User login
The cost of postponing medical care during the pandemic
Friends of mine who work in the ED have noticed a drop-off in patients. Granted, so has my office, but theirs is a little less expected.
It’s not just in my region. An article on this site last week mentioned the same phenomenon. Not just minor stuff but visits for more serious conditions also have decreased. This means that either people are currently choosing to ignore those things entirely or are trying to get them handled at a later date in the outpatient setting.
Neither one is good.
One friend pointed out that since a fair percentage of visits to the ED aren’t really “emergencies” maybe this is part of the reason. With all the news about COVID-19, the risk of going to the ED for something minor isn’t worth it. This may apply to some, but not all. Certainly, if it clarifies to people what is and isn’t an emergency, that would be helpful to prevent ED overuse in the future.
Every day we all face a countless number of decisions, each with its own risks and benefits. When the question of whether or not to go to an ED comes up, usually the only perceived drawbacks are costs in time and money, compared with the benefit of believing you’re going to get the problem “fixed.”
In the era of coronavirus, with daily news reports on its spread and casualties, the risk of going to the ED is perceived to be higher, and so people are more willing to stay away. If you were going in for a sinus infection, this is probably a good idea. If you’re having a more serious problem and staying home ...
A cost of the pandemic that will come to light in the future will be people who unknowingly survived mild cardiac events, strokes, and other potentially serious problems. While they may do okay in the short term, in the long run they may not be aware they had a problem and so it will continue to go untreated. Coronary or cerebrovascular arteries that need to be reopened won’t be. People with poorly controlled hypertension, dyslipidemia, or diabetes won’t be started on medications they need until it may be too late to avoid more serious outcomes.
Likewise, I worry about an uptick in cancer-related deaths down the road. With the shutdown of many nonurgent procedures, patients may have missed a window for early diagnosis of a malignancy, either because the procedure wasn’t available or they were reluctant to venture out.
Medical data from 2020 will be analyzed many times in the coming years, not just for coronavirus, but for its effects on medical care as a whole. As the first worldwide pandemic of the information age, there will be a lot of lessons to be learned as to how medicine, science, and society in general should and should not respond. Both good and bad things will be learned, but whatever knowledge is gained will be critical for the inevitable next pandemic.
The future world is watching.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Friends of mine who work in the ED have noticed a drop-off in patients. Granted, so has my office, but theirs is a little less expected.
It’s not just in my region. An article on this site last week mentioned the same phenomenon. Not just minor stuff but visits for more serious conditions also have decreased. This means that either people are currently choosing to ignore those things entirely or are trying to get them handled at a later date in the outpatient setting.
Neither one is good.
One friend pointed out that since a fair percentage of visits to the ED aren’t really “emergencies” maybe this is part of the reason. With all the news about COVID-19, the risk of going to the ED for something minor isn’t worth it. This may apply to some, but not all. Certainly, if it clarifies to people what is and isn’t an emergency, that would be helpful to prevent ED overuse in the future.
Every day we all face a countless number of decisions, each with its own risks and benefits. When the question of whether or not to go to an ED comes up, usually the only perceived drawbacks are costs in time and money, compared with the benefit of believing you’re going to get the problem “fixed.”
In the era of coronavirus, with daily news reports on its spread and casualties, the risk of going to the ED is perceived to be higher, and so people are more willing to stay away. If you were going in for a sinus infection, this is probably a good idea. If you’re having a more serious problem and staying home ...
A cost of the pandemic that will come to light in the future will be people who unknowingly survived mild cardiac events, strokes, and other potentially serious problems. While they may do okay in the short term, in the long run they may not be aware they had a problem and so it will continue to go untreated. Coronary or cerebrovascular arteries that need to be reopened won’t be. People with poorly controlled hypertension, dyslipidemia, or diabetes won’t be started on medications they need until it may be too late to avoid more serious outcomes.
Likewise, I worry about an uptick in cancer-related deaths down the road. With the shutdown of many nonurgent procedures, patients may have missed a window for early diagnosis of a malignancy, either because the procedure wasn’t available or they were reluctant to venture out.
Medical data from 2020 will be analyzed many times in the coming years, not just for coronavirus, but for its effects on medical care as a whole. As the first worldwide pandemic of the information age, there will be a lot of lessons to be learned as to how medicine, science, and society in general should and should not respond. Both good and bad things will be learned, but whatever knowledge is gained will be critical for the inevitable next pandemic.
The future world is watching.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Friends of mine who work in the ED have noticed a drop-off in patients. Granted, so has my office, but theirs is a little less expected.
It’s not just in my region. An article on this site last week mentioned the same phenomenon. Not just minor stuff but visits for more serious conditions also have decreased. This means that either people are currently choosing to ignore those things entirely or are trying to get them handled at a later date in the outpatient setting.
Neither one is good.
One friend pointed out that since a fair percentage of visits to the ED aren’t really “emergencies” maybe this is part of the reason. With all the news about COVID-19, the risk of going to the ED for something minor isn’t worth it. This may apply to some, but not all. Certainly, if it clarifies to people what is and isn’t an emergency, that would be helpful to prevent ED overuse in the future.
Every day we all face a countless number of decisions, each with its own risks and benefits. When the question of whether or not to go to an ED comes up, usually the only perceived drawbacks are costs in time and money, compared with the benefit of believing you’re going to get the problem “fixed.”
In the era of coronavirus, with daily news reports on its spread and casualties, the risk of going to the ED is perceived to be higher, and so people are more willing to stay away. If you were going in for a sinus infection, this is probably a good idea. If you’re having a more serious problem and staying home ...
A cost of the pandemic that will come to light in the future will be people who unknowingly survived mild cardiac events, strokes, and other potentially serious problems. While they may do okay in the short term, in the long run they may not be aware they had a problem and so it will continue to go untreated. Coronary or cerebrovascular arteries that need to be reopened won’t be. People with poorly controlled hypertension, dyslipidemia, or diabetes won’t be started on medications they need until it may be too late to avoid more serious outcomes.
Likewise, I worry about an uptick in cancer-related deaths down the road. With the shutdown of many nonurgent procedures, patients may have missed a window for early diagnosis of a malignancy, either because the procedure wasn’t available or they were reluctant to venture out.
Medical data from 2020 will be analyzed many times in the coming years, not just for coronavirus, but for its effects on medical care as a whole. As the first worldwide pandemic of the information age, there will be a lot of lessons to be learned as to how medicine, science, and society in general should and should not respond. Both good and bad things will be learned, but whatever knowledge is gained will be critical for the inevitable next pandemic.
The future world is watching.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Frontal lobe glucose abnormalities may indicate increased SUDEP risk
, new research suggests.
“The data provide initial evidence that hypometabolism in certain parts of the frontal cortex may be associated with higher SUDEP risk,” said lead author Maysaa M. Basha, MD, associate professor of neurology and director of the Adult Comprehensive Epilepsy Program, Wayne State University/Detroit Medical Center, in Michigan.
If this research is validated, “it potentially can be used to screen patients for higher SUDEP risk,” she said. The idea is to identify those at high risk and then reduce that risk with more aggressive management of seizures or closer monitoring in certain cases, she added.
The research is being presented online as part of the 2020 American Academy of Neurology (AAN) Science Highlights.
Hypometabolism
Dr. Basha and colleagues were encouraged to pursue this new line of research after a pilot [18F]fluorodeoxyglucose positron-emission tomography (FDG-PET) study revealed frontal lobe hypometabolism among patients who subsequently died.
“We wanted to determine if such a metabolic abnormality is associated with SUDEP risk,” said Dr. Basha. She noted that no PET studies have addressed this question, only MRI studies.
In this new study, researchers aimed to identify specific patterns of objectively detected brain glucose metabolic abnormalities in patients with refractory focal epilepsy who were at risk for SUDEP.
The study included 80 patients (45 female patients) aged 16 to 61 years (mean age, 37 years) who underwent FDG-PET as part of their presurgical evaluation for epilepsy surgery. Patients with large brain lesions, such as an infarct or a large tumor, were excluded from the study; such lesions can affect the accuracy of an objective PET analysis, explained Dr. Basha.
The researchers assessed risk for SUDEP using the seven-item SUDEP inventory (SUDEP-7), which was developed as a marker of clinical SUDEP risk. The 0- to 10-point scale is used to evaluate the frequency of tonic-clonic and other seizures, the duration of epilepsy, the use of antiepileptic drugs, and intellectual disability.
The researchers calculated SUDEP-7 inventory scores as closely as possible to FDG-PET assessments. The mean score in the patient population was 3.6.
The investigators divided participants into two subgroups: 22 patients had a SUDEP score of 5 or greater; and 58 had a score of less than 5 (higher scores indicate higher risk for SUDEP).
The researchers compared PET scans of each of these subgroups to PET scans from healthy adults to determine whether they showed common areas of metabolic abnormality. For this, they used an image analytic software program called Statistical Parametric Mapping, which compares group values of metabolic activity measured in small units of the brain (voxels) with statistical methods.
The analysis showed that the higher-risk group displayed a common pattern of hypometabolism in certain brain areas.
“The epilepsy patient subgroup with high SUDEP risk showed areas of decreased metabolism, as compared to the control group, in portions of the frontal cortex,” said Dr. Basha. “The statistically most significant decreases were in the right frontal lobe area—both lateral convexity and medial cortex.”
Dr. Basha added that these group abnormalities were “remarkably similar” to the individual metabolic abnormalities found in the four SUDEP patients in the previous pilot study who underwent PET scanning and who subsequently died.
A similar group analysis showed that the group at low SUDEP risk displayed no common metabolic abnormalities.
MRI findings were normal for 40 patients.
Dr. Basha and colleagues believe that “this is the first PET study assessing the metabolic correlates of SUDEP risk on the group level.”
Common feature
Interictal glucose hypometabolism is “common in and around epileptic foci,” noted Dr. Basha. However, this could extend into nonepileptic regions—for example, to remote connected regions where seizures can spread from the primary focus and into subcortical gray matter structures, such the thalamus.
Some of these metabolic abnormalities may indicate subtle, microscopic, structural abnormalities in the affected brain, said Dr. Basha.
Abnormalities that are induced by epilepsy and that result from purely metabolic changes could be partly or fully reversed if seizures are controlled on a long-term basis, she said. “Some metabolic abnormalities can be reversed after better seizure control with antiepileptic drugs, epileptic surgery, or other antiepileptic treatment,” she said.
It’s “quite possible” that the same brain pattern would be evident in children with epilepsy, although her team has not performed the same analysis in a younger pediatric group, said Dr. Basha. She noted that it would be unethical to administer PET scans, which involve radiation, to young, healthy control persons.
It’s too early to recommend that all epilepsy patients undergo FDG-PET scanning to see whether this pattern of brain glucose hypometabolism is present, said Dr. Basha. “But if this is proven to be a good biomarker, the next step would be a prospective study” to see whether this brain marker is a true signal of SUDEP risk.
“I don’t think our single study would do that, but ultimately, that would be the goal,” she added.
One more piece of the SUDEP puzzle
Commenting on the study, William Davis Gaillard, MD, president of the American Epilepsy Society and chief of neurology, Children’s National Medical Center, Chevy Chase, Maryland, said this new information provides one more piece of the SUDEP puzzle but doesn’t complete the picture.
The study authors assessed PET scans of a group of patients and found common abnormalities that implicate the right medial frontal cortex. “That’s a pretty reasonable method” of investigation, said Dr. Gaillard.
“The challenge is that they’re looking at people they believe have a risk of SUDEP as opposed to people who died,” said Dr. Gaillard.
But he agreed that the results might signal “a biomarker” that “allows you to identify who’s at high risk, and then you may be able to intervene to save them.”
It’s not clear that people with frontal lobe epilepsy are at greater risk for SUDEP than those with temporal lobe epilepsy, he said.
“What you don’t know is whether this represents people with a seizure focus in that area or this represents a common network implicated in people with diverse forms of focal epilepsy; so you need to do some more work,” he said.
Dr. Gaillard pointed out that other research has implicated regions other than the mesial frontal cortex in SUDEP risk. These regions include the insula, the amygdala, the hippocampus, and the brain stem.
He also noted that the SUDEP-7, which has not been thoroughly validated, is designed for use only in adults.
In his own practice, he asks patients about the frequency of tonic-clonic seizures and whether they occur at night. The number of antiepileptic medications a patient takes reflects the difficulty of controlling seizures and may not be “an independent variable for risk,” said Dr. Gaillard.
“It’s clear one needs a better assessment and better idea of who is at risk,” he said.
The researchers have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Basha A et al. AAN 2020. Abstract P5.001.
, new research suggests.
