User login
COVID-19 quarantine: Managing pediatric behavioral issues
We are living through unprecedented challenges, faced with profound uncertainties about the public health, the economy, the safety of our workplaces, the risks of gathering with friends and family, and even about the rhythm of the school year. Parents always have sought guidance from their pediatric providers when they are uncertain about their children’s health, behavior, and development. We want to share some guidance with you about several of the most common questions we have been hearing in the past few months, in the hope that it may prove useful in your conversations with patients and families.
What happens when we are so busy at home that our 2-year-old is ignored for much of the day?
If they are fortunate enough to be able to work from home, but have lost their child care, many parents are suddenly facing the sustained challenge of parenting while working. Even older children will have a tough time remembering that home is now a workplace, and they can’t interrupt their parents during a Zoom meeting. But older children will understand. Younger children (preschoolers) simply will not be able to understand that their parents are in sight but not fully available to them. They are exquisitely sensitive to their parents’ attention. If they are consistently ignored, behavioral problems can emerge. If both parents are at home, they should try to arrange a schedule taking turns so that one of them could turn their full attention to their kids if need be. If a working parent can be out of sight (i.e., in another room), it makes the situation easier for everyone.
If there is only one parent at home, that mom or dad should consider arranging a babysitter or sharing child care with a friend, with some reasonable safety provisions in place. The small risk of exposure to the virus is balanced by the risk of sustained invalidation in a developing child. Help parents set reasonable expectations for how productive they can be at home. If possible, they can manage their employer’s expectations, so that they do not find themselves in the impossible bind of choosing between a crying child and a crucial deadline. If they can work near the child (and be prepared for interruptions) when reading emails or writing, that may be enough availability for the child. And parents should not be discouraged when they have to repeatedly remind their children that they adore them, but also have to work while they are at home right now. Using age-appropriate screen time as a babysitter for a few hours each day is a perfectly acceptable part of a plan. Simply planning regular breaks when their children can have their attention will make the day easier for everyone at home.
What can I do about my 13-year-old who is lying around the house all day?
This is a time to pick your battles. If children can keep their regular sleep schedule, get their schoolwork done, and do some physical exercise every day, they are doing great. And if parents are continuously complaining that they are being lazy, it will probably cease to mean much to them. Instead, focus on clear, simple expectations, and parents should live by them, too. If parents can exercise with them, or try a new activity, that is a wonderful way to model self-care and trying new things. It is important to remember that the developmental task for a 13-year-old is to establish new avenues of independence that they will drive down further with each passing year. Give them some leeway to experiment and figure out their own way of handling this challenge, although it is bound to create some tension. Parents should always acknowledge how hard it is to stick with schoolwork without school, exercise without a team, practice music without a band, or do your work without an office!
What do we do about our 16-year-old who is staying up all night and sleeping until the late afternoon?
Adolescents naturally have their sleep cycle shift, so they are sleepy later and sleep longer. But staying up all night is usually about texting with friends or playing video games. The problem is that their sleep schedule can flip. They will not be able to participate in online class or enjoy exercise in the sun, and they rarely get enough sleep during the daytime, making them more irritable, anxious, inattentive, and tired. This will only make managing their schoolwork harder and increase the chances of conflict at home. So it is important to preserve rules around sleep. You might extend bedtime by an hour or so, but preserve rules and bedtime routines. Sleep is essential to health, well-being, and resilience, and all are critical during times of uncertainty and change.
We think our 17-year-old is using marijuana, and it might be a problem.
When parents think their children may have a problem with drugs, the children almost certainly do, as parents are typically the last to know about the extent of their use. Sheltering in place together may make their drug use much more apparent, and offer an opportunity for parents to respond. Talk with them about it. Let them know what you have noticed. See if they can tell you honestly about their drug use. Kids who are only experimenting socially are unlikely to be using drugs at home under quarantine. If you are truly calm and curious, they are more likely to be honest, and it could be a relief for them to discuss it with you. Find out what they think it helps, and what – if anything – they are worried about. Then share your concerns about marijuana use and the developing brain, and the risk of addiction. If they think it is “medical” use, remind them that anxiety or mood symptoms get better with therapy, whereas drugs (including marijuana) and alcohol actually worsen those problems. It is also a time to establish home rules, explain them, and enforce them. They will have your support while stopping and may learn that they are actually sleeping and feeling better after a few weeks without marijuana.
Parents should not hesitate to reach out to pediatric providers for guidance on local resources for assessment and treatment for substance abuse and addiction. These are medical problems, and they can become serious if untreated.
My 12-year-old perfectionist is very stressed about getting her work done well now that she is home schooling. How do I help her relax?
Some children, especially our anxious perfectionists, may respond to the switch to home school with great effort and organization. These kids usually are not the ones parents worry about. But they are very prone to expanding anxiety without the regular support and feedback of teachers. The school environment naturally encourages their taking chances and normalizes the setbacks and failures that are an essential part of learning something new. At home, parents are inclined to let these kids work independently. But they benefit from regular check-ins that are not focused on work completion or scores. Instead, ask about what they are doing that is hardest, and let them teach you about it. Model how you approach a new challenge, and how you regroup and try again when you don’t get it right. Finally, this is a good age to start discussing “reasonable expectations.” No one can “do their best” all the time; not parents, not professional athletes, not even machines can sustain long bursts of maximum speed without problems. Help them to start experimenting with different speeds and levels of effort, and see how it feels.
My 10-year-old is very anxious about catching coronavirus or one of us catching it. How do I help ease her anxiety when there is no certainty about how to prevent it?
Anxiety is a normal response to a situation with as much uncertainty as this one. But some are prone to more profound anxiety, and parents may find they are doing a lot of reassuring throughout the day. For especially anxious children (and adults), accommodating the anxiety by avoiding the stressful situation is a common response that provides temporary relief. But accommodation and avoidance actually fuel anxiety, and make it harder and harder to manage. It is important to talk about the “accommodations” we all are doing, how masks are recommended to protect others (not ourselves) and to slow down the spread of a new illness so our hospitals aren’t overwhelmed. It can seem counterintuitive, but rather than jumping to reassurance or dismissing their sense of risk, ask your children to play the full movie of what they are most worried about. What happens if they get sick? If you get sick? If they are worried about dying, go ahead and ask what they think happens then. You are demonstrating that you have confidence they can handle these feelings, and you are modeling curiosity – not avoidance – yourself. Correct any misunderstandings, check on facts together, acknowledge uncertainty. It also is very important for parents to assess whether their own anxiety level makes this task especially hard or may even be contributing to their children’s level of worry. Each of us is managing anxiety right now, and this moment presents an opportunity for all of us to learn about how we can face and bear it, learn to manage, and even master it.
We are all getting cabin fever at home and snapping at each other constantly. How do we keep the peace without just hiding in our rooms all day?
Cabin fever seems inevitable when a family is suddenly at home together all day every day with no end in sight. But if we establish some simple and realistic routines and preserve some structure without being rigid, it can go a long way to helping each member of a family to find their equilibrium in this new normal. Structure can be about preserving normal sleep and meal times. Ensuring everyone is getting adequate, restful sleep and is not hungry is probably the most powerful way to keep irritability and conflict low. It is also helpful to establish some new routines. These should be simple enough to be memorable and should be realistic. You might identify predictable blocks of time that are dedicated to school (or work), exercise, creative time, and family time. While much of the day may find each family member doing some independent activity, it helps when these “blocks” are the same for everybody. Try to consistently do one or two things together, like a walk after the family dinner or family game time. And also remember that everyone needs some alone time. Respect their need for this, and it will help you to explain when you need it. If someone wants to sit out the family Yahtzee tournament, don’t shame or punish them. Just invite them again the next night!
What are going to be the consequences of all this screen time?
The great majority of kids (and parents) will not suffer any adverse consequences from the increased amount of time spent in front of screens when these activities are varied and serve a useful purpose – including distraction, senseless fun, and social time. Beyond letter or email writing, screen and phone time are the only ways to stay socially connected while physically distant. But parents are the experts on their kids. Youth who are depressed and have in the past wanted to escape into long hours of video games or YouTube videos should not be allowed to do that now. Youth with attentional issues who have a hard time stopping video games will still have that difficulty. If they are getting adequate sleep and regular exercise, and are doing most of their school work and staying socially connected, screens are not dangerous. They are proving to be a wonderful tool to help us visit libraries and museums, take dance classes, learn new languages, follow the news, order groceries, or enjoy a movie together. If we stay connected to those we care about and to the world, then this time – although marked by profound suffering and loss – may prove to be a time when we were able to slow down and remember what truly matters in our lives.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. They have no relevant financial disclosures. Email them at [email protected].
We are living through unprecedented challenges, faced with profound uncertainties about the public health, the economy, the safety of our workplaces, the risks of gathering with friends and family, and even about the rhythm of the school year. Parents always have sought guidance from their pediatric providers when they are uncertain about their children’s health, behavior, and development. We want to share some guidance with you about several of the most common questions we have been hearing in the past few months, in the hope that it may prove useful in your conversations with patients and families.
What happens when we are so busy at home that our 2-year-old is ignored for much of the day?
If they are fortunate enough to be able to work from home, but have lost their child care, many parents are suddenly facing the sustained challenge of parenting while working. Even older children will have a tough time remembering that home is now a workplace, and they can’t interrupt their parents during a Zoom meeting. But older children will understand. Younger children (preschoolers) simply will not be able to understand that their parents are in sight but not fully available to them. They are exquisitely sensitive to their parents’ attention. If they are consistently ignored, behavioral problems can emerge. If both parents are at home, they should try to arrange a schedule taking turns so that one of them could turn their full attention to their kids if need be. If a working parent can be out of sight (i.e., in another room), it makes the situation easier for everyone.
If there is only one parent at home, that mom or dad should consider arranging a babysitter or sharing child care with a friend, with some reasonable safety provisions in place. The small risk of exposure to the virus is balanced by the risk of sustained invalidation in a developing child. Help parents set reasonable expectations for how productive they can be at home. If possible, they can manage their employer’s expectations, so that they do not find themselves in the impossible bind of choosing between a crying child and a crucial deadline. If they can work near the child (and be prepared for interruptions) when reading emails or writing, that may be enough availability for the child. And parents should not be discouraged when they have to repeatedly remind their children that they adore them, but also have to work while they are at home right now. Using age-appropriate screen time as a babysitter for a few hours each day is a perfectly acceptable part of a plan. Simply planning regular breaks when their children can have their attention will make the day easier for everyone at home.
What can I do about my 13-year-old who is lying around the house all day?
This is a time to pick your battles. If children can keep their regular sleep schedule, get their schoolwork done, and do some physical exercise every day, they are doing great. And if parents are continuously complaining that they are being lazy, it will probably cease to mean much to them. Instead, focus on clear, simple expectations, and parents should live by them, too. If parents can exercise with them, or try a new activity, that is a wonderful way to model self-care and trying new things. It is important to remember that the developmental task for a 13-year-old is to establish new avenues of independence that they will drive down further with each passing year. Give them some leeway to experiment and figure out their own way of handling this challenge, although it is bound to create some tension. Parents should always acknowledge how hard it is to stick with schoolwork without school, exercise without a team, practice music without a band, or do your work without an office!
What do we do about our 16-year-old who is staying up all night and sleeping until the late afternoon?
Adolescents naturally have their sleep cycle shift, so they are sleepy later and sleep longer. But staying up all night is usually about texting with friends or playing video games. The problem is that their sleep schedule can flip. They will not be able to participate in online class or enjoy exercise in the sun, and they rarely get enough sleep during the daytime, making them more irritable, anxious, inattentive, and tired. This will only make managing their schoolwork harder and increase the chances of conflict at home. So it is important to preserve rules around sleep. You might extend bedtime by an hour or so, but preserve rules and bedtime routines. Sleep is essential to health, well-being, and resilience, and all are critical during times of uncertainty and change.
We think our 17-year-old is using marijuana, and it might be a problem.
When parents think their children may have a problem with drugs, the children almost certainly do, as parents are typically the last to know about the extent of their use. Sheltering in place together may make their drug use much more apparent, and offer an opportunity for parents to respond. Talk with them about it. Let them know what you have noticed. See if they can tell you honestly about their drug use. Kids who are only experimenting socially are unlikely to be using drugs at home under quarantine. If you are truly calm and curious, they are more likely to be honest, and it could be a relief for them to discuss it with you. Find out what they think it helps, and what – if anything – they are worried about. Then share your concerns about marijuana use and the developing brain, and the risk of addiction. If they think it is “medical” use, remind them that anxiety or mood symptoms get better with therapy, whereas drugs (including marijuana) and alcohol actually worsen those problems. It is also a time to establish home rules, explain them, and enforce them. They will have your support while stopping and may learn that they are actually sleeping and feeling better after a few weeks without marijuana.
Parents should not hesitate to reach out to pediatric providers for guidance on local resources for assessment and treatment for substance abuse and addiction. These are medical problems, and they can become serious if untreated.
My 12-year-old perfectionist is very stressed about getting her work done well now that she is home schooling. How do I help her relax?
Some children, especially our anxious perfectionists, may respond to the switch to home school with great effort and organization. These kids usually are not the ones parents worry about. But they are very prone to expanding anxiety without the regular support and feedback of teachers. The school environment naturally encourages their taking chances and normalizes the setbacks and failures that are an essential part of learning something new. At home, parents are inclined to let these kids work independently. But they benefit from regular check-ins that are not focused on work completion or scores. Instead, ask about what they are doing that is hardest, and let them teach you about it. Model how you approach a new challenge, and how you regroup and try again when you don’t get it right. Finally, this is a good age to start discussing “reasonable expectations.” No one can “do their best” all the time; not parents, not professional athletes, not even machines can sustain long bursts of maximum speed without problems. Help them to start experimenting with different speeds and levels of effort, and see how it feels.
My 10-year-old is very anxious about catching coronavirus or one of us catching it. How do I help ease her anxiety when there is no certainty about how to prevent it?
Anxiety is a normal response to a situation with as much uncertainty as this one. But some are prone to more profound anxiety, and parents may find they are doing a lot of reassuring throughout the day. For especially anxious children (and adults), accommodating the anxiety by avoiding the stressful situation is a common response that provides temporary relief. But accommodation and avoidance actually fuel anxiety, and make it harder and harder to manage. It is important to talk about the “accommodations” we all are doing, how masks are recommended to protect others (not ourselves) and to slow down the spread of a new illness so our hospitals aren’t overwhelmed. It can seem counterintuitive, but rather than jumping to reassurance or dismissing their sense of risk, ask your children to play the full movie of what they are most worried about. What happens if they get sick? If you get sick? If they are worried about dying, go ahead and ask what they think happens then. You are demonstrating that you have confidence they can handle these feelings, and you are modeling curiosity – not avoidance – yourself. Correct any misunderstandings, check on facts together, acknowledge uncertainty. It also is very important for parents to assess whether their own anxiety level makes this task especially hard or may even be contributing to their children’s level of worry. Each of us is managing anxiety right now, and this moment presents an opportunity for all of us to learn about how we can face and bear it, learn to manage, and even master it.
We are all getting cabin fever at home and snapping at each other constantly. How do we keep the peace without just hiding in our rooms all day?
Cabin fever seems inevitable when a family is suddenly at home together all day every day with no end in sight. But if we establish some simple and realistic routines and preserve some structure without being rigid, it can go a long way to helping each member of a family to find their equilibrium in this new normal. Structure can be about preserving normal sleep and meal times. Ensuring everyone is getting adequate, restful sleep and is not hungry is probably the most powerful way to keep irritability and conflict low. It is also helpful to establish some new routines. These should be simple enough to be memorable and should be realistic. You might identify predictable blocks of time that are dedicated to school (or work), exercise, creative time, and family time. While much of the day may find each family member doing some independent activity, it helps when these “blocks” are the same for everybody. Try to consistently do one or two things together, like a walk after the family dinner or family game time. And also remember that everyone needs some alone time. Respect their need for this, and it will help you to explain when you need it. If someone wants to sit out the family Yahtzee tournament, don’t shame or punish them. Just invite them again the next night!
What are going to be the consequences of all this screen time?
The great majority of kids (and parents) will not suffer any adverse consequences from the increased amount of time spent in front of screens when these activities are varied and serve a useful purpose – including distraction, senseless fun, and social time. Beyond letter or email writing, screen and phone time are the only ways to stay socially connected while physically distant. But parents are the experts on their kids. Youth who are depressed and have in the past wanted to escape into long hours of video games or YouTube videos should not be allowed to do that now. Youth with attentional issues who have a hard time stopping video games will still have that difficulty. If they are getting adequate sleep and regular exercise, and are doing most of their school work and staying socially connected, screens are not dangerous. They are proving to be a wonderful tool to help us visit libraries and museums, take dance classes, learn new languages, follow the news, order groceries, or enjoy a movie together. If we stay connected to those we care about and to the world, then this time – although marked by profound suffering and loss – may prove to be a time when we were able to slow down and remember what truly matters in our lives.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. They have no relevant financial disclosures. Email them at [email protected].
We are living through unprecedented challenges, faced with profound uncertainties about the public health, the economy, the safety of our workplaces, the risks of gathering with friends and family, and even about the rhythm of the school year. Parents always have sought guidance from their pediatric providers when they are uncertain about their children’s health, behavior, and development. We want to share some guidance with you about several of the most common questions we have been hearing in the past few months, in the hope that it may prove useful in your conversations with patients and families.
What happens when we are so busy at home that our 2-year-old is ignored for much of the day?
