User login
Women thrive on baroreflex activation for heart failure
The striking gains in functional capacity and quality of life conferred by baroreflex activation therapy in patients with heart failure, as shown in the pivotal phase 3 clinical trial for this novel intervention, were at least as great in women as in men, JoAnn Lindenfeld, MD, said at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
The results of the multicenter, prospective, randomized BeAT-HF trial led to marketing approval of the BaroStim Neo system for improvement in symptoms of heart failure with reduced ejection fraction (HFrEF) by the Food and Drug Administration in August 2019. Dr. Lindenfeld presented a fresh breakdown of the results by gender which showed, intriguingly, that the improvement in all study endpoints was consistently numerically greater in the women – sometimes startlingly so – although these gender differences in response didn’t achieve statistical significance. The 6-month randomized trial was underpowered for drawing definitive conclusions on that score, with a study population of only 53 women and 211 men. So the investigator remained circumspect.
“We think that what this study shows us is that women have at least equivalent improvement as men in this population. I don’t think we can conclude from this study yet that it’s better, but it’s certainly in all these parameters as least as good. And I think this is a population in which we’ve seen that improving symptoms and functional capacity is very important,” said Dr. Lindenfeld, professor of medicine and director of advanced heart failure/cardiac transplantation at Vanderbilt University, Nashville, Tenn.
The FDA approval was restricted to patients like those enrolled in BeAT-HF: that is, individuals with New York Heart Association functional class III heart failure, a left ventricular ejection fraction of 35% or less while on stable optimal medical therapy, and ineligibility for cardiac resynchronization therapy according to current guidelines. Seventy-eight percent of BeAT-HF participants had an implantable cardioverter-defibrillator.
Participants were randomized to baroreflex activation therapy (BAT) plus optimal medical therapy or to optimal medical therapy alone. The three coprimary endpoints were change from baseline to 6 months in 6-minute hall walk distance (6MHW), scores on the Minnesota Living with Heart Failure Questionnaire (MLHF), and N-terminal pro-B-type natriuretic peptide (NT-proBNP).
In the overall study population, 6MHW increased by 60 m in the BAT group and decreased by 8 m in controls; MLHF scores dropped by 14 and 6 points, respectively; and NT-proBNP fell by an average of 25% with BAT while rising by 3% in controls.
Very often, just a 5-point reduction in MLHF score is considered a clinically meaningful improvement in quality of life, the cardiologist noted.
The gender-based analysis is where things got particularly interesting.
The investigators defined a clinically relevant response as a greater than 10% increase from baseline on the 6MHW, at least a one-class improvement in NYHA class, or a reduction of 5 points or more on the MLHF. Among subjects in the BAT group, 70% of women and 60% of men met the clinically relevant response standard in terms of 6MHW, as did 70% of women and 64% of men for improvement in NYHA class, and 78% of women and 66% of men for MLHF score.
Eighty-seven percent of women and 68% of men on BAT had a clinically relevant response on at least one of these endpoints, as did about 28% of controls. Moreover, 31% of women in the BAT group were clinically relevant responders on at least two endpoints, compared with 19% of BAT men and 4% and 9% of controls.
Women dominate super-responder category
In order to be classified as a super responder, a patient had to demonstrate a greater than 20% increase in 6MHW, improvement in NYHA class I status, or at least a 10-point improvement in MLHF score. Ninety-one percent of women on BAT achieved super-responder status for at least one of these endpoints, compared with 76% of men. Forty-three percent of women and 24% of men in the BAT group were super responders in at least two domains, as were 8% and 11% of female and male controls, Dr. Lindenfeld continued.
Discussant Ewa Anita Jankowska, MD, PhD, deemed the BeAT-HF results on the therapeutic benefits of this autonomic modulation strategy “quite convincing.”
“We need to acknowledge that in recent years we have been spoiled a bit by the huge trials in heart failure where the ultimate goal was a reduction in mortality. But I think this is the time when we should think about the patients who want to live – here, now – with a better life. Patients expect symptomatic benefits. There is a substantial group of patients who are symptomatic even though they receive quite extensive neurohormonal blockage and who are not suitable for CRT. This study demonstrates that, for this group of patients, BAT can bring really significant symptomatic benefits,” she said.
“If you think about a treatment that provides patients who are NYHA class III an increase in 6MHW of 60 meters, that’s really something. And 20% of patients went from NYHA class III to class I – that’s really something, too,” added Dr. Jankowska, professor of medicine and head of the laboratory of applied research on the cardiovascular system at Wroclaw (Poland) University.
How baroreflex activation therapy works
The BaroStim system consists of a 2-mm unipolar electrode on a 7-mm backer that is placed over the carotid sinus. It is supported by a small generator with a 4- to 5-year battery life implanted under the collarbone, along with radiofrequency telemetry capability and programming flexibility.
Stimulation of the carotid baroreceptor promotes an integrated autonomic nervous system response which enhances parasympathetic activity and inhibits sympathetic nervous system activity. The result, as shown in numerous earlier proof-of-concept studies, is a reduced heart rate, decreased ventricular remodeling, enhanced diuresis, increased vasodilation, a drop in elevated blood pressure, and decreased renin secretion – all achieved nonpharmacologically.
The study was sponsored by CVRx. Dr. Lindenfeld reported serving as a consultant to CVRx, Abbott, AstraZeneca, Boehringer Ingelheim, Edwards Lifesciences, Impulse Dynamics, and VWave.
The striking gains in functional capacity and quality of life conferred by baroreflex activation therapy in patients with heart failure, as shown in the pivotal phase 3 clinical trial for this novel intervention, were at least as great in women as in men, JoAnn Lindenfeld, MD, said at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
The results of the multicenter, prospective, randomized BeAT-HF trial led to marketing approval of the BaroStim Neo system for improvement in symptoms of heart failure with reduced ejection fraction (HFrEF) by the Food and Drug Administration in August 2019. Dr. Lindenfeld presented a fresh breakdown of the results by gender which showed, intriguingly, that the improvement in all study endpoints was consistently numerically greater in the women – sometimes startlingly so – although these gender differences in response didn’t achieve statistical significance. The 6-month randomized trial was underpowered for drawing definitive conclusions on that score, with a study population of only 53 women and 211 men. So the investigator remained circumspect.
“We think that what this study shows us is that women have at least equivalent improvement as men in this population. I don’t think we can conclude from this study yet that it’s better, but it’s certainly in all these parameters as least as good. And I think this is a population in which we’ve seen that improving symptoms and functional capacity is very important,” said Dr. Lindenfeld, professor of medicine and director of advanced heart failure/cardiac transplantation at Vanderbilt University, Nashville, Tenn.
The FDA approval was restricted to patients like those enrolled in BeAT-HF: that is, individuals with New York Heart Association functional class III heart failure, a left ventricular ejection fraction of 35% or less while on stable optimal medical therapy, and ineligibility for cardiac resynchronization therapy according to current guidelines. Seventy-eight percent of BeAT-HF participants had an implantable cardioverter-defibrillator.
Participants were randomized to baroreflex activation therapy (BAT) plus optimal medical therapy or to optimal medical therapy alone. The three coprimary endpoints were change from baseline to 6 months in 6-minute hall walk distance (6MHW), scores on the Minnesota Living with Heart Failure Questionnaire (MLHF), and N-terminal pro-B-type natriuretic peptide (NT-proBNP).
In the overall study population, 6MHW increased by 60 m in the BAT group and decreased by 8 m in controls; MLHF scores dropped by 14 and 6 points, respectively; and NT-proBNP fell by an average of 25% with BAT while rising by 3% in controls.
Very often, just a 5-point reduction in MLHF score is considered a clinically meaningful improvement in quality of life, the cardiologist noted.
The gender-based analysis is where things got particularly interesting.
The investigators defined a clinically relevant response as a greater than 10% increase from baseline on the 6MHW, at least a one-class improvement in NYHA class, or a reduction of 5 points or more on the MLHF. Among subjects in the BAT group, 70% of women and 60% of men met the clinically relevant response standard in terms of 6MHW, as did 70% of women and 64% of men for improvement in NYHA class, and 78% of women and 66% of men for MLHF score.
Eighty-seven percent of women and 68% of men on BAT had a clinically relevant response on at least one of these endpoints, as did about 28% of controls. Moreover, 31% of women in the BAT group were clinically relevant responders on at least two endpoints, compared with 19% of BAT men and 4% and 9% of controls.
Women dominate super-responder category
In order to be classified as a super responder, a patient had to demonstrate a greater than 20% increase in 6MHW, improvement in NYHA class I status, or at least a 10-point improvement in MLHF score. Ninety-one percent of women on BAT achieved super-responder status for at least one of these endpoints, compared with 76% of men. Forty-three percent of women and 24% of men in the BAT group were super responders in at least two domains, as were 8% and 11% of female and male controls, Dr. Lindenfeld continued.
Discussant Ewa Anita Jankowska, MD, PhD, deemed the BeAT-HF results on the therapeutic benefits of this autonomic modulation strategy “quite convincing.”
“We need to acknowledge that in recent years we have been spoiled a bit by the huge trials in heart failure where the ultimate goal was a reduction in mortality. But I think this is the time when we should think about the patients who want to live – here, now – with a better life. Patients expect symptomatic benefits. There is a substantial group of patients who are symptomatic even though they receive quite extensive neurohormonal blockage and who are not suitable for CRT. This study demonstrates that, for this group of patients, BAT can bring really significant symptomatic benefits,” she said.
“If you think about a treatment that provides patients who are NYHA class III an increase in 6MHW of 60 meters, that’s really something. And 20% of patients went from NYHA class III to class I – that’s really something, too,” added Dr. Jankowska, professor of medicine and head of the laboratory of applied research on the cardiovascular system at Wroclaw (Poland) University.
How baroreflex activation therapy works
The BaroStim system consists of a 2-mm unipolar electrode on a 7-mm backer that is placed over the carotid sinus. It is supported by a small generator with a 4- to 5-year battery life implanted under the collarbone, along with radiofrequency telemetry capability and programming flexibility.
Stimulation of the carotid baroreceptor promotes an integrated autonomic nervous system response which enhances parasympathetic activity and inhibits sympathetic nervous system activity. The result, as shown in numerous earlier proof-of-concept studies, is a reduced heart rate, decreased ventricular remodeling, enhanced diuresis, increased vasodilation, a drop in elevated blood pressure, and decreased renin secretion – all achieved nonpharmacologically.
The study was sponsored by CVRx. Dr. Lindenfeld reported serving as a consultant to CVRx, Abbott, AstraZeneca, Boehringer Ingelheim, Edwards Lifesciences, Impulse Dynamics, and VWave.
The striking gains in functional capacity and quality of life conferred by baroreflex activation therapy in patients with heart failure, as shown in the pivotal phase 3 clinical trial for this novel intervention, were at least as great in women as in men, JoAnn Lindenfeld, MD, said at the European Society of Cardiology Heart Failure Discoveries virtual meeting.
The results of the multicenter, prospective, randomized BeAT-HF trial led to marketing approval of the BaroStim Neo system for improvement in symptoms of heart failure with reduced ejection fraction (HFrEF) by the Food and Drug Administration in August 2019. Dr. Lindenfeld presented a fresh breakdown of the results by gender which showed, intriguingly, that the improvement in all study endpoints was consistently numerically greater in the women – sometimes startlingly so – although these gender differences in response didn’t achieve statistical significance. The 6-month randomized trial was underpowered for drawing definitive conclusions on that score, with a study population of only 53 women and 211 men. So the investigator remained circumspect.
“We think that what this study shows us is that women have at least equivalent improvement as men in this population. I don’t think we can conclude from this study yet that it’s better, but it’s certainly in all these parameters as least as good. And I think this is a population in which we’ve seen that improving symptoms and functional capacity is very important,” said Dr. Lindenfeld, professor of medicine and director of advanced heart failure/cardiac transplantation at Vanderbilt University, Nashville, Tenn.
The FDA approval was restricted to patients like those enrolled in BeAT-HF: that is, individuals with New York Heart Association functional class III heart failure, a left ventricular ejection fraction of 35% or less while on stable optimal medical therapy, and ineligibility for cardiac resynchronization therapy according to current guidelines. Seventy-eight percent of BeAT-HF participants had an implantable cardioverter-defibrillator.
Participants were randomized to baroreflex activation therapy (BAT) plus optimal medical therapy or to optimal medical therapy alone. The three coprimary endpoints were change from baseline to 6 months in 6-minute hall walk distance (6MHW), scores on the Minnesota Living with Heart Failure Questionnaire (MLHF), and N-terminal pro-B-type natriuretic peptide (NT-proBNP).
In the overall study population, 6MHW increased by 60 m in the BAT group and decreased by 8 m in controls; MLHF scores dropped by 14 and 6 points, respectively; and NT-proBNP fell by an average of 25% with BAT while rising by 3% in controls.
Very often, just a 5-point reduction in MLHF score is considered a clinically meaningful improvement in quality of life, the cardiologist noted.
The gender-based analysis is where things got particularly interesting.
The investigators defined a clinically relevant response as a greater than 10% increase from baseline on the 6MHW, at least a one-class improvement in NYHA class, or a reduction of 5 points or more on the MLHF. Among subjects in the BAT group, 70% of women and 60% of men met the clinically relevant response standard in terms of 6MHW, as did 70% of women and 64% of men for improvement in NYHA class, and 78% of women and 66% of men for MLHF score.
Eighty-seven percent of women and 68% of men on BAT had a clinically relevant response on at least one of these endpoints, as did about 28% of controls. Moreover, 31% of women in the BAT group were clinically relevant responders on at least two endpoints, compared with 19% of BAT men and 4% and 9% of controls.
Women dominate super-responder category
In order to be classified as a super responder, a patient had to demonstrate a greater than 20% increase in 6MHW, improvement in NYHA class I status, or at least a 10-point improvement in MLHF score. Ninety-one percent of women on BAT achieved super-responder status for at least one of these endpoints, compared with 76% of men. Forty-three percent of women and 24% of men in the BAT group were super responders in at least two domains, as were 8% and 11% of female and male controls, Dr. Lindenfeld continued.
Discussant Ewa Anita Jankowska, MD, PhD, deemed the BeAT-HF results on the therapeutic benefits of this autonomic modulation strategy “quite convincing.”
“We need to acknowledge that in recent years we have been spoiled a bit by the huge trials in heart failure where the ultimate goal was a reduction in mortality. But I think this is the time when we should think about the patients who want to live – here, now – with a better life. Patients expect symptomatic benefits. There is a substantial group of patients who are symptomatic even though they receive quite extensive neurohormonal blockage and who are not suitable for CRT. This study demonstrates that, for this group of patients, BAT can bring really significant symptomatic benefits,” she said.
“If you think about a treatment that provides patients who are NYHA class III an increase in 6MHW of 60 meters, that’s really something. And 20% of patients went from NYHA class III to class I – that’s really something, too,” added Dr. Jankowska, professor of medicine and head of the laboratory of applied research on the cardiovascular system at Wroclaw (Poland) University.
How baroreflex activation therapy works
The BaroStim system consists of a 2-mm unipolar electrode on a 7-mm backer that is placed over the carotid sinus. It is supported by a small generator with a 4- to 5-year battery life implanted under the collarbone, along with radiofrequency telemetry capability and programming flexibility.
Stimulation of the carotid baroreceptor promotes an integrated autonomic nervous system response which enhances parasympathetic activity and inhibits sympathetic nervous system activity. The result, as shown in numerous earlier proof-of-concept studies, is a reduced heart rate, decreased ventricular remodeling, enhanced diuresis, increased vasodilation, a drop in elevated blood pressure, and decreased renin secretion – all achieved nonpharmacologically.
The study was sponsored by CVRx. Dr. Lindenfeld reported serving as a consultant to CVRx, Abbott, AstraZeneca, Boehringer Ingelheim, Edwards Lifesciences, Impulse Dynamics, and VWave.
FROM ESC HEART FAILURE 2020
School daze
A few weeks ago I was asked by the head of our local parks and recreation department for my opinion on whether the town should open its summer recreation camps program. He had been receiving multiple inquiries from parents who in the past had relied on the day camps for day care. The director already had surveyed health care administrators and other providers in the town and his team had crafted a plan based on what guidelines they could glean from state and federal advisory groups. The feedback he had received from town officials and health care representatives was that they felt opening would be a bad decision. One physician observed that there is just “so much we don’t know about the virus at this point.”
I certainly agreed that we still have much to learn about COVID-19, but I told the director that we know enough that I would feel comfortable with opening the day camps, which have traditionally been held outdoors under open-sided tents. If group sizes were kept small, staff personnel were dedicated to just one group, and temperatures were taken at the beginning and at the midpoint of each daily session, I felt that the risk of triggering an outbreak was small. I told him that in my mind the Achilles heel of the plan was whether the camp staff, who are generally high school and college-age young people, could be trusted to follow rigorous social distancing in their off-work hours.
Eventually the decision was made by the traditionally risk-averse town officials to open the camps. I hope that this step forward will spur the process of reopening the schools in the fall by demonstrating that, at least in an open-air environment, some simple common sense measures could create a safe environment for children to congregate in. Unfortunately, the long delay in formulating the plan and a basic hesitancy on the part of some parents has resulted in disappointing enrollment figures so far.
I suspect that many of you have been asked to participate in the planning and decision-making processes for opening the school systems in your community or at least have some thoughts of your own about how best to begin the reopening process.
