 2). The epidermis showed mild spongiosis, lymphocytic exocytosis, and rare necrotic keratinocytes. A T-cell gene rearrangement assay was negative for a monoclonal population of T lymphocytes. Based on the clinical and histologic features, the diagnosis was most consistent with papuloerythroderma of Ofuji (PEO); however, a lymphoproliferative disorder needed to be excluded. Further workup included a peripheral smear, complete blood cell count with differential, comprehensive metabolic panel, IgE level, and hepatitis panel; all were normal, except for an elevated serum IgE level. Human immunodeficiency virus and age-appropriate malignancy screening were negative. The patient was prescribed betamethasone dipropionate cream 0.05% twice daily, which resulted in near-complete resolution of the rash and marked improvement in pruritus.
In 1984, PEO was described as an entity of generalized pruritic erythroderma characterized by flat-topped, red to brown, coalescing papules with sparing of the skinfolds, later coined the deck-chair sign.1,2 Papuloerythroderma of Ofuji commonly presents in elderly Asian males with a male to female ratio of 4:1.3 Papuloerythroderma of Ofuji is a T cell-mediated skin disease; however, the etiology of the signature rash remains unclear. One explanation includes circulating factors in the skin that elicit an inflammatory response, which does not occur in areas of external pressure.3 The deck-chair sign may occur more frequently in elderly individuals due to increased skin laxity, which creates crease lines that are spared from rubbing and excoriations.4
Although Ofuji et al2 initially reported 4 idiopathic cases, subsequent authors described PEO in association with other conditions, including cutaneous T-cell lymphoma (CTCL) and atopic diathesis, and infections, as well as secondary to medications. Some authors have suggested that PEO may be an early variant of mycosis fungoides; therefore, physicians should monitor patients closely.5-7 Maher et al6 commented that multiple causative factors including CTCL underlie the development of papuloerythroderma.
In a review of PEO, Torchia et al3 proposed diagnostic criteria and an etiologic classification to address whether PEO represents an independent entity or an unusual manifestation of other dermatoses. They established 4 categories of papuloerythroderma--primary, secondary, papuloerythrodermalike CTCL, and pseudopapuloerythroderma--and proposed that primary PEO is a diagnosis of exclusion. If no secondary association is found, they proposed 10 criteria for primary PEO: 5 major criteria include coalescing flat-topped papules, the deck-chair sign, pruritus, histopathologic exclusion of diseases such as CTCL, and a negative workup to exclude other causes.3 In 2018, Maher et al6 recommended workup to rule out cutaneous malignancy, including skin biopsy, flow cytometry, Sézary cell count, T-cell rearrangement, lactate dehydrogenase, and human T-cell lymphotropic virus 1. The 5 minor criteria proposed by Torchia et al3 include age older than 55 years, male sex, eosinophilia, elevated IgE level, and lymphopenia. Our patient fulfilled all 5 major criteria and 3 minor criteria; eosinophilia and lymphopenia were absent.
Clinically, PEO has been associated with the deck-chair sign, a pattern of selective sparing of skinfolds, including axillary, inguinal, submammary, and other flexural areas. Although the deck-chair sign was originally considered pathognomonic for PEO, this clinical pattern also has been observed in other entities, such as angioimmunoblastic lymphoma, cutaneous Waldenström macroglobulinemia, and acanthosis nigricans.5,8,9
Specific characteristics of the rash and certain clinical symptoms may help to distinguish the deck-chair sign of PEO from its other causes. Although malignant acanthosis nigricans may spare the skinfolds, lesions have a classic velvety thickening and brownish hyperpigmentation, which is not characteristic of the reddish brown, flat-topped papules of PEO.9 Pai et al5 described a patient with parthenium dermatitis presenting with the deck-chair sign that developed years after repeated exposure to the allergen. Our patient did not have a history of repeated episodes of allergic contact dermatitis. In addition, areas of sparing may mimic the appearance of pityriasis rubra pilaris. As in our patient, those with PEO generally lack the follicular hyperkeratotic papules, palmoplantar keratoderma, widespread orange-red erythema, and characteristic histopathologic finding of hyperkeratosis with alternating orthokeratosis and parakeratosis, allowing these entities to be easily distinguished in most instances.10
Histopathologically, primary PEO shows a nonspecific spongiotic dermatitis-like pattern characterized by slight epidermal hyperplasia with spongiosis and a predominantly perivascular dermal infiltrate with lymphocytes, histiocytes, and eosinophils.3 These histologic findings may at times show some overlap with CTCL, and therefore T-cell gene rearrangement and flow cytometry may be performed in those instances.6
Treatment includes the management of any underlying condition causing the papuloerythroderma.3,6 There are no large clinical trials of treatment options for primary PEO due to its rarity. Topical or systemic corticosteroids remain the mainstay of treatment.3 Alternative treatments used with variable success include phototherapy, interferon, etretinate, cyclosporine, and azathioprine.11 Allegue et al11 successfully used methotrexate to treat a patient with primary PEO and postulated that methotrexate may act through an immunosuppressive mechanism on activated T cells due to the involvement of helper T cells TH2 and TH22 in its pathogenesis.
Although the cutaneous manifestations of PEO may respond well to topical steroids, it is important to consider the possible presence of an underlying malignancy and other associated systemic conditions.
- Farthing CF, Staughton RC, Harper JI, et al. Papuloerythroderma--a further case with the 'deck chair sign.' Dermatologica. 1986;172:65-66.
- Ofuji S, Furukawa F, Miyachi Y, et al. Papuloerythroderma. Dermatologica. 1984;169:125-130.
- Torchia D, Miteva M, Hu S, et al. Papuloerythroderma 2009: two new cases and systematic review of the worldwide literature 25 years after its identification by Ofuji et al. Dermatology. 2010;220:311-320.
- Ochi H, Ang CC. Novel association of a papuloerythroderma of Ofuji phenotype with dermatitis herpetiformis. Int J Dermatol. 2018;57:856-857.
- Pai S, Shetty S, Rao R. Parthenium dermatitis with deck-chair sign. JAMA Dermatol. 2015;151:906-907.
- Maher AM, Ward CE, Glassman S, et al. The importance of excluding cutaneous T-cell lymphomas in patients with a working diagnosis of papuloerythroderma of Ofuji: a case series. Case Rep Dermatol. 2018;10:46-54.
- Grob JJ, Collet-Villette AM, Horchowski N, et al. Ofuji papuloerythroderma. report of a case with T cell skin lymphoma and discussion of the nature of this disease. J Am Acad Dermatol. 1989;20(5 pt 2):927-931.
- Ferran M, Gallardo F, Baena V, et al. The 'deck chair sign' in specific cutaneous involvement by angioimmunoblastic T cell lymphoma. Dermatology. 2006;213:50-52.
- Murao K, Sadamoto Y, Kubo Y, et al. Generalized malignant acanthosis nigricans with "deck-chair sign." Int J Dermatol. 2013;52:377-378.
- Regina G, Paramita L, Radiono S, et al. Papuloerythroderma of Ofuji in Indonesia: the first case report. JDVI. 2016;1:93-98.
- Allegue F, Fachal C, Gonzalez-Vilas D, et al. Papuloerythroderma of Ofuji successfully treated with methotrexate. Dermatol Ther. 2018;31:E12638.
The Diagnosis: Papuloerythroderma of Ofuji
The patient presented with a characteristic finding of skinfold sparing, known as the "deck-chair sign" (Figure 1).1 A repeat biopsy at our institution revealed a dermal perivascular and bandlike infiltrate with lymphocytes and occasional eosinophils (Figure 2). The epidermis showed mild spongiosis, lymphocytic exocytosis, and rare necrotic keratinocytes. A T-cell gene rearrangement assay was negative for a monoclonal population of T lymphocytes. Based on the clinical and histologic features, the diagnosis was most consistent with papuloerythroderma of Ofuji (PEO); however, a lymphoproliferative disorder needed to be excluded. Further workup included a peripheral smear, complete blood cell count with differential, comprehensive metabolic panel, IgE level, and hepatitis panel; all were normal, except for an elevated serum IgE level. Human immunodeficiency virus and age-appropriate malignancy screening were negative. The patient was prescribed betamethasone dipropionate cream 0.05% twice daily, which resulted in near-complete resolution of the rash and marked improvement in pruritus.


In 1984, PEO was described as an entity of generalized pruritic erythroderma characterized by flat-topped, red to brown, coalescing papules with sparing of the skinfolds, later coined the deck-chair sign.1,2 Papuloerythroderma of Ofuji commonly presents in elderly Asian males with a male to female ratio of 4:1.3 Papuloerythroderma of Ofuji is a T cell-mediated skin disease; however, the etiology of the signature rash remains unclear. One explanation includes circulating factors in the skin that elicit an inflammatory response, which does not occur in areas of external pressure.3 The deck-chair sign may occur more frequently in elderly individuals due to increased skin laxity, which creates crease lines that are spared from rubbing and excoriations.4
Although Ofuji et al2 initially reported 4 idiopathic cases, subsequent authors described PEO in association with other conditions, including cutaneous T-cell lymphoma (CTCL) and atopic diathesis, and infections, as well as secondary to medications. Some authors have suggested that PEO may be an early variant of mycosis fungoides; therefore, physicians should monitor patients closely.5-7 Maher et al6 commented that multiple causative factors including CTCL underlie the development of papuloerythroderma.
In a review of PEO, Torchia et al3 proposed diagnostic criteria and an etiologic classification to address whether PEO represents an independent entity or an unusual manifestation of other dermatoses. They established 4 categories of papuloerythroderma--primary, secondary, papuloerythrodermalike CTCL, and pseudopapuloerythroderma--and proposed that primary PEO is a diagnosis of exclusion. If no secondary association is found, they proposed 10 criteria for primary PEO: 5 major criteria include coalescing flat-topped papules, the deck-chair sign, pruritus, histopathologic exclusion of diseases such as CTCL, and a negative workup to exclude other causes.3 In 2018, Maher et al6 recommended workup to rule out cutaneous malignancy, including skin biopsy, flow cytometry, Sézary cell count, T-cell rearrangement, lactate dehydrogenase, and human T-cell lymphotropic virus 1. The 5 minor criteria proposed by Torchia et al3 include age older than 55 years, male sex, eosinophilia, elevated IgE level, and lymphopenia. Our patient fulfilled all 5 major criteria and 3 minor criteria; eosinophilia and lymphopenia were absent.
Clinically, PEO has been associated with the deck-chair sign, a pattern of selective sparing of skinfolds, including axillary, inguinal, submammary, and other flexural areas. Although the deck-chair sign was originally considered pathognomonic for PEO, this clinical pattern also has been observed in other entities, such as angioimmunoblastic lymphoma, cutaneous Waldenström macroglobulinemia, and acanthosis nigricans.5,8,9
Specific characteristics of the rash and certain clinical symptoms may help to distinguish the deck-chair sign of PEO from its other causes. Although malignant acanthosis nigricans may spare the skinfolds, lesions have a classic velvety thickening and brownish hyperpigmentation, which is not characteristic of the reddish brown, flat-topped papules of PEO.9 Pai et al5 described a patient with parthenium dermatitis presenting with the deck-chair sign that developed years after repeated exposure to the allergen. Our patient did not have a history of repeated episodes of allergic contact dermatitis. In addition, areas of sparing may mimic the appearance of pityriasis rubra pilaris. As in our patient, those with PEO generally lack the follicular hyperkeratotic papules, palmoplantar keratoderma, widespread orange-red erythema, and characteristic histopathologic finding of hyperkeratosis with alternating orthokeratosis and parakeratosis, allowing these entities to be easily distinguished in most instances.10
Histopathologically, primary PEO shows a nonspecific spongiotic dermatitis-like pattern characterized by slight epidermal hyperplasia with spongiosis and a predominantly perivascular dermal infiltrate with lymphocytes, histiocytes, and eosinophils.3 These histologic findings may at times show some overlap with CTCL, and therefore T-cell gene rearrangement and flow cytometry may be performed in those instances.6
Treatment includes the management of any underlying condition causing the papuloerythroderma.3,6 There are no large clinical trials of treatment options for primary PEO due to its rarity. Topical or systemic corticosteroids remain the mainstay of treatment.3 Alternative treatments used with variable success include phototherapy, interferon, etretinate, cyclosporine, and azathioprine.11 Allegue et al11 successfully used methotrexate to treat a patient with primary PEO and postulated that methotrexate may act through an immunosuppressive mechanism on activated T cells due to the involvement of helper T cells TH2 and TH22 in its pathogenesis.
Although the cutaneous manifestations of PEO may respond well to topical steroids, it is important to consider the possible presence of an underlying malignancy and other associated systemic conditions.
The Diagnosis: Papuloerythroderma of Ofuji
The patient presented with a characteristic finding of skinfold sparing, known as the "deck-chair sign" (Figure 1).1 A repeat biopsy at our institution revealed a dermal perivascular and bandlike infiltrate with lymphocytes and occasional eosinophils (Figure 2). The epidermis showed mild spongiosis, lymphocytic exocytosis, and rare necrotic keratinocytes. A T-cell gene rearrangement assay was negative for a monoclonal population of T lymphocytes. Based on the clinical and histologic features, the diagnosis was most consistent with papuloerythroderma of Ofuji (PEO); however, a lymphoproliferative disorder needed to be excluded. Further workup included a peripheral smear, complete blood cell count with differential, comprehensive metabolic panel, IgE level, and hepatitis panel; all were normal, except for an elevated serum IgE level. Human immunodeficiency virus and age-appropriate malignancy screening were negative. The patient was prescribed betamethasone dipropionate cream 0.05% twice daily, which resulted in near-complete resolution of the rash and marked improvement in pruritus.


In 1984, PEO was described as an entity of generalized pruritic erythroderma characterized by flat-topped, red to brown, coalescing papules with sparing of the skinfolds, later coined the deck-chair sign.1,2 Papuloerythroderma of Ofuji commonly presents in elderly Asian males with a male to female ratio of 4:1.3 Papuloerythroderma of Ofuji is a T cell-mediated skin disease; however, the etiology of the signature rash remains unclear. One explanation includes circulating factors in the skin that elicit an inflammatory response, which does not occur in areas of external pressure.3 The deck-chair sign may occur more frequently in elderly individuals due to increased skin laxity, which creates crease lines that are spared from rubbing and excoriations.4
Although Ofuji et al2 initially reported 4 idiopathic cases, subsequent authors described PEO in association with other conditions, including cutaneous T-cell lymphoma (CTCL) and atopic diathesis, and infections, as well as secondary to medications. Some authors have suggested that PEO may be an early variant of mycosis fungoides; therefore, physicians should monitor patients closely.5-7 Maher et al6 commented that multiple causative factors including CTCL underlie the development of papuloerythroderma.
In a review of PEO, Torchia et al3 proposed diagnostic criteria and an etiologic classification to address whether PEO represents an independent entity or an unusual manifestation of other dermatoses. They established 4 categories of papuloerythroderma--primary, secondary, papuloerythrodermalike CTCL, and pseudopapuloerythroderma--and proposed that primary PEO is a diagnosis of exclusion. If no secondary association is found, they proposed 10 criteria for primary PEO: 5 major criteria include coalescing flat-topped papules, the deck-chair sign, pruritus, histopathologic exclusion of diseases such as CTCL, and a negative workup to exclude other causes.3 In 2018, Maher et al6 recommended workup to rule out cutaneous malignancy, including skin biopsy, flow cytometry, Sézary cell count, T-cell rearrangement, lactate dehydrogenase, and human T-cell lymphotropic virus 1. The 5 minor criteria proposed by Torchia et al3 include age older than 55 years, male sex, eosinophilia, elevated IgE level, and lymphopenia. Our patient fulfilled all 5 major criteria and 3 minor criteria; eosinophilia and lymphopenia were absent.
Clinically, PEO has been associated with the deck-chair sign, a pattern of selective sparing of skinfolds, including axillary, inguinal, submammary, and other flexural areas. Although the deck-chair sign was originally considered pathognomonic for PEO, this clinical pattern also has been observed in other entities, such as angioimmunoblastic lymphoma, cutaneous Waldenström macroglobulinemia, and acanthosis nigricans.5,8,9
Specific characteristics of the rash and certain clinical symptoms may help to distinguish the deck-chair sign of PEO from its other causes. Although malignant acanthosis nigricans may spare the skinfolds, lesions have a classic velvety thickening and brownish hyperpigmentation, which is not characteristic of the reddish brown, flat-topped papules of PEO.9 Pai et al5 described a patient with parthenium dermatitis presenting with the deck-chair sign that developed years after repeated exposure to the allergen. Our patient did not have a history of repeated episodes of allergic contact dermatitis. In addition, areas of sparing may mimic the appearance of pityriasis rubra pilaris. As in our patient, those with PEO generally lack the follicular hyperkeratotic papules, palmoplantar keratoderma, widespread orange-red erythema, and characteristic histopathologic finding of hyperkeratosis with alternating orthokeratosis and parakeratosis, allowing these entities to be easily distinguished in most instances.10
Histopathologically, primary PEO shows a nonspecific spongiotic dermatitis-like pattern characterized by slight epidermal hyperplasia with spongiosis and a predominantly perivascular dermal infiltrate with lymphocytes, histiocytes, and eosinophils.3 These histologic findings may at times show some overlap with CTCL, and therefore T-cell gene rearrangement and flow cytometry may be performed in those instances.6
Treatment includes the management of any underlying condition causing the papuloerythroderma.3,6 There are no large clinical trials of treatment options for primary PEO due to its rarity. Topical or systemic corticosteroids remain the mainstay of treatment.3 Alternative treatments used with variable success include phototherapy, interferon, etretinate, cyclosporine, and azathioprine.11 Allegue et al11 successfully used methotrexate to treat a patient with primary PEO and postulated that methotrexate may act through an immunosuppressive mechanism on activated T cells due to the involvement of helper T cells TH2 and TH22 in its pathogenesis.
Although the cutaneous manifestations of PEO may respond well to topical steroids, it is important to consider the possible presence of an underlying malignancy and other associated systemic conditions.
- Farthing CF, Staughton RC, Harper JI, et al. Papuloerythroderma--a further case with the 'deck chair sign.' Dermatologica. 1986;172:65-66.
- Ofuji S, Furukawa F, Miyachi Y, et al. Papuloerythroderma. Dermatologica. 1984;169:125-130.
- Torchia D, Miteva M, Hu S, et al. Papuloerythroderma 2009: two new cases and systematic review of the worldwide literature 25 years after its identification by Ofuji et al. Dermatology. 2010;220:311-320.
- Ochi H, Ang CC. Novel association of a papuloerythroderma of Ofuji phenotype with dermatitis herpetiformis. Int J Dermatol. 2018;57:856-857.
- Pai S, Shetty S, Rao R. Parthenium dermatitis with deck-chair sign. JAMA Dermatol. 2015;151:906-907.
- Maher AM, Ward CE, Glassman S, et al. The importance of excluding cutaneous T-cell lymphomas in patients with a working diagnosis of papuloerythroderma of Ofuji: a case series. Case Rep Dermatol. 2018;10:46-54.
- Grob JJ, Collet-Villette AM, Horchowski N, et al. Ofuji papuloerythroderma. report of a case with T cell skin lymphoma and discussion of the nature of this disease. J Am Acad Dermatol. 1989;20(5 pt 2):927-931.
- Ferran M, Gallardo F, Baena V, et al. The 'deck chair sign' in specific cutaneous involvement by angioimmunoblastic T cell lymphoma. Dermatology. 2006;213:50-52.
- Murao K, Sadamoto Y, Kubo Y, et al. Generalized malignant acanthosis nigricans with "deck-chair sign." Int J Dermatol. 2013;52:377-378.
- Regina G, Paramita L, Radiono S, et al. Papuloerythroderma of Ofuji in Indonesia: the first case report. JDVI. 2016;1:93-98.
- Allegue F, Fachal C, Gonzalez-Vilas D, et al. Papuloerythroderma of Ofuji successfully treated with methotrexate. Dermatol Ther. 2018;31:E12638.
- Farthing CF, Staughton RC, Harper JI, et al. Papuloerythroderma--a further case with the 'deck chair sign.' Dermatologica. 1986;172:65-66.
- Ofuji S, Furukawa F, Miyachi Y, et al. Papuloerythroderma. Dermatologica. 1984;169:125-130.
- Torchia D, Miteva M, Hu S, et al. Papuloerythroderma 2009: two new cases and systematic review of the worldwide literature 25 years after its identification by Ofuji et al. Dermatology. 2010;220:311-320.
- Ochi H, Ang CC. Novel association of a papuloerythroderma of Ofuji phenotype with dermatitis herpetiformis. Int J Dermatol. 2018;57:856-857.