“The data provide initial evidence that hypometabolism in certain parts of the frontal cortex may be associated with higher SUDEP risk,” said lead author Maysaa M. Basha, MD, associate professor of neurology and director of the Adult Comprehensive Epilepsy Program, Wayne State University/Detroit Medical Center, in Michigan.
If this research is validated, “it potentially can be used to screen patients for higher SUDEP risk,” she said. The idea is to identify those at high risk and then reduce that risk with more aggressive management of seizures or closer monitoring in certain cases, she added.
The research is being presented online as part of the 2020 American Academy of Neurology (AAN) Science Highlights.
Hypometabolism
Dr. Basha and colleagues were encouraged to pursue this new line of research after a pilot [18F]fluorodeoxyglucose positron-emission tomography (FDG-PET) study revealed frontal lobe hypometabolism among patients who subsequently died.
“We wanted to determine if such a metabolic abnormality is associated with SUDEP risk,” said Dr. Basha. She noted that no PET studies have addressed this question, only MRI studies.
In this new study, researchers aimed to identify specific patterns of objectively detected brain glucose metabolic abnormalities in patients with refractory focal epilepsy who were at risk for SUDEP.
The study included 80 patients (45 female patients) aged 16 to 61 years (mean age, 37 years) who underwent FDG-PET as part of their presurgical evaluation for epilepsy surgery. Patients with large brain lesions, such as an infarct or a large tumor, were excluded from the study; such lesions can affect the accuracy of an objective PET analysis, explained Dr. Basha.
The researchers assessed risk for SUDEP using the seven-item SUDEP inventory (SUDEP-7), which was developed as a marker of clinical SUDEP risk. The 0- to 10-point scale is used to evaluate the frequency of tonic-clonic and other seizures, the duration of epilepsy, the use of antiepileptic drugs, and intellectual disability.
The researchers calculated SUDEP-7 inventory scores as closely as possible to FDG-PET assessments. The mean score in the patient population was 3.6.
The investigators divided participants into two subgroups: 22 patients had a SUDEP score of 5 or greater; and 58 had a score of less than 5 (higher scores indicate higher risk for SUDEP).
The researchers compared PET scans of each of these subgroups to PET scans from healthy adults to determine whether they showed common areas of metabolic abnormality. For this, they used an image analytic software program called Statistical Parametric Mapping, which compares group values of metabolic activity measured in small units of the brain (voxels) with statistical methods.
The analysis showed that the higher-risk group displayed a common pattern of hypometabolism in certain brain areas.
“The epilepsy patient subgroup with high SUDEP risk showed areas of decreased metabolism, as compared to the control group, in portions of the frontal cortex,” said Dr. Basha. “The statistically most significant decreases were in the right frontal lobe area—both lateral convexity and medial cortex.”
Dr. Basha added that these group abnormalities were “remarkably similar” to the individual metabolic abnormalities found in the four SUDEP patients in the previous pilot study who underwent PET scanning and who subsequently died.
A similar group analysis showed that the group at low SUDEP risk displayed no common metabolic abnormalities.
MRI findings were normal for 40 patients.
Dr. Basha and colleagues believe that “this is the first PET study assessing the metabolic correlates of SUDEP risk on the group level.”
Common feature
Interictal glucose hypometabolism is “common in and around epileptic foci,” noted Dr. Basha. However, this could extend into nonepileptic regions—for example, to remote connected regions where seizures can spread from the primary focus and into subcortical gray matter structures, such the thalamus.
Some of these metabolic abnormalities may indicate subtle, microscopic, structural abnormalities in the affected brain, said Dr. Basha.
Abnormalities that are induced by epilepsy and that result from purely metabolic changes could be partly or fully reversed if seizures are controlled on a long-term basis, she said. “Some metabolic abnormalities can be reversed after better seizure control with antiepileptic drugs, epileptic surgery, or other antiepileptic treatment,” she said.
It’s “quite possible” that the same brain pattern would be evident in children with epilepsy, although her team has not performed the same analysis in a younger pediatric group, said Dr. Basha. She noted that it would be unethical to administer PET scans, which involve radiation, to young, healthy control persons.
It’s too early to recommend that all epilepsy patients undergo FDG-PET scanning to see whether this pattern of brain glucose hypometabolism is present, said Dr. Basha. “But if this is proven to be a good biomarker, the next step would be a prospective study” to see whether this brain marker is a true signal of SUDEP risk.
“I don’t think our single study would do that, but ultimately, that would be the goal,” she added.
One more piece of the SUDEP puzzle
Commenting on the study, William Davis Gaillard, MD, president of the American Epilepsy Society and chief of neurology, Children’s National Medical Center, Chevy Chase, Maryland, said this new information provides one more piece of the SUDEP puzzle but doesn’t complete the picture.
The study authors assessed PET scans of a group of patients and found common abnormalities that implicate the right medial frontal cortex. “That’s a pretty reasonable method” of investigation, said Dr. Gaillard.
“The challenge is that they’re looking at people they believe have a risk of SUDEP as opposed to people who died,” said Dr. Gaillard.
But he agreed that the results might signal “a biomarker” that “allows you to identify who’s at high risk, and then you may be able to intervene to save them.”
It’s not clear that people with frontal lobe epilepsy are at greater risk for SUDEP than those with temporal lobe epilepsy, he said.
“What you don’t know is whether this represents people with a seizure focus in that area or this represents a common network implicated in people with diverse forms of focal epilepsy; so you need to do some more work,” he said.
Dr. Gaillard pointed out that other research has implicated regions other than the mesial frontal cortex in SUDEP risk. These regions include the insula, the amygdala, the hippocampus, and the brain stem.
He also noted that the SUDEP-7, which has not been thoroughly validated, is designed for use only in adults.
In his own practice, he asks patients about the frequency of tonic-clonic seizures and whether they occur at night. The number of antiepileptic medications a patient takes reflects the difficulty of controlling seizures and may not be “an independent variable for risk,” said Dr. Gaillard.
“It’s clear one needs a better assessment and better idea of who is at risk,” he said.
The researchers have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Basha A et al. AAN 2020. Abstract P5.001.
, new research suggests.
“The data provide initial evidence that hypometabolism in certain parts of the frontal cortex may be associated with higher SUDEP risk,” said lead author Maysaa M. Basha, MD, associate professor of neurology and director of the Adult Comprehensive Epilepsy Program, Wayne State University/Detroit Medical Center, in Michigan.
If this research is validated, “it potentially can be used to screen patients for higher SUDEP risk,” she said. The idea is to identify those at high risk and then reduce that risk with more aggressive management of seizures or closer monitoring in certain cases, she added.
The research is being presented online as part of the 2020 American Academy of Neurology (AAN) Science Highlights.
Hypometabolism
Dr. Basha and colleagues were encouraged to pursue this new line of research after a pilot [18F]fluorodeoxyglucose positron-emission tomography (FDG-PET) study revealed frontal lobe hypometabolism among patients who subsequently died.
“We wanted to determine if such a metabolic abnormality is associated with SUDEP risk,” said Dr. Basha. She noted that no PET studies have addressed this question, only MRI studies.
In this new study, researchers aimed to identify specific patterns of objectively detected brain glucose metabolic abnormalities in patients with refractory focal epilepsy who were at risk for SUDEP.
The study included 80 patients (45 female patients) aged 16 to 61 years (mean age, 37 years) who underwent FDG-PET as part of their presurgical evaluation for epilepsy surgery. Patients with large brain lesions, such as an infarct or a large tumor, were excluded from the study; such lesions can affect the accuracy of an objective PET analysis, explained Dr. Basha.
The researchers assessed risk for SUDEP using the seven-item SUDEP inventory (SUDEP-7), which was developed as a marker of clinical SUDEP risk. The 0- to 10-point scale is used to evaluate the frequency of tonic-clonic and other seizures, the duration of epilepsy, the use of antiepileptic drugs, and intellectual disability.
The researchers calculated SUDEP-7 inventory scores as closely as possible to FDG-PET assessments. The mean score in the patient population was 3.6.
The investigators divided participants into two subgroups: 22 patients had a SUDEP score of 5 or greater; and 58 had a score of less than 5 (higher scores indicate higher risk for SUDEP).
The researchers compared PET scans of each of these subgroups to PET scans from healthy adults to determine whether they showed common areas of metabolic abnormality. For this, they used an image analytic software program called Statistical Parametric Mapping, which compares group values of metabolic activity measured in small units of the brain (voxels) with statistical methods.
The analysis showed that the higher-risk group displayed a common pattern of hypometabolism in certain brain areas.
“The epilepsy patient subgroup with high SUDEP risk showed areas of decreased metabolism, as compared to the control group, in portions of the frontal cortex,” said Dr. Basha. “The statistically most significant decreases were in the right frontal lobe area—both lateral convexity and medial cortex.”
Dr. Basha added that these group abnormalities were “remarkably similar” to the individual metabolic abnormalities found in the four SUDEP patients in the previous pilot study who underwent PET scanning and who subsequently died.
A similar group analysis showed that the group at low SUDEP risk displayed no common metabolic abnormalities.
MRI findings were normal for 40 patients.
Dr. Basha and colleagues believe that “this is the first PET study assessing the metabolic correlates of SUDEP risk on the group level.”
Common feature
Interictal glucose hypometabolism is “common in and around epileptic foci,” noted Dr. Basha. However, this could extend into nonepileptic regions—for example, to remote connected regions where seizures can spread from the primary focus and into subcortical gray matter structures, such the thalamus.
Some of these metabolic abnormalities may indicate subtle, microscopic, structural abnormalities in the affected brain, said Dr. Basha.
Abnormalities that are induced by epilepsy and that result from purely metabolic changes could be partly or fully reversed if seizures are controlled on a long-term basis, she said. “Some metabolic abnormalities can be reversed after better seizure control with antiepileptic drugs, epileptic surgery, or other antiepileptic treatment,” she said.
It’s “quite possible” that the same brain pattern would be evident in children with epilepsy, although her team has not performed the same analysis in a younger pediatric group, said Dr. Basha. She noted that it would be unethical to administer PET scans, which involve radiation, to young, healthy control persons.
It’s too early to recommend that all epilepsy patients undergo FDG-PET scanning to see whether this pattern of brain glucose hypometabolism is present, said Dr. Basha. “But if this is proven to be a good biomarker, the next step would be a prospective study” to see whether this brain marker is a true signal of SUDEP risk.
“I don’t think our single study would do that, but ultimately, that would be the goal,” she added.
One more piece of the SUDEP puzzle
Commenting on the study, William Davis Gaillard, MD, president of the American Epilepsy Society and chief of neurology, Children’s National Medical Center, Chevy Chase, Maryland, said this new information provides one more piece of the SUDEP puzzle but doesn’t complete the picture.
The study authors assessed PET scans of a group of patients and found common abnormalities that implicate the right medial frontal cortex. “That’s a pretty reasonable method” of investigation, said Dr. Gaillard.
“The challenge is that they’re looking at people they believe have a risk of SUDEP as opposed to people who died,” said Dr. Gaillard.
But he agreed that the results might signal “a biomarker” that “allows you to identify who’s at high risk, and then you may be able to intervene to save them.”
It’s not clear that people with frontal lobe epilepsy are at greater risk for SUDEP than those with temporal lobe epilepsy, he said.
“What you don’t know is whether this represents people with a seizure focus in that area or this represents a common network implicated in people with diverse forms of focal epilepsy; so you need to do some more work,” he said.
Dr. Gaillard pointed out that other research has implicated regions other than the mesial frontal cortex in SUDEP risk. These regions include the insula, the amygdala, the hippocampus, and the brain stem.
He also noted that the SUDEP-7, which has not been thoroughly validated, is designed for use only in adults.
In his own practice, he asks patients about the frequency of tonic-clonic seizures and whether they occur at night. The number of antiepileptic medications a patient takes reflects the difficulty of controlling seizures and may not be “an independent variable for risk,” said Dr. Gaillard.
“It’s clear one needs a better assessment and better idea of who is at risk,” he said.
The researchers have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Basha A et al. AAN 2020. Abstract P5.001.
Dermatologic changes with COVID-19: What we know and don’t know
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
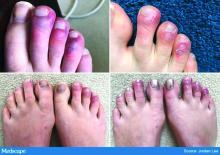
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
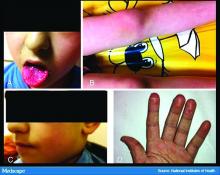
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
FDA approves ripretinib for advanced GISTs
The U.S. Food and Drug Administration approved ripretinib (Qinlock, Deciphera Pharmaceuticals) tablets as the first-ever drug specifically approved as a fourth-line treatment for advanced gastrointestinal stromal tumors (GISTs).
A new kinase inhibitor, ripretinib is indicated for patients previously treated with three or more other kinase inhibitor therapies, including imatinib.