I suspect you agree that, if the number of new cases detected each day in your state is still rising and/or your state’s ability to test, track, case find, and quarantine is inadequate, reopening schools is probably just asking for trouble. However, a recent study has found that children and young adults under the age of 20 years were almost half as likely to become infected as those over the age of 20 (Nat Med. 2020 Jun 16. doi: 10.1038/s41591-020-0962-9). We already know that, in general, children are presenting with less severe illness. Although the authors observe that we still need to learn more about the transmissibility of subclinical infections, particularly in children, they suggest that “interventions aimed at children might have relatively little impact on reducing SARS-CoV-2 transmission.” It is sounding like reopening schools will place the children at relatively low risk. However, until we know more about transmissibility we have to assume reopening schools may place the community at an increased risk.
If this new information is confirmed by other studies, how would this change the recommendations you would make to the community about reopening its schools? What about masks? We are learning that they make a difference for adults, but is this true for very young children as well? Masks should probably remain part of the hygiene education program as well for at least the foreseeable future.
Do you think your school system can broaden its focus beyond surface cleaning to air handling and ventilation? Here in Maine, keeping the windows open for more than a few weeks a year is going to present problems that may be expensive to remedy.
There are always more questions than answers, but my hope is that here in Maine our apparent success on a state level will allow us to reopen the schools as long as we remain vigilant for the first signs that we need to return to lock down. How do you feel about your community’s situation?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A few weeks ago I was asked by the head of our local parks and recreation department for my opinion on whether the town should open its summer recreation camps program. He had been receiving multiple inquiries from parents who in the past had relied on the day camps for day care. The director already had surveyed health care administrators and other providers in the town and his team had crafted a plan based on what guidelines they could glean from state and federal advisory groups. The feedback he had received from town officials and health care representatives was that they felt opening would be a bad decision. One physician observed that there is just “so much we don’t know about the virus at this point.”
I certainly agreed that we still have much to learn about COVID-19, but I told the director that we know enough that I would feel comfortable with opening the day camps, which have traditionally been held outdoors under open-sided tents. If group sizes were kept small, staff personnel were dedicated to just one group, and temperatures were taken at the beginning and at the midpoint of each daily session, I felt that the risk of triggering an outbreak was small. I told him that in my mind the Achilles heel of the plan was whether the camp staff, who are generally high school and college-age young people, could be trusted to follow rigorous social distancing in their off-work hours.
Eventually the decision was made by the traditionally risk-averse town officials to open the camps. I hope that this step forward will spur the process of reopening the schools in the fall by demonstrating that, at least in an open-air environment, some simple common sense measures could create a safe environment for children to congregate in. Unfortunately, the long delay in formulating the plan and a basic hesitancy on the part of some parents has resulted in disappointing enrollment figures so far.
I suspect that many of you have been asked to participate in the planning and decision-making processes for opening the school systems in your community or at least have some thoughts of your own about how best to begin the reopening process.
I suspect you agree that, if the number of new cases detected each day in your state is still rising and/or your state’s ability to test, track, case find, and quarantine is inadequate, reopening schools is probably just asking for trouble. However, a recent study has found that children and young adults under the age of 20 years were almost half as likely to become infected as those over the age of 20 (Nat Med. 2020 Jun 16. doi: 10.1038/s41591-020-0962-9). We already know that, in general, children are presenting with less severe illness. Although the authors observe that we still need to learn more about the transmissibility of subclinical infections, particularly in children, they suggest that “interventions aimed at children might have relatively little impact on reducing SARS-CoV-2 transmission.” It is sounding like reopening schools will place the children at relatively low risk. However, until we know more about transmissibility we have to assume reopening schools may place the community at an increased risk.
If this new information is confirmed by other studies, how would this change the recommendations you would make to the community about reopening its schools? What about masks? We are learning that they make a difference for adults, but is this true for very young children as well? Masks should probably remain part of the hygiene education program as well for at least the foreseeable future.
Do you think your school system can broaden its focus beyond surface cleaning to air handling and ventilation? Here in Maine, keeping the windows open for more than a few weeks a year is going to present problems that may be expensive to remedy.
There are always more questions than answers, but my hope is that here in Maine our apparent success on a state level will allow us to reopen the schools as long as we remain vigilant for the first signs that we need to return to lock down. How do you feel about your community’s situation?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
A few weeks ago I was asked by the head of our local parks and recreation department for my opinion on whether the town should open its summer recreation camps program. He had been receiving multiple inquiries from parents who in the past had relied on the day camps for day care. The director already had surveyed health care administrators and other providers in the town and his team had crafted a plan based on what guidelines they could glean from state and federal advisory groups. The feedback he had received from town officials and health care representatives was that they felt opening would be a bad decision. One physician observed that there is just “so much we don’t know about the virus at this point.”
I certainly agreed that we still have much to learn about COVID-19, but I told the director that we know enough that I would feel comfortable with opening the day camps, which have traditionally been held outdoors under open-sided tents. If group sizes were kept small, staff personnel were dedicated to just one group, and temperatures were taken at the beginning and at the midpoint of each daily session, I felt that the risk of triggering an outbreak was small. I told him that in my mind the Achilles heel of the plan was whether the camp staff, who are generally high school and college-age young people, could be trusted to follow rigorous social distancing in their off-work hours.
Eventually the decision was made by the traditionally risk-averse town officials to open the camps. I hope that this step forward will spur the process of reopening the schools in the fall by demonstrating that, at least in an open-air environment, some simple common sense measures could create a safe environment for children to congregate in. Unfortunately, the long delay in formulating the plan and a basic hesitancy on the part of some parents has resulted in disappointing enrollment figures so far.
I suspect that many of you have been asked to participate in the planning and decision-making processes for opening the school systems in your community or at least have some thoughts of your own about how best to begin the reopening process.
I suspect you agree that, if the number of new cases detected each day in your state is still rising and/or your state’s ability to test, track, case find, and quarantine is inadequate, reopening schools is probably just asking for trouble. However, a recent study has found that children and young adults under the age of 20 years were almost half as likely to become infected as those over the age of 20 (Nat Med. 2020 Jun 16. doi: 10.1038/s41591-020-0962-9). We already know that, in general, children are presenting with less severe illness. Although the authors observe that we still need to learn more about the transmissibility of subclinical infections, particularly in children, they suggest that “interventions aimed at children might have relatively little impact on reducing SARS-CoV-2 transmission.” It is sounding like reopening schools will place the children at relatively low risk. However, until we know more about transmissibility we have to assume reopening schools may place the community at an increased risk.
If this new information is confirmed by other studies, how would this change the recommendations you would make to the community about reopening its schools? What about masks? We are learning that they make a difference for adults, but is this true for very young children as well? Masks should probably remain part of the hygiene education program as well for at least the foreseeable future.
Do you think your school system can broaden its focus beyond surface cleaning to air handling and ventilation? Here in Maine, keeping the windows open for more than a few weeks a year is going to present problems that may be expensive to remedy.
There are always more questions than answers, but my hope is that here in Maine our apparent success on a state level will allow us to reopen the schools as long as we remain vigilant for the first signs that we need to return to lock down. How do you feel about your community’s situation?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Telehealth and medical liability
The COVID-19 pandemic has led to the rapid uptake of telehealth nationwide in primary care and specialty practices. Over the last few months many practices have actually performed more telehealth visits than traditional in-person visits. The use of telehealth, which had been increasing slowly for the last few years, accelerated rapidly during the pandemic. Long term, telehealth has the potential to increase access to primary care and specialists, and make follow-up easier for many patients, changing how health care is delivered to millions of patients throughout the world.
Since telehealth will be a regular part of our practices from now on, it is important for clinicians to recognize how telehealth visits are viewed in a legal arena.
As is often the case with technological advances, the law needs time to adapt. Will a health care provider treating a patient using telemedicine be held to the same standard of care applicable to an in-person encounter? Stated differently, will consideration be given to the obvious limitations imposed by a telemedicine exam?
Standard of care in medical malpractice cases
The central question in most medical malpractice cases is whether the provider complied with the generally accepted standard of care when evaluating, diagnosing, or treating a patient. This standard typically takes into consideration the provider’s particular specialty as well as all the circumstances surrounding the encounter.1 Medical providers, not state legislators, usually define the standard of care for medical professionals. In malpractice cases, medical experts explain the applicable standard of care to the jury and guide its determination of whether, in the particular case, the standard of care was met. In this way, the law has long recognized that the medical profession itself is best suited to establish the appropriate standards of care under any particular set of circumstances. This standard of care is often referred to as the “reasonable professional under the circumstances” standard of care.
Telemedicine standard of care
Despite the fact that the complex and often nebulous concept of standard of care has been traditionally left to the medical experts to define, state legislators and regulators throughout the nation have chosen to weigh in on this issue in the context of telemedicine. Most states with telemedicine regulations have followed the model policy adopted by the Federation of State Medical Boards in April 2014 which states that “[t]reatment and consultation recommendations made in an online setting … will be held to the same standards of appropriate practice as those in traditional (in-person) settings.”2 States that have adopted this model policy have effectively created a “legal fiction” requiring a jury to ignore the fact that the care was provided virtually by telemedicine technologies and instead assume that the physician treated the patient in person, i.e, applying an “in-person” standard of care. Hawaii appears to be the lone notable exception. Its telemedicine law recognizes that an in-person standard of care should not be applied if there was not a face-to-face visit.3
Proponents of the in-person telemedicine standard claim that it is necessary to ensure patient safety, thus justifying the “legal fiction.” Holding the provider to the in-person standard, it is argued, forces the physician to err on the side of caution and require an actual in-person encounter to ensure the advantages of sight, touch, and sense of things are fully available.4 This discourages the use of telemedicine and deprives the population of its many benefits.
Telemedicine can overcome geographical barriers, increase clinical support, improve health outcomes, reduce health care costs, encourage patient input, reduce travel, and foster continuity of care. The pandemic, which has significantly limited the ability of providers to see patients in person, only underscores the benefits of telemedicine.
The legislatively imposed in-person telemedicine standard of care should be replaced with the “reasonable professional under the circumstances” standard in order to fairly judge physicians’ care and promote overall population health. The “reasonable professional under the circumstances” standard has applied to physicians and other health care professionals outside of telemedicine for decades, and it has served the medical community and public well. It is unfortunate that legislators felt the need to weigh in and define a distinctly different standard of care for telemedicine than for the rest of medicine, as this may present unforeseen obstacles to the use of telemedicine.
The in-person telemedicine standard of care remains a significant barrier for long-term telemedicine. Eliminating this legal fiction has the potential to further expand physicians’ use of telemedicine and fulfill its promise of improving access to care and improving population health.
Mr. Horner (partner), Mr. Milewski (partner), and Mr. Gajer (associate) are attorneys with White and Williams. They specialize in defending health care providers in medical malpractice lawsuits and other health care–related matters. Dr. Skolnik is professor of family and community Medicine at the Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Follow Dr. Skolnik, and feel free to submit questions to him on Twitter: @neilskolnik. The authors have no financial conflicts related to the content of this piece.
References
1. Cowan v. Doering, 111 N.J. 451-62,.1988.
2. Model Policy For The Appropriate Use Of Telemedicine Technologies In The Practice Of Medicine. State Medical Boards Appropriate Regulation of Telemedicine. April 2014..
3. Haw. Rev. Stat. Ann. § 453-1.3(c).
4. Kaspar BJ. Iowa Law Review. 2014 Jan;99:839-59.
The COVID-19 pandemic has led to the rapid uptake of telehealth nationwide in primary care and specialty practices. Over the last few months many practices have actually performed more telehealth visits than traditional in-person visits. The use of telehealth, which had been increasing slowly for the last few years, accelerated rapidly during the pandemic. Long term, telehealth has the potential to increase access to primary care and specialists, and make follow-up easier for many patients, changing how health care is delivered to millions of patients throughout the world.
Since telehealth will be a regular part of our practices from now on, it is important for clinicians to recognize how telehealth visits are viewed in a legal arena.
As is often the case with technological advances, the law needs time to adapt. Will a health care provider treating a patient using telemedicine be held to the same standard of care applicable to an in-person encounter? Stated differently, will consideration be given to the obvious limitations imposed by a telemedicine exam?
Standard of care in medical malpractice cases
The central question in most medical malpractice cases is whether the provider complied with the generally accepted standard of care when evaluating, diagnosing, or treating a patient. This standard typically takes into consideration the provider’s particular specialty as well as all the circumstances surrounding the encounter.1 Medical providers, not state legislators, usually define the standard of care for medical professionals. In malpractice cases, medical experts explain the applicable standard of care to the jury and guide its determination of whether, in the particular case, the standard of care was met. In this way, the law has long recognized that the medical profession itself is best suited to establish the appropriate standards of care under any particular set of circumstances. This standard of care is often referred to as the “reasonable professional under the circumstances” standard of care.
Telemedicine standard of care
Despite the fact that the complex and often nebulous concept of standard of care has been traditionally left to the medical experts to define, state legislators and regulators throughout the nation have chosen to weigh in on this issue in the context of telemedicine. Most states with telemedicine regulations have followed the model policy adopted by the Federation of State Medical Boards in April 2014 which states that “[t]reatment and consultation recommendations made in an online setting … will be held to the same standards of appropriate practice as those in traditional (in-person) settings.”2 States that have adopted this model policy have effectively created a “legal fiction” requiring a jury to ignore the fact that the care was provided virtually by telemedicine technologies and instead assume that the physician treated the patient in person, i.e, applying an “in-person” standard of care. Hawaii appears to be the lone notable exception. Its telemedicine law recognizes that an in-person standard of care should not be applied if there was not a face-to-face visit.3
Proponents of the in-person telemedicine standard claim that it is necessary to ensure patient safety, thus justifying the “legal fiction.” Holding the provider to the in-person standard, it is argued, forces the physician to err on the side of caution and require an actual in-person encounter to ensure the advantages of sight, touch, and sense of things are fully available.4 This discourages the use of telemedicine and deprives the population of its many benefits.
Telemedicine can overcome geographical barriers, increase clinical support, improve health outcomes, reduce health care costs, encourage patient input, reduce travel, and foster continuity of care. The pandemic, which has significantly limited the ability of providers to see patients in person, only underscores the benefits of telemedicine.
The legislatively imposed in-person telemedicine standard of care should be replaced with the “reasonable professional under the circumstances” standard in order to fairly judge physicians’ care and promote overall population health. The “reasonable professional under the circumstances” standard has applied to physicians and other health care professionals outside of telemedicine for decades, and it has served the medical community and public well. It is unfortunate that legislators felt the need to weigh in and define a distinctly different standard of care for telemedicine than for the rest of medicine, as this may present unforeseen obstacles to the use of telemedicine.
The in-person telemedicine standard of care remains a significant barrier for long-term telemedicine. Eliminating this legal fiction has the potential to further expand physicians’ use of telemedicine and fulfill its promise of improving access to care and improving population health.
Mr. Horner (partner), Mr. Milewski (partner), and Mr. Gajer (associate) are attorneys with White and Williams. They specialize in defending health care providers in medical malpractice lawsuits and other health care–related matters. Dr. Skolnik is professor of family and community Medicine at the Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Follow Dr. Skolnik, and feel free to submit questions to him on Twitter: @neilskolnik. The authors have no financial conflicts related to the content of this piece.
References
1. Cowan v. Doering, 111 N.J. 451-62,.1988.
2. Model Policy For The Appropriate Use Of Telemedicine Technologies In The Practice Of Medicine. State Medical Boards Appropriate Regulation of Telemedicine. April 2014..
3. Haw. Rev. Stat. Ann. § 453-1.3(c).
4. Kaspar BJ. Iowa Law Review. 2014 Jan;99:839-59.
The COVID-19 pandemic has led to the rapid uptake of telehealth nationwide in primary care and specialty practices. Over the last few months many practices have actually performed more telehealth visits than traditional in-person visits. The use of telehealth, which had been increasing slowly for the last few years, accelerated rapidly during the pandemic. Long term, telehealth has the potential to increase access to primary care and specialists, and make follow-up easier for many patients, changing how health care is delivered to millions of patients throughout the world.
Since telehealth will be a regular part of our practices from now on, it is important for clinicians to recognize how telehealth visits are viewed in a legal arena.
As is often the case with technological advances, the law needs time to adapt. Will a health care provider treating a patient using telemedicine be held to the same standard of care applicable to an in-person encounter? Stated differently, will consideration be given to the obvious limitations imposed by a telemedicine exam?
Standard of care in medical malpractice cases
The central question in most medical malpractice cases is whether the provider complied with the generally accepted standard of care when evaluating, diagnosing, or treating a patient. This standard typically takes into consideration the provider’s particular specialty as well as all the circumstances surrounding the encounter.1 Medical providers, not state legislators, usually define the standard of care for medical professionals. In malpractice cases, medical experts explain the applicable standard of care to the jury and guide its determination of whether, in the particular case, the standard of care was met. In this way, the law has long recognized that the medical profession itself is best suited to establish the appropriate standards of care under any particular set of circumstances. This standard of care is often referred to as the “reasonable professional under the circumstances” standard of care.