- Pai S, Shetty S, Rao R. Parthenium dermatitis with deck-chair sign. JAMA Dermatol. 2015;151:906-907.
- Maher AM, Ward CE, Glassman S, et al. The importance of excluding cutaneous T-cell lymphomas in patients with a working diagnosis of papuloerythroderma of Ofuji: a case series. Case Rep Dermatol. 2018;10:46-54.
- Grob JJ, Collet-Villette AM, Horchowski N, et al. Ofuji papuloerythroderma. report of a case with T cell skin lymphoma and discussion of the nature of this disease. J Am Acad Dermatol. 1989;20(5 pt 2):927-931.
- Ferran M, Gallardo F, Baena V, et al. The 'deck chair sign' in specific cutaneous involvement by angioimmunoblastic T cell lymphoma. Dermatology. 2006;213:50-52.
- Murao K, Sadamoto Y, Kubo Y, et al. Generalized malignant acanthosis nigricans with "deck-chair sign." Int J Dermatol. 2013;52:377-378.
- Regina G, Paramita L, Radiono S, et al. Papuloerythroderma of Ofuji in Indonesia: the first case report. JDVI. 2016;1:93-98.
- Allegue F, Fachal C, Gonzalez-Vilas D, et al. Papuloerythroderma of Ofuji successfully treated with methotrexate. Dermatol Ther. 2018;31:E12638.
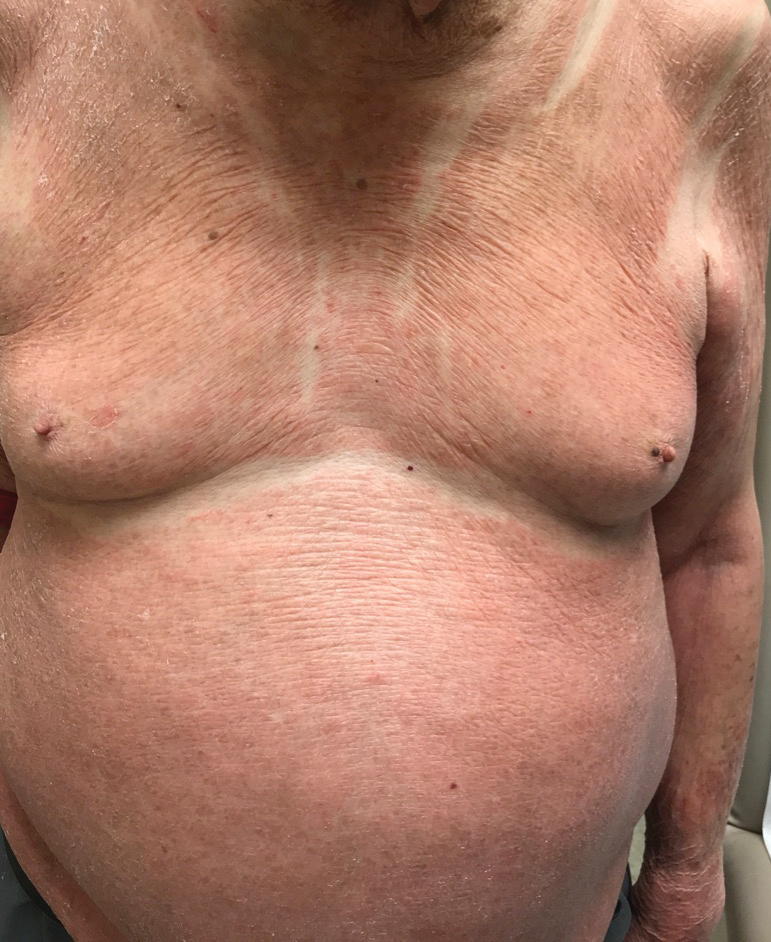
An 89-year-old Asian man presented with a generalized pruritic eruption of 2 months' duration. The rash started on the flanks and later spread to the arms and legs, abdomen, and back; the face and palms were spared. Physical examination revealed numerous erythematous papules coalescing into large scaly plaques on the trunk, arms, and legs. There were noticeable areas of sparing of the skinfolds, especially the axillary, inframammary, and inguinal folds, as well as the midline of the back. A biopsy performed by an outside physician showed findings consistent with a possible pityriasiform drug eruption; however, there were no recent changes in medication history.
COVID 19: Confessions of an outpatient psychiatrist during the pandemic
It seems that some glitches would be inevitable. With a sudden shift to videoconferencing in private psychiatric practices, there were bound to be issues with both technology and privacy. One friend told me of such a glitch on the very first day she started telemental health: She was meeting with a patient who was sitting at her kitchen table. Unbeknownst to the patient, her husband walked into the kitchen behind her, fully naked, to get something from the refrigerator. “There was a full moon shot!” my friend said, initially quite shocked, and then eventually amused. As we all cope with a national tragedy and the total upheaval to our personal and professional lives, the stories just keep coming.
I left work on Friday, March 13, with plans to return on the following Monday to see patients. I had no idea that, by Sunday evening, I would be persuaded that for the safety of all I would need to shut down my real-life psychiatric practice and switch to a videoconferencing venue. I, along with many psychiatrists in Maryland, made this decision after Amy Huberman, MD, posted the following on the Maryland Psychiatric Society (MPS) listserv on Sunday, March 15:
“I want to make a case for starting video sessions with all your patients NOW. There is increasing evidence that the spread of coronavirus is driven primarily by asymptomatic or mildly ill people infected with the virus. Because of this, it’s not good enough to tell your patients not to come in if they have symptoms, or for you not to come into work if you have no symptoms. Even after I sent out a letter two weeks ago warning people not to come in if they had symptoms or had potentially come in contact with someone with COVID-19, several patients with coughs still came to my office, as well as several people who had just been on trips to New York City.
If we want to help slow the spread of this illness so that our health system has a better chance of being able to offer ventilators to the people who need them, we must limit all contacts as much as possible – even of asymptomatic people, given the emerging data.
I am planning to send out a message to all my patients today that they should do the same. Without the president or the media giving clear advice to people about what to do, it’s our job as physicians to do it.”
By that night, I had set up a home office with a blank wall behind me, windows in front of me, and books propping my computer at a height that would not have my patients looking up my nose. For the first time in over 20 years, I dusted my son’s Little League trophies, moved them and a 40,000 baseball card collection against the wall, carried a desk, chair, rug, houseplant, and a small Buddha into a room in which I would have some privacy, and my telepsychiatry practice found a home.
After some research, I registered for a free site called Doxy.me because it was HIPAA compliant and did not require patients to download an application; anyone with a camera on any Internet-enabled phone, computer, or tablet, could click on a link and enter my virtual waiting room. I soon discovered that images on the Doxy.me site are sometimes grainy and sometimes freeze up; in some sessions, we ended up switching to FaceTime, and as government mandates for HIPAA compliance relaxed, I offered to meet on any site that my patients might be comfortable with: if not Doxy.me (which remains my starting place for most sessions), Facetime, Skype, Zoom, or Whatsapp. I have not offered Bluejeans, Google Hangouts, or WebEx, and no one has requested those applications. I keep the phone next to the computer, and some sessions include a few minutes of tech support as I help patients (or they help me) navigate the various sites. In a few sessions, we could not get the audio to work and we used video on one venue while we talked on the phone. I haven’t figured out if the variations in the quality of the connection has to do with my Comcast connection, the fact that these websites are overloaded with users, or that my household now consists of three people, two large monitors, three laptops, two tablets, three cell phone lines (not to mention one dog and a transplanted cat), all going at the same time. The pets do not require any bandwidth, but all the people are talking to screens throughout the workday.
As my colleagues embarked on the same journey, the listserv questions and comments came quickly. What were the best platforms? Was it a good thing or a bad thing to suddenly be in people’s homes? Some felt the extraneous background to be helpful, others found it distracting and intrusive.
How do these sessions get coded for the purpose of billing? There was a tremendous amount of confusion over that, with the initial verdict being that Medicare wanted the place of service changed to “02” and that private insurers want one of two modifiers, and it was anyone’s guess which company wanted which modifier. Then there was the concern that Medicare was paying 25% less, until the MPS staff clarified that full fees would be paid, but the place of service should be filled in as “11” – not “02” – as with regular office visits, and the modifier “95” should be added on the Health Care Finance Administration claim form. We were left to wait and see what gets reimbursed and for what fees.
Could new patients be seen by videoconferencing? Could patients from other states be seen this way if the psychiatrist was not licensed in the state where the patient was calling from? One psychiatrist reported he had a patient in an adjacent state drive over the border into Maryland, but the patient brought her mother and the evaluation included unwanted input from the mom as the session consisted of the patient and her mother yelling at both each other in the car and at the psychiatrist on the screen!
Psychiatrists on the listserv began to comment that treatment sessions were intense and exhausting. I feel the literal face-to-face contact of another person’s head just inches from my own, with full eye contact, often gets to be a lot. No one asks why I’ve moved a trinket (ah, there are no trinkets) or gazes off around the room. I sometimes sit for long periods of time as I don’t even stand to see the patients to the door. Other patients move about or bounce their devices on their laps, and my stomach starts to feel queasy until I ask to have the device adjusted. In some sessions, I find I’m talking to partial heads, or that computer icons cover the patient’s mouth.
Being in people’s lives via screen has been interesting. Unlike my colleague, I have not had any streaking spouses, but I’ve greeted a few family members – often those serving as technical support – and I’ve toured part of a farm, met dogs, guinea pigs, and even a goat. I’ve made brief daily “visits” to a frightened patient in isolation on a COVID hospital unit and had the joy of celebrating the discharge to home. It’s odd to be in a bedroom with a patient, even virtually, and it is interesting to note where they choose to hold their sessions; I’ve had several patients hold sessions from their cars. Seeing my own image in the corner of the screen is also a bit distracting, and in one session, as I saw my own reaction, my patient said, “I knew you were going to make that face!”
The pandemic has usurped most of the activities of all of our lives, and without social interactions, travel, and work in the usual way, life does not hold its usual richness. In a few cases, I have ended the session after half the time as the patient insisted there was nothing to talk about. Many talk about the medical problems they can’t be seen for, what they are doing to keep safe (or not), how they are washing down their groceries, and who they are meeting with by Zoom. Of those who were terribly anxious before, some feel oddly calmer – the world has ramped up to meet their level of anxiety and they feel vindicated. No one thinks they are odd for worrying about germs on door knobs or elevator buttons. What were once neurotic fears are now our real-life reality. Others have been triggered by a paralyzing fear, often with panic attacks, and these sessions are certainly challenging as I figure out which medications will best help, while responding to requests for reassurance. And there is the troublesome aspect of trying to care for others who are fearful while living with the reality that these fears are not extraneous to our own lives: We, too, are scared for ourselves and our families.
For some people, stay-at-home mandates have been easier than for others. People who are naturally introverted, or those with social anxiety, have told me they find this time at home to be a relief. They no longer feel pressured to go out; there is permission to be alone, to read, or watch Netflix. No one is pressuring them to go to parties or look for a Tinder date. For others, the isolation and loneliness have been devastating, causing a range of emotions from being “stir crazy,” to triggering episodes of major depression and severe anxiety.
Health care workers in therapy talk about their fears of being contaminated with coronavirus, about the exposures they’ve had, their fears of bringing the virus home to family, and about the anger – sometimes rage – that their employers are not doing more to protect them.
Few people these past weeks are looking for insight into their patterns of behavior and emotion. Most of life has come to be about survival and not about personal striving. Students who are driven to excel are disappointed to have their scholastic worlds have switched to pass/fail. And for those struggling with milder forms of depression and anxiety, both the patients and I have all been a bit perplexed by losing the usual measures of what feelings are normal in a tragic world and we no longer use socializing as the hallmark that heralds a return to normalcy after a period of withdrawal.
In some aspects, it is not all been bad. I’ve enjoyed watching my neighbors walk by with their dogs through the window behind my computer screen and I’ve felt part of the daily evolution as the cherry tree outside that same window turns from dead brown wood to vibrant pink blossoms. I like the flexibility of my schedule and the sensation I always carry of being rushed has quelled. I take more walks and spend more time with the family members who are held captive with me. The dog, who no longer is left alone for hours each day, is certainly a winner.
Some of my colleagues tell me they are overwhelmed – patients they have not seen for years have returned, people are asking for more frequent sessions, and they are suddenly trying to work at home while homeschooling children. I have had only a few of those requests for crisis care, while new referrals are much quieter than normal. Some of my patients have even said that they simply aren’t comfortable meeting this way and they will see me at the other end of the pandemic. A few people I would have expected to hear from I have not, and I fear that those who have lost their jobs may avoiding the cost of treatment – this group I will reach out to in the coming weeks. A little extra time, however, has given me the opportunity to join the Johns Hopkins COVID-19 Mental Health team. And my first attempt at teaching a resident seminar by Zoom has gone well.
For some in the medical field, this has been a horrible and traumatic time; they are worked to exhaustion, and surrounded by distress, death, and personal fear with every shift. For others, life has come to a standstill as the elective procedures that fill their days have virtually stopped. For outpatient psychiatry, it’s been a bit of an in-between, we may feel an odd mix of relevant and useless all at the same time, as our services are appreciated by our patients, but as actual soldiers caring for the ill COVID patients, we are leaving that to our colleagues in the EDs, COVID units, and ICUs. As a physician who has not treated a patient in an ICU for decades, I wish I had something more concrete to contribute to the effort, and at the same time, I’m relieved that I don’t.
And what about the patients? How are they doing with remote psychiatry? Some are clearly flustered or frustrated by the technology issues. Other times sessions go smoothly, and the fact that we are talking through screens gets forgotten. Some like the convenience of not having to drive a far distance and no one misses my crowded parking lot.
Kristen, another doctor’s patient in Illinois, commented: “I appreciate the continuity in care, especially if the alternative is delaying appointments. I think that’s most important. The interaction helps manage added anxiety from isolating as well. I don’t think it diminishes the care I receive; it makes me feel that my doctor is still accessible. One other point, since I have had both telemedicine and in-person appointments with my current psychiatrist, is that during in-person meetings, he is usually on his computer and rarely looks at me or makes eye contact. In virtual meetings, I feel he is much more engaged with me.”
In normal times, I spend a good deal of time encouraging patients to work on building their relationships and community – these connections lead people to healthy and fulfilling lives – and now we talk about how to best be socially distant. We see each other as vectors of disease and to greet a friend with a handshake, much less a hug, would be unthinkable. Will our collective psyches ever recover? For those of us who will survive, that remains to be seen. In the meantime, perhaps we are all being forced to be more flexible and innovative.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
It seems that some glitches would be inevitable. With a sudden shift to videoconferencing in private psychiatric practices, there were bound to be issues with both technology and privacy. One friend told me of such a glitch on the very first day she started telemental health: She was meeting with a patient who was sitting at her kitchen table. Unbeknownst to the patient, her husband walked into the kitchen behind her, fully naked, to get something from the refrigerator. “There was a full moon shot!” my friend said, initially quite shocked, and then eventually amused. As we all cope with a national tragedy and the total upheaval to our personal and professional lives, the stories just keep coming.
I left work on Friday, March 13, with plans to return on the following Monday to see patients. I had no idea that, by Sunday evening, I would be persuaded that for the safety of all I would need to shut down my real-life psychiatric practice and switch to a videoconferencing venue. I, along with many psychiatrists in Maryland, made this decision after Amy Huberman, MD, posted the following on the Maryland Psychiatric Society (MPS) listserv on Sunday, March 15:
“I want to make a case for starting video sessions with all your patients NOW. There is increasing evidence that the spread of coronavirus is driven primarily by asymptomatic or mildly ill people infected with the virus. Because of this, it’s not good enough to tell your patients not to come in if they have symptoms, or for you not to come into work if you have no symptoms. Even after I sent out a letter two weeks ago warning people not to come in if they had symptoms or had potentially come in contact with someone with COVID-19, several patients with coughs still came to my office, as well as several people who had just been on trips to New York City.
If we want to help slow the spread of this illness so that our health system has a better chance of being able to offer ventilators to the people who need them, we must limit all contacts as much as possible – even of asymptomatic people, given the emerging data.
I am planning to send out a message to all my patients today that they should do the same. Without the president or the media giving clear advice to people about what to do, it’s our job as physicians to do it.”
By that night, I had set up a home office with a blank wall behind me, windows in front of me, and books propping my computer at a height that would not have my patients looking up my nose. For the first time in over 20 years, I dusted my son’s Little League trophies, moved them and a 40,000 baseball card collection against the wall, carried a desk, chair, rug, houseplant, and a small Buddha into a room in which I would have some privacy, and my telepsychiatry practice found a home.
After some research, I registered for a free site called Doxy.me because it was HIPAA compliant and did not require patients to download an application; anyone with a camera on any Internet-enabled phone, computer, or tablet, could click on a link and enter my virtual waiting room. I soon discovered that images on the Doxy.me site are sometimes grainy and sometimes freeze up; in some sessions, we ended up switching to FaceTime, and as government mandates for HIPAA compliance relaxed, I offered to meet on any site that my patients might be comfortable with: if not Doxy.me (which remains my starting place for most sessions), Facetime, Skype, Zoom, or Whatsapp. I have not offered Bluejeans, Google Hangouts, or WebEx, and no one has requested those applications. I keep the phone next to the computer, and some sessions include a few minutes of tech support as I help patients (or they help me) navigate the various sites. In a few sessions, we could not get the audio to work and we used video on one venue while we talked on the phone. I haven’t figured out if the variations in the quality of the connection has to do with my Comcast connection, the fact that these websites are overloaded with users, or that my household now consists of three people, two large monitors, three laptops, two tablets, three cell phone lines (not to mention one dog and a transplanted cat), all going at the same time. The pets do not require any bandwidth, but all the people are talking to screens throughout the workday.
As my colleagues embarked on the same journey, the listserv questions and comments came quickly. What were the best platforms? Was it a good thing or a bad thing to suddenly be in people’s homes? Some felt the extraneous background to be helpful, others found it distracting and intrusive.
How do these sessions get coded for the purpose of billing? There was a tremendous amount of confusion over that, with the initial verdict being that Medicare wanted the place of service changed to “02” and that private insurers want one of two modifiers, and it was anyone’s guess which company wanted which modifier. Then there was the concern that Medicare was paying 25% less, until the MPS staff clarified that full fees would be paid, but the place of service should be filled in as “11” – not “02” – as with regular office visits, and the modifier “95” should be added on the Health Care Finance Administration claim form. We were left to wait and see what gets reimbursed and for what fees.
Could new patients be seen by videoconferencing? Could patients from other states be seen this way if the psychiatrist was not licensed in the state where the patient was calling from? One psychiatrist reported he had a patient in an adjacent state drive over the border into Maryland, but the patient brought her mother and the evaluation included unwanted input from the mom as the session consisted of the patient and her mother yelling at both each other in the car and at the psychiatrist on the screen!
Psychiatrists on the listserv began to comment that treatment sessions were intense and exhausting. I feel the literal face-to-face contact of another person’s head just inches from my own, with full eye contact, often gets to be a lot. No one asks why I’ve moved a trinket (ah, there are no trinkets) or gazes off around the room. I sometimes sit for long periods of time as I don’t even stand to see the patients to the door. Other patients move about or bounce their devices on their laps, and my stomach starts to feel queasy until I ask to have the device adjusted. In some sessions, I find I’m talking to partial heads, or that computer icons cover the patient’s mouth.
Being in people’s lives via screen has been interesting. Unlike my colleague, I have not had any streaking spouses, but I’ve greeted a few family members – often those serving as technical support – and I’ve toured part of a farm, met dogs, guinea pigs, and even a goat. I’ve made brief daily “visits” to a frightened patient in isolation on a COVID hospital unit and had the joy of celebrating the discharge to home. It’s odd to be in a bedroom with a patient, even virtually, and it is interesting to note where they choose to hold their sessions; I’ve had several patients hold sessions from their cars. Seeing my own image in the corner of the screen is also a bit distracting, and in one session, as I saw my own reaction, my patient said, “I knew you were going to make that face!”
The pandemic has usurped most of the activities of all of our lives, and without social interactions, travel, and work in the usual way, life does not hold its usual richness. In a few cases, I have ended the session after half the time as the patient insisted there was nothing to talk about. Many talk about the medical problems they can’t be seen for, what they are doing to keep safe (or not), how they are washing down their groceries, and who they are meeting with by Zoom. Of those who were terribly anxious before, some feel oddly calmer – the world has ramped up to meet their level of anxiety and they feel vindicated. No one thinks they are odd for worrying about germs on door knobs or elevator buttons. What were once neurotic fears are now our real-life reality. Others have been triggered by a paralyzing fear, often with panic attacks, and these sessions are certainly challenging as I figure out which medications will best help, while responding to requests for reassurance. And there is the troublesome aspect of trying to care for others who are fearful while living with the reality that these fears are not extraneous to our own lives: We, too, are scared for ourselves and our families.