If they are fortunate enough to be able to work from home, but have lost their child care, many parents are suddenly facing the sustained challenge of parenting while working. Even older children will have a tough time remembering that home is now a workplace, and they can’t interrupt their parents during a Zoom meeting. But older children will understand. Younger children (preschoolers) simply will not be able to understand that their parents are in sight but not fully available to them. They are exquisitely sensitive to their parents’ attention. If they are consistently ignored, behavioral problems can emerge. If both parents are at home, they should try to arrange a schedule taking turns so that one of them could turn their full attention to their kids if need be. If a working parent can be out of sight (i.e., in another room), it makes the situation easier for everyone.
If there is only one parent at home, that mom or dad should consider arranging a babysitter or sharing child care with a friend, with some reasonable safety provisions in place. The small risk of exposure to the virus is balanced by the risk of sustained invalidation in a developing child. Help parents set reasonable expectations for how productive they can be at home. If possible, they can manage their employer’s expectations, so that they do not find themselves in the impossible bind of choosing between a crying child and a crucial deadline. If they can work near the child (and be prepared for interruptions) when reading emails or writing, that may be enough availability for the child. And parents should not be discouraged when they have to repeatedly remind their children that they adore them, but also have to work while they are at home right now. Using age-appropriate screen time as a babysitter for a few hours each day is a perfectly acceptable part of a plan. Simply planning regular breaks when their children can have their attention will make the day easier for everyone at home.
What can I do about my 13-year-old who is lying around the house all day?
This is a time to pick your battles. If children can keep their regular sleep schedule, get their schoolwork done, and do some physical exercise every day, they are doing great. And if parents are continuously complaining that they are being lazy, it will probably cease to mean much to them. Instead, focus on clear, simple expectations, and parents should live by them, too. If parents can exercise with them, or try a new activity, that is a wonderful way to model self-care and trying new things. It is important to remember that the developmental task for a 13-year-old is to establish new avenues of independence that they will drive down further with each passing year. Give them some leeway to experiment and figure out their own way of handling this challenge, although it is bound to create some tension. Parents should always acknowledge how hard it is to stick with schoolwork without school, exercise without a team, practice music without a band, or do your work without an office!
What do we do about our 16-year-old who is staying up all night and sleeping until the late afternoon?
Adolescents naturally have their sleep cycle shift, so they are sleepy later and sleep longer. But staying up all night is usually about texting with friends or playing video games. The problem is that their sleep schedule can flip. They will not be able to participate in online class or enjoy exercise in the sun, and they rarely get enough sleep during the daytime, making them more irritable, anxious, inattentive, and tired. This will only make managing their schoolwork harder and increase the chances of conflict at home. So it is important to preserve rules around sleep. You might extend bedtime by an hour or so, but preserve rules and bedtime routines. Sleep is essential to health, well-being, and resilience, and all are critical during times of uncertainty and change.
We think our 17-year-old is using marijuana, and it might be a problem.
When parents think their children may have a problem with drugs, the children almost certainly do, as parents are typically the last to know about the extent of their use. Sheltering in place together may make their drug use much more apparent, and offer an opportunity for parents to respond. Talk with them about it. Let them know what you have noticed. See if they can tell you honestly about their drug use. Kids who are only experimenting socially are unlikely to be using drugs at home under quarantine. If you are truly calm and curious, they are more likely to be honest, and it could be a relief for them to discuss it with you. Find out what they think it helps, and what – if anything – they are worried about. Then share your concerns about marijuana use and the developing brain, and the risk of addiction. If they think it is “medical” use, remind them that anxiety or mood symptoms get better with therapy, whereas drugs (including marijuana) and alcohol actually worsen those problems. It is also a time to establish home rules, explain them, and enforce them. They will have your support while stopping and may learn that they are actually sleeping and feeling better after a few weeks without marijuana.
Parents should not hesitate to reach out to pediatric providers for guidance on local resources for assessment and treatment for substance abuse and addiction. These are medical problems, and they can become serious if untreated.
My 12-year-old perfectionist is very stressed about getting her work done well now that she is home schooling. How do I help her relax?
Some children, especially our anxious perfectionists, may respond to the switch to home school with great effort and organization. These kids usually are not the ones parents worry about. But they are very prone to expanding anxiety without the regular support and feedback of teachers. The school environment naturally encourages their taking chances and normalizes the setbacks and failures that are an essential part of learning something new. At home, parents are inclined to let these kids work independently. But they benefit from regular check-ins that are not focused on work completion or scores. Instead, ask about what they are doing that is hardest, and let them teach you about it. Model how you approach a new challenge, and how you regroup and try again when you don’t get it right. Finally, this is a good age to start discussing “reasonable expectations.” No one can “do their best” all the time; not parents, not professional athletes, not even machines can sustain long bursts of maximum speed without problems. Help them to start experimenting with different speeds and levels of effort, and see how it feels.
My 10-year-old is very anxious about catching coronavirus or one of us catching it. How do I help ease her anxiety when there is no certainty about how to prevent it?
Anxiety is a normal response to a situation with as much uncertainty as this one. But some are prone to more profound anxiety, and parents may find they are doing a lot of reassuring throughout the day. For especially anxious children (and adults), accommodating the anxiety by avoiding the stressful situation is a common response that provides temporary relief. But accommodation and avoidance actually fuel anxiety, and make it harder and harder to manage. It is important to talk about the “accommodations” we all are doing, how masks are recommended to protect others (not ourselves) and to slow down the spread of a new illness so our hospitals aren’t overwhelmed. It can seem counterintuitive, but rather than jumping to reassurance or dismissing their sense of risk, ask your children to play the full movie of what they are most worried about. What happens if they get sick? If you get sick? If they are worried about dying, go ahead and ask what they think happens then. You are demonstrating that you have confidence they can handle these feelings, and you are modeling curiosity – not avoidance – yourself. Correct any misunderstandings, check on facts together, acknowledge uncertainty. It also is very important for parents to assess whether their own anxiety level makes this task especially hard or may even be contributing to their children’s level of worry. Each of us is managing anxiety right now, and this moment presents an opportunity for all of us to learn about how we can face and bear it, learn to manage, and even master it.
We are all getting cabin fever at home and snapping at each other constantly. How do we keep the peace without just hiding in our rooms all day?
Cabin fever seems inevitable when a family is suddenly at home together all day every day with no end in sight. But if we establish some simple and realistic routines and preserve some structure without being rigid, it can go a long way to helping each member of a family to find their equilibrium in this new normal. Structure can be about preserving normal sleep and meal times. Ensuring everyone is getting adequate, restful sleep and is not hungry is probably the most powerful way to keep irritability and conflict low. It is also helpful to establish some new routines. These should be simple enough to be memorable and should be realistic. You might identify predictable blocks of time that are dedicated to school (or work), exercise, creative time, and family time. While much of the day may find each family member doing some independent activity, it helps when these “blocks” are the same for everybody. Try to consistently do one or two things together, like a walk after the family dinner or family game time. And also remember that everyone needs some alone time. Respect their need for this, and it will help you to explain when you need it. If someone wants to sit out the family Yahtzee tournament, don’t shame or punish them. Just invite them again the next night!
What are going to be the consequences of all this screen time?
The great majority of kids (and parents) will not suffer any adverse consequences from the increased amount of time spent in front of screens when these activities are varied and serve a useful purpose – including distraction, senseless fun, and social time. Beyond letter or email writing, screen and phone time are the only ways to stay socially connected while physically distant. But parents are the experts on their kids. Youth who are depressed and have in the past wanted to escape into long hours of video games or YouTube videos should not be allowed to do that now. Youth with attentional issues who have a hard time stopping video games will still have that difficulty. If they are getting adequate sleep and regular exercise, and are doing most of their school work and staying socially connected, screens are not dangerous. They are proving to be a wonderful tool to help us visit libraries and museums, take dance classes, learn new languages, follow the news, order groceries, or enjoy a movie together. If we stay connected to those we care about and to the world, then this time – although marked by profound suffering and loss – may prove to be a time when we were able to slow down and remember what truly matters in our lives.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. They have no relevant financial disclosures. Email them at [email protected].
COVID-19 will likely change docs’ incentive targets, bonuses: Survey
“Employed physicians are often getting a guaranteed salary for a month or two, but no bonuses or extra distributions,” Joel Greenwald, MD, a financial adviser for physicians in St. Louis Park, Minn., told Medscape Medical News.
“This amounts to salary reductions of 10% to 30%,” he said.
The COVID-19 crisis dramatically reversed the consistent upward trajectory of physician compensation, according to a Medical Group Management Association (MGMA) survey, as reported by Medscape Medical News.
The survey, conducted April 7-8, found that practices have reported an average 55% drop in income. The report also found an average decrease in patient volume of 60%.
Before pandemic, salaries were rising
The pandemic interrupted a steady gain in compensation for this year compared to last, according to the Medscape Physician Compensation Report 2020.
The report reflects data gathered from October 4, 2019, to February 10, 2020, and includes online survey responses from 17,000 physicians in more than 30 specialties.
Before the pandemic, primary care physician (PCP) pay was up 2.5%, to $243,000, from the previous year’s average of $237,000. Specialists saw a 1.5% increase, from $341,000 in 2019 to $346,000 this year.
Reported compensation for employed physicians included salary, bonus, and profit-sharing contributions. For those self-employed, compensation includes earnings after taxes and deductible business expenses before income tax.
This report reflects only full-time salaries. But most physicians work more than full time. The report notes that physicians overall spent 37.8 hours a week seeing patients. Add to that the 15.6 average hours spent on paperwork, and doctors are averaging 53.4 hours a week.
Administrative demands varied widely by specialty. Physicians in critical care, for example, spent the most hours on paperwork (19.1 per week), and ophthalmologists spent the least on those tasks, at 9.8.
Orthopedists top earners again
The top four specialties were the same this year as they were last year and were ranked in the same order: orthopedists made the most, at $511,000, followed by plastic surgeons, at $479,000, otolaryngologists, at $455,000, and cardiologists, at $438,000.
Pediatricians and public health/preventive medicine physicians made the least, at $232,000, followed by family physicians ($234,000) and diabetes/endocrinology specialists ($236,000).
Despite the low ranking, public health/preventive medicine providers had the biggest compensation increase of all physicians, up 11% from last year. Two specialties saw a decrease: otolaryngology salaries dropped 1%, and dermatology pay dropped 2%. Pay in gastroenterology and diabetes/endocrinology was virtually unchanged from last year.
Kentucky has highest pay
Ranked by state, physicians in Kentucky made the most on average ($346,000). Utah, Ohio, and North Carolina were new to the top 10 in physician pay this year, pushing out Connecticut, Arkansas, and Nevada.
More than half of all physicians receive incentive bonuses (58% of PCPs and 55% of specialists).
The average incentive bonus is 13% of salary, but that varies by specialty. Orthopedists got an average $96,000 bonus, whereas family physicians got $24,000.
According to the report, “Among physicians who have an incentive bonus, about a third of both PCPs and specialists say the prospect of an incentive bonus has encouraged them to work longer hours.”
Gender gap similar to previous year
Consistent with Medscape compensation reports over the past decade, this year’s report shows a large gender gap in pay. Among PCPs, men made 25% more than women ($264,000 vs. $212,000); among specialists, they made 31% more than their female colleagues ($375,000 vs. $286,000).
Some specialties report positive changes from growing awareness of the gap.
“Many organizations have been carefully analyzing their culture, transparency, and pay practices to make sure they aren’t unintentionally discriminating against any group of employees,” Halee Fischer-Wright, MD, pediatrician and CEO of MGMA, told Medscape Medical News.
She added that the growing physician shortage has given all physicians more leverage in salary demands and that increased recognition of the gender gap is giving women more confidence and more evidence to use in negotiations.
Three specialties have seen large increases in the past 5 years in the percentage of women physicians. Obstetrics/gynecology and pediatrics both saw increases from 50% in 2015 to 58% in 2020. Additionally, women now account for 54% of rheumatologists, up from 29% in 2015.
Would you choose your specialty again?
Of responding physicians who were asked if they would choose their specialty again, internists were least likely to say yes (66%), followed by nephrologists (69%) and family physicians (70%).
Orthopedists were most likely to say they would choose the same specialty (97%), followed by oncologists (96%) and ophthalmologists and dermatologists (both at 95%).
Most physicians overall (77%) said they would choose medicine again.
Despite aggravations and pressures, in this survey and in previous years, physicians have indicated that the top rewards are “gratitude/relationships with patients,” “being very good at what I do/finding answers, diagnoses,” and “knowing that I make the world a better place.” From 24% to 27% ranked those rewards most important.
“Making good money at a job I like” came in fourth, at 12%.
This article first appeared on Medscape.com.
“Employed physicians are often getting a guaranteed salary for a month or two, but no bonuses or extra distributions,” Joel Greenwald, MD, a financial adviser for physicians in St. Louis Park, Minn., told Medscape Medical News.
“This amounts to salary reductions of 10% to 30%,” he said.
The COVID-19 crisis dramatically reversed the consistent upward trajectory of physician compensation, according to a Medical Group Management Association (MGMA) survey, as reported by Medscape Medical News.
The survey, conducted April 7-8, found that practices have reported an average 55% drop in income. The report also found an average decrease in patient volume of 60%.
Before pandemic, salaries were rising
The pandemic interrupted a steady gain in compensation for this year compared to last, according to the Medscape Physician Compensation Report 2020.
The report reflects data gathered from October 4, 2019, to February 10, 2020, and includes online survey responses from 17,000 physicians in more than 30 specialties.
Before the pandemic, primary care physician (PCP) pay was up 2.5%, to $243,000, from the previous year’s average of $237,000. Specialists saw a 1.5% increase, from $341,000 in 2019 to $346,000 this year.
Reported compensation for employed physicians included salary, bonus, and profit-sharing contributions. For those self-employed, compensation includes earnings after taxes and deductible business expenses before income tax.
This report reflects only full-time salaries. But most physicians work more than full time. The report notes that physicians overall spent 37.8 hours a week seeing patients. Add to that the 15.6 average hours spent on paperwork, and doctors are averaging 53.4 hours a week.
Administrative demands varied widely by specialty. Physicians in critical care, for example, spent the most hours on paperwork (19.1 per week), and ophthalmologists spent the least on those tasks, at 9.8.
Orthopedists top earners again
The top four specialties were the same this year as they were last year and were ranked in the same order: orthopedists made the most, at $511,000, followed by plastic surgeons, at $479,000, otolaryngologists, at $455,000, and cardiologists, at $438,000.
Pediatricians and public health/preventive medicine physicians made the least, at $232,000, followed by family physicians ($234,000) and diabetes/endocrinology specialists ($236,000).
Despite the low ranking, public health/preventive medicine providers had the biggest compensation increase of all physicians, up 11% from last year. Two specialties saw a decrease: otolaryngology salaries dropped 1%, and dermatology pay dropped 2%. Pay in gastroenterology and diabetes/endocrinology was virtually unchanged from last year.
Kentucky has highest pay
Ranked by state, physicians in Kentucky made the most on average ($346,000). Utah, Ohio, and North Carolina were new to the top 10 in physician pay this year, pushing out Connecticut, Arkansas, and Nevada.
More than half of all physicians receive incentive bonuses (58% of PCPs and 55% of specialists).
The average incentive bonus is 13% of salary, but that varies by specialty. Orthopedists got an average $96,000 bonus, whereas family physicians got $24,000.
According to the report, “Among physicians who have an incentive bonus, about a third of both PCPs and specialists say the prospect of an incentive bonus has encouraged them to work longer hours.”
Gender gap similar to previous year
Consistent with Medscape compensation reports over the past decade, this year’s report shows a large gender gap in pay. Among PCPs, men made 25% more than women ($264,000 vs. $212,000); among specialists, they made 31% more than their female colleagues ($375,000 vs. $286,000).
Some specialties report positive changes from growing awareness of the gap.
“Many organizations have been carefully analyzing their culture, transparency, and pay practices to make sure they aren’t unintentionally discriminating against any group of employees,” Halee Fischer-Wright, MD, pediatrician and CEO of MGMA, told Medscape Medical News.
She added that the growing physician shortage has given all physicians more leverage in salary demands and that increased recognition of the gender gap is giving women more confidence and more evidence to use in negotiations.
Three specialties have seen large increases in the past 5 years in the percentage of women physicians. Obstetrics/gynecology and pediatrics both saw increases from 50% in 2015 to 58% in 2020. Additionally, women now account for 54% of rheumatologists, up from 29% in 2015.
Would you choose your specialty again?
Of responding physicians who were asked if they would choose their specialty again, internists were least likely to say yes (66%), followed by nephrologists (69%) and family physicians (70%).
Orthopedists were most likely to say they would choose the same specialty (97%), followed by oncologists (96%) and ophthalmologists and dermatologists (both at 95%).
Most physicians overall (77%) said they would choose medicine again.
Despite aggravations and pressures, in this survey and in previous years, physicians have indicated that the top rewards are “gratitude/relationships with patients,” “being very good at what I do/finding answers, diagnoses,” and “knowing that I make the world a better place.” From 24% to 27% ranked those rewards most important.
“Making good money at a job I like” came in fourth, at 12%.
This article first appeared on Medscape.com.
“Employed physicians are often getting a guaranteed salary for a month or two, but no bonuses or extra distributions,” Joel Greenwald, MD, a financial adviser for physicians in St. Louis Park, Minn., told Medscape Medical News.
“This amounts to salary reductions of 10% to 30%,” he said.
The COVID-19 crisis dramatically reversed the consistent upward trajectory of physician compensation, according to a Medical Group Management Association (MGMA) survey, as reported by Medscape Medical News.
The survey, conducted April 7-8, found that practices have reported an average 55% drop in income. The report also found an average decrease in patient volume of 60%.
Before pandemic, salaries were rising
The pandemic interrupted a steady gain in compensation for this year compared to last, according to the Medscape Physician Compensation Report 2020.
The report reflects data gathered from October 4, 2019, to February 10, 2020, and includes online survey responses from 17,000 physicians in more than 30 specialties.