“Despite the progress that has been made over the past 20 years in developing treatments for GIST … some patients don’t respond to treatment and their tumors continue to progress. Today’s approval provides a new treatment option for patients who have exhausted all FDA-approved therapies for GIST,” said Richard Pazdur, MD, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
Dr. Pazdur explained that the FDA has previously approved four targeted therapies for GISTs – imatinib in 2002, sunitinib in 2006, regorafenib in 2013, and avapritinib in 2020.
The new approval of ripretinib is based on the results of a multicenter, randomized, double-blind, placebo-controlled clinical trial that enrolled 129 patients with advanced GISTs whose disease progressed despite prior treatment with the other targeted therapies.
In the trial, patients received ripretinib or placebo once a day in 28-day cycles, until disease progression, or intolerable toxicity.
Median progression-free survival was 6.3 months in the ripretinib arm versus 1 month in the placebo arm.
The most common side effects with ripretinib were alopecia, fatigue, nausea, abdominal pain, constipation, myalgia, diarrhea, decreased appetite, palmar-plantar erythrodysesthesia syndrome, and vomiting.
Ripretinib also can cause skin cancer, hypertension, and cardiac dysfunction manifested as ejection fraction decrease.
The FDA collaborated with the Australian Therapeutic Goods Administration and Health Canada on the review of this application as part of Project Orbis.
This article first appeared on Medscape.com.
The U.S. Food and Drug Administration approved ripretinib (Qinlock, Deciphera Pharmaceuticals) tablets as the first-ever drug specifically approved as a fourth-line treatment for advanced gastrointestinal stromal tumors (GISTs).
A new kinase inhibitor, ripretinib is indicated for patients previously treated with three or more other kinase inhibitor therapies, including imatinib.
“Despite the progress that has been made over the past 20 years in developing treatments for GIST … some patients don’t respond to treatment and their tumors continue to progress. Today’s approval provides a new treatment option for patients who have exhausted all FDA-approved therapies for GIST,” said Richard Pazdur, MD, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
Dr. Pazdur explained that the FDA has previously approved four targeted therapies for GISTs – imatinib in 2002, sunitinib in 2006, regorafenib in 2013, and avapritinib in 2020.
The new approval of ripretinib is based on the results of a multicenter, randomized, double-blind, placebo-controlled clinical trial that enrolled 129 patients with advanced GISTs whose disease progressed despite prior treatment with the other targeted therapies.
In the trial, patients received ripretinib or placebo once a day in 28-day cycles, until disease progression, or intolerable toxicity.
Median progression-free survival was 6.3 months in the ripretinib arm versus 1 month in the placebo arm.
The most common side effects with ripretinib were alopecia, fatigue, nausea, abdominal pain, constipation, myalgia, diarrhea, decreased appetite, palmar-plantar erythrodysesthesia syndrome, and vomiting.
Ripretinib also can cause skin cancer, hypertension, and cardiac dysfunction manifested as ejection fraction decrease.
The FDA collaborated with the Australian Therapeutic Goods Administration and Health Canada on the review of this application as part of Project Orbis.
This article first appeared on Medscape.com.
The U.S. Food and Drug Administration approved ripretinib (Qinlock, Deciphera Pharmaceuticals) tablets as the first-ever drug specifically approved as a fourth-line treatment for advanced gastrointestinal stromal tumors (GISTs).
A new kinase inhibitor, ripretinib is indicated for patients previously treated with three or more other kinase inhibitor therapies, including imatinib.
“Despite the progress that has been made over the past 20 years in developing treatments for GIST … some patients don’t respond to treatment and their tumors continue to progress. Today’s approval provides a new treatment option for patients who have exhausted all FDA-approved therapies for GIST,” said Richard Pazdur, MD, acting director of the Office of Oncologic Diseases in the FDA’s Center for Drug Evaluation and Research.
Dr. Pazdur explained that the FDA has previously approved four targeted therapies for GISTs – imatinib in 2002, sunitinib in 2006, regorafenib in 2013, and avapritinib in 2020.
The new approval of ripretinib is based on the results of a multicenter, randomized, double-blind, placebo-controlled clinical trial that enrolled 129 patients with advanced GISTs whose disease progressed despite prior treatment with the other targeted therapies.
In the trial, patients received ripretinib or placebo once a day in 28-day cycles, until disease progression, or intolerable toxicity.
Median progression-free survival was 6.3 months in the ripretinib arm versus 1 month in the placebo arm.
The most common side effects with ripretinib were alopecia, fatigue, nausea, abdominal pain, constipation, myalgia, diarrhea, decreased appetite, palmar-plantar erythrodysesthesia syndrome, and vomiting.
Ripretinib also can cause skin cancer, hypertension, and cardiac dysfunction manifested as ejection fraction decrease.
The FDA collaborated with the Australian Therapeutic Goods Administration and Health Canada on the review of this application as part of Project Orbis.
This article first appeared on Medscape.com.
Energy-based therapy plus oxymetazoline proves safe for rosacea
according to results from a phase 4 study.
“The current study was designed to evaluate the safety and tolerability of oxymetazoline when used as an adjunctive treatment with energy‐based therapy for patients with moderate to severe facial erythema associated with rosacea,” wrote Emil A. Tanghetti, MD, of the Center for Dermatology and Laser Surgery in Sacramento, and coauthors. The findings were published in Lasers in Surgery and Medicine.
The open-label, interventional study included 46 patients with rosacea, with moderate to severe facial erythema. Study participants received treatment with one of four energy-based devices: pulsed‐dye laser Vbeam Perfecta (PDL-Vbeam), pulsed‐dye laser Cynergy (PDL-Cynergy), intense pulsed-light therapy (IPL), or potassium titanyl phosphate laser (KTP laser), in combination with adjunctive oxymetazoline hydrochloride cream (1%).
On days 3-27 and 31-56, oxymetazoline, an alpha1A adrenoceptor agonist was applied once daily, while energy-based therapy was provided on day 1 and day 29.
The primary safety endpoints were the incidence of treatment‐emergent adverse events (TEAEs) and serious adverse events; the exploratory efficacy endpoint was the change in clinician erythema assessment (CEA) score from start of therapy measured over a 6-hour period post treatment.
Among 43 evaluable patients (who completed the study), CEA score was improved in 39 (90.7%) patients 6 hours post treatment on day 56 and in 30 (68.2%) patients pretreatment.
On day 31, of the 43 evaluable patients, “one‐grade or greater improvement was observed” in 26 (60.5%) patients before application of oxymetazoline, and in 38 (88.4%) of patients 6 hours post treatment, they wrote.
Overall, patient satisfaction increased over the course of the study, with 28 (65.1%) of patients reporting they were satisfied or very satisfied with the treatment on day 56.
Among 46 patients who received at least one treatment, 5 (10.9%) patients had one or more TEAEs (KTP laser, n = 1; PDL-Vbeam, n = 4), and 4 patients had one or more treatment‐related TEAEs (PDL-Vbeam, n = 4); All TEAEs were considered mild or moderate. “Three (6.5%) patients experienced TEAEs related to oxymetazoline; all led to study discontinuation,” the researchers reported.
The researchers acknowledged that a key limitation of the study was the use of multiple energy-based devices, delivered by different providers, which could have caused inconsistency in the results.
“Prospective clinical studies assessing the long‐term safety and efficacy of combined treatment with oxymetazoline and energy‐based therapies are needed,” they concluded.
The manuscript was funded by oxymetazoline manufacturer Aclaris Therapeutics. Several authors disclosed being an investigator, consultant, and/or laser manufacturers. One author was an employee of Aclaris at the time of the study.
SOURCE: Tanghetti EA et al. Lasers Surg Med. 2020 May 6. doi: 10.1002/lsm.23253.
according to results from a phase 4 study.
“The current study was designed to evaluate the safety and tolerability of oxymetazoline when used as an adjunctive treatment with energy‐based therapy for patients with moderate to severe facial erythema associated with rosacea,” wrote Emil A. Tanghetti, MD, of the Center for Dermatology and Laser Surgery in Sacramento, and coauthors. The findings were published in Lasers in Surgery and Medicine.
The open-label, interventional study included 46 patients with rosacea, with moderate to severe facial erythema. Study participants received treatment with one of four energy-based devices: pulsed‐dye laser Vbeam Perfecta (PDL-Vbeam), pulsed‐dye laser Cynergy (PDL-Cynergy), intense pulsed-light therapy (IPL), or potassium titanyl phosphate laser (KTP laser), in combination with adjunctive oxymetazoline hydrochloride cream (1%).
On days 3-27 and 31-56, oxymetazoline, an alpha1A adrenoceptor agonist was applied once daily, while energy-based therapy was provided on day 1 and day 29.
The primary safety endpoints were the incidence of treatment‐emergent adverse events (TEAEs) and serious adverse events; the exploratory efficacy endpoint was the change in clinician erythema assessment (CEA) score from start of therapy measured over a 6-hour period post treatment.
Among 43 evaluable patients (who completed the study), CEA score was improved in 39 (90.7%) patients 6 hours post treatment on day 56 and in 30 (68.2%) patients pretreatment.
On day 31, of the 43 evaluable patients, “one‐grade or greater improvement was observed” in 26 (60.5%) patients before application of oxymetazoline, and in 38 (88.4%) of patients 6 hours post treatment, they wrote.
Overall, patient satisfaction increased over the course of the study, with 28 (65.1%) of patients reporting they were satisfied or very satisfied with the treatment on day 56.
Among 46 patients who received at least one treatment, 5 (10.9%) patients had one or more TEAEs (KTP laser, n = 1; PDL-Vbeam, n = 4), and 4 patients had one or more treatment‐related TEAEs (PDL-Vbeam, n = 4); All TEAEs were considered mild or moderate. “Three (6.5%) patients experienced TEAEs related to oxymetazoline; all led to study discontinuation,” the researchers reported.
The researchers acknowledged that a key limitation of the study was the use of multiple energy-based devices, delivered by different providers, which could have caused inconsistency in the results.
“Prospective clinical studies assessing the long‐term safety and efficacy of combined treatment with oxymetazoline and energy‐based therapies are needed,” they concluded.
The manuscript was funded by oxymetazoline manufacturer Aclaris Therapeutics. Several authors disclosed being an investigator, consultant, and/or laser manufacturers. One author was an employee of Aclaris at the time of the study.
SOURCE: Tanghetti EA et al. Lasers Surg Med. 2020 May 6. doi: 10.1002/lsm.23253.
according to results from a phase 4 study.
“The current study was designed to evaluate the safety and tolerability of oxymetazoline when used as an adjunctive treatment with energy‐based therapy for patients with moderate to severe facial erythema associated with rosacea,” wrote Emil A. Tanghetti, MD, of the Center for Dermatology and Laser Surgery in Sacramento, and coauthors. The findings were published in Lasers in Surgery and Medicine.
The open-label, interventional study included 46 patients with rosacea, with moderate to severe facial erythema. Study participants received treatment with one of four energy-based devices: pulsed‐dye laser Vbeam Perfecta (PDL-Vbeam), pulsed‐dye laser Cynergy (PDL-Cynergy), intense pulsed-light therapy (IPL), or potassium titanyl phosphate laser (KTP laser), in combination with adjunctive oxymetazoline hydrochloride cream (1%).
On days 3-27 and 31-56, oxymetazoline, an alpha1A adrenoceptor agonist was applied once daily, while energy-based therapy was provided on day 1 and day 29.
The primary safety endpoints were the incidence of treatment‐emergent adverse events (TEAEs) and serious adverse events; the exploratory efficacy endpoint was the change in clinician erythema assessment (CEA) score from start of therapy measured over a 6-hour period post treatment.
Among 43 evaluable patients (who completed the study), CEA score was improved in 39 (90.7%) patients 6 hours post treatment on day 56 and in 30 (68.2%) patients pretreatment.
On day 31, of the 43 evaluable patients, “one‐grade or greater improvement was observed” in 26 (60.5%) patients before application of oxymetazoline, and in 38 (88.4%) of patients 6 hours post treatment, they wrote.
Overall, patient satisfaction increased over the course of the study, with 28 (65.1%) of patients reporting they were satisfied or very satisfied with the treatment on day 56.
Among 46 patients who received at least one treatment, 5 (10.9%) patients had one or more TEAEs (KTP laser, n = 1; PDL-Vbeam, n = 4), and 4 patients had one or more treatment‐related TEAEs (PDL-Vbeam, n = 4); All TEAEs were considered mild or moderate. “Three (6.5%) patients experienced TEAEs related to oxymetazoline; all led to study discontinuation,” the researchers reported.
The researchers acknowledged that a key limitation of the study was the use of multiple energy-based devices, delivered by different providers, which could have caused inconsistency in the results.