For some people, stay-at-home mandates have been easier than for others. People who are naturally introverted, or those with social anxiety, have told me they find this time at home to be a relief. They no longer feel pressured to go out; there is permission to be alone, to read, or watch Netflix. No one is pressuring them to go to parties or look for a Tinder date. For others, the isolation and loneliness have been devastating, causing a range of emotions from being “stir crazy,” to triggering episodes of major depression and severe anxiety.
Health care workers in therapy talk about their fears of being contaminated with coronavirus, about the exposures they’ve had, their fears of bringing the virus home to family, and about the anger – sometimes rage – that their employers are not doing more to protect them.
Few people these past weeks are looking for insight into their patterns of behavior and emotion. Most of life has come to be about survival and not about personal striving. Students who are driven to excel are disappointed to have their scholastic worlds have switched to pass/fail. And for those struggling with milder forms of depression and anxiety, both the patients and I have all been a bit perplexed by losing the usual measures of what feelings are normal in a tragic world and we no longer use socializing as the hallmark that heralds a return to normalcy after a period of withdrawal.
In some aspects, it is not all been bad. I’ve enjoyed watching my neighbors walk by with their dogs through the window behind my computer screen and I’ve felt part of the daily evolution as the cherry tree outside that same window turns from dead brown wood to vibrant pink blossoms. I like the flexibility of my schedule and the sensation I always carry of being rushed has quelled. I take more walks and spend more time with the family members who are held captive with me. The dog, who no longer is left alone for hours each day, is certainly a winner.
Some of my colleagues tell me they are overwhelmed – patients they have not seen for years have returned, people are asking for more frequent sessions, and they are suddenly trying to work at home while homeschooling children. I have had only a few of those requests for crisis care, while new referrals are much quieter than normal. Some of my patients have even said that they simply aren’t comfortable meeting this way and they will see me at the other end of the pandemic. A few people I would have expected to hear from I have not, and I fear that those who have lost their jobs may avoiding the cost of treatment – this group I will reach out to in the coming weeks. A little extra time, however, has given me the opportunity to join the Johns Hopkins COVID-19 Mental Health team. And my first attempt at teaching a resident seminar by Zoom has gone well.
For some in the medical field, this has been a horrible and traumatic time; they are worked to exhaustion, and surrounded by distress, death, and personal fear with every shift. For others, life has come to a standstill as the elective procedures that fill their days have virtually stopped. For outpatient psychiatry, it’s been a bit of an in-between, we may feel an odd mix of relevant and useless all at the same time, as our services are appreciated by our patients, but as actual soldiers caring for the ill COVID patients, we are leaving that to our colleagues in the EDs, COVID units, and ICUs. As a physician who has not treated a patient in an ICU for decades, I wish I had something more concrete to contribute to the effort, and at the same time, I’m relieved that I don’t.
And what about the patients? How are they doing with remote psychiatry? Some are clearly flustered or frustrated by the technology issues. Other times sessions go smoothly, and the fact that we are talking through screens gets forgotten. Some like the convenience of not having to drive a far distance and no one misses my crowded parking lot.
Kristen, another doctor’s patient in Illinois, commented: “I appreciate the continuity in care, especially if the alternative is delaying appointments. I think that’s most important. The interaction helps manage added anxiety from isolating as well. I don’t think it diminishes the care I receive; it makes me feel that my doctor is still accessible. One other point, since I have had both telemedicine and in-person appointments with my current psychiatrist, is that during in-person meetings, he is usually on his computer and rarely looks at me or makes eye contact. In virtual meetings, I feel he is much more engaged with me.”
In normal times, I spend a good deal of time encouraging patients to work on building their relationships and community – these connections lead people to healthy and fulfilling lives – and now we talk about how to best be socially distant. We see each other as vectors of disease and to greet a friend with a handshake, much less a hug, would be unthinkable. Will our collective psyches ever recover? For those of us who will survive, that remains to be seen. In the meantime, perhaps we are all being forced to be more flexible and innovative.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
It seems that some glitches would be inevitable. With a sudden shift to videoconferencing in private psychiatric practices, there were bound to be issues with both technology and privacy. One friend told me of such a glitch on the very first day she started telemental health: She was meeting with a patient who was sitting at her kitchen table. Unbeknownst to the patient, her husband walked into the kitchen behind her, fully naked, to get something from the refrigerator. “There was a full moon shot!” my friend said, initially quite shocked, and then eventually amused. As we all cope with a national tragedy and the total upheaval to our personal and professional lives, the stories just keep coming.
I left work on Friday, March 13, with plans to return on the following Monday to see patients. I had no idea that, by Sunday evening, I would be persuaded that for the safety of all I would need to shut down my real-life psychiatric practice and switch to a videoconferencing venue. I, along with many psychiatrists in Maryland, made this decision after Amy Huberman, MD, posted the following on the Maryland Psychiatric Society (MPS) listserv on Sunday, March 15:
“I want to make a case for starting video sessions with all your patients NOW. There is increasing evidence that the spread of coronavirus is driven primarily by asymptomatic or mildly ill people infected with the virus. Because of this, it’s not good enough to tell your patients not to come in if they have symptoms, or for you not to come into work if you have no symptoms. Even after I sent out a letter two weeks ago warning people not to come in if they had symptoms or had potentially come in contact with someone with COVID-19, several patients with coughs still came to my office, as well as several people who had just been on trips to New York City.
If we want to help slow the spread of this illness so that our health system has a better chance of being able to offer ventilators to the people who need them, we must limit all contacts as much as possible – even of asymptomatic people, given the emerging data.
I am planning to send out a message to all my patients today that they should do the same. Without the president or the media giving clear advice to people about what to do, it’s our job as physicians to do it.”
By that night, I had set up a home office with a blank wall behind me, windows in front of me, and books propping my computer at a height that would not have my patients looking up my nose. For the first time in over 20 years, I dusted my son’s Little League trophies, moved them and a 40,000 baseball card collection against the wall, carried a desk, chair, rug, houseplant, and a small Buddha into a room in which I would have some privacy, and my telepsychiatry practice found a home.
After some research, I registered for a free site called Doxy.me because it was HIPAA compliant and did not require patients to download an application; anyone with a camera on any Internet-enabled phone, computer, or tablet, could click on a link and enter my virtual waiting room. I soon discovered that images on the Doxy.me site are sometimes grainy and sometimes freeze up; in some sessions, we ended up switching to FaceTime, and as government mandates for HIPAA compliance relaxed, I offered to meet on any site that my patients might be comfortable with: if not Doxy.me (which remains my starting place for most sessions), Facetime, Skype, Zoom, or Whatsapp. I have not offered Bluejeans, Google Hangouts, or WebEx, and no one has requested those applications. I keep the phone next to the computer, and some sessions include a few minutes of tech support as I help patients (or they help me) navigate the various sites. In a few sessions, we could not get the audio to work and we used video on one venue while we talked on the phone. I haven’t figured out if the variations in the quality of the connection has to do with my Comcast connection, the fact that these websites are overloaded with users, or that my household now consists of three people, two large monitors, three laptops, two tablets, three cell phone lines (not to mention one dog and a transplanted cat), all going at the same time. The pets do not require any bandwidth, but all the people are talking to screens throughout the workday.
As my colleagues embarked on the same journey, the listserv questions and comments came quickly. What were the best platforms? Was it a good thing or a bad thing to suddenly be in people’s homes? Some felt the extraneous background to be helpful, others found it distracting and intrusive.
How do these sessions get coded for the purpose of billing? There was a tremendous amount of confusion over that, with the initial verdict being that Medicare wanted the place of service changed to “02” and that private insurers want one of two modifiers, and it was anyone’s guess which company wanted which modifier. Then there was the concern that Medicare was paying 25% less, until the MPS staff clarified that full fees would be paid, but the place of service should be filled in as “11” – not “02” – as with regular office visits, and the modifier “95” should be added on the Health Care Finance Administration claim form. We were left to wait and see what gets reimbursed and for what fees.
Could new patients be seen by videoconferencing? Could patients from other states be seen this way if the psychiatrist was not licensed in the state where the patient was calling from? One psychiatrist reported he had a patient in an adjacent state drive over the border into Maryland, but the patient brought her mother and the evaluation included unwanted input from the mom as the session consisted of the patient and her mother yelling at both each other in the car and at the psychiatrist on the screen!
Psychiatrists on the listserv began to comment that treatment sessions were intense and exhausting. I feel the literal face-to-face contact of another person’s head just inches from my own, with full eye contact, often gets to be a lot. No one asks why I’ve moved a trinket (ah, there are no trinkets) or gazes off around the room. I sometimes sit for long periods of time as I don’t even stand to see the patients to the door. Other patients move about or bounce their devices on their laps, and my stomach starts to feel queasy until I ask to have the device adjusted. In some sessions, I find I’m talking to partial heads, or that computer icons cover the patient’s mouth.
Being in people’s lives via screen has been interesting. Unlike my colleague, I have not had any streaking spouses, but I’ve greeted a few family members – often those serving as technical support – and I’ve toured part of a farm, met dogs, guinea pigs, and even a goat. I’ve made brief daily “visits” to a frightened patient in isolation on a COVID hospital unit and had the joy of celebrating the discharge to home. It’s odd to be in a bedroom with a patient, even virtually, and it is interesting to note where they choose to hold their sessions; I’ve had several patients hold sessions from their cars. Seeing my own image in the corner of the screen is also a bit distracting, and in one session, as I saw my own reaction, my patient said, “I knew you were going to make that face!”
The pandemic has usurped most of the activities of all of our lives, and without social interactions, travel, and work in the usual way, life does not hold its usual richness. In a few cases, I have ended the session after half the time as the patient insisted there was nothing to talk about. Many talk about the medical problems they can’t be seen for, what they are doing to keep safe (or not), how they are washing down their groceries, and who they are meeting with by Zoom. Of those who were terribly anxious before, some feel oddly calmer – the world has ramped up to meet their level of anxiety and they feel vindicated. No one thinks they are odd for worrying about germs on door knobs or elevator buttons. What were once neurotic fears are now our real-life reality. Others have been triggered by a paralyzing fear, often with panic attacks, and these sessions are certainly challenging as I figure out which medications will best help, while responding to requests for reassurance. And there is the troublesome aspect of trying to care for others who are fearful while living with the reality that these fears are not extraneous to our own lives: We, too, are scared for ourselves and our families.
For some people, stay-at-home mandates have been easier than for others. People who are naturally introverted, or those with social anxiety, have told me they find this time at home to be a relief. They no longer feel pressured to go out; there is permission to be alone, to read, or watch Netflix. No one is pressuring them to go to parties or look for a Tinder date. For others, the isolation and loneliness have been devastating, causing a range of emotions from being “stir crazy,” to triggering episodes of major depression and severe anxiety.
Health care workers in therapy talk about their fears of being contaminated with coronavirus, about the exposures they’ve had, their fears of bringing the virus home to family, and about the anger – sometimes rage – that their employers are not doing more to protect them.
Few people these past weeks are looking for insight into their patterns of behavior and emotion. Most of life has come to be about survival and not about personal striving. Students who are driven to excel are disappointed to have their scholastic worlds have switched to pass/fail. And for those struggling with milder forms of depression and anxiety, both the patients and I have all been a bit perplexed by losing the usual measures of what feelings are normal in a tragic world and we no longer use socializing as the hallmark that heralds a return to normalcy after a period of withdrawal.
In some aspects, it is not all been bad. I’ve enjoyed watching my neighbors walk by with their dogs through the window behind my computer screen and I’ve felt part of the daily evolution as the cherry tree outside that same window turns from dead brown wood to vibrant pink blossoms. I like the flexibility of my schedule and the sensation I always carry of being rushed has quelled. I take more walks and spend more time with the family members who are held captive with me. The dog, who no longer is left alone for hours each day, is certainly a winner.
Some of my colleagues tell me they are overwhelmed – patients they have not seen for years have returned, people are asking for more frequent sessions, and they are suddenly trying to work at home while homeschooling children. I have had only a few of those requests for crisis care, while new referrals are much quieter than normal. Some of my patients have even said that they simply aren’t comfortable meeting this way and they will see me at the other end of the pandemic. A few people I would have expected to hear from I have not, and I fear that those who have lost their jobs may avoiding the cost of treatment – this group I will reach out to in the coming weeks. A little extra time, however, has given me the opportunity to join the Johns Hopkins COVID-19 Mental Health team. And my first attempt at teaching a resident seminar by Zoom has gone well.
For some in the medical field, this has been a horrible and traumatic time; they are worked to exhaustion, and surrounded by distress, death, and personal fear with every shift. For others, life has come to a standstill as the elective procedures that fill their days have virtually stopped. For outpatient psychiatry, it’s been a bit of an in-between, we may feel an odd mix of relevant and useless all at the same time, as our services are appreciated by our patients, but as actual soldiers caring for the ill COVID patients, we are leaving that to our colleagues in the EDs, COVID units, and ICUs. As a physician who has not treated a patient in an ICU for decades, I wish I had something more concrete to contribute to the effort, and at the same time, I’m relieved that I don’t.
And what about the patients? How are they doing with remote psychiatry? Some are clearly flustered or frustrated by the technology issues. Other times sessions go smoothly, and the fact that we are talking through screens gets forgotten. Some like the convenience of not having to drive a far distance and no one misses my crowded parking lot.
Kristen, another doctor’s patient in Illinois, commented: “I appreciate the continuity in care, especially if the alternative is delaying appointments. I think that’s most important. The interaction helps manage added anxiety from isolating as well. I don’t think it diminishes the care I receive; it makes me feel that my doctor is still accessible. One other point, since I have had both telemedicine and in-person appointments with my current psychiatrist, is that during in-person meetings, he is usually on his computer and rarely looks at me or makes eye contact. In virtual meetings, I feel he is much more engaged with me.”
In normal times, I spend a good deal of time encouraging patients to work on building their relationships and community – these connections lead people to healthy and fulfilling lives – and now we talk about how to best be socially distant. We see each other as vectors of disease and to greet a friend with a handshake, much less a hug, would be unthinkable. Will our collective psyches ever recover? For those of us who will survive, that remains to be seen. In the meantime, perhaps we are all being forced to be more flexible and innovative.
Dr. Miller is coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, both in Baltimore.
Preoperative nivo/ipi yields high response rate in early-stage colon cancer
Preoperative nivolumab plus ipilimumab appears safe and feasible for patients with early-stage colon cancer, according to researchers.
The combination produced few grade 3/4 toxicities in a phase 2 trial. It also produced pathological responses in 100% of patients with mismatch repair-deficient (dMMR) tumors and in 27% of patients with MMR-proficient (pMMR) tumors. Myriam Chalabi, MD, of the Netherlands Cancer Institute in Amsterdam, and colleagues reported these results in Nature Medicine.
The open-label, exploratory trial included 40 patients with resectable, early-stage colon adenocarcinoma, 21 of whom had dMMR tumors and 20 of whom had pMMR tumors (1 patient had both tumor types). The patients underwent surgery within 6 weeks of study enrollment.
Prior to surgery, patients received ipilimumab (1 mg/kg) on day 1 plus nivolumab (3 mg/kg) on days 1 and 15. Those with pMMR tumors also received celecoxib (200 mg) from day 1 until the day leading up to surgery. The primary endpoints were safety and feasibility. Efficacy was evaluated using histopathological response.Grade 3-4 treatment‐related adverse events (AEs) were reported in 5 patients (13%). These AEs included rash, colitis, and asymptomatic rises in laboratory parameters. Grade 3 surgery-related AEs occurred in 8 patients (20%).
“This treatment is both safe and feasible, with few treatment-related AEs and without compromising surgery,” the authors wrote.
There were 35 patients evaluable for response. Among patients with dMMR tumors, pathological responses occurred in 100% (n = 20), major pathological responses (<10% residual vital tumor) occurred in 95% (n = 19), and complete responses occurred in 60% (n = 12).
Among patients with pMMR tumors, 27% (n = 4) had a pathological response, 20% (n = 3) had a major pathological response, and 7% (n = 1) had a partial response.
The researchers acknowledged that the small sample size and short duration of postoperative follow-up were key limitations of this study, but they said this combination should be studied further.
“Neoadjuvant immunotherapy in early-stage colon cancers warrants further research and, when validated in larger studies with longer follow-up, may become a new standard of care in dMMR and possibly a subgroup of pMMR colon cancers,” the authors concluded.
This study was sponsored by the Netherlands Cancer Institute in collaboration with Bristol-Myers Squibb. Authors reported financial relationships with Bristol-Myers Squibb and many other companies.
SOURCE: Chalabi M et al. Nat Med. 2020 Apr 6. doi: 10.1038/s41591-020-0805-8.
Preoperative nivolumab plus ipilimumab appears safe and feasible for patients with early-stage colon cancer, according to researchers.
The combination produced few grade 3/4 toxicities in a phase 2 trial. It also produced pathological responses in 100% of patients with mismatch repair-deficient (dMMR) tumors and in 27% of patients with MMR-proficient (pMMR) tumors. Myriam Chalabi, MD, of the Netherlands Cancer Institute in Amsterdam, and colleagues reported these results in Nature Medicine.
The open-label, exploratory trial included 40 patients with resectable, early-stage colon adenocarcinoma, 21 of whom had dMMR tumors and 20 of whom had pMMR tumors (1 patient had both tumor types). The patients underwent surgery within 6 weeks of study enrollment.
Prior to surgery, patients received ipilimumab (1 mg/kg) on day 1 plus nivolumab (3 mg/kg) on days 1 and 15. Those with pMMR tumors also received celecoxib (200 mg) from day 1 until the day leading up to surgery. The primary endpoints were safety and feasibility. Efficacy was evaluated using histopathological response.Grade 3-4 treatment‐related adverse events (AEs) were reported in 5 patients (13%). These AEs included rash, colitis, and asymptomatic rises in laboratory parameters. Grade 3 surgery-related AEs occurred in 8 patients (20%).
“This treatment is both safe and feasible, with few treatment-related AEs and without compromising surgery,” the authors wrote.
There were 35 patients evaluable for response. Among patients with dMMR tumors, pathological responses occurred in 100% (n = 20), major pathological responses (<10% residual vital tumor) occurred in 95% (n = 19), and complete responses occurred in 60% (n = 12).
Among patients with pMMR tumors, 27% (n = 4) had a pathological response, 20% (n = 3) had a major pathological response, and 7% (n = 1) had a partial response.
The researchers acknowledged that the small sample size and short duration of postoperative follow-up were key limitations of this study, but they said this combination should be studied further.
“Neoadjuvant immunotherapy in early-stage colon cancers warrants further research and, when validated in larger studies with longer follow-up, may become a new standard of care in dMMR and possibly a subgroup of pMMR colon cancers,” the authors concluded.
This study was sponsored by the Netherlands Cancer Institute in collaboration with Bristol-Myers Squibb. Authors reported financial relationships with Bristol-Myers Squibb and many other companies.
SOURCE: Chalabi M et al. Nat Med. 2020 Apr 6. doi: 10.1038/s41591-020-0805-8.
Preoperative nivolumab plus ipilimumab appears safe and feasible for patients with early-stage colon cancer, according to researchers.
The combination produced few grade 3/4 toxicities in a phase 2 trial. It also produced pathological responses in 100% of patients with mismatch repair-deficient (dMMR) tumors and in 27% of patients with MMR-proficient (pMMR) tumors. Myriam Chalabi, MD, of the Netherlands Cancer Institute in Amsterdam, and colleagues reported these results in Nature Medicine.
The open-label, exploratory trial included 40 patients with resectable, early-stage colon adenocarcinoma, 21 of whom had dMMR tumors and 20 of whom had pMMR tumors (1 patient had both tumor types). The patients underwent surgery within 6 weeks of study enrollment.
Prior to surgery, patients received ipilimumab (1 mg/kg) on day 1 plus nivolumab (3 mg/kg) on days 1 and 15. Those with pMMR tumors also received celecoxib (200 mg) from day 1 until the day leading up to surgery. The primary endpoints were safety and feasibility. Efficacy was evaluated using histopathological response.Grade 3-4 treatment‐related adverse events (AEs) were reported in 5 patients (13%). These AEs included rash, colitis, and asymptomatic rises in laboratory parameters. Grade 3 surgery-related AEs occurred in 8 patients (20%).
“This treatment is both safe and feasible, with few treatment-related AEs and without compromising surgery,” the authors wrote.
There were 35 patients evaluable for response. Among patients with dMMR tumors, pathological responses occurred in 100% (n = 20), major pathological responses (<10% residual vital tumor) occurred in 95% (n = 19), and complete responses occurred in 60% (n = 12).