Before the pandemic, primary care physician (PCP) pay was up 2.5%, to $243,000, from the previous year’s average of $237,000. Specialists saw a 1.5% increase, from $341,000 in 2019 to $346,000 this year.
Reported compensation for employed physicians included salary, bonus, and profit-sharing contributions. For those self-employed, compensation includes earnings after taxes and deductible business expenses before income tax.
This report reflects only full-time salaries. But most physicians work more than full time. The report notes that physicians overall spent 37.8 hours a week seeing patients. Add to that the 15.6 average hours spent on paperwork, and doctors are averaging 53.4 hours a week.
Administrative demands varied widely by specialty. Physicians in critical care, for example, spent the most hours on paperwork (19.1 per week), and ophthalmologists spent the least on those tasks, at 9.8.
Orthopedists top earners again
The top four specialties were the same this year as they were last year and were ranked in the same order: orthopedists made the most, at $511,000, followed by plastic surgeons, at $479,000, otolaryngologists, at $455,000, and cardiologists, at $438,000.
Pediatricians and public health/preventive medicine physicians made the least, at $232,000, followed by family physicians ($234,000) and diabetes/endocrinology specialists ($236,000).
Despite the low ranking, public health/preventive medicine providers had the biggest compensation increase of all physicians, up 11% from last year. Two specialties saw a decrease: otolaryngology salaries dropped 1%, and dermatology pay dropped 2%. Pay in gastroenterology and diabetes/endocrinology was virtually unchanged from last year.
Kentucky has highest pay
Ranked by state, physicians in Kentucky made the most on average ($346,000). Utah, Ohio, and North Carolina were new to the top 10 in physician pay this year, pushing out Connecticut, Arkansas, and Nevada.
More than half of all physicians receive incentive bonuses (58% of PCPs and 55% of specialists).
The average incentive bonus is 13% of salary, but that varies by specialty. Orthopedists got an average $96,000 bonus, whereas family physicians got $24,000.
According to the report, “Among physicians who have an incentive bonus, about a third of both PCPs and specialists say the prospect of an incentive bonus has encouraged them to work longer hours.”
Gender gap similar to previous year
Consistent with Medscape compensation reports over the past decade, this year’s report shows a large gender gap in pay. Among PCPs, men made 25% more than women ($264,000 vs. $212,000); among specialists, they made 31% more than their female colleagues ($375,000 vs. $286,000).
Some specialties report positive changes from growing awareness of the gap.
“Many organizations have been carefully analyzing their culture, transparency, and pay practices to make sure they aren’t unintentionally discriminating against any group of employees,” Halee Fischer-Wright, MD, pediatrician and CEO of MGMA, told Medscape Medical News.
She added that the growing physician shortage has given all physicians more leverage in salary demands and that increased recognition of the gender gap is giving women more confidence and more evidence to use in negotiations.
Three specialties have seen large increases in the past 5 years in the percentage of women physicians. Obstetrics/gynecology and pediatrics both saw increases from 50% in 2015 to 58% in 2020. Additionally, women now account for 54% of rheumatologists, up from 29% in 2015.
Would you choose your specialty again?
Of responding physicians who were asked if they would choose their specialty again, internists were least likely to say yes (66%), followed by nephrologists (69%) and family physicians (70%).
Orthopedists were most likely to say they would choose the same specialty (97%), followed by oncologists (96%) and ophthalmologists and dermatologists (both at 95%).
Most physicians overall (77%) said they would choose medicine again.
Despite aggravations and pressures, in this survey and in previous years, physicians have indicated that the top rewards are “gratitude/relationships with patients,” “being very good at what I do/finding answers, diagnoses,” and “knowing that I make the world a better place.” From 24% to 27% ranked those rewards most important.
“Making good money at a job I like” came in fourth, at 12%.
This article first appeared on Medscape.com.
Video coaching may relieve anxiety and distress for long-distance cancer caregivers
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
Anxiety and distress related to caring for a cancer patient who lives far away may be alleviated through an intervention that includes video-based coaching sessions with a nurse practitioner or social worker, a randomized study suggests.
About 20% of long-distance caregivers had a significant reduction in anxiety and 25% had a significant reduction in distress when they received video coaching sessions, attended oncologist visits via video, and had access to a website specifically designed for their needs.
Adding the caregiver to oncologist office visits made the patients feel better supported and didn’t add a significant amount of time to the encounter, said Sara L. Douglas, PhD, RN, of Case Western Reserve University, Cleveland.
Taken together, these results suggest that fairly simple technologies can be leveraged to help caregivers cope with psychological strains related to supporting a patient who doesn’t live nearby, Dr. Douglas said.
Distance caregivers, defined as those who live an hour or more away from the patient, can experience high rates of distress and anxiety because they lack first-hand information or may have uncertainty about the patient’s current condition, according to Dr. Douglas and colleagues.
“Caregivers’ high rates of anxiety and distress have been found to have a negative impact not only upon their own health but upon their ability to provide high quality care to the patient,” Dr. Douglas said.
With this in mind, she and her colleagues conducted a 4-month study of distance caregivers. Dr. Douglas presented results from the study at the American Society of Clinical Oncology virtual scientific program during a press briefing in advance of the meeting. This year, ASCO’s annual meeting is split into two parts. The virtual scientific program will be presented online on May 29-31, and the virtual education program will be available Aug. 8-10.
Study details
The study enrolled 441 distance caregivers of cancer patients, and Dr. Douglas presented results in 311 of those caregivers. (Data in the presentation differ from the abstract.) The caregivers were, on average, 47 years of age. Most were female (72%), white (67%), the child of the patient (63%), currently employed (81%), and new to the distance caregiver role (89%).
The caregivers were randomized to one of three study arms.
One arm received the full intervention, which consisted of four video-coaching sessions with an advanced practice nurse or social worker, videoconference office visits with the physician and patient, and access to a website with information for cancer distance caregivers. A second arm received no video coaching but had access to the website and participated in video visits with the physician and patient. The third arm, which only received access to the website, served as the study’s control group.
Results
Dr. Douglas said that the full intervention had the biggest impact on caregivers’ distress and anxiety.
Among distance caregivers who received the full intervention, 19.2% had a significant reduction in anxiety (P = .03), as measured in online surveys before and after the intervention using the PROMIS Anxiety instrument. Furthermore, 24.8% of these caregivers had a significant reduction in distress (P = .02) from preintervention to post intervention, as measured by the National Comprehensive Cancer Network Distress Thermometer. Overall, distress and anxiety scores decreased in this arm.
Distance caregivers who only had physician-patient video visits and website access had a “moderate” reduction in distress and anxiety, Dr. Douglas said. Among these caregivers, 17.3% had an improvement in anxiety from baseline, and 19.8% had an improvement in distress. Overall, distress scores decreased, but anxiety scores increased slightly in this arm.
In the control arm, 13.1% of caregivers had an improvement in anxiety from baseline, and 18% had an improvement in distress. Overall, both anxiety and distress scores increased in this arm.
“While the full intervention yielded the best results for distance caregivers, we recognize that not all health care systems have the resources to provide individualized coaching sessions to distance caregivers,” Dr. Douglas said. “Therefore, it is worth noting that videoconference office visits alone are found to be of some benefit in improving distress and anxiety in this group of cancer caregivers.”
The study results suggest videoconferencing interventions can improve the emotional well-being of remote caregivers who provide “critical support” for cancer patients, said ASCO President Howard A. “Skip” Burris III, MD.
“As COVID-19 forces separation from loved ones and increases anxiety for people with cancer and their caregivers, providing emotional support virtually is more important than ever,” Dr. Burris said in a news release highlighting the study.
This study was funded by the National Institutes of Health and Case Comprehensive Cancer Center. Dr. Douglas reported having no disclosures. Other researchers involved in the study disclosed relationships with BridgeBio Pharma, Cardinal Health, Apexigen, Roche/Genentech, Seattle Genetics, Tesaro, Array BioPharma, Abbvie, Bristol-Myers Squibb, and Celgene. A full list of Dr. Burris’s financial disclosures is available on the ASCO website.
SOURCE: Douglas SL et al. ASCO 2020, Abstract 12123.
FROM ASCO 2020
Consider COVID-19–associated multisystem hyperinflammatory syndrome
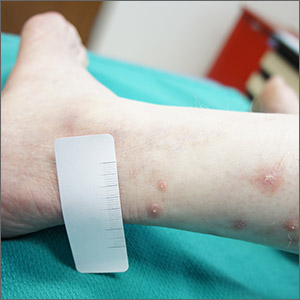
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
A 21-year-old young adult presented to the ED with a 1-week history of high fever, vomiting, diarrhea, and abdominal pain. His mother was SARS-CoV-2 positive by polymerase chain reaction approximately 3 weeks prior; his PCR was negative for SARS-CoV-2.
Following admission, he became hypotensive and tachycardic with evidence of myocarditis. His chest x-ray was normal and his O2 saturation was 100% on room air. His clinical presentation was initially suggestive of toxic shock syndrome without a rash, but despite aggressive fluid resuscitation and broad-spectrum antibiotics, he continued to clinically deteriorate with persistent high fever and increasing cardiac stress. Echocardiography revealed biventricular dysfunction. His laboratory abnormalities included rising inflammatory markers and troponin I and B-type natriuretic peptide (BNP). A repeat PCR for SARS-CoV-2 was negative on day 2 of illness. He was diagnosed as likely having macrophage-activation syndrome (MAS) despite the atypical features (myocarditis), and he received Anakinra with no apparent response. He also was given intravenous immunoglobulin (IVIg) for his myocarditis and subsequently high-dose steroids. He became afebrile, his blood pressure stabilized, his inflammatory markers declined, and over several days he returned to normal. His COVID-19 antibody test IgG was positive on day 4 of illness.
This case challenged us for several reasons. First, the PCR from his nasopharynx was negative on two occasions, which raises the issue of how sensitive and accurate these PCR tests are for SARS-CoV-2 or are patients with COVID-19–associated hyperinflammatory syndrome still PCR positive? Second, although we have seen many adult cases with a cytokine storm picture similar to this patient, nearly all of the prior cases had chest x-ray abnormalities and hypoxia. Third, the severity of the myocardial dysfunction and rising troponin and BNP also was unusual in our experience with COVID-19 infection. Lastly, the use of antibody detection to SARS-CoV-2 enabled us to confirm recent COIVD-19 disease and see his illness as part of the likely spectrum of clinical syndromes seen with this virus.
The Lancet reported eight children, aged 4-14 years, with a hyperinflammatory shock-like syndrome in early May.1 The cases had features similar to atypical Kawasaki disease, KD shock syndrome, and toxic shock syndrome. Each case had high fever for multiple days; diarrhea and abdominal pain was present in even children; elevated ferritin, C-reactive protein, d-dimer, increased troponins, and ventricular dysfunction also was present in seven. Most patients had no pulmonary involvement, and most tested negative for SARS-CoV-2 despite four of the eight having direct contact with a COVID-positive family member. All received IVIg and antibiotics; six received aspirin. Seven of the eight made a full recovery; one child died from a large cerebrovascular infarct.
Also in early May, the New York Times described a “mysterious” hyperinflammatory syndrome in children thought to be linked to COVID-19. A total of 76 suspected cases in children had been reported in New York state, three of whom died. The syndrome has been given the name pediatric multisystem inflammatory syndrome. The syndrome can resemble KD shock syndrome with rash; fever; conjunctivitis; hypotension; and redness in the lips, tongue and mucous membranes . It also can resemble toxic shock syndrome with abdominal pain, vomiting, and diarrhea. However, the degree of cardiac inflammation and dysfunction is substantial in many cases and usually beyond that seen in KD or toxic shock.
The syndrome is not limited to the United States. The Royal College of Pediatrics and Child Health has created a case definition:2
- A child presenting with persistent fever, inflammation (elevated C-reactive protein, neutrophilia, and lymphopenia) and evidence of single or multiorgan dysfunction (shock, cardiac, respiratory, renal, gastrointestinal, or neurologic) with additional features.
- Exclusion of any other microbial causes such as bacterial sepsis or staphylococcal or streptococcal shock syndromes, infections known to be associated with myocarditis (such as enterovirus).
- SARS-CoV-2 testing may or may not be positive.
As with our young adult, treatment is supportive, nonspecific, and aimed at quieting the inflammatory response. The current thinking is the syndrome is seen as antibody to SARS-CoV-2 appears and frequently the nasopharyngeal PCR is negative. It is hypothesized that the syndrome occurs in genetically predisposed hosts and potentially is a late-onset inflammatory process or potentially an antibody-triggered inflammatory process. The negative PCR from nasopharyngeal specimens reflects that the onset is later in the course of disease; whether fecal samples would be COVID positive is unknown. As with our case, antibody testing for IgG against SARS-CoV-2 is appropriate to confirm COVID-19 disease and may be positive as early as day 7.
The approach needs to be team oriented and include cardiology, rheumatology, infectious diseases, and intensive care specialists working collaboratively. Such cases should be considered COVID positive despite negative PCR tests, and full personal protective equipment should be used as we do not as yet know if live virus could be found in stool. We initiated treatment with Anakinra (an interleukin-1 type-1 receptor inhibitor) as part of our treatment protocol for MAS; we did not appreciate a response. He then received IVIg and high-dose steroids, and he recovered over several days with improved cardiac function and stable blood pressure.
What is the pathogenesis? Is SARS-CoV-2 causative or just an associated finding? Who are the at-risk children, adolescents, and adults? Is there a genetic predisposition? What therapies work best? The eight cases described in London all received IVIg, as did our case, and all but one improved and survived. In adults we have seen substantial inflammation with elevated C-reactive protein (often as high as 300), ferritin, lactate dehydrogenase, triglycerides, fibrinogen, and d-dimers, but nearly all have extensive pulmonary disease, hypoxia, and are SARS-CoV-2 positive by PCR. Influenza is also associated with a cytokine storm syndrome in adolescents and young adults.3 The mechanisms influenza virus uses to initiate a cytokine storm and strategies for immunomodulatory treatment may provide insights into COVID-19–associated multisystem hyperinflammatory syndrome.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician in pediatric infectious diseases at Boston Medical Center. Dr. Camelo is a senior fellow in pediatric infectious diseases at Boston Medical Center. They have no relevant financial disclosures. Email them at [email protected].
References
1. Riphagen S et al. Lancet. 2020 May 6. doi: 10.1016/S0140-6736(20)31094-1.
2. Royal College of Paediatrics and Child Health Guidance: Paediatric multisystem inflammatory syndrome temporally associated with COVID-19.
3. Liu Q et al.Cell Mol Immunol. 2016 Jan;13(1):3-10.
Secondary acute lymphoblastic leukemia more lethal than de novo
The application of improved chemotherapy regimens and novel chemotherapy for acute lymphoblastic leukemia (ALL) has increased the complete remission rate to 85%-90%, however, secondary ALL is common, and the prolonged long-term survival rate is only 30%-50% among ALL patients.
Favorable outcomes decrease with increasing age, and overall survival is greater for adult patients with de novo ALL, compared with patients with secondary ALL, according to the Jiansheng Zhong of the department of hematology, Guangzhou Red Cross Hospital, Jinan University, Guangzhou, Guangdong, China, and colleagues in a new study published online in Clinical Lymphoma, Myeloma & Leukemia.
The researchers retrospectively analyzed the results of 8,305 ALL patients undergoing chemotherapy from the Surveillance, Epidemiology, and End Results (SEER) database during 1975 to 2015, of which 7,454 (80.1%) cases were in the de novo ALL group, and 851 (19.9%) cases were in the secondary acute lymphoblastic leukemia (sALL) group. They used propensity matching before assessing overall survival between the two groups.
Demographically, the results showed that women ALL patients had a lower risk of death than men [hazard ratio (HR) = .93, P < .01], and that the mortality in blacks was higher than that of whites (HR = 1.29, P < .001).
For both ALL groups, patients aged 45-75 years and patients 75 years and older had a higher risk of death than younger patients (HR = 1.82, P < .001 and HR = 3.85, P < .001, respectively).
Although the mean age of de novo ALL group was significantly less than that of the sALL group (51.1 vs. 60.3 years, P < .001), after the propensity matching, the 1-, 2-, 3-, 4- and 5-year overall survival of the de novo ALL group was higher than that of the sALL group at all ages (18-75 years, P < .001).
The authors speculated that one reason for the across-the-board increased mortality in sALL, compared with de novo ALL, might be the fact that sALL patients have been reported to have more MLL gene rearrangements and chromosomal aberrations than are found in de novo ALL. This has previously been suggested as the reason for poor prognosis in secondary ALL patients.
One limitation of the study mentioned by the authors was the lack of individualized chemotherapy data available for analysis. “Considering that the features of sALL and chemotherapeutic modalities or therapy protocols may affect the mortality of sALL, more work is needed to be done in the future to demonstrate the association between chemotherapy and the prognosis of ALL patients, and the influence of cytogenetic lesions and molecular characteristics on sALL,” they concluded.
The authors declared they had no conflicts of interest.
SOURCE: Zhong J et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 30; doi.org/10.1016/j.clml.2020.04.013.
The application of improved chemotherapy regimens and novel chemotherapy for acute lymphoblastic leukemia (ALL) has increased the complete remission rate to 85%-90%, however, secondary ALL is common, and the prolonged long-term survival rate is only 30%-50% among ALL patients.
Favorable outcomes decrease with increasing age, and overall survival is greater for adult patients with de novo ALL, compared with patients with secondary ALL, according to the Jiansheng Zhong of the department of hematology, Guangzhou Red Cross Hospital, Jinan University, Guangzhou, Guangdong, China, and colleagues in a new study published online in Clinical Lymphoma, Myeloma & Leukemia.
