User login
Surgical Comanagement for Hip Fracture: Time for a Randomized Trial
The growth in the hospitalist workforce has been one of the major trends shaping US (and international) inpatient medicine over the last 25 years.1 Hospitalists’ clinical work is typically split among serving as the primary attending for admitted patients (termed “most responsible physician,” or MRP, in Canada), outpatient clinics, medical consults, and comanagement.2,3 Comanagement typically involves the cooperative efforts of hospitalists and subspecialists ranging from general surgery to orthopedics to medical oncology. Comanagement differs from typical medical consultation because comanaging hospitalists are commonly given broad discretion to directly write orders, manage intercurrent medical illness (eg, hyperglycemia), and even discharge patients from the hospital when appropriate. There can be significant heterogeneity in how comanagement is implemented across institutions.4
With respect to hip fractures, literature suggests that subspecialists value comanagement and that comanagement is associated with reductions in hospital length of stay, timelier surgical repair, and potential cost savings for hospitals.5-7 Some studies have found reductions in in-hospital and 1-year mortality (including one meta-analysis on ortho-geriatric comanagement)8 and complications,9 but others have found no such benefits.10,11
In the current issue of the Journal of Hospital Medicine, Maxwell and Mirza used data from the National Surgical Quality Improvement Program (NSQIP) Participant Use Data File (PUF)—specifically, from the Hip Fracture PUF—to investigate the relationship between comanagement and mortality and major morbidity among more than 15,000 patients hospitalized with hip fracture.12 The investigators did not find that comanagement was associated with a reduction in either morbidity or mortality.
Several factors give gravitas to their analysis. First, the NSQIP PUF is an extremely rigorous data source for evaluating surgical outcomes. Originally developed in the US Veterans Health Administration in the 1980’s to standardize data elements needed for quality improvement and hospital benchmarking, today NSQIP involves more than 600 hospitals in 9 different countries submitting hundreds of thousands of cases annually.13 Second, the authors recognized that the comanagement and noncomanagement groups differed substantially and used propensity score matching in an effort to account for these differences. Surprisingly, they found that the comanagement had significantly higher mortality and morbidity than the noncomanagement group, even after propensity score matching.
These results are important in testing the assumption of the inherent “good” of comanagement. Does this study provide definitive evidence that surgical comanagement does not improve outcomes? We would suggest that this study be interpreted in light of certain considerations.
First, comanagement is a broad term including a variety of operationalizations, such as geriatrician vs hospitalist comanagement, involvement before vs after surgery, and varying divisions of responsibility between the surgical and medical services. Research indicates that successful comanagement models tend to incorporate multidisciplinary teams, embrace the “dual primary caregiver” nature of comanagement, and shared goals among primary caregivers, specifically anticipating prevention of complications.5 The NSQIP data do not provide sufficient granularity to allow for investigation of these crucial nuances that may ultimately determine whether comanagement programs are effective. Additionally, comanagement often (but not always) coexists with a care pathway, and so deficiencies in or absence of a care pathway add additional heterogeneity to the comanagement group which is not captured in the NSQIP PUF.
Second, it is important to consider the potential for unmeasured confounding. The propensity score matching did seem to achieve balance in the distribution of most baseline variables between the comanagement and noncomanagement groups, though differences remain for certain covariates. A key assumption in propensity score matching (and in observational research more broadly) is the principle of “no unmeasured confounders” (ie, the assumption that all variables that might influence treatment assignment and outcomes are measured).14 For the NSQIP PUF this absence of unmeasured confounders is clearly not the case because hospital and surgeon variables are omitted from the PUF for reasons of confidentiality. Inclusion of hospital and surgeon variables could well be important because outcomes may vary by hospital or by surgeon, and simultaneously, different hospitals and different surgeons will have different protocols and preferences regarding comanagement. Furthermore, confounding is virtually guaranteed to the extent that hospitals and surgeons do not randomly assign hip fracture patients to comanagement or usual care. The finding of higher mortality in the comanagement group, even after adjustment and matching, suggests the presence of residual confounding. Even if residual confounding is the explanation for the worse outcomes observed in the comanagement group, the finding of a lack of benefit of comanagement is noteworthy and should not be dismissed out of hand.
Limitations aside, these results suggest a need for humility among strong proponents of comanagement, at least in the hip fracture population. While it may still be reasonable to claim that comanagement improves efficiency and may enhance certain aspects of patient or physician satisfaction, the lack of an impact on mortality highlights a need to examine the benefits of these programs more carefully. From a clinical perspective, hospitalists and orthopedic surgeons should consider which hip fracture patients might be most likely to benefit from comanagement.4 From a research perspective, the current study highlights the pressing need for a randomized trial of comanagement to definitively address the effectiveness of these programs.
1. Wachter RM, Goldman L. Zero to 50,000 — the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
3. Soong C, Eddy Fan, Eric E Howell, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag . 2009;16(2):69
4. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
5. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: an economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4
6. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
7. Soong C, Cram P, Chezar K, et al. Impact of an integrated hip fracture inpatient program on length of stay and costs. J Orthop Trauma. 2016;30(12):647-652. https://doi.org/10.1097/BOT.0000000000000691
8. Grigoryan KV, Javedan H, Rudolph JL. Ortho-geriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49-e55. https://doi.org/10.1097/BOT.0b013e3182a5a045
9. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x
10. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
11. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. http://doi.org/10.1001/archinte.167.17.1869
12. Maxwell B, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score matched retrospective cohort analysis of the national surgical quality improvement project. J Hosp Med. 2020;15:468-474. http://doi.org/10.12788/jhm.3343
13. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336–46.e1. https://doi.org/10.1016/j.jamcollsurg.2013.02.027
14. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786
The growth in the hospitalist workforce has been one of the major trends shaping US (and international) inpatient medicine over the last 25 years.1 Hospitalists’ clinical work is typically split among serving as the primary attending for admitted patients (termed “most responsible physician,” or MRP, in Canada), outpatient clinics, medical consults, and comanagement.2,3 Comanagement typically involves the cooperative efforts of hospitalists and subspecialists ranging from general surgery to orthopedics to medical oncology. Comanagement differs from typical medical consultation because comanaging hospitalists are commonly given broad discretion to directly write orders, manage intercurrent medical illness (eg, hyperglycemia), and even discharge patients from the hospital when appropriate. There can be significant heterogeneity in how comanagement is implemented across institutions.4
With respect to hip fractures, literature suggests that subspecialists value comanagement and that comanagement is associated with reductions in hospital length of stay, timelier surgical repair, and potential cost savings for hospitals.5-7 Some studies have found reductions in in-hospital and 1-year mortality (including one meta-analysis on ortho-geriatric comanagement)8 and complications,9 but others have found no such benefits.10,11
In the current issue of the Journal of Hospital Medicine, Maxwell and Mirza used data from the National Surgical Quality Improvement Program (NSQIP) Participant Use Data File (PUF)—specifically, from the Hip Fracture PUF—to investigate the relationship between comanagement and mortality and major morbidity among more than 15,000 patients hospitalized with hip fracture.12 The investigators did not find that comanagement was associated with a reduction in either morbidity or mortality.
Several factors give gravitas to their analysis. First, the NSQIP PUF is an extremely rigorous data source for evaluating surgical outcomes. Originally developed in the US Veterans Health Administration in the 1980’s to standardize data elements needed for quality improvement and hospital benchmarking, today NSQIP involves more than 600 hospitals in 9 different countries submitting hundreds of thousands of cases annually.13 Second, the authors recognized that the comanagement and noncomanagement groups differed substantially and used propensity score matching in an effort to account for these differences. Surprisingly, they found that the comanagement had significantly higher mortality and morbidity than the noncomanagement group, even after propensity score matching.
These results are important in testing the assumption of the inherent “good” of comanagement. Does this study provide definitive evidence that surgical comanagement does not improve outcomes? We would suggest that this study be interpreted in light of certain considerations.
First, comanagement is a broad term including a variety of operationalizations, such as geriatrician vs hospitalist comanagement, involvement before vs after surgery, and varying divisions of responsibility between the surgical and medical services. Research indicates that successful comanagement models tend to incorporate multidisciplinary teams, embrace the “dual primary caregiver” nature of comanagement, and shared goals among primary caregivers, specifically anticipating prevention of complications.5 The NSQIP data do not provide sufficient granularity to allow for investigation of these crucial nuances that may ultimately determine whether comanagement programs are effective. Additionally, comanagement often (but not always) coexists with a care pathway, and so deficiencies in or absence of a care pathway add additional heterogeneity to the comanagement group which is not captured in the NSQIP PUF.
Second, it is important to consider the potential for unmeasured confounding. The propensity score matching did seem to achieve balance in the distribution of most baseline variables between the comanagement and noncomanagement groups, though differences remain for certain covariates. A key assumption in propensity score matching (and in observational research more broadly) is the principle of “no unmeasured confounders” (ie, the assumption that all variables that might influence treatment assignment and outcomes are measured).14 For the NSQIP PUF this absence of unmeasured confounders is clearly not the case because hospital and surgeon variables are omitted from the PUF for reasons of confidentiality. Inclusion of hospital and surgeon variables could well be important because outcomes may vary by hospital or by surgeon, and simultaneously, different hospitals and different surgeons will have different protocols and preferences regarding comanagement. Furthermore, confounding is virtually guaranteed to the extent that hospitals and surgeons do not randomly assign hip fracture patients to comanagement or usual care. The finding of higher mortality in the comanagement group, even after adjustment and matching, suggests the presence of residual confounding. Even if residual confounding is the explanation for the worse outcomes observed in the comanagement group, the finding of a lack of benefit of comanagement is noteworthy and should not be dismissed out of hand.
Limitations aside, these results suggest a need for humility among strong proponents of comanagement, at least in the hip fracture population. While it may still be reasonable to claim that comanagement improves efficiency and may enhance certain aspects of patient or physician satisfaction, the lack of an impact on mortality highlights a need to examine the benefits of these programs more carefully. From a clinical perspective, hospitalists and orthopedic surgeons should consider which hip fracture patients might be most likely to benefit from comanagement.4 From a research perspective, the current study highlights the pressing need for a randomized trial of comanagement to definitively address the effectiveness of these programs.
The growth in the hospitalist workforce has been one of the major trends shaping US (and international) inpatient medicine over the last 25 years.1 Hospitalists’ clinical work is typically split among serving as the primary attending for admitted patients (termed “most responsible physician,” or MRP, in Canada), outpatient clinics, medical consults, and comanagement.2,3 Comanagement typically involves the cooperative efforts of hospitalists and subspecialists ranging from general surgery to orthopedics to medical oncology. Comanagement differs from typical medical consultation because comanaging hospitalists are commonly given broad discretion to directly write orders, manage intercurrent medical illness (eg, hyperglycemia), and even discharge patients from the hospital when appropriate. There can be significant heterogeneity in how comanagement is implemented across institutions.4
With respect to hip fractures, literature suggests that subspecialists value comanagement and that comanagement is associated with reductions in hospital length of stay, timelier surgical repair, and potential cost savings for hospitals.5-7 Some studies have found reductions in in-hospital and 1-year mortality (including one meta-analysis on ortho-geriatric comanagement)8 and complications,9 but others have found no such benefits.10,11
In the current issue of the Journal of Hospital Medicine, Maxwell and Mirza used data from the National Surgical Quality Improvement Program (NSQIP) Participant Use Data File (PUF)—specifically, from the Hip Fracture PUF—to investigate the relationship between comanagement and mortality and major morbidity among more than 15,000 patients hospitalized with hip fracture.12 The investigators did not find that comanagement was associated with a reduction in either morbidity or mortality.
Several factors give gravitas to their analysis. First, the NSQIP PUF is an extremely rigorous data source for evaluating surgical outcomes. Originally developed in the US Veterans Health Administration in the 1980’s to standardize data elements needed for quality improvement and hospital benchmarking, today NSQIP involves more than 600 hospitals in 9 different countries submitting hundreds of thousands of cases annually.13 Second, the authors recognized that the comanagement and noncomanagement groups differed substantially and used propensity score matching in an effort to account for these differences. Surprisingly, they found that the comanagement had significantly higher mortality and morbidity than the noncomanagement group, even after propensity score matching.
These results are important in testing the assumption of the inherent “good” of comanagement. Does this study provide definitive evidence that surgical comanagement does not improve outcomes? We would suggest that this study be interpreted in light of certain considerations.
First, comanagement is a broad term including a variety of operationalizations, such as geriatrician vs hospitalist comanagement, involvement before vs after surgery, and varying divisions of responsibility between the surgical and medical services. Research indicates that successful comanagement models tend to incorporate multidisciplinary teams, embrace the “dual primary caregiver” nature of comanagement, and shared goals among primary caregivers, specifically anticipating prevention of complications.5 The NSQIP data do not provide sufficient granularity to allow for investigation of these crucial nuances that may ultimately determine whether comanagement programs are effective. Additionally, comanagement often (but not always) coexists with a care pathway, and so deficiencies in or absence of a care pathway add additional heterogeneity to the comanagement group which is not captured in the NSQIP PUF.