Telemedicine standard of care
Despite the fact that the complex and often nebulous concept of standard of care has been traditionally left to the medical experts to define, state legislators and regulators throughout the nation have chosen to weigh in on this issue in the context of telemedicine. Most states with telemedicine regulations have followed the model policy adopted by the Federation of State Medical Boards in April 2014 which states that “[t]reatment and consultation recommendations made in an online setting … will be held to the same standards of appropriate practice as those in traditional (in-person) settings.”2 States that have adopted this model policy have effectively created a “legal fiction” requiring a jury to ignore the fact that the care was provided virtually by telemedicine technologies and instead assume that the physician treated the patient in person, i.e, applying an “in-person” standard of care. Hawaii appears to be the lone notable exception. Its telemedicine law recognizes that an in-person standard of care should not be applied if there was not a face-to-face visit.3
Proponents of the in-person telemedicine standard claim that it is necessary to ensure patient safety, thus justifying the “legal fiction.” Holding the provider to the in-person standard, it is argued, forces the physician to err on the side of caution and require an actual in-person encounter to ensure the advantages of sight, touch, and sense of things are fully available.4 This discourages the use of telemedicine and deprives the population of its many benefits.
Telemedicine can overcome geographical barriers, increase clinical support, improve health outcomes, reduce health care costs, encourage patient input, reduce travel, and foster continuity of care. The pandemic, which has significantly limited the ability of providers to see patients in person, only underscores the benefits of telemedicine.
The legislatively imposed in-person telemedicine standard of care should be replaced with the “reasonable professional under the circumstances” standard in order to fairly judge physicians’ care and promote overall population health. The “reasonable professional under the circumstances” standard has applied to physicians and other health care professionals outside of telemedicine for decades, and it has served the medical community and public well. It is unfortunate that legislators felt the need to weigh in and define a distinctly different standard of care for telemedicine than for the rest of medicine, as this may present unforeseen obstacles to the use of telemedicine.
The in-person telemedicine standard of care remains a significant barrier for long-term telemedicine. Eliminating this legal fiction has the potential to further expand physicians’ use of telemedicine and fulfill its promise of improving access to care and improving population health.
Mr. Horner (partner), Mr. Milewski (partner), and Mr. Gajer (associate) are attorneys with White and Williams. They specialize in defending health care providers in medical malpractice lawsuits and other health care–related matters. Dr. Skolnik is professor of family and community Medicine at the Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Follow Dr. Skolnik, and feel free to submit questions to him on Twitter: @neilskolnik. The authors have no financial conflicts related to the content of this piece.
References
1. Cowan v. Doering, 111 N.J. 451-62,.1988.
2. Model Policy For The Appropriate Use Of Telemedicine Technologies In The Practice Of Medicine. State Medical Boards Appropriate Regulation of Telemedicine. April 2014..
3. Haw. Rev. Stat. Ann. § 453-1.3(c).
4. Kaspar BJ. Iowa Law Review. 2014 Jan;99:839-59.
COVID-19: Medicare data show long hospital stays, disparities
according to a new analysis by the Centers for Medicare & Medicaid Services.
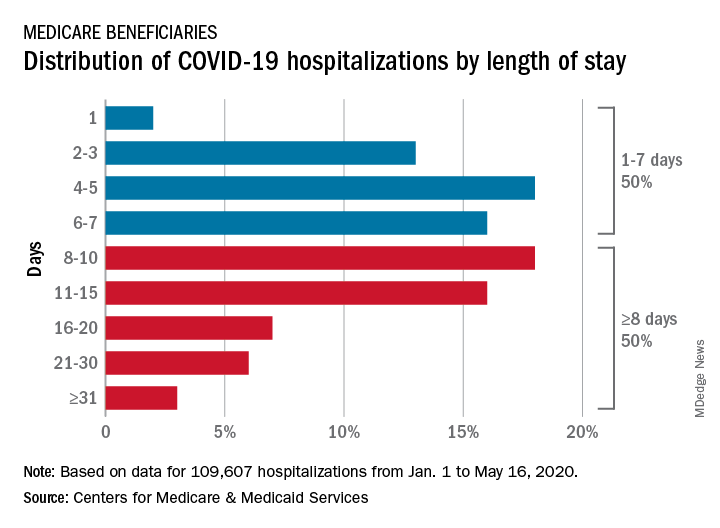
CMS encounter and claims data show almost 110,000 hospital stays for COVID-19 from Jan. 1 to May 16, 2020. Of the longer admissions, 18% were 8-10 days, 16% were 11-15 days, and another 16% were 16 days or longer, the CMS reported in a preliminary data snapshot released June 22.
The hospitalization rate for the Medicare population was 175 per 100,000 as of May 16, but the CMS data show a number of disparities involving race/ethnicity and other demographic characteristics were uncovered, such as the following:
- Black patients were hospitalized for COVID-19 at a much higher rate, at 465 per 100,000 beneficiaries, than were Hispanics (258), Asians (187), and whites (123).
- Residents of urban/suburban areas had a much higher hospitalization rate than did those living in rural areas: 205 versus 57 per 100,000.
- Beneficiaries enrolled in both Medicare and Medicaid had 473 hospitalizations per 100,000, but the rate for those with Medicare only was 112.
“The disparities in the data reflect longstanding challenges facing minority communities and low-income older adults, many of whom face structural challenges to their health that go far beyond what is traditionally considered ‘medical,’ ” CMS Administrator Seema Verma said in a separate statement.
according to a new analysis by the Centers for Medicare & Medicaid Services.

CMS encounter and claims data show almost 110,000 hospital stays for COVID-19 from Jan. 1 to May 16, 2020. Of the longer admissions, 18% were 8-10 days, 16% were 11-15 days, and another 16% were 16 days or longer, the CMS reported in a preliminary data snapshot released June 22.
The hospitalization rate for the Medicare population was 175 per 100,000 as of May 16, but the CMS data show a number of disparities involving race/ethnicity and other demographic characteristics were uncovered, such as the following:
- Black patients were hospitalized for COVID-19 at a much higher rate, at 465 per 100,000 beneficiaries, than were Hispanics (258), Asians (187), and whites (123).
- Residents of urban/suburban areas had a much higher hospitalization rate than did those living in rural areas: 205 versus 57 per 100,000.
- Beneficiaries enrolled in both Medicare and Medicaid had 473 hospitalizations per 100,000, but the rate for those with Medicare only was 112.
“The disparities in the data reflect longstanding challenges facing minority communities and low-income older adults, many of whom face structural challenges to their health that go far beyond what is traditionally considered ‘medical,’ ” CMS Administrator Seema Verma said in a separate statement.
according to a new analysis by the Centers for Medicare & Medicaid Services.

CMS encounter and claims data show almost 110,000 hospital stays for COVID-19 from Jan. 1 to May 16, 2020. Of the longer admissions, 18% were 8-10 days, 16% were 11-15 days, and another 16% were 16 days or longer, the CMS reported in a preliminary data snapshot released June 22.
The hospitalization rate for the Medicare population was 175 per 100,000 as of May 16, but the CMS data show a number of disparities involving race/ethnicity and other demographic characteristics were uncovered, such as the following:
- Black patients were hospitalized for COVID-19 at a much higher rate, at 465 per 100,000 beneficiaries, than were Hispanics (258), Asians (187), and whites (123).
- Residents of urban/suburban areas had a much higher hospitalization rate than did those living in rural areas: 205 versus 57 per 100,000.
- Beneficiaries enrolled in both Medicare and Medicaid had 473 hospitalizations per 100,000, but the rate for those with Medicare only was 112.
“The disparities in the data reflect longstanding challenges facing minority communities and low-income older adults, many of whom face structural challenges to their health that go far beyond what is traditionally considered ‘medical,’ ” CMS Administrator Seema Verma said in a separate statement.
Could jump in opioid overdoses be linked to COVID?
Early evidence suggests that opioid overdoses and deaths are on the rise this year, the director of the National Institute on Drug Abuse warned colleagues, although it’s not clear whether the coronavirus pandemic is responsible for the trend.
The picture is complicated since COVID-19 could have both positive and negative effects on substance use, Nora D. Volkow, MD, said in a plenary session at the virtual annual meeting of the College on Problems of Drug Dependence. However, she said, one thing is clear: The pandemic marks an opportunity to investigate new strategies and potentially reform treatment.
“We are being faced with an unknown world, and the lack of information curtails our capacity to implement interventions in the most effective way,” Dr. Volkow said. “There’s an urgency to obtain these data. All of you out there in the trenches have an opportunity to help gather this information in a way that can be integrated and deployed rapidly for us to guide practices and treatment.”
It’s too early to know for certain how the pandemic is affecting substance use in the United States, since statistics are sparse and COVID-19 is still relatively new. Still, local news reports have suggested overdose deaths have risen, Dr. Volkow said.
And, she noted, the Office of National Drug Control Policy’s Overdose Detection Mapping Application Program – which tracks overdoses nationwide – issued 191% more “spike alerts” from January to April this year, compared with the same time period in 2019. However, the spike alerts started going up in January, several weeks before mass numbers of COVID-19 cases began to be diagnosed.
Dr. Volkow noted the uncertainty about the numbers but said several factors could cause the pandemic to boost overdoses:
- Stress and isolation. “My first fear was that overdoses are going to go up because the stress is actually extraordinarily difficult,” she said. “Social distancing is making it very difficult for individuals with substance use disorder or opioid use disorder to get the community support that keeps them from relapsing,” such as methadone clinics and syringe exchange programs.
- Unwitnessed opioid overdoses. Social distancing could “lead to overdoses that nobody has observed, so no one can administer naloxone,” she said.
- Treatment decisions affected by stigma. “Our health systems will be overburdened, and they have to make decisions about which patients to treat,” she said. Stigma could play a very important role in interfering with the treatment of individuals with substance use disorders.”
- Drug-related vulnerabilities. On another front, she said, substance users may be especially vulnerable to the pandemic, because the drugs target multiple body systems that worsen COVID-19 outcomes. These include not only the lungs but also the cardiac and metabolic systems, she said.
For example, “if you have a long history of drug use, you’re going to be much more likely to have a pulmonary disease,” she said. “We know that pulmonary disease is a risk factor for getting COVID and for much worse outcomes.”
But the pandemic could also help in the fight against substance use. For one thing, she said, the pandemic could disrupt drug markets and make it harder for users to get illicit products.
In yet another complication, there is an ongoing debate over whether tobacco use could actually be protective against COVID-19. Research into nicotine patches as a treatment is in the works, she said.
What now? Dr. Volkow said one priority going forward should be an evaluation of virtual medicine. “We have virtual technologies that have enabled us to do telemedicine to provide mental health support and hotlines, as well as virtual support meetings,” she said. “These have proliferated and have served to a certain extent to compensate for some of the deficit from the erosion of the community support systems that exist.”
Now, she said, we should evaluate which interventions are effective, which patients they help, and the components that make them work.
There are other opportunities for useful investigations, she said. For example, researchers could examine the effects of COVID-related changes in policy, such as the federal government allowing more methadone users to take doses home and expanded telemedicine policy allowing more remote prescriptions.
“If we can show that the outcomes are as good or better [than before] then we may be able to transform these practices that make it so very difficult for so many patients to get access to treatment and to sustain treatment – but have not been questioned for years and years.”
Dr. Volkow reported no relevant disclosures.
Early evidence suggests that opioid overdoses and deaths are on the rise this year, the director of the National Institute on Drug Abuse warned colleagues, although it’s not clear whether the coronavirus pandemic is responsible for the trend.
The picture is complicated since COVID-19 could have both positive and negative effects on substance use, Nora D. Volkow, MD, said in a plenary session at the virtual annual meeting of the College on Problems of Drug Dependence. However, she said, one thing is clear: The pandemic marks an opportunity to investigate new strategies and potentially reform treatment.
“We are being faced with an unknown world, and the lack of information curtails our capacity to implement interventions in the most effective way,” Dr. Volkow said. “There’s an urgency to obtain these data. All of you out there in the trenches have an opportunity to help gather this information in a way that can be integrated and deployed rapidly for us to guide practices and treatment.”
It’s too early to know for certain how the pandemic is affecting substance use in the United States, since statistics are sparse and COVID-19 is still relatively new. Still, local news reports have suggested overdose deaths have risen, Dr. Volkow said.
And, she noted, the Office of National Drug Control Policy’s Overdose Detection Mapping Application Program – which tracks overdoses nationwide – issued 191% more “spike alerts” from January to April this year, compared with the same time period in 2019. However, the spike alerts started going up in January, several weeks before mass numbers of COVID-19 cases began to be diagnosed.
Dr. Volkow noted the uncertainty about the numbers but said several factors could cause the pandemic to boost overdoses:
- Stress and isolation. “My first fear was that overdoses are going to go up because the stress is actually extraordinarily difficult,” she said. “Social distancing is making it very difficult for individuals with substance use disorder or opioid use disorder to get the community support that keeps them from relapsing,” such as methadone clinics and syringe exchange programs.
- Unwitnessed opioid overdoses. Social distancing could “lead to overdoses that nobody has observed, so no one can administer naloxone,” she said.
- Treatment decisions affected by stigma. “Our health systems will be overburdened, and they have to make decisions about which patients to treat,” she said. Stigma could play a very important role in interfering with the treatment of individuals with substance use disorders.”
- Drug-related vulnerabilities. On another front, she said, substance users may be especially vulnerable to the pandemic, because the drugs target multiple body systems that worsen COVID-19 outcomes. These include not only the lungs but also the cardiac and metabolic systems, she said.
For example, “if you have a long history of drug use, you’re going to be much more likely to have a pulmonary disease,” she said. “We know that pulmonary disease is a risk factor for getting COVID and for much worse outcomes.”
But the pandemic could also help in the fight against substance use. For one thing, she said, the pandemic could disrupt drug markets and make it harder for users to get illicit products.
In yet another complication, there is an ongoing debate over whether tobacco use could actually be protective against COVID-19. Research into nicotine patches as a treatment is in the works, she said.
What now? Dr. Volkow said one priority going forward should be an evaluation of virtual medicine. “We have virtual technologies that have enabled us to do telemedicine to provide mental health support and hotlines, as well as virtual support meetings,” she said. “These have proliferated and have served to a certain extent to compensate for some of the deficit from the erosion of the community support systems that exist.”
Now, she said, we should evaluate which interventions are effective, which patients they help, and the components that make them work.
There are other opportunities for useful investigations, she said. For example, researchers could examine the effects of COVID-related changes in policy, such as the federal government allowing more methadone users to take doses home and expanded telemedicine policy allowing more remote prescriptions.
“If we can show that the outcomes are as good or better [than before] then we may be able to transform these practices that make it so very difficult for so many patients to get access to treatment and to sustain treatment – but have not been questioned for years and years.”
Dr. Volkow reported no relevant disclosures.
Early evidence suggests that opioid overdoses and deaths are on the rise this year, the director of the National Institute on Drug Abuse warned colleagues, although it’s not clear whether the coronavirus pandemic is responsible for the trend.
The picture is complicated since COVID-19 could have both positive and negative effects on substance use, Nora D. Volkow, MD, said in a plenary session at the virtual annual meeting of the College on Problems of Drug Dependence. However, she said, one thing is clear: The pandemic marks an opportunity to investigate new strategies and potentially reform treatment.
“We are being faced with an unknown world, and the lack of information curtails our capacity to implement interventions in the most effective way,” Dr. Volkow said. “There’s an urgency to obtain these data. All of you out there in the trenches have an opportunity to help gather this information in a way that can be integrated and deployed rapidly for us to guide practices and treatment.”
It’s too early to know for certain how the pandemic is affecting substance use in the United States, since statistics are sparse and COVID-19 is still relatively new. Still, local news reports have suggested overdose deaths have risen, Dr. Volkow said.
And, she noted, the Office of National Drug Control Policy’s Overdose Detection Mapping Application Program – which tracks overdoses nationwide – issued 191% more “spike alerts” from January to April this year, compared with the same time period in 2019. However, the spike alerts started going up in January, several weeks before mass numbers of COVID-19 cases began to be diagnosed.
Dr. Volkow noted the uncertainty about the numbers but said several factors could cause the pandemic to boost overdoses:
- Stress and isolation. “My first fear was that overdoses are going to go up because the stress is actually extraordinarily difficult,” she said. “Social distancing is making it very difficult for individuals with substance use disorder or opioid use disorder to get the community support that keeps them from relapsing,” such as methadone clinics and syringe exchange programs.
- Unwitnessed opioid overdoses. Social distancing could “lead to overdoses that nobody has observed, so no one can administer naloxone,” she said.
- Treatment decisions affected by stigma. “Our health systems will be overburdened, and they have to make decisions about which patients to treat,” she said. Stigma could play a very important role in interfering with the treatment of individuals with substance use disorders.”
- Drug-related vulnerabilities. On another front, she said, substance users may be especially vulnerable to the pandemic, because the drugs target multiple body systems that worsen COVID-19 outcomes. These include not only the lungs but also the cardiac and metabolic systems, she said.
For example, “if you have a long history of drug use, you’re going to be much more likely to have a pulmonary disease,” she said. “We know that pulmonary disease is a risk factor for getting COVID and for much worse outcomes.”
But the pandemic could also help in the fight against substance use. For one thing, she said, the pandemic could disrupt drug markets and make it harder for users to get illicit products.
In yet another complication, there is an ongoing debate over whether tobacco use could actually be protective against COVID-19. Research into nicotine patches as a treatment is in the works, she said.
What now? Dr. Volkow said one priority going forward should be an evaluation of virtual medicine. “We have virtual technologies that have enabled us to do telemedicine to provide mental health support and hotlines, as well as virtual support meetings,” she said. “These have proliferated and have served to a certain extent to compensate for some of the deficit from the erosion of the community support systems that exist.”