“Prospective clinical studies assessing the long‐term safety and efficacy of combined treatment with oxymetazoline and energy‐based therapies are needed,” they concluded.
The manuscript was funded by oxymetazoline manufacturer Aclaris Therapeutics. Several authors disclosed being an investigator, consultant, and/or laser manufacturers. One author was an employee of Aclaris at the time of the study.
SOURCE: Tanghetti EA et al. Lasers Surg Med. 2020 May 6. doi: 10.1002/lsm.23253.
FROM LASERS IN SURGERY AND MEDICINE
FDA approves chemo-free combo for lung cancer
The US Food and Drug Administration (FDA) today approved the combination of nivolumab (Opdivo, Bristol-Myers Squibb) plus ipilimumab (Yervoy, Bristol-Myers Squibb) as first-line treatment for patients with metastatic non–small cell lung cancer (NSCLC) whose tumors express PD-L1 (≥1%).
Use is limited to patients with no epidermal growth factor receptor or anaplastic lymphoma kinase genomic tumor aberrations.
The FDA also approved a companion diagnostic device, the PD-L1 IHC 28-8 pharmDx (Agilent Technologies), for identifying patients appropriate for the combination treatment.
The approval is based on results from the CHECKMATE-227 study, a randomized, open-label, multipart trial in patients with metastatic or recurrent NSCLC and no prior anticancer therapy.
The findings were first presented at the 2019 European Society of Medical Oncology (ESMO 2019) annual meeting, and simultaneously published online in the New England Journal of Medicine.
In part 1a of the trial, 793 patients were randomly assigned to receive either the combination of nivolumab plus ipilimumab (n = 396) or platinum-doublet chemotherapy (n = 397). Median overall survival was 17.1 months versus 14.9 (hazard ratio, 0.79; 95% confidence interval, 0.67, 0.94; P = .006). Confirmed overall response rate was 36% and 30%.
Median response duration was 23.2 months in the nivolumab-plus-ipilimumab group versus 6.2 months in the platinum-doublet-chemotherapy group.
The most common adverse reactions in 20% or more of patients receiving the combination of nivolumab plus ipilimumab in CHECKMATE-227 were fatigue, rash, decreased appetite, musculoskeletal pain, diarrhea/colitis, dyspnea, cough, pruritus, nausea, and hepatitis.
At ESMO 2019, study investigator Solange Peters, MD, PhD, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, called the results “practice changing.”
But Marina C. Garassino, MD, head of thoracic medical oncology at the National Cancer Institute of Milan, Italy, had a different opinion. She said that although the results “show we have a new treatment option for the first-line treatment of metastatic NSCLC ... we don’t yet know if the findings are practice changing.”
Garassino added that more work is needed to determine which patients are optimally treated with two immunotherapies, with a combination of chemotherapy and immunotherapy, or just with a single agent.
The recommended doses for metastatic NSCLC are nivolumab 3 mg/kg every 2 weeks and ipilimumab 1 mg/kg every 6 weeks until disease progression or unacceptable toxicity, or up to 2 years in patients without disease progression.
More information about the approval is available on the FDA website.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) today approved the combination of nivolumab (Opdivo, Bristol-Myers Squibb) plus ipilimumab (Yervoy, Bristol-Myers Squibb) as first-line treatment for patients with metastatic non–small cell lung cancer (NSCLC) whose tumors express PD-L1 (≥1%).
Use is limited to patients with no epidermal growth factor receptor or anaplastic lymphoma kinase genomic tumor aberrations.
The FDA also approved a companion diagnostic device, the PD-L1 IHC 28-8 pharmDx (Agilent Technologies), for identifying patients appropriate for the combination treatment.
The approval is based on results from the CHECKMATE-227 study, a randomized, open-label, multipart trial in patients with metastatic or recurrent NSCLC and no prior anticancer therapy.
The findings were first presented at the 2019 European Society of Medical Oncology (ESMO 2019) annual meeting, and simultaneously published online in the New England Journal of Medicine.
In part 1a of the trial, 793 patients were randomly assigned to receive either the combination of nivolumab plus ipilimumab (n = 396) or platinum-doublet chemotherapy (n = 397). Median overall survival was 17.1 months versus 14.9 (hazard ratio, 0.79; 95% confidence interval, 0.67, 0.94; P = .006). Confirmed overall response rate was 36% and 30%.
Median response duration was 23.2 months in the nivolumab-plus-ipilimumab group versus 6.2 months in the platinum-doublet-chemotherapy group.
The most common adverse reactions in 20% or more of patients receiving the combination of nivolumab plus ipilimumab in CHECKMATE-227 were fatigue, rash, decreased appetite, musculoskeletal pain, diarrhea/colitis, dyspnea, cough, pruritus, nausea, and hepatitis.
At ESMO 2019, study investigator Solange Peters, MD, PhD, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, called the results “practice changing.”
But Marina C. Garassino, MD, head of thoracic medical oncology at the National Cancer Institute of Milan, Italy, had a different opinion. She said that although the results “show we have a new treatment option for the first-line treatment of metastatic NSCLC ... we don’t yet know if the findings are practice changing.”
Garassino added that more work is needed to determine which patients are optimally treated with two immunotherapies, with a combination of chemotherapy and immunotherapy, or just with a single agent.
The recommended doses for metastatic NSCLC are nivolumab 3 mg/kg every 2 weeks and ipilimumab 1 mg/kg every 6 weeks until disease progression or unacceptable toxicity, or up to 2 years in patients without disease progression.
More information about the approval is available on the FDA website.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) today approved the combination of nivolumab (Opdivo, Bristol-Myers Squibb) plus ipilimumab (Yervoy, Bristol-Myers Squibb) as first-line treatment for patients with metastatic non–small cell lung cancer (NSCLC) whose tumors express PD-L1 (≥1%).
Use is limited to patients with no epidermal growth factor receptor or anaplastic lymphoma kinase genomic tumor aberrations.
The FDA also approved a companion diagnostic device, the PD-L1 IHC 28-8 pharmDx (Agilent Technologies), for identifying patients appropriate for the combination treatment.
The approval is based on results from the CHECKMATE-227 study, a randomized, open-label, multipart trial in patients with metastatic or recurrent NSCLC and no prior anticancer therapy.
The findings were first presented at the 2019 European Society of Medical Oncology (ESMO 2019) annual meeting, and simultaneously published online in the New England Journal of Medicine.
In part 1a of the trial, 793 patients were randomly assigned to receive either the combination of nivolumab plus ipilimumab (n = 396) or platinum-doublet chemotherapy (n = 397). Median overall survival was 17.1 months versus 14.9 (hazard ratio, 0.79; 95% confidence interval, 0.67, 0.94; P = .006). Confirmed overall response rate was 36% and 30%.
Median response duration was 23.2 months in the nivolumab-plus-ipilimumab group versus 6.2 months in the platinum-doublet-chemotherapy group.
The most common adverse reactions in 20% or more of patients receiving the combination of nivolumab plus ipilimumab in CHECKMATE-227 were fatigue, rash, decreased appetite, musculoskeletal pain, diarrhea/colitis, dyspnea, cough, pruritus, nausea, and hepatitis.
At ESMO 2019, study investigator Solange Peters, MD, PhD, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland, called the results “practice changing.”
But Marina C. Garassino, MD, head of thoracic medical oncology at the National Cancer Institute of Milan, Italy, had a different opinion. She said that although the results “show we have a new treatment option for the first-line treatment of metastatic NSCLC ... we don’t yet know if the findings are practice changing.”
Garassino added that more work is needed to determine which patients are optimally treated with two immunotherapies, with a combination of chemotherapy and immunotherapy, or just with a single agent.
The recommended doses for metastatic NSCLC are nivolumab 3 mg/kg every 2 weeks and ipilimumab 1 mg/kg every 6 weeks until disease progression or unacceptable toxicity, or up to 2 years in patients without disease progression.
More information about the approval is available on the FDA website.
This article first appeared on Medscape.com.
Not the Neck-tar of the Gods
ANSWER
The correct answer is cutaneous sinus tract of odontogenic origin (choice “d”).
DISCUSSION
These uncommon lesions are known by several names, including odontogenic fistula. Invariably, they are misdiagnosed as an “infection” and treated with antibiotics, which only calm these lesions until they inevitably return to their pretreatment appearance. Though bacteria (mostly Peptostreptococcus—the anaerobe predominating in the mouth) are involved, this is not an infection as it usually manifests.
The underlying process of this condition is caused by a periapical abscess: as it grows in size and pressure, it enters into the mouth or fistulizes out through the buccal tissue, continuing until it penetrates the skin and begins to release its pustular contents. Eighty percent manifest on the submental or chin area, while 20% tunnel inwards toward the oral cavity.
They initially manifest as papules with a 2-to-3-mm surface that soon drain pus from a central sinus. As this continues, the epithelium responds to the chronic inflammation by forming a mass of pseudoepitheliomatous hyperplasia. Biopsy would reveal that the mass also shows signs of chronic inflammation. Ordinary bacterial culture often shows nothing because the predominant organism is an anaerobe. At most, one might see a polymicrobial result.
TREATMENT
For affected patients, a dentist can be considered for radiography of the area to confirm the location of the periapical abscess. Then the tooth is usually extracted, resulting in a cure. No further treatment of the sinus tract is necessary because it will essentially disappear over time. The tract does not require excision because it is lined with reactive granulation tissue and not epithelium (as is the case for many other fistular processes).
If the dental exam and radiograph fail to show the expected result, the other diagnoses—thyroglossal duct cyst, branchial cleft cyst, squamous cell carcinoma—would have to be considered.
ANSWER
The correct answer is cutaneous sinus tract of odontogenic origin (choice “d”).
DISCUSSION
These uncommon lesions are known by several names, including odontogenic fistula. Invariably, they are misdiagnosed as an “infection” and treated with antibiotics, which only calm these lesions until they inevitably return to their pretreatment appearance. Though bacteria (mostly Peptostreptococcus—the anaerobe predominating in the mouth) are involved, this is not an infection as it usually manifests.
The underlying process of this condition is caused by a periapical abscess: as it grows in size and pressure, it enters into the mouth or fistulizes out through the buccal tissue, continuing until it penetrates the skin and begins to release its pustular contents. Eighty percent manifest on the submental or chin area, while 20% tunnel inwards toward the oral cavity.
They initially manifest as papules with a 2-to-3-mm surface that soon drain pus from a central sinus. As this continues, the epithelium responds to the chronic inflammation by forming a mass of pseudoepitheliomatous hyperplasia. Biopsy would reveal that the mass also shows signs of chronic inflammation. Ordinary bacterial culture often shows nothing because the predominant organism is an anaerobe. At most, one might see a polymicrobial result.
TREATMENT
For affected patients, a dentist can be considered for radiography of the area to confirm the location of the periapical abscess. Then the tooth is usually extracted, resulting in a cure. No further treatment of the sinus tract is necessary because it will essentially disappear over time. The tract does not require excision because it is lined with reactive granulation tissue and not epithelium (as is the case for many other fistular processes).
If the dental exam and radiograph fail to show the expected result, the other diagnoses—thyroglossal duct cyst, branchial cleft cyst, squamous cell carcinoma—would have to be considered.
ANSWER
The correct answer is cutaneous sinus tract of odontogenic origin (choice “d”).
DISCUSSION
These uncommon lesions are known by several names, including odontogenic fistula. Invariably, they are misdiagnosed as an “infection” and treated with antibiotics, which only calm these lesions until they inevitably return to their pretreatment appearance. Though bacteria (mostly Peptostreptococcus—the anaerobe predominating in the mouth) are involved, this is not an infection as it usually manifests.
The underlying process of this condition is caused by a periapical abscess: as it grows in size and pressure, it enters into the mouth or fistulizes out through the buccal tissue, continuing until it penetrates the skin and begins to release its pustular contents. Eighty percent manifest on the submental or chin area, while 20% tunnel inwards toward the oral cavity.
They initially manifest as papules with a 2-to-3-mm surface that soon drain pus from a central sinus. As this continues, the epithelium responds to the chronic inflammation by forming a mass of pseudoepitheliomatous hyperplasia. Biopsy would reveal that the mass also shows signs of chronic inflammation. Ordinary bacterial culture often shows nothing because the predominant organism is an anaerobe. At most, one might see a polymicrobial result.
TREATMENT
For affected patients, a dentist can be considered for radiography of the area to confirm the location of the periapical abscess. Then the tooth is usually extracted, resulting in a cure. No further treatment of the sinus tract is necessary because it will essentially disappear over time. The tract does not require excision because it is lined with reactive granulation tissue and not epithelium (as is the case for many other fistular processes).