Among patients with pMMR tumors, 27% (n = 4) had a pathological response, 20% (n = 3) had a major pathological response, and 7% (n = 1) had a partial response.
The researchers acknowledged that the small sample size and short duration of postoperative follow-up were key limitations of this study, but they said this combination should be studied further.
“Neoadjuvant immunotherapy in early-stage colon cancers warrants further research and, when validated in larger studies with longer follow-up, may become a new standard of care in dMMR and possibly a subgroup of pMMR colon cancers,” the authors concluded.
This study was sponsored by the Netherlands Cancer Institute in collaboration with Bristol-Myers Squibb. Authors reported financial relationships with Bristol-Myers Squibb and many other companies.
SOURCE: Chalabi M et al. Nat Med. 2020 Apr 6. doi: 10.1038/s41591-020-0805-8.
FROM NATURE MEDICINE
A case of neutrophilic eccrine hidradenitis attributed to HIV treatment
arising in an affected patient, Jessica Kalen, MD, advised during a virtual meeting held by the George Washington University department of dermatology.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
In a presentation entitled, “When HAART [highly active antiretroviral therapy] Hurts,” Dr. Kalen, a dermatology resident at the university, presented a case report involving a 65-year-old man who presented with juicy red edematous papules and plaques on his scalp and ears. He was on the three-drug combination of rilpivirine (a non-nucleoside reverse transcriptase inhibitor), and the NRTIs tenofovir, and emtricitabine (Odefsey) for treatment of HIV infection, which was well controlled, with no detectable viral load.
The patient was also on insulin detemir for diabetes; pravastatin, amlodipine, and lisinopril for hypertension; and episodic acyclovir for recurrent herpes simplex outbreaks. However, none of those drugs has been associated with NEH. In contrast, Dr. Kalen found three published case reports describing a link between NRTIs and NEH.
Lesional biopsy of her patient showed the classic features of NEH: a dermal neutrophilic infiltrate surrounding the eccrine secretory coils and ducts, with vacuolar degeneration that spared the acrosyringium.
The most common causes of NEH, a rare dermatologic disorder first described in 1982, are hematologic malignancies and some of the chemotherapeutic agents used in treating them. Particularly prominent are acute myelogenous leukemia and cytarabine, which are often prescribed for that cancer. Carbamazepine, granulocyte-colony stimulating factor, and BRAF inhibitors have also been associated with NEH.
The pathogenesis of NEH is not fully worked out; however, NRTIs are secreted via eccrine structures, and that close contact could potentially promote an environment favoring inflammation and destruction of the eccrine coils. Also, NRTIs inhibit DNA polymerase, as does cytarabine, Dr. Kalen noted.
Her patient’s NEH was treated with triamcinolone. His skin condition resolved completely while he remained on NRTI therapy, with no relapses to date.
Dr. Kalen reported having no financial conflicts regarding her presentation.
arising in an affected patient, Jessica Kalen, MD, advised during a virtual meeting held by the George Washington University department of dermatology.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
In a presentation entitled, “When HAART [highly active antiretroviral therapy] Hurts,” Dr. Kalen, a dermatology resident at the university, presented a case report involving a 65-year-old man who presented with juicy red edematous papules and plaques on his scalp and ears. He was on the three-drug combination of rilpivirine (a non-nucleoside reverse transcriptase inhibitor), and the NRTIs tenofovir, and emtricitabine (Odefsey) for treatment of HIV infection, which was well controlled, with no detectable viral load.
The patient was also on insulin detemir for diabetes; pravastatin, amlodipine, and lisinopril for hypertension; and episodic acyclovir for recurrent herpes simplex outbreaks. However, none of those drugs has been associated with NEH. In contrast, Dr. Kalen found three published case reports describing a link between NRTIs and NEH.
Lesional biopsy of her patient showed the classic features of NEH: a dermal neutrophilic infiltrate surrounding the eccrine secretory coils and ducts, with vacuolar degeneration that spared the acrosyringium.
The most common causes of NEH, a rare dermatologic disorder first described in 1982, are hematologic malignancies and some of the chemotherapeutic agents used in treating them. Particularly prominent are acute myelogenous leukemia and cytarabine, which are often prescribed for that cancer. Carbamazepine, granulocyte-colony stimulating factor, and BRAF inhibitors have also been associated with NEH.
The pathogenesis of NEH is not fully worked out; however, NRTIs are secreted via eccrine structures, and that close contact could potentially promote an environment favoring inflammation and destruction of the eccrine coils. Also, NRTIs inhibit DNA polymerase, as does cytarabine, Dr. Kalen noted.
Her patient’s NEH was treated with triamcinolone. His skin condition resolved completely while he remained on NRTI therapy, with no relapses to date.
Dr. Kalen reported having no financial conflicts regarding her presentation.
arising in an affected patient, Jessica Kalen, MD, advised during a virtual meeting held by the George Washington University department of dermatology.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
In a presentation entitled, “When HAART [highly active antiretroviral therapy] Hurts,” Dr. Kalen, a dermatology resident at the university, presented a case report involving a 65-year-old man who presented with juicy red edematous papules and plaques on his scalp and ears. He was on the three-drug combination of rilpivirine (a non-nucleoside reverse transcriptase inhibitor), and the NRTIs tenofovir, and emtricitabine (Odefsey) for treatment of HIV infection, which was well controlled, with no detectable viral load.
The patient was also on insulin detemir for diabetes; pravastatin, amlodipine, and lisinopril for hypertension; and episodic acyclovir for recurrent herpes simplex outbreaks. However, none of those drugs has been associated with NEH. In contrast, Dr. Kalen found three published case reports describing a link between NRTIs and NEH.
Lesional biopsy of her patient showed the classic features of NEH: a dermal neutrophilic infiltrate surrounding the eccrine secretory coils and ducts, with vacuolar degeneration that spared the acrosyringium.
The most common causes of NEH, a rare dermatologic disorder first described in 1982, are hematologic malignancies and some of the chemotherapeutic agents used in treating them. Particularly prominent are acute myelogenous leukemia and cytarabine, which are often prescribed for that cancer. Carbamazepine, granulocyte-colony stimulating factor, and BRAF inhibitors have also been associated with NEH.
The pathogenesis of NEH is not fully worked out; however, NRTIs are secreted via eccrine structures, and that close contact could potentially promote an environment favoring inflammation and destruction of the eccrine coils. Also, NRTIs inhibit DNA polymerase, as does cytarabine, Dr. Kalen noted.
Her patient’s NEH was treated with triamcinolone. His skin condition resolved completely while he remained on NRTI therapy, with no relapses to date.
Dr. Kalen reported having no financial conflicts regarding her presentation.
Expert discusses her approach to using systemic agents in children and adolescents with severe skin disease
In the clinical opinion of Kaiane A. Habeshian, MD, dermatologists shouldn’t think twice about using systemic agents in pediatric patients with severe dermatologic diseases.
“By the time patients come to us pediatric dermatologists, they have been treated by multiple other doctors, and are frustrated,” Dr. Habeshian said during a virtual meeting held by the George Washington University department of dermatology. “Childhood eczema affects not only patients, but the whole family. For instance, if the child is not sleeping due to itch, their parents are probably not sleeping, either. Parental well-being and workplace productivity are affected, and finances are affected.”
Only a limited number of medications are Food and Drug Administration approved in pediatric patients for common dermatologic indications. These include dupilumab for atopic dermatitis (AD), etanercept and ustekinumab for psoriasis, adalimumab for hidradenitis suppurativa, and omalizumab for chronic idiopathic urticaria. “The approvals are mainly for the adolescent age group, except for etanercept, which is approved at the age of 4 years and above,” said Dr. Habeshian of the department of dermatology at Children’s National Hospital, Washington.
. “These agents are approved for other indications in infants and have many years of data to describe their use in these other conditions, although comprehensive randomized, controlled studies in pediatric patients for dermatologic conditions are lacking,” she said. “What’s in clinical trials for pediatric skin disease? There are multiple ongoing clinical studies of biologic agents in pediatric dermatology, mainly for psoriasis and also for dupilumab in younger patients, as well as a JAK [Janus kinase] inhibitor for alopecia areata.”
Dr. Habeshian noted that while some clinicians may have a knee-jerk reaction to go straight to dupilumab, which was approved in March of 2019 for adolescents with moderate to severe AD, that agent is not currently approved for the most sizable pediatric population with this condition – those under 12 years of age. “FDA approval is important in part because it helps establish safety and optimal dosing, which is often different and weight based in children,” she said. “In addition, FDA approval significantly impacts access to these newer, more expensive medications.”
Speaking from her experience treating patients in the DC/Maryland/Virginia area, Medicaid has consistently denied dupilumab coverage in children under age 12, “even in severe eczema that is suboptimally controlled with both methotrexate and cyclosporine, despite multiple levels of appeal, including letters of medical necessity and peer-to-peer evaluation,” she said. “This can vary across the country among states. However, dupilumab has been completely unattainable in those under 12 in our practice.”
When dupilumab is approved, most insurers first require step therapy with off-label agents for at least 3 months, as well as documented failure of topical corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if done). “It’s important to document an objective measure of severity at the very first visit with the SCORAD [scoring atopic dermatitis] or IGA [investigator global assessment],” she said. “Often, that is required if there is any hope for coverage. A familiarity with these requirements is often acquired through trial and error, and may change over time. This can lead to many delays in getting patients these treatments.” Additional information to consider documenting include the disease impact on quality of life, sleep, and school attendance, any hospitalizations for AD flares or secondary infections, and comorbid disease such as asthma.
Meanwhile, dupilumab is under priority review for children aged 6-11 years with moderate to severe AD, with a target action date of May 26, 2020. “It’s unclear how recent events [with the COVID-19 pandemic] will impact that, but there is something to look forward to, and give us hope for our patients,” she said.
Typically, Dr. Habeshian starts her pediatric patients with moderate to severe AD on methotrexate, which she characterized as “a time-tested, affordable, and very accessible option. It requires a little bit less monitoring upon initiation than cyclosporine, and it can be used for longer periods of time before weaning is required.”
In cases when disease is severe or intolerable, she often starts methotrexate and cyclosporine together. “I will usually start right at the 0.5 mg/kg per week rather than titrating up, because this maximizes the response and reduces the amount of blood work needed, unless they have an underlying risk factor for GI distress, or obese patients who are at increased risk for LFT [liver function test] elevation,” she noted. “Patients will note some improvement as early as 2 weeks on methotrexate, but I counsel them to expect 4-6 weeks for maximum improvement. We do not do a test dose of methotrexate at our institution. If there is a slight LFT elevation upon checking labs, ensure that the labs were done at least 4-6 days after the dose, because transient LFT dose elevations are common in 3-4 days.”
GI distress is by far the most common clinical side effect of methotrexate. “We do not do much intramuscular injection of methotrexate, so we rely a lot on folic acid, which reduces the risk of GI distress and elevated LFTs without reducing efficacy,” she said. “We recommend daily folic acid for simplicity, or folic acid 6 days per week.”
Dr. Habeshian said that many pediatric patients can swallow the 2.5 mg tablets of methotrexate “because they’re quite small, and most patients don’t have a problem taking the methotrexate when it’s crushed and mixed with food such as apple sauce or pudding. However, it is critical to discuss proper handling to avoid lung toxicity.” This includes placing the pills in a plastic bag prior to crushing, avoiding inhalation, and avoiding handling near pregnant women and pets, she noted. In addition, she said, “in adolescents, we need to consider the teratogenicity of methotrexate, as well as the possibility of alcohol consumption worsening liver complications. If I prescribe methotrexate in patients of childbearing age, I will counsel them extensively regarding the risk of fetal death and birth defects. If needed, I will start combined oral contraceptives. Ultimately, I’m willing to use these medicines safely, with significant counseling.”
When addressing the risk of methotrexate overdose, she reminds parents to store the medication in a safe place, out of the reach of children. “Patients are at the highest risk of overdose complications if they are given the medication multiple days in a row rather than a one-time, single high dose,” she said. “The literature suggests that one-time overdoses of methotrexate – deliberate or accidental – are unlikely to cause acute bone marrow suppression or hepatitis. This is probably because GI absorption of methotrexate reaches a saturation point, and the kidneys passively and actively excrete the medication at quite a rapid pace so that the methotrexate is often undetectable in the blood at 24 hours post ingestion. I do prescribe a limited supply to help prevent accidental overdoses. In part, this is because if the patient is receiving the medication daily, they’ll run out very quickly, and it will come the family’s attention and to your attention that it’s not being administered correctly.”
Another treatment option to consider for cases of moderate to severe AD is cyclosporine, “which works extremely quickly,” Dr. Habeshian said. “It is very good to rapidly control severe disease while methotrexate or other modes of treatment kick in. It’s best used as a bridge, given the risks of renal damage with long-term use. I like to limit its use to 6 months.”
Cyclosporine comes in two formulations: a modified oral formulation and a nonmodified oral formulation. The modified formulation is absorbed much better than the unmodified formulation. “We start at 5 mg/kg divided b.i.d., which is higher than the recommended dosing for dermatologic conditions in adults,” she said. “This is because children may not absorb the medication as well and may have improved renal clearance. Higher doses may be needed to achieve the desirable effect. In contrast to methotrexate, cyclosporine is available in a capsule, so it cannot be crushed.”
The choice of medication for psoriasis is generally guided by insurance step therapy requirements and is limited in the pediatric population (new guidelines on the care of pediatric psoriasis patients can be found at J Am Acad Dermatol 2020; 82[1]:161-201). In Dr. Habeshian’s experience, methotrexate is the go-to for most patients. “It treats concomitant psoriatic arthritis and can be used as monotherapy or combined with biologics,” she said. “Cyclosporine is useful for erythrodermic, pustular, and severe plaque psoriasis as a bridge. Other options include etanercept weekly in patients age 4-17 years and ustekinumab weekly dosing in patients age 12-17 years.”
Acitretin can be a useful adjunct for younger patients who are unable to obtain biologic agents. “It is most useful in widespread guttate and pustular psoriasis, but can be used be used in plaque psoriasis as well,” Dr. Habeshian said. “It is usually dosed as 0.1-1 mg/kg per day. Improvement in plaque disease is generally seen in 2-3 months of therapy, so it has a slow onset, whereas improvement in pustular psoriasis is seen within 3 weeks.” The most common side effects are dry skin and mucous membranes, while an important consideration is the potential for inducing premature bone toxicity. “It is thought that the risk is relatively low if the daily and total doses are kept low,” she said. “There is no consensus for monitoring bone health. Some clinicians will consider radiography periodically.”
Dr. Habeshian concluded her talk by noting that clinicians should give vaccinations/boosters before starting systemic therapy in young children. “The safety and efficacy of live immunization administered to children on biologics is not known,” she said. “Therefore, if live vaccination is needed, it’s generally recommended to postpone initiating biologic treatment.” The MMR and varicella vaccines are given at 12-15 months of life, with a booster at 4-6 years. The varicella vaccine should be given at least 6 weeks before starting immunosuppressive therapy, and the MMR vaccine at least 4 weeks before starting therapy.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Habeshian reported having no disclosures.
In the clinical opinion of Kaiane A. Habeshian, MD, dermatologists shouldn’t think twice about using systemic agents in pediatric patients with severe dermatologic diseases.
“By the time patients come to us pediatric dermatologists, they have been treated by multiple other doctors, and are frustrated,” Dr. Habeshian said during a virtual meeting held by the George Washington University department of dermatology. “Childhood eczema affects not only patients, but the whole family. For instance, if the child is not sleeping due to itch, their parents are probably not sleeping, either. Parental well-being and workplace productivity are affected, and finances are affected.”
Only a limited number of medications are Food and Drug Administration approved in pediatric patients for common dermatologic indications. These include dupilumab for atopic dermatitis (AD), etanercept and ustekinumab for psoriasis, adalimumab for hidradenitis suppurativa, and omalizumab for chronic idiopathic urticaria. “The approvals are mainly for the adolescent age group, except for etanercept, which is approved at the age of 4 years and above,” said Dr. Habeshian of the department of dermatology at Children’s National Hospital, Washington.
. “These agents are approved for other indications in infants and have many years of data to describe their use in these other conditions, although comprehensive randomized, controlled studies in pediatric patients for dermatologic conditions are lacking,” she said. “What’s in clinical trials for pediatric skin disease? There are multiple ongoing clinical studies of biologic agents in pediatric dermatology, mainly for psoriasis and also for dupilumab in younger patients, as well as a JAK [Janus kinase] inhibitor for alopecia areata.”
Dr. Habeshian noted that while some clinicians may have a knee-jerk reaction to go straight to dupilumab, which was approved in March of 2019 for adolescents with moderate to severe AD, that agent is not currently approved for the most sizable pediatric population with this condition – those under 12 years of age. “FDA approval is important in part because it helps establish safety and optimal dosing, which is often different and weight based in children,” she said. “In addition, FDA approval significantly impacts access to these newer, more expensive medications.”
Speaking from her experience treating patients in the DC/Maryland/Virginia area, Medicaid has consistently denied dupilumab coverage in children under age 12, “even in severe eczema that is suboptimally controlled with both methotrexate and cyclosporine, despite multiple levels of appeal, including letters of medical necessity and peer-to-peer evaluation,” she said. “This can vary across the country among states. However, dupilumab has been completely unattainable in those under 12 in our practice.”
When dupilumab is approved, most insurers first require step therapy with off-label agents for at least 3 months, as well as documented failure of topical corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if done). “It’s important to document an objective measure of severity at the very first visit with the SCORAD [scoring atopic dermatitis] or IGA [investigator global assessment],” she said. “Often, that is required if there is any hope for coverage. A familiarity with these requirements is often acquired through trial and error, and may change over time. This can lead to many delays in getting patients these treatments.” Additional information to consider documenting include the disease impact on quality of life, sleep, and school attendance, any hospitalizations for AD flares or secondary infections, and comorbid disease such as asthma.
Meanwhile, dupilumab is under priority review for children aged 6-11 years with moderate to severe AD, with a target action date of May 26, 2020. “It’s unclear how recent events [with the COVID-19 pandemic] will impact that, but there is something to look forward to, and give us hope for our patients,” she said.
Typically, Dr. Habeshian starts her pediatric patients with moderate to severe AD on methotrexate, which she characterized as “a time-tested, affordable, and very accessible option. It requires a little bit less monitoring upon initiation than cyclosporine, and it can be used for longer periods of time before weaning is required.”
In cases when disease is severe or intolerable, she often starts methotrexate and cyclosporine together. “I will usually start right at the 0.5 mg/kg per week rather than titrating up, because this maximizes the response and reduces the amount of blood work needed, unless they have an underlying risk factor for GI distress, or obese patients who are at increased risk for LFT [liver function test] elevation,” she noted. “Patients will note some improvement as early as 2 weeks on methotrexate, but I counsel them to expect 4-6 weeks for maximum improvement. We do not do a test dose of methotrexate at our institution. If there is a slight LFT elevation upon checking labs, ensure that the labs were done at least 4-6 days after the dose, because transient LFT dose elevations are common in 3-4 days.”
GI distress is by far the most common clinical side effect of methotrexate. “We do not do much intramuscular injection of methotrexate, so we rely a lot on folic acid, which reduces the risk of GI distress and elevated LFTs without reducing efficacy,” she said. “We recommend daily folic acid for simplicity, or folic acid 6 days per week.”
Dr. Habeshian said that many pediatric patients can swallow the 2.5 mg tablets of methotrexate “because they’re quite small, and most patients don’t have a problem taking the methotrexate when it’s crushed and mixed with food such as apple sauce or pudding. However, it is critical to discuss proper handling to avoid lung toxicity.” This includes placing the pills in a plastic bag prior to crushing, avoiding inhalation, and avoiding handling near pregnant women and pets, she noted. In addition, she said, “in adolescents, we need to consider the teratogenicity of methotrexate, as well as the possibility of alcohol consumption worsening liver complications. If I prescribe methotrexate in patients of childbearing age, I will counsel them extensively regarding the risk of fetal death and birth defects. If needed, I will start combined oral contraceptives. Ultimately, I’m willing to use these medicines safely, with significant counseling.”
When addressing the risk of methotrexate overdose, she reminds parents to store the medication in a safe place, out of the reach of children. “Patients are at the highest risk of overdose complications if they are given the medication multiple days in a row rather than a one-time, single high dose,” she said. “The literature suggests that one-time overdoses of methotrexate – deliberate or accidental – are unlikely to cause acute bone marrow suppression or hepatitis. This is probably because GI absorption of methotrexate reaches a saturation point, and the kidneys passively and actively excrete the medication at quite a rapid pace so that the methotrexate is often undetectable in the blood at 24 hours post ingestion. I do prescribe a limited supply to help prevent accidental overdoses. In part, this is because if the patient is receiving the medication daily, they’ll run out very quickly, and it will come the family’s attention and to your attention that it’s not being administered correctly.”