Now, she said, we should evaluate which interventions are effective, which patients they help, and the components that make them work.
There are other opportunities for useful investigations, she said. For example, researchers could examine the effects of COVID-related changes in policy, such as the federal government allowing more methadone users to take doses home and expanded telemedicine policy allowing more remote prescriptions.
“If we can show that the outcomes are as good or better [than before] then we may be able to transform these practices that make it so very difficult for so many patients to get access to treatment and to sustain treatment – but have not been questioned for years and years.”
Dr. Volkow reported no relevant disclosures.
FROM CPDD 2020
Inside Mercy’s mission to care for non-COVID patients in Los Angeles
When the hospital ship USNS Mercy departed San Diego’s Naval Station North Island on March 23, 2020, to support the Department of Defense efforts in Los Angeles during the coronavirus outbreak, Commander Erin Blevins remembers the crew’s excitement was palpable.
“We normally do partnerships abroad and respond to tsunamis and earthquakes,” said Cdr. Blevins, MD, a pediatric hematologist-oncologist who served as director of medical services for the mission. “This was a slight change in situation, but still disaster relief in the form of a pandemic. We switched our mindset to putting together the best experts for an infectious disease pandemic versus an earthquake disaster relief.”
A new mission
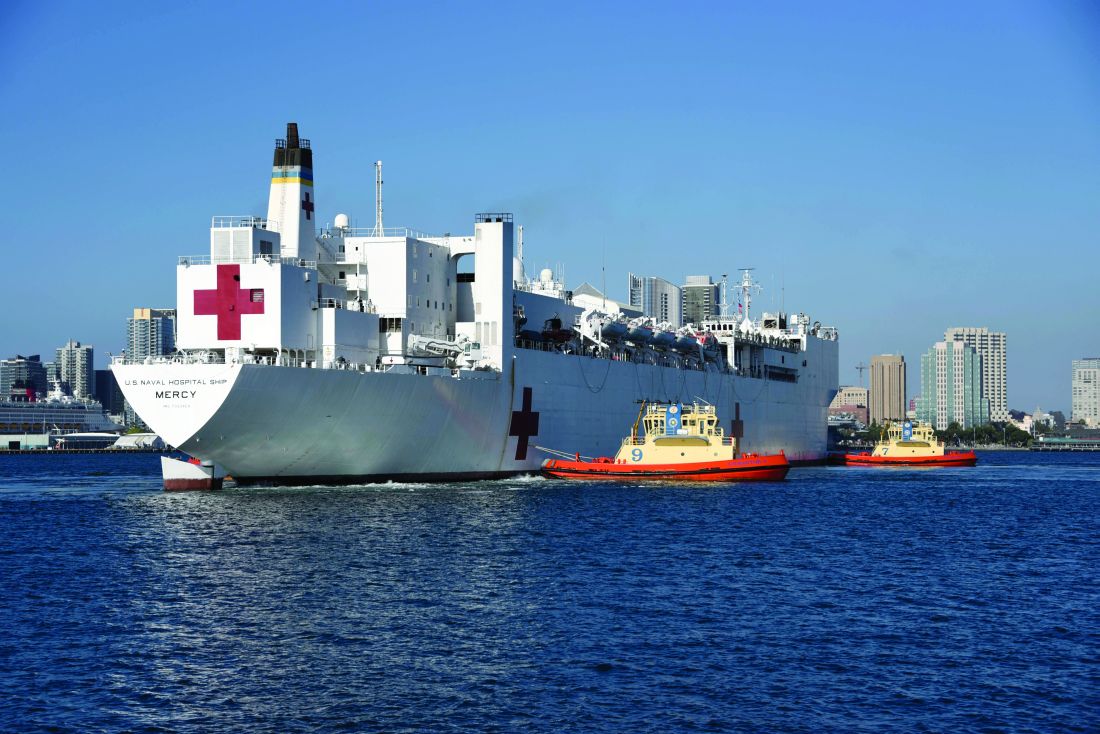
The 1,000-bed Mercy ship – a converted San Clemente–class oil tanker that was delivered in 1986 – spent nearly 50 days pier side in Los Angeles as a referral hospital for non–COVID-19 patients, so that clinicians at Los Angeles area hospitals could care for an anticipated surge of COVID-19 patients. “We went into it with expectations of, ‘We’ll treat as many patients as you need us to take,” Cdr. Blevins recalled. “I don’t even think Los Angeles [health officials] knew exactly where they were going to peak and what the need was going to be.”
Between March 29 and May 15, about 1,071 medical personnel aboard the Mercy cared for 77 patients with an average age of 53 years who were referred from 11 Los Angeles area hospitals. The physicians, nurses, and other medical support personnel were drawn from military treatment facilities across the country. “We had additional people join us as we scoped the mission to be more medically heavy and surgically light,” said Captain John Rotruck, MD, an anesthesiologist who is commanding officer of Mercy’s medical treatment facility. “We did adjust to make sure that we had the right staffing mix to meet the parameters that we were assigned. That was the crux of the change: a change in flavors of staffing to ensure that we focused on ICU and ward medical care as opposed to very heavy surgical care in support of a combat operation.”
About 10% of the team consisted of reservists who volunteered for the mission. “There’s no way you could have walked around the ship and known who was active duty and who was reservist,” said Capt. Rotruck, who was formerly chief of staff at Walter Reed National Military Medical Center, Bethesda, Md. “They worked together so well, and I think that marriage of active duty who are used to working in a military medical treatment facility – in our case, a Navy medical treatment facility – together with our reservist physician colleagues who work in civilian facilities around the country, was beneficial. It was a synergistic relationship. I think both sides walked away learning quite a bit from each other.”
Start with screening
All crew members underwent a temperature check and completed a health screening questionnaire: once before departing their home of record and again before boarding Mercy. Based on those results, crew members and medical staff were screened for COVID-19 and tested as needed in order to minimize the risk of an outbreak aboard the ship.
Fewer than 1% of crew members developed COVID-19 or tested positive for the virus during the mission, according to Capt. Rotruck. Affected individuals were isolated and quarantined. “All staff have recovered and are doing well,” he said.
Mercy personnel worked with local health officials to ensure that all patients transferred to the ship tested negative for COVID-19. Physicians aboard the Mercy then worked directly with the patients’ civilian physician to ensure a safe and thorough turnover process before the patients were transferred.
From basic medical to trauma care
Care aboard the ship, which consists of open-bay medical wards, ranged from basic medical and surgical care to critical care and trauma. The most common procedures were cholecystectomies and orthopedic procedures, and the average length of stay was 4-5 days, according to Cdr. Blevins. Over the course of the mission, the medical professionals conducted 36 surgeries, 77 x-ray exams, 26 CT scans, and administered hundreds of ancillary studies ranging from routine labs to high-end x-rays and blood transfusion support.
“Within our ICU, we did have some end-of-life patients who ended up dying on our ship in comfort care,” Cdr. Blevins said. “Fortunately, we had a wonderful ICU team who had a great deal of experience with end-of-life care and were able to take care of these patients very comfortably and ensure good communication with family and loved ones during that time. In most instances we tried to make sure that people got to FaceTime or video chat with their loved one before they passed away.”
The Mercy, which includes 12 operating rooms, four x-ray units, and one CAT-scan unit, was not equipped to deliver pediatric or obstetrical care. Other unavailable services included psychiatry, oncology, cardiac and thoracic surgery, nuclear medicine, MRI, mammography, electrophysiology, cardiac catheterization, negative-pressure isolation, speech therapy, and occupational therapy.
Not your typical hospital experience
But for patients who did receive medical care aboard the Mercy – which made three 150-day deployments in recent years for the military-led humanitarian response known as Pacific Partnership in 2015, 2016, and 2018 – it was an experience that they are unlikely to forget.
“Every time a patient left the ship, our team on the ground surveyed them to see how their experience was and see what we could do to improve,” Cdr. Blevins said. “Across the board, they were all very appreciative of the medical care. We had a couple of veterans on board. They got [USNS Mercy] hats on their way out and seemed to very much enjoy a slightly different experience than they would get at a regular hospital.”
Capt. Rotruck added that the enthusiasm crew members had for supporting fellow Americans “really energized our team and really saturated that caring aspect of the people who interacted directly with patients,” he said. “It wasn’t just the physicians and nurses, but it was the staff delivering the food and coming to take blood samples and every other interaction that the patients had with our team. I think they really felt that enthusiasm for being there and supporting our neighbors in LA [Los Angeles].”
Crew life aboard the Mercy
Just as with any hospital on shore, personnel aboard the Mercy practiced preventive hygiene measures recommended by the Centers for Disease Control and Prevention to help prevent the spread of COVID-19, such as wearing cloth face masks, spacing out tables in the dining hall, closing indoor gyms, and devising creative ways to stay physically fit. Popular options included jogging around the perimeter of the ship and practicing yoga and calisthenics on the deck, “making sure you were physically distanced appropriately, and when you were done, putting your mask back on,” Cdr. Blevins said. Others supplemented their workouts with a pull-up bar on the deck. “In addition, we have a series of ramps that run on the starboard side of the ship that we can use for patient movement with litters on wheels or patient beds,” Capt. Rotruck said. “The uphill portion of those ramps represents a good workout opportunity as well.”
Downtime in an era of physical distancing also afforded crew members the opportunity to call or FaceTime with loved ones, watch streamed TV shows and movies, and work on their own professional development. Some continued with coursework for online degree programs offered by colleges and universities they were enrolled in, while some enlisted personnel used the time to complete the Navy Enlisted Warfare Qualification Programs Instruction, which issues the basic overarching requirements for the qualification and designation of all enlisted warfare programs.
“As you can imagine, people spend a lot of time learning how the ship works and how it integrates into larger naval forces and so forth,” Capt. Rotruck said. “Not just our ship but also other ships: their weapons systems and defense mechanisms and navigation systems. We had people spending a significant amount of time working on that. We had people complete their Enlisted Surface Warfare qualification while we were on the mission.”
End of the mission
Mercy returned to its home base in San Diego on May 15, but about 60 medical personnel stayed behind in Los Angeles to support Federal Emergency Management Agency (FEMA), state, and local health care professionals. Some worked at a site where clinicians provided care for COVID-19–positive patients who had been transferred from area skilled nursing facilities.
In addition, a team consisting of one nurse and five corpsmen “would go out to individual skilled nursing facilities and mainly conduct assessments and training, such as training in donning proper PPE [personal protective equipment] and determining what needs they had,” Capt. Rotruck said. “They met those needs if possible or [communicated with California officials] and let them know what the requirements were and what the needs were in that facility.” The assignment for those who stayed behind ended on May 31.
On the opposite coast, Mercy’s sister ship, USNS Comfort, arrived in New York Harbor from Norfolk, Va., on March 30 and spent 3½ weeks assisting area hospitals in the COVID-19 pandemic fight. A few days into the mission, Comfort’s internal spaces were reconfigured to create separate COVID-negative and COVID-positive sections. Medical teams aboard the ship cared for a total of 182 patients during the assignment.
Looking back on Mercy’s mission, Cdr. Blevins marveled at the sense of teamwork that unfolded. “We have quarterly training exercises with a core set of personnel, [and] we train getting ready for activation in 5 days,” she said. “All of that training kicks in and it comes to fruition in a mission like this. It was terrific to see a group of very disparate subject matter experts from all over the country come together with one purpose: which was to serve our own country during the pandemic.”
Capt. Rotruck pointed out that the experience enabled enlisted and nonenlisted physicians to maintain their skill sets during a time when military and civilian hospitals had stopped doing elective procedures and routine appointments. “The fact that those people were able to come on board the ship and continue to conduct their medical practice and maintain their skills and competencies in an environment that they weren’t quite used to is great,” he said. “Otherwise, some of those medical personnel would have been sitting idle, wherever they were from. This is the power of Navy medicine on behalf of our country.”
When the hospital ship USNS Mercy departed San Diego’s Naval Station North Island on March 23, 2020, to support the Department of Defense efforts in Los Angeles during the coronavirus outbreak, Commander Erin Blevins remembers the crew’s excitement was palpable.
“We normally do partnerships abroad and respond to tsunamis and earthquakes,” said Cdr. Blevins, MD, a pediatric hematologist-oncologist who served as director of medical services for the mission. “This was a slight change in situation, but still disaster relief in the form of a pandemic. We switched our mindset to putting together the best experts for an infectious disease pandemic versus an earthquake disaster relief.”
A new mission

The 1,000-bed Mercy ship – a converted San Clemente–class oil tanker that was delivered in 1986 – spent nearly 50 days pier side in Los Angeles as a referral hospital for non–COVID-19 patients, so that clinicians at Los Angeles area hospitals could care for an anticipated surge of COVID-19 patients. “We went into it with expectations of, ‘We’ll treat as many patients as you need us to take,” Cdr. Blevins recalled. “I don’t even think Los Angeles [health officials] knew exactly where they were going to peak and what the need was going to be.”
Between March 29 and May 15, about 1,071 medical personnel aboard the Mercy cared for 77 patients with an average age of 53 years who were referred from 11 Los Angeles area hospitals. The physicians, nurses, and other medical support personnel were drawn from military treatment facilities across the country. “We had additional people join us as we scoped the mission to be more medically heavy and surgically light,” said Captain John Rotruck, MD, an anesthesiologist who is commanding officer of Mercy’s medical treatment facility. “We did adjust to make sure that we had the right staffing mix to meet the parameters that we were assigned. That was the crux of the change: a change in flavors of staffing to ensure that we focused on ICU and ward medical care as opposed to very heavy surgical care in support of a combat operation.”
About 10% of the team consisted of reservists who volunteered for the mission. “There’s no way you could have walked around the ship and known who was active duty and who was reservist,” said Capt. Rotruck, who was formerly chief of staff at Walter Reed National Military Medical Center, Bethesda, Md. “They worked together so well, and I think that marriage of active duty who are used to working in a military medical treatment facility – in our case, a Navy medical treatment facility – together with our reservist physician colleagues who work in civilian facilities around the country, was beneficial. It was a synergistic relationship. I think both sides walked away learning quite a bit from each other.”
Start with screening
All crew members underwent a temperature check and completed a health screening questionnaire: once before departing their home of record and again before boarding Mercy. Based on those results, crew members and medical staff were screened for COVID-19 and tested as needed in order to minimize the risk of an outbreak aboard the ship.
Fewer than 1% of crew members developed COVID-19 or tested positive for the virus during the mission, according to Capt. Rotruck. Affected individuals were isolated and quarantined. “All staff have recovered and are doing well,” he said.
Mercy personnel worked with local health officials to ensure that all patients transferred to the ship tested negative for COVID-19. Physicians aboard the Mercy then worked directly with the patients’ civilian physician to ensure a safe and thorough turnover process before the patients were transferred.
From basic medical to trauma care

Care aboard the ship, which consists of open-bay medical wards, ranged from basic medical and surgical care to critical care and trauma. The most common procedures were cholecystectomies and orthopedic procedures, and the average length of stay was 4-5 days, according to Cdr. Blevins. Over the course of the mission, the medical professionals conducted 36 surgeries, 77 x-ray exams, 26 CT scans, and administered hundreds of ancillary studies ranging from routine labs to high-end x-rays and blood transfusion support.
“Within our ICU, we did have some end-of-life patients who ended up dying on our ship in comfort care,” Cdr. Blevins said. “Fortunately, we had a wonderful ICU team who had a great deal of experience with end-of-life care and were able to take care of these patients very comfortably and ensure good communication with family and loved ones during that time. In most instances we tried to make sure that people got to FaceTime or video chat with their loved one before they passed away.”

The Mercy, which includes 12 operating rooms, four x-ray units, and one CAT-scan unit, was not equipped to deliver pediatric or obstetrical care. Other unavailable services included psychiatry, oncology, cardiac and thoracic surgery, nuclear medicine, MRI, mammography, electrophysiology, cardiac catheterization, negative-pressure isolation, speech therapy, and occupational therapy.
Not your typical hospital experience
But for patients who did receive medical care aboard the Mercy – which made three 150-day deployments in recent years for the military-led humanitarian response known as Pacific Partnership in 2015, 2016, and 2018 – it was an experience that they are unlikely to forget.
“Every time a patient left the ship, our team on the ground surveyed them to see how their experience was and see what we could do to improve,” Cdr. Blevins said. “Across the board, they were all very appreciative of the medical care. We had a couple of veterans on board. They got [USNS Mercy] hats on their way out and seemed to very much enjoy a slightly different experience than they would get at a regular hospital.”
Capt. Rotruck added that the enthusiasm crew members had for supporting fellow Americans “really energized our team and really saturated that caring aspect of the people who interacted directly with patients,” he said. “It wasn’t just the physicians and nurses, but it was the staff delivering the food and coming to take blood samples and every other interaction that the patients had with our team. I think they really felt that enthusiasm for being there and supporting our neighbors in LA [Los Angeles].”
Crew life aboard the Mercy
Just as with any hospital on shore, personnel aboard the Mercy practiced preventive hygiene measures recommended by the Centers for Disease Control and Prevention to help prevent the spread of COVID-19, such as wearing cloth face masks, spacing out tables in the dining hall, closing indoor gyms, and devising creative ways to stay physically fit. Popular options included jogging around the perimeter of the ship and practicing yoga and calisthenics on the deck, “making sure you were physically distanced appropriately, and when you were done, putting your mask back on,” Cdr. Blevins said. Others supplemented their workouts with a pull-up bar on the deck. “In addition, we have a series of ramps that run on the starboard side of the ship that we can use for patient movement with litters on wheels or patient beds,” Capt. Rotruck said. “The uphill portion of those ramps represents a good workout opportunity as well.”