The researchers retrospectively analyzed the results of 8,305 ALL patients undergoing chemotherapy from the Surveillance, Epidemiology, and End Results (SEER) database during 1975 to 2015, of which 7,454 (80.1%) cases were in the de novo ALL group, and 851 (19.9%) cases were in the secondary acute lymphoblastic leukemia (sALL) group. They used propensity matching before assessing overall survival between the two groups.
Demographically, the results showed that women ALL patients had a lower risk of death than men [hazard ratio (HR) = .93, P < .01], and that the mortality in blacks was higher than that of whites (HR = 1.29, P < .001).
For both ALL groups, patients aged 45-75 years and patients 75 years and older had a higher risk of death than younger patients (HR = 1.82, P < .001 and HR = 3.85, P < .001, respectively).
Although the mean age of de novo ALL group was significantly less than that of the sALL group (51.1 vs. 60.3 years, P < .001), after the propensity matching, the 1-, 2-, 3-, 4- and 5-year overall survival of the de novo ALL group was higher than that of the sALL group at all ages (18-75 years, P < .001).
The authors speculated that one reason for the across-the-board increased mortality in sALL, compared with de novo ALL, might be the fact that sALL patients have been reported to have more MLL gene rearrangements and chromosomal aberrations than are found in de novo ALL. This has previously been suggested as the reason for poor prognosis in secondary ALL patients.
One limitation of the study mentioned by the authors was the lack of individualized chemotherapy data available for analysis. “Considering that the features of sALL and chemotherapeutic modalities or therapy protocols may affect the mortality of sALL, more work is needed to be done in the future to demonstrate the association between chemotherapy and the prognosis of ALL patients, and the influence of cytogenetic lesions and molecular characteristics on sALL,” they concluded.
The authors declared they had no conflicts of interest.
SOURCE: Zhong J et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 30; doi.org/10.1016/j.clml.2020.04.013.
The application of improved chemotherapy regimens and novel chemotherapy for acute lymphoblastic leukemia (ALL) has increased the complete remission rate to 85%-90%, however, secondary ALL is common, and the prolonged long-term survival rate is only 30%-50% among ALL patients.
Favorable outcomes decrease with increasing age, and overall survival is greater for adult patients with de novo ALL, compared with patients with secondary ALL, according to the Jiansheng Zhong of the department of hematology, Guangzhou Red Cross Hospital, Jinan University, Guangzhou, Guangdong, China, and colleagues in a new study published online in Clinical Lymphoma, Myeloma & Leukemia.
The researchers retrospectively analyzed the results of 8,305 ALL patients undergoing chemotherapy from the Surveillance, Epidemiology, and End Results (SEER) database during 1975 to 2015, of which 7,454 (80.1%) cases were in the de novo ALL group, and 851 (19.9%) cases were in the secondary acute lymphoblastic leukemia (sALL) group. They used propensity matching before assessing overall survival between the two groups.
Demographically, the results showed that women ALL patients had a lower risk of death than men [hazard ratio (HR) = .93, P < .01], and that the mortality in blacks was higher than that of whites (HR = 1.29, P < .001).
For both ALL groups, patients aged 45-75 years and patients 75 years and older had a higher risk of death than younger patients (HR = 1.82, P < .001 and HR = 3.85, P < .001, respectively).
Although the mean age of de novo ALL group was significantly less than that of the sALL group (51.1 vs. 60.3 years, P < .001), after the propensity matching, the 1-, 2-, 3-, 4- and 5-year overall survival of the de novo ALL group was higher than that of the sALL group at all ages (18-75 years, P < .001).
The authors speculated that one reason for the across-the-board increased mortality in sALL, compared with de novo ALL, might be the fact that sALL patients have been reported to have more MLL gene rearrangements and chromosomal aberrations than are found in de novo ALL. This has previously been suggested as the reason for poor prognosis in secondary ALL patients.
One limitation of the study mentioned by the authors was the lack of individualized chemotherapy data available for analysis. “Considering that the features of sALL and chemotherapeutic modalities or therapy protocols may affect the mortality of sALL, more work is needed to be done in the future to demonstrate the association between chemotherapy and the prognosis of ALL patients, and the influence of cytogenetic lesions and molecular characteristics on sALL,” they concluded.
The authors declared they had no conflicts of interest.
SOURCE: Zhong J et al. Clin Lymphoma Myeloma Leuk. 2020 Apr 30; doi.org/10.1016/j.clml.2020.04.013.
FROM Clinical Lymphoma, Myeloma & Leukemia
High-intensity exercise builds bone in older men
A high-intensity exercise program, already shown effective in improving bone density and performance in women, is also effective in older men with low bone density, according to the LIFTMOR-M study, published in Bone. The protocol incorporates barbell-based weightlifting and impact training involving jumping chin-ups.
“When you’ve got a condition primarily in one of the sexes, the other sex often gets ignored, and that’s absolute the case with osteoporosis,” said lead author Belinda Beck, PhD, a professor at Griffith University, Gold Coast, Australia, in an interview.
In older adults with low bone density, when it comes to building bone and reducing fracture, a review of the literature suggests that exercise doesn’t work. That’s not really true though, according to Dr. Beck. An unpublished analysis of studies of high-intensity exercise only at her institution shows promise. “It looks like exercise doesn’t work. It’s not that, it’s that the wrong kind of exercise doesn’t work,” she stressed.
The original LIFTMOR trial, in women, was inspired by a collaboration with Lisa Weis, an Olympic weightlifter who specialized in training older women, who subsequently showed improvements on bone scans. “That’s what jump-started it, because just like every other scientist, I would have been too scared to do this kind of loading in this fragile population, and that’s the reason why people haven’t been doing it. They don’t want to break people,” said Dr. Beck.
The investigators “cherry-picked some of those exercises and tested them in the LIFTMOR trial. I was nervous about the study because the weights we were lifting were much heavier than most people had applied for people with osteoporosis. The risk was, we would cause the fractures we were trying to prevent,” said Dr. Beck. Her team tested a high-intensity resistance and impact (HiRIT) protocol in postmenopausal women with low bone mass (J Bone Miner Res. 2019 Mar;34[3]:572. Controls underwent a home-based, low-intensity exercise program. They found improvements in bone density and functional performance, compared with controls.
“The exercise was effective and safe for this population if practiced with proper technique under close supervision,” said Dr. Beck, but she emphasized that the exercises must be led by experienced coaches because of the potential for injury.
The investigators then looked at men. “There are still one in five men over 50 who are going to fracture,” Dr. Beck said.
Her team launched LIFTMOR-M, which enrolled 93 men (mean age, 67.1 years) with a lower than average proximal femur areal bone mineral density. Of them, 34 were randomized to HiRIT, 33 to supervised machine-based isometric axial compression (IAC) exercise training, and 26 were designated as controls and self-selected to usual activities.
The intervention included 8 months of twice-weekly, supervised, 30-minute HiRIT sessions, which included five sets of five repetitions, using more than 85% the weight of the single repetition maximum. The routine included the deadlift, squat, and overhead press. The impact component included five sets of five repetitions of jumping chin-ups followed by a firm, flat-footed landing.
After 8 months, there was no difference in compliance between the two intervention groups. Those in the HiRIT group had improved medial femoral neck cortical thickness, compared with controls (5.6% vs. –0.1%; P = .028) and IAC (5.6% vs. 0.7%; P = .044). Those in the HiRIT group maintained distal tibia trabecular area, while the control group experienced a loss (0.2% vs. –1.6%; P = .013). The IAC group did not show any improvement in bone strength in any of the sites examined, though some findings suggest it may counteract age-related loss in bone strength indices in the distal tibia and radius.
The program requires a fluid movement that maintains a neutral spine throughout. Dr. Beck has developed the Onero program (theboneclinic.com.au/onero/) based on the routine, and licenses it to physical therapists and exercise physiologists.
The study was funded by the Australian Research Foundation and the Australian Government Research Training Program. Dr. Beck owns the Bone Clinic, which sells licenses to the Onero program based on the exercise program used in the study.
SOURCE: Beck B et al. Bone. 2020 April 11. doi: 10.1016/j.bone.2020.115362.
A high-intensity exercise program, already shown effective in improving bone density and performance in women, is also effective in older men with low bone density, according to the LIFTMOR-M study, published in Bone. The protocol incorporates barbell-based weightlifting and impact training involving jumping chin-ups.
“When you’ve got a condition primarily in one of the sexes, the other sex often gets ignored, and that’s absolute the case with osteoporosis,” said lead author Belinda Beck, PhD, a professor at Griffith University, Gold Coast, Australia, in an interview.
In older adults with low bone density, when it comes to building bone and reducing fracture, a review of the literature suggests that exercise doesn’t work. That’s not really true though, according to Dr. Beck. An unpublished analysis of studies of high-intensity exercise only at her institution shows promise. “It looks like exercise doesn’t work. It’s not that, it’s that the wrong kind of exercise doesn’t work,” she stressed.
The original LIFTMOR trial, in women, was inspired by a collaboration with Lisa Weis, an Olympic weightlifter who specialized in training older women, who subsequently showed improvements on bone scans. “That’s what jump-started it, because just like every other scientist, I would have been too scared to do this kind of loading in this fragile population, and that’s the reason why people haven’t been doing it. They don’t want to break people,” said Dr. Beck.
The investigators “cherry-picked some of those exercises and tested them in the LIFTMOR trial. I was nervous about the study because the weights we were lifting were much heavier than most people had applied for people with osteoporosis. The risk was, we would cause the fractures we were trying to prevent,” said Dr. Beck. Her team tested a high-intensity resistance and impact (HiRIT) protocol in postmenopausal women with low bone mass (J Bone Miner Res. 2019 Mar;34[3]:572. Controls underwent a home-based, low-intensity exercise program. They found improvements in bone density and functional performance, compared with controls.
“The exercise was effective and safe for this population if practiced with proper technique under close supervision,” said Dr. Beck, but she emphasized that the exercises must be led by experienced coaches because of the potential for injury.
The investigators then looked at men. “There are still one in five men over 50 who are going to fracture,” Dr. Beck said.
Her team launched LIFTMOR-M, which enrolled 93 men (mean age, 67.1 years) with a lower than average proximal femur areal bone mineral density. Of them, 34 were randomized to HiRIT, 33 to supervised machine-based isometric axial compression (IAC) exercise training, and 26 were designated as controls and self-selected to usual activities.
The intervention included 8 months of twice-weekly, supervised, 30-minute HiRIT sessions, which included five sets of five repetitions, using more than 85% the weight of the single repetition maximum. The routine included the deadlift, squat, and overhead press. The impact component included five sets of five repetitions of jumping chin-ups followed by a firm, flat-footed landing.
After 8 months, there was no difference in compliance between the two intervention groups. Those in the HiRIT group had improved medial femoral neck cortical thickness, compared with controls (5.6% vs. –0.1%; P = .028) and IAC (5.6% vs. 0.7%; P = .044). Those in the HiRIT group maintained distal tibia trabecular area, while the control group experienced a loss (0.2% vs. –1.6%; P = .013). The IAC group did not show any improvement in bone strength in any of the sites examined, though some findings suggest it may counteract age-related loss in bone strength indices in the distal tibia and radius.
The program requires a fluid movement that maintains a neutral spine throughout. Dr. Beck has developed the Onero program (theboneclinic.com.au/onero/) based on the routine, and licenses it to physical therapists and exercise physiologists.
The study was funded by the Australian Research Foundation and the Australian Government Research Training Program. Dr. Beck owns the Bone Clinic, which sells licenses to the Onero program based on the exercise program used in the study.
SOURCE: Beck B et al. Bone. 2020 April 11. doi: 10.1016/j.bone.2020.115362.
A high-intensity exercise program, already shown effective in improving bone density and performance in women, is also effective in older men with low bone density, according to the LIFTMOR-M study, published in Bone. The protocol incorporates barbell-based weightlifting and impact training involving jumping chin-ups.
“When you’ve got a condition primarily in one of the sexes, the other sex often gets ignored, and that’s absolute the case with osteoporosis,” said lead author Belinda Beck, PhD, a professor at Griffith University, Gold Coast, Australia, in an interview.
In older adults with low bone density, when it comes to building bone and reducing fracture, a review of the literature suggests that exercise doesn’t work. That’s not really true though, according to Dr. Beck. An unpublished analysis of studies of high-intensity exercise only at her institution shows promise. “It looks like exercise doesn’t work. It’s not that, it’s that the wrong kind of exercise doesn’t work,” she stressed.
The original LIFTMOR trial, in women, was inspired by a collaboration with Lisa Weis, an Olympic weightlifter who specialized in training older women, who subsequently showed improvements on bone scans. “That’s what jump-started it, because just like every other scientist, I would have been too scared to do this kind of loading in this fragile population, and that’s the reason why people haven’t been doing it. They don’t want to break people,” said Dr. Beck.
The investigators “cherry-picked some of those exercises and tested them in the LIFTMOR trial. I was nervous about the study because the weights we were lifting were much heavier than most people had applied for people with osteoporosis. The risk was, we would cause the fractures we were trying to prevent,” said Dr. Beck. Her team tested a high-intensity resistance and impact (HiRIT) protocol in postmenopausal women with low bone mass (J Bone Miner Res. 2019 Mar;34[3]:572. Controls underwent a home-based, low-intensity exercise program. They found improvements in bone density and functional performance, compared with controls.
“The exercise was effective and safe for this population if practiced with proper technique under close supervision,” said Dr. Beck, but she emphasized that the exercises must be led by experienced coaches because of the potential for injury.
The investigators then looked at men. “There are still one in five men over 50 who are going to fracture,” Dr. Beck said.
Her team launched LIFTMOR-M, which enrolled 93 men (mean age, 67.1 years) with a lower than average proximal femur areal bone mineral density. Of them, 34 were randomized to HiRIT, 33 to supervised machine-based isometric axial compression (IAC) exercise training, and 26 were designated as controls and self-selected to usual activities.
The intervention included 8 months of twice-weekly, supervised, 30-minute HiRIT sessions, which included five sets of five repetitions, using more than 85% the weight of the single repetition maximum. The routine included the deadlift, squat, and overhead press. The impact component included five sets of five repetitions of jumping chin-ups followed by a firm, flat-footed landing.
After 8 months, there was no difference in compliance between the two intervention groups. Those in the HiRIT group had improved medial femoral neck cortical thickness, compared with controls (5.6% vs. –0.1%; P = .028) and IAC (5.6% vs. 0.7%; P = .044). Those in the HiRIT group maintained distal tibia trabecular area, while the control group experienced a loss (0.2% vs. –1.6%; P = .013). The IAC group did not show any improvement in bone strength in any of the sites examined, though some findings suggest it may counteract age-related loss in bone strength indices in the distal tibia and radius.
The program requires a fluid movement that maintains a neutral spine throughout. Dr. Beck has developed the Onero program (theboneclinic.com.au/onero/) based on the routine, and licenses it to physical therapists and exercise physiologists.
The study was funded by the Australian Research Foundation and the Australian Government Research Training Program. Dr. Beck owns the Bone Clinic, which sells licenses to the Onero program based on the exercise program used in the study.
SOURCE: Beck B et al. Bone. 2020 April 11. doi: 10.1016/j.bone.2020.115362.
FROM BONE
COVID-19 fears tied to dangerous drop in child vaccinations
The social distancing and sheltering in place mandated because of the COVID-19 pandemic are keeping parents and kids out of their doctors’ offices, and that has prompted a steep decline in recommended routine vaccinations for U.S. children, according to Centers for Disease Control and Prevention researchers.
Pediatric vaccinations dropped sharply after the national emergency was declared on March 13, suggesting that some children may be at increased risk for other serious infectious diseases, such as measles.
The researchers compared weekly orders for federally funded vaccines from Jan. 6 to April 19, 2020, with those during the same period in 2019.
They noted that, by the end of the study period, there was a cumulative COVID-19–related decline of 2.5 million doses in orders for routine noninfluenza pediatric childhood vaccines recommended by the Advisory Committee on Immunization Practices, as well as a cumulative decline in orders of 250,000 doses of measles vaccines.
Although the overall decrease in vaccinations during the study period was larger, according to CDC spokesperson Richard Quartarone, the above figures represent declines clearly associated with the pandemic.
The weekly number of measles vaccines ordered for children aged 24 months or older fell dramatically to about 500 during the week beginning March 16, 2020, and fell further to approximately 250 during the week beginning March 23. It stayed at that level until the week beginning April 13. By comparison, more than 2,500 were ordered during the week starting March 2, before the emergency was declared.
The decline was notably less for children younger than 2 years. For those children, orders dropped to about 750 during the week starting March 23 and climbed slightly for 3 weeks. By comparison, during the week of March 2, about 2,000 vaccines were ordered.
The findings, which were published in the CDC’s Morbidity and Mortality Weekly Report, stem from an analysis of ordering data from the federal Vaccines for Children (VFC) Program, as well as from vaccine administration data from the CDC’s Vaccine Tracking System and the collaborative Vaccine Safety Datalink (VSD).
The VFC provides federally purchased vaccines at no cost to about half of persons aged 18 years or younger. The VSD collaborates on vaccine coverage with the CDC’s Immunization Safety Office and eight large health care organizations across the country. Vaccination coverage is the usual metric for assessing vaccine usage; providers’ orders and the number of doses administered are two proxy measures, the authors explained.
“The substantial reduction in VFC-funded pediatric vaccine ordering after the COVID-19 emergency declaration is consistent with changes in vaccine administration among children in the VSD population receiving care through eight large U.S. health care organizations,” wrote Jeanne M. Santoli, MD, and colleagues, of the immunization services division at the National Center for Immunization and Respiratory Diseases. “The smaller decline in measles-containing vaccine administration among children aged ≤24 months suggests that system-level strategies to prioritize well child care and immunization for this age group are being implemented.”