If the dental exam and radiograph fail to show the expected result, the other diagnoses—thyroglossal duct cyst, branchial cleft cyst, squamous cell carcinoma—would have to be considered.
For 4 years, the lesion on this 66-year-old woman’s chin has been growing slowly, causing little or no pain. However, a foul-smelling cloudy liquid drains from the site about once a week and—understandably—causes her considerable distress. Her primary care provider (PCP) regarded the problem as an infection and prescribed an antibiotic, which reduced the lesion’s size for a time. However, it soon grew again after the patient completed the treatment.
The patient denies any other serious health conditions such as diabetes or immunosuppression. She states she never had an injury to this area. Concerned about the possibility of skin cancer, the PCP refers the patient to dermatology.
Physical exam reveals a patient in no particular distress, afebrile, and oriented in all 3 spheres. She is cooperative with the history and exam. Her husband, who has accompanied her, helps to corroborate her answers.
The lesion is striking both in size (2.8 cm) and mixed morphology. A deeply retracted 1.5-cm dimple is located on the right lower chin/submental interface, situated evenly with the right lateral oral commissure. There is no erythema in this area or elsewhere in or around the lesion.
A fleshy, vermicular, 2 × 4–mm, soft, friable linear mass protrudes from the dimple, extending toward the submental region. Gentle pressure produces a small amount of pustular material issuing from the center of the retracted area. There are no other nodes in the region. There is little or no evidence of past overexposure to ultraviolet sources (dyschromia, weathering, actinic keratoses, or telangiectasias).
Proteins in urine may predict active lupus nephritis
according to a cross-sectional study published in Nature Communications. The proteins that best differentiate active lupus nephritis from inactive systemic lupus erythematosus (SLE) vary across ethnicities, the researchers wrote.
“A longitudinal study is warranted to investigate how these molecules relate to disease pathology and progression over time,” said senior study author Chandra Mohan, MD, PhD, of the department of biomedical engineering at the University of Houston, and colleagues. In addition, researchers should investigate the roles of protein biomarkers ALCAM, PF-4, properdin, VCAM-1, and sE-selectin in mediating lupus nephritis.
Limitations of renal biopsy
About 60% of patients with SLE will develop lupus nephritis, and 10%-15% of patients who develop lupus nephritis progress to end-stage renal disease. Although renal biopsy is the gold standard for the diagnosis of renal involvement in SLE, biopsies are invasive, not serially repeatable, and may not represent the entire kidney, Dr. Mohan and colleagues wrote.
To identify potential urinary biomarkers of lupus nephritis using an unbiased, proteomic approach, the investigators screened urine samples from 23 participants – 7 with active lupus nephritis, 8 with inactive SLE, and 8 healthy controls. They used an aptamer-based screen to investigate more than 1,100 proteins. The researchers then validated biomarker candidates using enzyme-linked immunosorbent assays. Independent cross-sectional cohorts included 127 patients with inactive SLE, 107 patients with active lupus nephritis, 67 patients with active nonrenal lupus, and 74 healthy controls. The cohorts included patients who were African American, Caucasian, and Asian. The researchers excluded patients with renal failure and pediatric patients.
Of the 12 urine proteins studied, 10 outperformed traditional laboratory measures, such as C3/C4 and anti–double stranded DNA, in discriminating active lupus nephritis from inactive SLE, wrote Dr. Mohan and colleagues. A Lasso regression analysis found that the best predictive model included 8 of the 12 urine proteins as well as race. The model discriminated active lupus nephritis from inactive SLE with an area under the receiver operating characteristic curve (AUC) of 0.98.
Among African Americans, urine proteins that best distinguished active lupus nephritis from inactive disease included PF-4 (AUC, 0.88), VCAM-1 (AUC, 0.87), properdin (AUC, 0.85), and ALCAM (AUC, 0.84). Among Caucasians, they included sE-selectin (AUC, 0.87), VCAM-1 (AUC, 0.84), BFL-1 (AUC, 0.81), and hemopexin (AUC, 0.80). Among Asians, they included ALCAM (AUC, 0.93), VCAM-1 (AUC, 0.92), TFPI (AUC, 0.88), and PF-4 (AUC, 0.83).
The study is “unique in highlighting the importance of tailoring the biomarkers to patient ethnicity,” the researchers wrote.
Basing subgroups on race rather than phenotypic profiles
“This is an important study because it confirms the ability to predict active lupus nephritis from urine samples and utilized advanced technologies to find key markers for that,” said Joan T. Merrill, MD, of the Oklahoma Medical Research Foundation in Oklahoma City. “It is unfortunate that investigators with access to such advanced technology are still using an outdated and extremely questionable method for distinguishing subgroups of patients, that of race.”
Grouping patients by phenotypic profiles that reflect current disease states “would be a more accurate method for finding optimal urinary markers for active nephritis,” and is “likely to prove more accurate for individuals in all races,” Dr. Merrill said. Certain racial subgroups may be more likely to have particular disease phenotypes, “which are usually identified based on gene pathway coexpression patterns.” Still, “people who self-identify as a given race are not genetically identical,” Dr. Merrill added. “In fact, this is a very blunt instrument, compared to phenotypic profiling now available for lupus patients.”
SLE and lupus nephritis are “heavily influenced by genetics,” and African Americans are three times more likely than Caucasians to develop SLE and are more like to develop end-stage renal disease, Dr. Mohan and colleagues wrote. Nevertheless, “influence from environmental triggers or socioeconomic factors cannot be ruled out,” they added. “Although patient demographics are widely known to affect SLE disease manifestations and outcomes, there are virtually no studies investigating this phenomenon in the context of disease biomarkers; most SLE biomarkers studies focus on one demographic group or all ethnic groups combined, which yield results that may not be equally predictive in all demographic groups of SLE patients.”
Dr. Mohan is collaborating with a biotechnology company to study drugs that may block ALCAM, according to a University of Houston news release. ALCAM is involved in immune and inflammatory responses, the researchers noted. “When all SLE patients were combined, urine ALCAM levels had the strongest bearing on disease activity status, in an unsupervised Bayesian network analysis,” they wrote. “Urine ALCAM also emerged as one of the few proteins that distinguished active [lupus nephritis] from active nonrenal lupus.”
National Institutes of Health grants supported the research. The investigators had no competing interests.
SOURCE: Stanley S et al. Nat Commun. 2020 May 4. doi: 10.1038/s41467-020-15986-3.
according to a cross-sectional study published in Nature Communications. The proteins that best differentiate active lupus nephritis from inactive systemic lupus erythematosus (SLE) vary across ethnicities, the researchers wrote.
“A longitudinal study is warranted to investigate how these molecules relate to disease pathology and progression over time,” said senior study author Chandra Mohan, MD, PhD, of the department of biomedical engineering at the University of Houston, and colleagues. In addition, researchers should investigate the roles of protein biomarkers ALCAM, PF-4, properdin, VCAM-1, and sE-selectin in mediating lupus nephritis.
Limitations of renal biopsy
About 60% of patients with SLE will develop lupus nephritis, and 10%-15% of patients who develop lupus nephritis progress to end-stage renal disease. Although renal biopsy is the gold standard for the diagnosis of renal involvement in SLE, biopsies are invasive, not serially repeatable, and may not represent the entire kidney, Dr. Mohan and colleagues wrote.
To identify potential urinary biomarkers of lupus nephritis using an unbiased, proteomic approach, the investigators screened urine samples from 23 participants – 7 with active lupus nephritis, 8 with inactive SLE, and 8 healthy controls. They used an aptamer-based screen to investigate more than 1,100 proteins. The researchers then validated biomarker candidates using enzyme-linked immunosorbent assays. Independent cross-sectional cohorts included 127 patients with inactive SLE, 107 patients with active lupus nephritis, 67 patients with active nonrenal lupus, and 74 healthy controls. The cohorts included patients who were African American, Caucasian, and Asian. The researchers excluded patients with renal failure and pediatric patients.
Of the 12 urine proteins studied, 10 outperformed traditional laboratory measures, such as C3/C4 and anti–double stranded DNA, in discriminating active lupus nephritis from inactive SLE, wrote Dr. Mohan and colleagues. A Lasso regression analysis found that the best predictive model included 8 of the 12 urine proteins as well as race. The model discriminated active lupus nephritis from inactive SLE with an area under the receiver operating characteristic curve (AUC) of 0.98.
Among African Americans, urine proteins that best distinguished active lupus nephritis from inactive disease included PF-4 (AUC, 0.88), VCAM-1 (AUC, 0.87), properdin (AUC, 0.85), and ALCAM (AUC, 0.84). Among Caucasians, they included sE-selectin (AUC, 0.87), VCAM-1 (AUC, 0.84), BFL-1 (AUC, 0.81), and hemopexin (AUC, 0.80). Among Asians, they included ALCAM (AUC, 0.93), VCAM-1 (AUC, 0.92), TFPI (AUC, 0.88), and PF-4 (AUC, 0.83).
The study is “unique in highlighting the importance of tailoring the biomarkers to patient ethnicity,” the researchers wrote.
Basing subgroups on race rather than phenotypic profiles
“This is an important study because it confirms the ability to predict active lupus nephritis from urine samples and utilized advanced technologies to find key markers for that,” said Joan T. Merrill, MD, of the Oklahoma Medical Research Foundation in Oklahoma City. “It is unfortunate that investigators with access to such advanced technology are still using an outdated and extremely questionable method for distinguishing subgroups of patients, that of race.”
Grouping patients by phenotypic profiles that reflect current disease states “would be a more accurate method for finding optimal urinary markers for active nephritis,” and is “likely to prove more accurate for individuals in all races,” Dr. Merrill said. Certain racial subgroups may be more likely to have particular disease phenotypes, “which are usually identified based on gene pathway coexpression patterns.” Still, “people who self-identify as a given race are not genetically identical,” Dr. Merrill added. “In fact, this is a very blunt instrument, compared to phenotypic profiling now available for lupus patients.”
SLE and lupus nephritis are “heavily influenced by genetics,” and African Americans are three times more likely than Caucasians to develop SLE and are more like to develop end-stage renal disease, Dr. Mohan and colleagues wrote. Nevertheless, “influence from environmental triggers or socioeconomic factors cannot be ruled out,” they added. “Although patient demographics are widely known to affect SLE disease manifestations and outcomes, there are virtually no studies investigating this phenomenon in the context of disease biomarkers; most SLE biomarkers studies focus on one demographic group or all ethnic groups combined, which yield results that may not be equally predictive in all demographic groups of SLE patients.”
Dr. Mohan is collaborating with a biotechnology company to study drugs that may block ALCAM, according to a University of Houston news release. ALCAM is involved in immune and inflammatory responses, the researchers noted. “When all SLE patients were combined, urine ALCAM levels had the strongest bearing on disease activity status, in an unsupervised Bayesian network analysis,” they wrote. “Urine ALCAM also emerged as one of the few proteins that distinguished active [lupus nephritis] from active nonrenal lupus.”
National Institutes of Health grants supported the research. The investigators had no competing interests.
SOURCE: Stanley S et al. Nat Commun. 2020 May 4. doi: 10.1038/s41467-020-15986-3.
according to a cross-sectional study published in Nature Communications. The proteins that best differentiate active lupus nephritis from inactive systemic lupus erythematosus (SLE) vary across ethnicities, the researchers wrote.
“A longitudinal study is warranted to investigate how these molecules relate to disease pathology and progression over time,” said senior study author Chandra Mohan, MD, PhD, of the department of biomedical engineering at the University of Houston, and colleagues. In addition, researchers should investigate the roles of protein biomarkers ALCAM, PF-4, properdin, VCAM-1, and sE-selectin in mediating lupus nephritis.
Limitations of renal biopsy
About 60% of patients with SLE will develop lupus nephritis, and 10%-15% of patients who develop lupus nephritis progress to end-stage renal disease. Although renal biopsy is the gold standard for the diagnosis of renal involvement in SLE, biopsies are invasive, not serially repeatable, and may not represent the entire kidney, Dr. Mohan and colleagues wrote.
To identify potential urinary biomarkers of lupus nephritis using an unbiased, proteomic approach, the investigators screened urine samples from 23 participants – 7 with active lupus nephritis, 8 with inactive SLE, and 8 healthy controls. They used an aptamer-based screen to investigate more than 1,100 proteins. The researchers then validated biomarker candidates using enzyme-linked immunosorbent assays. Independent cross-sectional cohorts included 127 patients with inactive SLE, 107 patients with active lupus nephritis, 67 patients with active nonrenal lupus, and 74 healthy controls. The cohorts included patients who were African American, Caucasian, and Asian. The researchers excluded patients with renal failure and pediatric patients.