Downtime in an era of physical distancing also afforded crew members the opportunity to call or FaceTime with loved ones, watch streamed TV shows and movies, and work on their own professional development. Some continued with coursework for online degree programs offered by colleges and universities they were enrolled in, while some enlisted personnel used the time to complete the Navy Enlisted Warfare Qualification Programs Instruction, which issues the basic overarching requirements for the qualification and designation of all enlisted warfare programs.
“As you can imagine, people spend a lot of time learning how the ship works and how it integrates into larger naval forces and so forth,” Capt. Rotruck said. “Not just our ship but also other ships: their weapons systems and defense mechanisms and navigation systems. We had people spending a significant amount of time working on that. We had people complete their Enlisted Surface Warfare qualification while we were on the mission.”
End of the mission
Mercy returned to its home base in San Diego on May 15, but about 60 medical personnel stayed behind in Los Angeles to support Federal Emergency Management Agency (FEMA), state, and local health care professionals. Some worked at a site where clinicians provided care for COVID-19–positive patients who had been transferred from area skilled nursing facilities.
In addition, a team consisting of one nurse and five corpsmen “would go out to individual skilled nursing facilities and mainly conduct assessments and training, such as training in donning proper PPE [personal protective equipment] and determining what needs they had,” Capt. Rotruck said. “They met those needs if possible or [communicated with California officials] and let them know what the requirements were and what the needs were in that facility.” The assignment for those who stayed behind ended on May 31.
On the opposite coast, Mercy’s sister ship, USNS Comfort, arrived in New York Harbor from Norfolk, Va., on March 30 and spent 3½ weeks assisting area hospitals in the COVID-19 pandemic fight. A few days into the mission, Comfort’s internal spaces were reconfigured to create separate COVID-negative and COVID-positive sections. Medical teams aboard the ship cared for a total of 182 patients during the assignment.
Looking back on Mercy’s mission, Cdr. Blevins marveled at the sense of teamwork that unfolded. “We have quarterly training exercises with a core set of personnel, [and] we train getting ready for activation in 5 days,” she said. “All of that training kicks in and it comes to fruition in a mission like this. It was terrific to see a group of very disparate subject matter experts from all over the country come together with one purpose: which was to serve our own country during the pandemic.”
Capt. Rotruck pointed out that the experience enabled enlisted and nonenlisted physicians to maintain their skill sets during a time when military and civilian hospitals had stopped doing elective procedures and routine appointments. “The fact that those people were able to come on board the ship and continue to conduct their medical practice and maintain their skills and competencies in an environment that they weren’t quite used to is great,” he said. “Otherwise, some of those medical personnel would have been sitting idle, wherever they were from. This is the power of Navy medicine on behalf of our country.”
When the hospital ship USNS Mercy departed San Diego’s Naval Station North Island on March 23, 2020, to support the Department of Defense efforts in Los Angeles during the coronavirus outbreak, Commander Erin Blevins remembers the crew’s excitement was palpable.
“We normally do partnerships abroad and respond to tsunamis and earthquakes,” said Cdr. Blevins, MD, a pediatric hematologist-oncologist who served as director of medical services for the mission. “This was a slight change in situation, but still disaster relief in the form of a pandemic. We switched our mindset to putting together the best experts for an infectious disease pandemic versus an earthquake disaster relief.”
A new mission

The 1,000-bed Mercy ship – a converted San Clemente–class oil tanker that was delivered in 1986 – spent nearly 50 days pier side in Los Angeles as a referral hospital for non–COVID-19 patients, so that clinicians at Los Angeles area hospitals could care for an anticipated surge of COVID-19 patients. “We went into it with expectations of, ‘We’ll treat as many patients as you need us to take,” Cdr. Blevins recalled. “I don’t even think Los Angeles [health officials] knew exactly where they were going to peak and what the need was going to be.”
Between March 29 and May 15, about 1,071 medical personnel aboard the Mercy cared for 77 patients with an average age of 53 years who were referred from 11 Los Angeles area hospitals. The physicians, nurses, and other medical support personnel were drawn from military treatment facilities across the country. “We had additional people join us as we scoped the mission to be more medically heavy and surgically light,” said Captain John Rotruck, MD, an anesthesiologist who is commanding officer of Mercy’s medical treatment facility. “We did adjust to make sure that we had the right staffing mix to meet the parameters that we were assigned. That was the crux of the change: a change in flavors of staffing to ensure that we focused on ICU and ward medical care as opposed to very heavy surgical care in support of a combat operation.”
About 10% of the team consisted of reservists who volunteered for the mission. “There’s no way you could have walked around the ship and known who was active duty and who was reservist,” said Capt. Rotruck, who was formerly chief of staff at Walter Reed National Military Medical Center, Bethesda, Md. “They worked together so well, and I think that marriage of active duty who are used to working in a military medical treatment facility – in our case, a Navy medical treatment facility – together with our reservist physician colleagues who work in civilian facilities around the country, was beneficial. It was a synergistic relationship. I think both sides walked away learning quite a bit from each other.”
Start with screening
All crew members underwent a temperature check and completed a health screening questionnaire: once before departing their home of record and again before boarding Mercy. Based on those results, crew members and medical staff were screened for COVID-19 and tested as needed in order to minimize the risk of an outbreak aboard the ship.
Fewer than 1% of crew members developed COVID-19 or tested positive for the virus during the mission, according to Capt. Rotruck. Affected individuals were isolated and quarantined. “All staff have recovered and are doing well,” he said.
Mercy personnel worked with local health officials to ensure that all patients transferred to the ship tested negative for COVID-19. Physicians aboard the Mercy then worked directly with the patients’ civilian physician to ensure a safe and thorough turnover process before the patients were transferred.
From basic medical to trauma care

Care aboard the ship, which consists of open-bay medical wards, ranged from basic medical and surgical care to critical care and trauma. The most common procedures were cholecystectomies and orthopedic procedures, and the average length of stay was 4-5 days, according to Cdr. Blevins. Over the course of the mission, the medical professionals conducted 36 surgeries, 77 x-ray exams, 26 CT scans, and administered hundreds of ancillary studies ranging from routine labs to high-end x-rays and blood transfusion support.
“Within our ICU, we did have some end-of-life patients who ended up dying on our ship in comfort care,” Cdr. Blevins said. “Fortunately, we had a wonderful ICU team who had a great deal of experience with end-of-life care and were able to take care of these patients very comfortably and ensure good communication with family and loved ones during that time. In most instances we tried to make sure that people got to FaceTime or video chat with their loved one before they passed away.”

The Mercy, which includes 12 operating rooms, four x-ray units, and one CAT-scan unit, was not equipped to deliver pediatric or obstetrical care. Other unavailable services included psychiatry, oncology, cardiac and thoracic surgery, nuclear medicine, MRI, mammography, electrophysiology, cardiac catheterization, negative-pressure isolation, speech therapy, and occupational therapy.
Not your typical hospital experience
But for patients who did receive medical care aboard the Mercy – which made three 150-day deployments in recent years for the military-led humanitarian response known as Pacific Partnership in 2015, 2016, and 2018 – it was an experience that they are unlikely to forget.
“Every time a patient left the ship, our team on the ground surveyed them to see how their experience was and see what we could do to improve,” Cdr. Blevins said. “Across the board, they were all very appreciative of the medical care. We had a couple of veterans on board. They got [USNS Mercy] hats on their way out and seemed to very much enjoy a slightly different experience than they would get at a regular hospital.”
Capt. Rotruck added that the enthusiasm crew members had for supporting fellow Americans “really energized our team and really saturated that caring aspect of the people who interacted directly with patients,” he said. “It wasn’t just the physicians and nurses, but it was the staff delivering the food and coming to take blood samples and every other interaction that the patients had with our team. I think they really felt that enthusiasm for being there and supporting our neighbors in LA [Los Angeles].”
Crew life aboard the Mercy
Just as with any hospital on shore, personnel aboard the Mercy practiced preventive hygiene measures recommended by the Centers for Disease Control and Prevention to help prevent the spread of COVID-19, such as wearing cloth face masks, spacing out tables in the dining hall, closing indoor gyms, and devising creative ways to stay physically fit. Popular options included jogging around the perimeter of the ship and practicing yoga and calisthenics on the deck, “making sure you were physically distanced appropriately, and when you were done, putting your mask back on,” Cdr. Blevins said. Others supplemented their workouts with a pull-up bar on the deck. “In addition, we have a series of ramps that run on the starboard side of the ship that we can use for patient movement with litters on wheels or patient beds,” Capt. Rotruck said. “The uphill portion of those ramps represents a good workout opportunity as well.”
Downtime in an era of physical distancing also afforded crew members the opportunity to call or FaceTime with loved ones, watch streamed TV shows and movies, and work on their own professional development. Some continued with coursework for online degree programs offered by colleges and universities they were enrolled in, while some enlisted personnel used the time to complete the Navy Enlisted Warfare Qualification Programs Instruction, which issues the basic overarching requirements for the qualification and designation of all enlisted warfare programs.
“As you can imagine, people spend a lot of time learning how the ship works and how it integrates into larger naval forces and so forth,” Capt. Rotruck said. “Not just our ship but also other ships: their weapons systems and defense mechanisms and navigation systems. We had people spending a significant amount of time working on that. We had people complete their Enlisted Surface Warfare qualification while we were on the mission.”
End of the mission
Mercy returned to its home base in San Diego on May 15, but about 60 medical personnel stayed behind in Los Angeles to support Federal Emergency Management Agency (FEMA), state, and local health care professionals. Some worked at a site where clinicians provided care for COVID-19–positive patients who had been transferred from area skilled nursing facilities.
In addition, a team consisting of one nurse and five corpsmen “would go out to individual skilled nursing facilities and mainly conduct assessments and training, such as training in donning proper PPE [personal protective equipment] and determining what needs they had,” Capt. Rotruck said. “They met those needs if possible or [communicated with California officials] and let them know what the requirements were and what the needs were in that facility.” The assignment for those who stayed behind ended on May 31.
On the opposite coast, Mercy’s sister ship, USNS Comfort, arrived in New York Harbor from Norfolk, Va., on March 30 and spent 3½ weeks assisting area hospitals in the COVID-19 pandemic fight. A few days into the mission, Comfort’s internal spaces were reconfigured to create separate COVID-negative and COVID-positive sections. Medical teams aboard the ship cared for a total of 182 patients during the assignment.
Looking back on Mercy’s mission, Cdr. Blevins marveled at the sense of teamwork that unfolded. “We have quarterly training exercises with a core set of personnel, [and] we train getting ready for activation in 5 days,” she said. “All of that training kicks in and it comes to fruition in a mission like this. It was terrific to see a group of very disparate subject matter experts from all over the country come together with one purpose: which was to serve our own country during the pandemic.”
Capt. Rotruck pointed out that the experience enabled enlisted and nonenlisted physicians to maintain their skill sets during a time when military and civilian hospitals had stopped doing elective procedures and routine appointments. “The fact that those people were able to come on board the ship and continue to conduct their medical practice and maintain their skills and competencies in an environment that they weren’t quite used to is great,” he said. “Otherwise, some of those medical personnel would have been sitting idle, wherever they were from. This is the power of Navy medicine on behalf of our country.”
Daily Recap: ED visits for life-threatening conditions plummet; COVID-19 imaging strategies for kids
Here are the stories our MDedge editors across specialties think you need to know about today:
ED visits drop for life-threatening conditions
Emergency department visits for myocardial infarction, stroke, and hyperglycemic crisis dropped substantially in the 10 weeks after COVID-19 was declared a national emergency, according to new research from the Centers for Disease Control and Prevention.
Compared with the 10-week period from Jan. 5 to March 14, ED visits were down by 23% for MI, 20% for stroke, and 10% for hyperglycemic crisis from March 15 to May 23.
“A short-term decline of this magnitude … is biologically implausible for MI and stroke, especially for older adults, and unlikely for hyperglycemic crisis, and the finding suggests that patients with these conditions either could not access care or were delaying or avoiding seeking care during the early pandemic period,” the researchers wrote in the Morbidity and Mortality Weekly Report. Read more.
Expert recommendations for pediatric COVID-19 imaging
A team of pulmonologists has synthesized the clinical and imaging characteristics of COVID-19 in children, and has issued recommendations for ordering imaging studies in suspected cases of the infection.
Current recommendations from the American College of Radiology (ACR) do not include chest computed tomography (CT) or chest radiography (CXR) as an upfront test to diagnose pediatric COVID-19, but the tests may still have a role in clinical monitoring, especially in patients with a moderate to severe disease course. The potential benefits of utilizing radiologic evaluation – such as establishing a baseline for monitoring disease progression – must be balanced with potential drawbacks, including radiation exposure and reduced availability of imaging resources owing to necessary cleaning and air turnover time.
Based on the most recent international guidelines for pediatric COVID-19 patient management, the authors developed an algorithm for performing imaging studies in suspected cases of COVID-19 pneumonia. The purpose of the tool is to support clinical decision-making around the utilization of CXR and CT to evaluate pediatric COVID-19 pneumonia. “The step by step algorithm addresses the selection, sequence and timing of imaging studies with multiple images illustrating key findings of COVID-19 pneumonia in the pediatric age group,” said Mary Cataletto, MD, of NYU Langone Health in Mineola, N.Y. Read more.
Cortisol levels on COVID-19 admission may be a marker of severity
Patients with COVID-19 who have high levels of the steroid hormone cortisol on admission to the hospital have a substantially increased risk of dying, according to new study findings.
Researchers assessed 535 patients admitted to major London hospitals. Of these, 403 patients were diagnosed with COVID-19 based on a positive result on real-time polymerase chain reaction testing or a strong clinical and radiological suspicion, despite a negative test. Mean cortisol concentrations in patients with COVID-19 were significantly higher than those not diagnosed with the virus and as of May 8, significantly more patients with COVID-19 died than those without (27.8% vs 6.8%).
Measuring cortisol on admission is potentially “another simple marker to use alongside oxygen saturation levels to help us identify which patients need to be admitted immediately, and which may not,” said Waljit S. Dhillo, MBBS, PhD, head of the division of diabetes, endocrinology and metabolism at Imperial College London.
“Having an early indicator of which patients may deteriorate more quickly will help us with providing the best level of care as quickly as possible. In addition, we can also take cortisol levels into account when we are working out how best to treat our patients,” he said. Read more.
Normal-weight prediabetes patients can benefit from lifestyle changes
Adults of normal weight with prediabetes may derive at least as much benefit from lifestyle health coaching programs as adults who are overweight or obese, results of a recent nonrandomized, real-world study show.
Fasting plasma glucose (FPG) normalized in about 63% of prediabetic adults with normal body mass index (BMI) participating in a personalized coaching program that emphasized exercise, nutrition, and weight management. In contrast, FPG normalized in about 52% of overweight and 44% of obese prediabetic individuals participating in the program.
“It is interesting to note that, although the normal weight group lost the least amount of weight, they still benefited from the lifestyle health coaching program... having a resultant greatest decrease in fasting plasma glucose and normalization to a range of someone without prediabetes,” said researcher Mandy Salmon, MS, a medical student at the University of Pennsylvania, Philadelphia. She presented the findings at the virtual annual scientific sessions of the American Diabetes Association. Read more.
Diabetes-related amputations rise in older adults
The recent resurgence in diabetes-related lower-extremity amputations in the United States is not limited to younger adults, according to the author of a recent study that documents similar increases among an older population of Medicare beneficiaries.
While the rate of amputations fell among these older adults from 2000 to 2009, it increased significantly from 2009 to 2017, albeit at a “less severe rate” than recently reported in younger populations, according to study investigator Jessica Harding, PhD, an assistant professor in the department of surgery at Emory University, Atlanta. Dr. Harding reported the results at the virtual annual scientific sessions of the American Diabetes Association.
The rate of nontraumatic lower extremity amputation (NLEA) was ticking upward by more than 1% per year over the 2009-2017 period. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
ED visits drop for life-threatening conditions
Emergency department visits for myocardial infarction, stroke, and hyperglycemic crisis dropped substantially in the 10 weeks after COVID-19 was declared a national emergency, according to new research from the Centers for Disease Control and Prevention.
Compared with the 10-week period from Jan. 5 to March 14, ED visits were down by 23% for MI, 20% for stroke, and 10% for hyperglycemic crisis from March 15 to May 23.
“A short-term decline of this magnitude … is biologically implausible for MI and stroke, especially for older adults, and unlikely for hyperglycemic crisis, and the finding suggests that patients with these conditions either could not access care or were delaying or avoiding seeking care during the early pandemic period,” the researchers wrote in the Morbidity and Mortality Weekly Report. Read more.
Expert recommendations for pediatric COVID-19 imaging
A team of pulmonologists has synthesized the clinical and imaging characteristics of COVID-19 in children, and has issued recommendations for ordering imaging studies in suspected cases of the infection.
Current recommendations from the American College of Radiology (ACR) do not include chest computed tomography (CT) or chest radiography (CXR) as an upfront test to diagnose pediatric COVID-19, but the tests may still have a role in clinical monitoring, especially in patients with a moderate to severe disease course. The potential benefits of utilizing radiologic evaluation – such as establishing a baseline for monitoring disease progression – must be balanced with potential drawbacks, including radiation exposure and reduced availability of imaging resources owing to necessary cleaning and air turnover time.