Dr. Santoli, who is an Atlanta-based pediatrician, and associates stressed the importance of maintaining regular vaccinations during the pandemic. “The identified declines in routine pediatric vaccine ordering and doses administered might indicate that U.S. children and their communities face increased risks for outbreaks of vaccine-preventable diseases,” they wrote. “Parental concerns about potentially exposing their children to COVID-19 during well child visits might contribute to the declines observed.” Parents should therefore be reminded of the necessity of protecting their children against vaccine-preventable diseases.
In 2019, a Gallup survey reported that overall support for vaccination continued to decline in the United States.
The researchers predicted that, as social distancing relaxes, unvaccinated children will be more susceptible to other serious diseases. “In response, continued coordinated efforts between health care providers and public health officials at the local, state, and federal levels will be necessary to achieve rapid catch-up vaccination,” they concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The social distancing and sheltering in place mandated because of the COVID-19 pandemic are keeping parents and kids out of their doctors’ offices, and that has prompted a steep decline in recommended routine vaccinations for U.S. children, according to Centers for Disease Control and Prevention researchers.
Pediatric vaccinations dropped sharply after the national emergency was declared on March 13, suggesting that some children may be at increased risk for other serious infectious diseases, such as measles.
The researchers compared weekly orders for federally funded vaccines from Jan. 6 to April 19, 2020, with those during the same period in 2019.
They noted that, by the end of the study period, there was a cumulative COVID-19–related decline of 2.5 million doses in orders for routine noninfluenza pediatric childhood vaccines recommended by the Advisory Committee on Immunization Practices, as well as a cumulative decline in orders of 250,000 doses of measles vaccines.
Although the overall decrease in vaccinations during the study period was larger, according to CDC spokesperson Richard Quartarone, the above figures represent declines clearly associated with the pandemic.
The weekly number of measles vaccines ordered for children aged 24 months or older fell dramatically to about 500 during the week beginning March 16, 2020, and fell further to approximately 250 during the week beginning March 23. It stayed at that level until the week beginning April 13. By comparison, more than 2,500 were ordered during the week starting March 2, before the emergency was declared.
The decline was notably less for children younger than 2 years. For those children, orders dropped to about 750 during the week starting March 23 and climbed slightly for 3 weeks. By comparison, during the week of March 2, about 2,000 vaccines were ordered.
The findings, which were published in the CDC’s Morbidity and Mortality Weekly Report, stem from an analysis of ordering data from the federal Vaccines for Children (VFC) Program, as well as from vaccine administration data from the CDC’s Vaccine Tracking System and the collaborative Vaccine Safety Datalink (VSD).
The VFC provides federally purchased vaccines at no cost to about half of persons aged 18 years or younger. The VSD collaborates on vaccine coverage with the CDC’s Immunization Safety Office and eight large health care organizations across the country. Vaccination coverage is the usual metric for assessing vaccine usage; providers’ orders and the number of doses administered are two proxy measures, the authors explained.
“The substantial reduction in VFC-funded pediatric vaccine ordering after the COVID-19 emergency declaration is consistent with changes in vaccine administration among children in the VSD population receiving care through eight large U.S. health care organizations,” wrote Jeanne M. Santoli, MD, and colleagues, of the immunization services division at the National Center for Immunization and Respiratory Diseases. “The smaller decline in measles-containing vaccine administration among children aged ≤24 months suggests that system-level strategies to prioritize well child care and immunization for this age group are being implemented.”
Dr. Santoli, who is an Atlanta-based pediatrician, and associates stressed the importance of maintaining regular vaccinations during the pandemic. “The identified declines in routine pediatric vaccine ordering and doses administered might indicate that U.S. children and their communities face increased risks for outbreaks of vaccine-preventable diseases,” they wrote. “Parental concerns about potentially exposing their children to COVID-19 during well child visits might contribute to the declines observed.” Parents should therefore be reminded of the necessity of protecting their children against vaccine-preventable diseases.
In 2019, a Gallup survey reported that overall support for vaccination continued to decline in the United States.
The researchers predicted that, as social distancing relaxes, unvaccinated children will be more susceptible to other serious diseases. “In response, continued coordinated efforts between health care providers and public health officials at the local, state, and federal levels will be necessary to achieve rapid catch-up vaccination,” they concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The social distancing and sheltering in place mandated because of the COVID-19 pandemic are keeping parents and kids out of their doctors’ offices, and that has prompted a steep decline in recommended routine vaccinations for U.S. children, according to Centers for Disease Control and Prevention researchers.
Pediatric vaccinations dropped sharply after the national emergency was declared on March 13, suggesting that some children may be at increased risk for other serious infectious diseases, such as measles.
The researchers compared weekly orders for federally funded vaccines from Jan. 6 to April 19, 2020, with those during the same period in 2019.
They noted that, by the end of the study period, there was a cumulative COVID-19–related decline of 2.5 million doses in orders for routine noninfluenza pediatric childhood vaccines recommended by the Advisory Committee on Immunization Practices, as well as a cumulative decline in orders of 250,000 doses of measles vaccines.
Although the overall decrease in vaccinations during the study period was larger, according to CDC spokesperson Richard Quartarone, the above figures represent declines clearly associated with the pandemic.
The weekly number of measles vaccines ordered for children aged 24 months or older fell dramatically to about 500 during the week beginning March 16, 2020, and fell further to approximately 250 during the week beginning March 23. It stayed at that level until the week beginning April 13. By comparison, more than 2,500 were ordered during the week starting March 2, before the emergency was declared.
The decline was notably less for children younger than 2 years. For those children, orders dropped to about 750 during the week starting March 23 and climbed slightly for 3 weeks. By comparison, during the week of March 2, about 2,000 vaccines were ordered.
The findings, which were published in the CDC’s Morbidity and Mortality Weekly Report, stem from an analysis of ordering data from the federal Vaccines for Children (VFC) Program, as well as from vaccine administration data from the CDC’s Vaccine Tracking System and the collaborative Vaccine Safety Datalink (VSD).
The VFC provides federally purchased vaccines at no cost to about half of persons aged 18 years or younger. The VSD collaborates on vaccine coverage with the CDC’s Immunization Safety Office and eight large health care organizations across the country. Vaccination coverage is the usual metric for assessing vaccine usage; providers’ orders and the number of doses administered are two proxy measures, the authors explained.
“The substantial reduction in VFC-funded pediatric vaccine ordering after the COVID-19 emergency declaration is consistent with changes in vaccine administration among children in the VSD population receiving care through eight large U.S. health care organizations,” wrote Jeanne M. Santoli, MD, and colleagues, of the immunization services division at the National Center for Immunization and Respiratory Diseases. “The smaller decline in measles-containing vaccine administration among children aged ≤24 months suggests that system-level strategies to prioritize well child care and immunization for this age group are being implemented.”
Dr. Santoli, who is an Atlanta-based pediatrician, and associates stressed the importance of maintaining regular vaccinations during the pandemic. “The identified declines in routine pediatric vaccine ordering and doses administered might indicate that U.S. children and their communities face increased risks for outbreaks of vaccine-preventable diseases,” they wrote. “Parental concerns about potentially exposing their children to COVID-19 during well child visits might contribute to the declines observed.” Parents should therefore be reminded of the necessity of protecting their children against vaccine-preventable diseases.
In 2019, a Gallup survey reported that overall support for vaccination continued to decline in the United States.
The researchers predicted that, as social distancing relaxes, unvaccinated children will be more susceptible to other serious diseases. “In response, continued coordinated efforts between health care providers and public health officials at the local, state, and federal levels will be necessary to achieve rapid catch-up vaccination,” they concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Itchy leg papules
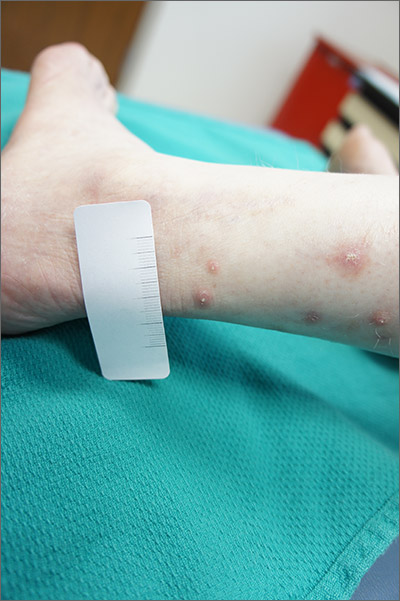
Punch biopsy revealed collagen extravasation consistent with acquired reactive perforating collagenosis, an uncommon acquired disease that is seen in patients with longstanding renal disease or diabetes mellitus. The patient’s history of end-stage renal disease, paired with the eruptive nature of the pink papules with central firm plugs, pointed to the diagnosis.
The differential diagnosis for lesions like these includes prurigo nodularis and eruptive keratoacanthomas. Prurigo nodules would itch but likely lack a central plug. Eruptive keratoacanthomas would have a central keratinaceous plug but would be less likely to itch. A biopsy can help distinguish these entities.
Reactive perforating collagenosis can affect up to 10% of hemodialysis patients. It also can be associated with human immunodeficiency virus, hyperparathyroidism, hypothyroidism, liver disease, and sclerosing cholangitis. One theory of the etiology is that underlying disease causes skin itching and the subsequent trauma from scratching causes reactivity.
The work-up consists of a punch biopsy of the entire lesion or central plug. A biopsy limited to the edge of the lesion, or one that is shallow, may fail to connect altered dermal collagen with its follicular elimination and be misread as dermatitis.
Lesions often resolve spontaneously, but disease can be widespread. Topical steroids and antihistamines may reduce itching. Narrowband UVB, topical or systemic retinoids, tetracyclines, and cryotherapy all have had reported success. Narrowband UVB is especially helpful for uremic pruritus and, if available, may be the treatment of choice.
This patient was treated with topical steroids, oral cetirizine 10 mg/d, and cryotherapy to the most stubborn lesions. Over 3 months, the number of lesions and severity of symptoms improved. She continued hemodialysis and awaits a renal transplant.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660.

Punch biopsy revealed collagen extravasation consistent with acquired reactive perforating collagenosis, an uncommon acquired disease that is seen in patients with longstanding renal disease or diabetes mellitus. The patient’s history of end-stage renal disease, paired with the eruptive nature of the pink papules with central firm plugs, pointed to the diagnosis.
The differential diagnosis for lesions like these includes prurigo nodularis and eruptive keratoacanthomas. Prurigo nodules would itch but likely lack a central plug. Eruptive keratoacanthomas would have a central keratinaceous plug but would be less likely to itch. A biopsy can help distinguish these entities.
Reactive perforating collagenosis can affect up to 10% of hemodialysis patients. It also can be associated with human immunodeficiency virus, hyperparathyroidism, hypothyroidism, liver disease, and sclerosing cholangitis. One theory of the etiology is that underlying disease causes skin itching and the subsequent trauma from scratching causes reactivity.
The work-up consists of a punch biopsy of the entire lesion or central plug. A biopsy limited to the edge of the lesion, or one that is shallow, may fail to connect altered dermal collagen with its follicular elimination and be misread as dermatitis.
Lesions often resolve spontaneously, but disease can be widespread. Topical steroids and antihistamines may reduce itching. Narrowband UVB, topical or systemic retinoids, tetracyclines, and cryotherapy all have had reported success. Narrowband UVB is especially helpful for uremic pruritus and, if available, may be the treatment of choice.
This patient was treated with topical steroids, oral cetirizine 10 mg/d, and cryotherapy to the most stubborn lesions. Over 3 months, the number of lesions and severity of symptoms improved. She continued hemodialysis and awaits a renal transplant.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Punch biopsy revealed collagen extravasation consistent with acquired reactive perforating collagenosis, an uncommon acquired disease that is seen in patients with longstanding renal disease or diabetes mellitus. The patient’s history of end-stage renal disease, paired with the eruptive nature of the pink papules with central firm plugs, pointed to the diagnosis.
The differential diagnosis for lesions like these includes prurigo nodularis and eruptive keratoacanthomas. Prurigo nodules would itch but likely lack a central plug. Eruptive keratoacanthomas would have a central keratinaceous plug but would be less likely to itch. A biopsy can help distinguish these entities.
Reactive perforating collagenosis can affect up to 10% of hemodialysis patients. It also can be associated with human immunodeficiency virus, hyperparathyroidism, hypothyroidism, liver disease, and sclerosing cholangitis. One theory of the etiology is that underlying disease causes skin itching and the subsequent trauma from scratching causes reactivity.
The work-up consists of a punch biopsy of the entire lesion or central plug. A biopsy limited to the edge of the lesion, or one that is shallow, may fail to connect altered dermal collagen with its follicular elimination and be misread as dermatitis.
Lesions often resolve spontaneously, but disease can be widespread. Topical steroids and antihistamines may reduce itching. Narrowband UVB, topical or systemic retinoids, tetracyclines, and cryotherapy all have had reported success. Narrowband UVB is especially helpful for uremic pruritus and, if available, may be the treatment of choice.
This patient was treated with topical steroids, oral cetirizine 10 mg/d, and cryotherapy to the most stubborn lesions. Over 3 months, the number of lesions and severity of symptoms improved. She continued hemodialysis and awaits a renal transplant.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660.
Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritis! Am J Med. 2019;132:E658-E660.
COVID-19: What will happen to physician income this year?
“At a combined system and hospital board meeting yesterday, there was a financial presentation,” said a cardiologist in Minnesota, who declined to be named. “We have ‘salary support’ through May 16, which means we will be receiving base pay at our 2019 level. After May 16, I think it’s fairly certain salaries will be decreased.”
A general internist in the same area added: “The system has decided to pay physicians and other employees for 8 weeks, until May 15, and they are borrowing about $150 million to do this. We don’t know what will happen after May 15, but we are supposed to have an update in early May.”
Physician income is of huge interest, and many aspects of it are discussed in Medscape’s Physician Compensation Report 2020, just released.
The worst may be yet to come
Of all the categories of physicians, “I am worried about private practices the most,” said Travis Singleton, senior vice president at Merritt Hawkins, a physician search firm. “They don’t have a financial cushion, and will start seeing big drops in revenue at the end of May.”
“A lot of the A/R [accounts receivables] for practices come within 30 days, and very little comes in after 90 days,” said Terrence R. McWilliams, MD, chief clinical consultant at HSG Advisors, a consultancy for not-for-profit hospitals and their employed physician networks around the country. “So private practices are reaching the point where prior A/R will start to dwindle and they will start feeling the decline in new claims submissions.”
Large practices may have a bigger financial cushion, but in many cases, they also have more liabilities. “We don’t know the financial loss yet, but I think it’s been devastating,” said Paul M. Yonover, MD, a urologist at UroPartners, a large single-specialty practice in Chicago with 62 urologists. “In fact, the financial loss may well be larger than our loss in volume, because we have to support our own surgery center, pathology lab, radiation center, and other in-house services.”
Employed physicians in limbo
In contrast to physicians in private practices, many employed physicians at hospitals and health systems have been shielded from the impact of COVID-19 – at least for now.
“The experiences of employed physicians are very mixed,” said Mr. Singleton at Merritt Hawkins. “Some health systems have reduced physicians’ pay by 20%, but other systems have been putting off any reductions.”
Hospitals and health systems are struggling. “Stopping elective surgeries deeply affected hospitals,” said Ryan Inman, founder of Physician Wealth Services in San Diego. “With fewer elective surgeries, they have much less income coming in. Some big hospitals that are pillars of their community are under great financial stress.”
“Hospitals’ patient volumes have fallen by 50%-90%,” Mr. McWilliams reported. “Lower volume means lower pay for employed physicians, who are paid by straight productivity or other models that require high volumes. However, some health systems have intervened to make sure these physicians get some money.”
Base pay is often safe for now, but quarterly bonuses are on the chopping block. “Employed physicians are often getting a guaranteed salary for a month or two, but no bonuses or extra distributions,” said Joel Greenwald, MD, a financial adviser for physicians in St. Louis Park, Minn., a state mecca for physician employment. “They’ve been told that they will continue to get their base salary but forget about the quarterly bonuses. This amounts to salary reductions of 10%-30%.”
Ensuring payment for these doctors means lowering their productivity benchmarks, but the benchmarks might still be too high for these times. An internist at a large health system in Minneapolis–St. Paul reports that, at a lunch meeting, employed doctors learned that payment benchmarks will be reduced to 70% of their 2019 monthly average.
“I am seeing nowhere near 70% of what I was seeing last year,” he said in an interview, asking that his name not be used. “Given how slow things have been, I am probably closer to 30%, but have not been given any data on this, so I am guessing at this point.”
Adapting to a brave new world
Even as they face a dark financial future, physicians have had to completely revamp the way they practice medicine – a cumbersome process that, in itself, incurred some financial losses. They had to obtain masks and other PPE, reposition or even close down their waiting rooms, cut back on unneeded staff, and adapt to telemedicine.
“It’s been an incredibly challenging time,” said Dr. Yonover, the Chicago urologist. “As a doctor. I cannot avoid contact, and it’s not totally clear yet how the virus spreads. But I don’t have the option of closing the door. As a practice owner, you’re responsible for the health and well-being of employees, patients, and the business.”
“A practice’s daily routine is somewhat slower and costlier,” said David N. Gans, MSHA, senior fellow at the Medical Group Management Association (MGMA), which represents group practices. “Between each patient, you have to clean a lot more than previously, and you have to stock up on PPE such as masks and gowns. PPE used to be limited to infectious patients, but now it’s universal.”
At PA Clinical Network, a clinically integrated network in Pennsylvania, volume fell 40%-50% and income fell 30%-50% from late March to late April, according to Jaan Sidorov, MD, an internist who is CEO of the network, which has 158 physicians in a variety of specialties working in 54 practices around the state.