Of the 12 urine proteins studied, 10 outperformed traditional laboratory measures, such as C3/C4 and anti–double stranded DNA, in discriminating active lupus nephritis from inactive SLE, wrote Dr. Mohan and colleagues. A Lasso regression analysis found that the best predictive model included 8 of the 12 urine proteins as well as race. The model discriminated active lupus nephritis from inactive SLE with an area under the receiver operating characteristic curve (AUC) of 0.98.
Among African Americans, urine proteins that best distinguished active lupus nephritis from inactive disease included PF-4 (AUC, 0.88), VCAM-1 (AUC, 0.87), properdin (AUC, 0.85), and ALCAM (AUC, 0.84). Among Caucasians, they included sE-selectin (AUC, 0.87), VCAM-1 (AUC, 0.84), BFL-1 (AUC, 0.81), and hemopexin (AUC, 0.80). Among Asians, they included ALCAM (AUC, 0.93), VCAM-1 (AUC, 0.92), TFPI (AUC, 0.88), and PF-4 (AUC, 0.83).
The study is “unique in highlighting the importance of tailoring the biomarkers to patient ethnicity,” the researchers wrote.
Basing subgroups on race rather than phenotypic profiles
“This is an important study because it confirms the ability to predict active lupus nephritis from urine samples and utilized advanced technologies to find key markers for that,” said Joan T. Merrill, MD, of the Oklahoma Medical Research Foundation in Oklahoma City. “It is unfortunate that investigators with access to such advanced technology are still using an outdated and extremely questionable method for distinguishing subgroups of patients, that of race.”
Grouping patients by phenotypic profiles that reflect current disease states “would be a more accurate method for finding optimal urinary markers for active nephritis,” and is “likely to prove more accurate for individuals in all races,” Dr. Merrill said. Certain racial subgroups may be more likely to have particular disease phenotypes, “which are usually identified based on gene pathway coexpression patterns.” Still, “people who self-identify as a given race are not genetically identical,” Dr. Merrill added. “In fact, this is a very blunt instrument, compared to phenotypic profiling now available for lupus patients.”
SLE and lupus nephritis are “heavily influenced by genetics,” and African Americans are three times more likely than Caucasians to develop SLE and are more like to develop end-stage renal disease, Dr. Mohan and colleagues wrote. Nevertheless, “influence from environmental triggers or socioeconomic factors cannot be ruled out,” they added. “Although patient demographics are widely known to affect SLE disease manifestations and outcomes, there are virtually no studies investigating this phenomenon in the context of disease biomarkers; most SLE biomarkers studies focus on one demographic group or all ethnic groups combined, which yield results that may not be equally predictive in all demographic groups of SLE patients.”
Dr. Mohan is collaborating with a biotechnology company to study drugs that may block ALCAM, according to a University of Houston news release. ALCAM is involved in immune and inflammatory responses, the researchers noted. “When all SLE patients were combined, urine ALCAM levels had the strongest bearing on disease activity status, in an unsupervised Bayesian network analysis,” they wrote. “Urine ALCAM also emerged as one of the few proteins that distinguished active [lupus nephritis] from active nonrenal lupus.”
National Institutes of Health grants supported the research. The investigators had no competing interests.
SOURCE: Stanley S et al. Nat Commun. 2020 May 4. doi: 10.1038/s41467-020-15986-3.
FROM NATURE COMMUNICATIONS
Planning for a psychiatric COVID-19–positive unit
Identifying key decision points is critical
Reports have emerged about the unique vulnerability of psychiatric hospitals to the ravages of COVID-19.
In a South Korea psychiatric hospital, 101 of 103 patients contracted SARS-CoV-2 during an outbreak; 7 eventually died.1,2 This report, among a few others, have led to the development of psychiatric COVID-19–positive units (PCU). However, it remains highly unclear how many are currently open, where they are located, or what their operations are like.
We knew that we could not allow a medically asymptomatic “covertly” COVID-19–positive patient to be introduced to the social community of our inpatient units because of the risks of transmission to other patients and staff.
In coordination with our health system infection prevention experts, we have therefore required a confirmed negative COVID-19 polymerase chain reaction nasal swab performed no more than 48 hours prior to the time/date of acute psychiatric inpatient admission. Furthermore, as part of the broad health system response and surge planning, we were asked by our respective incident command centers to begin planning for a Psychiatric COVID-19–positive Unit (PCU) that might allow us to safely care for a cohort of patients needing such hospitalization.
It is worth emphasizing that the typical patient who is a candidate for a PCU is so acutely psychiatrically ill that they cannot be managed in a less restrictive environment than an inpatient psychiatric unit and, at the same time, is likely to not be medically ill enough to warrant admission to an internal medicine service in a general acute care hospital.
We have identified eight principles and critical decision points that can help inpatient units plan for the safe care of COVID-19–positive patients on a PCU.
1. Triage: Patients admitted to a PCU should be medically stable, particularly with regard to COVID-19 and respiratory symptomatology. PCUs should establish clear criteria for admission and discharge (or medical transfer). Examples of potential exclusionary criteria to a PCU include:
- Respiratory distress, shortness of breath, hypoxia, requirement for supplemental oxygen, or requirement for respiratory therapy breathing treatments.
- Fever, or signs of sepsis, or systemic inflammatory response syndrome.
- Medical frailty, significant medical comorbidities, delirium, or altered mental status;
- Requirements for continuous vital sign monitoring or of a monitoring frequency beyond the capacity of the PCU.
Discharge criteria may also include a symptom-based strategy because emerging evidence suggests that patients may be less infectious by day 10-14 of the disease course,3 and viral lab testing is very sensitive and will be positive for periods of time after individuals are no longer infectious. The symptom-based strategy allows for patients to not require retesting prior to discharge. However, some receiving facilities (for example residential or skilled nursing facilities) may necessitate testing, in which case a testing-based strategy can be used. The Centers for Disease Control and Prevention provides guidelines for both types of strategies.4
2. Infection control and personal protective equipment: PCUs require modifications or departures from the typical inpatient free-ranging environment in which common areas are provided for patients to engage in a community of care, including group therapy (such as occupational, recreational, Alcoholics Anonymous, and social work groups).
- Isolation: PCUs must consider whether they will require patients to isolate to their rooms or to allow modified or limited access to “public” or “community” areas. While there do not appear to be standard recommendations from the CDC or other public health entities regarding negative pressure or any specific room ventilation requirements, it is prudent to work with local infectious disease experts on protocols. Important considerations include spatial planning for infection control areas to don and doff appropriate personal protective equipment (PPE) and appropriate workspace to prevent contamination of non–COVID-19 work areas. Approaches can include establishing clearly identified and visually demarcated infection control “zones” (often referred to as “hot, warm, and cold zones”) that correspond to specific PPE requirements for staff. In addition, individuals should eat in their own rooms or designated areas because use of common areas for meals can potentially lead to aerosolized spread of the virus.
- Cohorting: Generally, PCUs should consider admitting only COVID-19–positive patients to a PCU to avoid exposure to other patients. Hospitals and health systems should determine protocols and locations for testing and managing “patients under investigation” for COVID-19, which should precede admission to the PCU.
- PPE: It is important to clearly establish and communicate PPE requirements and procedures for direct physical contact versus no physical contact (for example, visual safety checks). Identify clear supply chains for PPE and hand sanitizer.
3. Medical management and consultation: PCUs should establish clear pathways for accessing consultation from medical consultants. It may be ideal, in addition to standard daily psychiatric physician rounding, to have daily internal medicine rounding and/or medical nursing staff working on the unit. Given the potential of COVID-19–positive patients to rapidly devolve from asymptomatic to acutely ill, it is necessary to establish protocols for the provision of urgent medical care 24/7 and streamlined processes for transfer to a medical unit.
Clear protocols should be established to address any potential signs of decompensation in the respiratory status of a PCU unit, including administration of oxygen and restrictions (or appropriate precautions) related to aerosolizing treatment such as nebulizers or positive airway pressure.
4. Code blue protocol: Any emergent medical issues, including acute respiratory decompensation, should trigger a Code Blue response that has been specifically designed for COVID-19–positive patients, including considerations for proper PPE during resuscitation efforts.
5. Psychiatric staffing and workflows: When possible, it may be preferable to engage volunteer medical and nursing staff for the PCU, as opposed to mandating participation. Take into consideration support needs, including education and training about safe PPE practices, processes for testing health care workers, return-to-work guidance, and potential alternate housing.
- Telehealth: Clinicians (such as physicians, social workers, occupational therapists) should leverage and maximize the use of telemedicine to minimize direct or prolonged exposure to infectious disease risks.
- Nursing: It is important to establish appropriate ratios of nursing and support staff for a COVID-19–positive psychiatry unit given the unique work flows related to isolation precautions and to ensure patient and staff safety. These ratios may take into account patient-specific needs, including the need for additional staff to perform constant observation for high-risk patients, management of agitated patients, and sufficient staff to allow for relief and break-time from PPE. Admission and routine care processes should be adapted in order to limit equipment entering the room, such as computer workstations on wheels.
- Medication administration procedures: Develop work flows related to PPE and infection control when retrieving and administering medications.
- Workspace: Designate appropriate workspace for PCU clinicians to access computers and documents and to minimize use of non–COVID-19 unit work areas.
6. Restraints and management of agitated patients: PCUs should develop plans for addressing agitated patients, including contingency plans for whether seclusion or restraints should be administered in the patient’s individual room or in a dedicated restraint room in the PCU. Staff training should include protocols specifically designed for managing agitated patients in the PCU.
7. Discharge processes: If patients remain medically well and clear their COVID-19 PCR tests, it is conceivable that they might be transferred to a non–COVID-19 psychiatric unit if sufficient isolation time has passed and the infectious disease consultants deem it appropriate. It is also possible that patients would be discharged from a PCU to home or other residential setting. Such patients should be assessed for ability to comply with continued self-quarantine if necessary. Discharge planning must take into consideration follow-up plans for COVID-19 illness and primary care appointments, as well as needed psychiatric follow-up.
8. Patients’ rights: The apparently highly infectious and transmissible nature of SARS-CoV-2 creates novel tensions between a wide range of individual rights and the rights of others. In addition to manifesting in our general society, there are potentially unique tensions in acute inpatient psychiatric settings. Certain patients’ rights may require modification in a PCU (for example, access to outdoor space, personal belongings, visitors, and possibly civil commitment judicial hearings). These discussions may require input from hospital compliance officers, ethics committees, risk managers, and the local department of mental health and also may be partly solved by using video communication platforms.
A few other “pearls” may be of value: Psychiatric hospitals that are colocated with a general acute care hospital or ED might be better situated to develop protocols to safely care for COVID-19–positive psychiatric patients, by virtue of the close proximity of full-spectrum acute general hospital services. Direct engagement by a command center and hospital or health system senior leadership also seems crucial as a means for assuring authorization to proceed with planning what may be a frightening or controversial (but necessary) adaptation of inpatient psychiatric unit(s) to the exigencies of the COVID-19 pandemic.
The resources of a robust community hospital or academic health system (including infection prevention leaders who engage in continuous liaison with local, county, state, and federal public health expertise) are crucial to the “learning health system” model, which requires flexibility, rapid adaptation to new knowledge, and accessibility to infectious disease and other consultation for special situations. Frequent and open communication with all professional stakeholders (through town halls, Q&A sessions, group discussions, and so on) is important in the planning process to socialize the principles and concepts that are critical for providing care in a PCU, reducing anxiety, and bolstering collegiality and staff morale.
References
1. Kim MJ. “ ‘It was a medical disaster’: The psychiatric ward that saw 100 patients with new coronavirus.” Independent. 2020 Mar 1.
2. Korean Society of Infectious Diseases et al. J Korean Med Sci. 2020 Mar 16;35(10):e112.
3. Centers for Disease Control and Prevention. Symptom-based strategy to discontinue isolation for persons with COVID-19. Decision Memo. 2020 May 3.
4. He X et al. Nature Medicine. 2020. 26:672-5.
Dr. Cheung is associate medical director and chief quality officer at the Stewart and Lynda Resnick Neuropsychiatric Hospital at the University of California, Los Angeles. He has no conflicts of interest. Dr. Strouse is medical director, UCLA Stewart and Lynda Resnick Neuropsychiatric Hospital and Maddie Katz Professor at the UCLA department of psychiatry/Semel Institute. He has no conflicts of interest. Dr. Li is associate medical director of quality improvement at Yale-New Haven Psychiatric Hospital in Connecticut. She also serves as medical director of clinical operations at the Yale-New Haven Health System. Dr. Li is a 2019-2020 Health and Aging Policy Fellow and receives funding support from the program.
Identifying key decision points is critical
Identifying key decision points is critical
Reports have emerged about the unique vulnerability of psychiatric hospitals to the ravages of COVID-19.