Second, it is important to consider the potential for unmeasured confounding. The propensity score matching did seem to achieve balance in the distribution of most baseline variables between the comanagement and noncomanagement groups, though differences remain for certain covariates. A key assumption in propensity score matching (and in observational research more broadly) is the principle of “no unmeasured confounders” (ie, the assumption that all variables that might influence treatment assignment and outcomes are measured).14 For the NSQIP PUF this absence of unmeasured confounders is clearly not the case because hospital and surgeon variables are omitted from the PUF for reasons of confidentiality. Inclusion of hospital and surgeon variables could well be important because outcomes may vary by hospital or by surgeon, and simultaneously, different hospitals and different surgeons will have different protocols and preferences regarding comanagement. Furthermore, confounding is virtually guaranteed to the extent that hospitals and surgeons do not randomly assign hip fracture patients to comanagement or usual care. The finding of higher mortality in the comanagement group, even after adjustment and matching, suggests the presence of residual confounding. Even if residual confounding is the explanation for the worse outcomes observed in the comanagement group, the finding of a lack of benefit of comanagement is noteworthy and should not be dismissed out of hand.
Limitations aside, these results suggest a need for humility among strong proponents of comanagement, at least in the hip fracture population. While it may still be reasonable to claim that comanagement improves efficiency and may enhance certain aspects of patient or physician satisfaction, the lack of an impact on mortality highlights a need to examine the benefits of these programs more carefully. From a clinical perspective, hospitalists and orthopedic surgeons should consider which hip fracture patients might be most likely to benefit from comanagement.4 From a research perspective, the current study highlights the pressing need for a randomized trial of comanagement to definitively address the effectiveness of these programs.
1. Wachter RM, Goldman L. Zero to 50,000 — the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
3. Soong C, Eddy Fan, Eric E Howell, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag . 2009;16(2):69
4. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
5. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: an economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4
6. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
7. Soong C, Cram P, Chezar K, et al. Impact of an integrated hip fracture inpatient program on length of stay and costs. J Orthop Trauma. 2016;30(12):647-652. https://doi.org/10.1097/BOT.0000000000000691
8. Grigoryan KV, Javedan H, Rudolph JL. Ortho-geriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49-e55. https://doi.org/10.1097/BOT.0b013e3182a5a045
9. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x
10. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
11. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. http://doi.org/10.1001/archinte.167.17.1869
12. Maxwell B, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score matched retrospective cohort analysis of the national surgical quality improvement project. J Hosp Med. 2020;15:468-474. http://doi.org/10.12788/jhm.3343
13. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336–46.e1. https://doi.org/10.1016/j.jamcollsurg.2013.02.027
14. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786
1. Wachter RM, Goldman L. Zero to 50,000 — the 20th anniversary of the hospitalist. N Engl J Med. 2016;375(11):1009-1011. https://doi.org/10.1056/NEJMp1607958
2. Hinami K, Whelan CT, Miller JA, Wolosin RJ, Wetterneck TB; Society of Hospital Medicine Career Satisfaction Task Force. Job characteristics, satisfaction, and burnout across hospitalist practice models. J Hosp Med. 2012;7(5):402-410. https://doi.org/10.1002/jhm.1907
3. Soong C, Eddy Fan, Eric E Howell, et al. Characteristics of hospitalists and hospitalist programs in the United States and Canada. J Clin Outcomes Manag . 2009;16(2):69
4. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. https://doi.org/10.1002/jhm.361
5. Swart E, Vasudeva E, Makhni EC, Macaulay W, Bozic KJ. Dedicated perioperative hip fracture comanagement programs are cost-effective in high-volume centers: an economic analysis. Clin Orthop Relat Res. 2016;474(1):222-233. https://doi.org/10.1007/s11999-015-4494-4
6. Bracey DN, Kiymaz TC, Holst DC, et al. An orthopedic-hospitalist comanaged hip fracture service reduces inpatient length of stay. Geriatr Orthop Surg Rehabil. 2016;7(4):171-177. https://doi.org/10.1177/2151458516661383.
7. Soong C, Cram P, Chezar K, et al. Impact of an integrated hip fracture inpatient program on length of stay and costs. J Orthop Trauma. 2016;30(12):647-652. https://doi.org/10.1097/BOT.0000000000000691
8. Grigoryan KV, Javedan H, Rudolph JL. Ortho-geriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49-e55. https://doi.org/10.1097/BOT.0b013e3182a5a045
9. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x
10. Gregersen M, Mørch MM, Hougaard K, Damsgaard EM. Geriatric intervention in elderly patients with hip fracture in an orthopedic ward. J Inj Violence Res. 2012;4(2):45-51. https://doi.org/10.5249/jivr.v4i2.96
11. Southern WN, Berger MA, Bellin EY, Hailpern SM, Arnsten JH. Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring. Arch Intern Med. 2007;167(17):1869-1874. http://doi.org/10.1001/archinte.167.17.1869
12. Maxwell B, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score matched retrospective cohort analysis of the national surgical quality improvement project. J Hosp Med. 2020;15:468-474. http://doi.org/10.12788/jhm.3343
13. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336–46.e1. https://doi.org/10.1016/j.jamcollsurg.2013.02.027
14. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. https://doi.org/10.1080/00273171.2011.568786
© 2020 Society of Hospital Medicine
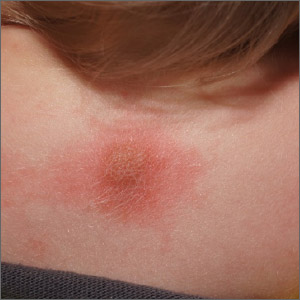
Fixed scaly lesion
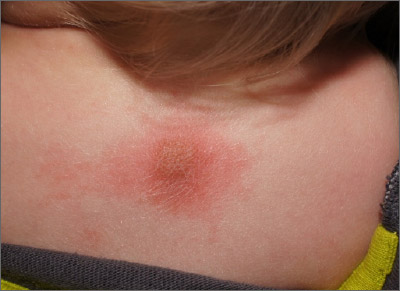
The patient’s history of a recurrent wheal was consistent with a diagnosis of mastocytoma, the most common and least concerning form of cutaneous mastocytosis. Mastocytomas commonly appear in infants as 1 to 3 firm 1- to 5-cm papules (or a plaque) caused by a histamine release from a group of mast cells with abnormal growth receptors. When flaring, the surface may have a prominent orange peel texture because of tethered adnexal structures. When uninflamed, the skin surface may be slightly raised and flesh-colored to pink.
When first noticed, mastocytomas are easily mistaken for insect bites or congenital nevi. However, mastocytomas don’t resolve completely, as would an insect bite, and they become recurrently inflamed (spontaneously or with trauma). Inflammation that can be elicited with pressure or scratching is called Darrier sign and is helpful in making the diagnosis and distinguishing these lesions from congenital nevi.
Dermoscopy of a mastocytoma lacks signs of a melanocytic nevi, which further adds to the clinical diagnosis. Blood tests and biopsy are unnecessary, but if a biopsy is performed, it is important to mention the possibility of mast cell disease to the lab so that appropriate immunostaining for mast cells can be carried out.
Mastocytomas that appear in infancy usually resolve spontaneously in early childhood or by puberty, at the latest. If there is any notable itching or discomfort, oral antihistamines are helpful, as are topical steroids and topical tacrolimus. In this case, the diagnosis was made clinically and the patient’s parents were content to observe the area.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46.

The patient’s history of a recurrent wheal was consistent with a diagnosis of mastocytoma, the most common and least concerning form of cutaneous mastocytosis. Mastocytomas commonly appear in infants as 1 to 3 firm 1- to 5-cm papules (or a plaque) caused by a histamine release from a group of mast cells with abnormal growth receptors. When flaring, the surface may have a prominent orange peel texture because of tethered adnexal structures. When uninflamed, the skin surface may be slightly raised and flesh-colored to pink.
When first noticed, mastocytomas are easily mistaken for insect bites or congenital nevi. However, mastocytomas don’t resolve completely, as would an insect bite, and they become recurrently inflamed (spontaneously or with trauma). Inflammation that can be elicited with pressure or scratching is called Darrier sign and is helpful in making the diagnosis and distinguishing these lesions from congenital nevi.
Dermoscopy of a mastocytoma lacks signs of a melanocytic nevi, which further adds to the clinical diagnosis. Blood tests and biopsy are unnecessary, but if a biopsy is performed, it is important to mention the possibility of mast cell disease to the lab so that appropriate immunostaining for mast cells can be carried out.
Mastocytomas that appear in infancy usually resolve spontaneously in early childhood or by puberty, at the latest. If there is any notable itching or discomfort, oral antihistamines are helpful, as are topical steroids and topical tacrolimus. In this case, the diagnosis was made clinically and the patient’s parents were content to observe the area.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

The patient’s history of a recurrent wheal was consistent with a diagnosis of mastocytoma, the most common and least concerning form of cutaneous mastocytosis. Mastocytomas commonly appear in infants as 1 to 3 firm 1- to 5-cm papules (or a plaque) caused by a histamine release from a group of mast cells with abnormal growth receptors. When flaring, the surface may have a prominent orange peel texture because of tethered adnexal structures. When uninflamed, the skin surface may be slightly raised and flesh-colored to pink.
When first noticed, mastocytomas are easily mistaken for insect bites or congenital nevi. However, mastocytomas don’t resolve completely, as would an insect bite, and they become recurrently inflamed (spontaneously or with trauma). Inflammation that can be elicited with pressure or scratching is called Darrier sign and is helpful in making the diagnosis and distinguishing these lesions from congenital nevi.
Dermoscopy of a mastocytoma lacks signs of a melanocytic nevi, which further adds to the clinical diagnosis. Blood tests and biopsy are unnecessary, but if a biopsy is performed, it is important to mention the possibility of mast cell disease to the lab so that appropriate immunostaining for mast cells can be carried out.
Mastocytomas that appear in infancy usually resolve spontaneously in early childhood or by puberty, at the latest. If there is any notable itching or discomfort, oral antihistamines are helpful, as are topical steroids and topical tacrolimus. In this case, the diagnosis was made clinically and the patient’s parents were content to observe the area.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46.
Leung AKC, Lam JM, Leong KF. Childhood solitary cutaneous mastocytoma: clinical manifestations, diagnosis, evaluation, and management. Curr Pediatr Rev. 2019;15:42-46.
Applications for the CUTIS 2021 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears ([email protected]) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
Survey: Most FPs live at or below their means
a Medscape survey has found.
According to the Medscape Family Physician Debt and Net Worth Report 2020, almost half of FPs (46%) reported having that amount as their net worth, compared with the 18% of gastroenterologists and 19% of urologists who fell into that category.
And whereas 19% of orthopedists reported at least $5 million in net worth, only 3% of FPs did.
A third are paying off student loans
FPs were also more likely, along with physical medicine and rehabilitation physicians, at 34%, to report that they are continuing to pay off student loans. Conversely, 14% of gastroenterologists and 15% of nephrologists and rheumatologists said they were still paying off the loans.
Student loan debt was third on the list for FPs. Two-thirds of FPs were paying off a mortgage, and 41% had car loan payments.
Overall, FPs appear to manage their finances well and are living within their means. Only 6% of FPs said they live above their means, whereas 51% said they live at their means, and 42% said they live below that threshold.
Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minn., said in an interview he recommends saving 20% of gross salary each year.
The survey was completed before Feb. 11 and before the financial effects of the COVID-19 pandemic could be known. The report is based on responses from more than 17,000 physicians across 30 specialties.
A lower level of net worth among FPs corresponds with their being close to the bottom among physicians in compensation. They made $234,000 on average, according to the report. By contrast, orthopedists made more than twice as much, at $511,000.
Smaller homes, less mortgage debt
FPs were among the least likely to indicate that they had a home of more than 5,000 square feet. That was true for only 6% of FPs; it was true for 22% of plastic surgeons and orthopedists. Most (61%) lived in dwellings of 3,000 square feet or less.
At the same time, FPs reported smaller mortgages than many of their colleagues.
Nearly half (49%) of FPs have mortgages of $300,000 or less; 26% have no mortgage at all. That figure was much higher than the 37% of physicians overall who had mortgages of $300,000 or less, although almost the same percentage had no mortgage at all.
Most had no financial loss in the past year
In further good news, most FPs (70%) said they did not experience a financial loss in the past year. Of those who did experience a loss, the top reasons were problems with their practice, such as reimbursement changes or changes in practice situations, or bad investments.
FPs socked away more into tax-deferred than taxable accounts, the survey showed.
More than half (54%) of FPs put at least $1,000 into tax-deferred accounts, such as college savings or retirement accounts, although 14% said they do not regularly contribute to such accounts.
Fewer (29%) contributed at least $1,000 to a taxable account.
As for who pays the day-to-day bills in households, 56% of FPs said they pool resources with a spouse or partner and pay bills from a common fund. Only 4% split the bills equally, no matter the income difference. One in four said they do not have joint finances with a spouse or partner.
FPs were divided as to whether they are currently working with a financial planner (38%) or had not worked with one (37%); the remainder said they had met with or used one in the past.
A version of this article originally appeared on Medscape.com.
a Medscape survey has found.
According to the Medscape Family Physician Debt and Net Worth Report 2020, almost half of FPs (46%) reported having that amount as their net worth, compared with the 18% of gastroenterologists and 19% of urologists who fell into that category.
And whereas 19% of orthopedists reported at least $5 million in net worth, only 3% of FPs did.
A third are paying off student loans
FPs were also more likely, along with physical medicine and rehabilitation physicians, at 34%, to report that they are continuing to pay off student loans. Conversely, 14% of gastroenterologists and 15% of nephrologists and rheumatologists said they were still paying off the loans.