Based on the most recent international guidelines for pediatric COVID-19 patient management, the authors developed an algorithm for performing imaging studies in suspected cases of COVID-19 pneumonia. The purpose of the tool is to support clinical decision-making around the utilization of CXR and CT to evaluate pediatric COVID-19 pneumonia. “The step by step algorithm addresses the selection, sequence and timing of imaging studies with multiple images illustrating key findings of COVID-19 pneumonia in the pediatric age group,” said Mary Cataletto, MD, of NYU Langone Health in Mineola, N.Y. Read more.
Cortisol levels on COVID-19 admission may be a marker of severity
Patients with COVID-19 who have high levels of the steroid hormone cortisol on admission to the hospital have a substantially increased risk of dying, according to new study findings.
Researchers assessed 535 patients admitted to major London hospitals. Of these, 403 patients were diagnosed with COVID-19 based on a positive result on real-time polymerase chain reaction testing or a strong clinical and radiological suspicion, despite a negative test. Mean cortisol concentrations in patients with COVID-19 were significantly higher than those not diagnosed with the virus and as of May 8, significantly more patients with COVID-19 died than those without (27.8% vs 6.8%).
Measuring cortisol on admission is potentially “another simple marker to use alongside oxygen saturation levels to help us identify which patients need to be admitted immediately, and which may not,” said Waljit S. Dhillo, MBBS, PhD, head of the division of diabetes, endocrinology and metabolism at Imperial College London.
“Having an early indicator of which patients may deteriorate more quickly will help us with providing the best level of care as quickly as possible. In addition, we can also take cortisol levels into account when we are working out how best to treat our patients,” he said. Read more.
Normal-weight prediabetes patients can benefit from lifestyle changes
Adults of normal weight with prediabetes may derive at least as much benefit from lifestyle health coaching programs as adults who are overweight or obese, results of a recent nonrandomized, real-world study show.
Fasting plasma glucose (FPG) normalized in about 63% of prediabetic adults with normal body mass index (BMI) participating in a personalized coaching program that emphasized exercise, nutrition, and weight management. In contrast, FPG normalized in about 52% of overweight and 44% of obese prediabetic individuals participating in the program.
“It is interesting to note that, although the normal weight group lost the least amount of weight, they still benefited from the lifestyle health coaching program... having a resultant greatest decrease in fasting plasma glucose and normalization to a range of someone without prediabetes,” said researcher Mandy Salmon, MS, a medical student at the University of Pennsylvania, Philadelphia. She presented the findings at the virtual annual scientific sessions of the American Diabetes Association. Read more.
Diabetes-related amputations rise in older adults
The recent resurgence in diabetes-related lower-extremity amputations in the United States is not limited to younger adults, according to the author of a recent study that documents similar increases among an older population of Medicare beneficiaries.
While the rate of amputations fell among these older adults from 2000 to 2009, it increased significantly from 2009 to 2017, albeit at a “less severe rate” than recently reported in younger populations, according to study investigator Jessica Harding, PhD, an assistant professor in the department of surgery at Emory University, Atlanta. Dr. Harding reported the results at the virtual annual scientific sessions of the American Diabetes Association.
The rate of nontraumatic lower extremity amputation (NLEA) was ticking upward by more than 1% per year over the 2009-2017 period. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Here are the stories our MDedge editors across specialties think you need to know about today:
ED visits drop for life-threatening conditions
Emergency department visits for myocardial infarction, stroke, and hyperglycemic crisis dropped substantially in the 10 weeks after COVID-19 was declared a national emergency, according to new research from the Centers for Disease Control and Prevention.
Compared with the 10-week period from Jan. 5 to March 14, ED visits were down by 23% for MI, 20% for stroke, and 10% for hyperglycemic crisis from March 15 to May 23.
“A short-term decline of this magnitude … is biologically implausible for MI and stroke, especially for older adults, and unlikely for hyperglycemic crisis, and the finding suggests that patients with these conditions either could not access care or were delaying or avoiding seeking care during the early pandemic period,” the researchers wrote in the Morbidity and Mortality Weekly Report. Read more.
Expert recommendations for pediatric COVID-19 imaging
A team of pulmonologists has synthesized the clinical and imaging characteristics of COVID-19 in children, and has issued recommendations for ordering imaging studies in suspected cases of the infection.
Current recommendations from the American College of Radiology (ACR) do not include chest computed tomography (CT) or chest radiography (CXR) as an upfront test to diagnose pediatric COVID-19, but the tests may still have a role in clinical monitoring, especially in patients with a moderate to severe disease course. The potential benefits of utilizing radiologic evaluation – such as establishing a baseline for monitoring disease progression – must be balanced with potential drawbacks, including radiation exposure and reduced availability of imaging resources owing to necessary cleaning and air turnover time.
Based on the most recent international guidelines for pediatric COVID-19 patient management, the authors developed an algorithm for performing imaging studies in suspected cases of COVID-19 pneumonia. The purpose of the tool is to support clinical decision-making around the utilization of CXR and CT to evaluate pediatric COVID-19 pneumonia. “The step by step algorithm addresses the selection, sequence and timing of imaging studies with multiple images illustrating key findings of COVID-19 pneumonia in the pediatric age group,” said Mary Cataletto, MD, of NYU Langone Health in Mineola, N.Y. Read more.
Cortisol levels on COVID-19 admission may be a marker of severity
Patients with COVID-19 who have high levels of the steroid hormone cortisol on admission to the hospital have a substantially increased risk of dying, according to new study findings.
Researchers assessed 535 patients admitted to major London hospitals. Of these, 403 patients were diagnosed with COVID-19 based on a positive result on real-time polymerase chain reaction testing or a strong clinical and radiological suspicion, despite a negative test. Mean cortisol concentrations in patients with COVID-19 were significantly higher than those not diagnosed with the virus and as of May 8, significantly more patients with COVID-19 died than those without (27.8% vs 6.8%).
Measuring cortisol on admission is potentially “another simple marker to use alongside oxygen saturation levels to help us identify which patients need to be admitted immediately, and which may not,” said Waljit S. Dhillo, MBBS, PhD, head of the division of diabetes, endocrinology and metabolism at Imperial College London.
“Having an early indicator of which patients may deteriorate more quickly will help us with providing the best level of care as quickly as possible. In addition, we can also take cortisol levels into account when we are working out how best to treat our patients,” he said. Read more.
Normal-weight prediabetes patients can benefit from lifestyle changes
Adults of normal weight with prediabetes may derive at least as much benefit from lifestyle health coaching programs as adults who are overweight or obese, results of a recent nonrandomized, real-world study show.
Fasting plasma glucose (FPG) normalized in about 63% of prediabetic adults with normal body mass index (BMI) participating in a personalized coaching program that emphasized exercise, nutrition, and weight management. In contrast, FPG normalized in about 52% of overweight and 44% of obese prediabetic individuals participating in the program.
“It is interesting to note that, although the normal weight group lost the least amount of weight, they still benefited from the lifestyle health coaching program... having a resultant greatest decrease in fasting plasma glucose and normalization to a range of someone without prediabetes,” said researcher Mandy Salmon, MS, a medical student at the University of Pennsylvania, Philadelphia. She presented the findings at the virtual annual scientific sessions of the American Diabetes Association. Read more.
Diabetes-related amputations rise in older adults
The recent resurgence in diabetes-related lower-extremity amputations in the United States is not limited to younger adults, according to the author of a recent study that documents similar increases among an older population of Medicare beneficiaries.
While the rate of amputations fell among these older adults from 2000 to 2009, it increased significantly from 2009 to 2017, albeit at a “less severe rate” than recently reported in younger populations, according to study investigator Jessica Harding, PhD, an assistant professor in the department of surgery at Emory University, Atlanta. Dr. Harding reported the results at the virtual annual scientific sessions of the American Diabetes Association.
The rate of nontraumatic lower extremity amputation (NLEA) was ticking upward by more than 1% per year over the 2009-2017 period. Read more.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
Five healthy lifestyle choices tied to dramatic cut in dementia risk
“I hope this study will motivate people to engage in a healthy lifestyle by not smoking, being physically and cognitively active, and having a high-quality diet,” lead investigator Klodian Dhana, MD, PhD, department of internal medicine, Rush University Medical Center, Chicago, said in an interview.
The study was published online June 17 in Neurology.
Risk-modifying behaviors
To help quantify the impact of a healthy life on risk for Alzheimer’s dementia, Dr. Dhana and colleagues reviewed data from two longitudinal study populations: the Chicago Health and Aging Project (CHAP), with 1,845 participants, and the Memory and Aging Project (MAP), with 920 participants.
They defined a healthy lifestyle score on the basis of the following factors: not smoking; engaging in 150 min/wk or more of physical exercise of moderate to vigorous intensity; light to moderate alcohol consumption (between 1 and less than 15 g/day for women and between 1 and less than 30 g/day for men); consuming a high-quality Mediterranean-DASH Diet Intervention for Neurodegenerative Delay diet (upper 40%); and engaging in late-life cognitive activities (upper 40%). The overall score ranged from 0 to 5.
At baseline, the mean age of participants was 73.2 years in the CHAP study and 81.1 years in the MAP study; 62.4% of the CHAP participants and 75.2% of the MAP participants were women.
During a median follow-up of 5.8 years in CHAP and 6.0 years in MAP, a total of 379 and 229 participants, respectively, developed Alzheimer’s dementia. Rates of dementia decreased with an increasing number of healthy lifestyle behaviors.
In multivariable-adjusted models across the two cohorts, the risk for Alzheimer’s dementia was 27% lower with each additional healthy lifestyle factor (pooled hazard ratio, 0.73; 95% confidence interval, 0.66-0.80).
Compared with individuals with a healthy lifestyle score of 0-1, the risk was 37% lower (pooled HR, 0.63; 95% CI, 0.47-0.84) for those with two or three healthy lifestyle factors and 60% lower (pooled HR, 0.40; 95% CI, 0.28-0.56) for those with four or five healthy lifestyle factors.
“From these findings and the fact that the lifestyle factors we studied are modifiable and in direct control of the individual, it is imperative to promote them concurrently among older adults as a strategy to delay or prevent Alzheimer’s dementia,” Dr. Dhana and colleagues concluded.
In a statement, Dallas Anderson, PhD, program director, division of neuroscience, National Institute on Aging, said the findings help “paint the picture of how multiple factors are likely playing parts in Alzheimer’s disease risk.”
“It’s not a clear cause-and-effect result, but a strong finding because of the dual data sets and combination of modifiable lifestyle factors that appear to lead to risk reduction,” Dr. Anderson added.
Essential questions remain
Commenting on the new study, Luca Giliberto, MD, PhD, neurologist with the Litwin-Zucker Research Center for Alzheimer’s Disease and Memory Disorders at the Feinstein Institutes for Medical Research in Manhasset, N.Y., said this analysis is “further demonstration that a healthy lifestyle is essential to overcome or curb” the risk for Alzheimer’s disease.
“What needs to be determined is how early should we start ‘behaving.’ We should all aim to score four to five factors across our entire lifespan, but this is not always feasible. So, when is the time to behave? Also, what is the relative weight of each of these factors?” said Dr. Giliberto.
Of note, he added, although addressing vascular risk factors such as hypertension, hyperlipidemia, and diabetes “may require an extensive mindful and logistic effort, a healthy diet is effortlessly achieved in some countries, where both the DASH and MIND diets do not need to be ‘prescribed’ but are rather culturally engraved in the population.
“This is, in part, related to the wide availability of high-quality food in these countries, which is not the same in the U.S. This work is one more demonstration of the need to revisit our take on quality of food in the U.S.,” said Dr. Giliberto.
Numerous clinical trials testing lifestyle interventions for dementia prevention are currently underway. The MIND Diet Intervention to Prevent Alzheimer’s Disease, for example, is an interventional clinical trial comparing parallel groups with two different diets. MIND has enrolled more than 600 participants and is ongoing. The anticipated completion date is 2021. Another is the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER), a multisite randomized clinical trial evaluating whether lifestyle interventions – including exercise, cognitively stimulating activities, and the MIND diet – may protect cognitive function in older adults who are at increased risk for cognitive decline.
Funding for the current study was provided by the National Institutes of Health and the National Institute on Aging. Dr. Dhana and Dr. Giliberto have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
“I hope this study will motivate people to engage in a healthy lifestyle by not smoking, being physically and cognitively active, and having a high-quality diet,” lead investigator Klodian Dhana, MD, PhD, department of internal medicine, Rush University Medical Center, Chicago, said in an interview.
The study was published online June 17 in Neurology.
Risk-modifying behaviors
To help quantify the impact of a healthy life on risk for Alzheimer’s dementia, Dr. Dhana and colleagues reviewed data from two longitudinal study populations: the Chicago Health and Aging Project (CHAP), with 1,845 participants, and the Memory and Aging Project (MAP), with 920 participants.
They defined a healthy lifestyle score on the basis of the following factors: not smoking; engaging in 150 min/wk or more of physical exercise of moderate to vigorous intensity; light to moderate alcohol consumption (between 1 and less than 15 g/day for women and between 1 and less than 30 g/day for men); consuming a high-quality Mediterranean-DASH Diet Intervention for Neurodegenerative Delay diet (upper 40%); and engaging in late-life cognitive activities (upper 40%). The overall score ranged from 0 to 5.
At baseline, the mean age of participants was 73.2 years in the CHAP study and 81.1 years in the MAP study; 62.4% of the CHAP participants and 75.2% of the MAP participants were women.
During a median follow-up of 5.8 years in CHAP and 6.0 years in MAP, a total of 379 and 229 participants, respectively, developed Alzheimer’s dementia. Rates of dementia decreased with an increasing number of healthy lifestyle behaviors.
In multivariable-adjusted models across the two cohorts, the risk for Alzheimer’s dementia was 27% lower with each additional healthy lifestyle factor (pooled hazard ratio, 0.73; 95% confidence interval, 0.66-0.80).
Compared with individuals with a healthy lifestyle score of 0-1, the risk was 37% lower (pooled HR, 0.63; 95% CI, 0.47-0.84) for those with two or three healthy lifestyle factors and 60% lower (pooled HR, 0.40; 95% CI, 0.28-0.56) for those with four or five healthy lifestyle factors.
“From these findings and the fact that the lifestyle factors we studied are modifiable and in direct control of the individual, it is imperative to promote them concurrently among older adults as a strategy to delay or prevent Alzheimer’s dementia,” Dr. Dhana and colleagues concluded.
In a statement, Dallas Anderson, PhD, program director, division of neuroscience, National Institute on Aging, said the findings help “paint the picture of how multiple factors are likely playing parts in Alzheimer’s disease risk.”
“It’s not a clear cause-and-effect result, but a strong finding because of the dual data sets and combination of modifiable lifestyle factors that appear to lead to risk reduction,” Dr. Anderson added.
Essential questions remain
Commenting on the new study, Luca Giliberto, MD, PhD, neurologist with the Litwin-Zucker Research Center for Alzheimer’s Disease and Memory Disorders at the Feinstein Institutes for Medical Research in Manhasset, N.Y., said this analysis is “further demonstration that a healthy lifestyle is essential to overcome or curb” the risk for Alzheimer’s disease.
“What needs to be determined is how early should we start ‘behaving.’ We should all aim to score four to five factors across our entire lifespan, but this is not always feasible. So, when is the time to behave? Also, what is the relative weight of each of these factors?” said Dr. Giliberto.
Of note, he added, although addressing vascular risk factors such as hypertension, hyperlipidemia, and diabetes “may require an extensive mindful and logistic effort, a healthy diet is effortlessly achieved in some countries, where both the DASH and MIND diets do not need to be ‘prescribed’ but are rather culturally engraved in the population.
“This is, in part, related to the wide availability of high-quality food in these countries, which is not the same in the U.S. This work is one more demonstration of the need to revisit our take on quality of food in the U.S.,” said Dr. Giliberto.
Numerous clinical trials testing lifestyle interventions for dementia prevention are currently underway. The MIND Diet Intervention to Prevent Alzheimer’s Disease, for example, is an interventional clinical trial comparing parallel groups with two different diets. MIND has enrolled more than 600 participants and is ongoing. The anticipated completion date is 2021. Another is the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER), a multisite randomized clinical trial evaluating whether lifestyle interventions – including exercise, cognitively stimulating activities, and the MIND diet – may protect cognitive function in older adults who are at increased risk for cognitive decline.
Funding for the current study was provided by the National Institutes of Health and the National Institute on Aging. Dr. Dhana and Dr. Giliberto have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
“I hope this study will motivate people to engage in a healthy lifestyle by not smoking, being physically and cognitively active, and having a high-quality diet,” lead investigator Klodian Dhana, MD, PhD, department of internal medicine, Rush University Medical Center, Chicago, said in an interview.
The study was published online June 17 in Neurology.
Risk-modifying behaviors
To help quantify the impact of a healthy life on risk for Alzheimer’s dementia, Dr. Dhana and colleagues reviewed data from two longitudinal study populations: the Chicago Health and Aging Project (CHAP), with 1,845 participants, and the Memory and Aging Project (MAP), with 920 participants.