In a South Korea psychiatric hospital, 101 of 103 patients contracted SARS-CoV-2 during an outbreak; 7 eventually died.1,2 This report, among a few others, have led to the development of psychiatric COVID-19–positive units (PCU). However, it remains highly unclear how many are currently open, where they are located, or what their operations are like.
We knew that we could not allow a medically asymptomatic “covertly” COVID-19–positive patient to be introduced to the social community of our inpatient units because of the risks of transmission to other patients and staff.
In coordination with our health system infection prevention experts, we have therefore required a confirmed negative COVID-19 polymerase chain reaction nasal swab performed no more than 48 hours prior to the time/date of acute psychiatric inpatient admission. Furthermore, as part of the broad health system response and surge planning, we were asked by our respective incident command centers to begin planning for a Psychiatric COVID-19–positive Unit (PCU) that might allow us to safely care for a cohort of patients needing such hospitalization.
It is worth emphasizing that the typical patient who is a candidate for a PCU is so acutely psychiatrically ill that they cannot be managed in a less restrictive environment than an inpatient psychiatric unit and, at the same time, is likely to not be medically ill enough to warrant admission to an internal medicine service in a general acute care hospital.
We have identified eight principles and critical decision points that can help inpatient units plan for the safe care of COVID-19–positive patients on a PCU.
1. Triage: Patients admitted to a PCU should be medically stable, particularly with regard to COVID-19 and respiratory symptomatology. PCUs should establish clear criteria for admission and discharge (or medical transfer). Examples of potential exclusionary criteria to a PCU include:
- Respiratory distress, shortness of breath, hypoxia, requirement for supplemental oxygen, or requirement for respiratory therapy breathing treatments.
- Fever, or signs of sepsis, or systemic inflammatory response syndrome.
- Medical frailty, significant medical comorbidities, delirium, or altered mental status;
- Requirements for continuous vital sign monitoring or of a monitoring frequency beyond the capacity of the PCU.
Discharge criteria may also include a symptom-based strategy because emerging evidence suggests that patients may be less infectious by day 10-14 of the disease course,3 and viral lab testing is very sensitive and will be positive for periods of time after individuals are no longer infectious. The symptom-based strategy allows for patients to not require retesting prior to discharge. However, some receiving facilities (for example residential or skilled nursing facilities) may necessitate testing, in which case a testing-based strategy can be used. The Centers for Disease Control and Prevention provides guidelines for both types of strategies.4
2. Infection control and personal protective equipment: PCUs require modifications or departures from the typical inpatient free-ranging environment in which common areas are provided for patients to engage in a community of care, including group therapy (such as occupational, recreational, Alcoholics Anonymous, and social work groups).
- Isolation: PCUs must consider whether they will require patients to isolate to their rooms or to allow modified or limited access to “public” or “community” areas. While there do not appear to be standard recommendations from the CDC or other public health entities regarding negative pressure or any specific room ventilation requirements, it is prudent to work with local infectious disease experts on protocols. Important considerations include spatial planning for infection control areas to don and doff appropriate personal protective equipment (PPE) and appropriate workspace to prevent contamination of non–COVID-19 work areas. Approaches can include establishing clearly identified and visually demarcated infection control “zones” (often referred to as “hot, warm, and cold zones”) that correspond to specific PPE requirements for staff. In addition, individuals should eat in their own rooms or designated areas because use of common areas for meals can potentially lead to aerosolized spread of the virus.
- Cohorting: Generally, PCUs should consider admitting only COVID-19–positive patients to a PCU to avoid exposure to other patients. Hospitals and health systems should determine protocols and locations for testing and managing “patients under investigation” for COVID-19, which should precede admission to the PCU.
- PPE: It is important to clearly establish and communicate PPE requirements and procedures for direct physical contact versus no physical contact (for example, visual safety checks). Identify clear supply chains for PPE and hand sanitizer.
3. Medical management and consultation: PCUs should establish clear pathways for accessing consultation from medical consultants. It may be ideal, in addition to standard daily psychiatric physician rounding, to have daily internal medicine rounding and/or medical nursing staff working on the unit. Given the potential of COVID-19–positive patients to rapidly devolve from asymptomatic to acutely ill, it is necessary to establish protocols for the provision of urgent medical care 24/7 and streamlined processes for transfer to a medical unit.
Clear protocols should be established to address any potential signs of decompensation in the respiratory status of a PCU unit, including administration of oxygen and restrictions (or appropriate precautions) related to aerosolizing treatment such as nebulizers or positive airway pressure.
4. Code blue protocol: Any emergent medical issues, including acute respiratory decompensation, should trigger a Code Blue response that has been specifically designed for COVID-19–positive patients, including considerations for proper PPE during resuscitation efforts.
5. Psychiatric staffing and workflows: When possible, it may be preferable to engage volunteer medical and nursing staff for the PCU, as opposed to mandating participation. Take into consideration support needs, including education and training about safe PPE practices, processes for testing health care workers, return-to-work guidance, and potential alternate housing.
- Telehealth: Clinicians (such as physicians, social workers, occupational therapists) should leverage and maximize the use of telemedicine to minimize direct or prolonged exposure to infectious disease risks.
- Nursing: It is important to establish appropriate ratios of nursing and support staff for a COVID-19–positive psychiatry unit given the unique work flows related to isolation precautions and to ensure patient and staff safety. These ratios may take into account patient-specific needs, including the need for additional staff to perform constant observation for high-risk patients, management of agitated patients, and sufficient staff to allow for relief and break-time from PPE. Admission and routine care processes should be adapted in order to limit equipment entering the room, such as computer workstations on wheels.
- Medication administration procedures: Develop work flows related to PPE and infection control when retrieving and administering medications.
- Workspace: Designate appropriate workspace for PCU clinicians to access computers and documents and to minimize use of non–COVID-19 unit work areas.
6. Restraints and management of agitated patients: PCUs should develop plans for addressing agitated patients, including contingency plans for whether seclusion or restraints should be administered in the patient’s individual room or in a dedicated restraint room in the PCU. Staff training should include protocols specifically designed for managing agitated patients in the PCU.
7. Discharge processes: If patients remain medically well and clear their COVID-19 PCR tests, it is conceivable that they might be transferred to a non–COVID-19 psychiatric unit if sufficient isolation time has passed and the infectious disease consultants deem it appropriate. It is also possible that patients would be discharged from a PCU to home or other residential setting. Such patients should be assessed for ability to comply with continued self-quarantine if necessary. Discharge planning must take into consideration follow-up plans for COVID-19 illness and primary care appointments, as well as needed psychiatric follow-up.
8. Patients’ rights: The apparently highly infectious and transmissible nature of SARS-CoV-2 creates novel tensions between a wide range of individual rights and the rights of others. In addition to manifesting in our general society, there are potentially unique tensions in acute inpatient psychiatric settings. Certain patients’ rights may require modification in a PCU (for example, access to outdoor space, personal belongings, visitors, and possibly civil commitment judicial hearings). These discussions may require input from hospital compliance officers, ethics committees, risk managers, and the local department of mental health and also may be partly solved by using video communication platforms.
A few other “pearls” may be of value: Psychiatric hospitals that are colocated with a general acute care hospital or ED might be better situated to develop protocols to safely care for COVID-19–positive psychiatric patients, by virtue of the close proximity of full-spectrum acute general hospital services. Direct engagement by a command center and hospital or health system senior leadership also seems crucial as a means for assuring authorization to proceed with planning what may be a frightening or controversial (but necessary) adaptation of inpatient psychiatric unit(s) to the exigencies of the COVID-19 pandemic.
The resources of a robust community hospital or academic health system (including infection prevention leaders who engage in continuous liaison with local, county, state, and federal public health expertise) are crucial to the “learning health system” model, which requires flexibility, rapid adaptation to new knowledge, and accessibility to infectious disease and other consultation for special situations. Frequent and open communication with all professional stakeholders (through town halls, Q&A sessions, group discussions, and so on) is important in the planning process to socialize the principles and concepts that are critical for providing care in a PCU, reducing anxiety, and bolstering collegiality and staff morale.
References
1. Kim MJ. “ ‘It was a medical disaster’: The psychiatric ward that saw 100 patients with new coronavirus.” Independent. 2020 Mar 1.
2. Korean Society of Infectious Diseases et al. J Korean Med Sci. 2020 Mar 16;35(10):e112.
3. Centers for Disease Control and Prevention. Symptom-based strategy to discontinue isolation for persons with COVID-19. Decision Memo. 2020 May 3.
4. He X et al. Nature Medicine. 2020. 26:672-5.
Dr. Cheung is associate medical director and chief quality officer at the Stewart and Lynda Resnick Neuropsychiatric Hospital at the University of California, Los Angeles. He has no conflicts of interest. Dr. Strouse is medical director, UCLA Stewart and Lynda Resnick Neuropsychiatric Hospital and Maddie Katz Professor at the UCLA department of psychiatry/Semel Institute. He has no conflicts of interest. Dr. Li is associate medical director of quality improvement at Yale-New Haven Psychiatric Hospital in Connecticut. She also serves as medical director of clinical operations at the Yale-New Haven Health System. Dr. Li is a 2019-2020 Health and Aging Policy Fellow and receives funding support from the program.
Reports have emerged about the unique vulnerability of psychiatric hospitals to the ravages of COVID-19.
In a South Korea psychiatric hospital, 101 of 103 patients contracted SARS-CoV-2 during an outbreak; 7 eventually died.1,2 This report, among a few others, have led to the development of psychiatric COVID-19–positive units (PCU). However, it remains highly unclear how many are currently open, where they are located, or what their operations are like.
We knew that we could not allow a medically asymptomatic “covertly” COVID-19–positive patient to be introduced to the social community of our inpatient units because of the risks of transmission to other patients and staff.
In coordination with our health system infection prevention experts, we have therefore required a confirmed negative COVID-19 polymerase chain reaction nasal swab performed no more than 48 hours prior to the time/date of acute psychiatric inpatient admission. Furthermore, as part of the broad health system response and surge planning, we were asked by our respective incident command centers to begin planning for a Psychiatric COVID-19–positive Unit (PCU) that might allow us to safely care for a cohort of patients needing such hospitalization.
It is worth emphasizing that the typical patient who is a candidate for a PCU is so acutely psychiatrically ill that they cannot be managed in a less restrictive environment than an inpatient psychiatric unit and, at the same time, is likely to not be medically ill enough to warrant admission to an internal medicine service in a general acute care hospital.
We have identified eight principles and critical decision points that can help inpatient units plan for the safe care of COVID-19–positive patients on a PCU.
1. Triage: Patients admitted to a PCU should be medically stable, particularly with regard to COVID-19 and respiratory symptomatology. PCUs should establish clear criteria for admission and discharge (or medical transfer). Examples of potential exclusionary criteria to a PCU include:
- Respiratory distress, shortness of breath, hypoxia, requirement for supplemental oxygen, or requirement for respiratory therapy breathing treatments.
- Fever, or signs of sepsis, or systemic inflammatory response syndrome.
- Medical frailty, significant medical comorbidities, delirium, or altered mental status;
- Requirements for continuous vital sign monitoring or of a monitoring frequency beyond the capacity of the PCU.
Discharge criteria may also include a symptom-based strategy because emerging evidence suggests that patients may be less infectious by day 10-14 of the disease course,3 and viral lab testing is very sensitive and will be positive for periods of time after individuals are no longer infectious. The symptom-based strategy allows for patients to not require retesting prior to discharge. However, some receiving facilities (for example residential or skilled nursing facilities) may necessitate testing, in which case a testing-based strategy can be used. The Centers for Disease Control and Prevention provides guidelines for both types of strategies.4
2. Infection control and personal protective equipment: PCUs require modifications or departures from the typical inpatient free-ranging environment in which common areas are provided for patients to engage in a community of care, including group therapy (such as occupational, recreational, Alcoholics Anonymous, and social work groups).
- Isolation: PCUs must consider whether they will require patients to isolate to their rooms or to allow modified or limited access to “public” or “community” areas. While there do not appear to be standard recommendations from the CDC or other public health entities regarding negative pressure or any specific room ventilation requirements, it is prudent to work with local infectious disease experts on protocols. Important considerations include spatial planning for infection control areas to don and doff appropriate personal protective equipment (PPE) and appropriate workspace to prevent contamination of non–COVID-19 work areas. Approaches can include establishing clearly identified and visually demarcated infection control “zones” (often referred to as “hot, warm, and cold zones”) that correspond to specific PPE requirements for staff. In addition, individuals should eat in their own rooms or designated areas because use of common areas for meals can potentially lead to aerosolized spread of the virus.