Student loan debt was third on the list for FPs. Two-thirds of FPs were paying off a mortgage, and 41% had car loan payments.
Overall, FPs appear to manage their finances well and are living within their means. Only 6% of FPs said they live above their means, whereas 51% said they live at their means, and 42% said they live below that threshold.
Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minn., said in an interview he recommends saving 20% of gross salary each year.
The survey was completed before Feb. 11 and before the financial effects of the COVID-19 pandemic could be known. The report is based on responses from more than 17,000 physicians across 30 specialties.
A lower level of net worth among FPs corresponds with their being close to the bottom among physicians in compensation. They made $234,000 on average, according to the report. By contrast, orthopedists made more than twice as much, at $511,000.
Smaller homes, less mortgage debt
FPs were among the least likely to indicate that they had a home of more than 5,000 square feet. That was true for only 6% of FPs; it was true for 22% of plastic surgeons and orthopedists. Most (61%) lived in dwellings of 3,000 square feet or less.
At the same time, FPs reported smaller mortgages than many of their colleagues.
Nearly half (49%) of FPs have mortgages of $300,000 or less; 26% have no mortgage at all. That figure was much higher than the 37% of physicians overall who had mortgages of $300,000 or less, although almost the same percentage had no mortgage at all.
Most had no financial loss in the past year
In further good news, most FPs (70%) said they did not experience a financial loss in the past year. Of those who did experience a loss, the top reasons were problems with their practice, such as reimbursement changes or changes in practice situations, or bad investments.
FPs socked away more into tax-deferred than taxable accounts, the survey showed.
More than half (54%) of FPs put at least $1,000 into tax-deferred accounts, such as college savings or retirement accounts, although 14% said they do not regularly contribute to such accounts.
Fewer (29%) contributed at least $1,000 to a taxable account.
As for who pays the day-to-day bills in households, 56% of FPs said they pool resources with a spouse or partner and pay bills from a common fund. Only 4% split the bills equally, no matter the income difference. One in four said they do not have joint finances with a spouse or partner.
FPs were divided as to whether they are currently working with a financial planner (38%) or had not worked with one (37%); the remainder said they had met with or used one in the past.
A version of this article originally appeared on Medscape.com.
a Medscape survey has found.
According to the Medscape Family Physician Debt and Net Worth Report 2020, almost half of FPs (46%) reported having that amount as their net worth, compared with the 18% of gastroenterologists and 19% of urologists who fell into that category.
And whereas 19% of orthopedists reported at least $5 million in net worth, only 3% of FPs did.
A third are paying off student loans
FPs were also more likely, along with physical medicine and rehabilitation physicians, at 34%, to report that they are continuing to pay off student loans. Conversely, 14% of gastroenterologists and 15% of nephrologists and rheumatologists said they were still paying off the loans.
Student loan debt was third on the list for FPs. Two-thirds of FPs were paying off a mortgage, and 41% had car loan payments.
Overall, FPs appear to manage their finances well and are living within their means. Only 6% of FPs said they live above their means, whereas 51% said they live at their means, and 42% said they live below that threshold.
Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minn., said in an interview he recommends saving 20% of gross salary each year.
The survey was completed before Feb. 11 and before the financial effects of the COVID-19 pandemic could be known. The report is based on responses from more than 17,000 physicians across 30 specialties.
A lower level of net worth among FPs corresponds with their being close to the bottom among physicians in compensation. They made $234,000 on average, according to the report. By contrast, orthopedists made more than twice as much, at $511,000.
Smaller homes, less mortgage debt
FPs were among the least likely to indicate that they had a home of more than 5,000 square feet. That was true for only 6% of FPs; it was true for 22% of plastic surgeons and orthopedists. Most (61%) lived in dwellings of 3,000 square feet or less.
At the same time, FPs reported smaller mortgages than many of their colleagues.
Nearly half (49%) of FPs have mortgages of $300,000 or less; 26% have no mortgage at all. That figure was much higher than the 37% of physicians overall who had mortgages of $300,000 or less, although almost the same percentage had no mortgage at all.
Most had no financial loss in the past year
In further good news, most FPs (70%) said they did not experience a financial loss in the past year. Of those who did experience a loss, the top reasons were problems with their practice, such as reimbursement changes or changes in practice situations, or bad investments.
FPs socked away more into tax-deferred than taxable accounts, the survey showed.
More than half (54%) of FPs put at least $1,000 into tax-deferred accounts, such as college savings or retirement accounts, although 14% said they do not regularly contribute to such accounts.
Fewer (29%) contributed at least $1,000 to a taxable account.
As for who pays the day-to-day bills in households, 56% of FPs said they pool resources with a spouse or partner and pay bills from a common fund. Only 4% split the bills equally, no matter the income difference. One in four said they do not have joint finances with a spouse or partner.
FPs were divided as to whether they are currently working with a financial planner (38%) or had not worked with one (37%); the remainder said they had met with or used one in the past.
A version of this article originally appeared on Medscape.com.
FDA approves low-sodium treatment option for narcolepsy
Xywav is a novel oxybate product with a unique composition of cations, resulting in 92% less sodium than sodium oxybate (Xyrem, Jazz Pharmaceuticals) at the recommended dosage range of 6 to 9 grams, the company said in a news release.
The FDA approved the drug based on a phase 3 trial involving 201 patients who had narcolepsy with cataplexy.
As reported by Medscape Medical News from the World Sleep 2019 meeting, Xywav demonstrated highly statistically significant differences (P < .0001) in weekly number of cataplexy attacks (primary efficacy endpoint) and Epworth Sleepiness Scale scores (key secondary outcome) vs placebo.
“Based on the efficacy demonstrated in the clinical program, the approval of Xywav is important for people living with cataplexy or EDS associated with narcolepsy,” lead investigator Richard K. Bogan, MD, said in the company’s news release.
He noted that the average American consumes too much sodium. “Excess sodium intake has been linked with increases in blood pressure, hypertension, stroke, and other cardiovascular disease,” said Dr. Bogan, associate clinical professor at the University of South Carolina School of Medicine, Columbia.
“Xywav makes it possible for patients to have a lower-sodium oxybate treatment option. This may help patients taking sodium oxybate better align with daily sodium intake recommendations, including those by the American Heart Association,” he added.
The overall safety profile of Xywav is in line with sodium oxybate, the company said. The most common adverse reactions in adults, occurring in at least 5% of participants, were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis (excessive sweating), anxiety, and vomiting.
Xywav has a boxed warning as a CNS depressant and for its potential for abuse and misuse. As a result, the drug is only available through a Risk Evaluation and Mitigation Strategy (REMS) program.
The US Drug Enforcement Agency has designated Xywav as a schedule III drug, meaning it has a moderate to low potential for physical and psychological dependence.
The company plans to launch Xywav by the end of the year. Full prescribing information and a medication guide are available online.
This article first appeared on Medscape.com.
Xywav is a novel oxybate product with a unique composition of cations, resulting in 92% less sodium than sodium oxybate (Xyrem, Jazz Pharmaceuticals) at the recommended dosage range of 6 to 9 grams, the company said in a news release.
The FDA approved the drug based on a phase 3 trial involving 201 patients who had narcolepsy with cataplexy.
As reported by Medscape Medical News from the World Sleep 2019 meeting, Xywav demonstrated highly statistically significant differences (P < .0001) in weekly number of cataplexy attacks (primary efficacy endpoint) and Epworth Sleepiness Scale scores (key secondary outcome) vs placebo.
“Based on the efficacy demonstrated in the clinical program, the approval of Xywav is important for people living with cataplexy or EDS associated with narcolepsy,” lead investigator Richard K. Bogan, MD, said in the company’s news release.
He noted that the average American consumes too much sodium. “Excess sodium intake has been linked with increases in blood pressure, hypertension, stroke, and other cardiovascular disease,” said Dr. Bogan, associate clinical professor at the University of South Carolina School of Medicine, Columbia.
“Xywav makes it possible for patients to have a lower-sodium oxybate treatment option. This may help patients taking sodium oxybate better align with daily sodium intake recommendations, including those by the American Heart Association,” he added.
The overall safety profile of Xywav is in line with sodium oxybate, the company said. The most common adverse reactions in adults, occurring in at least 5% of participants, were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis (excessive sweating), anxiety, and vomiting.
Xywav has a boxed warning as a CNS depressant and for its potential for abuse and misuse. As a result, the drug is only available through a Risk Evaluation and Mitigation Strategy (REMS) program.
The US Drug Enforcement Agency has designated Xywav as a schedule III drug, meaning it has a moderate to low potential for physical and psychological dependence.
The company plans to launch Xywav by the end of the year. Full prescribing information and a medication guide are available online.
This article first appeared on Medscape.com.
Xywav is a novel oxybate product with a unique composition of cations, resulting in 92% less sodium than sodium oxybate (Xyrem, Jazz Pharmaceuticals) at the recommended dosage range of 6 to 9 grams, the company said in a news release.
The FDA approved the drug based on a phase 3 trial involving 201 patients who had narcolepsy with cataplexy.
As reported by Medscape Medical News from the World Sleep 2019 meeting, Xywav demonstrated highly statistically significant differences (P < .0001) in weekly number of cataplexy attacks (primary efficacy endpoint) and Epworth Sleepiness Scale scores (key secondary outcome) vs placebo.
“Based on the efficacy demonstrated in the clinical program, the approval of Xywav is important for people living with cataplexy or EDS associated with narcolepsy,” lead investigator Richard K. Bogan, MD, said in the company’s news release.
He noted that the average American consumes too much sodium. “Excess sodium intake has been linked with increases in blood pressure, hypertension, stroke, and other cardiovascular disease,” said Dr. Bogan, associate clinical professor at the University of South Carolina School of Medicine, Columbia.
“Xywav makes it possible for patients to have a lower-sodium oxybate treatment option. This may help patients taking sodium oxybate better align with daily sodium intake recommendations, including those by the American Heart Association,” he added.
The overall safety profile of Xywav is in line with sodium oxybate, the company said. The most common adverse reactions in adults, occurring in at least 5% of participants, were headache, nausea, dizziness, decreased appetite, parasomnia, diarrhea, hyperhidrosis (excessive sweating), anxiety, and vomiting.
Xywav has a boxed warning as a CNS depressant and for its potential for abuse and misuse. As a result, the drug is only available through a Risk Evaluation and Mitigation Strategy (REMS) program.
The US Drug Enforcement Agency has designated Xywav as a schedule III drug, meaning it has a moderate to low potential for physical and psychological dependence.
The company plans to launch Xywav by the end of the year. Full prescribing information and a medication guide are available online.
This article first appeared on Medscape.com.
Heavy toll from ongoing cancer referral delays
Delays in cancer referrals caused by the COVID-19 pandemic and the ensuing shutdown in cancer services will lead to thousands of additional deaths and tens of thousands of life-years lost, suggest two new modeling studies from the United Kingdom.
Clearing the backlog in cancer diagnoses will require a coordinated effort from the government and the National Health Service (NHS), say the authors, inasmuch as services were already running at “full capacity” before the pandemic.
Both studies were published in The Lancet Oncology on July 20.
When the UK-wide lockdown to combat the COVID-19 pandemic was implemented on March 23, cancer screening and routine outpatient referrals in the NHS were suspended, and treatment of cancer patients either halted or slowed down.
Moreover, because of physical distancing measures, which are expected to continue for up to a year, urgent 3-week referrals for suspected cancer cases have fallen by as much as 80%.
To estimate the potential impact on cancer deaths, Ajay Aggarwal, MD, from the London School of Hygiene and Tropical Medicine, United Kingdom, and colleagues conducted a population-based modeling study.
They collected data on 32,583 patients with breast cancer, 24,975 with colorectal cancer, 6744 with esophageal cancer, and 29,305 with lung cancer. Patients were diagnosed between 2010 and 2012 and were followed to 2015.
The investigators used that data to estimate the impact of diagnostic delays resulting from 12 months of physical distancing.
For breast cancer, this would lead to a 7.9%-9.6% increase in the number of cancer deaths within 5 years after diagnosis, or to 281-344 additional deaths.
For colorectal cancer, there would be a 15.3%-16.7% increase in mortality over 5 years, or an additional 1,445-1,563 deaths.
For lung cancer, there would a 4.8%-5.3% increase in mortality, or an additional 1235-1372 deaths.
For esophageal cancer, the mortality increase over 5 years would be 5.8%-6.0%, leading to 330-342 additional deaths.
Across the four tumor types, 59,204-63,229 life-years would be lost because of physical distancing compared to the prepandemic era.
Resources need to be increased
These additional deaths are not inevitable, the researchers suggest.
To prevent the increase in colorectal cancer deaths, for example, Aggarwal said, “It is vital that more resources are made urgently available for endoscopy and colonoscopy services, which are managing significant backlogs currently.
“Whilst currently attention is being focused on diagnostic pathways where cancer is suspected, the issue is that a significant number of cancers are diagnosed in patients awaiting investigation for symptoms not considered related to be cancer,” he added in a statement.
“Therefore we need a whole system approach to avoid the predicted excess deaths.”
Coauthor Bernard Rachet, PhD, also from the London School of Hygiene and Tropical Medicine, added that “to absorb the cancer patient backlog, the healthcare community also needs to establish clear criteria to prioritise patients on clinical grounds, in order to maintain equitability in care delivery.”