Another treatment option to consider for cases of moderate to severe AD is cyclosporine, “which works extremely quickly,” Dr. Habeshian said. “It is very good to rapidly control severe disease while methotrexate or other modes of treatment kick in. It’s best used as a bridge, given the risks of renal damage with long-term use. I like to limit its use to 6 months.”
Cyclosporine comes in two formulations: a modified oral formulation and a nonmodified oral formulation. The modified formulation is absorbed much better than the unmodified formulation. “We start at 5 mg/kg divided b.i.d., which is higher than the recommended dosing for dermatologic conditions in adults,” she said. “This is because children may not absorb the medication as well and may have improved renal clearance. Higher doses may be needed to achieve the desirable effect. In contrast to methotrexate, cyclosporine is available in a capsule, so it cannot be crushed.”
The choice of medication for psoriasis is generally guided by insurance step therapy requirements and is limited in the pediatric population (new guidelines on the care of pediatric psoriasis patients can be found at J Am Acad Dermatol 2020; 82[1]:161-201). In Dr. Habeshian’s experience, methotrexate is the go-to for most patients. “It treats concomitant psoriatic arthritis and can be used as monotherapy or combined with biologics,” she said. “Cyclosporine is useful for erythrodermic, pustular, and severe plaque psoriasis as a bridge. Other options include etanercept weekly in patients age 4-17 years and ustekinumab weekly dosing in patients age 12-17 years.”
Acitretin can be a useful adjunct for younger patients who are unable to obtain biologic agents. “It is most useful in widespread guttate and pustular psoriasis, but can be used be used in plaque psoriasis as well,” Dr. Habeshian said. “It is usually dosed as 0.1-1 mg/kg per day. Improvement in plaque disease is generally seen in 2-3 months of therapy, so it has a slow onset, whereas improvement in pustular psoriasis is seen within 3 weeks.” The most common side effects are dry skin and mucous membranes, while an important consideration is the potential for inducing premature bone toxicity. “It is thought that the risk is relatively low if the daily and total doses are kept low,” she said. “There is no consensus for monitoring bone health. Some clinicians will consider radiography periodically.”
Dr. Habeshian concluded her talk by noting that clinicians should give vaccinations/boosters before starting systemic therapy in young children. “The safety and efficacy of live immunization administered to children on biologics is not known,” she said. “Therefore, if live vaccination is needed, it’s generally recommended to postpone initiating biologic treatment.” The MMR and varicella vaccines are given at 12-15 months of life, with a booster at 4-6 years. The varicella vaccine should be given at least 6 weeks before starting immunosuppressive therapy, and the MMR vaccine at least 4 weeks before starting therapy.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Habeshian reported having no disclosures.
In the clinical opinion of Kaiane A. Habeshian, MD, dermatologists shouldn’t think twice about using systemic agents in pediatric patients with severe dermatologic diseases.
“By the time patients come to us pediatric dermatologists, they have been treated by multiple other doctors, and are frustrated,” Dr. Habeshian said during a virtual meeting held by the George Washington University department of dermatology. “Childhood eczema affects not only patients, but the whole family. For instance, if the child is not sleeping due to itch, their parents are probably not sleeping, either. Parental well-being and workplace productivity are affected, and finances are affected.”
Only a limited number of medications are Food and Drug Administration approved in pediatric patients for common dermatologic indications. These include dupilumab for atopic dermatitis (AD), etanercept and ustekinumab for psoriasis, adalimumab for hidradenitis suppurativa, and omalizumab for chronic idiopathic urticaria. “The approvals are mainly for the adolescent age group, except for etanercept, which is approved at the age of 4 years and above,” said Dr. Habeshian of the department of dermatology at Children’s National Hospital, Washington.
. “These agents are approved for other indications in infants and have many years of data to describe their use in these other conditions, although comprehensive randomized, controlled studies in pediatric patients for dermatologic conditions are lacking,” she said. “What’s in clinical trials for pediatric skin disease? There are multiple ongoing clinical studies of biologic agents in pediatric dermatology, mainly for psoriasis and also for dupilumab in younger patients, as well as a JAK [Janus kinase] inhibitor for alopecia areata.”
Dr. Habeshian noted that while some clinicians may have a knee-jerk reaction to go straight to dupilumab, which was approved in March of 2019 for adolescents with moderate to severe AD, that agent is not currently approved for the most sizable pediatric population with this condition – those under 12 years of age. “FDA approval is important in part because it helps establish safety and optimal dosing, which is often different and weight based in children,” she said. “In addition, FDA approval significantly impacts access to these newer, more expensive medications.”
Speaking from her experience treating patients in the DC/Maryland/Virginia area, Medicaid has consistently denied dupilumab coverage in children under age 12, “even in severe eczema that is suboptimally controlled with both methotrexate and cyclosporine, despite multiple levels of appeal, including letters of medical necessity and peer-to-peer evaluation,” she said. “This can vary across the country among states. However, dupilumab has been completely unattainable in those under 12 in our practice.”
When dupilumab is approved, most insurers first require step therapy with off-label agents for at least 3 months, as well as documented failure of topical corticosteroids, calcineurin inhibitors, crisaborole ointment, and phototherapy (if done). “It’s important to document an objective measure of severity at the very first visit with the SCORAD [scoring atopic dermatitis] or IGA [investigator global assessment],” she said. “Often, that is required if there is any hope for coverage. A familiarity with these requirements is often acquired through trial and error, and may change over time. This can lead to many delays in getting patients these treatments.” Additional information to consider documenting include the disease impact on quality of life, sleep, and school attendance, any hospitalizations for AD flares or secondary infections, and comorbid disease such as asthma.
Meanwhile, dupilumab is under priority review for children aged 6-11 years with moderate to severe AD, with a target action date of May 26, 2020. “It’s unclear how recent events [with the COVID-19 pandemic] will impact that, but there is something to look forward to, and give us hope for our patients,” she said.
Typically, Dr. Habeshian starts her pediatric patients with moderate to severe AD on methotrexate, which she characterized as “a time-tested, affordable, and very accessible option. It requires a little bit less monitoring upon initiation than cyclosporine, and it can be used for longer periods of time before weaning is required.”
In cases when disease is severe or intolerable, she often starts methotrexate and cyclosporine together. “I will usually start right at the 0.5 mg/kg per week rather than titrating up, because this maximizes the response and reduces the amount of blood work needed, unless they have an underlying risk factor for GI distress, or obese patients who are at increased risk for LFT [liver function test] elevation,” she noted. “Patients will note some improvement as early as 2 weeks on methotrexate, but I counsel them to expect 4-6 weeks for maximum improvement. We do not do a test dose of methotrexate at our institution. If there is a slight LFT elevation upon checking labs, ensure that the labs were done at least 4-6 days after the dose, because transient LFT dose elevations are common in 3-4 days.”
GI distress is by far the most common clinical side effect of methotrexate. “We do not do much intramuscular injection of methotrexate, so we rely a lot on folic acid, which reduces the risk of GI distress and elevated LFTs without reducing efficacy,” she said. “We recommend daily folic acid for simplicity, or folic acid 6 days per week.”
Dr. Habeshian said that many pediatric patients can swallow the 2.5 mg tablets of methotrexate “because they’re quite small, and most patients don’t have a problem taking the methotrexate when it’s crushed and mixed with food such as apple sauce or pudding. However, it is critical to discuss proper handling to avoid lung toxicity.” This includes placing the pills in a plastic bag prior to crushing, avoiding inhalation, and avoiding handling near pregnant women and pets, she noted. In addition, she said, “in adolescents, we need to consider the teratogenicity of methotrexate, as well as the possibility of alcohol consumption worsening liver complications. If I prescribe methotrexate in patients of childbearing age, I will counsel them extensively regarding the risk of fetal death and birth defects. If needed, I will start combined oral contraceptives. Ultimately, I’m willing to use these medicines safely, with significant counseling.”
When addressing the risk of methotrexate overdose, she reminds parents to store the medication in a safe place, out of the reach of children. “Patients are at the highest risk of overdose complications if they are given the medication multiple days in a row rather than a one-time, single high dose,” she said. “The literature suggests that one-time overdoses of methotrexate – deliberate or accidental – are unlikely to cause acute bone marrow suppression or hepatitis. This is probably because GI absorption of methotrexate reaches a saturation point, and the kidneys passively and actively excrete the medication at quite a rapid pace so that the methotrexate is often undetectable in the blood at 24 hours post ingestion. I do prescribe a limited supply to help prevent accidental overdoses. In part, this is because if the patient is receiving the medication daily, they’ll run out very quickly, and it will come the family’s attention and to your attention that it’s not being administered correctly.”
Another treatment option to consider for cases of moderate to severe AD is cyclosporine, “which works extremely quickly,” Dr. Habeshian said. “It is very good to rapidly control severe disease while methotrexate or other modes of treatment kick in. It’s best used as a bridge, given the risks of renal damage with long-term use. I like to limit its use to 6 months.”
Cyclosporine comes in two formulations: a modified oral formulation and a nonmodified oral formulation. The modified formulation is absorbed much better than the unmodified formulation. “We start at 5 mg/kg divided b.i.d., which is higher than the recommended dosing for dermatologic conditions in adults,” she said. “This is because children may not absorb the medication as well and may have improved renal clearance. Higher doses may be needed to achieve the desirable effect. In contrast to methotrexate, cyclosporine is available in a capsule, so it cannot be crushed.”
The choice of medication for psoriasis is generally guided by insurance step therapy requirements and is limited in the pediatric population (new guidelines on the care of pediatric psoriasis patients can be found at J Am Acad Dermatol 2020; 82[1]:161-201). In Dr. Habeshian’s experience, methotrexate is the go-to for most patients. “It treats concomitant psoriatic arthritis and can be used as monotherapy or combined with biologics,” she said. “Cyclosporine is useful for erythrodermic, pustular, and severe plaque psoriasis as a bridge. Other options include etanercept weekly in patients age 4-17 years and ustekinumab weekly dosing in patients age 12-17 years.”
Acitretin can be a useful adjunct for younger patients who are unable to obtain biologic agents. “It is most useful in widespread guttate and pustular psoriasis, but can be used be used in plaque psoriasis as well,” Dr. Habeshian said. “It is usually dosed as 0.1-1 mg/kg per day. Improvement in plaque disease is generally seen in 2-3 months of therapy, so it has a slow onset, whereas improvement in pustular psoriasis is seen within 3 weeks.” The most common side effects are dry skin and mucous membranes, while an important consideration is the potential for inducing premature bone toxicity. “It is thought that the risk is relatively low if the daily and total doses are kept low,” she said. “There is no consensus for monitoring bone health. Some clinicians will consider radiography periodically.”
Dr. Habeshian concluded her talk by noting that clinicians should give vaccinations/boosters before starting systemic therapy in young children. “The safety and efficacy of live immunization administered to children on biologics is not known,” she said. “Therefore, if live vaccination is needed, it’s generally recommended to postpone initiating biologic treatment.” The MMR and varicella vaccines are given at 12-15 months of life, with a booster at 4-6 years. The varicella vaccine should be given at least 6 weeks before starting immunosuppressive therapy, and the MMR vaccine at least 4 weeks before starting therapy.
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Habeshian reported having no disclosures.
Do ObGyns think hormonal contraception should be offered over the counter?
In their advocacy column, “OTC hormonal contraception: An important goal in the fight for reproductive justice” (January 2020), Abby L. Schultz, MD, and Megan L. Evans, MD, MPH, discussed a recent committee opinion from the American College of Obstetricians and Gynecologists (ACOG) focused on improving contraception access by offering oral contraceptive pills, progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate over the counter (OTC). The authors agreed with ACOG’s stance and offered several reasons why.
OBG Management polled readers to see their thoughts on the question of whether or not hormonal contraception should be offered OTC.

In their advocacy column, “OTC hormonal contraception: An important goal in the fight for reproductive justice” (January 2020), Abby L. Schultz, MD, and Megan L. Evans, MD, MPH, discussed a recent committee opinion from the American College of Obstetricians and Gynecologists (ACOG) focused on improving contraception access by offering oral contraceptive pills, progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate over the counter (OTC). The authors agreed with ACOG’s stance and offered several reasons why.
OBG Management polled readers to see their thoughts on the question of whether or not hormonal contraception should be offered OTC.


In their advocacy column, “OTC hormonal contraception: An important goal in the fight for reproductive justice” (January 2020), Abby L. Schultz, MD, and Megan L. Evans, MD, MPH, discussed a recent committee opinion from the American College of Obstetricians and Gynecologists (ACOG) focused on improving contraception access by offering oral contraceptive pills, progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate over the counter (OTC). The authors agreed with ACOG’s stance and offered several reasons why.
OBG Management polled readers to see their thoughts on the question of whether or not hormonal contraception should be offered OTC.


AI could identify fracture risk
A natural language processing algorithm, designed to scour emergency department records for fracture cases, has the potential to improve treatment of osteoporosis and prevent future, more severe fractures.
The approach led to a notable increase in referrals to the osteoporosis refracture prevention service at the Prince of Wales Hospital in Sydney, where the work was done.
The strongest predictor of a future fracture is a recent previous fracture, said Christopher White, MBBS, the hospital’s director of research, who presented results of an analysis at a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“We have really effective therapies that can reduce the risk of [future] fractures by 50%, and yet 80% of osteoporotic patients leave the hospital untreated after fracture,” said Dr. White.
That, he explained, is because of a fundamental disconnect in fracture care – emergency department physicians tackle the immediate aftermath of a broken bone, but they are not tasked with treating the underlying condition. As a result, many patients who would be candidates for follow-up care are not referred.
The current work grew out of Dr. White’s frustration with not being able to recruit patients for osteoporosis clinical trials. In fact, he got so annoyed trying to recruit and not getting patients referred to him – even though he’d find they were actually in the hospital – that he decided “to start an AI [artificial intelligence] program that would read the radiology report and bypass the referrer,” he said.
To that end, with the help of an industry partner, he developed a software program called XRAIT (X-Ray Artificial Intelligence Tool), which analyzed the reports and, with Dr. White’s iterated guidance, learned to identify fractures.
The system performed a little too well. “You have to be careful what you wish for, because suddenly I went from 70 referrals to 339,” he said.
That influx is a potential downside, however, according to Angela Cheung, MD, PhD, director of the Centre of Excellence in Skeletal Health Assessment and Osteoporosis Program at the University of Toronto’s University Health Network. Natural language processing can help identify patients that a human reviewer would miss, because reviewers tend to focus on cases in which the fracture was the reason for the hospital visit, rather than on incidental findings. But not all incidental findings are clinically important. “A pneumonia patient might have had the fracture 30 years ago, falling off a tree as a college student. It may not pick up the highest-risk group in terms of fractures, because we know that recency of fractures matters,” Dr. Cheung, who was not associated with the research, said in an interview.
“It means the fracture liaison coordinator would need to review [more] numbers in trying to figure out whether the patient should get attention and whether they should be treated as well,” said Dr. Cheung, adding that more studies would need to be done to determine if the approach would be cost effective.
The researchers performed a technical evaluation of 2,445 nonfracture and 433 fracture reports, in which the tool performed with more than 99% sensitivity and specificity.
In a clinical validation, a fracture clinician and XRAIT reviewed 5,089 x-ray and computed tomography reports from ED patients who were older than 50 years. The ED referred 70 cases, leading to identification of 65 fractures. The combination of ED referral and a fracture clinician’s review of 224 cases revealed 98 fracture cases. By contrast, XRAIT nearly instantaneously analyzed 5,089 reports from 3,217 patients, and identified fractures in 349 patients – a nearly fivefold higher number than the manual case finding of 70. Of those 349 patients, results for 10 were false positives, leading to a total find of 339 patients.
In all, 57 cases were found both by XRAIT and the ED referral/fracture clinician, resulting in 282 unique cases identified by XRAIT alone. That translated to a 3.5-fold increase in cases that were identifiable using XRAIT.
In an external validation, the researchers tested the system on 327 reports from a subset of the Dubbo Osteoporosis Epidemiology Study, based in the city of Dubbo in New South Wales, Australia. In that cohort, XRAIT identified 97 positive cases, of which 87 were true fractures (10 false positives). Of 230 cases that it considered not to be fractures, there were 38 false negatives. Those numbers translated to a sensitivity of 69.6% and a specificity of 95.0%.
All of those hits have the potential to overwhelm osteoporosis services. “I now have to adjust to that, and further development will be to link the AI with clinical risk factors and treatment data to assist my fracture coordinators to target the right patients. We’ll increase the number of patients with osteoporosis on treatment, improve productivity and safety, and reduce the burden of care,” said Dr. White.
The study was funded by The Sydney Partnership for Health, Education, Research and Enterprise and the Musculoskeletal Consumer Advisory Group. The researchers reported no financial conflicts of interest, as did Dr. Cheung.
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
A natural language processing algorithm, designed to scour emergency department records for fracture cases, has the potential to improve treatment of osteoporosis and prevent future, more severe fractures.
The approach led to a notable increase in referrals to the osteoporosis refracture prevention service at the Prince of Wales Hospital in Sydney, where the work was done.
The strongest predictor of a future fracture is a recent previous fracture, said Christopher White, MBBS, the hospital’s director of research, who presented results of an analysis at a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“We have really effective therapies that can reduce the risk of [future] fractures by 50%, and yet 80% of osteoporotic patients leave the hospital untreated after fracture,” said Dr. White.
That, he explained, is because of a fundamental disconnect in fracture care – emergency department physicians tackle the immediate aftermath of a broken bone, but they are not tasked with treating the underlying condition. As a result, many patients who would be candidates for follow-up care are not referred.
The current work grew out of Dr. White’s frustration with not being able to recruit patients for osteoporosis clinical trials. In fact, he got so annoyed trying to recruit and not getting patients referred to him – even though he’d find they were actually in the hospital – that he decided “to start an AI [artificial intelligence] program that would read the radiology report and bypass the referrer,” he said.
To that end, with the help of an industry partner, he developed a software program called XRAIT (X-Ray Artificial Intelligence Tool), which analyzed the reports and, with Dr. White’s iterated guidance, learned to identify fractures.
The system performed a little too well. “You have to be careful what you wish for, because suddenly I went from 70 referrals to 339,” he said.
That influx is a potential downside, however, according to Angela Cheung, MD, PhD, director of the Centre of Excellence in Skeletal Health Assessment and Osteoporosis Program at the University of Toronto’s University Health Network. Natural language processing can help identify patients that a human reviewer would miss, because reviewers tend to focus on cases in which the fracture was the reason for the hospital visit, rather than on incidental findings. But not all incidental findings are clinically important. “A pneumonia patient might have had the fracture 30 years ago, falling off a tree as a college student. It may not pick up the highest-risk group in terms of fractures, because we know that recency of fractures matters,” Dr. Cheung, who was not associated with the research, said in an interview.
“It means the fracture liaison coordinator would need to review [more] numbers in trying to figure out whether the patient should get attention and whether they should be treated as well,” said Dr. Cheung, adding that more studies would need to be done to determine if the approach would be cost effective.
The researchers performed a technical evaluation of 2,445 nonfracture and 433 fracture reports, in which the tool performed with more than 99% sensitivity and specificity.
In a clinical validation, a fracture clinician and XRAIT reviewed 5,089 x-ray and computed tomography reports from ED patients who were older than 50 years. The ED referred 70 cases, leading to identification of 65 fractures. The combination of ED referral and a fracture clinician’s review of 224 cases revealed 98 fracture cases. By contrast, XRAIT nearly instantaneously analyzed 5,089 reports from 3,217 patients, and identified fractures in 349 patients – a nearly fivefold higher number than the manual case finding of 70. Of those 349 patients, results for 10 were false positives, leading to a total find of 339 patients.
In all, 57 cases were found both by XRAIT and the ED referral/fracture clinician, resulting in 282 unique cases identified by XRAIT alone. That translated to a 3.5-fold increase in cases that were identifiable using XRAIT.
In an external validation, the researchers tested the system on 327 reports from a subset of the Dubbo Osteoporosis Epidemiology Study, based in the city of Dubbo in New South Wales, Australia. In that cohort, XRAIT identified 97 positive cases, of which 87 were true fractures (10 false positives). Of 230 cases that it considered not to be fractures, there were 38 false negatives. Those numbers translated to a sensitivity of 69.6% and a specificity of 95.0%.