They defined a healthy lifestyle score on the basis of the following factors: not smoking; engaging in 150 min/wk or more of physical exercise of moderate to vigorous intensity; light to moderate alcohol consumption (between 1 and less than 15 g/day for women and between 1 and less than 30 g/day for men); consuming a high-quality Mediterranean-DASH Diet Intervention for Neurodegenerative Delay diet (upper 40%); and engaging in late-life cognitive activities (upper 40%). The overall score ranged from 0 to 5.
At baseline, the mean age of participants was 73.2 years in the CHAP study and 81.1 years in the MAP study; 62.4% of the CHAP participants and 75.2% of the MAP participants were women.
During a median follow-up of 5.8 years in CHAP and 6.0 years in MAP, a total of 379 and 229 participants, respectively, developed Alzheimer’s dementia. Rates of dementia decreased with an increasing number of healthy lifestyle behaviors.
In multivariable-adjusted models across the two cohorts, the risk for Alzheimer’s dementia was 27% lower with each additional healthy lifestyle factor (pooled hazard ratio, 0.73; 95% confidence interval, 0.66-0.80).
Compared with individuals with a healthy lifestyle score of 0-1, the risk was 37% lower (pooled HR, 0.63; 95% CI, 0.47-0.84) for those with two or three healthy lifestyle factors and 60% lower (pooled HR, 0.40; 95% CI, 0.28-0.56) for those with four or five healthy lifestyle factors.
“From these findings and the fact that the lifestyle factors we studied are modifiable and in direct control of the individual, it is imperative to promote them concurrently among older adults as a strategy to delay or prevent Alzheimer’s dementia,” Dr. Dhana and colleagues concluded.
In a statement, Dallas Anderson, PhD, program director, division of neuroscience, National Institute on Aging, said the findings help “paint the picture of how multiple factors are likely playing parts in Alzheimer’s disease risk.”
“It’s not a clear cause-and-effect result, but a strong finding because of the dual data sets and combination of modifiable lifestyle factors that appear to lead to risk reduction,” Dr. Anderson added.
Essential questions remain
Commenting on the new study, Luca Giliberto, MD, PhD, neurologist with the Litwin-Zucker Research Center for Alzheimer’s Disease and Memory Disorders at the Feinstein Institutes for Medical Research in Manhasset, N.Y., said this analysis is “further demonstration that a healthy lifestyle is essential to overcome or curb” the risk for Alzheimer’s disease.
“What needs to be determined is how early should we start ‘behaving.’ We should all aim to score four to five factors across our entire lifespan, but this is not always feasible. So, when is the time to behave? Also, what is the relative weight of each of these factors?” said Dr. Giliberto.
Of note, he added, although addressing vascular risk factors such as hypertension, hyperlipidemia, and diabetes “may require an extensive mindful and logistic effort, a healthy diet is effortlessly achieved in some countries, where both the DASH and MIND diets do not need to be ‘prescribed’ but are rather culturally engraved in the population.
“This is, in part, related to the wide availability of high-quality food in these countries, which is not the same in the U.S. This work is one more demonstration of the need to revisit our take on quality of food in the U.S.,” said Dr. Giliberto.
Numerous clinical trials testing lifestyle interventions for dementia prevention are currently underway. The MIND Diet Intervention to Prevent Alzheimer’s Disease, for example, is an interventional clinical trial comparing parallel groups with two different diets. MIND has enrolled more than 600 participants and is ongoing. The anticipated completion date is 2021. Another is the U.S. Study to Protect Brain Health Through Lifestyle Intervention to Reduce Risk (U.S. POINTER), a multisite randomized clinical trial evaluating whether lifestyle interventions – including exercise, cognitively stimulating activities, and the MIND diet – may protect cognitive function in older adults who are at increased risk for cognitive decline.
Funding for the current study was provided by the National Institutes of Health and the National Institute on Aging. Dr. Dhana and Dr. Giliberto have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM NEUROLOGY
Diabetes-related amputations on the rise in older adults
The recent resurgence in diabetes-related lower-extremity amputations in the United States is not limited to younger adults, according to the author of a recent study that documents similar increases among an older population of Medicare beneficiaries.
While the rate of amputations fell among these older adults from 2000 to 2009, it increased significantly from 2009 to 2017, albeit at a “less severe rate” than recently reported in younger populations, said study investigator Jessica Harding, PhD.
The rate of nontraumatic lower extremity amputation (NLEA) was ticking upward by more than 1% per year over the 2009-2017 period, according to Dr. Harding, assistant professor in the department of surgery at Emory University, Atlanta.
This latest report follows one from last year, published in Diabetes Care, that documented an annual percentage increase approaching 6% between 2009 and 2015, driven by larger increases among adults 18-64 years of age, as well as an increase among men.
It’s not clear why rates of NLEA would be on the rise among younger and older adults in the United States, Dr. Harding said, though factors she said could be implicated include changes in amputation practice, increased comorbidities, higher insulin costs, or shortcomings in early prevention programs.
“We need large-scale studies with granular data to tease out key risk factors that could help identify the drivers of these increases in amputations,” Dr. Harding said in a presentation at the virtual annual scientific sessions of the American Diabetes Association.
“In the interim, increased attention to preventive foot care across the age spectrum could benefit adults with diabetes,” she added.
Devastating complication in older adults
The latest findings from Dr. Harding and coauthors emphasize the importance of a “team approach” to early prevention in older adults with diabetes, said Derek LeRoith, MD, PhD, director of research in the division of endocrinology, diabetes, and bone diseases with Icahn School of Medicine at Mount Sinai, New York.
“If you take a 75-year-old or even an 80-year-old, their life expectancy can still be a good 10 years or more,” Dr. LeRoith said in an interview. “We shouldn’t give up on them – we should be treating them to prevent complications.”
Lower-extremity amputation is a “particularly devastating” complication that can compromise mobility, ability to exercise, and motivation, according to Dr. LeRoith, lead author of a recent Endocrine Society clinical practice guideline that urges referral of older adults with diabetes to a podiatrist, orthopedist, or vascular specialist for preventive care.
“Quite often, treating their glucose or high blood pressure will be much more difficult because of these changes,” he said.
Lower extremity amputation trends upward
Rates of NLEA declined for years, only to rebound by 50%, according to authors of a recent analysis of Nationwide Inpatient Sample (NIS) data reported last year. In their report, the age-standardized diabetes-related NLEA rate per 1,000 adults with diabetes went from 5.30 in 2000, down to 3.07 in 2009/2010, and back up to 4.62 by 2015 (Diabetes Care. 2019 Jan;42:50-4).
The resurgence was fueled mainly by an increased rate of amputations in younger and middle-aged adults and men, and through increases in minor amputations, notably the toe, according to the investigators. “These changes in trend are concerning because of the disabling and costly consequences of NLEAs as well as what they may mean for the direction of efforts to reduce diabetes-related complications,” authors of that report said at the time.
In the current study, Dr. Harding and colleagues included Medicare Parts A and B claims data for beneficiaries enrolled from 2000 to 2017. There were 4.6 million Medicare fee-for-service beneficiaries with diabetes in 2000, increasing to 6.9 million in 2017, she reported at the virtual ADA meeting.
Rates of NLEA followed a trajectory similar to what was seen in the earlier NIS report, falling from 8.5 per 1,000 persons in 2000 to 4.4 in 2009, for an annual percentage change of –7.9 (P < .001), Dr. Harding said. Then rates ticked upward again, to 4.8 in 2017, for an annual percentage change of 1.2 over that later period (P < .001).
While the trend was similar for most subgroups analyzed, the absolute rates were highest among men and black individuals in this older patient population, reported Dr. Harding and coauthors.
Dr. Harding said she and coauthors had no disclosures related to the research, which was performed as a collaboration between Emory University and the Centers for Disease Control and Prevention Division of Diabetes Translation.
SOURCE: Harding J. ADA 2020, Abstract 106-OR.
The recent resurgence in diabetes-related lower-extremity amputations in the United States is not limited to younger adults, according to the author of a recent study that documents similar increases among an older population of Medicare beneficiaries.
While the rate of amputations fell among these older adults from 2000 to 2009, it increased significantly from 2009 to 2017, albeit at a “less severe rate” than recently reported in younger populations, said study investigator Jessica Harding, PhD.
The rate of nontraumatic lower extremity amputation (NLEA) was ticking upward by more than 1% per year over the 2009-2017 period, according to Dr. Harding, assistant professor in the department of surgery at Emory University, Atlanta.
This latest report follows one from last year, published in Diabetes Care, that documented an annual percentage increase approaching 6% between 2009 and 2015, driven by larger increases among adults 18-64 years of age, as well as an increase among men.
It’s not clear why rates of NLEA would be on the rise among younger and older adults in the United States, Dr. Harding said, though factors she said could be implicated include changes in amputation practice, increased comorbidities, higher insulin costs, or shortcomings in early prevention programs.
“We need large-scale studies with granular data to tease out key risk factors that could help identify the drivers of these increases in amputations,” Dr. Harding said in a presentation at the virtual annual scientific sessions of the American Diabetes Association.
“In the interim, increased attention to preventive foot care across the age spectrum could benefit adults with diabetes,” she added.
Devastating complication in older adults
The latest findings from Dr. Harding and coauthors emphasize the importance of a “team approach” to early prevention in older adults with diabetes, said Derek LeRoith, MD, PhD, director of research in the division of endocrinology, diabetes, and bone diseases with Icahn School of Medicine at Mount Sinai, New York.
“If you take a 75-year-old or even an 80-year-old, their life expectancy can still be a good 10 years or more,” Dr. LeRoith said in an interview. “We shouldn’t give up on them – we should be treating them to prevent complications.”
Lower-extremity amputation is a “particularly devastating” complication that can compromise mobility, ability to exercise, and motivation, according to Dr. LeRoith, lead author of a recent Endocrine Society clinical practice guideline that urges referral of older adults with diabetes to a podiatrist, orthopedist, or vascular specialist for preventive care.
“Quite often, treating their glucose or high blood pressure will be much more difficult because of these changes,” he said.
Lower extremity amputation trends upward
Rates of NLEA declined for years, only to rebound by 50%, according to authors of a recent analysis of Nationwide Inpatient Sample (NIS) data reported last year. In their report, the age-standardized diabetes-related NLEA rate per 1,000 adults with diabetes went from 5.30 in 2000, down to 3.07 in 2009/2010, and back up to 4.62 by 2015 (Diabetes Care. 2019 Jan;42:50-4).
The resurgence was fueled mainly by an increased rate of amputations in younger and middle-aged adults and men, and through increases in minor amputations, notably the toe, according to the investigators. “These changes in trend are concerning because of the disabling and costly consequences of NLEAs as well as what they may mean for the direction of efforts to reduce diabetes-related complications,” authors of that report said at the time.
In the current study, Dr. Harding and colleagues included Medicare Parts A and B claims data for beneficiaries enrolled from 2000 to 2017. There were 4.6 million Medicare fee-for-service beneficiaries with diabetes in 2000, increasing to 6.9 million in 2017, she reported at the virtual ADA meeting.
Rates of NLEA followed a trajectory similar to what was seen in the earlier NIS report, falling from 8.5 per 1,000 persons in 2000 to 4.4 in 2009, for an annual percentage change of –7.9 (P < .001), Dr. Harding said. Then rates ticked upward again, to 4.8 in 2017, for an annual percentage change of 1.2 over that later period (P < .001).
While the trend was similar for most subgroups analyzed, the absolute rates were highest among men and black individuals in this older patient population, reported Dr. Harding and coauthors.
Dr. Harding said she and coauthors had no disclosures related to the research, which was performed as a collaboration between Emory University and the Centers for Disease Control and Prevention Division of Diabetes Translation.
SOURCE: Harding J. ADA 2020, Abstract 106-OR.
The recent resurgence in diabetes-related lower-extremity amputations in the United States is not limited to younger adults, according to the author of a recent study that documents similar increases among an older population of Medicare beneficiaries.
While the rate of amputations fell among these older adults from 2000 to 2009, it increased significantly from 2009 to 2017, albeit at a “less severe rate” than recently reported in younger populations, said study investigator Jessica Harding, PhD.
The rate of nontraumatic lower extremity amputation (NLEA) was ticking upward by more than 1% per year over the 2009-2017 period, according to Dr. Harding, assistant professor in the department of surgery at Emory University, Atlanta.
This latest report follows one from last year, published in Diabetes Care, that documented an annual percentage increase approaching 6% between 2009 and 2015, driven by larger increases among adults 18-64 years of age, as well as an increase among men.
It’s not clear why rates of NLEA would be on the rise among younger and older adults in the United States, Dr. Harding said, though factors she said could be implicated include changes in amputation practice, increased comorbidities, higher insulin costs, or shortcomings in early prevention programs.
“We need large-scale studies with granular data to tease out key risk factors that could help identify the drivers of these increases in amputations,” Dr. Harding said in a presentation at the virtual annual scientific sessions of the American Diabetes Association.
“In the interim, increased attention to preventive foot care across the age spectrum could benefit adults with diabetes,” she added.
Devastating complication in older adults
The latest findings from Dr. Harding and coauthors emphasize the importance of a “team approach” to early prevention in older adults with diabetes, said Derek LeRoith, MD, PhD, director of research in the division of endocrinology, diabetes, and bone diseases with Icahn School of Medicine at Mount Sinai, New York.
“If you take a 75-year-old or even an 80-year-old, their life expectancy can still be a good 10 years or more,” Dr. LeRoith said in an interview. “We shouldn’t give up on them – we should be treating them to prevent complications.”
Lower-extremity amputation is a “particularly devastating” complication that can compromise mobility, ability to exercise, and motivation, according to Dr. LeRoith, lead author of a recent Endocrine Society clinical practice guideline that urges referral of older adults with diabetes to a podiatrist, orthopedist, or vascular specialist for preventive care.
“Quite often, treating their glucose or high blood pressure will be much more difficult because of these changes,” he said.
Lower extremity amputation trends upward
Rates of NLEA declined for years, only to rebound by 50%, according to authors of a recent analysis of Nationwide Inpatient Sample (NIS) data reported last year. In their report, the age-standardized diabetes-related NLEA rate per 1,000 adults with diabetes went from 5.30 in 2000, down to 3.07 in 2009/2010, and back up to 4.62 by 2015 (Diabetes Care. 2019 Jan;42:50-4).
The resurgence was fueled mainly by an increased rate of amputations in younger and middle-aged adults and men, and through increases in minor amputations, notably the toe, according to the investigators. “These changes in trend are concerning because of the disabling and costly consequences of NLEAs as well as what they may mean for the direction of efforts to reduce diabetes-related complications,” authors of that report said at the time.
In the current study, Dr. Harding and colleagues included Medicare Parts A and B claims data for beneficiaries enrolled from 2000 to 2017. There were 4.6 million Medicare fee-for-service beneficiaries with diabetes in 2000, increasing to 6.9 million in 2017, she reported at the virtual ADA meeting.
Rates of NLEA followed a trajectory similar to what was seen in the earlier NIS report, falling from 8.5 per 1,000 persons in 2000 to 4.4 in 2009, for an annual percentage change of –7.9 (P < .001), Dr. Harding said. Then rates ticked upward again, to 4.8 in 2017, for an annual percentage change of 1.2 over that later period (P < .001).
While the trend was similar for most subgroups analyzed, the absolute rates were highest among men and black individuals in this older patient population, reported Dr. Harding and coauthors.
Dr. Harding said she and coauthors had no disclosures related to the research, which was performed as a collaboration between Emory University and the Centers for Disease Control and Prevention Division of Diabetes Translation.
SOURCE: Harding J. ADA 2020, Abstract 106-OR.
FROM ADA 2020
Guidance on infection prevention for health care personnel
As we reopen our offices we are faced with the challenge of determining the best way to do it safely – protecting ourselves, our staff, and our patients.
In this column we will focus on selected details of the recommendations from IDSA and the CDC that may be helpful in primary care offices.
Face masks
Many clinicians have asked whether a physician should use a mask while seeing patients without COVID-19 in the office, and if yes, which type. The IDSA guideline states that mask usage is imperative for reducing the risk of health care workers contracting COVID-19.1 The evidence is derived from a number of sources, including a retrospective study from Wuhan (China) University that examined two groups of health care workers during the outbreak. The first group wore N95 masks and washed their hands frequently, while the second group did not wear masks and washed their hands less frequently. In the group that took greater actions to protect themselves, none of the 493 staff members contracted COVID-19, compared with 10 of 213 staff members in the other group. The decrease in infection rate occurred in the group that wore masks despite the fact that this group had 733% more exposure to COVID-19 patients.2 Further evidence came from a case-control study done in hospitals in Hong Kong during the 2003 SARS-CoV outbreak.3 This study showed that mask wearing was the most significant intervention for reducing infection, followed by gowning, and then handwashing. These findings make it clear that mask usage is a must for all health care providers who may be caring for patients who could have COVID-19.
The guideline also reviews evidence about the use of surgical masks versus N95 masks. On reviewing indirect evidence from the SARS-CoV epidemic, IDSA found that wearing any mask – surgical or N95 – led to a large reduction in the risk of developing an infection. In this systematic review of five observational studies in health care personnel, for those wearing surgical masks, the odds ratio for developing an infection was 0.13 (95% CI, 0.03-0.62), and for those wearing N95 masks, the odds ratio was 0.12 (95% CI, 0.06-0.26). There was not a significant difference between risk reductions for those who wore surgical masks and N95 masks, respectively.1,4 The IDSA guideline panel recommended “that health care personnel caring for patients with suspected or known COVID-19 use either a surgical mask or N95 respirator ... as part of appropriate PPE.” Since there is not a significant difference in outcomes between those who use surgical masks and those who use N95 respirators, and the IDSA guideline states either type of mask is considered appropriate when taking care of patients with suspected or known COVID-19, in our opinion, use of surgical masks rather than N95s is sufficient when performing low-risk activities. Such activities include seeing patients who do not have a high likelihood of COVID-19 in the office setting.