It will not be easy “to pin down the exact number of additional cancer deaths we expect to see over the coming years, but studies like this help us to understand the devastating long-term effect a pandemic like COVID-19 will have on the lives of thousands of cancer patients,” commented Michelle Mitchell, chief executive of Cancer Research UK.
Underlining the “enormous backlog” of cancer care that has built up during the pandemic, she said: “Diagnosing and treating people swiftly is vital to give people with cancer the greatest chances of survival.
“The government must work closely with the NHS to ensure it has sufficient staff and equipment to clear the backlog while giving patients the care that they need, quickly and safely,” Mitchell added.
Increasing resources will not be easy. In an accompanying editorial, William Hamilton, MD, PhD, University of Exeter, United Kingdom, warns that many NHS imaging departments, for example, were “working at full capacity before the COVID-19 pandemic.”
Consequently, they “might not be able to meet the increase in demand” resulting from the backlog in patients, especially as “the need to keep patients separate and to clean equipment has reduced their efficiency.
“The UK has had a long-term shortage of diagnostic capacity, although this shortage is not simply of equipment, but also of personnel, which is not so easily improved,” he cautions.
Another study, similar estimates
For the second study, Clare Turnbull, PhD, Institute of Cancer Research, London, and colleagues obtained age- and stage-stratified 10-year cancer survival estimates for patients in England diagnosed with 20 common tumor types between 2008 and 2017.
They also gathered data on cancer diagnoses made via urgent 2-week referrals between 2013 and 2016. They estimate that 6,281 patients were diagnosed with cancer of stages I-III per month.
Of those, 1,691 (27%) would die within 10 years of their diagnosis, they found.
They then calculated that delays in 2-week referrals during a 3-month lockdown would lead to an average delay in presentation of 2 months per patient.
A resulting 25% backlog in referrals would lead to 181 additional lives and 3,316 life-years lost. With a 75% backlog in referrals, an additional 276 lives and 5,075 life-years would be lost.
The team says that additional diagnostic delays spread over 3-8 months after the lockdown could increase the impact of a 25% backlog in referrals to 401 additional lives and 14,873 life-years lost.
For a 75% backlog in referrals, the additional lives lost would rise to 1,231, and the number of life-years lost would reach 22,635.
“Substantial additional deaths from diagnostic delays on top of those expected from delays in presentation – because many people are simply too afraid to visit their GP or hospital – are likely, especially if rapid provision of additional capacity, including technical provision and increased staffing, is not forthcoming,” Turnbull commented in a statement.
The study by Aggarwal and colleagues was funded by the U.K. Research and Innovation Economic and Social Research Council. Several of the researchers were supported by Cancer Research UK and Breast Cancer Now. Turnbull reports receiving support from the Movember Foundation.
This article first appeared on Medscape.com.
Delays in cancer referrals caused by the COVID-19 pandemic and the ensuing shutdown in cancer services will lead to thousands of additional deaths and tens of thousands of life-years lost, suggest two new modeling studies from the United Kingdom.
Clearing the backlog in cancer diagnoses will require a coordinated effort from the government and the National Health Service (NHS), say the authors, inasmuch as services were already running at “full capacity” before the pandemic.
Both studies were published in The Lancet Oncology on July 20.
When the UK-wide lockdown to combat the COVID-19 pandemic was implemented on March 23, cancer screening and routine outpatient referrals in the NHS were suspended, and treatment of cancer patients either halted or slowed down.
Moreover, because of physical distancing measures, which are expected to continue for up to a year, urgent 3-week referrals for suspected cancer cases have fallen by as much as 80%.
To estimate the potential impact on cancer deaths, Ajay Aggarwal, MD, from the London School of Hygiene and Tropical Medicine, United Kingdom, and colleagues conducted a population-based modeling study.
They collected data on 32,583 patients with breast cancer, 24,975 with colorectal cancer, 6744 with esophageal cancer, and 29,305 with lung cancer. Patients were diagnosed between 2010 and 2012 and were followed to 2015.
The investigators used that data to estimate the impact of diagnostic delays resulting from 12 months of physical distancing.
For breast cancer, this would lead to a 7.9%-9.6% increase in the number of cancer deaths within 5 years after diagnosis, or to 281-344 additional deaths.
For colorectal cancer, there would be a 15.3%-16.7% increase in mortality over 5 years, or an additional 1,445-1,563 deaths.
For lung cancer, there would a 4.8%-5.3% increase in mortality, or an additional 1235-1372 deaths.
For esophageal cancer, the mortality increase over 5 years would be 5.8%-6.0%, leading to 330-342 additional deaths.
Across the four tumor types, 59,204-63,229 life-years would be lost because of physical distancing compared to the prepandemic era.
Resources need to be increased
These additional deaths are not inevitable, the researchers suggest.
To prevent the increase in colorectal cancer deaths, for example, Aggarwal said, “It is vital that more resources are made urgently available for endoscopy and colonoscopy services, which are managing significant backlogs currently.
“Whilst currently attention is being focused on diagnostic pathways where cancer is suspected, the issue is that a significant number of cancers are diagnosed in patients awaiting investigation for symptoms not considered related to be cancer,” he added in a statement.
“Therefore we need a whole system approach to avoid the predicted excess deaths.”
Coauthor Bernard Rachet, PhD, also from the London School of Hygiene and Tropical Medicine, added that “to absorb the cancer patient backlog, the healthcare community also needs to establish clear criteria to prioritise patients on clinical grounds, in order to maintain equitability in care delivery.”
It will not be easy “to pin down the exact number of additional cancer deaths we expect to see over the coming years, but studies like this help us to understand the devastating long-term effect a pandemic like COVID-19 will have on the lives of thousands of cancer patients,” commented Michelle Mitchell, chief executive of Cancer Research UK.
Underlining the “enormous backlog” of cancer care that has built up during the pandemic, she said: “Diagnosing and treating people swiftly is vital to give people with cancer the greatest chances of survival.
“The government must work closely with the NHS to ensure it has sufficient staff and equipment to clear the backlog while giving patients the care that they need, quickly and safely,” Mitchell added.
Increasing resources will not be easy. In an accompanying editorial, William Hamilton, MD, PhD, University of Exeter, United Kingdom, warns that many NHS imaging departments, for example, were “working at full capacity before the COVID-19 pandemic.”
Consequently, they “might not be able to meet the increase in demand” resulting from the backlog in patients, especially as “the need to keep patients separate and to clean equipment has reduced their efficiency.
“The UK has had a long-term shortage of diagnostic capacity, although this shortage is not simply of equipment, but also of personnel, which is not so easily improved,” he cautions.
Another study, similar estimates
For the second study, Clare Turnbull, PhD, Institute of Cancer Research, London, and colleagues obtained age- and stage-stratified 10-year cancer survival estimates for patients in England diagnosed with 20 common tumor types between 2008 and 2017.
They also gathered data on cancer diagnoses made via urgent 2-week referrals between 2013 and 2016. They estimate that 6,281 patients were diagnosed with cancer of stages I-III per month.
Of those, 1,691 (27%) would die within 10 years of their diagnosis, they found.
They then calculated that delays in 2-week referrals during a 3-month lockdown would lead to an average delay in presentation of 2 months per patient.
A resulting 25% backlog in referrals would lead to 181 additional lives and 3,316 life-years lost. With a 75% backlog in referrals, an additional 276 lives and 5,075 life-years would be lost.
The team says that additional diagnostic delays spread over 3-8 months after the lockdown could increase the impact of a 25% backlog in referrals to 401 additional lives and 14,873 life-years lost.
For a 75% backlog in referrals, the additional lives lost would rise to 1,231, and the number of life-years lost would reach 22,635.
“Substantial additional deaths from diagnostic delays on top of those expected from delays in presentation – because many people are simply too afraid to visit their GP or hospital – are likely, especially if rapid provision of additional capacity, including technical provision and increased staffing, is not forthcoming,” Turnbull commented in a statement.
The study by Aggarwal and colleagues was funded by the U.K. Research and Innovation Economic and Social Research Council. Several of the researchers were supported by Cancer Research UK and Breast Cancer Now. Turnbull reports receiving support from the Movember Foundation.
This article first appeared on Medscape.com.
Delays in cancer referrals caused by the COVID-19 pandemic and the ensuing shutdown in cancer services will lead to thousands of additional deaths and tens of thousands of life-years lost, suggest two new modeling studies from the United Kingdom.
Clearing the backlog in cancer diagnoses will require a coordinated effort from the government and the National Health Service (NHS), say the authors, inasmuch as services were already running at “full capacity” before the pandemic.
Both studies were published in The Lancet Oncology on July 20.
When the UK-wide lockdown to combat the COVID-19 pandemic was implemented on March 23, cancer screening and routine outpatient referrals in the NHS were suspended, and treatment of cancer patients either halted or slowed down.
Moreover, because of physical distancing measures, which are expected to continue for up to a year, urgent 3-week referrals for suspected cancer cases have fallen by as much as 80%.
To estimate the potential impact on cancer deaths, Ajay Aggarwal, MD, from the London School of Hygiene and Tropical Medicine, United Kingdom, and colleagues conducted a population-based modeling study.
They collected data on 32,583 patients with breast cancer, 24,975 with colorectal cancer, 6744 with esophageal cancer, and 29,305 with lung cancer. Patients were diagnosed between 2010 and 2012 and were followed to 2015.
The investigators used that data to estimate the impact of diagnostic delays resulting from 12 months of physical distancing.
For breast cancer, this would lead to a 7.9%-9.6% increase in the number of cancer deaths within 5 years after diagnosis, or to 281-344 additional deaths.
For colorectal cancer, there would be a 15.3%-16.7% increase in mortality over 5 years, or an additional 1,445-1,563 deaths.
For lung cancer, there would a 4.8%-5.3% increase in mortality, or an additional 1235-1372 deaths.
For esophageal cancer, the mortality increase over 5 years would be 5.8%-6.0%, leading to 330-342 additional deaths.
Across the four tumor types, 59,204-63,229 life-years would be lost because of physical distancing compared to the prepandemic era.
Resources need to be increased
These additional deaths are not inevitable, the researchers suggest.
To prevent the increase in colorectal cancer deaths, for example, Aggarwal said, “It is vital that more resources are made urgently available for endoscopy and colonoscopy services, which are managing significant backlogs currently.
“Whilst currently attention is being focused on diagnostic pathways where cancer is suspected, the issue is that a significant number of cancers are diagnosed in patients awaiting investigation for symptoms not considered related to be cancer,” he added in a statement.
“Therefore we need a whole system approach to avoid the predicted excess deaths.”
Coauthor Bernard Rachet, PhD, also from the London School of Hygiene and Tropical Medicine, added that “to absorb the cancer patient backlog, the healthcare community also needs to establish clear criteria to prioritise patients on clinical grounds, in order to maintain equitability in care delivery.”
It will not be easy “to pin down the exact number of additional cancer deaths we expect to see over the coming years, but studies like this help us to understand the devastating long-term effect a pandemic like COVID-19 will have on the lives of thousands of cancer patients,” commented Michelle Mitchell, chief executive of Cancer Research UK.
Underlining the “enormous backlog” of cancer care that has built up during the pandemic, she said: “Diagnosing and treating people swiftly is vital to give people with cancer the greatest chances of survival.
“The government must work closely with the NHS to ensure it has sufficient staff and equipment to clear the backlog while giving patients the care that they need, quickly and safely,” Mitchell added.
Increasing resources will not be easy. In an accompanying editorial, William Hamilton, MD, PhD, University of Exeter, United Kingdom, warns that many NHS imaging departments, for example, were “working at full capacity before the COVID-19 pandemic.”
Consequently, they “might not be able to meet the increase in demand” resulting from the backlog in patients, especially as “the need to keep patients separate and to clean equipment has reduced their efficiency.
“The UK has had a long-term shortage of diagnostic capacity, although this shortage is not simply of equipment, but also of personnel, which is not so easily improved,” he cautions.
Another study, similar estimates
For the second study, Clare Turnbull, PhD, Institute of Cancer Research, London, and colleagues obtained age- and stage-stratified 10-year cancer survival estimates for patients in England diagnosed with 20 common tumor types between 2008 and 2017.
They also gathered data on cancer diagnoses made via urgent 2-week referrals between 2013 and 2016. They estimate that 6,281 patients were diagnosed with cancer of stages I-III per month.
Of those, 1,691 (27%) would die within 10 years of their diagnosis, they found.
They then calculated that delays in 2-week referrals during a 3-month lockdown would lead to an average delay in presentation of 2 months per patient.
A resulting 25% backlog in referrals would lead to 181 additional lives and 3,316 life-years lost. With a 75% backlog in referrals, an additional 276 lives and 5,075 life-years would be lost.
The team says that additional diagnostic delays spread over 3-8 months after the lockdown could increase the impact of a 25% backlog in referrals to 401 additional lives and 14,873 life-years lost.
For a 75% backlog in referrals, the additional lives lost would rise to 1,231, and the number of life-years lost would reach 22,635.
“Substantial additional deaths from diagnostic delays on top of those expected from delays in presentation – because many people are simply too afraid to visit their GP or hospital – are likely, especially if rapid provision of additional capacity, including technical provision and increased staffing, is not forthcoming,” Turnbull commented in a statement.
The study by Aggarwal and colleagues was funded by the U.K. Research and Innovation Economic and Social Research Council. Several of the researchers were supported by Cancer Research UK and Breast Cancer Now. Turnbull reports receiving support from the Movember Foundation.
This article first appeared on Medscape.com.