All of those hits have the potential to overwhelm osteoporosis services. “I now have to adjust to that, and further development will be to link the AI with clinical risk factors and treatment data to assist my fracture coordinators to target the right patients. We’ll increase the number of patients with osteoporosis on treatment, improve productivity and safety, and reduce the burden of care,” said Dr. White.
The study was funded by The Sydney Partnership for Health, Education, Research and Enterprise and the Musculoskeletal Consumer Advisory Group. The researchers reported no financial conflicts of interest, as did Dr. Cheung.
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
A natural language processing algorithm, designed to scour emergency department records for fracture cases, has the potential to improve treatment of osteoporosis and prevent future, more severe fractures.
The approach led to a notable increase in referrals to the osteoporosis refracture prevention service at the Prince of Wales Hospital in Sydney, where the work was done.
The strongest predictor of a future fracture is a recent previous fracture, said Christopher White, MBBS, the hospital’s director of research, who presented results of an analysis at a virtual news conference held by the Endocrine Society. The study was slated for presentation during ENDO 2020, the society’s annual meeting, which was canceled because of the COVID-19 pandemic.
“We have really effective therapies that can reduce the risk of [future] fractures by 50%, and yet 80% of osteoporotic patients leave the hospital untreated after fracture,” said Dr. White.
That, he explained, is because of a fundamental disconnect in fracture care – emergency department physicians tackle the immediate aftermath of a broken bone, but they are not tasked with treating the underlying condition. As a result, many patients who would be candidates for follow-up care are not referred.
The current work grew out of Dr. White’s frustration with not being able to recruit patients for osteoporosis clinical trials. In fact, he got so annoyed trying to recruit and not getting patients referred to him – even though he’d find they were actually in the hospital – that he decided “to start an AI [artificial intelligence] program that would read the radiology report and bypass the referrer,” he said.
To that end, with the help of an industry partner, he developed a software program called XRAIT (X-Ray Artificial Intelligence Tool), which analyzed the reports and, with Dr. White’s iterated guidance, learned to identify fractures.
The system performed a little too well. “You have to be careful what you wish for, because suddenly I went from 70 referrals to 339,” he said.
That influx is a potential downside, however, according to Angela Cheung, MD, PhD, director of the Centre of Excellence in Skeletal Health Assessment and Osteoporosis Program at the University of Toronto’s University Health Network. Natural language processing can help identify patients that a human reviewer would miss, because reviewers tend to focus on cases in which the fracture was the reason for the hospital visit, rather than on incidental findings. But not all incidental findings are clinically important. “A pneumonia patient might have had the fracture 30 years ago, falling off a tree as a college student. It may not pick up the highest-risk group in terms of fractures, because we know that recency of fractures matters,” Dr. Cheung, who was not associated with the research, said in an interview.
“It means the fracture liaison coordinator would need to review [more] numbers in trying to figure out whether the patient should get attention and whether they should be treated as well,” said Dr. Cheung, adding that more studies would need to be done to determine if the approach would be cost effective.
The researchers performed a technical evaluation of 2,445 nonfracture and 433 fracture reports, in which the tool performed with more than 99% sensitivity and specificity.
In a clinical validation, a fracture clinician and XRAIT reviewed 5,089 x-ray and computed tomography reports from ED patients who were older than 50 years. The ED referred 70 cases, leading to identification of 65 fractures. The combination of ED referral and a fracture clinician’s review of 224 cases revealed 98 fracture cases. By contrast, XRAIT nearly instantaneously analyzed 5,089 reports from 3,217 patients, and identified fractures in 349 patients – a nearly fivefold higher number than the manual case finding of 70. Of those 349 patients, results for 10 were false positives, leading to a total find of 339 patients.
In all, 57 cases were found both by XRAIT and the ED referral/fracture clinician, resulting in 282 unique cases identified by XRAIT alone. That translated to a 3.5-fold increase in cases that were identifiable using XRAIT.
In an external validation, the researchers tested the system on 327 reports from a subset of the Dubbo Osteoporosis Epidemiology Study, based in the city of Dubbo in New South Wales, Australia. In that cohort, XRAIT identified 97 positive cases, of which 87 were true fractures (10 false positives). Of 230 cases that it considered not to be fractures, there were 38 false negatives. Those numbers translated to a sensitivity of 69.6% and a specificity of 95.0%.
All of those hits have the potential to overwhelm osteoporosis services. “I now have to adjust to that, and further development will be to link the AI with clinical risk factors and treatment data to assist my fracture coordinators to target the right patients. We’ll increase the number of patients with osteoporosis on treatment, improve productivity and safety, and reduce the burden of care,” said Dr. White.
The study was funded by The Sydney Partnership for Health, Education, Research and Enterprise and the Musculoskeletal Consumer Advisory Group. The researchers reported no financial conflicts of interest, as did Dr. Cheung.
The research will be published in a special supplemental issue of the Journal of the Endocrine Society. In addition to a series of news conferences on March 30-31, the society will host ENDO Online 2020 during June 8-22, which will present programming for clinicians and researchers.
FROM ENDO 2020
ASCO announces its own COVID-19 and cancer registry
Data will not be commercialized, unlike CancerLinQ
The American Society of Clinical Oncology (ASCO) has launched a registry to collect data on cancer patients with COVID-19 and is asking oncology practices across the United States to share information about their patients with the infection for educational purposes.
The new registry joins at least two other cancer and COVID-19 patient registries already underway in the U.S.
In a statement, ASCO President Howard “Skip” Burris III, MD said there is a need to know “how the virus is impacting our patients, their cancer treatment, and outcomes to inform current cancer care” and future care.
The web-based registry, known as the American Society of Clinical Oncology (ASCO) Survey on COVID-19 in Oncology Registry, is open to all U.S. oncology practices. Participating practices will receive an unspecified “nominal” payment for their data entry efforts.
The registry patient information will be stored on ASCO’s “Big Data” platform, known as CancerLinQ, but is being held apart from that pool of data. The registry information will not be available for commercial purposes, ASCO spokesperson Rachel Martin recently told Medscape Medical News.
Separately, CancerLinQ, which is a wholly owned subsidiary of ASCO, will continue to collect data from its participant oncology practices (as usual), including COVID-19 information.
CancerLinQ has been criticized by ethicists for allowing partner companies to sell access to its data (after stripping off patient identifiers), but without asking for patients’ permission, as reported last year by Medscape Medical News.
Eleven practices, including academic enterprises, have so far expressed interested in participating in the ASCO COVID-19 Registry.
Participating practices are requested to send in details about cancer patients with a confirmed COVID-19 diagnosis. As well as a baseline data capture form, they will need to provide details of subsequent status, treatment, and outcomes. Some patient-identifying data, including zip code, date of birth, gender, race, ethnicity, type of cancer, and comorbidities, will be collected for the purposes of analysis.
ASCO hopes to learn about characteristics of patients with cancer most impacted by COVID-19; estimates of disease severity; treatment modifications or delays; implementation of telemedicine in the cancer treatment setting; and clinical outcomes related to both COVID-19 and cancer.
ASCO says it will deliver periodic reports to the cancer community and the broader public on these and other “key learnings.” It also says that the registry is designed to capture point-in-time data as well as longitudinal data on how the virus will impact care and outcomes into 2021.
ASCO is not alone in its data collection efforts.
The COVID-19 and Cancer Consortium is already collecting information from more than 50 cancer centers and organizations on COVID-19 in patients with cancer. The American Society of Hematology (ASH) Research Collaborative COVID-19 Registry for Hematologic Malignancy is doing the same but with a focus on hematologic malignancies.
This article first appeared on Medscape.com.
Data will not be commercialized, unlike CancerLinQ
Data will not be commercialized, unlike CancerLinQ
The American Society of Clinical Oncology (ASCO) has launched a registry to collect data on cancer patients with COVID-19 and is asking oncology practices across the United States to share information about their patients with the infection for educational purposes.
The new registry joins at least two other cancer and COVID-19 patient registries already underway in the U.S.
In a statement, ASCO President Howard “Skip” Burris III, MD said there is a need to know “how the virus is impacting our patients, their cancer treatment, and outcomes to inform current cancer care” and future care.
The web-based registry, known as the American Society of Clinical Oncology (ASCO) Survey on COVID-19 in Oncology Registry, is open to all U.S. oncology practices. Participating practices will receive an unspecified “nominal” payment for their data entry efforts.
The registry patient information will be stored on ASCO’s “Big Data” platform, known as CancerLinQ, but is being held apart from that pool of data. The registry information will not be available for commercial purposes, ASCO spokesperson Rachel Martin recently told Medscape Medical News.
Separately, CancerLinQ, which is a wholly owned subsidiary of ASCO, will continue to collect data from its participant oncology practices (as usual), including COVID-19 information.
CancerLinQ has been criticized by ethicists for allowing partner companies to sell access to its data (after stripping off patient identifiers), but without asking for patients’ permission, as reported last year by Medscape Medical News.
Eleven practices, including academic enterprises, have so far expressed interested in participating in the ASCO COVID-19 Registry.
Participating practices are requested to send in details about cancer patients with a confirmed COVID-19 diagnosis. As well as a baseline data capture form, they will need to provide details of subsequent status, treatment, and outcomes. Some patient-identifying data, including zip code, date of birth, gender, race, ethnicity, type of cancer, and comorbidities, will be collected for the purposes of analysis.
ASCO hopes to learn about characteristics of patients with cancer most impacted by COVID-19; estimates of disease severity; treatment modifications or delays; implementation of telemedicine in the cancer treatment setting; and clinical outcomes related to both COVID-19 and cancer.
ASCO says it will deliver periodic reports to the cancer community and the broader public on these and other “key learnings.” It also says that the registry is designed to capture point-in-time data as well as longitudinal data on how the virus will impact care and outcomes into 2021.
ASCO is not alone in its data collection efforts.
The COVID-19 and Cancer Consortium is already collecting information from more than 50 cancer centers and organizations on COVID-19 in patients with cancer. The American Society of Hematology (ASH) Research Collaborative COVID-19 Registry for Hematologic Malignancy is doing the same but with a focus on hematologic malignancies.
This article first appeared on Medscape.com.
The American Society of Clinical Oncology (ASCO) has launched a registry to collect data on cancer patients with COVID-19 and is asking oncology practices across the United States to share information about their patients with the infection for educational purposes.
The new registry joins at least two other cancer and COVID-19 patient registries already underway in the U.S.
In a statement, ASCO President Howard “Skip” Burris III, MD said there is a need to know “how the virus is impacting our patients, their cancer treatment, and outcomes to inform current cancer care” and future care.
The web-based registry, known as the American Society of Clinical Oncology (ASCO) Survey on COVID-19 in Oncology Registry, is open to all U.S. oncology practices. Participating practices will receive an unspecified “nominal” payment for their data entry efforts.
The registry patient information will be stored on ASCO’s “Big Data” platform, known as CancerLinQ, but is being held apart from that pool of data. The registry information will not be available for commercial purposes, ASCO spokesperson Rachel Martin recently told Medscape Medical News.
Separately, CancerLinQ, which is a wholly owned subsidiary of ASCO, will continue to collect data from its participant oncology practices (as usual), including COVID-19 information.
CancerLinQ has been criticized by ethicists for allowing partner companies to sell access to its data (after stripping off patient identifiers), but without asking for patients’ permission, as reported last year by Medscape Medical News.
Eleven practices, including academic enterprises, have so far expressed interested in participating in the ASCO COVID-19 Registry.
Participating practices are requested to send in details about cancer patients with a confirmed COVID-19 diagnosis. As well as a baseline data capture form, they will need to provide details of subsequent status, treatment, and outcomes. Some patient-identifying data, including zip code, date of birth, gender, race, ethnicity, type of cancer, and comorbidities, will be collected for the purposes of analysis.
ASCO hopes to learn about characteristics of patients with cancer most impacted by COVID-19; estimates of disease severity; treatment modifications or delays; implementation of telemedicine in the cancer treatment setting; and clinical outcomes related to both COVID-19 and cancer.
ASCO says it will deliver periodic reports to the cancer community and the broader public on these and other “key learnings.” It also says that the registry is designed to capture point-in-time data as well as longitudinal data on how the virus will impact care and outcomes into 2021.
ASCO is not alone in its data collection efforts.
The COVID-19 and Cancer Consortium is already collecting information from more than 50 cancer centers and organizations on COVID-19 in patients with cancer. The American Society of Hematology (ASH) Research Collaborative COVID-19 Registry for Hematologic Malignancy is doing the same but with a focus on hematologic malignancies.
This article first appeared on Medscape.com.
TWILIGHT-COMPLEX: Tap ticagrelor monotherapy early after complex PCI
Patients who underwent complex PCI for acute coronary syndrome followed by 3 months of dual-antiplatelet therapy (DAPT) with ticagrelor plus aspirin fared significantly better by dropping aspirin at that point in favor of long-term ticagrelor monotherapy than with continued dual-antiplatelet therapy in the TWILIGHT-COMPLEX study.
The rate of clinically relevant bleeding was significantly lower at 12 months of follow-up in the ticagrelor monotherapy group than it was in patients randomized to continued DAPT. Moreover, this major benefit came at no cost in terms of ischemic events, which were actually numerically less frequent in the ticagrelor plus placebo group, George D. Dangas, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“We found that the aspirin just doesn’t add that much, even in complex patients – just bleeding complications, for the most part,” explained Dr. Dangas, professor of medicine and of surgery at the Icahn School of Medicine at Mount Sinai, New York.
The TWILIGHT-COMPLEX study was a secondary post hoc analysis of outcomes in 2,342 participants in the previously reported larger parent TWILIGHT randomized trial who underwent complex PCI. The main TWILIGHT trial included 7,119 patients in 11 countries who underwent PCI for acute coronary syndrome, successfully completed 3 months of DAPT with ticagrelor plus aspirin without incident, and were then randomized double blind to 12 months of ticagrelor plus placebo or to another 12 months of ticagrelor and aspirin.
In the overall TWILIGHT trial, ticagrelor alone resulted in a significantly lower clinically relevant bleeding rate than did long-term ticagrelor plus aspirin, with no increase in the risk of death, MI, or stroke (N Engl J Med 2019; 381:2032-42). But the results left many interventional cardiologists wondering if a ticagrelor monotherapy strategy was really applicable to their more challenging patients undergoing complex PCI given that the risk of ischemic events is known to climb with PCI complexity. The TWILIGHT-COMPLEX study was specifically designed to address that concern.
To be eligible for TWILIGHT-COMPLEX, patients had to meet one or more prespecified angiographic or procedural criteria for complex PCI, such as a total stent length in excess of 60 mm, three or more treated lesions, use of an atherectomy device, or PCI of a left main lesion, a chronic total occlusion, or a bifurcation lesion with two stents. These complex PCI patients accounted for one-third of the total study population in TWILIGHT; 36% of them met more than one criteria for complex PCI.
TWILIGHT-COMPLEX findings
In the 12 months after randomization, patients who received ticagrelor plus placebo had a 4.2% incidence of clinically significant Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding, which was significantly lower than the 7.7% rate in the group on long-term DAPT and represented a 46% relative risk reduction. Severe or fatal bleeding – that is, BARC type 3 or 5 – occurred in 1.1% of those on ticagrelor monotherapy and 2.6% of the DAPT group, for a significant 59% relative risk reduction.
The composite ischemic endpoint comprising cardiovascular death, MI, or ischemic stroke occurred in 3.6% of the ticagrelor monotherapy group and 4.8% of patients on long-term DAPT, a trend that didn’t achieve statistical significance. The all-cause mortality rate was 0.9% in the ticagrelor monotherapy group and 1.5% with extended DAPT, again a nonsignificant difference. Similarly, the rate of definite or probable stent thrombosis was numerically lower with ticagrelor monotherapy, by a margin of 0.4% versus 0.8%, a nonsignificant difference.
The results were consistent regardless of which specific criteria for complex PCI a patient had or how many of them.
Results are ‘reassuring’
At a press conference where Dr. Dangas presented the TWILIGHT-COMPLEX results, discussant Claire S. Duvernoy, MD, said she was “very impressed” with just how complex the PCIs were in the study participants.
“Really, these are the patients that in my own practice we’ve always been the most cautious about, the most worried about thrombotic risk, and the ones where we get down on our house staff when they drop an antiplatelet agent. So this study is very reassuring,” said Dr. Duvernoy, professor of medicine at the University of Michigan, Ann Arbor.
She identified two key differences between TWILIGHT-COMPLEX and earlier studies that showed a benefit for extended DAPT in higher-risk patients. In the earlier studies, it was the P2Y12 inhibitor that was dropped; TWILIGHT was the first major randomized trial to discontinue the aspirin instead. And patients in the TWILIGHT study received second-generation drug-eluting stents.
“That makes a huge difference,” Dr. Duvernoy said. “We have stents now that are much safer than the old ones were, and that’s what allows us to gain this incredible benefit of reduced bleeding.”
Dr. Dangas cautioned that since this was a secondary post hoc analysis, the TWILIGHT-COMPLEX study must be viewed as hypothesis-generating.
The TWILIGHT trial was funded by AstraZeneca. Dr. Dangas reported receiving institutional research grants from that company as well as Bayer and Daichi-Sankyo. He also served as a paid consultant to Abbott Vascular, Boston Scientific, and Biosensors.
Simultaneous with his presentation at ACC 2020, the TWILIGHT-COMPLEX results were published online (J Am Coll Cardiol. 2020 Mar 13. doi: 10.1016/j.jacc.2020.03.011).
SOURCE: Dangas GD. ACC 20, Abstract 410-09.
Patients who underwent complex PCI for acute coronary syndrome followed by 3 months of dual-antiplatelet therapy (DAPT) with ticagrelor plus aspirin fared significantly better by dropping aspirin at that point in favor of long-term ticagrelor monotherapy than with continued dual-antiplatelet therapy in the TWILIGHT-COMPLEX study.
The rate of clinically relevant bleeding was significantly lower at 12 months of follow-up in the ticagrelor monotherapy group than it was in patients randomized to continued DAPT. Moreover, this major benefit came at no cost in terms of ischemic events, which were actually numerically less frequent in the ticagrelor plus placebo group, George D. Dangas, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“We found that the aspirin just doesn’t add that much, even in complex patients – just bleeding complications, for the most part,” explained Dr. Dangas, professor of medicine and of surgery at the Icahn School of Medicine at Mount Sinai, New York.
The TWILIGHT-COMPLEX study was a secondary post hoc analysis of outcomes in 2,342 participants in the previously reported larger parent TWILIGHT randomized trial who underwent complex PCI. The main TWILIGHT trial included 7,119 patients in 11 countries who underwent PCI for acute coronary syndrome, successfully completed 3 months of DAPT with ticagrelor plus aspirin without incident, and were then randomized double blind to 12 months of ticagrelor plus placebo or to another 12 months of ticagrelor and aspirin.
In the overall TWILIGHT trial, ticagrelor alone resulted in a significantly lower clinically relevant bleeding rate than did long-term ticagrelor plus aspirin, with no increase in the risk of death, MI, or stroke (N Engl J Med 2019; 381:2032-42). But the results left many interventional cardiologists wondering if a ticagrelor monotherapy strategy was really applicable to their more challenging patients undergoing complex PCI given that the risk of ischemic events is known to climb with PCI complexity. The TWILIGHT-COMPLEX study was specifically designed to address that concern.
To be eligible for TWILIGHT-COMPLEX, patients had to meet one or more prespecified angiographic or procedural criteria for complex PCI, such as a total stent length in excess of 60 mm, three or more treated lesions, use of an atherectomy device, or PCI of a left main lesion, a chronic total occlusion, or a bifurcation lesion with two stents. These complex PCI patients accounted for one-third of the total study population in TWILIGHT; 36% of them met more than one criteria for complex PCI.
TWILIGHT-COMPLEX findings
In the 12 months after randomization, patients who received ticagrelor plus placebo had a 4.2% incidence of clinically significant Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding, which was significantly lower than the 7.7% rate in the group on long-term DAPT and represented a 46% relative risk reduction. Severe or fatal bleeding – that is, BARC type 3 or 5 – occurred in 1.1% of those on ticagrelor monotherapy and 2.6% of the DAPT group, for a significant 59% relative risk reduction.
The composite ischemic endpoint comprising cardiovascular death, MI, or ischemic stroke occurred in 3.6% of the ticagrelor monotherapy group and 4.8% of patients on long-term DAPT, a trend that didn’t achieve statistical significance. The all-cause mortality rate was 0.9% in the ticagrelor monotherapy group and 1.5% with extended DAPT, again a nonsignificant difference. Similarly, the rate of definite or probable stent thrombosis was numerically lower with ticagrelor monotherapy, by a margin of 0.4% versus 0.8%, a nonsignificant difference.
The results were consistent regardless of which specific criteria for complex PCI a patient had or how many of them.