“Revenue went down but it didn’t crash,” he said. “And our physicians pivoted very quickly. They adapted to telehealth and applied for the federal loan programs. They didn’t use waiting rooms. In some cases, staff was out in the parking lot, putting stethoscopes through patients’ windows.”
“None of the practices closed, not even temporarily,” Dr. Sidorov said. “But clearly this cannot go forever without having serious consequences.”
How much can telemedicine help?
Telemedicine has been a lifeline for many struggling practices. “As much as 20%-40% of a practice’s losses can be recouped through telemedicine, depending on variables like patients’ attitudes,” said Mr. Singleton at Merritt Hawkins.
The rise in telemedicine was made possible by a temporary relaxation of the limits on telemedicine payments by Medicare and many private payers. Medicare is currently paying the same rates for telemedicine as it does for in-office visits.
In a recent MGMA Stat survey, 97% of practices reported that they had taken up telemedicine, according to Mr. Gans. He estimates that 80% of primary care could be converted to telemedicine, including medication refills, ongoing care of chronic patients, and recording patients’ vital signs from home.
Some primary care physicians are now using telemedicine for 100% of their visits. “I voluntarily closed my practice weeks ago except for virtual visits due to the risk of exposure for my patients,” a doctor in South Carolina told the Primary Care Collaborative in mid-April. “I continue to pay my staff out of pocket but have reduced hours and am not receiving any income myself.”
However, Mr. Inman of Physician Wealth Services said family medicine clients using telemedicine for all of their patients are earning less per visit, even though the Medicare reimbursement is the same as for an office visit. “They earn less because they cannot charge for any ancillaries, such as labs or imaging,” he said.
“Telemedicine has its limits,” Mr. Singleton said. It cannot replace elective surgeries, and even in primary care practices, “there is a lot of work for which patients have to come in, such as physicals or providing vaccines,” he said. “I know of one doctor who has refrigerator full of vaccines to give out. That pays his bills.”
In many cases, “telemedicine” simply means using the phone, with no video. Many patients can only use the phone, and Medicare now reimburses for some kinds of phone visits. In a mid-April survey of primary care providers, 44% were using the telephone for the majority of their visits, and 14% were not using video at all. Medicare recently decided to pay physicians the same amount for telephone visits as in-person visits.
Financial boosts will run out soon
Many private practices are surviving only because they have managed to tap into new federal programs that can finance them for the short-term. Here are the main examples:
Receiving advance Medicare payments. Through the Medicare Accelerated and Advance Payment Program, physicians can be paid up to 3 months of their average Medicare reimbursement in advance. However, repayment starts 120 days after receiving the money and must be completed within 210 days.
Obtaining a federal loan. Under the Paycheck Protection Program (PPP), which is available to all kinds of small businesses, practices can apply for up to 2.5 times their average monthly payroll costs.
PPP money can be used for payroll, rent, mortgage interest, or utility payments for up to 8 weeks. The loan will be entirely forgiven as long as the rules are followed. For example, three quarters of the money must go to payroll, and laid-off employees must be rehired by June 30.
There was such a rush for the first round of PPP loans that many physicians failed to get the loan. “Many of my physician clients applied for the loan as soon as they could, but none of them got it,” said Mr. Inman, the San Diego financial adviser. “We are hoping that the next round of funding will provide them some relief.” The second round started on April 27.
Physicians who have already obtained the PPP loan are very relieved. “This loan made it possible for us to pay our employees,” said George W. Monks, MD, a dermatologist in Tulsa, Okla., and president of the Oklahoma Medical Association.
Staff benefiting from higher unemployment payments. Many practices and hospitals are laying off their staff so that they can collect unemployment benefits. This is a good time to do that because the federal government has boosted unemployment payments by $600 a week, creating a total benefit that is greater than many people earned at their regular jobs.
This extra boost ends in July, but practices with PPP loans will have to rehire their laid-off workers a month before that. Getting laid-off staffers to come back in is going to be critical, and some practices are already having a hard time convincing them to come back, said Michael La Penna, a physician practice manager in Grand Rapids, Mich.
“They are finding that those people don’t want to come back in yet,” he said. “In many cases they have to care for children at home or have been getting generous unemployment checks.”
The problem with all these temporary financial boosts is that they will disappear within weeks or months from now. Mr. La Penna is concerned that the sudden loss of this support could send some practices spinning into bankruptcy. “Unless volume gets better very soon, time is running out for a lot of practices,” he said.
Hospitals, which also have been depending on federal assistance, may run out of money, too. Daniel Wrenne, a financial planner for physicians in Lexington, Ky., said smaller hospitals are particularly vulnerable because they lack the capital. He said a friend who is an attorney for hospitals predicted that 25% of small regional hospitals “won’t make it through this.”
Such financial turmoil might prompt many physicians to retire or find a new job, said Gary Price, MD, a plastic surgeon in New Haven, Conn., and president of the Physicians Foundation, an advocacy group for the profession. In a survey of doctors by the Physicians Foundation and Merritt Hawkins, released on April 21, 18% planned to retire, temporarily close their practices, or opt out of patient care, and another 14%, presumably employed physicians, planned to change jobs.
Is recovery around the corner?
In early May, practices in many parts of the country were seeing the possibility of a return to normal business – or at least what could pass for normal in these unusual times.
“From mid-March to mid-April, hospitals and practices were in panic mode,” said MGMA’s Mr. Gans. “They were focusing on the here and now. But from mid-April to mid-May, they could begin looking at the big picture and decide how they will get back into business.”
Surgeons devastated by bans on elective surgeries might see a bounce in cases, as the backlog of patients comes back in. By late April, 10 states reinstituted elective surgeries, including California, Arizona, Georgia, Indiana, Colorado, and Oklahoma, and New York has reinstituted elective surgeries for some counties.
Dr. Price said he hopes to reopen his plastic surgery practice by the end of June. “If it takes longer than that, I’m not sure that the practice will survive.” His PPP loan would have run out and he would have to lay off his staff. “At that point, ongoing viability of practice would become a real question.”
Dr. Monks said he hopes a lot more patients will come to his dermatology practice. As of the end of April, “we’re starting to see an uptick in the number of patients wanting to come in,” he said. “They seem to be more comfortable with the new world we’re living in.
“Viewing the backlog of cases that haven’t been attended to,” Dr. Monks added, “I think we’ll be really busy for a while.”
But Mr. La Penna said he thinks the expected backlog of elective patients will be more like a trickle than a flood. “Many patients aren’t going to want to return that fast,” he said. “They may have a condition that makes exposure to COVID-19 more risky, like diabetes or high blood pressure, or they’re elderly, or they live in a household with one of these risk groups.”
Andrew Musbach, cofounder of MD Wealth Management in Chelsea, Mich., said he expects a slow recovery for primary care physicians as well. “Even when the lockdowns are over, not everyone is going to feel comfortable coming to a hospital or visiting a doctor’s office unless it’s absolutely necessary,” he said.
Getting back to normal patient volumes will involve finding better ways to protect patients and staff from COVID-19, Dr. Yonover said. At his urology practice, “we take all the usual precautions, but nothing yet has made it dramatically easier to protect patients and staff,” he said. “Rapid, accurate testing for COVID-19 would change the landscape, but I have no idea when that will come.”
Mr. Wrenne advises his physician clients that a financial recovery will take months. “I tell them to plan for 6 months, until October, before income returns to pre–COVID-19 levels. Reimbursement lags appointments by as much as 3 months, plus it will probably take the economy 2-3 months more to get back to normal.”
“We are facing a recession, and how long it will last is anyone’s guess,” said Alex Kilian, a physician wealth manager at Aldrich Wealth in San Diego. “The federal government’s efforts to stimulate the economy is keeping it from crashing, but there are no real signs that it will actually pick up. It may take years for the travel and entertainment industries to come back.”
A recession means patients will have less spending power, and health care sectors like laser eye surgery may be damaged for years to come, said John B. Pinto, an ophthalmology practice management consultant in San Diego. “[That kind of surgery] is purely elective and relatively costly,” he said. “When people get back to work, they are going to be building up their savings and avoiding new debt. They won’t be having [laser eye surgery].”
“There won’t be any quick return to normal for me,” said Dr. Price, the Connecticut plastic surgeon. “The damage this time will probably be worse than in the Great Recession. Back then, plastic surgery was off by 20%, but this time you have the extra problem of patients reluctant to come into medical offices.”
“To get patients to come in, facilities are going to have to convince patients that they are safe,” Mr. Singleton said. “That may mean undertaking some marketing and promotion, and hospitals tend to be much better at that than practices.”
What a new wave of COVID-19 would mean
Some states have begun reopening public places, which could signal patients to return to doctors’ offices even though doctors’ offices were never officially closed. Oklahoma, for example, reopened restaurants, movie theaters, and sports venues on May 1.
Dr. Monks, president of the Oklahoma Medical Association, said his group opposes states reopening. “The governor’s order is too hasty and overly ambitious,” he said. “Oklahoma has seen an ongoing growth in the number of cases, hospitalizations, and deaths in the past week alone [in late April].”
The concern is that opening up public places too soon would create a new wave of COVID-19, which would not only be a public health disaster, but also a financial disaster for physicians. Doctors would be back where they were in March, but unlike in March, they would not benefit from revenues from previously busy times.
Mr. Pinto said the number of COVID-19 cases will rise and fall in the next 2 years, forcing states to reenact new bans on public gatherings and on elective surgeries until the numbers subside again.
Mr. Pinto said authorities in Singapore have successfully handled such waves of the disease through short bans that are tantamount to tapping the brakes of a car. “As the car gathers speed down the hill, you tap the brake,” he said. “I suspect we’ll be seeing a lot of brake-tapping until a vaccine can be developed and distributed.”
Gary LeRoy, MD, president of the American Academy of Family Physicians, recalled the worldwide Spanish Flu pandemic a century ago. “People were allowed out of their houses after 2 months, and the flu spiked up again,” he said. “I hope we don’t make that mistake this time.”
Dr. LeRoy said it’s not possible to predict how the COVID-19 crisis will play out. “What will the future be like? I don’t know the answer,” he said. “The information we learn in next hours, days, or months will probably change everything.”
A version of this article originally appeared on Medscape.com.
“At a combined system and hospital board meeting yesterday, there was a financial presentation,” said a cardiologist in Minnesota, who declined to be named. “We have ‘salary support’ through May 16, which means we will be receiving base pay at our 2019 level. After May 16, I think it’s fairly certain salaries will be decreased.”
A general internist in the same area added: “The system has decided to pay physicians and other employees for 8 weeks, until May 15, and they are borrowing about $150 million to do this. We don’t know what will happen after May 15, but we are supposed to have an update in early May.”
Physician income is of huge interest, and many aspects of it are discussed in Medscape’s Physician Compensation Report 2020, just released.
The worst may be yet to come
Of all the categories of physicians, “I am worried about private practices the most,” said Travis Singleton, senior vice president at Merritt Hawkins, a physician search firm. “They don’t have a financial cushion, and will start seeing big drops in revenue at the end of May.”
“A lot of the A/R [accounts receivables] for practices come within 30 days, and very little comes in after 90 days,” said Terrence R. McWilliams, MD, chief clinical consultant at HSG Advisors, a consultancy for not-for-profit hospitals and their employed physician networks around the country. “So private practices are reaching the point where prior A/R will start to dwindle and they will start feeling the decline in new claims submissions.”
Large practices may have a bigger financial cushion, but in many cases, they also have more liabilities. “We don’t know the financial loss yet, but I think it’s been devastating,” said Paul M. Yonover, MD, a urologist at UroPartners, a large single-specialty practice in Chicago with 62 urologists. “In fact, the financial loss may well be larger than our loss in volume, because we have to support our own surgery center, pathology lab, radiation center, and other in-house services.”
Employed physicians in limbo
In contrast to physicians in private practices, many employed physicians at hospitals and health systems have been shielded from the impact of COVID-19 – at least for now.
“The experiences of employed physicians are very mixed,” said Mr. Singleton at Merritt Hawkins. “Some health systems have reduced physicians’ pay by 20%, but other systems have been putting off any reductions.”
Hospitals and health systems are struggling. “Stopping elective surgeries deeply affected hospitals,” said Ryan Inman, founder of Physician Wealth Services in San Diego. “With fewer elective surgeries, they have much less income coming in. Some big hospitals that are pillars of their community are under great financial stress.”
“Hospitals’ patient volumes have fallen by 50%-90%,” Mr. McWilliams reported. “Lower volume means lower pay for employed physicians, who are paid by straight productivity or other models that require high volumes. However, some health systems have intervened to make sure these physicians get some money.”
Base pay is often safe for now, but quarterly bonuses are on the chopping block. “Employed physicians are often getting a guaranteed salary for a month or two, but no bonuses or extra distributions,” said Joel Greenwald, MD, a financial adviser for physicians in St. Louis Park, Minn., a state mecca for physician employment. “They’ve been told that they will continue to get their base salary but forget about the quarterly bonuses. This amounts to salary reductions of 10%-30%.”
Ensuring payment for these doctors means lowering their productivity benchmarks, but the benchmarks might still be too high for these times. An internist at a large health system in Minneapolis–St. Paul reports that, at a lunch meeting, employed doctors learned that payment benchmarks will be reduced to 70% of their 2019 monthly average.
“I am seeing nowhere near 70% of what I was seeing last year,” he said in an interview, asking that his name not be used. “Given how slow things have been, I am probably closer to 30%, but have not been given any data on this, so I am guessing at this point.”
Adapting to a brave new world
Even as they face a dark financial future, physicians have had to completely revamp the way they practice medicine – a cumbersome process that, in itself, incurred some financial losses. They had to obtain masks and other PPE, reposition or even close down their waiting rooms, cut back on unneeded staff, and adapt to telemedicine.
“It’s been an incredibly challenging time,” said Dr. Yonover, the Chicago urologist. “As a doctor. I cannot avoid contact, and it’s not totally clear yet how the virus spreads. But I don’t have the option of closing the door. As a practice owner, you’re responsible for the health and well-being of employees, patients, and the business.”
“A practice’s daily routine is somewhat slower and costlier,” said David N. Gans, MSHA, senior fellow at the Medical Group Management Association (MGMA), which represents group practices. “Between each patient, you have to clean a lot more than previously, and you have to stock up on PPE such as masks and gowns. PPE used to be limited to infectious patients, but now it’s universal.”
At PA Clinical Network, a clinically integrated network in Pennsylvania, volume fell 40%-50% and income fell 30%-50% from late March to late April, according to Jaan Sidorov, MD, an internist who is CEO of the network, which has 158 physicians in a variety of specialties working in 54 practices around the state.
“Revenue went down but it didn’t crash,” he said. “And our physicians pivoted very quickly. They adapted to telehealth and applied for the federal loan programs. They didn’t use waiting rooms. In some cases, staff was out in the parking lot, putting stethoscopes through patients’ windows.”
“None of the practices closed, not even temporarily,” Dr. Sidorov said. “But clearly this cannot go forever without having serious consequences.”
How much can telemedicine help?
Telemedicine has been a lifeline for many struggling practices. “As much as 20%-40% of a practice’s losses can be recouped through telemedicine, depending on variables like patients’ attitudes,” said Mr. Singleton at Merritt Hawkins.
The rise in telemedicine was made possible by a temporary relaxation of the limits on telemedicine payments by Medicare and many private payers. Medicare is currently paying the same rates for telemedicine as it does for in-office visits.
In a recent MGMA Stat survey, 97% of practices reported that they had taken up telemedicine, according to Mr. Gans. He estimates that 80% of primary care could be converted to telemedicine, including medication refills, ongoing care of chronic patients, and recording patients’ vital signs from home.
Some primary care physicians are now using telemedicine for 100% of their visits. “I voluntarily closed my practice weeks ago except for virtual visits due to the risk of exposure for my patients,” a doctor in South Carolina told the Primary Care Collaborative in mid-April. “I continue to pay my staff out of pocket but have reduced hours and am not receiving any income myself.”
However, Mr. Inman of Physician Wealth Services said family medicine clients using telemedicine for all of their patients are earning less per visit, even though the Medicare reimbursement is the same as for an office visit. “They earn less because they cannot charge for any ancillaries, such as labs or imaging,” he said.
“Telemedicine has its limits,” Mr. Singleton said. It cannot replace elective surgeries, and even in primary care practices, “there is a lot of work for which patients have to come in, such as physicals or providing vaccines,” he said. “I know of one doctor who has refrigerator full of vaccines to give out. That pays his bills.”
In many cases, “telemedicine” simply means using the phone, with no video. Many patients can only use the phone, and Medicare now reimburses for some kinds of phone visits. In a mid-April survey of primary care providers, 44% were using the telephone for the majority of their visits, and 14% were not using video at all. Medicare recently decided to pay physicians the same amount for telephone visits as in-person visits.
Financial boosts will run out soon
Many private practices are surviving only because they have managed to tap into new federal programs that can finance them for the short-term. Here are the main examples:
Receiving advance Medicare payments. Through the Medicare Accelerated and Advance Payment Program, physicians can be paid up to 3 months of their average Medicare reimbursement in advance. However, repayment starts 120 days after receiving the money and must be completed within 210 days.
Obtaining a federal loan. Under the Paycheck Protection Program (PPP), which is available to all kinds of small businesses, practices can apply for up to 2.5 times their average monthly payroll costs.
PPP money can be used for payroll, rent, mortgage interest, or utility payments for up to 8 weeks. The loan will be entirely forgiven as long as the rules are followed. For example, three quarters of the money must go to payroll, and laid-off employees must be rehired by June 30.
There was such a rush for the first round of PPP loans that many physicians failed to get the loan. “Many of my physician clients applied for the loan as soon as they could, but none of them got it,” said Mr. Inman, the San Diego financial adviser. “We are hoping that the next round of funding will provide them some relief.” The second round started on April 27.