Abaloparatide shows no effect on cardiovascular risk in postmenopausal women
Osteoporosis treatment with abaloparatide in postmenopausal women does not lead to increased cardiovascular risk, according to a post hoc analysis of the pivotal ACTIVE and ACTIVExtend trials.
“Neither treatment with abaloparatide or teriparatide was associated with an increase in serious cardiac [adverse events],” wrote Felicia Cosman, MD, of Columbia University, New York, and coauthors. The study was published in the Journal of Clinical Endocrinology.
To assess the cardiovascular safety profile of abaloparatide, a synthetic analogue of parathyroid hormone–related peptide, the researchers analyzed data on heart rate, blood pressure and cardiovascular-related adverse events (AEs) from patients taking part in the Abaloparatide Comparator Trial in Vertebral Endpoints (ACTIVE) trial and its ACTIVExtend extension study.
The 2,460 participants in the ACTIVE trial were postmenopausal women between the ages of 49 and 86 years with osteoporosis; they were given 80 mcg of daily subcutaneous abaloparatide, 20 mcg of open-label daily subcutaneous teriparatide, or placebo in roughly equal numbers for 18 months. After a 1-month treatment-free period, 1,133 eligible participants from either the abaloparatide or placebo groups were enrolled in ACTIVExtend and given 70 mg of open-label alendronate once a week for 24 months. Because heart rate was only assessed pre- and post dose in the ACTIVE trial, an additional pharmacology study of abaloparatide involving 55 healthy volunteers (32 men and 23 women) was undertaken. After a dose of either abaloparatide or placebo, heart rate was measured at 15, 30, and 45 minutes and 1, 1.5, 2, 2.5, 4, 6, 8, and 12 hours.
Overall, treatment-emergent AEs were higher in the abaloparatide (165, 20.1%) and teriparatide (106, 13%) groups, compared with placebo (74, 9%), as were AEs that led to discontinuation of the study and were potentially associated with changes in heart rate or BP (27 in abaloparatide, 11 in teriparatide, and 5 in placebo). However, the percentage of patients with serious cardiac AEs was similar across groups (1%, 1%, and 0.9%, respectively).
During the ACTIVE trial, major cardiac adverse events plus heart failure were more common in the placebo group (1.7%) than the abaloparatide (0.5%) or teriparatide (0.6%) groups. During ACTIVExtend, major cardiac adverse plus heart failure were similarly common in the abaloparatide/alendronate (1.6%) and the placebo/alendronate (1.6%) groups.
On day 1 of treatment during ACTIVE, the mean change in heart rate from pretreatment to an hour post treatment was 7.9 bpm, 5.3 bpm, and 1.2 bpm for abaloparatide, teriparatide, and placebo, respectively (P < .0001 for abaloparatide and teriparatide vs. placebo; P < .05 for abaloparatide vs. teriparatide).
Subsequent visits saw similar changes. The mean maximum heart rate at 1 hour post dose was 80.7 bpm for abaloparatide, 79.0 bpm for teriparatide, and 73.7 bpm for placebo (P < .0001 for abaloparatide and teriparatide vs. placebo; P < .01 for abaloparatide vs. teriparatide). In the study of healthy volunteers, HR peaked at 15 minutes after dosing and then declined, resolving within 2.5-4 hours.
From predose to 1 hour post dose, small but significant decreases were observed in mean supine systolic and diastolic BP across groups (–2.7/–3.6 mm Hg with abaloparatide, –2.0/–3.6 with teriparatide, –1.5/–2.3 with placebo). During the first year of ACTIVE, the mean maximal decrease in BP from predose to 1 hour post dose was slightly higher (1-2 mm Hg) in the abaloparatide and teriparatide groups, compared with the placebo group (P < .05).
The authors acknowledged their study’s limitations, including the analysis of major cardiac adverse plus heart failure in ACTIVE being limited because of a low number of events and the trial not being designed in that regard.
Abaloparatide was approved by the Food and Drug Administration in 2017 on the basis of results from the ACTIVE and ACTIVExtend trials showing significant reductions in new vertebral and nonvertebral fractures, compared with placebo.
The analysis was partially funded by Radius Health. Its authors acknowledged numerous potential conflicts of interest, including receiving grants and research support from various organizations and pharmaceutical companies.
SOURCE: Cosman F et al. J Clin Endocrinol Metab. 2020 Jul 13. doi: 10.1210/clinem/dgaa450.
Osteoporosis treatment with abaloparatide in postmenopausal women does not lead to increased cardiovascular risk, according to a post hoc analysis of the pivotal ACTIVE and ACTIVExtend trials.
“Neither treatment with abaloparatide or teriparatide was associated with an increase in serious cardiac [adverse events],” wrote Felicia Cosman, MD, of Columbia University, New York, and coauthors. The study was published in the Journal of Clinical Endocrinology.
To assess the cardiovascular safety profile of abaloparatide, a synthetic analogue of parathyroid hormone–related peptide, the researchers analyzed data on heart rate, blood pressure and cardiovascular-related adverse events (AEs) from patients taking part in the Abaloparatide Comparator Trial in Vertebral Endpoints (ACTIVE) trial and its ACTIVExtend extension study.
The 2,460 participants in the ACTIVE trial were postmenopausal women between the ages of 49 and 86 years with osteoporosis; they were given 80 mcg of daily subcutaneous abaloparatide, 20 mcg of open-label daily subcutaneous teriparatide, or placebo in roughly equal numbers for 18 months. After a 1-month treatment-free period, 1,133 eligible participants from either the abaloparatide or placebo groups were enrolled in ACTIVExtend and given 70 mg of open-label alendronate once a week for 24 months. Because heart rate was only assessed pre- and post dose in the ACTIVE trial, an additional pharmacology study of abaloparatide involving 55 healthy volunteers (32 men and 23 women) was undertaken. After a dose of either abaloparatide or placebo, heart rate was measured at 15, 30, and 45 minutes and 1, 1.5, 2, 2.5, 4, 6, 8, and 12 hours.
Overall, treatment-emergent AEs were higher in the abaloparatide (165, 20.1%) and teriparatide (106, 13%) groups, compared with placebo (74, 9%), as were AEs that led to discontinuation of the study and were potentially associated with changes in heart rate or BP (27 in abaloparatide, 11 in teriparatide, and 5 in placebo). However, the percentage of patients with serious cardiac AEs was similar across groups (1%, 1%, and 0.9%, respectively).
During the ACTIVE trial, major cardiac adverse events plus heart failure were more common in the placebo group (1.7%) than the abaloparatide (0.5%) or teriparatide (0.6%) groups. During ACTIVExtend, major cardiac adverse plus heart failure were similarly common in the abaloparatide/alendronate (1.6%) and the placebo/alendronate (1.6%) groups.
On day 1 of treatment during ACTIVE, the mean change in heart rate from pretreatment to an hour post treatment was 7.9 bpm, 5.3 bpm, and 1.2 bpm for abaloparatide, teriparatide, and placebo, respectively (P < .0001 for abaloparatide and teriparatide vs. placebo; P < .05 for abaloparatide vs. teriparatide).
Subsequent visits saw similar changes. The mean maximum heart rate at 1 hour post dose was 80.7 bpm for abaloparatide, 79.0 bpm for teriparatide, and 73.7 bpm for placebo (P < .0001 for abaloparatide and teriparatide vs. placebo; P < .01 for abaloparatide vs. teriparatide). In the study of healthy volunteers, HR peaked at 15 minutes after dosing and then declined, resolving within 2.5-4 hours.
From predose to 1 hour post dose, small but significant decreases were observed in mean supine systolic and diastolic BP across groups (–2.7/–3.6 mm Hg with abaloparatide, –2.0/–3.6 with teriparatide, –1.5/–2.3 with placebo). During the first year of ACTIVE, the mean maximal decrease in BP from predose to 1 hour post dose was slightly higher (1-2 mm Hg) in the abaloparatide and teriparatide groups, compared with the placebo group (P < .05).
The authors acknowledged their study’s limitations, including the analysis of major cardiac adverse plus heart failure in ACTIVE being limited because of a low number of events and the trial not being designed in that regard.
Abaloparatide was approved by the Food and Drug Administration in 2017 on the basis of results from the ACTIVE and ACTIVExtend trials showing significant reductions in new vertebral and nonvertebral fractures, compared with placebo.
The analysis was partially funded by Radius Health. Its authors acknowledged numerous potential conflicts of interest, including receiving grants and research support from various organizations and pharmaceutical companies.
SOURCE: Cosman F et al. J Clin Endocrinol Metab. 2020 Jul 13. doi: 10.1210/clinem/dgaa450.
Osteoporosis treatment with abaloparatide in postmenopausal women does not lead to increased cardiovascular risk, according to a post hoc analysis of the pivotal ACTIVE and ACTIVExtend trials.
“Neither treatment with abaloparatide or teriparatide was associated with an increase in serious cardiac [adverse events],” wrote Felicia Cosman, MD, of Columbia University, New York, and coauthors. The study was published in the Journal of Clinical Endocrinology.
To assess the cardiovascular safety profile of abaloparatide, a synthetic analogue of parathyroid hormone–related peptide, the researchers analyzed data on heart rate, blood pressure and cardiovascular-related adverse events (AEs) from patients taking part in the Abaloparatide Comparator Trial in Vertebral Endpoints (ACTIVE) trial and its ACTIVExtend extension study.
The 2,460 participants in the ACTIVE trial were postmenopausal women between the ages of 49 and 86 years with osteoporosis; they were given 80 mcg of daily subcutaneous abaloparatide, 20 mcg of open-label daily subcutaneous teriparatide, or placebo in roughly equal numbers for 18 months. After a 1-month treatment-free period, 1,133 eligible participants from either the abaloparatide or placebo groups were enrolled in ACTIVExtend and given 70 mg of open-label alendronate once a week for 24 months. Because heart rate was only assessed pre- and post dose in the ACTIVE trial, an additional pharmacology study of abaloparatide involving 55 healthy volunteers (32 men and 23 women) was undertaken. After a dose of either abaloparatide or placebo, heart rate was measured at 15, 30, and 45 minutes and 1, 1.5, 2, 2.5, 4, 6, 8, and 12 hours.
Overall, treatment-emergent AEs were higher in the abaloparatide (165, 20.1%) and teriparatide (106, 13%) groups, compared with placebo (74, 9%), as were AEs that led to discontinuation of the study and were potentially associated with changes in heart rate or BP (27 in abaloparatide, 11 in teriparatide, and 5 in placebo). However, the percentage of patients with serious cardiac AEs was similar across groups (1%, 1%, and 0.9%, respectively).
During the ACTIVE trial, major cardiac adverse events plus heart failure were more common in the placebo group (1.7%) than the abaloparatide (0.5%) or teriparatide (0.6%) groups. During ACTIVExtend, major cardiac adverse plus heart failure were similarly common in the abaloparatide/alendronate (1.6%) and the placebo/alendronate (1.6%) groups.
On day 1 of treatment during ACTIVE, the mean change in heart rate from pretreatment to an hour post treatment was 7.9 bpm, 5.3 bpm, and 1.2 bpm for abaloparatide, teriparatide, and placebo, respectively (P < .0001 for abaloparatide and teriparatide vs. placebo; P < .05 for abaloparatide vs. teriparatide).
Subsequent visits saw similar changes. The mean maximum heart rate at 1 hour post dose was 80.7 bpm for abaloparatide, 79.0 bpm for teriparatide, and 73.7 bpm for placebo (P < .0001 for abaloparatide and teriparatide vs. placebo; P < .01 for abaloparatide vs. teriparatide). In the study of healthy volunteers, HR peaked at 15 minutes after dosing and then declined, resolving within 2.5-4 hours.
From predose to 1 hour post dose, small but significant decreases were observed in mean supine systolic and diastolic BP across groups (–2.7/–3.6 mm Hg with abaloparatide, –2.0/–3.6 with teriparatide, –1.5/–2.3 with placebo). During the first year of ACTIVE, the mean maximal decrease in BP from predose to 1 hour post dose was slightly higher (1-2 mm Hg) in the abaloparatide and teriparatide groups, compared with the placebo group (P < .05).
The authors acknowledged their study’s limitations, including the analysis of major cardiac adverse plus heart failure in ACTIVE being limited because of a low number of events and the trial not being designed in that regard.
Abaloparatide was approved by the Food and Drug Administration in 2017 on the basis of results from the ACTIVE and ACTIVExtend trials showing significant reductions in new vertebral and nonvertebral fractures, compared with placebo.
The analysis was partially funded by Radius Health. Its authors acknowledged numerous potential conflicts of interest, including receiving grants and research support from various organizations and pharmaceutical companies.
SOURCE: Cosman F et al. J Clin Endocrinol Metab. 2020 Jul 13. doi: 10.1210/clinem/dgaa450.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Stress, COVID-19 contribute to mental health concerns in college students
Socioeconomic, technological, cultural, and historical conditions are contributing to a mental health crisis among college students in the United States, according to Anthony L. Rostain, MD, MA, in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
A recent National College Health Assessment published in fall of 2018 by the American College Health Association found that one in four college students had some kind of diagnosable mental illness, and 44% had symptoms of depression within the past year.
The assessment also found that college students felt overwhelmed (86%), felt sad (68%), felt very lonely (63%), had overwhelming anxiety (62%), experienced feelings of hopelessness (53%), or were depressed to the point where functioning was difficult (41%), all of which was higher than in previous years. Students also were more likely than in previous years to engage in interpersonal violence (17%), seriously consider suicide (11%), intentionally hurt themselves (7.4%), and attempt suicide (1.9%). According to the organization Active Minds, suicide is a leading cause of death in college students.