The IDSA recommendation also discusses universal masking, defined as both patients and clinicians wearing masks. The recommendation is supported by the findings of a study in which universal mask usage was used to prevent the spread of H1N 1 during the 2009 outbreak. In this study of staff members and patients exposed to H1N1 who all wore masks, only 0.48% of 836 acquired infection. In the same study, not wearing a mask by either the provider or patient increased the risk of infection.5 Also, in a prospective study of hematopoietic stem cell transplant patients, universal masking caused infection rates to drop from 10.3% to 4.4%.6
The IDSA guideline states the following: “There may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCPs [health care providers] and visitors.”1
The CDC’s guidance statement says the following: “Continued community transmission has increased the number of individuals potentially exposed to and infectious with SARS-CoV-2. Fever and symptom screening have proven to be relatively ineffective in identifying all infected individuals, including HCPs. Symptom screening also will not identify individuals who are infected but otherwise asymptomatic or pre-symptomatic; additional interventions are needed to limit the unrecognized introduction of SARS-CoV-2 into healthcare settings by these individuals. As part of aggressive source control measures, healthcare facilities should consider implementing policies requiring everyone entering the facility to wear a cloth face covering (if tolerated) while in the building, regardless of symptoms.”7
It is our opinion, based on the CDC and IDSA recommendations, that both clinicians and patients should be required to wear masks when patients are seen in the office if possible. Many offices have instituted a policy that says, if a patient refuses to wear a mask during an office visit, then the patient will not be seen.
Eye protection
Many clinicians are uncertain about whether eye protection needs to be used when seeing asymptomatic patients. The IDSA acknowledges that there are not studies that have looked critically at eye protection, but the society also acknowledges “appropriate personal protective equipment includes, in addition to a mask or respirator, eye protection, gown and gloves.”1 In addition, the CDC recommends that, for healthcare workers located in areas with moderate or higher prevalence of COVID-19, HCPs should wear eye protection in addition to facemasks since they may encounter asymptomatic individuals with COVID-19.
Gowns and gloves
Gowns and gloves are recommended as a part of personal protective gear when caring for patients who have COVID-19. The IDSA guideline is clear in its recommendations, but does not cite evidence for having no gloves versus having gloves. Furthermore, they state that the evidence is insufficient to recommend double gloves, with the top glove used to take off a personal protective gown, and the inner glove discarded after the gown is removed. The CDC do not make recommendations for routine use of gloves in the care of patients who do not have COVID-19, even in areas where there may be asymptomatic COVID-19, and recommends standard precautions, specifically practicing hand hygiene before and after patient contact.8
The Bottom Line
When seeing patients with COVID-19, N-95 masks, goggles or face shields, gowns, and gloves should be used, with hand hygiene routinely practiced before and after seeing patients. For offices seeing patients not suspected of having COVID-19, the IDSA guideline clarifies that there is not a statistical difference in acquisition of infection with the use of surgical face masks vs N95 respirators. According to the CDC recommendations, eye protection in addition to facemasks should be used by the health care provider, and masks should be worn by patients. Hand hygiene should be used routinely before and after all patient contact. With use of these approaches, it should be safe for offices to reopen and see patients.
Neil Skolnik, MD, is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Jeffrey Matthews, DO, is a second-year resident in the Family Medicine Residency at Abington Jefferson Health. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Lynch JB, Davitkov P, Anderson DJ, et al. COVID-19 Guideline, Part 2: Infection Prevention. IDSA Home. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/. April 27, 2020. Accessed June 10, 2020.
2. J Hosp Infect. 2020 May;105(1):104-5.
3. Lancet. 2003;361(9368):1519-20.
4. Influenza Other Respir Viruses. 2020 Apr 4. doi: 2020;10.1111/irv.12745.
5. J Hosp Infect. 2010;74(3):271-7.
6. Clin Infect Dis. 2016;63(8):999-1006.
7. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed Jun 16, 2020.
8. Centers for Disease Control and Prevention. Healthcare Infection Prevention and Control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html. Accessed June 15, 2020.
As we reopen our offices we are faced with the challenge of determining the best way to do it safely – protecting ourselves, our staff, and our patients.
In this column we will focus on selected details of the recommendations from IDSA and the CDC that may be helpful in primary care offices.
Face masks
Many clinicians have asked whether a physician should use a mask while seeing patients without COVID-19 in the office, and if yes, which type. The IDSA guideline states that mask usage is imperative for reducing the risk of health care workers contracting COVID-19.1 The evidence is derived from a number of sources, including a retrospective study from Wuhan (China) University that examined two groups of health care workers during the outbreak. The first group wore N95 masks and washed their hands frequently, while the second group did not wear masks and washed their hands less frequently. In the group that took greater actions to protect themselves, none of the 493 staff members contracted COVID-19, compared with 10 of 213 staff members in the other group. The decrease in infection rate occurred in the group that wore masks despite the fact that this group had 733% more exposure to COVID-19 patients.2 Further evidence came from a case-control study done in hospitals in Hong Kong during the 2003 SARS-CoV outbreak.3 This study showed that mask wearing was the most significant intervention for reducing infection, followed by gowning, and then handwashing. These findings make it clear that mask usage is a must for all health care providers who may be caring for patients who could have COVID-19.
The guideline also reviews evidence about the use of surgical masks versus N95 masks. On reviewing indirect evidence from the SARS-CoV epidemic, IDSA found that wearing any mask – surgical or N95 – led to a large reduction in the risk of developing an infection. In this systematic review of five observational studies in health care personnel, for those wearing surgical masks, the odds ratio for developing an infection was 0.13 (95% CI, 0.03-0.62), and for those wearing N95 masks, the odds ratio was 0.12 (95% CI, 0.06-0.26). There was not a significant difference between risk reductions for those who wore surgical masks and N95 masks, respectively.1,4 The IDSA guideline panel recommended “that health care personnel caring for patients with suspected or known COVID-19 use either a surgical mask or N95 respirator ... as part of appropriate PPE.” Since there is not a significant difference in outcomes between those who use surgical masks and those who use N95 respirators, and the IDSA guideline states either type of mask is considered appropriate when taking care of patients with suspected or known COVID-19, in our opinion, use of surgical masks rather than N95s is sufficient when performing low-risk activities. Such activities include seeing patients who do not have a high likelihood of COVID-19 in the office setting.
The IDSA recommendation also discusses universal masking, defined as both patients and clinicians wearing masks. The recommendation is supported by the findings of a study in which universal mask usage was used to prevent the spread of H1N 1 during the 2009 outbreak. In this study of staff members and patients exposed to H1N1 who all wore masks, only 0.48% of 836 acquired infection. In the same study, not wearing a mask by either the provider or patient increased the risk of infection.5 Also, in a prospective study of hematopoietic stem cell transplant patients, universal masking caused infection rates to drop from 10.3% to 4.4%.6
The IDSA guideline states the following: “There may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCPs [health care providers] and visitors.”1
The CDC’s guidance statement says the following: “Continued community transmission has increased the number of individuals potentially exposed to and infectious with SARS-CoV-2. Fever and symptom screening have proven to be relatively ineffective in identifying all infected individuals, including HCPs. Symptom screening also will not identify individuals who are infected but otherwise asymptomatic or pre-symptomatic; additional interventions are needed to limit the unrecognized introduction of SARS-CoV-2 into healthcare settings by these individuals. As part of aggressive source control measures, healthcare facilities should consider implementing policies requiring everyone entering the facility to wear a cloth face covering (if tolerated) while in the building, regardless of symptoms.”7
It is our opinion, based on the CDC and IDSA recommendations, that both clinicians and patients should be required to wear masks when patients are seen in the office if possible. Many offices have instituted a policy that says, if a patient refuses to wear a mask during an office visit, then the patient will not be seen.
Eye protection
Many clinicians are uncertain about whether eye protection needs to be used when seeing asymptomatic patients. The IDSA acknowledges that there are not studies that have looked critically at eye protection, but the society also acknowledges “appropriate personal protective equipment includes, in addition to a mask or respirator, eye protection, gown and gloves.”1 In addition, the CDC recommends that, for healthcare workers located in areas with moderate or higher prevalence of COVID-19, HCPs should wear eye protection in addition to facemasks since they may encounter asymptomatic individuals with COVID-19.
Gowns and gloves
Gowns and gloves are recommended as a part of personal protective gear when caring for patients who have COVID-19. The IDSA guideline is clear in its recommendations, but does not cite evidence for having no gloves versus having gloves. Furthermore, they state that the evidence is insufficient to recommend double gloves, with the top glove used to take off a personal protective gown, and the inner glove discarded after the gown is removed. The CDC do not make recommendations for routine use of gloves in the care of patients who do not have COVID-19, even in areas where there may be asymptomatic COVID-19, and recommends standard precautions, specifically practicing hand hygiene before and after patient contact.8
The Bottom Line
When seeing patients with COVID-19, N-95 masks, goggles or face shields, gowns, and gloves should be used, with hand hygiene routinely practiced before and after seeing patients. For offices seeing patients not suspected of having COVID-19, the IDSA guideline clarifies that there is not a statistical difference in acquisition of infection with the use of surgical face masks vs N95 respirators. According to the CDC recommendations, eye protection in addition to facemasks should be used by the health care provider, and masks should be worn by patients. Hand hygiene should be used routinely before and after all patient contact. With use of these approaches, it should be safe for offices to reopen and see patients.
Neil Skolnik, MD, is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Jeffrey Matthews, DO, is a second-year resident in the Family Medicine Residency at Abington Jefferson Health. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Lynch JB, Davitkov P, Anderson DJ, et al. COVID-19 Guideline, Part 2: Infection Prevention. IDSA Home. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/. April 27, 2020. Accessed June 10, 2020.
2. J Hosp Infect. 2020 May;105(1):104-5.
3. Lancet. 2003;361(9368):1519-20.
4. Influenza Other Respir Viruses. 2020 Apr 4. doi: 2020;10.1111/irv.12745.
5. J Hosp Infect. 2010;74(3):271-7.
6. Clin Infect Dis. 2016;63(8):999-1006.
7. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed Jun 16, 2020.
8. Centers for Disease Control and Prevention. Healthcare Infection Prevention and Control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html. Accessed June 15, 2020.
As we reopen our offices we are faced with the challenge of determining the best way to do it safely – protecting ourselves, our staff, and our patients.
In this column we will focus on selected details of the recommendations from IDSA and the CDC that may be helpful in primary care offices.
Face masks
Many clinicians have asked whether a physician should use a mask while seeing patients without COVID-19 in the office, and if yes, which type. The IDSA guideline states that mask usage is imperative for reducing the risk of health care workers contracting COVID-19.1 The evidence is derived from a number of sources, including a retrospective study from Wuhan (China) University that examined two groups of health care workers during the outbreak. The first group wore N95 masks and washed their hands frequently, while the second group did not wear masks and washed their hands less frequently. In the group that took greater actions to protect themselves, none of the 493 staff members contracted COVID-19, compared with 10 of 213 staff members in the other group. The decrease in infection rate occurred in the group that wore masks despite the fact that this group had 733% more exposure to COVID-19 patients.2 Further evidence came from a case-control study done in hospitals in Hong Kong during the 2003 SARS-CoV outbreak.3 This study showed that mask wearing was the most significant intervention for reducing infection, followed by gowning, and then handwashing. These findings make it clear that mask usage is a must for all health care providers who may be caring for patients who could have COVID-19.
The guideline also reviews evidence about the use of surgical masks versus N95 masks. On reviewing indirect evidence from the SARS-CoV epidemic, IDSA found that wearing any mask – surgical or N95 – led to a large reduction in the risk of developing an infection. In this systematic review of five observational studies in health care personnel, for those wearing surgical masks, the odds ratio for developing an infection was 0.13 (95% CI, 0.03-0.62), and for those wearing N95 masks, the odds ratio was 0.12 (95% CI, 0.06-0.26). There was not a significant difference between risk reductions for those who wore surgical masks and N95 masks, respectively.1,4 The IDSA guideline panel recommended “that health care personnel caring for patients with suspected or known COVID-19 use either a surgical mask or N95 respirator ... as part of appropriate PPE.” Since there is not a significant difference in outcomes between those who use surgical masks and those who use N95 respirators, and the IDSA guideline states either type of mask is considered appropriate when taking care of patients with suspected or known COVID-19, in our opinion, use of surgical masks rather than N95s is sufficient when performing low-risk activities. Such activities include seeing patients who do not have a high likelihood of COVID-19 in the office setting.
The IDSA recommendation also discusses universal masking, defined as both patients and clinicians wearing masks. The recommendation is supported by the findings of a study in which universal mask usage was used to prevent the spread of H1N 1 during the 2009 outbreak. In this study of staff members and patients exposed to H1N1 who all wore masks, only 0.48% of 836 acquired infection. In the same study, not wearing a mask by either the provider or patient increased the risk of infection.5 Also, in a prospective study of hematopoietic stem cell transplant patients, universal masking caused infection rates to drop from 10.3% to 4.4%.6
The IDSA guideline states the following: “There may be some, albeit uncertain, benefit to universal masking in the absence of resource constraints. However, the benefits of universal masking with surgical masks should be weighed against the risk of increasing the PPE burn rate and contextualized to the background COVID-19 prevalence rate for asymptomatic or minimally symptomatic HCPs [health care providers] and visitors.”1
The CDC’s guidance statement says the following: “Continued community transmission has increased the number of individuals potentially exposed to and infectious with SARS-CoV-2. Fever and symptom screening have proven to be relatively ineffective in identifying all infected individuals, including HCPs. Symptom screening also will not identify individuals who are infected but otherwise asymptomatic or pre-symptomatic; additional interventions are needed to limit the unrecognized introduction of SARS-CoV-2 into healthcare settings by these individuals. As part of aggressive source control measures, healthcare facilities should consider implementing policies requiring everyone entering the facility to wear a cloth face covering (if tolerated) while in the building, regardless of symptoms.”7
It is our opinion, based on the CDC and IDSA recommendations, that both clinicians and patients should be required to wear masks when patients are seen in the office if possible. Many offices have instituted a policy that says, if a patient refuses to wear a mask during an office visit, then the patient will not be seen.
Eye protection
Many clinicians are uncertain about whether eye protection needs to be used when seeing asymptomatic patients. The IDSA acknowledges that there are not studies that have looked critically at eye protection, but the society also acknowledges “appropriate personal protective equipment includes, in addition to a mask or respirator, eye protection, gown and gloves.”1 In addition, the CDC recommends that, for healthcare workers located in areas with moderate or higher prevalence of COVID-19, HCPs should wear eye protection in addition to facemasks since they may encounter asymptomatic individuals with COVID-19.
Gowns and gloves
Gowns and gloves are recommended as a part of personal protective gear when caring for patients who have COVID-19. The IDSA guideline is clear in its recommendations, but does not cite evidence for having no gloves versus having gloves. Furthermore, they state that the evidence is insufficient to recommend double gloves, with the top glove used to take off a personal protective gown, and the inner glove discarded after the gown is removed. The CDC do not make recommendations for routine use of gloves in the care of patients who do not have COVID-19, even in areas where there may be asymptomatic COVID-19, and recommends standard precautions, specifically practicing hand hygiene before and after patient contact.8
The Bottom Line
When seeing patients with COVID-19, N-95 masks, goggles or face shields, gowns, and gloves should be used, with hand hygiene routinely practiced before and after seeing patients. For offices seeing patients not suspected of having COVID-19, the IDSA guideline clarifies that there is not a statistical difference in acquisition of infection with the use of surgical face masks vs N95 respirators. According to the CDC recommendations, eye protection in addition to facemasks should be used by the health care provider, and masks should be worn by patients. Hand hygiene should be used routinely before and after all patient contact. With use of these approaches, it should be safe for offices to reopen and see patients.
Neil Skolnik, MD, is professor of family and community medicine at the Thomas Jefferson University, Philadelphia, and associate director of the Family Medicine Residency Program at Abington (Pa.) Jefferson Health. Jeffrey Matthews, DO, is a second-year resident in the Family Medicine Residency at Abington Jefferson Health. For questions or comments, feel free to contact Dr. Skolnik on Twitter @NeilSkolnik.
References
1. Lynch JB, Davitkov P, Anderson DJ, et al. COVID-19 Guideline, Part 2: Infection Prevention. IDSA Home. https://www.idsociety.org/practice-guideline/covid-19-guideline-infection-prevention/. April 27, 2020. Accessed June 10, 2020.
2. J Hosp Infect. 2020 May;105(1):104-5.
3. Lancet. 2003;361(9368):1519-20.
4. Influenza Other Respir Viruses. 2020 Apr 4. doi: 2020;10.1111/irv.12745.
5. J Hosp Infect. 2010;74(3):271-7.
6. Clin Infect Dis. 2016;63(8):999-1006.
7. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed Jun 16, 2020.
8. Centers for Disease Control and Prevention. Healthcare Infection Prevention and Control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-faq.html. Accessed June 15, 2020.
