Physicians who have already obtained the PPP loan are very relieved. “This loan made it possible for us to pay our employees,” said George W. Monks, MD, a dermatologist in Tulsa, Okla., and president of the Oklahoma Medical Association.
Staff benefiting from higher unemployment payments. Many practices and hospitals are laying off their staff so that they can collect unemployment benefits. This is a good time to do that because the federal government has boosted unemployment payments by $600 a week, creating a total benefit that is greater than many people earned at their regular jobs.
This extra boost ends in July, but practices with PPP loans will have to rehire their laid-off workers a month before that. Getting laid-off staffers to come back in is going to be critical, and some practices are already having a hard time convincing them to come back, said Michael La Penna, a physician practice manager in Grand Rapids, Mich.
“They are finding that those people don’t want to come back in yet,” he said. “In many cases they have to care for children at home or have been getting generous unemployment checks.”
The problem with all these temporary financial boosts is that they will disappear within weeks or months from now. Mr. La Penna is concerned that the sudden loss of this support could send some practices spinning into bankruptcy. “Unless volume gets better very soon, time is running out for a lot of practices,” he said.
Hospitals, which also have been depending on federal assistance, may run out of money, too. Daniel Wrenne, a financial planner for physicians in Lexington, Ky., said smaller hospitals are particularly vulnerable because they lack the capital. He said a friend who is an attorney for hospitals predicted that 25% of small regional hospitals “won’t make it through this.”
Such financial turmoil might prompt many physicians to retire or find a new job, said Gary Price, MD, a plastic surgeon in New Haven, Conn., and president of the Physicians Foundation, an advocacy group for the profession. In a survey of doctors by the Physicians Foundation and Merritt Hawkins, released on April 21, 18% planned to retire, temporarily close their practices, or opt out of patient care, and another 14%, presumably employed physicians, planned to change jobs.
Is recovery around the corner?
In early May, practices in many parts of the country were seeing the possibility of a return to normal business – or at least what could pass for normal in these unusual times.
“From mid-March to mid-April, hospitals and practices were in panic mode,” said MGMA’s Mr. Gans. “They were focusing on the here and now. But from mid-April to mid-May, they could begin looking at the big picture and decide how they will get back into business.”
Surgeons devastated by bans on elective surgeries might see a bounce in cases, as the backlog of patients comes back in. By late April, 10 states reinstituted elective surgeries, including California, Arizona, Georgia, Indiana, Colorado, and Oklahoma, and New York has reinstituted elective surgeries for some counties.
Dr. Price said he hopes to reopen his plastic surgery practice by the end of June. “If it takes longer than that, I’m not sure that the practice will survive.” His PPP loan would have run out and he would have to lay off his staff. “At that point, ongoing viability of practice would become a real question.”
Dr. Monks said he hopes a lot more patients will come to his dermatology practice. As of the end of April, “we’re starting to see an uptick in the number of patients wanting to come in,” he said. “They seem to be more comfortable with the new world we’re living in.
“Viewing the backlog of cases that haven’t been attended to,” Dr. Monks added, “I think we’ll be really busy for a while.”
But Mr. La Penna said he thinks the expected backlog of elective patients will be more like a trickle than a flood. “Many patients aren’t going to want to return that fast,” he said. “They may have a condition that makes exposure to COVID-19 more risky, like diabetes or high blood pressure, or they’re elderly, or they live in a household with one of these risk groups.”
Andrew Musbach, cofounder of MD Wealth Management in Chelsea, Mich., said he expects a slow recovery for primary care physicians as well. “Even when the lockdowns are over, not everyone is going to feel comfortable coming to a hospital or visiting a doctor’s office unless it’s absolutely necessary,” he said.
Getting back to normal patient volumes will involve finding better ways to protect patients and staff from COVID-19, Dr. Yonover said. At his urology practice, “we take all the usual precautions, but nothing yet has made it dramatically easier to protect patients and staff,” he said. “Rapid, accurate testing for COVID-19 would change the landscape, but I have no idea when that will come.”
Mr. Wrenne advises his physician clients that a financial recovery will take months. “I tell them to plan for 6 months, until October, before income returns to pre–COVID-19 levels. Reimbursement lags appointments by as much as 3 months, plus it will probably take the economy 2-3 months more to get back to normal.”
“We are facing a recession, and how long it will last is anyone’s guess,” said Alex Kilian, a physician wealth manager at Aldrich Wealth in San Diego. “The federal government’s efforts to stimulate the economy is keeping it from crashing, but there are no real signs that it will actually pick up. It may take years for the travel and entertainment industries to come back.”
A recession means patients will have less spending power, and health care sectors like laser eye surgery may be damaged for years to come, said John B. Pinto, an ophthalmology practice management consultant in San Diego. “[That kind of surgery] is purely elective and relatively costly,” he said. “When people get back to work, they are going to be building up their savings and avoiding new debt. They won’t be having [laser eye surgery].”
“There won’t be any quick return to normal for me,” said Dr. Price, the Connecticut plastic surgeon. “The damage this time will probably be worse than in the Great Recession. Back then, plastic surgery was off by 20%, but this time you have the extra problem of patients reluctant to come into medical offices.”
“To get patients to come in, facilities are going to have to convince patients that they are safe,” Mr. Singleton said. “That may mean undertaking some marketing and promotion, and hospitals tend to be much better at that than practices.”
What a new wave of COVID-19 would mean
Some states have begun reopening public places, which could signal patients to return to doctors’ offices even though doctors’ offices were never officially closed. Oklahoma, for example, reopened restaurants, movie theaters, and sports venues on May 1.
Dr. Monks, president of the Oklahoma Medical Association, said his group opposes states reopening. “The governor’s order is too hasty and overly ambitious,” he said. “Oklahoma has seen an ongoing growth in the number of cases, hospitalizations, and deaths in the past week alone [in late April].”
The concern is that opening up public places too soon would create a new wave of COVID-19, which would not only be a public health disaster, but also a financial disaster for physicians. Doctors would be back where they were in March, but unlike in March, they would not benefit from revenues from previously busy times.
Mr. Pinto said the number of COVID-19 cases will rise and fall in the next 2 years, forcing states to reenact new bans on public gatherings and on elective surgeries until the numbers subside again.
Mr. Pinto said authorities in Singapore have successfully handled such waves of the disease through short bans that are tantamount to tapping the brakes of a car. “As the car gathers speed down the hill, you tap the brake,” he said. “I suspect we’ll be seeing a lot of brake-tapping until a vaccine can be developed and distributed.”
Gary LeRoy, MD, president of the American Academy of Family Physicians, recalled the worldwide Spanish Flu pandemic a century ago. “People were allowed out of their houses after 2 months, and the flu spiked up again,” he said. “I hope we don’t make that mistake this time.”
Dr. LeRoy said it’s not possible to predict how the COVID-19 crisis will play out. “What will the future be like? I don’t know the answer,” he said. “The information we learn in next hours, days, or months will probably change everything.”
A version of this article originally appeared on Medscape.com.
“At a combined system and hospital board meeting yesterday, there was a financial presentation,” said a cardiologist in Minnesota, who declined to be named. “We have ‘salary support’ through May 16, which means we will be receiving base pay at our 2019 level. After May 16, I think it’s fairly certain salaries will be decreased.”
A general internist in the same area added: “The system has decided to pay physicians and other employees for 8 weeks, until May 15, and they are borrowing about $150 million to do this. We don’t know what will happen after May 15, but we are supposed to have an update in early May.”
Physician income is of huge interest, and many aspects of it are discussed in Medscape’s Physician Compensation Report 2020, just released.
The worst may be yet to come
Of all the categories of physicians, “I am worried about private practices the most,” said Travis Singleton, senior vice president at Merritt Hawkins, a physician search firm. “They don’t have a financial cushion, and will start seeing big drops in revenue at the end of May.”
“A lot of the A/R [accounts receivables] for practices come within 30 days, and very little comes in after 90 days,” said Terrence R. McWilliams, MD, chief clinical consultant at HSG Advisors, a consultancy for not-for-profit hospitals and their employed physician networks around the country. “So private practices are reaching the point where prior A/R will start to dwindle and they will start feeling the decline in new claims submissions.”
Large practices may have a bigger financial cushion, but in many cases, they also have more liabilities. “We don’t know the financial loss yet, but I think it’s been devastating,” said Paul M. Yonover, MD, a urologist at UroPartners, a large single-specialty practice in Chicago with 62 urologists. “In fact, the financial loss may well be larger than our loss in volume, because we have to support our own surgery center, pathology lab, radiation center, and other in-house services.”
Employed physicians in limbo
In contrast to physicians in private practices, many employed physicians at hospitals and health systems have been shielded from the impact of COVID-19 – at least for now.
“The experiences of employed physicians are very mixed,” said Mr. Singleton at Merritt Hawkins. “Some health systems have reduced physicians’ pay by 20%, but other systems have been putting off any reductions.”
Hospitals and health systems are struggling. “Stopping elective surgeries deeply affected hospitals,” said Ryan Inman, founder of Physician Wealth Services in San Diego. “With fewer elective surgeries, they have much less income coming in. Some big hospitals that are pillars of their community are under great financial stress.”
“Hospitals’ patient volumes have fallen by 50%-90%,” Mr. McWilliams reported. “Lower volume means lower pay for employed physicians, who are paid by straight productivity or other models that require high volumes. However, some health systems have intervened to make sure these physicians get some money.”
Base pay is often safe for now, but quarterly bonuses are on the chopping block. “Employed physicians are often getting a guaranteed salary for a month or two, but no bonuses or extra distributions,” said Joel Greenwald, MD, a financial adviser for physicians in St. Louis Park, Minn., a state mecca for physician employment. “They’ve been told that they will continue to get their base salary but forget about the quarterly bonuses. This amounts to salary reductions of 10%-30%.”
Ensuring payment for these doctors means lowering their productivity benchmarks, but the benchmarks might still be too high for these times. An internist at a large health system in Minneapolis–St. Paul reports that, at a lunch meeting, employed doctors learned that payment benchmarks will be reduced to 70% of their 2019 monthly average.
“I am seeing nowhere near 70% of what I was seeing last year,” he said in an interview, asking that his name not be used. “Given how slow things have been, I am probably closer to 30%, but have not been given any data on this, so I am guessing at this point.”
Adapting to a brave new world
Even as they face a dark financial future, physicians have had to completely revamp the way they practice medicine – a cumbersome process that, in itself, incurred some financial losses. They had to obtain masks and other PPE, reposition or even close down their waiting rooms, cut back on unneeded staff, and adapt to telemedicine.
“It’s been an incredibly challenging time,” said Dr. Yonover, the Chicago urologist. “As a doctor. I cannot avoid contact, and it’s not totally clear yet how the virus spreads. But I don’t have the option of closing the door. As a practice owner, you’re responsible for the health and well-being of employees, patients, and the business.”
“A practice’s daily routine is somewhat slower and costlier,” said David N. Gans, MSHA, senior fellow at the Medical Group Management Association (MGMA), which represents group practices. “Between each patient, you have to clean a lot more than previously, and you have to stock up on PPE such as masks and gowns. PPE used to be limited to infectious patients, but now it’s universal.”
At PA Clinical Network, a clinically integrated network in Pennsylvania, volume fell 40%-50% and income fell 30%-50% from late March to late April, according to Jaan Sidorov, MD, an internist who is CEO of the network, which has 158 physicians in a variety of specialties working in 54 practices around the state.
“Revenue went down but it didn’t crash,” he said. “And our physicians pivoted very quickly. They adapted to telehealth and applied for the federal loan programs. They didn’t use waiting rooms. In some cases, staff was out in the parking lot, putting stethoscopes through patients’ windows.”
“None of the practices closed, not even temporarily,” Dr. Sidorov said. “But clearly this cannot go forever without having serious consequences.”
How much can telemedicine help?
Telemedicine has been a lifeline for many struggling practices. “As much as 20%-40% of a practice’s losses can be recouped through telemedicine, depending on variables like patients’ attitudes,” said Mr. Singleton at Merritt Hawkins.
The rise in telemedicine was made possible by a temporary relaxation of the limits on telemedicine payments by Medicare and many private payers. Medicare is currently paying the same rates for telemedicine as it does for in-office visits.
In a recent MGMA Stat survey, 97% of practices reported that they had taken up telemedicine, according to Mr. Gans. He estimates that 80% of primary care could be converted to telemedicine, including medication refills, ongoing care of chronic patients, and recording patients’ vital signs from home.
Some primary care physicians are now using telemedicine for 100% of their visits. “I voluntarily closed my practice weeks ago except for virtual visits due to the risk of exposure for my patients,” a doctor in South Carolina told the Primary Care Collaborative in mid-April. “I continue to pay my staff out of pocket but have reduced hours and am not receiving any income myself.”
However, Mr. Inman of Physician Wealth Services said family medicine clients using telemedicine for all of their patients are earning less per visit, even though the Medicare reimbursement is the same as for an office visit. “They earn less because they cannot charge for any ancillaries, such as labs or imaging,” he said.
“Telemedicine has its limits,” Mr. Singleton said. It cannot replace elective surgeries, and even in primary care practices, “there is a lot of work for which patients have to come in, such as physicals or providing vaccines,” he said. “I know of one doctor who has refrigerator full of vaccines to give out. That pays his bills.”
In many cases, “telemedicine” simply means using the phone, with no video. Many patients can only use the phone, and Medicare now reimburses for some kinds of phone visits. In a mid-April survey of primary care providers, 44% were using the telephone for the majority of their visits, and 14% were not using video at all. Medicare recently decided to pay physicians the same amount for telephone visits as in-person visits.
Financial boosts will run out soon
Many private practices are surviving only because they have managed to tap into new federal programs that can finance them for the short-term. Here are the main examples:
Receiving advance Medicare payments. Through the Medicare Accelerated and Advance Payment Program, physicians can be paid up to 3 months of their average Medicare reimbursement in advance. However, repayment starts 120 days after receiving the money and must be completed within 210 days.
Obtaining a federal loan. Under the Paycheck Protection Program (PPP), which is available to all kinds of small businesses, practices can apply for up to 2.5 times their average monthly payroll costs.
PPP money can be used for payroll, rent, mortgage interest, or utility payments for up to 8 weeks. The loan will be entirely forgiven as long as the rules are followed. For example, three quarters of the money must go to payroll, and laid-off employees must be rehired by June 30.
There was such a rush for the first round of PPP loans that many physicians failed to get the loan. “Many of my physician clients applied for the loan as soon as they could, but none of them got it,” said Mr. Inman, the San Diego financial adviser. “We are hoping that the next round of funding will provide them some relief.” The second round started on April 27.
Physicians who have already obtained the PPP loan are very relieved. “This loan made it possible for us to pay our employees,” said George W. Monks, MD, a dermatologist in Tulsa, Okla., and president of the Oklahoma Medical Association.
Staff benefiting from higher unemployment payments. Many practices and hospitals are laying off their staff so that they can collect unemployment benefits. This is a good time to do that because the federal government has boosted unemployment payments by $600 a week, creating a total benefit that is greater than many people earned at their regular jobs.
This extra boost ends in July, but practices with PPP loans will have to rehire their laid-off workers a month before that. Getting laid-off staffers to come back in is going to be critical, and some practices are already having a hard time convincing them to come back, said Michael La Penna, a physician practice manager in Grand Rapids, Mich.
“They are finding that those people don’t want to come back in yet,” he said. “In many cases they have to care for children at home or have been getting generous unemployment checks.”
The problem with all these temporary financial boosts is that they will disappear within weeks or months from now. Mr. La Penna is concerned that the sudden loss of this support could send some practices spinning into bankruptcy. “Unless volume gets better very soon, time is running out for a lot of practices,” he said.
Hospitals, which also have been depending on federal assistance, may run out of money, too. Daniel Wrenne, a financial planner for physicians in Lexington, Ky., said smaller hospitals are particularly vulnerable because they lack the capital. He said a friend who is an attorney for hospitals predicted that 25% of small regional hospitals “won’t make it through this.”
Such financial turmoil might prompt many physicians to retire or find a new job, said Gary Price, MD, a plastic surgeon in New Haven, Conn., and president of the Physicians Foundation, an advocacy group for the profession. In a survey of doctors by the Physicians Foundation and Merritt Hawkins, released on April 21, 18% planned to retire, temporarily close their practices, or opt out of patient care, and another 14%, presumably employed physicians, planned to change jobs.
Is recovery around the corner?
In early May, practices in many parts of the country were seeing the possibility of a return to normal business – or at least what could pass for normal in these unusual times.
“From mid-March to mid-April, hospitals and practices were in panic mode,” said MGMA’s Mr. Gans. “They were focusing on the here and now. But from mid-April to mid-May, they could begin looking at the big picture and decide how they will get back into business.”
Surgeons devastated by bans on elective surgeries might see a bounce in cases, as the backlog of patients comes back in. By late April, 10 states reinstituted elective surgeries, including California, Arizona, Georgia, Indiana, Colorado, and Oklahoma, and New York has reinstituted elective surgeries for some counties.
Dr. Price said he hopes to reopen his plastic surgery practice by the end of June. “If it takes longer than that, I’m not sure that the practice will survive.” His PPP loan would have run out and he would have to lay off his staff. “At that point, ongoing viability of practice would become a real question.”
Dr. Monks said he hopes a lot more patients will come to his dermatology practice. As of the end of April, “we’re starting to see an uptick in the number of patients wanting to come in,” he said. “They seem to be more comfortable with the new world we’re living in.
“Viewing the backlog of cases that haven’t been attended to,” Dr. Monks added, “I think we’ll be really busy for a while.”
But Mr. La Penna said he thinks the expected backlog of elective patients will be more like a trickle than a flood. “Many patients aren’t going to want to return that fast,” he said. “They may have a condition that makes exposure to COVID-19 more risky, like diabetes or high blood pressure, or they’re elderly, or they live in a household with one of these risk groups.”