This shift in mental health for individuals in Generation Z, those born between the mid-1990s and early 2010s, can be attributed to historical events since the turn of the century, Dr. Rostain said at the meeting, presented by Global Academy for Medical Education. The Sept. 11, 2001, attacks, wars in Iraq and Afghanistan, the financial crisis of 2007-2008, school shootings, globalization leading to economic uncertainty, the 24-hour news cycle and continuous media exposure, and the influence of the Internet have all influenced Gen Z’s identity.
“Growing up immersed in the Internet certainly has its advantages, but also maybe created some vulnerabilities in our young people,” he said.
Concerns about climate change, the burden of higher education and student debt, and the COVID-19 pandemic also have contributed to anxiety in this group. In a spring survey of students published by Active Minds about COVID-19 and its impact on mental health, 91% of students reported having stress or anxiety, 81% were disappointed or sad, 80% said they felt lonely or isolated, 56% had relocated as a result of the pandemic, and 48% reported financial setbacks tied to COVID-19.
“Anxiety seems to have become a feature of modern life,” said Dr. Rostain, who is director of education at the department of psychiatry and professor of psychiatry at the Hospital of the University of Pennsylvania in Philadelphia.
“Our culture, which often has a prominent emotional tone of fear, tends to promote cognitive distortions in which everyone is perceiving danger at every turn.” in this group, he noted. While people should be washing their hands and staying safe through social distancing during the pandemic, “we don’t want people to stop functioning, planning the future, and really in college students’ case, studying and getting ready for their careers,” he said. Parents can hinder those goals through intensively parenting or “overparenting” their children, which can result in destructive perfectionism, anxiety and depression, abject fear of failure and risk avoidance, and a focus on the external aspects of life rather than internal feelings.
Heavier alcohol use and amphetamine use also is on the rise in college students, Dr. Rostain said. Increased stimulant use in young adults is attributed to greater access to prescription drugs prior to college, greater prevalence of attention-deficit/hyperactivity disorder (ADHD), peer pressure, and influence from marketing and media messaging, he said. Another important change is the rise of smartphones and the Internet, which might drive the need to be constantly connected and compete for attention.
“This is the first generation who had constant access to the Internet. Smartphones in particular are everywhere, and we think this is another important factor in considering what might be happening to young people,” Dr. Rostain said.
Developing problem-solving and conflict resolution skills, developing coping mechanisms, being able to regulate emotions, finding optimism toward the future, having access to mental health services, and having cultural or religious beliefs with a negative view of suicide are all protective factors that promote resiliency in young people, Dr. Rostain said. Other protective factors include the development of socio-emotional readiness skills, such as conscientiousness, self-management, interpersonal skills, self-control, task persistence, risk management, self-acceptance, and having an open mindset or seeking help when needed. However, he noted, family is one area that can be both a help or a risk to mental health.
“Family attachments and supportive relationships in the family are really critical in predicting good outcomes. By the same token, families that are conflicted, where there’s a lot of stress or there’s a lot of turmoil and/or where resources are not available, that may be a risk factor to coping in young adulthood,” he said.
Individual resilience can be developed through learning from mistakes and overcoming mindset barriers, such as feelings of not belonging, concerns about disappointing one’s parents, worries about not making it, or fears of being different.
On campus, best practices and emerging trends include wellness and resiliency programs, reducing stigma, engagement from students, training of faculty and staff, crisis management plans, telehealth counseling, substance abuse programs, postvention support after suicide, collaboration with mental health providers, and support for diverse populations.
“The best schools are the ones that promote communication and that invite families to be involved early on because parents and families can be and need to be educated about what to do to prevent adverse outcomes of young people who are really at risk,” Dr. Rostain said. “It takes a village to raise a child, and it takes the same village to bring someone from adolescence to young adulthood.”
Family-based intervention has also shown promise, he said, but clinicians should watch for signs that a family is not willing to undergo therapy, is scapegoating a college student, or there are signs of boundary violations, violence, or sexual abuse in the family – or attempts to undermine treatment.
Specific to COVID-19, campus mental health services should focus on routine, self-care, physical activity, and connections with other people while also space for grieving lost experiences, facing uncertainty, developing resilience, and finding meaning. In the family, challenges around COVID-19 can include issues of physical distancing and quarantine, anxiety about becoming infected with the virus, economic insecurity, managing conflicts, setting and enforcing boundaries in addition to providing mutual support, and finding new meaning during the pandemic.
“I think these are the challenges, but we think this whole process of people living together and handling life in a way they’ve never expected to may hold some silver linings,” Dr. Rostain said. “It may be a way of addressing many issues that were never addressed before the young person went off to college.”
Global Academy and this news organization are owned by the same parent company. Dr. Rostain reported receiving royalties from Routledge/Taylor Francis Group and St. Martin’s Press, scientific advisory board honoraria from Arbor and Shire/Takeda, consulting fees from the National Football League and Tris Pharmaceuticals, and has presented CME sessions for American Psychiatric Publishing, Global Medical Education, Shire/Takeda, and the U.S. Psychiatric Congress.
Socioeconomic, technological, cultural, and historical conditions are contributing to a mental health crisis among college students in the United States, according to Anthony L. Rostain, MD, MA, in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
A recent National College Health Assessment published in fall of 2018 by the American College Health Association found that one in four college students had some kind of diagnosable mental illness, and 44% had symptoms of depression within the past year.
The assessment also found that college students felt overwhelmed (86%), felt sad (68%), felt very lonely (63%), had overwhelming anxiety (62%), experienced feelings of hopelessness (53%), or were depressed to the point where functioning was difficult (41%), all of which was higher than in previous years. Students also were more likely than in previous years to engage in interpersonal violence (17%), seriously consider suicide (11%), intentionally hurt themselves (7.4%), and attempt suicide (1.9%). According to the organization Active Minds, suicide is a leading cause of death in college students.
This shift in mental health for individuals in Generation Z, those born between the mid-1990s and early 2010s, can be attributed to historical events since the turn of the century, Dr. Rostain said at the meeting, presented by Global Academy for Medical Education. The Sept. 11, 2001, attacks, wars in Iraq and Afghanistan, the financial crisis of 2007-2008, school shootings, globalization leading to economic uncertainty, the 24-hour news cycle and continuous media exposure, and the influence of the Internet have all influenced Gen Z’s identity.
“Growing up immersed in the Internet certainly has its advantages, but also maybe created some vulnerabilities in our young people,” he said.
Concerns about climate change, the burden of higher education and student debt, and the COVID-19 pandemic also have contributed to anxiety in this group. In a spring survey of students published by Active Minds about COVID-19 and its impact on mental health, 91% of students reported having stress or anxiety, 81% were disappointed or sad, 80% said they felt lonely or isolated, 56% had relocated as a result of the pandemic, and 48% reported financial setbacks tied to COVID-19.
“Anxiety seems to have become a feature of modern life,” said Dr. Rostain, who is director of education at the department of psychiatry and professor of psychiatry at the Hospital of the University of Pennsylvania in Philadelphia.
“Our culture, which often has a prominent emotional tone of fear, tends to promote cognitive distortions in which everyone is perceiving danger at every turn.” in this group, he noted. While people should be washing their hands and staying safe through social distancing during the pandemic, “we don’t want people to stop functioning, planning the future, and really in college students’ case, studying and getting ready for their careers,” he said. Parents can hinder those goals through intensively parenting or “overparenting” their children, which can result in destructive perfectionism, anxiety and depression, abject fear of failure and risk avoidance, and a focus on the external aspects of life rather than internal feelings.
Heavier alcohol use and amphetamine use also is on the rise in college students, Dr. Rostain said. Increased stimulant use in young adults is attributed to greater access to prescription drugs prior to college, greater prevalence of attention-deficit/hyperactivity disorder (ADHD), peer pressure, and influence from marketing and media messaging, he said. Another important change is the rise of smartphones and the Internet, which might drive the need to be constantly connected and compete for attention.
“This is the first generation who had constant access to the Internet. Smartphones in particular are everywhere, and we think this is another important factor in considering what might be happening to young people,” Dr. Rostain said.
Developing problem-solving and conflict resolution skills, developing coping mechanisms, being able to regulate emotions, finding optimism toward the future, having access to mental health services, and having cultural or religious beliefs with a negative view of suicide are all protective factors that promote resiliency in young people, Dr. Rostain said. Other protective factors include the development of socio-emotional readiness skills, such as conscientiousness, self-management, interpersonal skills, self-control, task persistence, risk management, self-acceptance, and having an open mindset or seeking help when needed. However, he noted, family is one area that can be both a help or a risk to mental health.
“Family attachments and supportive relationships in the family are really critical in predicting good outcomes. By the same token, families that are conflicted, where there’s a lot of stress or there’s a lot of turmoil and/or where resources are not available, that may be a risk factor to coping in young adulthood,” he said.
Individual resilience can be developed through learning from mistakes and overcoming mindset barriers, such as feelings of not belonging, concerns about disappointing one’s parents, worries about not making it, or fears of being different.
On campus, best practices and emerging trends include wellness and resiliency programs, reducing stigma, engagement from students, training of faculty and staff, crisis management plans, telehealth counseling, substance abuse programs, postvention support after suicide, collaboration with mental health providers, and support for diverse populations.
“The best schools are the ones that promote communication and that invite families to be involved early on because parents and families can be and need to be educated about what to do to prevent adverse outcomes of young people who are really at risk,” Dr. Rostain said. “It takes a village to raise a child, and it takes the same village to bring someone from adolescence to young adulthood.”
Family-based intervention has also shown promise, he said, but clinicians should watch for signs that a family is not willing to undergo therapy, is scapegoating a college student, or there are signs of boundary violations, violence, or sexual abuse in the family – or attempts to undermine treatment.
Specific to COVID-19, campus mental health services should focus on routine, self-care, physical activity, and connections with other people while also space for grieving lost experiences, facing uncertainty, developing resilience, and finding meaning. In the family, challenges around COVID-19 can include issues of physical distancing and quarantine, anxiety about becoming infected with the virus, economic insecurity, managing conflicts, setting and enforcing boundaries in addition to providing mutual support, and finding new meaning during the pandemic.
“I think these are the challenges, but we think this whole process of people living together and handling life in a way they’ve never expected to may hold some silver linings,” Dr. Rostain said. “It may be a way of addressing many issues that were never addressed before the young person went off to college.”
Global Academy and this news organization are owned by the same parent company. Dr. Rostain reported receiving royalties from Routledge/Taylor Francis Group and St. Martin’s Press, scientific advisory board honoraria from Arbor and Shire/Takeda, consulting fees from the National Football League and Tris Pharmaceuticals, and has presented CME sessions for American Psychiatric Publishing, Global Medical Education, Shire/Takeda, and the U.S. Psychiatric Congress.
Socioeconomic, technological, cultural, and historical conditions are contributing to a mental health crisis among college students in the United States, according to Anthony L. Rostain, MD, MA, in a virtual meeting presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
A recent National College Health Assessment published in fall of 2018 by the American College Health Association found that one in four college students had some kind of diagnosable mental illness, and 44% had symptoms of depression within the past year.
The assessment also found that college students felt overwhelmed (86%), felt sad (68%), felt very lonely (63%), had overwhelming anxiety (62%), experienced feelings of hopelessness (53%), or were depressed to the point where functioning was difficult (41%), all of which was higher than in previous years. Students also were more likely than in previous years to engage in interpersonal violence (17%), seriously consider suicide (11%), intentionally hurt themselves (7.4%), and attempt suicide (1.9%). According to the organization Active Minds, suicide is a leading cause of death in college students.
This shift in mental health for individuals in Generation Z, those born between the mid-1990s and early 2010s, can be attributed to historical events since the turn of the century, Dr. Rostain said at the meeting, presented by Global Academy for Medical Education. The Sept. 11, 2001, attacks, wars in Iraq and Afghanistan, the financial crisis of 2007-2008, school shootings, globalization leading to economic uncertainty, the 24-hour news cycle and continuous media exposure, and the influence of the Internet have all influenced Gen Z’s identity.
“Growing up immersed in the Internet certainly has its advantages, but also maybe created some vulnerabilities in our young people,” he said.
Concerns about climate change, the burden of higher education and student debt, and the COVID-19 pandemic also have contributed to anxiety in this group. In a spring survey of students published by Active Minds about COVID-19 and its impact on mental health, 91% of students reported having stress or anxiety, 81% were disappointed or sad, 80% said they felt lonely or isolated, 56% had relocated as a result of the pandemic, and 48% reported financial setbacks tied to COVID-19.
“Anxiety seems to have become a feature of modern life,” said Dr. Rostain, who is director of education at the department of psychiatry and professor of psychiatry at the Hospital of the University of Pennsylvania in Philadelphia.
“Our culture, which often has a prominent emotional tone of fear, tends to promote cognitive distortions in which everyone is perceiving danger at every turn.” in this group, he noted. While people should be washing their hands and staying safe through social distancing during the pandemic, “we don’t want people to stop functioning, planning the future, and really in college students’ case, studying and getting ready for their careers,” he said. Parents can hinder those goals through intensively parenting or “overparenting” their children, which can result in destructive perfectionism, anxiety and depression, abject fear of failure and risk avoidance, and a focus on the external aspects of life rather than internal feelings.