Results are ‘reassuring’
At a press conference where Dr. Dangas presented the TWILIGHT-COMPLEX results, discussant Claire S. Duvernoy, MD, said she was “very impressed” with just how complex the PCIs were in the study participants.
“Really, these are the patients that in my own practice we’ve always been the most cautious about, the most worried about thrombotic risk, and the ones where we get down on our house staff when they drop an antiplatelet agent. So this study is very reassuring,” said Dr. Duvernoy, professor of medicine at the University of Michigan, Ann Arbor.
She identified two key differences between TWILIGHT-COMPLEX and earlier studies that showed a benefit for extended DAPT in higher-risk patients. In the earlier studies, it was the P2Y12 inhibitor that was dropped; TWILIGHT was the first major randomized trial to discontinue the aspirin instead. And patients in the TWILIGHT study received second-generation drug-eluting stents.
“That makes a huge difference,” Dr. Duvernoy said. “We have stents now that are much safer than the old ones were, and that’s what allows us to gain this incredible benefit of reduced bleeding.”
Dr. Dangas cautioned that since this was a secondary post hoc analysis, the TWILIGHT-COMPLEX study must be viewed as hypothesis-generating.
The TWILIGHT trial was funded by AstraZeneca. Dr. Dangas reported receiving institutional research grants from that company as well as Bayer and Daichi-Sankyo. He also served as a paid consultant to Abbott Vascular, Boston Scientific, and Biosensors.
Simultaneous with his presentation at ACC 2020, the TWILIGHT-COMPLEX results were published online (J Am Coll Cardiol. 2020 Mar 13. doi: 10.1016/j.jacc.2020.03.011).
SOURCE: Dangas GD. ACC 20, Abstract 410-09.
Patients who underwent complex PCI for acute coronary syndrome followed by 3 months of dual-antiplatelet therapy (DAPT) with ticagrelor plus aspirin fared significantly better by dropping aspirin at that point in favor of long-term ticagrelor monotherapy than with continued dual-antiplatelet therapy in the TWILIGHT-COMPLEX study.
The rate of clinically relevant bleeding was significantly lower at 12 months of follow-up in the ticagrelor monotherapy group than it was in patients randomized to continued DAPT. Moreover, this major benefit came at no cost in terms of ischemic events, which were actually numerically less frequent in the ticagrelor plus placebo group, George D. Dangas, MD, reported at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. ACC organizers chose to present parts of the meeting virtually after COVID-19 concerns caused them to cancel the meeting.
“We found that the aspirin just doesn’t add that much, even in complex patients – just bleeding complications, for the most part,” explained Dr. Dangas, professor of medicine and of surgery at the Icahn School of Medicine at Mount Sinai, New York.
The TWILIGHT-COMPLEX study was a secondary post hoc analysis of outcomes in 2,342 participants in the previously reported larger parent TWILIGHT randomized trial who underwent complex PCI. The main TWILIGHT trial included 7,119 patients in 11 countries who underwent PCI for acute coronary syndrome, successfully completed 3 months of DAPT with ticagrelor plus aspirin without incident, and were then randomized double blind to 12 months of ticagrelor plus placebo or to another 12 months of ticagrelor and aspirin.
In the overall TWILIGHT trial, ticagrelor alone resulted in a significantly lower clinically relevant bleeding rate than did long-term ticagrelor plus aspirin, with no increase in the risk of death, MI, or stroke (N Engl J Med 2019; 381:2032-42). But the results left many interventional cardiologists wondering if a ticagrelor monotherapy strategy was really applicable to their more challenging patients undergoing complex PCI given that the risk of ischemic events is known to climb with PCI complexity. The TWILIGHT-COMPLEX study was specifically designed to address that concern.
To be eligible for TWILIGHT-COMPLEX, patients had to meet one or more prespecified angiographic or procedural criteria for complex PCI, such as a total stent length in excess of 60 mm, three or more treated lesions, use of an atherectomy device, or PCI of a left main lesion, a chronic total occlusion, or a bifurcation lesion with two stents. These complex PCI patients accounted for one-third of the total study population in TWILIGHT; 36% of them met more than one criteria for complex PCI.
TWILIGHT-COMPLEX findings
In the 12 months after randomization, patients who received ticagrelor plus placebo had a 4.2% incidence of clinically significant Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding, which was significantly lower than the 7.7% rate in the group on long-term DAPT and represented a 46% relative risk reduction. Severe or fatal bleeding – that is, BARC type 3 or 5 – occurred in 1.1% of those on ticagrelor monotherapy and 2.6% of the DAPT group, for a significant 59% relative risk reduction.
The composite ischemic endpoint comprising cardiovascular death, MI, or ischemic stroke occurred in 3.6% of the ticagrelor monotherapy group and 4.8% of patients on long-term DAPT, a trend that didn’t achieve statistical significance. The all-cause mortality rate was 0.9% in the ticagrelor monotherapy group and 1.5% with extended DAPT, again a nonsignificant difference. Similarly, the rate of definite or probable stent thrombosis was numerically lower with ticagrelor monotherapy, by a margin of 0.4% versus 0.8%, a nonsignificant difference.
The results were consistent regardless of which specific criteria for complex PCI a patient had or how many of them.
Results are ‘reassuring’
At a press conference where Dr. Dangas presented the TWILIGHT-COMPLEX results, discussant Claire S. Duvernoy, MD, said she was “very impressed” with just how complex the PCIs were in the study participants.
“Really, these are the patients that in my own practice we’ve always been the most cautious about, the most worried about thrombotic risk, and the ones where we get down on our house staff when they drop an antiplatelet agent. So this study is very reassuring,” said Dr. Duvernoy, professor of medicine at the University of Michigan, Ann Arbor.
She identified two key differences between TWILIGHT-COMPLEX and earlier studies that showed a benefit for extended DAPT in higher-risk patients. In the earlier studies, it was the P2Y12 inhibitor that was dropped; TWILIGHT was the first major randomized trial to discontinue the aspirin instead. And patients in the TWILIGHT study received second-generation drug-eluting stents.
“That makes a huge difference,” Dr. Duvernoy said. “We have stents now that are much safer than the old ones were, and that’s what allows us to gain this incredible benefit of reduced bleeding.”
Dr. Dangas cautioned that since this was a secondary post hoc analysis, the TWILIGHT-COMPLEX study must be viewed as hypothesis-generating.
The TWILIGHT trial was funded by AstraZeneca. Dr. Dangas reported receiving institutional research grants from that company as well as Bayer and Daichi-Sankyo. He also served as a paid consultant to Abbott Vascular, Boston Scientific, and Biosensors.
Simultaneous with his presentation at ACC 2020, the TWILIGHT-COMPLEX results were published online (J Am Coll Cardiol. 2020 Mar 13. doi: 10.1016/j.jacc.2020.03.011).
SOURCE: Dangas GD. ACC 20, Abstract 410-09.
FROM ACC 2020
Children’s Hospitals Caring for Adults During a Pandemic: Pragmatic Considerations and Approaches
Health systems around the world have been called upon to expand acute care capacity to manage the current and projected surge of adults with COVID-19, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 There has been mixed guidance on how pediatric facilities should consolidate and coordinate pediatric care in a way that optimizes the capacity of hospital beds, staff, and supplies, such as ventilators and medications, for both adults and children in a community.2 Furthermore, if and how these pediatric facilities should expand capacity to care for adult patients safely is uncertain.
For the last 5 years, both Boston Children’s Hospital and Cincinnati Children’s Hospital Medical Center have been caring for specific adult populations in free-standing pediatric hospitals because of the increasing prevalence of young adults with rare, complex, and historically fatal conditions (eg, chromosomal abnormalities). In the past, low life expectancies for children with such conditions contributed to the evolution of specialized care in pediatric health systems that often does not exist in adult health systems. Our teams in Boston and Cincinnati have gained insight into the multifaceted infrastructure and teams necessary to provide safe care for adults hospitalized in a pediatric setting.
In this perspective piece, we will highlight important principles that pediatric facilities and providers should prioritize if they anticipate caring for hospitalized adults during this pandemic. Designing and implementing an adult care model requires iteratively addressing the following key areas: development of a multistakeholder team, system readiness for intensive care unit (ICU) care of adults, institutional situation awareness, scope of practice, staffing considerations, patient safety, and patient populations and special considerations (eg, adults with chronic conditions of childhood onset). With these areas in mind, pediatric facilities should then consider whether they have the capacity to manage hospitalized adults.
DEVELOPMENT OF A MULTISTAKEHOLDER TEAM
Providing care for any hospitalized patient requires engagement with many health system stakeholders. By involving key stakeholders early in the planning process for our adult care model, we were able to anticipate potential obstacles when caring for a unique subset of patients and gain support of multidisciplinary partners. For instance, inclusion of bedside and support staff highlighted specific needs, such as nurses with adult training and a revised formulary to include common adult medications (eg, clopidogrel for adults with a drug-eluting stent).
Responding to the surge of hospitalized adult patients will require increasing hospital capacity.3 In pediatric settings, this will require consideration of innovative care models. These care models may include pediatric systems flexing to care for adult patients. We recommend hospital leaders from both pediatric and adult facilities have formal discussions on the best ways for pediatric facilities to respond to serve their local population. Inclusion of other key stakeholders will ensure factors imperative to the safe care of adults will not be missed.
SYSTEM READINESS FOR ICU CARE OF ADULTS
There were three levels of consideration for the use of our local pediatric ICU for these patients. First, our institutional policies allow care for adults throughout the system, which we describe in more detail later, in the “Scope of Practice” section. Second, our free-standing pediatric hospital ICUs have accreditation for the care of adults. Third, we developed clear guidelines for subspecialists regarding when adults can safely be admitted or transferred to the pediatric ICU.
Responding to a crisis still necessitates establishing a clear care-escalation plan. An initial barrier may be that some systems do not have a pediatric ICU accredited for care of patients above a certain age. During a crisis, however, as hospital volumes and mortalities rise, states may pursue executive orders, as New York State did, that ease these age restrictions.4 Otherwise, we recommend a clear transfer plan to an adult ICU or emergency credentialing and privileging of adult intensivists. Both of these options may pose challenges during a pandemic because adult ICUs will likely be full.
INSTITUTIONAL SITUATION AWARENESS
Institutional situation awareness for the identification and mitigation of risks inherent in adult care in a pediatric setting is essential for patient safety. Tracking of admitted adult patients via our electronic health record (EHR) occurs daily by an adult care–team member. Our adult care teams partner with physician safety officers and attend daily institutional multidisciplinary safety huddles to create a shared mental model for the care of adult patients. Daily huddle reports include discussion regarding the number of admitted adults, review of illness acuity, consultative advice on management, and contingency planning for potential decompensation.5,6 This integration into institutional huddles has been instrumental in proactively identifying hospitalized adults who are at risk for clinical decompensation and mitigating those risks.
Should a pediatric system admit adults to new sites or units, we recommend leveraging preexisting patient safety infrastructure similarly to identify and mitigate risks. If possible, any institutional communication about adult patients should involve adult-trained staff. Mechanisms for tracking patients will depend on local EHRs but are important to guide regular check-ins with providers caring for those patients.
SCOPE OF PRACTICE
Multiple levels of regulation affect a provider’s scope of practice. The most general of these regulations are state guidelines, followed by local institutional policy. Our institutions require consults for older adults—age varies at our specific institutions—by our adult-care team for assessment of risk and comanagement of adult-specific comorbidities. Additionally, we have agreements with our affiliated adult health facilities that allow in-person adult subspecialty consultation.
While state and institutional policies lay the foundation for pediatric systems considering new adult-care models, provider-level considerations are also needed. Often the patient’s age is a primary consideration, but comorbid conditions also affect the provider’s comfort and ability to care for these patients. We urge practitioners to exercise the full range of their capacities, but also to think critically about the ethical scope of one’s practice. As healthcare providers, it is our duty to hold each other accountable, voice concerns, and advocate to increase health system capacity equitably.7 It’s paramount that channels of communication, in-person or virtual, be arranged for supportive adult subspecialist consultation.
STAFFING CONSIDERATIONS
Med-Peds physicians and advanced practice providers are the foundation of the clinical care provided to adults at our institutions. Our Med-Peds providers practice in both the free-standing pediatric hospital and an affiliated adult health system. They offer expertise in adult clinical care and navigate between pediatric and adult systems when the need arises (eg, adult requiring urgent intervention for an acute myocardial infarction). Adult competencies of other staff must be addressed. For example, our cardiac ICUs include nurses with adult clinical care experience because critically ill adults with congenital heart disease are admitted. Advanced Care Life Support (ACLS) training is also required for staff caring for adults throughout the hospital.
There are many ways, even during a crisis, to develop an adult care model in a pediatric setting. Depending on workforce availability, internal medicine, Med-Peds, family medicine, critical care, and emergency medicine physicians could serve on either a primary service or as a consultant to support pediatrics-trained providers in caring for adults should the patient volume and acuity require staffing restructuring. Adult subspecialty access must be addressed. Telehealth may play a significant role in extending clinicians in all of these clinical roles both during the current crisis but also in underresourced settings.8 A clear process and indication for emergency or temporary credentialing and privileging necessitates understanding and addressing such challenges early. Training in adult care, or lack thereof, for other staff, such as nurses and respiratory therapists, is also crucial to consider.
PATIENT SAFETY
Adults are more likely than children to have comorbidities and clinical deterioration while hospitalized. At our institutions, when a rapid response team is called for an adult patient, an adult care–team provider responds to aid in clinical management and determines the appropriate care setting. Additionally, given that the incidence of coronary artery disease increases starting at age 35 years,9 our systems have developed procedures for managing time-sensitive conditions seen more commonly in adults, such as acute myocardial infarction, stroke, and pulmonary embolism. Despite simulation training for pediatric providers and staff, it is clear that implementing these procedures is highly dependent on involvement of the adult care team.
With the urgency of implementation, pediatric systems should consider increasing the number of providers and staff with ACLS training, especially for rapid response and code teams. Many pediatric systems may need to evaluate how their code carts are stocked and ensure they are equipped with appropriate medication dosages and sizes of supplies. Emergent and accessible adult care will be needed, especially for issues with time-to-intervention criteria like acute myocardial infarction and stroke. Hospitalized adults with COVID-19 may also have a higher incidence of arrhythmia, cardiac ischemia, and stroke.10 Consider proactively simulating common COVID-19–related scenarios to build interdisciplinary teamwork in emergency scenarios. Interhospital agreements and pathways exist for sharing medications. Outreach to pharmacies may be indicated to ensure accessibility for medications not commonly found in pediatric systems.
PATIENT POPULATIONS AND SPECIAL CONSIDERATIONS
Our children’s hospitals care for certain adult populations with chronic conditions of childhood origin because of the availability of subspecialty clinical expertise. Our adult care team aids in contingency planning to help determine place of admission (adult vs pediatric hospital) depending on patient clinical needs and system expertise. For instance, an adult with congenital heart disease may have two cardiologists—one for congenital heart disease and one for coronary artery disease. Patients with an acute issue such as new-onset arrhythmia may be admitted to our pediatric hospital; however, for a stroke they would be admitted to the adult hospital.
While important and tempting to address this issue first, creating criteria to determine which patient population to admit should be a last consideration during a pandemic. Consider if the decision to admit should be determined based on COVID-19 infection status. From there, types of conditions thought to be within the purview of pediatric practice can be considered. These include basic infectious diseases pathology (eg, skin/soft-tissue infections and pyelonephritis) and chronic conditions of childhood origin (eg, cystic fibrosis, diabetes, and inflammatory bowel disease), which have specialty providers who could work across an extended age range. Conditions potentially more challenging to safely care for in pediatric facilities include acute cardiac conditions (eg, angina, acute coronary syndrome, and arrhythmias), alcohol withdrawal, end-stage liver or kidney disease, and gastrointestinal bleeds. Considerations need to be made for research protocols and novel therapies only available at adult institutions. Through this whole process, it is especially crucial to note care equity and ensure that all patients have access to the highest attainable care possible.
CONCLUSION
Policymakers at pediatric facilities should think critically about their institution’s capacity to manage adults. In some circumstances, the decision might be to not admit adult patients based on the factors discussed in this paper or other contextual factors of the local healthcare systems. Our role in providing care for adults in pediatric hospitals involves not only ensuring age-appropriate care, but also in supporting patients and other healthcare providers to navigate a fragmented health system. Our adult-care models required building relationships between pediatric and adult health systems. Building these relationships in the setting of crisis can strengthen health systems and healthcare communities beyond the era of COVID-19. Because it’s promoted enhanced collaboration between pediatric and adult facilities, COVID-19 can be a platform to build a better system to support our already vulnerable young adults with chronic conditions of childhood origin for years to come.
1. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) Pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. https://jamanetwork.com/channels/health-forum/fullarticle/2763353. Accessed March 30, 2020.
2. Children’s Hospital Association. Consolidating Pediatric Hospital Care to Increase Capacity for Adults with COVID-19. https://www.childrenshospitals.org/Quality-and-Performance/COVID19/Resources/Consolidating-Pediatric-Hospital-Care-Increase-Capacity-Adults-COVID19. Accessed March 28, 2020.
3. Campbell J. Andrew Cuomo’s order to hospitals: expand capacity or face state takeover. Democrat & Chronicle. April 1, 2020. https://www.democratandchronicle.com/story/news/politics/albany/2020/04/01/coronavirus-cuomo-order-state-hospital-takeover/5100134002/. Accessed April 2, 2020.
4. New York State Education Department, Office of the Professions. COVID-19 Executive Orders. http://www.op.nysed.gov/COVID-19_EO.html. Accessed April 2, 2020.
5. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364.
6. Conway-Habes EE, Herbst BF, Herbst LA, et al. Using quality improvement to introduce and standardize the National Early Warning Score (NEWS) for adult inpatients at a children’s hospital. Hosp Pediatr. 2017;7(3):156-163. https://doi.org/10.1542/hpeds.2016-0117.
7. Berry JG, Bloom S, Foley S, Palfrey JS. Health inequity in children and youth with chronic health conditions. Pediatrics. 2010;126(Suppl 3):S111-S119. https://doi.org/10.1542/peds.2010-1466D.
8. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020:1357633X20916567. https://doi.org/10.1177/1357633X20916567.
9. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-e596. https://doi.org/10.1161/CIR.0000000000000757.
10. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
Health systems around the world have been called upon to expand acute care capacity to manage the current and projected surge of adults with COVID-19, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 There has been mixed guidance on how pediatric facilities should consolidate and coordinate pediatric care in a way that optimizes the capacity of hospital beds, staff, and supplies, such as ventilators and medications, for both adults and children in a community.2 Furthermore, if and how these pediatric facilities should expand capacity to care for adult patients safely is uncertain.
For the last 5 years, both Boston Children’s Hospital and Cincinnati Children’s Hospital Medical Center have been caring for specific adult populations in free-standing pediatric hospitals because of the increasing prevalence of young adults with rare, complex, and historically fatal conditions (eg, chromosomal abnormalities). In the past, low life expectancies for children with such conditions contributed to the evolution of specialized care in pediatric health systems that often does not exist in adult health systems. Our teams in Boston and Cincinnati have gained insight into the multifaceted infrastructure and teams necessary to provide safe care for adults hospitalized in a pediatric setting.
In this perspective piece, we will highlight important principles that pediatric facilities and providers should prioritize if they anticipate caring for hospitalized adults during this pandemic. Designing and implementing an adult care model requires iteratively addressing the following key areas: development of a multistakeholder team, system readiness for intensive care unit (ICU) care of adults, institutional situation awareness, scope of practice, staffing considerations, patient safety, and patient populations and special considerations (eg, adults with chronic conditions of childhood onset). With these areas in mind, pediatric facilities should then consider whether they have the capacity to manage hospitalized adults.
DEVELOPMENT OF A MULTISTAKEHOLDER TEAM
Providing care for any hospitalized patient requires engagement with many health system stakeholders. By involving key stakeholders early in the planning process for our adult care model, we were able to anticipate potential obstacles when caring for a unique subset of patients and gain support of multidisciplinary partners. For instance, inclusion of bedside and support staff highlighted specific needs, such as nurses with adult training and a revised formulary to include common adult medications (eg, clopidogrel for adults with a drug-eluting stent).
Responding to the surge of hospitalized adult patients will require increasing hospital capacity.3 In pediatric settings, this will require consideration of innovative care models. These care models may include pediatric systems flexing to care for adult patients. We recommend hospital leaders from both pediatric and adult facilities have formal discussions on the best ways for pediatric facilities to respond to serve their local population. Inclusion of other key stakeholders will ensure factors imperative to the safe care of adults will not be missed.