- Cohorting: Generally, PCUs should consider admitting only COVID-19–positive patients to a PCU to avoid exposure to other patients. Hospitals and health systems should determine protocols and locations for testing and managing “patients under investigation” for COVID-19, which should precede admission to the PCU.
- PPE: It is important to clearly establish and communicate PPE requirements and procedures for direct physical contact versus no physical contact (for example, visual safety checks). Identify clear supply chains for PPE and hand sanitizer.
3. Medical management and consultation: PCUs should establish clear pathways for accessing consultation from medical consultants. It may be ideal, in addition to standard daily psychiatric physician rounding, to have daily internal medicine rounding and/or medical nursing staff working on the unit. Given the potential of COVID-19–positive patients to rapidly devolve from asymptomatic to acutely ill, it is necessary to establish protocols for the provision of urgent medical care 24/7 and streamlined processes for transfer to a medical unit.
Clear protocols should be established to address any potential signs of decompensation in the respiratory status of a PCU unit, including administration of oxygen and restrictions (or appropriate precautions) related to aerosolizing treatment such as nebulizers or positive airway pressure.
4. Code blue protocol: Any emergent medical issues, including acute respiratory decompensation, should trigger a Code Blue response that has been specifically designed for COVID-19–positive patients, including considerations for proper PPE during resuscitation efforts.
5. Psychiatric staffing and workflows: When possible, it may be preferable to engage volunteer medical and nursing staff for the PCU, as opposed to mandating participation. Take into consideration support needs, including education and training about safe PPE practices, processes for testing health care workers, return-to-work guidance, and potential alternate housing.
- Telehealth: Clinicians (such as physicians, social workers, occupational therapists) should leverage and maximize the use of telemedicine to minimize direct or prolonged exposure to infectious disease risks.
- Nursing: It is important to establish appropriate ratios of nursing and support staff for a COVID-19–positive psychiatry unit given the unique work flows related to isolation precautions and to ensure patient and staff safety. These ratios may take into account patient-specific needs, including the need for additional staff to perform constant observation for high-risk patients, management of agitated patients, and sufficient staff to allow for relief and break-time from PPE. Admission and routine care processes should be adapted in order to limit equipment entering the room, such as computer workstations on wheels.
- Medication administration procedures: Develop work flows related to PPE and infection control when retrieving and administering medications.
- Workspace: Designate appropriate workspace for PCU clinicians to access computers and documents and to minimize use of non–COVID-19 unit work areas.
6. Restraints and management of agitated patients: PCUs should develop plans for addressing agitated patients, including contingency plans for whether seclusion or restraints should be administered in the patient’s individual room or in a dedicated restraint room in the PCU. Staff training should include protocols specifically designed for managing agitated patients in the PCU.
7. Discharge processes: If patients remain medically well and clear their COVID-19 PCR tests, it is conceivable that they might be transferred to a non–COVID-19 psychiatric unit if sufficient isolation time has passed and the infectious disease consultants deem it appropriate. It is also possible that patients would be discharged from a PCU to home or other residential setting. Such patients should be assessed for ability to comply with continued self-quarantine if necessary. Discharge planning must take into consideration follow-up plans for COVID-19 illness and primary care appointments, as well as needed psychiatric follow-up.
8. Patients’ rights: The apparently highly infectious and transmissible nature of SARS-CoV-2 creates novel tensions between a wide range of individual rights and the rights of others. In addition to manifesting in our general society, there are potentially unique tensions in acute inpatient psychiatric settings. Certain patients’ rights may require modification in a PCU (for example, access to outdoor space, personal belongings, visitors, and possibly civil commitment judicial hearings). These discussions may require input from hospital compliance officers, ethics committees, risk managers, and the local department of mental health and also may be partly solved by using video communication platforms.
A few other “pearls” may be of value: Psychiatric hospitals that are colocated with a general acute care hospital or ED might be better situated to develop protocols to safely care for COVID-19–positive psychiatric patients, by virtue of the close proximity of full-spectrum acute general hospital services. Direct engagement by a command center and hospital or health system senior leadership also seems crucial as a means for assuring authorization to proceed with planning what may be a frightening or controversial (but necessary) adaptation of inpatient psychiatric unit(s) to the exigencies of the COVID-19 pandemic.
The resources of a robust community hospital or academic health system (including infection prevention leaders who engage in continuous liaison with local, county, state, and federal public health expertise) are crucial to the “learning health system” model, which requires flexibility, rapid adaptation to new knowledge, and accessibility to infectious disease and other consultation for special situations. Frequent and open communication with all professional stakeholders (through town halls, Q&A sessions, group discussions, and so on) is important in the planning process to socialize the principles and concepts that are critical for providing care in a PCU, reducing anxiety, and bolstering collegiality and staff morale.
References
1. Kim MJ. “ ‘It was a medical disaster’: The psychiatric ward that saw 100 patients with new coronavirus.” Independent. 2020 Mar 1.
2. Korean Society of Infectious Diseases et al. J Korean Med Sci. 2020 Mar 16;35(10):e112.
3. Centers for Disease Control and Prevention. Symptom-based strategy to discontinue isolation for persons with COVID-19. Decision Memo. 2020 May 3.
4. He X et al. Nature Medicine. 2020. 26:672-5.
Dr. Cheung is associate medical director and chief quality officer at the Stewart and Lynda Resnick Neuropsychiatric Hospital at the University of California, Los Angeles. He has no conflicts of interest. Dr. Strouse is medical director, UCLA Stewart and Lynda Resnick Neuropsychiatric Hospital and Maddie Katz Professor at the UCLA department of psychiatry/Semel Institute. He has no conflicts of interest. Dr. Li is associate medical director of quality improvement at Yale-New Haven Psychiatric Hospital in Connecticut. She also serves as medical director of clinical operations at the Yale-New Haven Health System. Dr. Li is a 2019-2020 Health and Aging Policy Fellow and receives funding support from the program.
FDA approves pomalidomide for Kaposi sarcoma
The Food and Drug Administration has granted accelerated approval to pomalidomide (Pomalyst, Bristol-Myers Squibb) for the treatment of AIDS-related Kaposi sarcoma that is resistant to highly active antiretroviral therapy (HAART) or that occurs in HIV-negative patients.
Pomalidomide is the only oral agent and first new treatment option for Kaposi sarcoma in more than 20 years, according to the company.
The drug, a thalidomide analogue, is already marketed for the treatment of multiple myeloma.
Pomalidomide has “shown positive results in Kaposi sarcoma patients, regardless of their HIV status,” said Robert Yarchoan, MD, chief of the HIV and AIDS Malignancy Branch, National Cancer Institute, in a press statement.
The conditional approval is based on the 71% overall response rate observed in a phase 1/2 open-label, single-arm clinical trial that involved 28 patients, 18 of whom were HIV positive and 10 of whom were HIV negative.
Most of the responses were partial (57%; 16/28); 14% (4/28) were complete. Median duration of response was 12.1 months. Additionally, for half of the patients who showed a response, that response was maintained for more than 12 months.
Patients received 5 mg of pomalidomide once daily for 21 of 28-day cycles until disease progression or unacceptable toxicity occurred.
Permanent discontinuation because of an adverse reaction occurred in 11% (3/28) of patients.
Adverse reactions (≥20%) included maculopapular rash (71%), constipation (71%), fatigue (68%), nausea (36%), diarrhea (32%), cough (29%), dyspnea (29%), peripheral edema (29%), upper respiratory tract infection (29%), muscle spasms (25%), hypothyroidism (21%), dry skin (21%), and chills (21%).
Grade 3 or 4 adverse reactions included maculopapular rash (3.6%), diarrhea (3.6%), and peripheral edema (3.6%).
Grade 3 or 4 laboratory abnormalities (≥5%) that worsened from baseline included decreased absolute neutrophil count (50%), decreased phosphate level (25%), elevated glucose level (7%), and elevated creatine kinase level (7%).
As a thalidomide analogue, pomalidomide includes a boxed warning in the prescribing information; thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. Deep vein thrombosis, pulmonary embolism, myocardial infarction, and stroke can occur in patients treated with pomalidomide; thromboprophylaxis is recommended.
Pomalidomide is available only through a restricted distribution program, Pomalyst REMS.
This article first appeared on Medscape.com.
The Food and Drug Administration has granted accelerated approval to pomalidomide (Pomalyst, Bristol-Myers Squibb) for the treatment of AIDS-related Kaposi sarcoma that is resistant to highly active antiretroviral therapy (HAART) or that occurs in HIV-negative patients.
Pomalidomide is the only oral agent and first new treatment option for Kaposi sarcoma in more than 20 years, according to the company.
The drug, a thalidomide analogue, is already marketed for the treatment of multiple myeloma.
Pomalidomide has “shown positive results in Kaposi sarcoma patients, regardless of their HIV status,” said Robert Yarchoan, MD, chief of the HIV and AIDS Malignancy Branch, National Cancer Institute, in a press statement.
The conditional approval is based on the 71% overall response rate observed in a phase 1/2 open-label, single-arm clinical trial that involved 28 patients, 18 of whom were HIV positive and 10 of whom were HIV negative.
Most of the responses were partial (57%; 16/28); 14% (4/28) were complete. Median duration of response was 12.1 months. Additionally, for half of the patients who showed a response, that response was maintained for more than 12 months.
Patients received 5 mg of pomalidomide once daily for 21 of 28-day cycles until disease progression or unacceptable toxicity occurred.
Permanent discontinuation because of an adverse reaction occurred in 11% (3/28) of patients.
Adverse reactions (≥20%) included maculopapular rash (71%), constipation (71%), fatigue (68%), nausea (36%), diarrhea (32%), cough (29%), dyspnea (29%), peripheral edema (29%), upper respiratory tract infection (29%), muscle spasms (25%), hypothyroidism (21%), dry skin (21%), and chills (21%).
Grade 3 or 4 adverse reactions included maculopapular rash (3.6%), diarrhea (3.6%), and peripheral edema (3.6%).
Grade 3 or 4 laboratory abnormalities (≥5%) that worsened from baseline included decreased absolute neutrophil count (50%), decreased phosphate level (25%), elevated glucose level (7%), and elevated creatine kinase level (7%).
As a thalidomide analogue, pomalidomide includes a boxed warning in the prescribing information; thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. Deep vein thrombosis, pulmonary embolism, myocardial infarction, and stroke can occur in patients treated with pomalidomide; thromboprophylaxis is recommended.
Pomalidomide is available only through a restricted distribution program, Pomalyst REMS.
This article first appeared on Medscape.com.
The Food and Drug Administration has granted accelerated approval to pomalidomide (Pomalyst, Bristol-Myers Squibb) for the treatment of AIDS-related Kaposi sarcoma that is resistant to highly active antiretroviral therapy (HAART) or that occurs in HIV-negative patients.
Pomalidomide is the only oral agent and first new treatment option for Kaposi sarcoma in more than 20 years, according to the company.
The drug, a thalidomide analogue, is already marketed for the treatment of multiple myeloma.
Pomalidomide has “shown positive results in Kaposi sarcoma patients, regardless of their HIV status,” said Robert Yarchoan, MD, chief of the HIV and AIDS Malignancy Branch, National Cancer Institute, in a press statement.
The conditional approval is based on the 71% overall response rate observed in a phase 1/2 open-label, single-arm clinical trial that involved 28 patients, 18 of whom were HIV positive and 10 of whom were HIV negative.
Most of the responses were partial (57%; 16/28); 14% (4/28) were complete. Median duration of response was 12.1 months. Additionally, for half of the patients who showed a response, that response was maintained for more than 12 months.
Patients received 5 mg of pomalidomide once daily for 21 of 28-day cycles until disease progression or unacceptable toxicity occurred.
Permanent discontinuation because of an adverse reaction occurred in 11% (3/28) of patients.
Adverse reactions (≥20%) included maculopapular rash (71%), constipation (71%), fatigue (68%), nausea (36%), diarrhea (32%), cough (29%), dyspnea (29%), peripheral edema (29%), upper respiratory tract infection (29%), muscle spasms (25%), hypothyroidism (21%), dry skin (21%), and chills (21%).
Grade 3 or 4 adverse reactions included maculopapular rash (3.6%), diarrhea (3.6%), and peripheral edema (3.6%).
Grade 3 or 4 laboratory abnormalities (≥5%) that worsened from baseline included decreased absolute neutrophil count (50%), decreased phosphate level (25%), elevated glucose level (7%), and elevated creatine kinase level (7%).
As a thalidomide analogue, pomalidomide includes a boxed warning in the prescribing information; thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. Deep vein thrombosis, pulmonary embolism, myocardial infarction, and stroke can occur in patients treated with pomalidomide; thromboprophylaxis is recommended.
Pomalidomide is available only through a restricted distribution program, Pomalyst REMS.
This article first appeared on Medscape.com.