Heavier alcohol use and amphetamine use also is on the rise in college students, Dr. Rostain said. Increased stimulant use in young adults is attributed to greater access to prescription drugs prior to college, greater prevalence of attention-deficit/hyperactivity disorder (ADHD), peer pressure, and influence from marketing and media messaging, he said. Another important change is the rise of smartphones and the Internet, which might drive the need to be constantly connected and compete for attention.
“This is the first generation who had constant access to the Internet. Smartphones in particular are everywhere, and we think this is another important factor in considering what might be happening to young people,” Dr. Rostain said.
Developing problem-solving and conflict resolution skills, developing coping mechanisms, being able to regulate emotions, finding optimism toward the future, having access to mental health services, and having cultural or religious beliefs with a negative view of suicide are all protective factors that promote resiliency in young people, Dr. Rostain said. Other protective factors include the development of socio-emotional readiness skills, such as conscientiousness, self-management, interpersonal skills, self-control, task persistence, risk management, self-acceptance, and having an open mindset or seeking help when needed. However, he noted, family is one area that can be both a help or a risk to mental health.
“Family attachments and supportive relationships in the family are really critical in predicting good outcomes. By the same token, families that are conflicted, where there’s a lot of stress or there’s a lot of turmoil and/or where resources are not available, that may be a risk factor to coping in young adulthood,” he said.
Individual resilience can be developed through learning from mistakes and overcoming mindset barriers, such as feelings of not belonging, concerns about disappointing one’s parents, worries about not making it, or fears of being different.
On campus, best practices and emerging trends include wellness and resiliency programs, reducing stigma, engagement from students, training of faculty and staff, crisis management plans, telehealth counseling, substance abuse programs, postvention support after suicide, collaboration with mental health providers, and support for diverse populations.
“The best schools are the ones that promote communication and that invite families to be involved early on because parents and families can be and need to be educated about what to do to prevent adverse outcomes of young people who are really at risk,” Dr. Rostain said. “It takes a village to raise a child, and it takes the same village to bring someone from adolescence to young adulthood.”
Family-based intervention has also shown promise, he said, but clinicians should watch for signs that a family is not willing to undergo therapy, is scapegoating a college student, or there are signs of boundary violations, violence, or sexual abuse in the family – or attempts to undermine treatment.
Specific to COVID-19, campus mental health services should focus on routine, self-care, physical activity, and connections with other people while also space for grieving lost experiences, facing uncertainty, developing resilience, and finding meaning. In the family, challenges around COVID-19 can include issues of physical distancing and quarantine, anxiety about becoming infected with the virus, economic insecurity, managing conflicts, setting and enforcing boundaries in addition to providing mutual support, and finding new meaning during the pandemic.
“I think these are the challenges, but we think this whole process of people living together and handling life in a way they’ve never expected to may hold some silver linings,” Dr. Rostain said. “It may be a way of addressing many issues that were never addressed before the young person went off to college.”
Global Academy and this news organization are owned by the same parent company. Dr. Rostain reported receiving royalties from Routledge/Taylor Francis Group and St. Martin’s Press, scientific advisory board honoraria from Arbor and Shire/Takeda, consulting fees from the National Football League and Tris Pharmaceuticals, and has presented CME sessions for American Psychiatric Publishing, Global Medical Education, Shire/Takeda, and the U.S. Psychiatric Congress.
EXPERT ANALYSIS FROM CP/AACP 2020 PSYCHIATRY UPDATE
Acute EVALI remains a diagnosis of exclusion
according to a synthesis of current information presented at the virtual Pediatric Hospital Medicine.
Respiratory symptoms, including cough, chest pain, and shortness of breath are common but so are constitutive symptoms, including fever, sore throat, muscle aches, nausea and vomiting, said Yamini Kuchipudi, MD, a staff physician at Cincinnati Children’s Hospital, during the session at the virtual meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
If EVALI is not considered across this broad array of symptoms, of which respiratory complaints might not be the most prominent at the time of presentation, the diagnosis might be delayed, Dr. Kuchipudi warned during the virtual meeting.
Teenagers and young adults are the most common users of e-cigarettes and vaping devices. In these patients or in any individual suspected of having EVALI, Dr. Kuchipudi recommended posing questions about vaping relatively early in the work-up “in a confidential and nonjudgmental way.”
Eliciting a truthful history will be particularly important, because the risk of EVALI appears to be largely related to vaping with tetrahydrocannabinol (THC)-containing products rather than with nicotine alone. Although the exact cause of EVALI is not yet completely clear, this condition is now strongly associated with additives to the THC, according to Issa Hanna, MD, of the department of pediatrics at the University of Florida, Jacksonville.
“E-liquid contains products like hydrocarbons, vitamin E acetate, and heavy metals that appear to damage the alveolar epithelium by direct cellular inflammation,” Dr. Hanna explained.
These products are not only found in THC processed for vaping but also for dabbing, a related but different form of inhalation that involves vaporization of highly concentrated THC waxes or resins. Dr. Hanna suggested that the decline in reported cases of EVALI, which has followed the peak incidence in September 2019, is likely to be related to a decline in THC additives as well as greater caution among users.
E-cigarettes were introduced in 2007, according to Dr. Hanna, but EVALI was not widely recognized until cases began accruing early in 2019. By June 2019, the growing number of case reports had attracted the attention of the media as well as public health officials, intensifying the effort to isolate the risks and causes.
Consistent with greater use of e-cigarettes and vaping among younger individuals, nearly 80% of the 2,807 patients hospitalized for EVALI in the United States by February of this year occurred in individuals aged less than 35 years, according to data released by the Centers for Disease Control and Prevention. The median age was less than 25 years. Of these hospitalizations, 68 deaths (2.5%) in 29 states and Washington, D.C., were attributed to EVALI.
Because of the nonspecific symptoms and lack of a definitive diagnostic test, EVALI is considered a diagnosis of exclusion, according to Abigail Musial, MD, who is completing a fellowship in hospital medicine at Cincinnati Children’s. She presented a case in which a patient suspected of EVALI went home after symptoms abated on steroids.
“Less than 24 hours later, she returned to the ED with tachypnea and hypoxemia,” Dr. Musial recounted. Although a chest x-ray at the initial evaluation showed lung opacities, a repeat chest x-ray when she returned to the ED showed bilateral worsening of these opacities and persistent elevation of inflammatory markers.
“She was started on steroids and also on antibiotics,” Dr. Musial said. “She was weaned quickly from oxygen once the steroids were started and was discharged on hospital day 3.”
For patients suspected of EVALI, COVID-19 testing should be part of the work-up, according to Dr. Kuchipudi. She also recommended an x-ray or CT scan of the lung as well as an evaluation of inflammatory markers.
Dr. Kuchipudi said that more invasive studies than lung function tests, such as bronchoalveolar lavage or lung biopsy, might be considered when severe symptoms make aggressive diagnostic studies attractive.
Steroids and antibiotics typically lead to control of acute symptoms, but patients should be clinically stable for 24-48 hours prior to hospital discharge, according to Dr. Kuchipudi. Follow-up after discharge should include lung function tests and imaging 2-4 weeks later to confirm resolution of abnormalities.
Dr. Kuchipudi stressed the opportunity that an episode of EVALI provides to induce patients to give up nicotine and vaping entirely. Such strategies, such as a nicotine patch, deserve consideration, but she also cautioned that e-cigarettes for smoking cessation should not be recommended to EVALI patients.
The speakers reported no potential conflicts of interest relevant to this study.
according to a synthesis of current information presented at the virtual Pediatric Hospital Medicine.
Respiratory symptoms, including cough, chest pain, and shortness of breath are common but so are constitutive symptoms, including fever, sore throat, muscle aches, nausea and vomiting, said Yamini Kuchipudi, MD, a staff physician at Cincinnati Children’s Hospital, during the session at the virtual meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
If EVALI is not considered across this broad array of symptoms, of which respiratory complaints might not be the most prominent at the time of presentation, the diagnosis might be delayed, Dr. Kuchipudi warned during the virtual meeting.
Teenagers and young adults are the most common users of e-cigarettes and vaping devices. In these patients or in any individual suspected of having EVALI, Dr. Kuchipudi recommended posing questions about vaping relatively early in the work-up “in a confidential and nonjudgmental way.”
Eliciting a truthful history will be particularly important, because the risk of EVALI appears to be largely related to vaping with tetrahydrocannabinol (THC)-containing products rather than with nicotine alone. Although the exact cause of EVALI is not yet completely clear, this condition is now strongly associated with additives to the THC, according to Issa Hanna, MD, of the department of pediatrics at the University of Florida, Jacksonville.
“E-liquid contains products like hydrocarbons, vitamin E acetate, and heavy metals that appear to damage the alveolar epithelium by direct cellular inflammation,” Dr. Hanna explained.
These products are not only found in THC processed for vaping but also for dabbing, a related but different form of inhalation that involves vaporization of highly concentrated THC waxes or resins. Dr. Hanna suggested that the decline in reported cases of EVALI, which has followed the peak incidence in September 2019, is likely to be related to a decline in THC additives as well as greater caution among users.
E-cigarettes were introduced in 2007, according to Dr. Hanna, but EVALI was not widely recognized until cases began accruing early in 2019. By June 2019, the growing number of case reports had attracted the attention of the media as well as public health officials, intensifying the effort to isolate the risks and causes.
Consistent with greater use of e-cigarettes and vaping among younger individuals, nearly 80% of the 2,807 patients hospitalized for EVALI in the United States by February of this year occurred in individuals aged less than 35 years, according to data released by the Centers for Disease Control and Prevention. The median age was less than 25 years. Of these hospitalizations, 68 deaths (2.5%) in 29 states and Washington, D.C., were attributed to EVALI.
Because of the nonspecific symptoms and lack of a definitive diagnostic test, EVALI is considered a diagnosis of exclusion, according to Abigail Musial, MD, who is completing a fellowship in hospital medicine at Cincinnati Children’s. She presented a case in which a patient suspected of EVALI went home after symptoms abated on steroids.
“Less than 24 hours later, she returned to the ED with tachypnea and hypoxemia,” Dr. Musial recounted. Although a chest x-ray at the initial evaluation showed lung opacities, a repeat chest x-ray when she returned to the ED showed bilateral worsening of these opacities and persistent elevation of inflammatory markers.
“She was started on steroids and also on antibiotics,” Dr. Musial said. “She was weaned quickly from oxygen once the steroids were started and was discharged on hospital day 3.”
For patients suspected of EVALI, COVID-19 testing should be part of the work-up, according to Dr. Kuchipudi. She also recommended an x-ray or CT scan of the lung as well as an evaluation of inflammatory markers.
Dr. Kuchipudi said that more invasive studies than lung function tests, such as bronchoalveolar lavage or lung biopsy, might be considered when severe symptoms make aggressive diagnostic studies attractive.
Steroids and antibiotics typically lead to control of acute symptoms, but patients should be clinically stable for 24-48 hours prior to hospital discharge, according to Dr. Kuchipudi. Follow-up after discharge should include lung function tests and imaging 2-4 weeks later to confirm resolution of abnormalities.
Dr. Kuchipudi stressed the opportunity that an episode of EVALI provides to induce patients to give up nicotine and vaping entirely. Such strategies, such as a nicotine patch, deserve consideration, but she also cautioned that e-cigarettes for smoking cessation should not be recommended to EVALI patients.
The speakers reported no potential conflicts of interest relevant to this study.
according to a synthesis of current information presented at the virtual Pediatric Hospital Medicine.
Respiratory symptoms, including cough, chest pain, and shortness of breath are common but so are constitutive symptoms, including fever, sore throat, muscle aches, nausea and vomiting, said Yamini Kuchipudi, MD, a staff physician at Cincinnati Children’s Hospital, during the session at the virtual meeting, sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
If EVALI is not considered across this broad array of symptoms, of which respiratory complaints might not be the most prominent at the time of presentation, the diagnosis might be delayed, Dr. Kuchipudi warned during the virtual meeting.
Teenagers and young adults are the most common users of e-cigarettes and vaping devices. In these patients or in any individual suspected of having EVALI, Dr. Kuchipudi recommended posing questions about vaping relatively early in the work-up “in a confidential and nonjudgmental way.”
Eliciting a truthful history will be particularly important, because the risk of EVALI appears to be largely related to vaping with tetrahydrocannabinol (THC)-containing products rather than with nicotine alone. Although the exact cause of EVALI is not yet completely clear, this condition is now strongly associated with additives to the THC, according to Issa Hanna, MD, of the department of pediatrics at the University of Florida, Jacksonville.
“E-liquid contains products like hydrocarbons, vitamin E acetate, and heavy metals that appear to damage the alveolar epithelium by direct cellular inflammation,” Dr. Hanna explained.
These products are not only found in THC processed for vaping but also for dabbing, a related but different form of inhalation that involves vaporization of highly concentrated THC waxes or resins. Dr. Hanna suggested that the decline in reported cases of EVALI, which has followed the peak incidence in September 2019, is likely to be related to a decline in THC additives as well as greater caution among users.
E-cigarettes were introduced in 2007, according to Dr. Hanna, but EVALI was not widely recognized until cases began accruing early in 2019. By June 2019, the growing number of case reports had attracted the attention of the media as well as public health officials, intensifying the effort to isolate the risks and causes.