SYSTEM READINESS FOR ICU CARE OF ADULTS
There were three levels of consideration for the use of our local pediatric ICU for these patients. First, our institutional policies allow care for adults throughout the system, which we describe in more detail later, in the “Scope of Practice” section. Second, our free-standing pediatric hospital ICUs have accreditation for the care of adults. Third, we developed clear guidelines for subspecialists regarding when adults can safely be admitted or transferred to the pediatric ICU.
Responding to a crisis still necessitates establishing a clear care-escalation plan. An initial barrier may be that some systems do not have a pediatric ICU accredited for care of patients above a certain age. During a crisis, however, as hospital volumes and mortalities rise, states may pursue executive orders, as New York State did, that ease these age restrictions.4 Otherwise, we recommend a clear transfer plan to an adult ICU or emergency credentialing and privileging of adult intensivists. Both of these options may pose challenges during a pandemic because adult ICUs will likely be full.
INSTITUTIONAL SITUATION AWARENESS
Institutional situation awareness for the identification and mitigation of risks inherent in adult care in a pediatric setting is essential for patient safety. Tracking of admitted adult patients via our electronic health record (EHR) occurs daily by an adult care–team member. Our adult care teams partner with physician safety officers and attend daily institutional multidisciplinary safety huddles to create a shared mental model for the care of adult patients. Daily huddle reports include discussion regarding the number of admitted adults, review of illness acuity, consultative advice on management, and contingency planning for potential decompensation.5,6 This integration into institutional huddles has been instrumental in proactively identifying hospitalized adults who are at risk for clinical decompensation and mitigating those risks.
Should a pediatric system admit adults to new sites or units, we recommend leveraging preexisting patient safety infrastructure similarly to identify and mitigate risks. If possible, any institutional communication about adult patients should involve adult-trained staff. Mechanisms for tracking patients will depend on local EHRs but are important to guide regular check-ins with providers caring for those patients.
SCOPE OF PRACTICE
Multiple levels of regulation affect a provider’s scope of practice. The most general of these regulations are state guidelines, followed by local institutional policy. Our institutions require consults for older adults—age varies at our specific institutions—by our adult-care team for assessment of risk and comanagement of adult-specific comorbidities. Additionally, we have agreements with our affiliated adult health facilities that allow in-person adult subspecialty consultation.
While state and institutional policies lay the foundation for pediatric systems considering new adult-care models, provider-level considerations are also needed. Often the patient’s age is a primary consideration, but comorbid conditions also affect the provider’s comfort and ability to care for these patients. We urge practitioners to exercise the full range of their capacities, but also to think critically about the ethical scope of one’s practice. As healthcare providers, it is our duty to hold each other accountable, voice concerns, and advocate to increase health system capacity equitably.7 It’s paramount that channels of communication, in-person or virtual, be arranged for supportive adult subspecialist consultation.
STAFFING CONSIDERATIONS
Med-Peds physicians and advanced practice providers are the foundation of the clinical care provided to adults at our institutions. Our Med-Peds providers practice in both the free-standing pediatric hospital and an affiliated adult health system. They offer expertise in adult clinical care and navigate between pediatric and adult systems when the need arises (eg, adult requiring urgent intervention for an acute myocardial infarction). Adult competencies of other staff must be addressed. For example, our cardiac ICUs include nurses with adult clinical care experience because critically ill adults with congenital heart disease are admitted. Advanced Care Life Support (ACLS) training is also required for staff caring for adults throughout the hospital.
There are many ways, even during a crisis, to develop an adult care model in a pediatric setting. Depending on workforce availability, internal medicine, Med-Peds, family medicine, critical care, and emergency medicine physicians could serve on either a primary service or as a consultant to support pediatrics-trained providers in caring for adults should the patient volume and acuity require staffing restructuring. Adult subspecialty access must be addressed. Telehealth may play a significant role in extending clinicians in all of these clinical roles both during the current crisis but also in underresourced settings.8 A clear process and indication for emergency or temporary credentialing and privileging necessitates understanding and addressing such challenges early. Training in adult care, or lack thereof, for other staff, such as nurses and respiratory therapists, is also crucial to consider.
PATIENT SAFETY
Adults are more likely than children to have comorbidities and clinical deterioration while hospitalized. At our institutions, when a rapid response team is called for an adult patient, an adult care–team provider responds to aid in clinical management and determines the appropriate care setting. Additionally, given that the incidence of coronary artery disease increases starting at age 35 years,9 our systems have developed procedures for managing time-sensitive conditions seen more commonly in adults, such as acute myocardial infarction, stroke, and pulmonary embolism. Despite simulation training for pediatric providers and staff, it is clear that implementing these procedures is highly dependent on involvement of the adult care team.
With the urgency of implementation, pediatric systems should consider increasing the number of providers and staff with ACLS training, especially for rapid response and code teams. Many pediatric systems may need to evaluate how their code carts are stocked and ensure they are equipped with appropriate medication dosages and sizes of supplies. Emergent and accessible adult care will be needed, especially for issues with time-to-intervention criteria like acute myocardial infarction and stroke. Hospitalized adults with COVID-19 may also have a higher incidence of arrhythmia, cardiac ischemia, and stroke.10 Consider proactively simulating common COVID-19–related scenarios to build interdisciplinary teamwork in emergency scenarios. Interhospital agreements and pathways exist for sharing medications. Outreach to pharmacies may be indicated to ensure accessibility for medications not commonly found in pediatric systems.
PATIENT POPULATIONS AND SPECIAL CONSIDERATIONS
Our children’s hospitals care for certain adult populations with chronic conditions of childhood origin because of the availability of subspecialty clinical expertise. Our adult care team aids in contingency planning to help determine place of admission (adult vs pediatric hospital) depending on patient clinical needs and system expertise. For instance, an adult with congenital heart disease may have two cardiologists—one for congenital heart disease and one for coronary artery disease. Patients with an acute issue such as new-onset arrhythmia may be admitted to our pediatric hospital; however, for a stroke they would be admitted to the adult hospital.
While important and tempting to address this issue first, creating criteria to determine which patient population to admit should be a last consideration during a pandemic. Consider if the decision to admit should be determined based on COVID-19 infection status. From there, types of conditions thought to be within the purview of pediatric practice can be considered. These include basic infectious diseases pathology (eg, skin/soft-tissue infections and pyelonephritis) and chronic conditions of childhood origin (eg, cystic fibrosis, diabetes, and inflammatory bowel disease), which have specialty providers who could work across an extended age range. Conditions potentially more challenging to safely care for in pediatric facilities include acute cardiac conditions (eg, angina, acute coronary syndrome, and arrhythmias), alcohol withdrawal, end-stage liver or kidney disease, and gastrointestinal bleeds. Considerations need to be made for research protocols and novel therapies only available at adult institutions. Through this whole process, it is especially crucial to note care equity and ensure that all patients have access to the highest attainable care possible.
CONCLUSION
Policymakers at pediatric facilities should think critically about their institution’s capacity to manage adults. In some circumstances, the decision might be to not admit adult patients based on the factors discussed in this paper or other contextual factors of the local healthcare systems. Our role in providing care for adults in pediatric hospitals involves not only ensuring age-appropriate care, but also in supporting patients and other healthcare providers to navigate a fragmented health system. Our adult-care models required building relationships between pediatric and adult health systems. Building these relationships in the setting of crisis can strengthen health systems and healthcare communities beyond the era of COVID-19. Because it’s promoted enhanced collaboration between pediatric and adult facilities, COVID-19 can be a platform to build a better system to support our already vulnerable young adults with chronic conditions of childhood origin for years to come.
Health systems around the world have been called upon to expand acute care capacity to manage the current and projected surge of adults with COVID-19, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 There has been mixed guidance on how pediatric facilities should consolidate and coordinate pediatric care in a way that optimizes the capacity of hospital beds, staff, and supplies, such as ventilators and medications, for both adults and children in a community.2 Furthermore, if and how these pediatric facilities should expand capacity to care for adult patients safely is uncertain.
For the last 5 years, both Boston Children’s Hospital and Cincinnati Children’s Hospital Medical Center have been caring for specific adult populations in free-standing pediatric hospitals because of the increasing prevalence of young adults with rare, complex, and historically fatal conditions (eg, chromosomal abnormalities). In the past, low life expectancies for children with such conditions contributed to the evolution of specialized care in pediatric health systems that often does not exist in adult health systems. Our teams in Boston and Cincinnati have gained insight into the multifaceted infrastructure and teams necessary to provide safe care for adults hospitalized in a pediatric setting.
In this perspective piece, we will highlight important principles that pediatric facilities and providers should prioritize if they anticipate caring for hospitalized adults during this pandemic. Designing and implementing an adult care model requires iteratively addressing the following key areas: development of a multistakeholder team, system readiness for intensive care unit (ICU) care of adults, institutional situation awareness, scope of practice, staffing considerations, patient safety, and patient populations and special considerations (eg, adults with chronic conditions of childhood onset). With these areas in mind, pediatric facilities should then consider whether they have the capacity to manage hospitalized adults.
DEVELOPMENT OF A MULTISTAKEHOLDER TEAM
Providing care for any hospitalized patient requires engagement with many health system stakeholders. By involving key stakeholders early in the planning process for our adult care model, we were able to anticipate potential obstacles when caring for a unique subset of patients and gain support of multidisciplinary partners. For instance, inclusion of bedside and support staff highlighted specific needs, such as nurses with adult training and a revised formulary to include common adult medications (eg, clopidogrel for adults with a drug-eluting stent).
Responding to the surge of hospitalized adult patients will require increasing hospital capacity.3 In pediatric settings, this will require consideration of innovative care models. These care models may include pediatric systems flexing to care for adult patients. We recommend hospital leaders from both pediatric and adult facilities have formal discussions on the best ways for pediatric facilities to respond to serve their local population. Inclusion of other key stakeholders will ensure factors imperative to the safe care of adults will not be missed.
SYSTEM READINESS FOR ICU CARE OF ADULTS
There were three levels of consideration for the use of our local pediatric ICU for these patients. First, our institutional policies allow care for adults throughout the system, which we describe in more detail later, in the “Scope of Practice” section. Second, our free-standing pediatric hospital ICUs have accreditation for the care of adults. Third, we developed clear guidelines for subspecialists regarding when adults can safely be admitted or transferred to the pediatric ICU.
Responding to a crisis still necessitates establishing a clear care-escalation plan. An initial barrier may be that some systems do not have a pediatric ICU accredited for care of patients above a certain age. During a crisis, however, as hospital volumes and mortalities rise, states may pursue executive orders, as New York State did, that ease these age restrictions.4 Otherwise, we recommend a clear transfer plan to an adult ICU or emergency credentialing and privileging of adult intensivists. Both of these options may pose challenges during a pandemic because adult ICUs will likely be full.
INSTITUTIONAL SITUATION AWARENESS
Institutional situation awareness for the identification and mitigation of risks inherent in adult care in a pediatric setting is essential for patient safety. Tracking of admitted adult patients via our electronic health record (EHR) occurs daily by an adult care–team member. Our adult care teams partner with physician safety officers and attend daily institutional multidisciplinary safety huddles to create a shared mental model for the care of adult patients. Daily huddle reports include discussion regarding the number of admitted adults, review of illness acuity, consultative advice on management, and contingency planning for potential decompensation.5,6 This integration into institutional huddles has been instrumental in proactively identifying hospitalized adults who are at risk for clinical decompensation and mitigating those risks.
Should a pediatric system admit adults to new sites or units, we recommend leveraging preexisting patient safety infrastructure similarly to identify and mitigate risks. If possible, any institutional communication about adult patients should involve adult-trained staff. Mechanisms for tracking patients will depend on local EHRs but are important to guide regular check-ins with providers caring for those patients.
SCOPE OF PRACTICE
Multiple levels of regulation affect a provider’s scope of practice. The most general of these regulations are state guidelines, followed by local institutional policy. Our institutions require consults for older adults—age varies at our specific institutions—by our adult-care team for assessment of risk and comanagement of adult-specific comorbidities. Additionally, we have agreements with our affiliated adult health facilities that allow in-person adult subspecialty consultation.
While state and institutional policies lay the foundation for pediatric systems considering new adult-care models, provider-level considerations are also needed. Often the patient’s age is a primary consideration, but comorbid conditions also affect the provider’s comfort and ability to care for these patients. We urge practitioners to exercise the full range of their capacities, but also to think critically about the ethical scope of one’s practice. As healthcare providers, it is our duty to hold each other accountable, voice concerns, and advocate to increase health system capacity equitably.7 It’s paramount that channels of communication, in-person or virtual, be arranged for supportive adult subspecialist consultation.
STAFFING CONSIDERATIONS
Med-Peds physicians and advanced practice providers are the foundation of the clinical care provided to adults at our institutions. Our Med-Peds providers practice in both the free-standing pediatric hospital and an affiliated adult health system. They offer expertise in adult clinical care and navigate between pediatric and adult systems when the need arises (eg, adult requiring urgent intervention for an acute myocardial infarction). Adult competencies of other staff must be addressed. For example, our cardiac ICUs include nurses with adult clinical care experience because critically ill adults with congenital heart disease are admitted. Advanced Care Life Support (ACLS) training is also required for staff caring for adults throughout the hospital.
There are many ways, even during a crisis, to develop an adult care model in a pediatric setting. Depending on workforce availability, internal medicine, Med-Peds, family medicine, critical care, and emergency medicine physicians could serve on either a primary service or as a consultant to support pediatrics-trained providers in caring for adults should the patient volume and acuity require staffing restructuring. Adult subspecialty access must be addressed. Telehealth may play a significant role in extending clinicians in all of these clinical roles both during the current crisis but also in underresourced settings.8 A clear process and indication for emergency or temporary credentialing and privileging necessitates understanding and addressing such challenges early. Training in adult care, or lack thereof, for other staff, such as nurses and respiratory therapists, is also crucial to consider.
PATIENT SAFETY
Adults are more likely than children to have comorbidities and clinical deterioration while hospitalized. At our institutions, when a rapid response team is called for an adult patient, an adult care–team provider responds to aid in clinical management and determines the appropriate care setting. Additionally, given that the incidence of coronary artery disease increases starting at age 35 years,9 our systems have developed procedures for managing time-sensitive conditions seen more commonly in adults, such as acute myocardial infarction, stroke, and pulmonary embolism. Despite simulation training for pediatric providers and staff, it is clear that implementing these procedures is highly dependent on involvement of the adult care team.
With the urgency of implementation, pediatric systems should consider increasing the number of providers and staff with ACLS training, especially for rapid response and code teams. Many pediatric systems may need to evaluate how their code carts are stocked and ensure they are equipped with appropriate medication dosages and sizes of supplies. Emergent and accessible adult care will be needed, especially for issues with time-to-intervention criteria like acute myocardial infarction and stroke. Hospitalized adults with COVID-19 may also have a higher incidence of arrhythmia, cardiac ischemia, and stroke.10 Consider proactively simulating common COVID-19–related scenarios to build interdisciplinary teamwork in emergency scenarios. Interhospital agreements and pathways exist for sharing medications. Outreach to pharmacies may be indicated to ensure accessibility for medications not commonly found in pediatric systems.
PATIENT POPULATIONS AND SPECIAL CONSIDERATIONS
Our children’s hospitals care for certain adult populations with chronic conditions of childhood origin because of the availability of subspecialty clinical expertise. Our adult care team aids in contingency planning to help determine place of admission (adult vs pediatric hospital) depending on patient clinical needs and system expertise. For instance, an adult with congenital heart disease may have two cardiologists—one for congenital heart disease and one for coronary artery disease. Patients with an acute issue such as new-onset arrhythmia may be admitted to our pediatric hospital; however, for a stroke they would be admitted to the adult hospital.
While important and tempting to address this issue first, creating criteria to determine which patient population to admit should be a last consideration during a pandemic. Consider if the decision to admit should be determined based on COVID-19 infection status. From there, types of conditions thought to be within the purview of pediatric practice can be considered. These include basic infectious diseases pathology (eg, skin/soft-tissue infections and pyelonephritis) and chronic conditions of childhood origin (eg, cystic fibrosis, diabetes, and inflammatory bowel disease), which have specialty providers who could work across an extended age range. Conditions potentially more challenging to safely care for in pediatric facilities include acute cardiac conditions (eg, angina, acute coronary syndrome, and arrhythmias), alcohol withdrawal, end-stage liver or kidney disease, and gastrointestinal bleeds. Considerations need to be made for research protocols and novel therapies only available at adult institutions. Through this whole process, it is especially crucial to note care equity and ensure that all patients have access to the highest attainable care possible.
CONCLUSION
Policymakers at pediatric facilities should think critically about their institution’s capacity to manage adults. In some circumstances, the decision might be to not admit adult patients based on the factors discussed in this paper or other contextual factors of the local healthcare systems. Our role in providing care for adults in pediatric hospitals involves not only ensuring age-appropriate care, but also in supporting patients and other healthcare providers to navigate a fragmented health system. Our adult-care models required building relationships between pediatric and adult health systems. Building these relationships in the setting of crisis can strengthen health systems and healthcare communities beyond the era of COVID-19. Because it’s promoted enhanced collaboration between pediatric and adult facilities, COVID-19 can be a platform to build a better system to support our already vulnerable young adults with chronic conditions of childhood origin for years to come.
1. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) Pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. https://jamanetwork.com/channels/health-forum/fullarticle/2763353. Accessed March 30, 2020.
2. Children’s Hospital Association. Consolidating Pediatric Hospital Care to Increase Capacity for Adults with COVID-19. https://www.childrenshospitals.org/Quality-and-Performance/COVID19/Resources/Consolidating-Pediatric-Hospital-Care-Increase-Capacity-Adults-COVID19. Accessed March 28, 2020.
3. Campbell J. Andrew Cuomo’s order to hospitals: expand capacity or face state takeover. Democrat & Chronicle. April 1, 2020. https://www.democratandchronicle.com/story/news/politics/albany/2020/04/01/coronavirus-cuomo-order-state-hospital-takeover/5100134002/. Accessed April 2, 2020.
4. New York State Education Department, Office of the Professions. COVID-19 Executive Orders. http://www.op.nysed.gov/COVID-19_EO.html. Accessed April 2, 2020.
5. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364.
6. Conway-Habes EE, Herbst BF, Herbst LA, et al. Using quality improvement to introduce and standardize the National Early Warning Score (NEWS) for adult inpatients at a children’s hospital. Hosp Pediatr. 2017;7(3):156-163. https://doi.org/10.1542/hpeds.2016-0117.
7. Berry JG, Bloom S, Foley S, Palfrey JS. Health inequity in children and youth with chronic health conditions. Pediatrics. 2010;126(Suppl 3):S111-S119. https://doi.org/10.1542/peds.2010-1466D.
8. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020:1357633X20916567. https://doi.org/10.1177/1357633X20916567.
9. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-e596. https://doi.org/10.1161/CIR.0000000000000757.
10. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
1. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) Pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. https://jamanetwork.com/channels/health-forum/fullarticle/2763353. Accessed March 30, 2020.
2. Children’s Hospital Association. Consolidating Pediatric Hospital Care to Increase Capacity for Adults with COVID-19. https://www.childrenshospitals.org/Quality-and-Performance/COVID19/Resources/Consolidating-Pediatric-Hospital-Care-Increase-Capacity-Adults-COVID19. Accessed March 28, 2020.
3. Campbell J. Andrew Cuomo’s order to hospitals: expand capacity or face state takeover. Democrat & Chronicle. April 1, 2020. https://www.democratandchronicle.com/story/news/politics/albany/2020/04/01/coronavirus-cuomo-order-state-hospital-takeover/5100134002/. Accessed April 2, 2020.
4. New York State Education Department, Office of the Professions. COVID-19 Executive Orders. http://www.op.nysed.gov/COVID-19_EO.html. Accessed April 2, 2020.
5. Brady PW, Muething S, Kotagal U, et al. Improving situation awareness to reduce unrecognized clinical deterioration and serious safety events. Pediatrics. 2013;131(1):e298-e308. https://doi.org/10.1542/peds.2012-1364.
6. Conway-Habes EE, Herbst BF, Herbst LA, et al. Using quality improvement to introduce and standardize the National Early Warning Score (NEWS) for adult inpatients at a children’s hospital. Hosp Pediatr. 2017;7(3):156-163. https://doi.org/10.1542/hpeds.2016-0117.
7. Berry JG, Bloom S, Foley S, Palfrey JS. Health inequity in children and youth with chronic health conditions. Pediatrics. 2010;126(Suppl 3):S111-S119. https://doi.org/10.1542/peds.2010-1466D.
8. Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020:1357633X20916567. https://doi.org/10.1177/1357633X20916567.
9. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139-e596. https://doi.org/10.1161/CIR.0000000000000757.
10. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. https://doi.org/10.1001/jama.2020.2648.
© 2020 Society of Hospital Medicine