Andrew Musbach, cofounder of MD Wealth Management in Chelsea, Mich., said he expects a slow recovery for primary care physicians as well. “Even when the lockdowns are over, not everyone is going to feel comfortable coming to a hospital or visiting a doctor’s office unless it’s absolutely necessary,” he said.
Getting back to normal patient volumes will involve finding better ways to protect patients and staff from COVID-19, Dr. Yonover said. At his urology practice, “we take all the usual precautions, but nothing yet has made it dramatically easier to protect patients and staff,” he said. “Rapid, accurate testing for COVID-19 would change the landscape, but I have no idea when that will come.”
Mr. Wrenne advises his physician clients that a financial recovery will take months. “I tell them to plan for 6 months, until October, before income returns to pre–COVID-19 levels. Reimbursement lags appointments by as much as 3 months, plus it will probably take the economy 2-3 months more to get back to normal.”
“We are facing a recession, and how long it will last is anyone’s guess,” said Alex Kilian, a physician wealth manager at Aldrich Wealth in San Diego. “The federal government’s efforts to stimulate the economy is keeping it from crashing, but there are no real signs that it will actually pick up. It may take years for the travel and entertainment industries to come back.”
A recession means patients will have less spending power, and health care sectors like laser eye surgery may be damaged for years to come, said John B. Pinto, an ophthalmology practice management consultant in San Diego. “[That kind of surgery] is purely elective and relatively costly,” he said. “When people get back to work, they are going to be building up their savings and avoiding new debt. They won’t be having [laser eye surgery].”
“There won’t be any quick return to normal for me,” said Dr. Price, the Connecticut plastic surgeon. “The damage this time will probably be worse than in the Great Recession. Back then, plastic surgery was off by 20%, but this time you have the extra problem of patients reluctant to come into medical offices.”
“To get patients to come in, facilities are going to have to convince patients that they are safe,” Mr. Singleton said. “That may mean undertaking some marketing and promotion, and hospitals tend to be much better at that than practices.”
What a new wave of COVID-19 would mean
Some states have begun reopening public places, which could signal patients to return to doctors’ offices even though doctors’ offices were never officially closed. Oklahoma, for example, reopened restaurants, movie theaters, and sports venues on May 1.
Dr. Monks, president of the Oklahoma Medical Association, said his group opposes states reopening. “The governor’s order is too hasty and overly ambitious,” he said. “Oklahoma has seen an ongoing growth in the number of cases, hospitalizations, and deaths in the past week alone [in late April].”
The concern is that opening up public places too soon would create a new wave of COVID-19, which would not only be a public health disaster, but also a financial disaster for physicians. Doctors would be back where they were in March, but unlike in March, they would not benefit from revenues from previously busy times.
Mr. Pinto said the number of COVID-19 cases will rise and fall in the next 2 years, forcing states to reenact new bans on public gatherings and on elective surgeries until the numbers subside again.
Mr. Pinto said authorities in Singapore have successfully handled such waves of the disease through short bans that are tantamount to tapping the brakes of a car. “As the car gathers speed down the hill, you tap the brake,” he said. “I suspect we’ll be seeing a lot of brake-tapping until a vaccine can be developed and distributed.”
Gary LeRoy, MD, president of the American Academy of Family Physicians, recalled the worldwide Spanish Flu pandemic a century ago. “People were allowed out of their houses after 2 months, and the flu spiked up again,” he said. “I hope we don’t make that mistake this time.”
Dr. LeRoy said it’s not possible to predict how the COVID-19 crisis will play out. “What will the future be like? I don’t know the answer,” he said. “The information we learn in next hours, days, or months will probably change everything.”
A version of this article originally appeared on Medscape.com.
Managing Trichomonas vaginalis infections
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18
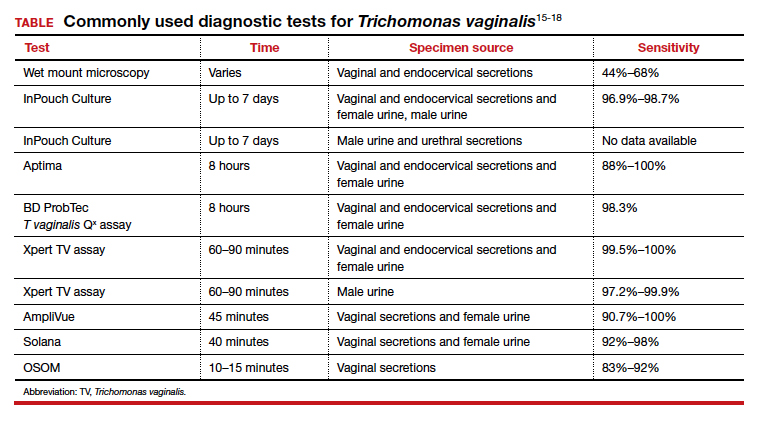
Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18

Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
CASE Woman with malodorous vaginal discharge
A 26-year-old nulligravid woman with 2 current sexual partners requests evaluation because she has a yellow-green frothy vaginal discharge that is slightly malodorous. One of her sexual partners has noted a similar discharge from his urethra. On physical examination, the clinician notes that the patient’s discharge emanates from the vaginal mucosa, and the exocervix has multiple punctate hemorrhages. Considerations in this case include:
- What is the most likely diagnosis?
- How should this patient be evaluated and treated?
- Should the patient’s sexual partners be treated?
This clinical scenario is most consistent with a trichomonas infection, although other conditions, including bacterial vaginosis, gonorrhea, and chlamydia infection, must be considered in the differential diagnosis.
In this article, we examine the microbiology, epidemiology, clinical manifestations, and diagnosis and treatment of this common sexually transmitted infection (STI).
The causative microbe
Trichomonas vaginalis is a free-living flagellated protozoan that accounts for almost half of all nonviral STIs globally. It has a predilection for the mucosal epithelium of the genitourinary tract, including the vagina and urethra. Humans are the only known host for T vaginalis. The infection is transmitted through sexual intercourse, and the organism reproduces through binary fission in the lower genital tract of women and in the urethra and prostate of men.
This anaerobic trophozoite has 4 flagella anteriorly and 1 flagellum that projects posteriorly, with an undulating membrane that gives its characteristic motile appearance on saline microscopy.1
T vaginalis infection causes major mechanical stress on epithelial cells, which results in disruption of the plasma cell membrane and, ultimately, cell death. The necrotic cell fragments are then phagocytosed by trichomonads, thus accelerating the infection.2
Groups at risk
Trichomonal infections are not reportable to public health authorities, which makes assessing the true prevalence of infection difficult.
The World Health Organization estimated the incidence of infection to be more than 156 million cases globally in 2016, with a prevalence of 110.4 million people at any one time.3
The 2013-2014 National Health and Nutrition Examination Survey tested 4,057 men and women aged 18 to 59 years for T vaginalis and found a prevalence of 0.5% among men and 1.8% among women.4 The prevalence increased with age. There was a disproportionate burden of trichomonas infections in the non-Hispanic black population, with 4.2% of black men and 8.9% of black women affected.4
Targeted screening of urogenital samples for T vaginalis in a population of US women undergoing Chlamydia trachomatis/Neisseria gonorrhoeae screening demonstrated prevalence rates of 8.7%, 6.7%, and 1.7% for T vaginalis, C trachomatis, and N gonorrhoeae, respectively.5
Differences in prevalence estimates may be due to differences in the varying sensitivity of each testing modality and patient populations. In one study, nucleic acid amplification testing (NAAT) for T vaginalis detected rates as high as 11.3% in women and 6.1% in men undergoing evaluations at STI clinics.6
Continue to: Clinical manifestations of infection...
Clinical manifestations of infection
Most cases of T vaginalis remain in an asymptomatic carrier state, with up to 85% of women and 77% of men reporting no clinical symptoms.1 However, approximately one-third of asymptomatic carriers will experience symptoms within 6 months of infection acquisition. This latency in appearance of clinical symptoms certainly contributes to the high transmission rate of T vaginalis.
Infected men may experience purulent urethritis, dysuria, and postcoital pruritus. Common clinical symptoms in women include abnormal vaginal discharge that may be malodorous, purulent, thin, frothy, and yellow-green, as well as symptoms of dyspareunia and vulvar irritation. Punctate hemorrhages in the cervix (colpitis macularis) and vaginal walls (macular vaginitis) give the characteristic “strawberry appearance,” but these findings are seen in only 2% of affected women.7
Complications in ObGyn patients
Although T vaginalis once was regarded as more of an annoyance than a public health issue, awareness of the infection’s ramifications has increased in recent years. Because of these complications, treatment of both symptomatic and asymptomatic patients is clearly indicated.
Complications of trichomonal infection in men include balanoposthitis, epididymitis, prostatitis, urethritis, and infertility.7 In women, complications include infections of the adnexa, endometrium, and vestibular glands, as well as cervical neoplasia and increased co-infection rates with other STIs, such as bacterial vaginosis, chlamydia infection, gonorrhea, syphilis, and herpes simplex virus type 2.1
Infection in pregnancy. Adverse outcomes in pregnant women with T vaginalis infections at mid-gestation include low birth weight, preterm premature rupture of membranes, preterm delivery, and postpartum endometritis.8 A disproportionately larger share of the low birth weight rate associated with T vaginalis infections occurs in black women compared with white and Hispanic women.8 Perinatal transmission to newborns can cause fever; respiratory difficulties; urinary tract infections; nasal discharge; and, in female infants, vaginal discharge.9,10
Co-infection concerns. The increased rate of co-infection with human immunodeficiency virus type 1 (HIV-1) and T vaginalis is a major concern.11 One study found a higher concentration of HIV-1 in semen samples from men with T vaginalis and symptomatic urethritis.12 Further, T vaginalis was found in 17.4% of women with HIV screened at a public clinic in California, with almost 38% of black women affected.13 Trichomoniasis can increase the risk of HIV-1 acquisition by 1.52-fold (95% confidence interval, 1.04- to 2.24-fold), pointing toward a potential amplifying effect of T vaginalis on HIV transmission rates.14 This association may be based at least in part on the organism’s ability to cause microulcerations in the genital and urinary tract epithelium, thus creating pathways for other microorganisms to enter the vascular system.
Making the diagnosis
The nonspecific symptoms of T vaginalis create a wide differential to consider. Vaginal discharge may be due to bacterial vaginosis, vulvovaginal candidiasis, physiologic discharge, atrophy, and nonspecific inflammation. The presence of malodorous and discolored discharge increases the likelihood of bacterial vaginosis or T vaginalis infection. Pruritus often is associated with candidiasis co-infection.
The diagnosis of trichomoniasis can be confirmed in the outpatient office with the use of saline microscopy, an inexpensive test that is based on observation of motile trichomonads in a wet mount of vaginal fluid. The sensitivity of the wet mount ranges from 44% to 68% compared with culture. Culture, traditionally using Diamond’s medium, has a sensitivity of 81% to 94% and was long the gold standard; however, culture has been replaced largely by molecular and antigen testing.
Three US Food and Drug Administration (FDA)-approved NAATs for T vaginalis currently are on the market; all can detect co-infection with gonorrhea and chlamydia from the same specimen. These tests include the Aptima T vaginalis rRNA target assay (Hologic, Bedford, Massachusetts) and the BD ProbTec T vaginalis Qx (TVQ) amplified DNA assay (BD Diagnostics, Baltimore, Maryland), both of which require up to 8 hours to yield results. The Xpert T vaginalis (TV) assay (Cepheid, Sunnyvale, California) is the first NAAT that is FDA approved for use with male urine (in addition to female urine), and it yields results in 60 to 90 minutes. Sensitivity for these NAAT assays ranges from 88% to 100%.15
Point-of-care testing is preferred for rapid diagnosis and for helping the clinician provide same-visit treatment for STIs. The Solana trichomonas assay (Quidel, San Diego, California) detects T vaginalis DNA and can yield results within 40 minutes, but it requires specialized equipment for running the samples. The AmpliVue trichomonas assay (Quidel, San Diego, California) is similar to the Solana assay but it is contained within a small handheld cartridge that does not require additional equipment. Sensitivities are 92% to 98% for Solona and 90.7% to 100% for AmpliVue. The OSOM trichomonas rapid test (Sekisui, Framingham, Massachusetts) uses antigen-detection immunochromatography to provide results in 10 to 15 minutes, with 83% to 92% sensitivity and 99% specificity for vaginal specimens.15,16
Continue to: The TABLE provides a summary...
The TABLE provides a summary of the clinical performance of the various tests for T vaginalis. 15-18

Treatment options
The 5-nitroimidazole agents, which include metronidazole and tinidazole, are the preferred agents for the treatment of trichomoniasis.
Dosing regimen. While a single oral dose of metronidazole 2 g has long been the mainstay of treatment for T vaginalis, this regimen recently has been questioned, at least in women, due to the high posttreatment positive rate of T vaginalis, which ranges from 5% to 37%.19,20 These cases may be due to reinfection by untreated sexual partners. They also may result from treatment failure, however, specifically inadequate treatment time.21 Overall, patients treated with single-dose metronidazole are 1.87 times more likely to experience treatment failure compared with those treated with a multidose regimen.19 Since many cases of T vaginalis infection are associated with bacterial vaginosis co-infection, recommending metronidazole 500 mg twice daily for 7 days is beneficial because this course provides optimal treatment for both infections.
Treatment during pregnancy. In the minds of some investigators, treatment of T vaginalis in asymptomatic pregnant women is problematic. One study demonstrated a similar to slightly increased risk of preterm delivery for metronidazole-treated patients compared with a placebo-treated group.22 Limitations of the study included atypical treatment dosing (2 doses of metronidazole 2 g given 48 hours apart at 16 to 23 weeks’ gestation and repeated at 24 to 29 weeks’ gestation) and a latency between the last dose of metronidazole and preterm delivery.22
We believe that all pregnant women, symptomatic or asymptomatic, should be treated because of the sexually transmitted nature of the infection and the probability that most asymptomatic carriers ultimately become symptomatic.
Cost of treatment. Generic oral metronidazole is very inexpensive. The approximate retail price for 14 metronidazole 500-mg tablets is $15.69 (www.goodrx.com). By contrast, a single-dose course of tinidazole (four 500-mg tablets) costs approximately $45. Accordingly, we reserve tinidazole for patients who have experienced a treatment failure with metronidazole or who cannot tolerate metronidazole.
Drug‒alcohol interaction. With both metronidazole and tinidazole, patients must abstain from alcohol during treatment and for 72 hours after completing therapy because these drugs have a disulfiram-like reaction with ethanol.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.
- Kissinger P. Epidemiology and treatment of trichomoniasis. Curr Infect Dis Rep. 2015;17:484.
- Midlej V, Benchimol M. Trichomonas vaginalis kills and eats—evidence for phagocytic activity as a cytopathic effect. Parasitology. 2010;137:65-76.
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P.
- Patel EU, Gaydos CA, Packman ZR, et al. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211-217.
- Ginocchio CC, Chapin K, Smith JS, et al. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601-2608.
- Schwebke J, Merriweather A, Massingale S, et al. Screening for Trichomonas vaginalis in a large high-risk population: prevalence among men and women determined by nucleic acid amplification testing. Sex Transm Dis. 2018;45:e23-e24.
- Petrin D, Delgaty K, Bhatt R, et al. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev. 1998;11:300-317.
- Cotch MF, Pastorek JG II, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex Transm Dis. 1997;24:353-360.
- Smith LM, Wang M, Zangwill K, et al. Trichomonas vaginalis infection in a premature newborn. J Perinatol. 2002;22:502-503.
- Temesvári P, Kerekes A, Tege A, et al. Demonstration of Trichomonas vaginalis in tracheal aspirates in infants with early respiratory failure. J Matern Fetal Neonatal Med. 2002;11:347-349.
- Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transm Infect. 2013;89:426-433.
- Cohen MS, Hoffman IF, Royce RA, et al; AIDSCAP Malawi Research Group. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868-1873.
- Sorvillo F, Kovacs A, Kerndt P, et al. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am J Trop Med Hyg. 1998;58:495-500.
- McClelland RS, Sangare L, Hassan WM, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698-702.
- Van Gerwen OT, Muzny CA. Recent advances in the epidemiology, diagnosis, and management of Trichomonas vaginalis infection. F1000Res. 2019;8:F1000 Faculty Rev-1666.
- Gaydos CA, Klausner JD, Pai NP, et al. Rapid and point-of-care tests for the diagnosis of Trichomonas vaginalis in women and men. Sex Transm Infect. 2017;93(S4):S31-S35.
- Rivers CA, Muzny CA, Schwebke JR. Diagnostic rates differ on the basis of the number of read days with the use of the InPouch culture system for Trichomonas vaginalis screening. J Clin Microbiol. 2013;51:3875-3876.
- Van Der Pol B, Williams JA, Taylor SN, et al. Detection of Trichomonas vaginalis DNA by use of self-obtained vaginal swabs with the BD ProbeTec Qx assay on the BD Viper System. J Clin Microbiol. 2014;52:885-889.
- Howe K, Kissinger P. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44:29-34.
- Duff P. Should the length of treatment for trichomoniasis in women be reconsidered? OBG Manag. 2017;29(3):48-49.
- Krashin JW, Koumans EH, Bradshaw-Sydnor AC, et al. Trichomonas vaginalis prevalence, incidence, risk factors and antibiotic-resistance in an adolescent population. Sex Transm Dis. 2010;37:440-444.
- Klebanoff MA, Carey JC, Hauth JC, et al; National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Failure of metronidazole to prevent preterm delivery among pregnant women with asymptomatic Trichomonas vaginalis infection. N Engl J Med. 2001;345:487-493.