Consistent with greater use of e-cigarettes and vaping among younger individuals, nearly 80% of the 2,807 patients hospitalized for EVALI in the United States by February of this year occurred in individuals aged less than 35 years, according to data released by the Centers for Disease Control and Prevention. The median age was less than 25 years. Of these hospitalizations, 68 deaths (2.5%) in 29 states and Washington, D.C., were attributed to EVALI.
Because of the nonspecific symptoms and lack of a definitive diagnostic test, EVALI is considered a diagnosis of exclusion, according to Abigail Musial, MD, who is completing a fellowship in hospital medicine at Cincinnati Children’s. She presented a case in which a patient suspected of EVALI went home after symptoms abated on steroids.
“Less than 24 hours later, she returned to the ED with tachypnea and hypoxemia,” Dr. Musial recounted. Although a chest x-ray at the initial evaluation showed lung opacities, a repeat chest x-ray when she returned to the ED showed bilateral worsening of these opacities and persistent elevation of inflammatory markers.
“She was started on steroids and also on antibiotics,” Dr. Musial said. “She was weaned quickly from oxygen once the steroids were started and was discharged on hospital day 3.”
For patients suspected of EVALI, COVID-19 testing should be part of the work-up, according to Dr. Kuchipudi. She also recommended an x-ray or CT scan of the lung as well as an evaluation of inflammatory markers.
Dr. Kuchipudi said that more invasive studies than lung function tests, such as bronchoalveolar lavage or lung biopsy, might be considered when severe symptoms make aggressive diagnostic studies attractive.
Steroids and antibiotics typically lead to control of acute symptoms, but patients should be clinically stable for 24-48 hours prior to hospital discharge, according to Dr. Kuchipudi. Follow-up after discharge should include lung function tests and imaging 2-4 weeks later to confirm resolution of abnormalities.
Dr. Kuchipudi stressed the opportunity that an episode of EVALI provides to induce patients to give up nicotine and vaping entirely. Such strategies, such as a nicotine patch, deserve consideration, but she also cautioned that e-cigarettes for smoking cessation should not be recommended to EVALI patients.
The speakers reported no potential conflicts of interest relevant to this study.
FROM PHM20
New oral anticoagulants drive ACC consensus on bleeding
Patients on oral anticoagulants who experience a bleeding event may be able to discontinue therapy if certain circumstances apply, according to updated guidance from the American College of Cardiology.
The emergence of direct-acting oral anticoagulants (DOACs) to prevent venous thromboembolism and the introduction of new reversal strategies for factor Xa inhibitors prompted the creation of an Expert Consensus Decision Pathway to update the version from 2017, according to the ACC. Expert consensus decision pathways (ECDPs) are a component of the solution sets issued by the ACC to “address key questions facing care teams and attempt to provide practical guidance to be applied at the point of care.”
In an ECDP published in the Journal of the American College of Cardiology, the writing committee members developed treatment algorithms for managing bleeding in patients on DOACs and vitamin K antagonists (VKAs).
Bleeding was classified as major or nonmajor, with major defined as “bleeding that is associated with hemodynamic compromise, occurs in an anatomically critical site, requires transfusion of at least 2 units of packed red blood cells [RBCs]), or results in a hemoglobin drop greater than 2 g/dL. All other types of bleeding were classified as nonmajor.
The document includes a graphic algorithm for assessing bleed severity and managing major versus nonmajor bleeding, and a separate graphic describes considerations for reversal and use of hemostatic agents according to whether the patient is taking a VKA (warfarin and other coumarins), a direct thrombin inhibitor (dabigatran), the factor Xa inhibitors apixaban and rivaroxaban, or the factor Xa inhibitors betrixaban and edoxaban.
Another algorithm outlines whether to discontinue, delay, or restart anticoagulation. Considerations for restarting anticoagulation include whether the patient is pregnant, awaiting an invasive procedure, not able to receive medication by mouth, has a high risk of rebleeding, or is being bridged back to a vitamin K antagonist with high thrombotic risk.
In most cases of GI bleeding, for example, current data support restarting oral anticoagulants once hemostasis is achieved, but patients who experience intracranial hemorrhage should delay restarting any anticoagulation for at least 4 weeks if they are without high thrombotic risk, according to the document.
The report also recommends clinician-patient discussion before resuming anticoagulation, ideally with time allowed for patients to develop questions. Discussions should include the signs of bleeding, assessment of risk for a thromboembolic event, and the benefits of anticoagulation.
“The proliferation of oral anticoagulants (warfarin and DOACs) and growing indications for their use prompted the need for guidance on the management of these drugs,” said Gordon F. Tomaselli, MD, chair of the writing committee, in an interview. “This document provides guidance on management at the time of a bleeding complication. This includes acute management, starting and stopping drugs, and use of reversal agents,” he said. “This of course will be a dynamic document as the list of these drugs and their antidotes expand,” he noted.
“The biggest change from the previous guidelines are twofold: an update on laboratory assessment to monitor drug levels and use of reversal agents,” while the acute management strategies have otherwise remained similar to previous documents, said Dr. Tomaselli.
Dr. Tomaselli said that he was not surprised by the biological aspects of recent research while developing the statement. However, “the extent of the use of multiple anticoagulants and antiplatelet agents was a bit surprising and complicates therapy with each of the agents,” he noted.
The way the pathways are presented may make them challenging to follow in clinical practice, said Dr. Tomaselli. “The pathways are described linearly and in practice often many things have to happen at once,” he said. “The other main issue may be limitations in the availability of some of the newer reversal agents,” he added.
“The complication of bleeding is difficult to avoid,” said Dr. Tomaselli, and for future research, “the focus needs to continue to refine the indications for anticoagulation and appropriate use with other drugs that predispose to bleeding. We also need better methods and testing to monitor drugs levels and the effect on coagulation,” he said.
In accordance with the ACC Solution Set Oversight Committee, the writing committee members, including Dr. Tomaselli, had no relevant relationships with industry to disclose.
SOURCE: Tomaselli GF et al. J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.04.053.
Patients on oral anticoagulants who experience a bleeding event may be able to discontinue therapy if certain circumstances apply, according to updated guidance from the American College of Cardiology.
The emergence of direct-acting oral anticoagulants (DOACs) to prevent venous thromboembolism and the introduction of new reversal strategies for factor Xa inhibitors prompted the creation of an Expert Consensus Decision Pathway to update the version from 2017, according to the ACC. Expert consensus decision pathways (ECDPs) are a component of the solution sets issued by the ACC to “address key questions facing care teams and attempt to provide practical guidance to be applied at the point of care.”
In an ECDP published in the Journal of the American College of Cardiology, the writing committee members developed treatment algorithms for managing bleeding in patients on DOACs and vitamin K antagonists (VKAs).
Bleeding was classified as major or nonmajor, with major defined as “bleeding that is associated with hemodynamic compromise, occurs in an anatomically critical site, requires transfusion of at least 2 units of packed red blood cells [RBCs]), or results in a hemoglobin drop greater than 2 g/dL. All other types of bleeding were classified as nonmajor.
The document includes a graphic algorithm for assessing bleed severity and managing major versus nonmajor bleeding, and a separate graphic describes considerations for reversal and use of hemostatic agents according to whether the patient is taking a VKA (warfarin and other coumarins), a direct thrombin inhibitor (dabigatran), the factor Xa inhibitors apixaban and rivaroxaban, or the factor Xa inhibitors betrixaban and edoxaban.
Another algorithm outlines whether to discontinue, delay, or restart anticoagulation. Considerations for restarting anticoagulation include whether the patient is pregnant, awaiting an invasive procedure, not able to receive medication by mouth, has a high risk of rebleeding, or is being bridged back to a vitamin K antagonist with high thrombotic risk.
In most cases of GI bleeding, for example, current data support restarting oral anticoagulants once hemostasis is achieved, but patients who experience intracranial hemorrhage should delay restarting any anticoagulation for at least 4 weeks if they are without high thrombotic risk, according to the document.
The report also recommends clinician-patient discussion before resuming anticoagulation, ideally with time allowed for patients to develop questions. Discussions should include the signs of bleeding, assessment of risk for a thromboembolic event, and the benefits of anticoagulation.
“The proliferation of oral anticoagulants (warfarin and DOACs) and growing indications for their use prompted the need for guidance on the management of these drugs,” said Gordon F. Tomaselli, MD, chair of the writing committee, in an interview. “This document provides guidance on management at the time of a bleeding complication. This includes acute management, starting and stopping drugs, and use of reversal agents,” he said. “This of course will be a dynamic document as the list of these drugs and their antidotes expand,” he noted.
“The biggest change from the previous guidelines are twofold: an update on laboratory assessment to monitor drug levels and use of reversal agents,” while the acute management strategies have otherwise remained similar to previous documents, said Dr. Tomaselli.
Dr. Tomaselli said that he was not surprised by the biological aspects of recent research while developing the statement. However, “the extent of the use of multiple anticoagulants and antiplatelet agents was a bit surprising and complicates therapy with each of the agents,” he noted.
The way the pathways are presented may make them challenging to follow in clinical practice, said Dr. Tomaselli. “The pathways are described linearly and in practice often many things have to happen at once,” he said. “The other main issue may be limitations in the availability of some of the newer reversal agents,” he added.
“The complication of bleeding is difficult to avoid,” said Dr. Tomaselli, and for future research, “the focus needs to continue to refine the indications for anticoagulation and appropriate use with other drugs that predispose to bleeding. We also need better methods and testing to monitor drugs levels and the effect on coagulation,” he said.
In accordance with the ACC Solution Set Oversight Committee, the writing committee members, including Dr. Tomaselli, had no relevant relationships with industry to disclose.
SOURCE: Tomaselli GF et al. J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.04.053.
Patients on oral anticoagulants who experience a bleeding event may be able to discontinue therapy if certain circumstances apply, according to updated guidance from the American College of Cardiology.
The emergence of direct-acting oral anticoagulants (DOACs) to prevent venous thromboembolism and the introduction of new reversal strategies for factor Xa inhibitors prompted the creation of an Expert Consensus Decision Pathway to update the version from 2017, according to the ACC. Expert consensus decision pathways (ECDPs) are a component of the solution sets issued by the ACC to “address key questions facing care teams and attempt to provide practical guidance to be applied at the point of care.”
In an ECDP published in the Journal of the American College of Cardiology, the writing committee members developed treatment algorithms for managing bleeding in patients on DOACs and vitamin K antagonists (VKAs).
Bleeding was classified as major or nonmajor, with major defined as “bleeding that is associated with hemodynamic compromise, occurs in an anatomically critical site, requires transfusion of at least 2 units of packed red blood cells [RBCs]), or results in a hemoglobin drop greater than 2 g/dL. All other types of bleeding were classified as nonmajor.
The document includes a graphic algorithm for assessing bleed severity and managing major versus nonmajor bleeding, and a separate graphic describes considerations for reversal and use of hemostatic agents according to whether the patient is taking a VKA (warfarin and other coumarins), a direct thrombin inhibitor (dabigatran), the factor Xa inhibitors apixaban and rivaroxaban, or the factor Xa inhibitors betrixaban and edoxaban.
Another algorithm outlines whether to discontinue, delay, or restart anticoagulation. Considerations for restarting anticoagulation include whether the patient is pregnant, awaiting an invasive procedure, not able to receive medication by mouth, has a high risk of rebleeding, or is being bridged back to a vitamin K antagonist with high thrombotic risk.
In most cases of GI bleeding, for example, current data support restarting oral anticoagulants once hemostasis is achieved, but patients who experience intracranial hemorrhage should delay restarting any anticoagulation for at least 4 weeks if they are without high thrombotic risk, according to the document.
The report also recommends clinician-patient discussion before resuming anticoagulation, ideally with time allowed for patients to develop questions. Discussions should include the signs of bleeding, assessment of risk for a thromboembolic event, and the benefits of anticoagulation.
“The proliferation of oral anticoagulants (warfarin and DOACs) and growing indications for their use prompted the need for guidance on the management of these drugs,” said Gordon F. Tomaselli, MD, chair of the writing committee, in an interview. “This document provides guidance on management at the time of a bleeding complication. This includes acute management, starting and stopping drugs, and use of reversal agents,” he said. “This of course will be a dynamic document as the list of these drugs and their antidotes expand,” he noted.
“The biggest change from the previous guidelines are twofold: an update on laboratory assessment to monitor drug levels and use of reversal agents,” while the acute management strategies have otherwise remained similar to previous documents, said Dr. Tomaselli.
Dr. Tomaselli said that he was not surprised by the biological aspects of recent research while developing the statement. However, “the extent of the use of multiple anticoagulants and antiplatelet agents was a bit surprising and complicates therapy with each of the agents,” he noted.
The way the pathways are presented may make them challenging to follow in clinical practice, said Dr. Tomaselli. “The pathways are described linearly and in practice often many things have to happen at once,” he said. “The other main issue may be limitations in the availability of some of the newer reversal agents,” he added.
“The complication of bleeding is difficult to avoid,” said Dr. Tomaselli, and for future research, “the focus needs to continue to refine the indications for anticoagulation and appropriate use with other drugs that predispose to bleeding. We also need better methods and testing to monitor drugs levels and the effect on coagulation,” he said.
In accordance with the ACC Solution Set Oversight Committee, the writing committee members, including Dr. Tomaselli, had no relevant relationships with industry to disclose.
SOURCE: Tomaselli GF et al. J Am Coll Cardiol. 2020. doi: 10.1016/j.jacc.2020.04.053.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY